Note: Descriptions are shown in the official language in which they were submitted.
WO 2021/041407
PCT/US2020/047791
ALKYL QUARTERNARY AMMONIUM TRYPTAMI NES AND THEIR THERAPEUTIC USES
Related Applications
[001] This application claims priority to US application 62/891,388, filed
August 25, 2019; to US
application 63/007,653, filed April 9, 2020; and to US application 63/053,976,
filed July 20, 2020; each of
which are incorporated herein by reference.
Technical Field
[002] This disclosure relates to alkyl quaternary ammonium tryptamines,
compositions and
pharmaceutical corn positions containing them as well as their use in treating
various diseases.
Background
[003] N,N-dimethyltryptamine (DMT) and its derivatives have been used by
humans for centuries
because of their psychoactive, entheogenic, or hallucinogenic effects, or
combinations thereof
(Cameron & Olson, 2018). Psilocybin, the 4-phosphate variant of DMT, is
arguably its most studied
derivative. Psilocybin is one of several naturally occurring psychoactive
tryptamines found in "magic"
mushrooms. When consumed by humans, psilocybin serves as a prodrug of
psilocin. Upon digestion,
psilocybin hydrolyses to generate psilocin, the 4-hydroxy derivative of DMT.
Psilocin is a potent
serotonin 2a-agonist, which is responsible for its psychoactive properties
(Dinis-Oliveira, 2017; Nichols,
2012).
[004] Psychoactive tryptamines like DMT and psilocin have garnered significant
interest recently
because of their potential for treating mood disorders, including depression,
anxiety, addiction, and
post-traumatic stress disorder (PTSD) (Johnson & Griffiths, 2017; Carhart-
Harris & Goodwin, 2017).
Altering the chemical structure within this class of compounds can
dramatically influence the potency
and action of the drugs. For example, merely changing the N,N-dialkyl groups
on DMT can modify its
psychoactive properties: increasing the chain length of the two alkyl groups
of the tryptamine to larger
than n-butyl dramatically reduces or eliminates the psychoactive effects
(Bradley & Johnston, 1970).
[005] The synthesis of N-methyl-N-isopropyltryptamine (MiPT) was reported in
1981 (Repke et aL,
1981). In 1985, Repke and co-workers reported that of the compounds in the
series of N,N-dialky1-4-hy-
droxytryptamines, the N-methyl-N-isopropyl derivative (4-HO-Mi PT) is the most
potent based upon
qualitative effects on humans (Repke et aL, 1985). Later quantitative studies
showed the N-methyl-N-
isopropyl derivatives of DMT and psilocin to be more potent as serotonin-1A, -
2A and -2B receptors
compared to the analogous dimethyl compounds (McKenna et aL, 1990).
1
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/04 7791
Njt
"Ann -0-
Hick34
cis
[006] New psychoactive tryptamines have been identified in "magic
mushrooms" as
recently as 2017. (Lentz, et al., 2017.) Until this year, there was no general
synthetic method for
producing useful amounts of the minor psychoactive tryptamines. (Sherwood,
Halberstadt, et al.)
One of these minor components is aeruginascin, (Jensen, et al., 2006) the N-
trimethyl analogue of
psilocybin. The limited exposure of humans to Inocybe aeruginascens mushrooms,
the only known
species in which aeruginascin has been found, has resulted in hallucinations
that exhibited only
euphoric experiences. (Gartz, 1989). This is in contrast to psilocybin and
psilocin mushrooms,
which often lead to dysphoric moods during the psychedelic experience. Despite
these
observations, the pharmacological activity of aeruginascin has remained
unexplored.
[007] Even with this previous work, there is a need to develop new psilocybin
derivatives with
improved properties for treatment of psychological disorders.
Summary of the Invention
[008] The invention relates to a compound of formula (I):
R R2
W 3
X-
R4
Rg
R6
R
8
R7
(I)
wherein
R1, R2 and R3 are each independently a straight chain or branched C1-05 alkyl
or C2- C6 alkenyl,
R4 is hydrogen, hydroxyl, C1-C6 alkoxy, -0C(0)Rs, or-0C(0)0R5,
Rs is a straight chain or branched C1-C6 alkyl,
Rs is 114 or a straight chain or branched Ci-Cs alkyl with the proviso that Rs
is not hydroxyl when RI, R2 and
R3 are all methyl,
2
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
117., Its and R9 are each independently hydrogen or a straight chain or
branched Ci-C6 alkyl, and
X- is a pharmaceutically acceptable anion. The invention also provides a
method of making compounds
of formula (I).
[009] The invention relates to compositions comprising, consisting essentially
of, or consisting of a
compound of formula (I) and an excipient. The invention also relates
pharmaceutical compositions
comprising a therapeutically effective amount of a compound of formula (I)
where the excipient is a
pharmaceutically acceptable carrier. The invention further relates to a method
of preventing or treating
a psychological disorder comprising the step of administering to a subject in
need thereof a
therapeutically effective amount of a compound of formula (I) or of a
pharmaceutical composition
containing the compound.
[010] The invention also relates to a composition comprising, consisting
essentially of, or consisting of
as a first active component: a compound of formula (I) of the disclosure; and
as a second active
component selected from (a) a sertonergic drug, (b) a purified psilocybin
derivative, (c) one or two
purified cannabinoids and (d) a purified terpene; and a pharmaceutically
acceptable excipient.
[011] The invention also relates to methods of preventing or treating
inflammation and/or pain
comprising the step of administering to a subject in need thereof a
therapeutically effective amount of a
compound of formula (I), and to administering a pharmaceutical composition or
a composition
according to the invention.
Brief Description of the Figures
[012] FIG. 1 is the fully labelled displacement ellipsoid representation (50%)
of the asymmetric unit of
crystalline 4-AcO-TMT iodide.
[013] FIG. 2 is the simulated X-ray Powder Diffraction Pattern (XRPD) for 4-
AcO-TMT iodide from its
single crystal structure.
[014] FIG. 3 is the fully labelled displacement ellipsoid representation (50%)
of the asymmetric unit of
crystalline 4-HO-TMT iodide.
[015] FIG. 4 is the simulated X-ray Powder Diffraction Pattern (XRPD) for 4-
AcO-TMT iodide from its
single crystal structure.
[016] FIG. 5 shows the molecular structure of crystalline DM PT iodide.
[017] FIG. 6 shows the packing of crystalline DMPT iodide.
[018] FIG. 7 is a simulated x-ray powder diffraction (XRPD) pattern of
crystalline DMPT iodide from its
single crystal data.
3
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
[019] FIG. 8 shows the molecular structure of crystalline DMALT iodide.
[020] FIG. 9 shows the packing of crystalline DMALT iodide.
[021] FIG. 10 is a simulated x-ray powder diffraction (XRPD) pattern of
crystalline DMALT iodide from
its single crystal data.
[022] FIG. 11 shows the molecular structure of crystalline 4-AcO-DMET iodide
hemihydrate showing
the atomic labelling.
[023] FIG. 12 is a simulated x-ray powder diffraction (XRPD) pattern of
crystalline 4-AcO-DMET iodide
hemihydrate from its single crystal data.
[024] FIG. 13 shows the molecular structure of crystalline 4-AcO-DMPT iodide
showing the atomic
labelling.
[025] FIG. 14 is a simulated x-ray powder diffraction (XRPD) pattern of
crystalline 4-AcO-DMPT iodide
from its single crystal data.
[026] FIG. 15 shows the molecular structure of 4-HO-DM PT iodide showing the
atomic labelling.
[027] FIG. 16 is a simulated x-ray powder diffraction (XRPD) pattern of
crystalline 4-HO-DM PT iodide
from its single crystal data.
Detailed Description
[028] Compounds of the Invention
[029] This invention relates to alkyl quaternary ammonium tryptamine compounds
of formula (I):
R1
2
\ R
nr--- 3
X-
R4
R9
Re
R8
R7
In formula (I), R1, R2 and R3 are each independently a straight chain or
branched Ci-C6 alkyl, for example
a straight chain Ci-C6 alkyl, or a C2-C6alkenyl, for example allyl. In some
embodiments, R1, R2 and R3 are
each independently a straight chain or branched Ci-C4 alkyl, for example a
straight chain C1-C4 alkyl, or a
C2-C4 alkenyl. R1, R2 and R3 may each independently be selected from methyl,
ethyl, n-propyl, isopropyl,
n-butyl, isobutyl and tert-butyl. In other embodiments, RI, R2 and R3 are each
methyl, are each ethyl, or
a mixture of methyl and ethyl groups. R4 is hydrogen, hydroxyl, C1-05alkoxy., -
0C(0)R5 or -0C(0)0115.
4
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
When R4 is a Ci-C6alkoxy group, or in some embodiments a C1-C4 alkoxy group,
it may be a straight chain
or branched Ci-C6alkoxy group or Ci-C4 alkoxy group, for example a straight
chain, and may be methoxy
or ethoxy. Rs is a straight chain or branched Cr-Cs alkyl or C1-C4 alkyl, for
example a straight chain Ci-C4
alkyl. In some embodiments, Rs is selected from methyl, ethyl, n-propyl or n-
butyl, and for example is
methyl or ethyl. R6 is R4 or a straight chain or branched Cr-C4 alkyl, with
the exemplary embodiments
just discussed and with the proviso that R6 is not hydroxyl when R1, R2 and R3
are all methyl. R7, Rs and
Rg are each independently hydrogen or a straight chain or branched C1-C4
alkyl, for example a straight
chain CrC4 alkyl. In some embodiments, 116, R7, R8 and Rg are each
independently selected hydrogen,
methyl, ethyl, n-propyl, isopropyl, n-butyl and isobutyl. In other
embodiments, R6, R7, R8 and Rg are each
independently hydrogen, methyl, ethyl. The anion, X-, may be any
pharmaceutically acceptable anion,
for example, Cl-, r, Br-, ascorbate, hydrofumarate, fumarate, maleate, and the
like. A preferred anion. X-,
is iodide, F. When the pharmaceutically acceptable anion is a di-anion it
balances two of the ammonium
cations.
[030] Preferred compounds of formula (I) are those where RI, R2 and R3 are
each independently a Ci-
Cis alkyl or a C2-C4 alkenyl; 114 is hydrogen, hydroxyl, Ci-C4 alkoxy, -
0C(0)R5 or -0C(0)0R5; Rs is selected
from methyl, ethyl, n-propyl and n-butyl; R6, R7, R8 and Rg are each
independently hydrogen, methyl, or
ethyl; and X- is Cl-, l-, Br, ascorbate, hydrofumarate, fumarate, or maleate.
Other preferred compounds
are those where R1, R2 and 113 are each independently methyl, ethyl, propyl or
allyl; Its is hydrogen,
hydroxyl, or -0C(0)R5; Rs is methyl or ethyl; 116, R7, and R9 are each
independently hydrogen; R8 is
hydrogen or methyl; and X- is r.
[0311 Exemplary compounds of formula (I) are:
N
0>
H (la),
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
\ /
N14---
OH I-
0 \
11 (lb)
o
\NI
--Ao i-
0 \
VI 00,
o
N
0 \
ri (Id),
\ 0----
Kr
I
0 \
ri (le),
6
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
Lre
\
(If),
se
k.
\
Ogb
1,
ii
.k)
N,
les
Nir
/
rfCeN tre
32 ;A
(11)/
\
p8
\ ..........................................
zr.:7-tt4N
rtE
Ckt 11 V,
-
(0), and
7
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
-------------------------------- 7 \
-c*E.
(1k).
[032] A compound of formula (I)
R R2
1\
rR3
X-
R0
Re
Re
R
8
R7
(I)
may be prepared formula (II) (below) with XR3, where X is I, by refluxing in
an appropriate organic
solvent, such as methanol or THF, under an inert atmosphere.
R2
Ri\N
R4
R3 opRe
R7
(II)
In a preferred embodiment, XR3 is ICH3 or ICH2CH3. When R4 or Rfi are hydroxyl
they can be converted to
esters, -0C(0)R5, or carbonate esters, -0C(0)0R5, as is known in the art.
Hydroxyl groups may be
introduced by hydrolysis of a corresponding ester. Other pharmaceutically
acceptable salts may be
prepared by anion exchange techniques known in the art to exchange the iodide
anion for a desired
pharmaceutically acceptable anion. For example, the iodide anion may be
exchanged using an anion
exchange resin.
[0331 Methods of Treatment and Therapeutic Uses
[034] In one embodiment, the compounds of formula (I), the methods, and the
pharmaceutical
compositions of the invention are used to regulate the activity of a
neurotransmitter receptor by
8
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
administering a therapeutically effective dose of a compound formula (I).
Methods of the invention
administer a therapeutically effective amount of a compound of formula (I) to
prevent or treat a
psychological disorder such as those discussed below. Compounds of formula (I)
may be administered
neat or as a pharmaceutical composition comprising a compound of formula (I)
as discussed below.
[035] Compounds of formula (I) may be used to prevent and/or treat a
psychological disorder. The
invention provides a method for preventing and/or treating a psychological
disorder by administering to
a subject in need thereof a therapeutically effective amount of a compound of
formula (I), including the
preferred embodiments discussed above. The psychological disorder may be
chosen from depression,
psychotic disorder, schizophrenia, schizophreniform disorder (acute
schizophrenic episode);
schizoaffective disorder; bipolar I disorder (mania, manic disorder, manic-
depressive psychosis); bipolar
II disorder; major depressive disorder; major depressive disorder with
psychotic feature (psychotic
depression); delusional disorders (paranoia); Shared Psychotic Disorder
(Shared paranoia disorder); Brief
Psychotic disorder (Other and Unspecified Reactive Psychosis); Psychotic
disorder not otherwise
specified (Unspecified Psychosis); paranoid personality disorder; schizoid
personality disorder;
schizotypal personality disorder; anxiety disorder; social anxiety disorder;
substance-induced anxiety
disorder; selective mutism; panic disorder; panic attacks; agoraphobia;
attention deficit syndrome, post-
traumatic stress disorder (PTSD), premenstrual dysphoric disorder (PMDD), and
premenstrual syndrome
(PMS).
[036] Compounds of formula (I) may be used to prevent and/or treat a brain
disorder. The invention
provides a method for preventing and/or treating a brain disorder by
administering to a subject in need
thereof a therapeutically effective amount of a compound of formula (I),
including the preferred
embodiments discussed above. The brain disorder may be chosen from
Huntington's disease,
Alzheimer's disease, dementia, and Parkinson's disease.
[037] Compounds of formula (I) may be used to prevent and/or treat
developmental disorders,
delirium, dementia, amnestic disorders and other cognitive disorders,
psychiatric disorders due to a
somatic condition, drug-related disorders, schizophrenia and other psychotic
disorders, mood disorders,
anxiety disorders, somatoform disorders, factitious disorders, dissociative
disorders, eating disorders,
sleep disorders, impulse control disorders, adjustment disorders, or
personality disorders. The invention
provides a method for preventing and/or treating these disorders by
administering to a subject in need
thereof a therapeutically effective amount of a compound of formula (I),
including the preferred
embodiments discussed above.
9
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
[038] A compound of formula (I) according to the invention may be used to
prevent and/or treat
inflammation and/or pain, such as, for example, inflammation and/or pain
associated with inflammatory
skeletal or muscular diseases or conditions. Accordingly the invention relates
to a method for
preventing and/or treating inflammation and/or pain by administering to a
subject in need thereof a
therapeutically effective amount of a compound of formula (I), including the
preferred embodiments
discussed herein. Generally speaking, treatable "pain" includes nociceptive,
neuropathic, and mix-type.
A method of the invention may reduce or alleviate the symptoms associated with
inflammation,
including, but not limited to, treating localized manifestation of
inflammation characterized by acute or
chronic swelling, pain, redness, increased temperature, or loss of function in
some cases. A method of
the invention may reduce or alleviate the symptoms of pain regardless of the
cause of the pain,
including, but not limited to, reducing pain of varying severity, i.e. mild,
moderate and severe pain,
acute pain and chronic pain. A method of the invention is effective in
treating joint pain, muscle pain,
tendon pain, burn pain, and pain caused by inflammation such as rheumatoid
arthritis. Skeletal or
muscular diseases or conditions which may be treated include, but are not
limited to, musculoskeletal
sprains, musculoskeletal strains, tendinopathy, peripheral radiculopathy,
osteoarthritis, joint
degenerative disease, polymyalgia rheumatica, juvenile arthritis, gout,
ankylosing spondylitis, psoriatic
arthritis, systemic lupus erythematosus, costochondritis, tendonitis,
bursitis, such as the common lateral
epicondylitis (tennis elbow), medial epicondylitis (pitchers elbow) and
trochanteric bursitis,
temporomandibular joint syndrome, and fibromyalgia.
[039] Compositions
[040] The invention relates to compositions comprising, consisting essentially
of, or consisting of an
effective amount of a compound of formula (I), including its preferred
embodiments discussed above,
and an excipient. In another embodiment of the invention, the invention also
relates to pharmaceutical
compositions comprising, consisting essentially of, or consisting of a
therapeutically effective amount of
a compound of formula (I) according to the invention, including its preferred
embodiments discussed
above, and a pharmaceutically acceptable excipient (also known as a
pharmaceutically acceptable
carrier). As discussed above, a compound of formula (I) according to the
invention may be, for example,
therapeutically useful to prevent and/or treat the psychological disorders,
brain disorders, pain and
inflammation as well as the other disorders discussed above.
[041] A composition or a pharmaceutical composition of the invention may be in
any form which
contains a compound of formula (I) according to the invention. The composition
may be, for example, a
tablet, capsule, liquid suspension, injectable, topical, or transdermal. The
compositions generally
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
contain, for example, about 1% to about 99% by weight of a compound of formula
(I) and, for example,
99% to 1% by weight of at least one suitable pharmaceutically acceptable
excipient. In one
embodiment, the composition may be between about 5% and about 75% by weight of
a compound of
formula (I) with the rest being at least one suitable pharmaceutically
acceptable excipient or at least one
other adjuvant, as discussed below.
[042] Published US applications US 2018/0221396 Al and US 2019/0142851 Al
disclose compositions
comprising a combination of a first purified psilocybin derivative with a
serotonergic drug, a second
purified psilocybin derivative, with one or two purified cannabinoids or with
a purified terpene. Various
ratios of these components in the composition are also disclosed. The
disclosures of US 2018/0221396
Al and US 2019/0142851 Al are incorporated herein by reference. According to
this invention, a
compound of formula (I) of the invention, and the preferred embodiments
described herein, may be
used as the "first purified psilocybin derivative" in the compositions
described in US 2018/0221396 Al
and US 2019/0142851 Al. Accordingly, this invention provides a composition
comprising as a first
component: a compound of formula (I) of the disclosure; and as a second
component selected from (a) a
sertonergic drug, (b) a purified psilocybin derivative, (c) one or two
purified cannabinoids and (d) a
purified terpene; with the rest being at least one suitable pharmaceutical
excipient or at least one other
adjuvant, as discussed below. Such a composition may be a pharmaceutical
composition wherein the
components are present individually in therapeutic effective amounts or by
combination in a
therapeutically effective amount to treat a disease, disorder or condition as
described herein.
[043] A serotonergic drug refers to a compound that binds to, blocks, or
otherwise influences (e.g., via
an allosteric reaction) activity at a serotonin receptor as described in
paragraphs [0245]-[0253] of US
2018/0221396 Al and [0305140311] US 2019/0142851 Al as well as the disclosed
preferred
embodiments, incorporated here by reference. Some exemplary serotonergic drugs
include the
following molecules: 6-Allyl-N,N-diethyl-NL, N,N-Dibutyl-T, N,N-Diethyl-T, N,N-
Diisopropyl-T, 5-
Methyoxy-alpha-methyl-T, N,N-Dimethyl-T, 2,alpha-Dimethyl-T, alpha,N-Dimethyl-
T, N,N-Dipropyl-T, N-
Ethyl-N-isopropyl-T, alpha-Ethyl-T, 6,N,N-Triethyl-NL, 3,4-Dihydro-7-methoxy-l-
methyl-C, 7-Methyoxy-1-
methyl-C, N,N-Dibuty1-4-hydroxy-T, N,N-Diethyl-4-hydroxy-T, N,N-Diisopropy1-4-
hydroxy-T, N,N-
Dimethy1-4-hydroxy-T, N,N-Dimethy1-5-hydroxy-T, N, N-Dipropy1-4-hydroxy-T, N-
Ethy1-4-hydroxy-N-
methyl-T, 4-Hydroxy-N-isopropyl-N-methyl-T, 4-Hydroxy-N-methyl-N-propyl-T, 4-
Hydroxy-N,N-
tetramethylene-T lbogaine, N,N-Diethyl-L, N-Butyl-N-methyl-T, N,N-Diisopropy1-
4,5-methylenedioxy-T,
N,N-Diisopropy1-5,6-methylenedioxy-T, N,N-Dimethy1-4,5-methylenedioxy-T, N,N-
Dimethy1-5,6-
methylenedioxy-T, N-Isopropyl-N-methyl-5,6-methylenedioxy-T, N,N-Diethyl-2-
methyl-T, 2,N,N-
11
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
Trimethyl-T, N-Acetyl-5-methoxy-T, N,N-Diethyl-5-methoxy-T, N,N-Diisopropy1-5-
methoxy-T, 5-Methoxy-
N,N-dimethyl-T, N-Isopropyl-4-methoxy-N-methyl-T, N-Isopropy1-5-methoxy-N-
methyl-T, 5,6-
Dimethoxy-N-isopropyl-N-methyl-T, 5-Methoxy-N-methyl-T, 5-Methoxy-N,N-
tetramethylene-T, 6-
Methoxy-1-methy1-1,2,3,4-tetrahydro-C, 5-Methoxy-2,N,N-trimethyl-T, N,N-
Dimethy1-5-methylthio-T, N-
Isopropyl-N-methyl-T, alpha-Methyl-T, N-Ethyl-T, N-Methyl-T, 6-Propyl-N 1_,
N,N-Tetramethylene-T,
Tryptamine, and 7-Methoxy-1-methy1-1,2,3,4-tetrahydro-C, alpha,N-Dimethy1-5-
methoxy-T. For
additional information regarding these compounds See Shulgin, A. T., &
Shulgin, A. (2016). Tihkal: The
Continuation. Berkeley, Calif.: Transform Press. In one embodiment, a
serotonergic drug is chosen from
alprazolam, amphetamine, aripiprazole, azapirone, a barbiturate, bromazepam,
bupropion, buspirone, a
cannabinoid, chlordiazepoxide, citalopram, clonazepam, clorazepate,
dextromethorphan, diazepam,
duloxetine, escitalopram, fluoxetine, flurazepam, fluvoxamine, lorazepam,
lysergic acid diethylamide,
lysergamide, 3,4-methylenedioxymethamphetamine, milnacipran, mirtazapine,
naratriptan, paroxetine,
pethidine, phenethylamine, psic.aine, oxazepam, reboxetine, serenic,
serotonin, sertraline, temazepam,
tramadol, triazolam, a tryptamine, venlafaxine, vortioxetine, and/or
derivatives thereof.
[044] Exemplary psilocybin derivatives include but are not limited to
psilocybin itself and the
psilocybin derivates described in paragraphs [0081]-[0109] of US 2018/0221396
Al and [082]-[0110] US
2019/0142851 Al as well as the disclosed preferred embodiments, incorporated
here by reference. In
one embodiment, the compositions disclosed herein comprise one or more
purified psilocybin
derivatives chosen from: [3-(2-Dimethylaminoethyl)-1H-indol-4-y11 dihydrogen
phosphate, 4-
hydroxytryptamine, 4-hydroxy-N,N-dimethyltryptamine, [3-(2-methylaminoethyl)-
1H-indol-4-yl]
dihydrogen phosphate, 4-hydroxy-N-methyltryptamine, [3-(aminoethyl)-1H-indo1-4-
yl] dihydrogen
phosphate, [3-(2-trimethylaminoethyl)-1H-indo1-4-yl] dihydrogen phosphate, and
4-hydroxy-N,N,N-
trimethyltryptamine.
[045] Exemplary cannabinoids include but are not limited to the cannabinoids
described in paragraphs
[0111]40159] of US 2018/0221396 Al and [0112]10160] US 2019/0142851 Al as well
as the disclosed
preferred embodiments, incorporated here by reference. Examples of
cannabinoids within the context
of this disclosure include the following molecules: Cannabichromene (CRC),
Cannabichromenic acid
(CBCA), Cannabichromevarin (CBCV), Cannabichromevarinic acid (CBCVA),
Cannabicyclol (CBL),
Cannabicyclolic acid (CBLA), Cannabicyclovarin (CBLV), Cannabidiol (CBD),
Cannabidiol monomethylether
(CBDM), Cannabidiolic acid (CBDA), Cannabidiorcol (CBD-C1), Cannabidivarin
(CBDV), Cannabidivarinic
acid (CBDVA), Cannabielsoic acid B (CBEA-B), Cannabielsoin (CBE),
Cannabielsoin acid A (CBEA-A),
Cannabigerol (CBG), Cannabigerol monomethylether (CBGM), Cannabigerolic acid
(CBGA),
12
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
Cannabigerolic acid monomethylether (CBGAM), Cannabigerovarin (CBGV),
Cannabigerovarinic acid
(CBGVA), Cannabinodiol (CBND), Cannabinodivarin (CBDV), Cannabinol (CBN),
Cannabinol methylether
(CBNM), Cannabinol-C2 (CBN-C2), Cannabinol-C4 (CBN-C4), Cannabinolic acid
(CBNA), Cannabiorcool
(CBN-C1), Cannabivarin (CBV), Cannabitriol (CBT), Cannabitriolvarin (CBTV), 10-
Ethoxy-9-hydroxy-delta-
6a-tetrahydrocannabinol, Cannbicitran (CRT), Cannabiripsol (CBR), 8,9-
Dihydroxy-delta-6a-
tetrahydrocannabinol, Delta-8-tetrahydrocannabinol (.DELTA.8-THC), Delta-8-
tetrahydrocannabinolic
acid (.DELTA.8-THCA), Delta-9-tetrahydrocannabinol (THC), Delta-9-
tetrahydrocannabinol-C4 (THC-C4),
Delta-9-tetrahydrocannabinolic acid A (THCA-A), Delta-9-tetrahydrocannabinolic
acid B (THCA-B), Delta-
9-tetrahydrocannabinolic acid-C4 (THCA-C4), Delta-9-tetrahydrocannabiorcol
(THC-C1), Delta-9-
tetrahydrocannabiorcolic acid (THCA-C1), Delta-9-tetrahydrocannabivarin
(THCV), Delta-9-
tetrahydrocannabivarinic acid (THCVA), 10-0xo-delta-6a-tetrahydrocannabinol
(OTHC),
Cannabichromanon (CBCF), Cannabifuran (CBF), Cannabiglendol, Delta-9-cis-
tetrahydrocannabinol (cis-
THC), Tryhydroxy-delta-9-tetrahydrocannabinol (tri0H-THC), Dehydrocannabifuran
(DCBF), and 3,4,5,6-
Tetrahydro-7-hydroxy-alpha-alpha-2-trimethy1-9-n-propy1-2,6-metha- no-2H-1-
benzoxocin-5-methanol.
In one embodiment the purified cannabinoid is chosen from THC, THCA, THCV,
THCVA, CBC, CBCA,
CBCV, CBCVA, CBD, CBDA, CBDV, CBDVA, CBG, CBGA, CBGV, or CBGVA.
[046] Exemplary terpenes include but are not limited to the terpenes described
in paragraphs [0160]-
[0238] of US 2018/0221396 Al and [0161]40300] US 2019/0142851 Al as well as
the disclosed
preferred embodiments, incorporated here by reference. In one embodiment, a
purified terpene is
chosen from acetanisole, acetyl cedrene, anethole, anisole, benzaldehyde,
hornyl acetate, borneol,
cadinene, cafestol, caffeic acid, camphene, camphor, capsaicin, carene,
carotene, carvacrol, carvone,
alpha-caryophyllene, beta-caryophyllene, caryophyllene oxide, cedrene, cedrene
epoxide, cecanal,
cedrol, cembrene, cinnamaldehyde, cinnamic acid, citronella!, citronellol,
cymene, eicosane, elemene,
estragole, ethyl acetate, ethyl cinnamate, ethyl maltol, eucalypto1/1,8-
cineole, eudesmol, eugenol,
euphol, farnesene, farnesol, fenchone, geraniol, geranyl acetate, guaia-
1(10),11-diene, guaiacol, guaiol,
guaiene, gurjunene, herniarin, hexanaldehyde, hexanoic acid, humulene, ionone,
ipsdienol, isoamyl
acetate, isoamyl alcohol, isoamyl formate, isoborneol, isomyrcenol, isoprene,
isopulegol, isovaleric acid,
lavandulol, limonene, gamma-linolenic acid, linalool, longifolene, lycopene,
menthol, methyl butyrate, 3-
mercapto-2-methylpentanal, beta-mercaptoethanol, mercaptoacetic acid, methyl
salicylate,
methylbutenol, methyl-2-methylvalerate, methyl thiobutyrate, beta-myrcene,
gamma-muurolene,
nepetalactone, nerol, nerolidol, neryl acetate, nonanaldehyde, nonanoic acid,
ocimene, octanal,
octanoic acid, pentyl butyrate, phellandrene, phenylacetaldehyde, phenylacetic
acid, phenylethanethiol,
13
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
phytol, pinene, propanethiol, pristimerin, pulegone, retinol, rutin, sabinene,
squalene, taxadiene,
terpineol, terpine-4-ol, terpinolene, thujone, thymol, umbelliferone,
undecanal, verdoxan, or vanillin. In
one embodiment, a purified terpene is chosen from bornyl acetate, alpha-
bisabolol, borneol, camphene,
camphor, carene, beta-caryophyllene, cedrene, cymene, elemene, eucalyptol,
eudesmol, farnesene,
fenchol, geraniol, guaiacol, humulene, isoborneol, limonene, linalool,
menthol, beta-myrcene, nerolidol,
ocimene, phellandrene, phytol, pinene, pulegone, sabinene, terpineol,
terpinolene, or valencene.
[047] A "therapeutically effective amount" of a compound of formula (I)
according to the invention is
generally in the range of about 0.1 to about 100 mg daily (oral dose), of
about 0.1 to about 50 mg daily
(oral dose) of about 0.25 to about 25 mg daily (oral dose), of about 0.1 to
about 5 mg daily (oral dose) or
of about 0.5 to about 2.5 mg daily (oral dose). The actual amount required for
treatment of any
particular patient may depend upon a variety of factors including, for
example, the disease being
treated and its severity; the specific pharmaceutical composition employed;
the age, body weight,
general health, sex, and diet of the patient; the mode of administration; the
time of administration; the
route of administration; and the rate of excretion; the duration of the
treatment; any drugs used in
combination or coincidental with the specific compound employed; and other
such factors well known
in the medical arts. These factors are discussed in Goodman and Gilman's 'The
Pharmacological Basis of
Therapeutics," Tenth Edition, A. Gilman, J. Hardman and L. Limbird, eds.,
McGraw-Hill Press, 155-173
(2001), which is incorporated herein by reference. A compound of formula (I)
according to the invention
and pharmaceutical compositions containing it may be used in combination with
other agents that are
generally administered to a patient being treated for psychological and other
disorders discussed above.
They may also be co-formulated with one or more of such agents in a single
pharmaceutical
composition.
[048] Depending on the type of pharmaceutical composition, the
pharmaceutically acceptable carrier
may be chosen from any one or a combination of carriers known in the art. The
choice of the
pharmaceutically acceptable carrier depends upon the pharmaceutical form and
the desired method of
administration to be used. Preferred carriers include those that do not
substantially alter the salt of
ritonavir or produce undesirable biological effects or otherwise interact in a
deleterious manner with
any other component(s) of the pharmaceutical composition.
[049] The pharmaceutical compositions of the invention may be prepared by
methods know in the
pharmaceutical formulation art, for example, see Remington's Pharmaceutical
Sciences, 18th Ed., (Mack
Publishing Company, Easton, Pa., 1994), which is incorporated herein by
reference. In a solid dosage
form, a compound of formula (I) may be admixed with at least one
pharmaceutically acceptable
14
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
excipient such as, for example, sodium citrate or dicalcium phosphate or (a)
fillers or extenders, such as,
for example, starches, lactose, sucrose, glucose, mannitol, and silicic acid,
(b) binders, such as, for
example, cellulose derivatives, starch, alignates, gelatin,
polyvinylpyrrolidone, sucrose, and gum acacia,
(c) humectants, such as, for example, glycerol, (d) disintegrating agents,
such as, for example, agar-agar,
calcium carbonate, potato or tapioca starch, alginic acid, croscarmellose
sodium, complex silicates, and
sodium carbonate, (e) solution retarders, such as, for example, paraffin, (f)
absorption accelerators, such
as, for example, quaternary ammonium compounds, (g) wetting agents, such as,
for example, cetyl
alcohol, and glycerol monostearate, magnesium stearate and the like, (h)
adsorbents, such as, for
example, kaolin and bentonite, and (i) lubricants, such as, for example, talc,
calcium stearate,
magnesium stearate, solid polyethylene glycols, sodium lauryl sulfate, or
mixtures thereof. In the case
of capsules, tablets, and pills, the dosage forms may also comprise buffering
agents.
[1:1501 Excipients or pharmaceutically acceptable adjuvants known in the
pharmaceutical formulation
art may also be used in the pharmaceutical compositions of the invention.
These include, but are not
limited to, preserving, wetting, suspending, sweetening, flavoring, perfuming,
emulsifying, and
dispensing agents. Prevention of the action of microorganisms may be ensured
by inclusion of various
antibacterial and antifungal agents, for example, parabens, chlorobutanol,
phenol, sorbic acid, and the
like. It may also be desirable to include isotonic agents, for example,
sugars, sodium chloride, and the
like. If desired, a pharmaceutical composition of the invention may also
contain minor amounts of
auxiliary substances such as wetting or emulsifying agents, pH buffering
agents, antioxidants, and the
like, such as, for example, citric acid, sorbitan monolaurate, triethanolamine
oleate, butylated
hydroxytoluene, etc.
[0511 Solid dosage forms as described above may be prepared with coatings and
shells, such as enteric
coatings and others well known in the art. They may contain pacifying agents
and can also be of such
composition that they release the active compound or compounds in a certain
part of the intestinal tract
in a delayed manner. Non-limiting examples of embedded compositions that may
be used are polymeric
substances and waxes. The active compounds may also be in microencapsulated
form, if appropriate,
with one or more of the above-mentioned excipients.
[052] Suspensions, in addition to the active compounds, may contain suspending
agents, such as, for
example, ethoxylated isostearyl alcohols, polyoxyethylene sorbitol and
sorbitan esters, microcrystalline
cellulose, aluminum metahydroxide, bentonite, agar-agar and tragacanth, or
mixtures of these
substances, and the like.
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
[053] Solid dosage forms for oral administration, which includes capsules,
tablets, pills, powders, and
granules, may be used. In such solid dosage forms, the active compound may be
mixed with at least one
inert, pharmaceutically acceptable excipient (also known as a pharmaceutically
acceptable carrier).
[054] Administration of a compound of formula (I) in pure form or in an
appropriate pharmaceutical
composition may be carried out via any of the accepted modes of administration
or agents for serving
similar utilities. Thus, administration may be, for example, orally, buccally,
nasally, parenterally
(intravenous, intramuscular, or subcutaneous), topically, transdermally,
intravaginally, intravesically, or
intrasystemically, in the form of solid, semi-solid, lyophilized powder, or
liquid dosage forms, such as, for
example, tablets, suppositories, pills, soft elastic and hard gelatin
capsules, powders, solutions,
suspensions, or aerosols, or the like, such as, for example, in unit dosage
forms suitable for simple
administration of precise dosages. One route of administration may be oral
administration, using a
convenient daily dosage regimen that can be adjusted according to the degree
of severity of the disease-
state to be treated.
[055] Examples
[056] Example 1: 4-Acetoxy-N,N,N-trimethyltryptammonium Iodide (4-AcO-TMT
iodide)
[057] 4-acetoxy-N,N-dimethyltryptammine (4-AcO-DMT) fumarate was stirred in a
1:1 solution of
methanol and iodomethane and heated to reflux for four hours. Solvent is
removed in vacua, and a white
powder is obtained after triturating and washing the resulting residue with
tetrahydrofuran. 1H NMR (400
MHz, D20): 57.46 (d, J = 8.1 Hz, 1H, ArH), 7.33 (s, 1H, ArH), 7.27 (t, I = 8.0
Hz, 1H, ArH), 6.90 (d, J = 7.8 Hz,
1H, ArH), 3.63 (t, J = 7.5 Hz, 2H, Cl-!2), 3.27 (t, J = 8.6 Hz, 2H, CH2), 321
(s, 9H, NC!-!3), 2.47 (s, 3H, C(0)CH4.
Unit Cell: a = 7.8459(9) A, b = 9.8098(12) A, c = 11.0823(12) A, a = 909, f3 =
101.069(3)9, y = 90`1, V =
837.10(17) A3.
[058] Second Preparation of 4-AcO-TMT iodide: 250 mg of 4-acetoxy-N,N-
dimethyltryptammonium (4-
AcO-DMT) fumarate was dissolved in 10 mL of methanol in a 50 mL round bottom
flask, and 10 ml_ of
iodomethane was then added. The mixture was stirred for 24 hours under an
atmosphere of dinitrogen.
The solvent was removed in vacuo. The resulting powder was washed with diethyl
ether and filtered to
yield 313 mg of yellow powder. This powder was dissolved in 75 mL of acetone.
The solution was heated
with stirring and reduced in volume to 40 mL. The mixture was cooled in an ice
bath, yielding a white
precipitate. The powder was filtered to yield 142 mg of white powder (53.01%
yield). 1H NMR (400 MHz,
D20): 6 7.46 (d, 3 = 8.6 Hz, 1 H, ArH), 7.34 (s, 1 H, ArH), 7.22 (t, J = 7.8
Hz, 1 H, ArH), 6.95 (d, 3 = 8.3 Hz,
1H, ArH) 3.63 (t, J = 7.9 Hz, 2 H, Cl-i2), 3.28 (t,1 = 8.0 Hz, 2 H, Cl-I2),
3.21 (s, 9 H, CH3), 2.47 (s, 3 H,
C(0)C1-13); 13C NMR (100 MHz, D20): 6 174.32 (C=0), 143.59 (ArC), 139.28
(ArC), 125.82 (ArC), 123.01
16
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
(ArC), 119.11 (ArC), 112.92 (ArC), 111.13 (ArC), 107.32 (ArC), 67.64 (NCH2),
53.73 (NCH3), 21.25 (CH2),
20.48 (C(0)CH3). Elemental analysis calcd. for CisH21N2021: C 46.40, H 5.45, N
7.22; Found: C 46.17, H
5.35, N 7.11.
[059] X-Ray data collection and refinement details for 4-AcO-TMT iodide:
Crystals suitable for X-ray
diffraction studies were grown from the slow evaporation of an aqueous
solution. All operations were
performed on a Bruker D8 Venture CMOS diffractometer, using Mo Ka radiation
with a TRIUMPH
monochromator at a temperature of 298 K. Data collection was carried out using
the Bruker APEX3
software. Cell refinement and data reduction were performed with the SAINT
program. The structure
solution was done with SHELKS and structure refinement was performed with
SHELXL. Further
refinement and molecular graphics were generated using the OLEX2 and Mercury
CSD software.
[060] All non-hydrogen atoms were refined anisotropically (XL) by full matrix
least squares on F2.
Hydrogen atom H1 was found from a Fourier difference map, and refined with a
fixed distance of 0.87 A.
Isotropic displacement parameters were set to 1.20 times Ueci of the parent N
atoms. The remaining
hydrogen atoms were placed in calculated positions and then refined with a
riding model with C-H
lengths of 0.93 A (sp2), 0.96 A (0-6) and 0.97 A (cm) with isotropic
displacement parameters set to 1.20
(sp2 and CH2) and 1.50 (CH3) times IJE.q of the parent C atom. Further details
are in Table 1. FIG. 11s the
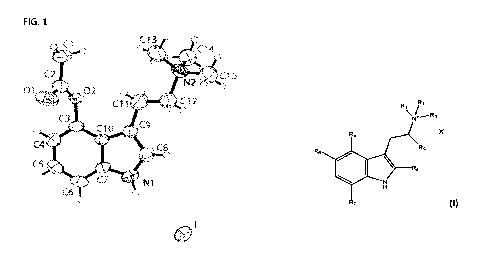
fully labelled displacement ellipsoid representation (50%) of the asymmetric
unit of 4-AcO-TMT iodide.
Table 1. Crystal data and structure refinement for 4-AcO-TMT iodide.
Empirical formula C15H21IN202
Formula weight 388.24
Temperature/K 299.05
Crystal system Monoclinic
Space group P21
a/A 7.8459(9)
b/A 9.8098(12)
c/A 11.0823(12)
PP 101.069(3)
VP 90
Volume/A3 837.10(17)
2
pcakg/cm3 1.540
Rim ncl 1.916
F(000) 388.0
Crystal size/mm3 0.2 x 0.1 x 0.05
Radiation MoKa (A. = 0.71073)
17
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
20 range for data collection/9 5.866 to 50.852
Index ranges -9 .1-19,-11
Reflections collected 18328
Independent reflections 3076 [Rim = 0.0424,
Rsigma = 0.02981
Data/restraints/parameters 3076/2/189
Goodness-of-fit on F2 1.064
Final R indexes [I 2o (I)] R1 = 0.0300, wR2 =
0.0548
Final R indexes [all data] R1= 0.0396, wR2 = 0.0583
Largest cliff. peak/hole / e 0.51/-0.40
Flack parameter -0.001(11)
[061] FIG. 2 is a simulated x-ray powder diffraction (XRPD) pattern of
crystalline 4-Acetoxy-N,N,N-
trimethyltryptammonium Iodide (4-AcO-TMT iodide) from its single crystal data.
Crystalline 4-Acetoxy-
N,N,N-trimethyltryptammonium Iodide (4-AcO-TMT iodide) may be characterized by
the XRPD peaks at
8.1, 14.6 and 19.8 -n 0.29213 as well as by an XRPD pattern substantially
similar to Fla 2.
[062] Example 2: 4-Hydroxy-N,N,N-trimethyltryptammonium (4-HO-TMT) Iodide
[063] 4-acetoxy-N,N,N-trimethyltryptammonium (4-AcO-TMT) iodide was stirred in
a 1:1 solution of
water and acetic acid in air for 12 hours. Solvent is removed in vacua, and a
white powder is obtained
after triturating and washing the resulting residue with tetrahydrofuran. 1H
NMR (400 MHz, D20): 6 7.18
(s, 1H, ArH), 7.12-7.06 (m, 2H, ArH), 6.57 (dd, J = 6.0, 2.4 Hz, 1H, ArH),
3.62 (t, J = 7.8 Hz, 2H, CH4, 3.37
(t, 3 = 8.1 Hz 2H, CH2), 3.19 (s, 9H, NCH3). Unit Cell: a = 11.3057(9) A, b =
11.2370(10) A, c= 12.7785(10)
A, a = 909, p = 113.087(2)9, y = 909, V = 1493.4(2) A3.
[064] Second preparation of 4-HO-TMT iodide: 95 mg of 4-Ac0 TMT iodide was
dissolved in 4 mL of
deionized (DI) water in a 50 mL round bottom flask. 20 mL of acetic acid was
added to the mixture, and
it was refluxed under an atmosphere of dinitrogen for 2 days. Solvent was
removed in vacuo to obtain a
green/blue oil. 3 mL of methanol and 20 mL of ethyl acetate were added to the
green/blue oil, leaving a
green/blue powder that was removed via filtration. Solvent was removed in
vacua. The resulting oil was
dissolved n 5 mL of ethanol, and 30 mL of pentane was added to generate a
precipitate. The resulting
powder was isolated via filtration to give 53.1 g (60% yield) to give an off-
white powder. 1H NMR (400
MHz, D20): 6 7.19 (s, 1 H, ArH), 7.12-7.07 (m, 2 H, ArH), 6.56 (dd, J = 5.9,
2.5 Hz, 1 H, ArH), 3.66-3.62 (m,
2 H, CH2), 3.40-3.36 (m, 2 H, CH2), 3.20 (s, 9 H, CH4; nc NMR (100 MHz, D20):
5 150.62 (ArC), 139.37
(ArC), 123.89 (ArC), 123.86 (ArC), 116.56 (ArC), 108.72 (ArC), 105.23 (ArC),
104.43 (ArC), 68.20 (NCH2),
53.59 (NCH3), 21.20 (Cl-I2). Elemental analysis calcd. For C13H19N201: C
45.10, H 5.53, N 8.09; Found: C
44.84, H 5.23, N 7.98.
18
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
[065] X-Ray data collection and refinement details for 4-HO-TMT iodide:
Crystals suitable for X-ray
diffraction studies were grown from the slow evaporation of an aqueous
solution. All operations were
performed on a Bruker D8 Venture CMOS diffractometer, using Mo Ka radiation
with a TRIUMPH
monochromator at a temperature of 200 K. Data collection was carried out using
the Bruker APEX3
software. Cell refinement and data reduction were performed with the SAINT
program. The structure
solution was done with SHELXS and structure refinement was performed with
SHEXL. Further
refinement and molecular graphics were generated using the OLEX2 and Mercury
CSD software.
[066] All non-hydrogen atoms were refined anisotropically (XL) by full matrix
least squares on F2.
Hydrogen atom H1 and HlA were found from a Fourier difference map, and refined
with a fixed distance
of 0.86 A and 0.85 A respectively. Isotropic displacement parameters were set
to 1.20 times Ueq of the
parent N atom, and 1.50 times Unq of the parent 0 atom. The remaining hydrogen
atoms were placed in
calculated positions and then refined with a riding model with C¨H lengths of
0.95 A (spl, 0.98 A (Cl-I2)
and 0.99 A (GO with isotropic displacement parameters set to 1.20 (sp2 and Cl-
h) and 1.50 (CH3) times
Uet, of the parent C atom. Further details are in Table 2. FIG. 3 is the fully
labelled displacement ellipsoid
representation (50%) of the asymmetric unit of 4-HO-TMTI.
Table 2. Crystal data and structure refinement for 4-HO-TMT iodide.
Empirical formula C13H19IN20
Formula weight 346.20
Temperature/K 200.0
Crystal system Monoclinic
Space group P2i/n
a/A 11.3057(9)
b/A 11.2370(10)
c/A 12.7785(10)
a.r 90
13P 113.087(2)
Yie 90
Volume/A3 1493.4(2)
Z 4
pcakg/cm3 1.540
it/mm_i 2.133
F(000) 688.0
Crystal size/mm3 0.4 x 0.2 x 0.1
Radiation MoKa (X = 0.71073)
20 range for data collection/. 6.164 to 52.884
Index ranges ¨13 5 h 5 14, ¨14 5
k 5 14, ¨15 5 I 5 15
Reflections collected 29269
Independent reflections 3063 [Rim = 0.0564,
Rsignia = 0.0277]
19
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
Data/restraints/parameters 3063/2/161
Goodness-of-fit on F2 1.073
Final R indexes [I 2, (I)] R1 = 0.0368, wR2 =
0.0797
Final R indexes [all data] Ri = 0.0574, wR2 =
0.0885
Largest cliff. peak/hole / e A-3 1.05/-0.93
[067] FIG. 4 is a simulated x-ray powder diffraction (XRPD) pattern of
crystalline 4-Hydroxy-N,N,N-
trimethyltryptammonium Iodide (4-HO-TMT iodide) from its single crystal data.
Crystalline 4-Hydroxy-
N,N,N-trimethyltryptammonium Iodide (4-HO-TMT iodide) may be characterized by
the XRPD peaks at
17.0, 18.1 and19.5 20 0.2 20 as well as by an XRPD pattern substantially
similar to FIG. 4.
[068] Example 3: Cellular Assays
[069] Cellular assays were performed by Eurofins CEREP SA, Celle-Levescault,
France. All receptors
were separately expressed in HEK-293 cells. Cell membrane homogenates (30 lig
protein) are incubated
for 60 min (5-HT1A, 5-EIT2A, 5-HT2B) or 120 min (5-HT3) at 22 C with
radiolabeled ligand in the absence or
presence of the test compound in a buffer containing 50 mM Tris-HCI (pH 7.4),
5 mM MgCl2, 10 p.M
pargyline and 0.1% ascorbic acid. For 5-HT3, the buffer contained 50 mM Tris-
HCI (pH 7.4), 5 mM MgCl2,
and 1mM EDTA. Binding was reported as the K1 for the inhibition of binding of
well-characterized
orthosteric ligands. The ligands used for each receptor were:
5-HT1A: [3H] 8-0H-DPAT
5-HT2A: [125.] tri = 1
D01
5-HT2R: [1251] ( )D01
5-HT3: [3H] BRL 43694
[070] Nonspecific binding was determined in the presence of 1 p.M unlabeled
ligand listed above.
Following incubation, the samples were filtered rapidly under vacuum through
glass fiber filters (GF/B,
Packard) presoaked with 0.3% PEI and rinsed several times with ice-cold 50 mM
Tris-HCI using a 96-
sample cell harvester (Unifilter, Packard). The filters were dried then
counted for radioactivity in a
scintillation counter (Topcount, Packard) using a scintillation cocktail
(Microscint 0, Packard). The results
are expressed as a percent inhibition of the control radioligand specific
binding.
[071] The IC50 values and Hill coefficients (nH) were determined by non-linear
regression analysis of
the competition curves generated with mean replicate values using Hill
equation curve fitting Y=D-F[ (A-
D) / (1+(C/C50) nil) ] where Y = specific binding, A = left asymptote of the
curve, D = right asymptote of the
curve, C = compound concentration, Co = ICso, and nH = slope factor.
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
[072] Analysis was performed using software developed at Cerep (Hill software)
and validated by
comparison with data generated by the commercial software SigmaPlot 4.0 for
Windows (0 1997 by
SPSS Inc.). The inhibition constants (KO were calculated using the Cheng
Prusoff equation: K= ICso
(1+LAD), where L = concentration of radioligand in the assay, and KD =
affinity of the radioligand for the
receptor. A scatchard plot was used to determine the Kr).
[073] The results in terms of inhibition constants (K) are shown in Table 3.
The aeruginascin active
metabolite, 4-HO-TMTI, shows activity at 5-HT1A, 5-HT2A and 5-HT2B. Counter to
the prevailing theory
that aeruginascin should function as a powerful 5-1-11-3 agonist, there is no
activity observed at this
receptor. The aeruginascin functional analogue, 4-AcO-TMTI shows no activity
at any of the receptors.
For comparison, psilocybin, the pro-drug of psilocin, shows no activity at 5-
HT1A, 5-HT2A, nor 5-HT3, but
does show itself to be a potent 5-HT2B agonist. Psilocin, its active
metabolite, shows activity at 5-HT1A
and 5-HT2A that is more active though comparable to 4-HO-TMTI. (Roth et al.)
It is significantly more
potent than 4-HO-TMTI at the 5-HT2B receptor, and in fact, psi locybin is more
active at this receptor as
well.
Table 3. Inhibition constants (K) in nM units.
Compound 5-HTIA 5-HT2A 5-HT2B 5-HT3
4-HO-TMTI 4,400 670 120 >10,000
4-Ac0-
>10,000 >10,000 >10,000 >10,000
TMTI
Psilocint 567.4 107.2 4.6
>10,000
Psilocybint >10,000 >10,000 98.7 >10,000
[074] Example 4: N,N-di-methyl-N-propyl-tryptammonium (DMPT) iodide
[075] N,N-di-methyl-N-propyl-tryptammonium (DMPT) iodide was prepared by
mixing 101 mg of a
commercial sample of N-methyl-N-propyl-tryptamine (The Ind le Shop) and 4 ml
of methyl iodide in 4
mL of methanol. The mixture was refluxed for twelve hours under an atmosphere
of nitro-gen. The
solvent was removed in vacuo, and the remaining residue was recrystallized
from ethanol to yield
colorless single crystals suitable for X-ray diffraction studies. The product
was also characterized by
nuclear magnetic resonance. 1H N MR (400 MHz, D20): d 7.69 (d, I = 8.0 Hz, 1
H, ArH), 7.55 (d, J = 8.2 Hz,
1 H, ArH), 7.33-7.28 (m, 2 H, Ad-1), 7.22 (t, J = 7.0 Hz, 1 H, ArH), 3.60 (m,
2 H, Cl-h), 3.36 (m, 4 H, Cl-I2),
3.17 (s, 6 H, CH3), 1.82 (m, 2 H, Cl-I2), 0.97 (t, J = 7.0 Hz, 3 H, Cl-I3).
[076] The molecular structure of crystalline DMPT iodide is shown in FIG. 5.
Crystal data, data
collection and structure refinement details are summarized in Table 4. The
asymmetric unit contains
one N,N-di-methyl-N-n-propyl tryptammonium (C15H23N21 cation and one iodide
anion. The indole ring
21
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
of the cation is near, planar with a mean deviation from planarity of 0.011 A.
The ethyl-ammonium arm
is turned away from the plane with a C7¨C8¨C9¨C10 torsion angle of 89.1 (4) .
The DMPT cation and
the iodide anion are held together in the asymmetric unit via N(1)¨H(1)-1(1)
hydrogen bonds, between
the indole nitrogen and the iodide. The packing of crystalline DMPT iodide is
shown in FIG. 6.
[077] Crystal data, data collection and structure refinement details are
summarized in Table 4.
Table 4: Crystalline DM PT Iodide
Chemical formula I-C3H23N2
Mr 358.25
Crystal system, space group Monoclinic, P21/c
Temperature (K) 303
a, 13, C (A) 7.4471 (6), 9.9016 (9),
22.052 (2)
[3 el 94.184(3)
V (A3) 1621.8 (2)
Z 4
Radiation type Mo Ka
m (mm-1) 1.96
Crystal size (mm) 0_40 x 0.14 x 0_12
Diffractometer Bruker D8 Venture CMOS
Absorption correction Multi-scan
SADABS201612 (Bruker,2016/2) was used for absorption
correction. wR2(int) was 0.0600 before and 0.0507 after
correction. The Ratio of minimum to maximum transmission is
0.8362. The I/2 correction factor is Not present.
Trniny Trnax 0.470, 0.562
No. of measured, independent and 44530, 3071, 2362
observed [I> 2s(I)] reflections
Rint 0.036
(sin q/l)max (k1) 0.611
RIF2> 2s(F2)], wR(F2), S 0.028, 0.054, 1.13
No. of reflections 3071
No. of parameters 170
No. of restraints 1
H-atom treatment H atoms treated by a
mixture of independent and constrained
22
CA 03149602 2022-2-25
WO 20211041407
PCT/US2020/047791
refinement
DiPmax,13Pmin (e A-3) 0.53, -0.47
Absolute structure ¨
Absolute structure parameter ¨
Computer programs: APEX3 (Bruker, 2018), SAINT (Bruker, 2018), SHELXT2014
(Sheldrick, 2015a),
SHELXL2018 (Sheldrick, 2015b), 01ex2 (Dolomanov et at, 2009), pubICIF
(Westrip, 2010).
Table 5 Hydrogen-bond geometry (A, 2) for Crystalline DMPT Iodide
D¨H---A D¨H H---A D---
A D¨H---A
N1¨H1===11 0.86 (1) 2.91 (2)
3.733 (3) 162 (3)
[078] FIG. 7 is a simulated x-ray powder diffraction (XRPD) pattern of
crystalline N,N-dimethyl-N-
propyl (DMPT) iodide from its single crystal data. Crystalline N,N-dimethyl-N-
propyl (DMPT) iodide may
be characterized by the XRPD peaks at 8.0, 17. 9 and 23.3 20 0.2 20 as well
as by an XRPD pattern
substantially similar to FIG. 7.
[079] Example 5: N,N-di-methyl-N-allyl-tryptammonium (DMALT) iodide
[080] N,N-di-methyl-N-allyl-tryptammonium (DMALT) iodide was prepared by
mixing 101 mg of a
commercial sample of N-allyl-N-methyl-tryptamine (The Indole Shop) with 4 mL
of methyl iodide in 4 mL
of methanol. The mixture was refluxed for twelve hours under an atmosphere of
nitrogen. The solvent
was removed in vacuo, and the remaining residue was recrystallized from
acetone to yield colorless
crystals suitable for X-ray diffraction studies. The product was also
characterized by nuclear magnetic
resonance. 1H NMR (400 MHz, D20): d 7.69 (d, J = 7.8 Hz, 1 H, ArH), 7.55 (d, J
= 8.2 Hz, 1 H, ArH), 7.32-
7.28 (m, 1 H, ArH), 7.22 (t, 1 = 7.2 Hz, 1 H, ArH), 6.13-6.03 (m, 1 H, CH),
5.77-5.71 (m, 2 H, CH2), 4.04 (d, 1
= 7.3 Hz, 1 H, CH2), 3.61-3.56 (m, 2 H, CH2), 3.37-3.32 (m, 2 H, CH2), 3.17
(s, 6 H, CH3).
[0811 The molecular structure of crystalline DMALT iodide is shown in FIG. 8.
The asymmetric unit
contains one N-allyl-N,N-di-methyl-tryptammonium (C15E1211421 cation and one
iodide anion. The indole
ring of the cation is near planar, with a mean deviation from planarity of
0.013 A. The ethyl-ammonium
arm is turned away from the plane with a C7¨C8¨C9¨C10 torsion angle of 101.9
(9)*. The allyl group is
disordered over two orientations with a 0.30(4) to 0.70(4) occupancy ratio for
C14, C15 and C14A,
C15A, respectively. The DMALT structure is very similar to that of DMPT, with
the ions held together in
the asymmetric unit through N(1)¨H(1)-1(1) hydrogen bonds. The packing of
crystalline DMALT iodide is
shown in FIG. 9.
[082] Crystal data, data collection and structure refinement details are
summarized in Table 6.
23
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
Table 6: Crystalline DMALT Iodide
Chemical formula 0.5(1)Ø5(C151-121N2)
Mr 178.12
Crystal system, space group Monoclinic, P21
Temperature (K) 303
a, b, c (A) 73471 (8), 9.9672 (9),
10.9499 (11)
fi (e) 94.671 (3)
V (A3) 799.20 (14)
Z 4
Radiation type Mo Ka
m (mm-1) 1.99
Crystal size (mm) 0.39 x 0.22 x 0.15
Diffractometer Bruker 08 Venture CMOS
Absorption correction Multi-scan SADABS2016/2
(Bruker,2016/2) was used for
absorption correction. wR2(int) was 0.0671 before and 0.0484
after correction. The Ratio of minimum to maximum
transmission is 0.8154. The 1/2 correction factor is Not present.
;lin/ Tmax 0.608, 0.745
No. of measured, independent and 26314, 3038, 2868
observed [I> 25(1)] reflections
Rint 0.031
(sin q/l)max (k1) 0.611
RIF2> 2s(F2)1, wR(F2), S 0.027, 0.071, 1.13
No. of reflections 3038
No. of parameters 174
No. of restraints 5
H-atom treatment H-atom parameters
constrained
DPmax, DPmm (e k3) 0.46, -0.48
Absolute structure Refined as an inversion
twin.
Absolute structure parameter 0.29 (5)
Computer programs: APEX3 (Bruker, 2018), SAINT (Bruker, 2018), SHELXT2014
(Sheldrick, 2015a),
SHELXL2018 (Sheldrick, 2015b), Olex2 (Dolomanov et at, 2009), pubICIF
(Westrip, 2010).
Table 7 Hydrogen-bond geometry (A, 2) for Crystalline MALT Iodide
D¨H---A D¨H H---A D--A
D¨H---A
24
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
N1-H1-11 0.86 2.95
3.727 (6) 152
[083] FIG. 10 is a simulated x-ray powder diffraction (XRPD) pattern of
crystalline NN-dimethyl-N-ally1
(DMALT) iodide from its single crystal data. Crystalline N,N-dimethyl-N-
allyl(DMALT) iodide may be
characterized by the XRPD peaks at 12.0, 18.5 and 23.4 020 0.2 29 as well as
by an XRPD pattern
substantially similar to FIG.10.
[084] Example 6: 4-acetoxy-NN-dimethyl-N-ethyltryptammonium (4-AcO-DMET)
iodide
[085] Synthesis: 300 mg of 4-acetoxy-N,N-dimethyl- tryptammonium (4-AcO-DMT)
fumarate was
dissolved in 30 ml of tetrahydrofuran, and 6 ml of iodoethane was added. The
mixture was refluxed
overnight under an atmosphere of nitrogen. This resulted in the precipitation
of a white powder from
the yellow solution. The precipitate was isolated via vacuum filtration to
give white powder, which was
washed with diethyl ether to yield 303 mg of pure product (91% yield). 1H NMR
(400 MHz, D20): 5 7.46
(dd, J = 8.2, 0.7 Hz, 1 H, ArH), 7.33 (s, 1 H, ArH), 7.25 (t, 1 = 7.9 Hz, 1 H,
ArH), 6.90 (dd, 1 = 7.7, 0.7 Hz, 1 H,
ArH), 3.58-3.54 (m, 2 H, CH2), 3.46 (q, 1 = 7.3 Hz, 2 H, CH2), 3.25-3.20 (m, 2
H, CH2), 3.12 (s, 6 H, CH3), 2.47
(s, 3 H, (CO)CH4, 1.38-1.35 (m, 3 H, CH4; 13C NMR (100 MHz, 020): 5 174.3(C0),
143.6 (ArC), 139.2(ArC),
125.7 (ArC), 123.0 (ArC), 119.1 (Ara 112.9 (ArC), 111.1 (ArC), 107.4 (ArC),
64.6 (AkC), 60.6 (Aka 50.7
(AkC), 21.3 (AkC), 19.9 (AkC), 8.1 (AkC).
[086] 4-AcO-DMET iodide was recrystallized by slow evaporation of an ethanol
solution to yield
crystals suitable for X-ray diffraction studies. Crystal data, data collection
and structure refinement
details are summarized in Table 8. FIG. 11 shows the molecular structure of
crystalline 4-AcO-DMET
iodide hemihydrate showing the atomic labelling. Displacement ellipsoids are
drawn at the 50%
probability level. There are two distinct tryptammonium cations and two
iodides in the asymmetric unit.
The solvate water molecule is modeled at 50% occupancy.
Table 8
Crystal data
2(1)-2(C16H23N202)-0.5(H20) F(000) = 818
Mr= 81333 Dx = 1.471 Mg
m-3
Monoclinic, P21 Mo Ka
radiation, I = 0.71073 A
a = 11.8538 (8) A Cell
parameters from 9080 reflections
Li = 10.3179 (7) A q = 3.0-25.7
c = 15.0132 (10) A m = 1.75 mm-1
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
= 90.611 (2) T= 297 K
V= 1836.1 (2) A3 BLOCK,
colourless
Z= 2 0.23 x 0.22 x
0.21 mm
Data collection
Bruker D8 Venture CMOS 6527
reflections with I> 2s(!)
diffractometer
f and w scans Rint = 0.029
Absorption correction: multi-scan qmax = 25.7 ,
gram = 2.7
SADABS2016/2 (Bruker,2016/2) was used
for absorption correction. wR2(int) was
0.0602 before and 0.0524 after correction.
The Ratio of minimum to maximum
transmission is 0.9438. The 1/2 correction
factor is Not present.
Trnm = 0.531, Tmax = 0.562 h = -141 14
47080 measured reflections k = -12.12
6821 independent reflections I = -18 18
Refinement
Refinement on F2 Hydrogen site
location: mixed
Least-squares matrix: full H atoms
treated by a mixture of
independent and constrained refinement
R1F2> 2s(F2)] = 0.029 w = 1/[52(F02)
+ (0.0298P)2+ 1.2326P]
where P= (F02 + 2F/)/3
wR(F2) = 0.072 (D/s). = 0.001
S = 1.05 Drimax = 0.66
e k3
6821 reflections Mims, = -0.46
e k3
410 parameters Absolute
structure: Flack x determined
using 2968 quotients [(1+)-(1-)1/[(1-04-(1-)]
(Parsons, Flack and Wagner, Ada Cryst.
B69 (2013) 249-259).
6 restraints Absolute
structure parameter: -0.009 (5)
[087] FIG. 12 is a simulated x-ray powder diffraction (XRPD) pattern of
crystalline 4-acetoxy-N,N-
dimethyl-N-ethyltryptammonium (4-AcO-DMET) iodide hemihydrate from its single
crystal data.
26
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
Crystalline 4-acetoxy-N,N-dimethyl-N-ethyltryptammonium (4-Ac0-DMET) iodide
hemihydrate may be
characterized by the XRPD peaks at 11.4, 14.6 and 19.2 020 0.2 20 as well as
by an XRPD pattern
substantially similar to FIG. 12.
[088] Example 7: 4-hydroxy-N,N-dimethyl-N-ethyltryptammonium (4-HO-DMET)
iodide
[089] Synthesis: 150 mg of 4-AcO-DMET iodide was dissolved in 2 mL of DI
water, and 10 ml_ of acetic
acid was added. The mixture was refluxed overnight under an atmosphere of
nitrogen. The solvent was
removed via distillation, yielding an orange sticky oil. The oil was dissolved
in a small volume of
tetrahydrofuran and acetone. Hexanes was added to the solution, producing a
white precipitate. The
powder was isolated via vacuum filtration to give 90 mg (67% yield) of pure
product. 1H NMR (400 MHz,
D20):45 7.19 (s, 1 H, ArH), 7.12-7.07 (m, 2 H, ArH), 6.59-6.54 (m, 1 H, ArH),
3.60-3.56 (m, 2 H, CH2), 3.46
(q, l = 7.3 Hz, 2 H, CH2), 3.35-3.31 (m, 2 H, CH2), 3.12 (s, 6 H, CH3), 1.39
(t, 1 = 7.3 Hz, 3 H, CH4; 13C NMR
(100 MHz, D20): 5 150.6 (ArC), 139.3(ArC), 1219 (ArC), 116.6 (ArC), 108.8
(ArC), 1052 (ArC), 104.4 (Ara
65.0 (AkC), 60.3 (AkC), 50.7 (AkC), 20.7 (AkC), 8.0 (AkC).
[090] Example 8: 4-acetoxy-N,N-dimethyl-N-n-propyltryptammonium (4-AcO-DMPT)
iodide
[091] Synthesis: 323 mg of 4-AcO-DMT fumarate was dissolved in 30 mL of
tetrahydrofuran, and 6 mL
of 1-iodopropane was added. The mixture was refluxed overnight under an
atmosphere of nitrogen. This
resulted in the precipitation of a white powder from the yellow solution. The
precipitate was isolated via
vacuum filtration to give a white powder, which was washed with diethyl ether
to yield 314 mg of pure
product (85% yield). 3-H NMR (400 MHz, 020): 57.46 (dd, I = 8.2, 0.5 Hz, 1 H,
ArH), 7.33 (s, 1 H, ArH), 7.26
(t, .1 = 7.9 Hz, 1 H, ArH), 6.90 (d, J = 7.7 Hz, 1 H, ArH), 3.61-3.57 (m, 2 H,
CH2), 3.35-3.29 (m, 2 H, CH2),
3.27-3.21 (m, 2 H, CH2), 3.14 (s, 6 H, CH3), 2.47 (s, 3 H, (CO)CH3), 1.81-1.72
(m, 2 H, CH2), 0.91 (t, 1= 7.3
Hz, 3 H, CH3); 13C NMR (100 MHz, D20): 5 174.3(C0), 143.6 (ArC), 139.3(ArC),
125.8 (ArC), 123.0 (ArC),
119.1 (ArC), 112.9 (ArC), 111.1 (ArC), 107.5 (ArC), 66.4 (AkC), 65.1 (AkC),
51.3 (AkC), 21.3 (AkC), 20.1
(AkC), 16.3 (AkC), 10.4 (AkC).
[092] 4-AcO-DMPT iodide was recrystallized by slow evaporation of an ethanol
solution to yield
crystals suitable for X-ray diffraction studies. Crystal data, data collection
and structure refinement
details are summarized in Table 9. FIG. 13 shows the molecular structure of
crystalline 4-AcO-DMPT
iodide showing the atomic labelling. Displacement ellipsoids are drawn at the
50% probability level.
Dashed bonds indicate a disordered component in the structure.
Table 9
Crystal data
27
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
1=C17H25N202 F(000) = 420
Mr= 416.29 Dx = 1.495 Mg
m-3
Monoclinic, P21 Mo Ka
radiation, I = 0.71073 A
a = 7.7067 (4) A Cell
parameters from 9480 reflections
b = 10.3424 (4) A q = 2.7-25.7
c = 11.6302(6) A m = 1.74 mm-1
13= 94.222 (2r T= 273 K
V= 924.48 (8) A3 BLOCK,
colourless
Z=2 0.23 x 0.2 x
0.07 mm
Data collection
Bruker D8 Venture CMOS 3251
reflections with I> 25(/)
diffractometer
f and w scans flint = 0.030
Absorption correction: multi-scan climax = 25.8%
chain = 2.7
SADA852016/2 (Bruker,2016/2) was used
for absorption correction. wR2(int) was
0.0659 before and 0.0527 after correction.
The Ratio of minimum to maximum
transmission is 0.8900. The 1/2 correction
factor is Not present.
Trnbn = 0.663, Tmax = 0.745 h = 9 9
32264 measured reflections k = -12.12
3428 independent reflections / = -149.4
Refinement
Refinement on F2 Hydrogen site
location: mixed
Least-squares matrix: full H atoms
treated by a mixture of
independent and constrained refinement
R1F2> 2s(F2)] = 0.035 w = 1/Es2u,-
02.
1 + (0.0233P)2+ 1.32821'1
where P = (F02 + 2Fc2)/3
wR(P)= 0.081 (D/s)max
<0.001
S = 1.04 Minim = 1.09 e
A-3
3428 reflections Dtimin = -0.78
e A-3
243 parameters Absolute
structure: Flack x determined
28
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
using 1473 quotients [(l+)-(I-)]/[(14-)+(1-)]
(Parsons, Flack and Wagner, Acta Cryst_
B69 (2013) 249-259).
35 restraints Absolute
structure parameter: 0.009 (7)
[093] FIG. 14 is a simulated x-ray powder diffraction (XRPD) pattern of
crystalline 4-acetoxy-N,N-
dimethyl-N-n-propyltryptammonium (4-AcO-DMPT) iodide from its single crystal
data. Crystalline 4-
acetoxy-N,N-dimethyl-N-n-propyltryptammonium (4-AcO-DMPT) iodide may be
characterized by the
XRPD peaks at 11.5, 16.7 and 19.8 '20 0.2 28 as well as by an XRPD pattern
substantially similar to FIG.
14.
[094] Example 9: 4-hydroxy-NN-dimethyl-N-n-propyltryptammonium (4-HO-DMPT)
iodide
[095] Synthesis: 200 mg of 4-AcO-DMPT iodide was dissolved in 3 mL of
deionized (DI) water, and 10
mL of acetic acid was added. The mixture was refluxed overnight under an
atmosphere of nitrogen. The
solvent was removed via distillation, yielding an orange sticky oil. The oil
was dissolved in a small volume
of tetrahydrofuran and acetone. Hexanes was then added to the solution,
producing a light green
precipitate. This powder was isolated via vacuum filtration and washed with
diethyl ether to give 157 mg
(87 A yield) of pure product. 1H NMR (400 MHz, D20): 6 7.17 (s, 1 H, ArH),
7.12-7.07 (m, 2 H, ArH), 6.57-
6.55 (m, 1 H, ArH), 3.57-3.53 (in, 2 H, CH2), 3.32-3.26 (m, 4 H, CH2), 3.11
(s, 6 H, CH3), 1.85-1.75 (m, 2 H,
CH2), 0.95 (t, .1 = 7.3 Hz, 3 H, CH4; 13C NMR (100 MHz, D20): 5 150.6 (ArC),
139.3(ArC), 123.9 (ArC), 116.7
(ArC), 108.9 (ArC), 105.2 (ArC), 104.4 (ArC), 66.1 (AkC), 65.4 (AkC), 51.3
(AkC), 20.8 (AkC), 16.2 (AkC),
10.4 (AkC).
[096] 4-HO-DMPT iodide was recrystallized by slow evaporation of an ethanol
solution to yield crystals
suitable for X-ray diffraction studies. Crystal data, data collection and
structure refinement details are
summarized in Table 10. FIG. 15 shows the molecular structure of 4-HO-DMPT
iodide showing the
atomic labelling. Displacement ellipsoids are drawn at the 50% probability
level.
Table 10
Crystal data
1-C151-123N20 F(000) = 752
Mr= 374.25 Dx = 1.487 Mg
m-3
Monoclinic, P21/c Mo Ka
radiation, I = 0.71073 A
a = 9.4296 (8) A Cell
parameters from 9954 reflections
b = 14.1816 (11) A q = 2.7-25.7
29
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
c = 13.2586 (10) A m = 1.91 mm'
13= 109.423 (3) T= 297 K
V= 1672.1 (2) A3 BLOCK,
colourless
Z=4 0.25 x 0.2 x
0.19 mm
Data collection
Bruker D8 Venture CMOS 2808
reflections with I> 25(/)
diffractometer
f and w scans Rint = 0.025
Absorption correction: multi-scan climax = 25.7%
chain = 2.7*
5ADA852016/2 (Bruker,2016/2) was used
for absorption correction. wR2(int) was
0.0578 before and 0.0440 after correction.
The Ratio of minimum to maximum
transmission is 0.9109. The 1/2 correction
factor is Not present.
Trnir = 0.679, Tmax = 0.745 h = -11 11
38248 measured reflections k = -17 17
3102 independent reflections / = -16 16
Refinement
Refinement on F2 Hydrogen site
location: mixed
Least-squares matrix: full H atoms
treated by a mixture of
independent and constrained refinement
R[F2> 2s(F2)] = 0.027 w = 1/(52(F02)
+ (0.0259P)2+ 1.432211
where P = (F02 + 2Fc2)13
wR(F2)= 0.069 (D/s), = 0.001
S = 1.05 Dñmax = 0.85 e
A-3
3102 reflections Dñmm = -0.64 e
A-3
182 parameters Extinction
correction: SHELXL201813
(Sheldrick 2018),
Fe=kFc[1+0.001xFc213/sin(2q)14/4
16 restraints Extinction
coefficient: 0.0082 (16)
Primary atom site location: structure-
invariant direct methods
CA 03149602 2022-2-25
WO 2021/041407
PCT/US2020/047791
[097] FIG. 16 is a simulated x-ray powder diffraction (XRPD) pattern of
crystalline 4-hydroxy-N,N-
dimethyl-N-n-propyltryptammonium (4-HO-DMPT) iodide from its single crystal
data. Crystalline 4-
hydroxy-N,N-dimethyl-N-n-propyltryptammonium (4-HO-DMPT) iodide may be
characterized by the
XRPD peaks at 115, 18.9 and 19.9 20 0.2 20 as well as by an XRPD pattern
substantially similar to FIG.
16.
[098] Example 10: 4-acetoxy-N,N-dimethyl-N-isopropyltryptammonium (4-AcO-
DM1PT) iodide
[099] Synthesis: 320 mg of 4-AcO-DMT fumarate was dissolved in 30 ml of
tetrahydrofuran, and 12
mL of 2-iodopropane was added. The mixture was retluxed overnight under an
atmosphere of nitrogen.
A mixture of orange/yellow solid and yellow liquid was obtained. The liquid
was decanted and the
remaining solid was triturated with ethyl acetate to yield a white powder. The
powder was isolated via
vacuum filtration to give 151 mg of pure product (41% yield). NMR (400 MHz,
D20): 67.46 (dd, J=
8.2, 0.7 Hz, 1 H, ArH), 7.33 (s, 1 H, ArH), 7.26 (t, 1= 7.9 Hz, 1 H, ArH),
6.89 (dd, 3 = 7.7, 0.6 Hz, 1 H, ArH),
3.86-3.76 (sep, 3= 6.6 Hz, 1 H, CH), 3.57-3.53 (m, 2 H, CH2), 3.26-3.22 (m, 2
H, CH2), 3.07 (s, 6 H, CH3),
2.47 (s, 3 H, (CO)CH4, 1.41 (d, J= 6.6 Hz, 6 H, CH3); 13C NMR (100 MHz, D20):
5 174.2(C0), 143.6 (ArC),
139.2 (ArC), 125.7 (ArC), 123.0 (ArC), 119.1 (ArC), 113.0 (ArC), 111.1 (ArC),
107.5 (ArC), 66.0 (AkC), 63.5
(AkC), 48.1 (AkC), 21.3 (AkC), 19.7 (AkC), 16.2 (AkC).
References
Bradley, R. J. & Johnston, V. S. (1970). Origin and Mechanism of
Hallucinations, edited by W. Keup, pp.
333-344. New York: Plenum Press.
Cameron, L P. & Olson, D. E. (2018). AG Chem. Neurosci. 9, 2344-2357.
Carhart-Harris, R. L. & Goodwin, G. M. (2017). Neuropsychopharmacology, 42,
2105-2113.
Dinis-Oliveira, R. J. (2017). Drug Metab. Rev. 49,84-91.
Johnson, M. W. & Griffiths, R. R. (2017). Neurotherapeutics 14, 734-740.
McKenna, D. J., Repke, D. B., Lo, L & Peroutka, S. J. (1990).
Neuropharmacology, 29, 193-198.
Nichols, D. E. (2012). WiREs Membr. Transp. Signal. 1, 559-579.
Repke, D. B., Grotjahn, 11 B. & Shulgin, A. T. (1985). J. Med. Chem. 28, 892-
896.
C. Lenz, J. Wick and D. Hoffmeister, J. Nat. Prod., 2017, 80, 2835-2838.
A. M. Sherwood, A. L. Halberstadt, A. K. Klein, J. D. McCorvy, K. W. Kaylo, R.
B. Kargbo and P.
Meisenheimer, J. Nat. Prod., Article ASAP, DOI: 10.1021/acs.jnatprod.9b01061.
N. Jensen, J. Gartz and H. Laatsch, Planta Med., 2006, 72, 665-666.
J. Gartz, kit. J. Crude Drug Res., 1989, 27, 141-144.
B. L. Roth, W. K. Kroeze, S. Patel and E. Lopez, The Neuroscientist, 2000, 6,
252-262.
31
CA 03149602 2022-2-25