Note: Descriptions are shown in the official language in which they were submitted.
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
INTERCELLULAR ADHESION MOLECULE 1 (ICAM1) ANTIBODY DRUG
CONJUGATE AND USES THEREOF
RELATED APPLICATION
This Application claims the benefit under 35 U.S.C. 119(e) of U.S.
Provisional
Application Serial No. 62/891,170, entitled "INTERCELLULAR ADHESION MOLECULE 1
(ICAM1) ANTIBODY DRUG CONJUGATE AND USES THEREOF" filed on August 23,
2019, the entire contents of which are incorporated herein by reference.
BACKGROUND
Pancreatic cancer (PC) remains one of the most lethal diseases and accounts
for 56,770
people death in the United States within 2019, representing 7% of all cancer
mortality. The
prognosis for PC patients is strikingly poor with a 5yr survival less than 8%
despite of the
recent intensified studies of immunotherapy and nanomedicine therapy.
SUMMARY
The present disclosure is based, at least in part, on the surprising finding
that
intercellular adhesion molecule 1 (ICAM1) can be targeted to improve
pancreatic cancer
treatment and stratify patient populations for precision medicine. The
immunosuppressive
microenvironment of pancreatic cancer tumors presents several challenges to
effective
treatment. For example, the tumor microenvironment of pancreatic cancer tumors
is often
characterized by desmoplastic stroma and poor vascularization, which create
physical barriers
that prevent T-cells or drugs from efficiently infiltrating the tumors. These
limitations are
addressed, at least in part, by the present disclosure.
Provided herein, in some aspects, antibody-drug conjugates (ADCs) that
comprise an
intercellular adhesion molecule 1 (ICAM1), which are useful for treatment of
pancreatic
cancer. As described below, use of the ADCs comprising an ICAM1 antibody
allowed for
preferential targeting of pancreatic cancer cells over non-cancerous cells,
which can improve
the therapeutic window of drugs and limit toxicity. Predicting therapeutic
sensitivity among
patient populations is also challenging given the high genetic heterogeneity
of pancreatic
cancer. Accordingly, further aspects of the present disclosure provide methods
of identifying
patient populations for treatment with an ICAM1 antibody or an ADC comprising
an ICAM1
antibody in a subject with pancreatic cancer.
Aspects of the present disclosure provide methods of treating pancreatic
cancer
comprising administering to a subject in need thereof an effective amount of
an antibody drug
1
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
conjugate (ADC) comprising an intercellular adhesion molecule 1 (ICAM1)
antibody
conjugated to a drug.
In some embodiments, the drug is selected from the group consisting of: N2'-
Deacetyl-
N2'-(3-mercapto-1-oxopropyl)mertansine (DM1), monomethyl auristatin E (MMAE),
monomethyl auristatin F (MMAF), and duocarmycin. In some embodiments, the drug
is DM1.
In some embodiments, the ICAM1 antibody and the drug is conjugated via a
linker.
In some embodiments, the linker is a cleavable linker. In some embodiments,
the
cleavable linker is selected from the group consisting of: N-succinimidyl 4-(2-
pyridyldithio)pentanoate (SPP), N-succinimidyl 3-(2-pyridyldithio)butanoate
(SPDB), Sulfo-
SPDB, valine-citrulline (Val-cit), acetyl butyrate, and CL2A. In some
embodiments, the
cleavable linker is Val-cit.
In some embodiments, the linker is a non-cleavable linker. In some
embodiments, the
non-cleavable linker is a selected from the group consisting of N-succinimidyl
4-
(Nmaleimidomethyl)cyclohexane-1-carboxylate (SMCC) and maleimidomethyl
cyclohexane-
1-carboxylate (MCC). In some embodiments, the non-cleavable linker is a N-
succinimidyl 4-
(Nmaleimidomethyl)cyclohexane-1-carboxylate (SMCC) linker.
In some embodiments, the ICAM1 antibody is selected from the group consisting
of an
IgG, an Ig monomer, a Fab fragment, a F(ab')2 fragment, a Fd fragment, a scFv,
a scAb, a
dAb, a Fv, an affibody, a diabody, a single domain heavy chain antibody, and a
single domain
light chain antibody.
In some embodiments, the ICAM1 antibody is Enlimomab or HCD54.
In some embodiments, the ratio of the ICAM1 antibody and the drug in the ADC
is 1:1
to 1:10. In some embodiments, the ratio of the ICAM1 antibody and the drug in
the ADC is
1:4.
In some embodiments, the ADC is administered via injection. In some
embodiments,
the injection is intravenous injection or intratumoral injection.
Further aspects of the present disclosure provide methods of treating
pancreatic cancer
comprising administering to a subject in need thereof an effective amount of
an antibody drug
conjugate (ADC) comprising an intercellular adhesion molecule 1 (ICAM1)
antibody
conjugated to N2'-Deacetyl-N2'-(3-mercapto-1-oxopropyl)mertansine (DM1) via a
N-
succinimidyl 4-(Nmaleimidomethyl)cyclohexane-1-carboxylate (SMCC) linker.
Further aspects of the present disclosure provide methods of predicting the
responsiveness of treatment with an ICAM1 antibody or an antibody drug
conjugate (ADC)
comprising an intercellular adhesion molecule 1 (ICAM1) antibody conjugated to
a drug in a
2
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
subject having pancreatic cancer comprising: (i) administering to the subject
an effective
amount of an ICAM1 antibody labeled with an imaging agent; and (ii)
visualizing the tumor
via imaging; (iii) determining the level of ICAM1 on the tumor, wherein a
higher level of
ICAM1 indicates that the subject is more responsive to treatment with the
ICAM1 antibody or
the ADC compared to a subject having a lower level of ICAM1(e.g., identifying
the subject as
being more responsive to the treatment if the level of ICAM1 is higher,
compared to a subject
with a tumor having a lower level of ICAM1).
In some embodiments, the ICAM1 antibody in (i) is labeled with DTPA-Gd.
In some embodiments, the visualizing in (ii) is via magnetic resonance imaging
(MRI).
In some embodiments, the method further comprises administering an effective
amount
of the ICAM1 antibody or the ADC to the subject predicted to be responsive to
the treatment to
treat the pancreatic cancer.
In some embodiments, the drug is selected from the group consisting of: N2'-
Deacetyl-
N2'-(3-mercapto-1-oxopropyl)mertansine (DM1), monomethyl auristatin E (MMAE),
monomethyl auristatin F (MMAF), and duocarmycin. In some embodiments, the drug
is DM1.
In some embodiments, the ICAM1 antibody and the drug is conjugated via a
linker.
In some embodiments, the linker is a cleavable linker. In some embodiments,
the
cleavable linker is selected from the group consisting of: N-succinimidyl 4-(2-
pyridyldithio)pentanoate (SPP), N-succinimidyl 3-(2-pyridyldithio)butanoate
(SPDB), Sulfo-
SPDB, valine-citrulline (Val-cit), acetyl butyrate, and CL2A. In some
embodiments, the
cleavable linker is Val-cit.
In some embodiments, the linker is a non-cleavable linker. In some
embodiments, the
non-cleavable linker is a selected from the group consisting of N-succinimidyl
4-
(Nmaleimidomethyl)cyclohexane-1-carboxylate (SMCC) and maleimidomethyl
cyclohexane-
1-carboxylate (MCC). In some embodiments, the non-cleavable linker is a N-
succinimidyl 4-
(Nmaleimidomethyl)cyclohexane-1-carboxylate (SMCC) linker.
In some embodiments, the ICAM1 antibody is selected from the group consisting
of an
IgG, an Ig monomer, a Fab fragment, a F(ab')2 fragment, a Fd fragment, a scFv,
a scAb, a
dAb, a Fv, an affibody, a diabody, a single domain heavy chain antibody, and a
single domain
light chain antibody.
In some embodiments, the ICAM1 antibody is Enlimomab or HCD54.
In some embodiments, the ratio of the ICAM1 antibody and the drug in the ADC
is 1:1
to 1:10.
In some embodiments, the ratio of the ICAM1 antibody and the drug in the ADC
is 1:4.
3
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
Further aspects of the present disclosure provide an antibody drug conjugate
(ADC)
comprising an intercellular adhesion molecule 1 (ICAM1) antibody conjugated to
a drug.
In some embodiments, the drug is selected from the group consisting of: N2'-
Deacetyl-
N2'-(3-mercapto-1-oxopropyl)mertansine (DM1), monomethyl auristatin E (MMAE),
monomethyl auristatin F (MMAF), and duocarmycin. In some embodiments, the drug
is DM1.
In some embodiments, the ICAM1 antibody and the drug is conjugated via a
linker.
In some embodiments, the linker is a cleavable linker. In some embodiments,
the
cleavable linker is selected from the group consisting of: N-succinimidyl 4-(2-
pyridyldithio)pentanoate (SPP), N-succinimidyl 3-(2-pyridyldithio)butanoate
(SPDB), Sulfo-
SPDB, valine-citrulline (Val-cit), acetyl butyrate, and CL2A. In some
embodiments, the
cleavable linker is Val-cit.
In some embodiments, the linker is a non-cleavable linker. In some
embodiments, the
non-cleavable linker is a selected from the group consisting of N-succinimidyl
4-
(Nmaleimidomethyl)cyclohexane-1-carboxylate (SMCC) and maleimidomethyl
cyclohexane-
1-carboxylate (MCC). In some embodiments, the non-cleavable linker is a N-
succinimidyl 4-
(Nmaleimidomethyl)cyclohexane-1-carboxylate (SMCC) linker.
In some embodiments, the ICAM1 antibody is selected from the group consisting
of an
IgG, an Ig monomer, a Fab fragment, a F(ab')2 fragment, a Fd fragment, a scFv,
a scAb, a
dAb, a Fv, an affibody, a diabody, a single domain heavy chain antibody, and a
single domain
light chain antibody.
In some embodiments, the ICAM1 antibody is Enlimomab or HCD54.
In some embodiments, the ratio of the ICAM1 antibody and the drug in the ADC
is 1:1
to 1:10. In some embodiments, the ratio of the ICAM1 antibody and the drug in
the ADC is
1:4.
Further aspects of the present disclosure provide an antibody drug conjugate
(ADC)
comprising an intercellular adhesion molecule 1 (ICAM1) antibody conjugated to
N2'-
Deacetyl-N2'-(3-mercapto-1-oxopropyl)mertansine (DM1) via a N-succinimidyl 4-
(Nmaleimidomethyl)cyclohexane-1-carboxylate (SMCC) linker.
BRIEF DESCRIPTION OF THE DRAWINGS
The accompanying drawings are not intended to be drawn to scale. In the
drawings,
each identical or nearly identical component that is illustrated in various
FIGs. is represented
by a like numeral. For purposes of clarity, not every component may be labeled
in every
drawing.
4
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
In the drawings:
FIGs. 1A-1H show that ICAM1 is differentially overexpressed in human PC
tissues
and cells. FIG. 1A is a heatmap of membrane proteins expression in human PC
cells,
compared with pancreatic normal epithelial cells. FIG. 1B is a Venn diagram
showing the
overlaps between the selected target sets for PC cells. FIG. 1C shows the top
10 upregulated
surface proteins in PC cells, and ICAM1 expression level in human PC cell
lines. FIG. 1D
contains images showing IF staining of ICAM1 in human PC and normal pancreatic
epithelial
cells. FIG. 1E contains representative images of IHC staining of ICAM1 in
human PC tumor
tissues with different stages and normal pancreas tissues. FIG. 1F shows a
comparison of
ICAM1 IHC staining score between PC and normal tissues. FIG. 1G shows
pathological
scores for tumor microarrays correlated with TNM stages. FIG. 1H shows a
Kaplan-Meier
analysis of overall survival of 84 PC patients according to different ICAM1
levels. *P<0.05,
**P<0.01, ***P<0.001.
FIGs. 2A-2G show that the ICAM1 antibody selectively recognizes and targets PC
tumor in vivo. FIG. 2A is a schematic diagram of the orthotopic PC model
injected PANC-1-
LUC at Day 0, receiving ICAM-AF or IgG-AF 28 days post tumor inoculation (n=6
per
group), and performing fluorescence imaging at Day 29. FIG. 2B shows ex vivo
fluorescence
imaging of PC tumors with surrounding normal pancreas tissues. FIG. 2C shows
corresponding quantification of fluorescence intensity in tumors. FIG. 2D
contains
representative imaging flow cytometry images showing the PC-specific
internalization of
ICAM1 Ab in PANC-1, BxPC-3 and HPNE cells. FIG. 2E shows signal intensity
analysis for
ICAM1 antibody-mediated cell internalization. FIG. 2F shows cell proliferation
of PANC-1
and BxPC-3 with treatment of ICAM1 Ab or IgG. FIG. 2G illustrates cell motion
trajectories
showing the response of PANC-1 and BxPC-3 after 24 h treatment of ICAM1 Ab.
*P<0.05,
**P<0.01, ***P<0.001.
FIGs 3A-3L show that ICAM1-DM1 selectively ablates PC cells in vitro and in
vivo.
FIG. 3A is a schematic illustration of ICAM1-DM1 ADC. FIG. 3B shows results of
screening
of cytotoxic payload with different linkers that conjugated to ICAM1 Ab. FIGs.
3C-3E show
results of cell viability assays to measure the anti-tumor activity of ICAM1-
DM1 to PANC-1
(FIG. 3C) and BxPC-3 (FIG. 3D), and normal HPNE (FIG. 3E), compared with IgG-
DM1
and GEM. FIG. 3F is a schematic diagram of the orthotopic PC model injected
LUC-PANC-1-
GFP at Week 0, receiving ICAM1-DM1, IgG-DM, gemcitabine or PBS 2 weeks post
tumor
inoculation (n=5,6 per group). IVIS was performed every week. FIG. 3G shows ex
vivo
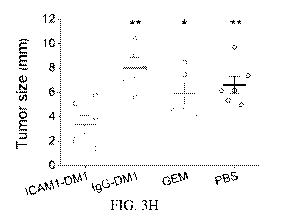
fluorescence imaging of GFP-expressing PC tumors. FIG. 3H shows corresponding
tumor
5
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
diameters. FIG. 31 shows the total flux of bioluminescence of the tumors in
different treatment
groups. FIG. 3J contains images showing treatment of ICAM1-DM1 ADC inhibits
tumor cell
proliferation. FIG. 3K shows corresponding quantification of Ki67+ cell
percentage. FIG. 3L
shows that treatment of ICAM1-DM1 ADC inhibits metastasis. *P<0.05, **P<0.01,
***P<0.001.
FIGs. 4A-4C show results of non-invasive MRI to assess ICAM1-expressing PC
tumor
in vivo. FIG. 4A is a schematic diagram of the orthotopic PC model injected
PANC-1-LUC at
Day 0, receiving ICAM-Gd or IgG-Gd 28 days post tumor inoculation (n=3 per
group), and
performing MRI at Day29. FIG. 4B shows representative in vivo Ti- and T2-
weighted MR
images of mice bearing orthotopic PC tumors received ICAM1-Gd and IgG-Gd.
Tumor was
circled and magnified in the insets. FIG. 4C shows quantitative changes of MRI
signal-to-
noise ratio in PC tumors. Bar graphs are shown as mean SD. *P<0.05, **P<0.01,
***P<0.001.
FIG. 5 shows representative H&E staining of mouse organs from different
treatment
groups. Scale bar, 100 pm. Micrometastasis sites are indicated by large
asterisks. Escalated
lymphocytes in spleen are indicated by small asterisks.
FIG. 6 shows ICAM1-DM1 treatment reduced PDAC metastasis.
FIG. 7 shows ICAM1-DM1 has no long term toxicity in mice received treatment.
DETAILED DESCRIPTION OF CERTAIN EMBODIMENTS
To date, pancreatic cancer (PC) remains among the most lethal diseases that
accounts
for 56,770 people death in the United States within 2019, representing 7% of
all cancer
mortality. The prognosis for PC patients is strikingly poor with a 5yr
survival less than 8%
despite of the recent intensified studies of immunotherapy and nanomedicine
therapy. These
undesirable results are largely due to the immunosuppressive tumor
microenvironment (TME)
of PC tumors, which is characterized by desmoplastic stroma and poor
vascularization. Such
TME creates physical barriers that prevent T-cells or nanomedicines
efficiently infiltrating
tumors and directly interacting with PC cells, leading to unfavorable
efficacies. It highlights a
critical need to develop novel targeted therapeutics that can better
infiltrated PC tumors while
maintaining potent tumor-specific efficacy.
Antibody drug conjugates (ADCs) have been shown to be promising clinical
efficacy
against several types of cancers including aggressive solid tumors like breast
cancer, which
response poorly to T-cell immunotherapy. Though several PC-targeted ADCs have
been
developed utilizing conventional PC targets (e.g., EGFR, EpHA2, and
Mesothelin), there still
6
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
lack a systematic and quantitative comparison of established PC targets and
other candidates at
their cell surface protein levels.
Provided herein, in some aspects, is an unbiased and quantitative screening of
cell
surface proteins to discover more optimal PC immunotherapeutic targets and
promot the
development of PC-targeted ADCs. ICAM1 was identfiied as a potential PC
immunotherapeutic target. The ICAM1 ADC described herein induced potent and
durable PC
tumor regression in vivo. The present disclosure also envisions the use of
ICAM1 as an
immunotherapy target for pancreatic cancer, including, without limitation, T
cell-based
immunotherapies such as CART, and checkpoint blockade.
Further, the present disclsoure provides a a non-invasive MRI approach to
identify
ICAM1-expressing tumors suitable for ICAM1-targeting immunotherapy Such
patients, in
some embodiments, are administered the ICAM1 ADC for the treatment of the PC.
Aspects of the present disclosure provide antibody-drug conjugates (ADCs)
comprising
intercellular adhesion molecule 1 (ICAM1) and a drug, methods of using the
same in treatment
of pancreatic cancer, and methods of predicting the responsiveness of
treatment with an
ICAM1 antibody or ICAM1 antibody-drug conjugate (ADC).
Antibody-drug conjugates (ADCs)
I. ICAM1 Antibodies
Antibody-drug conjugates (ADCs) are a class of immunotherapeutics that
comprise an
antibody conjugated to a drug. The ADCs of the present disclosure can target
cells expressing
ICAM1. ICAM1 is a cell surface glycoprotein that has been shown to bind
integrins of type
CD11 a / CD18, or CD1lb / CD18 and has been implicated in mediating cell-cell
interactions
and promoting leukocyte endothelial transmigration. ICAM1 is also referred to
as ICAM-1,
BB2, Cluster of Differentiation 54 (CD54), and P3.58.
Non-limiting examples of amino acid sequences encoding ICAM1 include UniProtKB
Accession Nos. P13597 and P05362.
UniProtKB Accession No. P13597 encodes ICAM1 from Mus Muscu/us has the
sequence of:
MAS TRAKP TLP LLLALVTVVIP GP GDAQVS
IHPREAFLPQGGSVQVNCSSSCKEDLSLGLETQWLKDELESGPNWK
LFEL SE I GEDS SP LCFENCGTVQS SASAT I TVYSFPESVELRP LPAWQQVGKDL
TLRCHVDGGAPRTQL SAVLLRG
EE I L SRQPVGGHPKDPKE I TFTVLASRGDHGANF SCRTELDLRPQGLALF SNVSEARSLRTFDLPAT
IPKLDTPDL
LEVGTQQKLFC SLEGLFPASEARI YLELGGQMP TQES TNS SDSVSATALVEVTEEFDRTLP LRCVLELADQ
I LETQ
RTLTVYNFSAPVLTLSQLEVSEGSQVTVKCEAHSGSKVVLLSGVEPRPPTPQVQFTLNASSEDHKRSFFCSAALEV
AGKFLFKNQTLELHVLYGPRLDETDCLGNWTWQEGSQQTLKCQAWGNP SPKMTCRRKADGALLP I
GVVKSVKQEMN
7
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
GTYVCHAFSSHGNVTRNVYLTVLYHSQNNWTIIILVPVLLVIVGLVMAASYVYNRQRKIRIYKLQKAQEEAIKLKG
QAPPP (SEQ ID NO: 1).
UniProtKB Accession No. P05362 encodes ICAM1 from Homo Sapiens and has the
sequence:
MAPSSPRPALPALLVLLGALFPGPGNAQTSVSPSKVILPRGGSVLVTCSTSCDQPKLLGIETPLPKKELLLPGNNR
KVYELSNVQEDSQPMCYSNCPDGQSTAKTFLTVYWTPERVELAPLPSWQPVGKNLTLRCQVEGGAPRANLTVVLLR
GEKELKREPAVGEPAEVTTTVLVRRDHHGANFSCRTELDLRPQGLELFENTSAPYQLQTFVLPATPPQLVSPRVLE
VDTQGTVVCSLDGLFPVSEAQVHLALGDQRLNPTVTYGNDSFSAKASVSVTAEDEGTQRLTCAVILGNQSQETLQT
VTIYSFPAPNVILTKPEVSEGTEVTVKCEAHPRAKVTLNGVPAQPLGPRAQLLLKATPEDNGRSFSCSATLEVAGQ
LIHKNQTRELRVLYGPRLDERDCPGNWTWPENSQQTPMCQAWGNPLPELKCLKDGTFPLPIGESVTVTRDLEGTYL
CRARSTQGEVTRKVTVNVLSPRYEIVIITVVAAAVIMGTAGLSTYLYNRQRKIKKYRLQQAQKGTPMKPNTQATPP
(SEQ ID NO: 2).
In some embodiments, a ICAM1 protein comprises a sequence that is at least
50%,
55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%,
98%,
99%, or is 100% identical to SEQ ID NO: 1 or to SEQ ID NO: 2. Additional ICAM1
proteins
are well known and may be identified using publically available databases
including, e.g.,
GenBank. An ICAM1 protein may be from any species, including homo sapiens.
Antibodies of the present disclosure are capable of binding ICAM1. In some
embodiments, the ICAM1 antibody is a monoclonal antibody. In some embodiments,
the
ICAM1 antibody is a polyclonal antibody. In some embodiments, the ICAM1
antibody is a
murine antibody. In some embodiments, the ICAM1 antibody is a humanized
antibody.
Non-limiting examples of ICAM1 antibodies include clone HCD54 ("HCD54,"
commercially available at BioLegend, catalog # 322702), UV3, RR1.1, R6.5 (BIRR-
1 or
Enlimomab, commercially available at Thermo Fisher Scientific, catalog #
BMS1011) and BI-
505. R6.5 (Enlimomab) is a monoclonal murine antibody produced by ATCC HB-9580
hybridoma cells, e.g., as described in United States Patent 5,324,510, which
is herein
incorporated by reference.
UV3 is a monoclonal antibody and has been shown to bind to ICAM-1 on myeloma
cells. In some embodiments, the ICAM1 antibody is a F(ab)'2 fragment of UV3.
See, e.g.,
Huang et al., Hybridoma. 1993 Dec;12(6):661-75; and Coleman et al., J
Immunother. 2006
Sep-Oct;29(5):489-98, which is each herein incorporated by reference. RR1.1 is
a monoclonal
ICAM1 antibody. See, e.g., Rothlein and Springer, 1986 J. Exp. Med. 163, 1132-
1149, which
is herein incorporated by reference. HCD54 is a monoclonal ICAM1 antibody. BI-
505 is a
fully human ICAM1 monoclonal antibody. See, e.g., Hansson et al., Clin Cancer
Res. 2015
Jun 15;21(12):2730-6, which is herein incorporated by reference.
8
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
The term "bind" refers to the association of two entities (e.g., two
proteins). Two
entities (e.g., two proteins) are considered to bind to each other when the
affinity (KD)
between them is <104 M, <10-5 M, <10-6 M, <10-7 M, <10-8 M, <10-9 M, <10-10M,
<10-11 M,
or <10-12 M. One skilled in the art is familiar with how to assess the
affinity of two entities
(e.g., two proteins).
The term "antibody" encompasses whole antibodies (immunoglobulins having two
heavy chains and two light chains), antibody mimetics, and antibody fragments.
An
"immunoglobulin (Ig)" is a large, Y-shaped protein produced mainly by plasma
cells that is
used by the immune system to neutralize an exogenous substance (e.g., a
pathogens such as
bacteria and viruses). Antibodies may be classified as IgA, IgD, IgE, IgG, and
IgM. "Antibody
fragments" include any antigen binding fragment (i.e., "antigen-binding
portion") or single
chain thereof. In some embodiments, an "antibody" refers to a glycoprotein
comprising at least
two heavy (H) chains and two light (L) chains inter-connected by disulfide
bonds, or an antigen
binding portion thereof. Each heavy chain is comprised of a heavy chain
variable region
(abbreviated herein as VH) and a heavy chain constant region. The heavy chain
constant region
is comprised of three domains, CH1, CH2 and CH3. Each light chain is comprised
of a light
chain variable region (abbreviated herein as VL) and a light chain constant
region. The light
chain constant region is comprised of one domain, CL. The VH and VL regions
can be further
subdivided into regions of hypervariability, termed complementarity
determining regions
(CDR), interspersed with regions that are more conserved, termed framework
regions (FR).
Each VH and VL is composed of three CDRs and four FRs, arranged from amino-
terminus to
carboxy-terminus in the following order: FR1, CDR1, FR2, CDR2, FR3, CDR3, FR4.
The
variable regions of the heavy and light chains contain a binding domain that
interacts with an
antigen. The constant regions of the antibodies may mediate the binding of the
immunoglobulin
to host tissues or factors, including various cells of the immune system
(e.g., effector cells) and
the first component (C lq) of the classical complement system. In some
embodiments, an
antibody is an immunoglobulin (Ig) monomer. An antibody may be a polyclonal
antibody or a
monoclonal antibody.
In some embodiments, an antibody is a heterotetrameric glycoprotein composed
of two
identical L chains and two H chains (an IgM antibody consists of 5 of the
basic heterotetramer
unit along with an additional polypeptide called J chain, and therefore
contain 10 antigen
binding sites, while secreted IgA antibodies can polymerize to form polyvalent
assemblages
comprising 2-5 of the basic 4-chain units along with J chain). In the case of
IgGs, the 4-chain
unit is generally about 150,000 daltons. Each L chain is linked to a H chain
by one covalent
9
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
disulfide bond, while the two H chains are linked to each other by one or more
disulfide bonds
depending on the H chain isotype. Each H and L chain also has regularly spaced
intrachain
disulfide bridges. Each H chain has at the N-terminus, a variable domain (VH)
followed by
three constant domains (CH) for each of the a and y chains and four CH domains
forll and
isotypes. Each L chain has at the N-terminus, a variable domain (VL) followed
by a constant
domain (CL) at its other end. The VL is aligned with the VH and the CL is
aligned with the first
constant domain of the heavy chain (CH1). Particular amino acid residues are
believed to form
an interface between the light chain and heavy chain variable domains. The
pairing of a VH and
VL together forms a single antigen-binding site. For the structure and
properties of non-limiting
examples of different classes of antibodies, see, e.g., Basic and Clinical
Immunology, 8th
edition, Daniel P. Stites, Abba I. Terr and Tristram G. Parslow (eds.),
Appleton & Lange,
Norwalk, Conn., 1994, page 71 and Chapter 6, incorporated herein by reference.
In some
embodiments, an antibody is an IgG.
The L chain from any vertebrate species can be assigned to one of two clearly
distinct
types, called kappa and lambda, based on the amino acid sequences of their
constant domains.
Depending on the amino acid sequence of the constant domain of their heavy
chains (CH),
immunoglobulins can be assigned to different classes or isotypes. There are
five classes of
immunoglobulins: IgA, IgD, IgE, IgG, and IgM, having heavy chains designated
a, 6, , y and
11, respectively. The y and a classes are further divided into subclasses on
the basis of relatively
minor differences in CH sequence and function, e.g., humans express the
following subclasses:
IgGl, IgG2, IgG3, IgG4, IgAl, and IgA2.
The V domain mediates antigen binding and define specificity of a particular
antibody
for its particular antigen. However, the variability is not evenly distributed
across the 110-
amino acid span of the variable domains. Instead, the V regions consist of
relatively invariant
stretches called framework regions (FRs) of 15-30 amino acids separated by
shorter regions of
extreme variability called "hypervariable regions" that are each 9-12 amino
acids long. The
variable domains of native heavy and light chains each comprise four FRs,
largely adopting a (3-
sheet configuration, connected by three hypervariable regions, which form
loops connecting,
and in some cases forming part of, the 13-sheet structure. The hypervariable
regions in each
chain are held together in close proximity by the FRs and, with the
hypervariable regions from
the other chain, contribute to the formation of the antigen-binding site of
antibodies (see, e.g.,
Kabat et al., Sequences of Proteins of Immunological Interest, 5th Ed. Public
Health Service,
National Institutes of Health, Bethesda, Md. (1991), incorporated herein by
reference). The
constant domains are not involved directly in binding an antibody to an
antigen, but exhibit
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
various effector functions, such as participation of the antibody in antibody
dependent cellular
cytotoxicity (ADCC).
In some embodiments, the antibody is a monoclonal antibody. A "monoclonal
antibody" is an antibody obtained from a population of substantially
homogeneous antibodies,
i.e., the individual antibodies comprising the population are identical except
for possible
naturally occurring mutations that may be present in minor amounts. Monoclonal
antibodies are
highly specific, being directed against a single antigenic site. Furthermore,
in contrast to
polyclonal antibody preparations which include different antibodies directed
against different
determinants (epitopes), each monoclonal antibody is directed against a single
determinant on
the antigen. In addition to their specificity, the monoclonal antibodies are
advantageous in that
they may be synthesized uncontaminated by other antibodies. The modifier
"monoclonal" is not
to be construed as requiring production of the antibody by any particular
method. For example,
the monoclonal antibodies useful in the present invention may be prepared by
the hybridoma
methodology first described by Kohler et al., Nature, 256:495 (1975), or may
be made using
recombinant DNA methods in bacterial, eukaryotic animal or plant cells (see,
e.g., U.S. Pat. No.
4,816,567). Monoclonal antibodies may also be isolated from phage antibody
libraries, e.g.,
using the techniques described in Clackson et al., Nature, 352:624-628 (1991)
and Marks et al.,
J. Mol. Biol., 222:581-597 (1991), incorporated herein by reference.
The monoclonal antibodies described herein encompass "chimeric" antibodies in
which
a portion of the heavy and/or light chain is identical with or homologous to
corresponding
sequences in antibodies derived from a particular species or belonging to a
particular antibody
class or subclass, while the remainder of the chain(s) is identical with or
homologous to
corresponding sequences in antibodies derived from another species or
belonging to another
antibody class or subclass, as well as fragments of such antibodies, so long
as they exhibit the
desired biological activity (see U.S. Pat. No. 4,816,567; and Morrison et al.,
Proc. Natl. Acad.
Sci. USA, 81:6851-6855 (1984)). Chimeric antibodies of interest herein include
"primatized"
antibodies comprising variable domain antigen-binding sequences derived from a
non-human
primate (e.g. Old World Monkey, Ape etc.), and human constant region
sequences.
In some embodiments, the antibody is a polyclonal antibody. A "polyclonal
antibody"
is a mixture of different antibody molecules which react with more than one
immunogenic
determinant of an antigen. Polyclonal antibodies may be isolated or purified
from mammalian
blood, secretions, or other fluids, or from eggs. Polyclonal antibodies may
also be recombinant.
A recombinant polyclonal antibody is a polyclonal antibody generated by the
use of
recombinant technologies. Recombinantly generated polyclonal antibodies
usually contain a
11
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
high concentration of different antibody molecules, all or a majority of
(e.g., more than 80%,
more than 85%, more than 90%, more than 95%, more than 99%, or more) which are
displaying a desired binding activity towards an antigen composed of more than
one epitope.
In some embodiments, the antibodies are "humanized" for use in human (e.g., as
therapeutics). "Humanized" forms of non-human (e.g., rodent) antibodies are
chimeric
antibodies that contain minimal sequence derived from the non-human antibody.
Humanized
antibodies are human immunoglobulins (recipient antibody) in which residues
from a
hypervariable region of the recipient are replaced by residues from a
hypervariable region of a
non-human species (donor antibody) such as mouse, rat, rabbit or non-human
primate having
the desired antibody specificity, affinity, and capability. In some instances,
framework region
(FR) residues of the human immunoglobulin are replaced by corresponding non-
human
residues. Furthermore, humanized antibodies may comprise residues that are not
found in the
recipient antibody or in the donor antibody. These modifications are made to
further refine
antibody performance. In general, the humanized antibody will comprise
substantially all of at
least one, and typically two, variable domains, in which all or substantially
all of the
hypervariable loops correspond to those of a non-human immunoglobulin and all
or
substantially all of the FRs are those of a human immunoglobulin sequence. The
humanized
antibody optionally also will comprise at least a portion of an immunoglobulin
constant region
(Fc), typically that of a human immunoglobulin. For further details, see Jones
et al., Nature
321:522-525 (1986); Riechmann et al., Nature 332:323-329 (1988); and Presta,
Curr. Op.
Struct. Biol. 2:593-596 (1992).
In some embodiments, the antibody is an "antibody fragment" containing the
antigen-
binding portion of a full-length ICAM1 antibody. In some embodiments, an
antibody is a single
domain heavy chain antibody. In some embodiments, an antibody is a single
domain light
chain antibody. The antigen-binding portion of an antibody refers to one or
more fragments of
an antibody that retain the ability to specifically bind to an antigen. It has
been shown that the
antigen-binding function of an antibody can be performed by fragments of a
full-length
antibody. Examples of binding fragments encompassed within the term "antigen-
binding
portion" of an antibody include (i) a Fab fragment, a monovalent fragment
consisting of the
VL, VH, CL and CH1 domains; (ii) a F(ab')2 fragment, a bivalent fragment
comprising two
Fab fragments linked by a disulfide bridge at the hinge region; (iii) a Fd
fragment consisting of
the VH and CH1 domains; (iv) a Fv fragment consisting of the VL and VH domains
of a single
arm of an antibody, (v) a dAb fragment (e.g., as described in Ward et al.,
(1989) Nature
341:544-546, incorporated herein by reference), which consists of a VH domain;
and (vi) an
12
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
isolated complementarity determining region (CDR). Furthermore, although the
two domains of
the Fv fragment, VL and VH, are coded for by separate genes, they can be
joined, using
recombinant methods, by a synthetic linker that enables them to be made as a
single protein
chain in which the VL and VH regions pair to form monovalent molecules (known
as single
chain Fv (scFv); see e.g., Bird et al. (1988) Science 242:423-426; and Huston
et al. (1988) Proc.
Natl. Acad. Sci. USA 85:5879-5883, incorporated herein by reference). Such
single chain
antibodies are also intended to be encompassed within the term "antigen-
binding portion" of an
antibody. These antibody fragments are obtained using conventional techniques
known to those
with skill in the art, and the fragments are screened for utility in the same
manner as are full-
length antibodies.
In some embodiments, an antibody fragment may be a Fc fragment, a Fv fragment,
or a
single-change Fv fragment. The Fc fragment comprises the carboxy-terminal
portions of both H
chains held together by disulfides. The effector functions of antibodies are
determined by
sequences in the Fc region, which region is also the part recognized by Fc
receptors (FcR)
found on certain types of cells.
The Fv fragment is the minimum antibody fragment which contains a complete
antigen-
recognition and -binding site. This fragment consists of a dimer of one heavy-
and one light-
chain variable region domain in tight, non-covalent association. From the
folding of these two
domains emanate six hypervariable loops (3 loops each from the H and L chain)
that contribute
the amino acid residues for antigen binding and confer antigen binding
specificity to the
antibody. However, even a single variable domain (or half of an Fv comprising
only three
CDRs specific for an antigen) has the ability to recognize and bind antigen,
although at a lower
affinity than the entire binding site.
Single-chain Fv also abbreviated as "sFv" or "scFv" are antibody fragments
that
comprise the VH and VL antibody domains connected into a single polypeptide
chain.
Preferably, the sFy polypeptide further comprises a polypeptide linker between
the VH and VL
domains which enables the sFy to form the desired structure for antigen
binding (e.g., as
described in Pluckthun in The Pharmacology of Monoclonal Antibodies, vol. 113,
Rosenburg
and Moore eds., Springer-Verlag, New York, pp. 269-315 (1994); Borrebaeck
1995,
incorporated herein by reference). In some embodiments, an antibody is a
dimerized scFV (a
diabody), a scFV timer (a triabody), or a scFV tetrameter (a tetrabody).
Antibodies of the present disclosure include antibody mimetics, including
affibody
molecules. An affibody is a small protein comprising a three-helix bundle that
functions as an
antigen binding molecule (e.g., an antibody mimetic). Generally, affibodies
are approximately
13
CA 03151800 2022-02-16
WO 2021/041171 PCT/US2020/047300
58 amino acids in length and have a molar mass of approximately 6 kDa.
Affibody molecules
with unique binding properties are acquired by randomization of 13 amino acids
located in two
alpha-helices involved in the binding activity of the parent protein domain.
Specific affibody
molecules binding a desired target protein can be isolated from pools
(libraries) containing
billions of different variants, using methods such as phage display.
In some embodiments, a ICAM1 antibody binds to an epitope that is present in
the
extracellular portion of an ICAM1. An "extracellular portion" of an ICAM1
refers to the
portion of the ICAM1 that is outside of the cytosol and on the surface of the
cell (as opposed to
the portion that is inside the cytosol. The extracellular portion of an ICAM1
typically
comprises
Methods of producing antibodies (e.g., monoclonal antibodies or polyclonal
antibodies)
are known in the art. For example, a polyclonal antibody may be prepared by
immunizing an
animal, preferably a mammal, with an allergen of choice followed by the
isolation of antibody-
producing B-lymphocytes from blood, bone marrow, lymph nodes, or spleen.
Alternatively,
antibody-producing cells may be isolated from an animal and exposed to an
allergen in vitro
against which antibodies are to be raised. The antibody-producing cells may
then be cultured to
obtain a population of antibody-producing cells, optionally after fusion to an
immortalized cell
line such as a myeloma. In some embodiments, as a starting material B-
lymphocytes may be
isolated from the tissue of an allergic patient, in order to generate fully
human polyclonal
antibodies. Antibodies may be produced in mice, rats, pigs (swine), sheep,
bovine material, or
other animals transgenic for the human immunoglobulin genes, as starting
material in order to
generate fully human polyclonal antibodies. In some embodiments, mice or other
animals
transgenic for the human immunoglobulin genes (e.g. as disclosed in U.S. Pat.
No. 5,939,598),
the animals may be immunized to stimulate the in vivo generation of specific
antibodies and
antibody producing cells before preparation of the polyclonal antibodies from
the animal by
extraction of B lymphocytes or purification of polyclonal serum.
Monoclonal antibodies are typically made by cell culture that involves fusing
myeloma
cells with mouse spleen cells immunized with the desired antigen (i.e.,
hyrbidoma technology).
The mixture of cells is diluted and clones are grown from single parent cells
on microtitre
wells. The antibodies secreted by the different clones are then assayed for
their ability to bind
to the antigen (with a test such as ELISA or Antigen Microarray Assay) or
immuno-dot blot.
The most productive and stable clone is then selected for future use.
II. Drugs
14
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
Drugs suitable for use in the ADCs include agents that are therapeutically
active against
pancreatic cancer. Non-limiting examples of drugs include chemotherapies. In
some
instances, a drug is a small molecule. In some embodiments, a drug is a
cytotoxic small
molecule. In some embodiments, a drug is a cytostatic small molecule.
Non-limiting examples of drugs suitable for use in the ADCs include N2'-
Deacetyl-
N2'-(3-mercapto-1-oxopropyl)mertansine (DM1), monomethyl auristatin E (MMAE),
monomethyl auristatin F (MMAF), and duocarmycin, paclitaxel, everolimus,
fluorouracil (5-
FU), gemcitabine, gemcitabine hydrochloride, mitomycin C, and derivatives
thereof. In some
embodiments, the drug is maytansine or an analog thereof. In some embodiments,
the drug is
DM1. DM1 is a cytotoxic maytansine analog that has been shown to inhibit
tubulin
polymerization. In some embodiments, the maytansine analog is DM4.
The term "small molecule" refers to molecules, whether naturally-occurring or
artificially created (e.g., via chemical synthesis) that have a relatively low
molecular weight.
Typically, a small molecule is an organic compound (e.g., it contains carbon).
The small
molecule may contain multiple carbon-carbon bonds, stereocenters, and other
functional
groups (e.g., amines, hydroxyl, carbonyls, and heterocyclic rings, etc.). In
certain
embodiments, the molecular weight of a small molecule is not more than about
1,000 g/mol,
not more than about 900 g/mol, not more than about 800 g/mol, not more than
about 700
g/mol, not more than about 600 g/mol, not more than about 500 g/mol, not more
than about
400 g/mol, not more than about 300 g/mol, not more than about 200 g/mol, or
not more than
about 100 g/mol. In certain embodiments, the molecular weight of a small
molecule is at least
about 100 g/mol, at least about 200 g/mol, at least about 300 g/mol, at least
about 400 g/mol, at
least about 500 g/mol, at least about 600 g/mol, at least about 700 g/mol, at
least about 800
g/mol, or at least about 900 g/mol, or at least about 1,000 g/mol.
Combinations of the above
ranges (e.g., at least about 200 g/mol and not more than about 500 g/mol) are
also possible.
Any known chemotherapeutic drugs may be used as the drug in the ADC descirbed
herein. Non-limiting exemplary chemotherapetic drugs include: Actinomycin, All-
trans
retinoic acid, Azacitidine, Azathioprine, Bleomycin, Bortezomib, Carboplatin,
Capecitabine,
Cisplatin, Chlorambucil, Cyclophosphamide, Cytarabine, Daunorubicin,
Docetaxel,
Doxifluridine, Doxorubicin, Epirubicin, Epothilone, Etoposide, Fluorouracil,
Gemcitabine,
Hydroxyurea, Idarubicin, Imatinib, Irinotecan, Mechlorethamine,
Mercaptopurine,
Methotrexate, Mitoxantrone, Oxaliplatin, Paclitaxel, Pemetrexed, Teniposide,
Tioguanine,
Topotecan, Valrubicin, Vinblastine, Vincristine, Vindesine, and Vinorelbine.
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
III. Linkers
One or more drugs may be conjugated to an ICAM1 antibody using techniques
known
in the art. In some embodiments, multiple (e.g., e.g., 2, 3, 4, 5, 6, 7, 8, 9,
10, or more) drugs
are conjugated to an ICAM1 antibody. The ratio of the ICAM1 antibody and the
drug in the
ADC may be 1:1 to 1:10 (e.g., 1:1, 1:2, 1:3, 1:4, 1:5, 1:6, 1:7, 1:8, 1:9, or
1:10). In some
embodiments, the ratio of the ICAM1 antibody and the drug in the ADC is 1:4.
An ICAM1 antibody may be conjugated to a second entity either directly or via
a
linker. As used herein, "conjugated" or "attached" means two entities are
associated,
preferably with sufficient affinity that the therapeutic or diagnostic benefit
of the association
between the two entities is realized. In some embodiments, a linker conjugates
an ICAM1
antibody to a drug in an ADC. The N-terminus or C-terminus of an ICAM1
antibody may be
conjugated to a drug. In some embodiments, a linker can be used to conjugate
an ICAM1
antibody to an imaging agent. The N-terminus or C-terminus of an ICAM1
antibody may be
conjugated to an imaging agent.
In some embodiments, a linker is a cleavable linker. As used herein, a
cleavable linker
is capable of releasing a conjugated moiety in response to a stimulus. In some
embodiments,
the stimulus is a physiological stimulus. Non-limiting examples of stimuli
include the
presence of an enzyme, acidic conditions, basic conditions, or reducing
conditions. For
example, cleavable linkers include peptide linkers, P-glucuronide linkers,
glutathione-sensitive
linkers (or disulfide linkers) and pH-sensitive linkers. In some embodiments,
a pH-sensitive
linker is cleaved at a pH between 5.0 and 6.5 or between a pH of 4.5 and 5Ø
In some
embodiments, a pH-sensitive linker is not cleaved when the pH is between 7 and
7.5. In some
embodiments, a pH-sensitive linker is not cleaved when the pH is between 7.3
and 7.5. In
some embodiments, a cleavable linker is a protease-sensitive linker.
Examples of cleavable linkers include N-succinimidyl 4-(2-
pyridyldithio)pentanoate
(SPP), N-succinimidyl 3-(2-pyridyldithio)butanoate (SPDB), Sulfo-SPDB, valine-
citrulline
dipeptide (Val-cit), acetyl butyrate, and CL2A. In some embodiments, the
cleavable linker is
Val-cit. See also, e.g., Donaghy, MAbs. 2016 May-Jun;8(4):659-71.
In some embodiments, a linker is non-cleavable. In some embodiments, a non-
cleavable linker is a linker that is not cleaved within systemic circulation
in a subject. In some
embodiments, a non-cleavable linker is a linker that is resistant to protease
cleavage. Non-
cleavable linkers include N-succinimidyl 4-(Nmaleimidomethyl)cyclohexane-1-
carboxylate
(SMCC) and maleimidomethyl cyclohexane-l-carboxylate (MCC). In some
embodiments, a
16
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
non-cleavable linker is a N-succinimidyl 4-(Nmaleimidomethyl)cyclohexane-1-
carboxylate
(SMCC) linker.
Any of the antibody-drug conjugates may be synthesized using methods known in
the
art. See, e.g., Yao et al., Int J Mol Sci. 2016 Feb 2;17(2). pii: E194.
The ADCs comprising ICAM1 antibody conjugated to a drug are also advantageous
to
use therapeutically, in part because the drugs (e.g., chemotherapeutic drugs)
are toxic and
cause severe side effects. By conjugate the drug (e.g., DM1) to the ICAM1
antibody, the
toxicity of the ADC may be reduced by at least 20%, at least 30%, at least
40%, at least 50%,
at least 60%, at least 70%, at least 80%, at least 90%, at least 99%, compared
to the drug in its
free from.
Other ICAMI Antibody Conjugates
ICAM1 antibodies and/or any of the ADCs of the present disclosure may be
conjugated
to an imaging agent, which may be useful for predicting the therapeutic
sensitivity of a subject
.. with pancreatic cancer. For example, imaging agents for computed tomography
(CT), positron
emission tomography (PET), magnetic resonance imaging (MRI), and endoscopic
detection
(e.g., endoscopic ultrasound) may be used and can include contrast agents.
See, e.g., Bird-
Lieberman et al., Nat Med. 2012;18(2):315-21; Van den Brande et al., Gut.
2007;56(4):509-
17, which is each herein incorporated by reference. In some embodiments, the
contrast agent
is administered as a salt. In some embodiments, the imaging agent is a
gadolinium-based MRI
contrast agent. For example, an imaging agent may be a gadolinium-
diethylenetriamine
pentaacetic acid (Gd-DTPA or DTPA-Gd). See, e.g., Can et al., AJR Am J
Roentgenol. 1984
Aug;143(2):215-24.
One or more imaging agents may be conjugated to an ICAM1 antibody or an ADC
described herein using techniques known in the art. In some embodiments,
multiple (e.g., e.g.,
2, 3, 4, 5, 6, 7, 8, 9, 10, or more) imaging agents are conjugated to an ICAM1
antibody. The
ratio of the ICAM1 antibody or ADC and the imaging agent may be 1:1 to 1:10
(e.g., 1:1, 1:2,
1:3, 1:4, 1:5, 1:6, 1:7, 1:8, 1:9, or 1:10). In some embodiments, the ratio of
the ICAM1
antibody or ADC and the imaging agent is 1:4. Any of the linkers disclosed
herein may be
.. used to conjugate an imaging agent to an ICAM1 antibody or to an ADC
described herein.
An imaging agent may be visualized with a suitable detection method (e.g., by
CT,
PET, MRI, ultrasound, and/or endoscopic detection).
Pharmaceutical Compositions and Uses Thereof
17
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
Compositions comprising any of the ADCs or other ICAM1 antibody conjugates
disclosed herein are encompassed by the present disclosure. In some
embodiments, the
composition is formulated as a pharmaceutical composition for administration
to a subject.
A subject may have, be suspected of having, or be at risk for pancreatic
cancer.
.. Pancreatic cancers are classified based on the cell type that starts the
tumor. The most
common type of pancreatic cancer are pancreatic adenocarcinomas, which are
cancers of the
exocrine pancreas. In contrast, pancreatic neuroendocrine tumors (NETs), or
islet cell tumors,
start in neuroendocrine cells.
Pancreatic cancers may also be stratified based on whether or not the cancer
has
metastasized. A pancreatic cancer may be stage 0 (carcinoma in situ), stage I,
stage II (e.g.,
stage IIA or stage JIB), stage III, or stage (IV). A non-limiting staging
method is the TNM
system, which evaluates the extent of the tumor (T), the spread of the cancer
to nearby lymph
nodes (N), and whether the cancer has spread to distant sites (M). The various
T, N, and M
levels (e.g., Table 1) may then be used to determine the stage of pancreatic
cancer (e.g., Table
2). Tables 1-2 show pancreatic tumor classification based on the Eighth
Edition of the
AJCC/UICC TNM staging system and as described by Cong et al. Sci Rep. 2018 Jul
10;8(1):10383.
Table 1. Non-limiting examples of TNM staging definitions
T1 Maximum tumor diameter <2 cm
T2 Maximum tumor diameter >2, <4 cm
T3 Maximum tumor diameter >4 cm
T4 Tumor involves the celiac axis, common
NO No regional lymph node metastasis
N1 Metastasis in 1-3 regional lymph nodes
N2 Metastasis in 4 regional lymph nodes
MO No distant metastasis
M1 Distant metastasis
Table 2. Pancreatic Staging Levels
IA Ti NO 1\40 Ti NO 1\40
TB T2 NO MO T2 NO MO
!IA T3 NO MO T3 NO MO
11B T1--T3 Nfl MO T1--T3 Nfl MO
HI T4 any N MO T4 any N MO
IV any T any N M1 any T Any N M1
18
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
In some embodiments, any of the pharmaceutical compositions disclosed herein
comprising an imaging agent is administered in an effective amount to a
subject to determine
the level of ICAM1 in a tumor of a subject with pancreatic cancer (e.g., CT,
PET, MRI, and
endoscopic detection (e.g., endoscopic ultrasound)). The imaging methods for
determining the
level of ICAM1 described herein are advantageous compare to conventional
methods (e.g.,
biopsy and analyzing the tissue obtained from the biopsy). The imaging methods
(e.g., MRI)
is non-invasive, and provides a comprehensive view of the tumor for ICAM1
level, providing
more accurate assessment of the tumor for prediction of outcome and/or
responsiveness to
treatment (e.g., treatment with ICAM1 antibody or ADC comprising ICAM1
antibody).
In some embodiments, the level of ICAM1 is detected in a subject with
pancreatic
cancer who has been administered a pharmaceutical composition of the present
disclosure
comprising an ICAM1 antibody and an imaging agent. In some embodiments, the
ICAM1
level detected in the tumor of the subject is at least 5%, at least 10%, at
least 20%, at least
30%, at least 40%, at least 50%, at least 60%, at least 70%, at least 80%, at
least 90%, at least
100%, at least 200%, at least 300%, at least 400%, at least 500%, at least
600%, at least 700%,
at least 800%, at least 900%, or at least 1,000% higher than a control. In
some embodiments,
the ICAM1 level detected in the tumor of the subject is substantially similar
to the control.
In some embodiments, a control is a subject with a tumor having a known level
of
ICAM1. In some embodiments, a control is the level of ICAM1 in the pancreas of
a subject
who does not have a tumor. In some embodiments, a control is a subject with a
tumor having a
low level of ICAM1. In some embodiments, a low level of ICAM1 is not
detectable. In some
embodiments, a control is a subject with a tumor having a high level of ICAM1.
In some
embodiments, a high level of ICAM1 is at least 5%, at least 10%, at least 20%,
at least 30%, at
least 40%, at least 50%, at least 60%, at least 70%, at least 80%, at least
90%, at least 100%, at
least 200%, at least 300%, at least 400%, at least 500%, at least 600%, at
least 700%, at least
800%, at least 900%, or at least 1,000% higher than the level of ICAM1
detected in a pancreas
of a healthy subject.
In some embodiments, the level of ICAM1 detected in a tumor using a method
disclosed herein is indicative of a subject with pancreatic cancer responding
to treatment with
.. an of treatment with an ICAM1 antibody or an antibody drug conjugate (ADC)
comprising an
intercellular adhesion molecule 1 (ICAM1) antibody conjugated to a drug. In
some
embodiments, a higher level of ICAM1 detected in a tumor as compared to the
tumor of a
subject with a lower level of ICAM1 is identified as being more responsive to
treatment with
an ICAM1 antibody or an ADC disclosed herein. In some embodiments, a subject
with a
19
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
higher level of ICAM1 in a tumor as compared to a subject with a lower level
of ICAM1 in a
tumor is at least 5%, at least 10%, at least 20%, at least 30%, at least 40%,
at least 50%, at least
60%, at least 70%, at least 80%, at least 90%, at least 100%, at least 200%,
at least 300%, at
least 400%, at least 500%, at least 600%, at least 700%, at least 800%, at
least 900%, or at
.. least 1,000% more responsive to treatment with a composition comprising an
ICAM1 antibody
(e.g., an ICAM1 ADC and/or an ICAM1 antibody not conjugated to a drug) In some
embodiments, a method disclosed herein comprises administering an ICAM1
antibody or an
ADC antibody disclosed herein after identifying the subject as being
responsive.
In some embodiments, the level of ICAM1 detected in a tumor using a method
disclosed herein is indicative of the stage of pancreatic cancer. In some
embodiments, the
level of ICAM1 detected in a tumor is indicative of stage 0, stage I, stage
II, stage III, or stage
IV.
Without being bound by a particular theory, in some embodiments,
administration of an
ICAM1 antibody conjugated to an imaging agent or an ICAM1 ADC conjugated to an
imaging
agent may serve a dual purpose of visualizing a pancreatic tumor and treating
the tumor.
In some embodiments, administration of an ICAM1 antibody and/or an ADC
comprising an ICAM1 antibody or a pharmaceutical composition thereof inhibits
the growth of
a tumor. In some embodiments, administration of an ICAM1 antibody and/or an
ADC
comprising an ICAM1 antibody or a pharmaceutical composition thereof results
in regression
of a tumor. In some embodiments, administration of an ICAM1 antibody and/or an
ADC
comprising an ICAM1 antibody or a pharmaceutical composition thereof decreases
the size of
a tumor by at least 5%, at least 10%, at least 20%, at least 30%, at least
40%, at least 50%, at
least 60%, at least 70%, at least 80%, at least 90%, at least 100%, at least
200%, at least 300%,
at least 400%, at least 500%, at least 600%, at least 700%, at least 800%, at
least 900%, or at
least 1,000% as compared to a control. In some embodiments, the control is a
subject who has
not been treated with a composition that comprises an ICAM1 antibody.
In some embodiments, administration of an ICAM1 antibody and/or an ADC
comprising an ICAM1 antibody or a pharmaceutical composition thereof disclosed
herein
decreases proliferation by at least 5%, at least 10%, at least 20%, at least
30%, at least 40%, at
least 50%, at least 60%, at least 70%, at least 80%, at least 90%, at least
100%, at least 200%,
at least 300%, at least 400%, at least 500%, at least 600%, at least 700%, at
least 800%, at
least 900%, or at least 1,000% higher than a control. In some embodiments,
proliferation is
measured using Ki67 staining. In some embodiments, the control is a subject
who has not
been treated with a composition that comprises an ICAM1 antibody.
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
In some embodiments, administration of an ICAM1 antibody and/or an ADC
comprising an ICAM1 antibody or a pharmaceutical composition thereof disclosed
herein
decreases metastasis of a tumor by at least 5%, at least 10%, at least 20%, at
least 30%, at least
40%, at least 50%, at least 60%, at least 70%, at least 80%, at least 90%, at
least 100%, at least
200%, at least 300%, at least 400%, at least 500%, at least 600%, at least
700%, at least 800%,
at least 900%, or at least 1,000% as compared to a control. In some
embodiments, the control
is a subject who has not been treated with a composition that comprises an
ICAM1 antibody.
In some embodiments, administration of an ICAM1 antibody and/or an ADC
comprising an ICAM1 antibody or a pharmaceutical composition thereof disclosed
herein does
not decrease the viability of healthy cells. In some embodiments,
administration of an ADC or
a pharmaceutical composition comprising an ADC disclosed herein allows for the
effective
amount (e.g., concentration) of a drug to be lower than if the drug was not
conjugated to an
ICAM1 antibody. In some embodiments, the effective amount of a drug is lowered
by at least
5%, at least 10%, at least 20%, at least 30%, at least 40%, at least 50%, at
least 60%, at least
70%, at least 80%, at least 90%, at least 100%, at least 200%, at least 300%,
at least 400%, at
least 500%, at least 600%, at least 700%, at least 800%, at least 900%, or at
least 1,000% as
compared to administration of the drug alone.
In some embodiments, the pharmaceutical composition further comprises a
pharmaceutically acceptable carrier. "Pharmaceutically acceptable" refers to
those
compounds, materials, compositions, and/or dosage forms which are, within the
scope of
sound medical judgment, suitable for use in contact with the tissues of human
beings and
animals without excessive toxicity, irritation, allergic response, or other
problem or
complication, commensurate with a reasonable benefit/risk ratio. A
"pharmaceutically
acceptable carrier" may be a pharmaceutically acceptable material, composition
or vehicle,
such as a liquid or solid filler, diluent, excipient, solvent or encapsulating
material, involved in
carrying or transporting the subject agents from one organ, or portion of the
body, to another
organ, or portion of the body. Each carrier must be "acceptable" in the sense
of being
compatible with the other ingredients of the formulation and not injurious to
the tissue of the
patient (e.g., physiologically compatible, sterile, physiologic pH, etc.). The
term "carrier"
denotes an organic or inorganic ingredient, natural or synthetic, with which
the active
ingredient is combined to facilitate the application. The components of the
pharmaceutical
compositions also are capable of being co-mingled with the molecules of the
present
disclosure, and with each other, in a manner such that there is no interaction
which would
substantially impair the desired pharmaceutical efficacy. Some examples of
materials which
21
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
can serve as pharmaceutically-acceptable carriers include: (1) sugars, such as
lactose, glucose
and sucrose; (2) starches, such as corn starch and potato starch; (3)
cellulose, and its
derivatives, such as sodium carboxymethyl cellulose, methylcellulose, ethyl
cellulose,
microcrystalline cellulose and cellulose acetate; (4) powdered tragacanth; (5)
malt; (6) gelatin;
(7) lubricating agents, such as magnesium stearate, sodium lauryl sulfate and
talc; (8)
excipients, such as cocoa butter and suppository waxes; (9) oils, such as
peanut oil, cottonseed
oil, safflower oil, sesame oil, olive oil, corn oil and soybean oil; (10)
glycols, such as
propylene glycol; (11) polyols, such as glycerin, sorbitol, mannitol and
polyethylene glycol
(PEG); (12) esters, such as ethyl oleate and ethyl laurate; (13) agar; (14)
buffering agents, such
as magnesium hydroxide and aluminum hydroxide; (15) alginic acid; (16) pyrogen-
free water;
(17) isotonic saline; (18) Ringer's solution; (19) ethyl alcohol; (20) pH
buffered solutions; (21)
polyesters, polycarbonates and/or polyanhydrides; (22) bulking agents, such as
polypeptides
and amino acids (23) serum component, such as serum albumin, HDL and LDL; (22)
C2-C12
alcohols, such as ethanol; and (23) other non-toxic compatible substances
employed in
pharmaceutical formulations. Wetting agents, coloring agents, release agents,
coating agents,
sweetening agents, flavoring agents, perfuming agents, preservative and
antioxidants can also
be present in the formulation.
The pharmaceutical compositions may conveniently be presented in unit dosage
form
and may be prepared by any of the methods well-known in the art of pharmacy.
The term "unit
dose" when used in reference to a pharmaceutical composition of the present
disclosure refers
to physically discrete units suitable as unitary dosage for the subject, each
unit containing a
predetermined quantity of active material calculated to produce the desired
therapeutic effect in
association with the required diluent; i.e., carrier, or vehicle.
The formulation of the pharmaceutical composition may dependent upon the route
of
administration. Injectable preparations suitable for parenteral administration
or intratumoral,
peritumoral, intralesional or perilesional administration include, for
example, sterile injectable
aqueous or oleaginous suspensions and may be formulated according to the known
art using
suitable dispersing or wetting agents and suspending agents. The sterile
injectable preparation
may also be a sterile injectable solution, suspension or emulsion in a
nontoxic parenterally
acceptable diluent or solvent, for example, as a solution in 1,3 propanediol
or 1,3 butanediol.
Among the acceptable vehicles and solvents that may be employed are water,
Ringer's
solution, U.S.P. and isotonic sodium chloride solution. In addition, sterile,
fixed oils are
conventionally employed as a solvent or suspending medium. For this purpose
any bland fixed
oil may be employed including synthetic mono- or di-glycerides. In addition,
fatty acids such
22
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
as oleic acid find use in the preparation of injectables. The injectable
formulations can be
sterilized, for example, by filtration through a bacterial-retaining filter,
or by incorporating
sterilizing agents in the form of sterile solid compositions which can be
dissolved or dispersed
in sterile water or other sterile injectable medium prior to use.
Compositions suitable for oral administration may be presented as discrete
units, such
as capsules, tablets, lozenges, each containing a predetermined amount of the
anti-
inflammatory agent. Other compositions include suspensions in aqueous liquids
or non-
aqueous liquids such as a syrup, elixir or an emulsion.
In some embodiments, the pharmaceutical compositions used for therapeutic
administration must be sterile. Sterility is readily accomplished by
filtration through sterile
filtration membranes (e.g., 0.2 micron membranes). Alternatively,
preservatives can be used to
prevent the growth or action of microorganisms. Various preservatives are well
known and
include, for example, phenol and ascorbic acid. The pharmaceutical composition
ordinarily
will be stored in lyophilized form or as an aqueous solution if it is highly
stable to thermal and
oxidative denaturation. The pH of the preparations typically will be about
from 6 to 8, although
higher or lower pH values can also be appropriate in certain instances.
"A therapeutically effective amount" or "effective amount" as used herein
refers to the
amount of each therapeutic agent (e.g., therapeutic agents for treating any of
the brain disease
described herein) of the present disclosure required to confer therapeutic
effect on the subject,
either alone or in combination with one or more other therapeutic agents.
Effective amounts
vary, as recognized by those skilled in the art, depending on the particular
condition being
treated, the severity of the condition, the individual subject parameters
including age, physical
condition, size, gender and weight, the duration of the treatment, the nature
of concurrent
therapy (if any), the specific route of administration and like factors within
the knowledge and
expertise of the health practitioner. These factors are well known to those of
ordinary skill in
the art and can be addressed with no more than routine experimentation. It is
generally
preferred that a maximum dose of the individual components or combinations
thereof be used,
that is, the highest safe dose according to sound medical judgment. It will be
understood by
those of ordinary skill in the art, however, that a subject may insist upon a
lower dose or
tolerable dose for medical reasons, psychological reasons or for virtually any
other reasons.
Empirical considerations, such as the half-life, generally will contribute to
the
determination of the dosage. For example, therapeutic agents that are
compatible with the
human immune system, such as polypeptides comprising regions from humanized
antibodies
or fully human antibodies, may be used to prolong half-life of the polypeptide
and to prevent
23
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
the polypeptide being attacked by the host's immune system. Frequency of
administration may
be determined and adjusted over the course of therapy, and is generally, but
not necessarily,
based on treatment and/or suppression and/or amelioration and/or delay of a
disease.
Alternatively, sustained continuous release formulations of a polypeptide may
be appropriate.
Various formulations and devices for achieving sustained release are known in
the art.
In some embodiments, dosage is daily, every other day, every three days, every
four
days, every five days, or every six days. In some embodiments, dosing
frequency is once
every week, every 2 weeks, every 4 weeks, every 5 weeks, every 6 weeks, every
7 weeks,
every 8 weeks, every 9 weeks, or every 10 weeks; or once every month, every 2
months, or
every 3 months, or longer. The progress of this therapy is easily monitored by
conventional
techniques and assays. The dosing regimen (including the anti-cancer agent
used) can vary
over time.
In some embodiments, for an adult subject of normal weight, doses ranging from
about
0.01 to 1000 mg/kg may be administered. In some embodiments, the dose is
between 1 to 200
mg. The particular dosage regimen, i.e., dose, timing and repetition, will
depend on the
particular subject and that subject's medical history, as well as the
properties of the anti-cancer
agent (such as the half-life of the anti-cancer agent, and other
considerations well known in the
art).
For the purpose of the present disclosure, the appropriate dosage of a
therapeutic agent
as described herein will depend on the specific agent (or compositions
thereof) employed, the
formulation and route of administration, the type and severity of the disease,
whether the anti-
cancer agent is administered for preventive or therapeutic purposes, previous
therapy, the
subject's clinical history and response to the antagonist, and the discretion
of the attending
physician. Typically the clinician will administer an anti-cancer agent until
a dosage is
reached that achieves the desired result. Administration of one or more anti-
cancer agents can
be continuous or intermittent, depending, for example, upon the recipient's
physiological
condition, whether the purpose of the administration is therapeutic or
prophylactic, and other
factors known to skilled practitioners. The administration of an anti-cancer
agent may be
essentially continuous over a preselected period of time or may be in a series
of spaced dose,
e.g., either before, during, or after developing a disease.
As used herein, the term "treating" refers to the application or
administration of an anti-
cancer agent to a subject in need thereof. "A subject in need thereof', refers
to an individual
who has a disease, a symptom of the disease, or a predisposition toward the
disease, with the
24
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
purpose to cure, heal, alleviate, relieve, alter, remedy, ameliorate, improve,
or affect the
disease, the symptom of the disease, or the predisposition toward the disease.
A "subject" to which administration is contemplated refers to a human (i.e.,
male or
female of any age group, e.g., pediatric subject (e.g., infant, child, or
adolescent) or adult
subject (e.g., young adult, middle¨aged adult, or senior adult)) or non¨human
animal. In some
embodiments, the non¨human animal is a mammal (e.g., rodent (e.g., mouse or
rat), primate
(e.g., cynomolgus monkey or rhesus monkey), commercially relevant mammal
(e.g., cattle,
pig, horse, sheep, goat, cat, or dog), or bird (e.g., commercially relevant
bird, such as chicken,
duck, goose, or turkey)). The non-human animal may be a male or female at any
stage of
development. The non-human animal may be a transgenic animal or genetically
engineered
animal.
In some embodiments, the subject is a companion animal (a pet). "A companion
animal," as used herein, refers to pets and other domestic animals. Non-
limiting examples of
companion animals include dogs and cats; livestock such as horses, cattle,
pigs, sheep, goats,
and chickens; and other animals such as mice, rats, guinea pigs, and hamsters.
In some
embodiments, the subject is a research animal. Non-limiting examples of
research animals
include: rodents (e.g., rats, mice, guinea pigs, and hamsters), rabbits, or
non-human primates.
Alleviating a disease (e.g., cancer) includes delaying the development or
progression of
the disease, or reducing disease severity. Alleviating the disease does not
necessarily require
curative results. As used therein, "delaying" the development of a disease
means to defer,
hinder, slow, retard, stabilize, and/or postpone progression of the disease.
This delay can be of
varying lengths of time, depending on the history of the disease and/or
individuals being
treated. A method that "delays" or alleviates the development of a disease, or
delays the onset
of the disease, is a method that reduces probability of developing one or more
symptoms of the
disease in a given time frame and/or reduces extent of the symptoms in a given
time frame,
when compared to not using the method. Such comparisons are typically based on
clinical
studies, using a number of subjects sufficient to give a statistically
significant result.
"Development" or "progression" of a disease means initial manifestations
and/or
ensuing progression of the disease. Development of the disease can be
detectable and assessed
using standard clinical techniques as well known in the art. However,
development also refers
to progression that may be undetectable. For purpose of this disclosure,
development or
progression refers to the biological course of the symptoms. "Development"
includes
occurrence, recurrence, and onset. As used herein "onset" or "occurrence" of a
disease
includes initial onset and/or recurrence.
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
Conventional methods, known to those of ordinary skill in the art of medicine,
can be
used to administer the pharmaceutical composition the subject, depending upon
the type of
disease to be treated or the site of the disease. The pharmaceutical
composition can also be
administered via other conventional routes, e.g., administered orally,
parenterally, by
inhalation spray, topically, rectally, nasally, buccally, vaginally or via an
implanted reservoir.
The term "parenteral" as used herein includes subcutaneous, intracutaneous,
intravenous,
intramuscular, intraarticular, intraarterial, intrasynovial, intrasternal,
intrathecal, intralesional,
and intracranial injection or infusion techniques. In some embodiments, the
pharmaceutical
composition is administered via intravenous injection or infusion. In
addition, it can be
administered to the subject via injectable depot routes of administration such
as using 1-, 3-, or
6-month depot injectable or biodegradable materials and methods. In some
embodiments, the
pharmaceutical composition is administered via injection. In some embodiments,
injection is
intravenous injection or intratumoral injection.
EXAMPLES
Introduction
To date, pancreatic cancer (PC) remains among the most lethal diseases that
accounts
for 56,770 people death in the United States within 2019, representing 7% of
all cancer
mortality. The prognosis for PC patients is strikingly poor with a 5 year
survival less than 8%
despite of the recent intensified studies of immunotherapy and nanomedicine
therapy. For
instance, immune checkpoint blockade therapies including cytotoxic T-
lymphocyte-associated
antigen 4 (CTLA4) inhibitor or Programmed death-ligand 1 (PD-L1) antibody have
yet to
show enough clinical efficacy in treating PC patients to date. Innovative
nanomedicine
formulations (e.g., EphA2-targeted liposomal docetaxel) also failed to bring
clinical benefits in
treating advanced PC. These undesirable results are largely due to the
immunosuppressive
tumor microenvironment (TME) of PC tumors, which is characterized by
desmoplastic stroma
and poor vascularization. Such TME creates physical barriers that prevent T-
cells or
nanomedicines efficiently infiltrating tumors and directly interacting with PC
cells, leading to
unfavorable efficacies. It highlights a critical need to develop novel
targeted therapeutics that
can better infiltrated PC tumors while maintaining potent tumor-specific
efficacy.
Antibody-drug conjugates (ADCs) are a rapidly growing class of
immunotherapeutics
that have shown promising clinical efficacy against several types of cancers
including
aggressive solid tumors like breast cancer, which respond poorly to T-cell
immunotherapy.
Unlike conventional chemotherapeutics, ADCs utilizes chemical linkers to
conjugate cytotoxic
26
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
drugs to tumor-homing antibodies, which are capable of selectively homing
tumors while
sparing normal tissues via recognizing tumor surface antigens, subsequently
internalizing and
delivering cytotoxic drugs into targeted tumor cells. Compared to T-cell
immunotherapy (e.g.,
chimeric antigen receptor-T cell (CAR-T) or immune checkpoint blockade) or
nanomedicines
(e.g., liposomes or exosomes), ADC features a superior tumor tissue
penetration due to its
ultrasmall size (<10 nm), which is ¨1,000 fold smaller than the size of a T-
cell, creating an
attractive opportunity to increase drug delivery into stroma dense PC tumors.
However, a major hurdle in developing PC-targeted ADC is identifying suitable
immunotherapeutic targets that effectively distinguish PC and normal tissues.
To meet the
safety and efficacy criteria for optimal ADC, such targets need to be
abundantly presented on
the cell surface of PC tumors with undetectable levels in normal tissues,
making it assessable
to the tumor-homing antibody of ADCs. Meanwhile, it is also required to
facilitate a rapid and
robust cell internalization of cytotoxic payloads conjugated on ADCs. Though
several PC-
targeted ADCs have been developed utilizing conventional PC targets (e.g.,
EGFR, EpHA2,
and Mesothelin), there still lacks a systematic and quantitative comparison of
established PC
targets and other candidates at their cell surface protein levels. The present
disclosure shows
that performing such unbiased and quantitative screening of cell surface
proteins leads to the
discovery of more optimal PC immunotherapeutic targets and promoting the
development of
PC-targeted ADCs.
ICAM1, also called CD54, is a transmembrane glycoprotein of immunoglobulin
superfamily, which is aberrantly overexpressed in multiple types of cancers
(e.g., triple
negative breast cancer) and is frequently associated with an aggressive
phenotype and worse
prognosis. In PC, ICAM1 is directly induced on pancreatic acinar cells by
KRASG12D mutation,
the most common oncogenic mutation in 70-95% PC patients, and drives the
formation of
pancreatic neoplastic lesions, leading to PC tumor initiation. The present
disclosure describes
the identification and application of ICAM1 as a potential PC
immunotherapeutic target based
on an unbiased and quantitative screening algorithm. As such, ICAM1 ADCthat
induces potent
and durable PC tumor regression in vivo was developed. To develop a precision
medicine, a
non-invasive MRI approach to identify ICAM1-expressing tumors suitable for
ICAM1-
targeting immunotherapy was designed.
Results and discussion
ICAMI is a rationally identified cell surface protein target for human PC.
27
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
To identify suitable protein targets distinguishing malignant PC tumors from
normal
tissues, a rationally-designed cell surface protein target discovery algorithm
was
developed(FIG. 1A). First, an unbiased and quantitative screening of a panel
of 72 cancer-
related surface antigens in four established human PC cell lines (PANC-1, BxPC-
3, Capan-1,
and Capan-2) was performed in comparison with two normal human pancreatic duct
epithelial
cells (HPDE and HPNE) as normal controls (FIG. 1B). Of the 68 screened
targets, 31
candidates were found to be commonly overexpressed in all four PC cell lines
and were
selected for further evaluation. By comparing their levels in human PC cells
and normal
pancreatic cells, ICAM1 emerged as the most overexpressed PC targets among the
top 10
candidates with almost no expression in non-neoplastic HPNE and HPDE cells
(FIG. 1C). The
cell surface density of ICAM1 ranges from 3x105 to lx106 molecules/cell on
four PC cell
lines, significantly higher than those of established PC targets (e.g., EGFR,
MUC1, or EphA2).
Additionally, ICAM1 is ubiquitously overexpressed across all four tested human
PC cell lines,
suggesting a broad target population in PC patients. The overexpression of
ICAM1 in human
PC cells was further confirmed using immunofluorescent (IF) staining. ICAM1
was
predominantly expressed on the plasma membranes of four PC cell lines (PANC-1,
BxPC-3,
Capan-1 and Capan-2) but absent in normal HNPE and HPNE cells (FIG. 1D). This
strong cell
surface expression of ICAM1 on human PC cells makes it readily assessable for
ICAM1-
targeting immunotherapeutics (e.g., ADCs or CAR-T cells).
To investigate whether high ICAM1 expression is a clinically relevant finding
in
human PC, an immunohistochemical (IHC) staining of ICAM lwas conducted in 80
human PC
tumor tissues and 20 normal pancreas tissues. In FIG. 1E and FIG. 1F, ICAM1
was
consistently overexpressed on plasma membrane and in cytoplasm of PC cells
from tumor
tissues at different disease stages, and ICAM1 is completely absent in the
normal human
.. pancreas tissues. The extent of staining and the pathological scores of
ICAM1 showed that
ICAM1 level was positively correlated to the disease TNM stages (FIG. 1G). The
on-target,
off-tumor sites for ICAM1-targeting immunotherapeutics in normal tissues were
also
evaluated. The protein levels of ICAM1 were examined in a comprehensive cohort
of 45
normal human organs by querying Human Protein Atlas database
(proteinatlas.or0. It was
found that ICAM1 expression was absent in most normal tissues by IHC analysis,
and only 4%
(2/45, lung and kidney) of normal tissues show high positive staining of
ICAM1, respectively.
This suggested that lung and kidney are potential on-target, off-tumor sites
for ICAM1-
targeting immunotherapeutics. Moreover, previous work has shown ICAM1-
targeting T-cell
28
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
immunotherapy does not induce any acute or delayed toxicity in both male and
female mice of
advanced thyroid cancer model.
The impact of ICAM1 overexpression on clinical outcomes of PC patients was
investigated by querying the R2: Genomics Analysis and Visualization Platform
database
(115(senierl.anksmil, Datasheet: Mixed Pancreas Tumor-Zhang). The overall
survival of PC
patients with high ICAM1 expression was significantly worse than those with
low ICAM1
expression (FIG. IH, P=0.021, log-rank test), suggesting that ICAM1 may serve
as a clinical
biomarker of poor prognosis in PC patients.
ICAMI antibody recognizes and targets PC tumor in vivo.
To assess ICAM1 as a potential immunotherapeutic target, the in vivo tumor-
specificity
of ICAM1 antibody was first determined in an orthotopic PC tumor model(FIG.
2A). ICAM1
monoclonal antibodies were fluorescently labeled with AF-647, a red
fluorescent dye, and
intravenously injected into PANC-1 tumor-bearing mice. AF-647 labeled IgG (IgG-
AF) was
used as a non-targeting control. Due to the fact that in vivo fluorescent
signal was interfered by
the intraperitoneal location of orthotopic PC tumors and abdominal skin
absorption, the
animals were euthanized at 24 hours-post injection and PC tumors and their
surrounding
pancreatic tissues were excised. Then, ex vivo imaging was performed to
determine the tumoral
accumulation of ICAM1-AF antibodies. As observed in Fig 2B, ICAM1 antibody
selectively
recognized and targeted orthotopic PC tumors with high affinity compared with
non-targeting
IgG controls. Normal pancreatic tissues adjacent to PC tumors were not
targeted by ICAM1
antibody, further confirming its PC tumor-specificity. Quantified fluorescent
signals (FIG. 2C)
confirmed that the tumoral accumulation of ICAM1 antibody was approximately 6-
fold higher
than that of non-targeting IgG-AF after one single dose of tail-vein
administration. These in
vivo findings strongly support the development of ICAM1 antibody-based
immunotherapeutics
for PC-targeted therapy.
Given that cell entry activity is a critical factor in ADC design, cell
internalization of
ICAM1 antibodies in human PC cells was investigated using an imaging flow
cytometry assay.
As shown in FIG. 2D, phycoerythrin (PE)-conjugated ICAM1 antibodies were
robustly
internalized by both PANC-1 and BxPC-3 cells via ICAM1 antigen-mediated
endocytosis,
whereas almost no PE-ICAM1 antibodies were internalized by normal HPNE cells
due to the
significant lack of ICAM1 antigen expression. The internalized amount of PE-
ICAM1
antibody by human PC cells was quantified as approximately 300-fold higher
than that of
HPNE cells (FIG. 2E).
29
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
Next, the therapeutic consequences of blocking ICAM1 signaling cascades in
human
PC cells was investigated using its neutralizing antibody. In FIG. 2F,
treatment with ICAM1
neutralizing antibody (2 i.t.g/mL) did not obviously alter PANC-1 or BxPC-3
cell proliferation.
However, ICAM1 neutralizing antibodies did potently inhibit PANC-1 and BxPC-3
cell
migration in comparison with IgG controls, which reduced cell migration of
PANC-1 and
BxPC-3 cells by 39% and 44%, respectively (FIG. 2G). Correlatively, ICAM1
neutralizing
antibodies have also been reported to potently inhibit PC tumor initiation in
vivo. These
findings indicate that ICAM1 can serve as a PC tumor-homing target,.They also
indicate that
that targeting ICAM1 signaling cascades can also hinder disease progression.
Rational design of ICAM1 antibody-drug conjugates
To translate ICAM1 target into PC therapy, a rationally-designed ICAM1 ADC was
designed as an immunotherapeutic for PC-targeted therapy (FIG. 3A). Given that
chemical
linker and cytotoxic payload substantially affect the efficacy of ADC, the
first step was to
select the optimal ADC formulation for PC treatment using an unbiased and
quantitative
screening approach. A series of ICAM1 ADCs was engineered using four
clinically-tested
ADC linkers and cytotoxic payloads (SMCC-DM1, Vc-MMAE, Mc-MMAF, Duocarmycin)
at
equivalent drug-to-antibody ratio (DAR) of 1 and compared their cytotoxicity
against human
PC cells, in comparison with non-targeting IgG ADC controls. As shown in FIG.
3B, ICAM1-
SMCC-DM1 showed the lowest IC50 (38.1 nM) among four tested ADC formulations
(other
IC50: 83.9-240.4 nM) in treating PANC-1 cells. The IC50 of ICAM1-SMCC-DM1 is
over
2,000-fold lower than Gem (89.1 t.M), the first-line chemotherapeutic for PDAC
therapy.
SMCC-DM1 is a clinically validated ADC formulation consisting of a non-
cleavable chemical
linker and a potent microtubule inhibitor, Mertansine (DM1). Thus, SMCC-DM1
was selected
as the optimized ADC formulation and subsequently synthesized ICAM1-SMCC-DM1
(ICAM1-DM1) as the optimized ICAM1 ADC for PC-targeted therapy. IgG-SMCC-DM1
(IgG-DM1) was also prepared under the same experimental conditions as a non-
targeting
control. The drug-to-antibody ratios (DARs) for ICAM1-DM1 and IgG-DM1 were
controlled
by the input amounts of DM1 and antibodies and achieved 3.4 for ICAM1-DM1 and
3.2 for
IgG-DM1 as determined using an UV/VIS spectroscopy assay.
ICAM1-DM1 selectively ablates PC cells in vitro and in vivo.
The PC-specific cytotoxicity of ICAM1-DMlwas determined in two human PC cells
(PANC-1 and BxPC-3) and normal HPNE cells (FIGs. 3C-3E). First-line chemodrug
GEM
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
and non-targeting IgG-DM1 were used as controls. As observed in FIG. 3D and
FIG. 3E,
ICAM1-DM1 showed potent cytotoxicity against PANC-1 and BxPC-3 cells. The IC50
of
ICAM1-DM1 is determined as 9.8 nM for PANC-1 and 4.0 nM for BxPC-3,
significantly
lower than those of GEM and IgG-DM1 (30 nM-88 p.m). Moreover, ICAM1-DM1 shows
no
cytotoxicity in normal HPNE cells due to their lack of expression of ICAM1
(FIG. 3E). These
in vitro results strongly support to evaluate anti-tumor activity of ICAM1-DM1
in the in vivo
settings of PC models.
The anti-tumor activity of ICAM1-DM1 was examined in suppressing orthotopic PC
tumor growth in vivo (FIG. 3F). ICAM1-DM1 or IgG-DM1 (non-targeting control)
were
intravenously administered in PANC-1-Luc tumor-bearing mice at 15 mg/kg every
3 weeks. In
comparison, GEM was weekly intravenously administered at a dose of 5 mg/kg due
to its short
circulation half-life (0.28 hr). After two ADC injections, ICAM1-DM1-treated
group
exhibited a potent and durable tumor regression compared to other groups
(FIGs. 3G-3H). The
quantified tumor mass showed that ICAM1-DM1 significantly reduced PC tumor
growth by
49% in comparison with PBS (sham) group (FIG. 31). The mechanism of ICAM1-DM1
induced toxicity was further examined by measuring cell proliferation marker
Ki67 expression
in PC tumor tissues. As observed in FIG. 3K and FIG. 3L, Ki67-positive cell
population in
ICAM1-DM1-treated group was significantly reduced compared with other groups,
contributing to the potent and persistent tumor suppression. This potent anti-
tumor activity of
ICAM1-DM1 also effectively inhibited spontaneous PC metastasis to normal
organs including
lung, liver, and spleen (FIG. 3M and FIG. 5). There was no evidence of
histopathological
damage to the normal vital organs collected from the ICAM1-DM1 treated group.
Non-invasively evaluating tumoral ICAMI expression by MRI.
To build a precision medicine, a MRI-based molecular imaging approach was
developed for non-invasively and rapidly identifying PC patients that may
benefit from
ICAM1-targeting immunotherapy. In clinic practice, a needle biopsy is commonly
adopted
prior to targeted therapy to examine the adequacy of target expression in
tumor tissues, but this
approach is limited by its invasiveness and the lack of accuracy (<50%) due to
the intratumoral
.. complexity and heterogeneity. To overcome these obstacles, an ICAM1-
targeting MRI probe
was developed and used to map the tumoral ICAM1 expression in an orthotopic PC
model
using MRI (FIG. 4A). The ICAM1-targeting MRI probe was first engineered by
covalently
conjugating ICAM1 antibody with DTPA-Gd, a clinically-used MRI contrast agent.
IgG-Gd
was prepared as a non-targeting control. Then ICAM1-Gd or IgG-Gd was
intravenously
31
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
administered into ICAM1-expressing PANC-1 tumor-bearing mice at a dosage of 5
mg/kg
mouse weight. At pre- and 24 hour-post injection of MRI probes, in vivo MRI
was performed
on PC tumor-bearing mice with a set of MRI sequences, including Ti, T2-
weighted spin echo
imaging. In FIG. 4B, high-resolution T2-weighted MRI images were analyzed to
locate PC
tumors (yellow circle) in peritoneal cavity. Once the PC tumor was located, Ti-
weighted MRI
images were used to quantitatively measure MRI signal changes in the area of
PC tumor
(yellow circle) as a function of intratumoral accumulation of gadolinium from
administered
MRI probes. In FIG. 4C, the tumoral Ti MRI signal increased ¨50% in ICAM1-Gd
group,
while no MRI signal changes were observed in non-targeting IgG-Gd group
(n=3/group).
These MRI signal changes are positively correlated with the level of antigen
expression on
targeted tumors, which can be used to identify ICAM1-positive patients that
may benefits from
ICAM1-targeting immunotherapy.
In summary, the present disclosure provides experimental evidence that ICAM1
is a
suitable ADC target for human PC. The utility of this target can be extended
to developing
other immunotherapeutics including CAR T cells or bi-specific antibodies
directed toward
ICAM1.
Materials and Methods
Materials.
Purified anti-human CD54 Antibody (Clone:HCD54), phycoerythrin (PE)-conjugated
mouse anti-human ICAM-1 antibody (PE-ICAM1) and PE conjugated mouse IgG
isotype (PE-
IgG) were purchased from BioLegend(San Diego, CA, USA). ADC prescreening G-
DM1, G-
MMAE, G-MMAF, G-Duoca were purchased from Levena Biopharma (San Diego, CA).
SMCC-DM1 was purchased from Medkoo(Morrisville, NC, USA). ZebaTM Spin
Desalting
Columns, (7K MWCO), Alexa Fluor 647 NHS ester, the Lab-Tek II Chamber Slide
System,
ProLong Gold Antifade Mountant was obtained from Thermo Fisher Scientific.
Gemcitabine
hydrochloride (GEM), Gadolinium(III) chloride hexahydrate (GdC13-6H20),
diethylenetriaminepentaacetic dianhydride (DTPAA), sodium bicarbonate, sodium
citrate
tribasic dihydrate were purchased from Sigma-Aldrich (St. Louis, MO).
Dulbecco's PBS,
DAPI, Quant-iT RNA Assay Kit, 0.25% trypsin/2.6 mM EDTA solution, Gibco DMEM,
Gibco DMEM/F12(1:1), Roswell Park Memorial Institute (RPMI)-1640 medium, and
McCoy's 5A medium were purchased from Invitrogen (Carlsbad, CA). MEGM Mammary
Epithelial Cell Growth Medium was purchased from Lonza (Basel, Switzerland).
Quantum
Simply Cellular microbeads were purchased from Bangs Laboratory (Fishers, IN).
The
32
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
Dojindo cell counting kit CCK-8 was purchased from Dojindo Molecular
Technologies
(Rockville, MD, USA). Human pancreatic cancer tissue and normal tissue arrays
(PA1002a)
were purchased from US Biomax (Derwood, MD).
Cell Culture.
PANC-1, BxPC-3, Capan-1, Capan-2 and HPNE cells were purchased from ATCC
(Manassas, VA). HPDE cells were purchased from Kerafast (Boston, MA). PANC-1,
Dulbecco's Modified Eagle's Medium with 10% FBS, BxPC-3, RPMI-1640 Medium with
10%
FBS; Capan-1, Iscove's Modified Dulbecco's Medium with 20% FBS, Capan-2,
Modified
McCoy's 5a Medium with 10% FBS; HPNE, 75% DMEM without glucose with additional
2
mM L-glutamine and 1.5 g/L sodium bicarbonate, 25% Medium M3 Base, FBS 5%, 10
ng/ml
human recombinant EGF, 5.5 mM D-glucose (lg/L), 750 ng/ml puromycin; HPDE,
Keratinocyte Basal Medium + supplied supplements (Lonza, Clonetics KBM, Cat#CC-
3111).
All cells were maintained at 37 C in a humidified incubator with 5% (vol/vol)
CO2.
Quantification of ICAM-1 Surface Expression.
Pancreatic cancer cell ICAM1 surface protein expression was evaluated by a BD
FACSCalibur flow cytometer (BD Biosciences) as described previously.
Quantification of the
ICAM-1 density on the cell surface was determined with reference to Quantum
Simply
.. Cellular microbeads, using the protocol as provided by the manufacturer.
106 cells were
collected and rinsed twice through suspension¨spin cycles. Cells were blocked
by 1% BSA in
PBS for 30 min in an ice bath. After BSA blockage, cells were incubated with
phycoerythrin
(PE) conjugated ICAM1 antibody for 1 hour at room temperature. Cells were
rinsed with 1%
BSA in PBS three times, resuspended in PBS, and evaluated by flow cytometry.
Immunohistological Staining.
Immunohistochemical studies were conducted on paraffin-embedded human PDAC and
normal tissue microarrays (PA1002a, US Biomax). Forty cases of human PDAC
tissue and ten
cases of human normal tissue microarray samples were evaluated for ICAM-1
expression as
described previously.The individual tissue cores in the microarrays were
scored by a surgical
pathologist, with no knowledge of sample identity. Immunostains were scored by
calculating
H-scores in which the percent of cells staining strong (3+), moderate (2+),
and weak (1+) were
multiplied according to the formula: H-score = 3 x (% of cells staining 3+) +
2 x (% of cells
33
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
staining 2+) + 1 x (% of cells staining 1+). Photomicrographs were taken on an
Olympus
BX41 microscope by using an Olympus Q-Color5 digital camera (Olympus America
Inc.).
In vitro binding and internalization of ICAM-1 Ab
The in vitro specific binding of ICAM1 antibody to the human pancreatic cancer
cell
lines was assessed using PE-ICAM1 antibody. IgG was used as the control. Cells
were seeded
in 8-well chamber slides at a density of 5x103 cells/well. After recovering
for 24 hours, the full
media was replaced with that containing PE-ICAM1 antibody, with 1% FBS. Cells
were
incubated with the PE-ICAM1-containing media at 37 C for an additional 4
hours. The cell
monolayer was then rinsed with cold phosphate-buffered saline (PBS) and fixed
with 4%
paraformaldehyde in the PBS solution. Cell nuclei were counterstained with
4',6'-diamidino-2-
phenylindole hydrochloride (DAPI) using ProLong Gold Antifade Mountant.
Fluorescence
images were acquired and analyzed using a Zeiss LSM 880 confocal microscope
(Oberkochen,
Germany).
In the imaging flow cytometry studies, cells were seeded in 6-well chamber
slides at a
density of lx106 cells/well. After cell recovery for 24 hours, the full media
was replaced with
that containing PE-ICAM1 antibody, with 1% FBS. Cells were incubated with the
PE-ICAM1-
containing media at 37 C forl hour. The cell monolayer was then collected and
rinsed with
cold PBS twice, resuspended and evaluated using an Amnis imagestreamX Mark II
imaging
flow cytrometry (Luminex, Austin, TX, USA).
Therapeutic effect of ICAM-1 Ab
The in vitro therapeutic effect of ICAM1 antibody to the human pancreatic
cancer cell
lines was assessed using quantitative phase imaging. IgG was used as the
control. Cells were
seeded in a 6-well plate at a density of 5x104 cells/well. After recovering
for 24 hours, the full
media was replaced with that containing ICAM1 antibody or IgG at a dosage of 2
1.tg/mL. Cells
were incubated with the ICAM1- or IgG-containing media at 37 C for 24 hours.
After that, the
plate was placed under a quantitative phase imaging microscope (Holomonitor
M4, Phase
Holographic Imaging Phi AB, Lund, Sweden) setting in an incubator and imaged
for an
additional 24 hours with a 5 min interval. Cell motion, morphology and
proliferation were then
analyzed using Hstutio4.
Preparation and Characterization of ADCs Ab-Gd.
34
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
IgG-DM1, ICAM1-DM1 were prepared by mix IgG or ICAM1 Ab (2.4 mg, 16 nmol)
with SMCC-DM1 (1.2 mg, 1.1umol) in a phosphate buffer (pH 7.2), rotating at
room
temperature for 1 h. Free SMCC-DM1 was removed by Ultra-4 Centrifugal Filter
(30K
MWCO). IgG-DM1, ICAM1-DM1 were washed with PBS (pH 7.4) for several times and
redispersed in PBS.
The ADCs were characterized by UV/Vis spectroscopy and antibody drug ratios
were
calculated according to the equation:
ADR = Cdrug/CAb
=( Az-v 280
6),(D)mAb¨A),(D)6280mAb) (A2806),(D)drug¨A),(D)6280drug)
/[(280drugE),(D)mAb¨C),(D)drugE280mAb)(6280mAbC),(D)drug¨C),(D)MAbC280drug)]
IgG-DTPA-Gd, ICAM1-DTPA-Gd were prepared as reported previously. DTPAA (0.4
mg, 1.1 Ilmol) was slowly added to IgG or ICAM1 Ab (0.2 mg, 1.3 nmol) in
NaHCO3 buffer
(pH 9.0, 0.1 M), and the mixture was rotated at room temperature overnight.
The DTPA-
conjugated IgG or ICAM1 Ab was purified by Ultra-4 Centrifugal Filter (30K
MWCO) and
redispersed in citrate buffer (pH 6.5, 0.1 M). Then GdC13 (0.1 mg, 0.27 Ilmol)
in 0.1 M citrate
buffer (pH 6.5) was mixed with DTPA-conjugated IgG or ICAM1 Ab for 24 h
rotated at room
temperature. The free Gd3+ was removed by Ultra-4 Centrifugal Filter (30K
MWCO), and IgG-
or ICAM1-DTPA-Gd was redispersed in PBS for subsequent use. The Ab-Gd were
characterized by liquid chromatography electrospray ionization mass
spectrometry LC-MS.
Cytotoxicity assays.
Human pancreatic cancer cell lines were seeded in a 96-well plate at a density
of 5x103
cells/well and allowed to adhere overnight. The culture medium was then
replaced with
medium containing free GEM, IgG or ICAM1 conjugated DM1, Duo, MMAE, MMAF at
different drug concentrations. After cells were cultured for another 72 hours,
the cytotoxicity
was determined by CCK-8 assay following the vendor-provided protocol. Cells
were carefully
rinsed with PBS after the drug-containing medium was removed, and this was
followed by
adding the CCK-8 containing medium solution. The cells were then incubated
with the CCK-8
medium for 4 hours. The plate was read at the absorbance wavelength of 450 nm
using a
microplate reader (5ynergy2; BioTek, Winooski, VT, USA). Cell viability was
determined by
comparing the absorbance of cells incubated with drugs to that of the control
cells incubated
without the presence of the drug.
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
Orthotopic PDAC mouse models.
All animal experiments were conducted following a protocol approved by the
Institutional Animal Care and Use Committee (IACUC) at Boston Children's
Hospital. PANC-
1 cells were transfected with a plasmid expressing the luciferase and GFP
genes according to
the manufacturer's instructions (MGH, Boston). Successful gene transfer was
confirmed
72 hours after infection, by the visualization of GFP on fluorescence
microscopy. Stably
transfected cells are sorted twice by flow cytometry for GFP signal using a
FACSAria II flow
cytometry (BD Biosciences), and maintained in DMEM-10% FBS. The orthotopic
pancreatic
cancer model was prepared by injecting PANC-1 cells into the pancreas of 8-
week-old male
athymic nude mice (Charles River; n = 6 for each group) using an established
surgical method.
Mice were anesthetized by isofluorane (5% with 02) during the surgery.
Incision was made on
the left flank of the abdominal region where the pancreas is typically
located, behind the
middle of the spleen. The pancreas was then gently pulled out using forceps
and 50 pt lx106
PANC-1 cells was carefully injected into the pancreas. After injection, the
pancreas was placed
back in the abdominal cavity before the abdominal muscle and the skin were
closed with 4-0
Polysorb sutures and surgical staples. Treatment was started after two weeks
of recovery.
Animals were randomly divided into four groups (n=6): PBS control, treated
with gemcitabine
(GEM), treated with nontargeted IgG-DM1, and treated with ICAM1-DM1. The mice
were
treated through intravenous tail vein injection at a dose of 12 mg/kg mouse
weight per three
weeks for IgG-DM1 and ICAM1-DM1, 80 mg/kg mouse weight twice a week for GEM,
while
the control group received only PBS injection. In total, there were two
injections with 3-week
intervals for ADC treated and control groups, and 12 injections with 3- or 4-
day intervals for
GEM. The body weights were measured twice a week, and tumor growth was
monitored using
the IVIS Spectrum Imaging System (PerkinElmer) after mice received i.p.
injection of D-
luciferin.
In Vivo MRI.
In vivo MRI was performed on the tumor-bearing mice in two groups, which were
injected intravenously with IgG-Gd and ICAM-Gd (at the dosage of 5 mg/kg mouse
weight),
respectively. Images were obtained at pre- and 24 hours-post injection with a
9.4 T Bruker
Horizontal Bore MRI with turbo spin echo sequence for Ti- and T2-weighted MRI.
The
imaging parameters were as follows: repetition time (TR) of 1,523 ms, TE of 33
ms, 340 x 220
matrix, 40 x 28-mm2 field of view, 180 flip angle, and 0.6-mm slice thickness
for T2-
weighted imaging; TR of 700 ms and TE of 22 ms for Ti-weighted imaging. To
quantify the
36
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
signal intensity for tumor, regions of interest (ROIs) were drawn around the
whole tumor at the
same slice with the same imaging depth. The pixel intensity was calculated and
normalized to
the area of ROIs by ImageJ software.
Histology.
The organs (liver, spleen, kidney, pancreas, heart, lung and muscle) and tumor
samples
were collected at the end point. Pathologies of orthotopic PANC-1 tumors
treated with ICAM-
DM1, IgG-DM1, GEM and PBS were investigated by H&E staining, Ki67 staining,
and
ICAM1 immunohistological staining. All staining was performed for the tumor
slices
following the standard protocol.
Statistical Analysis.
Quantitative data are presented as means SD. Differences were compared using
an
unpaired t test. Statistics were performed using Microsoft Excel software. P
values <0.05 were
considered statistically significant.
Examples of the structures of the linker and drug in the ADCs
The linker and drug structures used in the present disclosure are provided
below.
Name: SMCC-DM1
Chemical Name: N2'-deacetyl-N243-M-P4[(2,5-dioxo-1-pyrrolidinyl)oxy]carbonyl]
cyclohexyl]methy1]-2,5-dioxo-3-pyrrolidinyl]thio]-1-oxopropyl]-maytansine
Chemical Structure:
No
o
e,
'\
d N
r
Name: VC-MMAE (MC-VC-PAB-MMAE)
Chemical Name: 4-((S)-2-((S)-2-(6-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-
yl)hexanamido)-3-
methylbutanamido)-5-ureidopentanamido)benzyl ((5)-1-(((S)-1-(((3R,45,55)-1-
((S)-2-
37
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
((1R,2R)-3-(((lS,2R)-1-hydroxy-1-phenylpropan-2-y1)amino)-1-methoxy-2-methyl-3-
oxopropyl)pyrrolidin-1- y1)-3 -methoxy-5-methyl-1 -oxoheptan-4-
yl)(methyl)amino)-3 -methyl-
1-oxobutan-2-yl)amino)-3-methyl-1-oxobutan-2-y1)(methyl)carbamate
Chemical Structure:
112
0
`.1 HW =":-." 'I<
ty0L0 0
õ
o r ,
ky k 9 H
Name: MC-MMAF
Chemical Name: ((2R,3R)-3-((S)-1-((3R,45,55)-4-((S)-2-((S)-2-(6-(2,5-dioxo-2,5-
dihydro-1H-
pyrrol-1-y1)-N-methylhexanamido)-3-methylbutanamido)-N,3-dimethylbutanamido)-3-
methoxy-5-methylheptanoyl)pyrrolidin-2-y1)-3-methoxy-2-methylpropanoy1)-L-
phenylalanine
Chemical Structure:
0
(3k)
, A
';)
Name: Mal-PEG4-VC-PAB-DMEA-Seco-Duocarmycin
Chemical Name: methyl (85)-4424[4-[[(25)-5-(carbamoylamino)-2-[[(25)-
24342424242-
[3-(2,5-dioxopyrrol-1-
y1)propanoylamino]ethoxy]ethoxy]ethoxy]ethoxy]propanoylamino]-3-
methylbutanoyl]amino]pentanoyl]amino]phenyl]methoxycarbonyl-methylamino]ethyl-
methylcarbamoyl]oxy-8-(chloromethyl)-6-(5,6,7-trimethoxy-1H-indole-2-carbony1)-
7,8-
dihydro-3H-pyrrolo[3,2-e]indole-2-carboxylate
Chemical Structure:
38
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
0
,
0
z
Ne V
s \,..."õ=;:fA, ,N---1..,s;n=ksoõ":w,s,\,,,N, õ
0 \1..4 0=
0,714
9 0
0
0
0
EQUIVALENTS AND SCOPE
Those skilled in the art will recognize, or be able to ascertain using no more
than
routine experimentation, many equivalents of the embodiments described herein.
The scope of
the present disclosure is not intended to be limited to the above description,
but rather is as set
forth in the appended claims.
Articles such as "a," "an," and "the" may mean one or more than one unless
indicated
to the contrary or otherwise evident from the context. Claims or descriptions
that include "or"
between two or more members of a group are considered satisfied if one, more
than one, or all
of the group members are present, unless indicated to the contrary or
otherwise evident from
the context. The disclosure of a group that includes "or" between two or more
group members
provides embodiments in which exactly one member of the group is present,
embodiments in
which more than one members of the group are present, and embodiments in which
all of the
group members are present. For purposes of brevity those embodiments have not
been
individually spelled out herein, but it will be understood that each of these
embodiments is
provided herein and may be specifically claimed or disclaimed.
It is to be understood that the disclosure encompasses all variations,
combinations, and
permutations in which one or more limitation, element, clause, or descriptive
term, from one or
more of the claims or from one or more relevant portion of the description, is
introduced into
another claim. For example, a claim that is dependent on another claim can be
modified to
include one or more of the limitations found in any other claim that is
dependent on the same
base claim. Furthermore, where the claims recite a composition, it is to be
understood that
methods of making or using the composition according to any of the methods of
making or
using disclosed herein or according to methods known in the art, if any, are
included, unless
otherwise indicated or unless it would be evident to one of ordinary skill in
the art that a
contradiction or inconsistency would arise.
39
CA 03151800 2022-02-16
WO 2021/041171
PCT/US2020/047300
Where elements are presented as lists, e.g., in Markush group format, it is to
be
understood that every possible subgroup of the elements is also disclosed, and
that any element
or subgroup of elements can be removed from the group. It is also noted that
the term
"comprising" is intended to be open and permits the inclusion of additional
elements or steps.
It should be understood that, in general, where an embodiment, product, or
method is referred
to as comprising particular elements, features, or steps, embodiments,
products, or methods
that consist, or consist essentially of, such elements, features, or steps,
are provided as well.
For purposes of brevity those embodiments have not been individually spelled
out herein, but it
will be understood that each of these embodiments is provided herein and may
be specifically
claimed or disclaimed.
Where ranges are given, endpoints are included. Furthermore, it is to be
understood
that unless otherwise indicated or otherwise evident from the context and/or
the understanding
of one of ordinary skill in the art, values that are expressed as ranges can
assume any specific
value within the stated ranges in some embodiments, to the tenth of the unit
of the lower limit
of the range, unless the context clearly dictates otherwise. For purposes of
brevity, the values
in each range have not been individually spelled out herein, but it will be
understood that each
of these values is provided herein and may be specifically claimed or
disclaimed. It is also to
be understood that unless otherwise indicated or otherwise evident from the
context and/or the
understanding of one of ordinary skill in the art, values expressed as ranges
can assume any
.. subrange within the given range, wherein the endpoints of the subrange are
expressed to the
same degree of accuracy as the tenth of the unit of the lower limit of the
range.
Where websites are provided, URL addresses are provided as non-browser-
executable
codes, with periods of the respective web address in parentheses. The actual
web addresses do
not contain the parentheses.
In addition, it is to be understood that any particular embodiment of the
present
disclosure may be explicitly excluded from any one or more of the claims.
Where ranges are
given, any value within the range may explicitly be excluded from any one or
more of the
claims. Any embodiment, element, feature, application, or aspect of the
compositions and/or
methods of the disclosure, can be excluded from any one or more claims. For
purposes of
brevity, all of the embodiments in which one or more elements, features,
purposes, or aspects
is excluded are not set forth explicitly herein.