Note: Descriptions are shown in the official language in which they were submitted.
WO 2021/080923
PCT/US2020/056359
ABSORBABLE VASCULAR FILTER
CROSS-REFERENCE TO RELATED APPLICATIONS
100011 This application claims priority to: CIP Patent Application No.
16/659,536, filed
October 21, 2019, which is a continuation in part of U.S. Patent Application
Serial No.
13/403,790 entitled "Absorbable Vascular Filter" to Mitchell Eggers,
electronically filed
February 23, 2012, which is a continuation in part of U.S. Patent Application
Serial No.
13/096,049 entitled "Vascular Filter Stent" to Mitchell Eggers, electronically
filed April 28,
2011, which is a continuation in part of U.S. Patent Application Serial No.
13/036,351 entitled
"Absorbable Vascular Filter" to Mitchell Eggers, electronically filed on
February 28, 2011, all
of which are expressly incorporated herein by reference in their entirety.
FIELD OF THE INVENTION
[0002] The present invention relates generally to a vascular filter and more
particularly to an
absorbable vascular filter deployed within a vessel for temporary filtering of
body fluids. An
embodiment is configured for the placement of such absorbable vascular filter
within the
inferior vena cava (IVC) for the prevention of pulmonary embolisms for a
specific duration of
time determined by the absorption properties of the filter.
BACKGROUND OF THE INVENTION
100031 Between 100,000 to 300,000 Americans die annually from pulmonary
embolism (PE)
¨ more than breast cancer and AIDS combined ¨ representing the 3rd leading
cause of death
in the US [1-5]. A similar incidence of PE is found in Europe with
approximately 370,000
annual deaths [6]. Moreover, PE is the 3rd most common cause of death in
trauma patients that
survive the first 24 hours. An estimated 25% of all hospitalized patients have
some form of
deep vein thrombosis (DVT) which is often clinically unapparent unless PE
develops [7]. On
average, 33% of DVT will progress to symptomatic PE of which 10% will be fatal
[6].
100041 The US Surgeon General has recognized this alarming statistic and in
2008 issued a
formal Call to Action to Prevent DVT and PE [1]. Unfortunately, DVT/PE
disproportionately
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
2
affects the elderly, in part due to prolonged periods of inactivity following
medical treatment.
The incidence is relatively low under the age of 50 (1/100,000), then
accelerates exponentially
reaching 1000/100,000 by the age of 85 [8]. Consequently the US Surgeon
General has
proclaimed that the growth in number of DVT/PE cases with an aging US
population may
outpace the population growth in the absence of better prevention [1].
[0005] Risk factors for PE arising from DVT follow Virchow's Triad [9]: (i)
endothelial injury,
(ii) hypercoaguability, and (iii) hemodynamic changes (stasis or turbulence).
Hence specific
risk factors include hip and knee arthroplasty, abdominal, pelvic and
extremity surgeries, pelvic
and long bone fractures, prolonged immobility such as prolonged hospital stays
and air travel,
paralysis, advanced age, prior DVT, cancer, obesity, COPD, diabetes and CHF.
Orthopedic
surgeons are especially concerned since their patients carry a 40%-80% risk
for DVT and PE
following knee and hip surgeries in the absence of prophylactic treatment [10-
12].
[0006] The American Academy of Orthopaedic Surgeons (AAOS) has issued
guidelines for
PE prophylaxis. Basically, patients at standard risk should be considered for
chemoprophylactic agents such as aspirin, low molecular weight heparin (LMWH),
synthetic
pentassaccharides, or warfarin, in addition to intra-operative and/or
inunediate postoperative
mechanical prophylaxis [13].
[0007] Aspirin has a 29% relative risk reduction in symptomatic DVT and a 58%
relative risk
reduction in fatal PE [14]. LMWH carries a 30% risk reduction in DVT and has
been proven
more effective than unfractionated heparin in high risk groups such as hip and
knee arthroplasty
[7]. Warfarin started within 24 to 48 hours of initiating heparin with a goal
of achieving
international normalized ratio (INR) results between 2 and 3 as secondary
thromboprophylaxis
for 3 months reduces the risk of recurrent venous thromboembolism (VTE) by 90%
as
compared with placebo [15,16]. Mechanical prophylaxis, consisting of pneumatic
compression
devices that repeatedly compress the legs with an air bladder, are also
utilized in conjunction
with anticoagulants to reduce the occurrence of PE.
[0008] The duration of prophylaxis depends on the source of potential DVT.
Current
recommendations for prophylaxis consist of a minimum 7-10 days for moderate to
high risk
surgeries and up to 28-35 days for many orthopedic surgeries. Specifically for
orthopedic
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
3
trauma, DVT prophylaxis is continued until patient mobilization (32%),
inpatient discharge
(19%), 3 weeks postop (16%), 6 weeks postop (27%), and in rare circumstances
greater than 6
weeks (7%) [17]. Studies indicate that hypercoaguability persists for at least
one month after
injury in 80% of trauma patients [18]. Regarding total knee and hip
arthroplasty and cancer
surgeries, 35 day prophylactic treatment is recommended [12, 19]. Overall,
prophylactic
treatment for possible VTE is often warranted for up to 6 weeks following
trauma or major
surgery.
100091 Contraindications for chemoprophylaxis include active bleeding,
hemorrhagic
diathesis, hemorrhagic stroke, neurologic surgery, excessive trauma,
hemothorax, pelvic or
lower extremity fractures with intracranial bleeding, anticoagulation
interruption, and recent
DVT/PE patients undergoing surgery.
[0010] For patients who are contraindicated for the above-mentioned anti-
coagulation
prophylaxis, or where anti-coagulation therapy has failed, the AAOS, American
College of
Physicians, and the British Committee of Standards in Haematology all
recommend the use of
inferior vena cava (IVC) filters [13, 20, 21]. These intravascular metal
filters are deployed via
catheter into the IVC to essentially catch emboli arising from DVT before
reaching the lungs
resulting in PE. Furthermore, the British Committee of Standards in Hematology
recommends
nrc filter placement in pregnant patients who have contraindications to
anticoagulation and
develop extensive VTE shortly before delivery (within 2 weeks).
100111 The Eastern Association for Surgery of Trauma further recommends
prophylactic IVC
filters placed in trauma patients who are at increased risk of bleeding and
prolonged
immobilization 1221 Such prophylactic recommendation follows studies that
demonstrate a
low rate of PE in patients with severe polytraturia who underwent IVC
placement [23-25]. In
fact the fastest growing indication of overall NC filter usage, from 49,000 in
1999 to 167,000
in 2007 with a projected 259,000 units for 2012, is the prophylactic market
utilizing retrievable
IVC filters [26, 27].
100121 Example vascular filters primarily for IVC placement are disclosed in
U.S. Pat No.
4,425,908; U.S. Pat. No. 4,655,771, U.S. Pat. No. 4,817,600;U.S. Pat. No.
5,626,605;U.S. Pat.
No. 6,146,404; U.S_ Pat. No. 6,217,600 Bl; U.S. Pat No. 6,258,026 Bl;U.S. Pat.
No. 6,497,709
CA 03155354 2022-4-20
WO 20211080923
PCT/US2020/056359
4
Bl;U.S. Pat. No. 6,506,205 82;U.S. Pat. No. 6,517,559 Bl;U.S. Pat. No.
6,620,183 B2; U.S.
Pat App. Pub. No. 2003/0176888;U.S. Pat App. Pub. No. 2004/0193209;U.S. Pat
App. Pub.
No. 2005/0267512;U.S. Pat. App. Pub. No. 2005/0267515;U.S. Pat. App. Pub. No.
2006/0206138 Al; U.S. Pat. App. Pub. No. 2007/0112372 Al; U.S. Pat. App. Pub.
No.
2008/0027481 Al; U.S. Pat. App. Pub. No. 2009/0192543 Al; U.S. Pat. App. Pub.
No.
2009/0299403 Al;U.S. Pat. App. Pub. No. 2010/0016881 Al; U.S. Pat. App. Pub.
No.
2010/0042135 Al; and U.S. Pat. App. Pub. No. 2010/0174310 Al.
100131 INC filter efficacy has been demonstrated in several class I and II
evidence studies [22,
28-30]. Most of the earlier filters installed were expected to be permanent
fixtures since
endothelialization occurs within 7-10 days making most models impractical to
remove without
irreversible vascular damage leading to life threatening bleeding, dissection
of the INC. and
thrombosis. Although these permanent filters have prevented PE, they have been
shown to
actually increase the risk of recurrent DVT over time.
100 141 Specifically, a Cochrane review [31] on the use of IVC filters for the
prevention of PE
cites a level I randomized prospective clinical trial by Decousus et al. [32]
wherein the
incidence of DVT with the IVC filter cohort increased almost 2-fold: (i) 21%
incidence of
recurrent DVT in the filter cohort vs. 12% in the non-filter LMWH cohort at 2
years (p = 0.02),
and (ii) 36% incidence of recurrent DVT in the filter cohort vs. 15% in the
non-filter group at
8 years (p = 0.042) [33]. However, the filters did reduce the occurrence of
PE; the filter cohort
experiencing only 1% PE vs. the non-filter cohort posting 5% PE in the first
12 days (p =0.03)..
No statistically significant difference in mortality rate was seen in any time
frame investigated.
Apparently the initial benefit of reduced PE with permanent IVC filters is
offset by an increase
in DVT, without any difference in mortality.
100151 In addition to increased incidence of DVT for prolonged IVC filter
deployment, filter
occlusion has been reported with a 6% to 30% occurrence, as well as filter
migration (3% to
69%), venous insufficiency (5% to 59%), and post thrombotic syndrome (13% to
41%) [34-
36]. Complications from insertion including hematoma, infection, pneumothorax,
vocal cord
paralysis, stroke, air embolism, misplacement, tilting arteriovenous fistula,
and inadvertent
carotid artery puncture have an occurrence rate of 4% - 11% [37].
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
[0016] Temporary or retrievable IVC filters have been marketed more recently
intended to be
removed once the risk of PE subsides, and hence circumvent many of the
deleterious
complications of permanent filters. The retrievable filters feature flexible
hooks, collapsing
components, and unrestrained legs to ease retrieval. Unfortunately these same
features have
led to unwanted filter migration, fatigue failure, INC penetration, fragment
migration to hepatic
veins and pulmonary arteries, filter tilt, and metallic emboli [38-43]. Since
2005, 921 adverse
filter events have been reported to the FDA including 328 device migrations,
146 device
detachments (metallic emboli), 70 perforations of the IVC, and 56 filter
fractures [44]. Some
retrievable brands post alarming failure rates such as the Bard Recovery
filter with 25%
fracturing over 50 months which embolized end organs. 71% of the fractures
embolized to the
heart caused life threatening ventricular tachycardia, tamponade, and sudden
death in some
cases. An alternative retrievable model, Bard 62, resulted in 12% fractures
over 24 months
[45]. Such prevalence of device fractures is postulated to be directionally
proportional to
indwell time.
[0017] These failures and others prompted the FDA in August 2010 to issue a
formal
communication stating that "FDA recommends that implanting physicians and
clinicians
responsible for the ongoing care of patients with retrievable INC filters
consider removing the
filter as soon as protection from PE is not longer needed" [44]. Even though
these types of
retrievable filters are intended to be removed in months time, several studies
indicate that
approximately 70%-81% of patients with retrievable IVC filters fail to return
to the hospital
for filter removal, thereby exposing hundreds of thousands of patients to the
life-threatening
adverse events of prolonged retrievable IVC filter placement [41, 44, 46-48].
These patients
are either lost to follow-up, or refuse to have the filters removed in the
absence of
complications.
BRIEF SUMMARY OF THE INVENTION
[0018] The present invention comprises systems and methods for filtering
fluids. Certain
embodiments comprise a novel absorbable vascular filter that temporarily
prevents pulmonary
embolism by capturing and restraining emboli within a body vessel. The
absorbable vascular
filter, according to certain aspects of the invention, possesses various
advantages over all
conventional vascular filters, including permanent, temporary, and optional
INC filters. Most
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
6
importantly, the absorbable vascular filter disclosed herein is slowly
biodegraded within the
vessel according to a planned schedule engineered by the choice of absorbable
filter materials
which prevents the requirement of filter removal. Moreover, the absorbable
vascular filter
elements are manufactured from non-metallic synthetic polymers which do not
adversely
impact end organs upon carefully planned degradation as exhibited by
conventional metal IVC
filters that migrate and often become fractionated. Also due to the relative
short indwell time
(months) of the absorbable vascular filter, the paradoxical increase in DVT
seen with
conventional long-term IVC filters is likely circumvented.
BRIEF DESCRIPTION OF THE DRAWINGS
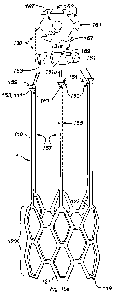
[0019] Fig. 1 a is a cut-away isometric view of one embodiment of the
absorbable vascular
filter that includes phased sequential biodegradation of the absorbable
capture elements.
[0020] Fig. lb features the capture elements of Fig. la in detail.
[0021] Fig. lc features the capture elements of Fig. lb at a later point in
time wherein the
proximal portion of the capture elements has been bioabsorbed/biodegraded.
[0022] Fig. Id features the capture elements of Fig lc at a later point in
time wherein the
proximal and middle sections of the capture elements have been
bioabsorbed/biodegraded,
leaving only the distal section.
100231 Fig. le represents complete bioabsorptiontbiodegradation of the capture
elements of
Fig. lb at the most distant point in time.
[0024] Fig. 2a is a cross-sectional schematic of another embodiment of the
absorbable vascular
filter that also features phased sequential biodegradation of the absorbable
capture elements.
[0025] Fig. 2b is an enlarged end-view of the absorbable capture elements of
the absorbable
filter depicted in Fig. 2a.
[0026] Fig. 2c depicts the capture elements of Fig. 2b at the time of filter
installation in a vessel.
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
7
[0027] Fig. 2d depicts the capture elements of Fig. 2c at a later point in
lime wherein the inner
capture ring element has been bioabsorbed/biodegraded.
[0028] Fig. 2e depicts the capture elements of Fig. 2d at a later point in
time wherein a
circumferential-mounted capture element has been bioabsorbed/biodegraded.
[0029] Fig. 2f depicts the capture elements of Fig. 2e at a later point in
time wherein two
circumferential-mounted capture elements have been bioabsorbed/biodegraded.
[0030] Fig. 2g depicts the capture elements of Fig. 2f at a later point in
time wherein only one
circumferential-mounted capture element remains following bioabsotption/
biodegradation.
[0031] Fig. 2h depicts the capture elements of Fig 2b which have completely
been
bioabsorbed/biodegraded at the most distant point in time.
[0032] Fig. 3a is a cut-away isometric view of one embodiment of the vascular
filter that
includes a plurality of capture elements attached to the stent for filtering
substances such as
emboli.
[0033] Fig. 3b features the capture elements of Fig. 3a in detail.
[0034] Fig. 4a is an absorbable vascular filter constructed from polydioxanone
suture sizes 3-
0, 2-0, 0, and 1 in a webbed pattern that features sequential degradation
based on the varying
diameters and expiration dates of the capture elements.
[0035] Fig. 4b is an absorbable vascular filter constructed from polydioxanone
suture similar
in design to the webbed design in Fig. 4a except that only size 2-0 is
utilized.
[0036] Fig. 4c is an absorbable vascular filter constructed from polydioxanone
suture size 2-0
in a radial pattern typical of traditional IVC filters.
CA 03155354 2022-4-20
WO 20211080923
PCT/US2020/056359
8
100371 Fig. 4d is an absorbable vascular filter constructed from polydioxanone
suture sizes 3-
0, 2-0, 0, and 1 in a radial pattern that features sequential degradation
based on the varying
diameters of the capture elements.
100381 Fig. 5 displays photographs of the absorbable filter presented in Fig.
4a during in-vitro
testing at weeks 0, 7, 13-22 to reveal the sequential degradation of the
filter loosing 1 to 2
capture elements per week beginning in week 13 and reaching final
disintegration by week 22.
100391 Fig. 6 is a graph of the mean load at break (kg/strand) of
polydioxanone capture
elements vs. time during the in-vitro testing.
[0040] Fig. 7 is a graph of polydioxanone capture element strength retention
as a percentage
of the original strength vs. time.
[0041] Fig. 8 is a graph of Young's modulus for polydioxanone capture elements
vs. time
during the in-vitro testing.
[0042] Fig. 9a is a cross-sectional schematic revealing a method for
installing the absorbable
vascular filter using a catheter-based system with the filter in compressed
mode.
100431 Fig. 9b is a cross-sectional schematic detailing the deployment of the
absorbable
vascular filter using a catheter-based system with sliding outer sheath to
deploy the filter in the
fully expanded mode.
[0044] Fig. 9c is a cross-sectional schematic detailing the removal of the
central stabilizing rod
or piston used to stabilize the absorbable vascular filter while removing the
outer sheath of the
catheter-based installation system.
[0045] Fig. 9d illustrates the operation of the absorbable vascular filter in
the presence of an
embolus in the vessel.
[0046] Fig. 9e represents the vessel following complete
biodegradation/bioabsorption of the
absorbable vascular filter.
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
9
100471 Fig. 10a represents an embodiment of the absorbable vascular filter
constructed of a
braided or woven stent integrated with a capture basket.
[0048] Fig. 10b is the associated top view of the absorbable vascular filter
shown in Fig. 10a.
[0049] Fig. 11 is an expanded view of the braid or weave of absorbable
elements comprising
the stent section of the absorbable vascular filter.
[0050] Fig. 12 is an expanded view of the braid or weave of absorbable
elements comprising
both the stent section and capture basket for the integrated absorbable
vascular filter.
[0051] Fig. 13a is a photograph of an integrated absorbable IVC filter woven
with a single
synthetic filament.
[0052] Fig. 13b is an end-view photograph of the integrated absorbable IVC
filter presented in
Fig. 13a.
[0053] Fig. 14a is an isometric view of one embodiment of an absorbable
vascular filter that is
cut from a generally tubular material whereby the filter apex is formed by
securing capture
elements with a filament.
100541 Fig. 14b is a corresponding isometric view of the embodiment of the
absorbable
vascular filter that is cut from a generally tubular material whereby the
filter apex is formed by
securing capture elements with a filament.
[0055] Fig.15a is an isometric view of one embodiment of an absorbable
vascular filter that is
cut from a generally tubular material whereby the filter apex is formed by
securing capture
elements with an end plate having splines.
[0056] Fig. 15b is a corresponding isometric view of the embodiment of the
absorbable
vascular filter that is cut from a generally tubular material whereby the
filter apex is formed by
securing capture elements with an end plate having splines.
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
100571 Fig. 16a is an isometric view of one embodiment of an absorbable
vascular filter that is
cut from a generally tubular material whereby the filter apex is formed by
securing capture
elements with an end plate having mating connection shafts.
100581 Fig. 16b is a corresponding isometric view of the embodiment of the
absorbable
vascular filter that is cut from a generally tubular material whereby the
filter apex is formed by
securing capture elements with an end plate having mating connection shafts.
100591 Fig. 17a is an isometric view of one embodiment of an absorbable
vascular filter that is
cut from a generally tubular material whereby the filter apex is formed by
securing capture
elements with an end plate.
100601 Fig. 17b is a corresponding isometric view of the embodiment of the
absorbable
vascular filter that is cut from a generally tubular material whereby the
filter apex is formed by
securing capture elements with an end plate.
100611 Fig. 18a is an isometric view of one embodiment of an absorbable
vascular filter where
the circumferential element is cut from a generally tubular material and the
filter basket is
formed from absorbable filament capture elements that are linked together and
secured at the
apex with an end plate.
100621 Fig. 18b is a corresponding isometric view of the embodiment of the
absorbable
vascular filter where the circumferential element is cut from a generally
tubular material and
the filter basket is formed from absorbable filament capture elements that are
linked together
and secured at the apex with an end plate.
DETAILED DESCRIPTION OF THE INVENTION
100631 Embodiments of the present invention will now be described in detail
with reference to
the drawings and pictures, which are provided as illustrative examples so as
to enable those
skilled in the art to practice the invention. Notably, the figures and
examples below are not
meant to limit the scope of the present invention to a single embodiment, but
other
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
11
embodiments are possible by way of interchange of some or all of the described
or illustrated
elements. Wherever convenient, the same reference numbers will be used
throughout the
drawings to refer to same or like parts. Where certain elements of these
embodiments can be
partially or fully implemented using known components, only those portions of
such known
components that are necessary for an understanding of the present invention
will be described,
and detailed descriptions of other portions of such known components will be
omitted so as not
to obscure the invention. In the present specification, an embodiment showing
a singular
component should not be considered limiting; rather, the invention is intended
to encompass
other embodiments including a plurality of the same component, and vice-versa,
unless
explicitly stated otherwise herein. Moreover, applicants do not intend for any
term in the
specification or claims to be ascribed an uncommon or special meaning unless
explicitly set
forth as such. Further, the present invention encompasses present and future
known equivalents
to the components referred to herein by way of illustration.
[0064] Referring to the embodiment depicted in Figs. la-e, an absorbable
vascular filter 1
comprises an outer, circumferential element 2 for supporting a plurality of
absorbable filter
capture elements (30-32, 40-41). The capture elements are purposely designed
to be
biologically absorbed and/or degraded in a sequential manner to avoid
simultaneous
detachment of the entire filter causing an unexpected embolus. Sequential
degradation can be
controlled by the choice of absorbable polymers that possess different
absorption profiles,
diameter, and/or expiration dates. Additionally, absorptive linkages may be
incorporated to
serves as detachment points during absorption. The sequential
bioabsorption/biodegradation
is illustrated in Figs. 1 b-e where decomposition begins with the proximal
capture elements 30,
progressing to the middle section capture elements 31, and finally full
bioabsorption/biodegradation as depicted in Fig. le.
100651 Such engineered, sequential bioabsorption/biodegradation of the capture
elements can
be achieved with numerous synthetic materials. The goal is to select the
absorbable filter
materials to match a desired filter indwell time. Per the prior background
section, a filter
indwell time of 6 weeks would be suitable for an IVC filter to prevent PE
following trauma or
in conjunction with major surgeries. Synthetic materials which can be used to
form the capture
elements include:
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
12
100661 Polydioxanone (PDO, PDS) ¨ colorless, crystalline, biodegradable
synthetic polymer
of multiple repeating ether-ester units. In suture form, PDS II (Ethicon,
Somerville, NJ) size
4/0 and smaller maintains 60%, 40%, and 35% of its tensile strength at 2, 4,
and 6 weeks
respectively. For PDS II size 3/0 and larger, it retains 80%, 70%, and 60% of
its tensile strength
at 2, 4, and 6 weeks respectively. In addition to providing wound support for
6 weeks, PDS II
suture is fully absorbed in 183-238 days via hydrolysis making it a strong
candidate for IVC
filter applications. Basically absorption is minimal in the first 90 days and
is essentially
complete in 6 months. Finally, PDS has a low affinity for microorganisms and
possesses
minimal tissue reaction.
100671 Polytrimethylene carbonate (Maxon) - similar to PDS in absorption
profile yet with
slightly higher breaking strength. Maxon (Covidien, Mansfield, MA) maintains
81%, 59%,
and 30% of its tensile strength at 2,4, and 6 weeks respectively, and is fully
hydrolyzed in 180-
210 days.
100681 Polyglactin 910 (Viciyl) ¨ braided multifilament coated with a
copolymer of lactide
and glycolide (polyglactin 370). In suture form, Vicryl (Ethicon) size 6/0 and
larger maintains
75%, 50%, and 25% of its tensile strength at 2,3, and 4 weeks respectively and
is fully absorbed
in 56-70 days.
100691 Polyglycolic acid (Dexon) ¨ similar to Polyglactin, made from
polyglycolic acid and
coated with polycaprolate. Dexon has similar tensile strength and absorption
profile as
Polyglactin.
100701 Poliglecaprone 25 (Monocryl) ¨ synthetic copolymer of glycolide and e-
caprolactone.
Monocryl (Ethicon) maintains 50%-70% and 20%-40% of its tensile strength at 1
and 2 weeks
respectively and is fully absorbed in 91-119 days.
100711 Polylacticoglycolic acid (PLGA) copolymer of monomers glycolic acid and
lactic acid.
Different forms and properties of PLGA can be fabricated by controlling the
ratio of lactide to
glycolide for polymerization. Like the other synthetic absorbable materials,
PLGA degrades
by hydrolysis with the absorption profile dependent on the monomer ratio; the
higher content
of glycolide, the faster degradation. However, the 50:50 copolymer exhibits
the fastest
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
13
degradation at 2 months. Since the polymer degrades in the body to produce
lactic acid and
glycolic acid, both being normal physiological substances, PLGA poses minimal
systemic
toxicity.
100721 Poly L-lactic Acid (PLA) is also a polymer made from lactic acid yet
with considerable
longevity. In soft tissue approximation, PLA remains intact for 28 weeks, and
is fully absorbed
within 52 weeks.
100731 As an example of engineering capture elements to sequentially degrade
following the
period of PE protection, the proximal capture elements 30,41 could be
fabricated with PDS II
size 4/0 (0.15mm dia.), while the middle capture elements 31,40 fabricated
with size 2/0
(0.3mm dia.), and finally the distal capture elements 32 fabricated with size
2 (0.5mm) PDS II
suture.
100741 As an alternative to assembling a plurality of capture elements, the
vascular filter can
be fabricated with absorbable or non-absorbable composite mesh. Candidates for
a mesh
capture system include polypropylene such as C-QUR (Atrium Medical Corp.
Hudson NH),
polypropylene encapsulated by polydioxanone as in PROCEED (Ethicon,
Somerville, NJ),
polypropylene co-knitted with polyglycolic acid fibers as in Bard Sepramesh IP
Composite
(Davol, Inc., Warwick, RI), polyethylene terephathalate as in Parietiex
Composite (Covidien,
Mansfield, MA), and ePTFE used in DUALAMESH (W. Gore & Assoc. Inc., Flagstaff,
AZ).
100751 Regarding the circumferential element 2 in Figs. 1, 2, and 3 that
serves to support the
capture elements of the absorbable vascular filter and maintain filter
positioning within the
vessel upon expansion from a catheter, either an absorbable material such as
described above
or non-absorbable material can be utilized. A non-absorbable material would
essentially serve
as a permanent steal, lasting well beyond the life of the absorbable capture
elements. This may
be an important option in cases where the vessel needs assistance in
maintaining patency. Both
types of circumferential elements 2 may incorporate barbs 79 (refer Fig. 2) to
maintain filter
positioning upon deployment. Plausible non-absorbing materials for
constructing the
circumferential element include: Nitinol, Elgiloy, Phynox, 316 stainless
steel, MP35N alloy,
titanium alloy, platinum alloy, niobium alloys, cobalt alloys, and tantalum
wire.
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
14
[0076] Figs, 2a-2h illustrate another embodiment of the absorbable vascular
filter wherein the
absorbable capture elements 60-Mare mounted to a simple circumferential
element 2 held
against the vessel wall 70 with optional barbs 79. Here again the
circumferential element 2 can
be fabricated with absorbable or non-absorbable materials of the like
described above. An
enlarged cross-sectional view of the capture element assembly 65 is shown in
Fig. 2b. Notice
that the sequential degradation of the capture elements is achieved by varying
the diameter of
the chosen absorbable material. For example, the inner capture element 60
could be PDS II 4/0
(0.15min dia.) resulting in the fastest absorption as illustrated in Fig. 2d
at time ti, followed by
capture element 61 degradation being PDS II 3/0 (0.20mm dia.) at time b in
Fig. 2e, followed
by capture element 62 degradation being PDS II 2/0 (0.30mm dia.) at time t3 in
Fig. 2f, followed
by capture element 63 degradation being PDS II 0 (0.35mm dia.) at time ta in
Fig. 2g, and
finally the degradation of the last capture element 64 constructed of PDS Ill
(0.40mm dia.) at
time t5 in Fig. 211. Although these dimensions represent a specific example,
any diameters
within approximately 0.1mm to 0.7mm would suffice. Overall, a gradual
progression of
degradation is designed purposely following a prophylactic window of 6 weeks
for trauma and
major surgery applications.
[0077] Referring to the embodiment depicted in Figs. 3a and b, a vascular
filter 1 comprises
an outer, circumferential stent 2 for supporting a plurality of collapsible
filter capture elements
(60-64) and to maintain vessel patency. The capture elements are purposely
designed to be
collapsible for catheter-based installation and to avoid end organ damage. The
supporting stent
2 is shown to be fabricated as an artificial vascular graft supported by
undulating supporting
structures 3. This vascular filter, which can be comprised of absorbable or
non-absorbable filter
capture elements, possesses various advantages over all conventional vascular
filters, including
permanent, temporary, and optional IVC filters. Most importantly, the vascular
filter is
fabricated with a stent that serves as a circumferential mount for the capture
elements in
addition to providing vessel patency, and avoids endothelialization
characteristic of metal
filters with barbed struts. Hence the increased incidence of DVT observed with
metal IVC
filters due to inherent vessel damage from the metal struts is likely
obviated.
[0078] The circumferential stent element 2 in Fig. 3a serves to support the
capture elements of
the vascular filter, in addition to maintaining vessel patency and maintaining
stationary filter
positioning within the vessel upon expansion. Numerous types of stents
conventionally
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
employed as thoracic endoprostheses can be utilized. Such stents would include
Gore TAG,
Medtronic Talent and Valiant Systems, and Cook Zenith TX2 System. In
particular, the Gore
TAG is comprised of an artificial vascular graft fabricated with a
fluoropolymer (expanded
polytetrafluoroethylenee PTFE and fluorinated ethylene propylene or FEP)
combined with a
Nitinol supporting structure. Alternatively, the stent component of the
vascular filter can be
fabricated with only the supporting structure (without the artificial vascular
graft) utilizing
nickel-titanium alloy (Nitinol), cobalt-chromium-nickel alloy (Elgiloy),
cobalt-chromium-
nickel-molybdenum alloy (Phynox), 316 stainless steel, MP35N alloy, titanium
alloy, platinum
alloy, niobium alloys, cobalt alloys, and tantalum wire.
100791 A specific embodiment of an absorbable vascular filter with sequential
degradation was
constructed, tested, and evaluated with assorted polydioxanone sutures (sizes
3-0, 2-0, 0, and
1) and is shown in Fig. 4a. The filter featured higher density webbing than
shown in Fig. 2b to
catch smaller emboli. Polydioxanone was a candidate polymer based on tension
retention and
absorption properties proven in wound approximation applications. Tygon long
flex lifetime
tubing (Saint-Gobain Performance Plastics, Akron, OH) with 25.4mm id similar
to the 1VC
was utilized for the vessel wall wherein polydioxanone was fabricated into the
various filter
patterns shown.
100801 Fig. 4a sports webbed capture elements that are purposely designed for
sequential or
phased absorption to avoid simultaneous detachment of the entire filter during
absorption. Here
varying diameter strands of polydioxanone (size 3-0, 2-0, 0 and 1) were
utilized to vary the
time to complete absorption, in addition to varying the expiration dates.
Since the absorbable
polymers initially break at the stress points during absorption, the webbed
filters were designed
to disintegrate into 8 pieces at length D/2, and 8 pieces sized D/4, where D
is the inside diameter
of the vessel. The objective is piecemeal disintegration, phased or
sequential, to minimize free
floating exposure of the polymer filter capture elements in circulation. Fig.
4b is the same
webbed design but with uniformly sized polydioxanone suture for comparison.
Fig. 4c is a
radial filter design similar to conventional metal IVC filters yet sports the
varying diameter
sutures for sequential absorption. Finally, Fig. 4d is a radial design
constructed exclusively
with polydioxanone size 2-0.
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
16
[0081] The primary endpoint for evaluating the absorbable polymers for
vascular filter
application was load at break as a function of time. In addition to the
absorbable filters pictured
in Fig. 4, several test cells were fabricated with the various absorbable
polymer candidates for
weekly destructive tensile testing. Polymer characterization was performed
utilizing the
ADMET eXpert 7601 tensile testing machine with MTESTQuattro software (Norwood,
MA)
at weekly intervals to yield stress vs. strain graphs in addition to the
primary endpoint of load
at break, and several secondary endpoints: (i) maximum stress (tensile
strength), (ii) maximum
strain (% elongation at break), (iii) energy at break, and (iv) Young's
modulus of elasticity.
The ADMET machine was operated with a crosshead speed of 3cm/min and outfitted
with a
high resolution 100lb load cell and 21C.N pneumatic grippers.
[0082] The candidate absorbable polymers (representing capture elements) sewn
into the test
cells were embedded in a closed circulation system engineered to mimic human
cardio
physiology. At weekly intervals, the system was shut down to extract sutures
of each size and
type to perform destructive tensile testing. As a control, identical
absorbable sutures were
submerged into a static buffer bath (StableTemp digital utility bath, Cole-
Parmer, Vernon Hill,
IL) held at 37 C and also tested on a weekly basis. The hypothesis being that
the increased
thermodynamics of the circulation system accelerates both absorption rate and
tensile strength
loss of the capture elements.
[0083] The closed circulation system was constructed with thin walled 3/4" PVC
with od 261
mm that fit snug inside the flexible 25.4 mm id Tygon tubing that simulated
the IVC. The heart
of the system was a Harvard Apparatus large animal pulsatile blood pump
(Holliston, MA) that
simulated the ventricular action of the heart. The Harvard Apparatus blood
pump was operated
near continuously for 22 weeks (913K L pumped) with minor preventative
maintenance.
[0084] The heart rate was adjusted to 60 bpm, stroke volume between 60 and 70
ml,
systolic/diastolic duration ratio 35%/65%, and systolic blood pressure varied
from 120 mmHg
(simulated conditions for an arterial filter to prevent cerebral and systemic
embolism) to
.5rnmHg (simulated conditions for an IVC filter to prevent PE).
[0085] Real time measurements were available from the upstream and downstream
sensor
manifolds. The sensors upstream from the absorbable filters under test
included digital
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
17
temperature, flow rate (Lint), total flow (L), and pressure (mmHg). Downstream
instrumentation included real rime measurement of % oxygen, total dissolved
solids (TDS in
ppt), and pH. TDS monitoring was included to evaluate the absorption by-
products less than
20 microns in size, while the downstream 80 micron in-line filter would catch
fragments of
suture from the filters and test cells.
100861 The four candidate absorbable vascular filters introduced in Fig. 4
were installed in
series along the upstream tubing, whereas 5 test cells containing absorbable
suture for weekly
destructive testing were installed in series along the downstream section of
the in-vitro cardio
test system. A 288W heating tape with thermostat was utilized to maintain 37 C
within the
closed circulation system. Finally, the circulating fluid was pH 7.4 phosphate
buffer
(Invitrogen, Carlsbad, CA) with a similar electrolyte profile as human blood.
Buffer was
replaced weekly in an effort to maintain stable pH.
100871 Absorption and tensile properties of the selected polymers were
determined as a
function of time until compete strength degradation in both the circulation
system and control
bath. The phosphate buffer in the circulation system was changed weekly as the
pH decreased
from 74 to an average 6.6 during each week. Buffer was changed in the control
bath only
monthly due to better pH stability in the static environment. Mean flow was
4.7 L/min while
oxygen averaged 30% and TDS 8.8 ppt.
100881 The phased or sequential absorption of the webbed absorbable filter
design is illustrated
in the collage of Figure 5. Notice the filter begins to disintegrate during
the 13th week and
continues in a phased manner, losing only 1 or 2 capture elements per week
thereafter, until
complete disintegration in 22 weeks. Initial fractures detected in the 13th
week were located at
the high stress points within the capture elements. Since the apex of a
capture element mounted
to the circumferential support experiences twice the stress in comparison to
the base of the
capture element, the initial break will be at the apex. The capture elements
that formed loops
extending from the vessel wall to the center of the filter were constructed of
polydioxanone
size 1 and 0 with expiration date Jan 2012, while the shorter capture elements
that extended a
quarter of the diameter were constructed of size 3-0 polydioxanone suture with
an expiration
date of Jan 2015. The expiration date was seen to play a greater role than
suture diameter in
the rate of absorption since the smaller diameter suture fractured in week 17,
versus the larger
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
18
diameter suture that fractured in week 13. The planned disintegration of 8
elements of length
D/2 and 8 elements of length D/4 for the webbed filter actually yielded
smaller brittle fragments
due to splintering and fragmenting. In fact the largest filter element
captured from the webbed
design by the downstream 80um filter revealed a maximum sized fragment of 5 mm
x 0.3 ram.
100891 Perhaps the paramount characteristic under consideration for use in an
absorbable
vascular filter is the strength retention profile of the absorbable polymers
as depicted in Fig. 6
for polydioxanone in the in-vitro circulation system. As shown, polydioxanone
initially
exhibits moderate strength degradation, less than approximately 5% per week
for the initial 5
to 6 weeks, followed by rapid decline approaching 20% per week thereafter. As
a conservative
summary for the initial 5 weeks in circulation, polydioxanone size 1
maintained about 10kg
strength, size 0 maintained 6kg, size 2-0 maintained 4kg, and size 4-0
maintained 1.5kg.
Similar results were obtained from a buffer bath control for the initial 5
weeks. However,
statistical difference was achieved at week 5 for size 0 (p <0.014), week 6
for sizes 2-0 and 1
(Pc 0.021), and week 7 for size 4-0 (p <0.011).
100901 The proposed filter designs employ multiple strands serving as capture
elements, hence
the emboli load is distributed across N strands. Therefore, assuming equal
distribution, the net
emboli load that can be accommodated by the filter is a multiple, N, of the
per strand load at
break. Consequently, a polydioxanone size 2-0 filter with 8 capture elements
secured at the
circumferential support would accommodate a net emboli load of 32kg.
100911 An alternative method for accessing strength retention for the polymers
is to chart the
percentage strength retention as a function of time as shown in Fig. 7. Here
all polydioxanone
sizes slowly lost strength for the first 5 weeks, then rapidly absorbed to
negligible strength by
the 10th week. Specifically, polydioxanone within the in-vitro circulation
system retained
average strength for sizes 2-0 and larger of 88% at 2 weeks, 85% at 4 weeks,
and 68% at 6
weeks vs. Ethicon's in-vivo animal tissue approximation applications that
yielded 80% at 2
weeks, 70% at 4 weeks and 60% at 6 weeks per Ethicon product literature.
100921 Young's modulus of elasticity ranged from 1.0 ¨ 2.3 GPa for
polydioxanone as shown
in Fig. 8 for the absorbable filter elements. Notice that Young's modulus
initially decreased
(polymer became more elastic) as it was subjected to the buffer, reached a
minimum at 6 weeks,
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
19
then increased to approximately twice the initial value. This increase in
Young's modulus for
polydioxanone is indicative of the increased brittleness as it reached zero
terminal strength,
and was further observed during disintegration. This property may well be
advantageous for
the absorbable filter application. For example, as polydioxanone reached zero
terminal strength
and disintegrated, it splintered and fractured into smaller, brittle fragments
thereby being
potentially less harmful to downstream organs. Further studies are required to
determine the
exact size of the terminal fragments in-vivo and evaluate potential pulmonary
micro-infarcts.
100931 In conclusion from the in-vitro absorbable filter study, polydioxanone
appears to be a
strong candidate for absorbable vascular filters with sufficient strength
retention to capture
emboli for at least 6 weeks, then absorb rapidly over the next 16 weeks via
hydrolysis into
carbon dioxide and water. Specifically polydioxanone size 2-0 was shown to
conservatively
maintain 4 kg load at break per strand throughout 5 weeks in circulation.
100941 Hence a filter incorporating 8 capture elements would trap an embolus
load of 32 kg;
or equivalently, an embolism would have to deliver 1600 kgrrun of energy to
break through the
filter which is highly unlikely given that the pressure in the IVC is a mere 5
mmHg (about
0.1psi). Moreover, the webbed filter geometry with varied diameter capture
elements and
expiration dates was shown to disintegrate in a sequential or phased marmer,
releasing 1 or 2
small brittle filter fragments (less than 5 mm x 0.3 mm each) weekly in
circulation from weeks
14 through 22. Together with polydioxanone being FDA-approved and proven to be
nonallergenic and nonpyrogenic, a catheter-deployed polydioxanone absorbable
vascular filter
would likely be an efficient and effective device for the prevention of
pulmonary embolism.
100951 An installation of the absorbable vascular filter is via intravenous
insertion with a
catheter requiring only a local anesthetic as illustrated in Figs. 9a-e. Here
the filter is collapsed
and compressed within a delivery catheter comprised of an outer sheath 71 and
internal
applicator or stabilizer piston 73 on a central rod as illustrated in Fig. 9a.
For IVC filter
deployment, the delivery catheter is inserted into the patient's vasculature
of convenient
location, such as the femoral vein or internal jugular. Subsequently, the
delivery catheter is fed
through the vasculature typically over a guide wire until reaching the desired
deployment
location, often inferior to the renal veins. Next the compressed filter 50 is
allowed to expand
upon sliding the exterior sheath 71 in the proximal direction while
simultaneously pushing the
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
stabilizer rod and piston 72 in the distal direction (refer Fig. 9b). Once the
exterior sheath 71 is
withdrawn away from the filter, the stabilizing piston 73 can also be
retracted as depicted in
Fig. 9c. Consequently as a thrombosis event releases an embolus 80, the
embolus is captured
by the vascular filter and is prevented from traveling to the heart and lungs
thereby preventing
a potentially fatal PE (refer Fig. 9d). Following the desired prophylactic
time window for filter
utilization (approximately 6 weeks in many applications), the filter is
biologically absorbed
resulting in the absence of any foreign material in the vessel as depicted in
Fig. 9e.
100961 An alternative embodiment of the absorbable vascular filter 1 is
portrayed in Fig. 10a
with an integrated circumferential support 102 and capture basket 101. Here
the circumferential
support 102 and capture basket 101 are braided or woven much like a radial
expansible stent
that can be compressed in a catheter as described above prior to deployment.
Fig. 10b is a top
view of the absorbable vascular filter that displays the weave or braid of the
capture basket
101. The weave is shown to maintain a patent center 104 to allow insertion of
a guide wire
during catheter deployment. The appeal of this particular embodiment is that
the entire
absorbable vascular filter (circumferential support and capture basket
composed of the capture
elements) can be fabricated from a single filament with a designed radial
force to prevent filter
migration as described below.
100971 The integrated absorbable vascular filter shown in Figs. 10a and b
yields a diametrically
expandable and compressible tubular filter that exhibits a radial force with
magnitude
dependent on the materials chosen, angle phi (p) of the crossing elements of
the weave, and
the amount of diameter over sizing employed. Specifically, the angle important
to establishing
radial force is depicted as cp in Fig. 11. The larger the angle cp as it
approaches 180 , the greater
the amount of radial force provided by the weave. Typically, ç is an obtuse
angle, chosen
between 90 and 180 .
100981 For illustration, a simple cylindrical braided weave (L = 7, P = 4) is
shown in Fig. 11
cut in the longitudinal direction and placed flat on a surface revealing the
looping pins 110 and
braiding filament 103. Considering the weave as a series of sinusoid waveforms
of period PT
(see bold section of weave in Fig. 11), where P is the number of looping pins
traversed for one
cycle of the sinusoid and T is the pin-to-pin spacing, an algorithm can be
derived to ensure that
for a given set of parallel looping pins L that equidistantly span the
circumference of the
CA 03155354 2022-4-20
WO 2021/080923 PCT/US2020/056359
21
intended diameter of the vascular filter, each pin will be looped once and the
final loop ending
at the origin.
100991 The algorithm can be visualized by a table as shown in Table 1 to
indicate the
relationship between L, P and the angle p for any desired number of
circumferential loops (L).
L/P represents the fractional number of sinusoids traversed per circumference,
and N represents
the total number of turns around the circumference of the cylinder.
Essentially the weave
creates sinusoids that are out of phase by a fixed increment until the final
loop is achieved for
which the final sinusoid is desired to be in-phase with the initial sinusoid.
The in-phase
condition requires the product Nx(L/P) to be an integer. Moreover, to ensure
all pins are looped,
the first integer to be formed by the product Nx(L/P) must occur where N = P.
Table 1. Relationship between braiding parameters.
L= 7
phi P LIP 1 2 3 4
5 6 7 8 9 10
41.0 2 330 3.50 7.00 10.50 14.00 17.50 21.00 24.50 28.00 31.50 35.00
73.6 4 1.75 1.75 3.50 5.25 7.00 8.75 10.50 12.25 14.00 15.75 17.50
96.6 6 1_17 1_17 2.33 3.50 467 5.83 7.00 8.17 9_33 10_50 1167
112.5 a 0.88 0.88 1.75 2.63 3.50 4.38 5.25
6.13 7.00 7.88 8.75
123.7 10 0.70 0.70 1.40 2.10 2.80 3.50 4.20
4.90 5.60 6.30 7.00
1001001 For example with L = 7 and P = 4, the first
integer that appears in the row
corresponding to P = 4 of Table 1 is where N = 4 so this combination of L, P.
and N will provide
a successful braid wherein all pins will be utilized (7 across the top, 7
across the bottom) and
the final weave will terminate at the origin. It can be demonstrated that L
must be an odd integer
for a successful braid. It can further be shown that the angle 9 can be
expressed as 9 = 2tan-
l(Pnr/L1) where r andl is the radius and length of the desired filter
circumferential support 102.
The values for r and I used for calculating 9 in Table 1 were 0.625 and 1.5
inches respectively.
Also, T is easily computed from the relationship LT = 27tr or T = 27r/L.
1001011 Fig. 12 depicts another braid combination where L = 7 and P =6. Notice
that the first
integer to appear in the row for P = 6 in Table 1 corresponds to N = 6 hence
the braid will
terminate successfully at the origin and all L pins looped once. Further Fig.
12 illustrates a
method for forming the capture basket 101 as a simple continuous extension of
the filament
beyond the circumferential support 102. As shown at the alternating looping
points across the
top of the circumferential support, the conical capture basket 101 is weaved
by sequentially
CA 03155354 2022- 4- 20
WO 2021/080923
PCT/US2020/056359
22
interlocking loops from adjacent loops 105 and extending a loop to the apex
106. The apical
loops from each extension 106 can be bonded together revealing a conical
capture basket as
shown in Fig. 10b with a patent center apex 104. Clearly other braided
patterns can be
employed to yield the pattern resolution sufficient to trap emboli of a
desired size.
[00102] Although only a set of 7 looping pins were considered for simplicity
in the above
illustrations, a more likely number useful for an absorbable vascular filter
for the IVC may well
be 17 or 19 with p> 100'. Specifically, an absorbable IVC filter with
integrated circumferential
support and capture basket was fabricated with a single 10ft synthetic
filament (0.5nrim
diameter) as shown in Figs. 13a and b with L = 17, P = 16, 9 = 1020, 1 = 1.5",
r = 0.625", and
= 0.23". The self-expandable 1VC filter provides sufficient radial force to
maintain placement
in the IVC by the choice of the obtuse weave angle, 25% oversized diameter (to
fit 1" IVC
diameter), and wide diameter filament (0.5min). Alternatively, the above
described integrated
absorbable vascular filter can be constructed with multiple bonded filaments,
although a single
continuous filament may be used.
[00103] Referring to the embodiment depicted in Figs. 14a and 14b, an
absorbable vascular
filter 1 comprises an outer, circumferential element 120 (similar to and/or
the same as
circumferential element 2 described above) for supporting a plurality of
filter capture elements
110 (e.g., similar to and/or the same as the capture elements described above)
and to maintain
position within the vessel. The capture elements 110 may be configured for
capturing or
retarding substances flowing in the vessel for a limited duration in time.
Here the
circumferential element 120 and capture elements 110 are both laser cut from a
generally
circular tube of absorbable polymer. This means the circumferential element
120 and the
capture elements 110 form a unitary piece, without joints, seams, and/or other
attachment
points between the circumferential element 120 and the capture elements 110.
The unitary
piece may be a substantially continuous structure having a smooth surface
without protrusions
that may be caused by joints, seams, and/or other attachment points. In some
embodiments,
the capture elements 110 and the circumferential element 120 may be woven from
a single
strand or fiber or cut from a sheet of material. For example, the filter may
be cut from a sheet
of absorbable polymer film and then formed into a tubular shape. In some
embodiments, the
capture elements 110 and the circumferential element 120 may be coupled
together as separate
pieces to form absorbable vascular filter 1.
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
23
1001041 The pattern for the circumferential element can be designed through
finite element
analysis and/or using other methods to produce a desired amount of radial
force (or given
amounts of radial force) for a given diameter (or diameters) upon deployment
to ensure caval
apposition. The proximal end 119 of the circumferential element 120 includes
undulating
features 121 (e.g., similar to and/or the same as undulating features formed
by the weaving as
described above) while the distal end 122 terminates with capture elements
110. In some
embodiments, the circumferential element has a lattice spacing (e.g., a
lattice design that
creates the spaces between members 117 of the circumferential element 120)
that is smaller
than a lattice spacing of the capture elements 110 (e.g., there is less space
between members
117 in the circumferential element 120 than between individual capture
elements 110). In some
embodiments, the lattice spacing of the circumferential element 120 is
configured such that the
filter 1 generates the desired amount of radial force as described above. In
some embodiments,
the lattice spacing of the capture elements 110 is configured such that emboli
or other
particulates of a target size are captured by the filter, without stopping the
overall fluid flow
through the vessel. In some embodiments, the members 117 of the
circumferential element
120 and/or the capture elements 110 may have a substantially rectangular cross
section, and/or
other cross sections that contribute to the radial force and/or capture
characteristics of the filter
1. In some embodiments, the members 117 of the circumferential element 120
and/or the
capture elements 110 may have radiused, chamfered, and/or other shaped edges
to facilitate
fluid flow through the filter 1.
1001051 In some embodiments, the capture elements comprise loops 113 at the
distal ends 111
that can be secured with an absorbable coupler (e.g., such as a filament) 130
to form the filter
apex at a distal end 141 of filter 1. In some embodiments, individual loops
113 may be formed
along a longitudinal axis of a corresponding capture element 110. In some
embodiment there
may be one loop 113 per capture element 110. Loops 113 may be formed such that
open areas
115 of loops 113 face a lumen of filter 1. Loops 113 and/or open areas 115 may
have a
generally circular shape and/or other shapes that facilitate the closure of
the distal end 141 of
filter 1 (e.g., as described below).
1001061 In this example, the absorbable coupling filament 130 may be a suture
and/or other
filaments. In some embodiments, the absorbable coupling filament 130 may be
pre-threaded
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
24
and/or otherwise looped through loops 113 before the filter is implanted (or
loaded into a
catheter for implant. The absorbable coupling filament 130 may be configured
to move
between an expanded configuration 130a and a contracted configuration 1306. In
the expanded
configuration 130a, absorbable coupling filament 130 is configured to allow
capture elements
110 to remain in an open configuration (Fig. 14a) that would not trap (or
would only trap very
large) emboli in a vessel_ In the contracted configuration 130b, absorbable
coupling filament
130 is configured to pull distal ends 111 of capture elements 110 toward each
other to form the
filter. In some embodiments, absorbable coupling filament 130 may be
configured to be moved
from the expanded configuration 130a to the contracted configuration
responsive to a pulling
force applied to an end 129 of filament 130. In some embodiments, filament 130
may include
a knot 127 and/or other tightening mechanisms that cause an open end 135 of
filament 130 to
reduce in size (e.g., thus causing ends 111 to move toward each other). For
example, pulling
on end 129 may cause a slip knot 127 to cinch down and close open end 135.
Other cinching
mechanisms are contemplated.
1001071 Referring to the embodiment depicted in Figs. 15a and b (where like
reference
numerals correspond to reference numerals in other figures described above),
absorbable
vascular filter 1 comprises outer, circumferential element 120 for supporting
a plurality of filter
capture elements 110 and to maintain position within the vessel. In this
example embodiment,
the capture elements 110 include spline features 151 at the distal ends 111
that can be fastened
and/or otherwise coupled to a complimentary spline receptacle 131a within an
absorbable
coupler (e.g., such as an end plate) 130 to form the filter apex. In some
embodiments, spline
features 151 comprise shaped distal ends 111. The shapes of distal ends may be
configured to
couple with corresponding spline receptacles 131a such that spline features
151 are not released
from receptacles 131a when filter 1 is deployed and/or in service.
1001081 In this example, spline features 151 comprise substantially
trapezoidal shapes. The
trapezoidal shapes may have corresponding edges 153 that extend in a
circumferential direction
from a width 155 of a given capture element 110. This makes the distal ends
111 wider than
the bodies 157 of the capture elements 110. This also makes the distal tips
159 of spine features
151 wider than the portions of spline features 151 that begin extending from
capture elements
110.
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
[00109] In this example, the corresponding spline receptacles 131a comprise
trapezoidal
shaped channels configured to receive spline features 151 (e.g., such that
pieces fit together
like a puzzle). Receptacles 131a may be positioned around an outer surface 161
of absorbable
coupler 130 such that the channels have a narrow end 163 at a proximal side
165 of coupler
130 and extend axially along outer surface 161 of coupler 130 to a distal side
167 of coupler
130. In some embodiments, the channels become wider (e.g., to match the shape
of spline
features 151) as the channel extends along outer surface 161 such that the
channels have a wide
end 169 at or near distal side 167. In some embodiments, the channels become
wider (e.g., to
match the shape of spline features 151) as the channel extends towards a
center of coupler 130
such that the channels have a wide side toward the center of coupler 130.
These shapes may
be configured to prevent separation of spline features 151 and receptacles
131a during
deployment and/or service of filter 1. These shapes are not intended to be
limiting. Spline
features 151 and/or receptacles 131 may have any shape and/or size that allows
them to
function as described herein.
[00110] In some embodiments, the end plate 130 may include a center hole 132
to
accommodate a (e.g., cylindrical) radiopaque marker and/or guidewire. Center
hole 132 may
be round as shown, or have other shapes. In some embodiments, center hole 132
may be
located at or near a center of absorbable coupler 130 and/or in other
locations. In some
embodiments, center hole 132 may be sized such that a radiopaque marker causes
hole 132 to
stretch and exert compressive force on the radiopaque marker once it is
inserted. In some
embodiments, center hole 132 may be sized to pass a guidewire.
[00111] In some embodiments, capture elements 110 may be configured to flex
such that a
spline feature 151 passes through or near an axial centerline of filter 1 and
is coupled to a
receptacle 131a on an opposite side of coupler 130 (without blocking hole
132). When the
individual capture elements are coupled to coupler 130 in this way, forces
from the individual
capture elements (e.g., trying to return to their as cut from a tube
straightened orientation) may
act substantially evenly around coupler 130 (e.g., each pushing on coupler 130
toward a center
of the filter), and prevent any individual spline feature from releasing from
its respective
channel.
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
26
[00112] In some embodiments, the end plate may be attached to the capture
elements during
manufacturing, when the filter is assembled on a catheter for eventual
deployment, and/or at
other times before an implant procedure.
[00113] Referring to the embodiment depicted in Figs. 16a and b (where like
reference
numerals correspond to reference numerals in other figures described above),
an absorbable
vascular filter 1 comprises an outer, circumferential element 120 for
supporting a plurality of
filter capture elements 110 and to maintain position within the vessel. The
capture elements
include loops 171 at the distal ends 111 that can be fastened to mating
connection shafts 133
within a recessed portion 131b of a coupler (e.g., such as an end plate) 130
to form the filter
apex.
[00114] In some embodiments, loops 171 may be similar and/or the same as loops
113
described above. In some embodiments, individual loops 171 may be formed along
a
longitudinal axis of a corresponding capture element 110. In some embodiment
there may be
one loop 171 per capture element 110, or less than one loop per capture
element 110 such as
having loops on alternating capture elements 110. Loops 171 may be formed such
that open
areas 173 of loops 171 face a lumen of filter 1. Loops 171 and/or open areas
173 may have a
generally circular shape and/or other shapes configured to couple with shafts
133 (e.g., as
described below).
[00115] In some embodiments, shafts 133 may by cylindrically shaped and have
circular cross
sectional shapes (as shown in Fig. 16a) and/or shafts 133 may have other
shapes (with the
shapes of open areas 173 corresponding to the shapes of shafts 133). In some
embodiments, a
diameter (and/or otherwise a size) of a shaft 133 may be the same as or
slightly larger than a
diameter (and/or otherwise a size) of a corresponding open area 173 such that
a loop 171 forms
a friction fit on a shaft 133 when the two are coupled. Shafts 133 extend from
abutting surfaces
183 of recessed portions 131b. Abutting surfaces 183 are configured to receive
corresponding
surfaces of loops 171.
[00116] Recessed portions 131b may be recessed from outer surface 161 of
coupler 130. In
some embodiments, recessed portions 131b may have a depth (e.g., from outer
surface 161 to
an abutting surface 183) that corresponds to a thickness of capture elements
110 (e.g., a wall
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
27
thickness of a tube from which filter 1 is cut). In some embodiments, recessed
portions 131b
may have open neck regions 181 configured to facilitate coupling between loops
171 and shafts
133. Open neck regions 181 may have a width that corresponds to width 155 of
capture
elements 110, for example. Open neck regions 181 may facilitate a flush or
nearly flush
coupling between capture elements 110 and coupler 130, and/or have other
purposes.
[00117] In some embodiments, capture elements 110 may be configured to flex
such that a
loop 171 passes through Of near an axial centerline of filter 1 and is coupled
to a shaft 133 on
an opposite side of coupler 130 (without blocking hole 132). When the
individual capture
elements are coupled to coupler 130 in this way, forces from the individual
capture elements
(e.g., trying to return to their as cut from a tube straightened orientation)
may act substantially
evenly around coupler 130 (e.g., each pushing on coupler 130 toward a center
of the filter), and
prevent any individual loop from releasing from its respective shaft (e.g.,
see Fig. 16b).
[00118] Referring to the embodiment depicted in Figs. 17a and b (where like
reference
numerals correspond to reference numerals in other figures described above),
an absorbable
vascular filter 1 comprises an outer, circumferential element 120 for
supporting a plurality of
filter capture elements 110 and to maintain position within the vessel. In
some embodiments,
the capture elements 110 include barbed features 190 at or near the distal
ends 111 of capture
elements 110 that can be inserted through holes 131c of a coupler (e.g., such
as an end plate)
130 to form the filter apex. In some embodiments, holes 131c comprise
cylindrical through
holes. In some embodiments, an axis of a through hole 131c is aligned with an
axis of coupler
130, hole 132, and/or other features of filter 1. In some embodiments, holes
131c may have a
conical cross section ancUor other cross sections, and/or be oriented along
axis that are not
aligned with an axis of coupler 130, hole 132, and/or other features of filter
1 (e.g., such that
holes 131c provide resistance against distal ends 111 passing through holes
131c and/or prevent
withdrawal of distal ends 111 through holes 131c).
[00119] In some embodiments, an individual capture element 110 may have one
barbed
feature 190, two barbed features 190, three barbed features 190, and/or other
numbers of barbed
features. The example in Fig. 17a and 17b shows two barbed features 190 on
each individual
capture element 110, but this is not intended to be limiting. In some
embodiments, the barbed
features 190 comprise protrusions 191, 195 from the bodies 157 of capture
elements 110.
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
28
Protrusions 191, 195 may comprise pointed or nearly pointed tips and/or other
dimensional
shapes, for example. In some embodiments, protrusions 191, 195 may protrude
the same
amount. In some embodiments, different protrusions 191, 195 on a given capture
element 110
may protrude different amounts. In some embodiments, protrusions 191, 195 may
protrude
different amounts on different individual capture elements 110.
1001201 The protrusions 191 may protrude from bodies 157 in a radial direction
(e.g., around
a circumference of filter 1) and/or in other directions. In some embodiments,
the protrusions
191 on the different capture elements may protrude from the respective bodies
157 in the same
radial direction. In some embodiments, the protrusions 191 on the different
capture elements
may protrude from the respective bodies 1157 in alternating radial directions
and/or in other
configurations. The protrusions 195 may protrude from bodies 157 in an axial
direction (e.g.,
along a long axis of filter 1) and/or in other directions. This may facilitate
insertion of distal
ends 111 into holes 131c and/or have other purposes, for example.
1001211 In some embodiments, barbed features 190 may include channels 193
between
protrusions 191. Channels 193 may have a width and/or depth that facilitate
coupling with
coupler 130, for example, and/or other coupling features. For example,
channels 193 may have
a width that corresponds to a thickness of coupler 130 and/or have other
dimensions. Channels
193 and/or protrusions 191 may be configured such that, as shown in Fig. 17a
and 17b, a first
protrusion 191 on a given capture element 110 passes through a corresponding
hole 131c but
a second protrusion 191 does not, such that the channel 193 between the
protrusions 191 is
positioned in hole 131c (e.g., as shown in Fig. 17b).
1001221 Referring to the embodiment depicted in Figs. 18a and b (where like
reference
numerals correspond to reference numerals in other figures described above),
an absorbable
vascular filter 1 comprises an outer, circumferential element 200 (similar to
and/or the same as
circumferential elements 2 and/or 120 described herein) for supporting a
plurality of filter
capture elements 110 and to maintain position within the vessel. The proximal
end of the
circumferential element includes undulating features 210 (similar to and/or
the same as
undulating features 121 described herein) while the distal end 220 terminates
with loops 221
and/or other features configured to facilitate securement of the proximal ends
of the absorbable
capture elements 110.
CA 03155354 2022-4-20
WO 20211080923
PCT/US2020/056359
29
1001231 In some embodiments, as shown in Fig. 18a, capture elements 110 may be
and/or
include capture filaments. The capture filaments may include a plurality of
individual
filaments that are linked together, and/or the capture filament may be formed
by a continuous
strand of material. The plurality of absorbable capture elements are linked
with adjacent
absorbable capture elements to establish the capture basket 100 (e.g., similar
to and/or the same
as capture basket 101 described above). The capture elements may intersect, be
wrapped
around each other, be braided together, and/or be coupled in other ways. The
apex of the filter
1 is formed by looping the capture elements through holes 310 in the end plate
300 (Fig. 18b,
e.g., similar to and/or the same as end plates 130 described above). An end
141 view 231 of
filter 1 (without plate 300) is shown in Fig. 18a. As shown in view 231, the
intersecting and
weaving of the capture filaments may form a petal structure configured to
capture emboli
and/or other particulate flowing through a vessel. The petal structure may
cover the lumen of
a vessel more densely closer to a center of a vessel and less densely toward
an outside of the
vessel, for example.
1001241 In some embodiments, the capture filaments are woven through the
peripheral holes
310 of the end plate 30010 form the apex of the filter, while in other
embodiments the proximal
ends of the capture filaments could be fastened at the peripheral hole
locations 310. In some
embodiments, the end plate includes a center hole 132 to accommodate a
cylindrical
radiopaque marker and/or guidewire, and/or for other purposes.
1001251 Although the present invention has been described with reference to
specific
exemplary embodiments, it will be evident to one of ordinary skill in the art
that various
modifications and changes may be made to these embodiments without departing
from the
broader spirit and scope of the invention. Accordingly, the specification and
drawings are to
be regarded in an illustrative rather than a restrictive sense.
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
REFERENCES
[1] Goldhaber SZ, Oriel TL. The Surgeon General's Call to Action to Prevent
Deep Vein
Thrombosis and Pulmonary Embolism, Office of the Surgeon General (US),
National Heart,
Lung, and Blood Institute (US) Rockville (MI)). 2008
[2] Spencer FA, Emery C, Lessard D, Anderson F, Emani 5, Aragam J et al. The
Worcester
Venous Thromboembolism study; a population-based study of the clinical
epidemiology of
venous thromboembolism. J Gen Intern Med 2006 Jul; 21(7); 722-7.
[3] Dick RL. Hereditary and acquired thrombophilia: preface. SeminThrombHemost
1999;
25;251-3.
[4] Agudelo JF, Morgan SJ, Smith WR. Venous Thromboembolism in Orthopedic
Trauma
Patients, Orthopedics. 2005 Oct;28(10):1164-71.
[5] Tapson VF. Acute pulmonary embolism. N Engl J Med, 2008, 358, 10. 1037-52.
[6] Goldhaber SZ, Visani L, De Rosa M. Acute PE: clinical outcomes in the
International
Cooperative PE Registry (ICOPER). Lancet 1999. 353. 1386-9.
[7] Geerts WH, Jay RNI, Code K1, et al. A comparison of low-dose heparin with
low-molecular
weight heparin as prophylaxis against venous thromboembolism after major
trauma N Engl J
Med. 1996; 335:701-7.
[8] Silverstein DM, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Meltron LJ,
3`d. Trends
in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year
population-based
study. Arch Intern Med 1998;158(6). 585-93.
[9] Von VR. Weitereuntersuchungenueber die verstopfung der lungenarterien und
ihrefolge.
Traube'sBeitraegeexp Path u Physiol, 1846; 2:21-31.
[10] Goldhaber SZ, Savage DD, Garrison R.J, et al. Risk factors for pulmonary
embolism: The
Framingham Study. Am J Med. 1983; 74: 1023-1028.
[11] Coon WW. Epidemiology of venous thromboembolism. Ann Surg. 1977; 186:149-
164.
[12] Muntz JE, Michota FA. Prevention and management of venous thromboembolism
in the
surgical patient: options bye surgery type and individual patient risk
factors, Am J of Surg,
2010; 199, 511-20.
[13] American Academy of Orthopaedic Surgeons Clinical Guideline on Prevention
of
Pulmonary Embolism in Patients Undergoing Total Hip or Knee Arthroplasty,
American
Academy of Orthopaedic Surgeons, May 2007.
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
31
[14] Pulmonary Embolism Prevention (PEP) Trial Collaborative Group: Prevention
of
pulmonary embolism and DVT with low dose aspirin: pulmonary embolism
prevention (PEP)
trial. Lancet. 2000; 355: 1295-1302
[15] Prins ME, Hutten BA, Koopman MM,et at. Longterm treatment of venous
thromboembolic disease. Thromb Haemost 1999;82:892-8.
[16] Tran H, McRae S, Ginsberg J. Anticoagulant Treatment of Deep Vein
Thrombosis and
Pulmonary Embolism. Cardiology Clinics, 2008, 26, 235-50.
[17] Morgan SJ, Jeray KJ, Phieffer LS, Grisby JH, Bosse MJ, Kellam JF.
Attitudes of
orthopaedic trauma surgeons regarding current controversies in the management
of pelvic and
acetabular fractures. J Orthop Trauma.2001; 15:526-32.
[18] Meissner MI-I, Chandler WL, Elliot JS. Venous thromboembolism in trauma:
a local
manifestation of systemic hypercoagulability? J Trauma.2003; 4:224-31.
[19] Geerts WH, Bergqvist D, Pineo OF, et al. Prevention of venous
thromboembolism:
American College of Chest Physicians evidence-based clinical practice
guidelines (8th
edition). Chest. 2008;133:381S-453S.
[20] Huo ME, Spyropoulos AC. The eighth American college of chest physicians
guidelines
on venous thromboembolism prevention: implications for hospital prophylaxis
strategies. J
Thromb Thrombolysis. 2011, Feb;31(2): 196-208.
[21] Baglin TP, Brush J, Streiff M. Guidelines on the use of vena cava
filters. British
Committee for Standard in Haematology, British J of Haematology, 2006, 134,
590-5.
[22] Rogers FR, Cipolle MD, Velmahos 6, et al. Practice management guidelines
for the
prevention of venous thromboembolism in trauma patients: the East practice
management
guidelines work group. J Trauma 2002; 53:142-164.
[23] Rosenthal D, Wellons ED, Lai KM, et al. Retrievable inferior venal cava
filters: initial
clinical results. Ann Vasc Surg 2006; 20:157-165.
[24] Gosin IS, Graham AM, Ciocca RG, et al. Efficacy of prophylactic vena cava
filters in high
risk trauma patients. Ann Vase Surg 1997; 11:100-05.
[25] Spain DA, Richardson JD, Polk HC, et al. Routine prophylactic vena cava
filter insertion
in severely injured patients decreases the incidence of pulmonary embolism. J
Am Coll Surg
1995; 180:641-47.
[26] Stein PD, Kayali F, Olson RE. Twenty-one year trends in the use of
inferior vena cava
filters. Arch Intern 1VIed 2004; 164:1541-5.
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
32
[27] Kaufman JA, Kinney TB, Streiff MD, et al. Guidelines for the use of
retrievable and
convertible vena cava filters: report from the Society of Interventional
Radiology
multidisciplinary consensus conference. J Vasc Intery Radio'. 2006;17:449-459.
[28] Rodriquez JL, Lopez ,11114, Proctor MC, et at. Early placemen of
prophylactic vena cava
filters in injured patients at high risk for pulmonary embolism. J Trauma1996;
40:797-804.
[29] Langan EM III, Miller RS, Case! WJ III, Carsten CG III, Graham RM, Taylor
SM.
Prophylactic inferior vena cava filters in trauma patients at high risk:
follow-up examination
and risk/benefit assessment. J Vasc Surg. 1999; 30:484-90.
[30] Greenfield LJ, Proctor MC, Rodriquez JL, Luchette FA, Cipolle MD, Cho J.
Posttrauma
thromboembolism prophylaxis. J Trauma. 1997,42:100-03.
[31] Young T, Tang H, Hughes R. Vena cava filters for the prevention of
pulmonary embolism
(Review). The Cochrane Library 2010, Issue 2.
[32] Decousus H, Leizorovics A, Parent F, et al. A clinical trial of vena
caval filters in the
prevention of pulmonary embolism in patients with proximal deep-vein
thrombosis, N England
J Med. 338,7:409-15.
[33] The PREPIC Study Group. Eight-year follow-up of patients with permanent
vena cava
filters in the prevention of pulmonary embolism: the PREPIC randomized study.
Circulation
2005; 112:416-22.
[34] Ray CE, Kaufman JA. Complications of vena cava filters. Abdom Imaging
1996; 21:368-
74.
[35] Ballew KA, Philbrick JT, Becker DM, Vona cava filter devices, Clin Chest
Med 1995;
16:295-305.
[36] Streiff MB. Vena cava filters: a comprehensive review. Blood 2000;
95:3669-77.
[37] Pons M, Riglietti A, van den Berg JC. The role of vena cava filters in
the management of
venous thromboembolism. J Cardiovasc Surg 2010;51: 355-64.
[38] Usoh F, Hignorani A, Ascher E, et al. Long-term follow-up for superior
vena cava filter
placement. Ann Vasc Surg. 2009;23:350-4.
[39] Turba UC, Arsian B, Meuse M, et al. Gunther Tulip filter retrieval
experience: predictors
of successful retrieval. Cardiovasc hitervent Radiol. 2009
[40] Kinney TB. Update on inferior vena cava filters. J Yaw Inters, Radio',
2003;14:425-40.
[41] Grande WJ, Trerotola SO, Reilly PM, et at. Experience with the recovery
filter as a
retrievable inferior vena cava filter. J Vasc Intery Radiol 2005; 161189-93.
CA 03155354 2022-4-20
WO 2021/080923
PCT/US2020/056359
33
[42] Kirilculc NN, Herget ET, Dicker RA, et al. Are temporary inferior vena
cava fillers really
temporary? Am J Surg 2005; 190:858-63.
[43] Kumar BC, Chakraverty Z, Zealley I. Failed retrieval of potentially
retrievable IVC filters:
a report of two cases. Cardiovasc Intervent Radial 2006; 29: 126-7.
[44] Removing Retrievable Inferior Vena Cava Filters: Initial Communication.
FDA Division
of Small Manufacturers, International and Consumer Assistance, Aug 9, 2010.
[45] Nicholson W, Nicholson WJ, Tolerico P. et al. Prevalence of fracture and
fragment
embolization of Bard retrievable vena cava filters and clinical implications
including cardiac
perforation and tamponade. Arch Intern Med. 2010.
[46] Karmy-Jones R, Jurkovich OH, Velmahos GC, et al. Practice patterns and
outcomes of
retrievable vena cava filters in trauma patients: an AAST multicenter study. J
Traurna.2007;
62: 17-25.
[47] Tschoe M, Kim HS, Brotman DJ, et al. Retrievable vena cava filters: a
clinical review. J
Hosp Med 2009, 4;7: 441-8.
[48] Dabbagh 0, Nagam N, Chitima-Matsiga R, et al. Retrievable inferior vena
cava filters are
not getting retrieved Where is the gap? Thrombosis Rsch 2010. 126: 493-7.
CA 03155354 2022-4-20