Note: Descriptions are shown in the official language in which they were submitted.
GAL676-1CA
1
Device for Automatically Inserting and Manipulating a Medical Tool Into and
Within a Bodily Lumen
TECHNICAL FIELD
The present invention, in some embodiments thereof, relates to automated
actuation of
surgical tools inserted into a bodily lumen.
BACKGROUND
US Patent No. 10,543,047 discloses "A robotic instrument driver for elongate
members
includes a first elongate member, and at least one manipulator mechanism
configured to
manipulate the first elongate member, and at least one articulating drive
configured to articulate
the first elongate member, positionable on a bed and beside a patient access
site. The
manipulator and articulating drive are positioned relative to each other a
distance less than the
insertable length of the first elongate member, stationary in position."
SUMMARY OF THE INVENTION
According to an aspect of some embodiments there is provided a compact robotic
device
for driving movement of two or more elongate surgical tools when the two or
more elongate
surgical tools are at least partially received within the device, the device
comprising:
a housing comprising walls which define a shared inner volume; the housing
encasing,
within the shared inner volume:
at least two inner pathways for accommodating at least a portion of each of
the
two or more elongate surgical tools;
a plurality of motors;
two or more tool actuation assemblies, each of the two or more actuation
assemblies configured at a position of one of the two or more inner pathways;
each of
the two or more actuation assemblies driven by at least one of the plurality
of motors,
each of the two or more actuation assemblies configured to operably contact at
least one
of the two or more elongate surgical tools when the two or more elongate
surgical tools
are at least partially received in the at least two inner pathways
respectively to at least
one of advance, retract and/or roll the elongate surgical tools.
Date Recue/Date Received 2022-04-14
GAL676-1CA
2
In some embodiments, the shared inner volume has no inner barriers which
separate the
plurality of motors from the two or more actuation assemblies.
In some embodiments, no wall, drape, shield or sterile protection separate the
plurality
of motors from the two or more actuation assemblies.
In some embodiments, each of the two or more inner pathways extends across the
inner
volume between an entry aperture and an exit aperture, the entry aperture and
the exit aperture
being configured on opposite walls of the device housing and in communication
with the inner
volume.
In some embodiments, each of the actuation assemblies comprises a plurality of
wheel
pairs, each wheel pair comprising a set of opposing wheels arranged to define
the inner pathway
therebetween.
In some embodiments, at least some of the opposing wheels are configured to
rotate to
advance and retract the elongate surgical tool within the inner pathway, and
to roll the elongate
surgical tool about a long axis of the elongate surgical tool.
In some embodiments, the tool actuation assemblies are both confined within
the walls
of the housing, and only portions of the two or more elongate surgical tools,
when received
within the device, extend outwardly from the walls of the housing to a
distance of at least 1 cm
away from the housing.
In some embodiments, at least one fixation location is defined externally to
the walls of
the housing for securing a proximal end of at least one of the two or more
elongate surgical
tools to the housing, while a more distal portion of the elongate surgical
tool is received inside
the housing, within one of the two or more inner pathways.
In some embodiments, the at least one fixation location is located at an exit
aperture
from the housing, such that the elongate surgical tool exiting the inner
volume through the exit
aperture is led into a lumen of a proximal end of a second elongate surgical
tool of the two or
more elongate surgical tools, forming a telescopic arrangement of the two
elongate surgical
tools.
In some embodiments the at least one fixation location defines a cavity shaped
and
configured to accommodate a proximal handle of the at least one elongate
surgical tool.
In some embodiments the inner volume is smaller than 2800 cm^3; and the device
has
a weight of less than 850 grams.
Date Recue/Date Received 2022-04-14
GAL676-1CA
3
In some embodiments, dimensions of the housing include a height shorter than
30 cm,
a width shorter than 30 cm, a length shorter than 30 cm; each of the at least
two inner pathways
extending axially along the length.
In some embodiments, the two or more elongate surgical tools include a
guidewire and
a microcatheter, the guidewire configured to at least partially extend through
a lumen of the
microcatheter.
In some embodiments, the device comprises a controller configured to control
the
plurality of motors for driving the two or more actuation assemblies.
In some embodiments, the controller is controlled remotely by an external
remote
control device.
In some embodiments, one or more of the two or more elongate surgical tools,
when
received within the inner pathway, extends outwardly from the walls of the
housing and forms
a curve externally to the device housing.
In some embodiments, each of the actuation assemblies comprises a designated
elongate
shaft extending axially along at least a portion of a length of the inner
pathway for the elongate
surgical tool to extend through.
In some embodiments, the robotic device a third actuation assembly, the third
actuation
assembly coupled to the housing and actuated by motors residing inside the
housing to move a
third elongate surgical tool.
In some embodiments, there is provided a kit comprising:
a robotic device for example as described herein;
a guidewire for loading onto the device such that at least a portion of the
guidewire
extends along one of the at least two inner pathways;
a microcatheter for loading onto the device such that at least a portion of
the
microcatheter extends along a second of the at least two inner pathways.
In some embodiments, there is provided a surgical system comprising:
a robotic device for example as described herein;
an add-on unit for driving movement of a guiding catheter, the add-on unit
mechanically
attachable to the housing of the robotic device.
According to an aspect of some embodiments there is provided a compact robotic
device
for driving movement of two or more elongate surgical tools when the two or
more elongate
surgical tools are at least partially received within the device, the device
comprising:
Date Recue/Date Received 2022-04-14
GAL676-1CA
4
a housing including at least two inner pathways for accommodating the two or
more
elongate surgical tools;
two or more tool actuation assemblies, each configured at a position of one of
the at
least two inner pathways; each of the two or more actuation assemblies
configured to operably
contact an elongate surgical tool of the two or more elongate surgical tools
which is at least
partially received in one of the at least two inner pathways to at least one
of advance, retract
and/or roll the respective elongate surgical tool; and
at least one external fixation location defined at walls of the housing; the
fixation
location including a holder which secures a proximal end of at least one of
the two or more
elongate surgical tools to the housing while a more distal segment of the at
least one elongate
surgical tool is received inside the housing within one of the at least two
inner pathways.
In some embodiments, the at least one fixation location is located at an exit
aperture of
the housing, such that the elongate surgical tool exiting the housing through
the exit aperture is
led into a lumen of a proximal end of a second elongate surgical tool of the
two or more elongate
surgical tools, forming a telescopic arrangement of the two elongate surgical
tools.
In some embodiments, the housing further encases a plurality of motors for
driving the
two or more tool actuation assemblies.
In some embodiments, at least one fixation location and at least one entry
aperture
leading into the housing are defined along a same wall of the housing such
that the elongate
surgical tool secured to the device at the at least one fixation location
forms a curve before
entering the housing through the at least one entry aperture.
In some embodiments, the at least one fixation location defines a cavity
shaped and
configured to accommodate a proximal handle of the at least one elongate
surgical tool.
In some embodiments, the holder comprises a luer.
In some embodiments, the holder is configured to provide for roll of the
respective
elongate surgical tool about a long axis of the tool.
According to an aspect of some embodiments there is provided a compact robotic
device
for driving movement of two or more elongate surgical tools when the two or
more elongate
surgical tools are at least partially received within the device, the device
comprising:
a housing comprising walls which define an inner volume including at least two
inner
pathways for accommodating the two or more elongate surgical tools; wherein
each of the at
least two inner pathways extends across the inner volume between an entry
aperture and an exit
Date Recue/Date Received 2022-04-14
GAL676-1CA
aperture, the entry aperture and the exit aperture being configured on
opposite walls of the
device housing and in communication with the inner volume; the at least two
inner pathways
being parallel to each other and having a similar axial extent.
In some embodiments, dimensions of the housing include a height shorter than
30 cm,
a width shorter than 30 cm, a length shorter than 30 cm; wherein each of the
two or more inner
pathways extends axially along the length.
In some embodiments, the housing comprises a removable or movable cover
providing
access to at least one of the two or more elongate surgical tools loaded onto
the device and
extending along at least a portion of the inner pathways.
In some embodiments, a distance between long axes of the inner pathways is
shorter
than 10 cm.
In some embodiments, the inner volume is smaller than 2800 cm^3 and wherein
the
device has a weight of less than 850 grams.
In some embodiments, two or more elongate surgical tools include a guidewire
and a
microcatheter, the guidewire configured to at least partially extend through a
lumen of the
microcatheter.
According to an aspect of some embodiments there is provided a compact robotic
device
for driving and manipulating movement of one or more elongate surgical tools,
comprising:
at least one motor;
at least one tool-moving element driven by the at least one motor, the tool-
moving
5 element positioned and configured to operably contact a tool at least
partially received in the
robotic device to advance, retract and/or rotate the elongate surgical tool;
and
a device housing shaped and sized to encase the at least one motor and the at
least one
tool-moving element.
In some embodiments, the at least one motor and the at least one tool-moving
element
are confined within walls of the housing, and wherein only the one or more
elongate surgical
tools, when received within the device, extend outwardly from the walls of the
housing.
In some embodiments, walls of the housing define an inner volume of less than
2800
cm^3 and wherein the device has a weight of less than 850 grams.
Date Recue/Date Received 2022-04-14
GAL676-1CA
6
In some embodiments, walls of the housing define at least one entry aperture
through which the
elongate surgical tool is inserted into the device and at least one exit
aperture through which
the elongate surgical tool exits the device.
In some embodiments, walls of the housing define at least two entry apertures
and at
least two exit apertures for at least two elongate surgical tools.
In some embodiments, the device comprises an anchoring location for a proximal
portion of
the elongate surgical tool, wherein the anchoring location and an entry
aperture for the elongate
surgical tool are aligned along a similar wall of the housing so that a
segment of the elongate
surgical tool extending externally to the housing and between the anchoring
location and the
entry aperture forms a U-shaped curve outside the housing.
In some embodiments, the housing comprises a designated elongate shaft for the
elongate surgical tool to extend through, the at least one tool moving element
positioned
adjacent the shaft and protruding inside the shaft to operably contact the
elongate surgical tool.
In some embodiments, the at least one tool-moving element comprises a set of
opposing
wheels configured to rotate to advance or retract the elongate surgical tool
within the shaft.
In some embodiments, the shaft is connected to a gear which when rotated
rotates the
shaft along with the at least one tool-moving element and the tool received
therein about the
shaft long axis, thereby rolling the tool with the at least one tool-moving
element.
In some embodiments, an inner contour of the shaft is shaped to match an outer
contour
of the at least one tool-moving element at their interface.
In some embodiments, the device comprises an anchoring location for a proximal
portion of the elongate surgical tool, the anchoring location including a
holder for holding a
proximal portion of the elongate surgical tool, while a more distal portion of
the elongate
surgical tool is received within the designated elongate shaft.
In some embodiments, one of the motors is configured to drive rotation of the
holder
and of the elongate shaft, thereby rolling the elongate surgical tool at two
spaced apart locations
along the length of the elongate surgical tool.
In some embodiments, a bottom wall of the housing is saddle shaped.
In some embodiments, a bottom wall of the housing is flat.
In some embodiments, dimensions of the housing include a height shorter than
30 cm,
a width shorter than 30 cm, a length shorter than 30 cm.
Date Recue/Date Received 2022-04-14
GAL676-1CA
7
In some embodiments, the housing, at the entry aperture and/or at the exit
aperture,
comprises a conically shaped protrusion having a rounded external lip.
In some embodiments, the housing comprises a removable or movable cover
providing
access to the one or more elongate surgical tools loaded onto the device.
In some embodiments, the device is configured to drive and manipulate movement
of
at least one of a guidewire and a microcatheter.
According to an aspect of some embodiments there is provided a surgical system
comprising:
a robotic device for example as described herein, and an add-on unit for
driving
movement of a guiding catheter, the add-on unit mechanically attachable to the
housing of the
robotic device.
In some embodiments, the system comprises a remote control device in
communication
with a controller of the robotic device.
In some embodiments, the system comprises an imaging modality in communication
with a
controller of the robotic device.
According to an aspect of some embodiments there is provided an assembly for
driving
linear movement and rotational movement of an elongate surgical tool,
comprising:
a shaft comprising a slot in communication with a central lumen of the shaft,
the lumen
extending along the shaft long axis;
a set of wheels positioned opposing each other and aligned on two sides of the
slot, the
wheels at least partially extending through apertures in the elongate shaft
and into the slot to
contact an elongate surgical tool received therein;
a gear positioned and configured, when rotated, to rotate the shaft along with
the set of
wheels about the shaft long axis.
In some embodiments, the gear is linearly aligned with the shaft and is co-
axial with the
shaft.
In some embodiments, the assembly comprises a motor positioned and configured
to
drive rotation of the wheels, the motor positioned and configured to rotate
with the shaft when
the shaft is rotated.
In some embodiments, the gear comprises a slot on its circumference, the slot
linearly
aligned with the slot of the shaft.
Date Recue/Date Received 2022-04-14
GAL676-1CA
8
In some embodiments, inner walls of the shaft which define the central lumen
are
contoured to match at least a portion of an external contour of at least one
of the wheels of the
set of wheels.
In some embodiments, the assembly comprises motor transmission in contact with
the
gear and configured to rotate the gear.
In some embodiments, each wheel of the set of wheels is arranged to lie on a
plane that
is substantially perpendicular to a plane defined by the slot.
In some embodiments, when the assembly is rotated about the shaft long axis,
the set
of wheels rotates along so that each wheel of the set of wheels remains lying
on the plane that
is substantially perpendicular to the plane defined by the slot.
According to an aspect of some embodiments there is provided a method of using
a
surgical robotic device for manipulation of at least one elongate surgical
tool, comprising:
providing a robotic device shaped and sized to be placed adjacent or on a
surgical bed;
loading at least one elongate surgical tool onto the device;
controlling manipulation of the at least one elongate surgical tool by the
robotic device
via a remote control interface to carry out a surgical procedure; and
disposing the robotic device along with the at least one elongate surgical
tool following
the surgical procedure.
In some embodiments, the robotic device comprises:
one or more motors;
one or more tool-moving elements driven by the one or more motors;
wherein loading places the at least one elongate surgical tool in direct
operable contact
with the one or more tool-moving elements, and the one or more tool-moving
elements are in
direct operable contact with the one or more motors.
In some embodiments, the robotic device is not covered by a sterile drape.
In some embodiments, the method comprises introducing the at least one
elongate
surgical tool into the body and allowing body fluids through the elongate
surgical tool and into
the robotic device.
According to an aspect of some embodiments there is provided a method of using
a
surgical robotic device for manipulation of at least one elongate surgical
tool, comprising:
providing a robotic device shaped and sized to be attached to a patient's
limb;
attaching the robotic device onto the patient's limb;
Date Recue/Date Received 2022-04-14
GAL676-1CA
9
loading the at least one elongate surgical tool onto the device; and
controlling manipulation of the at least one elongate surgical tool by the
robotic device
to carry out a surgical procedure.
In some embodiments, the limb is one of: a patient's leg where the robotic
device is
attached to the thigh, a patient's arm where the robotic device is attached
adjacent the wrist.
In some embodiments, the method comprises forming an incision in the patient's
groin
and introducing, using the robotic device, the at least one elongate surgical
tool through the
incision.
In some embodiments, attaching comprises strapping the robotic device onto the
limb.
According to an aspect of some embodiment there is provided a method of
controlling
a usable length of an elongate surgical tool, comprising:
providing a robotic device comprising a housing;
loading the elongate surgical tool onto the robotic device such that the
elongate surgical
tool is held at a first location along the length of the elongate surgical
tool and slidably held at
a second location along the length of the elongate surgical tool; wherein a
segment of the tool
extending between the first and second locations forms a curve; and
sliding the elongate surgical tool at the second location to shorten or
lengthen a distance
between a maximal point of the curve and the housing of the robotic device to
control the length
of the elongate surgical tool.
In some embodiments, the method comprises controlling, via the shortening or
lengthening, a length of a distal segment of the elongate surgical tool which
extends from the
robotic device housing to a target point inside the patient's body.
According to an aspect of some embodiments there is provided a compact robotic
device
for driving and manipulating movement of at least two elongate surgical tools,
comprising:
a housing comprising:
at least one motor;
at least two assemblies, each assembly configured for driving linear movement
and/or rotation of one of the at least two elongate surgical tools, each
assembly
comprising tool-moving elements driven by the at least one motor or associated
transmission;
wherein the housing defines a volume of less than 2800 cm^3 and has a weight
of less
than 850 grams.
Date Recue/Date Received 2022-04-14
GAL676-1CA
According to an aspect of some embodiments there is provided a compact robotic
device
for driving and manipulating movement of at least one elongate surgical tool,
comprising:
a housing comprising:
at least one motor;
5 a first tool-moving element driven by the at least one motor, the
tool-moving
element positioned and configured to operably contact an elongate surgical
tool at least
partially received in the robotic device to advance or retract the elongate
surgical tool;
and
a second tool-moving element driven by the at least one motor and configured
to to roll the elongate surgical tool about the long axis of the elongate
surgical tool.
In some embodiments, the housing comprises a shaft for the elongate surgical
tool to
extend through, the first tool-moving element at least partially protruding
into the shaft to
contact the elongate surgical tool.
In some embodiments, inner walls of the shaft are contoured to match at least
a portion
of an external contour of the first tool-moving element.
In some embodiments, the first tool-moving element comprises at least one pair
of
wheels which advance or retract the elongate surgical tool dependent on the
wheel direction of
rotation.
In some embodiments, the second tool-moving element comprises a gear aligned
linearly along the shaft and configured to rotate the shaft.
According to some embodiments, there are provided advantageous medical devices
for
inserting and advancing a medical tool within bodily lumen(s), wherein the
devices are
configured to advance the medical tool in a linear movement and/or rotational
movement. In
some embodiments, the advantageous devices disclosed herein allow the
insertion and
advancement of more than one medical tool, separately or simultaneously, while
being small in
size, thereby configured to be mounted onto the subject body, or at least in
close proximity
thereto. In some embodiments, the devices disclosed herein are configured to
operate
automatically and/or controlled manually by a user, utilizing a remote
controller. In some
embodiments, further provided are systems which include the disclosed devices
and methods
of using the same in various medical procedures.
According to some embodiments, there is provided a medical device for
advancing and
inserting a medical tool into a bodily lumen, the device being configured to
be mounted on the
Date Recue/Date Received 2022-04-14
GAL676-1CA
11
subject's body or to be positioned in close proximity thereto, and including:
a housing
configured for positioning the medical device on the body of the subject or in
close proximity
to the subject's body; at least one movement control unit comprising at least
one actuator
configured for linearly advancing the medical tool and at least one rotational
actuator
configured for rotating the medical tool; wherein the at least one rotational
actuator and the at
least one linear actuator are activated simultaneously and/or and
independently from each other.
According to some embodiments, the device may further include a controller
configured
to activate the at least one linear actuator and the at least one rotational
actuator. According to
some embodiments, the controller may be configured for manual operation by a
user. According
to some embodiments, the controller may be configured for receiving commands
from a
processor. In some embodiments, the device may be autonomously computer
controlled.
According to some embodiments, the at least one linear actuator and the at
least one
rotational actuator may have one or more common actuators.
According to some embodiments, the at least one linear actuator may include an
actuator
selected from: a DC motor, an AC motors, a stepper motors, an electromagnetic
actuator, a
piezoelectric actuator, a pneumatic actuator, an hydraulic actuator, or any
combination thereof.
According to some embodiments, the at least one rotational actuator may
include an
actuator selected from: a DC motor, an AC motors, a stepper motors, an
electromagnetic
actuator, a piezoelectric actuator, a pneumatic actuator, an hydraulic
actuator, or any
combination thereof. In some embodiments, the medical device is disposable. In
some
embodiments, the medical device is miniature in size. In some embodiments, the
medical device
is lightweight.
According to some embodiments, the medical tool may be selected from: a
guidewire,
micro-catheter, balloon catheter, guiding catheter, stenting catheter,
embolization catheter, stent
retriever device, and the like, or any combination thereof.
According to some embodiments, the body lumen may be selected from a blood
vessel,
urethra and trachea, gastric anatomy, and the like. According to some
embodiments, the device
may include more than one movement control unit, wherein each control unit may
be
configured to linearly advance and/or rotate a separate medical tool or
combination of two or
more motors can perform a decoupled or combined motion of the medical tools.
According to some embodiments, the device may include two movement control
units,
wherein a first movement control unit is configured to linearly advance and/or
rotate a first
Date Recue/Date Received 2022-04-14
GAL676-1CA
12
medical tool, and a second movement control unit configured to linearly
advance and/or rotate
a second medical tool.
According to some embodiments, the first medical tool may be a guidewire and
the
second medical tool may a catheter.
According to some embodiments, the first medical tool may be configured to
advance
through a lumen of the second medical tool.
According to some embodiments, the device may be further configured to allow
control
over the tip parameters of the medical tool.
According to some embodiments, the movement control unit may include at least
two
discs opposing each other along a portion of their external circumference,
such that the medical
tool is capable of being placed in a space formed therebetween, while
maintaining at least partial
contact with at least one of the discs, whereby upon spinning of said discs,
the medical tool
linearly advances. The surface of the external circumference of the discs may
be rough, soft,
smooth, coated, spongy, hydrophilic, hydrophobic, or with other
characteristics that may
optimize the interaction with the medical tool. The driving discs may be
assembled in such a
way that the medical tool is not actuated along a straight line, but along a
curved route, thus
allowing for higher driving force and higher rotational moment.
According to some embodiments, the medical device may further include a power
source.
According to some embodiments, the device may be configured to linearly
advance the
medical tool at a constant or varying rate (velocity).
According to some embodiments, the device may be configured to automatically
insert
and advance the medical tool into the bodily lumen.
According to some embodiments, there is provided a system for inserting a
medical tool
into a bodily lumen, the system includes: a medical device for inserting the
medical tool into
the bodily lumen, the device being configured for positioning on or in close
proximity to a body
of a subject, and comprising: at least one movement control unit comprising at
least one actuator
configured for linearly advancing the medical tool and at least one rotational
actuator
configured for rotating the medical tool; a controller configured to activate
the at least one linear
actuator and the at least one rotational actuator, said controller is
configured to activate the at
least one rotational actuator and the at least one linear actuator at least
one of simultaneously
Date Recue/Date Received 2022-04-14
GAL676-1CA
13
and independently from each other; and a processor configured to provide
commands to said
controller.
According to some embodiments, the controller may be configured for manual
operation by a user.
According to some embodiments, the controller may include activating buttons,
selected
from: press buttons, sliding buttons, joystick, or any combination thereof.
According to some embodiments, the system disclosed herein is used for
automatically
inserting and advancing the medical tool into the bodily lumen in a medical
procedure.
According to some embodiments, the medical procedure may include an
endovascular
procedure, selected from coronary, peripheral and cerebral endovascular
procedures, gastric
procedures, procedures in the urinal tract and in procedures in the
respiratory tract.
According to some embodiments, the system may further include or be configured
to
operate in conjunction with an imaging device. According to some embodiments,
the imaging
device may be selected from: X-ray device, fluoroscopy device, CT device, cone
beam CT
device, CT fluoroscopy device, MRI device and ultrasound device. According to
some
embodiments, there is provided a method for inserting and advancing a medical
tool into a
bodily lumen, the method comprising: mounting and securing the medical device
disclosed
herein on a subject's body or positioning the medical device in close
proximity to the subject's
body, and advancing the medical tool into the bodily lumen of the subject. In
some
embodiments, the method is automatic (i.e. advancing of the medical tool is
performed
automatically by the medical device).
According to some embodiments, there is provided a body mountable medical
device
for inserting a medical tool into a bodily lumen, the device includes: a
housing configured for
positioning on a body of a subject and securing thereto; at least one linear
actuator configured
for linearly advancing the medical tool; at least one rotational actuator
configured for rotating
the medical tool; a controller configured to activate the at least one linear
actuator and the at
least one rotational actuator; wherein the controller is configured to
activate the at least one
rotational actuator and the at least one linear actuator at least one of
simultaneously and
independently from each other.
According to some embodiments, the guidewire and microcatheter, entering and
exiting
the device from the rear and front end, advantageously allow the motion of a
microcatheter over
the guidewire without having the microcatheter drive impair the guidewire
drive.
Date Recue/Date Received 2022-04-14
GAL676-1CA
14
Certain embodiments of the present disclosure may include some, all, or none
of the
above advantages. One or more other technical advantages may be readily
apparent to those
skilled in the art from the figures, descriptions, and claims included herein.
Moreover, while
specific advantages have been enumerated above, various embodiments may
include all, some,
or none of the enumerated advantages.
According to an aspect of some embodiments there is provided a medical device
for
advancing and inserting a medical tool into a bodily lumen, comprising: a
housing configured
for positioning the medical device on a or in close proximity to a body of a
subject and securing
thereto; at least one movement control unit comprising at least one actuator
configured for
linearly advancing the medical tool and at least one rotational actuator
configured for rotating
the medical tool; wherein the at least one rotational actuator and the at
least one linear actuator
are activated simultaneously and/or and independently from each other.
In some embodiments, the device comprises a controller configured to activate
the at
least one linear actuator and the at least one rotational actuator.
In some embodiments, the controller is configured for manual operation by a
user.
In some embodiments, the controller is configured for receiving commands from
a
processor.
In some embodiments, the at least one linear actuator and the at least one
rotational
actuator have one or more common actuators.
In some embodiments, the at least one linear actuator comprises an actuator
selected
from: a DC motor, an AC motors, a stepper motors, an electromagnetic actuator,
a piezoelectric
actuator, pneumatic actuator, hydraulic actuator, or any combination thereof.
In some embodiments, the at least one rotational actuator comprises an
actuator selected
from: a DC motor, an AC motors, a stepper motors, an electromagnetic actuator,
a piezoelectric
actuator, pneumatic actuator, hydraulic actuator, or any combination thereof.
In some embodiments, the medical device is disposable.
In some embodiments, the medical tool is selected from: a guide wire, micro-
catheter,
balloon catheter, a guiding catheter, stent, retrieval device, or any
combination thereof.
In some embodiments, the body lumen is selected from, a blood vessel, urethra,
trachea
and gastrointestinal.
Date Recue/Date Received 2022-04-14
GAL676-1CA
In some embodiments, the device comprises more than one movement control unit,
wherein each control unit is configured to linearly advance and/or rotate a
separate medical
tool.
In some embodiments, the device comprises two movement control units, wherein
a
5 first movement control unit is configured to linearly advance and/or
rotate a first medical tool,
and a second movement control unit configured to linearly advance and/or
rotate a second
medical tool.
In some embodiments, the first medical tool is a guidewire and the second
medical tool
is a catheter.
10 In some embodiments, the first medical tool is configured to advance
through a lumen
of the second medical tool.
In some embodiments, the device is further configured to allow control over
the tip
parameters using additional actuator of the medical tool.
In some embodiments, the movement control unit comprises at least two discs
opposing
15 each other along a portion of their external circumference, such that
the medical tool is capable
of being placed in a space formed therebetween, while maintaining at least
partial contact with
at least one of the wheels whereby upon spinning of the discs, the medical
tool linearly
advances. In some embodiments, the device comprises a power source.
In some embodiments, the device is configured to linearly advance the medical
tool at
a constant or varying rate (velocity).
In some embodiments, the device is configured to automatically insert and
advance the
medical tool into the bodily lumen.
According to an aspect of some embodiments there is provided a system for
inserting a
medical tool into a bodily lumen, the system comprising: a medical device for
inserting the
medical tool into the bodily lumen, the device comprising: a housing
configured for positioning
the medical device on a body of a subject or in close proximity thereto, and
securing thereto; at
least one movement control unit comprising at least one actuator configured
for linearly
advancing the medical tool and at least one rotational actuator configured for
rotating the
medical tool; a controller configured to activate the at least one linear
actuator and the at least
one rotational actuator, the controller is configured to activate the at least
one rotational actuator
and the at least one linear actuator at least one of simultaneously and
independently from each
other; and a processor configured to provide commands to the controller.
Date Recue/Date Received 2022-04-14
GAL676-1CA
16
In some embodiments, the controller is configured for manual operation by a
user.
In some embodiments, the controller comprises activating buttons, selected
from: press
buttons, sliding buttons, joystick, or any combination thereof.
In some embodiments, the at least one linear actuator and the at least one
rotational
actuator have one or more common actuators.
In some embodiments, the at least one linear actuator comprises an actuator
selected
from: a DC motor, an AC motors, a stepper motors, an electromagnetic actuator,
a piezoelectric
actuator, a pneumatic actuator, an hydraulic actuator, or any combination
thereof.
In some embodiments, the at least one rotational actuator comprises an
actuator selected
from: a DC motor, an AC motors, a stepper motors, an electromagnetic actuator,
a piezoelectric
actuator, a pneumatic actuator, an hydraulic actuator, or any combination
thereof.
In some embodiments, the medical device is disposable.
In some embodiments, the medical tool is selected from: a guide wire, micro-
catheter,
a guiding catheter and balloon catheter.
In some embodiments, the body lumen is selected from, a blood vessel, urethra,
gastric
and trachea.
In some embodiments, the system comprises two movement control units, wherein
a
first movement control unit is configured to linearly advance and/or rotate a
first medical tool,
and a second movement control unit configured to linearly advance and/or
rotate a second
medical tool. In some embodiments, the first medical tool is a guidewire and
the second medical
tool is a catheter.
In some embodiments, the system is configured for automatically inserting and
advancing the medical tool into the bodily lumen in a medical procedure.
In some embodiments, the medical procedure is selected from coronary,
peripheral, and
cerebral endovascular procedures, gastric procedure, urinal procedures and
respiratory tract
procedures.
In some embodiments, the system further comprises an imaging device.
In some embodiments, the imaging device is selected from: Xray device,
fluoroscopy
device, CT device, cone beam CT device, CT fluoroscopy device, MRI device and
ultrasound
device.
According to an aspect of some embodiments there is provided a method for
inserting
and advancing a medical tool into a bodily lumen, the method comprising:
positioning a medical
Date Recue/Date Received 2022-04-14
GAL676-1CA
17
device on or in close proximity to a body of a subject, the device comprising:
a housing
configured for positioning the medical device on or in close proximity to a
body of a subject
and securing thereto; at least one movement control unit comprising at least
one actuator
configured for linearly advancing the medical tool and at least one rotational
actuator
configured for rotating the medical tool; wherein the at least one rotational
actuator and the at
least one linear actuator are activated simultaneously and/or and
independently from each other;
and; advancing the medical tool into the bodily lumen of the subject.
In some embodiments, the medical tool is selected from: a guidewire, a micro-
catheter,
a guiding catheter and a balloon catheter.
to In some
embodiments, the body lumen is selected from, a blood vessel, urethra and
trachea.
In some embodiments, advancing of the medical tool is performed automatically
by the
medical device.
According to an aspect of some embodiments there is provided a medical device
for
inserting a medical tool into a bodily lumen, comprising: a housing configured
for positioning
on a body of a subject or in close proximity to the subject and securing
thereto; at least one
linear actuator configured for linearly advancing the medical tool; at least
one rotational
actuator configured for rotating the medical tool; a controller configured to
activate the at least
one linear actuator and the at least one rotational actuator; wherein the
controller is configured
to activate the at least one rotational actuator and the at least one linear
actuator at least one of
simultaneously and independently from each other.
In some embodiments, the controller is configured for manual operation by a
user.
In some embodiments, the controller is configured for receiving commands from
a
processor.
In some embodiments, the controller is configured to receive commands from
wireless
remote controller.
In some embodiments, the wireless remote controller is a Wi-Fi remote
controller, and
Bluetooth remote controller.
In some embodiments, the at least one linear actuator and the at least one
rotational
actuator have one or more common actuators.
In some embodiments, the at least one linear actuator comprises at least one
piezoelectric actuator.
Date Recue/Date Received 2022-04-14
GAL676-1CA
18
In some embodiments, the at least one rotational actuator comprises at least
one
piezoelectric actuator.
According to an aspect of some embodiments there is provided a compact robotic
device
for driving movement of two or more elongate surgical tools when the two or
more elongate
surgical tools are at least partially received within the device, the device
comprising:
a housing comprising walls which define an inner volume including at least two
inner
pathways for accommodating the two or more elongate surgical tools
the housing encasing:
a plurality of motors;
to two or more tool actuation assemblies configured at a position of
each of the two
or more inner pathways; the actuation assemblies driven by the plurality of
motors and
configured to operably contact an elongate surgical tool at least partially
received in the
inner pathway to at least one of advance, retract and/or roll the elongate
surgical tool.
In some embodiments, each of the two or more inner pathways extends across the
inner
volume between an entry aperture and an exit aperture, the entry aperture and
the exit aperture
being configured on opposite walls of the device housing and in communication
with the inner
volume.
In some embodiments, no inner barrier exists between the two or more inner
pathways
such that the two or more tool actuation assemblies and the plurality of
motors all share the
inner volume with no separation therebetween.
In some embodiments, at least one fixation location is defined externally to
the walls of
the housing for securing a proximal end of an elongate surgical tool to the
housing.
In some embodiments, the at least one fixation location is located at one of
the exit
apertures, such that an elongate surgical tool exiting the inner volume
through the exit aperture
is led into a lumen of a proximal end of a second elongate surgical tool,
forming a telescopic
arrangement of the two tools.
In some embodiments, the at least one fixation location and one of the at
least two entry
apertures are defined along the same wall of the housing such that an elongate
surgical tool
secured to the device at the at least one fixation location forms a curve
before entering the inner
-- volume through the at least one entry aperture.
In some embodiments, the two or more inner pathways are parallel to each other
and
have a similar axial extent.
Date Recue/Date Received 2022-04-14
GAL676-1CA
19
In some embodiments, a distance between long axes of the inner pathways is
shorter
than 10 cm.
In some embodiments, the tool actuation assemblies are both confined within
the walls
of the housing, and wherein only portions of the two or more elongate surgical
tools, when
received within the device, extend outwardly from the walls of the housing to
a distance of at
least 1 cm away from the housing.
In some embodiments, the inner volume is smaller than 2800 cm^3 and wherein
the
device has a weight of less than 850 grams.
In some embodiments, the plurality of motors comprises 3-5 motors.
In some embodiments, each of the actuation assemblies comprises:
a designated elongate shaft extending axially along at least a portion of a
length of the
inner pathway for the elongate surgical tool to extend through; and
at least one pair of wheels positioned adjacent the shaft and protruding
inside the shaft
to operably contact the elongate surgical tool received within the shaft.
In some embodiments, each of the actuation assemblies comprises a plurality of
wheels
pairs, each wheel pair comprising a set of opposing wheels arranged to define
the inner pathway
therebetween.
In some embodiments, the at least one pair of wheels comprises a set of
opposing wheels
configured to rotate to advance or retract the elongate surgical tool within
the shaft.
In some embodiments, the shaft is connected to a gear which when rotated
rotates the
shaft along with the plurality of wheels and with the elongate surgical tool
received therein
about the shaft long axis, thereby rolling the elongate surgical tool.
In some embodiments, dimensions of the housing include a height shorter than
30 cm,
a width shorter than 30 cm, a length shorter than 30 cm; wherein each of the
inner pathways
.. extends axially along the length.
In some embodiments, the housing, at least one of the entry aperture and/or at
least one
of the exit aperture, comprises a conically shaped protrusion having a rounded
external lip.
In some embodiments, the housing comprises a removable or movable cover
providing
access to the one or more elongate surgical tools loaded onto the device and
extending along at
least a portion of the inner pathways.
Date Recue/Date Received 2022-04-14
GAL676-1CA
In some embodiments, the device is configured to drive movement of a guidewire
and
a microcatheter, the guidewire configured to at least partially extend through
a lumen of the
microcatheter.
In some embodiments, the device comprises a controller configured to control
the
5 plurality of motors for driving the two or more actuation assemblies.
In some embodiments, the controller is controlled remotely by an external
remote
control device.
In some embodiments, there is provided a kit comprising: a device for example
as
described herein; a guidewire for loading onto the device such that at least a
portion of the
10 guidewire extends along one of the inner pathways; and microcatheter for
loading onto the
device such that at least a portion of the microcatheter extends along a
second of the inner
pathways.
In some embodiments, there is provided a surgical system comprising: a robotic
device
for example as described herein; and an add-on unit for driving movement of a
guiding catheter,
15 the add-on unit mechanically attachable to the housing of the robotic
device.
Unless otherwise defined, all technical and/or scientific terms used herein
have the same
meaning as commonly understood by one of ordinary skill in the art to which
the invention
pertains. Although methods and materials similar or equivalent to those
described herein can
20 be used in the practice or testing of embodiments of the invention,
exemplary methods and/or
materials are described below. In case of conflict, the patent specification,
including definitions,
will control. In addition, the materials, methods, and examples are
illustrative only and are not
intended to be necessarily limiting.
Implementation of the method and/or system of embodiments of the invention can
involve performing or completing selected tasks manually, automatically, or a
combination
thereof. Moreover, according to actual instrumentation and equipment of
embodiments of the
method and/or system of the invention, several selected tasks could be
implemented by
hardware, by software or by firmware or by a combination thereof using an
operating system.
For example, hardware for performing selected tasks according to embodiments
of the
invention could be implemented as a chip or a circuit. As software, selected
tasks according to
embodiments of the invention could be implemented as a plurality of software
instructions
being executed by a computer using any suitable operating system. In an
exemplary
Date Recue/Date Received 2022-04-14
GAL676-1CA
21
embodiment of the invention, one or more tasks according to exemplary
embodiments of
method and/or system as described herein are performed by a data processor,
such as a
computing platform for executing a plurality of instructions. Optionally, the
data processor
includes a volatile memory for storing instructions and/or data and/or a non-
volatile storage,
for example, a magnetic hard-disk and/or removable media, for storing
instructions and/or data.
Optionally, a network connection is provided as well. A display and/or a user
input device such
as a keyboard or mouse are optionally provided as well.
BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWING(S)
Some embodiments of the invention are herein described, by way of example
only, with
reference to the accompanying drawings. With specific reference now to the
drawings in detail,
it is stressed that the particulars shown are by way of example and for
purposes of illustrative
discussion of embodiments of the invention. In this regard, the description
taken with the
drawings makes apparent to those skilled in the art how embodiments of the
invention may be
practiced.
In the drawings:
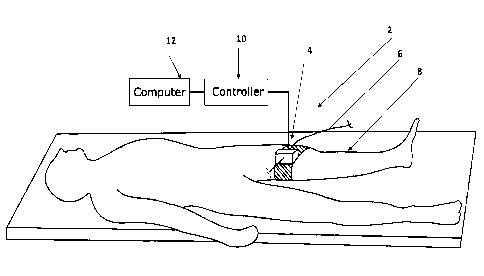
FIG. 1 shows a schematic diagram of a medical system comprising an insertion
device
secured to a subject's body, according to some embodiments;
FIGs. 2A-2B illustrate schematic perspective views (front and rear,
respectively), of an
insertion device, according to some embodiments;
FIGs. 3A-3B illustrate schematic perspective views of an insertion device,
according to
some embodiments;
FIGs. 4A-4B show schematic perspective cross-sectional views of the insertion
device
shown in Figs. 3A-3B, according to some embodiments;
FIG. 5 illustrates a schematic perspective top view of movement control units
of an
insertion device, according to some embodiments;
FIG. 6A illustrates a schematic perspective view of an insertion device,
according to
some embodiments;
FIG. 6B shows a perspective view of a movement control unit, according to some
embodiments;
FIG. 6C shows a side view of a movement control element, according to some
embodiments;
Date Recue/Date Received 2022-04-14
GAL676-1CA
22
FIG. 7 shows a longitudinal cross section view of a movement control element
of Fig.
6C;
FIG. 8 schematically illustrates a movement control unit, according to some
embodiments;
FIGs. 9A-9B illustrate moving units for linear advancement and/or rotational
movement
of a medical instrument, according to some embodiments. FIG. 9A shows
schematically a
piezoelectric actuated mechanism for linear translation of a medical tool,
according to some
embodiments, and FIG. 9B shows schematically a piezoelectric actuated
mechanism for
rotating a medical tool, according to some embodiments;
FIG. 10 depicts schematic illustrations of an exemplary device capable of
imparting
both linear and rotational motion on a medical tool, according to some
embodiments;
FIG. 11 illustrates a movement control unit, according to some embodiments.
FIG. 12 illustrates an assembly of movement control units, for controlling
movement of
more than one medical instrument, according to some embodiments;
FIG. 13 is a block diagram of a surgical robotic system, according to some
embodiments;
FIG. 14 is a flowchart of a general method of using a surgical robotic device,
according
to some embodiments;
FIG. 15 is a flowchart of a method of loading a plurality of surgical tools
onto the
surgical robotic device, according to some embodiments;
FIGs. 16A-D are various configurations of a remote control device of the
surgical
robotic system, according to some embodiments;
FIG. 17 is a schematic example of a screen interface associated with the
surgical robotic
system, according to some embodiments;
FIGs. 18A-B are different views of a robotic device, according to some
embodiments;
FIGs. 19A-B schematically illustrates a surgical robotic device including or
attached
to a guiding catheter driving unit, according to some embodiments;
FIGs. 20A-C are an example of an isolated mechanism of the guiding catheter
driving
unit, an example of a guiding catheter driving unit housing, and a guiding
catheter driving unit
assembled onto the robotic surgical system, according to some embodiments;
FIG. 21A-C show mechanisms for actuating rotation (roll) and/or linear
movement of
a tool actuated by the robotic surgical system, according to some embodiments;
Date Recue/Date Received 2022-04-14
GAL676-1CA
23
FIG. 22 shows an exemplary arrangement of mechanisms driving movement of a
guidewire, according to some embodiments;
FIGs. 23A-B are a schematic diagram and a flowchart pertaining to controlling
a length
and/or position of a tool by adjusting a curved portion of the tool, according
to some
embodiments;
FIG. 24 shows a system configuration defining an arrangement of tools in which
a tool
length can be adjusted, according to some embodiments;
FIG. 25 schematically illustrates tool-movement driving mechanisms of the
system,
according to some embodiments;
FIGs. 26A-B are examples of a device configuration including elastic elements
(e.g.
springs) for selectively engaging tools received by the system, according to
some
embodiments;
FIG. 27 is a schematic block diagram of a robotic device configured for
manipulating
two or more elongate surgical tools, according to some embodiments;
FIG. 28 schematically illustrates a robotic device for manipulation of a
guidewire and
a microcatheter, the guidewire extending at least in part within the
microcatheter lumen,
according to some embodiments; and
FIG. 29 schematically illustrates a robotic device for manipulation of three
or more
elongate surgical tools configured for a telescopic arrangement, according to
some
embodiments.
Date Recue/Date Received 2022-04-14
GAL676-1CA
24
DETAILED DESCRIPTION
The present invention, in some embodiments thereof, relates to automated
actuation of
elongate surgical tools inserted into a bodily lumen.
A broad aspect of some embodiments relates to a compact robotic device for
manipulating movement of elongate surgical endoluminal tools which extend and
curve outside
of the device housing. Some embodiments described herein pertain to
structural, functional
and/or design features suitable for manipulating the tool using a compact
sized robotic device
which dimensions are not affected by the length of the tool being manipulated.
In some
embodiments, properties of the robotic device such as volume, weight are
dictated solely by the
electrical and mechanical components of the device and substantially not by
the tools being
manipulated.
An aspect of some embodiments relates to a compact robotic device shaped and
sized
to be mounted onto a patient's body and/or onto a surgical bed. In some
embodiments, a volume
of the device is smaller than 3000 cm^3, 2800 cm^3, 2500 cm^3, or
intermediate, larger or
smaller volume. In some embodiments, a weight of the device is less than 1000
grams, 850
grams, 500 grams or intermediate, larger or smaller weight.
In some embodiments, the device comprises a plurality of actuation mechanisms
for
moving one or more elongate surgical tools (e.g. a guidewire, a
microcatheter), for example,
for advancing or retracting the tool linearly, for rolling the tool. In some
embodiments, a device
housing encapsulates the actuation mechanisms, while walls of the housing
define a plurality
of entry and/or exit apertures and/or anchoring locations for the tool. In
some embodiments, an
anchoring location (e.g. a holder) at which a proximal end portion of the tool
is coupled to the
housing, and an entry aperture for a tool leading to the inner side of the
housing are aligned
with respect to each other along a similar horizontal or vertical axis, so
that a tool segment
extending between the anchoring location and the entry aperture forms a curve
externally to the
device housing. In some embodiments, an anchoring location and an entry
aperture of a tool are
defined in a similar face (or wall) of the device housing. In some
embodiments, an entry
aperture and an exit aperture for the same tool are configured on opposing
walls of the housing,
so that a tool entering the housing extends across the inner space defined by
the housing, to the
exit aperture.
In some embodiments, no device portions protrude outwardly from the housing,
and
optionally only the tools loaded onto the device extend outwardly from the
housing.
Date Recue/Date Received 2022-04-14
GAL676-1CA
In some embodiments, a maximal dimension of the robotic device housing (e.g. a
width,
a height, such as in a box shaped device) is a function of a distance between
exit and entry
apertures of a tool that curves externally to the device. The distance between
the exit and entry
apertures may be set, for example, in accordance with a minimal radius of
curvature that the
5 tool
can withstand. In an example, a maximal dimension of the device housing is
between 2- 6
times, 2-10 times, 2-5 times or intermediate, higher or lower number of times
a minimal radius
of curvature of a tool manipulated by the device and curving outside of the
housing. A potential
advantage of a device housing where a maximal dimension is determined in
accordance with a
minimal radius of curvature of a tool that bends upon exiting and re-entering
the housing may
10 include
providing for a compact, minimalized size housing. In an example, for a tool
having a
minimal radius of curvature of X, a minimal distance between entry and exit
apertures of the
tool would be 2X. In such situation, a wall of the housing through which the
tool exits and
enters comprises a width of, for example, 2X, 2.1X, 3X, 5X or intermediate,
larger or smaller
dimension.
15 In some
embodiments, a minimal radius of curvature of an elongate tool includes a
maximal bend of the tool which still allows for the tool to function, for
example allows the
transferring of torque along the length of the tool. In some embodiments, a
minimal radius of
curvature of an elongate tool includes a bend in which the tool remains intact
(for example, not
broken).
20 In some
embodiments, exit and entry apertures from and to the housing are shaped to
reduce or avoid friction between the tool and the edges of the aperture, for
example by having
a conical profile and/or rounded lip of the aperture. A potential advantage of
apertures formed
with no sharp edges may include reducing friction contact between the tool and
the walls of the
housing, which may reduce a risk of wear or tear of the tool, especially when
the tool extends
25 and curves externally to the housing, before re-entering the housing.
In some embodiments, a shape and/or size of the housing is dictated by the
mechanical
and/or electrical components within the housing, for example, motor(s), motor
transmission
(e.g. gears), tool actuation mechanisms (e.g. tool-moving elements, such as
wheels). In some
embodiments, the housing is sized to be as small as possible while still fully
encasing the
mechanical components inside it. Optionally, no mechanical components of the
robotic device
protrude outwardly from the housing. Optionally, no additional mechanical
components from
outside the housing are required for performing actuation of the tools. In
some embodiments,
Date Recue/Date Received 2022-04-14
GAL676-1CA
26
the housing is shaped and configured so that only the elongate surgical tools
extend into and
out of the housing. In some embodiments, extending out of the housing
comprises extending at
least 1 cm, at least 2 cm, at least 4 cm or intermediate, longer or shorter
distance away from a
wall of the housing, such as from a wall throughout which the tool exits the
housing. For
example, extending of a surgical tool such as a guidewire or microcatheter at
least 1 cm away
from the housing.
In some embodiments, components which are integral to the housing, for example
protrusions which define lips of entry and/or exit apertures of the housing,
extend out of the
housing to a distance of less than 1 cm, less than 0.5 cm, less than 0.3 cm,
or intermediate,
longer or shorter distance.
In some embodiments, a housing of the robotic device is not limited to a
certain
orientation, for example, so that the housing can be positioned in at least a
first orientation and
in a second orientation, for example where the second orientation is 90
degrees or 180 degrees
to the first orientation. In some embodiments, a symmetry exists so that at
least two opposing
faces of the housing are similar in contour and in size, allowing for
positioning the device in
one of two "flipped" orientations.
In some embodiments, a plurality of pathways are defined through an inner
volume of
the device, where actuation mechanism(s) for driving movement of a tool
received within a
pathway are configured along the pathway. In some embodiments, a pathway
extends between
an entry aperture leading into the inner volume of the device housing, and an
exit aperture
leading out of the inner volume. In some embodiments, the entry and exit
apertures are defined
on opposing walls of the housing. In some embodiments, the device includes
multiple pathways
(e.g. 2, 3, 4, 6, or intermediate, larger or smaller number of pathways) for
receipt of a
corresponding number of elongate surgical tools, each tool received within a
pathway. In some
embodiments, long axes of pathways are parallel. In some embodiments,
actuation mechanisms
of multiple pathways are aligned side-by-side, and optionally extend along a
similar axial
extent. In some embodiments, no barrier (e.g. wall, shielding, drape, and the
like) exists
between actuation mechanisms of the multiple pathways, and the actuation
mechanism share a
similar space.
An aspect of some embodiments relates to a single-use robotic device for
manipulation
of elongate surgical tools. In some embodiments, the device is disposed
(optionally along with
the tools manipulated by it) following the surgical procedure. In some
embodiments, the single-
Date Recue/Date Received 2022-04-14
GAL676-1CA
27
use device does not need to be covered by a sterile drape or cover. In some
embodiments, no
additional mechanical components are needed to be operably connected to the
single-use
robotic device for driving and/or manipulating tools loaded within the device.
In some
embodiments, the device is provided packaged and pre-sterilized, optionally
with one or more
pre-loaded tools. Additionally or alternatively, tools are loaded onto the
device in the surgical
room.
In some embodiments, a tool that is loaded onto the device comes in direct
operable
contact with one or more tool-moving elements that manipulate it. In some
embodiments, one
or more tool-moving elements are in direct operable contact with the one or
more motors. In
some embodiments, the one or more motors and the one of more tool-moving
elements are
encased in a single housing, and the housing, together with its content, are
disposed when the
clinical procedure is completed.
In some embodiments, components of the robotic device such as the tool driving
assemblies, and optionally the robotic device as a whole, are disposed
following use along with
the tools which were manipulated by the device. A potential advantage of a
disposable device
may include that the tools manipulated by the device are allowed to come into
direct contact
and/or exist in a similar shared volume with device components, including
movement driving
components such as motors and/or transmission gears.
In some embodiments, no bordering element or barrier exists between the tool
and its moving
elements and/or driving motors within the housing. This is enabled, in some
embodiments, due
to that the device is disposed following use, therefore risk of contamination
which may occur,
for example, upon re-use, is avoided. Some potential advantages of a device in
which the loaded
tool may contact the device's tool-moving elements (and/or other device
components, e.g.
motors) directly may include simplifying use, potentially reducing loading
time, and potentially
improving the mechanical engagement with the tool (for example since no
"bordering"
elements are needed), thereby reducing or avoiding unwanted tool movements
such as slippage,
twisting, or kinking of the tool.
In some embodiments, no sterile barriers are required between the device
actuation
components and the tools manipulated by the device. In some cases, existence
of the tools and
device actuation components in a same shared volume may imply that during
operation, fluid
(e.g. blood, saline) contacted by and/or flowing within the tool may also come
in contact with
the device actuation components, yet a risk of contamination would be reduced
or prevented
Date Recue/Date Received 2022-04-14
GAL676-1CA
28
since the device is supplied in a sterile state and does not require cleaning
or re-sterilization
following its use.
In some embodiments, the device is constructed from durable, lightweight,
disposable
and optionally recyclable materials, such as plastic, aluminum, steel, copper,
and/or other
suitable metals.
An aspect of some embodiments relates to a dual-function assembly in which
both linear
movement and rotational movement (e.g. roll) of an elongate tool are carried
out at a same
physical location. In some embodiments, the assembly is configured for
linearly moving the
tool while the tool is being rolled; or vice versa- rolling the tool while the
tool is being moved
.. linearly.
In some embodiments, the assembly comprises an elongate shaft with a central
lumen
in which the tool is received. A set of wheels are positioned adjacent the
shaft and each of the
wheels at least partially extends into the central lumen to operably contact
the tool inside. In
some embodiments, a motor which drives rotation of the wheels is mounted
adjacent the wheels,
for example, under the shaft. In some embodiments, rotation of the wheels
pushes or retracts
the tool, depending on the direction of rotation. In some embodiments, the
motor which drives
rotation of the wheels is configured as a part of the assembly. Alternatively,
driving force is
transferred to the wheels via motor transmission.
In some embodiments, the inner walls of the shaft, which define the central
lumen, are
.. contoured to match an outer contour of at least some of the wheels. In such
construction, the
central lumen extends into a space in between the wheels, feeding the tool
into close contact
with the wheels. In an example, in a 4-wheel assembly, the inner walls of the
shaft may be
contoured to match at least one, two, three or all four of the wheels, at the
central lumen segment
which is closest to a contact point where the tool contacts the wheel(s).
In some embodiments, a gear that is co-axial with the shaft is connected along
the shaft
and/or at a proximal or distal end of the shaft, so that upon rotation of the
gear, the shaft and
wheel set are rotated by the gear as a single unit, thereby rolling the tool
(e.g. guidewire,
steerable microcatheter) that is within the central lumen of the shaft.
A potential advantage of an assembly which drives linear and rotational
movement of a
tool at a same physical location (such as a specific physical location within
the device housing
and/or a specific location of engagement with the tool) may include reducing
or avoiding
unwanted tool movement such as slippage, kinking, twisting which may occur for
example if
Date Recue/Date Received 2022-04-14
GAL676-1CA
29
two spaced apart mechanisms were to each drive linear movement and rotational
movement
respectively, and the tool would need to extend in between- where the unwanted
movement
may occur. Another potential advantage is the compact design enabled by
assigning two
functions, such as rotation and advancement/retraction of the tool, to the
same site.
An aspect of some embodiments relates to driving rotation (roll) of an
elongate tool, at
two spaced apart engagement locations along the length of the tool, using the
same motor. In
some embodiments, the tool is engaged by elements which rotate the tool at two
or more points
along the length of the tool, for example, at a proximal portion of the tool
(e.g. adjacent a handle
of the tool), and at a more distal portion. In an exemplary construction, a
first gear rotates a
to holder
which holds a proximal portion of the tool; rotation of the first gear then
rotates a second
gear which is a part of the linear movement assembly (such as described
herein), where the
second gear rotates a shaft in which a more distal portion of the tool is
received. In such
arrangement, actuation of a single motor drives rotation of both the first and
second gears,
generating rotation (roll) of the tool at both engagement locations.
A potential advantage of driving rotational movement at two spaced apart
engagement
locations along the length of the tool using a single motor may include
improved control over
the tool, for example as compared to use of two different motors for driving
rotation at the two
locations, where actuation timing and/or speed and/or direction of the two
motors would need
to be synchronized to ensure uniform roll of the tool along its length.
In some embodiments, one or more tools which are manipulated by the device are
engaged and manipulated only from their proximal portion (e.g. from a tool
handle); while one
or more additional tools are engaged at a more distal segment thereof (i.e.
not from the tool
handle).
An aspect of some embodiments relates to controlling a usable length of an
elongate
surgical tool by modifying a size of a curve of the tool outside of the
robotic device. In some
embodiments, a tool manipulated by the device extends in a curved manner
(bends) outside of
the housing one or more times. In some embodiments, when a length of a more
distal segment
(e.g. a tool segment extending between an exit aperture from the device
housing and a target
within the patient's body) changes, the curve is expanded or contracted in
size. In some
embodiments, a tool passes into and out from the device housing several times,
forming more
than one curve outside the housing. For example, a guidewire is curved twice-
once
independently, optionally between a proximal handle and a more distal portion,
and a second
Date Recue/Date Received 2022-04-14
GAL676-1CA
time while being received within a lumen of a curved microcatheter. In some
embodiments, the
curve is a "U" shaped curve, which can be modified, for example, by
lengthening or shortening
a distance of a maximal point of the "U" shape relative to the closest wall of
the device housing.
The invention, in accordance with some embodiments, relates to automated
devices for
5
inserting an elongate surgical medical tool into a bodily lumen, and more
specifically to body-
mountable automated devices for inserting elongate surgical medical tools,
such as guidewires
and microcatheters into blood vessels.
Many medical procedures, such as catheterization for diagnostic and/or
therapeutic
purposes, require insertion of a catheter into the patient's blood vessels and
other body lumens.
to
Typically, the physician first inserts a guidewire into an artery, such as the
femoral
artery, or a vein, and navigates it through the torturous vasculature until it
reaches the target,
which may be the heart, an artery, a peripheral blood vessel, the brain etc.
Once properly
positioned, the physician places a catheter over the guidewire, and pushes the
catheter until it
too reaches the target. In some cases, the procedure requires use of a small
radius catheter,
15
typically known as a microcatheter. In such cases, the physician may insert
the microcatheter
directly, without use of a guidewire. Manual insertion and navigation of
guidewires/microcatheters through the torturous vasculature is not only
challenging for the
physician, but it may also be hazardous to the patient, as even subtle
erroneous movements may
result in unintentional perforation of the blood vessel wall. Further, manual
procedures require
20 the
physician and additional medical personnel to be present at the procedure room
during the
entire procedure. Since most invasive procedures are done under imaging, such
as X-ray, CT,
etc., the medical personnel, as well as the patient, are exposed to radiation.
Remotely manipulated automated (robotic) devices have been developed in recent
years, however, existing robotic devices are cumbersome and expensive.
Therefore, there is a
25 need
for a small, inexpensive and easy to use automated device for inserting
guidewires and/or
microcatheters into bodily lumens, such as blood vessels, and navigating
therethrough to a
target region.
According to some embodiments, the insertion device may include a power
source. In
some embodiments, the power source may be a battery, a power supply, and the
like. In some
30
embodiments, the battery is disposable. In some embodiments, the battery is
reusable. In some
embodiments, the battery is rechargeable. In some embodiments, the power
supply may be
directly or indirectly connected to mains power. In some embodiments,
insertion device may
Date Recue/Date Received 2022-04-14
GAL676-1CA
31
include one or more printed circuit boards (PCBs), configured to
relay/process/convey
instructions and/or electrical connection between various components of the
device.
According to some embodiments, the insertion device may allow the linear
and/or
rotational advancement/movement of the medical instrument. In some
embodiments, the
insertion device may be configured to automatically advance the insertion
device and/or further
automatically allow the rotational movement thereof by rotating the insertion
device. In some
embodiments, when the medical tool is a guidewire, the insertion device may
allow controlling
the linear and/or rotation and/or tip parameters of the guidewire. In some
embodiments, when
the medical tool is a guidewire, the insertion device may allow automatically
and/or remotely
controlling the linear and/or rotation and/or tip parameters of the guidewire.
In some
embodiments, the medical instrument may be preloaded onto the medical device,
prior to being
used for a medical procedure. In some embodiments, the medical instrument may
be preloaded
onto the medical device, prior to being placed on the subject's body.
According to some embodiments, there is provided an insertion device
configured to
remotely and automatically linearly advance one or more medical tools (such as
a guidewire
and catheter) into and within bodily lumens, such as blood vessels, for
endovascular procedures,
including coronary, peripheral and cerebral endovascular procedures. In some
embodiments,
the insertion device is configured to further automatically and/or remotely
control/allow the
rotational movement of the one or more medical tools. In some embodiments, the
insertion
device is further configured to control parameters of the one or more medical
tools, such as, tip
stiffness. In some embodiments, the device is configured to control a force
applied by a distal
tip of the tool, for example by controlling one or more of: speed of
advancement of the tool, a
stiffness of the tool. Optionally, the tool is manipulated such that its
distal tip applies a constant
force or a varying force onto structures encountered by the tip (e.g. tissue
such as a vessel wall).
According to some embodiments, there is provided an insertion device
configured to
remotely and automatically linearly advance one or more medical tools (such as
a guidewire
and catheter) into and within bodily lumens, for various endoluminal
procedures. According to
some embodiments, when the first tool is a guide wire and the second medical
tool is a catheter,
the insertion device may allow the linear, rotational and/or tip parameters
control of the
guidewire, and the linear motion (over the guidewire) of the catheter, and
rotation motion
thereof (relative to the insertion device).
Date Recue/Date Received 2022-04-14
GAL676-1CA
32
According to some embodiments, the linear velocity of advancement of the
medical
instrument may be in the range of about 0-100 mm/sec or any subranges thereof.
In some
exemplary embodiments, the linear velocity of the medical instrument may be in
the range of
about 0-50 mm/sec, 1-100 mm/sec, 5-50 mm/sec or intermediate, higher or lower
velocity. The
velocity may be constant and/or in varying increments and may be adjusted
(manually and/or
automatically), during the procedure. In some embodiments, the velocity may be
in the range
of about 0-25 mm/sec with increments of about 0.1mm/sec. In some embodiments,
the velocity
may be in the range of about 25-50 mm/sec with increments of about lmm/sec. In
some
embodiments, the position holding stability at actuator is about 0.1mm.
According to some
embodiments, the rotational movement may be in anywhere in the range of 360
degrees.
According to some embodiments, the rotational movement may be continuous in
the
range of 360 degrees. In some embodiments, the number of full revolutions may
be limited. In
some embodiments, the number of full revolutions may be limited to about 5-10
revolutions in
each direction from the neutral (starting) setting.
According to some embodiments, the rotation position resolution may be in
increments
of 1-5, 0.5-10 degrees, 0.1-1 degrees or intermediate, higher or lower
resolution. In some
exemplary embodiments, the rotational position resolution may be about +/-
2degrees, +/- 1
degree, +/- 0.5 degrees or intermediate, higher or lower resolution.
According to some embodiments, the controller of the device may be a remote
controller. In some embodiments, the controller of the device may be
integrated with the device.
In some embodiments, the controller of the device may be connected by wired or
wireless
means. In some embodiments, the controller may be configured to allow control
over the
operation of the medical device. In some embodiments, the controller may be
configured to
allow control of advancement of the medical instrument, including, but not
limited to: linear
direction of advancement, velocity of advancement, increment of advancement,
rotational
movement, degree of rotational movement, and the like or any combination
thereof. In some
embodiments, the controller may include one or more operating buttons. In some
embodiments,
the buttons may include pressure buttons, slider buttons, joystick, and the
like, or any
combination thereof. In some embodiments the system may have means for
injecting a contrast
agent into the lumen, e.g., the vasculature. The injection mechanism may be
remotely operated,
so as to allow the surgeon/physician to perform the entire procedure from a
remote location. In
Date Recue/Date Received 2022-04-14
GAL676-1CA
33
some embodiments, the system may be configured to control linear and/or
rotational
movements of a guiding catheter, if used in the procedure.
As referred to herein, a "robotic device" or "device" may refer to the device
housing
inclusive of mechanical and/or electrical components accommodated inside the
housing. In
some embodiments, the "device" is not meant to cover add-ons or external
components such as
a guiding catheter driving unit (when coupled externally to the housing and
not integrated in
it), a mounting of the device, a remote control of the device, and the like.
As referred to herein, an "assembly" or "actuation assembly" may include tool-
moving
elements, such as wheels, and/or a coupling for the tool, such as an elongate
shaft in which the
tool is received. In some embodiments, an "assembly" or "actuation assembly"
further
comprises one or more motors and/or transmission (e.g. gears) which transfer
force from one
or more motors external to the assembly.
Before explaining at least one embodiment of the invention in detail, it is to
be
understood that the invention is not necessarily limited in its application to
the details of
construction and the arrangement of the components and/or methods set forth in
the following
description and/or illustrated in the drawings and/or the Examples. The
invention is capable of
other embodiments or of being practiced or carried out in various ways.
Before explaining at least one embodiment of the invention in detail, it is to
be
understood that the invention is not necessarily limited in its application to
the details set forth
in the following description or exemplified by the Examples. The invention is
capable of other
embodiments or of being practiced or carried out in various ways.
Reference is made to FIG. 1, which shows a schematic diagram of an exemplary
medical
system, according to some embodiments. As illustrated in FIG. 1, system 2
includes a body-
mountable miniature automated insertion device 4, configured to insert a
medical instrument,
such as a guidewire 6, into a subject's 8 lumen (such as, a blood vessel).
According to some
embodiments, depending on the location of the target tissue (for example, the
heart, a peripheral
blood vessel in the lower extremities, brain, liver, and the like) and the
purpose of the procedure,
the entry point may be selected from, but not limited to, at the patient's
groin (i.e., the femoral
artery), arm (i.e., the radial artery) or neck (i.e., the jugular vein).
Accordingly, the location of
the insertion device 4 on the patient's body may vary. In the example shown in
FIG. 1, the
device is attached to the patient's thigh, to allow access to the patient's
femoral artery. It can
be appreciated that the device may additionally or alternatively be attached
to the patient's arm,
Date Recue/Date Received 2022-04-14
GAL676-1CA
34
or any other desired location on the patient's body, depending on the selected
entry point.
According to some embodiments, the device may be attached/mounted/secured to
the patient's
body using any suitable attachment element. For example, the device may be
attached to the
patient's body using a band, which can be pulled over the patient's leg up to
his/her thigh. The
band may be flexible, such that it stretches according to the circumference of
the thigh, or it
may be substantially rigid or semi-flexible and include a length-adjusting
mechanism.
Alternatively, one or more straps may be wrapped around the patient's thigh
directly. Such
straps may be substantially rigid or semi-flexible, having a length-adjusting
mechanism, and
provided with connectors (e.g., buckles) at their opposite ends, for fastening
the straps and
securing them to the patient's thigh. The bands/straps may include one or more
sensors, such
as force sensor, disposed thereon.
According to some embodiments, the insertion device is not body mountable, but
configured for positioning in close proximity to the patient's body, e.g.,
using a robotic arm, a
base structure configured for securing to the patient's bed, etc.
In some embodiments, the insertion device may be disposable, either partially,
such that
some of its components are discarded and replaced between procedures, or
entirely, such that
the entire insertion device is disposed of once the procedure has been
completed, i.e., a single-
use device. In other embodiments, the insertion device may be reusable, such
that it can be used
repeatedly with new medical instruments (e.g., guidewires and/or catheters).
In some embodiments, the device may be configured such that it can be used to
insert
into bodily lumens a variety of different medical instruments, of varying
lengths and diameters,
including, for example, guidewire, catheter, microcatheters, and the like. In
some exemplary
embodiments, without limitation, the device may be adapted to insert into a
blood vessel, a
guidewire such as that disclosed in co-owned U.S. Patent 15 No. 9,586,029,
titled "Guidewire
Having Selectively Adjustable Stiffness and Tip Curvature", and/or in co-owned
U.S. Patent
Application Publication No. US 2018/214,675, titled "Double Concentric
Guidewire", both to
Shekalim et al.
According to some embodiments, the system may further include a controller 10
for
controlling the operation of the device, in particular, the insertion and/or
steering of the medical
instrument/s (such as, a guidewire and/or a catheter) toward the target (e.g.,
heart chamber,
blocked artery, etc.). The controller 10 may be coupled to the insertion
device 4 via a wired
connection or a wireless connection, and it may be either manually operated by
a physician (for
Date Recue/Date Received 2022-04-14
GAL676-1CA
example, the controller may be in a form of a joystick), or automatically
operated using a
dedicated software. In the latter case, the system may further comprise a
computer 12, which
may include at least one processor, user interface and a display. The computer
12 may be a
personal computer (PC), a laptop, a tablet, a smai _______________________
(phone or any other processor-based device.
5 In some embodiments, the controller 10 is disposable. In some
embodiments, the controller 10
is reusable. In some embodiments, the controller 10 is configured to
interact/couple to more
than one insertion device.
In some embodiments, the system 2 may further include an imaging device, or it
may
be used in conjunction with an imaging device. The utilized imaging modality
may be any one
10 of X-ray fluoroscopy, CT, cone beam CT, CT fluoroscopy, MRI, ultrasound,
or any other
suitable imaging modality. According to some embodiments, the insertion device
may be
capable of advancing the medical instrument linearly within the bodily lumen.
In some
embodiments, the device may further be capable of rotating the medical
instrument within the
lumen alternatively or in addition to linearly advancing the medical
instrument. In some
15 embodiments, the device may further be capable of rotating the medical
instrument within the
vessel separately and/or also simultaneously, while linearly advancing the
medical instrument.
For example, in some exemplary embodiments, the insertion device may be
capable of
advancing a guidewire and/or catheter linearly within the blood vessel. In
some embodiments,
the device may further be capable of rotating a guidewire and/or catheter
within the vessel
20 alternatively or in addition to linearly advancing the guidewire and/or
catheter. In some
embodiments, the device may further be capable of rotating a guidewire and/or
catheter within
the vessel separately and/or also simultaneously, while linearly advancing the
guidewire and/or
catheter. According to some embodiments, as further exemplified herein, the
insertion device
is configured to allow the linear advancement of the medical instrument within
the bodily lumen
25 .. along with the rotational movement thereof, by utilizing one or more
actuators that further
advantageously enable the smooth movement of the medical instrument, without
deforming the
medical instrument (i.e., without forming tension or twists along the length
of the medical
instrument). According to some embodiments, as further exemplified herein, the
linear and
rotational movements of the medical instrument (such as a guidewire and/or
microcatheter)
30 may be generated by separate actuators, or by one or more dual-purpose
actuators, configured
to allow both rotational and linear movement of the instrument/s.
Date Recue/Date Received 2022-04-14
GAL676-1CA
36
Reference is now made to FIGs 2A-2B, which illustrate schematic perspective
views
(front and rear, respectively), of an insertion device, according to some
embodiments. As shown
in FIG. 2A, insertion device includes elements for advancing a first medical
instrument (shown
as guidewire 22), in a linear direction and optionally in rotational direction
(as indicated by the
movement arrows). As illustrated in Fig. 2A, the proximal end of the guidewire
22 may be
secured to a dedicated holder 34, that may further allow control over tip
parameters of the
guidewire 22, as described below. The guidewire 22 advances from a first
opening 35 in the
holder (on the front face of the device 20), to enter the insertion device 20
via a second opening
36, and re-exit the device 20 from a different opening (first rear opening
(not shown)) at the
rear (back) face of the device 20. The guidewire 22 can then re-enter the
insertion device 20
through another opening (second rear opening (not shown)) at the rear face of
the device 20,
and can re-exit the insertion device 20 from a third (front) opening 37, such
that the distal end
24 of the guidewire 22 after exiting the third opening 37 can be configured to
be inserted into
a body of a subject, more particularly to a bodily lumen, such as a blood
vessel.
In some embodiments, as illustrated in FIG. 2A, the guidewire 22 exits the
insertion
device 20 into the lumen of a second medical instrument (shown as catheter
32), which can be
connected/attached/associated with the first rear opening, re-enter the
insertion device 20 via
the second rear opening and exit the front face of the insertion device 20 via
the third front
opening 37. In some embodiments, the second medical instrument is configured
to be inserted
into the bodily lumen. In some embodiments, the second medical instrument
(such as, catheter
32) may be inserted into the bodily lumen together with and/or following the
advancing of the
first medical instrument (e.g., guidewire 22) by the automated medical device
20. The above-
described winding path of the guidewire 22 and/or the catheter 32 enables a
compact spatial
arrangement (e.g., side by side) of the movement control units (described
below), thus
minimizing the device's overall size. In some embodiments, pathways (e.g.
shafts) through
which the tools extend inside the housing are aligned side by side, and are
optionally parallel
to each other. A lateral alignment in which the movement actuation mechanisms
are positioned
substantially side by side may provide for a smaller device size, such as a
thinner device width.
The small size of the device allows, in some embodiments, positioning of the
device
.. on the subject's body.
In some embodiments, the medical device 20 includes one or more
actuators/elements
configured to allow the linear and/or rotation movement/advancement of the
medical
Date Recue/Date Received 2022-04-14
GAL676-1CA
37
instrument/s. In some embodiments, as illustrated in FIG. 2A, device 20
includes a first
movement control unit 26 configured to allow linear and/or rotational movement
of the
guidewire 22. The first movement control unit 26 may include one or more
actuators/motors
allowing the movement of the guidewire 22, as further detailed herein below.
Device 20 may
further include a second movement control unit 28) configured to allow linear
and/or rotational
movement of the catheter 32. The second movement control unit 32 may include
one or more
actuators/motors allowing the movement of the catheter 32, as further detailed
herein below.
Optionally, device 20 may further include at least one additional movement
control
unit, for example, in instances in which the guidewire is comprised of a
hollow outer wire and
an inner wire disposed within a lumen of the outer wire, as disclosed, for
example, in
abovementioned U.S. Patent Application Publication No. US 2018/214,675. In
such instances,
an additional movement control unit 29 may be used to allow the controlling of
the movement
of the inner wire of the guidewire 22 relative to the outer wire of the
guidewire 22, to control
tip parameters of the guidewire 22, such as stiffness and/or curvature. The
movement of the
inner wire relative to the outer wire may be achieved by means of an
adjuster/slider 33 attached
to the inner wire, a non-rotating nut 30 and a lead screw 31 threaded therein.
Rotation of the
screw 31 by a motor/actuator causes linear movement of the nut 30 along the
length of the lead
screw 31, which in turn causes linear movement of the adjuster/slider 33 and
the inner wire
attached thereto. In some embodiments, the movement control unit 29 may allow
one or more
of the following relative states between the inner and outer wires of the
guidewire 22: 1) the
distal tip of the inner wire extends distally beyond the distal tip of the
outer wire, 2) the distal
tip of the inner wire is translated proximally such that it resides within the
outer wire (i.e., the
distal tip of the outer wire extends beyond the distal tip of the inner wire,
and/or 3) the distal
tips of the inner and outer wires are aligned. In some embodiments, rotation
of the guidewire
22 and the holder 34 to which it is attached, at its proximal end, may be
controlled by the
movement control unit 26. In some embodiments, in order to ensure that the
holder 34 smoothly
rotates together with guidewire 22, so as to prevent twisting/kinking of the
guidewire 22 (as the
guidewire 22 may not be able to rotate relative to the holder 34), the
movement control unit 29
may include an additional actuator/motor, e.g., coupled to the proximal end of
the holder 34, to
further control the rotation of the holder 34.
Reference is now made to FIG. 2B, which shows a perspective rear view of
insertion
device 20. As shown in FIG. 2B, insertion device 20, includes elements/units
for advancing a
Date Recue/Date Received 2022-04-14
GAL676-1CA
38
first medical instrument (shown as guidewire 22), in a linear direction and
optionally in
rotational direction (as indicated by the movement arrows). As illustrated in
FIG. 2B, the
proximal end of the guidewire 22 may be secured to a dedicated holder 34. The
guidewire 22
may advance from the first opening 35 in the holder 34 (on the front face of
the device), to enter
the insertion device 20 via a second opening (not shown in Fig. 2B), and re-
exit the device 20
from a first rear opening 38 at the rear (back) face of the device 20. The
guidewire 22 may then
re-enter the insertion device 20 through a second rear opening 39 at the rear
face of the device
20, and then re-exit the insertion device 20 from a third (front) opening (not
shown in Fig. 2B),
such that the distal end 24 of the guidewire 22, after exiting the third
opening, may be
configured to be inserted into a body of a subject, more particularly to a
bodily lumen, such as
a blood vessel.
In some embodiments, as illustrated in FIG. 2B, the guidewire 22 may exit the
insertion
device 20 from the first rear opening 38 into the lumen of a second another
medical instrument
(shown as catheter 32), which may be connected/attached/associate with the
first rear opening
38, re-enter the insertion device 20 via the second rear opening 39 and exit
the front face of the
insertion device 20 via the third front opening. In some embodiments, the
second medical
instrument 32 is configured to be inserted into the bodily lumen. In some
embodiments, the
second medical instrument (such as, catheter 32) may be inserted into the
bodily lumen together
with and/or following the advancing of the first medical instrument (e.g.,
guidewire 22) by the
automated medical device.
Reference is now made to FIGs. 3A-3B, which illustrate schematic perspective
top
views, of an insertion device, according to some embodiments. As shown in FIG.
3A, insertion
device 50 includes a casing 52 and a top cover 53, which is shown in an open
configuration.
Further shown is a holder 54 which holds the proximal end of the guidewire 58
and may further
allow, in some embodiments, to adjust the tip parameters of the guidewire 58.
In some
embodiments, the top cover 53 is intended to allow access 14 to the holder 54,
such that the
holder 54, with the guidewire 58 attached thereto, may be inserted into and/or
removed from
the casing 52. As shown in FIG. 3A, the guidewire 58 may advance from a first
front opening
55 in the holder 54, to enter the casing 52 via a second front opening 56, and
re-exit the casing
52 from a first rear opening (not shown)) at the rear face of the casing. The
guidewire 58 can
then re-enter the casing through a second rear opening (not shown) on the rear
face of the casing
52, and re-exit the casing 52 from a third front opening 57. In some
embodiments, as illustrated
Date Recue/Date Received 2022-04-14
GAL676-1CA
39
in FIG. 3A, the guidewire 58 exits the first rear opening of the casing 52,
while being threaded
within the lumen of another medical instrument (shown as catheter 62), which
may be
connected/attached/associated with the first rear opening, re-enter the casing
52 via the second
rear opening and exit the front face of the casing 52 via the third front
opening 57. In some
embodiments, the second medical instrument 62 is configured to be inserted
into the bodily
lumen. In some embodiments, the second medical instrument (such as, catheter
62) may be
inserted into the bodily lumen together with and/or following the advancing of
the first medical
instrument (e.g., guidewire 58) by the automated medical device, i.e. the
guidewire 58 may
serve as a rail on which the catheter 62 rides.
The above-described winding path of the guidewire 58 and/or the catheter 62
enables a
compact spatial arrangement of the movement control units of the device 50, as
described
below, thus minimizing the device's overall size. The small size of the device
allows, in some
embodiments, positioning of the device 50 on the subject's body. In some
embodiments, the
medical device includes one or more actuators/elements/units configured to
allow the linear
and/or rotation movement/advancement of the first and second medical
instruments.
Reference is made to FIG. 3B, which schematically illustrates the medical
device of
FIG. 3A, with the top cover 53 and a top portion of the casing 52 removed. As
illustrated in
Fig. 3B, the device 50 may include a first movement control unit 66 configured
to allow linear
and/or rotational movement of the guidewire 58. The device 50 may further
include a second
movement control unit 68, configured to allow linear and/or rotational
movement of the
catheter. The first movement control unit 66 and the second movement control
unit 68 may
include one or more: actuators/motors, gears, racks shafts, rotational screws,
allowing the
movement (linear and/or rotational) of the guidewire and/or catheter,
respectively, as further
detailed herein below. In some embodiments, the device 50 may include one or
more additional
movement control units. For example, in instances in which the guidewire is
secured at its
proximal end to a holder 54, the device 50 may further include a movement
control unit having
at least a motor/actuator and a gear 65 which control the rotation of the
holder 54 about its axis.
As shown in FIG. 3B, in instances wherein the guidewire 58 includes a hollow
outer
wire and an inner wire disposed within the lumen of the outer wire, the device
may include an
additional movement control unit comprised of a non-rotating nut 63 attached
to an
adjuster/slider 61 of the holder, which is rigidly attached to the proximal
end of the inner wire,
and a lead screw (not shown) threaded within the nut 63, to allow the
controlling of the
Date Recue/Date Received 2022-04-14
GAL676-1CA
movement of the inner wire relative to the outer wire, thus controlling the
tip parameters of the
guidewire (such as, adjusting the stiffness and/or curvature thereof).
Rotation of the lead screw
by a motor/actuator (not shown) causes linear movement of the nut 63 along the
length of the
lead screw, which in turn causes linear movement of the adjuster/slider 61 and
the inner wire
5 attached thereto.
In some embodiments, one or more of the following relative states between the
inner
and outer wires of the guidewire may be enabled by the above movement control
unit: 1) the
distal tip of the inner wire extending distally beyond the distal tip of the
outer wire, 2) the distal
tip of the inner wire being translated proximally so as to be disposed within
the outer wire (i.e.,
10 the distal tip of the outer wire extending beyond the distal tip of the
inner wire, and/or 3) the
distal tips of the inner and outer wires being aligned.
Reference is now made to FIGs. 4A-4B, which show perspective views of cross
sections
of the insertion device of FIGs. 3A-3B, according to some embodiments. Shown
in Fig. 4A, is
a longitudinal cross-section view of insertion device 50 (illustrated in FIGs.
3A-3B), cross
15 sectioned at a line between the first movement control unit (66 in FIG.
3B), and the second
movement control unit (68 in Fig. 3B). As shown in FIG. 4A, the first movement
control unit
66 includes at least one motor (shown as motor 75), a shaft 76, through which
the first medical
instrument (shown as guidewire 58) is moved. Also shown are gears (such as
exemplary gear
78). In addition, movement element 80 is indicated. As further elaborated
below, movement
20 element 80 includes at least two opposing round discs/wheels/rings
placed one above the other
and/or placed on adjacent the other and having a space therebetween, such that
the medical
instrument (shown as guidewire 58) is located in this space.
Further shown in FIG. 4A is the rear end opening 82, through which the
guidewire 58
can exit the device, for example into a catheter lumen, which is configured to
be connected to
25 the rear end opening. Reference is now made to Fig. 4B, which
illustrates a longitudinal cross
section of the first movement control unit 66.
As shown in FIG. 4B, the movement element 80 includes two opposing spinning
wheels/discs/rings (86A, 86B), placed one over the other and/or one adjacent
the other, with a
space therebetween. In the space formed between the wheels, guidewire 58 is
located, such that
30 upon spinning of the wheels (which is actuated, for example, by various
interconnected gears),
linear movement of the guidewire 58 within the shaft 76 towards the rear
opening 82 is
facilitated. By controlling the velocity of the spinning, the velocity of
advancement of the
Date Recue/Date Received 2022-04-14
GAL676-1CA
41
guidewire 58 may be controlled. In some embodiments, the movement control unit
66 and/or
the movement element 80 may be rotated along a longitudinal axis, thereby
further allowing
the rotational movement of the guidewire 58. In some embodiments, the wheels
may be similar
or different in size, shape, stiffness, material or composition.
As can be further observed in FIGs. 4A-B, in some embodiments, an aperture
through
which a tool passes into and/or out of the housing is structured to reduce
friction between the
tool and the walls of the housing. For example, aperture 81 (through which
guidewire 48 re-
enters the housing) defines a conically shaped protrusion ending with a
rounded lip. A potential
advantage of an aperture of the housing being formed with a rounded shape and
no sharp corners
may include reducing friction between the tool and the walls of the housing,
thereby potentially
reducing a risk of tear or wear of the tool (e.g. due to the tool rubbing
against the wall). This
may be especially advantage for devices such as described herein, in which the
tool extends
and curves outside of the housing, and may therefore be more prone to touching
the aperture
walls, for example as compared to tool which is held solely along a single
straight linear axis.
Reference is now made to FIG. 5, which illustrates a schematic perspective top
view of
movement control units of an exemplary insertion device, according to some
embodiments. As
shown in FIG. 5, insertion device 100, includes several movement control
units. A first
movement control unit 110 is configured to allow advancement of the guidewire
108, which is
inserted therethrough after having been re-inserted into the insertion device
(as detailed above).
A second movement control unit 120 is configured to allow advancing of a
second medical tool
(such as a catheter) after the guidewire 108 has re-entered the insertion
device, while being
threaded within the lumen of the second medical tool (catheter), via a second
rear opening,
towards the front face of the insertion device (via a corresponding front
opening), as detailed
above. A third, optional, movement control unit 102 is configured to allow
controlling the
rotation of the holder 104, in instances in which a holder 104 is used for
holding the proximal
end of the 30 guidewire 108, depending on the type of guidewire being used, so
as to prevent
twisting/kinking/tangling of the guidewire 108 while the guidewire is being
rotated.
As shown in FIG. 5, the first movement control unit 110 may include a
channel/shaft
113, through which the guidewire 108 is passed, and a movement element 114.
Further shown
are a motor 111) and one or more gears (representative gear 112 is shown),
which allow
controlling the operation of the movement control unit 110. The movement
element 114 may
include a rotating disc/ring/wheel 115, which is positioned so as to be in
contact with the
Date Recue/Date Received 2022-04-14
GAL676-1CA
42
guidewire 108, whereupon spinning/rotation thereof, the guidewire 108 may
linearly advance
along its route. The guidewire 108 may be pushed toward the rotating
disc/ring/wheel 115 by
means of a spring/screw preloaded pinion. In some embodiments, the guidewire
is pushed to
the rotating disc/ring/wheel 115 by means of a pair of spring/screw preloaded
pinions.
As shown in FIG. 5, facing the wheel 115 may be a groove, which forms a bent
in the
guidewire 108. The built-in bent in the guidewire's path increases the
perpendicular distance of
the line of action of force from the axis of rotation, which would be equal to
the radius of the
guidewire if the guidewire were to follow a linear path, thus enabling to
exert a sufficient
rotating moment (torque) on the thin guidewire, without having to apply a high
normal force
on the guidewire.
As further shown in FIG. 5, the channel/shaft 113 may have an opening/slit 116
along
its length, to allow access to the guidewire 108 and further to allow
placement/removal of the
guidewire 108, if need be. In some embodiments, the first movement control
unit 110 may
rotate about an axis (for example, by the control of actuators 118), thereby
allowing the
rotational movement of the guidewire 108 (and the holder 104). In instances
where rotational
movement is actuated, the opening 113 may accordingly face another direction.
As further shown in FIG. 5, the second movement control unit 120 includes at
least a
channel 123, through which the medical instrument(s) are passed, and a
movement element
124. Movement element 124 may include a rotating disc/ring/wheel 125, which is
in contact
with the medical instrument (e.g., the catheter with the guidewire threaded
therein) placed in
the channel 123, whereupon rotation thereof, the medical instrument can
advance along its
route.
As shown in FIG. 5, the channel 123 may have an opening/slit 126 along its
length, to
allow access to the medical instrument and further allow placement/removal of
the medical
instrument, if need be. In some embodiments, the second movement control unit
120 may be
configured to rotate about its axis, thereby allowing the rotational movement
of the second
medical instrument (e.g., the catheter).
As further shown in FIG. 5, the third, optional, movement control unit 102 may
include
at least one gear 130, allowing the rotation of the holder 104. In some
embodiments, in which
the guidewire 108 comprises a double concentric guidewire (i.e., an inner wire
disposed within
the lumen of an outer hollow wire), the device 100 may further include
actuators/element to
allow controlling of the relative movement between the inner and outer wires
of the guidewire,
Date Recue/Date Received 2022-04-14
GAL676-1CA
43
so as to control parameters of the tip of the guidewire (for example, the
stiffness and/or
curvature of the guidewire). in some embodiments, the device may include a non-
rotating nut
103 attached to an adjuster/slider of the holder 104, which is rigidly
attached to the proximal
end of the inner wire, and a lead screw 105 threaded within the nut 103, to
allow the controlling
of the movement of the inner wire relative to the outer wire, thus controlling
the tip parameters
of the guidewire (such as, adjusting the stiffness and/or curvature thereof).
Rotation of the lead
screw 105 causes linear movement of the nut 103 along the length of the lead
screw 105, which
in turn causes linear movement of the adjuster/slider and the inner wire
attached thereto.
In some embodiments, one or more of the following relative states between the
inner
and outer wires of the guidewire may be enabled by the above movement
mechanism: 1) the
distal tip of the inner wire extending distally beyond the distal tip of the
outer wire, 2) the distal
tip of the inner wire being translated proximally so as to be disposed within
the outer wire (i.e.,
the distal tip of the outer wire extending beyond the 20 distal tip of the
inner wire), and/or 3)
the distal tips of the inner and outer wires being aligned.
Reference is now made to FIG. 6A, which illustrates a schematic perspective
view of
an exemplary insertion device, according to some embodiments. As shown in FIG.
6A, the
insertion device 150 may include a housing (shown as semi-transparent housing
158) which
encases movement control units 156 configured for advancing a medical
instrument (such as a
guidewire 154), in a linear direction and, optionally, in rotational movement.
As illustrated in
FIG. 6A, the proximal end of the guidewire 154 may be secured to a dedicated
holder 152 that
may further allow control over tip parameters of the guidewire 154. The
guidewire 154 can
advance from the holder 152 to enter the insertion device via an opening, and
re-exit the device
from a different opening at the opposite face of the device. In some
embodiments, the guidewire
154 may exit the insertion device 150 into the lumen of another medical
instrument (such as a
catheter), which can be connected/attached/associated with an opening of the
device.
Reference is now made to FIG. 6B, which shows a perspective view of the
movement
control unit 156. As shown in FIG. 6B, the movement control unit 156 may
include a
shaft/channel 162, through which the medical tool (such as guidewire 154) can
pass/advance.
Movement control unit 156 further includes a medical instrument linear drive
(168) and
optionally a rotational drive (164). The movement control unit 156 may further
include a slip
ring 160 configured to allow rotational movement. The movement control unit
156 may further
include one or more rotating/spinning elements (such as wheels and gears),
configured to
Date Recue/Date Received 2022-04-14
GAL676-1CA
44
mediate mechanical movement of various moving parts, as detailed below.
Reference is now
made to FIG. 6C, which shows a side view of the movement control unit 156.
Shown in FIG.
6C is shaft 162, guide wire 154, rotational drive 164, as well as slip ring
160.
Reference is now made to FIG. 7, which shows a longitudinal cross section view
of the
linear drive 168 of the movement control unit shown in FIG. 6C, essentially
along the center of
shaft 162.
As shown in FIG. 7, the linear drive 168 may include at least two
rings/wheels/discs
(170A, 170B), placed/situated/located one above the other and having a limited
space
therebetween. The medical instrument (such as guide wire 154) is configured to
pass via the
to tight
space between wheels 170A and 170B, such that upon spinning/rotation of the
wheels, the
guidewire, which is at least partially in contact with both wheels, advances
linearly.
In some embodiments, the wheels/rings/discs 170A and 170B may be identical in
size,
shape, composition, or form. In some embodiments, the wheels/rings/discs 170A
and 170B may
be different in size, shape, composition, stiffness, material or form. In some
embodiments, the
space between the wheels 170A and 170B is formed in a groove, such that the
medical
instrument 154 is slightly bent, to allow better rotation of the medical
instrument. A potential
advantage of a built-in bent in the guidewire's path may include increasing
the perpendicular
distance of the line of action of force from the axis of rotation (which would
be equal to the
radius of the guidewire if the guidewire were to follow a linear path), thus
enabling to exert a
sufficient rotating moment (torque) on the thin guidewire, without having to
apply a high
normal force on the guidewire.
Reference is now made to FIG. 8, which schematically illustrates a movement
control
unit, according to some embodiments. As shown in Fig. 8, movement control unit
is configured
to allow linear advancement and/or rotational movement of a medical instrument
(such as a
guidewire 202). In some embodiments, the medical instrument 202 can advance
along a path,
for example, as defined by a channel or shaft (shown as channel 204). In order
to allow linear
movement of the medical instrument 202, the movement control unit may comprise
a linear
drive element 200, which may include two or more spinning/rotating elements,
shown in Fig.
8 as wheels/discs/rings 206A and 206B.
As shown in FIG. 8, the wheels may be placed side by side, forming a tight
space
therebetween. The medical instrument 202 me be threaded between the wheels,
such that it may
pass below a first wheel 206A and above a second wheel 206B, so as to form an
S shape, or
Date Recue/Date Received 2022-04-14
GAL676-1CA
substantially an S shape. By this manner, since the medical instrument 202 is
at least partially
in contact with the wheels, spinning/rotation of the wheels in opposite
directions causes the
instrument 202 to linearly advance. The relative spinning direction of the
wheels 206A and
206B can determine the direction of the linear movement of the medical
instrument 202.
5 In some
embodiments, the movement control unit may further include a rotational drive
element 210, which can allow rotation (for example, in direction 212) of the
linear drive element
200 and hence of the medical instrument 202 intertwined therein. By utilizing
the intertwining
of the medical instrument around the wheels in an S shaped path as detailed
above, the medical
instrument can rotate freely around its axis, without slippage and without
forming bents along
10 its
length. In some embodiments, the movement control unit is located/placed on a
platform
(shown as platform 214), to allow free rotation of the unit.
Reference is now made to FIGs. 9A-9B, which illustrate moving units for linear
advancement and/or rotational movement of the medical instrument, according to
some
embodiments. In some embodiments, as shown in FIG. 9A-9B, linear and/or
rotational
15
movement of the guidewire may be generated by means of piezoelectric
actuators. Piezoelectric
elements are composed of ceramic material which changes its geometric
dimensions as a
function of the applied voltage. Piezoelectric elements enable activation at
high frequencies,
e.g., 50-150kHz, and they can produce relatively large forces, which are
linearly correlated to
the degree of lengthening of the element (stroke). Using piezoelectric
actuators in an automated
20 medical
device is advantageous as their activation does not generate a magnetic field,
which is
undesirable in medical applications. Further, piezoelectric actuators are MRI
compatible. In
some embodiments, other actuator types may be used, for example,
electromagnetic actuators
(solenoid), DC motors, stepper motors or AC motors.
According to some embodiments, the insertion device may include two separate
25
portions/units; a first portion for generating linear movement (also referred
to hereinafter as
"linear portion") and a second portion for generating rotational movement
(also referred to
hereinafter as "rotational portion"), to allow each movement type, i.e.,
linear and rotational, to
be generated independently of the other. A combined movement, i.e.,
simultaneous rotation and
linear advancement, may be generated by activating the two portions in an
ordered or alternate
30 manner.
In some embodiments, the linear portion may be in the form of an inchworm
motor, and
it may comprise three piezoelectric actuators, as shown in FIG. 9A. Piezo
actuators 301 and
Date Recue/Date Received 2022-04-14
GAL676-1CA
46
303 are used to grip the medical instrument 304 (e.g., a guidewire), by
extending (lengthening)
and relaxing (shortening) along the vertical axis when powered, and motion is
achieved by
piezo actuator 302 lengthening and shortening along the horizontal axis when
powered. In some
embodiments, piezo actuator 301 and/or 303, may include a single actuator
which presses the
guidewire 304 against a static element, when extending, to grip the guidewire
304. In other
embodiments, piezo actuators 301 and/or 303 are de facto a pair of piezo
actuators, positioned
on opposite sides of the guidewire 304, such that both extend and relax to
grip and release,
respectively, the guidewire 304. The actuation process of the linear portion
is a cyclic process.
In order to move the instrument 304 from left to right, for example, piezo
actuator 303, which
is the forward clutch piezo in this example, is first extended so as to grip
the instrument, as
shown in FIG. 9A. Next, piezo actuator 302, the lateral piezo, is extended,
resulting in piezo
actuator 1003, together with the instrument, moving a small distance to the
right. It should be
noted that the center of piezo actuator 302 is fixated, such that when power
is supplied to piezo
actuator 302, its extension is symmetrical to both sides, left and right.
Since at this stage of the
process piezo actuator 301, which is the aft clutch piezo in this example, is
in a relaxed state,
and does not grip the instrument, the instrument, which is gripped by piezo
actuator 303, moves
to the right. Next, piezo actuator 301 is extended so as to grip the
instrument, followed by the
relaxation of piezo actuator 303, so as to release its grip of the instrument.
Next, piezo actuator
302 is relaxed. Next, piezo actuator 303 is extended to re-grip the
instrument, followed by the
relaxation of piezo actuator 301.
As shown in FIG. 9B, the rotational portion/moving unit of the device may
include a
pair of piezo actuators 306, 307, which contact the instrument 308 on opposite
sides, parallel
to one another. Extending the two piezo actuators in opposite directions 309A
and 309B causes
the instrument to rotate. In some embodiments, at least one of the clutch
piezo actuators/pairs,
i.e., piezo actuator 301 and/or piezo actuator 303, may be part of the
rotational portion of the
device, as well as of the linear portion of the device, as described above. In
other embodiments,
an additional pair of piezo actuators may be used for rotating the guidewire.
Reference is now made to FIG. 10, depicting a schematic illustration of an
exemplary
device capable of imparting both linear and rotational motion on a medical
tool, according to
some embodiments. In some embodiments, the linear motion may be achieved in an
inchworm
manner using piezo motors 401, 402 and 403, essentially as described above
with respect of
FIGs. 9A-9B, but with additional piezo motors 404 and 405 serving as clutches
which are
Date Recue/Date Received 2022-04-14
GAL676-1CA
47
moved toward and away from the medical tool (shown as guidewire 408) by piezo
motor 403.
In order to rotate the guidewire clockwise ("CW"), for example, piezo motor
403 is
relaxed/contracted, such that it moves piezo motors 404 and 405 toward the
guidewire 408,
until they grip the guidewire, at opposite sides. Piezo motor 405 is then
extended (moved
downward), while piezo motor 404 is simultaneously relaxed/contracted (moved
upward),
causing the guidewire to rotate. Piezo motor 401 is then extended, so as to
grip the guidewire,
and piezo motor 403 is extended, so as to release the grip of the guidewire by
moving piezo
motors 404 and 405 away from the guidewire, to their original position. In
alternative
embodiments, an additional piezo motor may be coupled to one of piezo motors
404 and 405,
instead of piezo motor 403, to move it toward and away from the guidewire. In
such
embodiments, rotation of the guidewire may be achieved by both piezo motors
404 and 405
extending (or contracting), in opposite directions. The utilized piezo
actuator/s may be, for
example, the PICMAO Monolithic Multilayer PZT Actuator, manufactured by PI
Ceramic
GmbH, Germany. In some embodiments, the rotating piezo actuators can rotate
the entire linear
advancement assembly.
Reference is now made to FIG. 11, which illustrates a movement control unit
having
two concentric circular components, which can rotate one relative to the
other, according to
some embodiments. As shown in FIG. 11, movement control unit 500 includes a
first movement
control element 502 (such as a piezoelectric motor) configured to allow linear
motion
(advancement) of a medical instrument (such as guidewire 510), in any linear
desired direction
505. The first movement control element 502 is fixed to the inner concentric
circular component
530 (see also FIG. 12). Movement control unit 500 further includes a second
movement control
element 504 configured to allow rotational motion of the first movement
control element 502
by rotating the inner concentric circular component, in any desired clockwise
or counter-
clockwise direction 507.
Further shown in FIG. 11 is an optional setting, in which the proximal end of
the medical
instrument is secured to a dedicated holder 520. In some embodiments, for
example when the
guidewire comprises a double concentric guidewire (i.e., an inner wire
disposed within the
lumen of an outer hollow wire), the holder may include a mechanism which
allows controlling
-- parameters of the medical instrument, such as tip stiffness, which includes
at least an
adjuster/slider 503 configured to linearly move the inner wire relative to the
outer wire. Further,
Date Recue/Date Received 2022-04-14
GAL676-1CA
48
an additional movement control unit 506 may be present, which allows control
over the rotation
of the holder 520 with the instrument attached thereto.
Reference is now made to FIG. 12, which illustrates an assembly of movement
control
units, for controlling movement of more than one medical instrument, according
to some
embodiments. As shown in FIG. 12, movement control assembly 600, includes two
separate
moving control units 602 and 604 that may be utilized in conjugation, such
that each unit is
configured to allow actuating and controlling movement of a different medical
instrument.
As shown in FIG. 12, a first movement control unit 602 includes various moving
elements, allowing linear motion (advancement) and/or rotational motion of a
first medical
instrument (such as guidewire 610), essentially as detailed above with respect
of FIG. 11. A
second movement control unit 604, includes various moving elements, allowing
linear motion
(advancement) and/or rotational motion of second medical instrument (such as
microcatheter
612). In some embodiments, the movement (linear and/or rotational) of the
first medical
instrument 610 may be independent of the movement (linear and/or rotational)
of the second
medical instrument 612.
In some embodiments, the movement (linear and/or rotational) of the first and
second
medical instrument may be synchronized. In some exemplary embodiments, as
illustrated in
FIG. 12, the first medical instrument (for example, a guidewire) may pass and
advance through
the lumen of the second medical instrument (for example, a catheter).
According to some
embodiments, any suitable actuator type may be used in any of the movement
control units,
devices and systems disclosed herein, including, but not limited to: motors
(such as, DC motors,
AC motors, stepper motors, and the like), electromagnetic actuators
(solenoid), piezoelectric
actuators, pneumatic actuators, hydraulic actuators, and the like.
FIG. 13 is a block diagram of a surgical robotic system, according to some
embodiments.
In some embodiments, a robotic system 1301 is suitable for use in a surgical
room.
Optionally, one or more system components (such as controlling components,
imaging
components) are physically separate from the rest of the system and may be
used remotely.
In some embodiments, system 1301 is configured to receive one or more surgical
tools
(e.g. a guidewire, a microcatheter, a guiding catheter, an intermediate
catheter, and/or other
elongate surgical tool) and to actuate movement of the tools.
Date Recue/Date Received 2022-04-14
GAL676-1CA
49
In some embodiments, the system is configured to drive linear movement (e.g.
advancement and/or retraction) of a tool received therein, and/or drive
rotational movement
(e.g. axial rotation) of a tool received therein. In some embodiments, linear
and rotational
movements are actuated simultaneously.
In some embodiments, system 1301 includes a robotic device 1303 for driving
movement of one or more tools. In some embodiments, the device housing
accommodates
and/or is operably connected to one or more of the following components:
= one or more actuators such as one or more motors 1305, and optionally
associated
transmission of the motors.
= Tool moving elements 1317, such as wheels, configured to operably contact a
tool
received by the system to move the tool (e.g. advance, retract, rotate the
tool). In
some embodiments, the tool moving elements are driven directly (e.g. by
contacting) or indirectly (e.g. via one or more gears or other transmission)
by the
motors 1305. Optionally, only some tool moving elements are driven (directly
or
indirectly) by motors, while other tool moving elements move in response to
movement of the tool and/or in response movement of a motor-driven tool moving
element.
= a controller 1307, configured to receive and/or send operation signals to
and/or from
a general control unit 1309. General control unit 1309 may be configured as a
remote
control device, a console, a control unit physically attached to the system
base, or a
combination thereof. In some embodiments, the controller 1307 is configured to
coordinate manipulation (e.g. linear movement, rotation) of tools received and
operated by the robotic system.
= powering means 1311, including for example a battery and/or connection
means
for mains electricity.
= sensing means 1315, for example, one or more sensors configured for
detecting, for
example, whether a tool has been inserted; a relative position of the tool; a
position
of tool-moving elements (e.g. wheels); actual movement of the tool-moving
elements (e.g. by a counter counting the number of wheel rotations); sensors
for
communicating with other system sensors, and/or for other measurements and/or
indications. In some embodiments, sensors are configured for detecting motor
Date Recue/Date Received 2022-04-14
GAL676-1CA
status, for example, a motor position, a motor rotation rate. Sensors of
various types
may be used, such as optical sensors, pressure sensors, force measurement
sensors,
speed sensors, sensor for detecting electrical current, flow sensors, position
sensors
(e.g. optical, magnetic, electrical position sensors).
5 = a
memory 1313, which stores, for example, parameters related to tool movement,
such as speed of movement, rotation, translation, angulation, deflection
angle;
indications obtained by one or more system sensors, such as a measure of force
acting on the tool, stiffness of the tool; parameters related to the patient
body and
sensed by the inserted tools (e.g. heart rate, blood pressure, temperature,
10 oxygenation level, and/or other sensed parameters).
In some embodiments, the robotic device (also referred to herein as an
insertion device)
is compact and is small enough in dimensions so as to reduce interference to
surgical room
personnel (e.g. nurse, surgeon) and/or to surgical room equipment and/or to
the patient. In some
embodiments, the device footprint is smaller than 500 cm^2, 250 cm^2, 180 cm^2
or
15
intermediate, larger or smaller area. In some embodiments, a volume of the
device is less than
3500 cm^3, 2800 cm^3, 2000 cm^3 or intermediate, larger or smaller volume. In
some
embodiments, a weight of the device is less than 1.5 kg, less than 1 Kg, less
than 800 grams,
less than 500 grams or intermediate, higher or lower weight.
In some embodiments, the robotic device is substantially block shaped, for
example
20 having
a box shaped compact configuration. Other configurations may include a
cylindrical
configuration, a rounded (e.g. ball shaped) configuration, a saddle shape,
and/or other.
In some embodiments, system 1301 includes an integrated imaging modality 1319.
Alternatively, the system is configured to be operably attached to (for
example, communicate
with) an existing imaging modality. An imaging modality may include, for
example, X-ray
25
fluoroscopy, CT, cone beam CT, CT fluoroscopy, MRI, ultrasound, or any other
suitable
imaging modality.
In some embodiments, system 1301 comprises a mounting 1321 for placing device
303
relative to the patient and/or relative to the surgical bed. In some
embodiments, the mounting
comprises or is configured to attach to an adjustable fixture. Optionally, a
height and/or angle
30 and/or
distance of the system relative to the patient (e.g. relative to the location
of body entry)
and/or relative to the bed are adjustable.
Date Recue/Date Received 2022-04-14
GAL676-1CA
51
In some embodiments, system 1301 comprises or is configured to engage an
adaptor
1323 for operably engaging a tool's proximal portion, for example, a handle.
In some embodiments, the adaptor defines a mechanical engagement between the
one
or more motors 1305 and one or more components of the handle which move the
tool. For
example, the adaptor connects one or more motor(s) or associated transmission
with a slider
component of the handle which deflects the tool tip upon sliding; with a knob
component of the
handle which rolls the tool when rotated; and/or with other handle components.
Additionally or
alternatively, the adaptor itself includes one or more integrated motors for
driving movement
of the handle components.
FIG. 14 is a flowchart of a general method of using a surgical robotic device,
according
to some embodiments.
In some embodiments, a decision is made, for example by a physician, surgeon
and/or
other clinical personnel, to operate (1401). In some embodiments, the
operation is for
therapeutic purposes. Additionally or alternatively, the operation is for
diagnostic purposes.
In some embodiments, the operation involves catheterization. In some
embodiments,
the operation involves insertion of one or more tools into and/or through
vasculature and/or into
other non-vascular endoluminal structures. Examples of tools may include: a
guide wire, a
microcatheter, a rapid exchange catheter, a guiding catheter, a balloon
catheter, a stent or coil,
ablation tools, an intermediate catheter, a suction catheter, an ultrasound
catheter, a pressure
catheter and/or other tools. In some embodiments, the operation is a through-
lumen based
procedure. In some embodiments, the operation is an over-the-wire based
procedure.
In some embodiments, the device is positioned relative to the patient (1403).
In some
embodiments, the device is mounted onto the surgical bed, for example via a
fixation. In some
embodiments, the device is attached to the patient, for example mounted onto
the patient's leg
(e.g. to the thigh), to the patient's arm, and/or to other body parts.
Attachment of the device to
the surgical bed and/or to the patient may be carried out using straps, bands,
a rigid mounting,
and/or other attachment means.
In some embodiments, attachment to the bed is carried out using a stand which
is
stabilized relative to mattress and/or to the rail of the bed and/or to the
floor. The system can
then be mounted on the stand, for example attached via a snap fit mechanism,
magnetic means,
straps (e.g. Velcro), and/or other. In some embodiments, the stand is
adjustable so as to enable
use with patients of various sizes and/or different bed height and the like.
In some embodiments,
Date Recue/Date Received 2022-04-14
GAL676-1CA
52
when setting a position of the device, one or more of a height, entry angle to
the body, alignment
of the device relative to the patient are selected. The device position may be
defined with respect
to the patient body or parts thereof (e.g. relative to the surgical entry
point) and/or relative to
the surgical bed and/or relative to other surgical room equipment, e.g.
relative to imaging
modules.
A potential advantage of attaching the device to the patient's body, for
example to a
limb and/or other body portion (e.g. leg, arm (optionally the snuffbox of the
hand), neck, foot,
etc.) may include that the device can be positioned closer to the entry
opening into the body. In
such configuration, a length of a tool segment extending between the device
and the body may
to be reduced, potentially allowing for more efficient use of a tool's
length. In some embodiments,
the device is compact enough so as to fit on top of a patient's limb, for
example, without
protruding laterally from the limb when the device is attached onto the limb
(for example, the
device is sized not to extend laterally from a patient's thigh).
In some embodiments, loading of the tools is performed (1405). In some
embodiments,
loading of tools is performed after the device position (e.g. relative to the
patient and/or to the
bed) is set; alternatively, loading of tools is performed before the device
position is set.
Optionally, one or more tools are preloaded onto the device, and are
optionally provided along
with the device. In an example, the device is provided in a sterilized package
while already
being loaded with one or more tools. Additionally or alternatively, tools are
unwrapped in the
surgical room and are loaded onto the device, for example by a nurse,
technician and/or other
clinical personnel. In some embodiments, tools are loaded and/or replaced
during operation, for
example when switching from a navigational tool (e.g. a guidewire) to a
treating tool, such as
an embolization tool, a catheter balloon, and/or other treating tool.
In some embodiments, the device is constructed so that no shielding (e.g. no
physical
separation by a wall, a wrap, a drape) exists or is required between the tool-
moving element
and the tool being loaded, for example, such that direct contact is formed
between the tool and
the tool-moving elements (e.g. wheels, gears, and/or other actuators).
Optionally, no draping
by a sterile drape or other cover is required. For example, in a single-use
device that is disposed
following surgery, due to having no permanent components, there is no need to
cover the device
and/or specific components of it which contact the tools by a sterile drape. A
potential
advantage of a device in which the device is configured to engage the surgical
tools directly,
Date Recue/Date Received 2022-04-14
GAL676-1CA
53
without a separation or cover may include a simpler, more efficient, time
and/or cost effective
preparation process and/or cleaning process following surgery.
Alternatively, in some embodiments, the device (and/or selected components of
the
device, such as the tool moving elements) are at least partially covered by a
sterile drape or
sheath.
In some embodiments, operation is performed by controlling, via a user
interface of the
device, movement of the surgical tools received within the units (1407).
Exemplary
manipulation of tools controlled by the device may include: linear advancement
and/or
retraction of a tool; rotation of a tool (e.g. roll about the tool axis);
twisting of a tool; angular
orientation of a tool (e.g. by curving a distal tip of a tool); articulation
(e.g. of a distal tip of a
tool); changing of mechanical properties of a tool, such as stiffness, for
example by controlling,
from a proximal end of the tool, a distal tip structure or inner arrangement.
In some embodiments, manipulation of tools is performed remotely. Optionally,
the
surgeon operates the system from a different room. Alternatively, the surgeon
stays in the
surgical room, and may operate the system while being adjacent or far from the
bed.
In some embodiments, manipulation of tools involves maneuvering of tools that
are
attached to each other and/or inserted into one another and/or otherwise
assembled in a manner
in which movement of one tool may affect the other, for example, when a
guidewire extends
within a lumen of a microcatheter. In such situation, controlling movement may
involve
carrying out (via user control and/or automatically by the system, upon
identification of
movement) "compensation" movements of the guidewire and/or microcatheter with
respect to
each other, which may be required when both are driven together in an
assembled configuration
(such as when the guidewire is within the microcatheter lumen in the position
of the tool moving
elements of the unit, where the tool is manipulated). In an example, when the
microcatheter is
advanced or retracted, it may be desired to hold the guidewire in place
without having the
guidewire move along with the microcatheter. This may be carried out, for
example, by driving
the linear movement mechanisms of both tools, but in opposite directions (e.g.
advancing the
microcatheter distally while at the same time driving the guidewire mechanism
in a manner that
would retract the guidewire proximally). A potential advantage of synchronized
controlled
movement of tools that are used together (such as a guidewire extending within
a lumen of a
microcatheter) may include the ability to hold one tool while advancing the
other tool, for
Date Recue/Date Received 2022-04-14
GAL676-1CA
54
example by driving the actuation mechanisms of the tools in opposite
directions- one tool would
be advanced or retracted while the other tool would effectively remain in
place.
In some embodiments, the user interface is configured on the device itself
(e.g. as a
screen and/or buttons and/or a joystick attached to the system units and/or to
the base), and/or
.. on a separate physician console, and/or on a separate remote control
device. Control signals
may be communicated via wired and/or wireless communication (e.g. network
based
communication) to the device.
In some embodiments, the device (e.g. the device controller) is programmed to
include
a loading mode, for insertion and/or calibration of tools and/or of the device
motors; and an
operational mode, where movement of the tools is carried out.
In some embodiments, the device or specific components of it are disposed
following
operation (1409). Optionally, the device is disposed as a whole, optionally
including the tools
loaded on it.
FIG. 15 is a flowchart of a method of loading a plurality of surgical tools
onto the
surgical robotic device, according to some embodiments.
In some embodiments, a robotic device for example as described herein is
provided
(1501). In some embodiments, one or more elongate surgical tools such as a
guidewire, a
microcatheter, a guiding catheter, a rapid exchange catheter and/or other
surgical tools are
provided (1503).
In some embodiments, a proximal handle of a tool such as a guidewire is placed
in
engagement with a designated adaptor or holder (1505), for example as
described in co-filed
PCT titled "ROBOTIC MANIPULATION OF A SURGICAL TOOL HANDLE" (PCT Patent
Application No. PCT/IL2020/051225).
In some embodiments, the guidewire is threaded (such as from the distal end
direction)
into a designated shaft of the guidewire driving mechanism of the robotic
device (1507). Then,
at least a portion of the guidewire length which exits the shaft (existing the
device housing) is
threaded into a lumen of a microcatheter (1509).
In some embodiments, a proximal end of the microcatheter (which has not yet
been
physically attached to the device) is secured to the device at an exit port of
the guidewire from
the housing (1511). Then, at least a portion of the microcatheter length
(including the guidewire
received inside) is threaded into a designated shaft of the microcatheter
driving mechanism of
Date Recue/Date Received 2022-04-14
GAL676-1CA
the device (1513). The microcatheter is then passed (along with the guidewire
received inside)
through a lumen of a guiding catheter (1515).
Optionally, the guiding catheter is received or engaged by a guiding catheter
driving
mechanism, which may be externally operably coupled to the device housing or,
alternatively,
5 integrated inside the device.
Then, in some embodiments, the one or more tools are introduced into the
patient's
body (1517) and are manipulated using the device.
In an exemplary use, the robotic device is loaded with a guidewire and
optionally a
microcatheter. Optionally, a guiding catheter (a distal portion thereof) is
manually inserted into
10 the patient's body. Then, the robotic device is placed adjacent a
proximal end of the guiding
catheter, and the guiding catheter (optionally along with a microcatheter in
which the guidewire
is received) is inserted into the lumen of the guiding catheter. In some
embodiments, insertion
into the guiding catheter lumen is performed via a seal element, which may be
an integrated
part of the robotic device or, alternatively, separate from it. Then, in some
embodiments, the
15 user connects the proximal end of the guiding catheter to the robotic
device. From this point
on, manipulation (e.g. linear advancement/retraction, and/or rotation) of the
guidewire and/or
of the microcatheter inside the lumen of the guiding catheter and optionally
upon the guidewire
and/or microcatheter exiting the guiding catheter (such as into a lumen of a
vessel) may be
carried out robotically using the device (for example, through a remote
control interface). In
20 some embodiments, linear advancement and/or retraction of the guiding
catheter, for example
to a certain limited extent, is also carried out using the robotic device.
FIGs. 16A-D are various configurations of a remote control device of the
surgical
robotic system, according to some embodiments.
In some embodiments, the remote control device is shaped to be manually held
by a
25 user, e.g. a physician. Optionally, the remote control device is
lightweight and small enough to
be held by the user without blocking the user's view of visual aids such as a
screen showing the
results of imaging during operation. In some embodiments, the remote control
device includes
one or more portions shaped to be gripped by the user palm and/or engaged by
the user's fingers.
In some embodiments, the remote control device communicates with the modular
30 robotic system. In some embodiments, the communication is wireless,
performed for example
via wi-fl, infrared, Bluetooth, RF, and/or other wireless modules.
Date Recue/Date Received 2022-04-14
GAL676-1CA
56
In some embodiments, the remote control device includes or is in communication
with
a controller of the modular robotic system. In some embodiments, maneuvering
of tools
received by the system is performed via the remote control device. Examples of
tool movements
and/or other operational manipulations of the tools which are controlled by
the remote control
device may include: linear advancement and/or retraction of a tool; axial
rotation of a tool;
control of a tool distal tip; speed of movement; control of unique tool
functions (e.g.
inflation/deflation of a balloon in a balloon catheter, stent deployment
and/or advancement),
and/or other tool manipulation.
Other functions which may be controlled via the remote control device include,
for
example: automated injection of materials (e.g. contrast agents, washing
solutions) into and
through a tool lumen; linear and/or angular movement of the assembled system
as a whole (e.g.
sliding of the assembled system relative to a mounting); safety stop of the
system; on/off
actuation of the system; supply of electrical power to the system or to
specific components;
and/or other system functions.
FIGs. 16A-B show a first example of a remote control device 1601, and FIGs.
16C-D
show a second example of a remote control device 1603. In some embodiments,
the device
includes interfaces in the form of one or more of: push buttons 1605, joystick
handles 1607,
manual sliders 1609, rotating knobs, 1611 and the like.
In some embodiments, the remote control device comprises a screen, such as for
notifying a user regarding current controls and/or for receiving commands from
the user.
In some embodiments, the remote control device includes an interface (e.g. a
button)
for rapid retraction of tools. Such interface may be used in case of an
emergency, device failure,
or the like and/or for planned retraction of a tool, such as for replacing the
tool with a new tool.
In some embodiments, the remote control device is modular. Optionally,
specific
buttons and/or add-on interfaces are selectively attached (and/or are
uncovered to enable their
use). For example, buttons for controlling movement of a guiding catheter
(when a guiding
catheter receiving unit has been attached on the system) are exposed for use
only when required
(e.g. are positioned under a removable or movable cover). In another example,
an interface for
controlling injection of materials through one or more system junctions is
attached to the remote
control device and/or uncovered for use upon need.
The remote control device may be operated at a distance from the system.
Optionally,
the remote control device is operated by a surgeon located in a different
room. Optionally, the
Date Recue/Date Received 2022-04-14
GAL676-1CA
57
remote control device is operated by a surgeon located in the surgery room
(adjacent the bed or
at a distance from the bed).
In some embodiments, the remote control may be configured as a screen
interface, for
example for use in a cell phone, tablet, computer or the like, such as
described below.
FIG. 17 is a schematic example of a screen interface associated with the
surgical robotic
system, according to some embodiments.
In some embodiments, additionally or alternatively to a remote control device
for
example as described hereinabove, a screen interface 1701 in communication
with the system
may be used. In some embodiments, the screen interface is configured for
receiving data (such
as from the device and/or from imaging means and/or from the physician and/or
from a hospital
system), presenting data, sending and/or receiving commands to and from the
robotic device,
and/or other.
In some embodiments, the screen interface may be configured in a computer, a
laptop,
a tablet, as a cell phone application and/or other.
The user interface screen shown in this figure presents examples of functions
and/or
indications related to operation of the tools by the robotic device,
including, but not limited to:
tool movement type (e.g. guidewire roll, guidewire advancement/retraction,
microcatheter
advancement/retraction, guiding catheter roll, guiding catheter
advancement/retraction);
guidewire tip control (e.g. guidewire tip deflection); tool speed and/or
movement direction
(e.g. increasing the speed using "turbo" mode, initiating fast or partially
fast retraction);
emergency stop (in case of device failure, medical emergency situation or the
like; in some
embodiments, the emergency stop button stops power supply to the robotic
device); controlling
movement of two (or more) tools together; customized control of tool movement,
such as:
control of accessories including devices and/or add-on accessories used with
the system and/or
tools, such as: injection of material through a port; inflation of a balloon;
stent expansion; tip
curvature, tool stiffness.
FIGs. 18A-B are different views of a robotic device, according to some
embodiments.
In some embodiments, a robotic device 1801 is shaped and sized to be located
adjacent
the patient (e.g. attached to the bed) and/or located on the patient, for
example on a patient's
limb (e.g. on the patient's thigh). In the example shown, device 1801
comprises a compact
housing 1802 having a saddle shaped bottom portion 1803. Optionally, the
saddle shaped
portion is shaped and sized to be seated on a patient's limb, on a rail of the
bed, on a designated
Date Recue/Date Received 2022-04-14
GAL676-1CA
58
mounting (e.g. a mounting having a planar bottom for positioning on a planar
surface, not
shown), and/or other. In some embodiments, a second portion 1805 of the
housing extends
from the saddle bottom, the second portion accommodating the one or more tool
driving
mechanisms.
In some embodiments, a guidewire is loaded onto the device 1801 as follows: in
some
embodiments, a proximal portion (e.g. a handle) of the guidewire is received
within an
accessible compai ________________________________________________________
intent 1807, optionally covered by a lid 1809 (compartment 1807 may also
be referred to herein as an "adaptor" or "holder"). Optionally, manipulation
of one or more
guidewire handle components is performed within compai ___________________
intent 1807 by one or more movers
which engage the handle (e.g. engage a slide of the handle, a rotational knob
of the handle,
and/or other handle components).
In some embodiments, a more distal portion of the guidewire (adjacent the
handle) exits
compai ___________________________________________________________________
intent 1807 via aperture 1813. Then, in some embodiments, an even more distal
portion
of the guidewire (optionally, the distal most end of the guidewire) is then
inserted through an
entry aperture 1811 into the device housing, where the inserted guidewire is
received within a
designated shaft (not shown) of its driving mechanism. In some embodiments,
the guidewire
exits the housing again, optionally from an opposite wall of the housing, via
aperture 1815. In
some embodiments, a location of aperture 1815 also serves as a securing point
for a proximal
end of a microcatheter. Optionally, the microcatheter is threaded on a knob
1817 and/or other
suitable protrusion to be secured to the housing. When the guidewire exits
through aperture
1815, it is received within a lumen of the microcatheter.
In some embodiments, the microcatheter (along with the guidewire extending
inside)
is curved (e.g. to a "U" shape) outside the housing to be inserted, via an
aperture 1819, into a
designated shaft of the microcatheter driving mechanism. The microcatheter
(along with the
guidewire inside) then exits the housing on an opposite wall, via aperture
1820.
In some embodiments, the device housing is shaped and sized solely for
accommodating the tool driving mechanisms, without being affected by tool size
considerations, such as tool length, a tool width (e.g. diameter). Optionally,
the housing
protects the tool driving mechanism inside while only the tools themselves
remain visible
and/or contactable externally to the housing. Optionally, no driving
mechanisms are visible. A
potential advantage of such construction may include reducing a risk of damage
(e.g. by
unwanted contact) with the tool driving mechanisms.
Date Recue/Date Received 2022-04-14
GAL676-1CA
59
In some embodiments, a portion of a tool that extends within the housing
itself is less than
25%, less than 20%, less than 10%, less than 5% or intermediate, larger or
smaller percentage
of the total length of the tool. A potential advantage of a housing which
accommodates the
driving mechanisms and does not require a long portion of a tool to be
received inside may
include allowing for a relatively compact housing having small dimensions
and/or small
weight.
In some embodiments, the housing includes a removable or movable portion such
as a lid.
Optionally, the lid is opened in case of an emergency and/or robot
malfunction, for example to
manually release the tools. Alternatively, the lid is opened in case of a need
to replace the tools.
In some embodiments, opening of the lid automatically returns the device
motors to an
initial (home) position and/or orientation. Optionally, the actuation
mechanisms of the tools,
for example a designated shaft in which a tool is received, are rotated to be
aligned such that a
slot extending along the shaft faces upwards, in the direction of the open
lid. A potential
advantage of the motors and/or tool shafts being automatically aligned upon
opening of the lid
of the device housing may include that tools can be more easily approached for
adjustment
and/or removal of a tool from its mechanism.
Exemplary dimensions of upper portion 1805 of the device (without the saddle
shaped
bottom, which could alternatively be formed as a planar surface) may include:
an axial length
1821 of less than 12 cm, a width 1823 of less than 7 cm, a height 1825 of less
than 9 cm.
In some embodiments, housing 1802 is formed of a relatively lightweight yet
durable
material, such as plastic, aluminum, composite materials. Optionally, the
material is recyclable,
so that a disposed device (e.g. a single use device) may be at least partially
recycled.
FIGs. 19A-B schematically illustrates a surgical robotic device including or
attached to
a guiding catheter driving unit, according to some embodiments.
FIGs. 19A-B show robotic devices having different shaped housings. FIG. 19A
shows
a robotic device housing 1900 such as described above in FIGs. 18A-B, seated
on a mounting
1921 which defines a planar surface. FIG. 19B shows a substantially box shaped
housing 1902,
having a square or rectangular cross section profile.
In some embodiments, a guiding catheter driving mechanism 1901 is configured
as a
separate add-on unit configured to operably couple to the robotic device, for
example, to be
appended to device housing 1903.
Date Recue/Date Received 2022-04-14
GAL676-1CA
In some embodiments, the guiding catheter driving unit attaches to the housing
in a
manner in which a microcatheter existing the housing (such as via an aperture
1905) enters a
lumen of a guiding catheter loaded onto the guiding catheter unit. In some
embodiments,
attachment of the guiding catheter unit to the housing is by one or more of:
an interference fit
5 coupling (e.g. via respective protrusions and indentations of the device
housing and a housing
of the guiding catheter driving unit), a sliding attachment (e.g. including a
rail 1906, for
example as shown in figure 19A).
In some embodiments, rail 1906 moveably couples the guiding catheter driving
unit
1901 to one or more motors located inside the housing of the device, for
example so that a
10 motor drives back and forth movement of the unit for moving the guiding
catheter. In some
embodiments, the guiding catheter driving mechanism is configured to drive
linear movement
and/or rotational movement (i.e. roll) of the guiding catheter. In some
embodiments, the
guiding catheter driving unit is configured to electrically connect to the
robotic device and
receive power supply from it. Alternatively, the guiding catheter driving unit
includes an
15 independent power supply (e.g. a battery).
In some embodiments, the guiding catheter driving unit connects to the robotic
device
via mechanical connections, such as by a snap fit connection, an interference
fit connection,
pin and socket, and/or other suitable mechanical coupling.
Alternatively, in some embodiments, the guiding catheter driving mechanism is
inside
20 the robotic device housing, and forms an integral part of the robotic
device.
In some embodiments, the guiding catheter driving mechanism is configured for
driving linear movement of the guiding catheter within a selected distance
range, for example,
to advance and/or retract the catheter a distance of 3 cm, 5 cm, 10 cm, or
intermediate, longer
or shorter distance. In some embodiments, this provides for fine tuning of a
position of a
25 guiding catheter previously inserted into the patient.
In some embodiments, to ensure that a microcatheter within the guiding
catheter moves
along with the guiding catheter, the microcatheter driving mechanism is
controlled for
compensating for that movement, for example, the microcatheter is actuated to
move in an
opposite direction to the guiding catheter. Optionally, a guidewire within the
microcatheter
30 moves along with the microcatheter as a single unit and does not require
independent actuation.
Date Recue/Date Received 2022-04-14
GAL676-1CA
61
FIGs. 20A-C are an example of an isolated mechanism of the guiding catheter
driving
unit, an example of a guiding catheter driving unit housing, and a guiding
catheter driving unit
assembled onto the robotic surgical system, according to some embodiments.
In some embodiments, a guiding catheter mechanism (see FIG. 20A) includes one
or
more motors, such as a motor 2001 for driving linear movement, and a motor
2003 for driving
rotation. In some embodiments, a proximal portion of the guiding catheter 2005
is attached at
connector 2009. In some embodiments, in operation, motor 2001 rotates a lead
screw 2007
which in turn advances or retracts connector 2009, thereby advancing or
retracing the guiding
catheter 2005. In some embodiments, motor 2003 moves linearly along with
connector 2009.
In some embodiments, activation of motor 2003 rotates connector 2009, thereby
rotating (rolling) the guiding catheter 2005.
FIG. 20B is an external view of a guiding catheter unit 2000. In some
embodiments,
the unit comprises an elongate housing 2011, and the lead screw 2007 (such as
shown in FIG.
20A) extends throughout the housing. In some embodiments, housing comprises
one or more
ports leading into the guiding catheter lumen. For example, an injection port
2010 through
which materials (e.g. liquid agents, saline, etc.) can be injected into and
through the lumen of
the guiding catheter.
In some embodiments, housing 2011 is shaped for attaching to the robotic
device. In
an example, the housing defines an abutment 2012 which can lean against the
external housing
.. of the robotic device and/or at least partially connect to it, such as by
being received within a
respective recess or indentation defined at the robotic device housing.
FIG. 20C shows the guiding catheter unit 2000 connected to a robotic device
2013. In
some embodiments, the guiding catheter unit is coupled to an external wall of
the device
housing 2015. Optionally, the guiding catheter unit extends distally in the
direction of insertion
into the patient.
As further shown in this example: a guidewire 2019 extends from a guidewire
holder
2021 and into a designated shaft of the guidewire driving mechanism; the
guidewire then exits
the housing at 2025, which also serves as a securing point for the
microcatheter 2027, and the
guidewire enters the microcatheter lumen. Then, the microcatheter is bended to
enter the device
housing at 2029, being received within a designated shaft of the microcatheter
driving
mechanism. When the microcatheter (along with the guidewire received within)
exits the
Date Recue/Date Received 2022-04-14
GAL676-1CA
62
housing, it is received within a lumen of the guiding catheter 2005 held and
manipulated by
the guiding catheter unit 2000.
FIGs. 21A-C show mechanisms for actuating rotation (roll) and/or linear
movement of
a tool actuated by the robotic surgical system, according to some embodiments.
In some embodiments, as shown in the exemplary mechanism of FIG. 21A, a
guidewire
2101 inserted into a designated shaft is engaged by at least one pair of
driving wheels 2103
positioned opposite each other and contacting the guidewire that passes
between them. A motor
2105 for driving linear movement of the tool actuates rotation of the wheels
which, depending
on the rotation direction, cause the guidewire to move axially in a proximal
or distal direction.
In some embodiments, a motor 2107 is configured to drive rotation of a first
gear 2109
which in turn interferes with a second gear 2111 (positioned adjacent or on
top of gear 2109),
causing second gear 2111 to rotate. In some embodiments, rotation of second
gear 2111
produces rotation of the assembly which includes the driving wheels 2103 and
the linear motor
2105, rotating the assembly (along with the guidewire held therein) in its
entirety.
In some embodiments, when gear 2109 is rotated, it rotates a holder 2121 of
the
guidewire, rotating (rolling) the guidewire. Therefore, in some embodiments,
rotation (roll) of
the guidewire is carried out at two locations along the guidewire: a first
location at holder 2121,
and a second location at the assembly which includes the driving wheels and
linear motor-
which is rotated, along with the guidewire, as a whole. A potential advantage
of rolling the
guidewire at two locations along the guidewire, where optionally one location
is proximal to
the curve and the other is distal to the curve, may include reducing twisting
of the guidewire
during roll, for example by synchronized actuation of rotation at both
locations, optionally by
executing the roll movement of both sites by a single motor.
A potential advantage of driving rotation of the guidewire at two locations
along the
guidewire length using the same single motor (e.g. via motor 2107 which moves
gear 2109)
may include improved control over roll of the guidewire, for example as
compared to driving
rotation at the two (or more) locations using different motors, which may
require
synchronization between the motors direction and/or speed and/or actuation
timing. Another
potential advantage of using a same single motor for driving rotation at two
different guidewire
length locations may include providing for a more compact, smaller device
housing.
Additionally or alternatively, rotation of gear 2109 does not rotate the
guidewire
directly (such as by not rotating holder 2121), and actuates rotation of the
guidewire which
Date Recue/Date Received 2022-04-14
GAL676-1CA
63
starts only from the point of the assembly that is rotated by gear 2111 (as
gear 2111 is rotated
by gear 2109).
In some embodiments, one or more slip rings are used for supplying electrical
current
to the motors regardless of a current orientation (e.g. rotational
orientation) of the assembly.
For example, a slip ring constructed of a reel 2117 and base 2119 is located
at the attachment
of the second gear 2111 to the driving wheels and linear motor assembly. In
some
embodiments, the slip ring maintains an electrical coupling to the linear
motor so that the linear
motor may be actuated regardless of the rotational orientation of the
assembly.
In another exemplary construction, shown in FIG. 21B, a motor which drives
rotation
and/or a gear 2113 which transmits rotation from the motor may directly
interface with the
assembly of the driving wheels and/or motor 2105 which drives linear movement
and/or with
a shaft in which the tool is received. In an example, gear 2113 is positioned
along a similar
long axis as the assembly.
In some embodiments, gear 2113 is formed with a slot 2123 through which the
guidewire passes. Optionally, slot 2123 forms a direct extension of a slot
2125 in a designated
shaft 2127 in which the guidewire is received. In some embodiments, the slot
extends along a
5 degrees, 10 degrees, 20 degrees arc of the gear circumference. A potential
advantage of a slot
through the gear may include that removal of the guidewire from the actuation
mechanism may
be facilitated.
FIG. 21C is a cross section view showing an actuation assembly comprising a
shaft
2127 and wheels 2103 which drive linear movement of the guidewire. In some
embodiments,
shaft 2127 defines an elongate inner lumen 2129 in which guidewire is
received, the lumen
being in communication with slot 2125. In some embodiments, inner walls of
shaft 2127 are
constructed to match a contour of the wheels (see for example curvature 2128)
so that a
guidewire within lumen 2129 is guided right into (and then out from) a path in-
between the
wheels. In some embodiments, lumen 2129 extends in close proximity to the
wheels outer
contour, to bring the guidewire directly in-between the wheels.
In some embodiments, wheels 2103 are arranged (lie) on a plane that is
substantially
perpendicular to a plane defined by slot 2125.
Alternatively, the wheels may be arranged on a plane parallel to a plane
defined by slot 2125.
A potential advantage of shaft constructed to match a contour of the wheels
may include
improved control of the guidewire as it is fed into (and exits out from) the
path in between the
Date Recue/Date Received 2022-04-14
GAL676-1CA
64
driving wheels. Another potential advantage may include reducing a risk of
slippage and/or
other movement of the guidewire out of its designated path.
A potential advantage of an assembly which includes wheels for driving linear
movement of the guidewire and which is configured to rotate, as a whole, to
generate roll of
the guidewire may include that linear movement can be performed during roll
movement (or
vice versa). Another potential advantage of a dual-movement assembly where
linear movement
and rotation are actuated at the same physical location (inside the robotic
device) may include
reducing slippage or other undesired guidewire movement which may occur, for
example, if
two spaced-apart mechanisms were each to drive linear movement and roll
movement, and the
guidewire would need to extend between them. In spaced apart mechanisms where
one
mechanism actuates rotation and another spaced-apart mechanism actuates linear
movement,
rotation of the guidewire may cause slippage of the guidewire between the
rotation mechanism
and the linear movement mechanism (or vice versa- linear movement of the
guidewire may
cause it to slip from the rotation mechanism). Another disadvantage of
separate spaced apart
mechanisms is the possible induction of friction at the idle site (i.e. the
applying of friction
onto a tool segment at a mechanism which is currently not in use), which may,
require some
type of release mechanism that would disengage one mechanism while the other
is operated.
FIG. 22 shows an exemplary arrangement of mechanisms driving movement of a
guidewire, according to some embodiments.
In some embodiments, guidewire rotation is carried out by more than one
mechanism.
Optionally, two or more mechanisms which engage the guidewire are configured
to cause
rotation (roll) of the guidewire. In such situation, the two mechanisms are
controlled in a
synchronized manner, for example to ensure that the guidewire is not twisted
or kinked.
In some embodiments, a guidewire proximal portion or handle is held within an
adaptor
or holder 2201, suitable to generate rotation of the guidewire by either
rotating to rotate the
handle as a whole, and/or by actuating a handle component, such as a rotatable
knob (not
shown), which generates rotation (roll) of the guidewire (optionally, roll of
a distal tip of the
guidewire). The guidewire extends from the holder 2201 along an axis 2203
about which it
rotates, until exiting the housing. When the guidewire renters the housing, it
may be passed
through a second mechanism suitable to actuate rotation (and in this example-
linear movement
as well). The second mechanism, for example a mechanism as shown in FIG. 21B,
may be
configured to actuate rotation (roll) of the guidewire about an axis 2205
along which the
Date Recue/Date Received 2022-04-14
GAL676-1CA
guidewire extends. Optionally, axis 2205 is parallel to axis 2203, defining
parallel paths along
which tool actuation takes place. Alternatively, paths of the tools defined
along axes 2205 and
2203 are not parallel, for example, angling in or angling out relative to each
other.
In some embodiments, the mechanisms extend to a similar height and/or axial
length,
5 so that they can be fitted within a compact housing.
FIGs. 23A-B are a schematic diagram and a flowchart pertaining to controlling
a length
and/or position of a tool by adjusting a curved portion of the tool, according
to some
embodiments.
10 In some embodiments, as schematically illustrated in FIG. 23A, a tool
2301
manipulated by the robotic device 2302 is engaged at two or more locations
2303, 2305 that
are spaced apart from each other (along the length of the device), such that a
segment 2307 of
the tool extending in between the two locations can be adjusted (lengthened or
shortened). In
some embodiments, locations 2303, 2305 define attachment points of tool 2301
to the robotic
15 device housing 2302, while segment 2307 extends externally to the device
(i.e. externally to
the device housing).
In some embodiments, locations 2303, 2305 are arranged relative to each other
in a
manner that causes a bend or curvature of the segment 2307, for example, into
a "U" shape
curvature as shown. In an example, locations 2303 and 2305 are aligned side-by-
side.
20 Alternatively, locations 2303 and 2305 are not aligned side by side.
In some embodiments, for controlling a length of the tool, the curve (e.g. the
"U" shape)
is changed in size (e.g. expanded or contracted), changing a maximal distance
2309 between a
peak of the curve and the housing of device 2302.
In some embodiments, an extent of the curve (e.g. as defined by a radius of
curvature
25 2310) is set by linear movement of the tool (e.g. an extent in which the
tool is advanced or
retracted) and/or by manual loading of the tool, where a certain segment of
the tool length is
loaded into the system. In some embodiments, the extent of the curve depends
on a total length
of the tool.
In some embodiments, a distance 2312 between the attachment points of the tool
to the
30 housing is a function of the radius of curvature 2310 of the tool.
Optionally, distance 2312 is
twice the minimal radius of curvature to which the tool can be bent.
Date Recue/Date Received 2022-04-14
GAL676-1CA
66
In some embodiments, dimensions of housing 2302 such as an extent of a wall of
the
housing in which the entry and exit apertures for the tool are formed is sized
in accordance
with the radius of curvature of the tool, for example being at least twice a
minimal radius of
curvature of the tool, but no more than 5 times, 6 times, 8 times, 10 times or
intermediate,
.. larger or smaller times the minimal radius of curvature of the tool
intended for manipulation
by the device.
In some embodiments, a maximal dimension of the housing (such as a width of
the
housing or a height of the housing) is between 5-10 cm, 8-20 cm, 12-40 cm or
intermediate,
longer or shorter.
In some embodiments, the device includes more than two engagement locations
with
the tool, allowing for a plurality of curves (e.g. "U" curves) to be formed in
between the
locations.
A potential advantage of a device that defines tool engagement locations such
that a
tool segment extending in between the locations is adjustable in length may
include improving
control over a length of the tool being manipulated. Optionally, a length of a
most distal tool
segment, such as a segment extending between the last exit from the robotic
device housing
and a target point inside the patient's body, is controlled, thereby
potentially allowing fine
control of a tool distal tip position. In some embodiments, advancing the tool
towards the target
point inside the body reduces the size of the curve of the tool outside the
housing, and vice
versa: retracting the tool back from the target point increases the size of
the curve.
Another potential advantage of a device that defines tool engagement locations
such
that a tool segment extending in between the locations is adjustable in length
may include the
ability to receive and manipulate tools of various lengths.
Another potential advantage of a device that defines tool engagement locations
such
that a tool segment extending in between the locations is adjustable in length
may include that
the curved segment extends externally to the device housing, potentially
allowing for a device
of relatively small dimensions (e.g. axial length) which is substantially not
affected by the tool
length, enabling a compact housing of small dimensions.
The flowchart of FIG. 23B is an example of the mechanism described by the
diagram
of FIG. 23A. In some embodiments, a tool proximal end is secured to the
robotic device (2321).
For example, a handle of the tool is received by and/or attached to a
designated adaptor or
holder of the device. This attachment may be referred to as a first engagement
location, for
Date Recue/Date Received 2022-04-14
GAL676-1CA
67
example as described above. In some embodiments, a more distal portion of the
tool is threaded
or inserted into the robotic device (2323). For example, a more distal portion
of the tool is
threaded into a designated shaft of the manipulating mechanism (e.g. a
guidewire is inserted to
be engaged by the tool-moving wheels). This second attachment may be referred
to as the
second engagement location, for example as described above.
Then, optionally, a tool segment extending in between the securing location of
the tool
proximal end and the engagement location of the tool (e.g. by the tool-moving
wheels) is
adjusted in length (2325).
FIG. 24 shows a system configuration defining an arrangement of tools in which
a tool
length can be adjusted, according to some embodiments.
In the example shown, a robotic device 2401 including and/or being coupled to
a
guiding catheter unit 2403 is configured to receive and drive movement of: a
guidewire 2405,
a microcatheter 2407, and a guiding catheter 2409. In some embodiments, as
shown in this
example, two "U" shaped curves 2411 and 2413 are defined by tools passed
through the
system: curve 2411 of the guidewire alone, and curve 2413 of the guidewire as
it extends inside
the lumen of the curved microcatheter. In some embodiments, a change in a size
of curve 2413
results in joint movement of the microcatheter and guidewire at segments that
are distal to the
curve. In some embodiments, movement of the microcatheter (advancement or
retraction)
changes the size of curve 2413.
As can be observed in FIG. 24, the device housing 2402 (i.e. the walls of the
housing)
define the following apertures through which the tools pass into and/or out
from of the inner
device space defined by the housing: in some embodiments, a proximal end
portion of
guidewire 2405 is anchored at a holder 2404 to the device; the guidewire then
enters the
housing at an aperture 2406 and exits via an aperture 2408, where aperture
2408 is optionally
located at an opposing wall of the housing to a wall in which aperture 2406 is
defined. In some
embodiments, a proximal portion of the microcatheter 2407 is anchored to the
device at a
holder 2410, where also the guidewire is received within the microcatheter
lumen. Then, in
some embodiments, the microcatheter enters the housing at an aperture 2412 and
exits the
housing at an aperture 2414, which is optionally configured at an opposite
wall of the housing
to aperture 2412.
FIG. 25 schematically illustrates tool-movement driving mechanisms of the
system,
according to some embodiments.
Date Recue/Date Received 2022-04-14
GAL676-1CA
68
In some embodiments, as shown in this example, tool-movement mechanisms are
arranged parallel to each other, for example, aligned side-by-side. A
potential advantage of the
tool moving mechanisms being parallel to each other (and optionally aligned
along a similar
axial extent) may include that a tool extending throughout the mechanisms can
be adjustably
bent, thus providing for variable tool length. A potential advantage of the
tool moving
mechanisms being parallel to each other (and optionally aligned along the a
similar axial
extent) may include that the device housing which accommodates these
mechanisms can be
maintained at relatively small, compact dimensions which are not determined by
the tool actual
length.
The tool-movement mechanisms shown herein include a mechanism 2501 for holding
and optionally rotating a guidewire 2502 (see for example the description of
FIG. 21A); a
mechanism 2503 for actuating linear translation of the guidewire, including
for example a set
of wheels 2505; and a mechanism 2507 for actuating linear translation of a
microcatheter 2508,
including for example a set of wheels 2509.
In some embodiments, guidewire rotation may be carried out at one or both of
mechanisms 2501, 2503, optionally under synchronization (such as a by a device
controller).
FIGs. 26A-B are examples of a device configuration including elastic elements
(e.g.
springs) for selectively engaging tools received by the system, according to
some
embodiments.
In some embodiments, elastic elements (e.g. springs, bands) are positioned and
configured to move (e.g. push) the driving wheels towards a tool received
within the device,
bringing the wheels into close contact with the tool. Additionally or
alternatively, elastic
elements are positioned and configured to move (e.g. push, center) a tool
received within the
device into operable contact with the driving wheels.
In the example shown, a spring 2601 is mounted onto a lever 2603 holding the
driving
wheels 2605 so that upon exertion of force onto the spring, the lever moves
the wheels into
contact with the tool. In some embodiments, force is exerted onto the spring
by closure or
movement of a portion of the housing, such as closure of a lid. In some
embodiment, the spring
is configured to retract the lever to move the wheels away from the tool, for
example to allow
for removal of the tool. Optionally, the spring is pulled on when the lid (or
other portion of the
housing) is opened or otherwise moved, thereby moving the wheels away from the
tool.
Date Recue/Date Received 2022-04-14
GAL676-1CA
69
In some embodiments, the spring is pre-configured to exert a force selected
for a
specific tool or tool size (e.g. tool diameter), for example, to position the
wheels in contact
with a tool of a certain thickness.
FIG. 27 is a schematic block diagram of a robotic device configured for
manipulating
two or more elongate surgical tools, according to some embodiments.
In some embodiments, walls of a housing 2701 of the robotic device define an
inner
volume 2703 in which at least two distinct pathways such as 2705, 2707 for the
elongate
surgical tools are defined. In some embodiments, the pathways extend across
the inner volume,
.. for example, between two opposing walls of the housing, such as wall 2709
and wall 2711.
Optionally, the housing is shaped in an elongated form, for example having a
substantially
rectangular cross section profile, and the pathways extend along the length of
the housing.
In some embodiments, each of the pathways extends between an entry aperture
formed
at the wall of the housing, and an exit aperture formed at an opposite wall of
the housing. In
the example shown, pathway 2705 extends between entry aperture 2713 formed at
wall 2709
and an exit aperture 2715 formed at wall 2711; and pathway 2707 extends
between an entry
aperture 2717 formed at wall 2711 and an exit aperture 2719 formed at wall
2709.
In some embodiments, an aperture formed in a wall of the housing is shaped
and/or
sized according to the surgical tool that is passed through it. For example, a
rounded (e.g.
circular) aperture is sized for fitting a cylindrical tool, such as a
guidewire or microcatheter,
where the aperture diameter is optionally no more than 5%, 10%, 25% or
intermediate, higher
or smaller percentage larger than a diameter of the tool. In some embodiments,
an aperture is
sized for more than one tool to be passed through. Optionally, the aperture
profile is oval (e.g.
ellipsoid), rectangular, slot shaped and/or other. In some embodiments, a
single elongated slot
serves as an aperture for both inner pathways.
In some embodiments, a single tool passes through an entry aperture into the
inner
volume of the housing, and exits the housing through a respective exit
aperture. Alternatively
or additionally, in some embodiments, a plurality of tools telescopically
arranged (e.g. 2 tools,
such as a guidewire provided within the inner lumen of a microcatheter) pass
together through
the same entry aperture and exit the housing together through a respective
exit aperture. Thus,
in such an example, a first tool passes through a first inner pathway, exits
the housing into the
lumen of a second tool, and the telescopic assembly of both tools passes
through a second inner
Date Recue/Date Received 2022-04-14
GAL676-1CA
pathway. In some embodiments, the telescopic arrangement of the tools occurs
outside of the
housing, after both tools have passed through their inner pathways, for
example, in the case of
a rapid exchange catheter which can be interfaced with the guidewire after
each of the
guidewire and the rapid exchange catheter have passed independently through
their respective
5 actuation assemblies located in the inner pathways.
In some embodiments, the pathways extend in a similar plane, for example, a
similar
horizontal plane, a similar vertical plane, a similar plane extending
diagonally between the
walls of the housing. In some embodiments, the pathways extend along parallel
axes. A
distance 2721 between the parallel axes may range, for example, between 3-12
cm, 2-10 cm,
to 5-9 cm or intermediate, longer or shorter distance.
Alternatively, in some embodiments, the pathways are not parallel, for
example, one
pathway extends directly between opposite walls while another takes a diagonal
or other
indirect route.
In some embodiments, except for the aperture locations, the housing is sealed.
15 .. Optionally, the housing includes a removable or moveable cover or lid.
In some embodiments,
the housing is open at least in part, for example, shaped as a box with no top
face.
In some embodiments, all components which engage the tool to manipulate it
and/or to
drive its movement are fully encased inside the inner volume of the housing
and at least some
of these components are positioned along the pathway defined for the tool. In
some
20 embodiments, these components include an actuation assembly, for example
the tool-moving
elements described in FIGs. 21B-C.
In some embodiments, as shown, a plurality of motors 2722, 2723 is configured
to drive
the actuation assemblies, for example configured to drive tool-moving elements
2725 (e.g.
wheels) of each assembly. In some embodiments, the motor and the tool moving
elements are
25 positioned along the pathway defined for the tool. In some embodiments,
the actuation
assemblies of the two (or more) pathways are aligned side-by-side. A potential
advantage of
the actuation assemblies being aligned side-by side may include allowing for a
short or minimal
distance 2728 (optionally being the device width or height) between opposing
walls 2733,
2735. In an example, distance 2728 is smaller than 15 cm, 12 cm, 10 cm or
intermediate, longer
30 or shorter distance.
In some embodiments, the actuation assemblies of the two or more pathways have
a
similar axial extent (or do not extend beyond a certain axial extent). A
potential advantage of
Date Recue/Date Received 2022-04-14
GAL676-1CA
71
the actuation assemblies being positioned relative to each other and/or sized
such that they do
not extend beyond a certain axial extent may include that a distance 2730
between walls 2709
and 2711 (optionally being the device length) may be kept to a minimal axial
extent needed to
contain the movement driving components. In an example, distance 2730 is
smaller than 10
cm, 7 cm, 12 cm or intermediate, longer or shorter distance. In some
embodiments, the plurality
of motors 2722, 2723 are also positioned within the axial extent of the
actuation assemblies,
and in proximity to the actuation assemblies, to facilitate the compact design
of the device. The
ability to position the motor(s) in close proximity to the actuation
assemblies and potentially
in contact with at least a portion of the actuation assemblies is provided,
for example, due to
that no barriers (e.g. sterile protection or shield) are needed between the
actuation assembly,
the motor(s), and the surgical tool being manipulated.
In some embodiments, the actuation assemblies of the two or more pathways are
positioned within the same, shared inner volume defined by the walls of the
housing. In some
embodiments, no barriers (e.g. inner walls, shields, drapes, and the like)
exist between the
movement driving components of the two or more pathways. In some embodiments,
no barriers
(e.g. inner walls, shields, drapes, and the like) exist between the actuation
assemblies and the
tools that are being manipulated by them.
Alternatively, in some embodiments, a partial partition or barrier are
provided. For
example, the device housing may include an inner wall or protrusion which do
not fully block
the inner volume, leaving at least some regions of the pathways in
communication with each
other.
In some embodiments, an actuation assembly of an inner pathway (e.g. an
actuation
assembly that includes a shaft in which a tool is received and/or wheels which
drive linear
movement of the tool) is exposed to an actuation assembly of a different inner
pathway, for
example an adjacent pathway.
In some embodiments, actuation assemblies of a plurality of pathways are
arranged and
held with respect to each other on a chassis. Optionally, the chassis is
exposed and open to its
surroundings, for example, no housing is provided.
In some embodiments, an actuation assembly of a pathway at least partially
restrict
movement of the tool within the inner pathway, for example, restricting
lateral movement of a
tool received within the pathway. For example, movement of the tool out of
notional limits
defined by the elongate pathway is restricted. In some embodiments, the tool
is channeled
Date Recue/Date Received 2022-04-14
GAL676-1CA
72
through the pathway, for example, received within a slot of an elongate shaft
(such as the shaft
of an actuation assembly, e.g. shaft 2127, FIG. 21B). Alternatively or
additionally, the pathway
is defined by a path generated between a plurality of pairs of opposing
wheels.
In some embodiments, in addition to extending through the pathway, a tool
engages the
device at one or more additional fixation locations (also referred to herein
as "securing points",
"engagement points"). In some embodiments, a fixation location comprises a
holder (such as
2727, 2729) located outside the housing, inside the housing, or partially
inside the housing and
partially outside the housing. In some embodiments, a fixation location
couples a tool to the
housing and/or to one or more other tools. For example, at fixation location
2729 a first
elongate surgical tool 2731 which extends through pathway 2705 (e.g. a
guidewire) enters an
inner lumen of a second elongate surgical tool 2733 (e.g. a microcatheter),
which is coupled to
the housing at fixation location 2729. In some embodiments, a proximal end of
tool 2731 is
coupled to the housing at fixation location 2727.
In some embodiments, fixation location 2727 is shaped and configured to
accommodate
a proximal handle of tool 2731, for example, a handle that manipulates the
distal portion of the
tool in terms of bend and/or stiffness. In some embodiments, an additional
motor (not shown)
is configured for rotating tool 2731 through two locations, one of which is
the handle of the
tool (for example at fixation location 2727) and the other is a region more
distal of the tool.
For example, a motor configured for rotating tool 2731 by rotating an
actuation assembly which
is associated with a portion of the tool 2731, is also operably connected to
the handle of the
tool, optionally through a gear system. As such, the motor is configured for
rotating the tool
simultaneously from these two distinct locations. An advantage for commencing
roll
movement by the same motor in two different locations along the tool may
include enhancing
the torque applied on the tool and eliminating the risk of slippage of the
tool in its gripping
locations found in the actuation assembly.
In some embodiments, a fixation location of a tool with the housing (such as
2727) and
an entry aperture leading the tool into the inner volume (such as 2713) are
located on a same
wall of the housing, so that a section of the tool that is found outside the
housing forms a curve,
for example, a U-shaped curve. In some embodiments, for example as described
in FIGs. 23A-
B, the extent of the U-curve is dynamically adjustable. Optionally, linearly
moving the tool
(such as via the tool-moving elements, e.g. wheels) changes the extent of the
U-curve relative
to the external side of the wall of the housing.
Date Recue/Date Received 2022-04-14
GAL676-1CA
73
In some embodiments, the curve is defined along a path which extends from and
to the
same wall of the device housing.
In the example shown, the housing comprises sharp corners and straight edge
walls,
but other configurations are also contemplated, including, for example,
rounded corners,
curved walls, and the like.
In some embodiments, actuation of the actuation assembly (e.g. via a motor) of
each of
the pathways is controlled by a controller 2735. In some embodiments,
components of each
pathway are controlled independently, yet in a synchronized manner.
In some embodiments, controller 2735 is controlled remotely by an external
device, for
example by a remote control device such as described herein.
FIG. 28 schematically illustrates a robotic device for manipulation of two or
more
elongate surgical tools configured for a telescopic arrangement, such as in a
non-limiting
manner a guidewire and a microcatheter, the first elongate tool extending at
least in part within
the lumen of the second elongate tool, according to some embodiments.
In some embodiments, robotic device 2801 comprises a housing 2803 comprised of
a
plurality of walls which form an inner volume 2805 between them. In some
embodiments, two
or more inner pathways extend inside the inner volume, such that tools 2810,
2813 received
and operated by the device extend, at least in part, along the inner pathways.
In some embodiments, each of the inner pathways includes an actuation assembly
positioned at a position of the pathway, for example, axially extending along
at least a portion
of the pathway. In some embodiments, an actuation assembly, such as 2806,
2807, is
configured for linearly moving the tool, for example, one or more sets of
wheels configured to
advance and/or retract the tool. Alternatively or additionally, an actuation
assembly, such as
2806, is configured for moving the tool in a roll manner, for example by
rotating a set of wheels
gripping the tool therebetween.
In some embodiments, actuation assemblies are operably coupled to a plurality
of
motors, for example motors 2811, 2808, 2809. In some embodiments, the motors
are
configured for operating the actuation assemblies to generate linear movement
of the tools
received therein. Alternatively or additionally, the motors are configured to
generate a roll
movement of the received tool, optionally by generating a roll movement of the
tool's
associated actuation assembly as a whole. For example, motor 2809 is operably
connected to
linear movement mechanism 2807, optionally via a gear system, and is
configured to rotate
Date Recue/Date Received 2022-04-14
GAL676-1CA
74
linear movement mechanism 2807 together with motor 2811, thereby rolling tool
2810 which
is gripped within linear movement mechanism 2807. A potential advantage for
rotating the
entire linear movement mechanism along with the tool is a simplification of
the associated gear
system, and the enablement of simultaneous operation of linear and roll
movement together.
Rolling of motor 2811 together with the linear movement mechanism 2806 is
enabled, in some
embodiments, due to that no sterile barrier exists between the motors and the
actuation
assemblies.
In the example shown, a first elongate surgical tool 2810 (e.g. a guidewire)
extends
along a first inner pathway, for example between an entry aperture 2814 into
the housing and
an exit aperture 2816 from the housing.
In some embodiments, linear movement of tool 2810 is driven by motor 2811, and
roll
of tool 2810 is driven by motor 2809, both located and configured at a
position of the inner
pathway (e.g. along a notional axis defined by the pathway across the inner
volume).
In some embodiments, at the exit aperture 2816 of tool 2810 from the housing,
the tool
2810 is telescopically received within a lumen of a second elongate surgical
tool 2813, for
example, a microcatheter. Tool 2813, in turn, enters the housing at an entry
aperture 2815 and
extends along a second inner pathway to an exit aperture 2817, with tool 2810
extending inside
it.
In some embodiments, linear movement of the tool 2813 is driven by actuation
assembly 2807.
In some embodiments, the actuation mechanism(s) and the plurality of motors
all share
the same inner volume, with no barrier or other physical separation
therebetween.
FIG. 29 schematically illustrates another exemplary embodiment of the robotic
device
configured for receiving three telescopically arranged elongate surgical
tools, for example, a
guidewire, a microcatheter and a guiding catheter.
In some embodiments, robotic device 2901 comprises a housing 2903 having an
inner
volume 2905, wherein entry aperture 2914 and exit aperture 2916 define,
between them, a first
inner pathway for receiving a first elongated surgical tool 2910, and entry
aperture 2915 and
exit aperture 2917 define, between them, a second inner pathway for receiving
a second
elongate surgical tool 2913.
In some embodiments, actuation assemblies 2906, 2907 are positioned along the
inner
pathways and configured to come into contact with the tools received therein
for at least one
Date Recue/Date Received 2022-04-14
GAL676-1CA
of advance, retract and/or roll the tool. In some embodiments, a plurality of
motors, such as
motors 2909, 2911 and 2908, are positioned in proximity to the inner pathways
and are
operably connected to the actuation assemblies. In some embodiments, the
motors and the
actuation assemblies are found within the same inner volume accommodating the
inner
5 pathways, for example without barriers blocking the air circulating
between them.
In some embodiments, only one motor is operably connected to an actuation
assembly,
as exemplified by actuation assembly 2907 and motor 2908, which is operably
connected to
the actuation assembly to advance or retract elongate surgical tool 2913. In
some embodiments,
two or more motors are operably connected to an actuation assembly, as
exemplified by
10 actuation assembly 2906 and motors 2909 and 2911. In this example,
motors 2909 and 2911
are operably connected to actuation assembly 2906 to advance, retract and roll
elongate
surgical tool 2910. Optionally, motor 2909 rolls tool 2910 by rolling the
complex 2904,
wherein complex 2904 comprises at least actuation assembly 2906 and motor
2911.
In some embodiments, the proximal end of elongate surgical tool 2910 is
secured to a
15 fixation location 2920. In some embodiments, fixation location 2920
includes a protrusion
configured to attach to a luer (not shown) optionally found in the proximal
end of tool 2910.
Alternatively, fixation location 2920 comprises a cavity sized and shaped to
accommodate a
handle (not shown) optionally found at the proximal end of tool 2910. In some
embodiments,
the proximal end of tool 2910 is operably connected to adaptor 2950 which, in
some
20 embodiments, causes the tool to roll around its longitudinal axis, for
example by roll of a
proximal handle portion of the tool which is received at the adaptor. In some
embodiments, the
motor which is operably connected to the adaptor to induce the roll movement,
is the same
motor operably connected to the actuation assembly associated with the tool at
a more distal
location. For example, as shown and exemplified through motor 2909, which is
operably
25 connected to adaptor 2905 and at the same time operably connected to
complex 2904, to cause
roll actuation of tool 2910 from at least these two distinct locations.
In some embodiments, a U-shape curve is formed in tool 2910 between fixation
location 2920 and entry aperture 2914. In some embodiments, when tool 2910 is
moved
linearly in actuation assembly 2906 it causes the distal end 2930 of tool 2910
to advance or
30 retract, optionally when a distal portion has been introduced into the
patient's body. In some
embodiments, as tool 2910 is advanced or retracted, a distance between a
maximal point of the
U-shape curve and housing 2903 is shortened or lengthened. An advantage of the
U-shape
Date Recue/Date Received 2022-04-14
GAL676-1CA
76
curve being formed outside of housing 2903 is that the housing size does not
need to
accommodate this distance, and the device is capable of navigating a range of
tool lengths,
with no dependency on the size of the device.
In some embodiments, a fixation location of one elongate surgical tool is
found at the
exit aperture of another elongate surgical tool, as shown and exemplified in
fixation point 2922,
which overlaps with exit aperture 2916, and as such, causes elongate surgical
tool 2910 to exit
housing 2903 through exit aperture 2916 directly into the lumen of elongate
surgical tool 2913,
when tool 2913 is connected to fixation location 2922.
In some embodiments, a second U-shaped curve for tool 2910 and a first U-
shaped
curve for tool 2913 are formed between fixation location 2922 and entry
aperture 2915. In
some embodiments, when advancing or retracting the distal end 2940 of tool
2913, both tool
2910 and tool 2913 are moved to lengthen or shorten the distance between the
maximal point
of the joint curve and housing 2903. In some embodiments, when it is desired
to linearly
translate the distal end 2940 (of tool 2913) without translating distal end
2930 (of tool 2910),
motor 2911 linearly translates tool 2910 at an opposite direction to the
translation of motor
2907 which affects both tools, thereby, causing the distal end 2930 of tool
2910 to effectively
to stand in place.
In some embodiments, an elongate surgical tool (for example, a guide catheter,
or
sheath) connected to a fixation location from outside of housing 2903 is
configured to be
operated by motors residing inside housing 2903, for example, elongate
surgical tool 2919
connected to fixation location 2917 and can be linearly moved actuation
assembly 2927,
operably connected to motor 2928 and motor 2929 for linear and roll movement,
respectively.
In some embodiments, actuation assembly 2927 together with motors 2928 and
2929 all reside
in the same inner volume as motors 2909, 2911 and 2908 and in the same inner
volume as the
actuation assemblies they are operably connected to, 2906 and 2907. In such
exemplary
embodiments, at least 5 motors reside within the same inner volume as the
inner pathways of
the elongate surgical tools 2910 and 2913.
In some embodiments, fixation location 2924 overlaps with exit aperture 2917,
such
that the telescopically arranged elongate surgical tools 2910 and 2913 exit
housing 2903
through exit aperture 2917, directly into the lumen of elongate surgical tool
2919. In some
embodiments, actuation assembly 2927 is positioned along the same inner
pathway as that of
tool 2913.
Date Recue/Date Received 2022-04-14
GAL676-1CA
77
As used herein, the terms "insertion device" and "medical device", "robotic
device",
"robotic system", "device", "system" and the like may interchangeably be used.
In some
instances, a device is addressed as part of a system.
As used herein, the terms "medical instrument" and "medical tool", "surgical
tool",
"elongate tool" and the like may interchangeably be used.
Although some examples described throughout this disclosure mainly relate to
insertion
of a guidewire into the patient's blood vessel, this is done for simplicity
reasons alone, and the
scope of this disclosure is not limited to devices for insertion of guidewires
alone, but may
include insertion of additional medical tools/instruments, such as,
microcatheters, balloon
catheters, etc. Further, the scope of this disclosure is not limited to
insertion of medical tools
into blood vessels, but it may include insertion of medical tools into other
bodily lumens, such
as the urethra, gastrointestinal tract and the trachea. In the description and
claims of the
application, the words "include" and "have", and forms thereof, are not
limited to members in
a list with which the words may be associated.
The terms "comprises", "comprising", "includes", "including", "having" and
their
conjugates mean "including but not limited to".
The term "consisting of' means "including and limited to".
The term "consisting essentially of' means that the composition, method or
structure
may include additional ingredients, steps and/or parts, but only if the
additional ingredients,
steps and/or parts do not materially alter the basic and novel characteristics
of the claimed
composition, method or structure.
As used herein, the singular form "a", "an" and "the" include plural
references unless
the context clearly dictates otherwise. For example, the term "a compound" or
"at least one
compound" may include a plurality of compounds, including mixtures thereof.
Throughout this application, various embodiments of this invention may be
presented
in a range format. It should be understood that the description in range
format is merely for
convenience and brevity and should not be construed as an inflexible
limitation on the scope
of the invention. Accordingly, the description of a range should be considered
to have
specifically disclosed all the possible subranges as well as individual
numerical values within
that range. For example, description of a range such as from 1 to 6 should be
considered to
have specifically disclosed subranges such as from 1 to 3, from 1 to 4, from 1
to 5, from 2 to
Date Recue/Date Received 2022-04-14
GAL676-1CA
78
4, from 2 to 6, from 3 to 6 etc., as well as individual numbers within that
range, for example,
1, 2, 3, 4, 5, and 6. This applies regardless of the breadth of the range.
Whenever a numerical range is indicated herein, it is meant to include any
cited
numeral (fractional or integral) within the indicated range. The phrases
"ranging/ranges
between" a first indicate number and a second indicate number and
"ranging/ranges from" a
first indicate number "to" a second indicate number are used herein
interchangeably and are
meant to include the first and second indicated numbers and all the fractional
and integral
numerals therebetween.
As used herein the term "method" refers to manners, means, techniques and
procedures
for accomplishing a given task including, but not limited to, those manners,
means, techniques
and procedures either known to, or readily developed from known manners,
means, techniques
and procedures by practitioners of the chemical, pharmacological, biological,
biochemical and
medical arts.
As used herein, the term "treating" includes abrogating, substantially
inhibiting,
slowing or reversing the progression of a condition, substantially
ameliorating clinical or
aesthetical symptoms of a condition or substantially preventing the appearance
of clinical or
aesthetical symptoms of a condition.
It is appreciated that certain features of the invention, which are, for
clarity, described
in the context of separate embodiments, may also be provided in combination in
a single
embodiment. Conversely, various features of the invention, which are, for
brevity, described in
the context of a single embodiment, may also be provided separately or in any
suitable
subcombination or as suitable in any other described embodiment of the
invention. Certain
features described in the context of various embodiments are not to be
considered essential
features of those embodiments, unless the embodiment is inoperative without
those elements.
Although the invention has been described in conjunction with specific
embodiments
thereof, it is evident that many alternatives, modifications and variations
will be apparent to
those skilled in the art. Accordingly, it is intended to embrace all such
alternatives,
modifications and variations that fall within the spirit and broad scope of
the appended claims.
Citation or identification of any reference in this application shall not be
construed as
an admission that such reference is available as prior art to the present
invention. To the extent
that section headings are used, they should not be construed as necessarily
limiting.
Date Recue/Date Received 2022-04-14