Note: Descriptions are shown in the official language in which they were submitted.
CA 03157380 2022-04-07
WO 2021/072084 PCT/US2020/054796
1
SYSTEMS AND METHODS FOR COGNITIVE DIAGNOSTICS FOR
NEUROLOGICAL DISORDERS: PARKINSON'S DISEASE AND COMORBID
DEPRESSION
SPECIFICATION
BACKGROUND
TECHNICAL FIELD
The present disclosure relates generally to the field of cognitive
diagnostics. More
particularly, the present disclosure relates to systems and methods for
cognitive diagnostics
in connection with Parkinson's disease, comorbid major depressive disorder and
response to
antidepressants.
RELATED ART
Parkinson's Disease (PD) is a neurological disease that affects specific brain
cells
and produces symptoms that include muscle rigidity, tremors, and changes in
speech and
gait. Mental health is extremely important in PD. Although common in other
chronic
diseases, research suggests that depression and anxiety are even more common
in PD. It is
estimated that at least 50 percent of those diagnosed with PD will experience
some form of
comorbid major depressive disorder ("MDD") during their illness, and up to 40
percent will
experience anxiety disorders. Most current solutions for early or initial
diagnosis of
Parkinson's and comorbid MDD are performed using rating scales or
questionnaires with
tests performed by healthcare providers when patients report specific
symptoms.
MDD is characterized by a long-lasting depressed mood or marked loss of
interest
or pleasure in all or nearly all activities. Antidepressants, including
serotonin-specific
reuptake inhibitors (hereinafter "S SRI"), can remediate depressive symptoms
in a substantial
proportion of patients suffering from MDD. It has been hypothesized that SSRIs
achieve
their therapeutic effect, in part, by modifying synaptic availability of
serotonin and possibly
also by enhancing neurogenesis in the hippocampal region. Yet, little is known
about the
underlying brain structure and neurochemistry in MDD. As a result, MDD
diagnosis is based
primarily on overt behavioral symptoms. Moreover, such diagnoses are given in
a long
interview with a medical professional and/or based on a form that is filled
out by a patient
CA 03157380 2022-04-07
WO 2021/072084
PCT/US2020/054796
2
or caretaker. Despite being accurate, such procedures for diagnosing MDD can
take a long
time to complete and require regular visits to professionals. Moreover, most
patients with
MDD do not respond positively to antidepressants and the current procedures
for diagnosing
MDD do not predict whether a patient will respond to antidepressants at all.
PD and MDD are discussed in the paper entitled "Depression Reduces Accuracy
While Parkinsonism Slows Response Time for Processing Positive Feedback in
Patients
with Parkinson's Disease with Comorbid Major Depressive Disorder Tested on a
Probabilistic Category-Learning Task," by Herzallah, et al., Frontiers in
Psychiatry, June
2017, Vol. 8, Art. 84.
CA 03157380 2022-04-07
WO 2021/072084
PCT/US2020/054796
3
SUMMARY
The present disclosure provides a computer system and method which can collect
data from a participant. The participant can interact with a computer device
(e.g., a tablet or
smartphone) through a short (e.g., ¨10 minutes) feedback-based probabilistic
classification
cognitive task (hereinafter "FPCT") during which data can be collected. The
data can be
processed by the computer device or a remote device in communication with the
computer
device over a network. The processing of the data can determine attributes of
a patient in
connection with the dissociation of learning from positive versus negative
feedback or other
forms of feedback-based learning (e.g., correct feedback versus incorrect
feedback or
reinforcement learning). The computer device can make this determination based
on
mathematical models and artificial intelligence approaches to extract
additional measures.
Based on the output of the computer device, a diagnosis of Parkinson's disease
can be made.
In addition, the results thereby generated can be used to assess whether the
patient also has
a comorbid major depressive disorder.
CA 03157380 2022-04-07
WO 2021/072084 PCT/US2020/054796
4
BRIEF DESCRIPTION OF THE DRAWINGS
The foregoing features of the invention will be apparent from the following
Detailed
Description, taken in connection with the accompanying drawings, in which:
FIG. 1 is a drawing illustrating an embodiment of a flow diagram of a system
of the
present disclosure;
FIGS. 2A-B are drawings showing graphs of a result from testing a first
example
cognitive task of the system of the present disclosure;
FIGS. 3A-B are drawings showing graphs of a result from testing a second
example
cognitive task of the system of the present disclosure;
FIGS. 4A-D are drawings showing sample screens of a feedback-based
classification
task in the system of the present disclosure;
FIGS. 5A-H are drawings showing graphs of testing results of the system of the
present disclosure;
FIGS. 6A-B are drawings showing two classification graphs for tests conducted
in
connection with the system of the present disclosure;
FIGS. 7A-C are drawings showing graphs of results of another test performed on
the
system of the present disclosure;
FIG. 8 is a graph illustrating mean positive and negative bias before and
after
treatment in connection with a test of the system of the present disclosure;
FIG. 9 is a diagram illustrating hardware and software components of the
system of
the present disclosure;
FIG. 10 is a diagram illustrating hardware and software components of a
computer
system on which the system of the present disclosure could be implemented;
FIG. 11 is a drawing illustrating another aspect of a flow diagram of a system
of the
present disclosure;
FIG. 12 is a schematic illustration of the system and method of the present
disclosure
for use in connection with Parkinson's disease;
FIG. 13 is a drawing showing a classification graph for tests conducted in
connection
with the system of the present disclosure; and
FIG. 14 is a drawing showing a classification graph for tests conducted in
connection
with the system of the present disclosure.
CA 03157380 2022-04-07
WO 2021/072084 PCT/US2020/054796
DETAILED DESCRIPTION
The present disclosure relates to systems and methods for cognitive
diagnostics in
connection with major depressive disorder and response to antidepressants, as
discussed in
detail below in connection with FIGS. 1-14.
The present disclosure uses Major Depressive Disorder ("MDD") as an example of
a psychiatric disorder, however, the system of the present disclosure can be
used to diagnose
any psychiatric disorder, including, but not limited to, post-traumatic stress
disorder,
obsessive compulsive disorder, schizophrenia, and other anxiety spectrum
disorders.
Moreover, the present disclosure refers to antidepressants and/or serotonin-
specific reuptake
inhibitors (hereinafter "S SRI") as examples of treatment, however, the system
of the present
disclosure can be used to predict whether a patient will respond to any number
of other
treatment modalities such as psychotherapy and others.
The present disclosure provides a computer system and method which can collect
data from one or more patients. These patients can interact with a computer
device (e.g., a
tablet or smartphone) through a short (e.g., ¨10 minutes) feedback-based
probabilistic
classification cognitive task (hereinafter "FPCT") during which data can be
collected. The
data can be processed by the computer device or a remote device in
communication with the
computer device over a network. The computer device can be a local device for
a closed-
circuit system. The processing of the data can determine attributes of a
patient in connection
with the dissociation of learning from positive versus negative feedback. The
computer
device can make this determination based on mathematical models and artificial
intelligence
approaches to extract additional measures. Based on the output of the computer
device, a
diagnosis of major depressive disorder (hereinafter "MDD") can be made. In
addition, the
results thereby generated can be used to predict whether the patient will
respond to
antidepressants.
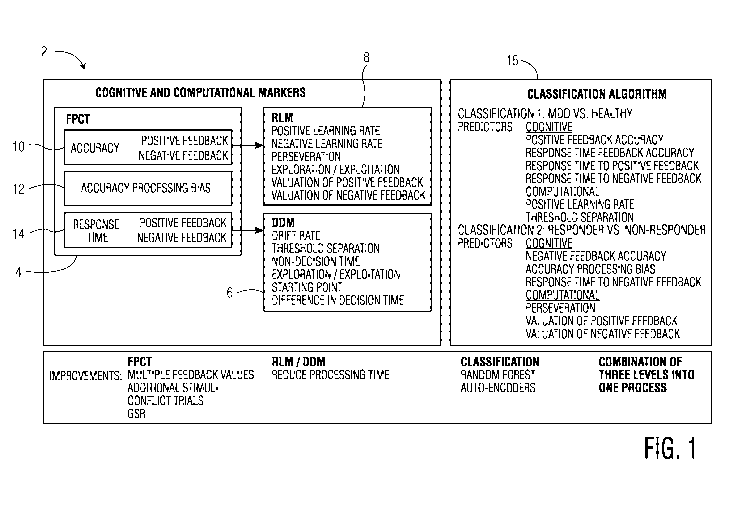
FIG. 1 is a drawing illustrating an embodiment of a flow diagram 2 of the
present
disclosure. The flow diagram 2 includes cognitive and computational and
artificial
intelligence markers having a FPCT step 4, variants of the Q-learning
reinforcement learning
model (RLM) 6, and variants of the drift-diffusion model (DDM) 8. The FPCT
step 4 can
be for collecting information relating to the results of the FPCT task a
patient performed.
The FPCT step 4 can include an accuracy component 10, an accuracy processing
bias
component 12, and a response time component 14. The accuracy component 10 can
include
factors relating to positive and negative feedback as will be explained in
greater detail below.
CA 03157380 2022-04-07
WO 2021/072084 PCT/US2020/054796
6
The response time component 14 can also include factors relating to positive
and negative
feedback as will be discussed in greater detail below.
The FPCT step 4 can output its collected cognitive data such as the accuracy
component 10 and the response time component 14 for processing by various
computational
models and artificial intelligence approaches. In particular, the accuracy
component 10 can
output its data for processing by the RLM models 8 which can be used to assess
parameters
related to learning accuracy. Moreover, the response time component 14 can
output its data
for processing by the DDM models 6 which can be used for assessing parameters
related to
response time distributions. Cognitive data from the FPCT step 4 and outputs
from the DDM
computational models 6 and the RLM computational models 8 can be sent to a
binomial or
multinomial logistic regression model 16 which can accurately determine MDD
patients
from healthy subjects. Further, the binomial or multinomial logistic
regression model 16
can use the same data to accurately determine responders and non-responders to
antidepressants. The multinomial logistic regression model 16 can include one
or more
classification algorithms and artificial intelligence approaches to make these
determinations.
For example, with respect to diagnosing a patient with MDD, cognitive
predictors can
include, but are not limited to, learning accuracy from positive feedback,
response time to
positive feedback, learning accuracy from negative feedback, and response time
to negative
feedback. With respect to diagnosing a patient with MDD, computational
predictors can
include, but are not limited to, positive learning rate, negative learning
rate, separation
threshold, difference in the speed of response for the execution of responses,
and drift rate
for negative feedback. With respect to determining whether a patient will
respond to
treatment, cognitive parameters include, but are not limited to, learning
accuracy from
negative feedback, accuracy processing bias, response time to negative
feedback, and
response time to positive feedback. With respect to determining whether a
patient will
respond to treatment, computational parameters include, but are not limited
to, preservation,
valuation of positive feedback, valuation of negative feedback, separation
threshold, and
starting point of evidence for decision making.
Examples of cognitive tasks will now be explained in greater detail. This
learning
task requires participants to learn a sequence of events leading to reward.
One example of
a cognitive task can be sequence learning and context generalization. It
should be noted that
the sequence learning and context generalization and chaining tasks are merely
examples of
a type of task that can be used. The present disclosure is not limited to the
exact
CA 03157380 2022-04-07
WO 2021/072084
PCT/US2020/054796
7
methodologies of the sequence learning and context generalization tasks
described herein.
Other variations of the tasks can be used, and the following sequence learning
and context
generalization task is for illustrative purposes. In the first phase of the
task, a computer
device can generate a screen which shows a first room (Room 1) with three
doors (Al, A2,
A3), each identified by its own color. The computer device can allow a
participant to choose
one of the doors. The computer device can set the correct response as door Al,
which can
lead to a reward, such as a treasure chest. The incorrect responses can be set
as doors A2 or
A3, which can lead to a locked door. If the participant selects an incorrect
door, the subjects
can be prompted to try again. Once the participant learns that door Al is
associated with a
reward, the computer device can present the participant with another room
(Room 2). This
room can have three new colored doors (B1, B2, B3). The computer device can
set the
incorrect responses to doors B2 and B3 which can lead to a locked door. The
computer
device can also set the correct response to door B1 which can lead to Room 1,
in which the
participant would again be presented with the doors Al, A2, and A3 where the
same door as
previously presented would lead to the reward and the other doors would lead
to locked
doors. This will allow the participant to learn an association where selecting
B1 and then
Al leads to a reward. Once this new association is learned, a new room (Room
3) can be
added to the sequence where doors Cl, C2, and C3 are presented to a
participant. C2 and
C3 can be set to lead to a locked door while Cl can lead to Room 2 as
discussed above. Now
the participant will learn an association where selecting Cl, Bl, and Al leads
to a reward.
Once this association is learned, the participant can be presented with Room 4
with doors
D1, D2, and D3. D2 and D3 can be set as incorrect responses and D1 (the
correct response)
can lead to Room 3. Here, the participant can learn an association that
selecting D1, Cl, Bl,
and Al leads to a reward. It should be noted that the above process is not
limited to a three-
door situation with a specific number door having the reward. The above
cognitive task is
merely an example task that can be used in the system of the present
disclosure.
Nevertheless, the system of the present disclosure can include other cognitive
tasks for
chaining and sequence mechanisms with context generalization. The above
process can be
seen in Table 1 below.
CA 03157380 2022-04-07
WO 2021/072084
PCT/US2020/054796
8
The Sequence Learning with Context Generalization Paradigm
Phase Description Doors shown Correct response
Practice Cue-association P1P2P3 P1¨*reward
Sequence- Chain step A Ai A2A3 A1¨> reward
Learning Chain step B Bi B2B3 B1 ¨>A1 ¨> reward
Chain step C Ci C2C3 C1 ¨>B1 ¨>A1 ¨> reward
Chain step D Di D2D3 D1 ¨>C1 ¨>B1 ¨>A1 ¨>
reward
Context Example Di BiXi D1 ¨>C1 ¨>B1 ¨>A1 ¨>
Generalization generalization trial reward
Retest Cue-association Yi Y2Y3 Y1¨> reward
Table 1
In the context generalization phase as shown above in Table 1, generalization
to
novel task demands can be tested by presenting various novel incorrect doors
as distractors
along with a correct door choice in each room. This can require participants
to learn the
correct response and context associations to obtain the reward as shown in
Table 1.
FIGS. 2A-B are graphs which show an example result of testing the above
cognitive
task. As can be seen, FIG. 2A shows performance on the sequence learning and
context
generalization task such as the mean number of errors on the sequence-learning
phase of the
task (chain steps A-D as shown in Table 1). FIG. 2B also shows the mean
numbers of errors
on the context generalization phase. In the graphs of FIGS. 2A-B, MDD
represents
medication naïve patients, MDD-T represents patients on medication, and HC
represents
healthy control subjects. The results show that persons with MDD that are not
being treated
with medication tend to make many errors on the initial learning/chaining
phase, but persons
with MDD on medication treatment make many errors in the contextual
generalization
phase. Univariate ANOVA (alpha=0.05) indicated a significant group difference
in the
chaining phase results [F(2,24)=4.25, p=0.026, partial n2=0.2611 as well as
the context
generalization phase results [F(2,24)=16.90, p<0.001, partial n2=0.591. In the
sequence-
learning phase results, an HSD post hoc test revealed a significant difference
between MDD
and HCs and between MDD and MDD-T (p<0.05), but not between HCs and MDD-T. In
the context generalization phase, an HSD post hoc test revealed a significant
difference
between MDD-T and HCs, and between MDD-T and MDD (p<0.001), but not between
HCs
and MDD. An a priori power analysis of one-way ANOVA, done to compute the
number
of subjects required per group to get a power of 95%, showed that a sample of
48 subjects
CA 03157380 2022-04-07
WO 2021/072084 PCT/US2020/054796
9
(16 per group) can be needed to achieve the mentioned power level on the
chaining ANOVA,
and a total sample of 15 subjects (5 per group) to achieve 95% power level on
the context
generalization ANOVA.
A second example cognitive task will now be explained in greater detail. The
second
cognitive task can use a reward-and-punishment-based computer-learning task
for weather
prediction. In each phase of the task, a computer device can generate four
stimuli such as
abstract geometric paintings. A participant can view a painting and the device
can ask the
participant whether that painting predicts rainy weather or sunny weather. The
computer
device can be programmed so that choosing an answer with respect to two of the
stimuli
(e.g., paintings) provide feedback for correct answers and incorrect answers
result in no
feedback. The computer device can also be programmed so that choosing an
answer in
connection with the other two stimuli provide feedback for incorrect answers
and no
feedback is given for correct answers. Among both the reward-trained and
punishment-
trained cues, equal numbers can be associated with rainy weather and sunny
weather. All
four cues can be intermixed during training. This task is not limited to any
specific
methodology and can include other tasks related to reward-and-punishment
mechanisms.
The cognitive tasks described in the present disclosure can also have the
ability to
change based on user input providing a dynamic functionality. In particular,
the cognitive
tasks can change a stimulus or task or question based on a user's prior
response(s). For
example, if a user is answering questions correctly, the system can increase
the difficulty of
a subsequent question. Moreover, if a user is answering questions incorrectly,
the system
can decrease the difficulty of a subsequent question. In this way, the
cognitive tasks of the
present disclosure are tailored to a user's abilities. Furthermore, the system
can change a
task to a different task based on the user's input. The system can take into
account a plurality
of different trials and present a tailored subsequent trial to a user.
Accordingly, the systems
and methods of the present disclosure can function as a closed loop system for
diagnosing
mental health conditions and responsiveness to treatments.
FIGS. 3A-B are graphs which show an example result of testing the above
cognitive
task. The results tested 13 medication-naïve MDD, 18 MDD-T (Treated, on
medication),
and 22 healthy controls (HC). FIGS. 3A-B show performance on the two types of
trials of
the reward and punishment learning task. For example, the mean number of
correct
responses in the four phases for the reward stimuli is shown in FIG. 3A and
the mean number
of correct responses in the four phases for the punishment stimuli is shown in
FIG. 3B. As
CA 03157380 2022-04-07
WO 2021/072084
PCT/US2020/054796
noted above, MDD represents patients who are medication naïve and MDD-T
represents
patients on medication. As can be seen in FIG. 3A, the results show no
difference in
performance on reward training between MDD and MDD-T being impaired in that
phase
using one-sample t-test to assess learning higher than chance. In a one-way
ANOVA
(Bonferroni correction of a=0.025 to protect the level of significance), using
the 4th block
of reward and punishment trials as the dependent variable, there existed a
significant effect
of group on learning from punishment [F(2,27)=4.821, p=0.016, n2=0.2491 but
not on
learning from reward [F(2,27)=0.49, p=0.6181. A post hoc analysis of the group
effect on
4th-b10ck punishment learning revealed a significant difference between MDD-T
patients
and MDD patients, and between MDD-T patients and HC (p<0.05). A priori power
analysis
for ANOVA revealed that the test can have a total of 51 subjects (17 per
group) to obtain a
power of 95%.
As noted above, the system of the present disclosure can collect data of the
participants progress in the above example cognitive tasks and variations
thereof The
system of the present disclosure can process this data using a binomial or
multinomial
logistic regression algorithm to classify subjects as either having MDD or
not, and if they
do have MDD, whether the subjects would respond to certain medications such as
antidepressants. Other classification approaches can be used such as random
forest, auto-
encoders, or other artificial intelligence and machine learning approaches.
Random forest
or auto-encoders can offer, in some circumstances, better and quicker results,
and can utilize
a greater number of predictors. Furthermore, the system of the present
disclosure can use
the Softmax function in making its classification determinations. It should be
noted that the
above tasks can be performed in a relatively short period of time (e.g., 15
minutes).
The system of the present disclosure can collect data relating to the time it
takes for
a participant to respond to the scenarios discussed herein. Depending on the
time it takes
for the participant to respond, the classification algorithm of the system of
the present
disclosure can take this information as an input and make determinations
regarding MDD
and ability to respond to treatments for MDD. As noted above, the data
gathered during the
cognitive tasks and used by the classification algorithms and artificial
intelligence
approaches can include, but is not limited to, accuracy of correct answers,
incorrect answers,
response time, response time as the task progresses, learning progress, and
how much the
participants value positive and negative feedback. These data points can be
processed by
the classification algorithm and artificial intelligence approaches to make a
determination as
CA 03157380 2022-04-07
WO 2021/072084 PCT/US2020/054796
11
to whether a patient has a particular psychiatric disorder and whether that
patient will
respond to treatment.
The system of the present disclosure can vary the amount of positive/negative
feedback associated with stimuli. With learning accuracy in positive and
negative feedback
being one of the key cognitive predictors, and valuation of feedback being one
of the key
computational predictors, the system can add new stimuli to the current FPCT
with various
amounts of positive and negative feedback to get clearer results related to
feedback
processing.
The system of the present disclosure can also use conflict trials while
diagnosing
MDD and responsiveness to medications. For example, in some cases, there can
be a
feedback processing bias that can differentiate clinically depressed vs. non-
depressed
subjects as well as responders and non-responders. The subject can learn the
feedback
associated with each stimulus, and it can be expected that subjects develop
preferences to
stimuli associated with particular feedback. Accordingly, conflict trials can
be used to
account for these factors.
The system of the present disclosure can also add multiple phases with more
stimuli.
In particular, the MDD state and potential response to treatment can be
expressed cognitively
as preferential learning of particular stimuli with particular feedback.
Therefore, adding
more stimuli while escalating the level of complexity of the FPCT can refine
the underlying
factors for preferential learning which improves the efficiency of the
classification model.
The system of the present disclosure can add galvanic skin response (GSR) or
an
eye-tracker to assess eye movements as well as pupil size as additional
predictors. By adding
GSR, eye-tracking, or electroencephalography (EEG), the system can present an
unbiased
physiological measure to accompany the cognitive measures from the FPCT.
Sensors and
electrodes can be placed on a patient's body, their eyes, and/or their scalp
which can gather
physiological data which can be communicated to a computer device in the
system of the
present disclosure. This computer device can process the data from the sensor
to determine
the emotions (e.g., happiness, fear, etc.) felt by the patient while
completing the tasks
described herein. Data from the eye-tracker can also be analyzed to specify
the points of
focus as well as changes in pupil size. Data from EEG can track changes in
brain electrical
activity during the FPCT or at baseline (before/after cognitive testing). The
classification
algorithm can receive these data as input and can use such information in
providing enhanced
CA 03157380 2022-04-07
WO 2021/072084 PCT/US2020/054796
12
classifications as to a diagnosis and whether a patient will respond to
treatment and the best
treatment to offer.
The system of the present disclosure can also apply the above processes and
cognitive
tasks to diagnose other psychiatric disorders including, but not limited to,
post-traumatic
stress disorder, obsessive compulsive disorder, schizophrenia, and other
anxiety spectrum
disorders.
The system of the present disclosure can also test patients after they have
received
antidepressants to determine whether they responded to the treatment or
whether they are
still depressed. This can be done by leveraging the cognitive tasks discussed
above.
The system can also predict a patient's response to psychotherapy in addition
to
antidepressants. The classification algorithm and artificial intelligence
approaches as
discussed above can use the data captured from the tasks and make a
determination as to
whether a patient will respond to psychotherapy. The system can also determine
whether
antidepressants or psychotherapy will be better for a given patient based on
the cognitive
tasks discussed above.
A test with respect to the system of the present disclosure will now be
described in
greater detail. This test includes 67 medication-naïve patients with MDD and
16 matched
healthy controls from various clinics in the Palestine area. A positive and
negative feedback
classification task for weather prediction was used given by Table 2 below:
Probability Probability
Stimulus Feedback
category A (%) category B (%)
Si 90 10 If correct: +25
S2 10 90 If incorrect: no feedback
S3 90 10 If correct: no feedback
S4 10 90 If incorrect: -25
Table 2
FIGS. 4A-B are drawings showing sample screens of a feedback-based
classification
task. These are the screens that were used in the above trial. On each trial,
a participant saw
one of four stimuli and was asked whether this stimulus predicts rainy or
sunny weather. In
screen 4B, no feedback is given for incorrect answers in positive feedback
stimuli or correct
answers in negative feedback stimuli. As shown in screen 4C, for positive
feedback stimuli,
correct responses receive positive feedback with visual feedback and twenty
five points of
CA 03157380 2022-04-07
WO 2021/072084
PCT/US2020/054796
13
winnings. As shown in screen 4D, for negative feedback, incorrect responses
get negative
feedback with visual feedback and the loss of 25 points. In the FCPT task, the
subject sees
one of four stimuli (abstract geometric paintings) and is asked to make a
prediction. For
example, the subject is asked whether that stimulus predicts Rain or Sun. Two
of the stimuli
are trained using only positive feedback for correct answers (incorrect
answers result in no
feedback). The other two stimuli are trained using only negative feedback for
incorrect
answers (correct answers result in no feedback). Among both the positive-
feedback-trained
and negative-feedback-trained cues, one is more strongly associated with Rain
and the other
with Sun. These associations are probabilistic, so that, for example, a rain-
preferred cue is
associated with 90% Rain and 10% Sun.
The above test used a variant of the Q-learning trial-by-trial computational
analysis
to calculate estimates for the following parameters: learning rate with
positive prediction
error (LR+); learning rate from negative prediction error (LR-); preservation;
noise (beta);
and valuation of feedback (RO+, RO-). It also used a variant of the DDM trial-
by-trial
computational analysis to calculate estimates for the following parameters:
drift rate (v) for
positive-feedback and negative-feedback; threshold separation (a); relative
starting point
(zr); non-decision time (t0); and difference in decision time for correct and
incorrect
responses (d). The results of the above test can be seen in FIGS. 5A-H. As can
be seen in
FIGS. 5A and 5B, cognitive and computational analysis results show learning
accuracy in
positive and negative feedback trials. FIGS. 5C and 5D show response time to
positive and
negative feedback stimuli. FIGS. 5E and 5F show positive/negative accuracy
bias. FIG. 5G
shows parameter estimates using a 6-parameter Q-learning model. FIG. 5H shows
parameter
estimates using a 6-parameter DDM analysis.
FIGS. 6A-B are drawings showing classification graphs for the above test
conducted
in connection with the system of the present disclosure. As can be seen, a
forward binomial
logistic regression classification graph shows a predicted probability of
membership for
MDD SSRI responder vs. non-responder in FIG. 6A and MDD vs. healthy in FIG.
6B. The
cutoff value can be 0.50. In FIG. 6A, R denotes a responder and N denotes a
non-responder.
In FIG. 6B, M denotes MDD and H denotes healthy subject. Each symbol
represents two
and a half cases. Four symbols on the graph represent one case.
The above test shows learning accuracy and response time to positive feedback
and
learning accuracy and response time to negative feedback can differentiate
potential patients
with MDD from healthy subjects. It also shows learning accuracy and response
time to
CA 03157380 2022-04-07
WO 2021/072084
PCT/US2020/054796
14
negative feedback can a priori differentiate potential SSRI-responders and non-
responders
at the medication-naïve level. These results provide an easy to use diagnostic
tool that can
have immediate clinical relevance. Moreover, it shows lower positive learning
rate and
learning noise in patients with MDD than healthy subjects. SSRI non-responders
exhibit
higher levels of preservation during learning. Further, SSRI non-responders
value no-
feedback in negative feedback trials as negative, which can explain the
deficit in negative
feedback learning accuracy. It also shows higher threshold separation (a),
higher difference
in decision time for correct and incorrect responses (d), lower non-decision
time (st0), and
lower drift rate for negative feedback (v-p). This could explain the slower
response time in
patients with MDD. In addition, MDD is associated with a selective deficit in
learning from
positive feedback. SSRI non-responders have balanced learning from positive
and negative
feedback at the medication-naïve state similar to healthy subjects.
Another test with respect to a positive and negative feedback probabilistic
classification task was conducted in connection with the system of the present
disclosure. In
particular, 67 medication naïve patients with MDD and 16 matched healthy
controls
participated in Palestine. Patients with MDD were retested 4-6 weeks after
starting
paroxetine regimen. Healthy controls were also retested at a similar time
interval. Response
to paroxetine was considered positive if a patient's Beck Depression Inventory
II score
dropped 50 percent from baseline, and the patient screened negative for MDD on
the Mini
International Neuropsychiatric Interview. The same positive and negative
feedback
probabilistic feedback classification task for weather prediction can be used
with a feedback
structure given by Table 2 above. A similar user interface can also be used as
shown in
FIGS. 4A-D.
FIGS. 7A-C shows results of the test discussed above. Performance on the
positive
and negative feedback learning task is shown. In FIG. 7A, the graph shows that
the mean
number of optimal responses in the four phases for the positive feedback
stimuli. In FIG.
7B, the graph shows the mean number of optimal responses in the four phases
for the
negative feedback stimuli. In FIG. 7C, the graph shows the mean difference
between
percentage optimal responses in positive and negative feedback trials per
block. MDD.R-
MN represents participants that are medication-naïve with MDD and who are SSRI
responders. MDD.R-T represents participants who are SSRI responders. MDD.NR-MN
represents participants who are medication-naïve with MDD who are SSRI non-
responders.
CA 03157380 2022-04-07
WO 2021/072084 PCT/US2020/054796
MDD.NR-T represents participants who are SSRI non-responders. HC test
represents
healthy controls at baseline and HC retest are healthy controls after 4-6
weeks.
FIG. 8 is a graph illustrating mean positive and negative bias before and
after SSRI
treatment. As can be seen, FIG. 8 shows a mean difference between percentage
optimal
responses in positive and negative feedback trials across blocks per testing
session before
and after SSRI treatment for MDD and at-test and retest for healthy subjects.
MDD-mn
(test) is medication-naive baseline for MDD and baseline for healthy subjects.
MDD-t
(retest) is SSRI-treated retesting for MDD patients 4-6 weeks after SSRI
administration and
retesting at 4-6 weeks for healthy subjects. The conclusions from this test
shows that
learning from negative feedback can differentiate potential SSRI-responders
and non-
responders at the medication-naive level. Moreover, S SRI-responsive MDD is
associated
with a selective deficit in learning from positive feedback. Further, SSRI non-
responders
have balanced learning from positive and negative feedback at the medication-
naive state.
Finally, SSRI administration suppresses learning from negative feedback in
responders only,
thereby bringing positive and negative feedback learning into balance.
FIG. 9 is diagram illustrating hardware and software components of the system
of
the present disclosure. A system 100 can include a mental health diagnostics
computer
system 102. The mental health diagnostics computer system can include a
database 104 and
a mental health diagnostics processing engine 106. The system 100 can also
include a
computer system(s) 108 for communicating with the mental health diagnostics
computer
system 102 over a network 110. The computer systems 108 can be computer
devices in
which the participants perform the tasks as described above. Network
communication could
be over the Internet using standard TCP/IP communications protocols (e.g.,
hypertext
transfer protocol (HTTP), secure HTTP (HTTPS), file transfer protocol (FTP),
electronic
data interchange (EDI), etc.), through a private network connection (e.g.,
wide-area network
(WAN) connection, emails, electronic data interchange (EDI) messages,
extensible markup
language (XML) messages, file transfer protocol (FTP) file transfers, etc.),
or any other
suitable wired or wireless electronic communications format. The computer
system 108
can also be a smartphone, tables, laptop, or other similar device. The
computer system 108
could be any suitable computer server (e.g., a server with an INTEL
microprocessor,
multiple processors, multiple processing cores) running any suitable operating
system (e.g.,
Windows by Microsoft, Linux, etc.). Alternatively, the computer system could
be a field-
programmable gate array (FPGA) that can run the mathematical models and
artificial
CA 03157380 2022-04-07
WO 2021/072084
PCT/US2020/054796
16
intelligence approaches simultaneously upon receipt of the cognitive data in a
closed-loop
system.
FIG. 10 is a diagram illustrating hardware and software components of a
computer
system on which the system of the present disclosure could be implemented. The
system
100 comprises a processing server 102 which could include a storage device
104, a network
interface 118, a communications bus 110, a central processing unit (CPU)
(microprocessor)
112, a random access memory (RAM) 114, and one or more input devices 116, such
as a
keyboard, mouse, etc. The server 102 could also include a display (e.g.,
liquid crystal display
(LCD), cathode ray tube (CRT), etc.). The storage device 104 could comprise
any suitable,
computer-readable storage medium such as disk, non-volatile memory (e.g., read-
only
memory (ROM), erasable programmable ROM (EPROM), electrically-erasable
programmable ROM (EEPROM), flash memory, field-programmable gate array (FPGA),
etc.). The server 102 could be a networked computer system, a personal
computer, a smart
phone, tablet computer etc. It is noted that the server 102 need not be a
networked server,
and indeed, could be a stand-alone computer system.
The functionality provided by the present disclosure could be provided by a
mental
health diagnostics program/engine 106, which could be embodied as computer-
readable
program code stored on the storage device 104 and executed by the CPU 112
using any
suitable, high or low level computing language, such as Python, Java, C, C++,
C#, .NET,
MATLAB, etc. The network interface 108 could include an Ethernet network
interface
device, a wireless network interface device, or any other suitable device
which permits the
server 102 to communicate via the network. The CPU 112 could include any
suitable single-
or multiple-core microprocessor of any suitable architecture that is capable
of implementing
and running the mental health diagnostics engine 106 (e.g., Intel processor).
The random
access memory 114 could include any suitable, high-speed, random access memory
typical
of most modern computers, such as dynamic RAM (DRAM), etc.
FIG. 11 is a drawing of a flow diagram 200 of another aspect of the system of
the
present disclosure. The flow diagram 200 illustrates a cognitive component
210, a
computational component 220, a classifier component 230 and an output 240. As
shown in
FIG. 11, an emphasis on the dynamic interaction between the cognitive
component 210 and
the computational component 220 of the system provides for maximizing an
accuracy of the
classifier component 230.
CA 03157380 2022-04-07
WO 2021/072084 PCT/US2020/054796
17
The cognitive component 210 includes a plurality of trial blocks 212a, 212b
and
212c. Each trial block 212a, 212b, and 212c can include a specified number of
trials, a
specified number of trial types and a working memory test. Additionally, trial
blocks 212b
and 212c can include additional features including, but not limited to,
outcome reversal,
outcome devaluation, gain/loss value modification and delay discounting. These
additional
features provide for a trial block following a preceding trial block to
explore non-dispositive
results from the preceding trial block. For example, trial block 212b could be
designed with
additional features such as gain/loss value modification and delay discounting
to explore
non-dispositive results from trial block 212a or other cognitive demands
related to
mental/psychiatric disorders.
The computational component 220 can analyze the cognitive results of each
trial
block 212a, 212b and 212c utilizing a plurality of modeling and artificial
intelligence
approaches on a trial by trial basis in real time. Specifically, upon
initiation of a cognitive
task of a trial block 212a-c, the computational component 220 performs the
trial-by-trial
computational analysis in real-time while the subject is performing the
cognitive task. The
plurality of modeling approaches can include, but are not limited to,
prediction error learning
(PEL) 222a-c, gain learning (GL) 224a-c, loss learning (LL) 226a-c and
stimulus-by-
stimulus learning (SSL) 228a-c. DDM trial-by-trial analysis of cognitive data
can be
conducted in parallel. Each of the plurality of modeling approaches can
include a set of
operating parameters. For example, PEL 222a-c can include operating parameters
such as
positive learning rate, negative learning rate, and noise, and GL 224a-c can
include operating
parameters such as gain learning rate, noise, preservation, and valuation of
no-feedback.
Additionally, LL can include operating parameters such as loss learning rate,
noise,
preservation, and valuation of no-feedback, and SSL can include operating
parameters such
as positive learning rate, negative learning rate, noise, preservation and
valuation of no-
feedback.
Conventional computer-based cognitive tasks suffer from static design that
typically
does not change throughout an execution of a cognitive task. As such, the
system utilizes
the cognitive component 210 to design and generate a dynamic cognitive task
wherein the
performance of the subject influences a design of a subsequent trial block, an
addition of
various features, and/or the repetition of some of the previously used trial
types for further
analysis. By fine-tuning a measurement of the cognitive features and the
computational
CA 03157380 2022-04-07
WO 2021/072084 PCT/US2020/054796
18
parameters, the system can maximize the classification abilities of the
classifier component
230.
Specifically, the system utilizes dynamic cognitive task-computational model
coupling to maximize the classification abilities of the classifier component
230. For
example, for a trial, the cognitive task can transmit a trial type, accuracy,
and response time
to the various computational models 222a-c, 224a-c, 226a-c and 228a-c to
extract parameters
of the learning process. Accordingly, over a course of 10-20 trials per trial
type, measures
of central tendency (e.g., mean and median) as well as variability (e.g.,
standard deviation,
skewness, and kurtosis) can be evaluated and compared to parameters extracted
from a large
pool of healthy subject data (e.g., a pool of approximately 1000 subjects).
Upon ascertaining
a difference or a lack of a difference between parameters of the tested
subject, the cognitive
results and the computational parameters can be adjusted. If the cognitive
results and the
computational parameters are not adjusted, additional testing of the same type
of trials can
be resumed in a subsequent trial block. According to the fixed cognitive
results and
computational parameters, the subsequent trial block can be programmed to test
the
cognitive dimensions of the subject according to resulting combinations.
The classifier component 230 can execute a plurality of algorithms and
artificial
intelligence approaches for synthesizing acquired data. For example, the
system can
implement a multi-layered convolutional neural network (CNN) classifier to
emphasize the
multi-dimensionality of the dynamic cognitive task-computational model
coupling approach
and acquired data. Then, according to the cognitive results and computational
parameters
232a, 232b and 232c extracted from the subject data, the CNN classifier can
assess
similarities between results of the subject and pre-defined
cognitive/computational patterns
that signify respective domains of mental/psychiatric disorders. Subsequently,
the system
can utilize Random Forest to assign final probabilities.
The present disclosure can be applied to Parkinson's disease and other
neurological
disorders. It can also be used to diagnose comorbid psychiatric manifestations
that affect
patients with Parkinson's disease, such as MDD, known as comorbid MDD.
Parkinson's
disease is diagnosed by the system by varying the amount of positive and / or
negative
feedback associated with stimuli during feedback-based probabilistic
classification
cognitive task (FPCT); utilizing reversal trials to potentially implicate the
involvement of
frontal regions in the disorder; and adding more stimuli while escalating the
level of
complexity of the FPCT. The system and method of the present disclosure allows
a subject
CA 03157380 2022-04-07
WO 2021/072084
PCT/US2020/054796
19
play a computer game on a phone/tablet/PC to receive a score for a potential
diagnosis with
a neurological disorder. This system and method provides an efficient and
convenient
diagnosis neurological disorders and comorbid mental disorders. This can help
the patients
and their treating physicians address neurological and mental complaints.
FIG. 12 is a schematic flow illustration of the system and method of the
present
disclosure for use in connection with Parkinson's disease. As can be seen, the
flow of FIG.
12 is similar to the that shown in FIG. 1 and like portions function in a like
manner. The
flow diagram 200 shows a cognitive module 201 and a computational module 202.
The
cognitive module 201 shows feedback-based probabilistic classifications (FPCT)
204
including accuracy 210 with positive feedback and negative feedback, accuracy
processing
bits 212 and response time 214 with positive feedback and negative feedback.
The cognitive
module 201 is in the form of a cognitive computer task. The computational
module 202
includes a reinforcement learning module (RLM) 208 that calculates positive
learning rates,
negative learning rates, perseveration, exploration / exploitation, valuation
of positive
feedback and valuation of negative feedback. The drift diffusion module (DDM)
208
calculates drift rate, threshold separation, non-decision time, exploration /
exploitation,
starting point and difference in decision time. The computational module 202
scales up the
data from the cognitive module 201 to generate more dimensions or features.
The classification algorithm 216 distinguishes between subjects with
Parkinson's
disease and healthy subjects based on cognitive predictors, including positive
feedback
accuracy, negative feedback accuracy and response time to negative feedback,
and based on
computational predictors, including learning noise, perseveration and positive
feedback drift
rate. The classification algorithm also distinguishes between subjects with
Parkinson's
disease that have and do not have comorbid mental disorder based on cognitive
predictors
including response time to negative feedback, and based on computational
predictors,
including perseveration and positive feedback drift rate. An algorithm
training component
220 is used to process and store data acquired over time and includes
attributes 222 for
FPCT, RLM and DDM, dimension modulation 224 such as random tree embedding to
determine the separation line of those having Parkinson's disease and those
that do not, and
cross validation 226 where the process is repeated to increase certainty.
Other components 230 can include, for FPCT, multiple feedback values wherein
the
reward / punishment values can be modified to accumulate more data for quicker
and more
efficient diagnosis, multitude of stimuli wherein the computer task can be
changed based on
CA 03157380 2022-04-07
WO 2021/072084 PCT/US2020/054796
the performance of the subject, conflict trials wherein the computer task can
be optimized
for a subject, and reversal trials which can assess control issues and control
of inhibitions.
Other components 230 can include RLM stimulus and feedback and DDM stimulus
and
feedback. Other components can also include classification algorithms,
including logistic
regression, support vector machine (SVM) which looks for plane of separation
of subjects
and random forest which includes multiple decision trees.
When a subject performs the computer-based task in the cognitive module 201,
five
cognitive attributes are generated, including: positive feedback accuracy,
negative feedback
accuracy, feedback bias accuracy, positive feedback response time, and
negative feedback
response time. These cognitive attributes are analyzed by RLM and DDM
computational
models in the computational module 202 to produce 12 computational attributes.
In
particular, RLM models 208 produce the following attributes: positive learning
rate,
negative learning rate, perseveration, noise, and valuation of feedback. DDM
models 206
produce the following attributes: drift rate, threshold separation, non-
decision time,
difference in decision time, response speed difference, and starting point.
The cognitive and
computational results from the training dataset are then used to train a
classification
algorithm, such as logistic regression, support-vector machines, decision
trees, or random
forest. Training confirms the attributes that will contribute to the most
efficient classification
process. Cross-validation approaches are then used to confirm that the trained
model can
sufficiently classify all of the assigned categories and can be generalized to
new data with
similar properties. The trained algorithm is then used as the classification
algorithm on new
data from new subjects.
Using data collected from a short (-10 minutes) FPCT that allows for the
dissociation
of learning from positive versus negative feedback alongside mathematical
models to extract
additional measures, PD can be diagnosed and an assessment can be made about
whether
patients have comorbid clinical depression (PD-MDD). The collected cognitive
data
(accuracy of choices and response time) are processed using two computational
models: (1)
A Q-learning RLM to assess parameters related to learning accuracy, and (2) A
DDM to
assess parameters related to response time distributions. Cognitive data from
the FPCT and
parameters from the two computational models are then fed into a multinomial
logistic
regression model that can differentiate PD patients from healthy subjects in
virtually all of
the cases. Further, these results can differentiate PD patients with PDD in
virtually all of the
CA 03157380 2022-04-07
WO 2021/072084 PCT/US2020/054796
21
cases. If there is a determination that the subject has PD and/or PD-MDD, the
subject can
be further evaluated and/or provided with medical treatment.
The parameters that differentiate healthy and PD subjects include:
COGNITIVELY (1) Learning accuracy from positive feedback, (2) Learning
accuracy from negative feedback, and (3) Response time to negative feedback;
COMPUTATIONALLY: (1) Learning noise, (2) Perseveration, (3) Positive
feedback drift-rate, and (4) Non-decision time parameters.
The parameters that differentiate PD patients with PDD include:
COGNITIVELY: (1) Response time to negative feedback;
COMPUTATIONALLY: (1) Learning noise, (2) Positive feedback drift-rate
parameters.
FIG. 13 is a drawing showing a classification graph for tests conducted in
connection
with the system of the present disclosure to differentiate PD from PD-MDD and
FIG. 14 is
a drawing showing a classification graph for tests conducted in connection
with the system
of the present disclosure to differentiate PD from a healthy subject. As can
be seen in FIG.
13, a forward binomial logistic regression classification graph shows a
predicted probability
of membership for PD-MDD where the cutoff value can be .50 and each symbol
represents
two cases. In FIG. 13, M denotes PD-MDD and P denotes PD. As can be seen in
FIG. 14,
a forward binomial logistic regression classification graph shows a predicted
probability of
membership for PD where the cutoff value can be .50 and each symbol represents
two cases.
In FIG. 14, P denotes PD and H denotes a healthy subject.
Having thus described the system and method in detail, it is to be understood
that the
foregoing description is not intended to limit the spirit or scope thereof It
will be understood
that the embodiments of the present disclosure described herein are merely
exemplary and
that a person skilled in the art may make any variations and modification
without departing
from the spirit and scope of the disclosure. All such variations and
modifications, including
those discussed above, are intended to be included within the scope of the
disclosure.