Note: Descriptions are shown in the official language in which they were submitted.
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
NANO-ENABLED IMMUNOTHERAPY IN CANCER
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims benefit of and priority to USSN
16/687,368 , filed on
November 18, 2019, and to USSN 62/914,950, filed on October 14, 2019 both of
which are
incorporated herein by reference in their entirety for all purposes.
STATEMENT OF GOVERNMENTAL SUPPORT
[0002] This invention was made with government support under Grant
Number
CA198846, awarded by the National Institutes of Health. The Government has
certain rights
in the invention.
BACKGROUND
[0003] While treatment of patients with localized breast cancer (BC)
has a survival
rate of ¨98%, the Breast Cancer Coalition has pointed out that there is
marginal improvement
on mortality rate since 1975 (DeSantis et al. (2017) CA Cancer J Clin. 67: 439-
448). This is
particularly true for metastatic disease, where none of the current treatments
(e.g., radiation,
chemotherapy, and estrogen blockers) are capable of eliminating BC once
metastatic spread
has taken place (Howlader et al. (eds). SEER Cancer Statistics Review, 1975-
2010, Nat.
Cancer Inst. Bethesda, MD, seer.cancer.govicsr/1975_2010/, based on November
2012 SEER
data submission, posted to the SEER web site, April 2013). Consequently, there
is no
recognized cure for metastatic disease, which is responsible for ¨90% of BC
mortality.
[0004] Pancreatic ductal adenocarcinoma (PDAC) is an almost uniformly fatal
disease with a 5-year survival outcome of less than 6% (American Cancer
Society, Cancer
Facts & Figures 2014, Atlanta: American Cancer Society; 2014). In spite of
this dismal
prognosis, the introduction of commercial nanocarriers providing paclitaxel
(PTX) or
irinotecan delivery has had some survival impact (Frese et al. 92012) Cancer
Discov. 2(3):
260-269; Passero et al. (2016) Exp. Rev. Anticancer Therap., 16(7): 697-703).
Thus, while PTX
delivery by an albumin-nanocarrier can suppress the drug-resistant tumor
stroma, allowing
increased gemcitabine uptake, the delivery of irinotecan by a liposome can
improve drug
pharmacokinetics. Moreover, our own studies using mesoporous silica
nanoparticles
(MSNP) have shown in a robust orthotopic PDAC animal model that it is
possible, in one
formulation, to include smart-design features to improve irinotecan loading
efficacy, carrier
1
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
stability and safety over a commercial liposomal equivalent, while a second
approach was to
develop a ratiometric-designed drug carrier for contemporaneous and
synergistic delivery of
PTX and gemcitabine (Liu et al. (2016) ACS Nano, 10(2): 2702-2715; Meng et al.
(2015)
ACS Nano, 9(4): 3540-3557).
[0005] In spite of this bleak picture, newfound optimism has emerged with
the advent
of cancer immunotherapy, where the power of T-cell immunity can be invoked to
treat solid
cancers, including, inter alia, breast cancer, and pancreatic cancer. This is
best exemplified
by the use of immune checkpoint blocking antibodies, that have changed the
treatment
landscape for melanoma and non-small cell lung cancer (NSCLC). However, in
spite of this
accomplishment, the overall response rate is only 20-30%, without clear
guidance to identify
responders.
SUMMARY
[0006] To increase the number of responders in the treatment of
cancers (e.g., breast
cancer), an important strategy that we exploited is to to convert immune
deplete into immune
replete ("hot") tumors as a prelude to further immunomodulatory therapy. One
approach was
to induce immunogenic conditions at the tumor site by via induced cell death
(ICD). ICD is a
specialized form of tumor cell death (Kroemer et al. (2013) Ann. Rev.
Immunol., 31: 51-72)
that can be triggered by specific chemotherapeutic drugs (e.g. anthracyclines,
taxanes,
oxaliplatin, mitoxantrone), radiation therapy, or cytotoxic viruses. ICD
facilitates tumor
antigen cross-presentation in. dendritic cells as a result of calreticulin
(CRT) expression on
the dying tumor cell surface (see, e.g., Figure 1). CRT expression provides an
"eat-me"
signal for dendritic cell uptake via the CD91 receptor. In addition, the
stepwise release of
adjuvant stimuli, including HMGB-1 (a TLR-4 ligand) and/or ATP (a signal that
activates the
NRLP3 inflammasome), allows dendritic cell maturation and antigen presentation
to naive T-
cells at the tumor site and regional lymph nodes (Kroemer et al. (2013) Ann.
Rev. Immunol.,
31: 51-72; Kepp et al. (2014) Oncoimmunol., 3(9): e955691). This response is
frequently
accompanied by a reduced number of Tregs.
[0007] We proposed that ICD will allow more predictable induction of
an immune
replete status to allow receptor-mediated blockade or perturbation of other
immune
surveillance pathways to induce durable anti-tumor immunity, which also takes
care of
metastases. As such, ICD can strengthen the effect of immune checkpoint
blocking
antibodies as well as indoleamine 2,3-dioxygenase (IDO) inhibitors that
interfere in this
2
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
metabolic immune surveillance pathway. Thus, ICD provides a deliberate means
of initiating
an immune "hot" start for subsequent response boosting by metabolic and immune
checkpoint inhibitors.
[0008] In addition to reversal of immune suppression by receptor
blocking antibodies
to CTLA-4, PD-1 and PD-L1, the IDO pathway is a relevant metabolic immune
checkpoint
pathway in breast cancer (and other cancers such as pancreatic and colon
cancer) because of
its overexpression at the tumor site. IDO-1 is the first and rate-limiting
enzymatic step in the
catabolism of tryptophan in the kynurenine pathway, and exerts potent
immunosuppressive
effects as a result of the metabolic disturbance of the amino acid ratios
(see, e.g., Prendergast
et al. (2017) Canc. Res., 77(24): 6795-6811; Lob et al. (2009) Nat. Rev.
Cancer, 9:445-452).
This allows the IDO effector pathway to control the activity of the mTOR
pathway (T-cell
activation); activation of the aryl hydrocarbon receptor (AhR) pathway;
activation of GCN2
(general control nondereressible), a serine/threonine-protein kinase that
senses amino acid
deficiency; and development of Tregs. As a result, IDO exerts strong
immunosuppressive
effects in the TME and regional lymph nodes, culminating in T-cell anergy,
decreased
cytotoxic killing, and increased accumulation of Tregs at the tumor site
(Prendergast et al.
(2014) Cancer lmmunol. Immunother. 63: 721-735; Lob et al. (2009) Nat. Rev.
Cancer, 9:
445-452). The increased expression of IDO is closely associated with the
clinical stage and
lymph node metastases in patients with breast cancer.
[0009] While a number of small molecule IDO pathway inhibitors have
emerged, one
of the best studied examples is 1-methyl-tryptophan, a.k.a. indoximod (IND).
Although IND
has been shown to improve the impact of paclitaxel in a mouse BC model, its
modest impact
as an adjuvant in human cancer studies has raised concerns about its clinical
efficacy. Our
own animal studies have demonstrated that the water insolubility of IND
contributes to an
unfavorable PK, short, circulatory half-life and inadequate tumor retention to
effectively
interfere in in the activity of MO, which is overexpressed at the tumor site.
This served as
the impetus to design nanocarriers into which IDO pathway inhibitors (e.g.,
IND) could be
co-delivered with ICD inducers (e.g., doxorubicin, mitoxantrone, etc.).
[0010] In one illustrative, but non-limiting embodiment, this goal was
accomplished
by synthesizing an IDO pathway inhibitor prodrug where the IDO inhibitor
(e.g., indoximod)
was conjugated to a lipid moiety (e.g., cholesterol or a phospholipid) that
can be assembled
into a lipid bilayer which can in turn be incorporated into a drug delivlery
vehicle (e.g., a
liposome). In one illustrative embodiment, this can be accomplished by
synthesizing IND as
3
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
a phospholipid-conjugated (or cholesterol-conjugated) prodrug that can self-
assembles to
form a nanovesicle (e.g., a liposome). Not only did the conlugated IND-lipid
bilayer exert a
major effect on tumor cell IND levels, but it also provided the lipid bilayer
backbone of the
carrier into which an ICD inducer (e.g.. DOX, mitoxantrone (MTX), etc.) can be
loaded, e.g.,
by remote import.
[0011] It is believed that a doxorubicin (DOX) or mitoxantrone (MTX)
encapsulating
nanocarrier provides a more potent ICD stimulus than the free drug, and can do
so
synergistically with a small molecule inhibitor (e.g., indoximod) of the IDO-1
pathway. It is
believed the nanocarrier is capable of facilitating this task, in part, by
improving the PK of
DOX and indoximod (IND) at the tumor site. This can provide a next generation
nanocarrier
providing an ICD stimulus and an IDO inhibitor as a promising synergistic
immunotherapy
platform for BC, including triple negative BC (TNBC) (most responsive to
immune
checkpoint inhibitors) as well as ER-positive tumors (numerically the largest
BC subtype
responsible for mortality) and other cancers (e.g., PDAC, and colon cancer).
[0012] Accordingly, in certain embodiments, compositions and methods are
provided
for systemic and/or for local (pen- or intratumoral) delivery of one or more
ICD-inducing
agents (e.g., doxorubicin, oxaliplatin, etc.) in conjunction with delivery of
an inhibitor of the
IDO pathway (e.g., indoximod). In certain embodiments the IDO inhibitor is
conjugated to a
nanovesicle-forming moiety (e.g., comprising a phospholipid bilayer). In still
another
embodiment, methods and compositions are provided where an ICD-inducing agent
(e.g.,
oxaliplatin, doxorubicin, mitoxantrone, irinotecan etc.) and an IDO inhibiting
agent (e.g., an
IDO inhibitor -prodrug) are integrated into a nanocarrier, that allows
systemic delivery to a
cancer site. Additioanlly, in certain embodiments, compositions and methods
are provided
for the treatment or prevention of a cancer via vaccination (e.g.,
subcutaneous vaccination),
utilizing certain cancer cells (e.g., drug-treated cancer cells) in which ICD
has been induced
ex vivo.
[0013] Various embodiments contemplated herein may include, but need
not be
limited to, one or more of the following:
[0014] Embodiment 1: A composition comprising an IDO inhibitor
conjugated to a
moiety that forms a nanovesicle in aqueous solution.
4
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0015] Embodiment 2: The composition of embodiment 1, wherein said IDO
inhibitor is conjugated to a moiety selected from the group consisting of a
lipid, a
phospholipid, vitamin E, cholesterol, and a fatty acid.
[0016] Embodiment 3: The composition according to any one of
embodiments 1-2,
wherein IDO inhibitor is conjugated directly to said moiety.
[0017] Embodiment 4: The composition according to any one of
embodiments 1-2,
wherein IDO inhibitor is conjugated to said moiety via a linker.
[0018] Embodiment 5: The composition of embodiment 4, wherein said IDO
inhibitor is conjugated to said moiety via an HO-(CH2)õ=2_5-0H linker.
[0019] Embodiment 6: The composition according to any one of embodiments 2-
5,
wherein said IDO inhibitor is conjugated to cholesterol (CHOL).
[0020] Embodiment 7: The composition according to any one of
embodiments 1-6,
wherein said IDO inhibitor comprises an agent selected from the group
consisting of D-1-
methyl-tryptophan (indoximod, D-1MT), L-1-methyl-tryptophan (L-1MT), a mixture
of D-
1MT and L-1MT, 1-methyl-L-tryptophan (L-1MT), methylthiohydantoin-dl-
tryptophan
(MTH-Trp, Necrostatin), 0-carbolines (e.g., 3-buty143-carboline),
Naphthoquinone-based
(e.g., annulin-B), S-allyl-brassinin, S-benzyl-brassinin, N-[2-(Indo1-3-
yeethyll-S-methyl-
dithiocarbamate, N-[2-(benzo[b]thiophen-3-yl)ethyll-S-methyl-dithiocarbamate,
N43-(Indo1-
3-yl)propyl]-S-methyl-dithiocarbamate, S-hexyl-brassinin, N-[2-(indo1-3-
yl)ethyl]-S-benzyl-
dithiocarbamate, N-[2-(indo1-3-yl)ethyll-S[(naphth-2-y1)methyll-
dithiocarbamate, N-[2-
(indo1-3-ypethyll-S-[(pyrid-3-yl)methyll-dithiocarbamate, N-[2-(indo1-3-
yl)ethyll-S-[(pyrid-
4-y1)methyll-dithiocarbamate, 5-bromo-brassinin, Phenylimidazole-based IDO
inhibitors
(e.g., 4-phenylimidazole), Exiguamine A, imidodicarbonimidic diamide,N-methyl-
N-9-
phenanthrenyl-monohydrochloride (NSC401366), INCB024360 (Epacadostat), 1-
cyclohexy1-2-(5H-imidazo115,1-alisoindol-5-yl)ethanol (GDC-0919), ID01-derived
peptide,
NLG919, Ebselen, Pyridoxal Isonicotinoyl Hydrazone, Norharmane, CAY10581, 2-
Benzy1-
2-thiopseudourea hydrochloride, and 4-phenylimidazole.
[0021] Embodiment 8: The composition of embodiment 7, wherein said IDO
inhibitor comprises 1-methyl-tryptophan.
[0022] Embodiment 9: The composition of embodiment 8, wherein said IDO
inhibitor comprises a D isomer of 1-methyl-tryptophpan.
5
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0023] Embodiment 10: The composition of embodiment 8, wherein said
IDO
inhibitor comprises an L isomer of 1-methyl-tryptophpan.
[0024] Embodiment 11: The composition of embodiment 8, wherein said
IDO
inhibitor comprises a mixture of D and L isomers of 1-methyl-tryptophpan.
[0025] Embodiment 12: The composition of embodiment 8, wherein the IDO
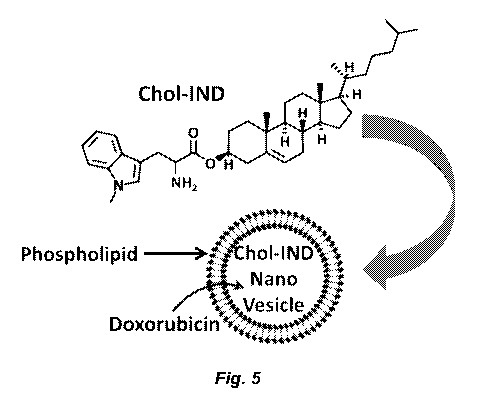
inhibitor conjugated to cholesterol comprises a compound having the structure:
Chol-IND-NH2 H
(free base) H
(4.
H 1-1
01-v)
N NHz
=
[0026] Embodiment 13: The composition of embodiment 8, wherein the IDO
inhibitor conjugated to cholesterol comprises a compound having the structure:
Chol-IND-N H2 H
(free base) ,*H
0
H H
0 H
N NH2
fl
6
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0027] Embodiment 14: The composition of embodiment 8, wherein the IDO
inhibitor conjugated to cholesterol comprises a compound having the structure:
Chol-IND-NH2 40 H
.<õ
(free base) , H
filil' ,
0 .,
H H
OH
NH2
i .
[0028] Embodiment 15: The composition according to any one of
embodiments 1-14,
wherein the conjugated IDO inhibitor forms a component of a vesicle.
[0029] Embodiment 16: A nanovesicle drug carrier for the combined
delivery of an
IDO inhibitor and an inducer of immunogenic cell death (ICD), said nanovesicle
drug carrier
comprising: a lipid vesicle wherein said lipid vesicle comprises a lipid
effective to form a
vesicle comprising a lipid bilayer in an aqueous solution, where said lipid
bilayer comprises a
composition according to any one of embodiments 1-15; and a cargo within said
vesicle
where said cargo comprises an agent that induces immunogenic cell death (ICD)
(ICD-
inducer).
[0030] Embodiment 17: The nanovesicle drug carrier of embodiment 16,
wherein
said drug carrier contains a predefined ratio of IDO inhibitor to ICD-inducer.
[0031] Embodiment 18: The nanovesicle drug carrier of according to any one
of
embodiments 16-17, wherein said lipid bilayer comprises a phospholipid, and
cholesterol
(CHOL).
[0032] Embodiment 19: The nanovesicle drug carrier according to any
one of
embodiments 16-18, wherein said lipid bilayer comprises a phospholipid, and
cholesterol-
IND (Chol-IND).
[0033] Embodiment 20: The nanovesicle drug carrier according to any
one of
embodiments 18-19, wherein said phospholipid comprises a saturated fatty acid
with a C14-
C20 carbon chain, and/or an unsaturated fatty acid with a C14-C20 carbon
chain, and/or a
natural lipid comprising a mixture of fatty acids with C12-C20 carbon chains.
7
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0034] Embodiment 21: The nanovesicle drug carrier of embodiment 20,
wherein
said phospholipid comprises a phospholipid selected from the group consisting
of
phosphatidylcholine (DPPC), 1,2-dimyristoleoyl-sn-glycero-3-phosphocholine
(DMPC), 1,2-
dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-distearoyl-sn-glycero-3-
phospho-rac-
glycerol (DSPG), 1,2-dipalmitoyl-sn-glycero-3-phosphoglycerol (DPPG),
distearoylphosphatidylcholine (DSPC), 1,2-dieicosenoyl-sn-glycero-3-
phosphocholine, and
diactylphosphatidylcholine (DAPC).
[0035] Embodiment 22: The nanovesicle drug carrier of embodiment 20,
wherein
said phospholipid comprises a natural lipid selected from the group consisting
of egg
phosphatidylcholine (egg PC), and soy phosphatidylcholine (soy PC).
[0036] Embodiment 23: The nanovesicle drug carrier of embodiment 20,
wherein
said phospholipid comprises distearoylphosphatidylcholine (DSPC).
[0037] Embodiment 24: The nanovesicle drug carrier according to any
one of
embodiments 19-23, wherein said lipid bilayer comprises an mPEG phospholipid
with a
phospholipid C14-C18 carbon chain, and a PEG molecular weight ranging from
about 350
Da to 5000 Da.
[0038] Embodiment 25: The nanovesicle drug carrier of embodiment 24,
wherein
said lipid bilayer comprises 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-
PEG (DSPE-
PEG).
[0039] Embodiment 26: The nanovesicle drug carrier of embodiment 25,
wherein
said DSPE-PEG comprises DPSE-PEG2K.
[0040] Embodiment 27: The nanovesicle drug carrier of embodiment 25,
wherein
said DSPE-PEG comprises DPSE-PEG5K.
[0041] Embodiment 28: The nanovesicle drug carrier according to any
one of
embodiments 23-27, wherein said lipid bilayer comprises DSPC : Chol-IND : DSPE-
PEG.
[0042] Embodiment 29: The nanovesicle drug carrier of embodiment 28,
wherein the
ratio of DSPC:Chol-IND:DSPE-PEG ranges from 40-90% DSPC: 10%-50% Chol-IND :
1%-10% DSPE-PEG (molar ratio).
[0043] Embodiment 30: The nanovesicle drug carrier of embodiment 29,
wherein the
ratio of DSPC:Chol-IND:DSPE-PEG is about 50:40:5 (molar ratio).
8
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0044] Embodiment 31: The nanovesicle drug carrier according to any
one of
embodiments 23-27, wherein said lipid bilayer comprises DPPG : Chol-IND : DSPE-
PEG.
[0045] Embodiment 32: The nanovesicle drug carrier of embodiment 31,
wherein the
ratio of DPPG:Chol-IND:DSPE-PEG ranges from 40-90% DPPG : 10%-50% Chol-IND :
1%-10% DSPE-PEG (molar ratio).
[0046] Embodiment 33: The nanovesicle drug carrier of embodiment 32,
wherein the
ratio of DPPG:Chol-IND:DSPE-PEG is about 50:40:5 (molar ratio).
[0047] Embodiment 34: The nanovesicle drug carrier according to any
one of
embodiments 18-33, whrein said lipid bilayer comprises a cholesterol
derivative selected
from the group consisting of cholesterol hemisuccinate (CHEMS), lysine-based
cholesterol
(CHLYS), and PEGylated cholesterol (Chol-PEG).
[0048] Embodiment 35: The nanovesicle drug carrier of embodiment 34,
wherein
said lipid bilayer comprises CHEMS.
[0049] Embodiment 36: The nanovesicle drug carrier of embodiment 35,
wherein
.. said bilayer comprises CHEMS ranging from about 5% (mol percent) up to
about 30% total
lipid.
[0050] Embodiment 37: The nanovesicle drug carrier of embodiment 36,
wherein
said bilayer comprise about 10% or about 20% CHEMS or about 30% CHEMS or about
40%
CHEMS.
[0051] Embodiment 38: The nanovesicle drug carrier according to any one of
embodiments 16-37, wherein the IDO inhibitor conjugated to cholesterol
comprises a
compound having the structure
Cho1-IND-N112 H
(free base)
O
Of
0 H
NH2
9
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0052] Embodiment 39: The nanovesicle drug carrier of embodiment 38,
wherein the
IDO inhibitor conjugated to cholesterol comprises a compound having the
structure:
Cho-ND-NH2 H
(free base)WI
H
0
0 H H H
NH2
=
[0053] Embodiment 40: The nanovesicle drug carrier of embodiment 38,
wherein the
IDO inhibitor conjugated to cholesterol comprises a compound having the
structure:
Choi-IND-NH2
(free base)
WI"
0 H H
L
0 H
NH2
=
[0054] Embodiment 41: The nanovesicle drug carrier according to any
one of
embodiments 16-40, wherein said cargo within said vesicle comprises an agent
selected from
the group consisting of mitoxantrone (MTX), doxorubicin (DOX), oxaliplatin,
.. anthracenedione, bleomycin, bortezomib, cisplatin, daunorubicin, docetaxel,
epirubicin,
idarubicin, paclitaxel, R2016, cyclophosphamide, irinotecan and a bioreactive
nanomaterial
that induces ICD.
[0055] Embodiment 42: The nanovesicle drug carrier of embodiment 41,
wherein
said cargo comprises mitoxantrone (MTX).
[0056] Embodiment 43: The nanovesicle drug carrier of embodiment 41,
wherein
said cargo comprises oxaliplatin.
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0057] Embodiment 44: The nanovesicle drug carrier of embodiment 41,
wherein
said cargo comprises doxorubicin.
[0058] Embodiment 45: The nanovesicle drug carrier of embodiment 41,
wherein
said cargo comprises a bioreactive nanomaterial that induces ICD and/or innate
immune
activation.
[0059] Embodiment 46: The nanovesicle drug carrier of embodiment 45,
wherein
said cargo comprises a nanomaterial that induces ICD where said nanomaterial
is selected
from the group consisting of CuO, Cu2O, Sb203, As203, Bi203, P203, ZnO, TiO2,
graphene
oxide, and bioreactive 2D materials other than graphene or graphene oxide.
[0060] Embodiment 47: The nanovesicle drug carrier according to any one of
embodiments 16-46, wherein when the cargo in the nanocarrier is a weak base,
said carrier
comprises a cargo-trapping agent.
[0061] Embodiment 48: The nanovesicle drug carrier of embodiment 47,
wherein
said cargo trapping agent before reaction with the cargo drug loaded in the
vesicle, is selected
from the group consisting of citric acid, triethylammonium sucrose octasulfate
(TEA8SOS).
(NH4)9SO4, an ammonium salt, a trimethylammonium salt, and a triethylammonium
salt.
[0062] Embodiment 49: The nanovesicle drug carrier of embodiment 48,
wherein
said cargo-trapping agent before reaction with said drug is citric acid.
[0063] Embodiment 50: The nanovesicle drug carrier of embodiment 48,
wherein
said cargo-trapping agent before reaction with said drug is ammonium sulfate.
[0064] Embodiment 51: The nanovesicle drug carrier according to any
one of
embodiments 16-50, wherein said drug carrier is conjugated to a moiety
selected from the
group consisting of a targeting moiety, a fusogenic peptide, and a transport
peptide.
[0065] Embodiment 52: The nanovesicle drug carrier of embodiment 51,
wherein
said drug carrier is conjugated to a peptide that binds a receptor on a cancer
cell or tumor
blood vessel.
[0066] Embodiment 53: The nanovesicle drug carrier of embodiment 52,
wherein
said drug carrier is conjugated to an iRGD peptide.
[0067] Embodiment 54: The nanovesicle drug carrier of embodiment 52,
wherein
said drug carrier is conjugated to a targeting peptide shown in Table 5.
11
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0068] Embodiment 55: The nanovesicle drug carrier according to any
one of
embodiments 51-54, wherein said drug carrier is conjugated to transferrin,
and/or ApoE,
and/or folate.
[0069] Embodiment 56: The nanovesicle drug carrier according to any
one of
embodiments 51-55, wherein said drug carrier is conjugated to a targeting
moiety that
comprises an antibody that binds to a cancer marker.
[0070] Embodiment 57: The nanovesicle drug carrier of embodiment 56,
wherein
said drug carrier is conjugated to a targeting moiety that comprises an
antibody that binds a
cancer marker shown in Table 4.
[0071] Embodiment 58: The nanovesicle drug carrier according to any one of
embodiments 56-57, wherein said antibody is selected from the group consisting
of an intact
immunoglobulin, an F(ab)'2, a Fab, a single chain antibody, a diabody, an
affibody, a
unibody, and a nanobody.
[0072] Embodiment 59: The nanovesicle drug carrier according to any
one of
embodiments 16-58, wherein said drug carriers in suspension are stable for at
least 1 month,
or at least 2 months, or at least 3 months, or at least 4 months, or at least
5 months, or at least
6 months when stored at 4 C.
[0073] Embodiment 60: The nanovesicle drug carrier according to any
one of
embodiments 16-59, wherein said nanoparticle drug carrier forms a stable
suspension on
rehydration after lyophilization.
[0074] Embodiment 61: The nanovesicle drug carrier according to any
one of
embodiments 16-60, wherein said nanoparticle drug carriers, show reduced drug
toxicity as
compared to free drug.
[0075] Embodiment 62: The nanovesicle drug carrier according to any
one of
embodiments 16-61, wherein said nanoparticle drug carrier has colloidal
stability in
physiological fluids with pH 7.4 and remains monodisperse to allow systemic
biodistribution
and is capable of entering a disease site by vascular leakage (EPR effect) or
transcytosis.
[0076] Embodiment 63: The nanovesicle drug carrier drug carrier
according to any
one of embodiments 16-62, wherein said carrier is colloidally stable.
12
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0077] Embodiment 64: The nanovesicle drug carrier according to any
one of
embodiments 16-63, wherein the IDO inhibitor and the ICD inducer are
synergistic in their
activity against a cancer.
[0078] Embodiment 65: The nanovesicle drug carrier according to any
one of
embodiments 16-64, wherein said drug carrier, when administered systemically,
delivers an
amount of an ICD inducer effective to induce or to facilitate induction of
immunogenic cell
death of a cancer cell at a tumor site.
[0079] Embodiment 66: The nanovesicle drug carrier according to any
one of
embodiments 16-65, wherein said drug carrier, when administered systemically,
delivers an
amount of an IDO inhibitor to partially or fully inhibit the IDO enzyme or IDO
pathway at a
cancer site.
[0080] Embodiment 67: A nanoparticle drug carrier for the combined
delivery of an
IDO inhibitor and an inducer of immunogenic cell death (ICD), said
nanoparticle drug carrier
comprising: a mesoporous silica nanoparticle having a surface and defining a
plurality of
pores that are suitable to receive molecules therein; a lipid bilayer coating
the surface where
said lipid bilayer comprises a composition according to any one of embodiments
1-15; and a
cargo comprising an agent that induces immunogenic cell death (ICD) (ICD-
inducer)
disposed within said mesoporous silica particle; wherein the lipid bilayer is
substantially
continuous and encapsulates said nanoparticle stably sealing the plurality of
pores.
[0081] Embodiment 68: The nanoparticle drug carrier of embodiment 67,
wherein
said nanoparticle drug carrier contains a predefined ratio of IDO inhibitor to
ICD-inducer.
[0082] Embodiment 69: The nanoparticle drug carrier according to any
one of
embodiments 67-68, wherein the IDO inhibitor and the ICD inducer are
synergistic in their
activity against a cancer.
[0083] Embodiment 70: The nanoparticle drug carrier according to any one of
embodiments 67-69, wherein said lipid bilayer comprises a phospholipid, and
cholesterol
(CHOL).
[0084] Embodiment 71: The nanoparticle drug carrier according to any
one of
embodiments 67-70, wherein said lipid bilayer comprises a phospholipid, and
cholesterol-
IND (Chol-IND).
13
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0085] Embodiment 72: The nanoparticle drug carrier according to any
one of
embodiments 70-71, wherein said phospholipid comprises a saturated fatty acid
with a C14-
C20 carbon chain, and/or an unsaturated fatty acid with a C14-C20 carbon
chain, and/or a
natural lipid comprising a mixture of fatty acids with C12-C20 carbon chains.
[0086] Embodiment 73: The nanoparticle drug carrier of embodiment 72,
wherein
said phospholipid comprises a phospholipid selected from the group consisting
of
phosphatidylcholine (DPPC), 1,2-dimyristoleoyl-sn-glycero-3-phosphocholine
(DMPC), 1,2-
dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-distearoyl-sn-glycero-3-
phospho-rac-
glycerol (DSPG), 1,2-dipahnitoyl-sn-glycero-3-phosphoglycerol (DPPG),
distearoylphosphatidylcholine (DSPC), 1,2-dieicosenoyl-sn-glycero-3-
phosphocholine, and
diactylphosphatidylcholine (DAPC).
[0087] Embodiment 74: The nanoparticle drug carrier of embodiment 72,
wherein
said phospholipid comprises a natural lipid selected from the group consisting
of egg
phosphatidylcholine (egg PC), and soy phosphatidylcholine (soy PC).
[0088] Embodiment 75: The nanoparticle drug carrier of embodiment 72,
wherein
said phospholipid comprises distearoylphosphatidylcholine (DSPC).
[0089] Embodiment 76: The nanoparticle drug carrier according to any
one of
embodiments 71-75, wherein said lipid bilayer comprises an mPEG phospholipid
with a
phospholipid C14-C18 carbon chain, and a PEG molecular weight ranging from
about 350
Da to 5000 Da.
[0090] Embodiment 77: The nanoparticle drug carrier of embodiment 76,
wherein
said lipid bilayer comprises 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-
PEG (DSPE-
PEG).
[0091] Embodiment 78: The nanoparticle drug carrier of embodiment 77,
wherein
said DSPE-PEG comprises DPSE-PEG2K.
[0092] Embodiment 79: The nanoparticle drug carrier of embodiment 77,
wherein
said DSPE-PEG comprises DPSE-PEG5K.
[0093] Embodiment 80: The nanoparticle drug carrier according to any
one of
embodiments 75-79, wherein said lipid bilayer comprises DSPC: Chol-IND : DSPE-
PEG.
14
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0094] Embodiment 81: The nanoparticle drug carrier of embodiment 80,
wherein
the ratio of DSPC:Chol-IND:DSPE-PEG ranges from 40-90% DSPC : 10%-50% Chol-IND
:
1%-10% DSPE-PEG (molar ratio).
[0095] Embodiment 82: The nanoparticle drug carrier of embodiment 81,
wherein
the ratio of DSPC:Chol-IND:DSPE-PEG is about 50:40:5 (molar ratio).
[0096] Embodiment 83: The nanoparticle drug carrier according to any
one of
embodiments 75-79, wherein said lipid bilayer comprises DPPG: Chol-IND : DSPE-
PEG.
[0097] Embodiment 84: The nanoparticle drug carrier of embodiment 83,
wherein
the ratio of DPPG:Chol-IND:DSPE-PEG ranges from 40-90% DSPC : 10%-50% Chol-IND
:
.. 1%-10% DPPG-PEG (molar ratio).
[0098] Embodiment 85: The nanoparticle drug carrier of embodiment 84,
wherein
the ratio of DPPG:Chol-IND:DSPE-PEG is about 50:40:5 (molar ratio).
[0099] Embodiment 86: The nanoparticle drug carrier according to any
one of
embodiments 67-85, whrein said lipid bilayer comprises a cholesterol
derivative selected
from the group consisting of cholesterol hemisuccinate (CHEMS), lysine-based
cholesterol
(CHLYS), and PEGylated cholesterol (Chol-PEG),
[0100] Embodiment 87: The nanoparticle drug carrier of embodiment 86,
wherein
said lipid bilayer comprises CHEMS.
[0101] Embodiment 88: The nanoparticle drug carrier of embodiment 87,
wherein
said bilayer comprises CHEMS ranging from about 5% (mol percent) up to about
30% total
lipid.
[0102] Embodiment 89: The nanoparticle drug carrier of embodiment 88,
wherein
said bilayer comprises about 10% or about 20% CHEMS or about 30% CHEMS or
about
40% CHEMS.
[0103] Embodiment 90: The nanoparticle drug carrier according to any one of
embodiments 67-89, wherein the IDO inhibitor conjugated to cholesterol
comprises a
CA 03157508 2022-04-08
WO 2021/076630 PCT/US2020/055585
compound having the structure:
Chol-IND-NH2
(free base) t H
0 . ..,
,...:õ..
I
N N H 2
i .
[0104] Embodiment 91: The nanoparticle drug carrier of embodiment 90,
wherein
the IDO inhibitor conjugated to cholesterol comprises a compound having the
structure:
Chol-IND-NH2 ,,,,, H
0
(free base) A H
0 1ft 0
A H
I õ.OH
N ' NH2
i
=
[0105] Embodiment 92: The nanoparticle drug carrier of embodiment 90,
wherein
the IDO inhibitor conjugated to cholesterol comprises a compound having the
structure:
ChOHND-NH2
(free base) H
. .... ilk
el H H
0 H
I N H2
=
[0106] Embodiment 93: The nanoparticle drug carrier according to any
one of
embodiments 67-92, wherein said cargo within said mesoporous silica
nanoparticle
16
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
comprises an agent selected from the group consisting of mitoxantrone (MTX),
doxorubicin
(DOX), oxaliplatin, anthracenedione, bleomycin, bortezomib, cisplatin,
daunorubicin.
docetaxel, irinotecan epirubicin, idarubicin, paclitaxel, R2016,
cyclophosphamide, and a
bioreactive nanomaterial that induces ICD.
[0107] Embodiment 94: The nanoparticle drug carrier of embodiment 93,
wherein
said cargo comprises mitoxantrone (MTX).
[0108] Embodiment 95: The nanoparticle drug carrier of embodiment 93,
wherein
said cargo comprises oxaliplatin.
[0109] Embodiment 96: The nanoparticle drug carrier of embodiment 93,
wherein
said cargo comprises doxorubicin.
[0110] Embodiment 97: The nanoparticle drug carrier according to any
one of
embodiments 67-96, wherein when the cargo in the nanocarrier is a weak base,
said carrier
comprises a cargo-trapping agent.
[0111] Embodiment 98: The nanoparticle drug carrier of embodiment 97,
wherein
said cargo trapping agent before reaction with the cargo drug loaded in the
vesicle, is selected
from the group consisting of citric acid, triethylammonium sucrose octasulfate
(TEA8SOS),
(NH4)2SO4, an ammonium salt, a trimethylammonium salt, and a triethylammonium
salt.
[0112] Embodiment 99: The nanoparticle drug carrier of embodiment 98,
wherein
said cargo-trapping agent before reaction with said drug is citric acid.
[0113] Embodiment 100: The nanoparticle drug carrier of embodiment 98,
wherein
said cargo-trapping agent before reaction with said drug is ammonium sulfate.
[0114] Embodiment 101: The nanoparticle drug carrier according to any
one of
embodiments 67-100, wherein said drug carrier is conjugated to a moiety
selected from the
group consisting of a targeting moiety, a fusogenic peptide, and a transport
peptide.
[0115] Embodiment 102: The nanoparticle drug carrier of embodiment 101,
wherein
said drug carrier is conjugated to a peptide that binds a receptor on a cancer
cell or tumor
blood vessel.
[0116] Embodiment 103: The nanoparticle drug carrier of embodiment
102, wherein
said drug carrier is conjugated to an iRGD peptide.
17
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0117] Embodiment 104: The nanoparticle drug carrier of embodiment
102, wherein
said drug carrier is conjugated to a targeting peptide shown in Table 5.
[0118] Embodiment 105: The nanoparticle drug carrier according to any
one of
embodiments 101-104, wherein said drug carrier is conjugated to transferrin,
and/or ApoE,
and/or folate.
[0119] Embodiment 106: The nanoparticle drug carrier according to any
one of
embodiments 101-105, wherein said drug carrier is conjugated to a targeting
moiety that
comprises an antibody that binds to a cancer marker.
[0120] Embodiment 107: The nanoparticle drug carrier of embodiment
106, wherein
said drug carrier is conjugated to a targeting moiety that comprises an
antibody that binds a
cancer marker shown in Table 4.
[0121] Embodiment 108: The nanoparticle drug carrier according to any
one of
embodiments 106-107, wherein said antibody is selected from the group
consisting of an
intact immunoglobulin, an F(ab)'2, a Fab, a single chain antibody, a diabody,
an affibody, a
unibody, and a nanobody.
[0122] Embodiment 109: The nanoparticle drug carrier according to any
one of
embodiments 67-108, wherein said drug carriers in suspension are stable for at
least 1 month,
or at least 2 months, or at least 3 months, or at least 4 months, or at least
5 months, or at least
6 months when stored at 4 C.
[0123] Embodiment 110: The nanoparticle drug carrier according to any one
of
embodiments 67-109, wherein said nanoparticle drug carrier forms a stable
suspension on
rehydration after lyophilization.
[0124] Embodiment 111: The nanoparticle drug carrier according to any
one of
embodiments 67-110, wherein said nanoparticle drug carriers, show reduced drug
toxicity as
compared to free drug.
[0125] Embodiment 112: The nanoparticle drug carrier according to any
one of
embodiments 67-111, wherein said nanoparticle drug carrier has colloidal
stability in
physiological fluids with pH 7.4 and remains monodisperse to allow systemic
biodistribution
and is capable of entering a disease site by vascular leakage (EPR effect) or
transcytosis.
[0126] Embodiment 113: The nanoparticle drug carrier drug carrier according
to any
one of embodiments 67-112, wherein said carrier is colloidally stable.
18
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0127] Embodiment 114: The nanoparticle drug carrier according to any
one of
embodiments 67-113, wherein the IDO inhibitor and the ICD inducer are
synergistic in their
activity against a cancer.
[0128] Embodiment 115: The nanoparticle drug carrier according to any
one of
.. embodiments 67-114, wherein said drug carrier, when administered
systemically, delivers an
amount of an ICD inducer effective to induce or to facilitate induction of
immunogenic cell
death of a cancer cell at a tumor site.
[0129] Embodiment 116: The nanoparticle drug carrier according to any
one of
embodiments 67-115, wherein said drug carrier, when administered systemically,
delivers an
amount of an IDO inhibitor to partially or fully inhibit the IDO enzyme or IDO
pathway at a
cancer site.
[0130] Embodiment 117: A nanomaterial carrier for the combined
delivery of an
IDO inhibitor and an inducer of immunogenic cell death (ICD), said
nanomaterial carrier
comprising: a nanomaterial that induces ICD; and a lipid or lipid formulation
comprising a
composition according to any one of embodiments 1-15, where said lipid or
lipid formulation
is disposed on the surface of said nanomaterial.
[0131] Embodiment 118: The nanomaterial carrier of embodiments 117,
wherein
said nanomaterial comprises a material selected from the group consisting of
CuO, Cu2O,
Sb203, As203, Bi203, P203, ZnO, TiO2, graphene oxide, and 2D materials other
than
graphene or graphene oxide.
[0132] Embodiment 119: The nanomaterial carrier according to any one
of
embodiments 117-118, wherein said lipid or lipid formulation fully
encapsulates said
nanomaterial.
[0133] Embodiment 120: The nanomaterial carrier according to any one
of
.. embodiments 117-119, wherein said lipid or lipid formulation is not a lipid
bilayer.
[0134] Embodiment 121: The nanomaterial carrier according to any one
of
embodiments 117-119, wherein said lipid or lipid formulation comprises a lipid
bilayer.
[0135] Embodiment 122: The nanomaterial carrier of according to any
one of
embodiments 117-121, wherein said lipid or lipid formulation comprises a
phospholipid, and
cholesterol (CHOL).
19
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0136] Embodiment 123: The nanomaterial carrier according to any one
of
embodiments 117-122, wherein said lipid or lipid formulation comprises a
phospholipid, and
cholesterol-IND (Chol-IND).
[0137] Embodiment 124: The nanomaterial carrier according to any one
of
embodiments 18-123, wherein said phospholipid comprises a saturated fatty acid
with a C14-
C20 carbon chain, and/or an unsaturated fatty acid with a C14-C20 carbon
chain, and/or a
natural lipid comprising a mixture of fatty acids with C12-C20 carbon chains.
[0138] Embodiment 125: The nanomaterial carrier of embodiment 124,
wherein said
phospholipid comprises a phospholipid selected from the group consisting of
phosphatidylcholine (DPPC), 1,2-dimyristoleoyl-sn-glycero-3-phosphocholine
(DMPC), 1,2-
dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-distearoyl-sn-glycero-3-
phospho-rac-
glycerol (DSPG), 1,2-dipahnitoyl-sn-glycero-3-phosphoglycerol (DPPG),
distearoylphosphatidylcholine (DSPC), 1,2-dieicosenoyl-sn-glycero-3-
phosphocholine, and
diactylphosphatidylcholine (DAPC).
[0139] Embodiment 126: The nanomaterial carrier of embodiment 124, wherein
said
phospholipid comprises a natural lipid selected from the group consisting of
egg
phosphatidylcholine (egg PC), and soy phosphatidylcholine (soy PC).
[0140] Embodiment 127: The nanomaterial carrier of embodiment 124,
wherein said
phospholipid comprises distearoylphosphatidylcholine (DSPC).
[0141] Embodiment 128: The nanomaterial carrier according to any one of
embodiments 123-127, wherein said lipid or lipid formulation comprises an mPEG
phospholipid with a phospholipid C14-C18 carbon chain, and a PEG molecular
weight
ranging from about 350 Da to 5000 Da.
[0142] Embodiment 129: The nanomaterial carrier of embodiment 128,
wherein said
lipid or lipid formulation comprises 1,2-distearoyl-sn-glycero-3-
phosphoethanolamine-PEG
(DSPE-PEG).
[0143] Embodiment 130: The nanomaterial carrier of embodiment 129,
wherein said
DSPE_PEG comprises DPSE-PE&K.
[0144] Embodiment 131: The nanomaterial carrier of embodiment 129,
wherein said
DSPE_PEG comprises DPSE-PEG5K.
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0145] Embodiment 132: The nanomaterial carrier according to any one
of
embodiments 127-131, wherein said lipid or lipid formulation comprises DSPC :
Chol-IND :
DSPE-PEG.
[0146] Embodiment 133: The nanomaterial carrier of embodiment 132,
wherein the
ratio of DSPC:Chol-IND:DSPE-PEG ranges from 40-90% DSPC : 10%-50% Chol-IND :
1%-10% DSPE-PEG (molar ratio).
[0147] Embodiment 134: The nanomaterial carrier of embodiment 133,
wherein the
ratio of DSPC:Chol-IND:DSPE-PEG is about 50:40:5 (molar ratio).
[0148] Embodiment 135: The nanomaterial carrier according to any one
of
embodiments 127-131, wherein said lipid or lipid formulation comprises DPPG :
Chol-IND :
DSPE-PEG.
[0149] Embodiment 136: The nanomaterial carrier of embodiment 135,
wherein the
ratio of DPPG:Chol-IND:DSPE-PEG ranges from 40-90% DPPG : 10%-50% Chol-IND :
1%-10% DSPE-PEG (molar ratio).
[0150] Embodiment 137: The nanomaterial carrier of embodiment 136, wherein
the
ratio of DPPG:Chol-IND:DSPE-PEG is about 50:40:5 (molar ratio).
[0151] Embodiment 138: The nanomaterial carrier according to any one
of
embodiments 122-137, whrein said lipid or lipid formulation comprises a
cholesterol
derivative selected from the group consisting of cholesterol hemisuccinate
(CHEMS), lysine-
based cholesterol (CHLYS), and PEGylated cholesterol (Chol-PEG).
[0152] Embodiment 139: The nanomaterial carrier of embodiment 138,
wherein said
lipid or lipid formulation comprises CHEMS.
[0153] Embodiment 140: The nanomaterial carrier of embodiment 139,
wherein said
bilayer comprises CHEMS ranging from about 5% (mol percent) up to about 30%
total lipid.
[0154] Embodiment 141: The nanomaterial carrier of embodiment 140, wherein
said
bilayer comprise about 10% or about 20% CHEMS.
[0155] Embodiment 142: The nanomaterial carrier according to any one
of
embodiments 117-141, wherein said drug carrier is conjugated to a moiety
selected from the
group consisting of a targeting moiety, a fusogenic peptide, and a transport
peptide.
21
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0156] Embodiment 143: The nanomaterial carrier of embodiment 142,
wherein said
drug carrier is conjugated to a peptide that binds a receptor on a cancer cell
or tumor blood
vessel.
[0157] Embodiment 144: The nanomaterial carrier of embodiment 143,
wherein said
.. drug carrier is conjugated to an iRGD peptide.
[0158] Embodiment 145: The nanomaterial carrier of embodiment 143,
wherein said
drug carrier is conjugated to a targeting peptide shown in Table 5.
[0159] Embodiment 146: The nanomaterial carrier according to any one
of
embodiments 142-145, wherein said drug carrier is conjugated to transferrin,
and/or ApoE,
.. and/or folate.
[0160] Embodiment 147: The nanomaterial carrier according to any one
of
embodiments 142-146, wherein said drug carrier is conjugated to a targeting
moiety that
comprises an antibody that binds to a cancer marker.
[0161] Embodiment 148: The nanomaterial carrier of embodiment 147,
wherein said
drug carrier is conjugated to a targeting moiety that comprises an antibody
that binds a cancer
marker shown in Table 4.
[0162] Embodiment 149: The nanomaterial carrier according to any one
of
embodiments 147-148, wherein said antibody is selected from the group
consisting of an
intact immunoglobulin, an F(ab)'2, a Fab, a single chain antibody, a diabody,
an affibody, a
unibody, and a nanobody.
[0163] Embodiment 150: The nanomaterial carrier according to any one
of
embodiments 117-149, wherein said drug carriers in suspension are stable for
at least 1
month, or at least 2 months, or at least 3 months, or at least 4 months, or at
least 5 months, or
at least 6 months when stored at 4 C.
[0164] Embodiment 151: The nanomaterial carrier according to any one of
embodiments 117-150, wherein said nanoparticle drug carrier forms a stable
suspension on
rehydration after lyophilization.
[0165] Embodiment 152: The nanomaterial carrier according to any one
of
embodiments 117-151, wherein said nanoparticle drug carriers, show reduced
drug toxicity as
compared to free drug.
22
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0166] Embodiment 153: The nanomaterial carrier according to any one
of
embodiments 117-152, wherein said nanoparticle drug carrier has colloidal
stability in
physiological fluids with pH 7.4 and remains monodisperse to allow systemic
biodistribution
and is capable of entering a disease site by vascular leakage (EPR effect) or
transcytosis.
[0167] Embodiment 154: The nanomaterial carrier drug carrier according to
any one
of embodiments 117-153, wherein said carrier is colloidally stable.
[0168] Embodiment 155: The nanomaterial carrier according to any one
of
embodiments 117-154, wherein the IDO inhibitor and the ICD inducer are
synergistic in their
activity against a cancer.
[0169] Embodiment 156: The nanomaterial carrier according to any one of
embodiments 117-155, wherein said drug carrier, when administered
systemically, delivers
an amount of an ICD inducer effective to induce or to facilitate induction of
immunogenic
cell death of a cancer cell at a tumor site.
[0170] Embodiment 157: The nanomaterial carrier according to any one
of
embodiments 117-156, wherein said drug carrier, when administered
systemically, delivers
an amount of an IDO inhibitor to partially or fully inhibit the IDO enzyme or
IDO pathway at
a cancer site.
[0171] Embodiment 158: A pharmaceutical formulation comprising: a
composition
according to any one of embodiments 1-15 and a pharmaceutically acceptable
carrier; and/or
a nanovesicle drug carrier according to any one of embodiments 16-66 and a
pharmaceutically acceptable carrier; and/or a nanoparticle drug carrier
according to any one
of embodiments 67-116 and a pharmaceutically acceptable carrier; and/or a
nanomaterial
carrier according to any one of embodiments 117-157 and a pharmaceutically
acceptable
carrier.
[0172] Embodiment 159: The pharmaceutical formulation of embodiment 158,
wherein said formulation comprises a composition according to any one of
embodiments 1-
15 and a pharmaceutically acceptable carrier.
[0173] Embodiment 160: The pharmaceutical formulation of embodiment
158,
wherein said formulation comprises a nanovesicle drug carrier according to any
one of
embodiments 16-66 and a pharmaceutically acceptable carrier.
23
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0174] Embodiment 161: The pharmaceutical formulation of embodiment
158,
wherein said formulation comprises a nanoparticle drug carrier according to
any one of
embodiments 67-116 and a pharmaceutically acceptable carrier.
[0175] Embodiment 162: The pharmaceutical formulation of embodiment
158,
wherein said formulation comprises a nanomaterial carrier according to any one
of
embodiments 117-157 and a pharmaceutically acceptable carrier.
[0176] Embodiment 163: The pharmaceutical formulation according to any
one of
embodiments 158-162, wherein said formulation is an emulsion, dispersion, or
suspension.
[0177] Embodiment 164: The pharmaceutical formulation of embodiment
163,
wherein said suspension, emulsion, or dispersion is stable for at least 1
month, or at least 2
months, or at least 3 months, or at least 4 months, or at least 5 months, or
at least 6 months
when stored at 4 C.
[0178] Embodiment 165: The pharmaceutical formulation according to any
one of
embodiments 158-164, wherein the nanovesicle drug carriers, and/or the a
nanoparticle drug
carriers, and/or the a nanomaterial carriers in said formulation show a
substantially unimodal
size distribution; and/or show a PDI less than about 0.2, or less than about
0.1.
[0179] Embodiment 166: The pharmaceutical formulation according to any
one of
embodiments 158-165, wherein said formulation is formulated for administration
via a route
selected from the group consisting of intravenous administration,
intraarterial administration,
intracerebral administration, intrathecal administration, oral administration,
aerosol
administration, administration via inhalation (including intranasal and
intratracheal delivery,
intracranial administration via a cannula, and subcutaneous or intramuscular
depot
deposition.
[0180] Embodiment 167: The pharmaceutical formulation according to any
one of
embodiments 158-165, wherein said formulation is a sterile injectable.
[0181] Embodiment 168: The pharmaceutical formulation according to any
one of
embodiments 158-167, wherein said formulation is a unit dosage formulation.
[0182] Embodiment 169: A method of treating a cancer, said method
comprising:
administering to a subject in need thereof an effective amount of: a
composition according to
any one of embodiments 1-15; and/or a nanovesicle drug carrier according to
any one of
24
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
embodiments 16-66; and/or a nanoparticle drug carrier according to any one of
embodiments
67-116; and/or a nanomaterial carrier according to any one of embodiments 117-
157.
[0183] Embodiment 170: The method of embodiment 169, wherein said
method
comprises administering to a subject in need thereof an effective amount of a
nanovesicle
drug carrier according to any one of embodiments 16-66.
[0184] Embodiment 171: The method of embodiment 169, wherein said
method
comprises administering to a subject in need thereof an effective amount of a
nanoparticle
drug carrier according to any one of embodiments 67-116.
[0185] Embodiment 172: The method of embodiment 169, wherein said
method
comprises administering to a subject in need thereof an effective amount of a
nanomaterial
carrier according to any one of embodiments 117-157.
[0186] Embodiment 173: The method according to any one of embodiments
170-
172, wherein the ICD inducer and the IDO inhibitor are synergistic in their
activity against
said cancer.
[0187] Embodiment 174: The method according to any one of embodiments 170-
173, wherein said ICD-inducer is in an amount effective to elevate
calreticulin (CRT)
expression in cells of said cancer.
[0188] Embodiment 175: The method according to any one of embodiments
170-
174, wherein said ICD-inducer is in an amount effective to elevate expression
and/or release
of HMGB1 and/or induction of ATP release.
[0189] Embodiment 176: The method according to any one of embodiments
170-
175, wherein said method comprises a primary therapy in a chemotherapeutic
regimen.
[0190] Embodiment 177: The method according to any one of embodiments
170-
175, wherein said method comprises an adjunct therapy in a treatment regime
that
additionally comprises chemotherapy using another chemotherapeutic agent,
and/or surgical
resection of a tumor mass, and/or radiotherapy.
[0191] Embodiment 178: The method according to any one of embodiments
170-
177, wherein said composition, a nanovesicle drug carrier, a nanoparticle drug
carrier
according, and/or nanomaterial carrier is a component in a multi-drug
chemotherapeutic
regimen.
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0192] Embodiment 179: The method according to any one of embodiments
170-
178, wherein said cancer is pancreatic ductal adenocarcinoma (PDAC).
[0193] Embodiment 180: The method according to any one of embodiments
170-
178, wherein said cancer is a cancer selected from the group consisting of
breast cancer, lung
cancer, melanoma, pancreas cancer, liver cancer, acute lymphoblastic leukemia
(ALL), acute
myeloid leukemia (AML), adrenocortical carcinoma, AIDS-related cancers (e.g.,
Kaposi
sarcoma, lymphoma), anal cancer, appendix cancer, astrocytomas, atypical
teratoid/rhabdoid
tumor, bile duct cancer, extrahepatic cancer, bladder cancer, bone cancer
(e.g., Ewing
sarcoma, osteosarcoma, malignant fibrous histiocytoma), brain stem glioma,
brain tumors
(e.g., astrocytomas, brain and spinal cord tumors, brain stem glioma, central
nervous system
atypical teratoid/rhabdoid tumor, central nervous system embryonal tumors,
central nervous
system germ cell tumors, craniopharyngioma, ependymoma, burkitt lymphoma,
carcinoid
tumors (e.g., childhood, gastrointestinal), cardiac tumors, cervical cancer,
chordoma, chronic
lymphocytic leukemia (CLL), chronic myelogenous leukemia (CML), chronic
myeloproliferative disorders, colon cancer, colorectal cancer,
craniopharyngioma, cutaneous
t-cell lymphoma, duct cancers e.g. (bile, extrahepatic), ductal carcinoma in
situ (DCIS),
embryonal tumors, endometrial cancer, ependymoma, esophageal cancer,
esthesioneuroblastoma, extracranial germ cell tumor, extragonadal germ cell
tumor,
extrahepatic bile duct cancer, eye cancer (e.g., intraocular melanoma,
retinoblastoma), fibrous
histiocytoma of bone, malignant, and osteosarcoma, gallbladder cancer, gastric
(stomach)
cancer, gastrointestinal carcinoid tumor, gastrointestinal stromal tumors
(GIST), germ cell
tumors (e.g., ovarian cancer, testicular cancer, extracranial cancers,
extragonadal cancers,
central nervous system), gestational trophoblastic tumor, brain stem cancer,
hairy cell
leukemia, head and neck cancer, heart cancer, hepatocellular (liver) cancer,
histiocytosis,
langerhans cell cancer, Hodgkin lymphoma, hypopharyngeal cancer, intraocular
melanoma,
islet cell tumors, pancreatic neuroendocrine tumors, kaposi sarcoma, kidney
cancer (e.g.,
renal cell, Wilm's tumor, and other kidney tumors), langerhans cell
histiocytosis, laryngeal
cancer, leukemia, acute lymphoblastic (ALL), acute myeloid (AML), chronic
lymphocytic
(CLL), chronic myelogenous (CML), hairy cell, lip and oral cavity cancer,
liver cancer
(primary), lobular carcinoma in situ (LCIS), lung cancer (e.g., childhood, non-
small cell,
small cell), lymphoma (e.g., AIDS-related, Burkitt (e.g., non-Hodgkin
lymphoma), cutaneous
T-Cell (e.g., mycosis fungoides, Sezary syndrome), Hodgkin, non-Hodgkin,
primary central
nervous system (CNS)), macroglobulinemia, Waldenstrom, male breast cancer,
malignant
26
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
fibrous histiocytoma of bone and osteosarcoma, melanoma (e.g., childhood,
intraocular
(eye)), merkel cell carcinoma, mesothelioma, metastatic squamous neck cancer,
midline tract
carcinoma, mouth cancer, multiple endocrine neoplasia syndromes, multiple
myeloma/plasma cell neoplasm, mycosis fungoides, myelodysplastic syndromes,
Myelogenous Leukemia, Chronic (CML), multiple myeloma, nasal cavity and
paranasal sinus
cancer, nasopharyngeal cancer, neuroblastoma, oral cavity cancer, lip and
oropharyngeal
cancer, osteosarcoma, ovarian cancer, pancreatic cancer, pancreatic
neuroendocrine tumors
(islet cell tumors), papillomatosis, paraganglioma, paranasal sinus and nasal
cavity cancer,
parathyroid cancer, penile cancer, pharyngeal cancer, pheochromocytoma,
pituitary tumor,
plasma cell neoplasm, pleuropulmonary blastoma, primary central nervous system
(CNS)
lymphoma, prostate cancer, rectal cancer, renal cell (kidney) cancer, renal
pelvis and ureter,
transitional cell cancer, rhabdomyosarcoma, salivary gland cancer, sarcoma
(e.g., Ewing,
Kaposi, osteosarcoma, rhadomyosarcoma, soft tissue, uterine), Sezary syndrome,
skin cancer
(e.g., melanoma, merkel cell carcinoma, basal cell carcinoma, nonmelanoma),
small intestine
cancer, squamous cell carcinoma, squamous neck cancer with occult primary,
stomach
(gastric) cancer, testicular cancer, throat cancer, thymoma and thymic
carcinoma, thyroid
cancer, trophoblastic tumor, ureter and renal pelvis cancer, urethral cancer,
uterine cancer,
endometrial cancer, uterine sarcoma, vaginal cancer, vulvar cancer,
Waldenstrom
macroglobulinemia, and Warn's tumor.
[0194] Embodiment 181: The method according to any one of embodiments 170-
180, wherein said administration is via a route selected from the group
consisting of
intravenous administration, intraarterial administration, intracerebral
administration,
intrathecal administration, oral administration, aerosol administration,
administration via
inhalation (including intranasal and intratracheal delivery, intracranial
administration via a
cannula, and subcutaneous or intramuscular depot deposition.
[0195] Embodiment 182: The method according to any one of embodiments
170-
180, wherein said administration comprises systemic administration via
injection or cannula.
[0196] Embodiment 183: The method according to any one of embodiments
170-
180, wherein said administration is administration to an intra-tumoral or peri-
tumoral site.
[0197] Embodiment 184: The method according to any one of embodiments 170-
183, wherein said mammal is a human.
27
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0198] Embodiment 185: The method according to any one of embodiments
170-
183, wherein said mammal is a non-human mammal.
[0199] Embodiment 186: The method according to any one of embodiments
170-
185, wherein said nanovesicle drug carrier is administered in conjunction with
administration
of an immune checkpoint inhibitor.
[0200] Embodiment 187: The method of embodiment 186, wherein said
immune
checkpoint inhibitor comprises an inhibitor of PD-1, PD-L1, PD-L2, PD-L3, PD-
L4, CTLA-
4, LAG3, B7-H3, B7-H4, KIR and/or TIM3.
[0201] Embodiment 188: The method of embodiment 187, wherein said
checkpoint
inhibitor comprises an antibody that inhibits a moiety selected from the group
consisting of
PD-1, PD-L1, and CTLA4.
[0202] Embodiment 189: The method of embodiment 188, wherein said
antibody
comprises an antibody that inhibits PD-1.
[0203] Embodiment 190: The method of embodiment 189, wherein said
antibody
comprises Pembrolizumab (Keytruda), or Nivolumab (Opdivo).
[0204] Embodiment 191: The method of embodiment 188, wherein said
antibody
comprises an antibody that inhibits PD-Li.
[0205] Embodiment 192: The method of embodiment 191, wherein said
antibody
comprises Atezolizumab (Tecentriq), Avelumab (Bavencio), or Durvalumab
(Imfinzi).
[0206] Embodiment 193: The method of embodiment 188, wherein said antibody
comprises an antibody that inhibits CTLA-4.
[0207] Embodiment 194: The method of embodiment 193, wherein said
antibody
comprises Ipilimumab (Yervoy).
[0208] Embodiment 195: The method according to any one of embodiments
186-
194, wherein the activity of said composition according to any one of
embodiments 1-15; or
said nanovesicle drug carrier according to any one of embodiments 16-66; or
said
nanoparticle drug carrier according to any one of embodiments 67-116; or said
a
nanomaterial carrier according to any one of embodiments 117-157 and said
immune
checkpoint inhibitor is synergistic.
28
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0209] Embodiment 196: A method of treating a cancer in a mammal, said
method
comprising: administering to an intra-tumoral or peri-tumoral site an
effective amount of: a
composition according to any one of embodiments 1-15; and/or a nanovesicle
drug carrier
according to any one of embodiments 16-66; and/or a nanoparticle drug carrier
according to
any one of embodiments 67-116; and/or a nanomaterial carrier according to any
one of
embodiments 117-157.
[0210] Embodiment 197: A kit for the treatment or prophylaxis of a
cancer said kit
comprising: a container containing: a composition according to any one of
embodiments 1-
15; and/or a nanovesicle drug carrier according to any one of embodiments 16-
66; and/or a
nanoparticle drug carrier according to any one of embodiments 67-116; and/or a
nanomaterial
carrier according to any one of embodiments 117-157.
[0211] Embodiment 198: A liposome comprising a lipid bilayer, where
said liposome
contains mitoxantrone.
[0212] Embodiment 199: The liposome of embodiment 198, wherein said
lipid
bilayer comprises a phospholipid, and cholesterol (CHOL) and/or a cholesterol
derivative.
[0213] Embodiment 200: The liposome of embodiment 199, wherein said
phospholipid comprises a saturated fatty acid with a C14-C20 carbon chain,
and/or an
unsaturated fatty acid with a C14-C20 carbon chain, and/or a natural lipid
comprising a
mixture of fatty acids with C12-C20 carbon chains.
[0214] Embodiment 201: The liposome of embodiment 200, wherein said
phospholipid comprises a phospholipid selected from the group consisting of
phosphatidylcholine (DPPC), 1,2-dimyristoleoyl-sn-glycero-3-phosphocholine
(DMPC), 1,2-
dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-distearoyl-sn-glycero-3-
phospho-rac-
glycerol (DSPG), 1,2-dipahnitoyl-sn-glycero-3-phosphoglycerol (DPPG),
distearoylphosphatidylcholine (DSPC), 1,2-dieicosenoyl-sn-glycero-3-
phosphocholine, and
diactylphosphatidylcholine (DAPC).
[0215] Embodiment 202: The liposome of embodiment 200, wherein said
phospholipid comprises a natural lipid selected from the group consisting of
egg
phosphatidylcholine (egg PC), and soy phosphatidylcholine (soy PC).
[0216] Embodiment 203: The liposome of embodiment 200, wherein said
phospholipid comprises distearoylphosphatidylcholine (DSPC).
29
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0217] Embodiment 204: The liposome according to any one of
embodiments 199-
203, wherein said lipid bilayer comprises an mPEG phospholipid with a
phospholipid C14-
C18 carbon chain, and a PEG molecular weight ranging from about 350 Da to 5000
Da.
[0218] Embodiment 205: The liposome of embodiment 204, wherein said
lipid
bilayer comprises 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-PEG (DSPE-
PEG).
[0219] Embodiment 206: The liposome of embodiment 205, wherein said
DSPE-
PEG comprises DPSE-PEG2x.
[0220] Embodiment 207: The liposome of embodiment 205, wherein said
DSPE-
PEG comprises DPSE-PEG5x.
[0221] Embodiment 208: The liposome according to any one of embodiments 203-
207, wherein said lipid bilayer comprises DSPC : Chol : DSPE-PEG.
[0222] Embodiment 209: The liposome of embodiment 208, wherein the
ratio of
DSPC:Chol-IND:DSPE-PEG ranges from 40-90% DSPC : 10%-50% Chol: 1%-10% DSPE-
PEG (molar ratio).
[0223] Embodiment 210: The liposome of embodiment 209, wherein the ratio of
DSPC:Chol:DSPE-PEG is about 50:40:5 (molar ratio).
[0224] Embodiment 211: The liposome according to any one of
embodiments 203-
207, wherein said lipid bilayer comprises DPPG : Chol : DSPE-PEG.
[0225] Embodiment 212: The liposome of embodiment 211, wherein the
ratio of
DPPG:Chol-IND:DSPE-PEG ranges from 40-90% DPPG : 10%-50% Chol: 1%-10% DSPE-
PEG (molar ratio).
[0226] Embodiment 213: The liposome of embodiment 212, wherein the
ratio of
DPPG:Chol:DSPE-PEG is about 50:40:5 (molar ratio).
[0227] Embodiment 214: The liposome according to any one of
embodiments 199-
213, whrein said lipid bilayer comprises a cholesterol derivative selected
from the group
consisting of cholesterol hemisuccinate (CHEMS), lysine-based cholesterol
(CHLYS), and
PEGylated cholesterol (Chol-PEG).
[0228] Embodiment 215: The liposome of embodiment 214, wherein said
lipid
bilayer comprises CHEMS.
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0229] Embodiment 216: The liposome of embodiment 215, wherein said
bilayer
comprises CHEMS ranging from about 5% (mol percent) up to about 30% total
lipid.
[0230] Embodiment 217: The liposome of embodiment 216, wherein said
bilayer
comprise about 10% or about 20% CHEMS or about 30% CHEMS or about 40% CHEMS.
[0231] Embodiment 218: A pharmaceutical formulation comprising: a liposome
according to any one of embodiments 198-217; and a pharmaceutically acceptable
carrier.
[0232] Embodiment 219: The pharmaceutical formulation of embodiment
218,
wherein said formulation is an emulsion, dispersion, or suspension.
[0233] Embodiment 220: The pharmaceutical formulation of embodiment
219,
wherein said suspension, emulsion, or dispersion is stable for at least 1
month, or at least 2
months, or at least 3 months, or at least 4 months, or at least 5 months, or
at least 6 months
when stored at 4 C.
[0234] Embodiment 221: The pharmaceutical formulation according to any
one of
embodiments 218-220, wherein the liposomes in said formulation show a
substantially
unimodal size distribution; and/or show a PDI less than about 0.2, or less
than about 0.1.
[0235] Embodiment 222: The pharmaceutical formulation according to any
one of
embodiments 218-221, wherein said formulation is formulated for administration
via a route
selected from the group consisting of intravenous administration,
intraarterial administration,
intracerebral administration, intrathecal administration, oral administration,
aerosol
administration, administration via inhalation (including intranasal and
intratracheal delivery,
intracranial administration via a cannula, and subcutaneous or intramuscular
depot
deposition.
[0236] Embodiment 223: The pharmaceutical formulation according to any
one of
embodiments 218-221, wherein said formulation is a sterile injectable.
[0237] Embodiment 224: The pharmaceutical formulation according to any one
of
embodiments 218-223, wherein said formulation is a unit dosage formulation.
[0238] Embodiment 225: A method of treating a cancer, said method
comprising:
administering to a subject in need thereof an effective amount a liposome
according to any
one of embodiments 198-217.
[0239] Embodiment 226: The method of embodiment 169, wherein said method
comprises a primary therapy in a chemotherapeutic regimen.
31
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0240] Embodiment 227: The method of embodiment 226, wherein said
method
comprises an adjunct therapy in a treatment regime that additionally comprises
chemotherapy
using another chemotherapeutic agent, and/or surgical resection of a tumor
mass, and/or
radiotherapy.
[0241] Embodiment 228: The method of embodiment 169, wherein said
composition,
a nanovesicle drug carrier, a nanoparticle drug carrier according, and/or
nanomaterial carrier
is a component in a multi-drug chemotherapeutic regimen.
[0242] Embodiment 229: The method according to any one of embodiments
169-
228, wherein said cancer is triple negative breast cancer.
[0243] Embodiment 230: The method according to any one of embodiments 169-
228, wherein said cancer is a cancer selected from the group consisting of
breast cancer, lung
cancer, melanoma, pancreas cancer, liver cancer, acute lymphoblastic leukemia
(ALL), acute
myeloid leukemia (AML), adrenocortical carcinoma, AIDS-related cancers (e.g.,
Kaposi
sarcoma, lymphoma), anal cancer, appendix cancer, astrocytomas, atypical
teratoid/rhabdoid
.. tumor, bile duct cancer, extrahepatic cancer, bladder cancer, bone cancer
(e.g., Ewing
sarcoma, osteosarcoma, malignant fibrous histiocytoma), brain stem glioma,
brain tumors
(e.g., astrocytomas, brain and spinal cord tumors, brain stem glioma, central
nervous system
atypical teratoid/rhabdoid tumor, central nervous system embryonal tumors,
central nervous
system germ cell tumors, craniopharyngioma, ependymoma, burkitt lymphoma,
carcinoid
tumors (e.g., childhood, gastrointestinal), cardiac tumors, cervical cancer,
chordoma, chronic
lymphocytic leukemia (CLL), chronic myelogenous leukemia (CML), chronic
myeloproliferative disorders, colon cancer, colorectal cancer,
craniopharyngioma, cutaneous
t-cell lymphoma, duct cancers e.g. (bile, extrahepatic), ductal carcinoma in
situ (DCIS),
embryonal tumors, endometrial cancer, ependymoma, esophageal cancer,
esthesioneuroblastoma, extracranial germ cell tumor, extragonadal germ cell
tumor,
extrahepatic bile duct cancer, eye cancer (e.g., intraocular melanoma,
retinoblastoma), fibrous
histiocytoma of bone, malignant, and osteosarcoma, gallbladder cancer, gastric
(stomach)
cancer, gastrointestinal carcinoid tumor, gastrointestinal stromal tumors
(GIST), germ cell
tumors (e.g., ovarian cancer, testicular cancer, extracranial cancers,
extragonadal cancers,
central nervous system), gestational trophoblastic tumor, brain stem cancer,
hairy cell
leukemia, head and neck cancer, heart cancer, hepatocellular (liver) cancer,
histiocytosis,
langerhans cell cancer, Hodgkin lymphoma, hypopharyngeal cancer, intraocular
melanoma,
islet cell tumors, pancreatic neuroendocrine tumors, kaposi sarcoma, kidney
cancer (e.g.,
32
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
renal cell, Wilms tumor, and other kidney tumors), langerhans cell
histiocytosis, laryngeal
cancer, leukemia, acute lymphoblastic (ALL), acute myeloid (AML), chronic
lymphocytic
(CLL), chronic myelogenous (CML), hairy cell, lip and oral cavity cancer,
liver cancer
(primary), lobular carcinoma in situ (LCIS), lung cancer (e.g., childhood, non-
small cell,
small cell), lymphoma (e.g., AIDS-related, Burkitt (e.g., non-Hodgkin
lymphoma), cutaneous
T-Cell (e.g., mycosis fungoides, Sezary syndrome), Hodgkin, non-Hodgkin,
primary central
nervous system (CNS)), macroglobulinemia, Waldenstrom, male breast cancer,
malignant
fibrous histiocytoma of bone and osteosarcoma, melanoma (e.g., childhood,
intraocular
(eye)), merkel cell carcinoma, mesothelioma, metastatic squamous neck cancer,
midline tract
-- carcinoma, mouth cancer, multiple endocrine neoplasia syndromes, multiple
myeloma/plasma cell neoplasm, mycosis fungoides, myelodysplastic syndromes,
Myelogenous Leukemia, Chronic (CML), multiple myeloma, nasal cavity and
paranasal sinus
cancer, nasopharyngeal cancer, neuroblastoma, oral cavity cancer, lip and
oropharyngeal
cancer, osteosarcoma, ovarian cancer, pancreatic cancer, pancreatic
neuroendocrine tumors
(islet cell tumors), papillomatosis, paraganglioma, paranasal sinus and nasal
cavity cancer,
parathyroid cancer, penile cancer, pharyngeal cancer, pheochromocytoma,
pituitary tumor,
plasma cell neoplasm, pleuropulmonary blastoma, primary central nervous system
(CNS)
lymphoma, prostate cancer, rectal cancer, renal cell (kidney) cancer, renal
pelvis and ureter,
transitional cell cancer, rhabdomyosarcoma, salivary gland cancer, sarcoma
(e.g., Ewing,
Kaposi, osteosarcoma, rhadomyosarcoma, soft tissue, uterine), Sezary syndrome,
skin cancer
(e.g., melanoma, merkel cell carcinoma, basal cell carcinoma, nonmelanoma),
small intestine
cancer, squamous cell carcinoma, squamous neck cancer with occult primary,
stomach
(gastric) cancer, testicular cancer, throat cancer, thymoma and thymic
carcinoma, thyroid
cancer, trophoblastic tumor, ureter and renal pelvis cancer, urethral cancer,
uterine cancer,
-- endometrial cancer, uterine sarcoma, vaginal cancer, vulvar cancer,
Waldenstrom
macroglobulinemia, and Wilms tumor.
[0244] Embodiment 231: The method according to any one of embodiments
169-
230, wherein said administration is via a route selected from the group
consisting of
intravenous administration, intraarterial administration, intracerebral
administration,
-- intrathecal administration, oral administration, aerosol administration,
administration via
inhalation (including intranasal and intratracheal delivery, intracranial
administration via a
cannula, and subcutaneous or intramuscular depot deposition.
33
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0245] Embodiment 232: The method according to any one of embodiments
169-
230, wherein said administration comprises systemic administration via
injection or cannula.
[0246] Embodiment 233: The method according to any one of embodiments
169-
230, wherein said administration is administration to an intra-tumoral or peri-
tumoral site.
[0247] Embodiment 234: The method according to any one of embodiments 169-
233, wherein said mammal is a human.
[0248] Embodiment 235: The method according to any one of embodiments
169-
233, wherein said mammal is a non-human mammal.
[0249] In certain embodiments the agent(s) that induce ICD exclude
cisplatin, and/or
in certain embodiments the agent(s) that induce ICD exclude doxorubicin. In
the
embodiments above, where a lipid/lipid bilayer comprises a an IDO inhibitor
conjugated to
cholesterol (e.g., IND-Chol), the use of an IDO inhibitor conjugated to a
cholesterol
deriviative (e.g., IND-CHEMS) is contemplated. In certain embodiments where
the lipid
bilayer contains both cholesterol and CHEMS and a conjugated IDO inhibitor,
the IDO can
be conjugated to the cholesterol (IND-Chol), to the cholesterol derivative
(e.g., IND-
CHEMS), or to both the cholesterol and to the cholesterol deriavative.
DEFINITIONS
[0250] The terms "subject," "individual," and "patient" may be used
interchangeably
and refer to humans, as well as non-human mammals (e.g., non-human primates,
canines,
equines, felines, porcines. bovines, ungulates, lagomorphs, and the like). In
various
embodiments, the subject can be a human (e.g., adult male, adult female,
adolescent male,
adolescent female, male child, female child) under the care of a physician or
other health
worker in a hospital, as an outpatient, or other clinical context. In certain
embodiments, the
subject may not be under the care or prescription of a physician or other
health worker.
[0251] As used herein, the phrase "a subject in need thereof refers to a
subject, as
described infra, that suffers from, or is at risk for a cancer as described
herein. Thus, for
example, in certain embodiments the subject is a subject with a cancer (e.g.,
pancreatic ductal
adenocarcinoma (PDAC), breast cancer (e.g., drug-resistant breast cancer),
colon cancer,
brain cancer, and the like). In certain embodiments the methods described
herein are
prophylactic and the subject is one in whom a cancer is to be inhibited or
prevented. In
34
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
certain embodiments the subject for prophylaxis is one with a family history
of cancer and/or
a risk factor for a cancer (e.g., a genetic risk factor, an environmental
exposure, and the like).
[0252] The term "treat" when used with reference to treating, e.g., a
pathology or
disease refers to the mitigation and/or elimination of one or more symptoms of
that pathology
or disease, and/or a delay in the progression and/or a reduction in the rate
of onset or severity
of one or more symptoms of that pathology or disease, and/or the prevention of
that
pathology or disease. The term treat can refer to prophylactic treatment which
includes a
delay in the onset or the prevention of the onset of a pathology or disease.
[0253] The terms "coadministration" or" administration in conjunction
with or
"cotreatment" when used in reference to the coadministration of a first
compound (or
component) (e.g., an ICD inducer) and a second compound (or component) (e.g.,
an IDO
inhibitor) indicates that the first compound (or component) and the second
compound (or
component) are administered so that there is at least some chronological
overlap in the
biological activity of first compound and the second compound in the organism
to which they
are administered. Coadministration can include simultaneous administration or
sequential
administration. In sequential administration there may even be some
substantial delay (e.g.,
minutes or even hours) between administration of the first compound and the
second
compound as long as their biological activities overlap. In certain
embodiments, the
coadminstration is over a time frame that permits the first compound and
second compound
to produce an enhanced therapeutic or prophylactic effect on the organism. In
certain
embodiments the enhanced effect is a synergistic effect.
[0254] The term "immunogenic cell death" or "ICD" refers to a unique
form of cell
death caused by some cytostatic agents such as anthracyclines (Obeid et al.
(2007) Nature
Med.,13(1): 54-61), anthracenedione (mitoxantrone, aka MTX), oxaliplatin,
irinotecan, and
bortezomib, or radiotherapy and/or photodynamic therapy (PDT). Unlike regular
apoptosis,
which is mostly non-immunogenic or even tolerogenic, immunogenic apoptosis of
cancer
cells can induce an effective antitumor immune response through activation of
dendritic cells
(DCs) and consequent activation of specific T cell response (Spisek and
Dhodapkar (2007)
Cell Cycle, 6(16): 1962-1965). Endoplasmic reticulum (ER) stress, reactive
oxygen species
(ROS) production and induction of autophagy are key intracellular response
pathways that
govern ICD (Krysko et al. (2012) Nat. Rev. Canc. 12(12): 860-875). In addition
to
facilitating tumor cell death that facilitates antigen presentation by
dendritic cells, ICD is
characterized by secretion or release of damage-associated molecular patterns
(DAMPs),
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
which exert additional immune adjuvant effects. Calreticulin (CRT), one of the
DAMP
molecules, which is normally in the lumen of the ER, is translocated to the
surface of dying
cell where it functions as an "eat me" signal for phagocytes. Other important
surface exposed
DAMPs are heat-shock proteins (HSPs), namely HSP70 and HSP90, which under
stress
condition are also translocated to the plasma membrane. On the cell surface
they have an
immunostimulatory effect, based on their interaction with number of antigen-
presenting cell
(APC) surface receptors like CD91 and CD40 and also facilitate cross-
presentation of
antigens derived from tumor cells on MHC class I molecule, which then triggers
CD8+ T cell
activation and expansion. Other important DAMPs, characteristic for ICD are
secreted
amphoterin (HMGB1) and ATP (see, e.g., Apetoh et al. (2007) Nature Med. 13(9):
1050-
1059; Ghiringhelli et al. (2009) Nature Med. 15(10): 1170-1178). HMGB1 is
considered to
be a late apoptotic marker and its release to the extracellular space appears
to be required for
the optimal release and presentation of tumor antigens to dendritic cells. It
binds to several
pattern recognition receptors (PRRs) such as Toll-like receptor (TLR) 2 and 4,
which are
expressed on APCs. The most recently found DAMP released during immunogenic
cell
death is ATP, which functions as a "find-me" signal for monocytes when
secreted and
induces their attraction to the site of apoptosis (see, e.g., Garg et al.
(2012) EMBO J. 31(5):
1062-1079). ATP binds to purinergic receptors on APCs.
[0255] The terms "IDO inhibitor", "IDO pathway inhibitor", and
"inhibitor of the IDO
pathway) are used interchangeably and refer to an agent (a molecule or a
composition) that
either partially or fully blocks the activity of indoleamine-2,3-dioxygenase
(IDO) and/or
partially or fully suppresses the post-enzymatic signaling cascade(s) in the
IDO pathway.
IDO is an intracellular heme-containing enzyme that initiates the first and
rate-limiting step
of tryptophan degradation along the kynurenine pathway. The indoleamine 2,3-
dioxygenase
(IDO) pathway regulates immune response by suppressing cytotoxic T cell
function,
enhancing regulatory T cell activity (Tregs) and enabling tumor immune escape,
either at the
tumor or regional lympnode sites. An IDO pathway inhibitor can inhibit the IDO
enzyme
directly or by interfering or perturbing IDO effector pathway components. Such
components
include, but are not limited to: ID02, tryptophan 2,3-dioxygenase (TDO), the
mammalian
target of rapamycin (mTOR) pathway, arylhydrocarbon receptor (AhR) pathway,
the general
control nonderepressible 2 (GCN2) pathway, and the AhR/IL-6 autocrine loop.
[0256] The terms "nanocarrier" and "nanoparticle drug carrier" are
used
interchangeably and refer to a nanostructure having a porous interior core
(e.g., a "porous
36
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
nanoparticle"). In certain embodiments the nanocarrier comprises a lipid
bilayer encasing (or
surrounding or enveloping) the porous particle core. In certain embodiments
the nanoparticle
is a porous silica nanoparticle (e.g., mesoporous silica nanoparticle or
"MSNP").
[0257] As used herein, the term "lipid" refers to conventional lipids,
phospholipids,
cholesterol, chemically functionalized lipids for attachment of PEG and
ligands, etc.
[0258] As used herein, the terms "lipid bilayer" or "LB" refers to any
double layer of
oriented amphipathic lipid molecules in which the hydrocarbon tails face
inward to form a
continuous non-polar phase.
[0259] As used herein, the terms "liposome" or "lipid vesicle" or
"vesicle" are used
interchangeably to refer to an aqueous compartment enclosed by a lipid
bilayer, as being
conventionally defined (see, e.g., Stryer (1981) Biochemistry, 2d Edition, W.
H. Freeman &
Co., p. 213).
[0260] A "nanovesicle" refers to a "lipid vesicle" having a diameter
(or population of
vesicles having a mean diameter) ranging from about 10 nm, or from about 20
nm, or from
about 30 nm, or from about 40 nm, or from about 50 nm up to about 500 nm, or
up to about
400 nm, or up to about 300 nm, or up to about 200 nm, or up to about 150 nm,
or up to about
100 nm, or up to about 80 nm. In certain embodiments a nanovesicle has a
diameter ranging
from about 10 nm up to about 80 nm, or from about 50 nm up to about 70 nm.
[0261] Compared with the lipid bilayer coated on mesoporous silica
nanopaticles, the
lipid bilayer in a lipid vesicle or liposome can be referred to as an
"unsupported lipid bilayer"
and the lipid vesicle itself (when unloaded) can be referred to as an "empty
vesicle". The
lipid bilayer coated on mesoporous silica nanopaticles can be referred to as a
"supported lipid
bilayer" because the lipid bilayer is located on the surface and supported by
a porous particle
core. In certain embodiments, the lipid bilayer can have a thickness ranging
from about 6 nm
to about 7 nm which includes a 3-4 nm thickness of the hydrophobic core, plus
the hydrated
hydrophilic head group layers (each about 0.9 nm) plus two partially hydrated
regions of
about 0.3 nm each. In various embodiments, the lipid bilayer surrounding the
silica
nanoparticle comprises a continuous bilayer or substantially continuous
bilayer that
effectively encapsulates and seals the nanoparticle.
[0262] As used herein, the term "selective targeting" or "specific binding"
refers to
use of targeting ligands on the surface of a drug delivery nanocarrier (e.g.,
a LB-coated
nanoparticle). In certain embodiments the targeting ligand(s) are on the the
surface of a lipid
37
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
bilayer or LB-coated nanoparticle. Typically, the ligands interact
specifically/selectively
with receptors or other biomolecular components expressed on the target, e.g.,
a cell surface
of interest. The targeting ligands can include such molecules and/or materials
as peptides,
antibodies, aptamers, targeting peptides, polysaccharides, and the like.
[0263] A coated mesoporous silica nanopaticle, having targeting ligands can
be
referred to as a "targeted nanoparticle or a targeted drug delivery
nanocarrier (e.g., LB-coated
nanoparticle).
[0264] The term "about" or "approximately" as used herein refers to
being within an
acceptable error range for the particular value as determined by one of
ordinary skill in the
art, which will depend in part on how the value is measured or determined,
i.e. the limitations
of the measurement system, i.e. the degree of precision required for a
particular purpose, such
as a pharmaceutical formulation. For example, "about" can mean within 1 or
more than 1
standard deviation, per the practice in the art. Alternatively, "about" can
mean a range of up
to 20%, preferably up to 10%, more preferably up to 5% and more preferably
still up to 1%
of a given value. Alternatively, particularly with respect to biological
systems or processes,
the term can mean within an order of magnitude, preferably within 5-fold, and
more
preferably within 2-fold, of a value. Where particular values are described in
the application
and claims, unless otherwise stated, the term "about" meaning within an
acceptable error
range for the particular value should be assumed.
[0265] The term "drug" as used herein refers to a chemical entity of
varying
molecular size, small and large, naturally occurring or synthetic, that
exhibits a therapeutic
effect in animals and humans. A drug may include, but is not limited to, an
organic molecule
(e.g., a small organic molecule), a therapeutic protein, peptide, antigen, or
other biomolecule,
an oligonucleotide, an siRNA, a construct encoding CRISPR cas9 components and,
optionally one or more guide RNAs, and the like.
[0266] A "pharmaceutically acceptable carrier" as used herein is
defined as any of the
standard pharmaceutically acceptable carriers. The pharmaceutical compositions
of the
subject invention can be formulated according to known methods for preparing
pharmaceutically useful compositions. The pharmaceutically acceptable carrier
can include
diluents, adjuvants, and vehicles, as well as carriers, and inert, non-toxic
solid or liquid
fillers, diluents, or encapsulating material that does not react with the
active ingredients of the
invention. Examples include, but are not limited to: phosphate buffered
saline, physiological
38
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
saline, water, and emulsions, such as oil/water emulsions. The carrier can be
a solvent or
dispersing medium containing, for example, ethanol, polyol (for example,
glycerol, propylene
glycol, liquid polyethylene glycol, and the like), suitable mixtures thereof,
and vegetable oils.
Formulations are described in a number of sources that are well known and
readily available
to those skilled in the art. For example, Remington's Pharmaceutical Sciences
(Martin E W
[1995] Easton Pa., Mack Publishing Company, 19th ed.) describes formulations
which can be
used in connection with the drug delivery nanocarrier(s) (e.g., LB-coated
nanoparticle(s))
described herein.
[0267] As used herein, an "antibody" refers to a protein consisting of
one or more
polypeptides substantially encoded by immunoglobulin genes or fragments of
immunoglobulin genes or derived therefrom that is capable of binding (e.g.,
specifically
binding) to a target (e.g., to a target polypeptide). The recognized
immunoglobulin genes
include the kappa, lambda, alpha, gamma, delta, epsilon and mu constant region
genes, as
well as myriad immunoglobulin variable region genes. Light chains are
classified as either
kappa or lambda. Heavy chains are classified as gamma, mu, alpha, delta, or
epsilon, which
in turn define the immunoglobulin classes, IgG, IgM, IgA, IgD and IgE,
respectively.
[0268] A typical immunoglobulin (antibody) structural unit is known to
comprise a
tetramer. Each tetramer is composed of two identical pairs of polypeptide
chains, each pair
having one "light" (about 25 kD) and one "heavy" chain (about 50-70 kD). The N-
terminus
of each chain defines a variable region of about 100 to 110 or more amino
acids primarily
responsible for antigen recognition. The terms variable light chain (VL) and
variable heavy
chain (VH) refer to these light and heavy chains respectively.
[0269] Antibodies exist as intact immunoglobulins or as a number of
well
characterized fragments produced by digestion with various peptidases. Thus,
for example,
.. pepsin digests an antibody below the disulfide linkages in the hinge region
to produce F(ab)'2,
a dimer of Fab which itself is a light chain joined to VH-CH1 by a disulfide
bond. The F(ab)12
may be reduced under mild conditions to break the disulfide linkage in the
hinge region
thereby converting the (Fab1)2 dimer into a Fab' monomer. The Fab' monomer is
essentially a
Fab with part of the hinge region (see, Fundamental Immunology, W.E. Paul,
ed., Raven
Press, N.Y. (1993), for a more detailed description of other antibody
fragments). While
various antibody fragments are defined in terms of the digestion of an intact
antibody, one of
skill will appreciate that such Fab' fragments may be synthesized de novo
either chemically or
by utilizing recombinant DNA methodology. Thus, the term antibody, as used
herein also
39
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
includes antibody fragments either produced by the modification of whole
antibodies or
synthesized de novo using recombinant DNA methodologies. Certain preferred
antibodies
include single chain antibodies (antibodies that exist as a single polypeptide
chain), more
preferably single chain Fv antibodies (sFy or scFv) in which a variable heavy
and a variable
light chain are joined together (directly or through a peptide linker) to form
a continuous
polypeptide. The single chain Fv antibody is a covalently linked VH_VL
heterodimer which
may be expressed from a nucleic acid including VH- and VL- encoding sequences
either
joined directly or joined by a peptide-encoding linker. Huston, et al. (1988)
Proc. Nat. Acad.
Sci. USA, 85: 5879-5883. While the VH and VL are connected to each as a single
polypeptide chain, the VH and VL domains associate non-covalently. The first
functional
antibody molecules to be expressed on the surface of filamentous phage were
single-chain
Fv's (scFv), however, alternative expression strategies have also been
successful. For
example Fab molecules can be displayed on a phage if one of the chains (heavy
or light) is
fused to g3 capsid protein and the complementary chain exported to the
periplasm as a
soluble molecule. The two chains can be encoded on the same or on different
replicons; the
important point is that the two antibody chains in each Fab molecule assemble
post-
translationally and the dimer is incorporated into the phage particle via
linkage of one of the
chains to, e.g., g3p (see, e.g., U.S. Patent No: 5733743). The scFv antibodies
and a number
of other structures converting the naturally aggregated, but chemically
separated light and
heavy polypeptide chains from an antibody V region into a molecule that folds
into a three-
dimensional structure substantially similar to the structure of an antigen-
binding site are
known to those of skill in the art (see e.g., U.S. Patent Nos. 5,091,513,
5,132,405, and
4,956,778). In certain embodiments antibodies should include all that have
been displayed on
phage (e.g., scFv, Fv, Fab and disulfide linked Fv (see, e.g, Reiter et al.
(1995) Protein Eng.
8: 1323-1331) as well as affibodies, unibodies, and the like.
[0270] The term "specifically binds", as used herein, when referring
to a biomolecule
(e.g., protein, nucleic acid, antibody, etc.), refers to a binding reaction
that is determinative of
the presence of a biomolecule in heterogeneous population of molecules (e.g.,
proteins and
other biologics). Thus, under designated conditions (e.g. immunoassay
conditions in the case
of an antibody or stringent hybridization conditions in the case of a nucleic
acid), the
specified ligand or antibody binds to its particular "target" molecule and
does not bind in a
significant amount to other molecules present in the sample.
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0271] "Two-dimensional materials (2D materials) are materials that do
not require a
substrate to exist. In other words, they can be isolated as freestanding one
atom thick sheets.
As a practical matter, this definition can be relaxed to include materials
with a thickness of a
few atoms (e.g., less than about 10 atoms).
[0272] The term "substantially pure isomer" refers to a formulation or
composition
wherein among various isomers of a compound a single isomer is present at 70%,
or greater
or at 80% or greater, or at 90% or greater, or at 95% or greater, or at 98% or
greater, or at
99% or greater, or said compound or composition comprises only a single isomer
of the
compound.
[0273] A "bioreactive nanomaterial" refers to an engineered biomaterial
that induces
or catalyzes a biological response. In certain embodiments the nanomaterial
induces a
response by virtue of one or more properties selected from the group
consisting of
composition, size, shape, aspect ratio, dissolution, electronic, redox,
surface display, surface
coating, hydrophobic, hydrophilic, an atomically thin nanosheet. or
functionalized surface
groups" to catalyze the biological response at various nano/bio interfaces. In
certain
embodiments the bioreactive nanomaterial has the ability to induce ICD
biological responses
in cells (e.g., in tumor cells) and/or as well as activating the innate immune
system through
delivery of "danger signal" and adjuvant effects.
BRIEF DESCRIPTION OF THE DRAWINGS
[0274] Figure 1 shows a schematic that illustrates how dual delivery of ICD
inducing
chemo and IND prodrug may impact the anti-cancer immune response. We
hypothesize that
nano-enabled co-delivery of a chemotherapeutic agent, which provides an ICD
stimulus, and
IND, which interferes in the IDO pathway, may combine to trigger a robust PDAC
immune
response. ICD chemo such as MTO (#1) induces an ICD response (#2) in which CRT
expression on the dying tumor cell surfaces provides an "eat-me" signal for DC
uptake, as
well as the release of HMGB-1 that delivers adjuvant stimuli to DC (#3).
Following uptake of
the dying tumor cells by DC, their maturation and cross-presentation of
endogenous tumor-
associated antigens (TAAs) (#4), the recruitment and activation of CD8+ T
cells (#5) will
lead to granulysin and perforin mediated killing of primary (#6) and
metastatic cancer cells
(#7). The concomitant delivery of IND prodrug (e.g. IND-Cholesterol) (#8)
interferes in the
IDO metabolic pathway, which can lead to strengthening the ICD effect by
interfering in
Treg development and overcome other immunomodulatory effects (#9). The ICD
pathway
41
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
also allows the activation of helper and memory T cells, which may prevent
disease
recurrence (#10). Following proof-of-principle testing of this scheme, we
propose that a co-
delivery nanocarrier (i.e. liposome) that contains IND which is capable of
synergistically
enhancing the ICD effect, providing more than just an additive outcome (#11).
[0275] Figure 2 illustrates the structure of indoximod and various other
IDO pathway
inhibitors.
[0276] Figure 3 illustrates representative examples to show the use of
an ester bond to
make IDO inhibitor (e.g., indoximod) pro-drug conjugates. As a general
strategy, the NH2
group (highlighted by circle) in the indoximod is protected before the
conjugation reaction.
The ¨COOH (green box) in indoximod can then robustly react with the ¨OH (blue
box) in,
for example, vitamin E or cholesterol, leading to a list of pro-drugs, that
can self-assemble as
vesicles (or micellar structures) in aqueous solution. It can also be used in
the lipid mixture
for MSNP coating.
[0277] Figure 4 illustrates representative examples to show the
combined use of HO-
(CH2)=2_5-0H linker and ester bond to make IDO inhibitor (e.g., indoximod) pro-
drug
conjugates. As illustrated in this example, the NH2 group (highlighted by red
circle) is
protected in the indoximod before the conjugation reaction. The ¨COOH (in box)
in
indoximod can robustly react with one ¨OH group (blue box) in the linker
compound, which
can also readily react with -COOH in the oleic acid or DHA molecule via the
other ¨OH
group.
[0278] Figure 5 shows construction of an IND nanovesicle by self-
assembly of Chol-
IND + Phospholipid.
[0279] Figure 6. Panel A) Flow cytometry experiment showing the
induction of the
ICD marker, CRT, in cultured KPC pancreatic cancer cells in the presence of
PBS, DOX
(20 LtM), OX (500 M), and activated DOX (a.k.a. DACHPt, 500 M) for 24 h. HMBG1
release was measured using ELISA. Panel B) Animal experimentation using 2
rounds of
vaccination one week apart. followed by injecting live KPC cells SC on the
contralateral side.
The details of the animal vaccination experiment are provided in the methods
section.
Tumors were collected on day 26 for size measurement and IHC analysis. Panel
C) Tumor
size measurement on the contralateral side. Panel D) Explanted tumor at the
contralateral
side. Panel E) Spaghetti curves to show KPC tumor growth in the contralateral
flank. Panel
F) Tumor collection was performed after euthanizing the animal to conduct IHC.
IHC
42
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
staining of CD8 and Foxp3 T cells was used to calculate CD8/FoxP3 T cells
ratio in each
group. *p < 0.05; **p < 0.01
[0280] Figure 7 illustrates the development of a dual delivery carrier
for OX plus IND
using lipid-bilayer coated mesoporous silica nanoparticles (OX/IND-MSNP). A
schematic
shows the structure of OX-laden MSNP, in which the drug is trapped by a lipid
bilayer
containing IND-Chol. This leads to stable entrapment of OX in the pores, with
IND-Chol
trapped in the bilayer. The coating procedure provides uniform and
instantaneous sealing of
all particle pores.
[0281] Figure 8 illustrates a Chol-IND prodrug (Formula II) as well as
the R
enantiomer (Formula Ha) and L enantiomer (Formula Hb).
[0282] Figure 9 illustrates synthesis of lysolipid conjugated 1-MT
prodrugs: Stage I-
II.
[0283] Figure 10 illustrates synthesis of lysolipid conjugated 1-MT
prodrugs: Stage
[0284] Figure 11 illustrates synthesis of lysolipid conjugated 1-MT
prodrugs: Stage
V-VI. Through the use of the full synthetic approach, one should be able to
obtain 16:0
LysoPC-indoximod (IND-PL) (see box 6c) that was tested in our previous studies
described
in PCT Publication No: WO/2018/213631 (PCT/U52018/033265). Because we now
disclosed a total synthetic approach, this strategy has opened up the whole
library for all other
similar conjugations, which have also been delineated as below.
[0285] Figure 12 illustrates synthesis of fatty acid and cholesterol
conjugated 1-MT
prodrugs.
[0286] Figure 13 illustrates a Steglich esterification (see, e.g.,
Steglich (1078) Agnew.
Chem. Int. Ed, 17(7): 522-524).
[0287] Figure 14 illustrates an alternative synthetic strategy.
[0288] Figure 15 illustrates and alternative Step 1 for the synthesis
strategy shown in
Fig. 14.
[0289] Figure 16 illustrates the synthesis of Chol-IND.
[0290] Figure 17 shows ESI-MS results for synthesis of Boc-Indoximod
(B0c-IND).
43
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0291] Figure 18 shows ESI-MS results for synthesis of cholesteryl-
indoximod-Boc
(Chol-IND-Boc).
[0292] Figure 19 shows. ESI-MS data of Chol-IND-NH3+TFA- salt. Thin
layer
chromatography profile of Chol-IND-NH3+TFA- salt was showed on the top right
corner.
Chol-IND-NH3+TFA- salt is a yellow color paste.
[0293] Figure 20 illustrates one design of Chol-IND liposome for ICD
inducing agent
delivery via a trapping agent mediated approach.
[0294] Figure 21 shows the successful synthesis of MTX laden Chol-IND
liposome.
The synthesis involved preparation of lipid biofilm, rehydration in citrate
solution (trapping
agent), extrusion (100 nm pore size), removal of free citrate acid, drug
import, and
purification. CyroEM visualization is provided. The final prodrug shows single
peak in the
DLS analysis, suggesting the formation of a liposome formulation with low PDI.
The MTX
loading is 14.1 wt%. The loading efficiency is determined to be 78%. The
liposome exhibits
slight positive charge, i.e. +4 mV. Note: This sample was made using the IND
prodrug as a
Chol-IND TFA salt. We are currently also making a liposome with salt free Chol-
IND.
[0295] Figure 22. Successful synthesis of DOX laden Chol-IND liposome.
The
synthesis is similar to MTX Chol/IND liposome. In DOX formulation, the
trapping agent is
(NH4)2SO4, similar to Doxil. We used TFA-free Chol-IND in this case. Our
synthesis led to
a size controlled liposome around ¨95 nm. The liposome exhibits positive
charge, i.e. +15
mV, which has promoted us to consider introducing charge reversal strategy
because we
prefer to inject neutral or slightly negatively charged liposome systemically
[0296] Figure 23. We synthesized MTX laden liposome using pristine
cholesterol or
Chol-IND at the identical (40%) molar ratio. The particle charge was measured
at different
stage during synthesis. Use of prodrug led to positive charge across the
board.
[0297] Figure 24. A list of MTX laden liposomes was made using pristine
cholesterol, CHEMS and Chol-IND. We reasoned that the inclusion of CHEMS in in
the
Chol-IND formulation should adjust the particle charge from positive to the
neutral or
slightly anionic. The design of the formulation is provided in the inserted
table. Four
formulations (C1-C4) were made, in which C3 contains 10% CHEMS and C4 contains
20%
CHEMS. Controls include MTX-only liposome using pristine cholesterol (Cl) and
DSPC:Cho-IND/DSPE-PEG2K=50:40:5 (molar ratio) without CHEMS (C2). The
particles
size, charge and PDI were summarized in the lower panel. As expected, use of
10% and 20%
44
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
CHEMS led to zeta potential values of +1 and -10 mV, respectively. Particle
size
measurement and PDI were not significantly changed among these formulations.
The sizes
of these particles are 100-130 nm; the PDI values are <0.15. The loading
efficiency of C3
and C4 formulations are 93 4% and 87 17%. respectively. The loading capacity
values are
14 0.5% and 13 2.5%, respectively . These values are similar to the
results in Cl and C2.
[0298] Figure 25. Demonstration of MTX/IND-Chol liposome is capable of
ICD
induction and IDO inhibition in vitro. 4T1 cells were treated by MTX/IND-Chol
liposome at
MTX dose of 2.5 [tM/mL for 8 h. Representative flow cytometry data was shown
to
demonstrate effective induction of calreticulin (CRT), a major biomarker
during ICD. 4T1
cells were treated with free IND or IND-Chol liposome at the indicated
concentrations for 3 h
in tryptophan-deficient DMEM. Western blot assays showing the enhanced effect
of IND-PL
on mTOR signaling, which can be conveniently studied by assessing the
phosphorylation of
P-S6K.
[0299] Figure 26. Panel A) Determination of MTD doses for free MTX and
liposomal
MTX in normal mice. MTD doses for free MTX and liposomal MTX were 3 and 15
mg/kg
for single IV administration. Due to the high MTD of liposomal MTX, we tested
the efficacy
of the MTX liposome at low dose (1 mg/Kg x 3 times) and high dose (3 mg/Kg x 3
times).
Panel B) Pilot tumor size measurement in 4T1 orthotopic tumor-bearing mice (n
= 6)
receiving IV free drug or MTX-only liposome. For free drug treatment, we have
included 2
two schedules namely single IV at 3 mg/kg at Day 8 or IV injections at 1 mg/kg
at Day 8, 11
and 14. Since MTX liposome has a 5x higher MTD dose, the tumor mice received
single IV
at 3 mg/kg at Day 8 or IV injections at Day 8, 11 and 14.
[0300] Figure 27 shows synthetic steps, NMR, MS and HPLC data of Chol-
IND.
[0301] Figure 28 shows 3 month stability of an MTX/IND co-delivery
liposome.
[0302] Figure 29 illustrates mechanisms of immunogenic cell death.
[0303] Figure 30, panels A-B, shows that MTX encapsulated in an IND-
Chol
liposome leads to stronger ICD induction and immune activation at tumor site.
Panel A) IHC
study to confirm the effect of ICD induction (e.g. CRT, HMGB1 and LC3) and
immune
activation (e.g. CD8/Foxp3 ratio, perforin). Panel B) The tumor tissues were
fixed and used
for IHC staining of CD8, FoxP3, CRT and HMGB1. The IHC staining intensity was
quantified using computer software. Panel D) Representative IHC staining of
CRT and
Perforin were provided.
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0304] Figure 31. Panel A) Orthotopic tumor-bearing 4T1 mice were IV
injected
with the encapsulated MTX liposomes to deliver indicated MTX dose every 3
days, for a
total of 3 administrations. This includes the treatment using IND-Chol/MTX co-
delivery
(IND: 3 mg/kg; MTX: 3 mg/kg). The animals were sacrificed at day 23. The
formulation
was provided on the right panel. Panel B) All the particles were fully
characterized
abiotically and biotically. The capability of CRT induction was confirmed in
4T1 cells. Panel
C) Tumor size measurement in the efficacy study. At the conclusion of the
experiment,
primary tumor and major organs were collected for weighing. Organ index values
were
calculated. The tumor tissues were fixed and used for IHC staining of CD8,
FoxP3, CRT and
HMGB1. The IHC staining results are pending. Panel D) In a separate
experiment, we also
performed an official survival study using these treatments in the same 4T1
orthotopic model.
N=10. *, p<0.05; **, p<0.01; ***, p<0.001. Panel E) We have obtained some IHC
data in
Experiment (panel C). This includes CRT and perforin. MTX/IND co-delivery
(LCIM) led
to the strongest CRT induction and perforin staining at tumor site. Panel F)
Representative
CRT staining was provided.
[0305] Figure 32, panels A-B, illustrates results of an animal study
in a CT26 colon
cancer model. Panel A) Subcutaneous CT26 colon cancer bearing mice were IV
injected
with MTX/IND co-delivery liposome to deliver 3 mg/kg MTX and 3 mg/kg IND every
3
days, for a total of 4 administrations. Detailed treatment schedule and group
information are
discussed in panel A. Panel B) Tumor size measurement in the animal study. A
statistically
significant difference (p<0.001) emerged as early as day 20 between dual
delivery (LCIM) vs
MTX only liposome. In the MTX/IND liposome group, five out of eight mice have
tumor
less than 150 mm3, which outperformed all the control groups including MTX-
only liposome
w/w CHEMS. The addition of empty IND liposome interfered the effect of co-
delivery via a
tumor access competition mechanism.
[0306] Figure 33, panels A-B. KPC pancreatic cells were treated by
using PBS
(negative control), OX (positive control) and indicated engineered
nanomaterials at low and
high concentrations. The choice of particle concentration is based on an MTS
assay (panel
A). Twenty-four hours post incubation, the total cells were harvested for CRT
analysis
through flow cytometry. This suggested a highly strong CRT induction effects
(more potent
than OX chemo) by nano-sized Ag, Cu, SiO2, V205, ZnO and graphene (panel B).
46
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
DETAILED DESCRIPTION
[0307] In various embodiments three treatment modalities are provided
to generate an
anti-cancer response premised, inter alio. on the induction of immunogenic
cell death (ICD)
in cancer cells. ICD is responsible for enhanced tumor antigen presentation as
well as
providing stimulatory effects to the participating DCs. This can trigger the
activation of
cytotoxic T cells and anti-cancer (e.g., anti-PDAC, anti-colon cancer)
immunity that can be
synergistically enhanced by an intervention in the IDO pathway.
[0308] A first treatment modality involves the combination of an ICD
inducer (e.g.,
oxaliplatin or MTX) in combination with an IDO inhibitor (e.g., indoximod)
into a single
nanocarrier that allows systemic (or local) biodistribution and drug delivery
to tumor sites. It
is believed the dual-delivery approach can provide synergistic enhancement of
adaptive and
innate immunity (e.g., anti-PDAC immunity), with a significant improvement in
animal
survival. In certain embodiments the nanocarrier comprises a vesicle (i.e., a
lipid bilayer
enclosing a fluid). In certain embodiments the nanocarrier comprises a
nanoparticle (e.g., a
mesoporous silica nanoparticle (MSNP) surrounded (encapusulated) by a lipid
bilayer.
[0309] A second treatment modality involves local delivery to a tumor
or peri-
tumoral region, of an agent that induces ICD (e.g., oxaliplatin) in
combination with a lipid
(e.g., a nanovesicle) that comprises an inhibitor of the IDO pathway (e.g.,
indoximod).
Without being bound by a particular theory, it is believed that such local
delivery of an ICD
inducer in combination with an IDO inhibitor induces recruitment of cytotoxic
CDS+
lymphocytes, depletion of Tregs, reversal of the CD r/Foxp3+ ratio, cytotoxic
tumor killing,
and tumor shrinkage at the local site. These adaptive immune responses can be
accompanied
by boosting of the innate immune system, as reflected by CRT and HMGB1
expression, as
well as the activation of a DC population, particularly well-suited for
generating cytotoxic T
cell responses.
[0310] A third treatment modality involves vaccination utilizing dying
cancer cells
(e.g., KPC cells) in which ICD is induced ex vivo. Such vaccination can
generate a systemic
immune response that can interfere with tumor growth at a remote site as well
as allowing
adoptive transfer to non-immune animals.
[0311] In various embodiments, methods and compositions for performing
these
treatment modalities are provided.
Approach 1 -- Systemic treatment of a cancer by combined delivery of ICD and
IDO
47
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
inhibition.
[0312] The first approach approach combines an ICD-inducer (e.g.,
doxirubicin,
oxaliplatin, MTX, etc.) and an inhibitor of the IDO pathway (e.g., indoximod)
into a single
nanocarrier, that can provide systemic biodistribution and drug delivery to
orthotopic tumor
sites.
[0313] In certain embodiments this dual-delivery approach involves the
formation of
lipid vesicles where a component of the lipid bilayer comprising the vesicle
incorporates or is
conjugated to an inhibitor of the IDO pathway (e.g., an indoximod prodrug such
Chol-IND)
and the vesicle contains an ICD inducer (e.g., doxorubicin (DOX), mitoxantrone
(MTX), and
the like). This approach is illustrated herein in Examples 2 and 3.
[0314] In another illustrative, but non-limiting embodiment, the
nanocarrier
comprises a mesoporous silica nanoparticle (MSNP) containing the ICD inducer
(e.g.,
oxaliplatin) where the silica nanoparticle is surrounded by (encapsulated by)
a lipid bilayer
containing (or conjugated to) an IDO inhibitor (e.g., indoximod provided as
the prodrug
Chol-IND (Formula I, Fig. 8). The lipid bilayer (LB) coated MSNP, also known
as a
silicasome (see, e.g., PCT Patent Application No: PCT/US2017/012625) is
designed to
provide effective dual delivery of two (or more therapeutics) and can be
exploited to provide
dual delivery of the ICD inducer and IDO inhibitor. Without being bound by a
particular
theory, it is believe that this dual-delivery approach achieved synergistic
enhancement of
.. adaptive and innate anti-PDAC or anti-colon cancer (CT26) immunity
immunity, leading to a
significant improvement in animal survival.
[0315] A third dual-delivery approach exploits the discovery that
certain
nanomaterials (e.g., CuO, graphene oxide) can induce immunogenic cell death
(ICD) (see,
e.g., Example 5). It is also believed that other nanomaterials such as CuO,
Cu2O, Sb203,
As203, Bi203, P203, ZnO, TiO2, and 2D materials other than graphene or
graphene oxide
(e.g., graphene, graphyne, borophene, germanene, silicene, Si2BN, stanene,
phosphorene,
bismuthene, molybdenite, metals, 2D supracrystals, and the like may also
induce
immunogenic cell death. Nanoparticles formed from these ICD inducers, or
combinations
thereof, can readily be coated with a lipid that contains (or is conjugated
to) an IDO inhibitor
(e.g., indoximod provided as the prodrug, Chol-IND (Formula I), and the like).
The lipid
coated nanomaterial thus forms a dual delivery vehicle for delivery of both an
ICD-inducer
and an IDO-inhibitor. Accordingly, in certain embodiments, the following dual-
delivery
vehicles are contemplated herein:
48
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0316] 1) ICD-inducer/IDO-inhibitor vesicle;
[0317] 2) ICD-inducer/IDO-inhibitor silicasome (LB-coated
nanoparticle);
[0318] 3) ICD-inducer nanomaterial (bioreactive nanomaterial)
coated with 'DO-
inhibitor lipid (phospholipid prodrug).
[0319] It will be recognized, that in addition to systemic administration,
any of these
carriers may be considered for local treatment of a tumor. Thus, for example,
any of these
carriers can be administered topically (e.g., for skin tumors), or directly,
e.g., to an intra-
tumoral or peri-tumoral site, e.g., via injection or during a surgical
procedure.
Dual-Delivery Lipid Vesicles (e.g., ICD/IDO inhibitorVesicles)
[0320] In certain embodiments dual-delviery nanovesicles are provided for
the
delivery of an ICD-inducer in combination with an inhibitor of the IDO pathway
and/or for
the delivery of an ICD inducer and a pharmacological agent other than an ICD
inducer or in
combination with an ICD inducer in addition to the inhibitor of the IDO
pathway.
[0321] Accordingly, in certain embodiments, a nanovesicle drug carrier
for the
combined delivery of an inhibitor of an IDO pathway and an inducer of
immunogenic cell
death (ICD), is provided where the nanovesicle drug carrier comprises a lipid
vesicle where a
lipid bilayer effectively forms a vesicle in an aqueous solution, and the
lipid or lipid
formuation comprising the vesicle is associated with (or conjugated to) an
inhibitor of the
indoleamine 2,3-dioxygenase (IDO) pathway (IDO pathway inhibitor); and a cargo
within the
vesicle where the cargo comprises an agent that induces immunogenic cell death
(ICD) (ICD-
inducer). It is noted that while this embodiment is described with respect to
a cargo that
induces immunogenic cell death, other cargos are contemplated as an
alternative or in
addition to the ICD inducer. Such cargos include, inter alia, various cancer
chemotherapeutics as described herein. The lipid vesicle is typically formed
from a lipid
bilayer. However in certain embodiments, a lipid micelle (which does not
comprise a lipid
bilayer) is contemplated. Thus, for example, in certain embodiments a lipid
micelle can be
comprise a phospholipid prodrug (e.g., lipid-IDO pathway inhibitor conjugate)
and a cargo
(typically a lipophilic) cargo can be disposed inside the micelle. In certain
embodiments the
nanovesicle provides an IDO inhibitor and an ICD inducer that are synergistic
in their activity
against a cancer. In certain embodiments the nanovesicle drug carrier, when
administered
systemically, delivers an amount of an ICD inducer effective to induce or to
facilitate
induction of immunogenic cell death of cancer cells at the tumor site. In
certain
49
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
embodiments the nanovesicle drug carrier, when administered systemically,
delivers an
amount of IDO inhibitor to partially or fully inhibit an IDO pathway at a
cancer site.
[0322] In certain embodiments the inhibitor of the IDO pathway
comprises an agent
selected from the group consisting of 1-methyl-D-tryptophan (indoximod, D-
1MT), L-1MT,
methylthiohydantoin-dl-tryptophan (MTH-Trp, Necrostatin), P-carbolines (e.g.,
3-buty143-
carboline), naphthoquinone-based (e.g., annulin-B), S-allyl-brassinin, S-
benzyl-brassinin, N-
[2-(Indo1-3-yl)ethyll-S-methyl-dithiocarbamate, N-[2-(benzo[b]thiophen-3-
yl)ethyll-S-
methyl-dithiocarbamate, N[3-(Indo1-3-yl)propylFS-methyl-dithiocarbamate, S-
hexyl-
brassinin, N-[2-(indo1-3-yl)ethyll-S-benzyl-dithiocarbamate, N-[2-(indo1-3-
yl)ethyll-
SRnaphth-2-yemethyll-dithiocarbamate, N-[2-(indo1-3-yl)ethyll-S-Rpyrid-3-
y1)methyll-
dithiocarbamate, N-[2-(indo1-3-yl)ethyll-S-Rpyrid-4-yOmethyll-dithiocarbamate,
5-bromo-
brassinin, Phenylimidazole-based IDO inhibitors (e.g., 4-phenylimidazole),
Exiguamine A,
imidodicarbonimidic diamide,N-methyl-N'-9-phenanthrenyl-monohydrochloride
(NSC401366), INCB024360 (Epacadostat), 1-cyclohexy1-2-(5H-imidazo[5,1-
alisoindol-5-
yl)ethanol (GDC-0919), ID01-derived peptide, NLG919, Ebselen, Pyridoxal
Isonicotinoyl
Hydrazone, Norharmane, CAY10581, 2-Benzy1-2-thiopseudourea hydrochloride, and
4-
phenylimidazole. In certain embodiments the IDO inhibitor comprises indoximod.
In certain
embodiments the IDO inhibitor comprises substantially pure "L" indoximod or
substantially
pure "R" indoximod, or a racemic mixture of "D" and "L" indoximod.
[0323] In certain embodiments the inhibitor of the IDO pathway, is disposed
in a lipid
comprising the vesicle and/or conjugated to a lipid, or other component,
comprising the
vesicle. In certain embodiments the vesicle comprises a phospholipid. In
certain
embodiments the vesicle comprises a phospholipid, and cholesterol (CHOL). In
certain
embodiments the phospholipid comprises a saturated fatty acid with a C14-C20
carbon chain,
and/or an unsaturated fatty acid with a C14-C20 carbon chain, and/or a natural
lipid
comprising a mixture of fatty acids with C12-C20 carbon chains. In certain
embodiments the
phospholipid comprises a saturated fatty acid selected from the group
consisting of
phosphatidylcholine (DPPC), dimyristoylphosphatidylcholine (DMPC), 1,2-
dioleoyl-sn-
glycero-3-phosphocholine (DOPC), 1,2-Distearoyl-sn-glycero-3-phospho-rac-
glycerol
(DSPG), dipalmitoylphosphatidylglycerol (DPPG), distearoylphosphatidylcholine
(DSPC),
diactylphosphatidylcholine (DAPC), and the like. In certain embodiments the
phospholipid
comprises a natural lipid selected from the group consisting of egg
phosphatidylcholine (egg
PC), and soy phosphatidylcholine (soy PC). In certain embodiments the
phospholipid
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
comprises an unsaturated fatty acid selected from the group consisting of 1,2-
dimyristoleoyl-
sn-glycero-3-phosphocholine, 1,2-dipalmitoleoyl-sn-glycero-3-
phosphocholine,1,2-dioleoyl-
sn-glycero-3-phosphocholine (DOPC), and 1,2-dieicosenoyl-sn-glycero-3-
phosphocholine.
In certain embodiments the vesicle comprises an mPEG phospholipid with a
phospholipid
C14-C18 carbon chain, and a PEG molecular weight ranging from about 350 Da to
5000 Da.
In certain embodiments the vesicle comprises 1,2-distearoyl-sn-glycero-3-
phosphoethanolamine-PEG (DSPE-PEG). In certain embodiments the vesicle
comprises
DPSE-PEG2K. In certain embodiments the IDO inhibitor is conjugated to a
component of
said vesicle. In certain embodiments the IDO inhibitor is conjugated to a
moiety selected
from the group consisting of a lipid, PHGP, vitamin E, cholesterol, and a
fatty acid. In
certain embodiments the IDO inhibitor is conjugated directly to the moiety,
while in other
embodimetns, the IDO inhibitor is conjugated to the moiety via a linker. In
certain
embodiments the IDO inhibitor is conjugated to a phospholipid. In certain
embodiments the
IDO inhibitor is conjugated to vitamin E. In certain embodiments the IDO
inhibitor is
conjugated to cholesterol (CHOL (see, e.g., Formula II)) or to CHEMs, or to
squalene. In
certain embodiments the IDO inhibitor is conjugated to a fatty acid (e.g.,
oleic acid or
docosahexaenoic acid). In certain embodiments the inhibitor of the IDO pathway
is
conjugated to oleic acid or docosahexaenoic acid via an HO-(CH2)õ.2_5-0H
linker. In certain
embodiments the inhibitor of the IDO pathway is conjugated to a lipid. In
certain
embodiments the inhibitor of the IDO pathway is conjugated to a phospholipid
comprising
the lipid vesicle. In certain embodiments the inhibitor of the IDO pathway is
conjugated to
cholesterol (e.g., IND-Chol, Figure 8, Formula I).
[0324] In certain embodiments the bilayered vesicle comprises PL/IND-
Chol/DSPE-
PEG. In certain embodiments the vesicle comprises about 75% PL, about 20% IND-
cholesterol, and about 5% DSPE-PEG2K. In certain embodiments the ICD inducer
comprises
a chemotherapeutic agent selected from the group consisting of doxorubicin
(DOX),
mitoxantrone (MTX), oxaliplatin, anthracenedione, bleomycin, bortezomib,
cisplatin,
daunorubicin, docetaxel, epirubicin, idarubicin, mitoxanthrone, paclitaxel,
R2016, irinotecan
and cyclophosphamide. In certain embodiments the ICD inducer comprises
doxorubicin. In
certain embodiments the ICD inducer comprises mitoxantrone.
[0325] In certain embodiments the bilayered vesicle comprises PL/Chol-
IND/DSPE-
PEG. In certain embodiments the bilayered vesicle comprises DSPC/Chol-IND/DSPE-
PEG2K. In certain embodiments the bilayered vesicle comprises DSPC/Chol-
IND/DSPE-
51
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
PEG2K in the molar ratio 50:40:5. It will also be recognized that in certain
embodiments the
bilyaerd vesicle (e.g., liposome) can additionally include cholesteryl
hemisuccinate
(CHEMS) and/or the cholesteryl hemisuccinate can be conjugated to an IDO
pathway
inhibitor (indoximod). In certain embodiments, for systemic administration, a
liposome is
formatted that is about 100 nm in size, with slightly negative charge, and
about 5 to about
20% drug loading capacity. Different lipid compositions can be optimized by,
for example,
varying the molar ratios of IND-Chol, cholesterol, CHEMS, DSPE, and the like.
In certain
embodiments the bilayered vesicle comprises IND-Chol (salt free) 30%: CHEMS
20% :
DSPC 45% : DSPE-PEG2K 5%. This liposome formulation is now subjected to animal
experiment.
Dual-Delivery (ICD-inducer/IDO-inhibitor) LB Coated MSNPs (ICD/IDO
Silicasomes).
[0326] As noted above, in certain embodiments a dual delivery carrier
for an ICD
inducer (e.g., oxaliplatin, mitoxantrone (MTX), etc.) and an IDO inhibitor
(e.g., indoximod)
is contemplated where the carrier comprises lipid-bilayer coated nanoparticles
(e.g.,
mesoporous silica nanoparticles). In various illustrative embodiments, the IDO
inhibitor
(e.g., indoximod) is provided disposed in and/or conjugated to a component of
the lipid
bilayer (e.g. conjugated to cholesterol) while the ICD inducer is provided on
or in (e.g.,
within the pores) of the nanoparticle, e.g., effectively sealed/encapsulated
by the lipid bilayer.
However, it will be recognized that in certain embodiments the ICD inducer can
be provided
in or conjugated to the lipid bilayer while the IDO inhibitor is contained on
or within the
nanoparticle. Such lipid bilayer coated nanoparticle drug delivery systems
(aka silicasomes),
are capable of delivering two (or more) active agents in precise concentration
ratios as
desired.
[0327] In one illustrative, but non-limiting embodiment the "dual-delivery
carrier"
comprises indoximod conjugated to a component of the lipid bilayer (e.g., as
IND-
Cholesterol (IND-Chol) (Formula I) or IND-Cholesterol hemisuccinate (IND-
CHEMS),
while the ICD inducer (e.g., doxorubicin (DOX), mitoxantrone (MTX),
oxaliplatin, irinotecan
etc.) is disposed within the nanoparticle. This leads to stable entrapment of
the ICD-inducer
(e.g., doxorubicin (DOX), mitoxantrone (MTX), oxaliplatin (OX)) in the pores,
with Chol-
IND trapped in the bilayer. The coating, procedure(s) described herein provide
uniform and
instantaneous sealing of all particle pores.
52
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0328] Accordingly in certain embodiments, a nanoparticle drug carrier
for the
combined delivery of an inhibitor of an IDO pathway and an inducer of
immunogenic cell
death (ICD) is provided where the nanoparticle drug carrier comprises: a
mesoporous silica
nanoparticle having a surface and defining a plurality of pores that are
suitable to receive
molecules therein; a lipid bilayer coating the surface; a first cargo
comprising an inhibitor of
the indoleamine 2,3-dioxygenase (IDO) pathway (IDO inhibitor); and a second
cargo
comprising an agent that induces immunogenic cell death (ICD) (ICD-inducer);
where the
lipid bilayer is substantially continuous and encapsulates the nanoparticle
stably sealing the
plurality of pores. In certain embodiments the nanoparticle drug carrier
contains a predefined
ratio of IDO inhibitor to ICD-inducer. As illustrated herein in the Examples,
in certain
embodiments, the IDO inhibitor and the ICD inducer are synergistic in their
activity against a
cancer (e.g., against PDAC).
[0329] In various embodiments the drug carrier, when administered
systemically, is
effective to deliver an amount of an ICD inducer effective to initiate or to
facilitate induction
of immunogenic cell death of a cancer cell. In certain embodiments the drug
carrier, when
administered systemically, will effectively deliver an amount of IDO inhibitor
to partially or
fully inhibit an IDO pathway at a cancer site. In certain embodiments, where
the activity of
the ICD inducer and IDO inhibitor is synergistic, the drug carrier can
contain/provide a lower
dose ICD inducer and/or IDO inhibitor than when these agents are used
individually. In
certain embodiments the combination of the ICD inducer and the IDO inhibitor
can achieve
an anti-cancer activity that cannot be achieved by the use of either agent
alone.
[0330] In certain embodiments the IDO inhibitor is disposed in the
lipid bilayer
and/or conjugated to a component (e.g., PL, Chol, Chol derivative (e.g.,
cholesterol
hemisuccinate), etc.) comprising the lipid bilayer while the ICD inducer is
disposed in the
plurality of pores. In certain embodiments the ICD-inducer comprises a
chemical or
biological agent described in Table 2, above. In certain embodiments the ICD-
inducer
comprises a chemotherapeutic agent selected from the group consisting of
doxorubicin
(DOX), mitoxantrone (MTX), oxaliplatin (OX) anthracenedione, bleomycin,
bortezomib,
cisplatin, daunorubicin, docetaxel, epirubicin, idarubicin, paclitaxel, R2016,
irinotecan and
cyclophosphamide. In certain embodiments the ICD-inducer comprises doxirubicin
(DOX).
In certain embodiments the ICD-inducer comprises mitoxantrone (MTX). In
certain
embodiments the ICD-inducer comprises oxaliplatin (OX).
53
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0331] In certain embodiments the ICD inducer comprises an ICD
inducing
nanomaterial (e.g., CuO Cu2O, Sb203, As203 Bi203, P203, ZnO TiO2, graphene
oxide, 2D
materials other than graphene or graphene oxide (e.g., graphene, graphyne,
borophene,
germanene, silicene, Si2BN, stanene, phosphorene, bismuthene, molybdenite,
metals, 2D
supracrystals, and the like) as described above or in Example 10. In certain
embodiments, the
ICD-inducing nanomaterial can be contained on or within the nanoparticle. In
certain
embodiments an ICD-inducing nanomaterial can be coated with a lipid or with a
lipid bilayer.
In certain embodiments the ICD-inducing nanomaterial can incorporate one or
more drugs as
described herein. In certain embodiments, where the ICD-inducing nanomaterial
is within a
lipid bilayer the nanomaterial may contain the IDO inhibitor, both of which
can be released at
a target site (e.g., cancer cell). In certain embodiments, where the ICD-
inducing
nanomaterial comprises graphene oxide, the surface can be functionalized to
deliver the IDO-
inhibitor.
[0332] In certain embodiments, the IDO inhibitor comprises an agent
selected from
the group consisting of 1-methyl-D-tryptophan (indoximod, D-1MT), L-1MT,
methylthiohydantoin-dl-tryptophan (MTH-Trp, Necrostatin), [3-carbolines (e.g.,
3-butyl-[3-
carboline), Naphthoquinone-based (e.g., annulin-B), S-allyl-brassinin, S-
benzyl-brassinin,
N-[2-(Indo1-3-yeethy1]-S-methyl-dithiocarbamate, N-[2-(benzo[b]thiophen-3-
yl)ethy1]-S-
methyl-dithiocarbamate, N[3-(Indo1-3-yepropyll-S-methyl-dithiocarbamate, S-
hexyl-
brassinin, N-[2-(indo1-3-yl)ethyll-S-benzyl-dithiocarbamate, N-[2-(indo1-3-
yl)ethyll-
S [(naphth-2-yemethyll-dithiocarbamate, N-[2-(indo1-3-yl)ethyll-S-Rpyrid-3-
y1)methyll-
dithiocarbamate, N-[2-(indo1-3-yl)ethyll-S-Rpyrid-4-y1)methyll-
dithiocarbamate, 5-bromo-
brassinin, Phenylimidazole-based IDO inhibitors (e.g., 4-phenylimidazole),
Exiguamine A,
imidodicarbonimidic diamide,N-methyl-N'-9-phenanthrenyl-monohydrochloride
(NSC401366), INCB024360 (Epacadostat), 1-cyclohexy1-2-(5H-imidazo[5,1-
alisoindol-5-
yl)ethanol (GDC-0919), ID01-derived peptide, NLG919, Ebselen, Pyridoxal
Isonicotinoyl
Hydrazone, Norharmane, CAY10581, 2-Benzy1-2-thiopseudourea hydrochloride, and
4-
phenylimidazole. In certain embodiments the IDO inhibitor comprises an agent
shown in
Table 3, above. In certain embodiments the IDO inhibitor comprises indoximod.
[0333] In certain embodiments, the nanoparticle drug carrier(s) can be
fabricated so
that a population of the drug carriers in suspension shows essentially a
substantially unimodal
size distribution; and/or shows a PDI less than about 0.2, or less than about
0.1; and/or shows
a coefficient of variation in size less than about 0.1 or less than about
0.05. In certain
54
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
embodiments, the nanoparticle drug carriers may distribute to developing tumor
sites on IV
injection. In certain embodiments the nanoparticle drug carrier forms a stable
suspension on
rehydration after lyophilization. In certain embodiments the nanoparticle drug
carriers, show
reduced drug toxicity as compared to free drug and/or drug in liposomes. In
certain
embodiments the nanoparticle drug carrier has colloidal stability in
physiological fluids with
pH 7.4 and remains monodisperse to allow systemic biodistribution and is
capable of entering
a disease site by vascular leakage (EPR effect) or transcytosis.
[0334] Various nanoparticle (e.g., mesoporous silica core), lipid
bilayer formulations,
and methods of synthesis are described in the sections below and in the
examples.
Nanoparticles.
[0335] In various embodiments silicasome drug carriers described
herein comprise a
porous silica (or other material) nanoparticle (e.g., a silica body having a
surface and defining
a plurality of pores that are suitable to receive molecules therein) coated
with a lipid bilayer.
For example, in certain embodiments the silica nanoparticle can be a
mesoporous silica
nanoparticle. The fact that the nanoparticle is referred to as a silica
nanoparticle does not
preclude materials other than silica from also being incorporated within the
silica
nanoparticle. In some embodiments, the silica nanoparticle may be
substantially spherical
with a plurality of pore openings through the surface providing access to the
pores. However,
in various embodiments the silica nanoparticle can have shapes other than
substantially
.. spherical shapes. Thus, for example, in certain embodiments the silica
nanoparticle can be
substantially ovoid, rod-shaped, a substantially regular polygon, an irregular
polygon, and the
like.
[0336] Generally, the silica nanoparticle comprises a silica body that
defines an outer
surface between the pore openings, as well as side walls within the pores. The
pores can
extend through the silica body to another pore opening, or a pore can extend
only partially
through the silica body such that that it has a bottom surface of defined by
the silica body.
[0337] In some embodiments, the silica body is mesoporous. In other
embodiments,
the silica body is microporous. As used herein, "mesoporous" means having
pores with a
diameter between about 2 nm and about 50 nm, while "microporous" means having
pores
with a diameter smaller than about 2 nm. In general, the pores may be of any
size, but in
typical embodiments are large enough to contain one or more therapeutic
compounds therein.
In such embodiments, the pores allow small molecules, for example, therapeutic
compounds
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
such as anticancer compounds to adhere or bind to the inside surface of the
pores, and to be
released from the silica body when used for therapeutic purposes. In some
embodiments, the
pores are substantially cylindrical.
[0338] In certain embodiments the nanoparticles comprise pores having
pore
diameters between about 1 nm and about 10 nm in diameter or between about 2 nm
and about
8 nm. In certain embodiments the nanoparticles comprise pores having pore
diameters
between about 1 nm and about 6 nm, or between about 2 nm and about 5 nm. Other
embodiments include particles having pore diameters less than 2.5 nm. In other
embodiments, the pore diameters are between 1.5 and 2.5 nm. Silica
nanoparticles having
.. other pore sizes may be prepared, for example, by using different
surfactants or swelling
agents during the preparation of the silica nanoparticles.
[0339] In various embodiments the nanoparticles can include particles
as large (e.g.,
average or median diameter (or other characteristic dimension) as about 1000
nm. However,
in various embodiments the nanoparticles are typically less than 500 nm or
less than about
.. 300 nm as, in general. particles larger than 300 nm may be less effective
in entering living
cells or blood vessel fenestrations. In certain embodiments the nanoparticles
range in size
from about 40 nm, or from about 50 nm, or from about 60 nm up to about 100 nm,
or up to
about 90 nm, or up to about 80 nm, or up to about 70 nm. In certain
embodiments the
nanoparticles range in size from about 60 nm to about 70 nm. Some embodiments
include
nanoparticles having an average maximum dimension between about 50 nm and
about 1000
nm. Other embodiments include nanoparticles having an average maximum
dimension
between about 50 nm and about 500 nm. Other embodiments include nanoparticles
having
an average maximum dimension between about 50 nm and about 200 nm. In some
embodiments, the average maximum dimension is greater than about 20nm, greater
than
about 30nm, greater than 40nm, or greater than about 50nm. Other embodiments
include
nanoparticles having an average maximum dimension less than about 500 nm, less
than about
300nm, less than about 200nm, less than about 100 nm or less than about 75 nm.
As used
herein, the size of the nanoparticle refers to the average or median size of
the primary
particles, as measured by transmission electron microscopy (TEM) or similar
visualization
technique.
[0340] Illustrative mesoporous silica nanoparticles include, but are
not limited to
MCM-41, MCM-48, and SBA-15 (see, e.g., Katiyaret a/. (2006)1 Chrornatog.
1122(1-2):
13-20).
56
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0341] Methods of making porous silica nanoparticles are well known to
those of skill
in the art. In certain embodiments mesoporous silica nanoparticles are
synthesized by
reacting tetraethyl orthosilicate (TEOS) with a template made of micellar
rods. The result is
a collection of nano-sized spheres or rods that are filled with a regular
arrangement of pores.
The template can then be removed by washing with a solvent adjusted to the
proper pH (see,
e.g., Trewyn et al. (2007) Chem. Eng. J. 137(1): 23-29. In certain embodiments
mesoporous
particles can also be synthesized using a simple sol-gel method (see, e.g.,
Nandiyanto, et al.
(2009) Microporous and Mesoporous Mat. 120(3): 447-453, and the like). In
certain
embodiments tetraethyl orthosilicate can also be used with an additional
polymer monomer
(as a template). In certain embodiments 3-mercaptopropyl)trimethoxysilane
(MPTMS) is
used instead of TEOS.
[0342] In certain embodiments the mesoporous silica nanoparticles are
cores are
synthesized by a modification of the sol/gel procedure described by Meng et
al. (2015) ACS
Nano, 9(4): 3540-3557. To synthesize a batch of ¨500 mg of MSNP, 50 mL of CTAC
is
.. mixed with 150 mL of H20 in a flask (e.g., a 500 mL conical flask),
followed by stirring
(e.g., at 350 rpm for 15 min at 85 C). This us followed by the addition of 8
mL of 10%
triethanolamine for 30 min at the same temperature. Then, 7.5 mL of the silica
precursor.
TEOS, is added dropwise at a rate of 1 mL/min using a peristaltic pump. The
solution is
stirred at 350 rpm at 85 C for 20 min, leading to the formation particles with
a primary size
.. of ¨65 nm. The surfactant can be removed by washing the particles with a
mixture of
methanol/HC1 (500:19 v/v) at room temperature for 24 h. The particles can be
centrifuged at
10 000 rpm for 60 min and washed three times in methanol.
[0343] While the methods described herein have been demonstrated with
respect to
porous silica nanoparticles (e.g., mesoporous silica), it will be recognized
that similar
methods can be used with other porous nanoparticles. Numerous other mesoporous
materials
that can be used in drug delivery nanoparticles are known to those of skill in
the art. For
example, in certain embodiments mesoporous carbon nanoparticles could be
utilized.
Mesoporous carbon nanoparticles are well known to those of skill in the art
(see, e.g., Huang
et al. (2016) Carbon, 101: 135-142; Zhu et al. (2014) Asian J. Pharm. Sci.,
9(2): 82-91; and
the like).
[0344] Similarly, in certain embodiments, mesoporous polymeric
particles can be
utilized. The syntheses of highly ordered mesoporous polymers and carbon
frameworks from
organic¨organic assembly of triblock copolymers with soluble, low-molecular-
weight
57
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
phenolic resin precursors (resols) by an evaporation induced self-assembly
strategy have been
reported by Meng et al. (2006) Chem. Mat. 6(18): 4447-4464 and in the
references cited
therein.
[0345] The nanoparticles described herein are illustrative and non-
limiting. Using the
teachings provided herein numerous other lipid bilayer coated nanoparticles
will be available
to one of skill in the art.
Lipid Bilayer and Methods of Coating Nanoparticles With a Lipid
Bilayer.
[0346] The drug carrier nanoparticles described herein comprise a
porous
nanoparticle (e.g. a mesoporous silica nanoparticle (MSNP)) coated with a
lipid bilayer. In
certain embodiments the bilayer composition is optimized to provide a rapid
and uniform
particle coating, to provide colloidal and circulatory stability, and to
provide effective cargo
retention, while also permitting a desirable cargo release profile.
[0347] In certain embodiments the lipid bilayer comprises a
combination of a
phospholipid, cholesterol, and in certain embodiments, a IDO-lipid conjugate,
a pegylated
lipid (e.g., DSPE-PEG2000), or a factionalized pegylated lipid (e.g., DSPE-
PEG2000-
maleimide) to facilitate conjugation with targeting or other moieties.
[0348] To attach a surface LB coating, a coated lipid film procedure
can be utilized in
which MSNP suspensions are added to a large lipid film surface, coated on,
e.g., a round-
bottom flask. Using different lipid bilayer compositions, a series of
experiments can be
performed to find a composition and optimal lipid/particle ratio that provides
rapid and
uniform particle wrapping, coating and effective cargo retention and/or
release upon
sonication. It is believed that this lipid composition and wrapping cannot be
achieved by
liposomal fusion to the particle surface under low energy vortexing
conditions.
[0349] In certain embodiments, the mesoporous silica nanoparticles are
coated with a
lipid bilayer that incorporates the IDO inhibitor coupled to a lipid (e.g., a
phospholipid) or to
cholesterol. In one illustrated embodiment the mesoporous silica nanoparitcles
are coated
with a lipid bilayer comprising IND-Chol, as well as serving to encapsulate
the ICD inducer
(e.g., doxorubicin (DOX), mitoxantrone (MTX), oxaliplatin, etc.) in the porous
interior (see,
e.g,. Figure 7, panel a). In another illustrated embodiment the mesoporous
silica
nanoparitcles are coated with a lipid bilayer comprising Chol-IND, as well as
serving to
encapsulate dwdrubicin, or oxaliplatin in the porous interior (Figure 7, panel
a).
58
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0350] In various embodiments lipid bilayer composition can be
optimized for an
OX/IND or for an MTX/IND drug delivery carrier (e.g., a bilayer coated
nanoparticle). This
can accomplished, for example, by using a DSPC/Cho-IND/DSPE-PEG2K or a
DSPC/Chol-
IND/CHEMS/DSPE-PEG2k mixture at various ratios and measuring the incorporated
IND.
The biofilm can be laid down at the bottom of a round bottom flask, to which
the OX-soaked
or MTX-soaked, or other ICD inducer soaked) MSNPs are added, followed by
sonication
(see, e.g., Liu et al. (2016) ACS Nano, 10(2): 2702-2715; Meng et al. (2015)
ACS Nano, 9(4):
3540-3557).
[0351] The lipid bilayer formulation described above and in the
Examples is
illustrative and non-limiting. Depending on the drug(s) being loaded into the
drug delivery
carrier and the desired release profile, in various embodiments different
lipid bilayer
formulations can be used and an optimal formulation can be determined.
[0352] Accordingly, in certain embodiments the lipid bilayer can
comprise: 1) one or
more saturated fatty acids with C14-C20 carbon chain, such as
dimyristoylphosphatidylcholine (DMPC), dipalmitoylphosphatidylcholine (DPPC),
distearoylphosphatidylcholine (DSPC), and diactylphosphatidylcholine (DAPC);
and/or 2)
One or more unsaturated fatty acids with a C14-C20 carbon chain, such as 1,2-
dimyristoleoyl-sn-glycero-3-phosphocholine, 1,2-dipalmitoleoyl-sn-glycero-3-
phosphocholine,1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-
dieicosenoyl-sn-
glycero-3-phosphocholine; and/or 3) Natural lipids comprising a mixture of
fatty acids with
C12-C20 carbon chain, such as Egg PC, and Soy PC, sphingomyelin, and 4) a
modified
cholesterol (e.g., cholesterol hemisuccinate (CHEMS)) the like. It is noted
that, in certain
embodiments, in order to compensate a positive charge that introduce during
Chol-IND
conjugation chemistry, it is possible to use cholesteryl hemisuccinate (CHEMS)
that carryies
one negative charge at pH >6.5 in the formulation. These lipids are
illustrative but non-
limiting and numerous other lipids are known and can be incorporated into a
lipid bilayer for
formation of a drug delivery nanocarrier (e.g., a bilayer-coated
nanoparticle).
[0353] In certain embodiments the drug carrier comprises bilayer
comprising a lipid
(e.g., a phospholipid), cholesterol (e.g., IND-Chol), and a PEG functionalized
lipid (e.g., a
mPEG phospholipid). In certain embodiments the mPEG phospholipids comprises a
C14-
C18 phospholipid carbon chain from, and a PEG molecular weight from 350-5000
(e.g.,
MPEG 5000, MPEG 3000, MPEG 2000, MPEG 1000, MPEG 750, MPEG 550, MPEG 350,
and the like). In certain embodiments the mPEG phospholipid comprises DSPE-
PEG5000,
59
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
DSPE-PEG3000, DSPE-PEG2000, DSPE-PEG1000, DSPE-PEG750, DSPE-PEG550, or
DSPE-PEG350. MPEGs are commercially available (see, e.g.,
//avantilipids.com/product-
category/products/polymers-polymerizable-lipids/mpeg-phospholipids).
[0354] In certain embodiments lipid bilayer comprises an mPEG
phospholipid with a
phospholipid C14-C18 carbon chain, and a PEG molecular weight ranging from
about 350
Da to 5000 Da. In certain embodiments the lipid bilayer comprises DPSE-PE&K.
[0355] In certain embodiments the lipid bilayer comprises 1,2-
distearoyl-sn-glycero-
3-phosphoethanolamine-PEG (DSPE-PEG).
[0356] In certain embodiments the IDO inhibitor is conjugated to a
moiety that forms
a component of a vesicle structure in aqueous solution and is provided in the
lipid bilayer
(see, e.g., conjugated IDO inhibitors, supra.). In certain embodiments the IDO
inhibitor is
conjugated to a moiety such as a lipid, vitamin E, cholesterol, and a fatty
acid (see, e.g.,
Examples 1 and 2). In various embodiments the IDO inhibitor is conjugated
directly to the
vesicle-forming moiety and in other embodiments the IDO inhibitor is
conjugated to the
vesicle-forming moiety via a linker (e.g., via a homo-bifunctional or hetero-
bifunctional
linker). In certain embodiments the linker comprises an HO-(CH2)õ=2_5-0H
linker.
[0357] In certain embodiments the inhibitor of the IDO pathway is
conjugated to a
lipid, and/or to vitamin E, and/or to cholesterol (CHOL), and/or to a fatty
acid (e.g., oleic
acid, docosahexaenoic acid, etc.). In certain embodiments the IDO inhibitor is
conjugated to
a cholesterol.
[0358] In certain embodiments the IDO inhibitor is conjugated to a
phospholipid
comprising the lipid bilayer or to cholesterol comprising said lipid bilayer.
In certain
embodiments the IDO inhibitor is directly conjugated to cholesterol.
[0359] In certain embodiments the ratio of phospholipid: IND-CHOL:PEG,
is about
phospholipid (50-90 mol%): CHOL (10-50 mol%) : PEG (1-10 mol%). In certain
embodiments the bilayer comprises DSPC/Cho-IND/DSPE-PEG2K. In certain
embodiments
the bilayer comprises DSPC/Cho-IND/DSPE-PEG2K in the molar ratio 50:40:5.
[0360] In certain embodiments the lipid bilayer is formulated to form
a substantially
uniform and intact bilayer encompassing the entire nanoparticle. In certain
embodiments the
lipid bilayer is formulated so that the mesoporous silica nanoparticle is
colloidally stable.
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
Dual Delivery Lipid-Coated ICD-Inducing Nanomaterials.
[0361] It was discovered that certain nanomaterials are effective ICD
inducers (see,
e.g., Example 5). In certain embodiments these ICD-inducing nanomaterials can
be
administered simply as nanoparticles. However, in other embodiments, the nano
particles
can be combined with a lipid where the lipid is associated with (e.g.,
complexed with or
conjugated to) an IDO pathway inhibitor (e.g., indoximod). In certain
embodiments the lipid
can compire an IND conjugated phospholipid (IND-PL) or IND conjugated
cholesterol
(Chol-IND) (Formula I). The lipid readily coats all or a part of the surface
of the
nanoparticle.
[0362] Accordingly in certain embodiments, a nanomaterial carrier for the
combined
delivery of an inhibitor of an IDO pathway and an inducer of immunogenic cell
death (ICD),
is provided where the nanomaterial carrier comprises a nanomaterial that
induces ICD; and a
lipid or lipid formulation comprising an IDO pathway inhibitor where the lipid
or lipid
formulation is disposed on the surface of said nanomaterial. In certain
embodiments the lipid
or lipid formulation fully encapsulates the nanomaterial, while in other
embodiments, the
lipid or lipid formulation is disposed on a surface of the nanoparticle, but
does not fully
encapsulate the nanoparticle. In certain embodiments the lipid or lipid
formulation can form
a lipid bilayer, while more typically, the lipid or lipid formulation is not a
lipid bilayer.
[0363] In certain embodiments the ICD-inducing nanomaterial comprises
one or more
ICD-inducing nanomaterials selected from the group consisting of CuO, Cu2O,
Sb203, As203,
Bi203, P203, ZnO, TiO2, graphene oxide, 2D materials other than graphene or
graphene oxide
(e.g., graphene, graphyne, borophene, germanene, silicene, Si2BN, stanene,
phosphorene,
bismuthene, molybdenite, metals, 2D supracrystals, and the like) and other ICD-
inducing
nanomaterials as described herein. In certain embodiments the nanomaterial
comprises
copper oxide (Cu0). In certain embodiments the nanomaterial comprises graphene
oxide
(GO).
[0364] In certain embodiments the IDO pathway inhibitor associated
with the lipid or
lipid formulation comprises an agent selected from the group consisting of 1-
methyl-D-
tryptophan (indoximod, D-1MT), L-1MT, methylthiohydantoin-dl-tryptophan (MTH-
Trp,
Necrostatin), 13-carbolines (e.g., 3-butyl-r3-carboline), naphthoquinone-based
(e.g., annulin-
B), S-allyl-brassinin, S-benzyl-brassinin, N-[2-(Indo1-3-yl)ethyll-S-methyl-
dithiocarbamate,
N-[2-(benzo[b[thiophen-3-yl)ethyll-S-methyl-dithiocarbamate, N43-(Indo1-3-
yl)propyll-S-
methyl-dithiocarbamate, S-hexyl-brassinin, N-[2-(indo1-3-yl)ethyll-S-benzyl-
61
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
dithiocarbamate, N-[2-(indo1-3-yl)ethyll-S[(naphth-2-y1)methyll-
dithiocarbamate, N-[2-
(indo1-3-yeethy1]-S-[(pyrid-3-y1)methyl]-dithiocarbamate, N-[2-(indo1-3-
yl)ethyl]-S-Rpyrid-
4-yl)methyll-dithiocarbamate, 5-bromo-brassinin, Phenylimidazole-based IDO
inhibitors
(e.g., 4-phenylimidazole), Exiguamine A, imidodicarbonimidic diamide,N-methyl-
N-9-
phenanthrenyl-monohydrochloride (NSC401366), INCB024360 (Epacadostat), 1-
cyclohexy1-2-(5H-imidazo[5,1-a]isoindol-5-yl)ethanol (GDC-0919), ID01-derived
peptide,
NLG919, Ebselen, Pyridoxal Isonicotinoyl Hydrazone, Norharmane, CAY10581, 2-
Benzy1-
2-thiopseudourea hydrochloride, and 4-phenylimidazole. In certain embodiments
the IDO
pathway inhibitor associated with the lipid or lipid formulation comprises 1
methyl-
tryptophan (1MT)). In certain embodiments the 1 methyl-tryptophan is a
substantially pure
"D" isomer of 1-methyl-tryptophan (D-1MT), while in other embodiments, the 1-
methyl-
tryptophan is a substantially pure "L" isomer of 1-methyl-tryptophan "L-1MT.
In certain
embodiments the 1-methyl-tryptophan comprises a mixture of the D and L
isomers.
[0365] In certain embodiments the IDO pathway inhibitor is conjugated
to a lipid or
to a component of the lipid formulation. In certain embodiments the IDO
pathway inhibitor
is conjugated to a moiety selected from the group consisting of a lipid (e.g.,
phospholipid),
vitamin E, cholesterol, cholesterol derivative (e.g., cholesterol
hemisuccinate (CHEMS)) and
a fatty acid. In certain embodiments the IDO inhibitor is conjugated directly
to the moiety,
while in other emobodiments, the IDO inhibitor is conjugated to the moiety via
a linker.
[0366] In certain embodiments the IDO pathway inhibitor is conjugated to
PGHP,
vitamin E, cholesterol (CHOL), a fatty acid, (e.g., oleic acid or
docosahexaenoic acid), or to a
lipid (e.g., a phospholipid). In certain embodiments the IDO pathway inhibitor
is conjugated
to a phospholipid. Illustrative phospholipids include, but are not limited to
phospholipids
comprising a saturated fatty acid with a C14-C20 carbon chain, and/or an
unsaturated fatty
acid with a C14-C20 carbon chain, and/or a natural lipid comprising a mixture
of fatty acids
with C12-C20 carbon chains. In certain embodiments the phospholipid comprises
a saturated
fatty acid selected from the group consisting of phosphatidylcholine (DPPC),
dimyristoylphosphatidylcholine (DMPC), distearoylphosphatidylcholine (DSPC),
and
diactylphosphatidylcholine (DAPC). In certain embodiments the phospholipid
comprises a
natural lipid selected from the group consisting of egg phosphatidylcholine
(egg PC), and soy
phosphatidylcholine (soy PC). In certain embodiments the phospholipid
comprises an
unsaturated fatty acid selected from the group consisting of 1,2-
dimyristoleoyl-sn-glycero-3-
phosphocholine, 1,2-dipalmitoleoyl-sn-glycero-3-phosphocholine,1,2-dioleoyl-sn-
glycero-3-
62
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
phosphocholine (DOPC), and 1,2-dieicosenoyl-sn-glycero-3-phosphocholine. In
certain
embodiments the phospholipid comprises 1-palmitoy1-2-hydroxy-sn-glycero-3-
phosphocholine.
[0367] In certain embodiments the IDO pathway inhibitor comprises an
agent
selected from the group consisting of 1-methyl-D-tryptophan (indoximod), 1-
methyl-L-
tryptophan, methylthiohydantoin-dl-tryptophan, Necrostatin-1, Ebselen,
Pyridoxal
Isonicotinoyl Hydrazone, Norharmane, CAY10581, 2-Benzy1-2-thiopseudourea
hydrochloride, Norharmane hydrochloride, INCB024360, S-allyl-brassinin, S-
benzyl-
brassinin, 5-Bromo-brassinin. 4-phenylimidazole Exiguamine A, and NSC401366.
In certain
embodiments the IDO pathway inhibitor comprises indoximod. In certain
embodiments the
IDO pathway inhibitor comprises substantially pure "L" isomer of 1-methyl-
tryptophan, or a
substantially pure "D" isomer of 1-methyl-tryptophan, or a racemic mixture of
"D" and "L"
isomers of 1-methyl-tryptophan. In certain embodiments the 1-methyl-tryptophan
is
conjugated to cholesterol (e.g., Chol-IND, Formula I) and/or to cholesterol
hemisuccinate
(CHEMS-IND). In certain embodiments where the lipid bilayer comprises both
cholesterol
and a cholesterol derivative (e.g., CHEMS), the 1-methyl-tryptophan conjugated
to the
cholesterol or to CHEMS, or to both cholesterol and to CHEMS.
Approach 2 --Local treatment of a tumor or peritumor site to inhibit the IDO
pathway
and to induce ICD.
[0368] A second treatment modality involves local delivery to a tumor or
peri-
tumoral region, of an agent that induces ICD (e.g., doxirubicin, oxaliplatin,
etc.) in
combination with an inhibitor of the IDO pathway (e.g., indoximod). In certain
embodiments, the IDO inhibitor can be complexed with or conjugated to a moiety
(e.g., a
lipid) that forms a vesicle (e.g., a nanovesicle). It is believed that such
local delivery of an
ICD inducer in combination with an IDO inhibitor induces recruitment of
cytotoxic CD8+
lymphocytes, depletion of Tregs, reversal of the CD8+/Foxp3+ ratio, cytotoxic
tumor killing,
and tumor shrinkage at the local site. It is believed that these adaptive
immune responses can
be accompanied by boosting of the innate immune system, as reflected by CRT
and HMGB1
expression, as well as the activation of a DC population, particularly well-
suited for
generating cytotoxic T cell responses.
[0369] Accordingly in certain embodiments, a method of treating a
cancer in a
mammal is provided where the method involves administering to an intra-tumoral
or pen-
63
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
tumoral site an effective amount of an inhibitor of the indoleamine 2,3-
dioxygenase (IDO)
pathway (an IDO inhibitor) in conjunction with an effective amount of an agent
that induces
immunogenic cell death (ICD) (an ICD-inducer). In certain embodiments, the
effective
amount of the ICD-inducer is an amount effective to elevate calreticulin (CRT)
expression
and/or to elevate expression and/or release of HMGB1 and/or introduce ATP
release in cells
of the cancer.
[0370] ICD inducers are well known to those of skill in the art and
ICD inducers
suitable for this method will readily be recognized in view of the teachings
provided herein.
Illustrative ICD inducers include, but are not limited to chemotherapeutic
agent(s) that induce
ICD such as oxaliplatin, anthracenedione, bleomycin, bortezomib, cisplatin,
daunorubicin,
docetaxel, doxorubicin, epirubicin, idarubicin, mitoxanthrone, oxaliplatin,
paclitaxel, R2016
(a heterocyclic quinolone derivative described by Son et al. (2017) Plos One,
DOI:10.1371,
which is incorporated herein by reference for the compounds described
therein), irinotecan
and cyclophosphamide.
[0371] Other suitable ICD inducers include oncolytic viruses (see, e.g.,
Angelova et
al. (2014) J. Virol., 88(10): 5263-52760. One illustrative suitable oncolytic
virus is an
oncolytic parvovirus (e.g., H-PV).
[0372] As explained above, and in Example, 2, it was discovered that
certain
nanomaterials can induce ICD. In certain embodiments the ability to induce ICD
is an
intrinsic property of the nanomaterial (e.g., chemical reaction of the
material and/or receptor
binding of the nanomaterial is not required for induction of ICD).
Accordingly, in certain
embodiments the tumor or peritumoral space is treated with a nanomaterial that
induces ICD.
Such materials include, but are not limited to e.g., CuO, Cu2O. Sb203, As203,
Bi203, P203,
ZnO, TiO2, graphene oxide, 2D materials other than graphene or graphene oxide
(e.g.,
graphene, graphyne, borophene, germanene, silicene, Si2BN, stanene,
phosphorene,
bismuthene, molybdenite, metals, 2D supracrystals, etc.) and the like) (see,
e.g., Example 2)
nanoparticles comprising such materials. In certain embodiments the
nanoparticle is entirely
fabricated from said materials. In certain embodiments the nanoparticle
comprises a doped
material containing said materials. In certain embodiments the nanoparticle
comprises a
core-shell structure compmrising said ICD inducing materials. Accordingly, in
certain
embodiments ICD is induced by contacting the cancer cells with a nanomaterial
(e.g., CuO,
Sb203, ZnO, TiO2, and graphene oxide) that induced ICD.
64
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0373] It will also be recognized that in various embodiments, two or
more ICD
inducers can be used to induce ICD via local delivery.
[0374] In certain embodiments, the ICD inducer comprises at least
oxaliplatin, or
doxirubicin e.g., as described in Examples 3 and 4.
[0375] As noted above, the ICD inducer can be used in conjunction with an
IDO
inhibitor. Numerous IDO inhibitors are known to those of skill in the art
(see, discussion
below) and the use of one or more of these IDO inhibitors is contemplated. In
certain
embodiments the IDO inhibitor(s) comprise a conjugated IDO inhibitor as
described herein.
In certain embodiments the IDO inhibitors comprise indoximod or a conjugated
indoximod as
described below and in Examples 1 and 2. In certain embodiments the IDO
inhibitors
comprise substantially pure "D" indoximod, or substantially pure "L"
indoximod, or
conjugated substantially pure "D" indoximod, or conjugated substantially pure
"L"
indoximod.
[0376] In certain embodiments the ICD inducer and the inhibitor of the
IDO pathway
are delivered locally to a target site. In certain embodiments the ICD inducer
and the
inhibitor of the IDO pathway can be delivered directly to a tumor site, e.g.,
by injection, or
through a cannula. In certain embodiments the ICD inducer and the inhibitor of
the IDO
pathway are delivered into a tumor mass and/or into a peritumoral site. In
certain
embodiments the ICD inducer and the inhibitor of the IDO pathway can be
delivered as
separate reagents. Alternatively, they can be delivered as a combined
formulation. In certain
embodiments the combined formulation comprise nanovesicles and/or lipid
bilayer coated
silica nanoparticles, e.g. as described herein, or suitable other dual
delivery carriers that
contain an IDO inhibitor plus a nanomaterial capable of inducing ICD.
[0377] In certain embodiments the ICD inducer and the IDO pathway
inhibitor are
delivered via an implantable depot delivery system (e.g., encapsulated in a
controlled release
polymer, a hydrogel, and the like). In certain embodiments both the ICD
inducer and the the
IDO pathway inhibitor are in implantable depot delivery systems and in other
embodiments
only the the IDO pathway inhibitor or the ICD inducer is in an implantable
depot delivery
system.
[0378] In certain embodiments the ICD inducer and the IDO pathway inhibitor
are
used in combination as a primary therapy. In certain embodiments the ICD
inducer and the
IDO pathway inhibitor are used as an adjunct therapy, e.g., in combination
with other
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
chemotherapeutics, and/or surgery, and/or radio therapy. In certain
embodiments the ICD
inducer and the the IDO pathway inhibitor are delivered to a surgical site
during or after
removal of a tumor mass.
[0379] In view of the examples and teachings provided herein, it will
be recognized
that the co-delivery of an ICD inducer and the IDO pathway inhibitor will find
use in the
treatment of a number of cancers. Illustrative cancers include, but are not
limited to
pancreatic ductal adenocarcinoma (PDAC), acute lymphoblastic leukemia (ALL),
acute
myeloid leukemia (AML), Adrenocortical carcinoma, Kaposi sarcoma, anal cancer,
appendix
cancer, astrocytoma, atypical teratoid/rhabdoid tumor, bile duct cancer,
extrahepatic cancer,
bladder cancer, bone cancer (e.g., Ewing sarcoma, osteosarcoma, malignant
fibrous
histiocytoma), brain stem glioma, brain tumors (e.g., astrocytomas, brain and
spinal cord
tumors, brain stem glioma, central nervous system atypical teratoid/rhabdoid
tumor, central
nervous system embryonal tumors, central nervous system germ cell tumors,
craniopharyngioma, ependymoma, breast cancer, bronchial tumors, burkitt
lymphoma,
carcinoid tumors (e.g., childhood, gastrointestinal), cardiac tumors, cervical
cancer,
chordoma, chronic lymphocytic leukemia (CLL), chronic myelogenous leukemia
(CML),
chronic myeloproliferative disorders, colon cancer, colorectal cancer,
craniopharyngioma,
cutaneous t-cell lymphoma, duct cancers e.g. (bile, extrahepatic), ductal
carcinoma in situ
(DCIS), embryonal tumors, endometrial cancer, ependymoma, esophageal cancer,
esthesioneuroblastoma, extracranial germ cell tumor, extragonadal germ cell
tumor,
extrahepatic bile duct cancer, eye cancer (e.g., intraocular melanoma,
retinoblastoma), fibrous
histiocytoma of bone, malignant, and osteosarcoma, gallbladder cancer, gastric
(stomach)
cancer, gastrointestinal carcinoid tumor, gastrointestinal stromal tumors
(GIST), germ cell
tumors (e.g., ovarian cancer, testicular cancer, extracranial cancers,
extragonadal cancers,
central nervous system), gestational trophoblastic tumor, brain stem cancer,
hairy cell
leukemia, head and neck cancer, heart cancer, hepatocellular (liver) cancer,
histiocytosis,
langerhans cell cancer, Hodgkin lymphoma, hypopharyngeal cancer, intraocular
melanoma,
islet cell tumors, kaposi sarcoma, kidney cancer (e.g., renal cell, Wilms
tumor, and other
kidney tumors), langerhans cell histiocytosis, laryngeal cancer, leukemia,
acute
lymphoblastic (ALL), acute myeloid (AML), chronic lymphocytic (CLL), chronic
myelogenous (CML), hairy cell, lip and oral cavity cancer, liver cancer
(primary), lobular
carcinoma in situ (LCIS), lung cancer (e.g., childhood, non-small cell, small
cell), lymphoma
(e.g., AIDS-related, Burkitt (e.g., non-Hodgkin lymphoma), cutaneous T-Cell
(e.g., mycosis
66
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
fungoides, Sezary syndrome), Hodgkin, non-Hodgkin, primary central nervous
system
(CNS)), macroglobulinemia, Waldenstrom, male breast cancer, malignant fibrous
histiocytoma of bone and osteosarcoma, melanoma (e.g., childhood, intraocular
(eye)),
merkel cell carcinoma, mesothelioma, metastatic squamous neck cancer, midline
tract
carcinoma, mouth cancer, multiple endocrine neoplasia syndromes, multiple
myeloma/plasma cell neoplasm, mycosis fungoides, myelodysplastic syndromes,
Myelogenous Leukemia, Chronic (CML), multiple myeloma, nasal cavity and
paranasal sinus
cancer, nasopharyngeal cancer, neuroblastoma, oral cavity cancer, lip and
oropharyngeal
cancer, osteosarcoma, ovarian cancer, pancreatic cancer, pancreatic
neuroendocrine tumors
(islet cell tumors), papillomatosis, paraganglioma, paranasal sinus and nasal
cavity cancer,
parathyroid cancer, penile cancer, pharyngeal cancer, pheochromocytoma,
pituitary tumor,
plasma cell neoplasm, pleuropulmonary blastoma, primary central nervous system
(CNS)
lymphoma, prostate cancer, rectal cancer, renal cell (kidney) cancer, renal
pelvis and ureter,
transitional cell cancer, rhabdomyosarcoma, salivary gland cancer, sarcoma
(e.g., Ewing,
Kaposi, osteosarcoma, rhadomyosarcoma, soft tissue, uterine), Sezary syndrome,
skin cancer
(e.g., melanoma, merkel cell carcinoma, basal cell carcinoma, nonmelanoma),
small intestine
cancer, squamous cell carcinoma, squamous neck cancer with occult primary,
stomach
(gastric) cancer, testicular cancer, throat cancer, thymoma and thymic
carcinoma, thyroid
cancer, trophoblastic tumor, ureter and renal pelvis cancer, urethral cancer,
uterine cancer,
endometrial cancer, uterine sarcoma, vaginal cancer, vulvar cancer,
Waldenstrom
macroglobulinemia, and Wilm's tumor.
[0380] In certain embodiments the cancer to be treated is cancer
pancreatic ductal
adenocarcinoma (PDAC) and in certain embodiments, the ICD inducer comprises
oxaliplatin
and the IDO inhibitor comprises indoximod or a conjugated indoximod as
described below in
in Example 1.
Approach 3 -- Vaccination to prevent or treat a cancer.
[0381] In various embodiments, methods are provided for the prevention
or treatment
of a cancer that involve vaccinating a subject (e.g., a human, or a non-human
mammal) to
induce an immune response directed against one or more cancers. It was a
surprising
discovery that vaccination of a mammal with cancer cells in which ICD has been
induced ex
vivo is sufficient to generate a systemic immune response that can interfere
with tumor
growth at a remote site as well as allowing adoptive transfer to non-immune
animals.
67
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0382] Without being bound to a particular theory it is believed that
such vaccination
methods can be used for the treatment of an existing cancer or
prophylactically to prevent or
inhibit the formation of a cancer in a subject. In the latter case, for
example, subjects that
have a family history for cancer in general or for particular cancers, and/or
that have a genetic
risk for a cancer (e.g., mutations in BRCA1, and/or BRCA2, and/or P53) may be
vaccinated
prophylactically to prevent the development of a cancer.
[0383] In certain embodiments, the vaccination is used as a primary
therapy in the
treatment of a cancer. In certain embodiments the vaccination is used as an
adjunct therapy,
e.g., in combination with surgery, and/or other chemotherapy regimen, and/or
radiation
therapy.
[0384] Accordingly, in various embodiments, a method for the treatment
and/or
prevention of a cancer in a mammal is provided where the method comprises
providing
cancer cells in which immunogenic cell death (ICD) has been induced ex vivo,
and
vaccinating the mammal with these cells, where the vaccination induces an anti-
cancer
immunogenic response.
[0385] In certain embodiments, the cancer cells are cells derived from
an existing
cancer, e.g., obtained during a biopsy, or after surgical resection of a tumor
mass). In certain
embodiments the cancer cells are cells obtained from the subject that is to be
treated and
comprise an autologous transplant. In certain embodiments the cells are
obtained from a
different subject of the same species or can even be obtained from a different
species.
[0386] In certain embodiments, the cancer cells are cells from a
cancer cell line.
Typically, where a non-human animal is to be treated (veterinary use) the cell
line is an
animal cell line from the same species that is to be treated. Similalry, where
a human is to be
treated a human cell line will typically be used. Numerous cancer cell lines
are known to
those of skill in the art. Illustrative, but non-limiting examples of suitable
cell lines are
shown in Table 1.
Table 1. Illustrative, but non-limiting, cell lines that can be used to
produce dying cancer
cells in which immunogenic cell death (ICD) has been induced.
KPC Mouse pancreatic ductal adenocarcinoma (for
research
purpose only)
Patient-derived cancer cells Primary cancer cells obtained via various
diagnosis (i.e.
obtained by fine needle biopsy) or surgical procedures
SH-SY5Y Human neuroblastoma
Hep G2 Human Caucasian hepatocyte carcinoma
68
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
293 (HEK 293) Human Embryo Kidney
HeLa Human cervix epitheloid carcinoma
MRC-5 (PD 19) Human foetal lung
A2780 Human ovarian carcinoma
CACO-2 Human Caucasian colon adenocarcinoma
THP 1 Human monocytic leukaemia
A549 Human Caucasian lung carcinoma
MRC-5 (PD 30) Human foetal lung
MCF7 Human Caucasian breast adenocarcinoma
Jurkat E6.1 Human leukaemic T cell lymphoblast
U937 Human Caucasian histiocytic lymphoma
HL60 Human Caucasian promyelocytic leukaemia
HT29 Human Caucasian colon adenocarcinoma
0E33 Human Caucasian oesophageal carcinoma
0E19 Human Caucasian oesophageal carcinoma
MDA-MB-231 Human Caucasian breast adenocarcinoma
K562 Human Caucasian chronic myelogenous leukaemia
U-87 MG Human glioblastoma astrocytoma
MRC-5 (PD 25) Human fetal lung
A2780cis Human ovarian carcinoma
1321N1 Human brain astrocytoma
A431 Human squamous carcinoma
U-2 OS Human Osteosarcoma
HCT 116 Human colon carcinoma
BEAS-2B Human bronchial epithelium, normal
T47D Human breast tumour
1301 Human T-cell leukaemia
PNT2 Human prostate normal, immortalised with SV40
TF1 Human erythroleukaemia
NCI-H322 Human Caucasian bronchioalveolar carcinoma
SK.N.SH Human Caucasian neuroblastoma
LNCaP.FGC Human Caucasian prostate carcinoma
0E21 Human Caucasian oesophageal squamous cell carcinoma
PSN1 Human pancreatic adenocarcinoma
MFE-280 Human caucasian endometrial adenocarcinoma
MG-63 Human osteosarcoma
EoL-1 cell Human eosinophilic leukaemia
VCaP Human Prostate Cancer Metastasis
tsA201 Human embryonal kidney. SV40 transformed
HT 1080 Human fibrosarcoma
PANC-1 Human Caucasian pancreas
Saos-2 Human primary osteogenic sarcoma
ATCC Pancreatic cell lines:
Capan-2 ATCC HTB -80
Panc 10.05 ATCC CRL-2547
CFPAC-1 ATCC CRL-1918
HPAF-II ATCC CRL-1997
SW 1990 ATCC CRL-2172
69
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
BxPC-3 ATCC CRL-1687
AsPC-1 ATCC CRL-1682
ATCC colon cancer lines
SNU-Cl ATCC CRL-5972
SK-CO-1 ATCC HTB-39
SW1116 ATCC CCL-233
SW948 ATCC CCL-237
T84 ATCC CCL-248
LS123 ATCC CCL-255
LoVo ATCC CCL-229
SW837 ATCC CCL-235
SNU-Cl ATCC CRL-5972
SW48 ATCC CCL-231
RKO ATCC CRL-2577
COLO 205 ATCC CCL-222
SW1417 ATCC CCL-238
LS411N ATCC CRL-2159
NCI-H508 ATCC CCL-253
HT-29 ATCC HTB-38
CRL-1718Tm CCF-STTG1 Human Brain Astrocytoma
HTB-12Tm SW 1088 Human Brain Astrocytoma
HTB-13Tm SW 1783 Human Brain Astrocytoma
CRL-3020TM CHLA-02- Human Brain Atypical Teratoid Rhabdoid Tumor
ATRT (ATRT)
CRL-1620Tm A172 Human Brain Glioblastoma
HTB-16Tm U-138 MG Human Brain Glioblastoma
CRL-2610Tm LN-18 Human Brain Glioblastoma
CRL-2611Tm LN-229 Human Brain Glioblastoma
HTB-14Tm U-87 MG Human Brain Glioblastoma, astrocytoma
HTB-15Tm U-118 MG Human Brain Glioblastoma, astrocytoma
CRL-1690Tm T98G Human Brain Glioblastoma, multiforme
HTB-138Tm Hs 683 Human Brain Glioma
CRL-3021 TM CHLA-01- Human Brain Medullomyoblastoma
MED
CRL-2273 TM CHP-212 Human Brain Neuroblastoma
HTB-148Tm H4 Human Brain Neuroglioma
HTB-187Tm D341 Med Human Brain, cerebellum Medulloblastoma
HTB-186Tm Daoy Human Brain, cerebellum Medulloblastoma,
desmoplastic cerebellar
CRL-2060TM PFSK-1 Human Brain, cerebellum Tumor, malignant primitive
neuroectodermal
CRL-2020TM DBTRG- Human Brain, glial cell Glioblastoma
05MG
CRL-2365TM M059K Human Brain, glial cell Glioblastoma
CRL2366TM M059J Human malignant glioblastoma
[0387] Although not required, in typical embodiments the cancer cells used
in the
vaccination are of the same type of cancer that is to be treated and/or
prevented. It will be
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
recognized however, that vaccination with cells of one type of cancer may
generate an
immune response directed against a different cancer and/or against multiple
cancers. In
certain embodiments the vaccination is with cells from multiple different
types (e.g., 2 or
more cancers, 3 or more cancers, 4 or more cancers, 5 or more cancers, 6 or
more cancers, 7
or more cancers, 8 or more cancers, 9 or more cancers, 10 or more cancers,
etc.) in which
ICD is induced.
[0388] In certain embodiments illustrative cancers to be treated or
prevented include,
but are not limited to pancreatic ductal adenocarcinoma (PDAC), acute
lymphoblastic
leukemia (ALL), acute myeloid leukemia (AML), Adrenocortical carcinoma, Kaposi
sarcoma, anal cancer, appendix cancer, astrocytoma, atypical teratoid/rhabdoid
tumor, bile
duct cancer, extrahepatic cancer, bladder cancer, bone cancer (e.g., Ewing
sarcoma,
osteosarcoma, malignant fibrous histiocytoma), brain stem glioma, brain tumors
(e.g.,
astrocytomas, brain and spinal cord tumors, brain stem glioma, central nervous
system
atypical teratoid/rhabdoid tumor, central nervous system embryonal tumors,
central nervous
system germ cell tumors, craniopharyngioma, ependymoma, breast cancer,
bronchial tumors,
burkitt lymphoma, carcinoid tumors (e.g., childhood, gastrointestinal),
cardiac tumors,
cervical cancer, chordoma, chronic lymphocytic leukemia (CLL), chronic
myelogenous
leukemia (CML), chronic myeloproliferative disorders, colon cancer, colorectal
cancer,
craniopharyngioma, cutaneous t-cell lymphoma, duct cancers e.g. (bile,
extrahepatic), ductal
carcinoma in situ (DCIS), embryonal tumors, endometrial cancer, ependymoma,
esophageal
cancer, esthesioneuroblastoma, extracranial germ cell tumor, extragonadal germ
cell tumor,
extrahepatic bile duct cancer, eye cancer (e.g., intraocular melanoma,
retinoblastoma), fibrous
histiocytoma of bone, malignant, and osteosarcoma, gallbladder cancer, gastric
(stomach)
cancer, gastrointestinal carcinoid tumor, gastrointestinal stromal tumors
(GIST), germ cell
tumors (e.g., ovarian cancer, testicular cancer, extracranial cancers,
extragonadal cancers,
central nervous system), gestational trophoblastic tumor, brain stem cancer,
hairy cell
leukemia, head and neck cancer, heart cancer, hepatocellular (liver) cancer,
histiocytosis,
langerhans cell cancer, Hodgkin lymphoma, hypopharyngeal cancer, intraocular
melanoma,
islet cell tumors, kaposi sarcoma, kidney cancer (e.g., renal cell, Wilm's
tumor, and other
kidney tumors), langerhans cell histiocytosis, laryngeal cancer, leukemia,
acute
lymphoblastic (ALL), acute myeloid (AML), chronic lymphocytic (CLL), chronic
myelogenous (CML), hairy cell, lip and oral cavity cancer, liver cancer
(primary), lobular
carcinoma in situ (LCIS), lung cancer (e.g., childhood, non-small cell, small
cell), lymphoma
71
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
(e.g., AIDS-related, Burkitt (e.g., non-Hodgkin lymphoma), cutaneous T-Cell
(e.g., mycosis
fungoides, Sezary syndrome), Hodgkin, non-Hodgkin, primary central nervous
system
(CNS)), macroglobulinemia, Waldenstrom, male breast cancer, malignant fibrous
histiocytoma of bone and osteosarcoma, melanoma (e.g., childhood, intraocular
(eye)),
merkel cell carcinoma, mesothelioma, metastatic squamous neck cancer, midline
tract
carcinoma, mouth cancer, multiple endocrine neoplasia syndromes, multiple
myeloma/plasma cell neoplasm, mycosis fungoides, myelodysplastic syndromes,
Myelogenous Leukemia, Chronic (CML), multiple myeloma, nasal cavity and
paranasal sinus
cancer, nasopharyngeal cancer, neuroblastoma, oral cavity cancer, lip and
oropharyngeal
cancer, osteosarcoma, ovarian cancer, pancreatic cancer, pancreatic
neuroendocrine tumors
(islet cell tumors), papillomatosis, paraganglioma, paranasal sinus and nasal
cavity cancer,
parathyroid cancer, penile cancer, pharyngeal cancer, pheochromocytoma,
pituitary tumor,
plasma cell neoplasm, pleuropulmonary blastoma, primary central nervous system
(CNS)
lymphoma, prostate cancer, rectal cancer, renal cell (kidney) cancer, renal
pelvis and ureter,
transitional cell cancer, rhabdomyosarcoma, salivary gland cancer, sarcoma
(e.g., Ewing,
Kaposi, osteosarcoma, rhadomyosarcoma, soft tissue, uterine), Sezary syndrome,
skin cancer
(e.g., melanoma, merkel cell carcinoma, basal cell carcinoma, nonmelanoma),
small intestine
cancer, squamous cell carcinoma, squamous neck cancer with occult primary,
stomach
(gastric) cancer, testicular cancer, throat cancer, thymoma and thymic
carcinoma, thyroid
cancer, trophoblastic tumor, ureter and renal pelvis cancer, urethral cancer,
uterine cancer,
endometrial cancer, uterine sarcoma, vaginal cancer, vulvar cancer,
Waldenstrom
macroglobulinemia, and Wilms tumor.
[0389] In various embodiments the cells used in the vaccination
include cells of one
or more of these cancers.
[0390] Methods of inducing immunogenic cell death (ICD) are well known to
those
of skill in the art. In certain embodiments ICD is induced by contacting the
cells (e.g.,
primary tumor cells, cancer cell lines, etc.) with one or more
chemotherapeutic agent(s) that
induce ICD. Such agents include, but are not limited to oxaliplatin,
anthracenedione,
bleomycin, bortezomib, cisplatin, daunorubicin, docetaxel, doxorubicin,
epirubicin,
idarubicin, mitoxanthrone, paclitaxel, irinotecan, R2016 (a heterocyclic
quinolone derivative
described by Son et al, (2017) Plos One, DOI:10.1371, which is incorporated
herein by
reference for the compounds described therein), and cyclophosphamide. In
certain
embodiments the ICD chemo reagents may also include the drug derivatives, i.e.
prodrugs,
72
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
that are capable of releasing the abovementioned chemotherapeutics in
biological
environments.
[0391] Another method of inducing ICD involves infecting the cells
with an oncolytic
virus. Illustrative, but non-limiting oncoviruses that induce ICD include, but
are not limited
to Parvovirus (e.g., H-PV (see, e.g., Angelova et al. (2014) J. Virol.,
88(10): 5263-5276), and
the like), Adenovirus (AD) (e.g., hTERT-Ad (see, e.g., Boozari et al. (2010)
Gut. 59: 1416-
1426), Ad5/3-D24-GMCSF (see, e.g., Liikanen et al. (2013) Mol. Ther. 21: 1212-
1223), and
the li.ke), Herpes simplex virus (HSV) (e.g., G207 (see, e.g., Toda et al.
(1999) Hum. Gene.
Ther. 10: 385-393), HSV-1716 (see, e.g., Benencia et al. (2005) Mol. Ther.,
12: 789-8020, T-
VEC (see, e.g., Hu et al. (2006) Clin. Cancer Res. 12: 6737-67470), HSV-2 APK
mutant
(see, e.g., Colunga et al. (2010) Gene Ther., 17: 315-327), and the like),
Poxvirus (e.g., vSP
(see, e.g., Guo et al. (2005) Cancer Res. 65: 9991-9998, vvDD (see, e.g., John
et al. (2012)
Cancer Res., 72: 1651-1660), Pexa-Vec (see, e.g., Heo et al. (2013) Nat. Med.,
19: 329-336),
and the like), Arbovirus (see, e.g., VSV-GFP (Indiana serotype) (see, e.g.,
Wongthida et al.
(2010) Cancer Res. 70: 4539-4549), VSVgm-icv (see, e.g., Lemay et al. (2012)
Mot. Ther.,
20: 1791-1799), and the like), Paramyxovirus (e.g., MV-eGFP (Edmonston strain)
(see, e.g.,
Donnelly et al. (2013) Gene Ther. 20: 7-15), and the like). A review of such
oncoviruses is
found in Bartlett et al. (2013) Mol. Cancer. 12: 103).
[0392] Other methods of inducing ICD involve exposure to radiation
(e.g., gamma
radiation, UVC radiation).
[0393] In certain embodiments ICD induction is accomplished using any
of the
compounds and/or modalities described in Table 2.
Table 2. Illustrative compounds and/or modalities to induce immunogenic cell
death (ICD).
ICD Inducer DAMPs released
Mitoxantrone
Pre-apoptotic ecto-CRT and ERp57; early
Oxaliplatin
apoptotic secreted ATP; mid to late
UVC irradiation
apoptotic ecto-HSP70; late apoptotic
y-irradiation
passively released HMGB1
anthracyclines (e.g., Daunorubicin,
Doxorubicin, Epirubicin, Idarubicin)
Early to mid apoptotic ecto-CRT; early to
mid apoptotic ecto-HSP70; early to mid
Shikonin
apoptotic ecto-GRP78
Pre-apoptotic ecto-CRT and ERp57; early
7A7 (EGFR-specific antibody)
to mid apoptotic ecto-HSP70; early to mid
apoptotic ecto-HSP90
73
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
Pre-apoptotic ecto-CRT; late apoptotic
Cyclophosphamide passively released HMGB1
Bortezomib Early to mid apoptotic ecto-HSP90
Pre-apoptotic ecto-CRT; early to mid
Cardiac glycosides apoptotic ATP release; late apoptotic
passively released HMGB1
Pre-apoptotic ecto-CRT; pre-apoptotic
secreted ATP; pre-apoptotic ecto-HSP70;
Hypericin-based PDT
tate apoptotic passively released HSP70,
HSP90 and CRT
Early apoptotic ecto-CRT; early apoptotic
Coxsackievirus B3 secreted ATP; late apoptotic passively
released HMGB1
Oncolytic parvovirus (e.g., H-PV)
Anthracenedione
Bleomycin
Docetaxel
Paclitaxel
R2016
Irinotecan
[0394] In other embodiments, the methods of inducing ICD can involve
contacting
the cells with materials, e.g., nanomaterials that induce ICD. It was a
surprising discovery
that certain materials (e.g., nanomaterials), as a result of intrinsic
nanomaterial properties, are
capable of inducing ICD, e.g., as determined by CRT induction, in a manner
comparable to
the positive control, oxaliplatin. Such materials include, but are not limited
to CuO, graphene
oxide, and certain others (see, e.g., Example 3). Accordingly, in certain
embodiments ICD is
induced by contacting the cancer cells with a nanomaterial that induces ICD
(e.g., CuO,
Cu2O, As203,Bi203, P203, ZnO, TiO2, graphene oxide, 2D materials other than
graphene or
graphene oxide (e.g., graphene, graphyne, borophene, germanene, silicene,
Si2BN, stanene,
phosphorene, bismuthene, molybdenite, metals, 2D supracrystals, and the
like)). In certain
embodiments, the nanomaterial comprises copper oxide. In certain embodiments,
the
nanomaterial comprises graphene oxide (GO), CuO, Cu2O, Sb203, As203, Bi203,
P203, ZnO,
Ti02, graphene oxide. and 2D materials other than graphene or graphene oxide
[0395] Extensive high throughput screening of a large number of
nanomaterial
libraries (including metals, metal oxides, rare earth oxides, graphene,
graphene oxide, multi-
and single walled carbon nanotubes, fumed silica, long aspect ratio
nanomaterials, redox
74
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
active nanomaterials, nanomaterials with functionalized catalytic surfaces and
coatings etc) in
our nanomaterial safety screening laboratory in the California Nano Systems
Institute at
UCLA have demonstrated a variety of mechanisms, involving intrinsic
nanomaterial
properties, that can induce a wide variety of different types of cell death,
including apoptosis,
necrosis, pyroptosis, and immunogenic cell death.
[0396] In view of the results demonstrated herein, it is believed that
numerous other
2-dimensional (2D) materials can similarly induce ICD.
[0397] In this regard, it is noted that a number of 2D materials other
than graphene
are known to those of skill in the art (see, e.g., Mas Balleste et al. (2011)
Nanoscale, 3: 20-
30). Such materials include, but are not limited to graphene, graphyne,
borophene,
germanene, silicene, Si2BN, stanene, phosphorene, bismuthene, molybdenite,
metals, 2D
supracrystals, and the like. Other 2D materials include, but are not limited
to BN, MoS2,
NbSe2, Bi2Sr2CaCi20õ (Id.), single layers of single layers of manganese (see,
e.g., Omomo et
al. (2003)1 Am. Chem. Soc., 125: 3568-3575), oxides of cobalt (see, e.g., Kim
et al. (2009)
Chem. Fur. J., 15: 10752-10761). tantalum (Fukuda et al. (2007) Inorg. Chem.
46: 4787-
4789), ruthenium (Fukuda et al. (2010) Inorg. Chem. 49: 4391-4393), and
titanium (Tanaka
et al. (2003) Chem. Mater. 15: 3564-3568) as well as sheets of several
perovskite type
structures, e.g., H2IAn_1BnO3n+11 where A is Na, CA, Sr, or LA, and B is Ta or
Ti,
K2LN2Ti3010A, KLnNb207, or RbLNTa207 where Ln is lanthanide ion, MWO6 where M
is
.. Nb or Ta, KCa2Nb3010, KSr2Nb3010, Bi2SrTa209, and the like.
[0398] It is noted that these ICD-inducing nanomaterials exhibit a
range of tunable
physicochemical properties that can readily be adapted to achieve the optimal
ICD-inducing
catalytic outcomes. For example, for graphene oxide these properties include,
inter alia,
nanosheet size, surface oxidation status, and the like, while for metal oxides
these properties
include, inter alia, the particle size, dissolution characteristics, zeta
potential, and the like.
[0399] The list of nanomaterials above that induce immunogenic cell
death is
illustrative and non-limiting. It is believed there are numerous other
materials that have the
capability of inducing ICD based on property-activity relationships, such as
the induction of
oxidative stress, mitochondrial damage, lysosomal damage, surface membrane
damage, DNA
damage, photo activation, oxygen radical generation, activation of the NRLP3
inflammasome, induction or interference in autophagy flux, etc.
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0400] It will also be recognized that in various embodiments, two or
more agents can
(e.g., two or more of the agents or modalities described above) can be used to
induce ICD.
[0401] Methods of determining whether IDC is induced in the cells are
known to
those of skill in the art. For example, ICD is characterized by elevated
expression of
calreticulin (CRT), and/or elevated expression and/or release of e.g.. HMGB1
or ATP as
compared to the same cells in which ICD is not induced. Illustrative, but non-
limiting
methods of inducing ICD in cancer cells (e.g., KPC cells) and evaluation of
the ICD are
described in Example 1.
[0402] These methods and agents for inducing ICD are illustrative and
non-limiting.
Numerous other agents and compositions for inducing ICD are known.
Modes of vaccination.
[0403] Methods of vaccination of humans or non-human mammals are well
known to
those of skill in the art. Most typically, the vaccination will be by
intramuscular,
subcutaneous, or intradermal injection. In various embodiments injection may
be performed
by needle or pressure.
[0404] In certain embodiments mucosal immunization can be performed
and such
modalities include, but are not limited to intraocular, intranasal and/or
oral.
[0405] In certain embodiments jet injectors, such as Antares Pharma's
MediJector
VISION, deliver medication through high-speed, pressurized liquid penetration
of the skin
without a needle. These have been developed as single-use devices and multiuse
systems. A
high peak pressure behind the liquid is required so it can drill a hole in the
skin, and then the
pressure is reduced to allow the rest of the liquid to enter the skin.
[0406] Other transdermal approaches deliver the antigen in a solid
form. These
approaches have the added benefit that the therapeutic agent is more stable
and therefore may
not need cold storage.
[0407] Another illustrative, but non-limiting approach uses the
pharmaceutical
formulation itself to puncture the skin. Glide Pharma has developed a low-
velocity, spring-
powered administrator that pushes a pointed rod of pharmaceutical material
through the skin
in a fraction of a second. This administrator enables constant, reliable
delivery of a solid
dosage form and could be applied to various vaccines including vaccines
comprising cancer
ICD-induced cancer cells as described herein.
76
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0408] In another illustrative, but non-limiting embodiment, the
antigen (e.g., ICD-
induced cancer cells) can be delivered by injection or implantation in a
hydrogel. In certain
embodiments the hydrogel is an injectible hydrogel.
[0409] Injectable hydrogels can be prepared using a wide range of
materials. Cyto-
and bio-compatibility as well as reactive chemistries are typical factors
considered for
selecting base materials that can be used in hydrogels for cell delivery.
Material crosslinking
(formation and concentration of physical or covalent linkages),
biodegradability, and
biochemical properties can influence the structural, mechanical, and
biological properties of
the hydrogels initially and over time. Hydrophilic polymers used for hydrogel
construction
generally can be divided into two categories: natural polymers derived from
tissues or other
natural sources and synthetic polymers fabricated using organic chemistry and
molecular
engineering principles. Biocompatible natural polymers such as hyaluronic
acid, chitosan,
heparin, alginate, fibrin, collagen, chondroitin sulfate, and silk, mimic
aspects of the native
microenvironment, including its mechanical and biochemical properties for
modulating cell
adhesion, migration, and other functions (see, e.g., Munarin et al. (2012) J.
Appl. Biomater,
Funct. Mater. 10(2): e67-81). These natural polymers have been used as
building blocks for
injectable hydrogel formation by physical (e.g., ionic, hydrogen bonding) or
covalent
crosslinking (e.g., reaction of functional groups on modified polymers) (see,
e.g., Kharkar et
al. (2013) Chem. .Soc. Rev. 42(17): 7335-7372.
[0410] Synthetic polymers such as poly(ethylene glycol) (PEG), poly(vinyl
alcohol)
(PVA), poly(N-isopropylacrylamide) (PNIPAAm), and polycaprolactone (PCL) have
frequently been used for the design of injectable, cell-compatible hydrogels
due to their
commercial availability, low batch-to-batch variation, versatility for
chemical modification,
and consequently, the ease of tuning the mechanical properties of the
resulting hydrogels.
Since synthetic polymers lack the inherent biochemical cues for interaction
with cells, In
certain embodiments they can be used in combination with natural polymers or
biomimetic
peptides to facilitate cell adhesion, migration, and protein secretion.
[0411] In certain illustrative, but non-limiting embodiments, the
cells can be delivered
by use of an injectable (or implantable) cryogel. Cryogels are a type of
hydrogel made up of
cross-linked hydrophilic polymer chains that can hold up to 99 percent water.
They are
created by freezing a solution of the polymer that is in the process of
gelling. When thawed
back again to room temperature, the substance turns into a highly
interconnected pore-
containing hydrogel, which is similar in composition to bodily soft tissues in
terms of their
77
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
water content, structure, and mechanics. By adjusting their shape, physical
properties, and
chemical composition sponge-like, porous cryogels can be formed that can be
infused with
living cells, biological molecules or drugs. One illustrative, but non-
limiting cyrogel is
formed from methacrylated alginate (MA-alginate) as described by Bencherif et
al. (2016)
Nat. Comm., 6: 7556.
Adjuvants.
[0412] In certain embodiments the vaccination utilizing cancer cells
in which ICD has
been induced is performed using one or more adjuvants to increase the
subject's immune
response to the vaccination. Typically, adjuvants enhance and direct the
adaptive immune
response to vaccine antigens.
[0413] Adjuvants may exert their effects through different mechanisms.
Some
adjuvants, such as alum and emulsions (e.g., MF59 ), function as delivery
systems by
generating depots that trap antigens at the injection site, providing slow
release in order to
continue the stimulation of the immune system. These adjuvants enhance the
antigen
persistence at the injection site and increase recruitment and activation of
antigen presenting
cells (APCs). Particulate adjuvants (e.g., alum) have the capability to bind
antigens to form
multi-molecular aggregates that encourage uptake by APCs (see, e.g., Leroux-
Roels (2010)
Vaccine. 28S(3) :C25-3).
[0414] Some adjuvants are also capable of directing antigen
presentation by the major
.. histocompatibility complexes (MHC) (Id.). Other adjuvants, essentially
ligands for pattern
recognition receptors (PRR), act by inducing the innate immunity,
predominantly targeting
the APCs and consequently influencing the adaptive immune response. AlOOH
described
below is one such example. Members of nearly all of the PRR families are
potential targets
for adjuvants. These include Toll-like receptors (TLRs), NOD-like receptors
(NLRs), RIG-I-
like receptors (RLRs) and C-type lectin receptors (CLRs). They signal through
pathways that
involve distinct adaptor molecules leading to the activation of different
transcription factors.
These transcription factors (e.g., NF-KB, IRF3) induce the production of
cytokines and
chemokines that play a key role in the priming, expansion and polarization of
the immune
responses. Activation of some members of the NLR family, such as NLRP3 and
NLRC4,
triggers the formation of a protein complex, called inflammasome, implicated
in the induction
of the pro-inflammatory cytokines IL-113 (see, e.g., Li et al. (2008) J.
Immunol. 181(1): 17-
21.) and IL-18. The NLRP3 and NLRC4 inflammasomes have been involved in the
innate
78
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
immunity induced by certain adjuvants. Much of our high throughput discovery
on material
such as AO0H, multiwall carbon nanotubes, singlewall carbon nanotubes,
graphene, rare
earth oxide nanoparticles, metal oxide nanorods, and the like function via the
NRLP3
pathway. Most of the adjuvant effects fit into the category of stimulating
DAMP pathways,
which overlaps with the concept of ICD.
Alum & emulsions
[0415] Alum is the most commonly used adjuvant in human vaccination.
Alum
provokes a strong Th2 response. Alum induces the immune response by a depot
effect and
activation of APCs. The NLRP3 inflammasome has been linked to the
immunostimulatory
properties of alum.
[0416] In certain embodiments a high aspect ratio AlOOH variant of
alum can be
used as an adjuvant. We have also made a much-improved variant of alum by high
throughput screening that identified high aspect ration AlOOH for use as an
adjuvant. The
high aspect ratio AlOOH that is 1-2 orders of magnitude better than Alum,
based, inter alia,
on the principle that the long aspect ratio of the material and its surface
reactivity provide
superior stimulation to the NRLP3 inflammasome in dendritic cells (see, e.g.,
Sun et al.
(2013) ACS Nano, 7(12): 10834-10849).
[0417] Additionally, emulsions (either oil-in-water or water-in-oil),
such as Freund's
Incomplete Adjuvant (IFA) and MF59 , can trigger depot generation and
induction of MHC
responses. IFA induces a predominantly Th2 biased response with some Thl
cellular
response. MF59 is a potent stimulator of both cellular (Thl) and humoral
(Th2) immune
responses.
PRR Ligands
[0418] New adjuvants are being developed that are natural ligands or
synthetic
agonists for PRRs, either alone or with various formulations. PRR activation
stimulates the
production of pro-inflammatory cytokines/chemokines and type I IFNs that
increase the
host's ability to eliminate the pathogen. Thus, the incorporation of pathogens
associated
molecular patterns (PAMPs) in vaccine formulations can improve and accelerate
the
induction of vaccine-specific responses. A number of these agonists are now in
clinical or
late preclinical stages of development (see, e.g., Steinhagen et al. (2011)
29(17): 3341-3355;
Mbow et al. (2010) Curr. Opin. linmunol. 22(3): 411-416). When used in
combination with
79
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
alum or classical emulsion adjuvants, the immune response can be biased
towards a Thl
response (see, e.g., Didierlaurent et al. (2009) J. Immunol. 183(10): 6186-
6197).
TLR3 and RLR Ligands
[0419] Double-stranded RNA (dsRNA), which is produced during the
replication of
most viruses, is a potent inducer of innate immunity. Synthetic analogs of
dsRNA, such as
poly(I:C), have been utilized as adjuvants. They act through TLR 3 and RIG-
I/MDA-5,
inducing IL-12 and type I IFNs production, facilitating antigen cross-
presentation to MHC
class II molecules, and improving generation of cytotoxic T cells.
TLR4 Ligands
[0420] Bacterial lipopolysaccharides (LPS), which are ligands for TLR4,
have long
been recognized as potent adjuvants. The development of less toxic derivatives
led to the
production of MPLA (monophosphoryl lipid A), which formulated with alum (AS04)
triggers
a polarized Thl response and is approved for clinical use in Europe. We also
have
demonstrated that graphene oxide can interact with TLR4.
TLR5 Ligands
[0421] The TLR5 ligand, bacterial flagellin, is a potent T-cell
antigen and has been
utilized as a vaccine adjuvant. Unlike other TLR agonists, flagellin tends to
produce mixed
Thl and Th2 responses rather than strongly Thl responses. Flagellin can be
used as an
adjuvant mixed with the antigen.
TLR7/8 Ligands
[0422] The TLR7/8 pathway, specialized in the recognition of single
stranded viral
RNA, has also been explored for use as vaccine adjuvants. Imidazoquinolines
(e.g.,
imiquimod, gardiquimod, and R848) are synthetic compounds that activate TLR7/8
in
multiple subsets of dendritic cells leading to the production of IFN-cc and IL-
12 thus
promoting a Thl response. In this regard, is is noted that the formulations
and/or drug
delivery nanocarriers described herein can can easily include imiquimod.
TLR9 Ligands
[0423] Oligodeoxynucleotides containing specific CpG motifs (CpG ODNs
such as
ODN 1826 and ODN 2006) are recognized by TLR9. They enhance antibody
production and
strongly polarize the cell responses to Th1 and away from Th2 responses. In
this regard, it is
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
noted that various drug delivery nanocarriers described herein (e.g., a
bilayer-coated
nanoparticle) can readily be modified to present CPG oligonucleotides on the
surface (e.g.,
LB-coated nanoparticles can present CPG oligo's on the lipid bilayer).
NOD2 Ligands
[0424] Fragments of bacterial cell walls, such as muramyl dipeptide (MDP),
have
long been recognized as adjuvants. More recently, it was discovered that MDP
triggers the
activation of NOD2 and the NLRP3 inflammasome.
[0425] Adjuvants may be combined to achieve a stronger effect or a
more potent
skewing of immune responses. For example, alum has been combined with TLR9
agonists
(see, e.g., Siegrist et al. (2004) Vaccine, 23(5): 615-622). In experimental
models,
administration of other combinations such as CpG ODNs with MDP or MPLA has
proven
effective (see, e.g., Kim et al. (2000) Vaccine, 19: 530-537).
[0426] In various embodiments, any one or more of the these adjuvants
may be used
to enhance response to the vaccination with cancer cells in which ICD has been
induced.
[0427] The foregoing vaccination methods are illustrative and non-limiting.
Using
the teachings provided herein, numerous other methods and compositions for
vaccinating
subjects with cancer cells in which ICD is induced will be available to one of
skill in the art.
IDO inhibitors
[0428] A number of IDO inhibitors are well-known to those of skill in
the art and
useful in the methods described herein. Illustrative, but non-limiting
examples of IDO
inhibitors are shown in Table 3 and the structures of several of these are
shown in Figure 2.
Table 3. Illustrative, but non-limiting IDO inhibitors.
IDO Inhibitor Mechanism Reference
Indoximod Tryptophan mimetic; D Metz et al. (2012)
(D-1MT) isoform of MT; Oncoimmunology, 1(9):
Transcriptional suppressor 1460-1468
of IDO
L-1MT Tryptophan mimetic; L Opitz et al. (2011)
Nature,
isoform of MT; selective 478(7368): 197-203
IDO1 inhibitor
methylthiohydantoin-dl- Tryptophan mimetic; Okamoto (2007)
tryptophan transcriptional suppressor of Cytotechnology,
54(2): 107-
(MTH-Trp) IDO 113
(Necrostatin)
81
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
13-carbolines Tryptophan mimetic; IDO Eguchi et al. (1984) Arch.
(e.g., 3-butyl-13-carboline) and TDO inhibitor Biochem.
Biophys. 232(2):
602-609
Naphthoquinone-based Pharmacophore of natural Kumar et al. (2008) J.
Med.
(e.g., annulin-B) product annulin B; indole Chem., 51(6): 1706-1718
mimetic; IDO inhibitor
S-allyl-brassinin Phytoalexin; indole mimetic U.S. Patent 7,705,022
S-benzyl-brassinin Phytoalexin; indole mimetic U.S. Patent 7,705,022
N-[2-(Indo1-3-yeethyll-S- U.S. Patent 7,705,022
methyl-dithiocarbamate
N-[2-(benzo[b]thiophen-3- U.S. Patent 7,705,022
yl)ethyll-S-methyl-
dithiocarbamate
N-[3-(Indo1-3-yl)propyll-S- U.S. Patent 7,705,022
methyl-dithiocarbamate
S-Hexyl-brassinin U.S. Patent 7,705,022
N-[2-(indo1-3-yeethy11-S- U.S. Patent 7,705,022
benzyl-dithiocarbamate
N-[2-(indo1-3-yeethy11- U.S. Patent 7,705,022
S Rnaphth-2-yl)methyll-
dithiocarbamate
N-[2-(indo1-3-yl)ethy11-S- U.S. Patent 7,705,022
[(pyrid-3-yl)methy11-
dithiocarbamate
N-[2-(indo1-3-ypethyll-S- U.S. Patent 7,705,022
[(pyrid-4-yl)methy11-
dithiocarbamate
5-Bromo-brassinin Phytoalexin; indole mimetic Banerjee et al. (2008)
Oncogene, 27(20): 2851-
2857
Phenylimidazole-based Heme ligand in IDO enzyme Sono et al. (1989)
(e.g., 4-phenylimidazole) Biochemistry (Mosc), 8(13):
5392-5399
Exiguamine A Non-tryptophan analogue Brastianos et al. (2006) J.
Am. Chem. Soc. /28(50):
16046-1647
imidodicarbonimidic Non-indolic IDO inhibitor Vottero et al. (2006)
diamide,N-methyl-N'-9- Biotechnol. J. 1(3): 282-
phenanthrenyl-, 288
monohydrochloride
(NSC401366)
INCB024360
(Epacadostat)
1-cyclohexy1-2-(5H-
imidazo[5,1-a]isoindo1-5-
yl)ethanol
(GDC-0919)
ID 01 -derived peptide
82
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
NLG919
Ebselen
Pyridoxal Isonicotinoyl
Hydrazone
Norharmane
(3R,4S)-4-(benzylamino)-
3-hydroxy-2,2-dimethyl-
3,4-
dihydrobenzo[g]chromene-
5,10-dione
(CAY 10581)
2-Benzy1-2-thiopseudourea
hydrochloride
[0429] Still other IDO inhibitors include, but are not limited to the
inhibitors
described in U.S. Patent Publication Nos: US 2016/0362412, US 2016/0289171, US
2016/0200674, US 2016/0143870, US 2016/0137595, US 2016/0060237, US
2016/0002249,
US 2014/0323740, US 2014/0066625, US 2013/0289083, US 2013/0183388, US
2012/0277217, US 2011/0136796, US 2011/0112282, US 2011/0053941, US
2010/0233166,
US 2010/0166881, US 2010/0076066, US 2009/0042868, US 2007/0173524, US
2007/0105907, which are all incorporated herein by reference for the IDO
inhibitors
described therein.
[0430] It is contemplated that the methods described herein can use
one or more of
these IDO inhibitors and/or any other IDO inhibitors known to those of skill
in the art. In
certain embodiments the one or more IDO inhibitors comprise indoximod.
Conjugated IDO inhibitors and vesicles thereof.
[0431] In certain embodiments one or more MO inhibitors (e.g., any one
or more of
the IDO inhibitors shown in Table 3) are conjugated to a moiety that forms a
vesicle (e.g., a
liposome or a micelle) structure in aqueous solution or that can form a
component of a lipid
bilayer comprising a liposome. The conjugated IDO inhibitors can be used
directly (e.g.,
described in approach 2 above), provided as components in a combined
formulation (e.g., in
combination with an ICD inducer), and in certain embodiments, the IDO
inhibitor is
conjugated to a moiety that forms a component of a lipid bilayer that can be
disposed on a
nanoparticle, e.g., as described below and in Example 1).
[0432] In certain embodiments the moiety that is conjugated to the the
IDO pathway
inhibitor comprises a lipid (e.g., a phospholipid), vitamin E, cholesterol,
and/or a fatty acid.
83
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
In certain embodiments the IDO pathway inhibitor can be conjugated directly to
the moiety
(see, e.g., Figure 3), while in other embodiments the IDO inhibitor can be
conjugated to the
moiety using a linker (e.g., a HO-(CH2)õ=2_5-0H linker as shown in Figure 4).
[0433] In the illustrative embodiments shown in Figure 3, the an ester
bond is used to
make the conjugate. As a general strategy in the case of indoximod, the NH2
group in the
indoximod is protected before the conjugation reaction. The ¨COOH in indoximod
can then
robustly react with the in the conjugating moiety (e.g., phospholipid, Vitamin
E, cholesterol,
a fatty acid, etc.). Similarly, Figure 34 illustrates representative examples
to show the
combined use of HO-(CH2)õ=2_5-0H linker and ester bond to make IDO inhibitor
(e.g.,
indoximod) pro-drug conjugates. Again, as illustrated. the NH2 group can be
protected.
[0434] Examples 8 and 9 illustrate various conjugation strategies.
These reactions,
however, are illustrative and non-limiting. Numerous IDO inhibitors have other
groups
readily available for conjugation directly to a vesicle-forming moiety or to a
linker. Such
groups include for example, H, OH, CH2, and the like (see, e.g., Figure 2).
[0435] In certain embodiments, particularly for rapid and easy
incorporation into a
lipid bilayer the IDO pathway inhibitor can be conjugated to a lipid (e.g., a
phospholipid), or
cholesterol. Of course, in certain embodiments, the other vesicle-forming
agents having
conjugated IDO inhibitor(s) can also be incorporated into a lipid bilayer.
[0436] In certain embodiments, the inhibitor of the IDO pathway is
conjugated to
cholesterol or to a modified cholesterol (e.g., cholesterol hemisuccinate
(CHEMS), lysine-
based cholesterol (CHLYS), PEGylated cholesterol (Chol-PEG), and the like). In
certain
embodiments the IDO pathway inhibitor is conjugated to cholesterol by a
linker. In certain
embodiments the IDO pathway inhibitor is conjugated directly to cholesterol
(see, e.g.,
Formulas II, Ha, and III) in Figure 8).
[0437] In certain embodiments, the inhibitor of the IDO pathway is
conjugated to a
phospholipid comprising a saturated fatty acid with a C14-C20 carbon chain,
and/or an
unsaturated fatty acid with a C14-C20 carbon chain, and/or a natural lipid
comprising a
mixture of fatty acids with C12-C20 carbon chains. In certain embodiments, the
phospholipid comprises a saturated fatty acid selected from the group
consisting of
phosphatidylcholine (DPPC), dimyristoylphosphatidylcholine (DMPC),
distearoylphosphatidylcholine (DSPC), and diactylphosphatidylcholine (DAPC).
In certain
embodiments, the phospholipid comprises a natural lipid selected from the
group consisting
84
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
of egg phosphatidylcholine (egg PC), and soy phosphatidylcholine (soy PC). In
certain
embodiments, the phospholipid comprises an unsaturated fatty acid selected
from the group
consisting of 1,2-dimyristoleoyl-sn-glycero-3-phosphocholine, 1,2-
dipalmitoleoyl-sn-
glycero-3-phosphocholine,1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), and
1,2-
dieicosenoyl-sn-glycero-3-phosphocholine, and the like.
[0438] It will be recognized that as shown above, in various
embodiments, the 1-
methyl-tryptophan component of the conjugated IND (e.g., Chol-IND, or any
other moiety
conjugated IND), can be a "D" isomer or an L isomer.
[0439] In certain embodiments the IDO pathway inhibitors can be
incorporated into
the lipid bilayer forming the vesicle witout conjugation to a lipid bilayer
component. For
example, epacadostat is a potent direct IDO enzyme inhibitor with an IC50 of
¨125 nM in a
whole blood assay (Yue et al. (2017) ACS Med. Chem. Letts. 8: 486-491).
Although the drug
showed good synergy with anti-PD1 antibody (nivolumab) in a phase II clinical
trial in
melanoma patients, the success could not be duplicated in a recent phase 3
clinical trial for
the same disease. This has raised questions about the exact role and efficacy
of IDO
inhibitors, their pharmacology and explaining the divergent effects.
Epacadostat is highly
soluble in ethanol (>20 mg/mL), which allows its incorporation into a
liposomal membrane
through the use of the ethanol injection method ((see, e.g., Pons, et al.
(1993) J.
Pharmaceutic, 95: 51-56). The ethanol injection method produces homogeneous
unilamellar
liposomes (Pereira et al, (2016) Int. J.. Pharmaceutics, 514: 150-159). In
this method, water
is poured into a concentrated lipid-ethanol solution (e.g., containing
docetaxel and possibly
IND-Chol in a ratiometric designed strategy), following which ethanol is
removed in an
evaporator (Id.). Dilution with water causes spontaneous formation of small
and
homogenous unilamellar liposomes from the micellar aggregate. The size of the
liposomes
can be controlled by the ratio of ethanol to water.
[0440] It will be recognized that the foregoing conjugates and lipid
bilayers
incorporating IDO inhibitors are illustrative and non-limiting. Using the
teaching provided
herein, numerous other IDO inhibitor conjugates and/ro IDO-containign lipid
bilayers
(vesicles) will be available to one of skill in the art.
MTX Only liposomes.
[0441] It is noted that the the use of both of the mitoxantrone-only
and
mitoxantrone/IND liposomes were extremely effective in a 4T1 breast cancer
model (see,
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
e.g., Example 8), and much better than the results with a Doxil equivalent
liposome
delivering doxorubicin only. Without being bound to a particular theory, it is
believed that
the effect can be attributed to the superior ICD inducing effect of
mitoxantrone over
doxirubicin, rendering a liposomal mitoxantrone candidate that can be used for
multiple
cancer types. Additionally, in the 4T1 model, the mitoxantrone-only liposome
was so
effective that an additional effect for cholesterol-IND was not observed,
reflecting the
possibility that the 4T1 triple negative breast cancer model may represent a
TN cancer subset
in which IDO-1 does not play a major role. In this regard, it is noted that
the same triple
negative cancer also fails to respond to anti-PD1, the ligand of which is
controlled by the
same IFN-gamma response pathway that is responsible for the expression of PD-1
ligand. In
this sense sense, TN breast cancer may be no different from a series of solid
cancers in
which there is only a 25-30% response rate to checkpoint inhibitors, likely
due to a variable
contribution by different immune escape mechanisms.
[0442] We have clear evidence that in spite of the lack of a
synergistic effect for IND
in the 4T1 model, that there is a strong ICD response in the
Immunohistochemistry data,
implying that the contribution of turning the cold tumor hot provides a strong
contribution
irrespective of an apparent lack of IDO-1 cinvolvement.
[0443] Without being bound by a particular theory, it is believed that
a potent ICD
agent such as mitoxantrone can exert similar effects on other solid tumors,
increasing the
25% response rate.
[0444] In view of this observation, it is believed that there is also
a role for a
mitoxantrone-only liposome in addition to a mitoxantrone/IND liposome. While
not
required, the use of an MTX-only liposome would be facilitated by the
identification of a
biomeraker to identify whether tumors are potentially IDO-1 responsive,
similar to the
.. manner in which the expression of PD-1 ligand is currently used to decide
who should
receive anti-PD1 therapy for lung cancer.
[0445] Accordingly, in certain embodiments, the use of liposomes
containing
mitoxantrone where the lipid bilayer does not contain IND or other IDO
inhibitor. In certain
embodiments the liposome formulations are the same as liposome formulations
described
herein comrpsing IND, but the lipid bilayer components do not comprise a
conjugated IDO
inhibitor.
86
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
Remote loading of Silicasomes, and Vesicles/Liposomes
[0446] In certain embodiments, the encapsulation of, e.g., the ICD
inducer in the
nanoparticle and/or in the nanovesicle can be optimized by using a "remote
loading" strategy
in which the addition of the drug (e.g., ICD-inducer such as doxorubicin) to
preformed
vesicles or silicasomes (LB-coated nanoparticles) which achieves high loading
levels using a
a pH gradient or an ion gradient capable of generating a pH gradient (see,
e.g., Ogawa et al.
(2009) J. Control. Rel. 1(5): 4-10; Fritze et al. (2006) Biochimica et Biophys
Acta. 1758:
1633-1640). In general, the remote loading method involves adding a cargo-
trapping reagent
(e.g., protonating reagent such as TEA8SOS, ammonium sulfate, etc.) which can
be added to
the lipid biofilm prior to the sonication in the formation of silicasomes, or
can be
incorporated into the nanovesicle lipids prior to the formation of the
nanovesicle e.g., as
described in Example 2.
[0447] Thus for example, using an IND-Cholesterol (IND-Chol) prodrug,
a
DOX/IND nanovesicle can be prepared as follows: 1) a total of 50 mg lipids of
IND-Chol
plus other vesicle-forming lipids (e.g., DPPC/Chol-IND/DPPG/DSPE-PEG (e.g.,
DSPE-
PEG2k, DSPE-PEG5k, and the like), in certain embodiments at a molar ratio of -
40%
(DPPC): -35% (Chol-IND): -20%DPPG: -5% DSPE-PEG) can be dissolved in 5 mL
chloroform in a 50 mL round bottom glass flask. The solvent can be evaporated
under a
rotatory vacuum to form a uniform thin lipid film that can be dried further
under vacuum
overnight. 2) The film can be hydrated with a cargo-trapping agent (e.g., with
2 mL of
ammonium sulfate (123 mM) and probe sonicated, e.g., for 1 h, then
subsequently extruded,
e.g., 15 times, through a Mini-Extruder (Avanti Polar Lipids), using, e.g., a
polycarbonate
membrane with 100 nm pores (Avanti Polar Lipids) at 80 C. IND nanovesicle
(1ND-NV)
size and morphology can be assessed by dynamic light scattering and cryoEM,
respectively
as desired. Unincorporated cargo-trapping agent (e.g., ammonium sulfate) can
be removed,
e.g., by running through a PD-10 size exclusion column. The drug to be loaded
(e.g., 6.4 mg
of DOX=HC1 (10 mg/mL) in DI water) can be incubated with the above prepared
IND-NVs,
e.g., at 65 C for 40 mm. The nanovesicles can be fractionated across a PD-10
column,
allowing the removal of free DOX. Their size and morphology can be assessed by
dynamic
light scattering, cryoEM and UPLC/MS-MS, respectively. In an other
illustrative, but non-
limiting embodiments, citrate can be used to load mitoxantrone.
87
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0448] Of course, this protocol is illustrative and non-limiting.
Using this teaching,
numerous other nanovesicles comprising an ICD-inducer and various lipid
formulatiosn can
be produced by one of skill in the art.
[0449] Similarly, preparation and remote-loading of a silicasome
comprising an IDO
pathway inhibitor and an ICD-inducer is illustrated in example 2. A DOX/IND-
MSNP dual-
delivery carrier is designed by trapping DOX in the mesoporous interior of a
¨65 nm MSNP,
using a lipid bilayer into which IND-Chol can be incorporated. In order to
apply the lipid
coating, we use the previously described biofilm method for rapid
encapsulation, by
sonication (Meng et al. (2015) ACS Nano, 9(4): 540-3557; Liu et al. (2016) ACS
Nano, 10:
2702-2715). DOX was then remotely loaded using the protocol as previously
described (Id).
[0450] Typically this involves preparing the MSNPs, e.g., by a sol-gel
synthesis
process (see. e.g., Meng et al. (2015) ACS Nano, 9(4): 540-3557). The MSNPs
are then
soaked in the cargo-trapping agent (e.g., ammonium sulfate) to load the agent
into the pores
of the MSNPs. The lipid formulation that will comprise the bilayer surrounding
the
silicasome is prepared, e.g., as described in Example 2, where the lipid
formulation
incorporates the IDO inhibitor (e.g., IND-Chol). The cargo-trapping agent
loaded MSNPs
are added to the IDO-inhibitor lipid film followed by sonication (e.g., 30 min
probe
sonication) to provide the trapping agent (e.g., ammonium sulfate)-loaded IND-
Chol coated
MSNP. To remove the free ammonium sulfate, the particle suspension can be
passed through
a PD-10 size exclusion column, Ammonium sulfate-containing IND-LB coated MSNPs
will
elute from column faster than free ammonium sulfate due to its large size.
Remote Dox
loading can be accomplished by incubating 6.5-32.4 mg of DOX=FIC1 (10 mg/mL)
in DI
water with cargo-trapping agent loaded laden IND-LB components coated MSNP at
65 C
for 40 min. The pure MSNPs can be collected by centrifuging at 15,000 rpm for
15 min,
three times.
[0451] This protocol also is illustrative and non-limiting. Using this
teaching,
numerous other silicasomes comprising an IDO pathway inhibitor and ICD-inducer
and
various lipid formulatiosn can be produced by one of skill in the art.
[0452] In this regard, it is noted that the lipid conjugation
technology described herein
.. can be used to make prodrugs out of chemo agents, which can be folded into
a liposome.
Thus, for example, ICD chemo agents like the taxanes can be incorporated into
a
phospholipid bilayer based on hydrophobicity, and this has been demonstrated
for a MSNP
88
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
where we used paclitaxel incorporation into the encapsulating phospholipid
bilayer. The
same can be done for a liposome.
[0453] Thus, the versatility of the liposomal platform described
herein allows the
encapsulation of ICD-inducing drugs such as paclitaxel, docetaxel,
mitroxantrone, irinotecan
.. and etoposide through the use different loading strategies that depend on
the chemical
structure of the drugs. For example, it is believed that mitoxantrone, which
is a weak basic
molecule with MW of 444.4, water solubility of 89 mg/mL and log P value of -
3.1
(mitoxantrone. www.drugbank.ca/drugs/DB01204) , can be remotely loaded into
the Chol-
IND liposome via a proton gradient, using (NH4)2SO4 or citric acid. The same
is possible for
etoposide. Since docetaxel has high ethanol solubility (-100 mg/mL), this
lends itself to
constructing liposomes by an ethanol injection method that can produce
homogeneous
unilamellar liposomes as described. In this method, water is poured into a
concentrated lipid-
ethanol solution (containing docetaxel and possibly Chol-IND in a ratiometric
designed
strategy), following which ethanol is removed in an evaporator (see, e.g.,
Pereira etal. (2016)
Int. J. Pharmaceuticsõ 514: 150-159). Dilution with water causes spontaneous
formation of
small and homogenous unilamellar liposomes from the micellar aggregate. The
size of the
liposomes can be controlled by the ratio of ethanol to water. While paclitaxel
(PTX) is
moderately soluble in ethanol (1.5 mg/mL), up to ¨5 wt% PTX can be loaded into
the
liposomal membrane by ethanol injection (Koudelka & Turanek(2012) J Control.
Release,
163: 322-334).
[0454] These embodiments are illustrative and non-limiting. Using the
teachings
provided herein numerous variants will be available to one of skill in the
art.
Cargo trapping reagents.
[0455] As explained above, in certain embodiments a cargo-trapping
reagent can be
utilized to facilitate incorporation of a cargo (e.g., DOX, MTX, OX,
irinotecan etc. (see, e.g.,
Table 2)) into the dual-delivery (ICD-inducer/MO-inhibitor) LB coated MSNP
(ICD/IDO
silicasome), and/or the dual-delivery lipid vesicles (e.g., ICD/IDO-lipid
vesicles). The cargo-
trapping reagent can be selected to interact with a desired cargo. In some
embodiments, this
interaction can be an ionic or protonation reaction, although other modes of
interaction are
contemplated. The cargo-trapping agent can have one or more ionic sites, i.e.,
can be mono-
ionic or poly-ionic. The ionic moiety can be cationic, anionic, or in some
cases, the cargo-
trapping agent can include both cationic and anionic moieties. The ionic sites
can be in
89
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
equilibrium with corresponding uncharged forms; for example, an anionic
carboxylate
(-COO-) can be in equilibrium with its corresponding carboxylic acid (-COOH);
or in another
example, an amine (-NH2) can be in equilibrium with its corresponding
protonated
ammonium form (-NH3). These equilibriums are influenced by the pH of the local
environment. Certain ICD-inducing weak-base reagents, such as doxorubicin, can
be loaded
using a trapping agent mediated approach for loading (see, e.g., Example 2).
[0456] Likewise, in certain embodiments, the cargo can include one or
more ionic
sites. The cargo-trapping agent and cargo can be selected to interact inside
the dual-delivery
(ICD-inducer/IDO-inhibitor) LB coated MSNP (ICD/IDO silicasome), and/or the
dual-
delivery lipid vesicle (e.g., ICD/IDO-lipid vesicle). This interaction can
help retain the cargo
within the nanoparticle until release of the cargo is desired. In some
embodiments, the cargo
can exist in a pH-dependent equilibrium between non-ionic and ionic forms. The
non-ionic
form can diffuse across the lipid bilayer and enter the vesicle or the pores
of the MSNP.
There, the cargo-trapping agent (e.g., a polyionic cargo-trapping agent) can
interact with the
ionic form of the cargo and thereby retain the cargo within the nanocarrier,
e.g., within the
vesicle or within the pores of the MSNP (provided the ionic forms of the cargo
and cargo-
trapping agent have opposite charges). The interaction can be an ionic
interaction, and can
include formation of a precipitate. Trapping of cargo within the nanocarrier
can provide
higher levels of cargo loading compared to similar systems, e.g., nanocarriers
that omit the
cargo-trapping agent, or liposomes that do include a trapping agent. Release
of the cargo can
be achieved by an appropriate change in pH to disrupt the interaction between
the cargo and
cargo-trapping agent, for example, by returning the cargo to its non-ionic
state which can
more readily diffuse across the lipid bilayer. In one embodiment, the cargo is
irinotecan and
the cargo-trapping agent is TEA8SOS.
[0457] The cargo trapping agent need not be limited to TEAsSOS. In certain
embodiments the cargo trapping comprises small molecules like citric acid,
(NH4)2SO4, and
the like (see, e.g., Examples 2 and 9). Other trapping agents include, but are
not limited to,
ammonium salts (e.g., ammonium sulfate, ammonium sucrose octasulfate, ammonium
a-
cyclodextrin sulfate, ammonium13-cyclodextrin sulfate, ammonium y-cyclodextrin
sulfate,
ammonium phosphate, ammonium a-cyclodextrin phosphate, ammonium 0-cyclodextrin
phosphate, ammonium y-cyclodextrin phosphate, ammonium citrate, ammonium
acetate, and
the like), trimethylammonium salts (e.g., trimethylammonium sulfate,
trimethylammonium
sucrose octasulfate, trimethylammonium a-cyclodextrin sulfate,
trimethylammonium 13-
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
cyclodextrin sulfate, trimethylammonium y-cyclodextrin sulfate,
trimethylammonium
phosphate, trimethylammonium a-cyclodextrin phosphate, trimethylammonium 13-
cyclodextrin phosphate, trimethylammonium y-cyclodextrin phosphate,
trimethylammonium
citrate, trimethylammonium acetate, and the like), triethylammonium salts
(e.g.,
triethylammonium sulfate, triethylammonium sucrose octasulfate,
triethylammonium a-
cyclodextrin sulfate, triethylammonium P-cyclodextrin sulfate,
triethylammonium y-
cyclodextrin sulfate, triethylammonium phosphate, triethylammonium a-
cyclodextrin
phosphate, triethylammonium P-cyclodextrin phosphate, triethylammonium y-
cyclodextrin
phosphate, triethylammonium citrate, triethylammonium acetate, and the like).
[0458] It is also worth pointing out that, in addition to TEA8SOS,
transmembrane pH
gradients can also be generated by acidic buffers (e.g. citrate) (Chou et al.
(2003) J. Biosci.
Bioengineer., 95(4): 405-408; Nichols et al. (1976) Biochimica et Biophysica
Acta (BBA)-
Biomembranes, 455(1): 269-271), proton-generating dissociable salts (e.g.
(NH4)2SO4)
(Haran et al. (1993) Biochimica et Biophysica Acta (BBA)-Biomembranes,
1151(2): 201-215;
Maurer-Spurej et at (1999) Biochimica et Biophysica Acta (BBA)-Biomembranes,
1416(1):
1-10; Fritze et al. (2006) Biochimica et Biophysica Acta (BBA)-Biomembranes,
1758(10):
1633-1640), or ionophore-mediated ion gradients from metal salts (e.g. A23187
and MnSO4)
(Messerer et al. (2004) Clinical Cancer Res. 10(19): 6638-6649; Ramsay et al.
(2008) Eur. I
Pharmaceut. Biopharmaceut. 68(3): 607-617; Fenske et al. (1998) Biochimica et
Biophysica
Acta (BBA)-Biomembranes, 1414(1): 188-204). Moreover, it is possible to
generate reverse
pH gradients for drug loading, such as use a calcium acetate gradient to
improve amphiphilic
weak acid loading in LB-MSNP, a strategy that has been utilized in liposomes
(Avnir et al.
(2008) Arthritis & Rheumatism, 58(1): 119-129).
[0459] In certain embodiments the cargo-trapping reagent is particular
suitable for use
with a cargo that comprises an organic compound that includes at least one
primary amine
group, or at least one secondary amine group, or at least one tertiary amine
group, or at least
one quaternary amine group, or any combination thereof, capable of being
protonated.
[0460] In certain embodiments the general characteristics of these
cargo molecules
include the following chemical properties:
[0461] (i) organic molecular compounds that include primary, secondary,
tertiary or quaternary amine(s);
[0462] (ii) a pKa <11 to allow protonation and entrapment behind
the LB
(Zucker et al. (2009) J. Control. Release, 139(1): 73-80; Cern et al. (2012)
J. Control.
91
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
Release, 160(2): 147-157; Xu et al. (2014) Pharmaceut. Res. 31(10): 2583-
2592);
[0463] (iii) a water solubility index of 5-25 mg/mL and
amphipathic
characteristics that allow diffusion across the LB;
[0464] (iv) an octanol/water partition coefficient or logP value
of -3.0 to 3.0
.. (Zucker et al. (2009) J. Control. Release, 139(1): 73-80; Cern et al.
(2012) J. Control.
Release, 160(2): 147-157);
[0465] (v) suitable molecular weight with a geometric size less
than MSNP
pore size (2-8 nm), to allow entry into the MSNP pores (Li et al. (2012) Chem.
Soc. Rev.
41(7): 2590-2605; Tang et al. (2012) Adv. Mat, 24(12): 1504-1534; Tarn et al.
(2013) Acc.
Chem. Res. 46(3): 792-801).
[0466] Remote loading utilizing doxorubicin, with ammonium sulfate as
a cargo
trapping agent is described in Example 2. This is illustrative, but non-
limiting. In addition to
DOX loading into nanovesicles or silicasomes, there are other possible drugs
that can be
imported across the lipid bilayer of these carriers. These include, but are
not limited to, weak
basic compounds, with medicinal chemical features. Such compounds include, but
are not
limited to alkaloids (e.g. irinotecan, topotecan, 10-hydroxycamptothecin,
belotecan,
rubitecan, vinorelbine, LAQ824, vinblastine, vincristine, homoharringtonine,
trabectedin),
anthracyclines (e.g. doxorubicin, epirubicin, pirarubicin, daunorubicin,
rubidomycin,
valrubicin, amrubicin), alkaline anthracenediones (e.g. mitoxantrone),
alkaline alkylating
agents (e.g. cyclophosphamide, mechlorethamine, temozolomide), purine or
pyrimidine
derivatives (e.g. 5-fluorouracil, 5'-deoxy-5-fluorouridine, gemcitabine,
capecitabine) and
protein kinase inhibitors (e.g., pazopanib, enzastaurin, vandetanib erlotinib,
dasatinib,
nilotinib, sunitinib, osimertinib, palbociclib, ribociclib), and the like.
[0467] Using the teachings provided herein, numerous other agents can
be remote
loaded (e.g., loaded using a cargo trapping agent) into the silicasomes (e.g.,
dual-delivery
(ICD-inducer/IDO-inhibitor) LB coated MSNP (ICD/IDO silicasome)), and vesicles
(e.g., the
dual-delivery lipid vesicles (e.g., ICD/IDO-lipid vesicles)) described herein.
Targeting ligands and Immunoconjugates.
[0468] In certain embodiments the dual-delivery (ICD-inducer/IDO-
inhibitor) LB
coated MSNPs (ICD/IDO silicasomes), and/or the dual-delivery lipid vesicles
(e.g.,
ICD/IDO-lipid vesicles), and/or dual delivery lipid-coated ICD-inducing
nanomaterial
carriers can be conjugated to one or more targeting ligands, e.g., to
facilitate specific delivery
92
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
in endothelial cells, to cancer cells, to fusogenic ligands, e.g., to
facilitate endosomal escape,
ligands to promote transport across the blood-brain barrier, and the like.
[0469] In one illustrative, but non-limiting embodiment, the
nanocarrier (e.g.,
ICD/IDO silicasome, ICD/IDO lipid vesicle, ICD-inducing nanomaterial carrier,
etc.) is
conjugated to a fusogenic peptide such as histidine-rich H5WYG (H2N-
GLFHAIAHFIHGGWHGLIHGWYG-COOH, (SEQ ID NO:1)) (see, e.g., Midoux et al.,
(1998) Bioconjug. Chem. 9: 260-267).
[0470] In certain embodiments the nanocarrier (e.g., ICD/IDO
silicasome, ICD/IDO
lipid vesicle, ICD-inducing nanomaterial carrier, etc.) is conjugated to one
or more targeting
ligand(s) that can include antibodies as well as targeting peptides. Targeting
antibodies
include, but are not limited to intact immunoglobulins, immunoglobulin
fragments (e.g.,
F(ab)12, Fab, etc.) single chain antibodies, diabodies, affibodies, unibodies,
nanobodies, and
the like. In certain embodiments antibodies will be used that specifically
bind a cancer
marker (e.g., a tumor associated antigen). A wide variety of cancer markers
are known to
those of skill in the art. The markers need not be unique to cancer cells, but
can also be
effective where the expression of the marker is elevated in a cancer cell (as
compared to
normal healthy cells) or where the marker is not present at comparable levels
in surrounding
tissues (especially where the chimeric moiety is delivered locally).
[0471] Illustrative cancer markers include, for example, the tumor
marker recognized
by the ND4 monoclonal antibody. This marker is found on poorly differentiated
colorectal
cancer, as well as gastrointestinal neuroendocrine tumors (see, e.g., Tobi et
al. (1998) Cancer
Detection and Prevention, 22(2): 147-152). Other important targets for cancer
immunotherapy are membrane bound complement regulatory glycoproteins CD46,
CD55 and
CD59, which have been found to be expressed on most tumor cells in vivo and in
vitro.
Human mucins (e.g. MUC1) are known tumor markers as are gp100, tyrosinase, and
MAGE,
which are found in melanoma. Wild-type Wilms tumor gene WT1 is expressed at
high levels
not only in most of acute myelocytic, acute lymphocytic, and chronic
myelocytic leukemia,
but also in various types of solid tumors including lung cancer.
[0472] Acute lymphocytic leukemia has been characterized by the TAAs
HLA-Dr,
CD1, CD2, CD5, CD7, CD19, and CD20. Acute myelogenous leukemia has been
characterized by the TAAs HLA-Dr, CD7, CD13, CD14, CD15, CD33, and CD34.
Breast
cancer has been characterized by the markers EGFR, HER2, MUC1, Tag-72. Various
93
CA 03157508 2022-04-08
WO 2021/076630 PCT/US2020/055585
carcinomas have been characterized by the markers MUC1, TAG-72, and CEA.
Chronic
lymphocytic leukemia has been characterized by the markers CD3, CD19, CD20,
CD21,
CD25, and HLA-DR. Hairy cell leukemia has been characterized by the markers
CD19,
CD20, CD21, CD25. Hodgkin's disease has been characterized by the Leu-M1
marker.
Various melanomas have been characterized by the HMB 45 marker. Non-hodgkins
lymphomas have been characterized by the CD20, CD19, and Ia marker. And
various
prostate cancers have been characterized by the PSMA and SE10 markers.
[0473] In addition, many kinds of tumor cells display unusual antigens
that are either
inappropriate for the cell type and/or its environment, or are only normally
present during the
organisms' development (e.g., fetal antigens). Examples of such antigens
include the
glycosphingolipid GD2, a disialoganglioside that is normally only expressed at
a significant
level on the outer surface membranes of neuronal cells, where its exposure to
the immune
system is limited by the blood-brain barrier. GD2 is expressed on the surfaces
of a wide
range of tumor cells including neuroblastoma, medulloblastomas, astrocytomas,
melanomas,
small-cell lung cancer, osteosarcomas and other soft tissue sarcomas. GD2 is
thus a
convenient tumor-specific target for immunotherapies.
[0474] Other kinds of tumor cells display cell surface receptors that
are rare or absent
on the surfaces of healthy cells, and which are responsible for activating
cellular signaling
pathways that cause the unregulated growth and division of the tumor cell.
Examples include
(ErbB2) HER2/neu, a constitutively active cell surface receptor that is
produced at
abnormally high levels on the surface of breast cancer tumor cells.
[0475] Other useful targets include, but are not limited to CD20,
CD52, CD33,
epidermal growth factor receptor and the like.
[0476] An illustrative, but not limiting list of suitable tumor
markers is provided in
Table 4. Antibodies to these and other cancer markers are known to those of
skill in the art
and can be obtained commercially or readily produced, e.g. using phage-display
technology.
Such antibodies can readily be conjugated to the drug delivery nanocarrier
(e.g., LB-coated
nanoparticle) described herein, e.g., in the same manner that iRGD peptide is
conjugated in
Example 3.
[0477] Table 4. Illustrative cancer markers and associated references, all
of which are
incorporated herein by reference for the purpose of identifying the referenced
tumor markers.
Marker Reference
94
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
alpha reductase Delos et al. (1998) Int J Cancer, 75:6 840-846
a-fetoprotein Esteban et al. (1996) Tumour Biol., 17(5): 299-305
AM-1 Harada et al. (1996) Tohoku J Exp Med., 180(3): 273-288
APC Dihlmannet al. (1997) Oncol Res., 9(3) 119-127
APRIL Sordat et al. (998) J Exp Med., 188(6): 1185-1190
BAGE Boel et al. (1995) Immunity, 2: 167-175.
13-catenin Hugh et al. (1999) Int J Cancer, 82(4): 504-11
Bc12 Koty et al. (1999) Lung Cancer, 23(2): 115-127
bcr-abl (b3a2) Verfaillie et al. (996) Blood, 87(11): 4770-4779
CA-125 Bast et al. (998) Int J Biol Markers, 13(4): 179-187
CASP-8/FLICE Mandruzzato et al. (1997) J Exp Med., 186(5): 785-793.
Cathepsins Thomssen et a/.(1995) Clin Cancer Res., 1(7): 741-746
CD19 Scheuermann et al. (1995) Leuk Lymphoma, 18(5-6): 385-397
CD20 Knox et al. (1996) Clin Cancer Res., 2(3): 457-470
CD21, CD23 Shubinsky et al. (1997) Leuk Lymphoma, 25(5-6): 521-530
CD22, CD38 French et al. (1995) Br J Cancer,71(5): 986-994
CD33 Nakase et al. (1996) Am J Clin Pathol., 105(6): 761-768
CD35 Yamakawa et al. Cancer, 73(11): 2808-2817
CD44 Naot et al. (1997) Adv Cancer Res., 71: 241-319
CD45 Buzzi et al. (1992) Cancer Res., 52(14): 4027-4035
CD46 Yamakawa et al. (1994) Cancer, 73(11): 2808-2817
CD5 Stein et al. (1991) Clin Exp Immunol., 85(3): 418-423
CD52 Ginaldi et al. (1998) Leuk Res., 22(2): 185-191
CD55 Spendlove et al. (1999) Cancer Res., 59: 2282-2286.
CD59 (791Tgp72) Jarvis et al. (1997) Int J Cancer, 71(6): 1049-1055
CDC27 Wang et al. (1999) Science, 284(5418): 1351-1354
CDK4 Wolfe' et al. (1995) Science, 269(5228): 1281-1284
CEA Kass et al. (1999) Cancer Res., 59(3): 676-683
c-myc Watson et al. (1991) Cancer Res., 51(15): 3996-4000
Cox-2 Tsujii et al. (1998) Cell, 93: 705-716
DCC Gotley et al. (1996) Oncogene, 13(4): 787-795
DcR3 Pitti et al. (1998) Nature, 396: 699-703
E6/E7 Steller et al. (1996) Cancer Res., 56(21): 5087-5091
EGI-R Yang et al. (1999) Cancer Res., 59(6): 1236-1243.
EMBP Shiina et al. (1996) Prostate, 29(3): 169-176.
Ena78 Arenberg et al. (1998) J. Clin. Invest., 102: 465-472.
FGF8b and FGF8a Dorkin et al. (1999) Oncogene, 18(17): 2755-2761
FLK-1/KDR Annie and Fong (1999) Cancer Res., 59: 99-106
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
Folic Acid Receptor Dixon et al. (1992) J Biol Chem., 267(33): 24140-72414
G250 Divgi et al. (1998) Clin Cancer Res., 4(11): 2729-2739
GAGE-Family De Backer et al. (1999) Cancer Res., 59(13): 3157-3165
gastrin 17 Watson et al. (1995) Int J Cancer, 61(2): 233-240
Gastrin-releasing Wang et al. (1996) Int J Cancer, 68(4): 528-534
hormone (bombesin)
GD2/GD3/GM2 Wiesner and Sweeley (1995) Int J Cancer, 60(3): 294-299
GnRH Bahk et al. (1998) Urol Res., 26(4): 259-264
GnTV Hengstler et al. (1998) Recent Results Cancer Res., 154: 47-
85
gp100/Pme117 Wagner et al. (1997) Cancer Immunol Immunother., 44(4): 239-
247
gp-100-in4 Kirkin et al. (1998) APMIS, 106(7): 665-679
gp15 Maeurer et al. (1996) Melanoma Res., 6(1): 11-24
gp75/TRP-1 Lewis et a/.(1995) Semin Cancer Biol., 6(6): 321-327
hCG Hoermann et al. (1992) Cancer Res., 52(6): 1520-1524
Heparanase Vlodaysky et al. (1999) Nat Med., 5(7): 793-802
Her2/neu Lewis et al. (1995) Semin Cancer Biol., 6(6): 321-327
Her3
HMTV Kahl et a/.(1991) Br J Cancer, 63(4): 534-540
Hsp70 Jaattela et al. (1998) EMBO J., 17(21): 6124-6134
hTERT Vonderheide et al. (1999) Immunity, 10: 673-679. 1999.
(telomerase)
IGFR1 Ellis et al. (1998) Breast Cancer Res. Treat., 52: 175-184
IL-13R Murata et al. (1997) Biochem Biophys Res Commun., 238(1):
90-94
iNOS Klotz et al. (1998) Cancer, 82(10): 1897-1903
Ki 67 Gerdes et al. (1983) Int J Cancer, 31: 13-20
KIAA0205 Gueguen et al. (1998) J Immunol., 160(12): 6188-6194
K-ras, H-ras, Abrams et al. (1996) Semin Oncol., 23(1): 118-134
N-ras
KSA Zhang et al. (1998) Clin Cancer Res., 4(2): 295-302
(C017-1A)
LDLR-FUT Caruso et al. (1998) Oncol Rep., 5(4): 927-930
MAGE Family Marchand et al. (1999) Int J Cancer, 80(2): 219-230
(MAGE1,
MAGE3, etc.)
Mammaglobin Watson et al. (1999) Cancer Res., 59: 13 3028-3031
MAP17 Kocher et al. (1996) Am J Pathol., 149(2): 493-500
Melan-A/ Lewis and Houghton (1995) Semin Cancer Biol., 6(6): 321-327
MART-1
96
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
mesothelin Chang et al. (1996) Proc. Natl. Acad. Sci., USA, 93(1): 136-
140
MIC A/B Groh et al. (1998) Science, 279: 1737-1740
MT-MMP's, such as Sato and Seiki (1996) J Biochem (Tokyo), 119(2): 209-215
MMP2, MMP3,
MMP7, MMP9
Moxl Candia et al. (1992) Development, 116(4): 1123-1136
Mucin, such as MUC- Lewis and Houghton (1995) Semin Cancer Biol., 6(6): 321-
327
1, MUC-2, MUC-3,
and MUC-4
MUM-1 Kirkin et al. (1998) APMIS, 106(7): 665-679
NY-ESO-1 Jager et al. (1998) J. Exp. Med., 187: 265-270
Osteonectin Graham et al. (1997) Eur J Cancer, 33(10): 1654-1660
p15 Yoshida et al. (1995) Cancer Res., 55(13): 2756-2760
P170/MDR1 Trock et al. (1997) J Natl Cancer Inst., 89(13): 917-931
p53 Roth et al. (1996) Proc. Natl. Acad. Sci., USA, 93(10):
4781-4786.
p97/melanotransferrin Furukawa et al. (1989) J Exp Med., 169(2): 585-590
PAI-1 Grondahl-Hansen et al. (1993) Cancer Res., 53(11): 2513-
2521
PDGF Vassbotn et al. (1993) Mol Cell Biol,, 13(7): 4066-4076
Plasminogen (uPA) Naitoh et al. (1995) Jpn J Cancer Res., 86(1): 48-56
PRAME Kirkin et al. (1998) APMIS, 106(7): 665-679
Probasin Matuo et al. (1985) Biochem Biophys Res Commun., 130(1):
293-
300
Progenipoietin
PSA Sanda et al. (1999) Urology, 53(2): 260-266.
PSM Kawakami et al. (1997) Cancer Res., 57(12): 2321-2324
RAGE-1 Gaugler et al.(1996) Immunogenetics, 44(5): 323-330
Rb Dosaka-Akita et al. (1997) Cancer, 79(7): 1329-1337
RCAS1 Sonoda et al. (1996) Cancer, 77(8): 1501-1509.
SART-1 Kikuchi et al.(1999( Int J Cancer, 81(3): 459-466
SSX gene Gure et al. (1997) Int J Cancer, 72(6): 965-971
Family
STAT3 Bromberg et al. (1999) Cell, 98(3): 295-303
STn Sandmaier et al. (1999) J Irnmunother., 22(1): 54-66
(mucin assoc.)
TAG-72 Kuroki et al. (1990)Cancer Res., 50(16): 4872-4879
TGF-a Imanishi et al. (1989) Br J Cancer, 59(5): 761-765
TGF-r3 Picon et al. (1998) Cancer Epidemiol Biomarkers Prey, 7(6):
497-
504
Thymosin [3 15 Bao et al. (1996) Nature Medicine. 2(12), 1322-1328
97
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
IFN-a Moradi et al. (1993) Cancer, 72(8): 2433-2440
TPA Maulard et al. (1994) Cancer, 73(2): 394-398
TPI Nishida et al.(1984) Cancer Res 44(8): 3324-9
TRP-2 Parkhurst et al. (1998) Cancer Res., 58(21) 4895-4901
Tyrosinase Kirkin et al. (1998) APMIS, 106(7): 665-679
VEGF Hyodo et al. (1998) Eur J Cancer, 34(13): 2041-2045
ZAG Sanchez et al. (1999) Science, 283(5409): 1914-1919
p16INK4 Queue et al. (1995) Oncogene Aug. 17, 1995; 11(4): 635-
645
Glutathione Hengstler (1998) et al. Recent Results Cancer Res., 154:
47-85
S-transferase
[0478] Any of the foregoing markers can be used as targets for the
targeting moieties
comprising the nanocarrier (e.g., ICD/IDO silicasomes, ICD/IDO lipid vesicles,
ICD-
inducing nanomaterial carriers, etc.) constructs described herein. In certain
embodiments the
target markers include, but are not limited to members of the epidermal growth
factor family
(e.g., HER2, HER3, EGF, HER4), CD1, CD2, CD3, CD5, CD7, CD13, CD14, CD15,
CD19,
CD20. CD21, CD23, CD25, CD33, CD34, CD38, 5E10, CEA, HLA-DR, HM 1.24, HMB 45,
la, Leu-M1, MUC1, PMSA, TAG-72, phosphatidyl serine antigen, and the like.
[0479] The foregoing markers are intended to be illustrative and not
limiting. Other
tumor associated antigens will be known to those of skill in the art.
[0480] Where the tumor marker is a cell surface receptor, a ligand to that
receptor can
function as targeting moieties. Similarly, mimetics of such ligands can also
be used as
targeting moieties. Thus, in certain embodiments peptide ligands can be used
in addition to
or in place of various antibodies. An illustrative, but non-limiting list of
suitable targeting
peptides is shown in Table 5. In certain embodiments any one or more of these
peptides can
be conjugated to a drug delivery vehicle described herein.
Table 5. Illustrative, but non-limiting peptides that target membrane
receptors expressed or
overexpressed by various cancer cells.
Target Membrane Targeting Peptide SEQ ID
Receptor NO
Integrin receptor A,133 c(RGDfK) 2
c(RGDfC) 3
c(RGDyC) 4
RGD
GM( GEll (YHWYGYTPQNVI) 5
98
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
GFR GS G-KCCYSL 6
SSTR2 Ostreotide
GRP QWAVGHML 7
CCK DYMGWMDF 8
NT RRPYIL 9
RRPYILQLYENKPRRPYIL 10
LHRH Gondaorelin
GPRC family members Antagonist G
c() indicates cyclopeptide. Lower case indicates "D" amino acid.
[0481] In certain embodiments the nanocarrier (e.g., ICD/IDO
silicasome, ICD/IDO
lipid vesicle, ICD-inducing nanomaterial carrier, etc.) can be conjugated to
moieties that
facilitate stability in circulation and/or that hide the nanocarrier from the
reticuloendothelial
system (REC) and/or that facilitate transport across a barrier (e.g., a
stromal barrier, the blood
brain barrier, etc.), and/or into a tissue. In certain embodiments the
nanocarriers are
conjugated to transferrin or ApoE to facilitate transport across the blood
brain barrier. In
certain embodiments the nanocarriers are conjugated to folate.
[0482] Methods of coupling the nanocarrier (e.g., ICD/IDO silicasome,
ICD/IDO
lipid vesicle, ICD-inducing nanomaterial carrier, etc.) to targeting (or
other) agents are well
known to those of skill in the art. Examples include, but are not limited to
the use of biotin
and avidin or streptavidin (see, e.g., U.S. Patent No: US 4,885,172 A), by
traditional
chemical reactions using, for example, bifunctional coupling agents such as
glutaraldehyde,
diimide esters, aromatic and aliphatic diisocyanates, bis-p-nitrophenyl esters
of dicarboxylic
acids, aromatic disulfonyl chlorides and bifunctional arylhalides such as 1,5-
difluoro-2,4-
.. dinitrobenzene; p,p'-difluoro m,m'-dinitrodiphenyl sulfone, sulfhydryl-
reactive maleimides,
and the like. Appropriate reactions which may be applied to such couplings are
described in
Williams et al. Methods in Immunology and Immunochemistry Vol. 1, Academic
Press, New
York 1967. In one illustrative but non-limiting approach a peptide (e.g.,
iRGD) is coupled to
the (e.g., ICD/IDO silicasome, ICD/IDO lipid vesicle, ICD-inducing
nanomaterial carrier,
etc.) by substituting a lipid (e.g., DSPE-PEG2000) with a lipid coupled to a
linker (e.g., DSPE-
PEG2000-maleimide), allowing thiol-maleimide coupling to the cysteine-modified
peptide. It
will also be recognized that in certain embodiments the targeting (and other)
moieties can be
conjugated to other moieties comprising the lipid bilayer on a silicasome or
vesicle, or
comprising the nanomaterial carrier. It is also possible to improve tumor
delivery of the IDO
99
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
inhibitor-ICD inducing nanoparticle, (e.g., OX laden IND-Lipid bilayer-MSNP
(IND-LB-
MSNP), MTX loaded Chol-IND-MSNP, etc.), through co-administration (not
conjugated) of
the iRGD peptide to enhance particle transcytosis.
[0483] The former conjugates and coupling methods are illustrative and
non-limiting.
Using the teachings provided herein, numerous other moieties can be conjugated
to, for
instance, ICD/IDO silicasome, ICD/IDO lipid vesicle, ICD-inducing nanomaterial
carrier,
etc., described herein by any of a variety of methods.
Pharmaceutical Formulations, Administration and Therapy
Pharmaceutical formulations.
[0484] In some embodiments, the nanocarrier (e.g., ICD/IDO silicasome,
ICD/IDO
lipid vesicle, ICD-inducing nanomaterial carrier, etc.) and/or the ICD-
inducing nanomaterials
are administered alone or in a mixture with a physiologically-acceptable
carrier (such as
physiological saline or phosphate buffer) selected in accordance with the
route of
administration and standard pharmaceutical practice. For example, when used as
an
injectable, the nanocarriers can be formulated as a sterile suspension,
dispersion, or emulsion
with a pharmaceutically acceptable carrier. In certain embodiments normal
saline can be
employed as the pharmaceutically acceptable carrier. Other suitable carriers
include, e.g.,
water, buffered water, 0.4% saline, 0.3% glycine, 5% glucose and the like,
including
glycoproteins for enhanced stability, such as albumin, lipoprotein, globulin,
etc. In
compositions comprising saline or other salt-containing carriers, the carrier
is preferably
added following nanocarrier formation. Thus, after the nanocarrier is formed
and loaded with
suitable drug(s), the nanocarrier can be diluted into pharmaceutically
acceptable carriers such
as normal saline.
[0485] Similarly, the ICD-inducing nanomaterials can be introduced
into carriers that
facilitate suspension of the nanomaterials (e.g., emulsions, dilutions, etc.).
[0486] The pharmaceutical compositions may be sterilized by
conventional, well-
known sterilization techniques. The resulting aqueous solutions, suspensions,
dispersions,
emulsions, etc., may be packaged for use or filtered under aseptic conditions.
In certain
embodiments the drug delivery nanocarriers (e.g., LB-coated nanoparticles) are
lyophilized,
the lyophilized preparation being combined with a sterile aqueous solution
prior to
administration. The compositions may also contain pharmaceutically acceptable
auxiliary
100
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
substances as required to approximate physiological conditions, such as pH-
adjusting and
buffering agents, tonicity adjusting agents and the like, for example, sodium
acetate, sodium
lactate, sodium chloride, potassium chloride, calcium chloride, etc.
[0487] Additionally, in certain embodiments, the pharmaceutical
formulation may
include lipid-protective agents that protect lipids against free-radical and
lipid-peroxidative
damage on storage. Lipophilic free-radical quenchers, such as alpha-tocopherol
and water-
soluble iron-specific chelators, such as ferrioxamine, are suitable.
[0488] The concentration of the nanocarrier (e.g., ICD/IDO silicasome,
ICD/IDO
lipid vesicle, ICD-inducing nanomaterial carrier, etc.) (or ICD-inducing
nanomaterial
particles) in the pharmaceutical formulations can vary widely, e.g., from less
than
approximately 0.05%, usually at least approximately 2 to 5% to as much as 10
to 50%, or to
40%, or to 30% by weight and are selected primarily by fluid volumes,
viscosities, etc., in
accordance with the particular mode of administration selected. For example,
the
concentration may be increased to lower the fluid load associated with
treatment. This may
be particularly desirable in patients having atherosclerosis-associated
congestive heart failure
or severe hypertension. Alternatively, nanocarriers composed of irritating
lipids may be
diluted to low concentrations to lessen inflammation at the site of
administration. The
amount of nanocarriers administered will depend upon the particular drug used,
the disease
state being treated and the judgment of the clinician but will generally be
between
approximately 0.01 and approximately 50 mg per kilogram of body weight,
preferably
between approximately 0.1 and approximately 5 mg per kg of body weight.
[0489] In some embodiments, e.g., it is desirable to include
polyethylene glycol
(PEG)-modified phospholipids in the LB-coated nanoparticles or vessicles.
Alternatively, or
additionally, in certain embodiments, PEG-ceramide, or ganglioside Gm-modified
lipids can
be incorporated in the nanocarrier (e.g., ICD/IDO silicasome, ICD/IDO lipid
vesicle, ICD-
inducing nanomaterial carrier, etc.). Addition of such components helps
prevent nanocarrier
aggregation and provides for increasing circulation lifetime and increasing
the delivery of the
loaded nanocarriers to the target tissues. In certain embodiments the
concentration of the
PEG-modified phospholipids, PEG-ceramide, or Gm-modified lipids in the
nanocarriers will
be approximately 1 to 15%.
[0490] In some embodiments, overall nanocarrier charge is an important
determinant
in nanocarrier clearance from the blood. It is believed that highly charged
nanocarriers (i.e.
101
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
zeta potential > +35 mV) will be typically taken up more rapidly by the
reticuloendothelial
system (see, e.g., Juliano (1975) , Biochem. Biophys. Res. Commun. 63: 651-658
discussing
liposome clearance by the RES) and thus have shorter half-lives in the
bloodstream.
Nanocarriers with prolonged circulation half-lives are typically desirable for
therapeutic uses.
.. For instance, in certain embodiments, drug delivery nanocarriers (e.g., LB-
coated
nanoparticles) that are maintained from 8 hrs, or 12 hrs, or 24 hrs, or
greater are desirable.
[0491] In another example of their use, nanocarriers (e.g., ICD/IDO
silicasome,
ICD/IDO lipid vesicle, ICD-inducing nanomaterial carrier. etc.) can be
incorporated into a
broad range of topical dosage forms including but not limited to gels, oils,
emulsions, and the
like, e.g., for the treatment of a topical cancer. For instance, in some
embodiments the
suspension containing the nanocarrier is formulated and administered as a
topical cream,
paste, ointment, gel, lotion, and the like.
[0492] In some embodiments, pharmaceutical formulations comprising
nanocarrier
(e.g., ICD/IDO silicasome, ICD/IDO lipid vesicle, ICD-inducing nanomaterial
carrier, etc.)
described herein additionally incorporate a buffering agent. The buffering
agent may be any
pharmaceutically acceptable buffering agent. Buffer systems include, but are
not limited to
citrate buffers, acetate buffers, borate buffers, and phosphate buffers.
Examples of buffers
include, but are not limited to citric acid, sodium citrate, sodium acetate,
acetic acid, sodium
phosphate and phosphoric acid, sodium ascorbate, tartaric acid, maleic acid,
glycine, sodium
lactate, lactic acid, ascorbic acid, imidazole, sodium bicarbonate and
carbonic acid, sodium
succinate and succinic acid, histidine, and sodium benzoate, benzoic acid, and
the like.
[0493] In some embodiments, pharmaceutical formulations comprising
nanocarrier
(e.g., ICD/IDO silicasome, ICD/IDO lipid vesicle, ICD-inducing nanomaterial
carrier, etc.)
described herein additionally incorporate a chelating agent. The chelating
agent may be any
.. pharmaceutically acceptable chelating agent. Chelating agents include, but
are not limited to
ethylene diaminetetraacetic acid (also synonymous with EDTA, edetic acid,
versene acid, and
sequestrene), and EDTA derivatives, such as dipotassium edetate, disodium
edetate, edetate
calcium disodium, sodium edetate, trisodium edetate, and potassium edetate.
Other chelating
agents include citric acid (e.g., citric acid monohydrate) and derivatives
thereof. Derivatives
of citric acid include anhydrous citric acid, trisodiumcitrate-dihydrate, and
the like. Still
other chelating agents include, but are not limited to, niacinamide and
derivatives thereof and
sodium deoxycholate and derivatives thereof.
102
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0494] In some embodiments, pharmaceutical formulations comprising
nanocarrier
(e.g., ICD/IDO silicasome, ICD/IDO lipid vesicle, ICD-inducing nanomaterial
carrier, etc.)
described herein additionally incorporate an antioxidant. The antioxidant may
be any
pharmaceutically acceptable antioxidant. Antioxidants are well known to those
of ordinary
.. skill in the art and include, but are not limited to, materials such as
ascorbic acid, ascorbic
acid derivatives (e.g., ascorbylpalmitate, ascorbylstearate, sodium ascorbate,
calcium
ascorbate, etc.), butylated hydroxy anisole, buylated hydroxy toluene,
alkylgallate, sodium
meta-bisulfate, sodium bisulfate, sodium dithionite, sodium thioglycollic
acid, sodium
formaldehyde sulfoxylate, tocopherol and derivatives thereof, (d-alpha
tocopherol, d-alpha
tocopherol acetate, dl-alpha tocopherol acetate, d-alpha tocopherol succinate,
beta tocopherol,
delta tocopherol, gamma tocopherol, and d-alpha tocopherol polyoxyethylene
glycol 1000
succinate) monothioglycerol, sodium sulfite and N-acetyl cysteine. In certain
embodiments
such materials, when present, are typically added in ranges from 0.01 to 2.0%.
[0495] In some embodiments, pharmaceutical formulations comprising
nanocarrier
(e.g., ICD/IDO silicasome, ICD/IDO lipid vesicle, ICD-inducing nanomaterial
carrier, etc.)
described herein are formulated with a cryoprotectant. The cryoprotecting
agent may be any
pharmaceutically acceptable cryoprotecting agent. Common cryoprotecting agents
include,
but are not limited to, histidine, polyethylene glycol, polyvinyl pyrrolidine,
lactose, sucrose,
mannitol, polyols, and the like.
[0496] In some embodiments, pharmaceutical formulations comprising
nanocarrier
(e.g., ICD/IDO silicasome, ICD/IDO lipid vesicle, ICD-inducing nanomaterial
carrier, etc.)
described herein are formulated with an isotonic agent. The isotonic agent can
be any
pharmaceutically acceptable isotonic agent. This term is used in the art
interchangeably with
iso-osmotic agent, and is known as a compound that is added to the
pharmaceutical
.. preparation to increase the osmotic pressure, e.g., in some embodiments to
that of 0.9%
sodium chloride solution, which is iso-osmotic with human extracellular
fluids, such as
plasma. Illustrative isotonicity agents include, but are not limited to,
sodium chloride,
mannitol, sorbitol, lactose, dextrose and glycerol.
[0497] In certain embodiments pharmaceutical formulations of the
nanocarrier (e.g.,
ICD/IDO silicasome, ICD/IDO lipid vesicle, ICD-inducing nanomaterial carrier,
etc.)
described herein may optionally comprise a preservative. Common preservatives
include, but
are not limited to, those selected from the group consisting of chlorobutanol,
parabens,
thimerosol, benzyl alcohol, and phenol. Suitable preservatives include but are
not limited to:
103
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
chlorobutanol (e.g., 0.3-0.9% w/v), parabens (e.g., 0.01-5.0%), thimerosal
(e.g., 0.004-0.2%),
benzyl alcohol (e.g., 0.5-5%), phenol (e.g., 0.1-1.0%), and the like.
[0498] In some embodiments, pharmaceutical formulations comprising the
nanocarriers (e.g., ICD/IDO silicasome, ICD/IDO lipid vesicle, ICD-inducing
nanomaterial
carrier, etc.) described herein are formulated with a humectant, e.g., to
provide a pleasant
mouth-feel in oral applications. Humectants known in the art include, but are
not limited to,
cholesterol, fatty acids, glycerin, lauric acid, magnesium stearate,
pentaerythritol, and
propylene glycol.
[0499] In some embodiments, an emulsifying agent is included in the
formulations,
for example, to ensure complete dissolution of all excipients, especially
hydrophobic
components such as benzyl alcohol. Many emulsifiers are known in the art,
e.g., polysorbate
60.
[0500] For some embodiments related to oral administration, it may be
desirable to
add a pharmaceutically acceptable flavoring agent and/or sweetener. Compounds
such as
.. saccharin, glycerin, simple syrup, and sorbitol are useful as sweeteners.
Administration
[0501] The nanocarrier (e.g., ICD/IDO silicasome, ICD/IDO lipid
vesicle, ICD-
inducing nanomaterial carrier, etc.) described herein can be administered to a
subject (e.g.,
patient) by any of a variety of techniques.
[0502] In certain embodiments the nanocarrier (e.g., ICD/IDO silicasome,
ICD/IDO
lipid vesicle, ICD-inducing nanomaterial carrier, etc.) and/or pharmaceutical
formulations
thereof are administered parenterally, e.g., intraarticularly, intravenously,
intraperitoneally,
subcutaneously, or intramuscularly. In some embodiments, the pharmaceutical
compositions
are administered intravenously, intraarteraly, or intraperitoneally by a bolus
injection (see,
.. e.g., U.S. Pat. Nos. 3,993354; 4,145,410; 4,235,871; 4,224,179; 4,522,803;
and 4,588,578
describing administration of liposomes). Particular pharmaceutical
formulations suitable for
this administration are found in Remington's Pharmaceutical Sciences, Mack
Publishing
Company, Philadelphia, Pa., 17th ed. (1985). Typically, the formulations
comprise a solution
of the drug delivery nanocarrier suspended in an acceptable carrier,
preferably an aqueous
carrier. As noted above, suitable aqueous solutions include, but are not
limited to
physiologically compatible buffers such as Hanks solution, Ringer's solution,
or physiological
(e.g., 0.9% isotonic) saline buffer and/or in certain emulsion formulations.
The solution(s)
104
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
can contain formulatory agents such as suspending, stabilizing and/or
dispersing agents. In
certain embodiments the active agent(s) can be provided in powder form for
constitution with
a suitable vehicle, e.g., sterile pyrogen-free water, before use. For
transmucosal
administration, and/or for blood/brain barrier passage, penetrants appropriate
to the barrier to
be permeated can be used in the formulation. These compositions may be
sterilized by
conventional, well-known sterilization techniques, or may be sterile filtered.
The resulting
aqueous solutions may be packaged for use as is, or lyophilized, the
lyophilized preparation
being combined with a sterile aqueous solution prior to administration. The
compositions
may contain pharmaceutically acceptable auxiliary substances as required to
approximate
physiological conditions, such as pH adjusting and buffering agents, tonicity
adjusting agents,
wetting agents and the like, for example, sodium acetate, sodium lactate,
sodium chloride,
potassium chloride, calcium chloride. sorbitan monolaurate, triethanolamine
oleate, etc., e.g.,
as described above.
[0503] In other methods, the pharmaceutical formulations containing
the nanocarrier
(e.g., ICD/IDO silicasome, ICD/IDO lipid vesicle, ICD-inducing nanomaterial
carrier, etc.)
described herein may be contacted with the target tissue by direct application
of the
preparation to the tissue. The application may be made by topical. "open" or
"closed"
procedures. By "topical" it is meant the direct application of the
pharmaceutical preparation
to a tissue exposed to the environment, such as the skin, oropharynx, external
auditory canal,
.. and the like. Open procedures are those procedures that include incising
the skin of a patient
and directly visualizing the underlying tissue to which the pharmaceutical
formulations are
applied. This is generally accomplished by a surgical procedure, such as a
thoracotomy to
access the lungs, abdominal laparotomy to access abdominal viscera, or other
direct surgical
approaches to the target tissue. Closed procedures are invasive procedures in
which the
internal target tissues are not directly visualized, but accessed via
inserting instruments
through small wounds in the skin. For example, the preparations may be
administered to the
peritoneum by needle lavage. Likewise, the pharmaceutical preparations may be
administered to the meninges or spinal cord by infusion during a lumbar
puncture followed
by appropriate positioning of the patient as commonly practiced for spinal
anesthesia or
metrizamide imaging of the spinal cord. Alternatively, the preparations may be
administered
through endoscopic devices. In certain embodiments the pharmaceutical
formulations are
introduced via a cannula.
105
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0504] In certain embodiments the pharmaceutical formulations
comprising the
nanocarrier (e.g., ICD/IDO silicasome, ICD/IDO lipid vesicle, ICD-inducing
nanomaterial
carrier, etc.) described herein are administered via inhalation (e.g., as an
aerosol). Inhalation
can be a particularly effective delivery route for administration to the lungs
and/or to the
brain. For administration by inhalation, the drug delivery nanocarriers are
conveniently
delivered in the form of an aerosol spray from pressurized packs or a
nebulizer, with the use
of a suitable propellant, e.g., dichlorodifluoromethane,
trichlorofluoromethane,
dichlorotetrafluoroethane, carbon dioxide or other suitable gas. In the case
of a pressurized
aerosol the dosage unit can be determined by providing a valve to deliver a
metered amount.
Capsules and cartridges of e.g. gelatin for use in an inhaler or insufflator
may be formulated
containing a powder mix of the compound and a suitable powder base such as
lactose or
starch.
[0505] In certain embodiments, the nanocarrier (e.g., ICD/IDO
silicasome, ICD/IDO
lipid vesicle, ICD-inducing nanomaterial carrier, etc.) described herein are
formulated for
oral administration. For oral administration, suitable formulations can be
readily formulated
by combining the drug delivery nanocarriers) with pharmaceutically acceptable
carriers
suitable for oral delivery well known in the art. Such carriers enable the
active agent(s)
described herein to be formulated as tablets, pills, dragees, caplets,
lozenges, gelcaps,
capsules, liquids, gels, syrups, slurries, suspensions and the like, for oral
ingestion by a
patient to be treated. For oral solid formulations such as, for example,
powders, capsules and
tablets, suitable excipients can include fillers such as sugars (e.g.,
lactose, sucrose, mannitol
and sorbitol), cellulose preparations (e.g., maize starch, wheat starch, rice
starch, potato
starch, gelatin, gum tragacanth, methyl cellulose, hydroxypropylmethyl-
cellulose, sodium
carboxymethylcellulose), synthetic polymers (e.g., polyvinylpyrrolidone
(PVP)), granulating
agents; and binding agents. If desired, disintegrating agents may be added,
such as the cross-
linked polyvinylpyrrolidone, agar, or alginic acid or a salt thereof such as
sodium alginate. If
desired, solid dosage forms may be sugar-coated or enteric-coated using
standard techniques.
The preparation of enteric-coated particles is disclosed for example in U.S.
Pat. Nos.
4,786,505 and 4,853,230.
[0506] In various embodiments the nanocarrier (e.g., ICD/IDO silicasome,
ICD/IDO
lipid vesicle, ICD-inducing nanomaterial carrier, etc.) described hereien can
be formulated in
rectal or vaginal compositions such as suppositories or retention enemas,
e.g., containing
conventional suppository bases such as cocoa butter or other glycerides.
Methods of
106
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
formulating active agents for rectal or vaginal delivery are well known to
those of skill in the
art (see, e.g., Allen (2007) Suppositories, Pharmaceutical Press) and
typically involve
combining the active agents with a suitable base (e.g., hydrophilic (PEG),
lipophilic materials
such as cocoa butter or Witepsol W45). amphiphilic materials such as Suppocire
AP and
polyglycolized glyceride, and the like). The base is selected and compounded
for a desired
melting/delivery profile.
[0507] The route of delivery of the nanocarrier (e.g., ICD/IDO
silicasome, ICD/IDO
lipid vesicle, ICD-inducing nanomaterial carrier, etc.) described herein can
also affect their
distribution in the body. Passive delivery of nanocarrier (e.g., ICD/IDO
silicasome, ICD/IDO
lipid vesicle, ICD-inducing nanomaterial carrier, etc.) involves the use of
various routes of
administration e.g., parenterally, although other effective administration
forms, such as
intraarticular injection, inhalant mists, orally active formulations,
transdermal iontophoresis,
or suppositories are also envisioned. Each route produces differences in
localization of the
drug delivery nanocarrier.
[0508] Because dosage regimens for pharmaceutical agents are well known to
medical practitioners, the amount of the liposomal pharmaceutical agent
formulations that is
effective or therapeutic for the treatment of a disease or condition in
mammals and
particularly in humans will be apparent to those skilled in the art. The
optimal quantity and
spacing of individual dosages of the formulations herein will be determined by
the nature and
extent of the condition being treated, the form, route and site of
administration, and the
particular patient being treated, and such optima can be determined by
conventional
techniques. It will also be appreciated by one of skill in the art that the
optimal course of
treatment, e.g., the number of doses given per day for a defined number of
days, can be
ascertained by those skilled in the art using conventional course of treatment
determination
tests.
[0509] Typically, the nanocarrier (e.g., ICD/IDO silicasome, ICD/IDO
lipid vesicle,
ICD-inducing nanomaterial carrier, etc.) and/or pharmaceutical formations
thereof described
herein are used therapeutically in animals (including man) in the treatment of
various cancers.
In certain embodiments the nanocarriers and/or pharmaceutical formations
thereof described
herein are particularly well suited in conditions that require: (1) repeated
administrations;
and/or (2) the sustained delivery of the drug in its bioactive form; and/or
(3) the decreased
toxicity with suitable efficacy compared with the free drug(s) in question. In
various
embodiments the nanocarriers and/or pharmaceutical formations thereof are
administered in a
107
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
therapeutically effective dose. The term "therapeutically effective" as it
pertains to the
nanocarriers described herein and formulations thereof means that the
combination of ICD
inducer and IDO inhibitor produces a desirable effect on the cancer. Such
desirable effects
include, but are not limited to slowing and/or stopping tumor growth and/or
proliferation
and/or slowing and/or stopping proliferation of metastatic cells, reduction in
size and/or
number of tumors, and/or elimination of tumor cells and/or metastatic cells,
and/or prevention
of recurrence of the cancer following remission.
[0510] Exact dosages will vary depending upon such factors as the
particular ICD
inducer(s) and IDO inhibitors and the desirable medical effect, as well as
patient factors such
as age, sex, general condition, and the like. Those of skill in the art can
readily take these
factors into account and use them to establish effective therapeutic
concentrations without
resort to undue experimentation.
[0511] For administration to humans (or to non-human mammals) in the
curative,
remissive, retardive, or prophylactic treatment of diseases the prescribing
physician will
ultimately determine the appropriate dosage of the drug for a given human (or
non-human)
subject, and this can be expected to vary according to the age, weight, and
response of the
individual as well as the nature and severity of the patient's disease. In
certain embodiments
the dosage of the drug provided by the nanocarrier(s) can be approximately
equal to that
employed for the free drug. However as noted above, the nanocarriers described
herein can
significantly reduce the toxicity of the drug(s) administered thereby and
significantly increase
a therapeutic window. Accordingly, in some cases dosages in excess of those
prescribed for
the free drug(s) will be utilized.
[0512] In certain embodiments, the dose of each of the drug(s) (e.g.,
ICD inducer,
IDO inhibitor) administered at a particular time point will be in the range
from about 1 to
about 1,000 mg/m2/day, or to about 800 mg/m2/day, or to about 600 mg/m2/day,
or to about
400 mg/m2/day. For example, in certain embodiments a dosage (dosage regiment)
is utilized
that provides a range from about 1 to about 350 mg/m2/day, 1 to about 300
mg/m2/day, 1 to
about 250 mg/m2/day, 1 to about 200 mg/m2/day, 1 to about 150 mg/m2/day, 1 to
about 100
mg/m2/day, from about 5 to about 80 mg/m2/day, from about 5 to about 70
mg/m2/day, from
about 5 to about 60 mg/m2/day, from about 5 to about 50 mg/m2/day, from about
5 to about
mg/m2/day, from about 5 to about 20 mg/m2/day, from about 10 to about 80
mg/m2/day,
from about 10 to about 70 mg/m2/day, from about 10 to about 60 mg/m2/day, from
about 10
to about 50 mg/m2/day, from about 10 to about 40 mg/m2/day, from about 10 to
about 20
108
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
mg/m2/day, from about 20 to about 40 mg/m2/day, from about 20 to about 50
mg/m2/day,
from about 20 to about 90 mg/m2/day, from about 30 to about 80 mg/m2/day, from
about 40
to about 90 mg/m2/day, from about 40 to about 100 mg/m2/day, from about 80 to
about 150
mg/m2/day, from about 80 to about 140 mg/m2/day, from about 80 to about 135
mg/m2/day,
from about 80 to about 130 mg/m2/day, from about 80 to about 120 mg/m2/day,
from about
85 to about 140 mg/m2/day, from about 85 to about 135 mg/m2/day, from about 85
to about
135 mg/m2/day, from about 85 to about 130 mg/m2/day, or from about 85 to about
120
mg/m2/day. In certain embodiments the does administered at a particular time
point may also
be about 130 mg/m2/day, about 120 mg/m2/day, about 100 mg/m2/day, about 90
mg/m2/day,
about 85 mg/m2/day, about 80 mg/m2/day, about 70 mg/m2/day, about 60
mg/m2/day, about
50 mg/m2/day, about 40 mg/m2/day, about 30 mg/m2/day, about 20 mg/m2/day,
about 15
mg/m2/day, or about 10 mg/m2/day.
[0513] Dosages may also be estimated using in vivo animal models, as
will be
appreciated by those skill in the art. In this regard, with respect to the
irinotecan-loaded drug
delivery nanocarriers described herein, it is noted that the effective
therapeutic dose of the
OX/IND nanocarrier in a KPC-derived orthotopic animal model is about 5 mg
OX/kg with 50
mg IND/kg, which is equivalent to 15.5 mg OX/m2 IND 150 mg/m2in a 60 kg human
subject.
Fibonacci analysis indicates this dose can be achieved by starting and
intermediary OX doses
of 37.5 and 75 mg/m2. It is noted that 75 mg/m2 OX is quite conservative and
higher dosages
are contemplated.
[0514] The dose administered may be higher or lower than the dose
ranges described
herein, depending upon, among other factors, the bioavailability of the
composition, the
tolerance of the individual to adverse side effects, the mode of
administration and various
factors discussed above. Dosage amount and interval may be adjusted
individually to provide
plasma levels of the composition that are sufficient to maintain therapeutic
effect, according
to the judgment of the prescribing physician. Skilled artisans will be able to
optimize
effective local dosages without undue experimentation in view of the teaching
provided
herein.
[0515] Multiple doses (e.g., continuous or bolus) of the compositions
as described
herein may also be administered to individuals in need thereof of the course
of hours, days,
weeks, or months. For example, but not limited to, 1, 2, 3, 4, 5, or 6 times
daily, every other
day, every 10 days, weekly, monthly, twice weekly, three times a week, twice
monthly, three
109
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
times a month, four times a month, five times a month, every other month,
every third month,
every fourth month, etc.
Methods of treatment.
[0516] In various embodiments methods of treatment using the
nanocarrier (e.g.,
ICD/IDO silicasome, ICD/IDO lipid vesicle, ICD-inducing nanomaterial carrier,
etc.) and/or
pharmaceutical formulation(s) comprising nanoparticle drug carriers described
herein are
provided. In certain embodiments the method(s) comprise a method of treating a
cancer. In
certain embodiments the method can comprise administering to a subject in need
thereof an
effective amount of a nanocarrier (e.g., ICD/IDO silicasome, ICD/IDO lipid
vesicle, ICD-
inducing nanomaterial carrier, etc.), and/or a pharmaceutical formulation
comprising a
nanocarrier as described herein, where the drug(s) comprising the nanocarrier
and/or said
pharmaceutical formulation comprises an anti-cancer drug.
[0517] In certain embodiments the nanocarrier (e.g., ICD/IDO
silicasome, ICD/IDO
lipid vesicle, ICD-inducing nanomaterial carrier, etc.) and/or pharmaceutical
formulation is a
primary therapy in a chemotherapeutic regimen. In certain embodiments the
nanoparticle
drug carrier and/or pharmaceutical formulation is a component in an adjunct
therapy in
addition to chemotherapy using one or more other chemotherapeutic agents,
and/or surgical
resection of a tumor mass, and/or radiotherapy.
[0518] In certain embodiments the nanocarrier (e.g., ICD/IDO
silicasome, ICD/IDO
lipid vesicle, ICD-inducing nanomaterial carrier, etc.) and/or pharmaceutical
formulation is a
component in a multi-drug chemotherapeutic regimen. In certain embodiments the
multi-
drug chemotherapeutic regimen comprises at least two drugs selected from the
group
consisting of irinotecan (IRIN), oxaliplatin (OX), 5-fluorouracil (5-FU), and
leucovorin (LV).
In certain embodiments the multi-drug chemotherapeutic regimen comprises at
least three
drugs selected from the group consisting of irinotecan (IRIN), oxaliplatin
(OX), 5-
fluorouracil (5-FU), and leucovorin (LV). In certain embodiments the multi-
drug
chemotherapeutic regimen comprises at least irinotecan (IRIN), oxaliplatin
(OX), 5-
fluorouracil (5-FU), and leucovorin (LV).
[0519] In various embodiments nanocarrier (e.g., ICD/IDO silicasome,
ICD/IDO lipid
vesicle, ICD-inducing nanomaterial carrier, etc.) and/or pharmaceutical
formulation(s)
threeof described herein are effective for treating any of a variety of
cancers. In certain
embodiments the cancer is pancreatic ductal adenocarcinoma (PDAC). In certain
110
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
embodiments the cancer is a cancer selected from the group consisting of acute
lymphoblastic
leukemia (ALL), acute myeloid leukemia (AML), adrenocortical carcinoma, AIDS-
related
cancers (e.g., Kaposi sarcoma, lymphoma), anal cancer, appendix cancer,
astrocytomas,
atypical teratoid/rhabdoid tumor, bile duct cancer, extrahepatic cancer,
bladder cancer, bone
.. cancer (e.g., Ewing sarcoma, osteosarcoma, malignant fibrous histiocytoma),
brain stem
glioma, brain tumors (e.g., astrocytomas, glioblastoma, brain and spinal cord
tumors, brain
stem glioma, central nervous system atypical teratoid/rhabdoid tumor, central
nervous system
embryonal tumors, central nervous system germ cell tumors, craniopharyngioma,
ependymoma, breast cancer, bronchial tumors, burkitt lymphoma, carcinoid
tumors (e.g.,
childhood, gastrointestinal), cardiac tumors, cervical cancer, chordoma,
chronic lymphocytic
leukemia (CLL), chronic myelogenous leukemia (CML), chronic myeloproliferative
disorders, colon cancer, colorectal cancer, craniopharyngioma, cutaneous t-
cell lymphoma,
duct cancers e.g. (bile, extrahepatic), ductal carcinoma in situ (DCIS),
embryonal tumors,
endometrial cancer, ependymoma, esophageal cancer, esthesioneuroblastoma,
extracranial
germ cell tumor, extragonadal germ cell tumor, extrahepatic bile duct cancer,
eye cancer
(e.g., intraocular melanoma, retinoblastoma), fibrous histiocytoma of bone,
malignant, and
osteosarcoma, gallbladder cancer, gastric (stomach) cancer, gastrointestinal
carcinoid tumor,
gastrointestinal stromal tumors (GIST), germ cell tumors (e.g., ovarian
cancer, testicular
cancer, extracranial cancers, extragonadal cancers, central nervous system),
gestational
trophoblastic tumor, brain stem cancer, hairy cell leukemia, head and neck
cancer, heart
cancer, hepatocellular (liver) cancer, histiocytosis, langerhans cell cancer,
Hodgkin
lymphoma, hypopharyngeal cancer, intraocular melanoma, islet cell tumors,
pancreatic
neuroendocrine tumors, kaposi sarcoma, kidney cancer (e.g., renal cell, Wilm's
tumor, and
other kidney tumors), langerhans cell histiocytosis, laryngeal cancer,
leukemia, acute
.. lymphoblastic (ALL), acute myeloid (AML), chronic lymphocytic (CLL),
chronic
myelogenous (CML), hairy cell, lip and oral cavity cancer, liver cancer
(primary), lobular
carcinoma in situ (LCIS), lung cancer (e.g., childhood, non-small cell, small
cell), lymphoma
(e.g., AIDS-related, Burkitt (e.g., non-Hodgkin lymphoma), cutaneous T-Cell
(e.g., mycosis
fungoides, Sezary syndrome), Hodgkin, non-Hodgkin, primary central nervous
system
(CNS)), macroglobulinemia, Waldenstrom, male breast cancer, malignant fibrous
histiocytoma of bone and osteosarcoma, melanoma (e.g., childhood, intraocular
(eye)),
merkel cell carcinoma, mesothelioma, metastatic squamous neck cancer, midline
tract
carcinoma, mouth cancer, multiple endocrine neoplasia syndromes, multiple
myeloma/plasma cell neoplasm, mycosis fungoides, myelodysplastic syndromes,
chronic
111
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
myeloid leukemia (CML), multiple myeloma, nasal cavity and paranasal sinus
cancer,
nasopharyngeal cancer, neuroblastoma, oral cavity cancer, lip and
oropharyngeal cancer,
osteosarcoma, ovarian cancer , pancreatic cancer, pancreatic neuroendocrine
tumors (islet cell
tumors), papillomatosis, paraganglioma, paranasal sinus and nasal cavity
cancer, parathyroid
cancer, penile cancer, pharyngeal cancer, pheochromocytoma, pituitary tumor,
plasma cell
neoplasm, pleuropulmonary blastoma, primary central nervous system (CNS)
lymphoma,
prostate cancer, rectal cancer, renal cell (kidney) cancer, renal pelvis and
ureter, transitional
cell cancer, rhabdomyosarcoma, salivary gland cancer, sarcoma (e.g., Ewing,
Kaposi,
osteosarcoma, rhadomyosarcoma, soft tissue, uterine), Sezary syndrome, skin
cancer (e.g.,
melanoma, merkel cell carcinoma, basal cell carcinoma, nonmelanoma), small
intestine
cancer, squamous cell carcinoma, squamous neck cancer with occult primary,
stomach
(gastric) cancer, testicular cancer, throat cancer, thymoma and thymic
carcinoma, thyroid
cancer, trophoblastic tumor, ureter and renal pelvis cancer, urethral cancer,
uterine cancer,
endometrial cancer, uterine sarcoma, vaginal cancer, vulvar cancer,
Waldenstrom
macroglobulinemia, and Wilms tumor.
[0520] In certain embodiments the nanocarrier (e.g., ICD/IDO
silicasome, ICD/IDO
lipid vesicle, ICD-inducing nanomaterial carrier, etc.) described herein is
not conjugated to
an iRGD peptide and the nanocarrier is administered in conjunction with an
iRGD peptide
(e.g., the nanocarrier and the iRGD peptide are co-administered as separate
formulations).
[0521] In various embodiments of these treatment methods, the nanocarrier
(e.g.,
ICD/IDO silicasome, ICD/IDO lipid vesicle, ICD-inducing nanomaterial carrier,
etc.) and/or
pharmaceutical formulation is administered via a route selected from the group
consisting of
intravenous administration, intraarterial administration, intracerebral
administration,
intrathecal administration, oral administration, aerosol administration,
administration via
inhalation (including intranasal and intratracheal delivery, intracranial
administration via a
cannula, and subcutaneous or intramuscular depot deposition. In certain
embodiments the
nanocarrier and/or pharmaceutical formulation is administered as an injection,
from an IV
drip bag, or via a drug-delivery cannula. In various embodiments the subject
is a human and
in other embodiments the subject is a non-human mammal.
Combined treatment with checkpoint inhibitors.
[0522] It is believed that the nanocarriers described herein (e.g.,
comprising an
inducer of immunogenic cell death (ICD), and an IDO inhibitor) showed
synertistic anti-
112
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
cancer activity when administered in combination with one or more checkpoint
inhibitors,
and this has been demonstrated for an Irinotecan silicasome and anti-PD1.
Accordingly,
certain embodiments, methods contemplated herein include the administration of
a drug
delivery nanovesicle and/or a drug delivery nanocarrier as described herein in
conjunction
with one or more checkpoint inhibitors.
[0523] Illustrative checkpoint inhibitors include, but are not limited
to inhibitors of
PD-1, PD-L1, PD-L2, PD-L3, PD-L4, CTLA-4, LAG3, B7-H3, B7-H4, KIR and/or TIM3
receptors.
[0524] In some embodiments, the immune checkpoint inhibitor can be a
small peptide
agent that can inhibit regulatory T cell function, including any one or a
combination of the
inhibitory receptors listed above. In some embodiments, the immune checkpoint
inhibitor
can be a small molecule (e.g. less than 500 Daltons) that can inhibit T
regulatory cell
function. including the immune checkpoint receptors listed above. In some
embodiments, the
immune checkpoint inhibitor can be a molecule providing co-stimulation of T-
cell activation.
In some embodiments, the immune checkpoint inhibitor can be a molecule
providing co-
stimulation of natural killer cell activation. In some embodiments, the immune
checkpoint
inhibitor can be an antibody. In some embodiments, the immune checkpoint
inhibitor is a PD-
1 antibody. In some embodiments, the immune checkpoint inhibitor is a PD-Li
antibody. In
some embodiments, the immune checkpoint inhibitor is a PD-L2 antibody. In some
embodiments, the immune checkpoint inhibitor is a PD-L3 antibody. In some
embodiments,
the immune checkpoint inhibitor is a PD-L4 antibody. In some embodiments, the
immune
checkpoint inhibitor is a CTLA-4 antibody. In some embodiments, the immune
checkpoint
inhibitor is an antibody of CTLA-4, LAG3, B7-H3, B7-H4, KIR, or TIM3.
[0525] In certain embodiments the antibody can be selected from a-CD3-
APC, a-
CD3-APC-H7, a-CD4-ECD, a-CD4-PB, a-CD8-PE-Cy7, a-CD-8-PerCP-Cy5.5, a-CD11c-
APC, a-CD11b-PE-Cy7, a-CD11b-AF700, a-CD14-FITC, a-CD16-PB, a-CD19-AF780, a-
CD19-AF700. a-CD2O-PO, a-CD25-PE-Cy7, a-CD40-APC, a-CD45-Biotin, Streptavidin-
BV605, a-CD62L-ECD, a-CD69-APC-Cy7, a-CD8O-FITC, a-CD83-Biotin, Streptavidin-
PE-Cy7, a-CD86-PE-Cy7, a-CD86-PE, a-CD123-PE, a-CD154-PE, a-CD161-PE, a-
CTLA4-PE-Cy7, a-FoxP3-AF488 (clone 259D), IgGl-isotype-AF488, a-ICOS (CD278)-
PE,
a-HLA-A2-PE, a-HLA-DR-PB, a-HLA-DR-PerCPCy5.5, a-PD1-APC, VISTA, co-
stimulatory molecule 0X40, CD137, and the like.
113
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0526] Any of a variety of antibodies can be used in the methods
described herein,
including, but nor limited to antibodies having high-affinity binding to PD-1
PD-L1, PD-L2,
PD-L3, or PD-L4. Human mAbs (HuMAbs) that bind specifically to PD-1 (e.g.,
bind to
human PD-1 and may cross-react with PD-1 from other species, such as
cynomolgus
monkey) with high affinity have been disclosed in U.S. Pat. No. 8.008,449,
which is
incorporated herein by reference for the antibodies described herein. HuMAbs
that bind
specifically to PD-Li with high affinity have been disclosed in U.S. Pat. No.
7,943,743,
which is incorporated herein by reference for the antibodies described herein.
Other anti-PD-
1 mAbs have been described in, for example, U.S. Pat. Nos. 6,808,710,
7,488,802 and
8,168,757, and PCT Publication No. WO 2012/145493, all of which are
incorporated herein
by reference for the antibodies described herein. Anti-PD-Li mAbs have been
described in,
for example, U.S. Pat. Nos. 7,635,757 and 8,217,149, U.S. Publication No.
2009/0317368,
and PCT Publication Nos. WO 2011/066389 and WO 2012/14549, all of which are
incorporated herein by reference for the antibodies described herein.
[0527] In some embodiments, the anti-PD-1 HuMAbs can be selected from 17D8,
2D3, 4H1. 5C4 (also referred to herein as nivolumab), 4A1 1, 7D3 and 5F4, all
of which are
described in U.S. Pat. No. 8,008,449. In some embodiments, the anti-PD-1
HuMAbs can be
selected from 3G10, 12A4 (also referred to herein as BMS-936559), 10A5, 5F8,
10H10,
1B12, 7H1, 1 1E6, 12B7, and 13G4, all of which are described in U.S. Pat. No.
7,943,743.
[0528] In certain embodiments the antibodies comprises antibodies that are
are
approved for clinical use. Such antibodies include, but are not limited to
antibodies that
target PD-1 (e.g., Pembrolizumab (Keytruda), Nivolumab (Opdivo)), antibodies
that target
PD-Li (e.g., Atezolizumab (Tecentriq), Avelumab (Bavencio), Durvalumab
(Imfinzi), and
the like), and/or antibodies that target CTLA-4 (e.g., Ipilimumab (Yervoy)).
[0529] The foregoing checkpoint inhibitors are illustrative and not
limiting. Using
the teaching provided herein numerous other checkpoint inhibitors can be used
in conjunction
with the delivery vehicles described herein.
Kits.
[0530] In certain embodiments, kits are provided containing reagents
for the practice
of any of the methods described herein. In certain embodiments the kit
comprises a container
containing an inhibitor of the indoleamine 2,3-dioxygenase (IDO) pathway (IDO
inhibitor);
and/or a container containing an agent that induces immunogenic cell death
(ICD) (ICD-
114
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
inducer). In certain embodiments the IDO inhibitor comprises an agent selected
from the
group consisting of 1-methyl-D-tryptophan (indoximod), 1-methyl-L-tryptophan,
methylthiohydantoin-dl-tryptophan, Necrostatin-1, Ebselen, Pyridoxal
Isonicotinoyl
Hydrazone, Norharmane, CAY10581, 2-Benzy1-2-thiopseudourea hydrochloride,
.. Norharmane hydrochloride, INCB024360, S-allyl-brassinin, S-benzyl-
brassinin, 5-Bromo-
brassinin, 4-phenylimidazole Exiguamine A, and NSC401366. In certain
embodiments the
IDO inhibitor comprises an agent shown in Table 3, supra. In certain
embodiments the IDO
inhibitor comprises indoximod. In certain embodiments the IDO inhibitor is
conjugated to an
agent that forms a vesicle. In certain embodiments the agent is selected from
the group
.. consisting of a lipid, PHGP, vitamin E, cholesterol, and a fatty acid. In
certain embodiments
the agent comprises a phospholipid. In certain embodiments the IDO inhibitor
is IDO-PL.
[0531] In certain embodiments the ICD inducer comprises a
chemotherapeutic agent
selected from the group consisting of oxaliplatin, cisplatin, doxorubicin,
epirubicin,
idarubicin, mitoxantrone, anthracenedione, bleomycin, bortezomib, R2016,
irinotecan and
cyclophosphamide. In certain embodiments the ICD inducer comprises
oxaliplatin. In
certain embodiments the ICD inducer is a compound or a biological agent in
Table 2.
[0532] In certain embodiments the kit contains both an IDO inhibitor
and an ICD
inducer. In certain embodiments the IDO inhibitor and the ICD inducer are in
separate
containers. In certain embodiments the IDO inhibitor and said ICD inducer are
in the same
container. In certain embodiments the IDO inhibitor and said ICD inducer are
provided as a
nanoparticle drug carrier (e.g., a drug delivery nanocarrier) as described
herein.
[0533] In certain embodiments the kit contains an ICD inducer that
comprise a
nanomaterial or a formulation thereof (e.g., a sterile formulation). In
certain embodiments
the nanomaterial comprises a material selected form the group consisting of
CuO, Sb203,
ZnO, TiO2, and graphene oxide.
[0534] In certain embodiments the kit comprises a container containing
a nanocarrier
(e.g., ICD/IDO silicasome, ICD/IDO lipid vesicle, ICD-inducing nanomaterial
carrier, etc.)
described herein.
[0535] Additionally, in certain embodiments, the kits can include
instructional
materials disclosing the means of the use of the ICD inducer to induce
immunogenic death in
cancer cells for vaccination, and/or the use of the ICD inducer and the IDO
inhibitor as a
cancer therapeutic for local administration, and/or the use of a drug-loaded
drug delivery
115
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
nanocarrier (e.g., LB-coated nanoparticle) or nanocarrier immunoconjugate as a
therapeutic
for a cancer (e.g., a pancreatic cancer, gastric cancer, cervical cancer,
ovarian cancer, etc.).
[0536] In addition, the kits optionally include labeling and/or
instructional materials
providing directions (e.g., protocols) for the use of the materials described
herein, e.g., alone
or in combination for the treatment of various cancers. Instructional
materials can also
include recommended dosages, description(s) of counterindications, and the
like.
[0537] While the instructional materials in the various kits typically
comprise written
or printed materials they are not limited to such. Any medium capable of
storing such
instructions and communicating them to an end user is contemplated by this
invention. Such
media include, but are not limited to electronic storage media (e.g., magnetic
discs, tapes,
cartridges, chips), optical media (e.g., CD ROM), and the like. Such media may
include
addresses to interne sites that provide such instructional materials.
EXAMPLES
[0538] The following examples are offered to illustrate, but not to
limit the claimed
invention.
Example 1
IDO Inhibitor Prodrugs
[0539] Indo1eamine-2,3-dioxygenase (IDO) is an intracellular heme-
containing
enzyme that initiates the first and rate-limiting step of tryptophan
degradation along the
kynurenine pathway. In mammalian organisms, tryptophan is an essential amino
acid for cell
survival; it cannot be synthesized de novo. IDO was shown to be expressed in
normal tissues
such as the endothelial cells in the placenta and lung, the epithelial cells
in the female genital
tract, and the lymphoid tissues in mature dendritic cells. Munn et al. showed
that IDO has a
central role in preventing T cell-driven rejection of allogeneic fetuses
during pregnancy as
.. trophoblast expressing IDO was found to induce maternal tolerance to fetal
allograft (see,
e.g., Munn et al. (1998) Science, 281(5380): 1191-1193). This discovery broke
ground for
further research addressing the immunomodulatory potential of IDO, including
the discovery
of IDO inhibitor for cancer treatment. The immunosuppressive roles of IDO have
also been
investigated for elucidation of therapeutic targets in the management of many
diseases
including cancer (Gajewski et al. (2013) Nature Immunol. 14: 1014-1022; Moon
et al. (2015)
J. ImmunoTherapy Cancer, 3: 51).
116
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0540] Based on a summary of clinical trials (Vacchelli et al. (2014)
Oncoimmunology, 3(10): e957994), we found that:
[0541] 1) Use of indoximod as a standalone agent often fails to
cause tumor
eradication; and
[0542] 2) Combination therapy, i.e. chemo + indoximod, showed promising
results. This includes the use of an IDO inhibitor plus many standard
chemoagents, such as
MTX, paclitaxel, docetaxel, etc. In PDAC, a clinical trial using IDO inhibitor
plus GEM and
PTX is ongoing.
Synthesis of IDO inhibitor indoximod prodrug
[0543] Indoximod is a potent IDO pathway inhibitor. It is currently used as
its free
form in NaOH solution and/or pellets in clinical trials. However, in order to
achieve an
effective therapeutic dose, extremely high concentrations of Indoximod are
required to be
used (e.g. oral formulation, 1200-2000 mg/day). We propose to use a bio-
conjugation or
supramolecular assembly approach to further improve the PK/PD, local retention
and potency
of Indoximod in vivo, either via local intratumoral injection or systemic IV
injection.
Bio-conjugation approach
[0544] Indoximod has a functional carboxyl group (see, e.g., Figure
2), that can be
readily conjugated to other compounds containing a hydroxyl moiety. A few
representative
compounds are provided (see, e.g., Figure 3). The resulting pro-drugs can form
nanovesicles
in an aqueous solution at certain concentrations (e.g. >CMC) or be used as a
component to
coat MSNPs as described herein, leading to a variety of immunotherapy drug
delivery
nanocarrier(s) (e.g., LB-coated nanoparticle(s)). Since the resulting
conjugates are
amphipathic molecules, they are readily incorporated into lipid vesicles and
can also self-
assemble as micellar structures, both of which are pharmaceutically active.
[0545] In certain embodiments a little more complicated ester-mediated
conjugation
could include the use of linkers such as an HO-(CH2)õ=2_5-0H as a linker in
the reaction. The
cases of oleic acid and docosahexaenoic acid (DHA) fall into this category
(see, e.g., Figure
4).
Supramolecular approach:
[0546] It is possible to take advantage of the chemical structure of
indoximod or other
IDO inhibitors, allowing the supramolecular assembly of this compound onto a
117
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
nanostructured surface mediated by individual or combined molecule-
nanomaterial
interactions, such as pi-pi stacking, electrostatic interactions, van der
Waals' force and/or
physical absorption. One example is graphene oxide, which is also an inducer
of 1CD in our
HTS studies.
[0547] While the above-identified methods are illustrated with respect to
indoximod,
it will be recognized that these or similar methods can be utilized with
numerous other IDO
inhibitors (see, e.g., Table 3, above, and Figure 2).
Example 2
Self-Asssembled Nanovesicles for the Co-Delivery of an IDO Pathway Inhibitor
Prodrug and Remote Loading of an Immunogenic Cell Death Inducing Agent.
[0548] A potential limitation of the OX/IND-MSNP carrier is its
relatively low
loading capacity for Pt-based drugs, such as OX (i.e. <10% wt). Since Pt-drugs
are
coordination complex compounds, they are usually not suitable for remote
loading by a
proton gradient, such as has been reported for irinotecan encapsulation in LB-
coated MSNPs
(see, e.g., Liu et al. (2016) ACS Nano, 10: 2702-2715). We therefore developed
new particle
iterations capable of achieving a higher loading capacity for ICD-inducing
chemo agents.
Synthesis of a DOX nanovesicle carrier that co-delivers IND-PL.
[0549] DOX is chosen to illustrate remote loading of IND-NVs based on
its
composition as a weak basic substance. Following its import into the vesicles,
DOX typically
precipitates as crystals, yielding a carrier that morphologically resembles
the DOXIL
liposome. We consider the DOX/IND liposome or an MTX/IND liposome as leading
carrier
prototypes for initiating antitumor immunotherapy in settings such as breast
cancer and other
cancer types.
Synthesis of DOX/IND nanovesicle:
[0550] The IND-Chol prodrug synthesis and preparation of liposomes
comprising
IND-Chol is described in Example7. Using the IND-Chol liposome prodrug, a
DOX/IND
nanovesicle can be prepared as follows: : 1) a total of 50 mg lipids of IND-
Chol plus other
vesicle-forming lipids (e.g., DPPC/Chol-IND/DPPG/DSPE-PEG (e.g., DSPE-PEG2k,
DSPE-
PEG5k, and the like), in certain embodiments at a molar ratio of ¨40% (DPPC):
¨35% (Chol-
IND): ¨20%DPPG: ¨5% DSPE-PEG) can be dissolved in 5 mL chloroform in a 50 mL
round
bottom glass flask. The solvent is evaporated under a rotatory vacuum to form
a uniform thin
118
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
lipid film, which is dried further under vacuum overnight. 2) The film is
hydrated with 2 mL
of ammonium sulfate (123 mM) and probe sonicated for 1 h, which is
subsequently extruded
15 times through a Mini-Extruder (Avanti Polar Lipids), using a polycarbonate
membrane
with 100 nm pores (Avanti Polar Lipids) at 80 C. IND-NV size and morphology
were
assessed by dynamic light scattering and cryoEM, respectively. 3)
Unincorporated
ammonium sulfate can be removed by running through a PD-10 size exclusion
column. 4)
6.4 mg of DOX=HC1 (10 mg/mL) in DI water is incubated with the above prepared
IND-NVs
at 65 C for 40 min. 5) The nanovesicles are fractionated across a PD-10
column, allowing
the removal of free DOX. Their size and morphology can be assessed by dynamic
light
scattering, cryoEM and UPLC/MS-MS, respectively. The final product is was
stored at 4 C
in the dark prior to biological testing.
[0551] The DOX/IND nanovesicle can be constructed by self-assembly of
IND-
Chol/LP (see, e.g., Figure 5). The prodrug is amphipathic, allowing self-
assembly into
nanovesicles (IND-NV) in the presence of an aqueous biological buffer.
Moreover, the
entrapment of a protonating agent (such as ammonium sulfate) at the time of
self-assembly,
permits the nanovesicle to import DOX from the surrounding drug suspension.
DOX can
precipitate as crystals in the nanovesicle. This provides a nanocarrier that
morphologically
resembles the DOXIL liposome.
Additional possible weak base laden co-delivery IND-NVs.
[0552] In addition to DOX loading into nanovesicles, there are other
possible drugs
that can be imported across the lipid bilayer of this carrier. These include,
but are not limited
to weak basic compounds with medicinal chemical features. Such copounds
include, but are
not limited to alkaloids (e.g. irinotecan, topotecan, 10-hydroxycamptothecin,
belotecan,
rubitecan, vinorelbine, LAQ824, vinblastine, vincristine, homoharringtonine,
trabectedin),
anthracyclines (e.g. doxorubicin, epirubicin, pirarubicin, daunorubicin,
rubidomycin,
valrubicin, amrubicin), alkaline anthracenediones (e.g. mitoxantrone),
alkaline alkylating
agents (e.g. cyclophosphamide, mechlorethamine, temozolomide), purine or
pyrimidine
derivatives (e.g. 5-fluorouracil, 5'-deoxy-5-fluorouridine, gemcitabine,
capecitabine) and
protein kinase inhibitors (e.g., pazopanib, enzastaurin, vandetanib erlotinib,
dasatinib,
nilotinib, sunitinib, osimertinib. palbociclib, ribociclib), etc.
Example 3
Doxorubicin is an ICD-inducing chemoagent in breast cancer leading to
development of
119
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
a co-delivery liposome for breast cancer nano-immunotherapy by contemporaneous
triggering of immunogenic cell death and restraining the IDO pathway
[0553] While treatment of patients with localized breast cancer (BC)
has a survival
rate of ¨98%, the Breast Cancer Coalition has pointed out that there is
marginal improvement
on mortality rate since 1975 (DeSantis et al. (2017) CA Cancer J Clin. 67: 439-
448). This is
particularly true for metastatic disease, where none of the current treatments
(e.g., radiation,
chemotherapy, and estrogen blockers) are capable of eliminating BC once
metastatic spread
has taken place (Howlader et al. (eds). SEER Cancer Statistics Review, 1975-
2010, Nat.
Cancer Inst. Bethesda, MD, seer.cancer.gov/csr/1975_2010/, based on November
2012 SEER
data submission, posted to the SEER web site, April 2013). Newfound optimism
has
emerged with the advent of cancer immunotherapy, where the power of T-cell
immunity can
be invoked to treat solid cancers, including breast cancer (Emens (2018) Clin.
Canc. Res. 24:
511-520). This is best exemplified by the use of immune checkpoint blocking
antibodies,
which have changed the treatment landscape for melanoma and non-small cell
lung cancer
(NSCLC) (Id.). However, in spite of this accomplishment, the overall response
rate is only
20-30%, without clear guidance to identify responders (see, e.g., Solinas et
al. (2017) ESMO
Open, 2: e000255).
[0554] The overarching challenge that we address to improve BC
mortality is to
improve the response rate to immunotherapy through the delivery of immunogenic
cell death
(ICD) stimuli by nanocarriers (see, e.g., Figure 1). Our data show
reproducible induction of
tumor infiltrating lymphocytes (TILs) in an orthotopic BC animal model by an
ICD-inducing
nanocarrier. The advantage of using a nanocarrier to deliver ICD-inducing
chemotherapy to
the cancer site lies in its improved pharmacokinetics, and decreased toxicity
of the drugs.
This will eliminate the guesswork to find responders, who are postulated to be
patients with a
high mutational load, in whom non-synonymous mutations generate a "hot" immune
environment (TME) (Nagarsheth et al. (2017) Nat. Rev. Immunol. 17: 559). This
facilitates
boosting of the immune response by antibodies that block CTLA-4, PD-1 and, PD-
Li
receptors.
[0555] We propose that ICD will allow more predictable induction of an
immune
replete status to allow receptor-mediated blockade or perturbation of other
immune
surveillance pathways to induce durable anti-tumor immunity, which also takes
care of
metastases. As such, ICD could strengthen the effect of immune checkpoint
blocking
120
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
antibodies as well as indoleamine 2,3-dioxygenase (IDO) inhibitors that
interfere in this
metabolic immune surveillance pathway.
[0556] It is believed that a doxorubicin (DOX) encapsulating
nanocarrier provides a
more potent ICD stimulus than the free drug, and can do so synergistically
with a small
molecule inhibitor (indoximod) of the IDO-1 pathway. The nanocarrier is
capable of
facilitating this task by improving the PK of DOX and indoximod (IND) at the
tumor site.
This provides us with a first generation nanocarrier providing an ICD stimulus
and an IDO
inhibitor as a promising synergistic immunotherapy platform for BC, including
triple
negative BC (TNBC) (most responsive to immune checkpoint inhibitors) as well
as ER-
positive tumors (numerically the largest BC subtype responsible for
mortality).
Doxorubicin (DOX) and mitoxantrone (MTX) are ICD-inducing chemoagents in
breast
cancer
[0557] In addition to improved intratumor drug content, we envisage
the use of
nanocarriers to deliver chemotherapy with a view to also implement breast
cancer (BC)
immunotherapy. One possible approach is to use chemotherapy to induce
immunogenic cell
death or ICD. Consensus guidelines have been developed is to identify drug and
chemo
agents that can trigger ICD in vitro and in vivo (Kepp et al. (2014)
Oncolmmunol. 3:
e955691). This allowed us to identify doxorubicin (DOX) and MTX as potent ICD
inducing
chemotherapeutic agents for BC immunotherapy, using 4T1 cells in a syngeneic
BALB/c
vaccination model. Multi-parameter cellular screening demonstrated that DOX
and MTX can
induce calreticulin (CRT expression), and release of HMGB1 during nuclear
disintegration of
cancer cells. In addition to DOX and MTX, we also identified paclitaxel (PTX)
as an ICD
inducer in 4T1 cells. In contrast, cisplatin (Cis) and 5-FU failed to induce
the same effect.
[0558] In certain embodiments the ability of the DOX- or PTX-treated
cells to
significantly suppress tumor growth at the challenge site is compared to the
negative control.
Additional in vitro ICD profiling (HMGB1 and ATP release as well as CRT cell
surface
visualization) has been determined. In certain embodiments tumors are excised
from the
mice from each group and the averaged tumor weight determined. Additionally,
bioluminescence visualization of 4T1 tumor development in the vaccination
experiment can
be performed using IVIS imaging at different time points. Mouse body weight
monitoring
can be provided.
121
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
Synthesis of a DOXIL look-like DOX-laden IND-Liposome (DOX/IND-Liposome).
[0559] In certain embodiments synthesis of a DOXIL look-like DOX-
laden IND-
Liposome (DOX/IND-Liposome) is contemplated. DOXIL is a PEGylated liposome
for
the delivery of DOX and has been in the marketplace for two decades.
Encapsulated DOX
delivery holds significant advantages over free DOX in patients with Kaposi's
sarcoma,
ovarian carcinoma and BC (Barenholz et al. (2012) J. Control. Rel. 160: 117-
134). This
advantage is in part derived from the improved PK of DOX at the tumor site as
well as a
reduction in cardiovascular and systemic DOX toxicity (Id.). DOX is loaded
into DOXIL
by using a trapping agent, which generates a proton gradient that allows the
import of weak-
basic DOX through the liposomal lipid bilayer. One potential downside of DOXIL
is the
preferential concentration of DOX in the skin, which can result in the hand-
foot syndrome
(redness and inflammation) (Id.). Clinical guidelines to avoid this side
effect by adapting the
DOXIL dosing schedule exist. Against this background of this FDA-approved
technology,
we asked whether it was possible to develop a liposome for dual DOX and
indoximod (IND)
delivery. In addition to the improving the PK of DOX, we hypothesized that we
would also
be able to improve the circulatory half-life (Tip) and tumor levels of
indoximod (IND).
[0560] This challenge can be met by using bio-conjugation chemistry to
synthesize a
cholesterol-conjugated IND prodrug (IND-Chol). IND (D-1-methyl tryptophan or D-
1MT)
can be covalently linked to cholesterol which is then incorporated into a
liposome.
[0561] Briefly, to construct the DOX/IND-liposome, a classic DOX remote
loading
strategy can be employed using ammonium sulfate as gradient. Following the
evaporation of
organic solvent that contains phospholipids, IND-Cholesterol, and DSPE-PEG2K,
a uniform
lipid film is formed along the bottom of the round flask. A protonating agent
(NH4)2SO4 can
be added into the flask afterwards, followed by probe sonication and PD-10
desalting column
purification to render the pure (NH4)2SO4-loaded IND-Liposome. Then DOX-I-IC1
solution is
incubated with (NH4)/SO4-loaded IND-Liposome at 65 C for the active loading
of DOX into
the hydrophilic pocket of IND-Liposome.
[0562] DOX, a weak basic molecule, can easily be loaded into the
liposome by using
ammonium sulfate as a protonating agent in the self-assembly solution. The
proton gradient
allows amphiphilic DOX to be imported across the liposomal membrane. This
provides dual
drug delivering liposome that visually resembles DOXIL . To determine the
optimal
DOX/IND-Liposome, a mixture of lipids containing varied molar ratios of IND-
Chol, PL,
and DSPE-PEG2K can be tested when encapsulating a fixed amount of DOX.
122
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
Example 4
Pancreas Cancer Immunotherapy Using Synergistic Immunogenic Cell Death and
Immunomodulatory Responses
[0563] While chemotherapy delivery by nanocarriers has modestly
improved the
survival prospects of pancreatic ductal adenocarcinoma (PDAC), additional
engagement of
the immune response could be game changing. We describe a nano-enabled
approach for
accomplishing robust anti-PDAC immunity in syngeneic mice through the
induction of
immunogenic cell death (ICD) as well as interfering in the immunosuppressive
indoleamine
2,3-dioxygenase (IDO) pathway (see, e.g., Figure 1).
[0564] This can be accomplished by conjugating the IDO inhibitor, indoximod
(IND),
to Cholesterol (Chol) or another component of a lipid bilayer that allows the
prodrug to self-
assemble into nanovesicles (IND-NV) or to be incorporated into a lipid bilayer
that
encapsulates mesoporous silica nanoparticles (MSNP). The porous MSNP interior
allows
contemporaneous delivery of the ICD-inducing chemotherapeutic agent,
oxaliplatin (OX). It
is believed that IND-NV plus free OX or OX/IND-MSNP can induce effective
innate and
adaptive anti-PDAC immunity when used in a vaccination approach, direct tumor
injection or
intravenous biodistribution to an orthotopic PDAC site. It is believed that
significant tumor
reduction or eradication cajn accomplished by recruited cytotoxic T
lymphocytes,
concomitant with downregulation of FoxP3+ T-cells.
[0565] In this Example, we report the design of nanocarriers to facilitate
the induction
of ICD and interference in the Kynurenin pathway, either through the
development of
nanovesicle that delivers an IND pro-drug or a lipid-coated MSNP, that co-
delivers
cholesterol-conjugated IND plus OX. It is believed that the synergy between
ICD and
interference in the IDO pathway can boost innate and adaptive immunity in the
syngeneic
.. animal KPC model. It is believed that this leads to effective killing of
pancreatic cancer cells
by CD8+ cytotoxic T cells at the tumor site, as well as interfering in
metastatic spread. The
cytotoxic response can be accompanied by disappearance of Tregs at the tumor
site. The
systemic immune response could also be adoptively transferred to non-immune
animals.
Results
Oxaliplatin-induced ICD provides a successful vaccination approach for PDAC
[0566] ICD is a modified form of apoptosis that can be used to
initiate an effective
immune response against endogenous tumor antigens (Kroemer et al. (2013) Ann.
Rev.
123
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
Immunol., 31: 51-72).1 Since this model was 1st proposed against the backdrop
of a select
number of cancer drugs (Id.), we focused on the use of OX, because it is FDA-
approved for
PDAC treatment. As a component of the FOLFIRINOX regimen (in combination with
irinotecan, 5-FU and folinic acid). For comparison, we also included the
anthracycline
antibiotic, DOX, as a positive control and cisplatin (Cis) as a negative
control for the
screening of PDAC cell lines, using cell surface CRT expression (Obeid et al.
(2017) Nat.
Med., 13(1): 54-61; Casares et al. (2005) J. Exp. Med. 202(12): 1691-1701;
Fucikova et al.
(2011) Canc. Res. 71(14): 4821-4833; Tesniere et aL (2010) Oncogene, 29(4):
482-491;
Galluzzi et al. (2012) Oncogene, 31(15): 1869-1883; Martins et al. (2011)
Oncogene, 30(10):
1147-1158). CRT is an endoplasmic reticulum (ER) stress protein that
translocates to the
surface membrane of cancer cells undergoing ICD (Obeid et al. (2017) Nat.
Med., 13(1): 54-61;
Fucikova et al. (2011) Canc. Res. 71(14): 4821-4833). Screening for CRT
expression was
performed in murine KPC cells, derived from a spontaneous tumor that developed
in a
transgenic KrasLSL-G12D-Firrp53LSL-R172H/+/Pdx-1-Cre (KPC) mouse (Hingorani et
al. (2005)
Cancer cell, 7(5): 469-483).
[0567] The KPC model recapitulates many of disease features of human
PDAC,
including oncogene expression, development of a robust cancer stroma,
extensive local
invasion and distant metastases (Torres et al. (2013) PloS one, 8(11): e80580;
Tseng et al.
(2010) Clin. Canc. Res. 16(14): 3684-3695). DOX and OX and activated DOX
induced the
ICD marker CRT in cultured KPC pancreatic cancer cells (Figure 6, panel A).
Similarly, in
the presence of DOX, OX, and activated DOX (a.k.a. DACHPt) HMGB1 release was
increased (Figure 6, panel A).
[0568] Animial experiments were performed using two rounds of
vaccination one
week apart, followed by injecting live KPC cells SC on the contralateral side
(Figure 6, panel
B). Tumors were collected on day 26 for size measurement and IHC analysis. KPC
tumor
size showd significant reduction on treatment with DOX, OX, and activated DOX
(Figure 6,
panel C).
[0569] Figure 6, panel D shows explanted tumors at the contralateral
side. Spaghetti
curves show a decrease in KPC tumor growth in the contralateral flank in
animals treated
with DOX, OX, and acitivated DOX (6, panel E).
124
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0570] Tumor collection was performed after euthanizing the animals to
conduct
IHC. IHC staining of CD8 and Foxp3 T cells was used to calculate CD8/FoxP3 T
cell ratio in
each group (see, e.g., Figure 6, panel F).
Discussion
[0571] PDAC is an often-fatal and notoriously treatment-resistant disease,
in
desperate need of new treatment approaches for dealing with the primary tumor
growth as
well as metastatic spread. We demonstrate a first treatment modality to
generate an anti-
PDAC response, premised on the ability of OX to induce ICD. ICD is responsible
for
enhanced tumor antigen presentation as well as providing stimulatory effects
to the
.. participating DCs. This triggers the activation of cytotoxic T cells and
anti-PDAC immunity
that was synergistically enhanced by an intervention in the IDO pathway. The
first treatment
modality comprises a subcutaneous vaccination approach that utilizes ex vivo
induction of
ICD by OX in a KPC cell line, it is sufficient to a generate systemic immune
response that
can interfere with tumor growth at a remote site as well as allowing adoptive
transfer to non-
immune animals.
[0572] In view of these results it is believed that two additional
treatment modalities
are available. The second treatment modality involves local injection of OX
plus an IND-
nanovesicle (e.g., and IND-Chol nanovesicle) to induce the recruitment of
cytotoxic CD8+
lymphocytes, depletion of Tregs, reversal of the CD8+/Foxp3+ ratio, cytotoxic
tumor killing,
and tumor shrinkage at the local injection site. It is believed these adaptive
immune
responses are accompanied by boosting of the innate immune system, as
reflected by CRT
and HMGB1 expression, as well as the activation of a DC population,
particularly well-suited
for generating cytotoxic T cell responses. The 3rd treatment approach combines
OX and an
IND-nanovesicle (e.g., and IND-Chol nanovesicle) into a single MSNP-based
nanocarrier,
that allows systemic biodistribution and drug delivery to orthotopic KPC tumor
sites. It is
believed the dual delivery approach can achieved synergistic enhancement of
adaptive and
innate anti-PDAC immunity, leading to a significant improvement in animal
survival.
[0573] Our proposed nano-enabled approach for boosting immunotherapy
offers
distinct advantages over current immunotherapy strategies for PDAC, including
peptide and
protein vaccines (e.g., mutant Kras, survivin, vascular endothelial growth
factor receptor,
gastrin and heat shock proteins) (Paniccia et al. (2015) Chinese J. Canc.
Res., 27(4): 376-
391), whole-cell vaccination approaches (e.g., PDAC cell lines engineered to
express GM-
125
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
CSF)[181, dendritic cell vaccines (Koido et al. (2014) Clin. Canc. Res.,
20(16): 4228-4239),
microorganisms (e.g., expression of antigenic peptides by vaccinia virus or
heat-killed
Mycobacterium obuense) (Strug et al. (2008) J Proteome Res., 7(7): 2703-2711)
and
immune checkpoint blockade (e.g., anti-CTLA-4 or anti-PD1 or monoclonal
antibodies)
(McCormick et al. (2016) Hum. Vacc. Immunother., 12(3): 563-575). While most
of these
approaches rely on select antigens chosen from the large repertoire of
potential immunogenic
PDAC components, the reality is that there is a dynamic interplay between the
tumor and the
immune system, which could render the use of specific antigens redundant,
including through
the process of immune editing or the display of T cell antigen receptors (TCR)
of sub-optimal
.. affinity or on/off rates (Dunn et al. (2004) Ann. Rev. Immunol. 22: 329-
360). In contrast, the
use of ICD prepares the dying cancer cells for uptake and processing by local
APCs, with the
possibility that the full complement of mutant or neo-antigens can participate
in dynamically
fashion in T cell selection, allowing effective TCR proofreading for immune
activation. This
allows the cognitive immune system to adapt to an array of continuously
evolving tumor
antigens rather than restricting the immune response to selected antigens.
[0574] The idea that ICD could be advantageous to mounting an anti-
PDAC immune
response is reflected by studies employing the whole cell vaccine,
Algenpantucel-L; this
vaccine is comprised of two irradiated PDAC cells, genetically engineered to
express the
murine enzyme, a (1, 3)-galactosyltransferase (aGT) (McCormick et al. (2016)
Hum. Vacc.
Immunother., 12(3): 563-575). aGT is responsible for the synthesis of the aGal
epitope, e.g.,
in normal gut flora. This immune challenge leads to a constitutive anti-aGal
response in the
human host, in the form of a high titer of aGT antibodies. Thus, vaccination
with the aGal-
expressing cell lines leads to the induction of These antibodies lead to a
hyper-acute immune
response upon vaccination with Algenpantucel-L. The death of these cell lines
leads to CRT-
mediated tumor cell uptake and processing by DCs, which also receive adjuvant
input in
subsequent phases of tumor cell death (Obeid et al. (2017) Nat. Med., 13(1):
54-61; Tesniere et
al. (2010) Oncogene, 29(4): 482-491). Noteworthy, data from a phase II
clinical trial, using the
aGal vaccine, have demonstrated the ability to induce a high titer of anti-CRT
antibodies,
which correlates with increased survival in PDAC patients (Rossi et al. (2014)
J. Clin. Oncol.
32(5s): Suppl: abstr 3029).
[0575] Instead of using genetically engineered PDAC cells, we propose
that ICD
induction by a an already FDA-approved chemotherapeutic agent (such as OX or
irinotecan)
constitutes a more effective means to achieve anti-PDAC immunity because it
targets
126
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
autologous cancer cells rather than preselected PC cell lines (which may not
dynamically
display the full complement of tumor antigens). We also propose that it may be
easier to
adjust the dosimetry of chemotherapy-induced ICD rather than relying on a
hyper-acute
immune response that may not always induce ICD. Good experimental data have
recently
been collected to show the feasibility of using chemotherapy to induce ICD in
lung or colon
carcinoma, with the ability to amplify these responses by immune checkpoint
blockade
(Pfirschke et al.(2016) Immunity, 44(2): 343-354; Rossi et al. (2014) J. Clin.
Oncol. 32(5s):
Suppl: abstr 3029). Also, for colon cancer it has been demonstrated that core-
shell
nanoparticles, comprised of an OX core and a photosensitizing pyrolipid
conjugate in the
shell, can synergize to deliver an ICD response, which may be useful for a
vaccination
approach or an abscopal effect (He et al. (2016) Nat. Comm. 7: 12499).
[0576] It is believed that the third approach described herein is the
first to use an ICD
approach in PDAC through the use of nanocarriers. Our work also introduces the
novel
principle of using a nanocarrier to simultaneously induce ICD and
immunomodulation. OX is
an integral component of the FOLFIRINOX regimen, and constitutes one of a
short list of
chemotherapeutics capable of inducing ICD, other than anthracyclines (Kepp et
al. (2014)
Oncatarget, 5(14): 5190-5191). The unique ability of these chemotherapeutics
to induce ICD
is dependent on their ability to initiate a sequence of events that differ
from regular apoptosis.
Integral to ICD, is triggering of ER stress, which leads to CRT expression at
the pre-mortem
stage (Id.). CRT expression serves as an "eat me" signal for antigen-
presenting DCs, which
also receive adjuvant signals at subsequent stages of ICD by the release of
the nuclear
protein, HMGB1, and ATP from the dying tumor cells (Obeid et cd. (2017) Nat.
Med., 13(1):
54-61; Kroemer et al. (2013) Ann. Rev. Immunol., 31: 51-72). CRT and HMGB-1
interacts
with CD91 and TLR4, respectively.
[0577] Immune activation in the PDAC microenvironment has to overcome a
number
of immune suppressive mechanisms, including the presence of CD4+/Foxp3+ Tregs,
secretion
of anti-inflammatory cytokines, expression of checkpoint inhibitors and
overproduction of
IDO. While our results indicate that OX alone is capable of increasing the
CD8+/Foxp3+
ratio at local and systemic tumor sites, it is believed the co-administration
of a vesicle-
conjugated IDO inhibitor, (e.g., IND-Chol liposome) can significantly enhance
this
integrative response parameter, which reflects the transition from an immune
suppressive to
an immune stimulatory TME. This synergy reflects the importance of the IDO
metabolic
127
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
effect in the TME, in much the same way as regional expression of this enzyme
please an
immune surveillance role in the placenta to protect the fetus.
[0578] IDO inhibitors are currently undergoing clinical trials in
several cancer types,
including breast, prostate, melanoma, brain and pancreas. This includes the
use of IND
together with gemcitabine, nab-paclitaxel and anti-PDL1 antibody. [311 It is
believed a major
advantage of our nanocarrier approach is the improvement of the PK and
intratumoral
accumulation of IND-Chol (or other IND-prodrug). Free IND is relatively water
insoluble
and has unfavorable PK characteristics. In contrast, it is believed an IND-NV
can
significantly increase the uptake and release of IND in tumor cells which
translates to a more
robust interference in IDO-mediated immune suppressive signaling pathways in
vitro and in
vivo. In addition to improving the circulatory t112 and PK of IND, it is
believed the dual
delivery carrier can also improve the PK of OX (Figure 7, panel c). It is also
believed that
the harmonized PK and contemporaneous delivery can further contribute to the
in vivo
synergy of the OX/IND-MSNP at the tumor site.
[0579] How can this discovery be practically implemented to provide PDAC
immunotherapy in the clinic? Based on our animal studies, possible ways to
improve
immunotherapy in patients could include: (i) tumor cell harvesting from
resected cancer
tissues during surgery, with the possibility of developing a cell culture-
based vaccine
approach; (ii) local injection of OX and IND-Chol (or other IND conjugated
prodrug) into the
.. tumor under remote guidance, during collection of biopsies or direct
visualization during
surgery; (iii) systemic administration of one or a combination of treatment
modalities, which
may include the use of free drugs, IND-NV or the dual-delivery carrier. In
addition, it is also
possible to enhance treatment efficacy by nanomaterials that exhibit intrinsic
nanoscale
properties and functions that lead to sequential induction of ER stress, ICD,
autophagy and
.. the release of adjuvants. It is also possible to use nanocarriers to
deliver other FDA-approved
drugs (e.g., cardiac glycosides, GADD34/PP1 inhibitors, Ca2+-activated K-
channel agonists,
poly-VC, etc. )[141 to achieve ICD, individually or in combination with
chemotherapeutics or
ICD-inducing nanoparticles. Another approach could be to combine chemotherapy
and IND
delivering nanoparticles with immune checkpoint blockers, irradiation,
photodynamic therapy
.. or cytotoxic viruses to achieve additional immune response enhancement. The
same
principles could also apply to the treatment of a host of other cancers.
Example 5
128
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
Nanomaterial ICD Inducers
[0580] A number of immunogenic cell death (ICD) inducers are known to
those of
skill in the art. Illustrative ICD inducers include, but are not limited to
oxaliplatin,
anthracenedione, bleomycin, bortezomib, cisplatin, daunorubicin, docetaxel,
doxorubicin,
doxorubicin, epirubicin, idarubicin, mitoxanthrone, oxaliplatin, paclitaxel,
R2016, irinotecan
and cyclophosphamide (see, e.g., Moon et al. (2015) J. ImmunoTherapy Cancer,
3: 51; Bezu
et al. (2015) Front. Immunol., 6:187).
Use of nanoparticles for the induction of immunogenic cell death.
[0581] The principles according to which various drugs listed are
capable of inducing
immunogenic cell death is the induction of an apoptosis-like cell death, which
is
accompanied by an early cell stress response and effects on autophagy. This
combination of
cell stress with apoptosis (which is generally non-immunogenic), leads to a
cell death process
where there is an early expression of the cell stress response marker,
calreticulum, which
serves as an "eat me" signal for dendritic cells. This changes the response
from non-
immunogenic to immunogenic, further assisted by the release of HMGB1 from the
nucleus
and ATP from the endoplasmic reticulum, which serves as immune adjuvants that
stimulate
the TLR4 and pure magic receptors, respectively.
[0582] Through high throughput screening discovery aimed at
understanding the
hazard and safety of a vase number of nanomaterials in our nanomaterial safety
laboratory,
have taught us important lessons about nanomaterial physicochemical properties
that can
trigger cell death response pathways. These include nanomaterial properties
(e.g., from
transition metal oxides, rare earth oxides, graphene oxide) that induce
oxidative stress, which
can induce mitochondrial triggering and the initiation of apoptosis. Another
example are rare
earth oxide nanoparticles that can trigger a cell death response pathway by
triggering
lysosomal damage and interference in autophagy flux. These particles can
induce cellular
pyroptosis, which is a different form of inflammatory cell death. There are
also nanoparticles
such as fumed silica that could trigger cell death through disruption of the
surface membrane.
We used our nanomaterial libraries, to screen for materials that can induce
immunogenic cell
death, which can be assayed by following calreticulin (CRT) expression, HMGB1
release etc.
[0583] Figure 33 shows the results of screening of nanomaterials (NMs) for
induced
immunogenic cell death (ICD) in KPC pancreatic cancer cell after 24 h
treatment with
engineered nanoparticles. Calreticulin (CRT), one of the hallmarks dictating
ICD, is
129
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
translocated onto the cell surface membrane from endoplasmic reticulum
following ICD
inducer treatment. Flow cytometry analysis was performed to quantitatively
measure the
induction of CRT level compared to control group. This suggested a highly
strong CRT
induction effects (more potent than OX chemo) by nano-sized Ag, Cu, SiO2,
V205, ZnO,
graphene, and the like. Illustrative nanomaterials believed to induce
immunogenic cell death
include, but are not limited to A1203, Ce02, CoO, Co304, Cr2O3, CuO, Dy203,
Er203, Eu203,
Fe2O3, Fe304, Gd203, Hf02, Ln203, La203, Mn203, Nd203, NiO, Ni203, SiO2,
Sm203, Sn02,
TiO2, W03, Y203, Yb203, ZnO, ZrO2, AP-WMCNT, PF108-MWCNT, COOH-MWCNT,
GO-S, GO-L, and the like.
[0584] Figures 33 shows the results of vaccination experiment using metal
and metal
oxide. Animal were treated using 2 rounds of vaccination (dying KPC cells
treated with
metal oxide nanoparticles) one week apart, followed by injecting live KPC
cells SC on the
contralateral side. Figure 33, panel A, shows spaghetti curves to show KPC
tumor growth in
the contralateral flank. Figure 33, panel B, shows percent CRT.
Example 6
[0585] Regarding the transition from lab scale synthesis to industrial
scale production
of lysolipid-conjugated 1-MT (either D- or L- form), we have conceptualized a
total synthesis
approach as a more economic approach for prodrug synthesis. This results in
better
scalability, reduced cost by using more economical synthetic building blocks,
reduction of the
number of potential side reactions that provides improved yield, as well as
better-quality
control. By using the total synthesis approach, it is possible to adjust the
position of the drug
(1-MT) conjugation to the glycerol backbone of the phospholipid or other lipid
bilayer
component (e.g., cholesterol).
[0586] In addition, we further expanded the prodrug conjugation
strategies from
lysolipid conjugation (saturated and non-saturated lipid with various chain
length) to fatty
acid (both saturated and non-saturated lipid with various chain length) as
well as cholesterol
conjugations (via ester or carbamate or amide conjugations). These are known
excipients
commonly being used in liposomal formulations (US FDA approved pharmaceutical
excipient), and other nano-formulations, e.g. emulsions, micelles, polymers,
hydrogels,
polymersomes, solid lipid nanoparticles, PLGA PEG nanoparticles/nanocapsules,
and other
lipid, amphiphilic, hydrophilic/hydrophobic formulation blends (see, e.g.,
Figures 23-27).
130
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
1. Total synthesis of lipid-conjugated 1-MT
[0587] The lysolipid (single chain phosphatidylcholine, a.k.a. LysoPC)
conjugated 1-
MT is essentially a conjugation of 4 building blocks: i) the drug (1-MT), ii)
a glycerol
backbone, iii) a fatty acid lipid chain, and iv) a phosphocholine head group.
The following
idealized example can be used for block-by-block assembly of 1-MT, glycerol,
fatty acid, and
phosphocholine, wherein the order of the building blocks can be swapped around
if needed.
The following idealized example consists of six synthesis steps, linking
together the key
building blocks. The sequence of procedures could be carried out commercially
or
synthesized in-house, using the most economic acquisition of the required
starting materials
as needed. Through the use of the full synthetic approach, one is able to
obtain the desired
molecule (e.g., "6c", shown in Fig. 11, which is the 16:0 LysoPC-indoximod
(IND-PL); this
is the material tested in our previous disclosure and patent
(PCT/US2018/033265). With the
invention of a total synthetic approach, this strategy allows the design and
construction of a
series of conjugations that can be used for design of indoximod prodrugs as
described below.
Stage
[0588] In brief, the first stage of the total synthesis starts from
the protection of the
amine (-NH2) group of the 1-(D/L)-MT using di-tert-butyl decarbonate (Boc20)
or other
amine protection groups (e.g. fluorenylmethyloxycarbonyl chloride (Fmoc-C1))
to obtain
Boc-1MT (or Fmoc-1MT). The amine protection avoids self-reaction when
conjugating to
other building blocks via ester bonds (1-MT can undergo amidation to form
amide bond
between to -NH2 and -COOH of two 1-MT molecules). Boc20 protection was shown
in the
following example, yielding compound (1) (Figure 9, Stage I).
Stage H - a/b
[0589] The second stage of the synthesis is to conjugate compound (1)
with a glycerol
building block via Steglich esterification reaction (Figure 9). In the
approach of Stage II ¨ a,
mono-hydroxyl protected glycerol, e.g. 2 Benzyloxy-1,3-propanediol and 3-
Benzyloxy-1,2-
propanediol were used yielding compound (2a) and compound (2b/2c) mixture,
respectively.
In the approach of Stage II ¨ b, dual-hydroxyl protected glycerol, e.g. 1,3-
Dibenzyloxy-2-
propanol and 1,3-0-benzylideneglycerol were used, both lead to compound (2f).
[0590] Common alcohol protecting groups, e.g. benzyloxy or suitable
alternatives can
be used when desired. Double-(hydroxyl) protected glycerol via the formation
of
isopropylidene acetal (e.g. solketal), or 1,3-0-benzylidene, or mono-choloro
substituted
131
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
double-hydroxyl protected glycerol (e.g. 4-chloromethy1-2,2-dimethy1-1,3-
dioxolane) can
also be used for sequential lipid prodrug conjugation through similar
approaches.
Stage III-IV
[0591] The next stage of the synthesis aims for the installation of a
lipid (fatty acid)
chain on to the 1-MT-conjugated glycerol backbone (Stage II compounds). This
was
achieved via a nucleophilic addition/elimination reaction between the alcohol
(hydroxyl
group from the glyceryl) and an acyl chloride yielding an ester, or can be
achieved by using
an acid anhydride. The acyl chloride used here is a fatty acid chloride,
either saturated fatty
acid chlorides with a general formula of CH3(CH2)nC0C1, n = 0-22, e.g.,
butyryl/valeroyl/hexanoyl/heptanoyl/octanoyl/pelargonic/caprylic/...stearic
chloride, or non-
saturated fatty acid chloride, e.g. oleic chloride. The fatty acid chloride
equivalent version of
fatty acid anhydride could also be used as a source of reactive acyl groups.
Once the fatty
acid chain was installed on compound (2a), (2b). and (2c), yielding compound
(3a), (3b), and
(3c), the glycerol was deprotected (benzyloxy removal) to free up the third
conjugation site of
the glycerol backbone, yielding compound (4a), (4b), and 4(c), respectively.
(Figure 10).
Compound (2f) will generate compound (4c) directly the glycerol backbone was
deprotected
in Stage II - b.
Stage V-VI
[0592] Stage V focuses on two synthesis options: Stage V ¨ a, to
install a
phosphatidylcholine head group onto the 1MT conjugated glycerol backbone
yielding lyso-
phosphatidylcholine (LysoPC) derivative drug conjugates); alternatively, in
Stage V ¨ b, a
secondary lipid (fatty acid) chain was conjugated, yielding mono-substitute
triglyceride (TG)
derivative drug conjugates, i.e. drug conjugated diglyceridediacylglycerol
(DAG) derivatives.
Stage V - a is achieved by first attaching a dioxaphospholane ring via 2-
Chloro-1,3,2-
dioxaphospholane 2-oxide or 2-chloro-1,3,2-dioxaphospholane followed by a ring
opening
reaction and the addition of a choline head via trimethylamine (Me3N, a.k.a.
TMA), yielding
compound (5a), (5b), and (5c). Stage V ¨ b is essentially the same reaction as
Stage III,
which the second fatty acid (FA-2) could be the same as the first fatty acid
installed (FA-1) or
different from FA-1 to mimic natural triglyceride configurations, yielding
compound (5d),
(5e), and (51). Compounds (5d) and (5e) will be the same if FA-1 is identical
to FA-2.
Finally, the amine protection on the 1MT was removed by suitable methods, e.g.
to use
132
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
TFA/TIPS for Boc removal yielding compounds (6a-f (Stage VI¨ a/b). The overall
reactions and the general formula of compounds (6a-f) is shown in Figure 11.
Single Fatty acid-conjugated and cholesterol-conjugated 1-MT
[0593] In addition to the 1MT-LysoPC and 1MT-DAG conjugations, we also
devised
single fatty acid drug-conjugation and cholesterol conjugation derivatives
(Figure 12). The
single fatty acid conjugation is simply achieved by performing a Steglich
esterification
reactions between compound (1), the Boc-1MT and a fatty alcohol (saturated or
poly-/mono-
unsaturated), yielding compound (7) followed by amine deprotection to afford
compound (8)
as the final drug conjugate. Ester conjugated 1MT-cholesterol is simply
synthesized by
conjugating Boc-1MT and cholesterol followed by amine deprotection, yielding
compound
(9) and (10), respectively. Alternatively, 1MT end be directly conjugated with
a cholesteryl
chloroformate yielding a 1MT-Cholesterol conjugate via carbamate as compound
(11).
Finally, we prepared the 1MT-amide4spacerl-Cholesterol conjugations by using
cholesteryl-
[spacerl-OH via amidation, where the spacer is [-00-(CH2)n-00-I, or by using a
cholesteryl-
[spacerl-C1, yielding compound (12).
Conclusion.
[0594] The resulting lipid/chol prodrugs could apply to the
preparation of all
nanomedicine formulations. To name a few, we can use the prodrug to coat
nanoparticle
surface or make self-assembly nanocarrier in which the interior or surface can
be used for
payload delivery.
Issues and solutions.
[0595] One difficulty for certain embodiments is that the Steglich
Esterification (Fig.
13) works for targets that have only one ester bonds in the end, otherwise,
trans-esterification
will take place resulting in many different esters.
[0596] For academic discovery research this is acceptable, but it can be
problematic
for for industrial scale production. An alternative synthetic strategy is a
total synthesis
approach (e..g, as described above). In this approach one estermification step
is used to
produce the intermediate compound where trans-esterification side reactions
less likely. The
largue components can be built first and the small components attached later
(see, e.g., Fig.
14, Steps 1-6 and alternative Step 1.
133
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
Example 7
Synthesis and Testing of Chol-Ind Liposomes
[0597] This example describes the synthesis of a cholesterol-based
indoximod
prodrug. The design is based on the use of medicinal chemistry criteria such
as presence of
reactive functional groups, steric hindrance, analysis of side products,
product yield and
avoidance of toxic/unstable/expensive chemicals to identify API candidate(s)
to direct
prodrug design. This prodrug can be used to formulate dual delivery liposomes,
capable of
co-delivery of mitoxantrone (MTX) and doxorubicin (DOX), which have the
potential to act
as ICD stimuli.
Synthesis of cholesterol-indoximod through an ester bond (Chol-IND)
[0598] The major steps for making Chol-IND are summarized in Fig. 16.
The
synthesis involves 4 simple steps: Boc-protection of IND, Cholesterol
conjugation, Boc
removal and de-salting. The raw materials and supplies that were used to make
Chol-IND are
summarized in Table 6. The overall yield is 20-30%.
Table 6. Raw Materials and supplies for making Chol-IND.
Catalog
Name Supplier
Number
1 Cholesterol from sheep wool C8503
2 Indoximod (1-methyl-D-tryptophan) 452483
3 Di-tert-butyl decarbonate (Boc20) 205249
4 4-(dimethylamino)pyridine (DMAP) S igma-
8204990025
5 Sand, 50-70 mesh 274739
Aldrich
6 MgSO4 (magnesium sulfat) anhydrous M7506
7 TEA (triethalamine) T0886
8 Sodium bicarbonate (NaHCO3) S6014
9 Triisopropylsilane (TIPS), 98% 233781
10 Tetrahydrofuran (THF)
11 Hydrochloric Acid (HC1) 12N A144-500
12 Dichloromethane, anhydrous, >99.7%, Alfa AesarTM Fisher AA41835K2
13 Ethyl acetate (Et0Ac) Scientific
14 Hexane
15 Acetonnitrile (MeCN)
1 -ethy1-3-(3-dimethylaminopropyl)carb odiimide
16 ThermalFisher 22980
hydrochloride (EDC.HC1)
17 Trifluoroacetic acid, 99% (TFA), Alfa AesarTM Scientific L06374
TLC Siliga gel 60 F254 x 25 Aluminum sheets (20 x 20
18 Millipore 1.05554.0001
cm)
Microcapillary Pipettes Disposable Soda-lime Glass, 20 Kimble Glass
19 71900-20
uL, 250 pipets Inc.
134
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
Disposable culture tubes (250 borosilicate glass tubes, 13 Globe
20 1510
x 100 mm) Scientific Inc.
Column, Chromatography, 24/40 Outer Joint, 250mL
21 CG-1197-14
Reservoir, lin ID X 12in E.L., 2mm Stpk
Column, Chromatography, 24/40 Outer Joint, 500mL Chemglass
Life Sciences
22 Reservoir, CG-1197-17
1 1/2in ID X 12in E.L., 2mm Stpk
SilicaFlash P60 40-63nm (230-400 mesh) for <1kDa,
23 R12030B
UltraPure Silica Gel
Silicycle
SPE-
24 SiliaPrepTm Carbonate (or Si-0O3)
R66030B
PoraPakTM Rxn CX Cartridges (alternative for SiliaPrep 1860045-
25 Waters
carbonate) 41/42/43/44
Step 1: Synthesis of Boc-Indoximod (Boc-IND).
[0599] Indoximod (1-Methyl-D-tryptophan, 95%) powder 3.2 g (13.93
mmol) and
sodium bicarbonate (NaHCO3) were suspended in 80 mL THF/H20 (1:1 v/v) and
chilled on
ice. Di-tert-butyl decarbonate (Boc20 anhydride) 4.16 g (19 mmol) was pre-
dissolved in 20
mL THF/H20 (1:1 v/v) and added drop-wise to the suspension. The ice-bath was
removed,
and the mixture was stirred overnight at room temperature under nitrogen in
which Boc-IND
was precipitated by adding 0.1N HCl without stirring. This yielded a sticky,
brownish
precipitate. The precipitate was recovered by filtration, dried in vacuum, and
recrystallized in
MeCN to yield pale-yellow crystalline compound 3.779g (11 mmol) = 78.98%.
Accurate
mass measurement by ESI-MS (Thermo ScientificTM Q Exactivem hybrid quadrupole-
Orbitrap mass spectrometer) was performed. We observed multiple MS peaks that
represent
to the complexes that contain one sodium (Na) together with 1-4
C17H22N204molecules.
This includes: i) K17H22N204Nal (theoretical: 341.1477. experiential:
341.1467), ii)
1(Ci7H22N204)2Nal (theoretical: 659.3056, experiential 659.3040), iii)
1(C17H22N204)3Na1
(theoretical: 977.4636, experimental: 977.4617) and iv) 1(Ci7H22N204)4Nal
(theoretical:
1295.6216, found: 1295.6202) (Fig. 17).
Step 2: Synthesis of Cholesteryl-Indoximod-Boc (Chol-IND-Boc):
[0600] Recrystallized Boc-IND (purity ¨90%, 900 mg. 1 mmol) was loaded
into a
100 mL round bottom flask containing anhydrous dichloromethane (25 mL) with a
magnetic
stir. This was followed by the addition of a catalytic amount of DMAP (12 mg,
0.1 mmol),
and cholesterol (purity 92.5%, 836 mg, 2 mmol) powder to form a solution that
was chilled
on ice. The solution was kept under nitrogen during the reaction. EDC HC1 (210
mg, 1.1
mmol) was pre-dissolved in anhydrous dichloromethane (5 mL) and triethylamine
(202
135
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
mg/279 L, 2 mmol) in a glass vial, chilled on ice, and then loaded drop lies
into the reaction
solution. The reaction was allowed to proceed at room temperature for 3 h.
Formation of the
Ch-IND-Boc can be monitored by TLC (mobile phase: EtOAC:Me0H = 9:1 v/v, Rf
0.73-
0.93, UV and iodine double positive). The reaction mixture was purified by
sequential
.. extraction with 0.5N HCl (50 mL x 2), saturated NaHCO3 (50 mL x 2), and an
optional brine
wash (50 mL x 1). The residual water in the organic phase was absorbed by
anhydrous
MgSO4, from which the MgSO4 hydrate was removed by filtration. The
dichloromethane
was evaporated in a rotary evaporator, the crude residual was dissolved in
minimal solvents
and purified by flush chromatography (mobile phase: Et0Ac:Hexane = 4:6 v/v, Rf
= 0.67,
UV and iodine double positive). The elution fractions containing the Ch-IND-
Boc were
pooled and solvent was removed in a rotary evaporator. This yielded a
transparent gel, which
was further dried in high vacuum overnight to afford a dried transparent solid
that turned into
white powder when scrapped off. Batch #1 yielding 421.71 mg (59.44%), Batch#2
yielding
469.09 mg (66.12%). Average yield = 62.78% (n = 2). Accurate mass measurement
by ESI-
MS (Thermo ScientificTM Q ExactiveTm hybrid quadrupole-Orbitrap mass
spectrometer):
[C44H66N204Na = M+Na1+ theoretical: 709.49203, found 709.4905;
[(C44H66N204)2Na
= 2M+Na1+ theoretical 1935.9943, found ¨1395.99. (Fig. 18).
Step 3: Synthesis of Ch-IND-NH3+TFA- (TFA salt).
[0601] Chol-IND-NH2-Boc (700 mg, ¨1 mmol) was dissolved in a mixture
solution
.. of triisopropylsilane (TIPS), CH2C12, and TFA (10 mL, 5:15:85 v/v) and
stirred for 1 h.
CH2C12 and the TFA excess was removed in a rotary evaporator. Residuals were
purified by
flush chromatography to obtain the Ch-IND-NH3+TFA- salt (mobile phase: Et0Ac
for
impurity removal, and Et0Ac plus TEA 99:1 v/v for Ch-IND-NH3+TFA- salt, Rf =
0.15, UV
and iodine double positive). Accurate mass measurement by ESI-MS (Thermo
ScientificTM
Q ExactiveTM hybrid quadrupole-Orbitrap mass spectrometer): [C39H59N202+= M+]
theoretical: 587.457652, found 587.4552; [(C39H59N202)2 -H = 2M+-H]
theoretical
1173.907481, found 1173.9032; [(C39H59N202)3 -H = 3M+-H] theoretical
1761.365134,
found 1761.3541 (Fig. 19).
Step 4: TFA removal.
[0602] TFA removal can be done using a commercially available resin
(siliaPrepTm
Carbonate (or Si-0O3) resin) or purification column, according to the
manufacturer's
136
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
instructions (see Item #24 in Table 6). Using a different industrial protocol
a TFA removal %
of 99.96% was observed.
Use of Chol-IND to make dual delivery liposome for concurrent ICD induction
and IDO
inhibition
[0603] We have experimentally demonstrated the feasibility of making co-
delivery
liposomes that incorporate indoximod (IND) as well as MTX and DOX. The ICD
inducing
chemo agents are loaded into the liposome by trapping agents, which include
citric acid for
MTX and ammonium sulfate for DOX (Fig. 20). The data are summarized in Figures
21 and
22.
Use of cholesteryl hemisuccinate (CHEMS) to reduce the positive charge in the
co-
delivery liposome.
[0604] Liposomal construction with the Chol-IND salt leads to the
formation of a
cationic liposome. The cationic charge is due to the presence of an amine
group in the
prodrug. For drug delivery purposes, we prefer a liposomal charge close to
neutral.
Construction of a liposome containing MTX-only (without the prodrug) yields a
negatively
charged carrier (Fig. 23). However, a liposome formulation containing MTX and
Chol-IND
yields a positively charged formulation at each step of construction as shown
in Fig. 23.
[0605] Cholesteryl hemisuccinate (CHEMS) is a cholesterol derivative
that is
frequently used in formulation studies (see, e.g,. Serpe et al. (2004) Elm J.
Pharm.
Biopharm. 58: 673-680; Ding et al. (2005) Int. J. Pharam. 300: 38-47). It
carries one
negative charge at a pH greater than 6.5. Interestingly, a simulation study
(membrane protein
crystallization) suggested that protonated form of CHEMS mimics many of the
membrane
properties of cholesterol quite well (Kulig et al. (2014) J. Mol. Model. 20:
2121). We
therefore introduced 10% and 20% CHEMS in our co-delivery liposome (i.e.
MTX/IND
liposome) with the view to reducing the surface charge in the Chol-IND
particles (Fig. 24).
Update on biological experiments
[0606] As shown in Figure 25, the MTX/IND-chol liposome is capable of
ICD
induction and IDO inhibition in vitro. Figure 26, panel A, shows a
determination of MTD
doses for free MTX and liposomal MTX in normal mice. MTD doses for free MTX
and
liposomal MTX were 3 and 15 mg/kg for single IV administration. Figure 26,
panel B, shows
137
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
pilot tumor size measurement in 4T1 orthotopic tumor-bearing mice receiving IV
free drug or
MTX-only liposome.
"Industrial" synthesis of Chol-IND.
[0607] Using an "industrial" process to implement the steps shown in
Fig. 27, a batch
of Chol-IND was synthesized to repeat the protocols described abovel. Two
grams of the
Chol-IND prodrug were successfully synthesized in a 2 week period (see, e.g.,
Fig. 27).
[0608] A portion of MTX/IND liposome (that was used for animal study)
was saved
for a stability check. The particles were used for size, morphology and charge
measurements
right after the synthesis and stored at 4 C for 3 months. We found minimal
change with the
respect to liposome morphology, size and charge after storage for 3 months
(see, e.g., Fig.
28). At 3 moths, majority MTX (quantified via fluorescence) and IDO
(quantified via HPLC)
remained encapsulated in the liposome. The leakage of MTX and IND was 0.06%
and
1.75%, respectively.
Example 8
Anmal Study in a 4T1 Orthotopic Breast Cancer model
[0609] Due to the multifunctionality of nanocarriers described herein
it is possible to
design a long list of co-delivery carriers that deliver an ICD stimulus plus
an immunological
agent. The scheme illustrated in Figure 29 illustrates the principles
underlying the design of
such a co-delivery carrier including the underling cancer biology. By way of
illustration,
mitoxatrone (MTX) was selected as an ICD inducer because MTX leads to a very
strong ICD
effect in multiple cancer types. In illustrative, but non-limiting
formulation, we used
Cholesterol-IND as an immunological modulation agent based on the formulation
work (see,
e.g., Example 7).
[0610] In this example we show that the use of both of the
mitoxantrone-only and
mitoxantrone/IND liposomes led to significan anticancer effect in the 4T1
breast cancer
model. Moreover these results were dramatically beter than the results
obtained with a Doxil
equivalent doxorubicin only liposome).
[0611] Without being bound to a particular theory, it is believed the
effect can be
attributed to the superior 1CD introducing effect of mitoxantrone over
doxorubicin, rendering
a liposomal mitoxantrone candidate that can be used for multiple cancer types.
138
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0612] It is worth noting that in the 4T1 model, the mitoxantrone-only
liposome was
so effective that an additional effect for cholesterol-IND could not be
observed. Without
being bound to a particular theory, it is believed this reflects the
possibility that the 4T1 triple
negative breast cancer model may represent a TN breast cancer subset in which
IDO-1 does
not play a major role. In that sense, TN breast cancer is no different from a
series of solid
cancers in which there is only a 25-30% response rate to checkpoint
inhibitors, likely due to a
variation on the theme of participation by different immune escape mechanisms.
We have
clear evidence, however, that in spite of a synergistic effect for IND in the
4T1 model, that
there is a strong ICD response in the immunohistochemistry data, implying that
the
contribution of turning the "cold" tumor "hot" is a valid approach
irrespective of the IDO-1
contribution. One could argue that a potent ICD agent such as mitoxantrone
could exert
similar effects on other solid tumors, increasing the 25% response rate.
[0613] In certain embodiments of these observations, in certain
embodiments
liposomes containing mitoxantrone (MTX), but not containing an IDO inhibitor.
[0614] Use of such MTX liposomes would be facilitated by development of a
biomarker to determine whether tumors are potentially IDO-1 responsive,
similar to the
mannter in which the expression of PD-1 ligand is currently used, to decide
who should
receive anti-PD1 therapy for lung cancer.
[0615] As illustrated in Figure 29 immunogenic cell death (ICD) is a
specialized form
of tumor cell death in response to specific chemotherapeutic drugs (e.g.
anthracyclines, MTX,
oxaliplatin), radiation therapy, photodynamic therapy or certain engineered
nanomaterials.
Our data showed MTX and certain nanomaterials led to very strong ICD in
multiple models
such as breast cancer and colon cancer models. ICD facilitates tumor antigen
cross-
presentation in dendritic cells as a result of calreticulin (CRT) expression
on the dying tumor
cell surface. CRT provides an "eat-me" signal for DC uptake via the CD91
receptor. In
addition, the stepwise release of adjuvant signals, including high mobility
group box 1
(HMGB-1) protein (a TLR-4 ligand) and ATP (activates the NRLP3 inflammasome),
allows
DC maturation and antigen presentation to naive T cells at the tumor site and
regional lymph
nodes. Following activation of naïve T-cells, a permissive immune response
with recruitment
of CTLs (from regional lymph node) can follow if powerful regional
immunosuppressive
pathways in the TME are overcome or removed. CTLs induce primary and
metastatic tumor
cell death by perforin and granzyme B release. For certain tumor (e.g., CT26),
the reason for
using a combinatorial regimen is that the expression of IDO-1 and PD-L1 is
paradoxically
139
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
increased by the recruitment of CTLs at the ICD site. It is also possible to
use certain
engineered nanomaterials (e.g. metal oxide and graphene) to trigger an ICD
effect.
[0616] Before investigating the efficacy of MTX/IND co-delivery
liposome in the
4T1-luc orthotopic model, we revisited the tumor samples in Figure 26 and
performed an
IHC study to confirm the effect of ICD induction (e.g. CRT, HMGB1 and LC3) and
immune
activation (e.g. CD8/Foxp3 ratio, perforin) at tumor site (Figure 30). The
overall conclusion
was the liposomal MTX introduced more potent ICD and immune activation effect
compared
to free drug at 4T1 tumor site in a dose-dependent fashion.
[0617] We continued to test the anti-cancer effect using MTX/IND
liposome in a 4T1
orthotopic model. Two experiments were performed. The 1st one is to look at
the anticancer
efficacy with the objective to investigate the ICD induction and immune
activation at 4T1
tumor site. We also performed an independent survival study to compare the
survival
outcome of each treatment. The results are shown in Figure 17.
[0618] We continued to test the anti-cancer effect using MTX/IND
liposome in a 4T1
.. orthotopic model. Two experiments were performed. The 1st one is to look at
the anticancer
efficacy with the objective to investigate the ICD induction and immune
activation at 4T1
tumor site. We also performed an independent survival study to compare the
survival
outcome of each treatment. The existing results are shown in Figure 31.
[0619] In particular, Figure 31 illustrates the efficacy of the dual
MTX plus Chol-IND
delivery by a liposome in the 4T1 model, along with survival data. Orthotopic
tumor-bearing
4T1 mice were IV injected with the encapsulated MTX liposomes to deliver 3
mg/kg IND
plus 3 mg/kg MTX every 3 days, for a total of 3 administrations, as
illustrated in panel A.
Flow cytometry was used to assess CRT induction in 4T1 tumor cells, showing
the
generation of an ICD response by MTX (Figure 31, panel B). At the conclusion
of the
experiment, primary tumor and major organs were collected for weighing. Organ
index
values were calculated (Figure 31, panel C). The tumor tissues were fixed and
used for IHC
staining of CD8, FoxP3, CRT and HMGB1 (Figure 31, panel D). In a separate
experiment,
we also performed an official survival study using these treatments in the
same 4T1
orthotopic model (see, e.g., Figure 31, panel E).
[0620] While we observed an impressive outcome using co-delivery liposome
in the
4T1 orthotopic model, we did not achieve statistical significance between
"DSPC : CHEMS :
Chol-IND : DSPE-PEG2kDa" (treatment #11 in Fig. 17) vs "DSPC : CHEMS : Chol :
DSPE-
140
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
PEG2kDa" (treatment #10 in Fig 31) in the 4T1 model. It is believed that this
is partially due
to the fact that the MTX-only liposome is so effective that we cannot see an
additional effect
for cholesterol-IND, reflecting the possibility that the 4T1 triple negative
breast cancer model
may represent a TN breast cancer subset in which IDO-1 does not play a major
role. This has
prompted us to test our liposome in a more immune responsive CT26 colon cancer
model
(see Example
Example 9
Animal Study in CT26 Colon Cancer Model
[0621] We proceeded to test the MTX/IND liposome in a CT26 colon
cancer, a model
believed to be more immunologically responsive than the 4T1 orthotopic model.
In this case,
CT26 subcutaneous tumor bearing mice received 4 IV injection of MTX/IND co-
delivery
liposome at indicated time points (Figure 32, panel A). The co-delivery
liposome is labeled
as "LCIM", in which "I" means IND-Cholesterol; "M" stands for MTX; "C.'
denotes
CHEMS; and "L" means liposome) (Figure 32). Both MTX and IDO doses in the co-
delivery liposome were 3 mg/kg. For comparison, the controls were untreated
mice (UT) and
MTX only liposomes with or without CHEMS (L5OM and LCM). We also included a
group
called "LCI2M", meaning the LCIM co-delivery plus empty IND liposome.
[0622] A stasticial significant difference (p<0.001) emerged as early
as day 20
between dual delivery (LCIM) vs MTX only liposome (Figure 32, panel B). In the
MTX/IND liposome group, five out of eight mice have tumor less than 150 mm3,
which
outperformed all the control groups including MTX-only liposome w/w CHEMS. The
addition of empty IND liposome appeared to interfere with the effect of co-
delivery via a
tumor access compition mechanism.
Example 10
Use of Engineeried Nanometerials to Trigger ICD
[0623] Various examples described above illustrate chemo-induced ICD,
which is
usually a "Type 1" ICD agent that primarily induces cell death by impacting
the cell nucleus,
with secondary effects on the endoplasmic reticulum (ER). It is also possible
to use another
approach to trigger ICD through a "Type 2" mechanism by which the ICD inducing
agent
primarily induces ER stress. We envisage that it is possible to use engineered
nanomaterials,
such as metal oxide and graphene, to induce ICD through "Type 1" or "Type 2"
or even both.
Accordingly, a CRT cellular screening was performed (see, e.g., Figure 33).
141
CA 03157508 2022-04-08
WO 2021/076630
PCT/US2020/055585
[0624] KPC pancreatic cells were treated by using PBS (negative
control), OX
(positive control) and indicated engineered nanomaterials at low and high
concentrations.
The choice of particle concentration is based on an MTS assay (Figure 33,
panel A). Twenty-
four hours post incubation, the total cells were harvested for CRT analysis
through flow
cytometry. This suggested a highly strong CRT induction effects (more potent
than OX
chemo) by nano-sized Ag, Cu, SiO2, V205, ZnO and graphene (Figure 33, panel
B).
[0625] Without being bound to a particular theory, we predict an even
better efficacy
outcome in a more immune responsive animal model, such as CT26 colon cancer
model.
[0626] It is understood that the examples and embodiments described
herein are for
illustrative purposes only and that various modifications or changes in light
thereof will be
suggested to persons skilled in the art and are to be included within the
spirit and purview of
this application and scope of the appended claims. All publications, patents,
and patent
applications cited herein are hereby incorporated by reference in their
entirety for all
purposes.
142