Note: Descriptions are shown in the official language in which they were submitted.
WO 2021/113757
PCT/US2020/063471
USE OF A KV7 POTASSIUM CHANNEL OPENER FOR TREATING PAIN
1. BACKGROUND
10001] Pain is a major medical problem that affects nearly 120 million people
in the United
States. Drug therapy is the mainstay of management for acute and chronic pain
in all age
groups, including neonates, infants, and children. Pain drugs are classified
by the American
Pain Society into three main categories: 1) non-opioid analgesics (e.g.,
acetaminophen) and
non-steroidal anti-inflammatory drugs (NSAIDs) (e.g., aspirin), 2) opioid
analgesics, and 3)
co-analgesics.
[0002] Sodium channel blockers have been shown to be useful in the treatment
of pain,
including acute, chronic, inflammatory, and neuropathic pain (see, e.g., Wood,
J.N., et al,
Neuroblot (2004), 61(1), 55-71). Preclinical evidence demonstrates that sodium
channel
blockers can suppress neuronal firing in peripheral and central sensory
neurons, and it is via
this mechanism that they are considered to be useful for relieving pain.
10003] Many pain sufferers, particularly those suffering from chronic pain,
cannot be treated
effectively. The consequences of ineffective pain treatment include reduced
mobility, limited
function, poor sleep, and an overall low quality of life. There remains a need
in the art for
novel and effective treatments of pain, including neuropathic pain and
nociceptive pain, such
as inflammatory pain. The present disclosure addresses this need by providing
compositions
and methods and uses for treating pain, and offers other related advantages.
10004] Citation of any reference in the Background section of this application
is not to be
construed as an admission that such reference is prior art to the present
application.
2. SUMMARY
[0005] The present disclosure describes certain methods and uses for the small
molecule N-
4-(6-Fluoro-3,4-dihydro-1H-isoquinolin-2-y1)-2,6-dimethylpheny1]-3,3-
dimethylbutanamide
(herein referred to as "Compound A").
[0006] In one embodiment, the present disclosure is directed to a method of
treating pain in a
subject (preferably, a mammal, such as a human) in need thereof, comprising
administering a
therapeutically effective amount of Compound A to the subject. In certain
instances, the pain
treated by the administration of Compound A is nociceptive pain, neuropathic
pain, or a
combination thereof In certain embodiments, the pain treated by the
administration of
Compound A is nociceptive pain, such as radicular pain, somatic pain, visceral
pain, soft
-1-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
tissue pain, inflammatory pain, post-operative pain, or a combination thereof,
particularly
post-operative pain.
[0007] In an additional embodiment, the method of treating pain comprising
administering a
therapeutically effective amount of Compound A further comprises enhancing the
opening of
a Kv7 potassium channel in the subject (e.g., human).
[0008] In another embodiment, the present disclosure is directed to a method
of opening or
enhancing the opening of a Kv7 potassium channel in a subject (preferably, a
mammal, such
as a human), comprising administering an effective amount of Compound A to the
subject,
wherein the subject is suffering from pain such as the various types of pain
described herein,
including nociceptive pain, neuropathic pain, or a combination thereof,
particularly
inflammatory pain.
[0009] In some aspects, the Kv7 potassium channel is one or more of Kv7.2,
Kv7.3, Kv7.4,
or Kv7.5. In certain instances, the opening or enhanced opening of one or more
of the Kv7.2,
Kv7,3, Kv7.4, or Kv7.5 potassium channels is selective over Kv7.1, In other
instances, the
method comprises opening or enhanced opening of the Kv7.2/Kv7.3 (KCNQ2/3)
potassium
channel.
[0010] In one embodiment, the present disclosure provides a method of treating
pain in a
subject (preferably, a mammal, such as a human) in need thereof, wherein
Compound A is
administered (preferably orally) to the subject In certain instances, the
administration to the
subject comprises a dose of 2 to 200 mg of Compound A per administration. In
other
instances, the administration to the subject comprises a dose of 5-1000 mg per
day, In further
instances, the administration to the subject comprises a dose of 0.05-20
mg/kg, such as 0.1-
mg/kg.
100111 In some embodiments of the present methods and uses, Compound A is
orally
administered to the subject (preferably, a mammal, such as a human) from
between about 30
minutes before to about 2 hours after eating a meal, for example, Compound A
may be orally
administered to the subject during a meal or within 15 minutes after eating a
meal.
[0012] In certain embodiments, the present disclosure provides a method of
treating pain in a
subject (preferably, a mammal, such as a human) in need thereof, comprising
administering
(e.g., orally) a therapeutically effective amount of Compound A to the subject
in combination
with one or more additional analgesic agents, such as an opioid analgesic.
-2-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
100131 In additional embodiments, the present disclosure provides a method of
reducing the
dose (e.g., a maintenance dose) of an opioid analgesic administered to a
subject (preferably, a
mammal, such as a human) in need thereof comprising administering (e.g.,
orally) a
therapeutically effective amount of Compound A to the subject, for example,
whereby the
effective amount of Compound A reduces the dose of the opioid analgesic needed
to achieve
pain relief in the subject.
100141 Compound A is a small molecule currently being developed for the
treatment of
seizure disorders, and its use as a potassium channel modulator is disclosed
in U.S. Patent
Nos. 8,293,911 and 8,993,593 as well as U.S. Application Serial Nos.
16/409,684 and
16/410,851, the disclosures of which are hereby incorporated by reference in
their entireties.
100151 These and other aspects of this disclosure will be apparent upon
reference to the
following detailed description. To this end, various references are set forth
herein which
describe in more detail certain background information and procedures and are
each hereby
incorporated by reference in their entirety.
3. BRIEF DESCRIPTION OF THE DRAWINGS
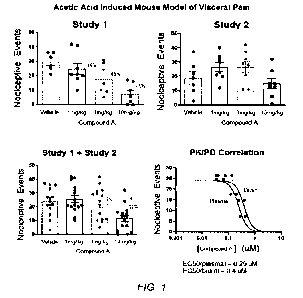
100161 FIG. 1 shows results of the acetic acid induced mouse model of visceral
pain showing
nociceptive events (y-axis) and vehicle, 1 mg/kg, 3 mg/kg, and 10 mg/kg dosing
of
Compound A (x-axis) for Study 1 (top left), Study 2 (top right), the
combination of Study 1
and Study 2 (bottom left), and PK/PD correlation (bottom right) showing a
PK/PD correlation
between Compound A concentrations in brain and plasma to the observed
efficacy.
100171 FIG. 2 shows results from the electronic von Frey test on non-lesion
and lesion paws
in groups of rats on Day 13, prior to treatment, showing force inducing paw-
withdrawal (g)
(y-axis) and the future treatment to be administered: vehicle, 8 mg/kg, 16
mg/kg, and 24
mg/kg Compound A, 20 mg/kg retigabine, and 128 mg/kg morphine p.o. dosing (x-
axis).
Paired student's t-test (versus non-lesioned paw): NS = Not Significant; * = p
<0.05; ** = p
<0.01; ''`** = p < 0.001.
100181 FIG. 3 shows results from the electronic von Frey test on lesioned paw
(tactile
allodynia evaluation on Day 14 and Day 18) in rats showing variation (delta
from baseline) of
force inducing paw-withdrawal (g) (y-axis) and vehicle, 8 mg/kg, 16 mg/kg, and
24 mg/kg
Compound A, 20 mg/kg retigabine, and 128 mg/kg morphine p.o. dosing (x-axis).
Inter-
group comparison (versus vehicle (p.o.)): NS = Not Significant; * = p <0.05;
** = p <0.01;
*** = p < 0.001.
-3-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
100191 FIG. 4 shows results from the cold plate test on lesioned paw (thermal
allodynia
evaluation on Day 14 at 2 h) in rats showing latency to the first paw-
withdrawal (s) (y-axis)
and vehicle, 8 mg/kg, 16 mg/kg, and 24 mg/kg Compound A, 20 mg/kg retigabine,
and
128 mg/kg morphine p_o_ dosing (x-axis). Inter-group comparison (versus
vehicle (p.o.)):
NS =Not Significant; * = p < 0.05; ** = p < 0.01; *** = p < 0.001.
100201 FIG. 5 shows results from the cold plate test on lesioned paw (thermal
allodynia
evaluation on Day 14 at 2 h) in rats showing number of withdrawal responses (y-
axis) and
vehicle, 8 mg/kg, 16 mg/kg, and 24 mg/kg Compound A, 20 mg/kg retigabine, and
128
mg/kg morphine p.o. dosing (x-axis). Inter-group comparison (versus vehicle
(p.o.)): NS =
Not Significant; * = p <0.05; ** = p <0.01; *** = p <0.001.
100211 FIG. 6 shows results from the cold plate test on lesioned paw (thermal
allodynia
evaluation on Day 14 at 2 h) in rats showing total duration of withdrawal
responses (s) (y-
axis) and vehicle, 8 mg/kg, 16 mg/kg, and 24 mg/kg Compound A, 20 mg/kg
retigabine, and
128 mg/kg morphine p.o. dosing (x-axis). Inter-group comparison (versus
vehicle (p.o.)):
NS =Not Significant; * = p < 0.05; ** = p < 0.01; *** = p < 0.001.
100221 FIG. 7 shows results from the cold plate test on lesioned paw (thermal
allodynia
evaluation on Day 18 at 2 h) in rats showing latency to the first paw-
withdrawal (s) (y-axis)
and vehicle, 8 mg/kg, 16 mg/kg, and 24 mg/kg Compound A, 20 mg/kg retigabine,
and
128 mg/kg morphine p.o. dosing (x-axis). Inter-group comparison (versus
vehicle (p.o.)):
NS =Not Significant; * = p < 0.05; = p < 0.01; *** = p < 0.001.
100231 FIG. 8 shows results from the cold plate test on lesioned paw (thermal
allodynia
evaluation on Day 18 at 2 h) in rats showing number of withdrawal responses (y-
axis) and
vehicle, 8 mg/kg, 16 mg/kg, and 24 mg/kg Compound A, 20 mg/kg retigabine, and
128
mg/kg morphine p.o. dosing (x-axis). Inter-group comparison (versus vehicle
(p.o.)): NS =
Not Significant; * = p < 0.05; ** = p < 0.01; *** = p < 0.001,
100241 FIG. 9 shows results from the cold plate test on lesioned paw (thermal
allodynia
evaluation on Day 18 at 2 h) in rats showing total duration of withdrawal
responses (s) (y-
axis) and vehicle, 8 mg/kg, 16 mg/kg, and 24 mg/kg Compound A, 20 mg/kg
retigabine, and
128 mg/kg morphine pm. dosing (x-axis). Inter-group comparison (versus vehicle
(p.o.)): NS
Not Significant; * = p < 0.05; = p <0.01; *** = p < 0.001.
-4-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
4. DETAILED DESCRIPTION
100251 The present disclosure relates to novel and improved methods and uses
for Compound
A, particularly for treatment of pain by administering Compound A to a subject
(preferably, a
mammal, such as a human) in need thereof by oral administration or by other
routes.
100261 In the following disclosure, certain specific details are set forth in
order to provide a
thorough understanding of various embodiments. However, one skilled in the art
will
understand that the methods and uses described herein may be practiced without
these details.
In other instances, well-known structures have not been shown or described in
detail to avoid
unnecessarily obscuring descriptions of the embodiments. Unless the context
requires
otherwise, throughout the specification and claims which follow, the word
"comprise" and
variations thereof, such as, "comprises" and "comprising" are to be construed
in an open,
inclusive sense, that is, as "including, but not limited to." Further,
headings provided herein
are for convenience only and do not interpret the scope or meaning of the
claimed invention.
100271 Reference throughout this specification to "one embodiment" or "an
embodiment"
means that a particular feature, structure, or characteristic described in
connection with the
embodiment is included in at least one embodiment. Thus, the appearances of
the phrases "in
one embodiment" or "in an embodiment" in various places throughout this
specification are
not necessarily all referring to the same embodiment. Furthermore, the
particular features,
structures, or characteristics may be combined in any suitable manner in one
or more
embodiments. Also, as used in this specification and the appended claims, the
singular forms
"a," "an," and "the" include plural referents unless the content clearly
dictates otherwise. It
should also be noted that the term "or" is generally employed in its sense
including "and/or"
unless the content clearly dictates otherwise.
4.1. Definitions
100281 As used in the specification and appended claims, unless specified to
the contrary, the
following terms and abbreviations have the meaning indicated:
100291 "Compound A" refers to the compound having the following formula:
H
mit
F N 40 N...1n<
=,---
0
;
-5-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
and having a chemical name of N44-(6-fluoro-3,4-dihydro-1H-isoquinolin-2-y1)-
2,6-
dimethylpheny11-3,3-dimethylbutanamide. Preparation of Compound A and its use
as a
Kv7.2/1Cv7.3 (KCNQ2/3) opener is disclosed in U.S. Patent Nos. 8,293,911 and
8,993,593 as
well as U.S. Application Serial Nos. 16/409,684 and 16/410,851. Compound A
potentiates
and enhances opening of the voltage-gated potassium channels Kv7.2 and Kv7.3
(Kv7.2/Kv7.3), which are important in controlling neuronal excitability.
Compound A is
used in the methods and uses described herein.
100301 "Acute pain" as used herein means pain that has a recent onset. Acute
pain
commonly declines over a short time (e.g., days, hours, or minutes) and
follows injury to the
body, and generally disappears when the bodily injury heals.
100311 "Breakthrough path" as used herein means a transitory increase in pain
above the
baseline or background pain experienced by a patient. In this context,
"baseline pain" means
the pain that is experienced or reported by a patient as the average pain
intensity experienced
for 12 or more hours.
100321 "Chronic pain" as used herein means pain persisting for at least a
week. Typically,
chronic pain persists for three to six months or longer
100331 The phrase "in combination" as used herein in the context of
administering
Compound A refers to the simultaneous or sequential administration of Compound
A with
one or more additional therapeutic agents, such as one or more other pain
treatments,
regimens, or analgesic agents_ For example, administering Compound A in
combination with
another therapeutic agent means that Compound A may be administered with
another
therapeutic agent simultaneously or sequentially in separate unit dosage forms
(e.g., as part of
a multiple dosage regimen) or together in a single unit dosage form. If the
additional
therapeutic agent and Compound A are administered sequentially, then this
could be within a
period of time up to 24 hours from the other, such 0.25, 0,5, 1, 2, 3, 4, 5,
6,7, 8, 9, 10, 11, 12,
13, 14, 15, 16, 17, 18, 18, 20, 21, 22, 23, or 24 hours or less from the
other.
100341 "Pain" as used herein refers to all categories of pain and includes,
but is not limited to,
neuropathic pain, inflammatory pain, nocic,eptive pain, idiopathic pain,
neuralgic pain,
orofacial pain, bum pain, burning mouth syndrome, somatic pain, visceral pain,
myofacial
pain, dental pain, cancer pain, chemotherapy pain, trauma pain, surgical pain,
post-surgical
pain, childbirth pain, labor pain, reflex sympathetic dystrophy, brachial
plexus avulsion,
neurogenic bladder, acute pain (e.g., musculoskeletal and post-operative
pain), chronic pain,
-6-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
persistent pain, peripherally mediated pain, centrally mediated pain, chronic
headache,
migraine headache, familial hemiplegic migraine, conditions associated with
cephalic pain,
sinus headache, tension headache, phantom limb pain, peripheral nerve injury,
pain following
stroke, thalamic lesions, radiculopathy, HIV pain, post-herpetic pain, non-
cardiac chest pain,
irritable bowel syndrome and pain associated with bowel disorders and
dyspepsia, and
combinations thereof.
[0035] "Therapeutically effective amount" as used herein refers to an amount
of Compound
A that is sufficient to treat the stated disease, disorder, or condition or
have the desired stated
effect on the disease, disorder, or condition or one or more mechanisms
underlying the
disease, disorder, or condition in a subject In certain embodiments, when
Compound A is
administered for the treatment of pain, therapeutically effective amount
refers an amount of
Compound A which, upon administration to a subject, treats or ameliorates pain
in the
subject, or exhibits a detectable therapeutic effect in the subject that
results in reduction in
pain. Changes in pain experienced by a patient can be measured through the use
of a pain
rating scale, and such scales are used in daily clinical practice to measure
pain intensity.
Commonly used pain measurement scales include the Visual Analog Scale (VAS),
the
Graphic Rating Scale ((IRS), the Simple Descriptor Scale (SDS), the Numerical
Rating Scale
(NRS), and the Faces Rating Scale (FRS). All of these scales have been
documented as being
valid measures of pain intensity.
[0036] "Treatment" as used herein refers to therapeutic applications
associated with
administering Compound A that ameliorate the indicated disease, disorder, or
condition (e.g.,
pain) or one or more underlying mechanisms of said disease, disorder, or
condition, including
slowing or stopping progression of the disease, disorder, or condition or one
or more of the
underlying mechanisms in a subject. In certain embodiments, when Compound A is
administered for the treatment of pain, treatment refers to therapeutic
applications to slow or
stop the increase of pain (i.e., to stabilize the level of pain) and/or
reduction or elimination of
pain. In some embodiments, the treatment of pain comprising the administration
of
Compound A is accompanied by an alteration of the cellular activity of one or
more Kv7
potassium channels (e.g., Kv7.2, Kv7.3, Kv7.4, and/or Kv7.5, particularly
Kv7.2 and/or
Kv7.3, optionally over Kv7.1) toward a normal level that would be observed in
the absence
of the pain.
[0037] "Under fed conditions" refers to the condition of having consumed food
during the
time period between from about 4 hours prior to the oral administration of an
effective
-7-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
amount (e.g., within the therapeutically effective dose range) of Compound A
to about 4
hours after the administration of Compound A. The food may be a solid, liquid,
or mixture of
solid and liquid food with sufficient bulk and fat content that it is not
rapidly dissolved and
absorbed in the stomach. In some instances, the food is a meal, such as
breakfast, lunch,
dinner or, alternatively, baby food (e.g., formula or breast milk). The
therapeutically
effective amount of Compound A may be orally administered to the subject, for
example,
between about 30 minutes before to about 2 hours after eating a meal, most
advantageously,
Compound A is orally administered during a meal or within 15 minutes after
eating a meal.
100381 "Under fasted conditions" refers to the condition of not having
consumed food during
the time period between from at least 4 hours prior to the oral administration
of a
therapeutically effective amount of Compound A to about 4 hours after
administration of
Compound A.
4.2. Embodiments
100391 In some embodiments, the present disclosure is directed to a method of
treating pain
in a subject (preferably, a mammal, such as a human) in need thereof,
comprising
administering (e.g., orally) a therapeutically effective amount of Compound A
to the subject.
In certain instances, the pain treated by administering Compound A is
nociceptive pain,
neuropathic pain, or a combination thereof
100401 In some instances, the pain is nociceptive pain, such as radicular
pain, somatic pain,
visceral pain, soft tissue pain, inflammatory pain, or a combination thereof,
particularly
inflammatory pain, including inflammatory pain associated with an inflammatory
disease or
condition, such as organ transplant rejection; reoxygenation injury resulting
from organ
transplantation (see Grupp et al., Mot Cell Cardiot 31:297-303 (1999))
including, but not
limited to, transplantation of the heart, lung, liver, or kidney; chronic
inflammatory diseases
of the joints, including arthritis, rheumatoid arthritis, osteoanthritis and
bone diseases
associated with increased bone resorption; inflammatory bowel diseases, such
as ileitis,
ulcerative colitis, Barrett's syndrome, and Crohn's disease; inflammatory lung
diseases, such
as asthma and adult respiratory distress syndrome; inflammatory diseases of
the eye,
including corneal dystrophy, trachoma, onchocerciasis, uveitis, sympathetic
ophthahnitis, and
endophthalmitis; chronic inflammatory disease of the gum, including gingivitis
and
periodontitis; tuberculosis; leprosy; inflammatory diseases of the kidney,
including uremic
complications, glomerulonephritis and nephrosis; inflammatory disease of the
skin, including
sclerodermatitis, psoriasis and eczema; inflammatory diseases of the central
nervous system,
-8-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
including chronic demyelinating diseases of the nervous system, multiple
sclerosis, AIDS-
related neurodegeneration, infectious meningitis, encephalomyelitis,
Parkinson's disease,
Huntington's disease, arnyotrophic lateral sclerosis and viral or autoimmune
encephalitis;
autoirnmune diseases, including Type I and Type II diabetes mellitus; diabetic
complications,
including, but not limited to, glaucoma, retinopathy, nephropathy (such as
microaluminuria
and progressive diabetic nephropathy), gangrene of the feet, atherosclerotic
coronary arterial
disease, peripheral arterial disease, foot ulcers, joint problems, and a skin
or mucous
membrane complication (such as an infection, a shin spot, a candidal infection
or necrobiosis
lipoidica diabeticorum), immune-complex vasculitis, and systemic lupus
erythematosus
(SLE); inflammatory disease of the heart, such as cardiomyopathy, ischemic
heart disease
hypercholesterolemia, and pericarditis; as well as various other diseases that
can have
significant inflammatory components, including preeclampsia, chronic liver
failure, brain and
spinal cord trauma, and cancer. The present methods and uses of Compound A can
also be
used to treat pain associated with an inflammatory disease that can, for
example, be a
systemic inflammation of the body, exemplified by gram-positive or gram
negative shock,
hemorrhagic or anaphylactic shock, or shock induced by cancer chemotherapy in
response to
pro-inflammatory cytokines, e.g., shock associated with pro-inflammatory
cytokines.
100411 In certain embodiments, the pain is neuropathic pain, including
neuropathic pain
selected from pain associated with spinal cord injury, spinal or brain stroke,
multiple
sclerosis, cancer, shingles, post-herpetic neuralgia, erythromelalgia
(including inherited
erythromelalgia), chemotherapy-induced neuropathy, oxaliplatin-induced
neuropathy,
trigeminal neuralgia, phantom pain, phantom limb pain, radiculopathy, complex
regional pain
syndrome, causalgia, reflex sympathetic dystrophy, lower back pain, peripheral
nerve trauma,
herpes virus infection, diabetes mellitus, diabetic neuropathy, plexus
avulsion, neuroima, limb
amputation, vasculitis, chronic alcoholism, human immunodeficiency virus (HIV)
infection,
uremia, vitamin deficiency, pelvic pain, or a combination thereof In some
embodiments, the
neuropathic pain is chronic neuropathic pain, such as pain resulting from
injury to the
peripheral or central nervous tissue. In some embodiments, the neuropathic
pain is a
neuropathy, such as one of those described herein.
100421 In some embodiments, the neuropathic pain is selected from pain
associated with
spinal cord injury, spinal or brain stroke, post-herpetic neuralgia,
erythromelalgia (including
inherited erythromelalgia), trigeminal neuralgia, radiculopathy, complex
regional pain
-9-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
syndrome, causalgia, reflex sympathetic dystrophy, peripheral nerve trauma,
diabetic
neuropathy, plexus avulsion, neuroma, vasculitis, or a combination thereof.
[0043] In certain embodiments, the neuropathic pain is selected from pain
associated with
shingles, multiple sclerosis, cancer, chemotherapy-induced neuropathy,
oxaliplatin-induced
neuropathy, herpes virus infection, diabetes mellitus, human immunodeficiency
virus (HIV)
infection, hypothyroidism, uremia, or a combination thereof, particularly pain
associated with
multiple sclerosis or cancer. In certain embodiments, the neuropathic pain is
selected from
pain associated with shingles or herpes virus infection. In some embodiments,
the
neuropathic pain is selected from pain associated with cancer, chemotherapy-
induced
neuropathy, or oxaliplatin-induced neuropathy.
[0044] In some embodiments, the neuropathic pain is selected from pain
associated with
phantom pain, phantom limb pain, lower back pain, limb amputation, chronic
alcoholism,
vitamin deficiency, pelvic pain, or a combination thereof, particularly
phantom pain, phantom
limb pain, or pain associated with limb amputation.
[0045] In certain instances, the pain treated by administering a
therapeutically effective
amount of Compound A to the subject (preferably, a mammal, such as a human) is
acute
pain. In some embodiments, the pain is chronic pain. Such administration may
be, e.g., by
oral, sublingual, buccal, occur, otic, vaginal, rectal, cutaneous, topical, or
transdermal
administration; by intravenous, intramuscular, intrathecal, or subcutaneous
injection; or by
implantation.
[0046] In some instances, the pain treated by administering a therapeutically
effective
amount of Compound A to the subject (preferably, a mammal, such as a human) is
mild,
moderate, or severe pain. In certain embodiments, the pain is moderate or
severe pain, or
moderate to severe pain. Such administration may be, e.g., by oral,
sublingual, buccal, occur,
otic, vaginal, rectal, cutaneous, topical, or transdermal administration; by
intravenous,
intramuscular, intrathecal, or subcutaneous injection; or by implantation.
[0047] In certain instances, the pain treated by administering (e.g., orally)
a therapeutically
effective amount of Compound A to the subject (e.g., a human) is associated
with a disease
state or other condition, such as cancer pain, rheumatic pain, arthritic pain,
bone pain, labor
pain, myocardial infarction pain, pancreatic pain, colic pain, post-operative
pain, headache
pain, muscle pain, pain associated with a periodontal disease (including
gingivitis and
periodontitis), or a combination thereof In some embodiments, the pain is of
tumor origin.
-10-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
In other embodiments, the pain is of non-tumor origin. In certain embodiments,
the pain is
associated with a migraine, including migraine without aura ("common
migraine"), migraine
with aura ("classic migraine"), migraine without headache, basilar migraine,
familial
hemiplegic migraine, migrainous infarction, migraine with prolonged aura, or a
combination
thereof.
[0048] In some embodiments, the pain treated by administering (e.g., orally) a
therapeutically
effective amount of Compound A to the subject (e.g., a human) is breakthrough
pain.
[0049] In some embodiments, the method of treating pain by administering a
therapeutically
effective amount of Compound A comprises enhancing the opening of a Kv7
potassium
channel in the subject (preferably, a mammal, such as a human).
[0050] In certain embodiments, the present disclosure provides a method or use
comprising
opening or enhancing the opening of a Kv7 potassium channel, such as the
Kv7.2, Kv7.3,
Kv7.4, and/or Kv7.5 potassium channel, particularly the Kv7.2/Kv7.3 (KCNQ2/3)
potassium
channel in a subject in need thereof by administering an effective amount of
Compound A.
In some of such embodiments, the subject suffers from pain, such as the types
of pain
described herein, including neuropathic pain or nociceptive pain, such as
inflammatory pain.
[0051] In certain instances, the method or use described herein comprises
selectively opening
or enhancing the opening of a Kv7 potassium channel, such as one or more of
Kv7.2, Kv7.3,
Kv7.4, or Kv7.5 over Kv7.1. In some embodiments, the method or use is
selective for Kv7.2,
over Kv7.1. In other embodiments, the method or use is selective for Kv7.3,
over Kv7.1.. In
yet other embodiments, the method or use is selective for Kv7.4, over Kv7.1.
In yet further
other embodiments, the method or use is selective for Kv7.5, over Kv7.1. In
certain
embodiments, the method or use is selective for Kv7.2 and Kv7.3, over Kv7.1.
In certain
embodiments, the method or use is selective for Kv7.2 and Kv7.3 over other Kv7
potassium
channels. In certain embodiments, the method or use is selective for Kv7.2 and
Kv7.3 over
Kv7.4 and Kv7.5.
[0052] As an alternative to oral administration, in certain instances other
routes of
administration of Compound A can be employed in the methods and used described
herein,
such as parenteral administration. Parenteral administration routes include
subcutaneous,
intravenous, intramuscular, intra-articular, intra-synovial, intrasternal,
intrathecal,
intrahepatic, intralesional, and intracranial injection or infusion techniques
or by
implantation. For example, Compound A can be administered by injection, such
as by
-11-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
intravenous, intramuscular, intrathecal, or subcutaneous injection. In certain
embodiments,
the above-discussed doses of Compound A are intended for oral administration
and can be
converted to doses suitable for parenteral administration, including
administration by
injection, by reducing the oral dose, for example by about half.
100531 Other administration routes suitable for administration of Compound A
according to
the methods and uses described herein include sublingual and buccal (e.g.,
with a film or
other composition that dissolves in the mouth under the tongue or on the
inside of the cheek),
ocular (e.g., eye drops), otic (e.g., by ear drops), oral or nasal inhalation
(e.g., by insufflation
or nebulization), cutaneous or topical (e.g., by creams or lotions), or
transdermal (e.g., by
skin patches). Besides oral administration, other enteral administration
routes can be used for
Compound A, including vaginal and rectal (e.g., by ointment, suppository,
enema).
100541 The presently described methods and uses involving administering
Compound A for
treating pain may also include administering Compound A in combination with
one or more
additional therapeutic agents, such as one or more other pain treatments,
regimens, or
analgesic agents. For instance, in some embodiments, the present disclosure
provides a
method of treating pain in a subject (e.g., a human) in need thereof,
comprising administering
(e.g., orally) a therapeutically effective amount of Compound A to the subject
in combination
with one Of more additional analgesic agents.
100551 In some embodiments, the one or more additional analgesic agents
include opioid
analgesics, such as opioid agonists, mixed agonist-antagonists, or partial
agonists including
but not limited to alfentanil, allylprodine, alphaprodine, anileridine,
benzylmorphine,
bezitramide, buprenorphine, butorphanol, clonitazene, codeine, desomorphine,
dextromoramide, dezocine, diampromide, diamorphone, dihydrocodeine,
dihydromorphine,
dimenoxadol, dimepheptanol, dimethyl-thiambutene, dioxaphetyl butyrate,
dipipanone,
eptazocine, ethoheptazine, ethylmethylthiambutene, ethylmorphine, etonitazene,
fentanyl,
heroin, hydrocodone, hydromorphone, hydroxypethidine, isomethadone,
ketobetnidone,
levorphanol, levophenacylmorphan, lofentanil, meperidine, meptazinol,
metazocine,
methadone, metopon, morphine, myrophine, narceine, nicomorphine,
norlevorphanol,
normethadone, nalorphine, nalbuphene, normorphine, norpipanone, opium,
oxycodone,
oxymorphone, papavereturn, pentazocine, phenadoxone, phenomorphan,
phenazocine,
phenoperidine, piminodine, piritramide, propheptazine, promedol, properidine,
propoxyphene, sufentanil, tilidine, and tramadol, including mixtures of any of
the foregoing
and pharmaceutically acceptable salts of any of the foregoing. In particular
embodiments, the
-12-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
additional analgesic agent includes an opioid agonist, such as buprenoirphine,
codeine,
hydrocodone, hydromorphone, methadone, morphine, oxycodone, oxymorphone,
tilidine, and
tramadol including mixtures of any of the foregoing and pharmaceutically
acceptable salts of
any of the foregoing. In some embodiments, the additional analgesic agent is
oxycodone or a
pharmaceutically acceptable salt thereof, such as oxycodone HO.
100561 On other embodiments the one or more additional analgesic agents
include non-opioid
treatments, such as aspirin; acetaminophen; non-steroidal anti-inflammatory
drugs
("NSAIDS"), e.g., ibuprofen, ketoprofen, naproxen, etc.; N-methyl-D-aspartate
(NMDA)
receptor antagonists, e.g., a morphinan such as dextromethorphan or
dextrorphan, or
ketamine; cyclooxygenase-2 inhibitors ("COX-II inhibitors"), such as
celecoxib, rofecoxib,
and etoricoxib; and/or glycine receptor antagonists.
100571 In some embodiments, administering Compound A in combination with one
or more
additional analgesic agents permits a reduction in the dosage of the
additional analgesic agent
without a reduction in the level of pain relief or analgesic efficacy
provided. For instance, in
some embodiments, the present disclosure provides a method of treating pain in
a subject
(e.g., a human) in need thereof, comprising administering (e.g., orally) a
therapeutically
effective amount of Compound A to the subject in combination with an amount of
one or
more additional analgesic agents, wherein the amount of the additional
analgesic agent is less
than the amount of the additional analgesic agent that would be needed to
achieve the same or
a similar level of pain relief or analgesic efficacy in the absence of
administering
Compound A. In certain of such embodiments, the one or more additional
analgesic agents is
an opioid analgesic, such as buprenorphine, codeine, hydrocodone,
hydromorphone,
methadone, morphine, oxycodone, oxymorphone, tilidine, and tramadol including
mixtures of
any of the foregoing and pharmaceutically acceptable salts of any of the
foregoing. In some
embodiments, the additional analgesic agent is oxycodone or a pharmaceutically
acceptable
salt thereof, such as oxycodone HCl.
100581 In related embodiments, the present disclosure provides a method of
reducing the
dose (e.g., maintenance dose) of an opioid analgesic administered to a subject
(preferably, a
mammal, such as a human) in need thereof comprising administering (e.g.,
orally) a
therapeutically effective amount of Compound A to the subject, for example,
whereby the
effective amount of Compound A offsets the reduction in the dose of the opioid
analgesic
such that the level of pain relief or analgesic efficacy experienced by the
subject is
maintained. In certain of such embodiments, the opioid analgesic is selected
from
-13-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
buprenorphine, codeine, hydrocodone, hydromorphone, methadone, morphine,
oxycodone,
oxymorphone, tilidine, and tramadol, including mixtures of any of the
foregoing and
pharmaceutically acceptable salts of any of the foregoing. In some
embodiments, the opioid
analgesic is oxycodone or a pharmaceutically acceptable salt thereof, such as
oxycodone HC1.
100591 In additional embodiments, the present disclosure provides a method of
reducing the
dose (e.g., a maintenance dose) of an opioid analgesic administered to a
subject (preferably, a
mammal, such as a human) in need thereof comprising administering (e.g.,
orally) a
therapeutically effective amount of Compound A to the subject, for example,
whereby the
effective amount of Compound A reduces the dose of the opioid analgesic needed
to achieve
pain relief in the subject. In certain of such embodiments, the opioid
analgesic is selected
from buprenorphine, codeine, hydrocodone, hydromorphone, methadone, morphine,
oxycodone, oxymorphone, tilidine, and tramadol, including mixtures of any of
the foregoing
and pharmaceutically acceptable salts of any of the foregoing. In some
embodiments, the
opioid analgesic is oxycodone or a pharmaceutically acceptable salt thereof,
such as
oxycodone HC1.
100601 In one embodiment, the methods and uses described herein, such as the
method of or
use in treating pain in a subject (preferably, a mammal, such as a human) in
need thereof, is
achieved by administering (e.g., orally) a therapeutically effective amount of
Compound A,
such as from about 0.05 mg/kg to about 20 mg/kg, including from about 0.05
mg/kg to about
mg/kg, from about 0.1 mg/kg to about 20 mg/kg, from about 0.1 mg/kg to about
10 mg/kg,
from about 0.05 mg/kg to about 5 mg/kg, from about 0.1 mg/kg to about 5 mg/kg,
from about
0.05 mg/kg to about 2 mg/kg, or from about 0.1 mg/kg to about 2 mg/kg. More
specific
representative amounts include 0.05 mg/kg, 0.1 mg/kg, 0.2 mg/kg, 0.3 mg/kg,
0.4 mg/kg, 0.5
mg/kg, 0.6 mg/kg, 0.7 mg/kg, 0,8 mg/kg, 0.9 mg/kg, 1 mg/kg, 1.1 mg/kg, 1.2
mg/kg,
1.3 mg/kg, L4 mg/kg, 1.5 mg/kg, 1.6 mg/kg, 1.7 mg/kg, 1.8 mg/kg, 1.9 mg/kg, 2
mg/kg, 5
mg/kg, 8 mg/kg, 10 mg/kg, 12 mg/kg, 15 mg/kg, 18 mg/kg, or 20 mg/kg or any
range of
amounts created by using two of the aforementioned amounts as endpoints. In
some aspects,
the method or use includes administering (e.g., orally) 0.1-1 mg/kg of
Compound A. In
certain aspects, the method includes administering (e.g., orally) 0.2-0.5
mg/kg of Compound
A. In some aspects, the method or use includes administering (e.g., orally)
0.05-20 mg/kg of
Compound A. In certain aspects, the method includes administering (e.g.,
orally) 1-10 mg/kg
of Compound A.
-14-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
[0061] In certain instances, the present disclosure provides a method of
treating pain in a
subject (preferably, a mammal, such as a human) in need thereof comprising
administering
(e.g., orally) a therapeutically effective amount of Compound A to the
subject, wherein the
pain is nociceptive pain, such as those described herein, including
inflammatory pain, and
wherein Compound A is administered at a dose of 0.05-5 mg/kg to the subject,
such as 0.1-
mg/kg, 0.05-2 mg/kg, or 0.1-2 mg/kg, including about 0.05 mg/kg, 0.1 mg/kg,
0.15 mg/kg,
0.2 mg/kg, 0.24 mg/kg, 025 mg/kg, 0.3 mg/kg, 0.35 mg/kg, 0.4 mg/kg, 0.5 mg/kg,
0.6
mg/kg, 0.7 mg/kg, 0.75 mg/kg, 0.8 mg/kg, 0.81 mg/kg, 0.85 mg/kg, 0.9 mg/kg, 1
mg/kg, 1.2
mg/kg, 1.5 mg/kg, 1.8 mg/kg,, 2 mg/kg, 3 mg/kg, 4 mg/kg, or 5 mg/kg or any
range of
amounts created by using two of the aforementioned amounts as endpoints.
[0062] In some instances, the present disclosure provides a method of treating
pain in a
subject (e.g., human) in need thereof comprising administering (e.g., orally)
a therapeutically
effective amount of Compound A to the subject, wherein the pain is neuropathic
pain, such as
those described herein, and wherein Compound A is administered at a dose of
0.5-10 mg/kg
to the subject, such as 0.5-8 mg/kg, 1-10 mg/kg, or 1-8 mg/kg, including about
0.5 mg/kg, 0.8
mg/kg, 1 mg/kg, 1.5 mg/kg, 2 mg/kg, 2.5 mg/kg, 2.6 mg/kg, 2.8 mg/kg, 3 mg/kg,
3.5 mg/kg,
4 mg/kg, 4.5 mg/kg, 5 mg,/kg, 5.2 mg/kg, 5.5 mg/kg, 6 mg/kg, 6.5 mg/kg, 7
mg/kg, 8 mg/kg,
9 mg/kg, or 10 mg/kg or any range of amounts created by using two of the
aforementioned
amounts as endpoints.
[0063] In some embodiments, the methods and uses described herein, such as the
method of
or use in treating pain in a subject (e.g., human) in need thereof, is
achieved by administering
(e.g., orally) a therapeutically effective amount of Compound A, such as 2 to
200 mg of
Compound A in a single or multiple dosage units. For example, the method can
include
administering (e.g., orally), in a single or multiple dosage units, about 2
mg, about 3 mg,
about 4 mg, about 5 mg, about 6 mg, about 7 mg, about 8 mg, about 9 mg, about
10 mg,
about 11 mg, about 12 mg, about 13 mg, about 14 mg, about 15 mg, about 16 mg,
about 17
mg, about 18 mg, about 19 mg, about 20 mg, about 21 mg, about 22 mg, about 23
mg, about
24 mg, about 25 mg, about 26 mg, about 27 mg, about 29 mg, about 30 mg, about
31 mg,
about 32 mg, about 33 mg, about 34 mg, about 35 mg, about 36 mg, about 37 mg,
about 38
mg, about 39 mg, about 40 mg, about 41 mg, about 42 mg, about 43 mg, about 44
mg, about
45 mg, about 46 mg, about 47 mg, about 48 mg, about 49 mg, about 50 mg, about
51 mg,
about 52 mg, about 53 mg, about 54 mg, about 55 mg, about 56 mg, about 57 mg,
about 58
mg, about 59 mg, about 60 mg, about 61 mg, about 62 mg, about 63 mg, about 64
mg, about
-15-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
65 mg, about 66 mg, about 67 mg, about 68 mg, about 69 mg, about 70 mg, about
71 mg,
about 72 mg, about 73 mg, about 74 mg, about 75 mg, about 76 mg, about 77 mg,
about 78
mg, about 79 mg, about 80 mg, about 81 mg, about 82 mg, about 83 mg, about 84
mg, about
85 mg, about 86 mg, about 87 mg, about 88 mg, about 89 mg, about 90 mg, about
91 mg,
about 92 mg, about 93 mg, about 94 mg, about 95 mg, about 96 mg, about 97 mg,
about 98
mg, about 99 mg, about 100 mg, about 101 mg, about 102 mg, about 103 mg, about
104 mg,
about 105 mg, about 106 mg, about 107 mg, about 108 mg, about 109 mg, about
110 mg,
about 111 mg, about 112 mg, about 113 mg, about 114 mg, about 115 mg, about
116 mg,
about 117 mg, about 118 mg, about 119 mg, about 120 mg, about 121 mg, about
122 mg,
about 123 mg, about 124 mg, about 125 mg, about 126 mg, about 127 mg, about
129 mg,
about 130 mg, about 131 mg, about 132 mg, about 133 mg, about 134 mg, about
135 mg,
about 136 mg, about 137 mg, about 138 mg, about 139 mg, about 140 mg, about
141 mg,
about 142 mg, about 143 mg, about 144 mg, about 145 mg, about 146 mg, about
147 mg,
about 148 mg, about 149 mg, about 150 mg, about 151 mg, about 152 mg, about
153 mg,
about 154 mg, about 155 mg, about 156 mg, about 157 mg, about 158 mg, about
159 mg,
about 160 mg, about 161 mg, about 162 mg, about 163 mg, about 164 mg, about
165 mg,
about 166 mg, about 167 mg, about 168 mg, about 169 mg, about 170 mg, about
171 mg,
about 172 mg, about 173 mg, about 174 mg, about 175 mg, about 176 mg, about
177 mg,
about 178 mg, about 179 mg, about 180 mg, about 181 mg, about 182 mg, about
183 mg,
about 184 mg, about 185 mg, about 186 mg, about 187 mg, about 188 mg, about
189 mg,
about 190 mg, about 191 mg, about 192 mg, about 193 mg, about 194 mg, about
195 mg,
about 196 mg, about 197 mg, about 198 mg, about 199 mg, or about 200 mg or
administering
(e.g., orally) any range of amounts created by using two of the aforementioned
amounts as
endpoints. In some aspects, the method or use includes oral administration of
5 to 50 mg of
Compound A in a single or multiple dosage units to a subject (e.g., a human).
In some
aspects, the method or use includes the oral administration of 10, 20, or 25
mg of Compound
A in a single or multiple dosage units to a subject (e.g., a human). In some
aspects, the
method or use includes oral administration of 20 mg of Compound A in a single
or multiple
dosage units to a subject (e.g., a human).
100641 In some aspects, the methods and uses described herein, such as the
method of or use
in treating pain in a subject (e.g., human) in need thereof, is achieved by
administering (e.g.,
orally) at least 20 mg of Compound A, such as at least 25, 30, 35, 50, 75, or
100 mg of
Compound A. In some embodiments, the methods and uses described herein, such
as the
-16-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
method of or use in treating pain in a subject in need thereof, is achieved by
administering
(e.g., orally) at least 50 mg of Compound A per day, such as at least 60, '75,
85, 100, 125,
150, 175, or 200 mg of Compound A per day to a subject (e.g., a human).
[0065] In some embodiments, the methods and uses described herein, such as the
method of
or use in treating pain in a subject (e.g., a human) in need thereof, is
achieved by
administering (e.g., orally) a therapeutically effective amount of Compound A
per day, such
as 5 to 1000 mg of Compound A per day, such as 5 to 500 mg or 5 to 250 mg of
Compound
A per day. For example, the method or use can include administering (e.g.,
orally) about 5
mg, about 10 mg, about 15 mg, about 20 mg, about 25 mg, about 30 mg, about 35
mg, about
40 mg, about 45 mg, about 50 mg, about 55 mg, about 60 mg, about 65 mg, about
70 mg,
about 75 mg, about 80 mg, about 85 mg, about 90 mg, about 95 mg, about 100 mg,
about 105
mg, about 110 mg, about 115 mg, about 120 mg, about 125 mg, about 130 mg,
about 135 mg,
about 140 mg, about 145 mg, about 150 mg, about 155 mg, about 160 mg, about
165 mg,
about 170 mg, about 175 mg, about 180 mg, about 185 mg, about 190 mg, about
195 mg,
about 200 mg, about 205 mg, about 210 mg, about 215 mg, about 220 mg, about
225 mg,
about 230 mg, about 235 mg, about 240 mg, about 245 mg, about 250 mg, about
255 mg,
about 260 mg, about 265 mg, about 270 mg, about 275 mg, about 280 mg, about
285 mg,
about 290 mg, about 295 mg, about 300 mg, about 305 mg, about 310 mg, about
315 mg,
about 320 mg, about 325 mg, about 330 mg, about 335 mg, about 340 mg, about
345 mg,
about 350 mg, about 355 mg, about 360 mg, about 365 mg, about 370 mg, about
375 mg,
about 380 mg, about 385 mg, about 390 mg, about 395 mg, about 400 mg, about
405 mg,
about 410 mg, about 415 mg, about 420 mg, about 425 mg, about 430 mg, about
435 mg,,
about 440 mg, about 445 mg, about 450 mg, about 455 mg, about 460 mg, about
465 mg,
about 470 mg, about 475 mg, about 480 mg, about 485 mg, about 490 mg, about
495 mg,
about 500 mg, or about 1000 mg of Compound A per day, or administering (e.g.,
orally) per
day a range of amounts created by using two of the aforementioned amounts as
endpoints. In
some aspects, the method or use includes orally administering 10 to 200 mg of
Compound A
per day, such as 10, 15, 20,25, 30, 35, or 40 mg to 75, 100, 125, 150, 175, or
200 mg of
Compound A per day, including 20 to 150 mg per day to a subject (e.g., a
human). In some
aspects, the oral administration includes 50, 75, 100, or 125 mg of Compound A
per day,
such as 100 mg per day to a subject (e.g., a human).
[0066] In certain instances, the above daily doses of Compound A are
administered (e.g.,
orally) as multiple doses per day, such as in two, three, four, or five doses
per day. For
-17-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
Example, a daily dose of 100 mg, maybe administered in five 20 mg, four 25 mg,
three 33.3
mg, or two 50 mg doses throughout the day.
[0067] In some embodiments, the above daily doses of Compound A are
administered (e.g.,
orally) as a single dose. For example, about 5, 10, 15, 20, 25, or 30 mg to
about 50, 65, 75,
100, 125, or 150 mg of Compound A per day can be orally administered as a
single dose,
including 10-25 mg, 10-30 mg, and 10-40 mg per day as a single dose, such as
10-25 mg per
day as a single dose. Relatedly, any of the doses of Compound A discussed in
the preceding
paragraphs may be included in a single unit dosage form or in multiple unit
dosage forms,
such as two, three, or four unit dosage forms.
100681 In certain embodiments, the methods and uses described herein, when
using the daily
dosing disclosed herein, achieve a steady state for Compound A within 6 to 9
days, such as in
about 1 week.
100691 In some embodiments, the methods and uses described herein for treating
pain by
administering (e.g., orally) Compound A include administering according to a
12-hour (i.e.,
twice-a-day), 24-hour (La, once-a-day), 48-hour (i.e., once-per-two-days), 72-
hour, 96-hour,
5-day, 6-day, 1-week, or 2-week dosing regimen, particularly 12-hour, 24-hour,
or 48-hour
dosing regimens. Such regimens can involve administering any of the above-
described doses
or daily doses. For instance, the present disclosure provides methods of
treating pain in a
subject (e.g., a human) in need thereof, comprising administering (e.g.,
orally) a
therapeutically effective amount of Compound A to the subject according to 12-
hour, 24-
hour, 48-hour, 72-hour, 96-hour, 5-day, 6-day, 1-week, or 2-week intervals,
particularly 12-
hour, 24-hour, or 48-hour intervals, wherein the amount of Compound A
corresponds to any
of the above-described doses or daily doses. In certain such embodiments,
Compound A is
orally administered to a human subject under fed conditions, e.g., from
between about 30
minutes before to about 2 hour after eating a meal, including during a meal or
within 15
minutes after eating a meal.
100701 In additional embodiments, the above-discussed methods or uses of
treating pain by
administering a therapeutically effective amount of Compound A comprises oral
administration of Compound A to a human subject under fed conditions, e.g.,
from between
about 30 minutes before to about 2 hour after eating a meal, including during
a meal or within
15 minutes after eating a meal. In some embodiments, the oral administration
of Compound
A to a human subject under fed conditions significantly enhances the
bioavailability and
-18-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
exposure of Compound A as compared to the oral administration of Compound A to
the
subject under fasted conditions. In some embodiments, the oral administration
of
Compound A to a human subject under fed conditions increases one or more
pharmacokinetic
parameters for Compound A (e.g., Cifia., AUCuir, Minx, t'/n, etc.) as compared
to when the
same amount of Compound A is orally administered to the subject under fasted
conditions.
100711 In certain embodiments, the methods and uses described herein
administer
Compound A in the form of a pharmaceutically acceptable oral composition that
comprises
Compound A and one or more pharmaceutically acceptable carriers or excipients.
The
amount of Compound A included in these compositions may correspond to one or
more of
the amounts described herein_ In some embodiments, the compositions are a unit
dose.
100721 Examples of pharmaceutically acceptable oral compositions that comprise
Compound A include solid formulations (such as tablets, capsules, lozenges,
dragees,
granules, powders, wafers, multi-particulates, and films), liquid formulations
(such as
aqueous solutions, elixirs, tinctures, slurries, suspensions, and
dispersions), and aerosolized
formulations (such as mists and sprays). In one embodiment, a pharmaceutically
acceptable
oral composition of Compound A includes a pediatric suspension or granulate.
All above-
noted amounts of Compound A may be included in such formulations, e.g., a
capsule
comprising 5, 10, 15, 10, 25, 30, or 35 mg of Compound A.
100731 Examples of compositions suitable for parenteral administration of
Compound A,
include sterile injectable solutions, suspensions, or dispersions, including
aqueous or
oleaginous preparations, particularly aqueous. In some embodiments, Compound A
is
administered according to a method or use described herein in an injectable
sterile aqueous
formulation that includes a parenterally-acceptable diluent or solvent, such
as water, Ringer's
solution, isotonic sodium chloride solution, buffered aqueous solutions, and
aqueous
solutions containing a miscible alcohol, such as 1,3-butanediol. Additional
suitable
excipients for parenteral formulations of Compound A include, mono- or di-
glycerides; fatty
acids, such as oleic acid and its glyceride derivatives; natural
pharmaceutically-acceptable
oils, such as olive oil or castor oil, including their polyoxyethylated
versions; long-chain
alcohol diluents or dispersants, such as alkyl celluloses, including
carboxymethyl cellulose;
and surfactants, such as Tweens, Spans and other emulsifying agents or
bioavailability
enhancers.
-19-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
100741 In another embodiment, kits are provided for oral administration of
Compound A for
the treatment of pain. Such kits comprise a plurality of oral unit dosage
forms of Compound
A in combination with instructions for orally administering Compound A.
100751 Additional embodiments and examples of the present disclosure are
described herein.
These embodiments and examples are illustrative and should not be construed as
limiting the
scope of the claimed invention.
5. EXAMPLES
100761 Studies were conducted to determine the effect of Compound A in rodent
models of
pain (e.g., acetic acid induced mouse model of visceral pain and rat spinal
nerve
ligation/Chung model of neuropathic pain). Additional studies are conducted to
determine
the effect, if any, of Compound A in accepted models of pain.
5.1. Example 1. Acetic Acid Induced Mouse Model of Visceral Pain
100771 Objective: The acetic acid test was performed to assess the potential
efficacy of
Compound A in the acetic acid writhing (AAW) model of inflammatory pain. The
AAW test
was performed as described previously (Yeping Bi et al., "Visceral
hyperalgesia induced by
forebrain-specific suppression of native Kv7/KCNQ/M-current in mice." Mot Pain
2011,
7:84; Gabriela F. Pavao-de-Souza et al., "Acetic acid- and phenyl-p-
benzoquinone-induced
overt pain-like behavior depends on spinal activation of MAP kinases, PI3K and
inicroglia in
mice." Pharmacol. Biochem. Behay. 101 (2012) 320-328; Kazuftuni Hirano et al.,
"Kv7.2-
7.5 voltage-gated potassium channel (KCNQ2-5) opener, retigabine, reduces
capsaicin-
induced visceral pain in mice." Neurosct Lett. 413 (2007) 159-162; Mosad A.
Ghareeb et al.,
"HPLC-ESI-MS/MS Profiling of Polyphenolics of a Leaf Extract from Alpinia
zerumbet
(Zingiberaceae) and Its Anti-Inflammatory, Anti-Nociceptive, and Antipyretic
Activities In
Vivo." Molecules 23 (2018) 3238; and Yaroslav A. Andreev et al., "Analgesic
Activity of
Acid-Sensing Ion Channel 3 (ASIC3) Inhibitors: Sea Anemones Peptides Ugr9-1
and
APETx2 versus Low Molecular Weight Compounds." Mar. Drugs 16 (2018), 500).
100781 Study Design: Briefly, acetic acid was injected intraperitoneally at
0.4%
concentration in 6-7 week old CD1 mice. After the injection of acetic acid,
animals were
placed in a chamber and their subsequent writhing behavior was video recorded.
A writhe is
defined as contraction of abdominal muscles accompanied by elongation of the
body and
extension of hind limbs or rotation of the trunk. Writhes were counted between
5-15 min
after the injection of acetic acid.
-20-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
100791 Selection of Acetic Acid Dose: Based on the concentration response (0.2-
0.8%) of
acetic acid in CD1 mice, EC75 concentration (0.4%) was selected to produce
optimal
writhing response. Diclofenac was used as positive control in the acetic acid
model.
100801 Results: The first study (FIG 1, Study 1) shows a dose-responsive
decrease in the
number of nociceptive events was observed through the dose groups (vehicle, 1
mg/kg, 3
mg/kg, and 10 mg/kg Compound A). The second study (FIG. 1, Study 2) shows a
decrease in
the number of nociceptive events at 10 mg/kg Compound A. A PK/PD correlation
was
shown between Compound A concentrations in brain and plasma to the observed
efficacy
(FIG.1, PIC/PD Correlation). The EC50 (effective concentration to give a 50%
reduction in
nociceptive events) was 0.25 LIM and 0.4 jtM in plasma and brain respectively.
5.2. Example 2. Spinal Nerve Ligation-induced Model of Neuropathic Pain in
Rats
100811 Objective: The efficacy of Compound A was assessed using the rat spinal
nerve
ligation (SNL)/Chung model of neuropathic pain. The SNL model in rats was
developed as
described previously (Chung JM, Kim HK, Chung IC "Segmental spinal nerve
ligation model
of neuropathic pain." Methods Mot Med. 99 (2004) 35-45).
Table 1. Materials for SNL Model
Route
Appear
Dose Vol. of
Substance Formulation of
Ptta
awe
expression Admin
Admin
Once daily
0.5% (w/v)
from Day 14
CMC-Na salt
to Day 18 mg/kg of 5 mL/kg
(Vehicle) and 0.1% (WO
11 - (120 min supplied body
substance
weight
Twecn 80 in
before the first
distilled water
test on Days
14 and 18)
Dispersed in
Once daily
0.5% (w/v)
carboxymethyl-
from Day 14
mg/kg of 5 mL/kg
white cellulose
to Day 18 supplied body
Compound A p.o.
(120 min
Powder (CMC)-Na salt substance weight
before the first
and 0.1% (v/v)
Tween 80 in
test on Days
14 and 18)
distilled water
Dispersed Once daily
in
Retigabine 0.5% (w/w)
from Day 14 mg/kg of 5 mukg
white
to Day 18
methykellulose supplied body
(Selleckehem) p.o.
powder and 0.2% (v/y) (120 min substance weight
before the first
Tween 80 in
distilled water
test on Days
14 and 18)
-21-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
60 min before
the first test on
Morphine
Days 14 and 5 mL/kg
hydrochloride white Dissolved in
18 (distilled mg/kgrbody
(Caesar Loretz Powder distilled water P'
' water salt weight
(3mb
administered
H)
once daily
from Day 15
to Day 17)
a Pretreatment time.
100821 Study Design: Briefly, on Day 0, von Frey and cold plate tests were
used for baseline
measurements of tactile and cold allodynia respectively. Post-baseline
measurements, rats
(171-209 g male Sprague Dawley) were anesthetized and an incision at the L4-52
level was
performed to expose the left L5 nerve. A ligature was tied tightly around the
L5 nerve
(Chung et al.; R. Dost et al., "The anti-hyperalgesic activity of Retigabine
is mediated by
KCNQ potassium channel activation." Naunyn-Schmiedeberg's Arch. Pharmacol. 369
(2004)
382-390; Gordon Blackburn-Munro et at, "The anticonvulsant retigabine
attenuates
nociceptive behaviors in rat models of persistent and neuropathic pain." Eur.
I Pharmacol.
460 (2003) 109-116; and Wu Yir et at, "Discovery of (S,E)-3-(2-fluoropheny1)-N-
(1-(3-
(pyridin-3-yloxy)phenyflethyl)-aciylamide as a potent and efficacious KCNQ2
(Kv7.2)
opener for the treatment of neuropathic pain." Bioorg Med. Chem. Lett 23
(2013) 6188-91).
The wound was then sutured and the rats were allowed to recover. On Day 13,
post-surgery
baselines for tactile and cold allodynia were measured to verify neuropathic
pain. Animals
were assigned to treatment groups based on the post-surgery baseline score.
Table 2 and
FIG. 2 show the results of the tactile allodynia von Frey test in rats on Day
13, prior to
treatment with vehicle, Compound A, retigabine or morphine.
Table 2. Pretreatment Test on Day 13: Electronic von Frey Test
Force inducing paw-withdrawal (g)
To be treated with Non-lesioned paw
Lesioned paw
Mean + s.e.m. Mean + s.e.m. Mean + s.e.m.
Vehicle (p.o.) 912+37
22.7 1.5
Compound A (8 mg/kg p.o.) 87.7 + GA
23.0 + 1.0 -74% ***
Compound A (16 mg/kg p.o.) 85.8 + 5.7
21.8 + 1.0 -75% ***
Compound A (24 mg/kg p.o.) 83.8 + 4A
22.1 + 1.7 -74% ***
Retigabine (20 mg/kg p.o.) 82.8 + 4.3
22.7 + 1.7 _73% ***
-22-
CA 03159436 2022- 5-25
WO 2021/113757
PCT/US2020/063471
Morphine (128 mg/kg p.o.) 90.1 +
2.4 22.6 1.6
Paired Student's t test: NS = Not Significant; * = p <0.05; ** = p <0.01; ***
= p <0.001
[0083] Dosing: For efficacy testing, Compound A was dosed orally once a day
for 5
consecutive days from Day 14-18. Animals were evaluated again for tactile and
thermal
allodynia on Day 14 and Day 18 two hours after the administration of Compound
A.
Morphine was used as positive control_ Table 3 shows the body weight of the
rats over the
duration of testing.
Table 3. Body Weight
Body weight (g) (mean
Treatment once
daily from Day
14 to Day 18a Day 0 Day 13 Day 14 Day 15 Day 16 Day 17 Day 18
194.4 309.4 314.5+ 327.1 335.2 345.6+ 351.6
Vehicle (p.o.)
2.0 4.1 3.7 3.8 4.2 4.5 4.7
Compound A (8 194.6 + 307.4 +
311.5+ 327.8 + 338.2 + 347.0 + 354.0 +
mg/kg p.o.) 2.4 6.6 6.7
7.8 7.6 8.2 8.0
Compound A (16 190.6 + 296.8
300.2 + 314.9 320.2 + 330.1 + 334.9
mg/kg p.o.) 2.5 4.0 5.1
5.3 5.2 6.7 6.4
Compound A (24 192.2 296.8
301.4 319.8 328.3 334.2 339.1
mg/kg p.o.) 1.8 4.5 5.1
5.7 5.5 6.4 5.5
Retigabine (20 190.6 297.8+
300.8+ 312.6+ 321.1+ 330.6+ 336.8+
mg/kg p.o.) 3.3 5.0 4.8
5.1 5.2 5.3 5.7
Morphine (128 188.4 293.9
295.9+ 312.8 302.0 298.9+ 306.7
mg/kg p.o.) 2.0 4.7 4.6
5.5 5.4 5.4 5.2
a Treatment once on Day 14 and on Day 18 for group treated with morphine.
5.2.1 Tactile Allodynia Evaluation using the Electronic von Frey Test
[0084] Rats were placed under an inverted acrylic plastic box on a grid floor.
The tip of an
electronic von Frey probe was then applied with increasing force to the
surgery hind paw and
the force required to induce paw-withdrawal was automatically recorded. The
procedure was
carried out 3 times and the mean paw withdrawal force was calculated. The
experimenter
was double blinded to treatment.
[0085] Results: Tables 4-5 and FIG. 3 show the results of the tactile
allodynia von Frey
evaluation on Day 14 and Day 18. Compared to morphine, neither Compound A nor
retigabine had much effect on the tactile allodynia endpoint. This was not
surprising for the
-23-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
Kv7.2 mechanism (see Blackburn-Munro and Jensen, Eur Pharmacol, 460(2-3): 109-
116
(2003)).
Table 4. Tactile Allodynia Evaluation on Day 14: Electronic von Frey Test on
Lesioned Paw
Force inducing paw-withdrawal (g)
Treatment once Baseline on Day 14 at
Day 14 at 2 h: Delta from baseline
daily from Day Day 14 2h
14 to Day 18'
Mean + Mean + Mean + Compared with Vehicle (p.o.)
s.e.m. s.e.m.
s.e.m. d A
Vehicle (p.o.) 22.9+ 1.2 23_2 + 1.7
+0.3 + 1.4
Compound A (8
21.5 + 1.6 28.5 + 3.4 +7.0 2.5
+6.7
mg/kg p.o.)
Compound A
19.9+ 1.9 25.2 1.9 +5.4 + 2.1
+5.1
(16 mg/kg p.o.)
NS
Compound A
22.4 + 1.4 29.9 + 2.9 +7.5+3,1
+7.2
(24 mg/kg p.o.)
Retigabine (20
22.5 1.6 26.5 + 2.5 +4.0 + 2.2
+4.0
mg/kg p.o.)
Morphine (128
23.0 1.8 50.6 5.0 +27.7 5.6 *** +27.4
mg/kg mo.)t
Inter-group comparison: NS = Not Significant; * = Pc 0.05; ** = Pc 0.01; *** =
p <0.001.
'Treatment once on Day 14 and on Day 18 for group treated with morphine.
b Test 1 h after administration.
For test substances-treated groups: One-way ANOVA with group as factor.
d Dunnete s test when One-way ANOVA is significant.
Table 5. Tactile Allodynia Evaluation on Day 18: Electronic von Frey Test on
Lesioned Paw
Force inducing paw-withdrawal (g)
i Baselne on Day 18 at
Treatment once Day 18 at 2 h: Delta from baseline
daily from Day Day 14 2h
14 to Day 18' Mean + Mean +
mean + Compared with Vehicle (p.o.)
s.e.m. s.e.m. s.e.m.
Vehicle (p.o.) 22+9 1.2 25.7 1.8
+2.8 2.1
Compound A
21.5 + 1.6 32.1 + 3.6 +10.6 + 35
+7.8
(8 mg/kg p.o.)
NS
Compound A
19.9 1.9 25.2 + 2.0 +5.4 + 2.1
+2.6
(16 mg/kg p.o.)
-24-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
Compound A 22.4 + 1.4
33.8 + 3.6 +11.5+3.0 +8.7
(24 mg/kg p.o.)
Retigabine
22.5 1 1.6 31.8 1 2.2 +9.2 1 2.1
-1-6.4
(20 mg/kg p.o.)
Morphine
(128 mg/kg 23.0 1.8 50.0
4.5 +27.1 4.6 *** +24.3
P-o-)h
Inter-group comparison: NS = Not Significant; * = p <0.05; ** = p <0.01; *** =
p <0.001.
'Treatment once on Day 14 and on Day 18 for group treated with morphine.
b Test 1 h after administration.
For test substances-treated groups: One-way ANOVA with group as factor.
d Durmett's test when One-way ANOVA is significant.
5.2.2 Thermal Allodynia Evaluation using the Cold Plate Test
100861 The cold plate apparatus was maintained at 4 1 'IC and was surrounded
by an acrylic
glass enclosure. Rats were placed on the plate for a period of 5 minutes. The
latency to Is'
paw-withdrawal, total number of paw withdrawals, and total duration of
withdrawal
responses were recorded for the surgery hind paw.
100871 Results: Tables 6-7 and FIGs. 4-9 show the results of the thermal
allodynia von Frey
evaluation on Day 14 and Day 18. FIGs. 7-9 show that at both 16 and 24 mg/kg,
Compound
A outperformed morphine (128 mg/kg) in the thermal allodynia endpoint.
Table 6. Thermal Allodynia Evaluation on Day 14: Cold Plate Test on Lesioned
Paw
Latency to first Number of withdrawal Total
duration of
paw-withdrawal (s)
responses withdrawal responses (s)
Treatment
Day 14 at 2h
once daily
Compared
from Day 14 Compared with
Compared with
Mean Vehicle (p.o.) mean with Vehicle mean
Vehicle (p.o.)
to Day 182
(p.o.)
s.e.m. s.e.m. A s.e.m.
C d A% c d c d A%
130.0 10.1 2.2
Vehicle (p.o.) 25.5
3.1 1.0
Compound A
226.8 21+ 34+
(8 mg/kg29.4 * +74
0.8 ** _79
3.4 - +55
p.o.)
*** _________________________________________________________________________
** NS
Compound A
2149 3.0 08
(16 mg/kg 22 NS +65
* -70 0.7 - -64
1.7
P.O.)
-25-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
Compound A
280.0
**
_91 0'0 +
+115 - -100
(24 mg/kgg
1 20.0 *** 0'9 +
09
0.0
p.o.)
Retigabine
272..17 l'5 +
(20 mg/kg
***
20 +110
13
** _85 0,3
0.3 - -86
p.o.)
Morphine
201.3 7.9+
(128 mg/kg 35.8 NS +55
2.8 NS
-22 49+
2.4 NS +123
p.o.)b
Inter-group comparison: NS = Not Significant; * = p <0.05; ** = p <0.01; *** =
p <0.001.
' Treatment once on Day 14 and on Day 18 for group treated with morphine.
b Test 1 h after administration.
For test substances-treated groups: One-way ANOVA with group as factor. For
comparison-ireated
group: Unpaired Student's test.
d Dunnett's test when One-way ANOVA is significant.
Table 7. Thermal Allodynia Evaluation on Day 18: Cold Plate Test on Lesioned
Paw
Latency to first Number of
withdrawal Total duration of
paw-withdrawal (s)
responses withdrawal responses (s)
Treatment
once daily Day
18 at 2h
from Day 14 mean Compared with mean
Compared with mean Compared with
to Day 182 Vehicle (p.o.)
Vehicle (p.o.) Vehicle (p.a.)
+ +
s.e.m. C d A% s.e.m. c d A% s.e.m. c d A%
94.2 10.9
10.8
Vehicle (p.o.)
25.6 3.5
4.3
Compound A
180.4
NS
-29 1.3
NS +92
- -88
(8 mg/kg 26.5 7'7+
2.8
0.9
p.o.)
Compound A
257.0 1-3
** _88 0.0
(16 mg/kg 25 *** +173
2.8
0.0 - -100
p.o.)
*** _________________________________________________________________________
** NS
Compound A
263.8
**
-94 0'0
*** +180 - -100
(24 mg/kg
24.3 0'6
0.5
0.0
p.o.)
Retigabine
209.3 34
(20 mg/kg 36.1 * +122 '
13
NS -69 27'3
235 - +153
P.O.)
Morphine
235+8 4,6
(128 mg/kg 33.2 ** +150
2.8
NS -58 3.8
2.7 NS
-65
Inter-group comparison: NS = Not Significant; * = Pc 0.05; ** = p< 0.01; *** =
p <0.001.
a Treatment once on Day 14 and on Day 18 for group treated with morphine.
-26-
CA 03159436 2022- 5- 25
WO 2021/113757
PCT/US2020/063471
b Test 1 h after administration.
For test substances-treated groups: One-way ANOVA with group as factor. For
comparison-treated
group: Unpaired Student's test.
Dutmett's test when One-way ANOVA is significant.
5.3. Example 3. Crossover Study with Pharmacokinetic Analysis
[0088] The pharmacokinetics (PK), safety, and tolerability of single doses of
Compound A in
healthy right-handed male human subjects were investigated in a randomized,
double-blind,
placebo-controlled, transcranial magnetic stimulation (TMS) crossover study.
[0089] An objective of the study was to evaluate the safety, tolerability, and
pharmacokinetics of single doses of Compound A in healthy male subjects.
[0090] Twenty healthy right-handed male subjects were enrolled and randomized
in a
blinded fashion to receive a single oral dose of 20 mg Compound A or placebo
(1:1
randomization ratio) on Day 1, then were crossed over to receive a single dose
of the other
treatment on Day 7.
[0091] Subjects were screened within 27 days prior to entering the study on
Day 1. For
Period 1, subjects were admitted to the study unit and dosed on Day 1, and
discharged on
Day 2. For Period 2, following a washout of 6 days, the same subjects were
again admitted
to the study unit and dosed on Day 7, and discharged on Day 8. All subjects
returned to the
clinical unit for an outpatient visit on Day 14, and received a follow-up
telephone call on
Day 37.
100921 Subjects were dosed in a fed state, but the timing of dosing relative
to meals was
changed during the study, and varied between a high fat or standard meal eaten
either 2 h or
30 minutes prior to dosing, and a high fat or standard meal eaten 1 h or 2.5 h
after dosing.
[0093] PK variables included maximal plasma concentration (Cmax), time of
maximal plasma
concentration (T.,), terminal elimination half-life (tin), elimination rate
constant (X2), area
under the curve from 0 to 24 h (AUC0_240, area under the curve from time zero
to the last
quantifiable concentration (AUCo-dasi), area under the curve from time zero to
infinity
(AUCo-inc), the percentage of AUC that is due to extrapolation from tlast to
infinity
(%AUCe,map), apparent total body clearance following oral administration
(CL/F), CL/F
normalized by body weight, mean residence time from time zero to the last
quantifiable
concentration (MRTiasi), mean residence time extrapolated to infinity
(MRTint), apparent
-27-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
volume of distribution during the terminal phase (VzJF), and VzJF normalized
by body
weight.
5.3.1. Pharmacokinetic Analysis
100941 The PK parameters for this study were summarized in two ways. Firstly,
PK
parameters were calculated where possible using the PK samples collected
during each 24 h
sampling period for Period 1 and Period 2 separately. Secondly, PK parameters
were
determined using samples beyond the 24 h sampling period (i.e., from Day 7/8
and/or
Day 14). For subjects who received Compound A in the first period, PK samples
taken prior
to placebo treatment provided additional PK timepoints at >24 It For subjects
who received
Compound A in the second period, there was no >24 h PK timepoint until a Day
14 PK
sample was added. Thus, subjects randomized to receive Compound A in the
second period
who were enrolled prior to implementation of the additional PK sample at Day
14 did not
have PK data beyond 24 h. The full PK profile data set consists of the 16
subjects for whom
PK samples were taken at >24 h post-dose. For discussion of the PK parameters
below, the
full PK profile data set was generally used, because it allowed more accurate
estimation of
PK parameters.
100951 Initially, subjects were dosed 2 hours after a high fat meal with a
relatively high fat
lunch provided 1 hour after dosing. After blinded review of the PK profiles in
the initial 8
subjects, the fat content of the lunch was reduced in an attempt to reduce the
time to T,.. In
addition, the timing of the meal relative to dose was changed from 2 hours
prior, to 30
minutes prior to dose and subsequently the fat content of the breakfast was
reduced. The
timing and type of meal for each subject is specified in Table S. Overall,
there was no clear
difference in C.. or T. despite the changes in meal composition and timing
relative to
dose. As such the PK data is presented without categorization according to
meal content, or
relative timing of the meal.
Table 8. Type and Timing of Meals Relative to Dosing
Pre-dose Meal
Post-dose Meal
Subjects
Type Time
Type Time
901, 908, 910,
High fata 2h pre-dose
High fat lh post-dose
907
912, 919, 914,
High fat 2h pre-dose
Standard 1h post-dose
918
-28-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
927, 925, 928,
High fat 0.5h pre-dose
Standard 2.5h post-dose
924
930, 934, 933,
937, 938, 941, Standard 0.5h pre-dose Standard
2.5h post-dose
940, 942
3 Except Subject 910 had standard breakfast prior to dosing
5.3.1.1. Plasma Concentrations
100961 A plasma concentrations over time for the full PK profile were
recorded. At the 2 h,
4 hand 6 h timepoints, the mean SD plasma concentrations were 15.9 21.4
ng/mL, 30.2
21.1 ng/mL and 42.1 19.1 ng/mL, respectively.
100971 There was no difference in mean Cmax or Tmax between periods (Table 9).
The overall
time to peak plasma concentrations ranged from 1.9 to 12 h, with a median time
of 7.8 h.
100981 Subjects who received placebo in Period 2 had low but measurable
Compound A
levels at the start of the placebo treatment period, with a mean Cm.. of 5.84
ng/mL (range
3.34-9.61 ng/mL).
Table 9. Pharmacokinetic Parameters by Period, Overall, and for Full PK
Profile
20 mg Compound A
Parameter Statistic Period 1 Period 2
Overall Full PK
Profile
(N=10)
(N=10) (N=20)
(N=16)
Mean
60.2 + 17.3 58.3 + 9.94 59.2 + 13.8
60.1 + 14.9
C.. (ng/mL) SD
Range 29.9 - 77.1 46.2 -
79.4 29.9 - 79.4 29.9 - 79.4
Median 6.94
7.83 7.83 6.83
Tina. (h)
Range 1.92 - 12
1.92 - 8.15 1.92 - 12 1.92 - 12
Mean
693 + 184 681 + 142 687 + 160 692 + 151
AUCo-24 SD
(ngth/mL)
Range 383 - 951
358 - 869 358 - 951 383 - 951
Mean +
16.4 + 5.61 16.4 + 3.87 16.4 + 4.69
4.52 + 1.82
Clam (ng/mL) SD
Range 10.1 - 27.8
7.1 - 21.3 7.1 - 27.8 1.33 - 7.67
Mean 23.8
23.8 23.8
235 + 81.5
Ttast (h) SD 0.375
0.213 0.299
Range 23.1 -24.3 23.5 -
24.1 23.1 -24.3 142 - 360
-29-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
Mean
H.4 2.6
10.6 + 2.9 11.1 + 2.6 127 + 84.6
T1/2 (h) SD
Range
8.46 - 14.9 8.01 - 143 8.01 - 14.9 48.2 -306
5.3.1.2. Other Pharmacolcinetic Parameters for Full PK Profile
100991 A summary of other PK parameters is provided in Table 10. The mean
AUCtast was
2370 ng*h/mL, which included PK samples from follow-up visits when available.
The
AUCmf from the same data set was 3155 ng*h/mL and the median (range)
extrapolated area
was 19.9 % (range 10.6-40.5%). This relatively high level of extrapolated area
in some
subjects suggests that the parameters calculated from kz (such as half-life,
MRTinf, clearance,
and volume of distribution) should be analyzed with caution and may have
higher inherent
variance in their calculation.
101001 The mean normalized volume of distribution (Vz/F) of 16.3 LAcg was well
above total
blood volume for the mean body weight of 72.3 kg, indicating that the drug
distributes out of
plasma into surrounding tissues.
101011 Body weight normalized clearance (CL/F) was 97.5 mL/h/kg (equivalent to
approximately 1.6 mL/min/kg). This value is plasma clearance, not blood
clearance;
however, even adjusting for hematocrit, it is well below total hepatic blood
flow of 17
tnL/min/kg (Carlisle et at, Gut 1992, 33:92-97), suggesting a low extraction
drug.
Table 10. Pharmacokinetic Parameters (Full PK Data Set)
Parameter
20 mg Compound A Full PK Profile
Data Set (N=16)
Mean SD
Range
AUCiast (ng*h/mL) 2370 680
1583 - 4400
AUCini (ng*h/mL) 3155 1341
1923 - 7393
Vz/F normalized (L/kg) 16.3 9.06
6.4- 33.8
tin (1) 127
84.6 482- 306
NWT( h) 77.4 23.7
48.2 - 122
MRTfrif (h) 102
+ 84.8 33 - 304
CL/F normalized (mL/h/kg) 97.5 25.7 40.3 - 136
5.3.2. Pharmacokinetic Conclusions
101021 Compound A was slowly absorbed after a 20 mg oral dose with median peak
plasma
concentrations occurring approximately 8 hours after administration. Upon
absorption, it
-30-
CA 03159436 2022-5-25
WO 2021/113757
PCT/US2020/063471
distributed out of plasma into surrounding tissues and was slowly cleared from
systemic
circulation at rates well below hepatic blood flow, indicating minimal hepatic
extraction
(metabolism). It exhibited a mean half-life of 127 h (range 48.2-306 h) and
mean residence
time of 102 h (range 33-304 h) which may be an underestimation since a number
of subjects
had %AUCextrap values above 20% and as high as 40%.
101031 Washout between periods was not long enough to allow Compound A levels
fall
below the limit of quantitation in subjects who received placebo in Period 2
(mean 3.1
ng/mL, range 1.3-6.8 ng/mL).
* * * * *
101041 All of the U.S. patents, U.S. patent application publications, U.S.
patent applications,
foreign patents, foreign patent applications, and non-patent publications
referred to in this
specification, including U.S. Provisional Application Nos. 62/945,093, filed
December 6,
2019 and 62/948,010, filed December 13, 2019, are incorporated herein by
reference in their
entireties.
101051 Although the foregoing compositions, methods, and uses have been
described in some
detail to facilitate understanding, it will be apparent that certain changes
and modifications
may be practiced within the scope of the appended claims. Accordingly, the
described
embodiments are to be considered as illustrative and not restrictive, and the
claimed invention
is not to be limited to the details given herein, but may be modified within
the scope and
equivalents of the appended claims.
-31-
CA 03159436 2022-5-25