Note: Descriptions are shown in the official language in which they were submitted.
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
COMPOSITIONS AND METHODS FOR TREATING DISORDERS AMELIORATED
BY MUSCARINIC RECEPTOR ACTIVATION
[0001] This application claims the benefit of priority of United States
Provisional Patent
Application Serial No. 62/936,837 filed November 18, 2019, and also claims the
benefit of
priority of United States Provisional Patent Application Serial No. 63/030,780
filed May 27,
2020, the disclosures of which are each incorporated by reference in their
entireties for all
purposes.
[0002] The present disclosure relates to compositions and their application as
pharmaceuticals for treating disorders ameliorated by activating muscarinic
receptors in a
human or animal subject.
[0003] Schizophrenia affects about 0.5 to 1% of the population. The disease is
characterized
by a set of symptoms divided into positive symptoms (e.g., hallucinations,
delusional
thoughts, etc.), negative symptoms (e.g., social isolation, anhedonia, etc.),
and cognitive
symptoms (e.g., inability to process information, poor working memory, etc.).
Patients who
suffer from schizophrenia experience a major decline in quality of life. They
are at increased
risk for mortality due to many factors, such as an increased suicide rate. The
cost of
schizophrenia to society is high, as people living with schizophrenia are much
more likely to
be incarcerated, homeless, or unemployed.
[0004] Existing treatments for schizophrenia rely upon dopamine and serotonin
receptors, as
was the case with the first antipsychotic, chlorpromazine, discovered in 1952.
For more than
60 years, the same fundamental pharmacology has been the standard of care in
schizophrenia.
Current antipsychotics are only efficacious toward positive symptoms, leaving
negative and
cognitive symptoms untreated. Alzheimer's disease is another therapeutic area.
It has proven
extremely difficult to develop new therapies, with a success rate of only 0.4%
for molecules
that enter clinical development and receive marketing approval. Patients in
these areas
desperately need new treatments, but development has been extremely difficult
despite
substantial efforts from scientists and drug developers worldwide.
[0005] Activating the muscarinic system through muscarinic agonists may treat
several
diseases, such as schizophrenia, Alzheimer's disease, Parkinson's disease,
depression,
movement disorders, drug addiction, pain, and neurodegeneration, such as
tauopathies or
synucleinopathies. Muscarinic cholinergic receptors are G-protein coupled
receptors with five
different receptor subtypes (M1-M5), each of which is found in the CNS with
different tissue
distributions. M1 and M4 subtypes have been of interest as therapeutic targets
for various
1
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
diseases. For instance, mood stabilizers lithium and valproic acid, used for
treating bipolar
depression, may affect the muscarinic system, particularly through the M4
subtype receptor.
Genetic evidence directly links the muscarinic system and alcohol addiction.
[0006] In a double-blind placebo-controlled trial of schizophrenic patients
using xanomeline,
a muscarinic cholinergic receptor agonist with preferential activity at the M1
and M4 subtype
receptors, schizophrenia was alleviated. However, because xanomeline is also
bound to
muscarinic receptors outside the brain, it has many serious side effects,
including GI side
effects, cardiac side effects, and hypersalivation. Dose-limited adverse
events were
problematic and led to very high discontinuation rates (including a 56%
dropout rate in a 26-
week study of Alzheimer's disease) and eventually to discontinuation of
xanomeline
development. Despite the early promise, xanomeline development halted for more
than 15
years. Many companies attempted and failed to develop muscarinic receptor
agonists for
CNS disorders, which avoided these unacceptable side effects, but no such
agonist has
reached the market. Past development efforts focused on medicinal chemistry to
develop
molecules that would be more tolerable, typically selecting the M1 and M4
subtypes over the
M2 and M3 muscarinic receptor subtypes. However, M1 and M4 activation outside
the brain
may still cause muscarinic related intolerance. Very little progress has been
made to mitigate
adverse effects due to the activation of peripheral muscarinic receptors.
[0007] There remains a need in the art for a pharmaceutical composition with
increased
tolerability for xanomeline, especially to treat cognitive and psychotic
disorders. The
following embodiments and aspects thereof are described and illustrated with
compositions
and methods, which are meant to be exemplary and illustrative, not limiting in
scope. In
various embodiments, one or more of the above-described problems have been
reduced or
eliminated, while other embodiments are directed to other improvements.
[0008] Provided herein is a method of treating schizophrenia or a disease
related to
schizophrenia in a patient in need thereof, the method comprising: orally
administering to the
patient twice daily an oral pharmaceutical composition comprising a plurality
of xanomeline
beads comprising xanomeline or a salt thereof, and a plurality of trospium
beads comprising a
salt of trospium, via a titration scheme that comprises up-titration of the
xanomeline, or a salt
thereof, and the salt of trospium.
[0009] Also provided is a method of treating schizophrenia or a disease
related to
schizophrenia in a patient in need thereof, the method comprising orally
administering to the
patient twice daily an oral pharmaceutical composition comprising a plurality
of xanomeline
2
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
beads comprising xanomeline or a salt thereof, and a plurality of trospium
beads comprising a
salt of trospium, via a titration scheme that comprises up-titration of the
xanomeline, or a salt
thereof, and the salt of trospium until an amount equivalent to 125 mg
xanomeline free base
and an amount equivalent to 30 mg trospium chloride is administered.
[0010] The present disclosure further provides a method of treating acute
psychosis in a
patient in need thereof. The method comprises orally administering to the
patient twice daily
an oral pharmaceutical composition comprising xanomeline or a salt thereof,
and a salt of
trospium, to achieve at least a 10 point mean reduction in total Positive and
Negative
Syndrome Scale (PANSS) score compared to placebo.
[0011] Further aspects and advantages will be apparent to those of ordinary
skill in the art
from a review of the following detailed description. While the dosage form,
method of
making, and treatment method are susceptible of embodiments in various forms,
the
description hereafter includes specific embodiments to understand that the
disclosure is
illustrative and is not intended to limit the disclosure to the specific
embodiments described
herein.
BRIEF DESCRIPTION OF THE DRAWINGS
[0012] The disclosure will be readily understood by the following detailed
description in
conjunction with the accompanying drawings, wherein like reference numerals
designate like
structural elements. The drawings provide exemplary embodiments or aspects of
the
disclosure and do not limit the scope of the disclosure.
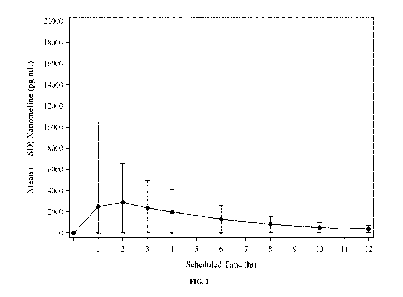
[0013] FIG. 1 depicts the mean ( standard deviation) xanomeline
pharmacokinetic
concentrations on Day 1 for KarXT 50/20 twice-daily treatment cohort of the
KAR-003
pharmacokinetic population.
[0014] FIG. 2 depicts the mean ( standard deviation) xanomeline
pharmacokinetic
concentrations by treatment on Day 3 for KarXT twice-daily treatment for all
cohorts of the
KAR-003 pharmacokinetic population.
[0015] FIG. 3 depicts the mean ( standard deviation) xanomeline
pharmacokinetic
concentrations by treatment on Day 7 for KarXT 100/20, 125/40, and 150/40
twice-daily
treatment cohorts of the KAR-003 pharmacokinetic population.
[0016] FIG. 4 depicts the mean ( standard deviation) xanomeline
pharmacokinetic
concentrations by treatment and visit for the KAR-003 pharmacokinetic
population.
[0017] FIG. 5 depicts the mean ( standard deviation) xanomeline
pharmacokinetic trough
concentrations by treatment for the KAR-003 pharmacokinetic population.
3
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
[0018] FIG. 6 depicts the mean ( standard deviation) trospium
pharmacokinetics
concentrations on Day 1 for the KarXT 50/20 twice-daily treatment cohort of
the KAR-003
pharmacokinetics population.
[0019] FIG. 7 depicts the mean ( standard deviation) trospium
pharmacokinetics
concentrations by treatment on Day 3 for the KAR-003 pharmacokinetics
population.
[0020] FIG. 8 depicts the mean ( standard deviation) trospium
pharmacokinetics
concentrations by treatment on Day 7 for the KAR-003 pharmacokinetics
population.
[0021] FIG. 9 depicts the mean ( standard deviation) trospium pharmacokinetic
concentrations by treatment and visit for the KAR-003 pharmacokinetic
population.
[0022] FIG. 10 depicts the mean ( standard deviation) trospium
pharmacokinetic trough
concentrations by treatment and visit for the KAR-003 pharmacokinetic
population.
[0023] FIG. 11 depicts total PANSS score change from baseline (LS mean
difference) of
subjects in the modified intent-to-treat (mITT) population of KAR-004 Phase II
study versus
time in weeks (***p<0.0001).
[0024] FIG. 12 depicts PANSS-positive subscore change from baseline (LS mean
difference)
of subjects in the mITT population of KAR-004 Phase II study versus time in
weeks
(***p<0.0001).
[0025] FIG. 13 depicts PANSS-negative subscore change from baseline (LS mean
difference)
of subjects in the mITT population of KAR-004 Phase II study versus time in
weeks
(*p<0.05, **1)0.001).
[0026] FIG. 14 depicts the PANS S Marder Factor score of subjects in the mITT
population of
KAR-004 Phase II study versus Visit day.
[0027] FIG. 15 depicts the statistically significant and clinically meaningful
improvement on
Clinical Global Impression-Severity (CGI-S) at baseline for patients on KarXT
versus
placebo.
[0028] FIG. 16 depicts the statistically significant and clinically meaningful
improvement on
CGI-S at the endpoint of Week 5 for patients on KarXT versus placebo.
[0029] FIG. 17 depicts that the rates of adverse events related to muscarinic
receptor agonism
(nausea and vomiting) decreased over time in KarXT-treated patients.
[0030] FIG. 18 depicts that the rates of a peripheral anticholinergic adverse
event (dry
mouth) decreased over time in KarXT-treated patients.
[0031] FIG. 19 depicts a box plot of standing heart rate (beats per mm, bpm)
from the KarXT
safety population plotted by the visit.
4
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
[0032] FIG. 20 depicts a box plot of orthostatic heart rate (beats per mm,
bpm) from the
KarXT safety population plotted by the visit.
[0033] FIG. 21 depicts a box plot of orthostatic diastolic pressure (mmHg)
from the KarXT
safety population plotted by the visit.
[0034] FIG. 22 depicts a box plot of the orthostatic systolic pressure (mmHg)
from the
KarXT safety population plotted by the visit.
DETAILED DESCRIPTION
[0035] Earlier development of xanomeline, a muscarinic receptor agonist, was
halted due to
peripheral cholinergic side effects. The current disclosure provides a dosage
form with
dissolution kinetics having a more effective therapeutic effect for both
active ingredients,
enhanced pharmacokinetics for trospium chloride, and greater dosing
compliance. The
current disclosure also provides dosage forms with different strengths or
different ratios of
the two actives.
[0036] Provided herein are the following specific embodiments:
[0037] Embodiment 1: A method of treating schizophrenia or a disease related
to
schizophrenia in a patient in need thereof, the method comprising: orally
administering to the
patient twice daily an oral pharmaceutical composition comprising a plurality
of xanomeline
beads comprising xanomeline or a salt thereof, and plurality of trospium beads
comprising a
salt of trospium, via a titration scheme that comprises up-titration of the
xanomeline, or a salt
thereof, and the salt of trospium.
[0038] Embodiment 2: A method of treating schizophrenia or a disease related
to
schizophrenia in a patient in need thereof, the method comprising: orally
administering to the
patient for at least five weeks twice daily an oral pharmaceutical composition
comprising a
plurality of xanomeline beads comprising xanomeline or a salt thereof, and
plurality of
trospium beads comprising a salt of trospium, wherein at least one adverse
event which
occurred at the start of oral administration is reduced to its pretreatment
level after five weeks
of treatment.
[0039] Embodiment 3: The method of Embodiment 1 or 2, wherein the
administration
occurs via a titration scheme that comprises up-titration of the xanomeline,
or the salt thereof,
and the salt of trospium until an amount equivalent to 125 mg xanomeline free
base and an
amount equivalent to 30 mg trospium chloride is administered twice daily.
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
[0040] Embodiment 4: The method of Embodiment 1 or 2, wherein the
administration
occurs via a titration scheme that comprises up-titration of the xanomeline,
or the salt thereof,
and the salt of trospium until an amount equivalent to 150 mg xanomeline free
base and an
amount equivalent to 30 mg trospium chloride is administered twice daily.
[0041] Embodiment 5: The method of Embodiment 1 or 2, wherein the
administration
occurs via a titration scheme that comprises up-titration of the xanomeline,
or the salt thereof,
and the salt of trospium until an amount equivalent to 175 mg xanomeline free
base and an
amount equivalent to 30 mg trospium chloride is administered twice daily.
[0042] Embodiment 6: The method of Embodiment 1 or 2, wherein the
administration
occurs via a titration scheme that comprises up-titration of the xanomeline,
or the salt thereof,
and the salt of trospium until an amount equivalent to 175 mg xanomeline free
base and an
amount equivalent to 40 mg trospium chloride is administered twice daily.
[0043] Embodiment 7: The method of any one of the proceeding Embodiments,
wherein the
patient has a diagnosis of schizophrenia.
[0044] Embodiment 8: The method of any one of the proceeding Embodiments,
wherein
prior to administration of the oral pharmaceutical composition, the patient
had a Clinical
Global Impression Severity Scale (CGI-S) score of 4-7, and after
administration the patient
had a CGI-S score equal to 1 or 2.
[0045] Embodiment 9: The method of any one of the proceeding Embodiments,
wherein the
xanomeline, or the salt thereof, is administered for a first period in a first
amount and then the
first amount is increased to a second amount.
[0046] Embodiment 10: The method of Embodiment 9, wherein the first amount of
xanomeline, or the salt thereof, is equivalent to 50 mg xanomeline free base.
[0047] Embodiment 11: The method of Embodiment 9 or 10, wherein the first
period for the
xanomeline administration is between 1 and 5 days.
[0048] Embodiment 12: The method of Embodiment 11, wherein the first period
for the
xanomeline administration is 2 days.
[0049] Embodiment 13: The method of any one of Embodiments 9 to 12, wherein
the
second amount of xanomeline, or the salt thereof, is equivalent to 100 mg
xanomeline free
base.
[0050] Embodiment 14: The method of any one of Embodiments 9 to 13, further
comprising
administering the xanomeline, or the salt thereof, for a second period in the
second amount
and then increasing the second amount to a third amount.
6
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
[0051] Embodiment 15: The method of Embodiment 14, wherein the second period
for
xanomeline administration is between three days and a week.
[0052] Embodiment 16: The method of Embodiment 14 or 15, wherein the third
amount of
xanomeline, or the salt thereof, is equivalent to 125 mg xanomeline free base.
[0053] Embodiment 17: The method of any of the preceding Embodiments, wherein
the salt
of trospium is administered for a first time period in a first amount and the
first amount is
increased to a second amount.
[0054] Embodiment 18: The method of Embodiment 17, wherein the first amount of
the salt
of trospium is equivalent to 20 mg trospium chloride.
[0055] Embodiment 19: The method of Embodiment 17 or 18, wherein the first
time period
for trospium administration is at least a week.
[0056] Embodiment 20: The method of any one of Embodiments 15 to 17, wherein
the
second amount of the salt of trospium is equivalent to 30 mg trospium
chloride.
[0057] Embodiment 21: The method of any one of the preceding Embodiments, at
least one
of vomiting, nausea and dry mouth which occurred at the start of oral
administration is
reduced to its pretreatment level after five weeks of treatment.
[0058] Embodiment 22: The method of any one of the preceding Embodiments,
wherein the
xanomeline, or the salt thereof, and the salt of trospium are administered
without inducing a
heart rate increase of more than about 5 beats per minute.
[0059] Embodiment 23: The method of any one of the preceding Embodiments,
wherein the
xanomeline, or the salt thereof, and the salt of trospium are administered
without inducing
syncope.
[0060] Embodiment 24: The method of any one of the preceding Embodiments,
wherein the
xanomeline, or the salt thereof, and the salt of trospium are administered
without inducing a
change in diastolic blood pressure of more than about 5 mmHg.
[0061] Embodiment 25: The method of any one of the preceding Embodiments,
wherein the
xanomeline, or the salt thereof, and the salt of trospium are administered
without inducing a
change in systolic blood pressure of more than about 5 mmHg.
[0062] Embodiment 26: The method of any one of the preceding Embodiments,
wherein the
xanomeline, or the salt thereof, and the salt of trospium are administered
without causing a
severe adverse event.
7
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
[0063] Embodiment 27: The method of any one of the preceding Embodiments,
wherein the
xanomeline, or the salt thereof, and the salt of trospium are administered
without causing a
severe adverse event related to heart rate.
[0064] Embodiment 28: The method of any one of the preceding Embodiments,
wherein the
xanomeline, or the salt thereof, and the salt of trospium are administered
without causing a
severe adverse event related to heart rate change.
[0065] Embodiment 29: The method of any one of the preceding Embodiments,
wherein the
xanomeline, or the salt thereof, and the salt of trospium are administered
without causing a
severe adverse event related to blood pressure.
[0066] Embodiment 30: The method of any one of the preceding Embodiments,
wherein the
xanomeline, or the salt thereof, and the salt of trospium are administered
without causing a
severe adverse event related to blood pressure change.
[0067] Embodiment 31: The method of any one of the preceding Embodiments,
wherein the
xanomeline, or the salt thereof, and the salt of trospium are administered
without increasing a
liver function test (LFT).
[0068] Embodiment 32: The method of any one of the preceding Embodiments,
wherein the
Positive and Negative Syndrome Scale (PANSS) total score for the patient
decreases by at
least 10 points compared to placebo after five weeks of treatment.
[0069] Embodiment 33: The method of any one of the preceding Embodiments,
wherein the
PANSS positive subscore decreases by at least 3 points compared to placebo
after five weeks
of treatment.
[0070] Embodiment 34: The method of any one of the preceding Embodiments,
wherein the
PANSS negative subscore decreases by at least 2 points compared to placebo
after five weeks
of treatment.
[0071] Embodiment 35: The method of any one of the preceding Embodiments,
wherein the
size of the xanomeline beads is between 0.425 mm and 1.18 mm.
[0072] Embodiment 36: The method of any one of the preceding Embodiments,
wherein the
size of the xanomeline beads is between 0.6 mm and 0.85 mm.
[0073] Embodiment 37: The method of any one of the preceding Embodiments,
wherein the
size of the trospium beads is between 0.425 mm and 1.18 mm.
[0074] Embodiment 38: The method of any one of the preceding Embodiments,
wherein the
size of the trospium beads is between 0.6 mm and 0.85 mm.
8
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
[0075] Embodiment 39: The method of any one of the preceding Embodiments,
wherein the
xanomeline beads contain about 2.5 times as much xanomeline free base as the
trospium
beads contain trospium salt.
[0076] Embodiment 40: The method of any one of the preceding Embodiments, the
plurality
of xanomeline and the plurality of trospium beads having a dissolution rate of
more than
about 95% within about the first 45 minutes following entry of the dosage form
into an
aqueous solution.
[0077] Embodiment 41: The method of Embodiment 40, having a dissolution rate
of more
than about 95% within about the first 20 minutes following entry of the dosage
form into an
aqueous solution.
[0078] Embodiment 42: The method of any one of the preceding Embodiments,
wherein the
salt of xanomeline is xanomeline tartrate.
[0079] Embodiment 43: The method of Embodiment 42, wherein the xanomeline
beads
comprise between 30 wt.% and 80 wt.% xanomeline tartrate.
[0080] Embodiment 44: The method of any Embodiment 43, wherein the xanomeline
beads
comprise 66 wt.% xanomeline tartrate.
[0081] Embodiment 45: The method of any one of the preceding Embodiments,
wherein the
xanomeline beads comprise between 15 wt.% and 65 wt.% microcrystalline
cellulose.
[0082] Embodiment 46: The method of Embodiment 45, wherein the xanomeline
beads
comprise 33.5 wt.% microcrystalline cellulose.
[0083] Embodiment 47: The method of any one of the preceding Embodiments,
wherein the
xanomeline beads comprise between 0 wt.% and 2 wt.% talc.
[0084] Embodiment 48: The method of Embodiment 46, wherein the xanomeline
beads
comprise 0.5 wt.% talc.
[0085] Embodiment 49: The method of any one of the preceding Embodiments,
wherein the
xanomeline beads comprise between 30 wt.% and 80 wt.% xanomeline tartrate,
between 15
wt.% and 65 wt.% microcrystalline cellulose, and between 0 wt.% and 2 wt.%
talc.
[0086] Embodiment 50: The method of Embodiment 49, wherein the xanomeline
beads
comprise 66 wt.% xanomeline tartrate, 33.5 wt.% microcrystalline cellulose,
and 0.5 wt.%
talc.
[0087] Embodiment 51: The method of any one of the preceding Embodiments,
wherein the
salt of trospium is trospium chloride.
9
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
[0088] Embodiment 52: The method of Embodiment 51, wherein the trospium beads
comprise between 8 wt.% and 35 wt.% trospium chloride.
[0089] Embodiment 53: The method of Embodiment 52, wherein the trospium beads
comprise 17.7 wt.% trospium chloride.
[0090] Embodiment 54: The method of any one of the preceding Embodiments,
wherein the
trospium beads comprise between 25 wt.% and 80 wt.% microcrystalline
cellulose.
[0091] Embodiment 55: The method of Embodiment 54, wherein the trospium beads
comprise 46.8 wt.% microcrystalline cellulose.
[0092] Embodiment 56: The method of any one of the preceding Embodiments,
wherein the
trospium beads comprise between 15 wt.% and 70 wt.% lactose monohydrate.
[0093] Embodiment 57: The method of Embodiment 56, wherein the trospium beads
comprise 35 wt.% lactose monohydrate.
[0094] Embodiment 58: The method of any one of the preceding Embodiments,
wherein the
trospium beads comprise between 0 wt.% and 2 wt.% talc.
[0095] Embodiment 59: The method of Embodiment 58, wherein the trospium beads
comprise 0.5 wt.% talc.
[0096] Embodiment 60: The method of any one of the preceding Embodiments,
wherein the
trospium beads comprise between 8 wt.% and 35 wt.% trospium chloride, between
25 wt.%
and 80 wt.% microcrystalline cellulose, between 15 wt.% and 70 wt.% lactose
monohydrate,
and between 0 wt.% and 2 wt.% talc.
[0097] Embodiment 61: The method of Embodiment 60, wherein the trospium beads
comprise 17.7 wt.% trospium chloride, 46.8 wt.% microcrystalline cellulose, 35
wt.% lactose
monohydrate, and 0.5 wt.% talc.
[0098] Embodiment 62: The method of any one of the preceding Embodiments,
wherein the
oral pharmaceutical composition further comprises ascorbic acid.
[0099] Embodiment 63: The method of Embodiment 62, wherein the oral
pharmaceutical
composition comprises between 0.2 wt.% and 1 wt.% ascorbic acid.
[0100] Embodiment 64: The method of Embodiment 63, wherein the oral
pharmaceutical
composition comprises about 0.5 wt.% ascorbic acid.
[0101] Embodiment 65: The method of any one of the preceding Embodiments,
wherein the
oral pharmaceutical composition further comprises butylated h3fdroxytoluene.
[0102] Embodiment 66: The method of Embodiment 64, wherein the oral
pharmaceutical
composition comprises between 0.01 wt.% and 0.1 wt.% butylated hydroxytoiuene.
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
[0103] Embodiment 67: The method of Embodiment 66, wherein the oral
pharmaceutical
composition comprises about 0.05 wt.% butylated hydroxytoluene.
[0104] Embodiment 68: The method of any one of the preceding Embodiments,
wherein the
oral pharmaceutical composition further comprises a capsule containing the
plurality of
xanomeline beads and the plurality of trospium beads.
[0105] Embodiment 69: A method of treating acute psychosis in a patient in
need thereof,
the method comprising: orally administering to the patient twice daily an oral
pharmaceutical
composition comprising xanomeline or a salt thereof, and a salt of trospium,
to achieve at
least a 10 point mean reduction in total Positive and Negative Syndrome Scale
(PANSS)
score compared to placebo.
[0106] Embodiment 70: The method of Embodiment 69, wherein at least an 11.6
point mean
reduction in total PANNS score is achieved.
[0107] Embodiment 71: The method of any Embodiment 69 or 70, wherein at least
a 3 point
mean reduction in PANS S positive subscore compared to placebo is achieved.
[0108] Embodiment 72: The method of any one of Embodiments 69 to 71, wherein
at least a
2 point reduction in the PANSS negative subscore compared to placebo is
achieved.
[0109] Embodiment 73: The method of any one of Embodiments 69 to 72, wherein
the
reduction in PANSS score is achieved within about 5 weeks.
[0110] Embodiment 74: The method of any one of Embodiments 69 to 73, wherein
before
administration of the oral pharmaceutical composition, the patient had a
Clinical Global
Impression Severity Scale (CGI-S) score of 4-7, and after administration, the
patient had a
CGI-S score equal to 1 or 2.
[0111] Embodiment 75: The method of any one of Embodiments 69 to 74, wherein
the
patient has a diagnosis of schizophrenia.
[0112] Embodiment 76: The method of any one of Embodiments 69 to 75, wherein
the
xanomeline is xanomeline tartrate and the salt of trospium is trospium
chloride.
[0113] Embodiment 77: The method of any one of Embodiments 69 to 76, at least
one
adverse event which occurred at the start of oral administration is reduced to
its pretreatment
level after five weeks of treatment.
[0114] Embodiment 78: The method of Embodiment 77, wherein at least one
adverse event
is chosen from vomiting, nausea and dry mouth.
11
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
[0115] The articles "a" and "an" refer to one or more than one (i.e., to at
least one) of the
grammatical object of the article. By way of example, "an element" means one
element or
more than one element.
[0116] The terms "comprise" and "comprising" are inclusive, open sense,
meaning that
additional elements may be included.
[0117] The term "consisting" limits the elements to those specified except for
impurities
ordinarily associated in addition to that.
[0118] The term "consisting essentially of' limits those specified elements
and those that do
not materially affect the basic and novel characteristics of the material or
steps.
[0119] All ranges set forth herein include all possible subsets of ranges and
any combinations
of such subset ranges. By default, ranges include the stated endpoints, unless
stated
otherwise, where a range of values is provided, each intervening value between
the upper and
lower limit of that range and any other stated or intervening value in that
stated range is
encompassed within the disclosure. The upper and lower limits of these smaller
ranges may
independently be included in the smaller ranges and encompassed within the
disclosure,
subject to any specifically excluded limit in the stated range. Where the
stated range includes
one or both limits, ranges excluding either or both of those included limits
are also
contemplated to be part of the disclosure.
[0120] The term "wt.%" is the weight percent based on the total weight, e.g.,
of the core, or
enteric coating, or total bead, as described in context. Unless stated
otherwise, the wt.% is
intended to describe the weight percent based on dry weight (e.g., for a core
following
drying).
[0121] The term "controlled release" is defined as a prolonged-release pattern
of one or more
drugs, such that the drugs are released over a period. A controlled release
formulation has
release kinetics that results in measurable serum levels of the drug over a
period longer than
what would be possible following intravenous injection or following
administration of an
immediate release oral dosage form. Controlled release, slow-release,
sustained-release,
extended-release, prolonged-release, and delayed-release have the same
definitions.
[0122] The term "including" means "including but not limited to." "Including"
and
"including but not limited to" are used interchangeably.
[0123] The term "mammal" is known in the art. Exemplary mammals include
humans,
primates, bovines, porcines, canines, felines, and rodents (e.g., mice and
rats).
12
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
[0124] A "patient," "subject," or "host" to be treated by the subject method
means either a
human or non-human mammal.
[0125] The term "pharmaceutically-acceptable carrier" is art-recognized. It
refers to a
pharmaceutically-acceptable material, composition or vehicle, such as a liquid
or solid filler,
diluent, excipient, solvent, or encapsulating material, involved in carrying
or transporting any
subject composition or component thereof from one organ, or portion of the
body, to another
organ, or portion of the body. Each carrier must be "acceptable" in the sense
of being
compatible with the subject composition and its components and not injurious
to the patient.
Some examples of materials that may serve as pharmaceutically acceptable
carriers include
sugars, such as lactose, glucose, and sucrose; starches, such as corn starch
and potato starch;
cellulose and its derivatives, such as sodium carboxymethyl cellulose,
ethylcellulose, and
cellulose acetate; powdered tragacanth; malt; gelatin; talc; excipients, such
as cocoa butter
and suppository waxes; oils, such as peanut oil, cottonseed oil, safflower
oil, sesame oil, olive
oil, corn oil, and soybean oil; glycols, such as propylene glycol; polyols,
such as glycerin,
sorbitol, mannitol, and polyethylene glycol; esters, such as ethyl oleate and
ethyl laurate;
agar; buffering agents, such as magnesium hydroxide and aluminum hydroxide;
alginic acid;
pyrogen-free water; isotonic saline; Ringer's solution; ethyl alcohol;
phosphate buffer
solutions; and other non-toxic compatible substances employed in
pharmaceutical
formulations.
[0126] The term "pharmaceutically-acceptable salt" or "salt" is art-
recognized. It refers to a
salt prepared from relatively nontoxic acids or bases, including inorganic
acids and bases and
organic acids and bases, including, for example, those contained in
compositions of the
present disclosure. Suitable non-toxic acids include inorganic and organic
acids such as
acetic, benzenesulfonic, benzoic, camphorsulfonic, citric, ethenesulfonic,
fumaric, gluconic,
glutamic, hydrobromic, hydrochloric, isethionic, lactic, maleic, malic,
mandelic,
methanesulfonic, mucic, nitric, pamoic, pantothenic, phosphoric, saccharinate,
succinic,
sulfuric, tartaric acid, p-toluenesulfonic, hydrochloric, hydrobromic,
phosphoric, and sulfuric
acids and the like.
[0127] The term "treating" is art-recognized and refers to curing as well as
ameliorating at
least one symptom of any condition or disorder.
[0128] In jurisdictions that forbid the patenting of methods practiced on the
human body, the
meaning of "administering" of a composition to a human subject shall be
restricted to
prescribing a controlled substance that a human subject will self-administer
by any technique
13
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
(e.g., orally, inhalation, topical application, injection, insertion, etc.).
The broadest reasonable
interpretation consistent with laws or regulations defining patentable subject
matter is
intended. In jurisdictions that do not forbid the patenting of methods
practiced on the human
body, the "administering" of compositions includes both methods practiced on
the human
body and the foregoing activities.
[0129] The term "therapeutic agent" is art-recognized and refers to any
chemical moiety that
is a biologically, physiologically, or pharmacologically active substance
acting locally or
systemically in a subject. Examples of therapeutic agents, also referred to as
"drugs," are
described in well-known literature references such as the Merck Index (14th
edition), the
Physicians' Desk Reference (64th edition), and The Pharmacological Basis of
Therapeutics
(12th edition). These therapeutic agents include without limitation
medicaments; vitamins;
mineral supplements; substances used for the treatment, prevention, diagnosis,
cure, or
mitigation of a disease or illness; substances that affect the structure or
function of the body,
or pro-drugs, which become biologically active or more active after they have
been placed in
a physiological environment.
[0130] The term "psychotherapy" refers to non-pharmacological therapies. Those
skilled in
the art use various techniques involving verbal and other interactions with a
patient to affect a
positive therapeutic outcome. Such techniques include, but are not limited to,
behavior
therapy, cognitive therapy, psychodynamic therapy, psychoanalytic therapy,
group therapy,
family counseling, art therapy, music therapy, vocational therapy, humanistic
therapy,
existential therapy, transpersonal therapy, client-centered therapy (also
called person-centered
therapy), Gestalt therapy, biofeedback therapy, rational emotive behavioral
therapy, reality
therapy, response-based therapy, Sandplay therapy, status dynamics therapy,
hypnosis, and
validation therapy. Psychotherapy may involve combining two or more
techniques. A
therapist can select and adjust the techniques based on the individual
patient's needs and the
patient's response.
[0131] The term "muscarinic disorder" refers to any disease or condition
ameliorated by
activating the muscarinic system. Such diseases include ones in which direct
activation of
muscarinic receptors themselves or inhibition of cholinesterase enzymes has
produced a
therapeutic effect.
[0132] The terms "diseases related to schizophrenia" and "disorders related to
schizophrenia"
include, but are not limited to, schizo-affective disorder, psychosis,
including acute psychosis,
delusional disorders, psychosis associated with Alzheimer's disease, psychosis
associated
14
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
with Parkinson's disease, psychotic depression, bipolar disorder, bipolar with
psychosis,
Huntington's disease, Lewy Body dementia, or any other disease with psychotic
features.
[0133] "Psychosis" refers to an abnormal condition of the mind that results in
difficulties
determining what is real and not. Symptoms of psychosis include, but are not
limited to, false
beliefs (delusions), seeing or hearing things that others do not see or hear
(hallucinations),
incoherent speech, behavior that is inappropriate for the situation, sleep
problems, social
withdrawal, lack of motivation, and difficulties carrying out daily
activities.
[0134] "Acute psychosis" refers to the quick or strong onset of psychotic
symptoms in a
patient, for example, as defined at "Acute and Transient Psychotic Disorder"
(International
Classification of Diseases-10) and "Brief Psychosis"(DSM-IV). A sharp striking
delusion
with quick changes in the structure occurs in the individual who has acute
psychosis after a
short preliminary period of anxiety, insomnia, and confusion. Acute psychosis
can include
acute psychotic exacerbation, when a patient may respond to hallucinations or
delusions.
Acute psychosis lasts for a short time, typically from one to two weeks.
[0135] The term "activator" means a molecule described as an agonist, partial
agonist, co-
agonist, physiological agonist, potentiator, stimulator, allosteric
potentiator, positive allosteric
modulator, allosteric agonist, or a molecule that increases the activity or
signaling of
receptors directly or indirectly.
[0136] The term "inhibitor" means a molecule described as an antagonist,
partial antagonist,
competitive antagonist, non-competitive antagonist, uncompetitive antagonist,
silent
antagonist, inverse agonist, reversible antagonist, physiological antagonist,
irreversible
antagonist, inhibitor, reversible inhibitor, irreversible inhibitor, negative
allosteric modulator,
allosteric antagonist, or a molecule that decreases the activity or signaling
of receptors
directly or indirectly.
[0137] As used herein, an "adverse event" is any untoward medical occurrence
associated
with treatment with a pharmaceutical composition described herein. A "mild
adverse event"
is easily tolerated by the subject, causes minimal discomfort, and does not
interfere with
everyday activities. A "moderate adverse event" is sufficiently discomforting
to interfere with
everyday activities; intervention may be needed. A "severe adverse event"
prevents everyday
activities; treatment or other intervention is usually needed. A "serious
adverse event" results
in death; is life-threatening (immediate risk of death from the event as it
occurred); requires
or prolongs inpatient hospitalization; results in persistent or significant
disability/incapacity;
or results in a congenital anomaly/disability, cancer, or drug overdose. An
adverse event is
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
incapacitating or disabling if it results in a substantial or permanent
disruption of the subject's
ability to carry out normal life functions.
[0138] As used herein, a patient is said to "tolerate" a dose of a compound if
administering
that dose to that patient does not result in an unacceptable adverse event or
an unacceptable
combination of adverse events. One of skill in the art will appreciate that
tolerance is a
subjective measure and that what may be tolerable to one patient may not be
tolerable to a
different patient. For example, one patient may not be able to tolerate a
headache. In contrast,
a second patient may find headache tolerable but is not able to tolerate
vomiting. For a third
patient, either headache alone or vomiting alone is tolerable. Still, the
patient cannot tolerate
the combination of headache and vomiting, even if the severity of each is less
than when
experienced alone.
[0139] The term "maximum tolerated dose" means the highest dose of a drug or
therapeutic
that a patient can take without the patient experiencing intolerable side
effects. The maximum
tolerated dose is typically determined empirically in clinical trials.
[0140] The term "muscarinic receptors" refers to G-protein linked receptors
that bind the
neurotransmitter acetylcholine. To date, five subtypes of the muscarinic
receptor have been
identified. "Ml" means the subtype one muscarinic receptor. "M2" means the
subtype two
muscarinic receptor. "M3" means the subtype three muscarinic receptor. "M4"
means the
subtype four muscarinic receptor. "M5" means the subtype five muscarinic
receptor.
[0141] The term "antipsychotic" refers to a drug that diminishes psychosis,
hallucinations, or
delusions. Antipsychotics include, but are not limited to haloperidol,
droperidol,
chlorpromazine, fluphenazine, perphenazine, prochlorperazine, thioridazine,
trifluoperazine,
mesoridazine, periciazine, promazine, triflupromazine, levomepromazine,
promethazine,
pimozide, chlorprothixene, flupenthixol, thiothixene, zuclopenthixol,
clozapine, olanzapine,
risperidone, quetiapine, ziprasidone, amisulpride, asenapine, paliperidone,
zotepine,
aripiprazole, bifeprunox, and tetrabenazine.
[0142] The term "anxiolytics" refers to drugs that reduce anxiety, fear,
panic, or related
feelings. Such drugs include, but are not limited to, benzodiazepines (e.g.,
alprazolam,
chlordiazepoxide, clonazepam, clorazepate, diazepam, lorazepam), buspirone,
barbiturates
(e.g., amobarbital, pentobarbital, secobarbital, phenobarbital), and
hydroxyzine.
[0143] The term "anti-depressants" refers to drugs that alleviate depression
and related
conditions (e.g., dysthymia). Such drugs include, but are not limited to,
selective serotonin-
reuptake inhibitors (SSRIs, e.g., citalopram, escitalopram, fluoxetine,
fluvoxamine,
16
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
paroxetine, sertraline), serotonin-norepinephrine reuptake inhibitors (SNRIs,
e.g.,
desvenlafaxine, duloxetine, milnacipran, venlafaxine), mianserin, mirtazapine,
norepinephrine reuptake inhibitors (e.g., atomoxetine, mazindol, reboxetine,
viloxazine),
bupropion, tianeptine, agomelatine, tricyclic antidepressants (e.g.,
amitriptyline,
clomipramine, doxepin, imipramine, trimipramine, desipramine, nortriptyline,
protriptyline),
and monoamine oxidase inhibitors (e.g., isocarboxazid, moclobemide,
phenelzine, selegiline,
tranylcypromine).
[0144] The terms "sedatives" or "tranquilizers" refer to drugs that induce
somnolence,
promote a feeling of being tired or desire to sleep, or promote a state of
unconsciousness.
Such drugs include, but are not limited to, benzodiazepines, barbiturates
(e.g., amobarbital,
pentobarbital, secobarbital, phenobarbital), eszopiclone, zaleplon, zolpidem,
and zopiclone.
Pharmaceutical Compositions
[0145] Provided herein is an oral pharmaceutical composition, comprising a
plurality of
xanomeline beads comprising xanomeline or a salt thereof; and a plurality of
trospium beads
comprising a salt of trospium. In certain embodiments, the salt of trospium is
chosen from
trospium chloride, trospium bromide, trospium iodide, and trospium
saccharinate.
[0146] In certain embodiments, the plurality of xanomeline beads has a core
comprising
xanomeline or a salt thereof. In certain embodiments, the plurality of
trospium beads has a
core comprising a trospium salt.
[0147] In certain embodiments, a capsule shell comprising hydroxypropyl
methylcellulose
(HPMC) containing separate populations of drug beads containing xanomeline
tartrate or
trospium chloride wherein the drug beads are of comparable size and release
the actives
rapidly and at substantially similar rates. Following the dissolution of the
capsule shell in the
stomach, the drug beads may dissolve in the stomach or pass through the
pyloric valve into
the duodenum intact or partially intact. Still, the two drugs ratio, both in
dissolved form and
in undissolved form, remains relatively constant in the gastrointestinal tract
until the drugs
are absorbed.
[0148] The formulation for each drug bead allows substantially similar
performance from
two actives at different dose ranges. The actives are released into the blood
serum at
substantially similar rates or achieve a substantially similar T.. In certain
embodiments, a
capsule comprises 50 mg xanomeline as the tartrate salt and 10 mg trospium
chloride. Fifty
mg xanomeline as free base corresponds to about 76 mg xanomeline tartrate.
17
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
[0149] A discrepancy in the number of drug beads in the capsule increases the
probability
that the drug beads ratio would not remain substantially constant after the
beads are released
and disperse. Thus, in certain embodiments, the trospium beads are formulated
with a lower
drug load. Effective doses of trospium and xanomeline are contained in roughly
equivalent
numbers of beads. Despite the differences in drug loads in certain
embodiments, the trospium
and xanomeline beads release at roughly similar rates. For example, if the
dissolution of the
capsules is assessed using a United States Pharmacopeia (USP) dissolution
apparatus, the
percentage of xanomeline dissolved is substantially equivalent to the
percentage of dissolved
trospium chloride, such as at 10 mm, 20 mm, or 30 mm.
[0150] The medicament may also include one or more pharmaceutically acceptable
salts. The
medicament may include one or more pharmaceutically-acceptable carriers. The
medicament
may be administered orally. The medicament may be delivered orally using
tablets, troches,
liquids, emulsions, suspensions, drops, capsules, caplets or gel caps, and
other methods of
oral administration known to one skilled in the art.
[0151] The medicament may be in a dosage form that immediately releases the
drug. In an
alternative embodiment, the medicament may have a controlled release dosage
form.
[0152] The medicament may be in dosage forms that use other controlled-release
formulations known to one in the art.
[0153] In another embodiment, the medicament is combined with one or more
therapies,
including psychotherapy and drugs. Therapeutic agents include, but are not
limited, to
antipsychotics, anxiolytics, anti-depressants, sedatives, tranquilizers,
analgesics, and other
pharmacological interventions known to one skilled in the art. A therapeutic
agent may fall
under the category of more than one drug. For instance, benzodiazepines can be
considered
anxiolytics, sedatives, and tranquilizers.
Bead / Core Excipients
[0154] The bead or core can comprise one or more excipients. In one
embodiment, the
excipients include one or more fillers, binders, and surfactants. Other
optional ingredients
include, but are not limited to, glidants, lubricants, disintegrants, swelling
agents, and
antioxidants. The xanomeline or a pharmaceutically acceptable salt thereof and
the salt of
trospium may be in separate matrices within the same medicament.
[0155] The amount of xanomeline free base in the core can be at least 10 wt.%
or at least 15
wt.%, or at least 20 wt.%, or at least 25 wt.%, or at least 30 wt.%. For
example, the amount of
18
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
xanomeline tartrate can be at least 50 wt.%, or at least 55 wt.%, or at least
60 wt.%, or at least
65 wt.%, or at least 70 wt.%, or at least 75 wt.%, or at least 80 wt.%, or at
least 85 wt.% of
the core, in a range of about 60 wt.% to about 90 wt.% or about 65 wt.% to
about 85 wt.%. It
is understood that all ranges including these values as endpoints are
contemplated, for
example, at least between about 15 wt.% and about 90 wt.%, between about 20
wt.% and
about 85 wt.%, between about 30 wt.% and about 85 wt.%, or between about 50
wt.% and
about 90 wt.%. In certain embodiments, the xanomeline beads comprise between
30 wt.%
and 80 wt.% xanomeline tartrate, such as 66 wt.% xanomeline tartrate.
[0156] The amount of trospium salt in the core can be at least 10 wt.% or at
least 15 wt.%, or
at least 20 wt.%, or at least 25 wt.%, or at least 30 wt.%. For example, the
amount of
trospium chloride can be at least 50 wt.%, or at least 55 wt.%, or at least 60
wt.%, or at least
65 wt.%, or at least 70 wt.%, or at least 75 wt.%, or at least 80 wt.%, or at
least 85 wt.% of
the core, in a range of about 60 wt.% to about 90 wt.% or about 65 wt.% to
about 85 wt.%. It
is understood that all ranges including these values as endpoints are
contemplated, for
example, at least between about 15 wt.% and about 90 wt.%, between about 20
wt.% and
about 85 wt.%, between about 30 wt.% and about 85 wt.%, or between about 50
wt.% and
about 90 wt.%. In certain embodiments, the trospium is trospium chloride. In
certain
embodiments, the trospium beads comprise between 8 wt.% and 35 wt.% trospium
chloride,
such as 17.7 wt.% trospium chloride.
[0157] In a further embodiment, the matrix comprises a polymer, for example,
to modify the
release profile of the active in the matrix. In a further embodiment, the
polymer comprises a
water-soluble polymer. In a further embodiment, the water-soluble polymer is
selected from
EudragitTM RL, polyvinyl alcohol, polyvinylpyrrolidone, methylcellulose,
hydroxypropyl
cellulose, hydroxypropylmethylcellulose, polyethylene glycol, and mixtures
thereof. In a
further embodiment, the polymer comprises a water-insoluble polymer. In a
further
embodiment, the water-insoluble polymer is selected from EudragitTM RS,
ethylcellulose,
cellulose acetate, cellulose propionate, cellulose acetate propionate,
cellulose acetate
butyrate, cellulose acetate phthalate, cellulose triacetate, poly(methyl
methacrylate),
poly(ethyl methacrylate), poly(butyl methacrylate), poly(isobutyl
methacrylate), poly(hexyl
methacrylate), poly(isodecyl methacrylate), poly(lauryl methacrylate),
poly(phenyl
methacrylate), poly(methyl acrylate), poly(isopropyl acrylate), poly(isobutyl
acrylate),
poly(octadecyl acrylate), poly(ethylene), poly(ethylene) low density,
poly(ethylene) high
19
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
density, poly(propylene), poly(ethylene terephthalate), poly(vinyl isobutyl
ether), poly(vinyl
acetate), poly(vinyl chloride), polyurethane, and mixtures thereof.
[0158] Fillers include, but are not limited to, lactose, saccharose, glucose,
starch,
microcrystalline cellulose, microfine cellulose, mannitol, sorbitol, calcium
hydrogen
phosphate, aluminum silicate, amorphous silica, and sodium chloride, starch,
and dibasic
calcium phosphate dihydrate. In one embodiment, the filler is not water-
soluble, although it
may absorb water. In one embodiment, the filler is a spheronization aid.
Spheronization aids
can include one or more of crospovidone, carrageenan, chitosan, pectinic acid,
glycerides, 13-
cyclodextrin (0-CD), cellulose derivatives, microcrystalline cellulose,
powdered cellulose,
polyplasdone crospovidone, and polyethylene oxide. In one embodiment, the
filler includes
microcrystalline cellulose.
[0159] The amount of filler in the xanomeline core is not particularly
limited. In
embodiments, the amount of filler (e.g., microcrystalline cellulose) can be in
a range of about
wt.% to about 70 wt.%, or about 16 wt.% to about 23 wt.%, or at least 19 wt.%
or at least
19.5 wt.%, for example about 20 wt.%. In certain embodiments, the xanomeline
beads
comprise between about 15 wt.% and about 65 wt.% microcrystalline cellulose,
such as
between about 15 wt.% and about 20 wt.%, between about 20 wt.% and about 25
wt.%,
between about 25 wt.% and about 30 wt.%, between about 30 wt.% and about 35
wt.%,
between about 35 wt.% and about 40 wt.%, between about 40 wt.% and about 45
wt.%,
between about 45 wt.% and about 50 wt.%, between about 50 wt.% and about 55
wt.%,
between about 55 wt.% and about 60 wt.%, or between about 60 wt.% and about 65
wt.%. In
certain embodiments, the xanomeline beads comprise 33.5 wt.% microcrystalline
cellulose.
[0160] The amount of filler in the trospium core is not particularly limited.
In embodiments,
the amount of filler (e.g., microcrystalline cellulose or lactose) can be in a
range of about 10
wt.% to about 80 wt.%, or about 16 wt.% to about 23 wt.%, or at least 19 wt.%
or at least
19.5 wt.%, for example about 20 wt.%. In certain embodiments, the trospium
beads comprise
between 25 wt.% and 80 wt.% microcrystalline cellulose, such as between about
25 wt.% and
30 wt.%, between about 30 wt.% and 35 wt.%, between about 35 wt.% and 40 wt.%,
between
about 40 wt.% and 45 wt.%, between about 45 wt.% and 50 wt.%, between about 50
wt.%
and 55 wt.%, between about 55 wt.% and 60 wt.%, between about 60 wt.% and 65
wt.%,
between about 65 wt.% and 70 wt.%, between about 70 wt.% and 75 wt.%, or
between about
75 wt.% and 80 wt.%. In certain embodiments, the trospium beads comprise 46.8
wt.%
microcrystalline cellulose.
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
[0161] In certain embodiments, the trospium beads comprise between 15 wt.% and
70 wt.%
lactose monohydrate, such as between about 15 wt.% and 20 wt.%, between about
20 wt.%
and 25 wt.%, between about 25 wt.% and 30 wt.%, between about 30 wt.% and 35
wt.%,
between about 35 wt.% and 40 wt.%, between about 40 wt.% and 45 wt.%, between
about 45
wt.% and 50 wt.%, between about 50 wt.% and 55 wt.%, between about 55 wt.% and
60
wt.%, between about 60 wt.% and 65 wt.%, or between about 65 wt.% and 70 wt.%.
In
certain embodiments, the trospium beads comprise 35 wt.% lactose monohydrate.
[0162] Binders include, but are not limited to, cellulose ethers,
methylcellulose,
ethylcellulose, hydroxyethylcellulose, propyl cellulose, hydroxypropyl
cellulose, lower-
substituted hydroxypropyl cellulose, hydroxypropylmethylcellulose
(hypromellose, e.g.,
hypromellose 2910, MethocelTM E), carboxymethyl cellulose, starch,
pregelatinized starch,
acacia, tragacanth, gelatin, polyvinyl pyrrolidone (povidone), cross-linked
polyvinyl
pyrrolidone, sodium alginate, microcrystalline cellulose, and lower-alkyl-
substituted
hydroxypropyl cellulose. In one embodiment, the binders are selected from wet
binders. In
one embodiment, the binder is selected from cellulose ethers, e.g.,
hypromellose.
[0163] The amount of binder in the xanomeline core is not particularly
limited. In
embodiments, the amount of binder (e.g., hypromellose) can be in a range
between about 1
wt.% and about 10 wt.%, between about 2 wt.% and about 8 wt.%, or between
about 4 wt.%
and about 6 wt.%, for example about 5 wt.%.
[0164] The amount of binder in the trospium core is not particularly limited.
In embodiments,
the amount of binder (e.g., hypromellose) can be in a range between about 1
wt.% and about
wt.%, between about 2 wt.% and about 8 wt.%, or between about 4 wt.% and about
6
wt.%, for example about 5 wt.%.
[0165] Surfactants include, but are not limited to, anionic surfactants,
including sodium
lauryl sulfate, sodium deoxycholate, dioctyl sodium sulfosuccinate, and sodium
stearyl
fumarate, nonionic surfactants, including polyoxyethylene ethers, and
polysorbate 80, and
cationic surfactants, including quaternary ammonium compounds. In one
embodiment, the
surfactant is selected from anionic surfactants, e.g., sodium lauryl sulfate.
[0166] The amount of surfactant, e.g., as a processing aid, is not
particularly limited in the
xanomeline core. In embodiments, the amount of surfactant (e.g.,
microcrystalline cellulose)
can be in a range between about 0.1 wt.% and about 1 wt.%, between about 0.2
wt.% and
about 0.8 wt.%, or between about 0.4 wt.% and about 0.6 wt.%, for example
about 0.5 wt.%.
21
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
[0167] The amount of surfactant, e.g., as a processing aid, is not
particularly limited in the
trospium core. In embodiments, the amount of surfactant (e.g., sodium lauryl
sulfate) can be
in a range between about 0.1 wt.% and about 1 wt.%, between about 0.2 wt.% and
about 0.8
wt.%, or between about 0.4 wt.% and about 0.6 wt.%, for example about 0.5
wt.%.
[0168] Disintegrants include, but are not limited to, starch, sodium cross-
linked
carboxymethyl cellulose, carmellose sodium, carmellose calcium, cross-linked
polyvinyl
pyrrolidone, and sodium starch glycolate, low-substituted hydroxypropyl
cellulose, and
hydroxypropyl starch.
[0169] Glidants include, but are not limited to, polyethylene glycols of
various molecular
weights, magnesium stearate, calcium stearate, calcium silicate, fumed silicon
dioxide,
magnesium carbonate, magnesium lauryl sulfate, aluminum stearate, stearic
acid, palmitic
acid, cetanol, stearol, and talc.
[0170] Lubricants include, but are not limited to, stearic acid, magnesium
stearate, calcium
stearate, aluminum stearate, and siliconized talc. In certain embodiments, the
xanomeline
beads comprise between 0 wt.% and 2 wt.% talc, such as 0.5 wt.% talc. In
certain
embodiments, the trospium beads comprise between 0 wt.% and 2 wt.% talc, such
as 0.5
wt.% talc.
[0171] In certain embodiments, the formulation further comprises one or more
antioxidants.
Examples of pharmaceutically-acceptable antioxidants include: (1) water
soluble
antioxidants, such as ascorbic acid, cysteine hydrochloride, sodium bisulfate,
sodium
metabisulfite, sodium sulfite and the like; (2) oil-soluble antioxidants, such
as ascorbyl
palmitate, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT),
lecithin,
propyl gallate, alpha-tocopherol, and the like; and (3) metal chelating
agents, such as citric
acid, ethylenediamine tetraacetic acid (EDTA), sorbitol, tartaric acid,
phosphoric acid, and
the like. In certain embodiments, the formulation comprises less than 1 wt.%
antioxidant,
such as 0.9 wt.%, 0.8 wt.%, 0.7 wt.%, 0.6 wt.%, 0.5 wt.%, 0.4 wt.%, 0.3 wt.%,
0.2 wt.%, 0.1
wt.%, 0.09 wt.% , 0.08 wt.% , 0.07 wt.% , 0.06 wt.%, 0.05 wt.%, 0.04 wt.%,
0.03 wt.%, 0.02
wt.%, or 0.01 wt.%.
[0172] In certain embodiments, the oral pharmaceutical composition further
comprises
ascorbic acid. In certain embodiments, the oral pharmaceutical composition
comprises
between 0.2 wt.% and 1 wt.% ascorbic acid. In certain embodiments, the oral
pharmaceutical
composition comprises about 0.5 wt.% ascorbic acid. In certain embodiments,
the oral
pharmaceutical composition further comprises butylated hydroxywhierie. In
certain
22
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
embodiments, the oral pharmaceutical composition comprises between 0.01 wt.%
and 0.1
wt.% butylated hydroxytoluene. In certain embodiments, the oral pharmaceutical
composition
comprises about 0.05 wt.% butylated hydroxytoluenc. In certain embodiments,
the
formulation comprises about 0.05 wt.% BHT or 0.5 wt.% ascorbic acid. In
certain
embodiments, the antioxidant is present in the xanomeline core or the
xanomeline beads.
[0173] In certain embodiments, the xanomeline beads comprise between 30 wt.%
and 80
wt.% xanomeline tartrate, between 15 wt.% and 65 wt.% microcrystalline
cellulose, and
between 0 wt.% and 2 wt.% talc. In certain embodiments, the trospium beads
comprise
between 0.2 wt.% and 2 wt.% talc, such as 0.5 wt.% talc. In certain
embodiments, the
trospium beads comprise between 8 wt.% and 35 wt.% trospium chloride, between
25 wt.%
and 80 wt.% microcrystalline cellulose, between 15 wt.% and 70 wt.% lactose
monohydrate,
and between 0.2 wt.% and 2 wt.% talc.
[0174] In certain embodiments, the xanomeline tartrate drug beads comprise 66
wt.%
xanomeline tartrate, 33.5 wt.% microcrystalline cellulose and 0.5 wt.% talc.
In certain
embodiments, the trospium chloride beads comprise 17.7 wt.% trospium chloride,
46.8 wt.%
microcrystalline cellulose, 35 wt.% lactose monohydrate, and 0.5 wt.% talc. In
this example,
the xanomeline tartrate beads contain about 2.5 times as much xanomeline as
the trospium
chloride beads contain trospium chloride.
[0175] Depending on dosing requirements, capsules can be prepared with
different amounts
of xanomeline tartrate and trospium chloride beads. In various embodiments,
capsules
contain 25 mg xanomeline and 10 mg trospium chloride, 50 mg xanomeline and 10
mg
trospium chloride, 50 mg xanomeline and 20 mg trospium chloride, 75 mg
xanomeline and
mg trospium chloride, 75 mg xanomeline and 20 mg trospium chloride, 125 mg
xanomeline and 30 mg trospium chloride, or 125 mg xanomeline and 40 mg
trospium
chloride. In certain embodiments, the capsule contains 25 mg xanomeline as
xanomeline
tartrate and 10 mg trospium chloride. In certain embodiments, the capsule
contains 50 mg
xanomeline as xanomeline tartrate and 10 mg trospium chloride. In certain
embodiments, the
capsule contains 50 mg xanomeline as xanomeline tartrate and 20 mg trospium
chloride. In
certain embodiments, the capsule contains 75 mg xanomeline as xanomeline
tartrate and 10
mg trospium chloride. In certain embodiments, the capsule contains 75 mg
xanomeline as
xanomeline tartrate and 20 mg trospium chloride. In certain embodiments, the
capsule
contains 125 mg xanomeline as xanomeline tartrate and 20 mg trospium chloride.
In certain
embodiments, the capsule contains 125 mg xanomeline as xanomeline tartrate and
40 mg
23
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
trospium chloride. In certain embodiments, the capsule contains 150 mg
xanomeline and 20
mg trospium chloride. In certain embodiments, the capsule contains 150 mg
xanomeline and
30 mg trospium chloride. In certain embodiments, the capsule contains 150 mg
xanomeline
and 40 mg trospium chloride. In certain embodiments, the capsule contains 175
mg
xanomeline and 20 mg trospium chloride. In certain embodiments, the capsule
contains 175
mg xanomeline and 30 mg trospium chloride. In certain embodiments, the capsule
contains
175 mg xanomeline and 40 mg trospium chloride.
[0176] In another embodiment, the medicament contains from five milligrams to
700
milligrams of xanomeline. In an embodiment, the medicament contains from 25
milligrams to
300 milligrams of xanomeline.
[0177] In another embodiment, the medicament contains from one milligram to
400
milligrams of trospium chloride. In an embodiment, the medicament contains
from 6.5
milligrams to 200 milligrams of trospium chloride.
[0178] In one embodiment, trospium chloride extended-release is used as the
trospium
chloride in the medicament. In another embodiment, the medicament contains
from one
milligram to 400 milligrams of trospium chloride extended-release. In an
embodiment, the
medicament contains from 6.5 milligrams to 200 milligrams of trospium chloride
extended-
release.
[0179] In an embodiment, the medicament contains 75 mg or 225 milligrams of
xanomeline,
and the same medicament contains 20 mg or 40 milligrams of trospium chloride.
In another
embodiment, the medicament contains 75 mg or 225 milligrams of xanomeline, and
a
different medicament to be co-administered contains 20 mg or 40 milligrams of
trospium
chloride.
Bead Coatings
[0180] In other embodiments, the beads may be coated with functional or non-
functional
coatings, such as aesthetic, handling, or stability. In certain embodiments,
the beads might be
coated with a pH-sensitive coating so that they do not dissolve in the low pH
of the stomach.
A nonfunctional coating might be used to maintain chemical separation between
the beads or
for cosmetic reasons.
[0181] In a further embodiment, the controlled release formulation comprises a
semi-
permeable coating. The xanomeline and trospium chloride may be in different
coatings in the
same formulation. In another embodiment, the xanomeline and trospium chloride
can be in
different coatings in different formulations or dosing vehicles. In a further
embodiment, the
24
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
semi-permeable coating comprises a polymer. In a further embodiment, the
controlled release
formulation comprises a matrix that suspends the xanomeline and trospium
chloride.
[0182] In certain embodiments, the distribution of coating thicknesses can be
stated in the
weight gain of coating material based on the total weight of the coated beads.
Thus, in one
embodiment, the distribution of coating thicknesses is at least 2% based on
the total weight of
the coated beads. In another embodiment, the distribution of coating
thicknesses is at least
3%. In another embodiment, the distribution of coating thicknesses is at least
4%. In another
embodiment, the distribution of coating thicknesses is at least 5%. In another
embodiment,
the distribution of coating thicknesses is at least 6%. In another embodiment,
the distribution
of coating thicknesses is at least 7%. In another embodiment, the distribution
of coating
thicknesses is at least 8%. In another embodiment, the distribution of coating
thicknesses is at
least 9%. In another embodiment, the distribution of coating thicknesses is at
least 10%. In
another embodiment, the distribution of coating thicknesses is at least 11%.
In another
embodiment, the distribution of coating thicknesses is at least 12%. In
another embodiment,
the distribution of coating thicknesses is at least 13%. In another
embodiment, the
distribution of coating thicknesses is at least 14%.
[0183] For example, the difference in coating thickness from bead to bead can
be in a range
of +/- 1-7% based on the coated beads total weight. The distribution of
coating thicknesses
can between about 2% and about 14% based on the weight of the coated beads,
such as
between about 3% and about 13%, between about 4% and about 12%, between about
5% and
about 11%, between about 6% to about 10%, between about 7% and 9%, between
about 3%
and 14%, between about 4% and 14%, between about 4% and 13%, or between 4% and
about
12%.
[0184] In one embodiment, the absorption (area under the curve, AUC) of the
dosage form
when dosed orally is advantageously increased, compared to other dosage forms
of
xanomeline or trospium chloride. Without intending to be bound by any theory,
the increase
in absorption is influenced by the dosage form exhibiting a pseudo-extended
release profile.
The pseudo-extended release profile is influenced by one or more factors,
including
distribution of coating thicknesses when present, distribution of bead
particle sizes, and the
beads having irregular bead shapes. For example, in an embodiment wherein the
beads have a
distribution of coating thicknesses, for beads with a relatively thin coating,
the coating
completely dissolves at the trigger pH relatively quickly to release the
xanomeline and/or
trospium chloride compositions, whereas for beads having a relatively thick
coating the
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
coating takes somewhat longer to completely dissolve and release the
xanomeline and/or
trospium chloride compositions. In an embodiment where the beads have a
distribution of
particle sizes and/or irregular bead shapes, the gut transit time of the beads
could be varied
due to bead size and/or shape, such that the transit time until reaching the
coating dissolution
pH is varied, thus contributing to a pseudo-extended release profile. In
another embodiment,
the dosage form exhibits substantially equivalent (e.g., bioequivalent) C.
and/or AUC
characteristics when administered orally inside a capsule shell or without a
capsule shell.
[0185] In certain embodiments, the dosage form provides a progressive and
predictable
absorption curve. In one embodiment, the T. of the dosage form, when dosed
orally, is
more stable on a dose-to-dose basis because the beads are individually coated.
A predictable,
consistent T. is advantageous for accomplishing a more consistent, sustained
therapeutic
effect. For example, process-related variations in coating thickness or other
influences on
coating dissolution affect only a fraction of the xanomeline and trospium
chloride in the
dosage form. They tend to lead to pseudo-extended release behavior. In
contrast, coated
capsules comprising xanomeline and trospium chloride microspheres exhibit
significant
variability in absorption time from the capsule to capsule.
[0186] In certain embodiments, the oral pharmaceutical composition comprises
xanomeline
and/or a salt thereof and trospium chloride for treating a muscarinic disorder
in a patient in
need thereof, which when administered to the patient in need thereof, the
composition is
sufficient to provide an in-vivo plasma profile comprising a median T. for
xanomeline of 2
hours and a median T. for trospium of 1 hour. In certain embodiments, the in-
vivo plasma
profile further comprises a mean dose-normalized C. of between 48.5 and 121.3
pg/mL/mg. In certain embodiments, the in-vivo plasma profile further comprises
a mean
dose-normalized C. of trospium of between 156 and 375 pg/mL/mg. In certain
embodiments, the in-vivo plasma profile further comprises a mean dose-
normalized AUC0_12
of xanomeline of between 263 and 577 hr pg/mL/mg. In certain embodiments, the
in-vivo
plasma profile further comprises a mean dose-normalized AUC0_12 of trospium of
between
881 and 2024 hr pg/mL/mg. In certain embodiments, the in-vivo plasma profile
further
comprises a mean C. of trospium at 7850 3360 pg/mL. In certain embodiments,
the in-
vivo plasma profile further comprises a mean AUC0_12 of 41900 15500
hr.pg/mL.
[0187] In another embodiment, the dosage form exhibits advantageous storage
stability, e.g.,
measured by the amount of xanomeline present following storage and/or by the
total amount
of related substances. The storage stability can be assessed following storage
at typical
26
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
ambient conditions (e.g., 25 C and 60% relative humidity) or accelerated
stability conditions
involving increased temperature and/or humidity.
[0188] The dosage form and methods are contemplated to include embodiments of
any
combination of one or more of the additional optional elements, features, and
steps further
described below (including those shown in the figures and Examples) unless
stated otherwise.
Reference to a bead and properties thereof apply equally to a collection of
beads (e.g., a
plurality of such beads). Likewise, referring to a core and properties thereof
apply equally to
a collection of cores (e.g., a plurality of such cores).
[0189] The enteric (gastro-resistant) coating material, e.g., polymer, can be
one that will
dissolve in intestinal juices at a pH level higher than that of the stomach,
e.g., a pH of greater
than 4.5, such as within the small intestine, and therefore permit the release
of the active
substance in the regions of the small intestine and substantially not in the
upper portion of the
GI tract. In one embodiment, the enteric material begins to dissolve in an
aqueous solution at
pH between about 4.5 and about 5.5. In another embodiment, the enteric
material rapidly
dissolves in an aqueous solution at a pH of about 5. In another embodiment,
the enteric
material rapidly dissolves in an aqueous solution at a pH of about 5.5.
[0190] For example, pH-sensitive materials do not significantly dissolve until
the dosage
form has emptied from the stomach. The small intestine's pH gradually
increases from about
4.5 to about 6.5 in the duodenal bulb to about 7.2 in the distal portions of
the small intestine
(ileum). To provide predictable dissolution corresponding to the small
intestine transit time of
about 3 hours (e.g., 2-3 hours) and permit reproducible release therein, the
coating should
begin to dissolve within the pH range of the duodenum and continue to dissolve
at the pH
range within the small intestine. Therefore, the amount (thickness) of enteric
coating should
be substantially dissolved during the about three-hour transit time within the
small intestine
(e.g., the proximal and mid-small intestine).
[0191] Suitable enteric (gastro-resistant) materials include, but are not
limited to, cross-
linked polyvinyl pyrrolidone; non-crosslinked polyvinylpyrrolidone;
hydroxypropylmethyl
cellulose phthalate, hydroxypropylmethyl cellulose acetate succinate,
cellulose acetate
succinate; cellulose acetate phthalate, hydroxypropylmethyl cellulose acetate
succinate,
cellulose acetate trimellitate; starch acetate phthalate; polyvinyl acetate
phthalate;
carboxymethyl cellulose; methyl cellulose phthalate; methyl cellulose
succinate; methyl
cellulose phthalate succinate; methyl cellulose phthalic acid half ester;
ethyl cellulose
succinate; carboxymethylamide; potassium methacrylate divinylbenzene
copolymer;
27
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
polyvinyl alcohols; poly oxy ethylene glycols; polyethylene glycol; sodium
alginate;
galactomannan; carboxypolymethylene; sodium carboxymethyl starch; copolymers
of acrylic
acid and/or methacrylic acid with a monomer selected from the following:
methyl
methacrylate, ethyl methacrylate, ethyl acrylate, butyl methacrylate, hexyl
methacrylate,
decyl methacrylate, lauryl methacrylate, phenyl methacrylate, methyl acrylate,
isopropyl
acrylate, isobutyl acrylate, or octadecyl acrylate, e.g. EudragitTM -L and -S
series, including L
100-55, L 30 D-55, L 100, S 100, L 12.5, and S 12.5, available from Evonik
Industries;
polyvinyl acetate; fats; oils; waxes; fatty alcohols; shellac; zein; gluten;
ethylacrylate-maleic
acid anhydride copolymer; maleic acid anhydride-vinyl methyl ether copolymer;
styrol-
maleic acid copolymer; 2-ethyl-hexyl-acrylate maleic acid anhydride; crotonic
acid-vinyl
acetate copolymer; glutaminic acid/glutamic acid ester copolymer;
carboxymethylethylcellulose glycerol monooctanoate; polyarginine;
poly(ethylene);
poly(propylene); poly(ethylene oxide); poly(ethylene terephthalate);
poly(vinyl isobutyl
ether); poly(vinyl chloride); and polyurethane. A combination of enteric
materials may also
be used. In one embodiment, the enteric material rapidly dissolves at pH 5.5
and higher to
provide fast dissolution in the upper bowel. For example, the enteric material
can be selected
from a copolymer of methacrylic acid and methyl methacrylate and a copolymer
of
methacrylic acid and ethyl acrylate. For example, an enteric polymer is
poly(methacrylic acid
co-ethyl acrylate)1:1 (EudragitTM L 30 D-55 and EudragitTM L 100-55).
[0192] Other suitable examples of enteric coating coatings include beeswax and
glyceryl
monostearate; beeswax, shellac and cellulose; and cetyl alcohol, mastic and
shellac, and
shellac and stearic acid; polyvinyl acetate and ethyl cellulose; and a neutral
copolymer of
polymethacrylic acid esters (EudragitTM L 30D); copolymers of methacrylic acid
and
methacrylic acid methylester, or a neutral copolymer of polymethacrylic acid
esters
containing metallic stearates. Such coatings comprise mixtures of fats and
fatty acids, shellac
and shellac derivatives, and the cellulose acid phthalates, e.g., those with
free carboxyl
content.
[0193] One or more plasticizers can be added to enteric polymers to increase
their pliability
and reduce brittleness, as known in the art. Suitable plasticizers include,
for example, butyl
citrates, triethyl citrate, diethyl phthalate, dibutyl sebacate, polyethylene
glycols (PEGs, such
as PEG 6000), acetyl triethyl citrate, and triacetin. In one embodiment, the
plasticizer is
triethyl citrate. While some enteric materials are flexible and do not require
plasticizers, more
brittle polymers (e.g., EudragitTM L/S types, EudragitTM RL/RS, and EudragitTM
FS 30 D)
28
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
benefit from plasticizers, for example, ranging from between 5 wt.% and 30
wt.% based on
the dry polymer mass, between about 8 wt.% and about 12 wt.% triethyl citrate
with
poly(methacrylic acid co-ethyl acrylate) 1:1.
[0194] In certain embodiments, the enteric coatings comprise one or more anti-
tacking agents
(antiadherents) to reduce the film's tackiness and prevent agglomeration, as
it is known in the
art. Suitable anti-tacking agents include, but are not limited to, talc,
glyceryl monostearate,
fumed silica (e.g., AerosilTM 200), precipitated silica (e.g., SipernatTM PQ),
and magnesium
stearate. Anti-tacking agents can be used in any suitable quantity, for
example ranging
between about 10 wt.% and 100 wt.% based on dry polymer mass, between about 10
wt.%
and about 50 wt.%, between about 10 wt.% and about 30 wt. %, or between about
15 wt.%
and about 30 wt.%. For example, in one embodiment in ranges between 15 wt.%
and about
30 wt.% based on dry polymer mass.
[0195] One or more surfactants can also be added to an enteric coating mixture
to increase
substrate wettability and/or stabilize suspensions, as it is known in the art.
Surfactants include
Polysorbate 80, sorbitan monooleate, and sodium dodecyl sulfate, and other
surfactants
described herein.
[0196] Any suitable process can form the enteric coating. Coating processes
include pan
coating, fluid bed coating, and dry coating (e.g., heat dry coating and
electrostatic dry
coating). Pan coating and fluid bed coating using solvent are well-established
processes. In
liquid coating, the enteric material and optional excipients (e.g., pigments,
plasticizers, anti-
tacking agents) are mixed in an organic solvent or water to form a solution or
dispersion. The
coating solution or dispersion is sprayed into solid dosage forms in a pan
coater or a fluid bed
dryer and dried by hot air. For example, in a Wurster fluid bed coating
process, the coating
fluid is sprayed from the fluid bed apparatus's bottom. Alternatively, the
coating fluid is
applied by top spraying. In certain embodiments, a tangential spray is
applied.
[0197] The amount of enteric material applied is sufficient to achieve the
desired acid
resistance and release characteristics. For example, in one embodiment, the
amount of enteric
coating meets USP <711> requirements (USP 36-NF 31) for delayed-release dosage
forms,
thereby not releasing 10.0 wt.% of the drug after 2 hours in 0.1 N HC1. In
certain
embodiments, the formulation releases at least 80% of the active in 20 minutes
in pH 6.8
buffer solution, e.g., using a dissolution method of USP 36-NF 31 section
<711>.
[0198] In one embodiment, the enteric coating is present in an amount in a
range between
about 10% and 40%, or between 25% and about 35% as measured by the weight gain
29
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
compared to the uncoated particle cores, or ranging between about 25% and
about 31%
weight gain, between about 27% and about 31% weight gain, or between about
28.5% and
about 31% weight gain, based on the weight of the uncoated particle cores.
[0199] The formulation can include a capsule shell in which the beads are
disposed. Soft and
hard capsule shells are known. The capsule shell is a hard-capsule shell in
one embodiment,
e.g., a gelatin capsule shell or a vegetable-based hard capsule shell. In
certain embodiments,
the capsule shell comprises one or more enteric coatings described herein.
During accelerated
storage, gelatin capsules may collapse. Thus, in certain embodiments, the
formulation can
include a hydroxypropyl methylcellulose capsule shell.
[0200] Thus, for example, one embodiment combining various of the features
described
above includes a pharmaceutical dosage form comprising a plurality of
xanomeline beads, the
beads comprising a core comprising xanomeline tartrate, a filler (optionally
microcrystalline
cellulose), a binder (optionally hypromellose), and an enteric coating
(optionally EudragitTM
L 30 D-55) surrounding the core, wherein the plurality of beads has a
distribution of particle
sizes ranging between about 0.7 mm and about 2.5 mm, wherein the enteric
coating ranges
between about 20% and about 40% based on the weight of the bead cores, and
wherein the
beads are disposed in a capsule shell.
Bead size and shape
[0201] The plurality of beads has a distribution of particle sizes. The
plurality of beads has
bead shapes. The plurality of beads has a distribution of coating thicknesses
when present.
[0202] Beads having a distribution of particle sizes were shown to exhibit
advantageous
pharmacokinetics. Without intending to be bound by any theory, it is
contemplated that the
pharmacokinetics are influenced by the plurality of beads having a
distribution of core sizes.
[0203] In one embodiment, the particle sizes of the beads range between about
0.4 mm and
about 1.2 mm, such as between about 0.4 mm and about 0.5 mm, between about 0.5
mm and
about 0.6 mm, between about 0.6 mm and about 0.7 mm, between about 0.7 mm and
about
0.8 mm, between about 0.8 mm and about 0.9 mm, between about 0.9 mm and about
1.0 mm,
between about 1.0 mm and about 1.1 mm, or between about 1.1 mm and about 1.2
mm. In
certain embodiments, the size of the xanomeline beads is between about 0.425
mm and about
1.18 mm. In certain embodiments, the size of the xanomeline beads is between
about 0.6 mm
and about 0.85 mm. In certain embodiments, the size of the trospium beads is
between about
0.425 mm and about 1.18 mm. In certain embodiments, the size of the trospium
beads is
between about 0.6 mm and about 0.85 mm.
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
[0204] The beads or bead mixtures may be used, for example, in suspensions,
filled into
capsules, compressed into tablets, or filled into sachets. One or more types
of modified
release beads can be mixed and encapsulated or used as a sprinkle on the
subject's food. In
certain embodiments, the oral solid dosage form may be any of these forms. In
certain
embodiments, the dosage form is a capsule.
[0205] As the particle size of the beads becomes too small, the variability in
the content of
the active increases. As the particle size becomes too large, the beads are
too large for drug
products labeled to be administered via sprinkling (e.g., on applesauce or
other soft foods,
such as jellies) and swallowed without chewing or administered via an enteral
feeding tube.
Also, as the particle size increases, the larger particles get coated more
than the smaller
particles, resulting in lower relative assay than smaller particles.
Relatively more beads are
needed to meet the label strength per capsule. Filling a capsule shell with
enough large
particles to meet the label strength per capsule becomes difficult or
impossible (e.g., to fill a
size 0 capsule to a 75-mg strength of xanomeline free base).
[0206] In one embodiment, the beads are formulated into capsules, e.g., with
an
encapsulation machine. Various capsule sizes may accommodate the strength and
fill weight
of the target formulations. Capsule size ranges from 00 to 5 for fill weights
ranging between
about 15 mg and about 630 mg.
[0207] The beads can be sorted (e.g., via sieving) to the desired particle
size. In certain
embodiments, the particle size range is any particle size range or combination
described
above regarding the cores. In one embodiment, the particle size range is the
same as the
particle size range of the uncoated cores. For example, the beads can be
sieved such that 5%
or less of the bead cores by weight is retained on a #12 mesh (1.68 mm)
screen, and 10% or
less by weight pass through a #20 mesh (0.84 mm) screen.
Method of Making
[0208] Provided is a method for preparing an oral pharmaceutical composition
comprising
admixing beads comprising a plurality of xanomeline beads comprising
xanomeline or a
pharmaceutically acceptable salt thereof with a plurality of trospium beads
comprising a salt
of trospium, such as trospium chloride. In certain embodiments, the method
further comprises
formulating the admixed beads into capsules.
[0209] Also disclosed herein are a method for preparing the dosage form,
comprising coating
a core comprising xanomeline or a pharmaceutically acceptable salt thereof and
an excipient
with an enteric polymer to form the enteric coating, and coating a core
comprising trospium
31
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
chloride or a pharmaceutically acceptable salt thereof and an excipient with
an enteric
polymer to form the enteric coating. Optionally, the core can be formed by a
wet granulation
method. Optionally, drug beads are sorted (e.g., via sieving) to a desired
particle size range
before enteric coating, and optionally again following enteric coating.
[0210] The drug beads may be made by different processes, including, but not
limited to,
spheronizing an extruded wet mass and coating of inert core spheres in a
fluidized bed. In
certain embodiments, the beads are prepared by extrusion and spheronization.
[0211] The beads are formulated to flow freely and to be compatible with
modern
encapsulation equipment. In some embodiments, the beads are blended to form a
uniform
mixture filled into capsules in a single stage. In other embodiments, the
beads are filled
separately into capsules using a two-stage capsule filler.
[0212] Any suitable process can form the cores comprising xanomeline or
pharmaceutically
acceptable salts thereof. In one embodiment, the core is formed by granulating
a mixture of
xanomeline or a pharmaceutically acceptable salt thereof with an excipient and
milling to a
desired particle size range. In another embodiment, the core can be formed by
extrusion and
spheronization of a mixture of xanomeline or a pharmaceutically acceptable
salt thereof with
an excipient.
[0213] Any suitable process can form the cores comprising trospium chloride or
pharmaceutically acceptable salts thereof. In one embodiment, the core is
formed by
granulating a mixture of trospium chloride or a pharmaceutically acceptable
salt thereof with
an excipient and milling to a desired particle size range. In another
embodiment, the core can
be formed by extrusion and spheronization of a mixture of trospium chloride or
a
pharmaceutically acceptable salt thereof with an excipient.
[0214] Granulating processes can include fluid bed granulation, wet
granulation, hot melt
granulation, and spray congealing. Other processes include slugging and roller
compaction.
The mixtures to be granulated can first be dry-blended. The dry-blended dry
ingredients can
be mixed with water before extrusion.
[0215] Extrusion and spheronization of a mixture of xanomeline or a
pharmaceutically
acceptable salt thereof and trospium chloride with an excipient provide
desirable cores with a
distribution of particle sizes as described herein and one or more other
desirable properties. In
certain embodiments, short processing times can lead to a more stable product.
For example,
reducing spheronization reduces friction and related heat, reducing the time
the product is
exposed to air (either when moist and/or before packaging) diminishes
oxidation. On the
32
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
other hand, rapid processing by extrusion and spheronization can lead to a
poor-quality
product, such as having a large fraction of the bead cores falling outside a
desired particle
size range. The moisture absorbed by spheronization aids (which happens over
time)
influences the beads spheronization characteristics.
[0216] Accordingly, in one embodiment, the moisture content of the granulation
mixture,
before drying, ranging between about 20 wt.% and about 40 wt.%, such as
between 25 wt.%
and about 35 wt.%, between about 28 wt.% and about 32 wt.%, at least about 28
wt.%, at
least about 28.5, between about 20 wt.% and about 40 wt.%, between about 25
wt.% and
about 35 wt.%, between about 27 wt.% and about 31 wt.%, or between about 28.5
wt.% and
about 31 wt.%.
[0217] In certain embodiments, the wet mass can be held before extrusion,
allowing the
spheronization aid to swell with granulating fluid. The hold time can be at
least 15 minutes,
such as at least 30 minutes, at least 45 minutes, or at least 60 minutes. In
certain
embodiments, the hold time ranges between about 15 minutes and about 120
minutes, such as
between 30 and 100 minutes or between 60 and 90 minutes.
[0218] As described above relating to cores, the method can include a step of
sorting (e.g., by
sieving) the cores before optional coating to retain particles in a
predetermined size range, for
example, sizes ranging between about 0.7 mm and about 2.8 mm, such as between
about 0.7
mm and about 2.5 mm, between about 0.8 mm and about 1.7 mm, or any range
described
herein.
[0219] As described above relating to beads, the method can include a step of
sorting (e.g.,
by sieving) the beads after optional coating to retain particles in a size
range, for example,
sizes ranging between about 0.7 mm and about 2.8 mm, such as between about 0.7
mm and
about 2.5 mm, or between about 0.8 mm and about 1.7 mm, or any range described
herein.
[0220] In an extrusion and spheronization process, the following optional
features can be
employed, individually or in one or more combinations thereof. Water can be a
granulation
agent. Microcrystalline cellulose can be in the cores as a spheronization aid.
Hypromellose
can be included in the cores as a binder. The extrusion screen size can be 1.0
mm. The
friction plate of the spheronizer can be cross-hatched. The friction plate of
the spheronizer
can be cross-hatched with a square pitch of at least about 3 mm, or greater
than about 3 mm,
or at least about 4 mm, or greater than about 4 mm, or ranging between about 3
mm and
about 7 mm, or about 5 mm. The spheronization time can be less than about 5
minutes, or
less than about 4 minutes, or less than about 3 minutes, or less than about 2
minutes, or up to
33
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
1 minute. The spheronized particles can include non-spherical particles (i.e.,
irregular
shapes), for example, a substantial fraction thereof, such as at least about
20 wt.%, at least
about 30 wt.%, at least about 40 wt.%, at least about 50 wt.%, at least about
60 wt.%, or at
least about 70 wt.% thereof.
[0221] In certain embodiments, the pharmaceutical composition is stored with a
desiccant,
for example, pharmaceutical grades of silica gel, crystalline sodium,
potassium or calcium
aluminosilicate, colloidal silica, anhydrous calcium sulfate, and the like.
[0222] In certain embodiments, the pharmaceutical composition is stored with
an oxygen
absorber.
[0223] In certain embodiments, the pharmaceutical composition is stored under
a dry inert
gas such as nitrogen, helium, argon, neon, xenon, krypton, or a mixture
thereof.
[0224] In certain embodiments, the pharmaceutical composition is stored under
reduced
pressure compared to the external ambient air.
[0225] In certain embodiments, the pharmaceutical composition is stored at a
reduced
temperature, e.g., at refrigerated temperatures (e.g., 2 C to 8 C). In
certain embodiments,
the pharmaceutical composition is stored in such a manner have fewer
impurities, such as
Impurity A, than when stored at 25 C.
[0226] In certain embodiments, the pharmaceutical composition is stored by a
manufacturer,
a distributor, a pharmacy, or a hospital at a temperature of between about 2
C and about 8 C
before dispensing the oral pharmaceutical composition to the subject. In
certain
embodiments, after the oral pharmaceutical composition is dispensed to the
subject, the
pharmaceutical composition is stored at a temperature of between about 20 C
and about 25
C.
[0227] Also provided is a method of stabilizing a pharmaceutical dosage form
or composition
described herein, comprising storing the dosage form at a temperature of about
2 C to about
8 C.
[0228] In certain embodiments, a method for preparing a pharmaceutical dosage
form
comprising xanomeline beads comprises forming a wet mass comprising xanomeline
tartrate
and an excipient, optionally microcrystalline cellulose, with a moisture
content ranging
between about 20 wt.% and about 40 wt.%, extruding and spheronizing the wet
mass
comprising xanomeline tartrate and excipient to make cores, sorting the cores
to a target
particle size range, optionally between about 0.7 mm and about 2.5 mm, coating
the sorted
34
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
cores with a polymer to form beads comprising a core and a coating, and
sorting the bead
particles to a target particle size range, optionally between about 0.7 mm and
about 2.5 mm.
[0229] In certain embodiments, a method for preparing a pharmaceutical dosage
form
comprising trospium beads comprises forming a wet mass comprising trospium
chloride and
an excipient, optionally microcrystalline cellulose, with a moisture content
ranging between
about 20 wt.% and about 40 wt.%, extruding, spheronizing, and drying the wet
mass
comprising trospium chloride and excipient to make cores, sorting the cores to
a target
particle size range, optionally between about 0.7 mm and about 2.5 mm, coating
the sorted
cores with a polymer to form beads comprising a core and a coating, and
sorting the bead
particles to a target particle size range, optionally between about 0.7 mm and
about 2.5 mm.
Purity
[0230] Also provided is the compound 3-11(4-hexyloxy)-1,2,5-thiadizao1-3-y11-5-
hydroy1-1-
methylpyridin-1-ium.
[0231] Also provided is a pharmaceutical composition, comprising xanomeline
and/or a salt
thereof and less than 0.5 wt.% 3-11(4-hexyloxy)-1,2,5-thiadizao1-3-y11-5-
hydroy1-1-
methylpyridin-1-ium (Impurity A). In certain embodiments, the pharmaceutical
composition
comprises less than 0.30 wt.% of Impurity A, such as less than 0.25 wt.%, less
than 0.20
wt.%, less than 0.15 wt.%, less than 0.14 wt.% or less than 0.1 wt.%. Also
provided is a
pharmaceutical composition, comprising xanomeline and/or a salt thereof and
less than 0.15
wt.% 3-11(4-hexyloxy)-1,2,5-thiadizao1-3-y11-5-hydroy1-1-methylpyridin-1-ium
(Impurity A).
[0232] Also provided is an oral pharmaceutical composition, comprising a
plurality of
xanomeline beads comprising xanomeline or a salt thereof and less than 0.5
wt.% 34(4-
hexyloxy)-1,2,5-thiadizao1-3-y11-5-hydroy1-1-methylpyridin-1-ium; and a
plurality of
trospium beads comprising a salt of trospium. Also provided is an oral
pharmaceutical
composition, comprising a plurality of xanomeline beads comprising xanomeline
or a salt
thereof and less than 0.15 wt.% 3-11(4-hexyloxy)-1,2,5-thiadizao1-3-y11-5-
hydroy1-1-
methylpyridin-1-ium; and a plurality of trospium beads comprising a salt of
trospium.
[0233] In certain embodiments, the pharmaceutical composition comprises less
than 0.5
wt.% of Impurity A after the pharmaceutical composition is stored for at least
3 months at 40
C and 75% relative humidity.
[0234] In certain embodiments, the total impurities in the pharmaceutical
compositions
provided herein are no greater than about 5% by weight, no greater than about
4% by weight,
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
no greater than about 3% by weight, no greater than about 2.5% by weight, no
greater than
about 2% by weight, no greater than about 1.5% by weight, no greater than
about 1% by
weight, no greater than about 0.5% by weight, or no greater than about 0.1% by
weight.
Method of Treating
[0235] Further provided is a method of activating muscarinic receptors in a
biological
sample, the method comprising contacting the biological sample with any oral
pharmaceutical composition described herein. Also provided is a method for
treating a
disorder ameliorated by activating muscarinic receptors in a subject in need
thereof,
comprising administering to the subject in need thereof any oral
pharmaceutical composition
described herein.
[0236] While activators of M1 and M4 muscarinic receptors have been suggested
to be
efficacious treatments for schizophrenia, the activation of muscarinic
receptors located
outside the brain has resulted in side effects which barred xanomeline from
the clinic. For
instance, in both Phase I and subsequent trials, the muscarinic agonist
xanomeline had
unacceptable GI and other side effects linked to the binding of muscarinic
receptors in the
body's periphery. By combining a xanomeline with trospium chloride, the
desired therapeutic
effect is achieved while diminishing or eliminating the side effects of
activating muscarinic
receptors located outside the brain.
[0237] The tolerability of xanomeline, a muscarinic activator, is increased by
co-
administering trospium chloride, a muscarinic antagonist. The most common
adverse events
observed with administering xanomeline are nausea, vomiting, diarrhea,
excessive sweating,
and excessive salivation (so-called cholinergic adverse events). A common
anticholinergic
adverse event observed with administering trospium chloride is dry mouth
(xerostomia). The
disclosed compositions reduced the incidence of these adverse events in
humans, evincing
increased xanomeline tolerability. In certain embodiments, after at least 4
weeks of treatment,
the occurrence of a cholinergic or anticholinergic adverse event is not
statistically
distinguishable from a placebo control. In certain embodiments, after at least
4 weeks of
treatment, at least one of nausea, vomiting, and dry mouth occurs at about the
same rate as an
untreated patient. In certain embodiments, at least one adverse event which
occurred at the
start of oral administration is reduced to its pretreatment level after five
weeks of treatment.
36
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
[0238] In one embodiment, xanomeline combined with trospium chloride treats an
animal. In
a further embodiment, the animal is a mammal. In an embodiment, the mammal is
a human
being.
[0239] In one embodiment, trospium chloride decreases the side effects
associated with
xanomeline. Such side effects include, but are not limited to, GI side
effects, cardiac side
effects, excessive sweating, and excessive salivation. The use of trospium
with xanomeline
allows the xanomeline to be used clinically when the xanomeline would not
otherwise be
used clinically due to its side effects. In another embodiment, the use of
trospium chloride
with the xanomeline allows for the xanomeline to achieve a higher maximum
tolerated dose
than xanomeline would otherwise achieve.
[0240] Various time and resource-intensive methods demonstrated the efficacy
of the
combination of xanomeline and trospium chloride. For example, animal models
demonstrate
the efficacy of new therapeutics for schizophrenia, including pharmacological
models (e.g.,
ketamine model) and genetic models (e.g., DISCI mouse). Likewise, animal
models,
including rodents, dogs, and non-human primates, demonstrate the side effect
profile of
pharmacological agents. Animal models are an experimental proxy for humans but
may
suffer from deficiencies in the physiological differences between humans and
animals and
may have limited predictive power for human experiments, particularly for
central nervous
system disorders. Alternatively, the disclosed combination can be tried in
controlled clinical
trials of people. Standard measures based on patient self-report can be used
by those skilled
in the art to assess various side effects such as GI discomfort. As another
example, objective
physiological measures (e.g., EKGs) may be used by those skilled in the art. A
set of standard
measures has also been developed to assess schizophrenia symptoms, including
the Brief
Psychiatric Rating Scale (BPRS), the Positive and Negative Syndrome Scale
(PANSS), and
Clinical Global Impression (CGI). Typically, clinical trials are double-
blinded, where one
group of patients receives an inactive placebo, and the other group the active
intervention.
[0241] The Positive and Negative Syndrome Scale (PANSS) is a medical scale
used for
measuring symptom severity of patients with schizophrenia. The name refers to
the two types
of symptoms in schizophrenia, as defined by the American Psychiatric
Association: positive
symptoms, which refer to an excess or distortion of normal functions (e.g.,
hallucinations and
delusions), and negative symptoms, which represent a diminution or loss of
normal functions.
Some of these functions which may be lost include normal thoughts, actions,
the ability to tell
fantasies from reality, and the ability to properly express emotions
37
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
[0242] The PANSS is a relatively brief interview of about 45 to 50 minutes.
The interviewer
must be trained to a standardized level of reliability. The patient is rated
from 1 to 7 on 30
different symptoms in three categories based on the interview and reports of
family members
or primary care hospital workers. The first category of the PANSS is the
positive scale,
comprising 7 Items (minimum score = 7, maximum score = 49): delusions,
conceptual
disorganization, hallucinations, excitement, grandiosity,
suspiciousness/persecution, and
hostility. The second category is the negative scale, comprising 7 items
(minimum score = 7,
maximum score = 49): blunted affect, emotional withdrawal, poor rapport,
passive/apathetic
social withdrawal, difficulty in abstract thinking, lack of spontaneity, and
flow of
conversation, stereotyped thinking. The third category is the General
Psychopathology scale,
which comprises 16 items (minimum score = 16, maximum score = 112): somatic
concern,
anxiety, guilt feelings, tension, mannerisms and posturing, depression, motor
retardation,
uncooperativeness, unusual thought content, disorientation, poor attention,
lack of judgment
and insight, disturbance of volition, poor impulse control, preoccupation,
active social
avoidance.
[0243] PANSS Marder factor score is the sum of five negative scales and two
general scales
(Ni. Blunted affect; N2. Emotional withdrawal; N3. Poor rapport; N4.
Passive/apathetic
social withdrawal; N6. Lack of spontaneity; G7. Motor retardation; and G16.
Active social
avoidance). If a patient has a PANSS assessment recorded, but any of the items
are missing,
the last non-missing score for the individual item from previous assessments
will be carried
forward. If more than 30% of the items are missing at a particular visit, the
respective
positive score is not calculated. It is treated as missing data in the
analysis.
[0244] Because 1 rather than 0 is given the lowest score for each item, a
patient cannot score
lower than 30 for the total PANSS score. Subscores can be given separately for
the positive
items, negative items, and general psychopathology. The maximum possible total
score is
210. In the original publication on the PANSS scale, 101 adult patients (20-68
years-old) with
schizophrenia were ranked. Their mean scores were a positive scale of 18.20, a
negative scale
of 21.01, and general psychopathology of 37.74. The mean total PANSS score for
these
subjects was 76.95.
[0245] In certain embodiments, the Positive and Negative Syndrome Scale
(PANSS) total
score for the subject decreases by at least 10 points than the placebo, for
example, after five
treatment weeks. In certain embodiments, the PANSS positive subscore decreases
by at least
3 points than the placebo, for example, after five treatment weeks. In certain
embodiments,
38
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
the PANSS negative subscore decreases by at least 2 points than the placebo,
for example,
after five treatment weeks.
[0246] Another scale used to assess patients is the Clinical Global Impression
¨ Severity
scale (CGI-S). This 7-point scale requires the clinician to rate the severity
of the patient's
illness at the time of assessment, relative to the clinician's experience with
patients who have
the same diagnosis. Possible ratings are (1) Normal, not at all ill; (2)
Borderline mentally ill;
(3) Mildly ill, (4) Moderately ill; (5) Markedly ill; (6) Severely ill, and
(7) Among the most
extremely ill patients. Among schizophrenia patients, changes in the CGI-S
follow a
consistent pattern relative to more objective PANSS scoring.
[0247] Before administering the disclosed combinations, patients may have a
lead-in period
from one to fourteen days, during which lead-in period trospium chloride is
given alone. In
one embodiment, the trospium chloride is administered for one or more dose
periods before
administering xanomeline to accumulate trospium chloride in the body or for
the trospium
chloride to reach or approach steady-state exposure levels. This accumulation,
or higher
exposure levels of the trospium chloride, increases the blockade of muscarinic
receptors
outside of the brain and reduces adverse events when xanomeline is
administered. In another
embodiment, the trospium chloride is administered for one or more days before
xanomeline.
[0248] Before administering the disclosed combinations, patients may
discontinue any prior
use of antipsychotic drugs. In some embodiments, the patients will discontinue
such drugs
for at least one week, such as two weeks. In some embodiments, patients do not
discontinue
any prior use of such antipsychotic drugs, and the disclosed combinations are
co-
administered with such drugs.
[0249] In one embodiment, xanomeline and trospium chloride are administered to
a patient 6
times during a 24-hour period. In another embodiment, xanomeline and trospium
chloride are
administered to a patient 5 times during a 24-hour period. In another
embodiment,
xanomeline and trospium chloride are administered to a patient 4 times during
a 24-hour
period. In an embodiment, xanomeline and trospium chloride are administered to
a patient 3
times during a 24-hour period. In another embodiment, xanomeline and trospium
chloride are
administered to a patient twice during a 24-hour period. In another
embodiment, xanomeline
and trospium chloride are administered to a patient once during a 24-hour
period.
[0250] In one embodiment, an extended-release formulation of trospium chloride
is used in
combination with xanomeline. In another embodiment, trospium chloride extended-
release is
administered to a patient from one time to five times during a 24-hour period.
In an
39
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
embodiment, the extended release of trospium chloride is administered from one
to three
times during a 24-hour period. In another embodiment, from five milligrams to
400
milligrams of trospium chloride, extended-release is used during a 24-hour
period. In an
embodiment, from 20 milligrams to 200 milligrams of trospium chloride extended-
release is
used during a 24-hour period.
[0251] In one embodiment, 225 mg xanomeline and 40 mg trospium chloride are
administered to a patient in a 24-hour period. In another embodiment, 100 mg
xanomeline
and 20 mg trospium chloride are administered to a patient in a 24-hour period.
In another
embodiment, 125 mg xanomeline and 20 mg trospium chloride are administered to
a patient
in a 24-hour period. In another embodiment, 125 mg xanomeline and 30 mg
trospium
chloride are administered to a patient in a 24-hour period. In another
embodiment, 125 mg
xanomeline and 40 mg trospium chloride are administered to a patient in a 24-
hour period. In
another embodiment, 200 mg xanomeline and 40 mg trospium chloride are
administered to a
patient in a 24-hour period. In another embodiment, 200 mg xanomeline and 80
mg trospium
chloride are administered to a patient in a 24-hour period. In another
embodiment, 250 mg
xanomeline and 60 mg trospium chloride are administered to a patient in a 24-
hour period. In
another embodiment, 250 mg xanomeline and 80 mg trospium chloride are
administered to a
patient in a 24-hour period. In another embodiment, 300 mg xanomeline and 40
mg trospium
chloride are administered to a patient in a 24-hour period. In another
embodiment, 300 mg
xanomeline and 60 mg trospium chloride are administered to a patient in a 24-
hour period. In
another embodiment, 300 mg xanomeline and 80 mg trospium chloride are
administered to a
patient in a 24-hour period. In another embodiment, 350 mg xanomeline and 40
mg trospium
chloride are administered to a patient in a 24-hour period. In another
embodiment, 350 mg
xanomeline and 60 mg trospium chloride are administered to a patient in a 24-
hour period. In
another embodiment, 350 mg xanomeline and 80 mg trospium chloride are
administered to a
patient in a 24-hour period.
[0252] Treatment may be initiated with smaller dosages. After that, small
increments may
increase the dosage until a balance between therapeutic effect and side
effects is attained.
While the subject is being treated, the patient's health may be monitored by
measuring one or
more of the relevant indices at predetermined times during the treatment
period. Treatment,
including composition, amounts, administration, and formulation times, may be
adjusted per
such monitoring. The patient may be periodically reevaluated to determine
improvement by
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
measuring the same parameters. Adjustments to the disclosed composition
administered and
possibly to the administration time may be made based on these reevaluations.
[0253] Provided is a method of treating schizophrenia or a disease related to
schizophrenia in
a patient in need thereof, the method comprising: orally administering to the
patient twice
daily an oral pharmaceutical composition comprising a plurality of xanomeline
beads
comprising xanomeline or a salt thereof, and a plurality of trospium beads
comprising a salt
of trospium, via a titration scheme that comprises up-titration of the
xanomeline, or a salt
thereof, and the salt of trospium.
[0254] Also provided is a method of treating schizophrenia or a disease
related to
schizophrenia in a patient in need thereof, the method comprising: orally
administering twice
daily an oral pharmaceutical composition comprising a plurality of xanomeline
beads
comprising xanomeline or a salt thereof, and a plurality of trospium beads
comprising a salt
of trospium, via a titration scheme that comprises up-titration of the
xanomeline, or a salt
thereof, and the salt of trospium until an amount equivalent to 125 mg
xanomeline free base
and an amount equivalent to 30 mg trospium chloride is administered.
[0255] In certain embodiments, the xanomeline, or a salt thereof, is
administered for the first
period in a first amount, and then the first amount is increased to a second
amount. In certain
embodiments, the first amount of xanomeline is equivalent to 50 mg xanomeline
free base. In
certain embodiments, the first period for the xanomeline administration is
between 1 and 5
days, such as 2 days. In certain embodiments, the second amount of xanomeline
is equivalent
to 100 mg xanomeline free base.
[0256] In certain embodiments, the method further comprises administering the
xanomeline,
or a salt thereof, for the second period in the second amount and then
increasing the second
amount to a third amount. In certain embodiments, the second period for
xanomeline
administration is between three days and a week. In certain embodiments, the
third amount of
xanomeline is equivalent to 125 mg xanomeline free base.
[0257] In certain embodiments, the salt of trospium is administered for the
first period in a
first amount, and the first amount is increased to a second amount. In certain
embodiments,
the first amount of the salt of trospium is equivalent to 20 mg trospium
chloride. In certain
embodiments, the first period for trospium administration is at least a week.
In certain
embodiments, the second amount of the salt of trospium is equivalent to 30 mg
trospium
chloride.
[0258] In certain embodiments, if the patient is not tolerating the higher
dose of xanomeline,
41
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
or a salt thereof, and the salt of trospium, the amount of xanomeline, or a
salt thereof, and the
salt of trospium administered to the patient is decreased.
[0259] In certain embodiments, the xanomeline, or a salt thereof, and the salt
of trospium are
administered without causing a severe adverse event.
[0260] "Blood pressure" refers to the pressure of circulating blood on the
walls of blood
vessels. Most of this pressure is due to the heart pumping blood through the
circulatory
system. Used without further specification, "blood pressure" usually refers to
the pressure in
large arteries of the systemic circulation. Blood pressure is usually
expressed in terms of the
systolic pressure (maximum during one heartbeat) over diastolic pressure
(minimum in
between two heartbeats) and is measured in millimeters of mercury (mmHg),
above the
surrounding atmospheric pressure. Normal resting blood pressure in an adult is
about 120
mmHg (16 kPa) systolic and 80 mmHg (11 kPa) diastolic, abbreviated "120/80
mmHg."
[0261] An adverse event related to blood pressure involves untoward medical
occurrence
affecting the systolic or diastolic blood pressure or changes to systolic or
diastolic blood
pressure, including hypertension, hypotension, and syncope (fainting). In
certain
embodiments, the xanomeline, or a salt thereof, and the salt of trospium are
administered
without inducing a change in diastolic blood pressure of more than about 5
mmHg. In certain
embodiments, the xanomeline, or a salt thereof, and the salt of trospium are
administered
without inducing a change in systolic blood pressure of more than about 5
mmHg. In certain
embodiments, the xanomeline, or a salt thereof, and the salt of trospium are
administered
without causing a severe adverse event related to blood pressure. In certain
embodiments, the
xanomeline, or a salt thereof, and the salt of trospium are administered
without causing a
severe adverse event related to blood pressure change.
[0262] "Heart rate" refers to the speed of the heartbeat measured by the
number of
contractions (beats) of the heart per minute (bpm). It is usually equal or
close to the pulse
measured at any peripheral point. The American Heart Association states that
the normal
resting adult human heart rate is 60-100 bpm. Tachycardia is a fast heart
rate, defined as
above 100 bpm at rest. Bradycardia is a slow heart rate, defined as below 60
bpm at rest,
except during sleep, when a slow heartbeat with rates around 40-50 bpm is
common and
normal. When the heart is not beating in a regular pattern, this is referred
to as an arrhythmia.
[0263] An adverse event related to heart rate involves an untoward medical
occurrence,
including tachycardia, bradycardia, and arrhythmia. In certain embodiments,
the xanomeline,
or a salt thereof, and the salt of trospium are administered without causing a
severe adverse
42
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
event related to heart rate. In certain embodiments, the xanomeline, or a salt
thereof, and the
salt of trospium are administered without causing a severe adverse event
related to heart rate
change.
[0264] Liver function tests (LFTs or LFs), also referred to as a hepatic
panel, are groups of
blood tests that provide information about the state of a patient's liver.
These tests include
prothrombin time (PT/INR), aPTT, albumin, bilirubin (direct and indirect),
liver
transaminases aspartate transaminase (AST or SGOT), alanine transaminase (ALT
or SGPT),
and others. A patient's blood sample is tested for functionality (e.g.,
albumin), integrity (e.g.,
transaminase), and conditions linked to the biliary tract (gamma-glutamyl
transferase and
alkaline phosphatase).
[0265] In certain embodiments, the xanomeline, or a salt thereof, and the salt
of trospium are
administered without increasing a liver function test (LFT). In certain
embodiments, the
xanomeline, or a salt thereof, and the salt of trospium are administered
without causing
elevated LFT. In some embodiments, the liver function test is chosen from
prothrombin time
(PT/INR), aPTT, albumin, bilirubin (direct and indirect), liver transaminases
aspartate
transaminase (AST or SGOT), and alanine transaminase (ALT or SGPT). In some
embodiments, the xanomeline, or a salt thereof, and the salt of trospium are
administered
without increasing at least one of ALT, AST, Alk phos, or bilirubin. In some
embodiments,
the xanomeline, or a salt thereof, and the salt of trospium are administered
without increasing
ALT, AST, Alk phos, or bilirubin.
[0266] The present disclosure further provides a method of treating acute
psychosis in a
patient in need thereof. The method comprises orally administering to the
patient twice daily
an oral pharmaceutical composition comprising xanomeline or a salt thereof,
and a salt of
trospium.
[0267] In certain embodiments, at least about an 11.6 point mean reduction in
total PANNS
score is achieved. In certain embodiments, at least a 3 point mean reduction
in PANSS
positive subscore compared to placebo is achieved. In certain embodiments, at
least a 2 point
reduction in the PANSS negative subscore compared to placebo is achieved. In
certain
embodiments, the reduction in the PANSS score is achieved within about 5
weeks. In certain
embodiments, before administering the oral pharmaceutical composition, the
patient had a
Clinical Global Impression Severity Scale (CGI-S) score of 4-7.
[0268] In certain embodiments, the patient has a diagnosis of schizophrenia.
In certain
embodiments, the patient has acute psychosis. In certain embodiments, the
patient has
43
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
psychosis associated with Alzheimer's disease. In certain embodiments, the
patient has a
schizo-affective disorder. In certain embodiments, the patient has psychosis.
In certain
embodiments, the patient has a delusional disorder. In certain embodiments,
the patient has
psychosis associated with Parkinson's disease. In certain embodiments, the
patient has
psychotic depression. In certain embodiments, the patient has bipolar
disorder. In certain
embodiments, the patient has bipolar disorder with psychosis. In certain
embodiments, the
patient has Huntington's disease. In certain embodiments, the patient has Lewy
Body
dementia.
[0269] In certain embodiments, the patient previously had been administered
one or more
antipsychotics. In certain embodiments, the patient was an inadequate
responder to such
administration. In certain embodiments, the patient was treatment-resistant.
[0270] In certain embodiments, the patient is an adult. In certain
embodiments, the patient is
elderly, e.g., above the age of 65 years. In certain embodiments, the patient
has dementia-
related psychosis.
EXAMPLES
[0271] The following examples are provided for illustration and are not
intended to limit the
scope of the disclosure.
Example 1 ¨ Immediate Release Beads
[0272] Beads were prepared for xanomeline tartrate (Table 1) and trospium
chloride (Table
2).
Table 1: Xanomeline tartrate (66%) Bead without Talc
Ingredient % w/w (dry basis) gjibatch
Xanomeline tartrate 66 99
Microcrystalline cellulose 34 51
Purified water* (30) (45)
Total: 100 150
*Removed during drying.
Table 2: Trospium chloride (17.7%) Bead without Talc
Ingredient % w/w (dry basis) gjibatch
Trospium chloride 17.7 17.7
44
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
Microcrystalline cellulose 35 35
Lactose monohydrate 47.3 47.3
Purified water* (45) (45)
Total: 100 100
*Removed during drying.
[0273] The powders were screened using Quadro Comil Model 197 equipped with
457-um
round hole screen, 0.2-inch spacer at 1625 rpm and mixed for 2 mm in a Hobart
low shear
mixer/granulator (model N-50) at a fixed speed of 60 rpm. The dry blending
step is optional,
as blend uniformity is driven by subsequent wet granulation. Beads were
screened by hand
through a 40 mesh (425 um) sieve.
[0274] Wetting was carried out in Hobart. The water was added using a Cole-
Parmer
peristaltic pump. The water addition rate (amount of water /dose time) is a
process variable.
[0275] The wet mass was extruded through a perforated screen (dome
configuration) single
screw extruder using an LCI Multi Granulator MG-55 at 30 rpm (shaft speed).
The wet mass
was extruded directly after wetting. Hold time, shaft speed, and extrusion
rate (load) were
process variables.
[0276] The extrudates were placed into an LCI Marumerizer (spheronizer) QJ-
230T equipped
with a 2.0 mm friction plate. The extrudates were spheronized at different
plate speeds for a
total of not more than 4 minutes. Spheronization speed and time are process
variables.
[0277] The beads were dried using an AeromaticTM Strea-1 fluid bed at an inlet
temperature
of 60 C until a water content of not more than 3% was obtained. Because beads
melted after
a few minutes at 60 C, the beads were dried at 30 C.
[0278] Water content was evaluated gravimetrically by loss-on-drying (LOD)
using a Mettler
Toledo Halogen Moisture Analyser, type HR83. The beads were heated at 105 C
until the
weight loss rate dropped to less or equal to 0.0 % within 60 seconds.
Table 3: Extrusion/Spheronization Process Parameters
Xanomeline tartrate Trospium chloride
Parameter
(66% w/w) (17.7% w/w)
Wet massing
Powder (g) 150 100
Water (g) 45 45
% (w/w) dry basis 30 45
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
Dose time (min) 3 3
Total massing time (mm) 3.5 3.5
Liquid rate (g/min) 15 17
Extrusion
Hold time (min) 0 0
Die hole size (mm) 0.8 0.8
Shaft speed (rpm) 30 30
Load (Ap) 2.3 2.2-2.4
Spheronization
Plate speed (rpm) 900/1500 900
Spheronization time
1/1 2
(mm)
Drying
Inlet Temp.( C) 60 60
Outlet Temp. ( C) NMT 53 NMT 53
Drying time (min) 75 30
LOD (%) 3.5 2.5
Example 2 - Scaling up Immediate Release Bead Formulations
[0279] The beads from Example 1 were scaled-up with and without talc (Tables 4-
7).
Extrusion/Spheronization process parameters are shown in Table 8.
Table 4: Xanomeline Tartrate (66%) Beads Without Talc
Ingredient % w/w (dry basis) g/batch
Xanomeline tartrate 66 660
Microcrystalline cellulose 34 340
Purified water* (24) (240)
Total: 100 1000
*Removed during drying.
Table 5: Xanomeline tartrate (66%) Bead with Talc
Ingredient Purpose % wiw (dry basis)
gibatch
Xanomeline tartrate Active 66.0 3,465.0
46
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
Microcrystalline cellulose Binder, 33.5 1758.75
(USP, Ph. Eur.) disintegrant
Granulating (30.0) (1575,0)
Purified water* (USP)
fluid
Talc (USP, Ph. Eur.) Glidant 0.5 26.25
Total 100.0 5,250.0
Abbreviations: Ph. Eur = European Pharmacopeia, USP = United States
Pharmacopeia
* - Evaporated during the process thus not included in total weight
Table 6: Trospium Chloride (17.7%) Beads Without Talc
Ingredient % w/w (dry basis) gjbatch
Trospium chloride 17.7 88.7
Microcrystalline cellulose 35 175.0
Lactose monohydrate 47.3 236.3
Purified water* (59) (295)
Total: 100 500
*Removed during drying.
Table 7: Trospium chloride (17.7%) Bead with Talc
Ingredient Purpose % wily (dry basis)
g/batch
Trospiurn chloride (USP) Active 17.7 593.6
Microcrystalline cellulose Binder, 46.8 1567.15
(USP, Ph. Eur.) disintegrant
Lactose monohydrate (NF) Filler 35.0 1,172.5
Granulating (47.0) (1574.5)
Purified water* (USP)
fluid
Talc (USP, Ph. Eur.) Glidant 0.5 16.75
Total 100 3,350.0
Abbreviations: NF = National Formulary, Ph, Eur = European Pharrn.acopeia, USP
= United
States Pharmacopeia. * - Evaporated during process
Table 8: Extrusion/Spheronization Process Parameters
47
CA 03161952 2022-05-17
WO 2021/101875 PCT/US2020/060859
Xanomeline tartrate Trospium chloride
Parameter
(66% w/w) (17.7% w/w)
Wet massing
Powder (g) 1000 500
Water (g) 240 295
% (w/w) dry basis 24 59
Dose time (mm) 3 4
Total massing time (min) 3.5 4.5
Liquid rate (g/min) 80 82
Extrusion
Hold time (mm) 0 0
Die hole size (mm) 0.8 0.8
Shaft speed (rpm) 30 30
Load (Ap) 2.2-2.3 2.4-2.5
Spheronization
Plate speed (rpm) 900 900
Spheronization time (mm) 0.5 1
Drying
Inlet Temp.( C) 60 60
Outlet Temp. ( C) NMT 50 NMT 49
Drying time (min) 50 40
LOD (%) 2.3 2.4
Example 3 - Capsule Stability and Dissolution Testing
[0280] An oral pharmaceutical composition comprising a plurality of xanomeline
beads
comprising xanomeline or a salt thereof, and a plurality of trospium beads
comprising a salt
of trospium is referred to as "KarXT." KarXT may be formulated in many dosage
strengths,
for example, as exemplified below KarXT 50/10, KarXT 50/20, and KarXT 75/20,
wherein
the number before the slash is the milligrams of xanomeline free base in the
composition (X)
and the number after the slash is the milligrams of trospium chloride in the
composition (T).
[0281] Capsules were produced by weighing beads and filling into HPMC capsules
manually.
Beads were encapsulated by hand using an AccofilTM capsule filling machine
where beads
48
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
premixed with talc (0.5%) were filled individually/one-after-the-other in the
capsule, as
shown in Table 19.
Table 9: Composition of Xanomeline / Trospium Chloride Capsules. Ingredients
are
listed in milligrams per capsule.
Function 25 mg / 50 mg / 50 mg / 75 mg / 75 mg /
Ingredient
mg 10 mg 20 mg 10 mg 20 mg
Xanomeline drug beads Active ingredient 58,1 116.1 116.1 174.2
174.2
Xanomeline tartrate Drug substance 38.3 76.6 76.6 115.0 115.0
Rota! weight (freebase)1 (25.0) (50.0) (50.0) (75.0) (75.0)
tvlicrocrystalline cellulose Binder, 19.5 38.9 38.9 58.4
58.4
(USP, Ph.Eur.) disintegrant
Talc (USP, Ph. Eur.) Glidant 0,3 0,6 0,6 0.9 0.9
Trospium drug beads Active ingredient 56.5 56.5
113.0 56.5 113.0
Trospium chloride (USP) Drug substance 10 10 20 10 20
Microcrystalline cellulose Binder, 26.4 26.4 52.9 26.4 52.9
(USP, Ph, Eur.) disintegrant
Lactose monohydrate, NF Filler 19.8 19.8 39.6 19.8 39.6
Talc (USP, Ph. Eur.) Glidant 0,3 0,3 0,6 0.3 0.6
HPMC capsule shell Capsule 95.6 95.6 95.6 95.6 95.6
Hydroxypropyl Structure 93,7 93,7 93.7 93.7 93.7
methylcellulose (USP, Ph,
Eur.)
Titanium dioxide Colorant 1.9 1.9 1.9 1.9 1.9
(USP, Ph. Eur.)
Total 210.2 268.2 324.7 326.3 382.8
[0282] After drying, the beads were screened by shaking 5 min through 16 mesh
(1.18 mm)
and 40 mesh (0.425 mm) screens. The beads in size between sieves 1.18 mm and
0.425 mm
were retained for further analysis.
49
CA 03161952 2022-05-17
WO 2021/101875 PCT/US2020/060859
[0283] The morphology and surface characteristics of beads were examined by
scanning
electron microscopy (SEM) using a JSM-6010LV InTouchScopeTm (JEOL Ltd, Tokyo,
JP)
microscope with a back-scattered electron detector (BES). Samples were placed
on metallic
stubs using double-sided carbon conductive tape. The images were obtained with
accelerating
voltages of 20 kV under low vacuum (60 Pa) and magnification 30x.
[0284] Bulk and tapped density were determined in duplicate using the USP
<616> method
using a tapped density tester (JV 1000, Copley Scientific). The bulk density
was measured
from the volume of a known mass of powder sample in a graduated cylinder. The
tapped
density was measured by mechanically tapping the measuring cylinder until the
volume
changed no further.
[0285] The powder flow properties were evaluated using Carr's Compressibility
Index and
Hausner ratio. Both were derived using the measured values for bulk and tapped
density.
Carr's Compressibility Index (CI) was calculated using bulk and tapped density
data when
fitted into the equation: Compressibility Index = (Tapped density ¨ Bulk
density) / Tapped
density x 100%. Hausner Ratio (H) was calculated as the ratio of tapped to
bulk density.
Capsules were analyzed for appearance, assay, related substances, water
content, and
dissolution.
[0286] The beads were further sized between 0.6 mm and 0.85 mm. Some beads
exhibited
similar morphological properties. Modifications in some other beads decreased
the density of
beads and led to rough surfaces and sphericity loss. Scanning electron
microscope (SEM)
images of xanomeline tartrate 66% beads and trospium chloride 17.7% beads at
30x
magnification showed that the beads are sized between 0.6 mm and 0.85 mm.
These beads
were used in xanomeline/trospium capsules. Particle size distribution (PSD) of
beads was
determined by mechanical sieving. As shown in Table 10, most beads for both
APIs were
sized between 0.425 and 1.18 mm.
Table 10: Particle Size Distribution by Mechanical Sieving of Beads
Sieve No. (opening % Retained
diameter) 66% Xanomeline tartrate 17.7% Trospium
chloride
16 mesh (1.18 mm) 8.1 0.4
40 mesh (0.425 mm) 90.6 97.3
Receiver 1.3 2.3
Total: 100 100
CA 03161952 2022-05-17
WO 2021/101875 PCT/US2020/060859
[0287] Table 11 shows the densities and flow properties of beads collected
between 0.425
mm and 1.18 mm sieves. Xanomeline tartrate and trospium chloride IR beads
showed
different densities and flow properties, which can be critical when mixing
bead systems.
Table 11: Density and Flow Properties of 0.425-1.18 mm Beads
Bulk density Tapped density Carr Hausner
Sample ID
(g/cm3) (g/cm3) Index (%)
Ratio
Xanomeline tartrate (66%)
0.59/0.58 0.63/0.62 7/7 1.08/1.08
beads ¨ Example 1
Xanomeline tartrate (66%)
0.54/0.54 0.58/0.57 6/6 1.07/1.07
beads ¨Scale up
Trospium chloride (17.7%)
0.81/0.80 0.83/0.83 2/3 1.02/1.04
beads - Example 1
Trospium chloride (17.7%)
0.78/0.79 0.81/0.82 3/3 1.03/1.03
beads - Scale up
[0288] The analysis in Table 12 shows favorable results for assay and related
substances and
moisture content for 50 mg xanomeline and 20 mg trospium chloride capsules.
Data in Table
13 show that these attributes were retained during storage stability studies.
Similar data are
provided for the 50 mg xanomeline and 10 mg trospium chloride capsules in
Table 14.
Dissolution data for these two dosage forms are provided in Table 15 and Table
16.
Table 12: Analytical Results
Trospium Chloride/ Xanomeline Trospium
Chloride/ Xanomeline
Formulation
Tartrate Beads in Capsules Tartrate
Beads in Capsules
20 mg salt Trospium Chloride 10 mg
salt Trospium Chloride
Dose strength
50 mg Xanomeline free base 50 mg
Xanomeline free base
Description White opaque capsules White opaque capsules
Trospium chloride 98.9% Trospium chloride 97.1%
Assay (n=2: 99.2, 98.5) (n=2: 97.1, 97.1)
(%LC) Xanomeline free base 99.4%
Xanomeline free base 100.6%
(n=2: 100.1, 98.8) (n=2: 100.3, 101.0)
Related Substances
No impurities >0.1%LC No impurities >0.1%LC
(%LC)
51
CA 03161952 2022-05-17
WO 2021/101875 PCT/US2020/060859
Moisture (KF)
2.4% 2.2%
(% w/w)
Table 13: Stability of KarXT 50/20
T = 0 White opaque capsules
T = lm, 40 C/75%RH No change from initials
T = 2m, 40 C/75%RH No change from initials
Description
T = 3m, 25 C/60%RH No change from initials
T = 3m, 40 C/75%RH No change from initials
T = 6m, 40 C/75%RH No change from initials
Trospium chloride: 98.9 (99.2, 98.5)
T = 0
Xanomeline free base: 99.4 (100.1, 98.8)
T= lm Trospium chloride 100.4 (97.8, 103.1)
40 C/75%RH Xanomeline free base: 101.7 (101.6, 101.8)
T = 2m Trospium chloride: 98.2 (98.7, 97.7)
Assay ( %LC) 40 C/75%RH Xanomeline free base: 99.3 (100.3, 98.3)
T = 3m Trospium chloride: 99.1 (99.7, 98.4)
25 C/60%RH Xanomeline free base: 102.0 (103.7, 100.3)
T = 3m Trospium chloride: 98.4 (98.5, 98.3)
40 C/75%RH Xanomeline free base: 99.9 (99.8, 100.0)
T = 6m Trospium chloride: 96.0 (95.6, 96.4)
40 C/75%RH Xanomeline free base: 97.8 (97.6, 98.1)
T = 0 No impurities >0.1%LC
T = lm, 40 C/75%RH No impurities >0.1%LC
Related
T = 2m, 40 C/75%RH 0.14%
Substances
T = 3m, 25 C/60%RH No impurities >0.1%LC
(%LC)
T = 3m, 40 C/75%RH 0.14%
T = 6m, 40 C/75%RH 0.2%
T = 0 2.4%
Moisture (KF)
T = lm, 40 C/75%RH 3.0%
(% w/w)
T = 2m, 40 C/75%RH 3.3%
USP <921>
T = 3m, 25 C/60%RH 2.7%
Method la
T = 3m, 40 C/75%RH 2.6%
52
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
T = 6m, 40 C/75%RH 3.4%
Table 14: Dissolution of KarXT 50/20
Active Trospium
chloride Xanomeline free base
Time (min) % LC Range % LC Range
10 77 90,88,52 76 93,87,47
20 99 101,99,97 98 98,97,98
T = 0
30 100 101,99,99 98 99,97,99
45 101,100,9
100 98 98,97,99
9
60 (ramp) 100 101,99,99 98 98,97,99
Active Trospium chloride
Xanomeline free base
Time
Range % LC Range
(mm) LC
Dissolution T = lm
81 78, 78, 85 81 77, 86, 80
900 mL 0.1N 40 C!
100 102, 95, 102 97 99, 98, 93
HC1 75%RH
101 102, 97, 103 97 99, 99, 94
Paddles @ 50
45 101 102, 97, 103 97 99, 99,
93
rpm, ramp @ 200
60 (ramp) 101 102, 97, 103 97 99, 99,
93
rpm after 45 min
Active Trospium chloride
Xanomeline free base
(n=3)
Time (mm) % LC Range % LC Range
T = 2m
10 68 83, 74, 48 76 92, 82,
55
C/
20 95 98, 93, 94 98 101, 98,
96
75%RH
30 97 99, 95, 96 100 103,
99, 98
97 99, 95, 96 100 103, 99, 98
Active Trospium chloride
Xanomeline free base
Time (mm) % LC Range % LC Range
T = 3m
10 78 84, 80, 69 87 94, 93,
75
25 C/
20 96 99, 96, 91 101
104, 103, 97
60%RH
30 97 99, 97, 95 102
104, 104, 99
45 97 99, 97, 96 103 104,
104, 101
53
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
Active Trospium chloride
Xanomeline free base
Time (min) % LC Range % LC Range
T = 3m
10 84 90, 84, 78 90 95, 89, 87
40 C/
20 97 98, 98, 96 99 99, 98, 99
75%RH
30 97 97, 98, 96 99 99, 99, 100
45 97 97, 98, 96 99 99, 99, 100
Active Trospium chloride
Xanomeline free base
Time (mm) % LC Range % LC Range
T = 6m
10 72 85, 53, 78 79 92, 58, 86
40 C/
20 96 98, 92, 98 98 99, 94, 100
75%RH
30 98 99, 95, 99 99 99, 97, 101
45 99 100, 96, 99 100 100, 98, 101
Table 15: Assay and Related Substances of KarXT 50/10
T = 0 White opaque capsules
T = lm, 40 C/75%RH No change from initials
Description T = 2m, 40 C/75%RH No change from initials
T = 3m, 25 C/60%RH No change from initials
T = 3m, 40 C/75%RH No change from initials
Trospium chloride: 97.1 (97.1, 97.1)
T = 0
Xanomeline free base: 100.6 (100.3, 101.0)
T = lm Trospium
chloride: 98.5 (98.2, 98.9)
40 C/75%RH Xanomeline free base: 102.7 (104.4, 101.1)
T = 2m Trospium chloride: 96.7 (95.7, 97.6)
Assay (%LC)
40 C/75%RH Xanomeline free base: 98.8 (99.3, 98.3)
T = 3m Trospium
chloride: 98.5 (96.5, 100.5)
25 C/60%RH Xanomeline free base: 99.2 (98.2, 100.1)
T = 3m Trospium
chloride: 98.1 (97.6, 98.6)
40 C/75%RH Xanomeline free base: 99.4 (99.0, 99.8)
Related T = 0 No impurities >0.1%LC
Substances T = lm, 40 C/75%RH No impurities >0.1%LC
54
CA 03161952 2022-05-17
WO 2021/101875 PCT/US2020/060859
(%LC) T = 2m, 40 C/75%RH 0.14%
T = 3m, 25 C/60%RH No impurities >0.1%LC
T = 3m, 40 C/75%RH 0.14%
T = 0 2.2% (n = 2: 2.4, 2.1)
Moisture (KF)
T = lm, 40 C/75%RH 2.1% (n = 2: 2.4, 1.9)
(% w/w)
T = 2m, 40 C/75%RH 2.2% (n = 3: 1.8, 2.4, 2.4)
USP <921>
T = 3m, 25 C/60%RH 2.1% (n = 3: 1.9, 2.4, 2.1)
Method la
T = 3m, 40 C/75%RH 2.5% (n = 3: 2.3, 2.6, 2.4)
Table 16: Dissolution of KarXT 50/10
mg Trospium Chloride
Dose strength
50 mg Xanomeline free base
Active Trospium Chloride Xanomeline free base
Time (min) % LC Range % LC Range
10 84 85, 86, 82 89 88,
90, 88
T = 0 20 96 97, 96, 94 97 96,
96, 98
30 96 97, 97, 94 97 96,
97, 98
45 96 97, 96, 94 97 96,
96, 98
Dissolution 60 (ramp) 96 97, 97, 94 97 96,
96, 98
900m1 0.1N ____________________________________________________________
Active Trospium Chloride Xanomeline free base
HC1
Time (min) % LC Range % LC Range
Paddles @50
T = lm 10 88 83, 91, 89 88 87, 92,
85
rpm
40 C! 20 101 100,101,101 95 96, 97,
94
ramp @ 200
75%RH 30 101 101,101,101 96 97, 97,
94
rpm after 45
45 101 102,101,101 96 97, 97,
94
min
(n=3) 60 (ramp) 101 102,101,102 96 97, 97, 94
Active Trospium Chloride Xanomeline free base
T = 2m Time (min) % LC Range % LC Range
40 C/ ____________________________________________________________
10 88 89, 91, 83 93 94, 91,
93
75%RH _____________________________________________________________
98 97, 102, 96 99 99, 98, 101
99 98, 103, 97 99 99, 98, 101
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
45 99 97, 103, 96 99 99, 98,
101
Active Trospium
Chloride Xanomeline free base
Time (min) % LC Range % LC Range
T = 3m
88 79, 91, 94 93 86, 94, 99
25 C!
99 95, 99, 102 98 95, 97, 102
60%RH
99 95, 99, 102 98 95, 96, 102
45 99 95, 99, 102 98 95, 96,
102
Active Trospium
Chloride Xanomeline free base
Time (mm) % LC Range % LC Range
T = 3m
10 90 89, 90, 91 92 90, 95,
90
C/7
20 98 99, 95, 99 95 95, 97,
94
5%RH
30 98 99, 95, 99 95 95, 97,
94
98 99, 95, 99 95 95, 97, 94
[0289] Subsequent testing showed that KarXT 50/10, 50/20, and 75/20 in hard-
shell capsules
were stable for at least 12 months 25 C/60%RH. Based on available data, a
shelf-life of 15
months at 25 C/60%RH is proposed.
[0290] The dissolution results show that the two compounds release quickly,
which may
increase their bioavailability. They also release at comparable rates despite
substantial
differences in compositions between the two bead formulations. Both xanomeline
and
trospium chloride have low bioavailabilities, and rapid release can increase
bioavailability by
overwhelming saturable processes that limit absorption into the general
circulation.
[0291] An unknown xanomeline impurity with a relative retention time of about
1.09 was
observed during stability studies of the combination drug products. The
impurity was first
observed during testing at the three-month time point for the 50 mg
xanomeline/10 mg
trospium chloride drug product and at the initial time point for the other
three combination
products, both of which occurred at the same time. The impurity peak increased
both with
time and with increasing storage temperature. The impurity had not been
observed before the
present studies.
[0292] Preliminary studies suggest that the RRT 1.09 impurity is 34(4-
hexyloxy)-1,2,5-
thiadizao1-3-y11-5-hydroxyl-1-methylpyridin-1-ium (Ci4H2oN302S+, MW = 294.1271
Da):
56
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
N
µS¨N
[0293] The RRT 1.09 impurity is a hydroxylated version of Compound V
(Ci4H2oN30S+,
MW = 278.1322 Da), which is the penultimate intermediate in the synthesis of
xanomeline
with negative mutagenic potential:
N+
µS¨N
[0294] To reduce the presence of the impurity, the storage temperature for the
drug product
was lowered. Bottles were flushed with argon to minimize headspace oxygen
during
packaging. In certain embodiments, the xanomeline bead formulation was
formulated with an
antioxidant, such as 0.5 wt.% ascorbic acid or 0.05 wt.% BHT.
Example 4 - KAR-001 Phase I study of Combination of Xanomeline and Trospium
Chloride
[0295] A Phase I, double-blind, randomized multiple-dose pilot study was
conducted with
xanomeline administered alone compared to xanomeline administered with
trospium chloride
in normal healthy volunteers. The primary objectives of this study were (1) to
assess the
safety and tolerability of administering, for 7 days, 225 mg daily of
xanomeline with 40 mg
daily of trospium chloride, versus administering 225 mg daily of xanomeline
alone for 7
days; and (2) to determine whether adding trospium 40 mg daily (20 mg BID) to
xanomeline
225 mg daily (75 mg TID) over 7 days significantly reduces peripheral
cholinergic side
effects (nausea, diarrhea, vomiting, sweating, excess salivation) versus
xanomeline 225 mg
daily, alone. Table 17 lists the parameters from this study.
Table 17: Parameters of the KAR-001 study
Sample Size: N = 70 subjects
Study Population: Normal healthy volunteers; ages 18-60
Study Duration: Treatment: Nine days; a two-day run-in period of either
placebo or
trospium 40 mg/day, followed by 7 days of active treatment
Follow-up: 14 days following discharge from the clinic
57
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
Test product, Xanomeline, 75 mg capsules, TID, for a 225-mg total daily
dose
dose, and mode Trospium chloride, 20 mg tablet, over-encapsulated, for a 40-
mg total
of administration: daily dose, BID. Matching placebo.
Study Design The study was an inpatient study conducted in normal healthy
volunteers.
Between study days ¨21 to ¨7, normal healthy volunteers visited the
clinic to receive and sign Informed Consent and undergo screening
procedures.
Patients entered the clinic on Study Day 0 for baseline safety assessment
and enrollment in the study.
On the morning of Study Day 1 subjects began the administration of study
drugs. Subjects randomized to the xanomeline-only arm received a
placebo for the first two days and began TID xanomeline treatment on
Day 3. Subjects randomized to the xanomeline + trospium arm received
BID trospium chloride for the first two days, and then TID xanomeline
plus BID trospium starting on Day 3. Matching placebo was administered
to maintain the blind. Patients remained in the clinic under observation for
the full duration of treatment (9 days).
Main criteria for Age 18-60
inclusion: Female subjects had to be postmenopausal (at least 2 years
before dosing)
or agree to use an acceptable form of birth control from screening until 14
days after completing the study. If on birth control pills, they had to have
been on a stable dose for 12 months.
Good general health
Ability to give informed consent and understand verbal instructions.
Willingness to spend 10 days in an in-patient facility.
Main criteria for History or presence of clinically significant
cardiovascular, pulmonary,
exclusion: hepatic, renal, hematologic, gastrointestinal, endocrine,
immunologic,
dermatologic, neurologic, oncologic, or psychiatric disease or any other
condition that, in the opinion of the investigator, would jeopardize the
safety of the subject or the validity of the study results. (Subjects with any
history of resolved cancer that was >5 years passed could be included.)
Body Mass Index <18 or >40 kg/m2
58
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
History of or high risk of urinary retention, gastric retention, or narrow-
angle glaucoma.
History of alcohol or drug abuse within the last 24 months, or current
abuse as determined by urine toxicology screen.
Clinically significant abnormal finding on the physical exam, medical
history, ECG, or clinical laboratory results at screening.
Had participated in another clinical trial within 90 days before the first
dose of study medication.
Needed to take any prescription medication besides the investigational
product or those specifically noted above.
Use of any vitamins, herbs, supplements, or over-the-counter medications
are excluded within one week of enrollment, and during the trial.
Specifically, subjects were not permitted to take Benadryl for one week
before and during the study. Use of any tobacco products within the past
30 days.
Previous positive test for HIV 1 and/or 2, or Hepatitis A, B, or C, or a
positive test obtained at screening.
Selected Treatment-emergent signs and symptoms (adverse event incidence
rates).
Endpoints: Cholinergic treatment-emergent signs and symptoms (salivation,
sweating, nausea, vomiting, diarrhea) (cholinergic adverse event
incidence rates). These adverse events were observed at high rates in past
xanomeline studies and were drivers of subject discontinuation.
[0296] Seventy total study subjects were randomized, and of these, 68 study
subjects
received at least one assessment on day 3, which was the first day of
xanomeline
administration. Table 18 lists the demographics of the study subjects.
Table 18: Demographics of the KAR-001 study subjects
Xanomeline alone Xanomeline + Trospium
Characteristic (N = 33) (N = 35)
Age (years; Mean [SD]) 34.8 [8.8] 40.9 [12.3]
Gender (M/F; [%]) 21/12 27/8
64%/36% 77%/23%
Race (White/ 9/24 13/21
59
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
Non-White; [%]) 27%/70% 37%/60%
Weight (kg; Mean [SD]) 88 [17] 88 [16]
BMI (kg/m2; Mean [SD]) 29.1 [5.0] 28.8 [5.0]
[0297] The most common adverse events with xanomeline are the so-called
cholinergic
adverse events of nausea, vomiting, diarrhea, excessive sweating, and
excessive salivation. In
this study, the co-administration of trospium chloride with xanomeline led to
a statistically
significant (p = 0.016) 43% reduction in the incidence rate of cholinergic
adverse events
compared to xanomeline co-administered with placebo. In the xanomeline +
placebo arm of
the study, 63% of subjects reported at least one cholinergic adverse event,
compared to only
34% of subjects reporting such an event in the xanomeline + trospium chloride
arm of the
study.
[0298] Further, in the study, each kind of individual cholinergic adverse
event also had a
decreased incidence rate in subjects administered xanomeline + trospium
chloride, compared
to the incidence rate in subjects administered xanomeline + placebo. The
decrease in the
incidence rate of sweating was statistically significant on its own, at 20.0%
in the xanomeline
+ trospium chloride arm, down from 48.5% in the xanomeline + placebo arm,
which was a
59% reduction (p = 0.013).
[0299] The overall cholinergic adverse event rate in the xanomeline + trospium
chloride arm
of the study was very similar to the 32% incidence rate reported during the
two-day run-in
period for subjects on placebo + placebo. Although these two data points did
not occur during
different periods of the study, the fact that the cholinergic adverse event
rate was comparable
to that of placebo suggests that the 43% reduction in adverse events due to
trospium chloride
may have been close to the maximum reduction possible in this study.
[0300] Table 19 shows the incidence and number of cholinergic adverse events
in the
evaluable population of the study were as follows, with all p-values based on
a chi-squared
test, except those marked with an *, which were based on a Fisher's exact
test.
Table 19: Cholinergic adverse events
Xanomeline + Xanomeline + Trospium
placebo (n = 34) (n = 35)
Category (n [%1 [# of events]) (n [%1 [# of events]) P-
value for
difference Reduction
Any 21(63.6%) 64 12 (34.3%) 33 0.0155 46%
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
TEAEs
Nausea 8 (24.2%) 11 6 (17.1%) 8 0.4693 29%
Vomiting 5 (15.2%) 5 2 (5.7%) 2 0.2522* 62%
Diarrhea 7 (21.2%) 8 2 (5.7%) 4 0.0794* 73%
Sweating 16 (48.5%) 24 7 (20.0%) 8 0.0131 59%
Salivation 12 (36.4%) 16 9 (25.7%) 11 0.342 39%
[0301] There were no meaningful differences between treatment groups in heart
rate, resting
blood pressure, orthostatic blood pressure, or electrocardiogram (ECG)
parameters, including
QT. A small subset of subjects in both treatment arms had transient increases
in heart rate and
orthostatic blood pressure changes, which may have contributed to syncope and
postural
dizziness in those subjects. Two subjects (both in the xanomeline alone arm)
experienced
syncope. The incidence of orthostatic adverse events in the xanomeline +
trospium group was
about one-half of subjects in the xanomeline alone group. Only one subject
discontinued due
to a treatment-emergent adverse event in the xanomeline + trospium group
regarding blood
pressure.
[0302] In addition to evaluating whether adding trospium chloride increased
the tolerability
of xanomeline, the study also provided data about the overall safety and
tolerability of
xanomeline + trospium chloride. Table 20 shows that the combination was well
tolerated with
no severe adverse events and no serious adverse events, and with most adverse
events being
mild.
Table 20: Tolerability
Xanomeline + placebo Xanomeline + Trospium
Category (n (%) # events) (N = 33) (N = 35)
Subjects with any TEAE 27 (81.8) 108 23 (65.7) 73
Max Severity of TEAE
Mild 22 (66.7) N/A 20 (57.1) N/A
Moderate 5 (15.2) N/A 3 (8.6) N/A
Severe 0 (0.0) 0 (0.0)
Any clinically significant TEAE 5 (15.2) 5 3 (8.6) 6
Any study drug-related TEAE 23 (69.7) 92 18 (51.4) 57
Max severity of study drug-related TEAE
61
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
Mild 19 (57/6) N/A 15 (42.9) N/A
Moderate 4 (12.1) N/A 3 (8.6) N/A
Severe 0 (0.0) N/A 0 (0.0) N/A
Any SAE 0 (0.0) 0 (0.0)
AE leading to discontinuation 2 (6.1) 2 1(2.9) 1
(D/C)
Study drug related AE leading to 1 (3.0) 1 0 (0.0)
D/C
[0303] This study's tolerability profile allowed future studies of the
combination of
xanomeline and trospium chloride to proceed.
Example 5 ¨ KAR-003 Phase I Study of KarXT, a xanomeline + trospium combined
formulation
[0304] This study was a Phase 1, randomized, multiple-dose, adaptive design,
inpatient study
to assess the safety and tolerability of KarXT in normal healthy volunteers
aged 18 to 60
years. Subjects signed the informed consent and underwent Screening
assessments on
Days -21 to -1. Upon completing all Screening assessments, subjects returned
to the study
clinic on Day 0 for baseline safety assessments and enrollment into the study.
They were
randomized 3:1 in each cohort into one of two treatment arms: KarXT or
placebo. Subjects
were assigned to 1 of 4 cohorts (Cohort 1, 2, 3, or 4).
[0305] Study drug was administered BID on Days 1 through 7. A combination
dosage
formulation of both xanomeline and trospium was used in all cohorts. All
cohorts began with
a 2-day lead-in of KarXT 50/20 BID (for subjects randomized to active
treatment); after the
2-day lead-in period, the unblinded pharmacist dispensed the study drug to
each subject per
the subject's randomization assignment for 5 days of specified cohort dosing,
for a total of 7
days of treatment. A matching placebo was administered throughout the study to
maintain the
blind. A sentinel group was introduced to the study for Cohorts 2 to 4. It was
monitored for
safety and tolerability by the Data Safety Evaluation Group (DSEG), such that
about 30% of
the proposed cohort was treated and assessed for safety before the rest of the
cohort was
dosed. Subjects and study clinic staff were blinded to treatment. The Dose
Selection
Committee (DSC) was unblinded to decide to dose for subsequent treatment
groups.
[0306] Serial blood samples for the PK assessment of xanomeline and trospium
were drawn
on Days 1, 3, and 7. More blood was sampled at routine intervals for
monitoring trough
62
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
concentrations of xanomeline and trospium and clinical laboratory assessments.
On Day 1,
saliva volume was collected twice. A saliva volume was measured predose on Day
1 and then
daily (afternoon) on Days 1 through 7 at about the same time of day to avoid
diurnal
variations. Other assessments included pupil size measurements and Bristol
stool scale
assessments. Subjects remained in the study clinic for the full duration of
treatment (7 days).
Following a safety assessment on Day 8, subjects were discharged from the
study clinic and
asked to return about 14 days after administering the study drug for a final
safety assessment.
[0307] During the study, following the 2-day lead-in of KarXT 50/20 BID (for
subjects
randomized to active treatment) in each cohort, subjects were dosed as
follows:
= In Cohort 1, subjects completed Days 3 through 7 of dosing of KarXT
100/20 BID (total
daily dose (TDD) of 200 mg xanomeline plus 40 mg trospium) or placebo.
= In Cohort 2, the sentinel group (Group 2a) discontinued dosing after the
Day 4 morning
dose. The dosage for subjects in Cohort 2 was KarXT 150/20 BID (TDD of 300 mg
xanomeline plus 40 mg trospium) or placebo. Dosing of Cohort 2 was
discontinued
(DSEG decision based on observed tolerability concerns). The study dosing the
Cohort 3
sentinel group (Group 3a) as the DSC determined that further dosing of Cohort
2 with
KarXT 150/20 BID was unlikely to be tolerated well enough to warrant further
developing this dose combination for a clinical population.
= In Cohort 3, the sentinel group (Group 3a) completed Days 3 through 7 of
the dosing
of KarXT 150/40 BID (TDD of 300 mg xanomeline plus 80 mg trospium) or placebo.
The second group in Cohort 3 (Group 3b) discontinued dosing after the Day 5
morning
dose.
= In Cohort 4, the sentinel group (Group 4a), the second group (Group 4b),
and the
remaining group (Group 4c) completed Days 3 through 7 of the dosing of KarXT
125/40
BID (TDD of 250 mg xanomeline plus 80 mg trospium) or placebo.
[0308] Ninety-six subjects were planned, 248 subjects were screened, 69
subjects were
randomized, 51 subjects completed the study, and 18 subjects discontinued the
study. The
population included healthy male and female subjects aged 18 to 60 years at
screening with a
body mass index of 18 to 40 kg/m2. Subjects were excluded from the study if
they had a
history of irritable bowel syndrome or serious constipation requiring
treatment within 6
months before Screening. Subjects were also excluded from the study if they
had a history or
presence of any disease or condition, including psychiatric or neurological
diseases that
would have jeopardized the subject's safety or the study in the Investigator's
opinion's
63
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
validity. Table 21 summarizes the demographics and baseline characteristics by
treatment
group. The demographic and baseline characteristics were consistent between
the Safety
Population and the PK Population.
Table 21: Summary of Demographics and Baseline Characteristics by Treatment
Group - Safety Population
Characteristic Cohort 1
Cohort 2 Cohort 3 Cohort Placebo Total
Category/Statistic KarXT KarXT KarXT 4
100/20 150/20 150/40 KarXT
BID BID [1] BID [2] 125/40
BID
18 5 12 18 16 69
Mean (SD) 42.0 39.0 38.2 (9.4) 39.8 37.9
39.6
(12.9) (8.80) (9.56) (10.61) (10.51)
Gender - n (%)
Male 11 (61.1) 3 (60.0) 5 (41.7) 9 (50.0) 13
41
(81.3) (59.4)
Female 7 (38.9) 2 (40.0) 7 (58.3) 9 (50.0) 3
(18.8) 28
(40.6)
Race - n (%)
White 8 (44.4) 1(20.0) 7 (58.3) 6 (33.3) 4
(25.0) 26
(37.7)
Black or African 9(50.0) 4(80.0) 5(41.7) 12 (66.7) 12
42
American (75.0)
(60.9)
Asian 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0)
0 (0.0)
American Indian 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0)
0 (0.0)
or Alaska Native
Native Hawaiian 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0)
0 (0.0)
or Other Pacific
Islander
Other 1(5.6) 0 (0.0) 0 (0.0) 0 (0.0) 0 (0.0)
1 (1.4)
Ethnicity - n (%)
Hispanic or Latino 2 (11.1) 1(20.0) 2 (16.7) 2 (11.1)
1(6.3) 8
64
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
(11.6)
Not Hispanic or 16 (88.9) 4 (80.0) 10 (83.3) 16 (88.9) 15
61
Latino (93.8) (88.4)
Baseline weight (kg)
Mean (SD) 81.8 81.0 81.3 73.5 77.6 78.5
(15.0) (12.1) (13.6) (8.9) -- (10.3) -- (12.2)
Baseline height (cm)
Mean (SD) 172.5 168.8 170.7 166.1 172.1 170.1
(9.5) (5.8) (10.1) (6.8) (8.8) -- (8.8)
Baseline body mass index (kg/m2)
Mean (SD) 27.4 28.4 27.8 26.7 26.3 27.1
(3.8) (3.8) (3.7) (3.2) (3.7) -- (3.6)
1. Cohort 2 sentinel group (5 subjects randomized to KarXT 150/20 BID and 1
subject
randomized to placebo) was discontinued after the Day 4 morning dose.
2. During the study, Cohort 3 Group 3b (8 subjects randomized to KarXT 150/40
BID and
1 subject randomized to placebo) was discontinued after the Day 5 morning
dose.
[0309] Serial blood samples for assessing the PK of xanomeline and trospium
were collected
from all subjects in each cohort on Days 1, 3, and 7 before the morning dose
and at 1, 2, 3, 4,
6, 8, 10, and 12 hours after the morning dose. The PK parameters listed below
were
calculated from the individual xanomeline and trospium concentration-time
profiles by
standard non-compartmental methods. Dose-normalized parameters were calculated
for C.
and area under the concentration-time curve (AUC) values. During the study,
additional
blood samples for monitoring trough concentrations of xanomeline and trospium
were collected on Days 2, 4, 5, and 6 before the morning dose and before
discharge on Day 8.
[0310] Safety evaluations included spontaneously reported adverse events,
ECGs, laboratory
assessments, vital signs, assessments of saliva volumes, Bristol stool scale,
pupil size, and
physical examinations. Descriptive statistics (n, mean, standard deviation,
median, minimum,
and maximum) summarized the treatment group's continuous data. Geometric mean
(GM),
geometric percent coefficient of variation (CV%), quartiles, or box plots was
generated. The
count and frequency tabulated categorical measurements, although formal
statistics were not
conducted.
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
[0311] Treatment groups were summarized as follows unless otherwise specified:
KarXT
50/20 BID (for adverse events and Day 1 PK summaries only), KarXT 100/20 BID,
KarXT
125/40 BID, KarXT 150/20 BID, KarXT 150/40 BID, and placebo (Empty Vcaps Plus
Capsules and Capsugel ; all cohort placebo groups combined). The safety
evaluation was
based on reported adverse events, ECGs, laboratory assessments, and vital
signs. Exploratory
analyses of saliva volumes, Bristol stool scale, and pupil size were also
conducted.
[0312] Xanomeline was well absorbed into the systemic circulation following
oral
administration of the KAR-003 formulation at all dosages. Peak concentrations
of
xanomeline were observed at a median time of 2 hours across all treatment
groups and study
days.
[0313] Median t112 values for xanomeline were similar between treatment groups
and across
study days, indicating that t112 was not dose-dependent. Median t112 ranged
from 3.4 to 5.8
hours.
[0314] GM xanomeline exposures did not increase dose-proportionally on Day 3
from 100 to
150 mg when xanomeline was administered with 20 mg trospium or 125 to 150 mg
when
administered with 40 mg trospium. Lower xanomeline exposures were observed
following
treatment with KarXT 150/40 compared to KarXT 125/40. Day 3 GM xanomeline
exposures
(C., AUCo-iast, and AUCo-imr) were similar when the 150 mg xanomeline dose was
administered with 20 and 40 mg trospium. On Day 7, GM xanomeline exposures
increased
slightly more than dose-proportionally from 125 to 150 mg when xanomeline was
administered 40 mg trospium.
[0315] Minimal to no xanomeline accumulated in plasma from Day 3 to Day 7
following
treatment with KarXT 100/20 BID and KarXT 125/40 BID; however, there was
accumulation
following administration of KarXT 150/40 BID in 3 of the 4 subjects who
completed the
study. The KarXT 150/40 BID group's mean accumulation ratios were 366.2% for
RAUC and
445.4% for RCmax=
Example 6 ¨ Xanomeline pharmacokinetics of KAR-003 compared to KAR-001
[0316] Comparing xanomeline GM exposures between KAR-001 (75 mg xanomeline TID
20 mg trospium BID) and the KarXT 100/20 BID group from KAR-003 showed that C.
values and AUCo-6hr (KAR-003) or AUCo-tau (KAR-001) values were greater in KAR-
003
(Days 3 and 7) than the corresponding exposures from KAR-001 (Days 3 and 9).
The median
T. was observed at 2 hours in both studies and both days (Days 3 and 9 for KAR-
001, and
66
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
Days 3 and 7 for KAR-003). These data indicate that the KarXT formulation
enhanced
xanomeline exposures.
[0317] Trospium was absorbed into the systemic circulation following oral
administration of
the KarXT formulation at all dosages. Peak concentrations of trospium were
observed at a
median time of 1.0 hour across all treatment groups and study days.
[0318] Median t112 values for trospium were similar between treatment groups
on Day 3, with
values ranging between 4.1 and 4.8 hours. On Day 7, median t112 values were
similar for the
KarXT 100/20 BID (4.9 hours) and KarXT 125/40 BID (4.5 hours) treatments but
were
slightly longer for the KarXT 150/40 BID group (7.1 hours).
[0319] GM trospium exposures increased slightly less than dose-proportionally
on Day 3
from 20 to 40 mg when administered with 150 mg xanomeline. Day 3 GM trospium
exposures (C., AUCo-iast, and AUCo-imr) were greater when the 20 mg BID dose
of
trospium was administered with 100 mg BID xanomeline compared to 150 mg BID
xanomeline. Day 3 GM trospium exposures were similar when the 40 mg trospium
BID dose
was given 125 mg xanomeline BID and 150 mg xanomeline BID.
[0320] Trospium did not accumulate in plasma from Day 3 to Day 7 following
administration
of KarXT 100/20 BID, KarXT 125/40 BID, and KarXT 150/40 BID. Trospium
accumulated
in plasma from Day 1 to Day 7 for the KarXT 100/20 BID group. Mean Day 7/Day 1
accumulation ratios were 348.7% (RAUC) and 379.9% (RC.).
[0321] Comparing trospium GM exposures between KAR-001 and the KarXT 100/20
BID
group from KAR-003 showed that C. and AUCo-imr values from KAR-003 were
greater
than the corresponding exposures from KAR-001 on both days (Days 3 and 9 for
KAR-001
and Days 3 and 7 for KAR-003). The median T. for trospium was observed at 1.0
hour in
both studies on both days. These data indicate that the KarXT formulation
enhanced trospium
exposures.
[0322] All cohorts of KAR-003 started with a 2-day lead-in period of KarXT
50/20 BID for
subjects randomized to KarXT. FIG. 1 presents the mean ( SD) xanomeline PK
concentrations, and Table 22 summarizes xanomeline PK parameters on Day 1 for
KarXT 50/20 BID treatment of all cohorts for the PK Population. No sample
collected before
administering the first dose of xanomeline on Day 1 displayed measurable
concentrations of
xanomeline. Concentrations of xanomeline were quantifiable (>50 pg/mL) at all
time points
after administering the Day 1 morning dose through 12 hours.
67
CA 03161952 2022-05-17
WO 2021/101875 PCT/US2020/060859
Table 22: Xanomeline PK Parameters on Day 1 for KarXT 50/20 BID (All Cohorts)
Characteristic n Statistic
C. (pg/mL) 53 1972.3 (131.8)
T. (h) 53 2.0 (1.0, 8.0)
t112 (h) 48 3.4 (2.0, 4.6)
AUCo-iast (h*pg/mL) 53 10775.5
(102.2)
AUCo-i2m (h*pg/mL) 52 10810.3
(103.5)
AUCo-mt (h*pg/mL) 48 12836.1 (97.7)
[0323] FIG. 2 presents the mean ( SD) xanomeline PK concentrations by
treatment on Day
3 for the PK population, and Table 23 summarizes these parameters.
Concentrations of
xanomeline were quantifiable in samples before administering the morning dose
of the study
drug on Day 3 and at all time points after administering the Day 3 morning
dose through 12
hours for all cohorts, except for one subject who had a xanomeline plasma
concentration
<50.0 pg/mL at 12 hours post-dose. Inter-subject variability ranged from 23.7%
to 58.2%
(CV%) for T., 79.8% to 136.3% (geometric CV%) for C., 21.6% to 26.3% (CV%) for
ti/2, and 77.1% to 96.1% (geometric CV%) for AUCo-i2hr across the four
treatment groups.
The median T. for xanomeline on Day 3 was 2 hours for the KarXT 100/20 BID,
KarXT 125/40 BID, KarXT 150/20 BID, and KarXT 150/40 BID groups. Individual T.
values range from 1.0 to 6.0 hours across the four treatment groups. The tin,
was estimated in
51 of 53 subjects, in contrast to the previous study, KAR-001, where the
elimination phase
was not well characterized. The median tin, on Day 3 for xanomeline was
numerically similar
across the four treatment groups. Median t1/2 ranged from 3.4 to 4.3 hours.
Individual t1/2
values ranged from 2.4 to 8.6 hours across the four treatment groups.
Table 23: Xanomeline PK Parameters by Treatment on Day 3
Cohort 1 Cohort 2 Cohort 3 Cohort 4
KarXT KarXT KarXT KarXT
100/20 BID 150/20 BID 150/40 BID 125/40 BID
Statistic n Statistic n Statistic n Statistic n Statistic
[2] [2] [2] [2]
Cmax 18 7368.4 5 7270.0 12 7866.7 18 8098.8
(pg/mL) (106.2) (79.8) (136.3) (99.1)
Tmax (h) 18 2.0(1.0, 5 2.0(2.0, 12 2.0(2.0, 18
2.0(1.0,
68
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
3.0) 4.0) 6.0) 6.0)
(h) 17 3.9 (3.0, 5 3.4 (2.4, 12 3.6 (2.6,
17 4.3 (3.1,
5.8) 4.3) 6.1) 8.6)
AUC0_last 18 42003.4 5 48031.1 12 39092.3 18 43450.2
(h*pg/mL) (86.9) (92.0) (96.1) (74.4)
AUCo_ 12hr 17 40912.1 5 48132.2 12 39403.3 17
43164.7
(h*pg/mL) (88.8) (92.0) (96.1) (77.1)
Dose-normalized 18 73.7 5 48.5 12 52.4 18 64.8
C. (pg/mL/mg) (106.2) (79.8) (136.3) (99.1)
Dose-normalized 18 420.0 5 320.2 12 260.6 18 347.6
AUCo_last (86.9) (92.0) (96.1) (74.4)
(h*pg/mL/mg)
Dose-normalized 17 409.1 5 320.9 12 262.7 17 345.3
AUCo_ 12hr (88.8) (92.0) (96.1) (77.1)
(h*pg/mL/mg)
Geometric CV%=100*(exp(SD2)-1) 5 , SD was the SD of the log-transformed data.
1. Cohort 2 sentinel group (5 subjects randomized to KarXT 150/20 BID and 1
subject
randomized to placebo) was discontinued after the Day 4 morning dose.
2. During the study, Cohort 3 Group 3b (8 subjects randomized to KarXT 150/40
BID and
1 subject randomized to placebo) was discontinued after the Day 5 morning
dose.
[0324] When KarXT was administered BID, as the xanomeline dose increased from
100 mg
(Cohort 1) to 150 mg (Cohort 2) without changing the trospium dose (20 mg),
the Day 3
dose-normalized GM exposures (dose-normalized GM C. and dose-normalized GM
AUCo_
last and AUCo_i2hr) for xanomeline decreased. Similarly, as the xanomeline
dose increased
from 125 mg (Cohort 4) to 150 mg (Cohort 3) without changing the trospium
dosage (40
mg), the Day 3 dose-normalized GM exposures for xanomeline decreased slightly
(i.e.,
xanomeline exposures were lower following treatment with KarXT 150/40 BID
compared to
treatment with KarXT 125/40 BID). Comparing xanomeline exposures following 150
mg
xanomeline BID administration with either 20 or 40 mg trospium BID showed that
the Day 3
GM, C., AUCo-last, and AUCo-imr for xanomeline were similar.
[0325] FIG. 3 presents the mean ( SD) xanomeline PK concentrations by
treatment on Day
7 for the PK population, and Table 24 summarizes these parameters.
Concentrations of
69
CA 03161952 2022-05-17
WO 2021/101875 PCT/US2020/060859
xanomeline were quantifiable in samples collected before administering the
morning dose of
the study drug on Day 7 and at all time points after the Day 7-morning dose
through 12 hours
for the KarXT 100/20 BID, KarXT 125/40 BID, and KarXT 150/40 BID groups. Inter-
subject
variability ranged from 38.3% to 47.9% (CV%) for T., 81.4% to 106.8%
(geometric CV%)
for C., 15.4% to 42.1% (CV%) for t112, and 45.2% to 71.2% (geometric CV%) for
AUCo_
12hr across the KarXT 100/20 BID, KarXT 150/40 BID, and KarXT 125/40 BID
groups. The
median T. for xanomeline on Day 7 was 2.0 hours for the KarXT 100/20 BID,
KarXT 125/40 BID, and KarXT 150/40 BID groups. Individual T. values ranged
from 0.0
to 6.0 hours across the KarXT 100/20 BID, KarXT 150/40 BID, and KarXT 125/40
BID
groups. The median t112 for xanomeline on Day 7 was numerically similar for
the KarXT
100/20 BID, KarXT 125/40 BID, and KarXT 150/40 BID groups. Median tin, for
xanomeline
ranged from 4.6 to 5.8 hours. Individual tu2 values ranged from 3.6 to 14.0
hours across the
KarXT 100/20 BID, KarXT 150/40 BID, and KarXT 125/40 BID groups.
Table 24: Xanomeline PK Parameters by Treatment on Day 7
Cohort 1 Cohort 2 Cohort 3 Cohort 4
KarXT KarXT KarXT KarXT
100/20 BID 150/20 BID 150/40 BID 125/40 BID
Statistic n Statistic n Statistic n Statistic n Statistic
[1] [1] [1] [1]
C. (pg/mL) 16 8373.6 N/A N/A 4 18191.3 18 8112.7
(94.3) (81.4) (106.8)
Tmax (h) 16 2.0 N/A N/A 4 2.0 18 2.0
(0.0, (1.0, 3.0) (1.0,
3.0) 6.0)
t112(h) 15 5.4 N/A N/A 4 4.6 17 5.7
(3.6, (3.9, 5.6) (4.0,
9.9) 14.0)
AUCo_last 16 53810.8 N/A N/A 4 86347.8 18 52727.0
(h*pg/mL) (89.8) (45.3) (76.7)
AUCo_ 12hr 15 48138.3 N/A N/A 4 86540.9 17 59945.1
(h*pg/mL) (71.2) (45.2) (45.9)
Dose-normalized 16 83.7 N/A N/A 4 121.3 18 64.9
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
C. (pg/mL/mg) (94.3) (81.4) (106.8)
Dose-normalized 16 538.1 N/A N/A 4 575.7 18 421.8
AUCO-last (89.8) (45.3) (76.7)
(h*pg/mL/mg)
Dose-normalized 15 481.4 N/A N/A 4 576.9 17 479.6
AUCo_12hr (71.2) (45.2) (45.9)
(h*pg/mL/mg)
Geometric CV%=100*(exp(SD2)-1) 5, SD was the SD of the log-transformed data.
1. Cohort 2 sentinel group (5 subjects randomized to KarXT 150/20 BID and 1
subject
randomized to placebo) was discontinued after the Day 4 morning dose.
2. During the study, Cohort 3 Group 3b (8 subjects randomized to KarXT 150/40
BID
and 1 subject randomized to placebo) was discontinued after the Day 5 morning
dose.
[0326] When KarXT was administered BID, as the xanomeline dose increased from
125 mg
(Cohort 4) to 150 mg (Cohort 3) without changing the trospium dosage (40 mg),
the Day 7
dose-normalized GM exposures (dose-normalized GM C., AUCO-last and AUCo-imr)
for
xanomeline increased.
[0327] Table 25 summaries xanomeline PK accumulation ratios (Day 7/Day 3) by
treatment
for the PK population. Based upon mean accumulation ratios of xanomeline
following
treatment with KarXT 100/20 BID (Cohort 1) and KarXT 125/40 BID (Cohort 4),
minimal to
no xanomeline accumulated in plasma from Day 3 to Day 7. The KarXT 100/20 BID
group's
mean accumulation ratios were 133.4% for RAUC and 130.5% for RC., and for the
KarXT
125/40 BID group was 143.9% for RAUC and 151.0% for RC.. Only one subject in
the
KarXT 100/20 BID group showed lower exposures on Day 7 compared to Day 3. In
contrast,
xanomeline accumulated moderately in three of the four subjects in the KarXT
150/40 BID
group who completed the study. The other subject in the KarXT 150/40 BID group
showed
similar exposures on Days 3 and 7. The KarXT 150/40 BID group's mean
accumulation ratios
were 366.2% (RAUC) and 445.4% (RCmax).
71
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
Table 25: Xanomeline PK Accumulation Ratios (Day 7/Day 3) by Treatment
Cohort 1 Cohort 2 Cohort 3 Cohort 4
KarXT KarXT 150/20 KarXT KarXT
100/20 BID BID [1] 150/40 BID [2] 125/40 BID
Statistic n Mean n Mean n Mean n Mean
(SD) (SD) (SD) (SD)
RAUC (%) 14 133.4 N/A N/A 4 366.2 16 143.9
(45.1) (N/A) (321.3) (80.9)
RC. (%) 16 130.5 N/A N/A 4 445.4 18 151.0
(55.1) (N/A) (537.0) (122.7)
RAUC=100*Day 7 AUCo-i2m/Day 3 AUCo-i2m. RC.=100*Day 7 C./Day 3 Cmax-
1. Cohort 2 sentinel group (5 subjects randomized to KarXT 150/20 BID and 1
subject
randomized to placebo) was discontinued after the Day 4 morning dose.
2. During the study, Cohort 3 Group 3b (8 subjects randomized to KarXT 150/40
BID and
1 subject randomized to placebo) was discontinued after the Day 5 morning
dose.
[0328] FIG. 4 compares the mean ( SD) xanomeline PK concentration-time
profiles by
treatment and visit (Day) for the PK population. FIG. 5 presents mean ( SD)
xanomeline PK
trough concentrations by treatment for the PK population. Attaining a steady-
state was not
assessed.
[0329] Comparing xanomeline GM exposures between KAR-001 (75 mg xanomeline TID
20 mg trospium BID) (Table 23) and the KarXT 100/20 BID group from KAR-003
(Table 21)
showed that Cmax values and AUCo-6hr (KAR-003) or AUCo-tau (AUC from time 0 to
6 hours)
values (KAR-001) values on Day 3 for the KarXT 100/20 BID group (KAR-003) were
about
2.3 to 2.6-fold greater than corresponding exposures from KAR-001 on Day 3.
[0330] Comparing Day 7 GM exposures for xanomeline for the KarXT 100/20 BID
group
from KAR-003 (Table 22) with Day 9 exposures from the xanomeline alone and
xanomeline
+ trospium arms from KAR-001 (Table 23) showed that values on Day 7 for the
KarXT
100/20 BID group (KAR-003) were about 1.4 to 1.8-fold greater than
corresponding
exposures from KAR-001 on Day 9. The median T. was 2.0 hours on Day 3 and Day
7 for
KAR-003 (Table 22) and Day 3 and Day 9 for KAR-001 (Table 23). These data
indicate that
the KAR-003 formulation provided sufficient exposures and PK properties.
[0331] Table 26 summarizes a subset of KAR-003 xanomeline PK parameters for
the KarXT
100/20 BID group on Day 3 and Day 7 for the PK Population. Table 27 presents a
summary
72
CA 03161952 2022-05-17
WO 2021/101875 PCT/US2020/060859
of a subset of KAR-001 xanomeline PK parameters for the treatments of KAR-001
on Day 3
and Day 9 for the PK Population.
Table 26: Subset of Xanomeline PK Parameters KarXT 100/20 BID on Days 3 and 7
KAR-003 PK Cohort 1 - KarXT 100/20 BID Cohort 1 - KarXT 100/20 BID
Parameter Day 3 Day 7
Statistic n Statistic [1] n Statistic [1]
C. (pg/mL) 18 7368.4 (106.2) 16 8373.6 (94.3)
Tmax (h) 18 2.0 (1.0, 3.0) 16 2.0 (0.0, 3.0)
AUCo-air (h*pg/naL) 18 28564.2 (88.2) 16 35129.1 (85.2)
Table 27: Subset Xanomeline PK Parameters for KAR-001 on Days 3 and 9
KAR-001 PK Xanomeline Alone [1] Xanomeline + Trospium [2]
Parameter Day 3 Day 9 Day 3 Day 9
Statistic n Statistic n Statistic n Statistic n
Statistic [3]
[3] [3] [3]
C. (pg/mL) 32 2951.1 31 4572.6 34 3043.0 32
4698.5
(107.7) (123.5) (84.5) (99.5)
Tmax (h) 32 2.0(2.0, 31 2.0(0.0, 34 2.0(1.0,
32 2.0 (1.0, 4.0)
5.9) 5.9) 5.9)
AUC0_tati 11 12585.1 21 24808.6 17 11638.8 22 20347.9
(h*pg/mL) (132.4) (85.4) (71.3) (107.3)
Geometric CV%=100*(exp(SD2)-1) 5, SD was the SD of the log-transformed data.
In KAR-001, xanomeline dosing started on Day 3. Hence Day 3 is the first day
of
xanomeline dosing and Day 9 is the seventh day of xanomeline dosing.
1. In KAR-001, the xanomeline-alone treatment arm received 2 placebo capsules
TID
during the 2-day lead-in phase, then xanomeline 75 mg TID (TDD 225 mg) and
placebo
on Days 3 through 9.
2. In KAR-001, the xanomeline plus trospium arm received trospium 20 mg BID
(TDD 40
mg) and placebo BID; and 2 placebo capsules QD during the 2-day lead-in phase;
then
xanomeline 75 mg TID and trospium 20 mg BID (TDD 40 mg) and placebo QD on
Days 3 through 9.
3. Statistics for parameters presented as geometric mean (geometric CV%),
except for
T., which is presented as the median with minimum and maximum values.
73
CA 03161952 2022-05-17
WO 2021/101875 PCT/US2020/060859
[0332] FIG. 6 presents mean ( SD) trospium PK concentrations on Day 1 for the
KarXT
50/20 BID treatment (all cohorts) for the PK population, and Table 28
summarizes these
parameters. No samples collected before administering the first dose of
trospium on Day 1
displayed measurable concentrations of trospium. Concentrations of trospium
were
quantifiable (>20 pg/mL) at all time points after administration of the Day 1
morning dose
through 12 hours.
Table 28: Trospium PK Parameters on Day 1 for KarXT 50/20 BID (All Cohorts)
Statistic n Statistic [1]
C. (pg/mL) 53 1824.7 (98.7)
Tmax (h) 53 1.0 (1.0, 10.0)
t1/2 (h) 26 4.5 (3.2, 5.1)
AUCo-iast (h*pg/mL) 53 10286.5 (86.3)
AUCo-i2m (h*pg/mL) 49 10623.7 (78.5)
AUCo-mf (h*pg/mL) 26 16526.6 (70.6)
Geometric CV%=100*(exp(SD2)-1) 5, SD was the SD of the log-transformed data.
1. Statistics for parameters are presented as geometric mean (geometric CV%),
except for
t112 and T, presented as medians with minimum and maximum values.
[0333] FIG. 7 presents mean ( SD) trospium PK concentrations by treatment on
Day 3
for the PK population, and Table 29 summarizes these parameters.
Concentrations of
trospium were quantifiable in samples collected before administering the
morning dose of the
study drug on Day 3 and at all time points after administering the Day 3
morning dose
through 12 hours for all treatment groups (except for one subject who had a
trospium plasma
concentration <20.0 pg/mL at 12 hours post-dose. Inter-subject variability
ranged from 0.0%
to 83.0% (CV%) for T., 54.8% to 80.7% (geometric CV%) for C., 9.1% to 34.0%
(CV%)
for ti/2, and 59.0% to 67.6% (geometric CV%) for AUCo_i2hr across the four
treatment groups.
Table 29: Trospium PK Parameters by Treatment on Day 3
Cohort 1 Cohort 2 Cohort 3 Cohort 4
KarXT KarXT KarXT KarXT
100/20 BID 150/20 BID [1] 150/40
BID [2] 125/40 BID
Statistic [3] n Statistic n Statistic n Statistic n
Statistic
C. (pg/mL) 18 5705.6 5 3109.0 12 9838.7 18
8496.4
74
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
(80.7) (54.8) (67.3) (74.9)
Tmax (h) 18 1.0 5 1.0 12 1.0 18 1.0
(1.0, 3.0) (1.0, 1.0) (1.0, 2.0) (1.0,
6.0)
t112 (h) 18 4.8 5 4.6 12 4.1 18 4.2
(3.3, 7.6) (4.3, 5.3) (3.0, 8.0) (2.8,
9.0)
AUC0_last 18 29175.4 5 17560.8 12 43581.1 18 46214.2
(h*pg/mL) (59.0) (64.8) (64.4) (67.5)
AUCo_ 12hr 18 29253.9 5 17612.9 12 44072.6 18
46333.3
(h*pg/mL) (59.0) (64.8) (64.3) (67.6)
Dose-normalized 18 285.3 5 155.5 12 246.0 18 212.4
C. (pg/mL/mg) (80.7) (54.8) (67.3) (74.9)
Dose-normalized 18 1458.8 5 878.0 12 1089.5 18 1155.4
AUCo-last (59.0) (64.8) (64.4) (67.5)
(h*pg/mL/mg)
Dose-normalized 18 1462.7 5 880.6 12 1101.8 18 1158.3
AUCo_ 12hr (59.0) (64.8) (64.3) (67.6)
(h*pg/mL/mg)
Geometric CV%=100*(exp(SD2)-1)115, SD was the SD of the log-transformed data.
1. Cohort 2 sentinel group (5 subjects randomized to KarXT 150/20 BID and 1
subject
randomized to placebo) was discontinued after the Day 4 morning dose.
2. During the study, Cohort 3 Group 3b (8 subjects randomized to KarXT 150/40
BID and
1 subject randomized to placebo) was discontinued after the Day 5 morning
dose.
3. Statistics for parameters presented as geometric mean (geometric CV%),
except for tin,
and T., which are presented as medians with minimum and maximum values.
[0334] The median T. for trospium on Day 3 was 1.0 hour for the KarXT 100/20
BID,
KarXT 125/40 BID, KarXT 150/20 BID, and KarXT 150/40 BID groups. Individual T.
values ranged from 1.0 to 6.0 hours across the 4 treatment groups. The median
tin, for
trospium on Day 3 was numerically similar across the 4 treatment groups;
median t1/2 ranged
from 4.1 to 4.8 hours. Individual tin, values ranged from 2.8 to 9.0 hours
across the
4 treatment groups.
[0335] When KarXT was administered BID, as the trospium dose increased from 20
mg
(Cohort 2) to 40 mg (Cohort 3) without changing xanomeline dose (150 mg), the
Day 3 dose-
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
normalized GM exposures for trospium increased. Comparing Day 3 trospium
exposures
following administration of 20 mg trospium BID with either 100 mg (Cohort 1)
or 150 mg
(Cohort 2) xanomeline BID showed that GM C., AUCo_last and AUCo-imr for
trospium were
greater when the 20 mg BID dose of trospium was administered with 100 mg
xanomeline
BID compared to 150 mg xanomeline BID.
[0336] Similarly, comparing trospium exposures following administration of 40
mg trospium
BID with either 125 mg (Cohort 4) or 150 mg (Cohort 3) xanomeline BID showed
that the
GM C., AUCo-iast, and AUCo-in for trospium were generally similar when
trospium was
administered with 125 and 150 mg xanomeline BID on Day 3.
[0337] FIG. 8 presents mean ( SD) trospium PK concentrations by treatment on
Day 7
for the PK population, and Table 30 summarizes the parameters. Concentrations
of trospium
were quantifiable in samples collected before administering the morning dose
of the study
drug on Day 7 and at all time points after the Day 7 morning dose through 12
hours for the
KarXT 100/20 BID, KarXT 125/40 BID, and KarXT 150/40 BID groups. Inter-subject
variability ranged from 0.0% to 86.3% (CV%) for T., 51.2% to 93.8% (geometric
CV%)
for C., 23.0% to 44.5% (CV%) for t112, and 59.4% to 76.7% (geometric CV%) for
AUCo-imr across the KarXT 100/20 BID, KarXT 150/40 BID, and KarXT 125/40 BID
groups.
Table 30: Trospium PK Parameters by Treatment on Day 7
Cohort 1 Cohort 2 Cohort 3 Cohort 4
KarXT KarXT KarXT KarXT
100/20 BID 150/20 BID [1] 150/40
BID [2] 125/40 BID
Statistic [3] n Statistic n Statistic n Statistic
n Statistic
C. (pg/mL) 16 7494.9 N/A N/A 4 9588.0 1
7213.8
(88.3) (N/A) (51.2) 8 (93.8)
Tmax (h) 16 1.0 N/A N/A 4 1.0 1 1.0
(0.0, 1.0) (1.0, 1.0) 8
(0.0, 6.0)
t112(h) 16 4.9 N/A N/A 4 7.1 1 4.5
(3.1, 7.1) (4.4, 8.2) 8
(3.7, 11.9)
AUCo_last 16 40377.8 N/A N/A 4 41865.2 1 44998.6
(h*pg/mL) (69.3) (N/A) (59.4) 8 (76.7)
AUCo_ 12hr 16 40488.0 N/A N/A 4 41997.6 1
45137.6
76
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
(h*pg/mL) (69.3) (N/A) (59.4) 8 (76.7)
Dose-normalized 16 374.7 N/A N/A 4 239.7 1 180.3
Cmax (88.3) (N/A) (51.2) 8 (93.8)
(pg/mL/mg)
Dose-normalized 16 2018.9 N/A N/A 4 1046.6 1 1125.0
AUCo_last (69.3) (N/A) (59.4) 8 (76.7)
(h*pg/mL/mg)
Dose-normalized 16 2024.4 N/A N/A 4 1049.9 1 1128.4
AUCo_12hr (69.3) (N/A) (59.4) 8 (76.7)
(h*pg/mL/mg)
Geometric CV%=100*(exp(SD2)-1) 5, SD was the SD of the log-transformed data.
1. Cohort 2 sentinel group (5 subjects randomized to KarXT 150/20 BID and 1
subject
randomized to placebo) was discontinued after the Day 4 morning dose.
2. During the study, Cohort 3 Group 3b (8 subjects randomized to KarXT 150/40
BID and
1 subject randomized to placebo) was discontinued after the Day 5 morning
dose.
3. Statistics for parameters presented as geometric mean (geometric CV%),
except for t112
and T., which are presented as medians with minimum and maximum values.
[0338] The median T. for trospium on Day 7 was 1.0 hour for the KarXT 100/20
BID,
KarXT 125/40 BID, and KarXT 150/40 BID treatments. Individual T. values ranged
from
0.0 to 6.0 hours across the KarXT 100/20 BID, KarXT 150/40 BID, and KarXT
125/40 BID
groups.
[0339] The median t112 for trospium on Day 7 was similar for the KarXT 100/20
BID
(4.9 hours) and KarXT 125/40 BID (4.5 hours) groups. The median ti/2 was 7.1
hours for the
KarXT 150/40 BID group. Individual t1/2 values ranged from 3.1 to 11.9 hours
across the
KarXT 100/20 BID, KarXT 150/40 BID, and KarXT 125/40 BID groups.
[0340] As observed on Day 3, comparing Day 7 trospium exposures following
administration
of 40 mg trospium BID with either 125 mg (Cohort 4) or 150 mg (Cohort 3)
xanomeline BID
showed that the GM Cmax, AUCo-iast, and AUCo-i2m for trospium were similar
when trospium
was administered with 125 and 150 mg xanomeline BID.
[0341] Table 31 summarizes trospium PK accumulation ratios (Day 7/Day 3; Day
7/Day 1)
by treatment for the PK Population. Based upon mean trospium PK accumulation
ratios,
trospium accumulated minimally in the plasma from Day 3 to Day 7 following
administration
of KarXT 100/20 BID (Cohort 1) and had little to no accumulation following
administration
77
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
of KarXT 125/40 BID (Cohort 4) and KarXT 150/40 BID (Cohort 3). Two subjects
showed
lower exposures on Day 7 compared to Day 3 in the KarXT 100/20 BID group.
[0342] Accumulation ratios from Day 3 to Day 7 varied widely between subjects
in the
KarXT 125/40 BID and KarXT 150/20 BID groups. Mean accumulation ratios ranged
from
108.6% to 141.4% for RAUC and from 111.0% to 135.8% for RC.. Trospium
accumulated
moderately in the plasma from Day 1 to Day 7 for the KarXT 100/20 BID group.
All but one
subject showed higher trospium exposures on Day 7 compared to Day 1. Mean
accumulation
ratios were 348.7% for RAUC and 379.9% for RC.. The possible effect of the
increase in
xanomeline dose (from 50 mg BID to 100 mg BID beginning on Day 3) on the PK
and
bioavailability of trospium cannot be ruled out as contributing to the
increased exposures
from Day 1 to Day 7.
Table 31: Trospium PK Accumulation Ratios (Day 7/Day 3; Day7/Day 1) by
Treatment
Cohort 1 Cohort 2 Cohort 3 Cohort 4
KarXT KarXT KarXT KarXT
100/20 BID 150/20 BID 150/40 BID
[2] 125/40 BID
[I]
Statistic n Mean n Mean n Mean n Mean
(SD) (SD) (SD) (SD)
Day 7/Day 3
RAUC (%) 16 141.4 N/A N/A 4 108.6 18 125.0
(56.6) (N/A) (39.0) (84.4)
RCmax(%) 16 135.8
N/A N/A 4 111.0 18 119.9
(70.5) (N/A) (67.8) (91.0)
Day 7/Day 1
RAUC (%) 15 348.7 N/A N/A N/A N/A N/A
N/A
(242.9) (N/A) (N/A) (N/A)
RCmax(%) 16 379.89
N/A N/A N/A N/A N/A N/A
(266.0) (N/A) (N/A) (N/A)
1. Pharmacokinetic accumulation ratios of Day7/Day3: RAUC=100*Day 7 AUCo-
imr/Day
3 AUCo-imr. RC.=100*Day 7 Cmax/Day 3 Cmax-
2. Pharmacokinetic accumulation ratios of Day 7/Day 1: RAUC= 100*Day 7 AUC0_
12hr/Day 1 AUCo-imr- RC.=100*Day 7 C./Day 1 C.-
78
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
3. Cohort 2 sentinel group (5 subjects randomized to KarXT 150/20 BID and 1
subject
randomized to placebo) was discontinued after the Day 4 morning dose.
4. During the study, Cohort 3 Group 3b (8 subjects randomized to KarXT 150/40
BID and
1 subject randomized to placebo) was discontinued after the Day 5 morning
dose.
[0343] FIG. 9 compares mean ( SD) trospium PK concentration-time profiles by
treatment
and visit (Day) for the PK Population. FIG. 10 presents mean ( SD) trospium
PK trough
concentrations by treatment and visit (Day) for the PK Population. Attaining a
steady state
was not assessed.
Example 7 ¨ Trospium pharmacokinetics of KAR-003 compared to KAR-001
[0344] Comparing GM exposures for trospium from Day 1 of KAR-001 (first dose
of
trospium alone with no prior treatment) (Table 33) and Day 1 of KAR-003 (first
dose of
xanomeline + trospium with no prior treatment) (Table 32) shows that the
trospium exposures
from KAR-003 are about 2.1- to 2.5-fold higher than those obtained from KAR-
001.
Although the comparison of Day 3 GM exposures between studies is not a head-to-
head
comparison (xanomeline dosing did not start until Day 3 in the KAR-003 study),
the number
of doses and a daily dose of trospium administered to subjects is the same.
The Day 3 GM
trospium exposures from KAR-003 (Table 32) are also ¨2.4- to 3.3-fold higher
than those
obtained from KAR-001 (Table 33). Comparing Day 7 GM exposures for trospium
for the
KarXT 100/20 BID cohort (Cohort 1) from KAR-003 (Table 32) with Day 9
exposures from
the xanomeline + trospium arm from KAR-001 (Table 33) indicates that exposures
were once
again higher (by approximately 3.5- to 4.3-fold) than those obtained from KAR-
001.
[0345] The median T. for trospium was 1.0 hour on Day 3 and Day 7 for the
KarXT 100/20
BID group for KAR-003 and Day 3 and Day 9 for the xanomeline + trospium arm
for KAR-
001. Median T. for trospium was lower (1.0 hour) on Day 1 for the KarXT 50/20
BID
group (KAR-003) compared to the median T. for trospium (3.0 hours on Day 1 for
the
trospium alone arm (KAR-001).
[0346] Table 32 summarizes a subset of KAR-003 trospium PK parameters for the
KarXT 50/20 BID treatment (all cohorts) on Day 1 and the KarXT 100/20 BID
treatment Day
3 and Day 7 for the PK Population. Table 33 summarizes a subset of KAR-001
trospium PK
parameters for the trospium-alone treatment on Day 1 and the xanomeline +
trospium
treatment on Day 3 and Day 9 for the PK Population.
79
CA 03161952 2022-05-17
WO 2021/101875 PCT/US2020/060859
Table 32: Subset of KAR-003 Trospium PK Parameters for KarXT 50/20 BID (All
Cohorts) on Day 1 and KarXT 100/20 BID on Days 3 and 7
KAR-003 PK
KAR 50/20 BID Cohort 1 - KAR 100/20 BID
Parameter
Day 1 Day 3 Day 7
n Statistic [1] n Statistic [1] n Statistic
[1]
C. (pg/mL) 53 1824.7 (98.7) 18 5705.6 16
7494.9
(80.7) (88.3)
Tmax (h) 53 1.0 (1.0, 10.0) 18 1.0 (1.0, 3.0) 16
1.0 (0.0,
1.0)
AUCo_i2hr (h*pg/mL) 49 10623.7 (78.5) 18 29253.9 16
40488.0
(59.0) (69.3)
AUCo-inf (h*pg/mL) 26 16526.6 (70.6) N/A N/A N/A N/A
1. Statistics for parameters are presented as geometric mean (geometric CV%),
except for
Tmax, which is presented as the median with minimum and maximum values.
Table 33: Subset of Trospium PK Parameters for KAR-001 on Days 1, 3, and 9
Trospium Alone [1] Xanomeline + Trospium [1]
KAR-001 PK
Day 1 Day 3 Day 9
Parameter
n Statistic [2] n Statistic [2] n
Statistic [2]
C. (pg/mL) 33 721.9 (78.2) 34 1711.6 (89.8) 33 1733.6
(124.1)
Tmax (h) 33 3.0 (1.0, 5.9) 34 1.0 (1.0, 5.9) 33
1.0 (0.0, 4.0)
AUC0-tau 26 5028.6 (65.9) 23 12176.3 (61.6) 30 11395.2
(h*pg/mL) (105.9)
AUCo_mf (h*pg/mL) 26 7787.3 (55.4) 23 18149.4 (62.0) 30 17519.4 (93.2)
Geometric CV%=100*(exp(SD2)-1) 5, SD is the standard deviation of the log-
transformed data. In KAR-001, xanomeline dosing started on Day 3. Hence Day 3
is the
first day of xanomeline dosing and Day 9 is the seventh day of xanomeline
dosing.
1. In KAR-001, the xanomeline + trospium arm received 20 mg trospium BID (TDD
40
mg) and placebo BID; and 2 placebo capsules QD during the 2-day lead-in phase;
then
75 mg xanomeline TID and 20 mg trospium BID (TDD 40 mg) and placebo QD on
Days 3 through 9.
2. Statistics for parameters are presented as geometric mean (geometric CV%),
except
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
for Tma,õ which is presented as the median with minimum and maximum values.
[0347] Table 34 lists the incidence of cholinergic TEAEs by system organ class
(SOC) and
preferred term for the Safety Population in the KAR-001 study. The overall
subject incidence
of cholinergic TEAEs was similar between the xanomeline + trospium arm (12
[34.3%1
subjects) in KAR-001, the KarXT 100/20 BID group (7 [38.9%1 subjects), and the
KarXT
125/40 BID group (6 [33.3%1 subjects).
Table 34: KAR-001 Incidence of Cholinergic Treatment-Emergent Adverse Events
by
System Organ Class and Preferred Term ¨ Safety Population
Xanomeline Xanomeline +
Total
System Organ Class Alone [1] Trospium [2]
(n = 69)
Preferred Term (n = 34) (n = 35)
n (%) #
n (%) # n (%) #
Subjects with any TEAEs 21 (61.8) 64 12 (34.3) 33 33
(47.8) 97
Gastrointestinal disorders 18 (52.9) 40 12 (34.3) 25 30
(43.5) 65
Salivary hypersecretion 12 (35.3) 16 9 (25.7) 11 21 (30.4)
27
Nausea 8 (23.5) 11 6 (17.1) 8 14 (20.3)
19
Diarrhea 7 (20.6) 8 2 (5.7) 4 9 (13.0) 12
Vomiting 5 (14.7) 5 2 (5.7) 2 7 (10.1) 7
Skin and subcutaneous tissue disorders 16 (47.1) 24 7 (20.0) 8
23 (33.3) 32
Hyperhidrosis 16 (47.1) 24 7 (20.0) 8 23 (33.3)
32
The percentage was calculated from the number of subjects in the column header
as the
denominator. # was the number of individual occurrences of the TEAE. The TEAEs
were
defined as adverse events that happened for the first time after dosing of
study drug or
existed before but worsened in severity or relationship to study drug after
dosing. For
noncholinergic adverse events, the first dose of any study drug (Day 1) was
used, and for
cholinergic adverse events, the first dose of xanomeline (Day 3) was used.
Cholinergic
adverse events had the additional specification that the start of the adverse
event must have
been within 24 hours (inclusive) of the last dose to be treatment-emergent. At
each level of
summation (total, system organ class term, preferred term), subjects who
reported more
than one adverse event were counted only once. During the study, a subject
could have
contributed to more than one preferred term.
In KAR-001, xanomeline dosing started on Day 3. Hence Day 3 was the first day
of
81
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
xanomeline dosing and Day 9 was the seventh day of xanomeline dosing.
1. In KAR-001, the xanomeline-alone treatment arm received two placebo
capsules TID
during the 2-day lead-in phase, then xanomeline 75 mg TID (TDD 225 mg) and
placebo on Days 3 through 9.
2. In KAR-001, the xanomeline + trospium arm received trospium 20 mg BID (TDD
40
mg) and placebo BID; and two placebo capsules QD during the 2-day lead-in
phase;
then xanomeline 75 mg TID and trospium 20 mg BID (TDD 40 mg) and placebo QD
on Days 3 through 9.
[0348] Subject incidence of salivary hypersecretion, hyperhidrosis, and
diarrhea was higher
in the xanomeline + trospium arm in KAR-001 compared to the KarXT 100/20 BID
and
KarXT 125/40 BID groups. Salivary hypersecretion occurred in 25.7% of subjects
in the
xanomeline + trospium arm in KAR-001, 5.6% of subjects in the KarXT 100/20 BID
group,
and no subjects in the KarXT 125/40 BID group. Hyperhidrosis occurred in 20.0%
of
subjects in the xanomeline + trospium arm in KAR-001, 5.6% of subjects in the
KarXT
100/20 BID group, and 11.1% of subjects in the KarXT 125/40 BID group.
Diarrhea occurred
in 5.7% of subjects in the xanomeline + trospium arm in KAR-001, and no
subjects in the
KarXT 100/20 BID group or the KarXT 125/40 BID group.
[0349] The xanomeline + trospium arm in KAR-001 showed no other apparent
trends than
the KarXT 100/20 BID and KarXT 125/40 BID groups for nausea and vomiting.
Nausea
occurred in 17.1% of subjects in the xanomeline + trospium arm in KAR-001 and
22.2% of
subjects in each KarXT 100/20 BID and KarXT 125/40 BID groups. Vomiting
occurred in
5.7% of subjects in the xanomeline + trospium arm in KAR-001, 27.8% of
subjects in the
KarXT 100/20 BID group, and 5.6% of subjects in the KarXT 125/40 BID group.
[0350] Xanomeline and trospium were absorbed into the systemic circulation
following oral
administration of the KAR-003 formulation at all dosages. The PK results
suggest that neither
xanomeline nor trospium meaningfully impacted the PK behavior of the other
drug. The
KAR-003 formulation provided enhanced xanomeline and trospium blood levels
compared to
KAR-001, where both compounds were dosed apart.
[0351] No new safety signals were reported with the KarXT formulation. All
TEAEs were
mild or moderate in severity with no SAEs or deaths. Subject incidence of
salivary
hypersecretion, hyperhidrosis, and diarrhea was higher in the xanomeline +
trospium arm in
KAR-001 compared to the KarXT 100/20 BID and KarXT 125/40 BID groups in KAR-
003.
82
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
Example 8 ¨ KAR-004 Phase II Study
[0352] This Phase II, randomized, double-blind, placebo-controlled, inpatient
study was
designed to assess the efficacy of KarXT (a fixed combination of xanomeline
and trospium)
versus placebo in reducing Positive and Negative Syndrome Scale (PANSS) total
scores in
adult inpatients with a diagnosis of schizophrenia. The five secondary
objectives were to
assess overall safety and tolerability of KarXT in adult inpatients with a DSM-
5 diagnosis of
schizophrenia, to assess spontaneously reported adverse events (AEs) in
subjects treated with
KarXT versus placebo, to assess spontaneously reported cholinergic symptoms in
subjects
treated with KarXT versus placebo, to assess orthostatic vital signs in
subjects treated with
KarXT versus placebo, and to assess ECG parameters in subjects treated with
KarXT versus
placebo.
[0353] The total study duration was up to 7 weeks, including a 7-day screening
phase (up to a
7-day extension of the screening phase was allowed, if necessary), and a 5-
week treatment
period. Subjects were randomized in a 1:1 ratio to either KarXT or placebo
group. The key
inclusion and exclusion criteria for the Phase II study are shown in Table 35.
The
demographics and baseline characteristics of the enrolled patients are shown
in Table 36.
Table 35: Key Inclusion and Exclusion Criteria
Inclusion Exclusion
18-60 years of age with a diagnosis of Primary diagnosis other than
schizophrenia
schizophrenia
Exacerbation of psychotic symptoms History of treatment-resistant
schizophrenia
requiring hospitalization
PANSS total 80-120 with > 4 (moderate) on Serious medical comorbidity or
active
at least two key PANSSp items and CGI-S > substance use
4 at screening and baseline
Washout of prior oral lithium and/or High risk for suicidal or destructive
antipsychotics > 2 weeks behavior
Capable of providing consent and History of medically important sensitivity
to
cooperating with study procedures peripheral anticholinergics
Resides in a stable living situation and has BMI < 18 or? 40 kg/m2
an identifiable informant
83
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
Table 36: Demographics and Baseline Characteristics of the Enrolled Patients
Placebo (n = 92) KarXT (n = 90)
Mean age (years) 41.6 43.4
Sex, male (%) 74 80
Race (%white/%non-white) 19/81 22/78
[0354] The study employed a flexible-dose, two-arm trial with 1:1
randomization to KarXT
or placebo with a five-week treatment period:
- Days 1-2: 50/20 KarXT BID (50 mg xanomeline/ 20 mg trospium)
- Days 3-7: 100/20 KarXT BID
- Days 8-35: 100/20 KarXT BID with an optional increase to 125/30 KarXT
BID;
titration based only on tolerability.
[0355] The primary endpoint was a change in total PANSS score from baseline
versus
placebo at week 5. The other endpoints included CGI, PANSS-positive and
¨negative
subscales, PANSS Marder factor, cognitive battery, and others. A CGI-S
responder is defined
as a subject with a CGI-S score equal to 1 or 2. A CGI-S non-responder is
defined as a subject
with a CGI-S scale equal to 3 to 7. The subject required a CGI-S score of > 4
at screening
and baseline visits. CGI-S score legend rated 1 as normal, 2 as borderline
ill, 3 as mildly ill, 4
as moderately ill, 5 as markedly ill, 6 as severely ill, and 7 as extremely
ill. The safety
endpoints comprised monitoring for spontaneous adverse events, orthostatic
vital signs
(supine and standing after 2 minutes), blood pressure (systolic and diastolic)
and heart rate
(beats/minute), clinical laboratory evaluations (hematology, clinical
chemistry, coagulation,
urinalysis, and drug screen), 12-lead ECG, physical examination, and rating
suicidal ideation
with the Columbia Suicide Severity Rating Scale (C-SSRS).
[0356] The Intent-to-Treat (ITT) population comprised all subjects who were
randomized to
the study. The Safety population comprised all subjects who received at least
one dose of
study medication. The Safety population was used for all analyses of safety
endpoints. The
modified Intent-to-Treat (mITT) population comprised all subjects who were
randomized,
received at least one dose of study medication, and had a baseline and at
least one post-
baseline PANSS assessment. The mITT population was used for all analyses of
efficacy
endpoints. The PK population comprised all subjects who received at least one
dose of the
study drug and had at least one measurable PK concentration. If any subjects
were found to
be noncompliant with respect to dosing, have incomplete data, or other
clinical events
84
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
potentially interfering with the pharmacokinetic profile, exclusion from PK
analysis was
determined. The Completer population comprised all mITT subjects who had a
valid PANSS
total score at Visit 9. The Completer population was used for sensitivity
analysis of the
primary efficacy endpoint. Per-Protocol (PP) population comprised all subjects
who were
randomized, received at least one dose of study medication, had a baseline and
at least one
post-baseline PANSS assessment, and had no major protocol deviations. The PP
population
was used for sensitivity analysis of the primary efficacy endpoint. All
subjects were analyzed
according to randomized treatment.
[0357] The demographics from the ITT population are shown in Table 37. There
were no
significant differences between the treatment groups.
Table 37: Key Demographics and Baseline Characteristics of the mITT Population
Placebo (n = KarXT (n = 83)
87)
Mean age (years) 41.8 10.0 43.7 10.0
Gender, male (%) 73.6 80.7
Race (% non-white) 80.4 77.1
Mean Baseline PANSS Score 96.6 8.4 97.3 9.3
Mean Baseline PANSS-positive Score 26.3 3.3 26.3 3.4
Mean Baseline PANSS-negative Score 22.9 4.6 22.5 4.3
Mean Baseline PANSS Marder Negative Score 22.4 5.1 22.3 4.6
Mean Baseline CGI-S Score 4.9 0.6 5.0 0.5
Plus-minus values are mean SD
[0358] For the primary endpoint, the KarXT treatment group demonstrated
clinically
meaningful and statistically significant improvement in total PANSS score
versus placebo
(FIG. 11). The subjects improved by 11.6 points compared to placebo at week 5
(p<0.0001).
Statistical separation occurred at every assessment timepoint. Cohen's d
effect size was 0.75.
Historically, changes as small as 5 points in the total PANSS score have been
determined as
efficacious for the current antipsychotics used as the standard of care.
[0359] For the secondary endpoints, the KarXT treatment group demonstrated
clinically
meaningful and statistically significant improvement in total PANSS-positive
subscore versus
placebo (FIG. 12). The subjects improved by 3.2 points compared to placebo at
week 5
(p<0.0001). Statistical separation occurred at every assessment timepoint.
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
[0360] The KarXT treatment group also demonstrated a clinically meaningful and
statistically significant improvement in total PANSS-negative versus placebo
(FIG. 13). The
subjects improved by 2.3 points compared to placebo at week 5 (p<0.001).
Statistical
separation occurred at every assessment timepoint. FIG. 14 depicts the PANSS
Marder Factor
score of subjects in the mITT population of KAR-004 Phase II study versus
Visit day.
[0361] Moreover, CGI-S showed highly significant improvements in a consistent
pattern with
the PANSS. The non-parametric comparison of KarXT versus placebo using the
Mann-
Whitney Wilcoxon test showed that CGI-S scores shifted from baseline
(p<0.001). At
baseline, the percentage of patients with scores 5 or 6 for KarXT compared to
placebo 84%
vs. 80% (FIG. 15). At the endpoint, the percentage of patients with scores
rated 5-7 for
KarXT compared to placebo was 33% versus 60%, and the percentage of patients
rated
mildly ill or better (scores rated 1, 2, or 3) for KarXT compared to placebo
were 37% versus
11% (FIG. 16). Statistical separation occurred at every assessment time point
(weeks 2, 4,
and 5).
[0362] Overall, KarXT was safe and well-tolerated. The overall discontinuation
rate on
KarXT (20%) was similar to placebo (21%). The number of discontinuations due
to
treatment-emergent adverse events (TEAEs) was equal in the KarXT and placebo
arms (n = 2
in each group). Dose escalation rate on KarXT was high and similar to placebo:
91% of
KarXT subjects escalated to 125/30 KarXT (vs. 97% on placebo), 4% percent de-
escalated
back to 100/20 KarXT dose (vs. 1% on placebo).
[0363] Overall adverse event rate on KarXT was 54% versus 43% on placebo
(Table 38):
Table 38: Adverse Events over Course of Study
Placebo KarXT
Safety population
(n = 90) (n = 89)
Any AE (n, %) 39 (43.3%) 48 (53.9%)
Any SAE 0 1(1.1%)
.................................................... 1
AE(s) leading to 2(2.2%) 2 (2.2%)
drug withdrawal
____________________________________________________ 1
All AEs > 2% in KarXT group
Constipation 3 (3.3%) 15 (16.9%)
_____________________________ õ ......
Nausea 4 (4.4%) 15 (16.9%)
_____________________________ , ................ I
Dry mouth 1(1.1%) 8 (9.0%)
____________________________________________________ -
86
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
______________________________ , .....
Dyspepsia 4 (4.4%) 8 (9.0%)
. .................................... õ
Vomiting 4 (4.4%) 8 (9.0%)
. .................................... õ
Headache 5 (5.6%) 6 (6.7%)
. õ
Somnolence 4 (4.4%) 5 (5.6%)
. õ
Akathisia 0 1 3 (3.4%)
Dizziness 3 (3.3%) 3 (3.4%)
Weight increased 4 (4.4%) 3 (3.4%)
Tachycardia 2 (2.2%) 3 (3.4%)
= Sedation 2 (2.2%) 2 (2.2%)
Diarrhea 4 (4.4%) 2 (2.2%)
......................................... ,. .......
GGT elevation 0 2 (2.2%)
......................................... ,. .......
Agitation 1 (1.1%) 2 (2.2%)
......................................... ,. .......
Insomnia 2 (2.2%) 2 (2.2%)
......................................... ,. .......
Decreased appetite 0 2 (2.2%)
Values are number (percent) of patients. SAE = serious adverse event, GGT =
gamma-
glutamyltransferase
[0364] The most common adverse events were constipation, nausea, dry mouth,
dyspepsia,
and vomiting. These adverse events represent a balance of events caused by
xanomeline and
events caused by trospium. For example, xanomeline alone causes sweating,
nausea,
vomiting, diarrhea, and excess salivation, as well as orthostasis and syncope.
Trospium
causes constipation, dry mouth, and stomach upset. Unlike in earlier
xanomeline trials, no
sweating was reported or observed.
[0365] The majority of the most common cholinergic/anticholinergic adverse
events
associated with KarXT treatment decreased throughout the study. Specifically,
the rates of
cholinergic adverse events (nausea and vomiting shown in FIG. 17) and
anticholinergic
adverse events (dry mouth, shown in FIG. 18) consistently decreased in the
KarXT group
(gray bars). By the endpoint at week 5, the rates of nausea, vomiting, and dry
mouth for the
KarXT were statistically indistinguishable from the placebo group's rates. The
rates of
constipation in the KarXT group showed less of a downward trend (data not
shown). Black
bars represent corresponding AE rates for the placebo group throughout the
study.
87
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
[0366] There was no syncope in the KarXT treated group. The mean resting
orthostatic and
standing heart rate increased only 4.4 bpm over placebo (FIGS. 19 and 20). The
effect on
heart rate was markedly lower compared to prior studies and was trending
toward resolution.
There was no mean change in orthostatic systolic or diastolic blood pressures
(FIGS. 21 and
22). There were no notable postural effects between orthostatic and standing
measurements.
[0367] Somnolence, weight gain, and extrapyramidal symptoms/akathisia were
similar to
placebo. No significant changes were observed in the Barnes akathisia scale,
Simpson-Angus
scale, or the abnormal involuntary movement scale. These are the adverse
events typically
observed with the current standard of care. These adverse events were not
manifest in KarXT
treatment.
[0368] The liver enzymes in the LFT were comparable to placebo (Table 39). Two
KarXT-
treated patients had elevated GGT (> 2X ULN) and one placebo-treated patient
with elevated
ALT (> 3X ULN), AST (> 3X ULN), and GGT (> 2X ULN). Specifically, one subject
discontinued due to elevated gamma-glutamyltransferase (GGT) present in the
LFT.
Table 39: Adverse Events over Course of Study
Lab Test Placebo (n = 90) KarXT (n = 89)
ALT ¨ U/L 2.1 32.4 2.8 16.2
AST ¨ U/L -0.5 16.9 -0.4 10.9
Alk phos ¨ U/L -1.7 14.5 -0.6 15.3
GGT ¨ U/L 2.1 26.0 1.5 34.1
Bilirubin ¨ mmol/L -0.3 4.3 -0.4 3.2
Plus-minus values are mean SD; Abbreviations: ALT = alanine
aminotransferase; AST =
aspartate aminotransferase; Alk phos = alkaline phosphatase; GGT = gamma-
glutamyltransferase.
[0369] One serious adverse event on KarXT was recorded¨a patient discontinued
and
sought hospital care for worsening psychosis. Although this event met the
technical definition
of serious adverse events per regulation, psychosis was caused by
schizophrenia, not by
KarXT. The patient did not withdraw because of a symptom caused by KarXT
administration.
Thus, this result reflected the lack of efficacy of KarXT for one patient
rather than the lack of
tolerability caused by the drug making new symptoms.
[0370] This Phase 2 study showed robust antipsychotic efficacy and favorable
safety/tolerability of KarXT in hospitalized patients with schizophrenia.
KarXT showed early
(2 weeks) and sustained (entire 5 weeks) separation from the placebo arm on
the primary
88
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
efficacy measure (PANSS total) and four of the five secondary outcome
measures. The safety
profile consistent with prior work with KarXT combination. All but one
treatment-emergent
adverse event was rated mild or moderate. Most cholinergic and anticholinergic
adverse
events decreased throughout the study to levels statistically
indistinguishable from the
placebo group.
[0371] The foregoing description is given for clearness of understanding only,
and no
unnecessary limitations should be understood therefrom, as modifications
within the scope of
the disclosure may be apparent to those having ordinary skill in the art.
Throughout the
specification, where compositions are described as including components or
materials, it is
contemplated that the compositions can also consist essentially of, or consist
of, any
combination of the recited components or materials, unless described
otherwise. Likewise,
where methods are described as including steps, it is contemplated that the
methods can also
consist essentially of, or consist of, any combination of the recited steps,
unless described
otherwise. The disclosure illustratively disclosed herein suitably may be
practiced in the
absence of any element or step which is not specifically disclosed herein.
[0372] The practice of a method disclosed herein, and individual steps
thereof, can be
performed manually and/or with the aid of or automation provided by electronic
equipment.
Although processes have been described with reference to embodiments, a person
of ordinary
skill in the art will readily appreciate that other ways of performing the
acts associated with
the methods may be used. For example, the order of various of the steps may be
changed
without departing from the scope or spirit of the method, unless described
otherwise. In
addition, some of the individual steps can be combined, omitted, or further
subdivided into
additional steps.
[0373] It is appreciated that certain features of the invention, which are,
for clarity, described
in the context of separate embodiments, may also be provided in combination in
a single
embodiment. Conversely, various features of the invention, which are, for
brevity, described
in the context of a single embodiment, may also be provided separately or in
any suitable
subcombination. All combinations of the embodiments pertaining to the chemical
groups
represented by the variables contained within the generic chemical formulae
described herein
are specifically embraced by the present invention just as if each and every
combination was
individually explicitly recited, to the extent that such combinations embrace
stable
compounds (i.e., compounds that can be isolated, characterized and tested for
biological
activity). In addition, all subcombinations of the chemical groups listed in
the embodiments
89
CA 03161952 2022-05-17
WO 2021/101875
PCT/US2020/060859
describing such variables, as well as all subcombinations of uses and medical
indications
described herein, are also specifically embraced by the present invention just
as if each and
every subcombination of chemical groups and subcombination of uses and medical
indications was individually and explicitly recited herein.
[0374] All patents, publications and references cited herein are hereby fully
incorporated by
reference. In case of conflict between the present disclosure and incorporated
patents,
publications and references, the present disclosure should control.