Note: Descriptions are shown in the official language in which they were submitted.
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
VIABLE TISSUE FORMS AND METHODS FOR MAKING AND USING SAME
CROSS-REFERENCE TO RELATED APPLICATIONS
The present application claims the benefit of U.S. Provisional Application No.
62/948,905,
filed on December 17, 2019, the entire disclosure of which is hereby
incorporated by reference
herein.
FIELD OF THE INVENTION
The present invention relates generally to preserved tissue forms which
contain
endogenous viable cells and retain or promote biological activity after being
stored at temperatures
above freezing for extended periods of time. The present invention also
relates to methods for
making and using such preserved tissue forms.
BACKGROUND OF THE INVENTION
Various tissue forms which are derived from processed tissue samples recovered
from
donors (either living or deceased) are known and useful as grafts for tissue
repair and
reconstruction in recipients having tissue which is damaged, diseased,
atrophied or which could
otherwise benefit from such treatment. In some cases, it is beneficial for the
tissue form to retain
or promote biological activity which facilitates tissue healing, growth and/or
reconstruction. Such
biological activity may be provided by endogenous or exogenous growth factors,
proteins, viable
cells, or other biologically active substances present in the tissue form.
Similarly, viable and non-
viable cells and their products and components may create, participate in, or
facilitate biologic
signaling processes and networks which control or manage biological activity
or processes.
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
Viable cells often produce or recruit biologically active substances such as
growth factors
and proteins. However, many processing techniques for producing tissue forms
from recovered
tissue samples tend to devitalize cells such that they no longer produce or
recruit such biologically
active substances. Even when the processing techniques do not devitalize cells
present in the tissue
samples, storage of the resulting tissue forms at room temperature, or
temperatures above freezing,
often results in the loss of a portion or all of the viable cells.
Accordingly, much effort has been
spent developing processing and preservation techniques for producing tissue
forms containing
viable endogenous cells and for storing those tissue forms under conditions
which preserve the
viability of the cells, as well as their biologic activity and ability to
produce signaling factors and
participate in biologic signaling processes.
In some cases, recovered tissue samples or the tissue forms produced therefrom
are simply
frozen by exposure to temperatures at or below about 0 C, which is known to
devitalize cells. In
other cases, recovered tissue samples or the tissue forms produced therefrom
are cryopreserved by
contacting them with one or more cryopreservation agents and exposing the
tissue samples or
tissue forms and cryopreservation agent(s) to temperatures at or below about 0
C. Cryopreserving
is known to preserve a portion of the viable cells present in the tissue
sample or tissue form, but
requires that the resulting tissue form be stored at temperatures at or below
freezing, often at
temperatures of about -30 C or less, or even about -80 C or less, for long
term viability.
Cryopreserved tissue forms must also be thawed prior to implantation as
grafts.
In still other cases, recovered tissue samples or the tissue forms produced
therefrom are
preserved by lyophilizing (i.e., freeze-drying) by contacting them with one or
more lyophilizing
agents and freezing them (either controlled rate or not), followed by drying
under vacuum.
Lyophilization is traditionally known to devitalize cells, but when viable
tissues are lyophilized
2
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
under appropriate conditions, it may be possible for these tissues to still
retain viable cells as well
as biologically active substances, such as growth factors or proteins.
Lyophilized tissue forms can
be stored for extended periods of time above freezing temperatures (greater
than about 0 C), but
typically require rehydration prior to implantation as grafts.
It would be helpful to develop processing techniques that produce preserved
tissue forms
containing viable cells endogenous to the initial recovered tissue samples
from which the tissue
forms are prepared, where the preserved tissue forms can be stored above
freezing for extended
periods of time (for example, from at least 14 days to 365 days, or at least 1
year, 2 years, 3 years,
or even more). Such extended storage of viable tissue forms above freezing
temperatures would
also facilitate transport or shipping of the viable tissue forms over longer
distances and times than
currently possible in some cases. The present invention provides a combination
of particular
protectants and lyopreserving techniques which produce viable tissue forms
that can be stored for
extended time periods above freezing of from at least 14 days to 365 days, or
at least 1 year, 2
years, 3 years, or even more, while retaining a population of viable
endogenous cells.
SUMMARY OF THE INVENTION
The present invention relates to preserved tissue forms which contain
endogenous viable
cells and retain or promote biological activity after being stored at
temperatures above freezing for
extended periods of time. The present invention also relates to methods for
making and using such
preserved tissue forms.
In one embodiment, a preserved tissue form for implanting in or on a subject
is provided
which comprises a preserved tissue sample which is derived from a recovered
tissue sample and
3
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
contains a post-lyopreservation population of endogenous viable cells which is
a portion of a pre-
lyopreservation population of endogenous viable cells of the recovered tissue
sample.
In another embodiment, a preserved tissue form for implanting in or on a
subject is
provided which comprises a preserved tissue sample which is derived from a
recovered tissue
sample and contains a post-lyopreservation population of endogenous viable
cells which is a
portion of a pre-contact population of endogenous viable cells of the
recovered tissue sample.
In another embodiment, a preserved tissue form for implanting in or on a
subject is
provided which comprises a preserved tissue sample which is derived from a
recovered tissue
sample and is capable of storage at a temperature above freezing for an
extended period of time,
after which the preserved tissue sample contains a retained population of
endogenous viable cells
which is a portion of a post-lyopreservation population of endogenous viable
cells. In some
embodiments, the extended period of time is from 14 days to 5 years.
In still another embodiment, a preserved tissue form for implanting in or on a
subject is
provided which comprises a preserved tissue sample comprising a tissue type,
wherein, after
storage at a temperature above freezing for an extended period of time, the
preserved tissue sample
contains a post-lyopreservation population of endogenous viable cells, wherein
the post-
lyopreservation population of endogenous viable cells of the preserved tissue
sample is
substantially comparable to a post-cryopreservation population of endogenous
viable cells of a
cryopreserved tissue sample which comprises the same tissue type as the
preserved tissue sample.
In some embodiments, the extended period of time is from 14 days to 5 years.
In some
embodiments, the post-lyopreservation population of endogenous viable cells of
the preserved
tissue sample is 90% of the post-cryopreservation population of endogenous
viable cells of the
cryopreserved tissue sample.
4
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
The preserved tissue form of may further comprise one or more biocompatible
fluids,
wherein the lyopreserved tissue sample is rehydrated by contact with the one
or more
biocompatible fluids. After the preserved tissue sample is rehydrated and
implanted in or on a
subject at an implantation site, the preserved tissue form retains beneficial
biological activity,
promotes beneficial biological activity, or both. The beneficial biological
activity comprises
promoting, at the implantation site, at least one of: tissue healing, tissue
growth, and tissue
generation.
A method is also provided for preparing a preserved tissue sample, which
comprises the
steps of:
(A) recovering a tissue sample from a donor;
(B) optionally, cleaning the tissue sample;
(C) optionally, disinfecting the tissue sample;
(D) optionally, modifying one or more of the size, shape and other physical
characteristics of the tissue sample by applying one or more physical
treatments,
chemical treatments, or combinations thereof;
(E) contacting the tissue sample with one or more protectants for a period
of contacting
time, to form a tissue-protectant mixture comprising a quantity of tissue
sample and
one or more protectants;
(F) optionally, prior to lyopreserving, storing the tissue-protectant
mixture, for a period
of storage time, at a storage temperature (e.g., less than -80 C, or less
than -50 C);
(G) optionally, during or after the step of (E) contacting the tissue
sample with one or
more protectants and prior to lyopreserving, incubating the tissue-protectant
mixture at an incubation temperature, for a period of incubation time; and
5
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
(H)
lyopreserving the tissue-protectant mixture by first freezing the tissue-
protectant
mixture, and then drying the frozen tissue-protectant mixture (optionally
under
vacuum) to produce a preserved tissue sample having a post-lyopreservation
population of endogenous viable cells,
wherein the preserved tissue sample is capable of storage at a temperature
above freezing for an
extended period of time after which the preserved tissue sample contains a
retained population of
endogenous viable cells which is a portion of the post-lyopreservation
population of endogenous
viable cells. In some embodiments, wherein the extended period of time is from
14 days to 5 years,
such as from 14 to 150 days.
The one or more protectants are selected from the group consisting of: sugars,
polyphenols,
carotenoids, and combinations thereof. In some embodiments, the one or more
protectants
comprises: glucose, fructose, sucrose, trehalose, dextran, EGCG, and
combinations thereof In
some embodiments, the contacting step (E) comprises contacting the tissue
sample with a
protectant solution comprising the one or more protectants and at least one
biologically compatible
fluid.
In some embodiments, the incubation temperature, at which the step of (G)
incubating the
tissue-protectant mixture prior to lyopreserving is performed, is selected
from a room temperature,
a refrigerating temperature, a warming temperature, and combinations thereof.
In some
embodiments, the incubation temperature is a refrigerating temperature
comprising from about 2
C to about 8 C. The period of incubation time, for which the step of (G)
incubating the tissue-
protectant mixture prior to lyopreserving is performed, may be from greater
than zero seconds to
about 48 hours.
6
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
The tissue sample recovered from a donor comprises a tissue type selected
from: adipose,
amnion, artery, bone, cartilage, chorion, colon, dental, dermal, duodenal,
endothelial, epithelial,
fascial, gastrointestinal, growth plate, intervertebral disc, intestinal
mucosa, intestinal serosa,
ligament, liver, lung, mammary, meniscal, muscle, nerve, ovarian, parenchymal
organ, pericardial,
periosteal, peritoneal, placental, skin, spleen, stomach, synovial, tendon,
testes, umbilical cord,
urological, vascular, vein, and a combination thereof
BRIEF DESCRIPTION OF THE DRAWINGS
The present invention will be further explained with reference to the attached
figure, in
which:
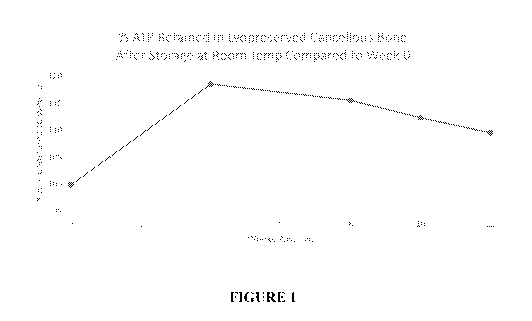
Figure 1 is a graph of the average ATP values obtained by ATP Assay testing
performed
on preserved cancellous bone tissue samples to confirm cell viability at 0, 4,
8, 10 and 12 weeks.
DETAILED DESCRIPTION OF THE INVENTION
Detailed descriptions of one or more embodiments of the present invention are
disclosed
herein. It should be understood that the disclosed embodiments are merely
illustrative of the
invention which may be embodied in various forms. In addition, each of the
examples given in
connection with the various embodiments of the invention is intended to be
illustrative, and not
restrictive. Further, the figures are not necessarily to scale, and some
features may be exaggerated
to show details of particular components. Measurements, specifications and the
like shown in the
figures are intended to be illustrative, and not restrictive. Therefore,
specific structural and
functional details disclosed herein are not to be interpreted as limiting, but
merely as examples for
teaching one skilled in the art to variously employ the present invention.
7
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
The articles "a" and "an" are used herein to refer to one or to more than one
(i.e., at least
one) of the grammatical object of the article. By way of example, "an element"
means one element
or more than one element.
For convenience, certain terms employed herein are enumerated below. Unless
defined
otherwise, all technical and scientific terms used herein have the same
meaning as commonly
understood by one of ordinary skill in the art to which this disclosure
belongs. The following
definitions are intended to enable persons of ordinary skill in the relevant
art to understand, make and
use the inventions described herein without undue experimentation, however,
the definitions should
not be construed to unreasonably limit the meaning or scope of the terms.
As used herein, the term "about" means within 20%, more preferably within 10%
and most
preferably within 5%. The term "substantial" means more than 50%, such as more
than 60%, or
more than 70%, preferably more than 80% or 85%, and most preferably more than
90%, or 95%,
or even 98%.
The term "substantially comparable" means within 90%, such as within 85%,
or within
80%, or within 75%, or within 70%, or within 65%, or within 60%, or
within 55%, or
within 50%, or within 45%, or within 40%, or within 35%, or within
30%, or within
25%, or within 20%, or within 15%, preferably within 10%, and most
preferably within
5%, including within any percent between 90% and 1%.
As used herein, "basal media" includes any medium which maintains the
viability and/or
supports the growth of cells (whether isolated, cultured, resident in tissue,
or otherwise positioned
or adhered on a substrate) and typically comprises a carbon source (e.g., a
simple sugar, such as
glucose, etc.), water, various salts (such as calcium chloride, potassium
chloride, magnesium
sulfate, sodium chloride, etc.), amino acids, and optionally vitamins (such as
thiamine, riboflavin,
8
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
folic acid, etc.). Suitable basal media include, without limitation, minimal
essential medium
(MEM), basal medium Eagle (BME), Dulbecco's Modified Eagle's Medium (DMEM),
etc.
"Biologically compatible fluid" and "biocompatible fluid" are used
interchangeably herein
and mean a fluid for combination with a protectant which will not produce a
toxic, injurious, or
immunologic response when contacted with living tissue. Examples of
biologically compatible
fluids suitable for combination with one or more protectants include, without
limitation: an
aqueous buffer (for example without limitation, phosphate buffered saline
("PBS")), a buffered or
non-buffered isotonic solution (for example without limitation, an aqueous
sodium chloride
solution (e.g., from about 0.1 weight % (wt%) to about 1 wt%, such as about
0.9 wt%), a lactated
Ringer's solution, a cell storage or culture media (for example without
limitation, DMEM, a basal
medium (e.g., Basal Medium Eagle (BME), or other similar media)), platelet
rich plasma (PRP),
lecithin, alginate, hyaluronic acid (HA), a derivative or salt of HA (e.g.,
sodium hyaluronate), bone
marrow, other suitable biologically compatible fluids known to persons of
ordinary skill in the
relevant art, and mixtures thereof.
"Cryopreservation," as used herein, means a preservation technique which
avoids
formation of ice crystals inside cells (whether isolated or contained in a
tissue sample) during
freezing by first contacting the cells, or tissue sample containing cells,
with one or more
cryopreservation agents which replace the water inside the cells, followed by
freezing the tissue
sample by reducing the temperature to 0 C or below. Thus, cryopreservation
methods generally
involve contacting a tissue sample recovered from a donor with one or more
cryopreservation
agents which means that the tissue sample contains a pre-contact population of
endogenous viable
cells (i.e., measured after performing all desired processing steps and prior
to contacting with the
cryopreservation agent(s)), and a post-contact (or pre-cryopreservation)
population of endogenous
9
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
viable cells (i.e., measured after performing all desired processing steps and
contacting the tissue
sample with one or more cryopreservation agents, but prior to cryopreserving
the processed tissue
sample). A cryopreserved tissue sample contains a preserved TO (or "Week 0,"
"post-
cryopreservation") population of endogenous viable cells. Depending on if and
how long after
cryopreservation the cryopreserved tissue sample is stored (at well below
freezing temperatures,
as necessary to maintain viability of the endogenous cells), a cryopreserved
tissue sample will also
contain a retained population of endogenous viable cells as measured after a
period of storage time
such as, without limitation, at least 14 days, or least 28 days, or at least
56 days, or at least 70 days,
at least 90 days, at least 180 days, at least 365 days, at least 1 year, 2
years, 3 years, or any point
.. in between, or even longer.
"Cryopreservation agents," as used herein means intracellular cryoprotectants
which
operate by permeating the cell membrane and replacing the intracellular water
to reduce the
intracellular water concentration and, thereby, reduce the amount of ice
formed within the cell
during freezing. Cryopreservation agents include, without limitation, glycols
(alcohols containing
.. at least two hydroxyl groups), such as ethylene glycol, propylene glycol,
glycerol, and propanediol,
as well as other compounds such as formamide, dimethylsulfoxide (DMSO),
methanol, dimethyl
acetamide, dimethyl formamide. It is known that intracellular cryoprotectants
are often harmful
and devitalize cells during performance of cryopreservation.
"Disinfection" and "disinfecting" as used herein, in all of their grammatical
forms, is any
process that reduces or minimizes bioburden (e.g., bacteria, fungi, etc.),
viral load, or both, of an
object (e.g., a tissue, a container for tissue, or an implement for processing
tissue) and typically,
but without limitation, involves contacting, rinsing or soaking the object
with one or more
disinfecting agents or a solution containing one or more disinfecting agents.
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
"Disinfecting agents" include, any substances, molecules or other materials
which
accomplish disinfection including, without limitation, chlorine and chlorine
compounds (e.g.,
hydrochloric acid), formaldehyde, glutaraldehyde, alcohols (e.g., ethanol,
isopropyl alcohol),
peracetic acid, surfactants (e.g., Triton X-100, Tween, Sodium Dodecyl
Sulfate) combinations
thereof, and solutions containing same.
The term "dormant" is used herein to describe tissue and cells that are in a
state in which
they have little to no metabolic activity, but such tissue or cells have
retained the ability to be
returned to a state in which evidence of metabolic activity can be detected.
"Dormant" cells may
be reinvigorated using methods such as by rehydrating with a biologically
compatible fluid. For
example, without limitation, tissue and cells may be described as "dormant"
after being subjected
to a preservation method and during subsequent storage for a period of time,
as described herein.
"Endogenous" as used herein refers to that which is naturally occurring,
incorporated
within, housed within, adherent to, attached to or resident in.
"Exogenous" as used herein, refers to that which is not originally naturally
present and,
therefore, is introduced from or produced outside, an organism, cell, tissue,
system, graft or
implant. Exogenous cells, materials or substances may be derived from the same
or a different
individual as is intended to receive the cells, materials, substances, tissue,
system, graft or implant.
"Freeze" and "freezing," as used herein, refers to a preservation technique
which involves
cooling a tissue sample, with or without a preservative or preservative
solution which may be
present, to a temperature below the freezing temperature of the tissue sample
or of the tissue
sample and preservative or preservative solution and results in the formation
of ice crystals within
the frozen tissue sample, and sometimes within cells contained therein. As
will be readily
recognized by persons of ordinary skill in the relevant art, the aforesaid
freezing temperature may
11
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
be about 0 C, or it may be less than or greater than about 0 C, depending on
the type of tissue
sample and the type of preservative or preservative solution, if present.
Before use, a preserved
tissue which has been frozen must be reconstituted by thawing.
"Freeze-drying" is synonymous and used interchangeably herein with the terms
"lyophilization" and "lyophilizing" (see below).
A "graft" as used herein means a tissue or organ used for transplantation to,
or other
placement in or on, a subject (e.g., a patient). Some grafts are made from or
otherwise include
tissue matrices produced by processing tissue samples recovered from one or
more donors. The
donor(s) and the receiving subject may each, independently, be: a mammal
(including humans and
non-human mammals), a reptile, an amphibian, fish, and/or a bird. Furthermore,
"grafts" include,
but are not limited to, a self-tissue transferred from one body site to
another in the same individual
("autologous graft"), a tissue transferred between genetically different
members of the same
species ("allograft"), and a tissue transferred between different species
("xenograft").
"Lyophilization" and "lyophilizing" (also referred to as "freeze-drying"), as
used herein
refers to a preservation technique which involves freezing a tissue sample
(either via controlled
rate or not), followed by drying under vacuum, which removes ice and other
frozen solvents from
the frozen tissue sample through the process of sublimation and removes bound
water molecules
through the process of desorption. The drying phase of a lyophilizing process
is typically
performed in two steps which include a primary drying step which removes the
majority of the ice
and frozen solvent (e.g., at least about 70 wt%, and often at least about 90
wt%), followed by a
secondary drying step which removes additional ice and frozen solvent from the
frozen tissue
sample to produce a lyophilized preserved tissue having less than about 10
wt%, such as less than
about 6 wt%, or even less than about 1 wt% water.
12
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
"Lyophilization agents" as used herein are extracellular lyoprotectants that
do not permeate
the cell membrane and include: monosaccharides (e.g., glucose, sucrose),
disaccharides (e.g.,
trehalose, lactose) and polysaccharides (e.g., dextran, hydroxyethyl starch
(HES), cellulose),
flavonoid polyphenols (e.g., catechins such as, without limitation,
epigallocatechin gallate
(EGCG), sugar alcohols (e.g., mannitol, sorbitol, xylitol, erythritol,
adonitol, etc.). Combinations
of these are also effective lyophilization agents.
"Lyopreserving" and "lyopreservation" as used herein refer to a preservation
technique
which involves freezing (either via controlled rate or not) a tissue sample in
the presence of one or
more specific protectants or a protectant solution containing them as
described herein, followed
by drying under vacuum which may be performed in a single step at a constant
temperature or at
temperatures varied within a single range, or in two steps including a primary
drying step at
temperatures within a first range which removes the majority of the ice and
frozen solvent (e.g., at
least about 70 wt% (based on the total weight of the tissue form prior to
lyopreservation), or at
least about 80 wt%, or at least about 90 wt%), followed by (2) a secondary
drying step at
temperatures within a second range which removes additional ice and frozen
solvent from the
frozen tissue sample to produce a preserved tissue sample or form having less
than about 15 wt%
residual moisture (based on the total weight of the preserved tissue form), or
having less than about
12 wt%, or less than about 10 wt%, or less than about 8 wt%, or less than
about 7 wt%, or less
than about 6 wt%, or less than about 5 wt%, or less than about 4 wt%, or less
than about 3 wt%,
or less than about 2 wt%, or less than about 1 wt% residual moisture.
Controlled rate
lyopreservation may be desired because it tends to avoid changes in the dried
product appearance
and characteristics.
13
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
"Molarity," abbreviated as "M," as used herein describes the molar
concentration of a
protectant or other solute in a biologically compatible fluid or other solvent
in units of moles of
the protectant or other solute per liter of solution (total of solute plus
solvent). Similarly, molar
concentration may be described as "millimolarity," abbreviated as "mM," in
units of millimoles
.. of the protectant or other solute per liter of solution (total of solute
plus solvent).
"Preservation" and "preserving," as used herein, generally means treating a
tissue sample
and the endogenous cells therein to minimize or prevent loss, injury, damage,
decay or change and
maintains or promotes viability of at least a portion of the endogenous cells.
Conventionally,
"preservation" techniques are understood to include cryopreservation, freezing
and lyophilization.
However, as used herein "preservation" techniques mean treatment of a tissue
sample and the
endogenous cells therein, with or without additional components, according to
lyopreserving
methods performed in the presence of one or more specific protectants or a
protectant solution
containing them, as described herein.
"Protectants," as used herein, means substances or compounds which, when
contacted with
a tissue sample containing a population of viable endogenous cells, promote,
facilitate, or enable
the preservation of the tissue sample and cells thereof to produce a preserved
tissue with at least a
portion of the population of cells being viable upon reconstitution. Suitable
protectants include,
without limitation, one or more substances selected from the group consisting
of: sugars,
polyphenols (i.e., flavonoids, stillbenes, lignans, phenolic acids), and
carotenoids. Suitable sugars
include, without limitation, monosaccharides (e.g., glucose, fructose),
disaccharides (e.g.,
trehalose) and polysaccharides (e.g., dextran). Among the suitable flavonoid
polyphenols are
catechins such as, without limitation, epigallocatechin gallate (EGCG),
epicatechin gallate (ECG),
epigallocatechin (EGC) and epicatechin (EC). Suitable carotenoids include,
without limitation, a-
14
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
carotene, 13-carotene, (3-cryptoxanthin, lycopene, lutein, and zeaxanthin. One
or more protectants
are often, but not necessarily, provided in a protectant solution with a
biologically compatible
fluid.
"Protectant solution," as used herein, means a mixture of at least one
protectant and a
biologically compatible fluid.
"Preserved tissue sample," and "preserved tissue form," as used herein, mean a
tissue
sample or tissue form, respectively, which contained a population of
endogenous viable cells prior
to preservation and which has been subjected to a preservation technique
according to the
invention described and contemplated herein, which includes contacting a
tissue sample with one
or more preservation agents, followed by lyopreserving according to the
methods described herein,
such that, when reconstituted (i.e., rehydrated by contact with a biologically
compatible fluid), at
least a portion of the population of endogenous viable cells remains viable
after preservation and
storage at temperatures above freezing for an extended period of time (e.g.,
from at least 14 days
to 365 days, or at least 1 year, 2 years, 3 years, 5 years, or even more, and
including any time
between 14 days and 5 years).
"Reconstitute" and "rehydrate" as used in connection with a preserved tissue
sample or
preserved tissue form comprising same mean the process by which a preserved
tissue sample and
cells therein are contacted with a biologically compatible fluid, which may
reinvigorate dormant
cells, e.g., bring cells from a dormant state to a state in which evidence of
metabolic activity can
be detected. Where a preserved tissue has been frozen, the preserved tissue
may be thawed before,
during, or after contacting with a biologically compatible fluid.
Additionally, a preserved tissue
sample or preserved tissue form may be reconstituted or rehydrated in situ,
such as after being
implanted in a subject, such as by bodily fluids of the subject (e.g., blood,
plasma, lymphatic fluid,
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
etc.) which are present at or recruited to the site of implantation, or with
biocompatible fluid
provided or added to the site of implantation (i.e., internal or external
implantation site) by a
medical practitioner or other user of the preserved tissue sample or preserved
tissue form
comprising same.
"Room temperature" as used herein is synonymous with ambient temperature and
means
from about 15 C to about 25 C, such as from about 19 C to about 25 C, or
from about 20 C
to about 23 C.
"Sterilization" and "sterilizing" as used herein, in all of their grammatical
forms, is any
process that renders an object (e.g., a tissue, a container for tissue, or an
implement for processing
tissue) essentially free from pathogenic organisms and/or viruses by
destroying them or otherwise
inhibiting their growth or vital activity. Such processes may include exposure
of the object to one
or more, without limitation, of gamma radiation, electron beam radiation,
chemical agents (e.g.,
alcohol, phenol, ethylene oxide gas, acids, bases, or peroxides), heat, or
ultraviolet radiation for
sufficient duration and dosages. When sterilization is performed on a finished
tissue product in its
.. final packaging, the process may be referred to as "terminal
sterilization".
As used herein, the term "storing," whether used for tissue samples recovered
from a donor
(i.e., before or after any processing steps, including contacting with one or
more protectants), or
preserved tissue samples or preserved tissue forms comprising same (i.e.,
after lyopreserving),
includes any periods of transport or shipping, regardless of temperature.
"Tissue sample" and "biological tissue sample" are used interchangeably herein
and mean
a piece, slice, slab, section, fragment, chunk, lump, wedge, or any other
shape, form or quantity,
of tissue which has been recovered, harvested or otherwise derived from one or
more donors, living
or deceased, human or non-human, which comprises a population of viable
endogenous cells and
16
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
may be fresh, refrigerated (i.e., at temperatures from greater than zero to
about 20 C, or frozen
(i.e., at temperatures of 0 C or below). Additionally, the tissue sample may
be, include, or be
derived from one or more of the following tissue types: adipose, amnion,
artery, bone, cartilage,
chorion, colon, dental, dermal, duodenal, endothelial, epithelial, fascial,
gastrointestinal, growth
plate, intervertebral disc, intestinal mucosa, intestinal serosa, ligament,
liver, lung, mammary,
meniscal, muscle, nerve, ovarian, parenchymal organ, pericardial, periosteal,
peritoneal, placental
(including amnion, chorion, amnionchorion, umbilical cord, and Whartons
jelly), skin, spleen,
stomach, synovial, tendon, testes, umbilical cord, urological, vascular, vein,
and a combination
thereof. In some embodiments, the tissue is derived from a human or non-human
mammal. In some
embodiments, the tissue is derived from a human donor. In some embodiments,
the human donor
is a cadaveric donor.
"Viable," "viability" and other grammatical forms thereof, as used herein to
describe tissue
or cells, means having the ability to live, grow, expand or develop, or which
are dormant and have
potential to live, grow, expand or develop upon reconstitution (e.g., such as
by thawing or
rehydrating). For example, viability, as applied to cells, can be
characterized by having directly or
indirectly observable, or measurable, metabolic activity, or which are dormant
and have potential
to become metabolically active upon reconstitution (e.g., such as by thawing
or rehydrating).
Compositions described and contemplated herein are preserved tissue forms
comprising
preserved tissue samples which are useful as grafts. The preserved tissue
forms comprise one or
more processed tissue samples which include a population of viable cells,
which are endogenous
to the tissue samples and at least a portion of which remains viable after the
preserved tissue
samples or tissue forms comprising them are stored at temperatures above
freezing for extended
periods of time and then rehydrated. The tissue samples are recovered from one
or more donors
17
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
and then processed with one or more processing steps and techniques which
include contacting the
tissue samples with one or more protectants, and lyopreserving the resulting
tissue-protectant
mixture (which may also be described as lyopreserving a tissue sample in the
presence of one or
more protectants or a protectant solution). The population of viable cells
comprise cells
endogenous to at least one of the processed tissue samples included in the
tissue form. The
population of viable cells may be supplemented or combined with exogenous
viable cells, which
may be autogenic, allogenic, xenogenic, or combinations thereof.
More particularly, prior to lyopreserving (but after other processing steps),
the preserved
tissue sample comprises a population of endogenous viable cells at least a
substantial portion (i.e.,
at least 98 %, or at least 90 %, or at least 80 %, etc.) of which remain
viable after convenient
storage of the preserved tissue forms at temperatures above freezing (i.e.,
greater than 0 C), for
extended periods of time (e.g., up to 14 days, or up to 90 days, or up to 180
days, or up to 365
days, or at least 1 year, 2 years, 3 years, 5 years, or even longer, and
including any time between
14 days and 5 years). Such characteristics mean that the tissue forms avoid
(1) safety issues
sometimes presented by exposure to ultra-low temperatures (i.e., less than
about -50 C) and (2)
the inconvenient necessity for thawing the preserved tissue form before use,
both of which are
encountered when using cryopreserved tissue forms.
The preserved tissue forms described and contemplated herein are useful for
tissue repair
and reconstruction in recipients having tissue which is damaged, diseased,
atrophied or which
could otherwise benefit from such treatment (including structural, functional
and aesthetic
benefits). The preserved tissue forms are useful for tissue repair and
reconstruction, at least in part,
because the endogenous viable cells retain and/or promote biological activity
which facilitates
tissue healing, growth and/or generation. Without intending to be limited by
theory, it is believed
18
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
that such biological activity may be provided by the endogenous viable cells
themselves and/or
growth factors, proteins, or other biologically active substances secreted by,
or recruited from
surrounding tissue by, the viable cells, or both. This biological activity is
in addition to any such
activity which may be provided by any growth factors, proteins or other
substances already present
and remaining in the tissue samples during and after processing.
Without intending to be limited be theory, it is believed that viable cells
such as, without
limitation, mesenchymal stem cells (MSCs), which are contained in several
types of tissue samples
(e.g., without limitation, bone, bone marrow, adipose, placenta, etc.) both
before and after being
subjected to the lyopreserving method described and contemplated herein, are
known to secrete
several types of biologically active substances including growth factors,
cytokines, chemokines, and
other proteins which cause or contribute to paracrine effects
(immunomodulatory, anti-apoptotic,
supportive (e.g., stimulation of mitosis, proliferation and differentiation),
angiogenic, anti-scaring,
chemoattractant, etc.) known to enhance wound healing and tissue
reconstruction. Specific examples
of such paracrine signaling substances secreted by one or more types of viable
cells, include, without
.. limitation, vascular endothelial growth factor (VEGF), hepatocyte growth
factor (HGF), insulin-like
growth factor-1 (IGF-1), placental growth factor (p1GF), transforming growth
factor beta (TGF-0),
basic fibroblast growth factor (bFGF), granulocyte-macrophage colony-
stimulating factor (GM-
CSF), prostaglandin E2 (PGE-2), indoleamine 2,3-dioxygenase (IDO), interleukin
(IL)-6, interleukin
(IL)-12, IL-1 receptor antagonist (IL-lra), tumor necrosis factor alpha (TNF-
a), monocyte
chemoattractant protein-1 (MCP-1 / also known as CCL2), macrophage
inflammatory proteins (MW-
la /CCL3, MIP-10 / CCL4, MIP-3a /CCL20, eotaxin-3 / CCL26, etc.), and many
others.
In accordance with the preserved tissue forms and methods for making and using
them which
are described and contemplated herein, after being subjected to the
lyopreserving methods described
19
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
and contemplated herein, preserved tissue forms contain both paracrine
signaling substances (growth
factors, cytokines, chemokines, etc.) already secreted by endogenous viable
cells of the tissue sample
(before and during lyopreserving), as well as viable cells which continue to
secrete such paracrine
signaling substances after lyopreserving and rehydration. Accordingly,
preserved tissue samples and
preserved tissue forms comprising them which are produced in accordance with
the lyopreserving
methods described and contemplated herein, upon rehydration, behave and
function as conditioned
media containing biologically active substances which cause or contribute to
paracrine effects and
other beneficial biological activities which cause, facilitate or enhance
wound healing and tissue
reconstruction when implanted in a host, patient or subject. Furthermore, upon
rehydration, such
preserved tissue samples and preserved tissue forms comprising them also
produce and provide
additional quantities of biologically active substances which continue to
provide such beneficial
healing and reconstructive activity at the site of implantation. As will be
understood by persons of
ordinary skill in the relevant art, the site of implantation may be within or
on an external surface of a
subject, or a combination or both.
The recovered tissue samples may be, include, or be derived from one or more
of the
following tissue types: adipose, amnion, artery, bone, cartilage, chorion,
colon, dental, dermal,
duodenal, endothelial, epithelial, fascial, gastrointestinal, growth plate,
intervertebral disc,
intestinal mucosa, intestinal serosa, ligament, liver, lung, mammary,
meniscal, muscle, nerve,
ovarian, parenchymal organ, pericardial, periosteal, peritoneal, placental
(including amnion,
chorion, amnionchorion, umbilical cord, and Wharton's jelly), skin, spleen,
stomach, synovial,
tendon, testes, umbilical cord, urological, vascular, vein, and a combination
thereof. Preferred
tissue types include, without limitation: adipose, fascia, dermis, bone,
cartilage, muscle, placenta
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
(including amnion, chorion, amniochorion, Wharton's jelly, and umbilical
cord), placental disk,
and combinations thereof.
As described below, embodiments of the present invention include preserved
tissue forms
comprising at least one processed tissue sample which has been contacted with
one or more
protectants and lyopreserved (i.e., at least one preserved tissue sample),
methods for producing
such preserved tissue forms, and methods for using such preserved tissue
forms. Although the
various embodiments are described in further detail in connection with tissue
samples comprising
bone (e.g., cancellous bone), cartilage, and placental (e.g., amnion and
chorion) tissues, it should
be clear and understood by persons of ordinary skill in the relevant art that
the present invention
contemplates and includes embodiments comprising other tissue types, such as
those listed above.
It should also be clear and understood that the present invention contemplates
and includes
embodiments comprising more than one tissue type and that those tissue types
may be processed
the same way or differently. Additionally, the present invention contemplates
and includes
embodiments in which the preserved tissue form further comprises materials,
substances or
components, which may be natural or synthetic, in addition to one or more
processed tissue
samples at least one of which has been contacted with one or more protectants
and lyopreserved.
All such embodiments, and other reasonably overlapping or derived from those
described herein
are within the scope of the present invention.
The one or more preserved tissue samples included in any particular preserved
tissue form
may be autografts (i.e., recovered from the same individual donor as the
intended recipient),
allografts (i.e., recovered from a different individual donor of the same
species as the intended
recipient), xenografts (i.e., recovered from an individual donor of a
different species as the
intended recipient), or combinations thereof.
21
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
As will be familiar to persons of ordinary skill in the relevant art, the
tissue samples are
processed by applying steps and techniques which are selected depending on the
type of tissue
recovered, the desired characteristics of the processed tissue samples, the
intended tissue form and
its intended use (i.e., the particular intended recipient, the tissue type to
be treated, the condition
to be treated and the desired results of said treatment). Accordingly, the
method for producing a
preserved tissue form comprises processing a tissue sample by performing one
or more of the
following steps:
(A) recovering a tissue sample from a donor according to accepted
ethical and sterile
procedures;
(B) optionally, cleaning the tissue sample;
(C) optionally, disinfecting the tissue sample;
(D) optionally, modifying one or more of the size, shape and other physical
characteristics of the tissue sample by applying one or more physical
treatments,
chemical treatments, or combinations thereof;
(E) contacting the tissue sample with one or more protectants, which may be
in solution
with a biologically compatible fluid, for a period of time, to form a tissue-
protectant
mixture comprising a quantity of tissue sample and one or more protectants;
(F) optionally, prior to lyopreserving, storing the tissue-
protectant mixture, for a period
of storage time, at a storage temperature (e.g., less than -80 C, or less
than -50 C),
optionally in contact with storage media, preservatives, priming media, or
combinations thereof;
22
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
(G) optionally, prior to lyopreserving, incubating the tissue-protectant
mixture at an
incubation temperature, for a period of incubation time, optionally in contact
with
storage media, preservatives, priming media, or combinations thereof;
(H) lyopreserving the tissue-protectant mixture by first freezing the
tissue-protectant
mixture, and then drying the frozen tissue-protectant mixture (optionally
under
vacuum) to produce a processed (lyopreserved) tissue sample.
The resulting processed tissue sample is a lyopreserved tissue sample which
may, by itself,
be suitably useful as a preserved tissue form and implanted into a subject.
Alternatively, the
method for producing a preserved tissue form may further comprise combining
the processed
preserved tissue sample with one or more additional components, either prior
to, during or after
the lyopreserving step (H) .
After recovery or harvest of the tissue sample from one or more donors, the
tissue sample
includes a fresh population of viable cells which are endogenous to the tissue
sample. Before
performing the step of contacting (E) the tissue sample with one or more
protectants, but after all
other desired processing steps are performed, the processed tissue sample
includes a pre-contact
(or Baseline) population of viable cells which is at least a portion of the
fresh population of viable
cells. After performing all desired processing steps and the step of
contacting (E) the tissue sample
with one or more protectants, but prior to performing the step of
lyopreserving (H), the processed
tissue form (with or without one or more additional components) includes a
post-contact (or pre-
lyopreservation / "pre-lyo") population of viable cells which is at least a
portion of the pre-contact
population of viable cells.
After lyopreserving (H), the resulting preserved tissue form (with or without
one or more
additional components, in addition to the preserved tissue sample) includes a
preserved TO (or
23
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
"Week 0," "post-lyopreservation," or "post-lyo") population of viable cells
which is at least a
portion of the post-contact population of viable cells. The TO ("Week 0,"
"post-lyopreservation,"
or "post-lyo") population of viable cells should be measured shortly after the
lyopreserving step
(H) is completed, such as within about 7 days, or within about 96 hours, or
within about 48 hours,
or even within about 24 hours, of completing the lyopreserving step (H). The
preserved tissue form
may be stored at temperatures above freezing, for an extended period of time,
after which the
preserved tissue form includes a retained population of viable cells, which is
at least a portion of
the TO population of viable cells. The fresh, pre-contact ("Baseline"), post-
contact (pre-
lyopreservation, or pre-lyo), TO ("Week 0," "post-lyopreservation," or "post-
lyo"), and retained
populations of viable cells each comprise viable cells endogenous to at least
one tissue sample
which has been subjected to processing as described and contemplated herein to
produce the
preserved tissue form. The viable endogenous cells of a preserved tissue
sample are those which
were present in the original unprocessed tissue sample and have not been
removed from that tissue
sample during processing and lyopreserving. In some embodiments, one or more
of the fresh, pre-
contact ("Baseline"), post-contact (pre-lyopreservation, or pre-lyo), TO
("Week 0," "post-
lyopreservation," or "post-lyo"), and retained populations of viable cells may
each be
supplemented or combined with exogenous viable cells, which may be,
independently of one
another, autogeneic, allogeneic, xenogeneic, or combinations thereof, and may
have been added
to the tissue form at any point during the processing and preservation steps.
In some embodiments,
the retained population of viable cells is more than 50% of the TO population
of viable cells, such
as more than 60%, or more than 70%, preferably more than 80% or 85%, and most
preferably
more than 90%, or 95%, or even 98%.
24
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
Storage of the preserved tissue form at temperatures above freezing includes,
for example
without limitation, storage at room temperatures (i.e., from about 19 C to
about 25 C), or
refrigeration temperatures (i.e., from greater than 0 C to about 10 C), or
intermediate
temperatures (i.e., from greater than 10 C to less than about 19 C).
Extended periods of time for
which preserved tissue forms may be stored and still have a retained
population of viable cells
includes, for example without limitation, from at least 14 days to 365 days or
more, such as at least
14 days, or at least 28 days, or at least 56 days, or at least 70 days, or at
least 90 days, or at least
180 days, or at least 270 days, or at least 365 days, or at least 1 year, 2
years, 3 years, or even
longer, and including any time between 14 days and 5 years. It is noted that
periods of storage may
include or overlap with one or more periods of time during which the preserved
tissue sample or
preserved tissue form comprising same is transported or shipped from one
location to another, and
during at least a portion of which the temperature to which the preserved
tissue sample or preserved
tissue form comprising same is exposed may be at or below freezing, or above
freezing.
The preserved tissue form provides several benefits and advantages, including
but not limited
to: (1) providing a preserved tissue form containing a (retained) population
of viable cells which may
be conveniently stored at temperatures above freezing until use, (2) resolving
customer safety concerns
presented by exposure to ultra-low temperatures (e.g., below -50 C or even -
80 C) relative to
conventional cryopreserved tissue forms, and (3) eliminates the need for
thawing time prior to use as
compared to conventional cryopreserved and frozen tissue forms. For tissue
processors and tissue
form producers, the ability to store the tissue forms above freezing
temperature eliminates the need
for costly storage resources (dry ice, liquid nitrogen, tanks and freezers,
etc.) and removes certain
limitations on tissue shipment (limit on shipment time and size due to dry
ice).
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
Each of the above-listed steps (A)-(H) of the method for producing a preserved
tissue form
comprising processing a tissue sample will now be described and explained in
further detail.
Tissue samples are recovered from a donor (A) according to accepted ethical
and sterile
procedures, which involve donor screening, obtaining donor releases and
informed consent, tissue
screening and testing and tissue transport to facilities for further
processing. The tissue samples
may be stored, such as in a biologically compatible fluid or not, for a period
of time after recovery
from the donor(s) (A) and prior to shipping / transport, or during shipping /
transport, or even for
a period of time after arriving at a processing facility but prior to
commencement of processing
steps (e.g., cleaning (B), disinfecting (C), modifying the size, shape, or
other physical
characteristics (D), contacting with one or more protectants (E), storing (F),
incubating (G),
lyopreserving (H), etc.). In some embodiments, recovered tissue samples may be
held or stored in
another kind of solution, such as, without limitation, a red cell lysis buffer
such as ACK
(Ammonium-Chloride-Potassium) Lysing Buffer which is intended to facilitate
removing
unwanted blood from the samples.
In various embodiments, the step of cleaning (B) a recovered tissue sample is
typically
intended to isolate the desired tissue type by removing unwanted tissue and
other materials and
may, for example, include one or more of the following processes: (1)
debriding or otherwise
separating the recovered tissue sample to remove and separate unwanted tissue
from desired
recovered tissue; and (2) removing unwanted materials and substances
including, without
limitation, blood, lipids, debris, and unwanted tissue by rinsing or washing
the recovered tissue
sample, for a period of cleaning time, at least once, with one or more fluids
such as, without
limitation, buffered or unbuffered saline solution, lactated Ringer's
solution, balanced salt
solution, basal medium, water, or combinations thereof.
26
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
A suitable period of cleaning time for rinsing or washing during the step of
cleaning (B)
the tissue sample is typically, for example without limitation, at least 2
seconds, or at least 10
minutes, or at least 20 minutes, or from about 2 seconds to about 2 hours,
such as from about 2
seconds to about 5 minutes, or from about 5 minutes to about 20 minutes, or
from about 20 minutes
to about 2 hours. The step of (B)(2) removing unwanted materials and
substances from a recovered
tissue sample may also, or alternatively, be performed by contacting the
recovered tissue sample
with supercritical carbon dioxide for a period of time of from about 2 minutes
to about 60 minutes,
such as from about 5 minutes to about 60 minutes, or from about 5 minutes to
about 40 minutes,
or from about 5 minutes to about 30 minutes, or from about 5 minutes to about
20 minutes.
In various embodiments, the step of disinfecting (C) a recovered tissue sample
typically,
but without limitation, includes contacting, rinsing, or soaking the tissue
sample, for a period of
disinfecting time, with one or more disinfecting agents or a disinfecting
solution comprising one
or more disinfecting agents. As will be recognized by persons of ordinary
skill in the relevant art,
a suitable period of disinfecting time for the step of disinfecting (C) will
depend on the type and
concentration of disinfecting agent or disinfecting solution and is typically,
for example without
limitation, at least 0.5 seconds, or from about 0.5 seconds to about 2 hours,
such as from about 0.5
seconds to about 5 minutes, or from about 5 minutes to about 20 minutes, or
from about 20 minutes
to about 2 hours, or from about 30 minutes to about 2 hours, or from about 30
minutes to about
1.5 hours, or from about 30 minutes to about 1 hour. Suitable disinfecting
agents include, without
limitation, chlorine and chlorine compounds (e.g., hydrochloric acid),
alcohols (e.g., ethanol,
isopropyl alcohol), peracetic acid, surfactants (e.g., Triton X-100, Tween,
Sodium Dodecyl
Sulfate), antibiotics (e.g., amoxicillin, penicillin, gentamicin,
amphotericin, doxycycline,
azithromycin, vancomycin, etc.), antimycotics (e.g., amphotericin B,
nystatin), combinations
27
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
thereof, and solutions containing same. In some embodiments, for example, the
step of disinfecting
(C) comprises soaking the tissue sample in a sufficient volume of peracetic
acid solution for a
sufficient amount of disinfecting time so as to produce a disinfected tissue
sample, wherein the
disinfected tissue sample has a sterility assurance level of at least 106. The
sterility of the
disinfected tissue sample is determined by the method described in
International Standards ISO
14937:2009. Furthermore, a sufficient volume of peracetic acid would provide,
for example
without limitation, a ratio of the tissue sample surface area (cm2) to the
volume of peracetic acid
solution (m1) of from about 0.01 cm2/m1 to about 0.65 cm2/ml.
The step (D) of modifying size or shape of the tissue sample includes, without
limitation,
increases or decreases in one or more dimensions (e.g., without limitation,
changes to one of more
of diameter, width, length, height, thickness, etc.) and formation or molding
recovered tissue into
any desired shape, whether by manual manipulation or with the use of a
container or mold. Possible
shapes for the tissue samples, processed tissue samples, and preserved tissue
forms include,
without limitation, particles, strips, chunks, pieces, blocks, sheets,
slivers, ribbons, branched and
.. unbranched elongated elements, filaments, fibers, three dimensional
geometric shapes such as
symmetric and asymmetric spheres, regular and irregular polyhedrons, cones,
pyramids, other
three dimensional forms having one or more planar or curved surfaces, and
irregular three
dimensional forms. As will be understood and practicable by persons of
ordinary skill in the
relevant art, processed tissue samples and preserved tissue forms comprising
them may be
produced in the form (i.e., have the shape) of, for example without
limitation, one or more of
particulates, fibers, chunks or pieces, etc. (i.e., a first shape) and then be
molded (reshaped) into
another desired shape, for example without limitation, sheets, blocks,
cylinders, plugs or other
three-dimensional shape (i.e., a second shape), such as by manual
manipulation, manipulation
28
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
using a device, or using a mold or other container to impart a desired shape,
with or without
agitation, drying, etc.. Modifications to other physical characteristics
include, without limitation,
removal of lipids such as for adipose tissue, demineralizing such as for bone
tissue, changes to the
molecular structure of collagens or proteins in a tissue sample (e.g.,
digestion, hydrolysis,
.. cleavage, crosslinking, etc.), and increasing or decreasing flexibility,
density, compressibility,
elasticity, etc.
In some embodiments, the optional step (D) of modifying size, shape or other
physical
characteristics of the tissue sample includes, for example without limitation,
one or more of the
following physical processes: cutting, slicing, cleaving, chopping, grating,
grinding, milling,
fragmenting, blending, homogenizing, extruding, fracturing, separating,
pressing, molding,
manual manipulation, heating, cooling, freezing, and the like, using devices
known now or in the
future to persons of ordinary skill in the relevant art. Selection of a
physical process and a device
for performing the selected physical process is within the ability of persons
of ordinary skill in the
relevant art based on this disclosure and the knowledge generally possessed by
and available to
.. such persons and will depend on various factors including, without
limitation, the type of tissue
sample being treated, the condition intended to be treated by the resulting
tissue form comprising
the processed tissue sample, and the desired result of such treatment.
In some embodiments, the step (D) of modifying size, shape or other physical
characteristics of the recovered tissue sample includes, for example without
limitation, one or more
of the following chemical processes: digesting, cleaving, dissolving,
disintegrate, dissociating,
hydrolyzing, fragmenting, separating, extracting, absorbing, desorbing,
aggregating, linking, and
the like, using agents and techniques known now or in the future to persons of
ordinary skill in the
relevant art. Suitable agents and techniques include, for example without
limitation, contacting the
29
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
tissue sample with an enzyme (e.g., a kinase, an amylase, telomerase, trypsin,
collagenase, pepsin,
lipase, etc.), an acid, a base, an ionic substance, a surfactant, and the
like, and combinations thereof,
with or without heating or cooling for a period of time sufficient to
accomplish the desired degree
of chemical alteration of the tissue sample. Selection of a chemical process
and an agent and/or
technique, including the quantity of the agent and length of time for
contacting the tissue sample
with the agent are within the ability of persons of ordinary skill in the
relevant art based on this
disclosure and the knowledge generally possessed by and available to such
persons and will
depend on various factors including, without limitation, the type of tissue
sample being treated,
the condition intended to be treated by the resulting tissue form comprising
the processed tissue
sample, and the desired result of such treatment.
Generally, any combination of one or more physical and chemical processes may
be
performed, in any order, to accomplish the step (D) of modifying size, shape
or other physical
characteristics of the tissue sample. In some embodiments, more than one
physical process may
be performed. In some embodiments, more than one chemical process may be
performed. In some
embodiments, the step (D) of modifying size, shape or other physical
characteristics of the tissue
sample may include at least one physical process and at least one chemical
process. In some
embodiments, one or more chemical processes may be performed prior to any
physical processes,
and in other embodiments, one or more physical processes may be performed
prior to any chemical
processes. In some embodiments, one or more chemical processes may be
performed both prior to
and after one or more physical processes. In some embodiments, one or more
physical processes
may be performed both prior to and after one or more chemical processes.
Additionally, the
physical and chemical processes of step (D) may be performed in any order as
desired.
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
As will be understood by persons of ordinary skill in the relevant art, in
addition to being
optional depending on the type and source of the tissue sample, the steps of
cleaning (B),
disinfecting (C), and modifying one or more of the size, shape and other
physical characteristics
of (D) the tissue sample may each be performed with one or more repetitions
and in any order, at
the discretion of persons of ordinary skill. For example, without limitation,
these steps may be
performed sequentially as listed above. In some embodiments, without
limitation, a recovered
tissue sample may first be disinfected (C) and then cleaned (B), with or
without subsequent
modifying one or more physical characteristics (D). In other embodiments, a
recovered tissue
sample may be cleaned (B), disinfected (C), then cleaned again (B), prior to,
optionally, modifying
one or more physical characteristics (D). In other embodiments, for example,
without limitation, a
recovered tissue sample may be cleaned (B), then cleaned (B) again (such as
with different
cleaning agents or by different cleaning techniques, etc.), followed by
disinfecting (C), with or
without subsequent modifying one or more physical characteristics (D). In
still other embodiments,
without limitation, a tissue sample may be subjected to a step of modifying
one or more of its
physical characteristics (D), followed by one or more cleaning steps (B), then
further modifying
one or more of physical characteristics (D) of the tissue sample, followed by
disinfecting (C), and
then an additional cleaning step (B). Any such number and order of the
optional steps of cleaning
(B), disinfecting (C), and modifying one or more physical characteristics (D),
as well as other
arrangements of such steps, are possible and suitable, as is within the
ability of persons of ordinary
skill in the relevant art to determine, depending on the source, type and
intended use of the
recovered tissue.
Furthermore, the tissue sample is contacted, in step (E) listed above, with
one or more
protectants (in solution or not) for a period of contacting time to form a
tissue-protectant mixture
31
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
comprising a quantity of tissue sample and the one or more protectants. The
period of contacting
time is from greater than zero seconds and extends until lyopreserving is
commenced, such as up
to about 48 hours. For example without limitation, the period of contacting
time for the step of (E)
contacting the tissue sample with one or more protectants may be at least
about 10 minutes, or at
least about 15 minutes, or at least about 20 minutes, or at least about 60
minutes, or at least about
120 minutes, or at least about 180 seconds, or at least 240 minutes, or at
least 6 hours, or at least
hours, or any value from greater than zero to about 48 hours. It is noted that
in some
embodiments, the tissue sample or portions thereof may be contacted with
(e.g., rinsed, soaked,
stored in, etc.) one or more protectants (in solution or not) at any time
during processing, before
10
during or after any of the steps of the method for producing the preserved
tissue forms described
herein. Any periods of time for which the tissue sample or portions thereof is
contacted with one
or more protectants before or during any of the steps of (A) recovering, (B)
cleaning, (C)
disinfecting, (D) modifying, (E) storing or (F) incubating, the tissue sample
are not necessarily
intended to be included in the period of contacting time which occurs during
the contacting step
(E) performed prior to lyopreserving as discussed above. In other words, in
some embodiments,
the tissue sample or a portion thereof may be contacted with one or more
protectants (in solution
or not) for a period of time longer than about 48 hours, particularly, when
the contact also occurs
before or concurrently with one or more of the steps performed prior to the
step of (H)
lyopreserving.
In some embodiments, such as those in which the tissue sample is a bone tissue
sample,
the period of contacting time may, without limitation, be at least about 20
minutes, or at least about
minutes, or at least about 60 minutes, or at least about 120 minutes, or at
least about 180
minutes, or at least 240 minutes, or any value between greater than zero and
up to about 6 hours,
32
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
or up to about 7 hours, or up to about 8 hours, or even up to about 9 hours.
In some embodiments,
such as those in which the tissue sample is a cartilage tissue sample, the
period of contacting time
may, without limitation, be at least about 10 minutes, or at least about 30
minutes, or at least about
60 minutes, or at least about 120 minutes, or at least about 180 minutes, or
at least 240 minutes, or
at least 300 minutes, or any value between greater than zero and up to about 6
hours, or up to about
7 hours, or up to about 8 hours, or even up to about 9 hours. In some
embodiments, such as those
in which the tissue sample is a placental tissue sample such as, without
limitation, one or a
combination of amnion and chorion tissues, the period of contacting time may,
without limitation,
be at least about 15 minutes, or at least about 30 minutes, or at least about
60 minutes, or at least
about 120 minutes, or at least about 180 minutes, or at least 200 minutes, or
any value between
greater than zero and up to about 4 hours, or up to about 5 hours, or up to
about 6 hours, or up to
about 7 hours, or up to about 8 hours, or even up to about 9 hours.
Examples of protectants suitable for use with the method described herein
include, without
limitation, one or more of: low molecular weight non-reducing sugars (e.g.,
trehalose, sucrose,
glucose, etc.), dextran, catechins (e.g., epigallocatechin (EGG),
epigallocatechin gallate (EGCG),
etc.), carotenoids (a-carotene, 13-carotene, (3-cryptoxanthin, lycopene,
lutein, zeaxanthin, and other
naturally occurring carotenoids), glycerol, antioxidants (e.g., ascorbic acid
(vitamin C), vitamin E,
etc.), and late embryogenesis abundant (LEA) proteins. Preferred protectants
include trehalose,
EGCG, and combinations thereof EGCG is also known to have antioxidant
properties and,
therefore, is a highly suitable protectant.
Where more than one protectant is employed, the protectants may be contacted
with the
tissue sample in any order and combination. For example, without limitation,
in some
embodiments, a first protectant may be contacted with the tissue sample, and
then an additional
33
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
one or more (i.e., second, third, fourth, etc.) protectants may be added
sequentially, or in
combinations. In some embodiments, two or more protectants may be contacted
with the tissue
sample concurrently, either by separate but concurrent addition to the tissue
sample, or by mixing
the protectants together and then adding the mixed protectants to the tissue
sample. Similarly, in
some embodiments, two or more protectants may be combined with one another to
form a first
protectant mixture, and two or more different protectants may be combined with
one another to
form a first protectant mixture, and then the first and second protectant
mixtures added to the tissue
sample, separately but concurrently, or sequentially, or by first mixing the
first and second
protectants mixtures together and then adding the resulting combined mixtures
to the tissue
sample.
The one or more protectants will typically, but do not have to, be in solution
with a
biologically compatible fluid. Suitable biologically compatible fluids
include, for example without
limitation, one or more of: saline, PBS, Hank's balanced salt solution (HBSS),
Hyclone growth
media containing fetal bovine serum (commercially available from Thermo Fisher
Scientific of
Carlsbad, California, U.S.A.), Dulbecco's Modified Eagle's medium (DMEM),
Basal Medium
Eagle (BME), and derivatives and combinations thereof In some embodiments,
after all
protectants, fluids and any additional ingredients have been combined to form
the protectant
solution, the solution may be sterilized, such as by exposure to ultraviolet
or gamma radiation,
filtering through a filter, such as a 0.2 um filter, or other techniques known
to persons of ordinary
skill in the art and discussed further hereinbelow.
In an exemplary embodiment, the protectant solution comprises trehalose and
EGCG
dissolved in PBS. Where trehalose is at least one of the protectants in the
protectant solution,
trehalose may, for example, be present in a concentration of from about 0.1 M
to about 1 M, such
34
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
as from about 0.2 M to about 0.6 M, or from about 0.3 M to about 0.5 M, or
about 0.4 M, or about
0.5 M. Where EGCG is at least one of the protectants in the protectant
solution, EGCG may, for
example, be present in a concentration of from about 0.5 mM to about 8 mM,
such as from about
1 mM to about 6 mM, or from about 3 mM to about 5 mM, or about 4 mM.
In some embodiments, additional ingredients may be combined with the
protectant(s) or
the protectant solution. Suitable additional ingredients for the protectant
solution include, without
limitation, one or more of: nonionic surfactants (e.g., Polysorbate 80),
glucose, mannitol, amino
acids (e.g., glycine), salts, vitamins, human serum albumin (HSA), bovine
serum albumin (BSA).
In some embodiments, for example, an exemplary protectant solution comprises
trehalose
and EGCG dissolved in either Hyclone media or phosphate buffered solution
(PBS) at a
concentration of from about 0.1 to about 0.5M and from about 1.0 to about
4.0mM, respectively.
In one embodiment, for example, the protectant solution may comprise lx PBS,
0.4M trehalose
and 4.0 mM EGCG. In some embodiments, the aforesaid exemplary protectant
solutions further
comprise dextran, glycerol, or a combination of both.
In some embodiments, the step (E) of contacting the tissue sample with one or
more
protectants may be performed at room temperature (i.e., from about 19 C to
about 25 C) and
ambient pressure (typically from about 0.8 atmosphere (atm) to about 1.05 atm,
which is from
about 85 kilopascals (kPa) to about 107 kPa, depending on altitude). In some
embodiments, the
tissue sample is contacted with one or more protectants and subjected to
heating (e.g., application
of or exposure to one or more temperatures above about 25 C) or cooling
(e.g., application of or
exposure to one or more temperatures below about 19 C), for all or a portion
of the contacting
time.
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
After performing the step of (E) contacting the tissue sample with one or more
protectants,
or protectant solution comprising same, the resulting tissue-protectant
mixture is subjected to the
step of (H) lyopreserving. In some embodiments, after (E) contacting with
protectants or protectant
solution, but before (H) lyopreserving, the tissue-protectant mixture may be
subjected to one or
more other intermediate treatment (i.e., processing) steps, such as one or
more of cleaning (B),
disinfecting (C), and modifying one or more of the size, shape and other
physical characteristics
(D), at the discretion of persons of ordinary skill. For example, without
limitation, in some
embodiments, the tissue-protectant mixture may be soaked or rinsed with a
biocompatible fluid
(e.g., PBS, a culture media, a buffered isotonic solution, etc.), or even with
a second protectant
solution (either of the same composition already used, or different
composition), prior to
performing a lyopreserving step (H).
The step (H) of lyopreserving the tissue-protectant mixture is typically
performed by first
freezing the tissue-protectant mixture, and then drying the frozen tissue-
protectant mixture under
vacuum to produce a processed tissue sample which has a retained population of
viable cells after
rehydration.
In some embodiments, the freezing phase of the lyopreserving step (H) may, for
example
without limitation, be performed at one or more temperatures in a range of
from about -80 C to
about -4 C, such as from about -70 C to about -4 C, or from about -50 C to
about -4 C. In some
embodiments, the freezing phase of the lyopreserving step (H) is performed at
a rate of from about
0.1 C/minute to about 10 C/minute, such as from about 0.1 C/minute to about
5 C/minute, or
from about 0.1 C/minute to about 2 C/minute. The freezing phase of the
lyopreserving step (H)
may be performed over a total freezing time of from about 5 minutes to about
300 minutes, or any
range therebetween, such as without limitation from about 5 minutes to about
200 minutes, or from
36
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
about 5 minutes to about 250 minutes, or from about 5 minutes to about 180
minutes, or from
about 5 minutes to about 130 minutes, or from about 5 minutes to about 100
minutes, or from
about 5 minutes to about 90 minutes, or from about 5 minutes to about 75
minutes, or from about
minutes to about 60 minutes, or from about 5 minutes to about 30 minutes, or
from about 10
5 minutes to about 300 minutes, or from about 15 minutes to about 200
minutes, or from about 20
minutes to about 300 minutes, or from about 30 minutes to about 300 minutes,
or from about 30
minutes to about 300 minutes, or from about 45 minutes to about 300 minutes,
or from about 60
minutes to about 300 minutes, or from about 75 minutes to about 300 minutes,
or from about 90
minutes to about 300 minutes, or from about 90 minutes to about 180 minutes,
or from about 90
minutes to about 135 minutes.
The drying phase of the lyopreserving step (H) removes water and other
solvents from the
frozen tissue sample and is performed for a period of total drying time. In
some embodiments, the
drying phase of the lyopreserving step (H) is performed under vacuum in a
single step, either at a
constant temperature or at varied temperatures (e.g., two or more steps)
within a single range. In
some embodiments, the drying phase of the lyopreserving step (H) is performed,
under vacuum or
not, in two steps including a primary drying step at one or more temperatures
within a first range
which removes the majority of the ice and frozen solvent (e.g., at least about
70 wt% of the frozen
water is removed), followed by (2) a secondary drying step at one or more
temperatures within a
second range which removes additional ice and frozen solvent from the frozen
tissue sample to
produce a preserved tissue sample having less than about 10 wt% water. In some
embodiments,
the drying phase of the lyopreserving step (H) is performed in three or more
steps, analogous to
those described above. Additionally, it is noted that the ranges of
temperatures employed during
the one or more steps of the drying phase (e.g., first range, second range,
etc.) may overlap or not.
37
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
Regardless of the number of drying steps or temperature ranges employed to
perform the drying
phase of the lyopreserving step (H), controlled rate lyopreserving may be
desired because it tends
to avoid changes in the dried product appearance and characteristics.
By application of a vacuum during the drying phase of lyopreserving the tissue
sample, the
drying phase may, for example without limitation, be performed at a pressure
of from about 0.013
kPa to about 0.13 kPa (i.e., from about 100 milliTorr (mTorr) to about 1000
mTorr), for example
from about 0.013 kPa to about 0.1 kPa (i.e., from about 100 mTorr to about 750
mTorr), or from
about 0.013 kPa to about 0.066 kPa (i.e., from about 100 mTorr to about 500
mTorr). The vacuum
and pressure during the drying phase of the lyopreserving step (H) need not
remain constant
throughout the drying phase. In some embodiments, the vacuum and pressure
during the drying
phase varies, by gradual change rates or by step changes, in a range of from
about 0.013 kPa to
about 0.13 kPa (i.e., from about 100 mTorr to about 1000 mTorr), with possible
exemplary sub-
ranges as stated above.
The total drying time for which the drying phase of the lyopreserving step (H)
is performed
may, for example without limitation, be up to about 48 hours, or from about 2
hours to about 48
hours, or any range therebetween, such as without limitation from about 2
hours to about 45 hours,
or from about 4 hours to about 40 hours, or from about 6 hours to about 36
hours, or from about
10 hours to about 40 hours, or from about 10 hours to about 36 hours, or from
about 10 hours to
about 30 hours, or from about 12 hours to about 40 hours, or from about 12
hours to about 36
hours, or from about 12 hours to about 30 hours, or from about 12 hours to
about 24 hours, or from
about 18 hours to about 40 hours, or from about 18 hours to about 36 hours, or
from about 18 hours
to about 24 hours.
38
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
In some embodiments, wherein the drying phase of the lyopreserving step (H) is
performed
under vacuum in a single step at a constant temperature, the constant
temperature may be in a
range of from about -50 C to about 25 C, for example without limitation,
from about -50 C to
about -15 C, or from about from about -50 C to about -10 C, or from about
from about -35 C to
about -10 C, or from about from about -25 C to about -10 C, or from about
from about -20 C to
about -10 C, or from about from about -20 C to about 0 C, or from about
from about -15 C to
about 25 C, or from about from about -10 C to about 25 C, or from about
from about -10 C to
about 20 C, or from about from about -10 C to about 10 C, or from about
from about -10 C to
about 20 C, or from about from about 0 C to about 20 C, or from about from
about 0 C to about
25 C. In some embodiments, wherein the drying phase of the lyopreserving step
(H) is performed
under vacuum varied temperatures (e.g., two or more steps), the varied
temperatures may be within
a single range of from about -50 C to about 25 C, for example without
limitation, from about -
50 C to about -15 C, or from about from about -15 C to about 25 C, or from
about from about
0 C to about 25 C. Whether a constant temperature or varied temperatures in
a single range are
used, the resulting preserved tissue form, which comprises a processed tissue
sample, with or
without additional components, has a water content of less than about 10 wt%,
or less than about
8 wt%, or less than about 7 wt%, or less than about 6 wt%, or less than about
5 wt%, or less than
about 4 wt%, or less than about 3 wt%, or less than about 2 wt%, or less than
about 1 wt%, based
on the total weight of the preserved tissue form.
In some embodiments, the drying phase of the lyopreserving step (H) is
performed under
vacuum in two steps which include a primary drying step at one or more
temperatures within a
first range which removes the majority of the ice and frozen solvent (e.g., at
least about 70 wt%,
or at least about 80 wt%, or at least about 90 wt%), followed by (2) a
secondary drying step at one
39
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
or more temperatures within a second range which removes additional ice and
frozen solvent from
the frozen tissue sample to produce a preserved tissue form, which comprises a
processed tissue
sample, with or without additional components, having less than about 10 wt%,
or less than about
8 wt%, or less than about 7 wt%, or less than about 6 wt%, or less than about
5 wt%, or less than
about 4 wt%, or less than about 3 wt%, or less than about 2 wt%, or less than
about 1 wt% water.
As mentioned above, the first and second ranges for drying temperatures may
overlap or
not. In some embodiments, for example without limitation, the first range of
drying temperatures
is from about -100 C to about 15 C, or any range therebetween, such as
without limitation from
about -80 C to about 0 C, or from about from about -60 C to about 0 C, or
from about from
about -50 C to about 0 C, or from about from about -50 C to about -15 C,
or from about from
about -50 C to about -10 C, or from about -45 C to about 0 C, or from
about -45 C to about -5
C, or from about -45 C to about -15 C, or from about -60 C to about 15 C,
or from about -45
C to about 5 C, or from about from about -30 C to about 15 C, or from about
from about -20 C
to about 15 C, or from about from about -15 C to about 15 C, or from about
from about -10 C
to about 15 C, or from about from about -10 C to about 15 C, or from about
from about -10 C
to about 10 C.
The time for which a primary drying step is performed may be, for example
without
limitation, up to about 40 hours, or from about 1 hour to about 40 hours, or
any range there
between, such as without limitation, or from about 2 hours to about 36 hours,
or from about 6 hours
to about 36 hours, or from about 12 hours to about 30 hours, or from about 12
hours to about 24
hours.
Generally, the secondary drying step is performed at one or more temperatures
higher than
drying temperatures employed during the primary drying step. In some
embodiments, the
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
secondary drying step is performed at one or more temperatures up to about 45
C, such as up to
about 40 C, or up to about 35 C. In some embodiments, for example without
limitation, the
second range of drying temperatures is from about -25 C to about 45 C, or
any range
therebetween, such as without limitation from about -25 C to about 45 C, or
from about -20 C
to about 40 C, or from about -25 C to about 35 C, or from about -15 C to
about 45 C, or from
about -15 C to about 35 C, or from about -15 C to about 25 C, or from
about -10 C to about 45
C, or from about -10 C to about 35 C, or from about -10 C to about 25 C,
or from about -5 C
to about 35 C, or from about -5 C to about 25 C, or from about 0 C to
about 35 C, or from
about 0 C to about 25 C.
The time for which a secondary drying step is performed may be, for example
without
limitation, up to about 40 hours, or from about 1 hour to about 40 hours, or
any range therebetween,
such as without limitation from about 2 hours to about 36 hours, or from about
6 hours to about
36 hours, or from about 6 hours to about 30 hours, or from about 6 hours to
about 20 hours, or
from about 6 hours to about 16 hours.
For example, in some embodiments, where the first range of drying temperatures
is from
about -50 C to about -15 C, the second range of drying temperatures may be
from about -15 C
to about 25 C, such as from about -10 C to about 25 C. In some embodiments,
where the second
range of drying temperatures is from about -50 C to about -10 C, the second
range of drying
temperatures may be from about -15 C to about 25 C, such as from about -10
C to about 25 C.
When lyopreserving is performed in accordance with the method described above,
in the
presence of one or more protectants described above, or a protectant solution
containing them, the
tissue form produced thereby comprises a processed tissue sample, with or
without additional
components, and contains a retained population of viable cells even after
storage at temperatures
41
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
above freezing for extended periods of time. Moreover, the retained population
of viable cells is a
substantial portion of the TO population of viable cells that was present in
the tissue sample
immediately after lyopreserving (H) and prior to storage at room or ambient
temperatures for a
period of time (e.g., from at least 14 days to 365 daysõ or at least 1 year, 2
years, 3 years, 5 years,
or even more, and including any time between 14 days and 5 years). In some
embodiments, the
retained population of viable cells in the preserved tissue form is measurably
viable after
rehydration of the tissue form. In some embodiments, at least a portion of the
retained population
of viable cells in the preserved tissue form are dormant and become measurably
viable after
reconstitution with a biologically compatible fluid. In some embodiments, the
retained population
of viable cells is more than 50% of the TO population of viable cells, such as
more than 60%, or
more than 70%, preferably more than 80% or 85%, and most preferably more than
90%, or 95%,
or even 98% of the TO population of viable cells.
Prior to lyopreserving (H), the tissue-protectant mixture may, optionally, be
subjected to a
storing step (F) in which the tissue-protectant mixture is held at a storage
temperature for a period
of storage time. For example, without limitation, the storage temperature may
be a freezing
temperature, such as at or below about 0 C, or below about -50 C, or even
below about -80 C.
A suitable period of storage time may, without limitation, be from about 60
minutes to about 7
days, or from about 60 minutes to about 14 days, or any period of time
therebetween).
Also prior to lyopreserving (H), the tissue-protectant mixture may,
optionally, be subjected
to an incubating step (G) in which the tissue-protectant mixture is held at an
incubation temperature
such as an ambient or room temperature (i.e., from about 19 C to about 25 C,
or from about 20
C to about 23 C), or a refrigerating temperature (i.e., from above 0 C to
about 18 C, or from
about 1 C to about 15 C, or from about 2 C to about 10 C, or from about 2
C to about 8 C,
42
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
or from about 2 C to about 6 C), or a warming temperature (i.e., from about
26 C to about 40
C, or from about 26 C to about 35 C, or from about 26 C to about 30 C, or
from about 28 C
to about 40 C). The incubating step (G) may, for example without limitation,
be advantageously
performed during or after the step of (E) contacting the processed tissue
sample with one or more
protectants and before any optional step of (F) storing the tissue-protectant
mixture, for a period
of storage time, at a storage temperature. Without wishing to be limited by
theory, it is believed
that performing the incubating step (G) wherein the tissue-protectant mixture
is stored at lower
temperature (e.g., from about 2 C to about 8 C, or from about 2 C to about
6 C) may improve
and increase viability of the tissue and cells therein, as well as
accelerating or otherwise assisting
with uptake of protectants (e.g., non-permeating protectants) by the tissue
and cells therein.
A suitable period of incubation time may, without limitation, be from greater
than zero
seconds to about 48 hours, such as at least about 20 minutes, or at least
about 60 minutes, or at
least about 120 minutes, or at least about 180 seconds, or at least 240
minutes, or at least 6 hours,
or at least 10 hours, or any value between zero and about 48 hours. In some
embodiments, such as
those in which the tissue sample is a bone tissue sample, the period of
incubating time may, without
limitation, be at least about 20 minutes, or at least about 30 minutes, or at
least about 60 minutes,
or at least about 120 minutes, or at least about 180 minutes, or at least 240
minutes, or any value
between zero and about 6 hours.
It is noted that any one or more of the contacting step (E), storing step (F),
and incubating
step (G) may be performed under vacuum, either constant, variable or pulsing,
or at pressures
greater than atmospheric or ambient pressures. Without being limited by
theory, varying the
pressures at which these steps are performed may also accelerate or otherwise
assisting with the
43
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
uptake of protectants (e.g., non-permeating protectants) by the tissue sample
or cells therein, and/or
may enhance or increase viability of the tissue or cells therein.
Additionally, in some embodiments, either of the storing (F) and incubating
(G) steps may
include contacting the tissue sample, or the tissue-protectant mixture, with
one or more storage
media, basal media, preservatives, or priming media which includes, without
limitation, one or
more of: amino acids, glucose, salts, vitamins, or other nutrients that
promote for cell survival, and
cell-preservative components. In some embodiments, the tissue sample may be
staged in such storage
media, basal media, preservatives, or priming media to increase cell
viability. Additionally, cell
viability may be enhanced through the inclusion of use known apoptosis
inhibitors, including
without limitation, dexamethasone, 3-aminobenzamide, aurintricarboxylic acid,
sodium
orthovanadate, and combinations thereof Such staging, storing or incubating
may occur during
one or more physical and chemical processes performed to accomplish the step
(D) of modifying
size, shape or other physical characteristics of the tissue sample. Use of
cooled storage or staging
media may be advantageous if applied to the tissue sample after cutting,
slicing and similar
techniques to decrease the temperature on the cut surface of the tissue
sample.
The preserved tissue forms may further include or be combined with one or more
additional
components, either prior to, during or after the lyopreserving step (H). Such
additional components
include, without limitation: mineralized or demineralized bone matrix
(cortical or cancellous) in
the form of chips, particles, fibers, powder, sheets, chunks, pieces,
geometric shapes, etc.;
decellularized tissue matrix in the form of chips, particles, fibers, powder,
sheets, chunks, pieces,
geometric shapes, etc.; other processed or unprocessed tissue forms (whether
viable or not) (e.g.,
cartilage allograft matrix (CAM), lyophilized decellularized delipidized
adipose-derived matrix,
cryopreserved matrices, etc.); a preservative; a biologically compatible
fluid; exogenous cells,
44
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
viruses, growth factors, proteins, or other biologically active substances; a
preservative; an
antioxidant; a pharmaceutically active compound; nutritional substances or
media; rheology
modifiers; crosslinking agents; pH modifiers (buffers); polymers (natural,
synthetic, or both);
biologically inert excipients (e.g., calcium carbonate, starch, cellulose,
glycol, glycerin, mineral
stearates, etc.).
In some embodiments, the preserved tissue form further includes one or more
natural or
synthetic polymers which may be biodegradable and present in proportions
selected to provide
preserved tissue grafts having various preferred rates of degradation and
resorption of the graft or
portions thereof. Suitable synthetic polymers include, but are not limited to,
bioabsorbable
polymers such as polylactic acid (PLA), polyglycolic acid (PGA), polylactic-
coglycolide acid
(PLGA), and other polyhydroxyacids, polycaprolactones, polycarbonates,
polyamides,
polyanhydrides, polyamino acids, polyortho esters, polyacetals, degradable
polycyanoacrylates
and degradable polyurethanes, as well as a polylactide-coglycolide (PLAGA)
polymer or a
polyethylene glycol-PLAGA copolymer. Examples of natural polymers include, but
are not limited
to, proteins such as albumin, collagen, fibrin, hyaluronic acid and its
derivatives, naturally
occurring polyamino acids, and polysaccharides such as alginate, heparin, and
other naturally
occurring biodegradable polymers of sugar units. The polymeric blend may also
include without
limitation polycarbonates, polyfumarates, and caprolactones.
The method for producing a preserved tissue form may further comprise an
optional step
of sterilizing the processed tissue sample or the tissue form comprising the
processed tissue
sample, either before, during or after the lyopreserving step (H). In some
embodiments, the
sterilizing step may be performed using one or more techniques including,
without limitation:
contacting with chemical sterilants, such as without limitation ethylene
oxide, highly acidic
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
solutions, highly basic solutions, and the like; and exposure to gamma
irradiation, electron beam
(E-beam) irradiation, microwave energy, and the like; and filtering with a
filter, such as a 0.2 um
filter; as will be familiar to persons of ordinary skill. In some embodiments,
the sterilizing step
may be performed by adding one or more sterilizing agents to the processed
tissue sample and/or
the preserved tissue form. In some embodiments, sterilizing is performed by
terminal sterilization
(i.e., performed after the lyopreserving step (H) and after the tissue form
has been placed and
sealed in a package). For terminally sterilized tissue forms, the protectant
could also contain a free
radical scavenger or other radio-protectant thus limiting or preventing cell
death.
After performing the step of lyopreserving (H) one or more processed tissue
samples, the
resulting preserved tissue form may be packaged in sterile packaging which may
include multiple
layers or components. Primary packaging for the tissue may consist of just a
single container (jar,
vial, bottle, pouch, syringe, cannula, tube, etc.) for both storage media and
preserved tissue form,
or multiple components such as one container for storage media and preserved
tissue form having
viable cells, and at least one other container for any non-viable tissue
components, and other
optional additional components. Packaging components or layers intended to
contain the preserved
tissue form, with or without storage or other media, should include a moisture
barrier for
preventing or minimizing moisture (water or other fluid) from entering or
escaping from the
packaging after sealing with the preserved tissue form therein. In some
embodiments, a processed
tissue sample may be placed in a first packaging component and subjected to
the lyopreserving
step (H) while in the first packaging component which is configured and/or
made of material which
is sufficiently permeable to allow moisture to escape during the lyopreserving
step (H) . In such
embodiments, after lyopreserving, the first packaging component and preserved
tissue sample
therein may be placed into a second packaging component, which may be
configured and/or made
46
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
of material which includes a moisture barrier for preventing or minimizing
moisture (water or
other fluid) from entering or escaping from the packaging after sealing with
the preserved tissue
form therein. In some embodiments, the components of the preserved tissue
form, including a
processed tissue sample, may be contacted with one or more preservatives (in
solution or not) in
packaging and then (H) lyopreserved while packaged. Packaging suitable for use
during
lyophilization is designed, configured and/or made of materials so as to be
compatible with the
types of tissue, cells, protectants and other components present in the tissue
sample or tissue form
to be preserved. Furthermore, packaging suitable for containing preserved
tissue samples or
preserved tissue forms containing same should be capable of preventing cross-
contamination
between tissue samples derived from different donors and also provide a
sterile barrier if needed
during lyopreserving (H). Suitable packaging may also include components which
facilitate
rehydration, handling of the tissue form (such as picking up with forceps,
laying flat, etc.), ., or
other preparation of the tissue form such as cutting, perforating, addition of
other ingredients such
as antibiotics, etc.
Lyopreserving (H) the tissue sample may facilitate molding or otherwise
forming the tissue
sample into an intentional predetermined shape which may, for example,
facilitate end use or
delivery to a surgical site or wound site of a subject, as well as providing
other physical
characteristics such as porosity which may enhance and/or accelerate
rehydration.
Clinical applications of the preserved tissue forms described and contemplated
herein
include use as a surgical graft for the treatment, repair or regeneration of
tissue. For example,
without limitation, when the preserved tissue forms are derived from tissue
samples comprising
bone, they will be useful for repair or treatment of bone defects. The
preserved tissue forms
comprising bone are also suitable for use in orthopedic surgery to promote
bone fusion where
47
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
needed such as, for example without limitation, intervertebral spinal fusion,
posterolateral spinal
fusion, long bone fusion, or anywhere bone fusion or bony defect filling is
required. The preserved
tissue forms provide an advantage over previously known cryopreserved cell-
containing bone
grafts which maintain long-term cell viability (up to five years), but also
require storage at
temperatures which are at or well below freezing, e.g., -80 C, until use.
Accordingly, such
cryopreserved bone grafts must be thawed prior to implantation and can also
present some
logistical issues for surgical centers that do not have the appropriate
freezer equipment.
Additionally, when the preserved tissue forms are derived from tissue samples
comprising
cartilage, they will be useful, for example without limitation, to promote
repair and/or regeneration
of damaged or worn cartilage where needed such as, for example without
limitation, in filling of
chondral defects, in filling of osteochondral defects, or in conjunction with
other cartilage repair
technologies such as, without limitation, microfracture, osteoarticular
transfer system (OATS)
surgery, and autologous chondrocyte implantation (ACT). Preserved tissue forms
derived from
tissue samples comprising cartilage would be useful, for example without
limitation, in the
treatment of j oints such as knees, hips, shoulders, elbows, wrists, ankles,
knuckles, etc.
When the preserved tissue forms are derived from tissue samples comprising one
or more
placental tissues (e.g., amnion, chorion, umbilical cord, amniochorion,
Wharton's Jelly, etc.), they
will be useful, for example without limitation, for use as a wound or tissue
covering to treat / repair
ulcers, burns, or other wounds; or to treat / repair interior wounds such as
tunneling wounds or
surgical wounds.
Exemplary methods for using a preserved tissue form prepared as described
above, for
repairing or reconstructing tissue in a subject, generally comprise:
48
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
- providing a preserved tissue form comprising one or more processed tissue
samples
which have been lyopreserved in the presence of one or more protectants and
stored at
temperatures above freezing;
- rehydrating the preserved tissue form by combining (e.g., by mixing,
attiring, etc.) the
preserved tissue form with a biologically compatible fluid such as, without
limitation,
saline, blood, bone marrow aspirate (BMA), platelet rich plasma (PRP), and the
like,
to produce a rehydrated tissue form; and
- treating living tissue of a subject by placing the rehydrated tissue form
on or in a subject,
proximate, adjacent or implanted within the living tissue of the subject.
The ability of the presently described preserved tissue forms, which contain a
population
of viable cells, to be stored at room-temperature or refrigeration
temperatures provides increased
efficiencies for both end users and tissue processors. Such preserved tissue
forms provide
improved ease-of-use for the user by removing the need to thaw and also offers
added convenience
to those users who are likely to otherwise have to use the graft shortly after
receipt and/or lack the
appropriate storage facilities for frozen tissue. For tissue processors, non-
frozen storage eliminates
the need for costly storage resources (dry ice, liquid nitrogen, tanks and
freezers, etc.) and removes
certain limitations on tissue shipment (limit on shipment time and size due to
dry ice). Additional
potential uses of these preserved tissue forms includes, without limitation,
implanting in extremities
for fusion of an ankle, a foot, a wrist, a hand, etc.
In some exemplary embodiments, the preserved tissue forms comprise bone
tissue, such as
cancellous bone, cortical bone or a combination of both. Such embodiments will
now be described
in further detail, with the understanding that the presently described and
contemplated preserved
tissue forms are not limited to those which include bone tissue, but rather
include preserved tissue
49
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
forms which comprise other tissue types as listed above (e.g., adipose,
cartilage, amnion, chorion,
etc.) and contain a population of viable cells which remain viable after
storage at temperatures
above freezing and rehydration.
In an exemplary embodiment, the preserved tissue form comprises processed bone
tissue
derived from cancellous bone samples and containing a population of endogenous
viable cells.
Optionally, demineralized cortical bone fibers or other allograft materials
may be added.
As will be recognized by persons of ordinary skill, the parameters and
performance of one
or more of the steps of the foregoing method for producing a processed tissue
sample and a
preserved tissue form comprising same may be modified, selected and optimized
depending on the
type of tissue sample being processed so as to preserve and retain the
beneficial bioactive
properties inherent to each tissue type. Descriptions of such specific
exemplary embodiments will
now be provided in connection with processing bone, cartilage and placental
tissue types, with the
understanding that these descriptions are not limiting and other variations
and modifications to the
steps of the method described herein are possible to optimize the processing
of other tissue types.
Specifically, processed viable cancellous bone tissue is produced by
contacting a bone
tissue sample with one or more protectants and then lyopreserving the bone
tissue sample and one
or more protectants to produce the processed bone tissue which remains viable
even after storage
at room temperature for extended periods of time. Stored under these
conditions, viable cells
including osteoblasts, osteoprogenitor cells, and mesenchymal stem cells in
the preserved tissue
form will remain viable for a period of at least 14 days, or at least 28 days,
and preferably greater
than 90 days after lyopreserving. An exemplary method for producing such a
preserved tissue form
comprises the steps of:
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
(A) Recovering or receiving a recovered cancellous bone tissue
sample (for example,
cancellous bone used in the graft may be recovered from the hemi-pelvis,
humeral
head, or other cancellous bone sites containing viable bone-forming cells or
mesenchymal stem cells);
(B) Cleaning the cancellous bone tissue sample;
1. Debriding the cancellous bone tissue sample to remove
soft tissue
2. Rinsing the cancellous bone tissue sample with buffered
saline to remove
blood and lipids;
(C) Disinfecting the cancellous bone tissue sample;
1. Rinsing the cancellous bone tissue sample with peracetic acid, mild
surfactant, and buffered saline to remove existing bioburden;
(D) Modifying one or more of the size, shape and other physical
characteristics of the
cancellous bone tissue sample by applying one or more physical treatments;
1. Cutting the cancellous bone tissue sample to form
cancellous blocks;
2. Optionally, storing the cancellous blocks in preservative or priming
media to
maintain cell viability (rinse and drain before further processing);
3. Milling the cancellous bone blocks to form cancellous
bone tissue granules;
a. Disinfecting (C) may be performed particularly
efficiently and
effectively after one or more size and/or shape modifying steps (D)
are performed on bone tissue samples, for examples using peracetic
acid;
51
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
(E) Contacting / adding cancellous bone tissue granules to a protectant
solution which
contains one or more protectants dissolved in PBS or other biocompatible fluid
to
form a cancellous bone tissue-protectant mixture;
(F) Optionally, storing the cancellous bone tissue-protectant mixture in a
freezer, such as
at a temperature of about -80 C, until lyopreserving; and
(H) Lyopreserving the cancellous bone tissue-protectant mixture to
produce a preserved
bone tissue form, by performing the following process;
1. if not already frozen in storage, freezing the
cancellous bone tissue-
protectant mixture at a rate of from 0.1 C/minute to 2 C/minute; and
2. drying the frozen cancellous bone tissue-protectant mixture
a. at either a constant temperature, or at a varied temperature in a
range of either from -50 C to -15 C, or from -15 C to 25 C,
preferably in a range of from 0 C to 25 C;
b. at a pressure of from 0.013 kPa to 0.13 kPa (i.e., from 100 mTorr to
1000 mTorr); and
c. for a period of time of at least 60 minutes, or at least 180 minutes,
or at least 180 minutes, or at least 5 hours, or at least 300 minutes,
or at least 600 minutes, or at least 900 minutes, or at least 1200
minutes, or at least 1800 minutes.
The preserved bone tissue form produced by the above described method
comprises
processed cancellous bone tissue sample and may be stored at temperatures
above freezing (e.g.,
refrigerated or room temperature), for at least 14 days, or at least 28 days,
or at least 56 days, or at
least 70 days, and preferably for at least 90 days, at least 180 days, at
least 365 days, at least 1
52
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
year, 2 years, 3 years, or even longer, and still contain viable endogenous
cells. More particularly,
the preserved bone tissue form comprising processed cancellous bone tissue may
be stored at room
temperatures (i.e., from about 19 C to about 25 C), or refrigeration
temperatures (i.e., from
greater than 0 C to about 10 C), or intermediate temperatures (i.e., from
greater than 10 C to
less than about 19 C). Viable cells in the preserved bone tissue form remain
viable after such
storage and after rehydration in saline, blood, bone marrow aspirate, PRP or
other solution /
compound.
All tissue processing steps are performed aseptically. At least one or more of
the processing
steps are designed to remove immunoreactive elements from the tissue sample,
which is demonstrated
to not elicit an immune response using an MLR assay, by methods known and
understood by persons
of ordinary skill in the relevant art. All selected preservation and
protectant agents are biologically
compatible.
In another exemplary embodiment, an additional component is prepared and added
to the
bone tissue sample prior to the steps of (E) contacting with one or more
protectants and (H)
lyopreserving, to produce a preserved tissue form comprising processed
cancellous bone tissue
and processed cortical bone tissue, as follows. The steps of (A) recovering /
receiving, (B) cleaning
and (D) modifying the size and shape of a cancellous bone tissue sample to
form cancellous bone
tissue granules, as described above, are performed. Separately, and possibly
but not necessarily
concurrently, the following additional steps are performed:
(AA) recovering or receiving recovered a cortical bone tissue sample;
(BB) modifying the size, shape and physical characteristics of the cortical
bone tissue
sample, by the following steps:
1.
cutting the cortical bone tissue sample to form smaller cortical bone
pieces;
53
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
2. milling one or more cortical bone pieces into cortical bone fibers;
3. demineralizing the cortical bone fibers by methods known now or in the
future to persons of ordinary skill in the relevant art; and
(X1) combining, by mixing, stirring, etc., the cancellous bone tissue granules
and
demineralized cortical bone fibers in a desired ratio (for example without
limitation, 70 wt%
cancellous bone tissue granules and 30 wt% demineralized cortical bone fibers
, or 60 wt%
cancellous bone tissue granules and 40 wt% demineralized cortical bone fibers,
or 50 wt%
cancellous bone tissue granules and 50 wt% demineralized cortical bone fibers,
or 40 wt%
cancellous bone tissue granules and 60 wt% demineralized cortical bone fibers
or 30 wt%
cancellous bone tissue granules and 70 wt% demineralized cortical bone fibers
based on the total
weight of the bone granules and bone fibers) to produce a bone tissue mixture.
Generally, cortical bone samples are recovered from a donor's femur, tibia,
humerus, radius,
ulna, and fibula, or other suitable long bones. The long bones are first
stripped of soft tissue and the
shaft cores are cleared of any cancellous bone. The cortical shafts are
cleaned using
detergents/surfactants to remove residual blood and lipids, then cut into
cross-sectional segments of the
appropriate length for milling. Milling of the shaft cross-sections results in
elongated fibers of cortical
bone. Following demineralization in dilute acid, calcium content of the bone
fibers is reduced and the
cortical bone fibers become putty-like in handling.
After the combining step (X1) in which the cancellous bone granules and
cortical bone
__ fibers are mixed in a desired ratio, the remaining method steps to complete
production of a
preserved bone tissue forms are analogous to those described above for the
cancellous-only tissue
form. More particularly, this exemplary embodiment further comprises the
following steps:
54
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
(E) Contacting / adding the bone tissue mixture to a protectant solution
which contains
one or more protectants dissolved in PBS or other biocompatible fluid to form
a bone
tissue-protectant mixture;
(F) Optionally, storing the bone tissue-protectant mixture in a freezer,
such as at a
temperature of about -80 C, until lyopreserving;
(H) Lyopreserving the bone tissue-protectant mixture to produce a
preserved bone tissue
form, by performing exemplary process delineated above in connection with the
cancellous-only tissue form, including;
1. freezing the bone tissue-protectant mixture;
2. drying the frozen cancellous bone tissue-protectant mixture
a. at either a constant temperature, or at a varied temperature; and
b. at a pressure of from 0.013 kPa to 0.13 kPa.
The preserved bone tissue form produced by the foregoing method may be
described as follows:
= Allograft Bone:
o 40 wt% Mineralized Cancellous Bone Granules (average size = 425 p.m to 4 mm)
containing viable endogenous cells, based on the total weight of the bone
granules and bone fibers;
o 60 wt% Demineralized Cortical Bone Fibers, based on the total weight of
the
bone granules and bone fibers (e.g., 50 wt % of fibers having thickness of
about
80 pm, and the other 50 wt % of fibers having thickness of about 150 p.m,
based
on the total weight of the Demineralized Cortical Bone Fibers),
o the wt% based on the total weight of the bone granules and bone fibers.
= Lyoprotectant solution
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
Tissue grafts are stored in a sealed, sterile container at a temperature above
freezing (e.g.,
refrigerated or room temp).
In an exemplary embodiment, the preserved tissue form comprises processed
cartilage tissue
derived from cartilage samples and containing a population of endogenous
viable cells. The type of
cartilage tissue suitable for use in the presently described preserved tissue
forms and methods for
making and using them is not particularly limited. The cartilage tissue may be
any type, including
without limitation, articular cartilage (such as recovered from condyles,
etc.), costal cartilage (such as
recovered from anterior ends of one or more ribs), fibrocartilage (such as
recovered from
intervertebral disks), elastic cartilage (such as recovered from ears), other
types of cartilage, and
combinations thereof The cartilage tissue, for example, may be obtained from
osteochondral grafts
recovered from one or more donors. Osteochondral grafts typically include a
bone portion and a
cartilage portion which may, but does not have to, be a condyle or
hemicondyle. Suitable
osteochondral grafts from which cartilage may be recovered include, without
limitation, femurs (distal
and proximal), talus, and patella. Although smaller, the patella tends to
provide more cartilage than
femurs because the layer of cartilage on the patella tends to be much thicker.
The cartilage samples, processed cartilage tissue, and preserved cartilage
tissue forms
comprising processed cartilage tissue, may have any size and shape suitable
for the intended use and
placement of the resulting preserved tissue form comprising the processed
cartilage tissue, including,
without limitation, particles, strips, chunks, pieces, blocks, sheets,
slivers, ribbons, branched and
unbranched elongated elements, filaments, fibers, three dimensional geometric
shapes such as
symmetric and asymmetric spheres, regular and irregular polyhedrons, cones,
pyramids, other
three dimensional forms having one or more planar or curved surfaces, and
irregular three
dimensional forms.
56
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
In some embodiments, the preserved tissue form may further comprise cartilage
allograft
matrix (CAM) such as non-viable freezer-milled cartilage particles, or other
materials. In some
embodiments, such a preserved tissue form comprising processed cartilage may
further comprise,
as an additional component, a population of cells selected from: autogenic
viable cells isolated
from a recipient's (patient's) own tissue, non-immunogenic allogenic viable
cells (e.g., cells
isolated from suitable allogenic sources such as allogenic cartilage),
isolated viable cells which
have been differentiated for cartilage, bone, etc. (e.g., added to tissue form
prior to lyopreserving
(H)), "dry cells" (which have been lyophilized, either in contact with a
protectant or not, separately
from the processed tissue and preserved tissue form).
Specifically, in an exemplary embodiment, a processed viable cartilage tissue
is produced
by contacting a cartilage tissue sample with one or more protectants and then
lyopreserving the
cartilage tissue sample and one or more protectants to produce a processed
cartilage tissue which
remains viable even after storage at room temperature for extended periods of
time. Stored under
these conditions, viable cells including chondrocytes, chondroblasts,
cartilage progenitor cells, and
mesenchymal stem cells in the preserved tissue form will remain viable for a
period of at least 14
days, or at least 28 days, and preferably greater than 90 days after
lyopreserving. An exemplary
method for producing such a preserved tissue form comprises the steps of:
(A) Recovering or receiving a recovered a cartilage tissue sample may
begin with
recovering or receiving a recovered bone tissue sample which typically
includes a
bone portion and a cartilage portion, where the cartilage portion further
contains one
or more endogenous viable cells including chondrocytes, cartilage-forming
cells,
and mesenchymal stem cells (for example, a femur having at least one intact
condyle
with a cartilage surface which contains viable cells);
57
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
(B1) Cleaning the bone tissue sample by debriding the bone tissue sample to
remove soft
tissue;
(D1) Modifying the size and shape of the bone tissue sample by cutting the
bone tissue
sample into at least two pieces, each comprising a bone portion and a
cartilage portion
(keep pieces moist by contacting with phosphate buffered saline (PBS));
(B2) Cleaning the resulting pieces by rinsing them one or more times (e.g.,
four times)
with buffered saline or similar biocompatible fluid (rinsed pieces may be held
in PBS
for up to 8 hours prior to further processing steps);
(C) Disinfecting the pieces by contacting them with one or more
antibiotics (e.g., place
them in a container with a mixture of penicillin, streptomycin, amphotericin
B);
1. for example, an ambient storage media containing one or
more antibiotics
may be used for concurrently storing and disinfecting the pieces, where such
an ambient storage media may contain:
a. DMEM,
b. Antibiotic-Antimycotic (penicillin / streptomycin / amphotericin B),
c. Non-Essential Amino Acid (NEAA) cell culture supplement,
d. L-glutamine,
e. insulin-transferrin-selenium (ITS)
S )
L-ascorbic acid (Vitamin C), and
g. dexamethasone;
2. In embodiments where cartilage tissue samples are (A)
recovered or
received "en block," or as one or more whole tissue sections, and not in an
ambient storage media or other stabilizing media, the cartilage tissue
58
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
samples may be subjected to a disinfecting (C) step (for example, by rinsing
with PBS, mild surfactant, and peracetic acid and water to remove existing
bioburden) prior to or during one or more cleaning (B) and modifying (D)
steps
(D2) Modifying the size and shape of the cartilage portion of each piece by
grating or
shaving with a grater to produce viable cartilage fibers containing one or
more types
of viable cells (as noted above);
1. Mount the bone
portion of the piece in a vise (avoid damage to cartilage
portion),
2. Using the
grater (e.g., an OXO brand steel grater Model #50581), grate the
cartilage portion of the shaft (e.g., until the cartilage has been removed
from
the bone portion),
3. Rinse the grater intermittently (e.g., after every 4 strokes) with a
biocompatible fluid (e.g., the ambient storage media which contains
antibiotics) to separate the resulting viable cartilage fibers from the grater
and
collect them in the biocompatible fluid,
4. Separate the viable cartilage fibers from the biocompatible fluid, e.g.,
by
using a mesh or similar filtering device to drain off the biocompatible fluid
and retain the viable cartilage fibers,
(B3) Cleaning and wetting the viable cartilage fibers by rinsing them one or
more times
(e.g., twice) with PBS or similar biocompatible fluid (gentle agitation during
the
second rinse with fresh PBS ensures all viable cartilage fibers are wetted
while
minimizing damage),
59
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
1. when the above-
described ambient storage media is used to collect the viable
cartilage fibers, it must be rinsed off the viable cartilage fibers within
three
hours from when the viable cartilage fibers are initially soaked in the
ambient
storage media, therefore, so this rinsing step must generally be performed
within three hours of when the grating is commenced (step (D2)));
(E) Contacting / adding the viable cartilage fibers to a protectant
solution which contains
one or more protectants dissolved in a biocompatible fluid (e.g., PBS or other
biocompatible fluid) to form a cartilage tissue-protectant mixture;
1. an exemplary protectant solution contains about 0.4 M trehalose and 0.4
mM
EGCG, dissolved in 1X PBS,
2. the protectant solution may, optionally, also contain one or more
additional
ingredients such as sugar alcohols, antioxidants, and other additives,
3. contacting the viable cartilage fibers with the protectant solution by
be
accomplished, for example, by combining a 0.75-0.85 gram quantity of
cartilage fibers with about 4-6 milliliters (mL) of protectant solution in a
30
mL jar,
(F) Optionally, storing the cartilage tissue-protectant mixture in a
freezer, such as at a
temperature of about -80 C, until lyopreserving; and
(H)
Lyopreserving the cartilage tissue-protectant mixture to produce a
preserved
cartilage tissue form (comprising viable cartilage fibers), by performing the
following process;
1. if not already
frozen in storage, freezing the cartilage tissue-protectant
mixture at a rate of from 0.1 C/minute to 2 C/minute; and
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
2. drying the frozen cartilage tissue-protectant mixture
a. at either a constant temperature, or at a varied temperature in a
range
of either from -50 C to -10 C, or from -10 C to 25 C, preferably
in a range of from 0 C to 25 C;
b. at a pressure of
from 0.013 kPa to 0.13 kPa (i.e., from 100 mTorr to
1000 mTorr); and
c. for a period of time of, for example, at least 60 minutes, or at
least
180 minutes, or at least 240 minutes, or at least 5 hours, or at least
400 minutes, or at least 600 minutes, or at least 900 minutes, or at
least 1200 minutes, or at least 1500 minutes, or at least 1800
minutes, or any period of time up to about 35 hours.
The preserved tissue form comprising a processed cartilage tissue sample
(i.e., the
preserved cartilage tissue form) which is produced by the above described
method may be stored
at temperatures above freezing (e.g., refrigerated or room temperature), for
at least 14 days, or at
least 28 days, or at least 56 days, or at least 70 days, and preferably for at
least 90 days, at least
180 days, at least 365 days, at least 1 year, 2 years, 3 years, or even
longer, and still contain viable
endogenous cells. More particularly, the preserved cartilage tissue form
comprising processed
cartilage tissue may be stored at room temperatures (i.e., from about 19 C to
about 25 C), or
refrigeration temperatures (i.e., from greater than 0 C to about 10 C), or
intermediate
temperatures (i.e., from greater than 10 C to less than about 19 C). Viable
endogenous cells in
the preserved cartilage tissue form remain viable after such storage and after
rehydration in saline,
blood, bone marrow aspirate, PRP or other solution / compound.
61
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
The cartilage processing method described above produces viable cartilage
fibers which
contain viable mesenchymal stem cells, viable chondrocytes, growth factors and
matrix proteins,
all of which are endogenous and were initially present in the original
cartilage tissue sample (i.e.,
bone and cartilage). Additionally, the viable cartilage fibers are cohesive
with one another and,
.. after shaping and implantation into a cartilage void or defect (with or
without additional
components), tend to remain in the cartilage void or defect, even when the
cartilage void or defect
and surrounding region are rinsed or irrigated with biocompatible fluid, or
gently wiped with a
finger, sponge or other suitable device.
The preserved tissue form comprising processed cartilage tissue (i.e., a
preserved cartilage
tissue form) may further comprise one or more additional components as
described above (e.g.,
CAM, fibrin glue, etc.). The preserved tissue form comprising processed
cartilage tissue may be
packaged, such as in a foil pouch, with or without a moisture barrier. In some
embodiments, the
components of the preserved tissue form, including a processed cartilage
tissue sample, may be
contacted with one or more preservatives (in solution or not) in packaging and
then (H)
.. lyopreserved while packaged.
All tissue processing steps are performed aseptically. At least one or more of
the processing
steps are designed to remove immunoreactive elements from the tissue sample,
which is demonstrated
to not elicit an immune response using an MLR assay, by methods known and
understood by persons
of ordinary skill in the relevant art. All selected preservation and
protectant agents are biologically
compatible.
A preserved cartilage tissue form comprising a processed cartilage tissue as
described herein
may be implanted into a recipient (patient, human or non-human) with damaged
or otherwise
inadequate cartilage as a result, for example without limitation, of disease
or naturally or surgically
62
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
created cartilage voids. In some embodiments, the preserved cartilage tissue
form further comprises
CAM and, when rehydrated with a biocompatible fluid, forms a putty and is
moldable, manually
or otherwise (e.g., using a mold or other container), to facilitate filling
any cartilage void in need
of repair. For example, in some embodiments, processed (viable) cartilage
fibers and one or more
protectants may be placed in a mold (i.e., a container having a cavity of
desired shape and size),
and lyophilized together and then packaged in a kit to be used in a syringe,
cannula, or other
delivery device. In some embodiments, one or more additional components such
as, without
limitation, CAM, may be combined with the processed cartilage fibers in the
mold and lyophilized
together to form a preserved cartilage tissue form having a predetermined size
and shape (i.e., the
aforesaid desired size and shape of the cavity). In some embodiments, the
processed cartilage fibers
are lyophilized (in a mold or not), then packaged in a kit, with one or more
additional components such
as, without limitation, CAM, also packaged in the kit, but separate from the
processed cartilage fibers,
with the intent that the processed cartilage fibers and additional components
are combined with one
another and, optionally, a biocompatible fluid, at the time of use and
implantation into (or onto) a
subject (patient). Such preserved cartilage tissue forms (i.e., the processed
cartilage fibers) contain
viable chondrocytes, chondrogenic growth factors, and extracellular matrix
components which
promote repair and healing of the cartilage void. More particularly, the
preserved cartilage tissue form
is expected to synthesize, and/or promote deposition of, hyaline cartilage and
proteoglycans.
In an exemplary embodiment, the preserved tissue form comprises processed
placental tissue
derived from placental samples and containing a population of endogenous
viable cells. In some
embodiments, such a preserved tissue form comprising processed placental
tissue, may further
comprise, as an additional component, a population of cells selected from:
autogenic viable cells
isolated from a recipient's (patient's) own tissue, non-immunogenic allogenic
viable cells (e.g.,
63
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
cells isolated from suitable allogenic sources such as allogenic cartilage),
isolated viable cells
which have been differentiated, "dry cells" (which have been lyophilized,
either in contact with a
protectant or not, separately from the processed tissue and preserved tissue
form).
Specifically, in an exemplary embodiment, a processed viable placental tissue
comprising
processed amnion tissue and processed chorion tissue, is produced by
contacting an amnion tissue
sample and a chorion tissue sample with one or more protectants and then
lyopreserving the tissue
samples and the one or more protectants to produce the processed placental
tissue which remains
viable even after storage at room temperature for extended periods of time.
Stored under these
conditions, viable cells, including one or more types of viable cells
including epithelial cells and
stromal cells (such as fibroblasts and mesenchymal stem cells) in the
preserved tissue form, will
remain viable for a period of at least 14 days, or at least 28 days, and
preferably greater than 90
days after lyopreserving. An exemplary method for producing such a preserved
tissue form comprises
the steps of:
(A) Recovering or receiving a recovered placenta sample which
includes at least an
amnion membrane and a chorion membrane;
a. Optionally, umbilical cord may also be included.
(B 1) Separating the amnion and chorion membranes from one another and other
portions
of the placenta sample, by:
1. manually peeling the amnion and chorion membranes apart,
2. cutting the amnion membrane free from the umbilical cord, if present,
3. cutting the chorion membrane free from the placental disk, if present;
4. Optionally, umbilical cord also may be cut free from the placental disk
and
then cleaned and processed in a similar manner as the subsequent steps.
64
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
(B2) Cleaning and removing blood and blood clots from each of the amnion and
chorion
membranes by:
1. manually
dabbing each of the amnion and chorion membranes with wetted
wipes,
2. contacting the
chorion membrane (one or more times), preferably with
agitation, with an RBC lysis solution containing ingredients which lyse red
blood cells and break up blood clots (e.g., a buffer solution comprising
streptokinase [commercially available as MP BIOMEDICALS brand from
Thermo Fisher Scientific of Waltham, Massachusetts, U.S.A.], and a red
blood cell lysis buffer [commercially available from Sigma Aldrich of St.
Louis, Missouri, U.S.A.]),
3. separating the chorion membrane from the RBC lysis solution, rinsing the
chorion membrane (one or more times) with a biocompatible fluid (e.g.,
HBSS) and dabbing with wetted wipes and/or sterile cotton applicators to
remove additional blood and blood clots,
4. soaking the amnion membrane (one or more times) in a biocompatible
buffer
solution (e.g., HBSS) and dabbing with wetted wipes (e.g., soak and wipe
twice for about 10 to about 20 minutes each time);
(C)
Disinfecting the amnion and chorion membranes (together or separately) by
contacting them with one or more antibiotics, preferably with agitation (e.g.,
place
them in a container with a mixture of vancomycin, gentamicin, amphotericin B
and
agitate for about 30 to about 60 minutes);
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
(B3) Cleaning the amnion and chorion membranes to remove the one or more
antibiotics
by soaking and rinsing, preferably with agitation (one or more times) with a
biocompatible fluid (e.g., perform a first rinse in a flask with HBSS and
shaking at
65 rpm for 5-10 min, repeat for a second rinse (preferably with fresh HBSS),
and
perform a third rinse in a flask with HBSS (preferably fresh) and shaking at
65 rpm
for 30-40 min),
(B4) maintaining hydration of the amnion and amnion and chorion membranes by
placing
and holding them (together or separate) in a biocompatible fluid (e.g., HBSS);
1. optionally, further dabbing and wiping with wetted wipes and/or sterile
cotton
applicators may be performed to remove any residual blood clots or remnants;
2. optionally, separating and removing the trophoblast layer from the
chorion
membrane by gently peeling, scraping, or sloughing portions of the
trophoblast layer using hands/fingers, a scraper, spatula or similar devices,
wetted wipes, or a combination of thereof;
(D1) Modifying the size and shape of the amnion and chorion membranes by
laying each
membrane flat and cutting with a blade or similar device,
1. for example, arranging the amnion and chorion membranes together, by
placing the amnion membrane on a backing material, optionally with the
epithelial side facing down and in contact with the backing material, and
placing the chorion membrane on top of the amnion membrane, optionally
with the trophoblast side (with or without the trophoblast layer present)
facing
up and not in contact with the amnion membrane, then cutting the layered
amnion and chorion membranes into smaller amnion-chorion pieces of
66
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
desired size and shape (still with backing material), and placing the cut
pieces
back into the biocompatible fluid (e.g., HBSS) to maintain hydration of the
cut pieces during the remaining cutting;
2. optionally, the cut amnion-chorion pieces may be soaked
or stored in a
biocompatible fluid (e.g., HBSS) to maintain hydration of the cut pieces
during the remaining cutting, and removed from the biocompatible fluid prior
to contacting with one or more protectants;
(E) Contacting / adding the cut amnion-chorion pieces to a
protectant solution which
contains one or more protectants dissolved in a biocompatible fluid (e.g.,
HBSS or
other culture media) to form an amnion-chorion tissue-protectant mixture;
1. an exemplary protectant solution contains about 0.5 M trehalose and 4.0
mM
EGCG, dissolved in HBSS,
2. the protectant solution may, optionally, also contain one or more
additional
ingredients such as sugar alcohols, antioxidants, and other additives,
3. the contacting time may be from about 15 to about 60 minutes,
4. the contacting temperature may be from greater than 0 C
to about 10 C,
(H) Lyopreserving the amnion-chorion tissue-protectant mixture to
produce a preserved
amnion-chorion tissue form (comprising both processed amnion and processed
chorion), by performing the following process;
1. if not already frozen in storage, freezing the amnion-chorion tissue-
protectant mixture at a rate of from 0.1 C/minute to 2 C/minute, optionally
with a period of constant temperature following the controlled-rate freezing,
67
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
for a total freezing time of from about 80 min to about 160 min (e.g., from
120 min to 150 min, or from 60 min to 90 min, or another time period); and
2. drying the frozen amnion-chorion tissue-protectant
mixture
a. at either a constant temperature, or at a varied temperature in a range
of either from -50 C to less than -10 C, or from -10 C to 25 C,
preferably in a range of from -10 C to 25 C;
b. at a pressure of from 0.013 kPa to 0.13 kPa (i.e., from 100 mTorr to
1000 mTorr); and
c. for a period of time of at least 60 minutes, or at least 180 minutes,
or at least 200 minutes, or at least 300 minutes, or at least 400
minutes, or at least 600 minutes, or at least 900 minutes.
As will be readily recognized by persons of ordinary skill in the relevant
art, several
modifications and additions may be made to customize and optimize the
foregoing method for
producing a preserved amnion-chorion tissue form as described herein. For
example, in some
embodiments, a color indicator, such as phenol red, may be added to the
biocompatible fluid to
impart a visible color to the amnion and chorion membranes to facilitate
visualization during cutting.
In some embodiments, to facilitate handling and protect the amnion-chorion
pieces from damage,
the cut amnion-chorion pieces may be placed into a retainer, netting, mesh, or
other type of
permeable container after the size modifying step (D) and prior to performing
one or more additional
processing steps (e.g., contacting with protectants, rinsing, etc.).
Furthermore, in some embodiments,
the step of (E) contacting the cut amnion-chorion tissue pieces with the
protectant solution may
include draining the protectant solution after the desired period of
contacting time, briefly (about 5
minutes) soaking or rinsing the cut amnion-chorion tissue pieces with a
biocompatible fluid (e.g.,
68
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
HB SS); draining excess fluid from the cut amnion-chorion pieces and placing
them into one or more
breathable pouches (such as without limitation, Tyvek pouches) and sealing the
pouch(es), and
perform the (H) lyopreserving step while the amnion-chorion pieces are sealed
in the pouches. After
(H) lyopreserving, the pouches containing lyopreserved amnion-chorion tissue
may be placed and
sealed in an external packaging material, such as moisture-barrier pouches.
The preserved tissue form comprising a processed amnion-chorion tissue (i.e.,
the
preserved amnion-chorion tissue form) produced by the above described method
may be stored at
temperatures above freezing (e.g., refrigerated or room temperature), for at
least 14 days, or at
least 28 days, or at least 56 days, or at least 70 days, and preferably for at
least 90 days, at least
180 days, at least 365 days, at least 1 year, 2 years, 3 years, or even
longer, and still contain viable
endogenous cells. More particularly, the preserved cartilage tissue form
comprising processed
cartilage tissue may be stored at room temperatures (i.e., from about 19 C to
about 25 C), or
refrigeration temperatures (i.e., from greater than 0 C to about 10 C), or
intermediate
temperatures (i.e., from greater than 10 C to less than about 19 C). Viable
endogenous cells in
the preserved cartilage tissue form remain viable after such storage and after
rehydration in saline,
blood, bone marrow aspirate, PRP or other solution / compound.
The placenta (e.g., amnion and chorion) processing method described above
produces
pieces of layered amnion and chorion membranes which contain viable epithelial
and stromal cells
(such as fibroblasts and mesenchymal stem stems), growth factors and matrix
proteins, all of which
are endogenous and were initially present in the original placenta tissue
sample (i.e., amnion and
chorion).
The preserved amnion-chorion tissue form may further comprise one or more
additional
components as described above (e.g., exogenous cells, other processed tissues
or grafts, etc.). The
69
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
preserved tissue form comprising processed amnion-chorion tissue may be
packaged, such as in a
pouch, preferably within a moisture barrier package. In some embodiments, the
components of the
preserved tissue form, including a processed cartilage tissue sample, may be
contacted with one or
more preservatives (in solution or not) in packaging and then (H) lyopreserved
while packaged.
All tissue processing steps are performed aseptically. At least one or more of
the processing
steps are designed to remove immunoreactive elements from the tissue sample,
which is demonstrated
to not elicit an immune response using an MLR assay, by methods known and
understood by persons
of ordinary skill in the relevant art. All selected preservation and
protectant agents are biologically
compatible.
In another exemplary embodiment, in which the preserved tissue form comprises
processed
placental tissue derived from placental samples and containing a population of
endogenous viable
cells, the placental tissue may comprise processed umbilical cord tissue
sample. Specifically, a
processed viable placental tissue comprising processed umbilical cord tissue
may be produced by
a method similar to that described above in connection with the processing of
amnion and chorion
tissues, except that at least some of the steps of (B) cleaning and (D)
modifying the size, shape and
other physical characteristics will be different due to the physical and other
differences between
amnion, chorion and umbilical cord.
More particularly, as will be understood by persons of ordinary skill in the
relevant art,
after (A) recovering or receiving a recovered placenta tissue sample, one or
more of several
cleaning steps (B) may be performed including, for example without limitation,
(B1) cutting and
separated the umbilical cord tissue sample from the remaining placental
tissues, (B2) separating
and removing blood vessels from the umbilical cord tissue sample, and (B3)
rinsing the umbilical
cord tissue sample with one or more biocompatible fluids to remove unwanted
materials (e.g.,
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
blood, debris, etc.). The umbilical cord tissue sample may also be subjected
to the step of (D)
modifying one or more of the size and shape of the umbilical cord tissue
sample by cutting the
umbilical cord longitudinally and spreading or flattening the umbilical cord
into a planar or sheet-
like configuration.
Such size and shape modification steps (D) may be followed by one or more
additional
cleaning steps (B) such as rinsing or soaking with one or more biocompatible
fluid. It should be
noted that the foregoing steps may be performed in a different order than
described above,
according to the judgment of persons or ordinary skill in the relevant art.
Thereafter, like the
method for processing amnion and chorion tissues, the umbilical cord tissue
sample is (E)
contacted with one or more protectants and then the resulting umbilical cord
tissue-protectant
mixture will be (H) lyopreserved to produce a processed umbilical cord tissue
which remains
viable even after storage at room temperature for extended periods of time.
Stored under these
conditions, viable cells, including one or types of viable cells including
epithelial cells and stromal
cells (such as fibroblasts and mesenchymal stem cells) in the preserved tissue
form, will remain
viable for a period of at least 14 days, or at least 28 days, and preferably
greater than 90 days after
lyopreserving.
Such a processed umbilical cord tissue form may further comprise other
processed tissues such
as, without limitation, processed amnion, processed chorion, or both, as well
as one or more additional
components, etc. For example, in some embodiments, such a preserved tissue
form comprising
processed umbilical cord tissue, may further comprise, as an additional
component, a population
of cells selected from: autogenic viable cells isolated from a recipient's
(patient's) own tissue, non-
immunogenic allogenic viable cells (e.g., cells isolated from suitable
allogenic sources such as
allogenic cartilage), isolated viable cells which have been differentiated,
"dry cells" (which have
71
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
been lyophilized, either in contact with a protectant or not, separately from
the processed tissue
and preserved tissue form).
It will be understood that the embodiments of the present invention described
hereinabove
are merely exemplary and that a person skilled in the art may make variations
and modifications
without departing from the spirit and scope of the invention. All such
variations and modifications
are intended to be included within the scope of the present invention.
EXAMPLES
The following descriptions, flowcharts and tables provide exemplary
embodiments of
methods for making the viable tissue forms from particular recovered tissue
types, including
human bone, human amnion, and human adipose.
72
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
Characterization Test Methods
The following descriptions provide explanations for each of the test methods
employed to
produce the characterization data and information provided below.
Analysis and assessment of cell presence and cell viability are measured using
the
following techniques. A tissue sample is set aside at any point during a
processing method at which
the presence and/or viability of the cells is desired to be measured, so that
cell viability/ biological
activity of the tissue can be evaluated at that point using commercially
available methods. While
such suitable methods include, but are not limited to, metabolic assays, such
as those involving
luciferase, tetrazolium salts (e.g., 3 -(4, 5-dimethy1-2-thiazoly1)-2, 5-
dipheny1-2H-tetrazolium
bromide (MT T), 3 -(4,5 -dim ethylthi azol-2-y1)-5 -(3 -carboxymethoxypheny1)-
2-(4-sulfopheny1)-
2H-tetrazolium (MTS), 2,3 -bi s-(2-m ethoxy-4-nitro-5 - sul
fopheny1)-2H-tetrazol ium-5 -
carb oxanili de (XTT), and other water soluble tetrazolium salts (e.g., WST-1,
-3, -4, -5, -8, -9, -10,
and -11)), live-dead assays, ATP assay, CCK-8 assay and dye exclusion assays
such as Trypan
Blue, for the presently described and contemplated preserved tissue forms and
methods for
producing them, metabolic activity is determined by performing an ATP assay,
as follows:
A viability assay based on the metabolic activity of a sample is performed by
measuring
the ATP content in the sample. In an embodiment, the assay is performed by
thawing frozen tissue
samples (if needed) and then transferring the tissue samples into a rinse
basin. The thawed and/or
rinsed tissue samples are combined with an assay reagent and incubated on an
orbital shaker. After
incubation, aliquots of the incubated reagent are transferred into a 96-well
plate and raw
luminescence values (in RLU), for example, are obtained using a plate reader.
A standard curve is
prepared by creating serial dilutions of known concentrations of an ATP
standard, combining with
assay reagent, incubating on an orbital shaker, and then reading the RLU of
each dilution. The
73
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
dilutions' RLUs are plotted against the known concentrations of each serial
dilution to generate an
equation, which is then used to convert RLU of the tissue samples to
concentrations of ATP. Finally,
depending on the type of tissue, the tested tissue samples may be rinsed in
water or a biologically
compatible fluid (such as, without limitation, 5% Dextrose in Lactated
Ringer's Solution (D5LR),
phosphate buffered saline (PBS), saline, HBSS, etc.), before being placed into
pre-weighed
weighing pans. The tissue sample may then be dried (e.g., if wet) and weighed,
and the
concentration of ATP per tissue sample is converted to amount of ATP per
weight of tissue sample.
Alternatively, the tissue sample may be undried, i.e., weighed in a wet
condition, or it may be
weighed dry prior to incubation with assay reagent. As will be understood by
persons of ordinary
skill in the relevant art, whether a tissue sample is wet or dry prior to
incubation with assay reagent,
and whether a tissue sample is rinsed (and with what type of fluid) prior to
drying and weighing,
will depend on the type of tissue. For example, bone tissue may be wet prior
to incubation with
assay reagent and not rinsed or weighed after RLU values are obtained, so that
the pre-testing wet
weight is used to calculate the amount of ATP per weight of tissue sample.
Alternatively, placental
(e.g., amnion, chorion, etc.) tissue may be rinsed after obtaining RLU values,
then dried and
weighed, as explained above. The tissue sample being tested for viable cell
population content may
be wet or dry and the foregoing ranges for the results of such testing are
applicable to both wet and
dry tissue samples.
As previously explained, a preserved tissue sample has a pre-lyopreservation
population of
.. endogenous viable cells and a post-lyopreservation (i.e., Week 0)
population of endogenous viable
cells. The post-lyopreservation population of endogenous viable cells is a
portion (e.g., a
percentage) of the pre-lyopreservation population of endogenous viable cells.
Additionally and
74
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
independently, the post-lyopreservation population of endogenous viable cells
is also a portion
(e.g., a percentage) of the pre-contact population of endogenous viable cells.
The preserved tissue samples (and preserved tissue forms comprising them)
contain
populations of endogenous viable cells which are substantially comparable in
quantity and
proportion to populations of endogenous viable cells contained in
cryopreserved tissue forms (and
cryopreserved tissue forms comprising them), where the preserved and
cryopreserved tissue
samples are of the same type of tissue and the populations of endogenous
viable cells of each of
the preserved and cryopreserved bone tissue samples are measured at the same
point of processing
or after the same period of time of storage after preservation and
cryopreservation. For example,
without limitation, a preserved bone tissue sample comprises a population of
endogenous viable
cells which is substantially comparable, i.e., within 15%, or within any
percent between 15%
and 1%, to the population of endogenous viable cells contained in a
cryopreserved bone tissue
sample, wherein both of the populations of endogenous viable cells are any one
of: post-contact
(i.e., measured after contacting with one or more protectants and after
contacting with one or more
cryopreservation agents, or "pre-lyopreservation" and "pre-cryopreservation,"
respectively)
populations of endogenous viable cells, post-lyopreservation and post-
cryopreservation
populations of endogenous viable cells, respectively (i.e., TO or Week 0
populations), or retained
populations of endogenous viable cells (i.e., measured after a period of
storage time). Similarly,
for example, without limitation, a preserved amnion tissue sample comprises a
population of
endogenous viable cells which is substantially comparable, i.e., within 85%,
or within any
percent between 85% and 1%, to the population of endogenous viable cells
contained in a
cryopreserved amnion tissue sample, wherein both of the populations of
endogenous viable cells
are measured at the same point of processing or after the same period of
storage time, as explained
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
above. Similarly, for example, without limitation, a preserved cartilage
tissue sample comprises a
population of endogenous viable cells which is substantially comparable, i.e.,
within 50%, or
within any percent between 50% and 1%, to the population of endogenous
viable cells contained
in a cryopreserved cartilage tissue sample, wherein both of the populations of
endogenous viable
cells are measured at the same point of processing or after the same period of
storage time, as
explained above.
It will be understood that the embodiments of the present invention described
hereinabove
and in the following examples are merely exemplary and that a person skilled
in the art may make
variations and modifications without departing from the spirit and scope of
the invention. All such
variations and modifications are intended to be included within the scope of
the present invention.
EXAMPLES
In the following experiments, all tissue samples were human tissues obtained
from eligible
donors after obtaining written informed consent, and applicable tissue
regulations for receipt and
disposition of tissues were strictly followed.
Furthermore, each of the following experiments which involved preservation
methods in
accordance with those described and contemplated above were performed using a
"Lyophilizing
Apparatus" (or "Lyophilizer") which were commercially available, at the time
of the experiments,
under the brand name VirTis from SP Scientific of Warminster, Pennsylvania,
U.S.A. and were
capable of the following operating parameters (for example, several of the
AdVantage models and
a Genesis Pilot model):
- Shelf Temperature range of from -40 C to +25 C;
76
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
- Capable of reaching Condenser Temperature of -60 C or less;; and
- Condenser Capacity of 3.5 liters or greater, and 2.5 liters or greater
capacity over 24
hours of condensation operation.
Example 1
Preserved Tissue Form Containing Both Viable Cancellous Bone Granules and
Demineralized Cortical Bone Fibers with Demonstrated Viability After
Lyopreservation and
Rehydration
Two experiments were performed and are reported below.
Experiment 1
In the first experiment, cancellous tissue samples were recovered from a
single donor, and
processed to provide cancellous granules. Multiple samples of the processed
cancellous granules
were preserved after contact with protectants in solution, in accordance with
the methods described
hereinabove, and analyzed for cell viability by measuring ATP. The ATP results
reported below
are based on the mathematical average of the measured ATP results for all of
the aforesaid samples
derived from the single donor of Experiment 1.
Experiment 2
In the second experiment, cancellous tissue samples comprising cancellous bone
were
recovered from a single donor (different from the donor of Experiment 1) and
processed to provide
cancellous granules, and cortical bone tissue samples were recovered from the
same single donor
of Experiment 2 and processed to provide cortical fibers. Multiple samples,
each comprising both
cancellous granules and cortical fibers, were preserved after contact with
protectants in solution,
in accordance with the methods described hereinabove, and analyzed for cell
viability by
77
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
measuring ATP. The ATP results reported below are based on the mathematical
average of the
measured ATP results for all of the aforesaid samples comprising both
cancellous granules and
cortical fibers and derived from the single donor of Experiment 2.
A. Protectant solution preparation:
1. Protectant solutions were prepared for each of Experiments 1 and 2 by
dissolving trehalose
and EGCG into PBS at concentrations of 0.4M and 4mM, respectively.
2. Following dissolution of the preservative ingredients (i.e., protectants
trehalose and
EGCG) in media, the solution was sterile filtered using a 0.21.tm membrane
filter.
B. Sample preparation (performed in sterile hood)
1. Viable cancellous tissue (granules) and demineralized cortical tissue
(fibers) were obtained
by sterile processing techniques as described hereinabove in connection with
processing
viable cancellous bone tissue (i.e., for cancellous granules: (A)
recovered/received
cancellous bone sample, (B) cleaned by debriding and rinsing, (D) modified by
(1) cutting
and then (3) milling to produce granules of average particle size from 425 p.m
to 4 mm),
followed by (C) disinfected to remove bioburden by rinsing with peracetic
acid, mild
surfactant, and buffered saline; and for cortical fibers: (AA)
recovered/received cortical
bone sample, (BB) modified by (1) cutting, (2) milling to produce fibers of
average width
from 80 um to 150 um and average length from 8 mm to 12 mm), and then (3)
demineralizing the fibers to a mineral content of less than 8 wt%).
2. Cancellous granules either alone or with demineralized cortical fibers were
packaged and
temporarily stored at refrigerated temperature (2-4 C) for a period of time
greater than 0
and up to 90 minutes, until time of use in experiment.
78
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
3. In a sterile hood, multiple samples were weighed out into 1 oz. jars, as
follows:
a. Experiment 1: each sample included 0.5g cancellous granules, and
b. Experiment 2: each sample included 0.5g cancellous granules and 0.8g
cortical
fibers.
4. 4mL of sterile protectant solution was added to each of the jars containing
tissue samples.
5. Tissue was allowed to incubate with protectant solution at room temperature
for 1 hour.
6. After the incubation period, jars containing tissue were placed on an
aluminum or stainless
steel tray and placed in the Lyopreserving Apparatus.
7. All tissue samples in Experiment 1 were lyopreserved according to the
recipe provided in
Table 1 below. All tissue samples in Experiment 2 were lyopreserved according
to the
recipe provided in Table 2 below.
Table 1 ¨ Experiment 1 Lyophilization Recipe
Thermal Treatment Temperature ( C) Time (min) Vacuum
(mTorr) Ramp/Hold
Step 1: Freeze -30 120 Ramp
Freeze, Condenser, and Vacuum Parameters Temperature ( C) Time (min) Vacuum
(mTorr) Ramp/Hold
Freeze Temperature ( C) -30 Hold
Additional Freeze (min) 180
Condenser Set Point ( C) -60
Vacuum Set Point (mTorr) 500
Drying Phase Parameters Temperature ( C) Time (min) Vacuum
(mTorr) Ramp/Hold
Step 1 0 40 200 Ramp
Step 2 0 360 200 Hold
Step 3 15 15 200 Ramp
Step 4 15 525 200 Hold
Step 5 25 15 200 Ramp
Step 6 25 360 200 Hold
79
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
Table 2 ¨ Experiment 2 Lyophilization Recipe
Thermal Treatment Temperature ( C) Time (min) Vacuum
(mTorr) Ramp/Hold
Step 1: Freeze -30 120 Ramp
Freeze, Condenser, and Vacuum Parameters Temperature ( C) Time (min) Vacuum
(mTorr) Ramp/Hold
Freeze Temperature ( C) -30 Hold
Additional Freeze (min) 180
Condenser Set Point ( C) -60
Vacuum Set Point (mTorr) 500
Drying Phase Parameters Temperature ( C) Time (min) Vacuum
(mTorr) Ramp/Hold
Step 1 -15 40 200 Ramp
Step 2 -15 900 200 Hold
Step 3 1 60 200 Ramp
Step 4 1 500 200 Hold
Key: "Ramp" indicates variable temperature over the time period indicated
(Time (min)).
"Hold" indicates temperature held constant over the time period indicated
(Time (min))
C. Sample testing
1. Prior to contact with the protectant solution (i.e., prior to step B.4.
above), a first set of
samples of about 0.5g of the cancellous tissue granules from Experiment 1, and
a second
set of samples of 0.5g the cancellous tissue and 0.8g of the cortical tissue
fibers (total of
1.3g combined bone tissue) from Experiment 2 were measured and separated, and
each of
the first and second pre-contact samples was tested per the ATP viability
assay procedures
described above, which provided "Baseline" (i.e., "pre-contact") ATP values.
2. Prior to lyopreservation but post-contact and incubation with the
protectant solution (i.e.,
prior to step B6), a first set of samples of about 0.5g of the cancellous
tissue granules from
Experiment 1, and a second set of samples of 0.5g the cancellous tissue and
0.8g of the
cortical tissue fibers (total of 1.3g combined bone tissue) from Experiment 2
were
measured and separated, and each of the first and second post-contact samples
was tested
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
per the ATP viability assay procedures described above, which provided "Pre-
Lyo" (i.e.,
"post-contact and incubation") ATP values.
3. In both of Experiments 1 and 2, following lyopreserving, each of the
samples was
rehydrated by adding 5mL of PBS 1X to each jar and letting sit at room
temperature for 5-
10 minutes (time varied based on experiment).
4. Following the 5-10 minute rehydration period, excess PBS solution was
decanted from all
jars.
5. 0.5g of rehydrated cancellous bone tissue (Experiment 1) and 1.3g of
rehydrated combined
cancellous bone tissue and cortical bone fibers (Experiment 2) were each
weighed out and
tested per the ATP viability assay procedures described above, which provided
Week 0
ATP values. The ATP data resulting from the foregoing experimental procedure
was
converted to % of Baseline ATP and % of Pre-Lyo ATP, as reported in Table 3
below,
using the following formulas:
% of Baseline ATP = (Average Week 0 ATP value / Average Baseline ATP value) x
100
% of Pre-Lyo ATP = (Average Week 0 ATP value / Average Pre-Lyo ATP value) x
100
Table 3
Experiment # Description % of % of
Baseline Pre-Lyo
ATP ATP
1 Cancellous granules 79% 85%
2 Cancellous granules with 56% 93%
demineralized cortical
fibers
Example 2
Preparation of Lyopreseryed Cancellous Bone Tissue Form (Granules) and Cell
Viability
After Storage at Room Temperature (up to 12 Weeks)
81
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
A. Materials and Equipment:
- Forceps
- Weigh pans
- Glass lyo vials and caps
- Stainless steel lyo tray
- Paper grid for lyo tray
- Chexall pouch, 12x18"
- Foil pouch
- PBS, 1X, without Ca and Mg
- Trehalose ¨ Cat # TO167, Sigma Aldrich
- Epigallocatechin gallate (EGCG) ¨ Cat # E4143, Sigma Aldrich
- CellTiter-Glo Luminescent Cell Viability Assay kit
- Lyophilizer (apparatus as described in Example 1 above)
B. Procedure:
Sample Preparation
1. Upon receiving processed milled cancellous bone tissue, weighed out a total
of 10 samples
of 0.5g milled cancellous tissue granules each, into each of 10 glass lyo
vials. (NOTE:
cancellous tissue granules produced by the same process as described in
Example 1 above,
Part B.1.)
2. Weighed an additional 2 samples of 0.5g each of untreated milled cancellous
tissue for pre-
contact (i.e., "Baseline") cell viability testing.
3. Prepared lyopreservation solution as follows:
a. Lyopreservation solution recipe:
82
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
i. To the desired volume of HYCLONE media, added EGCG at a
concentration of 0.945mg/mL and added trehalose at a concentration of
37.83mg/mL to form lyopreservation solution.
ii. Sterile filtered lyopreservation solution with a 0.2um filter after
preparation.
iii. Example: For 78mL of lyopreservation solution, added 0.0737g of EGCG
and 2.951g of trehalose to 78mL of HYCLONE media.
4. Added 2mL of lyopreservation solution to each of 10 vials containing the 10
weighed
samples of milled cancellous bone tissue to form tissue-protectant mixtures.
5. Placed the 10 lyo vials (containing tissue-protectant mixtures) on
stainless steel lyo tray
and placed caps loosely on top of vials without sealing.
6. Carefully sealed lyo tray containing samples in a large autoclave pouch
(paper side facing
up). Ensured that vials and caps do not get knocked over and that the vials
are not sealed.
7. Allowed cancellous tissue-protectant mixtures to incubate in
lyopreservation solution at
ambient temperature without agitation for a 3 ¨ 3.5 hours.
8. After incubation was complete, lyopreserved all 10 processed tissue-
protectant mixtures
using 24 hour recipe provided in Table 4 below. Upon completion of the
lyopreservation
cycle, samples stoppered under vacuum prior to releasing lyo vacuum.
83
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
Table 4
Thermal Treatment Temperature ( C) Time (min) Vacuum
(mTorr) Ramp/Hold
Step 1: Freeze -40 35 Ramp
Freeze, Condenser, and Vacuum Parameters Temperature ( C) Time (min) Vacuum
(mTorr) Ramp/Hold
Freeze Temperature ( C) -40 Hold
Additional Freeze (min) 180
Condenser Set Point ( C) -60
Vacuum Set Point (mTorr) 500
Drying Phase Parameters Temperature ( C) Time (min) Vacuum
(mTorr) Ramp/Hold
Step 1 0 40 200 Ramp
Step 2 0 360 200 Hold
Step 3 15 15 200 Ramp
Step 4 15 525 200 Hold
Step 5 25 15 200 Ramp
Step 6 25 360 200 Hold
9. Performed cell viability testing of 2 tissue samples set aside in step B.2
above, following
procedures described above for the CellTiterGlo ATP Assay (test kit
commercially
available from Promega, located in Madison, WI, USA), which provided two
"Baseline"
(pre-contact) ATP values which were averaged to provide a single "Average
Baseline ATP
Value" for use in calculating % of Baseline ATP and % retained populations of
viable cells
after 4, 8, 10 and 12 weeks of storage, as explained and reported below.
10. Sealed lyophilized samples in a Mangar foil pouch and stored at ambient
conditions.
C. Cell Viability Testing at 0, 4, 8, 10 and 12 weeks
1. Immediately (i.e., about 1 hour, which is TO or "Week 0") after
lyophilization and also at
each of Weeks 4, 8, 10, and 12 of post-lyopreservation storage, removed 2
preserved tissue
form samples for cell viability testing.
84
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
2. Prior to testing, rehydrated each of the preserved tissue form samples by
adding 4mL of
PBS 1X to each vial and allowing to sit for 10 min at ambient conditions.
3. Cell viability testing was performed on each pair of samples pulled at
each of Weeks 0, 4,
8, 10, and 12, following procedures described above for the CellTiterGlo ATP
Assay (same
as specified above). This viability testing provided two ATP values for each
of Weeks 0,
4, 8, 10 and 12. The two ATP values obtained for Week 0 were averaged to
provide an
"Average Week 0 ATP Value," and the same averaging calculation was performed
for each
of the Week 4, 8, 10 and 12 pairs of ATP values, to obtain Average Week X ATP
Values.
Results of cell viability testing are provided in Tables 5A and 5B below.
In particular, Table 5A below provides % of Baseline (pre-contact) ATP for the
two Week
0 samples of preserved cancellous bone tissue samples, which were calculated
as follows:
% of Baseline ATP = (Week 0 ATP Value / Average Baseline ATP Value) x 100
Table 5A
Cell Viability at Post-Lyo (Week 0) Compared to Baseline
% of
Baseline
Sample # ATP
1 38.5
2 35.8
Table 5B below and Figure 1 show the % Retained ATP at 4, 8, 10 and 12 weeks
as
compared to viability at Week 0, which is calculated as follows:
% Retained ATP = (Average Week X ATP Value / Average Week 0 ATP Value ) x 100,
where X = 4, 8, 10, 12, respectively.
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
Table 5B
% Retained
ATP
Timepoint
Week 4 119%
Week 8 116%
Week 10 112%
Week 12 110%
*Note: Samples were not sealed under vacuum due to a mistake in setting up the
samples in the lyophilizing
apparatus. % Retained ATP in Table 5B greater than 100% is attributed to
sample variability but otherwise
proves stability of cell viability overtime.
Example 3
Preserved Tissue Form Containing Both Viable Cancellous Bone Granules and
Demineralized Cortical Bone Fibers with Demonstrated Viability After
Lyopreservation and
Storage at Room Temperature (up to 12 Weeks).
For this experiment, cancellous and cortical bone tissue samples from 3
different donors
were obtained, processed and combined, as follows, to produce a total of 18
combined test samples.
From each donor, cancellous tissue samples comprising cancellous bone were
recovered and
processed to provide cancellous granules, and cortical bone tissue samples
were recovered and
processed to provide cortical fibers. Quantities of cancellous granules and
cortical fibers derived
from the same donor were measured and combined to produce three combined test
samples per
donor, each of which contained about 40 wt% cancellous granules and about 60
wt% cortical
fibers, the wt % being based on the total weight of the combined cancellous
granules and cortical
fibers of each sample. The total weights of the test samples (i.e., each
comprising both cancellous
granules and cortical fibers) ranged from 1.3g to 1.6g. The test samples were
preserved after
86
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
contact with protectants in solution, in accordance with the methods described
hereinabove, and
analyzed for cell viability by measuring ATP. The ATP results reported below
are based on the
mathematical average of the measured ATP results for each of the three test
samples comprising
both cancellous granules and cortical fibers and derived from a common donor.
A. Protectant solution preparation:
1. Protectant solution was prepared by dissolving trehalose and EGCG into PBS
1X at
concentrations of 0.4M and 4mM, respectively.
2. Following dissolution of preservative ingredients in media, the solution
was sterile filtered
using a 0.21.tm membrane filter.
B. Sample preparation (performed in sterile hood)
3. Viable cancellous tissue (granules) and demineralized cortical tissue
(fibers) were obtained
by sterile processing techniques as described in Example 1 above, Part B.1.
4. Bulk cancellous granules and demineralized cortical fibers were packaged
under sterile
conditions and temporarily stored at refrigerated temperature (2-4 C) for a
period of time
greater than 0 and up to 90 minutes, until time of use in experiment.
5. In a sterile hood, multiple samples were weighed out into 1 oz. jars, as
follows:
a. each sample included 0.5g cancellous granules and 0.8g cortical fibers.
6. 4mL of sterile protectant solution was added to each jar containing
tissue.
7. Tissue was allowed to incubate with protectant solution at room temperature
for 1 hour.
8. After the incubation period, jars containing tissue were placed on an
aluminum or stainless
steel tray and placed in the Lyopreserving Apparatus.
9. Tissue was lyopreserved generally according to the recipe provided below in
Table 6.
87
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
Table 6
Thermal Treatment Temperature ( C) Time (min) Vacuum
(mTorr) Ramp/Hold
Step 1: Freeze -30 120 Ramp
Freeze, Condenser, and Vacuum Parameters Temperature ( C) Time (min) Vacuum
(mTorr) Ramp/Hold
Freeze Temperature ( C) -30 Hold
Additional Freeze (min) 180
Condenser Set Point ( C) -60
Vacuum Set Point (mTorr) 500
Drying Phase Parameters Temperature ( C) Time (min) Vacuum
(mTorr) Ramp/Hold
Step 1 -15 40 200 Ramp
Step 2 -15 1000 200 Hold
Step 3 1 60 200 Ramp
Step 4 1 600 200 Hold
Secondary Drying Phase Parameters Temperature ( C) Time (min) Vacuum
(mTorr) Ramp/Hold
Secondary Set Point 2
Post Heat Step 2 5 100 Hold
Key: "Ramp" indicates variable temperature over the time period indicated
(Time (min)).
"Hold" indicates temperature held constant over the time period indicated
(Time (min))
C. Sample testing
10. Prior to contact with protectant solution (i.e., prior to step B.6.
above), one or more test
samples containing about 1.3-1.6g of total tissue were measured and tested per
the ATP
viability assay procedures described above, which provided "Baseline" ATP
values.
11. Prior to lyopreservation but post-contact and incubation with the
protectant solution (i.e.,
prior to step B8 above), one or more test samples containing about 1.3-1.6g of
total tissue
were measured and tested per the ATP viability assay procedures described
above, which
provided "Pre-Lyo" (i.e., "post-contact and incubation") ATP values.
12. Following lyopreserving, samples were rehydrated by adding 1X PBS to each
jar to the fill
line and letting sit at room temperature for 5 minutes.
13. Following the 5 minute rehydration period, excess PBS solution was
decanted from all jars.
88
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
14. For each sample, all the tissue (1.3-1.6g) was tested per the ATP
viability assay procedures
described above and values corrected based on their pre-lyo weight, which
provided Week
0 ATP values. The ATP data resulting from the foregoing experimental procedure
was
converted to % of Baseline ATP and % of Pre-Lyo ATP, as reported in Table 7A
below,
using the following formulas:
% of Baseline ATP = (Average Week 0 ATP value / Average Baseline ATP value) x
100
% of Pre-Lyo ATP = (Average Week 0 ATP value / Average Pre-Lyo ATP value) x
100
Table 7A
Donor # Description
% of Baseline ATP* % of Pre-Lyo ATP
1 Cancellous granules with demineralized cortical fibers 68%
84%
2 Cancellous granules with demineralized cortical fibers 36%
48%
3 Cancellous granules with demineralized cortical fibers 55%
75%
* Baseline samples = viable cancellous granules and demineralized bone fibers
tested immediately prior
to use in experiment, with no exposure to lyophilization solution, therefore,
indicative of "pre-contact"
population of viable cells
Table 7B below and shows the % Retained ATP 12 weeks as compared to viability
at Week
0, which is calculated for each donor using the average ATP values for the 3
test sample
replicates, as follows:
% Retained ATP = (Average Week 12 ATP Value / Average Week 0 ATP Value ) x 100
where,
Average Week 0 ATP Value = [Week 0 ATP Sample 1 + Week 0 ATP Sample 2 + Week
0 ATP Sample 3]! 3
Average Week 12 ATP Value = [Week 0 ATP Sample 1 + Week 0 ATP Sample 2 +
Week 0 ATP Sample 3]! 3
89
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
Table 7B
Donor # Description
% Retained ATP (compared to 0 week)*
1 12 Week Post-Lyo Cancellous
granules with demineralized 110%
cortical fibers
2 12 Week Post-Lyo Cancellous
granules with demineralized 107%
cortical fibers
3 12 Week Post-Lyo Cancellous
granules with demineralized 95%
cortical fibers
*. Retained ATP in Table 7B greater than 100% is attributed to sample
variability but otherwise
proves stability of cell viability overtime.
Example 4
Comparison of Cryopreserved (DMSO) Viable Cartilage Fibers and Lvopreserved
(trehalose/EGCG) Viable Cartilage Fibers
A. Preparation of Cryopreservation and Lyoprotectant Solutions
1. Cryopreservation Solution - 10% DMSO Hyclone
a. Add 100mL of sterile DMSO to 900mL of sterile Hyclone media inside the
biological safety cabinet.
b. Invert 3-5 times to homogenize the solution.
2. Lyopreservative Solution - 0.4M Trehalose (151.32mg/mL) / 4mM EGCG
(1.9mg/mL)
in PBS
a. Same preparation as in Example 1 (section A) above
B. Viable Cartilage Fibers Preparation Procedure:
1. Distal Femur osteochondral (OC) grafts (tissue samples) were received
and/or packaged
in ambient storage media
a. Composition of the ambient storage media was as follows:
i. 0.95X DMEM,
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
ii. supplemented with 0.125uM Dexamethasone, and
iii. 0.95X Antibiotic-Antimycotic (Penicillin/Streptomycin/Amphotericin B).
2. OC grafts in ambient storage media and necessary supplies and equipment
were passed
into a sterile hood (biological safety cabinet).
a. All following steps are performed aseptically
3. One Nalgene container was filled with one full bag (250mL) of ambient
storage media.
4. An OC Graft was removed from its packaging and the leftover solution
drained off, down
the drain.
5. The OC Graft was placed in ACT Vise by clamping the bone portion of the
graft into the
vise by turning the handle until secure. Care was taken not to clamp or damage
areas of
cartilage.
6. Grating cartilage to produce viable cartilage fibers was performed by
pressing firmly on
the cartilage portion of tissue with an OXO grater (Model #50581), using a
stroking
motion directed toward yourself Using two hands facilitates the grating
process. After
four strokes, the grater was dipped in the Nalgene container with ambient
storage media.
a. Care was taken not to grate any areas of damaged cartilage or bone
tissue
b. The OC graft was repositioned in the ACT Vise as necessary to accomplish
grating and formation of cartilage fibers
7. The time that the first portion of fibers are placed in the ambient
storage media was
recorded.
a. Viable cartilage fibers must be collected in ambient storage media and
drained
within 3 hours of when the first set of fibers were soaked.
8. Step 5 was repeated until all cartilage was grated into fibers.
91
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
9. Once all cartilage was grated, the mesh basket designated was placed, with
the lid off,
over a sieve. The grated fibers and ambient storage media were poured into the
basket.
Once the storage media drained, the lid of the mesh basket was closed and
secured. The
basket was lifted up and down to drain residual storage media.
10. 1 liter of PBS was poured over the cartilage fibers in the basket to rinse
off any excess
media not removed during draining.
11. A Nalgene container was filled with PBS and the mesh basket placed into
the container,
ensuring that the fibers were submerged completely.
12. A loz sample jar was tared on a scale.
13. The basket was lifted out of the container with PBS, bobbing it up and
down and tilting
back and forth until PBS no longer dripped from the basket.
14. The lid of the mesh basket was unsecured and opened.
15. Using forceps, the viable cartilage fibers were stirred and fluffed to
release any surface
tension from the PBS with the bottom of the mesh basket. This step is helpful
to ensure
that all the fibers are evenly wetted throughout the basket.
16. Using forceps, 0.3-0.4g of the viable cartilage fibers were aliquoted into
the loz jar.
a. Another quantity of fibers may be aliquoted. 0.3-0.4g of
fibers was sufficient for
ATP testing performed as described herein.
17. 2 samples of viable cartilage fibers without any solution were set aside
to be tested for
baseline ATP (as reported in Table 8 below).
a. Baseline samples were tested as described above (in Characterization Test
Methods section) with CellTiter-Glo Reagent by Promega (Madison, WI) to
obtain baseline cell viability.
92
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
18. Respective protectant media (Cryopreservation / Lyoprotectant Solution)
were
immediately poured into the jar for each cryo/lyo sample of cartilage fibers.
a. For Cryopreservation Solution ¨ solution was added to the fill line on the
inside of
the jar. The lid was tightly screwed shut.
b. For Lyoprotectant Solution ¨ solution was added until all fibers were
completely
submerged and lid screwed tightly shut.
19. For cryopreservation solution samples, the time the first jar was filled
was recorded. All
samples must be placed in the cryopreservation apparatus (Thermo Scientific
CryoMed
Model 7454, the "CryoMed") and the CryoMed must be started within 2 hours from
when the first sample jar was filled.
20. For lyoprotectant solution samples, the time the first jar was filled was
recorded. All
samples were incubated for at least one hour from when the first sample jar
was filled (at
ambient temperature, 19-23 C).
a. 3 samples were tested as described above (in Characterization Test Methods
section) with CellTiter-Glo Reagent for pre-lyo cell viability after the 1-
hour
incubation was complete and after aliquoting of all other samples for
cryopreservation was completed, representing a post-contact viability measure
but
prior to lyophilization.
21. Step 16 was repeated 5 times, and the mesh basket dipped back into the
Nalgene
container with PBS to re-wet the fibers in between. Again, steps 13-15 were
performed to
dry the fibers.
22. Fibers were aliquoted until all sample jars were filled or until all
cartilage fibers produced
from a particular OC graft were used up.
93
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
C. Preservation Techniques
Cryopreservation:
1. Each sample-containing jar to be cryopreserved was placed into a foil pouch
horizontally
and the pouches sealed.
2. The pouches were placed on the CryoMed rack and the rack was placed in the
CryoMed
when all samples were loaded.
3. The CryoMed was turned on and the desired cryopreservation recipe/procedure
was
selected, which is provided in Table 8 below:
Table 8
Target Rate of Change
Action Time
(minutes)
Temperature ( C/minute)
Hold (Chamber) 4 C 30
Sample
temperature 4 C -3
decrease A
Chamber
temperature -90 C -10
decrease
Hold (Chamber) -90 C 20
Hold (Chamber) -85 C 25
Sample temperature -100 C -5
decrease
Hold (Chamber) -100 C Until
complete
A NOTE: Rate of Change steps are all chamber temperature rates, with S and C
being either
Sample or Chamber target temperature
4. The CryoMed door was closed and the "Start" button pushed.
5. The CryoMed operating instructions to stop the cycle were performed after
completion of
the recipe.
94
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
6. With protective equipment; insulating gloves and face mask on, the rack was
removed
from the CryoMed and each foil pouch was opened and every sample-containing
jar
taken out of its foil pouch.
7. Samples were stored in a LN tank (-210 to -196 C).
8. To prepare for testing for ATP, the samples were thawed (still in jars) in
a water bath for
20-30 minutes.
9. Once completely thawed, for each sample, the cryopreservation solution was
decanted
from the jar and PBS added to the jar to the fill line to rinse
cryopreservation solution
from the fibers.
10. PBS was decanted from the jar to provide relatively dry fibers for cell
viability testing
with CellTiter Glo Reagent.
11. The cryopreserved samples were tested for cell viability as described
above (in
Characterization Test Methods section) with CellTiter-Glo Reagent.
Lyophilization:
1. Each sample-containing jar to be lyopreserved was placed onto an aluminum
or stainless
steel dish and inserted into the lyophilizer (apparatus as described in
Example 1 above).
2. The lyophilizer was turned on and the desired lyopreserving
recipe/procedure was
selected, which is provided in Table 9 below:
95
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
Table 9
Thermal Treatment Temperature ( C) Time (min) Vacuum
(mTorr) Ramp/Hold
Step 1: Freeze -30 120 Ramp
Freeze, Condenser, and Vacuum Parameters Temperature ( C) Time (min) Vacuum
(mTorr) Ramp/Hold
Freeze Temperature ( C) -30 Hold
Additional Freeze (min) 180
Condenser Set Point ( C) -60
Vacuum Set Point (mTorr) 500
Drying Phase Parameters Temperature ( C) Time (min) Vacuum
(mTorr) Ramp/Hold
Step 1 -15 40 200 Ramp
Step 2 -15 1000 200 Hold
Step 3 1 60 200 Ramp
Step 4 1 600 200 Hold
Secondary Drying Phase Parameters Temperature ( C) Time (min) Vacuum
(mTorr) Ramp/Hold
Secondary Set Point 2
Post Heat Step 2 5 100 Hold
3. When the recipe was complete, the sample-containing jars were removed from
the
lyophilizer and packaged in Mangar foil pouches to prevent moisture
absorption.
4. In preparation for cell viability testing, the lyopreserved viable
cartilage fibers were
rehydrated with PBS 1X by filling each loz jar to the fill line.
5. Allowed tissue to sit for 2 minutes before decanting excess PBS 1X using
gauze.
6. The lyopreserved samples were tested for cell viability as described above
(in
Characterization Test Methods section), and specifically as described below,
using
CellTiter-Glo reagent.
D. Cell Viability Testing ¨Cell Titer Glo Viability Assay:
1. The CellTiter-Glo reagent was thawed in a water bath at room temperature.
At least 20
minutes was allowed for a full 100mL set to thaw.
96
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
2. Once thawed, the bottle was inverted multiple times and rolled to
homogenize the
reagent. Reagent was stored away from light until use.
3. DMEM was aliquoted into a 50mL tube and allowed to reach room temperature.
4. 0.3-0.4g of viable cartilage fibers were placed into a 15mL tube.
5. 2000uL of DMEM was added into each tube with cartilage fibers.
6. 2000uL of CellTiter-Glo was added into each tube with cartilage fibers.
7. A blank was created by mixing 1000uL DMEM and 1000uL CellTiter-Glo into a
15mL
that does not contain any cartilage fibers.
8. All tubes were inverted 5 times in quick succession and placed in a rack.
9. Protected from light, the tubes were placed on an orbital shaker and shaken
for 5 minutes
at 150rpm at room temperature.
10. The tubes were then placed on the bench top for 20 minutes, still
protected from light.
11. After the 20 minute incubation, the tubes were centrifuges tubes at
3000rpm for 3
minutes.
12. The solution from each tube was distributed into an opaque 96-well plate.
a. 200uL from each tube was transferred to 6 wells of the 96-well plate.
b. 200uL of the blank was plated in quadruplicate.
13. The plate was covered with aluminum foil to protect from light and placed
and shaken on
an orbital shaker for 2 minutes at 150rpm.
14. After 2 minutes, the luminescence of the plate referencing WI-330 was read
and
recorded.
97
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
E. Cell-Viability Results:
This experiment sought to establish whether lyophilization of viable cartilage
fibers could preserve
cell viability. As described above, viable cartilage fibers were produced by
grating from
osteochondral grafts stored in ambient storage media. Post-grating, viable
cartilage fibers were
subsequently incubated in lyoprotectant media containing protectants, i.e.,
trehalose and EGCG,
for one hour and lyopreserved. Viability was assessed via ATP assay whereby
CellTiter-Glo by
Promega (Madison, WI) was used to measure ATP content. Results are shown below
in Tables
10A& 10B.
Table 10A
Week 0 Post-Lyo & Post-Cryo Cartilage Fiber Cell Viability Results
Sample % of Baseline ATP
% of Pre-Lyo ATP
TO Post-Lyopreservation 4% 16%
TO Post-Cryopreservation 10% 36%
Table 10B
% Retained ATP for 1 year timepoint Post-Lyo & Post-Cryo Cartilage Fiber
compared to week 0
Sample % Retained ATP
(compared to 0 week)*
Post-Lyo 1 Year 54%
Post-Cryo 1 Year 142%
*. % Retained ATP in Table 10B greater than 100% is attributed to sample
variability but otherwise proves
stability of cell viability overtime.
98
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
Example 5:
Comparison of Cryopreserved (DMSO) Viable Cancellous Bone Granules and
Lyopreserved
(trehalose/EGCG) Viable Cancellous Bone Granules
A. Preparation of Cryopreservation and Lyoprotectant Solutions
1. Cryopreservation Solution - 10% DMSO Hyclone
a. Add 100mL of sterile DMSO to 900mL of sterile Hyclone media inside the
biological safety cabinet.
b. Invert 3-5 times to homogenize the solution.
2. Lyopreservative Solution - 0.1M Trehalose (37.83mg/mL) / 2mM EGCG
(0.945/mL)
in Hyclone media.
a. Same preparation as in Example 1 (section A) above
B. Procedure (performed in sterile hood):
Sample Preparation ¨ Lyophilization
1. Upon receiving milled cancellous bone tissue, about 0.5g of milled
cancellous tissue
granules per sample were weighed and placed into 1 oz jars. (NOTE: cancellous
tissue
granules produced by the same process as described in Example 1 above, Part
B.1.)
2. Two additional samples of 0.5g milled cancellous tissue were weighed and
set aside for
Baseline cell viability testing.
3. Added 4mL of lyopreservation solution to each jar containing weighed
samples of milled
cancellous bone tissue designated for lyophilization to form tissue-protectant
mixtures.
4. Placed lyo jar (containing tissue-protectant mixtures) on stainless
steel lyo tray and tightly
sealed a vented cap with porous liner on the jar.
99
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
5. Carefully sealed lyo tray containing samples in a large autoclave pouch
(paper side facing
up).
6. Allowed cancellous tissue-protectant mixtures to incubate in
lyopreservation solution at
ambient temperature without agitation for 1 hour.
7. After incubation was complete, lyopreserved all processed tissue-protectant
mixtures using
24 hour recipe provided in Table 11 below. Upon completion of the
lyopreservation cycle,
samples stoppered under vacuum prior to releasing lyo vacuum.
Table 11 ¨Lyophilization Recipe for Example 5
Thermal Treatment Temperature ( C) Time (min) Vacuum
(mTorr) Ramp/Hold
Step 1: Freeze -30 120 Ramp
Freeze, Condenser, and Vacuum Parameters Temperature ( C) Time (min) Vacuum
(mTorr) Ramp/Hold
Freeze Temperature ( C) -30 Hold
Additional Freeze (min) 180
Condenser Set Point ( C) -60
Vacuum Set Point (mTorr) 500
Drying Phase Parameters Temperature ( C) Time (min) Vacuum
(mTorr) Ramp/Hold
Step 1 0 40 200 Ramp
Step 2 0 360 200 Hold
Step 3 15 15 200 Ramp
Step 4 15 525 200 Hold
Step 5 25 15 200 Ramp
Step 6 25 360 200 Hold
Sample Preparation - Cry opreservation
1. Upon receiving milled cancellous bone tissue, 0.5g of milled cancellous
tissue per sample
were weighed and placed into loz jars.
2. The same baseline samples as the lyopreservation sample preparation step
were utilized
whereby 0.5g of milled cancellous tissue were tested for baseline cell
viability.
100
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
3. For cryopreservation samples, cryopreservation solution was added to the
fill line on the
inside of the jar. The lid was tightly screwed shut.
4. The same cryopreservation technique as in Example 3 (Section C) was used.
C. Cell Viability Testing Post-Lyo and Post-Cryo
1. Cryopreservation samples were set out on a benchtop and thawed at room
temperature
2. Once thawed, cryopreservation samples were decanted of cryoprotectant
solution, rinsed
with PBS filling the jar up to the fill-in, and once again decanted.
3. At the same time, lyopreservation samples were rehydrated by adding 1X PBS
to each jar
until the fill line and allowed to rest for 5 minutes at ambient conditions.
4. After 5 minutes, excess solution was decanted from the lyopreservation
samples.
5. Cell viability testing was performed following procedures described above
for the
CellTiterGlo ATP Assay (same as specified above).
6. For each sample, decanted tissue (about 0.48g) was weighed out and tested
per the ATP
viability assay procedures described above, which provided Week 0 ATP values.
The ATP
data resulting from the foregoing experimental procedure was converted to % of
Baseline
ATP, as reported in Table 12 below, using the following formula:
% of Baseline ATP = (Average Week 0 ATP value / Average Baseline ATP value)
x 100
Table 12
Sample Set # Description
Average % of
Baseline ATP*
1 Two replicates of lyopreserved viable cancellous granules
41.6%
2 Two replicates of cryopreserved viable cancellous
granules 46.2%
101
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
Example 6
Lyopreseryed Viable Amnion / Chorion Bilayer Tissue Forms
A. Preparation of Lyopreserved Amnion / Chorion Bilayer with
Trophoblast Layer
1. A solution of 100 IU/mL Streptokinase in red blood cell lysis buffer was
prepared (SK-RBC
lysis buffer solution).
2. Lyoprotectant Solution was prepared as described in Example 1 above but
having the
following content: 0.5M trehalose, 4mM Epigallocatechin gallate (EGCG), in
HBSS
2.1. The lyoprotectant solution was protected from light and refrigerated
until time of use.
3. The amnion membrane was peeled apart from the chorion membrane by hand and
then cut
free from the umbilical cord with scissors.
4. The chorion membrane was cut free from the placental disk with scissors.
5. Large blood clots on the chorion were gently dabbed using wetted wipes and
the chorion was
placed into a flask of 500m1 SK-RBC lysis buffer solution.
6. The flask was placed onto an orbital shaker and agitated at 150RPM for 90
minutes.
7. Meanwhile, the amnion was manually cleaned, by dabbing and wiping with
wetted wipes
while soaking in HBSS to remove blood and blood clots, two times for 10-20
minutes each
time.
7.1. After cleaning, the amnion was left in the second HBSS solution while
waiting for
chorion agitation to finish.
8. After agitation in the SK-RBC lysis buffer solution, the chorion was
removed from the flask
and transferred into a container of HBSS.
9. The chorion was manually cleaned by dabbing and wiping with wetted wipes
and cotton
applicators while soaking in the HBSS to remove residual blood and blood
clots.
102
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
10. Antibiotic solution was prepared by making a solution of 50ug/mL
vancomycin, 50ug/mL
gentamycin, and 2.5ug/mL amphotericin B in HBSS.
11. The amnion and chorion were transferred into a flask with antibiotic
solution and agitated at
65RPM for 60 minutes.
12. The amnion and chorion were transferred into a flask with fresh HBSS and
agitated at
65RPM for 5 minutes.
13. The amnion and chorion were transferred into a flask with fresh HBSS and
agitated at
65RPM for 5 minutes.
14. The amnion and chorion were transferred into a flask with fresh HBSS with
phenol and
agitated at 65RPM for 30 minutes, to impart a reddish color to the tissue for
easier
visualization during cutting.
15. The amnion was transferred into a basin of fresh HBSS with phenol red to
keep the reddish
color.
16. The chorion was transferred into a basin of fresh HBSS and then manually
cleaned by
dabbing and wiping with wetted wipes and cotton applicators while soaking in
HBSS to
remove residual blood and blood clots.
17. The amnion was placed on a backing material with epithelial side facing
down, toward the
backing.
18. The chorion was placed on top of the amnion, with the trophoblast side
facing upward, away
from the amnion.
19. The layered amnion/chorion composite was cut using a rolling bladed cutter
into samples of
approximately 5x5cm.
103
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
20. The amnion/chorion sample still on backing was placed into a snap retainer
and then
immersed into HB S S until all cutting was completed.
20.1. Several tissue containment configurations were tested across donors,
such as placing
tissue on backing into a netting mesh, placing tissue on netting mesh into a
retainer,
and placing tissue on backing into a retainer.
21. The retainers containing amnion/chorion tissue were placed into
lyoprotectant solution and
refrigerated for 30-60 minutes.
22. After 30-60 minutes, the retainers were removed from solution and
transferred into regular
HB S S for 5 minutes.
23. Each retainer was drained of excess liquid by dabbing on its side against
sterile wipes and
then packaged and sealed into a breathable Tyvek pouch.
24. The sealed pouches were placed with breathable side up into a lyophilizer
(apparatus as
described in Example 1 above) and lyophilized using a programmed recipe as
described in
Tables 13-17 provided below.
25. After lyo cycle was completed, pouches were removed and sealed into foil
pouches as a
moisture barrier pouch.
B. Amnion / Chorion Bilayer without Trophoblast Layer
1. Placenta Tissue Sample was processed as previously described above in
Example 6, Part A,
until Step 16, when chorion was being cleaned after antibiotic soak.
2. Before spot cleaning residual blood and blood clots, the trophoblast
layer of the chorion was
removed by gently peeling loose the trophoblast layer using fingers, cotton
applicators,
instruments such as dissecting forceps, or wetted wipes.
104
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
2.1. The removal of the trophoblast layer also resulted in the removal of
residual blood and
blood clots embedded within the tissue, leaving very little residual blood on
the
remaining chorion.
3. Further processing with the amnion and thinner chorion was performed as
previously
described above in Example 6, Part A, Step 17 and onward.
The following Table 13 provides a summary of the features and conditions of
each of the above-
described Bilayer Lyopreserved Tissue Forms, both with and without trophoblast
layer, including
the lyopreservation recipe performed in the lyophilizer, the packaging
configuration and the tissue
.. configuration. The Iteration # corresponds to placental tissue samples
recovered from different
donors, or to different lyo recipes or packaging configurations within the
same donor.
Table 13
Tissue
Iteration # Lyo Recipe Packaging Configuration Configuration
1 RTT On backing, in netting AM and CM
separate
2 RTAC vi between 2 netting, in retainer AM/CM
composite
3 RTAC v2 between 2 netting, in retainer AM/CM
composite
4 RTAC v2 between 2 backing, in retainer AM/CM
composite
5 RTAC v2 on backing, in retainer AM/CM composite
6 RTAC v2 on backing, in retainer AM/CM composite
7 RTAC v3 on backing, in retainer AM/CM composite
8 RTAC v3 on backing, in retainer AM/CM composite
9 RTAC v3 on backing, in retainer AM/CM composite
10 RTAC v3 on backing, in retainer AM/CM composite
11 RTAC v3 on backing, in retainer AM/CM composite
The lyopreservation recipes identified in Table 13 above (i.e., RTT, RTAC vi,
RTAC v2, and
.. RTAC v3) as performed on the above-described Bilayer Lyopreserved Tissue
Forms are provided
in Tables 14, 15,16, and 17 below.
105
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
Table 14 ¨ RTT Recipe
Recipe Phase Temperature Time Vacuum
Ramp/Hold
( C) (min) (mTorr)
Thermal Treatment
Step 1 -30 120 Ramp
Freeze Temperature -30 Hold
Additional Freeze Time 180
Condenser Set Point -60
Vacuum Set Point 500
Drying Phase
Step 1 -15 40 200 Ramp
Step 2 -15 1000 200 Hold
Step 3 1 60 200 Ramp
Step 4 1 600 200 Hold
Secondary Drying Phase
Post Heat Step 2 5 100 Hold
Table 15 ¨ RTAC vl
Recipe Phase Temperature Time Vacuum
Ramp/Hold
( C) (min) (mTorr)
Thermal Treatment
Step 1 -40 60 Ramp
Freeze Temperature -40 Hold
Additional Freeze Time 30
Condenser Set Point -40
Vacuum Set Point 600
Drying Phase
Step 1 -10 720 600 Hold
Secondary Drying Phase
Post Heat Step 25 240 600 Hold
Table 16 ¨ RTAC v2
Recipe Phase Temperature Time Vacuum
Ramp/Hold
( C) (min) (mTorr)
Thermal Treatment
Step 1 -40 120 Ramp
Freeze Temperature -40 Hold
Additional Freeze Time 30
Condenser Set Point -40
Vacuum Set Point 600
106
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
Drying Phase
Step 1 -10 720 600 Hold
Secondary Drying Phase
Post Heat Step 25 240 600 Hold
Table 17 ¨ RTAC v3
Temperature Time Vacuum
Recipe Phase
Ramp/Hold
( C) (min) (mTorr)
Thermal Treatment
Step 1 20 15 - Hold
Step 2 -20 160 - Ramp
Step 3 -40 60 - Hold
Freeze Temperature -40 Hold
Additional Freeze Time 30
Condenser Set Point -40
Vacuum Set Point 600
Drying Phase
Step 1 -5 520 600 Hold
Secondary Drying Phase
Post Heat Step 25 300 600 Hold
C. Cell Viability Analysis via ATP Assay
A unit of lyopreserved viable amnion/chorion bilayer tissue form (prepared as
described above in
Example 6, Parts A-B) was cut into approximately 4cm2 pieces for the ATP
assay. Each piece was
rehydrated by immersing in PBS for 10 minutes, and the amnion and chorion
layers were peeled
apart using dissecting forceps to test the layers by ATP assay separately.
Each approximately 4cm2
rehydrated tissue piece was combined with 0.5mL DMEM and 0.5mL assay reagent
in a well plate
107
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
and incubated on an orbital shaker in the dark for 20 minutes at room
temperature. After
incubation, aliquots of the incubated reagent were transferred into a 96-well
plate and raw
luminescence values (in RLU) were obtained using a plate reader. A standard
curve was prepared
by creating serial dilutions of known concentrations of an ATP standard,
combining with assay
reagent, incubating on an orbital shaker, and then reading the RLU of each
dilution. The dilutions'
RLUs were plotted against the known concentrations of each serial dilution to
generate an
equation, which was then used to convert RLU of the tissue pieces to
concentrations of ATP.
Finally, the tested tissue pieces were rinsed in water, placed into pre-
weighed weighing pans, and
dried in a gravity oven. The dried tissue and pans were then weighed to obtain
dry tissue weights,
and the concentration of ATP per sample was converted to amount of ATP per dry
weight, and the
amounts of ATP converted to % Pre-Lyo ATP retained in the Lyopreserved Amnion
/ Chorion
Bilayer Tissue Form with Trophoblast Layer (Example 6, Part A), are reported
in Table 18, using
the following formula:
% of Pre-Lyo ATP = (Week 0 ATP value / Pre-Lyo ATP value) x 100
Table 18
Iteration # % of Pre-Lyo ATP, AM+ % of Pre-Lyo ATP, CM+
1 3.90% 1.46%
2 7.47% 1.87%
3 7.72% 1.67%
4 27.06% 1.52%
5 25.04% 2.10%
* "AM+" and "CM+" refer to "Amnion" and "Chorion" with trophoblast layer,
lyophilized as a
bilayer, rehydrated for 10min in PBS then peeled apart prior to testing ATP
separately
Similarly, the amounts of ATP converted to % Pre-Lyo ATP retained in the
Lyopreserved Amnion
/ Chorion Bilayer Tissue Form without Trophoblast Layer (Ex. 4B),are reported
in Table 19 below.
108
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
Table 19
Iteration # % of Pre-Lyo ATP, AM- % of Pre-Lyo ATP, CM-
1 N/A 13.12%
2 5.92% 1.82%
3 N/A 2.61%
* "AM-" and "CM-" refer to "Amnion" and "Chorion" without trophoblast layer,
lyophilized as a
bilayer, rehydrated for 10min in PBS then peeled apart prior to testing ATP
separately
A AM/CM- could not be separated for testing for Iteration #3
Example 7
Cell Viability Evaluation via Enzymatic Digestion and Live/Dead Fluorescent
Staining
List of Materials and Equipment Used:
1. Collagenase Type II lgram (Worthington Biochemicals, Cat. L5004196)
2. 0.25% Trypsin EDTA 1X (Mediatech, Cat. 25-053-CI)
3. Phosphate Buffered Saline 1X (Mediatech, Cat. 21-040-CM)
4. Dulbecco's Modification of Eagle's Medium/Ham's F-12 50/50 Mix without
phenol red
(Mediatech, Cat. 16-405-CV)
5. Dulbecco's Phosphate-Buffered Saline, lx (Mediatech, Cat. 21-031-CM)
6. Penicillin-Streptomycin Solution, 100X (Mediatech, Cat. 30-002-CI)
7. Fetal Bovine Serum, Premium (Heat Inactivated) (Mediatech, Cat. 35-016-CV
or
equivalent)
8. Corning glutaGRO, Liquid (Mediatech, Cat. 25-015-CI)
9. LIVE/DEAD Viability/Cytotoxicity Kit (Invitrogen, Cat. L3224)
10. CO2 Incubator (NuAire)
11. 6-well; Standard tissue culture; Flat-bottom (Fisher Scientific, Cat. 08-
772-1B)
12. 1001.tm cell strainer (Fisher Scientific, Cat. 22-363-549)
109
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
Tissue Processing:
1. Amnion/Chorion Bilayer with Trophoblast (AM+/CM+) and Amnion/Chorion
Bilayer
without Trophoblast (AM-/CM-) 5cm x 5cm samples were prepared as described
above
in Example 6, but Lyoprotectant Solution was made with 0.3M Trehalose and no
EGCG.
One sample of each tissue configuration was lyopreserved using RTACv2 lyo
recipe
(Iteration #6) and one sample of each tissue configuration was lyopreserved
using
RTACv3 lyo recipe (Iteration #7).
Tissue Rehydration and Equilibration:
1. Supplemented DMEM/F12 media was prepared by adding 10% FBS, 1% PenStrep
solution, and 1% glutaGRO solution final v/v to DMEM/F12 media without phenol
red
(i.e. 10mL FBS, lmL PenStrep solution, lmL glutaGRO solution to 88mL of
DMEM/F12). The prepared media was stored at 4 C in the dark. Immediately
before
use, the prepared media was warmed in a 37 C water bath.
2. 12 days after completion of lyo recipe, each lyopreserved 5cm x 5cm
amnion/chorion
bilayer sheet sample was removed from the outer foil pouch, inner Tyvek pouch,
retainer,
and backing, and immersed in a weigh pan of room temperature PBS for
approximately
10 minutes. After rehydration, the amnion and chorion membranes were peeled
apart and
each membrane from each sample was placed into a separate well of a 6 well
tissue
culture plate, to which 5mL of supplemented DMEM/F12 culture media was added.
Each 5cm x 5cm amnion or chorion membrane was allowed to equilibrate in media
overnight in a CO2 incubator at 37 C and 5% CO2 level.
Enzymatic Tissue Digestion:
110
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
1. A 0.75% collagenase II solution was prepared by adding lg of powdered
collagenase II
per 133mL of DMEM/F12 media without phenol red. The collagenase II solution
and
0.25% trypsin solution were each warmed in a 37 C water bath immediately prior
to their
use in the subsequent steps.
2. Each 5cm x 5cm amnion (AM+, AM-) and chorion sample (CM+, CM-) was placed
into
a separate 50mL conical tube containing 40mL of collagenase II solution. Each
conical
tube was capped and laid sideways on an incubator shaker at 65rpm for 40
minutes at
37 C, and then centrifuged at 2000rpm for 10 minutes at ambient temperature.
Taking
care not to aspirate tissue, liquid in each tube was aspirated down to 5mL
remaining, and
then 40mL of 0.25% trypsin was added. Each conical tube was recapped and laid
sideways on an incubator shaker at 65rpm for 15 minutes at 37 C. After
shaking, 5mL of
Fetal Bovine Serum was added to each tube to neutralize the trypsin.
3. Each tube's contents was poured through a separate 1001.tm cell strainer
and into a new
50mL conical tube, to remove remaining tissue and debris. The new conical
tubes were
centrifuged at 2000rpm for 10 minutes at ambient temperature. The liquid in
each tube
was aspirated down to 5mL remaining, and then the last 5mL of solution was
transferred
into a 15mL conical tube. The 15mL conical tubes were centrifuged at 2000rpm
for 10
minutes at ambient temperature. The supernatant from each conical tube was
aspirated
out of each tube and lmL of DMEM/F12 without phenol red was added to each. The
contents of each tube was pipetted up and down for a minimum of 10 times to
resuspend
the cells, until no visible cell pellets were visible.
4. The cell suspension was transferred into a separate microcentrifuge tube
for performing
live/dead staining, as described below.
111
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
Live/Dead Staining and Cell Count:
1. Because the live/dead stain is light sensitive, all live/dead staining
steps were performed
in the dark. The reagents from a live/dead viability kit (LIVE/DEAD
Viability/Cytotoxicity Kit, Invitrogen) were thawed at room temperature. 20 L
of 2mM
ethidium homodimer-1 (EthD-1) was added to 10mL Dulbecco's Phosphate-Buffered
Saline (DPBS) to form an EthD-1 solution, which was vortexed to mix. 5 L of
4mM
calcein AM was added to the EthD-1 solution and vortexed to mix. The live/dead
working solution (4 M EthD-1, 2 L calcein AM) was kept in foil to avoid
exposing the
solution to light before it was applied to the samples of enzymatically
digested cell
suspension, as follows.
2. Each microcentrifuge tube containing enzymatically digested cell suspension
was
centrifuged in a microcentrifuge for 6 minutes at 300x g. The resulting
supernatant was
removed by aspirating. lmL of the live/dead working solution was added to each
microcentrifuge tube, followed by pipetting up and down to resuspend cells.
The
microcentrifuge tubes were incubated in the dark for 20 minutes at room
temperature.
3. The tubes were centrifuged for 6 minutes at 300x g, supernatant was removed
from each
tube by aspirating, followed by adding lmL of fresh DPBS to each
microcentrifuge tube
and pipetting up and down to resuspend cells again. The foregoing centrifuge-
aspirate-
pipette cycle was repeated twice more, for a total of three cycles. 504, of
each cell
suspension was pipetted onto a separate microscope slide and carefully covered
with a
microscope coverslip, avoiding creating air bubbles.
4. Because the live/dead stain is light sensitive, the following live/dead
cell enumeration
steps to produce a representative image for each sample were also performed in
the dark.
112
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
Cells on each slide were visualized using a dual FITC/TRITC filter on the
microscope, at
100% light power and using a 0.14-second manual exposure. EthD-1 (indicating
dead
cells) was red and calcein AM (indicating live cells) was green. Each
coverslip was
divided approximately evenly into multiple smaller sampling areas (shown
below) and a
representative image taken from each area.
1 2 3
4 5 6
7 8 9
5. A public domain, Java-based image processing program known as IMAGEJ
Version
1.51j8 was used to analyze each representative image produced by the foregoing
steps
and determine the number of viable cells and nonviable cells. IMAGEJ was
obtained
from the National Institutes of Health, of Bethesda, Maryland, USA. The
software was
downloaded from the NIH website at https://imagej.nih.gov/ij/.
6. Using IMAGEJ, for each representative image, the image was opened and a
conversion
scale applied by drawing a line over the scale bar and going to Analyze->Set
Scale. The
known distance and units were entered. The "Global" option was checked to set
a global
scale, which applied a size limit for particle analysis at a later step. Menu
options Image-
>Adjust->Threshold were successively selected. A hue filter (50-255 pass) and
a
brightness filter (150-255 pass) with B&W threshold color to filter out low
intensity
and/or red stains were selected/applied. Menu options Analyze->Analyze
Particles were
successfully selected. A size limit of 30-800 um2 was applied to filter out
small artifacts
and larger debris and then the "Summarize" option was checked.
7. For each representative image processed as described above using IMAGEJ,
the number
of viable cells as indicated by a relatively strong green fluorescence with a
stain size
113
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
appropriate for a cell were reported. The nonviable cells were either manually
counted,
or the foregoing procedure was repeated for red stains (i.e., 0-50 pass
instead of 50-255
pass hue filter, 100-255 pass brightness filter, and 10-300um^2 particle
size). However,
whenever possible, nonviable cells were manually counted because automated
counts by
IMAGEJ were known to sometimes return false positives because EthD-1 stains
nuclei,
not whole cells, and the stained nuclei are closer in size to non-cellular
artifacts. The %
Viable Cells for each representative image was calculated as follows, which
provides a
snapshot look at cell viability in each sample at the time of enzymatic
digestion:
Viable Cells
Viable cells + Nonviable Cells
The two iterations, both with (Table 20) and without trophoblast layer (Table
21) included,
showed that a high % cell viability could be obtained in the lyopreserved
amnion/chorion
bilayer after 12 days in storage.
Table 20
Iteration
% Viable Cells, AM+ % Viable Cells, CM+
6 91.59% 88.39%
7 92.78% 93.06%
* "AM+" and "CM+" refer to "Amnion" and "Chorion" with trophoblast layer,
lyophilized as a
bilayer, rehydrated for 10min in PBS then peeled apart prior to testing as
described
Table 21
Iteration
% Viable Cells, AM- % Viable Cells, CM-
6 N/A 89.65%
7 95.84% 96.38%
* "AM-" and "CM-" refer to "Amnion" and "Chorion" without trophoblast layer,
lyophilized as a
bilayer, rehydrated for 10min in PBS then peeled apart prior to testing as
described
114
CA 03165002 2022-06-16
WO 2021/127230 PCT/US2020/065688
** Insufficient cells were recovered to perform counting of AM- digested cells
for Iteration #6
Example 8
Cell Viability Testing after Storage, at 0, 1, 2, 3 and 4 weeks
1. Amnion/Chorion Bilayer with Trophoblast (AM+/CM+) samples were processed
and
preserved as previously described in Example 6, but with Lyoprotectant
Solution
containing 0.3M trehalose with either OmM EGCG or 2mM EGCG, lyopreserved using
RTAC v3 lyo recipe, and then sealed into individual foil pouches. Immediately
(i.e., about
1 hour) after lyophilization (referred to as "0 week"), or at 1, 2, 3 and 4
week timepoints,
removed 1 preserved AM/CM+ sample per EGCG condition for cell viability
testing as
described in Cell Viability Analysis via ATP Assay above in Example 6. Results
of cell
viability testing are provided in Tables 22 and 23 below, as a % of Week 0 ATP
values.
Several samples of CM+ reported average ATP values above 100%, most likely due
to the
highly variable nature of the metabolic activity of the placental tissue as
well as variability
in the trophoblast tissue layer thickness for each sample, which would impact
the average
cell density per tissue volume and thus the normalized nnmol of ATP/g of
tissue.
Table 22
OmM EGCG
Iteration #8 Iteration #9 Iteration #10
AM+ CM+ AM+ CM+ AM+ CM+
0 weeks 100.00% 100.00% 100.00%
100.00% 100.00% 100.00%
1 week 50.87% 106.62% 62.04% 125.53% 104.13% 84.23%
2 weeks 57.84% 163.10% 93.47% 120.54% 84.49% 64.73%
3 weeks 54.12% 293.83% 65.17% 126.18% 123.91% 63.92%
4 weeks 71.66% 382.72% 102.29% 125.90%
108.88% 26.74%
115
CA 03165002 2022-06-16
WO 2021/127230 PCT/US2020/065688
Table 23
2mM EGCG
Iteration #8 Iteration #9 Iteration #10
AM+ CM+ AM+ CM+ AM+ CM+
0 weeks 100.00% 100.00% 100.00%
100.00% 100.00% 100.00%
1 week 46.79% 86.47% 67.53% 68.63% 76.34% 86.98%
2 weeks 50.31% 129.91% 76.89% 70.46% 62.91% 50.39%
3 weeks 39.60% 138.04% 60.79% 77.26% 86.63% 60.35%
4 weeks 41.94% 53.59% 35.65% 41.84% 36.62% 27.74%
Example 9
Comparison of Cell Viability Testing Post-Lyo vs. Post-Cryo
1. Amnion/Chorion Bilayer with Trophoblast (AM+/CM+) samples were processed
and
either lyopreserved as previously described in Example 6 but with
Lyoprotectant Solution
containing 0.3M trehalose and no EGCG, or cryopreserved as described below.
Additionally, Amnion single layer and Chorion single layer with Trophoblast
samples were
also processed and either lyopreserved in the same manner as the lyopreserved
bilayer
samples, or cryopreserved in the same manner as the cryopreserved bilayer
samples.
Cryopreservation:
1. Tissue was processed as described in Example 6 up through Step 20 (cutting
5cm x 5cm
samples and placing each into a retainer in HB SS until all cutting was
complete).
2. After all samples were cut, the samples in retainers designated for
cryopreservation were
each placed into a film pouch. Each film pouch was filled with approximately
22.5mL of
cryopreservation media (Hyclone media with 10% DMSO), sealed, and then placed
into
an outer pouch. The samples and a representative probe sample were then
cryopreserved
using a controlled-rate freezer (Thermo Scientific CryoMed Model 7454) per the
recipe
provided in Table 24 below and stored then at -70 C or colder.
116
CA 03165002 2022-06-16
WO 2021/127230
PCT/US2020/065688
Table 24
CryoMed recipe
Step # Parameter
1 Cool chamber 2 C/min until Sample reaches -4 C
2 Cool chamber 25 C/min until Chamber reaches -60 C
3 Heat chamber 10 C/min until Chamber reaches -12 C
4 Cool chamber 1.5 C/min until Chamber reaches -40 C
Cool chamber 2 C/min until Sample reaches -100 C
6 Hold chamber at -100 C
7 End
Cell Viability Analysis via ATP Assay
5 1. Lyopreserved samples were rehydrated and went through cell viability
testing as
described in Cell Viability Analysis via ATP Assay in Example 6.
2. Cryopreserved samples were thawed in 0.9% normal saline and then rinsed in
0.9%
saline for 5 minutes instead of rehydration preparation, before continuing
with cell
viability testing as described in Cell Viability Analysis via ATP Assay above.
3. Results of cell viability testing are provided in Table 25 below.
Table 25
Post-Lyo & Post-Cryo Amnion/Chorion Cell Viability Results
Iteration #11
Post-Cryo Post-Lyo
AM+ CM+ AM+ CM+
From
100.0% 100.0% 18.7% 17.8%
Bilayer
Single
100.0% 100.0% N/A 26.4%
Layer
*The Post-Lyo AM+ Single Layer sample could not be retrieved from the
packaging and tested
On average the lyopreserved samples contained about 20-30% of the ATP content
of the
corresponding cryopreserved samples.
117