Note: Descriptions are shown in the official language in which they were submitted.
WO 2021/163602
PCT/US2021/018007
RECOMBINANT THERAPEUTIC INTERVENTIONS FOR CANCER
CROSS-REFERENCE TO RELATED APPLICATIONS
100011 This application claims the benefit of priority under 35
U.S.C. 119 (c) of U.S. Patent
Application No. 16/790,161 filed on February 13, 2020, which is a continuation-
in-part (CIP) of
16/638,943 filed on February 13,2020, that is a 35 U.S.C. 371 U.S. national
entry of International
Application PCT/US2019/022341, having an international filing date of March
14, 2019, which
claims the benefit of U.S. Provisional Application No. 62/658,661, filed April
17, 2018, the content
of each of the aforementioned applications is herein incorporated by reference
in their entirety.
INCORPORATION OF SEQUENCE LISTING
[0002] The material in the accompanying sequence listing is
hereby incorporated by reference
into this application. The accompanying sequence listing text file, named
JHU4280_2W0 Sequence Listing.txt, was created on February 11, 2021, and is 157
kb. The file
can be assessed using Microsoft Word on a computer that uses Windows OS.
STATEMENT OF GOVERNMENTAL INTEREST
[0003] This invention was made with government support under
grant nos. AI036973,
AI037856, awarded by the National Institutes of Health. The government has
certain rights in the
invention.
BACKGROUND OF THE INVENTION
[0004] Urothelial cancer of the bladder is the most common type
of bladder cancer (BC) in
North America, South America, Europe and Asia. Non-Muscle Invasive Bladder
Cancer (NMIBC)
is associated with a high recurrence rate, frequent intravesical treatments,
risk of progression to
advanced stages and the highest lifetime treatment among all cancers.
Intravesical BCG (bacillus
Calmette Guerin) instillation has been the standard of care treatment for
NMIBC for 30 years. It
is effective in 60-70% patients. BCG has shown to be a very effective vehicle
for delivery of
antigens. Many studies corroborating an underlying immune response skewed
towards a Type I
interferon and Th I induced mediated immune response show promise. Efforts to
generate
recombinant BCG (rBCG) strains for NMIBC have focused on developing strains
that augment
these anti-tumor immune responses. To date such efforts have not yielded
demonstrable
improvement over traditional BCG.
1
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
SUMMARY OF THE INVENTION
[0005] One embodiment of the present invention provides a vector
including a nucleic acid
sequence expressing a protein or functional part thereof that makes a STING
agonist including c-
di-AMP (also known as 3'-5' c-di-AMP); c-di-GMP (also known as 3"-5' c-di-
GMP); 3'-
3'cGAMP (also known as 3'-5', 3'-5'cGAMP, the product of the Vibrio cholerae
DncV protein);
2' -3'cGAMP (also known as 2' -5' , 3'-5' cGAMP, the product of the human cGAS
protein) and a
combination thereof, as examples. Some vectors of the present invention
include a nucleic acid
sequence selected from the group consisting of a first nucleic acid sequence
encoding a Ry1354c
protein, or a functional part thereof; a second nucleic acid sequence encoding
a 3'-3' cyclic GMP-
AMP synthase (DncV) protein, or a functional part thereof; a third nucleic
acid sequence encoding
a 2'-3'cyclic GMP-AMP synthase (cGAS) protein, or a functional part thereof; a
fourth nucleic
acid sequence encoding a DNA integrity scanning (disA) protein, or a
functional part thereof and
a combination thereof. Each of these nucleic acid sequences express proteins
that make one or
more of the STING agonist as described in the definition section of the
specification. Some vectors
of the present invention include in addition to one or more of the sequences
listed above a fifth
nucleic acid sequence encoding a PanC protein and a PanD protein or functional
part thereof.
Vectors including a nucleic acid sequence encoding a PanC protein and a PanD
protein or
functional part thereof are typically free of an antibiotic resistance gene.
Suitable vectors used in
the present invention may include vectors that replicate episomally in
multiple copies, or vectors
that integrate into a bacterial chromosome in single copy or are otherwise
present in the bacterial
cell. A vector of the present invention may stably integrate into a bacterial
genome or it may stably
replicate as an episomal plasmid. Suitable third nucleic acid sequences
include those that
overexpress the cyclase domains of the cyclic GMP-AMP synthase (cGAS) protein.
Other suitable
third nucleic acid sequence may express a cyclic GMP-AMP synthase (cGAS)
protein haying a
regulatory DNA recognition capability that is non-functional. Vectors of the
present invention may
also include nucleic acid sequences that encode sequences or proteins that
knock out the expression
of PDE genes of a strain of Mycobacteria used in the present invention.
[0006] Another embodiment of the present invention provides a
strain of Mycobacleria
including any one of the vectors of the present invention including a vector
comprising a protein
2
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
or functional part thereof that makes a STING agonist. As mentioned above,
examples of STING
agonist include c-di-AMP (also known as 3'-5' c-di-AMP); c-di-GMP (also known
as 3'-5' e-di-
GMP); 3'-3'cGAMP (also known as 3'-5', 3'-5'cGAMP, the product of the Vibrio
cholerae DncV
protein); 2'-3'cGAMP (also known as 2'-5', 3'-5' cGAMP, the product of the
human cGAS
protein) and a combination thereof, as examples. Examples of suitable nucleic
acid sequence
includes a nucleic acid sequence selected from the group consisting of a first
nucleic acid sequence
encoding a Ry1354c protein, or a functional part thereof; a second nucleic
acid sequence encoding
a 3' -3' cyclic GMP-AMP synthase (DncV) protein, or a functional part thereof;
a third nucleic acid
sequence encoding a 2'-3' cyclic GMP-AMP synthase (cGAS) protein, or a
functional part thereof;
a fourth nucleic acid sequence encoding a DNA integrity scanning (dis A)
protein, or a functional
part thereof and a combination thereof. Examples of suitable strains of
Mycobacterium used in the
present invention include Mycobacterium tuberculosis, Mycobacterium bovis, or
a combination
thereof, for example. Another strain used in the present invention is
Mycobacterium bacillus
Calmette Guerin (BCG). A strain of Mycobacteria used in the present invention
may be a
panthothenate auxotroph of BCG lacking its pan CD genetic operon. pan CD
auxotoph strains lack
genomic sequences able to encode functional PanC and/or PanD protein. In some
embodiments,
strains of Mycobacteria that are pantothenate auxotrophs comprise vectors of
the present invention
including apanCD nucleic acid encoding the PanC and PanD proteins or
functional parts thereof.
Vectors of the present invention that include panCD nucleic acid sequences are
preferably free of
antibiotic resistant genes or nucleic acid sequences that encode functional
proteins providing
antibiotic resistance. Mycobacteria that are pantothenate auxotrophs of the
present invention are
preferably free of a genomic antibiotic resistant gene or unable to encode
functional proteins that
provide antibiotic resistance.
[0007] Another embodiment of the present invention provides a
pharmaceutical composition,
including any one of the strains of Mycobacteria of the present invention, and
a pharmaceutically
acceptable carrier.
[0008] Another embodiment of the present invention provides a
method of eliciting a type 1
interferon response, enhancing the expression of pro-inflammatory cytokine,
and/or eliciting
trained immunity in a subject including the steps of: administering a
pharmaceutical composition
including anyone of the strains of the present invention into a subject; and
eliciting a type 1
3
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
interferon response, enhancing the expression of pro-inflammatory cytokine,
and/or eliciting
trained immunity in the subject. In one aspect, the pharmaceutical composition
is administered
into the bladder of the subject by a catheter.
100091 Another embodiment provides a method of using a strain of
Mycobacteria of the
present invention to treat or prevent cancer in a subject. The method includes
the steps of:
administering a pharmaceutical composition including a strain of Mycobucteriu
including a vector
expressing a protein that makes a STING agonist or a functional part thereof
to a subject having
cancer; and treating or preventing cancer in the subject. The present
invention may be used to treat
or prevent cancers including epithelial cancers, breast cancer, non-muscle
invasive bladder cancer,
as examples. In some aspects, the cancer is a BCG-unresponsive non-muscle
invasive bladder
cancer (BCG-unresponsive NMIBC) and the pharmaceutical composition is
administered by
intravesical instillation. In some aspects, the cancer is a BCG-ntifive non-
muscle invasive bladder
cancer (BC G -n alve NM IBC) and the pharmaceutical composition is
administered by i ntrav es i c al
instillation. In other aspects, the cancer is selected from the group
consisting of colon cancer,
uterine cancer, cervical cancer, vaginal cancer, esophageal cancer,
nasopharyngeal cancer,
endobronchial cancer, and a combination thereof and the pharmaceutical
composition is
administered to a luminal surface of the epithelial cancer. In some aspetcs,
the cancer is selected
from a solid tumor or a liquid tumor and the pharmaceutical composition is
administered by
intratumoral injection and/or by systemic infusion. The methods of the present
invention may
include the step of administering a checkpoint inhibitor, such as an anti-PD1
antibody, an anti-
PDL1 antibody, or a combination thereof, as example. In another aspect, the
cancer is bladder
cancer and the pharmaceutical composition is administered via a catheter.
100101 One embodiment of the present invention provides an
expression vector including a
first nucleic acid sequence encoding a Rv1354c protein, or a functional part
thereof; a second
nucleic acid sequence encoding a cyclic GMP-AMP synthase (DncV) protein, or a
functional part
thereof; a third nucleic acid sequence encoding a cyclic GMP-AMP synthase
(cGAS) protein, or a
functional part thereof; a fourth nucleic acid sequence encoding a DNA
integrity scanning (disA)
protein which functions as a diadenylate cyclase, or a functional part
thereof, or a combination
thereof Some expression vectors of the present invention include a first
nucleic acid sequence that
overexpresses the cyclase domains of the Rv1354c protein when compared to the
expression of a
4
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
native Rv1354c protein as a reference. Some expression vectors of the present
invention include a
second nucleic acid sequence that overexpresses the cyclic GMP-AMP synthase
(DncV) protein,
when compared to the expression of a native DncV protein. Some expression
vectors of the present
invention include a third nucleic acid sequence that vet-expresses the
cyclasc domains of the cyclic
GMP-AMP synthase (cGAS) protein when compared to the expression of a native
cGAS protein.
Suitable Rv1354 proteins used in the present invention include a Mycobacterium
tuberculosis
Rv1354 protein. Suitable DncV proteins used in the present invention include a
Vibrio cholera
DncV protein. Suitable cGAS proteins used in the present invention include a
Homo sapiens cGAS
protein. Suitable DisA proteins used in the present invention include a
Mycobacterium tuberculosis
disA protein.
[0011] Another embodiment of the present invention provides a
strain of BCG including a
cdnP gene, an Rv1354c gene, an Rv1357c gene, or a combination thereof, wherein
the cdnP gene
is unable to express a functional cyclic di-nucleotide phosphodiesterase
(CdnP) protein, the
Rv1354e gene is unable to express a functional Rv1345c protein, and/or the
Rv1357c gene is
unable to express a functional Rv1357 protein. Some BCG strains of the present
invention may
have an Rv1354c gene that includes a non-functional EAL domain. The BCG
strains of the present
invention may include any of the expression vectors of the present invention.
[0012] Another embodiment of the present invention provides a
method of treating or
preventing bladder cancer including the steps of: administering a
pharmaceutical composition
including a strain of BCG including an expression vector of the present
invention into the bladder
of a subject; and treating or preventing bladder cancer in the subject when
compared to a reference
subject who was not administered the pharmaceutical composition. The
pharmaceutical
composition may be administered by any suitable means including by a catheter.
[0013] Another embodiment of the present invention provides a
method of eliciting a type 1
interferon response in a subject including the steps of: administering a
pharmaceutical composition
including a strain of BCG including an expression vector of the present
invention into the subject
such as the subject's bladder; and enhancing a type 1 interferon response in
the subject compared
to a reference subject not administered the pharmaceutical composition.
[0014] Another embodiment of the present invention provides a
method of treating or
preventing cancer in a subject including the steps of: administering a
pharmaceutical composition
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
comprising a strain of BCG including an expression vector of the present
invention into a tumor
of a subject having cancer; and treating or preventing cancer in the subject
when compared to a
reference subject not administered the pharmaceutical composition. The
pharmaceutical
composition may be administered by any suitable means including injection into
the tumor.
Cancers that may be treated or prevented by this method include, but are not
limited to, breast
cancer, and/or non-muscle invasive bladder cancer.
[0015] Examples of Mycobacteria used in the present invention
include Mycobacterium
tuberculosis, Mycobacterium bovis, Mycobacterium bovis Bacillus Calmette
Guerin (referred to a
BCG), Mycobacterium smegmatis, Mycobacterium avium complex, and other non-
tuberculous
mycobacteria (NTM). Examples of BCG strains used in the present invention
including those that
overexpress STING agonists, include BCG Pasteur, BCG-Pasteur-Aeras, BCG Tice
(also known
as BCG Chicago), BCG-Connaught (also known as BCG Toronto), BCG Danish, BCG-
Prague
(also known as BCG Czechoslovakian), BCG Russia (also known as BCG Moscow),
BCG Moreau
(also known as BCG Brazil), BCG Japan (also known as BCG Tokyo), BCG Sweden
(also known
as BCG Gothenburg), BCG Birkhaug, BCG Glaxo, BCG Frappier (also known as BCG
Montreal),
BCG Phipps, or other available BCG strains.
[0016] Another embodiment of the present invention provides a
method of treating diabetes
including the steps of: administering a pharmaceutical composition including a
strain of
Mycobacteria including a vector expressing a protein or a functional part
thereof that makes a
STING agonist to a subject having diabetes; and treating or preventing
diabetes in the subject by
providing trained immunity. Trained immunity refers to the ability of one
antigenic stimulus to
elicit more potent immune responses to a second, different antigenic stimulus
introduced at a later
time. Trained immunity is antigen independent, based on heterologous CD4 and
CD8 memory
activation, cytokine mediated, and is associated with epigenetic and metabolic
changes. The
method results in the upregulation of glycolysis mediated by the trained
immunity. The
aforementioned up-regulation of glyeolysis is beneficial in preventing and
treating type 1 and type
2 diabetes mellitus.
[0017] Another embodiment of the present invention provide a
method of stimulating trained
immunity in a subject including the steps of: administering a pharmaceutical
composition
including a strain of Mycohacteria including a vector expressing a protein or
a functional part
6
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
thereof that makes a STING agonist to a subject; and stimulating trained
immunity in the subject.
Upregulating glycolysis in the subject and/or stimulating episomal changes in
hi stone methylation
in the subject mediate trained immunity in the subject.
[0018] Another embodiment of the present invention provides a
method of treating or
preventing a viral infection in a subject including the steps of:
administering a pharmaceutical
composition including a strain of Mycobacteria including a vector expressing a
protein or a
functional part thereof that makes a STING agonist to a subject; and treating
or preventing the
viral infection in the subject. Stimulating trained immunity in the subject
treats or prevents the
viral infection in the subject. Upregulating glycolysis in the subject and/or
stimulating episomal
changes in histone methylation in the subject mediate trained immunity in the
subject.
[0019] Another embodiment of the present invention provides a
method of treating or
preventing a bacterial infection, or a drug-resistant bacterial infection in a
subject including the
steps of: administering a pharmaceutical composition including a strain of /14-
ycobacteria including
a vector expressing a protein or a functional part thereof that makes a STING
agonist to a subject;
and treating or preventing the bacterial infection or the drug-resistant
bacterial infection in the
subject. Stimulating trained immunity in the subject treats or prevents the
bacterial infection in
the subject. Upregulating glycolysis in the subject and/or stimulating
episomal changes in histone
methylation in the subject mediate trained immunity in the subject. The
methods of the present
invention may use one or more of the vectors of the present invention or one
or more strain of
bacteria including a vector of the present invention.
[0020] Another embodiment of the present invention provides a
method of suppressing the
expression of myeloid-derived suppressor cells (MDSCs), M2 macrophages, and
Treg cells in a
tumor and inducing the expression of macrophages, dendritic cells (DCs), and T
effector cells in a
tumor. The method includes the steps of administering a pharmaceutical
composition including a
strain of Mycobacteria including a vector expressing a protein that makes a
STING agonist or a
functional part thereof to a subject having a tumor; suppressing the
expression of MDSCs, M2
macrophages, and Treg cells in the tumor; and inducing the expression of
macrophages, DCs, and
T effector cells in the tumor. An example of Ml macrophages having induced
expression in a
tumor includes Ml macrophages. An example of T effector cells having induced
expression in a
tumor includes CD4+ T cells and CDS+ T cells. Suppressing the expression of
MDSCs, M2
7
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
macrophages, and Treg cells in the tumor of subjects administered a
Mycobacteria including a
vector of the present invention is observed when compared to the expression of
MDSCs, M2
macrophages, and Treg cells in a tumor of a referenced subject not
administered a pharmaceutical
composition including the strain of Mycobacteria. Inducing the expression of
macrophages, DCs,
and T effector cells in a tumor is observed when compared to the expression of
macrophages, DCs,
and T effector cells in a tumor of a referenced subject not administered a
pharmaceutical
composition comprising the strain of Mycobacteria. Examples of suitable STING
agonist include
3'-5' c-di-AMP (also known as c-di-AMP); 3'-5' c-di-GMP (also known as c-
diGMP); 3'-
3' cGAMP; 2'-3'eGAMP and a combination thereof. A suitable vector of the
present invention
may include a nucleic acid sequence selected from the group consisting of a
first nucleic acid
sequence encoding a Ry1354c protein, or a functional part thereof; a second
nucleic acid sequence
encoding a 3'-3'cyclic GMP-AMP synthase (DncV) protein, or a functional part
thereof; a third
nucleic acid sequence encoding a 2'-3' cyclic GMP-AMP synthase (cGAS) protein,
or a functional
part thereof; a fourth nucleic acid sequence encoding a DNA integrity scanning
(DisA) protein, or
a functional part thereof and a combination thereof. The tumor may be a
epithelial cancer, a breast
cancer, or a non-muscle invasive bladder cancer, and melanoma as examples. In
some aspects, the
tumor may be a non-muscle invasive bladder cancer such as a BCG-unresponsive
non-muscle
invasive bladder cancer (BCG-unresponsive NMIBC) and the pharmaceutical
composition can be
administered by intravesical instillation. In other aspects, the tumor may be
a non-muscle invasive
bladder cancer such as a BCG-naive non-muscle invasive bladder cancer (BCG-
nalve NMIBC)
and the pharmaceutical composition can be administered by intravesical
instillation. In other
aspects, the tumor may be an epithelial cancer selected from the group
consisting of colon cancer,
uterine cancer, cervical cancer, vaginal cancer, esophageal cancer,
nasopharyngeal cancer,
endobronchial cancer, and a combination thereof and the pharmaceutical
composition can be
administered to a luminal surface of the epithelial cancer. In other aspects,
the tumor is a solid
tumor and the pharmaceutical composition is administered by intratumoral,
intravenous,
intradermal, transdermal, intravesical topical, intramuscular or subcutaneous
injection. In other
aspects, the tumor is a liquid tumor and the pharmaceutical composition is
administered by
intravenous, intradermal, transdermal, intravesical topical, intramuscular or
subcutaneous
injection. Methods of the present invention may further comprise the step of
administering a
8
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
checkpoint inhibitor. Suitable checkpoint inhibitors that may be used in the
present invention
include ipilimumab (anti-CTLA-4 antibody), nivolumab (anti-PD-1 antibody),
pembrolizumab
(anti-PD-1 antibody), cemiplimab (anti-PD-1 antibody), atezolizumab (anti-PD-
L1 antibody),
avclumab (anti-PD-Li antibody), durvalumab (anti-PD-L1 antibody) and a
combination thereof
BRIEF DESCRIPTION OF THE DRAWINGS
[0021] This application is continuation- in- part (CIP) of
16/638,943, all the figures from US
serial number 16/638,943 are herein incorporated by reference in their
entirety.
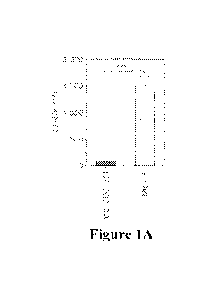
[0022] FIGs 1A-1B Mycobacteria overexpressing disA from the pSD5B
Phsp6o::disA plasmid
construct release large amounts of c-di-AMP into the macrophage cytosol and
transcribe high
levels of disA mRNA. FIG. 1A. J774 macrophages infected with M.tb harboring
the pSD5B
Phsp60::disA plasmid or wild type M.tb (CDC1551) at all MOI of 1:20.
Intramacrophage levels of
c-di-AMP were determined by LC-MS/MS after 24 hours of infection. As can be
seen, the M.tb-
disA-OE strain produces ¨15-fold more c-di-AMP than wild type M.tb (CDC1551).
The BCG-
disA-OE would be expected to show similarly high levels of c-di-AMP. (Data are
from Dey B,
Dey RJ, Cheung LS, Pokkali S, Guo H, Lee JH, Bishai WR. A bacterial cyclic
dinucleotide
activates the cytosolic surveillance pathway and mediates innate resistance to
tuberculosis. Nat
Med. 2015;21:401-6. PMID: 25730264.) FIG. 1B. BCG-Pasteur harboring the pSD5B
Phsp6o::disA
plasmid or BCG-Pasteur-WT were grown to mid-exponential phase. Bacteria were
lysed and
mRNA was prepared. The levels of disA mRNA were determined by quantitative RT-
PCR. The
BCG-disA-OE strain produces ¨50-fold more disA mRNA than BCG-Pasteur-WT.
[0023] FIG. 2. BCG overexpressing disA augments pro-inflammatory
eytokines. Gene
expression profiling (qPCR) of pro-inflammatory cytokines and IFNA3 in mouse
BMDMs
challenged with wild-type and disA overexpression strains of BCG-Pasteur.
[0024] FIG. 3. BCG overexpressing disA augments IRF3 signaling.
Effect of disA
overexpression on activation of IRE pathway measured by IRF-SEAP QUANTI Blue
reporter
assay. The culture supernatants of infected RAW-Blue ISG cells were assayed
for IRF activation.
The image below the IRF-activation graph represents QUANTI Blue assay plate
and sample wells;
treatment parameters for column of wells correspond to those defined for the
bars above aligned
with the wells. BCG-disA-OE in this figure is derived from BCG Pasteur.
9
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
[0025] FIG s 4A-4C. Increased pro-inflammatory cytokines in
response to disA
overexpression. FIG. 4A shows differential expression of TNF-a. FIG. 4B shows
differential
expression of IL-6. FIG. 4C shows differential expression of IL-113.Mouse
BMDMs were
challenged with wild-type and disA overexpression strains of BCG-Pasteur.
Culture supernatants
were assayed by ELISA for different cytokines.
[0026] FIG. 5. BCG overexpressing disA induces differential
immune response in human
bladder cancer cells (RT4). Differential gene expression in human RT4 bladder
cancer cells
challenged with wild-type BCG-Pasteur, wild-type BCG-Tice strain, and BCG-
Pasteur-disA-OE
Expression levels of mRNA was measured using a SYBR green-based quantitative
real-time PCR.
[0027] FIG. 6. Schematic workflow of testing relative therapeutic
efficacy of wild-type and
BCG-disA-OE strains.
[0028] FIG. 7. Tumor involvement index of tumor-bearing rats
untreated or treated with WT
BCG or rBCG overexpressing disA (rBCG = BCG-Pasteur-disA-0E; wtBCG = BCG-
Pasteur).
[0029] FIG. 8. Immune profiling of MNU-induced Fisher rat urinary
bladder tumors in
response to intravesical therapy using different strains of BCG. Differential
gene expression in rat
bladder tumor cells after therapy with wild-type and disA overexpression
strains of Mycobacterium
bovis BCG-Pasteur. Expression levels of mRNA were measured using a TaqMan-
based
quantitative real-time PCR. BCG-WT is BCG Pasteur and BCG-disA-OE was derived
from BCG
Pasteur.
[0030] FIG. 9. Gene expression profiling of bladders from MNU
tumor bearing rats untreated
or treated with WT or rBCG overexpressing disA.
[0031] FIG. 10. Summary of relative gene expression by BCG-disA-
OE versus BCG-WT in
different cells or tissues. Mouse bone marrow-derived macrophages (BMDM),
human
immortalized bladder cancer cell lines RT4 and 5637, and rat immortalized
bladder cancer cell
lines were infected with BCG-disA-OE and BCG-WT for 24 hours and mRNA was
prepared from
the cells. Rats were exposed to MNU by intravesical instillation over 8 weeks
and then treated
with either BCG-disA-OE or BCG-WT by intravesical instillation for 8 weeks.
Bladders were
removed upon necropsy at week 16, and mRNA was prepared. Quantitative RT-PCR
for the
cytokine or chemokine genes indicated was performed. The changes shown are the
fold-induction
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
or reduction observed with BCG-disA-OE normalized to that seen with BCG-WT.
BCG-WT is
BCG Pasteur and BCG-disA-OE was derived from BCG Pasteur.
[0032] FIG. 11. Diagram of two cyclic dinucleotide cyclase and
phosphodiesterase proteins
present in BCG: BCG_RS07340 and BCG_AHM07112. BCG_RS07340 is a bifunctional
protein
with both CDN cyclase and CDN PDE activities. BCG AHM07112 is a CDN PDE. The
domains
are: GAF (regulatory), GGDEF (diguanylate cyclase), and EAL diguanylate
phosphodiesterase.
[0033] FIG. 12. M. tuberculosis harboring the pSD5B Phsp60::disA
plasmid (Mtb-disA-OE or
Mtb-OE) is significantly attenuated for virulence in mice compared to wild
type M.tb (Mtb-
CDC1551). 6-7-week-old female BALB/c mice (n = 10 per group) were infected as
described
above with ¨3.5 logio CFU by aerosol infection. Day 1 CFU counts were
performed on 3 mice
in each group and confirmed the implantation of 3.5 log10 CFU units. Mice were
held until
death. As can be seen, the median time to death for wild-type M tuberculosis
infection was
150.5 days. In contrast, mice infected with the same inoculum of Mtb-disA-OE
(Mtb-OE) had
a median time to death of 321.5 days (p < 0.001). The BCG-disA-OE is expected
to show similar
loss of virulence in mice compared with BCG-WT. (Data are from Dey B, Dey RJ,
Cheung LS,
Pokkali S, Guo H, Lee JH, and Bishai WR. A bacterial cyclic dinucleotide
activates the cytosolic
surveillance pathway and mediates innate resistance to tuberculosis. Nat Med.
2015; 21: 401-6.
PMID: 25730264.)
[0034] FIGs 13A-13B. Other BCG strains are also active: BCG Tice
strain overexpressing
disA also shows induction of proinflammatory cytokincs similar to BCG Pasteur
overexpressing
disA. Bone marrow derived macrophages were challenged with wild-type and disA
overexpressing
strains of both BCG Pasteur and BCG Tice strains at an M.O.I of 1:20 for 15 h.
Culture
supernatants were harvested and probed for cytokines using EL1SA. FIG. 13A
shows Bdifferential
expression pattern of TNF-a. FIG. 13B shows differential expression of IL-6.
Mouse BMDM
were challenged with the two different strains of BCG. The BCG-Tice strain was
from the
commerci ally avail able Onco-Tice product.
[0035] FIG. 14. Type I interferon responses in macrophages in
response to BCG-disA-OE are
STING-dependent. Bone marrow-derived macrophages from STING-ablated (KO) and
control
mouse were challenged with wild-type and disA OE strains of BCG Pasteur for 24
h. Culture
supernatants were probed for 1EN- 13 levels using EL1SA.
11
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
[0036] FIG. 15 shows that intravesical instillation of BCG-disA-
OE displays greatest
antitumor efficacy (statistically significant improvement in pathology) in the
MNU carcinogen
model of non-muscle invasive bladder cancer (NIMBC). Groups of rats received 4
intravesical
treatments with MN U over the first 8 weeks (one treatment every 2 weeks) to
elicit NIMBC. Over
the next 8 weeks they received 4 intravesical treatments with either PBS
(untreated), BCG-WT, or
BCG-disA-OE (one treatment every 2 weeks). At the end of the 16-week
experiment, rats were
sacrificed, and their bladders were removed. A portion of the bladder was
fixed and subjected to
H&E staining and then interpreted in a blinded fashion by a Board-certified
urologic pathologist.
The tumor involvement score and cancer stage (T2-3, Ti, CIS + papillary
lesions, CIS alone, or
normal-dysplastic) were determined and are shown. As may be seen BCG-disA-OE
instillation
resulted into statistically significantly lower tumor involvement index than
PBS (untreated) while
BCG-WT was not statistically significantly superior to PBS. This 16 -week
experiment was
performed twice. The data in Fig 7 represent the results of Experiment 1. The
data in this figure
(Figure 15) represent the combined results of Experiment 1 plus Experiment 2.
The qPCR data
shown in Figure 8 and Figure 9 were obtained using bladder tissue at necropsy
from the end of
Experiment 1.
[0037] FIG. 16 shows that BCG-disA-OE reduces Tregs
(CD4+CD25+Foxp3 ) in murine
syngeneic bladder cancer tumors. Mice were implanted on the flank with 5 x 106
BBN975 murine
bladder cancer tumor cells. When the tumors were 1.5 cm in diameter, mice
received 3 intratum oral
injections of either PBS (control), BCG-WT, or BCG-disA-OE (one treatment
every 2 days). Two
days after the last intratumoral treatment, mice were sacrificed, and their
spleens and tumors were
removed. After tumor cell dispersal, the cell preparations were stained and
subjected to flow
cytometry. As may be seen BCG-disA-OE led to reduced tumor C134 Tregs, reduced
tumor CD8
Tregs, and reduced spleen CD4+ Tregs.
[0038] FIGs 17A-17B show that BCG-disA-OE is safer than BCG-WT in
two mouse models.
FIG. 17A shows that groups of BALB/e mice (immunocompetent) were exposed to 1
x 103 CFU
(confirmed by sacrificing a group of mice and determining day 1 lung CFU
counts) of either BCG-
WT or BCG-disA-OE using a Glas-Col aerosolization chamber. After 4 weeks, the
mice were
sacrificed from each group, their lungs were removed, homogenized, and plated
on 7H11 agar
plates. The figure shows the mean CFU counts for the BCG-WT and BCG-disA -OE-
infected
12
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
mouse lungs. As may be seen a statistically significantly lower lung CFU
burden was observed
with BCG-disA -OE compared with BCG-WT. FIG. 17B shows that groups of SCID
mice
(immunosuppressed) were exposed to 1 x 102 CFU (confirmed by sacrificing a
group of mice and
determining day 1 lung CFU counts) of either BCG-WT or BCG-disA-OE using a
Glas-Col
aerosolization chamber. A third group was uninfected. The figure shows a
Kaplan-Meier survival
curve for the groups of mice. As may be seen BCG-disA-0E-infected mice had a
statistically
significantly longer survival time than BCG-WT-infected mice.
[0039] FIG. 18 shows that BCG-disA-OE elicits statistically
significantly higher levels of
"Trained Immunity immunological and epigenetic marks" in CD14+ human monocytes
than does
BCG-WT. "Trained Immunity" refers to the ability of a first immunologic
stimulus to induce
increased immune responses to a second antigenically different stimulus give
subsequently. In this
experiment, CD lzkhuman monocytes were prepared from LeukoPaks collected by
apheresis. On
day 0 they were infected with either BCG-WT or BCG-disA -OE at a MOT of 5:1
for 3 hours. A
third group of cells were not infected. After infection, cells were washed
multiple times (every two
days). After a 6-day rest period, the monocytes were re-stimulated with the
TLR1/2 agonist
PAM3CSK4 for 2 hours. Cells were washed repeatedly and were subsequently
incubated for 24 h.
Th levels of secreted IL-1 13 were measured in the culture supernatants by
ELISA. As may be seen,
while BCG-WT itself elicited statistically significantly higher levels of
immune response to the
second stimulus compared to uninfected cells, BCG-disA-OE elicit statistically
significantly more
of a response than either BCG-WT or uninfected cells.
[0040] FIG. 19 shows that BCG-disA-OE elicits a greater histone
activation mark (H3K4-
trimethylation) in the IL6 and TNF gene promoter regions than BCG-WT. "Trained
Immunity"
refers to the ability of a first immunologic stimulus to induce increased
immune responses to a
second antigenically different stimulus give subsequently. Trained immunity
has been associated
with epigenetic modifications, such as histone methylation, in the promoter
region of cytokines
and other immune mediators. The experiment shown in Figure 19 was performed in
the same set
of cells and exactly the same way as that described in Figure 18 except that
after the second
stimulus with the TLR1/2 agonist PAM3CSK4 (abbreviated PAM3), cells were
harvested fixed,
chromatins were cross-linked and DNA was collected for chromatin
immunoprecipitation analysis
(ChM) using an antibody specific for the H3K4-me3 histone methylati on mark.
H3K4-me3 is
13
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
known to be a gene activating mark. The graph shows the relative fold change
in abundance of
immunoprecipitated DNA as measured by quantitative PCR using primers for the
IL6 and 'TNF
gene promoter region. As may be seen both BCG-Pasteur-di.sA-OE and BCG-Tice-
disA-OE led to
significantly greater levels of H3K4 histonc trimethylation in thc 1L6 and TNF
promoter regions
than did their corresponding BCG-WT strains following challenge with the
second stimulus,
PAM3C SK4.
[0041] FIG. 20 shows the successful construction of BCG-Tice-disA-
0E. The inventors'
previous work had utilized BCG-Pasteur to construct BCG-Pasteur-disA-0E. This
strain was
provided to one of the inventors by Dr. Frank Collins in 1995. It is the same
strain known as BCG-
Pasteur-Aeras. BCG-Tice is manufactured and sold by Merck and is the sole FDA-
approved BCG
available in the United States. The inventors purchased BCG-Tice, prepared
electrocompetent
BCG-Tice, and electroporated the pSD5-hsp60-MT3692 plasmid into BCG-Tice. The
drawing
shows the results of colony PCRs for 5 kanamycin-resistant candidate clones of
transformed BCG-
Tice and confirms the successful preparation of BCG-Tice-disA-OE by
electroporation of the
pSD5-hsp65-MT3692 plasmid into BCG-Tice. Note on nomenclature, the inventors
had
previously referred to this same plasmid pSD5-hsp60-MT3692. However, the
actual promoter in
this strain is the promoter for the hsp65 gene of M leprae. Thus, the
inventors now more correctly
refer to the plasmid as pSD5-hsp65-MT3692.
[0042] FIG. 21 shows that clone 2 ofBCG-Tice-disA -OE from the
transformation experiment
shown in Figure 20 strongly expresses the disA gene. Real time PCR was used to
show differential
disA expression in four different BCG-Tice-disA-OE clones. Gene expression was
measured in
total RNA isolated from the late log phase cultures using log phase cultures
using SYBR green
based quantitative real-time PCR. The graphical data points represent the mean
of 3 independent
experiments standard error mean (SEM). M tuberculosis sigA (Rv2703) was used
as an internal
control. Data analysis was performed using 2-AAcT method. Student's t test
followed by Welch
correction (***P<0.001; **P<0.01). The inventors created seedlots of BCG-Tice-
disA-OE clone
2 and refer to this clone as simply "BCG-Tice-disA-0E" in all subsequent work.
[0043] FIG. 22 shows potent, statistically significantly enhanced
1RF3 induction in mouse
bone marrow-derived macrophages infected with BCG-Pasteur-disA-OE compared
with BCG-
Pasteur-WT. Mouse (C57BL/6) bone marrow-derived macrophages were infected with
wild-type
14
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
and disA overexpressing strains of BCG Pasteur (20 MOI) for 3h. Cells were
washed with warm
DPBS to remove non-internalized bacilli and were subsequently incubated for
another 3 hours.
IRF3 expression was measured in total RNA isolated from the cell lysate using
SYBR green based
quantitative real-time PCR. The graphical data points represent the mean of 3
independent
experiments standard error mean (SEM). Mouse beta-actin was used as an
internal control. Data
analysis was performed using 2-'2T method. Student's t test followed by Welch
correction
(***P<0 001 ; **P<0.01).
[0044] FIG. 23 shows that STING is required for enhanced type I
IFN (TENT) induction in
response to BCG-WT and BCG-disA-OE. Mouse (C57BL/6) bone marrow-derived
macrophages
from STING ablated (STING-KO) wild-type animals were infected with different
strains of BCG
(MOI=1:20) for 3 h. Cell were washed using warm DPBS to removed non-
internalized bacilli and
were subsequently incubated in for another 24 h before culture supernatants
were harvested.
ELISA for IFN-p was performed in culture supernatants as per the
manufacturer's instruction.
Data points represent the mean of three independent biological experiments
standard error mean
(S.E.M.). Student's t test followed by Welch correction (**P<0.01).
[0045] FIGs 24A-24C shows that interferon-3 is induced murine
BMDMs, BMDCs and
J774.1 macrophages in upon exposure to disA overexpressing BCG strains and
that the TENT
response is statistically significantly greater for BCG-Pasteur-disA-OE and
BCG-Ti ce-disA -OE
than for the corresponding BCG-WT strains. Mouse (C57BL/6) bone marrow-derived
macrophages (BMDMs), and J774.1 macrophages were infected for 3h using
different strains of
BCG (MOI: 20). Non-internalized bacilli were washed using warm DPBS and cell
were incubated
for another 24 hours. IFN-P levels were quantified in culture supernatants
using ELISA as per
manufacturer's instruction. Data points represent three independent biological
experiments+
standard error mean (S.E.M.). Data analysis was performed using unpaired t-
test (***P<0.001;
**P<0.01; *P<0.05). FIG. 24A shows interferon-13 levels in murine BMDMs. FIG.
24B shows
interferon-I3 levels in murine BMDCs. FIG. 24C shows intcrferon-P levels in
murine J774.1
macrophages.
[0046] FIGs 25A-25C show that IL-6 is induced in mouse BMDMs,
BMDCs and J774.1
macrophages in response to exposure to disA overexpressing BCG strains and
that the IL-6
response is statistically significantly greater for BCG-Pasteur-disA-OE and
BCG-Tice-disA-OE
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
than for the corresponding BCG-WT strains. Mouse (C57BL/6) bone marrow-derived
macrophages (BMDMs), and J774.1 macrophages were infected for 3 h using
different strains of
BCG (MOI: 20). Non-internalized bacilli were washed using warm DPBS and cell
were incubated
for another 24 hours. 1L-6 levels were quantified in culture supernatants
using EL1SA as per
manufacturer's instruction. Data points represent three independent biological
experiments
standard error mean (S.E.M.). Data analysis was performed using unpaired t-
test (***P<0.001;
**13<0.01; *P<0.05). FIG. 25A shows IL-6 levels in murine BMDMs. FIG. 25B
shows IL-6 levels
in murine BMDCs. FIG. 25C shows IL-6 levels in murine J774.1 macrophages.
[0047] FIGs 26A-26C shows that TNF is induced in mouse BMDMs,
BMDCs and J774.1
macrophages in response to exposure to disA overexpressing BCG strains and
that the responses
are statistically significantly greater for BCG-Pasteur-disA-OE and BCG-Tice-
disA-OE than for
the corresponding BCG-WT strains. Mouse (C57BL/6) bone marrow-derived
macrophages
(BMDMs), and J774.1 macrophages were infected for 3h using different strains
of BCG (MOT:
20). Non-internalized bacilli were washed using warm DPBS and cell were
incubated for another
24 hours. TNF levels were quantified in culture supernatants using ELISA as
per manufacturer's
instruction. Data points represent three independent biological experiments
standard error mean
(S.E.M.). Data analysis was performed using unpaired t-test (***P<0.001;
**P<0.01; *P<0.05).
FIG. 26A shows TNF levels in murine BMDMs. FIG. 26B shows TNF levels in murine
BMDCs.
FIG. 26C shows TNF levels in murine J774.1 macrophages.
[0048] FIGs 27A-27B shows that TNF and IFN-y are induced in the
rat bladder carcinoma
NBT-II cell line in response to exposure to disA overexpressing BCG strains
and that the two
responses are statistically significantly greater for BCG-Pasteur-disA-OE and
BCG-Tice-disA-OE
than for the corresponding BCG-WT strains. NBT-1I cells were infected with
wild-type and
recombinant strains of BCG for 3h. Non-internalized bacilli were repeatedly
washed using warm
DPBS and cells were incubated for another 24 h. Culture supernatants were used
for quantification
of TNF and IFN-y. Data points represent three independent biological
experiments standard error
mean (S.E.M.). Data analysis was performed using unpaired t-test (***P<0.0001;
**P<0.001;
*P<0.05). FIG. 27A shows TNF levels in NBT-II cells. FIG. 27B shows IFN-y
levels in NBT-II
cells.
16
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
[0049] FIGs 28A-28D shows that of IFN-13, IFN-y, TNF and IL-113
in are induced the in the
human transitional cell papilloma RT4 bladder cancer cell line in response to
exposure to disA
overexpressing BCG strains and that the two responses are greater for BCG-
Pasteur-disA-OE and
BCG-Tice-disA-OE than for thc corresponding BCG-WT strains. RT4 cells were
infected with
wild-type and recombinant strains of BCG for 3h. Non-internalized bacilli were
repeatedly washed
using warm DPBS and cells were incubated for another 24 h. Culture
supernatants were used for
quantification of cytokines as per manufacturer's instruction. Data points
represent two
independent biological experiments+ standard error mean (S.E.M.). Data
analysis was performed
using unpaired t-test (***P<0.001; **P<0.01; *P<0.05). FIG. 28A shows IFN-13
levels in RT4
cells. FIG. 28B shows IFN-y levels in RT4 cells. FIG. 28C shows TNF levels in
RT4 cells. FIG.
28D shows IL-1[3 levels in RT4 cells.
[0050] FIG. 29 shows that BCG-disA-OE stimulates increased IFN-13
levels in multiple
bladder cancer cell lines to a greater degree than BCG-WT. The drawing shows
the levels of -ITN-
13 mRNA (relative expression by the 2-A cT method) following exposure to BCG-
WT, BCG-disA-
OE, and LPS. 5637 cells are human muscle-invasive bladder cancer cells, RT4
cells are human
transitional cell papilloma bladder cancer cells, and NBT-II cells are rat
bladder carcinoma cells
induced by N-butyl N ( 4 hydroxybutyl) nitrosamine.
[0051] FIGs 30A-30D shows the cytokine responses for IFN-13, IFN-
y, IL-6, and TNF in BCG-
WT and BCG-disA -OE-infected mouse lungs at different time points following
aerosol infection.
The drawing reveals that at most time points for most cytokines, the responses
are greater for BCG-
Pasteur-disA-OE and BCG-Tice-disA-OE than for the corresponding BCG-WT
strains. BALB/c
mice were infected by the aerosol route as described in Figure 19. Groups of
mice were sacrificed
at 2, 4, and 6 weeks after infection. Lung homogenates were prepared, and
cytokine levels were
quantified using ELISA as per manufacturer's protocol (n=4 animals/treatment
group S.E.M.).
Data analysis was performed using paired t-test (***P<0.001; **P<0.01;
*P<0.05). FIG_ 30A
shows IFN-[3 levels in BCG-WT and BCG-c/isA-0E-infected mouse lungs. FIG. 30B
shows IFN-
y levels in BCG-WT and BCG-disA-0E-infected mouse lungs. FIG. 30C shows IL-6
levels in
BCG-WT and BCG-disA-0E-infected mouse lungs. FIG. 30D shows TNF levels in BCG-
WT and
BCG-disA -OE-infected mouse lungs.
17
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
[0052] FIG s 31A-31D shows the cytokine responses for
IFN-y, IL-6, and TNF in BCG-
WT and BCG-disA-0E-infected mouse spleens at 4 weeks following aerosol
infection. The
drawing reveals that for most cytokines, the responses are greater for BCG-
Pasteur-disA-OE and
BCG-Ticc-disA-OE than for the corresponding BCG-WT strains. BALB/c mice were
infected by
the aerosol route as described in Figure 17. Groups of mice were sacrificed at
4 weeks after
infection. Spleen homogenates were prepared, and cytokine levels were
quantified using ELISA
as per manufacturer's protocol (n=4 animals/treatment group S.E.M.). Data
analysis was
performed using paired t-test (***P<0.001; **P<0.01; *P<0.05). FIG. 31A shows
IFN43 levels in
BCG-WT and BCG-disA-0E-infected mouse spleens. FIG. 31B shows IFNI, levels in
BCG-WT
and BCG-disA-0E-infected mouse spleens. FIG. 31C shows IL-6 levels in BCG-WT
and BCG-
disA-0E-infected mouse spleens. FIG. 31D shows TNF levels in BCG-WT and BCG-
disA-0E-
infected mouse spleens.
[0053] FIG. 32 shows the strategy used to generate "pSD5.hsp65-
disA.panCD--No Kan"
(SEQ ID NO: 31). The scheme replaces Kan cassette "pSD5.hsp65-disA.Kan" (SEQ
ID NO:30)
with the panCD operon to generate "pSD5.hsp65-disA.panCD--No Kan" (SEQ ID
NO:31).
[0054] FIG. 33 shows the molecular structure of the pJV53, the
recombineering plasmid
which is SEQ ID NO:32
[0055] FIG. 34 shows the molecular structure of the pUC-Hyg, a
plasmid with dif sites
flanking a Hyg cassette which is SEQ TD NO:35. pUC-Hyg is used to generate the
plasmid "pUC-
Hyg-panCD-KO" (SEQ ID NO:36).
[0056] FIG. 35 shows the molecular structure of the plasmid "pUC-
Hyg-panCD-KO" which
is SEQ ID NO:36. "pUC-Hyg-panCD-KO" is generated by cloning 500 bp of the
panCD 5'UTR
on one flank of the Hyg cassette, and cloning 500 bp of the panCD 3' UTR the
other flank.
[0057] FIG. 36 shows the molecular structure of the plasmid
"pSD5.hsp65-disA.Kan" which
is SEQ ID NO:30.
[0058] FIG. 37 shows the molecular structure of the plasmid
"pSD5.hsp65-disA.panCD¨No
Kan" which is SEQ ID NO:31. This plasmid is generated using the scheme
illustrated in Figure
32.
[0059] FIG. 38 shows the number of positive specimens.
18
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
[0060] FIGs 39A-39C shows confirmation of Mtb-disA overexpression
phenotype of BCG-
disA -OE and induction of TRF signaling. Fig. 39A shows colony PCR using
Kanamycin gene
specific primer confirms the presence of recombinant plasmid pSD5-hsp60-MT3692
in the BCG-
disA-OE (Tice) clones selected against Kanamycin (25 mg/mL). Fig. 39B shows
real time PCR
showing differential disA expression in different clones of BCG Tice BCG-disA-
OE Tice.
Transcript levels were measured in total RNA isolated from the late log phase
cultures using log
phase culture. M tuberculosis sigA (Rv2703) was used as a reference gene, and
relative expression
was calculated by 2AACT method. Fig. 39C shows measurement of IRF activation
BY quantification
of IRF induction based on ISRE (RLU, relative light units) in 24-h-post
infection (MOI = 1:20)
culture supernatants of RAW-Lucia ISG cells. Data represent mean SEM (n = 3
replicates).
Student's t-test (two-tailed) *P<0.05, **P<0.01, ***P<0.001, ****P< 0.0001
[0061] FIGs 40A-40C show BCG strains overexpressing c-di-AMP as
strong inducers of type
I interferon in STING-dependent manner. Fig. 40A shows quantitative
measurement of IFN-p in
culture supernatants of wild-type C57BL/6-derived BMDMs and STING-KO BMDM
(C57BL/6J-
Tmem173gt/J) 24 h after BCG-disA -OE (Tice) infection. Fig. 40B shows
quantitative
measurement of IFN-p in culture supernatants of wild-type C57BL/6-derived
BMDMs, BMDCs,
J774.1 macrophages and human monocyte-derived macrophages (RMDMs) 24 h post-
infection.
Fig. 40C shows quantitative measurement of IFNI!, in culture supernatants of
wild-type C57BL/6-
derived BMDMs, BMDCs, J774.1 macrophages and human monocyte-derived
macrophages
(HMDMs) 24 h post-infection. Macrophage to BCG infection ratio = 1:20. Data
represent mean
SEM (n = 3 replicates). Student's t-test (two-tailed) *P<0.05, **P<0.01,
***P<0.001, ****1)<
0.0001
[0062] FIGs 41A-41D show BCG strains overexpressing c-di-AMP are
strong inducers of
proinflammatory cytokines, TNF-a, and IL-6. (A-B) Quantitative measurement of
TNF-ct in
culture supernatants of wild-type C57BL/6-derived BMDMs, BMDCs, J774.1
macrophages and
human monocyte-derived macrophages (HMDMs). Fig. 41A shows measurements 24 h
post-
infection with BCG-disA-OE (Tice). Fig. 41B shows measurements 24 h post-
infection with BCG-
disA-OE (Pasteur). (C-D) Quantitative measurement of IL-6 in culture
supernatants of wild-type
C57BL/6-derived BMDMs, BMDCs, J774.1 macrophages and human monocyte-derived
macrophages (HMDMs). Fig. 41C shows measurements 24 h post-infection with BCG-
disA-OE
19
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
(Tice). Fig. 41D shows measurements 24 h post-infection with BCG-disA-OE
(Pasteur).
Macrophage to BCG infection ratio = 1:20. Data represent mean SEM (n = 3
replicates).
Student's t-test (two-tailed) *P<0.05, **P<0.01, ***P<0.001, ****P< 0.0001
[0063] FIG. 42 shows BCG overexpressing c-di-AMP strongly induces
proinflammatory
cytokine INF-a in STING-dependent manner. Quantitative measurement of TNF-a in
culture
supernatants of wild-type C57BL/6-derived BMDMs and STING-KO BMDM (C57BL/6J-
Tmem173gt/J) 24 h after BCG-disA-OE (Tice) infection. Macrophage to BCG
infection ratio
1:20. Data represent mean SEM (n = 3 replicates). Student's t-test (two-
tailed) *P<0.05,
**P<0.01, ***P<0.001, ****P< 0.0001
[0064] FIGs 43A-43E show BCG-disA-OE induces significantly higher
Thl cytokines and
chemokines as compared to WT BCG. Fig. 43A shows relative gene expression
analyses of
different cytokines and chemokines in IFN-y activated macrophages at 6 h post-
infection by wild-
type BCG (Tice) and BCG-disA-OE (Tice) strains. (B-D) Relative gene expression
analyses of IL-
6, 1L-12 and MCP-1 1FN-y activated macrophages at 6 h post-infection by wild-
type BCG
(Pasteur) and BCG-disA -OE (Pasteur) strains. ft-actin was used as a reference
gene, and relative
expression was calculated by 2AACT method. Data represent mean SEM (n = 3
replicates).
Student's t-test (two-tailed). Fig. 43B shows IL-6 relative gene expression.
Fig. 43C shows IL-12
relative gene expression. Fig. 43D shows MCP-1 relative gene expression. Fig.
43E shows
quantitative measurement of MCP-1 in culture supernatants of wild-type C57BL/6-
derived
BMDMs 24 h after BCG-disA-OE (Tice) infection. Macrophage to BCG infection
ratio = 1:20.
Data represent mean SEM (n = 3 replicates). Student's t-test (two-tailed)
*P<0.05, **P<0.01,
****P< 0.0001
[0065] FIGs 44A-44C show differential apoptotie induction in
murine BMDMs and J774.1
macrophage after infection with different BCG strains. (A-B) Murine BMDMs and
J774.1
macrophages were challenged with WT or BCG-disA-OE strains of BCG at a MOI of
1:10 for 24
h and apoptotic cell death was accessed by Annexin and PI staining.
Representative data from
individual infection assay. Fig. 44A shows measurements in murine BMDMs. Fig.
44B shows
measurements in J774.1 macrophages. Fig. 44C shows a bar diagram showing
quantifications of
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
late apoptotic cell death following infection. Data represent mean SEM (n =
3 replicates).
Student's t-test (two-tailed) *P<0.05
[0066] FIGs 45A-45C show internalization and differential
toxicity of WT and BCG-disA-OE
strains in human urothelial carcinoma cells. (A-C) Cell viability of RT4
(Human bladder cancer
cell line representing grade I carcinoma), 5637 (Human bladder cancer cell
line representing grade
II carcinoma) and J82 (Human bladder cancer cell line representing grade III)
cells exposed to
different MOIs of wild-type BCG. Cell viability was measured using CellTiter-
Glo Luminescent
Cell Viability assay. Fig. 45A shows cell viability of RT4 cells. Fig. 45B
shows cell viability of
5637 cells. Fig. 45C shows cell viability of J82 cells.
[0067] FIGs 46A-46D show BCG Tice overexpressing c-di-AMP as a
stronger inducer of
antitumor cytokine response in urothelial carcinoma cells. (A-D) Quantitative
measurement of
differential TNF-a, IL-6, IL-113 and IFN-y levels using ELISA in different
urothelial carcinoma
cells 24 h after infection with different wild-type BCG (Tice) and BCG-disA-OE
(Tice) strains.
Cells to BCG infection ratio = 1:20, data represent mean SEM (n = 3
replicates). Student's t-test
(two-tailed) *P<0.05, **P<0.01, ***P<0.001, ****P< 0.0001. Fig. 46A shows TNF-
a levels. Fig.
46B shows IL-6 levels. Fig. 46C shows IL-1f3 levels. Fig. 46D shows IFN-y
levels.
[0068] FIGs 47A-47G show BCG Pasteur overexpressing c-di-AMP as a
stronger inducer of
antitumor cytokine response in urothelial carcinoma cells. (A-G) Quantitative
measurement of
differential cytokine levels using ELISA in different urothelial carcinoma
cells 24 h after infection
with different wild-type BCG (Pasteur) and BCG-diszl-OE (Pasteur) strains.
Cells to BCG
infection ratio = 1:20. Data represent mean SEM (n = 3 replicates).
Student's t-test (two-tailed)
*P<0.05, **P<0.01, ***P<0.001, ****P< 0.0001. Fig. 47A shows TNF-a levels in
5637 cells.
Fig. 47B shows TNF-a levels in BBN975 cells. Fig. 47C shows 11-6 levels in
UPPL1595 cells.
Fig 47D shows IL-113 levels in MB49 cells. Fig. 47E shows IL-113 levels in
UPPL1595 cells. Fig.
47F shows IFN-y levels in BBN975 cells. Fig. 47G shows IFN-y levels in NBT-II
cells.
[0069] FIGs 48A-48B show stronger macrophage reprogramming
towards M1 phenotype
after infection with BCG strains overexpressing c-di-AMP. Fig. 48A shows wild-
type BMDMs
infected with different BCG strains and for 24 h at 1:10 MOIs. Cell surface
and intracellular
straining was carried out and cells were analyzed using flow-cytometry (BD LSR
II flow
21
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
cytometer. Bar diagram showing percentage of TNE-oc positive antigen
presenting mouse
macrophages (MHC Class II+CD 1 1b+F4/80+) following infection with wild-type
and c-di-AMP
overexpressing BCG Tice and Pasteur strains. Data were processed using FlowJo
software (Tree
Star v10). Fig. 48B shows representative flow plots showing different cell
phenotypes of antigen
producing M1 macrophages. Data represent mean SEM (n = 3 replicates).
Student's t-test (two-
tailed) *P<0.05, **P<0.01, ***P<0.001, ****13< 0.0001
[0070] FIGs 49A-49B show stronger reprogramming of M2 macrophages
after infection with
BCG strains overexpressing c-di-AMP. Fig. 49A shows percentage of M2
macrophage surface
markers (CD2061CD124' ) positivity on mouse BMDM macrophages (CD1lb F4/80')
after
infection with wild-type. Fig. 49B shows percentage of IL-10 producing
macrophages of M2
macrophage (CD206 CD124+ mouse macrophages) population. Briefly, wild-type
BMDMs were
generated in presence of murine M-CSF. Macrophages were infected with
different BCG strains
and for 24h at 1:10 MOIs. Cell surface and intracellular straining were
carried out and cells were
analyzed using flow-cytometry (BD LSR II flow cytometer. Data were processed
using FlowJo
software (Tree Star v10). Data represent mean SEM (n = 3 replicates).
Student's t-test (two-
tailed) *P<0.05, **P<0.01, ***P<0.001, ****P< 0.0001
[0071] FIGs 50A-50B show stronger induction of monocytic MDSCs
secreting IL-10 in
murine BMDMs after infection with wild-type and c-di-AMP overexpressing BCG
strains. Fig.
50A shows the percentage of M-MDSCs of total myeloid cells (CD45+). Fig. 50B
shows the
percentage of IL-10 producing M-MDSCs after infection of murine BMDMs with WT
and BCG-
disA-OE strains. Briefly, wild-type BMDM macrophages were infected with
different BCG strains
and for 24 h at 1:10 MOIs. Cell surface and intracellular straining was
carried out and cells were
analyzed using flow-cytometry (BD LSR TT flow cytometer. Data was processed
using FlowJo
software (Tree Star v10). Data represent mean SEM (n = 3 replicates).
Student's t-test (two-
tailed) *P<0.05, **P<0.01, ***P<0.001, ****P< 0.0001
[0072] FIGs 51A-51B show differential induction of classical
(inflammatory) monocytes after
infection with wild-type and c-di-AMP overexpressing BCG Tice. Fig_ 51A shows
a bar diagram
showing percentage of classical monocytes (CD14 CD16 ) of CD 1 lb populations.
Briefly, human
monocytes were isolated from PBMCs drawn from different healthy blood donors.
Negatively
22
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
selected human monocytes were infected with wild-type and BCG-disA-OE strains
for 24 h (1:10
MOTs). Fig. 51B shows representative flow-cytometry plots showing different
percentage of
monocyte populations. Cell surface staining was carried out and cells were
analyzed using flow-
cytomctry (BD LSR 11 flow cytometcr). Data were processed using FlowJo
software (Tree Star
v10). Student's t-test (two-tailed) *P<0.05, **P<0.01, ***p< P< 0.0001
[0073] FIGs 52A-52B show BCG overexpressing c-di-AMP as a potent
inducer of
proinflammatory cytokines in human monocyte-derived macrophages. Fig. 52A is a
bar diagram
showing percentage of MHC class II positive classical macrophages producing
TNF-a (TNF-
a+HLA-DR+/CD14+CD16 ) and IL-6 (IL-6+HLA-DR+/CD14+CD16 ). Briefly, human
monocytes
were isolated from PBMCs drawn from different healthy blood donors. Negatively
selected human
monocytes were differentiated into macrophages. Macrophages were infected with
wild-type and
BCG-disA-OE strains for 24 h (1:10 MOIs). Fig. 52B shows representative flow-
cytometry plots
showing different percentage of macrophage populations. Cell surface staining
was carried out and
cells were analyzed using flow-cytometry (BD LSR IT flow cytometer. Data were
processed using
FlowJo software (Tree Star v10). Student's t-test (two-tailed) *P<0.05,
**P<0.01, ***P<0.001,
****P< 0.0001
[0074] FIGs 53A-53C show BCG overexpressing c-di-AMP strongly
suppressing M2
macrophage phenotypes. Fig. 53A shows percentage of immunosuppressive M2
(CD206+CD163+) macrophages of total transitional (CD14+CD16+) macrophages.
Fig. 53B
shows percentage of IL-10 producing macrophages of total M2 macrophages
(CD206+CD163+)
after infection of HMDMs with WT and BCG-disA-OE Tice strains. Fig. 53C shows
representative
flow-cytometry plots showing M2 cell surface phenotypes and IL-10 producing
cells of M2
macrophages. Briefly, human monocytes were isolated from PBMCs drawn from
different healthy
blood donors. Negatively selected human monocytes were differentiated into M2
macrophages in
presence of M-CSF. Infections were carried out for 24 h (1:10 MOIs), cell
surface staining and
intracellular staining was performed. Data are mean SEM (n = 3 replicate
experiments performed
on inonocytes-derived inacrophages from healthy human donors). Student's t-
test (two-tailed).
*P<0.05, **P<0.01, ***13<0.001, ****P< 0.0001
[0075] FIGs 54 shows BCG overexpressing c-di-AMP infected
macrophages enhanced
phagocytosis. HMDMs were infected with WT BCG and BCG-disA-OE strains for 6 h
and
23
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
phagocytic activity was measured by quantifying intracellular FITC-labeled IgG-
opsonized latex
beads. Images were acquired on live cells. Nuclear staining was done using
Hoechst . Image
acquisition was carried out using LSM700 confocal microscope at 63X
magnification. Images
were process using Fiji software. Cells to BCG infection ratio = 1:10. Data
are mean SEM (n =
3 replicate experiments performed on monocytes-derived macrophages from
healthy human
donors). Student's t-test (two-tailed). *P<0.05, **P<0.01, ***P<0.001, ****P<
0.0001. Fig. 54 is
a bar graph showing the quantification of the fluorescence.
[0076] FIGs 55A-55B show BCG overexpressing c-di-AMP as a potent
inducer of
proinflammatory cytokines in primary human monocytes. (A-B) BCG-dis,4-0E
induces
significantly higher gene expression of TNF-a and IL-6 in primary human
monocytes as compared
to WT BCG. TNF-a and IL-6 expression was accessed in primary human monocytes
isolated from
different healthy donors using qRT-PCR. RNU6A was used as reference gene, and
relative
expression was calculated by 2AACT method. Data represent mean SEM (n = 6
different healthy
donors). Student's t-test (two-tailed) *P<0.05, **P<0.01, ***P<0.001, ****P
0.0001. Fig. 55A
shows TNF-a levels. Fig. 55B shows IL-6 levels.
[0077] FIGs 56A-56E show BCG overexpressing c-di-AMP as a
stronger inducer of trained
immunity epigenetic marks in human monocytes after training. (A-B) Bar diagram
showing
epigenetic active chromatin mark H3K4me3 on TNF-a and IL-6 gene promoters.
Fold enrichment
of epigenetically modified TNF-c. and IL-6 gene promoters in BCG-trained human
monocytes
isolated from different donors (n = 4 different healthy donors) following re-
stimulation by
Pam3CSK4. H3K4-trimethylated promoters were enriched and quantified using ChIP-
PCR. Fig.
56A shows H3K4me3 levels on TNF-a gene promoter. Fig. 56B shows H3K4me3 levels
on TL-6
gene promoter. (C-D) Bar diagram showing epigenetic inactive chromatin mark,
H3K9me3 on
TNF-a and IL-6 gene promoters. Fold enrichment of epigenetically modified TNF-
a and IL-6
gene promoters in BCG-trained human monocytes isolated from different donors
(n = 4 different
healthy donors) following re-stimulation by Pam3CSK4. H3K9-trimethylated
promoters were
enriched and quantified using ChIP-PCR. Student's 1-test (two-tailed) *P<0.05,
**P<0.01,
***P<0.001, ****P 0.0001. Fig. 56C shows H3K9me3 levels on TNF-a gene
promoter. Fig.
24
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
56D shows H3K9me3 levels on IL-6 gene promoter. Fig. 56E shows schematic
representation of
ex-vivo BCG training.
[0078] FIGs 57A-57C shows BCG overexpressing c-di-AMP
demonstrating improved
antitumor activity in the MN U carcinogen model of NM1BC. Fig. 57A Schematic
of intravcsical
treatment strategy of BCG in MNU carcinogen model of NMIBC. Fig. 57B is a
graph bar showing
tumor involvement index. Fig. 57C is a graph showing staging of tumors.
Student's t-test (two-
tailed) *P<0.05, **P<0.01, ***P<0.001, ****p< 0.0001
[0079] FIGs 58A-58C show BCG-disA-OE strains attenuated for
virulence in vivo. Fig. 58A
illustrates BALB/c BCG aerosol challenge model. Fig. 58B shows BALB/c mice
lung bacillary
burden of wild-type and BCG-disA-OE strains in mouse lungs 4-week post
infection. Data are
mean S.E.M. (n = 5 animals/group). Fig. 58C shows implantation (day 01) of
BCG strains
following aerosol challenge in BALB/c mice. Student's t-test (two-tailed)
*P<0.05, **P<0.01,
***P<0.001, ****P< 0.0001
[0080] FIGs 59A-59C show BCG strain overexpressing c-di-AMP
attenuated for virulence in
a severely immunocompromised (SCID) mouse model of aerosol infection. Fig. 59A
illustrates
SCID mice model of BCG aerosol infection. Fig. 59B shows survival of SCID mice
(n =10) after
infection with different BCG strains. Fig. 59C shows implantation (day 01) of
BCG strains
following aerosol challenge in SCID mice. Student's 1-test (two-tailed)
*P<0.05, **P<0.01,
***P<0.001, ****P< 0.0001
[0081] FIGs 60A-60D show BCG overexpressing c-di-AMP as a
stronger inducer of Thl
cytokines in vivo. (A-B) Quantitative levels of IFN-P, TNF-a, IL-6 and IFN-y
in lung homogenates
from BALB/c mice at 4 weeks after infection with different BCG strains using
ELISA. Fig. 60A
shows results with WT BCG (Tice) and BCD-disA-OE (Tice). Fig. 60B shows
results with WT
BCG (Pasteur) and BCD-disA-OE (Pasteur). (C-D) Quantitative levels of IFN-p,
TNF-a, IL-6 and
IFN-y in lung homogenates from BALB/c mice at 4 weeks after infection with
different BCG
strains using ELISA. Fig. 60C shows results with WT BCG (Tice) and BCD-disA -
OE (Tice). Fig.
60D shows results with WT BCG (Pasteur) and BCD-disA-OE (Pasteur). Results are
represented
as the mean (pg/ml) SEM (n = 4 animals/group). Student's t-test. Student's t-
test (two-tailed)
*P<0.05, **P<0.01, ***P<0.001, ****P< 0.0001
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
[0082] FIGs 61A-61B show therapeutic intratumoral injection of
BCG overexpressing c-di-
AMP leading to greater antitumor activities in MB49 model of bladder cancer.
Fig. 61A shows a
schematic representation of intratumoral injection of tumors. Mice were
implanted with lx105
MB49 cells on day 0, then accessed for tumor volume until the group averaged
¨40mm3. At that
time, mice were treated with 5x106 wild-type or BCG-disA-OE strain in a total
volume of 50 L
or with PBS alone every 3rd day for a total of 3 treatments. Fig. 61B shows
tumor outgrowth of
MB49 bearing animals treated with vehicle (PBS), wild-type and BCG-disA -OE
following
treatments as shown in figure 61A. Two-way ANOVA.
[0083] FIGs 62A-62D show BCG overexpressing c-di-AMP inducing
stronger infiltration of
IFN-y at tumor site following intratumoral administration. (A-B) Dot plot
showing relative
abundance of percentage of CD4 or CD8 of total CD3 populations in single cells
isolated from
tumor. Fig. 62A shows percentage of CD4 cells. Fig. 62B shows percentage of
CD8 cells. (C-D)
Dot plot showing higher percentage of interferon-y producing CD4 T cells
inside single cells
isolated from tumors receiving BCG-disA -OE. Fig. 62C shows percentage of INF-
y+ cells among
CD4 cells. Fig. 6211 shows percentage of INF-y cells among CD8 cells. No
significant changes
in percentage of interferon-y producing CD8 T cells were observed. Briefly,
single cells were
prepared from excised tumors after intratumoral administration of PBS or BCG
strains. Cell
surface and intracellular cytokine staining was performed, and cells were
analyzed using flow
cytometry. Student's t-test. Student's t-test (two-tailed) *P<0.05, **P<0.01,
***P<0.001, ****P<
0.0001
DETAILED DESCRIPTION OF THE INVENTION
[0084] Unless defined otherwise, all technical and scientific
terms used herein have the
meaning commonly understood by a person skilled in the art to which this
invention belongs. The
following references provide one of skill with a general definition of many of
the terms used in
this invention: Singleton et al., Dictionary of Microbiology and Molecular
Biology (2nd ed. 1994);
The Cambridge Dictionary of Science and Technology (Walker ed., 1988); The
Glossary of
Genetics, 5th Ed., R. Rieger et al. (eds.), Springer Verlag (1991); and Hale &
Marham, The Harper
26
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
Collins Dictionary of Biology (1991). As used herein, the following terms have
the meanings
ascribed to them below, unless specified otherwise.
[0085] By "agent" is meant any small molecule chemical compound,
antibody, nucleic acid
molecule, or polypeptide, or fragments thereof.
[0086] By "alteration" is meant a change (increase or decrease)
in the expression level or
activity of a gene or polypeptide as detected by standard methods known in the
art such as those
described herein. As used herein, an alteration includes a 10% change in
expression level,
preferably a 25% change, more preferably a 40% change, and most preferably a
50% or greater
change in expression level.
[0087] By "ameliorate" is meant decrease, suppress, attenuate,
diminish, arrest, or stabilize the
development or progression of a disease.
[0088] By "analog" is meant a molecule that is not identical but
has analogous functional or
structural features. For example, a polypeptide analog retains the biological
activity of a
corresponding naturally occurring polypeptide, while having certain
biochemical modifications
that enhance the analog's function relative to a naturally occurring
polypeptide. Such biochemical
modifications could, for example, increase the analog's protease resistance,
membrane
permeability, or half-life, without altering, for example, ligand binding. An
analog may include an
unnatural amino acid, in another example.
[0089] By "cdnP" is meant either 1) a cdnP gene or nucleic acid
sequence that encodes a cyclic
di-nucleotide phosphodiesterase (cdnP) protein or 2) the cyclic di-nucleotide
phosphodiesterase
protein. Examples include the AI tuberculosis cdnP gene in H37Rv, Rv2837c,
having NCBI Gene
ID 888920, and a cdnP protein of UniProtKB/Swiss-Prot P71615.2.
100901 By "cGas" is meant either 1) a cGas gene or nucleic acid
sequence that encodes a cyclic
GMP-AMP synthase (cGAS) protein, or 2) the cyclic GMP-AMP synthase protein.
Examples of
cGas include the H. sapiens cGAS gene (NCBI Gene ID: 115004) and the protein
encoded by this
gene (UniProtKB/Swiss-Prot: Q8N884.2). The cGas protein is a cyclic GMP-AMP
synthase from
humans that makes 2'3' cGMP. 2'3' cGMP is a STING agonist in humans.
[0091] "Cyclase domains" of cGAS for example, refers to a portion
or fragment of the 522
amino acids of the human cGAS protein described in Kranzusch et al. (Cell
Reports 2013; 3:1362-
1368 PMID 23707061). A cyclase domain may be described as having an NTase core
situated
27
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
from amino acid 160-330, and a regulatory-sensor domain that is the C-domain
situated from
amino acids 330-522. Mutants of the NTase core sequence as well as mutants of
the regulatory-
sensor domain can be used to generate constitutively active variants of cGAMP
designed to
produce high levels of cGAMP without the normal requirement for activation by
DNA binding.
Another example of a cyclase domain includes M. tuberculosis Rv1354c of NCBI
Gene ID:
887485, and the protein encoded by this gene (UniProtl(B/Swiss-Prot: P9WM13)
that encodes a
623 amino acid-long protein capable of both c-di-GMP (cyclic diguanylate or
cyclic di-GMP)
synthesis (via its GGDEF domain, amino acids 201-400) and degradation (via its
EAL domain,
amino acids 401-623). The GAF domain (amino acids 1-200) is a regulatory
domain. The GGDEF
domain as well as mutants of the regulatory-sensor GAF domain and polypeptides
truncated to
remove the EAL domain (phosphodiesterase activity) can be used to generate
constitutively active
variants of Rv1354c designed to produce high levels of c-di-GMP.
[0092] By "DisA" or "disA" is meant either 1) a Dis A gene or
nucleic acid sequence that
encodes a DNA integrity scanning (DisA) protein or 2) the DNA integrity
scanning protein.
Examples include M. tuberculosis disA gene Rv3586 of NCBI Gene ID: 887485, and
the protein
encoded by this gene is UniProtKB/Swiss-Prot: P9WNW5.1. The protein is a 358
amino acid-long
diadenylate cyclase as described by Dey & Bishai et al. Nature Medicine
2015;21:401-6. PMID:
25730264. A DisA protein is a diadenylate cyclase that makes c-di-AMP. c-di-
AMP is a STING
agoni st.
[0093] By "disease" is meant any condition or disorder that
damages or interferes with the
normal function of a cell, tissue, or organ. Examples of diseases include, but
are not limited to,
bladder cancer.
100941 By "dncV" is meant a gene that encodes a Cyclic GMP-AMP
synthase that catalyzes
the synthesis of 3'3'-cyclic GMP-AMP (3'3'-cGAMP) from GTP and ATP, a second
messenger in
cell signal transduction. dncV isalso able to produce c-di-AMP and c-di-GMP
from ATP and GTP,
respectively; however, 33-cGAMP is the dominant molecule produced by DncV in
vivo, contrary
to the 2'3'-cGAMP produced by eukaryotes. dncV isrequired for efficient
V.cholerae intestinal
colonization, and down-regulates the colonization-influencing process of
chemotaxis. dncV is not
active with dATP, TTP, UTP, and CTP. The DncV protein is a cyclic GMP-AMP
synthase from
V.cholerae that makes 3'3'cGAMP. 3'3'cGAMP is a STING agonist.
28
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
[0095] -EAL domain" means a conserved protein domain that is
found in diverse bacterial
signaling proteins. The EAL domain may function as a diguanylate
phosphodiesterase and has
been shown to stimulate degradation of a second messenger, cyclic di-GMP. A
non-functional
EAL domain will not have one or more of these functions. An example of an EAL
domain includes
M tuberculosis Rv1357c gene of NCBI Gene ID: 886815, and the 307 amino acid-
long protein
encoded by this gene is UniProtKB/Swiss-Prot: P9WM07 that encodes a c-di-GMP
phosphodiesterase (PDE) and is comprised of a sole EAL domain. This enzyme's
activity is to
serve as a c-di-GMP phosphodiesterase, cleaving the cyclic dinucleotide (which
has signaling
activity) into 2 GMP molecules (which lack signaling activity), as described
in the article titled,
"A full-length bifunctional protein involved in c-di-GMP turnover is required
for long-term
survival under nutrient starvation in Mycobacterium smegmatis," Bharati BK,
Sharma IM, Kasetty
S, Kumar M, Mukherjee R, Chatterji D. Microbiology. 2012 Jun;158(Pt 6):1415-
27. doi:
10.1099/micØ053892-0. Epub 2012 Feb 16.PMID: 22343354. Another example of an
EAL
domain includes the 336 amino acid-long protein encoded by Al. tuberculosis
cdnP gene in H37Rv
(Rv2837c), a c-di-AMP phosphodiesterase comprising an EAL domain with the
capability of
hydrolyzing human 2'-3'cGAMP (the product of the human cGAS enzyme) as shown
by Jain-Dey
Bishai et al. Nat Chem Biol. 2017;13:210-217 PMID 28106876. The structural
characteristics of
the EAL domains (cyclic dinucleotide phosphodiesterase activity) and GGDEF
domains (cyclic
dinucleotide cyclization-biosynthetic activity) are known and well described
(for example, in
Schirmer T, Jenal U. Structural and mechanistic determinants of c-di-GMP
signaling. Nat Rev
Microbiol. 2009;7:724-35. PMID: 19756011).
[0096] By "effective amount" is meant the amount required to
ameliorate the symptoms of a
disease relative to an untreated patient. The effective amount of active
compound(s) used to
practice the present invention for therapeutic treatment of a disease varies
depending upon the
manner of administration, the age, body weight, and general health of the
subject. Ultimately, the
attending physician or veterinarian will decide the appropriate amount and
dosage regimen. Such
amount is referred to as an "effective" amount.
[0097] By -dncV" is meant either 1) a dncV gene or nucleic acid
sequence that encodes a
cyclic GMP-AMP synthase (DncV) protein, or 2) the Cyclic GMP-AMP synthase
protein.
29
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
Examples include, but are not limited to, the Vibrio cholerae dncV gene of
NCBI Gene ID:
2614190 and the protein encoded by this gene is UniProtKB/Swiss-Prot: Q9KVG7.1
[0098] By "fragment" is meant a portion of a polypeptide or
nucleic acid molecule. This
portion contains, preferably, at least 10%, 20%, 300/u, 40%, 50%, 60%, 70%,
800/u, or 90% of the
entire length of the reference nucleic acid molecule or polypeptide. A
fragment may contain, for
example, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 200, 300, 400, 500, 600,
700, 800, 900, or 1000
nucleotides or amino acids.
[0099] By "gene deletion" is meant using allelic exchange
methodologies well-known to one
skilled in the art to delete the full gene coding region of the gene of
interest from the chromosome
of BCG. Gene replacement with selectable markers such as antibiotic resistance
cassettes is a form
of allelic exchange and may be performed. Technologies are also available to
generate unmarked
deletions (no selectable marker) in which the gene is entirely deleted, and no
selectable marker is
introduced in its place.
[0100] By "gene domain deletion" is meant using the above allelic
exchange methodologies
to remove the portion of a gene encoding a particular domain (in the case of
the present invention
the EAL domain of Rv1354c which encodes the CDN phosphodiesterase domain of a
multifunctional polypeptide) leaving the other portions of the polypeptide
intact and in frame.
[0101] By "H. sapiens" is meant Homo sapiens.
[0102] By "obtaining" as in "obtaining an agent" is meant
synthesizing, purchasing, or
otherwise acquiring the agent.
[0103] By "overexpression" is meant, in a general sense, a gene
expressing its corresponding
protein in a greater quantity than a wild type or reference gene. An example
of creating a gene
overexpressing a protein in the present invention includes fusing the DNA
encoding the gene of
interest to a strong promoter in BCG such as Phsp60 or to a strong
conditionally active promoter
such as PtetOFF. In PtetOFF, gene expression is turned off in the presence of
tetracycline,
anhydrotetracycline, or doxycycline; however, when the recombinant BCG is
administered as an
immunotherapy in a human or an animal model, the gene of interest will be
turned on. This
conditionally active strategy has the advantage of preventing any deleterious
effects on viability
or growth rate that strong overexpression of cyclic dinucleotide producing
enzyme might have on
the BCG organisms while the BCG is being grown, and it allows for strong
expression
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
("overexpression") only when the BCG immunotherapy is given as a therapeutic
to a mammalian
host.
[0104] By "Mib" is meant Mycobacleriurn lubercitlosis.
[0105] The terms "polypeptide," "peptide" and "protein" are used
interchangeably herein to
refer to a polymer of amino acid residues. The terms apply to amino acid
polymers in which one
or more amino acid residue is an analog or mimetic of a corresponding
naturally occurring amino
acid, as well as to naturally occurring amino acid polymers. Polypeptides can
be modified, e.g., by
the addition of carbohydrate residues to form glycoproteins. The terms
"polypeptide," "peptide"
and "protein" include glycoproteins, as well as non-glycoproteins.
[0106] By "reduce" or "decrease" is meant a negative alteration
of at least about 100/u, 25%,
50%, 75%, or 100%, for example, or any percentage in between.
[0107] By "increase" is meant a positive alteration of at least
about 10%, 25%, 50%, 75%, or
100%, for example, or any percentage in between.
[0108] By "reference" is meant a standard or control condition.
[0109] A "reference sequence" is meant a defined sequence used as
a basis for sequence
comparison. A reference sequence may be a subset of or the entirety of a
specified sequence, for
example, a segment of a full-length cDNA or gene sequence, or the complete
cDNA or gene
sequence. For polypeptides, the length of the reference polypeptide sequence
will generally be at
least about 16 amino acids, preferably at least about 20 amino acids, more
preferably at least about
25 amino acids, and even more preferably about 35 amino acids, about 50 amino
acids, or about
100 amino acids. For nucleic acids, the length of the reference nucleic acid
sequence will generally
be at least about 50 nucleotides, preferably at least about 60 nucleotides,
more preferably at least
about 75 nucleotides, and even more preferably about 100 nucleotides or about
300 nucleotides or
any integer thereabout or there between.
[0110] By "reference BCG strain" is meant, for example, a
conventional BCG strain that does
not contain the expression vectors of the present invention and/or the
endogenous genes unable to
express a cdnP functional protein, a Rv1354c functional protein, a Rv1357c
functional protein, or
a combination thereof.
[0111] By "regulatory DNA recognition capability" is meant the
ability of a protein to detect
or bind DNA. For example, a cGAS protein is known to bind DNA, such as
cytosolic DNA, and
31
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
triggers the reaction of GTP and ATP to form cyclic GMP-AMP (cGAMP). cGAMP
binds to the
Stimulator Interferon Genes (STING) which triggers phosphorylation of IRF3 via
TBK1.
[0112] By "Rv1354c" is meant either 1) a Rv1354c gene or nucleic
acid sequence that encodes
a Rv1354c protein or 2) the Rv1354c protein (e.g., Gupta, Kumar, and
Chatterji; PLoS ONE
(November, 2010); Vol. 5; Issue 11; and Bhariati, Sharma, Kasetty, Kumar,
Mukherjee, and
Chatterji; Microbiology (2012), 158, 1415-1427). The Rv1354c protein is a
diguanylate cyclase
that mkes c-di-GMP. C-di-GMP is a STING agonist.
[0113] By "Rv1357c" is meant either 1) a Rv1357 gene or nucleic
acid sequence that encodes
a cyclic di-GMP phosphodiesterase protein (Rv1357) protein or 2) the cyclic di-
GMP
phosphodiesterase protein (e.g., Gupta, Kumar, and Chatterji; PLoS ONE
(November, 2010); Vol.
5; Issue 11; and Bhariati, Sharma, Kasetty, Kumar, Mukherjee, and Chatterji;
Microbiology(2012),
158, 1415-1427). The Rv1357c protein is a diguanylate cyclase that mkes c-di-
GMP. C-di-GMP
is a STING agonist.
[0114] By "STING agonist" is meant a molecule which binds to
STING (stimulator of
interferon genes, or TMEM173), activates it, and triggers activation of the
IRF3-TBK1 pathway
leading to increased transcription of type 1 interferon and other genes.
[0115] By -CDN" is meant cyclic dinuculeotide such as 3'-5' c-di-
AMP, 3'-5' c-di-GMP,
3'-3' cGAMP (also known as 3'-5', 3'-5'cGAMP, the product of the Vibrio
eholerae DncV
protein), or 2'-3' cGAMP (also known as 2'-5', 3'-5' cGAMP, the product of the
human cGAS
protein).
[0116] By -PAMP" is meant pathogen associated molecular pattern.
PAMPs are microbial
products including small molecules which are recognized by innate immune
sensors. Examples of
PAMPs are 3'-5' c-di-AMP, 3'-5' c-di-GMP, 3'-3' cGAMP.
[0117] By "DAMP" is meant danger associated molecular pattern.
DAMPs are host-derived
(that is human, mouse, or other mammalian model of disease) molecules that are
produced to signal
danger such as infection or other derangement of normal physiology. An example
of a DAMP is
2'-3' cGAMP which is produced by the host sensor enzyme cGAS upon detection of
double-
stranded DNA in the cytosol as occurs during viral or certain intracellular
bacterial infections.
[0118] By "panCD" is meant the genetic operon from bacteria or
other species the encodes the
biosynthetic gene panC (encoding the PanC protein which has pantoate-beta-
alanine ligase
32
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
enzymatic activity) and the biosynthetic gene panD (encoding the PanD protein
which has
aspartate 1-decarboxylase enzymatic activity). The PanC and PanD proteins are
required for the
biosynthesis of pantothenic acid or pantothenate also called vitamin B5 (a B
vitamin). Pantothenic
acid, a water-soluble vitamin, is an essential nutrient for bacteria and for
all mycobacteria including
BCG. Pantothenic acid is required in order to synthesize coenzyme-A (CoA), as
well as to
synthesize and metabolize proteins, carbohydrates, and fats.
[0119] By "specifically binds" is meant a compound, nucleic acid,
peptide, protein, or
antibody, for example, that recognizes and binds a polypeptide or nucleic acid
sequence, but which
does not substantially recognize and bind other molecules in a sample.
[0120] By "substantially identical" is meant a polypeptide or
nucleic acid molecule exhibiting
at least 50% identity to a reference amino acid sequence (for example, any one
of the amino acid
sequences described herein) or nucleic acid sequence (for example, any one of
the nucleic acid
sequences described herein). Preferably, such a sequence is at least 60%, more
preferably 80% or
85%, and more preferably 90%, 95% or even 99% identical at the amino acid
level or nucleic acid
to the sequence used for comparison. Sequence identity is typically measured
using sequence
analysis software (for example, Sequence Analysis Software Package of the
Genetics Computer
Group, University of Wisconsin Biotechnology Center, 1710 University Avenue,
Madison, Wis.
53705, BLAST, BESTFIT, GAP, or PILEUP/PRETTYBOX programs). Such software
matches
identical or similar sequences by assigning degrees of homology to various
substitutions, deletions,
and/or other modifications. Conservative substitutions typically include
substitutions within the
following groups: glycine, alanine; valine, isoleucine, leucine; aspartic
acid, glutamic acid,
asparagine, glutamine; senile, threonine; lysine, arginine; and phenylalanine,
tyrosine. In an
exemplary approach to determining the degree of identity, a BLAST program may
be used, with a
probability score between e-3 and e-m indicating a closely related sequence.
[0121] By "subject" is meant a mammal, including, but not limited
to, a human or non-human
mammal, such as a bovine, equine, canine, ovine, or feline.
[0122] By "sensitivity" is meant the percentage of subjects with
a particular disease.
[0123] By -specificity" is meant the percentage of subjects
correctly identified as having a
particular disease, i.e., normal or healthy subjects. For example, the
specificity is calculated as the
33
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
number of subjects with a particular disease as compared to normal healthy
subjects (e.g. ,non-
cancer subjects).
[0124] By "trained immunity" is meant the ability of one
antigenic stimulus to elicit more
potent immune responses to a second, different antigen administered at a later
time. Trained
immunity is antigen-independent, based on heterologous CD4 and CD8 memory
activation,
cytokine mediated, and is associated with epigenetic and metabolic changes.
[0125] By "Phsp60" or "Phsp65" is meant a strong mycobacterial
promoter derived from the
Mycobacterium leprae Hsp65 5'UTR.
[0126] By "5'UTR" is meant the 5' untranslated region of a gene.
[0127] By "3'UTR" is meant the 3' untranslated region of a gene.
[0128] By "WT" is meant wild type.
[0129] By "BCG-WT" is meant a wild type strain of Mycobacterium
bovis bacillus Calmette
Guerin.
[0130] Ranges provided herein are to be understood to be
shorthand for all of the values within
the range. For example, a range of 1 to 50 is to be understood to include any
number, combination
of numbers, or sub-range from the group consisting of 1,2, 3,4, 5, 6, 7, 8, 9,
10, 11, 12, 13, 14,
15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33,
34, 35, 36, 37, 38, 39, 40,
41, 42, 43, 44, 45, 46, 47, 48, 49, or 50.
[0131] As used herein, the terms "treat," "treating,"
"treatment," and the like refer to reducing
or ameliorating a disorder and/or symptoms associated therewith. It will be
appreciated that,
although not precluded, treating a disorder or condition does not require that
the disorder, condition
or symptoms associated therewith be completely eliminated.
101321 Unless specifically stated or obvious from context, as
used herein, the term "or" is
understood to be inclusive. Unless specifically stated or obvious from
context, as used herein, the
terms "a", "an", and "the" are understood to be singular or plural.
[0133] Unless specifically stated or obvious from context, as
used herein, the term "about" is
understood as within a range of normal tolerance in the art, for example
within 2 standard
deviations of the mean. About can be understood as within 10%, 9%, 8%, 7%, 6%,
5%, 4%, 3%,
2%, 1%, 0.5%, 0.1%, 0.05%, or 0.01% of the stated value. Unless otherwise
clear from context,
all numerical values provided herein are modified by the term about.
34
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
[0134] As used herein, "comprises," "comprising," "containing"
and "having" and the like can
have the meaning ascribed to them in U.S. Patent law and can mean "includes,"
"including," and
the like; "consisting essentially of or "consists essentially" likewise has
the meaning ascribed in
U.S. Patent law and the term is open-ended, allowing for the presence of more
than that which is
recited so long as basic or novel characteristics of that which is recited is
not changed by the
presence of more than that which is recited, but excludes prior art
embodiments.
[0135] Any compositions or methods provided herein can be
combined with one or more of
any of the other compositions and methods provided herein.
[0136] As used herein, the terms "prevent," "preventing,"
"prevention," "prophylactic
treatment" and the like refer to reducing the probability of developing a
disorder or condition in a
subject, who does not have, but is at risk of or susceptible to developing a
disorder or condition.
[0137] Such treatment (surgery and/or chemotherapy) will be
suitably administered to
subjects, particularly humans, suffering from, having, susceptible to, or at
risk for bladder cancer
or disease, disorder, or symptom thereof. Determination of those subjects "at
risk" can be made by
any objective or subjective determination by a diagnostic test or opinion of a
subject or health care
provider (e.g., genetic test, enzyme or protein marker, a marker (as defined
herein), family history,
and the like). In some embodiments, determination of subjects susceptible to
or having a urothelial
cancer is determined by measuring levels of at least one of the markers.
[0138] In some embodiments, the present invention relates to
genetic alterations of
Mycobacterium bovis BCG (hereafter, "BCG") which generate recombinant BCG
(hereafter
"rBCG") strains. These strains have greater potency as (i) tuberculosis
vaccines and/or (ii)
immunotherapies for non-muscle invasive bladder cancer (NMIBC). Some
embodiments of the
present invention relate to BCG strains that synthesize and secrete high
levels of cyclic
dinucleotides (CDNs) which are known to elicit valuable immunomodulatory
responses from
-human p-hagocyti c cells such as macrophages, den dri ti c cells, and others.
Another embodiment of
this invention is to combine genetic modifications of BCG to generate
multivalent CDN-
overexpression modifications that include addition of novel CDN-synthesizing
genetic material
and/or mutations of endogenous BCG phosphodi esterase genes or genetic domains
that will
enhance the accumulation and release of CDNs.
[0139] BCG
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
[0140] BCG (bacillus Calmette Guerin) is a mutant version of
Mycobacterium bovis generated
by the French microbiologists Calmette and Guerin in 1921 by 13 years of
serial passage of virulent
M bovis. Between 1921 and 1960 BCG was carried by serial passage in numerous
world
laboratories, until defined seedlots were established and banked in reference
laboratories. As such,
many dozen variants of BCG exist worldwide such as BCG Pasteur, BCG Tice, BCG
Tokyo, BCG
Danish, BCG Montreal, etc. The majority of existing BCG strains have now been
defined by whole
genome sequencing. Major differences between virulent M bovis and the various
BCG strains
include the deletion of at least 15 regions of difference that comprise
genomic deletions in BCG
compared with virulent M. tuberculosis. Key regions of difference in the
development of BCG
were RD1 (9.5 kb deletion leading to loss of the Esx-1 secretion system and
inability to release
antigens ESAT-6 and CFP-10) and RD3 (9.2 kb deletion). Regions of difference
RD4-RD11 are
absent in all BCG strains compared with virulent M tuberculosis.
[0141] Since the 1920s, BCG has been used as a vaccine for
prevention of tuberculosis (TB).
In 2004 it was estimated that BCG was given to about 100 million children,
hence since its
introduction BCG has been given to approximately 5 billion humans and as such
is the most widely
utilized vaccine in history. It is most commonly given intradermally at birth,
and to date it is still
given in most countries except the United States, Canada, and parts of Europe.
BCG has been
shown to reduce the incidence of childhood disseminated TB, but BCG-vaccinated
individuals are
not fully protected from the risk of TB.
[0142] Since 1977, BCG has also achieved wide use as a cancer
immunotherapy for non-
muscle invasive bladder cancer (NMIBC). It is given intravesically weekly for
six weeks and in
some instances, such as high-risk disease, it is given as maintenance therapy
weekly for three
weeks at 3, 6, 12, 18, 24, 30, and 36 months after initial therapy.
Intravesical BCG has been shown
to (i) induce a mononuclear cell infiltrate comprised predominantly of CD4 T
cells and
macrophages, (ii) increase the expression of interferon gamma (IFNy) in the
bladder, and (iii)
increase urinary cytokine levels of IL-1, IL-2, IL-6, IL-8, IL-12, 1FNy, and
TNFa.
[0143] Despite the wide global use of BCG as (i) a vaccine for TB
and (ii) an immunotherapy
for NMIBC, there is considerable room for improvement in its efficacy. For TB,
BCG gives only
partial protection predominantly against childhood disseminated tuberculosis.
For NMIBC,
approximately 30% of patients have BCG-resistant disease. These individuals
require riskier
36
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
treatments with systemic chemotherapy and have higher rates of progression to
more invasive
forms of bladder cancer.
[0144] Urothelial cancer
[0145] Urothclial cancer of the bladder is the most common
malignancy of the urinary tract. It
is the fourth most common cancer in males and 11th most common in females. It
is estimated that
approximately 79,000 new cases of bladder cancer will be diagnosed in the USA
in 2017,
associated with 19,870 deaths. Although the estimated five-year survival for
bladder cancer
patients is 78%, the rates decline dramatically for patients with locally
advanced or metastatic
disease. Approximately 75% of patients with bladder cancer present with a
disease that is confined
to the mucosa (stage Ta, carcinoma in situ) or submucosa (stage Ti), known as
non-muscle
invasive bladder cancer (NMIBC). Transurethral resection is the initial
treatment of choice for
NMIBC. For patients with muscle invasive bladder cancer (MIBC; T2 or greater),
the first-line
treatment option is platinum-containing chemotherapy followed by bladder
removal. For those
patients with NMIBC who do not respond to intravesical treatments, there is
high risk of
progression to MIBC. Thus, the high rates of recurrence and significant risk
of progression
mandate that additional therapy be implemented. Improving clinical outcomes
for patients with
high risk-NMIBC therefore requires the development of novel treatments.
[0146] Intra-vesical administration of Bacillus-Calmette Guerin
(BCG), developed in the
1970s for NMIBC, provided the first successful immunotherapy against an
established solid
cancer, and it remains the standard of care for patients with NMIBC. (Askeland
EJ, Newton MR,
O'Donnell MA, Luo Y. Bladder Cancer Immunotherapy: BCG and Beyond. Adv Urol.
2012;2012:181987. PMID: 22778725. Morales A. BCG: A throwback from the stone
age of
vaccines opened the path for bladder cancer immunothcrapy. Can J Urol.
2017;24:8788-8793.
PMID: 28646932). The exact mechanism of the anti-tumor effects of BCG, which
is an attenuated
strain of Mycobacterium bovis, remains unclear, but it is believed to
orchestrate a vigorous immune
cellular and humoral immune response, predominantly Th 1 response, after
binding to the
urothelium through fibronectin and integrin a5131 (Redelman-Sidi G, Glickman
MS, Bochner BH.
The mechanism of action of BCG therapy for bladder cancer--a current
perspective. Nat Rev Urol.
2014;11:153-62. PMID: 24492433). However, typical complete response rates for
BCG treatment
are 55-65% for papillary tumors and 70-75% for carcinoma in situ (CIS). (A
skel and ET, Newton
37
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
MR, O'Donnell MA, Luo Y. Bladder Cancer Immunotherapy: BCG and Beyond. Adv
Urol.
2012;2012:181987. PMED: 22778725. Morales A. BCG: A throwback from the stone
age of
vaccines opened the path for bladder cancer immunotherapy. Can J Urol.
2017;24:8788-8793.
PM1D: 28646932). The burden of patients with BCG unresponsive and relapsing
disease and of
those intolerant to treatment has therefore prompted the need for further
improving the efficacy of
BCG against NMIBC.
[0147] CDNs are important PAMPs and DAMPs that generate valuable
immune
responses for TB and NMIBC.
[0148] Bacterial pathogen-associated molecular patterns (PAMPs).
Human cells utilize an
innate immune monitoring system known as the cytosolic surveillance program
(CSP) to detect
nucleic acid including cyclic dinucleotides in the cytosol. Originally
characterized as a viral
defense system, the CSP has now been shown to be important in anti-bacterial
defenses particularly
against intracellular bacteria such as Mycobacterium tuberculosis, Listeria
monocytogenes,
Salmonella species, and others. Cytosolic pattern recognition receptors (PRRs)
including STING,
cGAS, DDX41 and many others are capable of binding to cytosolic CDNs and
nucleic acids
leading to their activation. A key signaling event is STING activation which
leads to activation of
TBK1 and IRF3 and subsequent upregulation of type I interferon expression.
STING activation by
cyclic dinucleotides also leads to the induction of STAT6 which induces
chemokines such as
CCL2 and CCL20 independently of the TBK1-IRF3 pathway. STING activation is
also believed
to activate the transcription factor NFicB through the IicB kinase (IKK)
activation.
[0149] Human danger associated molecular patterns (DAMPs). Cyclic
cGAMP (cGAS)
synthase is a cytosolic PRR which recognized cytosolic DNA. Upon binding to
DNA it
undergoes a conformational change that activates its core enzymatic activity
which is to catalyze
the formation of 2'3' cGAMP. 2'3' cGAMP in turn is a potent DAMP which
activates the
STING-TBK1-IRF3 axis leading to increased type 1 interferon expression as well
as the STAT6
activation and IKK activation.
[0150] STING-mediated mechanism of CDN-triggered immune
responses. Type I IFNs,
produced both by innate immune cells in the tumor microenvironment and by the
tumor cells
themselves, are known to mediate anti-tumor effects against several
malignancies, due to their
ability to intervene in all phases of cancer immune-editing. (Zitvogel L,
Galluzzi L, Kepp 0,
38
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nat Rev
Immunol.
2015;15:405-14. PMID: 26027717). STING (stimulator of interferon genes), is a
major regulator
of type I IFN innate immune responses to pathogens, following recognition of
eytosolic DNA by
the sensor cyclic GMP-AMP synthase (cGAS). cGAS catalyzes the synthesis of
cyclic GMP-AMP
(cGAMP), which in turn functions as a second messenger that binds to and
activates STING. (Zhao
GN, Jiang DS, Li H. Interferon regulatory factors: at the crossroads of
immunity, metabolism, and
disease. Biochim Biophys Acta. 2015;1852:365-78. PMID:24807060). Novel
anticancer
immunotherapies based on recombinant type I IFNs, type I IFN-encoding vectors,
type I IFN-
expressing cells, and STING agonists are therefore currently being developed
as novel tumor
immunotherapies.
101511 Overexpression of the PAMP immunomodulator, 3'-5" c-di-
AMP. 3"-5" c-di-AMP is a
strong inducer of the STING-TBK1-IRF3 axis. It is produced by mycobacteria
including BCG by
the disA gene which encodes the DisA protein (BCG protein WP_010950916.1 in
BCG,
tuberculosis protein Rv3586 or P9WNW5.1). Mycobacterium tuberculosis (M.tb)
synthesizes and
secretes c-di-AMP, which activates the interferon regulatory factor (IRF)
pathway and type I IFN
responses through STING-signaling and cGAS. (Ahmed D, Cassol E. Role of
cellular metabolism
in regulating type I interferon responses: Implications for tumour immunology
and treatment.
Cancer Lett. 2017;409:20-29. PMID: 28888999.). c-di-AMP overexpressing M.tb
strains showed
attenuation of TB in a mouse model. As a mucosal adjuvant, c-di-AMP exerts
immune stimulatory
effects causing maturation of dendritic cells, up-regulation of co-stimulatory
molecules and
production of pro-inflammatory cytokines, and strong Thl, Th17 and CD8 T cell
responses against
pathogens. A c-di-AMP¨overexpressing BCG strain (rBCG-disA or BCG-disA-0E) has
been
constructed and it was surprisingly found that it produced a significantly
higher 1RF and IFN-13
response than BCG itself, indicating that bacteria-derived c-di-AMP gains
access to the host cell
cytosol despite the absence of the ESX-1 protein secretion system. (Ahmed D,
Cassol E. Role of
cellular metabolism in regulating type I interferon responses: Implications
for tumour immunology
and treatment. Cancer Lett. 2017;409:20-29. PMID: 28888999.). These findings
suggest that
rBCG strains modified to overexpress c-di-AMP could induce better protective
immunity against
bladder tumors than BCG itself
39
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
[0152] Induction of pro-inflammatory Thl cytokines in mouse bone
marrow-derived
macrophages (BMDMs) in response to BCG oyerexpressing nth disA (MT3692): Mil)
genome encodes a di-adenylate cyclase enzyme (DisA, also called DacA, P9WNW5.1
in the
UniProtKB/Swiss-Prot databases) that synthesizes e-di-AMP from ATP or ADP. The
BCG protein
WP 010950916.1 (NCBI reference number) is 100% identical to M. tuberculosis
DisA. M.tb
strains overexpressing disA intoxicate macrophages by releasing excessive c-di-
AMP, a unique
bacterial PAMP that activates STING-dependent IFN-13 production. (Ahmed D,
Cassol E. Role of
cellular metabolism in regulating type I interferon responses: Implications
for tumour immunology
and treatment. Cancer Lett. 2017;409:20-29. PMID: 28888999.). To expand the
antigenic
repertoire of a non-pathogenic vaccine strain, BCG Pasteur was transformed
with a kanamycin-
resistance (Kan-R)-conferring plasmid that harbors the disA gene (M
tuberculosis Rv3586 or
MT3692) from M.tb (the M.tb and BCG disA genes are 100% identical) fused to
the strong
mycobacterial promoter, Phsp60. Addition of this plasmid to BCG-Pasteur
increased the level of
disA mRNA by 50-fold (Fig. lb). The closely related M.tb-disA-OE strain
releases 15-fold more
c-di-AMP into the macrophage cytosol than wild type M tb. (Fig. la), and hence
it is expected
that BCG-disA-OE also releases significantly more c-di-AMP into the host
cytosol. These disA
overexpressor recombinants (rBCG or BCG-disA-0E) were better inducers of STING-
dependent
IFN-(3 as compared to the parental strain. Most importantly as reported in
PCT/US2016/017248,
filed February 10, 2016, guinea pigs vaccinated with rBCG were significantly
better protected
against aerosol infection with virulent M.tb, suggesting improved protective
efficacy over existing
BCG strain.
[0153] As shown in Fig. 2, immune responses elicited by BCG-
Pasteur disA-OE were tested
in an in vitro macrophage infection model. BMDMs from C57BL/6 mice infected
with BCG-
Pasteur disA-OE showed significant upregulation of IFN-f3, TNF-a, IL-6 and IL-
2 in comparison
to uninfected or wild-type BCG infected macrophages.
[0154] As shown in Fig. 3, augmented c-di-AMP-based STING
activation was confirmed in
RAWBlue ISG macrophages. RAWBlue macrophages showed increased IRF3 levels when
infected with BCG-Pasteur disA-0E, as compared to parental control.
[0155] As shown in Figs 4A-4C, a significant increase in secreted
pro-inflammatory cytokines
(TNF-a, IL-6 and IL-1 was found in culture supernatants of BCG-Pasteur-disA -
OE infected
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
mouse BMDMs. These findings indicate that BCG-Pasteur-disA-OE with increased
antigenic
repertoire acts like a STING agonist, and hence a potent inducer of STING-
dependent type T IFNs.
Furthermore, the immune responses in macrophages in response to BCG-Pasteur
disA-OE were
skewed towards Thl, a phenotype largely attributed for control of NMIBC by BCG
immunotherapy.
[0156] As shown in Fig. 5, BCG-disA-OE elicits anti-tumor immune
responses in human
bladder carcinoma (RT4) cells. BCG-Pasteur-disA-OE was tested to determine
whether it elicits
similar immune responses in bladder cancer (BC) cells, in comparison to WT
strains BCG-Pasteur
and OncoTICE (the current immunotherapeutic BCG strain). Human RT4 BC cells,
derived from
human NMIBC tumors, were challenged with the wild-type (both Pasteur and TICE)
and
recombinant BCG Pasteur disA-OE strain at 1:20 (RT4 BCG) for 3h, and
differential gene
expression profile was determined in comparison to uninfected cells. Key
immune mediators such
as, monocyte chemoattractant protein 1 (MCP-1)/CCL2, TEN-13 and IL-113 were
found to be
significantly increased in bladder cancer cells exposed to BCG-Pasteur-disA-OE
compared to
responses to wild type strains.
[0157] As shown in Fig. 6, an experimental system was set up to
test whether intravesical
BCG-disA-OE immunotherapy leads to heightened Thl responses and anti-tumor
efficacy in the
MNU carcinogen model of NMIBC. Results from the aforementioned experiments
with RT4 cells
encouraged the inventors to test the relative therapeutic efficacy of BCG-
Pasteur di sA OE in an in
vivo rat NMIBC model, pioneered in Bivalacqua lab. (Kates M, Nirschl T, Sopko
NA, Matsui H,
Kochel CM, Reis LO, Netto GJ, Hogue MO, Hahn NM, McConkey DJ, Baras AS, Drake
CG,
Bivalacqua TJ. Intravesical BCG Induces CD4(+) T-Cell Expansion in an Immune
Competent
Model of Bladder Cancer. Cancer Immunol Res. 2017;5:594-603. PMID: 28588015).
In this
model, N-methyl-N-nitrosourea (MNU), a carcinogenic alkylating agent, is used
to induce
urothelial cancer in female Fischer rats.
[0158] As can be seen in Fig. 7, BCG-disA-OE has significant
immunotherapeutic effects in
the rat bladder cancer model. Urothelial dysplasia develops within eight weeks
of MNU
instillation, and by the 16th week after the first instillation, all rats
display carcinoma-in-situ,
papillary Ta, or high-grade Ti urothelial carcinoma with histopathologic and
immunophenotypic
features similar to those observed in human urothelial cancer. Using this
model, it was showed
41
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
that intravesical BCG immunotherapy lead to a large, transient rise in the CD4
T cell population
in the urothelium. (Kates M, Nirschl T, Sopko NA, Matsui H, Kochel CM, Reis
LO, Netto GJ,
Roque MO, Hahn NM, McConkey DJ, Baras AS, Drake CG, Bivalacqua TJ.
Intravesical BCG
Induces CD4(+) T-Cell Expansion in an Immune Competent Model of Bladder
Cancer. Cancer
Immunol Res. 2017;5:594-603. PMID: 28588015). Intravesical instillation of BCG-
disA-OE strain
was performed in MNU-treated rats, administered sequentially every week for 6
weeks starting
eight weeks after MNU induction when tumors are visible. Bladder tumors were
staged by a GU
pathologist according to WHO-ISUP classifications with percent tumor
involvement (sum of Ta,
Ti and CIS) calculated for each group according to criteria as described.
(Kates M, Nirschl T,
Sopko NA, Matsui H, Kochel CM, Reis LO, Netto GJ, Hogue MO, Hahn NM, McConkey
DJ,
Baras AS, Drake CG, Bivalacqua TJ. Intravesical BCG Induces CD4(+) T-Cell
Expansion in an
Immune Competent Model of Bladder Cancer. Cancer Immunol Res. 2017;5:594-603.
PMID:
28588015). A significant decrease in tumor involvement index in rats treated
with BCG-Pasteur
disA-OE was found in comparison to bladders from untreated or BCG-Pasteur
treated rats.
[0159] As can be seen in Fig. 8, BCG-disA-OE induces a
characteristic cytokine and
chemokine signature in rat bladders undergoing immunotherapy. Rat urinary
bladders from rats
treated with BCG-disA-OE showed a significant induction of IFN-a/[3, IFN-y, IL-
113, INF-a, IGF-
Pp, iNOS, IP-10, MCP-1 and MIP-la in comparison to untreated or BCG-Pasteur
treated rats.
[0160] As shown in Fig. 9, evidence was found for increased
infiltration of CCL2+
macrophages, Nos2 and IL-113' M1 macrophages, accompanied by increased IL-6
and IFN-
expression in bladders of rats treated with BCG-Pasteur-disA-0E.
Interestingly, increased levels
of IP-10 were found, which together with increased IFN-y is known to promote a
strong T cell
recruitment at the site of infection and inflammation.
[0161] Fig. 10 shows a summary of the cytokine expression level
changes observed with BCG-
disA-OE versus BCG-WT in primary cells, cancer cell lines, and in rat bladder
cancer tissues. As
can be seen, cytokines associated with Thl T cell and M1 macrophage expansion,
two type 1
interferons, and three pro-inflammatory chemokines were significantly
upregulated by BCG-disA-
OE compared to BCG-WT (2-fold to 30-fold) across these cells, cell lines and
tissues. In contrast,
cytokines associated with Th2 T cell and M2 macrophage expansion were
generally down-
regulated by BCG-disA-OE in comparison to BCG-WT (1-fold to 10-fold).
42
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
[0162] BCG immunotherapy may be effective via three immune
mechanisms: (i) increased
generation of tumor-specific cytotoxic CDS T cells, (ii) cytokine environment
which promotes
macrophage-mediated CD4 cell activation against tumor antigens, and (iii)
macrophage MI shift
promoting enhanced tumoricidal activity. The findings reported herein strongly
indicate that BCG
overexpressing c-di-AMP is taken up by bladder tumor cells, and myeloid cells
that are either
resident or recruited to the tumor microenvironment, and induces host immune
responses,
including activation of STING and type I IFN responses, and NF-KB signaling,
that promotes
secretion of cytokines and chemokines, macrophage recruitment and apoptotic
mechanisms, all of
which collectively reduce tumor progression.
[0163] In addition to overexpression of disA generating increased
levels of the PAMP
molecule c-di-AMP, there are additional recombinant DNA modification which may
be made to
BCG to enhance its production of other PAMP and DAMP molecules. Genes for
other CDN
cyclases--(i) the GGDEF domain of the BCG_R507340 protein or /14. tuberculosis
Rv1354c
protein (100% identical to each other), (ii) the Vibrio cholerae DncV protein,
Q9KVG7 in Swiss-
Prot, which is a 2'-5'c-GAMP synthase, and (iii) the human cGAS protein Q8N884
in Swiss-Prot
which is a 2'-3' cGAMP synthase--may be added to BCG. These added CDN cyclase
genes may
be added alone or in combination. Such combinations would represent
multivalent CDN
overexpressing BCG. Also, as shown in Fig. 11, BCG possess several CDN
phosphodiesterase
genes or genes which contain phosphodiesterase domains. Recombinant technology
methods to
remove these endogenous phosphodiesterase genes and intragenic
phosphodiesterase domains: (i)
the BCG WP_003414507 gene which encodes a CDN PDE in BCG that is 100%
identical to the
M tuberculosis Rv2837c (also called CdnP or CnpB), (ii) the DNA encoding the
EAL domain of
protein BCG RS07340 (previously BCG 1416c) which is 100% identical to the
known CDN PDE
M. tuberculosis Rv1354c protein, and (iii) the gene encoding BCG AHM07112
which is
homologous the known CDN PDE M. tuberculosis Rv1357c. Removal of the genes
encoding these
PDEs will serve to further increase the levels of CDN PAMP and DAMP molecules
produced by
the rBCG strains disclosed herein.
[0164] SEQ ID NO:1
43
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
[0165] Diadenylate cyclase DisA from BCG and other related
mycobacteria, amino acid
sequence (358 amino acids; BCG protein A0Q92_RS18745; NCBT Reference Sequence:
NZ CUWL01000001.1). The identical sequence is present in other strains of BCG,
e.g.,
Mycobacterium tuberculosis as protein Rv3586 or MT3692, and in Mycobacterium
bovis as
protein Mb3617.
MHAVTRPTLREAVARLAPGTGLRDGLERILRGRTGALIVLGHDENVEATCDGGESLDVRY
AATRLRELCKMDGAVVLSTDGSRIVRANVQLVPDPSIPTDESGTRHRSAERAATQTGYPV
1SVSHSMNIVTVYVRGERHVLTDSATILSRANQAIATLERYKTRLDEVSRQLSRAEIEDF
VTLRDVMTVVQRLELVRRIGLVIDYDVVELGTDGRQLRLQLDELLGGNDTARELIVRDYH
ANPEPPSTGQINATLDELDALSDGDLLDFTALAKVFGYPTTTEAQDSTLSPRGYRAMAGI
PRLQFAHADLLVRAFGTLQGLLAASAGDLQSVDGIGAMWARHVREGLSQLAESTISDQ
[0166] SEQ ID NO:2
[0167] Diadenylate cyclase disii from BCG and other related
mycobacteria, DNA sequence
(1077 nucleotides 1358 codons, 1 stop codonl; encodes BCG gene A0Q92 RS18745;
NCBI
Reference Sequence: NZ CUWL01000001.1) Identical sequence is present in other
strains of
BCG, e.g., Mycobacterium tuberculosis as gene Rv3586 or MT3692, Mycobacterium
bovis as gene
Mb3617.
1 atgcacgctg tgactcgtcc gaccctgegt gaggctgtcg cccgcclagc cccgggcact
61 gggctgcggg acggcctgga gcgtatcctg cgcggccgca ctggtgccct gatcgtgctg
121 ggccatgacg agaatgtcga ggccatctgc gatggtggct tctccctcga tgtccgctat
181 gcagcaaccc ggctacgcga gctgtgcaag atggacggcg ccgtggtgct gtccaccgac
241 ggcagccgca tcgtgcgggc caacgtgcaa ctggtaccgg atccgtcgat ccccaccgac
301 gaatcgggga cccggcaccg ctcggccgag cgggccgcga tccagaccgg ttacccggtg
361 atctcagtga gccactcgat gaacatcgtg accgtctacg tccgcgggga acgt, cacgta
421 ttgaccgact cggca.accat cctgtcgcgg gccaaccagg ccatcgcaac cctggagcgg
481 tacaaaacca ggctcgacga ggtcagccgg caactgtcca gggcagaaat cgaggacttc
541 gtcacgctgc gcgatgtgat gacggtggtg caacgcctcg a.gctggfccg gcga.atcggg
601 ctggtgatcg actacgacgt ggtcgaactc ggcactgatg gtcgtcagct gcggctgcag
661 ctcgacgagt tgctcggcgg caacgacacc gcccgggaat tgatcgtgcg cgattaccac
721 gccaacccgg aaccaccgtc cacggggcaa atcaatgcca ccctggacga actggacgcc
781 ctgtcggacg gcgacctcct cgatttcacc gcgctggcaa aggttttcgg atatccgacg
841 accacggaag cgcaggattc ggcgctgagc ccgcgtggct accgcgcgat ggccggtatc
901 ccccggctcc agttcgccca tgccgacctg ctggtccggg cgttcggaac gttgcagggt
961 ctgctggcgg ccagcgccgg cgatctgcaa tcagtggacg gcatcggcgc catgtgggcc
1021 cgtcatgtgc gcgatgggtt gtcacagctg gcggaatcga ccatcagcga tcaataa
[0168] SEQ ID NO:3.
[0169] Plasmid pSD5B-Phsp6o::dis,4 is an episomally replicating
E. coli-mycobacterial shuttle
plasmid that overexpresses the BCG disil gene from the Phsp60 promoter, DNA
sequence. (7742
44
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
nucleotides; promoter Phsp6o DNA comprised of a portion of the M. leprae hsp65
gene nucleotides
13 to 180 is underlined; disA coding sequence nucleotides 242 to 131 g; ATG
start codon and TAA
stop codon shown in boldface, underline).
GGATCCTTCTAGAATTCCGGAATTGCACTCGCCTTAGGGGAGTGCTAAAAATGATCCTGGCACTCGCGATCAGCGAG
1-77
TGCCAGGTCGGGACGGTGAGACCCAGCCAGCAAGCTGTGGTCGTCCGTCGCGGGCACTGCACCCGGCCAGCGTAAGT
78-154
AATGGGGGTTGTCGGCACCCGGTGACCTAGACACATGCATGCATGCTTAATTAATTAAGCGATATCCGGAGGAATCA
155-231
CTTCCATATGATCCACGCTGTGACTCGTCCGACCCTGCGTGAGGCTGTCGCCCGCCTAGCCCCGGGCACTGGGCTGC
232-308
GGGACGGCCTGGAGCGTATCCTGCGCGGCCGCACTGGTGCCCTGATCGTGCTGGGCCATGACGAGAATGTCGAGGCC
309-385
ATCTGCGATGGTGGCTTCTCCCTCGATGTCCGCTATGCAGCAACCCGGCTACGCGAGCTGTGCAAGATGGACGGCGC
386-462
CGTGGTGCTGTCCACCGACGGCAGCCGCATCGTGCGGGCCAACGTGCAACTGGTACCGGATCCGTCGATCCCCACCG
463-539
ACGAATCGGGGACCCGGCACCGCTCGGCCGAGCGGGCCGCGATCCAGACCGGTTACCCGGTGATCTCAGTGAGCCAC
540-616
TCGATGAACATCGTGACCGTCTACGTCCGCGGGGAACGTCACGTATTGACCGACTCGGCAACCATCCTGTCGCGGGC
617-693
CAACCAGGCCATCGCAACCCTGGAGCGGTACAAAACCAGGCTCGACGAGGTCAGCCGGCAACTGTCCAGGGCAGAAA
694-770
TCCiAGGACTTCGTCACGCTGCGCGATCiTGATGACCiGTGGTGCAACGCCTCCiAGCTGGTCCGGCGAATCGGGCTli
GTG 771-847
ATCGACTACGACGTGGTCGAACTCGGCACTGATGGICGTCAGCTGCGGCTGCAGCTCGACGAGTTGCTCGGCGGCAA
848-924
CGACACCGCCCGGGAATIGATCGIGCGCGATTACCACGCCAACCCGGAACCACCGICCACGGGGCAAATCAATGCCA
925-1001
CCCIGGACGAACTGGACGCCCIGICGGACGGCGACCICCTCGATTICACCGCGCIGGCAAAGGTTITCGGATATCCG
1002-1078
ACGACCACGGAAGCGCAGGATTCGACGCTGAGCCCGCGTGGCTACCGCGCGATGGCCGGTATCCCCCGGCTCCAGTT
1079-1155
CGCCCATGCCGACCTGCTGGTCCGGGCGTTCGGAACGTTGCAGGGTCTGCTGGCGGCCAGCGCCGGCGATCTGCAAT
1156-1232
CAGTGGACGGCATCGGCGCCATGTGGGCCCGTCATGTGCGCGAGGGGTTGTCACAGCTGGCGGAATCGACCATCAGC
1233-1309
GATCAATAAACGCGTTCTGGCGTAATAGCGAAGAGGCCCGCACCGATCGCCCTTCCCAACAGTTGCGCAGCCTGAAT
1310-1386
GGCGAATGGCGCTTTGCCTGGTTTCCGGTCGAAGCTTGGCCGGATCTAAAGTTTTGTCGTCTTTCCAGACGTTAGTA
1387-1463
AATGAATTTTCTGTATGAGGTTTTGCTAAACAACTTTCAACAGTTTCAGCGGAGTGAGAATAGAAAGGAACAACTAA
1464-1540
AGGAATTGCGAATAATAATTTTTTCACGTTGAAAATCTCCAAAAAAAAAGGCTCCAAAAGGAGCCTTTAATTGTATC
1541-1617
GGTTTATCAGCTTGCTTTCGAGGTGAATTTCTTAAACAGCTTGATACCGATAGTTGCGCCGACAATGACAACAACCA
1618-1694
TCGCCCACGCATAACCGATATATTCGGTCGCTGAGGCTTGCAGGGAGTCAAAGGCCGCTTTTGCGGGGATCCGCTC1i
1695-1771
GAGGCGCGGICGCGGCGCGGCTGTGGCATGTCGGGGCGTGCCGCTCCECCGGCGCCGCCCATCGGCCCGCCCATTGG
1772-1848
CATICCGCCCATGCCGCCCATCATICCIGIGGAGCCAGAACTGATCCAGCCIGIGCCACAGCCGACAGGATGGIGAC
1849-1925
CACCATTTGCCCCATATCACCGTCGGTACTGATCCCGTCGTCAATAAACCGAACCGCTACACCCTGAGCATCAAACT
1926-2002
CTTTTATCAGTTGGATCATGTCGGCGGTGTCGCGGCCAAGACGGTCGAGCTTCTTCACCAGAATGACATCACCTTCC
2003-2079
TCCACCTTCATCCTCAGCAAATCCAGCCCTTCCCGATCTGTTGAACTGCCGGATGCCTTGTCGGTAAAGATGCGGTT
2080-2156
AGCTTTTACCCCTGCATCTTTGAGCGCTGAGGTCTGCCTCGTGAAGAAGGTGTTGCTGACTCATACCAGGCCTGAAT
2157-2233
CGCCCCATCATCCAGC CAGAAAG TGAGGGAGC CAC GG TTGATGAGAGCTTTGTTG TAGG
TGGACCAGTTGGTGATTT 2234-2310
TGAACTTTTGCTTTGCCACGGAACGGTCTGCGTTGTCGGGAAGATGCGTGATCTGATCCTTCAACTCAGCAAAAGTT
2311-2387
CGATTTATTCAACA A AGCCGCCGTCCCGTCAAGTCAGCGTA ATGCTCTGCCACiTGTTACA ACCA ATT A
ACCA ATTCT 2388-2464
GATTAGAAAAACTCATCGAGCATCAAATGAAACTGCAATTTATTCATATCAGGATTATCAATACCATATTTTTGAAA
2465-2541
AAGCCGTTTCTGTAATGAAGGAGAAAACTCACCGAGGCAGTTCCATAGGATGGCAAGATCCTGGTATCGGTCTGCGA
2542-2618
TTCCGACTCGTCCAACATCAATACAACCTATTAATTTCCCCTCGTCAAAAATAAGGTTATCAAGTGAGAAATCACCA
2619-2695
TGAGTGACGACTGAATCCGGTGAGAATGGCAAAAGCTTATGCATTTCTTTCCAGACTTGTTCAACAGGCCAGCCATT
2696-2772
ACGCTCGTCATCAAAATCACTCGCATCAACCAAACCGTTATTCATTCGTGATTGCGCCTGAGCGAGACGAAATACGC
2773-2849
GATCGCTGTTAAAAGGACAATTACAAACAGGAATCGAATGCAACCGGC GCAGGAACACTGCCAGCGCATCAACAATA
2850-2926
TTTTCACCTGAATCAGGATATTCTTCTAATACCTGGAATGCTGTTTTCCCGGGGATCGCAGTGGTGAGTAACCATGC
2927-3003
ATCATCAGGAGTACGGATAAAATGCTTGATGGTCGGAAGAGGCATAAATTCCGTCAGCCAGTTTAGTCTGACCATCT
3004-3080
CATCTGTAACATCATTGGCAACGCTACCTTTGCCATGTTTCAGAAACAACTCTGGCGCATCGGGCTTCCCATACAAT
3081-3157
CGATAGATTGTCGCACCTGATTGCCCGACATTATCGCGAGCCCATTTATACCCATATAAATCAGCATCCATGTTGGA
3158-3234
ATTTAATCGCGGCCTCGAGCAAGACGTTTCCCGTTGAATATGGCTCATAACACCCCTTGTATTACTGTTTATGTAAG
3235-3311
CAGACAGTTTTATTGTTCATGATGATATATTTTTATCTTGTGCAATGTAACATCAGAGATTTTGAGACACAACGTGG
3312-3388
CTTTGTTGAATAAATCGAACTTTTGCTGAGTTGAAGGATCAGATCACGCATCTTCCCGACAACGCAGACCGTTCCGT
3389-3465
GGCAAAGCAAAAGTTCAAAATCACCAACTGGTCCACCTACAACAAAGCTCTCATCAACCGTGGCTCCCTCACTTTCT
3466-3542
GGCTGGATGATGGGGCGATTCAGGCCTGGTATGAGTCAGCAACACCTTCTTCACGAGGCAGACCTCAGCGCTAGCGli
3543-3619
AGTGTATACTOGCTTACTATGTTGOCACTGATGAGGGTGTCAGTOA AGTGCTTCATOT(1( i(1,Ati(iA(
1A,A AA AAtiCiCTri 3620-3696
CACCGGIGCGICAGCAGAATAIGIGATACAGGATATATICCGCTICCTCGLICACTGACICC1CIACGCTCGGICGIT
3697-3773
CGACTGCGGCGAGCGGAAATGGCTTACGAACGGGGCGGAGATTTC CTGGAAGATGCCAGGAAGATACTTAACAGGGA
3774-3850
AGTGAGAGGGCCGCGGCAAAGCCGTTTTTCCATAGGCTCCGCCCCCCTGACAAGCATCACGAAATCTGACGCTCAAA
3851-3927
TCAGTGGTGGCGAAACCCGACAGGACTATAAAGATACCAGGCGTTTCCCCCTGGCGGCTCCCTCGTGCGCTCTCCTG
2928-4004
TTCCTGCCTTTCGGTTTACCGGTGTCATTCCGCTGTTATGGCCGCGTTTGTCTCATTCCACGCCTGACACTCAGTTC
4005-4081
CGGGTAGGCAGTTCGCTCCAAGCTGGACTGTATGCACGAACCCCCCGTTCAGTCCGACCGCTGCGCCTTATCCGGTA
4082-4158
ACTATCGTCTTGAGTCCAACCCGGAAAGACATGCAAAAGCACCACTGGCAGCAGCCACTGGTAATTGATTTAGAGGA
4159-4235
GTTAGTCTTGAAGTCATGCGCCGGTTAAGGCTAAACTGAAAGGACAAGTTTTGGTGACTGCGCTCCTCCAAGCCAGT
4236-4312
TACCTCGGTTCAAAGAGTTGGTAGCTCAGAGAACCTTCGAAAAACCGCCCTGCAAGGCGGTTTTTTCGTTTTCAGAG
4313-4389
CAAGAGATTACGCGCAGACCAAAACGATCTCAAGAAGATCATCTTATTAAGGGGTCTGACGCTCAGTGGAACGAAAA
4390-4466
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
CTCACGTTAAGGGATTTTGGTCATGAGATTATCAAAAAGGATCTTCACCTAGATCCTTTTAAAAGTGCTCATCATTG
4467-4543
GAAAACGTTCTTCGGGGCGAAAACTCTCAAGGATCTTACCGCTGTTGAGATCCAGTTCGATGTAACCCACTCGTGCA
4544-4620
CCCA A CTGA TCTTCA CiCA TCTTTTAC TTTC ACC AGCCITTTCTGCiGTGA GCA A AA ACAGGA
ACICiCA A A A TGCCGCA A A 4621-4697
AAAGGGAATAAGGGCGACACGGAAATGITCiAATACTCATACTCTICCTTITTCAATATTATTGAAGCATTTATCACiG
4698-4774
GrlATTGICTCATGAGCGGATACATAFFYGAATGTATITAGAAAAATAAACAAATAGGGGITCCGCGCACATFICCC
4775-4851
CGAAAAGTGCCACCTGACGTCTAAGAAACCATTATTATCATGACATTAACCTATAAAAATAGGCGTATCACGAGGCC
4852-4928
CTTTCGTCTTCAAGAATTCCCAGGCATCAAATAAAACGAAAGGCTCAGTCGAAAGACTGGGCCTTTCGTTTTATCTG
4929-5005
TTGTTTGTCGGTGAACGCTCTCCTGAGTAGGACAAATCCGCCGGGAGCGGATTTGAACGTTGCGAAGCAACGGCCCG
5006-5082
GAGGGTGGCGGGCAGGACGCCCGCCATAAACTGCCAGGGAATTCCCATCGAGCCGAGAACGTTATCGAAGTTGGTCA
5083-5159
TGTGTAATCCCCTCGTTTGAACTTTGGATTAAGCGTAGATACACCCTTGGACAAGCCAGTTGGATTCGGAGACAAGC
5160-5236
AAATTCAGCCTTAAAAAGGGCGAGGCCCTGCGGTGGTGGAACACCGCAGGGCCTCTAACCGCTCGACGCGCTGCACC
5237-5313
AACCAGCCCGCGAACGGCTGGCAGCCAGCGTAAGGCGCGGCTCATCGGGCGGCGTTCGCCACGATGTCCTGCACTTC
5314-5390
GAGCCAAGCCTCGAACACCTGCTGGTGTGCACGACTCACCCGGTTGTTGACACCGCGCGCGGCCGTGCGGGCTCGGT
5391-5467
GOGGCGGCTCTGTCGCCCTTGCCAGCGTGAGTAGCGCGTACCTCACCTCGCCCAACAGGTCGCACACAGCCGATTCG
5468-5544
TACGCCATAAAGCCAGGTGACiCCCACCAGCTCCGTAAGTTCGGGCGCTGTGTGGCTCCiTACCCGCGCATTCAGGCGG
5545-5621
CAGGGGGTCTAACGGGICLAAGGCGGCCiTGTACGCGGCCACAGCGGCTCTCAGCGGCCCGGAAACGTCCTCGAAACG
5622-5698
ACGCATGIGTICCTCCTGGITGGTACAGGIGGTIGGGGGTGCTCGGCTGICGCGGTTGTICCACCACCAGGGCTCGA
5699-5775
CGGGAGAGCGGGGGAGTGTGCACTTGTGGGGTGGCCCCTCAGCGAAATATCTGACTTGGAGCTCGTGTCGGACCATA
5776-5852
CACCGGTGATTAATCGTGGTCTACTACCAAGCGTGAGCCACGTCGCCGACGAATTTGAGCAGCTCTGGCTGCCGTAC
5853-5929
TGGCCGCTGGCAAGCGACGATCTGCTCGAGGGGATCTACCGCCAAAGCCGCGCGTCGGCCCTAGGCCGCCGGTACAT
5930-6006
CGAGGCGAACCCAACAGCGCTGGCAAACCTGCTGGTCGTGGACGTAGACCATCCAGACGCAGCGCTCCGAGCGCTCA
6007-6083
GCGCCCGGGGGTCCCATCCGCTGCCCAACGCGATCGTGGGCAATCGCGCCAACGGCCACGCACACGCAGTGTGGGCA
6084-6160
CTCAACGCCCCTGTTCCACGCACCGAATACGCGCGGCGTAAGCCGCTCGCATACATGGCGGCGTGCGCCGAAGGCCT
6161-6237
TCGGCGGCCGTCGACGGCGACCGCAGTTACTCAGGCCTCATGACCAAAAACCCCGGCCACATCGCCTGGGAAACGGA
6238-6314
ATGGCTCCACTCAGATCTCTACACACTCAGCCACATCGAGGCCGAGCTCGGCGCGAACATGCCACCGCCGCGCTGGC
6315-6391
GTCAGCAGACCACGTACAAAGCGGCTCCGACGCCGCTAGGGCGGAATTGCGCACTGTTCGATTCCGTCAGGTTGTGG
6392-6468
GCCTATCGTCCCGCCCTCATCiCGGATCT ACCTGCCG ACCCGG A A CG TGG A CGG A CTCGGCCGCGCG
A TCTA TGCCG A 6469-6545
GTGCCACGCGCGAAACGCCGAATTCCCGTGCAACGACGTGTGTCCCGGACCGCTACCGGACAGCGAGGTCCGCGCCA
6546-6622
TCGCCAACAGCATITGGCGTIGGATCACAACCAAGTCGCGCATTIGGGCGGACGGGATCGIGGICIACGAGGCCACA
6623-6699
CICAGTGCGCGCCAGICGGCCATCTCGCGGAAGGGCGCAGCAGCGCGCACGGCGGCGAGCACAGITGCGCGGCGCGC
6700-6776
AAAGTCCGCGTCAGCCATGGAGGCATTGCTATGAGCGACGGCTACAGCGACGGCTACAGCGACGGCTACAACCGGCA
6777-6853
GCCGACTGTCCGCAAAAAGCCGTGACGCGCCGAAGGCGCTCGAATCACCGGACTATCCGAACGCCACGTCGTCCGGC
6854-6930
TCGTGGCGCAGGAACGCAGCGAGTGGCTCGCCGAGCAGGCTGCACGCGCGCGAAGCATCCGCGCCTATCACGACGAC
6931-7007
GAG GGCCACTCTTGGCCGCAAACGGCCAAACATTTCGGGCTGCATCTGGACACCGTTAAGCGACTCGGCTATCGGGC
7008-7084
GAGGAAAGAGCGTGCGGCAGAACAGGAAGCGGCTCAAAAGGCCCACAACGAAGCCGACAATCCACCGCTGTTCTAAC
7085-7161
GCAATTGGGGACGGGTGTCGCGGGGGTTCCGTGGGGGGTTCCGTTGCAACGGGTCGGACAGGTAAAAGTCCTGGTAG
7162-7238
ACGCTAGTTTTCTGGTTTGGGCCATGCCTGTCTCGTTGCGTGTTTCGTTGCGCCGTTTTGAATACCAGCCAGACGAG
7239-7315
ACGGGGTTCTACGAATCTTGGTCGATACCAAGCCATTTCCGCTGAATATCGGGGAGCTCACCGCCAGAATCGGTGGT
7316-7392
TGTGGTGATGTACGTGGCGAACTCCGTTGTAGTGCCTGTGGTGGCATCCGTGGCCACTCTCGTTGCACGGTTCGTTG
7393-7469
TGCCGTTACACiGCCCCGTTGACAGCTCACCGA ACGTAGTTA A AACATGCTGGTCA A ACTAGGTTTACCA
ACCiATACG 7470-7546
AGICAGCTCATCTAGGGCCAGITCIAGGCGITGITCGTYGCGCGGTICGTIGCGCATUFLICGIGTGULIGCTAGAT
7547-7623
GGCTCCGCAACCACACGCTTCGAGGTTGAGTGCTTCCAGCACGGGCGCGATCCAGAAGAACTTCGTCGTGCGACTGT
7624-7700
CCTCGTTGGGATCTAGCCCGCCTAATGAGCGGGCTTTTTTTT 7701-7742
[0170]
Mycobacteria overexpressing disA are attenuated for virulence. As shown
in Fig. 12,
when mice are infected with 3.5 logio units by the aerosol route of either M.
tuberculosis harboring
the pSD5B Phsp60::disA plasmid
tb-disA-OE or Mth-OE) or wild type M. tuberculosis (Mtb-
CDC1551), there are profound differences in the median time to death (MTD) of
the animals. As
can be seen, wild type M tuberculosis (Mtb-CDC1551) gave an MTD of 150.5 days,
while M.
tuberculosis harboring the pSD5B Phsp6o::disA plasmid (M.tb-disA-OE or Mtb-OE)
was a
significantly weaker pathogen giving an MTD of 321.5 days. A similar reduction
in the
pathogenicity is to be expected with BCG-disA-OE compared with BCG-WT. Hence,
it is likely
that should BCG-disA-OE be used as a cancer immunotherapy, one would
anticipate reduced rates
46
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
of bloodstream dissemination, reduced dysuria, reduced urgency and reduced
malaise compared
with BCG-WT.
[0171] Addition of CDN cyclase genes to rBCG other than disA
[0172] Overexpression of the PAMP immunomodulator, 3'-5' c-di-GMP
by overexpressint
the GGDEF domain of protein BCG RS07340. 3'-5' c-di-GMP is a strong inducer of
the STING-
TBK1-IRF3 axis. It is produced by mycobacteria including BCG by the GGDEF
domain of protein
BCG RS07340 (previously BCG_1416c) and by the M. tuberculosis Rv1354c gene.
The
BCG RS07340 protein (100% identical to the M. tuberculosis Rv1354c protein)
encodes a
bifunctional diguanylate cyclase/diguanylate phosphodiesterase. Hence the
portion that functions
as a diguanylate cyclase is an endogenous CDN-producing enzyme in BCG. The
full-length
BCG RS07340 polypeptide is 623 amino acids in length, and its domain structure
is: N-terminus-
GAF-GGDEF-EAL-C-terminus. The GAF domain (approximately amino acids 1-190) is
a
regulatory domain which influences the activity of the other domains. The
GGDEF domain
(approximately amino acids 190-350) is a diguanylate cyclase catalyzing the
reaction 2 GTP 4 c-
di-GMP + 2 pyrophosphates. The EAL domain (approximately amino acids 350-623)
is a
diguanylate phosphodiesterase catalyzing the reaction c-di-GMP
2 GMP. By genetically
removing the DNA sequences that encode the C-terminal EAL domain, it is
possible to use the
DNA encoding the GGDEF domain to generate a recombinant BCG that will
overexpress
diguanulate cyclase activity. This may be accomplished by also deleting the
DNA encoding the
regulatory-sensor GAF domain and/or the use of mutations in the DNA encoding
the GAF domain
to relieve any cyclase inhibitory activity it may possess. Such techniques to
generate constitutively
active recombinant forms of the BCG R507340 protein will produce high levels
of c-di-GMP in
recombinant BCG.
[0173] SEQ ID NO:4
[0174] Bifunctional diguanylate cyclase/phosphodiesterase
BCG_RS07340 from BCG and
other related mycobacteria, amino acid sequence (623 amino acids; BCG protein
BCG_RS07340;
NCBI Reference Sequence: NC 008769.1; Protein ID WP 003898837.1; old locus tag
BCG 1416c). The identical sequence is present in other strains of BCG, e.g.,
Mycobacterium
tuberculosis as protein Rv1354c or MT1397, and in Mycobacterium bovis as
protein Mb1389c.
The EAL domain is from amino acid 354 to 623 and is underlined.
47
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
MCNDTATPQLEELVTIVANQLMTVDAATSAEVSQRVLAYLVEQL
GVDVSFLRHNDRDRRATRLVAEWPPRLNIPDPDPLRLIYFADADPVFALCEHAKEPLV
FRPEPATHDYQRLIHEARGVPVTSAAAVPLVSGEITTGLLGFIKFGDRKWHEAELNAL
MTIATLFAQVQARVAAEARLRYLADHDDLTGLHNRRALLQHLDQRLAPGQPGPVAALF
LDLDRLKAINDYLGHAAGDQFIHVFAQRIGDALVGESLIARLGGDEFVLIPASPMSAD
AAQPLAERLRDQLKDHVAIGGEVLTRTVSIGVASGTPGQHTPSDLLRRADQAALAAKH
AGGDSVAIFTADMSVSGELRNDIELHLRRGIESDAL RLVYLPEVDL RTGDIVGTEALV
RWQHPTRGLLAPGCFIPVAESINLAGELDRWVLRRACNEFSEWQSAGLGHDALLRINV
SAGQLVTGGFVDFVADTIGQHGLDASSVCLEITENVVVQDLHTARATLARLKEVGVHI
AIDDFGTGYSAISLLQTLPIDTLKIDKTFVRQLGTNTSDLVIVRGIMTLAEGFQLDVV
AEGVETEAAARILLDQRCYRAQGFLFSRPVPGEAMRHMLSARRLPPTCIPATDPALS
[0175] SEQ ID NO:5
[0176] Bifunctional diguanylate cyclase/phosphodiesterase
BCG_RS07340 from BCG and
other related mycobacteria, DNA sequence (1872 nucleotides [623 codons 1
stop codons];
encodes BCG protein BCG RS07340; NCBT Reference Sequence: NC 008769.1; Protein
ID WP
003898837.1; old locus tag BCG_1416e; DNA from NC_008769.1:e1548390-1546519
Mycobacterium bovis BCG Pasteur 1173P2). The identical sequence is present in
other strains of
BCG, e.g., M,vcobacierium tuberculosis as protein Rv1354c or MT1397, and in
Mycobacterium
bovis as protein Mb1389c. EAL domain is encoded from nucleotide 1060 to 1872
and is
underlined.
ATGTGCAACGACACCGCGACGCCGCAGCTTGAGGAGCTCGTCACCACCGTAGCCAACCAGCTCATGACAG
TCGACGCTGCCACGTCAGCCGAAGTCAGTCAGCGCGTTTTGGCCTATCTAGTGGAACAGCTGGGCGTAGA
TGTCAGCTTTTTGCGTCATAACGATCGCGACAGGCGCGCGACGAGGCTGGTGGCCGAATGGCCACCTCGC
CTCAACATACCGGACCCCGATCCGCTCAGGCTGATCTACTTCGCTGATGCCGACCCGGTGTTTGCGCTAT
GCGAACACGCCAAAGAGCCTCTCGTGTTCCGGCCCGAGCCGGCCACCGAGGACTATCAACGCCTCATCGA
AGAAGCCCGCGGGGTTCCGGTAACGTCGGCTGCCGCCGTGCCGCTGGTATCTGGCGAGATCACCACTGGA
CTGCTGGGGTTCATCAAGTTCGGTGATCGGAAATGGCACGAGGCCGAGCTTAACGCCCTCATGACCATCG
CTACACTCTTCGCCCAGGTGCAGGCTCGCGTCGCC GCCGAGGCGCGGCTTCGCTATCTGGCCGACCATGA
CGATCTGACCGGACTCiCA l'AACCGTCGCGCGTIGCTGCAGCACCTGGACCAAAGACTIGGCCCCCGOACAA
CCTGGCCCGGTCGCGGCGCTATTTCTCGACTTGGACCGCCTCAAGGCCATCAACGACTACCTGGGCCACG
CCGCCGGTGACCAGTTCATCCATGTGTTCGCCCAACGGATC GGTGACGCACTCGTTGGCGAGAGCCTGAT
CGCCCGACTCGGCGGCGACGAATTCGTCCTCATACCCGCATCTCCAATGAGTGCCGATGCCGCTCAACCG
CTCGCCGAACGTCTTCGCGACCAGCTCAAGGACCACGTCGCTATCGGCGGTGAGGTGCTCACCCGCACCG
TCAGTATCGGTGTCGCCTCAGGGACTCCCGGACAGCACACAC CGTCGGAC CTCCTGC GCCGAGCCGACCA
AGCCGCTCTGGCAGCCAAACACGCCGGCGGAGATAGCGTCGCGATTTTCACCGCGGACATGTCGGTCAGC
GGCGAACTGCGCAACGATATTGAACIACACCITCGACGI'GGTATCGAATCGGACGCCCATCGCCI'GGTCT
ACCTACCCGAGGTCGACCTACGGACCGGCGACATTGTCGGGACCGAGGCATTGGTCCGGTGGCAGCACCC
CACCCGTGGGCTGCTGGCACCGGGCTGCTTCATCCCTGTGGCCGAATCCATCAACCTTGCAGGCGAATTG
GATAGATOGGTOCTOCGGAGGGCCTOCAATGAATTCTCCGAGIGGCAGTCAGCCOUTITOGGCCACGACG
CGCTGCTGCGTATCAACGTCTCAGCTGGACAGCTGGTGACGGGCGGGTTTGTTGACTTCGTCGCAGACAC
GATCGGCCAGCACGGTCTGGACGCCTCGTCCGTGTGTTTGGAAATCACC GAAAACGTTGTGGTGCAAGAC
CTACATACCGCCAGAGCCACCCTGGCTCGACTCAAAGAAGTCGGCGTTCACATCGCTATCGACGATTTCG
GCACCGGCTATAGCGCCATATCACTGTTGCAGAC GCTACCGATCGACACGCTCAAGATCGACAAAACATT
CGTCiCGGC A ACTCGGA ACC A AC ACTA GCCiA TCTCiGTC ATTGTGCCiCGCiCA TC
ATGACACTCGCCGA A GCiC
TTCCAACTCGATGTAGTAGCCGAAGGCGTCGAGACCGAGGCTGCCGCCAGAATTCTATTGGATCAGCGCT
GTTACCGTGCGCAAG GCTTCTTGTTCTCCCGGCCTGTCCCCGGG GAGGCCATGCGG CACATGTTGTCCGC
48
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
ACGACGACTACCGCCGACCTGCATACCTGCAACTGACCCGGCGTTATCTTGA
[0177] SEQ ID NO:6
[0178] Modified bifunctional diguanylate
cyclase/phosphodiesterase from BCG and other
related mycobacteria, with its EAL domain deleted so that it acts as a
monofunctional diguanylate
cyclase, amino acid sequence (353 amino acids; a fragment of BCG protein
BCG_RS07340; NCB1
Reference Sequence: NC_008769.1; Protein ID WP 003898837.1; old locus tag
BCG_1416c).
The identical sequence fragment is present in other strains of BCG, e.g.,
Mycobacterium
tuberculosis as protein Rv1354c or MT1397, and in Mycobacterium bovis as
protein Mb1389c.
MCNDTATPQLEELVTTVANQLMTVDAATSAEVSQRVLAYLVEQL
GVDVSF LRHNDRDRRATRLVAE WPPRLNIPDPDPLRLIYEADADPVFALCEHAKEPLV
FRPEPATEDYQRLIEEARGVPVTSAAAVPLVSGEITTGL LGFIKFGDRKWHEAELNAL
MTTATLFAQVQARVA A EA RL RYL A DHDDLTGLHNRR A LLQHLDQRL A PGQPGPVA A LF
LDLDRLKAINDYLGHAAGDQFTHVFAQRIGDALVGESLIARLGGDEFVLIPASPM SAD
AAQPLAERLRDQLKDHVAIGGEVLTRTVSIGVASGTPGQHTP SDLLRRADQAALAAKH
AGGDSVAIFTADMSVSGEL
[0179] SEQ ID NO:7
[0180] Modified, bifunctional diguanylate
cyclase/phosphodiesterase from BCG and other
related mycobacteria, with sequences encoding its EAL domain deleted so that
it encodes a
monofunctional diguanylate cyclase, DNA sequence (1059 nucleotides 1353 codons
0 stop
codons]; encodes a fragment of BCG protein BCG_RS07340; NCBI Reference
Sequence:
NC 008769.1; Protein ID WP 003898837.1; old locus tag BCG 1416c; DNA from
NC 008769.1:c1548390-1546519 Mycobacterium bovis BCG Pasteur 1173P2). The
identical
sequence is present in other strains of BCG, e.g., Mycobacteriutn tuberculosis
as a fragment of
gene Rv1354c or MT1397, and in Mycobacterium bovis as a fragment of gene
Mb1389c.
ATGTGCAACGACACCGCGACGCCGCAGCTTGAGGAGCTCGTCACCACCGTAGCCAACCAGCTCATGACAG
TCGACGCTGCCACGTCAGCCGAAGTCAGTCAGCGCGTTTTGGCCTATCTAGTGGAACAGCTGGGCGTAGA
TGTCAGCTTTTTGCGTCATAACGATCGCGACAGGCGCGCGACGAGGCTGGTGGCCGAATGGCCACCTCGC
C TCAACATACCGGACCCCGATCCGCTCAGGCTGATCTACTIVGCTGATGCCGACCCGGTUITMCGCTAT
GCGAACACGCCAAAGAGCCTCTCGTGTTCCGGCCCGAGCCGGCCACCGAGGACTATCAACGCCTCATCGA
AGAAGCCCGCGGGGTTCCGGTAACGTCGGCTGCCGCCGTGCCGCTGGTATCTGGCGAGATCACCACTGGA
C IGC1'GGGGITCATCAAGITCGGTGATCGGAAATGGCACGAGGCCGAGCTI'AACGCCCTCATGACCATCG
CTACACTCTTCGCCCAGGTGCAGGCTCGCGTCGCCGCCGAGGCGCGGCTTCGCTATCTGGCCGACCATGA
CGATCTGACCGGACTGCATAACCGTCGCGCGTTGCTGCAGCACCTGGACCAAAGACTGGCCCCCGGACAA
CCIGGCCCOGTCGCGGCOCIAI"ITCTCGACTIGOACCGCCTCAAGGCCAICAACGACTACCTOGCiCCACG
CCGCCGGTGACCAGTTCATCCATGTGTTCGCCCAACGGATC GGTGACGCACTCGTTGGCGAGAGCCTGAT
MCCCGACTCGGCGGCGACGA ATTMTCCTCA TA CCCGC ATCTCC A A TGA GTGCCGA TGCCGCTCA ACCG
49
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
CTCGCCGAACGTCTTCGCGACCAGCTCAAGGACCACGTCGCTATCGGCGGTGAGGTGCTCACCCGCACCG
TCAGTATCGGTGTCGCCTCAGGGACTCCCGGACAGCACACACCGTCGGACCTCCTGCGCCGAGCCGACCA
AGCCGCTCTGGCAGCCAAACACGCCGGCGGAGATAGCGTCGCGATTTTCACCGCGGACATGTCGGTCAGC
GGCGAACTG
[0181] Overexpression of the PAMP immunomodulator, 2'-5'c-GAMP
synthase: Q9KVG7
(Swiss-Prot). 2'-5' c-GAMP is a strong inducer of the STING-TBK1-IRF3 axis.
The Vibrio
cholerae Q9KVG7 protein (436 amino acids) encoded by the dncV gene is a known
2'-5'c-GAMP
synthase. It is possible to generate a recombinant dncV gene which is codon-
optimized for BCG.
The codon-optimized structural gene may be overexpressed in BCG by fusion to a
strong promoter
(such as Phsp60) or a conditionally active strong promoter such as PTET-off.
Such techniques to
generate a constitutively active recombinant forms of the Q9KVG7 protein will
produce high
levels of 2'-5'c-GAMP in recombinant BCG.
[0182] SEQ ID NO:8
[0183] Cyclic GMP-AMP synthase, DncV, from Vibrio cholerae, amino
acid sequence (436
amino acids; UniProtKB/Swiss-Prot Protein ID Q9KVG7.1).
MRMTWNFHQYYTNRNDGLMGKLVLTDEEKNNLKALRKIIRLRTRDVFEEAKGIAKAVKKSALTFEIIQEK
VSTTQIKHL SD SEQREVAKLIYEMDDDARDEFLGLTP RFWTQ GSFQYDTLNRPFQPGQEMDIDDGTYMPM
PIFESEPKIGHSLLILLVDASLKSLVAENHGWKFEAKQTCGRIKIEAEKTHIDVPMYAIPKDEFQKKQIA
LE ANR SFVKGA TFESYVA DSITDDSETYE LDSENVNL A LR EGDR K WINS DPKTVEDWFND SCTR
TGKHLR
KVC RFMKAWRDAQWDVGGPS SI S LMAATVNILDSVAHDASDLGETMKIIAKHLP SEFARGVESP DSTDEK
PLFPPSYKHGPREMDIMSKLERLPEIL SSAESADSKSEALKKINMAFGNRVTNSELIVLAKALPAFAQEP
SSASKPEKISSTMVSG
[0184] SEQ ID NO: 9
[0185] Cyclic GMP-AMP synthase, DncV, from Vibrio cholerae, DNA
sequence (1311
nucleotides [436 eodons 1 stop codon]; encodes UniProtKB/Swiss-Prot Protein ID
Q9KVG7.1;
NCBI Reference Sequence: NC 002505.1: Vibrio cholerae 01 biovar El Tor str.
N16961
chromosome I, complete sequence, and nucleotides 180419-181729)
Ci IGAGAA TGAC 1TGGAAC f 1TCACCAGIAC 1ACACAAACCGAAA TGAIGGC 1TGA IGGGCAAGC lAG
ITC
TTACAGACGAGGAGAAGAACAATCTAAAGGCATTGCGTAAGATCATCCGCTTAAGAACACGAGATGTATT
TGAAGAAGCTAAGGGTATTGCCAAGGCTGTGAAAAAAAGT GCTCTTACGTTTGAAATTATTCAGGAAAAG
G IGICAACGACCCAAAITAAGCACC ITTC 1 GACAGCGAACAACGAGAAG IGGrC TAAGC 1TA 1TACGAGA
TGGATGATGATGCTCGTGATGAGTTTTTGGGATTGACACCTCGCTTTTGGACTCAGGGAAGCTTTCAGTA
TGACACGCTGAATCGCCCGTTTCAGCCTGGTCAAGAAATGGATATTGATGATGGAACCTATATGCCAATG
CCTATTTTTGAGTCAGACiCCTAAGATTCiGTCATTCTTTACTAATTCTTCTTCiTTCiACCiCCiTCACTTAAGT
CACTTGTAGCTGAAAATCATGGCTGGAAATTTGAAGCTAAGCAGACTTGTGGGAGGATTAAGATTGAGGC
AGAGAAA ACACATATTGATGTACCAATGTATGCAATCCCTAA AGATGAGTTCCAGA AA AAGCA A ATAGCT
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
TTAGAAGCAAATAGATCATTTGTTAAAGGTGCCATTITTGAATCATATGTTGCAGATTCAATTACTGACG
ATAGTGAAACTTATGAATTAGATTCAGAAAACGTAAACCTTGCTCTTCGTGAAGGTGATCGGAAGTGGAT
CAATAGCGACCCCAAAATAGTTGAAGATTGGTTCAACGATAGTTGTATACGTATTGGTAAACATCTTCGT
AAGGTTTGTCGCTTTATGAAAGCGTGGAGAGATGCGCAGTGGGATGTTGGAGGTCCGTCATCGATTAGTC
TTATGGCTGCAACGGTAAATATTCTTGATAGCGTTGCTCATGATGCTAGTGATCTCGGAGAAACAATGAA
GATAATTGCTAAGCATTTACCTAGTGAGTTTGCTAGGGGAGTAGAGAGCCCTGACAGTACCGATGAAAAG
CCACTCTTCCCACCCTCTTATAAGCATGGCCCTCGGGAGATGGACATTATGAGCAAACTAGAGCGTTTGC
CAGAGATTCTGTCATCTGCTGAGTCAGCTGACTCTAAGTCAGAGGCCTTGAAAAAGATTAATATGGCGTT
TGGGAATCGTGTTACTAATAGCGAGCTTATTGTTTTGGCAAAGGCTTTACCGGCTTTCGCTCAAGAACCT
AGTICAGCCTCGAAACCTGAAAAAATCAGCAGCACAATGGTAAGTGGCTGA
[0186] Overexpression of the DAMP immunomodulator, 2'-3' cGAMP
synthase: Q8N884
(Swiss-Prot). 2'-3' cGAMP is a strong inducer of the STING-TBK1-IRF3 axis. The
cGAS protein
is produced by the human cGAS gene to yield a 522 amino acid polypeptide which
senses cytosolic
DNA and functions as a 2'-3' cGAMP synthase. The synthase or cyclase domain of
cGAS becomes
activated when cGAS binds to DNA. It is possible to generate a recombinant
cGAS gene which
contains only the cyclase domain and is hence constitutively active. This
recombinant gene can
also be codon-optimized for BCG. The codon-optimized structural gene may be
overexpressed in
BCG by fusion to a strong promoter (such as Phsp60) or a conditionally active
strong promoter
such as PTET-off. Such techniques to generate a constitutively active
recombinant forms of the
cGAS protein will produce high levels of 2'-3'c-GAMP in recombinant BCG.
[0187] SEQ ID NO:10
[0188] Cyclic 2'3'-GMP-AMP synthase, cGAS, from Homo sapiens,
amino acid sequence
(522 amino acids, UniProtKB/Swiss-Prot Protein ID Q81\1884.2).
MQPWHGKAMQRASEAGATAPKASARNARGAPMDPTESPAAPEAALPKAGKEGPARKSGSRQKKSAPDT
QE
REPVRATGARAKKAPQRAQDTQPSDATSAPGAEGLEPPAAREPALSRACiSCRQRGARCSTKPRPPPGPWD
VP SPG L PVSAPILVRRDAAPG ASKL RAVLEKLKL SRDDISTAAG MVKGVVDHLLLRLKCDSAFRGVG LLN
TGSYYEHVKISAPNEFDVMFKLE VPRIQLEEYSNTRAYYFVKFKRNPKENPLSQFLEGEIL SASKMLSKF
RKIIKEEINDIKDTDVIMKRKRGG SPAVTLLISEKISVDITLALE SKS SWPASTQEGLRIQNWL SAKVRK
QLRL KPFYLVPKHAKEGNGFQEETWRL SF SHIEKEILNNHGKSKTCCENKEEKCC RKDCLKLMKYLLEQL
KERFKDKKHLDKFSSYHVKTAFFHVCTQNPQDSQWDRKDLGLCFDNCVTYFL QCLRTEKLENYFIPEFNL
FSSNLIDKRSKEFLTKQIEYERNNEFPVFDEF
[0189] SEQ ID NO: 11
[0190] Cyclic 2'3'-GMP-AMP synthasc, cGAS, from Homo sapiens, DNA
sequence of
mRNA with nucleotide T used in place of U (1802 nucleotides; encodes
UniProtKB/Swiss-Prot
Protein ID Q8N884.2; NCBI Reference Sequence: NM 138441.2. Coding sequence is
1569
51
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
nucleotides [522 codons, 1 stop codon], start codon ATG [bold underlined] at
nucleotide 140; Stop
codon TGA (bold, underlined) at nucleotide 1706]).
AGCCTGGGGTTCCCCTTCGGGTCGCAGACTCTTGTGTGCCCGCCAGTAGTGCTTGGTTTCCAACAGCTGC
TGC TGGC TCTTCCTCTTGCGGCCTTTTCCTGAAAC GGATTCTTCTTTCGGGGAACAGAAAGCGCCAGCCA
TGCAGCCTTGGCACGGAAAGGCCATGCAGAGAGCTTCCGAGGCC GGAGCCACTGCCCCCAAGGCTTCCGC
ACGGAATGCCAGOGGCGCCCCGATOGATCCCACCGAGTC ---------
ICCGGCTGCCCCCGAGGCCGCCCTGCCIAAG
GCGGGAAAGTTCGGCCCCGCCAGGAAGTCGGGATCCCGGCAGAAAAAGAGCGCCCCGGACACCCAGGAGA
GGCCGCCCGTCCGCGCAACTGGGGCCC GCGC CAAAAAGGCCCCTCAGCGCGCCCAGGACACGCAGCCGTC
TGACGCCACCAGCGCCCCIGGGGCAGAGGGGCTGGAGCCTCCTGCGGCTCGGGAGCCGGCTCTTTCCAGG
GCTGGTTCTTGCCGCCAGAGGGGCGCGCGCTGCTCCAC GAAGCCAAGACCTCCGCCCGGGCCCTGGGACG
TGCCCAGCCCCGGCCTGCCGGTCTCGGCCCCCATTCTCGTACGGAGGGATGCGGCGCCTGGGGCCTCGAA
GCTCCUGGCGGTTTTGGAGAAGTTGAAGCTCAGCCGCGATGATATCTCCACGGCGGCGGGGATGOTGAAA
GGGGTTGTGGACCACCTGCTGCTCAGACTGAAGTGCGACTCCGCGTTCAGAGGCGTCGGGCTGCTGAACA
CC GGGA CiC TA CT A TGA GC A CGTGA A GA TTTC TGC A CC T A A TG A A TTTGA TGTC
ATGTTT A A A CTGGA A GT
CCCCAGAATTCAACTAGAAGAATATTCCAACACTCGTGCATATTACTTTGTGAAATTTAAAAGAAATCCG
AAAGAAAATCCTCTGAGTCAGTTTTTAGAAGGTGAAATATTATCAGCTTCTAAGATGCTGTCAAAGTTTA
GGAAAATCATTAAGGAAGAAATTAACGACATTAAAGATACAGATGTCATCATGAAGAGGAAAAGAGGAGG
GAGCCCTGCTGTAACACTTCTTATTAGTGAAAAAATATCTGTGGATATAACCCTGGCTTTGGAATCAAAA
AGTAGCTGGCCTGCTAGCACCCAAGAAGGCCTGCGCATTCAAAACTGGCTTTCAGCAAAAGTTAGGAAGC
AACTACGACTAAAGCCATTTTACCTTGTACCCAAGCATGCAAAGGAAGGAAATGGTTTCCAAGAAGAAAC
ATGGCGGCTATCCTTCTCTCACATCGAAAAGGAAATTTTGAACAATCATGGAAAATCTAAAACGTGCTGT
GAAAACAAAGAAGAGAAATGTTGCAGGAAAGATTGTTTAAAACTAATGAAATACCTTTTAGAACAGCTGA
AAGAAAGGTTTAAAGACAAAAAACATCTGGATAAATTCTCTTCTTATCATGTGAAAACTGCCTTCTTTCA
CGTATGTACCCAGAACCCTCAAGACAGTCAGTGGGACCGCAAAGACCTGGGCCTCTGCTTTGATAACTGC
GTGACATACTTTCTTCAGTGCCTCAGGACAGAAAAACTTGAGAATTATTTTATTCCTGAATTCAATCTAT
TCTCTAGCAACTTAATTGACAAAAGAAGTAAGGAATTTCTGACAAAGCAAATTGAATATGAAAGAAACAA
TGAGTTTCCAGTTTTTGATGAATITTGAGATTGTATTTTTAGAAAGATCTAAGAACTAGAGTCACCCTAA
ATCCTGGA GA ATAC A A GA A A A ATTTGA A A A GGGGCCA GACGCTGTGGC TCAC
[0191] SEQ ID NO: 12
[0192] Cyclic 2'3'-GMP-AMP synthase, cGAS, from Homo sapiens with
mycobacterial
codon optimization, DNA sequence. (1569 nucleotides [522 codons, 1 stop
codon]; encodes
UniProtKB/Swiss-Prot Protein ID Q8N884.2).
A TCiCA A CCATGGCACCiGG A A A GCC A TG C A GCGT CiCCi A GCCiA ACiCCGGGGCG A
CGG CCCCC A A GGCGTCGGCGCGT A A CGCGCG
GGG TGC GCCCATGGACCCGACCiGAGTCCCCCGCGGCGCCGGAGGC GGCCCTCiCCGAAAGCGGGTAAGTTCGG
TCCAGCCiCGGA
AAAGCGGGAGCCGCCAAAAGAAGICCGCGCCCGACACCCAGGAGCGICCCCCGGICCGGGCCACCGGCGCGCGIGCCAA
AAAA
GCCCCGCAACGGGCGCAAGATACGCAGCCAAGCGATGCGACCTCCGCCCCCGGGGCGGAGGGTCTGGAGCCCCCGGCCG
CCCG
GGAGCCAGC GCTCTCGCGCGCGGGTTCCTGCCGTCAGCGGGGCG
CGCGGTGTTCCACGAAACCCCGTCCCCCACCAGGTCCCT
GGGACGTGCCGTCGCCGGGTTTGCCGGTGAGCGCGCCAATCCTGGTCCGGCGCGACGCGGCCCCGGGGGCGTCGAAATT
GCGT
GCGGTGCTCGAGAAATTGAAGTTGTCGCGCGACGACATCTCCACGGCCGOGGGTATGGTCAAGGGCGTGGTCGATCATT
TGTT
GTTGCGGCTCAAGTGTGATTCGGCGTTCCGCGGGGTGGGCTTGCTGAACACG
GGGTCCTACTATGAGCATGTCAAAATCAGCG
CCCCCAACGAATITGACGIGATGITTAAGCTUGAAGIGCCACGTATCCAATIGGAAGAGIATICCAATACCCGIGCGTA
TTAT
TTCGTCAAATTTAACiCGCAATCCCiAACiGAAAATCCACTCAGCCAATTCTIGGAGOGCGAAATTCTGICGGCCTCGA
AAATGCT
CTCCAAATTTCGTAAGATTATCAAGGAGGAGATCAACGACATTAAGGACACGGATGTGATCATGAAACGTAAACGTGGC
GGTT
CCCCCGCGGTGACGCTCCTCATTTCGGAAAAAATTTCGGTGGACATTACCCTGGCGTIGGAATCGAAGTCCAGCTGGCC
GGCG
TCCi A CCC A CiciA CiCiCiCCTGCCiCi A TTCA A AA CTC1CiTTGA GCGCCA AA CiTC1CCA1A
MiCACi CTGCciTCTCA A A CCCTTTTA TT TCA1TC
CCGAAACATGCCAAAGAGGGTAACGGTTTTCAAGAGGAAACCTGGCGTTTGAGCTTCTCCCACATTGAGAAGGAGATTT
TGAAC
AACCATtiGTAAGTCCAAAACCiTGCTGCGAGAATAAGCiAAGAAAAATGTIGTCGCAAAGATTGICICAAATTGATGA
AATAITTG
CTGGAACAACTCAAAGAGCGTTTTAAGGACAAGAAGCATCTCGACAAGTTCTCCTCGTATCACGTCAAGACCGCCT
TCTTTCAT
GTCTGTACGCAGAACCCGCAAGATAGCCAGTGGGATCGCAAGGACTTGGGGTTGTGTTTTGACAATTGCGTCACCTATT
TCTTG
CAATGTTTGCGGACCGAGAAATTGGAGAACTACTT
TATTCCAGAATTCAACTTGTTTTCCTCGAATCTGATTGACAAACG CTCC
AAAGAGTTTCTGACGAAGCAGATTGAATACGAGCGTAACAATGAGTTTCCGGTCTTTGACGAGTTTTGA
[0193] SEQ ID NO:13
52
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
[0194] Plasmid pMH94H-Phsp6o::disA::hcGASco::mCherry which is an
E. co/i-mycobacterial
shuttle plasmid that overexpresses the BCG disA gene, the human cGAS gene
(with mycobacterial
codon optimization), and mCherry from the Pl in
troduced
into promoter, DNA sequence.
When
into BUG, M. tuberculosis, M. bovis or highly related strains, this plasmid
integrates as a single
copy in the mycobacterial chromosome (10842 nucleotides; promoter Ph,p6o DNA
comprised of
a portion of the M. leproe hsp65 gene nucleotides 901 to 1068 is underlined;
disA coding sequence
are from nucleotides 1069 to 2145; human cGAS with mycobacterial codon
optimization
sequences are from nucleotides 2158 to 3726; ATG start codons and TAA or TGA
stop codons
are shown in boldface, underline).
TCGCGCOTTTCGGTGATGACCGTGAAAACCICTGACACATGCAGCTCCCGGAGACCGTCACACCTIGTCTGTAAGCGGA
TCCC 1-83
GGGAGCAGACAAGCCCGTCAGGGCGCGTCAGCGGGTGTTGGCGGGTGTCGGGGCTGGCTTAACTATGCGGCATCAGAGC
AGAT 84-166
TGTACTGAGAGTGCACCAAAATTGTAAACGTTAATATTTTGTTAAAATTCGCGTTA A A
TTTTTGTTAAATCAGCTCATTTTTT 167-249
AACCAATAGGCCGAAATCGGCAAAATCCCTTATAAATCAAAAGAATAGCCCGAGATAGGGTTGAGTGTTGTTCCAGTTT
GGA 250-331
ACAAGAGTCCACTATTAAAGAACGTGGACTCCAACGTCAAAGGGCGAAAAACCGTCTATCAGGGCGATGGCCCACTACG
TGAA 332-414
CC A TC A CCC A A A TC A A GTTTTTTCGGGTC GGT GCC GT A A A GC A CT A A A TCGG A
A CCC TA A A GGGA GCCCCCGA TTT A GA GC 415-497
TTGACCGGGAAAGCCGGCGAACCTGGCGAGAAAGGAAGGGAAGAAAGCGAAAGGACCGGGCGCTAGGGCGCTGGCAAGT
GTAG 498-580
CGGTCACGCTGCGCGTAACCACCACACCCGCCGCGCTTAATGCGCCGCTACAGGGCGCOTACTATGGTTGCTTTGACGT
GCGG 581-663
TGTGAAATACCGCACAGATGCGTAAGGAGAAAATACCGCATCAGGCGCCATTCGCCATTCAGGCTGCGCAACTGTTGGG
AAGG 664-746
GCGATCGGTGCGGGCCTCTTCGCT ATT ACGCCAGCT GGCGAAAGGGGGATGT GCT GC A GGCGATT
AAGTTGGGT AACGCCAG 747-829
GGTTTTCCCAGTCACGACGTTGTAAAACGACGGCCAGTGAATTCGAGCTCGGTACCCGGGGATCCTCTAGAAATTCCGG
AATT 830-912
GCAC TCGCCTTAGGGGAGTGC TAAAAATGATCCTGGCACTC GC GATCACC GAGT GCCAGGTC GG
GACCGTGAGAC CCAGCCAG 913-995
CAACCTGTGGTCGTCCGTCCCGGGCACTCCACCCGGCCAGCGTAAGTAATGGGCGTTGTCCGCACCCGCTGACATGCAC
CCTG 996-1078
TGACTCGTCC GACC CT GCGTGAGGC TGTCGCCC GCCTAGCC CC GGGCAC TGGGC TGC GGGAC
GGCCTGGAGCGTATCC TGCGC 1079-1161
GGCCGCACTGGTGCCCTGATCGTGCTGGGCCATGACGAGAATGTCGAGGCCATCTGCGATGGTGGCTTCTCCCTCGATG
TCCG 1162-1244
CT ATGCAGCAACCCGGCT
ACGCGAGCTGTGCAAGATGGACGGCGCCGTGGTGCTGTCCACCGACGGCAGCCGCATCGTGCGGG 1245-1327
CCAACGTGCAACTGGTACCGGATCCGTCGATCCCCACCGACGAATCGGGGACCCGCCACCGCTCGGCCGAGCGGGCCGC
GATC 1228-1410
CAGACCGGTTACCCGCTGATCTCAGTGAGCCACTCGATGAACATCGTGACCGTCTACGTCCGCCGGGAACCTCACGTAT
TGAC 1411-1493
CGACTCCGCAACCATCCTGTCGCGCGCCAACCAGGCCATCGCAACCCTCGAGCCGTACAAAACCAGGCTCGACGAGGTC
AGCC 1494-1576
GGCAACTGTCCAGGGCAGAAATCGAGGACTTCGTCACGCTGCGCGATGTGATGACCGTGGTGCAACGCCTCGAGCTGGT
CCGG 1577-1659
CGAATCGGGCTGGTGATCGACTACGACGTGGTC
GAACTCGGCACTGATGGTCGTCAGCTCCGGCTGCAGCTCGACGAGTTGCT 1660-1742
CGGCGGCAACGAC ACC GCC CGGGAATT G AT C GT GCGCG AT T ACCACGCC
AACCCGGAACCACCGTCC AC GGGGCAAAT C AAT G 1743-1825
C:CAC:CCTGOACCiA ACTOCrAC'OCC:C: R4 (1CA COG( 'GACC ITT ' ICOA: IT
ICACCGCGC:TOGC AA AGGIIIIVGGAT A IrCGACG 1826-1908
ACCACGGAAGCGCAGGATTCGACGCTGAGCCCGCGTGGCTACCGCGCGATGGCCGGTATCCCCCGGCTCCAGTTCGCCC
ATGC 1909-1991
CGACCTGCTGGTCCGGGCGTTCGGAACGTTGCAGGrGTCTGCTGGCGGCCAGCCCCGGCGATCTGCAATCAGTGGACGG
CATCG 1992-2074
GC GC CATGT GGGCC C GTCATGTGCGC GAGGGGT TGTCACAGC TGGC GGAATC GACCATCAGC
GATCAATAAGAGCACATCGAT 2075-2157
ATGCAACCATGGCACGGGAAAGCCATGCAGC GT GC GAGCGAAGCC GGGGCGACGGC CCCCAAGGC GTC GGC
GC GTAAC GCGCG 2158-2240
GGGTGCGCCCATGGACCCGAC7GGAGTCCCCCGC7GGCGCCGGAGGCGGCCCTGCCGAAACiCGGGTAAGTTCGCTCCA
GCGCGGA 2241-2323
AAAGCGGGAGCCGCCAAAAGAAGTCCGCGCCCGACACCCAGGAGCGTCCCCCGGTCCGGGCCACCGGCGCGCGTGCCAA
AAAA 2324-2406
GCCCCCiCA A CGOGCGCA AGA TA CGC A GCCA A GCGA TGCGA CC TCCGCCCCCGGGGCGG A
GGOTCTOCrA CiCCCCCGGCCGCCCCr 2407-2489
GGAGCCAGCGCTCTCGCGCGCCiGGTTCCTGCCGTCAGCGGGGCGCGCGOTOTTCCACGAAACCCCGTCCCCCACCAGG
TCCCT 2490-2572
GGGACGTGCCGTCGCCGGGTTTGCCGGTGAGCGCGCCAATCCTGGTCCGGCGCGACGCGGCCCCGGGGGCGTCGAA_AT
TGCGT 2573-2655
GCC4GIGCTCGAGAAATTGAAGTTGTCGCGCGACGACATCTCCACGGCCGCGGGTATGCTCAAGGGCGTGGTCGATCAT
TTGTT 2656-2738
ITiCOCCTCA A (i 1(1 INA It :GOCG FCCOCOGOOTOGGC OCIOA A C A :(1(10GTCC: IA (7
IA1 GA GC A 1:(1 IVA A AA IC AOCCi 2739-2821
CCCCCAACGAATTTGACGTGATGTTTAAGCTGGAAGTGCCAC
GTATCCAATTGGAAGAGTATTCCAATACCCGTGCGTATTAT 2822-2904
TTCGTCAAATTTAAGCGCAATCCGAAGGAAAATCCACTCAGCCAATTCTTGGAGGGCGAAATTCTGTCGGCCTCGAAAA
TGCT 2905-2987
CTCCAAATTTCGTAAGATTATCAAGGAGGAGATCAACGACATTAAGGACACGGATGTGATCATGAAACGTAAACGTGGC
GGTT 2988-3070
CCCCCGCGGTGACGCTCCTCATTTCGGAAAAAATTTCGGTGGACATTACCCTGGCGTTGGAATCGAAGTCCAGCTGGCC
GGCG 3071-3153
TCGACCCAGGAGGGCCTGCGGATTCAAAACTGGTTGAGCGCCAAAGTGCGGAAGCAGCTGCGTCTCAAACCCTTTTATT
TGGTC 3154-3237
CCOAAACA FOCCAAAOACOU TAACCUFT1 TCAAGAGOAAACC 1tiOCCi1116ACC1IC
1CCCACA11CTAGAAUGAGA11116AAC 3238-3321
AACCATGGTAAGTCCAAAACGTGCTGCGAGAATAAGGAAGAAAAATGTIGTCGCAAAGATTGTCTCAAATTGATGAAAT
ATTTG 3322-3405
CTGGAACAACTCAAAGAGCGTTTTAAGGACAAGAAGCATCTCGACAAGTTCTCCTCGTATCACGTCAAGACCGCCTTCT
TTCAT 3406-3489
GT CTGT ACGC A GA ACCCGC A A GAT AGC CA GTGGGA TCGCA A GG ACTTGGGGTT GTGTTTTGA
CA A TTGCGTCA CCTATTTCTTG 3490-3573
CAATGTTTGCGGACCGAGAAATTGGAGAACTACTTTATTCCAGAATTCAACTTGITTTCCTCGAATCTGATTGACAAAC
GCTCC 3574-3657
AAAGAGTTTCTGACGAAGCAGATTG A A
TACGAGCGTAACAATGAGTTTCCGGTCTTTGACGAGTTTTGAAAGCTTGAGATGGTG 3658-3741
AGCAAGGGCGAGGAGGATAACATGGCCATCATCAAGGACTICATGCGCTTCAAGGIGCACATGGAGGGCTCCGTGAACG
(3CCAC 3742-3825
GAGTTCGAGATCGAGGGCGAGGGCGAGGGCCGCCCCTACGAGGGCACCCAGACCGCCAAGCTGAAGGTGACCAAGGGTG
GCCCC 3826-3909
CTGCCCTTCGCCTGG CACATCCTCTCCCCTCAGTTCATCTACCG CTCCAAGC CCTACCTCAAGCACCCCC CC
GACATCCCCCAC 3919-3993
T ACTTGAAGCT GTCCTTCCCCGAGGGCTTCAAGT GGGAGCGC GT GATGAACTTCGAGGACGGCGGCG
TGGTGACCGT GACCCAG 39944077
GACTCCTCCCTGCAGGACGGCGAGTTCATCTACAAGGTGAAGCTGCGCGGCACCAACTTCCCCTCCGACGGCCCCGTAA
TGCAG 4078-4161
A AGA AGA( :( :A I CGGC ITiGGAGGC( T:1:( :( AGCGGA IA( :( :( :GAGGACGOCGC( :(
!CAA GGG( :GAGA: IC A AG( :A GA GGCTG 4162-4225
AAGCTGAAGGACCGCGGCCACTAC
GACGCTGAGGTCAAGACCACCTACAAGGCCAAGAAGCCCGTGCAGCTGCCCGGCGCGTAC 4226-4329
53
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
AACGTCAACATCAAGTTGGACATCACCTCCCACAACGAOGACTACACCATCGTGGAACAGTACOAACGCGCCGAGGGCC
GCCAC 4330-4413
TCCACCGGCGGCATGGACGAGCTGTACAAGTAGACTAGTTGCCTGGCGGCAGTAGCGCGGTGGTCCCACCTGACCCCAT
GCCGA 44144497
ACTCAGAAGTGAAACGCGGT AGCGCCGATGG TACT GT G GGGT CTCCCCAT GC
GAGAGTAGGGAACTGCCAGGCATCAAAT A AAA 4498-4581
CGAAAGGCTCAGTCGAAAGACTGGGCCTTTCGT TT
TATCTGTTGTTTGTCGGTGAAEGCTCTCCTGAGTAGGACAAATCCGCCG 4582-4665
GGAGCGGATTTGAACGTTGCGAAGCAACGGCCCGGAAGGGTGGCGGGCAGGACGCCCGCCATAAACTGCCAGGCATCAA
ATTAA 4667-4749
GCAGAAGGCCATCCTGACGGATGGCCTTTTTTCTAGAGTCGACCACCAAGGGCACCATCTCTGCTTGGGCCACCCCGTT
GGCCG 4750-4833
CAGCCAGCTCGCTGAGAGCCGTGAACGACAGGGCGAACGCCAGCCCGCCGACCIGCCIAGGGTTCCGACCGCTGCAACT
CCCGGTG 4834-4917
CAACCTTGTCCCGGTCTATTCTCTICACTGCACCAGCTCCAATCTGGTGTGAATGCCCCTCGTCTGTTCGCGCAGGCGG
GGGGC 4918-5001
TCTATTCGTTTGTCAGCATCGAAAGTAGCCAGATCAGGGATGCGTTGCAACCGCGTATGCCCAGGTCAGAAGAGTCGCA
CAAGA 5002-5085
GT TGCA GACCCCTGGA A AGA A AAA TGGCCAGAGGGCGA AA A CA CCCTCTGACCA GCGGA
GCGOGCGACGGGA ATCGA A CCCGCG 5086-5169
TAGCTAGTTTGGAAGAATGGGIGICTGCCGACCACATATGGGCCGGTCAAGATAGGTTITTACCCCCTCTCGGCTGCAT
CCTCT 5170-5253
AAGTGGAAAGAAATTGCAGGTCGTAGAAGCGCGTTGAAGCCTGAGAGTTGCACAGGAGTTGCAACCCGGTAGCCTTGTT
CACGA 5254-5337
CGAGAGGAGACCTAGTTGGCACGTCGCGGATGGCGATCGCTGAAGACTCAGCGCAGCGGGAGGATCCAAGCCTCATACG
TCAAC 5338-5421
CCGCAGGACGGTGTGAGGTACTACGCGCTGCAGACCTACGACAACAAGATGGACGCCGAAGCCTGGCTCGCGGGCGAGA
AGCGG 5422-5505
CTCATCGAGATGGAGACCTGGACCCCTCCACAGGACCGGGCGAAGAAGGCAGCCGCCAGCGCCATCACGCTGGAGGAGT
ACACC 5506-5589
CGGA A G
' KiCiA GC:GC:GMT'. !MC A GACCiGC A CC A CiGGA.I'r l'A CACCGGGC A CcieGC4A
GCGCCGC A. I I' A CCCGG 5590-5673
CTAGGTGAAGTGGCGGTCACAGAGATGACGCCAGCTCTGGTGCGTGCGTGGTGGGCCGGGATGGGTAGGAAGCACCCGA
CTGCC 5674-5757
CGCCGGCATGCCTACAACGTCCTCCCiGGCGGTGATGAACACAGCGGTCGAGGACAAGCTGATCGCAGAGAACCCGTGC
CGGATC 5768-5841
GAGCAGAAGGCAGCCGATGAGCGCGACGTAGAGGCGCTGACGCCTGAGGAGCTGGACATCGTCGCCGCTGAGATCTTCG
AGCAC 5842-5925
TACCGGATCGCGGCATACATCCIGGCGTGGACGAGCCICC
GGTTCGGAGAGCTGATCGAGCTTCGCCGCAAGGACATCGTGGAC 5926-6009
GACGGCATGACGATGAAGCTCCGGGTGCGCCGTGGCGCTTCCCGCGTGGGGAACAAGATCGTCGTTGGCAACGCCAAGA
CCGTC 6010-6093
CGGTCGAAGCGTCCIGTGACGGTTCCGCCTCACGTCGCGGAGATGATCCGAGCGCACATGAAGGACCGTACGAAGATGA
ACAAG 6094-6177
GOCCCC GA CrGC ATT CCT G ACC ACCIA CGC AGGGC A A CCGGCT GT C GA A
GTCCGCGTTCACCA AGTCGCTGA AGCGTGGCTAC 6175-6261
GCCAAGATCGGTCGGCCGGAACTCCGCATCCACGACCTCCGCGCTGTCGGCGCTACGTTCGCCGCTCAGGCAGGTGCGA
CGACC 6262-6345
AAGGAGCTGATGGCCCGTCTCGGTCACACGACTCCTAGGATGGCGATGAAGTACCAGATGGCGTCTGAGGCCCGCGACG
AGGCT 6346-6429
ATCGCTGAGGCGATGTCCAAGCTGGCCAAGACCTCCTGAAACGCAAAAAGCCCCCCTCCCAAGGACACTGAGTCCTAAA
GAGGG 6430-6513
(ierei FIR:FIVI( :A ( IA( :OCCiA AGA ACCACOCCTOGCCOC :(iA :(
AOC ACCOCC:0( :1(71(i FOCOGAGAC:C IGOOCACCAOCCC 6514-6597
CGCCGCCGCCAGGAGCATTGCCGTTCCCGCCAGCTGAGTTCTGTTGTGCGCCGCCTATGTAGAGCTGGTCGTTGTAGGT
CCGA 6598-6680
TCT CCAGGCG ACTTTCC GGCG AC GCTG AGGATGTC GAT C AC AGAGCC TCCGGGAC CGC C GGTT
GC GGT C AAACCTG ACCATCC 6681-6763
GACAGCGGACGCCGTGGTGTTTCCTCCAGGGCCTCCGGCCTIGCCTGAGAATACAGAGCCAGCTCCCGCTGCGCCTCCA
GCTC 6764-6846
CGACGAGCCCGGTGATCGTCTTGGTCGACCTGCAGGCATGCAAAAGCTGATCCTTGCCGAGCTGGGATGGAAGCCCGGC
CGAC 6847-6929
CCACCCTGGAGGAGAT GATC G AGGATGC C AGGGC CTTT C ACGCCC GCC GC T GCT GAGCGT CC
GCC GC C GGGC CCGCACCGCCG 6830-7012
FCGGCCOUCCCGC FCCGOCiC fCCiCAGCAGCGGGC fCGOCUCGGOCCCGGGCiC
ICCCCGOCCOCCOGOCCiCiGGCFCCGCCCGG 7013-7095
CGGCCGCCOGGOGCCGGGGGCGGCGCCGGGCGOCCCGOGGCGTCAGGCGCCGGGGGCGGTGTCCGGCGGCCCCCAGAGG
AACT 7096-7178
GCGCCAGTTCCTCCGGATCGGTGAAGCCGGAGAGATCCAGCGGGGTCTCCTCGAACACCTCGAAGTCGTGCAGGAAGGT
GAAG 7179-7261
GCGAGCAGTTCGCGGGCGAAGTCCTCGGTCCGCTTCCACTGC GCCCCGTCGAGCAGCGCGGCCAGGATCTCGC
GGTCGCCCCG 7262-7344
GAAGGCGTTGAGATGCAGTTGCACCAGGCTGTAGCGGGAGTCTCCCGCATAGACGICGGTGAAGTCGACGATCCCGGTG
ACCT 7345-7427
CGGTCGCGGCCAGGTCCACGAAGATGTTGGTCC CGTGCAGGTCGCCGTGGAC GAACCGGGGTTC
GCGGCCGGCCAGCAGCGTG 7428-7510
TCCACGTCC GGCAGCCAGTCCTC CAGGCGGTCCAGCAGCC GGGGC GAGAG GTAGC CC CACCC GC GGT
GGTCC TCGAC GGTCGC 7511-7593
CGC GCGGC GTTCCCGCAGCAGTTCC GGGAAGAC CTCGOAAT GGGGGGTGAGCAC G GT GTTCC
CGGTCAGC GGCACC CTGTGCA 7594-7676
GCCGCCCGAGCACCCGGCCGAGTTCGCGG GCCAC CGAGCAGC GC G T TC CGGTC G G TC G TGCC
TCCATCGCGGACCG CCAG 7677-7759
GT GGTGCCGGTCATCCGGCTC AT CACCAGGT
AGGGCCACGGCCAGGCTCCGGTGCCGGGCCGCAGCTCGCCGCGGOCGAGGAG 7760-7842
GCGGGGCACCGGC ACC GGGGCGTC CGCCAGGACCGCGT ACGC CT CC GAC T CCGAC GCGAGGCTC TCC
GGACCGCACCAGTGCT 7743-7925
CGCCGAACAGC f [GA! CACCGGGC CGGGC IC GC C GACCA6 fACGGGCi f _EGG TOCIC f
CGCCGGGCACCCGCAGCACCUGCGOC 7926-3008
ACCGGCAGCCCGAGCTCCTCCAGGGCTCGGCGGGCCAGCGGCTCCCAGAATTCCTGGTCGTTCCGCAGGCTCGCGTAGG
AATC 8009-8091
ATCCGAAICAA fACOG ICGAGAAG fAACAGO GA f IC! ICACAGC GOACCIC lA f ICACAGGG
IACGCOCCGOCY f 1092-8174
CCGCACGGCCGGTCGCGACACGGCCTGTCCGCACCGCGGATCAGGCGTTGACGATGACGGGCTGGTCGGCCACGTCGGG
GACG 8175-8257
TTCTCGGTGGTGCTGCGGTCGGGATCGCCAATCTCTACGGGCCGACCGAGGCGACGGTGTACGCCACCGCCTGGTTCTG
CGAC 8258-8340
GGCG A GGCGCCGTCCC A GGCCCC.GCC GA TCCCC GTCCCCC GC GTCGTCG A GCGCGGT GCCG
AC.G AC A CC GCCCrCG TGGC TCGT 8341-8473
CACGGAGGCCGTCCCCGGCGTCGCGGCGGCCGAGGAGTGGCCCGAGCACCAGCGGITCGCCGTGGTCGAGGCGATGGCG
GAGC 8424-8506
TGGCCCGCGCCCTCCACGAGCTGCCCGTGGAGGACTGCCCCTTCGACCGGCGCCTCGACGCGGCGGTCGCCGAGGCCCG
GCGG 8507-8589
AACGTCGCCGAGGGCCTGTGGACCTCCACGACCTGCAG
GCATGCAAGCTAGCTTTTGTTATCCGCTCACAATTCCACACAACA 8590-8672
TACGAGCCGGAAGCATAANGTGTAAAGCCTGGGGTGCCTAATGAGTGAGCTAACTCACATTAATTGCGTTGCGCTCACT
GCCC 8673-8755
GCTTTCCAGTCGGGAAACCTGTCGTGCCAGCTGCATTAATGAATCGGCCAACGCGCGGGGAGAGGCGGTTTGCGTATTG
GGCG 8756-8838
GEC r CGC [ fCC1CGC [CAC FGAC fCGC fGCGGIVGG fCG GGC IGCGGCGAGCGCiTAtCAGC [CAC
ICAAAGGCGG IAA 8839-8921
TACGGTTATCCACAGAATCAGGGGATAACGCAGGAAAGAACATGTGAGCAAAAGGCCAGCAAAAGGCCAGGAACCGTAA
AAAG 8922-9004
GCCGCGTTGCTGViCGITTTTCCATAGGCTCCCiCCCCCCTGACC_TAGCATCACAAAAATCGACGCTCAAGTCAGAGG
TGGCGAAA 9005-9087
CCCGACAGGACTATAAAGATACCAGGCGTTTCCCCCIGGAAGCTCCCTCGTGCGCTCTCCTGTTCCGACCCTGCCGCTT
ACCG 9088-9170
GATACCIGTCCGCCTTTCTCCCTTCGGGAAGCGTGGCGCTTTCTCAATGCTCACGCTGTAGGIATCTCAGTTCGGTGTA
GGTC 9171-9253
GT TCGCTCCAAGCT GGGCTGT GTGCACGAACCCCCCGTTCAGCC CGACCGCTGCGC
CTTATCCGGTAACTATCGTCTTGAGTC 9254-9336
CAACCCGGTAAGACACGAC
TTATCGCCACTGGCAGCAGCCACTGGTAACAGGATTAGCAGAGCGAGGTATGTAGGCGGTGC TA 9337-9419
CAGAGTTCTTGAAGTGGTGGCCTAACTACGGCTACACTAGAAGGACAGTATTTGGTATCTGCGCTCTGCTGAAGCCAGT
TACC 9420-9502
TTCGGAAAAAGAGTTGGTAGCTCTTGATCCGGCAAACAAACCACCGCTGGTAGCGGTGGTTTTTTTGTTTGCAAGCAGC
AGAT 9503-9585
TACGCGCAGAAAAAAAGGATCTCAAGAAGATCCTTTGATCTTTTCTACGGGGTCTGACGCTCAGTGGAACGAAAACTCA
CGTT 9586-9668
AAGGGATTTTGGTCATGAGAT TATCAAAAAGGATCTTCACCTAGATCCT TT
TAAATTAAAAATGAAGTTTTAAATCAATCTAA 9669-9751
AGTATATATGAGTAAACTTGGICTGACAGTTACCAATGCTTAATCAGTGAGGCACCTATCTCAGC7GATCTGTCTATTT
CGTTC 8752-9834
ATCCATAGTTGCCTGACTCCCCGTCGTGTAGATAACTACGATACGGGAGGGCTTACCATCTGGCCCCAGTGCTGCAATG
ATAC 9835-9917
CGCGAGACCCACGCTCACCGGCTCCAGATTTATCAGCAATAAACCAGCCAGCCGGAAGGGCCGAGCGCAGAAGTGGTCC
TGCA 9918-10000
ACTTTATC CGCCT C CATCCAGTC TATTAATTGTTGCC GGGAAGC
TAGAGTAAGTAGTTCGCCAGTTAATAGITTGC GCAAC GTT 10001-10085
GT TGCCATTGC TACAG GCATC GTGGTGTCACGCTC GTC GTTTGGTATGGC TTCAT TCAGC TCCGGTTC
CCAACGATCAAGGC GA 10086-10169
GT TACATGATCC CCC AT GTTGTGCAAAAAAGC GGT TAGC TCCTTC GGTCCTC
CGATCGTTGTCAGAAGTAAGTTGGCCGCAGTG 10170-10253
l'A IV AC ICA MG l'A IGGCA(TCAC IGCA IAA I IC FC !AC 1G ICA IGCCA I CCG IA AGA
INC IICIG !GAL IGGIGAGIAC 10254-10337
TCAACCAAGTCATTCTGAGAATAGTGTATGCGGCGACCGAGTTGCTCTTGCCCGCTCGTCAATACGGGATAATACCGCG
CCACAT 10338-10421
AGCAGAACTTTAAAAGTGCTCATCATTGGAAAACGTTCTTCGGGGCGAAAACTCTCAAGGATCTTACCGCTGTTGAGAT
CCAGT 10422-10505
TC GATGTAAC CC AC TC GTGCAC C CAAC TGATCTTCAGCATC TTTTAC TTTCACCAGC GTTTC
TGGGTGAGCAA A A A CAGGAAGG 10506-10589
CAAAATGC C GCAAAAAAGGGAATAAGGGC GACAC GGAAATGTTGAATACTCATACTCTTCC TTT TT
CAATATTATTGAAGCATT 10590-10673
TATCAGGGTTATTGTCTCATGAGCGGATACATATTTGAATGTATTTAGAAAAAT A A A CAAAT_AGGGGT T
CC GCGCACATTTCCC 10674-10757
54
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
CGAAAAGTGCCACCTGACGTCTAAGAAACCATTATTATCATGACATTAACCTATAAAAATAGGCGTATCACGAGGCCCT
TTCGT 10758-10841
10842-10842
[0195] Knocking out endogenous BCG phosphodiesterase genes and
intragenic segments
encoding phosphodiesterase domains in order to increase CDN PAMP and DAMP
levels
[0196] Overexpression of CDNs by knocking out an endogenous BCG
phosphodiesterase:
WP 003414507
[0197] The BCG AHM08589.1 protein encodes a 316 amino acid endogenous
bifunctional c-
di-AMP and eGAMP phosphodiesterase in BCG that is 100% identical to the Al
tuberculosis
Rv2837c over the C-terminal 316 amino acids (also called CdnP, CnpB, 3'-to-5
oligoribonuclease
A, bifunctional oligoribonuclease, or PAP phosphatase NrnA). The M
tuberculosis Rv2837c
protein is known to hydrolyze both 3'-S' c-di-AMP (bacterial PAMP molecule)
and 2' -3'cGAMP
(host DAMP molecule). Since the BCG protein is 100% identical over the C-
terminal 315 amino
acids, knockout (gene replacement) of the BCG AHM08589.1 protein will lead to
increased levels
of CDNs (3'-5' c-di-AMP and 2' -3'cGAMP) in recombinant BCG.
[0198] SEQ ID NO: 14
[0199] Bifunctional c-di-AMP and cGAMP phosphodiesterase CdnP
(also called CnpB, 3'-
to-5' oligoribonuclease A, bifunctional oligoribonuclease, PAP phosphatase
NrnA) from BCG,
amino acid sequence (316 amino acids; BCG protein AHM08589.1; NCBI Reference
DNA
Sequence: CP003494.1 from BCG strain ATCC 35743; NCBI Reference Protein
Identifier
WP_003414507). A similar sequence is present in Mycobacterium tuberculosis as
protein
Rv2837c or MT2903, and in Mycobacterium bovis as protein Mb2862c.
MDAVGAAALLSAAARVGVVCHVHPDADTIGAGLALALVLDGCGKRVEVSFAAPATLPESLRSLPGCHLL
VRPEVMRRDVDLVVTVDIPSVDRLGALGDLTDSGRELLVIDHHASNDLFGTANFIDPSADSTTTMVAEILD
AWGKPIDPRVAHCIYAGLATDTCiSFRWASVRGYRLAARLVEIGVDNATVSRTLMDSHPFTWLPLLSRVLG
SAQLVSEAVGGRGLVYVVVDNREWVAARSEEVESIVDIVRTTQQAEVAAVFKEVEPHRWSVSMRAKTVN
LAAVASGFGGGGHRLAAGYTTTGSIDDAVASLRAALG
[0200] SEQ ID NO: 15
[0201] Bifunctional c-di-AMP and cGAMP phosphodiesterase gene,
cdnP (also called cnpB
or gene for 3'-to-5' oligoribonuclease A, bifunctional oligoribonuclease, or
PAP phosphatase
NrnA) from BCG, DNA sequence (951 nucleotides [316 codons and 1 stop codon];
encodes BCG
protein AHM08589.1; NCBI Reference Sequence: CP003494.1 from BCG strain ATCC
35743).
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
A similar sequence is present in Mycobacterium tuberculosis encoding protein
Ry2837c or
MT2903, and in Mycobacterium hovis encoding protein Mb2 g 62e.
GTGGACGC CGTCGGTGCCGCTGCGCTGTTGTC GGCCGC TGCCAGGGTCGGGGTAGTCTGCCACGTC CA
CCCCGATGCCGACACCATCGGC GCCGGATTGGCATTGGCATTGGTGTTGGACGGGTGCGGCAAGCGGG
TAGAGGTCAGCTTTGCC GCGCCGGCGACACTGCCCGAGTCGCTGCGTTC GCTGCCGGGCTGC CATC TG
CTGGTCCGCCCTGAGGTGATGCGCCGCGATGTCGATTTGGTTGTGACTGTTGACATTCCGAGTGTTGAT
CGGCTCGGTGCTCTGGGCGATCTAACTGATTCCGGGCGGGAGCTCCTGGTAATCGACCATCACGCCTC
CAACGACCTGTTCGGCACCGCGAATTTCATTGACCCGTCGGCGGATTCCACCACGACGATGGTTGCCG
AGA TCCTCGACGCGTG GGGGA A ACCGA TAGACCCGCGCGTCGCGC A CTGC A TCTACGC CGGGTTGGCG
ACCGACACGGGGTCGTTTCGCTGGGCCAGTGTGCGGGGGTATC GGCTGGCGGCGCGGCTGGTAGAGAT
CGGTGTGGACAACGCCACCGTCAGCAGGACCTTGATGGACAGCCATC CCTTCACCTGGTTGCCGTTGC
TATC GC GGGTGTTGGGTTCGGCGCAGC TGGIGTCC GAGGCGGTC GGTGGCCGCGGGCTGGTTTACGTC
GTCGTCGACAACCGGGAGTGGGTCGCTGCGCGCTCGGAGGAAGTGGAAAGCATCGTCGACATCGTCC
GCACCACGCAACAAGCCGAGGTCGCGGCGGTGITCAAGGAGGTCGAACCGCATCGGTGGTCGGTGTC
GATGCGGGCTAAGACCGTGAATTTGGC CGCGGTTGCCTC TGGGTTCGGTGGCGGTGGTCAC C GGCTGG
CCGCGGGGTATACGACCACCGGCTCGATCGACGACGCTGTGGCGTCGTTGCGCGCGGCGCTTGGTTAG
[0202] SEQ ID NO: 16
[0203] Bifunctional c-di-AMP and cGAMP phosphodiesterase CdnP
(also called CnpB,
Ry2837c, or MT2903, 3'-to-5' oligoribonuclease A, bifunctional
oligoribonuclease, PAP
phosphatase NrnA) from Mycobacterium tuberculosis, amino acid sequence (336
amino acids; M.
tuberculosis protein WP_003905944.1; NCB I/GenBank Reference Sequence:
AL123456 from M.
tuberculosis strain H37Rv). The M. tuberculosis protein has 20 additional
amino acids at its N-
terminus compared with the BCG protein (SEQ ID NO:14) which are underlined and
boldfaced.
MTTIDPRSELVDGRRRAGARVDAVGAAALLSAAARVGVVCHVHPDADTIGAGLALALVLD
GCGKRVEVSFAAPATLPESLRSLPGCHLLVRPEVMRRDVDLVVTVDIPSVDRLGALGDLT
DS GRELLVIDHHASNDLF GTANFIDP SAD STTTMVAEILDAWGKPIDPRVAHCIYAGLAT
DTGSFR WA SVRGYRL A ARLVETGVDNATVS RTLMDSHPFTWLPLL SRVL GSA QLVSEAVG
GRGLVYVVVDNREWVAARS EEVESIVDIVRTTQQAEVAAVFKEVEPHRWSVSMRAKTVNL
AAVASGFGGGGHRLAAGYTTTGSIDDAVASLRAALG
[0204] Overexpression of CDNs by knocking out an endogenous BCG
phosphodiesterase
domain: EAL domain of protein BCG R507340 (previously BCG 1416c). The BCG
RS07340
protein (SEQ ID NO:4) is encoded by the DNA sequence shown in SEQ ID NO:5. The
BCG RS07340 protein is 100% identical to the M. tuberculosis Ry1354c protein
and is an
endogenous CDN PDE in BCG. The full-length polypeptide is 623 amino acids in
length, and it
encodes a bifunctional diguanylate cyclase/diguanylate phosphodiesterase. The
domain structure
56
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
is: N-terminus-GAF-GGDEF-EAL-C-terminus as shown. The GAF domain
(approximately amino
acids 1-190) is a regulatory domain which influences the activity of the other
domains. The
GGDFF domain (approximately amino acids 190-350) is a diguanylate cyclase
catalyzing the
reaction 2 GTP 4 c-di-GMP + 2 pyrophosphates. The EAL domain (amino acids 354
to 623,
highlighted in SEQ ID No: 4) is a diguanylate phosphodiesterase catalyzing the
reaction c-di-GMP
2 GMP. As the EAL domain of this protein is known to cleave 3'-5' c-di-GMP,
knockout of
this endogenous cyclic dinucleotide phosphodiesterase domain will increase the
levels of c-di-
GMP produced by BCG. Targeted knockout of the EAL domain may be accomplished
by gene
replacement of the full-length WT BCG RS07340 gene with one which encodes only
amino acids
1-353 (the GAF-GGDEF domains), that is truncating the coding sequence of the
gene to exclude
the sequences that encode amino acids 354-623 (shown as the underlined DNA
sequence in SEQ
ID NO:5) and including an appropriate stop codon and transcription termination
sequence.
Recombinant BCG lacking the EAL domain of BCG RS07340 will lead to increased
levels of the
CDN PAMP c-di-GMP.
[0205] Overexpression of CDNs by knocking out an endogenous BCG
phosphodiesterase:
BCG AHM07112. The BCG_AHM07112 protein is an endogenous diguanylate
phosphodiesterase
in BCG (homologous the 307 amino acid M tuberculosis Rv1357c protein). Some
strains of BCG
lack BCG_AHM07112 altogether while others such as BCG Tice harbor it. Among
the BCG
strains that have this polypeptide, the protein may be 288 amino acids in
length (such as in BCG
ATCC 35743) or 307 amino acids in length (such as in BCG Pasteur 1173 P2). The
BCG AHM07112 protein from BCG ATCC 35743 is 288 amino acids in length and is
100%
identical to the /1//. tuberculosis Rv1357c protein over its C-terminal 287
amino acids. The domain
structure of BCG AHM07112 is that of a single EAL domain. As the M.
tuberculosis Rv1357c
protein is known to cleave 3'-S' c-di-GMP, it is highly likely that the BCG
protein performs the
same reaction. Knockout of this endogenous cyclic dinucleotide
phosphodiesterase in BCG is
anticipated to increase the levels of c-di-GMP produced by BCG. Targeted
knockout of the EAL
domain may be accomplished by gene replacement of the full-length WT BCG
AHM07112 gene
and subsequent generation of an unmarked deletion.
[0206] SEQ ID NO: 17
57
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
[0207] Diguanylate phosphodiesterase AHM07112.1 from BCG and
other related
mycobacteria, amino acid sequence (288 amino acids; GenBank Reference
Sequence:
CP003494.1; from BCG strain ATCC 35743). AHM07112.1 is 100% identical to the C-
terminal
287 amino acids of the diguanylatc phosphodiesterasc of Mycobacterium
tuberculosis protein
Rv1357c or MT1400 and of Mycobacterium bovis as protein Mb1392c.
MID YEEMFRGAMQARAMVAN PDQ WAD S DRDQVNTRHY STSMRVALDRGEFFL V YQPIIRLADNRIIGAE
ALLRWEHPTLGTLL PGRFIDRAENNGL MVPLTAFVLEQACRHVRSWRDHSTDPQPFVSVNVSASTICDPG
FLVLVEGVLGETGLPAHALQLELAEDARLSRDEKAVTRLQELSALGVGIAIDDFGIGFSSLAYLPRLPVD
VVKLGGKFTECLDGDTQARLANEQTTRAMIDLODKLGTTVTAKLVESPSQA A RLR AFGCK A AQGWHF AK A
LPVDFFRE
[0208] SEQ ID NO: 18
[0209] Diguanylate phosphodiesterase AHM07112.1 from BCG and
other related
mycobacteria, DNA sequence (867 nucleotides [288 codons, 1 stop codon];
GenBank Reference
Sequence: CP003494.1; from BCG strain ATCC 35743). AHM07112.1 is 100%
identical to the
C-terminal 287 amino acids of the diguanylate phosphodiesterase of
Mycobacterium tuberculosis
protein Rv1357c or MT1400 and of Mycobacterium bovis as protein Mb1392c.
1 ttgatcgact acgaagagat gtttaggggc gcgatgcaag cgcgagcgat ggtagccaat
61 cctgaccaat gggcggactc cgaccgcgac caggtcaaca ctcgccatta tctgtccact
121 tcgatgcgcg tggcactgga tcgcggtgaa ttcttcctcg tctaccagcc aatcatccgg
181 cttgccgaca accgcatcat cggcgccgag gccctgctgc gctgggaaca cccgacgttg
241 ggcacgclac teccgggccg gticatcgac cgtgecgaga acaaeggact gatgglgccg
301 ctcacggcct tcgtgctcga gcaggcctgc cgccacgtcc gcagttggcg tgaccacagc
361 accgacccgc aaccgtttgt cagcgtcaac gtctccgcca gcaccatctg cgatcccggc
421 ttcctggtgc tggtcgaagg tgtgctcggc gaaaccggcc tgcccgccca tgccctgcag
481 ctcgaactgg ccgaggacgc gcgccttagc agagacgaga aggcggtgac caggctacaa
541 gaattgtccg ctctcggcgt cggcatcgcc atcgacgact tcggcattgg attctccagc
601 cicgcctacc accecgcct ccccgtcgac gtggtcaaac tcgggggaaa gttcatcgag
661 tgcctcgatg gcgacattca agctcggctg gccaacgaac agatcacccg ggcaatgatc
721 gaccttggcg acaagctcgg tatcaccgtc actgcaaagc tagtcgaaag ccccagccaa
781 gccgcccggt tgcgcgcctt eggctgtaaa gccgcacaag gctggcactt tgccaaggca
841 ctgccggtcg acttatcag agagtag
[0210] SEQ ID NO:19
[0211] Diguanylatc phosphodiesterase Rv1357c or MT1400 from Mycobacterium
tuberculosis and BCG Pasteur 1173 P2, amino acid sequence (307 amino acids,
NCBI/GenBank
58
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
Reference Sequence: AL123456 from M tuberculosis strain H37Rv). The 19 amino
acid N-
terminal extension is present in the Al tuberculosis and in BCG Pasteur strain
1173 P2 but absent
in several other BCG strains. The 19 amino acid N-terminal extension is
underlined and
boldfaced. The C-terminal 287 amino acids of M. tuberculosis Rv1357c arc 100%
identical to the
BCG diguanylate phosphodiesterase AHM07112.1.
MDRCCORATAFACALRPTKLIDY EEMFRGAMQARAMVAN PDQ WADSD RDQV N TRHY L ST S
MRVALDRGEFFLVYQPIIRLADNRIIGAEALLRWEHPTLGTLLPGRF IDRAENNGLMVPL
TAFVLEQACRHVRSWRDHSTDPQPITVSVNVSASTICDPGELVLVEG VLGETGLPAHALQL
ELAEDARL S RDEKAVTRLQEL SALGVGIAIDDF GIGF S SLAYLPRLPVDVVKLGGKFIEC
LDGDTQARL ANEQTTR A MIDLG DKLGITVTAKLVETPSQA ARLR AFGCK A A QGWHFA K A L
PVDF FRE
[0212] The sequences referenced in the application are summarized
in Table 1 below.
Table 1
SEQUENCE DESCRIPTION
NUMBER
SR() ID NO:] Diadenylate cyclase DisA from BCG and other
related mycobacteria, amino
acid sequence
SEQ ID NO:2 Diadenylate cyclase disA from BCG and other
related mycobacteria, DNA
sequence
SEQ ID NO:3 Plasmid pSD5B-Phspoo::disA which overexpresses the
disA gene, DNA
sequence
SEQ ID NO:4 Bifunctional diguanylate cyclase/phosphodiesterase
BCG R507340 from
BCG and other related mycobacteria, amino acid sequence
SEQ ID NO:5 Bifunctional diguanylate cyclase/phosphodiesterase
BCG R507340 from
BCG and other related mycobacteria, DNA sequence
SEQ ID NO:6 Modified, bifunctional diguanylate
cyclase/phosphodiesterase from BCG and
other related mycobacteria lacking the EAL domain so that it functions as a
monofunctional diguanylate cyclase, amino acid sequence
SEQ ID NO:7 Modified, bifunctional diguanylate
cyclase/phosphodiesterase from BCG and
other related mycobacteria lacking the EAL domain so that it functions as a
monofunctional diguanylate cyclase, DNA sequence
SEQ ID NO:8 Cyclic (IMP-AMP synthase DncV from Vibrio
cholerae, amino acid
sequence
SEQ ID NO:9 Cyclic GMP-AMP synthase dncV from Vibrio cholerae,
DNA sequence
SEQ ID NO:10 Cyclic (IMP-AMP synthase cGAS from Homo sapiens,
amino acid sequence
SEQ ID NO:11 Cyclic GMP-AMP synthase cGAS from Homo sapiens,
DNA sequence
SEQ ID NO:12 Cyclic GMP-AMP synthase cGAS gene from Homo
sapiens with
mycobacterial codon optimization, DNA sequence
59
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
SEQ ID NO:13 Plasmid pMH94H- Ph.sp60::disA::C0cGAS: :rnCherry
which overexpresses the
disA gene, the codon-optimized human cGAS gene, and mChen-y, DNA
sequence
SEQ ID NO:14 Bifunctional c-di-AMP & cGAMP phosphodiesterase
CdnP from BCG,
amino acid sequence
SEQ ID NO:15 Bifunctional c-di-AMP & cGAMP phosphodiesterase
CdnP from BCG,
DNA sequence
SEQ TD NO:16 Bifuncticmal c-di-AMP & cGAMP pliosphodiesterase
CdnP from M.
tuberculosis with 20 amino acid N-terminal extension, amino acid sequence.
SEQ ID NO:17 Diguanylate phosphodiesterase AII1V107112.1 from
BCG and other related
mycobacteria, amino acid sequence
SEQ ID NO:18 Diguanylate phosphodiesterase AIIM07112.1 from
BCCi and other related
mycobacteria, DNA sequence
SEQ ID NO:19 Diguanylate phosphodiesterase Ry1357c or MT1400
from Mycobacterium
tuberculosis and BCG Pasteur 1173 P2 with 19 amino acid N-tenninal
extension, amino acid sequence
SEQ ID NO: 20 DNA sequence for the 1350 bp panCD operon from BCG Pasteur
SEQ ID NO: 21 Protein sequence for the 301 aa PanC polypeptide
from BCG Pasteur
SEQ ID NO: 22 Protein sequence for the 139 aa PanD polypeptide from BCG
Pasteur
SEQ ID NO: 23 DNA sequence for the 2501 bp panCD-containing region from BCG
SEQ ID NO: 24 Protein sequence for the 724 aa mutant PanC polypeptide from BCG
SEQ ID NO: 25 Protein sequence for the 139 aa PanD polypeptide from BCG Tice
SEQ ID NO: 26 DNA sequence alignment of the BCG Pasteur and BCG Tice panC
SEQ ID NO: 27 DNA sequence alignment of the BCG Pasteur and BCG Tice panD
SEQ ID NO: 28 L primer to amplify the panCD operon (diagnostically)
SEQ ID NO: 29 R primer to amplify the panCD operon (diagnostically)
SEQ ID NO: 30 pSD5.hsp65-disA.Kan
SEQ ID NO: 31 pSD5.hsp65-disA.panCDa.'Ã-No Kan
SEQ ID NO: 32 DNA sequence for pJV53 (recombineering plasmid)
SEQ ID NO: 33 DNA fragment containing panCD allelic exchange substrate
cassette
SEQ ID NO: 34 dif-Hyg-dif cassette
SEQ ID NO: 35 pUC-Hyg Plasmid
SEQ ID NO: 36 pUC-Hyg-panCD KO plasmid
SEQ ID NO: 37 Left primer used to generate the backbone of "pSD5.hsp65-
SEQ ID NO: 38 Right primer used to generate the backbone of "pSD5.hsp65-
SEQ ID NO: 39 Left primer used to generate the panCD portion of "pSD5.1isp65-
SEQ ID NO: 40 Right primer used to generate the panCD portion of "pSD5.hsp65-
102131 In one embodiment, the present invention relates to an
expression cassette or expression
vector including a nucleic acid sequence encoding a Rv1354c protein, or a
functional part thereof;
a nucleic acid sequence encoding a cyclic GMP-AMP synthase (DncV) protein, or
a functional
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
part thereof; a nucleic acid sequence encoding a cyclic GMP-AMP synthase
(cGAS) protein, or a
functional part thereof; or a combination thereof. In some aspects, the
expression vector or
expression cassette further includes a nucleic acid sequence encoding a DNA
integrity scanning
(disA) protein which functions as a diadenylate cyclase, or a functional part
thereof. In other
aspects, the nucleic acid sequence encoding a Rv1354c protein does not contain
a
phosphodiesterase gene or phosphodiesterase domain. In some aspects, the
expression vector or
expression cassette does not contain a phosphodiesterase gene or
phosphodiesterase domain.
[0214] Methods for generating expression vectors and expression
cassettes, transforming
Mycobacteria and isolating the same have been described. In some embodiments,
an expression
vector or expression cassette of the invention includes one or more regulatory
sequences, e.g., a
promoter and/or enhancer element, operably linked to a nucleic acid of the
invention which
controls or influences transcription of the nucleic acid. In some aspects, an
expression vector or
expression cassette of the invention includes one or more sequences operably
linked to a nucleic
acid of the invention which direct termination of transcription, post-
transcriptional cleavage,
and/or polyadenylation. In some aspects, an expression vector or expression
cassette of the
invention includes a variable length intervening sequence and/or a selectable
marker gene operably
linked to a nucleic acid of the invention.
[0215] In one embodiment, the present invention relates to a
strain of Mycobacterium
including an expression vector or expression cassette of the invention
described herein. In some
aspects, the strain of Mycobacterium is Mycobacterium tuberculosis,
Mycobacterium bovis, or a
combination thereof. In other aspects, the strain of Mycobacteriwn is BCG. In
some aspects, the
strain includes the plasmid of SEQ ID NO:13.
102161 In another embodiment, the present invention relates to a
strain of Mycobacteriwn that
expresses or overexpresses diadenylate cyclase and/or expresses or
overexpresses one or more
other cyclase genes or domains (e.g., those described herein). In some
aspects, the expression or
overexpression results in the release of one or more STING agonists (e.g., c-
di-AMP, c-di-GMP,
2'-3' cGAMP, and/or 3'-3' cGAMP). In some aspects, the present invention
relates to a strain of
Mycobacterium that expresses or overexpresses diadenylate cyclase and/or does
not express a
phosphodiesterase (PDE) that hydrolyzes STING agonists (e.g., contains a
deletion of a PDE gene
that hydrolyzes STING agonists). In some aspects, the strain of Mycobacterium
is Illycobacterium
61
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
tuberculosis, Mycobacterium bovis, or a combination thereof. In some aspects,
the strain of
Mycobacterium is BCG.
[0217] Statistically significant anti-tumor effects with BCG-
di.sA-OE in the rat MNU bladder
cancer model
[0218] The rat MNU bladder cancer model is a validated model of
bladder cancer in which
administration of intravesical BCG can be shown to be therapeutic (Fig. 6 and
Kates et al. PMID
28588015). The inventors extended their previous findings of the therapeutic
effect of BCG-disA-
OE versus BCG-WT which were shown in Figure 7. The inventors have now
performed the 16-
week rat MNU model twice. Figure 7 was based on Experiment 1 and shows that
BCG-disA-OE
displays a trend towards a better outcome versus BCG-WT. After performing
Experiment 2 and
combining its data with Experminent 1, it is now shown that BCG-disA-OE is
statistically
significantly superior to no treatment (p = 0.048) whereas BCG-WT is not
statistically significantly
superior to no treatment (data shown in Figure 15).
[0219] Reduction of tumor-suppressive Treg cells by BCG-disA-OE
in a murine syngeneic
bladder cancer tumor model.
[0220] In the MNU rat bladder cancer model the amount of bladder
tissue at the end of the 16-
week experiment is insufficient to perform flow cytometry. In order to study
the cell population
changes elicited by BCG-disA-OE a murine syngeneic bladder cancer tumor model
using BBN975
cells was developped. The model allows for large tumors (>1.5 cm in diameter)
to develop on the
mouse flank. Mice were treated with BCG-disA-OE and BCG-WT by intratumoral
injection. As
is shown in Figure 16, the use of BCG-disA-OE led to reduced levels of tumor-
associated CD4+
Treg cells, tumor-associated CD8+ Treg cells, and splenic CD4+ Treg cells.
102211 BCG-disA-OE delivers sustained STING agonist from the
intracellular compartment.
[0222] Persistence of BCG in the bladder.
[0223] Bowyer et al (The persistence of bacilli Calmette-Guerin
in the bladder after
intravesical treatment for bladder cancer. Brit J Urol. 1995; 75: 188-192.
PMID 7850324)
evaluated 125 bladder cancer patients from 1986- 1992 who received
intravesical BCG. Patients
were asked to provide monthly urine samples which were then sent for
mycobacterial culture. 90
patients survived and were compliant with the monthly urine samples. 4/90
patients (4.4%) had
persistent BCG in their urine, one for up to 16.5 months. A fifth patient
required a cystectomy
62
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
7 weeks after completing intravesical BCG treatments and was found to have
microscopic
evidence of acid-fast bacilli in the bladder by microscopy.
[0224] Durek et al. (The fate of bacillus Calmette-Guerin after
intravesical instillation. J Urol.
2001; 165: 1765-1768. PM1D 11342972) studied 49 patients with serial urine
cultures following
intravesical BCG. BCG was in the urine detected in 96.4% of the specimens
after 2 hours and in
67.9% after 24 hours after instillation. The number of positive specimens
decreased, and was
27.1% on day 7 immediately before the next instillation (FIG. 38). The
investigators also evaluated
bladder biopsies by PCR for mycobacterial DNA within 1 week after the 6th
instillation
(instillations were given monthly). In 14 of 44 bladder biopsies (31.8%)
mycobacterial ribosomal
DNA was found. Additionally, positive PCRs for mycobacterial DNA was evident
up to 24 months
in between 4.2% and 37.5% of the investigated biopsies.
[0225] The fact that BCG is known to persist in bladder tissue
represents an important
advantage of the BCG-disA -OE strategy for STING agonist deliver in cancer.
While numerous
technologies have focus on generating small molecule STING agonists, such
agents have relatively
short exposure times. In contrast, as an intracellular microorganism and as
demonstrated by the
Bowyer and Durek studies, BCG persists in cells and tissues for many weeks.
The persistence of
BCG-disA-OE in tissue offers sustained long-term deliver of the STING agonist
in the tumor
microenvironment.
[0226] BCG-disA -OE is safer than BCG-WT in two separate mouse
models
[0227] Intravesical BCG treatment in humans is associated with
dysuria, fatigue, and malaise
in treated patients. Additional more severe adverse effects are persistent
cystitis with BCG and
disseminated BCGosis. The patient safety of BCG was reviewed extensively in
O'Donnell et al
(Up-to-date, 2019). The incidence of dissemination of BCG into the bloodstream
after intravesical
instillation is estimated at 1/15,000 patients.
[0228] To test the safety of BCG-disA-OE compared to BCG-WT the
inventors used two
mouse models of BCG infection where the BCG strains were aerosolized into the
lungs of
immunocompetent BABL/c mice or immunosuppressed SCID mice. As shown in Figures
17A-
17B, BCG-disA-OE was less capable of proliferating in immunocompetent mouse
lungs than
BCG-WT, and it was less lethal in a time-to-death assay in immunosuppressed
mice.
63
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
[0229] BCG has been shown to elicit trained immunity which has
been associated with its
therapeutic benefit in solid and liquid tumors and for diabetes. STING agonist
overexpressing
BCG strains elicit stronger trained immunity changes than BCG-WT
[0230] Trained immunity. Trained immunity refers to thc ability
of one antigenic stimulus to
elicit more potent immune responses to a second, different antigen. Trained
immunity is antigen
independent, based on heterologous CD4 and CD8 memory activation, cytokine
mediated, and is
associated with epigenetic and metabolic changes. BCG is a potent tool as the
first antigenic
stimulus to elicit trained immunity to subsequent antigenic stimuli such as
tumors, viral infection,
or drug-resistant bacterial infections (Netea et al. Trained immunity: a
program of innate immune
memory in health and disease. Science 2016. PMID 27102489; and Arts et al. BCG
vaccination
protects against experimental viral infection in humans through the induction
of cytokines
associated with trained immunity. Cell Host Microbe 2018. PMID 29324233).
[0231] BCG for solid and liquid tumors. BCG has a long history of
therapeutic benefit as an
immunotherapy for both solid and liquid tumors in humans (Hersh et al. BCG as
adjuvant
immunotherapy for neoplasia. Annu Rev Med 1977. PMID 324372). It has been used
both
systemically and intratumorally for malignancies that include melanoma, non-
small cell lung
cancer (NSCLC), and acute lymphoblastic leukemia (ALL). Recently there have
been trials of
BCG together with checkpoint inhibitors for forms of bladder cancer.
[0232] BCG for diabetes. BCG vaccination has recently been shown
to have therapeutic
benefits in glucose control for various forms of diabetes mellitus including
Type 1 diabetes
mellitus (Stienstra and Netea. Firing up glycolysis: BCG vaccination effects
on Type 1 diabetes
mellitus. Trends Endoc Metab 2018. PMID: 30327169). The effect is believed to
be mediated by
the trained immunity effects of BCG which have been shown to lead to
epigenetic modifications
which promote pro-inflammatory cytokine expression as well as the expression
of metabolic
enzymes such as those for glycolosis.
[0233] BCG-disA-OE and trained immunity. To investigate the
ability of STING agonist
overexpressing strains of BCG to stimulate trained immunity, the inventors
tested the ability of
BCG-WT versus BCG-disA-OE to elicit potentiation of second antigen stimulation
in rested
human monocytes following an exposure to the BCG strains six days prior. The
first antigen was
a BCG strain on day 0, and after six days of rest, the second antigen was the
unrelated TLR-1/2
64
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
antigen PAM3CSK4. As may be seen in Figure 18, upon receiving the second
stimulus, the
immune response tested (secretion of IL-1 p) was potentiated by both BCG-WT
and BCG-disA-
OE, but the degree of stimulation by BCG-disA-OE was statistically
significantly greater than that
of either no BCG first stimulus or BCG-WT as the first stimulus. This reveals
that STING
overexpressing BCG strains such as BCG-disA-OE are a more potent stimulators
of trained
immunity than BCG-WT.
[0234] In a related experiment, the inventors conduced the same
BCG-first stimulation/6 day
rest/ TLR-1, 2 second antigen stimulation with PAM3CSK4 experiment with human
monocytes.
At the end of the experiment cellular DNA was collected and subjected to
chromatin
immunoprecipitation (ChIP) using an antibody for the H3K4 histone methylation
mark. The H3K4
mark is a known transcriptional activation mark. Upon quantitative PCR
amplification of the IL-6
promoter region of the immunoprecipitated DNA, the results showed that BCG-
Pasteur-disA-OE
and BCG-Tice-disA -OE were statistically significantly more potent in
eliciting the H3K4 mark in
the IL-6 promoter (IL-6 is a pro-inflammatory cytokine) than their respective
BCG-WT strains.
These results showed that STING overexpressing BCG strains such as BCG-disA-OE
are a more
potent stimulators of epigenetic changes associated with trained immunity than
BCG-WT.
[0235] BCG-Tice-disA-OE expresses much higher levels of the disA
gene than BCG-WT
[0236] As may be seen in Figure 21, the relative expression of
BCG-Tice-disA-OE clone 2
(which was selected for seed-lot preparation and storage) was 300:1 using the
2-AACT method of
comparison. This indicates that disA is strongly overexpressed by being on a
multicopy plasmid
and driven by the M leprae hsp65 promoter in pSD5-hsp65-MT3692 plasmid. This
strong
overexpression leads to much higher levels of release of the STING agonist, c-
di-AMP.
102371 STING agonist oyerexpression BCG strains such as BCG-disA-
OE elicit pro-
inflammatory changes in signaling pathways and cytokine secretion profiles in
multiple
model systems.
[0238] The inventors tested STING agonist overexpressing strains
such as BCG-disA-OE
compared to BCG-WT in multiple model systems to evaluate its relative capacity
to elicit
proinflammatory cytokine changes. BCG-disA-OE was statistically significantly
superior than
BCG-WT in the majority of their tests. When the comparisons were not
statistically significant,
BCG-disA -OE gave the stronger of the two responses.
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
[0239] Figure 23 also shows that the elevation of type 1 IFN
secretion in both BCG-disA-OE
and BCG-WT is STING-dependent.
[0240] In summary, BCG-di.s,4-0E is a more potent stimulator of
pro-inflammatory cytokine
expression and proinflammatory pathway induction than BCG-WT
[0241] The table below summarizes the data:
[0242] Table 2:
Mouse BMDM in vitro IRF3 qRT-
PCR BCG-disA-OE > BCG-WT Figure 22
Mouse BMDM, BMDC, J774 IFN-fi ELISA BCG-
disA-OE > BCG-WT Figure 24
macrophage cell line in vitro
Mouse BMDM; BMDC. J774 IL-6 ELISA BCG-
disA-OE > BCG-WT Figure 25
macrophage cell line in vitro
Mouse BMDM, BMDC, J774 TNF ELISA BCG-
disA-OE > BCG-WT Figure 26
macrophage cell line in vitro
Rat bladder cancer NBT-II line TNF, IFN-y ELISA BCG-
disA-OE > BCG-WT Figure 27
in vitro
Human bladder cancer RT4 line IFN-f3, IFN-y, ELISA BCG-
disA-OE > BCG-WT Figure 28
in vitro TNF, IL-1f3
5637, RT4, NBT-II bladder TFN-fl qRT-
PCR BCG-disA-OE > BCG-WT Figure 29
cancer cell lines In vitro
Mouse lungs in vivo (different IFN-f3, IF N-7, IL- ELISA BCG-
disA -OE > BCG-WT Figure 30
time points) 6, TNF
In vivo
Mouse spleens in vivo IFN-f3; IFN-7 IL-
ELISA BCG-disA-OE > BCG-WT Figure 31
(4 weeks). In vivo 6; TNF
[0243] A method to produce an antibiotic gene cassette-free
recombinant BCG which
overexpresses a STING agonist biosynthetic gene.
[0244] The disA -overexpressing plasmid pSD5-hsp65-MT3692 carries
a Kan resistance gene
cassette conferring resistance to the antibiotic kanamycin. The inventors
disclose a method to
generate an antibiotic gene cassette-free recombinant BCG which overexpresses
a STING agonist
biosynthetic gene.
[0245] The mycobacterial genetic operon pan CD encodes for the
biosynthetic gene panC
(Pantoate--beta-alanine ligase gene) and panD (aspartate 1-decarboxylase
gene). The gene
products PanC and PanD are required for the biosynthesis of pantothenic acid
also called vitamin
66
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
B5 (a B vitamin). Pantothenic acid, a water-soluble vitamin, is an essential
nutrient for
mycobacteria such as BCG. Animals require pantothenic acid in order to
synthesize coenzyme-A
(CoA), as well as to synthesize and metabolize proteins, carbohydrates, and
fats. The anion is
called pantothenate.
[0246] Genetic deletion ofpanCD in mycobacteria has been shown to
yield mutant strains that
can only grow in the presence of added pantothenate. As such they are
auxotroph for pantothenate.
ApanCD mutants of Atvcobacteriuni tuberculosis, have been shown to be highly
attenuated in
animal infection, being rapidly cleared, because of their inability to grow in
mammalian tissues
where pantothenate is not available to them.
[0247] The inventors disclose a detailed method for generating an
unmarked (no antibiotic
gene cassettes) ApanCD deletion mutant of BCG. This mutant will only be able
to grow in the
presence of pantothenate and would not be expected to survive during infection
or be an effective
delivery vector for STING agonist expression.
[0248] The inventors disclose a detailed method for generating a
shuttle plasmid which harbors
the mycobacterial pan CD gene as well as an overexpression construct for the
biosynthesis of
STING agonists (such as the Phsp65::di,sil construct which overexpresses the
dinsA gene and
releases excess STING agonist, c-di-AMP). The shuttle plasmid is capable of
replication in E. colt
or in mycobacteria. It harbors an antibiotic cassette that can be conveniently
removed by cleavage
with a rare-cutting restriction enzyme and re-ligation. Alternatively, the
shuttle plasmid may be
generated by PCR amplification of the backbone of the plasmid excluding the
antibiotic resistance
cassette that generates unique restriction sites at the termini and ligating
in a PCR product
consisting of an amplified panCD operon with the same unique restriction sites
at its termini. In
either manner the antibiotic resistance gene-free shuttle plasmid (ligation
product) may be
electroporated into a BCG or E. coli auxotroph and selected for on
pantothenate-free agar plates.
[0249] In the final manifestation of this disclosure, the
inventors show a method to introduce
the antibiotic-cassette-free plasmid harboring the mycobacterial pan CD gene
as well as an
overexpression construct for the biosynthesis of STING agonists (such as the
Phsp65::disA
construct) into an unmarked BCG ApanCD mutant. The end result is a BCG strain
that harbors
no antibiotic resistance genes, and that strongly overexpresses a STING
agonist biosynthetic
67
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
gene(s). In a mammalian host or a human, such a BCG strain would be under
strong selective
pressure to retain the plasmid due to its requirement forpanCD complementation
from the plasmid.
[0250] In another manifestation of the disclosure, the panCD
cassette and the construct for the
biosynthesis of STING agonists (such as the Phsp65::disA construct) could be
introduced into a
chromosomally integrating vector such as pMH94. Using similar methods, the
antibiotic cassette
could be eliminated from pMH94. Introduction of this chromosomally integrating
plasmid into an
unmarked BCG ApanCD mutant would also yield a BCG strain that harbors no
antibiotic
resistance genes, and that strongly overexpresses a STING agonist biosynthetic
gene(s). A
disadvantage of this strategy is that the overexpression construct would be in
single copy on the
bacterial chromosome, rather than being in multicopy on a plasmid, and this
could result in lower
levels of STING agonist release.
[0251] BCG-Tice (ATCC 35743) is a natural pantothenate auxotroph.
[0252] The inventors disclose that the Mycobacterium hovis BCG
Tice strain (ATCC 35743)
is a natural pantothenate auxotroph. This strain carries a 5 bp DNA insertion
in its panC gene at
base pairs 739-743. This insertion mutation change leads to a frameshift
mutation after the 246th
amino acid of PanC (wild type PanC is 309 amino acids in length). As a result
of the 5 bp insertion
mutation, the mutant PanC polypeptide in the Mycobacterium bovis BCG Tice
strain (ATCC
35743) is comprised of 246 amino acids of the wild type PanC sequence at its N-
terminus followed
by a 475 amino acid nonsense polypeptide at its C-ten-ninus. This mutant PanC
polypeptide is
highly unlikely to retain any functional pantoate--beta-alanine ligase
activity (the normal
enzymatic function of PanC). Additionally, the PanD polypeptide in BCG Tice
(ATCC 35743) is
highly unlikely to be translated because the stop codon for the panC gene
(which overlaps with
the ATG for panD translation initiation in the wild type sequence) is out of
frame. Ribosomal
termination of PanC translation is coupled with ribosomal initiation of PanD
translation in the wild
type pan CD operon. Since there is no ribosomal termination immediately
upstream of the panD
start codon, ribosomal initiation of translation of the panD gene is highly
unlikely to occur.
[0253] The inventors disclose that this natural auxotrophy
enables the more rapid construction
of an antibiotic gene cassette-free recombinant BCG which overexpresses a
STING agonist
biosynthetic gene.
68
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
[0254] The inventors disclose a method for introducing an
antibiotic-cassette-free plasmid
harboring the mycobacterial panCD gene as well as an overexpression construct
for the
biosynthesis of STING agonists (such as the Phsp65::diAA construct) directly
into BCG-Tice
(ATCC 35743).
[0255] pSD5-hsp60-MT3692 is the same as pSD5-hsp65-MT3692. The
inventors had
previously referred to this same plasmid as pSD5-hsp60-MT3692. However, the
actual promoter
in this strain is the promoter for the hsp65 gene of M leprae. Thus, the
inventors may refer to the
plasmid pSD-hsp60-MT3692 as pSD5-hsp65-MT3692.
[0256] In one embodiment, the present invention relates to a
pharmaceutical composition
including an expression vector, expression cassette, or strain of the
invention described herein and
a pharmaceutically acceptable carrier.
[0257] In another embodiment, the present invention relates to
methods and/or compositions
for treating and/or preventing cancer comprising administration of an
expression vector,
expression cassette, strain or pharmaceutical composition described herein to
a subject. In some
aspects, the cancer is bladder cancer (e.g., non-muscle invasive bladder
cancer (NMIBC)), breast
cancer, or a solid tumor. Additional embodiments of the disclosure concern
methods and/or
compositions for treating and/or preventing a bladder cancer in which
modulation of a type 1
interferon (IFN) response is directly or indirectly related. In certain
aspects, individuals with a
bladder cancer such as NMIBC are treated with a modulator of the type 1
interferon response, and
in some aspects an individual with bladder cancer is provided a modulator of
expression type 1
interferon expression, such as an inducer of its expression.
[0258] In certain aspects, the level to which an inducer of type
1 interferon expression
increases type 1 interferon expression may be any level so long as it provides
amelioration of at
least one symptom of bladder cancer, including non-muscle-invasive bladder
cancer (NMIBC).
The level of expression of type 1 interferon may increase by at least 2, 3, 4,
5, 10, 25, 50, 100,
1000, or more fold expression compared to the level of expression in a
standard, in at least some
cases. An individual may monitor expression levels of type 1 interferon using
standard methods
in the art, such as northern assays or quantitative PCR, for example.
[0259] An individual known to have bladder cancer, suspected of
having bladder cancer, or at
risk for having bladder cancer may be provided an effective amount of an
inducer of type 1
69
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
interferon expression, including a BCG strain of the present invention
comprising an expression
vector of the present invention. The expression vector expresses a RV1 354c
protein, or functional
part thereof; a cyclic GMP-AMP synthase (DneV) protein, or functional part
thereof; a cyclic
GMP-AMP synthase (cGAS) protein, or functional part thereof; a DNA integrity
scanning (disA)
protein which functions as a denylate cyclase, or functional part thereof; or
a combination thereof.
It is preferred that a BCG strain of the present invention comprising an
expression vector of the
present invention be administered into the bladder of the subject and that the
expressed protein(s)
enhance type 1 interferon expression in the bladder. Those at risk for bladder
cancer may be those
individuals having one or more genetic factors, may be of advancing age,
and/or may have a family
history, for example.
[0260] In particular aspects of the disclosure, an individual is
given an agent for bladder cancer
therapy in addition to the one or more inducers of type 1 interferon of the
present invention. Such
additional therapy may include intravesi cal chemotherapies such as m i to myc
i n C,
cyclophosphamide, or a combination thereof, for example. When combination
therapy is employed
with one or more inducers of type 1 interferon (such as a BCG strain
expressing one or more of
the following proteins: a RV1354c protein, or functional part thereof; a
cyclic GMP-AMP synthase
(DncV) protein, or functional part thereof; a cyclic GMP-AMP synthase (cGAS)
protein, or
functional part thereof; a DNA integrity scanning (disA) protein which
functions as a denylate
cyclase, or functional part thereof) the additional therapy may be given prior
to, at the same time
as, and/or subsequent to the inducer of type 1 interferon.
[0261] In some aspects, an expression vector, expression
cassette, strain, pharmaceutical
composition, and/or method of the invention described herein has increased
safety, increased
tolerability (e.g., decreased dysuria, urgency, or malaise), and/or decreased
likelihood to cause
infection in the bloodstream or disseminated bloodstream infection compared to
non-recombinant
BCG.
[0262] In one embodiment, the present invention relates to a
method of treating and/or
preventing cancer, including administering to a subject an expression vector,
expression cassette,
strain, and/or pharmaceutical composition of the invention described herein,
wherein the
administration results in an increased safety profile, increased tolerability
(e.g., decreasing dysuria,
urgency, or malaise), and/or decreased likelihood of infection in the
bloodstream or disseminated
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
bloodstream infection compared to non-recombinant BCG. In some aspects, the
cancer is, for
example, bladder cancer (e.g., non-muscle-invasive bladder cancer (NMIBC)),
breast cancer, or a
solid tumor. In other aspects, the solid tumor is, for example, a sarcoma,
carcinoma, or lymphoma.
[0263] In some embodiments, the present invention relates to a
method of increasing the
safety, increasing the tolerability (e.g., decreasing dysuria, urgency, or
malaise), and/or decreasing
the likelihood to cause infection in the bloodstream or disseminated
bloodstream infection
compared to non-recombinant BCG, comprising administering an expression
vector, expression
cassette, strain, and/or pharmaceutical compositions of the invention
described herein to a subject.
[0264] Pharmaceutical Preparations
[0265] Pharmaceutical compositions of the present invention
include an effective amount of
one or more inducers of expression of type 1 interferon such as such as a BCG
strain expressing
one or more of the following proteins: a RV1354c protein, or functional part
thereof; a cyclic
(IMP-AMP synfhase (DncV) protein, or functional part thereof; a cyclic (IMP-
AMP synthase
(cGAS) protein, or functional part thereof; a DNA integrity scanning (disA)
protein which
functions as a denylate cyclase, or functional part thereof, dissolved or
dispersed in a
pharmaceutically acceptable carrier. The phrase "pharmaceutical" or
"pharmacologically
acceptable" refers to molecular entities and compositions that do not produce
an adverse, allergic
or other untoward reaction when administered to an animal, such as, for
example, a human, as
appropriate. The preparation of a pharmaceutical composition that comprises at
least one inducer
of expression of type 1 interferon or additional active ingredient will be
known to those of skill in
the art in light of the present disclosure, as exemplified by Remington: The
Science and Practice
of Pharmacy, 21 Ed. Lippincott Williams and Wilkins, 2005, incorporated herein
by reference.
Moreover, for animal (e.g., human) administration, it will be understood that
preparations should
meet sterility, pyrogenicity, general safety and purity standards as required
by FDA Office of
Biological Standards.
[0266] As used herein, "pharmaceutically acceptable carrier"
includes any and all solvents,
dispersion media, coatings, surfactants, antioxidants, preservatives (e.g.,
antibacterial agents,
antifungal agents), isotonic agents, absorption delaying agents, salts,
preservatives, drugs, drug
stabilizers, gels, binders, excipients, disintegration agents, lubricants,
sweetening agents, flavoring
agents, dyes, such like materials and combinations thereof, as would be known
to one of ordinary
71
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
skill in the art (see, for example, Remington's Pharmaceutical Sciences, 18th
Ed. Mack Printing
Company, 1990, pp. 1289-1329, incorporated herein by reference). Except
insofar as any
conventional carrier is incompatible with the active ingredient, its use in
the pharmaceutical
compositions is contemplated.
[0267] The inducer of expression of type 1 interferon (such as a
BCG strain expressing one or
more of the following proteins: a RV1354c protein, or functional part thereof;
a cyclic GMP-AMP
synthase (DncV) protein, or functional part thereof; a cyclic GMP-AMP synthase
(cGAS) protein,
or functional part thereof; a DNA integrity scanning (disA) protein which
functions as a denylate
cyclase, or functional part thereof) may comprise different types of carriers
depending on whether
it is to be administered in solid, liquid or aerosol form, and whether it need
to be sterile for such
routes of administration as injection. In some aspects, the present invention
(e.g., expression
vectors, strains, or pharmaceutical compositions) can be administered
intravenously,
i ntraderm ally, tran sd erm ally, i ntrath ec ally, Intraarteri ally, i
ntraperi ton eally, intranasally,
intravaginally, intrarectally, intravesically (e.g., administered directly
into the bladder, e.g., by
injection, or by intravesical instillation), intratumorally, topically,
intramuscularly,
subcutaneously, mucosally, orally, topically, locally, inhalation (e.g.,
aerosol inhalation),
injection, infusion, continuous infusion, localized perfusion bathing target
cells directly, via a
catheter, via a lavage, in cremes, in lipid compositions (e.g., liposomes), or
by other method or any
combination of the foregoing as would be known to one of ordinary skill in the
art (see, for
example, Remington's Pharmaceutical Sciences, 18th Ed. Mack Printing Company,
1990, and
Francica et al. TNFa and radio-resistant stromal cells are essential for
therapeutic efficacy of cyclic
dinucleotide STING agonists in non-immunogenic tumors. Cancer Immunol Res.
2018 Feb 22.
PMID: 29472271, incorporated herein by reference).
[0268] Pharmaceutically acceptable salts, include the acid
addition salts, e.g., those formed
with the free amino groups of a proteinaceous composition, or which are formed
with inorganic
acids such as for example, hydrochloric or phosphoric acids, or such organic
acids as acetic, oxalic,
tartaric or mandelic acid. Salts formed with the free carboxyl groups can also
be derived from
inorganic bases such as for example, sodium, potassium, ammonium, calcium or
ferric hydroxides;
or such organic bases as isopropylamine, trimethylamine, histidine or
procaine. Upon formulation,
solutions will be administered in a manner compatible with the dosage
formulation and in such
72
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
amount as is therapeutically effective. The formulations are easily
administered in a variety of
dosage forms such as formulated for parenteral administrations such as
injectable solutions, or
aerosols for delivery to the lungs, or formulated for alimentary
administrations such as drug release
capsules and the like.
[0269] Further in accordance with the present disclosure, the
composition of the present
invention suitable for administration is provided in a pharmaceutically
acceptable carrier with or
without an inert diluent. The carrier should be assimilable and includes
liquid, semi-solid, i.e.,
pastes, or solid carriers. Except insofar as any conventional media, agent,
diluent or carrier is
detrimental to the recipient or to the therapeutic effectiveness of a
composition contained therein,
its use in administrable composition for use in practicing the methods of the
present invention is
appropriate. Examples of carriers or diluents include fats, oils, water,
saline solutions, lipids,
liposomes, resins, binders, fillers and the like, or combinations thereof. The
composition may also
include various antioxidants to retard oxidation of one or more component.
Additionally, the
prevention of the action of microorganisms can be brought about by
preservatives such as various
antibacterial and antifungal agents, including but not limited to parabens
(e.g., methylparabens,
propylparabens), chlorobutanol, phenol, sorbic acid, thimerosal or
combinations thereof.
[0270] In accordance with the present invention, the composition
is combined with the carrier
in any convenient and practical manner, i.e., by solution, suspension,
emulsification, admixture,
encapsulation, absorption and the like. Such procedures are routine for those
skilled in the art.
[0271] In a specific aspect of the present invention, the
composition is combined or mixed
thoroughly with a semi-solid or solid carrier. The mixing can be carried out
in any convenient
manner such as grinding. Stabilizing agents can be also added in the mixing
process in order to
protect the composition from loss of therapeutic activity, i.e., denaturation
in the stomach.
Examples of stabilizers for use in the composition include buffers, amino
acids such as glycine
and lysine, carbohydrates such as dextrose, mannose, galactose, fructose,
lactose, sucrose, maltose,
sorbitol, mannitol, etc.
[0272] In further aspects, the present invention includes the use
of pharmaceutical lipid vehicle
compositions that include inducer of expression of type 1 interferon, one or
more lipids, and an
aqueous solvent. As used herein, the term "lipid" includes any of a broad
range of substances that
is characteristically insoluble in water and extractable with an organic
solvent. This broad class of
73
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
compounds are well known to those of skill in the art, and as the term -lipid"
is used herein, it is
not limited to any particular structure. Examples include compounds which
contain long-chain
aliphatic hydrocarbons and their derivatives. A lipid may be naturally
occurring or synthetic (i.e.,
designed or produced by man). However, a lipid is usually a biological
substance. Biological
lipids are well known in the art, and include for example, neutral fats,
phospholipids,
phosphoglycerides, steroids, terpenes, lysolipids, glycosphingolipids,
glycolipids, sulphatides,
lipids with ether and ester-linked fatty acids and polymerizable lipids, and
combinations thereof.
Of course, compounds other than those specifically described herein that are
understood by one of
skill in the art as lipids are also encompassed by the compositions and
methods of the present
invention.
[0273] One of ordinary skill in the art would be familiar with
the range of techniques that can
be employed for dispersing a composition in a lipid vehicle. For example, the
inducer of inducer
of expression of Type 1 interferon of the present invention may be dispersed
in a solution
containing a lipid, dissolved with a lipid, emulsified with a lipid, mixed
with a lipid, combined
with a lipid, covalently bonded to a lipid, contained as a suspension in a
lipid, contained or
complexed with a micelle or liposome, or otherwise associated with a lipid or
lipid structure by
any means known to those of ordinary skill in the art. The dispersion may or
may not result in the
formation of liposomes.
[0274] The actual dosage amount of a composition of the present
invention administered to an
animal patient can be determined by physical and physiological factors such as
body weight,
severity of condition, the type of disease being treated, previous or
concurrent therapeutic
interventions, idiopathy of the patient and on the route of administration.
Depending upon the
dosage and the route of administration, the number of administrations of a
preferred dosage and/or
an effective amount may vary according to the response of the subject. The
practitioner responsible
for administration will, in any event, determine the concentration of active
ingredient(s) in a
composition and appropriate dose(s) for the individual subject.
[0275] In certain aspects, pharmaceutical compositions may
include, for example, at least
about 0.1% of an active compound. In other aspects, the active compound may
include between
about 2% to about 75% of the weight of the unit, or between about 25% to about
60%, for example,
and any range derivable therein. Naturally, the amount of active compound(s)
in each
74
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
therapeutically useful composition may be prepared is such a way that a
suitable dosage will be
obtained in any given unit dose of the compound. Factors such as solubility,
bioavailability,
biological half-life, route of administration, product shelf life, as well as
other pharmacological
considerations will be contemplated by one skilled in the art of preparing
such pharmaceutical
foimulations, and as such, a variety of dosages and treatment regimens may be
desirable.
[0276] In other non-limiting examples, a dose may also comprise
from about 1
microgram/kg/body weight, about 5 microgram/kg/body weight, about 10
microgram/kg/body
weight, about 50 microgram/kg/body weight, about 100 microgram/kg/body weight,
about 200
microgram/kg/body weight, about 350 microgram/kg/body weight, about 500
microgram/kg/body
weight, about 1 milligram/kg/body weight, about 5 milligram/kg/body weight,
about 10
milligram/kg/body weight, about 50 milligram/kg/body weight, about 100
milligram/kg/body
weight, about 200 milligram/kg/body weight, about 350 milligram/kg/body
weight, about 500
milligram/kg/body weight, to about 1000 mg/kg/body weight or more per
administration, and any
range derivable therein. In non-limiting examples of a derivable range from
the numbers listed
herein, a range of about 5 mg/kg/body weight to about 100 mg/kg/body weight,
about 5
microgram/kg/body weight to about 500 milligram/kg/body weight, etc., can be
administered,
based on the numbers described above.
[0277] A. Alimentary Compositions and Formulations
[0278] In one embodiment of The present disclosure, the inducers
of expression of inducer of
expression of type 1 interferon of the present invention are formulated to be
administered via an
alimentary route. Alimentary routes include all possible routes of
administration in which the
composition is in direct contact with the alimentary tract. Specifically, the
pharmaceutical
compositions disclosed herein may be administered orally, buccally, rectally,
or sublingually. As
such, these compositions may be formulated with an inert diluent or with an
assimilable edible
carrier, or they may be enclosed in hard- or soft- shell gelatin capsule, or
they may be compressed
into tablets, or they may be incorporated directly with the food of the diet.
[0279] In certain aspects, the active compounds may be
incorporated with excipients and used
in the form of ingestible tablets, buccal tables, troches, capsules, elixirs,
suspensions, syrups,
wafers, and the like (Mathiowitz E, Jacob JS, Jong YS, Carino GP, Chickering
DE, Chaturvedi P.
Santos CA, Vijayaraghavan K, Montgomery S, Bassett M, Morrell C. Biologically
erodable
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
microspheres as potential oral drug delivery systems. Nature. 1997;386:410-4.
PMID: 9121559;
Hwang MJ, Ni X, Waldman M, Ewig CS, Hagler AT. Derivation of class TI force
fields. VI.
Carbohydrate compounds and anomeric effects. Biopolymers. 1998;45:435-68.
PMID: 9538697;
Hwang JS, Chac SY, Lee MK, Bac YH. Synthesis of sulfonylurca conjugated
copolymer via PEO
spacer and its in vitro short-tell"' bioactivity in insulin secretion from
islets of Langerhans.
Biomaterials. 1998;19:1189-95. PMID: 9720902; Hwang SJ, Park H, Park K.
Gastric retentive
drug-delivery systems. Crit Rev Ther Drug Carrier Syst. 1998;15:243-84. PMID:
9699081; U.S.
Pat. Nos. 5,641,515; 5,580,579; and 5,792, 451, each specifically incorporated
herein by reference
in its entirety). The tablets, troches, pills, capsules and the like may also
contain the following: a
binder, such as, for example, gum tragacanth, acacia, cornstarch, gelatin or
combinations thereof;
an excipient, such as, for example, dicalcium phosphate, mannitol, lactose,
starch, magnesium
stearate, sodium saccharine, cellulose, magnesium carbonate or combinations
thereof; a
disintegrating agent, such as, for example, corn starch, potato starch,
alginic acid or combinations
thereof; a lubricant, such as, for example, magnesium stearate; a sweetening
agent, such as, for
example, sucrose, lactose, saccharin or combinations thereof; a flavoring
agent, such as, for
example peppermint, oil of wintergreen, cherry flavoring, orange flavoring,
etc. When the dosage
unit form is a capsule, it may contain, in addition to materials of the above
type, a liquid carrier.
Various other materials may be present as coatings or to otherwise modify the
physical form of
the dosage unit. For instance, tablets, pills, or capsules may be coated with
shellac, sugar, or both.
When the dosage form is a capsule, it may contain, in addition to materials of
the above type,
carriers such as a liquid carrier. Gelatin capsules, tablets, or pills may be
enterically coated. Enteric
coatings prevent denaturation of the composition in the stomach or upper bowel
where the pH is
acidic. See, e.g., U.S. Pat. No. 5,629,001. Upon reaching the small
intestines, the basic pH therein
dissolves the coating and permits the composition to be released and absorbed
by specialized cells,
e.g., epithelial enterocytes and Peyer's patch M cells. A syrup of elixir may
contain the active
compound sucrose as a sweetening agent methyl and propylparabens as
preservatives, a dye and
flavoring, such as cherry or orange flavor. Of course, any material used in
preparing any dosage
unit form should be phainfaceutically pure and substantially non-toxic in the
amounts employed.
In addition, the active compounds may be incorporated into sustained-release
preparation and
formulations.
76
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
[0280] For oral administration the compositions of the present
disclosure may alternatively be
incorporated with one or more excipients in the form of a mouthwash,
dentifrice, buccal tablet,
oral spray, or sublingual orally- administered formulation. For example, a
mouthwash may be
prepared incorporating the active ingredient in the required amount in an
appropriate solvent, such
as a sodium borate solution (Dobell's Solution). Alternatively, the active
ingredient may be
incorporated into an oral solution such as one containing sodium borate,
glycerin and potassium
bicarbonate, or dispersed in a dentifrice, or added in a therapeutically-
effective amount to a
composition that may include water, binders, abrasives, flavoring agents,
foaming agents, and
humectants. Alternatively, the compositions may be fashioned into a tablet or
solution form that
may be placed under the tongue or otherwise dissolved in the mouth.
[0281] Additional formulations which are suitable for other modes
of alimentary
administration include suppositories. Suppositories are solid dosage forms of
various weights and
shapes, usually medicated, for insertion into the rectum. After insertion,
suppositories soften, melt
or dissolve in the cavity fluids. In general, for suppositories, traditional
carriers may include, for
example, polyalkylene glycols, triglycerides or combinations thereof. In
certain aspects,
suppositories may be formed from mixtures containing, for example, the active
ingredient in the
range of about 0.5% to about 10%, and preferably about 1% to about 2%.
[0282] B. Parenteral Compositions and Formulations
[0283] In further embodiments, inducer of expression of type 1
interferon of the present
invention may be administered via a parenteral route. As used herein, the term
-parenteral"
includes routes that bypass the alimentary tract. Specifically, the
pharmaceutical compositions
disclosed herein may be administered, for example, intravenously,
intradermally, intramuscularly,
intraarterially, intrathccally, subcutaneous, or intraperitoneally. See, e.g.,
U.S. Pat. Nos.
6,7537,514; 6,613,308; 5,466,468; 5,543,158; 5,641,515; and 5,399,363 (each
specifically
incorporated herein by reference in its entirety).
[0284] Solutions of the active compounds as free base or
pharmacologically acceptable salts
may be prepared in water suitably mixed with a surfactant, such as
hydroxypropylcellulose.
Dispersions may also be prepared in glycerol, liquid polyethylene glycols, and
mixtures thereof
and in oils. Under ordinary conditions of storage and use, these preparations
contain a preservative
to prevent the growth of microorganisms. The pharmaceutical forms suitable for
injectable use
77
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
include sterile aqueous solutions or dispersions and sterile powders for the
extemporaneous
preparation of sterile injectable solutions or dispersions (U.S. Patent
5,466,468, specifically
incorporated herein by reference in its entirety). In all cases, the form must
be sterile and must be
fluid to the extent that easy injectability exists. It must be stable under
the conditions of
manufacture and storage and must be preserved against the contaminating action
of
microorganisms, such as bacteria and fungi. The carrier can be a solvent or
dispersion medium
containing, for example, water, ethanol, polyol (i.e., glycerol, propylene
glycol, and liquid
polyethylene glycol, and the like), suitable mixtures thereof, and/or
vegetable oils. Proper fluidity
may be maintained, for example, by the use of a coating, such as lecithin, by
the maintenance of
the required particle size in the case of dispersion and by the use of
surfactants. The prevention of
the action of microorganisms can be brought about by various antibacterial and
antifungal agents,
for example, parabens, chlorobutanol, phenol, sorbic acid, thimerosal, and the
like. In many cases,
it will be preferable to include isotonic agents, for example, sugars or
sodium chloride. Prolonged
absorption of the injectable compositions can be brought about by the use in
the compositions of
agents delaying absorption, for example, aluminum monostearate and gelatin.
[0285] For parenteral administration in an aqueous solution, for
example, the solution should
be suitably buffered if necessary and the liquid diluent first rendered
isotonic with sufficient saline
or glucose. These particular aqueous solutions are especially suitable for
intravenous,
intramuscular, subcutaneous, and i ntraperi ton e al administration. In this
conn ecti on, sterile
aqueous media that can be employed will be known to those of skill in the art
in light of the present
disclosure. For example, one dosage may be dissolved in isotonic NaCl solution
and either added
hypodermoclysis fluid or injected at the proposed site of infusion, (see, for
example, "Remington's
Pharmaceutical Sciences" 15th Edition, pages 1035-1038 and 1570-1580). Some
variation in
dosage will necessarily occur depending on the condition of the subject being
treated. The person
responsible for administration will, in any event, determine the appropriate
dose for the individual
subject. Moreover, for human administration, preparations should meet
sterility, pyrogenicity, and
general safety and purity standards as required by FDA Office of Biologics
standards.
[0286] Sterile injectable solutions are prepared by incorporating
the active compounds in the
required amount in the appropriate solvent with several of the other
ingredients enumerated above,
as required, followed by filtered sterilization. Generally, dispersions are
prepared by incorporating
78
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
the various sterilized active ingredients into a sterile vehicle which
contains the basic dispersion
medium and the required other ingredients from those enumerated above. In the
case of sterile
powders for the preparation of sterile injectable solutions, the preferred
methods of preparation are
vacuum-drying and freeze-drying techniques which yield a powder of the active
ingredient plus
any additional desired ingredient from a previously sterile-filtered solution
thereof. A powdered
composition is combined with a liquid carrier such as, e.g., water or a saline
solution, with or
without a stabilizing agent.
[0287] C. Miscellaneous Pharmaceutical Compositions and
Formulations
[0288] In some aspects of the invention, the active compound
inducer of expression of type 1
interferon of the present invention may be formulated for administration via
various miscellaneous
routes, for example, topical (i.e., transdermal) administration, mucosal
administration (intranasal,
vaginal, etc.) and/or inhalation. Phatmaceutical compositions for topical
administration may
include the active compound formulated for a medicated application such as an
ointment, paste,
cream or powder. Ointments include all oleaginous, adsorption, emulsion and
water-soluble based
compositions for topical application, while creams and lotions are those
compositions that include
an emulsion base only. Topically administered medications may contain a
penetration enhancer to
facilitate adsorption of the active ingredients through the skin. Suitable
penetration enhancers
include glycerin, alcohols, alkyl methyl sulfoxides, pyrrolidones and
luarocapram. Possible bases
for compositions for topical application include polyethylene glycol, lanolin,
cold cream and
petrolatum as well as any other suitable absorption, emulsion or water-soluble
ointment base.
Topical preparations may also include emulsifiers, gelling agents, and
antimicrobial preservatives
as necessary to preserve the active ingredient and provide for a homogenous
mixture. Transdermal
administration of the present invention may also include the use of a "patch".
For example, the
patch may supply one or more active substances at a predetermined rate and in
a continuous
manner over a fixed period of time.
[0289] In certain embodiments, the pharmaceutical compositions
may be delivered by eye
drops, intranasal sprays, inhalation, and/or other aerosol delivery vehicles.
Methods for delivering
compositions directly to the lungs via nasal aerosol sprays has been described
e.g., in U.S. Pat.
Nos. 5,756,353 and 5,804,212 (each specifically incorporated herein by
reference in its entirety).
Likewise, the delivery of drugs using intranasal microparticle resins
(Takenaga M, Serizawa Y,
79
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
Azechi Y, Ochiai A, Kosaka Y, Igarashi R, Mizushima Y. Microparticle resins as
a potential nasal
drug delivery system for insulin. J
[0290] Control Release. 1998;52:81-7. PMID: 9685938) and
lysophosphatidyl-glycerol
compounds (U.S. Pat. No. 5,725, 871, specifically incorporated herein by
reference in its entirety)
are also well-known in the phaimaceutical arts. Likewise, transmucosal drug
delivery in the form
of a polytetrafluoroetheylene support matrix is described in U.S. Pat. No.
5,780,045 (specifically
incorporated herein by reference in its entirety). The term aerosol refers to
a colloidal system of
finely divided solid of liquid particles dispersed in a liquefied or
pressurized gas propellant. The
typical aerosol of the present invention for inhalation will consist of a
suspension of active
ingredients in liquid propellant or a mixture of liquid propellant and a
suitable solvent. Suitable
propellants include hydrocarbons and hydrocarbon ethers. Suitable containers
will vary according
to the pressure requirements of the propellant. Administration of the aerosol
will vary according
to subject's age, weight and the severity and response of the symptoms.
[0291] Kits of the Disclosure
[0292] Any of the compositions described herein may be comprised
in a kit. In a non-limiting
example, an inducer of expression of type 1 interferon of the present
invention (such as a BCG
strain expressing one or more of the following proteins: a RV1354c protein, or
functional part
thereof; a cyclic GMP-AMP synthase (DncV) protein, or functional part thereof;
a cyclic GMP-
AMP synthase (cGAS) protein, or functional part thereof; a DNA integrity
scanning (disA) protein
which functions as a denylate cyclase, or functional part thereof) may be
comprised in a kit.
[0293] The kits may comprise a suitably aliquoted inducer of
expression of type 1 interferon
of the present invention and, in some cases, one or more additional agents.
The component(s) of
the kits may be packaged either in aqueous media or in lyophilized form. The
container means of
the kits will generally include at least one vial, test tube, flask, bottle,
syringe or other container
means, into which a component may be placed, and preferably, suitably
aliquoted. Where there is
more than one components in the kit, the kit also will generally contain a
second, third or other
additional container into which the additional components may be separately
placed. However,
various combinations of components may be included in a vial. The kits of the
present invention
also will typically include a means for containing the inducer of expression
of type 1 interferon of
the present invention and any other reagent containers in close confinement
for commercial sale.
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
Such containers may include injection or blow-molded plastic containers into
which the desired
vials are retained.
[0294] When the components of the kit are provided in one and/or
more liquid solutions, the
liquid solution is an aqueous solution, with a sterile aqueous solution being
particularly preferred.
The inducer of expression of type 1 interferon of the present invention
composition(s) may be
formulated into a syringeable composition. In which case, the container means
may itself be a
syringe, pipette, and/or other such like apparatus, from which the formulation
may be applied to
an infected area of the body, injected into an animal, and/or even applied to
and/or mixed with the
other components of the kit.
[0295] However, the components of the kit may be provided as
dried powder(s). When
reagents and/or components are provided as a dry powder, the powder can be
reconstituted by the
addition of a suitable solvent. It is envisioned that the solvent may also be
provided in another
container means.
[0296] The Examples above have been included to provide guidance
to one of ordinary skill
in the art for practicing representative embodiments of the presently
disclosed subject matter. In
light of the present disclosure and the general level of skill in the art,
those of skill can appreciate
that the Examples above are intended to be exemplary only and that numerous
changes,
modifications, and alterations can be employed without departing from the
scope of the presently
disclosed subject matter. The Examples above are offered by way of
illustration and not by way
of limitation.
[0297] All references, including publications, patent
applications, and patents, cited herein are
hereby incorporated by reference to the same extent as if each reference were
individually and
specifically indicated to be incorporated by reference and were set forth in
its entirety herein.
[0298] Recitation of ranges of values herein are merely intended
to serve as a shorthand
method of referring individually to each separate value falling within the
range, unless otherwise
indicated herein, and each separate value is incorporated into the
specification as if it were
individually recited herein. All methods described herein can be performed in
any suitable order
unless otherwise indicated herein or otherwise clearly contradicted by
context. The use of any and
all examples, or exemplary language (e.g., such as") provided herein, is
intended merely to better
illuminate the invention and does not pose a limitation on the scope of the
invention unless
81
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
otherwise claimed. No language in the specification should be construed as
indicating any non-
claimed element as essential to the practice of the invention.
[0299] Preferred embodiments of this invention are described
herein, including the best mode
known to the inventors for carrying out the invention. Variations of those
preferred embodiments
may become apparent to those of ordinary skill in the art upon reading the
foregoing description.
The inventors expect skilled artisans to employ such variations as
appropriate, and the inventors
intend for the invention to be practiced otherwise than as specifically
described herein.
Accordingly, this invention includes all modifications and equivalents of the
subject matter recited
in the claims appended hereto as permitted by applicable law. Moreover, any
combination of the
above-described elements in all possible variations thereof is encompassed by
the invention unless
otherwise indicated herein or otherwise clearly contradicted by context.
[0300] Presented below are examples discussing enhancement of
trained immunity by re-
engineered BCG overexpressing the PAMP molecule cyclic di-AMP, contemplated
for the
discussed applications. The following examples are provided to further
illustrate the embodiments
of the present invention but are not intended to limit the scope of the
invention. While they are
typical of those that might be used, other procedures, methodologies, or
techniques known to those
skilled in the art may alternatively be used.
EXAMPLES
EXAMPLE 1
MATERIAL AND METHODS
[0301] Referring specifically to Figs 39-62, the following
material and methods are provided
to details the methods used to obtain the presented results.
103021 Bacterial strains and culture conditions:
[0303] Mycobacterium bovis (M. bovis) Bacillus Calmette- Guerin
(BCG) Pasteur (BCG-WT
Pasteur) (a generous gift from Dr. Frank Collins [FDA] and identical to BCG-
Pasteur provided by
the Pasteur Institute to the Trudeau Institute in 1967 as TMC No. 1011) and
commercially available
BCG-Tice (Onco-Tice , Merck) were used for the generation of c-di-AMP
overexpressing
recombinant BCG strains. Genomic DNA from Mycobacterium tuberculosis (M. tb)
strain
CDC1551 was used for PCR amplification of disA (MT3692/Rv3586). Single
isolated bacterial
colonies growing on 7H11 plates supplemented with oleic-albumin-dextrose-
catalase (OADC)
82
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
(Cat. B11886, Fisher Scientific) were picked and propagated in 7H9 Middlebrook
liquid medium
(Cat. B271310, Fisher Scientific) supplemented with (OADC) (Cat. B11886,
Fisher Scientific),
0.5% glycerol (Cat. G55116, Sigma) and 0.05% Tween-80 (Cat. BP338, Fisher
Scientific).
Cloning experiments were performed using E. coli strain DH5-a (Cat. 18258012,
Fisher Scientific)
and was routinely maintained in LB broth. For generation of disA
overexpressing BCG, an E. coil-
mycobacterial shuttle vector (pSD5.hsp60) was used to clone M.tb gene MT3692
or Rv3586 under
the strong mycobacterial promoter hsp60. Clones were confirmed by gene
sequencing and were
used for bacterial transformation by electroporation method. Recombinant
strains were confirmed
using colony PCR against kanamycin cassette, subjected to whole genome
sequencing and qPCR
analyses. Details of all bacterial strains, plasmids and constructs are listed
in Table 3.
[0304] Table 3:
Name Description/Source
Bacterial strains
M. tuberculosis strain
Mtb-CDC1551 Wild-type M tuberculosis
M. bovis BCG strains
BCG Pasteur M. hovis BCG Pasteur
BCG-disA-OE (Pasteur) BCG Pasteur strain overexpressing disA
(MT3692) of Mtb
BCG Tice M. hovis BCG Tice
BCG-disA-OE (Tice) BCG Tice strain overexpressing disA
(MT3692) of M.tb
E. coil strain
DH5-a Competent E. coli (High Efficiency)
Cell lines
Urinary bladder carcinoma cells
RT4 (ATCC HTB-2Tm) Human low grade urothelial cancer
5637 (ATCC HTB-9Tm) Human high-grade urothelial cancer
NBT-II (ATCC CRL-1655TM) N-butyl-N-(4-hydroxybutyl) nitrosamine
induced tumor cell line
in Rattus norvegicus Nara Bladder Tumor No. 2
MB49 (Cat. SSC148, EMD DMBA [7,12-dimethylbenz[a]anthracene]
induced murine
Millipore) urothelial carcinoma cells,
UPPL-1595 Luminal cell line established from a
spontaneous primary bladder
tumor in an Uroplakin-Cre driven PTEN/P53 knockout genetically
engineered mouse model
BBN 975 Basal- cell line established from, (,0
N-B tyl-N -(4-
hydroxybu (813N) induced
murine urothelial
cancer model
J28 (ATCC HTB-1Tm) high grade urothelial cancer
83
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
Reporter cells
RAW-Lucia TSG (invivoGen) TFN Reporter Raw 264.7 mu-rine
macrophages
Macrophage cell lines
J774A.1 (ATCC TIB67Tm) Murine macrophage cell line
Plasmids
pSD5.hsp60 Mycobacterial expression plasmid with
hsp60 promoter
pSD5hsp60.MT3692 dis,4 over-expression plasmid
Confocal Microscopy Reagents
Primary Antibodies
LC3B NB100-2220, Novus Biologicals
P62/SQ SIMI P0067, Sigma
Secondary Antibodies
Goat anti-Rabbit IgG Alexa Fluor A32733, Thermo Fisher Scientific
Plus
647
Chemicals/Probes
Fluorescein 5(6)-isothiocyanate 46950, Sigma
(FITC)
Hoechst 33342 62249, Thermo Fisher Scientific
Flow Cytometry Reagents
Antibodies (mouse BMDM study)
anti-CD45 (clone 30-F11) Biolegend
anti-CD124 (I clone 015F8) Biolegend
anti-LA/1-E (clone 107630) Biolegend
anti-Ly6C (clone HK1.4) Biolegend
anti-CD1lb (clone M1/70) Biolegend
anti-F4/80 (clone BM8) Biolegend
anti-Ly6G (clone 1A8) Biolegend
anti CD206 (clone C068C2) Biolegend
anti-TNF (clone MP6-XT22) B iolegend
anti- IL-10 (clone JES5-16E3) eBioscience
Antibodies (HMDM study)
anti-CD16 (clone 3G8) Biolegend
anti-CD14 (clone 63D3) Biolegend
an ti-HLA -DR (clone L243) Biolegend
anti-CD! lb (clone ICRF44) Biolegend
anti-TLR4 (clone HTA125) Biolegend
anti-CD206 (clone 15-2) Biolegend
anti-CD163 (clone (iHI/61) Biolegend
anti-TNF (clone MAbl 1) Biolegend
anti-IL-6 (clone MQ2-13A5) Biolegend
84
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
Antibodies (myeloid cell panel, Syngeneic MB49 urothelial cancer model)
CD45 (clone 30-F11) Biolegend
CD124 (IL-4Ra) (clone I015F8) Biolegend
I-a/I-e (clone M5/114.15.2) Biolegend
F4/80 (clone BM8) Biolegend
CD206 (clone C068C2) Biolegend)
TNF (clone MP6-XT22) Thermo Fisher
IL-10 (clone JES5-16E3) Thermo Fisher
Antibodies (lymphoid cell panel, Syngeneic MB49 urothelial cancer model)
CD45 (clone PerCP) Biolegend
CD25 (clone PC61) Biolegend
CD3 (clone 17A2) Biolegend
CD4 (clone GK1.5) Biolegend
CD8a (clone 53-6.7) Biolegend
FOXP3 (clone MF-14) Biolegend
Mouse IFN-y (clone XMG1.2) Biolegend
FOXP3 (clone MF-14) Biolegend
Reagents/Kits
Protein transport inhibitor eBioscience, 00-4980-03
cocktail
Zombie AquaTM Fixable Viability Biolegend, 423101
Kit
TruStain FcXTM Biolegend, 101320
Fixation and Permeabilization Biolegend, 421403
Buffer Set
Human TruStain FcXTM Biolegend, 422302
True-Stain Monocyte BlockerTm Biolegend, 426102
ELISA
Mouse ELISA Kits
TNF- DuoSet DY410, R6000B, R and D Systems
1L-6 DuoSet DY406, R6000B, R and D Systems
IFN- DuoSet DY485, R6000B, R and D Systems
CCL2/JE/MCP-1 DuoSet DY479, R6000B, R and D Systems
LEGEND MAXTM Mouse IFN-13 439407, Biolegend
Human ELISA Kits
TNF- DuoSet DY210, R6000B, R and D Systems
1L-6 DuoSet DY206, R6000B, R and D Systems
IFN- ELISA Kit 41410-2, PBL Assay Science
Rat ELISA Kits
IFN- Quantikine RIFOO, R and D Systems
TNF- Quantikine RTA00, R and D Systems
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
1L-2 Quantikine R2000, R and D Systems
Chromatin Immunoprecipitation
ChIP Antibodies
Histone H3K9me3 (H3K9 (cat. A-4036-100, epigentek)
Trimethyl) Polyclonal Antibody
Anti-Histone H3 (tri methyl K4) (cat. ab8580, abeam)
antibody - ChIP Grade
ChIP Reagents
BSA (Cat. A3294, Sigma-Aldrich)
Salmon Sperm DNA (Cat. 15632011, ThermoFisher Scientific)
IIEPES (Cat. 113375, Sigma-Aldrich)
Formaldehyde (Cat. 252549, Sigma-Aldrich)
EGTA (Cat. 03777, Sigma-Aldrich)
EDTA (Cat. E6758, Sigma-Aldrich)
TritonX-100 (Cat. T8787, Sigma-Aldrich)
SDS (Cat. 71736, Sigma-Aldrich)
NaHCO3 (Cat. 5761, Sigma-Aldrich)
Nuclease free water (Cat. AM9930, ThermoFisher Scientific)
SYBR green dye (Cat. 4385614, Applied Biosystems)
[0305] Mammalian cell culture:
[0306] Cell lines: For cell-based in vitro infection assays
J774.1 (American Type Culture
Col I ection-ATCC T I B67 ' m, Manassas, VA, USA) murine macrophage cell
lines were cultivated
in RPMI-Glutamax (Cat. 61870-036, Fischer Scientific), supplemented with 10%
heat inactivated
fetal bovine serum (FBS) (Cat. 10082147, Fischer Scientific) with 1%
streptomycin/penicillin at
37 C with 5% CO2. Urothelial carcinoma cell lines 5637 (ATCC HTB-9 rm), a
human high grade
urothelial cancer; RT4 (ATCC HTB-2Tm), a human transitional cell low grade
urothelial cancer;
J82 (ATCC HTB-1Tm), a human high grade urothelial cancer; and NBT II (ATCC
CRL-
1655Tm), N-butyl-N-(4-hydroxybutyl) nitrosamine induced tumor cell line in
Rattus norvegicus
Nara Bladder Tumor No. 2, UPPL1595 (luminal cell line established from a
spontaneous primary
bladder tumor in an Uroplakin-Cre driven PTEN/P53 knockout genetically
engineered mouse
model and were generously provided by Dr. William Kim (UNC Chapel Hill).,
BBN975 (basal-
cell line established from , 0.05% N-Butyl-N-(4-hydroxybutyl) nitrosamine
(BBN) induced
murine urothelial cancer model and was generously provided by Dr. William Kim
(UNC Chapel
86
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
Hill), and MB49 (murine urothelial carcinoma cells, 7,12-
dimethylbenz[a]anthracene (DMBA,
EMD Millipore, Cat. SSC148) were maintained as monolayer in RPMTI 640 medium
supplemented with 10% heat inactivated fetal bovine serum (FBS) with 1%
streptomycin/penicillin at 37 C with 5% CO2. Mouse fibroblast cell line NCTC
clone 929 [L cell,
L-929, derivative of Strain L] (ATCCCD CCL-1TM) were routinely maintained as
monolayer in
DMEM media supplemented with 10% heat inactivated fetal bovine serum (FBS)
with 1%
streptomycin/penicillin at 37 C with 5% CO2. All cell lines were not
maintained more than 10
passage cycle and Mycoplasma testing was performed periodically while cells
were in culture.
Reporter mouse cell line, RAW-Lucia ISG (InvivoGen, CA, USA) was cultivated in
custom
prepared media as per manufacturer's instructions.
[0307] Primary cells (Macrophages and Dendritic Cells):
[0308] For generation of murine bone-marrow-derived macrophages
(BMDMs) and dendritic
cells (BMDCs), bone marrow (BM) cells were isolated from 4-week old wild-type
(WT)
C57BL/6J (Charles River laboratories, North Wilmington, Mass) and STING-KO
mice
(C57BL/6J-Tmem173gt/J, Jackson laboratories). Multiple vials of bone-marrow
cells were
preserved in cryopreservation media containing 10% DMS0 (Cat. D2650; Sigma)
and 90% heat
inactivated FBS (Cat. 10082147, Fischer Scientific) in liquid nitrogen. For
differentiation of BM
cells into macrophages or DCs, random cryopreserved vials were chosen and
differentiated for 6
days in BMDM-differentiation media made from DMEM containing 10% FBS, 1% MEM
amino
acids (Cat. 11130051, Thermo Fisher Scientific), 1% MEM non-essential amino
acids (Cat.
11140050, Thermo Fisher Scientific), 1% sodium pyruvate (Cat. 11360070, Thermo
Fisher
Scientific), 1% MEM vitamin (Cat. 11120052, Thermo Fisher Scientific) and
antibiotics
(Penicillin-Streptomycin solution) supplemented with 30% sterile mouse
fibroblast L929
(ATCCO CCL-1TM) conditioned media. Differentiation of BM cells into DCs was
carried out in
low attachment 10 mm cell culture dish in presence of bone marrow-
differentiation media in
presence of recombinant murine Granulocyte-Macrophage Colony-Stimulating
Factor (GM-CSF)
(Cat. 315-03, Peprotech) for 48 h. Non-adherent cells were washed and loosely
attached cells were
allowed to differentiate into BMDCs for next 6 days. Cells were characterized
for macrophage and
DC markers using cell-surface staining and flow cytometry analyses. Human
primary monocytes
and human monocyte-derived macrophages (HMDMs) were used for cell-based in
vitro infection
87
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
assays. Peripheral blood-derived mononuclear cells (PBMCs) isolated from
healthy male donors
(leukopacks) aged between 18-30 were used for isolation of human monocytes
(HM) or human
monocyte-derived macrophages (HMDM). To separate blood constituents and
isolation of buffy
coat density gradient centrifugation (400 x g at 18 C for 30 min) of RPM1-1640
diluted blood over
a Ficoll-PaqUeTM Plus reagent (Cat. 17-1440-02, GE Healthcare, Piscataway, NJ)
was performed.
Cells were washed several times using 1 x PBS and were counted using
hemocytometer. Once
counted CD14+ human monocytes were isolated from PBMCs using magnetic labeling
(Monocyte
Isolation Kit II, Cat. 130-091-153, Miltenyi Biotec, San Diego, CA) and
magnetic columns as per
manufacturer's instructions. The purity of isolated CD14+ cells was confirmed
using a fraction of
cells stained with a fluorochrome-conjugated antibody against a monocyte
marker as
recommended by manufacturer and cells were analyzed using BD-LSR2 flow
cytometer. Human
monocytes were seeded (2.0 - 3.0 X 105 cells / ml in RPMI 1640 medium
supplemented with 10%
FBS and 1% streptomycin/penicillin at 37 C with 5% CO2. Monolayers of CD14+
monocytes
were differentiated into M1 [GM-CSF (20 ng/ml, PeproTech, Rocky Hill, NJ) and
IFN-y(20 ng/ml,
PeproTech, Rocky Hill, NJ PeproTech)] or M2 [M-CSF (20 ng/ml, PeproTech, Rocky
Hill, NJ)
and IL-4 (20 ng/ml, PeproTech, Rocky Hill, NJ PeproTech)] for next 7 days.
[0309] Animals:
[0310] Experimental procedures involving live animals were
carried out in agreement with the
protocols approved by the Institutional Animal Care and Use Committee (TACUC)
at The Johns
Hopkins University School of Medicine. For animal infection protocols,
pathogen-free age 4-6
weeks female C57BL/6J (Charles River Laboratories, North Wilmington, Mass) and
Fox Chase
SCID mice (Charles River Laboratories North Wilmington, Mass.) were purchased
and housed
under pathogen-free conditions at an Animal Biosafety Level-3 animal facility
without cross-
ventilation. Fischer 344 female rats age 8 weeks (Harlan, avg. weight 160g)
were housed at an
BSL2 animal facility. Animals were given free access to water and standard
chow and were
monitored daily for general behavior and appearance by veterinary specialists.
[0311] In vitro infection assays:
[0312] For in vitro infection assays, cell lines or primary cells
were seeded at required cell
density in 6-well tissue culture plates or 10 mm petri dishes. For infection,
log-phase wild-type
and BCG-disA-OE strains were harvested by centrifugation and washed twice
using DPBS to
88
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
remove residual detergent and BSA then suspended in antibiotic-free RPMI 1640
media
supplemented with 10% FBS. For infection assays, the bacteria were deposited
at pre-calibrated
multiplicity of infection (MOI). Infection was allowed for next 4 hours,
followed by repeated
washing of infected cells using warm DPBS to remove non-internalized bacteria.
Infected cells
were incubated until endpoints in presence of RPMI-1640 medium supplemented
with 10% FBS
and antibiotics.
[0313] Toxicity assays:
[0314] Human urothelial cancer cell lines, RT4, 5637, and J82,
were cultured at 37 C under
5% CO2 in RPMI 1640 containing 10% FBS without antibiotics. For cell toxicity
assay, 3000 cells
for RT4 and 1500 cells for 5637 and J82 were seeded in a 96-well tissue-
treated plate in triplicate,
respectively. Twenty-four hours after seeding, cells were treated with the
indicated ratio of BCG
to cells for 72 hours. To measure cell viability, CellTiter-Glo Luminescent
Cell Viability Assay
(Promega, Madison, WI, USA) and FLUOstar OPTIMA (BMG Labtech, Ortenberg,
Germany)
were used according to manufacturer's protocols. Relative cell viability was
calculated by dividing
the viability of the indicated ratio by that of a control.
[0315] For Annexin-PI staining, 0.5 million J774.1 cell and BMDMs
were plated per well in
6-well plates for physical attachment. Cells were exposed at 1:10 MOIs for 24
hours using wild-
type and BCG-disA-OE strains of Tice and Pasteur to determine the BCG
cytotoxicity following
exposure. At the endpoint of infection or treatment cells were non-
enzymatically removed using
0.02% EDTA-PBS solution. Cells were washed twice with ice-cold PBS and FITC-
annexin-PI
was done as per manufacturer's instruction using FITC Annexin V Apoptosis
Detection Kit I (Cat.
556547, BD Biosciences). Flow cytometry was performed using a BD LSR II flow
cytometer of
the Flow Cytometry Core Facility at The Bloomberg School of Public Health,
Johns Hopkins
University). Data was processed using FlowJo software (Tree Star v10).
[0316] Quantitative real-time ca'CR:
[0317] Gene expression profiling was carried out using total RNA
isolated from cell lines or
primary cells. For RNA isolation from rat bladders, pieces of whole bladder
samples were excised,
snap frozen in liquid nitrogen immediately after harvesting and stored in
RNAlater (Cat. AM7021,
Ambion) at -80 C. Total RNA isolation was carried out using RNeasy system
(Cat. 74106,
Qiagen). Real-time qPCR was performed using the StepOnePlus system (Applied
Biosystems).
89
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
For gene expression analyses in cell lines and primary cells, SYBR Fast green
double stranded
DNA binding dye (Cat. 40g561 2, Applied Biosystems) was used. Gene expression
analyses in rat
bladder tissues were performed using TaqMan gene expression assays. Gene-
specific qPCR
primers were purchased from Integrated DNA Technologies and all TaqMan gene
expression
assays were purchased from Thermo Fischer Scientific. Amplification of RNU6,
13-actin, GAPDH
were used as endogenous control for RNA samples derived from human, mouse and
rat
cells/tissues respectively. All experiments were performed at least in
triplicate and data analyses
was done using 2-AACT method. Details of NCBI gene identifiers and primer
sequences are given
in the Table 4.
[0318] Table 4:
Cloning primers used in the study
Accession Gene Sequence (5'-3') HQ ID
NO:
Number
pSD5hsp60.MT3692 GCiGCATCATATGCACGCTGTGACTCGTC SEQ
ID
(F)
NO:107
pSD5hsp60.MT3692 GGGACGCGTTATTGATCGCTGATGGTCGATT SEQ ID
NO:42
(R)
Kanamyein cassette (F) GAGAAAACTCACCGAGGCAG SEQ
ID NO:43
Kanamycin cassette (R) GTATTTCGTCTCGCTCAGGC SEQ
ID NO:44
32287254 M. tb sigH (F) GCGATGGTGGCTTCTCCCTCG SEQ
ID NO:45
M.tb sigH (R) CCATCTTGCACAGCTCGCGTAG SEQ
ID NO:46
qPCR primers used in the study
Mouse Primers
11461 Mousc43 actin (F) TAAGGCCAACCGTGAAAAGATG SEQ
ID NO:47
Mouse.13 actin (R) CTGGATGGCTACGTACATGGCT SEQ
ID NO:48
21926 Mouse.TNF-a (F) GACCCTCACACTCAGATCATC SEQ
ID NO:49
Mouse.TNF-a (R) GCTGCTCCTCCACTTGGT SEQ
ID NO:50
15977 Mouse IFN-13 (F) CCACAGCCCTCTCCATCAAC SEQ
ID NO:51
Mouse.IFN-13 (R) CTCCGTCATCTCCATAGGGA SEQ
ID NO:52
16193 Mouse.IL6 (F) CTGCAAGAGACTTCCATCCAG SEQ
ID NO:53
Mouse.IL6 (R) CAGGICTGTIGGGAGTGG SEQ
ID NO:54
15978 Mouse.IFN (F) AGCGGCTGACTGAACTCAGATTGT SEQ
ID NO:55
Mousc.IFN (R) GTCACAGTTTTCAGCTGTATAGGG SEQ
ID NO:56
16176 Mouse.IL1 (F) GGAGAGTGTGGATCCCAA SEQ
ID NO:57
Mouse.IL1 (R) GTGGAGTTTGAGTCTGCAG SEQ
ID NO:58
20296 Mouse.MCP1 (F) GGCTCAGCCAGATGCAGTTAAC SEQ
ID NO:59
Mouse.MCP1 (R) GATCCTCTTGTAGCTCTCCAGC SEQ
ID NO:60
16160 Mouse.IL12b (F) GAAAGACGTTTATGTTGTAGAGG SEQ
ID NO:61
Mouse.IL12b (R) GACTCCATGTCTCTGGTCTG SEQ
ID NO:62
17329 Mouse.CXCL9 (F) GGAGTTCGAGGAACCCTAGTG SEQ
ID NO:63
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
Mouse.CXCL9 (R) GGGATTTGTAGTGGATCGTGC SEQ
ID NO:64
15945 Mouse.CXCL10 (F) GTGGGACTCAAGGGATCCCTCTC SEQ
ID NO:65
Mousc.CXCL10 (R) GCTTCCCTATGGCCCTCATTC SEQ
ID NO:66
18126 Mouse.NOS2 (F) GTTCTCAGCCCAACAATACAAG SEQ
ID NO:67
Mouse.NOS2 (R) GGAACATTCTGTGCTGTCCC SEQ
ID NO:68
20299 Mouse.CCL22 (F) CTCTGATGCAGGTCCCTATGGTG SEQ
ID NO:69
Mouse.CCL22 (R) GGCAGAGGGTGACGGATGTAG SEQ
ID NO:70
Human Primers
26827 Human. RNU6A (F) CTCGCTTCGGCAGCACATATAC SEQ
ID NO:71
Human. RNU6A (R) AATATGGAACGCTTCACGAATTTG SEQ
ID NO:72
3456 Human.IFN13 (F) CAACTTGCTTGGATTCCTACAAAG SEQ
ID NO:73
Human.IFN13 (R) TATTCAAGCCTCCCATTCAATTG SEQ
ID NO:74
3569 Human.IL6 (F) GGTACATCCTCGACGGCATCT SEQ
ID NO:75
Human.IL6 (R) GTGCCTCTTTGCTGCTTTCAC SEQ
ID NO:76
Rat Primers
64367 Rat.PPIB (F) CAGGATTCATGTGCCAGGGT SEQ
ID NO:77
Rat.PPIB (R) CCAAAGACCACATGCTTGCC SEQ
ID NO:78
24481 Rat.IFN-(3 (F) GAGTCTTCACACTCCTGGC SEQ
ID NO:79
Rat.IFN-(3 (R) GTCCTTCAGGCATGAGACAG SEQ
ID NO:80
298210 Rat.IFNLJ a (F) GCGTTCCTGCTGTGCTTCTC SEQ
ID NO:81
Rat.IFN-a (R) CCATTCAGCTGCCTCAGGAGC SEQ
ID NO:82
25712 Rat.IFNLJ y (F) CGTCTTGGTTTTGCAGCTCT SEQ
ID NO:83
Rat.IFN-y (R) CGTCCTTTTGCCAGTTCCTC SEQ
ID NO:84
24599 Rat. iNOS (F) 6 GTCiA GGGGA CTG(3 A CTTTTA G SEQ
ID NO:85
Rat. iNOS (R) TTGTTGGGCTGGGAATAGCA SEQ
ID NO:86
245920 Rat.IP10 (F) TCCACCTCCCTTTACCCAGT SEQ
ID NO:87
Rat.IP10 (R) AGAGCTAGGAGAGCCGTCAT SEQ
ID NO:88
24770 Rat.MCP-1 (F) CAGGICTCTGICACGCTTCTG SEQ
ID NO:89
Rat.MCP-1 (R) GCCAGTGAATGAGTACiCAGCAG SEQ
ID NO:90
25542 Rat.MIP-1 a (F) ACAAGCGCACCCTCTGTTAC SEQ
ID NO:91
Rat.MIP-1 a (R) CiCiTCAGGAAAATCiACACCCG SEQ
ID NO:92
24494 Rat.IL-1f3 (F) GACTTCACCATGGAACCCGT SEQ
ID NO:93
Rat.IL-1f3 (R) GGAGACTGCCCATTCTCGAC SEQ
ID NO:94
24835 Rat.TNF-a (F) CGTCCCTCTCATACACTGG SEQ
ID NO:95
Rat.TNF-a (R) CATGCTTTCCGTGCTCATG SEQ
ID NO:96
59086 Rat.TGF-fl (F) TGACGTCACTGGAGTTGTCC SEQ
ID NO:97
Rat.TGF-(3 (R) CCTCGACGTTTGGGACTGAT SEQ
ID NO:98
25325 Rat.IL-10 (F) CCTCTGGATACAGCTGCGAC SEQ
ID NO:99
Rat.IL-10 (R) TGCCGGGTGGTTCAATTTTTC SEQ
ID
NO:100
ChIP-PCR Primers
Human.GAPDH (F) TACTAG CCi GITTT AC Ci G Ci CG SEQ
ID
NO:101
91
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
Human.GAPDH (R) TCCiAACAGGAGGA GCAGAGAGC GA SEQ
ID
NO:102
Human.IL-6 (F) CGGTGAA G AATGGA TGACCT SEQ
ID
NO:103
Human.IL-6 (R) A_AACC1AGACCCTTGCACAAC SEQ
ID
NO:104
Human.TNF-cx (F) ATC A GICAGTGGCCCAG,A,A G A CC C SEQ
ID
NO:105
Human.TNF-ct (R) CCAC7GICC7CGGATCATGCTTC_AG SEQ
ID
NO:106
[0319] ELISA:
[0320] Sandwiched ELISA was performed for cytokine (TEN-y, TNF-a,
IL-6, IFN-13, IL-1[3
and MCP-1/CCL2) measurement in culture supernatants and animal tissues from
lung, spleen or
urinary bladder. Tissues and culture supernatants were flash frozen in liquid
nitrogen immediately
after harvest and stored at -80 C. Animal tissues were homogenized using
micro tissue
homogenizers (Cat. 1215D61, Kimble) and filter sterilized for measurement of
various cytokine
protein expression levels using sandwiched ELISA as per manufacturer's
recommendations.
Details of all ELISA kits and accessory reagents are given in Table 4.
[0321] Multicolor confocal microscopy:
[0322] Multicolor laser confocal microscopy experiments were
performed to determine
phagocytosis, autophagy, and colocalization studies in urothelial cancer cells
and primary
macrophages. Cells were allowed to adhere on sterile glass cover slips placed
in 6-well tissue
culture plates and infections were carried at pre-calibrated MOT. Log phase
bacterial cultures were
labeled using FITC (Cat. F7250, Sigma). Following infection and treatment
conditions, cells were
fixed, permeabilized and blocked followed by overnight incubation with a
primary antibody for
LC3B (Cat. NB100-2220, Novus) or p62/SQSTM1 (Cat. P0067, Sigma-Aldrich) at
recommended
dilutions at 4 C. Cells were washed and incubated in the dark with Alexa
Flour 647 conjugated
secondary antibody (Cat. A32733, Thermo Fisher Scientific) at 4 C for lhour.
DNA staining was
carried out using Hoechst 33342 (Cat. 62249, Thermo Fisher Scientific) for 5
minutes. Images
were acquired using Zeiss LSM700 single-point, laser scanning confocal
microscope at 63X
magnification at the Microscope Facility, Johns Hopkins School of Medicine.
Image processing
and analyses was carried out using open source Fiji software. For LC3B or p62
quantification,
92
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
perinuclear LC3B puncta (spot) was counted in a minimum 100 cells across
different fields using
and Tmaris 9.5Ø Quantification carried out using GraphPad Prism software.
[0323] Phaaocytosis assay:
[0324] IgG-FITC conjugated latex bead phagocytosis assay kit
(Item No. 500290, Cayman
Chemicals, USA) was used for phagocytosis studies. HMDMs were placed on
sterile glass cover
slip for attachment. Infection was carried out at 5:1 (HMDM versus BCG) ratio
for 3 hours
followed by addition of IgG-FITC beads in warm RPMI 1640 media at 1: 400
dilutions for 3 hours.
Nuclear staining was carried out using Hoechst 33342 (Cat. 62249, Thermo
Scientific) and cells
were visualized for bead phagocytosis using Zeiss LSM700 single-point, laser
scanning confocal
microscope. Quantification of beads was measured by mean fluorescence
intensity (M.F.I.)
calculations using open source Fiji Software.
[0325] Multicolor flow cNtometry:
[0326] The cell surface and intracellular staining was carried
out on J774.1, murine BMDMs,
human HMDMs and single cells derived from murine MB49 tumors and spleens. Flow
cytometry
panel were designed and if needed modified form murine myeloid and lymphoid
cells and human
myeloid cells. Details of all antibodies and the dilutions used are given in
the Table 3. For in vitro
infection assays, protein transport inhibitor cocktail (Cat. 00-4980-03,
eBioscience) at
recommended dilution, 12 hours before harvesting monolayer of cells. At the
endpoint cells were
harvested using a cell-detachment buffer (ice-cold PBS - 10 mM EDTA solution).
Single cell
isolation was performed using animal tissues by harvesting tumors and spleens
following
necropsy. Briefly, tissues were manually disrupted before incubating in
collagenase type I (Gibco)
and DNase (Roche) in RMPI for 30 minutes at 37 C. Tumor and spleen cells were
dissociated
through a 70- m filter and washed with PBS. RBC lysis was performed for 5
minutes using ACK
lysis buffer (Cat. A1049201, Thermo Fisher Scientific) at room temperature.
Cells were washed
twice using ice-cold PBS and stained using Zombie AqUaTM Fixable Viability Kit
(Cat. 423101,
Biolegend). Cells were washed and resuspended in FACS buffer (1% BSA, 2mM EDTA
in PBS),
Fe blocked (TruStain FcXTM, Cat. 101320, and True-Stain Monocyte BlockerTM
Cat. 426102
Biolegend) and stained with conjugated primary antibodies as per
manufacturer's protocol.
Intracellular staining was performed following fixation and permeabilization
(Fixation and
Permeabilization Buffer Set, eBioscience). Cells were washed and resuspended
in flow buffer and
93
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
acquired using BD LSRII with FACSDiva Software. analyses were performed using
FlowJo (v10)
(TreeStar).
[0327] The following antibodies were used to stain myeloid and
lymphoid cells:
[0328] Mouse BMDMs: Anti-CD45 (clone 30-F11), anti-CD124 (clone
1015F8), anti-I-A/1-E
(clone 107630), anti-Ly6C (clone HK1.4), anti-CD1lb (clone M1/70), anti-F4/80
(clone BM8),
anti-Ly6G (clone 1A8), anti CD206 (clone C068C2), anti-TNF (clone MP6-XT22)
all Biolegend
and anti- IL-10 (clone JES5-16E3 eBiosciences).
[0329] Human HMDMs: anti CD16 (clone 3G8), anti-CD14 (clone
63D3), anti-HLA-DR
(clone L243), anti-CD 1 lb (clone ICRF44), anti-CD206 (clone 15-2), anti-CD163
(clone GHI/61),
anti-TNF (clone MAb11), and anti-TNF (clone MAbll) all Biolegend.
[0330] Mouse macrophages (syngeneic MB49 model of urothelial
carcinoma): CD45 (clone
30-F11, Biolegend), CD124 (IL-4Ra) (clone 1015F8, Biolegend), I-a/I-e (clone
M5/114.15.2,
Biolegend), F4/80 (clone BM8, Biolegend), CD206 (clone C068C2, Biolegend), TNF
(clone MP6-
XT22, Thermo Fisher), IL-10 (clone JES5-16E3, Thermo Fisher)
[0331] Mouse T cells (syngeneic MB49 model of urothelial
carcinoma): CD45 (clone PerCP,
Biolegend), CD25 (clone PC61, Biolegend), CD3 (clone 17A2, Biolegend), CD4
(clone GK1.5,
Biolegend), CD8a (clone 53-6.7, Biolegend), FOXP3 (clone MF-14, Biolegend),
Mouse IFN-y
(clone XMG1.2, Biolegend) and FOXP3 (clone MF-14 Biolegend).
[0332] hi vitro monoeyte trained immunity experiment:
[0333] In vitro training of primary human monocytes was performed
as described ear1ier47.
PBMCs were isolated from healthy donors (leukopaks). Following magnetic
separation, CD14+
monocytes were seeded in 10 1111113 tissue culture dishes for 3 hours in warm
RPMI 1640 media
supplemented with 10% FBS at 37 C with 5% CO2. Non-adherent cells were removed
by washing
cells using warm PBS. Monolayer culture of human monocytes was infected with
BCG-WT and
BCG-disA-OE strains at 5:1 (monocyte versus BCG) MOIs for 4 hours in presence
of RPMI 1640
supplemented with 10% FBS. Non-internalized bacilli were washed out using warm
PBS and
subsequently incubated for 24 hours. Cells were again washed using warm PBS
and fresh warm
RPMI 1640 media was added. For the following 5 days, cells were allowed to
rest with a PBS
wash and addition of fresh media every 2nd day. Cells were re-stimulated on
day 6 with RPMI
1640 supplemented with 10% FBS (negative control, without training) or TLR1/2
agonist,
94
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
Pam3Cys (Cat. tlrl-pms, InvivoGen). Following stimulation, for 24 h, culture
supernatants were
collected, filter sterilized and quickly snap-frozen (-80 C) for cytokine
measurement Cells were
harvested for chromatin immunoprecipitation (ChIP) experiments to measure
epigenetic changes
on gene promoters.
[0334] Chromatin immunoprecipitation (ChIP): Human monocytes were
fixed with a final
concentration of 1% formaldehyde for 10 minutes at room temperature. Cell
fixation was stopped
using 125 mM glycine (Cat no. 50046, Sigma-Aldrich, USA), followed by
sonication to fragment
cellular DNA to an average size between 300 to 600 bp using Qsonica Sonicator
Q125 (Cat.
15338283, Their/to Fisher Scientific). Sonicated cell lysates were subjected
to
immunoprecipitation (IP) by overnight incubation with recommended
concentration of primary
antibodies [(Histone H3K9me3 (H3K9 Trimethyl) Polyclonal Antibody cat. A-4036-
100,
epigentek); Anti-Histone H3 (tri methyl K4) antibody - ChIP Grade (ab8580),
abeam)] in presence
of magnetic Dynabeads (Cat no. 10004D, Thermo Fisher Scientific, USA) at 4 C.
Non-bound
material was removed by sequentially washing the Dynabeads with lysis buffer,
chromatin IP
(ChIP) wash buffer and Tris-EDTA (TE buffer). DNA elution was done using ChIP
elution buffer.
Amplification of different segments of the regulatory regions of immunity
genes was carried out
using qPCR using specific primers. Reactions were normalized with input DNA
while beads
served as negative control. Details of all primary antibodies and sequence of
primers have been
given in Table 4.
[0335] Targeted Metabolite analysis with LC-MS/MS:
[0336] Targeted metabolite analysis was performed with liquid-
chromatography tandem mass
spectrometry (LC-MS/MS) as described ear1ier48. Metabolites from cells or snap-
frozen xenograft
tumor tissue were extracted with 80% (v/v) methanol solution equilibrated at
¨80 C, and the
metabolite-containing supernatants were dried under nitrogen gas. Dried
samples were re-
suspended in 50% (v/v) acetonitrile solution and 4m1 of each sample were
injected and analyzed
on a 5500 QTRAP triple quadrupole mass spectrometer (AB Sciex) coupled to a
Prominence ultra-
fast liquid chromatography (UFLC) system (Shimadzu). The instrument was
operated in selected
reaction monitoring (SRM) with positive and negative ion-switching mode as
described. This
targeted metabolomics method allows for analysis of over two hundred of
metabolites from a
single 25-min LC-MS acquisition with a 3-ms dwell time and these analyzed
metabolites cover all
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
major metabolic pathways. The optimized MS parameters were: ESI voltage was
+5,000V in
positive ion mode and ¨4,500V in negative ion mode; dwell time was 3ms per SRM
transition and
the total cycle time was 1.57 seconds. Hydrophilic interaction chromatography
(HILIC)
separations were performed on a Shimadzu UFLC system using an amide column
(Waters XBridge
BEH Amide, 2.1 x 150 mm, 2.5jtm). The LC parameters were as follows: column
temperature, 40
C; flow rate, 0.30 ml/min. Solvent A, Water with 0.1% formic acid; Solvent B,
Acetonitrile with
0.1% formic acid; A non-linear gradient from 99% B to 45% B in 25 minutes with
5min of post-
run time. Peak integration for each targeted metabolite in SR1VI transition
was processed with
MultiQuant software (v2.1, AB Sciex). The preprocessed data with integrated
peak areas were
exported from MultiQuant and re-imported into Metaboanalyst software for
further data analysis
including statistical and principle components analyses.
[0337] Histologic analyses and immunohistochemistry (IHC):
[0338] For histologic analyses, a portion of bladder was formal
in fixed and paraffin embedded.
Sections of 5vt in thickness on glass slides were stained with hematoxylin-
eosin for classification
according to the World Health Organization/International Society of Urological
Pathological
consensus as described ear1ier27. Tumor staging was performed by 2 board
certified genitourinary
pathologists (A.S.B., A.M.). Specimens were classified based on the percentage
of involvement of
abnormal tissue (1 = 10% involvement, 2 = 20% involvement, and so forth). For
IHC staining,
high-temperature antigen retrieval (18-23 psi/126 C) was performed by
immersing the slides in
Trilogy (Cell Marque). Endogenous peroxidase activity was blocked for 5 min in
using Dual
Endogenous Enzyme Block (Cat. S2003, Dako). Primary Antibodies used included
Ki67 (1:50,
Cat. ab16667; Abeam), CD68 (1:250, Cat. MCA341R; Serotec), CD86 (1:100, Cat.
bs-1035R;
Bioss) and CD206 (1:10K, Cat. ab64693; Abeam). For Ki67, slides were stained
with ImmPACT
DAB (Vector Labs) for 3 min and counterstained with haematoxylin (Richard-
Allen). Dual
staining for CD68/CD206 and CD68/CD86 was achieved by first staining for CD68
with Impact
DAB (Vector Labs) followed by secondary antigen retrieval and incubation as
above with either
CD86 or CD206 and visualized with ImmPACT AEC (Vector Labs). For each section,
Ki67
expression was scored as a percentage of positive cells in the urothelium.
Dual stains for
CD68/CD86 and CD68/CD206 were scored based on positive clusters of cells for
each marker (0=
96
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
no staining, 1 = rare isolated cells positive, 2 = clusters of up to 10
positive cells, 3= clusters of >
positive cells).
[0339] In vivo experiments:
[0340] Intravesical BCG treatment in carcinogen induced NMIBC rat
model:
[0341] The induction of urothelial cancer in rats and subsequent
treatment of intravesical BCG
were performed. N-methyl-N-nitrosourea (MNU) instillations were given every
other week for a
total of 4 instillations. Fischer 344 female rats age 7 weeks (Harlan, avg.
weight 160g) were
anesthetized with 3% isoflurane. After complete anesthesia, a 20G
angiocatheter was placed into
the rat's urethra. MNU (1.5mg/kg) (Spectrum) dissolved in 0.9.% sodium
chloride was then
instilled and the catheter removed, with continued sedation lasting for 60
minutes to prevent
spontaneous micturition and allow absorption. Eighteen weeks after the first
MNU instillation,
intravesical treatment with PBS or 5 x 106 CFU of each BCG strain (0.3m1 via a
20G angiocatheter)
was administered weekly for a total of 6 doses. Rodents were sacrificed 2 d
after the last
intravesical treatment, and bladders were harvested within 48 hours of the
last BCG instillation for
mRNA and protein expression analysis as well as histological evaluation.
[0342] BCG infection of BALB/c mice and CFU enumeration:
[0343] To determine the lung bacillary burden of wild-type and
BCG-disA-OE strains 6-week-
old female BALB/c mice were exposed using the aerosol route in a Glasscol
inhalation exposure
system (Glasscol). The inoculum implanted in the lungs at day 1 (n=3 mice per
group) in female
BALB/c mice was determined by plating the whole lung homogenate on 7H11
selective plates
containing carbenicillin (50 mg/ml), Trimethoprim (20 mg/ml), Polymyxin B (25
mg/ml) and
Cycloheximide (10 mg/ml). Following infection, mice lungs were harvested (n =
5 animals/group),
homogenized in their entirety in sterile PBS and plated on 7H11 selective
plates at different
dilutions. The 7H11 selective plates were incubated at 37 C and single
colonies were enumerated
at week 3 and 4. Single colonies were expressed at log CFU per organ.
[0344] SCID Mice time to death study:
[0345] The virulence testing of BCG-WT and BCG-disA-OE strains
was done in severely
compromised immunodeficient mice aerosol infection model as described
previously. The
inoculum implanted in the lungs at day 1 (n = 3 animals per group) was
determined by plating the
97
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
whole lung homogenate on 7H11 selective plates. For time to death analyses (n
= 10 animals per
group) infected animal were monitored until their death.
[0346] Syngeneic MB49 model of urothelial cancer:
[0347] MB49 tumor cells arc urothclial carcinoma line derived
from an adult C57BL/6 mouse
by exposure of primary bladder epithelial cell explant to 7,12-
dimethylbenz[a]anthracene (DMBA)
for 24 hours followed by a long-term cu1ture79. Before implantation, MB49
cells were cultured as
monolayers in RPMI 1640 media supplemented with 10% FBS and 1%
streptomycin/penicillin at
37 C with 5% CO2. Cells were harvested using Trypsinization and cell viability
was determined
using Trypan blue dye. Live MB49 cells were resuspended in sterile PBS and
adjusted at 1 x 105
live cells per 100 1. Female C57BL/6J mice, age 4-6 weeks (Charles River
Laboratories) were
subcutaneously injected with 1 x 105 MB49 cells in the right flank of hind
leg. Tumor growth was
monitored every 2nd day to observe the increase the tumor burden at the time
of treatment
initiation. Once palpable tumor developed (7 to 9 days, average volume ¨ 30
mm3), 1 x 106 bacilli
of BCG-WT or BCG-disA-OE in a total 50 l PBS was injected intratumorally (Fig.
41). A total
of 4 intratumoral injections of BCG was given every 3rd day. Tumors were
measured by electronic
caliper, and tumor volume was calculated using the following equation: tumor
volume = length x
width x height x 0.5326. Mice were killed at specified time, and tumors and
spleens were collected
after necropsy for single cell preparation.
EXAMPLE 2
BCG-DISA -OE ELICITS GREATER PRO-INFLAMMATORY
CYTOKINE RESPONSES IN MACROPHAGES THAN BCG-WT.
[0348] BCG-disA-OE is a genetically-engineered BCG strain in
which an endogenous
diadenylatc cyclase gene, disA, is fused to a strong promoter, leading to a
300-fold overcxpression
of disA and a 15-fold increase in production of cyclic di-AMP (Fig. 39).
Compared with BCG-
WT, BCG-disA-OE significantly increased STING pathway activation in
macrophages as
measured by IRF3 induction (Fig. 39). To control for the fact that numerous
BCG strains are used
worldwide and variabilities in their clinical efficacies have been described,
two versions of BCG-
disA-OE and a corresponding BCG-WT were generated: one using BCG-Tice and one
using BCG-
Pasteur. No significant differences between the Tice and Pasteur versions were
detected.
98
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
[0349] To characterize the trained immunity-inducing potential of
BCG-disA-OE versus BCG-
WT, their capacity to induce cytokine expression in human monocyte-derived
macrophages
(HMDMs), primary murine bone marrow-derived macrophages (BMDM), and dendritic
cells
(BMDC) as well as a macrophage cell line (J774.1) were evaluated. Consistent
induction of IRF3,
IFN-13, TNF-a and IL-6 in all myeloid cell types were found in response to BCG-
disA-OE that was
significantly higher than that seen with BCG-WT-exposed cells (Fig. 40 and Fig
43), and in human
MDM and murine BMDM this difference was observed even in when cells were IFN-
yprimed
(Fig. 43). These differences were strictly STING-dependent as confirmed using
BMDM from
STING-/- mice (Fig. 40). Since STING activation leads to upregulation of NF-KB
via the TBK1-
IRF3 pathway, it was found that expression of both TNF-a and IL-6 in the same
panel of cells
paralleled that of IFNI, and was significantly higher following exposure to
BCG-disA-OE
compared with BCG-WT (Figs 41 and 43). Cyclic dinucleotides including cyclic
di-AMP are
known to be potent inducers of several chemokines (CXCL9, CXCL10 [TP-10],
CXCL22, and
MCP-1) as well as iNOS; consistent with this, IFN-y-primed BMDMs showed a more
robust
induction of these chemokines and iNOS when challenged with BCG-disA-OE strain
than with
BCG-WT (Fig. 41). The cellular toxicity was also assessed using annexin-PI
staining and found
that whereas late apoptotic cell death remained at baseline with BCG-disA-OE
exposure in both
BMDM and J774.1 macrophages, BCG-WT exposure elicited significantly higher
levels of
apoptotic cell death (Fig. 44) in the BMDM cells. These observations
demonstrate that BCG-disA-
OE elicits pro-inflammatory cytokine expression more potently than BCG-WT in
primary human
MDM as well as murine primary macrophages and macrophage cell lines.
EXAMPLE 3
PRO-INFLAMMATORY POLARIZATION OF MACROPHAGES
IS GREATER WITH BCG-D/SA-OE THAN WITH BCG-WT
[0350] Trained immunity is associated with polarization of
macrophages towards
inflammatory phenotypes with a concomitant shift away from anti-inflammatory
states. To
investigate macrophage polarization, flow cytometry was used to monitor
phenotypic shifts of both
murine and human primary macrophages following a 24 h exposure to BCG-disA-OE
or BCG-WT.
First, the MHC class II-expressing CD45 CD1 1 b F4/80 murine BMDM population
were focused
on, following in vitro BCG exposure using the gating scheme. As may be seen in
Fig. 50 and Fig.
99
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
48, a significantly greater expansion of TNF-a-expressing CD1 1 F4/80-' (M1)
murine BMDMs
was observed following exposure to BCG-disA -OE than with BCG-WT. Next cells
expressing the
M2 surface receptors CD206f and CD124 among CD45f CD1 lb+ F4/80' macrophages
were gated
on and a greater reduction of this population with BCG-disA-OE than with to
BCG-WT was
observed (Fig. 50 and Fig. 49). Within this immunosuppressive cell population,
there was a higher
proportion of IL-10-expressing CD206+ CD124+ cells in BCG-WT-exposed
macrophages, while
IL-10-expressing cells were significantly reduced in response to BCG-disA-OE
exposure (Fig. 50
and Fig. 49). These results demonstrate that compared with BCG-WT, BCG-disA-OE
exposure
elicits more extensive macrophage reprogramming with expansion of pro-
inflammatory M1
macrophages displaying increased antigen presentation (MHC class II
expression) and TNF-a
expression and contraction of immunosuppressive M2 macrophages expressing IL-
10.
103511 Myeloid-derived suppressor cells (MDSCs) are a
heterogeneous population of
immature myeloid cells known to foster immunosuppression. Accordingly, the
induction of
monocytic-myeloid derived suppressor cells, M-MDSCs, was investigated (CD45-'
Ly6Chi Ly6G-
CD11b' F4/80) using primary murine BMDMs using the gating scheme shown.
Following BCG-
WT exposure a significant expansion of M-MDSCs was observed, while in contrast
this same
population showed minimal expansion following BCG-disA-OE exposure (Fig. 50).
Moreover,
the M-MDSCs elicited by BC G-WT exhibited higher IL-10 expression, whereas IL-
10-expressing
M-MDSCs were virtually absent after BCG-disA-OE exposure (Fig. 50). These
observations
suggest that BCG-WT contributes to an expansion of M-MDSCs which have
immunosuppressive
properties; however, forced overexpression of the pro-inflammatory PAMP cyclic
di-AMP by
BCG prevents M-MDSC expansion.
103521 The macrophage activation phenotypes in HMDMs isolated
from several independent
healthy human donors was next characterized. Both the BCG-WT and BCG-disA-OE
strains
elicited increases in the population of classical macrophages (CD1lb CD14-'
CD16), but these
inductions were comparatively higher in response to BCG-disA-OE (Fig. 51 and
Fig. 52).
classically activated antigen-presenting macrophages (CD14+ CD16- HLA-DR') and
their ability
to produce TNF-a or IL-6 were examined and it was found a significantly
increased proportion of
TNF-a and IL6- producing HLA-DR cells following exposure to BCG-disA-OE
compared to
BCG-WT (Fig. 52 and Fig. 53). The M2 surface markers, CD206+ and CD163-', were
also
100
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
investigated on transitional or intermediate macrophages (CD11b+ CD14+ CD16+)
and it was found
a consistently greater decrease in them following BCG-disA-OE exposure than
with BCG-WT
(Fig. 51, Fig. 53). The fraction of these intermediate macrophages expressing
M2 surface markers
and 1L-10 was also significantly lower in response to exposure to BCG-disA-OE
than with BCG-
WT (Fig. 53). In summary, using both mouse and human primary macrophage ex
vivo models, it
was found that, compared with BCG-WT, BCG-disA-OE promotes greater macrophage
activation
towards an M1 phenotype (inflammatory), and concomitantly reduces the
emergence of cells with
immunosuppressive abilities, including M-MDSCs.
EXAMPLE 4
MACROPHAGES EXPOSED TO BCG-D/SA-OE
ARE MORE PHAGOCYTIC THAN THOSE WITH BCG-WT
[0353] Cyclic clinucleotides have been reported to recruit
inflammatory macrophages which
display high phagocytic potential. Consistent with these observations it waswe
confirmed that
HMDMs transfected with cyclic di-AMP showed increased phagocytosis and
exhibited elongated
dendrites compared to mock-transfected populations. It was then evaluated the
phagocytic
properties of HMDMs following exposure to the different BCG strains and found
significantly
greater phagocytosis of IgG-opsonized FITC-latex beads by macrophages exposed
to
BCG-disA-OE compared to BCG-WT (Fig. 54). In keeping with the previously
established role of
STING pathway activation in augmenting autophagy, it was found that a majority
of intracellular
BCG-disA-OE bacilli were co-localized with LC3B in IFN-y-activated primary
BMDMs, while
autophagy induction in BCG-WT was significantly lower. It was also found a
significantly greater
co-localization of BCG-cli sA-OE bacilli with the autophagy adapter protein
p62 compared to that
observed with BCG-WT. These results reveal BCG-disA-OE increases the levels of
phagocytosis
and autophagic processing within macrophages to a greater degree than BCG-WT,
a phenomenon
associated with enhanced peptide antigen presentation to MHC class-II
molecules.
EXAMPLE 5
BCG-D/SA-OE REPROGRAMS MACROPHAGES EPIGENETICALLY
AND POTENTIATES TRAINED IMMUNITY TO A GREATER DEGREE THAN BCG-WT
[0354] In light of recent data showing BCG to be a potent inducer
of trained immunity through
epigenetic modifications of key pro-inflammatory genes, it was hypothesized
that the addition of
101
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
cyclic di-AMP overexpression to standard BCG might potentiate epigenetic
modifications in
primary human monocytes. Having already established that BCG-disA -OE is a
more potent
inducer of macrophage TNF-a and IL-6 secretion than BCG-WT, it was confirmed
this in primary
human monocytes from a group of 6 healthy human subjects. The ability of
traditional BCG to
elicit trained immunity has been correlated with changes in epigenetic marks
that increase pro-
inflammatory gene expression. Thus, it was asked if the enhanced induction of
TNF-a and IL-6
expression elicited by BCG-disA-OE compared with BCG-WT is epigenetically
mediated. To this
end, it was evaluated the promoter regions of the TNF-a and IL-6 genes for
durable, antigen-
independent epigenetic changes using an assay in which human monocytes exposed
to BCG strains
for 24 h were rested for five days prior to challenge with a heterologous
antigen, the TLR1/2
agonist Pam3CSK4 on day 6 (Fig. 56). Using chromatin immunoprecipitation-
polymerase chain
reaction (ChIP-PCR) assays the activating histone methylation mark H3K4me3
present in the
TNF-a and IL-6 promoters was quantified. It was observed that exposure to BCG-
disA-OE led to
greater enrichment of this mark than BCG-WT even without the heterologous
second stimulation
(i.e., adding RPMI media alone at day 6). Upon re-stimulation with Pam3CSK4 at
day 6, the
abundance of the activating epigenetic mark was further increased by both BCG
strains, but BCG-
disA-0E-pretreatment yielded notably more enrichment than BCG-WT (Fig. 56).
Similarly, it was
investigated the chromatin repression mark H3K9me3 at the same two promoters
and found that,
while both BCG strains led to reduced levels of H3K9me3 (which were further
accentuated by
addition of Pam3CSK4), the degree of reduction mediated by BCG-disA-OE was
consistently
greater than that mediated by BCG-WT, both upon initial exposure and after
rest and re-stimulation
(Fig. 56). Simultaneous measurement of TNF-a and IL-6 in BCG-trained culture
supernatant
following non-specific stimulation by Pam3CSK4 revealed that BCG-disA-0E-
trained
macrophages produced significantly higher levels of these pro-inflammatory
eytokines than did
those trained with BCG-WT. These results indicate that an augmented BCG which
overexpresses
the PAMP molecule cyclic di-AMP leads to significantly more robust epigenetic
changes
classically associated with trained immunity.
102
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
EXAMPLE 6
BCG-DLSA -OE REPROGRAMS THE MACROPHAGE IMMUNO-METABOLIC
STATE TOWARDS PRO-INFLAMMATORY SIGNATURES
TO A GREATER DEGREE THAN BCG-WT
[0355] BCG-training has been reported to stimulate glycolysis as
well as the tricarboxylic acid
cycle through glutamine replenishment with accumulation of fumarate. To
address whether the
addition of cyclic di-AMP overexpression alters the BCG-mediated metabolomic
shifts, LC-MS was
used to characterize key metabolites in primary human and murine macrophages
exposed to the two
BCG strains. HMDMs or BMDMs showed increased catabolic signatures (elevated
intracellular
glucose and lactate) to a greater degree following a 24 h exposure to BCG-disA-
OE than with BCG-
WT. Also, the TCA cycle metabolites itaconate and fumarate were also more
elevated with
BCG-disA-OE than with BCG-WT. These observations suggest greater catabolism of
carbon
substrates for ATP generation consistent with a pro -in fl ammatory bi
oenergeti c profile in
macrophages infected with BCG-disA-OE than with BCG-WT.
[0356] Excess tryptophan catabolism to kynurenine by tryptophan
dehydrogenase and
indoleamine 2,3-dioxygenase (IDO) has been strongly associated with
immunosuppression, and
IDO inhibitors have shown potential as immune activators in a variety of
infectious and oncologic
diseases. Kynurenine levels were dramatically lower in macrophages following
BCG-disA-OE
exposure than those seen with BCG-WT, and as would be expected tryptophan
levels were
elevated by BCG-disA-OE while BCG-WT led to tryptophan levels comparable to
the baseline
seen with heat-killed BCG controls. Citrulline levels were also higher while
putrescine levels were
lower with BCG-disA-OE than BCG-WT suggesting that nitric oxide synthase-
mediated
conversion of argininc to NO (pro-inflammatory) and citrullinc was more
strongly induced by
BCG-disA-0E. Finally, it was of interest that itaconate, an isocitrate lyase
inhibitor made by
macrophages that has been shown to have antibacterial activity, was more
potently induced by
BCG-disA-OE than BCG-WT. Thus, compared with BCG-WT, BCG-disA-OE elicited a
greater
pro-inflammatory metabolomic signature with reduced kynurenine accumulation
and increases in
glycolytic metabolites, NOS products, and itaconate production.
103
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
EXAMPLE 7
FUNCTIONAL EFFICACY TN VIVO: BCG-DLSA -OE DEMONSTRATES
SUPERIOR IMMUNOTHERAPEUTIC OUTCOMES
IN RELEVANT ANIMAL MODELS OF TRAINED IMMUNITY
[0357] In addition to being used as a TB vaccine, BCG has served
as a first-line
immunotherapy for the treatment of non-muscle invasive bladder cancer (NMIBC)
since the mid-
1970s. Recent studies have indicated that BCG exerts its antitumor effects via
a trained immunity
mechanism. Having demonstrated that augmenting BCG with excess cyclic di-AMP
release leads
to improved trained immunity parameters across a battery of in vitro assays,
it was sought to
determine if these effects could be demonstrated in vivo.
[0358] First, BCG-disA-OE versus BCG-WT was tested in a
carcinogen-induced model of
NMIBC in which intravesical therapies can be introduced into the bladder as
they are in humans
with non-invasive urothelial cancer. The rat tV-methyl-Ar-nitrosourea (MNU)
model of bladder
cancer (BC) is schematized in Fig. 57 In this model urothelial dysplasia
develops at week 14 after
the first intravesical instillation of MNU and by week 24 rats display a
different forms of urothelial
cancer severity including carcinoma-in-situ (CIS), papillary Ta (superficial),
or higher-grade Tl -
T2 urothelial carcinoma with histopathologic and immunophenotypic features
similar to those
observed in human bladder cancer. Following carcinogen-mediated tumor
induction with 4 cycles
of MNU (wk 0, wk 2, wk 4, wk6), groups of rats were treated with 6 weekly
doses of intravesical
BCG-disA-0E, BCG-WT, or no treatment from week 18-23. Upon sacrifice at wk 24
the rat
urinary bladders were divided into halves for (i) RT-PCR analysis, and (ii)
histologic analysis
including tumor staging by a blinded genitourinary pathologist.
Transcriptional analysis of the
whole excised bladders at week 24 showed that compared with BCG-WT, BCG-disA-
OE elicited
significantly increased levels of IFN-P, IFN-y, TNF-a, IL-113, CXCL10, MCP-I,
MIP-la, and
iNOS transcription while mRNA levels of the immunosuppressive cytokines IL-10
and TGF-p
were reduced by both BCG strains (Fig. 57). These patterns of cytokine
expression were confirmed
at the protein level using ELISA for TNF-a 1L-2, and IFN-y and noted that
intravesical BCG-disA-
OE, strongly increased the levels of IFN-y in rat spleens while BCG-WT did
not. Correspondingly,
it was found a significant decrease in highest pathology grade, tumor
involvement index and
highest tumor stage (Fig. 57) in rats treated with BCG-disA-OE in comparison
to untreated. By
104
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
tumor involvement index BCG-disA-OE was statistically significantly superior
to no treatment (p
<0.001) and to BCG-WT (p < 0.05), whereas BCG-WT showed only a trend towards
improvement
over no treatment. Importantly, the highest tumor stage observed in BCG-disA-
0E-treated rats
was CIS, whereas it was Ti in those receiving BCG-WT, and T2 in untreated
rats, and 53.3% of
BCG-disA-0E-treated rats were cancer free (p=0.009) compared with 31.2% of BCG-
WT and 0%
of the untreated rats (Fig. 57). Immunohistochemical analyses revealed a
significant reduction in
Ki67 staining in BCG-disA-0E-treated MNU rat bladders when compared to
untreated (p < 0.01)
and BCG-WT (p < 0.05) suggesting reduced tumor proliferation. CD68 staining of
rat bladder
showed significantly higher levels of macrophage recruitment with a trend
toward elevation of the
pro-inflammatory Ml-like CD86+ macrophages and a significant reduction in
CD206+ M2-like
macrophages that are associated with tumor promotion in the BC G-disA-0E-
treated rats compared
with untreated controls. These observations indicate that the enhanced
induction of type I IFN and
other proinflammatory signatures in bladders of tumor-bearing rats treated
with BCG-disA-OE
correlated with the enhanced antitumor activity of the recombinant BCG strain.
[0359] The functional efficacy of BCG-disA-OE was also tested in
a murine heterotopic,
syngeneic bladder cancer model using MB49 urothelial cancer cells. Following
flank engraftment
with MB49 tumor cells, mice received four intratumoral treatments over 9 days
as shown in Fig.
61. In this model BCG-disA-OE also showed more robust immunotherapeutic
efficacy than BCG-
WT as measured by tumor volume and weight after intraturnoral injection of BCG-
disA-OE when
compared with BCG-WT (Fig. 61). Histopathology demonstrated extensive necrosis
and
congestion in MB49 tumors treated with BCG-disA-OE when compared to BCG-WT and
untreated. There were no significant changes in body weights of mice receiving
BCG, however
splenic weight was significantly increased by both BCG strains. We further
characterized the
impact of the treatments on macrophage polarization and recruitment of
activated T cells in the
tumor microenvironment (TME). As shown in Fig. 61, compared with BCG-WT, BCG-
disA-OE
significantly reduced the abundance of immunosuppressive M2 macrophages when
compared to
untreated and BCG-WT and significantly (p < 0.01) increased proinflammatory M1
macrophages.
Similarly, BCG-disA-OE recruited significantly more IFN-y-producing CD4 T
cells when
compared to BCG-WT, and both BCG strains increased IFN-y-producing CD8 T
cells. While
both BCG strains recruited more CD4' and CD S' cells to the tumors, BCG-disA-
OE uniquely
105
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
recruited more CDS+ T cells to the spleens of treated animals. BCG-disA-OE
also significantly
reduced tumor-associated T-regulatory (Treg) cells to a greater degree than
BCG-WT in both
tumor and spleen. In keeping with our earlier findings in primary cells, we
also found that
compared with BCG-WT, BCG-disA-OE elicited more potent cytokinc responses and
autophagy
in human urothelial cancer cells representing various tumor stages. These
results indicate that in
this murine model of urothelial cancer, BCG-disA-OE has superior antitumor
efficacy than BCG-
WT, and its efficacy correlates with shift in polarization of macrophages to
Ml, increased
activation of both CD4 and CD8' T cells, and a reduction of local intratumoral
and systemic Treg
cell populations.
EXAMPLE 8
SAFETY: BCG-D/SA-OE IS LESS PATHOGENIC THAN BCG-WT
IN TWO MOUSE MODELS
[0360]
To address concerns that the enhanced pro-inflammatory immune responses
elicited by
BCG-disA-OE might lead to adverse effects, safety in two separate mouse models
was evaluated.
An immunocompetent BALB/c mouse model of aerosol exposure was used and
measured the lung
bacillary burden after four weeks when adaptive immune responses are maximal
(Fig. 58). While
the day 1 implantation of the two BCG strains was equivalent, we observed that
BCG-disA-OE
proliferated in murine lungs to a significantly lower degree than BCG-WT by a
margin of 0.43
logio colony forming units (Fig. 58). As previously observed in cell-based
models, pm-
inflammatory cytokine levels in both lungs and spleens were significantly
higher in BCG-dis,4-
OE-exposed mice than those receiving BCG-WT (Fig. 60).
the two strains in
immunocompromised SCID mice which do not survive infection with BCG were also
tested.
Again, using a low dose aerosol exposure model (Fig. 59), it was observed a
statistically significant
survival prolongation with BCG-disA-OE compared to BCG-WT (Fig. 59). Thus,
despite eliciting
more profound inflammatory signatures in numerous model systems, BCG-disA-OE
is less
pathogenic than BCG-WT in these two murine model systems.
EXAMPLE 9
DISCUSSION
[0361]
Numerous recombinant BCG strains have been generated and tested over the
years.
These studies were generally conducted with the goal of improving either TB
protective efficacy
106
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
or bladder cancer immunotherapy, but in certain cases the goal has been
prevention of other
infectious diseases. A common strategy has been to overexpress an antigen to
elicit disease-
specific immunity or a cytokine gene to boost local host responses. While many
modified BCGs
have shown efficacy in pre-clinical models, few have progressed to human
clinical trials. To date
only BCGAureC::hly (VPM1002), a BCG designed to enhance phagosome permeability
and
exposure of BCG antigens to cytosolic MHC class I antigen processing, has
advanced to late stage
clinical trials for tuberculosis. This is the first to specifically re-
engineer BCG with the specific
goal of improving trained immunity by overexpressing the PAMP molecular cyclic-
di-AMP to
increase STING pathway engagement.
[0362] To determine if trained immunity parameters may be
increased, BCG-disA-OE versus
BCG-WT were tested in a battery of in vitro assays. Cytokine release profiles,
macrophage
polarization, autophagy, phagocytosis, epigenetic modifications, and metabolic
remodeling in
-human and murine primary cells were evaluated. In each assay system, BCG-disA
-OE was a more
potent potentiator of pro-inflammatory responses than BCG-WT. the cyclic-di-
AMP expressing
BCG was further tested in a functional in vivo assay of trained immunity,
namely bladder cancer
immunotherapy. In two separate models of urothelial cancer, BCG-disil-OE has
greater
immunotherapeutic efficacy than did BCG-WT indicating that our in vitro
results were predictive
of functional efficacy in a relevant animal model. Interestingly, despite
eliciting a significantly
more potent pro-inflammatory responses in our in vitro assay systems, BCG-disA-
OE did not
produce excess pathogenicity in two animal models of BCG infection or BCGosis.
[0363] It was also observed that BCG-WT did not uniformly elicit
pro-inflammatory
responses. For example, it was observed that treatment of murine macrophages
with BCG-WT in
fact induced a higher percentage of M-MDSCs (anti-inflammatory) compared with
untreated
controls (Fig. 50), and similarly BCG-WT led to elevated levels of the anti-
inflammatory
metabolite kynurenine. These findings of certain anti-inflammatory
consequences of BCG-WT
may correlate with the observation that in countries which routinely use BCG
for TB prevention,
vaccinees display reduced levels of asthma and atopic dermatitis. In contrast,
this expansion of M-
MDSCs in macrophages by BCG-WT was reversed by cyclic di-AMP overexpression
which is in
keeping with recent studies showing that STING pathway activation reduces the
induction of
MDSCs in certain cancers.
107
CA 03167223 2022- 8-5
WO 2021/163602
PCT/US2021/018007
[0364] Trained immunity changes elicited by BCG may underlie the
immunotherapeutic
effects of BCG in cancer prevention. Therefore, another goal of this study was
to evaluate whether
the salutary effects of BCG-di.sA-OE as a NMIBC immunotherapy is mediated
through
engagement of STING pathway and modulates BCG-mcdiated trained immunity. In a
rat model
of NMIBC, it was found that whereas invasive tumors developed in untreated
tumor-bearing rats
(highest tumor grade of T2) as well as BCG-WT-treated animals (highest tumor
grade of Ti),
invasive bladder cancer was completely absent in rats treated with BCG-disA-
0E. Similarly, in
the MB49 mouse model of bladder cancer, BCG-dis24-0E was superior to BCG-WT in
reducing
tumor growth with associated increase in tumor necrosis, and these effects
were accompanied by
significantly higher recruitment of M1 macrophages, IFN-y-producing CD4 cells,
and reduced
accumulation of Treg cells in the tumors. Elevated levels of pro-inflammatory
cytokines and
chemokines were observed in bladders from tumor-bearing animals treated with
BCG-disA-OE
compared to BCG-WT. Since non-immune cells have also been shown to possess
immunological
memory, the possibility that this cytokine response may have originated from
myeloid cells in the
TME and/or the tumor cells themselves was considered. Indeed, it was found
that compared with
BCG-WT, BCG-di.sA-OE elicited more potent cytokine responses in both primary
macrophages
and human urothelial cancer cells representing various tumor stages. This
appeared to be a
downstream consequence of STING activation since we found dramatically reduced
expression in
BMDMs from STING' mice. In addition, robust induction of several chemokines as
has been
observed in other studies with stimulation using exogenous STING agonists was
found.
[0365] Although the invention has been described with reference
to the above examples, it will
be understood that modifications and variations are encompassed within the
spirit and scope of the
invention. Accordingly, the invention is limited only by the following claims.
[0366] Although the invention has been described with reference
to the above examples, it will
be understood that modifications and variations are encompassed within the
spirit and scope of the
invention. Accordingly, the invention is limited only by the following claims.
108
CA 03167223 2022- 8-5