Note: Descriptions are shown in the official language in which they were submitted.
CA 03168643 2022-07-26
[DESCRIPTION]
[Invention Title]
POLYPEPTIDE HAVING ANTIBACTERIAL ACTIVITY, COMPOSITION
FOR PREVENTING OR TREATING SEPSIS COMPRISING SAME, AND
ANTIBACTERIAL COMPOSITION
[Technical Field]
The present invention relates to a polypeptide having antibacterial activity,
a
composition for preventing or treating sepsis, which includes the same, and an
antibacterial composition, and the like.
This application claims priority to and the benefits of Korean Patent
Application No. 10-2019-0023534, filed on February 28, 2019, and Korean Patent
Application No. 10-2020-0023629, filed on February 26, 2020, the disclosures
of
which are incorporated herein by reference in their entirety.
[Background Art]
Sepsis is an inflammatory response caused by excessive activation of the
immune system of the body by lipopolysaccharide (LPS), which is a component of
a
cell wall, acting as a toxin when the body is infected by pathogenic gram-
negative
bacteria, and can cause infection in the whole body or can be accompanied by
shock
if there are severe symptoms. Specifically, sepsis is generally caused when
patients
with basic diseases such as malignant tumors, leukemia, malignant lymphoma,
acquired immunodeficiency syndrome (AIDS), collagen disease, renal failure,
liver
disease, cerebrovascular disorders, and diabetes or weakly resistant hosts
with humoral
or cellular immunodeficiency, such as the elderly and premature infants
undergo
1
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
chemotherapy with adrenal steroids or antitumor drugs, radiation therapy such
as
cobalt irradiation, or treatment and surgery such as indwelling catheters,
hemodialysis,
organ transplantation and heart surgery. Sepsis has become the leading cause
of
death of patients admitted to the intensive care unit of a hospital, and is a
very serious
disease with a fatality rate of usually 30% or more. Despite the advances in
medical
technology, there are many cases of sepsis due to infection as a sequela of
surgery
worldwide, and infection of a person with weak immunity in the body, such as a
newborn baby or the elderly, often develops to sepsis. Typically, it is known
that
neonatal sepsis occurs in approximately 3 out of 1,000 full-term infants, and
the
incidence thereof increases 3 to 4-fold in premature infants.
While sepsis is usually treated with antibiotics, it cannot be effectively
treated
only with antibiotics in the case of treatment being delayed such that a large
amount
of bacteria have proliferated or in the case of infection by a strain having
resistance to
antibiotics, and the number of pathogens resistant to various antibiotics is
gradually
increasing, . For this reason, there is an urgent demand for the development
of a
novel sepsis therapeutic agent.
Meanwhile, antimicrobial-resistant bacteria refer to bacteria that are
resistant
to a specific antibacterial agent and thus the agent does not work. For
example, the
antimicrobial-resistant bacteria include penicillin-resistant Staphylococcus
aureus,
which causes penicillin to be completely ineffective. In
addition, Methicillin-
Resistant Staphylococcus aureus (MRSA), which was first reported in academia
in
1961 and has become a major pathogenic infectious bacterium worldwide, is
known,
and vancomycin-resistant enterococcus (VRE) was first found in Europe in 1988.
In
2
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
addition, vancomycin-resistant staphylococcus aureus (VRSA) exhibiting high
resistance to vancomycin, known as the final therapeutic agent for
Staphylococcus
aureus , which is the most common cause of human infection, was first reported
by the
US Centers for Disease Control and Prevention in 2002. Taking all of the above
together, it can be seen that the spread of so-called super bacteria is highly
increasing.
[Disclosure]
[Technical Problem]
Therefore, as a result of repeated research to meet the above-described
demands, the inventors confirmed that a peptide having a specific amino acid
sequence
is effective in inhibiting bacterial proliferation as well as in treating
sepsis due to an
excellent effect of removing an endotoxin isolated from dead bacteria, and
selectively
exhibits excellent antibacterial activity against gram-positive/negative
bacteria, and
thus the present invention was completed.
Accordingly, technical problems to be solved in the present invention are
directed to providing a peptide having antibacterial activity, a composition
for
preventing or treating sepsis, which includes the same, and an antibacterial
composition, and the like.
However, technical problems to be solved in the present invention are not
limited to the above-described ones, and other problems which are not
described herein
will be fully understood by those of ordinary skill in the art from the
following
descriptions.
3
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
[Technical Solution]
To achieve the purpose of the present invention, the present invention
provides
a polypeptide represented by the following general sequence formula:
[General Formula]
Ln-X1-L-X2-V-X3-X4-X5-R-X6-L-X7
In the general formula,
n is 0 or 1;
L is leucine;
V is valine;
R is arginine;
X1 is lysine (K) or arginine (R);
X2 is glycine (G) or arginine (R);
X3 is glutamic acid (E) or lysine (K);
X4 is alanine (A) or leucine (L);
X5 is lysine (K) or arginine (R);
X6 is tyrosine (Y), alanine (A), tryptophan (W), lysine (K) or aspartic acid
(D);
and
X7 is aspartic acid (D) or arginine (R),
however, in the general formula, a polypeptide represented by the sequence of
K-L-G-V-E-A-K-R-Y-L-D is excluded.
In one embodiment of the present invention, any one of 9 types of polypeptides
consisting of 1) to 9) as follows is provided:
1) a polypeptide represented by the general formula, in which
4
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
n is 0;
X1 is lysine (K);
X2 is glycine (G);
X3 is glutamic acid (E);
X4 is alanine (A);
X5 is lysine (K);
X6 is tyrosine (Y), and
X7 is arginine (R);
2) a polypeptide represented by the general formula, in which
n is 1;
X1 is arginine (R);
X2 is glycine (G);
X3 is glutamic acid (E);
X4 is alanine (A);
X5 is lysine (K);
X6 is tyrosine (Y), and
X7 is arginine (R);
3) a polypeptide represented by the general formula, in which
n is 1;
X1 is arginine (R);
X2 is glycine (G);
X3 is glutamic acid (E);
5
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
X4 is leucine (L);
X5 is lysine (K);
X6 is tyrosine (Y), and
X7 is arginine (R);
4) a polypeptide represented by the general formula, in which
n is 0;
X1 is lysine (K);
X2 is glycine (G);
X3 is glutamic acid (E);
X4 is alanine (A);
X5 is leucine (L);
X6 is tyrosine (Y), and
X7 is aspartic acid (D);
5) a polypeptide represented by the general formula, in which
n is 0;
X1 is arginine (R);
X2 is arginine (R);
X3 is lysine (K);
X4 is leucine (L);
X5 is arginine (R);
X6 is tyrosine (Y), and
X7 is arginine (R);
6
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
6) a polypeptide represented by the general formula, in which
n is 0;
X1 is lysine (K);
X2 is arginine (R);
X3 is lysine (K);
X4 is leucine (L);
X5 is arginine (R);
X6 is tyrosine (Y), and
X7 is arginine (R);
7) a polypeptide represented by the general formula, in which
n is 0;
X1 is lysine (K);
X2 is arginine (R);
X3 is lysine (K);
X4 is leucine (L);
X5 is arginine (R);
X6 is alanine (A), and
X7 is arginine (R);
8) a polypeptide represented by the general formula, in which
n is 0;
X1 is lysine (K);
7
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
X2 is arginine (R);
X3 is lysine (K);
X4 is leucine (L);
X5 is arginine (R);
X6 is tryptophan (W), and
X7 is arginine (R); and
9) a polypeptide represented by the general formula, in which
n is 0;
XI is lysine (K);
X2 is arginine (R);
X3 is lysine (K);
X4 is leucine (L);
X5 is arginine (R);
X6 is lysine (K), and
X7 is arginine (R).
In one embodiment of the present invention, the polypeptide refers to a
peptidomimetic including an L-type peptide, a D-type peptide and a peptoid, or
non-
natural amino acids.
In one embodiment of the present invention, an end of the polypeptide is
alkylated, PEGylated, or amidated.
In one embodiment of the present invention, an amine group (NH2) is added to
the C-terminus of the polypeptide.
8
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
In one embodiment of the present invention, the polypeptide has one or more
of the following characteristics:
binding ability to lipopolysaccharide (LPS) of gram-negative bacteria;
selective disruption ability against bacterial cell membrane;
resistance to proteolytic enzymes;
cell membrane disruption ability against gram-positive bacteria; and
binding ability to gram-positive bacteria-derived lipoteichoic acid (LTA) or
lipoprotein (LPP).
In addition, the present invention provides an acetate salt substitution of
trifluoroacetic acid (TFA) of a polypeptide represented by the general
sequence
formula.
In addition, the present invention provides a composition for preventing,
alleviating or treating sepsis, which includes the polypeptide represented by
the
general sequence formula or an acetate salt substitution of trifluoroacetic
acid thereof
as an active ingredient.
In addition, the present invention provides a pharmaceutical/food/health
food/cosmetic/quasi-drug/feed composition for preventing, alleviating or
treating
sepsis, which includes the polypeptide represented by the general sequence
formula or
an acetate salt substitution of trifluoroacetic acid thereof as an active
ingredient.
The present invention provides a method of preventing or treating sepsis,
which
includes administering the polypeptide represented by the general sequence
formula
9
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
or an acetate salt substitution of trifluoroacetic acid thereof into a subject
in need
thereof
In addition, the present invention provides a use of the polypeptide
represented
by the general sequence formula or an acetate salt substitution of
trifluoroacetic acid
thereof for preventing, alleviating or treating sepsis.
Further, the present invention provides a use of the polypeptide represented
by
the general sequence formula or an acetate salt substitution of
trifluoroacetic acid
thereof for preparing a preparation for preventing, alleviating or treating
sepsis.
In addition, the present invention provides an antibacterial composition,
which
includes the polypeptide represented by the general sequence formula or an
acetate salt
substitution of trifluoroacetic acid thereof as an active ingredient.
In addition, the present invention provides an antibacterial
pharmaceutical/food/health food/cosmetic/quasi-drug/feed composition, which
includes the polypeptide represented by the general sequence formula or an
acetate salt
substitution of trifluoroacetic acid thereof as an active ingredient.
The present invention provides an antibacterial method, which includes
administering the polypeptide represented by the general sequence formula or
an
acetate salt substitution of trifluoroacetic acid thereof into a subject in
need thereof
In addition, the present invention provides an antibacterial use of the
polypeptide represented by the general sequence formula or an acetate salt
substitution
of trifluoroacetic acid thereof
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
Further, the present invention provides a use of the polypeptide represented
by
the general sequence formula or an acetate salt substitution of
trifluoroacetic acid
thereof for preparing an antibacterial agent.
In one embodiment of the present invention, bacteria targeted by the
antibacterial composition include one or more types selected from the group
consisting
of Escherichia coli DH5a, Escherichia coli Kl, Acinetobacter baumannii,
Pseudomonas aeruginosa, Salmonella enteritidis, Salmonella typhimurium,
Klebsiella
pneumoniae, Staphylococcus aureus and Staphylococcus epidermidis.
In one embodiment of the present invention, bacteria targeted by the
antibacterial composition include one or more types of antibiotic-resistant
bacteria
selected from the group consisting of Extended Spectrum Beta Lactamase (ESBL)-
resistant bacteria, carbapenem-resistant bacteria and Colistin-resistant
bacteria.
In one embodiment of the present invention, the antibiotic-resistant bacteria
include one or more types selected from the group consisting of ESBL(E. coli),
carbapenem-resistant (CR)-Acinetobactor baumannii, CR-Klebsiella pneumoniae,
CR-Pseudomonas aeruginosa, and Colistin-resistant Acinetobactor baumannii.
In addition, the present invention provides a composition for preventing,
alleviating or treating an infectious disease, which includes the polypeptide
represented by the general sequence formula or an acetate salt substitution of
trifluoroacetic acid thereof as an active ingredient.
In addition, the present invention provides a pharmaceutical/food/health
food/cosmetic/quasi-drug/feed composition for preventing, alleviating or
treating an
infectious disease, which includes the polypeptide represented by the general
sequence
11
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
formula or an acetate salt substitution of trifluoroacetic acid thereof as an
active
ingredient.
The present invention provides a method of preventing or treating an
infectious
disease, which includes administering the polypeptide represented by the
general
sequence formula or an acetate salt substitution of trifluoroacetic acid
thereof into a
subject in need thereof
In addition, the present invention provides a use of the polypeptide
represented
by the general sequence formula or an acetate salt substitution of
trifluoroacetic acid
thereof for preventing, alleviating or treating an infectious disease.
Further, the present invention provides a use of the polypeptide represented
by
the general sequence formula or an acetate salt substitution of
trifluoroacetic acid
thereof for preparing a preparation for preventing, alleviating or treating an
infectious
disease.
In one embodiment of the present invention, the infectious disease is a gram-
negative bacteria infection-related disease selected from the group consisting
of
pneumonia, peritonitis, meningitis, wound infection, osteoarthritis,
cholecystitis,
urinary tract infection, endocarditis, myocarditis, pericarditis, arthritis,
pharyngitis,
gonorrhea, bacterial dysentery, enteritis, conjunctivitis, gastritis, otitis
media, cystitis,
and lymphangitis.
In one embodiment of the present invention, the infection disease is gram-
positive bacteria-infected disease selected from the group consisting of
laryngopharyngitis, impetigo, rheumatic fever, glomerulonephritis, neonatal
sepsis,
meningitis, pharyngitis, pneumonia, endocarditis, scarlet fever, SSTI(skin and
soft
tissue infection), deep soft tissue infection, empyema, and vaginitis.
12
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
In addition, the present invention provides a polynucleotide encoding the
polypeptide represented by the general sequence formula.
In addition, the present invention provides a recombinant vector including the
polynucleotide.
In addition, the present invention provides a method of preparing a
polypeptide
represented by the general sequence formula, which includes culturing host
cells
transformed with the recombinant vector.
[Advantageous Effects]
A peptide according to the present invention has excellent effects of not only
inhibiting the proliferation of standard bacteria and antibiotic-resistant
bacteria, but
also removing a bacteria-derived endotoxin, thereby exhibiting an excellent
sepsis
treatment effect, and when used in combination with an antibiotic, the peptide
can
minimize side effects caused by the antibiotic and thus can be effectively
used in
preventing or treating sepsis.
In addition, the peptide according to the present invention selectively has
excellent antibacterial activity against gram-positive/negative bacteria, and
thus can
be effectively used in an antibacterial composition against gram-
positive/negative
bacteria or in preventing or treating various infectious diseases caused by
gram
positive/negative bacteria.
13
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
In addition, the peptide according to the present invention is not only safe
for
the human body, but also exhibits stability against a proteolytic enzyme, and
thus can
be used for a variety of purposes.
[Description of Drawings]
FIG. 1 is a set of graphs illustrating the result of measuring hemolytic
activity
according to Example 4 of the present invention:
From top left to right, FP12-NH2 (SEQ ID NO: 6) / allD FP12-NH2 (SEQ ID
NO: 8); and FP13-NH2 (SEQ ID NO: 7)/ allD FP13-NH2 (SEQ ID NO: 9).
From bottom left to right, allD FP13 9A (SEQ ID NO: 10)/ allD FP13 9D
(SEQ ID NO: 18); and allD FP13 9W (SEQ ID NO: 11)/ allD FP13 9K (SEQ ID
NO: 12).
FIG. 2 is a set of graphs illustrating the result of measuring the secondary
structures of peptides by circular polarization dichroism spectroscopy
according to
Example 5 of the present invention.
FIG. 3 is a set of graphs showing the result of measuring the disruption
ability
of peptides with respect to liposomes mimicking a bacterial cell membrane
(top) and
a red blood cell membrane (bottom) according to Example 6 of the present
invention.
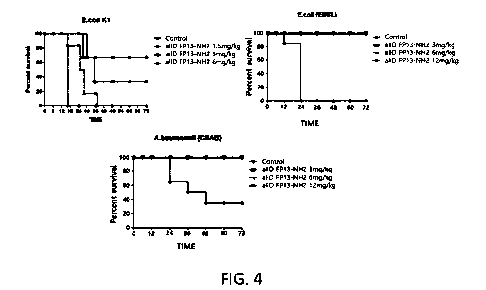
FIG. 4 illustrates the antibiotic effect of the allD FP13-NH2 peptide
according
to Example 8 of the present invention. From left to right, the antibiotic
effect of the
allD FP13-NH2 peptide against a standard strain Escherichia colt Kl; the
antibiotic
effect of the allD FP13-NH2 peptide against a clinical strain Escherichia colt
(ESBL);
and the antibiotic effect of the allD FP13-NH2 peptide against a clinical
strain
carbapenem-resistant Acinetobacter baummanii.
14
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
FIGS. 5A to 5C illustrate the antibiotic effect, toxic effect and
antiinflammatory cytokine-inhibitory effect of the allD FP13-NH2 (AcOH)
peptide
according to Example 9 of the present invention:
FIG. 5A shows the comparison of the antibiotic effects between AHD FP13-
NH2 (TFA)(top) and AHD FP13-NH2 (AcOH)(bottom) against Escherichia coil
(ESBL);
FIG. 5B shows the comparison of in vivo toxicity between AHD FP13-NH2
(TFA)(top) and AHD FP13-NH2 (AcOH)(bottom); and
FIG. 5C shows the comparison of anti-inflammatory cytokine (IL-6)-inhibitory
effects between AHD FP13-NH2 (TFA; top) and AHD FP13-NH2 (AcOH; bottom).
FIG. 6 is a set of graphs showing the result of measuring the disruption
ability
of peptides with respect to liposomes mimicking the cell membranes of gram-
positive
bacteria of embodiment 11 of the present invention.
[Modes of the Invention]
The present invention provides a polypeptide represented by the general
sequence formula as follows:
[General Formula]
Ln-X1-L-X2-V-X3-X4-X5-R-X6-L-X7
In the general formula,
n is 0 or 1;
L is leucine;
V is valine;
R is arginine;
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
X1 is lysine (K) or arginine (R);
X2 is glycine (G) or arginine (R);
X3 is glutamic acid (E) or lysine (K);
X4 is alanine (A) or leucine (L);
X5 is lysine (K) or arginine (R);
X6 is tyrosine (Y), alanine (A), tryptophan (W), lysine (K) or aspartic acid
(D);
and
X7 is aspartic acid (D) or arginine (R),
however, a polypeptide having an amino acid sequence of K-L-G-V-E-A-K-
R-Y-L-D is excluded.
In one embodiment of the present invention, one or more polypeptides of 9
types of the polypeptides represented by the general formula 1) to 9) as
follows:
1) a polypeptide represented by the general formula, in which
n is 0;
X1 is lysine (K);
X2 is glycine (G);
X3 is glutamic acid (E);
X4 is alanine (A);
X5 is lysine (K);
X6 is tyrosine (Y), and
X7 is arginine (R),
that is, a polypeptide having an amino acid sequence of KLGVEAKRYLR
(SEQ ID NO: 2);
16
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
2) a polypeptide represented by the general formula, in which
n is 1;
X1 is arginine (R);
X2 is glycine (G);
X3 is glutamic acid (E);
X4 is alanine (A);
X5 is lysine (K);
X6 is tyrosine (Y), and
X7 is arginine (R),
that is, a polypeptide having an amino acid sequence of LRLGVEAKRYLR
(SEQ ID NO: 3);
3) a polypeptide represented by the general formula, in which
nisi;
X1 is arginine (R);
X2 is glycine (G);
X3 is glutamic acid (E);
X4 is leucine (L);
X5 is lysine (K);
X6 is tyrosine (Y), and
X7 is arginine (R),
that is, a polypeptide having an amino acid sequence of LRLGVELKRYLR
(SEQ ID NO: 4);
17
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
4) a polypeptide represented by the general formula, in which
n is 0;
X1 is lysine (K);
X2 is glycine (G);
X3 is glutamic acid (E);
X4 is alanine (A);
X5 is leucine (L);
X6 is tyrosine (Y), and
X7 is aspartic acid (D),
that is, a polypeptide having an amino acid sequence of KLGVEALRYLD
(SEQ ID NO: 5);
5) a polypeptide represented by the general formula, in which
n is 0;
X1 is arginine (R);
X2 is arginine (R);
X3 is lysine (K);
X4 is leucine (L);
X5 is arginine (R);
X6 is tyrosine (Y), and
X7 is arginine (R),
that is, a polypeptide having an amino acid sequence of RLRVKLRRYLR
(SEQ ID NO: 15);
18
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
6) a polypeptide represented by the general formula, in which
n is 0;
X1 is lysine (K);
X2 is arginine (R);
X3 is lysine (K);
X4 is leucine (L);
X5 is arginine (R);
X6 is tyrosine (Y), and
X7 is arginine (R),
that is, a polypeptide having an amino acid sequence of KLRVKLRRYLR
(SEQ ID NO: 16);
7) a polypeptide represented by the general formula, in which
n is 0;
X1 is lysine (K);
X2 is arginine (R);
X3 is lysine (K);
X4 is leucine (L);
X5 is arginine (R);
X6 is alanine (A), and
X7 is arginine (R),
that is, a polypeptide having an amino acid sequence of KLRVKLRRALR
(SEQ ID NO: 10);
19
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
8) a polypeptide represented by the general formula, in which
n is 0;
X1 is lysine (K);
X2 is arginine (R);
X3 is lysine (K);
X4 is leucine (L);
X5 is arginine (R);
X6 is tryptophan (W), and
X7 is arginine (R),
that is, a polypeptide having an amino acid sequence of KLRVKLRRWLR
(SEQ ID NO: 11); and
9) a polypeptide represented by the general formula, in which
n is 0;
X1 is lysine (K);
X2 is arginine (R);
X3 is lysine (K);
X4 is leucine (L);
X5 is arginine (R);
X6 is lysine (K), and
X7 is arginine (R),
that is, a polypeptide having an amino acid sequence of KLRVKLRRKLR
(SEQ ID NO: 12).
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
The term "polypeptide" used herein refers to a linear molecule formed by
connecting amino acid residues by peptide bonds. The polypeptide of the
present
invention may be prepared by a chemical synthesis method known in the art
(e.g.,
solid-phase synthesis techniques) as well as a molecular biology method
(Merrifield,
I Amer. Chem. Soc. 85: 2149-54(1963); Stewart, et al., Solid Phase Peptide
Synthesis,
2nd. ed., Pierce Chem. Co.: Rockford, 111(1984)).
In addition, in the range of the polypeptide of the present invention, a
biologically functional equivalent having a variation in an amino acid
sequence
exhibiting equivalent biological activity to the polypeptide of the present
invention
may be included. The variation in an amino acid sequence may be generated
based
on the relative similarity, for example, hydrophobicity, hydrophilicity, a
charge or a
size, of an amino acid substituent. Through analyses on the size, shape and
type of
an amino acid side chain substituent, it can be seen that all of arginine,
lysine and
histidine are positively-charged residues; alanine, glycine and serine have
similar sizes;
and phenylalanine, tryptophan and tyrosine have similar shapes. Therefore,
based on
these considerations, the arginine, lysine and histidine; the alanine, glycine
and serine;
or the phenylalanine, tryptophan and tyrosine may be biologically functional
equivalents.
For introduction of the variation, a hydrophobicity index of amino acids may
be considered. A hydrophobicity index is assigned to each amino acid according
to
hydrophobicity and a charge. In addition, it is also known that substitution
between
amino acids having similar hydrophilicity result in peptides having equivalent
biological activity.
21
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
Amino acid exchange in peptides that do not entirely change molecular activity
is known in the art (H. Neurath, R. L. Hill, The Proteins, Academic Press, New
York,
1979). The most generally occurring exchange is the exchange between amino
acid
residues, for example, Ala/Ser, Val/Ile, Asp/Glu, Thr/Ser, Ala/Gly, Ala/Thr,
Ser/Asn,
Ala/Val, Ser/Gly, Thy/Phe, Ala/Pro, Lys/Arg, Asp/Asn, Leu/Ile, Leu/Val,
Ala/Glu,
and Asp/Gly.
Considering the variations with the above-described biologically equivalent
activity, the polypeptide of the present invention is construed to include a
sequence
exhibiting substantial identity with a sequence listed in the accompanying
sequence
listing. The substantial identity may refer to a sequence exhibiting at least
80%, 90%
or 95% homology when any different sequence is aligned as much as possible
with the
sequence of the present invention and is analyzed using an algorithm
conventionally
used in the art. An alignment method for sequence comparison is known in the
art
(Huang et al., Comp. App!. BioSci. 8:155-65(1992); Pearson et al., Meth. Mol.
Biol.
24:307-31(1994)).
In addition, in one embodiment of the present invention, the polypeptide
represented by the general sequence formula according to the present invention
may
be a peptidomimetic including an L-type peptide, a D-type peptide and a
peptoid, or
non-natural amino acids.
The D-type polypeptide is an enantiomer which has the same amino acid
sequence as the L-type polypeptide, and refers to an allomeric type
polypeptide.
22
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
In an exemplary embodiment of the present invention, for example, as an
allomeric type polypeptide which has the same amino acid sequence as FP12-NH2
(SEQ ID NO: 6), allD FP12-NH2 (SEQ ID NO: 8) is provided.
The term "allD" used to name a polypeptide herein refers to a D-type
polypeptide.
The polypeptide, which is a peptidomimetic including the L-type, D-type and
a peptoid, or non-natural amino acids, may be prepared by various methods
known in
the art according to a predetermined amino acid sequence.
In one embodiment of the present invention, an end of the polypeptide
represented by the general sequence formula according to the present invention
is
alkylated, PEGylated, or amidated.
In an exemplary embodiment of the present invention, in the polypeptide
according to the present invention, the N terminus of allD FP13-NH2 (AcOH)
(SEQ
ID NO: 13) was PEGylated, thereby providing PEG-allD FP13-NH2 (AcOH) (SEQ ID
NO: 14). For PEGylation, the polypeptide according to the present invention
may
react with Fmoc-NH-PEG2-CH2COOH, but the present invention is not limited
thereto.
Here, the molecular weight (MW) of polyethylene glycol is 385.4Da.
Conditions for PEGylation may be adjusted by those of ordinary skill in the
art
according to the selected polypeptide, and PEGylation may be performed by
various
methods known in the art.
Conditions for alkylation or amidation may also be adjusted by those of
ordinary skill in the art depending on the selected polypeptide, and the
alkylation or
amidation may be performed by various methods known in the art.
23
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
In one embodiment of the present invention, an amine group (NH2) may be
added to the C terminus of the polypeptide represented by the general sequence
formula according to the present invention.
In an exemplary embodiment of the present invention, as the polypeptide to
which an amine group (NH2) is added to the C terminus of FP12 (SEQ ID NO: 15)
in
the polypeptide according to the present invention, FP12-NH2 (SEQ ID NO: 6) is
provided.
The term "-N142" used to name a polypeptide herein refers to the C terminus of
the polypeptide to which an amine group (NH2) is added.
The addition of an amine group (NH2) to the C terminus of the polypeptide
may be performed by various methods known in the art.
In addition, in the range of the polypeptide of the present invention, a
pharmaceutically acceptable salt thereof may also be included.
The term "pharmaceutically acceptable" used herein refers to a peptide which
is suitable to be used in contact with tissue of a subject (e.g., a human) due
to a
reasonable benefit/risk ratio without excessive toxicity, stimulus, allergic
response or
other problems or complications, and is included in the scope of sound medical
judgment. The pharmaceutically acceptable salt includes, for example, an acid
addition salt formed by a pharmaceutically acceptable free acid and a
pharmaceutically
acceptable metal salt.
Examples of suitable acids may include hydrochloric acid, bromic acid,
sulfuric acid, nitric acid, perchloric acid, fumaric acid, maleic acid,
phosphoric acid,
24
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
glycolic acid, lactic acid, salicylic acid, succinic acid, p-toluenesulfonic
acid, tartaric
acid, acetic acid, citric acid, methanesulfonic acid, formic acid, benzoic
acid, malonic
acid, gluconic acid, naphthalene-2-sulfonic acid, and benzenesulfonic acid.
The acid
addition salt may be prepared by a conventional method, for example, by
dissolving a
compound in an excessive amount of acid aqueous solution, and precipitating
the salt
using a water-miscible organic solvent such as methanol, ethanol, acetone or
acetonitrile. In addition, the acid addition salt may be prepared by heating
equimolar
amounts of compound and an acid or alcohol in water, and evaporating and then
drying
the mixture, or suction filtering the precipitated salt.
Salts induced from suitable bases may include alkali metals such as sodium
and potassium, alkaline earth metals such as magnesium, and ammonium, but the
present invention is not limited thereto. The alkali metals or alkaline earth
metals
may be obtained by, for example, dissolving a compound in an excessive alkali
metal
hydroxide or alkaline earth metal hydroxide solution, filtering an undissolved
compound salt, and evaporating and drying the filtrate. Here, it is
pharmaceutically
suitable that a metal salt, particularly, a sodium, potassium or calcium salt
is prepared,
and in addition, a silver salt corresponding thereto may be obtained by
reacting an
alkali metal or alkaline earth metal salt with a suitable silver salt (e.g.,
silver nitrate).
As a pharmaceutically acceptable salt of the above-described polypeptide, the
present invention provides an acetate salt of the above-described polypeptide.
More specifically, an acetate salt substituent of trifluoroacetic acid (TFA)
of
the polypeptide according to the present invention is provided.
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
More specifically, the present invention provides an acetate salt of a
polypeptide represented by the above-described general formula for a sequence;
a
polypeptide, which is a peptidomimetic including an L-type peptide, a D-type
peptide
and a peptoid, or non-natural amino acids; a polypeptide which end of the
polypeptide
is alkylated, PEGylated or amidated; or a polypeptide to which an amine group
(NH2)
is added to the C terminus.
The term "AcOH" used to name a polypeptide herein means an acetate salt of
the polypeptide.
The polypeptide according to the present invention and a salt substitution
thereof have one or more characteristics as follows:
binding ability to lipopolysaccharide (LPS) of gram-negative bacteria;
selective disruption ability against bacterial cell membrane;
resistance to proteolytic enzymes;
cell membrane disruption ability against gram-positive bacteria; and
binding ability to gram-positive bacteria-derived lipoteichoic acid (LTA) or
lipoprotein (LPP).
In an exemplary embodiment of the present invention, the following
characteristics of the polypeptide according to the present invention and a
salt
substitution thereof were confirmed, but the present invention is not limited
thereto.
In an exemplary embodiment of the present invention, it was confirmed that
the polypeptide according to the present invention and a salt substitution
thereof has a
strong binding ability to lipopolysaccharide (LPS) (Example 1).
26
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
In addition, in an exemplary embodiment of the present invention, it was
confirmed that the polypeptide according to the present invention and a salt
substitution thereof has disruption ability against a bacterial cell membrane
(Examples
6 and 11). Particularly, it did not exhibit disruption ability against the
cell membrane
of a red blood cell, proving safety (Example 6).
In addition, in an exemplary embodiment of the present invention, it was
confirmed that the polypeptide according to the present invention and a salt
substitution thereof has excellent resistance to proteolytic enzymes (Example
7). Even
when treated together with a proteolytic enzyme, the polypeptide according to
the
present invention and a salt substitution thereof exhibits excellent
antibacterial activity,
confirming that stability against proteolytic enzymes is ensured.
In addition, in an exemplary embodiment of the present invention, it was
confirmed that the polypeptide according to the present invention and a salt
substitution thereof have excellent binding ability to lipoteichoic acid (LTA)
and
lipoprotein (LPP), which are components for the cell wall of gram-positive
bacteria
(Example 12).
In addition, in an exemplary embodiment of the present invention, it was
confirmed that the polypeptide according to the present invention and a salt
substitution thereof exhibit insignificant hemolytic activity, confirming that
toxicity is
very low (Example 4).
In addition, the present invention provides a composition for preventing,
alleviating or treating sepsis, which includes the polypeptide represented by
the
27
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
general sequence formula or an acetate salt substitution of trifluoroacetic
acid thereof
as an active ingredient.
In addition, the present invention provides a pharmaceutical/food/health
food/cosmetic/quasi-drug/feed composition for preventing, alleviating or
treating
sepsis, which includes the polypeptide represented by the general sequence
formula or
an acetate salt substitution of trifluoroacetic acid thereof as an active
ingredient.
The present invention provides a method of preventing or treating sepsis,
which
includes administering the polypeptide represented by the general sequence
formula
or an acetate salt substitution of trifluoroacetic acid thereof into a subject
in need
thereof
In addition, the present invention provides a use of the polypeptide
represented
by the general sequence formula or an acetate salt substitution of
trifluoroacetic acid
thereof for preventing, alleviating or treating sepsis.
Further, the present invention provides a use of the polypeptide represented
by
the general sequence formula or an acetate salt substitution of
trifluoroacetic acid
thereof for preparing a preparation for preventing, alleviating or treating
sepsis.
The term "sepsis" used herein refers to a condition in which a severe
inflammatory response occurs throughout the body through infection with
microorganisms. When two or more symptoms among fever in which a body
temperature increases to 38 C or more, hypothermia in which a body
temperature
decreases to 36 C or less, an increase in respiratory rate to 24 beats per
minute
(tachypnea), an increase in heart rate to 90 beats per minute (tachycardia),
or an
increase or significant decrease in the number of white blood cells are
observed by a
28
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
blood test, it is called systemic inflammatory response syndrome (SIRS). When
SIRS is caused by a microbial infection, it is called sepsis. Pathogens
continuously
or intermittently enter the bloodstream from infectious lesions of the body,
and settle
in various organ tissues, thereby forming lesions, and showing severe systemic
symptoms. Causative bacteria include Staphylococcus, Streptococcus,
Escherichia,
Pseudomonas aeruginosa, Mycobacterium tuberculosis, Klebsiella pneumoniae,
fungi,
and anaerobes, but the present invention is not limited thereto.
In addition, the present invention provides an antibacterial composition,
which
includes the polypeptide represented by the general sequence formula or an
acetate salt
substitution of trifluoroacetic acid thereof as an active ingredient.
In addition, the present invention provides an antibacterial
pharmaceutical/food/health food/cosmetic/quasi-drug/feed composition, which
includes the polypeptide represented by the general sequence formula or an
acetate salt
substitution of trifluoroacetic acid thereof as an active ingredient.
The present invention provides an antibacterial method which includes
administering the polypeptide represented by the general sequence formula or
an
acetate salt substitution of trifluoroacetic acid thereof into a subject in
need thereof
In addition, the present invention provides an antibacterial use of the
polypeptide represented by the general sequence formula or an acetate salt
substitution
of trifluoroacetic acid thereof
Further, the present invention provides a use of the polypeptide represented
by
the general sequence formula or an acetate salt substitution of
trifluoroacetic acid
thereof for preparing an antibacterial agent.
29
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
The term "antibacterial" or "antibacterial activity" used herein refers to a
characteristic of resistance against microorganisms such as bacteria or fungi,
and more
particularly, a characteristic of inhibiting the growth or proliferation of
bacterial by an
antibiotic material.
The term "antibacterial composition" used herein is a composition having
activity of suppressing the growth of microorganisms such as bacteria or
fungi, and
may include all types used in various fields requiring an antibacterial
effect, for
example, medicine, a cosmetic, a quasi-drug, a food additive or a feed
additive.
Specifically, such a composition may be used as an antibiotic or antifouling
agent in
medicines, for the purpose of a preservative or antibacterial purpose in food,
for the
purpose of an antibacterial, bactericidal or antiseptic effect in agriculture,
may be used
in products directly related to microorganisms for preventing dandruff,
preventing
athlete's foot, suppressing armpit odor, or anti-acne in cosmetics and
household
products, or used for the purpose of a preservative, antibacterial or
bactericidal effect
in a cleaning product or detergent for dishwashing, but the present invention
is not
limited thereto.
In one embodiment of the present invention, antibacterial targets of the
antibacterial composition according to the present invention include gram-
negative
bacteria, gram-positive bacteria, antibiotic-resistant gram-negative bacteria,
and
antibiotic-resistant gram-positive bacteria.
The term "gram negative bacteria" used herein refers to bacteria stained red
when stained by a gram staining method, and generally has strong pigment
resistance
and strong surfactant resistance. The gram-negative bacteria of the present
invention
include all types of gram-negative bacteria containing an endotoxin, for
example,
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
strains of the genus Escherichia, the genus Pseudomonas, the genus
Acinetobacter, the
genus Salmonella, the genus Klebsiella, the genus Neisseria, the genus
Enterobacter,
the genus Shigella, the genus Moraxella, the genus Helicobacter, the genus
Stenotrophomonas, the genus Bdellovibrio, and the genus Legionella, but the
present
invention is not limited thereto. Specifically, the gram-negative bacteria
include, but
is not limited to, Escherichia coli, Pseudomonas aeruginosa, Pseudomonas
jitiorescens, Pseudomonas putida, Pseudomonas chlororaphis, Pseudomonas
pertucinogena, Pseudomonas stutzeri, Pseudomonas syringae, Acinetobacter
baumannii, Acinetobacter lwoffii, Acinetobacter calcoaceticus, Acinetobacter
haemolyticus, Salmonella enterica, Salmonella bongori, Salmonella enteritidis,
Salmonella typhimurium, Salmonella gallinarum, Salmonella pullorum, Salmonella
mbandaka, Salmonella choleraesuis, Salmonella thompson, Salmonella infantis,
Salmonella derby, Klebsiella pneumoniae, Klebsiella granulomatis, Klebsiella
oxytoca, Klebsiella terrigena, Neisseria gonorrhoeae, Neisseria meningitidis,
Enterobacter aerogenes, Enterobacter cloacae, Shigella boydii, Shigella
dysenteriae,
Shigella jlexneri, Shigella sonnei, Moraxella catarrhalis, Moraxella lacunata,
lfforaxella bovis, Helicobacter pylori, Helicobacter heilmannii, Helicobacter
fells,
Helicobacter mustelae, Helicobacter fenelliae, Helicobacter rappini,
Helicobacter
hepaticus, Helicobacter bills, Helicobacter pullorum, Stenotrophomonas
maltophilia,
Stenotrophomonas nitritireducens, Bdellovibrio bacteriovorus, Legionella
pneumophila, Legionella anisa, Legionella birminghamensis, Legionella
bozemanii,
Legionella cincinnatiensis, Legionella dumoffii, Legionella feeleii,
Legionella
gormanii, Legionella hackelia, Legionella israelensis, Legionella jordanis,
Legionella
lansingensis, Legionella longbeachae, Legionella longbeachae, Legionella
micdadei,
31
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
Legionella oakridgensis, Legionella sainthelensi, Legionella tucsonensis, and
Legionella wadsw or thii
The term "gram positive bacteria" used herein refers to bacteria which are
stained royal blue or purple through gram staining, and may include strains of
the
genus Staphylococcus, the genus Enterococcus, the genus Streptococcus, and the
genus Clostridium, but the present invention is not limited thereto.
Specifically, the
gram-positive bacteria include Staphylococcus aureus, Staphylococcus
epidermidis,
Enterococcus faecium, Streptococcus pneumoniae, and Bacillus anthracis, but
the
present invention is not limited thereto.
The term "antibiotic-resistant gram-positive bacteria" used herein includes,
for
example, methicillin-resistant gram-positive bacteria, carbapenem-resistant
gram-
positive bacteria, vancomycin-resistant gram-positive bacteria, macrolide and
multidrug-resistant gram-positive bacteria, but the present invention is not
limited
thereto.
The "antibiotic-resistant gram-negative bacteria" used herein include, for
example, streptomycin-resistant gram-negative bacteria, colistin-resistant
gram-
negative bacteria, carbapenem-resistant gram-negative bacteria,
chloramphenicol-
resistant gram-negative bacteria, tetracyclines-resistant gram-negative
bacteria,
cefotaxime-resistant gram-negative bacteria, imipenem-resistant gram-negative
bacteria, ESBL-resistant gram-negative bacteria, tigecycline-resistant gram-
negative
bacteria and multidrug-resistant gram-negative bacteria, but the present
invention is
not limited thereto.
In one embodiment of the present invention, bacteria targeted by the
antibacterial composition may be one or more selected from the group
consisting of
32
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
Escherichia coli DH5 a, Escherichia coli Kl , Acinetobacter baumannii,
Pseudomonas
aeruginosa, Salmonella enteritidis, Salmonella typhimurium, Klebsiella
pneumoniae,
Staphylococcus aureus and Staphylococcus epidermidis
In addition, in one embodiment of the present invention, the bacteria targeted
by the antibacterial composition may be one or more antibiotic-resistant
bacteria
selected from the group consisting of ESBL-resistant bacteria, carbapenem-
resistant
bacteria and colistin-resistant bacteria. For example, the antibiotic-
resistant bacteria
may be ESBL(E. coli), carbapenem resistant (CR)-Acinetobactor baumannii, CR-
Klebsiella pneumoniae, CR-Pseudomonas aeruginosa, and colistin-resistant
Acinetobactor baumannii, but the present invention is not limited thereto.
In addition, the present invention provides a composition for preventing,
alleviating or treating an infectious disease, which includes the polypeptide
represented by the general sequence formula or an acetate salt substitution of
trifluoroacetic acid thereof as an active ingredient.
In addition, the present invention provides a pharmaceutical/food/health
food/cosmetic/quasi-drug/feed composition for preventing, alleviating or
treating an
infectious disease, which includes the polypeptide represented by the general
sequence
formula or an acetate salt substitution of trifluoroacetic acid thereof as an
active
ingredient.
The present invention provides a method of preventing or treating an
infectious
disease, which includes administering the polypeptide represented by the
general
sequence formula or an acetate salt substitution of trifluoroacetic acid
thereof into a
subject in need thereof
33
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
In addition, the present invention provides a use of the polypeptide
represented
by the general sequence formula or an acetate salt substitution of
trifluoroacetic acid
thereof for preventing, alleviating or treating an infectious disease.
Further, the present invention provides a use of the polypeptide represented
by
the general sequence formula or an acetate salt substitution of
trifluoroacetic acid
thereof for preparing a preparation for preventing, alleviating or treating an
infectious
disease.
The term "infectious disease" used herein refers to a disease caused by
disease-
causing pathogens such as viruses, bacteria, fungi, and parasites, which
spread to or
invade animals or humans, and for the purpose of the present invention, the
infectious
disease means all types of infectious disease caused by gram-negative bacteria
or
gram-positive bacteria.
For example, the infectious disease includes infectious diseases caused by
gram-negative bacteria, such as pneumonia, peritonitis, meningitis, wound
infection,
osteoarthritis, cholecystitis, urinary tract infection, endocarditis,
myocarditis,
pericarditis, arthritis, pharyngitis, gonorrhea, bacterial dysentery,
enteritis,
conjunctivitis, gastritis, otitis media, cystitis, and lymphangitis, but the
present
invention is not limited thereto.
For example, the infectious disease includes infectious diseases caused by
gram-positive bacteria, such as laryngopharyngitis, impetigo, rheumatic fever,
glomerulonephritis, neonatal sepsis, meningitis, pharyngitis, pneumonia,
endocarditis,
scarlet fever, SSTI(skin and soft tissue infection), deep soft tissue
infection, empyema,
and vaginitis, but the present invention is not limited thereto.
34
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
The pharmaceutical composition of the present invention may further include
suitable carrier, excipient and diluent, which are conventionally used in
preparation of
a pharmaceutical composition. The pharmaceutical composition according to the
present invention may be used in an oral formulation such as a powder, a
granule, a
tablet, a capsule, a suspension, an emulsion, a syrup, or an aerosol, an
external
preparation, a suppository, or a sterilized injectable solution according to a
conventional method.
Examples of carriers, excipients and diluents that can be included in the
pharmaceutical composition of the present invention may include lactose,
dextrose,
sucrose, sorbitol, mannitol, xylitol, erythritol, maltitol, starch, acacia
gum, alginate,
gelatin, calcium phosphate, calcium silicate, cellulose, methyl cellulose,
microcrystalline cellulose, polyvinyl pyrrolidone, water,
methylhydroxybenzoate,
propylhydroxybenzoate, talc, magnesium stearate, and mineral oil.
The pharmaceutical composition of the present invention may be formulated
with a diluent or an excipient such as a filler, a bulking agent, a binder, a
wetting agent,
a disintegrant, a surfactant, which are conventionally used. A solid
formulation for
oral administration may be a tablet, pill, powder, granules or a capsule, and
such a
solid formulation may be prepared by mixing at least one of excipients, for
example,
starch, calcium carbonate, sucrose, lactose and gelatin, with the active
ingredient.
Also, in addition to the simple excipient, lubricants such as magnesium
stearate and
talc may also be used. As a liquid formulation for oral administration, a
suspension,
oral liquids, an emulsion, or a syrup may be used, and a generally-used simple
diluent
such as water or liquid paraffin, as well as various types of excipients, for
example, a
wetting agent, a sweetener, a fragrance and a preservative may be included. A
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
formulation for parenteral administration may be a sterilized aqueous
solution, a non-
aqueous solvent, a suspension, an emulsion, a lyophilized product or a
suppository.
As the non-aqueous solvent or suspension, propylene glycol, polyethylene
glycol, a
vegetable oil such as olive oil, or an injectable ester such as ethyl oleate
may be used.
As a suppository base, Witepsol, macrogol, Tween 61, cacao butter, laurinum,
or
glycerogelatin may be used.
The term "prevention" used herein refers to all actions of preventing,
inhibiting
or delaying symptoms generated by a disease by administration of the
composition
according to the present invention.
The term "treatment" or "alleviation" used herein refers to all actions
involved
in improving or beneficially changing symptoms by administration of the
composition
according to the present invention.
The term "administration" used herein refers to the provision of the
composition of the present invention to a subject by a suitable method.
The term "subject" used herein refers to a target in need of prevention or
treatment. For example, the subject may be a mammal such as a human or a non-
human primate, a mouse, a dog, a cat, a horse, a sheep, or a cow.
The composition according to the present invention is administered at a
pharmaceutically effective amount. In the present invention, the
"pharmaceutically
effective amount" used herein refers to an amount sufficient for treating a
disease at a
reasonable benefit/risk ratio applicable for medical treatment, and an
effective dosage
may be determined by parameters including a type of a patient's disease,
severity, drug
activity, sensitivity to a drug, administration time, an administration route
and an
excretion rate, the duration of treatment and drugs simultaneously used, and
other
36
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
parameters well known in the medical field.
Specifically, the pharmaceutical
composition may be administered at a dose of 0.001 to 1,000 mg/kg, 0.01 to 100
mg/kg,
0.01 to 10 mg/kg, 0.1 to 10 mg/kg or 0.1 to 1 mg/kg once or in divided
portions a day.
In consideration of all of the above-mentioned parameters, it is important to
achieve
the maximum effect with the minimum dose without a side effect, and such a
dose may
be determined by one of ordinary skill in the art. Specifically, the effective
amount
of the pharmaceutical composition according to the present invention may be
determined according to a patient's age, sex, condition, body weight, an
absorption
rate of the active ingredient in the body, an inactivation rate, an excretion
rate, a type
of disease, or a concomitant drug.
The pharmaceutical composition of the present invention may be administered
into a subject via various routes. All administration routes may be expected,
and the
pharmaceutical composition of the present invention may be administered by,
for
example, orally, or intrarectal, intravenous, intramuscular, subcutaneous,
intrauterine,
intradural or intracerebrovascular injection.
The pharmaceutical composition according to the present invention may
further include one or more known materials effective in alleviating,
preventing or
treating sepsis or septic shock; preventing bacterial infection; and
alleviating,
preventing or treating an infectious disease.
The pharmaceutical composition of the present invention may further include
a bronchodilator, an antihistamine, or an anti-inflammatory analgesic drug in
addition
to the active ingredient.
For example, the bronchodilator may be a (3 agonist, an anticholinergic agent,
or methylxanthine, the antihistamine may be acrivastine, cetirizine,
desloratadine,
37
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
fexofenadine, levocetirizine, loratadine, mizolastine, alimemazine,
chlocertirizine,
clemastine, cyproheptadine, hydroxyzine, ketotifen or promethazine, and the
anti-
inflammatory analgesic drug may be aspirin, diclofenac, fenoprofen,
flurbiprofen,
ibuprofen, indomethacin, ketoprofen, naproxen, piroxicam, sulindac, celecoxib,
valdecoxib, or rofecoxib.
In an exemplary embodiment of the present invention, it was confirmed that
the polypeptide according to the present invention and a salt thereof have a
high
binding ability to LPS by measuring the binding ability of the polypeptide
according
to the present invention and a salt thereof with LPS (Example 1).
In addition, in an exemplary embodiment of the present invention, it was
confirmed that the polypeptide according to the present invention and a salt
thereof
exhibit excellent antibacterial activity (Examples 2, 3 and 10).
In addition, in an exemplary embodiment of the present invention, it was
confirmed that the polypeptide according to the present invention and a salt
thereof
exhibit a strong antibiotic effect (Examples 8 and 9).
In addition, the present invention provides a polynucleotide encoding the
polypeptide.
The term "polynucleotide" is a polymer of deoxyribonucleotides or
ribonucleotides, which are present in single or double strand(s). The
polynucleotide
encompasses an RNA genome sequence, DNA (gDNA and cDNA) and an RNA
sequence transcribed therefrom, and includes an analog of a natural
polynucleotide
unless otherwise specified.
38
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
The polynucleotide includes the nucleotide sequence, and also includes a
sequence complementary thereto. The complementary sequence includes not only a
perfectly complementary sequence, but also a substantially complementary
sequence.
it refers to a sequence capable of hybridizing with the nucleotide sequence
under
stringent conditions known in the art.
In addition, the polynucleotide may be modified. The modification includes
addition, deletion, non-conservative substitution or conservative substitution
of a
nucleotide. The polynucleotide encoding the amino acid sequence is interpreted
to
also include a nucleotide sequence exhibiting substantial identity to the
nucleotide
sequence. The substantial identity may be a sequence having at least 80%, 90%
or
95% homology when the nucleotide sequence is aligned to any different sequence
such
that they correspond to be maximal correspondence, and the aligned sequence is
analyzed using an algorithm conventionally used in the art.
In addition, the present invention provides a recombinant vector including the
polynucleotide.
The term "vector" used herein refers to a means for expressing a target gene
in
host cells. For example, the vector includes a plasmid vector, a cosmid
vector, a
bacteriophage vector, and viral vectors such as an adenovirus vector, a
retrovirus
vector and an adeno-associated virus vector. A vector that can be used as the
recombinant vector may be constructed by manipulating a plasmid often used in
the
art (e.g., pSC101, pGV1106, pACYC177, ColE1, pKT230, pME290, pBR322,
pUC8/9, pUC6, pBD9, pHC79, p1161, pLAFR1, pHV14, pGEX series, pET series or
pUC19), a phage (e.g., UkkA or M13) or a virus (e.g., CMV or SV40).
39
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
In the recombinant vector, a polynucleotide encoding the peptide may be
operatively linked to a promoter. The term "operatively linked" used herein
means a
function linkage between a nucleotide expression regulatory sequence (e.g.,
promoter
sequence) and a different nucleotide sequence. Therefore, the regulatory
sequence
may regulate the transcription and//or translation of the different nucleotide
sequence.
The recombinant vector may typically be constructed as a vector for cloning or
expression. The expression vector may be a conventional vector used to express
a
foreign protein in a plant, animal or microorganism in the art. The
recombinant
vector may be constructed through various methods known in the art.
In addition, the present invention provides a host cell transformed by the
recombinant vector.
As the host cell, any host cell known in the art may be used, and prokaryotic
cells, for example, strains of the family Enterobacteriaceae including E. coil
JM109,
E. coil BL21, E. coil RR1, E. coil LE392, E. coil B, E. coil X 1776, or E.
coil W3110,
a strain of the genus Bacillus such as Bacillus subtilis or Bacillus
thuringiensis,
Salmonella typhimurium, Serratia marcescens , and various Pseudomonas species
may
be used, and when transformed into eukaryotic cells, as host cells, yeast
cells
(Saccharomyces cerevisiae), insect cells, plant cells and animal cells, for
example,
SP2/0, Chinese hamster ovary (CHO) Kl, CHO DG44, PER.C6, W138, BHK, COS-
7, 293, HepG2, Huh7, 3T3, RN and MDCK cell lines may be used.
The present invention provides a method of preparing a polypeptide
represented by the general sequence formula according to the present
invention, which
includes culturing the host cells.
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
The insertion of the polynucleotide or a recombinant vector including the same
into host cells may be used by an insertion method widely known in the art.
The
delivery method may be, for example, a CaCl2 method or an electroporation
method
when the host cell is a prokaryotic cell, and microinjection, calcium
phosphate,
precipitation, liposome-mediated transfection and gene bombardment when the
host
cell is a eukaryotic cell, but the present invention is not limited thereto.
The method of selecting the transformed host cells may be easily carried out
by a method widely known in the art using a phenotype expressed by a selection
marker. For example, when the selection marker is a specific antibiotic-
resistant
gene, a transformant is cultured in a medium containing the antibiotic so that
the
transformant may be easily selected.
Hereinafter, to help in understanding the present invention, exemplary
examples will be suggested. However, the following examples are merely
provided
to more easily understand the present invention, and not to limit the present
invention.
Example 1: Measurement of LPS bindin2 ability of ADK-derived peptide
candidates for E. coli K1
FP1 (ADK 44-54) is denoted by wild type (WT), and peptides in which a point
mutation occurred at various sites in WT were manufactured (FP3, F135, FP6,
and FP9).
To increase the interaction with LPS in a residue sequence of FP3, peptides
were
designed based on FP3- and LPS-binding models (FP12-NH2 and FP13-NH2), and
peptides were designed by additionally introducing a non-natural amino acid
and an
amino acid isomer (allomeric D-type amino acid) (allD FP12-NH2, allD FP13-NH2,
41
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
allD FP-13-9a, allD FP-13-9w, allD FP-13-9k, and allD FP13-NH2 (AcOH)). In
addition, an N-terminus PEGylated peptide (PEG-alld FP13-NH2 (AcOH)) was
additionally designed, synthesized, and provided by ANYGEN, Co. Ltd. The
binding affinity between each of the provided peptides and lipopolysaccharide
(LPS)
was confirmed through measuring the binding affinity (Kd) using Isothermal
Titration
Calorimeter (ITC).
Specifically, a method of analyzing binding affinity (Kd) between a peptide
and LPS is as follows.
Measurement was carried out using a Malvern MicroCal PEAQ-ITC cell
instrument, and the following pretreatment was carried out to confirm
interaction with
LPS. The amount and morphology of a sample cell are 300 pL and coin-shaped,
fixed-in-place, respectively; a syringe rotation rate was 1,200 rpm; and a
temperature
was 30 C, 35 C or 25 C.
Before the experiment, LPS and the peptide were previously diluted with PBS,
thereby preparing 2 mM LPS and 0.2 mM peptide. 300 pL of the peptide was added
into cells in the ITC instrument, which had been washed, and 40 pL of LPS was
put
into a syringe. After measurement conditions for ITC (temperature, number of
injections) were set, the syringe was inserted into the cell, and ITC
measurement was
started. When the measurement was completed, analysis is performed, and the Kd
values of LPS and the peptide were calculated.
As a result of measurement, it was confirmed that all types of peptides of
FP3,
F135, FP6, FP9, FP12-NH2, FP13-NH2, allD FP12-NH2, allD FP13-NH2, allD FP-13-
9a, allD FP-13-9w, allD FP-13-9k, and allD FP13-NH2 (AcOH) have strong LPS
binding affinity, which is equal to or higher than that of FP1 (wild type,
WT).
42
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
[Table 1]
SequenceT .= 1
ID No. PePtide i Sequence,
Kd (calculated by ITC) :
1 F( W1) KLGVEAKRYLD 2,38X ID"' '
2 "r FP3 ___ KLGVEAKRYLR ______ 3,32X10-'
Fps LRL.GvEAKRyLiz 8.54X10-4
1 4 FP6 . LRLGVELKRYLR 1 11X10 5 :
' FP9 KLGVEALRYLD 18.26X10-4 .:
,
8. FP12-N.H2 RIRVKLRRYLR-NH2 .2,42X104
. '.7'.' , =FP13-M42 . KLRVKIRRYLR-NH2 1,18X lie
.
8 =iailD FP12-NH2 RLRVKLRRYLR-N Ii2
________________________________ fall d-forrro ______
KIRVKLRRYLP-NH2 '
.S4 :011D F17-i -NH,z . . ' ' 4.7forio--
,..., .. _, ,, (ail d-forieri--
. :/0 atiCY'FP-15'aap. :tdrv*rar-NH2
N=142 leg d-form)
all0 FP-13-947 kin/ ki rrd ir- NI-42
II 1,74X10
_______________ : _ Nii2 __ _ (ail d-formL
12 all D FP-139k -- Id rvklr rld r-
NI-12¨ 199X10.4
;=,i-I-12 (ail d-form)
[ 13 :MID Fpn-N1.12 KiRVKLRRYLR4IH2
1,95Xle
...1 .(1/401-1) j... (A d-form)
[Table 2]
Sequence
Pepti:de Sequence Kd (calculated by ix).
, 1 1 FP(WT) -KLGVEAKRYLD 2.88)0 CI4
PEG-KLRVKIRRYLR-NH2
= PEG-aild FP13-NH2
: 14 (a3d-forrn) 1.78Kie
(Ac01-1)
.. PEG: Frqoc-NH-PEG
5
(In Tables 1 and 2, allD indicates a D-type amino acid)
43
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
(In Tables 1 and 2, (AcOH) indicates a salt substitution in which
trifluoroacetic
acid (TFA) at a terminus of the 9th amino acid from the N terminus of a
peptide is
substituted with an acetate salt.)
(In Table 2, PEG indicates PEGylation. Information on peptide N-terminus
PEGylation: polyethylene glycol (PEG), molecular weight (Mw)=385.4Da, Fmoc-
NH-PEG2-CH2COOH)
Example 2: Evaluation of comparison in antibacterial activity of allD
FP13-NH2 and four commercially available antibiotics against gram-
positive/ne2ative standard strains
The antibacterial activity of one peptide (allD FP13-NH2) that had been
confirmed to have strong affinity for LPS among the peptides designed in
Example 1
and four commercially available antibiotics against gram-positive/negative
standard
strains was compared.
To compare the antibacterial activity between the four commercially available
antibiotics (ampicillin, gentamicin, levofloxacin and imipenem) and the allD
FP13-
NH2 (AcOH) peptide, an antibacterial activity test was performed to measure
the
minimum inhibitory concentrations (MIC50 and MIC80) for gram-negative and gram-
positive standard strains (Escherichia coil DH5a, Escherichia coil Kl,
Acinetobacter
baumannii, Pseudomonas aeruginosa, Salmonella enteritidis, Salmonella
typhimurium, and Klebsiella pneumoniae).
More specifically, 100 pL of a medium was dispensed into each well of a 96-
well plate, and in the first column, an antibiotic and a peptide in 200 pL of
the medium
were dispensed. 1/2 serial dilution was performed from columns 1 to 11, and
gram-
44
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
negative and gram-positive bacteria which had been previously cultured were
diluted
(OD600nm value in dilution: approximately 0.0025) and dispensed into each
well. The
plate was incubated in a 37 C incubator for 4 hours, and measured at OD600nm.
A
bacterial growth rate was determined as 100% with the OD600nm value of a blank
(well
containing only medium and bacteria), and MICso was determined by confirming
the
concentrations of the antibiotic and peptide having an OD600nm value at which
a growth
inhibition rate of 50% was shown, and MICso was determined by confirming the
concentrations (p.g/m1) at which a growth inhibition rate of 80% was shown.
As a result, by comparing the allD FP13-NH2 (AcOH) peptide with the four
commercially available antibiotics (ampicillin, gentamicin, levofloxacin and
imipenem), it was confirmed that the allD FP13-NH2 (AcOH) peptide exhibits the
same or superior antibacterial activity against all seven types strains of
gram-
positive/negative bacteria.
[Table 31
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
Organisms ma anlitnicrabfal agent titc. 41u.40)
Togmg .raopt :51P.,.'g 8.81,
Esµtheritiiia ti,418. IRLAniATCC PTA 4750
Ø:1,i-: 812E:- ige ..,=:40.
"
.",-.0,.
88cmi,,yrz f2i -428 ,,e,4.8 4. te.
elYa rt"141142 8,126-i28 41.8 -2.0
EstirettrNIF468 it
p8r8:r1
PpµA:Mi,..Iti 1:02in C:0,$. ji0
6.12?:: = pe ..4-ttr,!: :-10
Emice,f1.11) kEM. -:128 ii a..5 ===,&
el6FPfINP.2 8:126 ,:.128 .k.Z0 0:4.8
:Atiferamagoritioiagivi(Amdiitifii
limpic,i0. 0:125 , .i0
r&.:=-=.:..1)* 8:1r -114 $48 :21,
Lc, :.:!,.=:,:i0r1- q.1.,?.t ' 1:,48 ..:u: .vto
giarm=34v02 11.47s.-:425 k4
:PA.OttriiiikkA Mot (Ma:010pm
Alpi,:d,r, 0.2i, 4 ,IK,eltect -qoAca
4'?.÷.31i.>:10- 8121:,. - Rs =-k.- k5 = =
to
10w:fr.:Ili kaa , in *12. -.174.
staFt^l341142 0:121-,428 Alti. iet 8
SebOrteresiii8efirithe likTI8C:OM
krio::Aik:: tilz 7:i20 ...,=:48 ' 5',8
C.
Vveto:,3".81 iiili .. Oa 4= t$: = ,I-J3
en FP1S:M.d. 0:178;,:i72 ...ilila ,<AO
Sainivalialyptittaition.Vaet 5364i.
i:fp030::5, 8.124::=;::128 ,;v1; R *2,0
cierOmeil OS '4,21 ,-0..& :*13
Lt:c..1,:=:,:slai 812=:,:- 128 .(:8.$ = :t:ta
knit:c.;Eiti 842s, -128 .iAil is.:88
. . ..
measiek maimoniasfiacc ttote,)
Arapsc.,14 kilt,'128 -tuikfifec8 Oa
Old
Go.e.u::,:in 812:- -,t28 =kt.6 :'t 14
644 -In ==2,8 c8.8
811DTPUilh:2
Example 3: Evaluation of comparison in antibacterial activity of peptides
and three antibiotics a2ainst antibiotic-resistant bacteria (ESBL and
5 carbapenem-resistant bacteria)
To analyze the antibacterial activity of 9 types of peptides, the
antibacterial
activity against bacteria resistant to ampicillin (AMP), imipenem, colistin,
and other
five antibiotics (ESBL-E. coil, carbapenem resistant A. baumannn, carbapenem
46
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
resistant Klebsiella pneumoniae, carbapenem resistant Pseudomonas aeruginosa,
and
colistin resistant Acinetobactor baumannii) was confirmed.
The antibacterial activity was evaluated according to an antibacterial
activity
test method for measuring the minimum inhibitory concentration 50 (MIC5o; the
minimal concentration that kills 50% of bacteria).
More specifically, 100 pL of a medium was dispensed into each well of a 96-
well plate, and in the first column, an antibiotic and a peptide in 200 pL of
the medium
were dispensed. 1/2 serial dilution was performed from columns 1 to 11 and
five
types of antibiotic-resistant bacteria (ESBL-resistant E. colt, carbapenem-
resistant A.
baumannii, carbapenem-resistant Klebsiella pneumoniae, carbapenem-resistant
Pseudomonas aeruginosa, colistin-resistant Acinetobactor baumannii), which had
been previously cultured, were diluted (0D600nm value in dilution:
approximately
0.0025) and dispensed into each well. The plate was incubated in a 37 C
incubator
for 4 hours, and measured at OD600nm. A bacterial growth rate was determined
as
100% with the OD600nm value of a blank (well containing only medium and
bacteria),
and a MIC50 value was measured by confirming the concentrations of an
antibiotic and
a peptide which have an OD600nm value at which a growth rate of 50% was shown.
As a result, it was confirmed that the peptide according to the present
invention
has improved antibacterial activity compared to the three comparative
antibiotics
(ampicillin (AMP), imipenem and colistin). Particularly, it was confirmed that
the
peptide according to the present invention also has antibacterial activity
against
bacteria resistant to these antibiotics (particularly, a carbapenem or
colistin-resistant
strain).
47
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
[Table 4]
= Carbapenern
Drugs ESBL (E.
resistant (CR)= CR-Kiebsiella Colistin
resistant
col0 Aeinetobattor pneutnoniae AB
imomannii
AMP No effect < 16ug,im1 No effect
No effect
Imipenem No effect No effect No effect
No effect
Colistin < 0.Sugjml < lugjrni < 125ug/a
No effect
FP12 < 2ugfrni < 16ugirni < 62q/oil <
125tgimi
FPI 3 .< 2ogirni < 16ugjrn1 62ugirni <
62ugirni
1.7 FP-12-NH2----* < 0.5ugiml < 4ug/rni < 8LIg/m1 < 16ug/mi
MID FP12-NH2 < 0.5ug,fm1 4ug/rni < 32tig/tni
< 16uolmi
cUD FP13 NH2 < 0.5ugiml 44.ig/rn1 < 8tIcyrn1
< -IWO&
aliD FP21-NH2 < 0.54m1 < 1 ug/mi 4ug/m1 <
ailD 1P13-NH2 lugimi < lug/m1 < 32ugimi <
16ugirni
(AcOH)
Example 4: Measurement of hemolytic activity of peptide
The following experiment was carried out to determine the toxicity of a
peptide
through measurement of the red blood cell hemolytic activity in a human.
The red blood cells in a human were diluted with PBS for washing, and
centrifuged for 10 minutes, the washing process was repeated three times. The
8.0%
red blood cell solution diluted with PBS was loaded into a 96-well microtiter
plate by
100 p1_, and 100 p1_, of a peptide solution was mixed, the mixture was
incubated at 37 C
for 1 hour, and then the 96-well microtiter plate was centrifuged for 5
minutes. A
100 p.1_, of supernatant was taken and transferred to another 96-well
microtiter plate,
and then absorbance was measured at 405 nm. Here, a value obtained when
treated
with 0.1% Triton X-100 was calculated as 100% hemolysis, and the hemolytic
activity
of the peptide was calculated as % hemolysis by Equation 1 below.
[Equation 11
48
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
A ¨ C
Cell disruption rate (% Hemolysis) = _________________ x 100
B ¨ C
Here, A is the absorbance at 405 nm in a peptide solution, B is the absorbance
at 405 nm in a 0.1% Triton X-100, and C is the absorbance at 405 nm in a PBS
solution.
Here, as a peptide for a control, melittin, which is an antibacterial peptide
exhibiting strong antibacterial activity and strong hemolytic activity, was
used.
As a result, as shown in FIG. 1, it can be seen that the peptides according to
the present invention exhibited almost no hemolytic activity even at a high
concentration of 100 p.M. On the other hand, melittin as a control showed 100%
hemolytic activity even at a low concentration, indicating very high toxicity.
Example 5: Measurement of secondary structure of peptide usin2 circular
polarization dichroism spectroscopy
To measure the secondary structure of the FP12-NH2 and FP13-NH2 peptides,
an experiment was carried out as follows.
To study the secondary structure of the peptides in a biomembrane-like
environment, the peptides were dissolved in a 0.3 mL solvent such that the
concentration of the peptides became 100 p.M per sample in an aqueous
solution, 100
mM SDS, or 50 mM DPC. 1 mm path length cells were analyzed using a Jam) J-720
circular dichroism spectrophotometer, thereby obtaining an absorption value
per 0.1
nm at an injection rate of 100 nm/min, and six injections were averaged,
thereby
obtaining a measurement value.
Circular polarization dichroism spectroscopy shows a characteristic absorption
pattern depending on the secondary structure of the backbone of a polypeptide.
The
49
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
FP12-NH2 and FP13-NH2 peptides did not have a secondary structure in an
aqueous
solution, but had two minimal points at wavelengths of approximately 208 and
222
nm, which is the characteristic absorption pattern of an cc-helix structure in
DPC
micelles, which is a biomembrane-like environment (FIG. 2).
From the above results, it can be predicted that these peptides will exhibit
antibacterial activity while forming cc-helix structures in the biomembrane of
bacteria.
On the other hand, it can be seen that allD (FP12-NH2) and allD (FP13-NH2)
peptides,
which were subjected to D-amino acid substitution, exactly have mirror images
of a
parent peptide. In contrast to the CD spectrum of the parent peptide showing a
right-
handed cc-helix structure, the peptides have negative intensity, indicating
that they have
a left-handed cc-helix structure (FIG. 2).
Example 6: Measurement of disruption ability of peptides a2ainst
liposomes mimicking gram-negative bacteria cell membrane and red blood cell
membrane
To study an action mechanism for FP12-NH2, FP13-NH2, allD FP12-NH2, and
allD FP13-NI-12 peptides, the disruption ability of the peptides against
liposomes
mimicking a bacterial cell membrane and a red blood cell membrane was
measured.
To confirm whether the antibacterial action of the peptides exhibit the action
mechanism of killing bacteria by targeting the bacterial cell membrane,
liposomes
encapsulating a fluorescent dye, calcein, which consist of EYPC/EYPG (7:3,
w/w)
mimicking the cell membrane of gram-negative bacteria and EYPC/CH (1:10, w/w)
mimicking a human red blood cell were manufactured, respectively, to perform a
fluorescent dye leakage assay.
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
EYPG is an abbreviation for egg yolk L-a-phosphatidyl-DL-glycerol, and
EYPC is an abbreviation for egg yolk 1,2-diacyl-sn-glycero-3-phosphocholine.
CH
is an abbreviation for cholesterol. After dissolving the phospholipids
composed
according to the content of each component in chloroform, the chloroform was
removed using a rotary evaporator, and lyophilization was performed overnight.
Afterward, the dried lipid film was dissolved in a Tris-buffer solution, and
calcein was
dissolved in liquid nitrogen. After repeated freezing and thawing, the calcein-
encapsulated liposome was manufactured using an extruder. Subsequently, after
an
excitation wavelength of a spectrofluorimeter (Shimadzu RF 5301 PC
spectrofluorimeter, Japan) was set to 490 nm, and an emission wavelength was
set to
520 nm, the peptide was administered to the liposome to measure relative dye
leakage,
provided that a 100% dye leakage was expressed as fluorescence intensity when
0.01%
Triton-Xioo was added.
The result is shown in FIG. 3. The upper graphs of FIG. 3 show the % dye
leakage for an EYPC/EYPG (7:3, w/w) liposome mimicking bacterial cells,
encapsulating a fluorescent dye, calcein, by a peptide according to a peptide
concentration. Here, as a control (peptide exhibiting the action mechanism of
cell
membrane targeting), an antibacterial peptide, melittin, was used. The lower
graphs
of FIG. 3 show the % dye leakage for an EYPC/CH (10:1, w/w) liposome, which is
a
triglyceride mimicking a human red blood, encapsulating a fluorescent dye,
calcein,
by a peptide according to a peptide concentration. Here, the antibacterial
peptide,
melittin, was used as a control (peptide exhibiting the action mechanism of
cell
membrane targeting).
51
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
As shown in FIG. 3, a control peptide is responsible for calcein leakage from
a
liposome consisting of PC/PG (7:3, w/w) mimicking a bacterial cell membrane
even
at a low concentration. For a PC/CH (10:1, w/w) liposome mimicking a human red
blood cell, since almost 100% calcein leakage may be shown at 0.25 04
melittin, it
may be predicted that there is no selectivity for bacterial cells, and
toxicity is very high.
On the other hand, the FP12-NH2, FP13-NH2, allD FP12-NH2, and allD FP13-
NH2 peptides caused high dye leakage for a PC/PG (7:3, w/w) liposome mimicking
a
bacteria cell membrane, indicating high ability of disrupting a bacterial cell
membrane.
The FP12-NH2, FP13-NH2, allD FP12-NH2, and allD FP13-NH2 peptides selectively
show almost no dye leakage for a PC/CH (1:1, w/w) liposome mimicking a red
blood
cell membrane in a mammal, which is consistent with low hemolytic activity.
Therefore, it was seen that the peptides according to the present invention
have an
action mechanism of selectively disrupting a bacterial cell membrane.
Example 7: Evaluation of stability throu2h measurement of proteolytic
enzyme resistance of peptide
Stability in blood was confirmed through measurement of proteolytic enzyme
resistance of the designed allD FP12-NH2 and allD FP13-NH2 peptides.
More specifically, to confirm resistance to three types of proteolytic enzymes
such as trypsin, protease K, and chymotrypsin, a proteolytic enzyme was
treated, and
then the antibacterial activity of the peptides against E. coil, A. baumanna
and S.
aureus was measured.
52
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
As a result, it was confirmed that the allD FP12-NH2 and allD FP13-NH2
peptides designed through D-amino acid substitution, a non-natural amino acid,
have
much improved resistance to the proteolytic enzyme.
In the experimental data for E. coil and A. baumannn shown in Table 5, the
allD FP12-NH2 and allD FP13-NH2 peptides showed antibacterial activity which
is
similar or reduced 2- to 4-fold compared to a case without proteolytic enzyme
treatment, confirming that the peptides are highly functional peptides with
very high
stability.
In addition, in the experimental data for S. aureus shown in Table 6, it was
also
confirmed that the allD FP12-NH2 and allD FP13-NH2 peptides are highly
functional
peptides with very high stability.
[Table 5]
alID FP12-NH2 alID FP13-
NH2
MIC(uM)
MIC50 h/HC90 M1050 MIC90
Eco/i 2 4 2 4
E.co/i+ 0.8uM trypsin 4 8 8 16
Ecoli + 0.8uM protease K 2 4 4 6(4.-8)
Ecoll + 0.8uM Chymotrypsin 4 8 2 6(4-8)
Abaumannii 4 8 4 a
Abaumannii + 0.8uM trypsin 8 32 8 32
ABaumannll + 0.8uM protease K 8 16 8 , 16 ,
Abaumannii + 0.8uM Chymotrypsin 8 16 8 16
[Table 61
53
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
ailD FP12-NH2 ailD FP13-NH2
MIC(uM)
MICSO MIC90 MIC50 MIC90
S. aureus 4 8 4 8
S. aureus + 0.8a4 trypsin 4 8 4 8
S. aureus + 0.8uM protease K 4 8 4 8
S. aureus + 0.8uM Chymotrypsin 4 8 4 8
Example 8: Antibiotic effect of allD FP13-NH2 on standard strain E. coli
K1 and two types of clinical strains
To evaluate the antibiotic activity of a peptide, the antibiotic effect and
therapeutic effect of a peptide on E. colt K1 (standard strain), E. colt
(ESBL) and
carbapenem-resistant bacteria (carbapenem-resistant Acinetobacter baummanii
(CRAB)) were verified through an animal experiment.
Except E. colt K1 (standard strain), E. colt (ESBL) and carbapenem-resistant
bacteria (CRAB) were prepared from antibiotic-resistant bacteria obtained from
patients with severe infection provided from the Division of Infection Disease
of the
Korea University Medical Center. The experimental bacteria were obtained by
collecting 10 to 20 morphologically identical colonies, inoculated into a test
tube
containing 10 mL of a suitable liquid medium (Mueller Hinton Broth), and
cultured in
a 37 C incubator for 12 hours. The liquid cell culture was placed in
a sterilized
cryo tube by 1 mL and stored at -80 C.
The test strain was cultured for 2 days in a 37 C incubator [blood agar plate
(BAP)], prepared in a Mueller Hinton Broth (MHB) liquid medium according to
the
amount of the inoculated strain (1x108 or 8x107), and intraperitoneally
injected (1 mL)
into 6-week-old mice to induce infection. The mice were divided into groups
per
experiment (n=5 or 6 per group), and allD FP13-NH2 mixed with a HARTMANN'S
54
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
DEX solution (vehicle) was injected intraperitoneally into mice six times at
0, 1, 2, 3,
4, or 5 hours, followed by observing a survival rate at intervals of 12 hours
for 3 days.
As a result, in a sepsis animal model into which each of Escherichia coil Kl,
Escherichia coil (ESBL), and carbapenem-resistant Acinetobacter baumannii was
infected, an increase in survival rate (antibiotic effect) was confirmed
within a
concentration range from 3 mg to 12 mg per kg (FIG. 4). It was verified that
the
peptide designed in Example 1 also exhibits in vivo antibiotic and anti-septic
effects
in the standard strain and clinical strain-infected animal models.
Example 9: Acetate salt substitution of peptide
To confirm the therapeutic and pharmaceutical effects of an acetate salt
substitution of allD FP13-NH2, a septic animal model induced by
intraperitoneally
administering each of the Escherichia coil (standard strain and ESBL) strains
or a
normal mouse was treated with the peptide allD FP13-NH2 (AcOH). The antibiotic
effect, the toxicity, and the inhibitory activity of anti-inflammatory
cytokine IL-6 of
the salt substitution with those of the same peptide at the end of
trifluoroacetic acid
(TFA) were compared.
An experimental method is as follows:
Six-week-old female ICR mice (SPF mice, Samtako) with an average body
weight of 25 g were used as experimental animals. The experimental animals
were
provided with sterile feed and water and bred with less than 10 mice per cage,
and a
day/night cycle was 12 hours.
The test strain was cultured for 2 days in a 37 C incubator [blood agar plate
(BAP)], prepared in a Mueller Hinton Broth (MHB) liquid medium according to
the
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
amount of the inoculated strain (1x108), and intraperitoneally injected
(1x108/200 pL)
into 6-week-old mice to induce infection. The mice were divided into groups
per
experiment (n=5 or 6 per group), and intraperitoneally injected 6 times with
allD FP13-
NH2 (TFA) and allD FP13-NH2 (AcOH) mixed with HARTMANN'S DEX solution
(vehicle) at 0, 1, 2, 3, 4, and 5 hours, followed by observing a survival rate
at intervals
of 12 hours.
To evaluate in vivo toxicity, the mice were divided into groups (n=5 or 6 per
group) per experiment, and intraperitoneally treated six times with allD FP13-
NH2
(TFA) and allD FP13-NH2 (AcOH) mixed with HARTMANN'S DEX solution
(vehicle) at 0, 1, 2, 3, 4, or 5 hours, followed by observing a survival rate
at intervals
of 12 hours for 72 hours.
In addition, an IL-6 secretion test for bone marrow-derived dendrite cells
(BMDCs) was carried out by comparatively analyzing cytokines secreted due to
LPS
30 minutes after peptide treatment through the evaluation of LPS-mediated
immune
responses of allD FP13-NH2 (TFA) and allD FP13-NH2 (AcOH).
As a result, according to the antibiotic effect and toxicity test for a sepsis
animal
model (E. coil ESBL-induced model), since ED50 is approximately 1 mpk (mg/kg),
LD5o is 80 mpk, and a ratio of a drug dose exhibiting toxicity to a drug dose
for
exhibiting a desired response (therapeutic index = LD5o/ED5o) is greater than
80, it is
expected that a sufficient safety margin will be ensured, confirming that
there is an
excellent possibility of developing a novel antiseptic drug. In addition, it
was
confirmed that allD FP13-NH2 (AcOH) significantly reduces the secretion of IL-
6
increased due to LPS (FIGS. 5A to 5C).
56
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
Example 10: Evaluation of antibacterial activity of allD-type peptide
a2ainst three types of 2ram-positive bacteria (two types of S. aureus and S.
epidermidis)
To analyze the antibacterial activity of an allD-type peptide against gram-
positive bacteria, the antibacterial activity against three types of gram-
positive bacteria
(two types of S. aureus and S. epidermidis) was confirmed.
To measure minimum inhibitory concentrations (MIC50, minimum
concentration for killing 50% of bacteria; MICioo, minimum concentration
required
for completely killing bacteria), an antibacterial activity experiment method
was
evaluated.
More specifically, 100 pL of a medium was dispensed into each well of a 96-
well plate, and in the first column, an antibiotic and a peptide in 200 pL of
the medium
were dispensed. 1/2 serial dilution was performed from columns 1 to 11 and
three
types of gram-positive bacteria (two types of S. aureus and S. epidermidis)
which had
been previously cultured were diluted (0D600nm value in dilution:
approximately
0.0025) and dispensed into each well. The plate was incubated in a 37 C
incubator
for 4 hours, and measured at OD600nm. A bacterial growth rate was determined
as
100% with the OD600nm value of a blank (well containing only medium and
bacteria),
and the concentrations of the antibiotic and peptide having an OD600nm value
at which
the growth inhibition rate was 50% or 100% were determined to calculate MIC50
and
MIC 100 values.
Consequently, as a result of analyzing antibacterial activity against gram-
positive bacteria, it was confirmed that three types of peptides (allD FP12-
NH2, allD
FP13-NH2, and allD FP21-NH2) to which an non-natural amino acid and an amino
57
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
acid isomer (allomeric D-type amino acid) were further introduced exhibit
excellent
antibacterial activity against gram-positive bacteria at the same level as
melittin.
[Table 7]
8. auteus avreus S. epidermidis
Drugs KCTC10.21 CCARM3708 CCARMS700
MIC50 M1C100 MIC50 MIC100 MIC50 141C100
atID-FP12-NH2 4 10 4 8 1 2
aND-FP13-N112 4 8 2 4 <1
alID-FP21--N1-12 _______ 4 8 2 4 _________ 1 2
L Melttin 1 .8 05 1 0,5 1
Example 11: Measurement of disruption ability of peptide a2ainst
liposome mimicking gram-positive bacterial cell membrane
To study the action mechanisms for FP12-NH2, FP13-NH2, allD FP12-NH2,
and allD FP13-NH2 peptides, the disruption ability of the peptides against a
liposome
mimicking a bacterial cell membrane was measured.
To perform an experiment of confirming whether the antibacterial action of a
peptide exhibits an action mechanism of killing bacteria by targeting the cell
membrane of gram-positive bacteria, a liposome consisting of EYPG/EYPC (6:4,
w/w)
mimicking the cell membrane of gram-positive bacteria, encapsulating a
fluorescent
dye, calcein, was manufactured for a dye leakage assay.
As a result, the, FP12-NH2, FP13-NH2, allD FP12-NH2 and allD FP13-NH2
peptides caused high dye leakage for an EYPG/EYPC (6:4, w/w) liposome
mimicking
a bacterial cell membrane, indicating excellent ability of disrupting a
bacterial cell
membrane. Therefore, it can be seen that the FP12-NH2, FP13-NH2, allD FP12-
NH2,
and allD FP13-NH2 peptides according to the present invention have an action
mechanism of disrupting a gram-positive bacterial cell membrane (FIG. 6).
58
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
Example 12: Measurement of 2ram-positive bacteria-derived LTA and
LPP bindin2 ability of allD FP13-NH2
The following experiment was carried out to confirm the action mechanism by
confirming the antibacterial activity of a peptide against gram-positive
bacteria.
The binding affinity to lipoteichoic acid (LTA) and lipoprotein (LPP), which
are cell wall components of gram-positive bacteria (S. aureus), was confirmed
through
ITC analysis using allD FP13-NH2.
The binding affinity was confirmed using the ITC analysis method, and the
experimental method in Example 1 was applied mutatis mutandis.
As a result, it was confirmed that allD FP13-NH2 binds to LTA and LPP, which
are cell wall components of gram-positive bacteria. It was confirmed that LPP
is
included in LTA, but does not bind to LPP including mutated LTA, and it was
expected
that LPP plays a more important role in binding of FP13-NH2 and LTA.
Accordingly,
it was confirmed that the LPP binding will be important in the mechanism of
antibacterial activity of FP13-NH2 against gram-positive bacteria.
The foregoing description of the present invention is for illustrative
purposes
only, and those of ordinary skill in the art to which the present invention
pertains can
understand that the exemplary embodiments disclosed herein can be easily
modified
into other specific forms without departing from the technical spirit or
essential
features of the present invention. Therefore, the exemplary embodiments
described
above should be interpreted as illustrative in all aspects and not
restrictive.
59
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
[Industrial availability]
The peptide according to the present invention not only inhibits the
proliferation of standard bacteria and antibiotic-resistant bacteria, but also
has an
excellent effect of removing a bacteria-derived endotoxin, thereby exhibiting
an
excellent treatment effect against sepsis. Besides, when used in combination
with
antibiotics, the peptide may minimize the side effects caused by the
antibiotic, and thus
can be efficiently used for the prevention or treatment of sepsis. In
addition, the peptide
according to the present invention shows an excellent antibacterial activity
selectively
against gram-positive/negative bacteria, and thus may be effectively used in
preventing or treating various infectious diseases caused by gram-
positive/negative
bacteria or an antibacterial composition against gram positive/negative
bacteria.
Moreover, since the peptide according to the present invention is not only
safe for the
human body, but also exhibits stability against a proteolytic enzymes, its
usage might
be expanded for a variety of purposes.
Sequence Listing Free Text
Hereinafter, the sequence listing will be provided as follows:
[SEQ ID NO:
Name of polypeptide
Amino acid sequence]
1
FP 1 (WT)
Lys Leu Gly Val Glu Ala Lys Arg Tyr Leu Asp
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
2
FP3
Lys Leu Gly Val Glu Ala Lys Arg Tyr Leu Arg
3
FPS
Leu Arg Leu Gly Val Glu Ala Lys Arg Tyr Leu Arg
4
FP6
Leu Arg Leu Gly Val Glu Leu Lys Arg Tyr Leu Arg
5
FP9
Lys Leu Gly Val Glu Ala Leu Arg Tyr Leu Asp
6
FP12-NH2
Arg Leu Arg Val Lys Leu Arg Arg Tyr Leu Arg
7
FP 13 -NH2
Lys Leu Arg Val Lys Leu Arg Arg Tyr Leu Arg
61
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
8
allD FP12-NH2
Arg Leu Arg Val Lys Leu Arg Arg Tyr Leu Arg
9
allD FP13-NH2
Lys Leu Arg Val Lys Leu Arg Arg Tyr Leu Arg
10 allD FP-13-9a-NH2
Lys Leu Arg Val Lys Leu Arg Arg Ala Leu Arg
11
allD FP-13-9w-NH2
Lys Leu Arg Val Lys Leu Arg Arg Trp Leu Arg
12
allD FP-13-9k-NH2
Lys Leu Arg Val Lys Leu Arg Arg Lys Leu Arg
13
allD FP13-NH2 (AcOH)
Lys Leu Arg Val Lys Leu Arg Arg Tyr Leu Arg
62
Date Recue/Date Received 2021-08-16
CA 03168643 2022-07-26
14
PEG-allD FP13-NH2 (AcOH)
Lys Leu Arg Val Lys Leu Arg Arg Tyr Leu Arg
15
FP12
Arg Leu Arg Val Lys Leu Arg Arg Tyr Leu Arg
16
FP13
Lys Leu Arg Val Lys Leu Arg Arg Tyr Leu Arg
17
allD FP21-NH2
Arg Leu Arg Val Lys Leu Arg Arg Trp Leu Arg
18
allD FP-13-9d-NH2
Lys Leu Arg Val Lys Leu Arg Arg Asp Leu Arg
63
Date Recue/Date Received 2021-08-16