Note: Descriptions are shown in the official language in which they were submitted.
WO 2021/178774
PCT/US2021/021045
COMPOSITIONS AND SYSTEMS FOR DISINFECTION
Field of the Invention
[00011 This invention is related to the field of disinfection and
sterilization compositions and
systems.
Background of the imention
[0002j There is a need for an inexpensive, effective, yet safe
and convenient method to
minimize the microbial burden of objects we interact with, without leaving
behind microbes with
resistance to future treatment. This need is demonstrated by the coronavirus
(also known as SARS-
CoV-2 or COVID-19) pandemic. Consequently, disinfectant systems for killing
viruses, bacteria,
and fungi are needed that prevent them from developing resistance, while using
ingredient
compounds that are not hazardous to humans, pets and other beneficial life
that may be exposed to
them.
[00031 Combinations of hydrogen peroxide and acetic acid to form
peroxyacetic acid have
proven to be especially effective. Several methods, apparatuses, and
disinfecting systems utilizing
peracids, including peroxyacetic acid, are well known in the art.
100041 However, one of the biggest drawbacks with using peracids
is that they are easily
hydrolyzed, and consequently have limited storage stability and shelf life.
Peroxyacetic acid
instability is described in detail in U.S. Patent No. 8,034,759. Measures to
stabilize peracids in
solution, as described, for example, in U.S. Patent Nos. 8,110,538 and
8,716,339, can be unsafe and
require additional components that are expensive, relatively scarce, and have
undesirable
environmental effects.
100051 As a result, there is still a need for sterilization and
disinfecting methods utilizing
peracids that are simultaneously effective, convenient, and safe, while at the
same time using cheap
and readily available materials.
Summary_ of the Invention
[0006j The present invention provides systems of compositions for
disinfecting surfaces
using peracids, the system comprising a first aqueous composition comprising a
first peracid reactant
compound that is either a peroxide compound or an organic acid compound, and a
second aqueous
composition comprising a second peracid reactant compound that is the other of
the first peracid
1
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
reactant compound. The compositions are formulated such that when they are
applied separately to,
and combined upon, a surface in need of disinfecting, a peracid composition is
formed in situ,
thereby disinfecting the surface.
[0007] In some embodiments, the first aqueous composition and the
second aqueous
composition each comprise one or more alcohols comprising at least 0.05% by
weight, and up to
70% by weight of the aqueous composition. In some embodiments, each aqueous
composition
comprises a first alcohol selected from the group of lower-chain alcohols
consisting of ethanol,
isopropanol, i-butanol, and combinations thereof.
[0008] In some embodiments, the concentration and identity of
alcohol compounds within an
aqueous composition are selected to reduce the surface tension of a
composition and enhance its
spread upon a surface to be disinfected. In some embodiments, the surface
tension of each of the
aqueous compositions is less than 50 dyne/cm at 20 'C. In some embodiments,
the surface tension
of at least one of the aqueous compositions, preferably the aqueous
composition applied first to the
surface, is less than 30 dyne/cm at 20 "C.
[0009] In a related aspect, there are described herein aqueous
compositions that are
formulated to maintain a low surface tension, while possessing a flash point
elevated enough to be
safely energetically distributed as aerosols into rooms and other volumetric
spaces. In some
embodiments, the flash point of each of the aqueous compositions within a
disinfectant system is at
least 50 "C.
100101 In some embodiments, aqueous compositions that are
formulated to have a surface
tension less than 30 dyne/cm at 20 C while also having a flash point greater
than 50 C can
comprise an alcohol blend comprising the first alcohol, a second alcohol, and
optionally, one or
more additional alcohols. In some embodiments, the second alcohol is selected
based on its ability
to form a partition at the air/water interface of an aerosol droplet, while
simultaneously being soluble
enough to avoid forming a lens on the droplet surface. In some embodiments,
the second alcohol, in
its neat, unmixed form, can have a partition coefficient (Log P) of at least
1.20 and a water solubility
in range of at least 10 g/L, and up to 30 g/L, when measured at 25 C. In some
embodiments, the
second alcohol can also be selected to have a flash point greater than 40 "V,
in the event a lens is
formed on a droplet surface. In some embodiments, a mid-chain Cs-Cs aliphatic,
al i cycl lc, or
aromatic alcohol compound can be selected as the second alcohol in an alcohol
blend. In some
embodiments, the second alcohol is selected from the group consisting of n-
pentanol; 2-methyl-1-
2
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
butanol; 3,3-di methy1-2-butanol ; 4-methyl-2-pentanol; 2-m ethy1-3-pentanol ;
3 -m ethy1-2-pentanol ; 2-
hexanol; 3-hexanol, phenol; 4-methyl phenol; phenylethyl alcohol; and 1-
phenylethanol.
[0011] In a related aspect, the concentration and mass ratio of
the second alcohol can be
modulated to arrive at a desired composition surface tension and flash point.
In some embodiments,
aqueous compositions comprising an alcohol blend and a Cs-Cs aliphatic,
alicyclic, or aromatic
alcohol compound as the second alcohol can be formulated so that: the aqueous
composition has a
flash point of at least 50 C; the mass ratio of the first alcohol relative to
the second alcohol within
the aqueous composition is at least 1:10, and up to 4:1; the second alcohol
comprises up to 2.5% by
weight of the aqueous composition, the alcohol blend comprises at least 2.0%
by weight, and up to
4.5% by weight, of the aqueous composition; and the surface tension of the
aqueous composition
comprising the alcohol blend is less than 30 dyne/cm at 20 C. In some
embodiments, the first
aqueous composition and the second aqueous composition each comprise at least
0.05% by weight,
and up to 5% by weight, of alcohol.
100121 In a related aspect, any of the aqueous compositions
described herein can be
formulated to readily evaporate after the peracid is formed in sin, and the
surface is disinfected. In
some embodiments, at least 99.50/, and preferably at least 99.9%, by weight of
each composition is
comprised of components having a vapor pressure of at least 1.0 mm Hg at 20
C.
[0013] In a related aspect, when present in any of the aqueous
compositions described herein,
the peroxide compound can be hydrogen peroxide, and can comprise up to 25% by
weight of the
aqueous composition.
[0014] In a related aspect, when present in any of the aqueous
compositions described herein,
the organic acid compound can be acetic acid, and can comprise up to 50% by
weight of the aqueous
composition. In some embodiments, the aqueous composition comprising acetic
acid can have a pH
less than 7Ø
[0015] Also described herein are disinfectant systems in which
each aqueous composition
has: a flash point of at least 50 C; at least 0.05% by weight, and up to 5%
by weight, of alcohol; at
least 99.5% by weight of components having a vapor pressure of at least 1.0 mm
Hg at 20 C; and a
surface tension less than 50 dyne/cm at 20 'C. In some embodiments, the
disinfectant system
comprises a first aqueous composition that comprises: at least 0.5% by weight,
and up to 10% by
weight, of acetic acid; at least 1.0% by weight, and up to 3.5% by weight, of
ethanol; and at least
0.5% by weight, and up to 1.5% by weight, of at least one alcohol compound
selected from the
3
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
group consisting of 2-hexanol and 3-hexanol, and a second aqueous composition
that comprises: at
least 0.5% by weight, and up to 10% by weight, of hydrogen peroxide; and up to
4.5% by weight of
isopropanol. in some embodiments, the disinfectant system comprises a first
aqueous composition
that comprises: at least 0.5% by weight, and up to 10% by weight, of acetic
acid; at least 1.0% by
weight, and up to 3.5% by weight, of ethanol; and at least 0.5% by weight, and
up to 2.0% by weight,
of n-pentanol, and a second aqueous composition that comprises: at least 0.5%
by weight, and up to
10% by weight, of hydrogen peroxide; and up to 4.5% by weight of isopropanol.
In either of the
above aqueous compositions, the first aqueous composition can further comprise
a third alcohol,
isopropanol, at a concentration of at least 0.1% by weight, and up to 0.5% by
weight, and a fourth
alcohol, n-butanol, at a concentration of at least 0.1% by weight, and up to
0.5% by weight.
[0016] In a related aspect, any of the aqueous compositions
within any of the disinfectant
systems described herein can further comprise a natural biocidal blend, the
natural biocidal blend
comprising at least 0.001% by weight, and up to 0.5% by weight, of the
composition, wherein the
natural biocidal blend comprises one or more natural biocides or natural
biocidal compounds
selected from the group consisting of manuka honey, oregano oil, thyme oil,
lemongrass oil, lemon
oil, orange oil, anise oil, clove oil, aniseed oil, cinnamon oil, geranium
oil, rose oil, mint oil,
peppermint oil, lavender oil, citronella oil, eucalyptus oil, sandalwood oil,
cedar oil, rosmarin oil,
pine oil, vervain tleagrass oil, ratanhiae oil, methylglyoxal, carvacrol,
eugenol, linalool, thymol,
p-cymene, myrcene, bomeol, camphor, caryophillin, cinnamaldehyde, geraniol,
nerol, citronellol,
and menthol, including combinations thereof.
100171 In a related aspect, any of the aqueous compositions
within any of the disinfectant
systems described herein can be formulated to be substantially free, and
preferably completely free,
of surfactants, bleaches, polymers, chelators, metal colloids, and
nanoparticles.
100181 In a related aspect, any of the disinfectant systems
described herein can be formulated
to generate a peracid composition on a surface in situ, in which the peracid
composition exhibits
antimicrobial efficacy against at least one microorganism selected from the
group consisting of:
Staphylococcus aureus (pacc. #6538), Pseudomonas aeruginosa (ATCC #I5442), and
Candida
auris (CDC MR-0381). In some embodiments, the antimicrobial efficacy of the in
situ peracid
composition against Staphylococcus aureus and Pseudomonas aeruginosa can be
measured in
accordance with the protocols of the AOAC Germicidal Spray Method 961.02. In
some
embodiments, the antimicrobial efficacy of the in situ peracid composition
against Candida auris
4
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
can be measured in accordance with the protocols of the OECD Quantitative
Method for Evaluating
Efficacy of Liquid Antimicrobials.
[0019] In a related aspect, any of the disinfectant systems
described herein can be packaged
and/or configured to prevent contact between the first aqueous composition and
the second aqueous
composition until both aqueous compositions have been dispersed into the
volumetric space. In
some embodiments, any of the disinfectant systems described herein can be
packaged and/or
configured to prevent contact between the first aqueous composition and the
second aqueous
composition until both aqueous compositions have contacted the surface to be
disinfected.
[0020] These and other embodiments of the present invention will
be apparent to one of
ordinary skill in the art from the following detailed description.
Brief Description of the Figures
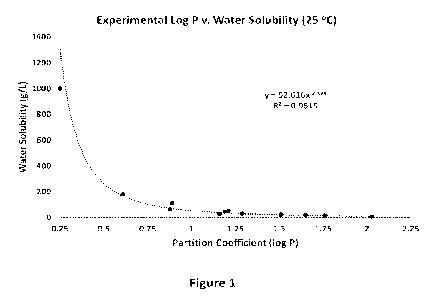
[0021] Figure 1 shows a standard curve of the water solubility of common
alcohol compounds as a
function of their Log P.
[0022] Figure 2 shows a standard curve of surface tensions of isopropanol
compositions measured
using a capillary tube-based method, overlaid with isopropanol composition
surface tensions from
the literature.
Detailed Description of the Invention
[0023] The present disclosure includes disinfectant systems
comprising multiple aqueous
compositions formulated to generate peracids on target surfaces in silu. Other
present methods and
systems require a peracid to be formed prior to application onto a surface. As
a result, conventional
peracid-based disinfecting systems require additional reactants or stabilizers
to be present. In
contrast, the compositions utilized in the systems of the present invention do
not require stabilizers
because compounds that form the peracid are dispersed separately, and they are
combined only upon
the surface(s) to be disinfected.
Definitions
[0024] As used in this specification and in the claims, the
singular forms "a," "an," and "the"
include plural referents unless the content clearly dictates otherwise.
[0025] The terms, "free" or "substantially free" refers to the
total absence or near total
absence of a particular compound in a composition, mixture, or ingredient.
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
100261 As used herein, the term "neat," with respect to an
alcohol compound, refers to the
pure, undiluted, and/or unmixed compound in a liquid phase and in the absence
of a solvent. As
described in further detail below, multiple physical properties of alcohol
compounds in their neat
form, particularly mid-chain Cs-Cs alcohol compounds, can be evaluated to
identify candidates for
inclusion in the alcohol blend of one or more of the aqueous disinfectant
precursor compositions of
the present invention.
[0027] As used herein, the phrase, "peracid reactant compound"
refers to a reactant
compound that will react to form a peracid on the target surface in sit u.
[0028] As used herein, the term, "reaction layer," refers to a
layer formed on a surface to be
disinfected when an aqueous composition comprising a second peracid reactant
compound is applied
onto a coalesced layer of an aqueous composition comprising first a first
peracid reactant compound
that is already on the surface. The product of the two reactant compounds is
formed in situ on the
reaction layer.
[0029] In describing embodiments of the disinfectant systems of
the present disclosure,
reference will be made to "first" or "second" as they refer to aqueous
compositions or peracid
reactant compounds. Except when there is clear context that a specific order
is intended, "first" and
"second" are merely relative terms, and a "first" composition or reactant
compound described could
just as easily and conveniently be referred to as a "second" composition or
reactant compound, and
such description is implicitly included herein.
100301 Concentrations, dimensions, amounts, and other numerical
data may be presented
herein in a range format. It is to be understood that such range format is
used merely for
convenience and brevity and should be interpreted flexibly to include not only
the numerical values
explicitly recited as the limits of the range, but also to include all the
individual numerical values or
sub-ranges encompassed within that range as if each numerical value and sub-
range is explicitly
recited. For example, a weight ratio range of about 0.5% to about 10% by
weight includes not only
the explicitly recited limits of 0.5% by weight and 10% by weight, but also
individual weights such
as 1% by weight and 5% by weight, and sub-ranges such as 2% to 8% by weight,
5% to 7% by
weight, etc.
Embodiments of the Invention
[0031] Without being limited by theory, it is believed that
peracids are so effective as
disinfectants because they are powerful oxidizing agents that can irreversibly
damage proteins and
6
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
DNA within microorganisms. Peracids are formed in an acid-catalyzed reaction
when a strong
oxidizing agent, such as a peroxide compound, comes into contact with an
organic acid. For
example, in a system that utilizes acetic acid as the organic acid, addition
of a peroxide compound
such as hydrogen peroxide can result in a reaction in which peracetic acid and
water are produced in
equilibrium as shown below:
H202 + CH3COOH CH3C00-0H + H20
100321 Once the peracid is formed on the surface to be
disinfected, it is strongly electrophilic.
If there are no electron-rich sources in solution with the peracid, the excess
water will drive
equilibrium toward hydrolysis of the peracid and back into production of the
parent acid.
Additionally, as the parent acid becomes increasingly acidic, the resultant
peracid similarly becomes
more reactive. Thus, even though the resultant peracid could become an even
better disinfectant
under those conditions, it is also more unstable and may potentially never
reach the target surface,
regardless of how immediately before application the individual components are
mixed.
Consequently, embodiments of this invention can similarly be more effective
than the present art in
industrial applications where stronger and more strictly-controlled components
are used and cost is
not an object.
100331 Generally, the disinfectant systems of the present
invention utilize at least two
aqueous compositions¨a first aqueous composition comprising a first peracid
reactant compound
that is either a peroxide compound or an organic acid compound, and a second
aqueous composition
comprising a second peracid reactant compound that is the other of the first
peracid reactant
compound. The compositions can either be separately dispersed into a
volumetric space and
deposited onto the surface(s) to be disinfected, or they can be separately
dispersed onto the
surface(s) directly. In some embodiments, the separately dispersed aqueous
compositions can make
contact with each other in the air within the volumetric space, forming a
peracid that deposits onto
the surfaces, thereby disinfecting them. In other embodiments, the two aqueous
compositions are
sequentially dispersed, such that upon the deposit of the first aqueous
composition onto the surface
to form a coalesced first aqueous composition layer, the second aqueous
composition is subsequently
dispersed and deposits onto the coalesced first aqueous composition layer,
forming a reaction layer
upon the surface, within which a peracid is formed in situ, disinfecting the
surface. In another
embodiment, the second aqueous composition can deposit into a coalesced second
aqueous
7
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
composition layer, which can be combined with the coalesced first aqueous
composition layer to
form the reaction layer.
100341 In embodiments in which the peracid is only formed within
a reaction layer formed
upon the surface, the effectiveness of such disinfectant systems is expected
to be independent of the
order in which the aqueous compositions are dispersed. Thus, the first peracid
reactant compound
can either be an organic acid compound or a peroxide compound, so long as the
second peracid
reactant compound is the opposite compound of that chosen to be the first
peracid reactant
compound. For example, the second peracid reactant compound is an organic acid
compound if a
peroxide compound is selected to be the first peracid reactant compound, and
the second peracid
reactant compound is a peroxide compound if an organic acid compound is
selected to be the first
peracid reactant compound. Although the compositions containing the peracid
reactant compounds
are generally mostly aqueous, water need not comprise the majority of the
composition.
Furthermore, any liquid carrier system that can facilitate the formation of
the peracid from a
peroxide compound and an organic acid can be used.
100351 In another embodiment, an aqueous composition containing a
peroxide compound,
non-limiting examples of which are hydrogen peroxide, metal peroxides, and
ozone, can comprise at
least 0.1%, or 0.5%, or 1%, or 2%, or 4%, or 6%, or 8%, or 10%, or 12%, or
14%, or 16%, or 18%,
or 20%, or 25% by weight of the peroxide compound. In other embodiments, an
aqueous
composition containing a peroxide compound can comprise less than or equal to
25%, or 20%, or
18%, or 16%, or 14%, or 12%, or 10%, or 8%, or 6%, or 4%, or 2%, or 1%, or
0.5%, or 0.1% by
weight of the peroxide compound. Useful ranges can be selected from any value
between and
inclusive of 0.1% to 25% by weight of the peroxide compound. Non-limiting
examples of such
ranges of the peroxide compound are: from 0.1% to 25%, from 0.5% to 25%, from
1% to 25%, from
2% to 25%, from 4% to 25%, from 6% to 25%, from 8% to 25%, from 10% to 25%,
from 0.5% to
10%, from 2% to 8%, or from 3% to 7% by weight of the aqueous composition. In
some
embodiments, the aqueous composition comprises about 10% by weight of the
peroxide compound.
In some embodiments, the aqueous composition comprises about 5% by weight of
the peroxide
compound. In preferred embodiments, the peroxide compound is hydrogen
peroxide.
100361 The organic acid compound can be any organic acid that can
effectively form a
peracid upon reacting with a peroxide compound. Generally, these will include,
but are not limited
to, carboxylic acids. Non-limiting examples of carboxylic acids which can be
used include formic
8
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
acid, acetic acid, citric acid, succinic acid, oxalic acid, propanoic acid,
lactic acid, benzoic acid,
butanoic acid, pentanoic acid, octanoic acid, amino acids, and mixtures
thereof. In some
embodiments, an aqueous composition containing an organic acid compound can
comprise at least
0.5%, or 1%, or 2%, or 5%, or 100/o, or 15%, or 20%, or 25%, or 30%, or 35%,
or 40%, or 45%, or
50%, by weight of the organic acid compound. in other embodiments, an aqueous
composition
containing an organic acid compound can comprise less than or equal to 50%, or
45%, or 40%, or
35%, or 30%, or 25%, or 20%, or 15%, or 10%, or 5%, or 2%, or 1%, or 0.5% by
weight of the
organic acid compound. Useful ranges can be selected from any value between
and inclusive of
0.5% and 50% by weight of the organic acid compound. Non-limiting examples of
such ranges of
the organic acid compound are: from 0.5% to 50%, from 1% to 50%, from 2% to
50%, from 5% to
50%, from 10% to 50%, from 0.5% to 20%, from 0.5% to 10%, from 1% to 20%, from
2% to 15%,
or from 5% to 10% by weight of the aqueous composition. In some embodiments,
the aqueous
composition comprises 10% by weight of the organic acid compound. In some
embodiments, the
aqueous composition comprises 8% by weight of the organic acid compound. In
some embodiments,
the organic acid compound is acetic acid. In some embodiments, the pH of the
aqueous composition
comprising acetic acid is less than 7Ø
[0037] In another embodiment, the aqueous compositions of any of
the disinfectant systems
described herein can be dispersed into a volumetric space and/or directly onto
a surface by any
means known in the art, including as a liquid stream or as a multiplicity of
droplets. Methods by
which a liquid composition can be dispersed as a multiplicity of droplets can
be selected from the
group consisting of: a coarse spray, mist, shower, aerosol, fog, and a vapor,
including combinations
thereof.
[0038] In another embodiment, one or more of the aqueous
compositions within the
disinfectant system can include non-aqueous compounds that lower the
composition's surface
tension, such as, as non-limiting examples, surfactants and/or alcohols. For
example, pure ethanol
has a surface tension of about 22.27 dyne/cm at 20 C, and relatively low
levels of many surfactants
have the ability to lower the surface tension of an aqueous composition to
about 30 dyne/cm at 20 C,
or lower. Without being limited by a particular theory, as the surface tension
of the droplets
decreases, the formed coalesced composition and/or reaction layers will spread
over a higher
proportion of the surface, at a less overall volume and effective uniform
thickness, than coalesced
composition and/or reaction layers having a higher suiface tension.
9
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
100391 Consequently, and in another embodiment, at least one, or
both of the aqueous
compositions further comprises at least one alcohol. The surface tension of an
aqueous composition
containing a peracid reactant compound and at least one alcohol, measured at
20 C, can be less than
or equal to 72, or 60, or 50, or 45, or 40, or 35, or 30, or 25, or 20
dyne/cm. In some embodiments,
the surface tension of an aqueous composition containing a peracid reactant
compound and at least
one alcohol, measured at 20 C, can be any value between and inclusive of 20
dyne/cm and 72
dyne/cm. In a further embodiment, at least one of the first aqueous
composition and the second
aqueous composition have a surface tension of less than or equal to about 30
dyne/cm at 20 'C. In
another embodiment, the surface tension of the first aqueous composition is
less than 30 dyne/cm,
the surface tension of the second aqueous composition can be less than or
equal to 60, or 55, or 50,
or 45, or 40, or 35, or 32.5 dyne/cm, when measured at 20 'C.
100401 Further, some alcohols also independently provide biocidal
activity separate from the
peracid. Consequently, and without being limited by a particular theory, using
alcohols in
combination with forming the peracid in situ on the surface to be disinfected
may provide additive
effects on the antimicrobial activity as compared to reaction layers which
only contain a peroxide
compound and an organic acid compound.
[0041] Advantageously, many alcohols have a sufficiently high
vapor pressure to promote
evaporation from a surface after the peracid has been formed and the surface
has been disinfected,
Accordingly, in some embodiments, aqueous compositions utilized in accordance
with disinfectant
systems of the present invention can have less than 0.01% by weight (100 ppm)
of surfactants, and
in further embodiments, are substantially free of surfactants, which generally
have low or negligible
vapor pressures, and can linger on a surface long after the surface has been
disinfected if the
surfactant residues aren't subsequently wiped off of the surface.
100421 In an aqueous composition comprising a peracid reactant
compound and one or more
alcohols, the one or more alcohols can comprise at least 0.05%, or 0.1%, or
1%, or 2%, or 3%, or 4%,
or 5%, or 10%, or 15%, or 20%, or 25%, or 30%, or 40%, or 50%, or 60%, or 70%
by weight of the
aqueous composition. In other embodiments, the one or more alcohols can
comprise less than or
equal to 70%, or 60%, or 50%, or 40%, or 30%, or 25%, or 20%, or 15%, or 10%,
or 5%, or 4%, or
3%, or 2%, or 1%, or 0.1%, down to equal to 0.05% by weight of the aqueous
composition. Useful
ranges can be selected from any value between and inclusive of about 0.05% to
70% by weight of
the alcohol. Non-limiting examples of such ranges of the alcohol are: from
0.05% to 70%, from
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
0.1% to 20%, from 1% to 15%, from 2% to 20%, from 3% to 5%, from 1% to 5%,
from 2% to 75 A,
or from 2.0% up to 4.5% by weight of the aqueous composition. In some
embodiments, an aqueous
composition can comprise 4% by weight of the alcohol. In some embodiments, an
aqueous
composition can comprise 2.5% by weight of the alcohol.
100431 The alcohol present in an aqueous composition can be a
single alcohol compound or a
combination of multiple alcohol compounds. Each alcohol compound can comprise
a primary,
secondary, or tertiary hydroxyl group, and can have aliphatic, alicyclic,
aromatic, or carbon-
containing structure having 1 to 24 carbon atoms, and in some embodiments, 2
to 8 carbon atoms.
Non-limiting examples of monohydric aliphatic alcohols, alicyclic, aromatic,
and unsaturated
alcohols that can be used include methanol; ethanol; propanol; isopropanol;
butanol; pentanol;
hexanol; heptanol; octanol; nonanol; and decanol, including all constitutional
isomers, stereoisomers,
denatured alcohols and combinations thereof. Further, each alcohol compound
can be straight-
chained or branched, saturated or unsaturated, and/or monohydric or
polyhydric. In some
embodiments, non-aliphatic alcohols may also be utilized.
100441 In another embodiment, for practical considerations, lower-
chain alcohol compounds
such as methanol, ethanol, isopropanol, t-butanol, and other aliphatic Cr-C4
alcohols, and denatured
alcohols thereof can be used because of their properties and cost. However,
many alcohols,
particularly primary alcohols, for example methanol and ethanol, can form low
levels of a peracid
upon reacting with hydrogen peroxide. Consequently, in some embodiments,
isopropanol and 1-
butanol, as secondary and tertiary alcohols, respectively, can be chosen as
alcohols because side
reactions with the hydrogen peroxide to form a peracid are not favored.
100451 However, although lower-chain alcohols are cheap, readily
available, and effectively
lower the surface tension of an aqueous composition, they can have
particularly low flash points, and
can combust when energetically dispersed in high concentrations as an aerosol
or vapor, e.g. droplets
having diameters less than 100 gm, less than 15 gm, or down to less than 1 gm.
In preferred
embodiments, aqueous compositions dispersed as an aerosol or a vapor have a
flash point greater
than about 40 C, more preferably greater than about 50 C, even more
preferably greater than about
55 "Cõ and still more preferably greater than about 60 'C. Yet, even at dilute
alcohol concentrations,
compositions containing lower-chain alcohol compounds such as ethanol, i
sopropanol , and i-butanol
can have a flash point of less than 40 C.
11
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
100461 Neat forms of alcohol compounds having five or more carbon
atoms generally have
flash points that are higher than lower-chain alcohols, while being able to
attain similar surface
tensions as lower-chain alcohols. However, the solubility of an alcohol in an
aqueous-based
composition decreases dramatically once the alcohol contains four or more
carbon atoms. For
example, at 25 "C, the solubility of n-butanol is 67 grams per liter, the
solubility of n-pentanol is 22
grams per liter, the solubility of n-hexanol is 5.9 grams per liter, and the
solubility of n-octanol are
0.5 grams per liter. Converted to percent by weight of an aqueous composition,
the solubility of
each compound is 6.7% by weight, 2.2% by weight, 0.59% by weight, and 0.05% by
weight of the
composition. As a result, many C5 or greater alcohol compounds are not soluble
enough to be added
at high enough concentrations to an aqueous composition to lower the surface
tension to less than or
equal to 30 dyne/cm at 20 C.
100471 However, and in some embodiments, a blend of alcohols
comprising a first alcohol
comprising at least one lower-chain alcohol compound, and a second alcohol
comprising at least one
C. or greater alcohol compound, can lower the surface tension of an aqueous
composition to less
than or equal to 30 dyne/cm at 20 'C while also maintaining a flash point
greater than 50 C.
Without being limited by a particular theory, it is believed that the addition
of a lower-chain alcohol
to a composition comprising a C5 or greater alcohol can increase the number of
alcohol compound
molecules within the composition, lowering the surface tension.
Simultaneously, the presence of the
C5 or greater alcohol can reduce the amount of lower-chain alcohol necessary
to lower the surface
tension without adversely decreasing a composition's flash point.
100481 In another embodiment, the one or more C5- or greater
alcohol compounds within the
alcohol blend can be soluble enough in water to minimize the amount of the
lower-chain alcohol
within the aqueous composition. For example, the solubility of the alcohol
compound in water,
measured at 25 C, can be greater than 5, or 10, or 15, or 20, or 25, or 30,
or 35 grams per liter (g/L),
up to 40 g/L. In another embodiment, the water solubility of the one or more
C5- or greater alcohol
compounds, measured at 25 C, can be less than 40, or 35, or 30, or 25, or 20,
or 15, or 10 g/L, down
to 5 g/L. In another embodiment, the water solubility of the one or more C5-
or greater alcohol
compounds within the alcohol blend, measured at 25 C, can be any value or
range between and
inclusive of 5 g/L and 40 g/L, for example, at least 10 g/L and up to 30 g/L.
100491 However, when the aqueous composition containing the
alcohol blend is volatilized,
it may be preferable to control the concentration of each of the alcohol to
avoid lens formation at the
12
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
composition surface. Without being limited by a particular theory, it is
believed that one or more
lenses can form when the concentration of a single alcohol, or a blend of two
or more alcohols, is no
longer miscible with the aqueous solvent once the alcohol compound(s)'
solubility limits are
exceeded. As a result, the non-miscible alcohol compounds congregate into a
lens, and the flash
point of that lens can approximately equivalent to the flash point of the pure
alcohol. Accordingly,
the benefit of using an alcohol blend to suppress a reduction in the flash
point of the composition can
be lost by adding too much alcohol to the composition.
[0050] In another embodiment, an alcohol compound can be
described by its partition
coefficient P, or the logarithm of its partition coefficient (Log P). A
partition coefficient is the ratio
of concentrations of a solute between two solvents, specifically for un-
ionized solvents, expressed as
a ratio of concentrations in octanol with respect to water, indicating a
measure of hydrophobicity of
the solute. Generally, the Log P of a compound increases as a compound's water
solubility
decreases. However, solubility and Log P are not directly proportional,
particularly as the alcohol
compound becomes more complex. In another embodiment, in their neat form, each
of the one or
more C5- or greater alcohol compounds within the alcohol blend can have a log
P of at least 1.20, or
1.30, or 1.40, or 1.50, or 1.60, or 1.70, or 1.80, or 1.90, or 2.00, or 2.10,
up to at least 2.20. In
another embodiment, in their neat form, each of the one or more C5- or greater
alcohol compounds
within the alcohol blend can have a log P of less than 2.20, or 2.10, or 2.00,
or 1.90, or 1.80, or 1.70,
or 1.60, or 1.50, or 1.40, or 1.30, down to 1.20. In another embodiment, the
Log P of the one or more
C5- or greater alcohol compounds within the alcohol blend can be any value or
range between and
inclusive of 1.20 and 2.20, for example, from at least 1.40, and up to 2Ø
[0051] Alcohol compounds having any of the above water solubility
and Log P values can be
identified using an online chemical database, such as PubMed or ChemSpider.
Within ChemSpider,
predicted physical properties are calculated by the ACD/Labs Percepta
platform. In particular, Log
P can be predicted via the ACD/Labs LogP platform or using the Molinspiration
Property
Calculation Service. Generally, experimental solubility values for each
alcohol compound in their
pure form were collected from Yalkwosky, S.H., et al., (2010) Handbook of
Aqueous Solubility
Data, Second Edition, CRC Press, Boca Raton, FL). Non-limiting examples of
compounds having a
Log P between 1.20 and 2.0 and both predicted and experimental water
solubility values within the
g/I., to 30 g/I., range are: n-pentanol; 2-methyl-1-butanol; 3-methyl-1-
butanol; 3,3-di rn eth y1-2-
1 3
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
butanol; 4-methyl-2-pentanol; 2-methyl-3-pentanol; 3-methyl-2-pentanol; 2-
hexanol; 3-hexanol;
phenol; 4-methyl phenol; phenylethyl alcohol; and 1-phenyl ethanol.
100521 Several aqueous compositions with alcohol blends
comprising ethanol and selected
C5-C8 alcohol compounds identified by the database search¨n-pentanol, 2-
hexanol, and 3-hexanol¨
were experimentally tested and/or modeled for both flash point and surface
tension, the details of
which are described in the Examples section, below. In another embodiment,
aqueous compositions
comprising any of the above alcohol blends, while having a flash point above
50 'V and a surface
tension below 30 dyne/cm at 20 C, have a total alcohol concentration of at
least 2.0%, and up to
4.5% by weight of the aqueous composition. As non-limiting examples, an
aqueous composition
comprising 8% by weight acetic acid, 0.2% by weight ethanol, and 2.0% by
weight n-propanol, and
an aqueous composition comprising 8% by weight acetic acid, 3.2% by weight
ethanol, and 0.9% by
weight n-propanol were both modeled and found to have a flash point above 50
'X; and a surface
tension below 30 dyne/cm at 20 C. Accordingly, in another embodiment, the
mass ratio of the
lower-chain alcohol relative to the C5-C8 alcohol compound can be in a range
from at least 1:10, and
up to 4:1.
100531 In another embodiment, some alcohol blends of the present
invention can comprise
more than two alcohol compounds. In further embodiments, an alcohol blend can
further comprise
isopropanol and n-butanol. As a non-limiting example, commercially-available
ethanol can be
obtained as a 90% (v/v) solution with isopropanol and water. In another non-
limiting example, n-
butanol can be used to solubilize a blend of one or more natural biocides or
natural biocidal
compounds to be included within an aqueous composition. In another embodiment,
either or both of
the isopropanol and the n-butanol can comprise at least 0.1% by weight, and up
to 0.5% by weight of
the aqueous composition.
100541 In another embodiment, aqueous compositions comprising
hydrogen peroxide can
further comprise any of the alcohol blends above. In another embodiment,
aqueous compositions
comprising a hydrogen peroxide can comprise a single alcohol compound. In
another embodiment,
the one or more alcohol compounds comprised within an aqueous composition
comprising hydrogen
peroxide can consist of secondary or tertiary alcohol compounds. In a further
embodiment, the one
or more secondary or tertiary alcohol compounds can be selected from the group
consisting of
isopropanol, 2-butanol, t-butanol, 3,3-di methyl-2-butanol; 4-m ethy1-2-pen
tanol ; 2-m ethy1-3-
pentanol; 3-methyl-2-pentanol; 2-hexanol; 3-hexanol; and I -phenylethanol,
including combinations
14
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
thereof. Without being limited by a particular theory, it is believed that the
use of a secondary or
tertiary alcohol precludes the formation of a peracid from the oxidation of a
primary alcohol by
hydrogen peroxide. It is also believed that as opposed to primary alcohols
such as ethanol and
benzyl alcohol, secondary and tertiary alcohols are more sterically-hindered,
and resistant to auto-
oxidation to form an aldehyde, which itself can be auto-oxidized to form a
carboxylic acid.
100551 In another embodiment, additional compounds can be
included in either aqueous
composition to enhance or supplement the effectiveness of the peracid
generated in situ on the
surface to be disinfected. Such compounds can include one or more natural
biocides, such as
manuka honey and essential oils, and/or natural biocidal compounds typically
found within manuka
honey and essential oils, such as methylglyoxal, carvacrol, eugenol, linalool,
thymol, p-cymene,
myrcene, borneol, camphor, caryophillin, cinnamaldehyde, geraniol, nerol,
citronellol, and menthol,
including combinations thereof. Non-limiting examples of essential oils that
can be included in one
or more of the aqueous compositions include the essential oils of oregano,
thyme, lemongrass,
lemons, oranges, anise, cloves, aniseed, cinnamon, geraniums, roses, mint,
peppermint, lavender,
citronella, eucalyptus, sandalwood, cedar, rosmarin, pine, vervain fleagrass,
and ratanhiae. In some
embodiments, one or more natural biocides or natural biocidal compounds,
particularly essential oils
and/or their chemical components, can be included in an aqueous composition at
a concentration of
at least 0.001%, or 0.005%, or 0.01%, or 0.05%, or 0.1%, or 0.25%, or 0.5% by
weight, up to 1% by
weight. In other embodiments, the natural biocidal blend can comprise less
than or equal to 1%, or
0.5%, or 0.25%, or 0.1%, or 0.05%, or 0.01%, or 0.005% by weight, down to
0.001% by weight.
Useful ranges can be selected from any value between and inclusive of 0.001%
to 1% by weight of
the aqueous composition, a non-limiting example of which is at least 0.001%,
and up to 0.5% by
weight.
100561 An advantage of many of the components described above,
particularly the peracid
reactant compounds, alcohol compounds, and natural biocidal blends, is that
they can be easily
volatilized after the sterilization is complete. Without being limited by a
particular theory, if the
selection of the components within the aqueous composition is controlled so
that substantially all of
the reaction layer is able to evaporate quickly, the post-disinfection wiping
down of surfaces, which
is typically required according to traditional practices, can be eliminated.
Formulating the aqueous
compositions to have a high volatility generally requires that non-volatile
salts, surfactants, high-
molecular weight materials, and other additives can be used sparingly or
omitted completely in order
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
to promote high turnover of the volumetric space containing the surfaces to be
disinfected. In some
embodiments, the aqueous compositions can be formulated to have a volatility
such that at least 90%,
or 95%, or 99%, or 99.5%, or 99.7%, or 99.9% by weight, up to 100% by weight
of the reaction
layer evaporates within 30 minutes.
10051 To enhance the volatility of the aqueous compositions
after they are deposited on one
or more surfaces, the individual components of each of the aqueous
compositions can be selected to
have a relatively higher standard vapor pressure compared to less labile
components that remain on
surfaces long after they are disinfected. Thus, in another embodiment, one or
both of the aqueous
compositions can be formulated so at least about 99.0% by weight, at least
about 99.5% by weight,
or at least about 99.9% by weight of the components have a standard vapor
pressure of at least 1.0
mm Hg at 20 C.
100581 In other embodiments, however, it can be advantageous to
include additional
components in at least one of the aqueous compositions in order to supplement
or enhance the
disinfection of surfaces within a volumetric space, particularly in situations
in which the volatility of
the aqueous compositions once they have been deposited onto surfaces is not a
concern. Such
additional components can include, but are not limited to surfactants, as
described above, as well as
polymers, chelators, metal colloids and/or nanoparticles, oxidizers, and other
chemical additives,
including combinations thereof
100591 In another embodiment, any of the disinfectant systems of
the present invention
described above can be used for a variety of user-identified biocidal
purposes, including
antimicrobial, bleaching, or sanitizing applications. In other aspects, the
disinfectant systems are the
present invention are effective against a wide variety of microorganisms, such
as Gram- positive
organisms (Listeria monocytogenes or Staphylococcus aureus); Gram-negative
organisms
(Escherichia coil or Pseudomonas aeruginosa); catalase-positive organisms
(Micrococcus luktis or
Staphylococcus epidermidis); spotulent organisms (Bacillus subtilis); drug-
resistant and non-drug-
resistant forms of one or more of the following organisms: Acinetobacter
baumannii; Enterococcus
faecium; Enterobacter aerogenes; Escherichict coil; Klebsiella pneumoniae;
norovirus; herpes
simplex virus; hepatitis; human immunodeficiency virus; severe acute
respiratory syndrome (SARS)
coronavirus; influenza; rhinovirus; Trichophyton interdigitale, Candida auris;
Clostridium difficile,
and SARS strains 1 and/or 2 (SARS-CoV-1 and SARS-CoV-2).
16
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
Examples
[0060] The following working and prophetic examples are offered
to illustrate, but not to
limit, the claimed invention. For avoidance of doubt, any examples relating to
subject-matter
outside the scope of the claims is included for reference only.
Example 1: Identification of C5-C8 Alcohol Compounds for Use in Alcohol Blends
100611 A study was conducted to identify monohydric alcohol
compounds having a Log P of
at least 1.20 and up to 2.00, and a water solubility, measured at 25 C.,
between 10 g/L and 30 g/L, as
candidates to be combined into alcohol blends that can lower the surface
tension of an aqueous
composition to below 30 dyne/cm at 20 C while also maintaining a composition
flash point above
50 C. Compounds were initially identified using the ChemSpider online
database. Search
limitations included an ACD/LogP of 1.20 to 2.00 and a single hydrogen bond
acceptor, and were
conducted serially using on the empirical formula CxHy0. Alcohol compounds
comprising 4 to 7
carbon atoms, as well as selected Cs-alcohol compounds, were queried.
[00621 Generally, each search hit included an ACD/Labs predicted
Log P, and an
experimental water solubility determined by the T.T.S. Environmental
Protection Agency's
EPISuiteTM, and some entries contained experimental Log P and/or solubility
values for the alcohol
compound. However, in a study that evaluated the accuracy of predicted Log P
values for non-ionic
compounds, such as alcohol compounds, the Molinspiration Property Calculation
Service (MPCS)
was determined to be one of the best freely-available prediction suites (see
Hodges, G., et al, (2019)
Environ. Sc!. Eur. 31:1-18, the disclosure of which is incorporated by
reference in its entirety. The
SMILES string for each alcohol compound identified within ChemSpider was
inserted as a query
within MPCS to determine its predicted Molinspiration Log P value. When the
ChemSpider entry
for a particular alcohol compound did not contain an experimentally-determined
water solubility, the
water solubility at 25 C was modeled by generating a standard curve of
several alcohol compounds
that had known Log P and solubility values, regardless of the number of
carbons present. The
alcohol compounds included in the standard curve, and their Log P and water
solubility values, are
shown below in Table 1.
17
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
TABLE I
Log P and Water Solubility of Common Alcohols
Compound Name Log P Solubility
(at 25 "C) (g/L1
n-pentanol 1.51
22.0
2-hexanol 1.76
13.7
3-hexanol 1 65
16.1
2-pentanol 1.19
44.6
3-pentanol 1.21
51.5
2-methyl -1-butanol 1.29
29.7
3-methyl-1-butanol 1.16
26.7
n-hexanol 2.03
5.9
n-butanol 0.88
63.2
2-butanol 0.61 181
n-propanol 0.25
1000*
i-amyl alcohol 0.89 110
[0063] The plot of Log P as a function of water solubility,
illustrated in Figure 1, indicates a
hyperbolic relationship, with an R2 of over 95% indicating a good fit.
Although alcohol compounds
that have a more complex structure than those in Table 1, particularly those
that are alicyclic,
aromatic, or unsaturated are likely to deviate at least somewhat, and
potentially significantly, for
some alcohol compounds, it is nonetheless believed that the equation from the
hyperbolic best fit
curve can reasonably predict the water solubility of many alcohol compounds
from the MPCS Log P.
[0064] In Table 2 below, the ACD/Labs predicted Log P, the MPCS
predicted Log P, and
estimated water solubility of 158 alcohol compounds are listed. Each compound
in the table has an
estimated water solubility between 10 g/L and 30 g/L, if it were measured at
25 C. If an alcohol
compound has an experimentally determined Log P or solubility, that is also
listed.
TABLE 2
Experimental and Predicted Physical Properties of Identified Alcohol Compounds
log P log P log P
Sol Sol
Name ACD Exp. MPCS (Est) (Exp)
1-Pentyn-1-01 2.30 1.34
26.65
3-Methyl-1,3-butadien-2-ol 1.39 1.81
13.25
2,4-Pentadien-2-ol 1.36 1.52
19.88
1,4-Pentadien-2-ol 1.56 1.54
19.29
3-Methy1-2-buten-1-ol 1.24 1.244 1.36
25.75
3-Methyl-2-buten-2-ol 1.89 2.03
10.15
I -Penten-1-ol 1.85 1.53
19.58
2-Penten-2-ol 1.87 1.75
14.33
18
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
2-Methyl-1-buten-1-ol 1.87 1.28
29.65
2-Penten-3-ol 1.87 1.75
14.33
3-Methyl-1-butanol 1.22 1.16 1.33 27.12 26.7
2-Methyl-I -butanol 1.22 1.29 1.3
28.60 29.7
2-Pentanol 1.22 1.19 1.48 21.16 44.6
3-Pentanol 1.22 1.21 1.43 22.91 51.5
n-pentanol 1.41 1.51 1.62 17.15 22
phenol 1.48 1.46 1.46 21.84 82
1,3,5-Hexatrien-3-ol 1.38 1.79
13.60
4-Methyl-4-penten-2-yn-1-ol 1.72 1.47
21.49
5-Hexen-3-yn-2-ol 1.52 1.28
29.65
1,3-Cyclohexadien-1-o1 1.55 1.66
16.20
1,5-Cyclohexadien-1-al 1.49 1.9
11.84
2-Methyl-l-penten-3-yn-1-ol 2.20 1.38
24.89
3-Methy1-1,4-pentadien-3 -01 1.22 1.4
24.07
3-Methylene-4-penten-2-ol 1.28 1.27
30.19
2,4-Hexadien-1-ol 1.36 1.334 1.09
43.07
2-Methyl-2,4-pentadien-1-ol 1.39 1.39
24.48
3-Methyl-2,4-pentadien-l-ol 1.39 1.39
24.48
4-m ethylpenta-2,3-di en-1-01 1.38 1.29
29.11
4-Methy1-2,4-pentadien-1-ol 1.39 1.39
24.48
3-Methyl-2-m ethylene-3-buten- I -ol 1.49 1.46
21.84
2-Methy1-1,4-pentadien-3-ol 1.43 1.5
20.51
1,3-Hexadien-l-ol 1.86 1.26
30.75
4-Methyl-1-pentyn-1-01 2.65 1.55
19.00
2-Methyl-1-cyclopenten-1-ol 1.94 1.72
14.92
1,5-Hexadien-2-ol 1.90 2.05
9.92
3-Methyl-1-cyclopenten-1-ol 1.84 1.65
16.43
1,5-Hexadien-1-ol 1.82 1.52
19.88
3-Methyl-l-pentyn-l-ol 2.65 1.52
19.88
4-Methyl-1,3-pentadien-1-01 1.88 1.53
19.58
(2-Ethylcyclopropyl)methanol 1.20 1.66
16.20
2-Ethylcyclobutanol 1.24 1.32
27.60
2-Ethyl-1-methylcyclopropanol 1.21 1.6
17.65
2-Propylcyclopropanol 1.20 1.72
14.92
2-(2-Methylcyclopropypethanol 1.20 1.43
22.91
1-Ethyl-2-methylcyclopropanol 1.21 1.6
17.65
2-Methylcyclopentanol 1.27 1.32
27.60
1-Ethylcyclobutanol 1.28 1.29
29.11
cyclohexanol 1.34 1.23 1.59
17.91
1-Methylcyclopentanol 1.32 1.53
19.58
3-Methyl-1-penten-3-ol 1.40 1.64
16.67 1
5-Hexen-2-ol 1.37 1.47
21.49 I
i
19
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
4-Methyl-1-penten-3-ol 1.40 1.43 22.91
30.6
3-Methy1-4-penten-1-ol 1.37 1.34
26.65
2-Methyl-4-penten-1-01 1.37 1.34
26.65
2,3-Di m ethy1-3-buten-2-ol 1.42 1.68
15.76
5-Hexen-3-ol 1.44 1.46
21.84
2-Ethy1-3-buten-1-ol 1.44 1.57
18.44
5-Hexen-1-ol 1.50 1.61
17.40
4-Hexen-1-ol 1.54 1.35
26.20
4-Methy1-4-penten-1-01 1.57 1.41
23.68
3-Hexen-1-ol 1.61 1.35
26.20
4-Methyl-3-penten-1-ol 1.63 1.63
16.90
2-Methy1-1-penten-3-o1 1.60 1.74
14.52
3-Methy1-3-penten-1-01 1.63 1.39
24.48
3-Methylene-2-pentanol 1.6 1.5
20.51
3-Methy1-2-methylene-1-butano1 1.6 1.39
24.48
2-Hexen-1-ol 1.75 1.88
12.13
2-Methyl-2-penten-1-ol 1.78 1.63
16.90
3-Methy1-2-penten-1-ol 1.78 1.63
16.90
2-Ethy1-2-buten-1-ol 1.78 1.39
24.48
2-Methylene-1-pentanol 1.78 1.7
15.33
1-Hexen-1-ol 2.38 2.04
10.04
2-Methyl-1-penten-1-01 2.4 1.84
12.76
3,3-Dimethy1-1-buten-1-01 2.02 1.56
18.72
3,3-Dimethy1-2-butanol 1.39 1.48 1.75 14.33 24.3
2,3 -Di m ethy1-2-butanol 1.39 1.61
17.40 43.5
3,3-Dimethy1-1-butanol 1.57 1.65 16.43
7.6
4-Methy1-2-pentanol 1.57 1.7 15.33
16.4
3-Methyl-3-pentanol 1.57 1.87 12.28
42.6
2,2-Dimethy1-1-butanol 1.57 1.72 14.92
7.6
2-Methy1-3-pentanol 1.57 1.67 15.98
20.1
3-Methyl-2-pentanol 1.57 1.67 15.98
19.4
4-Methyl-l-pentanol 1.75 1.6
17.65 7.6
2-Hexanol 1.75 1.76 1.99 10.63 13.7
2-Ethyl-1-butanol 1.75 1.81 13.25
4
3-hexanol 1.75 1.65 1.98 10.76 16.1
2-Methyl-I -pentanol 1.75 1.86
12.44 6
3-Methyl-i-pentanol 1.75 1.58 18.17
4.3
1,3,6-Cycloheptatrien-I-ol 1.44 1.67
15.98
Bicyclo[2.2.1]hepta-2,5-dien-2-ol 1.34 1.75
14.33
142,4 -Cyclopentadien-1-ylidene)ethanol 1.43 1.51
20.19
2,6-Heptadien-4-yn-1-01 1.70 1.44
22.55
6-Methylene-1,3-cyclohexadien-1-ol 1.59 1.73
14.72 1
4,6-Heptadien-2-yn-l-ol 1.72 1.44
22.55 I
i
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
3,5-Heptadlyn-1-ol 1.61 1.52
19.88
3,5-Heptadiyn-2-ol 1.79 1.61
17.40
5-Methyl ene-1,3-eyel ohexadi en-1-ol 1.63 1.73
14.72
4-methyl phenol 1.94 1.94 1.91
11.69 21.5
2-methyl phenol 1.94 1.95 2.33
7.37 25.9
3-methyl phenol 1.94 1.88
12.13
4,6-Heptadiyn-3-ol 2.44 1.3
28.60
2,4-Heptadiyn-l-al 1.98 1.75
14.33
1,6-Heptadien-4-yn-3-ol 2.15 1.55
19.00
3-Ethyl-1-penten-4-yn-3-ol 1.20 1.42
23.29
2,3-Dimethy1-1-penten-4-yn-3-ol 1.23 1.47
21.49
6-Hepten-3-yn-2-o1 1.25 1.55
19.00
4-Methy1-5-hexen-2-yn-1-01 1.25 1.67
15.98
1-Hepten-5-yn-3-al 1.27 1.55
19.00
4-Methy1-2,4-cyclohexadien-1-o1 1.24 1.39
24.48
5-Methy1-4-hexen-1-yn-3-ol 1.40 1.5
20.51
2-Methyl-1-hexen-5-yn-3-ol 1.36 1.29
29.11
6-Hepten-2-yn-1-ol 1.32 1.7
15.33
hept-1-en-5-yn-4-ol 1.33 1.55
19.00
2,4,6-Heptatrien-1-ol 1.34 1.36
25.75
2-Methylene-4-hexyn-1-ol 1.34 1.51
20.19
3-Methyl-2,4,5-hexatri en-1-01 1.40 1.32
27.60
5-Hepten-2-yn-1-ol 1.42 1.44
22.55
5-Methyl-5-hexen-2-yn-1-ol 1.45 1.74
14.52
2-Methy1-2,3,5-hexatrien-1-ol 1.47 1.32
27.60
1-Hepten-4-yn-3-ol 1.51 1.79
13.60
4-Methylene-1,5-hexadien-3-ol 1.51 1.54
19.29
6-Hepten-4-yn-1-ol 1.56 1.46
21.84
3-Viny1-2,4-pentadien-1-ol 1.53 1.43
22.91
2-Methy1-1,4,5-hexatrien-3-ol 1.56 1.44
22.55
4-Methyl-1,4,5-hexatrien-3-ol 1.56 1.44
22.55
6-Hepten-4-yn-2-ol 1.69 1.55
19.00
3-Hepten-5-yn-2-ol 1.70 1.53
19.58
2-Methy1-5-hexen-3-yn-1-01 1.69 1.67
15.98
1,4,6-Heptatrien-3-ol 1.67 1.48
21.16
Bicyclo[2.2.1]hept-2-en-2-01 1.76 1.77
13.96
3-Methylene-4-hexyn-1-ol 1.77 1.51
20.19
2-Methyl-5-hexen-3-yn-2-ol 1.87 1.73
14.72
5-Hepten-3-yn-1-01 1.86 1.44
22.55
2-Hepten-4-yn-i-ol 1.88 1.68
15.76
5-Methyl-5-hexen-3-yn-1-ol 1.89 1.74
14.52
1,3-Cyclohexadien-1-ylmethanol 1.91 1.3
28.60 1
2,3-Dimethy1-2-penten-4-yn-l-ol 1.91 1.45
22.19 I
i
21
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
3-methylhex-2-en-4-yn-1-ol 1.90 1.72
14.92
1-(1-Cyclopenten-l-yl)ethenol 1.92 1.75
14.33
2-Methyl-2-hexen-4-yn-1-ol 1.90 1.72
14.92
6-Methy1-1,4-cyclohexadien-1-ol 1.98 1.41
23.68
2-Vinyl-1,4-pentadien-1-01 1.91 1.35
26.20
5-Methy1-5-hexen-3-yn-2-ol 2.07 1.83
12.92
5-Hepten-3-yn-2-ol 2.04 1.53
19.58
6-Hepten-4-yn-3-ol 2.05 1.79
13.60
2,4-Dimethy1-3-pentanol 1.92 1.91 11.69
7
2,3,3-Trimethy1-2-butanol 1.74 2.19
8.51 22
2,4-Dimethy1-2-pentanol 1.92 2.14
8.98 13.6
4,4-Dimethy1-2-pentanol 1.92 2.02
10.27
2,3-Dimethy1-3-pentanol 1.92 2.12
9.18 16.4
2,3-Dimethy1-2-pentanol 1.92 2.12
9.18 15.7
2,2,3-Trimethyl-1-butanol 1.92 1.96
11.01
Bicyclo[4.2.0]octa-1,3,5-trien-3-ol 2.04 1.61
17.40
5-Cyclopropy1-2,4-pentadiyn-1-ol 1.81 1.74
14.52
(4-Methylene-2,5-cyclohexadien-1-
ylidene)metha.nol 1.54 1.34
26.65
(6-Methylene-2,4-cyclohexadien-1-
ylidene)methanol 1.54 1.29
29.11
Phenylethyl alcohol 1.36 1.49
20.83 22.4
1-Phenylethanol 1.38 1.5
20.51 14.7
100651 Among the 158 compounds listed in Table 2, there are 13
alcohol compounds,
n-pentanol, 2-methyl-1-butanol, 3-methy1-1-butanol, 3,3-dimethy1-2-butanol, 4-
methyl-2-pentanol,
2-methyl-3-pentanol, 3-methyl-2-pentanol, 2-hexanol, 3-hexanol, phenol, 4-
methyl phenol,
phenylethyl alcohol, and 1-phenylethanol, which had both estimated and
experimental water
solubility values between 10 g/I., and 30 g/L. Of these, aqueous compositions
comprising alcohol
blends having n-propanol, 2-hexanol, and/or 3-hexanol were formulated and
tested for flash point
and surface tension.
Example 2: Aqueous Compositions for Flash Point and Surface Tension
Determination
100661 Two separate aqueous compositions, one containing acetic
acid and one containing
hydrogen peroxide, were prepared. An aqueous composition comprising the acetic
acid was
prepared as the first aqueous composition, and contained both an alcohol blend
and a natural biocidal
blend. A composition comprising hydrogen peroxide was prepared that contained
just a single
alcohol compound, isopropanol.
22
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
First Aqueous Composition Second Aqueous
Composition
8% (w/w) acetic acid 5% (w/w)
hydrogen peroxide
1.9% (w/w) ethanol 2% (w/w)
isopropanol
0.1% (w/w) isopropanol 93% (w/w)
distilled water
0.9% (w/w) n-pentanol
0.3% (w/w) natural biocidal blend
87.1% (w/w) distilled water
Natural Biocidal Blend
66.44% (w/w) n-butanol
4.65% (w/w) cinnamic aldehyde
1.99% (w/w) isoeugenol
0.33% (w/w) thymol
0.50% (w/w) carvacrol
21.93% (w/w) phenyl ethyl alcohol
2.33% (w/w) hexyl acetate
1.83% (w/w) eucalyptus oil
100671 Based on the 0.3% by weight concentration of the natural
biocidal blend within the
first aqueous composition, the total concentration of n-butanol with the
composition was 0.2% by
weight. Both compositions were homogenous, with no visible lens formation.
Both compositions
were placed in separate containers for further testing.
Example 3: Flash Point Determination of Aqueous Compositions
[0068] The flash point of the aqueous compositions of Example 2
where determined, using a
Pensky-Martens Closed Cup flash point test conducted in accordance with
American Society for the
Testing of Materials (ASTM) D93, Standard Test Methods for Flash Point by
Pensky-Martens
Closed Cup Tester, which can be used for determining the flash point of
petroleum products over a
temperature range of 40 C to 360 C.
[0069] Observed flash point temperatures were corrected using the
equation, FP = T +
0.033(760 ¨ P), wherein T = Observed flash point temperature ( C) and P =
Barometric pressure
23
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
(mm Hg). The actual flash point of the sample was then reported as the
corrected temperature. The
corrected temperature was rounded to the nearest 0.5 C.
100701 Two samples were tested of the first aqueous composition
from Example 2. The
observed flash points were 56 C (+/- 1 C) and 55 C (+/- 1 C), which were
corrected to 56.5 C
(+/- 1 C) and 55.5 C (+/- 1 C), respectively. Two samples were also tested of
the second aqueous
composition from Example 2. The observed flash points for both samples were 63
C (+1- 1 C),
which were both corrected to 63.5 C (+/- 1 C).
Example 4: Determination of Aqueous Composition Surface Tension
10071.1 The surface tensions of the aqueous compositions of
Example 2 were determined by
measuring the height of a liquid column of the composition within a capillary
tube. Generally,
several drops of a composition were pipetted into one or more wells within a
multi-well porcelain
spot plate until the cavity was filled to within 1-mm from the top surface.
Upon dipping a capillary
tube, such as a micro-hematocrit capillary tube, into the center of the cavity
to draw up liquid, the
bottom of the tube was sealed with Sigilium wax and placed into a capillary
tube holding tray.
Several additional samples were prepared from other cavities within the spot
plate, using additional
capillary tubes. The height of the liquid in each tube was measured using a
caliper or other
measuring device capable of reading at 0.01-mm increments, from a point at the
top of the wax seal
to the meniscus of the fluid within the tube.
100721 The column height of compositions within multiple
capillary tubes in the same test
set were compared against each other to determine a mean and standard
deviation across the entire
data set. Most data sets contained at least 6 tubes. Data points that were
more than 2 standard
deviations away from the mean are discarded. Each data set also typically
contained standards
consisting of a deionized water composition (72 dyne/cm at 20 'C) and pure
ethanol (22 dyne/cm at
20 C). By including the two standards in each data set, a temperature-
corrected curve for the
change in surface tension as a function of ethanol concentration could be
generated, using surface
tensions reported by Vasquez, et al., above.
100731 The method was verified by comparing the column height of
experimentally prepared
dilutions of isopropanol, using deionized water and 100% (w/w) isopropanol
compositions,
respectively, as the two standards. A standard curve of the surface tension of
known concentrations
of dilute isopropanol compositions was generated, using data from Vasquez, et
al. The line of best
fit was a sixth-order polynomial, having an R2 value of 99.8%, as shown in
Figure 2. The column
24
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
heights of the two standards were plotted against the isopropanol
concentration (either 0% w/w or
100% w/w) to generate an equation defining the linear relationship between the
height of the liquid
inside each capillary tube and the surface tension of the liquid. Several sets
of compositions of
dilute isopropanol in water were formulated and tested according to the
procedure above, in which
each set tested a different concentration of isopropanol. Each set contained 6
tested samples. The
mean column height from each set was fit to the column height/surface tension
equation to
determine the mean surface tension of each set. The mean surface tension of
each set was then
plotted against the concentration of isopropanol of samples within each set,
to generate a curve that
can be overlaid with the curve generated from the literature data. The
overlaid curves shown in
Figure 2 indicate that the surface tension curve generated by the capillary
tube test method described
herein is in strong agreement with the surface tension curve generated by
Vasquez, et al. As a result,
the capillary tube test method can be utilized to determine the surface
tension of any of the aqueous
compositions described herein.
100741 The capillary tube test method was utilized to evaluate
the surface tension of the
compositions prepared in Example 2. A standard curve correlating the column
height of the liquid
inside the capillary tube as a function of surface tension was generated,
using deionized water and
100% (w/w) ethanol as the standards. Six samples were included in each set,
and the temperature of
the room was approximately 22 C. Using the capillary tube test method, it was
determined that the
surface tension of the first aqueous composition of Example 2 was 25 dyne/cm,
and the surface
tension of the second aqueous composition was 43 dyne/cm. Surprisingly, the 25
dyne/cm surface
tension of the first aqueous composition is approximately equivalent to the
surface tension of a
composition containing 80% (w/w) ethanol, even though the total alcohol
concentration within the
first aqueous composition is about 3.2% (w/w). it is expected that the
difference in the surface
tension of the compositions, if measured at 20 'C, would be insubstantial,
based on the data
disclosed in Vasquez, et at. Accordingly, the first aqueous composition of
Example 2, containing
both an alcohol blend and a natural biocidal blend, was shown to have both a
flash point above
50 C and a surface tension below 30 dyne/cm, in agreement with the modeling
in Example 3, above.
Example 5: Multidimensional Analysis of the Surface Tension and Flash Points
of Aqueous
Compositions Selected Alcohols
100751 A study was conducted in accordance with embodiments of
the present disclosure to
predict the surface tension and flash point of aqueous compositions containing
one or more alcohol
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
compounds, at various concentrations of each alcohol compound. The known
physical properties of
several alcohols were compiled into MP, a statistical analysis software
available from SAS Institute,
Inc, which is able to analyze, model, and visualize data over several
variables in order to determine
correlations between variables over several dimensions. JMP was utilized to
model the effect of the
identity and concentration of an alcohol compound, either within a blend or on
its own, on the
surface tension or flash point of an aqueous composition.
[0076] Alcohol compounds that were studied in IMP were: methanol,
ethanol, n-propanol,
isopropanol, n-butanol, 2-butanol, isobutanol, i-butanol, n-pentanol, 2-
pentanol, 3-pentanol, 1-
hexanol, 2-hexanol, 3-hexanol, 2-methylbutano-1-ol, 3-methylbutan-l-ol, 2-
methylbutan-2-ol, and
3-methylbutan-2-ol. Acetic acid and hydrogen peroxide were also included in
the .IMP model.
Physical properties of each compound that were compiled into JMP include:
molecular weight;
number of primary carbon atoms, number of secondary carbon atoms, number of
tertiary carbon
atoms, effective number of carbon atoms, total number of carbon atoms,
position of the alcohol
group, boiling point, density, flash point, surface tension, and aqueous
solubility. Physical
properties that were statistically correlated by JMP with a change in surface
tension or flash point
can then be utilized to predict the surface tension or flash points of aqueous
compositions having one
or more alcohol compounds at user-defined concentrations.
[0077] Statistically correlated variables in the determination of
surface tension of a
composition were: water concentration, concentration of alcohols by type
(primary, secondary, or
tertiary); and the concentration of acetic acid in the acetic acid-containing
composition. Statistically
correlated variables in the determination of flash point were water
concentration and concentration
of alcohols by type.
[0078] The concentrations of alcohol compounds within the first
aqueous composition of
Example 2 were studied to determine what concentration limits could still
result in a flash point
greater than about 50 'C, +/- 0.50. Generally, the concentration of n-butanol,
contained within the
natural biocidal blend, was maintained at a constant 0.2% (w/w). Additionally,
the concentration of
the isopropanol within the composition was maintained at approximately 5% of
the total ethanol
concentration, based on the 95:5 blend of ethanol to isopropanol used as an
ethanol stock solution.
JMP modeling indicated that ethanol and isopropanol concentrations within the
first aqueous
composition can be increased to 3.2% (w/w) and 0.16% (w/w), respectively,
while maintaining a
constant n-pentanol concentration of 0.9% (w/w), to yield a predicted flash
point of about 49.8 C.
26
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
The predicted surface tension of the composition having 3.2% (w/w) ethanol,
0.16% (w/w)
isopropanol, 0.2% (w/w) n-butanol and 0.9% (w/w) n-pentanol (total alcohol
concentration of about
4.5% (w/w) was 24.5 dyne/cm.
[0079] Similarly, the concentration of the n-pentanol can be
increased to 1.6% (w/w), while
maintaining a constant ethanol and isopropanol concentrations of 1.9% (w/w)
and 0.1% (w/w),
respectively, to yield a predicted flash point of about 49.5 C. The predicted
surface tension of the
composition having 1.9% (w/w) ethanol, 0.1% (w/w) isopropanol, 0.2% (w/w) n-
butanol and 1.6%
(w/w) n-pentanol was 21.7 dyne/cm.
Example 6: Antimicrobial Efficacy of Peracids Formed in situ against
Staphylococcus aureus,
Pseudontonas aeruginosa, and Candida auris
[0080] A study was conducted in accordance with embodiments of
the present disclosure to
determine the antimicrobial kill of peracids formed within reaction layers in
situ against selected
microorganisms Staphylococcus aureus, Pseudomonas aeruginosa, and Candida
auris, which are
known to cause hospital acquired infections. Antimicrobial efficacy against
Staphylococcus tutreus
(ATCC #6538) and Pseudomonas aeruginosa (ATCC #15442) were determined using
the
Association of Official Agricultural Chemists Germicidal Spray Method (AOAC
961.02). Test
substance exposure consisted of three sprays of the first aqueous composition
of Example 2 from a
first hand-sprayer, followed by three sprays of the second aqueous composition
of Example 2 from a
second hand-sprayer. Following exposure, the carriers were transferred to
vessels containing
neutralizing subculture medium comprising Letheen Broth, 0.28% (w/w) lecithin,
2.0% (w/w)
Tween 80, 0.2% (w/w) sodium thiosulfate, and 0.05% (w/w) catalase. The
subcultures were
incubated at 20 C for 48 hours and assayed for survivors. Appropriate culture
purity, viability,
organic soil load sterility, neutralizing subculture medium sterility, carrier
sterility, carrier
population and neutralization confirmation controls were performed in
parallel.
[0081] Glass carriers inoculated with Staphylococcus aureus (vrcc
#6538) were exposed to
the reaction layer formed by the first and second aqueous compositions for 9.5
minutes prior to
transferring the carriers to subculture medium. 120 glass carriers were
inoculated-60 that were
supplemented with FBS and 60 that were not supplemented with FBS. The average
number of
colony forming units (CFU) per carrier ranged from 3.87 x 105 to 6.5 x 105
(Logics = 5.59 to 5.81).
All 60 of the carriers without FBS present, as well as 59 of the 60 carriers
containing FBS,
demonstrated no growth of bacteria in the subculture medium, indicating over a
log-5 kill of the
27
CA 03170617 2022- 9- 2
WO 2021/178774
PCT/US2021/021045
Staphylococcus aureus in 119 of the 120 carriers upon contact with the peracid-
containing reaction
layer for 9.5 minutes.
100821
Sets of 60 glass carriers inoculated with Pseudomonas aeruginosa
(ATcc, /415442)
were exposed to the reaction layer formed by the first and second aqueous
compositions for multiple
time points ...... 30 seconds, 45 seconds, 60 seconds, 4 minutes, and 9.5
minutes ...... prior to transferring
the carriers to subculture media. All 300 of the glass carriers were
supplemented with FBS. The
average number of CFU per carrier ranged from 1.7 x 105 to 8.2 x 106 (Logic,
5.23 to 6.50). All of
the carriers (180 total) that were exposed to the peracid-containing reaction
layer for 60 seconds, 4
minutes, or 9.5 minutes demonstrated no growth of bacteria in the subculture
medium, indicating
over a log-5 kill, and in some instances over a log-6 kill, of the Pseudomonas
aeruginosa in those
carriers after being exposed for at least 60 seconds. 57 of the 60 carriers
that were exposed to the
peracid-containing reaction layer for 45 seconds demonstrated no growth of
bacteria in the
subculture medium, and 56 of the 60 carriers that were exposed to the peracid-
containing reaction
layer for 30 seconds demonstrated no growth of bacteria in the subculture
medium, indicating over a
log-5 kill, and in some instances over a log-6 kill, of the Pseudomonas
aeruginosa in those carriers.
100831
Antimicrobial efficacy against Candidly curls (CDC #AR-0381) was
determined
using the Organization for Economic Co-Operation and Development (OECD)
Quantitative Method
for Evaluating Efficacy of Liquid Antimicrobials. The procedure for testing is
similar to the
procedures described above for determining the efficacy against Staphylococcus
aureus and
Pseudomonas aeruginosa, in which: glass carriers inoculated with Candida curls
and supplemented
with 5% (w/w) FIBS are exposed to a peracid-containing reaction layer formed
from combining the
first aqueous composition and the second aqueous composition from Example 2 on
the carrier
surface; and transferring those carriers to subcultures to evaluate the growth
of CVU's within the
subculture medium. 25 IA, of each aqueous composition was separately dispensed
onto the carrier
surface and allowed to mix to form the reaction layer on the carrier. The
subculture medium for
evaluating growth of Candida auris is identical to the subculture medium
described above. The
average number of CFU per carrier was 3.02 x 105 (Logi = 5.48), and each
carrier was exposed to
the peracid-containing reaction layer for 9.5 minutes. 59 of the 60 carriers
demonstrated no growth
of bacteria in the subculture medium, indicating over a log-5 kill on those
carriers within 9.5
minutes.
28
CA 03170617 2022- 9- 2