Note: Descriptions are shown in the official language in which they were submitted.
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
SYSTEMS AND METHODS FOR BOTULINUM TOXIN OR OTHER DRUG
INJECTIONS FOR MEDICAL TREATMENT
BACKGROUND
This application is a continuation in part of application serial no.
16/570,515, filed September 13, 2019, which claims priority from provisional
application
serial no. 62/734,918, filed September 21, 2018. The entire contents of each
of these
applications are incorporated herein by reference.
Technical Field
This application relates to systems and methods for drug injections, and more
particularly, to customized patient masks, and processes for forming the
masks, for
facilitating injections of drugs such as botulinum toxin.
Background of Related Art
Botulinum toxin is a neurotoxin made from a toxin produced by the bacterium
Clostridium botulinum. Doctors use this drug in small doses in cosmetics for
temporary
smoothing of facial wrinkles and improving appearance. The injections work by
weakening or paralyzing certain muscles or by blocking certain nerves. The
effects last
about three to twelve months, depending on what is being treated. There are
various
types of botulinum toxin, marketed under brand names such as Botox
(onabotulinumtoxinA, Allergan) and Dysport/Azzalure (abobotulinumtoxinA,
Ipsen/Galdemia), Xeomin/Bocouture (incobotulinumtoxinA, Merz) and Jeuveau
(prabotulinumtoxinA, Evolus/Daewoong), along with several others in clinical
trials.
Botulinum toxin is typically injected in the human face. The effects of
current
botulinum toxin injections for glabellar lines (lines between the eyes)
typically last two to
four months, although this is patient, and in some cases, product-dependent,
with some
patients experiencing a longer duration of effect. Injection of botulinum
toxin into the
muscles under facial wrinkles causes relaxation of those muscles, resulting in
the
smoothing of the overlying skin. Smoothing of wrinkles is usually visible
three-five days
1
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
after injection, with maximum effect typically a week following injection.
Muscles can
be treated repeatedly to maintain the smoothed appearance.
Patients frequently complain about how their botulinum toxin (e.g., Botox,
Dysport, Xeomin) injections for wrinkles/frown lines differ due to variability
in where it
is injected at various practitioner's offices or even in some cases in the
same office by the
same practitioner. This variability, for example, can change the shape of
one's eyebrows,
making them too arched, too flat, drooping, etc., or not provide the desired
wrinkle
reduction. This variability also results in a different appearance than the
patient might
have expected. The variability in the patient anatomy and muscles under the
skin is the
reason there is variability in patient results with injections.
The need exists for achieving more consistent results for botulinum toxin
injections. The need further exists for botulinum injections which better
conform to the
individual patient's objectives. Such systems which deliver more consistent
results and
better conformance to desires would beneficially also have use in other
surgical
applications/procedures. Such systems would also be beneficial if they could
improve
results while also improving convenience and reducing costs to the patient.
SUMMARY OF THE INVENTION
The present invention advantageously overcomes the problems and deficiencies
of the prior art. The present invention provides various systems and methods
to achieve
more consistent results from botulinum toxin injections (or from other drug
injections).
The present invention also provides various systems and methods that are more
responsive to the patient's wishes. These systems and methods provide for
input of the
anatomical structure of the patient's face and/or scalp (or other body
regions), input of
location for injections, and/or creation of 3D masks with injection locators
in response to
the input. In alternate embodiments of the present invention, the 3D masks are
created
with openings for markers so that the physician can mark the patient's skin to
provide
consistent injection locations. Each of these systems and methods are
discussed in detail
below.
The present invention provides customized masks that can in their simplest
forms
provide locators for fluid injections by the physician. The present invention
in alternate
2
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
embodiments can provide customized masks with locators for attachable
injection
devices or with integral injection devices. The present invention in alternate
embodiments
provides customized masks with locators for skin markings on the patient.
These various
embodiments are discussed in detail below.
The present invention also provides in some embodiments systems and methods
that enable the patient rather than the doctor to inject the fluid, as
discussed in detail
below.
In accordance with one aspect of the present invention, a method for creating
a
customized cover (mask) for injection of drugs into a patient is provided, the
method
comprising a) marking on a skin of a patient indicators to identify locations
for drug
injections: b) taking an image of the skin with the markings; and c) inputting
the image to
create a cover with openings corresponding to the markings on the skin, the
openings
extending through the cover dimensioned for insertion of markers through the
cover to
enable repeated markings through the cover.
In accordance with another aspect of the present invention, a method for
creating
a customized cover for injection of drugs into a patient is provided based on
utilization of
a customized cover formed by data input of anatomical features of a patient,
the method
comprising a) marking on a skin of a patient indicators to identify locations
for drug
injections; b) taking an image of the skin with the markings thereon to
provide data input
for the cover; and c) after obtaining a customized cover with openings in the
cover,
inserting a marking device through at least one of the openings to mark the
skin for
injection locations.
In accordance with some embodiments, the method further comprises the step of
marking the skin with varied color markings to indicate a different dosage of
the drug for
injection.
In some embodiments, the method further comprises the step of removing the
cover after the markings are made through the openings with one or more
marking
devices and injecting the drug at the locations of markings.
In some embodiments, the cover has designations integrally formed thereon, or
alternatively applied to the cover after the cover is formed, to indicate
dosage, and the
method further comprises removing the cover and injecting the drug into the
patient in
3
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
accordance with the indicated dosage.
In some embodiments, the method further comprises the step of aligning the
cover
on the skin in accordance with at least one alignment marker on the cover.
In some embodiments, the method further comprises the step of editing the
image
to provide a revised image and uploading the revised image to provide a guide
for
forming the cover.
In accordance with another aspect of the present invention, a method for
providing consistent injection of a drugs through a skin of a patient is
provided, the
method comprising the steps of:
a) manually providing first markings on the skin of a patient to provide
locators for drug injection;
b) taking a first image of the skin with the first markings thereon;
c) storing the first image;
d) injecting a drug into the patient at the location of the first markings;
e) after a period of time, evaluating the result of the injection of the drug
and
either i) editing the first image to adjust one or more injection locations or
ii) not editing
the first image;
f) after obtaining a cover made in accordance with either the edited or not
edited first image, placing the cover on the skin and manually providing
second markings
through openings in the cover; and
g) removing the cover and injecting the drug at the second markings.
In some embodiments, the method further comprises the step of uploading either
the edited or unedited first image to a web portal for linking with a device
for
manufacturing the cover.
In accordance with another aspect of the present invention, a customized cover
for
placement over a portion of a body of a patient is provided, the cover having
a plurality
of openings extending therethrough configured and dimensioned for manual
insertion of
markers by a health care provider to provide marked locations on the patient
for injection
of one or more drugs. The cover is manufactured from a set of instructions
produced from
a software based application processing an image of an anatomical view of the
patient
with markings thereon. The cover is customized to the portion of the body
based on the
4
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
software based application and the plurality of openings are customized to the
desired
locations of markings through the cover for subsequent injection of one or
more drugs
independent of the cover.
In some embodiments, the cover is configured for placement over a face of a
patient. In some embodiments, the cover includes one or more alignment
markings to
align the cover on the body. In some embodiments, the cover includes dosage
indicators
thereon.
In some embodiments, the image is inputted (uploaded) to a website portal
linked
to a software application on a device which receives and processes data
corresponding to
desired locations of drug injection based on the manually marked locations to
provide the
set of instructions for manufacture of the cover. In some embodiments, the
image is
manipulated prior to input to a website portal; in other embodiments the image
is
uploaded to the website portal and then manipulated.
In some embodiments, the customized cover is formed by 3D printing.
In some embodiments, the cover is manufactured based on human editing on
the software program.
In accordance with another aspect, a method of manufacturing a customized
cover
for placement over a portion of a body of a patient is provided, comprising
interpreting a
set of instructions produced from a software based application processing an
image of an
anatomical view of the patient made with marked locations thereon, customizing
the
cover to the portion of the body based on the software based application, and
customizing
a plurality of openings in the cover to the desired locations of markings for
subsequent
injection of one or more drugs at the markings without the cover, i.e.,
independent of the
cover. The cover has a plurality of openings extending therethrough configured
and
dimensioned for manual insertion of markers by a health care provider to
provide marked
locations on a patient for injection of one or more drugs once the cover is
removed from
the patient
In some embodiments, the cover is manufactured based on an image inputted to a
website portal linked to a software application on a device which receives and
processes
data corresponding to desired locations of drug injection by the patient,
based on
manually marked locations, to provide instructions for manufacture of the
cover.
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
In accordance with another aspect of the present invention, a method for
making a
customized cover for placement over a portion of a body of a patient is
provided. The
cover is made with a plurality of openings extending therethrough configured
and
dimensioned for manual insertion of markers by a health care provider to
provide marked
locations on the patient for injection of one or more drugs. The cover is made
based on
input of a set of instructions produced from a software based application
processing an
image of an anatomical view of the patient with markings thereon. The cover is
made
customized to the portion of the body based on the software based application
and the
plurality of openings are made customized to the desired locations of markings
through
the cover for subsequent injection of one or more drugs independent of the
cover.
In accordance with another aspect of the present invention, a software based
system for enabling consistent injection of a drugs through the skin of a
patient is
provided comprising a) storing a first image of the skin having first markings
thereon
corresponding to select regions for one or more injection locations; b)
storing a revised
image, the revised image based on one of human input or software generated
input to
adjust the one or more injection locations; and c) uploading the revised image
for
processing by a manufacturer to create a customized cover for placement over
the skin of
the patient.
In some embodiments, the software provides instructions to manufacturer the
cover with openings solely to receive markers for marking the patient's skin
for direct
injection at the markings.
In some embodiments, the revised image is uploaded to a web portal for
processing by the manufacturer. In some embodiments, the revised image is not
editable
by the manufacturer.
In accordance with another aspect of the present invention, a cover (mask) for
placement over a portion of a body of a patient is provided. The cover has a
plurality of
openings extending therethrough for injection of a drug into the body, the
cover being
customized to the portion of the body and the plurality of openings being
customized to
the desired locations of injection of the drug through the openings and into
the body.
The openings in some embodiments are configured for passage of an injection
6
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
needle through the cover and into the body. The cover can be configured for
placement
over a face of a patient, an abdomen of the patient, or other body regions
such as the
chest, arms legs or back.
In some embodiments, a plurality of injection devices are attached to the
cover,
each of the injection devices communicating with one of the plurality of
openings for
injection of the drug through the openings. The injection devices can in some
embodiments be provided pre-filled with the drug.
In some embodiments, the cover includes a plurality of guides extending
proximally from the cover having a lumen to receive at least a portion of an
injection
device, and the guides communicate with respective openings of the cover. In
some
embodiments, the guides are removably attachable to the cover; in other
embodiments,
the guides are integral with the cover.
In accordance with another aspect of the present invention, a cover for
placement
over a portion of a body of a patient is provided. The cover has a plurality
of openings
for injection of a drug into the body, the cover being customized to the
portion of the
body. The cover has a plurality of guides extending proximally from the cover
in
alignment with the openings, the guides having a lumen configured to receive
at least a
portion of an injection device for injection of the drug.
In some embodiments, the guides are removably attachable to the cover; in
other
embodiments, the guides are integral with the cover. The cover can in some
embodiments, include one or more alignment markings to align the cover on the
body.
In accordance with another aspect of the present invention, a system for drug
injection into an anatomical region of a patient is provided. The system
includes an input
subsystem to provide data on the region of the patient and an output subsystem
to provide
a customized cover for the region of the patient, the customized cover having
openings to
enable passage of the drug through the cover into the region of the patient.
In some embodiments, the input subsystem includes images of the region of the
patient. The images can be digital photos, created by a 3D scanner or by other
methods.
In some embodiments, the input subsystem includes an app for receiving and
processing data corresponding to desired locations of drug injection by the
patient. In
some embodiments, the output subsystem includes a 3D printing of the
customized cover
7
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
with the openings pre-formed in the cover. In some embodiments, the input
subsystem
includes markings placed on the images or, alternatively, markings placed on
the region
prior to imaging, to provide opening (injection) locators.
The system can include a plurality of injection devices removably mountable to
the cover for injection of the drug into the region of the patient.
In accordance with another aspect of the present invention, a system for drug
injection into an anatomical region of a patient is provided. The system
includes an input
subsystem to provide data on the region of the patient and an output subsystem
to provide
a customized cover for the region of the patient, the customized cover having
openings to
enable passage of markers through the cover into the region of the patient.
In accordance with another aspect of the present invention, a kit for
injecting a
drug into a patient is provided comprising a) a cover for placement over a
portion of a
human body, the cover having a plurality of openings for injection of a drug
into the
body, the cover being customized to the patient; and b) a plurality of
injection devices for
connection to the cover to inject the drug through the openings in the cover.
In some embodiments, the injection devices are pre-filled with the drug.
In accordance with another aspect of the present invention, a method for
creating
a customized cover to provide location regions for injection of drugs into a
patient is
provided, the method comprising the steps of:
a) processing images of a region of the patient;
b) identifying locations for openings in the cover based on the images to
identify locations for drug injection; and
c) creating the customized cover with the openings.
In some embodiments, the method further comprises the step of taking images of
the region of the body for processing of the images.
In some embodiments, the method further comprises the step of receiving images
of the region of the body obtained by an outside source, the receiving step
occurring
before the step of processing the images. In some embodiments, the images are
taken via
a digital camera; in other embodiments, the images are taken via a scanner.
In some embodiments, the step of creating the cover comprises the steps of
providing a 3D image for processing for performing step (a) above and 3D
printing the
8
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
customized cover. In some embodiments, the method includes the step of
shipping the
cover to a doctor's office; in other embodiments, the method includes the step
of shipping
the cover directly to the patient.
In accordance with another aspect of the present invention, a method for
creating
a customized cover for injection of drugs into a patient is provided, the
method
comprising a) identifying locations for openings on a 3D image to identify
locations for
pre-formed openings in the cover for drug injection; and b) creating the cover
with the
openings. The method can in some embodiments include the step of creating the
cover
with a plurality of guides for receiving injection devices for injection of
the drugs.
In accordance with another aspect of the present invention, a method for
creating
input for a customized cover for injection of drugs into a patient is
provided, the method
comprising a) identifying locations for openings to identify locations for pre-
formed
openings in the cover for drug injection; and b) storing a 3D image with the
identified
locations.
In some embodiments, the step of identifying locations for openings comprises
marking the locations directly on the body region of the patient. In other
embodiments,
the step of identifying locations for openings comprises marking the locations
on a 3D
image of the body region of the patient.
The method can further include the step of transferring the stored data to a
system
for creating the customized mask. In some embodiments, the system is a 3D
printer. In
some embodiments, the method includes the step of taking digital photos to
create the 3D
image.
In accordance with another aspect of the present invention, a method for
creating
input for injection of drugs into body region of a patient is provided
comprising a)
identifying body region locations to identify locations for drug injection; b)
storing a 3D
image with the identified locations; and c) projecting the image onto the body
region. In
some embodiments, the image is projected from a mobile device.
In accordance with another aspect of the present invention, a method for
creating
a customized cover for injection of drugs into a patient is provided, the
method
comprising a) marking on a skin of a patient indicators to identify locations
for drug
injections: b) taking an image of the skin with the markings; and c) utilizing
the image to
9
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
create a cover with openings corresponding to the markings on the skin, the
openings
extending through the cover to enable drug injection through the cover.
In some embodiments, the method includes the step of providing a coded
designation adjacent the openings to indicate the desired dosage of the drug
injection
through the openings. In some embodiments, the coded designation is integral
with the
cover. In other embodiments, the coded designation is applied to the cover
after the
cover is formed.
In accordance with another aspect of the present invention, a method of
creating a
customized cover for drug injection is provided, the method comprising the
steps of
e) receiving, by an apparatus, input from a patient regarding cosmetic
parameters;
0 identifying, by the apparatus, in response to receiving the input, locations
for openings for the customized cover; and
g) storing, by the apparatus, the identified locations on a 3D image contained
by the apparatus.
In some embodiments, the method includes the step of creating a 3D model from
the stored image of step (c). The method can include printing via a 3D printer
the cover
from the 3D model. In some embodiments, the step of receiving input includes
responses
to questions regarding appearance objectives of the patient.
BRIEF DESCRIPTION OF THE DRAWINGS
Preferred embodiment(s) of the present invention are described herein with
reference to the drawings wherein:
Figure 1 A is a diagram of the overall system in accordance with one
embodiment
of the present invention;
Figure 1B is a diagram of the overall system in accordance with an alternate
embodiment of the present invention;
Figure 2A is a flow chart depicting in more detail the steps of the system of
Figure 1A;
Figure 2B is a flow chart depicting in more detail the steps of the system of
Figure
1B;
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
Figure 2C is a flow chart depicting an alternate system of the present
invention for
use by a physician;
Figure 2D is a flow chart depicting an alternate system of the present
invention
for use by a patient without the role of the physician;
Figure 2E is a flow chart depicting an alternate system of the present
invention;
Figure 2F is a flow chart depicting an alternate system of the present
invention;
Figure 2G is a flow chart depicting an alternate system of the present
invention;
Figure 2H is a flow chart depicting an alternate system of the present
invention;
Figure 21 is a flow chart depicting an alternate system of the present
invention;
Figure 2J is a block diagram depicting manufacture of the cover in accordance
with some embodiments of the present invention;
Figure 2K is flow chart depicting a software based system of the present
invention;
Figure 3 is a block diagram illustrating the alternate injection methods of
the
customized mask of the present invention;
Figure 4 is a block diagram illustrating the system of the embodiment of
Figure
IA;
Figure 5A is a front perspective view of one embodiment of a customized
forehead and lateral eye mask (cover) of the present invention with preformed
holes;
Figure 5B is a front perspective view of an alternate embodiment of a
customized
mask of the present invention having alignment lines;
Figure 5C is a perspective view of an alternate embodiment of the cover of the
present invention having dosage indicators.
Figure 6 is a front perspective view of an alternate embodiment of a
customized
full face mask of the present invention;
Figure 7A is a front perspective view of another alternate embodiment of a
customized forehead and lateral eye mask of the present invention having
replaceable
mini-syringes attachable/mountable to the mask;
Figure 7B is a perspective view of the mini-syringe used with the mask of
Figure
7A;
Figure 8 is a front perspective view of another alternate embodiment of a
11
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
customized forehead and lateral eye mask of the present invention having pre-
loaded
syringes attached to the mask;
Figure 9 is a front perspective view of another alternate embodiment of a
customized forehead and lateral eye mask of the present invention having an
external
injection mechanism;
Figure 10 is a front perspective view of another alternate embodiment of a
customized mask of the present invention positioned on a patient's forehead
and scalp;
Figure 11 is a rear perspective view of the customized mask of Figure 10;
Figure 12A is side perspective view of a mask in accordance with one
embodiment of the present invention;
Figure 12B is an enlarged view of the area of detail A-A identified in Figure
12A
showing the guide channels in accordance with one embodiment of the present
invention
prior to attachment to the mask;
Figure 12C is an enlarged view similar to Figure 12B showing an alternate
embodiment wherein the guide channels are built into (integral with) the mask;
Figure 12D is a perspective view showing a syringe (injection device) prior to
insertion into the syringe guide channel (extending from a portion of the
mask) of Figure
12B;
Figure 13 is a front view showing the syringe inserted into the guide channel
of
Figure 12B;
Figure 14 is a cross-sectional view taken along line A-A of Figure 13;
Figure 15 is a close up view of the area of detail B of Figure 13;
Figure 16 is a close up view similar to Figure 15 showing an alternate
embodiment of the syringe guide channel of the present invention;
Figure 17 is a close up perspective view of the guide channel of Figure 12B
prior
to insertion into the mask;
Figure 18 is a close up perspective view of the guide channel of Figure 12B
attached to the mask;
Figure 19 is a perspective view showing a syringe and a needle guide channel
in
accordance with an alternate embodiment of the present invention;
Figure 20 is a front view showing the syringe inserted into the guide channel
of
12
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
Figure 19;
Figure 21 is a cross-sectional view taken along line D-D of Figure 20;
Figure 22A is side perspective view of a mask in accordance with an alternate
embodiment of the present invention;
Figure 22B is an enlarged view of the area of detail B-B identified in Figure
22A
showing the guide channels in accordance with an alternate embodiment of the
present
invention;
Figure 22C is a perspective view showing a syringe prior to insertion into the
syringe guide channel (extending from a portion of the mask) of Figure 22B;
Figure 23 is a front view showing the syringe inserted into the guide channel
of
Figure 22, the needle shown in the retracted position;
Figure 24 is a cross-sectional view taken along line F-F of Figure 23;
Figure 25 is a front perspective view of an abdominal mask (cover) of the
present
invention shown wrapped around a portion of the patient's body;
Figure 26 is a rear perspective view of the abdominal mask of Figure 25
showing
the closed strap;
Figure 27 is a view similar to Figure 25 showing the syringe prior to
insertion into
the guide channel of the abdominal mask;
Figure 28 is a view similar to Figure 27 showing the syringe inserted into the
guide channel of the abdominal mask;
Figure 29 is a front view of an abdominal mask of Figure 25;
Figure 30 is a top view of the abdominal mask of Figure 25;
Figure 31 is a close up view of the area of detail H of Figure 29 showing the
guide
channel and umbilicus alignment hole of the mask of Figure 25, and further
showing a
syringe inserted into one of the guide channels;
Figure 32 is a cross-sectional view taken along line G-G of Figure 29;
Figure 33 is close up view of the area of detail J of Figure 32;
Figure 34 is a view similar to Figure 33 showing an alternate embodiment of
the
syringe guide channel having a shorter height to increase the depth of needle
insertion;
Figure 35A is side perspective view of a mask (cover) in accordance with
another
alternate embodiment of the present invention;
13
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
Figure 35B is an enlarged view of the area of detail K-K identified in Figure
35A
showing the guide channel supports (mask mating features) in accordance with
one
embodiment of the present invention, the supports shown prior to attachment to
the mask;
Figure 35C is an enlarged view similar to Figure 35B showing an alternate
embodiment wherein the guide channel supports are built into (integral) with
the mask;
Figure 35D is an enlarged view similar to Figure 35B showing the syringe guide
channels prior to insertion into the guide channel supports f Figure 35B;
Figure 35E is a perspective view of the support and the syringe guide channel
of
Figure 35D prior to insertion of the guide channel into the support;
Figure 36 is a perspective view showing the syringe guide channel of Figure
35E
inserted into the support of the mask;
Figure 37 is a perspective view showing the syringe prior to insertion into
the
guide channel of Figure 36;
Figure 38 is a perspective view showing the syringe inserted into the guide
channel of Figure 36;
Figure 39 is a side view showing the syringe guide channel of Figure 35D
clicked
into the support (mating feature) of the mask;
Figure 40 is a top view of the syringe guide channel of Figure 35D;
Figure 41 is a cross-sectional view taken along line L-L of Figure 40;
Figure 42 is a perspective view of the support of Figure 35D;
Figure 43 is a side view of the support of Figure 35D;
Figure 44 is a top view of the support of Figure 35D;
Figure 45 is a side view of the syringe inserted into the guide channel of
Figure
36;
Figure 46 is cross-sectional view showing the syringe inserted into the guide
channel of Figure 36;
Figure 47 is a top view of the syringe and guide channel of Figure 45; and
Figure 48 is an enlarged view of the area of detail N of Figure 46.
DESCRIPTION OF PREFFERED EMBODIMENTS
The present invention provides patient masks with pre-formed holes, and
14
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
processes for making such masks, for injection of botulinum toxin, or in
alternate
embodiments, other drugs or medicines. In some embodiments, the masks are
formed to
provide openings for insertion of markers to mark injection locations on the
skin; in other
embodiments, the masks are formed to provide openings for insertion of
injection
needles. In some embodiments, the masks are printed in a doctor's office or in
a health
clinic and provided to the patient's doctor; in other embodiments, the masks
are printed at
a central location and sent to the doctor or provided directly to the patient
who can bring
the mask to the doctor. In still other embodiments, the masks are printed at a
central
location and sent along with pre-filled syringes directly to the patient. In
this latter
embodiment, the patient is injecting the drug without the need for a
physician; in the
former embodiments, the patient relies on the physician to inject the drug.
Each of these
embodiments is discussed in detail below. Note the masks are also referred to
herein as
"covers" as they cover a portion of the patient's body, and are thus not
limited to
covering the patient's face or forehead. Thus, the term "mask" and "cover' are
used
interchangeably herein. The customized mask (cover) forms the output subsystem
of the
overall system of the present invention.
Note that injection by a physician as used herein is meant to differentiate
from
injection by a patient. It should be appreciated that injection by a physician
(also
considered a "health care provider") includes an injection by another health
care
provider, such as a nurse, physician assistant, pharmacist, etc. (and not by
the patients
themselves).
Various imaging devices can be utilized for the foundation for the custom
masks.
Examples of such imaging devices are described below.
In the embodiments described below, the locations for injections can be
provided
by physician input, e.g., the physician marking the particular locations of
the patient's
face or forehead or marking the locations on a 3D image. In alternate
embodiments, the
software algorithm of the app determines the injection locations based on
patient input in
the app. In either case, the mask is created customized to the patient's
anatomy and
goals. These various inputs form input subsystems of the system of the present
invention
to provide data on the anatomical region of the patient and desired injection
locations.
The masks can be of various shapes and configurations. Several examples are
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
shown in the illustrated drawings. The masks can be created by various methods
and in
some embodiments are provided with openings at the desired locations for
injection. In
some embodiments, the masks can also be created with indicators of the
recommended
dosage at each of the injection sites (openings). The syringes for injections
through the
masks can be provided separate from or alternatively in conjunction with the
mask, each
of these versions discussed below. In short, the customized masks of the
present
invention essentially provide a roadmap for drug or medicine injection to
facilitate
injection and provide more consistent injections to provide more consistent
and improved
results. The reuse of the mask on the patient provides a continuous roadmap
for the
multiple injections typically spaced over a determined time period. For reuse
on the
patient, the mask can be wiped down with for example an alcohol pad or an
antiseptic
wipe.
Note as used herein, the syringe forms one type of injection device, it being
understood that other types of injection devices can be utilized to inject the
drug.
Referring now to the drawings wherein like reference numerals identify similar
structural features of the apparatus disclosed herein, Figures 1A and 2A
illustrate one
embodiment of the system/method process of the present invention and Figures
1B and
2B illustrate an alternate embodiment of the system/method process of the
present
invention. The methods differ in how the data is collected for formation of
the
customized masks of the present invention, however, in both systems, the
face/scalp
mask is created with pre-formed holes to satisfy the needs of the individual
patient and
enable predictable and consistent cosmetic results of the treatment. Note the
discussion
of Figures 1A-48 herein is, for convenience, discussed in terms of botulinum
toxin for
cosmetic treatment; however, it should be understood that the same systems and
methods/processes can be utilized for injections of other fluids, e.g. drugs
or medicines,
such as Kybella (deoxycholic acid) by way of example. Thus, the present
invention is
not limited to botulinum injection. Also, the masks (covers) discussed below
which are
placed over the patient's face or forehead for injection locators or for
marker locators are
discussed for such use to provide one example of a cover for the patient for
injections, it
being understood that the covers for injection locators or for marker locators
can
alternatively be placed in other regions of the body, such as over the abdomen
as shown
16
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
in Figures 25-34 by way of example, or in other body regions such as the
chest, arms legs
or back. It should also be understood, that the injection devices and guide
channels
discussed herein can be used with any of the covers disclosed herein, or with
alternative
embodiments of the covers.
Note as used herein the term "distal" denotes components, regions or sections
closer to the patient's body and the term "proximal" denotes components,
regions or
sections further from the patient's body.
Turning initially to Figure 1A, a block diagram of the system of a first
embodiment of the present invention is illustrated. In this embodiment, the
patient takes
photos/pictures and then enters personal information into the smart device,
e.g., IPhone,
IPad, tablet etc. which includes the patient's objectives and desired results
from the
treatment as directed in the app. These inputted photos and information
regarding
cosmetic parameters are then analyzed and processed by the app algorithms (or
in
alternate embodiments by a physician, provider, or technician at a company
remote site)
which is then used to create a three dimensional (3D) representation on the
smart device,
stored for transmission and printing.
In the next step, the 3D representation is transmitted to a 3D printer which
prints a
customized mask or mold for the patient in accordance with the 3D
representation. The
customized mask is printed with openings, responsive to the patient input on
the app, for
fluid injection (or for markers) as described in more detail below. The fluid
injection can
be performed in various ways such as depicted in Figure 3 discussed below.
More detailed steps of the system and method of Figure 1 A are depicted in the
flow chart of Figure 2A which shows the process start to finish. In the first
step, the
patient, with a smart device, takes a photo or series of photos of his/her
face and/or scalp
and/or other body region, depending on the desired location for botulinum
injections. In
the next step, the patient answers a series of questions on an app as to what
the patient
desires with regard to the botulinum toxin injections, e.g., wrinkles,
eyebrows lifted,
natural appearance, etc. In the following steps, the photos are uploaded for
processing
and software on the smart device (or in alternate embodiments a central server
at a
company remote site) converts them to a 3D model. The 3D model, in the next
step, is
then inputted to a 3D printer which prints out a custom mask for the patient's
face and/or
17
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
scalp (or other body region) which has pre-formed holes/openings to guide
injections, the
holes created in response to the input on the app regarding the injections. In
some
embodiments, the software algorithm identifies the location of the holes in
response to
the patient's input regarding the desired treatment results. In other
embodiments, the
physician or technician utilizes the patient's input to map out the location
of the injection
holes on the program. In either case, the customized mask is created by the 3D
printer
and can be sent directly to the patient who can bring it to a physician's
office, or
alternatively, the mask can be sent directly to the physician/practitioner's
office. In some
versions, the physician office or health clinic can be equipped with the 3D
printer so the
masks can be printed at the office or clinic. In any of these arrangements,
when treatment
is scheduled, the mask is placed on the patient and then injections of the
botulinum toxin
are made by the physician in the pre-formed holes in the mask in accordance
with the last
step on the flow chart of Figure 2A. This system improves the
predictability/consistency
of treatment. Note the injections can be in accordance with the variations of
Figure 3
discussed below. Various features on the mask can aid injection as discussed
in detail
below.
The injections through the mask can be effected in several ways, as depicted
in
the diagram of Figure 3: 1) directly through the pre-formed holes (openings)
in the mask
wherein the physician utilizes fluid injection devices inserted through the
holes; 2) via
pre-loaded syringes extending into the mask which can be mounted to the mask
(see e.g.,
Figure 7); and/or 3) via replaceable syringes which are mounted on the mask.
These last
two methods provide a more automated system. The pre-formed holes not only
provide
locators for injections but also inform the physician of the number of units
to inject.
Depth control of injections can be provided by the mask as discussed below.
Various
masks are illustrated in the drawings and are discussed in more detail below.
In an alternate embodiment, depicted in the flow chart of Figure 2E, a
projected
image/hologram is used instead of a 3D printed mask. In this method/system,
the
physician marks the face (or other body region) of the patient with spots
(dots) for fluid
injection, e.g. Botox injection. A photo of the face is taken with a mobile
device or
camera. The saved dot locations are then projected back onto the patient's
face through a
free-standing projector or a projector out of a mobile device. The image with
the dot
18
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
locations and color/unit dosages are then projected back onto the person's
face (like a
hologram) the next time they get their injections to ensure that they are in
the correct
position. Thus, the system involves the method of collecting the data from the
dots on
the face, saving the data, and then "re-marking" the face for the next
injection, utilizing a
projected image/hologram instead of a 3D printed mask.
Figures 1B and 2B illustrate an alternate embodiment of the present invention.
In
this embodiment, instead of utilizing a smart device to create the 3D
representation via
photos and patient input where the software is sent to the 3D printer, a
medical imaging
device such as a three dimensional scanner is utilized. In this system, the
patient goes to
a designated location where the 3D scanner would create an image of the
patient's
face/scalp. The physician or technician identifies on the scanned image the
locations for
the injections in response to the patient's desires, and then the data (image
and location
marks) is transmitted to a 3D printer which prints a customized mask with the
holes
formed at the injection sites designated by the physician or technician. The
customized
mask is then printed with the pre-formed holes for direct injection through
the holes, via
preloaded syringes or through replaceable syringes as shown schematically in
Figure 3.
The mask, as in the embodiment of Figure 2A can be shipped to the patient or
to the
practitioner's office. Thus, the method/system of Figures 1B and 2B differs
from the
system/method of Figures IA and 2A in how the data is created and transmitted
to the 3D
printer. The fluid injection can be in various ways such as depicted in the
drawings
discussed below.
Note the customized mask formed in the manner of Figures 1B and 2B can in an
alternate embodiment, provide holes for the physician to insert a marker to
mark the
injection locations directly on the patient's skin, and the mask removed so
the markings
on the patient's skin provide locators for the syringe and fluid injection (as
explained in
conjunction with Figures 2G-2I). In this version, the fluid injections are not
conducted
through the mask, thereby providing full visibility for the physician during
injection.
Thus, in some systems of the present invention disclosed herein, the mask
(cover)
provides openings (holes) for insertion of injection needles. In alternate
systems of the
present invention, drug injection is not performed through the mask. Instead,
in these
alternate systems/methods/processes, the mask (cover) is provided with
openings for
19
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
insertion by the physician, or other health care provider, of a marker or
other locating
device. In these embodiments, the mask is used to enable markings to be made,
then the
mask is removed and the physician has direct visualization of the patient's
skin as
injections are made at the marked locations. In these embodiments, the same
mask can be
repeatedly used at multiple office visits by the patient so the marks can be
made through
the mask openings at the same location to provide consistent location of drug
injections.
Figure 2C illustrates an embodiment wherein the physician initially marks the
location for injection of Botox (or another drug) directly on the patient.
This
process/system differs from other processes disclosed herein in that instead
of utilizing
the 3D image to determine and mark mask opening locations, the opening
locations are
marked directly on the patient's body, e.g., face of forehead, and then the 3D
image is
taken with the markings.
More specifically, in this process of Figure 2C, dots are first placed by the
provider, e.g.. the physician/clinician, on the patient's skin to designate
injection
locations and dosing. Next an image (preferably a 3D image) is taken with the
marked
dots on the patient's body, the dots used as indicators for openings in the
customized
mask. The injection of the drug, e.g. botulinum toxin, occurs at the marked
locations
prior to the mask being made. The customized mask is not made until after the
botulinum toxin injection has sufficient time for it to fully work, for
example 1-2 weeks,
to confirm the dots were placed in the correct location. Before making the
mask,
feedback (and potentially photos) are obtained from the patient or provider
(possibly via
a questionnaire or input on a mobile app or website) to see how symmetric the
result is
from the injection 1-2 weeks prior. If the result is satisfactory and
symmetric and the
patient is satisfied, then the mask will be made based on the markings (dots)
on record on
the images. If there needs to be a touch-up or the patient is asymmetric, the
dots will be
moved/changed/added/deleted as needed to give what would be the logical
correction of
the asymmetry (based on known musculature under the skin). After the
correction, either
the mask can be made based on the correction or another injection can be made
with a 1-
2 week follow up to assess the results to see if they satisfy the performance,
e.g.,
cosmetic. objectives. In either event, the customized mask is made in
accordance with
the methods/processes disclosed herein.
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
Note in the above process of Figure 2C, optionally a first image of the
patient can
be taken before application of the dots to capture the face (or other body
region) at rest
and potentially in animation without any markings. This could be used for data
collection on wrinkles and facial aging over time. This initial 3D image can
be taken at
the doctor's office or alternatively the patient can take the photos and bring
or transmit
the photos to the doctor's office.
The flow chart of Figure 2E provides an example of the mask (cover) holes,
rather
than providing a site for injection by insertion of a syringe through the
holes, provide a
locator or guide for physician marking. More specifically, in this embodiment,
the
customized masks would be made in accordance with the various processes and
various
configurations disclosed herein, and placed over the patient. The physician
then inserts a
marker through the holes to mark the injection locations directly on the
patient's skin.
The mask is then removed, and the markings on the patient's skin provide
locators for the
syringe and fluid injection. In follow up visits, the mask is again placed
over the patient's
skin, the physician inserts a marker through the holes to mark the sites, and
then the mask
is removed and injections are made at the marked locations. Different colored
markers
can be used to correspond to different dosages and/or different drugs.
Figures 2G-2I provide examples of methods utilizing the mask (cover) holes as
a
guide for physician marking rather than providing for insertion of a syringe
through the
holes for injection. Note the image(s) can be taken in accordance with the
various ways to
take images as described herein (e.g., digital photos, scans, etc.), or by
other alternative
ways. (e.g., symmetric and the patient is satisfied)
Turning first to Figure 2G, in the first step, markings such as dots made with
markers, are manually placed on the patient's skin by the physician (or other
health care
provider) to provide locators for drug injection, e.g., injection of botulinum
toxin. An
image is then taken of the patient's skin, e.g., face, with the markings. The
image is
stored for later evaluation. The drug is then injected into the patient at the
regions of the
markers on the skin. The patient then waits a select period of time, two weeks
by way of
example, then returns to the physician. At this point, the physician can
evaluate the
results of the first treatment by the initial injection. If the results are
satisfactory (e.g.,
symmetric and the patient is satisfied), the first stored image is uploaded to
a web portal
21
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
for manufacture of the 3D mask (or alternatively transmitted directly for
manufacture of
the mask). On the other hand, if the result is not satisfactory (e.g., there
needs to be a
touchup or the patient is asymmetric and the markings, e.g., dots, need to be
moved/changed/added/deleted), the first stored image is edited on the screen
and the
location of the markers (e.g., dots) are adjusted accordingly. Once adjusted,
the edited
image is uploaded to the web portal for manufacture of the 3D mask (or
alternatively
transmitted directly for manufacture of the mask). Note the images can be
taken in
accordance with the various methods disclosed herein, with the software
application
(app) enabling editing, processing and formatting the image for 3D printing
(or other
forms of manufacture).
In the alternate embodiment of Figure 2H, the steps are the same as the method
of
Figure 2G, except a second image is taken of the edited markings. More
specifically, in
the first step, markings such as dots made with markers, are manually placed
on the
patient's skin by the physician (or other health care provider) to provide
locators for drug
injection, e.g., injection of botulinum toxin. An image is then taken of the
patient's skin,
e.g., face, with the markings. The image is stored for later evaluation. The
drug is then
injected into the patient at the markers on the skin. The patient then waits a
select period
of time, two weeks by way of example, then returns to the physician. At this
point, the
physician can evaluate the results of the first treatment by the initial
injection. If the
results are satisfactory, the first stored image is uploaded to a web portal
for manufacture
of the 3D mask. On the other hand, if the result is not satisfactory, new
markings are
made on the skin and a second image is taken to replace the first stored
image. This new
second image is uploaded to the web portal for manufacture of the 3D mask.
Note the
images can be taken in accordance with the various methods disclosed herein,
with the
software application enable editing, processing and formatting the image for
3D printing.
The markings in the foregoing embodiments can be made at a non-rest position
such as raised eyebrows, frown, etc. and then the later evaluation can also be
made at the
non-rest state. The image, e.g., scan, can be taken at a rest state in some
embodiments.
In the foregoing embodiments either the first image is edited if necessary or
a
second replacement image is taken. In an alternate embodiment, depicted in
Figure 21,
comparative images are taken. In this different approach, if the initial
results are not
22
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
satisfactory, a second image is taken and compared to the first image and the
software's
comparative analysis produces a desired image for the mask printing by editing
(adjusting) the first image. More specifically, as shown in the flow chart, in
the first step,
markings such as dots made with markers, are manually placed on the patient's
skin by
the physician (or health care provider) to provide locators for drug
injection, e.g.,
injection of botulinum toxin. An image is then taken of the patient's skin,
e.g., face, with
the markings. The image is stored for later evaluation. The drug is then
injected into the
patient at the markers on the skin. The patient then waits a select period of
time, two
weeks by way of example, then returns to the physician. At this point, the
physician can
evaluate the results of the first treatment by the initial injection. If the
results are
satisfactory, the first stored image is uploaded to a web portal for
manufacture of the 3D
mask. On the other hand, if the result is not satisfactory, a second image is
taken and
compared to the first stored image. This stored image is adjusted based on the
new image
and is uploaded to the web portal for manufacture of the 3D mask. Note the
images can
be taken in accordance with the various methods disclosed herein, with the
with the
software application enable editing, processing and formatting the image for
3D printing.
Figure 2J provides a diagram showing the process of mask manufacture for the
methods of Figures 2G-2I. In this diagram, the mask is made by 3D printing,
however, it
should be appreciated that the mask can be made by other ways as disclosed
herein. The
physician's images of the patient with markings as locators for injections are
uploaded to
a web portal. Based in these image(s), the manufacturer creates/manufactures
the mask
such as via 3D printing. The mask is made with holes corresponding to the
markings on
the skin. The mask can be made with dosage indicators adjacent the holes.
Alternatively
the dosage indicators are applied to the mask adjacent the holes after
manufacture. In
either case, the holes in the mask are dimensioned to accommodate a marker
therethrough
so the physician can mark the skin through the holes then remove the mask for
injection
at the markings. With the dosage indicators on the mask, the physician can
insert
different colored markers to correspond to the different dosages to mark the
skin prior to
removal of the mask for injection.
Note the diagram of Figure 2J shows the alternative where images can be
created
and formatted and sent directly to a mask manufacturer without use of the
portal.
23
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
Note the images can be processed and formatted by a software application
downloaded independent of the web portal e.g., on a mobile device or desktop.
The
images are then uploaded to the portal for access by the manufacturer, e.g.,
printer of the
mask, or in alternate embodiments, sent directly to the manufacturer.
Alternatively, the
images can be processed and formatted via a web app accessible on the web
portal where
it is subsequently uploaded for access by the manufacturer. (The web portal
can be
designed to block access to the software by the manufacturer and only allow
access to the
formatted images for manufacturing e.g., printing). Stated another way, the
customized
cover can be manufactured from a set of instructions provided from a software
based
application processing an image of an anatomical view of the patient made with
the
marked locations thereon. The cover is customized to the portion of the body
based on
the software based application and the plurality of openings in the cover are
customized
to the desired locations of markings through the cover for subsequent
injection of one or
more drugs independent of the cover, i.e., with the cover removed. The
software based
application can be downloaded to a computer or mobile device for the
physician. In other
embodiments it is accessed or downloaded through the web portal. In other
embodiments,
the physician sends the images to the manufacturer that accesses or downloads
the app.
In some embodiments, a method of manufacturing a customized cover for
placement over a portion of a body of a patient is provided comprising the
steps of
interpreting a set of instructions produced from a software based application
processing
an image of an anatomical view of the patient made with marked locations
thereon,
customizing the cover to the portion of the body based on the software based
application,
customizing a plurality of openings in the cover to the desired locations of
markings and
to accommodate markers for subsequent injection of one or more drugs
independent of
the cover. i.e., at the markings without the cover. The cover is manufactured
having a
plurality of openings extending therethrough configured and dimensioned for
manual
insertion of markers by a health care provider to provide marked locations on
a patient
for injection of one or more drugs once the cover is removed from the patient.
In some
embodiments, the cover is manufactured based on an image inputted to a website
portal
linked to a software application on a device which receives and processes data
corresponding to desired locations of drug injection by the patient, based on
manually
24
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
marked locations, to provide instructions for manufacture of the cover.
In some embodiments, the mask can be manufactured so that the openings
accommodate markers but do not accommodate injection devices. That is, the
openings
can be solely for insertion of markers. For example, a cloth or other material
can be
provided at the openings through which a marker can be inserted but an
injection device
could not safely be inserted. Other structure or configuration is also
contemplated in
these embodiments for contraindication and/or prevention of injection through
the
openings. The mask can be manufactured with labels or indicators regarding non-
injection through the mask, and these labels/indicators can be created by the
software as
part of the instructions for the mask manufacture.
A software based system for enabling consistent injection of a drugs through
the
skin of a patient is outlined in the flow chart of Figure 2K. The software
system
comprises a) storing a first image of the skin having first markings thereon
corresponding
to select regions for one or more injection locations; b) storing a revised
image, the
revised image based on one of human input or software generated input to
adjust the one
or more injection locations; and c) uploading the revised image for processing
by a
manufacturer to create a customized cover for placement over the skin of the
patient.
In some embodiments, the software provides instructions to manufacturer the
cover with openings solely to receive markers for marking the patient's skin
for direct
injection at the markings. The revised image can be uploaded to a web portal.,
storage
device, a cloud storage, etc. for processing by the manufacturer. As noted
herein, in
some embodiments, the revised image is not editable by the manufacturer.
Note in an alternate process of Figure 2C, instead of first injecting the drug
with
1-2 week follow up, after the markings are made and images taken, the mask can
be made
so that the first injections are through the mask. This is a different
approach since
injections are made through the mask without direct visibility. Also, in the
approach, the
mask would need to be made with openings that can accommodate injection
devices.
In the above process, coding, such as color coding and/or number/symbol
coding,
of marked locations before the 3D scan is taken can be made to designate units
to be
embossed or applied as stickers to the mask mold. More specifically, when the
physician/provider is marking the patient's face (or other body region) with
dots to show
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
the locations of the drug injections, e.g., botulinum toxin injections, they
can use different
colored markers/pencils to designate different doses based on a predetermined
key. For
example, red dots can signify a dose of 4 units and black dots can signify a
dose of 2
units of the drug to be later injected through the mask. (Other dosage and
other color or
symbols are also contemplated). Then the 3D photo is taken with the dots on
the body
which is translated into the hole (opening) locations on the mask. The number
of units
for the dots will then be designated on the final printed mask, either with
engraving,
embossing, stickers, or different color resin (if the mask is made of resin)
around each
opening in the mask. The designations can be made with and integral with the
mask or
provided on the mask after manufacture, e.g.. printing. This will be the
dosing guide for
the provider injecting later with the mask in place or for injecting at the
markings with
the mask removed in alternate approaches disclosed herein. Such coding to
indicate
dosage can also be utilized in the embodiments wherein the patient is
injecting the drug.
In an alternate embodiment, the physician is fully out of the loop and the
patient
deals directly with a centralized location to obtain the mask and syringes,
preferably, pre
filled. The centralized location can be one or more company locations which
print the
masks in response to patient input and provide the drugs/medicines directly to
the patient
so the patient can inject the drug. The specific steps of this embodiment are
depicted in
Figure 2D as follows. The patient takes photos outside the doctor's office,
the patient
answers questions on the app of the smart device regarding appearance
objectives, and
the photos with the inputted data are sent to a central location (e.g., a
company). Dots are
placed on the images via the algorithm (in accordance with the patient input)
at the
central location and the 3D model is created and the customized mask is
printed with the
injection holes corresponding to the dots. The customized mask is then sent to
the patient
with syringes, either separate from the mask or attached to (or integral with)
the mask,
and which can be preloaded with the fluid or empty and sent with the fluid,
for injection
by the patient. As can be appreciated, in this method/system, the patient
interacts directly
with a central location, leaving the physician out of the loop. Since multiple
injections
are provided over a period of time, additional preloaded syringes, or
additional syringes
and additional fluid, can be periodically sent to the patient for injection
through the same
customized mask. The patient's progress can be continuously or periodically
visually
26
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
monitored or via analysis of additional photos. Note guide channels for the
syringes in
accordance with the embodiments discussed herein can facilitate injection by
the patient.
The diagram of Figure 4 illustrates schematically the option of the system
wherein
the customized mask can be shipped to the physician or to the patient, as
shown by the
split line in the diagram. Such option is also applicable to the 3D scanner
embodiment of
Figure 2B and other embodiments disclosed herein. More specifically, as can be
appreciated from the diagram, the patient takes photos, answers questions
regarding
objectives, and uploads the photos to the app wherein the software provides a
3D
representation of injection locations. The customized mask is printed from the
3D
representation and shipped either directly to the patient or alternatively
directly to the
physician as described herein.
To facilitate imaging, a chinrest or other facial support for aligning the
face and
preventing movement can be utilized to get a proper 3D image.
As disclosed herein, the customized mask of the various embodiments disclosed
herein are made by 3D printing process. However, it should be understood that
the
customized masks of the present invention can alternately be made by other
processes
such as injection molding, hand made, etc.
Figures 5-11 illustrate various embodiments of the customized masks of the
present invention by way of example, it being understood that masks of other
shapes and
sizes and covering other sections of the face or scalp (or other body regions)
could be
provided. Additionally, it should be appreciated that since the holes formed
in the masks
would vary from patient to patient, the holes illustrated in the masks of
Figures 5-11 are
shown by way of example since the number and location of holes would vary from
patient to patient (mask to mask).
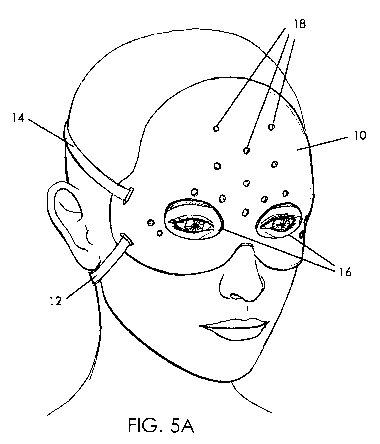
Figure 5A shows a first embodiment of a customized mask in the form of a
forehead and lateral eye mask. These are the most common areas injected with
botulinum toxin. The customized mask, produced by 3D printing as described
above (or
produced by other methods), reduces variability and is reproducible by
providing custom
masks of the forehead and sides of their eyes (crow's feet area). The mask is
designated
generally by reference numeral 10 and has face and eye slits (openings) 16.
Mask 10
includes straps 12, 14 extending around the head to help secure the mask to
the patient's
27
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
face, although other securement devices for mask 10, as well as for other
masks disclosed
herein, are also contemplated which can include, belts, Velcro, hooks, clasps,
etc. The
mask 10 aligns with the patient's eyebrows and also the midline of the nose.
The pre-
formed (pre-drilled) holes 18 (only a few of which are labeled for clarity)
are provided in
the printed mask 10 to provide locators or guides for fluid injection via
syringes or other
fluid injection devices placed into the holes to access the desired regions of
the patient's
forehead and face.
Figure 5B illustrates an alternate embodiment of a forehead and lateral eye
mask
having overlaid vertical alignment line 19a and overlaid horizontal alignment
line 19b on
mask 19 for the nose to mark the midline of the mask 19 and lateral canthus to
mark
vertical orientation. That is, the alignments lines 19a, 19b help to center
the mask 19
relative to the bridge of the nose and eyebrows with one line 19a down the
middle of the
nose for aligning side to side and lines 19b at the outer edge of the eyes to
align up and
down. Note such alignment lines can be provided, e.g., overlaid, on the other
masks
disclosed herein. The mask 19, like mask 10 of Figure 5A, has eye slits and a
strap (not
shown), or other securement mechanism, to hold mask 19 on the patient's face
and
forehead. Holes 19c provide injection site locators in the same manner as
holes 18 of
Figure 5.
Figure 5C illustrates an alternate embodiment of the mask with a central
(midline)
alignment marker between the eye openings and dosage designations, e.g., 21.1
and 4 .
Figure 6 illustrates an alternate embodiment of the mask forming a full face
mask.
Mask 20 is in the form of a full face mask with eye slits (openings) 26 and
mouth and
nose slits (openings 27) and includes straps 22, 24 extending around the head
to keep the
mask 20 in place. Pre-formed holes (injection spots/locations) 28 for the
forehead, lateral
eyes, nose, perioral and other regions of the face are provided. As in the
embodiment of
Figure 5A, the pre-formed (pre-drilled) holes 28 are provided in the printed
mask 20 to
provide locators for fluid injection via syringes or other fluid injection
devices placed
into the holes 28 to access the desired regions of the patient's forehead and
face. Note for
clarity, in this and in other embodiments, only a few of the holes are
designated with a
reference numerals.
28
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
In the embodiments described herein, straps, elastic bands, or other
mechanism,
can be provided to hold/secure the mask to the face. The masks can include for
example
nasal or chin fixation structures or forming the mask so it extends down to
the chin or
down the nose for additional securement. That is, the mask can be formed to
extend to
cover bony fixation points to enhance securement.
Figures 5A, 5B and 6 show pre-formed holes wherein the physician would insert
syringes or other injection devices directly through the holes for injection.
In the
alternate embodiment of Figure 7, instead of the pre-formed holes to receive
standard
syringes, replaceable mini-syringes are attached to the syringe mounts 33 for
multiple
use. More specifically, forehead and lateral eye mask, designated generally by
reference
numeral 30, has a series of syringe mounts 33 extending outwardly from the
mask 30 for
attachment of syringes 40. The syringe mounts 33 aid alignment, e.g.,
centering, of the
syringes to enhance accuracy and consistency of injection. Syringe 40 includes
a needle
and plunger for fluid injection. Syringe 40 is shown as one type of injection
device that
can be attached to the syringe mount 33. The mounts (receivers) 33 are at the
desired
(pre-selected) regions of injection and can be configured to enable snap on
attachment,
screw on attachment or other methods of attaching the syringes 40 to the mask
30. The
syringes are preferably pre-loaded (pre-filled) but alternatively can be empty
and require
filling with fluid prior to injection. The mask 30 is in the shape of mask 10
of Figure 5
with straps 32, 24 and eye slits (openings) 36. It should be appreciated that
the mounts
for the syringes is shown on mask 30 by way of example but can be provided on
mask 20
or on other masks disclosed herein.
In the alternate embodiment of Figure 8, instead of the predrilled holes of
Figure
5A to receive standard syringes, pre-loaded syringes (or other injection
devices) are
provided already mounted to the mask or built into the mask itself. More
specifically,
forehead and lateral eye mask 50 has as series of syringe mounts 53 extending
outwardly
from the mask 50 for securing syringes 60 (or other injection devices),
preferably pre-
filled (only some of which are labeled for convenience). The mounts
(receivers) 53 are at
the desired (pre-selected) regions of injection and the syringes 60 are
snapped on,
screwed on or attached by other methods of attachment for the syringes prior
to shipment
of the mask 50. In the built in version, the syringes can be non-removably
attached to the
29
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
mask 50. The syringe buttons can be pushed so the needles go in and then
automatically
retract. The mask 50 is in the form of mask 10 with straps 52, 54 and eye
slits (openings)
56. It should be appreciated that the already mounted or built in syringes are
shown on
mask 50 by way of example but can be provided on any of the other masks
disclosed
herein.
To facilitate insertion of the syringe into the preformed holes in the mask,
syringe
guide channels can be provided on the mask. The guide channels (also referred
to herein
as "channel guides" or "guides") align the syringe to center the syringe to
thereby center
the injection. For example, if there is a 4mm hole in the mask, the needle can
be aimed
upwards, downwards or sideways which will change the injection area. These
guides
ensure the syringes inserted through the mask are kept straight which provides
more
control and consistency of injection. These guide channels attach to the mask
in different
ways as described below. The guide channels provide a way to align the syringe
without
having the needle get in the way, without utilizing a self-advancing and self-
retracting
mechanism for the needle. These guide channels can be integral (monolithic)
with the
mask or alternatively separate components attached or secured to the mask. In
either
case, the guide channel openings are aligned with the pre-formed holes in the
mask so the
syringe needle can be inserted through the guide channel and mask for
injection of the
fluid into the patient's body. The guide channels can provide guides for
conventional
syringes or for the designed syringes disclosed herein, which can in some
embodiments
be prefilled. An example of such guide channels was discussed above in
conjunction
with mask 30 of the embodiment of Figure 7. Various alternate embodiments of
the
integral and separate syringe guide channels are discussed below in reference
to Figures
12A-48.
Turning initially to Figures 12A-12D and 13-17, a syringe guide channel 106 is
illustrated. The guide channel 106 (also referred to herein as a "channel
guide") extends
upwardly (proximally) from the upper surface 104a (also referred to herein as
top or
proximal surface) of the mask 104. As shown, the designated region 104 is a
section of
the overall mask cutaway for illustrative purposes (see detail A-A of Figure
12A) since in
this embodiment, the guide channels are separate components attached to the
mask 104.
Note that two guide channels are shown in the cutaway region 104a of the mask
104 in
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
Figure 12B and a single guide channel is shown in the region 104a in Figure
12D, it
being understood that a different number of guide channels can be provided for
securement to the mask 104. The number of guide channels, in this and in
alternate
embodiments disclosed herein, preferably correspond to the number of pre-
formed holes
in the mask. The guide channel 106 is shown cylindrically shaped, although
other
shapes/configurations and sizes are also contemplated.
Guide channel 106 is inserted into, e.g. pressed into, the preformed hole 116
of
mask 104. The guide channel 106 helps to align the syringe to center the
injection.
Guide channel 106 includes a proximal opening 114 communicating with the lumen
extending through guide channel 106. Guide channel 106 can be flexible and is
inserted
through the hole 116 of the mask 104 so circumferential cutout 112 (Figure 17)
is
engaged by the circumferential wall of the hole 116, with lower
(bottom/distal) portion
113 forming a flange or stop to prevent separation of the guide channel 106
from the
mask 104. With the guide channels 106 provided as separate components, they
can be
provided sterile and removed from the mask and discarded after use. Also, by
being
provided as separate units, they can be interchanged with guide channels of
different
sizes, shapes, depths, etc. For example, if a greater depth of injection is
required, a guide
channel to accommodate such increased depth can be inserted.
The top surface 106a of guide channel 106 forms a stop for the injection
syringe,
e.g., syringe. More specifically, the syringe, designated by reference numeral
102, has a
flange 103 and a plunger 105 movable distally to inject the fluid stored in
the chamber
113 of the barrel 115 of the syringe 102. Plunger 105 supports a needle 108
with a
penetrating tip so that distal movement of the plunger 105 injects the fluid
from the
syringe. Seal 109 seals off the inside of the syringe as the plunger 105 is
pressed to
deliver the medication.
Note in alternate embodiments, a mechanism can be provided to provide
retractability of the syringe needles.
The height of the guide channel 106 in this embodiment controls the depth of
injection. This can be appreciated by comparing Figures 15 and 16. A taller
guide
channel having a height Hl as shown in Figure 15 yields a shallower injection
depth D1
while a shorter guide channel 106' having a height 112 as shown in Figure 16
yields a
31
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
deeper injection depth D2. These depths can be customized for different
patients or
arranged in specified arrays for deeper injections in certain areas and
shallower injections
in other areas. By adjusting the height of the channel guides, the depth of
injection can
be controlled without changing any structure of the syringe. However, it is
also
contemplated, that alternatively, the injection depth can be controlled by
modifications to
the syringe, e.g., the distance the needle extends distally through the mask
as a result of
the syringe structure. For example, various gauge and needle lengths can be
used to
deliver medication deeper or more superficial.
In use, the syringe guide channels 106 are each inserted into one of the
preformed
holes in the mask 104 to thereby anchor it to the injection site. (Note
alternatively the
mask can be provided with the removable guide channels already positioned in
the mask).
The syringe 102 is then inserted through openings 106 in the guide channel
104. The
syringe 102 is inserted until the distalmost wall of distal portion 111
contacts (abuts) the .
upper (proximal) surface 106a of guide channel 106. This depth is designated
by Dl.
Thus, the guide channel 106 provides a stop for syringe insertion and thus
controls the
depth of insertion of the needle to control the depth of injection. When
attached to the
guide channel 106, the needle 108 extends through the lumen of the guide
channel 106
and past distal surface 104b of the customized mask 104 and past distal
surface 106b of
guide channel 106. To inject the fluid, the plunger 105 is depressed, thereby
injecting the
fluid, e.g., medicine or drug, into the predesignated injection locations for
the patient
previously determined and located by the pre-formed customized patient mask
created in
the processes discussed above. Markings or graduated lines 107 can be provided
on the
outer wall of the barrel 115 for dosing, i.e., to track the amount of fluid in
the syringe
102. Alternatively, the syringes can be color-coded to indicate dosing without
the use of
the markings or can be prefilled or pre-calibrated with the required dose.
Such markings,
color coding or pre-filling can be utilized with the other syringes disclosed
herein. An
insulin type syringe can also be calibrated for exact dosage to be used for
injections at
designated locations or holes. The syringe could come pre-filled or re-
fillable. The exact
total number of units can be placed onto the syringe based on the addition of
all injection
points.
In the embodiment of Figure 12D, the distal portion of the syringe 102 abuts
the
32
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
top of the guide channel 106. In the alternate embodiment of Figures 19-21, a
wider
guide channel is provided such that a distal portion of the syringe fits
inside the guide
channel. More specifically, guide channel 124 of Figures 19-21 extends
upwardly
(proximally) from the upper surface (proximal surface) of the mask 104, and
can be
integral with the mask 104 (as in Figure 12C) or a separate component with a
flexible
feature inserted through the pre-formed hole in the mask (as in Figure 12B).
The guide
channel 124 is identical to guide channel 106 except for its increased width,
i.e.,
diameter. Syringe 120, having plunger 125, seal 127, barrel 123 and fluid
chamber 129,
is inserted through opening 126 and through the lumen of the channel guide 126
and
through the hole 116 of the mask 104. The syringe 120 is inserted until the
bottom wall
123a of barrel 123 contacts, i.e., abuts, the wall 124a within the lumen of
guide channel
124. Thus, the distal portion of the barrel 123 is within the confines of wall
124b of
channel guide 124. Wall 124a of guide channel 124 acts as a stop to limit the
depth of
insertion of the syringe and thus the depth of extension of the needle, to
thereby control
the depth of injection. Thus, with this wider channel guide design, the
channel guide
encapsulates the body of the syringe to guide the needle through. The depth of
the needle
is controlled by the design of the channel guide component and how far it lets
the syringe
be inserted. The height H3 of the wall can be of various heights to provide
different
depths of insertion. It is also contemplated that the guide channel itself can
be provided
with an adjustable wall to adjust the height of the individual guide channel
to
accommodate varying desired depths of insertion. Except for the foregoing,
i.e., wider
opening for receipt of the syringe therein, guide channel 124 is identical to
guide channel
106 so the discussion of guide channel 106 of Figure 12A-18 and its functions
are fully
applicable to guide channel 124 of Figures 19-21.
In the embodiment of Figure 12B, the guide channels are inserted into the pre-
formed holes in the mask for securement thereto. In the alternate embodiment
of Figure
12C, the guide channels 106' are integral with the mask 104' and thus do not
require
separate insertion either in manufacture or by the user.
In alternate embodiments, the guide channel is provided on a flat support, and
the
support is attached to the mask. This differs from the foregoing embodiments
wherein
the guide channel is secured directly to the mask. Figures 22A-24 provide an
example of
33
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
such separate support which is secured to the mask by adhesive. Support 130
has a top
surface 130a from which guide channel 132 extends proximally. On the bottom or
distal
surface 130b of the support 130, an adhesive material is attached. Thus,
support 130
forms an adhesive pad which is secured to the mask 200 so that the opening 134
and
lumen 134a within guide channel are aligned with the pre-formed hole in the
mask. The
syringe is the same as the syringe of Figure 19 so has been labeled with the
same
reference numerals.
In use, the supports 130, with the exposed adhesive bottom surface, are
adhesively secured to the mask so that the channel opening 133 is in alignment
with the
hole in the customized mask. The syringe 120 (with optional gradations 128) is
inserted
through the channel, until the distal portion bottoms out on the wall 132b
(similar to wall
124a of guide 124 of Figure 21). This provides a stop for syringe insertion to
control the
depth of needle penetration. When inserted through the channel, the needle 122
extends
though the lumen 132a and distally beyond the support 130 into the body.
Advancement
of plunger 125 injects the fluid from the chamber 113 of the syringe 120. In
some
embodiments, the adhesive can be covered until ready for attachment wherein
the cover
can be peeled off to expose the adhesive surface.
The injection depth D3 is controlled by the height H3 of the channel guide
component 113 and this height can be varied to achieve different injection
depths.
Alternatively, the needle height could be varied to achieve different
injection depths.
Another way to control the depth is to vary the thickness of the mask, with a
thicker mask
providing a shorter injection depth and a thinner mask providing a greater
penetration
depth.
Note the guide channel 130 could alternatively be configured so that the
syringe
remains outside the guide channel (rather than positioned therein) as in the
embodiment
of Figure 15. Also, although adhesive is disclosed for attachment, other ways
to attach
the pad carrying guide channel are also contemplated.
In the alternate embodiment of Figures 35A-48, mini syringes are inserted into
guide channels having a click-in or snap-in component. This click in syringe
guide can
be inserted into the mating feature of the mask before the syringe or together
with the
syringe.
34
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
More specifically, the syringe guide channel 158 has an opening 160
communicating with the lumen to receive the syringe 160. The guide channel 158
is
inserted into the mating feature 152, also referred to herein as the guide
receiver. The
receiver 152 can be integral with mask 150 as shown in Figure 35C or a
separate
component attached to the mask as shown in Figure 358, which can be either
removable
or permanently (non-removably) attached. (Note the Figures illustrate a
section of the
mask in the same manner as detailed section 104a of the mask 104 of Figure 12A
and
thus only two receiver/guide channels are shown in Figures 35A-35D and a
single
receiver and guide channel are shown in Figures 35E-38). If a separate
component, the
receiver 152 can include a flange and a recess for insertion through the pre-
formed holes
in the customized mask 150. Whether integral or separately attached component,
receiver 152 extends proximally (outwardly) from upper (proximal) surface 150a
of mask
150 and is dimensioned to receive the guide channel 158 within its lumen.
Guide channel
158 is inserted through opening 156 in receiver 152 and into the lumen of the
receiver,
and protrudes proximally of receiver 152. The syringe guide 158 is clicked
into the
receiver 152 via the flexible tab 154 which is spring biased inwardly. When
the guide
channel 158 is inserted into receiver 152, it initially forces tab 154
outwardly as its outer
wall engages the inner surface 154a of the tab 154 until the inner surface
154a is aligned
with circumferential notch (recess) 162 of guide channel 158 as shown in
Figure 41.
When so aligned, the tab 154 can return to its initial position to secure the
guide channel
158 to the receiver 152. Thus, the flexible tab 154 of the mating component
152 allows
the user to insert new syringe guides and remove them once they have been
used. The
syringe guide 158 can include a collapsible material 159, such as a sterile
sponge, within
its lumen as shown in Figure 41.
The syringe guide 158 aligns the syringe 161 to inject at the right location
as can
be appreciated in Figures 45 and 46. As the user presses the syringe collar
down when
the syringe 161 is inserted into the guide 158, the collapsible material 159
will compress
(due to distal wall 161a) allowing the needle 165 to enter the tissue as it
extends through
the opening 157 in wall 158a of syringe guide 158 and through lumen 153 of
receiver 152
and past the distal surface of mask 150. The user then holds handle (flange)
164 and
presses the plunger 166 to inject the medication from the chamber 163 within
the syringe
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
161. The collapsible material 159 allows the user to safely align multiple
syringes
without the needle contacting the skin and can spring back up following the
injection. A
seal 167 similar to seal 127 of Figure 21 can be provided within the barrel of
the syringe
161.
As noted above, the customized masks (covers) of the present invention are not
limited to covering the face or forehead of the patient but can be applied to
other parts of
the body. Figures 25-34 illustrate such alternative by way of example, showing
a cover
(mask) placed over an abdominal section of the patient, which is contoured
around a
shape to align with the patient's body. The customized "abdominal mask"
(cover) 140
includes pre-formed openings for injection locations and is made in accordance
with the
processes described herein. The mask 140 can be used for example for abdominal
fat
dissolving/destruction. Cover 140 has a plurality of guide channels 144 which
can be
integral with the cover 140, i.e., built directly into the cover 140, as
shown, or,
alternatively, separately attached to the cover in the same ways as discussed
above with
respect to the other embodiments. These guide channels 144 are aligned with
the
openings in the customized mask 140 to provide passage of the syringes. The
guide
channels 144 can control the depth of the injections. For example, in Figure
33, a taller
guide channel (H4) yields a shallower injection (D4) while in Figure 34 a
shorter guide
channel (H5) yields a deeper injection (D5). The mask 140 can be provided with
different height channel guides to regulate different injection depths at
different sites, or
the needle lengths can be varied to achieve different injection depths or the
thickness of
the mask can vary.
The cover 140 has distal and proximal surfaces 140a, 140b and includes an
umbilical alignment hole 146 with arrows to ensure the user aligns the cover
140
correctly. Figures 27 and 31 show the alignment hole 146 for the umbilicus.
Strap 142 extends from the edge of the cover 140 and wraps around the abdomen,
and is secured at region 143 by Velcro, although other ways to secure the
cover 140 or
strap 142 are also contemplated such as clips, hooks, clasps, etc. The strap
142 tightens
the cover 140 to the body.
In use, the syringes 120 are inserted into the guide channels 144 as shown in
Figures 28 and 32 so that the needle 122 extends through the opening 144a and
lumen
36
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
144b of guide 144. Syringe 120 bottoms out at wall 144c so that the wall 144c
of guide
144 provides stop to limit the depth of insertion of the syringe 120 and thus
the depth of
penetration of the needle 122 and injection of the fluid. Once the syringes
are inserted,
the plunger 125 is depressed to inject the fluid from chamber 129 of the
syringe.
In the embodiments disclosed herein, the channel guides can be made from
silicone or rubber of varying durometer to safely ensure the passage of the
needle without
damaging the needle. The guide channels are preferably designed with a slight
lead-in
around the needle guide hole, preferably minimal, to reduce the likelihood of
the user
hitting the mask with the needle and bending or breaking the needle.
In alternate embodiments, the syringe alignment features can be dimensioned to
be much taller than the needle, in which case the entire body of the syringe
will be
aligned before the needle even gets to the surface of the needle guide
hole/mask.
In the embodiment of Figure 9, a built in pneumatic mechanism or other
mechanism is utilized to deliver the fluid from the syringes at the same time.
Mask 70
has a series of holes 78 which receive injection devices and are connected via
channels
77 to external fluid injection control device 80. Only some of the holes 78
and channels
77 are labeled for clarity. Tube 83 extends from control device 80 and is in
fluid
communication with channels 77 of mask 70 so that air (or other powering
fluid) can be
delivered through the channels 77 and into the injection devices mounted to
the holes 78.
In this manner, instead of actuating each syringe by individually injecting
each syringe by
pushing with the finger, the control device 80 provides a system to
simultaneously
actuate the syringes. The tube mount 85 in the illustrated embodiments is at
the side of
the mask, but could, in alternate embodiments, be provided in other regions of
the mask,
to provide fluid communication with the syringes mounted at the holes in the
mask to
effect injection. The mask 80 is in the form of mask 10 with straps 72, 74 and
eye slits
(openings) 76, although this "force" mechanism can be used with the other
masks
disclosed herein. The control unit 80 can be a pneumatic system or other
mechanism for
delivering a force to force the toxin from the syringes transdermally through
the
respective hole. The injection devices, e.g., syringes, can be preloaded
syringes or
replaceable syringes as in the foregoing embodiments. The arrangement and
communication of the interconnected channels 78 of Figure 9 is just one
example as the
37
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
channels can be arranged and communicate in other arrangements. The mask 70 is
in the
form of mask 10 with straps 72, 74 and eye slits (openings) 76. It should be
appreciated
that the system of Figure 9 with the built in channels is shown by way of
example as it
can be provided on mask 20 or other masks disclosed herein. Note in an
alternate
embodiment, the injection control device 80 can, in addition to containing the
pump or
other force mechanism, also contain the fluid, e.g., toxin, or, alternatively
contain the
force mechanism and be provided with another tube connected to the toxin
source, and
the channels, holes and/or syringes could be configured to control the dosage.
As can be appreciated, the system and methods/processes of the present
invention
address three variables: a) the customized mask controls injection location;
b) the
injection device controls the amount of fluid injected; and c) the mask
configuration (or
alternatively the injection device configuration) controls the depth of the
injection.
The customized masks of the present invention can be provided with
identification such as the name of the patient. Additionally, the customized
masks can
have pre-printed dosages (units) next to each guide hole to facilitate
injection. The
dosages can be based on the patient's answers to the questions used to
assemble the data
and create the mask, or alternatively provided by the physician/practitioner.
These
reusable masks would then be shipped to the physician's office or
alternatively shipped to
the patent and brought by the patient to the physician's office as described
above. The
pre-printed information on the mask can then be used to guide the injections.
This
advantageously reduces the variability in injection location as well as
dosage.
As described above, the customized masks of the present invention could
alternatively come with pre-filled small syringes built into the mask with
reconstituted
botulinum toxin. The dosage would be based on questions the patient answers.
This
custom mask could be shipped directly to a physician office or patient. This
could
expand the access of patients to these injections as any practitioner could
apply the mask
and push the buttons to inject with reproducible accuracy.
In some embodiments, kits of pre-loaded syringes of medication can be shipped
to
the physician office or shipped directly to the patient for home
administration. The
syringes can be disposable or reusable by being re-fillable. In some
embodiments, kits
containing the mask and injection devices (either pre-filled or empty shipped
with the
38
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
fluid separate) can be shipped to the physician office or patient.
The customized masks not only allow predictability but the masks in some
embodiments can be re-used so repeated treatment can be ensured to be in the
same
locations. Also, the shipments of medication to the physician or patient for
use with the
re-used mask can vary depending on the results of prior injections.
In the foregoing embodiments, the physician or healthcare provider. e.g.,
nurse,
physician assistant, pharmacist, performs the injection, however, it is also
contemplated
in alternate embodiments that the patient can perform the injection. Thus, in
these
embodiments, the botulinum toxin is self-injected/self-administered by the
patient. Thus,
for example, instead of the step of injections by the physician in the process
of Figures
2A and 2B, the step of injection would be performed by the patient. Pre-loaded
syringes
would facilitate patient injection.
In an alternate embodiment, instead of a 3D scanner, the physician takes 2D
photos in the office and makes the recommended markings on the photos based on
the
physician's knowledge about the patient and the patient's previous injections.
The
photos are then sent to another entity to do the 3D modeling and print the
mask, which is
then sent back to the physician or directly to the patient.
As noted above in the foregoing embodiments, the customized masks are created
via 3D printing, however, it is also contemplated that other methods processes
can be
utilized to create the 3D masks.
In the foregoing embodiments, 3D customized masks for the patient are printed.
In an alternate embodiment, instead of utilizing such 3D customized masks, for
simplification, various size mask templates can be created. Thus, various size
mask
templates can be made, not 3D fitted to individual faces, but close fits to
forehead/face
size. These templates could then be given to physicians or patients and then
brought to
physician offices where a physician/practitioner could mark them, i.e., punch
holes, to
individualize them based on where the patient should or usually gets
injections. This
could then be used as a template on its own or it could be used to make a more
permanent
template with or without the syringes built into it. Thus, such masks would
provide a
customizable facial/head template to guide injections of the botulinum toxin.
Color coding, number/symbol coding or other forms of
identification/designation
39
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
of marked locations can be inputted prior to creation of the mask to inform
the clinician
of the number of units (dosage) to be injected. Such dosage indicators can be
embossed
or otherwise imprinted on the masks during creation so the printing of the
mask includes
the dosage indicators or alternatively the dosage indicators can be applied as
stickers to
the mask after it is formed. For example, different colors can be printed on
the clear
mask or decals can be applied to the mask by the technician/clinician.
The present invention as described above in the various embodiments is used
for
injection of botulinum toxin for cosmetic surgery. However, the
concepts/systems of any
of the embodiments disclosed herein can also be used for other procedures. The
masks
can be created in the same way as in the systems/processes disclosed herein
(or other
ways, e.g., templates), except the masks can be created for placement on other
regions
such as for example the scalp and forehead. Figures 10 and 11 show by way of
example
a mask which extends over the scalp and can be used for treatment of
migraines. More
specifically, mask 90 covers the patient's scalp and has chin straps 92, 93 to
secure the
mask 90 and a series of holes 94 for fluid injection. The injections can be
made via
injection devices as described above, including, for example, the replaceable
syringes as
in Figure 7, the pre-loaded syringes as in Figure 8 or the channel system of
Figure 9. The
present invention also contemplates use of botulinum toxin for other parts of
the body,
e.g., underarms for sweating.
It should be appreciated that different botulinum toxins can be injected.
Botulinum toxin is the product of Clostridium botulinum. This growing bacteria
produces
the neurotoxin botulinum toxin, which inhibits the release of acetylcholine
and results in
the flaccid paralysis of the affected muscles. There are several distinct
types of
botulinwn toxin: A. B, Cl, D. E. F, and G. The various types of botulinum
toxin are
marketed under brand names such as Botox (onabotulinumtoxinA, Allergan) and
Dysport/Azzalure (abobotul inumtox inA,
Ipsen/Galderma), Xeomin/Bocouture
(incobotulinumtoxinA, Merz) and Jeuveau (prabotulinumtoxinA. Evolus/Daewoong),
along with several others in clinical trials. Revance Therapeutics, Inc. also
offers the
neuromodulator DaxibotulinumtoxinA for Injection (RT002)
Although botulinum toxin is described as the fluid injected through the masks
of
the foregoing embodiments, it is also contemplated that other fluids can be
utilized for
CA 03170985 2022-08-11
WO 2021/162954
PCT/US2021/016875
other treatments or surgical procedures. For example, Kybella (deoxycholic
acid), which
is used to reduce fat, can be used on multiple surface areas of the body to
target stubborn
fat areas after a 3D printout of the mask is made.
It should be appreciated that the various imaging methods and the various ways
to
manufacture the masks disclosed herein are applicable to the masks of the
present
invention in which injection needles are provided through openings in the mask
as well as
applicable to the alternative masks of the present invention in which markings
are made
through the mask and the mask is removed for drug injection.
While the above description contains many specifics, those specifics should
not
be construed as limitations on the scope of the disclosure, but merely as
exemplifications
of preferred embodiments thereof. Those skilled in the art will envision many
other
possible variations that are within the scope and spirit of the disclosure as
defined by the
claims appended hereto.
41