Note: Descriptions are shown in the official language in which they were submitted.
Description
Title of Invention: CELL PRESERVATION METHOD
Technical Field
[0001]
The present invention relates to cells, a cell suspension, a container, a
device, or a
pharmaceutical composition for regenerative medicine.
Background Art
[0002]
Temcell HS Injection and Stemirac Injection are marketed in Japan as stem cell-
based
products for regenerative medicine. These products are cryopreserved and
delivered to medical
settings, and are then thawed and administered to patients. The dosage form at
the time of
administration is a cell suspension.
[0003]
Temcell HS Injection contains 72 x 106 human mesenchymal stem cells, 1.08 mL
of dimethyl
sulfoxide (DMS0), and others in a bag (with a total volume of 10.8 mL).
Stemirac Injection
contains 0.5 to 2.0 x 108 autologous bone marrow mesenchymal stem cells, 2 mL
or 4 mL of DMSO,
and others in a bag (with a total volume of 20 mL or 40 mL). Here, DMS0 is a
cryoprotectant.
[0004]
Patent Literature 1 describes a pharmaceutical composition containing human
mesenchymal
stem cells and a method of producing the same. An application for registration
of extension of this
patent term concerning Temcell HS Injection has been filed. Patent Literature
2 describes a method
of producing a regenerative medicine containing bone marrow- or blood-derived
cells. An
application for registration of extension of this patent term concerning
Stemirac Injection has been
filed.
Citation List
Patent Literature
[0005]
Patent Literature 1: Japanese Patent No. 5394932
Patent Literature 2: Japanese Patent No. 4936341
Summary of the Invention
Technical Problem
[0006]
The above regenerative medicine products and the regenerative medicine
pharmaceutical
compositions described in the Patent Literatures are freeze-thawed and then
used. Thus, the cell
suspension contains a cry oprotectant. Unfortunately, the fact that the
concentration of the
cryoprotectant is high may cause adverse side effects.
Solution to Problem
[0007]
The present inventors have succeeded in producing a cell suspension with a low
concentration of cryoprotectant even after freeze-thawing. An aspect of the
present invention
provides a method of preserving cells, comprising the steps of (a) enriching
cells from a cell
suspension containing the cells and a cryoprotective solution to generate an
enriched fraction, and (b)
freezing the enriched fraction to prepare a frozen material. A cell suspension
with a low
concentration of cryoprotectant may be obtained after a composition containing
cells preserved by
1
Date Recue/Date Received 2022-09-21
this method is thawed and suspended in a solution.
[0008]
Another aspect of the present invention provides a method of freezing and
thawing cells,
comprising the steps of (a) enriching cells from a cell suspension containing
the cells and a
cryoprotective solution to generate an enriched fraction, (b) freezing the
enriched fraction to prepare
a frozen material, and (c) thawing the frozen material to prepare a thawed
material.
[0009]
Still another aspect of the present invention provides a method of producing a
frozen cell-
containing composition, comprising the steps of (a) enriching cells from a
cell suspension containing
the cells and a cryoprotective solution to generate an enriched fraction, and
(b) freezing the enriched
fraction to prepare a frozen material.
[0010]
Still another aspect of the present invention provides a method of producing a
cell suspension,
comprising the steps of (a) enriching cells from a cell suspension containing
the cells and a
cryoprotective solution to generate an enriched fraction, (b) freezing the
enriched fraction to prepare
a frozen material, (c) thawing the frozen material to prepare a thawed
material, and (d) mixing the
thawed material and a solution to produce a cell suspension.
[0011]
Still another aspect of the present invention provides a method of producing a
cell suspension,
comprising the steps of thawing a frozen material in a container including a
cell suspension containing
cells and a cryoprotective solution, placing, into the container, a needle
attached to a syringe, and
injecting a solution from the syringe into the container.
[0012]
Still another aspect of the present invention provides a container comprising
a frozen
composition containing a frozen cryoprotectant and a frozen single cell
population of 2.0 x 107
cells/mL or more cells.
[0013]
Still another aspect of the present invention provides a device comprising a
container
containing cells and a cryoprotective solution, and a syringe, wherein the
container and the syringe
are integrated.
[0014]
Still another aspect of the present invention provides a device comprising a
syringe
integrated with a container containing a pellet-like cell-containing
composition.
[0015]
Still another aspect of the present invention provides a pharmaceutical
composition for
regenerative medicine, comprising cells and a cryoprotectant, wherein the
cryoprotectant has a
content of 1% (v/v) or less.
Brief Description of Drawings
[0016]
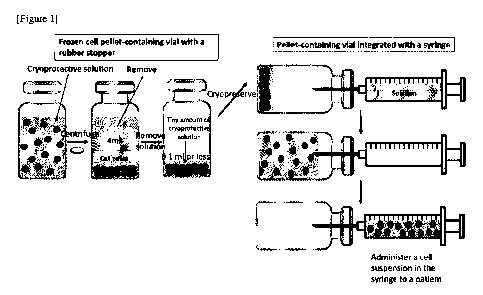
[Figure 1] Fig. 1 is a diagram schematically illustrating an example of the
pellet method
described in Example 1.
[Figure 21 Fig. 2 is a graph showing the results of the volume of
cryoprotective solution
remained in Example 1.
[Figure 3] Fig. 3 is a graph showing the results of the cell viability after
the cells were frozen
and then thawed in Example 1.
2
Date Recue/Date Received 2022-09-21
[Figure 4] Fig. 4 is graphs showing the results of checking the cell count
after thawing and
the cell count on day 3 of culturing in Example 1.
[Figure 5] Fig. 5 is graphs showing the results of checking the cell viability
and the cell count
after the cells were frozen and then thawed in Example 2.
[Figure 61 Fig. 6 is photographs of how a bag was placed in a centrifuge in
Example 3.
[Figure 7] Fig. 7 is a graph showing the results of examining the volume of
cryoprotective
solution remained in Example 3.
[Figure 81 Fig. 8 is a graph showing the results of the cell viability after
the cells were frozen
and then thawed in Example 3.
[Figure 9] Fig. 9 is a graph showing the results of checking the cell count
after thawing and
the cell count on day 3 of culturing in Example 3.
Description of Embodiments
[0017]
Hereinafter, embodiments of the invention will be described in detail. Note
that repeated
descriptions of the same content are omitted, if appropriate, so as to avoid
redundancy.
[0018]
An embodiment of the invention involves a method of preserving cells,
comprising the steps
of (a) enriching cells from a cell suspension containing the cells and a
cryoprotective solution to
generate an enriched fraction, and (b) freezing the enriched fraction to
prepare a frozen material. A
cell suspension with a low concentration of cryoprotectant may be obtained
after a composition
containing cells preserved by this method is thawed and suspended in a
solution. Cells under
preservation by this method can be stored in a container (e.g., in a freezer)
until use for their intended
purpose (e.g., administration of the cells to a patient(s) in regenerative
medicine). For example,
when used in regenerative medicine, cells preserved by this method can be
taken out from a freezer
before administered to a patient, thawed, suspended in a solution, and
administered to the patient.
In this case, depending on the volume of suspension, the cryoprotectant in the
suspension can be set
to a low concentration. This makes it possible to administer a cell suspension
with superior safety.
Also, since the cells are concentrated in advance, the cells at a desired
concentration can remain in
the suspension.
[0019]
An embodiment of the invention involves a method of preserving cells,
comprising the steps
of: centrifuging a container containing cells and a cryoprotective solution to
separate a supernatant
from a precipitate; removing the supernatant to generate an enriched fraction;
and freezing the
enriched fraction. A cell suspension with a low concentration of
cryoprotectant may be obtained
after a composition containing cells preserved by this method is thawed and
suspended in a solution.
[0020]
An embodiment of the invention includes a method of freezing and thawing
cells, comprising
the steps of (a) enriching cells from a cell suspension containing the cells
and a cryoprotective solution
to generate an enriched fraction, (b) freezing the enriched fraction to
prepare a frozen material, and
(c) thawing the frozen material to prepare a thawed material. A cell
suspension with a low
concentration of cryoprotectant may be obtained after cells preserved and
thawed by this method are
suspended in a solution.
[0021]
An embodiment of the invention includes a method of freezing and thawing
cells, comprising
the steps of suspending single cells in a cell cryoprotective solution,
centrifuging the cells to form a
3
Date Recue/Date Received 2022-09-21
pellet, and freezing and thawing the remaining cell pellet after a supernatant
has been removed from
the cryoprotective solution. A cell suspension with a low concentration of
cryoprotectant may be
obtained after cells preserved and thawed by this method are suspended in a
solution.
[0022]
An embodiment of the invention involves a method of producing a frozen cell-
containing
composition, comprising the steps of (a) enriching cells from a cell
suspension containing the cells
and a cryoprotective solution to generate an enriched fraction, and (b)
freezing the enriched fraction
to prepare a frozen material. A cell suspension with a low concentration of
cry oprotectant may be
obtained after a frozen cell-containing composition obtained by this
production method is thawed and
suspended in a solution. In this case, depending on the volume of suspension,
the cryoprotectant in
the suspension can be set to a low concentration. This makes it possible to
obtain a cell suspension
with superior safety. Also, since the cells are concentrated in advance, the
cells at a desired
concentration can remain in the suspension.
[0023]
An embodiment of the invention involves a method of producing a frozen cell-
containing
composition, comprising the steps of: centrifuging a container containing
cells and a cryoprotective
solution to separate a supernatant from a precipitate; removing the
supernatant to generate an enriched
fraction; and freezing the enriched fraction. A cell suspension with a low
concentration of
cryoprotectant may be obtained after a frozen cell-containing composition
obtained by this production
method is thawed and suspended in a solution.
[0024]
An embodiment of the invention includes a method of producing a cell
suspension,
comprising the steps of (a) enriching cells from a cell suspension containing
the cells and a
cryoprotective solution to generate an enriched fraction, (b) freezing the
enriched fraction to prepare
a frozen material, (c) thawing the frozen material to prepare a thawed
material, and (d) mixing the
thawed material and a solution to produce a cell suspension. This production
method allows for a
cell suspension with a low concentration of cryoprotectant.
[0025]
An embodiment of the invention involves a method of producing a cell
suspension,
comprising the steps of: centrifuging a container containing cells and a
cryoprotective solution to
separate a supernatant from a precipitate; removing the supernatant to
generate an enriched fraction;
freezing the enriched fraction; and mixing the resulting frozen material and a
solution to produce a
cell suspension. This production method allows for a cell suspension with a
low concentration of
cryoprotectant.
[0026]
An embodiment of the invention involves a method of producing a cell
suspension,
comprising the steps of freezing an enriched fraction obtained by enriching
cells to produce a frozen
material or thawing a frozen enriched fraction obtained by enriching cells to
produce a thawed
material. This production method allows for a cell suspension with a low
concentration of
cryoprotectant.
[0027]
An embodiment of the invention includes a method of producing a cell
suspension,
comprising the steps of thawing a frozen material in a container including a
cell suspension containing
cells and a cryoprotective solution, placing, into the container, a needle
attached to a syringe, and
injecting a solution from the syringe into the container. This production
method allows for a cell
suspension with a low concentration of cryoprotectant.
4
Date Recue/Date Received 2022-09-21
[0028]
An embodiment of the invention involves a method of producing a container
including a
frozen cell-containing composition, comprising the steps of (a) enriching
cells from a cell suspension
containing the cells and a cryoprotective solution to generate an enriched
fraction, and (b) freezing
the enriched fraction to prepare a frozen material. A cell suspension with a
low concentration of
cryoprotectant may be obtained after a frozen cell-containing composition in a
container as obtained
by this production method is thawed and suspended in a solution.
[0029]
An embodiment of the invention involves a method of producing a frozen cell-
containing
composition, comprising the steps of (a) enriching cells from a cell
suspension containing the cells
and a cryoprotective solution to generate an enriched fraction. A cell
suspension with a low
concentration of cryoprotectant may be obtained after a frozen cell-containing
composition obtained
by this production method is thawed and suspended in a solution.
[0030]
An embodiment of the invention involves a method of producing a cell pellet,
comprising
the steps of: centrifuging the above-described container containing cells and
a cryoprotective solution
to separate a supernatant from a precipitate; and removing the supernatant to
generate an enriched
fraction. The cell pellet obtained by this production method may be frozen and
thawed to produce
a cell pellet.
[0031]
An embodiment of the invention includes a device comprising: a rubber stopper-
attached
vial containing the above-described cell pellet; and a syringe having a cell
suspension, wherein the
syringe and the vial are combined and shaped such that a needle attached to
the syringe is pierced
into the rubber stopper.
[0032]
An embodiment of the invention includes a method of producing a cell-
containing
composition or a method comprising the step of (a) enriching cells from a cell
suspension containing
the cells and a cryoprotective solution to generate an enriched fraction, or
(b) freezing the enriched
fraction to prepare a frozen material.
[0033]
An embodiment of the invention includes a container comprising a frozen
composition
containing a frozen cryoprotectant and a frozen single cell population of 2.0
x 107 cells/mL or more
cells (e.g., stem cells). The frozen material contained in this container may
be thawed and then
mixed with a solution. In this case, a cell suspension can be prepared while
the cryoprotectant is
diluted according to the volume of solution. This container may be produced by
the production
method of one of the above embodiments of the invention. Here, the cell
concentration in the
composition may be 2.0 x 107, 3 x 107, 4 x 107, 5 x 107, 6 x 10, 7 x 107, 1 x
108, 3 x 108, 5 x 108, 7
x 108, 9 x 108, or 1 x 109/mL or higher, or may be a number between any two of
them. The above
composition may contain a cryoprotectant in a concentration of 20% (v/v) or
less. This
concentration may be, for example, 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, or
20% (v/v), or may be a
number between any two of them. The cryoprotectant may be, for example, DMSO,
glycerol,
glycerin, dextran, polyethylene glycol, ethylene glycol, propylene glycol, or
propanediol. If the
cryoprotectant is trehalose, sorbitol, or polyvinylpyrrolidone, the
concentration, namely the content
of the cryoprotectant may be 20% (w/v) or less. This concentration may be, for
example, 0.5, 1, 2,
3,4, 5, 6, 7, 8, 9, 10, 15, or 20% (w/v), or may be a number between any two
of them. The above
composition may contain 0.001 to 1 mL of cryoprotectant. This volume may be,
for example, 0.001,
Date Recue/Date Received 2022-09-21
0.005, 0.01, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, or 1 mL, or
may be a number between any
two of them.
[0034]
An embodiment of the invention includes a container containing a frozen
composition
containing frozen cells and a frozen cryoprotective solution, wherein the
volume of the frozen
composition is 30% or less of the volume of the container. The frozen
composition contained in this
container may be thawed and then mixed with a solution. In this case, a cell
suspension may be
prepared while the cryoprotectant is diluted according to the volume of
solution. At that time, the
solution can be injected into part that is in the container but is other than
the frozen composition.
Therefore, the cell suspension can be simply prepared.
[0035]
An embodiment of the invention includes a device comprising a container of one
of the above
embodiments of the invention and a syringe, wherein the container and the
syringe are integrated.
This device can be used to simply prepare a cell suspension. In addition, this
device may be used
to produce a cell suspension with a low concentration of cryoprotectant. In
one embodiment of the
invention, the integration includes a connected state. In one embodiment of
the invention, the
connection includes a form of direct or indirect connection. In the case of
direct connection, for
example, a surface of the container and the syringe may be directly connected.
In the case of indirect
connection, for example, the container and the syringe may be connected via a
coupling part (e.g., a
tube, a connector). At this time, a surface of the container is connected to
the coupling part, and the
coupling part may then be connected to the syringe.
[0036]
An embodiment of the invention includes a composition containing a
cryoprotectant and 2.0
x i07 cells/mL or more cells (e.g., stem cells). Here, the cells should be a
population of single cells.
At this time, the concentration of cryoprotectant is not limited as long as
the concentration is effective
in cryoprotecting cells. This concentration may be, for example, 0.5 to 20%
(v/v) or 0.5 to 20%
(w/v). When this composition is frozen and the resulting frozen composition is
thawed and then
mixed with a solution, a cell suspension can be prepared while the
cryoprotectant is diluted in
accordance with the volume of the solution. The concentrations of cells and
cryoprotectant in this
composition may each be in the range of values listed in the description of
the composition in the
container above. This composition can be produced by the production method of
one of the above
embodiments of the invention. An embodiment of the invention includes a
container containing the
above composition.
[0037]
An embodiment of the invention includes a device comprising a container
containing the
above composition and a syringe, wherein the container and the syringe are
integrated. This device
can be used to simply prepare a cell suspension. In addition, this device may
be used to produce a
cell suspension with a low concentration of cryoprotectant.
[0038]
An embodiment of the invention includes a device comprising a syringe and a
container
including a pellet-like cell (e.g., stem cell)-containing composition, wherein
the container and the
syringe are integrated. This device can be used to simply prepare a cell
suspension. An
embodiment of the invention includes a device comprising a syringe and a
container including a
frozen composition containing frozen cells (e.g., stem cells), wherein the
container and the syringe
are integrated. In addition, this device may be used to produce a cell
suspension with a low
concentration of cryoprotectant.
6
Date Recue/Date Received 2022-09-21
[0039]
An embodiment of the invention includes a frozen composition, container, or
device of one
of the above embodiments of the invention. This frozen material may be used to
produce a cell
suspension with a low concentration of cryoprotectant. The frozen container
including a frozen cell-
containing composition or a frozen device in which the container and a syringe
are integrated can be
distributed while kept in a frozen state. The frozen device in which the
container and a syringe are
integrated has an advantage of capable of omitting the step of connecting the
container and the syringe
at a medical site.
[0040]
An embodiment of the invention includes a composition (e.g., a pharmaceutical
composition
for regenerative medicine) containing cells (e.g., human stem cells) and a low
concentration of
cryoprotectant. The concentration of cryoprotectant in this composition may
be, for example, 1%
(v/v) or less or 1% (w/v) or less. This composition has a low concentration of
cryoprotectant and is
thus safe when administered to a subject. This concentration of cryoprotectant
may be, for example,
the concentration at the time of administration to a patient. In one
embodiment of the invention, 1%
(v/v) or less or 1% (w/v) or less may be, for example, 1 x 106, 1 x 10-5, 1 x
10, 1 x 10-3, 1 x 10-2, 1
x 10-1, 0.2, 0.3, 0.4, 0.5, or 1% (v/v) or % (w/v), or may be a number between
any two of them. An
embodiment of the invention involves use of cells for the manufacture of a
composition for
regenerative medicine, wherein the composition for regenerative medicine
contains a low
concentration of cryoprotectant. An embodiment of the invention includes a
composition
containing cells for use in regenerative medicine, wherein the composition
contains a low
concentration of cryoprotectant. In one embodiment of the invention, in
regenerative medicine, a
composition containing cells and a cryoprotectant (e.g., at a concentration of
1% (v/v) or less) may
be administered to a subject. The regenerative medicine also includes cell
therapy. The cell
therapy includes a treatment method comprising the step of administering cells
to a subject.
[0041]
An embodiment of the invention includes a method of regenerative medicine,
comprising
the step of administering to a patient a regenerative medicine-use composition
containing cells (e.g.,
human stem cells), wherein a cryoprotectant in the regenerative medicine-use
composition is at a low
concentration. The concentration of the cryoprotectant at that time may be,
for example, 1% (v/v)
or less or 1% (w/v) or less. This method of regenerative medicine has
excellent safety because the
concentration of cryoprotectant administered is low. The administration may
be, for example,
intravascular (e.g., intravenous or intra-arterial) administration. The
administration may be
conducted by infusion.
[0042]
An embodiment of the invention includes a method of regenerative medicine,
comprising
the steps of: enriching cells from a cell suspension containing the cells and
a cryoprotective solution
to generate an enriched fraction; freezing the enriched fraction to prepare a
frozen material; thawing
the frozen material to prepare a thawed material; mixing the thawed material
and a solution to produce
a cell suspension; and administering the cell suspension to a subject.
[0043]
An embodiment of the invention includes a method of regenerative medicine,
comprising
the steps of: thawing a frozen composition according to any one of the above
embodiments of the
invention in a container; adding a solution to the above container to suspend
cells; or administering
the above cell suspension to a subject.
[0044]
7
Date Recue/Date Received 2022-09-21
According to an embodiment of the invention, a method of preserving cells, a
method of
freezing and thawing cells, a method of producing a frozen cell-containing
composition, a method of
producing a cell suspension, a method of producing a container, or a method of
producing a cell pellet
may comprise the steps of: culturing the cells; dispersing the resulting cell
colony into single cells
(e.g., with a trypsin-containing solution); centrifuging the resulting
population of single cells to
generate a precipitate fraction; or mixing the precipitate fraction with a
cryoprotective solution.
[00451
According to an embodiment of the invention, a method of preserving cells, a
method of
freezing and thawing cells, a method of producing a frozen cell-containing
composition, a method of
producing a cell suspension, a method of producing a container, or a method of
producing a cell pellet
may comprise the step of mixing cells and a cryoprotective solution to produce
a cell suspension.
After this step, the cell suspension may be allowed to stand. The incubation
time may be 1, 2, 3, 4,
5, 10, 20, 30, or 60 mm or longer, or may be a number between any two of them.
From the viewpoint
of obtaining higher cell viability after freezing and thawing, the incubation
time should be at least 5
min. The temperature at that time may be, for example, 3, 4, 5, 6, 10, 20, 30,
or 37 C, or may be a
number between any two of them.
[0046]
According to an embodiment of the invention, the method or production method
(e.g., the
method of preserving cells, the method of freezing and thawing cells, the
method of producing a
frozen cell-containing composition, the method of producing a cell suspension,
the method of
producing a container, or the method of producing a cell pellet) may comprise
the steps of:
centrifuging a container containing cells and a cryoprotective solution to
separate a supernatant from
a precipitate; and removing the supernatant to prepare an enriched fraction.
This two steps may be
used to generate a cell-enriched fraction by a simple procedure. In addition,
highly concentrated
cells and an effective amount of cryoprotectant for cryoprotection may be
included in the enriched
fraction.
[0047]
According to an embodiment of the invention, the method or production method
(e.g., the
method of preserving cells, the method of freezing and thawing cells, the
method of producing a
frozen cell-containing composition, the method of producing a cell suspension,
the method of
producing a container, or the method of producing a cell pellet) may comprise
the step of using a
filter to enrich cells from a cell suspension containing the cells and a
cryoprotective solution. This
step makes it possible to include highly concentrated cells and an effective
amount of cryoprotectant
for cry oprotection in the enriched fraction.
[0048]
According to an embodiment of the invention, the method or production method
(e.g., the
method of preserving cells, the method of freezing and thawing cells, the
method of producing a
frozen cell-containing composition, the method of producing a cell suspension,
the method of
producing a container, or the method of producing a cell pellet) may comprise
the step of placing a
container containing the enriched fraction under a freezing point environment.
The method or
production method may comprise the step of connecting a syringe with a
container containing the
above-mentioned enriched fraction (e.g., by placing a syringe-attached needle
into the container) to
form a device where the above container and syringe are integrated. The method
or production
method may comprise the step of freezing the above device. The method or
production method may
comprise the step of placing the above device under a freezing point
environment. The method or
production method may comprise the step of storing the above frozen material
in a freezer. The
8
Date Recue/Date Received 2022-09-21
method or production method may comprise the step of injecting a solution from
the above syringe
into the above container. The method or production method may comprise the
step of repeatedly
conducting charge into and discharge from a syringe. The method or production
method may
comprise the step of aspirating, with a syringe, the above cell suspension in
a container. The method
or production method may comprise the step of separating the syringe from the
container. The
method or production method does not have to comprise, between steps (a) and
(b), the step of
culturing or washing the cells, adding a cryoprotectant to the container, or
adding culture medium,
buffer solution, or saline to the container.
[0049]
The terms described in the above embodiments are each explained in more detail
below.
[0050]
In one embodiment of the invention, the syringe may contain a pharmaceutically
acceptable
carrier or a frozen material thereof. The syringe may contain, for example,
saline, buffer solution
(e.g., PBS), or a frozen equivalent.
[0051]
In one embodiment of the invention, the enriched fraction may contain cells
and a
cryoprotective solution. The enriched fraction may be contained in a vial or
bag.
[0052]
In one embodiment of the invention, the enrichment may be five-fold or higher.
This fold
may be, for example, 5, 10, 20, 30, 40, 50, 60, 70, 100, 120, 150, 200, 300,
or 400 times or more, or
may be a number between any two of them.
[0053]
In one embodiment of the invention, the cells may exist as a cell population.
The cells may
also be a population of single cells. The population of single cells includes
cells that are not attached
to each other and are in a single state. The single-cell population may be
generated, for example,
by treating the cell population with a cell-dispersing agent (e.g., trypsin).
Examples of the single-
cell population include a cell population predominantly composed of single
cells. Examples of the
single-cell population-containing composition include a form of cell
dispersion. The single-cell
population may be observed, for example, under a microscope. The single-cell
population may also
be analyzed with a cell sorter. The single-cell population has higher cell
viability after thawing in
the method or production method according to one of the above embodiments of
the invention. The
cell viability after thawing may be, for example, 75, 80, 85, 90, 95, 96, 97,
98, 99, or 100%, or may
be a number between any two of them.
[0054]
In one embodiment of the invention, the cells may be, for example, mammalian
cells.
Examples of the mammal include animals such as humans, monkeys, or rodents
(e.g., mice, hamsters).
The cells include stem cells or somatic cells. Examples of the stem cells
include cells with self-
renewal potential and potential to differentiate into different cell types.
Examples of the stem cells
include pluripotent stem cells, multipotent stem cells, or unipotent stem
cells. Examples of the
pluripotent stem cells include ES cells or iPS cells. Examples of the
multipotent stem cells include
mesenchymal stem cells, adipose stem cells, hematopoietic stem cells, or
neural stem cells.
Examples of the unipotent stem cells include muscle stem cells or pigment stem
cells. Examples of
the somatic cells include cells derived from the heart, skin, liver, lung,
stomach, intestine, kidney,
uterus, brain, blood, or mesenchymal tissue. Other examples of the somatic
cells include fibroblasts
or blood cells (e.g., leukocytes (e.g., T cells, dendritic cells, NK cells),
erythrocytes, platelets).
These cells may be genetically modified cells (e.g., CAR-T cells). These cells
can be applied to the
9
Date Recue/Date Received 2022-09-21
method including steps (a) and (b) above, resulting in a cell suspension with
excellent safety and
favorable cry opreservati on efficiency.
[0055]
In one embodiment of the invention, examples of the cryoprotectant include
DMSO, glycerol,
dextran, polyethylene glycol, ethylene glycol, propylene glycol, glycerin,
polyvinylpyrrolidone,
propanediol, trehalose, or sorbitol. The cryoprotectant may be produced by
known procedures.
The cryoprotectant may be commercially available and may be purchased from
manufacturers (e.g.,
Zenoaq Resource Co., Ltd., FUJIFILM Wako Pure Chemical Corporation, TOKYO
CHEMICAL
INDUSTRY CO., LTD.). Examples of the trehalose include a,a-trehalose, a,[3-
trehalose, [343-
trehalose, glucosyl trehalose, maltosyl trehalose, or maltotriosyl trehalose.
Examples of the dextran
include dextran 40 or dextran 70.
[0056]
In one embodiment of the invention, the regenerative medicine includes medical
practice of
treating a disease by administering cells to a patient with the disease.
Examples of the regenerative
medicine include treatment of administering human mesenchymal stem cells to
each patient with
acute graft-versus-host disease (e.g., after hematopoietic stem cell
transplantation). Examples of the
regenerative medicine also include treatment of administering (e.g.,
autologous) bone-marrow
mesenchymal stem cells to each patient with neurological syndrome or
dysfunction associated with
spinal cord injury. The route of administration to the patient may be, for
example, intravenous.
[0057]
In one embodiment of the invention, the composition (e.g., a cell suspension,
a
pharmaceutical composition for regenerative medicine) may contain a
pharmaceutically acceptable
carrier. The composition may contain cells (e.g., stem cells) at 1.0 x 105
cells/mL or more. This
concentration may be, for example, 1.0 x 105, 1.0 x 106, 1.0 x 107, 1.0 x 108,
or 1.0 x 109 cells/mL or
higher, or may be a number between any two of them. The composition may
contain a
cryoprotectant at 1 x 10-6 to 0.5% (v/v) or 1 x 10-6 to 0.5% (w/v). This
concentration may be, for
example, 1 x 106, 1 x 10-5, 1 x 104, 1 x 10-3, 1 x 102, 1 x 10-1, 0.2, 0.3,
0.4, or 0.5% (v/v) or % (w/v),
or may be a number between any two of them. This composition may be contained
in a syringe or
bag. The concentration of cells or cryoprotectant in this composition may be,
for example, the
concentration at the time of administering the composition to a patient.
[0058]
In one embodiment of the invention, the freezing may be slow freezing or quick
freezing.
Examples of the slow freezing include a procedure for freezing cells by
cooling them at a slow cooling
rate over a long period of time. For the slow freezing, for example, a BICELL
(Japan Freezer Co.,
Ltd.) may be used; freezing control by a programmed freezer may be used; or a
heat insulator
Styrofoam box may be used. The slow freezing may be performed, for example, at
a rate of 0.1 to
1 C/min. The "0.1 to PC/min" may be, for example, 0.1, 0.2, 0.3, 0.4, 0.5,
0.6, 0.7, 0.8, 0.9, or
1 C/min, or may be a number between any two of them. Examples of the quick
freezing include a
procedure for quick freezing in liquid nitrogen and preservation. The freezing
is preferably slow
freezing. This case excels in terms of maintaining the number of viable cells
after freezing and
thawing. The freezing may be caused by placing or storing the above container,
suspension, or
composition below freezing point. The temperature below freezing point may be,
for example, -50,
-60, -80, -100, -120, or -140 C or less, or may be a number between any two of
them. The freezing
time may be, for example, 3, 6, 12, 18, 24, 30, 36, 42, 48, 72, or 96 h or
longer, or may be a number
between any two of them.
[0059]
Date Recue/Date Received 2022-09-21
In one embodiment of the invention, the step of removing the supernatant after
centrifugation
from the container may comprise the step of aspirating the supernatant in the
container with a pipette,
or aspirating the supernatant in the container with a syringe connected to the
container. When the
post-centrifugation supernatant is removed from the container, part of the
supernatant may be
removed. The part may be 80, 90, 95, 96, 97, 98, 99, 99.5% or more, may be a
number between any
two of them, and may be less than 100%.
[0060]
In one embodiment of the invention, the centrifugal force of the
centrifugation performed on
a container containing a mixture of cells and a cryoprotective solution may
be, for example, 150, 200,
250, 300, 350, 400, 450, 500, 600, or 700 g, or may be a number between any
two of them. The
centrifugation time may be, for example, 2, 3, 4, 5, 6, 7, 8, 9, 10, or 15 min
or less, or may be a
number between any two of them. A plate centrifuge may be used to centrifuge
the bag.
[0061]
In one embodiment of the invention, examples of the container include a vial,
a bag, or a
bottle. Examples of the container include a container having a stopper through
which a needle
attached to a syringe can penetrate. Examples of the stopper include a rubber
stopper. The rubber
stopper is likely to keep a closed system when the needle is placed. Examples
of a material for the
container include glass or plastic. Examples of the plastic include
polypropylene, polyethylene, or
an ethylene-vinyl acetate copolymer. When a solution is charged from the
syringe into the container,
the solution may be charged while releasing air (e.g., from the end of the
rubber stopper). Examples
of the vial include a bottle-type vessel that can hold a solution (e.g., a
cell suspension). Examples
of the vial include a sterile vial (e.g., a vial with a sterile solution
compartment) or a vial for
cryopreservation. The bag has a superior shape to keep a closed system. When
the bag is used,
the shape of the bag can be changed in response to the volume of liquid or gas
in the container. Thus,
the solution can be easily injected from the syringe into the container. When
the bag is used, the
amount of gas in the bag may be reduced before the cell-containing composition
in the bag is frozen.
Examples of the bag include a sterile bag (e.g., a bag with a sterile solution
compartment), a
cryopreservation bag, an infusion bag, a soft bag, or a bag with tubing
attached. The bag may be
connected directly or indirectly to the syringe. In the case of direct
connection, for example, an
opening of the bag and the syringe may be directly connected. In the case of
indirect connection,
for example, the bag and the syringe may be connected via a coupling part
(e.g., a tube, a connector).
At this time, an opening of the container is connected to the coupling part,
and the coupling part may
then be connected to the syringe. The volume of the solution with which the
container can be filled
may be, for example, 1, 2, 3, 4, 5, 10, 15, 20, 25, 30, 50, 100, 500, 1000, or
1500 mL or more, or may
be a number between any two of them.
[0062]
In one embodiment of the invention, a device where the container and syringe
are integrated
may be configured such that a needle attached to the syringe passes through a
surface of the container.
The container surface part through which the needle passes may be made of
rubber.
[0063]
In one embodiment of the invention, the size of injection needle may be, for
instance, 18G
or larger. This size may be, for example, 18, 19, 20, 21, 22, 23, 24, 25, 26,
or 27G, or may be a
number between any two of them. Preferred is from 21 to 23G from the viewpoint
of how easily
the cells can be suspended when a solution is injected from the syringe into
the container and how
low the cells receive stress. In one embodiment of the invention, the volume
of solution with which
the syringe can be filled may be, for example, 1, 5, 10, 15, 20, 50, 100, 200,
300, 400, or 500 inL or
11
Date Recue/Date Received 2022-09-21
more, or may be a number between any two of them. The syringe may be equipped
with a plunger.
[0064]
In one embodiment of the invention, the cell thawing time may be, for example,
1, 2, 3, 4,
or 5 min or longer, or may be a number between any two of them. The thawing
may include melting.
[0065]
All the literatures and (patent or patent application) publications cited
herein are incorporated
by reference in their entirety.
[0066]
As used herein, the Willi "or" is used when "at least one" matter listed in
the text is acceptable.
The same applies to "or". When the wording "number between any two" is
indicated herein, this
range encompasses the two numbers inclusive. The wording "from A to B" herein
means A or more
and B or less.
[0067]
Hereinabove, embodiments of the invention have been described. However, they
are
examples of the invention. Hence, various configurations other than the above
can be adopted. In
addition, the configurations described in the above embodiments may be
combined and adopted.
Examples
[0068]
Hereinbelow, the invention will be described in more detail with reference to
Examples.
However, the invention is not limited to them.
[0069]
<Example 1>
Four cell types (umbilical cord-derived mesenchymal stem cells (umbilical cord
MSCs,
provided by CET), bone marrow-derived mesenchymal stem cells (bone marrow
MSCs, Lonza, PT-
3001), mouse embryonic fibroblasts (MEFs, Chernicon International), or iPS
cell-derived
cardiomyocytes (iPS-CMs, Myoridge, H-013506)) were subjected to a conventional
freezing and
thawing method (hereinafter, referred to as the conventional method) or a
freezing and thawing
method using enriched cells (hereinafter, referred to as the pellet method).
Here, the cell viability
after thawing and the cell count after thawing were compared with the cell
count on day 3 of culturing.
[0070]
1.1 Experimental Procedure
1.1.1 Conventional method
Each conical tube containing various types of cells (1 x 10e7 cells) made into
single cells by
trypsin solution or TrypLETm Select solution (Thermo Fisher) was centrifuged
at 400 g X 5 min. The
trypsin solution in the supernatant was removed by decantation using a tip,
and replaced with 4 ml of
ZENOAQ's Stem Cell Banker (used for umbilical cord MSCs or bone marrow MSCs)
cry oprotectant
(10% (v/v) DMS0), or Cell Banker 1 plus (used for MEFs or iPS-CMs)
cryoprotectant (10% (v/v)
DMS0). The total volume of the resulting cell suspension was transferred to a
vial with a rubber
stopper (5-111-02, manufactured by Maruemu Corporation; volume: 5 ml), and
then subjected to slow
freezing overnight in a BICELL (Japan Freezer), which is a container for slow
freezing, in a freezer
at -80 C. The vial was then warmed for thawing at 37 C for several minutes.
Subsequently, 16
ml of PBS was added for dilution to the vial for recovery. The cell suspension
was transferred to a
centrifuge tube, and the cell viability and the cell count were measured with
a cell counter. Next,
the cell suspension was centrifuged at 400 g x 5min to continue cell culture
evaluation. The PBS in
the supernatant was then removed. After an appropriate amount of each cell
culture medium was
12
Date Recue/Date Received 2022-09-21
added, the cells were suspended by pipetting, seeded on a culture dish, and
cultured for 3 days. The
cells were made into single cells by using a trypsin solution, and the number
of cells was recounted.
[0071]
1.1.2 Pellet method
Fig. 1 is a schematic diagram illustrating an example of the pellet method.
Each conical
tube containing various types of cells (1 x 10e7 cells) made into single cells
by trypsin solution or
TrypLE' Select solution was centrifuged at 400 g x 5 min. The trypsin solution
in the supernatant
was removed by decantation using a tip, and replaced with 4 ml of ZENOAQ's
Stem Cell Banker
(used for umbilical cord MSCs or bone marrow MSCs) cryoprotectant (10% (v/v)
DMSO), or Cell
Banker 1 plus (used for MEFs or iPS-CMs) cryoprotectant (10% (v/v) DMSO). The
total volume
of the resulting cell suspension was transferred to a vial with a rubber
stopper (5-111-02,
manufactured by Maruemu Corporation; volume: 5 ml), allowed to stand in a
refrigerator at 4 C for
min, and then centrifuged at 400 g x 5 min to form a cell pellet. After that,
a 1000- 1 tip and a 20-
jil were
used to remove as much the cryoprotective solution in the supernatant as
possible (3.9 to
3.96 ml in total was removed), followed by overnight slow freezing in a -80 C
freezer in a BICELL,
which is a container for slow freezing. The vial was then warmed for thawing
at 37 C for several
minutes. A syringe filled with 16 ml of PBS and a 21G needle were used; the
needle was placed
through the rubber stopper; and PBS was then added. The vial was lightly
tapped, so that the pellet
was made to float. The cell suspension was then slowly aspirated with the
needle to collect the cells.
The cell suspension was transferred to a centrifuge tube, and the cell
viability and the cell count were
measured with a cell counter. Next, the cell suspension was centrifuged at 400
g x 5 min to continue
cell culture evaluation. The PBS in the supernatant was then removed. After an
appropriate
amount of each cell culture medium was added, the cells were suspended by
pipetting, seeded on a
culture dish, and cultured for 3 days. The cells were made into single cells
by using a trypsin
solution, and the number of cells was recounted.
[0072]
1.2 Results of experiments
1.2.1 Volume of cry oprotective solution remained
In the conventional method or the pellet method, the volume of cryoprotective
solution
remained at the time of cell freezing was calculated from the volume of
cryoprotective solution
removed from the vial, and plotted in a graph (Fig. 2). In the pellet method,
the volume of
cryoprotective solution remained was reduced to 1/40 to 1/100 of that in the
conventional method.
[0073]
1.2.2 Cell viability and cell count
In the conventional method and the pellet method, the cell viability after
cell thawing was
measured with a cell counter (Fig. 3). The results showed no significant
difference between the two
groups.
[0074]
In the conventional method and the pellet method, the cell count after thawing
and the cell
count on day 3 of culturing were measured with a cell counter (Fig. 4). The
results showed no
significant difference between the two groups.
[0075]
<Example 2>
2.1 Experimental Procedure
Instead of ZENOAQ's Stem Cell Banker of Example 1, 5% DMSO-containing fetal
bovine
serum (FBS) (5% DMSO/FBS solution) (DMSO (Sigma), FBS (Gibco)), 10% glycerol-
containing
13
Date Recue/Date Received 2022-09-21
fetal bovine serum (FBS) (10% glycerol/FBS solution) (glycerol (Sigma), FBS
(Gibco)), or Stem Cell
Banker DMSO-free GMP grade (ZENOAQ) was used as the cry oprotectant to freeze
and thaw
umbilical cord MSCs. The other experimental procedure was performed according
to Example 1.
[0076]
2.2 Results of experiments
Like in Example 1, the volume of cryoprotective solution remained at the time
of cell
freezing was much smaller in the pellet method than in the conventional
method. In each
cryoprotective solution, the cell viability and the cell count after thawing
were measured for the
conventional method or the pellet method, and no significant differences were
observed (Fig. 5).
[0077]
<Example 3>
3.1 Experimental Procedure
3.1.1 Conventional method
Umbilical cord MSCs were frozen and thawed using a FROZEBAG F-050 (NIPRO;
volume:
25 ml). Each conical tube containing umbilical cord MSCs (10 x 10e7 cells)
made into single cells
by TrypLET" Select solution was centrifuged at 400 g x 5 min. The trypsin
solution in the
supernatant was removed and then replaced with 20 ml of Stem Cell Banker
cryoprotective solution
(10% (v/v) DMSO). The entire cell suspension obtained was filled into a
FROZEBAG, placed in a
heat insulator Styrofoam box, and subjected to slow freezing overnight at a
rate of 0.1 to PC/min in
a -80 C freezer. The cells were thawed by warming the bag at 37 C for several
minutes. After the
thawing, a syringe was attached to a connector of the bag. The cell suspension
was then slowly
aspirated from the tube to collect the cells. The cell suspension was
transferred to a centrifuge tube,
and the cell viability and the cell count were measured with a cell counter.
Next, the cell suspension
was centrifuged at 400 g x 5 min to continue cell culture evaluation. The
cryoprotective solution in
the supernatant was then removed. After an appropriate amount of each cell
culture medium was
added, the cells were suspended by pipetting, seeded on a culture dish, and
cultured for 3 days. The
cells were made into single cells by using TrypLE' Select solution, and the
number of cells was
recounted.
[0078]
3.1.2 Pellet method
Umbilical cord MSCs were frozen and thawed using a FROZEBAG F-050 (NIPRO;
volume:
25 m1). Each conical tube containing umbilical cord MSCs (10 x 10e7 cells)
made into single cells
by TrypLET" Select solution was centrifuged at 400 g x 5 mm. The trypsin
solution in the
supernatant was removed and then replaced with 20 ml of Stem Cell Banker cry
oprotective solution
(10% (v/v) DMSO). The entire cell suspension obtained was filled into a
FROZEBAG, placed in a
4 C refrigerator for 20 min, and then centrifuged at 400 g x 10 min with the
bag laterally lying on a
multi-well plate rotor TS-41C of TOMY LCX-200 centrifuge. In this way, a cell
pellet was formed
over a side with a large area of the bag (Fig. 6). Subsequently, the FROZEBAG
was taken out
slowly so that the pellet did not float. A syringe was attached to a connector
of the bag to remove
as much the cryoprotective solution in the supernatant as possible from the
tube (about 19 ml in total
was removed). The resulting material was placed in a heat insulator Styrofoam
box, and subjected
to slow freezing overnight at a rate of 0.1 to 1 C/min in a -80 C freezer. For
cell thawing, the bag
was subjected to thawing at 37 C for several minutes. After that, a syringe
filled with 20 ml of PBS
was set to the tube of the bag. PBS was added to and injected into the bag,
and the bag was gently
loosened by hand to float the pellet. The cell suspension in the bag was then
slowly aspirated, from
the tube, with the syringe attached to the connector to collect the cells. The
cell suspension was
14
Date Recue/Date Received 2022-09-21
transferred to a centrifuge tube, and the cell viability and the cell count
were measured with a cell
counter. Next, the cell suspension was centrifuged at 400 g x 5 min to
continue cell culture
evaluation. The PBS in the supernatant was then removed. After an appropriate
amount of each
cell culture medium was added, the cells were suspended by pipetting, seeded
on a culture dish, and
cultured for 3 days. The cells were made into single cells by using TrypLE'
Select solution, and
the number of cells was recounted.
[0079]
3.2 Results of experiments
3.2.1 Volume of cryoprotective solution remained
The volume of cryoprotective solution remained at the time of freezing by the
conventional
method or the pellet method was calculated from the volume of cryoprotective
solution removed from
the bag, and plotted in a graph. In the pellet method, the volume remained was
reduced to 1/20 of
that in the conventional method (Fig. 7).
[0080]
3.2.2 Cell viability and cell count
The cell viability and the cell count after thawing by the conventional method
or the pellet
method were measured, and no significant differences were observed (Fig. 8).
The cell count after
thawing and the cell count on day 3 of culturing were measured with a cell
counter and compared
between the conventional method and the pellet method. Then, no significant
differences were
observed (Fig. 9).
[0081]
As described above, the pellet method has been demonstrated to have favorable
cell
cryopreservation efficiency while the volume of cryoprotective solution
remained is dramatically
decreased. In addition, this method allows cell thawing and syringe filling to
be performed in a
closed system or in a series of operations, which makes it easy to administer
cells to each patient.
Further, since the procedure after thawing of cells is only required at a
medical site, a cell suspension
obtained by this method, for example, can be administered to each patient even
in medical facilities
without any laboratory.
[0082]
Hereinabove, the Examples have been described. The Examples are just examples.
It
should be understood by those skilled in the art that various modifications
are allowed and such
modified embodiments are within the scope of the invention.
Date Recue/Date Received 2022-09-21