Note: Descriptions are shown in the official language in which they were submitted.
COMPOSITIONS AND METHODS FOR ENHANCED DELIVERY OF
ANTIVIRAL AGENTS
FIELD
Disclosed herein are methods, compositions and kits for treating a viral
infection, for
example, COVID-19, MERS and SARS. The disclosed compositions provide for
enhanced
delivery of the disclosed antiviral agents.
BRIEF DESCRIPTION OF THE FIGURES
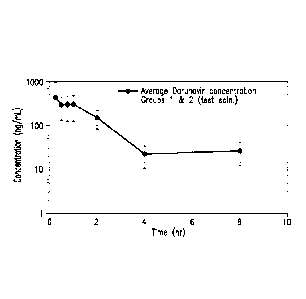
Figure 1 depicts the average plasma concentration of darunavir in the test
animals at
1 hour is approximately 306 ng/mL and at 2 hours is approximately 153 ng/mL.
Figure 2 depicts the average plasma concentration of danmavir in the animals
given
the control formulation at 1 hour is approximately 185 ng/mL and at 2 hours is
approximately 63 ng/mL.
Figure 3 depicts the average plasma concentration of efavirenz in the test
animals at
1 hour is approximately 133 ng/mL and at 2 hours is approximately 142 ng/mL.
Figure 4 depicts the average plasma concentration of efavirenz in the animals
given
the control formulation at 1 hour is approximately 139 ng/mL and at 2 hours is
approximately 117 ng/mL.
DETAILED DESCRIPTION OF THE DISCLOSURE
The materials, compounds, compositions, articles, and methods described herein
may be understood more readily by reference to the following detailed
description of
specific aspects of the disclosed subject matter and the Examples included
therein.
Also, throughout this specification, various publications are referenced.
General Definitions
In this specification and in the claims that follow, reference will be made to
a
number of terms, which shall be defined to have the following meanings:
All percentages, ratios and proportions herein are by weight, unless otherwise
specified. All temperatures are in degrees Celsius (0C) unless otherwise
specified.
1
8917050
Date Recue/Date Received 2023-11-15
The terms "a" and "an" are defined as one or more unless this disclosure
explicitly
requires otherwise.
Ranges may be expressed herein as from "about" one particular value, and/or to
"about" another particular value. When such a range is expressed, another
aspect includes
from the one particular value and/or to the other particular value. Similarly,
when values
are expressed as approximations, by use of the antecedent "about," it will be
understood that
the particular value foims another aspect. It will be further understood that
the endpoints of
each of the ranges are significant both in relation to the other endpoint, and
independently
of the other endpoint.
The terms "comprise" (and any form of comprise, such as "comprises" and
"comprising"), "have" (and any form of have, such as "has" and "having"),
"include" (and
any form of include, such as "includes" and "including") and "contain" (and
any form of
contain, such as "contains" and "containing") are open-ended linking verbs. As
a result, an
apparatus that "comprises," "has," "includes" or "contains" one or more
elements possesses
those one or more elements, but is not limited to possessing only those
elements. Likewise,
a method that "comprises," "has," "includes" or "contains" one or more steps
possesses
those one or more steps, but is not limited to possessing only those one or
more steps.
Any embodiment of any of the disclosed methods or compositions can consist of
or
consist essentially of¨ rather than comprise/include/contain/have ¨ any of the
described
steps, elements, and/or features. Thus, in any of the claims, the term
"consisting of' or
"consisting essentially of' can be substituted for any of the open-ended
linking verbs recited
above, in order to change the scope of a given claim from what it would
otherwise be using
the open-ended linking verb.
The feature or features of one embodiment may be applied to other embodiments,
even though not described or illustrated, unless expressly prohibited by this
disclosure or
the nature of the embodiments.
Any embodiment of any of the disclosed compounds or methods can consist of or
consist essentially of ¨ rather than comprise/include/contain/have ¨ any of
the described
steps, elements, and/or features. Thus, in any of the claims, the term
"consisting of' or
"consisting essentially of' can be substituted for any of the open-ended
linking verbs recited
above, in order to change the scope of a given claim from what it would
otherwise be using
the open-ended linking verb.
2
8917050
Date Recue/Date Received 2023-11-15
The feature or features of one embodiment may be applied to other embodiments,
even though not described or illustrated, unless expressly prohibited by this
disclosure or
the nature of the embodiments.
As used herein, the term "Coronaviridae" refers to a family of enveloped,
positive-
sense, single-stranded RNA viruses. The term "coronavirus" refers in the
methods
described herein specifically to SARS-CoV-2, which causes COVID-19, and which
originated in Wuhan China in 2019. The term coronavirus and variations thereof
are used
interchangeably througjh out the disclosure. Other Coronaviridae viruses are
used as
examples, targets and standards by which the presently disclosed compounds are
measured.
For example, MERS (Middle East Respiratory Syndrome) coronavirus.
As used herein, the term "subject" refers to a human or an animal that has
been
diagnosed with COVID-19 or one or more strains of SARS-CoV-2, or has tested
positive
for COVID-19 or one or more strains of SARS-CoV-2. The term subject also
includes
humans or animals that have been exposed to Wuhan coronavirus but are not
symptomatic.
As used herein, the term "Lassa virus" refers to a RNA virus belonging to the
family
of Arenaviridae. As used herein "Lassa fever" refers to an acute viral
haemorrhagic illness
caused by Lassa virus.
As used herein the term Poxiviridae refers to the poxvirus family. Four genera
of
poxviruses may infect humans: orthopoxvirus, parapoxvirus, yatapoxvirus,
molluscipoxvirus. Orthopox: smallpox virus (variola), vaccinia virus, cowpox
virus,
monkeypox virus; Parapox: orf virus, pseudocowpox, bovine papular stomatitis
virus;
Yatapox: tanapox virus, yaba monkey tumor virus; Molluscipox: molluscum
contagiosum
virus (MCV). The most common are vaccinia (seen on Indian subcontinent) and
molluscum
contagiosum, but monkeypox infections are rising (seen in west and central
African
rainforest countries).
As used herein, the tarn "Vaccinia" refers to a family of large, complex,
enveloped
virus belonging to the poxvirus family. It is characterized by having a linear
double-
stranded CAN genome approximately 190 kbp in length, which encodes
approximately 250
genes. The dimensions of the virion are roughly 360 x 270 x 250 nm, with a
mass of about
5-10 fg. Included with in the genus are the species buffalo virus, cantagalo
virus, rabbitpox
virus Utrecht, Vaccinia virus Ankara, Vaccinia virus Copenhagen and Vaccinia
virus WR.
HIV differs from many viruses in that it has very high genetic variability.
This
diversity is a result of its fast replication cycle, with the generation of
about 10' virions
3
8917050
Date Recue/Date Received 2023-11-15
every day, coupled with a high mutation rate of approximately 3 x 10-5 per
nucleotide
base per cycle of replication and recombinogenic properties of reverse
transcriptase.
As used herein then tent! "Orthomyxoviridae" refers to common "Influenza," for
example, illnesses caused by any of three types of Influenza viruses: Type A,
Type B, or
Type C. Influenza A virus has a large number of subtypes, for example, H1NI,
H5N1,
H7N9 ("bird flu"), and the like. The viruses are named after the specific
combination of
hemagglutinin and neuraminidase that comprises the viral package. Because
there are 18
known types of hemagglutinin and 11 known types of neuraminidase, in, in
theory, 198
different combinations of these proteins are possible. This type of virus is
the most
common in humans and animals.
As used herein the term Filoviridae refers to a virus family that includes the
following genera, including Cuevavirus, Dianlovirus, Ebolavirus and
Marburgvirus.
Importantly as it relates to Filoviridae, Ebolavirus includes the species
Bombali ebolavirus,
Bundibugyo ebolavirus, Reston ebolavirus, Sudan ebolavirus, Tai Forest
ebolavirus and
Zaire ebolavirus. Marburgvirus includes the species Marburg Marburgvirus.
These
viruses cause viral haemorrhagic fever in humans.
As used herein the teiin Herpesviridae refers to a family of viruses which
includes
the following genra found in humans: Cytomegalovirus , Rhadinovirus ,
Roseolovirus,
Simplexvirus, and Lymphocgtovirus. Species of Herpesviridae include Human
alphaherpesvirus I and Human alphaherpesvirus 1.
As used herein the term Flavivirdae refers to a family of viruses typically
spread via
insects, for examples, ticks and mosquitoes. Flaviridae includes the following
genra:
Flavivirus (Yellow fever, West Nile, Dengue and Zika viruses) and Gepacivirus
(hepatitis
viruses).
As used herein then tem'. "Orthoretrovirinae" refers to the virus family which
includes the Human Immunodeficiency viruses (HIV), for example, the two
species of
Lentivirus; Human immunodeficiency virus 1 and Human immunodeficiency virus 2.
As used herein, the teims "treat," "treating," "treatment," and the like refer
to
reducing or ameliorating a disorder and/or symptoms associated therewith. It
will be
appreciated that, although not precluded, treating a disorder or condition
does not require
that the disorder, condition or symptoms associated therewith be completely
eliminated.
As used herein, the terms "prevent," "preventing," "prevention," "prophylactic
treatment" and the like are encompassed within the term "treating," and refer
to reducing the
4
8917050
Date Recue/Date Received 2023-11-15
probability of developing a disorder or condition in a subject, who does not
have, but is at
risk of or susceptible to developing a disorder or condition.
As used herein, "pharmaceutically acceptable" means physiologically tolerable,
for
either human or veterinary applications. In addition, "pharmaceutically
acceptable" is
meant for a material that is not biologically or otherwise undesirable, i.e.,
the material may
be administered to a subject without causing any undesirable biological
effects or
interacting in a deleterious manner with any of the other components of the
pharmaceutical
composition in which it is contained. Essentially, the pharmaceutically
acceptable material
is nontoxic to the recipient. The carrier would naturally be selected to
minimize any
degradation of the active ingredient, to minimize any adverse side effects in
the subject, and
to optimize formulation for drug delivery and dosing to the target tissues
infected by
Coronaviridae as would be well known to one of skill in the art. For a
discussion of
pharmaceutically acceptable carriers and other components of pharmaceutical
compositions,
see, e.g., Remington's Pharmaceutical Sciences, 18th ed., Mack Publishing
Company, 1990.
The terms "composition" and "formulation" are used interchangeably throughout
the
Specification to refer to the disclosed combination of ingredients which
deliver increased
plasma level concentrations of anti-viral compounds.
As used herein the term "active base" refers to the two essential components
of the
disclosed compositions: one or more antiviral agents and a bioavailability
enhancing agent.
As disclosed herein below, an antiviral agent and a bioavailability enhancing
agent can be
given to a subject in need without any other active or inactive ingredients
present to achieve
the desired increase in antiviral plasma levels.
As used herein, the term "unit-dosage form" refers to a physically discrete
unit
suitable for administration to a human and animal subject, and packaged
individually as is
known in the art. Each unit-dose contains a predetermined quantity of an
active
ingredient(s) sufficient to produce the desired therapeutic effect, in
association with the
required pharmaceutical carriers or excipients. A unit-dosage form may be
administered in
fractions or multiples thereof. Examples of a unit-dosage foim include a
tablet, liquid
formulation or and individually packaged tablet and capsule.
As used herein, the term "multiple-dosage form" is a plurality of identical
unit-
dosage forms packaged in a single container to be administered in segregated
unit-dosage
form. Examples of a multiple-dosage fomi include a bottle of tablets or
capsules, or bottle
of a liquid composition.
8917050
Date Recue/Date Received 2023-11-15
As used herein, and unless otherwise specified, the terms "composition,"
"formulation," and "dosage form" are intended to encompass products comprising
the
specified ingredient(s), for example, and antiviral agent, bioavailability
agent (in the
specified amounts, if indicated), as well as any product(s) which result,
directly or
indirectly, from combination of the specified ingredient(s) in the specified
amount(s).
As disclosed herein the terms "bile acid," "bile salt," "bile acid/salt,"
"bile acids,"
"bile salts," and "bile acids/salts" are, unless otherwise indicated, utilized
interchangeably
herein. Any reference to a bile acid used herein includes reference to a bile
acid or a salt
thereof. Furthermore, it is to be understood that any singular reference to a
component (bile
acid or otherwise) used herein includes reference to one and only one, one or
more, or at
least one of such components. Similarly, any plural reference to a component
used herein
includes reference to one and only one, one or more, or at least one of such
components,
unless otherwise noted.
"Test agents" or otherwise "test compounds" as used herein refers to an agent
or
compound that is to be screened in one or more of the assays described herein.
Test agents
include compounds of a variety of general types including, but not limited to,
small organic
molecules, known pharmaceuticals, polypeptides; carbohydrates such as
oligosacchari des
and polysaccharides; polynucleotides; lipids or phospholipids; fatty acids;
steroids; or
amino acid analogs. Test agents can be obtained from libraries, such as
natural product
libraries and combinatorial libraries. In addition, methods of automating
assays are known
that permit screening of several thousands of compounds in a short period.
Unless defined otherwise, all technical and scientific terms used herein have
the
same meaning as commonly understood by one of ordinary skill in the art to
which this
invention belongs. Although any methods and materials similar or equivalent to
those
described herein can also be used in the practice or testing of the described
invention, the
preferred methods and materials are now described.
As used herein "stop-gap" refers to the administration of the disclosed
compounds to
ameliorate the spread of a coronavirus and emergence of drug resistant
strains. A stop-gap
administration is a temporary measure designed to control the spread of the
virus until
medical personnel can evaluate the extent of infection and/or the source.
Details associated with the embodiments described above and others are
described
below.
6
8917050
Date Recue/Date Received 2023-11-15
DETAILED DESCRIPTION
Disclosed herein are compositions or formulations that can deliver antiviral
agents
in a more effective manner. The disclosed compositions overcome the low
bioavailability
of known antiviral agents. Bioavailability refers to the extent and rate at
which the
disclosed antiviral agent enters systemic circulation, thereby accessing the
site of action.
Bioavailability for a given formulation provides an estimate of the relative
fraction of the
orally administered dose that is absorbed into the systemic circulation. Low
bioavailability
is most common with oral dosage forms of poorly water-soluble, slowly absorbed
drugs.
Insufficient time for absorption in the gastrointestinal tract is a common
cause of low
bioavailability. If the drug does not dissolve readily or cannot penetrate the
epithelial
membrane (e.g., in some examples, if it is highly ionized and polar), time at
the absorption
site may be insufficient. For orally administered drugs, passage through the
stomach to the
intestinal wall and then the portal circulation to the liver are causes of
poor bioavailability.
Antiviral agents not well absorbed will undergo first-pass metabolism, for
example,
glucuronidation, after which the antiviral agent as a metabolite is excreted
in the urine or
feces. The lack of bioavailability is therefore, due to inadequate absorption.
Bioavailability of an antiviral agent can usually be assessed by determining
the area
under the plasma concentration¨time curve (AUC). AUC is directly proportional
to the
total amount of unchanged drug that reaches systemic circulation. Plasma drug
concentration increases with extent of absorption; the maximum (peak) plasma
concentration is reached when drug elimination rate equals absorption rate.
Peak time is the
most widely used general index of absorption rate; the slower the absorption,
the later the
peak time.
Antiviral agents can be further administered by delivery to the oral,
oropharyngeal,
nasal, olfactory, pulmonary and/or genitourinary mucosa, whether intended for
systemic
perfusion or localized delivery.
COMPOSITIONS
For the purposes of the present disclosure, the terms "a pharmaceutically
effect
amount" or "an effective amount" is used interchangeably throughout the
Specification. An
effective amount is the amount of an antiviral agent that comprises a
disclosed composition
or formulation and which results in an increase in the plasma concentration of
a subject
being administered the antiviral agent in a disclosed composition or
formulation versus the
plasma level of the same antiviral agent administered in a non-disclosed
foimulation or
composition. For example, the study herein below demonstrates the increase in
plasma
7
8917050
Date Recue/Date Received 2023-11-15
levels of darunavir and efavirenz when administered in a disclosed formulation
versus the
same drugs administered without the benefit of the disclosed bioavailability
agents.
A general aspect of the disclosed compositions relates to the active base,
comprising:
a) one or more antiviral agents; and
b) a bioavailability enhancing agent.
In one embodiment of the general aspect of the disclosed compositions relates
to the
active base, comprising:
a) one or more anti-inflammatory agents; and
b) a bioavailability enhancing agent.
In another embodiment of the general aspect of the disclosed compositions
relates to
the active base, comprising:
a) one or more antiviral agents and an anti-inflammatory agent; and
b) a bioavailability enhancing agent
The administration of the active base without other active or inactive
ingredients
results in an increase in the plasma concentration of the one or more
antiviral agents. The
formulator will understand that administration of the antiviral agents and
bioavailability
enhancing agent will serve to promote increase formulatability, stability,
provide options for
safe and quantitative delivery, as well as provide a range of options for
deliver, for example,
via a comestible agent as described herein.
The following aspects relate to the composition of the active base prior to
admixing
or combining the active base with other ingredients as discussed herein below.
In one
aspect, the active base can comprise:
a) from about 20% to about 65% by weight of one or more antiviral agents;
b) from about 35% to about 80% by weight of a bioavailability enhancing
agent.
In another aspect, the active base can comprise:
a) from about 30% to about 60% by weight of one or more antiviral agents;
b) from about 40% to about 70% by weight of a bioavailability enhancing
agent.
In a further aspect, the active base can comprise:
a) from about 20% to about 50% by weight of one or more antiviral agents;
b) from about 50% to about 80% by weight of a bioavailability enhancing
agent.
8
8917050
Date Recue/Date Received 2023-11-15
In still further aspect, the active base can comprise:
a) from about 33% to about 67% by weight of one or more antiviral agents;
b) from about 33% to about 67% by weight of a bioavailability enhancing
agent.
In still yet further aspect, the active base can comprise:
a) from about 33% to about 50% by weight of one or more antiviral agents;
b) from about 50% to about 67% by weight of a bioavailability enhancing
agent.
The disclosed active base can comprise from about 20% to about 65% by weight
of
one or more antiviral agents. For example, 20%, 21%, 22%, 23%, 24%, 25%, 26%,
27%,
28%, 29%, 30% 31%, 32%, 33%, 34%, 35%, 36%, 37%, 38%, 39%, 40%, 41%, 42%, 43%,
44%, 45%, 46%, 47%, 48%, 49%, 50%, 51%, 52%, 53%, 54%, 55%, 56%, 57%, 58%,
59%, 60%, 61%, 62%, 63%, 64%, or 65% by weight of one or more antiviral
agents.
The disclosed active base can comprise from about 20% to about 65% by weight
of
one or more bioavailability enhancing agents. For example, 35%, 36%, 37%, 38%,
39%,
40%, 41%, 42%, 43%, 44%, 45%, 46%, 47%, 48%, 49%, 50%, 51%, 52%, 53%, 54%,
55%, 56%, 57%, 58%, 59%, 60%, 61%, 62%, 63%, 64%, 65%, 66%, 67%, 68%, 69%,
70%, 71%, 72%, 73%, 74%, 75%, 76%, 77%, 78%, 79%, or 80% by weight of a
bioavailability enhancing agent.
The formulator will recognize that the same increase in plasma level
concentration
cannot be expected from one antiviral agent to the next. An increase can be
expected when
a particular antiviral agent is foimulated as disclosed herein versus a
composition that does
not comprise the ingredients disclosed herein.
Antiviral Agents
The disclosed antiviral agent can be chosen from protease inhibitors,
endonuclease
inhibitors, integrase inhibitors, enzyme inhibitors, non-nucleoside reverse
transcriptase
inhibitors, fusion inhibitors, cell entry inhibitors, mRNA and protein
synthesis inhibitors,
viral replication blockers, uncoating inhibitors, reverse transcriptase
inhibitors,
topoisomerase inhibitors, assembly inhibitors, M2 inhibitors, DNA polymerase
inhibitors,
DNA terminase complex inhibitors, HCV protein inhibitors, neuraminidase
inhibitors,
virus-neutralizing antibodies, and the like.
One aspect of the present disclosure relates to the delivery of protease
inhibitors.
Non-limiting examples of protease inhibitors include amprenavir (or the pro-
drug
9
8917050
Date Recue/Date Received 2023-11-15
fosamprenavir), atazanavir, bepridil, boceprevir, darunavir, ebastine,
indinavir, lopinavir,
nelfinavir, ritonavir, rupintrivir, saquinavir, simeprevir, telaprevir, and
tipranavir.
A further aspect relates to the delivery of Nucleoside Reverse Transcriptase
Inhibitors (NNRTI). Non-limiting examples of NNRTI antiviral agents include
doravirine,
efavirenz, etravirine, loviride, and rilpivirine.
Other suitable antiviral agents include liver enzyme inhibitors, for example,
cobicistat; endonuclease inhibitors, for example, baloxavir marboxil;
integrase inhibitors,
for example, bictegravir and elvitegravir; HCV protein inhibitors, for
example, daclatasvir;
fusion/entry inhibitors, for example, maraviroc and umifenovir; cell entry
prohibitors, for
example, colchicine; inRNA synthesis inhibitors, for example, methisazone;
tromantadine,
which prevents viral DNA replication, prevents viral uncoating, prevents viral
entry; and
replication inhibitors, for example rimantadine.
Other antiviral compounds include GS-441524 having the formula:
NH,
N
HO N
HOP.
=õ,/
CN
GS-441524 is the precursor nucleotide to remdesivir having the formula:
NH2
0
H 0 N
NA
4P-0 N
N
CH3 0
HOW..
1/CN
H,
Remdesivir is an investigational intravenous drug with broad antiviral
activity that
inhibits viral replication through premature termination of RNA transcription
and has in
vitro activity against SARS-CoV-2 and in vitro and in vivo activity against
related
betacoronaviruses.
In addition to antivirus agents, virus neutralizing, fully human antibodies,
for
example, sarilumab can be used in the disclosed compositions, alone or in
combination with
the disclosed antiviral agents.
Other non-limiting examples of antiviral agents includes chloroquine (See,
Wang M
et al., "Remdesivir and chloroquine effectively inhibit the recently emerged
novel
8917050
Date Recue/Date Received 2023-11-15
coronavirus (2019-nCoV) in vitro," Cell Research 30, 269-271 (2020)) and
hydroxy-
chloroquine. Hydroxychloroquine and chloroquine are oral prescription drugs
that have
been used for treatment of malaria and certain inflammatory conditions.
Hydroxy -
chloroquine and chloroquine are under investigation in clinical trials for pre-
exposure or
post-exposure prophylaxis of SARS-CoV-2 infection, and treatment of patients
with mild,
moderate, and severe COVID-19.
The following are non-limiting examples of antivirals or compounds used to
treat
Covid-19 or that are directed to the treatment of HIV.
Indinavir
HIV-1 protease is an enzyme required for the proteolytic cleavage of the viral
polyprotein precursors into the individual functional proteins found in
infectious HIV-I.
This compound binds to the protease active site and inhibits the activity of
the enzyme. As
such, indinavir acts to regulate HIV by inhibiting the virus' critical
protease activity.
Raltegravir
Raltegravir in an integrase inhibitor. As such, raltegravir acts to regulate a
key
enzyme in the replication mechanism of HIV. The HIV integrase is responsible
for the
transfer of virally encoded DNA into the host chromosome which is a necessary
event in
retroviral replication.
Nevirapine
Nevirapine is a non-nucleoside reverse transcriptase inhibitor (NNRTI) of HIV-
I
which blocks HIV-1 RNA-dependent and DNA-dependent DNA polymerase activities
by
causing a disruption of the enzyme's catalytic site. Nevirapine does this by
binding directly
to the reverse transcriptase (RT).
Azidothymidine (AZT)
AZT, a thymidine analogue, works by selectively inhibiting HIV's reverse
transcriptase, the enzyme that the virus uses to make a DNA copy of its RNA.
Thus AZT
inhibits HIV replication without affecting the function of uninfected cells.
Colchicine
In addition to antiviral agents, anti-inflammatory agents can be used to treat
viral
infections either alone or in combination with antiviral agents. For example,
colchicine
which has the foimula:
11
8917050
Date Recue/Date Received 2023-11-15
H3C
HN
0
H3C0
H3CO OCH3
OCH3
Colchicine is used to treat inflammatory disorders. It is known to work via
several
mechanisms including reducing Intereukin-6, Interleukin-8, Tumour Necrosis
Factor-alpha.
Recently it has been prescribed to control the oxidative stress pathways which
is an
important component in the clinical course and outcome of patients infected
with COVID-
19. See Dalili N et al., "Adding Colchicine to the antiretroviral Medication ¨
Lopinavir/Ritonavir (Kaletra) in Hospitalized Patients with Non-Severe Covid-
19
Pneumonia: A structured Summary of a Study Protocol for a Randomized
Controlled Trial,"
Trials, 21: 489 (2020).
The disclosed compositions can comprise from about 25 mg to about 300 mg. In
one aspect the disclosed single dose compositions of a disclosed antiviral
agent can
comprise any amount from about 25 mg to about 200 mg.
In a further aspect the disclosed single dose compositions of a disclosed
antiviral
agent can comprise any amount from about 100 mg to about 200 mg. In a yet
further aspect
the disclosed single dose compositions of a disclosed antiviral agent can
comprise any
amount from about 75 mg to about 250 mg. In another further aspect the
disclosed single
dose compositions of a disclosed antiviral agent can comprise any amount from
about 50
mg to about 150 mg. In a yet another aspect the disclosed single dose
compositions of a
disclosed antiviral agent can comprise any amount from about 100 mg to about
200 mg. In a
yet still further aspect the disclosed single dose compositions of a disclosed
antiviral agent
can comprise any amount from about 200 mg to about 300 mg.
The disclosed compositions can comprise any amount of antiviral agent from
about
25 mg to about 300 mg. For example, the disclosed compositions can comprise 25
mg, 26
mg, 27 mg, 28 mg, 29 mg, 30 mg 31 mg, 32 mg, 33 mg, 34 mg, 35 mg, 36 mg, 37
mg, 38
mg, 39 mg, 40 mg, 41 mg, 42 mg, 43 mg, 44 mg, 45 mg, 46 mg, 47 mg, 48 mg, 49
mg, 50
mg, 51 mg, 52 mg, 53 mg, 54 mg, 55 mg, 56 mg, 57 mg, 58 mg, 59 mg, 60 mg, 61
mg, 62
mg, 63 mg, 64 mg, 65 mg, 66 mg, 67 mg, 68 mg, 69 mg, 70 mg, 71 mg, 72 mg, 73
mg, 74
mg, 75 mg, 76 mg, 77 mg, 78 mg, 79 mg, 80 mg, 81 mg, 82 mg, 83 mg, 84 mg, 85
mg, 86
12
8917050
Date Recue/Date Received 2023-11-15
mg, 87 mg, 88 mg, 89 mg, 90 mg, 90 mg, 91 mg, 92 mg, 93 mg, 94 mg, 95 mg, 96
mg, 97
mg, 98 mg, 99 mg, 100 mg, 101 mg, 102, mg, 103, mg, 104 mg, 105 mg, 106 mg,
107 mg,
108 mg, 109 mg, 110 mg, 111 mg, 112 mg, 113 mg, 114 mg, 115 mg, 116 mg, 117
mg, 118
mg, 119 mg, 120 mg, 121 mg, 122 mg, 123 mg, 124 mg, 125 mg, 126 mg, 127 mg,
128 mg,
129 mg, 130 mg 31 mg, 132 mg, 133 mg, 134 mg, 135 mg, 136 mg, 137 mg, 138 mg,
139
mg, 140 mg, 141 mg, 142 mg, 143 mg, 144 mg, 145 mg, 146 mg, 147 mg, 148 mg,
149 mg,
150 mg, 151 mg, 152 mg, 153 mg, 154 mg, 155 mg, 156 mg, 157 mg, 158 mg, 159
mg, 160
mg, 161 mg, 1 62 mg, 163 mg, 164 mg, 165 mg, 166 mg, 167 mg, 168 mg, 169 mg,
170
mg, 171 mg, 172 mg, 173 mg, 174 mg, 175 mg, 176 mg, 177 mg, 178 mg, 179 mg,
180 mg,
181 mg, 182 mg, 183 mg, 184 mg, 185 mg, 186 mg, 187 mg, 188 mg, 189 mg, 190
mg, 190
mg, 191 mg, 192 mg, 193 mg, 194 mg, 195 mg, 196 mg, 197 mg, 198 mg, 199 mg,
200 mg,
201 mg, 202, mg, 203, mg, 204 mg, 205 mg, 206 mg, 207 mg, 208 mg, 209 mg, 210
mg,
212 mg, 212 mg, 213 mg, 214 mg, 215 mg, 216 mg, 217 mg, 218 mg, 219 mg, 220
mg, 221
mg, 222 mg, 223 mg, 224 mg, 225 mg, 226 mg, 227 mg, 228 mg, 229 mg, 230 mg,
231 mg,
232 mg, 233 mg, 234 mg, 235 mg, 236 mg, 237 mg, 238 mg, 239 mg, 240 mg, 241
mg, 242
mg, 243 mg, 244 mg, 245 mg, 246 mg, 247 mg, 248 mg, 249 mg, 250 mg, 251 mg,
252 mg,
253 mg, 254 mg, 255 mg, 256 mg, 257 mg, 258 mg, 259 mg, 260 mg, 261 mg, 2 62
mg,
263 mg, 264 mg, 265 mg, 266 mg, 267 mg, 268 mg, 269 mg, 270 mg, 271 mg, 272
mg, 273
mg, 274 mg, 275 mg, 276 mg, 277 mg, 278 mg, 279 mg, 280 mg, 281 mg, 282 mg,
283 mg,
284 mg, 285 mg, 286 mg, 287 mg, 288 mg, 289 mg, 290 mg, 290 mg, 291 mg, 292
mg, 293
mg, 294 mg, 295 mg, 296 mg, 297 mg, 298 mg, 299 mg, or 300 mg of one or more
antiviral
agents.
The disclosed compositions can provide a single dose of a disclosed antiviral
agent
based upon the body mass of the subject being treated. Therefore, a single
dose of a
disclosed antiviral agent can range from about 0.35 mg/kg to about 20 mg/kg of
the
subject's body mass.
In one embodiment, the amount of a disclosed antiviral agent in a single dose
is
from about 1 mg/kg to about 8 mg/kg of the subject's body mass.
In another embodiment, the amount of a disclosed antiviral agent in a single
dose is
from about 2 mg/kg to about 5 mg/kg of the subject's body mass.
In a further embodiment, the amount of a disclosed antiviral agent in a single
dose is
from about 1.5 mg/kg to about 4 mg/kg of the subject's body mass.
In a yet further embodiment, the amount of a disclosed antiviral agent in a
single
dose is from about 4 mg/kg to about 10 mg/kg of the subject's body mass.
13
8917050
Date Recue/Date Received 2023-11-15
In a still further embodiment, the amount of a disclosed antiviral agent in a
single
dose is from about 5 mg/kg to about8 mg/kg of the subject's body mass.
For example, the dose can comprise any amount from about 0.5 mg/kg to about 10
mg/kg on the body mass of the subject being treated.
For example, 0.5 mg/kg, 0.6 mg/kg, 0.7 mg/kg, 0.8 mg/ kg, 0.9 mg/kg, 1 mg/kg,
1.1
mg/kg, 1.2 mg/kg, 1.3 mg/kg, 1.4 mg/kg, 1.5 mg/kg, 1.6 mg/kg, 1.7 mg/kg, 1.8
mg/kg, 1.9
mg/kg, 2.0 mg/kg, 2.1 mg/kg, 2.2 mg/kg, 2.3 mg/kg, 2.4 mg/kg, 2.5 mg/kg, 2.6
mg/kg, 2.7
mg/kg, 2.8 mg/kg, 2.9 mg/kg, 3.0 mg/kg, 3.1 mg/kg, 3.2 mg/kg, 3.3 mg/kg, 3.4
mg/kg, 3.5
mg/kg, 3.6 mg/kg, 3.7 mg/kg, 3.8 mg/kg, 3.9 mg/kg, 4.0 mg/kg, 4.1 mg/kg, 4.2
mg/kg, 4.3
mg/kg, 4.4 mg/kg, 4.5 mg/kg, 4.6 mg/kg, 4.7 mg/kg, 4.8 mg/kg, 4.9 mg/kg, or
5.0 mg/kg,
5.1 mg/kg, 5.2 mg/kg, 5.3 mg/kg, 5.4 mg/kg, 5.5 mg/kg, 5.6 mg/kg, 5.7 mg/kg,
5.8 mg/kg,
5.9 mg/kg, 6.0 mg/kg, 6.1 mg/kg, 6.2 mg/kg, 6.3 mg/kg, 6.4 mg/kg, 6.5 mg/kg,
6.6 mg/kg,
6.7 mg/kg, 6.8 mg/kg, 6.9 mg/kg, 7.0 mg/kg, 7.1 mg/kg, 7.2 mg/kg, 7.3 mg/kg,
7.4 mg/kg,
7.5 mg/kg, 7.6 mg/kg, 7.7 mg/kg, 7.8 mg/kg, 7.9 mg/kg, 8.0 mg/kg, 8.1 mg/kg,
8.2 mg/kg,
8.3 mg/kg, 8.4 mg/kg, 8.5 mg/kg, 8.6 mg/kg, 8.7 mg/kg, 8.8 mg/kg, 8.9 mg/kg,
90 mg/kg,
9.1 mg/kg, 9.2 mg/kg, 9.3 mg/kg, 9.4 mg/kg, 9.5 mg/kg, 9.6 mg/kg, 9.7 mg/kg,
9.8 mg/kg,
9.9 mg/kg, or 10.0 mg/kg of body mass.
Pharmaceutically Acceptable Salts
In some embodiments of the disclosed compositions, the antiviral agent is in
the
form of a pharmaceutically acceptable salt. Non-limiting examples of
pharmaceutically
acceptable salts include acetate, adipate, alginate, aspartate, benzoate,
benzenesulfonate
(besylate), bisulfate, butyrate, citrate, camphorate, camphorsulfonate,
cyclopentane-
propionate, digluconate, dodecylsulfate, 1,2-ethanedisulfonate (edisylate),
ethanesulfonate
(esylate), formate, fumarate, glucoheptanoate, glycerophosphate, glycolate,
hemisulfate,
heptanoate, hexanoate, hydrochloride, hydrobromide, hydroiodide, 2-
hydroxyethane-
sulfonate, lactate, maleate, malonate, methanesulfonate (mesylate), 2-
naphthalenesulfonate
(napsylate), nicotinate, nitrate, oxalate, palmoate, pectinate, persulfate, 3-
phenylpropionate,
phosphate, picrate, pivalate, propionate, salicylate, succinate, sulfate,
tartrate, thiocyanate,
tosylate, or undecanoate salts.
Bioavailability Enhancing Agent
The disclosed bioavailability enhancing agent comprises one or more
triglycerides.
In one aspect the disclosed triglycerides are edible oils. An edible oil is
defined herein as an
oil that is capable of undergoing de-esterification or hydrolysis in the
presence of pancreatic
lipase in vivo under normal physiological conditions. Specifically, digestible
oils comprise
14
8917050
Date Recue/Date Received 2023-11-15
glycerol triesters of C6-C22 fatty acids. The term "edible oil" refers to oils
derived from
plants, for example, corn oil, sunflower oil, oils derived from living
animals, for example,
fish oils. In addition, the oils can be derived from krill.
The disclosed edible oils can have a low percentage of saturated fatty acids,
for
example, hemp seed oil (7.0%) or a high percentage of saturated fatty acids,
for example,
coconut oil (82.5%) provided the solid content index is such that the oil is
liquid and
flowable at temperatures above about 15 C.
In one aspect of the disclosed bioavailability enhancing agents the
triglycerides
comprise less than or equal to about 5% by weight of free fatty acids, mono-
glycerides and
di-glycerides. The triglycerides of the disclosed bioavailability enhancing
agent are refined,
bleached and de-odorized.
Vegetable oils comprise the disclosed triglycerides. These oils are refined in
order
to remove the non-glyceride impurities that are present in the crude oil. Some
of these
impurities are naturally present in the seeds or formed during harvesting and
storage of
seeds or during extraction of crude oil and subsequently during its refining.
Oil refining
processes for vegetable oils are designed to remove these impurities from the
oil or reduce
them to a level where their deleterious effects on oil stability are minimal
and made suitable
for human consumption or for pharmaceutical formulation. Vegetable oil
undergoes
degradation almost immediately after the seed is crushed. The oil starts to
show the signs of
primary oxidation as measured by its peroxide value. Under certain
circumstances the oil
may develop a darker color or higher free fatty acids and eventually an
unpleasant odor or
viscosity. Gums, phosphatides and mucilaginous substances act as emulsifiers
increasing
loss of oil and can decompose at processing temperatures. Free fatty acids
increase
foaming and diminish the storage and formulating properties of the disclosed
oils.
Presence of compounds such as phosphatides, free fatty acids, odiferous
volatiles,
colourant, waxes and metal compounds in oil negatively affect the desired
properties for
compounding with the disclosed antiviral compounds and storage stability of
the refined oil
avoids the presence of any unwanted or reactive species being a part of the
final
composition. Refining processes have, therefore, been developed to remove
undesirable
compounds such as tocopherols, phenols, sterols and the like.
Chemical refining includes degumming, neutralizing, bleaching, winterizing and
de-
odorizing stages. The edible oils of the disclosed bioavailability enhancing
agents are
refined oils that have been winterized to prevent the precipitation of wax.
8917050
Date Recue/Date Received 2023-11-15
The disclosed oils are refined, bleached and deodorized to obtain as complete
a pure
triglyceride composition. There will be, however, some amount of mono- and
diglycerides
present in the final oil. Some of the disclosed oils can be obtained from the
host plant by
supercritical extraction or other harvesting means. The final oils used in the
disclosed
formulations comprise at least about 97% by weight of triglycerides and the
balance
compatible ingredients, inter alia, mono- and diglycerides and free fatty
acids or fatty acid
esters, phosphatides, sterols, fatty alcohols, fat soluble vitamins and other
color bodies.
Disclosed herein are non-limiting examples of edible oils suitable for use in
delivering antiviral agents. Plant based oils include borage seed oil,
syzigium aromaticum
oil, hempseed oil, herring oil, cod-liver oil, salmon oil, flaxseed oil, wheat
germ oil, evening
primrose oils, almond oil, babassu oil, borage oil, black currant seed oil,
canola oil, castor
oil, coconut oil, corn oil, cottonseed oil, emu oil, evening primrose oil,
flax seed oil,
grapeseed oil, groundnut oil (e.g., peanut), lanolin oil, linseed oil, mink
oil, mustard seed
oil, olive oil, palm oil, palm kernel oil, rapeseed oil, safflower oil, sesame
oil, shark liver
oil, soybean oil, sunflower oil, tree nut oil, hydrogenated castor oil,
hydrogenated coconut
oil, hydrogenated palm oil, hydrogenated soybean oil, hydrogenated vegetable
oil, glyceryl
trioleate, glyceryl trilinoleate, glyceryl trilinolenate, citrate thisocetyl
triglyceride having
10-18 carbon atoms, omega-3 polyunsaturated fatty acid triglyceride containing
oil, omega-
3 oil, omega-6 oil, and any combination thereof.
In one embodiment the edible oil is chosen from palm kernel oil, soybean oil,
rapeseed oil (canola), sunflower oil, groundnut oil, sesame oil, flaxseed oil,
palm oil, olive
oil and corn oil. In one iteration, the edible oil is chosen from soybean oil,
rapeseed oil
(canola), sunflower oil, palm oil, olive oil or corn oil. A non-limiting
example of an edible
oil used in the disclosed formulations includes sunflower oil. A further non-
limiting
example of an edible oil used in the disclosed fommlations includes palm oil.
A still further
non-limiting example of an edible oil used in the disclosed formulations
includes rapeseed
oil. A yet still further non-limiting example of an edible oil used in the
disclosed
formulations includes soybean oil. A further non-limiting example of an edible
oil used in
the disclosed formulations includes corn oil.
In one aspect the edible oils comprise one or more fish oils. Included within
fish oil
are algal oils. Non-limiting examples of fish oils include herring, sardines,
Spanish
mackerel, salmon, halibut, tuna, swordfish, tilefish, pollock, cod, catfish,
flounder, grouper
mahi mahi, orange roughy, red snapper, shark, king mackerel, hoki, and
gemfish.
16
8917050
Date Recue/Date Received 2023-11-15
Edible oils having a plurality of non-conjugated di-enes and tri-enes, for
example,
linoleic and linolenic acids, can by "touch hardened" to increase the amount
of mono-
olefins present. Touch harden refers to hydrogenation to a point wherein the
Iodine value of
the triglyceride is lowered to 1-107 or less.
The disclosed compositions can comprise from about 50 mg to about 600 mg. Ti
one aspect the disclosed single dose compositions of a disclosed
bioavailability enhancing
agent can comprise any amount from about 50 mg to about 400 mg.
In a further aspect the disclosed single dose compositions of a disclosed
bioavailability enhancing agent can comprise any amount from about 100 mg to
about 200
mg. In a yet further aspect the disclosed single dose compositions of a
disclosed
bioavailability enhancing agent can comprise any amount from about 150 mg to
about 250
mg. In a still further aspect the disclosed single dose compositions of a
disclosed
bioavailability enhancing agent can comprise any amount from about 200 mg to
about 400
mg
The disclosed single dose compositions can comprise any amount a disclosed
bioavailability enhancing agent from about 50 mg to about 600 mg. For example,
the
disclosed compositions can comprise 50 mg, 51 mg, 52 mg, 53 mg, 54 mg, 55 mg,
56 mg,
57 mg, 58 mg, 59 mg, 60 mg, 61 mg, 62 mg, 63 mg, 64 mg, 65 mg, 66 mg, 67 mg,
68 mg,
69 mg, 70 mg, 71 mg, 72 mg, 73 mg, 74 mg, 75 mg, 76 mg, 77 mg, 78 mg, 79 mg,
80 mg,
81 mg, 82 mg, 83 mg, 84 mg, 85 mg, 86 mg, 87 mg, 88 mg, 89 mg, 90 mg, 90 mg,
91 mg,
92 mg, 93 mg, 94 mg, 95 mg, 96 mg, 97 mg, 98 mg, 99 mg, 100 mg, 101 mg, 102,
mg, 103,
mg, 104 mg, 105 mg, 106 mg, 107 mg, 108 mg, 109 mg, 110 mg, 111 mg, 112 mg,
113 mg,
114 mg, 115 mg, 116 mg, 117 mg, 118 mg, 119 mg, 120 mg, 121 mg, 122 mg, 123
mg, 124
mg, 125 mg, 126 mg, 127 mg, 128 mg, 129 mg, 130 mg 131 mg, 132 mg, 133 mg, 134
mg,
135 mg, 136 mg, 137 mg, 138 mg, 139 mg, 140 mg, 141 mg, 142 mg, 143 mg, 144
mg, 145
mg, 146 mg, 147 mg, 148 mg, 149 mg, 150 mg, 151 mg, 152 mg, 153 mg, 154 mg,
155 mg,
156 mg, 157 mg, 158 mg, 159 mg, 160 mg, 161 mg, 1 62 mg, 163 mg, 164 mg, 165
mg,
166 mg, 167 mg, 168 mg, 169 mg, 170 mg, 171 mg, 172 mg, 173 mg, 174 mg, 175
mg, 176
mg, 177 mg, 178 mg, 179 mg, 180 mg, 181 mg, 182 mg, 183 mg, 184 mg, 185 mg,
186 mg,
187 mg, 188 mg, 189 mg, 190 mg, 190 mg, 191 mg, 192 mg, 193 mg, 194 mg, 195
mg, 196
mg, 197 mg, 198 mg, 199 mg, 200 mg, 201 mg, 202, mg, 203, mg, 204 mg, 205 mg,
206
mg, 207 mg, 208 mg, 209 mg, 210 mg, 211 mg, 212 mg, 213 mg, 214 mg, 215 mg,
216 mg,
217 mg, 218 mg, 219 mg, 220 mg, 221 mg, 222 mg, 223 mg, 224 mg, 225 mg, 226
mg, 227
mg, 228 mg, 229 mg, 230 mg, 231 mg, 232 mg, 233 mg, 234 mg, 235 mg, 236 mg,
237 mg,
17
8917050
Date Recue/Date Received 2023-11-15
238 mg, 239 mg, 240 mg, 241 mg, 242 mg, 243 mg, 244 mg, 245 mg, 246 mg, 247
mg, 248
mg, 249 mg, 250 mg, 251 mg, 252 mg, 253 mg, 254 mg, 255 mg, 256 mg, 257 mg,
258 mg,
259 mg, 260 mg, 261 mg, 2 62 mg, 263 mg, 264 mg, 265 mg, 266 mg, 267 mg, 268
mg,
269 mg, 270 mg, 271 mg, 272 mg, 273 mg, 274 mg, 275 mg, 276 mg, 277 mg, 278
mg, 279
mg, 280 mg, 281 mg, 282 mg, 283 mg, 284 mg, 285 mg, 286 mg, 287 mg, 288 mg,
289 mg,
290 mg, 290 mg, 291 mg, 292 mg, 293 mg, 294 mg, 295 mg, 296 mg, 297 mg, 298
mg, 299
mg, 300 mg, 301 mg, 302, mg, 303, mg, 304 mg, 305 mg, 306 mg, 307 mg, 308 mg,
309
mg, 310 mg, 311 mg, 312 mg, 313 mg, 314 mg, 315 mg, 316 mg, 317 mg, 318 mg,
319 mg,
320 mg, 321 mg, 323 mg, 323 mg, 324 mg, 325 mg, 326 mg, 327 mg, 328 mg, 329
mg, 330
mg, 331 mg, 332 mg, 333 mg, 334 mg, 335 mg, 336 mg, 337 mg, 338 mg, 339 mg,
340 mg,
341 mg, 342 mg, 343 mg, 344 mg, 345 mg, 346 mg, 347 mg, 348 mg, 349 mg, 350
mg, 351
mg, 352 mg, 353 mg, 354 mg, 355 mg, 356 mg, 357 mg, 358 mg, 359 mg, 360 mg,
361 mg,
362 mg, 363 mg, 364 mg, 365 mg, 366 mg, 367 mg, 368 mg, 369 mg, 370 mg, 371
mg, 372
mg, 373 mg, 374 mg, 375 mg, 376 mg, 377 mg, 378 mg, 379 mg, 380 mg, 381 mg,
382 mg,
383 mg, 384 mg, 385 mg, 386 mg, 387 mg, 388 mg, 389 mg, 390 mg, 390 mg, 391
mg, 392
mg, 393 mg, 394 mg, 395 mg, 396 mg, 397 mg, 398 mg, 399 mg, 400 mg, 401 mg,
402,
mg, 403, mg, 404 mg, 405 mg, 406 mg, 407 mg, 408 mg, 409 mg, 410 mg, 411 mg,
412
mg, 413 mg, 414 mg, 415 mg, 416 mg, 417 mg, 418 mg, 419 mg, 440 mg, 421 mg,
422 mg,
423 mg, 424 mg, 425 mg, 426 mg, 427 mg, 428 mg, 429 mg, 430 mg, 431 mg, 432
mg, 433
mg, 434 mg, 435 mg, 436 mg, 437 mg, 438 mg, 439 mg, 440 mg, 441 mg, 442 mg,
443 mg,
444 mg, 445 mg, 446 mg, 447 mg, 448 mg, 449 mg, 450 mg, 451 mg, 452 mg, 453
mg, 454
mg, 455 mg, 456 mg, 457 mg, 458 mg, 459 mg, 460 mg, 461 mg, 462 mg, 463 mg,
464 mg,
465 mg, 466 mg, 467 mg, 468 mg, 469 mg, 470 mg, 471 mg, 472 mg, 473 mg, 474
mg, 475
mg, 476 mg, 477 mg, 478 mg, 479 mg, 480 mg, 481 mg, 482 mg, 483 mg, 484 mg,
485 mg,
486 mg, 487 mg, 488 mg, 489 mg, 490 mg, 490 mg, 491 mg, 492 mg, 493 mg, 494
mg, 495
mg, 496 mg, 497 mg, 498 mg, 499 mg, 500 mg, 501 mg, 50q, mg, 503, mg, 504 mg,
505
mg, 506 mg, 507 mg, 508 mg, 509 mg, 510 mg, 511 mg, 51q mg, 513 mg, 514 mg,
515 mg,
516 mg, 517 mg, 518 mg, 519 mg, 520 mg, 551 mg, 525 mg, 553 mg, 554 mg, 555
mg, 556
mg, 557 mg, 558 mg, 559 mg, 530 mg, 531 mg, 532 mg, 533 mg, 534 mg, 535 mg,
536 mg,
537 mg, 538 mg, 539 mg, 540 mg, 541 mg, 542 mg, 543 mg, 544 mg, 545 mg, 546
mg, 547
mg, 548 mg, 549 mg, 550 mg, 551 mg, 552 mg, 553 mg, 554 mg, 555 mg, 556 mg,
557 mg,
558 mg, 559 mg, 560 mg, 561 mg, 562 mg, 563 mg, 564 mg, 565 mg, 566 mg, 567
mg, 568
mg, 569 mg, 570 mg, 571 mg, 572 mg, 573 mg, 574 mg, 575 mg, 576 mg, 577 mg,
578 mg,
579 mg, 580 mg, 581 mg, 582 mg, 583 mg, 584 mg, 585 mg, 586 mg, 587 mg, 588
mg, 589
18
8917050
Date Recue/Date Received 2023-11-15
mg, 590 mg, 590 mg, 591 mg, 592 mg, 593 mg, 594 mg, 595 mg, 596 mg, 597 mg,
598 mg,
599 mg, or 600 mg of one or more bioavailability enhancing agents.
Adjunct Ingredients
The disclosed compositions can comprise one or more adjunct ingredients.
Emulsifiers
The disclosed compositions can comprise from about 30% to about 80% one or
more emulsifiers. Non-limiting examples of emulsifiers are chosen from gum
arabic,
modified starch, pectin, xanthan gum, gum ghatti, gum tragacanth, fenugreek
gum,
mesquite gum, mono-glycerides and di-glycerides of long chain fatty acids,
sucrose
monoesters, sorbitan esters, polyethoxylated glycerols, stearic acid, palmitic
acid, mono-
glycerides, di-glycerides, propylene glycol esters, lecithin, lactylated mono-
and di-
glycerides, propylene glycol monoesters, polyglycerol esters, diacetylated
tartaric acid
esters of mono- and di-glycerides, citric acid esters of monoglycerides,
stearoy1-2-lactylates,
polysorbates, succinylated monoglycerides, acetylatal monoglycerides,
ethoxylated
monoglycerides, quillaia, whey protein isolate, casein, soy protein, vegetable
protein,
pullulan, sodium alginate, guar gum, locust bean gum, tragacanth gum, tamarind
gum,
carrageenan, furcellaran, Gellan gum, psyllium, curdlan, konjac mannan, agar,
and cellulose
derivatives, and combinations thereof, or a sugar alcohol that can optionally
have humectant
properties such as ethylene glycol, glycerol, erythritol, threitol, arabitol,
xylitol, ribitol,
mannitol, sorbitol, galactitol, frucitol, iditol, sucrose, fructose, isomalt,
maltitol, lactitol,
sorbitol, dextrose or inositol, or mixtures thereof.
The disclosed compositions can comprise an emulsifier system comprising an
emulsifier and a solubilizing carrier. For example:
i) an emulsifier; and
ii) a carrier.
Typically, after addition of the active base and emulsifier, one or more
components
may remain not fully dissolved. In that case the formulator can add a carrier
to achieve
composition homogeneity.
For example:
i) from about 85% to about 95% an emulsifier; and
ii) from about 5% to about 15 a carrier.
In one embodiment the compositions comprise from about 30% to about 80% of one
or more emulsifiers. In another embodiment the compositions comprise from
about 60% to
about 80% of one or more emulsifiers. In a further embodiment the compositions
comprise
19
8917050
Date Recue/Date Received 2023-11-15
from about 50% to about 70% of one or more emulsifiers. In in a yet another
embodiment
the compositions comprise from about 60% to about 70% of one or more
emulsifiers. In a
still further embodiment the compositions comprise from about 40% to about 70%
of one or
more emulsifiers. In a still yet further embodiment the compositions comprise
from about
35% to about 80% of one or more emulsifiers.
The compositions can comprise any amount of emulsifier from 30% to 80%, for
example, 30%, 31%, 32%, 33%, 34%, 35%, 36%, 37%, 38%, 39%, 40%, 41%, 42%, 43%,
44%, 45%, 46%, 47%, 48%, 49%, 50%, 51%, 52%, 53%, 54%, 55%, 56%, 57%, 58%,
59%, 60%, 61%, 62%, 63%, 64%, 65%, 66%, 67%, 68%, 69%, 70%, 71%, 72%, 73%,
74%, 75%, 76%, 77%, 78%, 79%, or 80%.
Bile Salts
The disclosed compositions can comprise from about 1% to about 15% of one or
more bile salts. The bile salts enhance the ability of the disclosed
compositions to target the
duodenum. Non-limiting examples of bile salts and/or bile acids includes
steroid acids
(and/or the carboxylate anion thereof) and salts thereof, found in the bile of
an animal (e.g.,
a human), including cholic acid, cholate, deoxycholic acid, deoxycholate,
hyodeoxycholic
acid, hyodeoxycholate, glycocholic acid, glycocholate, taurocholic acid,
taurocholate,
chenodeoxycholic acid, chenodeoxycholate, lithocholic acid, lithocolate, and
the like.
Taurocholic acid and/or taurocholate are referred to herein as TCA.
Bile salts are typically conjugated with glycine or taurine. For example, the
term
"bile acid" as used herein includes cholic acid conjugated with either glycine
or taurine:
glycocholate and taurocholate, respectively (and salts thereof). Any reference
to a bile salt
or bile acid used herein includes reference to an identical compound naturally
or
synthetically prepared.
In one embodiment the composition comprises from about 3% to about 12% of a
bile salt. In another embodiment the composition comprises from about 5% to
about 12%
of a bile salt. In a further embodiment the composition comprises from about
7% to about
12% of a bile salt. In a still further embodiment the composition comprises
from about 7%
to about 15% of a bile salt. In in a yet further embodiment the composition
comprises from
about 8% to about 15% of a bile salt. The compositions can comprise, for
example, 1%,
2%, 3%, 4%, 5%, 6%, 7%, 8%, 9%, 10%, 11%, 12%, 13%, 14%, or 15% of one or more
bile salts. In one non-limiting example the bile used herein is ox bile.
Carriers
8917050
Date Recue/Date Received 2023-11-15
The disclosed compositions can comprise from about 1% to about 10% by weight
of
one or more pharmaceutically acceptable carriers. In one embodiment the
compositions
comprise from about 1% to about 5% of a carrier. In another embodiment the
compositions
comprise from about 1% to about 4% of a carrier. In further embodiment the
compositions
comprise from about 3% to about 7% of a carrier. In a yet further embodiment
the
compositions comprise from about 4% to about 8% of a carrier. In a still
further
embodiment the compositions comprise from about 6% to about 10% of a carrier.
The
compositions can comprise, for example, 1%, 2%, 3%, 4%, 5%, 6%, 7%, 8%, 9%, or
10%
of one or more carriers.
The term "pharmaceutically acceptable carrier" refers to a diluent, adjuvant
or
excipient, with which the disclosed compositions can be admixed or otherwise
delivered.
Pharmaceutically acceptable carriers include any and all solvents, diluents,
or other liquid
vehicles, dispersions or suspension aids, surface active agents, isotonic
agents, thickening or
emulsifying agents, preservatives, solid binders, lubricants and the like, as
suited to the
particular dosage form desired. Remington's Pharmaceutical Sciences, Sixteenth
Edition, E.
W. Martin (Mack Publishing Co., Easton, Pa., 1980) discloses various carriers
used in
formulating pharmaceutically acceptable compositions and known techniques for
the
preparation thereof Except insofar as any conventional carrier medium is
incompatible with
the compounds of the disclosure such as by producing any undesirable
biological effect or
otherwise interacting in a deleterious manner with any other component(s) of
the
pharmaceutically acceptable composition, its use is contemplated to be within
the scope of
this disclosure. Examples of pharmaceutically acceptable carriers include, but
are not
limited to buffer substances such as phosphates, glycine, sorbic acid, or
potassium sorbate,
partial glyceride mixtures of saturated vegetable fatty acids, water, sugars
such as lactose,
glucose and sucrose; starches such as corn starch and potato starch; cellulose
and its
derivatives such as sodium carboxymethyl cellulose, ethyl cellulose and
cellulose acetate.
Non-limiting examples of carriers includes water, ethanol, glycerin, propylene
glycol, and sorbitol.
Surfactants
The disclosed compositions can comprise from about 1% to about 15% one or more
surfactants. In one embodiment the composition comprises from about 3% to
about 12% of
a surfactant. In another embodiment the composition comprises from about 5% to
about
12% of a surfactant. In a further embodiment the composition comprises from
about 7% to
about 12% of a surfactant. In a still further embodiment the composition
comprises from
21
8917050
Date Recue/Date Received 2023-11-15
about 7% to about 15% of a surfactant. In in a yet further embodiment the
composition
comprises from about 8% to about 15% of a surfactant. The compositions can
comprise, for
example, 1%, 2%, 3%, 4%, 5%, 6%, 7%, 8%, 9%, 10%, 11%, 12%, 13%, 14%, or 15%
of
one or more surfactants.
The following are non-limiting examples of surfactants.
Natural Extract Surfactants
One category of suitable surfactants includes compounds that are extracted
from
plant material that have surfactant activity. The compositions can comprise
from about
0.05% to about 0.5% by weight of one or more natural surfactants. Non-limiting
examples
include extracts of Gynostemma Pentapphyllum,Panax Ginseng, Sapindus
mukorossi,
cucumis sativus, Olea Europea , and the like. Also suitable for use are
mixtures of extracts
having surfactant properties.
Anionic Surfactants
The disclosed compositions can comprise one or more Cio-Cis alkyl alkoxy
sulfates having
the formula:
CH3(CH2)(OCH2C112)y0S03M
wherein the index x is from 9 to 17, y is from 1 to 7 and M is a water soluble
cation chosen
from ammonium, lithium, sodium, potassium and mixtures thereof. A non-limiting
example includes sodium dodecyl diethoxy sulfate having the formula:
CH 3 (CH2)11 (0012012)20 S 03Na.
Alkyl alkoxy sulfates are also commercially available as a mixture of
ethoxylates, for
example, sodium laureth sulfate is available as a mixture of ethoxylates,
i.e., the index y is
from 2 to 4. Other suitable examples include sodium laureth-2 sulfate having
an average of
2 ethoxylates and a C12 linear alkyl chain. Sodium laureth-2 is available as
Texaponrm N
56 from Cognis Corp. Further examples of alkyl alkoxy sulfates includes sodium
laureth-1
sulfate, sodium laureth-3 sulfate, sodium laureth-4 sulfate, sodium myreth-2
sulfate and
sodium myreth-3 sulfate.
The disclosed compositions can comprise one or more Cio-C18 alkyl alkoxy
carboxylates having the formula:
CH3(CH2)õ(OCH2CH2)yCO2M
wherein the index x is from 9 to 17, y is from 1 to 5 and M is a water soluble
cation chosen
from ammonium, lithium, sodium, potassium and mixtures thereof. A non-limiting
example includes sodium dodecyl diethoxy carboxylate having the formula:
CH3(042)11(OCH2CH2)2CO2Na.
22
8917050
Date Recue/Date Received 2023-11-15
Alkyl alkoxy carboxylates are also commercially available as a mixture of
ethoxylates, for
example, sodium laureth sulfate is available as a mixture of ethoxylates,i.e.,
the index y is
from 2 to 4. Other suitable examples include sodium laureth-2 sulfate having
an average of
2 ethoxylates and a C12 linear alkyl chain. Sodium laureth-2 is available as
Texaponrm N
56 from Cognis Corp. Further examples of alkyl alkoxy sulfates include sodium
laureth-1
sulfate, sodium laureth-3 sulfate, sodium laureth-4 sulfate, sodium myreth-2
sulfate and
sodium myreth-3 sulfate.
The disclosed compositions can comprise one or more Cio-C18 isethionate esters
of
alkyl alkoxy carboxylates having the formula:
CH3(CH2)(OCH2CH2)y0CH2C(0)0CH2CH2S03M
wherein the index x is from 9 to 17, the index y is froml to 5 and M is a
water soluble
cation. Isethionate esters of alkyl alkoxy carboxylates are described in U.S.
5,466,396 the
disclosure of which is included herein by reference in its entirety.
The disclosed compositions can comprise one or more Cio-Cis alkyl
carboxyamides
having the formula:
CH3(CH2),C(0)NR(CH2)yCO2M
wherein R is hydrogen or methyl the index x is from 9 to 17, the index y is
froml to 5 and
M is a water soluble cation. A non-limiting example of an alkyl carboxyamide
suitable for
use in the disclosed compositions includes potassium cocoyl glycinate
available as
AMILITCm GCK-12 from Ajinomoto. A further example includes compounds wherein R
is methyl, for example, sodium cocoyl sarcosinate.
Zwitterionic Surfactants
One category of zwitterionic surfactants relates to Cio-C16 alkyl amide
betaines
having the formula:
CH3(CH2)C(0)NH(CH2).10(CH3)2(CH2)tCO2-
wherein the index w is from 9 to 15, the index u is from 1 to 5 and the index
t is from 1 to 5.
Non-limiting examples of betaine surfactants includes {[3-
(decanoylamino)ethyll-
(dimethyl)-ammonio } acetate, { [3-(decanoylarnino)ethyl] (dimethypammoni o } -
acetate, { [3-
(dodecanoyl-amino)ethyll(dimethypanunonio} acetate, 1[3-
(dodecanoylamino)propy1]-
(dimethyl)-ammonio } acetate, {[3-(dodecanoylamino)-butyl](dimethyl)ammoniol
acetate,
[3-(tetra-decanoylarnino)ethyl](dimethyl)-ammoniol acetate, 1[3-
(tertadecanoylamino)-
propyll(dimethyl)ammoniolacetate, {[3-(hexadecanoylamino)ethyll(dimethyl)-
ammoniolacetate, andl[3-(hexa-decanoylamino)propyl](dimethypammoniol acetate.
23
8917050
Date Recue/Date Received 2023-11-15
Another category of zwitterionic surfactants relates to C io-C16 alkyl amide
sultaines
having the formula:
CH3(CH2)C(0)NH(CH2)1N F(CH3)2(CH2)tS03-
wherein the index w is from 9 to 15, the index u is from 1 to 5 and the index
t is from 1 to 5.
Non-limiting examples of sultaine surfactants includes {[3-
(decanoylamino)ethy1]-
(dimethyl)-ammonio } methan esulfonat e, { [3 -
(decanoylamino)ethyll(dimethypammoni o } -
methanesulfonate, {[3-(dodecanoyl-
amino)ethyl](dimethypammoniolmethanesulfonate,
[3-(dodecanoylamino)-propyll(dimethyl)arnmonio } methane sulfonate, 1[3 -
(dodecanoyl-
amino)butyl](dimethyl)-ammonio}methanesulfonate, {[3-
(tetradecanoylamino)ethy1]-
(dimethyDammonio}methane-sulfonate, {[3-(tertadecanoylamino)propyll(dimethyl)-
ammoniolmethanesulfonate, { [3 -(hexadecanoy lamino)ethyll(dimethypammonio } -
methanesulfonate, andf[3-(hexadecanoylamino)propyl](dimethypammoniol-
methanesulfonate.
A further category of zwitterionic surfactants relates to Cio-Ci6 alkyl
hydroxy
sultaines having the formula:
CH3(CH2)W(CH3)2CH2CHOHCH2S03"
wherein the index w is from 9 to 15. Non-limiting examples of alkyl hydroxy
sultaine
surfactants includes 3-[dodecyl(dimethyl)azaniumy1]-2-hydroxypropane-1-
sulfonate (lauryl
hydroxysultaine), 3-[tetradecyl(dimethypazaniumy1]-2-hydroxypropane-1-
sulfonate
(my ri styl hydroxy sultaine), (Z)- {dimethyl[3 -(octadec9-enami do)propyl]
ammoni o } -
methanesulfonate (oleyl hydroxysultaine), and the like.
Nonionic Surfactants
One category of nonionic surfactants relates to C8-C18 alkylglycosidyl
nonionic
surfactant having the formula:
CH3(CH2)q0[G]pH
wherein G represents a monosaccharide residue chosen from glucose, fructose,
mannose,
galactose, talose, allose, altrose, idose, arabinose, xylose, lyxose, ribose
and mixtures
thereof, the index p is from 1 to 4, the index q is from 7 to 17. The
following are non-
limiting examples of alkyl glucoside surfactants include (2R,3S,4S,5R,6R)-2-
(hydroxymethyl)-6-octooxyoxane-3,4,5-triol (octyl glucoside, n-octyl-P-D-
glucoside),
(2R,3R,4S,5S,6R)-2-decoxy-6-(hydroxymethyptetra-hydropyran-3,4,5-triol (decyl
glucoside, n-decyl-P-D-glucoside), and (2R,3R,4S,5S,6R)-2-dodecoxy-6-
(hydroxymethyptetrahydropyran-3,4,5-triol (dodecyl glucoside, lauryl
glucoside, n-
24
8917050
Date Revile/Date Received 2023-11-15
dodecy1-13-D-glucoside). One example of a suitable admixture of C8-C16
alkylglycosidyl
nonionic surfactants is PLANTACARE,Tm 818 UP available from Cogins Chemical
Co.
A further category of nonionic surfactants relates to polyoxyethylene glycol
alkyl
ethers having the formula:
RO(CH2CH20)nH
wherein R is a linear or branched alkyl group having from 6 to 20 carbon atoms
and n is an
integer of about 2 to about 20.
On example of suitable ethoxylate alcohol surfactants are the NEODOLTm
ethoxylated alcohols from Shell Chemicals. NEODOLTm 23-1 is a surfactant
comprising a
mixture of R units that are C12 and C13 in length with an average of 1 ethoxy
unit. Non-
limiting examples of ethoxylated alcohols include NEODOLTm 23-1, NEODOLTm 23-
2,
NEODOLTm 23-6.5, NEODOLTM 25-3, NEODOLTm 25-5, NEODOLTm 25-7, NEODOLTM
25-9, PLURONICTIvi 12R3, and PLURONICTm 25R2 available from BASF.
A still further category of nonionic surfactants relates to polyoxyethylene
glycol
alkyl ethers having the formula:
RO(CH2CH(CH3)0)H
wherein R is a linear or branched alkyl group having from 6 to 20 carbon atoms
and n is an
integer of about 2 to about 20.
Another category of nonionic surfactants suitable for use in the disclosed
compositions includes polyoxy ethylene polyoxypropylene block copolymers known
as
"poloxamers" having the formula:
HO(CH2CH2)yi(CH2CH2CH20)y2(CH2CH20)y3OH
these are nonionic block copolymers composed of a polypropyleneoxy unit
flanked by two
polyethyleneoxy units. The indices y1, y2, and y3 have values such that the
poloxamer has
an average molecular weight of from about 1000 g/mol to about 20,000 g/mol.
These
extracellular desiccants are also well known by the trade name PLURONICSTm.
These
compounds are commonly named with the word Poloxamer followed by a number to
indicate the specific co-polymer, for example Poloxamer 407 having two PEG
blocks of
about 101 units (y1 and y3 each equal to 101) and a polypropylene block of
about 56 units.
This category of nonionic surfactant is commercially, for example, under the
trade name
LUTKOLTm F-17 available from BASF.
Another category of adjunct ingredients includes flavor enhancing agents. Non-
limiting examples of flavor enhancing agents include vanilla, vanillin, ethyl
vanillin, orange
oil, lemon oil, peppermint oil, strawberry, raspberry, and mixtures thereof.
8917050
Date Recue/Date Received 2023-11-15
Another example of an adjunct ingredient is an emulsifier which provides a
homogeneous composition. Non-limiting examples of emulsifiers includes soy and
egg
lecithin, mono- and diglycerides, polysorbates, carrageenan, and guar gum. In
some
embodiments one or more of the bioavailability agents can serve a suitable
emulsifier.
The disclosed compositions can further comprise lipid soluble vitamins and
vitamin
precursors. For example, retinol, retinoic acid, 13-carotene, cholecalciferol,
tocopherols, and
vitamin K.
The disclosed compositions can also comprise one or more alkalizing agents to
improve intestinal motility. Non-limiting examples include extract of Cynara
cardunculus,
extract of Zingiber officinale, cholagogues, sodium carbonate, sodium
bicarbonate, as well
as other alkaline buffers.
The disclosed compositions can comprise one or more anti-inflammatory drugs or
agents with anti-inflammatory properties to reduce the immunological cascade
effect
associated with viral infections. For example, aspirin, ibuprofen, naproxen,
ketoprofen,
tolmetin, etodolac, diclofenac, piroxicam, indomethacin or others.
The disclosed compositions can comprise one or more anti-caking agents
COMPOSITIONS
One aspect of the disclosed compositions, comprises:
a) one or more active agents;
b) a bioavailability enhancing agent; and
c) an emulsifier.
In one iteration of this aspect, the compositions comprises:
a) an effective amount of one or more antiviral agents; and
b) an effective amount of an edible oil bioavailability enhancing agent
chosen
from palm kernel oil, soybean oil, rapeseed oil (canola), sunflower oil,
groundnut oil, sesame oil, flaxseed oil, palm oil, olive oil or corn oil; and
c) one or more emulsifiers chosen from gum arabic, modified starch, pectin,
xanthan gum, gum ghatti, gum tragacanth, fenugreek gum, mesquite gum,
mono-glycerides and di-glycerides of long chain fatty acids, sucrose
monoesters, sorbitan esters, polyethoxylated glycerols, stearic acid, palmitic
acid, mono-glycerides, di-glycerides, propylene glycol esters, lecithin,
lactylated mono- and di-glycerides, propylene glycol monoesters,
polyglycerol esters, diacetylated tartaric acid esters of mono- and di-
glycerides, citric acid esters of monoglycerides, stearoy1-2-lactylates,
26
8917050
Date Recue/Date Received 2023-11-15
polysorbates, succinylated monoglycerides, acetylated monoglycerides,
ethoxylated monoglycerides, quillaia, whey protein isolate, casein, soy
protein, vegetable protein, pullulan, sodium alginate, guar gum, locust bean
gum, tragacanth gum, tamarind gum, carrageenan, furcellaran, GelIan gum,
psyllium, curdlan, konjac mannan, agar, and cellulose derivatives, and
combinations thereof, or a sugar alcohol that can optionally have humectant
properties such as ethylene glycol, glycerol, erythritol, threitol, arabitol,
xylitol, ribitol, mannitol, sorbitol, galactitol, frucitol, iditol, sucrose,
fructose,
isomalt, maltitol, lactitol, sorbitol, dextrose or inositol, or mixtures
thereof.
In one embodiment of this iteration, the composition comprises:
a) an effective amount of one or more protease inhibitors chosen from
amprenavir (or the pro-drug fosamprenavir), bepridil, atazanavir, boceprevir,
darunavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir,
simeprevir,
telaprevir, or tipranavir;
b) an effective amount of an edible oil bioavailability enhancing agent
chosen
from palm kernel oil, soybean oil, rapeseed oil (canola), sunflower oil,
groundnut oil, sesame oil, flaxseed oil, palm oil, olive oil or corn oil; and
c) one or more emulsifiers chosen from gum arabic, modified starch, pectin,
xanthan gum, gum gjhatti, gum tragacanth, fenugreek gum, mesquite gum,
mono-glycerides and di-glycerides of long chain fatty acids, sucrose
monoesters, sorbitan esters, polyethoxylated glycerols, stearic acid, pahnitic
acid, mono-glycerides, di-glycerides, propylene glycol esters, lecithin,
lactylated mono- and di-glycerides, propylene glycol monoesters,
polyglycerol esters, diacetylated tartaric acid esters of mono- and di-
glycerides, citric acid esters of monoglycerides, stearoy1-2-lactylates,
polysorbates, succinylated monoglycerides, acetylated monoglycerides,
ethoxylated monoglycerides, quillaia, whey protein isolate, casein, soy
protein, vegetable protein, pullulan, sodium alginate, guar gum, locust bean
gum, tragacanth gum, tamarind gum, carrageenan, furcellaran, Gellan gum,
psylliiim curdlan, konjac mannan, agar, and cellulose derivatives, and
combinations thereof, or a sugar alcohol that can optionally have humectant
properties such as ethylene glycol, glycerol, erythritol, threitol, arabitol,
xylitol, ribitol, mannitol, sorbitol, galactitol, frucitol, iditol, sucrose,
fructose,
isomalt, maltitol, lactitol, sorbitol, dextrose or inositol, or mixtures
thereof.
27
8917050
Date Recue/Date Received 2023-11-15
In another embodiment of this iteration, the composition comprises:
a) an effective amount of protease inhibitors chosen from amprenavir (or
the
pro-drug fosamprenavir), bepridil, atazanavir, boceprevir, danmavir,
indinavir, lopinavir, nelfinavir, ritonavir, saquinavir, simeprevir,
telaprevir,
or tipranavir;
b) an effective amount of an edible oil bioavailability enhancing agent
chosen
from palm kernel oil, soybean oil, rapeseed oil (canola), sunflower oil,
groundnut oil, sesame oil, flaxseed oil, palm oil, olive oil or corn oil; and
c) gum arabic.
In one non-limiting example of this embodiment, the composition comprises:
i) from about 5% to about 20% by weight of an active base,
comprising:
a) from about 25% to about 50% by weight of darunavir;
b) from about 50% to about 75% by weight of sunflower oil; and
ii) from about 50% to about 70% gum arabic;
iii) the balance carriers and other adjunct ingredients.
In another non-limiting example of this embodiment, the composition comprises:
i) from about 1% to about 15% by weight of an active base,
comprising:
a) from about 25% to about 50% by weight of bepredil;
b) from about 50% to about 75% by weight of sunflower oil; and
ii) from about 50% to about 70% gum arabic.
In a further non-limiting example of this embodiment, the composition
comprises:
i) from about 1% to about 15% by weight of an active base,
comprising:
a) from about 25% to about 50% by weight of rupintrivir;
b) from about 50% to about 75% by weight of sunflower oil; and
ii) from about 50% to about 70% gum arabic
In a yet another non-limiting example of this embodiment, the composition
comprises:
i) from about 1% to about 15% by weight of an active base,
comprising:
a) from about 25% to about 50% by weight of ebastine;
b) from about 50% to about 75% by weight of sunflower oil; and
ii) from about 50% to about 70% gum arabic.
Another iteration of this aspect relates to compositions comprising:
28
8917050
Date Recue/Date Received 2023-11-15
a) one or more protease inhibitors chosen from amprenavir (or the pro-drug
fosamprenavir), atazanavir, boceprevir, darunavir, indinavir, lopinavir,
nelfinavir, ritonavir, saquinavir, simeprevir, telaprevir, or tipranavir;
b) a bioavailability enhancing agent chosen from palm kernel oil, soybean
oil,
rapeseed oil (canola), sunflower oil, groundnut oil, sesame oil, flaxseed oil,
palm oil, olive oil and corn oil;
c) one or more emulsifiers chosen from gum arabic, modified starch, pectin,
xanthan gum, gum gjhatti, gum tragacanth, fenugreek gum, mesquite gum,
mono-glycerides and di-glycerides of long chain fatty acids, sucrose
monoesters, sorbitan esters, polyethoxylated glycerols, stearic acid, palmitic
acid, mono-glycerides, di-glycerides, propylene glycol esters, lecithin,
lactylated mono- and di-glycerides, propylene glycol monoesters,
polyglycerol esters, diacetylated tartaric acid esters of mono- and di-
glycerides, citric acid esters of monoglycerides, stearoy1-2-lactylates,
polysorbates, succinylated monoglycerides, acetylated monoglycerides,
ethoxylated monoglycerides, quillaia, whey protein isolate, casein, soy
protein, vegetable protein, pullulan, sodium alginate, guar gum, locust bean
gum, tragacanth gum, tamarind gum, carrageenan, furcellaran, Gellan gum,
___________________ curdlan, konjac mannan, agar, and cellulose derivatives,
and
combinations thereof, or a sugar alcohol that can optionally have humectant
properties such as ethylene glycol, glycerol, erythritol, threitol, arabitol,
xylitol, ribitol, mannitol, sorbitol, galactitol, frucitol, iditol, sucrose,
fructose,
isomalt, maltitol, lactitol, sorbitol, dextrose or inositol, or mixtures
thereof;
and
d) one or more bile salts.
One embodiment of this iteration relates to compositions comprising:
a) one or more protease inhibitors chosen from amprenavir (or the pro-drug
fosamprenavir), atazanavir, boceprevir, darunavir, indinavir, lopinavir,
nelfinavir, ritonavir, saquinavir, simeprevir, telaprevir, or tipranavir;
b) a bioavailability enhancing agent is sunflower oil;
c) one or more emulsifiers chosen from gum arabic, propylene glycol, or
mixtures thereof; and
d) ox bile.
One non-limiting example of this embodiment is a composition comprising:
29
8917050
Date Recue/Date Received 2023-11-15
a) from about 3% to about 6% by weight of darunavir;
b) from about 5% to about 15% by weight of sunflower oil;
c) an emulsifier system containing:
i) from about 60% to about 70% by weight of the composition gum
arabic; and
ii) from about 3% to about 10% by weight of the composition propylene
glycol; and
d) from about 5% to about 12% by weight ox bile.
This example can further comprise one or more anti-caking agents. For example,
a composition containing silicon dioxide as an anti-caking agent, comprising:
a) 4.4% darunavir;
b) 8.9% sunflower oil;
c) 63.3% gum arabic;
d) 9% ox bile;
e) 10% silicon dioxide; and
f) 4.4% propylene glycol.
Another non-limiting example of this embodiment is a composition comprising:
a) from about 3% to about 6% by weight of bepredil;
b) from about 5% to about 15% by weight of sunflower oil;
c) an emulsifier system containing:
i) from about 60% to about 70% by weight of the composition gum
arabic; and
ii) from about 3% to about 10% by weight of the composition propylene
glycol; and
d) from about 5% to about 12% by weight ox bile.
This example can further comprise one or more anti-caking agents. For example,
a composition containing silicon dioxide as an anti-caking agent, comprising:
a) 4.4% bepredil;
b) 8.9% sunflower oil;
c) 63.3% gum arabic;
d) 9% ox bile;
e) 10% silicon dioxide; and
4.4% propylene glycol
A further non-limiting example of this embodiment is a composition comprising:
8917050
Date Recue/Date Received 2023-11-15
a) from about 3% to about 6% by weight of rupintrivir;
b) from about 5% to about 15% by weight of sunflower oil;
c) an emulsifier system containing:
i) from about 60% to about 70% by weight of the composition gum
arabic; and
ii) from about 3% to about 10% by weight of the composition propylene
glycol; and
d) from about 5% to about 12% by weight ox bile.
This example can further comprise one or more anti-caking agents. For example,
a composition containing silicon dioxide as an anti-caking agent, comprising:
a) 4.4% rupintrivir;
b) 8.9% sunflower oil;
c) 63.3% gum arabic;
d) 9% ox bile;
e) 10% silicon dioxide; and
f) 4.4% propylene glycol.
A still further non-limiting example of this embodiment is a composition
comprising:
a) from about 3% to about 6% by weight of ebastine;
b) from about 5% to about 15% by weight of sunflower oil;
c) an emulsifier system containing:
i) from about 60% to about 70% by weight of the composition gum
arabic; and
ii) from about 3% to about 10% by weight of the composition propylene
glycol; and
d) from about 5% to about 12% by weight ox bile.
This example can further comprise one or more anti-caking agents. For example,
a composition containing silicon dioxide as an anti-caking agent, comprising:
a) 4.4% ebastine;
b) 8.9% sunflower oil;
c) 63.3% gum arabic;
d) 9% ox bile;
e) 10% silicon dioxide; and
4.4% propylene glycol.
31
8917050
Date Recue/Date Received 2023-11-15
In another iteration of this aspect, the compositions comprises:
a) an effective amount of one or more nucleoside reverse transcriptase
inhibitors chosen from doravirine, efavirenz, etravirine, loviride, or
rilpivirine;
b) an effective amount of an edible oil bioavailability enhancing agent
chosen
from palm kernel oil, soybean oil, rapeseed oil (canola), sunflower oil,
groundnut oil, sesame oil, flaxseed oil, palm oil, olive oil or corn oil; and
c) one or more emulsifiers chosen from gum arabic, modified starch, pectin,
xanthan gum, gum ghatti, gum tragacanth, fenugreek gum, mesquite gum,
mono-glycerides and di-glycerides of long chain fatty acids, sucrose
monoesters, sorbitan esters, polyethoxylated glycerols, stearic acid, palmitic
acid, mono-glycerides, di-glycerides, propylene glycol esters, lecithin,
lactylated mono- and di-glycerides, propylene glycol monoesters,
polyglycerol esters, diacetylated tartaric acid esters of mono- and di-
glycerides, citric acid esters of monoglycerides, stearoy1-2-lactylates,
polysorbates, succinylated monoglycerides, acetylated monoglycerides,
ethoxylated monoglycerides, quillaia, whey protein isolate, casein, soy
protein, vegetable protein, pullulan, sodium alginate, guar gum, locust bean
gum, tragacanth gum, tamarind gum, carrageenan, furcellaran, Gellan gum,
psyllium, curdlan, konjac mannan, agar, and cellulose derivatives, and
combinations thereof, or a sugar alcohol that can optionally have humectant
properties such as ethylene glycol, glycerol, erythritol, threitol, arabitol,
xylitol, ribitol, mannitol, sorbitol, galactitol, frucitol, iditol, sucrose,
fructose,
isomalt, maltitol, lactitol, sorbitol, dextrose or inositol, or mixtures
thereof.
In one embodiment of this iteration, the composition comprises:
a) an effective amount of of one or more nucleoside reverse transcriptase
inhibitors chosen from doravirine, efavirenz, etravirine, loviride, or
rilpivirine;
b) an effective amount of an edible oil bioavailability enhancing agent
chosen
from palm kernel oil, soybean oil, rapeseed oil (canola), sunflower oil,
groundnut oil, sesame oil, flaxseed oil, palm oil, olive oil or corn oil; and
c) gum arabic.
In one non-limiting example of this embodiment, the composition comprises:
i) from about 5% to about 20% by weight of an active base,
comprising:
32
8917050
Date Recue/Date Received 2023-11-15
a) from about 25% to about 50% by weight of efavirenz;
b) from about 50% to about 75% by weight of sunflower oil; and
ii) from about 50% to about 70% gum arabic;
iii) the balance carriers and other adjunct ingredients.
In another non-limiting example of this embodiment, the composition comprises:
i) from about 1% to about 15% by weight of an active base,
comprising:
a) from about 25% to about 50% by weight of doravirine;
b) from about 50% to about 75% by weight of sunflower oil; and
ii) from about 50% to about 70% gum arabic.
In a further non-limiting example of this embodiment, the composition
comprises:
i) from about 1% to about 15% by weight of an active base,
comprising:
a) from about 25% to about 50% by weight of etravirine;
b) from about 50% to about 75% by weight of sunflower oil; and
ii) from about 50% to about 70% gum arabic
In a yet another non-limiting example of this embodiment, the composition
comprises:
i) from about 1% to about 15% by weight of an active base,
comprising:
a) from about 25% to about 50% by weight of loviride;
b) from about 50% to about 75% by weight of sunflower oil; and
ii) from about 50% to about 70% gum arabic.
In still further non-limiting example of this embodiment, the composition
comprises:
i) from about 1% to about 15% by weight of an active base,
comprising:
a) from about 25% to about 50% by weight of rilpivirine;
b) from about 50% to about 75% by weight of sunflower oil; and
ii) from about 50% to about 70% gum arabic
Another further iteration of this aspect relates to compositions comprising:
a) one or more one or more nucleoside reverse transcriptase inhibitors
chosen
from doravirine, efavirenz, etravirine, loviride, or rilpivirine;
b) a bioavailability enhancing agent chosen from palm kernel oil, soybean
oil,
rapeseed oil (canola), sunflower oil, groundnut oil, sesame oil, flaxseed oil,
palm oil, olive oil and corn oil;
c) one or more emulsifiers chosen from gum arabic, modified starch, pectin,
xanthan gum, gum ghatti, gum tragacanth, fenugreek gum, mesquite gum,
mono-glycerides and di-glycerides of long chain fatty acids, sucrose
33
8917050
Date Recue/Date Received 2023-11-15
monoesters, sorbitan esters, polyethoxylated glycerols, stearic acid, palmitic
acid, mono-glycerides, di-glycerides, propylene glycol esters, lecithin,
lactylated mono- and di-glycerides, propylene glycol monoesters,
polyglycerol esters, diacetylated tartaric acid esters of mono- and di-
glycerides, citric acid esters of monoglycerides, stearoy1-2-lactylates,
polysorbates, succinylated monoglycerides, acetylated monoglycerides,
ethoxylated monoglycerides, quillaia, whey protein isolate, casein, soy
protein, vegetable protein, pullulan, sodium alginate, guar gum, locust bean
gum, tragacanth gum, tamarind gum, carrageenan, furcellaran, Gellan gum,
psyllium, curdlan, konjac mannan, agar, and cellulose derivatives, and
combinations thereof, or a sugar alcohol that can optionally have humectant
properties such as ethylene glycol, glycerol, erythritol, threitol, arabitol,
xylitol, ribitol, mannitol, sorbitol, galactitol, frucitol, iditol, sucrose,
fructose,
isomalt, maltitol, lactitol, sorbitol, dextrose or inositol, or mixtures
thereof;
and
d) one or more bile salts.
One embodiment of this iteration relates to compositions comprising:
a) one or more one or more nucleoside reverse transcriptase inhibitors
chosen
from doravirine, efavirenz, etravirine, loviride, or rilpivirine;
b) a bioavailability enhancing agent is sunflower oil;
c) one or more emulsifiers chosen from gum arabic, propylene glycol, or
mixtures thereoff, and
d) ox bile.
One non-limiting example of this embodiment is a composition comprising:
a) from about 3% to about 6% by weight of doravirine;
b) from about 5% to about 15% by weight of sunflower oil;
c) an emulsifier system containing:
i) from about 60% to about 70% by weight of the composition gum
arabic; and
ii) from about 3% to about 10% by weight of the composition propylene
glycol; and
d) from about 5% to about 12% by weight ox bile.
This example can further comprise one or more anti-caking agents. For example,
a composition containing silicon dioxide as an anti-caking agent, comprising:
34
8917050
Date Recue/Date Received 2023-11-15
a) 4.4% doravirine;
b) 8.9% sunflower oil;
c) 63.3% gum arabic;
d) 9% ox bile;
e) 10% silicon dioxide; and
4.4% propylene glycol.
One non-limiting example of this embodiment is a composition comprising:
a) from about 3% to about 6% by weight of efavirenz;
b) from about 5% to about 15% by weight of sunflower oil;
c) an emulsifier system containing:
i) from about 60% to about 70% by weight of the composition
gum
arabic; and
from about 3% to about 10% by weight of the composition propylene
glycol; and
d) from about 5% to about 12% by weight ox bile.
This example can further comprise one or more anti-caking agents. For example,
a composition containing silicon dioxide as an anti-caking agent, comprising:
a) 4.4% efavirenz;
b) 8.9% sunflower oil;
c) 63.3% gum arabic;
d) 9% ox bile;
e) 10% silicon dioxide; and
4.4% propylene glycol
In another non-limiting example of this embodiment is a composition
comprising:
a) from about 3% to about 6% by weight of etravirine;
b) from about 5% to about 15% by weight of sunflower oil;
c) an emulsifier system containing:
i) from about 60% to about 70% by weight of the composition gum
arabic; and
ii) from about 3% to about 10% by weight of the composition propylene
glycol; and
d) from about 5% to about 12% by weight ox bile.
This example can further comprise one or more anti-caking agents. For example,
a composition containing silicon dioxide as an anti-caking agent, comprising:
8917050
Date Recue/Date Received 2023-11-15
a) 4.4% etravirine;
b) 8.9% sunflower oil;
c) 63.3% gum arabic;
d) 9% ox bile;
e) 10% silicon dioxide; and
f) 4.4% propylene glycol.
In as still further non-limiting example of this embodiment is a composition
comprising:
a) from about 3% to about 6% by weight of loviride;
b) from about 5% to about 15% by weight of sunflower oil;
c) an emulsifier system containing:
i) from about 60% to about 70% by weight of the composition gum
arabic; and
ii) from about 3% to about 10% by weight of the composition propylene
glycol; and
d) from about 5% to about 12% by weight ox bile.
This example can further comprise one or more anti-caking agents. For example,
a composition containing silicon dioxide as an anti-caking agent, comprising:
a) 4.4% loviride;
b) 8.9% sunflower oil;
c) 63.3% gum arabic;
d) 9% ox bile;
e) 10% silicon dioxide; and
4.4% propylene glycol.
A yet still further non-limiting example of this embodiment is a composition
comprising:
a) from about 3% to about 6% by weight of rilpivirine;
b) from about 5% to about 15% by weight of sunflower oil;
c) an emulsifier system containing:
i) from about 60% to about 70% by weight of the composition gum
arable; and
ii) from about 3% to about 10% by weight of the composition propylene
glycol; and
d) from about 5% to about 12% by weight ox bile.
36
8917050
Date Recue/Date Received 2023-11-15
This example can further comprise one or more anti-caking agents. For example,
a composition containing silicon dioxide as an anti-caking agent, comprising:
a) 4.4% rilpivirine;
b) 8.9% sunflower oil;
c) 63.3% gum arabic;
d) 9% ox bile;
e) 10% silicon dioxide; and
4.4% propylene glycol.
In a yet further iteration of this aspect, the compositions comprises:
a) an effective amount of one or more active agents;
b) an effective amount of an edible oil bioavailability enhancing agent
chosen
from palm kernel oil, soybean oil, rapeseed oil (canola), sunflower oil,
groundnut oil, sesame oil, flaxseed oil, palm oil, olive oil or corn oil; and
c) one or more emulsifiers chosen from gum arabic, modified starch, pectin,
xanthan gum, gum ghatti, gum tragacanth, fenugreek gum, mesquite gum,
mono-glycerides and di-glycerides of long chain fatty acids, sucrose
monoesters, sorbitan esters, polyethoxylated glycerols, stearic acid, palmitic
acid, mono-glycerides, di-glycerides, propylene glycol esters, lecithin,
lactylated mono- and di-glycerides, propylene glycol monoesters,
polyglycerol esters, diacetylated tartaric acid esters of mono- and di-
glycerides, citric acid esters of monoglycerides, stearoy1-2-lactylates,
polysorbates, succinylated monoglycerides, acetylated monoglycerides,
ethoxylated monoglycerides, quillaia, whey protein isolate, casein, soy
protein, vegetable protein, pullulan, sodium alginate, guar gum, locust bean
gum, tragacanth gum, tamarind gum, carrageenan, furcellaran, Gellan gum,
psyllium, curdlan, konjac mannan, agar, and cellulose derivatives, and
combinations thereof, or a sugar alcohol that can optionally have humectant
properties such as ethylene glycol, glycerol, erythritol, threitol, arabitol,
xylitol, ribitol, mannitol, sorbitol, galactitol, frucitol, iditol, sucrose,
fructose,
isomalt, maltitol, lactitol, sorbitol, dextrose or inositol, or mixtures
thereof.
In one embodiment of this iteration, the composition comprises:
a) an effective amount of one or more antivirals chosen from GS-
441524,
remdesivir, indinavir, raltegravir, nevirapine, or azidothymidine;
37
8917050
Date Recue/Date Received 2023-11-15
b) an effective amount of an edible oil bioavailability enhancing agent
chosen
from palm kernel oil, soybean oil, rapeseed oil (canola), sunflower oil,
groundnut oil, sesame oil, flaxseed oil, palm oil, olive oil or corn oil; and
c) one or more emulsifiers chosen from gum arabic, modified starch, pectin,
xanthan gum, gum ghatti, gum tragacanth, fenugreek gum, mesquite gum,
mono-glycerides and di-glycerides of long chain fatty acids, sucrose
monoesters, sorbitan esters, polyethoxylated glycerols, stearic acid, palmitic
acid, mono-glycerides, di-glycerides, propylene glycol esters, lecithin,
lactylatetl mono- and di-glycerides, propylene glycol monoesters,
polyglycerol esters, diacetylated tartaric acid esters of mono- and di-
glycerides, citric acid esters of monoglycerides, stearoy1-2-lactylates,
polysorbates, succinylated monoglycerides, acetylated monoglycerides,
ethoxylated monoglycerides, quillaia, whey protein isolate, casein, soy
protein, vegetable protein, pullulan, sodium alginate, guar gum, locust bean
gum, tragacanth gum, tamarind gum, carrageenan, furcellaran, Gellan gum,
psyllium, curdlan, konjac mannan, agar, and cellulose derivatives, and
combinations thereof, or a sugar alcohol that can optionally have humectant
properties such as ethylene glycol, glycerol, erythritol, threitol, arabitol,
xylitol, ribitol, mannitol, sorbitol, galactitol, frucitol, iditol, sucrose,
fructose,
isomalt, maltitol, lactitol, sorbitol, dextrose or inositol, or mixtures
thereof.
In another embodiment of this iteration, the composition comprises:
a) an effective amount of one or more antivirals chosen from GS-441524,
remdesivir, indinavir, raltegravir, nevirapine, or azidothymidine;
b) an effective amount of an edible oil bioavailability enhancing agent
chosen
from palm kernel oil, soybean oil, rapeseed oil (canola), sunflower oil,
groundnut oil, sesame oil, flaxseed oil, palm oil, olive oil or corn oil; and
c) gum arabic.
In one non-limiting example of this embodiment, the composition comprises:
i) from about 5% to about 20% by weight of an active base,
comprising:
a) from about 25% to about 50% by weight of GS-441524;
b) from about 50% to about 75% by weight of sunflower oil; and
ii) from about 50% to about 70% gum arabic;
iii) the balance carriers and other adjunct ingredients.
In another non-limiting example of this embodiment, the composition comprises:
38
8917050
Date Recue/Date Received 2023-11-15
i) from about 1% to about 15% by weight of an active base,
comprising:
a) from about 25% to about 50% by weight of remdesivir;
b) from about 50% to about 75% by weight of sunflower oil; and
ii) from about 50% to about 70% gum arabic.
In a further non-limiting example of this embodiment, the composition
comprises:
i) from about 1% to about 15% by weight of an active base,
comprising:
a) from about 25% to about 50% by weight of indinavir;
b) from about 50% to about 75% by weight of sunflower oil; and
ii) from about 50% to about 70% gum arabic
In another non-limiting example of this embodiment, the composition comprises:
i) from about 1% to about 15% by weight of an active base,
comprising:
a) from about 25% to about 50% by weight of raltegravir;
b) from about 50% to about 75% by weight of sunflower oil; and
ii) from about 50% to about 70% gum arabic.
In a yet another non-limiting example of this embodiment, the composition
comprises:
i) from about 1% to about 15% by weight of an active base,
comprising:
a) from about 25% to about 50% by weight of nevirapine;
b) from about 50% to about 75% by weight of sunflower oil; and
ii) from about 50% to about 70% gum arabic.
In a still further non-limiting example of this embodiment, the composition
comprises:
i) from about 1% to about 15% by weight of an active base,
comprising:
a) from about 25% to about 50% by weight of azidothymidine;
b) from about 50% to about 75% by weight of sunflower oil; and
ii) from about 50% to about 70% gum arabic.
In a still yet further non-limiting example of this embodiment, the
composition
comprises:
i) from about 5% to about 20% by weight of an active base,
comprising:
a) from about 25% to about 50% by weight of colchicine;
b) from about 50% to about 75% by weight of sunflower oil; and
ii) from about 50% to about 70% gum arabic;
iii) the balance carriers and other adjunct ingredients.
A still yet further iteration of this aspect relates to compositions
comprising:
39
8917050
Date Recue/Date Received 2023-11-15
a) an effective amount of one or more antivirals chosen from GS-441524,
remdesivir, indinavir, raltegravir, nevirapine, or azidothymidine;
b) a bioavailability enhancing agent chosen from palm kernel oil, soybean
oil,
rapeseed oil (canola), sunflower oil, groundnut oil, sesame oil, flaxseed oil,
palm oil, olive oil and corn oil;
c) one or more emulsifiers chosen from gum arabic, modified starch, pectin,
xanthan gum, gum ghatti, gum tragacanth, fenugreek gum, mesquite gum,
mono-glycerides and di-glycerides of long chain fatty acids, sucrose
monoesters, sorbitan esters, polyethoxylated glycerols, stearic acid, palmitic
acid, mono-glycerides, di-glycerides, propylene glycol esters, lecithin,
lactylated mono- and di-glycerides, propylene glycol monoesters,
polyglycerol esters, diacetylated tartaric acid esters of mono- and di-
glycerides, citric acid esters of monoglycerides, stearoy1-2-lactylates,
polysorbates, succinylated monoglycerides, acetylated monoglycerides,
ethoxylated monoglycerides, quillaia, whey protein isolate, casein, soy
protein, vegetable protein, pullulan, sodium alginate, guar gum, locust bean
gum, tragacanth gum, tamarind gum, carrageenan, furcellaran, Gellan gum,
psyllium, curdlan, konjac mannan, agar, and cellulose derivatives, and
combinations thereof, or a sugar alcohol that can optionally have humectant
properties such as ethylene glycol, glycerol, erythritol, threitol, arabitol,
xylitol, ribitol, mannitol, sorbitol, galactitol, frucitol, iditol, sucrose,
fructose,
isomalt, maltitol, lactitol, sorbitol, dextrose or inositol, or mixtures
thereof;
and
d) one or more bile salts.
One embodiment of this iteration relates to compositions comprising:
a) an effective amount of one or more antivirals chosen from GS-441524,
remdesivir, indinavir, raltegravir, nevirapine, or azidothymidine;
b) a bioavailability enhancing agent is sunflower oil;
c) an emulsifier chosen from gum &al*, propylene glycol, or mixtures
thereof;
and
d) ox bile.
One non-limiting example of this embodiment is a composition comprising:
a) from about 3% to about 6% by weight of GS-441524;
b) from about 5% to about 15% by weight of sunflower oil;
8917050
Date Recue/Date Received 2023-11-15
c) an emulsifier system containing:
i) from about 60% to about 70% by weight of the composition gum
arabic; and
ii) from about 3% to about 10% by weight of the composition propylene
glycol; and
d) from about 5% to about 12% by weight ox bile.
This example can further comprise one or more anti-caking agents. For example,
a composition containing silicon dioxide as an anti-caking agent, comprising:
a) 4.4% GS-441524;
b) 8.9% sunflower oil;
c) 63.3% gum arabic;
d) 9% ox bile;
e) 10% silicon dioxide; and
0 4.4% propylene glycol.
Another non-limiting example of this embodiment is a composition comprising:
a) from about 3% to about 6% by weight of remdesivir;
b) from about 5% to about 15% by weight of sunflower oil;
c) an emulsifier system containing:
i) from about 60% to about 70% by weight of the composition gum
arabic; and
ii) from about 3% to about 10% by weight of the composition propylene
glycol; and
d) from about 5% to about 12% by weight ox bile.
This example can further comprise one or more anti-caking agents. For example,
a composition containing silicon dioxide as an anti-caking agent, comprising:
a) 4.4% remdesivir;
b) 8.9% sunflower oil;
c) 63.3% gum arabic;
d) 9% ox bile;
e) 10% silicon dioxide; and
4.4% propylene glycol
A further non-limiting example of this embodiment is a composition comprising:
a) from about 3% to about 6% by weight of indinavir;
b) from about 5% to about 15% by weight of sunflower oil;
41
8917050
Date Recue/Date Received 2023-11-15
c) an emulsifier system containing:
i) from about 60% to about 70% by weight of the composition gum
arabic; and
ii) from about 3% to about 10% by weight of the composition propylene
glycol; and
d) from about 5% to about 12% by weight ox bile.
This example can further comprise one or more anti-caking agents. For example,
a composition containing silicon dioxide as an anti-caking agent, comprising:
a) 4.4% indinavir;
b) 8.9% sunflower oil;
c) 63.3% gum arabic;
d) 9% ox bile;
e) 10% silicon dioxide; and
0 4.4% propylene glycol.
A yet further non-limiting example of this embodiment is a composition
comprising:
a) from about 3% to about 6% by weight of raltegravir;
b) from about 5% to about 15% by weight of sunflower oil;
c) an emulsifier system containing:
i) from about 60% to about 70% by weight of the composition gum
arabic; and
ii) from about 3% to about 10% by weight of the composition propylene
glycol; and
d) from about 5% to about 12% by weight ox bile.
This example can further comprise one or more anti-caking agents. For example,
a composition containing silicon dioxide as an anti-caking agent, comprising:
a) 4.4% raltegravir;
b) 8.9% sunflower oil;
c) 63.3% gum arabic;
d) 9% ox bile;
e) 10% silicon dioxide; and
4.4% propylene glycol.
A yet another non-limiting example of this embodiment is a composition
comprising:
a) from about 3% to about 6% by weight of nevirapine;
42
8917050
Date Recue/Date Received 2023-11-15
b) from about 5% to about 15% by weight of sunflower oil;
c) an emulsifier system containing:
i) from about 60% to about 70% by weight of the composition gum
arabic; and
ii) from about 3% to about 10% by weight of the composition propylene
glycol; and
d) from about 5% to about 12% by weight ox bile.
This example can further comprise one or more anti-caking agents. For example,
a composition containing silicon dioxide as an anti-caking agent, comprising:
a) 4.4% nevirapine;
b) 8.9% sunflower oil;
c) 63.3% gum arabic;
d) 9% ox bile;
e) 10% silicon dioxide; and
4.4% propylene glycol.
A still yet further non-limiting example of this embodiment is a composition
comprising:
a) from about 3% to about 6% by weight of azidothymidine;
b) from about 5% to about 15% by weight of sunflower oil;
c) an emulsifier system containing:
i) from about 60% to about 70% by weight of the composition gum
arabic; and
ii) from about 3% to about 10% by weight of the composition propylene
glycol; and
d) from about 5% to about 12% by weight ox bile.
This example can further comprise one or more anti-caking agents. For example,
a composition containing silicon dioxide as an anti-caking agent, comprising:
a) 4.4% azidothymidine;
b) 8.9% sunflower oil;
c) 63.3% gum arabic;
d) 9% ox bile;
e) 10% silicon dioxide; and
0 4.4% propylene glycol.
43
8917050
Date Recue/Date Received 2023-11-15
All of the above disclosed aspects, iterations, embodiments and examples can
further comprise one or more of the disclosed adjunct ingredients.
Another still yet further iteration of this aspect relates to compositions
comprising:
a) an effective amount of colchicine;
b) a bioavailability enhancing agent chosen from palm kernel oil, soybean
oil,
rapeseed oil (canola), sunflower oil, groundnut oil, sesame oil, flaxseed oil,
palm oil, olive oil and corn oil;
c) one or more emulsifiers chosen from gum arabic, modified starch, pectin,
xanthan gum, gum ghatti, gum tragacanth, fenugreek gum, mesquite gum,
mono-glycerides and di-glycerides of long chain fatty acids, sucrose
monoesters, sorbitan esters, polyethoxylated glycerols, stearic acid, palmitic
acid, mono-glycerides, di-glycerides, propylene glycol esters, lecithin,
lactylated mono- and di-glycerides, propylene glycol monoesters,
polyglycerol esters, diacetylated tartaric acid esters of mono- and di-
glycerides, citric acid esters of monoglycerides, stearoy1-2-lactylates,
polysorbates, succinylated monoglycerides, acetylated monoglycerides,
ethoxylated monoglycerides, quillaia, whey protein isolate, casein, soy
protein, vegetable protein, pullulan, sodium alginate, guar gum, locust bean
gum, tragacanth gum, tamarind gum, carrageenan, furcellaran, Gellan gum,
psyllium, curdlan, konjac mannan, agar, and cellulose derivatives, and
combinations thereof, or a sugar alcohol that can optionally have humectant
properties such as ethylene glycol, glycerol, erythritol, threitol, arabitol,
xylitol, ribitol, mannitol, sorbitol, galactitol, frucitol, iditol, sucrose,
fructose,
isomalt, maltitol, lactitol, sorbitol, dextrose or inositol, or mixtures
thereof;
and
d) one or more bile salts.
One embodiment of this iteration relates to compositions comprising:
a) an effective amount of colchicine;
b) a bioavailability enhancing agent is sunflower oil;
c) an emulsifier chosen from gum arabic, propylene glycol, or mixtures
thereof;
and
d) ox bile.
One non-limiting example of this embodiment is a composition comprising:
a) from about 3% to about 6% by weight of colchicine;
44
8917050
Date Recue/Date Received 2023-11-15
b) from about 5% to about 15% by weight of sunflower oil;
c) an emulsifier system containing:
i) from about 60% to about 70% by weight of the composition
gum
arabic; and
from about 3% to about 10% by weight of the composition propylene
glycol; and
d) from about 5% to about 12% by weight ox bile.
This example can further comprise one or more anti-caking agents. For example,
a composition containing silicon dioxide as an anti-caking agent, comprising:
a) 4.4% colchicine;
b) 8.9% sunflower oil;
c) 63.3% gum arabic;
d) 9% ox bile;
e) 10% silicon dioxide; and
4.4% propylene glycol.
All of the above disclosed aspects, iterations, embodiments and examples can
further comprise one or more of the disclosed adjunct ingredientas.
METHODS
Disclosed herein are methods for treating a subject having a viral infection.
The
methods comprise administering to a person infected with a virus an effective
amount of a
composition comprising:
a) one or more antiviral agents; and
b) a bioavailability enhancing agent.
The disclosed methods can also comprise administering to a person infected
with a
virus an effective amount of a composition comprising:
a) one or more anti-inflammatories; and
b) a bioavailability enhancing agent.
The disclosed methods can further comprise administering to a person infected
with
a virus an effective amount of a composition comprising:
a) one or more antiviral agents and an anti-inflammatory agent; and
b) a bioavailability enhancing agent.
In a further aspect of the presently disclosed methods comprises administering
to a
subject infected with a virus and effective amount of a composition
comprising:
a) one or more antiviral agents;
8917050
Date Recue/Date Received 2023-11-15
b) a bioavailability enhancing agent; and
c) an emulsifier.
In a still further aspect of the presently disclosed methods comprises
administering
to a subject infected with a virus and effective amount of a composition
comprising:
a) one or more antiviral agents;
b) a bioavailability enhancing agent;
c) an emulsifier; and
d) one or more adjunct ingredients.
The methods include the use of any of the disclosed compositions. The methods
of
use relate to oral dosing of the compositions. The compositions will include,
as noted
above, an effective amount of a virus inhibitor in combination with a
pharmaceutically
acceptable carrier and, in addition, can include other medicinal agents,
pharmaceutical
agents, carriers, adjuvants, diluents, etc. as adjunct ingredients described
herein above
preferably in unit dosage form suitable for single administration of a precise
dosage.
EXAMPLES
Disclosed herein are the following non-limiting examples of the disclosed
compositions.
General Process
One or more antiviral agents are combined with a bioavailability enhancing
agent
and the ingredients are heated and thoroughly admixed to render a homogenous
composition wherein the triglycerides and the antiviral agents are in intimate
contact. A
comestible agent is added and the ingredients further admixed. The composition
is then
subjected to dehydration, lyophilization or other drying methods to remove all
water and
volatiles resulting in free flowing powder. The composition is then combined
with one or
more adjunct ingredients. The final powder can be further processed to produce
the desired
particle size range. The final powder can also be further combined with one or
more
emulsifiers and/or surfactants in an aqueous preparation together with
processing via high
pressure homogenization or ultrasonication to render a liquid nanoemulsified
format with a
desired particle size range. The final powder and/or liquid nanoemulsion can
also be further
combined with one or more ingredients used to formulate topical creams,
lotions, ointments,
gels, balms or other topical dosage forms to render a preparation suitable for
application to
the peripheral epidermal and/or mucosal membranes.
46
8917050
Date Recue/Date Received 2023-11-15
As disclosed herein above, the disclosed compositions can comprise an
emulsifier
system or solubilizing system comprising an emulsifier and a solubilizing
carrier. For
example:
i) an emulsifier; and
ii) a carrier.
Typically, after addition of the active base and emulsifier, one or more
components
may remain not fully dissolved. In that case the formulator can add a carrier
to achieve
composition homogeneity.
For example:
i) from about 85% to about 95% an emulsifier; and
ii) from about 5% to about 15 a carrier.
The formulator can combine the active base with the components the
solubilizing
system in any order. For example, combining an antiviral with an edible oil
followed by a
carrier to form a homogeneous admixture prior to addition of the emulsifier.
Non limiting examples of compositions can be found in the following Tables:
TABLE I
Percentage of Composition
1 2 3 4 5
efavirenz 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
TABLE II
milligrams
6 7 8 9 10
efavirenz 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
silicon dioxide 100 80 65 57 73
47
8917050
Date Recue/Date Received 2023-11-15
TABLE III
Percentage of Composition
11 12 13 14 15
darunavir 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
TABLE IV
milligrams
16 17 18 19 20
darunavir 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
silicon dioxide 100 80 65 57 73
TABLE V
Percentage of Composition
21 22 23 24 25
bepredil 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
TABLE VI
milligrams
26 27 28 29 30
bepridil 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
48
8917050
Date Recue/Date Received 2023-11-15
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
silicon dioxide 100 80 65 57 73
TABLE VII
Percentage of Composition
31 32 33 34 35
rupintrivir 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
TABLE VIII
milligrams
36 37 38 39 40
rupintrivir 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
silicon dioxide 100 80 65 57 73
TABLE IX
Percentage of Composition
41 42 43 44 45
ebastine 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
49
8917050
Date Recue/Date Received 2023-11-15
TABLE X
milligrams
46 47 48 49 50
ebastine 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
silicon dioxide 100 80 65 57 73
TABLE XI
Percentage of Composition
51 52 53 54 55
doravirine 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
TABLE XII
milligrams
56 57 58 59 60
doravirine 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
silicon dioxide 100 80 65 57 73
TABLE XIII
Percentage of Composition
61 62 63 64 65
etravirine 44 35.2 28.6 25.1 32.1
8917050
Date Recue/Date Received 2023-11-15
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
TABLE XIV
milligrams
66 67 68 69 70
etravirine 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
silicon dioxide 100 80 65 57 73
TABLE XV
Percentage of Composition
71 72 73 74 75
loviride 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
TABLE XVI
milligrams
76 77 78 79 80
loviride 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
silicon dioxide 100 80 65 57 73
51
8917050
Date Recue/Date Received 2023-11-15
TABLE XVII
Percentage of Composition
81 82 83 84 85
rilpivirine 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
TABLE XVIII
milligrams
86 87 88 89 90
rilprivirine 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
silicon dioxide 100 80 65 57 73
TABLE XIX
Percentage of Composition
91 92 93 94 95
GS-441524 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
TABLE XX
milligrams
96 97 98 99 100
GS-441524 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
52
8917050
Date Recue/Date Received 2023-11-15
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
silicon dioxide 100 80 65 57 73
TABLE XXI
Percentage of Composition
101 102 103 104 105
remdesivir 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
TABLE XXII
milligrams
106 107 108 109 110
remdesivir 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
silicon dioxide 100 80 65 57 73
TABLE XXIII
Percentage of Composition
111 112 113 114 115
indinavir 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
53
8917050
Date Recue/Date Received 2023-11-15
TABLE XXIV
milligrams
116 117 118 119 120
indinavir 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
silicon dioxide 100 80 65 57 73
TABLE XXV
Percentage of Composition
121 122 123 124 125
raltegravir 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
TABLE XXVI
milligrams
126 127 128 129 130
raltegravir 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
silicon dioxide 100 80 65 57 73
TABLE XXVII
Percentage of Composition
131 132 133 134 135
colchicine 44 35.2 28.6 25.1 32.1
54
8917050
Date Recue/Date Received 2023-11-15
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
ethanol 44 35.2 28.6 25.1 32.1
TABLE XXVIII
milligrams
136 137 138 139 140
colchicine 44 35.2 28.6 25.1 32.1
sunflower oil 89 71.2 57.9 50.7 65
gum arabic 633 506.4 411.5 360.8 462.1
ox bile 90 72 58.5 51.3 65.7
propylene glycol 44 35.2 28.6 25.1 32.1
ethanol 100 80 65 57 73
The disclosed oral solid compositions can be prepared by conventional methods
of
blending, filling or tableting. The blending operation can be repeated to
distribute the active
principle throughout compositions containing large quantities of adjunct
ingredients. Such
operations are conventional. Oral liquid preparations can be in the form of,
for example,
aqueous or oily suspensions, solutions, emulsions, syrups or elixirs, or can
be presented as a
dry product for reconstitution with water or with a suitable vehicle before
use. Such liquid
preparations can contain conventional additives such as suspending agents, for
example
sorbitol, syrup, methyl cellulose, gelatine, hydroxy ethyl cellulose,
carboxymethyl cellulose,
aluminium stearate gel, or hydrogenated edible fats; emulsifying agents, such
as lecithin,
sorbitan monooleate, or acacia; non-aqueous vehicles oily esters such as
esters of glycerine,
propylene glycol, or ethyl alcohol; preservatives, such as methyl or propyl p-
hydroxy-
benzoate or sorbic acid, and if desired, conventional flavoring or coloring
agents. Oral
formulations also include conventional slow-release formulations such as
enterically coated
tablets or granules.
The compounds and compositions, according to the disclosed methods can be
administered using any amount. The exact amount required will vary from
subject to
subject, depending on the age, and general condition of the subject, the
severity of the viral
infection, the particular antiviral agent. Compounds of the invention are
preferably
formulated in dosage unit form for ease of administration and uniformity of
dosage. The
8917050
Date Recue/Date Received 2023-11-15
expression "dosage unit form" as used herein refers to a physically discrete
unit of an agent
appropriate for the patient to be treated. It will be understood, however,
that the total daily
usage of the compounds and compositions disclosed herein will be decided by
the attending
physician within the scope of sound medical judgment. The specific effective
dose level for
any particular patient will depend upon a variety of factors including the
disorder being
treated and the severity of the disorder; the activity of the specific
compound employed; the
specific composition employed; the age, body weight, general health, sex and
diet of the
patient; the time of administration, and rate of excretion of the specific
antiviral agent
employed; the duration of the treatment; drugs used in combination or
coincidental with the
specific compound employed, and like factors well known in the medical arts.
Liquid Formulations
A composition comprising the active base is added to a "sugar syrup." The
sugar
syrup is prepared by dissolving in aqueous solution appropriate quantities of
sugar, citric
acid, preservatives, glycerin to obtain clear homogenous syrup. The filtered
solution of
extracts is added to syrup and stirred to obtain a homogenous syrup. The
sugars are any
pharmaceutically acceptable sugars, for example, monosaccharides include
fructose,
galactose glucose and dextrose. Disaccharides include lactose, maltose and
sucrose. The
disclosed syrups can also comprise sugar alcohols, for example, erythritol,
glycerol,
mannitol, sorbitol, or xylitol. One or more polysaccharides can also be added
to adjust the
viscosity of the resulting liquid formulation.
Colors, menthol and other flavoring agents along with other commonly known
pharmaceutical excipients may be added as required to make this syrup
palatable for
patients' acceptability.
In general, a liquid formulation can be prepared, for example, by combining a
dehydrated free-flowing solid composition comprising:
i) from about 5% to about 20% by weight of an active base,
comprising:
a) from about 25% to about 50% by weight of darunavir;
b) from about 50% to about 75% by weight of sunflower oil; and
ii) from about 50% to about 70% gum arabic;
and a sugar syrup, mixing the admixture until the solution is homogeneous. The
formulator
can adjust the amount of the active base in order to deliver a desired unit
dose of the anti-
viral agents, for example, from about 100 mg/dose to about 300 mg/dose.
A non-limiting example of a single dose liquid formulation comprises:
a) 20 % darunavir;
56
8917050
Date Recue/Date Received 2023-11-15
b) 40% sunflower oil;
c) 10% gum arabic; and
d) the balance sugar syrup.
Disclosed herein are nanoemulsions of the antiviral compositions disclosed
herein
above. Once the formulator has selected the active base to be delivered into a
liquid
formulation the nanoemulsion is then prepared. The nanoemulsion is obtained
according to
the procedures disclosed herein. The disclosed nanoemulsions are
thermodynamically
stable, for example high kinetic stability, with low viscosity and optical
transparency.
The term "saponin" refers to compounds derived from various plant species,
particularly amphipathic glycosides having emulsifier or surfactant
properties.
Saponins
The process for preparing the disclosed nanoemulsions uses emulsifiers and
surfactants to obtain the desired properties. In one aspect the disclosed
process utilizes
saponins for their emulsification properties.
The disclosed saponins are obtained from naturally occurring sources, for
example,
the genus Saponaria, of the family Caryophyllaceae; Sapindus of the family
Sapindaceae;
in the families Sapindaceae, Hippocastanaceae, Gynostemma (G. pentaphyllum
sp.), and
Cucurbitaceae. In addition, saponins can be derived from the genus Panax, for
example,
Panax quinquefolius, Panax vietnamensis, and Panax pseudoginseng. One non-
limiting
example of a suitable saponin is "soap bark" obtained from Quillaja saponaria,
herein
referred to as "quillaja."
In one aspect the disclosed nanoemulsions have an average droplet size from
about
50 nm to about 1,000 nm. In one embodiment the droplet size is from about 10
nm to about
500 nm. In a further embodiment the droplet size is from about 100 to about
500 nm. In a
yet further embodiment the droplet size is from about 200 to about 800 I1M. In
a still further
embodiment the droplet size is from about 400 to about 800 nm.
Disclosed herein is a nanoemulsion, comprising:
A) a first component containing:
i) from about 5% to about 20% by weight of an active base,
comprising:
a) from about 25% to about 50% by weight of one or more
antiviral agents;
b) from about 50% to about 75% by weight of a bioavailability
enhancing agent; and
57
8917050
Date Recue/Date Received 2023-11-15
ii) from about 50% to about 70% a base substrate; and
B) a second component containing:
a) an emulsifier; and
b) water;
wherein the average droplet size is from about 50 nm to about 1,000 nm.
General Process for Preparing the Disclosed Compositions in the Form of a
Nanoemulsion
One or more biologically active ingredients are combined with a
bioavailability
enhancing agent and the ingredients are heated and thoroughly admixed to
render a
homogenous composition wherein the triglycerides and the biologically active
ingredients
are in intimate contact. A base substrate is added and the ingredients further
admixed. The
composition is then subjected to dehydration, lyophilization or other drying
methods to
remove all water and volatiles resulting in free flowing powder. The
composition is then
combined with one or more optional adjunct ingredients. The final powder can
be further
processed to produce the desired particle size range.
The control composition comprises:
a) one or more antiviral agents;
b) a bioavailability enhancing agent;
wherein (a) and (b) are present in a ratio from about 1:1 to about 1:3; and
c) a the base substrate.
General Process for the Formation of Nanoemulsions
Disclosed herein is a general process for preparing a nanoemulsion,
comprising:
A) combining one or more antiviral agents and a bioavailability enhancing
agent
to form an enhanced delivery admixture;
B) combining the enhanced delivery admixture with a base substrate and
removing any water present to form a first component;
C) dissolving the first component in a aqueous solution of a saponin at a
temperature of from about 50 C to about 60 C to finial an admixture;
D) cooling the admixture in step (C) to a temperature of from about 40 C
to
about 50 C to form a cooled solution; and
E) high pressure homogenizing the cooled solution at 30,000 psi to form the
nanoemulsion.
In one aspect the process for converting the composition to a nanoemulsion,
comprises:
58
8917050
Date Recue/Date Received 2023-11-15
i) an aqueous solution of a saponin is heated to a temperature of from
about 50
C to about 60 C to form an aqueous emulsion;
ii) the fine powder composition is added to the emulsion formed in step (i)
and
the resulting solution admixed;
iii) the solution of step (ii) is cooled to a temperature of from about 40
C to
about 50 C; and
iv) the cooled solution was high pressure homogenized at 30,000 psi to form
the
nanoemulsion.
EXAMPLE I
A fine powder formulation of the active base containing composition was
prepared
according to the General Process. The composition comprised lactose
monohydrate powder
as a base substrate, one or more antiviral agents high oleic acid sunflower
oil in a 1:1 ratio.
A surfactant, polysorbate 80 was also added.
Once prepared, the powder formulation was then converted to a nanoemulsion
according to the following steps:
i) an aqueous solution of quillaja obtained from Quiilaja saponaria was
heated
to a temperature of from about 50 C to about 60 C to form an aqueous
emulsion;
ii) the fine powder composition was added to the emulsion formed in step
(i)
and the resulting solution admixed;
iii) the solution of step (ii) is cooled to a temperature of from about 40
C to
about 50 C; and
iv) the cooled solution was high pressure homogenized at 30,000 psi to form
the
nanoemulsion.
Homogenization
The homogenization step can include rnicrofluidization under high pressure.
For
example at pressures from about 10,000 psi to about 30,000 psi. In some
embodiments a
high shear rotostator processor and/or an ultrasonication processor can be
used. As known
to the formulator these processes vary in efficiency depending on the duration
and intensity
of the energy applied.
In one embodiment the formulator can apply microfluidization at 30,000 PSI for
a
"single pass" through the processor or multiple passes through the processor
which is more
time consuming of course but can lead to better particle size reduction and
size distribution
homogeneity than a single pass.
59
8917050
Date Recue/Date Received 2023-11-15
Once formed, the nanoemulsion can be dispersed into a sugar syrup. The
nanoemulsion-containing sugar syrup can further comprise colorants and/or
flavorants.
The disclosed nanoemulsion-containing liquid compositions, comprise:
I) from about 10% to about 15% of a nanoemulsion comprising:
A) a first component containing:
i) from about 5% to about 20% by weight of an active
base,
comprising:
a) from about 25% to about 50% by weight of one or
more antiviral agents;
b) from about 50% to about 75% by weight of a
bioavailability enhancing agent; and
ii) from about 50% to about 70% a base substrate; and
B) a second component containing:
a) an emulsifier; and
b) water; and
II) The balance a sugar syrup.
Evaluation of Compositions In Vivo
In this study, the exposure and distribution of darunavir and efavirenz were
evaluated following oral administration in male Sprague-Dawley rats. Four
cohorts of 10
rats each were used in this study. The first cohort involved darunavir using
the disclosed
formulations and the second cohort darunavir in a control formulation. The
third cohort
involved efavirenz using the disclosed formulations and the fourth cohort
efavirenz in a
control formulation.
Formulations were administered orally (PO) at 10 mg/kg of darunavir or
efavirenz.
Following dosing, blood, urine and fecal samples were collected up to 8-hour
post dose
(groups 1, 3, 5, and 7) or 24-hour post dose (groups 2, 4, 6, and 8). Brain
tissue was
collected at 8 hours or 24 hours, following the 8 hour or 24-hour urine and
feces sample
collection. Plasma, urine, feces, and brain tissue concentrations of the
analytes were
determined by LC-MS/MS. Plasma phannacokinetic parameters were determined
using
WinNonlin (v8.0) software. A summary of the average plasma exposures and brain
tissue
concentrations are shown in Table 2 and Table 3, respectively. A summary of
the average
darunavir or efavirenz recovery in urine and feces are shown in Table 4.
Test Solutions
8917050
Date Recue/Date Received 2023-11-15
The following test solutions were prepared for use in the study described
herein
below
1. Darunavir Test solution
Ingredient percent
Darunavir 4.4
Gum Arabic 63.3
High oleic sunflower oil 8.9
Propylene glycol 4.4
Bile salts 9
Silicon dioxide 10
The 8-hour dosage test solution (Group #1) was prepared as follows. The above
test
solution (476.13 mg) is charged to a 23 mL clear glass vial. Water (15 mL) is
added to the
vial and the solution is vortexed/sonicated, QS to 20.0 mL, resulting in a
fine suspension.
The 24-hour dosage test solution (Group #2) was prepared as follows. The above
test solution (502.05 mg) is charged to a 23 mL clear glass vial. Water (15
mL) is added to
the vial and the solution is vortexed/sonicated, QS to 21.1 mL, resulting in a
fine
suspension.
2. Darunavir Control solution
Ingredient percent
Darunavir 4.6
Gum Arabic 85.4
Silicon dioxide 10
The 8-hour dosage control solution (Group #3) was prepared as follows. The
above
test solution (448.68 mg) is charged to a 23 mL clear glass vial. Water (15
mL) is added to
the vial and the solution is vortexed/sonicated, QS to 20.0 mL, resulting in a
fine
suspension.
The 24-hour dosage control solution (Group #1) was prepared as follows. The
above test solution (452.47 mg) is charged to a 23 mL clear glass vial. Water
(15 mL) is
added to the vial and the solution is vortexed/sonicated, QS to 20.2 mL,
resulting in a fine
suspension.
3. Efavirenz Test Solution
Ingredient percent
61
8917050
Date Recue/Date Received 2023-11-15
Efavirenz 44
Gum Arabic 63.3
High oleic sunflower oil 8.9
Propylene glycol 4.4
Bile salts 9
Silicon dioxide 10
The 8-hour dosage test solution (Group #5) was prepared as follows. The above
test
solution (482.0 mg) is charged to a 21 mL clear glass vial. Water (15 mL) is
added to the
vial and the solution is vortexed/sonicated, QS to 20.4 mL, resulting in a
fine suspension.
The 24-hour dosage test solution (Group #2) was prepared as follows. The above
test solution (481.11 mg) is charged to a 21 mL clear glass vial. Water (15
mL) is added to
the vial and the solution is vortexed/sonicated, QS to 20.4 mL, resulting in a
fine
suspension.
4. Efavirenz Control solution
Ingredient percent
Efavirenz 4.6
Gum Arabic 85.4
Silicon dioxide 10
The 8-hour dosage control solution (Group #7) was prepared as follows. The
above
test solution (441.79 mg) is charged to a 21 mL clear glass vial. Water (15
mL) is added to
the vial and the solution is vortexed/sonicated, QS to 20.4 mL, resulting in a
fine
suspension.
The 24-hour dosage control solution (Group #8) was prepared as follows. The
above test solution (440.22 mg) is charged to a 21 mL clear glass vial. Water
(15 mL) is
added to the vial and the solution is vortexed/sonicated, QS to 20.3 mL,
resulting in a fine
suspension.
Table 1 below discloses the dosing protocol for the present study (No.1). For
example, the 8-hour group #1 and the 24-hour group #2 were dosed with the
darunavir test
solution.
62
8917050
Date Recue/Date Received 2023-11-15
TABLE 1
Nominal Con. Measured Con. Measured % of
Group # Test Formulation
(mg/mL) (mg/mL) Nominal
1 & 2 Darunavir Test 1 1.06+0.18 106
3 &4 Darunavir Control 1 0.99+0.17 99.2
& 6 Efavirenz Test 1 0.93+0.07 92.9
7 & 8 Efavirenz Control 1 0.91+0.05 91.3
Table 2 provides a summary of the average plasma exposures for darunavir and
efavirenz after oral administration of the disclosed formulations a 10 mg/kg
in male
Sprague-Dawley rats.
TABLE 2
Dose-
normalized
Group Test Cmax AUClast
t1/2 (hr) tmax (hr) AUClast
# Formulation (ng/mL) (hrng/mL)
(hrkg=ng
/mL/mg)
1 &2 DAR test 0.98+0.28 509+516 0.53+0.28
721+332 72.1+33.2
3 & 4 DAR cont. 0.72+0.21
472+200 0.38+0.24 469+251 46.9+25.1
5 & 6 EFA test 2.75+1.14 172+67.9
2.00+1.15 752+203 75.2+20.3
7 & 8 EFA cont. 2.78+1.19 153+46.8
1.20+0.57 650+148 65.0+14.8
Table 3 is a summary of the average brain tissue concentrations for dartmavir
and
efavirenz after oral administration of the disclosed foimulations at 10 mg/kg
in male
Sprague-Dawley rats.
TABLE 3
Brain Tissue Concentration (ng/g)
Group # Test Formulation
8 hours 24 hours
1 & 2 DAR test ND ND
3 & 4 DAR cont. ND ND
5 & 6 EFA test 166+19.1 ND
7 & 8 EFA cont. 211+56.2 ND
ND: not determined because analytical data were BLOQ (below limit of
quantification,10 ng/mL)
63
8917050
Date Recue/Date Received 2023-11-15
Table 4 is a summary of the average urine and feces % if dose recovered for
darunavir and efavirenz after oral administration of the disclosed
formulations at 10 mg/kg
in male Sprague-Dawley rats.
TABLE 4
Group Test Urine (%) Feces (%)
Formulation 8 hours 24 hours 8 hours 24 hours
1 &2 DAR test 0.0071+0.0028
0.0164 0.0118 2.00 2.24 8.24+1.32
3 &4 DAR cont. 0.0116+0.0035 0.0115 0.0049 6.31 4A3
10.7 2.33
& 6 EFA test ND ND 2.27 2.62 29.4
7.57
7 & 8 EFA cont. ND ND 3.10 2.70
31.4+10.0
Plasma, urine, feces, and brain tissue samples were extracted and analyzed
using the
methods described as follows.
Analytical Stock Solution Preparation
Analytical stock solutions (1.00 mg/mL of the free drug) were prepared in
DMSO.
Sample Homogenization Brain tissue and feces samples were homogenized with a
Virsonic
100 ultrasonic homogenizer. Each brain tissue sample was first weighed, and
then an
appropriate volume of 20:80 methanol:water was added to make a 2 mL/gram
sample.
Samples were then homogenized on ice, and stored frozen until analysis. Feces
samples
were homogenized with 20:80 MeOH:water (8 mL/gram of tissue) prior to
extraction.
Standard Preparation (Plasma, Urine, and Brain) Standards were prepared in rat
plasma
containing sodium heparin as the anticoagulant, or in blank tissue homogenate.
Working
solutions were prepared in 50:50 acetonitrile:water. Working solutions were
then added to
blank matrix to make calibration standards to final concentrations of 1000,
500, 250, 100,
75.0, 50.0, 25.0, and 10 ng/rriL. Standards were treated identically to the
study samples.
Sample Extraction (Plasma, Urine, Feces, and Brain) Plasma, urine, feces, and
brain tissue
samples were manually extracted via acetonitrile precipitation in a 96-well
plate. Urine
samples were diluted a minimum of 5-fold into blank rat plasma prior to
extraction. Diluted
urine samples were quantified against the rat plasma calibration curve. Brain
and feces
tissue homogenates were quantified against calibration curves prepared in
matched blank
tissue homogenate.
64
8917050
Date Recue/Date Received 2023-11-15
Table 5 shows the average plasma concentration (ng/mL) of the animals after
being
given a 10 mg/mL dose of one of the 8 test formulations. The values are the
mean plasma
level of each group over time.
TABLE 5
Mean plasma level in ng/mL
Time Groups Groups Groups Groups
(hr) 1 & 2 3 & 4 5 & 6 7 & 8
0.25 444 464 33.7 57.9
0.5 294 357 76.7 102.9
0.75 301 236 109 124
1 306 185 133 139
2 153 63.1 142 117
4 22.6 16.6 97.7 75.9
8 26.6 54.3 44.5
* Below the detectable limit
The results of Study No. 1 are summarized in Table 5 and Figure 1 to Figure 4.
Figure 1 depicts the average plasma concentration of darunavir in the test
solutions. As
seen in Table 5 and Figure 1 the average plasma concentration of darunavir in
the test
animals at 1 hour is approximately 306 ng/mL and at 2 hours is approximately
153 ng/mL.
In contrast, as seen in Table 5 and Figure 2, the average plasma concentration
of darunavir
in the animals given the control formulation at 1 hour is approximately 185
ng/mL and at 2
hours is approximately 63 ng/mL. The compositions disclosed herein provide
double the
plasma concentration of the anti-viral agent darunavir versus compositions
that do not
comprise the disclosed compositions.
As seen in Table 5 and Figure 3 the average plasma concentration of efavirenz
in
the test animals at 1 hour is approximately 133 ng/mL and at 2 hours is
approximately 142
ng/mL. As seen in Table 5 and Figure 4, the average plasma concentration of
efavirenz in
the animals given the control formulation at 1 hour is approximately 139 ng/mL
and at 2
hours is approximately 117 ng/mL. The foimulations disclosed herein provide
plasma
concentration levels of efavirenz at higher levels than control formulations.
While particular embodiments of the present disclosure have been illustrated
and
described, it would be obvious to those skilled in the art that various other
changes and
modifications can be made without departing from the spirit and scope of the
disclosure. It
8917050
Date Recue/Date Received 2023-11-15
is therefore intended to cover in the appended claims all such changes and
modifications
that are within the scope of this disclosure.
66
8917050
Date Recue/Date Received 2023-11-15