Note: Descriptions are shown in the official language in which they were submitted.
WO 2021/199009
PCT/1B2021/052801
MICROBIAL OILS WITH HIGH LEVELS OF OMEGA-3 FATTY
ACIDS
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims priority to U.S. Provisional Application No.
63/005,054,
filed April 3, 2020, which is incorporated by reference herein in its
entirety.
BACKGROUND
Oil from microorganisms is produced as a result of two parallel fatty acid
synthesis
pathways: the classical fatty acid synthesis (FAS) pathway and the
polyunsaturated fatty acid
(PUFA) synthase pathway. Medium chain fatty acids like myristic (C14:0) and
palmitic acid
(C16:0) are generally produced from the FAS pathway and long chain
polyunsaturated fatty
acids (LC-PUFA) like docosahexaenoic acid (DHA, C22:6 n-3) and
docosapentaenoic acid
(DPA, C22:5 n-6) are generally produced from the PUFA synthase pathway. The
resultant
fatty acid profile, however, varies greatly across microorganisms, depending
on the relative
activity of these parallel pathways.
BRIEF SUMMARY
Provided herein are microbial oils and methods of making and using microbial
oils
with high levels of omega-3 fatty acids. Specifically, provided is a microbial
oil comprising
at least 85% total fatty acids by weight, wherein the total fatty acids
comprise at least 50%
DHA by weight. Also provided is a method of making a biomass comprising
culturing an
oil-producing microorganism (e.g., Aurantiochytrium) in a culture medium
comprising a fatty
acid synthesis inhibitor, wherein the biomass comprises at least 500 mg/g oil.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure [is a graph showing fatty acid profiles of Anrantiochytrium sp. (G3)
utilizing
different initial fatty acid synthesis inhibitor dosing timepoints at 20 C.
Figure 2 is a graph showing fatty acid profiles of Aurantiochytrium sp. (G3)
in
varying concentrations of fatty acid synthesis inhibitor at 20 C.
Figure 3 is a graph showing fatty acid profiles of Aurantiochytrium sp. (G3)
in
various fatty acid synthesis inhibitor dosing strategies at 20 C. Treatments
indicating 3x and
6x are multiple additions of fatty acid synthesis inhibitor at equally-spaced
time intervals
CA 03172997 2022- 9- 22
WO 2021/199009
PCT/IB2021/052801
starting at 24 hours after inoculation. For the 3X experiment, the interval
was every 24
hours. For the 6X experiment, the interval was every 12 hours.
Figure 4 is a graph showing final product (DHA, total fatty acid (TFA), and
saturated
fatty acid (SFA)) concentration in Aurantiochytrium sp. (G3) in various fatty
acid synthesis
inhibitor dosing strategies at 20 C. Treatments indicating 3x and 6x are
multiple additions of
fatty acid synthesis inhibitor at equally-spaced time intervals (every 12
hours) starting 24
hours after inoculation.
Figure 5 is a graph showing yield of product (SFA and DHA) per unit of glucose
consumed in Aurantiochytrium sp. (G3) in various fatty acid synthesis
inhibitor dosing
strategies at 20 C. Treatments indicating 3x and 6x are multiple additions of
fatty acid
synthesis inhibitor at equally-spaced time intervals (every 12 hours) starting
at 24 hours after
inoculation.
Figure 6 is a graph showing fatty acid profiles of Aurantiochytrium sp (G3)
from
25 C and 20 C cultivation conditions at flask scale.
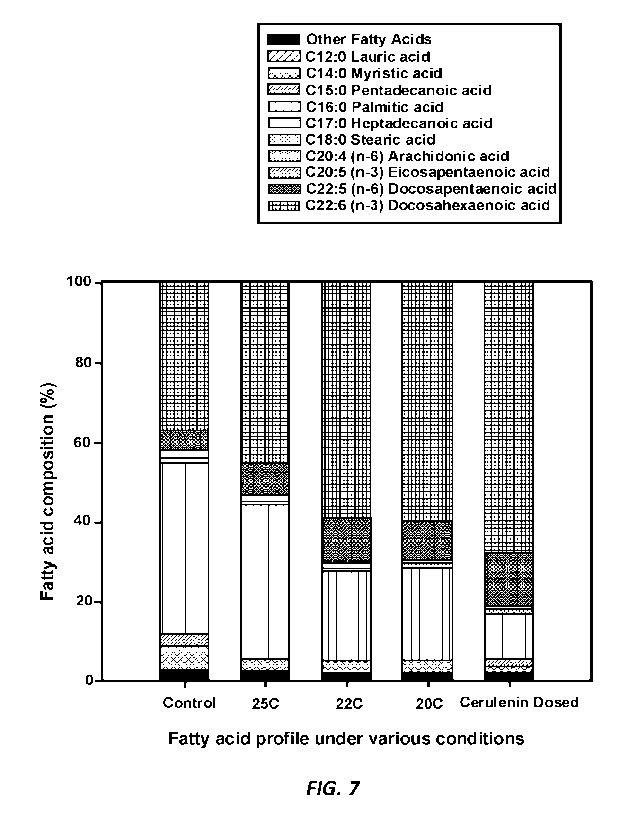
Figure 7 is a graph showing the fatty acid profiles of Aurantiochytrium sp.
(G3).
Controls were cultured in flask at 25 C with no chemical inhibitor. The full-
length
fermentation runs were fed-batch fermentations in 30L fermentor at the noted
temperature,
and the fatty acid synthesis inhibitor treatment was cultured at 20 C with a
pulsed addition of
121.iM total cerulenin at flask scale.
DETAILED DESCRIPTION
Omega-3 fatty acids, including docosahexaenoic acid (DHA, C22:6 n-3) and
eicosapentaenoic acid (EPA, C20:5 n-3) from the long chain-polyunsaturated
fatty acid (LC-
PUFA) family, are essential fatty acids for humans and non-human mammals. The
amount of
omega-3 fatty acids required in a microbial oil varies based on applications.
For example,
dietary supplements and pharmaceuticals typically prefer higher concentrations
of omega-3
fatty acids per unit of oil. To achieve such concentrations, the omega-3 fatty
acids are
typically concentrated by transesterification and molecular distillation
(Bonilla-Mendez and
Hoyos-Concha, Corpoica Cienc Tecnol Agropecuaria, Mosquera (Colombia),
19(3):645-668
(2018)). However, the final concentrated products are typically in ethyl-ester
(EE) chemical
form instead of the triglyceride (TG) form, the natural chemical structure
when the lipids are
synthesized by microorganisms. Not only does the EE form require additional
processing
that may compromise its olfactory characteristics, it has also been shown that
the EE form of
omega-3 is less bioavailable to the human body than the original TG form.
2
CA 03172997 2022- 9- 22
WO 2021/199009
PCT/IB2021/052801
Common strategies to increase omega-3 content include selecting high omega-3
microbial strains, classical mutagenesis, and genetic modification (Lian et
al., Appl Biochem
Biotechnol, 162:935-941 (2010)). Alternatively, chemicals with potential to
affect the fatty
acid synthesis metabolism under various mechanisms have been tested in
different
microorganisms. However, efficient and effective methods of applying such
principles to
achieve a commercially meaningful increase in DHA content while avoiding or
minimizing
any undesirable changes in other fatty acid components, including DPA and
saturated fatty
acids, are lacking.
Described herein are oils containing high levels of omega-3 fatty acid with
DHA
accounting for approximately 50-70% of the fatty acid profile. Also described
are methods
using fatty acid synthesis inhibitors to suppress less-desirable products of
the FAS pathway in
favor of the high-value PUFA products from the PUFA synthase pathway. The
provided oils
contain high concentrations of DHA without sacrificing the productivity of the
microorganism. Further, described are methods of using inhibitors that inhibit
the fatty acid
synthase multienzyme complex as described herein. The examples illustrate
fatty acid
profiles of G3, showing a substantial increase in DHA content (up to more than
83.3% when
compared to the control) and even more with pulsed-addition of a fatty acid
synthesis
inhibitor. The outcome of almost 70% DHA in TG form in an oil exceeds reported
DHA
concentrations.
Microorganisms, including Thraustochytrids, produce oil containing a variety
of
lipids, including fatty acids in various forms and amounts. As used herein,
the term lipid
includes phospholipids, free fatty acids, esters of fatty acids,
triacylglycerols, sterols and
sterol esters, carotenoids, xanthophylls (e.g., oxycarotenoids), hydrocarbons,
and other lipids
known to one of ordinary skill in the art. Fatty acids are hydrocarbon chains
that terminate in
a carboxyl group, being termed unsaturated if they contain at least one carbon-
carbon double
bond, and polyunsaturated when they contain multiple carbon-carbon double
bonds. For
example, microorganisms can produce (i) short-chain fatty acids (SCFA), which
are fatty
acids with aliphatic tails of fewer than six carbons (e.g., butyric acid);
(ii) medium-chain fatty
acids (MCFA), which are fatty acids with aliphatic tails of 6-12 carbons;
(iii) long-chain fatty
acids (LCFA), which are fatty acids with aliphatic tails of greater than 13
carbons. Various
microorganisms produce varying types and amounts of these fatty acids.
Provided herein are
microorganisms and methods that shift production of these fatty acids away
from medium-
chain fatty acids produced by the FAS pathway to long-chain fatty acids
produced by the
PUFA synthase pathway. Fatty acid synthesis (FAS) is defined as the creation
of fatty acids
3
CA 03172997 2022- 9- 22
WO 2021/199009
PCT/IB2021/052801
from acetyl-CoA and NADPH through the action of enzymes called fatty acid
synthases. The
PUFA synthase pathway enables the synthesis of polyunsaturated fatty acids de
novo from
malonyl-CoA by large multi-domain, multi-subunit enzymes. The major end-
product of the
FAS pathway is palmitate, while the major end-product of the PUFA synthases
are PUFAs
such as DHA and DPA.
Thus, provided herein are microbial oils and methods for making and using
microbial
oils. The oils include fatty acids in the form of monoglycerides,
diglycerides, and
triglycerides, as well as free fatty acids and phospholipids. Optionally, the
microbial oil
comprises at least 90% (e.g., 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99%)
triglycerides. Optionally, the microbial oil comprises at least 95%
triglycerides.
The oils also contain at least 85% (e.g., 86%, 87%, 88%, 89%, 90%, 91%, 92%,
93%,
94%, 95%, 96%, 97%, 98%, or 99%) by weight total fatty acids (TFA).
Optionally, the oil
contains between 85% to 99% total fatty acids by weight Optionally, the
microbial oil
comprises 85% to 95% total fatty acids by weight. Optionally, the microbial
oil comprises at
least 90% total fatty acids by weight.
The percentages in reference to oils or total fatty acids are recited
throughout by
weight percent. For example, when a microbial oil comprises at least 90% total
fatty acids,
the oil contains at least 90% total fatty acids by weight of the oil. Also,
total fatty acids
contain specific fatty acids and percentages of the specific fatty acid are
expressed throughout
as weight % of the total fatty acids. For example, when the total fatty acids
in the oil contain
DHA, the amount of DHA is expressed as weight % of the total fatty acids. For
example, the
total fatty acids comprise at least 50% DHA by weight.
As described, the total fatty acids of the provided oils contain DHA.
Optionally, the
total fatty acids comprise at least 35%, at least 40%, or at least 45% DHA.
Optionally, the
total fatty acids comprise at least 50% DHA. Optionally, the total fatty acids
include at least
60% DHA. Optionally, the total fatty acids include 50% to 70% DHA. Optionally,
the total
fatty acids comprise 60% to 70% DHA.
Optionally, the oil also contains between 6% to 18% DPA. Optionally, the oil
contains between 10% to 18% DPA. Optionally, the oil contains between 6% to
10% DPA.
Optionally, the total fatty acids comprise at least 60% DHA and between 10% by
weight to
18% DPA. Optionally, the ratio of DHA to DPA is less than or equal to 7:1 or
6:1.
Optionally, the total fatty acids comprise at least 50% DHA and the ratio of
DHA to DPA is
less than or equal to 7:1. Optionally, the total fatty acids comprise at least
50% DHA and the
ratio of DHA to DPA is less than or equal to 6:1.
4
CA 03172997 2022- 9- 22
WO 2021/199009
PCT/IB2021/052801
Optionally, the oil contains less than 1% stearic acid. Optionally, the total
fatty acids
comprise 0.01% to 1% stearic acid or 0.001% to 1% by weight stearic acid.
Optionally, the microbial oil comprises at least 85% total fatty acids,
wherein the total
fatty acids comprise at least 50% DHA, between 6% to 18% DPA, and less than 1%
stearic
acid. Optionally, the microbial oil comprises at least 85% total fatty acids,
wherein the total
fatty acids comprise at least 60% DHA.
Optionally, the total fatty acids comprise less than 3%, 2% or 1%
eicosapentaenoic
acid. Optionally, the total fatty acids comprise 0.01% to 1% eicosapentaenoic
acid or 0.001%
to 1% eicosapentaenoic acid. Optionally, the total fatty acids comprise 0.01%
to 2%
eicosapentaenoic acid or 0.001% to 2% eicosapentaenoic acid. Optionally, the
total fatty
acids comprise 0.01% to 3% eicosapentaenoic acid or 0001% to 3%
eicosapentaenoic acid.
Optionally, the total fatty acids comprise less than 5%, less than 4%, or less
than 3%
pentadecanoic acid. Optionally, the total fatty acids comprise 0.01% to 3%,
0.01% to 4%, or
0.01% to 5% pentadecanoic acid.
Optionally, the total fatty acids comprise less than 45%, 40%, 35%, 30%, or
25%
saturated fatty acids (SFAs). Saturated fatty acids in the oils produced by
the herein
described method include, but are not limited to, C12:0 (lauric acid), C14:0
(myristic acid),
C15:0 (pentadecanoic acid), C16:0 (palmitic acid), C17:0 (heptadecanoic acid),
and C18:0
(stearic acid). Optionally, the total fatty acids comprise between 10% and 45%
saturated
fatty acids (e.g., 10% and 40%, 10% and 30, 10% and 20%, 15% and 30%, 15% and
20%,
20% and 30%, or 20% and 25% saturated fatty acids).
Optionally, the total fatty acids comprise 0.001% to 2.0% (e.g., 0.01% to 2.0%
or
0.05% to 2.0%) arachidonic acid (C20:4 (n-6)). Optionally, the total fatty
acids comprise
0.001% to 1% (e.g., 0.01% to 1%) eicosatetraenoic acid (C20:4 (n-3)).
Optionally, the total fatty acids comprise less than 5% by weight myristic
acid.
Optionally, the total fatty acids comprise 0.001% to 5%, (e.g., 0.01% to 5%,
0.1% to 5%,
0.01% to 4%, 0.1% to 4% or 1% to 4%) by weight myristic acid.
Optionally, the total fatty acids comprise less than 40% by weight palmitic
acid.
Optionally, the total fatty acids comprise between 5% and 40%, 5% and 30%, 10%
and 40%,
10% and 30%, 20% and 40%, 20% and 30%, 20% and 40%, 20% and 25%, or 25% and
40%
by weight palmitic acid.
Optionally, the total fatty acids comprise 0.1% to 0.5% or 0.001% to 0.5% by
weight
heptadecanoic acid.
5
CA 03172997 2022- 9- 22
WO 2021/199009
PCT/IB2021/052801
Optionally, the fatty acids in the biomass or isolated therefrom comprise less
than 3%
by weight eicosapentaenoic acid, less than 5% by weight pentadecanoic acid,
and less than
45% saturated fatty acids.
Optionally, the fatty acids in the biomass or isolated therefrom comprise
0.001% to
2.0%, 0.01% to 2.0%, or 0.05% to 2.0% arachidonic acid, less than 5% by weight
myristic
acid, and less than 40% by weight palmitic acid.
Also provided are microbial biomasses comprising oil. The microbial biomass
comprises between 40 to 75% total fatty acids by weight of the total biomass.
Optionally, the
oil in the biomass comprises DHA and the biomass comprises 20% to 55% DHA by
weight
of the biomass. Optionally, the oil in the biomass comprises between 2.5% to
8% DPA by
weight of the biomass. Optionally, the oil contains between 4% to 8% DPA by
weight of the
biomass. Optionally, the oil contains between DPA 2.5% to 4% by weight of the
biomass.
Eukaryotic microorganisms useful for producing the provided microbial oils and
biomasses include, but are not limited to, microorganisms selected from the
genus
Oblongichytrium, Aurantiochytrium, Thraustochytrium, Schizochytrium, and
Ulkenia or any
mixture thereof Optionally, the oil-producing eukaryotic microorganisms are
microorganisms with an 18S sequence with at least 97%, 98%, 99% or 100%
identity to the
nucleic acid sequence set forth in SEQ ID NO: 1. Optionally, the oil-producing
microorganisms are microorganisms of the strain Aurantiochytriuin limacinum.
Optionally,
the eukaryotic microorganism is the same as the microorganism deposited with
the
International Depositary Authority of Canada (IDAC), National Microbiology
Laboratory,
Public Health Agency of Canada, 1015 Arlington Street, Winnipeg, Manitoba
Canada R3E
3R2, on July 22, 2016, having IDAC assigned Accession No. 220716-01. This
deposit will
be maintained under the terms of the Budapest Treaty on the International
Recognition of the
Deposit of Microorganisms for the Purposes of Patent Procedure. This deposit
is exemplary
and was made merely as a convenience for those of skill in the art and is not
an admission
that a deposit is required for patentability. The terms "G3," "G3-1" or "G3-1
strain" or
"strain G3-1" are used herein interchangeably to refer to the eukaryotic
microorganism
having IDAC Accession No. 220716-01
Nucleic acid, as used herein, refers to deoxyribonucleotides or
ribonucleotides and
polymers and complements thereof. The term includes deoxyribonucleotides or
ribonucleotides in either single- or double-stranded form. The term
encompasses nucleic
acids containing known nucleotide analogs or modified backbone residues or
linkages, which
are synthetic, naturally occurring, and non-naturally occurring, which have
similar binding
6
CA 03172997 2022- 9- 22
WO 2021/199009
PCT/IB2021/052801
properties as the reference nucleic acid, and which are metabolized in a
manner similar to the
reference nucleotides. Examples of such analogs include, without limitation,
phosphorothioates, phosphorami dates, methyl phosphonates, chiral-methyl
phosphonates, 2-
0-methyl ribonucleotides, and peptide-nucleic acids (PNAs). Unless otherwise
indicated,
conservatively modified variants of nucleic acid sequences (e.g., degenerate
codon
substitutions) and complementary sequences can be used in place of a
particular nucleic acid
sequence recited herein. Specifically, degenerate codon substitutions may be
achieved by
generating sequences in which the third position of one or more selected (or
all) codons is
substituted with mixed-base and/or deoxyinosine residues (Batzer et al.,
Nucleic Acid Res.
19:5081 (1991); Ohtsuka et al., J. Biol. Chem. 260:2605-2608 (1985); Rossolini
et al., Mol.
Cell. Probes 8:91-98 (1994)). The term nucleic acid is used interchangeably
with gene,
cDNA, mRNA, oligonucleotide, and polynucleotide
The terms identical or percent identity, in the context of two or more nucleic
acids
or polypeptide sequences, refer to two or more sequences or subsequences that
are the same
or have a specified percentage of amino acid residues or nucleotides that are
the same (i.e.,
about 60% identity, preferably 65%, 70%, 75%, 80%, 85%, 90%, 91%, 92%, 93%,
94%,
95%, 96%, 97%, 98%, 99%, or higher identity over a specified region, when
compared and
aligned for maximum correspondence over a comparison window or designated
region) as
measured using a BLAST or BLAST 2.0 sequence comparison algorithms with
default
parameters described below, or by manual alignment and visual inspection (see,
e.g., NCBI
web site or the like). Such sequences are then said to be substantially
identical. This
definition also refers to, or may be applied to, the compliment of a test
sequence. The
definition also includes sequences that have deletions and/or additions, as
well as those that
have substitutions. As described below, the preferred algorithms can account
for gaps and
the like. Preferably, identity exists over a region that is at least about 25
amino acids or
nucleotides in length, or more preferably over a region that is 50-100 amino
acids or
nucleotides in length.
For sequence comparison, typically one sequence acts as a reference sequence
to
which test sequences are compared. When using a sequence comparison algorithm,
test and
reference sequences are entered into a computer, subsequence coordinates are
designated, if
necessary, and sequence algorithm program parameters are designated.
Preferably, default
program parameters can be used, or alternative parameters can be designated as
appropriate.
The sequence comparison algorithm then calculates the percent sequence
identities for the
test sequences relative to the reference sequence based on the program
parameters.
7
CA 03172997 2022- 9- 22
WO 2021/199009
PCT/IB2021/052801
A comparison window, as used herein, includes reference to a segment of any
one
of the number of contiguous positions selected from the group consisting of
from 20 to 600,
usually about 50 to about 200, more usually about 100 to about 150 in which a
sequence may
be compared to a reference sequence of the same number of contiguous positions
after the
two sequences are optimally aligned. Methods of alignment of sequences for
comparison are
well-known in the art. Optimal alignment of sequences for comparison can be
conducted,
e.g., by the local homology algorithm of Smith & Waterman, Adv. Appl. Math.
2:482 (1981);
by the homology alignment algorithm of Needleman & Wunsch, J. Mol. Biol.
48:443 (1970);
by the search for similarity method of Pearson & Lipman, Proc. Nat'l. Acad.
Sci. USA
85:2444 (1988); by computerized implementations of these algorithms (GAP,
BESTFIT,
FASTA, and TFASTA in the Wisconsin Genetics Software Package, Genetics
Computer
Group, 575 Science Dr., Madison, WI); or by manual alignment and visual
inspection (see,
e g , Current Protocols in Molecular Biology (Ausubel et al., eds. 1995
supplement))
A preferred example of an algorithm that is suitable for determining percent
sequence identity and sequence similarity are the BLAST and BLAST 2.0
algorithms, which
are described in Altschul et al., Nuc. Acids Res. 25:3389-3402 (1977), and
Altschul et al., J.
Mol. Biol. 215:403-410 (1990), respectively. BLAST and BLAST 2.0 are used,
with the
parameters described herein, to determine percent sequence identity for
nucleic acids or
proteins. Software for performing BLAST analyses is publicly available through
the
National Center for Biotechnology Information, as known in the art. This
algorithm involves
first identifying high scoring sequence pairs (HSPs) by identifying short
words of a selected
length (W) in the query sequence, which either match or satisfy some positive-
valued
threshold score T when aligned with a word of the same length in a database
sequence. T is
referred to as the neighborhood word score threshold (Altschul et al.). These
initial
neighborhood word hits act as seeds for initiating searches to find longer
HSPs containing
them. The word hits are extended in both directions along each sequence for as
far as the
cumulative alignment score can be increased. Cumulative scores are calculated
using, for
nucleotide sequences, the parameters M (reward score for a pair of matching
residues; always
> 0) and N (penalty score for mismatching residues; always < 0). For amino
acid sequences,
a scoring matrix is used to calculate the cumulative score. Extension of the
word hits in each
direction are halted when: the cumulative alignment score falls off by the
quantity X from its
maximum achieved value; the cumulative score goes to zero or below, due to the
accumulation of one or more negative-scoring residue alignments; or the end of
either
sequence is reached. The BLAST algorithm parameters W, T, and X determine the
8
CA 03172997 2022- 9- 22
WO 2021/199009
PCT/IB2021/052801
sensitivity and speed of the alignment. The Expectation value (E) represents
the number of
different alignments with scores equivalent to or better than what is expected
to occur in a
database search by chance. The BLASTN program (for nucleotide sequences) uses
as
defaults a wordlength (W) of 11, an expectation (E) of 10, M=5, N=-4 and a
comparison of
both strands. For amino acid sequences, the BLASTP program uses as defaults a
wordlength
of 3, expectation (E) of 10, and the BLOSUM62 scoring matrix (see Henikoff &
Henikoff,
Proc. Natl. Acad. Sci. USA 89:10915 (1989)), alignments (B) of 50, expectation
(E) of 10,
M=5, N=-4, and a comparison of both strands.
Provided herein are methods of making biomass using oil-producing
microorganisms. Specifically, provided is a method of making a biomass
comprising
culturing an oil-producing Aurantiochytrium microorganism in a culture medium
comprising
a fatty acid synthesis inhibitor, wherein the biomass comprises at least 350,
400, 450 or 500
mg/g oil The biomass can be used for making an oil having the characteristics
described
herein. The method further comprises isolating the oil from the biomass.
The culturing step can be carried out at a temperature of 15 C to 28 C (e.g.,
15, 16,
17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 or 28 C). Optionally, the culture
medium is at a
temperature of 18 C to 22 C, 22 C to 28 C, 22 C to 25 C, or 25 C to 28 C.
The fatty acid synthesis inhibitor can be added to the culture medium before,
after or
concurrently with addition of the microorganisms to the culture medium.
Optionally, the
fatty acid synthesis inhibitor is fed to the culture medium either
continuously or
intermittently. Optionally, the method further comprises adding the
microorganisms to the
culture medium and then adding the fatty acid synthesis inhibitor to the
culture medium at
least 6, 12, 24 or 48 hours after addition of the microorganisms to the
culture medium.
Optionally, the method further comprises adding the microorganisms to the
culture medium
and then adding the fatty acid synthesis inhibitor to the culture medium
between 24 to 48
hours after addition of the microorganisms to the culture medium. Optionally,
the method
further comprises adding the microorganisms to the culture medium and then
adding the fatty
acid synthesis inhibitor to the culture medium between 6 to 24 hours after
addition of the
microorganisms to the culture medium. Optionally, the method further comprises
adding the
microorganisms to the culture medium and then adding the fatty acid synthesis
inhibitor to
the culture medium between 12 to 24 hours after addition of the microorganisms
to the
culture medium.
The fatty acid synthesis inhibitor can be added to the culture medium in one
or more
doses. Optionally, the method further comprises adding the microorganisms to
the culture
9
CA 03172997 2022- 9- 22
WO 2021/199009
PCT/IB2021/052801
medium and the culturing comprises adding the fatty acid synthesis inhibitor
in 1, 2, 3, 4, 5,
or 6 doses, for example, beginning 6, 12, 24, or 48 hours after addition of
the microorganisms
to the culture medium. Optionally, the one or more doses are added every 6,
12, or 24 hours.
By way of example, 3 doses of the fatty acid inhibitor can be added to the
culture medium
every 12 hours starting 24 hours after the microorganisms are added to the
culture medium.
The total amount of fatty acid synthesis inhibitor added to the culture medium
comprises between 3 M and 40 M. Optionally, the fatty acid synthesis
inhibitor is
administered in one or multiple doses (e.g., 2, 3, 4, 5, 6, 7, 8, 9, or 10
doses) amounting to a
total of inhibitor between 3 M and 40 M. For example, the fatty acid
synthesis inhibitor
can be added in 6 doses of 2 M amounting to a total of 12 MM. By way of other
examples,
the fatty acid synthesis inhibitor can be added 3 times at doses of 1, 2, 3,
or 8 M.
Optionally, the fatty acid synthesis inhibitor can be added 6 times at doses
of 0.5, 1, 2, 3, and
4 M Optionally, the total concentration of the fatty acid synthesis inhibitor
added to the
culture medium during the culturing comprises between 30 g and 700 jig of
inhibitor per
gram of biomass. Optionally, the fatty acid synthesis inhibitor is
administered in 1, 2, 3, 4, 5,
6, 7, 8, 9, or 10 doses amounting to between 30 g and 700 g per gram of
biomass. By way
of example, the fatty acid synthesis inhibitor can be added in 6 doses of 40
g per gram of
biomass amounting to a total of 240 [lg. By way of other examples, the fatty
acid synthesis
inhibitor can be added 3 times at doses of approximately 12, 24, 42, and 120
g per gram of
biomass. Optionally, the fatty acid synthesis inhibitor can be added 6 times
at doses of 5.5,
11, 25, 40 and 62 g per gram of biomass.
Suitable fatty acid synthesis inhibitors include, but are not limited to fatty
acid
synthase inhibitors. Optionally, the fatty acid synthase inhibitor is selected
from the group
consisting of quercetin, a-mangostin, thiolactomycin, triclosan, isoniazid,
decynoyl-N-
acetylcysteamine (NAC), and cerulenin.
As described above, eukaryotic microorganisms useful for the provided methods
include, but are not limited to, microorganisms selected from the genus
Oblongichytrium,
Aurantiochytriwn, Thraustochytriutn, Schizochytrium, and Ellkenia or any
mixture thereof
Optionally, the oil-producing eukaryotic microorganisms are microorganisms
with an 18S
sequence with at least 97%, 98%, 99% or 100% identity to the sequence set
forth in SEQ ID
NO:l. Optionally, the eukaryotic microorganism has IDAC Accession No. 220716-
01.
As described herein, the biomass comprises fatty acids, e.g., fatty acids
comprising at
least at least 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99%
triglycerides as
CA 03172997 2022- 9- 22
WO 2021/199009
PCT/IB2021/052801
described above. Oils in the biomass also contain at least 85%, total fatty
acids by weight as
described above.
Optionally, the fatty acids isolated from the biomass comprise at least 50%
DHA by
weight as described above.
The fatty acids in the biomass optionally comprise between 6% to 18% DPA by
weight as described above. Optionally, the ratio of DHA to DPA in the fatty
acids, the oil in
the biomass, or isolated therefrom is less than or equal to 7:1 or 6:1 as
described above.
The oil in the biomass or isolated therefrom optionally contains less than 1%
by
weight stearic acid as described above.
Optionally, the fatty acids in the biomass or isolated therefrom comprise less
than 3%
by weight eicosapentaenoic acid, less than 5% by weight pentadecanoic acid,
and less than
45% saturated fatty acids as described above.
Optionally, the fatty acids in the biomass or isolated therefrom comprise
0.001% to
2.0% or 0.01% to 2.0% or 0.05% to 2.0%, arachidonic acid, less than 5% by
weight myristic
acid, and less than 40% by weight palmitic acid. Optionally, the fatty acids
comprise 0.1% to
0.5% or 0.001% to 0.5% by weight heptadecanoic acid.
Culture medium as used in the described methods supplies various nutritional
components, including a carbon source and a nitrogen source, for the
microorganisms.
Medium for culture can include any of a variety of carbon sources. Examples of
carbon
sources include fatty acids, lipids, glycerols, triglycerols, carbohydrates,
polyols, amino
sugars, and any kind of biomass or waste stream. Fatty acids include, for
example, oleic acid.
Carbohydrates include, but are not limited to, glucose, cellulose,
hemicellulose, fructose,
dextrose, xylose, lactulose, galactose, maltotriose, maltose, lactose,
glycogen, gelatin, starch
(corn or wheat), acetate, m-inositol (e.g., derived from corn steep liquor),
galacturonic acid
(e.g., derived from pectin), L-fucose (e.g., derived from galactose),
gentiobiose, glucosamine,
alpha-D-glucose-1-phosphate (e.g., derived from glucose), cellobiose, dextrin,
alpha-
cyclodextrin (e.g., derived from starch), and sucrose (e.g., from molasses).
Polyols include,
but are not limited to, maltitol, erythritol, and adonitol. Amino sugars
include, but are not
limited to, N-acetyl-D-galactosamine, N-acetyl-D-glucosamine, and N-acetyl-
beta-D-
mannosamine. The carbon source can be present in the heterotrophic medium at a
concentration of 200 g/L, 175 g/L, 150 g/L, 100 g/L, 60 g/L or less, e.g., at
a concentration of
1 to 200 g/L, 5 to 200 g/L, 10 to 200 g/L, 50 to 200 g/L, or 100 to 200 g/L.
The microorganisms can be cultured in medium having a chloride concentration
from about 0.5 g/L to about 50.0 g/L (e.g., a chloride concentration from
about 0.5 g/L to
11
CA 03172997 2022- 9- 22
WO 2021/199009
PCT/IB2021/052801
about 35 g/L, from about 18 g/L to about 35 g/L, or from about 2 g/L to about
35 g/L). The
microorganisms described herein can be grown in low chloride conditions, e.g.,
from about
0.5 g/L to about 20 g/L, or from about 0.5 g/L to about 15 g/L.
The culture medium optionally includes NaCl. The culture medium can include
non-chloride-containing sodium salts as a source of sodium. Examples of non-
chloride
sodium salts suitable for use in accordance with the present methods include,
but are not
limited to, soda ash (a mixture of sodium carbonate and sodium oxide), sodium
carbonate,
sodium bicarbonate, sodium sulfate, and mixtures thereof See, e.g., U.S. Pat.
Nos. 5,340,742
and 6,607,900, the entire contents of each of which are incorporated by
reference herein.
Optionally, the medium comprises 9 g/L chloride when using 20 g/L of carbon,
20 g/L soy
peptone, and 5 g/L yeast extract. The medium can comprise 35 g/L chloride when
the
medium contains 10 g/L carbon, 5 g/L soy peptone, 5 g/L yeast extract and 10
g/L agar. The
medium can comprise 2 g/L chloride when the medium contains 20-40 g/L carbon,
1 g/L
yeast extract, 1-20 g/L monosodium glutamate (MSG), 0.3-2.0 g/L phosphates, 4
g/L
magnesium sulfate, 5-10 g/L ammonium sulfate, 1.5 mL/L trace elements
solution, 1 mL/L of
vitamin B solution, and 0.1 g/L CaCl2.
Medium for a microorganism culture can include any of a variety of nitrogen
sources.
Exemplary nitrogen sources include ammonium solutions (e.g., NH4 in H2O),
ammonium or
amine salts (e.g., (NH4)2SO4, (NH4)31304, NH4NO3, NH400CH2CH3 (NH4Ac)),
peptone, soy
peptone, tryptone, yeast extract, malt extract, fish meal, sodium glutamate,
soy extract,
casamino acids and distiller grains. Concentrations of nitrogen sources in
suitable medium
typically range between and including about 1 g/L and about 25 g/L (e.g.,
about 5 to 20 g/L,
about 10 to 15 g/L, or about 20 g/L). Optionally, the concentration of
nitrogen is about 10 to
15 g/L when yeast extract is the source of complex nitrogen in the medium.
Optionally, the
concentration of nitrogen is about 1 to 5 g/L when soy peptone is in the
medium along with
L-Glutamic acid monosodium salt hydrate (MSG) or ammonium sulfate.
The medium optionally includes a phosphate, such as potassium phosphate or
sodium-
phosphate (e.g., potassium phosphate monobasic).
Inorganic salts and trace nutrients in the medium can include ammonium
sulfate,
sodium bicarbonate, sodium orthovanadate, potassium chromate, sodium
molybdate, selenous
acid, nickel sulfate, copper sulfate, zinc sulfate, cobalt chloride, iron
chloride, manganese
chloride calcium chloride, and EDTA. Optionally, the medium includes at least
1.5 ml/L of a
trace element solution. Optionally, the trace element solution comprises 2
mg/mL copper (II)
sulfate pentahydrate, 2 mg/mL zinc sulfate heptahydrate, 1 mg/mL cobalt (II)
chloride
12
CA 03172997 2022- 9- 22
WO 2021/199009
PCT/IB2021/052801
hexahydrate, 1 mg/mL manganese (II) chloride tetrahydrate, 1 mg/mL sodium
molybdate
dihydrate, and 1 mg/mL nickel (II) sulfate.
The medium can include magnesium sulfate, optionally, with a trace element
solution
and/or potassium phosphate monobasic.
Vitamins such as pyridoxine hydrochloride, thiamine hydrochloride, calcium
pantothenate, p-aminobenzoic acid, riboflavin, nicotinic acid, biotin, folic
acid and vitamin
B12 can be included in the culture medium.
The pH of the medium can be adjusted to between and including 3.0 and 10.0
using
acid or base, where appropriate, and/or using the nitrogen source. Optionally,
the medium is
sterilized.
Generally a medium used for culture of a microorganism is a liquid medium.
However, the medium used for culture of a microorganism can be a solid medium.
In
addition to carbon and nitrogen sources as discussed herein, a solid medium
can contain one
or more components (e.g., agar and/or agarose) that provide structural support
and/or allow
the medium to be in solid form.
Cultivation of the microorganisms can be carried out using known conditions,
for
example, those described in International Publication Nos. WO 2007/069078 and
WO
2008/129358. For example, cultivation can be carried out for 1 to 30 days
(e.g., 1 to 21 days,
1 to 15 days, 1 to 12 days, 1 to 9 days, or 3 to 5 days). Cultivation can be
carried out at
temperatures between 4 to 30 C. Optionally, cultivation is carried out by
aeration-shaking
culture, shaking culture, stationary culture, batch culture, fed-batch
culture, continuous
culture, rolling batch culture, wave culture, or the like. Optionally,
cultivation is carried out
with a dissolved oxygen content of the culture medium between 1 and 20%,
between 1 and
10% , or between 1 and 5%.
The biomass as described herein can be incorporated into a final product
(e.g., food or
feed supplement, biofuel, etc.). Thus, provided is a method of using the
protein-rich biomass.
The method optionally includes incorporating the protein-rich biomass into a
foodstuff (e.g.,
a pet food, a livestock feed, or an aquaculture feed).
Oils or lipids can be isolated from the described microorganism culture and
used in
various food and feed supplements. Suitable food or feed supplements into
which the oils
can be incorporated include beverages such as milk, water, sports drinks,
energy drinks, teas,
and juices; confections such as candies, jellies, and biscuits; fat-containing
foods and
beverages such as dairy products; processed food products such as soft rice
(or porridge);
infant formulae; breakfast cereals; or the like. Optionally, one or more
produced lipids can
13
CA 03172997 2022- 9- 22
WO 2021/199009
PCT/IB2021/052801
be incorporated into a dietary supplement, such as, for example, a vitamin or
multivitamin.
Optionally, an oil produced according to the method described herein can be
included in a
dietary supplement and optionally can be directly incorporated into a
component of food or
feed (e.g., a food supplement).
Examples of feedstuffs into which oils or lipids produced by the methods
described
herein can be incorporated include pet foods such as cat foods; dog foods;
feeds for aquarium
fish, cultured fish or crustaceans, etc.; or feed for farm-raised animals
(including livestock
and fish or crustaceans raised in aquaculture). Food or feed material into
which the oils or
lipids produced according to the methods described herein can be incorporated
is preferably
palatable to the organism which is the intended recipient. This food or feed
material can have
any physical properties currently known for a food material (e.g., solid,
liquid, soft).
Optionally, one or more of the produced compounds (e.g., PUFAs) can be
incorporated into a nutraceutical or pharmaceutical product Examples of such a
nutraceutical or pharmaceutical forms include various types of tablets,
capsules, drinkable
agents, etc. Optionally, the nutraceutical or pharmaceutical is suitable for
topical application
(e.g., lotion form). Dosage forms can include, for example, capsules, oils,
tablets or the like.
The oil or lipids produced according to the methods described herein can be
incorporated into products in combination with any of a variety of other
agents. For instance,
such compounds can be combined with one or more binders or fillers, chelating
agents,
pigments, salts, surfactants, moisturizers, viscosity modifiers, thickeners,
emollients,
fragrances, preservatives, etc., or any combination thereof.
Disclosed are materials, compositions, and components that can be used for,
can be
used in conjunction with, can be used in preparation for, or are products of
the disclosed
methods and compositions. These and other materials are disclosed herein, and
it is
understood that when combinations, subsets, interactions, groups, etc. of
these materials are
disclosed that while specific reference of each various individual and
collective combinations
and permutations of these compounds may not be explicitly disclosed, each is
specifically
contemplated and described herein. For example, if a method is disclosed and
discussed and
a number of modifications that can be made to a number of molecules including
the method
are discussed, each and every combination and permutation of the method, and
the
modifications that are possible are specifically contemplated unless
specifically indicated to
the contrary. Likewise, any subset or combination of these is also
specifically contemplated
and disclosed. This concept applies to all aspects of this disclosure
including, but not limited
to, steps in methods using the disclosed compositions. Thus, if there are a
variety of
14
CA 03172997 2022- 9- 22
WO 2021/199009
PCT/IB2021/052801
additional steps that can be performed, it is understood that each of these
additional steps can
be performed with any specific method steps or combination of method steps of
the disclosed
methods, and that each such combination or subset of combinations is
specifically
contemplated and should be considered disclosed.
Publications cited herein and the material for which they are cited are hereby
specifically incorporated by reference in their entireties.
The examples below are intended to further illustrate certain aspects of the
methods
and compositions described herein, and are not intended to limit the scope of
the claims.
Examples
Example 1. Fatty Acid Synthesis Inhibitor Dose Timing Strategy.
To determine the ideal time for addition of the fatty acid synthase inhibitor
cerulenin
without excessive harm such as preventing cell growth, experiments were
conducted by
adding a set amount of cerulenin at various amounts of time after inoculation.
In all
experiments, the control experiment was performed with the same medium and
growth
conditions without the addition of FAS inhibitors. The results demonstrated
that cell growth
was not inhibited at any addition time, however it did have a significant
impact on the fatty
acid profile (Figure 1). Table 2 shows that adding cerulenin 24 hours after
inoculation
resulted in higher DHA and DPA content (% of TFA) with fatty acid synthesis
pathway
products C14:0 and C16:0 reduced by 21.1% and 27.9% respectively.
Table 1. Fatty acid content (in mg/g) of predominant fatty acids in
Aurantiochytrium sp. (G3)
at 20 C (except 48hr). Addition times represent the time of addition of 25 M
cerulenin after
inoculation.
Fatty Acid Content (mg/g of dry biomass)
Time of C14:0 C16:0 C17:0 C20:5 C22:5 C22:6
SFA TFA DHA:
addition (n-3) (n-6) (n-3)
DPA
Control 25.50 230.24 3.07 3.10 35.26 277.28 280.75 619.90 7.98
hr 23.45 183.73 4.07 1.97 45.07 276.37 232
31 579.14 6.13
24 hr 18.23 150.17 4.36 1.90 55.95 287.63
204.48 560.24 5.14
48 hr* 29.55 216.10 2.79 1.97 62.60 284.99
272.20 641.86 4.58
* From 25 C data
CA 03172997 2022- 9- 22
WO 2021/199009
PCT/IB2021/052801
Table 2. Fatty acid content (relative to total fatty acids) of predominant
fatty acids in
Aurantiochytrium sp. (G3) at 20 C (except 48hr). Addition times represent the
time of
addition of 2504 cerulenin after inoculation
Fatty Acid Content (% of TFA)
Time of C22:5 (n- C22:6
(n- SFA
C14:0 C16:0 C17:0 C20:5 (n-3)
addition 6) 3)
Control 4.12 37.16 0.50 0.50 5.67 44.71
45.32
0 hr 4.05 31.73 0.70 0.34 7.78 47.72
40.11
24 hr 3.25 26.80 0.76 0.33 9.99 51.34
35.35
48 hr* 4.62 33.62 0.44 0.31 9.73 44.42
42.39
* From 25 C data
Example 2. Fatty Acid Synthesis Inhibitor Concentration.
To determine the optimum concentration of cerulenin to provide the best
results, a
range of concentrations from 1p.M to 4404 were tested. For consistency, all
cerulenin
additions were made at 24 hours after inoculation. Figure 2 shows the fatty
acid profiles of
Aurantiochytrium sp. (G3) in varying concentrations of cerulenin at 20 C.
Table 5 shows
that increasing cerulenin concentration has a clear impact on the amount of
C16:0 and DPA
accumulated in the cells. In terms of percentage of the total fatty acids,
those changes in
specific fatty acids correspond to an increase in DHA content (% of TFA) from
44.71% to as
high as 50.92% when 25 M of cerulenin is used (Table 4).
Table 3. Fatty acid content (mg/g) of predominant fatty acids in
Aurantiochylrium sp. (G3) at
C with cerulenin added at 24 hours after inoculation.
Fatty Acid Content (mg/g of dry biomass)
Cerulenin C14:0 C16:0 C17:0 C20:5 C22:5 C22:6 SFA TFA DHA:
(n-3) (n-6) (n-3)
DPA
Control 25.50 230.24 2.93 2.69 35.26 277.28 280.75 619.90 7.98
littM 28.38 211.50 3.26 3.60 29.32 256.10
265.63 580.28 8.73
2ittM 26.95 196.70 3.15 3.58 31.82 270.39
248.72 580.90 8.50
31tM
24.49 216.49 2_95 2.15 47.79 323.48 265.09 658.72 6.77
6ittM 24.72 204.08 3.22 2.74 43.70 300.35
252.79 619.88 6.87
9ittM 25.94 181.97 3.48 2.49 47.27 296.00
233.91 603.79 6.26
25uM 19.16 160.27 4.36 1.90 55.84 294.31
204.48 578.10 5.27
401t1VI 21.71 138.29 4.94 1.87 59.05 268.00
188.40 540.11 4.54
16
CA 03172997 2022- 9- 22
WO 2021/199009
PCT/IB2021/052801
Table 4. Fatty acid content (relative to total fatty acids) of predominant
fatty acids in
Aurantiochytrium sp. (G3) at 20 C with cerulenin added at 24 hours after
inoculation.
Fatty Acid Content (/0 of TFA)
Cerulenin C22:5 (n- C22:6
(n-
C14:0 C16:0 C17:0 C20:5 (n-3)
SFA
6) 3)
Control 4.12 37.16 0.50 0.50 5.67 44.71
45.32
1 M 4.89 36.45 0.56 0.62 5.05 44.13
45.78
2ittM 4.64 33.86 0.54 0.62 5.48 46.55
42.82
3 M 3.72 32.87 0.45 0.33 7.26 49.11
40.24
6juLM 3.99 32.92 0.52 0.44 7.05 48.45
40.78
911M 4.30 30.14 0.58 0.41 7.83 49.02
38.74
25AM 3.31 27.70 0.76 0.33 9.67 50.92
35.35
40AM 4.02 25.60 0.91 0.35 10.93 49.62
34.88
Example 3. Fatty Acid Synthesis Inhibitor Dosing Regimen.
To investigate the effect of prolonged, repeated exposure to cerulenin, doses
were
spread equally across 3 or 6 times during the experiment. In each case, the
total amount
added was equivalent to the corresponding treatments that were added entirely
at 24 hours.
All additions began at 24 hours after inoculation. In Figures 3 and 4, a clear
trend of
increasing DHA and DPA was visible with both increasing cerulenin
concentration and by
dosing the cerulenin in multiple equivalent pulses. Furthermore, Table 5 shows
no negative
impact on total fatty acids when repeated dose addition is employed, while the
actual amount
of DHA (in mg/g) was increasing with increasing cerulenin. The highest DHA
observed was
using 6 equivalent doses of cerulenin, amounting to 12 M in total, resulting
in a DHA
increase of 51.6%. There was also a maximal decrease in C14:0 and C16:0 of
85.1% and
83.9%, suggesting very effective inhibition of the FAS pathway by cerulenin
under these
conditions.
Table 5. Fatty acid content (in mg/g) of Aurantiochytriurn sp. (G3) in various
cerulenin
dosing strategies at 20 C. Treatments indicating 3x and 6x are multiple
additions of cerulenin
at equally-spaced time intervals starting at 24 hours after inoculation. For
the 3x addition
experiment, the interval was 24 hours; while for the 6x addition experiment,
the interval was
12 hours.
17
CA 03172997 2022- 9- 22
WO 2021/199009
PCT/IB2021/052801
Fatty Acid Content (mg/g of dry biomass)
Cerulenin C14:0 C16:0 C17:0 C20:5 C22:5 C22:6 SFA TFA DHA:
(n-3) (n-6) (n-3)
DPA
Control 25.50 230.24 2.93 2.69 35.26 277.28
281.54 619.90 7.98
3uM
24.49 216.49 2.95 2.15 47.79 323.48 265.09 658.72 6.77
6uM
24.72 204.08 3.22 2.74 43.70 300.35 252.79 619.88 6.87
9uM 25.94 181.97 3.48 2.49 47.27 296.00
233.91 603.79 6.26
25uM 20.09 170.38 4.36 1.90 55.73 301.00
204.48 595.96 5.40
3x1uM 19.42 167.92 2.66 2.35 52.40 349.67 210.60 636.22 6.67
3x2uM 19.58 157.98 3.14 2.54 54.90 334.67
201.30 614.40 6.10
3x3uM 13.28 131.13 3.40 2.34 55.54 349.04
168.66 597.67 6.28
3x8.3uM 9.92 117.45 3.87 1.55 78.58 373.12
151.53 617.52 4.75
6x0.5uM 10.99 90.92 3.28 2.43 70.48 398.73
124.07 616.79 5.66
6x1uM 7.52 70.33 3.50 2.19 81.79 418.90
99.59 620.75 5.12
6x2uM 5.52 53.01 3.94 1.89 85.07 398.90
81.59 585.23 4.69
6x3uM 4.52 41.96 3.96 1.63 87.15 364.99
69.39 540.81 4.19
6x4.17uM 3.79 37.13 4.14 1.56 92.73 363.03
64.00 538.56 3.91
Table 6. Fatty acid content (relative to total fatty acids) of
Aurantiochytrium sp. (G3) in
various cerulenin dosing strategies at 20 C. Treatments indicating 3x and 6x
are multiple
additions of cerulenin at equally-spaced time intervals starting at 24 hours
after inoculation.
For the 3x addition experiment, the interval was 24 hours; while for the 6x
addition
experiment, the interval was 12 hours.
Fatty Acid Content (% of TFA)
Cerulenin C14:0 C16:0 C17:0 C20:5 (n-3) C22:5 (n-
C22:6 (n- SFA
6) 3)
Control 4.12 37.16 0.50 0.50 5.67 44.71
45.32
3uM 3.72 32.87 0.45 0.33 7.26 49.11
40.24
6uM 3.99 32.92 0.52 0.44 7.05 48.45
40.78
9uM 4.30 30.14 0.58 0.41 7.83 49.02
38.74
25uM 3.37 28.59 0.76 0.33 9.35 50.51
35.35
3x1uM 3.05 26.39 0.42 0.37 8.24 54.96
33.10
3x2uM 3.19 25.71 0.51 0.41 8.93 54.47
32.76
3x3uM 2.22 21.94 0.57 0.39 9.29 58.40
28.22
3x8.3uM 1.61 19.02 0.63 0.25 12.72 60.42
24.54
6x0.5uM 1.78 14.75 0.53 0.40 11.42 64.63
20.13
18
CA 03172997 2022- 9- 22
WO 2021/199009
PCT/IB2021/052801
6x1uM 1.21 11.34 0.56 0.35 13.17 67.47
16.06
6x2uM 0.94 9.06 0.67 0.32 14.53 68.16
13.95
6x3uM 0.84 7.76 0.73 0.30 16.11 67.49
12.82
6x4.17uM 0.70 6.88 077 0.29 17.23 67.39
11.88
Example 4. Fatty Acid Synthesis Inhibitor Effect on Product Yield.
To assess the impact of the herein described methods for PUFA enhancement
strategy, the yields of product (i.e. TFA, DHA or DPA) relative to the amount
of carbon
assimilated by the G3 cells was investigated. In Figure 5, the total amount of
product
synthesized (categorized as total fatty acids (TFA), saturated fatty acids
(SFA) or DHA) is
depicted. In the presence of fatty acid synthesis inhibitors, dosed at certain
frequency and
concentration, both TFA and DHA concentration increased while SFA
concentration
decreased. For example, under the condition where cerulenin was dosed 1 p.M
each time for 6
times at 12 hour intervals, TFA and DHA increased by 15% and 71%,
respectively, when
compared to the results of the control experiment (Figure 5). When the results
were
calculated as fatty acid yield (gram product/gram carbon consumed), DHA yield
generally
increased significantly while SFA yield reduced, under any cerulenin dosing
conditions. For
example, one of the optimal condition at 6x1 pM saw 53% increase in DHA yield
and 64%
reduction in SFA yield, when compared to the result of the control experiment,
thereby
suggesting that the cells were not only experiencing inhibition to the FAS
pathway but they
were up-regulating the PUFA synthase pathway to more efficiently utilize the
available
carbon.
Example 5. Effects of Temperature on DHA A. from Aurantiochytrium sp. (G3).
Flask scale experiments were executed to investigate the impact of decreasing
the
cultivation temperature (from 25 C to 20 C) on the fatty acid profile and the
productivity of
Aurantiochytrium sp. (G3). The data in Figure 6 illustrate the increasing
proportion of DHA
and DPA with a decreased culture temperature to 20 C. Tables 7 and 8 show
summary data
exhibiting an increase in DHA of 14.7% paired with a decrease in C14:0 and
C16:0 by 27.5%
and 10.6%, respectively.
19
CA 03172997 2022- 9- 22
WO 2021/199009
PCT/IB2021/052801
Table 7. Effect of different temperatures on predominant fatty acids (in mg/g)
and their
relationships present in Attrantiochytrium sp. (G3).
Fatty Acid Content (mg/g of dry biomass)
C14:0 C16:0 C17:0 C20:5 C22:5 C22:6 SFA TFA DHA:DPA
(n-3) (n-6) (n-3)
25 C 35.73 262.05 2.58 2.59 35.68 246.18
324.31 630.78 7.03
20 C 25.50 230.24 3.07 3.10 35.26 277.28
280.75 619.90 7.98
Table 8. Effect of different temperatures on predominant fatty acids (relative
to total fatty
acids) present in Aurantiochytrium sp. (G3).
Fatty Acid Content (% of TFA)
C14:0 C16:0 C17:0 C20:5 C22:5 C22:6
SFA
(n-3) (n-6) (n-3)
25 C 5.68 41.57 0.41 0.41 5.64 38.99
51.46
20 C 4.12 37.16 0.50 0.50 5.67 44.71
45.32
Example 6. Effects of Full-length Fermentation on DHA% from Aurantiochytrium
sp.
(G3).
Full-length fermentations of Aurantiochytrimn sp. G3 were carried out using
30L
stainless steel fermentors at varying temperatures (from 25 C to 20 C). These
fermentations
generally lasted from 150 hours to 200 hours with biomass and TFA reaching
over 130 g/L
and 55%, respectively. As can be seen in Figure 7, full-length fermentation at
a typical
temperature of 25 C was able to improve final DHA content over that from a
typical control
cultivation using flask; while full-length fermentations at lower temperatures
were able to
reach even higher DHA%. In the examples included in Figure 7, fermentation at
25 C
reached 45% DHA, about 8% higher than that of a flask control at 37%.
Fermentations at
22 C and 20 C achieved further DHA% increase at 59.7% and 60.0%, respectively.
However,
DHA content from the flask experiment using multiple and intermittent dosing
of cerulenin
was still the highest DHA% reached, at 68.7%.
Example 7. Effects of Elevated Temperature on Aurantiochytrium sp. (63).
G3 strains were cultured in 30-L fermenters for enhanced biomass and fatty
acid
production at 22 C, 25 C, and 28 C. G3 was pre-cultured in four Erlenmeyer
flasks
CA 03172997 2022- 9- 22
WO 2021/199009
PCT/IB2021/052801
containing 500 mL of basal media (50 g/L glucose, 6.25 g/L yeast extract, 4
g/L
MgSO4.7H20, 4 g/L, 2.5 g/L NaCl, 2 mg/L copper sulfate, 2 mg/L zinc sulfate, 1
mg/L
sodium molybdate, 1 mg/L cobalt (II) chloride, 1 mg/L manganese chloride and 1
mg/L
nickel sulfate). Flasks were incubated under agitation at 25 C and 200 rpm for
2 days. After
the incubation period, three of the flasks (1.5 L) were used to inoculate 20 L
of media in a 30
L bioreactor containing 175 g/L glucose, 9.66 g/L yeast extract, 2.57 g/L
MgSO4.7H20, 0.45
g/L sodium chloride, 6.44 g/L ammonium sulfate, 1.6 g/L potassium phosphate
monobasic,
1.74 g/L potassium phosphate dibasic, 12.87 g/L monosodium glutamate, 0.1 g/L
calcium
chloride dehydrate, 1 mg/L copper sulfate, 1 mg/L zinc sulfate, 0.5 mg/L
sodium molybdate,
0.5 mg/L cobalt (II) chloride, 0.5 mg/L manganese chloride, 0.5 mg/L nickel
sulfate, 0.03
mg/L vitamin 812, 0.03 mg/L biotin and 6 mg/L thiamin hydrochloride and
cultured in 30-L
fermenters under the conditions of 22 C, 25 C, and 28 C Agitation started at
325 rpm and
increased to 365 rpm, aeriation was maintained at 0.3 vvm with atmospheric
air, and pH 60.
pH was maintained by the addition of base (27% NH4OH). Vessels were fed to
maintain a
glucose consumption rate of approximately 3 g/L/h with a 750 g/L glucose
solution. Cells
were collected at various intervals and the biomass, TFA and lipid profiles
were measured.
Characteristics of the final G3 profiles at 25 and 28 C are shown in Table 9.
The lipid
profiles are shown in Tables 10, 11, and 12.
Table 9. G3 Final Results at 22, 25 and 28 C.
G3 at 22 C G3 at 25 C G3 at 28 C
Time, h 186.89 140.19 165.67
Biomass, g/L 121.83 107.92 104.16
TFA, % biomass 49.6 56.3 59.2
DHA, % TFA 66.78 64.1 58.6
SFA, % TFA 16.18 19 25
MUFA, % TFA 0.9 0.8
21
CA 03172997 2022- 9- 22
LO
Table 10. Fatty Acid Profile of G3 Cultured at 22 C.
ts.)
. ''''I5i 16;0 ... AI8:0
-201:4:111.:46J. .. = .C122.:604YI.DT44.'r.11
v:0
45h . 0.06 0.96 0.24 0.34 24.64 0.16 1.10
0.32 0.12 0.65 0,50 0.24 9.44 0.24 58.47
681i 0.08 1.35 0.29 0.24 21.17 0.23 0.86 0.39 0.14 0.65 0,38
0.16 10.25 0.16 61.51
901i 0.08 1.30 0.47 0.20 17.12 0.32 0.68 0.38 0.17 0.89 0,39
0.17 10.61 0.13 65.25
I 15It 0.08 1.17 0.63 0.12 14.17 0.38 0.53 0.37
0.16 1.08 0.42 0.14 10.91 0.12 68.07
I4IIfl 0.08 1.06 0.71 0.10 13.21 0.41 0.49 0.40
0.15 1.15 0.44 0.14 11.57 0.13 68.22
1.6311 0.08 1.11 0.66 0.10 13.71 0.40 0.51 0.41
0.15 1.08 0.46 0.14 11.80 0.14 67.50
t=.)
1871.1 0.08 1.11 0.62 0.09 14.32 0.37 0.52 0.43
0.15 1.02 0.47 0.15 12.02 0.15 66.69
7,1
NJ
LO
NJ
Table 11. Fatty Acid Profile of G3 Cultured at 25 C.
ts.)
6311 0.11 1.07 0.23 0.21 2093. 0.16 0.83 0.17
0.14 0.54 0.33 0.12 1202. 0.16 60.79
9211 " 0.15 1.29 0.35 0.17 19.66 0.22 0.78 0.19
0.16 0.62 0.29 0.17 12.00 0.12 60.78
1161W 0.08 1.19 0.49 0.12 16.18 0.25 0.59 0.19
0.15 0.81 0.33 0.12 12.94 0.12 64.60
.
1401t."] 0.08 1.22 0.43 0.11 16.85 0.25 0.56 0.21
0.13 0.75 0.35 0.10 12.99 0.14 64.13
:
Table 12. Fatty Acid Profile of G3 Cultured at 28 C.
LiPid"'42:u'NIT 14.P 15.() 16.0
20.4o1-6)- -203'ell.-3) EPA 120 12'7-(641-DPA:- 22.5e -; ) DPA 22
.RIPTID11A
631 0.10 1.24 0.22 0.21 19.28 0.16 0.71 0.13 0.13 0,50
0.32 0.12 13.66 0.19 60.76
921i 0.12 1.26 0.26 0.17 17.29 0.18 0.61 0,14 0,15 0,49
0,28 0,12 14,47 0,20 62,22
t!tt!t1--
11611 0,13 1,41 0,40 0,16 18,75 0,23 0,65 0,20
0,15 0,68 0,30 0,11 13,67 0,16 61,10
14011.- 0.08 1.60 0.39 0.15 21.05 0.25 0.67 0.23
0.12 0.74 0.33 0.10 13.02 0.15 59.36
166 0.09 1.66 0.31 0.14 22.10
0.23 0.66 0.22 0.10 0.75 0.44 0.08 12.84 0.16 58.55
"d
7,1
DdJI
:J
t=.)