Note: Descriptions are shown in the official language in which they were submitted.
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
USE OF BONE MORPHOGENETIC PROTEINS AND THEIR RECEPTORS FOR
AESTHETICS AND COSMETICS
CROSS REFERENCE TO RELATED APPLICATIONS
This application claims the benefit under 35 U.S.C. 119(e) of U.S.
Provisional
Application No. 63/078,174, filed September 14, 2020, entitled "USE OF BONE
MORPHOGENETIC PROTEINS AND THEIR RECEPTORS FOR AESTHETICS AND
COSMETICS," the entire disclosure of which is hereby incorporated by
reference.
REFERENCE TO A SEQUENCE LISTING SUBMITTED AS A TEXT FILE VIA
EFS-WEB
The instant application contains a Sequence Listing which has been submitted
in
ASCII format via EFS-Web and is hereby incorporated by reference in its
entirety. Said
ASCII copy, created on September 7, 2021, is named G091970069W000-SEQ-SXT, and
is
19,220 bytes in size.
FIELD OF THE INVENTION
The present disclosure relates to the use of bone morphogenetic proteins
(BMPs)
and/or proteins associated with BMPs and their cellular receptors in aesthetic
and cosmetic
applications such as enhancing or preserving desirable facial contours and/or
preventing or
reducing skin wrinkles and improving skin texture.
BACKGROUND
Anti-aging strategies have been widespread in the industries of dermatology
and
aesthetics for decades. Traditionally, plastic surgeries, invasive procedures,
and/or high
intensity chemical treatments have been implemented for reversing or delaying
the inevitable
signs of aging of the skin and improving attractive appearance. However, these
traditional
strategies can be cost prohibitive, intolerably invasive, and/or potentially
unsafe to human
subjects, especially given that multiple rounds of treatments and/or long-term
use are
frequently required.
1
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
SUMMARY
Aspects of the disclosure relate to methods of preventing or reducing skin
wrinkles or
improving skin texture or evenness of skin tone, comprising administering to a
subject in
need thereof an effective amount of a composition comprising one or more bone
morphogenetic proteins (BMPs), or variants or derivatives thereof.
Further aspects of the disclosure relate to methods of enhancing or preserving
facial
contours or improving the attractiveness of facial contours, comprising
administering to a
subject in need thereof an effective amount of a composition comprising one or
more bone
morphogenetic proteins (BMPs), or variants or derivatives thereof.
In some embodiments, the BMP or variant or derivative thereof, is selected
from the
group consisting of BMP2, BMP6, BMP7, and BMP9, or variants or derivatives
thereof. In
some embodiments, the BMP or variant or derivative thereof, is BMP2, or a
variant or
derivative thereof. In some embodiments, the BMP or variant or derivative
thereof, is BMP6,
or a variant or derivative thereof. In some embodiments, the BMP or variant or
derivative
thereof, is BMP7, or a variant or derivative thereof. In some embodiments, the
BMP or
variant or derivative thereof, is BMP9, or a variant or derivative thereof. In
some
embodiments, the composition comprises BMP2, BMP7, BMP6, and BMP9, or variants
or
derivatives thereof. In some embodiments, the composition further comprises
one or more
proteins associated with a BMP, or a variant or derivative thereof. In some
embodiments, the
protein associated with a BMP is Noggin, or a variant or derivative thereof.
In some
embodiments, the protein associated with a BMP is a BMP peptide, or a variant
or derivative
thereof. In some embodiments, the protein associated with a BMP is Activin, or
a variant or
derivative thereof.
In some embodiments, the subject is a human subject having, suspected of
having, or
at risk of developing, skin wrinkles. In some embodiments, the subject is a
human subject
who desires improving skin texture or evenness of skin tone. In some
embodiments, the skin
wrinkles are facial and/or neck wrinkles. In some embodiments, the subject is
a human
subject having, suspected of having, or at risk of having, loss of natural
facial contours. In
some embodiments, the subject is a human subject who desires a change in
facial contours to
improve attractiveness. In some embodiments, the subject is elderly. In some
embodiments,
the subject is not elderly.
In some embodiments, enhancing or preserving facial contours comprises
inhibiting
or reversing bone resorption. In some embodiments, the one or more BMPs, or
variants or
2
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
derivatives thereof, stimulates the formation of adipocytes in the skin. In
some embodiments,
the one or more BMPs, or variants or derivatives thereof, stimulates
dedifferentiation of
keratinocytes. In some embodiments, the one or more BMPs, or variants or
derivatives
thereof, reduces or modulates melanin formation by melanocytes. In some
embodiments, the
presence of Noggin, or a variant or derivative thereof, reduces potential side
effects of the
one or more BMPs, or variants or derivatives thereof.
In some embodiments, the composition is administered topically. In some
embodiments, the composition is administered in a cream or serum. In some
embodiments,
the composition is administered through subdermal injection. In some
embodiments, the
subdermal injection is near periosteum.
In some embodiments, the concentration of the one or more BMPs, or variants or
derivatives thereof, in the composition is less than about 3%. In some
embodiments, the
concentration of Noggin, or a variant or derivative thereof, in the
composition is less than
about 3%. In some embodiments, the concentration of the BMP peptide, or a
variant or
derivative thereof, in the composition is less than about 10%. In some
embodiments, the one
or more BMPs, or variants or derivatives thereof, are administered at a dose
of about 100-
1,000 ng/mL per administration site. In some embodiments, the method further
comprises
administering Noggin, or a variant or derivative thereof, at a dose of about
100-1,000 ng/mL
per administration site.
Further aspects of the disclosure relate to compositions comprising one or
more bone
morphogenetic proteins (BMPs), or variants or derivatives thereof, in a
pharmaceutically
acceptable carrier for use in preventing or reducing skin wrinkles or
improving skin texture or
evenness of skin tone.
Further aspects of the disclosure relate to compositions comprising one or
more bone
morphogenetic proteins (BMPs), or variants or derivatives thereof, in a
pharmaceutically
acceptable carrier for use in enhancing or preserving facial contours or
improving the
attractiveness of facial contours.
In some embodiments, the BMP, or variant or derivative thereof, is selected
from the
group consisting of BMP2, BMP7, and BMP9, or variants or derivatives thereof.
In some
embodiments, the BMP, or variant or derivative thereof, is BMP2, or a variant
or derivative
thereof. In some embodiments, the BMP, or variant or derivative thereof, is
BMP6, or a
variant or derivative thereof. In some embodiments, the BMP, or variant or
derivative
3
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
thereof, is BMP7, or a variant or derivative thereof. In some embodiments, the
BMP, or
variant or derivative thereof, is BMP9, or a variant or derivative thereof. In
some
embodiments, the composition comprises BMP2, BMP6, BMP7 and BMP9, or variants
or
derivatives thereof.
In some embodiments, the composition further comprises one or more proteins
associated with a BMP, or a variant or derivative thereof. In some
embodiments, the protein
associated with a BMP is Noggin, or a variant or derivative thereof. In some
embodiments,
the protein associated with BMP is a BMP peptide, or a variant or derivative
thereof. In
some embodiments, the protein associated with a BMP is Activin, or a variant
or derivative
thereof.
In some embodiments, the composition is a cream or serum. In some embodiments,
the concentration of the one or more BMPs, or variants or derivatives thereof,
in the
composition is less than about 3%. In some embodiments, the concentration of
Noggin, or a
variant or derivative thereof, in the composition is less than about 3%. In
some
embodiments, the concentration of the BMP peptide, or a variant or derivative
thereof, in the
composition is less than about 10%. In some embodiments, the composition is
formulated for
subdermal injection. In some embodiments, the composition further comprises a
dermal
filler. In some embodiments, the composition further comprises one or more
agents that
enhance penetration.
In some embodiments, the one or more BMPs, or variants or derivatives thereof,
are
recombinantly produced. In some embodiments, the one or more BMPs, or variants
or
derivatives thereof, are recombinantly produced in a mammalian cell. In some
embodiments,
the mammalian cell is a HEK 293 cell or a CHO cell. In some embodiments, the
one or more
BMPs, or variants or derivatives thereof, are recombinantly produced in a
yeast cell. In some
embodiments, the yeast cell is a Pichia pastoris cell. In some embodiments,
the one or more
BMPs, or variants or derivatives thereof, are recombinantly produced in an E.
coli cell.
Further aspects of the disclosure relate to kits comprising a composition
comprising
one or more bone morphogenetic proteins (BMPs), or variants or derivatives
thereof, and
instructions for use in administering the composition to prevent or reduce
skin wrinkles or to
improve skin texture or evenness of skin tone.
Further aspects of the disclosure relate to kits comprising a composition
comprising
one or more bone morphogenetic proteins (BMPs), or variants or derivatives
thereof, and
4
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
instructions for use in administering the composition to enhance or preserve
facial contours
or to improve the attractiveness of facial contours.
Each of the limitations of the invention can encompass various embodiments of
the
invention. It is, therefore, anticipated that each of the limitations of the
invention involving
any one element or combinations of elements can be included in each aspect of
the invention.
This invention is not limited in its application to the details of
construction and the
arrangement of components set forth in the following description or
illustrated in the
drawings. The invention is capable of other embodiments and of being practiced
or of being
carried out in various ways. Also, the phraseology and terminology used herein
is for the
purpose of description and should not be regarded as limiting. The use of
"including,"
"comprising," or "having," "containing," "involving," and variations of
thereof herein, is
meant to encompass the items listed thereafter and equivalents thereof as well
as additional
items. As used in this specification and the appended claims, the singular
forms "a," "an" and
"the" include plural referents unless the content clearly dictates otherwise.
BRIEF DESCRIPTION OF THE DRAWINGS
The following drawings form part of the present specification and are included
to
further demonstrate certain aspects of the present disclosure, which may be
better understood
by reference to one or more of these drawings in combination with the detailed
description of
specific embodiments presented herein. The accompanying drawings are not
intended to be
drawn to scale. The drawings are illustrative only and are not required for
enablement of the
disclosure. For purposes of clarity, not every component may be labeled in
every drawing. In
the drawings:
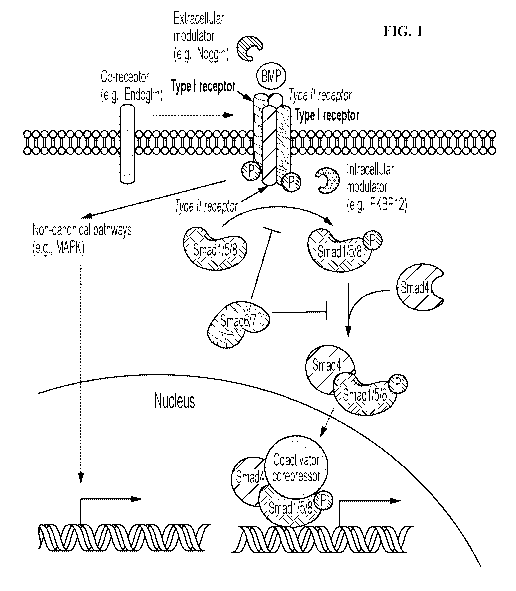
FIG. 1 shows a diagram depicting mechanisms of BMP signaling. The diagram is
adapted from Wang et al., Genes and Diseases, DOT:
10.1016/j.gendis.2014.07.005.
DETAILED DESCRIPTION OF THE INVENTION
This disclosure provides methods and compositions for the use of BMPs and/or
proteins associated with BMPs in aesthetic and cosmetic indications. For
example, BMPs
and/or proteins associated with BMPs described herein can be used for
preventing or
reducing skin wrinkles, improving skin texture or evenness of skin tone,
enhancing or
preserving facial contours, and/or improving the attractiveness of facial
contours. This
5
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
disclosure describes recombinant production of BMPs and/or proteins associated
with BMPs
in host cells and the use of recombinantly produced BMPs and/or proteins
associated with
BMPs for administration to human subjects for preventing or reversing various
signs of skin
aging. A strategy that uses BMPs and/or associated proteins and receptors may
provide an
advantageous approach to meet the increasing anti-aging demand in the
aesthetic and
cosmetic industries.
Bone Morphogenetic Proteins (BMPs) and Proteins Associated with BMPs
Bone morphogenetic proteins (BMPs) are a large subclass (more than 20 members)
of
the TGF-beta (Transforming Growth Factor-Beta) super family that is active in
many tissues
under normal physiologic conditions. BMPs are growth factors that are known as
cytokines
and as metabologens. They play a crucial role in bone and cartilage formation
as well as in
adult homeostasis of bone function. Initially discovered for their ability to
induce bone
formation, BMPs are now known to play crucial roles in all organ systems. BMPs
are also
considered to provide a group of pivotal morphogenetic signals, orchestrating
tissue
architecture throughout the body. Without wishing to be bound by any theory,
BMPs can be
regulated through reversible interactions with extracellular antagonists,
including Noggin,
Chordin, Follistatin and Gremlin. These interactions determine the
bioavailability of different
BMPs for binding to their cognate receptors and activation of downstream
responses. The
biological functions and signaling mechanisms of BMPs are further described,
for example,
in Wang et al. (Bone Morphogenetic Protein (BMP) signaling in development and
human
diseases; Genes and Diseases, (2014) 1, 87-105) and Katagiri and Watabe (Bone
Morphogenetic Proteins; Cold Spring Harb Perspect Biol. 2016, 8)6): a021899),
which are
incorporated herein by reference in their entireties.
Typically, BMPs interact with specific receptors on the cell surface, such as
bone
morphogenetic protein receptors (BMPRs). As illustrated in FIG. 1, signal
transduction
through BMPRs results in mobilization of members of the SMAD family of
proteins (e.g.,
Smad1/5/8 and Smad 4). Currently, more than 15 known BMPs are structurally
related and
can be further categorized into subgroups based on amino acid or nucleotide
similarity. For
example, BMPs include but are not limited to BMP1, BMP 2, BMP 3, BMP 4, BMP 5,
BMP
6, BMP 7, BMP 8a, BMP 8b, BMP 9, BMP10, BMP11, BMP12, BMP13, BMP14, and
BMP15. Specifically, BMP2/4, BMP5/6/7/8, BMP9/BMP10, and BMP12/13/14
(GDF5/6/7)
have been reported to be subgroups based on phylogenetic analysis. BMPs
typically have a
domain structure that includes at least a secretory leader sequence at the
amino terminus, a
6
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
protease domain, and three internal repeat Clr/Cls domains, wherein the second
and the third
internal repeat C lr/Cls domain are separated by an EGF domain. The structure
of BMPs and
their related biological functions is discussed further, for example, in
Wozney (Bone
Morphogenetic Proteins and Their Gene Expression; Cellular and Molecular
Biology of
Bone, 1993), which is incorporated herein by reference in its entirety.
Without wishing to be bound by any theory, each of the BMPs may have distinct
biological functions. For example, experimental knockout of BMP3 can result in
increased
bone density, whereas experimental knockout of BMP7 is associated with defects
in skeletal
patterning and decreased brown fat. BMPs are also linked to activation of
adipocyte
transcription factors due to neogenic hair follicle formation during
myofibroblast
reprogramming when the tissues are undergoing wound healing. Mechanisms of
adipocyte
formation triggered by BMPs are further described, for example, in Plikus et
al.
(Regeneration of fat cells from myofibroblasts during wound healing; Science,
2017,
355(6326): 748-752), which is incorporated herein by reference in its
entirety. In addition,
BMP2 and BMP4 have been reported to be regulators of skin stem cell
proliferation and
differentiation.
Methods and compositions described herein can also include one or more
proteins
associated with a BMP. As used in the present disclosure, "protein associated
with a BMP" or
"BMP associated protein," which are used interchangeably, includes any protein
that interacts
with a BMP or that impacts, directly or indirectly, a signaling pathway
involving a BMP
and/or the biological function of a BMP. A protein associated with a BMP may
be capable of
regulating (e.g., inhibiting, stimulating, activating, delaying) the signaling
pathways of a
BMP. Proteins associated with BMPs can include, for example, Activin, Noggin,
Chordin,
Folistatin, Gremlin, and BMP receptor 1 a-extracellular domain. Others may
include BMP
peptides, BMP receptors, and BMP or receptor decoys.
In some embodiments, methods and compositions described herein can also
include
modulation of one or more BMP receptors. BMP receptors are hetero-tetrameric
complex
transmembrane receptors and generally contain at least an N-terminal
extracellular ligand
binding domain, a transmembrane domain and an intracellular region. Non-
limiting examples
of BMP receptors include but are not limited to ALK1, ALK2, ALK3, ALK6,
ACVR2A,
ACVR2B, BMPR2, and AMHR2.
In some embodiments, receptor availability can be regulated. As non-limiting
examples, BMP receptors may be regulated by BMP3, LEFTYA/B monomers and/or
activin
13/inhibin a heterodimers. Without wishing to be bound by any theory, these
ligands may
7
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
occupy and therefore reduce or eliminate the availability of the receptors but
not activate the
receptors (e.g., ACVR2A or ACVR2B). The negative regulation may diminish
Activin and
BMP signaling pathways since ACVR2A and ACVR2B are shared receptors for these
two
ligand subtypes.
In general, activated BMP receptors promote the activation of Smad-dependent
or
independent pathways. Without wishing to be bound by any theory, BMPs
described herein,
including chimeric BMPs, may have increased binding specificity for receptors,
so that the
receptors are less likely to be negatively regulated by BMP antagonists (e.g.,
blocking BMP
signal pathway). BMPs described herein, including chimeric BMPs, may have
increased
binding affinity for receptors, which can result in increasing the potency of
the BMPs by
increasing activation of the BMP signal pathway. BMP receptors are discussed
further, for
example, in Carreira et al., (Bone Morphogenetic Proteins: Structure,
biological function and
therapeutic applications; Archives of Biochemistry and Biophysics 561 (2014)
64-73), which
is incorporated herein by reference in its entirety.
Without wishing to be bound by any theory, Activin may be able to act as an
"on"
switch for BMP signaling. Activin may include but is not limited to activin
PA, activin f3B,
activin PC, activin PE, myostatin, GDF1, GDF3, GDF11, and GDF15. Noggin, also
known as
NOG, on the contrary, may be able to act as an "off' switch for BMP signaling
by binding to
a BMP receptor. Other examples of proteins associated with BMPs that may
function as
endogenous BMP antagonists include Chordin, Cerberus, Glypixan-3 and
Follistatin. Proteins
associated with BMPs also include BMP peptides that may be able to function as
antagonists
or agonists for BMP signal transduction. Other examples of proteins associated
with BMPs
include receptor decoys, which may act as inhibitors of BMP signaling.
Proteins associated
with BMPs include BMP, type 1, which includes BMP receptor type la and BMP
receptor
type lb, as well as BMP, type 2.
BMPs, including recombinant BMPs, have previously been used in areas such as
surgical operations, orthopedics, dental implants, and dental tissue
regeneration. For instance,
BMP2 and BMP7 have been approved by the FDA. Discussion of the use of
recombinant
human BMP2 can be found, for example, in Lykissas and Gkiatas (World J Orthop.
2017.18;
8(7): 531-535), which is incorporated herein by reference in its entirety.
Methods of Use of BMPs and BMP-Associated Proteins for Aesthetics and
Cosmetics
Aspects of the present disclosure relate to administering one or more proteins
that
regulate morphogenesis to preserve or improve appearance (e.g., appearance of
human skin).
8
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
For example, proteins that regulate morphogenesis disclosed herein can be used
for
preventing or reducing skin wrinkles or improving skin texture or evenness of
skin tone.
Proteins that regulate morphogenesis disclosed herein can be used for
enhancing or
preserving facial contours or improving the attractiveness of facial contours.
In some
examples, proteins that regulate morphogenesis disclosed herein can be used
for any desired
improvements of appearance of the skin. In some embodiments, proteins that
regulate
morphogenesis are multi-functional growth factors. In some embodiments,
proteins that
regulate morphogenesis are cytokines. In some embodiments, proteins that
regulate
morphogenesis are growth and differentiation factors.
In some embodiments, proteins that regulate morphogenesis are BMPs, including
but
not limited to, BMP1, BMP 2, BMP 3, BMP 4, BMP 5, BMP 6, BMP 7, BMP 8a, BMP
8b,
BMP 9, BMP10, BMP11, BMP12, BMP13, BMP14, and BMP15. In some embodiments, the
BMP is BMP2, BMP6, BMP7, or BMP9. In some embodiments, the BMP is BMP2. In
some
embodiments, the BMP is BMP6. In some embodiments, the BMP is BMP7. In some
embodiments, the BMP is BMP9. The BMP may be any BMP known in the art that is
suitable for the compositions and methods disclosed herein.
In some embodiments, compositions comprise more than one BMP. In some
embodiments, compositions comprise BMP2 and BMP7. In some embodiments,
compositions comprise BMP2 and BMP6. In some embodiments, compositions
comprise
BMP2 and BMP9. In some embodiments, compositions comprise BMP6 and BMP7. In
some
embodiments, compositions comprise BMP6 and BMP9. In some embodiments,
compositions comprise BMP7 and BMP9. In some embodiments, compositions
comprise
BMP2, BMP7 and BMP9. In some embodiments, compositions comprise BMP2, BMP6 and
BMP7. In some embodiments, compositions comprise BMP6, BMP7 and BMP9. In some
embodiments, compositions comprise BMP2, BMP6, BMP7 and BMP9. In some
embodiments, compositions comprise any combination of BMPs that is able to
prevent or
reduce skin wrinkles and/or improve skin texture or evenness of skin tone. In
some
embodiments, compositions comprise any combination of BMPs that is able to
enhance or
preserve facial contours or improve the attractiveness of facial contours.
In some embodiments, proteins that regulate morphogenesis can be one or more
proteins associated with BMPs. In some embodiments, proteins associated with
BMPs
include Activin, Noggin, Chordin, Cerberus, Gremlin, Glypixan-3, Follistatin,
BMP peptides,
and receptor decoys. In some embodiments, a protein associated with BMPs is
Noggin. In
9
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
some embodiments, a protein associated with BMPs is a BMP peptide. In some
embodiments, a protein associated with BMPs is Activin.
BMPs associated with the disclosure may comprise wildtype sequences or may be
engineered. BMPs associated with the disclosure may comprise one or more amino
acid
substitutions, additions, deletions, or insertions. BMPs associated with the
disclosure may be
naturally occurring or may be synthetic. BMPs associated with the disclosure
can be in the
form of fusion proteins. BMPs associated with the disclosure can be in the
form of chimeric
proteins. BMPs associated with the disclosure can be fragments or peptides of
BMPs, such as
fragments or peptides that preserve some or all of the activity of a full-
length BMP. BMPs
associated with the disclosure can be truncated forms of BMPs, such as
truncated forms that
preserve some or all of the activity of a full-length BMP.
The sequence of human BMP2 is provided by UniProt Accession number P12643:
MVAGTRCLLALLLPQVLLGGAAGLVPELGRRKFAAASSGRPSSQPSDEVLSEFELRLLSM
FGLKQRPTPSRDAVVPPYMLDLYRRHSGQPGSPAPDHRLERAASRANTVRSFHHEESLEE
LPETSGKTTRRFFFNLS SIPTEEFITSAELQVFREQMQDALGNNS S FHHRINIYEIIKPA
TANSKFPVTRLLDTRLVNQNASRWESFDVTPAVMRWTAQGHANHGFVVEVAHLEEKQGVS
KRHVRISRS LHQDEHSWS QIRPLLVTFGHDGKGHPLHKREKRQAKHKQRKRLKS SCKRHP
LYVDFSDVGWNDWIVAPPGYHAFYCHGECPFPLADHLNSTNHAIVQTLVNSVNSKIPKAC
CVPTELSAISMLYLDENEKVVLKNYQDMVVEGCGCR (SEQ ID NO: 1)
The sequence of human BMP4 is provided by UniProt Accession number P12644:
MIPGNRMLMVVLLCQVLLGGASHASLIPETGKKKVAEIQGHAGGRRSGQSHELLRDFEAT
LLQMFGLRRRPQPSKS AVIPDYMRDLYRLQSGEEEEEQIHSTGLEYPERPASRANTVRSF
HHEEHLENIPGTSENS AFRFLFNLSSIPENEVISSAELRLFREQVDQGPDWERGFHRINI
YEVMKPPAEVVPGHLITRLLDTRLVHHNVTRWETFDVSPAVLRWTREKQPNYGLAIEVTH
LHQTRTHQGQHVRISRSLPQGSGNWAQLRPLLVTFGHDGRGHALTRRRRAKRSPKHHSQR
ARKKNKNCRRHS LYVDFS DVGWNDWIVAPPGYQAFYCHGDCPFPLADHLNS TNHAIVQTL
VNSVNSSIPKACCVPTELSAISMLYLDEYDKVVLKNYQEMVVEGCGCR (SEQ ID NO: 2)
The sequence of human BMP6 is provided by Uniprot Accession number P22004:
MPGLGRRAQWLCWWWGLLCSCCGPPPLRPPLPAAAAAAAGGQLLGDGGSPGRTEQPPPSPQSSSGFL
YRRLKTQEKREMQKEILS VLGLPHRPRPLHGLQQPQPPALRQQEEQQQQQQLPRGEPPPGRLKSAPLFM
LDLYNALSADNDEDGASEGERQQSWPHEAASSSQRRQPPPGAAHPLNRKSLLAPGSGSGGASPLTSAQ
DSAFLNDADMVMSFVNLVEYDKEFSPRQRHHKEFKFNLSQIPEGEVVTAAEFRIYKDCVMGSFKNQTF
LISIYQVLQEHQHRDSDLFLLDTRVVWASEEGWLEFDITATSNLWVVTPQHNMGLQLSVVTRDGVHV
HPRAAGLVGRDGPYDKQPFMVAFFKVSEVHVRTTRSASSRRRQQSRNRSTQSQDVARVS SASDYNS SE
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
LKTACRKHELYVSFQDLGWQDWIIAPKGYAANYCDGECSFPLNAHMNATNHAIVQTLVHLMNPEYVP
KPCCAPTKLNAISVLYFDDNSNVILKKYRNMVVRACGCH (SEQ ID NO: 3)
The sequence of human BMP7 is provided by UniProt Accession number P18075:
MHVRSLRAAAPHSFVALWAPLFLLRS ALADFSLDNEVHSSFIHRRLRSQERREMQREILS
ILGLPHRPRPHLQGKHNSAPMFMLDLYNAMAVEEGGGPGGQGFSYPYKAVFSTQGPPLAS
LQDSHFLTDADMVMSFVNLVEHDKEFFHPRYHHREFRFDLSKIPEGEAVTAAEFRIYKDY
IRERFDNETFRIS VYQVLQEHLGRESDLFLLD SRTLWASEEGWLVFDITATSNHWVVNPR
HNLGLQLSVETLDGQSINPKLAGLIGRHGPQNKQPFMVAFFKATEVHFRSIRSTGSKQRS
QNRSKTPKNQEALRMANVAENSS SDQRQACKKHELYVSFRDLGWQDWIIAPEGYAAYYCE
GECAFPLNSYMNATNHAIVQTLVHFINPETVPKPCCAPTQLNAIS VLYFDDS SNVILKKY
RNMVVRACGCH (SEQ ID NO: 4)
The sequence of BMP9, which is also called Growth/differentiation factor 2
(GDF2)
is provided by UniProt Accession number Q9UK05:
MCPGALWVALPLLSLLAGSLQGKPLQSWGRGSAGGNAHSPLGVPGGGLPEHTFNLKMFLE
NVKVDFLRS LNLS GVPSQDKTRVEPPQYMIDLYNRYTSDKS TTPASNIVRSFSMEDAISI
TATEDFPFQKHILLFNISIPRHEQITRAELRLYVSCQNHVDPSHDLKGS V VIYDVLDGTD
AWDSATETKTFLVSQDIQDEGWETLEVSS AVKRWVRSDSTKSKNKLEVTVESHRKGCDTL
DISVPPGSRNLPFFVVFSNDHSSGTKETRLELREMISHEQESVLKKLSKDGSTEAGESSH
EEDTDGHVAAGSTLARRKRS AGAGSHCQKTSLRVNFEDIGWDSWIIAPKEYEAYECKGGC
FFPLADDVTPTKHAIVQTLVHLKFPTKVGKACCVPTKLSPIS VLYKDDMGVPTLKYHYEG
MSVAECGCR (SEQ ID NO: 5)
Aspects of the present disclosure provide methods and compositions comprising
one
or more BMPs in combination with one or more proteins associated with a BMP.
For
example, the composition can comprise: BMP2, BMP6, BMP7 and/or BMP9 and
Noggin;
BMP2, BMP6, BMP7 and/or BMP9 and Activin; or BMP2, BMP6, BMP7 and/or BMP9 and
one or more BMP peptides. In some embodiments, compositions may include a
fusion
protein derived from sequences of two or more proteins or peptides (e.g.,
comprising portions
of BMP2 and BMP6), or a chimeric protein (e.g., comprising portions of BMP7
and Activin).
In some embodiments, chimeric proteins can comprise portions of different
BMPs. In
some embodiments, chimeric proteins may be heterodimers. In some embodiments,
chimeric
proteins can comprise portions of proteins associated with BMPs. In some
embodiments,
chimeric proteins can comprise portions of BMPs and portions of proteins
associated with
BMPs. For example, chimeric proteins can comprise portions of a BMP and an
Activin. In
some embodiments, chimeric proteins can comprise portions of BMP2 and BMP7.
Chimeric
11
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
proteins encompassed by the present disclosure can comprise portions of any
BMP and/or
any protein associated with BMPs. In some embodiments, chimeric proteins may
have one or
more improved properties relative to native BMPs and/or native proteins
associated with
BMPs. In some embodiments, chimeric proteins may have one or more amino acid
modifications such as amino acid substitutions, insertions, additions or
deletions. Without
wishing to be bound by any theory, heterodimers, chimeric proteins, and amino
acid
modifications may have increased binding affinity or specificity to BMP
receptors, which
may prohibit BMP antagonists from negatively regulating the BMP signaling
pathways. Any
methods known in the art for producing chimeric proteins can be used for
generating BMP
chimeric proteins. BMP chimeric proteins and their biological effects are
discussed further in,
for example, Seeherman et al., (A BMP/activin A chimera is superior to native
BMPs and
induces bone repair in nonhuman primates when delivered in a composite matrix;
Sci. Transl.
Med. 11, eaar4953 (2019)), which is incorporated herein by reference in its
entirety.
In some embodiments, compositions described herein comprise one or more
proteins
associated with a BMP and do not comprise one or more BMPs. For example, a
composition
can comprise Noggin, Activin, and/or one or more BMP peptides, or any other
protein
associated with a BMP disclosed herein.
Compositions described herein comprising one or more BMPs and/or one or more
proteins associated with BMPs can be administered to enhance or preserve
facial contours or
improve the attractiveness of facial contours. In some embodiments, enhancing
or preserving
facial contours or improving the attractiveness of facial contours involves
inhibiting or
reversing bone resorption. Bone resorption refers to resorption of bone
tissues by which
osteoclasts break down the tissue in bones. Bone structure and mineral density
is gradually
lost during this process. Inhibiting or reversing bone resorption can re-build
or strengthen the
natural facial contours. In some embodiments, enhancing or preserving facial
contours or
improving the attractiveness of facial contours involves bone augmentation. In
some
embodiments, enhancing or preserving facial contours or improving the
attractiveness of
facial contours involves cartilage augmentation. Bone augmentation or
cartilage
augmentation in the face can result in a fuller appearance of the face. In
some embodiments,
bone augmentation or cartilage augmentation can result in effects similar to
implanting facial
bones or cartilage tissues. In some embodiments, compositions described herein
comprising
one or more BMPs and/or one or more proteins associated with BMPs can be
administered to
produce bone augmentation and/or cartilage augmentation effects with or
without enhancing
or preserving facial contours.
12
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
In some embodiments, "enhancing" facial contours involves restoring facial
contours
during or after bone resorption. In some embodiments, "preserving" facial
contours involves
maintaining facial contours before actual or noticeable bone resorption has
occurred.
Compositions described herein comprising one or more BMPs can be administered
to
prevent or reduce skin wrinkles and/or improve skin texture or evenness of
skin tone. As used
herein, skin texture refers to skin surface conditions in general. Skin
texture can be generally
categorized into even or uneven skin texture. Even skin texture in some
embodiments can be
considered as soft, smooth, firm, or hydrated. Uneven skin texture in some
embodiments can
be considered as coarse, rough, bumpy, dull, or dry. Uneven skin texture can
also include the
appearance of "macro-texture" (e.g., fine lines and wrinkles) as disclosed in,
for example,
EP2640347A2, which is incorporated herein by reference in its entirety. In
some
embodiments, skin "evenness" can be determined based at least in part on the
criteria listed
above ¨ the degree of softness, smoothness, firmness, and/or hydration, or
coarseness,
roughness, bumpiness, dullness, and/or dryness.
Any technique known in the art can be used for assessing and determining skin
texture or the evenness of the skin. As an example, a digital camera equipped
with a suitable
lens for facial imaging (e.g., 60mm Nikor lens) can be used for capturing
facial images to
determine skin texture. The region of interest (ROT) can be marked manually
based on
predefined facial landmarks such as corners of the eyes and bridge of the
nose. The degree of
textured skin in the ROT can be quantified using image analysis algorithms
based on, for
example, an Optimus software platform. Other suitable software platforms can
also be used.
The software platforms can automatically locate each surface feature and can
quantify the
total number, length and area of facial features, based on a predetermined
standard (e.g.,
shorter than 5 mm and less than 0.16 mm wide), known magnification used to
convert pixel
data to actual length and area data. Thresholds can be based on clinically
important facial
texture. For example, in some embodiments, lines greater than 5 mm and broader
than 0.16
mm may be excluded. Because the ROT varies in shape and size, total textured
area can be
normalized to total ROT size to yield a Texture Area Fraction (TAF). In some
embodiments,
skin evenness can also be measured by analyzing the luminance homogeneity with
the
applications of Haralick homogeneity or chromaticity. Methods for measuring
skin evenness
are discussed further in, for example, Batres et al. (Cosmetics increase skin
evenness:
Evidence from perceptual and physical measures; Skin Res Technol, 2019, 25(5):
672-676),
which is incorporated herein by reference in its entirety.
13
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
In some embodiments, compositions described herein including one or more BMPs
and/or one or more proteins associated with BMPs reduce the thickness of the
stratum
corneum, and/or increase the thickness of the living epidermis, resulting in
less wrinkled,
smoother surface texture. In some embodiments, compositions described herein
including one
or more BMPs and/or one or more proteins associated with BMPs stimulate the
formation of
adipocytes in the skin, which may contribute to fuller, less wrinkled skin, or
improved texture
or evenness of skin tone. In some embodiments, compositions described herein
including one
or more BMPs and/or one or more proteins associated with BMPs stimulate
dedifferentiation
of keratinocytes. Keratinocytes are the primary type of cell found in the
epidermis, which is
the outermost layer of the skin. Keratinocytes function in the formation of a
barrier against
environmental damages such as UV light and radiation. Keratinocyte
dedifferentiation has
biological significance during wound healing when epidermis must rapidly
regenerate barrier
function. In some embodiments, compositions described herein including one or
more BMPs
and/or one or more proteins associated with BMPs reduces, evens out, or
modulates melanin
formation by melanocytes. Without wishing to be bound by any theory, BMPs may
be
capable of affecting or regulating melanin synthesis (or skin pigmentation),
so that
administration of BMPs can improve the skin tone or the evenness of the skin
tone (e.g.,
reduced pigmentation in the skin). In some embodiments, compositions described
herein
including one or more BMPs and/or one or more proteins associated with BMPs
contributes
to the appearance of more youthful or attractive skin.
In some embodiments, the presence of Noggin in a composition comprising one or
more BMPs can reduce potential side effects associated with the one or more
BMPs. The
presence of Noggin as an inhibitor of BMP can ensure that the anti-wrinkle
and/or pro-facial
contouring effects of BMPs can be appropriately regulated. In some
embodiments, Noggin
can be formulated with or without the presence of one or more BMPs. For
example, a Noggin
formulation can be administered to a subject separately from administration of
one or more
BMPs, such as a few days after the administration of one or more BMPs.
Subjects associated with the disclosure include human and non-human subjects.
In
some embodiments, a non-human subject is a non-human primate. In some
embodiments, a
non-human subject is a companion animal or a farm animal. In some embodiments,
the
subject is a subject that has exposed skin.
In some embodiments, the subject is a human subject having, suspected of
having, or
at risk of developing skin wrinkles. In some embodiments, the skin wrinkles
are facial
wrinkles. In some embodiments, the skin wrinkles are neck wrinkles. Skin
wrinkles include
14
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
any types of wrinkles, such as fine lines and creases that can be found or
observed on human
skins particularly facial and neck regions. Skin wrinkles include raised
portions of the
epithelial layer which are elevated above a normal surface of skin. Skin
wrinkles can be
caused by a variety of factors such as aging, UV light exposure, lifestyle,
environmental
factors, genetics, and medical treatments. In general, wrinkles start to form
at the dermis layer
of the skin, which constitutes fibroblast cells that synthesize the structural
proteins collagens
and elastin. During the formation of wrinkles, the dermis layer loses its
ability to effectively
repair and restore. Therefore, the skin's strength deteriorates and begins to
wrinkle.
Without wishing to be bound by any theory, wrinkles can in some embodiments be
classified as crinkle lines, permanent elastotic creases, dynamic expression
lines, and/or
gravitational folds. Crinkle lines are also known as atrophic crinkling
rhytids, which are
relatively shallow. They typically run in parallel to each other, often on the
forehead, and
disappear when the skin is stretched. Permanent elastotic creases can be
associated with pale
complexions and heavy sun exposure. Characterized by deep lines in the skin,
they can occur
at points where the skin creases naturally, such as the base of the neck, the
lips, and the
cheeks. Dynamic expression lines can be caused by habitual facial expressions,
combined
with the skin's loss of elasticity; for example, frown lines on the brow,
crow's feet around the
eyes, and laugh lines above the mouth. Gravitational folds can be associated
with the losses
of firmness of the skin with age during which the skin begins to sag, pulling
away from the
underlying fat and muscle. This can create folds that are particularly
prominent on the neck,
chin, and jowls.
A subject having, suspected of having, or at risk of developing skin wrinkles
can be
identified by routine examination, e.g., visual inspection, laboratory tests,
physical exams,
and/or dermatoscopy, as would be understood by one of ordinary skill in the
art. Numbers,
sites and depths of the wrinkles can be measured. The non-invasive digital
fringe
profilometry, which utilizes phase-shifted fringes being projected to the
skin, can be used.
Digital fringe profilometry is discussed further in Sari et al (Measurement of
skin wrinkles
differences using novel optical skin imaging system of digital fringes
profilometry; Proc.
SPIE 11044, Third International Seminar on Photonics, Optics, and Its
Applications (ISPh0A
2018), 1104406 (2019)), which is incorporated herein by reference in its
entirety.
In some embodiments, a subject is a human subject who desires to improve skin
texture or evenness of skin tone. In some embodiments, a human subject who
desires to
improve skin texture or evenness of skin tone does not have visible signs of
aging such as
wrinkles or uneven skin tone.
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
In some embodiments, a subject is a human subject having, suspected of having,
or at
risk of having a loss of natural facial contours. Facial contours can include
the shape and size
of the facial skeleton and structure of a subject. With aging, natural facial
contours may be
lost due to facial bone resorption. Many plastic surgery or aesthetic
procedures are used for
preserving or improving facial contours, such as implant insertion by facial
skeletal
augmentation surgery, e.g., to make the face more balanced by adding fullness,
making the
cheekbones higher, the chin more prominent, and/or the jawline stronger and
wider.
In some embodiments, a subject is a human subject who desires a change in his
or her
current facial contours to improve attractiveness of facial contours. A human
subject may
want to change his or her facial contours at least in part due to personal
choices. In some
embodiments, a human subject has never received any previous procedures or
treatments to
change facial contours. In other embodiments, a human subject has previously
received one
or more procedures or treatments to change facial contours.
Improved attractiveness can be assessed by subjective and/or objective
methods. In
some embodiments, improved attractiveness can be assessed by the human subject
who is
administered a composition disclosed herein. In other embodiments, improved
attractiveness
can be assessed by another human subject. In other embodiments, improved
attractiveness
can be assessed by a machine, such as a computer program or algorithm that can
predict
attractiveness, such as facial attractiveness. For example, a machine can
comprise a
computer-based system and an auto-encoder, creating a machine learning system
that can
automatically determine how attractive a certain facial image is. A set of
facial images that
are considered "attractive" or "non (less) attractive" can be provided to the
system for
modeling. After a composition associated with the disclosure is administered
to a human
subject, one or more facial images of the human subject can be uploaded to the
machine
learning system, which will assess whether the facial images are attractive
and/or whether the
facial images are more attractive than a control, such as facial images of the
same subject
before being administered the composition. In some embodiments, assessing
attractiveness
can be done by reviewing whether a facial image contains attractive or
unattractive features.
A facial image that lacks pre-determined unattractive features may be
considered as
attractive. A facial image that has less unattractive features relative to a
control may be
considered to have increased attractiveness relative to the control. Solely by
way of example,
unattractive features can include but are not limited to, acne, age spots/sun
damage, bruises,
bumps, cellulite, light spots, pitting, scars, freckles, including damaged
freckles, and
wrinkles. In some embodiments, unattractive features can be particularly
associated with the
16
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
appearance of facial contours. For example, such features may include sunken
cheeks, facial
asymmetry, or sunken eyebrow area. In some embodiments, attractiveness is
based on the
observations or opinions of a population (e.g., social standards and human
behaviors).
Methods and approaches for determining attractiveness of facial appearance are
further
described, for example, in US 10,726,601 and US 10,486,174, which are
incorporated herein
by reference in their entireties.
In some embodiments, a subject has been identified as having skin wrinkles or
loss of
natural facial contours. In other embodiments, a subject has not been
identified as having
skin wrinkles or loss of natural facial contours. A subject suspected of
having skin wrinkles
or loss of natural facial contours may be a subject that exhibits one or more
symptoms or
signs of skin wrinkles or loss of natural facial contours, e.g., small creases
on the skin, loss of
elasticity. In other embodiments, the subject has not exhibited any symptoms
or signs of skin
wrinkles or loss of natural facial contours and/or has no history of skin
wrinkles or loss of
natural facial contours. In other embodiments, the subject desires a change in
skin texture or
evenness of skin tone or facial contour in the belief that the change produces
a more attractive
appearance. A subject at risk for skin wrinkles or loss of natural facial
contours may be a
subject having one or more of the risk factors associated with the development
of skin
wrinkles and/or loss of natural facial contours. For example, risk factors
associated with skin
wrinkles or loss of natural facial contours can include in some embodiments:
(a) genetic
factors; (b) age; (c) family history; (d) habitual exposure to UV light;
and/or (e) smoking.
In some embodiments, a subject is a human subject who has undergone, is
undergoing, or will undergo gender reassignment procedures and/or surgeries.
Gender
reassignment procedures and/or surgeries refers to any procedures or surgeries
that change
the sex assigned at birth of a subject. In some embodiments, the human subject
is male
assigned at birth and undergoes gender reassignment procedures and/or
surgeries to change
the gender to female. In some embodiments, the human subject is female
assigned at birth
and undergoes gender reassignment procedures and/or surgeries to change the
gender to
male. For example, a male subject who wants to undergo gender reassignment
procedures
and/or surgeries can be administered a composition disclosed herein with the
goal of
achieving softer and more feminine facial features as part of facial
feminization surgery
during the male-to-female transition. In some embodiments, before and after
facial images
can verify whether administration of compositions disclosed herein achieves
the desired
effects.
17
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
In some embodiments, a subject is elderly (e.g., 65 years old or older).
Elderly
subjects are more prone to develop skin wrinkles and to suffer from the loss
of facial
contours. For instance, the quantities and the types of skin wrinkles may be
more prominent
in an elderly subject than in a subject who is younger than 65 years old. In
other
embodiments, a subject is not elderly (e.g., a subject is younger than 65
years old). A subject
who is not elderly may also be prone to developing skin wrinkles and to
suffering from the
loss of facial contours. As disclosed in the present application, many factors
other than aging
can cause or accelerate the appearance of skin wrinkles and undesirable facial
contours. In
some embodiments, a subject is not prone to developing skin wrinkles or to
suffering from
the loss of facial contours but is administered compositions disclosed herein
to prevent or
inhibit the formation of skin wrinkles and/or to prevent or inhibit the loss
of facial contours.
In some embodiments, compositions described here that contain one or more BMPs
and/or one or more proteins associated with BMPs prevent or reduce skin
wrinkles by at least
5%, 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 100%, or by at least 2-fold,
at least
5-fold, at least 10-fold, at least 20-fold, at least 50-fold, at least 100-
fold, or at least 1000-fold
compared to a control. In some embodiments, the one or more BMP used in the
methods
described in the present application enhances or preserves facial contours by
at least 5%,
10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 100%, or by at least 2-fold, at
least 5-
fold, at least 10-fold, at least 20-fold, at least 50-fold, at least 100-fold,
or at least 1000-fold
compared to a control. In some embodiments, a control is a subject or a skin
sample that is
not administered a composition that contains one or more BMPs and/or one or
more proteins
associated with BMPs.
In some embodiments, compositions described here that contain one or more BMPs
and/or one or more proteins associated with BMPs improve skin texture or
evenness of skin
tone by at least 5%, 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 100%, or by
at least
2-fold, at least 5-fold, at least 10-fold, at least 20-fold, at least 50-fold,
at least 100-fold, or at
least 1000-fold compared to a control. In some embodiments, compositions
described here
that contain one or more BMPs and/or one or more proteins associated with BMPs
improve
the attractiveness of facial contours by at least 5%, 10%, 20%, 30%, 40%, 50%,
60%, 70%,
80%, 90%, 100%, or by at least 2-fold, at least 5-fold, at least 10-fold, at
least 20-fold, at
least 50-fold, at least 100-fold, or at least 1000-fold compared to a control.
In some
embodiments, a control is a subject or a skin sample that is not administered
a composition
that contains one or more BMPs and/or one or more proteins associated with
BMPs.
18
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
In some embodiments, the control is the skin from the same subject at an
earlier time
period such as prior to the administration of a composition comprising a BMP
or a protein
associated with a BMP. In some embodiments, a control is a subject having the
same signs
of skin wrinkles but without administration of a composition comprising a BMP
or a protein
associated with a BMP. The changes to formation of skin wrinkles can be
determined with
methods known to one of skill in the art.
The term "effective amount" or "amount effective" in the context of a
composition or
dose for administration to a subject refers to an amount of the composition or
dose that
produces one or more desired responses in the subject, e.g., preventing or
reducing skin
wrinkles, improving skin texture or evenness of skin tone, enhancing or
preserving facial
contours, or improving the subject's perception of their own attractiveness.
In some
embodiment, one or more desired responses in the subject is the change of
facial appearance
during a male-to-female transition or a female-to-male transition. Therefore,
in some
embodiments, an effective amount is any amount of a composition or dose
provided in the
present application that produces one or more of the desired effects and/or
preventative
responses as provided in the present application. The amount can be one that a
clinician
would believe may have a clinical benefit for a subject in need thereof, or an
amount that a
clinician would believe would produce an improvement in attractiveness of the
subject in
need thereof, or in the subject's perception of their own attractiveness. Any
one of the
compositions or doses as provided in the present application can be in an
amount effective.
The amount can be one that is disclosed in the present application.
An effective amount can involve reducing the level of an undesired response,
although in some embodiments, it involves preventing an undesired response
altogether. An
effective amount can also involve delaying the occurrence of an undesired
response. An
amount that is effective can also be an amount that produces a desired
therapeutic endpoint or
a desired therapeutic result. In other embodiments, the amounts effective can
involve
enhancing the level of a desired response, such as a therapeutic endpoint or
result. An
effective amount, preferably, results in a preventative result or therapeutic
result or endpoint
with respect to signs of skin aging such as skin wrinkles and fine lines as
well as the changes
of facial contours in a subject. The achievement of any of the foregoing can
be monitored by
routine methods and the methods as disclosed in the present application.
Effective amounts will depend, of course, on the particular subject being
treated; the
severity of a condition; the individual patient parameters including age,
physical condition,
size and weight; the duration of the treatment; the nature of concurrent
therapy (if any); the
19
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
specific route of administration and like factors; the subject's perception of
their need for
improvement in appearance and the amount of change that would satisfy that
need.
Compositions, Kits, and Administration
The present disclosure provides compositions, including pharmaceutical or
cosmetic
compositions, comprising one or more BMPs and/or BMP-associated proteins, or
pharmaceutically acceptable salts thereof, and optionally a pharmaceutically
acceptable
excipient.
In certain embodiments, a BMP described in this application is provided in an
effective amount in a composition, such as a pharmaceutical or cosmetic
composition. In
certain embodiments, the effective amount is a therapeutically effective
amount. In certain
embodiments, the effective amount is a prophylactically effective amount. In
certain
embodiments, the effective amount is a cosmetically effective amount.
Compositions, such as
pharmaceutical or cosmetic compositions, described in this application can be
prepared by
any method known in the art. In general, such preparatory methods include
bringing a protein
or peptide (e.g., an active ingredient) described in this application into
association with a
carrier or excipient, and/or one or more other accessory ingredients, and
then, if necessary
and/or desirable, shaping, and/or packaging the product into a desired single-
or multi-dose
unit.
Pharmaceutical or cosmetic compositions can be prepared, packaged, and/or sold
in
bulk, as a single unit dose, and/or as a plurality of single unit doses. A
"unit dose" is a
discrete amount of a pharmaceutical or cosmetic composition comprising a
predetermined
amount of an active ingredient. The amount of the active ingredient is
generally equal to the
dosage of the active ingredient which would be administered to a subject
and/or a convenient
fraction of such a dosage, such as one-half or one-third of such a dosage.
Relative amounts of the active ingredient, the pharmaceutically acceptable
excipient,
and/or any additional ingredients in a pharmaceutical or cosmetic composition
described in
this application will vary, depending upon the identity, size, and/or
condition of the subject
treated and further depending upon the route by which the composition is to be
administered.
The composition may comprise, e.g., between 0.1% and 100% (w/w) active
ingredient.
The term "pharmaceutically acceptable excipient" or "pharmaceutically
acceptable
carrier" means a pharmacologically inactive material used together with a
pharmacologically
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
active material to formulate the compositions. Pharmaceutically acceptable
excipients
comprise a variety of materials known in the art, including but not limited to
saccharides
(such as glucose, lactose, and the like), preservatives such as antimicrobial
agents,
reconstitution aids, colorants, saline (such as phosphate buffered saline),
and buffers. Any
one of the compositions provided in the present application may include a
pharmaceutically
acceptable excipient or carrier
Pharmaceutically acceptable excipients used in the manufacture of
pharmaceutical or
cosmetic compositions can include inert diluents, dispersing and/or
granulating agents,
surface active agents and/or emulsifiers, disintegrating agents, binding
agents, preservatives,
buffering agents, lubricating agents, and/or oils. Excipients such as cocoa
butter and
suppository waxes, coloring agents, coating agents, sweetening, flavoring, and
perfuming
agents may also be present in the composition. Exemplary excipients include
diluents,
dispersing and/or granulating agents, surface active agents and/or
emulsifiers, disintegrating
agents, binding agents, preservatives, buffering agents, lubricating agents,
and/or oils (e.g.,
synthetic oils, semi-synthetic oils).
The term "pharmaceutically acceptable salt" refers to those salts which are,
within the
scope of sound medical judgment, suitable for use in contact with the tissues
of humans and
lower animals without undue toxicity, irritation, allergic response and the
like, and are
commensurate with a reasonable benefit/risk ratio. Pharmaceutically acceptable
salts are well
known in the art. For example, Berge et al., describe pharmaceutically
acceptable salts in
detail in J. Pharmaceutical Sciences, 1977, 66, 1-19, incorporated by
reference.
Pharmaceutically acceptable salts of the compounds disclosed in this
application include
those derived from suitable inorganic and organic acids and bases. Examples of
pharmaceutically acceptable, nontoxic acid addition salts are salts of an
amino group formed
with inorganic acids such as hydrochloric acid, hydrobromic acid, phosphoric
acid, sulfuric
acid, and perchloric acid or with organic acids such as acetic acid, oxalic
acid, maleic acid,
tartaric acid, citric acid, succinic acid, or malonic acid or by using other
methods known in
the art such as ion exchange. Other pharmaceutically acceptable salts include
adipate,
alginate, ascorbate, aspartate, benzenesulfonate, benzoate, bisulfate, borate,
butyrate,
camphorate, camphorsulfonate, citrate, cyclopentanepropionate, digluconate,
dodecylsulfate,
ethanesulfonate, formate, fumarate, glucoheptonate, glycerophosphate,
gluconate,
hemisulfate, heptanoate, hexanoate, hydroiodide, 2¨hydroxy¨ethanesulfonate,
lactobionate,
lactate, laurate, lauryl sulfate, malate, maleate, malonate, methanesulfonate,
2-
21
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
naphthalenesulfonate, nicotinate, nitrate, oleate, oxalate, palmitate,
pamoate, pectinate,
persulfate, 3¨phenylpropionate, phosphate, picrate, pivalate, propionate,
stearate, succinate,
sulfate, tartrate, thiocyanate, p-toluenesulfonate, undecanoate, valerate
salts, and the like.
Salts derived from appropriate bases include alkali metal, alkaline earth
metal, ammonium
and N+(C1-4 alky1)4- salts. Representative alkali or alkaline earth metal
salts include
sodium, lithium, potassium, calcium, magnesium, and the like. Further
pharmaceutically
acceptable salts include, when appropriate, nontoxic ammonium, quaternary
ammonium, and
amine cations formed using counterions such as halide, hydroxide, carboxylate,
sulfate,
phosphate, nitrate, lower alkyl sulfonate, and aryl sulfonate.
Exemplary diluents can include calcium carbonate, sodium carbonate, calcium
phosphate, dicalcium phosphate, calcium sulfate, calcium hydrogen phosphate,
sodium
phosphate lactose, sucrose, cellulose, microcrystalline cellulose, kaolin,
mannitol, sorbitol,
inositol, sodium chloride, dry starch, cornstarch, powdered sugar, and
mixtures thereof.
Exemplary granulating and/or dispersing agents can include potato starch, corn
starch,
tapioca starch, sodium starch glycolate, clays, alginic acid, guar gum, citrus
pulp, agar,
bentonite, cellulose, and wood products, natural sponge, cation-exchange
resins, calcium
carbonate, silicates, sodium carbonate, cross-linked poly(vinyl-pyrrolidone)
(crospovidone),
sodium carboxymethyl starch (sodium starch glycolate), carboxymethyl
cellulose, cross-
linked sodium carboxymethyl cellulose (croscarmellose), methylcellulose,
pregelatinized
starch (starch 1500), microcrystalline starch, water insoluble starch, calcium
carboxymethyl
cellulose, magnesium aluminum silicate (Veegum), sodium lauryl sulfate,
quaternary
ammonium compounds, and mixtures thereof.
Exemplary surface active agents and/or emulsifiers can include natural
emulsifiers
(e.g., acacia, agar, alginic acid, sodium alginate, tragacanth, chondrux,
cholesterol, xanthan,
pectin, gelatin, egg yolk, casein, wool fat, cholesterol, wax, and lecithin),
colloidal clays (e.g.,
bentonite (aluminum silicate) and Veegum (magnesium aluminum silicate)), long
chain
amino acid derivatives, high molecular weight alcohols (e.g., stearyl alcohol,
cetyl alcohol,
oleyl alcohol, triacetin monostearate, ethylene glycol distearate, glyceryl
monostearate, and
propylene glycol monostearate, polyvinyl alcohol), carbomers (e.g., carboxy
polymethylene,
polyacrylic acid, acrylic acid polymer, and carboxyvinyl polymer),
carrageenan, cellulosic
derivatives (e.g., carboxymethylcellulose sodium, powdered cellulose,
hydroxymethyl
cellulose, hydroxypropyl cellulose, hydroxypropyl methylcellulose,
methylcellulose),
22
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
sorbitan fatty acid esters (e.g., polyoxyethylene sorbitan monolaurate (Tween
20),
polyoxyethylene sorbitan (Tween 60), polyoxyethylene sorbitan monooleate
(Tween 80),
sorbitan monopalmitate (Span 40), sorbitan monostearate (Span 60), sorbitan
tristearate
(Span 65), glyceryl monooleate, sorbitan monooleate (Span 80),
polyoxyethylene esters
(e.g., polyoxyethylene monostearate (Myrj 45), polyoxyethylene hydrogenated
castor oil,
polyethoxylated castor oil, polyoxymethylene stearate, and Soluto1 ), sucrose
fatty acid
esters, polyethylene glycol fatty acid esters (e.g., Cremophor ),
polyoxyethylene ethers, (e.g.,
polyoxyethylene lauryl ether (Brij 30)), poly(vinyl-pyrrolidone), diethylene
glycol
monolaurate, triethanolamine oleate, sodium oleate, potassium oleate, ethyl
oleate, oleic acid,
ethyl laurate, sodium lauryl sulfate, Pluronic F-68, poloxamer P-188,
cetrimonium bromide,
cetylpyridinium chloride, benzalkonium chloride, docusate sodium, and/or
mixtures thereof.
Exemplary binding agents can include starch (e.g., cornstarch and starch
paste),
gelatin, sugars (e.g., sucrose, glucose, dextrose, dextrin, molasses, lactose,
lactitol, mannitol,
etc.), natural and synthetic gums (e.g., acacia, sodium alginate, extract of
Irish moss, panwar
gum, ghatti gum, mucilage of isapol husks, carboxymethylcellulose,
methylcellulose,
ethylcellulose, hydroxyethylcellulose, hydroxypropyl cellulose, hydroxypropyl
methylcellulose, microcrystalline cellulose, cellulose acetate, poly(vinyl-
pyrrolidone),
magnesium aluminum silicate (Veegum ), and larch arabogalactan), alginates,
polyethylene
oxide, polyethylene glycol, inorganic calcium salts, silicic acid,
polymethacrylates, waxes,
water, alcohol, and/or mixtures thereof.
Exemplary preservatives can include antioxidants, chelating agents,
antimicrobial
preservatives, antifungal preservatives, antiprotozoan preservatives, alcohol
preservatives,
acidic preservatives, and other preservatives. In certain embodiments, the
preservative is an
antioxidant. In other embodiments, the preservative is a chelating agent.
Exemplary antioxidants can include alpha tocopherol, ascorbic acid, acorbyl
palmitate, butylated hydroxyanisole, butylated hydroxytoluene,
monothioglycerol, potassium
metabisulfite, propionic acid, propyl gallate, sodium ascorbate, sodium
bisulfite, sodium
metabisulfite, and sodium sulfite.
Exemplary chelating agents can include ethylenediaminetetraacetic acid (EDTA)
and
salts and hydrates thereof (e.g., sodium edetate, disodium edetate, trisodium
edetate, calcium
disodium edetate, dipotassium edetate, and the like), citric acid and salts
and hydrates thereof
(e.g., citric acid monohydrate), fumaric acid and salts and hydrates thereof,
malic acid and
23
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
salts and hydrates thereof, phosphoric acid and salts and hydrates thereof,
and tartaric acid
and salts and hydrates thereof. Exemplary antimicrobial preservatives can
include
benzalkonium chloride, benzethonium chloride, benzyl alcohol, bronopol,
cetrimide,
cetylpyridinium chloride, chlorhexidine, chlorobutanol, chlorocresol,
chloroxylenol, cresol,
ethyl alcohol, glycerin, hexetidine, imidurea, phenol, phenoxyethanol,
phenylethyl alcohol,
phenylmercuric nitrate, propylene glycol, and thimerosal.
Exemplary antifungal preservatives can include butyl paraben, methyl paraben,
ethyl
paraben, propyl paraben, benzoic acid, hydroxybenzoic acid, potassium
benzoate, potassium
sorbate, sodium benzoate, sodium propionate, and sorbic acid.
Exemplary alcohol preservatives can include ethanol, polyethylene glycol,
phenol,
phenolic compounds, bisphenol, chlorobutanol, hydroxybenzoate, and phenylethyl
alcohol.
Exemplary acidic preservatives can include vitamin A, vitamin C, vitamin E,
beta-
carotene, citric acid, acetic acid, dehydroacetic acid, ascorbic acid, sorbic
acid, and phytic
acid.
Other preservatives can include tocopherol, tocopherol acetate, deteroxime
mesylate,
cetrimide, butylated hydroxyanisol (BHA), butylated hydroxytoluened (BHT),
ethylenediamine, sodium lauryl sulfate (SLS), sodium lauryl ether sulfate
(SLES), sodium
bisulfite, sodium metabisulfite, potassium sulfite, potassium metabisulfite,
Glydant Plus,
Phenonip , methylparaben, German 115, Germaben II, Neolone , Kathon , and
Euxyl .
Exemplary buffering agents can include citrate buffer solutions, acetate
buffer
solutions, phosphate buffer solutions, ammonium chloride, calcium carbonate,
calcium
chloride, calcium citrate, calcium glubionate, calcium gluceptate, calcium
gluconate, D-
gluconic acid, calcium glycerophosphate, calcium lactate, propanoic acid,
calcium levulinate,
pentanoic acid, dibasic calcium phosphate, phosphoric acid, tribasic calcium
phosphate,
calcium hydroxide phosphate, potassium acetate, potassium chloride, potassium
gluconate,
potassium mixtures, dibasic potassium phosphate, monobasic potassium
phosphate,
potassium phosphate mixtures, sodium acetate, sodium bicarbonate, sodium
chloride, sodium
citrate, sodium lactate, dibasic sodium phosphate, monobasic sodium phosphate,
sodium
phosphate mixtures, tromethamine, magnesium hydroxide, aluminum hydroxide,
alginic acid,
pyrogen-free water, isotonic saline, Ringer's solution, ethyl alcohol, and
mixtures thereof.
24
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
Exemplary lubricating agents can include magnesium stearate, calcium stearate,
stearic acid, silica, talc, malt, glyceryl behanate, hydrogenated vegetable
oils, polyethylene
glycol, sodium benzoate, sodium acetate, sodium chloride, leucine, magnesium
lauryl sulfate,
sodium lauryl sulfate, and mixtures thereof.
Exemplary natural oils can include almond, apricot kernel, avocado, babassu,
bergamot, black current seed, borage, cade, camomile, canola, caraway,
carnauba, castor,
cinnamon, cocoa butter, coconut, cod liver, coffee, corn, cotton seed, emu,
eucalyptus,
evening primrose, fish, flaxseed, geraniol, gourd, grape seed, hazel nut,
hyssop, isopropyl
myristate, jojoba, kukui nut, lavandin, lavender, lemon, litsea cubeba,
macademia nut,
mallow, mango seed, meadowfoam seed, mink, nutmeg, olive, orange, orange
roughy, palm,
palm kernel, peach kernel, peanut, poppy seed, pumpkin seed, rapeseed, rice
bran, rosemary,
safflower, sandalwood, sasquana, savoury, sea buckthorn, sesame, shea butter,
silicone,
soybean, sunflower, tea tree, thistle, tsubaki, vetiver, walnut, and wheat
germ oils. Exemplary
synthetic or semi-synthetic oils include, but are not limited to, butyl
stearate, medium chain
triglycerides (such as caprylic triglyceride and capric triglyceride),
cyclomethicone, diethyl
sebacate, dimethicone 360, isopropyl myristate, mineral oil, octyldodecanol,
oleyl alcohol,
silicone oil, and mixtures thereof. In certain embodiments, exemplary
synthetic oils comprise
medium chain triglycerides (such as caprylic triglyceride and capric
triglyceride).
Carriers used in the manufacture of pharmaceutical or cosmetic compositions
can
include human collagen, recombinant collagen, collagen sponges, hyaluronic
acid, or
composite matrices. Carriers used in the manufacture of pharmaceutical or
cosmetic
compositions can include liposomes, polymeric micelles, or microspheres. In
some
embodiments, carriers used in the manufacture of pharmaceutical or cosmetic
compositions
can include any known ingredients or materials that are suitable for
compositions described
in the present disclosure.
In some embodiments, compositions comprising one or more BMPs and/or BMP-
associated proteins are formulated for subdermal injection. In some
embodiments,
compositions comprising one or more BMPs and/or BMP-associated proteins are
formulated
for subcutaneous injection. In some embodiments, compositions comprising one
or more
BMPs and/or BMP-associated proteins are formulated for supraperiostial
injection. In some
embodiments, the subdermal injection is near periosteum. Periosteum is a dense
layer of
vascular connective tissue enveloping the bones except at the surfaces of the
joints. It is a
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
membrane that covers the outer surface of all bones. An injection around
periosteum
promotes localized effects of the BMPs and/or the proteins associated with a
BMP.
Compositions described herein can be administered via any route that is
suitable for the
composition and the subject in need thereof.
In some embodiments, the composition comprises a dermal filler. Liquid dosage
forms for subdermal, subcutaneous or supraperiostial administration include
pharmaceutically acceptable emulsions, microemulsions, solutions, suspensions,
syrups and
elixirs. In addition to the active ingredients (e.g., the BMPs), the liquid
dosage forms may
comprise inert diluents commonly used in the art such as, for example, water
or other
solvents, solubilizing agents and emulsifiers such as ethyl alcohol, isopropyl
alcohol, ethyl
carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate, propylene glycol,
1,3-butylene
glycol, dimethylformamide, oils (e.g., cottonseed, groundnut, corn, germ,
olive, castor, and
sesame oils), glycerol, tetrahydrofurfuryl alcohol, polyethylene glycols and
fatty acid esters
of sorbitan, and mixtures thereof. Besides inert diluents, the oral
compositions can include
adjuvants such as wetting agents, emulsifying and suspending agents,
sweetening, flavoring,
and perfuming agents. In certain embodiments for parenteral administration,
agents such as
BMPs can be mixed with solubilizing agents such as Cremophor , alcohols, oils,
modified
oils, glycols, polysorbates, cyclodextrins, polymers, and mixtures thereof.
Injectable preparations, for example sterile injectable aqueous or oleaginous
suspensions, can be formulated according to known methods using suitable
dispersing or
wetting agents and suspending agents. The sterile injectable preparation can
be a sterile
injectable solution, suspension, or emulsion in a nontoxic parenterally
acceptable diluent or
solvent, for example, as a solution in 1,3-butanediol. Among the acceptable
vehicles and
solvents that can be employed are water, Ringer's solution, U.S.P., and
isotonic sodium
chloride solution. In addition, sterile, fixed oils are conventionally
employed as a solvent or
suspending medium. For this purpose, any bland fixed oil can be employed,
including
synthetic mono- or di-glycerides. In addition, fatty acids such as oleic acid
can be used in the
preparation of injectables. The injectable formulations can be sterilized, for
example, by
filtration through a bacterial-retaining filter, or by incorporating
sterilizing agents in the form
of sterile solid compositions, which can be dissolved or dispersed in sterile
water or other
sterile injectable medium prior to use.
26
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
Dermal fillers may include but are not limited to hyaluronic acid, calcium
hydroxylapatite, poly-L-lactic acid, polymethylmethacrylate (PMMA), and
autologous fat
injections (facial fat grafting). In some embodiments, dermal fillers may
include sugar
threading (e.g., sugar thread lift work). Any of the dermal fillers that are
approved by the
FDA and are appropriate for the composition as disclosed in the present
application can be
used for aspects of the disclosure.
In some embodiments, compositions associated with the disclosure comprise one
or
more agents that enhance penetration. In some embodiments, the penetration
enhancers may
include but are not limited to ethanol, dimethyl sulfoxide, dimethyl
isosorbide, isopropyl
myristate and propylene glycol. In some embodiments, compositions can be
formulated as
nanoemulsions for enhancing the penetration of BMPs. In some embodiments,
encapsulating
compositions can be used for enhancing penetration, which includes the use of
polymeric
substances, waxes and amphipathic lipids capable of forming liposomes. Solid
compositions
of a similar type can be employed as fillers in soft and hard-filled gelatin
capsules using such
excipients as lactose or milk sugar as well as high molecular weight
polethylene glycols and
the like. The active ingredient can be in a micro-encapsulated form with one
or more
excipients as noted above.
Dosage forms for topical administration of a protein or peptide described in
this
application may include ointments, pastes, creams, serums, lotions, gels,
powders, solutions,
and/or patches. In some embodiments, the dosage form for topical
administration is a cream
or serum. Generally, the active ingredient is admixed under sterile conditions
with a
pharmaceutically acceptable carrier or excipient and/or any needed
preservatives and/or
buffers as can be required. Additionally, the present disclosure contemplates
the use of
dermal patches for providing controlled delivery of an active ingredient into
the skin. A
dermal patch, skin patch, or the like as used herein refers to a medicated
adhesive patch that
is placed on the skin to deliver a specific dose of a composition into the
skin. Dermal or skin
patches can include but are not limited to single-layer drug-in-adhesive,
multi-layer drug-in-
adhesive, reservoir, matrix, and vapour patches. Dosage forms, including for
dermal or skin
patches, can be prepared, for example, by dissolving and/or dispensing the
active ingredient
in the proper medium. Alternatively, or additionally, the rate can be
controlled by either
providing a rate controlling membrane and/or by dispersing the active
ingredient in a polymer
matrix and/or gel. In some embodiments, permeation enhancers can be used for
enhancing
the permeation of active ingredients in the patch (e.g., BMP proteins and/or
proteins
27
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
associated with BMPs). In some embodiments, hypodermic needles can be used for
dermal
administration. In some embodiments, microneedles can be used for dermal
administration.
In some embodiments, different types of administration methods can be used.
For instance, in
some embodiments, compositions disclosed herein can be administered via dermal
patch in
combination with hyaluronic acid microneedle injection. In some embodiments,
the
administration is not systemic administration (e.g., the active ingredient(s)
being delivered do
not enter the bloodstream). In some embodiments, administration of
compositions disclosed
herein delivers active ingredients only to the skin surface and/or to deeper
layers of the skin.
Formulations suitable for topical administration include, but are not limited
to, liquid
and/or semi-liquid preparations such as liniments, lotions, oil-in-water
and/or water-in-oil
emulsions such as creams, serums, ointments, and/or pastes, and/or solutions
and/or
suspensions. Topically administrable formulations may, for example, comprise
various
concentrations (w/w) of active ingredient, although the concentration of the
active ingredient
can be as high as the solubility limit of the active ingredient in the
solvent. Formulations for
topical administration may further comprise one or more of the additional
ingredients
described in this application.
Although the descriptions of pharmaceutical compositions provided in this
application
are principally directed to pharmaceutical or cosmetic compositions which are
suitable for
administration to humans, it will be understood by the skilled artisan that
such compositions
are generally suitable for administration to animals of all sorts.
Modification of
pharmaceutical or cosmetic compositions suitable for administration to humans
in order to
render the compositions suitable for administration to various animals is well
understood, and
the ordinarily skilled veterinary pharmacologist can design and/or perform
such modification
with ordinary experimentation.
Proteins and peptides provided in this application are typically formulated in
dosage
unit form for ease of administration and uniformity of dosage. It will be
understood, however,
that the total daily usage of the compositions described in this application
can be decided by a
physician within the scope of sound medical judgment. The specific
therapeutically effective
dose level for any particular subject or organism will depend upon a variety
of factors
including the volumes of skin wrinkles; the activity of the specific active
ingredient
employed; the specific composition employed; the age, body weight, general
health, and
gender of the subject; the time of administration, route of administration,
and rate of
28
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
excretion of the specific active ingredient employed; the duration of the
treatment; proteins or
peptides used in combination or coincidental with the specific active
ingredient employed;
and like factors well known in the medical arts.
In some embodiments, proteins or compositions disclosed in this application
are
formulated and/or administered in nanoparticles. Nanoparticles are particles
in the nanoscale.
In some embodiments, nanoparticles are less than 1 p.m in diameter. In some
embodiments,
nanoparticles are between about 1 and 100 nm in diameter. Nanoparticles
include organic
nanoparticles, such as dendrimers, liposomes, or polymeric nanoparticles.
Nanoparticles also
include inorganic nanoparticles, such as fullerenes, quantum dots, and gold
nanoparticles.
Compositions may comprise an aggregate of nanoparticles. In some embodiments,
the
aggregate of nanoparticles is homogeneous, while in other embodiments the
aggregate of
nanoparticles is heterogeneous.
The exact amount of a protein or peptide, or composition comprising a protein
or
peptide, required to achieve an effective amount will vary from subject to
subject, depending,
for example, on age, and general condition of a subject, identity of the
particular protein,
mode of administration, and the like. An effective amount may be included in a
single dose
(e.g., single oral dose) or multiple doses (e.g., multiple oral doses). In
certain embodiments,
when multiple doses are administered to a subject or applied to a tissue or
cell, any two doses
of the multiple doses include different or substantially the same amounts of a
protein
described in this application. Dosage forms may be administered at a variety
of frequencies.
In certain embodiments, when multiple doses are administered to a subject or
applied to a
tissue or cell, the frequency of administering the multiple doses to the
subject or applying the
multiple doses to the tissue or cell is three doses a day, two doses a day,
one dose a day, one
dose every other day, one dose every third day, one dose every week, one dose
every two
weeks, one dose every three weeks, or one dose every four weeks, or less
frequent than every
four weeks. In certain embodiments, the frequency of administering the
multiple doses to the
subject or applying the multiple doses to the tissue or cell is one dose per
day. In certain
embodiments, the frequency of administering the multiple doses to the subject
or applying the
multiple doses to the tissue or cell is two doses per day. In certain
embodiments, the
frequency of administering the multiple doses to the subject or applying the
multiple doses to
the tissue or cell is three doses per day. In certain embodiments, when
multiple doses are
administered to a subject or applied to a tissue or cell, the duration between
the first dose and
last dose of the multiple doses is one day, two days, four days, one week, two
weeks, three
29
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
weeks, one month, two months, three months, four months, six months, nine
months, one
year, two years, three years, four years, five years, seven years, ten years,
fifteen years,
twenty years, or the lifetime of the subject, tissue, or cell. In certain
embodiments, the
duration between the first dose and last dose of the multiple doses is three
months, six
months, or one year. In certain embodiments, the duration between the first
dose and last
dose of the multiple doses is the lifetime of the subject, tissue, or cell. In
some embodiments,
dose ranging studies can be conducted to establish optimal therapeutic or
effective amounts
of the component(s) (e.g., proteins or peptides) to be present in dosage
forms. In
embodiments, the component(s) are present in dosage forms in an amount
effective to
generate a preventative or therapeutic response to various signs of aging.
In some embodiments, the composition is provided to a subject in need thereof
preventatively; e.g., prior to the subject experiencing one or more signs of
aging. In some
embodiments, the composition is provided to a subject who perceives the need
thereof to
improve their own appearance. In some embodiments, the composition is provided
to a
subject about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or more than 10 years before the
onset of one or more
signs of aging. In some embodiments, the composition is provided to a subject
therapeutically, i.e., after the subject has one or more signs of aging. In
some embodiments,
the composition is provided to a subject about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10,
or more than 10
years after the onset of the one or more signs of aging. In some embodiments,
the
composition is provided both preventatively and, if necessary, therapeutically
(e.g., the
composition is administered prior to and following the onset of the one or
more signs of
aging). In some embodiments, the composition is provided to a subject about 1,
2, 3, 4, 5, 6,
7, 8, 9, 10, or more than 10 years before the onset of one or more signs of
aging and about 1,
2, 3, 4, 5, 6, 7, 8, 9, 10, or more than 10 years after the onset of one or
more signs of aging.
In some embodiments, more than one composition associated with the disclosure
is
administered to a subject. In some embodiments, the compositions are
administered
concomitantly. In other embodiments, the compositions are not administered
concomitantly.
In some embodiments, the first composition is not administered within 1 month,
1 week, 6
days, 5, days, 4 days, 3 days, 2 days, 1 day, 12 hour, 6 hours, 5 hours, 4
hours, 3 hours, 2
hours, or 1 hour of the second composition.
The term "concomitantly" refers to administering two or more materials/agents
to a
subject in a manner that is correlated in time, preferably sufficiently
correlated in time such
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
that a first composition has an effect on a second composition, such as
increasing the efficacy
of the second composition, preferably the two or more materials/agents are
administered in
combination. In some instance, a second composition has an effect on a first
composition,
such as regulating the efficacy of the first composition. In some embodiments,
concomitant
administration may encompass administration of two or more compositions within
a specified
period of time. In some embodiments, the two or more compositions are
administered within
1 month, within 1 week, within 1 day, or within 1 hour. In some embodiments,
concomitant
administration encompasses simultaneous administration of two or more
compositions. In
some embodiments, when two or more compositions are not administered
concomitantly,
there is little to no effect of the first composition on the second
composition.
The compositions provided in the present application may be administered
according
to a dosing schedule. As an example, any one of the subjects provided herein
may be treated
with a composition comprising one or more BMPs or proteins associated with a
BMP, such
as BMP2, BMP7, BMP9 and/or Noggin, according to any dosage schedule disclosed
herein.
In some embodiments, the concentration of the one or more BMPs in a
composition
for topical administration described in this application is less than about
1%, less than about
2%, less than about 3%, less than about 5%, less than about 10%, less than
about 15%, less
than about 20%, less than about 25%, less than about 30%, less than about 40%,
less than
about 50%, less than about 60%, less than about 70%, or less than about 80%,
of the
composition, inclusive of all ranges and subranges therebetween. In certain
embodiments, the
concentration of the one or more BMPs in the composition described in this
application is
less than about 3% of the composition. In some embodiments, the concentration
of the one or
more BMPs in the composition described in this application can be any
concentration suitable
for the method.
In some embodiments, the concentration of Noggin in a composition for topical
administration described in this application is less than about 1%, less than
about 2%, less
than about 3%, less than about 5%, less than about 10%, less than about 15%,
less than about
20%, less than about 25%, less than about 30%, less than about 40%, less than
about 50%,
less than about 60%, less than about 70%, less than about 80%, of the
composition, inclusive
of all ranges and subranges therebetween. In certain embodiments, the
concentration of
Noggin in the composition for topical administration described in this
application is less than
about 3% of the composition. In some embodiments, the concentration of Noggin
in the
31
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
composition for topical administration described in this application can be
any concentration
suitable for the method.
In some embodiments, the concentration of one or more BMP peptides in a
composition for topical administration described in this application is less
than about 1%, less
than about 2%, less than about 3%, less than about 5%, less than about 10%,
less than about
15%, less than about 20%, less than about 25%, less than about 30%, less than
about 40%,
less than about 50%, less than about 60%, less than about 70%, less than about
80%, of the
composition, inclusive of all ranges and subranges therebetween. In some
embodiments, the
concentration of the BMP peptide in the composition for topical administration
described in
this application is less than about 10% of the composition. In some
embodiments, the
concentration of the BMP peptide in the composition for topical administration
described in
this application can be any concentration suitable for the method.
In some embodiments, the concentration of a protein associated with a BMP in a
composition for topical administration described in this application is less
than about 1%, less
than about 2%, less than about 3%, less than about 5%, less than about 10%,
less than about
15%, less than about 20%, less than about 25%, less than about 30%, less than
about 40%,
less than about 50%, less than about 60%, less than about 70%, less than about
80%, of the
composition, inclusive of all ranges and subranges therebetween. In some
embodiments, the
concentration of proteins associated with a BMP in the composition for topical
administration
described in this application is less than about 10% of the composition. In
some
embodiments, the concentration of a protein associated with a BMP in the
composition for
topical administration described in this application can be any concentration
suitable for the
method.
In some embodiments, the one or more BMPs described in this application are
subdermally administered at a dose of about 50-1,500 ng/mL, about 100-1,000
ng/mL, about
150-950 ng/mL, about 200-900 ng/mL, about 250-850 ng/mL, about 300-800 ng/mL,
about
350-400 ng/mL, about 100-950 ng/mL, about 50-400 ng/mL, about 200-1,500 ng/mL,
about
500-600 ng/mL, about 550-850 ng/mL, or about 650-1,000 ng/mL per
administration site,
inclusive of all ranges and subranges therebetween, to a subject in need
thereof. In some
embodiments, the one or more BMPs described in this application are
subdermally
administered at a dose of about 100-1,000 ng/mL per administration site to a
subject in need
thereof. In some embodiments, the one or more BMPs described in this
application are
32
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
subdermally administered at any suitable dose per administration site to a
subject in need
thereof. In some embodiments, the one or more BMPs described in this
application are
subcutaneouslly or supraperiostially administered at any suitable dose per
administration site
to a subject in need thereof.
In some embodiments, Noggin is subdermally administered at a dose of about 50-
1,500 ng/mL, about 100-1,000 ng/mL, about 150-950 ng/mL, about 200-900 ng/mL,
about
250-850 ng/mL, about 300-800 ng/mL, about 350-400 ng/mL, about 100-950 ng/mL,
about
50-400 ng/mL, about 200-1,500 ng/mL, about 500-600 ng/mL, about 550-850 ng/mL,
or
about 650-1,000 ng/mL per administration site, inclusive of all ranges and
subranges
therebetween, to a subject in need thereof. In some embodiments, Noggin is
subdermally
administered at a dose of about 100-1,000 ng/mL per administration site to a
subject in need
thereof. In some embodiments, Noggin is subdermally administered at any
suitable dose per
administration site to a subject in need thereof. In some embodiments, Noggin
is
subcutaneouslly or supraperiostially administered at any suitable dose per
administration site
to a subject in need thereof.
In some embodiments, other proteins associated with a BMP described in this
application are subdermally administered at a dose of about 50-1,500 ng/mL,
about 100-
1,000 ng/mL, about 150-950 ng/mL, about 200-900 ng/mL, about 250-850 ng/mL,
about
300-800 ng/mL, about 350-400 ng/mL, about 100-950 ng/mL, about 50-400 ng/mL,
about
200-1,500 ng/mL, about 500-600 ng/mL, about 550-850 ng/mL, or about 650-1,000
ng/mL
per administration site, inclusive of all ranges and subranges therebetween,
to a subject in
need thereof. In some embodiments, other proteins associated with a BMP
described in this
application are subdermally administered at a dose of about 100-1,000 ng/mL
per
administration site to a subject in need thereof. In some embodiments, other
proteins
associated with a BMP described in this application are subdermally
administered at any
suitable dose per administration site to a subject in need thereof. In some
embodiments, a
protein associated with a BMP is subcutaneouslly or supraperiostially
administered at any
suitable dose per administration site to a subject in need thereof.
Dose ranges as described in this application provide guidance for the
administration
of provided pharmaceutical or cosmetic compositions to an adult. The amount to
be
administered to, for example, an elderly subject can be determined by a
medical practitioner
33
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
or person skilled in the art and can, in some embodiments, be lower or higher
than the
amount that is administered to a younger subject.
Compositions as described in this application can be administered in
combination
with one or more additional pharmaceutical or cosmetic agents (e.g.,
therapeutically and/or
prophylactically active agents). The compounds or compositions can be
administered in
combination with additional pharmaceutical agents that improve their activity,
improve
bioavailability, improve safety, reduce and/or modify metabolism, inhibit
excretion, and/or
modify distribution in a subject or cell. It will also be appreciated that the
therapy employed
may achieve a desired effect for the same purpose such as skin wrinkle
reduction, and/or it
may achieve different effects. In certain embodiments, a pharmaceutical or
cosmetic
composition described in this application, including a protein or peptide
described in this
application and an additional pharmaceutical agent, shows a synergistic effect
that is absent
in a pharmaceutical or cosmetic composition including one of the proteins or
peptides
described in this application and the additional pharmaceutical agent, but not
both.
The composition can be administered concurrently with, prior to, or subsequent
to one
or more additional pharmaceutical or cosmetic agents, which may be useful as,
e.g.,
combination therapies. Pharmaceutical agents include therapeutically active
agents.
Pharmaceutical agents also include prophylactically active agents.
Pharmaceutical agents
include small organic molecules such as drug compounds (e.g., compounds
approved for
human or veterinary use by the U.S. Food and Drug Administration as provided
in the Code
of Federal Regulations (CFR)), peptides, proteins, carbohydrates,
monosaccharides,
oligosaccharides, polysaccharides, nucleoproteins, mucoproteins, lipoproteins,
synthetic
polypeptides or proteins, small molecules linked to proteins, glycoproteins,
steroids, nucleic
acids, DNAs, RNAs, nucleotides, nucleosides, oligonucleotides, antisense
oligonucleotides,
lipids, hormones, vitamins, and cells. In certain embodiments, the additional
pharmaceutical
or cosmetic agent is a pharmaceutical or cosmetic agent useful for preventing
or alleviating
signs of aging (e.g., preventing or reducing skin wrinkles, enhancing or
preserving facial
contours). Each additional pharmaceutical or cosmetic agent may be
administered at a dose
and/or on a time schedule determined for that pharmaceutical or cosmetic
agent. The
additional pharmaceutical or cosmetic agents may also be administered together
with each
other and/or with the composition described in this application in a single
dose or
administered separately in different doses. The particular combination to
employ in a regimen
will take into account compatibility of the protein or peptide described in
this application
34
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
with the additional pharmaceutical or cosmetic agent(s) and/or the desired
therapeutic and/or
prophylactic effect to be achieved. In general, it is expected that the
additional
pharmaceutical or cosmetic agent(s) in combination be utilized at levels that
do not exceed
the levels at which they are utilized individually. In some embodiments, the
levels utilized in
combination will be lower than those utilized individually.
In some embodiments, one or more additional pharmaceutical or cosmetic agents
can
be any agents that are useful for anti-aging purposes. A non-limiting list of
pharmaceutical or
cosmetic agents can include botulinum toxin (Botox), fillers, sunscreens,
skincare products,
antioxidants such as polyphenols, flavonoids, Vitamins C, B3, E, and K,
hormone
replacement therapy, collagens, cell regulators, such as retinols and their
derivates (retinyl
palmitate, retinyl propionate, retinyl linoleate and other retinyl complex),
peptides and
growth factors, superficial peels (a-f3-, lipo-hydroxy acids (HA),
trichloroacetic acid (TCA)
10-30%), medium-depth peels (TCA above 30 to 50%), deep peels (TCA > 50%,
phenol),
placental extract and polycyclic aromatic hydrocarbons (PAHs), natural plant
such as
tragacanth, natural oils extracts such as pomegranate oil, fennel oil, citrus
oil, rosemary oil,
chamomile oil, jojoba oil, rosehip oil, biological active ingredients such as
acetyl
hexapeptide-8, aspartic acid, minerals such as Zn or Mg Silicates, platelet-
rich plasma (PRP),
and other natural additives such as glutathione, hyaluronic acid. One or more
aesthetic
procedures (invasive or non-invasive) can be implemented in combination with
compositions
disclosed herein comprising one or more BMPs and/or proteins associated with a
BMP. A
non-limiting list of aesthetic procedures can include visible light devices,
intense pulsed light
(HT), ablative and nonablative laser photo¨rejuvenation, radiofrequency (RF),
injectable skin
biostimulation and rejuvenation, prevention of dynamic wrinkles, correction of
static,
anatomical wrinkles, restoration (redistribution) of fat and volume loss, and
skin
augmentation and contouring.
In some embodiments, compositions can be administered concurrently with, prior
to,
or subsequent to one or more additional pharmaceutical or cosmetic agents by
using devices
for enhanced penetration through the skin. For example, devices that rely on
ultrasound,
microcurrent, or microneedles can be used. In some embodiments, the devices
are also used
for delivering the one or more additional pharmaceutical or cosmetic agents.
Also encompassed by the disclosure are kits (e.g., pharmaceutical or cosmetic
packs)
comprising a composition comprising one or more BMPs and/or one or more
proteins
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
associated with a BMP for use in administering the composition to prevent or
reduce skin
wrinkles and/or to enhance or preserve facial contours. The kits provided may
comprise a
composition, such as a pharmaceutical or cosmetic composition, or a protein or
peptide
described in this application, and a container (e.g., a vial, ampule, bottle,
syringe, and/or
dispenser package, or other suitable container). In some embodiments, provided
kits may
optionally further include a second container comprising a pharmaceutical
excipient for
dilution or suspension of a pharmaceutical or cosmetic composition or a
protein or peptide
described in this application. In some embodiments, the pharmaceutical or
cosmetic
composition or a protein or peptide described in this application provided in
a first container
and a second container are combined to form one unit dosage form. Thus, in one
aspect,
provided are kits including a first container comprising a composition,
protein, or peptide
described in this application. In certain embodiments, the kits are useful for
alleviating signs
of aging in a subject in need thereof. In certain embodiments, the kits are
useful for
preventing or reducing the appearance of skin wrinkles in a subject in need
thereof. In certain
embodiments, the kits are useful for enhancing or preserving facial contours
in a subject in
need thereof.
In certain embodiments, a kit described in this application further includes
instructions for using the kit. A kit described in this application may also
include information
as required by a regulatory agency such as the U.S. Food and Drug
Administration (FDA). In
certain embodiments, the information included in the kits is prescribing
information. In
certain embodiments, the kits and instructions provide for alleviating signs
of aging in a
subject in need thereof. In certain embodiments, the kits and instructions
provide for
preventing or reducing the appearance of skin wrinkles in a subject in need
thereof. In certain
embodiments, the kits and instructions provide for improving skin texture or
evenness of skin
tone in a subject in need thereof. In certain embodiments, the kits and
instructions provide for
enhancing or preserving facial contours in a subject in need thereof. In
certain embodiments,
the kits and instructions provide for improving the attractiveness of facial
contours in a
subject in need thereof. A kit described in this application may include one
or more additional
pharmaceutical agents described in this application as a separate composition
or one or more
additional aesthetic procedures as a separate instruction (e.g.,
recommendation for receiving
intense pulsed light).
Host cells
36
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
The term "host cell" refers to a cell that can be used to express a
polynucleotide, such
as a polynucleotide that encodes a BMP or a protein associated with a BMP. The
terms
"genetically modified host cell," "recombinant host cell," and "recombinant
strain" are used
interchangeably and refer to host cells that have been genetically modified
by, e.g., cloning
and transformation methods, or by other methods known in the art (e.g.,
selective editing
methods). Thus, the terms include a host cell (e.g., bacterial cell, yeast
cell, fungal cell, insect
cell, plant cell, mammalian cell, human cell, etc.) that has been genetically
altered, modified,
or engineered, so that it exhibits an altered, modified, or different genotype
and/or phenotype,
as compared to the naturally-occurring cell from which it was derived. It is
understood that
the term "cell," as used in this application, may refer to a single cell or a
population of cells,
such as a population of cells belonging to the same cell line or strain. Use
of the singular
term "cell" should not be construed to refer explicitly to a single cell
rather than a population
of cells.
The term "heterologous" with respect to a polynucleotide, such as a
polynucleotide
comprising a gene, is used interchangeably with the term "exogenous" and the
term
"recombinant" and refers to: a polynucleotide that has been artificially
supplied to a
biological system; a polynucleotide that has been modified within a biological
system, or a
polynucleotide whose expression or regulation has been manipulated within a
biological
system. A heterologous polynucleotide that is introduced into or expressed in
a host cell may
be a polynucleotide that comes from a different organism or species than the
host cell, or may
be a synthetic polynucleotide, or may be a polynucleotide that is also
endogenously expressed
in the same organism or species as the host cell. For example, a
polynucleotide that is
endogenously expressed in a host cell may be considered heterologous when it
is situated
non-naturally in the host cell; expressed recombinantly in the host cell,
either stably or
transiently; modified within the host cell; selectively edited within the host
cell; expressed in
a copy number that differs from the naturally occurring copy number within the
host cell; or
expressed in a non-natural way within the host cell, such as by manipulating
regulatory
regions that control expression of the polynucleotide. In some embodiments, a
heterologous
polynucleotide is a polynucleotide that is endogenously expressed in a host
cell but whose
expression is driven by a promoter that does not naturally regulate expression
of the
polynucleotide. In other embodiments, a heterologous polynucleotide is a
polynucleotide that
is endogenously expressed in a host cell and whose expression is driven by a
promoter that
does naturally regulate expression of the polynucleotide, but the promoter or
another
37
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
regulatory region is modified. In some embodiments, the promoter is
recombinantly
activated or repressed. For example, gene-editing based techniques may be used
to regulate
expression of a polynucleotide, including an endogenous polynucleotide, from a
promoter,
including an endogenous promoter. See, e.g., Chavez et al., Nat Methods. 2016
Jul; 13(7):
563-567. A heterologous polynucleotide may comprise a wild-type sequence or a
mutant
sequence as compared with a reference polynucleotide sequence.
Suitable host cells include, but are not limited to: yeast cells, bacterial
cells, algal
cells, plant cells, fungal cells, insect cells, and animal cells, including
mammalian cells.
Suitable yeast host cells include, but are not limited to: Candida, Hansenula,
Saccharomyces, Schizosaccharomyces, Pichia, Kluyveromyces, and Yarrowia. In
some
embodiments, the yeast cell is Hansenula polymorpha, Saccharomyces cerevisiae,
Saccaromyces carlsbergensis, Saccharomyces diastaticus, Saccharomyces
norbensis,
Saccharomyces kluyveri, Schizosaccharomyces pombe, Komagataella phaffii,
formerly
known as Pichia pastoris, Pichia finlandica, Pichia trehalophila, Pichia
kodamae, Pichia
membranaefaciens, Pichia opuntiae, Pichia thermotolerans, Pichia salictaria,
Pichia
quercuum, Pichia pijperi, Pichia stipitis, Pichia methanolica, Pichia angusta,
Kluyveromyces
lactis, Candida albicans, or Yarrowia lipolytica.
In some embodiments, the yeast strain is an industrial polyploid yeast strain.
Other
non-limiting examples of fungal cells include cells obtained from Aspergillus
spp.,
Penicillium spp., Fusarium spp., Rhizopus spp., Acremonium spp., Neurospora
spp., Sordaria
spp., Magnaporthe spp., Allomyces spp., Ustilago spp., Botrytis spp., and
Trichoderma spp.
In certain embodiments, the host cell is an algal cell such as, Chlamydomonas
(e.g., C.
Reinhardtii) and Phormidium (P. sp. ATCC29409).
In other embodiments, the host cell is a prokaryotic cell. Suitable
prokaryotic cells
include gram positive, gram negative, and gram-variable bacterial cells. The
host cell may be
a species of, but not limited to: Agrobacterium, Alicyclobacillus, Anabaena,
Anacystis,
Acinetobacter, Acidothermus, Arthrobacter, Azobacter, Bacillus,
Bifidobacterium,
Brevibacterium, Butyrivibrio, Buchnera, Campestris, Camplyobacter,
Clostridium,
Corynebacterium, Chromatium, Coprococcus, Escherichia, Enterococcus,
Enterobacter,
Erwinia, Fusobacterium, Faecalibacterium, Francisella, Flavobacterium,
Geobacillus,
Haemophilus, Helicobacter, Klebsiella, Lactobacillus, Lactococcus, Ilyobacter,
Micrococcus,
Microbacterium, Mesorhizobium, Methylobacterium, Methylobacterium,
Mycobacterium,
38
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
Neisseria, Pantoea, Pseudomonas, Prochlorococcus, Rhodobacter,
Rhodopseudomonas,
Rhodopseudomonas, Roseburia, Rhodospirillum, Rhodococcus, Scenedesmus,
Streptomyces,
Streptococcus, Synecoccus, Saccharomonospora, Saccharopolyspora,
Staphylococcus,
Serratia, Salmonella, Shigella, Thermoanaerobacterium, Tropheryma, Tularensis,
Temecula,
Thermos ynechococcus, Thermococcus, Ureaplasma, Xanthomonas, Xylella,
Yersinia, and Zymomonas.
In some embodiments, the bacterial host strain is an industrial strain.
Numerous
bacterial industrial strains are known and suitable for the methods and
compositions
described in this application. In some embodiments, the bacterial host cell is
of
the Agrobacterium species (e.g., A. radiobacter, A. rhizogenes, A. rubi),
the Arthrobacterspecies (e.g., A. aurescens, A. citreus, A. globformis, A.
hydrocarboglutamicus, A. mysorens, A. nicotianae, A. paraffineus, A.
protophonniae, A.
roseoparaffinus, A. sulfureus, A. ureafaciens), the Bacillus species (e.g., B.
thuringiensis, B.
anthracis, B. megaterium, B. subtilis, B. lentus, B. circulars, B. pumilus, B.
lautus, B.
coagulans, B. brevis, B. firmus, B. alkaophius, B. lichernformis, B. clausii,
B.
stearothermophilus, B. halodurans and B. amyloliquefaciens. In particular
embodiments, the
host cell will be an industrial Bacillus strain including but not limited to
B. subtilis, B.
pumilus, B. lichernformis, B. megaterium, B. clausii, B. stearothermophilus
and B.
amyloliquefaciens. In some embodiments, the host cell will be an industrial
Clostridium species (e.g., C. acetobutylicum, C. tetani E88, C. lituseburense,
C.
saccharobutylicum, C. perfringens, C. beijerinckii). In some embodiments, the
host cell will
be an industrial Corynebacterium species (e.g., C. glutamicum, C.
acetoacidophilum). In
some embodiments, the host cell will be an industrial Escherichia species
(e.g., E. coli). In
some embodiments, the host cell will be an industrial Erwinia species (e.g.,
E. uredovora, E.
carotovora, E. ananas, E. herbicola, E. punctata, E. terreus). In some
embodiments, the host
cell will be an industrial Pantoea species (e.g., P. citrea, P. agglomerans).
In some
embodiments, the host cell will be an industrial Pseudomonas species, (e.g.,
P. putida, P.
aeruginosa, P. mevalonii). In some embodiments, the host cell will be an
industrial Streptococcus species (e.g., S. equisimiles, S. pyo genes, S.
uberis). In some
embodiments, the host cell will be an industrial Streptomyces species (e.g.,
S. ambofaciens, S.
achromo genes, S. avermitilis, S. coelicolor, S. aureofaciens, S. aureus, S.
fun gicidicus, S.
griseus, S. lividans). In some embodiments, the host cell will be an
industrial Zymomonas
species (e.g., Z. mobilis, Z. lipolytica), and the like.
39
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
The present disclosure is also suitable for use with a variety of animal cell
types,
including mammalian cells, for example, human (including HEK 293, HEK 293T,
A549,
HepG2, HeLa, WI38, PER.C6 and Bowes melanoma cells), non-human primate
(including
COS-1, COS-7) mouse (including 3T3, C2C12, ROS 17/2.8 (osteosarcoma cells),
NSO, NS1,
Sp2/0), hamster (CHO, BHK), monkey (COS, FRhL, Vero), insect cells, for
example fall
armyworm (including Sf9 and Sf21), silkmoth (including BmN), cabbage looper
(including
BTI-Tn-5B1-4) and common fruit fly (including Schneider 2), and hybridoma cell
lines.
In some embodiments, the effect of BMPs and/or proteins associated with BMPs
is
tested in one or more cell types such as mesenchymal stem cells, preosteoblast
cells, and/or
osteoblast cells.
In various embodiments, strains that may be used in the practice of the
disclosure
including both prokaryotic and eukaryotic strains, and are readily accessible
to the public
from a number of culture collections such as American Type Culture Collection
(ATCC),
Deutsche Sammlung von Mikroorganismen and Zellkulturen GmbH (DSM),
Centraalbureau
Voor Schimmelcultures (CBS), and Agricultural Research Service Patent Culture
Collection,
Northern Regional Research Center (NRRL).
Culturing of Host Cells
Any of the cells disclosed in this application can be cultured in media of any
type
(rich or minimal) and any composition prior to, during, and/or after contact
and/or integration
of a nucleic acid. The conditions of the culture or culturing process can be
optimized through
routine experimentation as would be understood by one of ordinary skill in the
art. In some
embodiments, the selected media is supplemented with various components. In
some
embodiments, the concentration and amount of a supplemental component is
optimized. In
some embodiments, other aspects of the media and growth conditions (e.g., pH,
temperature,
etc.) are optimized through routine experimentation. In some embodiments, the
frequency
that the media is supplemented with one or more supplemental components, and
the amount
of time that the cell is cultured, is optimized.
Culturing of the cells described in this application can be performed in
culture vessels
known and used in the art. In some embodiments, an aerated reaction vessel
(e.g., a stirred
tank reactor) is used to culture the cells. In some embodiments, a bioreactor
or fermenter is
used to culture the cell. Thus, in some embodiments, the cells are used in
fermentation. As
used in this application, the terms "bioreactor" and "fermenter" are
interchangeably used and
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
refer to an enclosure, or partial enclosure, in which a biological,
biochemical and/or chemical
reaction takes place that involves a living organism or part of a living
organism. A "large-
scale bioreactor" or "industrial-scale bioreactor" is a bioreactor that is
used to generate a
product on a commercial or quasi-commercial scale. Large scale bioreactors
typically have
volumes in the range of liters, hundreds of liters, thousands of liters, or
more.
Non-limiting examples of bioreactors include: stirred tank fermenters,
bioreactors
agitated by rotating mixing devices, chemostats, bioreactors agitated by
shaking devices,
airlift fermenters, packed-bed reactors, fixed-bed reactors, fluidized bed
bioreactors,
bioreactors employing wave induced agitation, centrifugal bioreactors, roller
bottles, and
hollow fiber bioreactors, roller apparatuses (for example benchtop, cart-
mounted, and/or
automated varieties), vertically-stacked plates, spinner flasks, stirring or
rocking flasks,
shaken multi-well plates, MD bottles, T-flasks, Roux bottles, multiple-surface
tissue culture
propagators, modified fermenters, and coated beads (e.g., beads coated with
serum proteins,
nitrocellulose, or carboxymethyl cellulose to prevent cell attachment).
In some embodiments, the bioreactor includes a cell culture system where the
cell
(e.g., yeast cell) is in contact with moving liquids and/or gas bubbles. In
some embodiments,
the cell or cell culture is grown in suspension. In other embodiments, the
cell or cell culture
is attached to a solid phase carrier. Non-limiting examples of a carrier
system includes
microcarriers (e.g., polymer spheres, microbeads, and microdisks that can be
porous or non-
porous), cross-linked beads (e.g., dextran) charged with specific chemical
groups (e.g.,
tertiary amine groups), 2D microcarriers including cells trapped in nonporous
polymer fibers,
3D carriers (e.g., carrier fibers, hollow fibers, multicartridge reactors, and
semi-permeable
membranes that can comprising porous fibers), microcarriers having reduced ion
exchange
capacity, encapsulation cells, capillaries, and aggregates. In some
embodiments, carriers are
fabricated from materials such as dextran, gelatin, glass, or cellulose.
In some embodiments, industrial-scale processes are operated in continuous,
semi-
continuous or non-continuous modes. Non-limiting examples of operation modes
are batch,
fed batch, extended batch, repetitive batch, draw/fill, rotating-wall,
spinning flask, and/or
perfusion mode of operation. In some embodiments, a bioreactor allows
continuous or semi-
continuous replenishment of the substrate stock, for example a carbohydrate
source and/or
continuous or semi-continuous separation of the product, from the bioreactor.
41
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
In some embodiments, the bioreactor or fermenter includes a sensor and/or a
control
system to measure and/or adjust reaction parameters. Non-limiting examples of
reaction
parameters include biological parameters (e.g., growth rate, cell size, cell
number, cell
density, cell type, or cell state, etc.), chemical parameters (e.g., pH, redox-
potential,
concentration of reaction substrate and/or product, concentration of dissolved
gases, such as
oxygen concentration and CO2 concentration, nutrient concentrations,
metabolite
concentrations, concentration of an oligopeptide, concentration of an amino
acid,
concentration of a vitamin, concentration of a hormone, concentration of an
additive, serum
concentration, ionic strength, concentration of an ion, relative humidity,
molarity, osmolarity,
concentration of other chemicals, for example buffering agents, adjuvants, or
reaction by-
products), physical/mechanical parameters (e.g., density, conductivity, degree
of agitation,
pressure, and flow rate, shear stress, shear rate, viscosity, color,
turbidity, light absorption,
mixing rate, conversion rate, as well as thermodynamic parameters, such as
temperature, light
intensity/quality, etc.). Sensors to measure the parameters described in this
application are
well known to one of ordinary skill in the relevant mechanical and electronic
arts. Control
systems to adjust the parameters in a bioreactor based on the inputs from a
sensor described
in this application are well known to one of ordinary skill in the art in
bioreactor engineering.
In some embodiments, the method involves batch fermentation (e.g., shake flask
fermentation). General considerations for batch fermentation (e.g., shake
flask fermentation)
include the level of oxygen and glucose. For example, batch fermentation
(e.g., shake flask
fermentation) may be oxygen and glucose limited, so in some embodiments, the
capability of
a strain to perform in a well-designed fed-batch fermentation is
underestimated.
In some embodiments, the cells of the present disclosure are adapted to
produce
BMPs or proteins associated with a BMP in vivo. In some embodiments, the cells
are adapted
to secrete one or more BMPs or proteins associated with a BMP. In some
embodiments, the
cells of the present disclosure are lysed, and the remaining lysates are
recovered for
subsequent use. In some embodiments, any of the methods described in this
application may
include isolation and/or purification of BMPs or proteins associated with a
BMP. For
example, the isolation and/or purification can involve one or more of cell
lysis,
centrifugation, extraction, column chromatography, distillation,
crystallization, and
lyophilization.
42
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
Expression of Nucleic Acids in Host Cells
Aspects of the present disclosure relate to recombinant proteins, functional
modifications and variants thereof, as well as their uses. For example, the
methods described
in this application may be used to produce BMPs or proteins associated with a
BMP. The
methods may comprise using a host cell comprising a protein or peptide
disclosed in this
application, cell lysate, isolated protein or peptide, or any combination
thereof. Methods
comprising recombinant expression of genes encoding a protein or peptide
disclosed in this
application in a host cell are encompassed by the present disclosure.
A nucleic acid encoding any of the recombinant polypeptides (e.g., BMP2, BMP9,
Noggin) described in this application may be incorporated into any appropriate
vector
through any method known in the art. For example, the vector may be an
expression vector,
including but not limited to a viral vector (e.g., a lentiviral, retroviral,
adenoviral, or adeno-
associated viral vector), any vector suitable for transient expression, any
vector suitable for
constitutive expression, or any vector suitable for inducible expression
(e.g., a galactose-
inducible or doxycycline-inducible vector).
A vector encoding any of the recombinant polypeptides (e.g., BMP2, BMP9,
Noggin)
described in this application may be introduced into a suitable host cell
using any method
known in the art. Non-limiting examples of yeast transformation protocols are
described in
Gietz et al., Yeast transformation can be conducted by the LiAc/SS Carrier
DNA/PEG
method. Methods Mol Biol. 2006;313:107-20, which is hereby incorporated by
reference in
its entirety. Host cells may be cultured under any conditions suitable as
would be understood
by one of ordinary skill in the art. For example, any media, temperature, and
incubation
conditions known in the art may be used. For host cells carrying an inducible
vector, cells
may be cultured with an appropriate inducible agent to promote expression.
In some embodiments, a vector replicates autonomously in the cell. In some
embodiments, a vector integrates into a chromosome within a cell. A vector can
contain one
or more endonuclease restriction sites that are cut by a restriction
endonuclease to insert and
ligate a nucleic acid containing a gene described in this application to
produce a recombinant
vector that is able to replicate in a cell. Vectors are typically composed of
DNA, although
RNA vectors are also available. Cloning vectors include, but are not limited
to: plasmids,
fosmids, phagemids, virus genomes and artificial chromosomes. As used in this
application,
the terms "expression vector" or "expression construct" refer to a nucleic
acid construct,
43
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
generated recombinantly or synthetically, with a series of specified nucleic
acid elements that
permit transcription of a particular nucleic acid in a host cell. In some
embodiments, the
nucleic acid sequence of a gene described in this application is inserted into
a cloning vector
so that it is operably joined to regulatory sequences and, in some
embodiments, expressed as
an RNA transcript. In some embodiments, the vector contains one or more
markers, such as
a selectable marker as described in this application, to identify cells
transformed or
transfected with the recombinant vector. In some embodiments, a host cell has
already been
transformed with one or more vectors. In some embodiments, a host cell that
has been
transformed with one or more vectors is subsequently transformed with one or
more vectors.
In some embodiments, a host cell is transformed simultaneously with more than
one vector.
In some embodiments, a cell that has been transformed with a vector or an
expression
cassette incorporates all or part of the vector or expression cassette into
its genome. In some
embodiments, the nucleic acid sequence of a gene described in this application
is codon-
optimized. Codon optimization may increase production of the gene product by
at least 10%,
at least 15%, at least 20%, at least 25%, at least 30%, at least 35%, at least
40%, at least 45%,
at least 50%, at least 55%, at least 60%, at least 65%, at least 70%, at least
75%, at least 80%,
at least 85%, at least 90%, at least 95%, or 100%, including all values in
between) relative to
a reference sequence that is not codon-optimized.
In some embodiments, the nucleic acid encoding any of the proteins described
in this
application is under the control of regulatory sequences (e.g., enhancer
sequences). In some
embodiments, a nucleic acid is expressed under the control of a promoter. The
promoter can
be a native promoter, e.g., the promoter of the gene in its endogenous
context, which provides
normal regulation of expression of the gene. Alternatively, a promoter can be
a promoter that
is different from the native promoter of the gene, e.g., the promoter is
different from the
promoter of the gene in its endogenous context.
In some embodiments, the promoter is a eukaryotic promoter. Non-limiting
examples
of eukaryotic promoters include TDH3, PGK1, PKC1, PDC1, TEF1, TEF2, RPL18B,
SSA1,
TDH2, PYK1, TPI1, GAL1, GAL10, GAL7, GAL3, GAL2, MET3, MET25, HXT3, HXT7,
ACT1, ADH1, ADH2, CUP1-1, EN02, and SOD1, as would be known to one of ordinary
skill in the art (see, e.g., Addgene website: blog.addgene.org/plasmids-101-
the-promoter-
region). In some embodiments, the promoter is a prokaryotic promoter (e.g.,
bacteriophage
or bacterial promoter). Non-limiting examples of bacteriophage promoters
include Pls icon,
44
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
T3, T7, SP6, and PL. Non-limiting examples of bacterial promoters include
Pbad, PmgrB,
Ptrc2, Plac/ara, Ptac, and Pm.
In some embodiments, the promoter is an inducible promoter. As used in this
application, an "inducible promoter" is a promoter controlled by the presence
or absence of a
molecule. Non-limiting examples of inducible promoters include chemically
regulated
promoters and physically regulated promoters. For chemically regulated
promoters, the
transcriptional activity can be regulated by one or more compounds, such as
methanol,
alcohol, tetracycline, galactose, a steroid, a metal, an amino acid, or other
compounds. For
physically regulated promoters, transcriptional activity can be regulated by a
phenomenon
such as light or temperature. Non-limiting examples of tetracycline-regulated
promoters
include anhydrotetracycline (aTc)-responsive promoters and other tetracycline-
responsive
promoter systems (e.g., a tetracycline repressor protein (tetR), a
tetracycline operator
sequence (tet0) and a tetracycline transactivator fusion protein (tTA)). Non-
limiting
examples of steroid-regulated promoters include promoters based on the rat
glucocorticoid
receptor, human estrogen receptor, moth ecdysone receptors, and promoters from
the
steroid/retinoid/thyroid receptor superfamily. Non-limiting examples of metal-
regulated
promoters include promoters derived from metallothionein (proteins that bind
and sequester
metal ions) genes. Non-limiting examples of pathogenesis-regulated promoters
include
promoters induced by salicylic acid, ethylene or benzothiadiazole (BTH). Non-
limiting
examples of temperature/heat-inducible promoters include heat shock promoters.
Non-
limiting examples of light-regulated promoters include light responsive
promoters from plant
cells. In certain embodiments, the inducible promoter is a galactose-inducible
promoter. In
some embodiments, the inducible promoter is induced by one or more
physiological
conditions (e.g., pH, temperature, radiation, osmotic pressure, saline
gradients, cell surface
binding, or concentration of one or more extrinsic or intrinsic inducing
agents). Non-limiting
examples of an extrinsic inducer or inducing agent include amino acids and
amino acid
analogs, saccharides and polysaccharides, nucleic acids, protein
transcriptional activators and
repressors, cytokines, toxins, petroleum-based compounds, metal containing
compounds,
salts, ions, enzyme substrate analogs, hormones or any combination.
In some embodiments, the promoter is a constitutive promoter. As used in this
application, a "constitutive promoter" refers to an unregulated promoter that
allows
continuous transcription of a gene. Non-limiting examples of a constitutive
promoter include
TDH3, PGK1, PKC1, PDC1, TEF1, TEF2, RPL18B, SSA1, TDH2, PYK1, TPI1, HXT3,
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
HXT7, ACT1, ADH1, ADH2, EN02, and SOD1. Other inducible promoters or
constitutive
promoters, including synthetic promoters, that may be known to one of ordinary
skill in the
art are also contemplated.
The precise nature of the regulatory sequences needed for gene expression may
vary
between species or cell types, but generally include, as necessary, 5' non-
transcribed and 5'
non-translated sequences involved with the initiation of transcription and
translation
respectively, such as a TATA box, capping sequence, CAAT sequence, and the
like. In
particular, such 5' non-transcribed regulatory sequences will include a
promoter region which
includes a promoter sequence for transcriptional control of the operably
joined gene.
Regulatory sequences may also include enhancer sequences or upstream activator
sequences.
The vectors disclosed may include 5' leader or signal sequences. The
regulatory sequence
may also include a terminator sequence. In some embodiments, a terminator
sequence marks
the end of a gene in DNA during transcription. The choice and design of one or
more
appropriate vectors suitable for inducing expression of one or more genes
described in this
application in a heterologous organism is within the ability and discretion of
one of ordinary
skill in the art. Expression vectors containing the necessary elements for
expression are
commercially available and known to one of ordinary skill in the art (see,
e.g., Sambrook et
al., Molecular Cloning: A Laboratory Manual, Fourth Edition, Cold Spring
Harbor
Laboratory Press, 2012).
Variants
Aspects of the disclosure relate to nucleic acids encoding any of the
polypeptides
(e.g., BMP2, BMP7, BMP9, Noggin) described in this application. In some
embodiments, a
nucleic acid encompassed by the disclosure is a nucleic acid that hybridizes
under high or
medium stringency conditions to a nucleic acid encoding a protein or peptide
(e.g., BMP2,
BMP7, BMP9, Noggin) and is biologically active. For example, high stringency
conditions of
0.2 to 1 x SSC at 65 C followed by a wash at 0.2 x SSC at 65 C can be used.
In some
embodiments, a nucleic acid encompassed by the disclosure is a nucleic acid
that hybridizes
under low stringency conditions to a nucleic acid encoding a protein or
peptide (e.g., BMP2,
BMP7, BMP9, Noggin) and is biologically active. For example, low stringency
conditions of
6 x SSC at room temperature followed by a wash at 2 x SSC at room temperature
can be
used. Other hybridization conditions include 3 x SSC at 40 or 50 C, followed
by a wash in 1
or 2 x SSC at 20, 30, 40, 50, 60, or 65 'C.
46
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
Hybridizations can be conducted in the presence of formaldehyde, e.g., 10%,
20%,
30% 40% or 50%, which further increases the stringency of hybridization.
Theory and
practice of nucleic acid hybridization is described, e.g., in S. Agrawal (ed.)
Methods in
Molecular Biology, volume 20; and Tijssen (1993) Laboratory Techniques in
biochemistry
and molecular biology-hybridization with nucleic acid probes, e.g., part I
chapter 2
"Overview of principles of hybridization and the strategy of nucleic acid
probe assays,"
Elsevier, New York provide a basic guide to nucleic acid hybridization.
Variants of proteins or peptides (or derivatives thereof) described in this
application
(e.g., BMP2, BMP7, BMP9, Noggin) are also encompassed by the present
disclosure. A
variant may share at least 5%, at least 10%, at least 15%, at least 20%, at
least 25%, at least
30%, at least 35%, at least 40%, at least 45%, at least 50%, at least 55%, at
least 60%, at least
65%, at least 70%, at least 71%, at least 72%, at least 73%, at least 74%, at
least 75%, at least
76%, at least 77%, at least 78%, at least 79%, at least 80%, at least 81%, at
least 82%, at least
83%, at least 84%, at least 85%, at least 86%, at least 87%, at least 88%, at
least 89%, at least
90%, at least 91%, at least 92%, at least 93%, at least 94%, at least 95%, at
least 96%, at least
97%, at least 98%, at least 99%, or 100% sequence identity with a reference
sequence,
including all values in between.
Unless otherwise noted, the term "sequence identity," which is used
interchangeably
in this disclosure with the term "percent identity," as known in the art,
refers to a relationship
between the sequences of two polypeptides or polynucleotides, as determined by
sequence
comparison (alignment). In some embodiments, sequence identity is determined
across the
entire length of a sequence (e.g., BMP2, BMP7, BMP9, Noggin sequence). In some
embodiments, sequence identity is determined over a region (e.g., a stretch of
amino acids or
nucleic acids, e.g., the sequence spanning an active site) of a sequence
(e.g., BMP2, BMP7,
BMP9, Noggin). For example, in some embodiments, sequence identity is
determined over a
region corresponding to at least 30%, at least 40%, at least 50%, at least
60%, at least 70%, at
least 80%, at least 90%, at least 95%, or over 100% of the length of the
reference sequence.
Identity measures the percent of identical matches between the smaller of two
or more
sequences with gap alignments (if any) addressed by a particular mathematical
model,
algorithm, or computer program. Identity of related polypeptides or nucleic
acid sequences
can be readily calculated by any of the methods known to one of ordinary skill
in the art. The
percent identity of two sequences (e.g., nucleic acid or amino acid sequences)
may, for
47
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
example, be determined using the algorithm of Karlin and Altschul Proc. Natl.
Acad. Sci.
USA 87:2264-68, 1990, modified as in Karlin and Altschul Proc. Natl. Acad.
Sci. USA
90:5873-77, 1993. Such an algorithm is incorporated into the NBLAST and
XBLAST
programs (version 2.0) of Altschul et al., J. Mol. Biol. 215:403-10, 1990.
BLAST protein
searches can be performed, for example, with the XBLAST program, score=50,
wordlength=3 to obtain amino acid sequences homologous to the proteins
described in this
application. Where gaps exist between two sequences, Gapped BLAST can be
utilized, for
example, as described in Altschul et al., Nucleic Acids Res. 25(17):3389-3402,
1997. When
utilizing BLAST and Gapped BLAST programs, the default parameters of the
respective
programs (e.g., XBLAST and NBLAST ) can be used, or the parameters can be
adjusted
appropriately as would be understood by one of ordinary skill in the art.
Another local alignment technique which may be used, for example, is based on
the
Smith-Waterman algorithm (Smith, T.F. & Waterman, M.S. (1981) "Identification
of
common molecular subsequences." J. Mol. Biol. 147:195-197). A general global
alignment
technique which may be used, for example, is the Needleman¨Wunsch algorithm
(Needleman, S.B. & Wunsch, C.D. (1970) "A general method applicable to the
search for
similarities in the amino acid sequences of two proteins." J. Mol. Biol.
48:443-453), which is
based on dynamic programming. More recently, a Fast Optimal Global Sequence
Alignment
Algorithm (FOGSAA) was developed that purportedly produces global alignment of
nucleic
acid and amino acid sequences faster than other optimal global alignment
methods, including
the Needleman¨Wunsch algorithm. In some embodiments, the identity of two
polypeptides
is determined by aligning the two amino acid sequences, calculating the number
of identical
amino acids, and dividing by the length of one of the amino acid sequences. In
some
embodiments, the identity of two nucleic acids is determined by aligning the
two nucleotide
sequences and calculating the number of identical nucleotide and dividing by
the length of
one of the nucleic acids. For multiple sequence alignments, computer programs
including
Clustal Omega (Sievers et al., Mol Syst Biol. 2011 Oct 11;7:539) may be used.
In preferred embodiments, a sequence, including a nucleic acid or amino acid
sequence, is found to have a specified percent identity to a reference
sequence, when
sequence identity is determined using the algorithm of Karlin and Altschul
Proc. Natl. Acad.
Sci. USA 87:2264-68, 1990, modified as in Karlin and Altschul Proc. Natl.
Acad. Sci. USA
90:5873-77, 1993 (e.g., BLAST , NBLAST , XBLAST or Gapped BLAST programs,
using default parameters of the respective programs).
48
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
In some embodiments, a sequence, including a nucleic acid or amino acid
sequence, is
found to have a specified percent identity to a reference sequence when
sequence identity is
determined using the Smith-Waterman algorithm (Smith, T.F. & Waterman, M.S.
(1981)
"Identification of common molecular subsequences." J. Mol. Biol. 147:195-197)
or the
Needleman-Wunsch algorithm (Needleman, S.B. & Wunsch, C.D. (1970) "A general
method
applicable to the search for similarities in the amino acid sequences of two
proteins." J. Mol.
Biol. 48:443-453) using default parameters.
In some embodiments, a sequence, including a nucleic acid or amino acid
sequence, is
found to have a specified percent identity to a reference sequence when
sequence identity is
determined using a Fast Optimal Global Sequence Alignment Algorithm (FOGSAA)
using
default parameters.
In some embodiments, a sequence, including a nucleic acid or amino acid
sequence, is
found to have a specified percent identity to a reference sequence when
sequence identity is
determined using Clustal Omega (Sievers et al., Mol Syst Biol. 2011 Oct
11;7:539) using
default parameters.
As used in this application, a residue (such as a nucleic acid residue or an
amino acid
residue) in sequence "X" is referred to as corresponding to a position or
residue (such as a
nucleic acid residue or an amino acid residue) "Z" in a different sequence "Y"
when the
residue in sequence "X" is at the counterpart position of "Z" in sequence "Y"
when
sequences X and Y are aligned using amino acid sequence alignment tools known
in the art.
As used in this application, variant sequences may be homologous sequences. As
used
in this application, homologous sequences are sequences (e.g., nucleic acid or
amino acid
sequences) that share a certain percent identity (e.g., at least 5%, at least
10%, at least 15%, at
least 20%, at least 25%, at least 30%, at least 35%, at least 40%, at least
45%, at least 50%, at
least 55%, at least 60%, at least 65%, at least 70%, at least 71%, at least
72%, at least 73%, at
least 74%, at least 75%, at least 76%, at least 77%, at least 78%, at least
79%, at least 80%, at
least 81%, at least 82%, at least 83%, at least 84%, at least 85%, at least
86%, at least 87%, at
least 88%, at least 89%, at least 90%, at least 91%, at least 92%, at least
93%, at least 94%, at
least 95%, at least 96%, at least 97%, at least 98%, at least 99%, or 100%
percent identity,
including all values in between). Homologous sequences include but are not
limited to
paralogous or orthologous sequences. Paralogous sequences arise from
duplication of a gene
within a genome of a species, while orthologous sequences diverge after a
speciation event.
49
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
In some embodiments, a polypeptide variant (e.g., BMP2, BMP7, BMP9, Noggin
variant) comprises a domain that shares a secondary structure (e.g., alpha
helix, beta sheet)
with a reference polypeptide (e.g., a reference BMP2, BMP7, BMP9, Noggin). In
some
embodiments, a polypeptide variant (e.g., BMP2, BMP7, BMP9, Noggin variant)
shares a
tertiary structure with a reference polypeptide (e.g., a reference BMP2, BMP7,
BMP9,
Noggin enzyme). As a non-limiting example, a polypeptide variant (e.g., BMP2,
BMP7,
BMP9, Noggin enzyme) may have low primary sequence identity (e.g., less than
80%, less
than 75%, less than 70%, less than 65%, less than 60%, less than 55%, less
than 50%, less
than 45%, less than 40%, less than 35%, less than 30%, less than 25%, less
than 20%, less
than 15%, less than 10%, or less than 5% sequence identity) compared to a
reference
polypeptide, but share one or more secondary structures (e.g., including but
not limited to
loops, alpha helices, or beta sheets), or have the same tertiary structure as
a reference
polypeptide. For example, a loop may be located between a beta sheet and an
alpha helix,
between two alpha helices, or between two beta sheets. Homology modeling may
be used to
compare two or more tertiary structures.
Functional variants of the recombinant proteins disclosed in this application
are
encompassed by the present disclosure. For example, functional variants may
bind one or
more of the same substrates or produce one or more of the same products. In
other examples,
functional variants may bind one or more of the same receptors associated with
the BMP
signaling and/or regulatory pathways. Functional variants may be identified
using any
method known in the art. For example, the algorithm of Karlin and Altschul
Proc. Natl.
Acad. Sci. USA 87:2264-68, 1990 described above may be used to identify
homologous
proteins with known functions.
Putative functional variants may also be identified by searching for
polypeptides with
functionally annotated domains. Databases including Pfam (Sonnhammer et al.,
Proteins.
1997 Jul;28(3):405-20) may be used to identify polypeptides with a particular
domain.
Homology modeling may also be used to identify amino acid residues that are
amenable to mutation (e.g., substitution, deletion, and/or insertion) without
affecting
function. A non-limiting example of such a method may include use of position-
specific
scoring matrix (PSSM) and an energy minimization protocol.
Position-specific scoring matrix (PSSM) uses a position weight matrix to
identify
consensus sequences (e.g., motifs). PSSM can be conducted on nucleic acid or
amino acid
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
sequences. Sequences are aligned and the method takes into account the
observed frequency
of a particular residue (e.g., an amino acid or a nucleotide) at a particular
position and the
number of sequences analyzed. See, e.g., Stormo et al., Nucleic Acids Res.
1982 May
11;10(9):2997-3011. The likelihood of observing a particular residue at a
given position can
be calculated. Without being bound by a particular theory, positions in
sequences with high
variability may be amenable to mutation (e.g., substitution, deletion, and/or
insertion; e.g.,
PSSM score >0) to produce functional homologs. PSSM may be paired with
calculation of a
Rosetta energy function, which determines the difference between the wild-type
and the
single-point mutant. The Rosetta energy function calculates this difference as
(AAGca/c).
With the Rosetta function, the bonding interactions between a mutated residue
and the
surrounding atoms are used to determine whether an amino acid substitution,
deletion, or
insertion increases or decreases protein stability. For example, an amino acid
substitution,
deletion, or insertion that is designated as favorable by the PSSM score (e.g.
PSSM score
can then be analyzed using the Rosetta energy function to determine the
potential impact of
the mutation on protein stability. Without being bound by a particular theory,
potentially
stabilizing mutations are desirable for protein engineering (e.g., production
of functional
homologs). In some embodiments, a potentially stabilizing mutation has a
AAGcaic value of
less than -0.1 (e.g., less than -0.2, less than -0.3, less than -0.35, less
than -0.4, less than -0.45,
less than -0.5, less than -0.55, less than -0.6, less than -0.65, less than -
0.7, less than -0.75,
less than -0.8, less than -0.85, less than -0.9, less than -0.95, or less than
-1.0) Rosetta energy
units (R.e.u.). See, e.g., Goldenzweig et al., Mol Cell. 2016 Jul 21;63(2):337-
346. Doi:
10.1016/j.molce1.2016.06.012.
In some embodiments, the coding sequence of a BMP or a protein associated with
a
BMP comprises a mutation at 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15,
16, 17, 18, 19, 20,
21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39,
40, 41, 42, 43, 44, 45,
46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64,
65, 66, 67, 68, 69, 70,
71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89,
90, 91, 92, 93, 94, 95,
96, 97, 98, 99, 100 or more than 100 positions relative to a reference (e.g.,
a BMP or a protein
associated with a BMP) coding sequence. In some embodiments, the coding
sequence of a
BMP or a protein associated with a BMP comprises a mutation in 1, 2, 3, 4, 5,
6, 7, 8, 9, 10,
11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29,
30, 31, 32, 33, 34, 35,
36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54,
55, 56, 57, 58, 59, 60,
61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79,
80, 81, 82, 83, 84, 85,
51
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99,100 or more codons of
the coding
sequence relative to a reference (e.g., a BMP or a protein associated with a
BMP) coding
sequence. As will be understood by one of ordinary skill in the art, a
mutation within a codon
may or may not change the amino acid that is encoded by the codon due to
degeneracy of the
genetic code. In some embodiments, the one or more mutation in the coding
sequence do not
alter the amino acid sequence of the coding sequence (e.g., a BMP or a protein
associated
with a BMP) relative to the amino acid sequence of a reference polypeptide
(e.g., a BMP or a
protein associated with a BMP).
It should be appreciated that sequences disclosed in the present application
may or
may not contain signal sequences.
In some embodiments, the one or more mutations in the coding sequence of a
recombinant BMP or a protein associated with a BMP sequence alters the amino
acid
sequence of the polypeptide (e.g., a BMP or a protein associated with a BMP)
relative to the
amino acid sequence of a reference polypeptide (e.g., a BMP or a protein
associated with a
BMP). In some embodiments, the one or more mutations alters the amino acid
sequence of
the recombinant polypeptide (e.g., a BMP or a protein associated with a BMP)
relative to the
amino acid sequence of a reference polypeptide (e.g., a BMP or a protein
associated with a
BMP) and also alters (enhances or reduces) an activity of the polypeptide
relative to the
reference polypeptide.
The activity (e.g., specific activity) of any of the recombinant polypeptides
described
in this application (e.g., a BMP or a protein associated with a BMP) may be
measured using
routine methods.
The skilled artisan will also realize that mutations in a recombinant
polypeptide (e.g.,
a BMP or a protein associated with a BMP) coding sequence may result in
conservative
amino acid substitutions to provide functionally equivalent variants of the
foregoing
polypeptides, e.g., variants that retain the activities of the polypeptides.
As used in this
application, a "conservative amino acid substitution" refers to an amino acid
substitution that
does not alter the relative charge or size characteristics or functional
activity of the protein in
which the amino acid substitution is made.
In some instances, an amino acid is characterized by its R group. For example,
an
amino acid may comprise a nonpolar aliphatic R group, a positively charged R
group, a
negatively charged R group, a nonpolar aromatic R group, or a polar uncharged
R group.
52
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
Non-limiting examples of an amino acid comprising a nonpolar aliphatic R group
include
alanine, glycine, valine, leucine, methionine, and isoleucine. Non-limiting
examples of an
amino acid comprising a positively charged R group includes lysine, arginine,
and histidine.
Non-limiting examples of an amino acid comprising a negatively charged R group
include
aspartate and glutamate. Non-limiting examples of an amino acid comprising a
nonpolar,
aromatic R group include phenylalanine, tyrosine, and tryptophan. Non-limiting
examples of
an amino acid comprising a polar uncharged R group include serine, threonine,
cysteine,
proline, asparagine, and glutamine.
Non-limiting examples of functionally equivalent variants of polypeptides may
include conservative amino acid substitutions in the amino acid sequences of
proteins
disclosed in this application. Conservative substitutions of amino acids
include substitutions
made amongst amino acids within the following groups: (a) M, I, L, V; (b) F,
Y, W; (c) K, R,
H; (d) A, G; (e) S, T; (f) Q, N; and (g) E, D. Additional non-limiting
examples of
conservative amino acid substitutions are provided in Table 1.
In some embodiments, 1,2, 3,4, 5, 6,7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17,
18, 19,20
or more than 20 residues can be changed when preparing variant polypeptides.
In some
embodiments, amino acids are replaced by conservative amino acid
substitutions. In some
embodiments, amino acids are replaced by non-conservative amino acid
substitutions.
Table 1: Non-limiting examples of conservative amino acid substitutions
Original Residue R Group Type
Conservative Amino Acid Substitutions
Ala (A) nonpolar aliphatic R group Cys, Gly, Ser
Arg (R) positively charged R group His, Lys
Mn (N) polar uncharged R group Asp, Gin, Glu
Asp (D) negatively charged R group Asn, Gin, Giu
Cs (C) polar uncharged R group Ala, Ser
Gin (Q) polar uncharged R group A snõA.sp, Gin
Giu (E) negatively charged R group Asn, Asp, Gin
Gy (G) nonpolar aliphatic Rgroup Ma, Ser
Uis (H) positively charged R group Arg, Tyr, Trp
lie (I) nonpolar aliphatic R group Leu, Met, Val
53
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
Leu (1_,) nonpolar aliphatic .R group Ile, Met, 'Val
Lys (K) positively charged R group Arg, His
Met (Ind) nonpolar aliphatic R group lie, Lou, Phe, Val
.Pro (P) polar uncharged .R group
Phc. (F) nonpolar aromatic R group Met, Trp, Tyr
Ser (S) polar uncharged R group Ala, (ily, Thr
Thr (T) polar uncharged R group Ala., Asn, Ser
Trp (W) nonpolar aromatic R group His, Phe, Tyr, Met
Tyr (Y) nonpolar aromatic R group His. Phe, Trp
Val (V) nonpolar aliphatic R group Ile, Len, Met, Thr
Amino acid substitutions in the amino acid sequence of a polypeptide to
produce a
recombinant polypeptide (e.g., a BMP or a protein associated with a BMP)
variant having a
desired property and/or activity can be made by alteration of the coding
sequence of the
polypeptide (e.g., a BMP or a protein associated with a BMP). Similarly,
conservative amino
acid substitutions in the amino acid sequence of a polypeptide to produce
functionally
equivalent variants of the polypeptide typically are made by alteration of the
coding sequence
of the recombinant polypeptide (e.g., a BMP or a protein associated with a
BMP).
Mutations (e.g., substitutions, insertions, additions, or deletions) can be
made in a
nucleic acid sequence by a variety of methods known to one of ordinary skill
in the art. For
example, mutations (e.g., substitutions, insertions, additions, or deletions)
can be made by
PCR-directed mutation, site-directed mutagenesis according to the method of
Kunkel
(Kunkel, Proc. Nat. Acad. Sci. U.S.A. 82: 488-492, 1985), by chemical
synthesis of a gene
encoding a polypeptide, by gene editing methods, or by insertions, such as
insertion of a tag
(e.g., a HIS tag or a GFP tag). Mutations can include, for example,
substitutions, insertions,
additions, deletions, and translocations, generated by any method known in the
art. Methods
for producing mutations may be found in in references such as Molecular
Cloning: A
Laboratory Manual, J. Sambrook, et al., eds., Fourth Edition, Cold Spring
Harbor Laboratory
Press, Cold Spring Harbor, New York, 2012, or Current Protocols in Molecular
Biology,
F.M. Ausubel, et al., eds., John Wiley & Sons, Inc., New York, 2010.
54
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
General Techniques
The practice of the present disclosure will employ, unless otherwise
indicated,
conventional techniques of molecular biology (including recombinant
techniques),
microbiology, cell biology, biochemistry and immunology, which are within the
ordinary skill
in the art (e.g., as disclosed in: Molecular Cloning: A Laboratory Manual,
fourth edition
(Green, et al., 2012 Cold Spring Harbor Press); Oligonucleotide Synthesis (M.
J. Gait, ed.,
1984); Methods in Molecular Biology, Humana Press; Cell Biology: A Laboratory
Notebook,
Vol. 3 (J. E. Cellis, ed., 2005) Academic Press; Animal Cell Culture (R. I.
Freshney, ed.,
1987); Introduction to Cell and Tissue Culture (J. P. Mather and P. E.
Roberts, 1998) Plenum
Press; Cell and Tissue Culture: Laboratory Procedures (A. Doyle, J. B.
Griffiths, and D. G.
Newell, eds., 1993-8) J. Wiley and Sons; Methods in Enzymology (Academic
Press, Inc.);
Handbook of Experimental Immunology (D. M. Weir and C. C. Blackwell, eds.);
Gene
Transfer Vectors for Mammalian Cells (J. M. Miller and M. P. Cabs, eds.,
1987); Short
Protocols in Molecular Biology (F. M. Ausubel, et al., eds., 2002); PCR: The
Polymerase
Chain Reaction, (Mullis, et al., eds., 1994); Current Protocols in Immunology
(J. E. Coligan
et al., eds., 1991); Short Protocols in Molecular Biology (Wiley and Sons,
1999);
Immunobiology (C. A. Janeway and P. Travers, 1997); Antibodies (P. Finch,
1997);
Antibodies: a practical approach (D. Catty., ed., IRL Press, 1988-1989);
Monoclonal
antibodies: a practical approach (P. Shepherd and C. Dean, eds., Oxford
University Press,
2000); Using antibodies: a laboratory manual (E. Harlow and D. Lane (Cold
Spring Harbor
Laboratory Press, 1999); The Antibodies (M. Zanetti and J. D. Capra, eds.,
Harwood
Academic Publishers, 1995). It is believed that one skilled in the art can,
based on the above
description, utilize the present invention to its fullest extent. The
following specific
embodiments are, therefore, to be construed as merely illustrative, and not
limitative of the
remainder of the disclosure in any way whatsoever. All publications cited in
the present
application are incorporated by reference for the purposes or subject matter
referenced herein.
EXAMPLES
In order that the invention described in the present application may be more
fully
understood, the following examples are set forth. The examples described in
this application
are offered to illustrate the systems and methods provided herein and are not
to be construed
in any way as limiting their scope.
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
Example 1: Screen to Identify Functional Expression of BMPs, BMP Peptides,
Receptor
Decoys, and Regulatory Proteins
To identify BMPs, BMP peptides, receptor decoys, and/or regulatory proteins
that
could be functionally expressed in host cells, a library of BMPs (e.g., human
mature BMP1,
BMP 2, BMP 3, BMP 4, BMP 5, BMP 6, BMP 7, BMP 8a, BMP Sb, BMP 9, BMP10,
BMPI 1, BMP12, BMP1.3, BMPI4, and/or BMP15) and derivatives of BMPs such as
heterodimers and peptides derived from the above-listed BMPs, chim.eric BMPs,
and
rationally engineered BMPs is designed. The library may include naturally
occurring BMPs
and/or engineered versions of BM Ps. The library may also include one or more
of proteins
associated with BMPs including Activin. The library may include chimeric
proteins that
consist of domains from different BMPs or Activins, such. as a BMP2/7 chimeric
protein or a
BMP/Activin chimeric protein, for example. Proteins within the library,
including chimeric
proteins, may also include one or more amino acid modifications, such as amino
acid
substitutions, additions, deletions, or insertions. Without wishing to be
bound by any theory,
heterodimers, chimeric proteins, and amino acid modifications may increase the
binding
affinity of the ligands to BMP receptors, which include but are not limited to
ALKI, AL,K2,
ALIO, ALK6, ACVR2A, ACNR2B, BMPR2, and AMHR2, Engineered BMPs included in
the library may exhibit increased potency compared to naturally occurring or
wildtype 13 MPs.
Strategies for engineering BMPs may include -bioinformatics analysis of the
BMP protein
sequences. For example, bioinformatics analyses may be used to predict the
fitness of the
native amino acid at every position in the relevant wildtype protein sequences
and to suggest
favorable alternatives if the native amino acid is suboptimal. In some
instances, BMPs within
the library contain one or more point mutations relative to the corresponding
wildtype B MP.
Receptor decoys such as BMP3, Activin 13, and Inhibin a are also designed,
screened
and tested. Without wishing to be bound by any theory, receptor decoys may
bind to BMP
receptors without activating them. Receptor decoys may lower the activation of
the SMAD
pathway induced by 131VIPs. Regulatory proteins, such as Activin, Gremlin,
and/or Noggin,
are also designed, screened and tested. Noggin and Gremlin are known to act as
antagonists
of BMP proteins, Receptor decoys and regulatory proteins may be naturally
occurring
proteins or may be engineered.
The screening and construction process may include DNA synthesis of DNA
sequences within the library that are cloned into a plasmid for expression in
a host cell. For
example, a host cell may be a yeast cell such as a P. pastoris cell, a
bacterial cell such as an
.Escherichia coli cell, and/or a mammalian cell, such as a 1-IEK 293 or CU()
(Chinese hamster
56
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
ovary) cell. The plasmids are transformed into the host cell and cultured in
well plates. After
growth at a defined temperature and time period, the library proteins produced
by the host
cells are assayed for activity and quantified using a method known the art
such as ELBA
(enzyme-linked immunosorbent assay) or a -fluorescence detection assay.
Efficacy testing is
performed for determining the presence and the level of alkaline phosphatase
production, and
by performing a Bone Gla Protein (BGP; osteocalcin) assay. Protein.s from the
library are
selected for further testing based on expression compatibility in the host
cells as well as
activity in the in vitro efficacy assay. Further testing may include testing
the effects of
proteins identified in the library on different cell types, including, for
example, mesenchymal
stem cells, preosteoblast cells, and/or osteoblast cells.
Example 2: Host Strain Engineering and Recombinant BMP Production Using P.
pastoris
A P. pastoris strain is used that has been genetically altered for improved
production
of recombinant proteins. In general, strains will have a single amino acid
auxotrophy (e.g.,
his4 or Ade2) to allow for selection of expression constructs following
transformation. In
addition, a limited number of protease knockouts (e.g., pep4 & prbl) or
secretion pathway
modifications (e.g., PB/1 overexpression) previously shown to be useful in
general secretion
screening strains may be introduced. BMP protein family members are known to
retain
biological activity even without sugar chains. However, since BMPs are
relatively
hydrophobic, recombinant BMP without sugar chains can cause protein
aggregation. When
using P. pastoris as a host cell, post-translational, modifications such. as
glycosylation and
folding can be performed. The host strain can be further modified to perform
post-
translational modifications (e.g., glycosylation) similar to the post-
translational modifications
(e.g., glycosylation) of human BMP 2 and BMP 7. The post-translational
modifications will.
be evaluated by using proteoglycomics.
BMP2 and BMP7, and other candidate proteins identified in the screening are
inserted
into the genome of P pastoris. An episomal expression system is developed.
Various
solubility and/or secretion tags (e.g., the S. cerevisiae mating factor) and
codon optimization
is tested to ensure optimal expression and secretion of the proteins. P
pastoris strains
containing the candidate proteins are grown in 96-well plates. Cell lysates,
supernatants,
and/or purified proteins are assayed by quantification using ELISA or
fluorescence assays.
Efficacy testing is then performed for determining the presence and/or the
level of alkaline
phosphatase production. The BGP assay is also conducted. The cell lysates,
supernatants,
57
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
and/or purified proteins are tested with mammalian cells to determine cell
proliferation. A
fermentation process is developed for producing larger quantities of the
candidate proteins.
For example, a bioreactor or fermenter can be used for culturing the host
cells. Batch
fermentation methods (e.g., shake flask or well plate fermentation) can be
used.
Example 3: Host Strain Engineering and Recombinant BMP Production Using
Mammalian Cells
An advantage of the expression systems utilizing mammalian cells for
generating
recombinant proteins is the ability to introduce proper protein folding, post-
translational
modifications, and product assembly, which are important for complete and
functional
biological activities of mammalian proteins. A mammalian cell line capable of
stably
expressing BMPs and/or other candidate proteins is developed. The cell line is
engineered for
producing high protein titers. An expression system appropriate for mammalian
cells and
development of growth medium for optimal production is designed.
BMP2 and BMP7 and other potential candidate proteins are inserted into an
episomal
expression system for transient expression. Alternatively, BMP2 and BMP7 and
other
potential candidate proteins are inserted into a construct for stable
expression. A strong
promoter such as the CMV or CAG promoter is used to express the BMPs or other
potential
candidate proteins, which is optionally adjusted by adding enhancer elements.
Various
solubility and/or secretion tags (e.g., the S. cerevisiae mating factor) are
tested. Codon
optimization is also tested to ensure optimal expression and secretion of the
proteins, if
needed. The episomal expression system is transfected into mammalian cells
such as HEK-
293 or CHO cells. Any mammalian cells suitable for producing recombinant
proteins can be
used. The mammalian cells are grown in liquid medium. Cell lysates,
supernatants, or
purified proteins are assayed by quantification using ELISA or fluorescence
assays. Efficacy
testing is performed for determining the presence and/or the level of alkaline
phosphatase
production. The BGP assay is also conducted. The cell lysates, supernatants,
and/or purified
proteins are also tested with mammalian cells to determine cell proliferation.
A fermentation
process is developed for producing larger quantities of proteins. For example,
a bioreactor or
fermenter can be used for culturing the cell. Batch fermentation methods
(e.g., shake flask
fermentation) can be used.
Example 4: Administration of BMPs and/or BMP Associated Proteins in Human
Subjects
58
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
In order to examine the effects of BMPs and/or BMP associated proteins on anti-
aging and aesthetic related characteristics, male and female subjects who are
older than 18
years of age are recruited for the studies. Subjects who are at least 65 years
of old (e.g.,
elderly) are a preferred group in some instances. Subject candidates are first
screened for
their health history and condition (e.g., allergies, pregnancies, drug and
medications that may
contraindicate the use of BMPs and BMP associated protein formulations,
familial
inheritance related to skin conditions), their routine skin care, and other
aesthetic non-
invasive procedures they have received according to various criteria that will
be set forth.
Prior to any treatments, baseline profiles are developed for each recruited
subject. The
baseline profiles may include but are not limited to several headshots of the
subjects,
anthropometric facial skeleton measurements such as the skeleton features
surrounding
periorbital regions, midface regions, perinasal regions, and lower face
regions, and wrinkles
and fine lines assessments via dermatoscopy and digital fringe profilometry
techniques, for
example, and one or more questionnaires capturing the subjects self-assessment
of
attractiveness and desired improvements in facial contouring or skin texture
or evenness of
skin tone.
BMPs and/or BMP associated proteins are administered to the selected human
subjects for testing the aesthetic and/or cosmetic effects of the proteins. As
disclosed in the
present application, any BMPs and BMP associated proteins suitable for the
study designs
may be chosen. For example, Activin, BMP2, BMP6, BMP7 or BMP9 can be
administered to
the subjects. In some instances, more than one BMP can be formulated for
administration to
the subjects. BMPs can be administered to the subjects with BMP associated
proteins. For
example, any of the BMPs can be administered to subjects in combination with
Noggin for
regulating the functions of BMPs in vivo. As disclosed in the present
application, Noggin
may function as a "off switch" to BMPs, so that the effects of BMPs can be
attenuated or
delayed. BMPs and BMP associated proteins can be formulated separately (i.e.,
in two
different formulations) or prepared in the same formulation. When appropriate,
subjects may
receive administration of BMP associated proteins at the same time or after
the
administration of BMPs. Some subjects may be administered placebo treatments
which will
not contain any BMPs and/or BMP associated proteins.
BMPs and/or BMP associated proteins may be administered to the subject
topically,
such as in the form of a cream and/or serum, or via subdermal, subcutaneous or
supraperiostial injections. BMPs and/or BMP associated proteins will
optionally be
59
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
encapsulated in a formulation for enhancing penetration of the proteins
through the skin of
the subjects.
For topical administrations, BMPs and BMP regulatory proteins such as Noggin
may
be formulated at a concentration of less than about 3%. BMP peptides may be
formulated at a
concentration of less than about 10% for topical administrations. Subjects who
receive topical
administration may optionally receive skin pre-treatments such as exfoliations
or peels to
minimize potential inconsistency of absorption of the cream or serum. For
subdermal,
subcutaneous or supraperiostial administration, BMPs and BMP regulatory
proteins such as
Noggin may be formulated at a concentration of about 100 to about 1,000 ng/mL
for each
injection site. The doses of formulations for topical or subdermal,
subcutaneous or
supraperiostial administration can be adjusted based on the needs of a subject
such as the
estimated frequencies of the administrations and site of the administrations
(e.g., face versus
hand). For subdermal, subcutaneous or supraperiostial injections, BMPs and BMP
regulatory
proteins can be either administered in a pre-formulated composition or in a
formulation that
will be prepared right before the injections. Alternatively, BMPs and BMP
regulatory
proteins can be pre-mixed with dermal fillers prior to the injections. In some
instances, a
subject may receive both subdermal (or subcutaneous or supraperiostial)
injection and topical
administration.
Example 5: Effects of Administration of BMPs and BMP Associated Proteins on
the
Appearance of Skin of Subjects
As described in Example 4, various BMPs and BMP regulatory proteins are
administered to human subjects for determining anti-aging and aesthetic
characteristics. After
administration (e.g., topical or subdermal), the subjects are evaluated for
any changes of the
appearance of their skin and for changes in their perception of
attractiveness. A list of skin
characteristics and signs to be examined is developed. For example,
characteristics for
evaluation may include the appearance of skin wrinkles and fine lines around
the face and
neck (e.g., crinkle lines, permanent elastotic creases, dynamic expression
lines, and/or
gravitational folds), the appearance of facial contours, the appearance of
facial bone
structures, and/or the subjects' perception of improvement in attractiveness.
Depending on the type of administration, various time points post-
administrations are
assigned for conducting evaluations. For example, for subjects who receive
subdermal (or
subcutaneous or supraperiostial) injections, characteristics may be evaluated
as soon as two
CA 03176245 2022-09-20
WO 2022/056198
PCT/US2021/049768
days after the initial injection. In some instances, the subjects who receive
subdermal (or
subcutaneous or supraperiostial) injections are evaluated 10 or more days
after the initial
injection. For subjects who receive topical administration, characteristics
may be evaluated,
e.g., approximately 17 days after the initial application. In some instances,
subjects who
receive topical administration of cream and/or serum are evaluated 30 or more
days after the
initial application. Optionally, subjects are evaluated at multiple time
points to monitor any
longer-term effects. Subjects may be evaluated against their own baseline
profiles and/or
against skin conditions and changes of subjects who receive placebo
treatments.
EQUIVALENTS
Those skilled in the art will recognize or be able to ascertain using no more
than
routine experimentation, many equivalents to the specific embodiments of the
invention
described in the present application. Such equivalents are intended to be
encompassed by the
following claims. All references, including patent documents, disclosed in
this application are
incorporated by reference in their entirety, particularly for the disclosure
referenced in this
application.
61