Note: Descriptions are shown in the official language in which they were submitted.
WO 2021/216986
PCT/US2021/028821
HOLLOW CORE GRANULES, PRODUCTS INCORPORATING THE GRANULES, AND
METHODS OF PREP ARTNG THE GRANULES
FIELD OF THE DISCLOSURE
The present disclosure relates to hollow core granules. The hollow core
granules can
comprise at least one wall surrounding a cavity defining a hollow core. The at
least one wall can
comprise particles of at least one wall forming material and likewise may
include at least one
binder.
BACKGROUND
A variety of chemical compounds are known for a variety of uses in
substantially solid
forms. Many chemical compounds, when provided in a substantially particulate
form, may provide
uses that can be limited by the available surface area. For example, while
some chemical
compounds may be reactive, any reaction may take place substantially only at
the surface of the
particle, and much of the mass of the particle does not participate in the
reaction. Moreover, many
materials that are useful in a solid, substantially particulate form may be
excessively heavy. For
example, clay particles are commonly used in a variety of consumer products,
most notably in
typical animal litter compositions. Animal litters are often sold in
substantially large volumes as
may be needed to, for example, fill a litter tray. Because of this typical
arrangement, the volume of
animal litter that is needed for commercial packaging can be excessively
heavy. Still further, there
is an ongoing need for new forms of products that provide convenient handling
while still
exhibiting properties that are equal to or exceed typically achieved ranges.
Accordingly, there
remains a need in the field for means for providing chemicals, compounds, and
compositions in a
solid, substantially granular form while also providing improved properties.
SUMMARY OF THE DISCLOSURE
The present disclosure relates to hollow core granules. The hollow core
granules
specifically can be an engineered structure wherein a plurality of particles
of one or more wall
forming materials are aggregated, agglomerated, or otherwise brought together
in the form of at
least one wall that substantially surrounds a cavity that defines the hollow
core. The hollow core
Granules are differentiated from native forms of the wall forming material in
that the combination
of individual particles as a wall surrounding a hollow core can cause the
granules to exhibit
properties that are improved relative to the wall forming material in its
native foini (i.e., not present
as a plurality of particles surrounding a hollow core). This can make the
hollow core granules
available for a variety of uses in a variety of products including, at least
in part, a plurality of the
-1 -
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
hollow core granules. The disclosure also provides methods of forming such
hollow core structures
as well as a variety of products or articles of manufacture that can include
the hollow core granules.
In one or more embodiments, the present disclosure can relate to a hollow core
granule.
While the structure is described in relation to a granule in the singular
form, it is understood that
such terminology is utilized for convenience, and the various properties and
uses of the hollow core
granule are not limited to a single granule. Rather, a plurality of granules
exhibiting substantially
the same properties and having substantially the same uses are encompassed by
the present
disclosure Moreover, it is understood that, in use, a plurality of the
granules will typically be
utilized in forming a product or carrying out a specific use. Nevertheless,
the present subject matter
may be identified in a single granule or a plurality of granules.
In an example embodiment, a hollow core granule according to the present
disclosure can
comprise at least one wall substantially surrounding a cavity that is
substantially devoid of any
solid or liquid so as to define a hollow core, the at least one wall
comprising a plurality of
individual particles of at least one wall forming material, the plurality of
individual particles
sufficiently bound together so that the at least one wall is structurally self-
sustaining. The hollow
core granule (or a plurality of the hollow core granules) can be further
defined, in one or more
embodiments, in relation to any one or more of the following statements, which
statements can be
combined as desired in any number or order, the ability to make any specific
combination of the
following statements (or all of the possible combinations of the following
statements) being readily
evident from the further disclosure herein.
The at least one wall forming material can be selected from the group
consisting of clays,
glass, ceramics, aluminas, silicates, zeolites, carbon, metals, salts,
absorbents, adsorbents,
deodorizers, odor control agents, surfactants, enzymes, bleaches, oxidizers,
reducers, gellants,
flavors, fragrances, abrasives, fertilizers, insecticides, pesticides,
bactericides, herbicides,
antimicrobials, anti-sticking agents, fillers, binders, preservatives, optical
agents, disinfectants,
chelators, molecular binding agents, dyes, coloring agents, colored particles,
de-dusting agents, and
combinations thereof
The at least one wall forming material includes can include clay.
The clay can comprise bentonite.
The at least one wall forming material can include a salt.
The salt can be selected from the group consisting of calcium carbonate,
sodium chloride,
sodium carbonate, sodium bicarbonate, sodium percarbonate, sodium sulfate,
sodium carbonate
peroxide, potassium chloride, magnesium carbonate, magnesium sulfate, and
combinations thereof.
The salt can be sodium bicarbonate.
The hollow core granule of claim 6, wherein the salt can be sodium carbonate.
-2-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
The salt can be sodium chloride.
The at least one wall forming material can be a fabric care composition.
The fabric care composition can be selected from the group consisting of
laundry
detergents, bleaches, whiteners, brighteners, stain removers, deodorizers,
scent boosters, and
combinations thereof
The at least one wall forming material can be an additive for a fabric care
composition.
The at least one wall forming material can be a pet litter composition.
The at least one wall forming material can be an additive for a pet litter
composition.
The additive for the pet litter composition can be selected from the group
consisting of
fillers, clumping agents, binders, preservatives, de-dusting agents,
fragrances, and mixture thereof.
The at least one wall forming material can be configured for absorption,
adsorption, or other
binding of one or more odor causing chemicals in which the hollow core granule
comes in contact
The at least one wall forming material can be configured for absorption,
adsorption, or other
binding of an aqueous liquid in which the hollow core granule comes in
contact.
The at least one wall forming material can be configured for absorption,
adsorption, or other
binding of a non-aqueous liquid in which the hollow core granule comes in
contact.
The at least one wall forming material can be a pH adjusting agent.
The at least one wall forming material can include a fertilizer.
The fertilizer can be selected from the group consisting of a nitrogen source,
a phosphorus
source, a potassium source, a micronutrient source, and combinations thereof.
The hollow core granule as a fertilizer can be characterized by one or more of
the following
conditions can be met: the at least one wall forming material further can
include a clay, and at least
a portion of the fertilizer can be absorbed, adsorbed, or otherwise combined
with particles of the
clay; at least a portion of the fertilizer can be in a microencapsulated form;
the fertilizer can include
at least two different fertilizers; the fertilizer can be configured for
substantially immediate release;
the fertilizer can be configured for controlled release.
The at least one wall forming material can include a pesticide.
The pesticide can be an active agent selected from the group consisting of
bifenthrin,
acephate, carbaryl, cyfluthrin, 2,4-dichlorophenoxyacetic acid, trifluralin,
chlorpyrifos, allethrins,
cypermethrin, disulfoton, 2,6-dichlorobenzonitrile, metolachlor, cyhalothrin,
hydramethylnon,
atrazine, chlorothalonil, myclobutanil, dicamba, azadirachtin, captan,
diazinon, carbofuran,
methomyl, deltamethrin, propiconazole, borate, dinotefuran, dithiopyr,
isoxaben, prodiamine,
quinclorac, sethoxydim, iron(III) phosphate, mancozeb, thiophanate-methyl,
esfenvalerate,
tebuconazole, resmethrin, glyphosate, malathion, permethrin, imidacloprid,
fipronil, abamectin,
-3-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
spinosad, triclopyr, piperonyl butoxide, pendimethalin, oryzalin, oxadiazon,
and combinations
thereof.
The at least one wall forming material further can include a clay, and at
least a portion of
the pesticide is absorbed, adsorbed, or otherwise combined with particles of
the clay.
The at least one wall forming material can include an odor masking agent.
The hollow core granule can be hydrophilic.
The hollow core granule can be hydrophobic.
The hollow core granule further can comprise one or more coating layers
overlying at least
a portion of the at least one wall.
The hollow core granule further can comprise at least one binder material
present in at least
a portion of interstitial spaces present between the individual particles of
the at least one wall
forming material_
The at least one binder can be a hydrophilic material.
The at least one binder can include a polyethylene glycol (PEG) material.
The at least one binder can be a hydrophobic material.
The at least one binder can include a material selected from the group
consisting of wax,
paraffin, polycaprolactone, ethylene-vinyl acetate copolymers, polypropylene
carbonate,
poly(tetramethylene oxide), poly(ethylene adipate), poly(trans-butadiene),
thermoplastic
polyurethane, stearic acid, and combinations thereof.
The at least one binder can comprise about 1% to about 45% by weight, based on
the total
weight of the hollow core granule.
The hollow core granule can have a diameter of about 0.1 mm to about 20 mm.
The diameter of the hollow core granule can be about 0.5 mm to about 6 mm.
The hollow core can have a diameter that is about 10% to about 80% of the
diameter of the
hollow core granule.
The diameter of the hollow core can be about 25% to about 55% of the diameter
of the
hollow core granule.
The at least one wall can have an average thickness of about 0.05 mm to about
8 mm.
The average thickness can be about 0.1 mm to about 4 mm.
The hollow core granule can be configured so that the cavity that defines the
hollow core
has a volume that is about 01% to about 50% of a volume of the hollow core
granule
The volume of the cavity can be about 0.5% to about 10% of the volume of the
hollow core
granule.
The hollow core granule can have a density that is at least 20% lower than a
density of the
wall forming material.
-4-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
The density of the hollow core granule can be about 15% to about 50% lower
than the
density of the wall forming material.
The hollow core granule can be buoyant in water.
The at least one wall can be an agglomeration of the individual particles of
the wall forming
material.
The individual particles of the wall forming material can have an average
particle size of
about 0.01 mm to about 2 mm
The individual particles of the wall forming material can have an average
particle size of
about 0.05 mm to about 1.0 mm
The hollow core granule can exhibit a time to substantially complete
solubilization that is at
least 10% faster than a time to substantially complete solubilization of a
same weight of the at least
one wall forming material alone.
The hollow core granule of claim 1, wherein, upon application of an external
force, the
hollow core granule can be configured to break into a plurality of parts
comprising individual
groups of the particles of the wall forming material.
In example embodiments, the present disclosure can relate to a product that
comprises a
plurality of the hollow core granules. The plurality of the hollow core
granules can be defined in
relation to any one or more of the foregoing statements, as well as any
further description of the
hollow core granules as described herein. Moreover, the product comprising a
plurality of the
hollow core granules may be further defined in relation to any one or more of
the following
statements, which statements can be combined as desired in any number or
order, the ability to
make any specific combination of the following statements (or all of the
possible combinations of
the following statements) being readily evident from the further disclosure
herein.
The product can be configured as a cleaning product.
The cleaning product can be a fabric care product.
The fabric care product can be selected from the group consisting of laundry
detergents,
upholstery cleaners, brighteners, whiteners, stain removers, scent boosters,
and combinations
thereof.
The cleaning product can be a dishwashing detergent.
The cleaning product can be an abrasive cleaner.
The cleaning product can be a teeth cleaning product.
The cleaning product can be a formulation of a plurality of ingredients, and
wherein the
plurality of the hollow core granule can be configured as a single ingredient
of the plurality of
ingredients.
-5-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
The cleaning product can be a formulation of a plurality of ingredients, and
wherein two or
more of the plurality of ingredients are included as the wall forming material
of the plurality of the
hollow core granule.
All of the plurality of ingredients can be included as the wall forming
material of the
plurality of the hollow core granule.
The product can be configured as a nutritional supplement.
The product can be configured as a laxative
The product can be configured as a deodorizer.
The plurality of the hollow core granule can be configured so as to include a
material
selected from the group consisting of sodium bicarbonate, zeolites, activated
carbon, bentonite, and
combinations thereof as the at least one wall forming material.
The plurality of the hollow core granule can be configured so as to include
one or both of an
odor neutralizing agent and an odor masking agent.
The product can be configured as an animal litter.
The plurality of the hollow core granule can be configured so as to include
sodium
bicarbonate as the at least one wall forming material.
The plurality of the hollow core granule can be configured so as to include a
clay as the at
least one wall forming material.
The clay can comprise bentonite.
The plurality of the hollow core granule can comprise at least 5% by weight of
the animal
litter.
The product can be configured as a pet litter additive.
The pet litter additive can be selected from the group consisting of fillers,
clumping agents,
binders, preservatives, de-dusting agents, fragrances, and mixture thereof.
The product can be a fertilizer.
The plurality of the hollow core granule can be configured so as to include
one or more of a
nitrogen source, a phosphorus source, a potassium source, and a micronutrient
source as the at least
one wall forming material.
The plurality of the hollow core granule can be configured so as to include
individual
particles of a clay as the at least one wall forming material.
At least one fertilizer material can be absorbed, adsorbed, or otherwise
combined with the
individual particles of the clay.
The plurality of the hollow core granule can be configured so as to include
one or more
fertilizer materials in an encapsulated form as the at least one wall forming
material.
The product can be a pesticide.
-6-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
The plurality of the hollow core granule can be configured so as to include an
active agent
selected from the group consisting of bifenthrin, acephate, carbaryl,
cyfluthrin, 2,4-
dichlorophenoxyacetic acid, trifluralin, chlorpyrifos, allethrins,
cypermethrin, disulfoton, 2,6-
dichlorobenzonitrile, metolachlor, cyhalothrin, hydramethylnon, atrazine,
chlorothalonil,
myclobutanil, dicamba, azadirachtin, captan, diazinon, carbofuran, methomyl,
deltamethrin,
propiconazole, borate, dinotefuran, dithiopyr, isoxaben, prodiamine,
quinclorac, sethoxydim,
iron(III) phosphate, mancozeb, thiophanate-methyl, esfenvalerate,
tebuconazole, resmethrin,
glyphosate, malathion, permethrin, imidacloprid, fipronil, abamectin,
spinosad, triclopyr, piperonyl
butoxide, pendimethalin, oryzalin, oxadiazon, and combinations thereof.
The plurality of the hollow core granule can be configured so as to include
individual
particles of a clay as the at least one wall forming material.
At least one pesticide material can be absorbed, adsorbed, or otherwise
combined with the
individual particles of the clay.
In example embodiments, the present disclosure further can provide methods for
preparing
hollow core granules. In particular, such methods can comprise: combining a
binder having a
melting point of about 40 C to about 95 C with a plurality of individual
particles of at least one
wall forming material that is substantially insoluble in the binder and has a
melting point that is
greater than the melting point of the binder so as to form a mixture; heating
the mixture to a
maximum temperature that is at or above the melting point of the binder and
below the melting
point of the plurality of the individual particles of at least one wall
forming material to form
agglomerations of the plurality of the individual particles of at least one
wall forming material; and
cooling the agglomerations of the plurality of the individual particles of at
least one wall forming
material to form the hollow core granules. The methods of manufacture may be
further defined in
relation to any one or more of the following statements, which statements can
be combined as
desired in any number or order, the ability to make any specific combination
of the following
statements (or all of the possible combinations of the following statements)
being readily evident
from the further disclosure herein.
The hollow core granules that are formed can comprise at least one wall
substantially
surrounding a cavity that is substantially devoid of any solid or liquid so as
to define a hollow core,
the at least one wall comprising the plurality of the individual particles of
at least one wall forming
material, the plurality of the individual particles sufficiently bound
together so that the at least one
wall is structurally self-sustaining.
The binder and the plurality of the individual particles of at least one wall
forming material
can be combined such that an amount of the binder present in the at least one
wall of the hollow
-7-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
core granules is about 0.1% to about 50% by weight, based on the total weight
of the hollow core
granules.
The amount of the binder present in the at least one wall of the hollow core
granules can be
about 5% to about 30% by weight based on the total weight of the hollow core
granules.
The process can be carried out in a fluidized bed.
The cooling can comprise cooling to a temperature that is below the melting
point of the
binder.
The present disclosure, in one or more embodiments, further can relate to
products that
comprise one or more hollow core granules prepared according to the methods
specifically
provided above and/or as otherwise described herein. In certain, non-limiting
example
embodiments, the product can be selected from the group consisting of laundry
detergents, dish
detergents, fabric cleaners, fabric deodorizers, abrasive cleaners, teeth
cleaning compositions,
disinfectants, stain removers, whiteners, brighteners, bleaches, scent
boosters, absorbents,
adsorbents, deodorizers, odor control products, odor masking products,
fertilizers, pesticides,
animal litters, and animal litter additives.
The present disclosure further comprises methods of delivering one or more
materials to a
desired site of use, wherein the one or more materials are provided for
delivery as a plurality of
individual particles of the material included in at least one wall of a hollow
core granule as
described herein.
BRIEF SUMMARY OF THE DRAWINGS
FIG. 1 is a partially cut away, perspective view of a hollow core granule
according to
example embodiments of the present disclosure.
FIG. 2 is a partial cross-sectional of an enlarged portion of a wall of a
hollow core granule
according to example embodiments of the present disclosure.
FIG. 3 is a partial cross-sectional of an enlarged portion of a wall of a
hollow core granule
according to further example embodiments of the present disclosure.
FIG. 4 is a cross-sectional view of a hollow core granule incorporating a
plurality of
walls/layers according to example embodiments of the present disclosure.
FIG. 5 is a graph showing bulk density versus processing time of hollow core
granules
prepared according to example embodiments of the present disclosure
FIG. 6 is a graph showing content of the wall forming material versus
processing time of
hollow core granules prepared according to example embodiments of the present
disclosure.
FIG. 7 is a graph showing crush strength versus process time for hollow core
granules
prepared according to example embodiments of the present disclosure.
-8-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
FIG. 8A through FIG. SE are graphs showing attrition for hollow core granules
prepared
according to example embodiments of the present disclosure at different
residence times in a
fluidized bed apparatus.
FIG. 9 is a graph showing granule sizes and associated cavity sizes for hollow
core granules
prepared according to example embodiments of the present disclosure.
FIG. 10 is a graph showing fractional weights of hollow core granules prepared
according to
example embodiments of the present disclosure with different residence times
in a fluidized bed
apparatus.
FIG. 11 is a graph showing granule bulk density for hollow core granules with
PEG binder
and bentonite wall forming material prepared according to example embodiments
of the present
disclosure with different residence times in a fluidized bed apparatus.
FIG 12 is a graph showing dimensions and associated cavity dimensions of
hollow core
granules prepared according to example embodiments of the present disclosure
as a function of
processing time in a fluidized bed apparatus.
FIG. 13 is a graph showing percentage of cavity volume to total granule volume
for hollow
core granules prepared according to example embodiments of the present
disclosure.
FIG. 14 is a graph showing granule sizes and associated cavity sizes for
hollow core
granules prepared according to example embodiments of the present disclosure
as a function of
residence time is a fluidized bed apparatus.
FIG. 15 is a graph showing percentage of cavity volume to total granule volume
for hollow
core granules prepared according to example embodiments of the present
disclosure.
FIG. 16 is a graph showing attrition for hollow core granules prepared
according to example
embodiments of the present disclosure as a function of time spent in a sieve.
FIG. 17 is a table showing data related to a variety of hollow core granules
prepared
according to example embodiments of the present disclosure.
FIG. 18 is a table showing additional data related to a variety of hollow core
granules
prepared according to example embodiments of the present disclosure.
FIG. 19A and FIG. 19B are scanning electron microscope (SEM) images at
different
magnifications of hollow core particles having a zeolite as the wall forming
material according to
example embodiments of the present disclosure.
FIG_ 20A and FIG 20B are SEM images at different magnifications of hollow core
particles
having activated charcoal as the wall forming material according to example
embodiments of the
present disclosure.
-9-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
FIG. 21A, 21B, and 21C are SEM images at different magnifications of hollow
core
particles having sodium bicarbonate as the wall forming material according to
example
embodiments of the present disclosure.
FIG. 22 is a graph showing performance of hollow core particles according to
example
embodiments of the present disclosure versus sodium bicarbonate alone and
bentonite alone for
reducing malodor caused by release of ammonia from a quantity of the cat
litter mimicking
composition, Felinine, added to a mass of the tested materials.
FIG. 23 is a graph showing performance of hollow core particles according to
example
embodiments of the present disclosure versus sodium bicarbonate alone and
bentonite alone for
reducing malodor caused by release of sulfurous compounds from a quantity of
the cat litter
mimicking composition, Felinine, added to a mass of the tested materials.
FIG_ 24 is an image of a hollow core granule formed of sodium bicarbonate as
the wall
forming material and PEG as the binder according to example embodiments of the
present
disclosure, the granule having been cut in half.
FIG. 25 is an image of a hollow core granule formed of bentonite as the wall
forming
material and PEG as the binder according to example embodiments of the present
disclosure, the
granule having been cut in half.
FIG. 26 is an image of a bass of hollow core granules formed of sodium
bicarbonate and
bentonite as the wall forming material and PEG as the binder according to
example embodiments of
the present disclosure.
FIG. 27 is an image of a hollow core granule formed of sodium bicarbonate as
the wall
forming material and polyoxyethylene stearyl ether as the binder according to
example
embodiments of the present disclosure, the granule having been cut in half.
FIG. 28 is an image of a hollow core granule formed of bentonite as the wall
forming
material and polyoxyethylene stearyl ether as the binder according to example
embodiments of the
present disclosure, the granule having been cut in half.
DETAILED DESCRIPTION OF THE DISCLOSURE
The invention now will be described more fully hereinafter through reference
to various
embodiments. These embodiments are provided so that this disclosure will be
thorough and
complete, and will fully convey the scope of the invention to those skilled in
the art Indeed, the
invention may be embodied in many different forms and should not be construed
as limited to the
embodiments set forth herein; rather, these embodiments are provided so that
this disclosure will
satisfy applicable legal requirements. As used in the specification, and in
the appended claims, the
-10-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
singular forms "a", "an", "the", include plural referents unless the context
clearly dictates
otherwise.
The present disclosure relates to structures having substantially hollow
cores, compositions
incorporating such structures, methods of making such structures, and
uses/applications of such
structures and compositions. The structures provided herein may particularly
be hollow core
structures comprising at least one shell/wall surrounding a cavity, which is
the hollow core. The
shell/wall specifically can comprise at least one solid wall forming material
and a binder material.
A plurality of solid wall forming materials may be utilized. Likewise, a
plurality of binders may be
utilized. The at least one solid wall forming material can be configured as a
plurality of individual
particles that are bound together with the binder to define the shell/wall
surrounding the cavity
defining the hollow core. The shell/wall may be characterized as a
substantially continuous wall
that surrounds and encloses a hollow core or cavity. An individual hollow core
structure, the wall
being formed of a plurality of individual particles, may thus be referenced as
being a granule.
Thus, the term "granule" as used herein can reference a hollow core structure,
and the term
"particle" can reference individual pieces of the solid material(s) used as
the wall forming material
to form the shell/wall of the granule or hollow core structure. In some
embodiments, a plurality of
shells/walls can be present, and each shell/wall can independently have a
different composition
and/or thicknesses. Further, the hollow core may be configured such that one
or more components
are included therein in an amount such that the hollow core is not completely
filled and thus can
still be referenced as being a hollow core. Such hollow core structures may be
useful as a
standalone material and/or may be useful in preparing a variety of products
wherein the hollow core
structures may be mixed or otherwise combined with further components.
Hollow core granules according to the present disclosure can be configured
with specific
properties and specific uses. The exact nature of the properties and/or uses
can vary based upon,
among other factors, the nature of the material(s) forming the
shell(s)/wall(s), the size of the hollow
core structures, the nature of any material forming a component that is
included within the hollow
core, and the like. In some embodiments, the present hollow core structures
can be particularly
configured to provide one or more deodorization functions. This can include
exhibiting an ability
to absorb and/or entrain odor-causing compounds and can alternatively or
additionally include
exhibiting an odor neutralizing ability, such as by including and/or
delivering an odor neutralizing
agent In some embodiments, the present hollow core stnictures can be
particularly configured to
provide one or more absorbent and/or adsorbent functions. This can include
exhibiting an ability to
absorb liquids, which can include polar and/or non-polar liquids. Further, the
hollow core
structures can be configured to selectively absorb and/or adsorb in
terrestrial and/or aquatic
settings.
-11 -
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
In some embodiments, the present hollow core structures can be provided as an
engineered
form of one or more chemicals, compounds, compositions, or the like that has a
desired use, and the
provision of the one or more chemicals, compounds, compositions, or the like
in the hollow core
format can achieved improved properties (e.g., hollow core sodium carbonate
exhibiting improved
odor absorption and/or cleaning properties relative to "regular" sodium
bicarbonate that is not in
the re-engineered, hollow core format or hollow core clay exhibiting improved
liquid absorption
relative to "regular" clay that is not in the re-engineered, hollow core
format) The improved
properties particularly may be as related to a native form of the chemical,
compound, composition,
or the like, the native form being a form in which the chemical, compound, or
composition
naturally exists, or a form in which the chemical, compound, or composition is
typically made
and/or sold. The native form in particular can be a form that is not a hollow
core format.
The present hollow core structures can be useful as a stand-alone chemical or
compound
that can be made available for a variety of purposes. Likewise, such stand-
alone chemicals or
compounds may be used as one or more components of a more complex composition
(e.g., the
complex composition being a material this is formed of at least two different
chemicals,
compounds, or the like). Further, two or more chemicals, compounds, or the
like may be combined
to form hollow core granules, which granules may form part or all of a
composition. In example
embodiments, stand-alone chemicals, compounds, or the like can include
materials, such as sodium
bicarbonate, clays, surfactants, etc., and further examples of such materials
are further discussed
herein. Such materials can thus be provided as a product that is formed
entirely or in part of
granules prepared such material. For example, cleaning products, abrasives,
personal care
products, deodorizers, animal litters, and the like may be prepared in total
from hollow core
granules as described herein or, alternatively, such hollow core granules may
form one or more
components of such products. In some embodiments, the present hollow core
structures can be
particularly configured for use in delivery of a desired product to a desired
setting. For example,
fertilizers, pesticides, and the like can be provided as hollow core structure
to enable delivery of the
fertilizers, pesticides, and the like with improved properties. The foregoing
uses and products are
understood to be example embodiments and are not intended to be limiting of
the useful
applications of the presently disclosed hollow core structures.
Structures with Hollow Cores
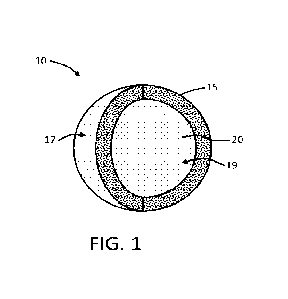
Referring to FIG. 1, a structure/granule 10 according to the present
disclosure may comprise
an outer wall 15 surrounding and substantially enclosing an inner core 20,
which inner core may be
substantially hollow and thus define a cavity. It is understood that the term
"wall" should not be
construed as limiting, and such term may be synonymous with similar terms,
such as "shell." Thus,
-12-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
while the term "wall" may be used throughout this disclosure, it is understood
that the wall
surrounds the cavity defining the hollow core. A substantially hollow core may
include a relatively
small content of material (e.g., solid or liquid) but is otherwise essentially
an open void within the
outer wall. Specifically, the phrase -substantially hollow" may indicate that
at least 90%, at least
95%, or at least 99% by volume of the core is void of any solid and/or liquid
material. The
structure 10 may be further defined in relation to having an outer wall
surface 17 and an inner wall
surface 19. The core of the hollow core structure thus may be defined as the
interior volume of the
hollow core structure that is bounded by the inner wall surface 19.
A hollow core granule according to the present disclosure particularly may be
described as
comprising at least one wall substantially surrounding a cavity that is
substantially devoid of any
solid or liquid so as to define a hollow core, the at least one wall
comprising a plurality of
individual particles of at least one wall forming material, the plurality of
individual particles
sufficiently bound together so that the at least one wall is structurally self-
sustaining. The at least
one wall that is substantially surrounding a cavity can indicate that the wall
completely surrounds
the cavity or may indicate the open porosity of the wall in that one more open
pores may define one
or more pathways between the inner cavity and the outer environment. In
addition to further
discussion provided herein, the nature of the at least on wall "substantially
surrounding" the cavity
particularly can mean that the wall completely surrounds the cavity (i.e.,
100% enclosure) or
surrounds the cavity with a minor portion of the wall being discontinuous,
such as through the
presence of open pores or other discontinuities in the wall providing an
opening between the cavity
and the outer environment (i.e., at least 90%, at least 95%, at least 98%, or
at least 99% enclosure
based on the area of the wall) may be present. Identifying the amount of
enclosure can be
calculated based on measurements of microscopic images. For example, in the
SEM images
provided in FIG. 19A to FIG. 21C, it is evident that open pores in the wall
can be visually
identified and measured. Other analytic methods may similarly be used. In some
embodiments, it
can be desirable to have somewhat less than 100% enclosure of the cavity in
order to achieve
improved properties as described herein. The cavity being "substantially
devoid" of any solid or
liquid can indicate that the core of the granule is not intentionally filled
with solid or liquid material
and there is an open space spanning across the hollow core granule when
measured from an interior
surface of the wall. This is particularly evident in the images shown in FIG.
24, FIG. 25, FIG. 27,
and FIG 2.8 of hollow core granules that have been cut in half to show the
interior cavity.
Substantially devoid thus can mean that internal volume of the core as defined
by the inner surface
of the wall is at least 90%, at least 95%, at least 97%, or at least 99% open
and absent of any solid
or liquid. The plurality of individual particles being "sufficiently bound"
can mean that particles
-13 -
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
retain their positioning relative to one another and do not exhibit any
significant degree of
rearrangement during normal handling of the hollow core granules.
The hollow core structure 10 may be provided in a variety of sizes, and an
average size may
be defined in relation to a diameter (e.g., for substantially spherical
structure) or in relation to the
largest dimension (e.g., a cross-wise or length-wise measurement for a
substantially elongated or
non-uniform structures) of the hollow core structure. The hollow core granules
can have an
average size of about 0.1 mm to about 20 mm, about 1 mm to about 10 mm, or
about 2 mm to
about 5 mm. In some embodiments, the hollow core structures may be
substantially small in size,
such as having an average size of about 0.1 mm to about 7 mm, about 0.5 mm to
about 6 mm, about
1 mm to about 5 mm, about 1.5 mm to about 4.5 mm, or about 2 mm to about 4 mm.
In other
embodiments, the hollow core structures may be substantially larger in size,
such as having an
average size of about 2 mm to about 20 mm, about 3 nun to about 15 mm, or
about 4 mm to about
12 mm. In still further embodiments, even larger sizes can be achieved, such
as about 5 mm to
about 50 mm, about 10 mm to about 45 mm, or about 15 mm to about 40 mm. The
foregoing sizes
may thus relate to an individual granule. Further, as is more evident from the
methods of
manufacture described below, the granule size achieved may be defined at least
in part by the
particle size of the binder material that is used. Thus, binder material may
be provided in larger
particle sizes to achieve larger hollow core granules, and binder material may
be provided in
smaller particle sizes to achieve smaller hollow core granules.
In some embodiments, individual granules of the hollow core structures may be
substantially spherical, substantially elliptical, or may otherwise have a
substantially rounded form.
In such embodiments, the wall may completely surround the cavity defining the
hollow core (i.e.,
the hollow core is completely separated from the surrounding environment).
Other shapes,
however, are not excluded. For example, in certain embodiments, a hollow core
structure 10 as
provided herein may be in an elongated form, such as a substantially fibrous
form or a tubular form,
which may have a closed end, an open end, or a partially closed end. Further,
the structures 10 may
be substantially irregular in form. For example, hollow core granules may have
a substantially
ellipsoid shape. Moreover, at least a portion of the wall of the hollow core
granule may be
concave. In some embodiments, a plurality of structures 10 may adhere one to
another to form
agglomerations of two, three, four, or more structures. Such agglomerates may
have a substantially
"pear" shape (e.g., where two adhered particles are of differing sizes) or may
have a substantially
"figure 8" shape (e.g., where two adhered particles are of substantially the
same size).
As illustrated in FIG. 1, the wall 15 of the structure 10 is substantially
uniform in thickness.
In some embodiments, however, the thickness of the wall 15 may vary. An
average wall thickness
(e.g., measured from the outer wall surface 17 to the inner wall surface 19)
may be in the range of
-14-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
about 0.05 mm to about 8 mm, about 0.1 mm to about 7 mm, about 0.5 mm to about
6 mm, about
1.0 mm to about 5 mm, or about 1.5 mm to about 2.5 mm. When small size
granules are prepared,
the average wall thickness thereof may be proportionally smaller, such as
about 0.1 mm to about 4
mm, about 0.25 mm to about 3.5 mm, about 1 mm to about 3 mm, or about 1.5 mm
to about 2.5
mm. The wall thickness and the overall size of the hollow core structures 10
can vary based upon
the types of materials used in forming the hollow core structures. In
particular, the nature of the
binder that is used can strongly influence the size of the cavity that defines
the core of the hollow
core structure. Likewise, the wall thickness can depend at least in part on
the size of individual
particles of wall forming material that are used. In some embodiments,
processing conditions, such
as the length of time spent in the fluidized bed, can also factor into the
dimensions of the hollow
core structures. Thus, one may customize the relative dimensions of the
overall size of the hollow
core structure, the thickness of the wall of the hollow core structure, and
the size of the cavity
defining the hollow core of the structure through choice of binder material,
choice of the type of
wall forming material, and size of the individual particles of the wall
forming material. In some
embodiments, such dimensions can be summarized in relation a diameter of the
cavity defining the
hollow core (i.e., the diameter across the hollow core at the largest
dimension as measured at inner
wall surface) of an individual granule relative to an overall diameter of the
individual granule (i.e.,
the diameter across the granule at the largest dimension as measured at the
outer wall surface). In
particular, the cavity diameter can be about 10% to about 80%, about 15% to
about 65%, about
20% to about 60%, about 25% to about 55%, or about 30% to about 50% of the
diameter of the
granule. In some embodiments, the relative dimensions can be summarized in
relation to a volume
of the cavity defining the hollow core to a volume of the overall granule. In
particular, the cavity
volume can be about 0.1% to about 50%, about 0.25% to about 25%, about 0.5% to
about 10%,
about 0.7% to about 7%, or about 1% to about 4% of the volume of the overall
granule. The
relative dimensions noted above can also affect the bulk density of the hollow
core structures. In
various embodiments, hollow core structures as described herein can have a
bulk density in the
range of about 200 grams per liter (g/L) to about 2000 g/L, about 250 g/L to
about 1200 g/L, about
200 g/L to about 900 g/L, about 400 g/L to about 850 g/L, about 450 g/L to
about 800 g/L, or about
500 g/L to about 750 g/L. As such, hollow core granules as described herein
can have a bulk
density that is significantly different from the bulk density of the wall
forming material itself. For
example, where sodium bicarbonate has a bulk density of about 1100 g/L, hollow
core granules as
described herein using sodium bicarbonate as the wall forming material can
have a bulk density of
about 700 g/L. Similarly, where bentonite has a bulk density of about 1000
g/L, hollow core
granules as described herein using bentonite as the wall forming material can
have a bulk density of
about 600 g/L. Accordingly, in some embodiments, hollow core granules of the
present disclosure
-15 -
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
can have a bulk density that is lower than the bulk density of the wall
forming material in its native
form (i.e., as found in nature or as sold as a commodity product) by at least
20%, at least 30%, or at
least 40%. Specifically, the hollow core granules can have a bulk density that
is about 10% to
about 75%, about 15% to about 50%, or about 20% to about 45% lower than the
bulk density of the
wall forming material in its native form. The comparison may be characterized
as being the density
of the formed, hollow core granule versus the density of the wall forming
material prior to being
incorporated into the hollow core granule.
The present hollow core structures, despite having an open or substantially
open cavity
bounded by a wall, can still retain a substantially consistent shape. This is
a surprising effect since
the at least one is formed of a plurality of individual particles of the wall
forming material without
an interior mass supporting the wall. Accordingly, the at least one wall can
be characterized as
being substantially self-sustaining in that the wall does not substantially
cave-in on itself but rather
maintains a granule shape as described above while having a central cavity
that, in some
embodiments, is substantially devoid of any solid or liquid material therein.
The hollow core granules, despite being hollow rather than being solid
throughout the
granule, can still exhibit significantly high strength. The strength in
particular may be a crush
strength, such as discussed in the appended examples. The strength can vary
based upon the choice
of wall forming material and the choice of binder. In some embodiments,
granule strength can be
at least 0.5 Newtons (N), at least 2 N, at least 3 N, at least 5 N, at least
10 N, or at least 15 N. The
maximum granule strength, in some embodiments, may have a maximum value of
about 50 N. In
certain embodiments, granule strength can be about 0.5 N to about 50 N, about
1 N to about 30 N,
about 2 N to about 25 N, or about 3 N to about 20 N.
The wall 15 of the hollow core structures 10 being configured as an
agglomeration of
individual particles 152 of one or more solid, wall-forming materials causes
the wall 15 to have
interstitial spaces 154 between the particles 152. This is seen in the partial
cross-section illustrated
in FIG. 2. As such, the wall 15 is a substantially continuous structure in
that it is formed of
individual particles that are sufficiently associated together to form a
stable, self-sustaining
structure, the interstitial spaces can provide certain properties to the
hollow core structures 10. As
seen in FIG. 2, the outer surface 17 and/or the inner surface 19 of the wall
15 is not necessarily
uniform and may exhibit a level of roughness or unevenness that may be
differentiated from a
substantially smooth wall surface In some embodiments, the interstitial space
154 may be at least
partially filled with a binder material. This is shown in FIG. 3, wherein the
particles 152 are
substantially surrounded by a binder 155. It is understood, however, that the
binder 155 may not
necessarily completely surround each and every particle 152. Likewise, the
binder 155 may be
-16-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
present in a discontinuous form, such as a particulate form such that
individual binder particles may
bind together two or more particles 152 of the wall-forming material.
A hollow core structure 10 according to the present disclosure may comprise a
single wall
15. In some embodiments, however, the structure 10 may be provided with a
plurality of walls,
which may be characterized as a wall with a multi-layer construction in some
embodiments. As
seen in the cross-section of FIG. 4, the structure 10 may comprise an inner
core or cavity 20 that
can be substantially empty or devoid of solid or liquid material and a
surrounding wall 15. The
wall 15 (which can be referenced as a first wall, first layer, inner wall, or
inner layer) may then be
substantially surrounded by another wall 25 (which may be referenced as a
second wall, second
layer, further wall, further layer, outer wall, or outer layer). As such, the
hollow core structure 10
may comprise a single wall or layer surrounding the substantially hollow inner
core 20 or may
comprise a plurality of walls or layers. When a plurality of walls or layers
is present, each
individual wall or layer may have a different average thickness, or the
relative average thicknesses
of the walls or layers may vary. In some embodiments, an outer wall or layer
may have a lesser
average thickness than an inner wall or layer. At least one of the plurality
of walls or layers will be
an agglomeration of individual particles of the wall-forming material. One or
more walls or layers,
however, and particularly an outer wall or outer layer may be configured as a
coating that is applied
to an inner wall or inner layer. Agglomeration may be referenced more
particularly in relation to
substantial adhesion of the individual particles of the wall forming material
to adjacent particles.
The adhesion may occur due to a variety of interactive forces and may be
achieved at least in part
due to the presence of the binder material that is one or more of at least
partially coating individual
particles of the wall forming material and/or that is at least partially
filling the interstitial spaces
between the individual particles of the wall forming material.
In some embodiments, a hollow core structure as described herein may be
defined in
relation to a porosity of a wall of the structure. Porosity may be at least
partially defined in relation
to the presence of the interstitial spaces 154 between particles 152 forming
the wall 15 of an
individual granule of the hollow core structures 10. Porosity may be
controlled in a variety of
manners, such as by altering the average size of individual particles 152
forming the wall 10, by
combining particles of two or more different average particle sizes, by
controlling the amount of
any binder that may be present, and the like. For example, particles used as
wall forming materials
may have an average size in the range of about 001 mm to about 2 mm, about 0
02 mm to about
1.5 mm, about 0.05 mm to about 1.0 mm, or about 0.1 mm to about 0.8 mm. In
some
embodiments, a range of particles sizes may be used to achieve a greater
packing density in the
wall with smaller particles filling in spaces between larger particles. Thus,
particles of the wall
-17-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
forming material may have an average size spanning a range so that the
smallest particle size
differs from the largest particle size by about 1 mm, about 0.8 mm, about 0.5
mm, or about 0.2 mm.
In some embodiments, porosity further may be at least partially controlled
through choice of
material used in forming the wall, such as utilizing a material with a high or
low porosity or
utilizing combinations of materials with different porosities. Example
materials useful in forming
the walls of the present, hollow core structures are discussed in detail
below. In some
embodiments, porosity may be defined in relation to any one or more of average
pore size, pore
distribution, and the like. For example, an average pore size of pores in the
wall of the structure
may be in the range of about 100 nm to about 200 um, about 250 nm to about 100
um, or about 500
nm to about 50 um.
In addition to the nature of the walls, hollow core structures according to
the present
disclosure likewise can be defined in relation to the nature of the hollow
cores As noted above, the
cavity (i.e., an open volume) defining the hollow core can vary, and the
cavity may be substantially
completely devoid of any solid or liquid materials (e.g., less than 10%, less
than 5%, less than 2%,
or less than 1% of the cavity volume including any solids or liquids therein
at the time of
manufacturing). In some embodiments, a hollow core structure may include a
content of a further
material present in the volume defined by the inner surface of the innermost
wall of the hollow core
structure. For example, structural scaffolding may be present in the cavity
defining the hollow
core. As other examples, liquids may be filled in the cavity defining the
hollow core. As such, the
hollow core structures may provide delivery articles whereby a material
present in the hollow core
can be delivered in a controlled manner via dissolution, breakage, or other
removal of the outer
wall to release the inner material.
Structures according to the present disclosure may be characterized utilizing
a variety of
testing techniques. For example, scanning electron microscopy (SEM) testing
may be useful in
characterizing the particle characteristics, particle morphology, porosity and
pore distribution, and
the like. Accordingly, the present structures and products incorporating such
structures may be
further defined in relation to one or more of the foregoing characteristics.
Porosity of the hollow
core granules can be seen, for example, in the scanning electron microscope
(SEM) images shown
in FIG. 19A to FIG. 21C. A hollow core granule comprising zeolite particles as
the wall forming
material is shown in the SEM images of FIG. 19A at 59X magnification and in
FIG. 19B at 270X
magnification A hollow core granule comprising activated charcoal particles as
the wall forming
material is shown in the SEM images of FIG. 20A at 68X magnification and in
FIG. 20B at 229X
magnification. A hollow core granule comprising sodium bicarbonate particles
as the wall forming
material is shown in the SEM images of FIG. 21A at 87X magnification, in FIG.
21B at 346X
magnification, and in FIG. 21C at 1,535X magnification. As seen in the
respective images, hollow
-18-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
core granules were consistently prepared with particles of different wall
forming materials.
Further, it is apparent from the images that the hollow core granules
consistently retain a similar
structure with the wall of the hollow core granule having a number of pores
between the individual
particles of the wall forming material. Open porosity is seen to be variable
with more of less of the
pores being filled with the binder material. Thus, the hollow core granules
can be configured with
greater or lesser open porosity by controlling processing so that more or less
of the binder is
retained in the walls of the hollow core granules The ability to control open
porosity can be
valuable in fine tuning the properties achieved, such as improved
solubilization,
absorption/adsorption properties, and other properties as further discussed
herein.
A variety of wall-forming materials may be used to prepare one or more walls
of a hollow
core granule according to the present disclosure. Wall forming materials may
be one or both of
functional and structural. A functional, wall forming material can be any
material that is included
in the hollow core granule to provide a desired function to a product
comprising the hollow core
granules. Accordingly, such materials may be used individually to form a
product exhibiting the
function of the functional material and/or may be used in any combination of
any number of such
materials to form a product exhibiting the combined functions. It is
understood that products
including hollow core granules having one or more of the functional materials
as a wall forming
material may also include other, non-functional components, such as fillers,
bulking agents, inert
components, and the like. Moreover, the hollow core granules themselves may
include fillers,
bulking agents, inert components, or the like as one or more wall forming
materials in combination
with one or more functional materials to achieve the proper dosing of the
functional material in the
overall hollow core granules. The functional material(s) may be available is a
solid form (e.g.,
particles) under conditions necessary for preparation of a hollow core granule
as described herein.
In such cases, the functional material may be additionally effective as a
structural component of the
wall(s) of the hollow core granule. In some embodiments, however, one or more
functional
materials for use in the hollow core granules may be typically available in a
liquid form under
conditions necessary for preparation of a hollow core granule as described
herein. In such
embodiments, the liquid material may be combined with a structural material to
provide the liquid
in a solid form. A structural material with which one or more liquid materials
may be combined
may also be a functional material. A structural material with which one or
more liquid materials
may be combined, however, may be non-functional in the hollow core granule to
be prepared, and
the structural material may thus be referenced as a carrier component or
particle, a filler, a bulking
agent, an inert component or particle, or the like Clays, ceramics, silicates,
zeolites, carbon, and
even other minerals or salts can be useful as carriers into or onto which a
desired liquid for
inclusion in the hollow core granules can be absorbed, adsorbed, or
impregnated. The carrier
-19-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
particles may be considered substantially inert to the delivery site (i.e.,
providing no desired benefit
but still safe for use) and may remain after delivery of the active agent or
may further dissolve or
disintegrate. In some embodiments, the carrier particles may provide an
additive effect so that the
efficacy of the liquid, functional material is improved through combination
with the carrier
particles or so that the carrier particles themselves provide a different,
desired effect at the delivery
site.
Liquid components may alternatively, or additionally, be provided in a form
suitable for use
in forming one or more walls of a hollow core granule as described herein
through use of
encapsulation technologies. Thus, capsules and/or microcapsules may be
utilized. Encapsulation
technologies likewise can be utilized with otherwise solid materials in order
to provide the
encapsulated component in a controlled release form whereby an encapsulating
shell must be
solubilized, degraded, or otherwise removed in order for the encapsulated
material to be released at
a delivery site.
Encapsulation of any material to be used as a wall forming material for the
present hollow
core granules can be carried out using any suitable technique. For example,
microcapsules can be
formed using any of various chemical encapsulation techniques such as solvent
evaporation,
solvent extraction, organic phase separation, interfacial polymerization,
simple and complex
coacervation, in-situ polymerization, liposome encapsulation, and
nanoencapsulation.
Alternatively, physical methods of encapsulation could be used, such as spray
coating, pan coating,
fluid bed coating, annular jet coating, spinning disk atomization, spray
cooling, spray drying, spray
chilling, stationary nozzle coextrusion, centrifugal head coextrusion, or
submerged nozzle
coextrusion. Regardless of the encapsulation methodology employed, materials
used to form the
capsules can vary. Classes of materials that are typically used as wall or
shell materials include
proteins, polysaccharides, starches, waxes, fats, natural and synthetic
polymers, and resins.
Exemplary materials for use in the microencapsulation process used to form the
microcapsules
include gelatin, acacia (gum arabic), polyvinyl acetate, potassium alginate,
carob bean gum,
potassium citrate, carrageenan, potassium polymetaphosphate, citric acid,
potassium
tripolyphosphate, dextrin, polyvinyl alcohol, povidone, dimethylpolysiloxane,
dimethyl silicone,
refined paraffin wax, ethylcellulose, bleached shellac, modified food starch,
sodium alginate, guar
gum, sodium, sodium citrate, carboxymethylcellulose, hydroxypropyl cellulose,
hydroxypropylmethylcellulose, sodium ferrocyanide, sodium polyphosphates,
locust bean gum,
methylcellulose, sodium trimetaphosphate, methyl ethyl cellulose, sodium
tripolyphosphate,
microcrystalline wax, tannic acid, petroleum wax, terpene resin, tragacanth,
polyethylene, xanthan
gum, and polyethylene glycol. Microcapsules are commercially available, and
exemplary types of
microcapsule technologies are of the type set forth in Gutcho, Microcapsules
and
-20-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
Microencapsulation Techniques (1976); Gutcho, Microcapsules and Other Capsules
Advances
Since 1975 (1979); Kondo, Microcapsule Processing and Technology (1979);
Iwamoto et al.,
AAPS Pharm. Sci. Tech. 2002 3(3): article 25; U.S. Pat. Nos. 5,004,595 to
Cherukuri etal.;
5,690,990 to Bonner; 5,759,599 to Wampler et al.; 6,039,901 to Soper et al.;
6,045,835 to Soper et
al.; 6,056,992 to Lew; 6,106,875 to Soper etal.; 6,117,455 to Takada et al.;
6,482,433 to DeRoos et
al.; and 6,929,814 to Bouwmeesters et al.; each of which is incorporated
herein by reference.
Non-limiting example embodiments of materials that can be suitable for use in
forming a
wall of a hollow core structure as described herein can include clays (e.g.,
bentonite), glass,
ceramics, aluminas, silicates, zeolites, carbon (e.g., activated charcoal),
metals, salts (e.g., sodium
bicarbonate or baking soda, sodium carbonate or soda ash, sodium chloride,
etc.), powdered
formulations (e.g., solid cleaning compositions, such as laundry detergents,
dish detergents, fabric
cleaners/deodorizers, abrasive cleaners, etc.), absorbents, adsorbents,
deodorizers, odor control
agents, health or beauty agents, surfactants, enzymes, bleaches, oxidizers
(e.g., peroxides),
reducers, gellants (e.g., gelatin, pectin, cellulosics, etc.), flavors,
fragrances, abrasives, fertilizers,
insecticides, pesticides, bactericides, herbicides, antimicrobials, anti-
sticking agents, fillers,
binders, preservatives, optical agents (e.g., brighteners), disinfectants,
chelators, molecular binding
agents, dyes, coloring agents, colored particles, de-dusting agents, and other
materials known for
use in consumer products and/or industrial settings to provide a specific
function to a product. Any
of the foregoing may be a functional material as referenced above and may also
be referenced as
additives in that they can be added to other products to impart the desired
function and/or may be
provided as a stand-alone product that can be combined as needed with other
products to achieve an
additive result. Such materials may be used in a solid form as a functional
and/or structural wall
forming material without modification or with modification to impart
controlled release and/or to
modify hydrophilicity/hydrophobicity of the material. Such materials may be
used in a liquid form
as a functional wall forming material when combined with a carrier or other
solid material and/or
with modification to be in a solid format, such as encapsulation techniques
noted above. The
foregoing list of wall forming materials is not intended to be all-inclusive,
and it is understood that
the skilled person, in light of the totality of the present disclosure, will
be able to identify other
chemicals, compounds, compositions, and the like that are used in or as
commercial products that
can likewise be utilized in forming hollow core structures as presently
disclosed.
In certain embodiments, bentonite or sodium bicarbonate may be particularly
used as a wall
forming material due to the extensive number of uses for such materials, and
either may be utilized
as a functional and/or structural component of the present hollow core
granules. Non-limiting
examples of bentonite clays that can be used include sodium bentonite,
potassium bentonite,
lithium bentonite, calcium bentonite and magnesium bentonite, or combinations
thereof Clay-
-21 -
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
based liquid absorbing materials are described, for example, in U.S. Patent
No. 8,720,375 to Miller
et al., the disclosure of which is incorporated herein by reference. Further,
non-limiting examples
of absorbent or adsorbent materials that are suitable for use in hollow core
granules in combination
with, or as an alternative to bentonite can include clay, quartz, feldspar,
calcite, illite, calcium
carbonate, carbon, mica, Georgia white clay, hectorite, smectite, opal,
kaolinite, pumice, tobermite,
slate, gypsum, vermiculite, halloysite, sepiolite, marls, diatomaceous earth,
dolomite, attapulgite,
montmorillonite, Monterey shale, Fuller's earth, silica, fossilized plant
materials, perlites, expanded
perlites, mixtures thereof, and like materials.
Preferably, the wall forming materials at the time of preparation of the
hollow core granules
will be in a solid, substantially particulate form and likewise may be adapted
to or configured to be
substantially insoluble in a binder that may be used in forming the walled
structure. This may
reference a naturally occurring state of the material or may arise through
combination of the desired
material with another structural material as already discussed above. In some
embodiments, the
wall-forming material, when used in preparation of the hollow core granules,
will be configured as
solid particles with a melting point of about 100 C or greater, about 110 C
or greater, about 120
C or greater, or about 130 C or greater.
Any functional material in a hollow core granule according to the present
disclosure may be
provided in a manner so as to provide controlled release of the material.
Controlled release can
specifically indicate any of the following: delayed release so that, after a
defined period,
substantially the entire amount of the material (i.e., a "bolus") is released;
delayed release so that,
after a defined period, release of the material begins and proceeds over a
second defined period
(i.e., "prolonged release"); or metered release so that release of the
material begins substantially
immediately after application, but the release proceeds over a defined period
of time. Controlled
release may be achieved through use of encapsulation methods discussed above.
Controlled release
may alternatively, or additionally, be achieved through selection of materials
that are configured as
"fast release" and "slow release" forms of the material. Further, controlled
release configurations
may be applied to any material, with any product, and/or with any use of the
hollow core granules
as otherwise described herein. While certain products discussed herein may be
specifically
described in relation to controlled release forms thereof, it is understood
that these controlled
release properties may be applied to any product described herein regardless
of whether such
feature is specifically called out in relation to the separate discussion of
the product herein.
In some embodiments, a wall of a hollow core structure as described herein may
be formed
from a gelled material. Such gelled materials may comprise at least one
hydrophilic long-chain
polymer and at least a water source. Hydrophilic, long-chain polymers useful
herein can include
long chain carbohydrates (e.g., polysaccharides) as well as various proteins.
The hydrophilic, long-
-22-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
chain polymer preferably is configured to thicken and form a gel upon
hydration (with or without
heating). Non-limiting examples of hydrophilic, long-chain polymers that may
be utilized in
forming a wall according to the present disclosure can include: gelatin,
pectin, carrageenan, gellan
gum, guar gum, locust bean gum, gum arabic, xanthan gum, starch,
methylcellulose, agar, konjac,
alginates, and combinations thereof (including single, binary, tertiary, or
quaternary blends). The
hydrophilic, long-chain polymer may comprise about 0.1% to about 20%, about 1%
to about 15%,
or about 2% to about 10% by weight of the gelled material used to form the
wall of the hollow core
structure. The gelled material may otherwise comprise about 80% to about
99.9%, about 85% to
about 99%, or about 90% to about 98% by weight of a water source, particularly
deionized water.
In some embodiments, a wall of a hollow core granule may comprise a lipidic
material.
Non-limiting examples of lipidic bases include oils, fats, and compositions
formed therewith. In
some embodiments, edible fats in particular may be used. Suitable lipidic
materials for use in
forming lipophilic compositions include fats and oils derived from one or more
of a vegetable
source, an animal source, a nut source, a seed source, and the like. Suitable
lipidic material may be
predominately or completely saturated, predominately or completely
unsaturated, or hydrogenated.
Non-limiting examples of suitable lipidic materials include fats and/or oils
derived from one or
more of the following: cocoa, palm, coconuts, almonds, cashews, hazelnuts,
macadamia nuts,
peanuts, pecans, pistachios, walnuts, pumpkin seeds, sesame seeds, soybeans,
rapeseed, corn,
safflower seeds, and the like. Specific, non-limiting examples of lipid based
materials that may be
used in preparing a composition as described herein include chocolates with
any cocoa
concentration (e.g., milk chocolate, dark chocolate, white chocolate), palm
fat, coconut fat, peanut
butter, hazelnut fats, vegetable oils, milk fats, confectionary fats (such as
available from AAK,
AB), and the like. Such materials may include additional components, such as
sugar, salt, other
oils, and the like. For example, chocolates may comprise sugar, cocoa butter,
cocoa processed with
alkali, milk fat, lactose (e.g., from milk), soy lecithin, emulsifier,
vanillin, artificial flavor, milk,
and/or other ingredients. Dairy components utilized in lipophilic compositions
can include fats,
proteins, and/or sugars derived from cow milk, goat milk, and the like.
As noted above, in one or more embodiments, a wall of a hollow core granule
will be
prepared though use of a binder, and the formed wall will retain a content of
the binder material. In
some embodiments, however, substantially all of the binder may be removed from
the structure
during processing of the stricture This may occur in particular when one or
more wall forming
materials as described above is of a nature whereby the particles thereof may
remain bound even
after removal of the binder material. In particular embodiments, at least a
portion of the binder will
be retained in the wall of the formed hollow core granules. For example,
formed granules may
include binder retained in the wall of the granule (e.g., in at least a
portion of interstitial spaces
-23 -
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
between individual particles of the wall forming material) in an amount of
about 0.1% to about
50% by weight, about 1% to about 45% by weight, about 2% to about 40% by
weight, or about 5%
to about 30% by weight, based on the total weight of the granules. The
remaining weight of the
granules may be accounted for by the wall forming material(s) alone or in
combination with any
coatings applied to the granules.
Binder materials particularly may be provided in particular formats for use in
processing to
form the hollow core granules Specifically, it can be beneficial for the
binders to be in particulate
form when added to the processing equipment. In this manner, the particles of
the wall forming
material can agglomerate or accumulate around the particles of the binder as
the solid binder
softens with heating. Thereafter, as the binder liquefies, the liquid binder
will flow out of the core
of the forming granules and into the walls forming with the wall forming
materials. To this end, it
can be particularly useful for the binder particles, seeds, or ciystals to
have a starting size in a range
of about 0 1 mm to about 5 mm, about 0.5 mm to about 4 mm, or about 0.8 mm to
about 3 mm.
A variety of materials may be utilized as a binder. In some embodiments, a
binder may be a
material that is substantially solid at a temperature of about 50 C or less,
about 45 C or less, or
about 40 C or less and is liquid above such temperature. In certain
embodiments, the binder may
be adapted to or configured to be a solid at a temperature in the range of
about 10 C to about 50
C, about 15 C to about 45 C, or about 20 C to about 40 'C. Additionally, or
alternatively, the
binder may be a material with a melting point in the range of about 40 C to
about 95 C, about 45
C to about 90 C, or about 50 C to about 90 C. The binder may also be chosen
for a defined
application, as further described herein, based upon whether the binder is
hydrophilic or
hydrophobic. For example, in some embodiments, hydrophobic binders may be
utilized, such as
paraffinic hydrocarbons, olefinic hydrocarbons, waxes, beeswax, or similar
materials exhibiting the
foregoing state change characteristics. Hydrophobic polymers may likewise be
utilized. Non-
limiting examples of suitable, hydrophobic binders can include wax, paraffin,
polycaprolactone,
ethylene-vinyl acetate copolymers, polypropylene carbonate,
poly(tetramethylene oxide),
poly(ethylene adipate), poly(trans-butadiene), thermoplastic polyurethane
(e.g., carbothane TPU),
stearic acid, and the like. Likewise, one or more lipidic materials described
above may be utilized
as a hydrophobic binder. In further embodiments, the binder may specifically
be a hydrophilic
material, such as a polyethylene glycol (PEG). Further examples of suitable
binders include
materials such as polyoxyethylene fatty ethers derived from various types of
alcohols (e.g., lauryl,
cetyl, stearyl, and oleyl alcohols), and such materials are available under
names such as BrijTm
S100 (polyoxyethylene stearyl ether) or Steareth-100. Such polyoxyethylene
fatty ethers may be
useful as a hydrophilic binder although being more hydrophobic in nature that
other hydrophilic
binders, such as PEG materials. Likewise, fatty acids with carbon chain
lengths in the range of C10
-24-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
to C30 may be useful as binders, one example embodiment being stearic acid. In
one or more
embodiments, binders may be a material having a melting temperature this is
less than a melting
temperature of the material used in forming the wall of the hollow core
structure. As such, suitable
binder materials may have a substantially high melting temperature, such as in
the range of about
90 C to about 200 C, about 100 C to about 180 C, or about 110 C to about
160 C. For
example, plastics (e.g., polyvinylchloride (PVC), high density polyethylene
(HDPE), etc.),
thermoplastics, rubbers, and similar materials may be utilized as binders in
some embodiments.
In some embodiments, binders may be chosen specifically in relation to the
viscosity of the
binder in the liquefied form. Binders with lower liquid viscosities can
achieve faster processing for
granule formation while binders with higher liquid viscosities can result in
longer processing
requirements for granule formation. Likewise, however, binder liquid viscosity
can affect one or
more properties of the finished granules. For example, binders with higher
liquid viscosities can
lead to relatively stronger granules. As such, binder choice can be a factor
of binder liquid
viscosity. In some embodiments, flow properties of the binder in liquid form
may be controlled, at
least in part, through selection of binder molecular weight. For example, PEG
materials can be
particularly useful as binders, and various grades so PEG materials can be
chosen based at least in
part on the molecular weight of the material. In various embodiments, suitable
PEG materials for
use as a binder in the hollow core granules particularly can have a molecular
weight of at least 400
Da, at least 1000 Da, at least 2000 Da, or at least 4000 Da. Maximum molecular
weight can be, for
example, no greater than 50000 Da, no greater than 45000 Da, or no greater
than 40000 Da. More
particularly, PEG molecular weight can be in the range of about 400 Da to
about 34,000 Da. In
specific embodiments, a lower range, such as about 400 Da to about 15000 Da,
about 500 Da to
about 12000 Da, or about 1000 Da to about 10000 Da may be used. In other
embodiments, a high
range, such as about 8000 Da to about 34000 Da, about 10000 Da to about 30000
Da, or about
12000 Da to about 25000 Da may be used.
Molecular weight can be expressed as a weight average molecular weight (M,) or
a number
average molecular weight (Mn). Both expressions are based upon the
characterization of
macromolecular solute containing solution as having an average number of
molecules (n) and a
molar mass for each molecule (Mi). Accordingly, number average molecular
weight is defined by
formula 1 below.
M,, = " (1)
n,
-25 -
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
Weight average molecular weight (also known as molecular weight average) is
directly measurable
using light scattering methods and is defined by formula 2 below.
(2)
n,i1/1
Molecular weight can also be expressed as a Z-average molar weight (Mz),
wherein the calculation
places greater emphasis on molecules with large molar weights. Z-average molar
weight is defined
by formula 3 below.
viz = n,M,3 (3)
n,M
Unless otherwise noted, molecular weight (MW) is expressed herein as weight
average molecular
weight.
Although a variety of solid, wall-forming materials arc described above along
with a variety
of binders, it is understood that the present disclosure contemplates all
combinations of wall-
forming materials and binders as described herein and as would be otherwise
recognized as useful
in light of the present disclosure. As such, the present disclosure
encompasses hollow core
structures wherein at least one wall or layer comprises any of the following:
particles of one or
more types of clay (e.g., bentonite) combined with at least one binder
described above; particles of
glass combined with at least one binder described above; particles of one or
more ceramics
combined with at least one binder described above; particles of one or more
aluminas combined
with at least one binder described above; particles of one or more silicates
combined with at least
one binder described above; particles of one or more zeolites combined with at
least one binder
described above, particles of carbon combined with at least one binder
described above; particles of
one or more metals combined with at least one binder described above;
particles of one or more
salts (e.g., sodium bicarbonate or baking soda, sodium carbonate or soda ash,
or sodium chloride)
combined with at least one binder described above, particles of one or more
cleaning compositions
combined with at least one binder described above; particles of one or more
fertilizers combined
with at least one binder described above; particles of one or more pesticides
combined with at least
one binder described above, particles of one or more absorbents and/or
adsorbents combined with
at least one binder described above; particles of one or more deodorizers
and/or odor control
agents combined with at least one binder described above; particles of one or
more bleaches or
-26-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
bleaching agents combined with at least one binder described above; particles
of one or more
oxidizers combined with at least one binder described above; particles of one
or more reducers
combined with at least one binder described above; particles of one or more
gellants combined with
at least one binder described above; particles of one or more fillers combined
with at least one
binder described above; and particles of one or more chelators combined with
at least one binder
described above. Of course, it is understood that any type of material as
described herein may be
used as a wall forming material alone or in combination with a solid where the
desired material is
not in a solid form.
As further discussed herein, choice of wall forming material and/or choice of
binder
material can be effective to customize the hollow core granules to exhibit
various properties. In
some embodiments, the hollow core granules may be defined in relation to water
absorption
capacity. This can be a characterizing feature particularly in relation to
hollow core granules
including appropriate wall forming materials and/or binders so that the hollow
core granules are
hydrophilic. In example embodiments, hollow core granules can have a water
absorption capacity
such that the hollow core granules will absorb a weight of water that is about
5% to about 80%,
about 10% to about 70%, or about 15% to about 60% of the initial weight of the
hollow core
granules. The hollow core granules likewise may exhibit greater water
absorption than the wall
forming material alone. For example, the hollow core granules may have a water
absorption that
exceeds the water absorption of the wall forming material used in forming the
hollow core granules
(i.e., when the wall forming material is in its native form, prior to
incorporation into the hollow
core granules) by an amount of about 2% to about 20%, about 2% to about 15%,
or about 3% to
about 10%.
In some embodiments, the hollow core granules as described herein may be
defined in
relation to oil absorption capacity. This can be a characterizing feature
particularly in relation to
hollow core granules including appropriate wall forming materials and/or
binders so that the hollow
core granules are hydrophobic. In example embodiments, hollow core granules
can have an oil
absorption capacity such that the hollow core granules will absorb a weight of
oil that is about 5%
to about 80%, about 10% to about 70%, or about 25% to about 65% of the initial
weight of the
hollow core granules. The hollow core granules likewise may exhibit greater
oil absorption than
the wall forming material alone. For example, the hollow core granules may
have an oil absorption
that exceeds the oil absorption of the wall forming material used in forming
the hollow core
granules (i.e., when the wall forming material is in its native form, prior to
incorporation into the
hollow core granules) by an amount of about 5% to about 50%, about 10% to
about 40%, or about
15% to about 35%.
-27-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
Methods of Preparation
Hollow core structures according to the present disclosure may be prepared
according to a
variety of methods. In one or more embodiments, a method for preparing a
structure with a
substantially hollow core may comprise combining a binder a described herein
with a plurality of
solid particles of a wall-forming material as described herein to form a
mixture. The wall-forming
material may particularly be a material that is substantially insoluble in the
binder and that has a
melting point that is greater than the melting point of the binder. In light
of the example
embodiments of solid, wall forming materials and the example embodiments of
binders provided
above, it will be readily evident which types of solid, wall forming materials
may be combined with
which types of binders to carry out such methods. In example embodiments, a
suitable binder may
be a material having a melting point of about 40 C to about 95 C (or a
further range as described
above), and suitable solid particles may be a material that has a melting
point of about 60 'V or
grater, about 70 C or greater, about 80 C or greater, about 100 C or
greater or about 110 C or
greater. Of course, it is understood that a suitable binder may be chosen so
that the binder has a
melting point that is less than the melting point of the wall forming material
by at least 5 C, at least
10 C, at least 15 C, or at least 20 C. The binder and solid particles may
be combined at a
temperature that is below the melting point of the binder, such as at room
temperature or ambient
temperature. The binder and the solid particles may be mixed at this
temperature for a certain time,
such as about 15 seconds to about 180 seconds, about 30 seconds to about 150
seconds, or about 45
seconds to about 120 seconds to provide a substantially uniform mixture.
Combination of the materials may be in a first container for transfer to a
second container
for heating. Alternatively, the process can be carried out in a single unit,
such as a fluidized bed
reactor. As such, a fluidizing gas, such as air, may flow upward through the
bed to provide mixing
and, optionally, to provide for heating and/or cooling of the mixture. Other
types of reactors may
also be used. When using a fluidized bed reactor, the particles of binder
material may be first
added to the fluidized bed followed by the particles of the wall forming
material.
The mixture of the binder and the solid particles can be heated to a maximum
temperature
to cause melting of the binder. As such, the maximum temperature can be a
temperature that is
above the melting point of the binder and below the melting point of the
plurality of solid particles.
Such heating can be adapted to or configured to form agglomerations of the
solid particles. In
some embodiments, the maximum temperature may be a temperature that exceeds
the melting point
of the binder by about 5 C or more, about 10 C or more, or about 20 C or
more. The binder may
alternatively, or additionally, be at least partially fluidized (e.g., melted)
when added to the wall
forming material. For example, binder in a liquid form may be sprayed onto
particles of the wall
forming material, such as through an atomizer or similar unit adapted to or
configured to provide
-28-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
the liquid binder in a substantially fine, spray or mist form. In some
embodiments, in situ melting
can be utilized where binder particles that are substantially larger than the
particles of the wall-
forming material are utilized. Specifically, hollow core particles may be
formed by immersion of
the particles of the wall-forming material into molten binder particles and
subsequent layering.
Preferably, however, materials will be supplied in a suitable configuration so
that particles of the
wall forming material with accumulate or agglomerate around seed particles or
crystals of the
binder so that, as heating continues, the binder will flow out of the center
of the forming granules
and into the interstitial spaces of the particles forming the wall.
In some embodiments, heating can be carried out utilizing a specified heating
rate. For
example, it can be desirable for heating to be carried out at a rate of about
5 C per minute to about
25 C per minute, about 7 C per minute to about 22 C per minute, or about 10
C per minute to
about 20 'C per minute. Heating may begin at ambient temperature, the heating
may be applied at
the noted rate until the maximum temperature is reached. In some embodiments,
the maximum
temperature may be maintained for a defined period of time. For example, the
maximum
temperature may be maintained for a time of about 30 seconds to about 1 hour,
about 30 seconds to
about 45 minutes, or about 2 minutes to about 30 minutes. As seen in the
appended examples,
residence time at the maximum heating temperature can affect the final granule
properties,
including wall thickness, granule size, and percentage of binder present in
the wall of the formed
granule.
In some embodiments, processing time in a fluidized bed reactor can be
controlled to adjust
the average size of individual granules of the hollow core structures that are
prepared. Processing
time may also be adjusted to control other properties, such as the size of the
cavity in individual
granules of the hollow core structures, the ratio of cavity diameter to
overall granule diameter, and
the bulk density of the granules. In some embodiments, processing time in a
fluidized bed reactor
can be adjusted to be within a range of about 10 minutes to about 20 minutes
or a range of about 12
minutes to about 18 minutes in order to maximize one or more the noted
properties. Lesser
processing times (e.g., about 1 minute to about 9 minutes or about 3 minutes
to about 7 minutes)
and/or greater processing times (e.g., about 22 minutes to about 30 minutes)
can be utilized to
provide lower values. Processing times may likewise be adjusted based upon the
viscosity of the
liquefied binder. Specifically, higher viscosities may require longer
residence times while lower
viscosities may require lesser residence times_
The formed agglomerations of the plurality of solid particles can be cooled to
provide the
plurality of granules that each have a substantially hollow core (i.e., an
internal cavity). In
particular, this can include cooling to a temperature that is below the
melting point of the binder. In
some embodiments, it can be beneficial to effect a substantially rapid cooling
of the solid particles,
-29-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
such as cooling to below the melting point of the binder within a time of
about 5 seconds to about 5
minutes, about 10 seconds to about 3 minutes, or about 15 seconds to about 2
minutes. In other
embodiments, longer cooling times may be utilized, such as about 5 minutes to
about 60 minutes,
about 10 minutes to about 50 minutes, about 20 minutes to about 40 minutes, or
about 25 minutes
to about 35 minutes.
As a non-limiting example, in some embodiments, preparation of structures
described
herein may be carried out in a multi-stage mixer, For example, in a first
stage mixer, particles of
the wall forming material may be combined with a binder and effectively form a
relatively thin
coating of the wall forming particles around a crystal or particle(s) of the
binder. The mixing may
continue while particles of the wall forming continue to flocculate around the
binder or otherwise
combine to increase the thickness of the wall. If desired, particles from the
first stage mixer may be
passed to a second stage mixer where flocculation or particle adherence may
continue for formation
of the wall. Structures having a desired wall thickness may then be passed to
a rotary dryer (or
similar structure) for removal of a portion or substantially all of the binder
from the structures to
leave the structures with the desired, hollow core configuration. Because of
such flocculation,
choice of binder material can be used to adjust the size of the cavity within
individual granules of
the hollow core structures. Binders that tend to exist as relatively smaller
particles or crystals can
thus be chosen to form individual granules with relatively small core
diameters, and binders that
tend to exist as relatively larger particles or crystals can thus be chosen to
form individual granules
with relatively large core diameters.
As discussed above, while particles of the wall forming material may initially
flocculate
around particles of the binder material, as the binder material liquefies, the
binder material can flow
out from the core of the forming granule and into accumulating particles of
the wall forming
material. The evacuation of the binder from the interior of the forming and/or
formed granules
results in the interior cavity of the granule. A portion of the binder may
remain at one or both of
the inner wall surface 19 and the outer wall surface 17 of the wall forming
the individual granule.
Likewise, a portion of the binder may remain in the interstitial spaces 154 as
discussed previously.
As non-limiting examples, a formed structure with a substantially hollow core
according to the
present disclosure may be configured so that the amount of binder present in
the wall of the hollow
core structure is about 0.1% to about 50% by weight, about 1% to about 45% by
weight, about 2%
to about 40% by weight, or about 5% to about 30% by weight based on the total
weight of the
granules.
In one or more embodiments, a structure with a substantially hollow core may
be prepared
in a gel-forming process. Such process can be particularly useful for forming
hollow core
structures with a substantially continuous phase outer wall that is a gel or
hydrogel and comprising
-30-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
mainly water and a gel-former. Such hollow core structures may be utilized as
formed or may be
further treated, such as to form a further, outer wall surrounding the gel
wall.
Methods for preparing structures according to such embodiments can comprise
providing a
solution of a gel-former in water. The gel-former particularly can be a
hydrophilic long chain
polymer as otherwise described herein. Preferably, the gel-former and water
can be at an increased
temperature or can be specifically heated to such temperature to expedite
polymer dissolution to
form a solution. For example, the solution of gel-former in water can be at a
temperature of about
50 C or greater, about 60 C or greater, or about 70 C or greater, such as
about 50 C to about 95
C, about 55 C to about 90 C, or about 60 C to about 85 'C. The solution may
be stirred or
simply left at the increased temperature until substantially all of the gel-
formed has dissolved ¨ e.g.,
as evidenced by visual inspection.
The methods further can comprise contacting a stream of the solution with a
hydrophobic
liquid in a manner adapted to or configured to form droplets of the gel-former
(e.g., the hydrophilic
long chain polymer). The contacting can be via a variety of means. For
example, a stream of the
solution and a stream of the hydrophobic liquid can be simultaneously poured
such that the two
streams may sufficient physical contact to cause the solution to separate into
the gel droplets. In
some embodiments, the hydrophobic liquid can be provided a container, and the
solution of the gel-
former in water can be poured or otherwise introduced into the container. If
desired, the solution
may be delivered for contact with the hydrophobic liquid in a substantially
droplet form or
relatively thin stream form. For example, the solution may be delivered
through a syringe pump or
similar device including one or a plurality of outlets of a desirably small
size, such as a diameter of
about 0.01 mm to about 2 mm, about 0.05 mm to about 1.5 mm, about 0.1 mm to
about 1.2 mm, or
about 0.2 mm to about 1 mm.
The solution may be at least partially cooled prior to combining with the
hydrophobic liquid
and/or may be cooled by contact with the hydrophobic liquid. In some
embodiments, pre-cooling
may be excluded. Preferably, the hydrophobic liquid is at a temperature that
is less than the
temperature of the solution of the gel-former. For example, the hydrophobic
liquid may be at a
temperature of about 45 C or less, about 40 C or less, or about 35 C or
less (e.g., about 5 C to
about 40 C, about 5 C to about 25 C, or about 5 C to about 20 C. In some
embodiments, the
hydrophobic liquid may be provided in a refrigerated tank or similar storage
unit.
Optionally, the method may comprise separating the gel droplets from the
hydrophobic
liquid. When two streams of the materials are simultaneously contacted, the
separation may occur
during the forming step ¨ e.g., by combining the streams over an appropriately
sized sieve or the
like so that the gel droplets are captured. Alternatively, when the stream of
the solution is added to
a hydrophobic liquid in a container, the mixture of hydrophobic liquid and
formed gel droplets may
-31 -
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
be processed through an appropriately sized sieve or the like to capture the
gel droplets. In some
embodiments, a conveyer or similar transport system can be used for collected
gel droplets (or
beads) to be moved out from the hydrophobic liquid tank.
In some embodiments, it can be useful to carry out washing of the gel
droplets, such as with
a soap, to provide substantially cleaned gel droplets. This may be achieved,
for example, by rinsing
with a soap solution, temporarily soaking the gel droplets in a soap solution
bath followed by
rinsing with substantially pure water, or any similar method. This can be
beneficial since residual
hydrophobic liquid on the gel droplets can render the gel droplets
substantially hydrophobic and
reduce final strength and water absorption properties of the gel droplets.
Washing with a soap or
the like can thus provide substantially cleaned gel droplets.
It can further be useful to at least partially coat the substantially cleaned
gel droplets with a
conditioning agent to form conditioned gel droplets. The conditioning agent
can be any material or
combination of materials that are adapted to or configured to substantially
prevent the gel droplets
from adhering to one another. As such, the conditioning agent may function as
a flow aid. Further,
the conditioning agent may be material or materials useful to improve the
adherence of a coating
layer/wall onto the gel droplets. In some embodiments, the conditioning agent
may be a mixture of
an inert powder and an oil For example, talcum powder, powdered starch(es)
(e.g., cornstarch,
tapioca starch, arrowroot starch, rice starch), grain flour(s) (e.g., oat
flour), fumed silica,
precipitated silica, confectionary sugar, calcium silicate, sodium
aluminosilicate, sodium
ferrocyanide, potassium ferrocyanide, calcium ferrocyanide, calcium carbonate,
magnesium
carbonate, cellulose powder, bone phosphate, sodium silicate, silicon dioxide,
magnesium
trisilicate, potassium aluminum silicate, bentonite, aluminum silicate, steric
acid,
polydimethylsiloxane, and the like may be utilized as an inert powder.
Suitable oils can include
silicone oil, mineral oil, dimethicone, and the like.
Addition of the conditioning agent may be particularly useful in relation to
the later addition
of a coating layer on the gel droplet. For example, a clay material in
particular may be formed into
a coating layer, and this can include contacting the conditioned gel droplets
with clay particles or
powdered clay (or other materials as already described herein). When a coating
layer it applied to
the gel droplets, in can be useful to carry out a drying step. For example,
the gel droplets with the
coating layer may be dried at ambient temperature or increased temperature and
alternatively,
forced air drying may be utilized. In some embodiments, the gel droplets with
the coating layer
may be dried at a temperature of about 90 C or greater, about 100 C or
greater, or about 110 C or
greater (e.g., about 90 C to about 150 C, about 100 C to about 140 C, or
about 110 C to about
130 C). Preferably, drying at increased temperature may be carried out after
coating is completed.
Coating may be carried out using a variety of coating units, such as a plate
granulator, drum
-32-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
granulator, or the like where the gel droplets may be substantially uniformly
intermixed with the
coating material.
In one or more embodiments, the present disclosure thus can provide for a
substantially
continuous process for manufacturing hollow core structures. Such process can
include forming
hydrogel beads/droplets, washing the formed hydrogel beads/droplets, and
coating the hydrogel
beads/droplets utilizing a powdered or particulate, solid coating material.
More particularly,
forming the bead/droplets can include contacting a hydrogel solution with a
hydrophobic liquid that
is optionally refrigerated, and this can include delivering the hydrogel
solution from a storage
container through a syringe pump or similar component that can include a
plurality of outlets. The
beads/droplets may form substantially spontaneously in the hydrophobic liquid,
and them may be
removed therefrom via a conveyor system or similar unit to a washing/rinsing
stage. In the
washing/rinsing stage, the beads/droplets may have a residual layer of the
hydrophobic liquid that
may be substantially or completely removed therefrom, such as through contact
with a detergent
solution that may be sprayed or otherwise contacted with the beads/droplets.
The washed/rinsed
beads/droplets optionally may be at least partially dried, such as through
passage through a heater
and/or air dryer. The washed/rinsed beads/droplets that are optionally at
least partially dried may
optionally be pre-conditioned as discussed above. As such, the beads/droplets
may be spray coated
or otherwise contacted with a suitable conditioning material. The
beads/droplets having undergone
washing/rinsing and any further, optional treatments, may then be passed
through a coating unit,
which may consist of one or a plurality of mixing stages wherein the
beads/droplets are contacted
with the powdered or particulate, solid coating material until reaching a
desired coating thickness.
The thus-coated beads/droplets can then be passed through a drying unit for
drying by heat and/or
forced air. The dried beads/droplets may be ready for use or may optionally be
passed through one
or more further mixing units for addition of further coating layers, such as
further conditioning
layers and/or further layers of the coating material (e.g., a bentonite powder
or other coating
material as described herein). Such process may be substantially continuous in
that the
beads/droplets may be continuously formed and transported along a conveying
system or similar,
suitable system, from one processing unit to the next to provide the finished,
hollow core structures.
Products and Articles of Manufacture
The hollow core structures/granules can be utilized in forming a variety of
products Such
products may be defined in relation to a functional aspect thereof and/or in
relation to a physical
property thereof that arises at least in part from the configuration of at
least one component of the
product as a hollow core structure as described herein. The methods of
manufacture described
above make it possible to configure a variety of solid materials (e.g.,
compounds, minerals, and
-33 -
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
mixtures of multiple components) into a hollow core form that can result in
improved properties as
compared to the same material when provided in its dense form (i.e., not
having an internal cavity
or hollow core). For example, provision of a material in a hollow core form as
described herein can
provide for increased uses and improved performance, such as reducing material
weight or bulk
density, improving product solubility, improving absorptive and/or adsorptive
properties,
improving release of a component, improving flowability of solid granules, or
similar properties,
and the like. In relation to mixtures of different materials, a single
component of the mixture may
be provided in the hollow core form and thus impart improved properties to the
overall mixture of
materials. Likewise, a plurality or even all of the components of a mixture
may be provided in the
hollow core form. For example, a mixture may include one or more components
separately
configured as hollow core granules (e.g., a first group of hollow core
granules where a first
component is the wall forming material and a second group of hollow core
granules where a second
component is the wall forming material, and optionally more groups of hollow
core granules, the
groups of hollow core granules being admixed). As another example, a mixture
may include one or
more components combined as hollow core granules (e.g., a group of hollow core
granules where
all of two or more components are used as the wall forming material). As a
further example, a
mixture may include any of the foregoing types of hollow core granules and one
or more
components not in the hollow core granule form
In some embodiments, products provided in the form of a hollow core structure
can exhibit
improved solubility versus the non-hollow core version of the material. The
improved
solubilization can be particularly pronounced when the materials are compared
on a size basis. A
granule prepared as a hollow core structure with an outer wall comprising a
plurality of individual
particles of a given material is significantly larger in size than the
individual particles of the
material that are present in the outer wall of the granule. The larger granule
can be configured to
readily break apart in the presence of a suitable solvent so that the smaller
particles forming the
wall of the granule will individually solubilize. A fully dense particle of
the material present in
substantially the same size as the hollow core granule will dissolve
significantly slower as the
solvent slowly penetrates through the surface. Thus, the granule formed of a
wall of individual
particles of the material will exhibit significantly greater surface area for
interaction with the
solvent. Likewise, the binder utilized in forming the granule can be chosen
for solubility in the
desired solvent_ For example, in relation to solid materials that are intended
for dissolution in
aqueous solvents or polar solvents, hydrophilic binders, such as various PEG
materials, may be
utilized, and the binder will this participate at least in part with rapid
dissolution of the granule in
the solvent. Similarly, in relation to solid materials that are intended for
dissolution in non-polar
solvents, hydrophobic binders, such as waxes or hydrophobic polymers, may be
utilized, and the
-34-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
binder again will participate at least in part with rapid dissolution of the
granule in the solvent. In
some embodiments, a time to substantially complete solubilization of a
specified weight of granules
having a hollow core structure as described herein can be at least 10%, at
least 25%, at least 50%,
or at least 75% faster than a time to substantially complete solubilization of
the same weight of the
same material in a fully dense form (i.e., not in a hollow core form). More
particularly, the hollow
core form of the material may be substantially solubilized at a rate that is
about 10% to about 99%,
about 15% to about 95%, about 20% to about 90%, or about 25% to about 80%
faster than the non-
hollow core form of the same material.
The nature of the hollow core structures described herein as being formed from
a plurality
of particles of one or more materials that are bound in a wall with a binder
material can provide a
variety of options for controlled release compositions. Different materials
will have different
dissolution rates in various solvents and solvent temperatures due to the
chemical and/or physical
nature of the materials. Based upon identified dissolution rates of the
materials, it is possible
according to the present disclosure to provide granules of hollow core
structures wherein the wall
thereof comprises particles of two or more different materials with two or
more different
dissolution rates. For example, as further discussed herein, the present
hollow core structures may
be utilized in fertilizer products. Various chemicals and compounds useful as
fertilizers can exhibit
different dissolution rate or release rates. In particular, there are
variously know "fast release"
fertilizers and "slow release- fertilizers Where it is desirable to provide
combinations of fertilizers
with different release rates, particles of a fast release fertilizer and
particles of a slow release
fertilizer can be combined in desired ratios and used as the wall forming
component for preparing
granules of hollow core structures as described above. Thus, the resulting
fertilizer granules will
have a wall surrounding a hollow core wherein the wall comprises the fast
release fertilizer
particles and the slow release fertilizer particles in the designed ratio.
Upon application to a site in
need of fertilization, the fast release fertilizer particles will provide
immediate fertilization, and the
slow release fertilizer particles will remain for the expected time for slow
release thereof. The
same principle can apply to any number of solid materials with differing
dissolution and/or release
rates so that many types of controlled release granules can be prepared.
Similarly, controlled release may be achieved by using two or more different
forms of the
same solid material. For example, a desired material may be provided as
particles in two or more
different forms that thus exhibit two or more different dissolution or release
rates The different
release rates may relate to particle size, particle purity, presence of an
encapsulating layer, or other
recognized manners for affecting dissolution or release rate. For example,
first particles of a first
size may have a first dissolution or release rate with second particles of a
second, different size may
have a second, different dissolution or release rate. As a further example, a
first set of particles that
-35 -
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
are substantially pure (i.e., formed entirely of a single material or having
only a minor amount of
impurities) may exhibit a first dissolution or release rate, and a second set
of particles may include
an additive (e.g., an inert material or a different, desired material that has
a different dissolution or
release rate) in an amount that causes the second set of particles to have a
dissolution or release rate
that differs from the first set of particles. As yet a further example, a
first set of particles may be
provided in an uncoated state, and a second set of particles of the same
material may be provided
with a coating or in an encapsulated form so that the coated or encapsulated
particles exhibit a
delayed release relative to the uncoated or non-encapsulated particles. These
or similar situations
can be applied to two, three, four, or even more sets of particles that then
can be mixed in desired
ratios and used as the wall forming material to prepare granules wherein the
wall surrounding the
hollow core comprises two, three, four, or even more sets of particles with
two, three, four, or even
more, different dissolution or release rates For example, in a laundry care
application, it may be
desirable to provide for immediate release of detergent materials in the wash
liquor but have a
delayed release of bleaching materials, brighteners, or the like. In such
cases, the laundry
components for immediate release may be provided in an unmodified form, and
the laundry
components for delated release may be provided in an encapsulated or coated
form, and the
different materials may then be mixed and used as the wall forming material to
prepare granules of
the laundry cleaning composition that, when added to the wash liquor, will
immediately release the
detergent components therefrom while delaying release of the further
components (i.e., the coated
or encapsulated components).
In some embodiments, the provision of materials in a hollow core form as
described herein
can be particularly beneficial in providing for reduced product weight without
limiting product
performance. For example, solid, particulate products that are often sold in
large quantities may
exhibit an undesirably high weight that can be troublesome for consumers to
carry and manipulate.
By providing such products in a hollow core form, the total weight can be
reduced while still
providing the product in a volume effective to achieve the desired end result
and thus avoid an
effective increase in cost to the consumer in order to achieve the same
result. In other words, the
effective volume of the product can still provide substantially the same end
result at approximately
the same product cost but with a reduced product weight.
In example embodiments, such desired reduction in overall weight can
particularly applied
in the field of animal litters, which are often formed at least in part from
dense products, such as
clays. Clays are often used in animal litters because they are a relatively
inexpensive and effective
liquid absorbing material. Clays, however, are relatively dense and cause
animal litter products to
be quite heavy with commercially sold quantities requiring as much as 30 to 40
pounds of a clay-
based litter to fill a large-size litter tray. The ability to provide hollow
core structures as described
-36-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
herein thus can be particularly useful in forming an animal litter having a
significantly lower weight
and even improved absorption properties. This can extend to clay-based hollow
core structures as
well as non-clay hollow-core structures.
A reduction in weight or mass of a given material by providing the material in
a hollow core
form as described herein can vary based upon the density of the substantially
pure product. Higher
density materials will exhibit a greater reduction in product mass or weight
when provided in a
hollow core form relative to materials with lesser densities. In some
embodiments, a specified
volume of a material provided in a hollow core form according to the present
disclosure can have a
mass or weight that is at least 5%, at least 10%, at least 15%, or at least
20% less than the mass or
weight of the same volume of the material when provided in its native or
typical non-hollow core
form. In certain embodiments, the hollow core version can have a mass or
weight that is about 5%
to about 60%, about 7% to about 40%, or about 10% to about 35% less than the
mass or weight of
the same volume of the non-hollow core version of the product.
In some embodiments, granules formed as hollow core structures can exhibit
improved
ability to absorb and or adsorb gases and liquids As such, materials that are
previously known to
exhibit good absorption and/or adsorption properties in their typical, dense
form can have such
properties improved by configuring particles of the material as a wall around
a hollow core
Likewise, materials that do not necessarily exhibit absorption and/or
adsorption properties in their
typical, dense form can be utilized for such purposes when particles of the
material are configured
as a wall around a hollow core. While not wishing to be bound by theory, it is
believed that
improvements in absorption and/or adsorption properties can arise at least in
part due to the
increased porosity achieved through binding together a significantly large
number of smaller
particles of the material into a wall surrounding a hollow core. Likewise, the
combination of a
large number of small particles in a shell structure can significantly
increase available surface area
to absorption and/or adsorption purposes. Still further, the addition of a
binder in the shell structure
can likewise provide absorption and/or adsorption properties that are additive
with such properties
existing in the particles of the solid, wall forming material itself. Such
properties can extend to
uses in odor absorption (i.e., uptake of odor causing chemicals that may exist
in a substantially
gaseous state) as well as liquid absorption (e.g., spill clean-up).
Improved absorption and/or adsorption particularly can indicate that the same
volume or
weight of a gas or liquid may be absorbed by a lesser weight of the hollow
core granules relative to
the same material in its native or typical, fully dense form (i.e., not in a
hollow core form). For
example, the present hollow core structures may provide for at least 10%, at
least 25%, at least
50%, or at least 75% greater absorbance of a gas and/or a liquid (based on the
volume of a gas or
based upon either of the volume or the mass of a liquid) compared to the same
weight of the
-37-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
material when not in the hollow core form as presently described. More
particularly, such
improvement may be in the range of about 10% to about 95%, about 15% to about
90%, about 20%
to about 85%, or about 25% to about 75%.
In example embodiments, hollow core granules with improved absorption and/or
adsorption
of gases and that are configured to be functional as a deodorizer (i.e.,
configured to absorb, adsorb,
or otherwise trap, bind, and/or neutralize odor causing compounds) can be
prepared with a variety
of wall forming materials, a variety of binders, and can include optional odor
neutralizing agents.
For instance, various clays (e.g., bentonite), salts (e.g., sodium
bicarbonate), carbon materials (e.g.,
activated carbon), and high porosity materials (e.g., zeolites) can be
effective at capturing odor
causing compounds, and any one or more of such materials, or other materials
exhibiting similar
efficacy, may be used as a wall forming material for the hollow core granules.
Suitable binders can
include materials such as PEGs of various molecular weights (e.g., PEG 8000,
PEG 12000, and/or
PEG 35000), saturated fatty acids (e.g., stearic acid), and polyoxyethylene
fatty ethers (e.g., Brij TM
S100). Odor neutralizing agents may be provided as solid that may be included
as a wall forming
material, may be liquids that are combined with solid, wall forming materials,
may be liquids that
are blended with the binder, or may be included in the hollow core granules in
any other suitable
manner. One example of a suitable odor neutralizing agent is lauryl
methacrylate. Odor masking
agents may likewise be utilized and can encompass fragrances and the like that
can deliver a
desired odor in an amount sufficient to mask an undesired odor.
The improved ability of a hollow core granule as described herein to mitigate
malodor by
absorbing, adsorbing, or other binding odor-causing chemicals or compounds is
illustrated in
Example 12 herein. Specifically, it has been shown that when a material
effective as an odor
controlling agent is used as a wall forming material in a hollow core granule
according to the
present disclosure, the hollow core form of the material will exhibit improved
functionality versus
the same material in its native state. For example, a hollow core form of an
odor reducing agent
can show improved malodor reduction versus the native form of the odor
reducing agent in that a
detectable concentration of an odor causing chemical or compound can be at
least 10% less, at least
25% less, at least 50% less, at least 75% less, or at least 90% less after a
defined time of contact of
the odor causing chemical or compound with the odor reducing agent. Testing in
Example 12
showed a continuing ability for the hollow core form of the odor reducing
agent to provide
improved malodor reduction that increased with time As such, the relevant time
period for the
above ranges may be as little as 1 hour or even as long as 100 hours.
In further example embodiments, hollow core granules with improved absorption
and/or
adsorption of liquids and that accordingly can be configured to be functional
for spill cleanup or
similar uses can be prepared with a variety of wall forming materials, a
variety of binders, and can
-38-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
include optional additives for achieving defined purposes, such as degradation
of organics. For
instance, various clays (e.g., bentonite), carbon materials (e.g., activated
carbon), and high porosity
materials (e.g., zeolites) can be effective for absorbing aqueous and/or non-
aqueous liquids at
various locations (terrestrial and/or water), and any one or more of such
materials, or other
materials exhibiting similar efficacy, may be used as a wall forming material
for the hollow core
granules. Suitable binders can be chosen based upon the desired use. For
example, in certain
embodiments, binders can be chosen specifically to prepare granules for
terrestrial use in
absorption of liquids, including hydrocarbons, such as various oils. Suitable
binders for such
purposes can include hydrophilic materials such as PEGs of various molecular
weights (e.g., PEG
8000, PEG 12000, and/or PEG 35000), saturated fatty acids (e.g., stearic
acid), and
polyoxyethylene fatty ethers (e.g., BrijTM S 100). Clays, such as bentonite,
may be particularly
useful as a wall forming material for such uses. In other, certain
embodiments, binders can be
chosen specifically to prepare granules for aquatic uses, such as cleanup of
oil spills and the like in
marine settings. Suitable binders for such purposes can include hydrophobic
materials such as
waxes, paraffins, polycaprolactone, ethylene-vinyl acetate copolymers,
polypropylene carbonate,
poly(tetramethylene oxide) poly(ethylene adipate), poly (trans-butadiene), and
thermoplastic
polyurethane (e.g., Carbothane TPU). Again, various clays may be particularly
useful as an
absorbent, wall forming material in such uses. Biological agents and similar
materials effective for
decomposition of hydrocarbons or effective for otherwise modifying spilled
liquids to improve ease
of cleanup may be included with the hollow core granules. Such components
provided as solid that
may be includes as a wall forming material, may be liquids that are combined
with solid, wall
forming materials, may be liquids that are blended with the binder, or may be
included in the
hollow core granules in any other suitable manner.
In some embodiments, the ability to provide a given material in the presently
described
hollow core form can be beneficial in relation to processing and use of the
material. For example,
many solid materials that are typically sold in a particulate form can exhibit
significant dusting
during handling due the presence of fines (i.e., a quantity of the material
that is significantly smaller
in size than the average size of the remaining quantity of the material).
Fines may be inherently
present in a mass of certain materials due to the manufacturing process, due
to unavoidable
crushing of the particles during storage and/or handling, or due to other
reasons. Reduced dusting
can be achieved according to the present disclosure since the individual
particles forming the
granules of the hollow core structures are retained in the shell/wall of the
individual granules due to
the presence of the binder. Since fine particles are bound or adhered together
in the wall of the
granules and/or in one or more layers of the wall, such fine particles are
less likely to become
airborne during movement of the particles. As such, the provision of
compositions in a hollow core
-39-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
structure where individual particles of a solid material are combined with a
binder to form one or
more walls or shells surrounding a hollow core, the amount of dust associated
with a given mass of
the material can be significantly reduced.
In addition to reducing dusting, the structure of the hollow core granules
(i.e., having a wall
of particles and binder surrounding a hollow core) can also lead to
improvements in flowability
and/or pourability of the material. Because the individual granules of the
hollow core structures are
formed through agglomeration around a binder particle, the individual granules
can exhibit a
substantial degree of uniformity in one or both of size and shape. This can
lead to an improved
appearance relative to particles of the same material in its typical, dense
form that may have a
significantly wide range of particle sizes and/or shapes. On the other hand,
the present hollow core
structures can be provided with a substantial uniformity of size so that the
average particle size may
vary, for example, by less than 20%, less than 15%, less than 10%, less than 5
A, or less than 2%
relative to the median particle size. Such uniformity can improve the manner
in which the
individual granules interact with one another during movement so that hollow
core structures flow
more easily along and around one another.
In some embodiments, hollow core granules according to the present disclosure
can be
configured to provide pH modification. As such, the hollow core granules can
be configured for
addition to a substantially acidic material or site (e.g., having a pH of less
than 7, less than 6, less
than 5, less than 4, or less than 3) in order to make the material or site
less acidic, substantially
neutral (e.g., in the range of about 6 to about 8 or about 6.5 to about 7.5),
or basic (e.g., pH greater
than 7, greater than 8, greater than 9, greater than 10, greater than 11, or
greater than 12).
Alternatively, the hollow core granules can be configured for addition to a
substantially neutral
material or site in order to make the material or site substantially acidic as
defined above or
substantially basic as defined above. Alternatively, the hollow core granules
can be configured for
addition to a substantially basic material or site as described above in order
to make the material or
site less basic, substantially neutral as defined above, or substantially
acidic as defined above.
Configuration for pH modification can be achieved by utilizing an acidic
component as the wall
forming material, using a basic component as the wall forming material,
utilizing a buffer as wall
forming material, or using some combination of acidic components, basic
components and buffers
as the wall forming material. Acidic components can include organic acids,
such as oxalic acid,
tartaric acid, citric acid, maleic acid, etc that are typically available in
solid form Various salts
likewise may be utilized in relation to the ability to release ions upon
solubilization that can be
effective at lowering pH of the surrounding environment. Basic components can
include materials
such as oxides of various metals, as well as various salts that release ions
upon solubilization that
can be effective to increase pH of the surrounding environment, such as
various carbonates,
-40-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
hydroxides, and the like. Buffers, for example, can be prepared with mixtures
of salts of similar
materials that will release ions in solution at an appropriate level to
maintain a substantially
consistent pH in a local environment. Once provided as hollow core granules,
the pH modifying
hollow core granules can be added to liquids, for example, and achieve rapid
solubilization to
modify pH in a manner as noted above.
In light of the foregoing, it can be seen that the present disclosure can
encompass a wide
variety of products that can exhibit very useful properties, including
improvements relative to
typical forms of the same material(s) when not in a hollow core form as
described herein. This can
extend to a number of chemicals and compounds that are typically useful in
various products in
their salt form. Many salts are made or found in nature in a solid form at
generally ambient
conditions and, as such, a wide variety of salts may be utilized as a wall
forming material in hollow
core granules according to the present disclosure. Salts that can be provided
in the form of hollow
core granules according to the present disclosure can be organic or inorganic.
In some
embodiments, salts suitable for preparation as hollow core granules can
include those with cationic
groups such as aluminum, ammonium, bismuth, calcium, chromium, copper,
germanium, iron,
lithium, magnesium, manganese, nickel, palladium, platinum, potassium, silver,
sodium, sulfur, tin,
titanium, tungsten, vanadium, zinc, and zirconium In further embodiments,
salts suitable for
preparation as hollow core granules can include those with anionic groups,
such as acetates,
aluminates, ammonium sulfates, benzoates, borides, bicarbonates, bromates,
bromides, carbides,
carbonates, chlorides, chromates, ferrites, fluorides, hydrides, hydroxides,
iodates, iodides, lactates,
manganates, nitrates, nitrides, oxalates, oxides, perchlorates, phosphates,
phosphides, silicates,
silicides, stearates, sulfates, sulfides, titanates, tungstates, vanadates,
and zirconates. Non-limiting
examples of specific salts that can be utilized in hollow core granules
include calcium carbonate,
sodium chloride, sodium carbonate, sodium bicarbonate, sodium percarbonate,
sodium sulfate,
sodium carbonate peroxide, potassium chloride, magnesium carbonate, magnesium
sulfate, and the
like.
The ability of compounds, such as salts, to exhibit surprisingly improved
properties when in
the form of a hollow core structure can be shown in relation to the example
embodiment of sodium
bicarbonate (NaHCO3) or baking soda. Sodium bicarbonate is known to have a
wide variety of
uses, one example of which is use as a deodorizer in light of the ability of
the material to absorb
odor causing compounds, such as sulfurous compounds As further described
herein, particles of
sodium bicarbonate can be combined with binders, such as PEG, paraffin, or
other binders, to form
granules wherein a hollow core is surrounded by one or more walls/shells
comprising the sodium
bicarbonate and the particular binder. The resulting hollow core sodium
bicarbonate granules can
provide improved odor-absorbing properties over known forms of sodium
bicarbonate where the
-41 -
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
material is provided simply as a powder or in a solid mass of larger size.
Hollow core sodium
bicarbonate granules thus can be particularly useful as deodorizing agents for
use in a variety of
environments, including refrigerators, trash cans, garbage cans, pet litter
boxes, and the like. This is
shown in Example 12 below where sodium bicarbonate in the hollow core form was
shown to
exhibit improved malodor reduction in relation to materials such as ammonia
and sulfur.
Hollow core sodium bicarbonate is thus an example embodiment of a
substantially pure
compound that can be upgraded for improved use through modification so that
the substantially
pure compound is combined with a binder to form hollow core granules. As such,
a hollow core
sodium bicarbonate granule would differ from the typical form of sodium
bicarbonate in that the
granules comprise particles of the sodium bicarbonate in a shell/wall with a
binder so that the
shell/wall surrounds a hollow core. The binder may be substantially inert in
relation to the desired
use of the sodium bicarbonate, however, in some embodiments, binders may be
chosen to
compliment the intended use and thus provide an additive effect to the sodium
bicarbonate itself.
The hollow core sodium bicarbonate would also differ from the typical form of
sodium bicarbonate
in relation to the improved properties as already discussed above ¨ e.g.,
improved absorption and/or
adsorption, improved solubilization, reduced weight, and other properties.
As seen from the example embodiment where sodium bicarbonate is used as the
wall
forming material for the hollow core structures, it is possible according to
the present disclosure to
structure the wall forming material into a higher order format so that
usefulness and efficacy of the
wall forming material can be improved as a standalone product. Such
improvements, however, are
not limited to sodium bicarbonate, and other wall forming materials as
described herein can
likewise benefit by the restructuring the material from its native format
(i.e., the typical, fully dense
form of the solid material) to the walled format where particles of the
material are positioned in a
wall with a binder surrounding a hollow core. Likewise, such improvements are
not limited to uses
as standalone products. Rather, individual materials, such as sodium
bicarbonate, that have been
upgraded into granules with the hollow core format can be used as components
of various mixtures
and compositions defining other types of products
For instance, using the example of hollow core sodium bicarbonate granules,
such upgraded form
of the material can be utilized as an ingredient is a number of useful
products. Presently, sodium
bicarbonate in its typical, fully dense form, finds use in other formulations,
such as laundry
detergents, dish detergents, carpet cleaners/deodorizers, animal litters, and
personal care products,
such as deodorants/antiperspirants, and dental care items (e.g., toothpastes).
Any one or more of
such products thus may be modified and improved by replacement of sodium
bicarbonate in its
typical form with hollow core sodium bicarbonate granules according to the
present disclosure.
The so-modified composition then can exhibit improvements arising at least
from the improved
-42-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
functional aspects of the hollow core sodium bicarbonate granules. Of course,
it is understood that
sodium bicarbonate is utilized as an example embodiment, and the ability to
provide improved
products is not limited to the use of hollow core sodium bicarbonate granules,
and such
improvements may be achieved through upgrading of chemicals, compounds, and
complex
mixtures and compositions that may or may not include sodium bicarbonate as a
component
thereof.
Because a wide variety of materials may be used as the wall forming material,
the present
hollow core granules can be configured as a wide variety of products. Non-
limiting examples of
products that may include, in part or in whole, the hollow core granules of
the present disclosure
include cleaning compositions (e.g., laundry detergents, dish detergents,
fabric cleaners, fabric
deodorizers, abrasive cleaners, teeth cleaning compositions, disinfectants,
etc.), cleaning
composition additives (e g , stain removers, whiteners, brighteners, bleaches,
scent boosters, etc.),
absorbents, adsorbents, deodorizers, odor control products, odor masking
products, fertilizers,
pesticides, animal litters, animal litter additives, and other consumer
products and/or industrial
products. Any of the foregoing may be a functional material as referenced
above and may also be
referenced as additives in that they can be added to other products to impart
the desired function
and/or may be provided as a stand-alone product that can be combined as needed
with other
products to achieve an additive result.
In one or more embodiments, products suitable for provision as hollow core
granules can
include one or more chemicals, compounds, or mixtures of materials that are
effective as
detergents/cleaners and/or as additives useful for combination with
detergents/cleaners. Many
cleaning products are provided in solid form, typically as powders or other
particulate forms.
Common examples of such compositions include fabric care items (e.g., laundry
detergents for use
in washing machines, upholstery cleaners, brighteners, whiteners, stain
removers, scent boosters,
and the like) and dishwashing detergents. According to the present disclosure,
pre-existing
cleaning compositions may be re-engineered into an upgraded format wherein one
or more
individual components of the mixture may be present in a hollow-core form. For
example, sodium
bicarbonate in such formulations may be replaced with hollow core granules of
sodium
bicarbonate. Other discrete components of the cleaning composition may
alternatively or
additionally be replaced with a hollow core version of the component. In other
embodiments, the
total powdered product may be modified so that the overall composition is in
the form of hollow
core granules. Powdered cleaning compositions may be a mixture of components
that are blended
into a substantially uniform powder or other particulate form. Rather than
being utilized in the
powdered form, the total mixture may be utilized as the wall forming material
and mixed with a
suitable binder so that the individual particles of the total cleaning
composition are agglomerated
-43 -
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
with the binder in one or more wall of the formed granules with the hollow
core format.
Alternatively, the granules with the hollow core format may be prepared to
have two or more
walls/shells. In such embodiments, a first portion of a cleaning composition
may be present as a
first, inner shell or wall, and a second (or more) portion of the cleaning
composition may be present
in a second (or more) wall or shell external to the inner shell. More
particularly, one or more
components of a cleaning composition may be present as a first, inner shell or
wall, and a second
(or more) component(s) of the cleaning composition may be present in a second
(or more) wall or
shell external to the inner shell. In this manner, a timed release of
individual components of the
cleaner may be provided. For example, one or more outer shells in a
dishwashing composition may
provide a detergency function, and one or more inner shells of the composition
may provide an
enzymatic or different function that is more desirable in a later portion of
the dishwashing cycle. In
this manner, a single composition can be provided with timed release of
different components of
the composition. Similar effects can be achieved through layering in other
compositions, such as
laundry cleaning compositions. In addition to providing timed release, the
provision of the
compositions in the hollow core format can provide further benefits. For
instance, re-engineering
of powdered laundry detergents and similar formulations may be desirable, for
example, to reduce
overall product weight, improve solubility (and thus reduce the likelihood of
detergent residue on
cleaned articles), and the like.
A cleaning composition according to the present disclosure may comprise
substantially only
hollow core granules according to the present disclosure. The hollow core
granules may include
one or more chemicals, compounds, or the like having one or more cleaning
application(s) as the
wall forming material, and the wall forming material optionally may also
include one or more
carriers, fillers, inert materials, or the like that do not necessarily
provide a cleaning function. The
cleaning composition comprising substantially only hollow core granules may
thus be configured
as a substantially complete formulation for a designed use (e.g., a laundry
detergent, a dishwashing
detergent, etc.), or the cleaning composition comprising substantially only
hollow core granules
may be configured as an additive (e.g., a bleach, brightener, whitener, stain
remover, deodorizer,
etc.) that can be added to another composition for a desired end use. A
cleaning composition
according to the present disclosure may comprise hollow core granules in
combination with non-
hollow core components. For example, a cleaning composition may be provided as
a mixture of
components, and one or more of the components may be provided in the hollow
core form while
one or more of the remaining components may be provided in a non-hollow core
form.
As is evident from the foregoing, a cleaning composition according to the
present disclosure
may be combination of materials defining the total cleaning composition or may
be a more
specialized product that is provided as an additive for cleaning products. Non-
limiting examples
-44-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
thereof include additives, such as brighteners, non-bleach whiteners
(including oxidizing materials),
scent-boosters, enzymes, deodorizers, stain removers, and other materials that
are useful in cleaning
products. Moreover, as already noted previously, the ability to provide the
compositions in the
hollow core format can extend to liquid or semi-solid components as well. In
particular, one or
more liquid or semi-solid components may be absorbed, adsorbed, or embedded in
or on a solid
component of the cleaning composition or on an inert carrier that may
harmlessly dissolve in the
washing liquor and be removed.
In some embodiments, a cleaning product or composition according to the
present
disclosure may be a fabric cleaner or fabric cleaning composition. A fabric
cleaner can be any
product that is configured at least for use with textiles or fabrics, such as
clothing, upholstery,
carpets, rugs, bedding (e.g., sheets, blankets, duvets, bedspreads, quilts,
mattresses, etc.), tapestries,
and the like_
A fabric cleaner specifically may be a laundry detergent. Such compositions
are known to
include a number of components, including polymers, surfactants, builders,
deodorizers, enzymes,
oxidizers, bleaching components, salts, fragrance, and the like. Salts such as
sodium sulfate,
sodium carbonate, sodium bicarbonate, sodium chloride, potassium chloride, and
the like
particularly may be included in laundry detergents. Example embodiments of
suitable polymers
include polyethylene glycol (PEG) polymers of various molecular weights.
Example embodiments
of suitable surfactants can include anionics, nonionics, zwitterionics,
ampholytics, cationics, and
combinations thereof. One example of a laundry detergent includes C12-15
ethoxylated alcohols,
sodium laureth sulfate, sodium sulfate, sodium carbonate, sodium bicarbonate,
disodium
distyrylbiphenyl disulfonate, modified acrylic copolymer, protease
enzyme/amylase enzyme,
sodium carbonate peroxide, potassium chloride, and fragrance. Such composition
may be provided
in a solid (e.g., powdered) format, and the solid detergent particles can be
used as the wall forming
material to provide the laundry detergent as hollow core granules. In some
embodiments, a
product according to the present disclosure can be a laundry detergent
prepared by a method as
described herein such that the laundry detergent comprises a mixture of hollow
core granules and
one or more further components that are effective in a laundry detergent
composition. In further
embodiments, a product according to the present disclosure can be a laundry
detergent prepared by
a method as described herein such that the laundry detergent comprises hollow
core granules
prepared such that a plurality of individual particles of at least one wall
forming material comprise
particles of a laundry detergent composition.
A fabric cleaner may also be provided in a more specialized form to provide a
designed
effect. Various functional formulations are possible to design products that
can be used as
additives in fabric cleaning, and particularly in laundry care. Example
embodiments of such
-45 -
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
additive formulations include scent boosters, stain removers, brighteners,
whiteners, bleaches, and
the like. One example of a scent booster includes a sodium chloride builder,
fragrance, a sodium
bicarbonate builder, a hydrated silica process aid, a sorbitan oleate
surfactant, and colorant. Such
composition may be provided in a solid (e.g., powdered) format, and the solid
particles can be used
as the wall forming material to provide the scent booster formulation as
hollow core granules. One
example of a stain remover includes a sodium carbonate, sodium percarbonate,
C12-15 linear
alcohol ethoxylate, fragrance, and blue salt. Such composition may be provided
in a solid (e.g.,
powdered) format, and the solid particles can be used as the wall forming
material to provide the
stain remover formulation as hollow core granules. Other additive formulations
for fabric care may
likewise be formulated for providing the product as hollow core granules.
Dishwashing detergents likewise can be formulated wherein powdered
compositions may
be provided in the form of hollow core particles. Any known, solid dishwashing
detergent may be
so formulated. Further, individual components of a dishwashing detergent may
be individually
formulated as hollow core granules that may be provided as additive or that
may be otherwise
admixed with other components of a dishwashing detergent that are not in a
hollow core format.
Other types of household cleaners may also be subject to such re-engineering.
For example,
in the field of fabric care, carpet cleaners or other upholstery cleaners are
often provided in a
powdered form, and such compositions can be improved by re-engineering into a
hollow core
format as described herein. For example, sodium bicarbonate may be used in
carpet cleaners to
remove odors as well as provide a cleaning effect, and the provision of sodium
bicarbonate as the
wall forming material of hollow core granules can be effective to improve
activity in the end use
because of the improved absorption and/or adsorption provided through such
format. Other
components of such cleaners may additionally or alternatively be included in
the product in the
hollow core format. Likewise, an entire carpet or upholstery cleaning
composition may be
provided as hollow core granules that can be applied to the material to be
cleaned. The applied
granules may be vacuumed or otherwise removed at the appropriate time or, in
some embodiments,
the hollow core granules may be acted upon through outside force (such as foot
traffic or use of
machinery) to effect breakdown of the hollow core granules into a finer,
powered form. Such
mechanical action can be effective to improve the cleaning effect, improve
odor removal, or the
like prior to removal of the composition, such as through vacuuming of carpet
or the like.
In some embodiments, the hollow core granules may be particularly configured
for
degradation upon application of an external force. The external force may be a
rubbing, wiping,
scrubbing, or other physical pressure typically applied during cleaning of a
surface. More
specifically, during the application of the external force, the hollow core
granules can be configured
to break into a plurality of parts comprising individual groups of the
particles of the wall forming
-46-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
material. In other words, the overall granule will break into a plurality of
subunits having a size
that is less than the size of the original granule but is greater than the
size of individual particles of
the wall forming material since a plurality of the particles will still be
agglomerated in each of the
plurality of subunits. It is possible, however, that during application of the
force, individual
particles may be freed along with the plurality of subunits. During further
application or continued
application of the force, the plurality of subunits can further degrade into
even smaller subunits
and/or into the individual particles of the wall forming material.
Similar to cleaning products as discussed above, usefulness of hollow core
structures can
particularly extend to abrasive-type cleaners. An abrasive-type cleaner as
used herein is intended to
mean a cleaner wherein cleaning is achieved at least in part through
mechanical action of the solid
particles physically removing deposits from a surface through a scrubbing
action. Such cleaners
may also achieve cleaning through detergency in addition to the mechanical
scrubbing of the
particles along the surface to be cleaned. As discussed herein, the granules
of the hollow core
structures have at least one wall formed of smaller particles of the wall
forming material. When the
wall forming material is effective as an abrasive-type cleaner, the hollow
core granules formed
therefrom can exist as relatively large particles that can provide a "rough"
abrasive surface, and the
mechanical scrubbing action can cause the hollow core granules to gradually
degrade into finer
particles. The result is similar to sanding of a surface wherein a low grit,
rough surface is initially
used for bulk removal of material from the surface, and higher grit surfaces
are used thereafter for
smoothing. The granules of the hollow core structure can similarly function as
a low grit, rough
abrasive for bulk removal of residues and buildup and, as the granules degrade
into the finer, wall
forming particles, such particles function as a higher grit abrasive to
provide a finer, cleaning effect
for removal of smaller traces of the residues and buildup. Further, the binder
material can be
chosen to control how easily the hollow core granules fracture, to control how
quickly the hollow
core granules will dissolve in a solvent, and also to provide additive
cleansing effects. In addition,
the hollow core format can impart haptic feedback to the user as efficacy of
the abrasive cleaning.
The larger hollow core granules will impart vibrations that are noticeably
different from the haptic
feel of the finer, wall forming particles. Likewise, since the hollow core
granules can be configured
to fracture under stress, such as application of pressure when cleaning, the
breaking of the granules
into the finer, wall forming particles will also provide haptic sensations of
how the cleaning action
is progressing Thus, the hollow core granules can be configured to break down
into successfully
smaller sized particles to provide a layered scouring efficacy due to
differences in cleaning ability
provided by the different sizes of the wall forming particles, the intact
hollow core granules, and
the intermediate sized sections of the granule wall as it breaks apart.
-47-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
Similar to abrasive cleaners as noted above, hollow core granules of the
present disclosure
likewise may be used as polishing agents. In particular, one or both of the
wall forming material
and the binder can be chosen to provide polishing attributes. Likewise, the
particles of the wall
forming material may be chosen in size to provide a desired level of
abrasiveness needed to achieve
the polishing effect without unduly scratching or marring the material being
polished. Otherwise,
hollow core polishing granules may be functionally similar to the abrasive
cleaning granules
described above
In some embodiments, the presently disclosed hollow core granules can be
utilized in
personal care items. A particular example is in the area of
deodorants/antiperspirants. A further
example can be exfoliating products where the hollow core granules can provide
a relatively rough
level of exfoliation in the initial, larger granule size and provide
continually smoother levels of
exfoliation as the hollow Care granules break down into the individual wall
forming particles that
are significantly smaller in size. The binder material in such applications
can be customized to
provide additional skin cleansing effects and/or to provide a lubricating
effect to the skin as the
granules break apart and/or as the granules are solubilized in water.
Dental care products are further examples of products that can exhibit
improvements
through utilization of hollow core granules More particularly, hollow core
granules as described
herein may be utilized in forming toothpaste compositions. One or more of the
individual
components of the toothpaste composition may be provided as hollow core
granules that are
incorporated into the overall paste, gel, or similar composition use for teeth
cleaning. For example,
sodium bicarbonate is a common ingredient in toothpaste compositions, and the
sodium bicarbonate
may be present in the composition as hollow core granules. Likewise, since
many teeth cleaning
compositions utilize at least mildly abrasive particles, such particles may be
incorporated into
hollow core granules as at least one of the wall forming materials. Further,
the binder material may
also be chosen to improve the activity of the wall forming material and/or to
improve solubilization
of the wall forming material for rapid deployment during brushing.
Alternatively, the overall teeth
cleaning composition may be re-engineered as a hollow core structure that then
can be combined
with a substrate or carrier material to from a paste, gel, or the like.
The use of hollow core granules can also provide for new teeth cleaning
formulations. For
example, rather than incorporating hollow core granules into a teeth cleaning
gel or paste, the
hollow core granules may comprise substantially the entirety of the teeth
cleaning composition. In
an example embodiment, a complete or substantially complete teeth cleaning
composition can be
utilized as the wall forming material so that the formed, hollow core granules
are effective as
"toothpaste bits" that can be poured into the mouth for teeth cleaning.
Similarly, a plurality of
hollow core teeth cleaning granules may be combined into a tablet form or
similar so that a single
-48-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
"tablet" may be inserted into the mouth for teeth cleaning. More particularly,
the toothpaste bits or
toothpaste tablet, once inserted into the mouth, may be chewed so that the
abrasive particles remove
debris and other materials from the teeth and/or gums of the user. Again, the
choice of binder
material may be effective to cause the teeth cleaning granules to break apart
easier or to persist
longer so that effective use time may be customized. Further, the binder may
be effective in
providing teeth cleaning properties so that the binder is at least partially
effective for removing
debris or other materials from the teeth and/or gums. As with other abrasive
cleaning hollow core
granules, the teeth cleaning hollow core granules can provide varying levels
of cleaning efficacy as
the hollow core structures break apart into successfully finer sized
particles.
Cleaners, detergents, and similar products may be prepared as substantially
"simple"
products with only a few ingredients, and one or more of the relatively few
components used in
such components may be present in a hollow core format, or substantially the
entire composition
defining the product may be present in the hollow core format. Others of such
types of products
may be relatively complex in relation to include a larger number of
components. Again, any one or
more of the components may be in the hollow core form or substantially the
entire composition
may be in the hollow core form. In some types of composition, however, it may
be more typical
for only primary components thereof to be in the hollow core form. As such,
only primary
components may be discussed herein in relation to being in a hollow core form.
It is understood,
however, that many consumer products may include a wide variety of classes of
materials, and any
further components that may be utilized in any product or article of
manufacture encompassed by
the present disclosure, including animal litters, laundry products,
dishwashing products, personal
care items, and the like, may be included in such products or articles of
manufacture in the hollow
core form as described herein. It is thus expressly intended that any of the
following additives may
be used in any product or article of manufacture wherein which components are
typically
understood to be used: fillers, binders, preservatives, fragrances, salts
(e.g., carbonates,
bicarbonates, chlorides, etc.), optical agents (e.g., brighteners and/or
whiteners), disinfectants,
enzymes, antimicrobials, oxidizers, deodorants, pH adjusters, dyes, coloring
agents, and the like.
In some embodiments, hollow core granules as described herein can be useful in
forming
nutritional supplements for oral ingestion. This can provide for a wide
variety of forms of the
nutritional supplements to provide for improved properties whether the
articles are configured to be
chewable or are configured for swallowing whole In relation to the latter
format, many nutritional
supplements suffer from poor release of the vitamin(s), mineral(s), fiber,
probiotics, enzymes,
amino acids, proteins, or other supplemental agent(s) typically found in
various nutritional
supplements. This often arises from poor solubility of the overall pill or
tablet form. As discussed
above, however, hollow core structures according to the present disclosure can
exhibit improved
-49-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
solubility because of the ability of the wall to rapidly break apart into the
significantly smaller
particles used as the wall forming material and because of the ability to
customize the binder to the
environment where dissolution will be carried out so that the binder itself
will readily solubilize in
a contacting solvent. Because of the much higher surface area afforded by the
small, wall forming
particles, the rapid break down of the larger hollow core granules into the
individual wall forming
particles can provide for rapid release and quick uptake of the nutritional
supplement(s) in the
digestive system of a user. Moreover, the hollow core format can enable
combination of various
components for timed release. As discussed otherwise herein, coatings,
encapsulation, and other
methods can be employed to provide a quantity of the individual particles of
one or more of the
wall forming materials in a delayed release or sustained release form. Thus,
when the nutritional
supplement hollow core granules are ingested, at least a portion of the
nutritional supplement used
as the wall forming particles may provide for substantially immediate release
(if desired), and at
least a portion of the nutritional supplement used as the wall forming
particles may provide for
delayed and/or sustained release (if desired). Similarly, as not all
nutritional supplements are
readily absorbed in the stomach and/or may be partially or completely degraded
in the stomach, the
present disclosure allows for providing at least part of the nutritional
supplement particles in a
coated or encapsulated form that will survive the high acid environment of the
stomach but be
released in the small intestine for necessary absorption. Thus, the ability to
provide different
nutritional components in different formats allows for highly customizable
nutritional supplement
compositions with the nutritional materials present as the wall forming
materials of the hollow core
granules.
Similar to nutritional supplements, the hollow core granules can be configured
as other
personal care products that are configured for oral ingestion. For example,
laxatives, antacids, and
similar materials may be used in the hollow core granules. Materials such as
PEG are known to be
functional as laxatives, and hollow core granules may be prepared using a PEG
binder for wall
forming materials that may be substantially inert, that may also be configured
as a laxative or stool
softener, or may provide additional benefits, such as being a fiber
supplement, being an antacid
(e.g., sodium bicarbonate), or the like.
Since some users may have difficulty with swallowing pills, tablets, capsules,
or the like,
the present hollow core granules can be configured so that the nutritional
supplement is in a
chewable format Specifically, the nutritional materials may again be used as
the wall forming
material of the hollow core granules, but the granules can be configured for
ease of chewing and/or
rapid dissolution in the mouth of a user so that the supplements can be
provided in a convenient
form (e.g., solid dosages versus liquid dosages) while still being easily
ingested. Moreover, a
variety of additives can be combined with the nutritional supplements to
provide the hollow core
-50-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
granules in a palatable configuration. For example, sweeteners, flavorants, or
other ingestible
materials may be used as a portion of the wall forming material so that
nutritional components that
may otherwise be bitter, sour, or the like may be masked by the additives.
Moreover, at least a
portion of the binder likewise may be configured to impart palatable qualities
to mask any
unpleasant tastes associated with the nutritional supplements themselves. The
nutritional
supplement including vitamin(s), mineral(s), fiber, probiotics, enzymes, amino
acids, proteins, or
other supplemental agent(s) typically found in various nutritional
supplements, can be provided in a
bulk format wherein a mass or volume of the hollow core granules are provided
with dosing
instructions for an amount of the hollow core granules to ingest to deliver
the daily recommended
dosage or other dosage of the supplement(s) included therein. Alternative, a
pre-dosed amount of
the hollow core granules can be combined into a single unit, such as through
the use of binding
agents so that the hollow Care granules are retained together as blocks,
wafers, or similar unitary
formats that a user may chew to release the hollow core granules therefrom.
An example embodiment of a nutritional supplement is a vitamin D supplement,
which
comprises dextrates, microcrystalline cellulose, magnesium stearate, chamomile
powdered extract,
flavor, and vitamin D. The components can be formulated and then used as the
wall forming
material in hollow core granules as described herein Any nutritional
supplement may be similarly
formulated for preparation of the supplement in the hollow core format.
In some embodiments, the present, hollow core structures can be particularly
useful in
forming animal litters. As previously mentioned herein, clays are often a
primary component of
animal litter products due to the relatively low cost thereof and the
particularly good efficacy for
liquid absorption. Clays, however, are relatively dense and cause animal
litter products to be quite
heavy with commercially sold quantities requiring as much as 30 to 40 pounds
of a clay-based litter
to fill a large-size litter tray. Clays, however, can be particularly amenable
for use as a wall
forming material to produce the clay as a hollow core granule having a wall
comprising smaller
particles of the clay and a binder. The resulting, hollow core clay granules
can thus be particularly
useful in forming an animal litter having a significantly lower weight and
even improved
absorption properties. This can extend to animal litters having hollow core
clay granules as well as
hollow core granules formed from different wall forming materials.
The present disclosure thus can provide animal litter compositions that
include at least one
component thereof in the form of hollow core granules and that can exhibit
improved properties
including, but not limited to, reduced overall composition weight. Hollow core
granules can be
present in the animal litter in a defined amount, such as an amount of about
1% or greater by
weight based on the total weight of the animal litter composition. In further
embodiments, one or
more types of hollow core granules may be present in the animal litter
composition in amounts
-51 -
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
(independent of one another) of about 1% to about 95% by weight, about 2% to
about 75% by
weight, about 3% to about 60%, or about 5% to about 50% by weight based on the
total weight of
the composition. In some embodiments, a material present as hollow core
granules may be present
in a relatively low concentration, such as about 1% to about 10%, about 1.25%
to about 7.5%, or
about 1.5% to about 5% by weight based on the total weight of the animal
litter composition. This
may be the case, for example, in relation to ingredients such as sodium
bicarbonate, which may be
useful as a deodorizing component, fragrances, or other components typically
present in animal
litters. In further embodiments, a material present as hollow core granules
may be present in a
relatively high concentration, such as about 10% to about 90%, about 20% to
about 85%, or about
25% to about 75% by weight based on the total weight of the animal litter
composition. This may
be the case, for example, in relation to ingredients such as liquid absorbents
(e.g., clay), fillers, or
the like. In other embodiments, the hollow Core granules in an animal litter
may be defined in
relation to the volume ratio of the material since the hollow core version is
expected to be
significantly lighter than a non-hollow core version of the same material. For
example, the total
content of hollow core granules in an animal litter may be in the range of
about 5% to about 98%,
about 10% to about 95%, about 20% to about 90%, or about 30% to about 80% by
volume based
on the total volume of the animal litter composition. Other concentration
ranges as already
described above may be utilized on a volume basis. This can include low
concentration
components and/or high concentration components.
Animal litters may include a variety of components, and it is understood that
animal litter
compositions according to the present disclosure may include one of the
following components in
the form of hollow core granules. Likewise, the present animal litter
compositions may include
two, three, four, or even more of the following components in any combination
in the form of
hollow core granules. Non-limiting examples of the types of components that
may be used in
animal litters and that may be present in the form of hollow core granules
include liquid absorbents,
fillers, clumping agents (or clump enhancing materials), binders,
preservatives, such as biocides
(e.g., benzisothiazolinone, methylisothiazolone), de-dusting agents,
fragrance, bicarbonates, and
combinations thereof
Fillers suitable for use in the present animal litter compositions can include
a variety of
materials that can be a non-absorbent, non-soluble substrate, or can be an
absorbent substrate. In
one or more embodiments, useful fillers can include absorbent substrates, such
as non-clumping
clays. Non-limiting examples of useful non-clumping clays include attapulgite,
Fuller's earth,
calcium bentonite, palygorskite, sepiolite, kaolinite, illite, halloysite,
hormite, vermiculite or
mixtures thereof. Suitable fillers according to the present disclosure also
can include a variety of
non-absorbent, non-soluble substrates, such as non-clay substances. Non-
limiting examples of non-
-52-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
clay materials that can be used include zeolites, crushed stone (e.g.,
dolomite and limestone),
gypsum, sand, calcite, recycled waste materials, and silica. As examples, the
animal litter
composition can comprise about 0% by weight to about 75% by weight, about 10%
by weight to
about 70% by weight, about 25% by weight to about 65% by weight, or about 40%
by weight to
about 60% by weight of one or more fillers based on the total weight of the
animal litter
composition or by volume based on the total volume of the animal litter
composition. Such fillers
may be present in a typical, non-hollow core format or may be present as
hollow core granules or
may be present as one of a plurality of components used as the wall forming
material for hollow
core granules.
Description of suitable clumping agents is provided in U.S. Patent No.
8,720,375 to Miller
et al., the disclosure of which is incorporated herein by reference. Useful
clumping agents are those
materials suitable to promote adhesion of the fine size particles of litter
granules to each other as
well as adhesion of the particles to form agglomerates when wetted.
Preferably, the clumping agent
allows the formation of a gelled agglomerate when exposed to a liquid, such as
animal urine. A
clumping agent may be provided in admixture (e.g., in particle form) with the
further components
of the animal litter. In some embodiments, the clumping agent can be provided
as a coating on at
least a portion of the other components forming the animal litter (e.g., as a
coating on at least a
portion of the filler material). Such coatings may be provided by any known
method, such as
spraying. If desired, a clumping agent may be provided as an outer layer/wall
on a hollow core
structure as already described above. For example, a clumping agent may be
coated on a hollow
core structure haying a clay wall and/or a sodium bicarbonate wall. Non-
limiting examples of
materials suitable for use as a clumping agent include naturally occurring
polymers, semisynthetic
polymers, and sealants. Example embodiments of naturally occurring clumping
agents include
various starches, including corn starch, various gums such as gum arabic, gum
karaya, gum
tragacanat, gum ghatti, guar gum, and xanthan gum, as well as alginates,
carrageenan, pectins,
dextran, gelatin, gluten, dried plants of the Plantago family, vinyl polymers,
including polyvinyl
alcohol, polyvinyl esters such as polyvinyl acetate, payvinylpyiTolidoneõ
polyvinyloxazoli done,
polyvinylmethyloxazolidone, copolymers and mixtures thereof Example
embodiments of
semisynthetie polymers include cellulose ethers (e.g., methvicellitiose,
hydroxyethyl c ellulose,
hydroxypropyl cellulose, ethy thydroxyethyi cel liLlose, tnethylltydroxypropyl
cellulose,
earboxynnethylcellulose, hydrox7Rropylmethyleellulose or mixtures thereof),
and guar gum
derivatives. The amount of any clumping agent that is present in the animal
litter composition can
vary based upon the total composition. For example, it can be useful to
include a greater amount of
clumping agents when a greater amount of non-absorbent fillers is used. In
some embodiments,
clumping agents can be present in a total amount of 0.1% by weight to about 6%
by weight, about
-53 -
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
0.2% by weight to about 5.5% by weight, about 0.3% by weight to about 5% by
weight, or about
0.5% by weight to about 4% by weight, based on the total weight of the litter
composition or by
volume based on the total volume of the litter composition.
To the extent that one or more binders, preservatives, de-dusting agents,
fragrances,
bicarbonates, or the like it included, such materials may be present
independently in any amount up
to about 5% by weight, up to about 2% by weight, up to about 1% by weight, or
up to about 0.5%
by weight, such as about 0.01% by weight to about 5% by weight, to about 4% by
weight, to about
3% by weight, to about 2% by weight, or to about 1% by weight based on the
total weight of the
animal litter composition. Such amounts alternatively may be by volume based
on the total volume
of the litter composition. Further, it is understood that any one or more of
such materials may be
expressly excluded from the present animal litter composition.
In some embodiments, a product according to the present disclosure can be an
animal litter
prepared by a method as described herein such that the animal litter comprises
a mixture of hollow
core granules and one or more further components that are effective in an
animal litter composition.
In further embodiments, a product according to the present disclosure can be
an animal litter
prepared by a method as described herein such that the animal litter comprises
hollow core granules
prepared such that a plurality of individual particles of at least one wall
forming material comprise
particles of a clay or particles of sodium bicarbonate.
In addition to litter compositions, the present disclosure further can extend
to a variety of
additives that may be used with cat litters. For example, various odor-masking
agents or
deodorizers may be available in solid form, and such materials may be provided
in the form of
hollow core granules as described herein. Non-limiting examples of litter
additives that may be
provided in the form of hollow core granules include deodorizers, clumping
agents, de-dusting
agents, fragrance, odor masking agents, and the like. Any one or more
materials useful in pet litters
may be formulated individually or together in the form of hollow core granules
to provide litter
additive products.
As already discussed herein, the hollow core granules can be particularly
useful in fertilizer
compositions. Fertilizers are typically recognized as compositions that
provide nitrogen,
phosphorus, potassium, or other minerals needed for plant health such as so-
called micronutrients
(e.g., boron, chlorine, copper, iron, manganese, molybdenum, and zinc). The
fertilizer particularly
can be a nitrogen source, and examples of nitrogen sources can include
materials wherein nitrogen
can be provided as one or more of: amides, such as urea, urea ammonium nitrate
(UAIVI), or
polymer encapsulated urea; ammonium compounds, such as ammonium bicarbonate,
ammonium
sulfate, ammonium chloride, and the like; and nitrates, such as sodium
nitrate, calcium nitrate, and
ammonium nitrate. The fertilizer particularly can be a phosphorus source, and
examples of
-54-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
phosphorus sources can include materials wherein phosphorus may be provided in
its elemental
form or in phosphate salts. The fertilizer particularly can be a potassium
source, and examples of
potassium sources can include materials wherein potassium can be provided in
an elemental form
or in a salt form, such as potash. The fertilizer particularly can be
micronutrient source, and
examples of micronutrient sources can include materials wherein micronutrients
as noted above or
otherwise accepted as such are provided. Other materials that may be
considered to fall within the
group of fertilizers as used herein can include materials that are commonly
used to modify soil pH.
Materials for increasing soil pH can include lime, which can more particularly
be present as
calcium carbonate, or calcitic limestone, or as dolomitic limestone, which is
a combination of
calcium carbonate and magnesium carbonate. Materials for reducing soil pH
include sulfur or
sulfur containing minerals or compounds.
Any one or more materials suitable for use as a fertilizer may be used as a
wall forming
material for hollow core granules as described herein. Fertilizer materials
available in a solid form
may thus be provided as relatively small particles that define one or more
walls/shells in a hollow
core granule. Upon delivery to a site of use, the fertilizer particles may be
released from the
relatively larger granules for solubilization in the soil. Fertilizer
materials available in a liquid form
may be combined with particles of an absorbent or adsorbent material, such as
clay, so that the
liquid fertilizers are entrained thereby. The particles of the combined
materials may then be used to
prepare hollow core granules of the fertilizer. Upon delivery to a site of
use, the fertilizer may be
released from the carrier particles, and the remaining clay particles may be
incorporated into the
soil or other site of delivery without harm.
Various chemicals and compounds useful as fertilizers can exhibit different
dissolution rate
or release rates. In particular, there are variously know -fast release"
fertilizers and -slow release"
fertilizers. Where it is desirable to provide combinations of fertilizers with
different release rates,
particles of a fast release fertilizer and particles of a slow release
fertilizer can be combined in
desired ratios and used as the wall forming component for preparing granules
of hollow core
structures as described above. Thus, the resulting fertilizer granules will
have a wall surrounding a
hollow core wherein the wall comprises the fast release fertilizer particles
and the slow release
fertilizer particles in the designed ratio. Upon application to a site in need
of fertilization, the fast
release fertilizer particles will provide immediate fertilization, and the
slow release fertilizer
particles will remain for the expected time for slow release thereof. The same
principle can apply
to any number of solid materials with differing dissolution and/or release
rates so that many types
of controlled release granules can be prepared. Alternatively, fertilizers
with different release rates
may be provided in separate hollow core granules. The separate hollow core
granules can then be
combined at the time of use in a desired ratio.
-55 -
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
In some embodiments, controlled release of fertilizers may be particularly
achieved through
use of encapsulation methods as already described above. For example, certain
fertilizers may be
provided in a microencapsulated or other encapsulated form so that the
fertilizer material is only
released after dissolution, degradation, of the like of the encapsulating
material. The encapsulated
fertilizer particles may be used as the wall forming material (alone or in
combination with other
fertilizer particles in a rapid release and/or controlled release form) to
provide hollow core fertilizer
granules that will exhibit controlled release. Coating technology may also be
utilized as a means
for provision of liquid components as the wall forming material. As an
example, particles of
polymer encapsulated urea may be used as a wall forming material that can be
combined with a
binder to prepare hollow core fertilizer granules. In further embodiments,
controlled release may
be achieved through choice of binder material. For example, binders may be
chosen based on a
specific solubility so that fertilizer particles in the wall of the hollow
core granules will only be
released upon solubilization of the binder. Likewise, hollow core fertilizer
granules may be
provided with multiple walls, shells, coatings, etc. with different properties
to control release of one
or more fertilizer components therefrom. The different walls, shells,
coatings, etc. may release
their fertilizer components only under specific conditions and/or may exhibit
different
solubilization properties so that a controlled release may be achieved based
upon the
solubilization/degradation of the multiple walls, shells, coatings, etc. This
can be characterized as a
multi-stage release fertilizer or controlled release fertilizer.
Fertilizer compositions may be applied to a site of use by a variety of
methods. Solid
fertilizer compositions may be applied directly to the soil for release of the
fertilizer component(s)
through solubilization over time. Other fertilizer compositions, however, may
be preferably
admixed with a solvent and then sprayed onto the delivery site. The improved
dissolution rates
provided by utilizing a hollow core format as described herein can be
beneficial for such uses. In
particular, the solid, hollow core fertilizer granules may be provided in bulk
and then the desired
mass or volume of the solid, hollow core fertilizer granules can be added to a
tank sprayer or
similar apparatus immediately prior to application. Because the hollow core
format can
significantly lessen the time to dissolution of the material, the hollow core
fertilizer granules can
rapidly dissolve in the tank sprayer or similar apparatus for application at
the site of use without
significant delay. The hollow core granules can thus be particularly useful in
large scale farming or
the like Likewise, since the hollow core format significantly reduces the
weight of the material,
this can further improve ease of application of the fertilizer.
Rapid dissolution of the hollow core granules can also be advantageous for
environmental
safety. Fertilizer run-off can be problematic due to passage into waterways
(e.g., rivers, streams,
ponds, or even wastewater drainage. Fertilizer particles can be easily washed
into such waterways
-56-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
by heavy rains if the particles have not substantially degraded or dissolved.
Since the hollow core
format can significantly hasten dissolution and/or degradation, the use of the
hollow core format for
fertilizers can reduce such potential problems. As seen in the appended
examples, hollow core
fertilizer granules formed using bentonite as a wall forming material and PEG
8000 as a binder
have been shown to dissolve in water in a matter of second. This illustrates
the ability according to
the present disclosure to provide hollow core fertilizer granules in a form
that are conveniently
stored and transported (solid versus liquid), are easier to handle (e.g.,
having a reduced weight), and
will dissolve rapidly once contacted with water to release the fertilizer
component.
In example embodiments, hollow core fertilizer granules can be prepared with a
variety of
wall forming materials and a variety of binders. For instance, various clays
(e.g., bentonite), salts
(e.g., sodium bicarbonate, magnesium sulfate, etc.), and similar materials can
be useful as a wall
forming material along with any specific, solid fertilizer materials that may
be included in the
granules. Clays are particularly useful since such components can effectively
bind various types of
fertilizers, release the fertilizers in the soil, and then remain in the soil
as an inert additive. Polymer
encapsulated fertilizers can particularly be utilized, encapsulated ureas and
encapsulated phosphates
being example embodiments. Suitable binders can include hydrophilic materials
such as PEGs of
various molecular weights (e.g., PEG 8000, PEG 12000, and/or PEG 3 5 000), or
similar materials
that will readily solubilize in soil for degradation of the granules and
release of the fertilizer
materials therefrom.
Other types of materials that may be desired for application in the
environment may
likewise benefit from provision in a hollow core format. Pesticides, for
example, can include a
wide variety of chemicals, compounds, and the like that are configured for
control of pests, insects,
and the like. The group of pesticides can include, for example, algaecides,
antimicrobials,
biopesticides, disinfectants, fungicides, herbicides, insecticides, miticides,
molluscicides, ovicides,
repellants, rodenticides, and the like. Use of the term "pesticide- herein may
thus be understood as
referencing any of the above, example embodiments of materials useful for
control of undesired
species (pests, insects, weeds, etc.). Many such materials are commonly sold
in particle form for
application with spreaders or for hand application. Pesticides may be designed
so that improved
performance is achieved by wetting following application. This can be
necessary to ensure that the
active ingredients are dispersed in the soil or other point of application
and/or to limit contact of the
active ingredients with humans, pets, or wild animals Moreover, wetting for
dispersion and soil
contact can be necessary to prevent unnecessary run-off of the potentially
dangerous chemicals into
waterways.
Any chemical, compound, or similar material effective as a pesticide may be
utilized in
forming a hollow core granule according to the present disclosure. The
pesticide specifically may
-57-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
be considered to be an active agent in light of previous recognition thereof
as exhibiting a pesticide
activity. Non-limiting examples of pesticides that may be used according to
the present disclosure
and that may be considered to be a pesticide active agent include bifenthrin,
acephate, carbaryl,
cyfluthrin, 2,4-dichlorophenoxyacetic acid, trifluralin, chlorpyrifos,
allethrins, cypermethrin,
disulfoton, 2,6-dichlorobenzonitrile, metolachlor, cyhalothrin,
hydramethylnon, atrazine,
chlorothalonil, myclobutanil, dicamba, azadirachtin, captan, diazinon,
carbofuran, methomyl,
deltamethrin, propiconazole, borate, dinotefuran, dithiopyr, isoxaben,
prodiamine, quinclorac,
sethoxydim, iron(III) phosphate, mancozeb, thiophanate-methyl, esfenvalerate,
tebuconazole,
resmethrin, glyphosate, malathion, permethrin, imidacloprid, fipronil,
abamectin, spinosad,
triclopyr, piperonyl butoxide, pendimethalin, oryzalin, and oxadiazon.
Any one or more pesticides may be utilized in a hollow core granule in a
manner similar as
already discussed above in relation to fertilizers. In particular, one or more
pesticides may be used
as a wall forming material for hollow core granules as described herein.
Pesticides available in a
solid form may thus be provided as relatively small particles that define one
or more walls/shells in
a hollow core granule via combination with one or more binders. Upon delivery
to a site of use, the
pesticide particles may be released from the relatively larger granules for
solubilization and/or
dispersion in the soil. Pesticides available in a liquid form may be combined
with particles of an
absorbent or adsorbent material, such as clay, so that the liquid pesticides
are entrained thereby.
The particles of the combined materials may then be used to prepare hollow
core granules of the
pesticides. Upon delivery to a site of use, the pesticide may be released from
the carrier particles,
and the remaining clay particles may be incorporated into the soil or other
site of delivery without
harm.
Pesticidal hollow core granules may be configured with a single pesticide or
with a
combination of pesticides having the same or different activities. When a
plurality of different
pesticides are used as the wall forming material in a hollow core structure,
the different pesticides
can be combined in desired ratios for combination with one or more binders for
preparing granules
of hollow core structures as described above. Thus, the resulting pesticide
granules will have a
wall surrounding a hollow core wherein the wall comprises the particles with
the different
pesticides in the designed ratio.
Pesticide compositions may be applied to a site of use by a variety of
methods. Solid
pesticide compositions may be applied directly to soil or other surface for
release of the pesticide
component(s). Other pesticide compositions, however, may be preferably admixed
with a solvent
and then sprayed onto the delivery site. The improved dissolution rates
provided by utilizing a
hollow core format as described herein can be beneficial for such uses. In
particular, the solid,
hollow core pesticide granules may be provided in bulk and then the desired
mass or volume of the
-58-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
solid, hollow core pesticide granules can be added to a tank sprayer or
similar apparatus
immediately prior to application. Because the hollow core format can
significantly lessen the time
to dissolution of the material, the hollow core pesticide granules can rapidly
dissolve in the tank
sprayer or similar apparatus for application at the site of use without
significant delay. This can
enable formation of lightweight compositions in a solid format that exhibit
ease of storage and
transportation while still allowing for rapid delivery at the point of use.
Pesticides that require delivery in a liquid format, such as in the case of
herbicides that
require direct plant contact, or such as in the case of pesticides that are
sprayed for application in
homes and the like, can be beneficially configured in a solid format by
combining the liquid
pesticide with a carrier particle prior to incorporating the pesticide-loaded
carrier particles as the
wall forming material in hollow core granules. The formed, hollow core
pesticide granules then
can be dissolved in a suitable solvent prior to application. Suitable carrier
particles and binder can
be chosen to likewise dissolve and be delivered with the pesticide as an inert
component or as an
additive. For example, particles of a solid pesticide may be used as a carrier
for a liquid pesticide.
Similarly, one or more pesticides may be used as the wall forming material to
prepare the pesticide
hollow core granules, and one or more further pesticides may be used as a
coating on the formed
particles to provide for combined delivery of multiple pesticides in a given
granule.
The ability to provide pesticide composition with different release properties
can be
particularly beneficial. For example, some pesticides may pose a risk if
ingested by wildlife or
domestic animals, and it can be beneficial for such pesticides to quickly
solubilize, degrade, or the
like to reduce the time during which non-desired interactions may occur. This
can be a problem
with many solid forms of pesticides, which can persist in the environment for
a significant length of
time. Such materials, however, may be provided according to the present
disclosure in a hollow
core form that exhibits rapid dissolution, degradation, or the like.
Hollow core pesticides may also be configured to exhibit specific properties
that make them
highly useful in various environments. For example, hollow core granules may
be prepared to
exhibit buoyance that can make them particularly useful for application in
water. Ponds or other
freshwater areas may require treatment for various pests, but in can be
difficult to provide solid
particles in a form that will persist at the surface rather than immediately
solubilizing or sinking.
Choice of binder and/or inclusion of additives in the wall forming material
can be effective to
render the overall granule buoyant for a sufficient time that the pesticide
agent can be released at
the surface of the water. For example, hydrophobic binders may be utilized for
this purpose, and
additives, such as cellulosics (e.g., cellulosic aerogels), straw (or similar,
buoyant plant materials),
and various clays may be utilized as additives and/or carriers for pesticides
in the wall forming
material of the hollow core granules to render the granules buoyant.
Controlled release options as
-59-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
already discussed herein may likewise be applied to ensure that the pesticide
material is released at
the appropriate time after application to the site of treatment.
In example embodiments, hollow core pesticide granules can be prepared with a
variety of
wall forming materials and a variety of binders. Clays (e.g., bentonite) can
be particularly useful as
wall forming materials in pesticide granules along with any specific, solid
pesticide materials that
may be included in the granules. Likewise, clays can be useful as carriers of
one or more pesticides
that may be typically provided in a liquid form. Suitable binders can include
hydrophilic materials
such as PEGs of various molecular weights (e.g., PEG 8000, PEG 12000, and/or
PEG 35000), or
similar materials that will readily solubilize for release of the pesticide
materials held in the
granules. PEGs and similar binders particularly may have one or more
pesticides admixed
therewith so that the binder functions as a vehicle for at least part of the
pesticide material(s), and
the clay particles Or similar materials may be utilized as an essentially
inert wall former.
In some embodiments, products formed as hollow core structures as described
herein may
be provided in unit dose forms. As already described above, a wide variety of
materials may be
used as wall forming materials for hollow core granules, and the resulting
product will be a
plurality of hollow core granules that can be provided in any mass or volume
desired. In some
embodiments, however, it may be beneficial for a defined mass or volume of
hollow core granules
to be provided in combination to achieve a desired dosage. For example, in the
field of
detergents/cleaners, it may be desirable to provide a defined mass or volume
of a laundry detergent
composition as a convenient, pre-dosed amount for a single load of laundry.
Similar benefits may
be achieved in other fields, such as nutritional supplements, fertilizers,
pesticides, and even further
areas where it may be more convenient for a consumer to have a pre-dosed
amount of the hollow
core granule products as an alternative to measuring out a desired dose of the
individual granules.
Any one or more hollow core granule products according to the present
disclosure thus may be
provided in a unit dose format in addition to or as an alternative to a mass
supply of individual
granules.
It is known, for example, to provide granular detergent compositions as wells
as pastes,
gels, slurries, and the like in water-soluble film pouches, which may be
referred to as pods. The
present compositions provided as hollow core granules likewise may be provided
in such unit dose
forms wherein a mass of the hollow core granules is provided in a pouch of
defined weight and/or
volume. Suitable technologies for providing hollow core granules as described
in unit dose forms
are described, for example, in U.S. Pat. No. 8,669,220 to Huber et al.; U.S.
Pat. App. Pub. No.
2002/0033004 to Edwards et al.; U.S. Pat. App. Pub. No. 2007/0157572 to Oehms
et al. U.S. Pat.
App. Pub. No. 2012/0097193 to Rossetto et al.; U.S. Pat. No. 4,973,416; U.S.
Pat. No. 7,915,213 to
Adamy et al.; and U.S. Pat. App. Pub. No. 2006/0281658 to Kellar et al.; all
of the foregoing
-60-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
documents being incorporated herein by reference. In an example embodiment,
hollow core
granules providing a defined product (e.g., laundry detergent, dishwashing
detergent, fertilizers,
pesticides, etc.) may be enclosed in poly (vinyl alcohol) film using, for
example, a simple Uline
heat sealer, thereby forming unit dose pods. Any suitable, water-soluble film
may be used, and any
suitable sealing technology may be utilized to form the pods in any desired
mass/volume suitable to
provide a defined quantity of the product for a desired end use. Unit doses
may likewise be
provided in other forms, such as fabric pouches, which may be formed of
dissolvable fibers or non-
dissolvable fibers.
Other types of unit dose forms are also encompassed by the present disclosure.
For
example, a unit dose may encompass a mass of solids that are compressed with
one or more
binding agents. Such unit dose forms may include a content of hollow core
granules as described
herein for a particular end use. For example, products such as nutritional
supplements, chewable
teeth cleaning compositions, or other products where it would be undesirable
for the product to be
enclosed in a film may be provided in such format. In particular embodiments,
a desired
mass/volume of hollow core granules may be combined with a binding agent that
will allow a
plurality of the hollow core granules to be retained together as a unit dose.
For example, gums
(e.g., guar gums or xanthan gums), cellulosic materials, starch materials,
and/or water soluble
adhesives may be used to create such blocks, tablets, pills, caplets, prills,
or other form factors
through agglomeration of a plurality of hollow core granules into a single,
unit dose form. These
blocks or the like formed from the hollow core structures can be significantly
lighter and/or
significantly faster dissolving in comparison to known unit dose powders that
do not include the
present hollow core structures
In light of the improved absorption properties discussed previously herein,
the hollow core
granules can be particularly effective in formation of one or more products
where absorption and/or
adsorption of a gas and/or liquid is desired. In some embodiments, hollow core
granules may be
configured for use in absorption and/or adsorption of one or more air
pollutants. Many materials
are classified, such as by the US Environmental Protection Agency, as air
pollutants. Non-limiting
examples of such materials are: carbon monoxide, lead, nitrogen oxides, ozone,
particulate matter,
sulfur dioxide, acrolein, asbestos, benzene, carbon disulfide, creosote, fuel
oils/kerosene, polycyclic
aromatic hydrocarbons, synthetic vitreous fibers, total petroleum
hydrocarbons, and the like. Many
materials are likewise known as being effective at absorbing, adsorbing, or
otherwise binding with
one or more of these or other types of air pollutants. Such materials can be
used as wall forming
materials in preparing hollow core granules according to the present
disclosure. Granules with
these air pollution capturing components can be deployed in various form
factors for interaction
with ambient air for capture of one or more air pollutants. Likewise, the
granules maybe embedded
-61 -
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
in articles, such as air filters, industrial pollution capture items (e.g.,
power plant gas filters and
exhaust cleaners), and the like that can be deployed in suitable locations for
capture of air pollutants
and then disposal. Such granules likewise may be utilized in personal items,
such as breathing
masks, to remove air pollutants for personal use. As a non-limiting example,
activated carbon,
zeolites, and other porous materials are known to be effective in capture of
various pollutants, and
such materials may be incorporated into hollow core granules as wall forming
materials and thus
provide granules effective at capturing one or more pollutants as well as
capturing odors, and the
like.
In some embodiments, hollow core granules may be configured for use in
absorption or
other capture of liquids. Because the hollow core granules can be configured
with excellent liquid
absorption properties, and because the granules may be configured to
preferentially uptake aqueous
or hydrophobic liquids, the hollow core granules can be used in a variety of
manners for removal of
liquids and/or remediation of liquid spill sites. In particular embodiments,
the hollow core granules
can be configured to absorb one or more types of liquids without
solubilization of the granule itself.
In this matter, liquids can be bound by the granules, which in turn may be
removed in a
substantially coherent mass and/or as individual granules that substantially
retain the granular
structure. This can be particularly useful in removal of organic spills (e.g.,
oil) in marine or other
water environments. The hollow core granules can be configured to
substantially float on the water
surface (e.g., exhibiting buoyancy as previously discussed herein) where
interaction with spilled
organics can be maximized. Again, the hollow core granules can be configured
to retain their
granular structure and/or agglomerate into masses that are relatively easy to
remove once the
binding activity has been completed. This can extend to preparation of unitary
items, such as spill
sleeves and the like, wherein the hollow core granules may be retained within
a fabric, mesh, or
otherwise porous item that will prevent dispersion of the individual granules
across the water
surface while still allowing influx of the organic material to be retained. In
some embodiments, the
granules likewise can incorporate components that provide functions in
addition to absorption
and/or adsorption. For example, biological components are known to be useful
to degrade organics
or render certain materials less viscous so as to improve the ability of the
material to be absorbed.
The presently disclosed, hollow core granules can be easily formulated for the
desired end
use through choice of wall forming material and/or binder material, and this
is shown in the
appended examples For example, it has been shown herein that utilization of a
hydrophobic binder
provides for hollow core granules that will float on the surface of water
(i.e., exhibiting buoyancy).
On the other hand, utilization of a hydrophilic binder, such as PEG, can
provide hollow core
granules that are hydrophilic and that will readily sink in water to quickly
dissolve. Moreover, it
has been shown herein that the use of the same wall forming material (e.g.,
bentonite), when used
-62-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
with a hydrophilic binder, such as PEG, or when used with a hydrophobic
binder, such as paraffin,
can result in two different types of hollow core granules that will behave
similarly in both aqueous
environments and non-aqueous environments. Thus, choice of a highly stable
wall forming
material, such as bentonite, can dictate granule properties irrespective of
choice of binder. Still
further, granules can be additionally modified through use of coating
materials to even further
adjust the properties. As an example, combination of a hydrophilic binder
(e.g., PEG) with a highly
stable wall forming material, such as bentonite, can result in a generally
hydrophilic granule, but
the granule can be modified through formation of a hydrophobic coating layer
to render the
otherwise hydrophilic granules buoyant and thus allow for floating in aquatic
settings to allow the
granules to carry out a designed function where buoyancy is desired. Thus, for
example, hollow
core granules formed of bentonite and PEG but coated with a hydrophobic layer,
such as paraffin,
can be rendered useful for oil spill cleanup in marine settings
EXPERIMENTAL
The present disclosure is more fully illustrated by the following examples,
which are set
forth to illustrate certain embodiments of the present disclosure and are not
to be construed as
limiting thereof.
Experimental Methods
A variety of samples of hollow core granules were prepared utilizing a
fluidized bed drier.
For each set of samples, 5 grams of the chosen binder in particulate form with
the chosen particle
size were loaded to the fluidized bed drier with 250 grams of the chosen wall
forming material in
particulate form with the chosen particle size. This provided an excess of the
amount of wall
forming material necessary to be bound together for formation of the hollow
core granules. After
processing, the formed granules were unloaded from the fluidized bed drier
(leaving behind any
remaining, unbound, wall forming material) and weighed. Since all of the
loaded binder was used
in the granule formation, but since not all the particles of the wall forming
material were used,
binder concentration of the formed granules was calculated as the total weight
of the formed
granules in grams divided by 5 grams (the initially loaded weight of the
binder). Granule formation
using 5 grams of binder and 250 grams of wall forming material (in these
examples, sodium
bicarbonate or bentonite clay) typically was effective to prepare
approximately 50 grams of the
hollow core granules.
Granule and Cavity Sizes
To measure average size of the formed granules, average size of the internal
cavity (i e , the
hollow core), and average wall thicknesses, 20 randomly selected granules from
each test set were
-63 -
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
cut in half using an Exact knife, and the half that was visually observed to
better retain the original
shape was measured microscopically using a microscopic ruler. For granules
exhibiting a
substantially elongated shape, three measurements were taken for each
dimension, and an average
of the sum of the three measurements was recorded.
Granule Density
To measure granule density, a cup with a known volume of 33.5 mL was filled
with
granules and weighed. The obtained weight was divided by the known volume to
establish granule
density, and a bulk density was obtained as an average of 5 measurements for a
given sample of
granules.
Granule Strength
Strength of the granules was measured as a maximum force needed to crush the
granule.
Testing was carried out using the Model 5 ST Benchtop Tester (5kN / 1k lbf)
from Tinius Olsen
The machine probe was set to move with a speed of 100 mm/min. Each measurement
was repeated
with ten randomly selected granules from the respective set of granules. The
resistant force was
recorded, and the maximum peak force was taken as a strength value. Ten
measurements were
averaged to provide the final number for the granule strength in the given
batch.
Water Absorption
To measure absorption of water by the granules, 1 gram of water was dropped on
a prepared
layer of granules having an average thickness of 1 cm. The combination was
allowed to sit for 5
minutes to form a clump prior to weighing. Percentage of water absorption was
calculated
according to the following formula: [(weight of clump ¨ 1g) / lg] x 100%.
Oil Absorption
To measure absorption of oil by the granules, 8 grams of granules were placed
in oil in a
sieve for 5 minutes. The combination was allowed to sit for 5 minutes to drain
excess oil and allow
any remaining, free oil to be absorbed by the filtering paper. The granules
were then weight, and
the percentage of oil absorption was calculated according to the following
formula: [(weight of wet
granules ¨ 8g) / 8g] x 100%.
Stability in Water
To evaluate water stability, 3 grams of granules were loaded in a beaker
filled with 0.03
liters of water at room temperature (approximately 22 C). The granules were
monitored to
determine when the granules begin to disintegrate The time between loading the
granules into the
water-filled beaker and the moment when they disintegrate was measured and
reported as time for
stability in water.
Stability in Oil
-64-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
To evaluate oil stability, 3 grams of granules were loaded in a beaker filled
with 0.03 liters
of Lukoil Standard 10W-40 multigrade mineral engine oil (API SF/CC) at room
temperature
(approximately 22 C). The granules were monitored to determine when the
granules begin to
disintegrate. The time between loading the granules into the oil-filled beaker
and the moment when
they disintegrate and appear as a sediment was measured and reported as time
for stability in oil.
Buoyancy
To evaluate ability of the granules to remain floating in water, 3 grams of
granules were
loaded in a beaker filled with 0.15 liters of water at room temperature
(approximately 22 C). The
combination was immediately evaluated, and granules were noted as being
buoyant in water in all
cases where a majority of the granules were observed to float.
Solubilization Time in Stirred Water
To further evaluate aqueous solubilization, 10 grams of granules were loaded
in a beaker
filled with 1.4 liters of deionized water at room temperature (approximately
22 C). An impeller
agitator was included in the beaker set at 500 rpm. The time between loading
the granules and the
moment when they disappear (i.e., the solution became substantially clear
indicating substantial
dissolution) was measured and reported as solubilization time. The
measurements were performed
only for samples where the wall forming materials was a water soluble solid
(i.e., sodium
bicarbonate).
EXAMPLE 1: Sodium Bicarbonate + PEG
Granules were prepared using 5 grams of PEG 8000 (1.2 mm ¨ 1.6 mm nominal
size) as
binder particles or crystals, which was loaded in the fluidized bed drier with
250 grams of sodium
bicarbonate (0.100 mm ¨ 0.400 mm nominal size). The fluidized bed dryer was
run at 65 C to
prepare five batches with different residence times at maximum temperature (5
min, 10 min, 15
min, 20 min, or 30 min). Respective batches were cooled to 30 C prior to
discharging the formed
granules. An image of one of the hollow core granules after being cut is shown
in FIG. 24.
Bulk density of the granules as a function of the processing time in the
fluidized bed drier
was found to substantially increase with increased residence time, and the
measured values are
shown in FIG. 5. Residence time was also found to be a factor in the total
amount of the sodium
bicarbonate particles present in the formed granules with the total content
increasing with
processing time but appearing to reach a plateau when the binder was fully
utilized, and this is
shown in FIG. 6. Granule strength was found to remain approximately unchanged
with only slight
reductions as processing time increased (see FIG. 7). A similar pattern was
seen in relation to
particle attrition (see FIG. 8A through FIG. 8E).
-65 -
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
The size of the cavity in the formed granule was determined to be strongly
influenced by the
initial size of the binder particle. The shell thickness was approximately the
same as diameter of
the cavity, as shown in FIG. 9, where A, B, and C are the outer dimensions of
the granules and a, b,
and c are the dimensions of the cavity. Therefore, the diameter of the cavity
was found to be
approximately 1/3 of the outer diameter of the granule. Cavity volume was thus
calculated to be
approximately 3-4% of the total volume of the granule. The fractional
composition of the typical
batch of hollow core granules is shown in FIG. 10, and this was found to again
be dependent on
residence time in the fluidized bed. The fractional composition was evaluated
in compliance with
ASTM E-11 using three sieves (1 mm, 2 mm, and 3.2 mm nominal sizes). It was
generally found
that larger granule sizes were obtained with a longer residence time in the
fluidized bed, allowing
longer periods for particles of the wall forming material to agglomerate with
the binder crystals (see
FIG. 10). Total granule properties are shown in the tables of FIG. 17 and FIG.
18.
EXAMPLE 2: Bentonite + PEG
Granules were prepared using 5 grams of PEG 8000 (1.2 mm ¨ 1.6 mm nominal
size) as
binder particles or crystals, which was loaded in the fluidized bed drier with
250 grams of bentonite
(0.100 mm ¨ 0.400 mm nominal size). The fluidized bed dryer was run at 65 C
to prepare two
batches with different residence times at maximum temperature (15 min and 30
min). Respective
batches were cooled to 30 C prior to discharging the formed granules. An
image of one of the
hollow core granules after being cut is shown in FIG. 25.
Bulk density of the granules as a function of the processing time in the
fluidized bed drier
was found to substantially decrease with increased residence time, and the
measured values are
shown in FIG. 11. Granule strength was found to be less than the measured
strength of the granules
of Example 1(3.6 N for the present granules versus 15 N for the granules of
Example 1). The shell
thickness of the bentonite hollow core granules was approximately equal to the
diameter of the
cavity (see FIG. 12). Thus, the diameter of the cavity was found to be
approximately 1/3 of the
total diameter of the granule. Therefore, the volume of the cavity was found
to be about 3-4% of
the total volume of the granule (see FIG. 13). Total granule properties are
shown in the tables of
FIG. 17 and FIG. 18.
EXAMPLE 3. Sodium Bicarbonate + Bentonite + PEG
Granules were prepared using 5 grams of PEG 8000 (1.2 mm ¨ 1.6 mm nominal
size) as
binder particles or crystals, which was loaded in the fluidized bed drier with
235 grams of bentonite
(0.100 mm ¨ 0.400 mm nominal size) and 235 grams of sodium bicarbonate (0.100
mm ¨ 0.400
mm nominal size). The fluidized bed dryer was run at 65 C for 15 minutes
prior to cooling to 30
-66-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
C before discharging the formed granules. The resulting granules had an
average granule size that
was similar to the bentonite granules of Example 2. The density of the
resulting granules was
approximately midway between the density of the sodium bicarbonate granules of
Example 1 and
the bentonite granules of Example 2. Strength of the granules was similar to
the strength of the
bentonite granules of Example 2. Total granule properties are shown in the
tables of FIG. 17 and
FIG. 18. An image of a plurality of the hollow core granules is shown in FIG.
26.
EXAMPLE 4: Sodium Bicarbonate + Brij TM S 1 00
Granules were prepared using 5 grams of BrijTM S100 (1.2 mm ¨ 1.6 mm nominal
size) as
binder particles or crystals, which was loaded in the fluidized bed drier with
250 grams of sodium
bicarbonate (0.100 mm ¨ 0.400 mm nominal size). The fluidized bed dryer was
run at 60 C.
Immediately after reaching the maximum temperature, the granules were cooled
to 30 C before
discharging the formed granules (i.e., residence time of substantially 0).
While the BrijTM S100 has
a similar melting temperature as PEG 8000, the resulting liquid is much less
viscous than the
liquefied PEG. This resulted in a significantly faster processing speed with
the liquefied BrijTM
S100 penetrating the agglomerated wall forming particles to leave behind the
cavity defining the
hollow core. The properties of the formed granules were substantially similar
to the PEG + Sodium
Bicarbonate granules with the exception of the solubility parameters since the
BrijTM S100 is
somewhat more hydrophobic than the PEG. Total granule properties are shown in
the tables of
FIG. 17 and FIG. 18. An image of one of the hollow core granules after being
cut is shown in FIG.
27.
EXAMPLE 5 Bentonite + BrijTM S100
Granules were prepared using 5 grams of BrijTM S100 (1.2 mm ¨ 1.6 mm nominal
size) as
binder particles or crystals, which was loaded in the fluidized bed drier with
250 grams of bentonite
(0.100 mm ¨ 0.400 mm nominal size). The fluidized bed dryer was run at 60 C.
Immediately after
reaching the maximum temperature, the granules were cooled to 30 C before
discharging the
formed granules (i.e., residence time of substantially 0). The properties of
the formed granules
were substantially similar to the PEG + Bentonite granules. The Brij TM S100 +
Bentonite granules
exhibited a somewhat higher water stability due to the BrijTM S100 being
somewhat more
hydrophobic than the PEG. Total granule properties are shown in the tables of
FIG. 17 and FIG
18. An image of one of the hollow core granules after being cut is shown in
FIG. 28.
EXAMPLE 6: Sodium Bicarbonate + Paraffin
-67-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
Granules were prepared using 5 grams of paraffin (1.2 mm ¨ 1.6 mm nominal
size) as
binder particles or crystals, which was loaded in the fluidized bed drier with
250 grams of sodium
bicarbonate (0.100 mm ¨ 0.400 mm nominal size). The fluidized bed dryer was
run at 55 C using
a residence time of 0, 5 min, and 10 min at maximum temperature. The granules
were cooled to 30
C before discharging the formed granules. Since paraffin has a lower melting
temperature than
PEG, granule formation was accordingly faster than observed using PEG as the
binder.
Bulk density of the formed granules was found to be lower than when using
sodium
bicarbonate wall forming particles with either PEG 8000 or Brij TM S 1 00 as
the binder ¨ i.e., 556 to
575 grams per liter for the present granules versus 671 to 716 grams per liter
for the granules
formed using PEG 8000 or BrijTM S100 as the binder. As seen in FIG. 14, the
total granule size and
the cavity size was slightly smaller with a 0 min residence time, but
increased with additional
residence time in the fluidized bed. The volume of' cavity was found to
account for approximately
2-5% of the total granule volume (see FIG. 15). The granules appeared to be
relatively weaker than
PEG based granules, which was demonstrated both by attrition testing (see FIG.
16) and crush
strength measurement (the present granules having a strength of 3.5 N). Total
granule properties are
shown in the tables of FIG. 17 and FIG 18.
EXAMPLE 7. Bentonite + Paraffin
Testing indicated that granules formed of bentonite and paraffin lacked
sufficient strength to
be survive the small amount of shear force encountered during preparation in a
fluidized bed
method as described above. Without wishing to be bound by theory, it is
believed that the
hydrophilic nature of the bentonite and the hydrophobic nature of the paraffin
was at least partially
responsible for sufficient incompatibility to allow for granule formation.
Granules, however, we
prepared using a drum-like forming process. Granules were prepared using 5
grams of paraffin (1.2
mm ¨ 1.6 mm nominal size) as binder particles or crystals. These were manually
mixed with
approximately 500 grams of bentonite (0.100 mm ¨ 0.400 mm nominal size) in a
heated pan at 55
C for 5 minutes. After cooling to 30 C, the formed granules we discharged for
evaluation.
The strength of these granules indeed was about 0.5 N (versus about 3.5 N for
samples
made using PEG or BrigTM S100). Because of hydrophobic nature of paraffin, in
the water
absorption testing, water did not propagate in the granulated material and
rather absorbed in the
bentonite particles on the surface, making the result of the measurement
artificially high in this
method ¨ 115%. Most of paraffin-bentonite granules initially floated in water
but disintegrated in
approximately 1 hour. The granules maintained their integrity in oil for
longer than 3 days.
Total granule properties are shown in the tables of FIG. 17 and FIG. 18.
-68-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
EXAMPLE 8: Bentonite + Stearic Acid
Granules were again formed using a manual, drum-like process using 5 grams of
stearic
acid (1.2 mm ¨ 1.6 mm nominal size) as binder particles or crystals. These
were manually mixed
with approximately 500 grams of bentonite (0.100 mm ¨ 0.400 mm nominal size)
in a heated pan at
75 C for 5 minutes. After cooling to 30 C, the formed granules we discharged
for evaluation.
Testing was not attempted using sodium bicarbonate as a wall forming material
since sodium
bicarbonate is known to decompose in the same temperature range as the melting
point of stearic
acid (i.e., around 70 C). The strength and bulk density of the formed
granules were similar to the
values obtained for the granules made with paraffin and bentonite. In this
case, stearic acid
behaved as hydrophobic binder very similar to paraffin. Total granule
properties are shown in the
tables of FIG. 17 and FIG. 18.
EXAMPLE 9. Bentonite + Polycaprolactone
Both the fluidized bed method and the manual mixing method described above
were utilized
in attempting to form hollow core granules using bentonite as a wall forming
material and
polycaprolactone as the binder, but hollow core granules were not successfully
formed.
Polycaprolactone is a hydrophobic polymer with a melting temperature of 60 C,
and even when
using temperatures up to 150 C, the resulting product was a thin coating of
bentonite particles
surrounding a polycaprolactone, solid core. Without wishing to be bound by
theory, it is believed
that the viscosity of the liquefied polycaprolactone was too high to allow
propagation of the liquid
into the bentonite shell. Therefore, in order to successfully form hollow core
granules, it was
determined that the viscosity of the liquefied binder should be sufficiently
low to allow for
propagation into the wall of the wall forming particles. It is likewise
believed that higher viscosity
materials, such as polycaprolactone, but still be utilized when admixed with
another binder material
and/or a viscosity modifying agent so that the total viscosity of the
liquefied binder composition is
sufficiently low for hollow granule formation.
EXAMPLE 10: Formation of Hollow Core Granules by Hydrogel Method
Hydrogel particles were prepared using a 2.4% by weight solution of agar in
water. The
solution was heated to approximately 100 C to dissolve the agar and then
cooled down to 60 C to
maintain a suitable solution viscosity for further processing
Droplets were formed by injecting a stream of the agar solution into a
vegetable oil bath
chilled to approximately 11 C. The droplets spontaneously formed upon contact
with the
vegetable oil, and the formed droplets were separated from the oil and washed
using a soap
solution.
-69-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
The separated and washed droplets were partially dried in air and then coated
with a
conditioning composition formed of talcum powder and silicon oil. The
conditioned gel droplets
were then mixed with bentonite powder until the particles were visually,
substantially uniformly
coated with the bentonite powder. The coated granules were dried under
applied, static heating at a
temperature of about 120 C It was noted that drying time was partially
dependent upon the
thickness of the granule layer. For example, a substantially single thickness
layer dried sufficiently
in approximately 1 hour while a layer of granules approximately 1 cm in
thickness required
approximately 10 hours for desired drying. Although static drying was
utilized, forced air may be
applied to reduce drying time. Some samples were dried at a temperature of
about 160 C, but it
was noted that such drying temperature led to undesired shrinkage/deformation
of the particles.
EXAMPLE 11, Hollow Core Detergent Granules
Hollow core granules were prepared using a powdered detergent composition, and
the
prepared, hollow core granules were tested against the detergent powder in its
native form for
changes in properties. The detergent used was a commercially available
composition sold under
the name Arm and Hammer Crisp Clean Detergent.
To prepare the hollow core granules, particles of Pluriol E 8000 PEG were
loaded into a
Sherwood M501 Fluidized Bed Dryer as a binder material with the powdered
detergent as the wall
forming material, and the materials were processed to form the hollow core
detergent granules.
Three separate runs were carried out to provide three samples of hollow core
detergent granules.
Processing was carried out similarly to the preparation methods described
above in Examples 1 to 3
to provide "lab scale" granule formation in an amount of about 100 grams of
the hollow core
granules (test samples 1 to 3 discussed below). Two additional samples were
prepared at a
manufacturing scale to provide granule formation in amounts of 4 Kg (test
sample 4) and 50 Kg
(test sample 5) to confirm that observed properties were consistently
maintained in large scale
production.
Solubility of the hollow core detergent granules was then compared with
solubility of the
detergent composition in the native form (i.e., "neat"). For each test sample
(hollow core granules
or neat detergent powder), 10 grams of the sample was dissolved in 1600 mL of
room temperature
water, and the time in which all granules dissolved was recorded as the
solubilization time. A 2000
mL glass beaker was used for this test as well as an IKA Werke ELTROSTAR Power-
B Overhead
Stirrer Mixer with a propeller, mixing at 700 rpm. A stopwatch was used to
measure time.
The comparative, neat sample of the detergent powder exhibited a
solubilization time of 9
minutes, 32 seconds. The five test samples of the hollow core detergent
granules exhibited the
following solubilization times: 1) 4 minutes, 30 seconds; 2) 4 minutes, 24
seconds; 3) 4 minutes, 21
-70-
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
seconds; 4) 4 minutes, 37 seconds; and 5) 6 minutes, 11 seconds. As verified
by the testing, the
presentation of the detergent composition in the hollow core format
significant reduced the time to
solubilization compared to the detergent composition in its native form. This
was surprising since
the particle size of the powdered detergent composition was unchanged in the
hollow core form
versus the native form. Rather, the solubilization time of the detergent
composition in the hollow
core form was reduced to be even lower than the solubilization time of the
binder material. This is
believed to be evidence that the overall nature of the hollow core granules
provides a more than
additive effect in improving solubilization properties, and it is expected
that such surprising
improvement in physical properties would extend to other functions, such as
absorptive properties.
Moreover, the significant reduction in solubilization time is believed to
support the expectation that
presentation of other chemicals, compounds, and compositions in the hollow
core format would
improve solubilization of such hollow core products and proportionally improve
release of
components thereof (e.g., cleaners, fertilizers, pesticides, and other
materials as discussed herein).
EXAMPLE 12: Hollow Core Granule Malodor Testing
Hollow core granules were prepared using a variety of different wall forming
materials to
evaluate ability to trap odor-causing chemicals and prevent or reduce
associated odor. Seven total
samples were evaluated: 1) hollow core granules with PEG binder and activated
carbon as the wall
forming material; 2) hollow core granules with PEG binder and zeolite
clinoptilolite as the wall
forming material; 3) hollow core granules with PEG binder and sepiolite clay
as the wall forming
material; 4) hollow core granules with PEG binder and a mixture of sepiolite
clay, zeolite
clinoptilolite, and activated carbon as the wall forming material; 5) hollow
core granules with PEG
binder and sodium bicarbonate as the wall forming material; 6) native sodium
bicarbonate powder;
and 7) native sodium bentonite clay from Bentonite Performance Minerals.
Samples 6 and 7 were
used as comparatives to compare performance of hollow core granules with
materials typically used
in products, such as pet litters, for odor reduction. For each of the hollow
core granule samples 1
through 5, the respective samples were prepared to comprise about 15% to about
25% by weight of
the PEG binder and about 85% to about 75% by weight of the respective wall
forming material.
SEM images of a zeolite clinoptilolite hollow core granule are provided in
FIG. 19A and FIG. 19B.
SEM images of an activated carbon hollow core granule are provided in FIG. 20A
and FIG. 20B.
SEM images of a sodium bicarbonate hollow core granule are provided in FIG 21A
to FIG 21C
To carry out the malodor testing, approximately 100 grams of the test sample
were placed in
an Erlenmeyer vacuum flask witted with a side valve and a one-hole stopper.
The stopper was
fitted with either an additional valve or a Drager ammonia sampling tube
(available from Drager,
Inc.). To each sample, about 20 mL of a synthetic urine (Felinine, which is an
amino acid
-71 -
CA 03176359 2022- 10- 20
WO 2021/216986
PCT/US2021/028821
compound [2-amino-3-propanoic acid] found in cat urine and a precursor via
microbial lyase of a
putative cat pheromone and thiol [3-mercapto-3-methylbutan-1-01]). Levels of
ammonia were
monitored directly from the Drager tube, and a Halimeter (available from
Interscan Corporation)
was used to periodically measure sulfurous gases. The samples were monitored
for approximately
100 hours to measure NH3 (in ppm) and S (in ppb) with lower concentrations of
the respective
odor-causing chemicals being indicative of better performance by the test
material in capturing the
odor-causing chemicals and with higher concentrations of the respective odor-
causing chemicals
being indicative of lesser performance by the test material in capturing the
odor-causing chemicals.
Performance for reducing malodor caused by NH3 is shown in FIG. 22, and
performance for
reducing malodor caused by S is shown in FIG. 23. For both NH3 and S gases,
the hollow core
zeolite clinoptilolite provided the best degree of odor mitigation. As seen in
FIG. 23, in a direct
comparison of hollow core sodium bicarbonate to native sodium bicarbonate for
sulfur odor
mitigation, the hollow core form of the material was shown to exhibit superior
performance.
Specifically, after approximately 100 hours, measured S concentration for the
hollow core sodium
bicarbonate was approximately 26 ppb while the measured S concentration for
native sodium
bicarbonate was approximately 100 ppb.
The "about'. or "substantially" as used herein can indicate that certain
recited values are
intended to be read as encompassing the expressly recited value and also
values that are relatively
close thereto. For example, a value of "about" a certain number or
"substantially" as certain value
can indicate the specific number or value as well as numbers or values that
vary therefrom (+ or -)
by 5% or less, 4% or less, 3% or less, 2% or less, or 1% or less. In some
embodiments, the values
may be defined as being express and, as such, the term "about" or
"substantially" (and thus the
noted variances) may be excluded from the express value.
Many modifications and other embodiments of the inventions set forth herein
will come to
mind to one skilled in the art to which these inventions pertain having the
benefit of the teachings
presented in the foregoing descriptions. Therefore, it is to be understood
that the inventions are not
to be limited to the specific embodiments disclosed and that modifications and
other embodiments
are intended to be included within the scope of the appended claims. Although
specific terms are
employed herein, they are used in a generic and descriptive sense only and not
for purposes of
limitation.
-72-
CA 03176359 2022- 10- 20