Note: Descriptions are shown in the official language in which they were submitted.
WO 2021/219849 PCT/EP2021/061401
1
AZETIDIN-3-YLMETHANOL DERIVATIVES AS CCR6 RECEPTOR MODULATORS
The present invention relates to novel compounds of Formula (I), or
pharmaceutically acceptable salts thereof, and
their use as CCR6 receptor modulators in the treatment or prevention of
various diseases, conditions or disorders
ameliorated by modulating said receptor. Furthermore, the present invention
concerns related aspects such as
pharmaceutical compositions containing one or more compounds of Formula (1)
and processes for the preparation of
said compounds.
Chemokine receptors comprise a family G-protein coupled receptors (GPCRs) that
recognize and bind to peptide
chemokine ligands. The predominant functions of chemokine receptors and their
ligands are to induce leukocyte
trafficking to-and-from lymphoid organs and tissues in the steady state, as
well as in the context of an infection or
inflammation. Additionally, chemokine signaling events can induce the
activation of integrin molecules on the surface
of immune cells, allowing firm adhesion to activated endothelium, facilitating
migration from blood into inflamed tissue
(Montresor A, Frontiers in Imm., 2012; Meissner A, Blood, 2003). Chemokine
receptor 6 (CCR6, aliases BN-1, C-C
CKR-6, CD196, CKRL3, CMKBR6, DCR2, DRY6, GPR29, GPRCY4, STRL22) is a GPCR
mainly expressed on effector
CD4-F T helper cells, but is also present on B cells, CD8-Ecytotoxic T cells,
regulatory T cells (Treg), immature dendritic
cells (DC) and type 3 innate lymphoid cells (IL03) (Cua DJ, Nat Rev lmmunol.
2010 Jul; 10(7):479-89. doi:
10.1038/nri2800). CCR6 binds to the chemokine CCL20 (chemokine (C-C motif)
ligand 20) (Greaves DR, J Exp Med.
1997 Sep 15; 186(6):837-44. doi: 10.1084/jem.186.6.837.). CCL20 is also called
macrophage inflammatory protein 3a
(MIP-3a), liver and activation-regulated chemokine (LARC), or Exodus-1
(Schutyser E, Cytokine Growth Factor Rev.
2003 Oct; 14(5):409-26. doi: 10.1016/s1359-6101(03)00049-2). CCR6/CCL20
interactions dictate the humoral
response in the intestinal mucosa and are required for lymphocyte homeostasis
in the mucosa of the small intestine
(Cook ON, Immunity. 2000 May; 12(5):495-503. doi: 10.1016/s1074-7613(00)80201-
0). Under steady state conditions,
CCR6 and CCL20 regulate production of IgA in the intestine, where CCL20
expressed in Peyer's patches guides
CCR6-FlgA+ B cells to the mucosa and secretory IgA can be released into the
gut lumen (Lin YL, Front lmmunol. 2017;
8:805. doi: 10.3389/fimmu.2017.00805; Reboldi A, Science. 2016 May 13;
352(6287):aaf4822. doi:
10.1126/science.aaf4822). Under inflammatory conditions, expression of CCL20
is highly upregulated by
proinflannmatory cytokines including IL-17A, TNFa and IL-1b in both
endothelial and epithelial cells (Hlarper EG, J Invest
Dermatol. 2009 Sep; 129(9):2175-83. doi: 10.1038/jid.2009.65; PLoS One. 2015;
10(11):e0141710. doi:
10.1371/journal.pone.0141710) and tissue fibroblasts (Hattori T, Mediators
Inflamm. 2015; 2015:436067. doi:
10.1155/2015/436067). Interleukin (IL)-17A expression is restricted to cells
expressing the transcription factor RORgt
(Cell. 2006 Sep 22; 126(6):1121-33. doi: 10.1016/j.ce11.2006.07.035). IL-17A
expression has been shown to segregate
with CCR6 expression on human T cells (Singh SP, J lmmunol. 2008 Jan 1;
180(1):214-21. doi:
10.4049/jimmuno1.180.1.214; Nat Immunol. 2007 Jun; 8(6):639-46. doi:
10.1038/ni1467). CCR6 was also described as
a target gene of RORgt (PLoS One. 2017; 12(8):e0181868. doi:
10.1371/journal.pone.0181868; Skepner J, J I mmunol.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
2
2014 Mar 15; 192(6):2564-75. doi: 10.4049/jimmuno1.1302190), thus clarifying
the co-expression of IL-17A and CCR6
in RORgt+ cell types.
Certain disclosures in the prior art may be regarded as relating to modulation
of CCR6. For instance, Tawaraishia et al.
(Bioorganic & Medicinal Chemistry Letters, Volume 28, Issue 18, 2018, Pages
3067-3072, ISSN 0960-894X,
https://doi.org/10.1016/j.bmc1.2018.07.042) disclose a series of
benzenesulfonyl-aminocyclohexane derivatives as
selective CCR6 inhibitors. CN103588697 teaches sulfonamide derivatives as CCR6
antagonists and their use in
treating CCR6-mediated diseases such as autoimmune diseases, inflammation,
psoriasis, multiple sclerosis or cancer.
W02014/075580 describes the use of aurintricarboxylic acid for targeting
chemokine receptors. W02015/084842
teaches certain sulfonamides which may be used in treating CCR6 related
diseases. W02017/087607,
W02010/131145, W02013/061004, W02013/061005, W02019/036374 and W02020/058869
provide certain
cyclobutenediones for use in the treatment of chemokine/CCR6 related diseases.
W02019/136370 teaches a method
of treating a certain type of psoriasis. W02019/147862 proposes azetidine
derivatives which may be used as chemokine
modulators.
Further, W01999/43664 discloses certain pyrrolidinones with anti-inflammatory
and analgesic properties. In
W02019/105915 certain heterocyclic compounds are provided which may be used as
MAGL inhibitors.
W02015/057626, US2015/0105366, W02014/062658, W02015/057205 and Tanis VM et
al. (Bioorg Med Chem Lett.
2019 Jun 15; 29(12):1463-1470. doi: 10.1016/j.bmc1.2019.04.021) relate to
modulators of the RORyt receptor which
may be used in treating rheumatoid arthritis or psoriasis. W003/022808
proposes certain azetidine derivatives for use
as pesticides. W02008/103426 and W02007/022351 disclose certain quaternary
ammonium compounds useful as
muscarinic receptor antagonists. W02006/136830 teaches certain heteroaryl-
alkylamines as protein kinase inhibitors.
W091/13359 proposes heterocyclic cholinergic enhancers. US28141 teaches
certain pyrrolidines which may be used
for treating depression. GB 1304650 discloses spasmolytic pyrrolidines.
US3479370, US3489769, US3499002, US
3542807 and US3651085 relate to certain pyrrolidines with
analgesic/tranquilizing activity.
The present CCR6 modulators may be useful, alone, or in combination in the
treatment or prevention of the following
diseases or disorders: Rheumatoid arthritis (RA) causes chronic inflammation
of the joints and chemokines regulate
infiltration of the inflamed synovium by inflammatory cells. RA is
characterized by the increased release of CCL20 and
the subsequent recruitment of CCR6-F T cells to the inflamed joints. CCL20 is
highly expressed in the synovial fluid of
RA (Hirota, J Exp Med. 2007 Nov 26; 204(12):2803-12. doi:
10.1084/jem.20071397; Matsui T, Clin Exp lmmunol. 2001
Jul; 125(1):155-61. doi: 10.1046/.1365-2249.2001.01542.x). In patients with
RA, CCR6+ Th cells have been found in
the inflamed synovium and increased proportions of peripheral blood CCR6+ Th
cells have been found in patients with
early RA (van Hamburg JP, Arthritis Rheum. 2011 Jan; 63(1):73-83. doi:
10.1002/art.30093; Leipe J Arthritis Rheum.
2010 Oct; 62(10):2876-85. doi: 10.1002/art.27622; Nistala K, Arthritis Rheum.
2008 Mar; 58(3):875-87. doi:
10.1002/art.23291). The production of CCL20 is known to be up-regulated in
synovium explants or fibroblast-like
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
3
synoviocytes from RA patients after stimulation of TNF-a, IL-lb and IL-17
(Matsui T, Olin Exp lmmunol. 2001 Jul;
125(1):155-61. doi: 10.1046/.1365-2249.2001.01542.x; J lmmunol. 2001 Nov 15;
167(10):6015-20. doi:
10.4049/jimmuno1.167.10.6015; Chevrel G, Ann Rheum Dis. 2002 Aug; 61(8)730-3.
doi: 10.1136/ard.61.8.730).
CCR6+ B cells in RA synovium have been reported, contributing to pathogenesis
by antigen presentation, autoantibody
production and/or inflammatory cytokine production. Furthermore, Rituximab is
an efficacious therapy for RA (Cohen
SB, Arthritis Rheum. 2006 Sep; 54(9):2793-806. doi: 10.1002/art.22025),
supporting a role for CCR6+ B cells in RA
pathogenesis. Additionally, CCR6-deficient mice have impaired IgG1-dependent
memory B cell responses (J Immunol.
2015 Jan 15; 194(2):505-13. doi: 10.4049/jimmuno1.1401553). Preclinical rodent
models showed that CCR6-deficient
mice developed a less severe joint inflammation in the collagen-induced
arthritis (CIA) model. Reduced production of
collagen-specific antibodies in 00R6-deficient mice were observed compared to
WT mice, and arthritic inflammation
was also reduced (J Cell Mol Med. 2018 Nov; 22(11):5278-5285. doi:
10.1111/jcmm.13783). Furthermore, depletion of
CCR6+ cells reduced the severity of SKG arthritis (Hirota K, J Exp Med. 2007
Nov 26; 204(12):2803-12. doi:
10.1084/jem.20071397).
CCR6+ Th17 are increased in peripheral blood in ankylosing spondylitis
patients (Shen H, Arthritis Rheum. 2009 Jun;
60(6):1647-56. doi: 10.1002/art.24568). Circulating interleukin-17-secreting
interleukin-23 receptor-positive y/6 T cells
were also reported in patients with active ankylosing spondylitis (Kenna TJ,
Arthritis Rheum. 2012 May; 64(5):1420-9.
doi: 10.1002/art.33507). Secukinumab, an IL-17A inhibitor, in was shown to be
efficacious in ankylosing spondylitis
(AS) (Baeten D, N Engl J Med. 2015 Dec 24; 373(26):2534-48. doi:
10.1056/NEJMoa1505066). CD32B expression on
memory B cells in AS was increased and was associated with disease activity.
Furthermore, CCR6* cytotoxic T-cells
and CD3213 memory B-cells were highly enriched within the synovial compartment
of AS patients (Sucur A, Olin Exp
Rheumatol. 2019 Nov 20; PMID: 31820725).
Psoriasis is a commonly occurring autoimmune skin disease. The role of Th17-
associated cytokines has been clinically
validated and their role in psoriatic inflammation confirmed (Paul C, J Fur
Acad Dermatol Venereol. 2015 Jun;
29(6):1082-90. doi: 10.1111/jdv.12751). An IL-17R-blocking antibody
(brodalumab, AMG 827) were shown to reduce
clinical manifestations of psoriasis and also to reduce CCL20 expression in
skin biopsies from psoriasis patients (Papp
KA, N Engl J Med. 2012 Mar 29; 366(13):1181-9. doi: 10.1056/NEJMoa1109017).
Also, an IL-23 neutralizing antibody
(guselkumab) was shown to be efficacious in reducing psoriatic inflammation
(Reich K, Lancet. 2019 Sep 7;
394(10201):831-839. doi: 10.1016/S0140-6736(19)31773-8). CCR6-deficient mice
failed to develop psoriasiform skin
lesions following intradermal IL-23 injections (Hedrick MN, J Olin Invest.
2009 Aug; 119(8):2317-29. doi:
10.1172/jci37378). Small molecule CCR6 antagonists have also been shown to be
efficacious in the Aldara and IL-36a-
injection mouse psoriasis models (Campbell JJ, J lmmunol. 2019 Mar 15;
202(6):1687-1692. doi:
10.4049/jimmuno1.1801519; Campbell JJ, J lmmunol. 2017 Nov 1; 199(9):3129-
3136. doi: 10.4049/jimmuno1.1700826).
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
4
Furthermore, CCR6-deficient mice have been shown to be protected from
imiquimod-induced ear swelling (Yu S, J
Invest Dermatol. 2019 Feb; 139(2): 485-488. doi: 10.1016/flid.2018.07.036).
Anti-CCR6 neutralizing antibodies have also shown efficacy in Aldara induced
ear swelling in mice (Robert R, JCI
Insight. 2017 Aug 3; 2(15): e94821. Published online 2017 Aug 3. doi:
10.1172/jci.insight.94821). An engineered
disulfide-linked CCL20 dimer, which binds CCR6 but inhibits T cell migration,
was shown to reduce skin swelling in an
IL-23¨dependent mouse model of psoriasis (Getschman AE, Proc Natl Acad Sci U S
A. 2017 Nov 21;114(47):12460-
12465. doi: 10.1073/pnas.1704958114). Collectively, these data show that a
positive feedback consisting of epidermal
and dermal production of CCL20, potent recruitment of CCR6+ T cells or into
inflamed psoriatic skin, their activation by
IL-23 and their expression of IL-17A and IL-22, drives a pathogenic Th17
response in psoriatic skin lesions. Inhibition
of CCR6 has therefore been recognized as a potential therapeutic pathway to
treat psoriasis (Hedrick MN, Expert Opin
Ther Targets. 2010 Sep;14(9):911-22. doi: 10.1517/14728222.2010.504716;
Mabuchi T, J Dermatol Sci. 2012
Jan;65(1):4-11. doi: 10.1016/j.jdermsci.2011.11.007). CCR6 expression was
shown to be upregulated in synovial
membranes of psoriatic arthritis (PsA) patients (Dolcino M, PLoS One. 2015 Jun
18;10(6):e0128262. doi:
10.1371/journal.pone.0128262). IL-17A- and GM-CSF-expressing CD4+ T cells
isolated from synovial fluid of PsA
patients also expressed CCR6 (Al-Mossawi et al., Nat Commun. 2017 Nov
15;8(1)1510. doi: 10.1038/s41467-017-
01771-2). CCL20 was shown to be highly upregulated in synovial fluid retrieved
from PsA patients (Melis L, Ann Rheum
Dis. 2010 Mar;69(3):618-23. doi: 10.1136/ard.2009.107649).
Additional inflammatory skin disorders including rosacea have been shown to
have highly elevated levels of CCL20 in
inflamed skin (Buhl T, JID, 2015).
CCR6 and CCL20 are highly elevated in active Crohn's disease (CD) and
ulcerative colitis (UC) (Skovdahl et al., PLoS
One. 2015 Nov 4;10(11):e0141710. doi: 10.1371/journal.pone.0141710). Increased
enterocyte CCL20 production has
been proposed to play an important role in lymphocyte recruitment to the
colonic epithelium in irritable bowel disease
(IBD) (Kwon JH, Gut. 2002 Dec; 51(6):818-26. doi: 10.1136/gut.51.6.818). CCL20
and CCR6 expression are also
correlated with histological severity in rectum resected from UC patients.
CCL20 expression in chronic UC is higher
than that in acute UC after pathological examination (Uchida K, Gastroenterol
Res Pract. 2015; 2015:856532. doi:
10.1155/2015/856532). Expression of CCL20 was significantly up-regulated in
the PBMCs of patients with UC
compared with those of normal healthy controls. UC groups treated with
sulfasalazine and GC showed decreases of
CCL20 expression in PBMCs, accompanied by ameliorated disease. TNFa or IL-113-
induced CCL20 secretion was
strongly reduced by sulfasalazine and/or GC treatment of human intestinal
epithelial cell lines (Lee HJ, 2 Inflamm Bowel
Dis. 2005 Dec; 11(12):1070-9. doi: 10.1097/01.mib.0000187576.26043.ac). CCR6
deficiency resulted in reduced
intestinal pathology in mice treated with dextran sodium sulfate (DSS) to
induce colonic inflammation (Varona R, Eur J
lmmunol. 2003 Oct; 33(10):2937-46. doi: 10.1002/eji.200324347).
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
Th17 cells expressing CCR6 were shown to be important effectors mediating dry
eye disease (DED), an inflammatory
state at the ocular surface, potentially resulting in corneal perforation.
Antibody-mediated neutralization of CCL20 in a
DED mouse model reduced Th17 recruitment into the ocular surface, resulting in
improved clinical readouts (Dohlman
TH, Invest Ophthalmol Vis Sci. 2013 Jun 12; 54(6):4081-91. doi:
10.1167/iovs.12-11216). Inhibition of the CCR6/CCL20
5 axis was therefore proposed as a therapeutic mechanism to treat DED.
CCR6 expression has been described on T cells isolated from the cerebrospinal
fluid of multiple sclerosis (MS) patients
(van Langelaar J, Brain, 2018 May 1; 141(5):1334-1349. doi:
10.1093/brain/awy069). CCR6 expression was also shown
on T cells infiltrating the inflamed CNS in experimental autoimmune
encephalomyelitis (EAE) (Mony JT, Front Cell
Neurosci. 2014; 8:187. doi: 10.3389/fnce1.2014.00187). Furthermore, CCL20 gene
polymorphisms have been shown
to be associated with MS patient cohorts (El Sharkav et al., Gene. 2019 Feb
15; 685:164-169. doi:
10.1016/j.gene.2018.11.006). Preclinical data has shown that CCR6 is important
for development of EAE (Reboldi A,
Nat lmmunol. 2009 May; 10(5):514-23. doi: 10.1038/ni.1716). This finding was
confirmed in later study, showing that
CCR6-deficient mice were resistant to disease induction with reduced peak
severity. In the same study, vaccination
with hCCL20 produced an anti-mouse CCL20 response in the host mice, which was
sufficient to reduce clinical scores
(Abraham M, Olin lmmunol. 2017 Oct; 183:316-324. doi:
10.1016/j.clim.2017.09.018). However, conflicting data exists
concerning the role for CCR6 in EAE development (J Neuroimmunol. 2009 Aug 18;
213(1-2):91-9. doi:
10.1016/j.jneuroim.2009.05.011). EAE severity and histopathology were
significantly reduced after injection of anti-
CCL20 upon first clinical manifestations (Kohler RE, J lmmunol. 2003 Jun 15;
170(12):6298-306. doi:
10.4049/jimmuno1.170.12.6298). Anti-CCR6 neutralizing antibodies were shown to
reduce the severity of EAE in mice
(Robert R, JCI Insight. 2017 Aug 3; 2(15): e94821. Published online 2017 Aug
3. doi: 10.1172/jci.insight.94821). IL-6
and IL-17 increase the expression of CCL20 from murine astrocytes (Meares GP,
Glia. 2012 May; 60(5):771-81. doi:
10.1002/glia.22307).
CCR6 and CCL20 are proposed to influence kinetics of germinal center (GC)
formation and B cell responses and CCR6
is considered a marker memory B cell precursors in both mouse and human
germinal centers (Suan D, Immunity. 2017
Dec 19; 47(6):1142-1153.e4. doi: 10.1016/j.immuni.2017.11.022). Expression of
CCR6 on naive, pre-GC, GC/plasma
cell and memory B cells in peripheral B cells of systemic lupus erythematosus
(SLE) patients was increased (Lee AYS,
Olin Rheumatol. 2017 Jun; 36(6):1453-1456. doi: 10.1007/s10067-017-3652-
3).CD4+CCR6+ cells may also contribute
to disease severity in SLE patients and were shown to be increased in anti-
DNA+ SLE patients, which correlated with
disease severity and erythrocyte sedimentation rate (Zhong W, PeerJ. 2018;
6:e4294. doi: 10.7717/peerj.4294).
Increased CCR6 expression in the salivary glands of patients with primary
Sj6gren's syndrome (pSS) was demonstrated
[Scand J lmmunol. 2020 Mar;91(3):e12852. doi: 10.1111/sji.12852]. A trend
towards increased CCL20 mRNA
expression was also observed. Significant reductions in CCR6+ Th cells (both
CCR9- and CCR9+) in the circulation of
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
6
patients with pSS as compared with healthy controls (HCs) were demonstrated
[Scand J lmmunol. 2020
Mar;91(3):e12852. doi: 10.1111/sji.12852].
In an animal model of autoimmune hepatitis (AIH), administering anti-TNF-a
suppressed hepatic CCL20 expression.
Mice receiving anti-CCL20 showed reduced AIH. Furthermore, TNFa stimulation
enhanced CCL20 expression in
hepatocytes. These findings suggest that TNFa is essential in the induction of
AIH through upregulation of hepatic
CCL20 expression, which recruits CCR6+ T cells which drive pathology (Clin
lmmunol. 2013 Jan; 146(1):15-25. doi:
10.1016/j.dim.2012.10.008).
The present CCR6 modulators may be useful, alone, or in combination in the
treatment or prevention of autoimmune
diseases or disorders including Posterior uveitis, allergic conjunctivitis,
allergic disease in the gastrointestinal tract, type
I diabetes and endometriosis (Medicina (Kaunas). 2018 Nov 16; 54(5). doi:
10.3390/medicina54050088). CCR6
modulators may also be useful, alone or in combination, to treat diseases of
the ocular surface in which elevated levels
of IL-17A have been recorded, including meibomian gland dysfunction; GVHD,
graft-versus host disease; autoimmune
keratitis, filamentary keratitis, dry eye syndrome with rheumatic arthritis;
dry eye syndrome without systemic disease;
Stevens-Johnson syndrome. (J Korean Med Sci. 2011 Jul;26(7):938-44. doi:
10.3346/jkms.2011.26.7.938).
The present CCR6 modulators may be useful, alone, or in combination in the
treatment or prevention of malignant
diseases. Modulation of the CCR6/CCL20 axis using siRNA, shRNA, CCR6 knock-out
animals, CCL20 ligand treatment
or antibodies has been shown to alter tumor growth and metastatic processes in
experimental disease models as single
agents, or in combination with immunotherapy (such as especially P01 and/or
PDL1 blockade) for the prevention /
prophylaxis or treatment of cancers.
The therapeutic potential of modulating this axis for the treatment of
malignancies has been described in tumor mouse
models using small interfering RNA (siRNA) or small hairpin RNA (shRNA)-
mediated silencing of CCR6 or CCL20.
Specifically, in a mouse model of cutaneous T cell lymphoma (My-La cells), Abe
et al. reported that the administration
of a CCR6-targeted siRNA prolonged survival of animals when compared with
control animals (Oncotarget. 2017 Jan
31; 8(5):7572-7585. doi: 10.18632/oncotarget.13810.). Using another approach,
Ito and colleagues demonstrated that
mice, injected with T lymphoma cells (My-La) harboring a CCR6 silencing siRNA
construct, survived significantly longer
than mice injected with control cells (Blood. 2014 Mar 6; 123(10):1499-511.
doi: 10.1182/blood-2013-09-527739.). Zhu
and co-workers demonstrated that, the average volume and weight of tumor
nodules in mice injected subcutaneously
with a set of colorectal cancer cell lines was decreased when CCR6 was
silenced in the cancer cells by means of
shRNA (PMID Biochim Biophys Acta Mol Basis Dis. 2018 Feb; 1864(2):387-397.
doi: 10.1016/j.bbadis.2017.10.033.).
In glioblastoma xenograft models using patient-derived glioblastoma cell
lines, mice injected with cells harboring a
shRNA construct silencing CCR6 expression survived longer than those injected
with control cells. In addition, histology
and immunohistochemistry revealed that tumors formed by glioma cells with CCR6-
targeting shRNA were much
smaller, and tumor vessel formation was significantly lower versus control
tumors. Collectively, these data further
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
7
support the notion that CCR6 signaling enhances the oncogenic potential of
malignancies including lymphoma,
colorectal tumors and glioblastoma (Oncogene. 2018 Jun; 37(23):3070-3087. doi:
10.1038/s41388-018-0182-7.).
Specifically, the implication of the CCR6/CCL20 axis in tumorigenesis using
CCR6 knock-out animals was reported in
the literature. In the CMT93 mouse model of colorectal cancer (CRC), the
infiltration of T regulatory cells was completely
prevented in tumors of mice deficient in CCR6 in comparison to wildtype
animals. The reported data further suggest
that the homing and trafficking of tumor-infiltrating T regulatory cells to
the tumor mass is dependent on the chemokine
receptor CCR6 in vivo (PLoS One. 2011 Apr 29; 6(4):e19495. doi:
10.1371/journal.pone.0019495.). According to Nandi
and colleagues, in a mouse model of spontaneous intestinal tumorigenesis
[APCMIN/-F mice, heterozygous for a
mutation in the adenomatous polyposis coli (APC) gene], mice deficient in CCR6
had a lower occurrence of
spontaneous intestinal tumorigenesis (PLoS One. 2014; 9(5):e97566. doi:
10.1371/journal.pone.0097566.).
The potential role of the CCR6/CCL20 axis in tumorigenesis was also
demonstrated by administrating the recombinant
CCL20 chemokine. Specifically, in a mouse model of colorectal cancer (CMT93
cells), Liu and colleagues showed that
tumor size was significantly increased in mice treated with recombinant mouse
CCL20 compared with PBS controls,
suggesting a critical role for CCL20 in colorectal cancer growth and
development (PLoS One. 2011 Apr 29; 6(4):e19495.
doi: 10.1371/journal.pone.0019495.).
Specifically, using neutralizing CCL20 antibodies, the potential role of the
CCR6/CCL20 axis in tumor promotion was
demonstrated in the literature using mouse models. Ikeda and co-workers used a
specific cutaneous T cell lymphoma
(CTCL) mouse model in which animals succumb to metastasis of CTCL cells into
multiple organs. However,
administration of a neutralizing CCL20 antibody significantly prolonged the
survival of the xenografted mice
(Oncotarget. 2016 Mar 22; 7(12):13563-74. doi: 10.18632/oncotarget.6916.). Lee
and co-workers described in a mouse
model of metastatic breast cancer (MDA-MB-231 cells were injected into the
left cardiac ventricles of nude mice) that
the administration of an anti-CCL20 antibody prevented the development of bone
metastasis, one of the major site of
breast cancer metastasis in human disease (Sci Rep. 2017 Aug 29; 7(1):9610.
doi: 10.1038/s41598-017-09040-4.). In
a humanized mouse model of nasopharyngeal carcinoma, Mrizak et al. observed a
significant decrease of T regulatory
cell recruitment into the tumor when mice were injected with anti-CCL20
monoclinal antibody in comparison to sham
treated animals (J Natl Cancer Inst. 2015 Jan; 107(1):363. doi:
10.1093/jnci/dju363.). In addition, in a mouse model of
hepatocarcinoma (Nepal-6 cells), blockade of CCL20 activity in immunocompetent
mice using an anti-CCL20 antibody,
attenuated tumor incidence, restrained tumor growth and distal metastasis.
Moreover, the authors reported that in this
mouse model, tumor angiogenesis was significantly inhibited upon CCL20
neutralization. (He at al., PMID 28560063 -
Am J Cancer Res. 2017; 7(5):1151-1163.). Using the same mouse model, the
administration of the anti-CCL20
neutralizing antibody remarkably reduced the infiltration of T regulatory
cells into the tumor, especially CCR6 positive T
regulatory cells, and significantly decreased tumor growth. Antitumor efficacy
was further enhanced when the mice
were co-treated with an anti-PDL-1 antibody. Collectively these data sets
suggest that CCL20 blockade could abrogate
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
8
anti-PD-L1 resistance in a mouse model of hepatocarcinoma by inhibiting T
regulatory recruitment to the tumor
(Hepatology. 2019 Jul; 70(1):198-214. doi: 10.1002/hep.30593.).
Specifically, the potential role of the CCR6/CCL20 axis in tumor metastasis
was described in the literature.
Dellacasagrande and colleagues reported that, in a mouse model of
plasmacytoma, tumor cells that disseminated to
the liver overexpressed functional CCR6 in comparison with tumor cells of the
primary tumor (from s.c. injection of
mouse plasmacytoma (MOPC315)). The same authors found that CCR6 was
overexpressed in small liver metastases
of colon, thyroid and ovarian carcinomas compared with normal liver (Scand J
lmmunol. 2003 Jun; 57(6):534-44.
doi: 10.1046/j.1365-3083.2003.01263.x.).
Furthermore, the present CCR6 modulators may be useful, alone, or in
combination in the treatment or prevention of
cancers where the expression of CCR6 and/or CCL20 correlates with disease
progression and resistance to standard
treatment care. Specifically, the correlation of CCR6 expression with disease
progression was described in the literature
for numerous cancer indications. For example, in renal cell carcinoma CCR6
expression is associated with a lower
overall survival (Cancers (Basel). 2019 Dec 30; 12(1). doi:
10.3390/cancers12010089.). In colorectal cancer, tumor
expression of CCR6 positively correlates with metastasis and upregulated CCR6
predicts poor survival, shorter disease-
free survival (PLoS One. 2014; 9(6):e101137. doi:
10.1371/journal.pone.0101137.), and poorer 5-year overall survival
(Biochim Biophys Acta Mol Basis Dis. 2018 Feb; 1864(2):387-397. doi:
10.1016/j.bbadis.2017.10.033.). In ovarian
cancer high CCR6 mRNA expression was also associated with a worse prognosis
(Cancer Lett. 2020 Mar 1; 472:59-
69. doi: 10.1016/j.canlet.2019.12.024.). CCR6 expression was associated with
rectal cancer aggressiveness, indeed,
high-level expression of CCR6 protein was more common in non-responders to
radiotherapy than in responders
(Cancer Res Treat. 2018 Oct; 50(4):1203-1213. doi: 10.4143/crt.2017.538.). The
expression level of CCR6 in prostate
cancer was associated with clinical and pathologic features of more advanced
and aggressive disease (J Cancer Res
Olin Oncol. 2008 Nov; 134(11):1181-9. doi: 10.1007/s00432-008-0403-5.). In non-
small cell lung cancer (NSCLC) high
CCR6 expression was associated with shorter disease-free survival and
conferred a disease stage-independent 5-fold
increased risk for disease recurrence (PLoS One. 2011; 6(9):e24856. doi:
10.1371/journal.pone.0024856.).
Hepatocarcinoma patients with increased infiltrated CCR6 positive immune cells
in tumor tissues showed a poorer
prognosis (Am J Cancer Res. 2017; 7(5):1151-1163.).
Analogous to CCR6, expression of its ligand CCL20 has been reported to
correlate with poorer disease outcome for
several indications. Specifically, in breast cancer, elevated CCL20 expression
significantly correlated with lower overall
free survival, lower percent metastasis free survival (Sci Rep. 2017 Aug 29;
7(1):9610. doi: 10.1038/s41598-017-
09040-4.), with higher histological grade, higher Ki67 index, and axillary
lymph node metastases. Moreover, breast
tumor CCL20 expression positively correlated with expression of FOXP3, a
marker of T regulatory cells. Patients with
axillary lymph node metastases, and concomitant elevation in CCL20 expression
and FOXP3-positive T regulatory
cells, had the worst overall survival. (Medicine
(Baltimore). 2019 Dec; 98(50):e18403.
CA 03177335 2022- 10- 28
WO 2021/219849 PCT/EP2021/061401
9
doi: 10.1097/MD.0000000000018403.). In NSCLC higher expression of CCL20 was
associated with a lower overall
survival (Biomed Pharmacother. 2015 Feb; 69:242-8. doi:
10.1016/j.biopha.2014.12.008.)(Cancer Lett. 2015 Jul
10; 363(1):60-70. doi: 10.1016/j.canlet.2015.04.005.). Analogous to NSCLC,
hepatocellular carcinoma patients with
high CCL20 expression had poorer overall survival and poorer recurrence-free
survival. The same authors described
that CCL20 expression was significantly associated with tumor size, tumor
number, vascular invasion, tumor
differentiation and tumor recurrence (J Gastrointest Surg. 2012 Apr; 16(4):828-
36. doi: 10.1007/s11605-011-1775-4.).
In addition to CCR6 or CCL20 alone, correlation of CCR6/CCL20 co-expression
with disease progression is stated in
literature. Indeed, overexpression of both, CCL20 and CCR6, was detected in
high-grade glioma tissues as compared
to low-grade tissues and increased with ascending tumor World Health
Organization (WHO) grades. Particularly glioma
patients with CCL20/CCR6 co-expression had the shortest overall survival (Med
Oncol. 2012 Dec; 29(5):3491-7.
doi: 10.1007/s12032-012-0314-9.).
Besides, CCR6 and/or CCL20 expression correlates with enhance chemotherapeutic
resistance and is associated with
metastasis. Indeed, CCL20 expression can increase the chemotherapeutic
resistance of breast cancer cells (PLoS
Biol. 2018 Jul; 16(7):e2005869. doi: 10.1371/journal.pbio.2005869.). Rubie and
colleagues describe that in colorectal
liver metastases (CRLM) and in human samples of hepatocellular carcinoma
(HCC), significant up-regulation of
CCL20/CCR6 was detected (RT-PCR). Moreover, CCL20 was significantly
overexpression in colorectal liver
metastases as compared to the primary HCC, indicating an involvement of the
CCL20/CCR6 ligand-receptor pair in the
carcinogenesis and progression of hepatic malignancies (World J Gastroenterol.
2006 Nov 7; 12(41):6627-33.
doi: 10.3748/wjg.v12.i41.6627.).
The present CCR6 modulators may be useful, alone, or in combination in the
treatment or prevention of diseases or
disorders where CCR6 and/or CCL20 are expressed or overexpressed in patient
samples or cancer cell lines.
Specifically, the chemokine receptor CCR6 is described to be expressed in
several cancer types or cancer cell lines in
the literature. Lu and coworkers describe that CCR6 expression was higher in
laryngeal cancer tissues compared with
their normal controls. The authors reported that CCR6 was also expressed in
commonly used laryngeal cancer cells
such as TU212, M4E, M2E and Hep-2
(Biomed Pharmacother. 2017 Jan; 85:486-492.
doi: 10.1016/j.biopha.2016.11.055.). Based on gene expression data from
malignant melanoma, among the biological
networks reported CCR6 gene was described and characterized as a valuable
factor involved in immune responses
and tumor progression (PLoS One. 2018; 13(1):e0190447. doi:
10.1371/journal.pone.0190447.) Whole exome
sequencing in 21 MALT lymphomas of the salivary gland and thyroid revealed
that CCR6 was expressed
(Haematologica. 2018 Aug; 103(8):1329-1336. doi:
10.3324/haemato1.2018.191601.). In samples of adult human T-cell
leukemia / lymphoma (ATLL) transcripts of CCR6 were detected, and CCR6 was
further found at the protein level using
flow cytometric analysis (Leuk Lymphoma. 2006 Oct; 47(10):2163-73. doi:
10.1080/10428190600775599.). In patient-
derived prostate cancer samples the gene expression of CCR6 (mRNA) was
significantly higher in tumor tissue as
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
compared to adjacent normal tissue (Cancer Res Treat. 2015 Apr; 47(2):306-12.
doi: 10.4143/crt.2014.015.). CCR6
expression was detected in commonly used cancer cell lines, indeed, according
to Mays and co-workers, in salivary
adenoid cystic carcinoma cells SACC-83, among other CC chemokine receptors,
CCR6 was expressed using RI-FOR
gene analysis (Anticancer Res. 2016 Aug; 36(8):4013-8.). According to Moller
and colleagues, in multiple myeloma
5 (MM) cell lines including U266 1970, U-266 1984, U-1958, Karpas 707, LP-
1,28 L-363, HL407E and HL407L.3, CCR6
was also expressed (Leukemia. 2003 Jan; 17(1):203-10. doi:
10.1038/sj.leu.2402717.).
Analogous to CCR6, the ligand CCL20 was reported to be expressed in multiple
tumor samples and tumor cell lines in
the literature. For example, Zhang and co-workers demonstrated that in samples
from NSCLC patients, using RT-PCR,
CCL20 showed higher expression in tumor samples than in samples of adjacent
tissue, this was also verified at the
10 protein level using immunohistochemistry
(Biomed Pharmacother. 2015 Feb; 69:242-8.
doi: 10.1016/j.bi0pha.2014.12.008.). Gene expression analysis of
cholangiocarcinoma samples and corresponding
normal tissue revealed CCL20 to be one of the genes most significantly over-
expressed in malignant vs healthy tissue
(EXCLI J. 2020; 19:154-166. doi: 10.17179/excli2019-1893.). CCL20 expression
was also reported in multiple myeloma
(MM) human samples (Cancer Res. 2008 Aug 15; 68(16):6840-50. doi: 10.1158/0008-
5472.CAN-08-0402.). Besides,
according to Rubies et al., CCL20 mRNA and protein was significantly up-
regulated in pancreatic carcinoma (8-fold) as
compared to matched normal pancreas in which CCL20 was weakly expressed (J
Transl Med. 2010 May 10; 8:45.
doi: 10.1186/1479-5876-8-45.). .). CCL20 is also expressed in oral squamous
cell carcinoma (IHC staining) and Lee et
al. reported that expression is enriched in human CCR6+ regulatory T cells
with superior suppressive activity (J
lmmunol. 2017 Jul 15; 199(2):467-476. doi: 10.4049/jimmuno1.1601815.).
In addition to CCR6 or CCL20 alone, the co-expression of both CCR6 and CCL20
is reported for samples of cancer
patients and cancer cells lines in literature. Both genes have been described
to be expressed in adult T-cell
leukemia/lymphoma patient samples (Microarray and IHC protein staining) (Int J
Oncol. 2014 Sep; 45(3):1200-8.
doi: 10.3892/ijo.2014.2524.) and in CTCL. In the latter, CCL20 and CCR6 were
detected at the mRNA and protein
levels (Olin Cancer Res. 2011 Dec 15; 17(24):7529-38. doi: 10.1158/1078-
0432.CCR-11-1192.). Transcriptomic
analysis (nanostring) of samples of hepatocellular carcinoma revealed CCR6 and
CCL20 expression. Moreover, a
chemotactic gradient between non-tumor and tumor tissues was reported and a
recruitment process of T regulatory
cells, tumor associated macrophages and natural killer cells involving the
CCR6/CCL20 axis suggested (Proc Natl Acad
Sci U S A. 2017 Jul 18; 114(29):E5900-E5909. doi: 10.1073/pnas.1706559114.).
Similarly, Guo and co-workers
reported CCR6 and CCL20 upregulation in hepatocarcinoma lesions compared to
healthy tissue as well as CCR6 and
CCL20 expression in hepatocarcinoma cell lines (L02, Li-/, Huh-7, SNU-387,
Hep3B) (Oncol Rep. 2019
Sep; 42(3):1075-1089. doi: 10.3892/or.2019.7221.). In human colorectal cancer,
both CCL20 and CCR6 are expressed
according to Nandi et al. (IHC protein staining). Both, CCR6 and CCL20, were
found to be highly expressed in samples
of NSCLC (protein and mRNA) (Oncol Lett. 2017 Dec; 14(6):8183-8189. doi:
10.3892/01.2017.7253). Using in situ
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
11
hybridization, both CCL20 and CCR6 mRNA moieties were strongly expressed in
all pancreatic cancer samples
analysed. In contrast, in healthy pancreas CCL20 and CCR6 expression was low
(Int J Cancer. 1999 May
17; 81(4):650-7. doi: 10.1002/(sici)1097-0215(19990517)81:4<650::aid-
ijc23>3Øco;2-#.). Jin and co-workers
examined CCR6 and CCL20 expression in glioblastoma using publicly available
datasets. The authors used the GEO
dataset GSE2223 to compare the mRNA levels of CCL20 and CCR6, between normal
brain and glioblastoma tissues.
Again, CCR6 and CCL20 expression levels were significantly higher in
glioblastoma tissues than in normal brain tissues
(Oncogene. 2018 Jun; 37(23):3070-3087. doi: 10.1038/s41388-018-0182-7.). In
addition, Wallace and colleagues
observed that in endometrial adenocarcinoma explants and cell lines,
expression of CCL20 and its receptor CCR6 were
higher compared to non-malignant endometrium (mRNA, RT-PCR) (Mol Cell
Endocrinol. 2011 Jan 1; 331(1):129-35.
doi: 10.1016/j.mce.2010.08.018.). CCL20/CCR6 axis may play a role in breast
cancer, cholangiocarcinoma, and thyroid
cancer since expression of CCR6/CCL20 genes and/or proteins was reported in
patient derived breast cancer cells
(Mol Carcinog. 2016 Jul; 55(7):1175-86. doi: 10.1002/mc.22360.), in HuCCT1 and
TFK-1 cholangiocarcinoma cell lines
() (Win et al., PMID 32194362) (EXCLI J. 2020; 19:154-166. doi:
10.17179/excli2019-1893.) and thyroid cancer cell
lines such as TPC-1, BCPAP, FTC-133, and SW1736 (Tumour Biol. 2016 Apr;
37(4):5569-75. doi: 10.1007/s13277-
015-4418-7.). Furthermore, the present CCR6 modulators may be useful, alone,
or in combination in the treatment or
prevention of cancers where the expression and/or evidence of CCR6/CCL20 axis
activity has been reported, or where
CCR6+ regulatory T cells have been identified inside the tumor
microenvironment.
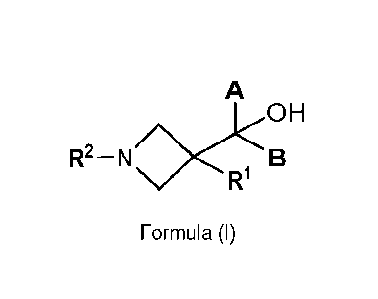
1) One aspect of the present invention relates to compounds of Formula (I),
wherein
A
H
R2 ¨ N
Formula (I)
A represents a 6-membered heteroaryl containing from one to three ring
nitrogen atom(s) (notably one or two ring
nitrogen atoms; especially one or two ring nitrogen atoms in meta-position(s)
and/or para-position of A with respect to
the point of attachment of A to the rest of the molecule), wherein said 6-
membered heteroaryl is independently
unsubstituted, mono-, di- or tri-substituted (notably mono- or di-substituted
in meta- and/or para-position of A with
respect to the point of attachment of A to the rest of the molecule), wherein
the substituent(s), if any, is(are)
independently selected from
= halogen (especially fluorine);
= cyano;
= hydroxy-C1_6-alkyl which is optionally further substituted with one to
three fluorine atoms (wherein notably the
hydroxy group is separated from any one fluorine atom by at least two carbon
atoms; especially such hydroxy-
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
12
01_6-alkyl represents 3-hydroxy-propyl, 4-hydroxy-butyl, 3-hydroxy-butyl, 3-
hydroxy-3-methyl-butyl, 3-hydroxy-
4-methyl-pentyl, 3-hydroxy-3-trifluoromethyl-butyl, or 2,2-difluoro-3-hydroxy-
prop-1-y1);
= 01_5-alkyl (notably methyl, ethyl, propyl, isopropyl, butyl, or isobutyl)
which is unsubstituted or mono-substituted
with
01_3-alkoxy (especially methoxy);
= 03_6-cycloalkyl which is optionally fused with a pyridine ring (notably
at positions 2 and 3 adjacent to
the nitrogen atom of said pyridine ring), wherein said 03_0-cycloalkyl is
unsubstituted or mono-
substituted with hydroxy (notably at the point of attachment of the 03_6-
cycloalkyl to the 01_4-alkyl);
= ¨0¨R 1, wherein R 1 represents 03_6-cycloalkyl (especially cyclopentyl)
or pyrrolidinyl (especially
pyrrolidin-2-y1) which is independently unsubstituted or mono-substituted with
01_3-alkyl (especially
methyl) or 01_4-alkyl-carbonyl (especially acetyl);
= phenyl¨L1¨, wherein said phenyl is unsubstituted or mono-substituted with
fluorine, 01_4-alkoxy-
carbonyl (especially methoxy-carbonyl), or hydroxy-01_4-alkyl (especially
hydroxy-methyl); wherein ¨
L1¨ represents a bond (i.e. the phenyl is attached directly to said alkyl),
oxygen (i.e. the phenyl is
attached via oxygen to said alkyl), or the group ¨CH2-0¨ (wherein the phenyl
is attached to the
methylene group);
= 04_6-heterocycly1 containing one or two ring heteroatoms independently
selected from nitrogen and
oxygen (notably azetidinyl, imidazolidinyl, pyrrolidinyl, oxazolidinyl,
piperidinyl, or tetrahydropyranyl;
especially azetidin-3-yl, tetrahydropyran-4-yl, imidazolidin-1-yl, pyrrolidin-
1-yl, oxazolidin-3-yl,
PYrrolidin-2-yl, pyrrolidin-3-yl, piperidin-4-y1), wherein said 04_6-
heterocycly1 is unsubstituted, mono-,
or di-substituted with oxo, hydroxy, 01_3-alkyl (especially methyl), 01_4-
alkyl-carbonyl (especially
acetyl), or 014-alkoxy-carbonyl (especially tert-butoxy-carbonyl);
[in particular such 04_6-heterocycly1 represents 1-(tert-butoxy-carbony1)-3-
hydroxy-azetidin-3-yl,
tetrahydropyran-4-yl, 4-hydroxy-tetrahydropyran-4-yl, 2-oxo-imidazolidin-1-yl,
2-oxo-3-methyl-
imidazolidin-1-yl, 2-oxo-pyrrolidin-1-yl, 2-oxo-oxazolidin-3-yl, 2-oxo-
oxazolidin-3-yl, N-acety1-2-
methyl-pyrrolidin-2-yl, N-(tert-butoxy-c2rbony1)-3-hydroxy-pyrrolidin-3-yl, N-
acetyl-piperidin-4-yl, or
N-(tert-butoxy-carbony1)-piperidin-4-yl]
). 5-membered heteroaryl containing one or two ring nitrogen atoms (notably
pyrazolyl; especially
pyrazol-4-y1), wherein said 5-membered heteroaryl is unsubstituted or mono-
substituted with 01_3-
alkyl (especially methyl);
_NRNiRm wherein RN1represents hydrogen and RN2 represents 01_3-alkyl-carbonyl
(especially acetyl)
or hydroxy-01_3-alkyl-carbonyl (especially hydroxymethyl-carbonyl) [in
particular such ¨NRN1RN2
represent acetyl-amino or hydroxymethyl-carbonyl-amino];
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
13
indolyl (especially indo1-2-y1);
= pyrrolopyridinyl (especially 1H-pyrrolo[2,3-b]pyridin-2-yI));
= N-(Ci_3-alkylyamino-carbonyl-oxy; or
= 1-hydroxy-1- 03_5-cycloal ky1-1-(pyridi nyI)-methy I (especially 1-
hydroxy-1-cyclopropy1-1-(pyridin-2-y1)-
methyl);
[in particular such 01_4-alkyl represents methyl, ethyl, isopropyl,
cyclopentyl-oxy-methyl, 2-(1-hydroxy-
cyclopropy1)-ethyl, 2-(1-hydroxy-cyclobutyI)-ethyl, 2-(1-hydroxy-cyclopentyI)-
ethyl, 3-methoxy-propyl, 4-fluoro-
phenoxy-methyl, 2-(2-(methoxy-carbonyI)-pheny1)-ethyl, benzyl-oxy-methyl, N-
(isopropy1)-amino-carbonyl-
oxy-methyl, 2-(1-(tert-butoxy-c2rbony1)-3-hydroxy2zetidin-3-y1)-ethyl, 3-
methy1-3-(2-oxo-i midazol idi n-1-yI)-
butyl, 3-methy1-3-(2-oxo-3-methyl-imidazolidin-1-y1)-butyl, 3-methyl-3-(2-oxo-
pyrrolidin-1-y1)-butyl, 2-(N-(tert-
butoxy-carbony1)-3-hydroxy-pyrrolidin-3-y1)-ethyl, 3-methyl-3-(2-oxo-
oxazolidin-3-y1)-butyl, 2-(1-methy1-1H-
pyrazol-4-y1)-ethyl, 2-(2-hydroxymethyl-pheny1)-ethyl,
2-(tetrahydropyran-4-yI)-ethyl, 2-(4-hydroxy-
tetrahydropyran-4-y1)-ethyl, 2-(indo1-2-y1)-ethyl, 2-(8-hydroxy-5,6,7,8-
tetrahydroguinolin-8-yI)-ethyl, 2-(7-
hydroxy-6,7-dihydro-5H-cyclopenta[b]pyridin-7-y1)-ethyl, 2-(N-acetyl-piperidin-
4-yI)-ethyl, 2-(N-(tert-butoxy-
carbony1)-piperidin-4-y1)-ethyl, N-acetyl-2-methyl-pyrrolidin-2-yl-oxy-methyl,
acetyl-amino-isopropyl, N-
(hydroxymethyl-carbony1)-amino-methyl, 3-hydroxy-3-cyclopropy1-3-(pyridin-2-
y1)-propyl, 2-(indo1-2-y1)-ethyl,
or 2-(1H-pyrrolo[2,3-b]pyridin-2-y1)-ethyl]
= Cm-alkyl (especially n-propyl, n-butyl, or n-pentyl) which is substituted
with hydroxy and RAI, wherein said
substituents are both at position 3 with respect to the point of attachment of
said Cm-alkyl to the rest of the
molecule; wherein
= RAI represents
= tetrahydropyranyl (especially tetrahydropyran-4-yI);
= phenyl which is unsubstituted or mono-substituted with fluorine
(especially 3-fluoro-phenyl)
or 013-alkoxy (especially 2-methoxy-phenyl or 4-methoxy-phenyl);
= 5- or 6-membered heteroaryl containing one or two ring heteroatom(s) being
independently
selected from nitrogen or sulfur (notably thiazolyl, pyrazolyl, pyridinyl,
pyrazinyl, or
pyrimidinyl; especially thiazol-4-yl, thiazol-5-yl, pyrazol-3-yl, pyrazol-4-
yl, pyridin-2-yl,
pyridin-3-yl, pyrimidin-2-yl, pyrazin-2-yl, or pyrimidin-4-y1), wherein said 5-
or 6-membered
heteroaryl is independently unsubstituted, mono- or di- substituted, and
wherein the
substituent(s), if any, is(are) independently selected from 01_3-alkyl
(especially methyl), 03_
5-cycloalkyl (especially cyclopropyl), or 01_3-alkoxy (especially methoxy); or
= indazolyl (especially indazol-3-y1);
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
14
[in particular such 03_5-alkyl which represents 3-hydroxy-3-(tetrahydropyran-4-
yI)-propyl, 3-hydroxy-3-phenyl-
propyl, 3-hydroxy-3-phenyl-butyl, 3-hydroxy-3-(3-fluoro-phenyI)-butyl, 3-
hydroxy-3-(2-methoxy-phenyI)-butyl,
3-hydroxy-3-(4-methoxy-phenyI)-butyl, 3-hydroxy-3-(1,5-dimethyl-pyrazol-3-y1)-
butyl, 3-hydroxy-3-(1-methyl-
pyrazol-3-y1)-butyl, 3-hydroxy-3-(1-cyclopropy1-1H-pyrazol-3-y1)-butyl, 3-
hydroxy-3-(1,5-dimethyl-pyrazol-3-
yI)-propyl, 3-hydroxy-3-(2-methyl-thiazol-4-y1)-butyl, 3-hydroxy-3-(2-methyl-
thiazol-5-y1)-butyl, 3-hydroxy-3-(6-
methoxy-pyridin-2-y1)-butyl, 3-hydroxy-3-(5-methyl-pyridin-3-yI)-butyl, 3-
hydroxy-3-(pyridin-2-yI)-butyl, 3-
hydroxy-3-(6-methyl-pyridin-2-y1)-butyl, 3-hydroxy-3-(pyrimidin-2-yI)-butyl, 3-
hydroxy-3-(6-methoxy-pyrimidin-
4-y1)-butyl, 3-hydroxy-3-(6-methyl-pyrimiclin-4-y1)-pentyl, 3-hydroxy-3-(2-
methyl-pyrimidin-4-yI)-butyl, 3-
hydroxy-3-(5-methyl-pyrazin-2-yI)-butyl, 3-hydroxy-3-(1H-indazol-3-y1)-
butyl, 3-hydroxy-3-(1,3-dimethyl-
pyrazol-4-y1)-propyl, 3-hydroxy-3-(2-methyl-thiazol-4-y1)-propyl, 3-hydroxy-3-
(pyridin-2-yI)-pentyl, or 3-
hydroxy-3-(6-methoxy-pyridin-2-yI)-pentyl]
= 035-alkenyl which is unsubstituted (especially isopropenyl) or mono-
substituted with hydroxy (especially 4-
hydroxy-but-1-en-2-y;
= 04_6-cycloalkenyl (notably cyclopentenyl; especially cyclopent-1-en-1-y1)
which is unsubstituted, mono-, or di-
substituted with C13-alkyl, oxo, or hydroxy; wherein optionally one ring
carbon atom of said C46-cycloalkenyl
is replaced by an oxygen atom;
[in particular such 04_6-cycloalkenyl represents cyclopent-1-en-1-yl, 3-oxo-
cyclopent-1-en-1-yl, 3-hydroxy-
cyclopent-1-en-1-yl, 3-hydroxy-3-methyl-cyclopent-1-en-1-yl, or 2,3-dihydro-
furan-3-yl]
C36-cycloalkyl which is unsubstituted, mono-, or di-substituted with C13-alkyl
(especially methyl, ethyl,
isopropyl), hydroxy or hydroxy-Ci_3-alkyl (especially 1-hydroxy-1-methyl-
ethyl), wherein optionally one ring
carbon atom of said 03_6-cycloalkyl is replaced by an oxygen atom;
[in particular such C3_6-cycloalkyl represents cyclopropyl, cyclopentyl, 3-
hydroxy-cyclopentyl, 3-hydroxy-3-
methyl-cyclopentyl, 3-hydroxy-3-ethyl-cyclopentyl,
3-hydroxy-3-isopropyl-cyclopentyl, 2-(2-hydroxy-
isopropylycyclopropyl, 4-hydroxy-cyclohexyl, 4-hydroxy-4-methyl-cyclohexyl,
oxetan-3-yl, tetrahydrofuran-3-
yl, tetrahydrofuran-2-yl, tetrahydropyran-4-yl, 5-methyl-tetrahydrofuran-2-yl,
or 5,5-dimethyl-tetrahydrofuran-
2-yl]
> -0-R02, wherein
> R 2 represents
= 014-alkyl (especially methyl, ethyl, isopropyl, sec-butyl, or isobutyl);
= C25-alkyl which is mono-substituted with hydroxy or C13-alkoxy
(especially methoxy);
[in particular such C2_5-alkyl represents 2-hydroxy-ethyl, 3-hydroxy-propyl, 2-
hydroxy-2-
methyl-propyl, 3-hydroxy-3-methyl-butyl, or 2-methoxy-ethyl]
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
= ¨L2¨CY2, wherein
+ ¨L2¨ independently represents a bond (i.e. the CY2 is directly attached
to the rest
of the molecule), -CH2-, or -CH2-CH2-; and
= CY2 independently represents
5 0
phenyl which is unsubstituted or mono-substituted with hydroxy-C1_3-alkyl
(especially 2-hydroxy-ethyl);
o benzyl-oxy;
O 5- to 6-membered heteroaryl containing one to three ring heteroatom(s)
being independently selected from nitrogen, oxygen, or sulfur (notably
10
pyridinyl, oxadiazolyl, or triazolyl); especially pyridin-2-yl, 1,2,4-
oxadiazol-
5-yl, or 1,2,4-triazol-1-y1), wherein said 5- or 6-membered heteroaryl is
independently unsubstituted, mono- or di- substituted; wherein the
substituent(s), if any, is(are) independently selected from 01_3-alkyl
(especially methyl) or Ci_3-cycloalkyl (especially cyclopropyl);
15
o 03_6-cycloalkyl, wherein optionally one carbon ring atom is replaced by
one heteroatom selected from oxygen and nitrogen (especially oxetanyl,
cyclopentyl or cyclohexyl); wherein said 03_6-cycloalkyl is unsubstituted,
mono-, or di-substituted, wherein the substituents are selected from 01_3-
alkyl (especially methyl), hydroxy, fluoro, oxo, 01_3-alkyl-carbonyl
(especially acetyl), and 01_3-alkoxy (especially methoxy);
o benzooxazolonyl (especially 3H-benzooxazol-2-on-6-y1);
o chromanyl (especially chroman-6-y;
[in particular such ¨L2¨CY2 represents phenyl, 4-(2-hydroxy-ethyl)-phenyl, 2-
(2-hydroxy-
ethyl)-phenyl, benzyl, 2-(benzyl-oxy)-ethyl, 2-(pyridin-2-yI)-ethyl, 3-
cyclopropy1-1,2,4-
oxadiazol-5-yl-methyl, 2-(3,5-dimethy1-1,2,4-triazol-1-y1)-ethyl, oxetan-3-yl-
methyl, 2,2-
dimethyl-cyclopentyl, 3,3-difluoro-cyclopentyl, 3-methoxy-cyclopentyl,
cyclohexyl, 4-
hydroxy-cyclohexyl, 4-methyl-4-hydroxy-cyclohexyl, 4-oxo-cyclohexyl,
tetrahydrofuran-3-yl,
tetrahydropyran-4-yl, tetrahydropyran-4-yl-methyl, N-acetyl-piperidin-4-yl-
methyl, 3H-
benzooxazol-2-on-6-yl, or chroman-6-yl]
[in particular such -0-1R02 represents methoxy, ethoxy, isopropoxy, isobutoxy,
sec-butoxy, phenoxy, 4-(2-
hydroxy-ethyl)-phenoxy, 2-(2-hydroxy-ethyl)-phenoxy, benzyl-oxy, 2-(benzyl-
oxy)-ethoxy, 2-methoxy-ethoxy,
3-hydroxy-propoxy, 2-hydroxy-2-methyl-propoxy, 2-hydroxy-ethoxy, 3-hydroxy-3-
methyl-butoxy, 2,2-dimethyl-
cyclopentyl-oxy, 3,3-difluoro-cyclopentyl-oxy, cyclohexyl-oxy, 4-hydroxy-
cyclohexyl-oxy, 4-methy1-4-hydroxy-
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
16
cyclohexyl-oxy,4-oxo-cyclohexyl-oxy, tetrahydrofuran-3-yl-oxy, tetrahydropyran-
4-yl-oxy, tetrahydropyran-4-
yl-methoxy, 3-methoxy-cyclopentyl-oxy, oxetan-3-yl-methoxy, 2-(pyridin-2-yI)-
ethyl-oxy, (3-cyclopropy1-1,2,4-
oxadiazol-5-y1)-methoxy, 2-(3,5-dimethy1-1,2,4-triazol-1-y1)-ethoxy, N-acetyl-
piperidin 4 yl methyl-oxy, 3H-
benzooxazol-2-on-6-yl, or chroman-6-yl-oxy]
= ¨CC-RT1, wherein
represents
= 01_4-alkyl (notably methyl, ethyl, isopropyl, or isobutyl), wherein said
01_4-alkyl independently
is mono-substituted with
+ hydroxy;
[in particular said 01_4-alkyl represents hydroxy-methyl, 1-hydroxy-ethyl, 2-
hydroxy-ethyl, 1-
hydroxy-2-methyl-propyl, 1-hydroxy-1-methyl-ethyl, or 1-hydroxy-1-
trifluoromethyl-ethyl];
+ 01_3-alkoxy (especially methoxy);
+ ¨S(=0)2¨RsoT, wherein Rscir represents 01_3-alkyl, 01_3-alkyl-amino,
or 3-5-
cycloalkyl (especially such ¨S(=0)2¨Rso-r represents methyl-sulfonyl, methyl-
amino-sulfonyl or cyclopropyl-sulfonyl);
_NRN-ri RNT2 wherein RNT1 represents hydrogen and RNT2 represents 01_3-alkyl-
carbonyl, 01_3-alkoxy-01_3-alkyl-carbonyl, or 03_6-cycloalkyl-carbonyl
(especially
such _NRNT1RNT2 represent acetyl-amino, isopropyl-carbonyl-amino, methoxy-
methyl-carbonyl-amino, or cyclopropyl-carbonyl-amino);
+ 04_6-heterocycly1 containing one or two ring heteroatom(s) independently
selected
from nitrogen and oxygen (notably oxazolidinyl, imidazolidinyl, or
pyrrolidinyl;
especially oxazolidin-3-yl, imidazolidin-3-yl, or pyrrolidin-111); wherein
said C4_6-
heterocycly1 is mono-substituted with oxo; or di-substituted with oxo and 01_3-
21ky1
(especially methyl); (especially such 04_6-heterocycly1 represents oxazolidin-
2-on-
3-yl, imidazolidin-2-on-3-yl, 1-methyl-innidazolidin-2-on-3-yl, or pyrrolidin-
2-on-1-
yl); or
+ N-(Ci_3-alkyl-carbonyI)-piperidinyl-Ci_3-alkyl (especially N-acetyl-
piperidin-4-yl-
methyl);
= C3_6-cycloalkyl (especially cyclopropyl) which is mono-substituted
(especially at the point of
attachment of the C3_6-cycloalkyl to the rest of the molecule) with
+ hydroxy;
+ amino-sulfonyl which is optionally di-substituted with methyl;
+ phenyl which is mono-substituted with halogen (especially 4-fluoro-
phenyl);
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
17
+ pyridinyl (especially pyridine-2-y1);
+ pyrimidinyl which is mono-substituted with Ci 3-alkyl (especially 6-
methyl-pyrimidin-
4-y1);
= oxazolidinonyl (especially oxazolidin-2-on-3-y1);
[in particular such 03_6-cycloalkyl represents 1-hydroxy-cyclopropyl, 1-
hydroxy-cyclobutyl, 1-
hydroxy-cyclopenty, 1-(amino-sulfony1)-cyclopropyl,
1-(dimethyl-amino-sulfony1)-
cyclopropyl, 1-(6-methyl-pyrimidin-4-y1)-cyclopropyl, 1-(pyridine-2-y1)-
cyclopropyl, 1-(4-
fluoro-pheny1)-cyclopropyl, 1-(pyridine-2-yI)-cyclopropyl, or 1-(oxazolidin-2-
on-3-y1)-
cyclopropyl];
= C36-cycloalkyl (notably cyclopentyl or cyclohexyl) fused with a pyridine
ring (notably at
positions 2 and 3 of the pyridine ring), wherein said Cm-cycloalkyl is mono-
substituted with
hydroxy (notably at position 1 of the C36-cycloalkyl ring); wherein optionally
one ring carbon
atom in said C3_6-cycloalkyl is replaced with one oxygen atom;
[in particular such C36-cycloalkyl represents 8-hydroxy-5,6,7,8-
tetrahydroquinolin-8-yl, 7-
hydroxy-6,7-dihydro-5H-cyclopenta[b]pyridin-7-yl, or 4-hydroxy-3,4-dihydro-2H-
pyrano[3,2-
b]pyridin-4-y1);
= C4_6-heterocycly1 containing one ring heteroatom independently selected
from nitrogen and
oxygen (notably azetidinyl, pyrrolidinyl, piperidinyl, tetrahydropyranyl;
especially azetidin-3-
yl, piperidin-4-yl, pyrrolidin-3-yl, pyrrolidin-2-yl, pyrrolidine-1-yl, or
tetrahydropyran-4-y1);
wherein said C4_6-heterocycly1 is mono-, di-, or tri-substituted (especially
mono- or di-
substituted), wherein the substituent(s) is(are) independently selected from
01_3-alkyl
(especially methyl), hydroxy, oxo, 01_3-alkyl-carbonyl (especially acetyl),
01_3-alkoxy-
carbonyl (especially tert-butoxy-carbonyl), 01_3-alkyl-sulfonyl (especially
methyl-sulfonyl),
and 01_3-alkyl-amino-sulfonyl (especially methyl-amino-sulfonyl);
[in particular such C4_6-heterocycly1 represents N-(isopropyl-carbony1)-3-
hydroxy-azetidin-3-
yl, N-(tert-butoxy-carbonyl)-3-hydroxy-azetidin-3-yl, N-methy1-3-hydroxy-
pyrrolidin-2-one-3-
yl, N-acetyl-2-methyl-pyrrolidin-2-yl, 3-hydroxy-N-(tert-butoxy-carbony1)-
pyrrolidin-3-yl, 2-
oxo-pyrrolidine-1-yl, N-acetyl-piperidin-4-yl, N-acetyl-4-methyl-piperidin-4-
yl, N-(methyl-
amino-sulfony1)-4-methyl-piperidin-4-yl, N-acetyl-4-hydroxy-
piperidin-4-yl, N-(methyl-
sulfonyI)-piperidin-4-yl, N-(tert-butoxy-carbony1)-piperidin-4-yl, 3-hydroxy-2-
oxo-1-methyl-
pyrrolidin-2-yl, or 4-hydroxy-tetrahydropyran-4-y1);
= pyrazolyl (notably 1H-pyrazol-4-y1) which is N-substituted with methyl;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
18
= indolyl (especially indo1-2-y1);
= 3-hydroxy-1-methy1-1,3-dihydro-indo1-2-on-3-y1; or
= 4-hydroxy-3,4-dihydro-2H-pyrano[3,2-b]pyridin-4-y1;
[in particular such -CC-RT1 represents 3-hydroxy-3-trifluoromethyl-but-1-yn-1-
yl, 3-hydroxy-prop-1-yn-1-yl, 4-
hydroxy-but-1-yn-1-yl, 3-hydroxy-but-1-yn-1-yl, 3-hydroxy-3-methyl-but-1-yn-1-
yl, 3-methy1-3-(pyrrolidin-2-on-
1-y1)-but-1-yn-1-yl, 3-methyl-3-(cyclopropyl-sulfony1)-but-1-yn-1-yl, 3-methyl-
3-(acetyl-amino)-but-1-yn-1-yl, 3-
methy1-3-(oxazolidin-2-on-3-y1)-but-1-yn-1-yl, 3-methyl-3-(imidazolidin-2-on-3-
y1)-but-1-yn-1-yl, 3-methy1-3-(1-
methyl-imidazolidin-2-on-3-y1)-but-1-yn-1-yl, 3-methy1-3-(2-oxo-pyrrolidine-
1-y1)-but-1-yn-1-yl, 3-methy1-3-
(methyl-sulfony1)-but-1-yn-1-yl, 3-methy1-3-(cyclopropyl-c2rb0ny1-2mino)-but-1-
yn-1-yl, 3-methyl-3-( isopropyl-
carbonyl-amino)-but-1-yn-1-yl, 3-methyl-3-(methoxy-methyl-carbonyl-amino)-but-
1-yn-1-yl, 3-hydroxy-4-
methyl-pent-1-yn-1-yl, 3-methoxy-prop-1-yn-1-yl, 3-(N-acetyl-piperidin-4-yI)-
prop-1-yn-1-yl, (1-hydroxy-
cyclopropy1)-ethynyl, (1-hydroxy-cyclobutyl)-ethynyl, (1-hydroxy-cyclopentyI)-
ethynyl, (8-hydroxy-5,6,7,8-
tetrahydroguinolin-8-y1)-ethynyl, (7-hydroxy-6,7-dihydro-5H-
cyclopenta[b]pyridin-7-y1)-ethynyl, (1-methyl-
pyrazol-4-y1)-ethynyl, (tetrahydropyran-4-yI)-ethynyl, (4-hydroxy-
tetrahydropyran-4-yI)-ethynyl, indo1-2-yl-
ethynyl, (1-(isopropyl-carbony1)-3-hydroxy-azetidin-3-y1)-ethynyl, (1-(tert-
butoxy-carbony1)-3-hydroxy-azetidin-
3-y1)-ethynyl, (1-(6-methyl-pyrimidin-4-y1)-cyclopropy1)-ethynyl, (N-acetyl-4-
methyl-piperidin-4-y1)-ethynyl, (N-
acety1-4-hydroxy-piperidin-4-y1)-ethynyl, (1-(4-fluoro-phenyI)-cyclopropy1)-
ethynyl, (N-acetyl-piperidin-4-yI)-
ethynyl, (N-(methyl-sulfony1)-piperidin-4-y1)-ethynyl, (1-(dimethyl-amino-
sulfonyI)-cyclopropy1)-ethynyl, (1-
(amino-sulfonyI)-cyclopropy1)-ethynyl,
(N-(methyl-amino-sulfony1)-4-methyl-piperidin-4-y1)-ethynyl, (1-
(pyridine-2-y1)-cyclopropy1)-ethynyl, (3-
hydroxy-N-(tert-butoxy-carbony1)-pyrrolidin-3-y1)-ethynyl, (1-
(oxazol idin-2-on-3-y1)-cyclopropy1)-ethynyl,
(3-hydroxy-1-methy1-1,3-dihydro-indo1-2-on-3-y1)-ethynyl, (4-
hydroxy-3,4-di hydro-2H-pyrano[3,2-b]pyridin-4-y1)-ethynyl,
(N-(tert-butoxy-carbonylypiperidin-4-y1)-ethynyl,
(N-acetyl-2-methyl-pyrrolidin-2-y1)-ethynyl, or (3-hydroxy-2-oxo-1-methyl-
pyrrolidin-3-yI)-ethynyl]
= -CEC-C(OH)(RT2)(RT3), wherein
RT2 represents hydrogen or C1_3-alkyl (notably methyl or ethyl; especially
methyl);
RT3 represents
= phenyl which is unsubstituted or mono-substituted, wherein the
substituent, if any, is
selected from 01_3-alkoxy (notably methoxy) or halogen (notably fluorine);
[in particular such phenyl which is unsubstituted or mono-substituted is 2-
fluoro-phenyl, 4-
methoxy-phenyl, or 2-methoxy-phenyl]
= 5- to 6-membered heteroaryl containing one or two ring heteroatom(s)
being independently
selected from nitrogen, oxygen, or sulfur (notably thiophenyl, thiazolyl,
pyrrolyl, pyrazolyl,
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
19
isoxazolyl, pyridinyl, pyrazinyl, or pyrimidinyl; especially thiophen-2-yl,
thiazol-4-yl, thiazol-
5-yl, pyrrol-2-yl, pyrazol-3-yl, pyrazol-4-yl, isoxazol-5-yl, pyridin-2-yl,
pyrimidin-2-yl, pyrazin-
2-yl, or pyrimidin-4-y1), wherein said 5- or 6-membered heteroaryl is
independently
unsubstituted, mono- or di- substituted, and wherein the substituent(s), if
any, is(are)
independently selected from C1_3-alkyl (especially methyl), C1_3-cycloalkyl
(especially
cyclopropyl), C1_3-fluoroalkyl (notably Cl-fluoroalkyl; especially
difluoromethyl or
trifluoromethyl), and 01_3-alkoxy (especially methoxy);
[in particular such 5- to 6-membered heteroaryl is 5-methyl-thiophen-2-yl, 2-
methyl-thi azol-
5-yl, 1-methyl-1H-pyrrol-2-yl, 1-cyclopropy1-1H-pyrazol-3-yl, 1-methyl-1H-
pyrazol-3-yl, 1,3-
dimethyl-pyrazol-4-yl, 1,5-dimethy1-1H-pyrazol-3-yl, 2-methyl-thiazol-4-yl, 3-
methyl-
isoxazol-5-yl, 1-methyl-1H-imidazol-2-yl, pyridin-2-yl, 6-methoxy-pyridin-2-
yl, 6-methyl-
pyridin-2-yl, pyrimidin-2-yl, 2-methoxy-pyrimidin-4-yl, 6-methoxy-pyrimidin-4-
yl, pyrimidin-4-
yl, 2-methyl-pyrimidin-4-yl, 6-methyl-pyrimidin-4-yl, 2,6-dimethyl-pyrimidin-4-
yl, 2,6-
dimethoxy-pyrimidin-4-yl, 2-methyl-6-methoxy-pyrimidin-4-
yl, 2-methoxy-6-methyl-
pyrimidin-4-yl, 5-methyl-pyrazin-2-yl, 1-cyclopropy1-1H-
pyrazol-3-yl, 6-cyclopropyl-
pyrimidin-4-yl, 6-difluoromethyl-pyrimidin-4-yl,
2-trifluoromethyl-pyrimidin-4-yl, 6-
trifluoromethyl-pyrimidin-4-yl, or 1,5-dimethy1-1H-pyrazol-3-yl]
= 04_7-heterocycly1 containing one ring heteroatom selected from nitrogen
and oxygen;
wherein said C4_7-heterocycly1 is unsubstituted, mono-, or di-substituted,
wherein the
substituent(s), if any, is(are) independently selected from C1_3-alkyl
(especially methyl) or
C1_3-alkyl-carbonyl (especially acetyl); or
[in particular such C4_7-heterocycly1 represents N-acetyl-piperidin-4-yl, N-
acety1-4-methyl-
piperidin-4-yl, or tetrahydropyran-4-yl]
= indazolyl (especially indazol-3-y1);
[in particular such -CEC-C(OH)(R12)(R13) represents 3-hydroxy-3-(5-methyl-
thiophen-2-y1)-but-1-yn-1-yl, 3-
hydroxy-3-(2-methyl-thi azol-5-y1)-but-1-yn-1-yl, 3-hydroxy-3-(1-methy1-1H-
pyrrol-2-y1)-but-1-yn-1-yl, 3-hydroxy-3-
(3-methyl-isoxazol-5-y1)-but-1-yn-1-yl, 3-hydroxy-3-(1-methy1-1H-pyrrol-2-y1)-
but-1-yn-1-yl, 3-hydroxy-3-(1-methy1-
1H-imidazol-2-y1)-but-1-yn-1-yl, 3-hydroxy-3-(5-methyl-pyrazin-2-y1)-but-1-yn-
1-yl, 3-hydroxy-3-(tetrahydropyran-
4-y1)-prop-1-yn-1-yl, 3-hydroxy-3-(N-acetyl-piperidi n-4-y1)-prop-1-yn-1-
yl, 3-hydroxy-3-(N-acety1-4-methyl-
piperidin-4-y1)-but-1-yn-1-yl, 3-hydroxy-3-phenyl-prop-1-yn-1-yl, 3-hydroxy-3-
(2-methyl-thiazol-4-y1)-prop-1-yn-1-
yl, 3-hydroxy-3-(1,3-dimethyl-pyrazol-4-y1)-prop-1-yn-1-yl, 3-hydroxy-3-phenyl-
but-1-yn-1-yl, 3-hydroxy-3-(1-
methy1-1H-pyrazol-3-y1)-but-1-yn-1-yl, 3-hydroxy-3-(1-cyclopropy1-1H-pyrazol-3-
y1)-but-1-yn-1-yl, 3-hydroxy-3-
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
(1,5-dimethy1-1H-pyrazol-3-y1)-but-1-yn-1-yl,
3-hydroxy-3-(1-methyl-pyrazol-3-y1)-but-1-yn-1-yl, 3-hydroxy-3-
(pyrimidin-2-y1)-but-1-yn-1-yl, 3-hydroxy-3-(1-methyl-pyrazol-3-y1)-but-1-yn-1-
yl, 3-hydroxy-3-(3-fluoro-pheny1)-but-
1-yn-1-yl, 3-hydroxy-3-(6-methyl-pyrimidin-4-y1)-but-1-yn-1-yl, 3-hydroxy-3-(2-
methoxy-pyrimidin-4-y1)-but-1-yn-1-
yl, 3-hydroxy-3-(2-methoxy-6-methyl-pyrimidin-4-y1)-but-1-yn-1-yl, 3-hydroxy-3-
(6-methoxy-pyrimidin-4-y1)-but-1-
5
yn-1-yl, 3-hydroxy-3-(2,6-dimethoxy-pyrimidin-4-y1)-but-1-yn-1-yl, 3-
hydroxy-3-(2-methyl-pyrimidin-4-yI)-but-1-yn-
l-yl, 3-hydroxy-3-(6-methoxy-2-methyl-pyrimidin-4-y1)-but-1-yn-1-yl, 3-hydroxy-
3-(pyridin-2-y1)-pent-1-yn-1-yl, 3-
hydroxy-3-(6-methoxy-pyridin-2-y1)-but-1-yn-1-yl, 3-hydroxy-3-(6-methyl-
pyridin-2-y1)-but-1-yn-1-yl, 3-hydroxy-3-
(6-methyl-pyridin-2-y1)-pent-1-yn-1-yl, 3-hydroxy-3-(2,6-dimethyl-pyrimidin-
4-y1)-but-1-yn-1-yl, 3-hydroxy-3-(6-
methoxy-pyridin-2-y1)-pent-1-yn-1-yl, 3-hydroxy-3-(1H-indazol-3-y1)-but-1-yn-1-
yl, 3-hydroxy-3-(6-cyclopropyl-
10 pyrimidin-4-y1)-but-1-yn-1-yl,
3-hydroxy-3-(6-difluoromethyl-pyrimidin-4-y1)-but-1-yn-1-yl, 3-hydroxy-3-
(2-
trifluoromethyl-pyrimidin-4-y1)-but-1-yn-1-yl,
3-hydroxy-3-(6-trifluoromethyl-pyrimidin-4-y1)-but-1-yn-1-yl, 3-
hydroxy-3-(indazol-3-y1)-but-1-yn-1-yl, 3-hydroxy-3-(4-methoxy-pheny1)-but-1-
yn-1-yl, or 3-hydroxy-3-(2-methoxy-
pheny1)-but-1-yn-1-yl]
= -NRoRN4 wherein
15 Ro represents C1_3-alkyl (especially methyl); and
R represents hydroxy-01_3-alkyl (especially 2-hydroxy-ethyl) or 2-(benzyl
oxy) 01_3-alkyl (especially
2-(benzyl-oxy)-ethyl); or
) R and RN4 form, together with the nitrogen to which they are
attached, a heterocyclic ring of 4 to 6
members (notably 5 to 6 members), wherein the members needed to complete said
heterocyclic ring
20
are each independently selected from -CH2-, -0-, -(0=0)-, -CHRx- and -
C(RY)2-; wherein said
heterocyclic ring does not contain more than one member independently selected
from the group
consisting of -0- and -(0=0)-; wherein said heterocyclic ring does not contain
more than two
members selected from the group consisting of -CHRx-; and wherein said
heterocyclic ring does not
contain more than two members selected from the group consisting of -C(RY)2-;
wherein Rx
independently represents fluorine, methyl, isopropyl, isobutyl, tert-butyl,
hydroxy, trifluoromethyl,
hydroxy-methyl, 1-hydroxy-ethyl, 1-hydroxy-1-methyl-ethyl, cyclopropyl, 2-
methoxy-ethyl, 2-methyl-
thiazol-5-yl, 4-methyl-thiazol-2-yl, phenyl, benzyl, tetrahydropyran-4-yl, N-
acetyl-piperidin-4-yl, 1,2,4-
oxadiazolyl, 3-methyl-1,2,4-oxadiazol-5-yl, 2-methyl-2H-[1,2,3]triazol-4-yl, 1-
methyl-1H-pyrazol-4-yl,
1-difluoromethy1-1H-pyrazol-4-yl, 1,3-dimethy1-1H-pyrazol-4-yl, pyridin-2-yl,
6-methyl-pyridin-3-yl, 6-
isopropyl-pyridin-2-yl, 6-trifluoromethyl-pyridin-3-yl, 2-isopropyl-pyrimidin-
4-yl, or 1-methoxy-methyl;
and wherein RY independently represents fluorine, hydroxy, cyclopropyl,
methyl, hydroxy-methyl, or
trifluoromethyl [notably such -NR R" is pyrrolidinyl; 2-pyrrolidonyl;
oxazolidinonyl (especially 1,3-
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
21
oxazolidin-2-on-3-y1); piperidinyl; or morpholinyl, optionally independently
substituted with one or two
substituents independently selected from a group consisting of Rx and RY];
[in particular such -NRN3RN4 represents pyrrolidin-1-yl, 3-fluoro-pyrrolidin-1-
yl, 3,3-difluoro-pyrrolidin-1-yl, 3,4-
difluoro-pyrrolidin-1-yl, 3-isopropyl-pyrrolidin-1-yl, 3,3-dimethyl-pyrrolidin-
1-yl, 3-hydroxy-pyrrolidin-1-yl, 2-
methyl-pyrrolidin-1-yl, 3-hydroxy-3-methyl-pyrrolidin-1-yl, 3-(hydroxy-methyl)-
pyrrolidin-1-yl, 2-(hydroxy-
methyl)-pyrrolidin-1-yl, 3-(1-hydroxy-ethyl)-pyrrolidin-1-yl, 3-hydroxy-3-
cyclopropyl-pyrrolidin-1-yl, 3-hydroxy-
3-trifluoromethyl-pyrrolidin-1-yl, 3-trifluoromethyl-pyrrolidin-1-yl, 3-(1-
hydroxy-1-methyl-ethyl)-pyrrolidin-1-yl,
3-(1-hydroxy-ethyl)-pyrrolidin-1-yl, 3-(hydroxy-methyl)-3-methyl-pyrrolidin-1-
yl, 1,3-oxazolidin-2-on-3-yl, 5-
(tert-buty1)-1,3-oxazolidin-2-on-3-yl, 5-pheny1-1,3-oxazolidin-2-on-3-yl, 5-
benzy1-1,3-oxazolidin-2-on-3-yl, 5-
isopropy1-1,3-oxazolidin-2-on-3-yl, 5-
(tetrahydropyran-4-y1)-1,3-oxazolidin-2-on-3-yl, 5,5-dimethy1-1,3-
oxazolidin-2-on-3-yl, morpholin-4-yl, piperidin-1-yl, 4-hydroxy-piperidin-1-
yl, 3-hydroxy-piperidin-1-yl, 3-
(tetrahydropyran-4-y1)-pyrrolid-2-on-1-yl, N-methyl-N-(2-(benzyl-oxy)-ethyl)-
amino, N-methyl-N-(2-hydroxy-
ethyl)-amino, pyrrolidin-2-on-1-yl, 4-phenyl-pyrrolidin-2-on-1-yl, 4-(N-acetyl-
piperidin-4-yI)-pyrrolidin-2-on-1-yl,
4-(pyridin-2-y1)-pyrrolidin-2-on-1-yl, 4-(6-methyl-pyridin-3-y1)-pyrrolidin-2-
on-1-yl, 4-(2-isopropyl-pyrimidin-4-
y1)-pyrrolidin-2-on-1-yl, 4-(6-
isopropyl-pyridin-2-y1)-pyrrolidin-2-on-1-yl, 4-(6-trifluoromethyl-pyridin-
3-y1)-
pyrrolidin-2-on-1-yl, 4-methyl-pyrrolidin-2-on-1-yl, 4-isopropyl-pyrrolidin-2-
on-1-yl, 3-isopropyl-pyrrolidin-2-on-
l-yl, 3,3-dimethyl-pyrrolidin-2-on-1-yl, 4,4-dimethyl-pyrrolidin-2-on-1-yl, 3-
(piperidin-4-yI)-pyrrolidin-2-on-1-yl,
H-pyrazol-4-y1)-pyrrolidin-2-on-1-yl, 4-(1-difluoromethy1-1H-pyrazol-4-y1)-
pyrrolidin-2-on-1-yl, 4-
(1,3-dimethy1-1H-pyrazol-4-y1)-pyrrolidin-2-on-1-yl, 4-(6-isopropyl-pyridin-2-
y1)-pyrrolidin-2-on-1-yl, 4-isobutyl-
pyrrolidin-2-on-1-yl, 4-cyclopropyl-pyrrolidin-2-on-1-yl, 4-trifluoromethyl-
pyrrolidin-2-on-1-yl, 3-(2-methoxy-
ethyl)-pyrrolidin-2-on-1-yl, 4-(2-methoxy-ethyl)-pyrrolidin-2-on-1-yl, 2,2,6,6-
tetrafluoro-morpholin-4-yl, 2,6-
dimethyl-morpholin-4-yl, 2-(methoxy-methyl)-morpholin-4-yl, 3-(3-methy1-1,2,4-
oxadiazol-5-y1)-pyrrolidin-1-yl,
4-(2-methyl-thiazol-5-y1)-pyrrolidin-2-on-1-yl, 4-(2-methyl-2H-[1,2,3]triazol-
4-y1)-pyrrolidin-2-on-1-yl, 3-(4-
methyl-thiazol-2-y1)-pyrrolidin-1-yl, or 3-(pheny1)-pyrrolidin-1-yl]
= -(0=0)-N(RN5)(RN6), wherein
RN5 represents hydrogen; and
RN6 represents C36-cycloalkyl (especially cyclopentyl) or tetrahydropyranyl
(especially
tetrahydropyran-4-y1); or
RN5and RN6form, together with the nitrogen to which they are attached,
pyrrolidinyl;
[in particular such -(C=0)-N(RN5)(RN6) represents N-cyclopentyl-amino-
carbonyl, N-(tetrahydropyran-
4-y1)-amino-carbonyl, or pyrrolidinyl-carbonyl]
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
22
piperidin-4-y1 or pyrrolidin-3-y1 which are mono-substituted at the nitrogen
ring atom, wherein the substituent
is independently selected from 01_4-alkoxy-carbonyl (especially tert-butoxy-
carbonyl), pyridinyl (especially
pyridin-2-y1), phenyl and (4-methylphenyI)-sulfonyl;
[in particular such piperidin-4-y1 or pyrrolidin-3-y1 are N-(tert-butoxy-
carbonyI)-piperidin-4-yl, N-(tert-butoxy-
carbonyI)-pyrrolidin-3-yl, N-(pyridin-2-yI)-piperidin-4-yl, N-(phenyl)-
piperidin-4-yl, or N4(4-methylpheny1)-
sulfonyl)-piperidin-4-yl]
5- or 6-membered heteroaryl containing from one to three (notably two or
three; especially three) ring
heteroatom(s) independently selected from nitrogen, oxygen and sulfur (notably
pyrazolyl, triazolyl, oxazolyl,
thiazolyl, oxadiazolyl, pyri midi nyl, or pyridinyl; especially pyrazol-1-yl,
1H-1,2,3-triazol-1-yl, oxazol-2-yl, thiazol-
2-yl, 1,2,4-oxadiazol-5-yl, 1,2,4-oxadiazol-3-yl, pyrimidin-2-yl, or pyridin-2-
y; wherein said 5-or 6-membered
heteroaryl is independently unsubstituted, mono-, di-, or tri-substituted
(notably mono-substituted; especially
mono-substituted in position 3 with respect to the point of attachment of said
5- or 6-membered heteroaryl to
A), wherein the substituent(s), if any, is(are) independently selected from
Cl-alkyl (notably methyl, ethyl, propyl, isopropyl, or tert-butyl) which is
= unsubstituted; or
= mono-substituted with
+ hydroxy;
+ 01_4-alkoxy (especially methoxy and tert-butoxy); or
= _N(:07)(RN8), wherein RN' represents hydrogen or C1_3-alkyl (especially
methyl);
and RN8 independently represent 03_5-cycloalkyl-carbonyl (especially
cyclopropyl-
carbonyl), C1_3-alkyl (especially methyl), C1_3-alkyl-carbonyl including
deuterated
01_3-alkyl-carbonyl (especially acetyl, ethyl-carbonyl, isopropyl-carbonyl, or
acetyl-
2,2,2-d3), C1-3-alkoxy-C1_3-alkyl-carbonyl (especially methoxy-methyl-
carbonyl),
tetrahydropyranyl-carbonyl (especially tetrahydropyran-4-yl-carbonyl), or
hydroxy-
01_3-alkyl-carbonyl (especially hydroxy-methyl-carbonyl);
= di-substituted, wherein one substituent is hydroxy, and the other
substituent is
trifluoromethyl; or both substituents are hydroxy; or
= di- or tri-substituted, wherein two substituents are fluorine and, if
present, a further
substituent is hydroxy (wherein especially the hydroxy group is separated by
at least two
carbon atoms from any of said fluorine substituents);
[in particular such 01_4-alkyl represents methyl, isopropyl, hydroxy-methyl, 1-
hydroxy-1-methyl-ethyl,
2-hydroxy-2-methyl-propyl, 2-hydroxy-1,1-dimethyl-ethyl, methoxy-methyl, 2-
methoxy-ethyl, 1-
methoxy-1-methyl-ethyl, 2-methoxy-2-methyl-propyl, 2-methoxy-1,1-dimethyl-
ethyl, tert-butoxy-
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
23
methyl; 1,2-dihydroxy-ethyl, N-acetyl-2-amino-ethyl, N-(acetyl-2,2,2-d3)-2-
amino-ethyl, 2-
(methylcarboxamido)-2-methyl-propyl, 2-(ethylcarboxamido)-2-methyl-
propyl, 2-(cyclopropyl-
carboxamido)-2-methyl-propyl, 2-(tetrahydropyran 4 yl carboxamido)-2-methyl-
propyl, 2-(methoxy-
methyl-carboxamido)-2-methyl-propyl, 2-(ethyl-carboxamido)-2-
methyl-propyl, 2-(isopropyl-
carboxamido)-2-methyl-propyl, 2-(methyl-d3-carboxamido)-2-methyl-propyl, N-
methyl-N-(hydroxy-
methyl-carbony1)-2-amino-ethyl, N-methyl-N-acetyl-2-amino-ethyl,
2,2,2-trifluoro-1-hydroxy-1-
methyl-ethyl, 1,1-difluoro-2-hydroxy-ethyl, 1,1-difluoro-ethyl, or N-methyl-N-
(acety1-2,2,2-d3)-2-
amino-ethyl; in particular such C1_4-alkyl group is 1-hydroxy-1-methyl-ethyl,
2-hydroxy-2-methyl-
propyl, or 2-hydroxy-1,1-dimethyl-ethyl]
> ¨L3¨CY3, wherein
= ¨L3¨ independently represents a bond (i.e. the CY3 is directly attached
to the rest of the
molecule), -CH2-, -CH2-CH2-, -C(CH3)2-, -CH(OH)-, or -0-CH2-, wherein when
¨L3¨ is -0-
CH2-, said CY3 is attached to the oxygen atom of said -0-CH2-; and
= CY3 independently represents 036-cycloalkyl or 046-heterocyclyl, said 046-
heterocycly1
containing one or two ring heteroatoms independently selected from nitrogen
and oxygen
(especially CY3 represents cyclopropyl, cyclobutyl, oxetanyl, azetidinyl,
pyrrolidinyl,
tetrahydropyranyl, tetrahydrofuranyl, morpholinyl, imidazolidinyl,
piperidinyl, or piperazinyl);
wherein said CY3independently is unsubstituted, mono-, di-, or tri-
substituted, wherein the
substituents is selected from
D halogen (especially fluorine);
oxo;
= hydroxy;
= C1_3-alkyl which is optionally mono-substituted with C1_3-alkoxy
(especially such C1_
3-alkyl represents methyl, ethyl, isopropyl, or methoxy-methyl);
> C13-alkoxy (especially methoxy);
> ¨(0=0)¨Rc0, wherein Rc represents
0 013-alkyl which is optionally mono-substituted with hydroxy or 013-
alkoxy
(especially methyl, ethyl, n-propyl, isopropyl, tert-butyl, hydroxy-methyl,
methoxy-methyl, benzyl-oxy, or 2-methoxy-ethyl);
o 01_3-fluoroalkyl (especially 2,2,2-trifluoro-ethyl);
o 01_3-alkoxy, wherein said 01_3-alkoxy is optionally mono-substituted with
01_3-alkoxy (especially methoxy, ethoxy, or 2-methoxy-ethoxy);
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
24
o 03_6-cycloalkyl-(0H2).-, wherein optionally one or two carbon ring
atom(s)
is/are replaced by one or two oxygen ring atom(s); wherein n represents
the integer 0, 1, or 2 (especially such 03_5-cycloalkyl-(0H2), represents
cyclopropyl, cyclopentyl, oxetan-3-yl, oxetan-3-yl-methyl, 1,4-dioxan-2-yl,
or tetrahydropyran-4-yI); or
o phenyl;
[especially such ¨(0=0)¨Rc0 represents acetyl, ethyl-carbonyl, n-propyl-
carbonyl,
isopropyl-carbonyl, tert-butyl-carbonyl, hydroxymethyl-carbonyl, 2,2,2-
trifluoro-
ethyl-carbonyl, methoxy-methyl-carbonyl, 2-methoxy-ethyl-carbonyl, methoxy-
carbonyl, ethoxy-carbonyl, 2-methoxy-ethoxy-carbonyl, cyclopropyl-carbonyl,
cyclopentyl-carbonyl, oxetan-3-yl-carbonyl, oxetan-3-yl-methyl-carbonyl,
phenyl-
carbonyl, or tetrahydropyran-4-yl-carbonyl, methoxy-methyl-carbonyl, or 1,4-
dioxan-2-yl-carbonyl]
¨N(RN9)(RNio), wherein RN9 represents hydrogen or 01_3-alkyl (especially
methyl);
and RN1 represents 01_3-alkyl (especially methyl), 01_3-alkyl-carbonyl
(especially
acetyl), C1_3-alkyl-sulfonyl (especially methyl-sulfonyl), 01_3-alkoxy-
carbonyl
(especially ethoxy-carbonyl), 01_3-alkoxy-01_3-alkyl-carbonyl (especially
methoxy-
methyl-carbonyl), or tetrahydropyranyl-carbonyl (especially tetrahydropyran-4-
yl-
carbonyl);
¨S(=0)2¨R90, wherein Rso represents
o C1_3-alkyl which is optionally mono-substituted with hydroxy, C1_3-
alkoxy,
or amino (especially such 01_3-alkyl is methyl, n-propyl, isopropyl, 2-
hydroxy-ethyl, 2-methoxy-ethyl, or methyl-amino); or
o C3_5-cycloalkyl, wherein optionally one carbon ring atom is replaced by
one oxygen ring atom (especially represents cyclopropyl, cyclopentyl, or
tetrahydropyranyl);
[especially such ¨S(=0)2¨Rs represents methyl-sulfonyl, n-propyl-sulfonyl,
isopropyl-sulfonyl, 2-hydroxyethyl-sulfonyl, cyclopropyl-sulfonyl, cyclopentyl-
sulfonyl, 2-methoxy-ethyl-sulfonyl, methyl-amino-sulfonyl, or tetrahydropyran-
4-yl-
sulfonyl]
)- 5-membered heteroaryl containing one ring heteroatom selected from
nitrogen,
oxygen, and sulfur (notably oxygen); wherein said 5-membered heteroaryl is
unsubstituted (notably furanyl; especially furan-2-y; and
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
phenyl-(CH2)p-, wherein p represents the integer 0, 1, or 2 (especially such
phenyl-
(CH2)p- represents phenyl or benzyl);
[in particular such -L3-CY3 represents1-hydroxy-cyclopropyl, 1-(methyl-amino)-
cyclopropyl, 1-
(acetyl-amino)-cycloprop-1-yl-methyl, 1-(N-acetyl-N-methyl-amino)-
cycloprop-1-yl-methyl, 1-(N-
5 (methoxy-methyl-carbonyI)-amino)-cycloprop-1-yl-methyl, 1-(N-
(ethoxy-carbonyI)-N-methyl-amino)-
cycloprop-1-yl, 1-(N-methylacetamido)-cycloprop-1-y1 (including 1-(N-
methylacet-d3-amido)-
cycloprop-1-y1), 1-(1-(methoxy-methyl)-cyclopropy1)-methyl,
1-(tetrahydropyran-4-yl-carbonyl-
amino)-cyclopropyl-methyl, cyclobutyl-oxy-methyl, 1-hydroxy-cyclobutyl, 3-
hydroxy-cyclobutyl, 1-
methoxy-cyclobutyl, 1-hydroxy-cyclopentyl, 3-hydroxy-3-methyl-cyclopentyl, 1-
hydroxy-cyclohexyl,
10 4-hydroxy-cyclohexyl, (1-hydroxy-cyclohexyl)-methyl, (1-hydroxy-
cyclobutyI)-methyl, 1-hydroxy-1-
cyclohexyl-methyl, 1-methoxy-cyclopentyl, oxetan-3-yl, tetrahydrofuran-2-yl-
methyl, tetrahydrofuran-
2-yl, tetrahydrofuran-3-yl, tetrahydropyran-3-yl, tetrahydropyran-4-yl, 4-
hydroxy-tetrahydropyran-4-yl,
4-hydroxy-tetrahydropyran-4-yl-methyl, 4-methyl-tetrahydropyran-4-
yl-methyl, 4-methyl-
tetrahydropyran-4-yl, 2,6-dimethyl-tetrahydropyran-4-yl, 4-methoxy-
tetrahydropyran-4-yl, 4-fluoro-
15 tetrahydropyran-4-yl, tetrahydropyran-4-yl-methyl, 2-
(tetrahydropyran-4-yI)-ethyl, 1-(tetrahydropyran-
4-y1)-1-methyl-ethyl, tetrahydropyran-4-yl-oxy-methyl, (4-methyl-
tetrahydropyran-4-yI)-oxy-methyl, N-
acety1-3-fluoro-azetidin-3-y1 (including N-acetyl-d3-3-fluoro-azetidin-3-y1),
N-acety1-3-methyl-azetidin-
3-y1 (including N-acetyl-d3-3-methyl-azetidin-3-y1), 2-oxo-azetidin-3-yl, 2-
oxo-azetidin-4-yl, N-acetyl-
azetidin-3-yl, 5-oxo-pyrrolidin-2-yl, N-phenyl-5-oxo-pyrrolidin-2-yl, N-phenyl-
5-oxo-pyrrolidin-3-yl, N-
20 benzy1-5-oxo-pyrrolidin-3-yl, N-benzy1-2-oxo-pyrrolidin-3-yl, 5-
oxo-pyrrolidin-3-yl, N-(furan-2-yl-
methyl)-5-oxo-pyrrolidin-3-yl, 3-methyl-5-oxo-pyrrolidin-3-yl, 1-methyl-5-oxo-
pyrrolidin-3-yl, 1-
isopropy1-5-oxo-pyrrolidin-3-yl, 1,3-dimethy1-2-oxo-pyrrolidin-3-yl, 2-oxo-
pyrrolidin-1-yl-methyl, 2-
oxo-pyrrolidin-1-yl-methyl, 1-ethyl-5-oxo-pyrrolidin-3-yl, 1-isobuty1-5-oxo-
pyrrolidin-3-yl, N-acetyl-
pyrrolidin-3-yl, N-acetyl-pyrrolidin-3-yl-methyl, 2,5-dioxo-3,3-dimethyl-
pyrrolidin-1-yl-methyl, 2-oxo-
25 imidazolidin-1-yl-methyl, 2-oxo-oxazolidin-3-yl-methyl,
piperidin-4-yl, 1-methy1-2,6-dioxo-piperidin-4-
yl, 4-methyl-piperidin-4-yl, 4-fluoro-piperidin-4-yl, 2-oxo-piperidin-4-yl, 6-
oxo-piperidin-2-yl, 6-oxo-
piperidin-3-yl, 1-methyl-6-oxo-piperidin-3-yl, 3-methyl-6-oxo-piperidin-3-yl,
N-acetyl-piperidin-4-yl, N-
acetyl-piperidin-4-yl-methyl, N-acetyl-2-methyl-piperidin-4-yl, N-acetyl-4-
fluoro-piperidin-4-yl, N-
acety1-3-methyl-piperidin-4-yl, N-acetyl-4-methyl-piperidin-4-yl, N-acetyl-4-
hydroxy-piperidin-4-yl, N-
acetyl-4-methoxy-piperidin-4-yl, N-acetyl-piperidin-3-yl, N-acetyl-piperidin-3-
yl-methyl, N-acety1-3-
methyl-piperidin-3-yl, N-acetyl-3-hydroxy-piperidin-3-yl, N-(isopropyl-
carbonyI)-4-hydroxy-piperidin-
4-yl, N-(tert-butyl-carbonyl)-piperidin-4-yl,
N-(2-methoxy-ethoxy-carbonyI)-piperidin-4-yl, N-
(hydroxymethyl-carbony1)-piperidin-4-yl, N-(cyclopropyl-sulfonyI)-piperidin-4-
yl, N-(methyl-sulfonyI)-
piperidin-4-yl, N-(n-propyl-sulfonyI)-piperidin-4-yl, N-(isopropyl-sulfonyI)-
piperidin-4-yl, N-(2-hydroxy-
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
26
ethyl-sulfonyI)-piperidin-4-yl, N-(cyclopentyl-sulfony1)-piperidin-4-yl, N-
(methoxy-carbony1)-piperidin-
4-yl, N-(cyclopropyl-carbonylypiperidin-4-yl, N-(cyclopentyl-carbonyI)-
piperidin-4-yl, N-(methyl-
amino-sulfonylypiperidin-4-yl, N-(2-methoxy-ethyl-sulfonyI)-piperidin-4-yl, N-
(ethyl-carbonyI)-4-
hydroxy-piperidin-4-yl, N-(methoxy-methyl-carbonyl)-piperidin-4-yl, N-(ethoxy-
carbonyI)-piperidin-4-
yl, N-acetyl-4-ethyl-piperidin-4-yl, N-(n-propyl-carbonyI)-piperidin-4-yl, N-
(2-methoxy-ethyl-carbonyI)-
piperidin-4-yl, N-(oxetan-3-yl-carbonyI)-piperidin-4-yl, N-(oxetan-3-yl-methyl-
carbonyI)-piperidin-4-yl,
N-(2,2,2-trifluoro-ethyl-carbonyI)-piperidin-4-yl, N-(tetrahydropyran-4-yl-
carbonyl)-piperidin-4-yl, N-
(phenyl-carbony1)-piperidin-4-yl, N-(tetrahydropyran-4-yl-sulfonyI)-piperidin-
4-yl, N-(benzyl-oxy-
carbony1)-piperidin-4-yl, N-(methoxy-methyl-carbonyl)-4-isopropyl-piperidin-4-
yl, N-(1,4-dioxan-2-yl-
carbonyl)-piperidin-4-yl, morpholin-4-yl, morpholin-4-yl-methyl, 1-methyl-1-
(morpholin-4-y1)-ethyl,
(2,6-dimethyl-morpholin-4-yI)-methyl, 2,4-dioxo-imidazolidin-1-yl-methyl, 2,5-
dioxo-imidazolidin-1-yl-
methyl, 2,5-dioxo-3-methyl-imidazolidin-1-yl-methyl, 2,4-dioxo-3-methyl-
imidazolidin-1-yl-methyl, 2-
oxo-3-methyl-imidazolidin-1-yl-methyl, 2-oxo-3-cyclopropyl-imidazolidin-1-yl-
methyl, 2,5-dioxo-
pyrrolidin 1 -yl-methyl, N-acetyl-pyrrolidin-3-yl, or 4-acetyl-piperazin-1-
yI];
> phenyl or 6-membered heteroaryl containing one or two ring nitrogen atom(s)
(notably pyridinyl or
pyrimidinyl; especially pyridin-3-y1 or pyrimidin-4-y; wherein said phenyl or
6-membered heteroaryl
is independently unsubstituted or mono-substituted with 01_3-alkyl (especially
methyl) or 01_3-alkoxy
(especially methoxy);
[in particular such phenyl or 6-membered heteroaryl is 3-methoxy-phenyl,
pyridin-3-yl, or 6-methyl-
pirimidin-4-yl]
= pyrazolyl-C13-alkyl (especially 2-(pyrazol-1-y1)-ethyl);
= 01_3-alkyl-sulfonyl-C1_3-alkyl (especially methyl-sulfonyl-methyl);
= 3-hydroxymethyl-bicyclo[1.1.1]pent-1-y1;
= 7-oxa-bicyclo[2.2.1]hept-2-y1; and
> 6-oxa-spiro[2.5]oct-1-y1;
= 5-oxo-4-oxa-6-azaspiro[2.4]hept-6-yl, 5-aza-spiro[2.4]heptan-6-on-5y1,
2,2-dimethy1-6-oxo-5-oxa-7-
azaspiro[3.4]oct-7-yl, 2-cyclopropy1-6-oxo-5-oxa-7-azaspiro[3.4]oct-7-yl, 2-
oxo-1-oxa-3-azaspiro[4.4]non-3-yl,
8,8-difluoro-2-oxo-1-oxa-3-azaspiro[4.5]clec-3-yl, or 8-oxo-7-oxa-9-
azadispiro[3.1.4.1]undec-9-y1;
= 7-aza-bicyclo[2.2.11hept-7-yl, 2-oxa-5-aza-bicyclo[2.2.11hept-5-yl, 6-oxa-
3-aza-bicyclo[3.1.11hept-3-yl, or 8-
oxa-3-azabicyclo[3.2.1]oct-3-y1;
= 5-oxo-6-azaspiro[3.4]oct-6-yl, 3-oxo-2-azaspiro[4.4]non-2-yl, 1-oxa-3-aza-
spiro[4.5]decan-2-on-3-yl, 1-oxo-2-
azaspiro[4.5]clec-2-yl, 1-oxo-8-oxa-2-azaspiro[4.5]clec-2-yl, 3-oxo-8-oxa-2-
azaspiro[4.5]clec-2-yl, or 4-oxo-
hexahydro-5H-furo[2,3-c]pyrrol-5-y1;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
27
= 3-(7-hydroxy-6,7-dihydro-5H-cyclopenta[b]pyridin-7-yl)propyl or 3-(8-
hydroxy-5,6,7,8-tetrahydroguinolin-8-
yl)propyl);
= 6-acetyl-7,8-dihydro-5H-pyrido[4,3-d]pyrimidin-2-y1;
= 6-acetyl-5,6,7,8-tetrahydro-1,6-naphthyridine-2-y1;
= 2-(6,7-dihydro-5H-[1]pyrindin-74)-ethyl,
= 2-(8-hydroxy-5,6,7,8-tetrahydro-guinolin-8-y1)-ethyl;
= 7,8-dihydro-5H-[1,6]naphthyridin-6-y1;
= 2,3-dihydro-isoindo1-1-on-2-y1;
= 7,8-dihydro-5H-pyrido[4,3-d]pyrimidin-6-yl, and
= 6-acetyl-7,8-dihydro-5H-pyrido[4,3-d]pyrimidin-2-y1;
B represents phenyl, which is unsubstituted, mono-, di- or tri-substituted,
wherein a first substituent (especially in para-
position with respect to the point of attachment of B to the rest of
molecule), if present, is selected from
)> halogen (especially bromine);
)- 01_5-alkyl (notably methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl,
tert-butyl, 1-ethyl-propyl, 2,2-dimethyl-
propyl, 1,1-dimethyl-propyl; especially methyl, ethyl, or isopropyl; in
particular isopropyl);
= C2_4-alkenyl (especially prop-1-en-2-y1);
= 01_3-alkoxy (especially methoxy, ethoxy, or isopropoxy);
)- C1_3-alkoxy-C1_4-alkyl (especially 3-methoxy-propyl);
Ci_4-fluoroalkyl (especially trifluoromethyl, 1-methyl-l-fluoro-ethyl, 1,1-
difluoro-ethyl, 2,2,2-trifluoro-ethyl, 1,1-
dimethy1-1-trifluoromethyl-methyl, 1,1,2,2,2-pentafluoro-ethyl, or 1,2,2,2-
tetrafluoro-1-trifluoromethyl-ethyl);
= C3_5-cycloalkyl (especially cyclopropyl or cyclobutyl) which
independently is unsubstituted or mono-substituted
(notably at the point of attachment of said 03_5-cycloalkyl to the rest of the
molecule) with 01_3-alkyl (especially
methyl) or 01_3-f1u0r021ky1 (especially trifluoromethyl);
= ¨SF5;
)- bicyclo[1.1.1]pent-1-y1;
= C3_b-cycloalkoxy (especially cyclopropoxy or cyclobutoxy); and
01_3-fluoroalkoxy (especially trifluoromethoxy);
and the remaining substituent/s of B, if present, independently is/are
selected from halogen (notably fluorine or chlorine;
especially said remaining substituent(s) are in meta-position with respect to
the point of attachment of B to the rest of
molecule) and C1_3-alkyl (especially methyl);
or B represents benzothiophenyl (notably 1-benzothiophenyl; especially 1-
benzothiophen-5-y1) or naphthalenyl
(especially naphthalen-2-y1);
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
28
[in particular such B is phenyl, 4-bromo-phenyl, 4-methyl-phenyl, 4-ethyl-
phenyl, 4-propyl-phenyl, 4-isopropyl-phenyl,
4-butyl-phenyl, 4-isobutyl-phenyl, 4-tert-butyl-phenyl, 4-(1-ethyl-propyI)-
phenyl, 4-(1,1-dimethyl-propyI)-phenyl, 4-(2,2-
dimethyl-propy1)-phenyl, 3-chloro-4-isopropyl-phenyl, 3-fluoro-4-isopropyl-
phenyl, 3,5-difluoro-4-isopropyl-phenyl, 3-
methyl-4-isopropyl-phenyl, 4-(prop-1-en-2-y1)-phenyl, 4-cyclopropyl-phenyl, 4-
methoxy-phenyl, 4-ethoxy-phenyl, 4-
isopropoxy-phenyl, 4-trifluoromethyl-phenyl, 4-(1-methy1-1-fluoro-ethyl)-
phenyl, 4-(1,1-difluoro-ethyl)-phenyl, 4-(2, 2,2-
trifluoro-ethyl)-phenyl, 4-(1,1-dimethyl-l-trifluoromethyl-methyl)-phenyl, 4-
(1,1,2,2,2-pentafluoro-ethyl)-phenyl, 4-
(1,2,2,2-tetrafluoro-l-trifluoromethyl-ethyl)-phenyl, 4-trifluoromethoxy-
phenyl, 4-(1-methyl-cyclopropyI)-phenyl, 4-
cyclobutyl-phenyl, 4-(pentafluoro-I6-sulfaneyI)-phenyl, 4-(bicyclo[1.1.1]pent-
l-y1)-phenyl, 4-cyclopropoxy-phenyl, 4-
cyclobutoxy-phenyl, 4-(trifluoromethoxy)-phenyl, 4-(3-methoxy-propyI)-phenyl,
4-(1-trifluoromethyl-cyclopropyI)-phenyl,
naphthalen-2-yl, or 1-benzothiophen-5-yl]
RI represents 01_3-alkyl (especially methyl or ethyl), cyano, or halogen
(especially fluorine);
R2 represents C1_4-alkyl (especially methyl, ethyl, n-propyl, isopropyl, tert-
butyl or isobutyl), C3_5-cycloalkyl (especially
cyclopropyl or cyclobutyl), C3_5-cycloalky1-01_3-alkyl (especially cyclopropyl-
methyl), hydroxy-C1_3-alkyl (especially 2-
hydroxy-ethyl), C1_3-alkoxy-C1_3-alkyl (especially 2-methoxy-ethyl), or C1_3-
fluoroalkyl (especially 2,2-difluoro-ethyl,
3,3,3-trifluoro-propyl, or 2-fl uoroethyl).
Definitions provided herein are intended to apply uniformly to the compounds
of Formula (I) as defined in any one of
embodiments 1) to 53), and, mutatis mutandis, throughout the description and
the claims unless an otherwise expressly
set out definition provides a broader or narrower definition. It is well
understood that a definition or preferred definition
of a term defines and may replace the respective term independently of (and in
combination with) any definition or
preferred definition of any or all other terms as defined herein. If not
explicitly defined otherwise in the respective
embodiment or claim, groups defined herein are unsubstituted.
The term "halogen", used alone or in combination, means fluorine, chlorine,
bromine, or iodine; notably fluorine or
chlorine; especially fluorine.
The term "cyano", used alone or in combination, refers to a group -ON.
The term "oxy", used alone or in combination, refers to a group -0-.
The term "oxo", used alone or in combination, refers to the group =0.
The term "amino", used alone or in combination, refers to the group -N H2.
The term "amino-carbonyl", used alone or in combination, refers to the group -
C(=0)-NH2.
The term "amino-carbonyl-oxy", used alone or in combination, refers to the
group -0-C(=0)-NH2 , wherein substitutions
to the amino group may be further defined. For example, N-(01_3-alkyl)-amino-
carbonyl-oxy- means that the amino group
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
29
of said amino-carbonyl-oxy group is substituted with a 01_3-alkyl group, said
01_3-alkyl group being defined hereinbelow.
An example of N-(Ci_ralkylyamino-carbonyl-oxy- is N-(isopropyI)-amino-carbonyl-
oxy.
The term "alkyl", used alone or in combination, refers to a saturated straight
or branched hydrocarbon chain group
containing one to six carbon atoms. The term "Cx_y-alkyl" (x and y each being
an integer), refers to an alkyl group as
defined before, containing x toy carbon atoms. In case a 0)(1-alkyl group is
used in combination with another substituent,
the term means that said substituent is linked through a 0)(1-alkyl group to
the rest of the molecule. For example, a Ci_
6-alkyl group contains from one to six carbon atoms. Examples of C1_6-alkyl
groups are the C1_4-alkyl groups methyl,
ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, tert-butyl, and isobutyl, as
well as n-pentyl, and isopentyl. A preferred
example of a C1_3-21ky1 as used for the substituent R1 is methyl. A preferred
example of a C1_4-21ky1 as used for the
substituent R2 is ethyl or methyl; most preferably methyl. A particularly
preferred example of a 01_6-alkyl as used for the
substituent of B is isopropyl.
The term "amino-alkyl", used alone or in combination, refers to an alkyl group
as defined before, wherein one hydrogen
atom has been replaced by an amino group. The term "amino-Cx_ralkyl" (x and y
each being an integer), used alone or
in combination, refers to an amino-alkyl group as defined before wherein the
alkyl group contains x to y carbon atoms.
For example, amino-01_4-alkyl is an amino-alkyl group containing from one to
four carbon atoms. Examples of such
amino-01_4-alkyl groups are amino-methyl, 1-amino-ethyl, 2-amino-ethyl and 2-
amino-2,2-dimethyl-ethyl.
The term "hydroxyalkyl", used alone or in combination, refers to an alkyl
group as defined before, wherein one hydrogen
atom has been replaced by a hydroxy group. The term "hydroxy-Cx_y-alkyl" (x
and y each being an integer), used alone
or in combination, refers to a hydroxyalkyl group as defined before wherein
the alkyl group contains x toy carbon atoms.
For example, a hydroxy-C1_6-alkyl group is a hydroxyalkyl group as defined
before which contains from one to six carbon
atoms.
The term "hydroxy-alkyl" refers to an alkyl group as defined herein, wherein
one hydrogen atom is replaced by one
hydroxy group.
The term "oxetanyl-alkyl", used alone or in combination, refers to an alkyl
group as defined before, wherein one
hydrogen atom has been replaced by an oxetanyl group; especially such oxetanyl
group is oxetan-3-yl. The term
"oxetanyl-Cx_y-alkyl" (x and y each being an integer), used alone or in
combination, refers to an oxetanyl-alkyl group as
defined before, wherein the alkyl group contains x to y carbon atoms. For
example, an oxetanyl-Ci_3-alkyl group is an
oxetanyl-alkyl group as defined before which contains from one to three carbon
atoms.
The term "pyridinyl-alkyl", used alone or in combination, refers to an alkyl
group as defined before, wherein one
hydrogen atom has been replaced by a pyridinyl group; especially such
pyridinyl group is pyridin-2-yl. The term
"pyridinyl-Cx_y-alkyl" (x and y each being an integer), used alone or in
combination, refers to a pyridinyl-alkyl group as
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
defined before, wherein the alkyl group contains x to y carbon atoms. For
example, a pyridiny1-01_3-alkyl group is a
pyridinyl-alkyl group as defined before which contains from one to three
carbon atoms.
The term "pyrazolyl-alkyl", used alone or in combination, refers to an alkyl
group as defined before, wherein one
hydrogen atom has been replaced by a pyrazolyl group; especially such
pyrazolyl group is pyrazol-1-yl. The term
5 "pyrazolyl-Cx_y-alkyl" (x and y each being an integer), used alone or in
combination, refers to a pyrazolyl-alkyl group as
defined before, wherein the alkyl group contains x to y carbon atoms. For
example, a pyrazolyl-C1_3-alkyl group is a
pyrazolyl-alkyl group as defined before which contains from one to three
carbon atoms.
The term "benzyl-oxy-alkyl", used alone or in combination, refers to an alkyl
group as defined before, wherein one
hydrogen atom has been replaced by the group 061-15-0H2-0-. The term "benzyl-
oxy-Cx_y-alkyl" (x and y each being an
10 integer), used alone or in combination, refers to a benzyl-oxy-alkyl
group as defined before, wherein the alkyl group
contains x to y carbon atoms. For example, a benzyl-oxy-01_3-alkyl group is
benzyl-oxy-alkyl group as defined before
which contains from one to three carbon atoms.
The term "alkyl-carbonyl", used alone or in combination, refers to an alkyl
group as defined before, wherein one
hydrogen atom has been replaced by the group -C(=0)-. The term "C-alkyl-
carbonyl" (x and y each being an integer),
15 used alone or in combination, refers to an alkyl-carbonyl group as
defined before, wherein the alkyl group contains x to
y carbon atoms. For example, a 01_3-alkyl-carbonyl group is an alkyl-carbonyl
group as defined before which contains
from one to three carbon atoms.
The term "amino-carbonyl-oxy", used alone or in combination, refers to the
group H2N-C(=0)-0-, wherein substitutions
to the amino group may be further defined.
20 The term "(hydroxy-alkyl)-carbonyl", used alone or in combination,
refers to a hydroxy-alkyl group as defined before,
wherein one hydrogen atom of the alkyl has been replaced by the group -0(=0)-.
The term "(hydroxy-Cx1-alky1)-
carbonyl" (x and y each being an integer), used alone or in combination,
refers to (hydroxy-alkyl)-carbonyl group as
defined before, wherein the alkyl group contains x to y carbon atoms. For
example, a (hydroxy-01_3-alkyl)-carbonyl
group is an (hydroxy-alkyl)-carbonyl group as defined before which contains
from one to three carbon atoms.
25 The term "alkyl-sulfonyl-alkyl", used alone or in combination, refers to
an alkyl group as defined before, wherein one
hydrogen atom has been replaced by an alkyl-sulfonyl group, wherein the alkyl
part of the said alkyl-sulfonyl is as
defined before. The term "Cx1_y1-alkyl-sulfonyl-Cx2_y2-alkyl" (x1, x2, y1 and
y2 each being an integer), used alone or in
combination, refers to an alkyl-sulfonyl-alkyl group as defined before,
wherein the alkyl groups contain independently
from one another x1 to y1 and x2 to y2 carbon atoms. For example, a C1_3-alkyl-
sulfonyl-C1_3-alkyl is an alkyl-sulfonyl-
30 alkyl group as defined before which contains in both alkyl pats
independently from one to three carbon atoms.
The term "fluoroalkyl", used alone or in combination, refers to an alkyl group
as defined before in which one or more
(and possibly all) hydrogen atoms have been replaced by fluorine. The term
"Cx_y-fluoroalkyl" (x and y each being an
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
31
integer) refers to a fluoroalkyl group as defined before containing x to y
carbon atoms. For example, a 01_3-fluoroalkyl
group contains from one to three carbon atoms in which one to seven hydrogen
atoms have been replaced with fluorine.
Examples of 01_3-fluoroalkyl groups are trifluoromethyl, 2-fluoroethyl, 2,2-
difluoroethyl and 2,2,2-trifluoroethyl.
The term "alkenyl", used alone or in combination, refers to a straight or
branched hydrocarbon chain an alkyl group as
defined before, wherein said chain comprises one double bond. The term "C-
alkenyl" (x and y each being an integer),
used alone or in combination, refers to an alkenyl group as defined before,
containing from x to y carbon atoms. For
example, the term C3_5-alkenyl, used alone or in combination, refers to an
alkenyl group as defined before containing
from 3 to 5 carbon atoms. Examples of 03_5-alkenyl groups are -CH=CH2, -CH=CH2-
CH3, -0H2-CH=CH2, and ¨
C(C H3)=C H2.
The term "alkylene", used alone or in combination, refers to a saturated,
branched or straight, bivalent aliphatic
hydrocarbon group, regarded as derived from an alkane by removal of two
hydrogen atoms. The term "C-alkylene" (x
and y each being an integer), used alone or in combination, refers to an
alkylene group as defined before containing x
to y carbon atoms. For example, a C1_2-alkylene is an alkylene group as
defined above containing one or two carbon
atoms. Examples of 01_2-alkylene groups are -CH2-, -(CH2)2- and -CH(CH3)-.
The term "hydroxy-alkylene", used alone or in combination, refers to an
alkylene group as defined before, wherein one
hydrogen atom has been replaced with a hydroxy group. The term "hydroxy-Cx_y-
alkylene" (x and y each being an
integer), used alone or in combination, refers to a hydroxy-alkylene group as
defined before containing x to y carbon
atoms. For example, a hydroxy-01_2-alkylene is a hydroxy-alkylene group as
defined above containing one or two carbon
atoms. Examples of hydroxy-01_2-alkylene groups are -CH(OH)-, -CH(OH)-CH2-,
and -C(OH)(CH3)-; preferably -
CH(OH)-.
The term oxy-01_2-alkylene, used alone or in combination, refers to the group -
0-01_2-alkylene, wherein 01_2-alkylene is
defined above. Preferably, oxy-01_2-alkylene is the group -0-CH2-.
The term "alkoxy", used alone or in combination, refers to an alkyl group as
defined before, wherein one hydrogen atom
has been replaced by -0-.The term "Cx_y-alkoxy" (x and y each being an
integer), used alone or in combination, refers
to an alkoxy group as defined before, wherein the alkoxy group contains x to y
carbon atoms. For example, a 01_3-
alkoxy group is an alkoxy group as defined before which contains from one to
three carbon atoms. Examples of C1-3-
alkoxy groups are methoxy, ethoxy, n-propoxy, or isopropoxy; notably methoxy.
The term "alkoxy-alkyl", used alone or in combination, refers to an alkyl
group as defined before, wherein one hydrogen
atom has been replaced by an alkoxy group as defined before. The term "Cxi_yi-
alkoxy-C2-alkyl" (x1, x2, y1 and y2
each being an integer), used alone or in combination, refers to an alkoxy-
alkyl group as defined before, wherein the two
hydrocarbon parts of the group contain independently from one another x1 to y1
and x2 to y2 carbon atoms. For
example, a C1_3-alkoxy-C1_3-alkyl is an alkoxy-alkyl group as defined before
which contains in the two hydrocarbon parts
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
32
of the group independently from one to three carbon atoms. Examples of 01_3-
alkoxy-01_3-alkyl groups are methoxy-
methyl or ethoxy-ethyl.
The term "fluoroalkoxy", used alone or in combination, refers to an alkoxy
group as defined before containing one to
three carbon atoms in which one or more (and possibly all) hydrogen atoms have
been replaced with fluorine. The term
"C-fluoroalkoxy" (x and y each being an integer) refers to a fluoroalkoxy
group as defined before containing x to y
carbon atoms. For example, a C1_3-fluoroalkoxy group contains from one to
three carbon atoms in which one to seven
hydrogen atoms have been replaced by fluorine. Examples of C1_3-fluoroalkoxy
groups include trifluoromethoxy,
difluoromethoxy, 2-fluoroethoxy, 2,2-difluoroethoxy and 2,2,2-trifluoroethoxy.
The term "01_4-alkoxy-carbonyl" refers to an alkoxy group as defined before
attached to a carbonyl group (i.e. 014-
alkoxy-C(=0)-).
The term "cycloalkyl", used alone or in combination, refers to a saturated
monocyclic hydrocarbon ring containing three
to seven carbon atoms (preferably three to six carbon atoms). The term "C-
cycloalkyl" (x and y each being an integer),
refers to a saturated monocyclic hydrocarbon ring containing x to y carbon
atoms. For example, a 03_6-cycloalkyl group
contains from three to six carbon atoms. Examples of C3_6-cycloalkyl groups
are cyclopropyl, cyclobutyl, cyclopentyl
and cyclohexyl.
Likewise, the term "Cx_y-heterocycly1" refers to a cycloalkyl group as define
above containing x to y ring atoms, wherein
in said group one or more ring carbon atoms are replaced by heteroatoms as
explicitly defined.
The term "cycloalkenyl", used alone or in combination, refers to an
unsaturated monocyclic hydrocarbon ring containing
one double carbon-carbon ring bond, said ring containing four to six carbon
(preferably five) atoms. The term "Cx_y-
cycloalkenyl" (x and y each being an integer), refers to a cycloalkenyl ring
as defined before containing x to y carbon
atoms. For example, a 04_6-cycloalkenyl group contains from four to six carbon
atoms. Examples of 04_6-cycloalkenyl
groups are cyclobutenyl, cyclopentenyl and cyclohexenyl; notably
cyclopentenyl; especially cyclopent-1-en-1-yl.
The term "cycloalkoxy", used alone or in combination, refers to an cycloalkyl
group as defined before, wherein one
hydrogen atom has been replaced by -0-.The term "C-cycloalkoxy" (x and y each
being an integer), used alone or in
combination, refers to a cycloalkoxy group as defined before, wherein the
cycloalkoxy group contains x to y carbon
atoms. For example, a C3_5-cycloalkoxy group is a cycloalkoxy group as defined
before which contains from three to
five carbon atoms. Examples of C3_5-cycloalkoxy groups are cyclopropoxy,
cyclobutoxy, or cyclopentoxy.
The term "cycloalkyl-sulfonyl", used alone or in combination, refers to a
cycloalkyl group as defined before, wherein one
hydrogen atom is replaced by a sulfonyl group (i.e. -S(=0)2-). The term "Cx_y-
cycloalkyl-sulfonyl" (x and y each being an
integer), refers to a cycloalkyl group containing x to y carbon atoms. For
example, a 03_5-cycloalkyl-sulfonyl group
contains from three to six carbon atoms. Examples of C3_5-cycloalkyl-sulfonyl
groups are cyclopropyl-sulfonyl,
cyclobutyl-sulfonyl and cyclopentyl-sulfonyl.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
33
The term "cycloalkyl-alkyl", used alone or in combination, refers to an alkyl
group as defined before, wherein one
hydrogen atom has been replaced by a cycloalkyl group as defined before. The
term" exi yi-cycloalkyl-Cx2y2-alkyl" (x1,
x2, y1 and y2 each being an integer), used alone or in combination, refers to
an cycloalkyl-alkyl group as defined before,
wherein the two hydrocarbon parts of the group contain independently from one
another x1 to yl and x2 to y2 carbon
atoms. For example, C3_5-cycloalkyl-C1_3-alkyl is a cycloalkyl-alkyl group as
defined before which contains in the
cycloalkyl part from three to five carbon atoms, and an alkyl part which
contains from one to three carbon atoms.
Examples of 035-cycloalkyl-01_3-alkyl groups are cyclopropyl-methyl or
cyclopropyl-ethyl.
The term "Cm-cycloalkyl fused with a pyridine ring", used alone or in
combination, refers to a 03_6-cycloalkyl group as
defined before which is fused with a pyridine ring. Examples of C36-cycloalkyl
fused with a pyridine ring are the following
groups:
(9 (9N N s.'=== N
I I I
; preferably the last two groups from left
to right. It is understood that when a substituent is referred to as "036-
cycloalkyl fused with a pyridine ring" the point of
attachment of said substituent to the rest of the molecule is in the Cm-
cycloalkyl part of the substituent and not in the
fused pyridine ring.
The term "hydroxy-azetidinyl", used alone or in combination, refers to an
azetidinyl group substituted with a hydroxy
group. Preferably, the term hydroxy-azetidinyl refers to 3-hydroxy-azetidin-3-
yl.
The term "heteroaryl", used alone or in combination, means a 5- to 10-membered
monocyclic or bicyclic aromatic ring
containing one to a maximum of four heteroatoms (notably containing one to a
maximum of three heteroatoms), each
independently selected from oxygen, nitrogen and sulfur. Examples of such
heteroaryl groups are furanyl, oxazolyl,
isoxazolyl, oxadiazolyl, thiophenyl, thiazolyl, isothiazolyl, thiadiazolyl,
pyrrolyl, imidazolyl, pyrazolyl, triazolyl, pyridinyl,
pyrimidinyl, pyridazinyl, pyrazinyl, indolyl, isoindolyl, benzofuranyl,
isobenzofuranyl, benzothiophenyl, indazolyl,
benzimidazolyl, benzoxamlyl, benzisoxazolyl, benzothiazolyl,
benzoisothiazolyl, benzotriazolyl, benzoxadiazolyl,
benzothiadiazolyl, quinolinyl, isoquinolinyl, naphthyridinyl, cinnolinyl,
quinazolinyl, quinoxalinyl, phthalazinyl,
pyrrolopyridinyl, pyrazolopyridinyl, pyrazolopyrimidinyl, pyrrolopyrazinyl,
imidazopyridinyl, imidazopyridazinyl, and
imidazothiazolyl. The above-mentioned heteroaryl / heteroarylene groups are
unsubstituted or substituted as explicitly
defined.
The term "5- to 6-membered heteroaryl", used alone or in combination, refers
to a 5- to 6-membered monocyclic
aromatic ring and containing one to a maximum of four ring heteroatoms
(preferably one to a maximum of three ring
heteroatoms), each independently selected from oxygen, nitrogen and sulfur.
Examples of 5-membered groups are 5-
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
34
membered heteroaryl groups such as furanyl, oxazolyl, isoxazolyl, oxadiazolyl,
thiophenyl, thiazolyl, isothiazolyl,
thiadiazolyl, pyrrolyl, imidazolyl, pyrazolyl, triazolyl, tetrazolyl; notably
pyrazolyl, triazolyl, oxazolyl, thiazolyl, oxadiazolyl;
especially pyrazol-1-yl, 1,2,3-triazol-1-yl, oxazol-2-yl, thiazol-2-yl, 1,2,4-
oxadiazol-5-y1 or 1,2,4-oxadiazol-3-y1; most
preferably 1,2,4-oxadiazol-5-y1 or 1,2,4-oxadiazol-3-yl. Examples of 6-
membered heteroaryl groups are groups such as
pyridinyl, pyrimidinyl, pyridazinyl, pyrazinyl. The above-mentioned heteroaryl
groups are unsubstituted or substituted
as explicitly defined.
In the context of mono-substituted 5-membered heteroaryl groups, it is
understood that a substitution in position 3 with
respect to the point of attachment of a 5-membered heteroaryl to A means that
the substituent in position 3 and the
point of attachment to A are in a relative 1,3-arr2ngement. Preferred examples
of such 5-membered heteroaryl groups
which are mono-substituted in position 3 with respect to the point of
attachment of said 5-membered heteroaryl to A
are selected from a group consisting of
*.
N
)31_
/1\1 I zo and
/11
N ** N N
**
Irk
wherein one asterisk (A) denotes the attachment point to the substituent and
two asterisks (AA) denote the attachment
point to A. Especially preferred are the last two (from left to right) 5-
membered heteroaryl groups.
Further embodiments of the invention are presented hereinafter:
2) One embodiment relates to compounds according to embodiment 1), wherein A
represents pyridinyl, pyrimidinyl,
pyrazinyl, or pyridazinyl (notably pyridazinyl or pyridinyl; especially
pyridin-2-yl, pyridin-3-yl, pyridin-4-yl, pyrimidin-5-yl,
pyrazin-2-yl, or pyridazin-4-y1), wherein A is independently unsubstituted,
mono-, di- or tri-substituted (notably mono-
or di-substituted in meta- and/or para-position of A with respect to the point
of attachment of A to the rest of the molecule;
especially mono-substituted in meta-position of A with respect to the point of
attachment of A to the rest of the molecule),
wherein the substituent(s), if any, is(are) as defined in embodiment 1).
3) Another embodiment relates to compounds according to embodiment 1), wherein
A represents pyridin-3-yl, pyridin-
4-yl, or pyridazin-4-y1 (in particular pyridin-3-y1 or pyridazin-4-y1),
wherein A is independently unsubstituted, mono-, di-
or tri-substituted (notably mono- or di-substituted in meta- and/or para-
position of A with respect to the point of
attachment of A to the rest of the molecule; especially mono-substituted in
meta-position of A with respect to the point
of attachment of A to the rest of the molecule), wherein the substituent(s),
if any, is(are) as defined in embodiment 1).
In a sub-embodiment, A is independently
unsubstituted;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
mono-substituted (notably in meta-position of A with respect to the point of
attachment of A to the rest of the
molecule), wherein the substituent is as defined in embodiment 1); or
di-substituted, wherein a first substituent is (notably in meta-position of A
with respect to the point of attachment
of A to the rest of the molecule) selected from the substituents defined in
embodiment 1) (especially excluding
5
halogen (especially fluorine) or cyano); and a second substituent is
(notably in para-position of A with respect
to the point of attachment of A to the rest of the molecule) selected from
halogen (especially fluorine) and
cyano.
4) Another embodiment relates to compounds according to embodiment 1), wherein
A represents pyridin-3-yl, pyridin-
4-yl, or pyrid2zin-4-y1 (especially pyridin-3-y1 or pyrid2zin-4-y1; in
particular pyridin-3-y1), wherein A is mono-substituted
10
(notably in meta-position of A with respect to the point of attachment of A
to the rest of the molecule), wherein the
substituent is as defined in embodiment 1).
5) Another embodiment relates to compounds according to any one of embodiments
1) to 4), wherein said substituent
of Al at least one of said substituents of A is
= ¨0¨R 2 as defined in embodiment 1).
15
6) Another embodiment relates to compounds according to embodiment 5),
wherein said substituent of Al at least one
of said substituents of A is
¨0¨R 2, wherein
Ro2 represents
= 01_4-alkyl (especially isopropyl, sec-butyl, or isobutyl);
20 = C25-alkyl which is mono-substituted with hydroxy or 013-
alkoxy (especially methoxy);
fin particular such C2_5-alkyl represents 2-hydroxy-ethyl, 3-hydroxy-propyl, 2-
hydroxy-2-
methyl-propyl, 3-hydroxy-3-methyl-butyl, or 2-methoxy-ethyl]
= ¨L2¨CY2, wherein
+ ¨L2¨ independently represents a bond (i.e. the CY2 is directly attached
to the rest
25 of the molecule); and
= CY2 independently represents
o 03_6-cycloalkyl (especially oxetanyl, cyclopentyl or cyclohexyl), wherein
optionally one carbon ring atom is replaced by one oxygen atom; wherein
said 03_6-cycloalkyl is unsubstituted, mono-, or di-substituted, wherein the
30
substituents are selected from 01_3-alkyl (especially methyl), hydroxy,
fluoro, oxo, 01_3-alkyl-carbonyl (especially acetyl), and 01_3-alkoxy
(especially methoxy);
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
36
o chromanyl (especially chroman-6-y1);
[in particular such -L2-CY2 represents 2-(benzyl-oxy)-ethyl, 2,2-dimethyl-
cyclopentyl, 3,3-
difluoro-cyclopentyl, 3-nnethoxy-cyclopentyl, cyclohexyl, 4-hydroxy-
cyclohexyl, 4-methy1-4-
hydroxy-cyclohexyl, 4-oxo-cyclohexyl,
tetrahydrofuran-3-yl, tetrahydropyran-4-yl,
tetrahydropyran-4-yl-methyl, or chroman-6-yl]
[in particular such -0-R 2 represents isopropoxy, isobutoxy, sec-butoxy, 2-
(benzyl-oxy)-ethoxy, 2-methoxy-
ethoxy, 3-hydroxy-propoxy, 2-hydroxy-2-methyl-propoxy, 2-hydroxy-ethoxy, 3-
hydroxy-3-methyl-butoxy, 2,2-
dimethyl-cyclopentyl-oxy, 3,3-difluoro-cyclopentyl-oxy, cyclohexyl-oxy, 4-
hydroxy-cyclohexyl-oxy, 4-methy1-4-
hydroxy-cyclohexyl-oxy,4-oxo-cyclohexyl-oxy, tetrahydrofuran-3-yl-oxy,
tetrahydropyran-4-yl-oxy,
tetrahydropyran-4-yl-methoxy, 3-methoxy-cyclopentyl-oxy, or chroman-6-yl-oxy]
7) Another embodiment relates to compounds according to any one of embodiments
1) to 4), wherein said substituent
of A / at least one of said substituents of A is
= 03_5-alkyl as defined in embodiment 1).
8) Another embodiment relates to compounds according to embodiment 7), wherein
said substituent of A / at least one
of said substituents of A is
= 03_5-alkyl (especially n-propyl, n-butyl, or n-pentyl) which is
substituted with hydroxy and RA1, wherein said
substituents are both at position 3 with respect to the point of attachment of
said C3_5-alkyl to the rest of the
molecule; wherein
>- RAI represents
= phenyl which is
unsubstituted or mono-substituted with fluorine (especially 3-fluoro-phenyl)
or 01_3-alkoxy (especially 2-methoxy-phenyl or 4-methoxy-phenyl);
= 6-membered heteroaryl containing one or two ring nitrogen heteroatom(s)
(notably pyridin-
2-yl, pyridin-3-yl, pyrimidin-2-yl, pyrazin-2-yl, or pyrimidin-4-y1), wherein
said 5- or 6-
membered heteroaryl is independently unsubstituted, mono- or di- substituted,
and wherein
the substituent(s), if any, is(are) independently selected from 01_3-alkyl
(especially methyl),
03_5-cycloalkyl (especially cyclopropyl), or 01_3-alkoxy (especially methoxy);
or
[in particular such C3_5-alkyl which represents 3-hydroxy-3-phenyl-propyl, 3-
hydroxy-3-phenyl-butyl, 3-hydroxy-
3-(3-fluoro-pheny1)-butyl, 3-hydroxy-3-(2-methoxy-pheny1)-butyl, 3-hydroxy-3-
(4-methoxy-phenyl)-butyl, 3-
hydroxy-3-(6-methoxy-pyridin-2-y1)-butyl, 3-hydroxy-3-(5-methyl-pyridin-3-y1)-
butyl, 3-hydroxy-3-(pyridin-2-y1)-
butyl, 3-hydroxy-3-(6-methyl-pyridin-2-y1)-butyl, 3-hydroxy-3-(pyrimidin-2-y1)-
butyl, 3-hydroxy-3-(6-methoxy-
pyrimidin-4-y1)-butyl, 3-hydroxy-3-(6-methyl-pyrimidin-4-y1)-pentyl,
3-hydroxy-3-(2-methyl-pyrimidin-4-y1)-
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
37
butyl, 3-hydroxy-3-(5-methyl-pyrazin-2-yI)-butyl, 3-hydroxy-3-(pyridin-2-yI)-
pentyl, or 3-hydroxy-3-(6-methoxy-
pyridin-2-y1)-pentyl]
9) Another embodiment relates to compounds according to any one of embodiments
1) to 4), wherein said substituent
of A/ at least one of said substituents of A is
= -
NRN3RN4, wherein Rio and RN4 form, together with the nitrogen to which they
are attached, a heterocyclic ring
as defined in embodiment 1).
10) Another embodiment relates to compounds according to embodiment 9),
wherein said substituent of A / at least
one of said substituents of A is
= -NRN3RN4 wherein
RN3 and RN4 form, together with the nitrogen to which they are attached, a
heterocyclic ring of 4 to 6
members (notably 5 to 6 members), wherein the members needed to complete said
heterocyclic ring
are each independently selected from -CH2-, -0-, -(0=0)-, -CHRx- and -C(RY)2-;
wherein said
heterocyclic ring does not contain more than one member independently selected
from the group
consisting of -0- and -(0=0)-; wherein said heterocyclic ring does not contain
more than two
members selected from the group consisting of -CHRx-; and wherein said
heterocyclic ring does not
contain more than two members selected from the group consisting of -C(RY)2-;
wherein Rx
independently represents fluorine, methyl, isopropyl, hydroxy,
trifluoromethyl, cyclopropyl, phenyl, 3-
methy1-1,2,4-oxadiazol-5-y1; and wherein RY independently represents fluorine,
hydroxy, cyclopropyl,
methyl, or trifluoromethyl [notably such -NRN3R"4 is pyrrolidinyl; 2-
pyrrolidonyl; oxazolidinonyl
(especially 1,3-oxazolidin-2-on-3-yI); piperidinyl; or morpholinyl, optionally
independently substituted
with one or two substituents independently selected from a group consisting of
Rx and RY].
[in particular such -NRN3RN4 represents pyrrolidin-1-yl, 3-fluoro-pyrrolidin-1-
yl, 3,3-difluoro-pyrrolidin-1-yl, 3,4-
difluoro-pyrrolidin-1-yl, 3-isopropyl-pyrrolidin-1-yl, 3,3-dimethyl-pyrrolidin-
1-yl, 3-hydroxy-pyrrolidin-1-yl, 2-
methyl-pyrrolidin-1-yl, 3-hydroxy-3-methyl-pyrrolidin-1-yl, 3-hydroxy-3-
trifluoromethyl-pyrrolidin-1-yl, 3-
trifluoromethyl-pyrrolidin-1-yl, morpholin-4-yl, 3-(tetrahydropyran-4-yI)-
pyrrolid-2-on-1-yl, pyrrolidin-2-on-1-yl,
4-phenyl-pyrrolidin-2-on-1-yl, 4-(pyridin-2-yI)-pyrrolidin-2-on-1-yl, 4-(6-
methyl-pyridin-3-yI)-pyrrolidin-2-on-1-yl,
4-(2-isopropyl-pyrimidin-4-yI)-pyrrolidin-2-on-1-yl,
4-(6-isopropyl-pyridin-2-yI)-pyrrolidin-2-on-1-yl, 4-(6-
trifluoromethyl-pyridin-3-y1)-pyrrolidin-2-on-1-yl, 4-methyl-pyrroliclin-2-on-
1-yl, 4-isopropyl-pyrrolidin-2-on-1-yl,
3-isopropyl-pyrrolidin-2-on-1-yl, 3,3-dimethyl-pyrrolidin-2-on-1-yl,
4,4-dimethyl-pyrrolidin-2-on-1-yl, 3-
(piperidin-4-yI)-pyrrolidin-2-on-1-yl, 4-(6-isopropyl-pyridin-2-yI)-pyrrolidin-
2-on-1-yl, 4-isobutyl-pyrrolidin-2-on-
l-yl, 4-cyclopropyl-pyrrolidin-2-on-1-yl, 4-trifluoromethyl-pyrrolidin-2-on-1-
yl, 2,2,6,6-tetrafluoro-morpholin-4-
yl, 2,6-dimethyl-morpholin-4-yl, 3-(3-methy1-1,2,4-oxadiazol-5-y1)-pyrrolidin-
1-yl, or 3-(phenyl)-pyrrolidin-1-yl]
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
38
11) Another embodiment relates to compounds according to any one of
embodiments 1) to 4), wherein said substituent
of Al at least one of said substituents of A is
5- or 6-membered heteroaryl as defined in embodiment 1), wherein the
substituent(s) of said 5- or 6-membered
heteroaryl, if any, is(are) independently selected from
Ci_4-alkyl as defined in embodiment 1).
12) Another embodiment relates to compounds according to embodiment 11),
wherein said substituent of A / at least
one of said substituents of A is
fr 5- or 6-membered heteroaryl containing from one to three (notably two or
three; especially three) ring
heteroatom(s) independently selected from nitrogen, oxygen and sulfur (notably
pyrazolyl, oxazolyl, thiazolyl,
oxadiazolyl, pyrimidinyl, or pyridinyl; especially pyrazol-1-yl, 1H-1,2,3-
triazol-1-yl, oxazol-2-yl, thiazol-2-yl,
1,2,4-oxadiazol-5-yl, 1,2,4-oxadiazol-3-yl, pyrimidin-2-yl, or pyridin-2-y1;
notably 1,2,4-oxadiazol-5-y1 or 1,2,4-
oxadiazol-3-y1; especially 1,2,4-oxadiazol-3-y1); wherein said 5- or 6-
membered heteroaryl is independently
unsubstituted, mono-, di-, or tri-substituted (notably mono-substituted;
especially mono-substituted in position
3 with respect to the point of attachment of said 5- or 6-membered heteroaryl
to A), wherein the substituent(s),
if any, is(are) independently selected from
fr Ci4-alkyl (especially methyl, ethyl, propyl, isopropyl, or tert-butyl)
which is
= unsubstituted;
= mono-substituted with
hydroxy; or
C14-alkoxy (especially methoxy and tert-butoxy); or
[in particular such 014-alkyl represents methyl, isopropyl, hydroxy-methyl, 1-
hydroxy-1-
methyl-ethyl, 2-hydroxy-2-methyl-propyl, 2-hydroxy-1,1-dimethyl-ethyl, methoxy-
methyl, 2-methoxy-ethyl, 1-methoxy-1-methyl-ethyl, 2-methoxy-2-methyl-propyl,
2-
methoxy-1,1-dimethyl-ethyl, or tert-butoxy-methyl; in particular such 014-
alkyl
represents 1-hydroxy-1-methyl-ethyl, 2-hydroxy-2-methyl-propyl, or 2-hydroxy-
1,1-
dimethyl-ethyl]
= di-substituted, wherein one substituent is hydroxy, and another
substituent is trifluoromethyl;
or two substituents are hydroxy;
= di- or tri-substituted, wherein two substituents are fluorine and, if
present, one substituent is
hydroxy (wherein the hydroxy group is separated by at least two carbon atoms
from any of
said fluorine substituents);
[in particular such C14-alkyl represents methyl, isopropyl, hydroxy-methyl, 1-
hydroxy-1-methyl-ethyl,
2-hydroxy-2-methyl-propyl, 2-hydroxy-1,1-dimethyl-ethyl, methoxy-methyl, 2-
methoxy-ethyl, 1-
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
39
methoxy-1-methyl-ethyl, 2-methoxy-2-methyl-propyl, 2-methoxy-1,1-dimethyl-
ethyl, tert-butoxy-
methyl; 1,2-dihydroxy-ethyl, N-acetyl-2-amino-ethyl, N-(acetyl-2,2,2-d3)-2-
amino-ethyl, 2-
(methylcarboxamido)-2-methyl-propyl, 2-(ethylcarboxamido)-2-methyl-
propyl, 2-(cyclopropyl-
carboxamido)-2-methyl-propyl, 2-(tetrahydropyran-4-yl-carboxamido)-2-methyl-
propyl, 2-(methoxy-
methyl-carboxamido)-2-methyl-propyl, 2-(ethyl-
carboxamido)-2-methyl-propyl, 2-(isopropyl-
carboxamido)-2-methyl-propyl, 2-(methyl-d3-carboxamido)-2-methyl-propyl, N-
methyl-N-(hydroxy-
methyl-carbony1)-2-amino-ethyl, N-methyl-N-acetyl-2-amino-ethyl,
2,2,2-trifluoro-1-hydroxy-1-
methyl-ethyl, 1,1-difluoro-2-hydroxy-ethyl, 1,1-difluoro-ethyl, or N-methyl-N-
(acety1-2,2,2-d3)-2-
amino-ethyl; in particular such 014-alkyl group is 1-hydroxy-1-methyl-ethyl, 2-
hydroxy-2-methyl-
propyl, or 2-hydroxy-1,1-dimethyl-ethyl]
13) Another embodiment relates to compounds according to embodiment 11),
wherein said substituent of A / at least
one of said substituents of A is
1,2,4-oxadiazol-5-y1 or 1,2,4-oxadiazol-3-y1 (especially 1,2,4-oxadiazol-3-
y1), wherein said oxadiazolyl groups
are mono-substituted, wherein the substituent is independently selected from
> C14-alkyl (especially methyl, ethyl, propyl, isopropyl, ot tetrt-butyl)
which is
= mono-substituted with
= hydroxy; or
+ 014-alkoxy (especially methoxy and tert-butoxy).
[in particular such 014-alkyl represents hydroxy-methyl, 1-hydroxy-1-methyl-
ethyl, 2-
hydroxy-2-methyl-propyl, 2-hydroxy-1,1-dimethyl-ethyl, methoxy-methyl, 2-
methoxy-
ethyl, 1-methoxy-1-methyl-ethyl, 2-methoxy-2-methyl-propyl, 2-methoxy-1,1-
dimethyl-
ethyl, or tert-butoxy-methyl; in particular such C14-alkyl represents 1-
hydroxy-1-methyl-
ethyl, 2-hydroxy-2-methyl-propyl, or 2-hydroxy-1,1-dimethyl-ethyl]
14) Another embodiment relates to compounds according to embodiment 11),
wherein said substituent of A / at least
one of said substituents of A is
1,2,4-oxadiazol-3-yl, which is mono-substituted (especially in position 3 with
respect to the point of attachment
of said oxadiazolyl to A), wherein the substituent is
Ci4-alkyl (especially methyl, ethyl, propyl, isopropyl, or tert-butyl) which
is
= mono-substituted with
hydroxy;
[in particular such 014-alkyl represents hydroxy-methyl, 1-hydroxy-1-methyl-
ethyl, 2-
hydroxy-2-methyl-propyl, 2-hydroxy-1,1-dimethyl-ethyl, in particular such 014-
alkyl
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
represents 1-hydroxy-1-methyl-ethyl, 2-hydroxy-2-methyl-propyl, or 2-hydroxy-
1,1-
dinnethyl-ethyl]
15) Another embodiment relates to compounds according to embodiment 11),
wherein A represents pyridin-3-yl,
wherein said pyridin-3-y1 is mono-substituted in meta-position with respect to
the point of attachment of said pyridin-3-
5 yl to the rest of the molecule, wherein the substituent is
1,2,4-oxadiazol-3-yl, which is mono-substituted (especially in position 3 with
respect to the point of attachment
of said oxadiazolyl to A), wherein the substituent is hydroxy-methyl, 1-
hydroxy-1-methyl-ethyl, 2-hydroxy-2-
methyl-propyl, or 2-hydroxy-1,1-dimethyl-ethyl.
[in particular such 1,2,4-oxadiazol-3-y1 represents 5-(hydroxy-methyl)-1,2,4-
oxadiazol-3-yl, 5-(1-hydroxy-1-
10 methyl-ethyl)-1,2,4-oxadiazol-3-yl, 5-(2-hydroxy-2-methyl-propy1)-
1,2,4-oxadiazol-3-yl, or 5-(2-hydroxy-1,1-
dimethyl-ethyl)-1,2,4-oxadiazol-3-yl]
16) Another embodiment relates to compounds according to any one of
embodiments 1) to 4), wherein said substituent
of Al at least one of said substituents of A is
5- or 6-membered heteroaryl as defined in embodiment 1), wherein the
substituent(s) of said 5- or 6-membered
15 heteroaryl, if any, is(are) independently selected from
¨L3¨CY3 as defined in embodiment 1).
17) Another embodiment relates to compounds according to embodiment 16),
wherein said substituent of A / at least
one of said substituents of A is
= 5- or 6-membered heteroaryl containing from one to three (notably two or
three; especially three) ring
20 heteroatom(s) independently selected from nitrogen, oxygen and sulfur
(notably pyrazolyl, oxazolyl, thiazolyl,
oxadiazolyl, pyrimidinyl, or pyridinyl; especially pyrazol-1-yl, 1H-1,2,3-
triazol-1-yl, oxazol-2-yl, thiazol-2-yl,
1,2,4-oxadiazol-5-yl, 1,2,4-oxadiazol-3-yl, pyrimidin-2-yl, or pyridin-2-y1;
notably 1,2,4-oxadiazol-5-y1 or 1,2,4-
oxadiazol-3-y1; especially 1,2,4-oxadiazol-3-y1); wherein said 5- or 6-
membered heteroaryl is independently
unsubstituted, mono-, di-, or tri-substituted (notably mono-substituted;
especially mono-substituted in position
25 3 with respect to the point of attachment of said 5- or 6-membered
heteroaryl to A), wherein the substituent(s),
if any, is(are) independently selected from
¨L3¨CY3, wherein
= ¨L3¨ independently represents a bond (i.e. the CY3 is directly attached
to the rest of the
molecule), -CH2-, -CH2-CH2-, -C(CH3)2-, -CH(OH)-, or -0-CH2-, wherein when
¨L3¨ is -0-
30 CH2-, said CY3 is attached to the oxygen atom of said -0-
CH2-; and
= CY3 independently represents piperidinyl; wherein said piperidinyl
independently is
unsubstituted, mono-, di-, or tri-substituted as defined in embodiment 1).
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
41
18) Another embodiment relates to compounds according to embodiment 16),
wherein said substituent of A / at least
one of said substituents of A is
1,2,4-oxadiazol-5-y1 or 1,2,4-oxadiazol-3-y1 (especially 1,2,4-oxadiazol-3-
y1), wherein said oxadiazoly1 groups
are mono-substituted, wherein the substituent is independently selected from
= -L3-CY3, wherein
= -L3- independently represents a bond (i.e. the CY3 is directly attached
to the rest of the
molecule), -CH2-, -CH2-CH2-, -C(CH3)2-, -CH(OH)-, or -0-CH2-, wherein when -L3-
is -0-
CH2-, said CY3 is attached to the oxygen atom of said -0-CH2-; and
= CY3 independently represents piperidinyl (notably piperidin-2-yl,
piperidin-3-yl, or piperidin-
4-y1; especially piperidin-4-y1) wherein said piperidinyl independently is
unsubstituted, mono-
, di-, or tri-substituted (especially unsubstituted, mono-, or di-substituted)
with
fr halogen (especially fluorine);
fr oxo;
= hydroxy;
Ci_3-alkyl which is optionally mono-substituted with 01_3-alkoxy (especially
such Ci_
3-alkyl represents methyl, ethyl, propyl, isopropyl, or methoxy-methyl);
fr C1_3-alkoxy (especially methoxy);
-(C=O)-R , wherein Rc represents
o C1_3-alkyl which is optionally mono-substituted with hydroxy or C1_3-
alkoxy
(especially methyl, ethyl, n-propyl, isopropyl, tert-butyl, hydroxy-methyl,
methoxy-methyl, benzyl-oxy, or 2-methoxy-ethyl; in particular said -
(C=0)-Rc is acetyl);
o C1_3-fluoroalkyl (especially 2,2,2-trifluoro-ethy1)1
o 01_3-alkoxy (especially methoxy);
o C3_6-cycloalkyl-(CH2).-, wherein optionally one or two carbon ring atom(s)
is/are replaced by one or two oxygen ring atom(s); wherein n represents
the integer 0, or 1 (especially such 035-cycloalkyl-(CH2)õ- represents
cyclopropyl, cyclopentyl, oxetan-3-yl, oxetan-3-yl-methyl, 1,4-dioxan-2-yl,
or tetrahydropyran-4-y; or
o phenyl;
[especially such -(C=0)-Rco represents acetyl, ethyl-carbonyl, n-propyl-
carbonyl,
isopropyl-carbonyl, tert-butyl-carbonyl, hydroxymethyl-carbonyl, 2,2,2-
trifluoro-
ethyl-carbonyl, methoxy-methyl-carbonyl, 2-methoxy-ethyl-carbonyl, methoxy-
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
42
carbonyl, ethoxy-carbonyl, 2-methoxy-ethoxy-carbonyl, cyclopropyl-carbonyl,
cyclopentyl-carbonyl, oxetan-3-yl-carbonyl, oxetan-3-yl-methyl-carbonyl,
phenyl-
carbonyl, or tetrahydropyran-4-yl-carbonyl, methoxy-methyl-carbonyl, or 1,4-
dioxan-2-yl-carbonyl]
-S(=0)2-Rso, wherein Rs represents
o C1_3-alkyl which is optionally mono-substituted with hydroxy, C1_3-
alkoxy,
or amino (especially methyl, n-propyl, isopropyl, 2-hydroxy-ethyl, 2-
methoxy-ethyl, or methyl-amino); or
o C35-cycloalkyl, wherein optionally one carbon ring atom is replaced by
one oxygen ring atom (especially represents cyclopropyl, cyclopentyl, or
tetrahydropyran-4-y;
[especially such -S(=0)2-Rs0 represents methyl-sulfonyl, n-propyl-sulfonyl,
isopropyl-sulfonyl, 2-hydroxyethyl-sulfonyl, cyclopropyl-sulfonyl, cyclopentyl-
sulfonyl, 2-methoxy-ethyl-sulfonyl, methyl-amino-sulfonyl, or tetrahydropyran-
4-yl-
sulfonyl]
[in particular such -L3-CY3 piperidin-4-yl, 1-methyl-2,6-dioxo-piperidin-4-yl,
4-methyl-piperidin-4-yl,
4-fluoro-piperidin-4-yl, 2-oxo-piperidin-4-yl, 6-oxo-piperidin-2-yl, 6-oxo-
piperidin-3-yl, 1-methy1-6-oxo-
piperidin-3-yl, 3-methyl-6-oxo-piperidin-3-yl, N-acetyl-piperidin-4-yl, N-
acetyl-piperidin 4 yl methyl,
N-acetyl-2-methyl-piperidin-4-yl, N-acetyl-4-fluoro-piperidin-4-yl, N-acetyl-3-
methyl-piperidin-4-yl, N-
acetyl-4-methyl-piperidin-4-yl, N-acetyl-4-hydroxy-piperidin-4-yl, N-acetyl-4-
methoxy-piperidin-4-yl,
N-acetyl-piperidin-3-yl-methyl, N-acetyl-3-methyl-piperidin-3-yl, N-acety1-3-
hydroxy-piperidin-3-yl, N-Osopropyl-carbony1)-4-hydroxy-
piperidin-4-yl, N-(tert-butyl-carbonyI)-
piperidin-4-yl, N-(2-methoxy-ethoxy-carbonyI)-piperidin-4-yl, N-(hydroxymethyl-
carbonyI)-piperidin-
4-yl, N-(cyclopropyl-sulfonyI)-piperidin-4-yl, N-(methyl-sulfonyI)-piperidin-4-
yl, N-(n-propyl-sulfonyI)-
piperidin-4-yl, N-(isopropyl-sulfonyI)-piperidin-4-yl, N-(2-hydroxy-ethyl-
sulfonyI)-piperidin-4-yl, N-
(cyclopentyl-sulfonylypiperidin-4-yl, N-(methoxy-carbonyl)-piperidin-4-yl, N-
(cyclopropyl-carbonyI)-
piperidin-4-yl, N-(cyclopentyl-carbonyI)-piperidin-4-yl, N-(methyl-amino-
sulfonyl)-piperidin-4-yl, N-(2-
methoxy-ethyl-sulfonyI)-piperidin-4-yl, N-(ethyl-carbonyI)-4-hydroxy-
piperidin-4-yl, N-(methoxy-
methyl-carbony1)-piperidin-4-yl, N-(ethoxy-carbonyI)-piperidin-4-yl, N-acetyl-
4-ethyl-piperidin-4-yl, N-
(n-propyl-carbonyl)-piperidin-4-yl, N-(2-
methoxy-ethyl-carbonyl)-piperidin-4-yl, N-(oxetan-3-yl-
carbony1)-piperidin-4-yl, N-(oxetan-3-yl-methyl-carbonyI)-
piperidin-4-yl, N-(2,2, 2-trifluoro-ethyl-
carbonyI)-piperidin-4-yl, N-(tetr2hydropyr2n-4-yl-c2rbonyI)-
piperidin-4-yl, N-(phenyl-carbonyI)-
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
43
piperidin-4-yl, N-(tetrahydropyran-4-yl-sulfonyI)-piperidin-4-yl, N-(benzyl-
oxy-carbonyI)-piperidin-4-yl,
N-(methoxy-methyl-carbonyI)-4-isopropyl-piperidin-4-yl, N-(1,4-dioxan-2-yl-
carbonylypiperidin-4-y1].
19) Another embodiment relates to compounds according to embodiment 16),
wherein said substituent of A / at least
one of said substituents of A is
1,2,4-oxadiazol-3-yl, wherein said oxadiazolyl group is mono-substituted,
wherein the substituent is
independently selected from
= ¨L3¨CY3 , wherein
= ¨L3¨represents a bond (i.e. the CY3 is directly attached to the rest of
the molecule); and
= CY3 independently represents piperidin-4-y1 which is unsubstituted, or
mono- or di-
substituted, wherein
> one substituent is attached to the nitrogen atom of said piperidine
ring, wherein the
substituent is ¨(C=0)¨Reo, wherein Re represents
o 01_3-alkyl optionally mono-substituted, wherein the substituent
represents
01_3-alkoxy or hydroxy (especially such 01_3-alkyl is methyl, ethyl, n-propyl,
isopropyl, tert-butyl, hydroxy-methyl, methoxy-methyl, 0r2-methoxy-ethyl;
in particular said ¨(C=0)¨Rc is acetyl);
o C1_3-fluoroalkyl (especially 2,2,2-trifluoro-ethyl);
O 01_3-alkoxy (especially methoxy);
O 03_6-cycloalkyl-(0H2).-, wherein optionally one or two carbon ring
atom(s)
is/are replaced by one or two oxygen ring atom(s); wherein n represents
the integer 0, or 1 (especially such 03_5-cycloalkyl-(CH2)õ- represents
cyclopropyl, cyclopentyl, oxetan-3-yl, oxetan-3-yl-methyl, 1,4-dioxan-2-yl,
or tetrahydropyran-4-y; or
[especially such ¨(C=0)¨Rc0 represents acetyl, ethyl-carbonyl, n-propyl-
carbonyl,
isopropyl-carbonyl, tert-butyl-carbonyl, hydroxymethyl-carbonyl, 2,2,2-
trifluoro-
ethyl-carbonyl, methoxy-methyl-carbonyl, 2-methoxy-ethyl-carbonyl, methoxy-
carbonyl, ethoxy-carbonyl, 2-methoxy-ethoxy-carbonyl, cyclopropyl-carbonyl,
cyclopentyl-carbonyl, oxetan-3-yl-carbonyl,
oxetan-3-yl-methyl-carbonyl,
tetrahydropyran-4-yl-carbonyl, methoxy-methyl-carbonyl, or 1,4-dioxan-2-yl-
carbonyl]
> and/or one substitutent is attached to a carbon atom of the piperidine ring,
wherein said
substituent is 013-alkyl (especially methyl), halogen (especially fluorine),
hydroxy, or 01 3-
alkoxy (especially methoxy).
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
44
20) Another embodiment relates to compounds according to any one of
embodiments 1) to 4), wherein said substituent
of Al at least one of said substituents of A independently is
= ¨CEC-R11; or
= ¨CEC-C(OH)(R12)(R13);
wherein said groups are as defined in embodiment 1).
21) Another embodiment relates to compounds according to embodiment 20),
wherein said substituent of A / at least
one of said substituents of A is
= ¨CEC-R11, wherein
y R-rr represents
= 014-alkyl (notably methyl, ethyl, isopropyl, or isobutyl), wherein said
014-alkyl independently
is mono-substituted with
= hydroxy;
[especially such 014-alkyl represents hydroxy-methyl, 1-hydroxy-ethyl, 2-
hydroxy-ethyl, 1-
hydroxy-2-methyl-propyl, or 1-hydroxy-1-methyl-ethyl];
Oi_3-alkoxy (especially methoxy);
= ¨S(=0)2¨Rs T, wherein RsoT represents C1_3-alkyl, C1_3-alkyl-amino, or C3-
b-
cycloalkyl (especially such ¨S(=0)2¨RsoT represents methyl-sulfonyl, methyl-
amino-sulfonyl or cyclopropyl-sulfonyl);
= C4_6-heterocycly1 containing one or two ring heteroatom(s) independently
selected
from nitrogen and oxygen (notably oxazolidinyl, imidazolidinyl, or
pyrrolidinyl;
especially oxazolidin-3-yl, imidazolidin-3-yl, or pyrrolidin-111); wherein
said 04_6-
heterocyclyl is mono-substituted with oxo; or di-substituted with oxo and one
C1-3-
alkyl (especially methyl); (especially such 04_6-heterocycly1 represents
oxazolidin-
2-on-3-yl, imidazolidin-2-on-3-yl, 1-methyl-imidazolidin-2-on-3-yl, or
pyrrolidin-2-
on-1-yI);
= 014-alkyl (notably methyl, ethyl, isopropyl, or isobutyl) which is
independently di-substituted,
wherein one substituent is hydroxy, and a second substituent is
trifluoromethyl (especially
1-hydroxy-1-trifluoromethyl-ethyl);
= 03_6-cycloalkyl (especially cyclopropyl) which is mono-substituted
(especially at the point of
attachment of the Cm-cycloalkyl to the rest of the molecule) with
= hydroxy;
= amino-sulfonyl which is optionally di-substituted with methyl;
= phenyl which is mono-substituted with halogen (especially 4-fluoro-
phenyl);
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
+ pyridinyl (especially pyridine-2-yl);
+ pyrimidinyl which is mono-substituted with 013-alkyl (especially 6-methyl-
pyrimidin-
4-yI);
= oxazolidinonyl (especially oxazolidin-2-on-3-y;
5 [in particular such 03_6-cycloalkyl represents 1-hydroxy-
cyclopropyl, 1-hydroxy-cyclobutyl, 1-
hydroxy-cyclopenty, 1-(amino-sulfonyI)-cyclopropyl,
1-(dimethyl-amino-sulfonyI)-
cyclopropyl, 1-(6-methyl-pyrimidin-4-yI)-cyclopropyl, 1-(pyridine-2-yI)-
cyclopropyl, 1-(4-
fluoro-pheny1)-cyclopropyl, 1-(pyridine-2-yI)-cyclopropyl, or 1-(oxazolidin-2-
on-3-yI)-
cyclopropyl];
10 = C46-heterocycly1 containing one ring heteroatom
independently selected from nitrogen and
oxygen (notably azetidinyl, pyrrolidinyl, piperidinyl, tetrahydropyranyl;
especially azetidin-3-
yl, piperidin-4-yl, pyrrolidin-3-yl, pyrrolidin-2-yl, pyrrolidine-1-yl, or
tetrahydropyran-4-y;
wherein said C4_6-heterocycly1 is mono-, di-, or tri-substituted (especially
mono- or di-
substituted), wherein the substituent(s) is(are) independently selected from
01_3-alkyl
15 (especially methyl), hydroxy, oxo, 01_3-alkyl-carbonyl
(especially acetyl), 01_3-alkoxy-
carbonyl (especially tert-butoxy-carbonyl), 01_3-alkyl-sulfonyl (especially
methyl-sulfonyl),
and 01_3-alkyl-amino-sulfonyl (especially methyl-amino-sulfonyl);
[in particular such C4_6-heterocycly1 represents N-Osopropyl-carbony1)-3-
hydroxy-azetidin-3-
yl, N-(tert-butoxy-carbonyl)-3-hydroxy-azetidin-3-yl, N-methy1-3-hydroxy-
pyrrolidin-2-one-3-
20 yl, N-acetyl-2-methyl-pyrrolidin-2-yl, 3-hydroxy-N-(tert-
butoxy-carbonyl)-pyrrolidin-3-yl, 2-
oxo-pyrrolidine-1-yl, N-acetyl-piperidin-4-yl, N-acetyl-4-methyl-piperidin-4-
yl, N-(methyl-
amino-sulfony1)-4-methyl-piperidin-4-yl, N-acetyl-4-hydroxy-
piperidin-4-yl, N-(methyl-
sulfony1)-piperidin-4-yl, N-(tert-butoxy-carbonyI)-piperidin-4-yl, 3-hydroxy-2-
oxo-1-methyl-
pyrrolidin-2-yl, or 4-hydroxy-tetrahydropyran-4-yI);
25 = indolyl (especially indo1-2-y1);;
= 3-hydroxy-1-methy1-1,3-dihydro-indo1-2-on-3-y1; or
[in particular such -CEC-R11 represents 3-hydroxy-3-trifluoromethyl-but-1-yn-1-
yl, 3-hydroxy-prop-1-yn-1-yl, 4-
hydroxy-but-1-yn-1-yl, 3-hydroxy-but-1-yn-1-yl, 3-hydroxy-3-methyl-but-1-yn-1-
yl, 3-hydroxy-4-methyl-pent-1-
yn-1-yl, (1-hydroxy-cyclopropyI)-ethynyl, (1-hydroxy-cyclobutyI)-ethynyl, (1-
hydroxy-cyclopenty1)-ethynyl, (8-
30 hydroxy-5,6,7,8-tetrahydroguinolin-8-yI)-ethynyl,
(7-hydroxy-6,7-dihydro-5H-cyclopenta[b]pyridin-7-yI)-
ethynyl, (4-hydroxy-tetrahydropyran-4-yI)-ethynyl, (1-(isopropyl-carbony1)-3-
hydroxy-azetidin-3-y1)-ethynyl, (1-
(tert-butoxy-carbony1)-3-hydroxy-azetidin-3-y1)-ethynyl, (N-acetyl-4-hydroxy-
piperidin- (3-hydroxy-N-(tert-
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
46
butoxy-carbony1)-pyrrolidin-3-y1)-ethynyl, (3-hydroxy-1-methyl-1,3-dihydro-
indo1-2-on-3-y1)-ethynyl, or (3-
hydroxy-2-oxo-1-methyl-pyrrol idin-3-y1)-ethynyl]
= -CEC-C(OH)(R12)(R13) is as defined in embodiment 1).
22) Another embodiment relates to compounds according to embodiment 20),
wherein said substituent of A / at least
one of said substituents of A is
= -CC-C(OH)(RT2)(RT3), wherein
= RT2 represents hydrogen or 01_3-alkyl (notably methyl or ethyl;
especially methyl);
= RT3 represents
= 6-membered heteroaryl containing one or two ring nitrogen atoms
(especially pyridinyl,
pyrazinyl, or pyrimidinyl; especially pyridin-2-yl, pyrimidin-2-yl, pyrazin-2-
yl, or pyrimidin-4-
yl; in particular pyrimidin-4-y1), wherein said 6-membered heteroaryl is
independently
unsubstituted, mono- or di- substituted; wherein the substituent(s), if any,
is(are)
independently selected from Ci_3-alkyl (especially methyl), 01_3-cycloalkyl
(especially
cyclopropyl), C1_3-fluoroalkyl (notably Ci-fluoroalkyl; especially
difluoromethyl or
trifluoromethyl), and 01_3-alkoxy (especially methoxy);
[in particular such 6-membered heteroaryl is pyridin-2-yl, 6-methoxy-pyridin-2-
yl, 6-methyl-
pyridin-2-yl, pyrimidin-2-yl, 2-methoxy-pyrimidin-4-yl, 6-methoxy-pyrimidin-4-
yl, pyrimidin-4-
yl, 2-methyl-pyrimidin-4-yl, 6-methyl-pyrimidin-4-yl, 2,6-dimethyl-pyrimidin-4-
yl, 2,6-
dimethoxy-pyrimidin-4-yl, 2-methyl-6-methoxy-pyri midi n-
4-y1 , 2-methoxy-6-methyl-
pyrimidin-4-yl, 5-methyl-pyrazin-2-yl, 6-cyclopropyl-pyrimidin-4-yl, 6-
difluoromethyl-
pyrimidin-4-yl, 2-trifluoromethyl-pyrimidin-4-yl, or 6-trifluoromethyl-
pyrimidin-4-yl]
[in particular such -CEC-C(OH)(R12)(R13) represents 3-hydroxy-3-(pyrimidin-2-
y1)-but-1-yn-1-yl, 3-hydroxy-3-(6-
methyl-pyrimidin-4-y1)-but-1-yn-1-yl, 3-hydroxy-3-(2-methoxy-pyrimidin-4-
y1)-butl-yn-1-yl, 3-hydroxy-3-(2-
methoxy-6-methyl-pyrimidin-4-y1)-but-1-yn-1-yl, 3-hydroxy-3-(6-methoxy-
pyrimidin-4-y1)-but-1-yn-1-yl, 3-hydroxy-
3-(2,6-dimethoxy-pyri midi n-4-y1)-but-1-yn-1-yl, 3-hydroxy-3-(2-methyl-
pyrimidin-4-yI)-but-1-yn-1-yl, 3-hydroxy-3-
(6-methoxy-2-methyl-pyrimidin-4-y1)-but-1-yn-1-yl,
3-hydroxy-3-(pyridin-2-y1)-pent-1-yn-1-yl, 3-hydroxy-3-(6-
methoxy-pyridin-2-y1)-but-1-yn-1-yl, 3-hydroxy-3-(6-methyl-pyridin-2-y1)-
but-1-yn-1-yl, 3-hydroxy-3-(6-methyl-
pyridin-2-y1)-pent-1-yn-1-yl, 3-hydroxy-3-(2,6-dimethyl-pyrimidin-4-y1)-but-1-
yn-1-yl, 3-hydroxy-3-(6-methoxy-
pyridin-2-y1)-pent-1-yn-1-yl, 3-hydroxy-3-(6-cyclopropyl-pyrimidin-4-yI)-
but-1-yn-1-yl, 3-hydroxy-3-(6-
difluoromethyl-pyrimidin-4-y1)-but-1-yn-1-yl, 3-hydroxy-3-(2-trifluoromethyl-
pyrimidin-4-y1)-but-1-yn-1-yl, or 3-
hydroxy-3-(6-trifluoromethyl-pyrimidin-4-y1)-but-1-yn-1-yl]
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
47
23) Another embodiment relates to compounds according to any one of
embodiments 1) to 22), wherein B represents
phenyl, which is mono-, di- or tri-substituted, wherein a first substituent is
attached in para-position with respect to the
point of attachment of B to the rest of molecule, wherein said substituent is
selected from
)- 01_5-alkyl (notably methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl,
tert-butyl, 1-ethyl-propyl, 2,2-dimethyl-
propyl, 1,1-dimethyl-propyl; especially methyl, ethyl, or isopropyl; in
particular isopropyl);
= C1_3-alkoxy-C1_4-alkyl (especially 3-methoxy-propyl);
= Ci_2-fluoroalkyl (especially trifluoromethyl or 2,2,2-trifluoro-ethyl);
= 03_5-cycloalkyl (especially cyclopropyl or cyclobutyl) which
independently is unsubstituted or mono-substituted
(notably at the point of attachment of said C3_5-cyc1021ky1 to the rest of the
molecule) with C1_3-21ky1 (especially
methyl) or 01_3-fluoroalkyl (especially trifluoromethyl); and
= Ci-fluoroalkoxy (especially trifluoromethoxy);
and the remaining substituent(s) of B (wherein especially said remaining
substituent(s) is/are attached in meta-position
with respect to the point of attachment of B to the rest of molecule), if
present, independently is/are selected from
halogen (notably fluorine or chlorine; especially fluorine).
[in particular such group B represents 4-methyl-phenyl, 4-ethyl-phenyl, 4-
propyl-phenyl, 4-isopropyl-phenyl, 4-butyl-
phenyl, 4-isobutyl-phenyl, 4-tert-butyl-phenyl, 4-(1-ethyl-propyI)-phenyl, 4-
(1,1-dimethyl-propyI)-phenyl, 4-(2,2-
dimethyl-propy1)-phenyl, 3-chloro-4-isopropyl-phenyl, 3-fluoro-4-isopropyl-
phenyl, 3,5-difluoro-4-isopropyl-phenyl, 4-
cyclopropyl-phenyl, 4-trifluoromethyl-phenyl, 4-(2,2,2-trifluoro-ethylyphenyl,
4-trifluoromethoxy-phenyl, 4-(1-methyl-
cyclopropy1)-phenyl, 4-cyclobutyl-phenyl, 4-(3-methoxy-propyI)-phenyl, or 4-(1-
trifluoromethyl-cyclopropyI)-phenyl]
24) Another embodiment relates to compounds according to any one of
embodiments 1) to 22), wherein B represents
phenyl, which is mono-, di- or tri-substituted, wherein a first substituent is
attached in para-position with respect to the
point of attachment of B to the rest of molecule, wherein said substituent is
selected from
)- 02_4-alkyl (notably n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl; in
particular isopropyl);
= trifluoromethyl or 2,2,2-trifluoro-ethyl;
03_5-cycloalkyl (especially cyclopropyl or cyclobutyl) which independently is
unsubstituted or mono-substituted
(notably at the point of attachment of said 03_5-cycloalkyl to the rest of the
molecule) with 01_3-alkyl (especially
methyl) or 01_3-fluoroalkyl (especially trifluoromethyl); and
= Ci-fluoroalkoxy (especially trifluoromethoxy);
and the remaining substituent(s) of B (wherein especially said remaining
substituent(s) is/are attached in meta-position
with respect to the point of attachment of B to the rest of molecule), if
present, independently is/are selected from
halogen (notably fluorine or chlorine; especially fluorine).
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
48
[in particular such group B represents 4-propyl-phenyl, 4-isopropyl-phenyl, 4-
butyl-phenyl, 4-isobutyl-phenyl, 4-tert-
butyl-phenyl, 3-chloro-4-isopropyl-phenyl, 3-fluoro-4-isopropyl-phenyl, 3,5-
difluoro-4-isopropyl-phenyl, 4-cyclopropyl-
phenyl, 4-trifluoromethyl-phenyl, 4-trifluoromethoxy-phenyl, 4-(2,2,2-
trifluoro-ethylyphenyl, 4-(1-methyl-cyclopropyI)-
phenyl, 4-cyclobutyl-phenyl, or 4-(1-trifluoromethyl-cyclopropyI)-phenyl]
25) Another embodiment relates to compounds according to any one of
embodiments 1) to 22), wherein B represents
phenyl, which is mono-substituted, wherein the substituent is attached in para-
position with respect to the point of
attachment of B to the rest of molecule, wherein said substituent is selected
from isopropyl (preferred), trifluoromethyl,
trifluoromethoxy, trifluoromethyl, cyclopropyl, cyclobutyl, 1-methyl-
cyclopropyl, and 1-trifluoromethyl-cyclopropyl.
[in particular such group B represents 4-isopropyl-phenyl, 4-cyclopropyl-
phenyl, 4-trifluoromethyl-phenyl, 4-
trifluoromethoxy-phenyl, 4-(1-methyl-cyclopropyI)-phenyl, 4-cyclobutyl-phenyl,
or 4-(1-trifluoromethyl-cyclopropyI)-
phenyl]
26) Another embodiment relates to compounds according to any one of
embodiments 1) to 25), wherein R1 represents
01_3-alkyl (notably methyl or ethyl; especially methyl);
27) Another embodiment relates to compounds according to any one of
embodiments 1) to 26), wherein R2 represents
014-alkyl (especially methyl (preferred), ethyl, n-propyl, isopropyl, tert-
butyl or isobutyl), C3_5-cycloalkyl (especially
cyclopropyl or cyclobutyl), C3_5-cycloalkyl-C1_3-alkyl (especially cyclopropyl-
methyl), or C1_3-fluoroalkyl (especially 2,2-
difluoro-ethyl, or 2-fluoroethyl).
28) Another embodiment relates to compounds according to any one of
embodiments 1) to 26), wherein R2 represents
methyl (preferred), ethyl, n-propyl, isopropyl, cyclopropyl, or cyclobutyl.
29) Another embodiment relates to compounds according to embodiment 1),
wherein A represents pyridin-3-yl, wherein
said pyridin-3-y1 is mono-substituted in meta-position with respect to the
point of attachment of said pyridin-3-y1 to the
rest of the molecule, wherein the substituent is 5-(hydroxy-methyl)-1,2,4-
oxadiazol-3-yl, 5-(1-hydroxy-1-methyl-ethyl)-
1,2,4-oxadiazol-3-yl, 5-(2-hydroxy-2-methyl-propy1)-1,2,4-oxadiazol-3-yl, or 5-
(2-hydroxy-1,1-dimethyl-ethyl)-1,2,4-
oxadiazol-3-y1;
B represents 4-propyl-phenyl, 4-isopropyl-phenyl, 4-isobutyl-phenyl, 4-tert-
butyl-phenyl, 3-fluoro-4-isopropyl-phenyl,
3,5-difluoro-4-isopropyl-phenyl, 4-cyclopropyl-phenyl, 4-trifluoromethyl-
phenyl, 4-trifluoromethoxy-phenyl, 4-(2,2,2-
trifluoro-ethylyphenyl, 4-cyclobutyl-phenyl, or 4-(1-trifluoromethyl-
cyclopropylyphenyl;
RI represents methyl; and
R2 represents methyl.
30) Another embodiment relates to compounds according to embodiment 1),
wherein A represents pyridin-3-yl, wherein
said pyridin-3-y1 is mono-substituted in meta-position with respect to the
point of attachment of said pyridin-3-y1 to the
rest of the molecule, wherein the substituent is
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
49
1,2,4-oxadiazol-3-yl, wherein said oxadiazolyl group is mono-substituted,
wherein the substituent is piperidin-
4-y1 which is unsubstituted, or mono- or di-substituted, wherein one
substituent is attached to the nitrogen
atom of said piperidine ring, wherein the substituent is
= acetyl, ethyl-carbonyl, n-propyl-carbonyl, isopropyl-carbonyl, tert-butyl-
carbonyl, hydroxymethyl-
carbonyl, 2,2,2-trifluoro-ethyl-carbonyl, methoxy-methyl-carbonyl, 2-methoxy-
ethyl-carbonyl,
methoxy-carbonyl, ethoxy-carbonyl, 2-methoxy-ethoxy-carbonyl, cyclopropyl-
carbonyl, cyclopentyl-
carbonyl, oxetan-3-yl-carbonyl, oxetan-3-yl-methyl-carbonyl, tetrahydropyran-4-
yl-carbonyl,
methoxy-methyl-carbonyl, or 1,4-dioxan-2-yl-carbonyl;
and/or one substituent is attached to a carbon atom of the piperidine ring,
wherein said substituent is 013-alkyl
(especially methyl), halogen (especially fluorine), hydroxy, or C1_3-alkoxy
(especially methoxy).
B represents 4-propyl-phenyl, 4-isopropyl-phenyl, 4-isobutyl-phenyl, 4-tert-
butyl-phenyl, 3-fluoro-4-isopropyl-phenyl,
3,5-difluoro-4-isopropyl-phenyl, 4-cyclopropyl-phenyl, 4-trifluoromethyl-
phenyl, 4-trifluoromethoxy-phenyl, 442,2,2-
trifluoro-ethyl)-phenyl, 4-cyclobutyl-phenyl, or 4-(1-trifluoromethyl-
cyclopropyI)-phenyl;
R1 represents methyl; and
R2 represents methyl.
31) Another embodiment relates to compounds according to embodiment 1),
wherein A represents pyridin-3-yl, wherein
said pyridin-3-y1 is mono-substituted in meta-position with respect to the
point of attachment of said pyridin-3-y1 to the
rest of the molecule, wherein the substituent is 3-hydroxy-3-(pyrimidin-2-yI)-
but-1-yn-1-yl, 3-hydroxy-3-(6-methyl-
pyrimidin-4-y1)-but-1-yn-1-yl, 3-hydroxy-3-(2-methoxy-pyrimidin-4-y1)-but-1-yn-
1-yl, 3-hydroxy-3-(2-methoxy-6-methyl-
pyrimidin-4-yI)-but-1-yn-1-yl, 3-
hydroxy-3-(6-methoxy-pyrimidin-4-yI)-but-1-yn-1-yl, 3-hydroxy-3-(2,6-
dimethoxy-
pyrimidin-4-y1)-but-1-yn-1-yl, 3-hydroxy-3-(2-methyl-pyrimidin-4-yI)-but-1-yn-
1-yl, 3-hydroxy-3-(6-methoxy-2-methyl-
pyrimidin-4-y1)-but-1-yn-1-yl, 3-hydroxy-3-(2,6-dimethyl-pyrimidin-4-yI)-
but-1-yn-1-yl, 3-hydroxy-3-(6-cyclopropyl-
pyrimidin-4-y1)-but-1-yn-1-yl, 3-hydroxy-3-(6-difluoromethyl-pyrimidin-4-
yI)-but-1-yn-1-yl, 3-hydroxy-3-(2-
trifluoromethyl-pyrimidin-4-y1)-but-1-yn-1-yl, or 3-hydroxy-3-(6-
trifluoromethyl-pyrimidin-4-yI)-but-1-yn-1-yl]
B represents 4-propyl-phenyl, 4-isopropyl-phenyl, 4-isobutyl-phenyl, 4-tert-
butyl-phenyl, 3-fluoro-4-isopropyl-phenyl,
3,5-difluoro-4-isopropyl-phenyl, 4-cyclopropyl-phenyl, 4-trifluoromethyl-
phenyl, 4-trifluoromethoxy-phenyl, 442,2,2-
trifluoro-ethylyphenyl, 4-cyclobutyl-phenyl, or 4-(1-trifluoromethyl-
cyclopropylyphenyl;
RI represents methyl; and
R2 represents methyl.
32) Another embodiment relates to compounds according to any one of
embodiments 1) to 31), which are also
compounds of Formula (II) (i.e. the asymmetric carbon atom to which A and B
are attached has the absolute
configuration depicted in Formula (ID)
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
A
R2-N B
R'
Formula (II)
33) Another embodiment relates to a compound according to embodiment 1)
selected from a group consisting of
(3-Fluoro-1-methyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-y1)-(4-
trifluoromethoxy-pheny1)-methanol;
5 3-[Hydroxy-(5-pyrrolidin-1-yl-pyridin-3-y1)-(4-trifluoromethoxy-phenyl)-
methy11-1-methyl-azetidine-3-carbonitrile;
(R)-(1-Ethy1-3-methyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-y1)-(4-
trifluoromethoxy-phenylymethanol;
(R)-(3-Methyl-1-propyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-y1)-(4-
trifluoromethoxy-pheny1)-methanol;
(R)-(1-lsopropy1-3-methyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-y1)-(4-
trifluoromethoxy-pheny1)-methanol;
(R)-(1-Cyclopropylmethy1-3-methyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-
y1)-(4-trifluoromethoxy-pheny1)-methanol;
10 (R)-(1-Cyclobuty1-3-methyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-
y1)-(4-trifluoromethoxy-pheny1)-methanol;
(R)-(1-lsobuty1-3-methyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-y1)-(4-
trifluoromethoxy-pheny1)-methanol;
(R)-(1-Cyclopropy1-3-methyl-azetidin-3-y1)-(5-pyrrolidin 1 yl pyridin-3-y1)-(4-
trifluoromethoxy-pheny1)-methanol;
(R)-0-(2-Fluoro-ethyl)-3-methyl-azetidin-3-y1]-(5-pyrrolidin-l-yl-pyridin-3-
y1)-(4-trifluoromethoxy-phenylymethanol;
(R)-0-(2,2-Difluoro-ethyl)-3-methyl-azetidin-3-y1]-(5-pyrrolidin-1-yl-pyridin-
3-y1)-(4-trifluoromethoxy-pheny1)-methanol;
15 (R)-(1-tert-Buty1-3-methyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-
y1)-(4-trifluoromethoxy-pheny1)-methanol;
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(3-methoxy-prop-1-yny1)-
pyridin-3-y11-methanol
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-0-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-prop-1-yn-1-ol;
4-{5-[(R)-(1,3-Dimethyl-szetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridin-3-y1}-2-methyl-but-3-yn-2-ol;
(R)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-
methyl]-pyridin-3-yll-but-3-yn-2-ol;
20 (S)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y11-but-3-yn-2-ol;
1-{5-[(R)-(1, 3-Di methyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridi n-3-ylethynylycyclopentanol ;
1-{5-[(R)-(1, 3-Di methyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridi n-3-ylethynylycyclopropanol ;
3-{5-[(R)-(l
carboxylic acid tert-butyl ester;
25 1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylFpyridin-3-ylethynylycyclobutanol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheng-methyl]-
pyridin-3-y11-but-3-yn-1-ol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(1-methy1-1H-pyrazol-4-
ylethyny1)-pyridin-3-y1]-methanol;
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-1-phenyl-prop-2-yn-1-ol,
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridin-3-y1}-2-phenyl-but-3-yn-2-ol;
30 1-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridi n-3-yI}-4-methyl-pent-1-yn-3-ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-ylethynylytetrahydro-pyran-4-ol;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
51
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-y1}-1-(tetrahydro-pyran-4-y1)-prop-
2-yn-1-01;
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridin-3-y1}-1-(1,3-dimethyl-1H-pyrazol-4-
y1)-prop-2-yn-1-ol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(tetrahydro-pyran-4-
ylethyny1)-pyridin-3-y1]-methanol;
3-{51(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyll-
pyridin-3-y11-1-(2-methyl-thiazol-4-y1)-prop-
2-yn-1-01;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-2-(3-fluoro-pheny1)-but-3-yn-2-
ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-2-(4-methoxy-pheny1)-but-3-
yn-2-ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-2-(2-methoxy-pheny1)-but-3-
yn-2-ol;
4-{51(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridi n-3-y1}-2-(1-methyl-1H-pyrazol-3-y1)-
but-3-yn-2-ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-2-(2-methyl-thiazol-4-y1)-but-3-
yn-2-ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyriclin-3-y1}-2-(6-methoxy-pyridin-2-y1)-but-
3-yn-2-ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyriclin-3-y1}-2-pyrimidin-2-yl-but-3-yn-2-ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-2-(1,5-dimethyl-1H-pyrazol-3-
y1)-but-3-yn-2-ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-2-(6-methyl-pyrimidin-4-y1)-
but-3-yn-2-ol;
3-151(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridin-3-y1}-1-(1,5-dimethyl-1H-pyrazol-3-
y1)-prop-2-yn-1-ol;
8-{5-[(R)-(1,3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-rnethyl]-
pyridi n-3-ylethyny1}-5,6,7,8-tetrahydro-
qui nolin-8-ol;
7-{5-[(R)-(1,3-Di methyl-azetidin-3-0-hydroxy-(4-isopropyl-pheny1)-rnethyl]-
pyridi n-3-ylethyny1}-6,7-di hydro-5H-
[1]pyrindin-7-ol;
1-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-y1}-3-pyridin-2-yl-pent-1-yn-3-ol;
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-3-(6-methoxy-pyridin-2-y1)-
pent-1-yn-3-ol;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
52
1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-0-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-ylethynyll-3-hydroxy-azetidin-
1-y1)-2-methyl-propan-1-one;
(R)-(1,3-Dimethyl-azetidin-3-y1)-[5-(1H-indo1-2-ylethyny1)-pyridin-3-y1]-(4-
isopropyl-phenyl)-methanol;
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(3-methoxy-propy1)-pyridin-
3-y11-methanol;
3-{5-[(R)-(1,3-Dimethyl-azetidin-311)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-propan-1-ol;
4-{5-[(R)-(1, 3-Di methyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-phenyl)-methyll-
pyridi n-3-yI}-2-methyl-butan-2-ol ;
(S)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylypyridin-3-ylybutan-2-ol;
(R)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-
methyl]-pyridin-3-yll-butan-2-ol;
1-(2-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-yll-ethyl)-cyclopentanol;
1-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-ylyhydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-ylyethyl)-cyclopropanol;
3-(2-{5-[(R)-(1,3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridi n-3-ylyethyl)-3-hydroxy-azetid ine-
1-carboxylic acid tert-butyl ester;
1-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-ylyhydroxy-(4-isopropyl-pheny1)-
methylFpyridin-3-ylyethyl)-cyclobutanol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-yll-butan-1-ol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{542-(1-methy1-1H-
pyrazol-4-y1)-ethyl]-pyridin-3-y1}-methanol;
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-1-phenyl-propan-1-ol;
4-{5-[(R)-(1, 3-Di methyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-phenyl)-methyll-
pyridi n-3-yI}-2-phenyl-butan-2-ol;
1-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridi n-3-yI}-4-methyl-pentan-3-ol;
4-(2-{5-[(R)-(1,3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methy1]-pyridi n-3-yll-ethylytetrahydro-pyran-4-
ol;
3-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridi n-3-y1}-1-(tetrahydro-pyran-4-y1)-
propan-1-ol;
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-1-(1,3-dimethyl-1H-pyrazol-4-
y1)-propan-1-ol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{542-(tetrahydro-pyran-4-
y1)-ethyl]-pyridin-3-yll-methanol;
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-1-(2-methyl-thiazol-4-y1)-
propan-1-ol;
4-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-y11-2-(3-fluoro-pheny1)-butan-2-ol;
4-{5-[(R)-(1,3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridi n-3-y1}-2-(4-methoxy-pheny1)-butan-2-
ol;
4-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-y1}-2-(2-methoxy-pheny1)-butan-2-
ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y11-2-(1-methy1-1H-pyrazol-3-y1)-
butan-2-ol;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
53
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyll-
pyridin-3-y1}-2-(2-methyl-thiazol-4-y1)-
butan-2-ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridin-3-y1}-2-(6-methoxy-pyridin-2-y1)-
butan-2-ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-2-pyrimidin-2-yl-butan-2-ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyll-
pyridin-3-y1}-2-(1,5-dimethyl-1H-pyrazol-3-
y1)-butan-2-ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-2-(6-methyl-pyrimidin-4-y1)-
butan-2-ol;
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-1-(1,5-dimethyl-1H-pyrazol-3-
y1)-propan-1-01;
8-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridin-3-y1}-ethyl)-5,6,7,8-tetrahydro-
quinolin-8-ol;
7-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-yll-ethyl)-6,7-dihydro-5H-
[1]pyrindin-7-ol;
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-3-pyridin-2-yl-pentan-3-ol;
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyll-
pyridin-3-y1}-3-(6-methoxy-pyridin-2-y1)-
pentan-3-ol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-{5-[2-(1H-indo1-2-y1)-ethyl]-pyridin-3-y11-(4-
isopropyl-phenyl)-methanol;
(R)-(4-Cyclopropyl-pheny1)-(1,3-dimethyl-azetidin-3-y1)-{543-(tetrahydro-pyran-
4-y1)41,2,41oxadiazol-5-y11-pyridin-3-yll-
methanol;
(R)-(4-Cyclopropyl-pheny1)-(1,3-dimethyl-azetidin-3-y1)-{543-(2-methoxy-1,1-
dimethyl-ethyl)-[1,2,4]oxadiazol-5-y1F
pyridin-3-yI}-methanol;
(R)45-(3-Cyclobutoxymethyl-[1,2,4]oxadiazol-5-y1)-pyridin-3-y1]-(4-cyclopropyl-
pheny1)-(1,3-dimethyl-azetidin-3-y1)-
methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-propyl-pheny1)-{5-[3-(tetrahydro-pyran-4-
yloxymethyl)-[1,2,4]oxadiazol-511]-pyridin-
3-yll-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-propyl-pheny1)-{543-(tetrahydro-pyran-4-
y1)41,2,4]oxadiazol-5-y11-pyridin-3-yll-
methanol;
(R)45-(3-Cyclobutoxymethyl-[1,2,4]oxadiazol-5-y1)-pyridin-3-y1]-(1,3-dimethyl-
azetidin-3-y1)-(4-propyl-phenyl)-
methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{543-(tetrahydro-pyran-4-
y1)41,2,4]oxadiazol-5-y1]-pyridin-3-yll-
methanol;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
54
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)4543-morpholin-4-ylmethyl-
[1,2,41oxadiazol-5-y1)-pyridi n-3-y11-
methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-{543-(2,6-dimethyl-morpholin-4-
ylmethyly[1,2,4]oxadiazol-5-y1]-pyridin-3-y11-(4-
isopropyl-phenyI)-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{543-(4-methyl-
tetrahydro-pyran-4-y1)41,2,4]oxadiazol-5-y1]-
pyridin-3-ylymethanol;
(R)-(1,3-D imethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-{5-[(1S,23,4R)-3-(7-
oxa-bicyclo [2.2. 1]hept-2-y1)41,2, 4]oxadi azol-
5-yI]-pyridi n-3-ylymethanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{543-(tetrahydro-pyran-4-
yloxymethy1)41 ,2, 41oxadiazol-5-y11-
pyridin-3-ylymethanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(3-morpholin-4-y1-0
,2,4]oxadiazo1-5-y1)-pyridin-3-y1]-methanol;
(R)-(4-Cyclopropyl-pheny1)-(1,3-dimethyl-azetidin-3-y1)-{543-(4-methoxy-
tetrahydro-pyran-4-y1)-[1,2,4]oxadiazol-5-A-
pyridin-3-ylymethanol;
(R)-(4-Cyclopropyl-phenyI)-(1, 3-dimethyl-azetidin-3-y1){5[343-hydroxymethyl-
bicyclo[1.1.1] pent-1-yI)-
[1,2, 4]oxadiazol-511]-pyridi n-3-yll-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-{543-(3-hydroxymethyl-bicyclo[1.1.1]pent-1-
y1)41,2,4]oxadiazol-5-y1]-pyridin-3-y11-(4-
isopropyl-phenylymethanol;
2-(5-{5-[(R)-(1, 3-Di nnethyl-azetidi n-3-y1 yhydroxy-(4-isopropyl-
phenylymethyl]-pyridi 4]oxadi azol-3-y1)-2-
methyl-propan-1-ol;
2-(5-{5-[(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-yly[1,2,41oxadi azol-3-y1)-
propan-2-ol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{543-(1-methoxy-1-methyl-
ethyl)41 , 2,4]oxadiazol-5-yli-pyridi n-3-
yll-methanol;
(R)-(1,3-D imethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-{543-(1-methoxy-
cyclobuty1)41,2, 4]oxadi azol-511]-pyridin-3-y11-
methanol;
1-(5-{54(R)-(1,
methyl-azetidi n-3-y1 yhydroxy-(4-isopropyl-phenylymethy1]-pyridi n-3-
yly[1,2,4]oxadi azol-3-y1)-2-
methyl-propan-2-ol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(5-
methanesulfonylmethy141 ,2,41oxadiazol-3-y1)-pyridin-3-y11-
methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{54542-methoxy-ethyl)-[1
,2, 4]oxadiazol-3-y1Fpyridin-3-y11-
methanol;
(R)-(1, 3-D imethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)45-(5-
methoxymethy141,2,4]oxadiazol-3-y1)-pyrid in-3-y1]-
methanol;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
(R)-(1,3-D imethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-{545-(tetrahydro-furan-
3-y1)41,2,41oxadi azol-3-y11-pyridi
methanol;
(R)-(1,3-D imethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-{5-[5-(tetrahydro-
pyran-4-y1)-[1,2, LI]oxadiazol-311]-pyridin-3-yll-
methanol;
5 1-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1141,2,4]oxadi azol-5-y1)-
cyclohexanol ;
(R)45-(5-tert-Butoxymethyl-[1,2,4]oxadiazol-3-y1)-pyridin-3-y1]-(1,3-dimethyl-
azetidin-3-y1)-(4-isopropyl-pheny1)-
methanol;
(R)-(1,3-D imethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-{545-(tetrahydro-pyran-
4-ylmethy1)41,2,41oxadi azol-3-y11-pyridin-
10 3-yll-methanol;
4-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y11-[1,2,4]oxadi azol-5-y1)-
cyclohexanol ;
1-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-
phenylymethylFpyridi n-3-y11-[1,2, il]oxadi azol-5-
yl methyl)-cyclohexanol ;
15 (R)-(1,3-D imethyl-ezetidi n-3-y1)-(4-isopropyl-pheny1)-{545-(1-methoxy-
cyclobuty1)41,2, 4]oxadi
methanol;
(R)-(1,3-D imethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-{5-[5-(6-oxa-
spiro[2.51oct-1-y1)-[1,2,41oxadiazol-3-y11-pyridi
methanol;
(R)-(1,3-D imethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-{545-(tetrahydro-pyran-
3-y1)41,2, 4]oxadiazol-3-y1]-pyridin-3-yll-
20 methanol;
(R)-(1,3-D imethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-(5-{5-[1-(tetrahydro-
furan-2-yl)methyl]-[1,2, 4]oxadiazol-3-yll-
pyridi n-3-yI)-methanol ;
(R)-2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1141,2,4]oxadiazol-5-y1)-
1,1,1-trifluoro-propan-2-ol;
25 (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{545-(1-methoxy-1-
methyl-ethyl)-[1,2,4]oxadiazol-3-A-pyridin-3-
yll-methanol;
(R)-{5-[5-((R)-Cyclohexyl-hydroxy-methyl)-[1,2, 4]oxadiazol-311]-pyridi n-3-
y1)-(1, 3-dimethyl-azetidin-3-yI)-(4-isopropyl-
phenyI)-methanol;
1-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1141,2,4]oxadi azol-5-y1)-
30 cyclopropanol;
2-(3-15-[(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-y1141,2,41oxadi azol-5-y1)-
propan-2-ol;
1-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1141,2,4]oxadi azol-5-y1)-
cyclopentanol;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
56
3-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-y1141,2,41oxadi azol-5-y1)-
cyclobutanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-{5-[5-(4-fluoro-tetrahydro-pyran-4-
y1)41,2,4]oxadiazol-3-y1]-pyridin-3-y11-(4-isopropyl-
pheny1)-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-{5454(2R,4R,6S)-2,6-dimethyl-tetrahydro-pyran-
4-y1)41,2,4]oxadiazol-3-y1]-pyridin-3-
y11-(4-isopropyl-pheny1)-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{545-(tetrahydro-pyran-4-
yloxymethy1)41,2,4]oxadiazol-3-y1]-
pyridin-3-yll-methanol;
(R)-(1,3-D imethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-(5-1541-methyl-1-
(tetrahydro-pyran-4-y1)-ethy1141,2, 41oxadiazol-
3-yll-pyriclin-3-y1)-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(5-{542-(tetrahydro-
pyran-4-y1)-ethyl]- [1 ,2,4]oxadiazol-3-yll-
pyridin-3-y1)-methanol;
1-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylFpyridin-3-y1141,2,4]oxadi azol-5-y1)-
cyclobutanol;
1-(3-{5-[(R)-(1, 3-Di methyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridi n-3-y11-[1,2, 4]oxadi azol-5-y1)-2-
methyl-propan-2-ol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{5-[5-(7-oxa-
bicyclo[2.2.1]hept-2-y1)-[1,2,4]oxadiazol-3-y11-pyridin-
3-y1}-methanol;
(R)-(1,3-D imethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-{545-(4-methyl-
tetrahydro-pyran-4-yloxymethy1)41, 2,4]oxadiazol-
3-yll-pyriclin-3-yll-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[5-(5-oxetan-3-y1-
[1,2,4]oxadiazol-3-y1)-pyridin-311]-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{545-(2-methoxy-1,1-di
methyl-ethyl)-[1,2,4]oxadiazol-3-yli-
pyridin-3-y1}-methanol;
2-(3-{5-[(R)-(1, 3-Di methyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-nheny1)-
methyl]-pyridi n-311141,2, 4]oxadi azol-5-y1)-2-
methyl-propan-1-ol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{5-[5-(2-methoxy-2-
methyl-propy1)-[1,2,4]oxadiazol-3-y1]-pyriclin-
3-yll-methanol;
1-(3-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-y1141,2,41oxadiazol-5-
ylmethyl)-cyclobutanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{545-(1-methoxymethyl-
cyclopropylmethyl)-[1,2,4]oxadiazol-3-y1]-
pyridin-3-yll-methanol;
(R)-(1,3-D imethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-{545-(2-pyrazol-1-yl-
ethy1)41,2, zfloxadiazol-3-y1]-pyridin-3-yll-
methanol;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
57
(R)-N-(2-(3-(54(1,3-dimethylazetidin-3-y1)(hydroxy)(4-
isopropylphenyl)methyppyridin-3-y1)-1,2,4-oxadiazol-5-
ypethypacetamide-2,2,2-d3;
(R)-N-(1-(3-(5-((1,3-dimethylazetidin-3-y1)(hydroxy)(4-
isopropylphenyl)methyppyridin-3-y1)-1,2,4-oxadiazol-5-y1)-2-
methylpropan-2-ypacetamide-2,2,2-d3;
144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1141,2,4]oxadiazol-5-y1)-
piperidin-1-y11-2-hydroxy-ethanone;
(R)-1-(4-(3-(54(1,3-dimethylazetidin-3-y1)(hydroxy)(4-
isopropylphenyl)methyppyridin-3-y1)-1,2,4-oxadiazol-5-
yl)piperidin-1-yl)ethan-1-one-2,2,2-d3;
N42-(3-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-y1141,2,41oxadiazol-5-y1)-
ethyl]-2-hydroxy-N-methyl-acetamide;
(R)-N-(2-(3-(5-((1,3-dimethylazetidin-3-y1)(hydroxy)(4-
isopropylphenyl)methyppyridin-3-y1)-1,2,4-oxadiazol-5-ypethyl)-
N-methylacetamide-d3;
(1,3-Dimethyl-azetidin-3-y1)-(6-methoxy-pyridin-3-y1)-(4-trifluoromethoxy-
phenylymethanol;
(1,3-Dimethyl-azetidin-3-y1)-(6-phenoxy-pyridin-3-y1)-(4-trifluoromethoxy-
pheny1)-methanol;
(1,3-Dimethyl-azetidin-3-y1)-(6-ethoxy-pyridin-3-y1)-(4-trifluoromethoxy-
pheny1)-methanol;
(1,3-Dimethyl-azetidin-3-y1)-(5-methyl-pyridin-3-y1)-(4-trifluoromethoxy-
pheny1)-methanol;
(1,3-Dimethyl-azetidin-3-y1)-(4-propyl-pheny1)-(5-pyrrolidin-1-yl-pyridin-3-
y1)-methanol;
(1,3-Dimethyl-azetidin-3-y1)-(4-methoxy-pheny1)-(5-pyrrolidin-1-yl-pyridin-3-
y1)-methanol;
(1,3-Dimethyl-azetidin-3-y1)-(4-ethyl-pheny1)-(5-pyrrolidin-1-yl-pyridin-3-y1)-
methanol;
(1,3-Dimethyl-azetidin-3-y1)-phenyl-(5-pyrrolidin-1-yl-pyridin-3-y1)-methanol;
(4-Cyclobutyl-pheny1)-(1,3-dimethyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-
3-y1)-methanol;
(4-Cyclobutoxy-pheny1)-(1,3-dimethyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-
3-y1)-methanol;
(1,3-Dimethyl-azetidin-3-y1)-(4-ethoxy-pheny1)-(5-pyrrolidin-1-yl-pyridin-3-
y1)-methanol;
(4-tert-Butyl-pheny1)-(1,3-dimethyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-
3-y1)-methanol;
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropoxy-pheny1)-(5-pyrrolidin-1-yl-pyridin-
3-y1)-methanol;
(1,3-Dimethyl-azetidin-3-y1)-(5-pyrrolidin 1 yl pyridin-3-y1)44-(1-
trifluoromethyl-cyclopropy1)-phenyl]-mothanol;
(1,3-Dimethyl-azetidin-3-y1)-[4-(1-methyl-cyclopropy1)-phenyl]-(5-pyrrolidin-1-
yl-pyridin-3-y1)-methanol;
(4-Cyclopropoxy-pheny1)-(1,3-dimethyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-
pyridin-3-y1)-methanol;
(S)-[2-(3,3-Difluoro-pyrrolidin-1-y1)-pyridin-4-y1]-(1,3-dimethyl-azetidin-3-
y1)-(4-isopropyl-phenyl)-methanol;
(S)-[24(3R,4S)-3,4-Difluoro-pyrrolidin-1-y1)-pyridin-4-y1]-(1,3-dimethyl-
azetidin-3-y1)-(4-isopropyl-pheny1)-methanol;
(S)-(1,3-Dimethyl-azetidin-3-y1)-(2-isobutoxy-pyridin-4-y1)-(4-isopropyl-
pheny1)-methanol;
4-{4-[(S)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-2-y1}-2-methyl-butan-2-ol;
(R)46-(3,3-Difluoro-pyrrolidin-1-y1)-pyridazin-4-y1]-(1,3-dimethyl-azetidin-3-
y1)-(4-isopropyl-pheny1)-methanol;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
58
(S)-5-tert-Buty1-3-{5-[(R)-(1,3-dimethyl-azetidin-3-0-hydroxy-(4-isopropyl-
pheny1)-methyll-pyridin-3-yll-oxazolidin-2-
one;
(R)-1 {5 [(R) (1,3 Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-
methyl]-pyridin-3-yll-pyrrolidin-3-ol;
(S)-1-{5-[(R)-(1,3-Dimethyl-azetidin-3-0-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-yll-pyrrolidin-3-ol;
(R)-(1,3-Dimethyl-azetidin-3-y1)454(S)-3-hydroxymethyl-pyrrolidin-1-y1)-
pyridin-3-y1]-(4-isopropyl-pheny1)-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(5-pyrrolidin-1-yl-
pyridin-3-y1)-methanol;
(S)-1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-01-3-methyl-pyrrolidin-3-ol;
3-Cyclopropy1-1-{5-[(R)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridin-3-yll-pyrrolidin-3-ol;
24(S)-1-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-yll-pyrrolidin-3-y1)-propan-
2-ol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(5-morpholin-4-yl-
pyridin-3-y1)-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(3,4,5,6-tetrahydro-2H-
[1,3]bipyridinyl-5'-y1)-methanol;
(R)45-(7-Aza-bicyclo[2.2.1]hept-7-y1)-pyridin-3-y1]-(1,3-dimethyl-azetidin-3-
y1)-(4-isopropyl-pheny1)-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[5-((S)-2-methyl-
pyrrolidin-1-y1)-pyridin-3-yI]-methanol;
1-{54(R)-(1,3-Dimethyl-azetidin-311)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-3-trifluoromethyl-pyrrolidin-3-
ol;
(R)-[5-(3,3-Difluoro-pyrrolidin-1-y1)-pyridin-3-y1]-(1,3-dimethyl-azetidin-3-
y1)-(4-isopropyl-phenyI)-methanol;
5'-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-methyl]-
3,4,5,6-tetrahydro-2H-[1,3]bipyridinyl-4-ol;
5'-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-methyl]-
3,4,5,6-tetrahydro-2H-[1,31bipyridinyl-3-ol:
(R)-{5-[(2-Benzyloxy-ethyl)-methyl-aminol-pyridin-3-y11-(1,3-dimethyl-azetidin-
3-y1)-(4-isopropyl-phenylymethanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-[4-(1-methyl-cyclopropy1)-phenyl]-(5-
pyrrolidin-1-yl-pyridin-3-y1)-methanol;
(R)-[54(3R,48)-3,4-Difluoro-pyrrolidin-1-0-pyridin-3-y1]-(1,3-dimethyl-
azetidin-3-044-(1-trifluoromethyl-cyclopropy1)-
phenyl]-methanol;
2-[(S)-1-(5-{(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-[4-(1-trifluoromethyl-
cyclopropy1)-phenyl]-methyl}-pyridin-3-y1)-
pyrrolidin-3-yI]-propan-2-ol;
(R)-(1,3-Dimethyl-azetidin-3-yI)-(5-pyrrolidin 1 yl pyridin-3-y1)-[4-(1-
trifluoromethyl-cyclopropylyphenyTmethanol;
(R)-(4-tert-Butyl-pheny1)-(1,3-dimethyl-azetidin-3-y1)-(3,4,5,6-tetrahydro-
2H41,3Thipyridinyl-51-y1)-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-y1)-(4-
trifluoromethoxy-phenyl)-methanol;
(R)-{545-(1-Cyclopropanesulfonyl-piperidin-4-y1)41,2,4]oxadiazol-311]-pyridin-
3-y1}-(1,3-dimethyl-azetidin-3-y1)-(4-
isopropyl-phenyI)-methanol;
2-(15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-yll-methyl-amino)-ethanol;
(R)-1-((S)-1-{5-[(R)-(1,3-Dimethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methy1]-pyridin-3-y1)-pyrrolidin-3-yI)-
ethanol;
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-oxazolidin-2-one;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
59
3-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyll-
pyridin-3-y11-5-phenyl-oxazolidi n-2-one;
5-Benzy1-3-{5-[(R)-(1, 3-dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyriclin-3-yll-oxazol id in-2-one;
3-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyq-
pyridin-3-y1}-5-isopropyl-oxazolidin-2-one;
6-{5-[(R)-(1, 3-Di methyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-phenylymethyll-
pyridi n-3-yI}-4-oxa-6-aza-spiro[2.4]heptan-
5-one;
3-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyll-
pyridi n-3-yI}-1-oxa-3-aza-spiro[4.4] nonan-
2-one;
3-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridi n-3-y11-5-(tetrahydro-pyran-4-y1)-
oxazolidin-2-one;
3-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyq-
pyridi n-3-yI}-5,5-di methyl-oxazol idin-2-one;
3-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridi n-3-yI}-8, 8-d ifluoro-1-oxa-3-aza-
spiro[4.5]clecan-2-one;
9-{5-[(R)-(1, 3-Di methyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-phenyl)-methyl]-
pyridi n-3-yI}-7-oxa-9-aza-
dispiro[3. 1.4.1]undecan-8-one;
2-Cyclopropy1-7-{5-[(R)-(1,3-dimethyl-ezetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyriclin-3-y11-5-oxa-7-eze-
spiro[3.4]octan-6-one;
7-{51(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyll-
pyridi n-3-y11-2,2-d imethy1-5-oxa-7-aza-
spiro[3.4]octan-6-one;
1-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-4-phenyl-pyrrolidi n-2-one;
1-{5-[(R)-(1, 3-Di methyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-phenylymethyll-
pyridi n-3-yI}-pyrrol idin-2-one;
1-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyq-
pyridi n-3-yI}-4-isopropyl-pyrrol idi n-2-one;
1-15-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyli-
pyridi n-3-yI}-3-isopropyl-pyrrol idi n-2-one;
1-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-4, 4-d imethyl-pyrrol idin-2-one;
5-(5-((R)-(1, 3-Di methyl-azetidi n-3-y1)(hydroxy)(4-isopropyl-
phenyl)methyl)pyricli n-3-yl)hexahydro-4H-furo[2,3-c] pyrrol-
4-one;
2-{5-[(R)-(1, 3-Di methyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyq-
pyridi n-3-yI}-2-aza-spi ro[4.4]nonan-3-one;
6-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridi n-3-yI}-6-aza-spi ro[3.4]octan-5-one;
2-{5-[(R)-(1, 3-Di methyl-azetidi n-311)-hydroxy-(4-isopropyl-pheny1)-rnethyl]-
pyridi n-3-y11-8-oxa-2-aza-spiro[4.5]decan-
3-one;
1-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyll-
pyridi n-3-y1}-3-(tetrahydro-pyran-4-y1)-
pyrrolidin-2-one;
2-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-methyl]-
pyridin-3-y11-2-aza-spi ro[4.5]clecan-1-one;
2-{5-[(R)-(1, 3-Di methyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridi n-311}-8-oxa-2-aza-spiro[4.5]decan-
1-one;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
(S)-1-{5-[(R)-(1, 3-Dimethyl-azetidin-3-y1)-hyd roxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-y11-4-isobutyl-pyrrol id in-2-one;
4-Cyclopropy1-1-{5-[(R)-(1,3-dimethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridi n-3-yll-pyrrolid in-2-one;
1-{5-[(R)-(1, 3-Di methyl-azetidin-3-0-hydroxy-(4-isopropyl-phenylymethyl]-
pyridi n-3-y1}-4-trifluoromethyl-pyrrol idin-2-
one;
5 1-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridi n-3-y1}-3-(2-methoxy-ethyl)-pyrrolidin-
2-one;
1-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridi n-3-y1}-4-(2-methoxy-ethyl)-pyrrolidin-
2-one;
1-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridazin-3-yll-pyrrolidin-2-one;
10 (1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-((R)-3-isopropyl-
pyrrolidi n-1-y1)-pyridin-3-y1]-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-[5-((2R,6S)-2,6-dimethyl-morpholin-4-y1)-
pyridin-3-y1]-(4-isopropyl-phenylymethanol;
(R)-(1,3-D imethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-[5-(6-oxa-3-aza-
bicyclo [3.1 .1]hept-3-y1)-pyridin-3-A-methanol;
(R)454(3R,4S)-3,4-Difluoro-pyrrolidin-1-y1)-pyridi n-3-y1]-(1,3-dimethyl-
azetidin-3-y1)-(4-isopropyl-pheny1)-methanol;
(R)-[54(38,48)-3,4-Difluoro-pyrrolidin-1-y1)-pyridin-3-y1]-(1,3-dimethyl-
azetidin-3-y1)-(4-isopropyl-pheny1)-methanol;
15 (R)-(1,3-Dimethyl-azetidin-3-y1)454(2S,6S)-2,6-dimethyl-morpholin-4-y1)-
pyridin-3-y1]-(4-isopropyl-pheny1)-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)454(2R,6R)-2,6-dimethyl-morpholin-4-y1)-
pyridin-3-y1]-(4-isopropyl-pheny1)-methanol;
(R)-(1,3-D imethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-{5-[3-(3-methyl-
[1,2,41oxadiazol-5-y1)-pyrrol idin-1-yll-pyridi
methanol;
(R)-(1,3-D imethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-{543-(4-methyl-thiazol-
2-y1)-pyrrol
20 methanol;
(R)-(1, 3-Di methyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[5-(3-phenyl-
pyrrolidin-1-y1)-pyri di n-3-y1]-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)45-(3,3-dimethyl-pyrrolidin-1-y1)-pyridin-3-
y1]-(4-isopropyl-pheny1)-methanol;
(R)-(1, 3-Dimethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-[(1S,4S)-5-(2-oxa-5-
aza-bicyclo[2.2. 1]hept-5-y1)-pyridin-3-y1]-
methanol;
25 (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[5-(2,2,6,6-
tetrafluoro-morpholin-4-y1)-pyridin-3-y1]-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[5-((R)-2-methoxymethyl-
morpholin-4-y1)-pyridin-311]-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[5-((S)-2-methoxymethyl-
morpholin-4-y1)-pyridin-3-y1]-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(3-trifluoromethyl-
pyrrolidin-1-y1)-pyridin-3-y11-methanol;
(R)-(1, 3-Dimethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-[(1R,4R)-5-(2-oxa-5-
aza-bicyclo[2.2. 1]hept-5-y1)-pyridin-3-y1]-
30 methanol;
(R)-(1, 3-Di methyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-[(1S,5R)-5-(8-oxa-3-
aza-bicyclo[3.2.1]oct-3-y1)-pyridin-3-y11-
methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-[(1S,4S)-5-(2-oxa-5-aza-bicyclo[2.2.1]hept-5-
y1)-pyridin-3-y1H4-(1-trifluoromethyl-
cyclopropy1)-phenyl]-methanol;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
61
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-ethyl-pheny1)-(5-pyrrolidin-1-yl-pyridin-3-
y1)-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-ethyl-pheny1)-[5-(6-oxa-3-aza-
bicyclo[3.1.1]hept-3-y1)-pyridin-3-y1]-methanol;
(R)-(1,3-D imethyl-azetidin-3-y1)-(4-ethyl-pheny1)-[(1S,4S)-5-(2-oxa-5-aza-
bicyclo[2.2.1]hept-5-y1)-pyridin-3-y1]-
methanol;
(R)-(5-Benzyloxy-pyridin-3-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-isopropyl-
pheny1)-methanol;
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[5-(2-pyridin-2-yl-ethoxy)-
pyridin-3-y11-methanol;
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(2-methoxy-ethoxy)-pyridin-
311]-methanol;
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(oxetan-3-ylmethoxy)-
pyridin-3-y1]-methanol;
3-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-yloxyl-propan-1-ol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(5-isopropoxy-pyridin-3-y1)-(4-isopropyl-
pheny1)-methanol;
(R)-(5-Cyclohexyloxy-pyridin-3-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-isopropyl-
pheny1)-methanol;
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-yloxy}-2-methyl-propan-2-ol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(3-methoxy-
cyclopentyloxy)-pyridin-3-y1]-methanol;
(R)-[5-(3-Cyclopropyl-[1,2,4]oxadiazol-5-ylmethoxy)-pyrid in-3-y1]-(1,3-di
methyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
methanol;
(R)-(1,3-D imethyl-azetidi n-3-y1)-{542-(3,5-dimethyl-[1, 2,4]triazol-1-y1)-
ethoxy]-pyridi n-3-y11-(4-isopropyl-pheny1)-
methanol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-yloxy}-2-methyl-butan-2-ol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(5-methoxy-pyridin-3-y1)-
methanol;
(R)45-(2-Benzyloxy-ethoxy)-pyridin-3-y1]-(1,3-dimethyl-azetidin-3-y1)-(4-
isopropyl-phenyl)-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[5-(tetrahydro-pyran-4-
yloxy)-pyridin-3-y1]-methanol;
2-15-[(R)-(1,3-Di methyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylFpyridin-3-yloxy}-ethanol ;
4-{5-[(R)-(1,3-D imethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridi n-3-yloxyl-cyclohexanol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-yloxyp -methyl-cyclohexanol;
(1,3-Dimethyl-azetidin-3-y1)-(2-phenoxy-pyrimidin-5-y1)-(4-trifluoromethoxy-
pheny1)-methanol;
(6-Benzyloxy-pyridin-3-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-trifluoromethoxy-
phenyl)-methanol;
(1,3-Dimethyl-azetidin-3-y1)-(5-pyrazol-1-yl-pyridin-3-y1)-(4-trifluoromethoxy-
phenylymethanol;
(1,3-Dimethyl-azetidin-3-y1)-(6-fluoro-5-pyrrolidin-1-yl-pyridin-3-y1)-(4-
trifluoromethoxy-pheny1)-methanol;
5-[(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-trifluoromethoxy-phenylymethyl]-3-
pyrrolidin-1-yl-pyridine-2-carbonitrile;
(1,3-Dimethyl-azetidin-3-y1)46-(tetrahydro-pyran-4-yloxy)-pyridin-3-y1]-(4-
trifluoromethoxy-pheny0-methanol;
(1,3-Dimethyl-azetidin-3-y1)-[6-(oxetan-3-ylmethoxy)-pyridin-3-y1]-(4-
trifluoromethoxy-pheny1)-methanol;
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(4-methyl-thiazol-2-y1)-
pyridin-3-y1]-methanol;
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(5-methyl-thiazol-2-y1)-
pyridin-311]-methanol;
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(2-methoxy-pyrimidin-5-y1)-
methanol;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
62
(S)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(2-pyrrolidin-1-yl-
pyridin-4-y1)-methanol;
(1,3-Dimethyl-azetidin-3-y1)42-((R)-2-hydroxymethyl-pyrrolidin-1-y1)-pyridin-4-
y1]-(4-isopropyl-phenylymethanol;
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(3,4,5,6-tetrahydro-2H-
[1,2]1Dipyridinyl-4'-y1)-methanol;
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(2-morpholin-4-yl-pyridin-4-
y1)-methanol;
(S)-(1,3-Dimethyl-azetidin-3-y1)-(2-ethyl-pyridin-4-y1)-(4-isopropyl-pheny1)-
methanol;
(S)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[2-(tetrahydro-pyran-4-
ylmethoxy)-pyridin-4-y11-methanol;
(S)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[2-(2-methoxy-ethoxy)-
pyridin-4-y1]-methanol;
(S)-(2-Cyclopentyl-pyridin-4-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-isopropyl-
pheny1)-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-y1)-(4-
trifluoromethyl-pheny1)-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)454(R)-3-hydroxymethy1-3-methyl-pyrrolidin-1-
y1)-pyridin-3-y1]-(4-isopropyl-pheny1)-
methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)454(S)-3-fluoro-pyrrolidin-1-y1)-pyridin-3-y1]-
(4-isopropyl-pheny1)-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{544-(tetrahydro-pyran-4-
y1)41,2,3]triazol-1-y1]-pyridin-3-yll-
methanol;
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(5-methyl-pyridin-3-y1)-
methanol;
(1,3-Dimethyl-azetidin-3-y1)-(5-isopropenyl-pyridin-3-y1)-(4-isopropyl-pheny1)-
methanol;
(5-Cyclopropyl-pyridin-3-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
methanol;
(5-Cyclopent-1-enyl-pyridin-3-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-isopropyl-
pheny1)-methanol;
3-{5-[(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-yll-cyclopent-2-enol;
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(5-isopropyl-pyridin-3-y1)-
methanol;
(5-Cyclopentyl-pyridin-3-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
methanol;
3-{5-[(1,3-Dimethyl-azetidin-3-0-hydroxy-(4-isopropyl-phenyl)-methy1]-pyridin-
3-yll-cyclopentanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-pyridin 3 yl methanol;
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-yll-cyclopent-2-enone;
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-1-methyl-cyclopent-2-enol;
(3S)-3-(5-((R)-(1,3-dimethylazetidin-3-y1)(hydroxy)(4-
isopropylphenyl)methyppyridin-3-y1)-1-methylcyclopentan-1-ol;
(3R)-3-(5-((R)-(1,3-dimethylazetidin-3-y1)(hydroxy)(4-
isopropylphenyl)methyppyridin-3-y1)-1-methylcyclopentan-1-ol;
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-0-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y11-1-ethyl-cyclopentanol;
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-1-isopropyl-cyclopentanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[5-(4-methyl-oxazol-2-
y1)-pyridin-3-y1]-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(5-ethyl-pyridin-3-y1)-(4-isopropyl-pheny1)-
methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(5-methyl-pyridin-3-y1)-
methanol;
(R)45-(4,5-Dihydro-furan-3-y1)-pyridin-3-y1]-(1,3-dimethyl-azetidin-3-y1)-(4-
isopropyl-pheny1)-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[5-(tetrahydro-furan-3-
y1)-pyridin-3-y1]-methanol;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
63
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-y1}-but-3-en-1-ol;
N-Cyclopenty1-5-[(R)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-nicotinamide;
{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridin-3-ylypyrrolidin-1-yl-methanone;
5-[(R)-(1,3-Dimethyl-azetidin-3-0-hydroxy-(4-isopropyl-pheny1)-methyll-N-
(tetrahydro-pyran-4-y1)-nicotinamide;
(1,3-Dimethyl-azetidin-3-y1)44-(3-methoxy-propy1)-pheny1]-(5-pyrrolidin-1-yl-
pyridin-3-y1)-methanol;
(R)-(4-Cyclopropyl-pheny1)-(1,3-dimethyl-azetidin-3-y1)-(5-pyrrolidin 1 yl
pyridin-3-yI)-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-y1)-p-tolyl-
methanol;
5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
3',4',5',6'-tetrahydro-2'H-[3,4']bipyridiny1-1'-
carboxylic acid tert-butyl ester;
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-pyrrolidine-1-carboxylic acid
tert-butyl ester;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[5-(tetrahydro-pyran-4-
y1)-pyridin-3-yI]-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(3',4',5',6'-tetrahydro-
2'H-[2,1';4',3"]terpyridin-5"-y1)-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(1'-phenyl-1 ',2',
3',4', 5',6'-hexahydro-[3, 4]bipyridiny1-5-y1)-
methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)411-(toluene-4-sulfony1)-
1',2',3',4',5',6'-hexahydro-[3,41]bipyridinyl-
5-y11-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[5-(tetrahydro-furan-2-
y1)-pyridin-3-yI]-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(5-methyl-tetrahydro-
furan-2-y1)-pyridin-3-y1]-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-[5-(5,5-dimethyl-tetrahydro-furan-2-y1)-
pyridin-3-y1]-(4-isopropyl-phenyI)-methanol;
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-2,2-difluoro-propan-1-ol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(5-methyl-oxazol-2-0-
pyridin-3-y1Fmethanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{545-(tetrahydro-pyran-4-
y1)-oxazol-2-y1]-pyridin-3-yll-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)45-(4-fluoro-phenoxymethyl)-pyridin-3-y1]-(4-
isopropyl-pheny1)-methanol;
Isopropyl-carbamic acid 5-[(R)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-pheny1)-methyl]-pyridin-3-ylmethyl
ester;
(R)-[5-(2-Benzyloxy-ethyl)-pyridin-3-y1]-(1,3-dimethyl-azetidin-3-y1)-(4-
isopropyl-phenylymethanol;
4-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-y1}-1-methyl-cyclohexanol;
2-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-ylyhydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-ylycyclopropy1)-propan-2-ol;
(S)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{245-(tetrahydro-pyran-4-
y1H1,2,4]oxadiazol-3-y1Fpyridin-4-y11-
methanol;
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(2-pyrrolidin-1-yl-pyrimidin-
5-y1)-methanol; and
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(6-pyrrolidin-1-yl-pyrazin-2-
y1)-methanol.
34) Another embodiment relates to a compound according to embodiment 1)
selected from a group consisting of
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
64
(R)-N-(1-(3-(54(1,3-dimethylazetidin-3-y1)(hydroxy)(4-
isopropylphenyl)methyppyridin-3-y1)-1,2,4-oxadiazol-5-
y1)cyclopropyl)acetamide-2,2,2-d3;
(R)-N-(1-(3-(5-((1,3-dimethylazetidin-3-y1)(hydroxy)(4-
isopropylphenyl)methyppyridin-3-y1)-1,2,4-oxadiazol-5-
y0cyclopropyl)-N-methylacetamide-d3;
(R)-N-((3-(54(1,3-dimethylazetidin-3-y1)(hydroxy)(4-
isopropylphenyl)methyppyridin-3-y1)-1,2,4-oxadiazol-5-yl)methyl)-
N-methylacetamide-d3;
N-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1H1,2,4]oxadiazol-5-
ylmethyl)-2-hydroxy-acetamide;
(R)-N-((3-(54(1,3-dimethylazetidin-3-y1)(hydroxy)(4-
isopropylphenyl)methyppyridin-3-y1)-1,2,4-oxadiazol-5-
yOmethypacetamide-2,2,2-d3;
1-(3-{5-[(R)-Hydroxy-(1-isopropy1-3-methyl-azetidin-3-y1)-(4-isopropyl-
phenylymethyl]-pyridin-3-yly[1,2,4]oxadiazol-5-
y1)-2-methyl-propan-2-ol;
1-(3-{5-[(R)-(1-Ethy1-3-methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethy1]-pyridin-3-y1H1,2,4]oxadiazol-5-y1)-2-
methyl-propan-2-ol;
2-(3-{5-[(R)-Hydroxy-(1-isopropy1-3-methyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1H1,2,4]oxadiazol-5-
y1)-propan-2-ol;
2-(3-{5-[(R)-(1-Ethy1-3-methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethy1]-pyridin-3-yly[1,2,41oxadiazol-5-y1)-
propan-2-ol;
4-(5-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1H1,2,4]oxadiazol-3-y1)-
piperidine-1-carboxylic acid benzyl ester;
1-[4-(5-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-yly[1,2,4]oxadiazol-3-y1)-
piperidin-1-y1Fethanone;
(R)-1-(3-(3-(54(1,3-dimethylazetidin-3-y1)(hydroxy)(4-
isopropylphenyl)methyppyridin-3-y1)-1,2,4-oxadiazol-5-
yl)azetidin-1-yl)ethan-1-one-2,2,2-d3;
(R)-1 (3 (3 (5 ((1,3-dimethylazetidin-3-y1)(hydroxy)(4-
isopropylphenyl)methyppyridin-3-y1)-1,2,4-oxadiazol-5-y1)-3-
methylazetidin-1-ypethan-1-one-2,2,2-d3;
(R)-1-(3-(3-(5-((1,3-dimethylazetidin-3-y1)(hydroxy)(4-
isopropylphenyl)methyppyridin-3-y1)-1,2,4-oxadiazol-5-y1)-3-
fluoroazetidin-1-yl)ethan-1-one-2,2,2-d3;
(R)-(1,3-D imethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-{5-[5-(1-methy1-1-
morphol in-4-yl-ethyl)-[1,2, 4]oxad iazol-3-y1]-
pyridin-3-yll-methanol;
4-(345-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-y1141,2,41oxadiazol-5-y1)-1-
methyl-piperidin-2-one;
1-[(S)-3-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylFpyridin-3-y1141,2,4]oxadiazol-5-
y1)-pyrrolidin-1-y1]-ethanone;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
1-[(R)-3-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-0-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-y1141,2,41oxadiazol-5-
y1)-pyrrolidin-1-y1]-ethanone;
N-[1-(3-{54(R)-(1,3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyriclin-3-y1H1 ,2,4]oxadiazol-5-y1)-1-
methyl-ethyll-acetamide;
5 1-Benzy1-3-(3-{5-[(R)-(1,3-dimethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyri di n-3-y1141,2,4]oxadiazol-5-
yI)-pyrrol idin-2-one;
3-(3-{5-[(R)-(1,3-Dinnethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1141,2,4]oxadiazol-5-y1)-1,3-
dimethyl-pyrrolidin-2-one;
4-(3-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-y1141,2,41oxadiazol-5-y1)-1-
10 isobutyl-pyrrolidin-2-one;
4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methy1]-
pyridin-3-y11-[1,2,4]oxadiazol-5-y1)-1-
furan-2-ylmethyl-pyrrolidin-2-one;
4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylFpyridin-3-y1141,2,4]oxadiazol-5-y1)-1-
phenyl-pyrrolidin-2-one;
15 1-Benzy1-4-(3-{5-[(R)-(1,3-dimethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyri di n-3-y1141 ,2,4]oxadiazol-5-
y1)-pyrrolidin-2-one;
5-(345-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyll-
pyridin-3-y1141,2,41oxadiazol-5-y1)-1-
phenyl-pyrrolidin-2-one;
4-(3-{5-[(R)-(1,3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y11-[1,2,4]oxadi azol-5-y1)-
20 pyrrolidin-2-one;
(S)-5-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y1}41,2,4]oxadiazol-5-y1)-
pyrrolidin-2-one;
(R)-5-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1141,2,4]oxadiazol-5-y1)-
pyrrolidin-2-one;
25 (S)-6-(345-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridin-3-yly[1,2,4]oxadiazol-5-y1)-
piperidin-2-one;
4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-0141,2,4]oxadiazol-5-y1)-
piperidin-2-one;
(S)-4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl
ymethy1]-pyrid in-3-y1}41,2,4]oxadiazol-5-y1)-
30 azetidin-2-one;
(S)-3-(3-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyrid [1 ,2,41oxadiazol-5-y1)-
azetidin-2-one;
(S)-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1H1 ,2,4]oxadiazol-5-y1)-1,4-
dimethyl-pyrrolidin-2-one;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
66
(R)-4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-y1141,2,41oxadiazol-5-y1)-
1,4-dimethyl-pyrrolidin-2-one;
(R) or (S)-4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y1141,2,41oxadiazol-
5-0-1,4-dimethyl-pyrrolidin-2-one;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-phenyl)45-(5-piperidin-4-
y141,2,4]oxadiazol-3-y1)-pyridin-3-y1]-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{545-(4-methyl-piperidin-
4-y1)-[1,2,41oxadiazol-3-y11-pyridin-3-y11-
methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-{545-(4-fluoro-piperidin-4-
y1)41,2,4]oxadiazol-3-y1]-pyridin-3-y11-(4-isopropyl-pheny1)-
methanol;
1-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-yly[1,2,4]oxadiazol-5-y1)-
piperidin-1-y1]-butan-1-one;
1-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y1)-[1,2,4]oxadiazol-5-y1)-
piperidin-1-yI]-2,2-dimethyl-propan-1-one;
144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1H1,2,4]oxadiazol-5-y1)-
piperidin-1-yI]-2-methoxy-ethanone;
144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1141,2,4]oxadiazol-5-y1)-
piperidin-1-yI]-3-methoxy-propan-1-one;
Cyclopropyl-[4-(3-{5-[(R)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyl]-pyridin-3-y1)-
[1,2,4]oxadiazol-5-y1)-piperidin-1-y1]-methanone;
Cyclopenty144-(3-{5-[(R)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyll-pyridin-3-y11-
[1,2,4]oxadiazol-5-y1)-piperidin-1-y1]-methanone;
[4-(3-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylFpyridin-3-y1}41,2,4]oxadiazol-5-y1)-
piperidin-1-y1]-(tetrahydro-pyran-4-y1)-methanone;
[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1141,2,4]oxadiazol-5-y1)-
piperidin-1-yI]-phenyl-methanone;
4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridin-3-y1141,2,4]oxadiazol-5-y1)-
piperidine-1-carboxylic acid methyl ester;
4-(3-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-y1141,2,41oxadiazol-5-y1)-
piperidine-1-carboxylic acid ethyl ester;
4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylFpyridin-3-y1141,2,4]oxadiazol-5-y1)-
piperidine-1-carboxylic acid 2-methoxy-ethyl ester;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-phenyl)-{545-(1-methanesulfonyl-
piperidin-4-y1)41,2,4]oxadiazol-3-4-
pyridin-3-y1}-methanol;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
67
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(5-{541-(propane-2-
sulfony1)-piperidin-4-y1141,2,41oxadiazol-3-y11-
pyridin-3-yI)-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(5-{5-[1-(propane-1-
sulfonylypiperidin-4-y1]-[1,2,4]oxadiazol-3-yly
pyridin-3-yI)-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(5-{541-(2-methoxy-
ethanesulfony1)-piperidin-4-y1]-
[1,2,4]oxadiazol-3-yll-pyridin-3-y1)-methanol;
244-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1141,2,4]oxadiazol-5-y1)-
piperidine-1-sulfonylFethanol;
(R)-{545-(1-Cyclopentanesulfonyl-piperidin-4-y1)41,2,41oxadiazol-3-y11-pyridin-
3-y11-(1,3-dimethyl-azetidin-3-y1)-(4-
isopropyl-phenyl)-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(5-{541-(tetrahydro-
pyran-4-sulfonylypiperidin-4-y1]-
[1,2,4]oxadiazol-3-y1}-pyridin-3-y1)-methanol;
4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylFpyridin-3-y1141,2,4]oxadiazol-5-y1)-
piperidine-1-sulfonic acid methylamide;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{545-(1-methylamino-
cyclopropy1)41,2,4]oxadiazol-3-y1]-pyridin-
3-y11-methanol;
[1-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-
phenylymethyll-pyridin-3-yly[1,2, 41oxadiazol-5-y1)-
cyclopropyI]-methyl-carbamic acid ethyl ester;
N41-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1141,2,4]oxadiazol-5-y1)-
cyclopropyI]-N-methyl-methanesulfonamide;
(R)-(1,3-Dimethyl-azetidin-3-y1)46-(2,2-dimethyl-cyclopentyloxy)-pyridazin-4-
y1]-(4-isopropyl-pheny1)-methanol;
(R)46-(3,3-Difluoro-cyclopentyloxy)-pyridazin-4-y1]-(1,3-dimethyl-azetidin-3-
y1)-(4-isopropyl-pheny1)-methanol;
2-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridazin-3-yloxyl-pheny1)-ethanol;
2-(4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridazin-3-yloxyl-pheny1)-ethanol;
(R)-[6-(Chroman-6-yloxy)-pyridazin-4-y1]-(1,3-dimethyl-azetidin-3-y1)-(4-
isopropyl-phenyl)-methanol;
6-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridazin-3-yloxyl-3H-benzooxazol-2-one;
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-3-(6-methyl-pyrimidin-4-y1)-
pent-1-yn-3-ol;
(S)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y11-2-(6-methyl-pyrimidin-4-
yI)-but-3-yn-2-ol;
(R)-4-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-y1)-2-(6-methyl-pyrimidin-4-
y1)-but-3-yn-2-ol;
(S)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y11-2-(1,5-dimethyl-1H-
pyrazol-3-y1)-but-3-yn-2-ol;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
68
(R)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-
methyll-pyridin-3-y11-2-(1,5-dimethy1-1H-
pyrazol-3-y1)-but-3-yn-2-ol;
(R) 4 {5 [(R) (1,3 Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-
methy1]-pyridin-3-y11-2-(1,5-dimethy1-1H-
pyrazol-3-y1)-but-3-yn-2-ol;
(S)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y11-2-(1,5-dimethy1-1H-
pyrazol-3-y1)-but-3-yn-2-ol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-{541-(4-fluoro-pheny1)-cyclopropylethyny1]-
pyridin-3-y11-(4-isopropyl-pheny1)-methanol;
(S)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y11-2-(1-methy1-1H-pyrazol-3-
y1)-but-3-yn-2-ol;
(R)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-
methy1]-pyridin-3-y1)-2-(1-methyl-1H-pyrazol-3-
y1)-but-3-yn-2-ol;
(R)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-
methyl]-pyridin-3-y1)-2-(1-methyl-1H-pyrazol-3-
y1)-but-3-yn-2-ol;
(S)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y11-2-(1-methy1-1H-pyrazol-3-
yI)-but-3-yn-2-ol;
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-ylethyny11-3-hydroxy-pyrrolidine-
1-carboxylic acid tert-butyl ester;
4-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methy1]-
pyridi n-3-ylethynylypiperidine-1-carboxylic
acid tert-butyl ester;
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-y1}-3-(6-methyl-pyrimidin-4-y1)-
pentan-3-ol;
2-(2-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyli-
pyridin-3-y1}-ethyl)-benzoic acid methyl
ester;
(R)-(1,3-Dimethyl-azetidin-3-y1)-{542-(2-hydroxymethyl-pheny1)-ethyl]-pyridin-
3-y11-(4-isopropyl-pheny1)-methanol;
4-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridin-3-y1}-2-(6-methoxy-pyrimidin-4-y1)-
butan-2-ol;
3-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-methyl]-
pyridin-3-ylyethyl)-3-hydroxy-
pyrrolidine-1-carboxylic acid tert-butyl ester;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{5-[2-(1H-pyrrolo[2,3-
b]pyridin-2-y1)-ethyl]-pyridin-3-yll-methanol;
4-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylFpyridin-3-yll-ethyl)-piperidine-1-
carboxylic acid tert-butyl ester;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-2-pyridin-2-yl-butan-2-ol;
1-Cyclopropy1-3-{5-[(R)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridin-3-y11-1-pyridin-2-yl-
propan-1-ol;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
69
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyriclin-3-y11-2-(5-methyl-pyridin-3-y1)-
butan-2-ol;
8-(2-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyriclazin-3-ylyethyl)-5,6,7, 8-tetrahydro-
qui nol in-8-ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyriclazin-3-y1}-2-(6-methoxy-pyridin-2-y1)-
butan-2-ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyriclazin-3-y1}-2-(6-methyl-pyridin-2-y1)-
butan-2-ol;
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(6-pyrrolidin-1-yl-pyridin-2-
y1)-methanol;
1-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-yly[1,2,4]oxadiazol-5-y1)-4-
fluoro-piperidin-1-y1]-ethanone;
1-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y1)-[1,2,4]oxadiazol-5-y1)-4-
methyl-piperidin-1-y1]-ethanone;
4-{5-[(R)-(1, 3-D imethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-yloxyl-cyclohexanone;
4-{5-[(R)-(1, 3-D imethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methy1]-
pyriclin-3-y1}-cyclohexanol;
(R)-(5-Cyclopentyloxymethyl-pyridin-3-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-
isopropyl-pheny1)-methanol;
1-(4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyll-
pyridin-3-ylmethoxyl-biperidin-1-y1)-
ethanone;
(R)-(1, 3-Di methyl-azetidin-3-y1)-(4-isopropyl-pheny1)46-(tetrahydro-pyran-4-
y1)-pyridazi n-4-y1]-methanol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyriclazin-3-y11-2-phenyl-butan-2-ol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{6-[(S)-(tetrahydro-
furan-3-yl)oxy]-pyridazi n-4-yll-methanol;
(R)-(1,3-D imethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-{6-[(R)-(tetrahydro-
furan-3-yl)oxy]-pyriclazi n-4-yll-methanol
1-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridazin-3-y1}-4-methyl-pyrrol idin-2-one;
1-{5-[(R)-(1, 3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyriclazin-3-y1}-3-isopropyl-pyrrolid in-2-one;
1-15-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyriclazin-3-y11-3,3-dimethyl-byrrolidi one;
1-{5-[(R)-(1, 3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyriclazin-3-y1}-4,4-dimethyl-pyrrolidi one;
5-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyriclazin-3-y1}-5-aza-spiro[2.4Theptan-6-
one;
1-15-[(R)-(1, 3-Di methyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyriclazi n-3-y1}-4-trifl uoromethyl-pyrrolidi n-
2-one;
4-Cyclopropy1-1-{5-[(R)-(1, 3-dimethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyl]-pyridazin-3-yll-pyrrolidi n-2-
one;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
2-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-methyll-
pyridazin-3-y11-8-oxa-2-aza-
spi ro[4.5]clecan-3-one;
3-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridazin-3-y11-1-oxa-3-aza-
spi ro[4.5]clecan-2-one;
5 1-{5-[(R)-(1, 3-Dimethyl-azetidin-311)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyriclazin-3-y1}-4-pyrid in-2-yl-pyrrol idin-2-
one;
1-{5-[(R)-(1, 3-Di methyl-azetidin-3-0-hydroxy-(4-isopropyl-pheny1)-rnethyl]-
pyridazi n-3-y11-3-(2-rnethoxy-ethyl)-
pyrrolidin-2-one;
1-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridazin-3-y11-4-phenyl-pyrrolidin-2-one;
10 2-{5-[(R)-(1, 3-Di rnethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
rnethyl]-pyridazi n-3-yI}-2, 3-di hydro-isoindol-1-one;
2-(3-{5-[(S)-(3-Fluoro-1-methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-
methyl]-pyridin-3-y1141,2,41oxadiazol-5-y1)-
propan-2-ol;
2-(3-{5-[(S)-(1-Cyclopropy1-3-fluoro-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethylFpyridin-3-y1141,2,4]oxadiazol-
5-y1)-propan-2-ol;
15 144-(3-{5-[(S)-(3-Fluoro-1-methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridin-3-y1)41,2,4]oxadiazol-5-
y1)-piperidin-1-y1]-ethanone;
1-[4-(3-{5-[(S)-(1-Cyclopropy1-3-fluoro-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl ymethy1]-pyrid
[1,2, zi]oxadiazol-5-y1)-piperidin-1-y1]-ethanone;
2-(3-{5-[(R)-(1-Cyclopropy1-3-methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-yly[1,2, oxadiazol-
20 5-yI)-propan-2-ol;
1-[4-(3-{5-[(R)-(1-Cycl opropy1-3-methyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-yly
[1 ,2,4]oxadiazol-5-y1)-piperidin-1-y1Fethanone;
(R)-(1-Cyclopropy1-3-methyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{545-
(tetrahydro-pyran-4-y1)41,2,4]oxadiazol-3-A-
pyridin-3-y1}-rnethanol;
25 trans-4-(3-15-[(R)-(1-Cyclopropy1-3-methyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-pheny1)-methyl]-pyridi n-3-y11-
[1 ,2,4]oxadiazol-5-y1)-cyclohexanol;
(R)-(1-Cyclopropy1-3-methyl-azetidi n-3-y1)-(4-isopropyl-pheny1)45-(5-oxetan-3-
y1-[1,2, 4]oxadi azol-3-y1)-pyridin-3-y1]-
methanol;
1-[(R)-3-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y1141,2,4]oxadiazol-5-
30 ylmethyl)-pyrrolidin-1-y1Fethanone;
143-(3-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-y1H1,2,41oxadiazol-5-y1)-
piperidin-1-yI]-ethanone;
143-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y1141,2,4]oxadiazol-5-y1)-3-
hydroxy-piperidin-1-yI]-ethanone;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
71
143-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-y1141,2,41oxadiazol-5-y1)-3-
methyl-piperidin-1-yI]-ethanone;
1-[3-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethylypyridin-3-y11-[1,2,4]oxadiazol-5-
ylmethyl)-piperidin-1-4-ethanone;
144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1141,2,4]oxadiazol-5-
ylmethyl)-4-hydroxy-piperidin-1-y11-ethanone;
N41-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1141,2,4]oxadiazol-5-
ylmethylycyclopropyl]-acetamide;
Tetrahydro-pyran-4-carboxylic acid [1-(3-{5-[(R)-(1, 3-di methyl-azetidin-3-
y1)-hydroxy-(4-isopropyl-phenyl)-methyll-
pyridin-3-y1}-[1,2,4]oxadiazol-5-ylmethyl)-cyclopropyl]-amide;
N41-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1141,2,4]oxadiazol-5-
ylmethyl)-cyclopropyl]-2-methoxy-acetamide;
N41-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylFpyridin-3-y1141,2,4]oxadiazol-5-
ylmethyl)-cyclopropyl]-N-methyl-acetamide;
N42-(3-{5-[(R)-(1,3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1141,2, 4]oxadi azol-5-y1)-
1,1-di methyl-ethyI]-propionamide;
N-[2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyll-pyridin-3-y1H1 ,2, 4Ioxadi azol-5-y1)-
1,1-di methyl-ethyl]-2-methoxy-acetami de;
Tetrahydro-pyran-4-carboxylic acid [2-(3-{5-[(R)-(1, 3-di methyl-azetidin-3-
y1)-hydroxy-(4-isopropyl-phenyl)-methyl]-
pyridin-3-y1141,2,41oxadiazol-5-y1)-1,1-dimethyl-ethyll-amide;
N-[2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1H1 ,2,4]oxadiazol-5-y1)-
1,1-dimethyl-ethylFisobutyramide;
Cyclopropanecarboxylic acid [2-(3-{5-[(R)-(1,3-di methyl-azetidi n-3-y1)-
hydroxy-(4-isopropyl-pheny1)-methyl]-pyridi n-3-
,2,4]oxadiazol-5-y1)-1,1-dirnethyl-ethyl]-arnide;
1-[cis-4-(3-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y11-[1,2,4]oxadiazol-5-y1)
-2-methyl-piperidin-1-yI]-ethanone;
1-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y11-[1,2,4]oxadiazol-5-y1)-4-
hydroxy-piperidin-1-yI]-2-methyl-propan-1-one;
1-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y11-[1,2,4]oxadiazol-5-y1)-4-
hydroxy-piperidin-1-y1Fpropan-1-one;
144-(3-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-y11-[1,2,41oxadiazol-5-y1)-4-
methoxy-piperidin-1-y1]-ethanone;
144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y1141,2,4]oxadiazol-5-y1)-
piperidin-1-yI]-3,3,3-trifluoro-propan-1-one;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
72
144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-y1141,2,41oxadiazol-5-y1)-
piperidin-1-yI]-2-oxetan-3-yl-ethanone;
1-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethylypyridin-3-yly[1,2,4]oxadiazol-5-y1)-4-
ethyl-pi pendi n-1-yll-ethanone;
[4-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1141,2, 4]oxadiazol-5-y1)-
pipendin-1-yll-oxetan-3-yl-methanone;
144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1141,2,4]oxadiazol-5-y1)-4-
isopropyl-pipendin-1-y1]-2-methoxy-ethanone;
1-[cis-4-(3-15-[(R)-(1, 3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-y1141,2, 41oxadiazol-5-y1)-
3-methyl-piperidin-1-yI]-ethanone;
5-(3-{5-[(R)-(1, 3-Di methyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridi n-3111-[1,2, 4]oxadi azol-5-y1)-1-
methyl-piperidin-2-one;
5-(3-{5-[(R)-(1, 3-Di methyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-
phenylymethylFpyridi n-3-y11-[1,2, 4]oxadi azol-5-y1)-5-
methyl-pipendin-2-one;
5-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1141,2,4]oxadi azol-5-y1)-
pipendin-2-one;
4-(3-{5-[(R)-(1, 3-Di methyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-
phenylymethyll-pyridi n-3-y1141,2, 41oxadi azol-5-y1)-4-
methyl-pyrrol idin-2-one;
1-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridi n-3111-[1,2, 4]oxadi azol-5-
ylmethyl)-3,3-dimethyl-pyrrolidine-2,5-dione;
1-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridi n-3-y11-[1,2, 4]oxadi azol-5-
ylmethyl)-imidazolidine-2, 4-dione;
1-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridi n-311141,2, 4]oxadi azol-5-
ylmethyl)-pyrrol idin-2-one;
3-(3-15-[(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridi n-3-y1141,2, 4]oxadi azol-5-
ylmethyl)-imidazolidine-2, 4-dione;
3-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridi n-3-y11-[1,2, 4]oxadi azol-5-
ylmethyl)-1-methyl-imidazol idine-2,4-dione;
1-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridi n-3-y11-[1,2, 4]oxadi azol-5-
ylmethyl)-3-methyl-imidazolidine-2,4-dione;
3-(3-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-y1141,2,41oxadiazol-5-
ylmethyl)-oxazolidin-2-one;
1-Cyclopropy1-3-(3-{5-[(R)-(1, 3-di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridi n-3-y11-
[1,2,4]oxadi azol-5-ylmethyl)-imidazolidi n-2-one;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
73
1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-y1141,2,41oxadiazol-5-
ylmethyl)-pyrrolidine-2,5-dione;
1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridin-3-y1111,2,4]oxadiazol-5-
ylmethyl)-3-methyl-imidazolidin-2-one;
1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-0141,2,4]oxadiazol-5-
ylmethyl)-imidazolidin-2-one;
2-(3-{5-[(R)-(1,3-Dirnethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-0141,2,4]oxadiazol-5-y1)-
propane-1,2-diol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(5-pyridin-3-
y141,2,41oxadiazol-3-y1)-pyridin-3-y1]-methanol;
1-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-yly[1,2,4]oxadiazol-5-
ylmethyl)-piperidin-1-y1]-ethanone;
(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridin-3-y1)41,2,4]oxadiazol-5-y1)-
acetonitrile;
144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1H1,2,4]oxadiazol-5-y1)-4-
hydroxy-piperidin-1-yI]-ethanone;
3-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-0141,2,4]oxadiazol-5-y1)-
propionitrile;
1-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y1)-[1,2,4]oxadiazol-5-y1)-3-
methoxy-piperidin-1-yI]-ethanone;
1-[(R)-3-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-0-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-0141,2,41oxadiazol-5-
y1)-piperidin-1-y1]-ethanone;
4-(3-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylFpyridin-3-y1}41,2,4]oxadiazol-5-y1)-
tetrahydro-pyran-4-ol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-{545-(3-hydroxymethyl-bicyclo[1.1.1]pent-1-
y1)41,2,4]oxadiazol-3-y1]-pyridin-3-y11-(4-
isopropyl-phenyl)-methanol;
4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridin-3-y1141,2,4]oxadiazol-5-y1)-1-
methyl-piperidine-2,6-dione;
2-(3-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-y1141,2,41oxadiazol-5-y1)-2,2-
difluoro-ethanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{545-(3-methoxy-
pheny1)41,2,4]oxadiazol-3-y1]-pyridin-3-y11-
methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)45-(5-isopropy141,2,4]oxadiazol-3-y1)-pyridin-
3-y1]-(4-isopropyl-pheny1)-methanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{545-(6-methyl-pyrimidin-
4-y1)41,2,4]oxadiazol-3-y1]-pyridin-3-yly
methanol;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
74
(R)-4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-y1141,2,41oxadiazol-5-y1)-1-
methyl-pyrrolidin-2-one;
(S)-4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethy1]-pyridin-3-y1141,2,4]oxadiazol-5-y1)-1-
methyl-pyrrolidin-2-one;
(S)-4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1)41,2,4]oxadiazol-5-y1)-1-
ethyl-pyrrolidin-2-one;
(R)-4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1141,2,4]oxadiazol-5-y1)-1-
ethyl-pyrrolidin-2-one;
(R)-4-(3-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-y1141,2,41oxadiazol-5-y1)-1-
ethyl-pyrrolidin-2-one;
(S)-4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-yly[1,2,4]oxadiazol-5-y1)-1-
ethyl-pyrrolidin-2-one;
(S)-4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y1141,2,4]oxadiazol-5-y1)-1-
isopropyl-pyrrolidin-2-one;
(R)-4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1141,2,4]oxadiazol-5-y1)-1-
isopropyl-pyrrolidin-2-one;
(R) 4 (3 {5 [(R) (1,3 Dimethyl-azetidin-3-ylyhydroxy-(4-isopropyl-
phenylymethyll-pyridin-3-y1141,2,41oxadiazol-5-y1)-1-
isopropyl-pyrrolidin-2-one;
(S)-4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y11-0,2,4]oxadiazol-5-y1)-1-
isopropyl-pyrrolidin-2-one;
1-[(S)-3-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-yly[1,2,4]oxadiazol-5-
ylmethyl)-pyrrolidin-1-y1Fethanone:
(S)-4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-
methy1]-pyridin-3-y1)41,2,4]oxadiazol-5-y1)-
piperidin-2-one;
(R) 4 (3 {5 [(R) (1,3 Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1111,2,4]oxadiazol-5-y1)-
piperidin-2-one;
(R)-4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y1141,2,4]oxadiazol-5-y1)-
piperidin-2-one;
(S)-4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y1)41,2,4]oxadiazol-5-y1)-
piperidin-2-one;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[5-(5-methyl-
[1,2,4]oxadiazol-3-y1)-pyridin-3-y11-methanol;
(R)-{545-(1,1-Difluoro-ethy1)41,2,4]oxadiazol-311]-pyridin-3-y11-(1,3-dimethyl-
azetidin-3-y1)-(4-isopropyl-pheny1)-
methanol;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
4-(5-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylypyridin-3-y1H1,2,41oxadiazol-3-y1)-
tetrahydro-pyran-4-ol;
(R)-[5-(3-tert-Butoxymethyl-[1,2,4]oxadiazol-5-y1)-pyridin-3-y1]-(1,3-dimethyl-
azetidin-3-y1)-(4-isopropyl-pheny1)-
methanol;
5 (R)-(1,3-Dimethyl-azetidin-3-y1)-[5-(3-hydroxymethyl-[1,2,4]oxadiazol-5-
y1)-pyridin-3-y1]-(4-isopropyl-pheny1)-methanol;
144-(5-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-yly[1,2,41oxadiazol-3-y1)-
piperazin-1-ylyethanone;
143-(5-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethylypyridin-3-y1H1,2,4]oxadiazol-3-
ylmethyl)-azetidin-1-ylyethanone;
10 4-(5-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethylypyridin-3-y1H1,2,4]oxadiazol-3-
ylmethyl)-tetrahydro-pyran-4-ol;
[4-(5-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethylypyridin-3-y1}-[1,2,4]oxadiazol-3-y1)-
piperidin-1-yly[1,4]dioxan-2-yl-methanone;
144-(5-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylYpyridin-3-y1Y[1,2,4]oxadiazol-3-y1)-
15 piperidin-1-y1]-2-methoxy-ethanone;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{543-(1-methanesulfonyl-
piperidin-4-y1)-[1,2,4]0x2di2z01-5-yly
pyridin-3-ylymethanol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(5-{3-[1-(2-methoxy-
ethanesulfonyl)-piperidin-4-y1]-
[1,2,4]oxadiazol-5-yll-pyridin-3-ylymethanol;
20 144-(5-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethylypyridin-3-y1H1,2,41oxadiazol-3-y1)-
piperidin-1-y1]-2-hydroxy-ethanone;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(541,2,41oxadiazol-3-yl-
pyridin-3-y1)-methanol;
144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylYpyridin-3-y1H1,2,4]oxadiazol-5-y1Y
piperidin-1-ylyethanone;
25 2-(3-{5-[(R)-(4-Bromo-pheny1)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-
methyl]-pyridin-3-y1}-[1,2,4]oxadiazol-5-y1)-propan-
2-01;
2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-naphthalen-2-yl-
methylypyridin-3-y1H1,2,4]oxadiazol-5-y1)-propan-2-
ol;
2-[3-(5-{(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-[4-(1-trifluoromethyl-
cyclopropyl)-phenyl]-methyll-pyridin-3-y1)-
30 [1,2,4]oxadiazol-5-ylYpropan-2-ol;
2-[3-(5-1(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-[4-(2,2,2-trifluoro-1,1-
dimethyl-ethyl)-phenyl]-methylYpyridin-3-y1)-
[1,2,4]oxadiazol-5-y1Ypropan-2-ol;
2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropoxy-
phenylymethylypyridin-3-y1}-[1,2,4]oxadiazol-511)-
propan-2-ol;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
76
2-[3-(5-{(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-[4-(1, 2,2,2-tetrafl uoro-
1 -trifl uoromethyl-ethyl)-phenylymethyll-pyridin-
3-y1)-[1,2,4]oxadiazol-5-y1]-propan-2-ol;
(R)-2-(3-(5-((1, 3-di methylazetidi n-3-y1)(hyd roxy)(4-(pentafluoro-X6-
sulfaneyl)phenyl) methyl)pyridin-3-y1)-1, 2, 4-
oxadiazol-5-yl)propan-2-ol;
2-(3-{5-[(R)-(1, 3-Di nnethyl-azetidin-3-y1)-(341 uoro-4-isopropyl-pheny1)-
hydroxy-methy1]-pyridi n-3-y1141,2,4] oxadiazol-5-
y1)-propan-2-ol;
2-(3-15-[(R)-(4-tert-Butyl-pheny1)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-
methyl]-pyridin-3-y11-[1,2,4]oxadiazol-5-y1)-
propan-2-ol;
2-(3-{5-[(R)-Benzo[b]thiophen-5-y1-(1,3-dimethyl-azetidin-3-y1)-hydroxy-
methy1]-pyridi n-3-y1}-[1,2, 4]oxadiazol-5-y1)-
propan-2-ol;
2-(3-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-pentafl uoroethyl-
pheny1)-methy1]-pyridin-3-y11-[1,2, 4]oxadiazol-5-
y1)-propan-2-ol ;
2-[3-(5-{(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-[4-(1-methyl-cyclopropy1)-
phenyl]-methyll-pyridi n-3-y1)-
[1,2, 4]0x2di2z01-5-y1]-prop2n-2-ol;
2-(3-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropy1-3-methyl-
phenyl)-methyl]-pyridi n-3-y1)-[1,2,4]oxadiazol-
5-y1)-propan-2-ol;
2-(3-{5-[(R)-(1, 3-Di methyl-azetidi n-3-y1)-hydroxy-(4-trifl uoromethoxy-
pheny1)-methy1]-pyridi n-3-y1)-[1, 2, 4]oxadiazol-5-
y1)-propan-2-ol;
1-[4-(3-{5-[(R)-(4-Bromo-pheny1)-(1, 3-dimethyl-azetid in-3-y1)-hydroxy-
methy1]-pyridi n-3-y11-[1,2, 4]oxadiazol-5-y1)-
piperidin-1-y1Fethanone;
243-(5-1(R)-(1, 3-Di methyl-azetidi n-3-y1)-[4-(1-fluoro-1-methyl-ethyl)-
phenyl]-hydroxy-methyll-pyridin-3-y1)-
[1,2, 4]oxadiazol-5-y1]-propan-2-ol;
2-(3-{5-[(R)-[4-(1,1-Difluoro-ethyl)-phenyl]-(1,3-dimethyl-azetidin-3-y1)-
hydroxy-methyl]-pyridin-3-y11-0 ,2,4]oxadiazol-5-
y1)-propan-2-ol;
2-(3-{5-[(R)-(4-Bicyclo[1. 1.1] pent-1-yl-pheny1)-(1,3-dimethyl-azetidi n-3-
y1)-hydroxy-methy1]-pyridin-3-y11-
[1,2, 4]oxadi azol-5-y1)-propan-2-ol;
(R)-(1,3-Dimethyl-azetidin-3-y1)-{5-[5-(tetrahydro-pyran-4-y1)41,2,4]oxadiazol-
311]-pyridin-3-y1144-(2,2,2-trifl uoro-
ethyl)-phenyl]-methanol;
2-[3-(5-{(R)-(1, 3-Di methyl-azetidin-3-y1)44-(1,1-dimethyl-propy1)-phenyll-
hydroxy-methyll-pyridi n-3-y1)-
[1,2, 4]oxadiazol-5-y1]-propan-2-ol;
144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-(3-fluoro-4-isopropyl-pheny1)-
hydroxy-methyl]-pyridin-3-y11-0 ,2,4]oxadiazol-
5-y1)-piperidin-1-y1]-ethanone;
1-{443-(5-{(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-[4-(1-trifl uoromethyl-
cyclopropy1)-pheny1]-methyll-pyridin-3-y1)-
,2, 4]oxadi azol-5-y1]-piperidin- -ylyethanone;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
77
144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-trifluoromethoxy-
pheny1)-methyll-pyriclin-3-y1}41,2,41oxadiazol-
5-y1)-pipendin-1-y1]-ethanone;
1-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropoxy-
phenylymethyl]-pyriclin-3-y1141,2,4]oxadiazol-5-y1)-
pipericlin-1-y11-ethanone;
2-(3-{5-[(R)-(3-Chloro-4-isopropyl-pheny1)-(1,3-dimethyl-azetidin-3-y1)-
hydroxy-methyl]-pyridin-3-y1141,2,4]oxadiazol-5-
y1)-propan-2-ol;
2-(3-{5-[(R)-(4-Cyclobutyl-phenyI)-(1 ,3-dimethyl-azetidin-3-y1)-hydroxy-
methy1]-pyriclin-3-y1141,2,4]oxadiazol-5-y1)-
propan-2-ol;
144-(3-15-[(R)-(4-Cyclobutyl-pheny1)-(1,3-dimethyl-azetidi n-3-y1)-hydroxy-
methyll-pyridin-3-y1141,2, 41oxadiazol-5-y1)-
piperidin-1-A-ethanone;
144-(3-{5-[(R)-(3, 5-Difluoro-4-isopropyl-phenyI)-(1, 3-d imethyl-azetidin-3-
y1)-hydroxy-methy1]-pyriclin-3-yll-
[1,2, Ll]oxadiazol-5-y1)-piperidin-1-y1]-ethanone;
2-(3-{5-[(R)-(3, 5-Difluoro-4-isopropyl-phenyI)-(1, 3-dimethyl-azetidin-3-y1)-
hydroxy-methy1]-pyrid
[1,2,4]oxadiazol-5-y1)-propan-2-ol;
144-(3-{5-[(R)-(4-Cyclobutoxy-pheny1)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-
methyl]-pyriclin-3-y1}41,2,4]oxadiazol-5-y1)-
piperidin-1-y1]-ethanone;
2-[3-(5-{(1,3-Dimethyl-azetidin-3-y1)44-(1-ethyl-propy1)-phenyll-hydroxy-
methyll-pyridin-3-y1)41,2,41oxadiazol-5-y11-
propan-2-ol;
1-{443-(5-{(1,3-Dimethyl-azetidi n-3-y1)-[4-(1-ethyl-propy1)-phenyl]-hydroxy-
methyll-pyridin-3-y1)-[1,2,4]oxadi azol-5-y1]-
pipenclin-1-y11-ethanone;
2-[3-(5-{(1,3-Dimethyl-azetidin-3-y1)-[4-(2,2-dimethyl-propy1)-phenyl]-hydroxy-
rnethyl}-pyridin-3-y1)41,2,4]oxadiazol-5-
yli-propan-2-ol;
144-P-(54(1,3-D imethyl-azetidi n-3-y1)44-(2,2-dimethyl-propy1)-phenyl]-
hydroxy-methyll-pyridin-3-y1)41,2,4]oxadi azol-
5-A-piperidin-1-yll-ethanone;
14113454(1,3-D imethyl-azetidi n-3-y1)-hydroxy-[4-(1-methyl-cyclopropy1)-
pheny1]-methyll-pyridin-3-y1)-
[1,2, Ll]oxadi azol-5-y1]-piperidin-1-ylyethanone;
2-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-(4-propyl-pheny1)-methyq-
pyridin-3-y1}41,2,4]oxadiazol-5-y1)-propan-
2-01;
2-(3-{5-[(R)-(4-Cyclopropyl-pheny1)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-
methyl]-pyriclin-3-y1H1,2, 4]oxadi azol-5-y1)-
propan-2-ol;
2-(3-15-[(R)-(1, 3-Di methyl-azetidin-3-y1)-(4-ethyl-pheny1)-hydroxy-methyll-
pyriclin-3-y11-[1, 2, 41oxadi azol-5-y1)-propan-2-
ol;
2-(3-{5-[(R)-(4-Butyl-pheny1)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-methyl]-
pyridin-3-y1141,2,4]oxadiazol-5-y1)-propan-2-
ol;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
78
2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-trifluoromethyl-pheny1)-
methyll-pyridin-3-y1141,2,41oxadiazol-5-y1)-
propan-2-ol;
2-[3-(5-{(R)-(1,3-Dirnethyl-azetidin-3-y1)-hydroxy-[4-(2,2,2-trifluoro-ethyl)-
phenyl]-methyll-pyridin-3-y1)41,2,4]oxadiazol-
5-y11-propan-2-ol;
2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isobutyl-pheny1)-methyl]-
pyridin-3-y1141,2,4]oxadiazol-5-y1)-
propan-2-ol;
2-(3-{5-[(R)-(4-Cyclobutoxy-pheny1)-(1,3-dirnethyl-azetidin-3-y1)-hydroxy-
rnethyl]-pyridin-3-y1141,2,4]oxadiazol-5-y1)-
propan-2-ol;
2-(3-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropenyl-pheny1)-
methyll-pyridin-3-y1H1 ,2,41oxadiazol-5-y1)-
propan-2-ol;
1-{443-(5-{(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-[4-(2,2,2-trifluoro-ethyl)-
phenyl]-methyll-pyriclin-3-y1)-
[1,2,4]oxadiazol-5-y1]-piperidin-1-y1}-ethanone;
144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-propyl-pheny1)-methyl]-
pyriclin-3-y1141,2,4]oxadiazol-5-y1)-
piperidin-1-y1]-ethanone;
144-(3-{5-[(R)-Hydroxy-(1-isopropy1-3-methyl-azetidin-3-y1)-(4-isopropyl-
pheny1)-methyl]-pyriclin-3-y1141,2,4]oxadiazol-
5-y1)-piperidin-1-y1]-ethanone;
1-[4-(3-{5-[(R)-0 -(2,2-Difluoro-ethyl)-3-methyl-azetidin-3-y11-hydroxy-(4-
isopropyl-phenylymethyll-pyridi
[1,2,4]oxadiazol-5-y1)-pipericlin-1-y1]-ethanone;
144-(3-{5-[(R)-Hydroxy-[1-(2-hydroxy-ethyl)-3-methyl-azetidi n-3-y1]-(4-
isopropyl-pheny1)-methy1]-pyridi
[1,2,4]oxadiazol-5-y1)-piperidin-1-y11-ethanone;
1-[4-(3-{5-[(R)-(1-Cyclopropylmethy1-3-methyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-phenylymethyl]-pyridi
[1,2,4]oxadiazol-5-y1)-pipericlin-1-y1Fethanone;
1-{443-(5-{(R)-Hydroxy-(4-isopropyl-pheny1)41-(2-methoxy-ethyl)-3-methyl-
azetidin-3-y1]-methyll-pyridin-3-y1)-
[1,2,4]oxadiazol-5-y1]-piperidin-1-yll-ethanone;
14113-(5-1(R)-Hydroxy-(4-isopropyl-pheny1)-[3-methyl-1-(3,3,3-trifluoro-
propy1)-azetidin-3-y1]-methyll-pyriclin-3-y1)-
[1,2,4]oxadiazol-5-y1]-piperidin-1-ylyethanone;
1-[4-(3-{5-[(R)-Hydroxy-(4-isopropyl-pheny1)-(3-methy1-1-propyl-azetidin-3-y1)-
methyl]-pyridin-3-y1}-[1,2,4]oxadiazol-5-
y1)-pipericlin-1-yll-ethanone;
1-[4-(3-{5-[(R)-(1-Ethy1-3-methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyriclin-3-yll- [1 ,2,4]oxadiazol-5-
y1)-pipericlin-1-y1Fethanone;
1-(4-15-[(R)-(1,3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridi n-3-ylethynyll-piperi di n-1-yI)-
ethanone;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyriclin-3-y11-1,1,1-trifluoro-2-methyl-but-3-
yn-2-ol;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
79
Cyclopropanecarboxylic acid (3-{5-[(R)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-pheny1)-methyll-pyridin-3-y11-
1,1-dimethyl-prop-2-ynylyamide;
N-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y11-1,1-di methyl-prop-2-ynyI)-
isobutyramide;
N-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y11-1,1-di methyl-prop-2-ynyI)-
2-methoxy-acetamide;
3-(3-{5-[(R)-(1, 3-Di nnethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
rnethyl]-pyridi n-3-01-1, 1-di methyl-prop-2-ynyI)-
oxazolidin-2-one;
1-(3-15-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridi n-3-y11-1, 1-di methyl-prop-2-ynyI)-
pyrrolidin-2-one;
1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-0-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y11-1,1-dimethyl-prop-2-ynyl)-3-
methyl-imidazolidin-2-one;
1-(3-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethylFpyridi n-3-yI}-1, 1-di methyl-prop-2-ynyI)-
imidazol idin-2-one;
3-(1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-methyl]-
pyridin-3-ylethynyll-cyclopropy1)-
oxazolidin-2-one;
1-((R)-2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyll-pyridin-3-ylethyny11-2-methyl-
pyrrolidin-1-y1)-ethanone;
1-(4-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridi n-3-ylethynyI}-4-hydroxy-piperidi n-
1-yI)-ethanone;
1-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y11-1-hydroxy-1-methyl-
prop-2-yny1)-piperidin-1-y1Fethanone;
144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y11-1-hydroxy-prop-2-yny1)-
piperidin-1-y1]-ethanone;
1-[4-(3-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-yll-prop-2-yny1)-piperidin-1-
y1]-ethanone;
1-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridi n-3-y11-1-hydroxy-prop-2-yny1)-
4-methyl-pi peridi n-1-yll-ethanone;
1-(4-{5-[(R)-(1, 3-Di methyl-azetidin-3-0-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-ylethynyll-4-rnethyl-pi peridi n-1-
yI)-ethanone;
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[5-(1-methanesulfonyl-
piperidin-4-ylethynyl)-pyridin-3-y1]-
methanol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylFpyridin-3-ylethyny11-4-methyl-piperidine-1-
sulfonic acid methyl amide;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(3-methanesulfony1-3-
methyl-but-1-yny1)-pyridin-3-y11-
methanol;
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridin-3-ylethynylycyclopropanesulfonic
acid dimethylamide;
5 1-{5-[(R)-(1,3-Dimethyl-azetidin-311)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-ylethynyll-cyclopropanesulfonic
acid amide;
(R)45-(3-Cyclopropanesulfony1-3-methyl-but-1-yny1)-pyridin-3-y1]-(1,3-dimethyl-
azetidin-3-y1)-(4-isopropyl-pheny1)-
methanol;
(R)-3-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-
methyll-pyridin-3-ylethyny11-3-hydroxy-1-
10 methyl-pyrrolidin-2-one;
(S)-3-{5-[(R)-(1,3-Dimethyl-azetidin-3-0-hydroxy-(4-isopropyl-phenyl)-methyl]-
pyridin-3-ylethyny11-3-hydroxy-1-
methyl-pyrrolidin-2-one;
4-{5-[(R)-(1,3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylFpyridi n-3-ylethyny1}-3,4-di hydro-2H-
pyrano[3,2-b]pyridin-4-ol;
15 3-{5-[(R)-(1,3-Di methyl-azetidi n-311)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridi n-3-ylethyny11-3-hydroxy-1-methyl-
1,3-di hydro-indo1-2-one;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-methyll-
pyridin-3-y1}-2-(1H-indazol-3-y1)-but-3-yn-2-
ol;
4-{5-[(R)-(1, 3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-methyl]-
pyridin-3-y1}-2-(1-methyl-1H-indazol-3-y1)-
20 but-3-yn-2-ol;
2-(1-Cyclopropy1-1H-pyrazol-3-y1)-4-{5-[(R)-(1,3-dimethyl-azetidin-3-y1)-
hydroxy-(4-isopropyl-phenyl)-methyl]-pyridin-3-
y1}-but-3-yn-2-ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-2-(2-methyl-pyrimidin-4-y1)-
but-3-yn-2-ol;
25 4-15-[(R)-(1,3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridi n-3-yI}-2-(5-methyl-pyrazi n-2-yI)-but-
3-yn-2-ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-2-(2-methyl-thiazol-5-y1)-but-3-
yn-2-ol;
2-(6-Cyclopropyl-pyrimidin-4-y1)-4-{5-[(R)-(1,3-dimethyl-azetidin-3-y1)-
hydroxy-(4-isopropyl-phenylymethyl]-pyridin-3-
30 yll-but-3-yn-2-ol;
(R)-4-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-y11-2-(6-methoxy-pyri midi n-4-
y1)-but-3-yn-2-ol;
(S)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hyd roxy-(4-isopropyl-pheny1)-
methy1]-pyridin-3-y1}-2-(6-methoxy-pyri midi n-4-
y1)-but-3-yn-2-ol;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
81
N-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-y11-1,1-dimethyl-prop-2-yny1)-
acetamide;
(S)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y11-2-(6-methoxy-pyrimidin-4-
y1)-but-3-yn-2-ol;
(R)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y11-2-(6-methoxy-pyri midi n-4-
yI)-but-3-yn-2-ol;
(R)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridin-3-y11-2-(2-methoxy-pyri midi n-4-
yI)-but-3-yn-2-ol;
(S)-4-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-y11-2-(2-methoxy-pyrimidin-4-
yI)-but-3-yn-2-ol;
(S)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-0-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y11-2-(2-methoxy-pyrimidin-4-
y1)-but-3-yn-2-ol;
(R)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridin-3-y11-2-(2-methoxy-pyri midi n-4-
yI)-but-3-yn-2-ol;
(S)-4-{5-[(R)-(1,3-Dimethyl-ezetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y11-2-(2,6-dimethyl-pyrimidin-
4-y1)-but-3-yn-2-ol;
(R) 4 {5 [(R) (1,3 Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyll-
pyridin-3-y11-2-(2,6-dimethyl-pyrimidin-
4-y1)-but-3-yn-2-ol;
(R)-(8)-2-(2,6-Dimethoxy-pyrimidin-4-y1)-4-{5-[(R)-(1,3-dimethyl-azetidin-3-
y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridin-3-yll-but-3-yn-2-ol;
(S)-2-(2,6-Dimethoxy-pyrimidin-4-y1)-4-{5-[(R)-(1,3-dimethyl-azetidin-3-y1)-
hydroxy-(4-isopropyl-phenylymethyl]-
(R)-2-(2,6-Dimethoxy-pyrimidin-4-y1)-4-{5-[(R)-(1,3-dimethyl-azetidin-3-y1)-
hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-but-3-yn-2-ol;
(S)-4-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y11-2-(2-methoxy-6-methyl-
pyrimidin-4-y1)-but-3-yn-2-ol;
(R)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridin-3-y1)-2-(2-methoxy-6-methyl-
pyrimidin-4-y1)-but-3-yn-2-ol;
(R)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridi n-3-yI)-2-(6-methoxy-2-methyl-
pyrimidin-4-yI)-but-3-yn-2-ol;
(S)-4-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-y11-2-(6-methoxy-2-methyl-
pyrimidin-4-y1)-but-3-yn-2-ol;
(S)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y11-2-(6-methoxy-2-methyl-
pyrimidin-4-y1)-but-3-yn-2-ol;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
82
(R)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyll-
pyridin-3-y1}-2-(6-methoxy-2-methyl-
pyrimidin-4-y1)-but-3-yn-2-ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridin-3-y1}-2-(6-trifluoromethyl-pyrimidin-
4-y1)-but-3-yn-2-ol;
2-(6-Difluoromethyl-pyrimidin-4-y1)-4-{5-[(R)-(1,3-dimethyl-azetidin-3-y1)-
hydroxy-(4-isopropyl-pheny1)-methyl]-pyridin-
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(1-pyridin-2-yl-
cyclopropylethyny1)-pyridin-3-y1]-methanol;
(R)-(1,3-Di methyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-{5-[1-(6-methyl-pyrim
idin-4-y1)-cyclopropyl ethyny1]-pyridin-3-yly
methanol;
1-[4-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-ylyethylypiperidin-1-y1]-
ethanone;
4-{5-[(R)-(1,3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridi uoro-2-methyl-butan-
2-01;
3-(3-{5-[(R)-(1,3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridi methyl-propy1)-
oxazolidin-2-one;
1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-01-1,1-dimethyl-propyl)-
pyrrolidin-2-one;
1-(3-{5-[(R)-(1,3-Dirnethyl-azetidin-3-yl)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y1}-1,1-dimethyl-propy1)-3-
methyl-imidazolidin-2-one;
1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-y11-1,1-dimethyl-propy1)-
imidazolidin-2-one;
1-[(8)-2-(2-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylFpyridin-3-y1}-ethyl)-2-methyl-
pyrrolidin-1-y1]-ethanone;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-2-(1H-indazol-3-y1)-butan-2-ol;
2-(1-Cyclopropy1-1H-pyrazol-3-y1)-4-15-[(R)-(1,3-dimethyl-azetidin-3-y1)-
hydroxy-(4-isopropyl-pheny1)-methyl]-pyridin-3-
ylybutan-2-ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-2-(2-methyl-pyrimidin-4-y1)-
butan-2-ol;
4-{5-[(R)-(1,3-Di methyl-azetidin-3-0-hydroxy-(4-isopropyl-pheng-methyl]-
pyridi n-3-y1}-2-(5-methyl-pyrazi n-2-y1)-
butan-2-ol;
4-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-y1}-2-(2-methyl-thiazol-5-y1)-
butan-2-ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y11-2-(3-methyl-isoxazol-5-y1)-but-
3-yn-2-ol;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
83
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-y11-2-(1-methyl-1H-imidazol-2-y1)-
but-3-yn-2-ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridin-3-y1}-2-(5-methyl-thiophen-2-y1)-but-
3-yn-2-ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-2-(1-methyl-1H-pyrrol-2-y1)-
but-3-yn-2-ol;
(R)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y11-2-(2-trifluoromethyl-
pyrimidin-4-yI)-but-3-yn-2-ol;
(S)-4-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hyd roxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-y11-2-(2-trifl uoromethyl-
pyrimidin-4-yI)-but-3-yn-2-ol;
(S)-(R)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y11-2-(2-trifluoromethyl-
pyrimidin-4-y1)-but-3-yn-2-ol;
(R)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y11-2-(2-trifluoromethyl-
pyrimidin-4-yI)-but-3-yn-2-ol;
4-(5-{(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-[4-(1 -trifluoromethyl-
cyclopropy1)-pheny1]-methyl}-pyridin-3-y1)-2-(6-
methyl-pyridin-2-y1)-but-3-yn-2-ol;
4-(5-{(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-[4-(1 -methyl-cyclopropy1)-
phenyll-methyll-pyridin-3-y1)-2-(6-methyl-
pyridin-2-y1)-but-3-yn-2-ol;
4-{5-[(R)-(1,3-Di methyl-azetidi n-3-yI)-hydroxy-(4-trifl uoromethyl-phenyI)-
methy1]-pyridi n-3-y11-2-(6-methyl-pyridin-2-y1)-
but-3-yn-2-ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-ethyl-pheny1)-hydroxy-methyl]-
pyridin-3-y1}-2-(6-methyl-pyridin-2-y1)-but-3-yn-
2-ol;
4-{5-[(R)-(4-tert-Butyl-pheny1)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-methyl]-
pyridin-3-y11-2-(6-methyl-pyridin-2-y1)-but-3-
yn-2-ol;
4-15-[(R)-(3-Ethy1-1-methyl-azetidi n-3-yI)-hyd roxy-(4-trifl uoromethoxy-
phenyI)-methy1]-pyridi n-3-y11-2-(6-methyl-pyrid in-
2-yI)-but-3-yn-2-ol;
4-(5-{(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-[4-(2,2,2-trifluoro-
ethylyphenyl]-methyll-pyridin-3-y1)-2-(6-methyl-
pyridin-2-y1)-but-3-yn-2-ol;
4-{5-[(S)-(3-Fluoro-1-methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y11-2-(6-methyl-pyridi n-2-yI)-
but-3-yn-2-ol;
1-(4-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridazin-3-ylethynyll-piperidin-1-ye-
ethanone;
N-(3-15-[(R)-(1,3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridazin-3-y11-1, 1-di methyl-prop-2-
ynyI)-acetamide;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
84
1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridazin-3-y11-1,1-dimethyl-prop-2-
yny1)-pyrrolidin-2-one;
4-{5-[(R)-Hydroxy-(1-isopropy1-3-methyl-azetidin-3-y1)-(4-isopropyl-
phenylymethyl]-pyridin 3 yll 2 (6 methyl-pyridi n-2-
y1)-but-3-yn-2-ol;
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-2-(6-methyl-pyridin-2-y1)-but-3-
yn-2-ol;
4-{5-[(R)-Hydroxy-[1-(2-hydroxy-ethyl)-3-methyl-azetidin-3-y1]-(4-isopropyl-
pheny1)-methyl]-pyridin-3-y11-2-(6-methyl-
pyridin-2-yI)-but-3-yn-2-ol;
4-15-[(R)41-(2,2-Difluoro-ethyl)-3-methyl-azetidi n-3-yll-hydroxy-(4-isopropyl-
pheny1)-methyll-pyridin-3-y11-2-(6-methyl-
pyridin-2-yI)-but-3-yn-2-ol;
1-(4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridin-3-yloxymethyll-piperidin-1-y1)-
ethanone;
4-{5-[(R)-(1-Cyclopropy1-3-methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridi n-3-yI}-2-(6-methyl-pyridin-
2-yI)-but-3-yn-2-ol;
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-4-(2-isopropyl-pyrimidin-4-y1)-
pyrrolidin-2-one;
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyll-
pyridin-3-y1}-4-(2-methyl-thiazol-5-y1)-
pyrrolidin-2-one;
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-4-(6-methyl-pyridin-3-y1)-
pyrrolidin-2-one;
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-4-(6-isopropyl-pyridin-2-y1)-
pyrrolidin-2-one;
1-{5-[(R)-(1,3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridi n-3-yI)-4-(6-trifluoromethyl-pyridi n-3-
y1)-pyrrolidin-2-one;
1-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-methyl]-
pyridin-3-y1}-4-(1-methyl-1H-pyrazol-4-y1)-
pyrrolidin-2-one;
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-y1}-4-(1,3-dimethyl-1H-pyrazol-4-
y1)-pyrrolidin-2-one;
4-(1-Difluoromethy1-1H-pyrazol-4-y1)-1-{5-[(R)-(1,3-dimethyl-azetidin-3-y1)-
hydroxy-(4-isopropyl-phenyl)-methyl]-
pyridin-3-yll-pyrrolidin-2-one;
1-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-
pyridin-3-y1}-4-(2-methyl-2H-[1,2,3]triazol-
4-y1)-pyrrolidin-2-one;
4-(1-Acetyl-piperidin-4-y1)-1-{5-[(R)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-pheny1)-methyl]-pyridazin-3-y1)-
pyrrolidin-2-one;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
5-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyll-
pyridazin-3-yll-hexahydro-furo[2,3-c]pyrrol-
4-one;
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-
pyridazin-3-y11-4-(6-isopropyl-pyridin-2-y1)-
pyrrolidin-2-one;
5 1-(2-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methy1]-pyridin-3-y11-7,8-dihydro-5H-pyrido[4, 3-
d]pyrimidin-6-yI)-ethanone;
1-(2-{5-[(R)-(1,3-D irnethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
rnethyl]-pyridi n-3-01-7,8-di hydro-5H-
[1,6]naphthyridin-6-yI)-ethanone;
144-(2-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-yll-pyrimidin-5-y1)-piperidin-
10 1-yI]-ethanone; and
144-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-yll-pyrimidin-4-y1)-piperidin-
1-yI]-ethanone.
35) Another embodiment relates to a compound according to embodiment 1)
selected from a group consisting of
(R) 2 (3 {5 [(R) (1,3 Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-0111,2,4]oxadiazol-5-y1)-
15 1,1,1-trifluoro-propan-2-ol;
2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-0141,2,4]oxadiazol-5-y1)-
propan-2-ol;
2-(3-{5-[(S)-(1-Cyclopropy1-3-fluoro-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y11-[1,2,4]oxadiazol-
5-y1)-propan-2-ol; and
20 2-(3-{5-[(R)-(4-Butyl-pheny1)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-
methyll-pyridin-3-y1141,2,41oxadiazol-5-y1)-propan-2-
ol.
36) Another embodiment relates to a compound according to embodiment 1)
selected from a group consisting of
4-{5-[(R)-Hydroxy-[1-(2-hydroxy-ethyl)-3-methyl-azetidin-3-y1]-(4-isopropyl-
pheny1)-methyl]-pyridin-3-y11-2-(6-methyl-
pyridin-2-yI)-but-3-yn-2-ol;
25 4-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-trifluoromethyl-
pheny1)-methyl]-pyridin-3-y11-2-(6-methyl-pyridin-2-y1)-
but-3-yn-2-ol;
(S)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y11-2-(6-methyl-pyrimidin-4-
y1)-but-3-yn-2-ol; and
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-ethyl-pheny1)-hydroxy-methyl]-
pyridin-3-y1}-2-(6-methyl-pyridin-2-y1)-but-3-yn-
30 2-ol.
37) Another embodiment relates to a compound according to embodiment 1)
selected from a group consisting of
1-[4-(3-{5-[(R)-(1-Cyclopropy1-3-methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridin-3-y11-
0,2,41oxadiazol-5-y1)-piperidin-1-y11-ethanone;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
86
144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-y1141,2,41oxadiazol-5-y1)-
piperidin-1-yI]-ethanone;
1-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-propyl-phenylymethyl]-
pyridin-3-y1141,2,4]oxadiazol-5-y1)-
piperidin-1-yll-ethanone; and
144-(3-{5-[(R)-Hydroxy-(1-isopropy1-3-methyl-azetidin-3-y1)-(4-isopropyl-
pheny1)-methyl]-pyridin-3-y1141,2,4]oxadiazol-
5-y1)-piperidin-1-y11-ethanone.
38) Another aspect of the present invention relates to compounds of embodiment
1), which are also compounds of
formula (Ip), wherein
A
OH
R2¨N
Formula (Ip)
A represents a 6-membered heteroaryl containing from one to three ring
nitrogen atom(s) (notably one or two ring
nitrogen atoms; especially one or two ring nitrogen atoms in meta-position(s)
and/or para-position of A with respect to
the point of attachment of A to the rest of the molecule), wherein said 6-
membered heteroaryl is independently
unsubstituted, mono-, di- or tri-substituted (notably mono- or di-substituted
in meta- and/or para-position of A with
respect to the point of attachment of A to the rest of the molecule), wherein
the substituent(s), if any, is(are)
independently selected from
D halogen (especially fluorine);
D cyano;
Ci4-alkyl (notably methyl, ethyl, propyl, or isopropyl; especially methyl,
ethyl, or propyl) which is unsubstituted
or mono-substituted with
= C1_3-alkoxy (especially methoxy);
= fluoro-phenoxy (especially 4-fluoro-phenoxy);
= benzyl-oxy;
= Cm-cycloalkyl which is optionally fused with a pyridine ring (notably at
positions 2 and 3 adjacent to the
nitrogen atom of said pyridine ring), wherein said Cm-cycloalkyl is
unsubstituted or mono-substituted with
hydroxy (notably at the point of attachment of the Cm-cycloalkyl to the 014-
alkyl);
= pyrazolyl (notably pyrazol-4-y1) which is unsubstituted or mono-
substituted with C1_3-alkyl (especially
methyl);
= tetrahydropyranyl (notably tetrahydropyran-4-y1) which is unsubstituted
or mono-substituted with hydroxy
(especially 4-hydroxy-tetrahydropyran-4-y;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
87
= indolyl (especially indo1-2-y1);
= N-(01_3-alkyl)-amino-carbonyl-oxy; or
= 1-(C14-alkyl-oxy-carbony1)-3-hydroxyazetidin-3-y1;
[in particular such 014-alkyl which is unsubstituted or mono-substituted as
defined above is methyl, ethyl,
isopropyl, 2-(1-hydroxy-cyclopropyI)-ethyl, 2-(1-hydroxy-cyclobutyl)-ethyl, 2-
(1-hydroxy-cyclopentyI)-ethyl, 3-
methoxy-propyl, 4-fluoro-phenoxy-methyl, benzyl-oxy-methyl, N-(isopropyI)-
amino-carbonyl-oxy-methyl, 2-0 -
(tert-butoxy-carbony1)-3-hydroxyazetidin-3-y1)-ethyl, 2-(1-methyl-pyrazol-4-
y1)-ethyl, 2-(tetrahydropyran-4-yI)-
ethyl, 2-(4-hydroxy-tetrahydropyran-4-yI)-ethyl, 2-(indo1-2-y1)-ethyl, 2-(8-
hydroxy-5,6,7,8-tetrahydroquinolin-8-
y1)-ethyl, or 2-(7-hydroxy-6,7-dihydro-5H-cyclopenta[b]pyridin-7-yI)-ethyl]
C35-alkenyl which is unsubstituted (especially isopropenyl) or mono-
substituted with hydroxy (especially 4-
hydroxy-but-1-en-2-yI);
tetrahydropyranyl (especially tetrahydropyran-4-yI);
hydroxy-016-alkyl which is optionally further substituted with one or more
fluorine atoms (especially 3-hydroxy-
propyl, 4-hydroxy-butyl, 3-hydroxy-butyl, 3-hydroxy-3-methyl-butyl, 3-hydroxy-
4-methyl-pentyl, or 2,2-difluoro-
3-hydroxy-prop-1-yI);
)> 03_5-alkyl (especially n-propyl, n-butyl, or n-pentyl) which is substituted
with hydroxy and RA1 (notably both
substituents at position 3 with respect to the point of attachment of said
C3_5-alkyl to the rest of the molecule),
wherein
= RA1 represents
= tetrahydropyranyl (especially tetrahydropyran-4-yI);
= phenyl which is unsubstituted or mono-substituted with fluorine
(especially 3-fluoro-phenyl) or
01_3-alkoxy (especially 2-methoxy-phenyl or 4-methoxy-phenyl); or
= 5- or 6-membered heteroaryl containing one or two ring heteroatom(s)
being independently
selected from nitrogen or sulfur (notably thiazolyl, pyrazolyl, pyridinyl, or
pyrimidinyl; especially
thiazol-4-yl, pyrazol-3-yl, pyrazol-4-yl, pyridin-2-yl, pyrimidin-2-yl, or
pyrimidin-4-y1), wherein said
5- or 6-membered heteroaryl is independently unsubstituted, mono- or di-
substituted, and
wherein the substituent(s), if any, is(are) independently selected from C13-
alkyl (especially
methyl) or 01_3-alkoxy (especially methoxy);
[in particular such C3_5-alkyl which is substituted as defined above is 3-
hydroxy-3-(tetrahydropyran-411)-propyl,
3-hydroxy-3-phenyl-propyl, 3-hydroxy-3-phenyl-butyl, 3-hydroxy-3-(m-fluoro-
pheny1)-butyl, 3-hydroxy-3-(o-
methoxy-pheny1)-butyl, 3-hydroxy-3-(p-methoxy-phenyI)-butyl, 3-hydroxy-3-(1,5-
dimethyl-pyrazol-3-y1)-butyl,
3-hydroxy-3-(1-methyl-pyrazol-3-y1)-butyl, 3-hydroxy-3-(1,5-dimethyl-pyrazol-3-
y1)-propyl, 3-hydroxy-3-(2-
methyl-thiazol-4-y1)-butyl, 3-hydroxy-3-(6-methoxy-pyridin-2-yI)-butyl, 3-
hydroxy-3-(pyrimidin-2-yI)-butyl, 3-
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
88
hydroxy-3-(6-methoxy-pyrimidin-4-yI)-butyl, 3-hydroxy-3-(1,3-dimethyl-pyrazol-
4-y1)-propyl, 3-hydroxy-3-(2-
methyl-thiazol-4-y1)-propyl, 3-hydroxy-3-(pyridin-2-yI)-pentyl, or 3-hydroxy-3-
(6-methoxy-pyridin-2-yI)-pentyl]
= 03_6-cycloalkyl which is unsubstituted, mono-, or di-substituted with
01_3-alkyl (especially methyl, ethyl,
isopropyl), hydroxy or hydroxy-C1_3-alkyl (especially 1-hydroxy-1-methyl-
ethyl), wherein optionally one ring
carbon atom of said Cm-cycloalkyl is replaced by an oxygen atom;
[in particular such 03_6-cycloalkyl which is unsubstituted, mono-, or di-
substituted as defined above is
cyclopropyl, cyclopentyl, 3-hydroxy-cyclopentyl, 3-hydroxy-3-methyl-
cyclopentyl, 3-hydroxy-3-ethyl-
cyclopentyl, 3-hydroxy-3-isopropyl-cyclopentyl, 2-(2-hydroxy-isopropyI)-
cyclopropyl, 4-hydroxy-4-methyl-
cyclohextyl, tetrahydrofuran-3-yl, tetrahydrofuran-2-yl, tetrahydropyran-4-yl,
5-methyl-tetrahydrofuran-2-yl, or
5,5-dimethyl-tetrahydrofuran-2-yl]
= 04_6-cycloalkenyl (notably cyclopentenyl; especially cyclopent-1-en-1-y1)
which is unsubstituted, mono-, or di-
substituted with C1_3-alkyl, oxo (i.e. =0), or hydroxy, wherein optionally one
ring carbon atom of said C4_6-
cycloalkenyl is replaced by an oxygen atom;
[in particular such C46-cycloalkenyl which is unsubstituted, mono-, or di-
substituted as defined above is 3-oxo-
cyclopent-1-en-1-yl, 3-hydroxy-3-methyl-cyclopent-1-en-1-yl, 2,3-dihydro-furan-
3-yl, or 5-methyl-furan-2-yl]
)=. -0-R , wherein
= Ro represents
= 014-alkyl (especially methyl, ethyl, isopropyl, sec-butyl, or isobutyl);
= hydroxy-C1_5-alkyl (especially 2-hydroxy-ethyl, 3-hydroxy-propyl, 2-
hydroxy-2-methyl-propyl, or 3-
hydroxy-3-methyl-butyl);
= Ci_ralkoxy-C2_3-alkyl (especially 2-methoxy-ethyl);
= C3_6-cycloalkyl (especially cyclopentyl or cyclohexyl) which is
unsubstituted, mono-, or di-substituted
with 01_3-alkyl (especially methyl), hydroxy or 01_3-alkoxy (especially
methoxy);
= tetrahydropyranyl (especially tetrahydropyran-4-y;
= tetrahydropyranyl-C1_3-alkyl (especially tetrahydropyran-4-yl-methyl);
= oxetanyl-C13-alkyl (especially oxetan-3-yl-methyl);
= phenyl;
= benzyl;
= benzyl-oxy-Ci_3-alkyl (especially 2-(benzyl-oxy)-ethyl);
= pyridinyl-C1_3-alkyl (especially 2-(pyridin-2-yI)-ethyl);
= (3-cyclopropy1-1,2,4-oxadiazol-5-y1)-methyl; or
= 2-(3,5-dimethy1-1,2,4-triazol-1-y1)-ethyl;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
89
[in particular such -0-R is methoxy, ethoxy, isopropoxy, isobutoxy, sec-
butoxy, phenoxy, benzyloxy, 2-
(benzyloxy)-ethoxy, 2-methoxy-ethoxy, oxetan-3-yl-methoxy, 3-hydroxy-propoxy,
cyclohexyl-oxy, 4-hydroxy-
cyclohexyl-oxy, 4-methyl-4-hydroxy-cyclohexyl-oxy, 2-hydroxy-2-methyl-propoxy,
2-hydroxy-ethoxy, 3-
hydroxy-3-methyl-butoxy, tetrahydropyran-4-yl-oxy, tetrahydropyran-4-yl-
methoxy, 3-methoxy-cyclopentyl-
oxy, (3-cyclopropy1-1,2,4-oxadiazol-5-y1)-methoxy, or 2-(3,5-dimethy1-1,2,4-
triazol-1-y1)-ethoxy]
> -CEC-R, wherein
RT1 represents
= hydroxy-014-alkyl (especially hydroxy-methyl, 1-hydroxy-ethyl, 2-hydroxy-
ethyl, 1-hydroxy-2-methyl-
propyl, or 1-hydroxy-1-methyl-ethyl);
= C1_3-alkoxy-C1_3-alkyl (especially methoxy-methyl);
= 03_6-cycloalkyl which is mono-substituted with hydroxy (notably at the
point of attachment of the Cm-
cycloalkyl to the rest of the molecule; especially 1-hydroxy-cyclopropyl, 1-
hydroxy-cyclobutyl, or 1-
hydroxy-cyclopentyl);
= 036-cycloalkyl (notably cyclopentyl or cyclohexyl) fused with a pyridine
ring (notably at positions 2
and 3 of the pyridine ring), wherein said C3_6-cycloalkyl is mono-substituted
with hydroxy (notably at
position 1 of the 03_6-cycloalkyl ring) (especially 8-hydroxy-5,6,7,8-
tetrahydroquinolin-8-y1 or 7-
hydroxy-6,7-dihydro-5H-cyclopenta[b]pyridin-7-yI);
= pyrazolyl (notably pyrazol-4-y1) which is mono- substituted with methyl
(especially 1-methyl-pyrazol-
4-yI);
=
tetrahydropyranyl (especially tetrahydropyran-4-y1) which is unsubstituted or
mono-substituted with
hydroxy (especially 4-hydroxy-tetrahydropyran-4-y;
= indolyl (notably indo1-2-y1); or
= hydroxy-azetidinyl (notably 3-hydroxy-azetidin-3-y1) which is N-
substituted with C1-4-alkoxy-carbonyl
(especially isopropyl-carbonyl or tert-butoxy-carbonyl);
[in particular such -CEO-R11 is 3-hydroxy-prop-1-yn-1-yl, 4-hydroxy-but-1-yn-1-
yl, 3-hydroxy-but-1-yn-1-yl, 3-
hydroxy-3-methyl-but-1-yn-1-yl, 3-hydroxy-4-methyl-pent-1-yn-1-yl, 3-methoxy-
prop-1-yn-1-yl, (1-hydroxy-
cyclopropy1)-ethynyl, (1-hydroxy-cyclobutyI)-ethynyl, (1-hydroxy-cyclopentyI)-
ethynyl, (8-hydroxy-5,6,7,8-
tetrahydroquinolin-8-y1)-ethynyl, (7-hydroxy-6,7-dihydro-5H-
cyclopenta[b]pyridin-7-yI)-ethynyl, (1-methyl-
pyrazol-4-y1)-ethynyl, (tetrahydropyran-4-yI)-ethynyl, (4-hydroxy-
tetrahydropyran-4-yI)-ethynyl, indo1-2-yl-
ethynyl, (1-(isopropyl-carbony1)-3-hydroxy-azetidin-3-y1)-ethynyl, or (1-(tert-
butoxy-carbony1)-3-hydroxy-
azetidin-3-y1)-ethynyl]
> -CEC-C(OH)(R12)(R13), wherein
RT2 represents hydrogen or 013-alkyl (especially methyl or ethyl);
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
RT3 represents
= phenyl which is unsubstituted or mono-substituted, wherein the
substituent(s), if any, is(are)
independently selected from 01_3-alkoxy (notably methoxy) or halogen (notably
fluorine);
[in particular such phenyl which is unsubstituted or mono-substituted is 3-
fluoro-phenyl, 4-methoxy-
5 phenyl, or 2-methoxy-phenyl]
= 5- to 6-membered heteroaryl containing one or two ring heteroatom(s)
being independently selected
from nitrogen or sulfur (notably thiazolyl, pyrazolyl, pyridinyl, or
pyrimidinyl; especially thiazol-4-yl,
pyrazol-3-yl, pyrazol-4-yl, pyridin-2-yl, pyrimidin-2-yl, or pyrimidin-4-y1),
wherein said 5- or 6-
membered heteroaryl is independently unsubstituted, mono- or di- substituted,
and wherein the
10
substituent(s), if any, is(are) independently selected from 013-alkyl
(especially methyl) and C 1 3-
alkoxy (especially methoxy); or
[in particular such 5- to 6-membered heteroaryl is 1-methyl-pyrazol-3-yl, 1,3-
dimethyl-pyrazol-4-yl,
1,5-dimethyl-pyrazol-3-yl, 2-methyl-thiazol-4-yl, pyridin-2-yl, 6-methoxy-
pyridin-2-yl, pyrimidin-2-yl,
pyrimidin-4-yl, or 1,5-dimethyl-pyrazol-3-yl]
15 = tetrahydropyranyl (especially tetrahydropyran-4-y;
[in particular such -CEC-C(OH)(R12)(R13) is 3-hydroxy-3-(tetrahydropyran-4-yI)-
prop-1-yn-1-yl, 3-hydroxy-3-
phenyl-prop-1-yn-1-yl, 3-hydroxy-3-(2-methyl-thiazol-4-y1)-prop-1-yn-1-yl, 3-
hydroxy-3-(1,3-dimethyl-pyrazol-
4-yI)-prop-1-yn-1-yl, 3-hydroxy-3-phenyl-but-1-yn-1-yl, 3-hydroxy-3-(1-methyl-
pyrazol-3-y1)-but-1-yn-1-yl, 3-
hydroxy-3-(1,5-dimethyl-pyrazol-3-y1)-but-1-yn-1-yl,
3-hydroxy-3-(1-methyl-pyrazol-3-y1)-but-1-yn-1-yl, 3-
20
hydroxy-3-(pyrimidin-2-yI)-but-1-yn-1-yl, 3-hydroxy-3-(1-methyl-pyrazol-3-
y1)-but-1-yn-1-yl, 3-hydroxy-3-(3-
fluoro-pheny1)-but-1-yn-1-yl, 3-hydroxy-3-(6-methyl-pyrimidin-4-yI)-but-1-yn-1-
yl, 3-hydroxy-3-(pyridin-2-yI)-
pent-1-yn-1-yl, 3-hydroxy-3-(6-methoxy-pyridin-2-yI)-but-1-yn-1-yl, 3-hydroxy-
3-(6-methoxy-pyridin-2-yI)-pent-
1-yn-1-yl, 3-hydroxy-3-(4-methoxy-phenyI)-but-1-yn-1-yl, or 3-hydroxy-3-(2-
methoxy-phenyI)-but-1-yn-1-yl]
-NRN1RN2 wherein
25 = Rio represents 01_3-alkyl (especially methyl);
= RN2 represents hydroxy-01_3-alkyl (especially 2-hydroxy-ethyl) or 2-
(benzyl-oxy)-C1_3-alkyl (especially 2-
(benzyl-oxy)-ethyl);
= or RN1 and RN2 form, together with the nitrogen to which they are
attached, a heterocyclic ring of 4 to 6
members (notably 5 to 6 members), wherein the members needed to complete said
heterocyclic ring are
30
each independently selected from -CH2-, -0-, -(C=0)-, -CHRx- and -C(RY)2-;
wherein said heterocyclic
ring does not contain more than one member independently selected from the
group consisting of -0- and
-(0=0)-; wherein said heterocyclic ring does not contain more than two members
selected from the group
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
91
consisting of -CHRx-; and wherein said heterocyclic ring does not contain more
than two members
selected from the group consisting of -C(RY)2-; wherein Rx independently
represents fluorine, methyl,
isopropyl, isobutyl, tert-butyl, hydroxy, trifluoromethyl, hydroxy-methyl, 1-
hydroxy-ethyl, 1-hydroxy-1-
methyl-ethyl, cyclopropyl, 2-methoxy-ethyl, 4-methyl-thiazol-2-yl, phenyl,
benzyl, tetrahydropyran-4-yl,
1,2,4-oxadiazolyl, 3-methyl-1,2,4-oxadiazol-5-yl, pyridin-2-yl, or 1-methoxy-
methyl; and wherein RY
independently represents fluorine, hydroxy, cyclopropyl, methyl, hydroxy-
methyl, or trifluoromethyl
[notably such -NRN1RN2 is pyrrolidinyl; 2-pyrrolidonyl; oxazolidinonyl
(especially 1,3-oxazolidin-2-on-3-yI);
piperidinyl; or morpholinyl, optionally independently substituted with one or
two substituents independently
selected from a group consisting of Rx and RY];
[in particular such -NRN1RN2 is pyrrolidin-1-yl, 3-fluoro-pyrrolidin-1-yl, 3,3-
difluoro-pyrrolidin-1-yl, 3,4-difluoro-
pyrrolidin-1-yl, 3-isopropyl-pyrrolidin-1-yl, 3,3-dimethyl-pyrrolidin-1-yl, 3-
hydroxy-pyrrolidin-1-yl, 2-methyl-
pyrrolidin-1-yl, 3-hydroxy-3-methyl-pyrrolidin-1-yl, 3-(hydroxy-methyl)-
pyrrolidin-1-yl, 2-(hydroxy-methyl)-
pyrrolidin-1-yl, 3-(1-hydroxy-ethyl)-pyrrolidin-1-yl, 3-hydroxy-3-cyclopropyl-
pyrrolidin-1-yl, 3-hydroxy-3-
trifluoromethyl-pyrrolidin-1-yl, 3-trifluoromethyl-pyrrolidin-1-yl, 3-(1-
hydroxy-1-methyl-ethyl)-pyrrolidin-1-yl, 3-
(1-hydroxy-ethyl)-pyrrolidin-1-yl, 3-(hydroxy-methyl)-3-methyl-pyrrolidin-1-
yl, 1,3-oxazolidin-2-on-3-yl, 5-(tert-
buty1)-1,3-oxazolidin-2-on-3-yl, 5-pheny1-1,3-oxazolidin-2-on-3-yl,
5-benzy1-1,3-oxazolidin-2-on-3-yl, 5-
isopropy1-1,3-oxazolidin-2-on-3-yl, 5-
(tetrahydropyran-4-yI)-1,3-oxazolidin-2-on-3-yl, .. 5,5-dimethy1-1,3-
oxazolidin-2-on-3-yl, morpholin-4-yl, piperidin-1-yl, 4-hydroxy-piperidin-1-
yl, 3-hydroxy-piperidin-1-yl, 3-
(tetrahydropyran-4-yI)-pyrrolid-2-on-1-yl, N-methyl-N-(2-(benzyl-oxy)-ethyl)-
amino, N-methyl-N-(2-hydroxy-
ethyl)-amino, pyrrolid-2-on-1-yl, 4-phenyl-pyrrolid-2-on-1-yl, 4-isopropyl-
pyrrolid-2-on-1-yl, 3-isopropyl-
pyrrolid-2-on-1-yl, 4,4-dimethyl-pyrrolid-2-on-1-yl, 3-(piperidin-4-yI)-
pyrrolid-2-on-1-yl, 4-isobutyl-pyrrolid-2-on-
1 -yl, 4-cyclopropyl-pyrrolid-2-on-1-yl, 4-trifluoromethyl-pyrrolid-2-on-1-yl,
3-(2-methoxy-ethyl)-pyrrolid-2-on-1-
yl, 4-(2-methoxy-ethyl)-pyrrolid-2-on-1-yl, 2,2,6,6-tetrafluoro-morpholin-4-
yl, 2,6-dimethyl-morpholin-4-yl, 2-
(methoxy-methyl)-morpholin-4-yl, 3-(3-methyl-1,2,4-oxadiazol-5-y1)-pyrrolidin-
1-yl, 3-(4-methyl-thiazol-2-y1)-
pyrrolidin-1-yl, or 3-(phenyI)-pyrrolidin-1-yl]
-(C=0)-N(RN3)(RN4), wherein
= RN3 represents hydrogen; and
RN4 represents Cm-cycloalkyl (especially cyclopentyl) or tetrahydropyranyl
(especially tetrahydropyran-4-
yl); or
= RN3 and RN4form,
together with the nitrogen to which they are attached, pyrrolidinyl;
[in particular such -(C=0)-N(RN3)(RN4) is N-cyclopentyl-amino-carbonyl, N-
(tetrahydropyran-4-yI)-amino-
carbonyl, or pyrrolidinyl-carbonyl]
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
92
piperidin-4-y1 or pyrrolidin-3-y1 which independently are mono-substituted at
the nitrogen ring atom, wherein
the substituent is selected from Ci a-al koxy-carbonyl (especially tert-butoxy-
carbonyl), pyridinyl (especially
pyridin-2-y1), phenyl and (4-methylphenyI)-sulfonyl;
[in particular such piperidin-4-y1 or pyrrolidin-3-y1 are N-(tert-butoxy-
carbonyI)-piperidin-4-yl, N-(tert-butoxy-
carbonyI)-pyrrolidin-3-yl, N-(pyridin-2-yI)-piperidin-4-yl, N-(phenyl)-
piperidin-4-yl, or N4(4-methylpheny1)-
sulfonyl)-piperidin-4-yl]
)>
5-membered heteroaryl containing from one to three (notably two or
three; especially three) ring heteroatom(s)
independently selected from nitrogen, oxygen and sulfur (notably pyrazolyl,
triazolyl, oxazolyl, thiazolyl,
0x2di2701y1; especially pyr2z01-1-yl, 1,2,3-tri2z01-1-yl, 0x2z01-2-yl, thi2z01-
2-yl, 1,2,4-ox2diazol-5-y1 or 1,2,4-
oxadiazol-3-y1); wherein said 5-membered heteroaryl is independently
unsubstituted, mono-, di-, or tri-
substituted (notably mono-substituted; especially mono-substituted in position
3 with respect to the point of
attachment of said 5-membered heteroaryl to A), wherein the substituent(s), if
any, is(are) independently
selected from
= Ci 4-alkyl which is unsubstituted or mono-substituted with hydroxy or 014-
alkoxy (especially methoxy and
tert-butoxy);
[in particular such C1_4-alkyl which is unsubstituted or mono-substituted as
defined above is methyl, 1-
hydroxy-1-methyl-ethyl, 2-hydroxy-2-methyl-propyl, 2-hydroxy-1,1-dimethyl-
ethyl, methoxy-methyl, 2-
methoxy-ethyl, 1-methoxy-1-methyl-ethyl, 2-methoxy-2-methyl-propyl, 2-methoxy-
1,1-di methyl-ethyl, tert-
butoxy-methyl; preferably such Ci_4-alkyl group is 1-hydroxy-1-methyl-ethyl, 2-
hydroxy-2-methyl-propyl,
or 2-hydroxy-1,1-dimethyl-ethyl]
= amino-C14-alkyl (especially 2-amino-ethyl or 2-amino-2,2-dimethyl-ethyl),
wherein the amino group is
mono- or di-substituted (especially mono-substituted) with C1_3-alkyl
(especially methyl), 01_3-alkyl-
carbonyl including deuterated
kyl-carbonyl (especially acetyl or acetyl-2,2,2-d3), or hydroxy-C 1_3 -
alkyl-carbonyl (especially hydroxy-methyl-carbonyl);
[in particular such amino-014-alkyl is N-acetyl-2-amino-ethyl, N-(acetyl-2,2,2-
d3)-2-amino-ethyl, N-acety1-
2-amino-2,2-dimethyl-ethyl, N-(acetyl-2,2,2-d3)-2-amino-2,2-dimethyl-ethyl, N-
methyl-N-(hydroxy-methyl-
carbony1)-2-amino-ethyl, N-methyl-N-acetyl-2-amino-ethyl, or N-methyl-N-
(acetyl-2,2,2-d3)-2-amino-ethyl]
= 03_6-cycloalkyl-L2-, wherein
= -L2- represents a bond (i.e. the 03_6-cycloalkyl is directly attached to
the rest of the molecule), oxygen,
01_3-alkylene (especially -CH2-, -CH2-CH2-, or -C(CH3)2-), hydroxy-01_2-
alkylene (especially -OH(OH)-
or oxy-C1_2-alkylene (especially -0-CH2-) (wherein the Cm-cycloalkyl is
attached to the oxygen atom
of said oxy-Ci_2-alkylene);
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
93
= 03_6-cycloalkyl is unsubstituted, mono-, or di-substituted with fluorine,
01_3-alkyl (especially methyl),
C13-alkoxy (especially methoxy), hydroxy, hydroxy-Ci 3-alkyl, or 013-alkoxy-
013-alkyl; wherein
optionally one ring carbon atom of said 03_6-cycloalkyl is replaced by an
oxygen atom;
[in particular such 03_6-cycloalkyl-L2 - is oxetan-3-yl, cyclobutoxy-methyl, 1-
hydroxy-cyclopropyl, 1-
hydroxy-cyclobutyl, 3-hydroxy-cyclobutyl, 1-hydroxy-cyclopentyl, 3-hydroxy-3-
methyl-cyclopentyl, 1-
hydroxy-cyclohexyl, 4-hydroxy-cyclohexyl, (1-hydroxy-cyclohexyl)-methyl, (1-
hydroxy-cyclobutyI)-methyl,
1-hydroxy-1-cyclohexylmethyl, 1-methoxy-cyclobutyl, 1-methoxy-cyclopentyl, 1-
(1-(methoxy-methyl)-
cyclopropylymethyl, tetrahydrofuran-2-yl-methyl,
tetrahydrofuran-2-yl, tetrahydrofuran-3-yl,
tetrahydropyran-3-yl, tetrahydropyran-4-yl, 4-methyl-tetrahydropyran-4-yl, 2,6-
dimethyl-tetrahydropyran-
4-yl, 4-methoxy-tetrahydropyran-4-yl, 4-fluoro-tetrahydropyran-4-yl,
tetrahydropyran-4-yl-methyl, 2-
(tetrahydropyran-4-yI)-ethyl, 1-(tetrahydropyran-4-yI)-1-methyl-ethyl,
tetrahydropyran-4-yl-oxy-methyl, or
(4-methyl-tetrahydropyran-4-yI)-oxy-methyl]
= piperidin-4-y1 which is N-substituted with 01_3-alkyl-carbonyl
(especially acetyl), (hydroxy-01_3-alkyl)-
carbonyl (especially hydroxymethyl-carbonyl), or 03_5-cycloalkyl-sulfonyl;
[in particular such piperidin-4-y1 is N-acetyl-piperidin-4-yl, N-
(hydroxymethyl-carbonyI)-piperidin-4-yl, or N-
(cyclopropyl-sulfony1)-piperidin-4-yl]
= morpholinyl-L3- (notably (morpholin-4-yI)-L3-), wherein -L3- represents a
bond (i.e. the morpholinyl is
directly attached to the rest of the molecule) or 012-alkylene; wherein said
morpholinyl is unsubstituted or
di-substituted with methyl;
[in particular such morpholinyl-L3- is morpholin-4-yl, morpholin-4-yl-methyl,
or (2,6-dimethyl-morpholin-4-
y1)-methyl]
D pyrazolyI-01_3-alkyl (especially 2-(pyrazol-1-y1)-ethyl);
D C1_3-alkyl-sulfonyl-C1_3-alkyl (especially methyl-sulfonyl-methyl);
D 2,2,2-trifluoro-1-hydroxy-1-methyl-ethyl;
) 3-hydroxymethyl-bicyclo[1.1.1]pent-1-y1;
= 7-oxa-bicyclo[2.2.1]hept-2-y1; and
= 6-oxa-spiro[2.5]oct-1-y1;
D 5-oxo-4-oxa-6-azaspiro[2.4]hept-6-yl, 2,2-dimethy1-6-oxo-5-oxa-7-
azaspiro[3.4]oct-7-yl, 2-cyclopropy1-6-oxo-
5-oxa-7-azaspiro[3.4]oct-7-yl, 2-oxo-1-oxa-3-azaspiro[4.4]non-3-yl,
8,8-difluoro-2-oxo-1-oxa-3-
azaspiro[4.5]dec-3-yl, or 8-oxo-7-oxa-9-azadispiro[3.1.4.1]undec-9-y1;
D 7-aza-bicyclo[2.2.1]hept-7-yl, 2-oxa-5-aza-bicyclo[2.2.1]hept-5-yl, 6-oxa-3-
aza-bicyclo[3.1.1]hept-3-yl, or 8-
oxa-3-azabicyclo[3.2.1]oct-3-y1;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
94
5-oxo-6-azaspiro[3.4]oct-6-yl, 3-oxo-2-azaspiro[4.4]non-2-yl, 1-oxo-2-
azaspiro[4.5]dec-2-yl, 1-oxo-8-oxa-2-
azaspiro[4.5]dec-2-yl, 3-oxo-8-oxa-2-azaspiro[4.5]dec-2-yl, or 4-oxo-hexahydro-
5H-furo[2,3-c]pyrrol-5-y1;
= 3-(7-hydroxy-6,7-dihydro-5H-cyclopenta[b]pyridin-7-yl)propyl or 3-(8-
hydroxy-5,6,7,8-tetrahydroquinolin-8-
yl)propyl); and
> 2-(6,7-dihydro-5H-Npyrindin-7-o1)-ethyl or 2-(8-hydroxy-5,6,7,8-
tetrahydro-quinolin-8-yI)-ethyl;
B represents phenyl, which is unsubstituted, mono-, di- or tri-substituted
(especially mono-substituted in para-position
with respect to the point of attachment of B to the rest of molecule), wherein
the substituent(s), if any, is(are)
independently selected from
= 01_5-alkyl (especially methyl, ethyl, n-propyl, isopropyl, or tert-butyl;
in particular isopropyl);
01_3-alkoxy (especially methoxy, ethoxy, or isopropoxy);
= 01_3-alkoxy-014-alkyl (especially 3-methoxy-propyl);
= C1_3-fluoroalkyl (especially trifluoromethyl);
= C3_5-cycloalkyl (especially cyclopropyl or cyclobutyl) which
independently is unsubstituted or mono-substituted
(notably at the point of attachment of said 03_5-cycloalkyl to the rest of the
molecule) with 01_3-alkyl (especially
methyl) or 01_3-fluoroalkyl (especially trifluoromethyl);
C3_5-cycloalkoxy (especially cyclopropoxy or cyclobutoxy); and
= Ci_3-fluoroalkoxy (especially trifluoromethoxy);
[in particular such B is phenyl, 4-methyl-phenyl, 4-ethyl-phenyl, 4-propyl-
phenyl, 4-isopropyl-phenyl, 4-tert-butyl-phenyl,
4-cyclopropyl-phenyl, 4-methoxy-phenyl, 4-ethoxy-phenyl, 4-isopropoxy-phenyl,
4-trifluoromethyl-phenyl, 4-(1-methyl-
cyclopropyI)-phenyl, 4-cyclobutyl-phenyl, 4-cyclopropoxy-phenyl, 4-cyclobutoxy-
phenyl, 4-(trifluoromethoxy)-phenyl, 4-
(3-methoxy-propyI)-phenyl, or 4-(1-trifluoromethyl-cyclopropyI)-phenyl]
RI represents C1_3-alkyl (especially methyl), cyano, or halogen (especially
fluorine);
R2 represents 014-alkyl (especially methyl, ethyl, n-propyl, isopropyl, tert-
butyl or isobutyl), 03_5-cycloalkyl (especially
cyclopropyl or cyclobutyl), 03_5-cycloalkyl-Ci_3-alkyl (especially cyclopropyl-
methyl), or 01_3-fluoroalkyl (especially 2,2-
difluoroethyl or 2-fl uoroethyl).
39) A further embodiment relates to compounds of Formula (Ip) according to
embodiment 38), wherein the
characteristics of any one of embodiments 2) to 32) apply mutatis mutandis.
40) One embodiment of the present invention relates to compounds according to
embodiment 38), wherein A represents
pyridinyl, pyrimidinyl, pyrazinyl, or pyridazinyl (notably pyridin-3-yl,
pyridin-4-yl, pyrimidin-5-yl, pyrazin-2-yl, or pyridazin-
4-y1; especially pyridin-3-y1), wherein A is independently unsubstituted, mono-
, di- or tri-substituted (notably mono- or
di-substituted in meta- and/or para-position of A with respect to the point of
attachment of A to the rest of the molecule;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
especially mono-substituted in meta-position of A with respect to the point of
attachment of A to the rest of the molecule),
wherein the substituent(s), if any, is(are) as defined in embodiment 38).
41) Another embodiment of the present invention relates to compounds according
to embodiment 38), wherein A
represents pyridin-3-y1 or pyridin-4-yl, wherein A is independently
unsubstituted, mono-, di- or tri-substituted (notably
5
mono- or di-substituted in meta- and/or para-position of A with respect to
the point of attachment of A to the rest of the
molecule; especially mono-substituted in meta-position of A with respect to
the point of attachment of A to the rest of
the molecule), wherein the substituent(s), if any, is(are) as defined in
embodiment 38).
42) Another embodiment of the present invention relates to compounds according
to embodiment 38), wherein A is
unsubstituted;
10
> mono-substituted (especially in meta-position of A with respect to the
point of attachment of A to the rest of
the molecule), wherein the substituent is as defined in embodiment 38).
di-substituted, wherein a first substituent is (notably in meta-position of A
with respect to the point of attachment
of A to the rest of the molecule) selected from the substituents defined in
embodiment 38) (notably excluding
halogen (especially fluorine) or cyano); and a second substituent is (notably
in para-position of A with respect
15
to the point of attachment of A to the rest of the molecule) selected from
halogen (especially fluorine) and
cyano.
[In a sub-embodiment of embodiment 4), A is pyridin-3-yl]
43) Another embodiment of the present invention relates to compounds according
to any one of embodiments 40) to
42), wherein A is mono-substituted in meta-position of A with respect to the
point of attachment of A to the rest of the
20 molecule, wherein the substituent of A is
5-membered heteroaryl containing from one to three (notably two or three;
especially three) ring heteroatom(s)
independently selected from nitrogen, oxygen and sulfur (notably pyrazolyl,
triazolyl, oxazolyl, thiazolyl,
oxadiazolyl; especially pyrazol-1-yl, 1,2,3-triazol-1-yl, oxazol-2-yl, thiazol-
2-yl, 1,2,4-oxadiazol-5-y1 or 1,2,4-
oxadiazol-3-y1; preferably 1,2,4-oxadiazol-5-y1 or 1,2,4-oxadiazol-3-y1);
wherein said 5-membered heteroaryl
25
is independently unsubstituted, mono-, di-, or tri-substituted (notably
mono-substituted; especially mono-
substituted in position 3 with respect to the point of attachment of said 5-
membered heteroaryl to A), wherein
the substituent(s), if any, is(are) independently selected from
Ci4-alkyl which is unsubstituted or mono-substituted with hydroxy or C14-
alkoxy (especially methoxy and
tert-butoxy);
30
[in particular such 014-alkyl which is unsubstituted or mono-substituted as
defined above is methyl, 1-
hydroxy-1-methyl-ethyl, 2-hydroxy-2-methyl-propyl, 2-hydroxy-1,1-dimethyl-
ethyl, methoxy-methyl, 2-
methoxy-ethyl, 1-methoxy-1-methyl-ethyl, 2-methoxy-2-methyl-propyl, 2-methoxy-
1,1-dimethyl-ethyl, tert-
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
96
butoxy-methyl; preferably such 01_4-alkyl group is 1-hydroxy-1-methyl-ethyl, 2-
hydroxy-2-methyl-propyl,
or 2-hydroxy-1,1-dimethyl-ethyl]
= amino-014-alkyl (especially 2-amino-ethyl or 2-amino-2,2-dimethyl-ethyl),
wherein the amino group is
mono- or di-substituted (especially mono-substituted) with 01_3-alkyl
(especially methyl), C1_3-alkyl-
carbonyl including deuterated C1_3-alkyl-carbonyl (especially acetyl or acetyl-
2,2,2-d3) or hydroxy-C 1-3-
alkyl-carbonyl (especially hydroxy-methyl-carbonyl);
[in particular such amino-C14-alkyl is N-acetyl-2-amino-ethyl, N-(acetyl-2,2,2-
d3)-2-amino-ethyl, N-acety1-
2-amino-2,2-dimethyl-ethyl, N-(acetyl-2,2,2-d3)-2-amino-2,2-dimethyl-ethyl, N-
methyl-N-(hydroxy-methyl-
c2rbony1)-2-2mino-ethyl, N-methyl-N-2cety1-2-2mino-ethyl, or N-methyl-N-
(2cety1-2,2,2-d3)-2-2mino-ethyl]
C3_6-cycloalkyl-L2-, wherein
= -L2- represents a bond (i.e. the 03_6-cycloalkyl is directly attached to
the rest of the molecule), oxygen,
C1_3-alkylene (especially -CH2-, -CH2-CH2-, or -C(CH3)2-), hydroxy-Ci_2-
alkylene (especially -CH(OH)-
or oxy-C1_2-alkylene (especially -0-CH2-) (wherein the C3_6-cycloalkyl is
attached to the oxygen atom
of said oxy-01_2-alkylene); and
= the C3_6-
cycloalkyl is unsubstituted, mono-, or di-substituted with fluorine, 01_3-
alkyl (especially
methyl), C1_3-alkoxy (especially methoxy), hydroxy, hydroxy-C1_3-alkyl, or
01_3-alkoxy-C1_3-alkyl;
wherein optionally one ring carbon atom of said 03_6-cycloalkyl is replaced by
an oxygen atom;
[in particular such 03_6-cycloalkyl-L2 - is oxetan-3-yl, cyclobutoxy-methyl, 1-
hydroxy-cyclopropyl, 1-
hydroxy-cyclobutyl, 3-hydroxy-cyclobutyl, 1-hydroxy-cyclopentyl, 3-hydroxy-3-
methyl-cyclopentyl, 1-
hydroxy-cyclohexyl, 4-hydroxy-cyclohexyl, (1-hydroxy-cyclohexyl)-methyl, (1-
hydroxy-cyclobutyI)-methyl,
1-hydroxy-1-cyclohexylmethyl, 1-methoxy-cyclobutyl, 1-methoxy-cyclopentyl, 1-
(1-(methoxy-methyl)-
cyclopropy1)-methyl, tetrahydrofuran 2 yl methyl,
tetrahydrofuran-2-yl, tetrahydrofuran-3-yl,
tetrahydropyran-3-yl, tetrahydropyran-4-yl, 4-methyl-tetrahydropyran-4-yl, 2,6-
dimethyl-tetrahydropyran-
4-yl, 4-methoxy-tetrahydropyran-4-yl, 4-fluoro-tetrahydropyran-4-yl,
tetrahydropyran-4-yl-methyl, 2-
(tetrahydropyran-4-yI)-ethyl, 1-(tetrahydropyran-4-yI)-1-methyl-ethyl,
tetrahydropyran-4-yl-oxy-methyl, or
(4-methyl-tetrahydropyran-4-yI)-oxy-methyl]
= piperidin-4-y1 which is N-substituted with 01_3-alkyl-carbonyl
(especially acetyl), (hydroxy-Ci_3-alkyl)-
carbonyl (especially hydroxymethyl-carbonyl), or 03_5-cycloalkyl-sulfonyl;
[in particular such piperidin-4-y1 is N-acetyl-piperidin-4-yl, N-
(hydroxymethyl-carbonyI)-piperidin-4-yl, or N-
(cyclopropyl-sulfonyI)-piperidin-4-yl]
= morpholinyl-L3 - (notably (morpholin-4-yI)-L3 -), wherein -L3 -
represents a bond (Le. the morpholinyl is
directly attached to the rest of the molecule) or Ci_2-alkylene; wherein said
morpholinyl is unsubstituted or
di-substituted with methyl;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
97
[in particular such morpholinyl-L3- is morpholin-4-yl, morpholin-4-yl-methyl,
or (2,6-dimethyl-morpholin-4-
y1)-methyl]
= pyrazolyl 01_3-alkyl (especially 2-(pyrazol-1-y1)-ethyl);
= C1_3-alkyl-sulfonyl-C1_3-alkyl (especially methyl-sulfonyl-methyl);
2,2,2-trifluoro-1-hydroxy-1-methyl-ethyl;
= 3-hydroxymethyl-bicyclo[1.1.1]pent-1-y1;
= 7-oxa-bicyclo[2.2.1]hept-2-y1; and
= 6-oxa-spiro[2.5]oct-1-yl.
44) Another embodiment of the present invention relates to compounds according
to any one of embodiments 40) to
42), wherein A is mono-substituted in meta-position of A with respect to the
point of attachment of A to the rest of the
molecule, wherein the substituent of A is
D 5-membered heteroaryl containing from one to three (notably two or
three; especially three) ring heteroatom(s)
independently selected from nitrogen, oxygen and sulfur (notably pyrazolyl,
triazolyl, oxazolyl, thiazolyl,
oxadiazolyl; especially pyrazol-1-yl, 1,2,3-triazol-1-yl, oxazol-2-yl, thiazol-
2-yl, 1,2,4-oxadiazol-5-y1 or 1,2,4-
oxadiazol-3-y1; most preferably 1,2,4-oxadiazol-5-y1 or 1,2,4-oxadiazol-3-y1);
wherein said 5-membered
heteroaryl is independently unsubstituted, mono-, di-, or tri-substituted
(notably mono-substituted; especially
mono-substituted in position 3 with respect to the point of attachment of said
5-membered heteroaryl to A),
wherein the substituent(s), if any, is(are) independently selected from
= amino-C1_4-alkyl (especially 2-amino-ethyl or 2-amino-2,2-dimethyl-
ethyl), wherein the amino group is
mono- or di-substituted (especially mono-substituted) with C1_3-alkyl
(especially methyl), 01_3-alkyl-
carbonyl including deuterated C1_3-alkyl-carbonyl (especially acetyl or acetyl-
2,2,2-d3), or hydroxy-C1_3-
alkyl-carbonyl (especially hydroxy-methyl-carbonyl);
[in particular such amino-C1_4-alkyl is N-acetyl-2-amino-ethyl, N-(acetyl-
2,2,2-d3)-2-amino-ethyl, N-acety1-
2-amino-2,2-dimethyl-ethyl, N-(acetyl-2,2,2-d3)-2-amino-2,2-dimethyl-ethyl, N-
methyl-N-(hydroxy-methyl-
carbonyI)-2-amino-ethyl, N-methyl-N-acetyl-2-amino-ethyl, or N-methyl-N-
(acetyl-2,2,2-d3)-2-amino-ethyl]
piperidin-4-y1 which is N-substituted with C1_3-alkyl-carbonyl (especially
acetyl), (hydroxy-Ci_3-alkyly
carbonyl (especially hydroxymethyl-carbonyl), or 03_5-cycloalkyl-sulfonyl.
[in particular such piperidin-4-y1 is N-acetyl-piperidin-4-yl, N-
(hydroxymethyl-carbonyI)-piperidin-4-yl, or N-
(cyclopropyl-sulfonylypiperidin-4-yl]
45) Another embodiment of the present invention relates to compounds according
to any one of embodiments 40) to
42), wherein A is mono-substituted in meta-position of A with respect to the
point of attachment of A to the rest of the
molecule, wherein the substituent is
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
98
1,2,4-oxadiazol-3-y1 which is mono-substituted, wherein the substituent is
independently selected from
amino-014-alkyl (especially 2-amino-ethyl or 2-amino-2,2-dimethyl-ethyl),
wherein the amino group is
= mono-substituted with 01_3-alkyl-carbonyl including deuterated 01_3-alkyl-
carbonyl (especially
acetyl or acetyl-2,2,2-d3);
= di-
substituted, wherein a first substituent is C1_3-alkyl (especially methyl) and
a second
substituent is C1_3-alkyl-carbonyl (especially acetyl) or C1_3-alkyl-carbonyl
including deuterated
01_3-alkyl-carbonyl (especially acetyl or acetyl-2,2,2-d3); or
= di-substituted, wherein a first substituent is C1_3-alkyl (especially
methyl) and a second
substituent is hydroxy-C1_3-alkyl-carbonyl (especially hydroxy-methyl-
carbonyl);
[in particular such amino-014-alkyl is N-acetyl-2-amino-ethyl, N-(acetyl-2,2,2-
d3)-2-amino-ethyl, N-acety1-
2-amino-2,2-dimethyl-ethyl, N-(acetyl-2,2,2-d3)-2-amino-2,2-dimethyl-ethyl, N-
methyl-N-(hydroxy-methyl-
carbony1)-2-amino-ethyl, N-methyl-N-acetyl-2-amino-ethyl, or N-methyl-N-
(acetyl-2,2,2-d3)-2-amino-ethyl]
piperidin-4-y1 which is N-substituted with C1_3-alkyl-carbonyl (especially
acetyl), (hydroxy-C1_3-alkyl)-
carbonyl (especially hydroxymethyl-carbonyl), or 03_5-cycloalkyl-sulfonyl.
[in particular such piperidin-4-y1 is N-acetyl-piperidin-4-yl, N-
(hydroxymethyl-carbony1)-piperidin-4-yl, or N-
(cyclopropyl-sulfony1)-piperidin-4-yl]
46) Another embodiment of the present invention relates to compounds according
to any one of embodiments 40) or
42), wherein A represents pyridinyl (especially pyridin-3-y1) which is mono-
substituted in meta-position with respect to
the point of attachment of A to the rest of the molecule, wherein the
substituent is oxadiazolyl (especially 1,2,4-
oxadiazol-3-y1) which is mono-substituted, wherein the substituent is 01_4-
alkyl which is unsubstituted or mono-
substituted with hydroxy or 01_4-alkoxy (especially such C1_4-alkyl is 1-
hydroxy-1-methyl-ethyl, 2-hydroxy-2-methyl-
propyl, or 2-hydroxy-1,1-dimethyl-ethyl).
47) Another embodiment of the present invention relates to compounds according
to any one of embodiments 40) to
42), wherein A is mono-substituted in meta-position of A with respect to the
point of attachment of A to the rest of the
molecule, wherein the substituent of A is
-CEC-R, wherein
represents
= hydroxy-01_4-alkyl (especially hydroxy-methyl, 1-hydroxy-ethyl, 2-hydroxy-
ethyl, 1-hydroxy-2-methyl-
propyl, or 1-hydroxy-1-methyl-ethyl);
= 01_3-alkoxy-Ci_3-alkyl (especially methoxy-methyl);
= C3_6-cycloalkyl which is mono-substituted with hydroxy (notably at the
point of attachment of the C3_6-
cycloalkyl to the rest of the molecule; especially 1-hydroxy-cyclopropyl, 1-
hydroxy-cyclobutyl, or 1-
hydroxy-cyclopentyl);
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
99
= 03_6-cycloalkyl (notably cyclopentyl or cyclohexyl) fused with a pyridine
ring (notably at positions 2
and 3 of the pyridine ring), wherein said 036-cycloalkyl is mono-substituted
with hydroxy (notably at
position 1 of the 03_6-cycloalkyl ring) (especially 8-hydroxy-5,6,7,8-
tetrahydroquinolin-8-y1 or 7-
hydroxy-6,7-dihydro-5H-cyclopenta[b]pyridin-7-yI);
= pyrazolyl (notably pyrazol-4-y1) which is mono- substituted with methyl
(especially 1-methyl-pyrazol-
4-yI);
= tetrahydropyranyl (especially tetrahydropyran-4-y1) which is
unsubstituted or mono-substituted with
hydroxy (especially 4-hydroxy-tetrahydropyran-4-y;
= indolyl (notably indo1-2-y1); or
^ hydroxy-azetidinyl (notably 3-hydroxy-azetidin-3-y1) which is N-
substituted with C14-alkoxy-carbonyl
(especially isopropyl-carbonyl or tert-butoxy-carbonyl);
[in particular such -CEC-R11 is 3-hydroxy-prop-1-yn-1-yl, 4-hydroxy-but-1-yn-1-
yl, 3-hydroxy-but-1-yn-1-yl, 3-
hydroxy-3-methyl-but-1-yn-1-yl, 3-hydroxy-4-methyl-pent-1-yn-1-yl, 3-methoxy-
prop-1-yn-1-yl, (1-hydroxy-
cyclopropy1)-ethynyl, (1-hydroxy-cyclobutylyethynyl, (1-hydroxy-cyclopentyI)-
ethynyl, (8-hydroxy-5,6,7,8-
tetrahydroquinolin-8-yI)-ethynyl, (7-hydroxy-6,7-dihydro-5H-
cyclopenta[b]pyridin-7-yI)-ethynyl, (1-methyl-
pyrazol-4-y1)-ethynyl, (tetrahydropyran-4-yI)-ethynyl, (4-hydroxy-
tetrahydropyran-4-yI)-ethynyl, indo1-2-yl-
ethynyl, (1-(isopropyl-carbony1)-3-hydroxy-azetidin-3-y1)-ethynyl, or (1-(tert-
butoxy-carbony1)-3-hydroxy-
azetidin-3-y1)-ethynyl]
-CEC-C(OH)(RT2)(RT3), wherein
RT2 represents hydrogen or 01_3-alkyl (especially methyl or ethyl);
RT3 represents
= phenyl which is unsubstituted or mono-substituted, wherein the
substituent, if any, is selected
from 01_3-alkoxy (notably methoxy) or halogen (notably fluorine);
[in particular such group is 3-fluoro-phenyl, 4-methoxy-phenyl, or 2-methoxy-
phenyl]
= 5- to 6-membered heteroaryl containing one or two ring heteroatom(s) being
independently selected
from nitrogen or sulfur (notably thiazolyl, pyrazolyl, pyridinyl, or
pyrimidinyl; especially thiazol-4-yl,
pyrazol-3-yl, pyrazol-4-yl, pyridin-2-yl, pyrimidin-2-yl, or pyrimidin-4-y1),
wherein said 5- or 6-
membered heteroaryl is independently unsubstituted, mono- or di- substituted,
and wherein the
substituent(s), if any, is(are) independently selected from C13-alkyl
(especially methyl) and 013-
alkoxy (especially methoxy); or
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
100
[in particular such 5- to 6-membered heteroaryl group is 1-methyl-pyrazol-3-
yl, 1,3-dimethyl-pyrazol-
4-yl, 1,5-dimethyl-pyrazol-3-yl, 2-methyl-thiazol-4-yl, pyridin-2-yl, 6-
methoxy-pyridin-2-yl, pyrimidin-2-
yl, pyrimidin-4-yl, or 1,5-dimethyl-pyrazol-3-yl]
= tetrahydropyranyl (especially tetrahydropyran-4-y;
[in particular such -CEC-C(OH)(R12)(R13) is 3-hydroxy-3-(tetrahydropyran-4-yI)-
prop-1-yn-1-yl, 3-hydroxy-3-
phenyl-prop-1-yn-1-yl, 3-hydroxy-3-(2-methyl-thiazol-4-y1)-prop-1-yn-1-yl, 3-
hydroxy-3-(1, 3-di methyl-pyrazol-
4-y1)-prop-1-yn-1-y1 , 3-hydroxy-3-phenyl-but-1-yn-1-yl, 3-hydroxy-3-(1-methyl-
pyrazo1-3-y1)-but-1-yn-1-yl, 3-
hydroxy-3-(1, 5-dimethyl-pyrazol-3-y1)-but-1-yn-1-y1 ,
3-hydroxy-3-(1-methyl-pyrazol-3-y1)-but-1-yn-1-yl, 3-
hydroxy-3-(pyrimidin-2-y1)-but-1-yn-1-y1 , 3-hydroxy-3-(1-methyl-pyrazol-3-y1)-
but-1-yn-1-yl, 3-hydroxy-3-(3-
fluoro-phenyI)-but-1-yn-1-yl, 3-hydroxy-3-(6-methyl-pyrimidin-4-yI)-but-1-yn-1-
yl, 3-hydroxy-3-(pyridin-2-yI)-
pent-1-yn-1-yl, 3-hydroxy-3-(6-methoxy-pyridin-2-yI)-but-1-yn-1-yl, 3-hydroxy-
3-(6-methoxy-pyridin-2-y1)-pent-
1-yn-1-yl, 3-hydroxy-3-(4-methoxy-phenyI)-but-1-yn-1-yl, or 3-hydroxy-3-(2-
methoxy-phenyl)-but-1-yn-1-yl]
48) Another embodiment of the present invention relates to compounds according
to any one of embodiments 38) to
42), wherein A is mono-substituted in meta-position of A with respect to the
point of attachment of A to the rest of the
molecule, wherein the substituent of A is as defined in embodiment 42) or 46).
49) Another embodiment of the present invention relates to compounds according
to any one of embodiments 38) to
48), wherein B represents phenyl, which is mono-substituted in para-position
with respect to the point of attachment of
B to the rest of molecule, wherein the substituent is selected from C15-alkyl
(especially ethyl, n-propyl, isopropyl, or tert-
butyl; in particular isopropyl).
[in particular such B is 4-ethyl-phenyl, 4-propyl-phenyl, 4-isopropyl-phenyl,
4-tert-butyl-phenyl; in particular 4-isopropyl-
phenyl]
50) Another embodiment of the present invention relates to compounds according
to any one of embodiments 38) to
49), wherein R1 represents C1_3-alkyl (especially methyl).
51) Another embodiment of the present invention relates to compounds according
to any one of embodiments 38) to
50), wherein R2 represents 01_4-alkyl (especially methyl, ethyl, n-propyl,
isopropyl, tert-butyl or isobutyl).
52) Another embodiment of the present invention relates to compounds according
to embodiment 38), wherein
A represents pyridinyl (especially pyridin-3-y1) which is mono-substituted in
meta-position with respect to the point of
attachment of A to the rest of the molecule, wherein the substituent is
oxadiazolyl (especially 1,2,4-oxadiazol-3-y1) which
is mono-substituted, wherein the substituent is 014-alkyl which is
unsubstituted or mono-substituted with hydroxy or
C14-alkoxy (especially such C14-alkyl which is unsubstituted or mono-
substituted is 1-hydroxy-1-methyl-ethyl, 2-
hydroxy-2-methyl-propyl, or 2-hydroxy-1, 1-di methyl-ethyl);
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
101
B represents phenyl which is mono-substituted in para-position with respect to
the point of attachment of B to the rest
of molecule, wherein the substituent is 01_5-alkyl (especially isopropyl);
R1 represents 01_3-alkyl (especially methyl); and
R2 represents 014-alkyl (especially methyl).
53) Another embodiment relates to compounds according to any one of
embodiments 38) to 52), which are also
compounds of Formula (lip) (i.e. the asymmetric carbon atom to which A and B
are attached has the absolute
configuration depicted in Formula (llp))
A
j\ LOH
R2-N
R1
Formula (lip)
It is understood that for A being pyridin-3-yl, pyrimidin-5-yl, pyrazin-2-yl,
or pyridazin-4-y1 (especially pyridin-3-y1), the
asymmetric carbon atom to which A and B are attached, as depicted in Formula
(llp), has absolute configuration (R).
In the case where A represents pyridin-4-yl, the asymmetric carbon atom to
which A and B are attached, as depicted
in Formula (H), has absolute configuration (S).
Based on the dependencies of the different embodiments 1) to 37) as disclosed
hereinabove, the following
embodiments are thus possible and intended, and herewith specifically
disclosed in individualized form:
2+1, 3+1, 4+1, 11+1, 11+2+1, 11+3+1, 11+4+1, 12+11+1, 12+11+2+1, 12+11+3+1,
12+11+4+1, 13+11+1, 13+11+2+1,
13+11+3+1, 13+11+4+1, 14+11+1, 14+11+2+1, 14+11+3+1, 14+11+4+1, 15+11+1,
15+11+2+1, 15+11+3+1, 15+11+4+1, 16+1,
16+2+1, 16+3+1, 16+4+1, 17+16+1, 17+16+2+1, 17+16+3+1, 17+16+4+1, 18+16+1,
18+16+2+1, 18+16+3+1, 18+16+4+1,
19+16+1, 19+16+2+1, 19+16+3+1, 19+16+4+1, 20+1, 20+2+1, 20+3+1, 20+4+1,
21+20+1, 21+20+2+1, 21+20+3+1, 21+20+4+1,
22+20+1, 22+20+2+1, 22+20+3+1, 22+20+4+1, 23+1, 23+2+1, 23+3+1, 23+4+1, 23+5,
23+6, 23+7, 23+8, 23+9, 23+10, 23+11+1,
23+11+2+1, 23+11+3+1, 23+11+4+1, 23+12+11+1, 23+12+11+2+1, 23+12+11+3+1,
23+12+11+4+1, 23+13+11+1,
23+13+11+2+1, 23+13+11+3+1, 23+13+11+4+1, 23+14+11+1, 23+14+11+2+1,
23+14+11+3+1, 23+14+11+4+1, 23+15+11+1,
23+15+11+2+1, 23+15+11+3+1, 23+15+11+4+1, 23+16+1, 23+16+2+1, 23+16+3+1,
23+16+4+1, 23+17+16+1, 23+17+16+2+1,
23+17+16+3+1, 23+17+16+4+1, 23+18+16+1, 23+18+16+2+1, 23+18+16+3+1,
23+18+16+4+1, 23+19+16+1, 23+19+16+2+1,
23+19+16+3+1, 23+19+16+4+1, 23+20+1, 23+20+2+1, 23+20+3+1, 23+20+4+1,
23+21+20+1, 23+21+20+2+1, 23+21+20+3+1,
23+21+20+4+1, 23+22+20+1, 23+22+20+2+1, 23+22+20+3+1, 23+22+20+4+1, 26+1,
26+2+1, 26+3+1, 26+4+1, 26+5, 26+6,
26+7, 26+8, 26+9, 26+10, 26+11+1, 26+11+2+1, 26+11+3+1, 26+11+4+1, 26+12+11+1,
26+12+11+2+1, 26+12+11+3+1,
26+12+11+4+1, 26+13+11+1, 26+13+11+2+1, 26+13+11+3+1, 26+13+11+4+1,
26+14+11+1, 26+14+11+2+1, 26+14+11+3+1,
26+14+11+4+1, 26+15+11+1, 26+15+11+2+1, 26+15+11+3+1, 26+15+11+4+1, 26+16+1,
26+16+2+1, 26+16+3+1, 26+16+4+1,
26+17+16+1, 26+17+16+2+1, 26+17+16+3+1, 26+17+16+4+1, 26+18+16+1,
26+18+16+2+1, 26+18+16+3+1, 26+18+16+4+1,
26+19+16+1, 26+19+16+2+1, 26+19+16+3+1, 26+19+16+4+1, 26+20+1, 26+20+2+1,
26+20+3+1, 26+20+4+1, 26+21+20+1,
26+21+20+2+1, 26+21+20+3+1, 26+21+20+4+1, 26+22+20+1, 26+22+20+2+1,
26+22+20+3+1, 26+22+20+4+1, 26+23+1,
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
102
26+23+2+1,26+23+3+1,26+23+4+1,26+23+5,26+23+6,26+23+7,26+23+8,26+23+9,26+23+10,
26+23+11+1,26+23+11+2+1,
26+23+11+3+1,26+23+11+4+1,26+23+12+11+1,26+23+12+11+2+1, 26+23+12+11+3+1,
26+23+12+11+4+1, 26+23+13+11+1,
26+23+13+11+2+1, 26+23+13+11+3+1, 26+23+13+11+4+1, 26+23+14+11+1,
26+23+14+11+2+1, 26+23+14+11+3+1,
26+23+14+11+4+1, 26+23+15+11+1,26+23+15+11+2+1,26+23+15+11+3+1,
26+23+15+11+4+1,26+23+16+1,26+23+16+2+1,
26+23+16+3+1,26+23+16+4+1,26+23+17+16+1,26+23+17+16+2+1, 26+23+17+16+3+1,
26+23+17+16+4+1, 26+23+18+16+1,
26+23+18+16+2+1, 26+23+18+16+3+1, 26+23+18+16+4+1, 26+23+19+16+1,
26+23+19+16+2+1, 26+23+19+16+3+1,
26+23+19+16+4+1, 26+23+20+1, 26+23+20+2+1, 26+23+20+3+1, 26+23+20+4+1,
26+23+21+20+1, 26+23+21+20+2+1,
26+23+21+20+3+1, 26+23+21+20+4+1, 26+23+22+20+1, 26+23+22+20+2+1,
26+23+22+20+3+1, 26+23+22+20+4+1, 26+24,
26+25, 28+1,28+2+1,28+3+1,28+4+1, 28+5,28+6,28+7, 28+8,28+9,
28+10,28+11+1,28+11+2+1,28+11+3+1,28+11+4+1,
28+12+11+1, 28+12+11+2+1, 28+12+11+3+1, 28+12+11+4+1, 28+13+11+1,
28+13+11+2+1, 28+13+11+3+1, 28+13+11+4+1,
28+14+11+1, 28+14+11+2+1, 28+14+11+3+1, 28+14+11+4+1, 28+15+11+1,
28+15+11+2+1, 28+15+11+3+1, 28+15+11+4+1,
28+16+1, 28+16+2+1, 28+16+3+1, 28+16+4+1, 28+17+16+1, 28+17+16+2+1,
28+17+16+3+1, 28+17+16+4+1, 28+18+16+1,
28+18+16+2+1, 28+18+16+3+1, 28+18+16+4+1, 28+19+16+1, 28+19+16+2+1,
28+19+16+3+1, 28+19+16+4+1, 28+20+1,
28+20+2+1, 28+20+3+1, 28+20+4+1, 28+21+20+1, 28+21+20+2+1, 28+21+20+3+1,
28+21+20+4+1, 28+22+20+1,
28+22+20+2+1,28+22+20+3+1,28+22+20+4+1,28+23+1,28+23+2+1,28+23+3+1,28+23+4+1,28
+23+5,28+23+6,28+23+7,
28+23+8, 28+23+9, 28+23+10, 28+23+11+1, 28+23+11+2+1, 28+23+11+3+1,
28+23+11+4+1, 28+23+12+11+1,
28+23+12+11+2+1, 28+23+12+11+3+1, 28+23+12+11+4+1, 28+23+13+11+1,
28+23+13+11+2+1, 28+23+13+11+3+1,
28+23+13+11+4+1, 28+23+14+11+1, 28+23+14+11+2+1, 28+23+14+11+3+1,
28+23+14+11+4+1, 28+23+15+11+1,
28+23+15+11+2+1, 28+23+15+11+3+1, 28+23+15+11+4+1, 28+23+16+1, 28+23+16+2+1,
28+23+16+3+1, 28+23+16+4+1,
28+23+17+16+1, 28+23+17+16+2+1, 28+23+17+16+3+1, 28+23+17+16+4+1,
28+23+18+16+1, 28+23+18+16+2+1,
28+23+18+16+3+1, 28+23+18+16+4+1, 28+23+19+16+1, 28+23+19+16+2+1,
28+23+19+16+3+1, 28+23+19+16+4+1,
28+23+20+1, 28+23+20+2+1, 28+23+20+3+1, 28+23+20+4+1, 28+23+21+20+1,
28+23+21+20+2+1, 28+23+21+20+3+1,
28+23+21+20+4+1, 28+23+22+20+1, 28+23+22+20+2+1, 28+23+22+20+3+1,
28+23+22+20+4+1, 28+24, 28+25, 28+26+1,
28+26+2+1,28+26+3+1,28+26+4+1,28+26+5,28+26+6,28+26+7,28+26+8,28+26+9,28+26+10,
28+26+11+1,28+26+11+2+1,
28+26+11+3+1,28+26+11+4+1,28+26+12+11+1,28+26+12+11+2+1,28+26+12+11+3+1,
28+26+12+11+4+1, 28+26+13+11+1,
28+26+13+11+2+1, 28+26+13+11+3+1, 28+26+13+11+4+1, 28+26+14+11+1,
28+26+14+11+2+1, 28+26+14+11+3+1,
28+26+14+11+4+1, 28+26+15+11+1,28+26+15+11+2+1,28+26+15+11+3+1,
28+26+15+11+4+1,28+26+16+1,28+26+16+2+1,
28+26+16+3+1,28+26+16+4+1,28+26+17+16+1,28+26+17+16+2+1, 28+26+17+16+3+1,
28+26+17+16+4+1,28+26+18+16+1,
28+26+18+16+2+1, 28+26+18+16+3+1, 28+26+18+16+4+1, 28+26+19+16+1,
28+26+19+16+2+1, 28+26+19+16+3+1,
28+26+19+16+4+1, 28+26+20+1, 28+26+20+2+1, 28+26+20+3+1, 28+26+20+4+1,
28+26+21+20+1, 28+26+21+20+2+1,
28+26+21+20+3+1, 28+26+21+20+4+1, 28+26+22+20+1, 28+26+22+20+2+1,
28+26+22+20+3+1, 28+26+22+20+4+1,
28+26+23+1, 28+26+23+2+1, 28+26+23+3+1, 28+26+23+4+1, 28+26+23+5, 28+26+23+6,
28+26+23+7, 28+26+23+8,
28+26+23+9,28+26+23+10,28+26+23+11+1,28+26+23+11+2+1,28+26+23+11+3+1,
28+26+23+11+4+1,28+26+23+12+11+1,
28+26+23+12+11+2+1, 28+26+23+12+11+3+1, 28+26+23+12+11+4+1, 28+26+23+13+11+1,
28+26+23+13+11+2+1,
28+26+23+13+11+3+1, 28+26+23+13+11+4+1, 28+26+23+14+11+1, 28+26+23+14+11+2+1,
28+26+23+14+11+3+1,
28+26+23+14+11+4+1, 28+26+23+15+11+1, 28+26+23+15+11+2+1, 28+26+23+15+11+3+1,
28+26+23+15+11+4+1,
28+26+23+16+1, 28+26+23+16+2+1, 28+26+23+16+3+1, 28+26+23+16+4+1,
28+26+23+17+16+1, 28+26+23+17+16+2+1,
CA 03177335 2022- 10- 28
W02021/219849
FI7I/EP2021/061401
103
28+26+23+17+16+3+1, 28+26+23+17+16+4+1, 28+26+23+18+16+1, 28+26+23+18+16+2+1,
28+26+23+18+16+3+1,
28+26+23+18+16+4+1, 28+26+23+19+16+1, 28+26+23+19+16+2+1, 28+26+23+19+16+3+1,
28+26+23+19+16+4+1,
28+26+23+20+1, 28+26+23+20+2+1, 28+26+23+20+3+1, 28+26+23+20+4+1,
28+26+23+21+20+1, 28+26+23+21+20+2+1,
28+26+23+21+20+3+1, 28+26+23+21+20+4+1, 28+26+23+22+20+1, 28+26+23+22+20+2+1,
28+26+23+22+20+3+1,
28+26+23+22+20+4+1,or28+26+24,28+26+25.
The invention relates to compounds of the Formula (I) as defined in embodiment
1), or to such compounds further
limited by the characteristics of any one of embodiments 2) to 53), under
consideration of their respective dependencies;
to pharmaceutically acceptable salts thereof; and to the use of such compounds
as medicaments especially in the
treatment of diseases or disorders where CCR6 receptors are involved as
described hereinbelow.
The present invention also includes isotopically labelled, especially 2H
(deuterium) labelled compounds of Formula (I),
which compounds are identical to the compounds of Formula (I) except that one
or more atoms have each been
replaced by an atom having the same atomic number but an atomic mass different
from the atomic mass usually found
in nature. Isotopically labelled, especially 2H (deuterium) labelled compounds
of Formula (I) and salts thereof are within
the scope of the present invention. Substitution of hydrogen with the heavier
isotope 2H (deuterium) may lead to greater
metabolic stability, resulting e.g. in increased in-vivo half-life or reduced
dosage requirements, or may lead to reduced
inhibition of cytochrome P450 enzymes, resulting e.g. in an improved safety
profile. In one embodiment of the invention,
the compounds of Formula (I) are not isotopically labelled, or they are
labelled only with one or more deuterium atoms.
In a sub-embodiment, the compounds of Formula (I) are not isotopically
labelled at all. Isotopically labelled compounds
of Formula (I) may be prepared in analogy to the methods described
hereinafter, but using the appropriate isotopic
variation of suitable reagents or starting materials.
Where the plural form is used for compounds, salts, pharmaceutical
compositions, diseases and the like, this is intended
to mean also a single compound, salt, pharmaceutical composition, disease or
the like.
Any reference to compounds of Formula (I) according to embodiments 1) to 53)
is to be understood as referring also to
the salts (and especially the pharmaceutically acceptable salts) of such
compounds, as appropriate 2nd expedient.
The term "pharmaceutically acceptable salts" refers to salts that retain the
desired biological activity of the subject
compound and exhibit minimal undesired toxicological effects. Such salts
include inorganic or organic acid and/or base
addition salts depending on the presence of basic and/or acidic groups in the
subject compound. For reference see for
example "Handbook of Pharmaceutical Salts. Properties, Selection and Use.", P.
Heinrich Stahl, Camille G. Wermuth
(Eds.), Wiley-VCH, 2008; and "Pharmaceutical Salts and Co-crystals", Johan
Wouters and Luc Quere (Eds.), RSC
Publishing, 2012.
Definitions provided herein are intended to apply uniformly to the compounds
of Formula (I), as defined in any one of
embodiments 1) to 53), and, mutatis mutandis, throughout the description and
the claims unless an otherwise expressly
set out definition provides a broader or narrower definition. It is well
understood that a definition or preferred definition
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
104
of a term defines and may replace the respective term independently of (and in
combination with) any definition or
preferred definition of any or all other terms as defined herein.
The compounds of Formula (I) may encompass compounds with one or more
asymmetric centers, such as one or more
asymmetric carbon atoms, which may be present in (R)- as well as (S)-
configuration. The compounds of Formula (I)
may further encompass compounds with one or more double bonds which are
allowed to be present in Z- as well as E-
configuration and/or compounds with substituents at a ring system which are
allowed to be present, relative to each
other, in cis- as well as trans-configuration. The compounds of Formula (I)
may thus be present as mixtures of
stereoisomers or preferably in stereoisomerically enriched form, especially as
essentially pure stereoisomers. Mixtures
of stereoisomers may be separated in a manner known to a person skilled in the
art.
In case a particular compound (or generic structure) is designated as (R)- or
(S)-enantiomer, such designation is to be
understood as referring to the respective compound (or generic structure) in
enriched, especially essentially pure,
enantiomeric form. Likewise, in case a specific asymmetric center in a
compound is designated as being in (R)- or (S)-
configuration or as being in a certain relative configuration, such
designation is to be understood as referring to the
compound that is in enriched, especially essentially pure, form with regard to
the respective configuration of said
asymmetric center. In analogy, cis- or trans-designations are to be understood
as referring to the respective
stereoisomer in enriched, especially essentially pure, form, Likewise, in case
a particular compound (or generic
structure) is designated as Z- or E-stereoisomer (or in case a specific double
bond in a compound is designated as
being in Z- or E-configuration), such designation is to be understood as
referring to the respective compound (or generic
structure) in enriched, especially essentially pure, stereoisomeric form (or
to the compound that is in enriched, especially
essentially pure, form with regard to the respective configuration of the
double bond).
The term "enriched", when used in the context of stereoisomers, is to be
understood in the context of the present
invention to mean that the respective stereoisomer is present in a ratio of at
least 70:30, especially of at least 90:10
(i.e., in a purity of at least 70% by weight, especially of at least 90% by
weight), with regard to the respective other
stereoisomer / the entirety of the respective other stereoisomers.
The term "essentially pure", when used in the context of stereoisomers, is to
be understood in the context of the present
invention to mean that the respective stereoisomer is present in a purity of
at least 95% by weight, especially of at least
99% by weight, with regard to the respective other stereoisomer / the entirety
of the respective other stereoisomers.
The compounds of Formula (I) according to embodiments 1) to 53) and their
pharmaceutically acceptable salts can be
used as medicaments, e.g. in the form of pharmaceutical compositions for
enteral (such especially oral) or parenteral
administration (including topical application or inhalation).
The production of the pharmaceutical compositions can be effected in a manner
which will be familiar to any person
skilled in the art (see for example Remington, The Science and Practice of
Pharmacy, 21st Edition (2005), Part 5,
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
105
"Pharmaceutical Manufacturing" [published by Lippincott Williams & Wilkins])
by bringing the described compounds of
Formula (I), or their pharmaceutically acceptable salts, optionally in
combination with other therapeutically valuable
substances, into a galenical administration form together with suitable, non-
toxic, inert, therapeutically compatible solid
or liquid carrier materials and, if desired, usual pharmaceutical adjuvants.
Whenever the word "between" is used to describe a numerical range, it is to be
understood that the end points of the
indicated range are explicitly included in the range. For example: if a
temperature range is described to be between 40
C and 80 C, this means that the end points 40 C and 80 C are included in
the range; or if a variable is defined as
being an integer between 1 and 4, this means that the variable is the integer
1, 2, 3, or 4.
Unless used regarding temperatures, the term "about" (or alternatively the
term "around") placed before a numerical
value "X" refers in the current application to an interval extending from X
minus 10% of X to X plus 10% of X, and
preferably to an interval extending from X minus 5% of X to X plus 5% of X. In
the particular case of temperatures, the
term "about" placed before a temperature "Y" refers in the current application
to an interval extending from the
temperature Y minus 10 C to Y plus 10 C, and preferably to an interval
extending from Y minus 5 C to Y plus 5 C.
The compounds of Formula (I) as defined hereinabove are useful for the
prevention or treatment of various diseases,
conditions or disorders ameliorated by modulating CCR6 receptors. Such
diseases, conditions or disorders where
CCR6 receptors are involved may be defined as inflammatory and/or autoimmune
diseases, conditions or disorders;
and cancer.
The compounds of Formula (I) as defined hereinabove are useful for the
prevention or treatment of of various diseases,
conditions or disorders ameliorated by modulating CCR6 receptors. Such
diseases, conditions or disorders where
CCR6 receptors are involved may be defined as including rheumatoid arthritis;
ankylosing spondylitis; spondyloarthritis;
psoriasis; psoriatic arthritis; inflammatory skin disorders such as rosacea;
Crohn's disease; ulcerative colitis;
inflammatory bowel disease; irritable bowel disease; dry eye disease; multiple
sclerosis; systemic lupus erythematosus;
Sjogren's disease; autoimmune hepatitis; Primary Sclerosing Cholangitis;
Posterior uveitis; allergic conjunctivitis;
allergic disease in the gastrointestinal tract; type I diabetes and
endometnosis; diseases of the ocular surface in which
elevated levels of IL-17A have been recorded such as meibomian gland
dysfunction; GVHD; graft-versus host disease;
autoimmune keratitis; filamentary keratitis; dry eye syndrome with rheumatic
arthritis; dry eye syndrome without
systemic disease; Stevens-Johnson syndrome; psoriasis including plaque
psoriasis, guttate psoriasis, inverse
psoriasis, pustular psoriasis, erythrodermic psoriasis; autoimmune keratitis;
filamentary keratitis; autoimmune uveitis;
allergic conjunctivitis; asthma; allergic disease of the gastrointestinal
tract; T1D; endometriosis; meibomian gland
dysfunction; graft-versus host disease; juvenile arthritis; juvenile
rheumatoid arthritis; systemic onset rheumatoid
arthritis; pauciarticular rheumatoid arthritis; pauciarticular juvenile
rheumatoid arthritis; polyarticular rheumatoid arthritis;
enteropathic arthritis; juvenile Reiter's Syndrome; ankylosing spondylitis;
juvenile ankylosing spondylitis; SEA
Syndrome; reactive arthritis (reactive arthropathy); psoriatic arthropathy;
juvenile enteropathic arthritis; polymyalgia
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
106
rheumatica; enteropathic spondylitis; juvenile idiopathic arthritis (JIA);
juvenile psoriatic arthritis; juvenile rheumatoid
arthritis; systemic onset juvenile rheumatoid arthritis; acute pancreatitis;
chronic pancreatitis; giant cell arteritis; and
secondary osteoarthritis from inflammatory diseases.
Further, such diseases, conditions or disorders where CCR6 receptors are
involved may be defined as including cancer
such as skin cancer e.g. melanoma (superficial spreading, nodular, lentigo
maligna and acral lentiginous melanoma);
advanced melanoma; metastatic melanoma; Merkel cell carcinoma; Kaposi sarcoma;
basal cell carcinoma; squamous
cell carcinoma; and pre-cancerous skin lesions such as actinic keratosis; lung
cancer including small cell lung cancer
and non-small (SOLO, NSCLC) such as squamous and non-squamous NSCLC,
pleuropulmonary blastoma and
tracheobronchial tumors; bladder cancer including urinary bladder cancer;
urothelial cell carcinoma; mesothelioma;
renal carcinomas including renal cell carcinoma (RCC) such as clear cell RCC;
papillary RCC; chromophobe RCC; non-
clear cell RCC; unclassified RCC; metastatic renal cell carcinoma; metastatic
renal clear cell carcinoma; renal
parenchymal carcinoma; gastro-intestinal cancers including colorectal cancer;
metastatic colorectal cancer; familial
adenomatous polyposis (FAP); rectum carcinoma; colon carcinoma; colorectal
adenoma; colorectal adenocarcinoma;
colorectal cancer liver metastases; hereditary non-polyposis colorectal
cancer; esophageal cancer; gastric cancer;
advanced gastric cancer; gallbladder cancer; cholangiocarcinoma;
hepatocellular carcinoma; pancreatic cancer such
as pancreatic adenocarcinoma or pancreatic ductal (adeno)carcinoma; pancreatic
neuroendocrine tumors; endometrial
cancer; ovarian cancer; prostate cancer including castrate-resistant prostate
cancer; brain tumors including brain
metastases, malignant gliomas, glioblastoma multiforme, medulloblastoma,
meningiomas, astrocytoma; peripheral
neuroectodermal tumors; oligoastrocytic tumors; oligodendrogliomas; ependymal
tumors; anaplastic astrocytoma;
pilocytic astrocytoma; craniopharyngioma; spinal cord tumors; brain stem
glioma; central nervous system atypical
teratoid/rhabdoid tumor; medulloblastoma; central nervous system germ cell
tumors; craniopharyngioma;
ependymonna; neuroblastonna; head and neck cancer such as
esthesioneuroblastoma; cervical cancer; advanced
cervical cancer; breast cancer including normal-like, basal-like, claudin-low,
HER2 positive, luminal-A, luminal-B and
triple negative breast carcinoma; pregnancy breast cancer and male breast
cancer; oral tumors; nasopharyngeal
tumors; heart tumors; thoracic cancer; lymphomas such as Hodgkin lymphoma, non-
Hodgkin lymphoma, Burkitt
lymphoma; primary intra-ocular B-Cell lymphoma; diffuse large B-cell lymphoma;
primary mediastinal large B-cell
lymphoma; mucosa-associated lymphoid tissue (MALT) lymphoma; gastric MALT
lymphoma; cutaneous T-cell
lymphoma; primary central nervous system lymphoma; Sezary syndrome and
Waldenstrom macroglobulinemia;
leukemia such as acute lymphoblastic leukemia; acute myeloid leukemia ;
chronic lymphocytic leukemia; chronic
myelogenous leukemia; hairy cell leukemia; chronic myeloid leukemia; adult T-
cell leukemia; carcinomas;
adenocarcinomas; thyroid carcinoma including papillary thyroid carcinoma and
medullary thyroid carcinoma
choriocarcinoma; sarcomas including Ewing's sarcoma; bone cancer such as
osteosarcoma; high-grade osteosarcoma;
rhabdomyosarcoma; Ewing sarcoma; malignant fibrous histiocytoma of the bone;
chordoma; soft tissue sarcoma;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
107
myeloma; multiple myelomas; labial carcinoma; larynx carcinoma; hypopharynx
carcinoma; tongue carcinoma; salivary
gland carcinoma; cervix carcinoma; uterine corpus carcinoma; endometrium
carcinoma; chorion carcinoma; testis
carcinoma; urinary carcinoma; bronchial carcinoma; basalioma; teratoma;
retinoblastoma; choroid melanoma;
seminoma; chondrosarcoma; myosarcoma; liposarcoma; fibrosarcoma; plasmacytoma;
hepatocarcinoma; advanced
liver cancer; gastrointestinal stromal tumors; neuroendocrine tumors; bile
duct cancer; appendix cancer; gastrointestinal
carcinoid tumor; carcinoid tumor; islet cell tumor; small intestine cancer;
stomach cancer; adrenocortical carcinoma;
parathyroid cancer; paraganglioma; pheochromocytoma; pituitary tumor; penile
cancer; renal pelvis and ureter cancer;
testicular cancer; urethral cancer; Wilms tumor; extracranial germ cell tumor;
extragonadal germ cell tumor; fallopian
tube cancer; gestational trophoblastic tumor; primary peritoneal cancer;
vaginal cancer; vulvar cancer; hypopharyngeal
cancer; laryngeal cancer; papillomatosis cancer; lip and oral cavity cancer;
metastatic squamous neck cancer; mouth
cancer; nasopharyngeal cancer; oropharyngeal cancer; paranasal sinus and nasal
cavity and paranasal sinus cancer;
parathyroid cancer; pharyngeal cancer; throat cancer; chronic
myeloproliferative neoplasm; Langerhans cell
histiocytosis; plasma cell neoplasm; myelodysplastic syndromes;
myeloproliferative neoplasm; midline tract carcinoma;
virally induced tumors; and diseases involving CCR6 and/or CCL20 mediated
metastasis, chemotaxis, cell adhesion,
trans-endothelial migration, cell proliferation and/or survival.
Notably, such diseases, conditions or disorders, where CCR6 receptors are
involved refer to rheumatoid arthritis;
ankylosing spondylitis; spondyloarthritis; psoriasis; psoriatic arthritis;
inflammatory skin disorders e.g. rosacea; Crohn's
disease; ulcerative colitis; irritable bowel disease; inflammatory bowel
disease; dry eye disease; multiple sclerosis;
systemic lupus erythematosus; Sjogren's disease; autoimmune hepatitis; Primary
Sclerosing Cholangitis; psoriasis
including plaque psoriasis, guttate psoriasis, inverse psoriasis, pustular
psoriasis, erythrodermic psoriasis; autoimmune
keratitis; filamentary keratitis; autoimmune uveitis; allergic conjunctivitis;
asthma; allergic disease of the gastrointestinal
tract; type 1 diabetes (T1D); endometriosis; meibomian gland dysfunction;
graft-versus host disease; lymphoma
including T cell lymphoma and primary mediastinal B-cell lymphoma; brain
cancer including glioma and glioblastoma;
breast cancer including triple negative breast cancer; colorectal cancer;
hepatocarcinoma; renal cell carcinoma; lung
cancer including non-small cell lung cancer and small cell lung cancer;
gastric cancer; melanoma including Merkel cell
carcinoma, cutaneous squamous cell carcinoma and malignant melanoma; bladder
cancer; head and neck cancer
including squamous cell head and neck carcinoma; Hodgkin's lymphoma; cervical
cancer; endometrial cancer; colon
cancer; gastrointestinal stromal tumors; pancreatic cancer; prostatic cancer;
leukemia including acute myeloid
leukemia; ovarian cancer; oesophageal carcinomas; mesothelioma; neuroblastoma;
sarcoma e.g. high-grade
osteosarcoma; astrocytoma; myeloma; urothelial cancer including locally
advanced and metastatic urothelial cancer;
MSI-H or dMMR cancer; rectal cancer; laryngeal cancer; salivary
adenocarcinoma; multiple myeloma;
cholangiocarcinoma; oral squamous cell carcinoma; thyroid cancer; and
esophagogastric junction cancer.
Especially, such diseases, conditions or disorders, where CCR6 receptors are
involved are selected from
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
108
= inflammatory/autoimmune diseases, conditions or disorders such as
psoriasis; psoriatic arthritis; rheumatoid
arthritis; ankylosing spondylitis; spondyloarthritis; inflammatory skin
disorders e.g. rosacea; Crohn's disease;
ulcerative colitis; irritable bowel disease; dry eye disease; multiple
sclerosis; systemic lupus erythematosus;
Sjogren's disease; autoimmune hepatitis; and Primary Sclerosing Cholangitis;
In particular, psoriasis or psoriatic
arthritis; and/or
= cancer such as lymphoma (e.g. T cell lymphoma); brain cancer (e.g. glioma
or glioblastoma); breast cancer;
colorectal cancer; hepatocarcinomas; renal cell carcinoma; lung cancer; and
gastric cancer.
For avoidance of any doubt, if compounds are described as useful for the
prevention or treatment of certain diseases,
conditions or disorders, such compounds are likewise suitable for use in the
preparation of a medicament for the
prevention or treatment of said diseases.
The present invention also relates to a method for the prevention or treatment
of diseases, conditions or disorders,
mentioned hereinabove and/or hereinbelow comprising administering to a subject
a pharmaceutically active amount of
a compound as described hereinabove or/and hereinbelow either alone or in
combination with other pharmacologically
active compounds and/or therapies.
The meaning of the term "prevention" may also be understood as "prophylaxis".
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
109
Preparation of compounds of Formula (I)
A further aspect of the invention is a process for the preparation of
compounds of Formula (I). Compounds according
to Formula (I) of the present invention can be prepared from commercially
available or well-known starting materials
according to the methods described in the experimental part; by analogous
methods; or according to the general
sequence of reactions outlined below. Optimum reaction conditions may vary
with the particular reactants or solvents
used, but such conditions can be determined by a person skilled in the art by
routine optimisation procedures. In the
schemes below, the generic groups A, B, R1 and R2 are as defined for the
compounds of Formula (I); the number of
carbon atoms "n" is 1. X represents a halogen atom, notably chlorine or
bromine. The meaning of the generic group R'
is derivable from the compounds of Formula (I) and/or the exemplified
embodiments. Other abbreviations used herein
are explicitly defined, or are as defined in the experimental section. In some
instances, the functional groups described
may be incompatible with the assembly illustrated in the schemes and so will
require the use of protecting groups (PG).
The use of protecting groups is well known in the art (see for example
"Protective Groups in Organic Synthesis", T.W.
Greene, P.G.M. Wuts, Wiley-lnterscience, 1999). For the purposes of this
discussion, it will be assumed that such
protecting groups as necessary are in place. The compounds obtained may also
be converted into salts, especially
pharmaceutically acceptable salts thereof in a manner known per se.
General preparation routes:
Compounds of Formula (I) can be prepared starting from an intermediate of
Formula (Al), which is reacted with N,0-
dimethylhydroxylamine hydrochloride under standard conditions (e.g. HATU,
DIPEA, DMF) to give the Weinreb amide
derivative of Formula (A2) (Scheme A). Upon reaction with a compound of
Formula (A3) wherein X is iodine or bromine,
in presence of n-butyl lithium or n-hexyl lithium in THF at a temperature
around -78 C, the ketone derivative of Formula
(A4) is produced, which can be further reacted with a compound of Formula (A5)
wherein X is a halogen atom, preferably
bromine, similarly using n-butyl lithium or n-hexyl lithium in THF at a
temperature around -78 C, to provide the tertiary
alcohol intermediate of Formula (A6). A chiral separation by HPLC over a
chiral stationary phase can be performed at
this stage to yield enantiomerically pure intermediates of Formula (A6).
Cleavage of the protecting group under standard
conditions such as treatment with HCI in dioxane in the case of a Boc
protecting group, or treatment with Pd/C (50%
water) in Et0H or EA under hydrogen atmosphere in case of a Cbz protecting
group, provides the free NH derivative
of Formula (A7). A reductive amination step can be performed with an aldehyde
of Formula (A8) or a ketone of Formula
(A9) under standard conditions such as using NaBH(OAc)3 or NaBH3CN as
reductive agent, in presence of a base such
as DIPEA or TEA, or in presence of an acid such as acetic acid, in a solvent
such as DCM, Me0H, THF or dioxane, or
a mixture thereof, and at a temperature around RT to provide compounds of
Formula (I). Alternatively, the intermediate
of Formula (A7) can be coupled to a reactant of Formula (A10) wherein X is
iodine or bromine, in presence of a base
such as TEA, DIPEA or Cs2CO3, in a solvent such as Me0H, THF or DMF, and
stirring at a temperature from 0 C to
70 C. Furthermore, the compounds of Formula (I) wherein R2 is cyclopropyl can
be prepared by coupling with (1-
CA 03177335 2022- 10- 28
WO 2021/219849 PCT/EP2021/061401
110
ethoxycyclopropoxy)trimethysilane, using NaBH(OAc)3 in presence AcOH in Et0H
and at RT. For the compounds of
Formula (I) wherein R2 is tert-butyl, specific conditions were used that are
fully described in the experimental part
(Example 12).
0 0 0
n(r5LL:OH n(r5c).L' B
N R '
PG, N R1 I X _ B PG, N R1
(Al) (A2) (A3) (A4)
A A 0 R 2 A
OH OH (A8) OH
B n( 0 n(6<)<B
nN6K-JIR R2
H N6<jR N
R1
X'A PG-
R2
IR'
(A5) (A6) (A7) (A9) (I)
X R2
(A10)
Scheme A
Alternatively, the compounds of Formula (I) can be prepared following the
route described in Scheme B. The protecting
group in intermediate (A4) can be removed and the free NH of the resulting
intermediate (B1) can be reacted with an
aldehyde of structure (A8), a ketone of structure (A9), a reactant of Formula
(A10), or (1-
ethoxycyclopropoxy)trimethysilane, as previously described. The resulting
intermediate of Formula (B2) can be reacted
with a compound of Formula (A5) wherein X is a halogen atom, preferably
bromine, using conditions described in
Scheme A to provide compounds of Formula (I), or using a iPrMgCl-LiCI-mediated
halogen-metal exchange protocol in
presence of LiCI in THF and heating at around 60 C.
0 0 0 A
n (([5<- B n(f5<11.' B (A8) n .. B
.. n(6<j<O13 H
R1 R` N R X N R1
PG . N R1 HN
R2- -A R2-
0 R2
(A4) (B1) (A9) (B2) (A5) (I)
X R2
(Al 0)
Scheme B
Alternatively, the compounds of Formula (I) can be prepared using the same
synthetic strategies as those described in
Schemes A and B, with the difference of performing the addition of the
compound of Formula (A5) prior to the addition
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
111
of the compound of Formula (A3) in the synthetic sequence (Scheme C), and
using the same conditions as those
reported previously.
0 0 A
A
n( N-0Me
n(r5<j(A OH
)t
n(/5B n
PG,Nr5 X PGõN X PG ( CH,N R1
HN R1
'A ,B
(A2) (A5) (Cl) (A3) (A6)
(A7)1
n A n IA
(A8), A(9) or (A10)
0 0 A
OH
<L
HN R,, ' (A8) 5 N R', X, N R1
R2' R2-
(A9)
(C2) or (A10) (C3) (A3) (I)
Scheme C
5 The intermediates of Formula (D1) (see Scheme D) wherein C and/or D
are/is a nitrogen atom and R is a protecting
group, or wherein C and/or D are/is a nitrogen atom and R is R2, can be
prepared starting from the appropriate
compound of Formula (A5) wherein A contains a cyano group in meta position to
the halogen atom X, X being iodine
or bromine, following the route described in Scheme A for the synthesis of
intermediates of Formula (A6), or of Formula
(I), respectively. Alternatively, an intermediate of Formula (D1) can be
prepared starting from a compound of Formula
(A5) wherein A contains a bromine atom in meta position to the halogen atom X,
X being iodine or bromine, and using
the same conditions as described above. The bromine atom may be further
transformed into a cyano group using zinc
cyanide, in presence of zinc and Pd2(dba)3, using a ligand such as 1,1'-
bis(diphenylphosphino)ferrocene, in a solvent
such as DMF and heating at around 150 C. An intermediate of Formula (D1) can
be transformed into a hydroxy-amidine
derivative of Formula (D2) by reaction with hydroxylamine hydrochloride in a
solvent such as Et0H or DMSO, in
presence of a base such as TEA or K2CO3, and at a temperature between 80 C and
100 C. The resulting hydroxy-
amidine intermediate (02) can be further reacted with a carboxylic acid of
Formula (03) to form an oxadiazole-containing
derivative of Formula (04) if R is a protecting group, or a compound of
Formula (I) if R is R2, using a coupling agent
such as HATU, PyBOP, EDC combined with HOBt, CDI, in presence of a base such
as DIPEA or K3PO4, optionally in
presence of molecular sieves 3A, in a solvent such as dioxane, DMSO or DMF,
and heating at a temperature between
80 C and 100 C. Alternatively, the formation of the oxadiazole ring can be
performed in two steps (i) coupling with the
carboxylic acid partner of Formula (D3) as described before at RI and (ii)
heating at a temperature between 80 C and
100 C in presence of molecular sieves 3A. Moreover, a compound of Formula (D2)
can be reacted with trimethyl
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
112
orthoformate, in presence of boron trifluoride and TEA, in a solvent such as
DMA, and heating at around 50 C to provide
a compound of Formula (D4) wherein R' is hydrogen. The intermediate of Formula
(04) can be further transformed into
a compound of Formula (I) following the two-step protocol described in Scheme
A.
,OH
N
4 N-R
N-R
3 ,D 5 CN ,D ,D-
,D-
C C -NH2 C C
-N
2
I 1 OH OH OH
n(
,R' R.-Nr5<,
RN R1
RN R1
HN R1
if R = PG
HOOC
(D1) (D2) (D3) R = PG: (D4)
(D5)
R = R2: (I)
(A8), A(9), or (A10)
N-R
C
N
,r5KOH
n
N
R1
R2
(I)
Scheme D
The intermediates of Formula (El) (see Scheme E) wherein C and/or D are/is a
nitrogen atom and R is a protecting
group, or wherein C and/or D are/is a nitrogen atom and R is R2, can be
prepared starting from the appropriate
compound of Formula (A5) wherein A contains a protected acid function such as
a tert-butyl carboxylic acid moiety in
meta position to the halogen atom X, following the route described in Scheme A
for the synthesis of intermediates of
Formula (A6), or compounds of Formula (I), respectively. An intermediate of
Formula (El) can be transformed into an
intermediate of Formula (E3) wherein R is a protecting group, or into a
compound of Formula (I) wherein R is R2, by
reaction with a hydroxyamidine derivative of Formula (E2) using conditions
described in Scheme (D). Hydroxyamidine
derivatives of Formula (E2), if not commercially available, can be prepared
using the same protocol as described in
Scheme (D).
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
113
,D COOH R' R'
N-OH
(6c,<OH OH
n B R' NH2
R,,N R1
R,,N R1 1-
11N60BH
if R = PG
(El) (E2) R = PG: (E3) (E4)
R = R2- (I)
(A8), A(9) or A(10)
c-13XL NI/ -R.
nN(/-5,0BH
R2'
(I)
Scheme E
Furthermore, the intermediates of Formula (D4) and (E3) wherein R is R2 and R'
contains a protected amine function
can be transformed into compounds of formula (I) following a two-step
protocol. Firstly, the amine protecting group can
be cleaved using standard conditions such as treatment with HCI in dioxane in
the case of a Boc protecting group. The
resulting amine-containing intermediate can be subsequently engaged in a
coupling reaction, with an acid chloride
reactant, in presence of a base such as DIPEA, in a suitable solvent such as
THE; or with an acid-containing reactant
of formula (D3), using HATU as coupling agent, in presence of a base such as
DBU, in a suitable solvent such as DMF;
or with a sulfonyl chloride reactant, in presence of a base such as DIPEA, in
a suitable solvent such as DOM. In case
diacylation was observed during the coupling reaction, subsequent treatment
with K2003 in Me0H can provide the
desired compound of formula (I).
The intermediates of Formula (F1) (see Scheme F) wherein X is a chlorine atom,
C is CH, and D is nitrogen; or wherein
X is a chlorine atom, C and D are nitrogen atoms; or wherein X is a bromine
atom, C is a nitrogen atom, and D is CH;
can be prepared following the route described in Scheme B using the
appropriate derivative of Formula (A5).
Compounds of Formula (I) can be prepared by reacting an intermediate of
Formula (F1) wherein X is a bromine atom,
C is a nitrogen atom, and D is CH; or wherein X is a chlorine atom, and C and
D are nitrogen atoms; with an NH-
containing reagent of Formula (F2), using standard conditions for a Buchwald
type reaction, using a palladium catalyst
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
114
such as Pd2(dba)3, in presence of a ligand such as Xantphos, RuPhos, or BINAP,
in presence of a base such as
NaOtBu, in toluene, and heating at a temperature around 100 C. Alternatively,
the coupling reaction can be performed
using a copper catalyst such as Cul, in presence of a ligand such as L-
proline, in presence of a base such as K2003,
in a solvent such as DMSO and heating at a temperature around 100 C.
Furthermore, compounds of Formula (I) can be prepared by reacting an
intermediate of Formula (F1), wherein X is a
chlorine atom, and C and D are nitrogen atoms; or wherein X is a chlorine
atom, C is CH, and D is a nitrogen atom;
with an NH-containing reagent of Formula (F2), using standard conditions for
an aromatic nucleophilic substitution type
reaction, optionally in presence of a base such as DIPEA, and heating at a
temperature between 100 C and 150 C in
a solvent such as n-butanol, NMP, or dioxane.
An intermediate of Formula (F1) wherein X is a bromine atom, C is a nitrogen
atom, and D is CH; or wherein X is a
chlorine atom, and C and D are nitrogen atoms; can be further reacted with an
amide-containing reagent of Formula
(F3), or with a carbamate-containing reagent of Formula (F4), using a copper
catalyst such as Cul, in presence of a
ligand such as N,N-dimethylenediamine, in presence of a base such as K2CO3, in
a solvent such as dioxane, and
heating at a temperature around 110 C.
In addition, an intermediate of Formula (F1) wherein X is a chlorine atom, C
is CH, and D is nitrogen; or wherein X is a
bromine atom, C is nitrogen, and D is CH; or wherein X is a bromine atom, and
C and D are CH can be reacted with an
alkyne-containing reagent of Formula (F5), using standard conditions for a
Sonogashira type reaction, using a palladium
catalyst such as Pd(PPh3)4, Pd(OAc)2, or PdC12(PPh3)2, optionally combined
with a copper catalyst such as Cul,
optionally in presence of a ligand such as PPh3, in presence of a base such as
piperidine, Et2NH, or K3PO4, in a solvent
such as THE, DMF, or DMSO/toluene mixture, and heating at a temperature
between 60 C and 80 C. The alkyne (F5)
reagents are either commercially available or accessible via multistep
synthesis as described in the experimental part.
The resulting alkyne-containing compound of Formula (I) can be further
transformed by hydrogenation of the alkyne
functionality into an alkane chain using Pd/C (50% water) in Et0H or Me0H and
under a hydrogen atmosphere.
Furthermore, an intermediate of Formula (F1) wherein X is a chlorine atom, C
is CH, and D is nitrogen; or wherein X is
a bromine atom, C is nitrogen, and D is CH; or wherein X is a bromine atom,
and C and D are CH can be reacted with
an boronic acid or boronate ester reagent of Formula (F6) using standard
conditions for a Suzuki type reaction, using
a palladium catalyst such as Pd(PPh3)4, Pd(dppf)2012 or PdC12(PPh3)2, in
presence of a base such as Na2003 or K3PO4,
in a solvent such as MeCN/water, DME/water or dioxane/water mixture, and
heating at a temperature around 80 C.
An intermediate of Formula (F1) wherein X is bromine can be converted by
Miyaura borylation using standard conditions
to the corresponding boronic acid or ester of Formula (F10) and subsequently
treated with a reagent of Formula (F9) in
a Suzuki type reaction as described previously.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
115
In addition, an intermediate of Formula (F1) wherein X is a chlorine atom, C
is CH, and D is nitrogen; or wherein X is a
bromine atom, C is nitrogen, and D is CH can be reacted with a alkyl zinc
reagent of Formula (F7) using standard
conditions for a Negishi type reaction, using a palladium catalyst such as
Pd(dppf)2012, in a solvent such as toluene,
and heating at a temperature around 70 C.
Furthermore, compounds of Formula (I) can be prepared by reacting an
intermediate of Formula (F1) wherein X is a
chlorine atom, C is CH, and D is a nitrogen atom, with an alcohol reagent of
Formula (F8), using standard conditions
for an aromatic nucleophilic substitution type reaction, in presence of a base
such as NaH, and heating at a temperature
between 100 C and 110 C, optionally in a solvent such as dioxane.
It will be understood by one skilled in the art that the steps described in
Scheme (F) can be performed with the protected
azetidine ring (n=1) prior to the introduction of the R2 group, and following
the two-step protocol from the intermediate
(A6) described in Scheme (A) to yield compounds of Formula (I).
CA 03177335 2022- 10- 28
WO 2021/219849 PCT/EP2021/061401
116
C -= R' C "- 1(
OH OH
, N
R2 R2-NrD<I CD "- y0R
I
(I) (I)
OH
B
H
A
R,N,R' /11C11,r0 R2
(I)
(F2) (F3) IR' H
,D X IR-Nf
C,D.,., "...---=-=--- R R
`-= C `--
k.,,, R'' U.,i...,_. OH OH OH
1-1(6<Bn(f<B -- n(f<B
N R1 N R1 N R1
R2- R2- R2-
(F5) (I) (I)
(F1)
,D 0,
I
Y Zn(R)2 n( B
R_B.0,R'
2, N R1
(F7)
R
(F6) ' (I)
B(OR') ,D R
CD-- R
C -- C --
U.,,õ.. RX L.,...,
OH OH (F9) OH
n(6(B n (r)--7<B
N R1 N-_/
R2- R2- R2-
(F10) (I)
(I)
Scheme F
The intermediates of Formula (G1) (see Scheme G) wherein C is CH, and D is a
nitrogen atom; or wherein C is a
nitrogen atom, and D is CH can be prepared following the route described in
Scheme B using the appropriate derivative
of Formula (A5) that contains a protected phenol group in the form of a
benzyloxy or methyloxy functionality.
Deprotection using Pd/C in Et0H under hydrogen atmosphere, or 2-diethylamino-
ethanethiol and KOtBu in DMF,
respectively, provides the intermediates of Formula (G1). Such intermediates
of Formula (G1) can be transformed into
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
117
compounds of Formula (I) by performing a Mitsunobu type reaction with a
hydroxy-containing derivative of Formula
(G2), using conditions such as cyanomethyltributylphosphorane in toluene and
heating at a temperature around 110
'C. It will be understood by one skilled in the art that the Mitsunobu
reaction can be performed with the protected
azetidine ring (n=1) prior to the introduction of the R2 group, and following
the synthetic sequence from the intermediate
(A6) described in Scheme (A) to yield compounds of Formula (I).
-D
C-DOH
C =-= 'R
OH
n(
NI, R1
R2 HO-RN R1
(G1) (G2) (I)
Scheme G
The intermediates of Formula (A5) (see Scheme H) wherein C is CH, and D is a
nitrogen atom; or wherein C is a
nitrogen atom, and D is CH can be prepared via a two-step procedure: (i)
treatment of an appropriate nitrile of Formula
(H1) with hydroxylamine hydrochloride in a solvent such as Et0H or DMSO, in
presence of a base such as K2003 or
TEA, and at a temperature between 80 C and 100 C and (ii) subsequent treatment
of the resulting hydroxy-amidine
derivative of Formula (H2) with a carboxylic acid of Formula (D3) using a
coupling agent such as HATU, PyBOP, EDC
combined with HOBt, or CDI in possible presence of a base such as DIPEA or
K3PO4, in a solvent such as dioxane,
DMF or DMSO, and heating at a temperature between 80 C and 100 C.
N0H WOCN \
C
_D-
C -NH2
y
R'
Br Br HOOC" Br
(H1) (D3)
(H2) (A5)
Scheme H
The intermediates of Formula (J1) (see Scheme J) can be prepared following the
route described in Scheme A using
the appropriate derivative of Formula (A3) that contains a bromo-phenyl group.
Such intermediates of Formula (J1) can
be transformed into compounds of Formula (I) wherein R represents an
appropriate C1_5-alkyl, C1_4-fluoroalkyl, or C2_4-
alkenyl group by Suzuki cross coupling with boron species of Formula (J2)
wherein R represents an appropriate C1-5-
alkyl, C1_3-alkoxy-C1_4-alkyl, C1_4-fluoroalkyl, C2_4-alkenyl group and BX
represents BF3K, Bpin or B(OH)2 in the presence
of a suitable palladium catalyst such as cataCXium A Pd G3 and a suitable base
such as Cs2CO3 and heating in a
suitable solvent such as a mixture of toluene and water at temperatures around
100 C.
CA 03177335 2022- 10- 28
WO 2021/219849 PCT/EP2021/061401
118
Alternatively, intermediates of Formula (J1) can be transformed into
intermediate of Formula (J3) by Miyaura borylation
using standard conditions such as treatment with bis(pinacolato)diboron in the
presence of a suitable palladium catalyst
such as Pd(dppf)012.DCM and a suitable base such as KOAc and heating in a
suitable solvent such as dioxane at
temperatures around 8000.
Such intermediates of Formula (J3) can be transformed into compounds of
Formula (1) wherein R represents a
trifluoromethyl group by copper catalyzed perfluoroalkylation with a
trifluoromethylation reagent of Formula (J4) such
as (phen)CuCF3 in the optional presence of a suitable base such as KF and
heating in a suitable solvent such as DMF
at temperatures around 50 C.
Alternatively, intermediates of Formula (J3) can be transformed into compounds
of Formula (1) wherein R represents a
C13-alkoxy or C35-cycloalkoxy group via a two-step procedure: (i) treatment
with an aq. solution of hydrogen peroxide
in the presence of NaOH in a solvent such as THF at temperatures between 0 C
and RI and (ii) subsequent treatment
of the resulting phenol intermediate of Formula (J5) with an appropriate 01_3-
alkyl or 03_5-cycloalkyl halide of formula
(J6) in the presence of suitable base such as K2CO3 and heating in a suitable
solvent such as DMF at temperatures
around 100 C.
A A
OH OH
n(
r-1,1\1 Ri I ¨Br
R2
XB' R2
R ,N
(J1) (J2) (I)
44) (;6.
A
OH A
OH
n( n(
,N R1 sl,õ.,,Bpin
R2 ¨).-R2,-N R1 ¨OH
(J3) (J5)
Scheme J
Reactants of Formula (A3), (A5), (A8), (A9), (03), (E2), (F2) to (F9), (G2),
(J2), (J4) and (J6) are either commercially
available or can be synthesized according to published protocols.
Whenever the compounds of Formula (1) are obtained in the form of mixtures of
enantiomers, the enantiomers can be
separated using methods known to one skilled in the art: e.g. by formation and
separation of diastereomeric salts or by
HPLC over a chiral stationary phase. Enantiomeric separation may be performed
at the stage of intermediate (A6) or
with compounds of Formula (1).
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
119
Experimental section:
Abbrevations (as used herein and in the description above):
Ac acetyl
aq. aqueous
BI NAP (2,2'-bis(diphenylphosphino)-1,11-binaphthyl)
Boc tert-butyloxycarbonyl
BSA Bovine serum albumin
Brine saturated aqueous NaCI solution
Bu butyl
cataCXium A Pd G3
mesylateRdi(1-adamantyl)-n-butylphosphine)-2-(2'-amino-1,1'-
bipheny1)]palladium(II)
Cbz benzyloxycarbonyl
CC column chromatography on silica gel
CDI 1,1'-carbonyldiimidazole
CV column volume
dba dibenzylideneacetone
DCM dichloromethane
DBU 1,8-diazabicyclo[5.4.0]undec-7-ene
DIPEA N-ethyldiisopropylamine
DMA N,N-Dimethylacetamide
DME 1,2-dimethoxyethane
DMAP 4-dimethylaminophenol
DMEM Dulbecco's modified eagle media
DMF dimethylformamide
DMP Dess Martin periodinane
DMSO dimethylsulfoxide
dppf 1,1'-bis(diphenylphosphino)ferrocene
EA ethyl acetate
EDC 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
eq Equivalent
Et ethyl
FCS fetal calf serum
FLIPR Fluorescent imaging plate reader
Fluo-8-AM acetyl oxymethyl 24N42-(acetyloxymethoxy)-2-oxoethyll-443-
(acetyloxymethoxy)-6-oxoxanthen-9-
y1]-2-[242-[bis[2-(acetyloxymethoxy)-2-
oxoethyl]amino]phenoxy]ethoxy]anilino]acetate
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
120
hour(s)
HATU 2-(7-Aza-1H-benzotriazole-1-y1)-1,1,3,3-
tetramethyluronium
HEK Human embryonic kidney
Hep heptanes
HOBt hydroxybenzotriazole
HV high vacuo
HPLC high performance liquid chromatography
LC liquid chromatography
LiHMDS lithium-bis(trimethylsilyl)amide
M molarity [mol L1]
Me methyl
MS mass spectrometry
min minute(s)
MTBE methyl tert-butyl ether
NMP N-methyl-2-pyrrolidone
NMR nuclear magnetic resonance spectroscopy
org. organic
Pd/C palladium on carbon
PG protecting group
Ph phenyl
phen phenanthroline
pin pinacol
Prep preparative
PyBOP (benzotriazol-1-yloxy)tripyrrolidinophosphonium
hexafluorophosphate
rpm rotations per minute
RT room temperature
RuPhos 2-dicyclohexylphosphino-2',6'-diisopropoxybiphenyl
sat. saturated
tert
TBAF tetrabutylammoniumfluorid
TEA triethylamine
TFA trifluoroacetic acid
THF tetrahydrofuran
TLC Thin layer chromatography
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
121
tR retention time
UPLC Ultra performance liquid chromatography
Xantphos 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene
I. Chemistry
The following Examples illustrate the preparation of compounds of the
invention but do not at all limit the scope thereof.
General: All temperatures are stated in degrees Celsius ( C). Unless otherwise
indicated, the reactions take place at
RT under an argon atmosphere and are run in a flame dried round-bottomed flask
or sealable tube equipped with a
magnetic stir bar.
Characterization methods used:
The LC-MS retention times have been obtained using the following elution
conditions:
I) LC-MS (A):
Zorbax RRHD SB-Aq, 1.8 Um, 2.1x50mm column thermostated at 40 C. The two
elution solvents were as follows:
solvent A= water 0.04%TFA; solvent B = MeCN. The eluent flow rate was 0.8
mL/min and the characteristics of the
eluting mixture proportion in function of the time t from start of the elution
are summarized in the table below (a linear
gradient being used between two consecutive time points):
t (min) 0 1.2 1.9 2.1
Solvent A (%) 95 5 5 95
Solvent B (%) 5 95 95 5
II) LC-MS (B):
Acquity UPLC CSH C18 1.7 um, 2.1x50 mm from Waters thermostated at 60 C. The
two elution solvents were as
follows: solvent A= water 0.05% HCOOH; solvent B = MeCN 0.045% HCOOH. The
eluent flow rate was 1 mL/min
and the characteristics of the eluting mixture proportion in function of the
time t from start of the elution are summarized
in the table below (a linear gradient being used between two consecutive time
points):
t (min) 0 2.0 2.1
Solvent A (%) 98 2 98
Solvent B (%) 2 98 2
The chiral SFC or HPLC retention times have been obtained using the following
elution conditions:
I) Chiral SFC (A):
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
122
CHIRALCEL OD-H, 5 m, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= 002; solvent B = Me0H. The eluent flow rate was 4 mL/min, the
duration of the run was 5min and the
isocratic solvent proportion was 92% (A) / 8% (B).
II) Chiral SFC (B):
CHIRALCEL OZ-H, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= CO2; solvent B = Me0H + 0.1% DEA. The eluent flow rate was 4
mL/min, the duration of the run was 4min
and the isocratic solvent proportion was 85% (A) / 15% (B).
III) Chiral SFC (C):
CHIRALPAK AD-H, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= CO2; solvent B = Et0H. The eluent flow rate was 4 mL/min, the
duration of the run was 3min and the isocratic
solvent proportion was 80% (A) / 20% (B).
IV) Chiral SFC (D):
CHIRALPAK I B, 5 Urn, 4.6x250mm column thermostated at 4000 was used. The two
elution solvents were as follows:
solvent A= CO2; solvent B = Et0H. The eluent flow rate was 4 mL/min, the
duration of the run was 5min and the isocratic
solvent proportion was 85% (A) / 15% (B).
V) Chiral SFC (E):
CHIRALPAK IC, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= CO2; solvent B = Me0H + 0.1% DEA. The eluent flow rate was 4
mL/min, the duration of the run was 5min
and the isocratic solvent proportion was 60% (A) / 40% (B).
VI) Chiral SFC (F):
CHIRALPAK I B, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= 002; solvent B = Me0H. The eluent flow rate was 4 mL/min, the
duration of the run was 4min and the
isocratic solvent proportion was 90% (A) / 10% (B).
VII) Chiral SFC (G):
CHIRALPAK AD-H, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= 002; solvent B = Et0H. The eluent flow rate was 4 mL/min, the
duration of the run was 4min and the isocratic
solvent proportion was 75% (A) / 25% (B).
VIII) Chiral SFC (H):
CHIRALCEL OZ-H, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= CO2; solvent B = Me0H + 0.1% DEA. The eluent flow rate was 4
mL/min, the duration of the run was 5min
and the isocratic solvent proportion was 85% (A) / 15% (B).
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
123
IX) Chiral SFC (I):
CHIRALCEL OD-H, 5 m, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= CO2; solvent B = Me0H. The eluent flow rate was 4 mL/min, the
duration of the run was 3.5min and the
isocratic solvent proportion was 90% (A) / 10% (B).
X) Chiral SFC (J):
CHIRALPAK AD-H, 5 Um, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= 002; solvent B = MeCN/Et0H 1/1 + 0.1%DEA. The eluent flow rate was
4 mL/min, the duration of the run
was 5min and the isocratic solvent proportion was 75% (A) / 25% (B).
XI) Chiral SFC (K):
CHIRALPAK AS-H, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= CO2; solvent B = MeCN/Et0H + 0.1%DEA. The eluent flow rate was 4
mL/min, the duration of the run was
3min and the isocratic solvent proportion was 70% (A) / 30% (B).
XII) Chiral SFC (L):
CHIRALCEL OD-H, 511m, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= 002; solvent B = Me0H + 0.1% DEA. The eluent flow rate was 4
mL/min, the duration of the run was 5min
and the isocratic solvent proportion was 85% (A) / 15% (B).
XIII) Chiral SFC (M):
CHIRALPAK AD-H, 5 Um, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= 002; solvent B = Et0H 1%DEA. The eluent flow rate was 4 mL/min, the
duration of the run was 3min and
the isocratic solvent proportion was 75% (A) / 25%(B).
XIV) Chiral SFC (N):
REGIS (R,R) Whelk-01, 5 Urn, 4.6x250mm column thermostated at 40 C. The two
elution solvents were as follows:
solvent A= 002; solvent B = DCM/Me0H 1/1 + 0.1%DEA. The eluent flow rate was 4
mL/min, the duration of the run
was 5min and the isocratic solvent proportion was 70% (A) / 30% (B).
XV) Chiral SFC (0):
CHIRALPAK ID, 5 Urn, 4.6x250mm column thermostated at 40 C. The two elution
solvents were as follows: solvent A=
002; solvent B = MeCN/Et0H 1/1 + 0.1%DEA. The eluent flow rate was 4 mL/min,
the duration of the run was 5min
and the isocratic solvent proportion was 65% (A) / 35% (B).
XVI) Chiral SFC (P):
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
124
CHIRALPAK I B, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= 002; solvent B = Me0H+0.1%DEA. The eluent flow rate was 4 mL/min,
the duration of the run was 5min
and the isocratic solvent proportion was 90% (A) / 10% (B).
XVII) Chiral SFC (Q):
CHIRALPAK I B, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= CO2; solvent B = Me0H. The eluent flow rate was 4 mL/min, the
duration of the run was 2.50min and the
isocratic solvent proportion was 80% (A) / 20% (B).
XVIII) Chiral SFC (R):
CHIRALPAK I H, 5 Urn, 4.6x250mm column thermostated at 4000 was used. The two
elution solvents were as follows:
solvent A= CO2; solvent B = MeCN + Et0H 1/1. The eluent flow rate was 4
mL/min, the duration of the run was 5min
and the isocratic solvent proportion was 85% (A) / 15% (B).
XIX) Chiral HPLC (S):
CHIRALPAK AY-H, 5 Urn, 4.6x250mm column thermostated at 25 C was used. The two
elution solvents were as follows:
solvent A= Hept; solvent B = 2-Propanol. The eluent flow rate was 1 mL/min,
the duration of the run was 15min and the
isocratic solvent proportion was 80% (A) / 20% (B).
XX) Chiral SFC (T):
CHIRALPAK I H, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= CO2; solvent B = Hept + Et0H 1/1. The eluent flow rate was 4
mL/min, the duration of the run was 5min and
the isocratic solvent proportion was 85% (A) / 15% (B).
XXI) Chiral SFC (U):
CHIRALCEL OD-H, 5 urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= CO2; solvent B = Et0H. The eluent flow rate was 4 mL/min, the
duration of the run was 2.5min and the
isocratic solvent proportion was 80% (A) / 20% (B).
XXII) Chiral SFC (V):
CHIRALPAK AD-H, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as
follows: solvent A= 002; solvent B = Et0H. The eluent flow rate was 4 mL/min,
the duration of the run was 5min and
the isocratic solvent proportion was 85% (A) / 15% (B).
XXIII) Chiral SFC (W):
CHIRALPAK AD-H, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= CO2; solvent B = Et0H. The eluent flow rate was 4 mL/min, the
duration of the run was 3.5min and the
isocratic solvent proportion was 90% (A) / 10% (B).
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
125
XXIV) Chiral HPLC (X):
CHIRALCEL OD-H, 5 m, 4.6x250mm column thermostated at 25 C was used. The two
elution solvents were as follows:
solvent A= Heptane; solvent B = Et0H. The eluent flow rate was 0.8 mL/min, the
duration of the run was 20min and the
isocratic solvent proportion was 80% (A) / 20% (B).
XXV) Chiral HPLC (Y):
CHIRALPAK IG, 5 rm, 4.6x250mm column thermostated at 25 C was used. The two
elution solvents were as follows:
solvent A= Hept + 0.05%DEA; solvent B = Me0H/Et0H 1/1 + 0.05%DEA. The eluent
flow rate was 1 mL/min, the
duration of the run was 15min and the isocratic solvent proportion was 30% (A)
/ 70% (B).
XXVI) Chiral SFC (Z):
CHIRALCEL OD-H, 5 rm, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= 002; solvent B = Me0H. The eluent flow rate was 4 mL/min, the
duration of the run was 2.5min and the
isocratic solvent proportion was 75% (A) / 25% (B).
XXVII) Chiral SFC (AA):
A (R,R) Whelk-01, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The
two elution solvents were as follows:
solvent A= CO2; solvent B = MeCN/iPrOH 1/1. The eluent flow rate was 4 mL/min,
the duration of the run was 3.5min
and the isocratic solvent proportion was 80% (A) / 20% (B).
XXVIII) Chiral SFC (AB):
A Chiralpak AD-H, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The
two elution solvents were as follows:
solvent A= 002; solvent B = Me0H. The eluent flow rate was 4 mL/min, the
duration of the run was 3.5min and the
isocratic solvent proportion was 85% (A) / 15% (B).
XXIX) Chiral SFC (AC):
A Chiralpak I B, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The
two elution solvents were as follows:
solvent A= 002; solvent B = MeCN/Et0H 1/1. The eluent flow rate was 4 mL/min,
the duration of the run was 5min and
the isocratic solvent proportion was 70% (A) / 30% (B).
XXX) Chiral SFC (AD):
A Chiralpak IG, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= 002; solvent B = Et0H. The eluent flow rate was 4 mL/min, the
duration of the run was 3min and the isocratic
solvent proportion was 85% (A) / 15% (B).
XXXI) Chiral SFC (AE):
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
126
CHIRALCEL OD-H, 5 m, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= 002; solvent B = Me0H. The eluent flow rate was 4 mL/min, the
duration of the run was 5.0min and the
isocratic solvent proportion was 90% (A) / 10% (B).
)00(II) Chiral SFC (AF):
CHIRALCEL OD-H, 5 m, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= CO2; solvent B = Me0H. The eluent flow rate was 4 mL/min, the
duration of the run was 5.0min and the
isocratic solvent proportion was 80% (A) / 20% (B).
)0001) Chiral SFC (AG):
CHIRALPAK AD-H, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= CO2; solvent B = Et0H. The eluent flow rate was 5 mL/min, the
duration of the run was 3.5min and the
isocratic solvent proportion was 95% (A) / 5% (B).
XXXIV) Chiral HPLC (AH):
CHIRALPAK AY-H, 5 Urn, 4.6x250mm column thermostated at 25 C was used. The two
elution solvents were as follows:
solvent A= CO2; solvent B = Et0H. The eluent flow rate was 1 mL/min, the
duration of the run was 5min and the isocratic
solvent proportion was 90% (A) / 10% (B).
)00(V) Chiral SFC (Al):
CHIRALPAK IC, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= CO2; solvent B = Et0H. The eluent flow rate was 4 mL/min, the
duration of the run was 5min and the isocratic
solvent proportion was 90% (A) / 10% (B).
XXXVI) Chiral SFC (AJ):
CHIRALPAK IC, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= CO2; solvent B = ACN/Et0H 1/1. The eluent flow rate was 4 mL/min,
the duration of the run was 3.5min and
the isocratic solvent proportion was 90% (A) / 10% (B).
XXXVII) Chiral HPLC (AK):
CHIRALPAK AY-H, 5 Urn, 4.6x250mm column thermostated at 25 C was used. The two
elution solvents were as follows:
solvent A= Hept; solvent B = Et0H. The eluent flow rate was 0.8 mL/min, the
duration of the run was 10min and the
isocratic solvent proportion was 75% (A) / 25% (B).
)0=111) Chiral HPLC (AL):
CHIRALPAK IC, 5 Urn, 4.6x250mm column thermostated at 25 C was used. The two
elution solvents were as follows:
solvent A= Hept; solvent B = Et0H. The eluent flow rate was 0.8 mL/min, the
duration of the run was 20min and the
isocratic solvent proportion was 95% (A) / 5% (B).
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
127
(XXXIX) Chiral SFC (AM):
CHIRALCEL OZ-H, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= CO2; solvent B = Me0H. The eluent flow rate was 4 mL/min, the
duration of the run was 4min and the
isocratic solvent proportion was 70% (A) / 30% (B).
(XL) Chiral SFC (AN):
CHIRALPAK AD-H, 5 Um, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= 002; solvent B = Et0H + 1%DEA. The eluent flow rate was 4 mL/min,
the duration of the run was 3min and
the isocratic solvent proportion was 85% (A) / 15%(B).
(XLI) Chiral SFC (AO):
A REGIS (R, R) Whelk 01, 5 Um, 4.6x250mm column thermostated at 40 C was used.
The two elution solvents were as
follows: solvent A= 002; solvent B = MeCN/iPrOH 1/1. The eluent flow rate was
4 mL/min, the duration of the run was
5min and the isocratic solvent proportion was 80% (A) / 20% (B).
(XLI I) Chiral SFC (AP):
CHIRALPAK OD-H, 57m, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= CO2; solvent B = Me0H + 0.1%DEA. The eluent flow rate was 4 mL/min,
the duration of the run was 5min
and the isocratic solvent proportion was 90% (A) / 10%(B).
(XLIII) Chiral SFC (AQ):
CHIRALPAK IC, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= 002; solvent B = Me0H. The eluent flow rate was 4 mL/min, the
duration of the run was 3.5min and the
isocratic solvent proportion was 80% (A) / 20%(B).
(XLIV) Chiral SFC (AR):
A CHIRALPAK IB, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= CO2; solvent B = MeCN/Me0H 1/1. The eluent flow rate was 4 mL/min,
the duration of the run was 3.5min
and the isocratic solvent proportion was 80% (A) / 20% (B).
(XLV) Chiral HPLC (AS):
CHIRALPAK 1E, 5 Urn, 4.6x250mm column thermostated at 25 C was used. The two
elution solvents were as follows:
solvent A= Hept; solvent B = Et0H. The eluent flow rate was 0.8mL/min, the
duration of the run was 15min and the
isocratic solvent proportion was 10% (A) / 90%(B).
(XLVI) Chiral HPLC (AT):
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
128
CHIRALCEL OZ-H, 5 Urn, 4.6x250mm column thermostated at 25 C was used. The two
elution solvents were as follows:
solvent A= Hept; solvent B = Et0H. The eluent flow rate was 0.8mUmin, the
duration of the run was 20min and the
isocratic solvent proportion was 10% (A) / 90%(B).
(XLVII) Chiral HPLC (AU):
CHIRALPAK AD-H, 5 Urn, 4.6x250mm column thermostated at 25 C was used. The two
elution solvents were as follows:
solvent A= Hept; solvent B = Et0H. The eluent flow rate was 0.8mL/min, the
duration of the run was 20min and the
isocratic solvent proportion was 80% (A) / 20%(B).
(XLVIII) Chiral SFC (AV):
A CHIRALPAK IB, 5 Urn, 4.6x250mm column thermostated at 4000 was used. The two
elution solvents were as follows:
solvent A= 002; solvent B = MeCN/Me0H 1/1. The eluent flow rate was 4 mL/min,
the duration of the run was 5min
and the isocratic solvent proportion was 85% (A) / 15% (B).
(XLIX) Chiral SFC (AW):
A CHIRALPAK IB, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= CO2; solvent B = MeCN/Me0H 1/1. The eluent flow rate was 4 mL/min,
the duration of the run was 5min
and the isocratic solvent proportion was 75% (A) / 25% (B).
(L) Chiral SFC (AX):
A CHIRALPAK 1E, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= CO2; solvent B = Et0H. The eluent flow rate was 4 mL/min, the
duration of the run was 5min and the isocratic
solvent proportion was 60% (A) / 40% (B).
(LI) Chiral SFC (AY):
A REGIS (R,R) Whelk 01, 5 Um, 4.6x250mm column thermostated at 40 C was used.
The two elution solvents were as
follows: solvent A= CO2; solvent B = MeCN/Et0H 1/1. The eluent flow rate was 4
mL/min, the duration of the run was
5min and the isocratic solvent proportion was 85% (A) / 15% (B).
(LI I) Chiral SFC (AZ):
A CHIRALPAK IB, 5 Urn, 4.6x250mm column thermostated at 40 C was used. The two
elution solvents were as follows:
solvent A= 002; solvent B = Et0H. The eluent flow rate was 4 mL/min, the
duration of the run was 5min and the isocratic
solvent proportion was 70% (A) / 30% (B).
Compound purity and identity was further confirmed by NMR spectroscopy (Bruker
Avance 11 400 MHz UltrashieldTM
or Bruker AscendTM 500 equipped with a 5mm DCH cryoprobe), 1H (400 MHz or 500
MHz). The chemical shifts are
reported in parts per million (ppm) relative to tetramethylsilane (TMS), and
multiplicities are given as s (singlet), d
(doublet), t (triplet), or m (multiplet).
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
129
Preparative LC-MS methods used:
The purifications by preparative LC-MS have been performed using the
conditions described hereafter.
I) Prep LC-MS (I):
AX-Bridge column (Waters 018, 10pm OBD, 50x150 mm) was used. The two elution
solvents were as follows: solvent
A = MeCN; solvent B = water + 0.5% NFI4OH (25%). The eluent flow rate and the
characteristics of the eluting mixture
proportion in function of the time t from start of the elution are summarized
in the tables below (a linear gradient being
used between two consecutive time points):
t (min) 0 0.3 0.8 7.5 7.7 9.5 10 11.5
12
Flow (mL/min) 75 75 150 150 150 150 150 150
75
Solvent A (%) 40 40 40 67 95 95 40 40
40
Solvent B (%) 60 60 60 33 5 5 60 60
60
II) Prep LC-MS (ID:
AX-Bridge column (Waters 018, 10pm OBD, 50x150 mm) was used. The two elution
solvents were as follows: solvent
A = water + 0.5% NH4OH (25%); solvent B = MeCN. The characteristics of the
eluting mixture proportion in function of
the time t from start of the elution are summarized in the tables below (a
linear gradient being used between two
consecutive time points):
t (min) 0 0.3 0.8 8 8.1 10.1 10.5 12.0
12.5
Flow (mL/min) 75 75 150 150 150 150 150 150
75
Solvent A (%) 50 50 50 15 5 5 50 50
50
Solvent B (%) 50 50 50 85 95 95 50 50
50
Ill) Prep LC-MS (III):
A Zorbax column (SB-AQ, 7pm OBD, 50x150 mm) was used. The two elution solvents
were as follows: solvent A =
water + 0.5% formic acid; solvent B = MeCN. The characteristics of the eluting
mixture proportion in function of the time
t from start of the elution are summarized in the tables below (a linear
gradient being used between two consecutive
time points):
t (min) 0 0.3 0.8 6 6.1 8.1 8.5 10.0
10.5
Flow 75 75 150 150 150 150 150 150
75
(mL/min)
Solvent A (%) 60 60 60 40 5 5 60 60
60
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
130
Solvent B (%) 40 40 40 60 95 95 40 40
40
IV) Prep LC-MS (IV):
AX-Bridge column (Waters 018, 10pm OBD, 50x150 mm) was used. The two elution
solvents were as follows: solvent
A = MeCN; solvent B = water + 0.5% NH4OH (25%). The eluent flow rate and the
characteristics of the eluting mixture
proportion in function of the time t from start of the elution are summarized
in the tables below (a linear gradient being
used between two consecutive time points):
t (min) 0 0.3 0.8 7.0 7.5 10 10.5 12
12.5
Flow (mL/min) 75 75 150 150 150 150 150 150
75
Solvent A (%) 40 40 40 85 95 95 40 40
40
Solvent B (%) 60 60 60 15 5 5 60 60
60
V) Prep LC-MS (V):
A X-Bridge column (Waters 018, 10pm OBD, 30x75 mm) was used. The two elution
solvents were as follows: solvent
A = water 0.5% NF140H (25%); solvent B = MeCN. The eluent flow rate was 75
mL/min and the characteristics of the
eluting mixture proportion in function of the time t from start of the elution
are summarized in the tables below (a linear
gradient being used between two consecutive time points):
t (min) 0 0.01 4.0 6.0 6.2
6.6
Solvent A (%) 90 90 5 5 90 90
Solvent B (%) 10 10 95 95 10 10
VI) Prep LC-MS (VI):
A X-Bridge column (Waters 018, 10pm OBD, 30x75 mm) was used. The two elution
solvents were as follows: solvent
A = water + 0.5% NI-140H (25%); solvent B = MeCN. The eluent flow rate was 75
mL/min and the characteristics of the
eluting mixture proportion in function of the time t from start of the elution
are summarized in the tables below (a linear
gradient being used between two consecutive time points):
t (min) 0 0.01 4.0 6.0 6.2 6.6
Solvent A (%) 80 80 5 5 80 80
Solvent B (%) 20 20 95 95 20 20
VII) Prep LC-MS (VII):
X-Bridge column (Waters 018, 10pm OBD, 30x75 mm) was used. The two elution
solvents were as follows: solvent A
= water + 0.5% NI-140H (25%); solvent B = MeCN. The eluent flow rate was 75
mL/min and the characteristics of the
eluting mixture proportion in function of the time t from start of the elution
are summarized in the tables below (a linear
gradient being used between two consecutive time points):
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
131
t (min) 0 0.01 3.5 6.0 6.2
6.6
Solvent A (%) 70 70 5 5 70
70
Solvent B (%) 30 30 95 95 30
30
VIII) Prep LC-MS (VIM):
X-Bridge column (Waters 018, 10pm OBD, 30x75 mm) was used. The two elution
solvents were as follows: solvent A
= water + 0.5% NI-140H (25%); solvent B = MeCN. The eluent flow rate was 75
mL/min and the characteristics of the
eluting mixture proportion in function of the time t from start of the elution
are summarized in the tables below (a linear
gradient being used between two consecutive time points):
t (min) 0 0.01 3.5 6.0 6.2 6.6
Solvent A (%) 50 50 5 5 50 50
Solvent B (%) 50 50 95 95 50 50
IX) Prep LC-MS (IX):
An Agilent column (Zorbax SB-Aq, 5pm OBD, 30x75 mm) was used. The two elution
solvents were as follows: solvent
A = water + 0.5% formic acid; solvent B = MeCN. The eluent flow rate was 75
mL/min and the characteristics of the
eluting mixture proportion in function of the time t from start of the elution
are summarized in the tables below (a linear
gradient being used between two consecutive time points):
t (min) 0 0.01 3 4.0 6.0 6.2
6.6
Solvent A (%) 95 95 50 5 5 95
95
Solvent B (%) 5 5 50 95 95 5
5
X) Prep LC-MS (X):
AX-Bridge column (Waters C18, 10pm OBD, 50x150 mm) was used. The two elution
solvents were as follows: solvent
A = MeCN; solvent B = water + 0.5% NH4OH (25%). The eluent flow rate and the
characteristics of the eluting mixture
proportion in function of the time t from start of the elution are summarized
in the tables below (a linear gradient being
used between two consecutive time points):
t (min) 0 0.3 0.8 8.0 8.1 10.1 10.5 12
12.5
Flow (mL/min) 75 75 150 150 150 150 150 150
75
Solvent A (%) 50 50 50 75 95 95 50 50
50
Solvent B (%) 50 50 50 25 5 5 50 50
50
XI) Prep LC-MS (XI):
CA 03177335 2022- 10- 28
WO 2021/219849 PCT/EP2021/061401
132
AX-Bridge column (Waters 018, 10pm OBD, 50x150 mm) was used. The two elution
solvents were as follows: solvent
A = MeCN; solvent B = water + 0.5% NH4OH (25%). The eluent flow rate and the
characteristics of the eluting mixture
proportion in function of the time t from start of the elution are summarized
in the tables below (a linear gradient being
used between two consecutive time points):
t (min) 0 0.3 0.8 10.0 15.0 15.5 17
17.5
Flow (mL/min) 75 75 150 150 150 150 150 75
Solvent A (%) 30 30 30 95 95 30 30 30
Solvent B (%) 70 70 70 5 5 70 70 70
XII) Prep LC-MS (XII):
A Zorbax SB-Aq column (Agilent, 5pm, 30x75 mm) was used. The two elution
solvents were as follows: solvent A =
water + 0.5% formic acid; solvent B = MeCN. The eluent flow rate was 75 mL/min
and the characteristics of the eluting
mixture proportion in function of the time t from start of the elution are
summarized in the tables below (a linear gradient
being used between two consecutive time points):
t (min) 0 0.01 3.5 6.0 6.2
6.6
Solvent A (%) 70 70 5 5 70
70
Solvent B (%) 30 30 95 95 30
30
XIII) Prep LC-MS (XIII):
A X-Bridge column (Waters 018, 10pm OBD, 30x75 mm) was used. The two elution
solvents were as follows: solvent
A = water + 0.5% formic acid; solvent B = MeCN. The eluent flow rate was 75
mL/min and the characteristics of the
eluting mixture proportion in function of the time t from start of the elution
are summarized in the tables below (a linear
gradient being used between two consecutive time points):
t (min) 0 0.01 3 4.0 6.0 6.2
6.6
Solvent A (%) 95 95 50 5 5 95
95
Solvent B (%) 5 5 50 95 95 5
5
XIV) Prep LC-MS (XIV):
An Agilent column (Zorbax SB-Aq, 5pm OBD, 30x75 mm) was used.The two elution
solvents were as follows: solvent
A = water + 0.5% formic acid; solvent B = MeCN. The eluent flow rate was 75
mL/min and the characteristics of the
eluting mixture proportion in function of the time t from start of the elution
are summarized in the tables below (a linear
gradient being used between two consecutive time points):
t (min) 0 0.01 4.0 6.0 6.2
6.6
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
133
Solvent A (%) 90 90 5 5 90
90
Solvent IR (%) 10 10 95 95 10
10
XV) Prep LC-MS (XV):
A X-Bridge column (Waters 018, 10pm OBD, 30x75 mm) was used. The two elution
solvents were as follows: solvent
A = water + 0.5% NFI4OH (25%); solvent B = MeCN. The eluent flow rate was 75
mL/min and the characteristics of the
eluting mixture proportion in function of the time t from start of the elution
are summarized in the tables below (a linear
gradient being used between two consecutive time points):
t (min) 0 0.01 3 4.0 6.0 6.2
6.6
Solvent A (%) 95 95 50 5 5 95
95
Solvent B (%) 5 5 50 95 95 5
5
XVI) Prep LC-MS (XVI):
AX-Bridge column (Waters 018, 10pm OBD, 50x150 mm) was used. The two elution
solvents were as follows: solvent
A = MeCN; solvent B = water + 0.5% NI-140H (25%). The eluent flow rate and the
characteristics of the eluting mixture
proportion in function of the time t from start of the elution are summarized
in the tables below (a linear gradient being
used between two consecutive time points):
t (min) 0 0.3 0.8 7.0 7.2 9.0 9.5 11
11.5
Flow (mL/min) 75 75 150 150 150 150 150 150
75
Solvent A (%) 35 35 35 65 95 95 35 35
35
Solvent B (%) 65 65 65 35 5 5 65 65
65
XVII) Prep LC-MS (XVII):
A Zorbax column (SB-AQ, 7pm OBD, 50x150 mm) was used. The two elution solvents
were as follows: solvent A =
water +0.5% formic acid; solvent B = MeCN. The characteristics of the eluting
mixture proportion in function of the time
t from start of the elution are summarized in the tables below (a linear
gradient being used between two consecutive
time points):
t (min) 0 0.3 0.8 7.5 7.7 9.5 10 11.5
12
Flow (mL/min) 75 75 150 150 150 150 150 150
75
Solvent A (%) 60 60 60 33 5 5 60 60
60
Solvent B (%) 40 40 40 67 95 95 40 40
40
XVIII) Prep LC-MS (XVIII):
CA 03177335 2022- 10- 28
WO 2021/219849 PCT/EP2021/061401
134
A X-Bridge column (Waters 018, 10pm OBD, 30x75 mm) was used. The two elution
solvents were as follows: solvent
A = water + 0.5% formic acid; solvent B = MeCN. The eluent flow rate was 75
mL/min and the characteristics of the
eluting mixture proportion in function of the time t from start of the elution
are summarized in the tables below (a linear
gradient being used between two consecutive time points):
t (min) 0 0.01 4.0 6.0 6.2
6.6
Solvent A (%) 90 90 5 5 90 90
Solvent B (%) 10 10 95 95 10 10
XIX) Prep LC-MS (XIX):
A X-Bridge column (Waters 018, 10pm OBD, 50x150 mm) was used. The two elution
solvents were as follows: solvent
A = MeCN; solvent B = water + 0.5% formic acid. The characteristics of the
eluting mixture proportion in function of the
time t from start of the elution are summarized in the tables below (a linear
gradient being used between two consecutive
time points):
t (min) 0 0.3 0.8 9 9.2 11 11.5 13
13.5
Flow (mL/min) 75 75 150 150 150 150 150 150
75
Solvent A (%) 40 40 40 72 95 95 40 40
40
Solvent B (%) 60 60 60 28 5 5 60 60
60
XX) Prep LC-MS (XX):
An Agilent column (Zorbax SB-Aq, 5pm OBD, 30x75 mm) was used. The two elution
solvents were as follows: solvent
A = water + 0.5% formic acid; solvent B = MeCN. The eluent flow rate was 75
mL/min and the characteristics of the
eluting mixture proportion in function of the time t from start of the elution
are summarized in the tables below (a linear
gradient being used between two consecutive time points):
t (min) 0 0.01 4.0 6.0 6.2 6.6
Solvent A (%) 80 80 5 5 80 80
Solvent B (%) 20 20 95 95 20 20
XXI) Prep LC-MS (XXI):
A X-Bridge column (Waters 018, 10pm OBD, 30x75 mm) was used. The two elution
solvents were as follows: solvent
A = water + 0.5% formic acid; solvent B = MeCN. The eluent flow rate was 75
mL/min and the characteristics of the
eluting mixture proportion in function of the time t from start of the elution
are summarized in the tables below (a linear
gradient being used between two consecutive time points):
t (min) 0 1.0 3.5 4.0 6.0 6.2 6.6
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
135
Solvent A (%) 100 100 80 5 5 100 100
Solvent IR (%) 0 0 20 95 95 0 0
XXII) Prep LC-MS (XXII):
An Agilent column (Zorbax SB-Aq, 5pm OBD, 30x75 mm) was used. The two elution
solvents were as follows: solvent
A = water + 0.5% formic acid; solvent B = MeCN. The eluent flow rate was 75
mL/min and the characteristics of the
eluting mixture proportion in function of the time t from start of the elution
are summarized in the tables below (a linear
gradient being used between two consecutive time points):
t (min) 0 0.01 3.0 6.0 6.2 6.6
Solvent A (%) 50 50 5 5 50 50
Solvent B (%) 50 50 95 95 50 50
XXIII) Prep LC-MS (XXIII):
An Agilent column (Zorbax SB-Aq, 5pm OBD, 30x75 mm) was used. The two elution
solvents were as follows: solvent
A = water + 0.5% formic acid; solvent B = MeCN. The eluent flow rate was 75
mL/min and the characteristics of the
eluting mixture proportion in function of the time t from start of the elution
are summarized in the tables below (a linear
gradient being used between two consecutive time points):
t (min) 0 1.0 3.5 4.0 6.0 6.2 6.6
Solvent A (%) 100 100 80 5 5 100 100
Solvent B (%) 0 0 20 95 95 0 0
XXIV) Prep LC-MS (XXIV):
A Zorbax column (SB-AQ, 7pm OBD, 50x150 mm) was used. The two elution solvents
were as follows: solvent A =
water +0.5% formic acid; solvent B = MeCN. The characteristics of the eluting
mixture proportion in function of the time
t from start of the elution are summarized in the tables below (a linear
gradient being used between two consecutive
time points):
t (min) 0 0.3 0.8 6 6.1 8.1 8.5 10.0
10.5
Flow 75 75 150 150 150 150 150 150
75
(mL/min)
Solvent A (%) 60 60 60 50 5 5 60 60
60
Solvent B (%) 40 40 40 50 95 95 40 40
40
)0(V) Prep LC-MS (XXV):
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
136
AX-Bridge column (Waters 018, 10pm OBD, 50x150 mm) was used. The two elution
solvents were as follows: solvent
A = water + 0.5% NH4OH (25%); solvent B = MeCN. The characteristics of the
eluting mixture proportion in function of
the time t from start of the elution are summarized in the tables below (a
linear gradient being used between two
consecutive time points):
t (min) 0 0.3 0.8 8 8.1 10.1 10.5 12.0
12.5
Flow (mL/min) 75 75 150 150 150 150 150 150
75
Solvent A (%) 45 45 45 30 5 5 45 45
45
Solvent B (%) 55 55 55 70 95 95 55 55
55
XXVI) Prep LC-MS (XXVI):
A Zorbax column (SB-AQ, 7pm OBD, 50x150 mm) was used. The two elution solvents
were as follows: solvent A =
MeCN; solvent B = water + 0.5% formic acid. The characteristics of the eluting
mixture proportion in function of the time
t from start of the elution are summarized in the tables below (a linear
gradient being used between two consecutive
time points):
t (min) 0 0.3 0.8 8 8.1 10.1 10.5 12.0
12.5
Flow (mL/min) 75 75 150 150 150 150 150 150
75
Solvent A (%) 40 40 40 65 95 95 40 40
40
Solvent B (%) 60 60 60 35 5 5 60 60
60
XXVII) Prep LC-MS (XXVII):
AX-Bridge column (Waters C18, 10pm OBD, 50x150 mm) was used. The two elution
solvents were as follows: solvent
A = water + 0.5% NH4OH (25%); solvent B = MeCN. The characteristics of the
eluting mixture proportion in function of
the time t from start of the elution are summarized in the tables below (a
linear gradient being used between two
consecutive time points):
t (min) 0 0.3 0.8 8 8.1 10.1 10.5 12.0
12.5
Flow (mL/min) 75 75 150 150 150 150 150 150
75
Solvent A (%) 60 60 60 25 5 5 60 60
60
Solvent B (%) 40 40 40 75 95 95 40 40
40
)(XVIII) Prep LC-MS (XXVIII)
A X-Bridge column (Waters 018, 10pm OBD, 50x150 mm) was used. The two elution
solvents were as follows:
solvent A = water + 0.5% formic acid ; solvent B = MeCN. The eluent flow rate
and the characteristics of the eluting
mixture proportion in function of the time t from start of the elution are
summarized in the tables below (a linear
gradient being used between two consecutive time points):
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
137
t (min) 0 0.3 0.8 7.0 7.5 10.0 10.5
12.0 12.5
Flow (mL/min) 75 75 150 150 150 150 150 150
75
Solvent A (%) 70 70 70 20 5 5 70 70
70
Solvent B (%) 30 30 30 80 95 95 30 30
30
XXIX) Prep LC-MS ()OKIX):
A X-Bridge column (Waters 018, 10pm OBD, 30x75 mm) was used. The two elution
solvents were as follows: solvent
A = water + 0.5% formic acid ; solvent B = MeCN. The eluent flow rate was 75
mL/min and the characteristics of the
eluting mixture proportion in function of the time t from start of the elution
are summarized in the tables below (a linear
gradient being used between two consecutive time points):
t (min) 0 0.01 4.0 6.0 6.2
6.6
Solvent A (%) 80 80 5 5 80 80
Solvent B (%) 20 20 95 95 20 20
XXX) Prep LC-MS (XXX):
A X-Bridge column (Waters C18, 10pm OBD, 30x75 mm) was used. The two elution
solvents were as follows: solvent
A = water + 0.5% formic acid ; solvent B = MeCN. The eluent flow rate was 75
mL/min and the characteristics of the
eluting mixture proportion in function of the time t from start of the elution
are summarized in the tables below (a linear
gradient being used between two consecutive time points):
t (min) 0 0.01 4.0 6.0 6.2
6.6
Solvent A (%) 70 70 5 5 70 70
Solvent B (%) 30 30 95 95 30 30
XXXI) Prep LC-MS (XXXI)
A X-Bridge column (Waters C18, 10pm OBD, 50x150 mm) was used. The two elution
solvents were as follows:
solvent A = water + 0.5% NI-140H (25%); solvent B = MeCN. The characteristics
of the eluting mixture proportion in
function of the time t from start of the elution are summarized in the tables
below (a linear gradient being used
between two consecutive time points):
t (min) 0 0.3 0.8 11.0 15.0 15.5 17.0
17.5
Flow (mL/min) 75 75 150 150 150 150 150 75
Solvent A (%) 80 80 80 5 5 80 80 80
Solvent B (%) 20 20 20 95 95 20 20 20
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
138
XXXII) Prep LC-MS (XXXII):
A X-Bridge column (Waters 018, 10pm OBD, 30x75 mm) was used. The two elution
solvents were as follows: solvent
A = water + 0.5% NFI4OH (25%); solvent B = MeCN. The eluent flow rate was 75
mL/min and the characteristics of the
eluting mixture proportion in function of the time t from start of the elution
are summarized in the tables below (a linear
gradient being used between two consecutive time points):
t (min) 0 1.0 3.5 4.0 6.0 6.2 6.6
Solvent A (%) 100 100 80 5 5 100 100
Solvent B (%) 0 0 20 95 95 0 0
)(XXIII) Prep LC-MS (XXXIII):
acidic large scale (Zorbax 50x150 mm, 7 um), 90%Water_150m1_to
75%_8min_RT_0,49
A Zorbax column (SB-AQ, 7pm OBD, 50x150 mm) was used. The two elution solvents
were as follows: solvent A =
MeCN; solvent B = water + 0.5% formic acid. The characteristics of the eluting
mixture proportion in function of the time
t from start of the elution are summarized in the tables below (a linear
gradient being used between two consecutive
time points):
t (min) 0 0.3 0.8 8 8.1 10.1 10.5 12.0
12.5
Flow (mL/min) 75 75 150 150 150 150 150 150
75
Solvent A (%) 10 10 10 25 95 95 10 10
10
Solvent B (%) 90 90 90 75 5 5 90 90
90
)00(1V) Prep LC-MS (>00(IV):
A Zorbax column (SB-AQ, 7pm OBD, 50x150 mm) was used. The two elution solvents
were as follows: solvent A -
MeCN; solvent B = water + 0.5% formic acid. The characteristics of the eluting
mixture proportion in function of the time
t from start of the elution are summarized in the tables below (a linear
gradient being used between two consecutive
time points):
t (min) 0 0.3 0.8 8 8.1 10.1 10.5 12.0
12.5
Flow (mL/min) 75 75 150 150 150 150 150 150
75
Solvent A (%) 20 20 20 35 95 95 20 20
20
Solvent B (%) 80 80 80 65 5 5 80 80
80
XXXV) Prep LC-MS (XXXV):
A Zorbax column (SB-AQ, 7pm OBD, 50x150 mm) was used. The two elution solvents
were as follows: solvent A =
water + 0.5% formic acid (25%); solvent B = MeCN. The characteristics of the
eluting mixture proportion in function of
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
139
the time t from start of the elution are summarized in the tables below (a
linear gradient being used between two
consecutive time points):
t (min) 0 0.3 0.8 8 8.1 10.1 10.5 12.0
12.5
Flow (mL/min) 75 75 150 150 150 150 150 150
75
Solvent A (%) 60 60 60 25 5 5 60 60
60
Solvent B (%) 40 40 40 75 95 95 40 40
40
XXXVI) Prep LC-MS (XXXVI):
A Zorbax column (SB-AQ, 7pm OBD, 50x150 mm) was used. The two elution solvents
were as follows: solvent A =
water + 0.5% formic acid (25%); solvent B = MeCN. The characteristics of the
eluting mixture proportion in function of
the time t from start of the elution are summarized in the tables below (a
linear gradient being used between two
consecutive time points):
t (min) 0 0.3 0.8 8 8.1 10.1 10.5 12.0
12.5
Flow (mL/min) 75 75 150 150 150 150 150 150
75
Solvent A (%) 80 80 80 50 5 5 80 80
80
Solvent B (%) 20 20 20 50 95 95 20 20
20
kOKVII) Prep LC-MS (XXXVII):
AX-Bridge column (Waters 018, 10pm OBD, 50x150 mm) was used. The two elution
solvents were as follows: solvent
A = water + 0.5% NH4OH (25%); solvent B = MeCN. The characteristics of the
eluting mixture proportion in function of
the time t from start of the elution are summarized in the tables below (a
linear gradient being used between two
consecutive time points):
t (min) 0 0.3 0.8 6 6.1 8.1 8.5 10.0
10.5
Flow 75 75 150 150 150 150 150 150
75
(mL/min)
Solvent A (%) 60 60 60 50 5 5 60 60
60
Solvent B (%) 40 40 40 50 95 95 40 40
40
Preparative chiral HPLC and SFC methods used:
The purifications by preparative chiral HPLC or SFC have been performed using
the conditions described hereafter.
I) Prep chiral SFC (I):
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
140
A ChiralCel OD-H column (51m, 30x250mm) thermostated at 40 C was used. The
elution solvent was 002/Me0H 92/8,
the run lasted for 6.50min and at a flow rate of 160mL/min.
II) Prep chiral SFC
A ChiralCel OZ-H column (am, 30x250mm) thermostated at 40 C was used. The
elution solvent was
CO2/MeOHA-0.1%DEA 85/15, the run lasted for 5.50min and at a flow rate of
160mL/min.
III) Prep chiral SFC (III):
A ChiralPak AD-H column (am, 30x250mm) thermostated at 40 C was used. The
elution solvent was CO2/Et0H 80/20,
the run lasted for 5min and at a flow rate of 160mL/min.
IV) Prep chiral SFC (IV):
A ChiralPak IB (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/Et0H 85/15,
run for 3.90min and at a flow rate of 160mL/min.
V) Prep chiral SFC (V):
A ChiralPak IC (51m, 30x250mm) column thermostated at 40 C was used. The
elution solvent was
CO2/MeOHA-0.1%DEA 60/40, run for 5min and at a flow rate of 160mL/min.
VI) Prep chiral SFC (VI):
A ChiralPak IB (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was CO2/MeOH 90/10,
run for 4min and at a flow rate of 160mL/min.
VII) Prep chiral SFC (VII):
A ChiralPak AD-H (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/Et0H 75/25,
run for 5min and at a flow rate of 160mL/min.
VIII) Prep chiral SFC (VIII):
A ChiralCel OD-H (5[Im, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/Me0H
90/10, run for 6min and at a flow rate of 160mL/min.
IX) Prep chiral SFC (IX):
A ChiralPak AD-H (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was CO2/50%MeCN-
50%Et0H-0.1%DEA 75/25, run for 5.5min and at a flow rate of 160mL/min.
X) Prep chiral SFC (X):
A ChiralPak AS-H (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/50%MeCN-
50%Et0H-0.1%DEA 72/28, run for 4.5min and at a flow rate of 160mL/min.
XI) Prep chiral SFC (XI):
A ChiralPak AS-H (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was CO2/50%MeCN-
50%Et0H-0.1%DEA 70/30, run for 4min and at a flow rate of 160mL/min.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
141
XII) Prep chiral SFC (XII):
A ChiralPak OD-H (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was
002/Me0H1-0.1%DEA 85/15, run for 9.30min and at a flow rate of 160mL/min.
XIII) Prep chiral SFC (XIII):
A ChiralPak AD-H (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was
002/Et0HA-0.1%DEA 75/25, run for 4min and at a flow rate of 160mL/min.
XIV) Prep chiral SFC (XIV):
A (R,R) Whelk (am, 30x250mm) column thermostated at 40 C was used. The elution
solvent was 002/50%DCM-
50%Me0H-0.1%DEA 70/30, run for 7.9min and at a flow rate of 160mL/min.
XV) Prep chiral SFC (XV):
A ChiralPak ID (5iim, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/50%MeCN-
50%Et0H-0.1%DEA 65/35, run for 4.75min and at a flow rate of 160mL/min.
XVI) Prep chiral SFC (XVI):
A ChiralPak IB (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was
CO2/MeOHA-0.1%DEA 90/10, run for 4.50min and at a flow rate of 160mL/min.
XVII) Prep chiral SFC (XVII):
A ChiralPak IB (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/Me0H 80/20,
run for 4min and at a flow rate of 160mL/min.
XVIII) Prep chiral SFC (XVIII):
A ChiralPak IH (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/50%MeCN-
50%Et0H 85/15, run for 6min and at a flow rate of 160mL/min.
XIX) Prep chiral SFC (XIX):
A ChiralPak AD-H (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/Et0H 85/15,
run for 5min and at a flow rate of 160mL/min.
XX) Prep chiral SFC (OX):
A ChiralPak AD-H (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/Et0H 90/10,
run for 4.8min and at a flow rate of 160mL/min.
XXI) Prep chiral HPLC (XXI):
A ChiralPak AY-H 30x250mm) column thermostated at 25 C was used. The
elution solvent was Hept/2-Propanol
80/20, run for 12min and at a flow rate of 38mL/min.
XXII) Prep chiral SFC (XXII):
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
142
A ChiralPak IH (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/50%Hept-
50%Et0H 85/15, run for 3.5min and at a flow rate of 160mL/min.
XXIII) Prep chiral SFC
A ChiralCel OD-H column (5lim, 30x250mm) thermostated at 40 C was used. The
elution solvent was CO2/Et0H 80/20,
the run lasted for 3.50min and at a flow rate of 160mL/min.
)0(1V) Prep chiral SFC ()OKIV):
A ChiralCel OD-H (alm, 30x250mm) column thermostated at 40 C was used. The
elution solvent was CO2/Me0H
85/15, run for 6min and at a flow rate of 160mL/min.
XXV) Prep chiral SFC (XXV)
A Chiralpak AD-H (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/Et0H 95/5,
run for 5.5min and at a flow rate of 160mL/min.
XXVI) Prep chiral HPLC (XXVI):
A ChiralPak IG (am, 20x250mm) column thermostated at 25 C was used. The
elution solvent was Hept/50%Me0H-
50%Et0H-0.1%DEA 30/70, run for 14min and at a flow rate of 20mL/min.
XXVII) Prep chiral SFC (XXVII):
A ChiralCel OD-H (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/Me0H
75/25, run for 4min and at a flow rate of 160mL/min.
XXVIII) Prep chiral SFC
A (R,R) Whelk-01 (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/50%iPr0H-
50%MeCN 80/20, run for 4min and at a flow rate of 160mL/min.
XXIX) Prep chiral SFC (XXIX):
A Chiralpak AD-H (5Um, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/Me0H
85/15, run for 4min and at a flow rate of 160mL/min.
XXX) Prep chiral SFC PON:
A Chiralpak IB (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/Me0H 70/30,
run for 6min and at a flow rate of 160mL/min.
)00(1) Prep chiral SFC 00((1):
A ChiralPak IB (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/Me0H 90/10,
run for 3.5min and at a flow rate of 160mL/min.
XXXII) Prep chiral SFC
A ChiralPak ID (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was CO2/Et0H-0.1%DEA
60/40, run for 6min and at a flow rate of 160mL/min.
XXXIII) Prep chiral SFC (X0(111):
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
143
A ChiralPak IC (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was CO2Et0H-O.1%DEA
60/40, run for 6min and at a flow rate of 160mL/min.
XXXIV) Prep chiral SEC (XXXIV):
A ChiralPak IH (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/Et0H 55/45,
run for 3.5min and at a flow rate of 160mL/min.
XXXV) Prep chiral SEC (XXXV):
A ChiralPak IG (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was CO2/Et0H 85/15,
run for 6min and at a flow rate of 160mL/min.
XXXVI) Prep chiral SFC (XXXVI):
A ChiralPak IC (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/Et0H 75/25,
run for 3.5min and at a flow rate of 160mL/min.
XXXVII) Prep chiral SFC (XXXVII):
A Chiralpak AY-H (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/Et0H 90/10,
run for 3.4min and at a flow rate of 160mL/min.
XXXVIII) Prep chiral SFC (XXXVIII):
A ChiralPak IC (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/Et0H 90/10,
run for 2.5min and at a flow rate of 160mL/min.
XXXIX) Prep chiral SFC (XXXIX):
A ChiralPak IC (am, 30x250mm) column thermostated at 4000 was used. The
elution solvent was 002/ACN:Et0H 1:1
90/10, run for 4.4min and at a flow rate of 160mL/min.
XL) Prep chiral HPLC (XL):
A ChiralPak AY-H (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was Hep/Et0H 75/25,
run for 11min and at a flow rate of 38mL/min.
XLI) Prep chiral HPLC (XLI):
A ChiralPak IC (am, 20x250mm) column thermostated at 40 C was used. The
elution solvent was heptane/Et0H 95/5,
run for 11.6min and at a flow rate of 16mL/min.
XLII) Prep chiral SFC (XLII):
A ChiralPak AD-H (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/Et0H 90/10,
run for 5.0min and at a flow rate of 160mL/min.
XLIII) Prep chiral HPLC (XLIII):
A ChiralCel OJ-H column (am, 20x250mm) thermostated at 40 C was used. The
elution solvent was Hept/Et0H 80/20,
the run lasted for 10.5min and at a flow rate of 16milmin.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
144
XLIV) Prep chiral SFC (XLIV):
A ChiralCel OJ-H column (am, 30x250mm) thermostated at 40 C was used. The
elution solvent was 002/Me0H 90/10,
the run lasted for 4min and at a flow rate of 16mL/min.
XLV) Prep chiral SFC (XLV):
A ChiralCel OZ-H column (am, 30x250mm) thermostated at 40 C was used. The
elution solvent was CO2/Me0H 70/30,
the run lasted for 4nnin and at a flow rate of 160mL/min.
XLVI) Prep chiral SFC (XLVI):
A ChiralPak AD-H (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was CO2/Et0H-0.1%
DEA 85/15, run for 5.0min and at a flow rate of 160mL/min.
XLVII) Prep chiral SFC (XLVII):
A Chiralpak IB (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/(MeCN:Et0H
1:1) 70/30, run for 6min and at a flow rate of 160mL/min.
XLVIII) Prep chiral SFC (XLVIII):
A Regis (R,R)-Whelk 01 (am, 30x250mm) column thermostated at 40 C was used.
The elution solvent was
002/(MeCN:iPrOH 1:1) 80/20, run for 5min and at a flow rate of 160mL/min.
XLIX) Prep chiral SFC (XLIX):
A ChiralPak OD-H (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/Me0H-
0.1% DEA 90/10, run for 9.0min and at a flow rate of 160mL/min.
L) Prep chiral SFC (L):
A ChiralPak IC (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/Me0H 80/20,
run for 4min and at a flow rate of 160mL/min.
LI) Prep chiral SFC (LI):
A Chiralpak IB (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/(MeCN:Me0H
1:1) 80/20, run for 6min and at a flow rate of 160mL/min.
LII) Prep chiral HPLC (LID:
A ChiralPak IE (am, 30x250mm) column thermostated at 25 C was used. The
elution solvent was Hept/Et0H 10/90,
run for 12.0min and at a flow rate of 34mL/min.
LIII) Prep chiral HPLC (LIII):
A Chiralcel OZ-H (am, 30x250mm) column thermostated at 25 C was used. The
elution solvent was Hept/Et0H 10/90,
run for 14.0min and at a flow rate of 34mL/min.
LIV) Prep chiral HPLC (LIV):
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
145
A Chiralpak AD-H (am, 30x250mm) column thermostated at 25 C was used. The
elution solvent was Hept/Et0H 80/20,
run for 10.0min and at a flow rate of 34mL/min.
LV) Prep chiral SFC (LV):
A Chiralpak IB (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was CO2/(MeCN:Me0H
1:1) 85/15, run for 6min and at a flow rate of 160mL/min.
LVI) Prep chiral SFC (LVI):
A Chiralpak IB (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/(MeCN:Me0H
1:1) 75/25, run for 7min and at a flow rate of 160mL/min.
LVII) Prep chiral SFC (LVII):
A Chiralpak IE (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was CO2/Et0H 60/40,
run for 5min and at a flow rate of 160mL/min.
LVIII) Prep chiral SFC (LVIII):
A Regis (R,R)-Whelk 01 (am, 30x250mm) column thermostated at 40 C was used.
The elution solvent was
002/(MeCN:Et0H 1:1) 85/15, run for 8min and at a flow rate of 160mL/min.
LIX) Prep chiral SFC (LIX):
A Chiralpak IB (am, 30x250mm) column thermostated at 40 C was used. The
elution solvent was 002/Et0H 70/30,
run for 6min and at a flow rate of 160mL/min.
Preparation of Intermediate Examples of Formula (A3), (A4), (A5), (A6), (A7),
(B2), (Cl), (C3), (D1), (D2), (D3),
(D4), (D5), (El), (E2), (F1), (F3), (F4), (F5), (G1), (J3) and (J5)
Example A3.1: 5-Bromo-1,3-difluoro-2-isopropylbenzene
A3.1.1: 2-(4-Bromo-2,6-difluoro-phenyl)-propan-2-ol
To a solution of 4-bromo-2,6-difluorobenzoic acid methyl ester (1 eq) in THF
(6.4 mL/mmol) was added at 0 C, a 3M
solution of methylmagnesium bromide in Et20 (3 eq). The ice bath was removed,
and the reaction mixture was stirred
for 1.5 h at RT. It was quenched with a half sat. solution of NI-14C1 and
extracted with EA. The combined org. phases
were dried over MgSO4 and concentrated in vacuo. The crude was purified by CC
using Sfar prepacked cartridges from
Biotage and eluting with Hept/EA to afford the title compound as colorless
liquid. 1H NMR (500 MHz, DMSO) 5: 7.34
(m, 2 H), 5.32 (s, 1 H), 1.56 (t, J = 2.0 Hz, 6 H).
A3.1.2: 5-Bromo-1,3-difluoro-2-isopropylbenzene
To a solution of 2-(4-bromo-2,6-difluoro-phenyI)-propan-2-ol (1 eq) and
triethylsilane (1.1 eq) in DCM (7.8 mL/mmol)
was added at 0 C, TFA (11 eq). The ice bath was removed, and the reaction
mixture was stirred for 3h at RT. It was
quenched with a half sat. solution of NaHCO3 and extracted with DCM. The
combined org. phases were dried over
MgSO4 and concentrated in vacuo. The crude was purified by CC using Sfar
prepacked cartridges from Biotage and
CA 03177335 2022- 10- 28
WO 2021/219849 PCT/EP2021/061401
146
eluting with pentane/EA to afford the title compound as colorless liquid. 1H
NMR (500 MHz, DMSO) 5: 7.40 (m, 2 H),
3.22-3.31 (m, 1 H), 1.27 (d, J = 7.1 Hz, 6 H).
Example A4.1: 3-Methyl-3-(4-trifluoromethoxy-benzoy1)-azetidine-1-carboxylic
acid tert-butyl ester
A4.1.1. 3-(Methoxy-methyl-carbamoy0-3-methyl-azetidine-1-carboxylic acid tert-
butyl ester
To a pale yellow solution of 1-Boc-3-methylazetidine-3-carboxylic acid (20g)
in DCM (500mL) were added N,0-
dimethylhydroxylamine hydrochloride (8.97g) and DIPEA (54mL). The mixture was
then cooled at 0 C and
propylphosphonic anhydride solution in EA (50% w/w, 68mL) was slowly added.
The resulting pale yellow solution was
stirred 18h at RI and quenched with aq. sat. NaHCO3 solution. The org. layer
was washed with citric acid (10%) and
water. The combined org. layers were dried (MgSO4), filtered off and
evaporated to dryness to afford 24.1g of the title
compound as yellowish resin which was used without further purification. LC-MS
(A): tR = 0.78min; [M+H]-: 259.32.
A4.1.2. 3-Methyl-3-(4-trifluoromethoxy-benzoy0-azetidine-1-carboxylic acid
tert-butyl ester
To a solution of 1-bromo-4-(trifluoromethoxy)benzene (12.2mL) in anhydrous THF
(150mL) under argon cooled down
to -78 C was added n-BuLi (2.5M in hexane, 29.7mL) dropwise over 45min so that
the internal temperature did not rise
above -70 C. The resulting mixture was stirred at -78 C for 20min. A solution
of Example A4.1.1 (16g) in anhydrous
THF (50mL) was added dropwise keeping the internal temperature below -70 C.
The resulting dark yellow solution was
allowed to warm up to RT and was stirred overnight. The reaction mixture was
quenched with water and extracted with
DCM. The org. layers were washed with brine, dried (MgSO4), filtered off and
evaporated to dryness. The resulting
crude material was purified by CC (Biotage, SNAP 340g, solvent A: Hep; solvent
B: EA; gradient in %B: 10 over 2CV,
10 to 30 over 3CV, 30 over 2CV) to afford 18g of the title compound as yellow
foam. LC-MS (A): tR = 1.07min; [M-
Me+H]-: 345.11.
Example A4.2 to Example A4.11 were synthesized starting from the appropriate
Weinreb amide of Formula (A2) and
bromo derivative of Formula (A3) and following the procedure described in
Example A4.1. LC-MS data of Example A4.2
to Example A4.11 are listed in the table below. The LC-MS conditions used were
LC-MS (A).
Example N Name tR [M-FH]-
A4.2 3-(4-lsopropyl-benzoy1)-3-methyl-azetidine-1-carboxylic acid tert-
butyl ester 1.09 318.26
A4.3 3-(4-tert-Butyl-benzoyI)-3-methyl-azetidine-1-carboxylic acid tert-
butyl ester 1.12 332.28
418.04
A4.4 3-(4-Ethyl-benzoyI)-3-methyl-azetidine-1-carboxylic acid tert-butyl
ester 1.03
[M-Me+H]
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
147
3-Methyl-3-(4-trifluoromethyl-benzoy1)-azetidine-1-carboxylic acid tert-butyl
A4.5 1.07 344.16
ester
3-Methy1-344-(1-methyl-cyclopropy1)-benzoy1Fazetidine-1-carboxylic acid tert-
A4.6 1.1 330.28
butyl ester
3-Methy1-3-[4-(1-trifluoromethyl-cyclopropy1)-benzoyll-azetidine-1-carboxylic
A4.7 1.1 384.28
acid tert-butyl ester
A4.8 3-(4-Bromo-benzoyI)-3-methyl-azetidine-1-carboxylic acid tert-butyl
ester 1.04 353.9
A4.9 3-Methy1-3-(4-propyl-benzoy1)-azetidine-1-carboxylic acid tert-butyl
ester 1.08 318.24
A4.10 3-(4-Cyclopropyl-benzoyI)-3-methyl-azetidine-1-carboxylic acid tert-
butyl ester 1.06 316.18
3-Fluoro-3-(4-trifluoromethoxy-benzoyI)-azetidine-1-carboxylic acid tert-butyl
A4.11 1.09 363.94
ester
Example A4.12: 3-Cyano-3-(4-trifluoromethoxy-benzoyI)-azetidine-1-carboxylic
acid tert-butyl ester
To a solution of 3-cyano-3-(methoxy-methyl-carbamoyI)-azetidine-1-carboxylic
acid tert-butyl ester (1.4g) in anhydrous
THF (40mL) under argon cooled down to 0 C was added 4-
(trifluoromethoxy)phenylmagnesium bromide (0.5M in THF,
20.8mL) dropwise. The resulting mixture was stirred at 0 C for 30 min, allowed
to warm up to RT and stirred overnight.
The reaction mixture was quenched with sat. aq. NI-141, stirred for 15min and
extracted with EA /water. The aq. layer
was back extracted twice with EA. The org. layers were dried (MgSO4), filtered
off and evaporated to dryness. The
resulting crude material was purified by CC (Biotage, SNAP 100g, solvent A:
Hep; solvent B: EA; gradient in %B: 0 over
30V, 0 to 10 over 5CV, 10 over 100V) to afford 1.2g of the title compound as
yellow solid. LC-MS (A): tR = 1.06min;
[M+H]': 371.15.
Example A4.13: 3-Fluoro-3-(4-isopropyl-benzoyI)-azetidine-1-carboxylic acid
tert-butyl ester
To a solution of 1-[(tert-butoxy)carbonyI]-3-fluoroazetidine-3-carboxylic acid
(500mg) in DCM (16mL) were added N,0-
dimethylhydroxylamine hydrochloride (221mg), DIPEA (1.36mL) and
propylphosphonic anhydride solution in DCM
(50% w/w, 1.64mL). The resulting mixture was stirred 40min at RT. Then it was
diluted with DCM and washed once
with aq. sat. NaHCO3 solution. The org. layer was washed with citric acid
(10%) and brine. The aq. layers were back
extracted with twice DCM. The combined org. layers were dried (MgSO4),
filtered off, evaporated and dried at HV to
afford 630mg of the title compound as yellow oil. LC-MS (A): tR = 0.62min;
[M+H]*: 263.13.
CA 03177335 2022- 10- 28
WO 2021/219849 PCT/EP2021/061401
148
Example A4.14 to A4.18, A4.20, A4.22 to A.4.33 were synthesized starting from
the appropriate Weinreb amide of
Formula (A2) and the appropriate bromo derivative of Formula (A3) and
following the procedure described in Example
A4.1. LC-MS data of Example A4.14 to A4.18, A4.20, and A4.22 to A4.33 are
listed in the table below. The LC-MS
conditions used were LC-MS (A).
Example N Name tR [M-F1-1]*
A4.14 1.08
311.04
3-Methyl-3-(naphthalene-2-carbony1)-azetidine-1-carboxylic acid tert-butyl
ester
A4.15 3-
Methy1-344-(1_trifluoromethyl_cyclopropy1)-benzoyll-azetidine-1-carboxylic
acid 1.12 383.97
tert-butyl ester
A4.16 3-Methy1-344-(2,2,2-trifluoro-1, 1-dinnethyl-ethyl)-benzoyl]-
azeticline-1-carboxylic 1.13 385.87
acid tert-butyl ester
A4.17 1.06
334.15
3-(4-lsopropoxy-benzoy1)-3-methyl-azetidine-1-carboxylic acid tert-butyl ester
A4.18 3-Methyl-3[441,2,2,2-tetrafluorol -trifluoromethyl-ethyl)-benzoyll-
azetidine-1- 1.16 443.90
carboxylic acid tert-butyl ester
A4.20 3-(3-
Fluoro-4-isopropyl-benzoyI)-3-methyl-azetidine-1-carboxylic acid tert-butyl
1.13 336.13
ester2
A4.22 1.14
394.04
3-Methyl-3-(4-pentafluoroethyl-benzoy1)-azetidine-1-carboxylic acid tert-butyl
ester
A4.23 3-(4-
lsopropy1-3-methyl-benzoy1)-3-methyl-azetidine-1-carboxylic acid tert-butyl
1.13 332.10
ester
A4.24 314-(1-
Fluoro-1-methykethyl)-benzoy11-3-methyl-azetidine-1-carboxylic acid tell- 1.08
336.10
butyl ester
A4.25 344-
(1,1-Difluoro-ethyl)-benzoy1]-3-methyl-azetidine-1-carboxylic acid tert-butyl
1.06 340.06
ester
A4.26 3-(4-
Bicyclo[1.1.1]pent-1-yl-benzoyI)-3-methyl-azetidine-1-c2rboxylic acid ten-
1.16 342.00
butyl ester
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
149
A4.27 3-[4-(1,1-Dimethyl-propyI)-benzoy1]-3-methyl-azetidine-1-carboxylic
acid tert-butyl 1.18 346.16
ester
A4.28 3-(3-
Chloro-4-isopropyl-benzoy1)-3-methyl-azetidine-1-carboxylic acid tert-butyl
1.15 352.08
ester
A4.29 1.13
330.12
3-(4-Cyclobutyl-benzoyI)-3-methyl-azetidine-1-carboxylic acid tert-butyl ester
A4.30 3-(3,5-
Difluoro-4-isopropyl-benzoyI)-3-methyl-azetidine-1-carboxylic acid tert-butyl
1.14 36316
ester
A4.31 1.09
346.12
3-(4-Cyclobutoxy-benzoyI)-3-methyl-azetidine-1-carboxylic acid tert-butyl
ester
A4.32 3-[4-(1-Ethyl-propyI)-benzoy1]-3-methyl-azetidine-1-carboxylic acid
tert-butyl ester 1.16 331.13
A4.33 344-
(2,2-Dimethyl-propy1)-benzoy1]-3-methyl-azetidine-1-carboxylic acid tert-butyl
1.17 346.16
ester
Example A4.19: 3-methy1-3-(4-(pentafluoro-X6-sulfaneyl)benzoyl)azetidine-1-
carboxylic acid tert-butyl ester
A4.19.1: 3-Formy1-3-methyl-azetidine-1-carboxylic acid tert-butyl ester
To a solution of Example A4.1.1 (1 eq) in THF (6.7 mL/mmol) was added dropwise
at -78 C, a 1M solution of
diisobutylaluminium hydride in DCM (1.2 eq). The reaction mixture was stirred
for 30 min at -78 C, quenched with
Me0H and partitioned between a sat. solution of potassium sodium tartrate
tetrahydrate and DCM. The org. phase was
dried over MgSO4 and concentrated in vacuo to provide the crude title compound
as yellowish oil. 1H NMR (400 MHz,
DMSO) 5: 9.69 (s, 1 H), 4.04 (d, J= 8.1 Hz, 2 H), 3.59 (d, J= 8.2 Hz, 2 H),
1.39 (s, 9 H), 1.35 (s, 3 H)
A4.19.2: 3-(Hydroxy(4-(pentafluoro-X6-sulfaneyl)phenyl)methyl)-3-
methylazetidine-1-carboxylic acid tert-butyl ester
To a solution of 4-bromophenylsulphur pentafluoride (1.5 eq) in anhydrous Et20
(7.5 mL/mmol) under argon and cooled
to -78 C, was added dropwise a 1.6M solution of tBuLi (1.1 eq). The reaction
mixture was stirred for 5 min and a solution
of 3-formy1-3-methyl-azetidine-1-carboxylic acid tert-butyl ester (1 eq) in
anhydrous THE (2 mL/mmol) was added
dropwise. The resulting dark yellow solution was stirred for 45 min at -78 C,
the dry ice bath was removed, and the
reaction mixture was additionally stirred for 45 min. It was quenched with
water and extracted with EA. The org. phase
was washed with brine, dried over MgSO4 and concentrated in vacuo. The crude
was purified by CC using Sfar
prepacked cartridges from Biotage and eluting with Hept/EA to afford the title
compound as white solid. LC-MS (A): tR
= 1.03 min; [M-F1-1]': 403.91
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
150
A4.19.3: 3-Methy1-3-(4-(pentafluoro-X6-sulfaneyl)benzoyl)azetidine-1-
carboxylic acid tert-butyl ester
To a solution of 3-(hydroxy(4-(pentafluoro-X6-sulfaneyl)phenyl)methyl)-3-
methylazetidine-1-carboxylic acid tert-butyl
ester (1 eq) in DCM (3.5 mL/mmol) was added at RT, DMP (1.2 eq). The reaction
mixture was stirred for 15 min at RT,
diluted with DCM, quenched with water and filtered. The filtrate was washed
with a sat. solution of NaHCO3 and the
org. phase was dried over MgSO4 and concentrated in vacuo. The crude was
purified by CC using Sfar prepacked
cartridges from Biotage and eluting with Hept/EA to afford the title compound
as colorless sticky oil. LC-MS (A): tR =
1.12 min; [M+H]: 401.73
Example A4.21: 3-(Benzo[b]thiophene-5-carbonyI)-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
The title compound was synthesized starting from the appropriate Weinreb amide
of Formula (A2) and the appropriate
bromo derivative of Formula (A3) and following the procedure described in
Example A4.1. It was isolated from the
resulting mixture of 3-(benzo[b]thiophene-2-carbonyI)-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester and 3-
(benzo[b]thiophene-5-carbonyI)-3-methyl-azetidine-1-carboxylic acid tert-butyl
ester by Prep chiral SFC (I11). LC-MS
(A): tR = 1.07 min; [M+H]-: 332.04.
Example A4.34 3-Ethyl-3-(4-trifluoromethoxy-benzoy1)-azetidine-1-carboxylic
acid tert-butyl ester
A4.34.1 3-Ethyl-azetidine-1,3-dicarboxylic acid 1-iced-butyl ester 3-ethyl
ester
To a solution of ethyl 1-Boc-azetidine-3-carboxylate (993 mg) and iodoethane
(0.338 mL) in THE (10 mL) was added
potassium bis(trimethylsilyl)amide (1.55 mL) as a solution in toluene (10 mL)
at -78 C. The reaction was stirred
overnight and allowed to slowly warm up to RT. The reaction was quenched by
the addition of aqueous aq. sat. NI-1.4C1
(20 mL) and diluted with water (20 mL) and EA (20 mL). The phases were
separated and the aqueous phase was
extracted with EA (3x, 30 mL). The combined organic phases were washed with
brine, dried over MgSO4 and
concentrated in vacuo. The product was obtained as 677mg yellow oil. LC-MS (A)
tR = 0.88min; [M-F1-1]': 257.16
A4.34.2 3-Ethyl-azetidine-1,3-dicarboxylic acid mono-tert-butyl ester
To the solution of A4.34.1 (676 mg) in water (3.72 mL) and dioxane (3.72 mL)
was added lithium hydroxide monohydrate
(780 mg) and the mixture was heated to 70 C (bath temperature) for 3h. The
reaction was diluted in water (ca 20 mL)
and EA (ca. 20 mL) and acidified (ca. pH = 4) with aqueous HCI (2 M). The
phases were separated, the aqueous phase
was extracted with EA (3x, 15 mL), the combined organic phases were washed
with brine, dried over MgSO4 and
concentrated in vacuo to give the pure product as 555mg yellowish solid. LC-MS
(A) tR = 0.65min, [M+H]*: 229.13
A4.34.3 3-Ethyl-3-(methoxy-methyl-carbamoy1)-azetidine-1 -carboxylic acid tert-
butyl ester
To a suspension of A4.34.2 (550 mg), N,0-dimethylhydroxylamine hydrochloride
(468 mg) and DMAP (2.93 mg) in
DCM (9 mL) was added N-(3-dimethylaminopropyI)-N'-ethylcarbodiimide
hydrochloride (929 mg) and triethylamine
(1.35 mL). The mixture was stirred at RT for 2h. The reaction was diluted with
water (10 mL) and and EA (10 mL) and
the pH was adjusted to 1. The phases were separated and the aqueous phase was
extracted with EA (3x, 10 mL). The
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
151
combined organic phases were washed with brine, dried over MgSO4 and
concentrated in vacuo to yield the product
as 570mg yellowish oil. LC-MS (A) tR = 0.77min; [M-F1-1]': 272.17
A4.34.4 3-Ethy1-3-(4-trifluoromethoxy-benzoy1)-azetidine-1-carboxylic acid
tert-butyl ester
The title compound was synthesized from Example A4.34.3 and following the
procedure described in Example A4.1
step A4.1.2. LC-MS (A): tR = 0.99min; [M-F1-1]+: 373.15.
Example A4.35 3-Methy1-344-(2,2,2-trifluoro-ethyl)-benzoy1Fazetidine-1-
carboxylic acid tert-butyl ester
A flask was charged with A4.8 (647mg), CsCO3 (1803mg), H20 (782pL) and toluene
(9.13mL). The suspension was
degassed with argon in ultrasonic bath for 5min, then t 4,4,5,5-Tetramethy1-2-
(2,2,2-trifluoroethyl)-1,3,2-dioxaborolane
(486pL) and cataCXium A Pd G3 (140mg) were added under argon. The flask was 3x
evacuated and backfilled with
argon. The resulting suspension was stirred at 100 C under argon overnight.
The reaction mixture was allowed to
cool down, filtrated, the filter was washed with EA, the filtrate was
evaporated to dryness and purified by CC (Biotage,
25g sphere duo, A: Hept, B: EA, gradient (in %B): 10 for 3CV, 10 to 30 over
1CV, 30 for 3CV, 30 to 50 over 2CV, 50
for 1CV) to give 569mg brown solid. LC-MS (A) tR = 1.06min; [M-FH]': 358.06
Example A5.1: 2-[3-(5-Bromo-pyridin-3-y1)41,2,4]oxadiazol-5-y1]-propan-2-ol
H2.1: 5-Bromo-N-hydroxy-nicotinamidine
To a solution of 5-bromonicotinonitrile (2g) in DMF (10mL) were sequentially
added hydroxylamine hydrochloride
(1.14g) and TEA (3.05 mL). The reaction mixture was heated to 85 C for 1h,
cooled to RT and quenched with water
(20mL). The resulting precipitate was filtered off and dried at 65 C under
vacuo to afford 2.1g of the title compound as
white solid. LC-MS (A): tR = 0.36 min; [M-FH]-: 215.98.
A5.1: 2-[3-(5-Bromo-pyridin-3-y1)-[1,2,4]oxadiazol-5-y1]-propan-2-ol
To a solution of CDI (11.6g) in DMF (35mL) under argon, was added dropwise at
RT a solution of 2-hydroxyisobutiric
acid (7.23g) in DMF (15mL). After stirring for 30 min, a suspension of
intermediate H2.1 (10g) in DMF (50mL) was
added slowly at RT. The mixture was heated to 90 C, stirred for 18h and cooled
to RT. It was quenched with dropwise
addition of water (100mL) at 000 and aged for 30 min at 0 C. The resulting
precipitate was filtered and dried at 65 C
under vacuo to afford 9.1g of the title compound as white solid. LC-MS (A): tR
= 0.77 min; [M+H]-: 283.98.
Example A5.2: 1-{4-[3-(5-Bromo-pyridin-3-y1)-[l,2,4]oxadiazol-5-A-piperidin-1-
ylyethanone
The title compound was synthesized following the procedure described in
Example A5.1 except that 2-hydroxyisobutiric
acid was replaced by 1-acetyl-4-piperidinecarboxylic acid in the second step.
LC-MS (A): tR = 0.84 min; [M+1-1]: 350.91.
Examples A6.1 to A6.25
Intermediate A6.1.1 to A6.30.1 were synthesized starting from the appropriate
precursor of Formula (A4) and the
appropriate bromo derivative of Formula (A5) and following the procedure
described in Example A7.1 step A7.1.1
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
152
except that nBuLi was replaced by HexLi. Prep LC-MS conditions and LC-MS data
are listed in the table below. The
LC-MS conditions used were LC-MS (A).
Example Prep
Precursor Precursor
Name tR [M+H]'
N LC-MS A4
A5
3-(Hydroxy-{545-(1-hydroxy-1-methyl-ethyl)-
[1,2,4]oxadiazol-3-y1Fpyridin-3-y11-
A6.1.1 1.01 531.08 XXXI A4.14 A5.1
naphthalen-2-yl-methyl)-3-methyl-azetidine-
1-carboxylic acid tert-butyl ester
3-{Hydroxy-{5-[5-(1-hydroxy-1-methyl-ethyl)-
[1,2,4]oxadiazol-3-q-pyridin-3-y1H4-(1-
A6.2.1 trifluoromethyl-cyclopropy1)-phenyl]-
methyl}- 1.05 589.08 VI A4.15 A5.1
3-methyl-azetidine-1-carboxylic acid tert-
butyl ester
3-{Hydroxy-{5-[5-(1-hydroxy-1-methyl-ethyl)-
[1,2,4]oxadiazol-311]-pyridin-3-y1H4-(2,2,2-
A6.3.1 trifluoro-1,1-dimethyl-ethyl)-phenyl]-
methyll- 1.05 590.97 VII A4.16 A5.1
3-methyl-azetidine-1-carboxylic acid tert-
butyl ester
3-[Hydroxy-{545-(1-hydroxy-1-methyl-ethyl)-
[1,2,4]oxadiazol-311]-pyridin-3-y11-(4-
A6.4.1 0.98 539.15 VI A4.17 A5.1
isopropoxy-phenyI)-methy1]-3-methyl-
azetidine-1-carboxylic acid tert-butyl ester
3-((3-Fluoro-4-isopropyl-phenyI)-hydroxy-{5-
[5-(1-hydroxy-1-methyl-ethyly
A6.5.1 [1,2,4]oxadiazol-3-y1]-pyridin-3-
ylymethyl)-3- 1.05 541.05 XXXI A4.20 A5.1
methyl-azetidine-1-carboxylic acid tert-butyl
ester
3-((4-tert-Butyl-phenyI)-hydroxy-{5-[5-(1-
hydroxy-1-methyl-ethyl)-[1,2,4]oxadiazol-3-
A6.6.1 1.05 537.00 VII A4.3 A5.1
yI]-pyridin-3-ylymethy1)-3-methyl-azetidine-
1-carboxylic acid tert-butyl ester
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
153
3-(Benzo[b]thiophen 5 yl hydroxy-{5-[5-(1-
hydroxy-1-methyl-ethyl)-[1,2,4]oxadiazol-3-
A6.7.1 0.99 537.04 VI A4.21 A5.1
y1]-pyridin-3-ylymethyl)-3-methyl-azetidine-
1-carboxylic acid tert-butyl ester
3-[Hydroxy-{545-(1-hydroxy-1-methyl-ethyl)-
[1,2,4]oxadiazol-3-y11-pyridin-3-y11-(4-
A6.8.1 1.06 599.07 XX A4.22 A5.1
pentafluoroethyl-phenylymethy1]-3-methyl-
azetidine-1-carboxylic acid tert-butyl ester
3-{Hydroxy-{545-(1-hydroxy-1-methyl-ethyl)-
[1,2,4]oxadiazol-311]-pyridin-3-y1H4-(1-
A6.9.1 methyl-cyclopropylyphenylymethy11-3- 1.04 535.10
VII A4.6 A5.1
methyl-azetidine-1-carboxylic acid tert-butyl
ester
3-[Hydroxy-{545-(1-hydroxy-1-methyl-ethyl)-
[1,2,4]oxadiazol-3-y1]-pyridin-3-y11-(4-
A6.10.1 isopropyl-3-methyl-phenylymethyl]-3- 1.06 537.13
VII A4.23 .. A5.1
methyl-azetidine-1-carboxylic acid tert-butyl
ester
3-[Hydroxy-{545-(1-hydroxy-1-methyl-ethyl)-
[1,2,4]oxadiazol-3-y1]-pyridin-3-y11-(4- VI +
A6.11.1 1.02 565.09
A4.1 A5.1
trifluoromethoxy-phenylymethy1]-3-methyl- XXIX
azetidine-1-carboxylic acid tert-butyl ester
3-([4-(1-Fluoro-1-methyl-ethyl)-pheny1]-
hydroxy-{545-(1-hydroxy-1-methyl-ethyl)-
A6.12.1 [1,2,4]oxadiazol-311]-pyridin-3-y11-methyl)-3- 1.00
541.13 VI .. A4.24 .. A5.1
methyl-azetidine-1-carboxylic acid tert-butyl
ester
3-([4-(1,1-Difluoro-ethyl)-pheny1]-hydroxy-{5-
[5-(1-hydroxy-1-methyl-ethyl)-
A6.13.1 [1,2,4]oxadiazol-311]-pyridin-3-y11-methyl)-3- 0.99
545.96 VI .. A4.25 .. A5.1
methyl-azetidine-1-carboxylic acid tert-butyl
ester
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
154
3-((4-Bicyclo[1.1.1]pent 1 yl pheny1)-
hydroxy-{5-[5-(1-hydroxy-1-methyl-ethyl)-
A6.14.1 [1,2,4]oxadiazol-311]-pyridin-3-
ylymethyl)-3- 1.08 547.19 VII A4.26 A5.1
methyl-azetidine-1-carboxylic acid tert-butyl
ester
3-([4-(1,1-Dimethyl-propylyphenyll-hydroxy-
{5-[5-(1-hydroxy-1-methyl-ethyl)-
A6.15.1 [1,2,4]oxadiazol-311]-pyridin-3-
ylymethyl)-3- 1.10 551.23 VII A4.27 A5.1
methyl-azetidine-1-carboxylic acid tert-butyl
ester
3-[{5-[5-(1-Acetyl-piperidin-4-y1)-
[1,2,4]oxadiazol-311]-pyridin-3-y11-(3-fluore-
A6.16.1 4-isopropyl-pheny1)-hydroxy-methyl]-3-
1.09 608.10 VI A4.20 A5.2
methyl-azetidine-1-carboxylic acid tert-butyl
ester
3-{{5-[5-(1-Acetyl-piperidin-4-yI)-
[1,2,4]exadiazol-3-y1]-pyridin-3-yll-hydroxy-
A6.17.1 [4-(1-trifluoromethyl-cyclopropyl)-
phenyl]- 1.09 656.23 VI A4.15 A5.2
methyl)-3-methyl-azetidine-1-carboxylic acid
tert-butyl ester
3-[{5-[5-(1-Acetyl-piperidin-4-y1)-
[1,2,4]oxadiazol-3-y1]-pyridin-3-yll-hydroxy-
A6.18.1 (4-trifluoromethoxy-pheny1)-methyl]-3-
1.06 632.20 VI A4.1 A5.2
methyl-azetidine-1-carboxylic acid tert-butyl
ester
3-[{5-[5-(1-Acetyl-piperidin-4-y1)-
[1,2,4]exadiazol-311]-pyridin-3-yll-hydroxy-
A6.19.1 1.03 606.25 VI A4.17 A5.2
(4-isopropoxy-pheny1)-methyl]-3-methyl-
azetidine-1-carboxylic acid tert-butyl ester
34(3-Chloro-4-isopropyl-pheny1)-hydroxy-{5-
A6.20.1 [5-(1-hydroxy-1-methyl-ethyly 1.07
557.15 VII A4.28 A5.1
[1,2,4]exadiazol-3-y1]-pyridin-3-y1}-methyl)-3-
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
155
methyl-azetidine-1-carboxylic acid tert-butyl
ester
3-((4-Cyclobutyl-pheny1)-hydroxy-{5-[5-(1-
hydroxy-1-methyl-ethy1)41,2,4]oxadiazol-3-
A6.21.1 1.05 535.11 VII A4.29 A5.1
yI]-pyridin-3-yll-methy1)-3-methyl-azetidine-
1-carboxylic acid tert-butyl ester
3-[{545-(1-Acetyl-piperidin-4-y1)-
[1,2,4]oxadiazol-3-A-pyridin-3-y11-(4-
A6.22.1 cyclobutyl-phenyI)-hydroxy-methy1]-3-
1.10 602.16 VI A4.29 A5.2
methyl-azetidine-1-carboxylic acid tert-butyl
ester
3-[{545-(1-Acetyl-piperidin-4-y1)-
[1,2,4]oxadiazol-3-A-pyridin-3-y1H3,5-
A6.23.1 difluoro-4-isopropyl-phenyl)-hydroxy-
1.11 626.11 VI A4.30 A5.2
methyI]-3-methyl-azetidine-1-carboxylic acid
tert-butyl ester
3-((3,5-Difluoro-4-isopropyl-phenyI)-
hydroxy-{545-(1-hydroxy-1-methyl-ethyl)-
A6.24.1 [1,2,4]oxadiazol-311]-pyridin-3-yll-
methyl)-3- 1.06 559.09 VII A4.30 A5.1
methyl-azetidine-1-carboxylic acid tert-butyl
ester
3-[{545-(1-Acetyl-piperidin-4-y1)-
[1,2,4]oxadiazol-311]-pyridin-3-y11-(4-
A6.25.1 cyclobutoxy-phenyl)-hydroxy-methyl]-3-
1.05 618.39 VI A4.31 A5.2
methyl-azetidine-1-carboxylic acid tert-butyl
ester
3-([4-(1-Ethyl-propy1)-pheny1]-hydroxy-{545-
(1-hydroxy-1-methyl-ethy1)41,2,4]oxadiazol-
A6.26.1 1.10 551.15 VII A4.32 A5.1
3-yll-pyridin-3-yll-methyl)-3-methyl-
azetidine-1-carboxylic acid tert-butyl ester
3-{{5-[5-(1-Acetyl-piperidin-4-y1)-
A6.27.1 1.16 618.30 VII A4.32 A5.2
[1,2,4]oxadiazol-3-y11-pyridin-3-y1144-(1-
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
156
ethyl-propylyphenyll-hydroxy-methy11-3-
methyl-azetidine-1-carboxylic acid tert-butyl
ester
3-([4-(2,2-Dimethyl-propyI)-pheny1]-hydroxy-
{5-[5-(1-hydroxy-1-methyl-ethyl)-
A6.28.1 [1,2,4]oxadiazol-3-y11-pyridin-3-ylymethyl)-3- 1.10
551.12 VII A4.33 A5.1
methyl-azetidine-1-carboxylic acid tert-butyl
ester
3-{{545-(1-Acetyl-piperidin-4-y1)-
[1,2,4]oxadiazol-311]-pyridin-3-y1144-(2,2-
A6.29.1 dimethyl-propylyphenyl]hydroxy-methyl}-3- 1.15
618.23 VII A4.33 A5.2
methyl-azetidine-1-carboxylic acid tert-butyl
ester
3-{{545-(1-Acetyl-piperidin-4-y1)-
[1,2,4]oxadiazol-3-y1]-pyridin-3-yll-hydroxy-
A6.30.1 [4-(1-methyl-cyclopropy1)-phenyl]-methyl}-3- 1.08
602.18 VI A4.6 A5.2
methyl-azetidine-1-carboxylic acid tert-butyl
ester
Example A6.1.1 to A6.25.1 were purified by Prep chiral SFC or HPLC to afford
the title compound of Formula (A6) as
pure enantiomer. The Prep chiral SFC or HPLC conditions and chiral SFC or HPLC
data are listed in the table below
Prep chiral
Example Chiral
Name tR SFC or
Precursor
N HPLC/SFC
HPLC
34(R)-Hydroxy-15-[5-(1-hydroxy-1-methyl-ethyl)-
[1,2,4]oxadiazol-3-y1]-pyridin-3-yll-naphthalen-2-
A6.1 AC 2.076
XLVI I A6.1.1
yl-methyl)-3-methyl-azetidine-1-carboxylic acid
tert-butyl ester
3-{(R)-Hydroxy-{545-(1-hydroxy-1-methyl-ethyl)-
[1,2,4]oxadiazol-3-y1]-pyridin-3-y1144-(1-
A6.2 I 3.088 VIII A6.2.1
trifluoromethyl-cyclopropy1)-phenyl]-methyll-3-
methyl-azetidine-1-carboxylic acid tert-butyl ester
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
157
3-{(R)-Hydroxy-{5-[5-(1-hydroxy-1-methyl-ethyl)-
[1,2,4]oxadiazol-3-y1]-pyridin-3-y1H4-(2,2,2-
A6.3 F 2.55 VI A6.3.1
trifluoro-1,1-dimethyl-ethylyphenyl]-methy1}-3-
methyl-azetidine-1-carboxylic acid tert-butyl ester
3-[(R)-Hydroxy-{5-[5-(1-hydroxy-1-methyl-ethyl)-
[1,2,4]oxadiazol-3-y11-pyridin-3-y11-(4-isopropoxy-
A6.4 AM 2.00 XLV A6.4.1
phenyI)-methy1]-3-methyl-azetidine-1-carboxylic
acid tert-butyl ester
3-((R)-(3-Fluoro-4-isopropyl-phenyI)-hydroxy-{5-
[5-(1-hydroxy-1-methyl-ethyl)-[1,2,4]oxadiazol-3-
A6.5 AN 1.884 XLVI A6.5.1
yq-pyridin-3-yll-methyl)-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
34(R)-(4-tert-Butyl-pheny1)-hydroxy-{545-(1-
hydroxy-1-methyl-ethyl)41,2,4]oxadiazol-3-y1]-
A6.6 C 1.47 III A6.6.1
pyridin-3-yll-methyl)-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
34(R)-Benzo[b]thiophen-5-yl-hydroxy-{545-(1-
hydroxy-1-methyl-ethy1)41,2,4]oxadiazol-3-y1]-
A6.7 AC 2.33 XLVI I A6.7.1
pyridin-3-yll-methyI)-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
3-[(R)-Hydroxy-{545-(1-hydroxy-1-methyl-ethyl)-
[1,2,4]oxadiazol-3-y1]-pyridin-3-y11-(4-
A6.8 AO 2.02 XLVIII A6.8.1
pentafluoroethyl-phenyI)-methy1]-3-methyl-
azetidine-1-carboxylic acid tert-butyl ester
3-{(R)-Hydroxy-{5-[5-(1-hydroxy-1-methyl-ethyl)-
[1,2,4]oxadiazol-3-y11-pyridin-3-y1114-(1-methyl-
A6.9 AV 3.05 LV A6.9.1
cyclopropy1)-phenylFmethyll-3-methyl-azetidine-
1-carboxylic acid tert-butyl ester
3-[(R)-Hydroxy-{5-[5-(1-hydroxy-1-methyl-ethyl)-
[1,2,4]oxadiazol-311]-pyridin-3-y11-(4-isopropyl-3-
A6.10 AP 3.40 XLIX A6.10.1
methyl-phenyl)-methyl]-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
158
3-[(R)-Hydroxy-{5-[5-(1-hydroxy-1-methyl-ethyl)-
[1,2,4]oxadiazol-311]-pyridin-3-y11-(4-
A6.11 V 1.36 XIX A6.11.1
trifluoromethoxy-phenyI)-methy1]-3-methyl-
azetidine-1-carboxylic acid tert-butyl ester
3-((R)44-(1-Fluoro-1-methyl-ethyl)-pheny1]-
hydroxy-{5-[5-(1-hydroxy-1-methyl-ethyl)-
A6.12 C 1.39 III A6.12.1
[1,2,4]oxadiazol-311]-pyridin-3-yll-methyl)-3-
methyl-azetidine-1-carboxylic acid tert-butyl ester
34(R)44-(1,1-Difluoro-ethyl)-phenyl]-hydroxy-{5-
[5-(1-hydroxy-1-methyl-ethyl)-[1,2,4]oxadiazol-3-
A6.13 AQ 2.25 L A6.13.1
yq-pyridin-3-yll-methyl)-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
34(R)-(4-Bicyclo[1.1.1]pent-1-yl-pheny1)-hydroxy-
{545-(1-hydroxy-1-methyl-ethy1)41,2,4]oxadiazol-
A6.14 AR 1.959 LI A6.14.1
311]-pyridin-3-yll-methyl)-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
34(R)44-(1,1-Dimethyl-propy1)-phenyl]-hydroxy-
{545-(1-hydroxy-1-methyl-ethy1)41,2,4]oxadiazol-
A6.15 AV 2.45 LV A6.15.1
311]-pyridin-3-yll-methyl)-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
34(R)-{545-(1-Acetyl-piperidin-4-y1)-
[1,2,4]oxadiazol-3-A-pyridin-3-y11-(3-fluoro-4-
A6.16 AS 9.894 LI I A6.16.1
isopropyl-phenyI)-hydroxy-methy1]-3-methyl-
azetidine-1-carboxylic acid tert-butyl ester
3-{(R)-{5-[5-(1-Acetyl-piperidin-4-yI)-
[1,2,4]oxadiazol-3-y11-pyridin-3-yll-hydroxy-[4-(1-
A6.17 AS 9.387 LI I A6.17.1
trifluoromethyl-cyclopropy1)-phenylFmethyll-3-
methyl-azetidine-1-carboxylic acid tert-butyl ester
3-KR)-{545-(1-Acetyl-piperidin-4-y1)-
[1,2,4]oxadiazol-3-y1]-pyridin-3-01-hydroxy-(4-
A6.18 AS 8.907 LI I A6.18.1
trifluoromethoxy-phenylymethy11-3-methyl-
azetidine-1-carboxylic acid tert-butyl ester
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
159
3-[(R)-{5-[5-(1-Acetyl-piperidin-4-yI)-
[1,2,4]oxadiazol-3-y1]-pyridin-3-ylyhydroxy-(4-
A6.19 AT 7.62 LIII A6.19.1
isopropoxy-phenyI)-methy1]-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
3-((R)-(3-Chloro-4-isopropyl-phenyI)-hydroxy-{5-
[5-(1-hydroxy-1-methyl-ethyly[1,2,4]oxadiazol-3-
A6.20 AU 4.807 LIV A6.20.1
y1]-pyridin-3-ylymethyl)-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
34(R)-(4-Cyclobutyl-pheny1)-hydroxy-{545-(1-
hydroxy-1-methyl-ethy1)41,2,4]oxadiazol-311]-
A6.21 AV 3.68 LV A6.21.1
pyridin-3-yll-methyI)-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
3-[(R)-{545-(1-Acetyl-piperidin-4-y1)-
[1,2,4]oxadiazol-3-A-pyridin-3-01-(4-cyclobutyl-
A6.22 AW 3.06 LVI A6.22.1
pheny1)-hydroxy-methyl]-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
3-[(R)-{545-(1-Acetyl-piperidin-4-y1)-
[1,2,4]oxadiazol-311]-pyridin-3-01-(3,5-difluoro-4-
A6.23 AX 2.94 LVI I A6.23.1
isopropyl-pheny1)-hydroxy-methy1]-3-methyl-
azetidine-1-carboxylic acid tert-butyl ester
34(R)-(3,5-Difluoro-4-isopropyl-pheny1)-hydroxy-
{545-(1-hydroxy-1-methyl-ethy1)41,2,4]oxadiazol-
A6.24 AY 3.15 LVIII A6.24.1
311]-pyridin-3-A-methyl)-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
3-[(R)-{545-(1-Acetyl-piperidin-4-y1)-
[1,2,4]oxadiazol-3-y11-pyridin-3-y11-(4-cyclobutoxy-
A6.25 AZ 2.785 LIX A6.25.1
phenyI)-hydroxy-methy1]-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
Example A6.26 to Example A6.28 were synthesized following the procedure
described in Example A7.1 step A7.1.1
using the ketone precursor and 3,5-dibromopyridine. The ketone precursor, LC-
MS (A) data, prep LC-MS method,
chiral preparative and analytical SEC data are listed in the table below.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
160
Example N Name Ketone tR [M-F1-1]' Prep
Chiral Chiral
precursor LC-MS prep
SFC analytical
LC-MS
A6.26 3-[(R)-(5-Bromo-pyridin-3- A4.34 1.12 530.92
(VII) (XLIV) 1.77 (AE)
yI)-hydroxy-(4-
trifluoromethoxy-phenyly
methyl]-3-ethyl-azetidine-1-
carboxylic acid tert-butyl
ester
A6.27 3-{(R)-(5-Bromo-pyridin-3- A4.35 1.07 515.02
(VII) (XIX) 1.70 (V)
ylyhydroxy-[4-(2,2,2-
trifluoro-ethyl)-phenyl]-
methyll-3-methyl-azetidine-
1-carboxylic acid tert-butyl
ester
A6.28 3-[(S)-(5-Bromo-pyridin-3-yI)- A4.13 1.14 478.96
(VII) (XXIII) 1.60 (AF)
hydroxy-(4-isopropyl- then
pheny1)-methyl]-3-fluoro- (XI I)
azetidine-1-carboxylic acid
tert-butyl ester
Example A6.29 3-[(R)-(5-Bromo-pyridin-3-y1)-hydroxy-(4-trifluoromethyl-
phenylymethyl]-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester was synthesized following the procedure
described Example A7.1 step A7.1.1 using
A4.5 and 3,5-dibromopyridine, purified by prep LC-MS ()ON) and chiral
separation conditions (XLII). LC-MS (A) tR =
1.07min; [M-FH]': 501.13; chiral SEC (W) tR = 1.792min.
Example A7.1: (R)-(3-Methyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-y1)-(4-
trifluoromethoxy-phenylymethanol,
hydrochloride salt
A7.1.1. 34Hydroxy-(5-pyrrolidin-1-yl-pyridin-3-y1)-(4-trifluoromethoxy-pheny1)-
methylp3-methyl-azetidine-1-carboxylic
acid tert-butyl ester
To a solution of Example A4.1 (15g) and 3-bromo-5-pyrrolidinopyridine (12.6g)
in anhydrous THF (150mL) under argon
cooled down to -78 C was added n-BuLi (2.5M in hexane, 20.04mL) dropwise over
20min so that the internal
temperature did not rise above -65 C. The resulting dark yellow solution was
stirred at -78 C for 1h30 and allowed to
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
161
warm up to RT. The reaction mixture was quenched with water and extracted with
DCM. The org. layers were dried
(MgSO4), filtered off and evaporated to dryness. The resulting crude material
was taken up in MeCN/Me0H (9/1 v/v)
and filtrated off. The solution was purified by Prep LC-MS (IV) to afford
6.67g of the title compound as yellow solid. LC-
MS (A): tR = 0.9 min; [M+H]: 508.31.
A7.1.2 3-1(R)-Hydroxy-P-pyrrolidin-1-yl-pyridin-3-0)-(4-trifluoromethoxy-
pheny1)-methyil-3-methyl-azetidine-1 -
carboxylic acid tert-butyl ester
Example A7.1.1 (6.67g) was purified by Prep chiral SFC (II) to afford the
title compound as pure enantiomer (3.35g) as
yellow solid. LC-MS (A): tR = 0.89min; [M+H]: 508.31. Chiral SFC (A): tR =
2.8min. The absolute configuration of the
compound of Example A7.1.2 was determined to be (R)- by obtaining a suitable
crystal of one of the two separated
enantiomers (solvent: methylcyclohexane) and performing single crystal X-ray
diffraction experiment.
A7.1.3. (R)-(3-Methyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-y1)-(4-
trifluoromethoxy-phenyl)-methanol, hydrochloride
salt
A pale-yellow solution of Example A7.1.2 (3.25g) in HCI in dioxane (4M, 15mL)
was stirred 1h at RT. The crude was
evaporated to dryness to give the title compound as yellow solid. LC-MS (A):
tR = 0.60min; [M+H]-: 408.33.
Example A7.2 and Example A7.3 were synthesized starting from the appropriate
Example of Formula (A4) and
following the procedure described in Example A7.1 but omitting the chiral
separation step A7.1.2. LC-MS data of
Example A7.2 and Example A7.3 are listed in the table below. The LC-MS
conditions used were LC-MS (A).
Example N Name tR
3-[Hydroxy-(5-pyrrol idin-1-yl-pyri di n-3-yI)-(4-trifl uoromethoxy-phenyI)-
A7.2 0.62 419.27
methyl]azetidine-3-carbonitrile, hydrochloride salt
(3-Fluoro-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-y1)-(4-
A7.3 0.61 412.20
trifluoromethoxy-phenyI)-methanol, hydrochloride salt
Example A7.4 to Example A7.33 were synthesized starting from the appropriate
precursor of Formula (A6) and
following the procedure described in Example A7.1 step A7.1.3 except that the
reaction mixture was stirred for 18h at
RT. LC-MS data of Example A7.4 to Example A7.33 are listed in the table below.
The LC-MS conditions used were LC-
MS (A).
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
162
Example
Precursor
Name tR
[M-FH]-
N
A6
2-(3-{5-[(R)-Hydroxy-(3-methyl-azetidin-3-y1)-naphthalen-2-yl-
A7.4 methy1]-pyridin-3-
y1141,2,4]oxadiazol-5-y1)-propan-2-ol, 0.65 431.08 A6.1
hydrochloride salt
243-(5-{(R)-Hydroxy-(3-methyl-azetidin-3-y1)44-(1-trifluoromethyl-
A7.5 cyclopropy1)-phenyl]-
methyl}-pyridin-3-y1)41,2,4]oxadiazol-511]- 0.71 489.03 A6.2
propan-2-ol, hydrochloride salt
243-(5-{(R)-Hydroxy-(3-methyl-azetidin-3-y1)-[4-(2,2,2-trifluoro-1,1-
A7.6 dimethyl-ethyl)-pheny1]-
methyl)-pyridin-3-y1)41,2,4]oxadiazol-511]- 0.70 490.95 A6.3
propan-2-ol, hydrochloride salt
2-(3-{5-[(R)-Hydroxy-(4-isopropoxy-pheny1)-(3-methyl-azetidin-3-
A7.7 ylymethyl]-pyridin-3-
yly[1,2,4]oxadiazol-5-y1)-propan-2-ol, 0.66 439.19 A6.4
hydrochloride salt
2-(3-{5-[(R)-(3-Fluoro-4-isopropyl-phenyI)-hydroxy-(3-methyl-
A7.8 azetidin-3-y1)-methy1]-
pyridin-3-yIH1,2,4]oxadiazol-5-y1)-propan-2- 0.68 441.11 A6.5
ol, hydrochloride salt
2-(3-{5-[(R)-(4-tert-Butyl-pheny1)-hydroxy-(3-methyl-azetidin-3-y1)-
A7.9 methy1]-pyridin-3-
y1H1,2,4]oxadiazol-5-y1)-propan-2-ol, 0.71 437.04 A6.6
hydrochloride salt
2-(3-{5-[(R)-Benzo[b]thiophen-5-yl-hydroxy-(3-methyl-azetidin-3-
A7.10 yl)-methyl]-pyridin-3-
y1H1,2,4]oxadiazol-5-y1)-propan-2-ol, 0.63 437.08 A6.7
hydrochloride salt
2-(3-{5-[(R)-Hydroxy-(3-methyl-azetidin-3-yI)-(4-pentafluoroethyl-
A7.11 phenylymethy1]-pyridin-3-
yly[1,2,4]oxadiazol-5-y1)-propan-2-ol, 0.70 499.03 A6.8
hydrochloride salt
243-(5-{(R)-Hydroxy-(3-methyl-azetidin-3-y1)44-(1-methyl-
A7.12 cyclopropy1)-phenyl]-
methyl}-pyridin-3-y1)41,2,4]oxadiazol-511]- 0.68 435.17 A6.9
propan-2-ol, hydrochloride salt
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
163
2-(345-[(R)-Hydroxy-(4-isopropy1-3-methyl-pheny1)-(3-methyl-
A7.13 azetidin-3-y1)-methyl]-pyridin-3-y11-[1,2,4]oxadiazol-5-y1)-
propan-2- 0.69 437.13 A6. 10
ol, hydrochloride salt
2-(3-{5-[(R)- Hydroxy-(3-methyl-azetidi n-3-y1)-(4-trifl uoromethoxy-
A7.14 phenylymethyl]-pyridin-3-y11-[1,2,4]oxadiazol-5-y1)-propan-
2-ol, 0.66 464.97 A6.11
hydrochloride salt
2-(3-{5-[(R)44-(1-Fluoro-1-methyl-ethyl)-phenyl]-hydroxy-(3-
A7.15 methyl-azetidin-3-y1)-methyl]-pyridin-3-yly[1,2,4]oxadiazol-
5-y1)- 0.64 441.15 A6.12
propan-2-ol, hydrochloride salt
2-(3-{5-[(R)- [4-(1, 1-Difl uoro-ethyl)-phenyThydroxy-(3-methyl-
A7.16 azetidin-3-y1)-methyll-pyridin-3-y1141,2,41oxadiazol-5-y1)-
propan-2- 0.64 445.15 A6.13
ol, hydrochloride salt
2-(3-{54(R)-(4-Bicyclo[1.1.1]pent 1 yl phenylyhydroxy-(3-methyl-
A7.17 azetidin-3-y1)-methyl]-pyridin-3-y11-[1,2,4]oxadiazol-5-y1)-
propan-2- 0.72 447.18 A6.14
ol, hydrochloride salt
2-(3-{5-[(R)44-(1,1-Dimethyl-propy1)-phenyl]-hydroxy-(3-methyl-
A7.18 azetidin-3-yl)-methy1]-pyridin-3-yly[1,2,4]oxadiazol-5-y1)-
propan-2- 0.76 451.22 A6.15
ol, hydrochloride salt
144-(3-{5-[(R)-(3-Fluoro-4-isopropyl-pheny1)-hydroxy-(3-methyl-
A7.19 azetidin-3-y1)-methy1]-pyridin-3-y1141,2,4]oxadiazol-5-y1)-
piperidin- 0.73 508.08 A6.16
1-y1]-ethanone, hydrochloride salt
1-{4-[3-(5-{(R)-Hydroxy-(3-methyl-azetidi n-3-y1)-[4-(1-
A7.20 trifluoromethyl-cyclopropy1)-phenyl]-methyl}-pyridin-3-y1)-
0.74 556.07 A6.17
[1,2,4]oxadiazol-511]-piperidin-1-y11-ethanone, hydrochloride salt
144-(3-{5-[(R)-Hydroxy-(3-methyl-azetidin-3-y1)-(4-
A7.21 trifluoromethoxy-phenyl)-methy1]-pyridin-3-
yly[1,2,4]oxadiazol-5- 0.71 532.03 AS. 18
y1)-piperidin-1-y1]-ethanone, hydrochloride salt
144-(3-{5-[(R)-Hydroxy-(4-isopropoxy-pheny1)-(3-methyl-azetidin-
A7.22 3-y1)-methy1]-pyridin-3-y1H1,2,4]oxadiazol-5-y1)-piperidin-
1-y1]- 0.69 506.12 A6.19
ethanone, hydrochloride salt
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
164
2-(3-15-[(R)-(3-Chloro-4-isopropyl-pheny1)-hydroxy-(3-methyl-
A7.23 azetidin-3-y1)-methyl]-pyridin-3-y11-[1,2,4]oxadiazol-5-y1)-
propan-2- 0.73 457.15 A6.20
ol, hydrochloride salt
2-(3-{5-[(R)-(4-Cyclobutyl-pheny1)-hydroxy-(3-methyl-azetidin-3-y1)-
A7.24 methy1]-pyridin-3-yly[1,2,4]oxadiazol-5-y1)-propan-2-ol,
0.71 435.10 A6.21
hydrochloride salt
144-(3-{5-[(R)-(4-Cyclobutyl-pheny1)-hydroxy-(3-methyl-azetidin-3-
A7.25 y1)-methyl]-pyridin-3-y1H1,2,4]oxadiazol-5-y1)-piperidin-1-
y1]- 0.74 502.21 A6.22
ethanone, hydrochloride salt
1-[4-(3-{5-[(R)-(3,5-Difluoro-4-isopropyl-pheny1)-hydroxy-(3-methyl-
A7.26 azetidin-3-y1)-methyll-pyridin-3-y1H1,2,41oxadiazol-5-y1)-
piperidin- 0.74 526.08 A6.23
1-y1]-ethanone, hydrochloride salt
2-(3-{5-[(R)-(3,5-0ifluoro-4-isopropyl-pheny1)-hydroxy-(3-methyl-
A7.27 azetidin-3-yl)-methy1]-pyridin-3-yly[1,2,4]oxadiazol-5-y1)-
propan-2- 0.72 459.17 A6.24
ol, hydrochloride salt
144-(3-{5-[(R)-(4-Cyclobutoxy-pheny1)-hydroxy-(3-methyl-azetidin-
A7.28 3-y1)-methyl]-pyridin-3-yly[1,2,4]oxadiazol-5-y1)-piperidin-
111]- 0.72 518.17 A6.25
ethanone, hydrochloride salt
2-(3-{54[4-(1-Ethyl-propy1)-pheny1]-hydroxy-(3-methyl-azetidin-3-
A7.29 ylymethy1]-pyridin-3-y1141,2,4]oxadiazol-5-y1)-propan-2-ol,
0.77 451.25 A6.26.1
hydrochloride salt
1-[4-(3-{54[4-(1-Ethyl-propy1)-phenyl]-hydroxy-(3-methyl-azetidin-
A7.30 3-y1)-methyll-pyridin-3-y1141,2,41oxadiazol-5-y1)-piperidin-
1-y11- 0.80 518.23 A6.27.1
ethanone, hydrochloride salt
2-(3-{54[4-(2,2-Dimethyl-propy1)-phenyl]-hydroxy-(3-methyl-
A7.31 azetidin-3-yl)-methy1]-pyridin-3-yly[1,2,4]oxadiazol-5-y1)-
propan-2- 0.76 451.25 A6.28.1
ol, hydrochloride salt
144-(3-{54[4-(2,2-Dimethyl-propy1)-pheny1]-hydroxy-(3-methyl-
A7.32 azetidin-3-y1)-methy1]-pyridin-3-y1H1,2,4]oxadiazol-5-y1)-
piperidin- 0.77 518.13 A6.29.1
1-y1]-ethanone, hydrochloride salt
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
165
1-14-[3-(5-{Hydroxy-(3-methyl-azetidin-3-y1)-[4-(1-methyl-
A7.33 cyclopropylyphenyl]-methyl}-pyridin-3-y1)-[1,2,4]oxadiazol-
511]- 0.72 502.13 A6.30.1
piperidin-1-ylyethanone, hydrochloride salt
Example A7.34 (RS)-4-{5-[(R)-Hydroxy-(4-isopropyl-pheny1)-(3-methyl-azetidin-3-
y1)-methyl]-pyridin-3-y11-2-(6-methyl-
pyridin-2-y1)-but-3-yn-2-ol
A7.34.11(R)-Hydroxy-{51(RS)-3-hydroxy-3-(6-methyl-pyridin-2-YO-but-1-
YnYIPPYridin-3-YU-(4-isoProPYI-PhenY0-
methyl]-3-methyl-azetidine-1-carboxylic acid tert-butyl ester
The title compound was synthesized starting from F1.11 using Example F5.27,
and following the procedure described
in Example 15, where the amount of Pd(PPh3)4 was adjusted to 0.1eq and the
base was changed to pyrrolidine (3.5eq).
Purified with prep LC-MS method (V). LC-MS (A) tR = 0.89; [MIN': 556.24
A7.34.2 (RS)-4-{5-0)-Hydroxy-(4-isopropyl-pheny1)-(3-methyl-azetidin-3-y1)-
methyll-pyridin-3-4-2-(6-methyl-pyridin-
2-yI)-but-3-yn-2-ol
To a solution of A7.34.1 (250mg)in dioxane (2mL) was added HCI 4M in dioxane
(473pL) dropwise at RT. The resulting
suspension was stirred for 23h before neutralized basified to pH=10 by the
dropwise addition of NaOH 1M, diluted with
water and DCM, extracted using a phase separator and was re-extracted 2x with
DCM. The org layer was concentrated
in vacuo and dried in HV to give 191mg yellowish solid. LC-MS (A) tR =
0.61min; [M-FH]t 465.22
Example A7.35 to Example A7.37 were synthesized from the protected amine
listed in the table below following the
procedure in Example 309 step 309.2. The LC-MS date can be found in the table
below. The method used was LC-
MS (A).
Example N Name tR [M+H]
Precursor
A7.35 (S)-(5-bromopyridin-3-y1)(3-ethylazetidin-3-y1)(4- 0.75
431.01 A6.26
(trifluoromethoxy)phenyl)methanol, hydrochloride salt
A7.36 (R)-(5-bromopyridin-3-y1)(3-methylazetidin-3-y1)(4- 0.71
415.01 A6.27
(2,2,2-trifluoroethyl)phenyl)methanol, hydrochloride
salt
A7.37 (S)-(5-bromopyridin-3-y1)(3-fluoroazetidin-3-y1)(4- 0.72
380.95 A6.28
isopropylphenyl)methanol, hydrochloride salt
Example A7.38: (R)-(5-Bromo-pyridin-3-y1)-(3-methyl-azetidin-3-y1)-(4-
trifluoromethyl-pheny1)-methanol
CA 03177335 2022- 10- 28
WO 2021/219849 PCT/EP2021/061401
166
The title compound was synthesized following the procedure described in
Example 309 step 309.2 using Example
A6.29. Instead of evaporation the reaction mixture was cooled with an ice-bath
and slowly basified to pH=8 with aq.
sat. NaHCO3 and extracted with 3x DCM. The combined org. layers were dried
over MgSO4, filtrated off, evaporated
and dried at HV to give 1.59g light yellow foam. LC-MS (A) tR = 0.71min;
[M+H]: 403.17
Example B2.1: (1,3-Dimethyl-azetidin-3-y1)-(4-trifluoromethoxy-
phenylymethanone
82.1.1. (3-Methyl-azetidin-3-y/)-(4-trifluoromethoxy-phenyl)-methanone; as
hydrochloric salt
A pale yellow solution of Example A4.1.2 (2g) in HCI in dioxane (4M, 10mL) was
stirred for 30min at RT. The crude was
evaporated to dryness and used in the next step without further purification.
LC-MS (A): tR = 0.66min; [M+H]: 260.29.
82.1.2. (1,3-Dimethyl-azetidin-3-yI)-(4-trifluoromethoxy-phenyl)-methanone
To a light yellow solution of Example B2.1.1 (1.5g) in Me0H (35mL) was added
AcOH (3.5mL), followed successively
by formaldehyde (37% in water, 2.27mL) and NaBH(OAc)3 (2.22g). The resulting
solution was stirred for 30min at RT
and evaporated in vacuo. The residue was diluted with water and the resulting
mixture was basified with aq. sat.
NaHCO3 solution. and extracted with DCM. The org. layers were dried (MgSO4),
filtered off and evaporated to dryness.
The resulting crude material was purified by CC (Biotage, SNAP 50g, solvent A:
DCM; solvent B: Me0H; gradient in
%B: 0 over 3CV, 0 to 3 over 5CV, 3 over 5CV) to afford 950mg of the title
compound as yellow sticky glue. LC-MS (A):
tR = 0.67min; [M-FH]': 274.02
Example B2.2 and Example B2.3 were synthesized following the procedure
described in Example B2.1, but using THF
instead of Me0H as solvent in the reductive amination step for the synthesis
of Example B2.3. LC-MS data of Example
B2.2 and Example B2.3 are listed in the table below. The LC-MS conditions used
were LC-MS (A).
Example N Name tR
B2.2 (1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-methanone 0.67
232.33
B2.3 (4-tert-Butyl-pheny1)-(1,3-dimethyl-azetidin-3-y1)-methanone 0.72
246.38
Example C1.1: 3-Methy1-345-pyrrolidin-1-yl-pyridine-3-carbony1)-azetidine-1-
carboxylic acid tert-butyl ester
The title compound was synthesized starting from 3-bromo-5-pyrrolidinopyridine
and Example A4.1.1, following the
synthesis procedure described in Example A7.1 step A7.1.1, and was purified by
Prep LC-MS (1). LC-MS (A): tR =
0.79min; [M+H]: 346.24
Example C1.2: 3-(5-Ethoxy-pyridine-3-carbonyI)-3-methyl-azetidine-1-carboxylic
acid tert-butyl ester
The title compound was synthesized starting from 3-bromo-5-ethoxypyridine and
Example A4.1.1, following the
synthesis procedure described in Example A7.1 step A7.1.1, and was purified by
CC (Biotage, SNAP 50g, solvent A:
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
167
Hep; solvent B: EA; gradient in %B: 0 over 2CV, 0 to 30 over 3CV, 30 over
5CV). LC-MS (A): tR = 0.94min; [M-F1-1]-:
321.19.
Example C3.1: (1,3-Dimethyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-y1)-
methanone
C3.1.1. (3-Methyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-y1)-methanone,
hydrochloride salt
A yellowish solution of Example 01.1 (1.45 g) in HCI in dioxane (4M, 10mL) was
stirred at RT for 45min and evaporated
in vacuo to afford the title compound (1.2g) as yellow solid. LC-MS (A): tR =
0.44min; [M+H]-: 246.34.
C3.1.2. (1,3-Dimethyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-y1)-
methanone
The title compound (170mg, yellow resin) was prepared starting from Example
03.1.1 (1.12g), and following the
procedure described in Example B2.1 step B2.1.2. The crude material was
purified by Prep LC-MS (XXIII). LC-MS (A):
tR = 1.09min; [M+H]: 422.40.
Example D1.1: 5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-nicotinonitrile
D1.1.1. 34(5-Bromo-pyridin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-3-methyl-
azetidine-1-carboxylic acid tert-butyl
ester
The title compound was synthesized starting from Example A4.2 and 3,5-
dibromopyridine, following the synthesis
procedure described in Example A7.1 step A7.1.1, and was purified by CC
(Biotage, SNAP 100g, solvent A: Hep;
solvent B: EA; gradient in %B: 0 over 30V, 0 to 60 over 150V). LC-MS (A): tR =
1.12min; [M+H]-: 475.22.
D1.1.2. 34(5-Cyano-pyridin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-3-methyl-
azetidine-1-carboxylic acid tert-butyl
ester
To a solution of Example D1.1.1 (11g) in anhydrous DMF (113mL) under argon
atmosphere were sequentially added
zinc (powder, 268mg), zinc cyanide (4.44g), Pd2(dba)3 (1.48g) and 1,1'-
bis(diphenylphosphino)ferrocene (7.37g). The
reaction mixture was refluxed for 20h, cooled down to RT, quenched with water
and extracted with EA. The combined
org. layers were dried over Na2SO4, filtered off and evaporated to dryness.
The crude was purified by CC (Biotage,
SNAP 100g, solvent A: Hep; solvent B: EA; gradient in %B: 10 over 4.40V, 10 to
22 over 2CV, 22 over 4CV, 22 to 40
over 1CV, 40 over 5.3CV) and by Prep LC-MS (XIX) to afford the title product
as white solid (5.8g). LC-MS (A): tR =
1.09min; [M-FH]-: 422.40.
D1.1.3. 34(R)-(5-Cyano-pyridin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methy11-3-
methyl-azetidine-1-carboxylic acid tert-
butyl ester
Example D1.1.2 (4.5g) was purified by Prep chiral SFC (VIII) to afford the
title compound as pure enantiomer (1.85g).
Chiral SFC (I): tR = 2.53min.
D1.1.4. 54(R)-Hydroxy-(4-isopropyl-pheny1)-(3-methyl-azetidin-3-y1)-
methylpnicotinonitrile, hydrochloride salt
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
168
Example D1.1.3 (1.5g) was dissolved in EA (15mL) and HCI in dioxane (4M,
8.9mL) was added. The reaction mixture
was stirred overnight at RT, concentrated in vacuo and dried under HV. The
residue was directly used in the next step
without further purification. LC-MS (A): tR = 0.69min; [M-FI-1]*: 321.99.
D1.1.5. 5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylknicotinonitrile
To a solution of Example D1.1.4 (1.27g) in dioxane (26mL) were added TEA
(1.49mL) and formaldehyde (37% in water,
0.93mL), followed by NaBH(OAc)3 (1.17g). The reaction mixture was stirred at
RT until completion of the reaction,
filtered off, and the filtrate was purified by Prep LC-MS (XVI) to afford the
title product as white powder (970mg). LC-
MS (A): tR = 0.70min; [M+H]: 336.13.
Example D1.2: 3-{(R)-(5-Cyano-pyridin-3-y1)-hydroxy-[4-(2,2,2-trifluoro-
ethylyphenyl]-methyll-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
The title compound was synthesized starting from Example D1.3 and 4,4,5,5-
tetramethy1-2-(2,2,2-trifluoroethyl)-1,3,2-
dioxaborolane, following the synthesis procedure described in Example 572, and
was purified by Prep LC-MS (XXIX).
LC-MS (A): tR = 1.03 min; [M-FH]': 462.15.
Example D1.3: 3-[(R)-(4-Bromo-pheny1)-(5-cyano-pyridin-3-y1)-hydroxy-methyl]-3-
methyl-azetidine-1-carboxylic acid
tert-butyl ester
01.3.1: 3-[(4-Bromo-pheny1)-(5-cyano-pyridin-3-y1)-hydroxy-methyl]-3-methyl-
azetidine-1-carboxylic acid tert-butyl
ester
The title compound was synthesized starting from Example A4.8 and 5-
bromonicotinonitrile, following the synthesis
procedure described in Example A7.1 step A7.1.1 except that nBuLi was replaced
by HexLi, and was purified by Prep
LC-MS (XXVIII). LC-MS (A): tR = 1.02 min; [M+H]: 459.87.
D1.3.2: 3-[(R)-(4-Bromo-pheny1)-(5-cyano-pyridin-3-y1)-hydroxy-methyl]-3-
methyl-azetidine-1-carboxylic acid tert-butyl
ester
Example D1.3.1 was purified by Prep chiral SEC (XXVII) to afford the title
compound as pure enantiomer. Chiral SEC
(Z): tR = 1.72 min.
Example D1.4: 3-{(5-Cyano-pyridin-3-y1)-hydroxy-[4-(1,2,2,2-tetrafluoro-1-
trifluoromethyl-ethyl)-phenyl]-methyll-3-
methyl-azetidine-1-carboxylic acid tert-butyl ester
The title compound was synthesized starting from Example A4.18 and 5-
bromonicotinonitrile, following the synthesis
procedure described in Example A7.1 step A7.1.1 except that nBuLi was replaced
by HexLi, and was purified by CC
(Biotage, SNAP, solvent A: Hep; solvent B: EA; gradient in %B: 10 over 3CV, 10
to 50 over 13CV). LC-MS (A): tR =
1.11 min; [M-FI-1]': 547.88.
Example D1.5: 34(5-cyanopyridin-3-y1)(hydroxy)(4-(pentafluoro-X6-
sulfaneyl)phenyl)methyl)-3-methylazetidi ne-1-
carboxylic acid tert-butyl ester
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
169
The title compound was synthesized starting from Example A4.19 and 5-
bromonicotinonitrile, following the synthesis
procedure described in Example A7.1 step A7.1.1 except that nBuLi was replaced
by HexLi, and was purified by CC
(Biotage, Star, solvent A: Hep; solvent B: EA; gradient in %B: 12 over 1CV, 12
to 100 over 10CV). LC-MS (A): tR = 1.05
min; [M+H]: 505.86.
Example D2.1: 5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-N-hydroxy-nicotinamidi ne
To a solution of Example D1.1 (970 mg) in Et0H (19 mL) were sequentially added
K2CO3 (1.60 g) and hydroxylamine
hydrochloride (609 mg). The reaction mixture was refluxed for 45 min, cooled
down to RI, and filtered off. The resulting
solution was evaporated in vacuo to afford 1.12 g of the title compound as off-
white solid. LC-MS (A): tR = 0.53min;
[M+H]': 369.25.
Example D2.2: 3-[(R)-Hydroxy-[5-(N-hydroxycarbamimidoy1)-pyridin-3-y1]-(4-
isopropyl-pheny1)-methy1]-3-methyl-
azetidine-1-carboxylic acid tert-butyl ester
The title compound was obtained starting from Example D1.1.3 and following the
procedure described in Example D2.1,
but stirring the reaction mixture at RT. LC-MS (A): tR = 0.81min; [M-FH]':
455.03.
Example D2.3: 3-{(R)-(4-Bromo-pheny1)-hydroxy-[5-(N-
hydroxycarbami midoy1)-pyridin-3-y1]-methy1}-3-methyl-
azetidine-1-carboxylic acid tert-butyl ester
The title compound was obtained starting from Example D1.3 and following the
procedure described in Example D2.1.
LC-MS (A): tR = 0.74min; [M-FH]': 492.95.
Example D2.4: 3-{Hydroxy-[5-(N-hydroxycarbamimidoy1)-pyridin-3-y1H4-(1,2,2,2-
tetrafluoro-1-trifluoromethyl-ethyl)-
phenyl]-methy1}-3-methyl-azetidine-1-carboxylic acid tert-butyl ester
The title compound was obtained starting from Example D1.4 and following the
procedure described in Example D2.1.
LC-MS (A): tR = 0.87min; [M-F1-1]': 580.89.
Example D2.5: 3-(hydroxy(5-(N'-hydroxycarbamimidoyOpyridin-3-y1)(4-
(pentafluoro-X6-sulfaneyl)phenyl)methyl)-3-
methylazetidine-1-carboxylic acid tert-butyl ester
The title compound was obtained starting from Example 01.5 and following the
procedure described in Example 02.1.
LC-MS (A): tR = 0.81 min; [M+H]: 538.93.
Example D2.6: 3-{(R)-Hydroxy-[5-(N-hydroxycarbamimidoy1)-pyridin-3-y1H4-(2,2,2-
trifluoro-ethyl)-phenyl]-methyl}-3-
methyl-azetidine-1-carboxylic acid tert-butyl ester
The title compound was obtained starting from Example 01.2 and following the
procedure described in Example 02.1.
LC-MS (A): tR = 0.79 min; [M+H]: 495.03.
Example D3.1: 1-(acetamido-2,2,2-d3)cyclopropane-1-carboxylic acid
D3.1.1 1-tert-Butoxycarbonylamino-cyclopropanecarboxylic acid benzyl ester
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
170
To a solution of Boc-1-aminocyclopropane-1-carboxylic acid (100mg) in DCM
(2mL) were added DCC (155mg) and
DMAP (31mg) followed by benzyl alcohol (51.5pL). The resulting solution was
stirred overnight at RT. The resulting
mixture was filtrated then basified with aq. sat. NaHCO3 solution and
extracted with DCM. The org. layers were dried
(MgSO4), filtered off and evaporated to dryness. LC-MS (A): tR = 0.94min;
[M+H]: 291.93.
D3.1.2 1-Amino-cyclopropanecarboxylic acid benzyl ester, hydrochloride salt
A solution of Example D3.1.1 (145mg) in HCI in dioxane (4M, 2mL) was stirred
30min at RT. The crude was evaporated
to dryness and used in the next step without further purification. LC-MS (A):
tR = 0.51min; [M-FH]-: 192.25.
D3.1.3 Benzyl 1-(acetamido-2,2,2-d3)cyclopropane-1-carboxylate
To a solution of of Example D3.1.2 (113mg) in DCM (2mL) were added DIPEA (257
pL), HOBt (81.1mg) and EDC.HCI
(115mg) followed by acetic acid-2,2,2-d3 (43pL). The resulting solution was
stirred overnight at RT. Crude was basified
with aq. sat. NaHCO3 solution and extracted with DCM over phase separator. The
resulting solution was evaporated to
dryness. LC-MS (A): tR = 0.66min; [M+H]-: 237.1.
D3.1.4 1-(acetamido-2,2,2-d3)cyclopropane-1-carboxylic acid
To a solution of Example D3.1.3 (117mg) in EA (2mL) was added Pd/C (10% w/w,
50% water, 41.3mg). The resulting
solution was stirred under H2 atmosphere was at RT for 5h, filtered over a
glass paper fiber filter and the filtrate was
concentrated in vacuo and dried under HV. LC-MS (A): tR = 0.41 min.
Example D3.2: 1-(N-methylacetamido-2,2,2-d3)cyclopropane-1-carboxylic acid
D3.2.1 1-(tert-Butoxycarbonyl-methyl-amino)-cyclopropanecarboxylic acid benzyl
ester
To a solution of 1-((tert-Butoxycarbonyl)(methypamino)cyclopropanecarboxylic
acid (100mg) in DMF (1mL) were added
K2CO3 (62.3mg) followed by benzyl bromide (54.6pL). The resulting solution was
stirred overnight at RT. The resulting
mixture was extracted with water and DCM. The org. layers were dried (MgSO4),
filtered off and evaporated to dryness.
LC-MS (A): tR = 1.02min; [M+H]: 305.93.
D3.2.2 1-(N-methylacetamido-2,2,2-d3)cyclopropane-1-carboxylic acid
The title compound was synthesized starting from Example D3.2.1 following the
three-step procedure described in
Example D3.1 steps D3.1.2 to D3.1.4. LC-MS (A): tR = 0.4min; [M-FH]-: 161.15.
Example D3.3 to Example D3.8 and Example D3.12 were synthesized starting from
the appropriate commercial amine
and acid reagents following the procedure described in Example D3.2. LC-MS
data of Example D3.3 to Example D3.8
and Example D3.12 are listed in the table below. The LC-MS conditions used
were LC-MS (A).
Example
Name tR
[M+H]-'
N
D3.3 N-(acetyl-d3)-N-methylglycine 0.27
135.42
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
171
D3.4 (2-Hydroxy-acetylamino)-acetic acid No UV trace
D3.5 (acetyl-d3)glycine No UV trace
D3.6 1-(acetyl-d3)azetidine-3-carboxylic acid 0.31 146.94
D3.7 1-(acetyl-d3)-3-methylazetidine-3-carboxylic acid 0.38 161.16
D3.8 1-(acetyl-d3)-3-fluoroazetidine-3-carboxylic acid 0.23 164.84
D3.12 1-(Acetyl-methyl-amino)-cyclopropanecarboxylic acid 0.4 158.16
Example D3.9: (S)-1-Acetyl-pyrrolidine-3-carboxylic acid
D3.9.1 (S)-Pyrrolidine-1,3-dicarboxylic acid 3-benzyl ester 1-tert-butyl ester
The title compound (275mg) was synthesized starting from (S) 1 N Boc beta
proline (200mg) following the procedure
described in Example D3.2 steps D3.2.1. LC-MS (A): tR = 0.99min; [M+H]:
305.84.
D3.9.2 (S)-Pyrrolidine-3-carboxylic acid benzyl ester
A solution of Example D3.9.1 (275mg) in HCI in dioxane (4M, 3mL) was stirred
2h at RT. The crude was evaporated to
dryness and used in the next step without further purification. LC-MS (A): tR
= 0.52min; [M-FH]-: 206.19.
D3.9.3 (6)-1-Acetyl-pyrrolidine-3-carboxylic acid benzyl ester
To a solution of Example 03.9.2 (185mg) in THF (3mL) were added DIPEA (308pL)
and acetyl chloride (64.7pL),
mixture was stirred 2h at RT. The crude was evaporated to dryness and placed
under HV. LC-MS (A): tR = 0.76min;
[M-F1-1]: 248.13.
D3.9.4 (S)-1-Acetyl-pyrrolidine-3-carboxylic acid
The title compound (141.6mg) was synthesized starting from Example D3.9.3
(223mg) following the procedure
described in Example 03.1 step 03.1.4. LC-MS (A): tR = 0.36min; [M-FH]-:
158.17.
Example D3.10 and Example D3.11 were synthesized starting from the appropriate
commercially available acid
following the procedure described in Example 03.9. LC-MS data of Example 03.10
and Example 03.11 are listed in
the table below. The LC-MS conditions used were LC-MS (A).
Example
Name tR
[M+H]
D3.10 (R)-1-Acetyl-pyrrolidine-3-carboxylic acid 0.35 158.15
D3.11 2-Acetylamino-2-methyl-propionic acid 0.31 146.23
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
172
Example D3.13: 1-Acetyl-4-hydroxy-piperidine-4-carboxylic acid
D3.13.1 4-Hydroxy-piperidine-4-carboxylic acid, hydrochloride salt
N-Boc-4-hydroxy-4-piperidinecarboxylic acid (124 mg) was treated with a
solution of HCI in dioxane (4 M, 1.25 mL) and
stirred at RT for 2.5 h. The mixture was concentrated and dried under HV to
give the desired product as a white solid
(87 mg). LC-MS (A): tR = 0.17 min; [M+H]': 146.09.
03.13.2 1-Acetyl-4-hydroxy-piperidine-4-carboxylic acid
To a mixture of Example 03.13.1 (87 mg) and 4-dimethylaminopyridine (59 mg) in
THF (0.5 mL) was added acetic
anhydride (55 mg) and NEt3 (0.20 mL). The mixture was heated at 60 C for 90
min. After cooling to RT, the reaction
was treated with aq. HCI (1 M) and extracted with EA/Me0H (9:1) five times.
The combined organic layers were dried
over MgS0.4, filtered, concentrated and dried under HV to give the title
compound as a white solid (87 mg). LC-MS (A):
tR = 0.47 min; [M+MeCN+H]-: 230.19.
Example D3.14: 1-Acetyl-3-methoxy-piperidine-4-carboxylic acid
D3.14.1 3-Methoxy-piperidine-4-carboxylic acid
1-[(tert-butoxy)carbonyI]-3-methoxypiperidine-4-carboxylic acid (200 mg) was
treated with a solution of HCI in dioxane
(4 M, 2 mL) and stirred for 30 min. The reaction mixture was concentrated
under vacuo to give the desired product as
a slightly yellow solid (193 mg). LC-MS (A): tR = 0.19 min; [M-FH]': 160.12.
D3.14.2 1-Acetyl-3-methoxy-piperidine-4-carboxylic acid
To a mixture of Example 03.14.1 (193 mg) and Et3N (0.16 mL) in DCM (2.5 mL)
was added acetic anhydride (0.11 mL).
The reaction was stirred at RT for 2 h, then concentrated under vacuo. The
crude was recrystallized from hot Et0H to
give the title compound as white crystals (61 mg). LC-MS (A): tR = 0.38 min;
[M+1-1]+: 202.32.
Example D3.15: (R)- or (S)-1-Methyl-5-oxo-pyrrolidine-3-carboxylic acid
D3.15.1 (R)- or (S)-1-Methyl-5-oxo-pyrrolidine-3-carboxylic acid ethyl ester
A mixture of rac-1-methyl-5-oxopyrrolidine-3-carboxylic acid (500 mg), H2SO4
(0.5 mL) in Et0H (5 mL) was stirred at
RT for 1 h. The solvent was evaporated and the residue was purified by prep LC-
MS (X001) to give the racemic
mixture. To obtain the enantiomeric pure compound the racemate was subjected
to prep chiral SFC (=KV). The
second eluting product was isolated to give the title compound. LC-MS (A): tR
= 0.50 min; [M+H]t 172.06. Chiral SFC
(AD): tR = 2.23 min.
03.15.2 (R)- or (S)-1-Methyl-5-oxo-pyrrolidine-3-carboxylic acid
To a solution of Example 03.15.1 (34 mg) in THF (1 mL) was added a solution of
LiOH in H20 (0.8 mL). The mixture
was stirred at RT for 16 h, extracted with DCM and EA. The combined organic
layers were concentrated and the residue
was used in the next step without further purification. LC-MS (A): tR = 0.32
min; [M+H]-: 144.12.
Example 03.16: rac-1-Ethyl-5-oxo-pyrrolidine-3-carboxylic acid
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
173
Example D3.16 was synthesized from rac-1-Ethy1-5-oxo-pyrrolidine-3-carboxylic
acid methyl ester through
saponification, which is described for Example 03.15, without chiral
separation. LC-MS (A): tR = 0.38 min; [M+1-1]-:
158.10.
Example D3.17: (S)- or (R)-1-lsopropy1-5-oxo-pyrrolidine-3-carboxylic acid
Example D3.17 was synthesized from rac-5-oxo-1-(propan-2-yl)pyrrolidine-3-
carboxylic acid, analogously to the 2-step
procedure described for Example 03.15. The racemic ester in step 1 was
purified by prep LC-MS (XV + )(X(IV), the
enantiomers were separated by prep chiral SFC (=NI). The ester of the title
compound was the first eluting
enantiomer. LC-MS (A): tR = 0.44 min; [M-FH]-: 171.99.
Example D3.18: (R)- or (S)-1-lsopropy1-5-oxo-pyrrolidine-3-carboxylic acid
Example 03.18 was synthesized from rac-5-oxo-1-(propan-2-yl)pyrrolidine-3-
carboxylic acid, analogously to the 2-step
procedure described for D3.15. The racemic ester in step 1 was purified by
prep LC-MS (XV + XXX I V) , the enantiomers
were separated by prep chiral SFC (XXXVI). The ester of the title compound was
the second eluting enantiomer. LC-
MS (A): tR = 0.44 min; [M+1-1]': 172.00.
Example 03.19: ((8)-1-Acetyl-pyrrolidin-3-y1)-acetic acid
D3.19.1 (S)-Pyrrolidin-3-yl-acetic acid, hydrochloride salt
(S)-(1-Boc-pyrrolidin-3-yI)-acetic acid (325 mg) was treated with a solution
of HCI in dioxane (4 M, 3.5 mL) and stirred
at RT for 1 h. The reaction was diluted with EA and stirred for 30 min. The
suspension was filtered and washed with EA
to give the desired compound as a white solid (182 mg). LC-MS (A): tR = 0.22
min; [M-FH]-: 130.07.
D3.19.2 ((S)-1-Acetyl-pyrrolidin-3-yI)-acetic acid
To a suspension of Example D3.19.1 (60 mg) in THF (2 mL) was added acetyl
chloride (30 mg) and NEt3 (0.10 mL).
The resulting mixture was stirred at RT for 30 min. The reaction was filtered,
the residue was washed with EA/dioxane
(3:1) and the combined filtrates were purified by prep LC-MS (IX) to give the
title compound as a white solid (27 mg).
LC-MS (A): tR = 0.42 min; [M+H]-: 172.02.
Example D4.1: 34(R)-(4-Bromo-pheny1)-hydroxy-{545-(1-hydroxy-1-methyl-
ethy1)41,2, 4]oxadiazol-3-y1]-pyridi
methyl)-3-methyl-azetidine-1-carboxylic acid tert-butyl ester
The title compound was synthesized starting from Example 02.3 and 2-
hydroxyisobutiric acid, following the procedure
described in Example 134, and was purified by CC (Biotage, Star, solvent A:
Hep; solvent B: EA; gradient in %B: 18
over 1CV, 18 to 100 over 6CV). LC-MS (A): tR = 0.99 min; [M+1-1]': 558.98.
Example 04.2: 3-{(R)-Hydroxy-{5-[5-(1-hydroxy-1-methyl-ethyly[1,2, 4]oxadiazol-
3-A-pyridi n-3-y11-[4-(1, 2,2,2-
tetrafluoro-1-trifluoromethyl-ethyl)-pheny1]-methy11-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
D42.1 3-{Hydroxy-{5-15-(1-hydroxy-1-methyl-ethyl)-11,2,47oxadiazol-3-yll-
pyridin-3-y1J-14-(1,2,22-tetrafluoro-1-
trifluoromethyl-ethyl)-phenyll-methyll-3-methyl-azetidine-1-carboxylic acid
tert-butyl ester
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
174
The title compound was synthesized starting from Example D2.4 and 2-
hydroxyisobutiric acid, following the procedure
described in Example 134, and was purified by by Prep LC-MS (XI). LC-MS (A):
tR = 1.09 min; [M-FI-1]': 649.04.
D4.2.2 3-{(R)-Hydroxy-{515-(1-hydroxy-1-methyl-ethy1)41 ,2,47oxadiazol-3-
ylrpyridin-3-014-(1 , 2,2, 2-tetraf uoro-1 -
trifluoromethyl-ethyl)-phenylpmethy11-3-methyl-azetidine-1-carboxylic acid
tert-butyl ester
Example D4.2.1 was purified by Prep chiral SFC (XXVIII) to afford the title
compound as pure enantiomer. Chiral SFC
(AA): tR = 1.79 min.
Example D4.3: (R)-3-(hydroxy(5-(5-(2-hydroxypropan-2-y1)-1,2,4-
oxadiazol-3-yl)pyridin-3-y1)(4-(pentafluoro-X6-
sulfaneyl)phenyl)methyl)-3-methylazetidine-1-carboxylic acid tert-butyl ester
D4.3.1 3-(hydroxy(5-(5-(2-hydroxypropan-2-y1)-1,2,4-oxadiazol-3-yOpyridin-3-
y1)(4-(pentafluoro-26- sulfaneyl)pheny0
methyl)-3-methylazetidine-1-carboxylic acid tert-butyl ester
The title compound was synthesized starting from Example D2.5 and 2-
hydroxyisobutiric acid, following the procedure
described in Example 134, and was purified by by Prep LC-MS (VI). LC-MS (A):
tR = 1.03 min; [M+H]: 607.04.
D4.3.2 (R)-3-(hydroxy(5-(5-(2-hydroxypropan-2-y1)-1,2,4-oxadiazol-3-yOpyridin-
3-y1)(4-(pentafluoro-26-sulfaney1)
phenyOrnethyl)-3-methylazetidine-1-carboxylic acid tert-butyl ester
Example D4.3.1 was purified by Prep chiral SFC (XXIX) to afford the title
compound as pure enantiomer. Chiral SEC
(AB): tR = 1.14 min.
Example D4.4: 3-[(R)-{545-(1-Acetyl-piperidin-4-y1)-[1,2,4]oxadiazol-3-A-
pyridin-3-y11-(4-bromo-pheny1)-hydroxy-
methyl]-3-methyl-azetidine-1-carboxylic acid tert-butyl ester
The title compound was synthesized starting from Example D2.3 and 1-acetyl-4-
piperidinecarboxylic acid, following the
procedure described in Example 134, and was purified by CC (Biotage, SNAP,
solvent A: DCM; solvent B: MeOH;
gradient in %B: 1 over 1CV, 1 to 5 over 100V5 over 3CV). LC-MS (A): tR = 1.03
min; [MA-1]: 627.99.
Example D4.5: 3-{(R)-Hydroxy-{5-[5-(tetrahydro-pyran-4-y1)-[1,2,4]oxadiazol-3-
y1]-pyridin-3-y1144-(2,2,2-trifluoro-
ethyl)-phenyl]-methyll-3-methyl-azetidine-1-carboxylic acid tert-butyl ester
The title compound was synthesized starting from Example D2.6 and
tetrahydropyran-4-carboxylic acid, following the
procedure described in Example 134, and was purified by by Prep LC-MS (XXX).
LC-MS (A): tR = 1.07 min; [M+FI]-:
589.18.
Example 05.1: 2-(3-{5-[(R)-(4-Bromo-pheny1)-hydroxy-(3-methyl-azetidin-3-y1)-
methyl]-pyridin-3-y11-[1,2,4]oxadiazol-
5-y1)-propan-2-ol, hydrochloride salt
The title compound was obtained starting from Example 04.1 and following the
procedure described in Example A7.1
step A7.1.3 except that the reaction mixture was stirred for 18h at RT. LC-MS
(A): tR = 0.62 min; [M-FI-1]': 458.95.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
175
Example D5.2: 243-(5-{(R)-Hydroxy-(3-methyl-azetidin-3-y1)44-(1,2,2,2-
tetrafluoro-1-trifluoromethyl-ethyl)-phenyll-
methyll-pyridin-3-y1)41,2,4]oxadiazol-5-y1]-propan-2-ol, hydrochloride salt
The title compound was obtained starting from Example D4.2 and following the
procedure described in Example A7.1
step A7.1.3 except that the reaction mixture was stirred for 18h at RT. LC-MS
(A): tR = 0.74 min; [M-FH]': 548.97.
Example D5.3: (R) 2 (3 (5 (hydroxy(3-methylazetidin-3-y1)(4-(pentafluoro-X6-
sulfaneyl)phenyl)methyppyridin-3-y1)-
1,2,4-oxadiazol-5-y1)propan-2-ol, hydrochloride salt
The title compound was obtained starting from Example 04.3 and following the
procedure described in Example A7.1
step A7.1.3 except that the reaction mixture was stirred for 18h at RT. LC-MS
(A): tR = 0.69 min; [M-FH]-: 506.93.
Example D5.4: 144-(3-15-[(R)-(4-Bromo-pheny1)-hydroxy-(3-methyl-
azetidin-3-y1)-methylFpyridin-3-y1}-0,2,41
oxadiazol-5-y1)-piperidin-1-y1]-ethanone, hydrochloride salt
The title compound was obtained starting from Example D4.4 and following the
procedure described in Example A7.1
step A7.1.3 except that the reaction mixture was stirred for 18h at RT. LC-MS
(A): tR = 0.66 min; [M-F1-1]+: 525.84.
Example 05.5: (R)-(3-Methyl-azetidin-3-y1)-{545-(tetrahydro-pyran-4-y1)-
[1,2,4]oxadiazol-3-y1]-pyridin-3-y1144-(2,2,2-
trifluoro-ethyl)-phenyl]-methanol, hydrochloride salt
The title compound was obtained starting from Example D4.5 and following the
procedure described in Example A7.1
step A7.1.3 except that the reaction mixture was stirred for 18h at RT. LC-MS
(A): tR = 0.70 min; [M+1-1]+: 489.12.
Example E1.1: 5-[(R)-(1-tert-Butoxycarbony1-3-methyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-pheny1)-methyll-nicotinic
acid
E1.1.1. 5-[(1-tert-Butoxycarbony1-3-methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methylknicotinic acid tert-butyl
ester
The title compound was synthesized starting from Example A4.2 and tert-butyl 5-
bromonicotinate, following the
synthesis procedure described in Example A4.1 step A4.1.2, and purified twice
by CC (Biotage, SNAP 330g, solvent
A: Hep; solvent B: EA; gradient in %B: 0 over 2min, 0 to 10 over 3min, 10 over
5min, 10 to 100 over 25min, 100 over
5min) followed by Prep LC-MS (II). LC-MS (A): tR = 1.14min; [M-FH]-: 497.26.
E1.1.2. 5-[(R)-(1-tert-Butoxycarbony1-3-methyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-pheny1)-methylknicotinic acid tert-
butyl ester
Example E1.1.1 (14.8g) was purified by Prep chiral SFC (1) to afford the title
compound as pure enantiomer (5.97g) as
beige solid. LC-MS (A): tR = 1.14min; [M+H]: 497.26. Chiral SEC (A): tR =
2.795 min.
E1.1.3. (R)-5-(hydroxy(4-isopropylphenyl)(3-methylazetidin-3-
yOrnethyOnicotinic acid, hydrochloride salt
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
176
A solution of Example E1.1.2 (5.97g) in HCI in dioxane (4M, 120mL) was stirred
at RT until completion of the reaction.
The mixture was concentrated to dryness and dried under HV. The resulting
white solid (5g) was directly used in the
next step without further purification. LC-MS (A): tR = 0.58min; [M-FH]-:
341.17.
E1.1.4. 5-[(R)-(1-tert-Butoxycarbonyl-3-methyl-azetidin-3-y0-hydroxy-(4-
isopropyl-phenyi)-methylknicotinic acid
To a suspension of Example E1.1.3 (5g) and di-tert-butyl dicarbonate (3.23g)
in THF (60mL) was added dropwise TEA
(3.4mL). The reaction mixture was stirred at RT for 1h and concentrated in
vacuo. The residue was purified by Prep
LC-MS (111) to afford the desired compound as white solid (4g). LC-MS (A): tR
= 0.92min; [M-F1-1]: 441.2.
Example E1.2 to Example E1.3 were synthesized starting from the appropriate
compound of Formula (A4) and
following the four-step procedure described in Example E1.1. LC-MS data of
Example E1.2 to Example E1.3 are listed
in the table below. The LC-MS conditions used were LC-MS (A). The preparative
and chiral SFC methods used in the
second step, and the preparative LC-MS methods used in the last step are
indicated in the table below.
Prep tR
Example Prep
Name tR [M-F1-1]* chiral
(Chiral
N
LC-MS
SFC SFC)
5-[(R)-(1-tert-Butoxycarbony1-3-methyl-
E1.2 azetidin-3-y1)-(4-cyclopropyl-pheny1)- 0.88 439.25 (IV)
2.01 (D) (XIV)
hydroxy-methyl]-nicotinic acid
5-[(R)-(1-tert-Butoxycarbony1-3-methyl-
E1.3 azetidin-3-y1)-hydroxy-(4-propyl-pheny1)- 0.92 441.26
(VI) 1.80 (F) (111)
methyl]-nicotinic acid
Example E2.1: N-Hydroxy-tetrahydro-pyran-4-carboxamidine
To a solution of 4-cyanotetrahydro-4H-pyran (389mg) in Et0H (9mL) and water
(2mL) were sequentially added K2003
(726mg) and hydroxylamine hydrochloride (730mg). The reaction mixture was
refluxed for 20h, cooled down to RT, and
filtered off. The solid was further washed with Et0H and the resulting
solution was concentrated in vacuo. EA was
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
177
added to the residue and the resulting mixture was concentrated and dried
under HV to afford the title compound as
yellow solid (494mg). LC-MS (A): tR = 0.21min; [M-FH]F: 145.27.
Example E2.2 to Example E2.13 were synthesized starting from the appropriate
cyano derivative and following the
procedure described in Example E2.1. LC-MS data of Example E2.2 to Example
E2.13 are listed in the table below.
The LC-MS conditions used were LC-MS (A).
Example
Name tR [M-
F1-1]*
N
E2.2 N-Hydroxy-3-methoxy-2,2-dimethyl-propionamidine 0.30 147.25
E2.3 N-Hydroxy-4-methoxy-tetrahydro-pyran-4-carboxamidine 0.25 175.39
E2.4 N-Hydroxy-3-hydroxymethyl-bicyclo[1.1.1]pentane-1-carboxamidine
0.22 157.15
E2.5 N-Hydroxy-2-morpholin-4-yl-acetamidine 0.20 160.22
E2.6 2-(2,6-Dimethyl-morpholin-4-y1)-N-hydroxy-acetamidine 0.26
188.40
E2.7 N-Hydroxy-4-methyl-tetrahydro-pyran-4-carboxamidine 0.24 159.26
E2.8 (1S,23,4R)-N-Hydroxy-7-oxa-bicyclo[2.2.1]heptane-2-carboxamidine
0.26 157.24
E2.9 3,N-Dihydroxy-2,2-dimethyl-propionamidine 0.20 133.23
E2.10 2,N-Dihydroxy-2-methyl-propionamidine 0.19 119.25
E2.11 N-Hydroxy-2-methoxy-2-methyl-propionamidine 0.21 133.23
E2.12 N-Hydroxy-1-methoxy-cyclobutanecarboxamidine 0.26 145.15
E2.13 3,N-Dihydroxy-3-methyl-butyramidine 0.20 133.26
Example E2.14: 4-(N-HydroxycarbamimidoyI)-piperidine-1-carboxylic acid benzyl
ester
To a solution of 1-N-Cbz-4-cyanopiperidine (100mg) in Et0H (4mL) were added
hydroxylamine hydrochloride (42.2mg)
and DIPEA (110pL), mixture was stirred 41h at 80 C. Reaction mixture was
evaporated to dryness, resulting crude
was extracted with EA and water, organic phase was washed with brine. The
combined org. layers were dried (MgSO4),
filtrated off, evaporated and dried under HV to give 104mg of the title
compound as a pale yellow resin. LC-MS (A): tR
= 0.59min; [M-F11-: 278.17.
Example E2.15: (E)-1-acetyl-N'-hydroxypiperidine-4-carboximidamide
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
178
To a solution of 1-acetylpiperidine-4-carbonitrile (955mg) in DMSO (5mL) were
added hydroxylamine hydrochloride
(621mg) and Et3N (1.66mL), the resulting mixture was stirred 10h at 90 C and
filtrated. Filtrate was lyophilisated,
resulting oil was stripped in MeCN, resulting precipitate was filtered and
washed with MeCN. The title compound was
obtained as 620mg of an off-white solid. LC-MS (A): tR = 0.24min; [M+H]:
186.28.
Example E2.16: 4,N-Dihydroxy-tetrahydro-pyran-4-carboxamidine
To a solution of 4-hydroxy-4-carbonitrile (200 mg) in Et0H (8 mL) were added
hydroxylamine hydrochloride (315 mg)
and K2003 (826 mg). The mixture was stirred at 80 C for 40 h, cooled to RT,
filtered, concentrated and dried under
HV to give the title compound as a pale sticky solid (231 mg). LC-MS (A): tR =
0.19 min; [M-FH]*: 161.07.
Example E2.17 to Example E2.20 were synthesized according to the procedure
described for Example E2.16 from the
corresponding nitriles. If necessary, purification was performed with prep LC-
MS. Retention times and observed masses
of the products, as well as purification methods (if applicable) are given in
the table below.
Example
prep LC-MS
Name tR [M+H]+
N
Method
E2.17 2-tert-Butoxy-N-hydroxy-acetamidine 0.33 147.20
-
E2.18 4-Acetyl-N-hydroxy-piperazine-1-carboxamidine 0.21
187.35 (XXXII)
4-(N-Hydroxycarbamimidoy1)-piperidine-1-carboxylic acid tert-
E2.19 0.51 244.28 -
butyl ester
E2.20 N-Hydroxy 2 (4 hydroxy-tetrahydro-pyran-4-yI)-acetamidine
0.20 175.34 -
Example E2.21: 1-[2-(tert-Butyl-diphenyl-silanyloxy)-acety1]-N-hydroxy-
piperidine-4-carboxamidine
E2,21.1 Piperidine-4-carbonitrile, hydrochloride salt
N-Boc-piperidine-4-carbonitrile (600 mg) was treated with a solution of HCI in
dioxane (4 M, 7.2 mL) and stirred at RT
for 2 h. The reaction mixture was concentrated and dried under HV to give the
desired product as a pale solid (455 mg).
LC-MS (A): tR = 0.20 min; [M+H]-: 111.17.
E2,21 .2 (tert-Butyl-diphenyl-silanyloxy)-acetic acid
To a solution of glycolic acid (2.00 g), N,N-dimethy1-4-aminopyridine (318
mg)and NEt3 (10.9 mL) in THF (100 mL) was
added tert-butyl(chloro)diphenylsilane (7.6 mL) dropwise at 0 C. The mixture
was allowed to warm to RT and was
stirred for 16 h. The reaction mixture was acidified with aq. HCI (1M) until
pH 1 was reached. The solution was extracted
with Et20 (3 times) and the combined organic layers were dried over MgSO4,
filtered and concentrated under vacua to
give a colorless oil, which was purified by CC (CombiFlash, RediSep 330 g,
gradient of n-Heptane/EA 100:0 to 70:30
over 25 min at 200 mL/min) to yield the desired product as a colorless oil
(7.05 g). LC-MS (A): tR = 1.05 min; product
mass was not observed; 1H-NMR (500 MHz, DMSO-d6): 5 = 12.56 (br s, 1 H), 7.67-
7.63 (m, 4 H), 7.51-7.41 (m, 6 H),
4.19 (s, 2 H), 1.02 (s, 9 H) ppm.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
179
E2,21.3 1-12-(tert-Butyl-diphenyl-silanyloxy)-acetyll-piperidine-4-
carbonitrile
To a suspension of Example E2.21.1 (455 mg), Example E2.21.2 (1.27 g) and HATU
(1.89 g) in THF (8.5 mL) was
added DIPEA (1.59 mL). The reaction was stirred at RT for 4 h. Then it was
diluted with EA, washed with aq. HCI (1
M), sat. aq. NaHCO3, water and brine, dried over Na2SO4, filtered and
concentrated under vacuo. The residue was
purified by CC (CombiFlash, RediSep 220 g, gradient of n-Heptane/EA 100:0 to
0:100 over 30 min at 150 mL/min) to
give the desired product as a colorless oil (968 mg). LC-MS (A): tR = 1.09
min; [M+H]: 407.27.
E2.21.4 142-(tert-Butyl-diphenyl-silanyloxy)-acetyll-N-hydroxy-piperidine-4-
carboxamidine
To a solution of Example E2.21.3 (968 mg) in Et0H (8 mL) were added
hydrodxylamine hydrochloride (501 mg) and
K2003 (1.32 g). The reaction mixture was stirred at 80 C for 16 h. The
reaction mixture was concentrated under vacuo
to give a yellow oil, which was purified by prep LC-MS (XIV) to give the title
compound as a white solid (79 mg). LC-MS
(A): tR = 0.83 min; [M-F1-1]': 440.32.
Example E2.22: 2-(1-Acetyl-azetidin-3-yI)-N-hydroxy-acetamidine
E2.22.1 Azetidin-3-yl-acetonitrile, hydrochloride salt
3-Cyanomethyl-azetidine-1-carboxylic acid tert-butyl ester (500 mg) was
treated with a solution of HCI in dioxane (4 M,
6.1 mL). The mixture was stirred for 1 h, concentrated and dried under HV to
give the desired product as a yellow
viscous oil (474 mg). 1H-NMR (500 MHz, DMSO-d6): 5 = 9.18 (br s, 2 H) 4.06-
3.98 (m, 2 H), 3.75-3.67 (m, 2 H), 3.10
(hept, J = 7.6 Hz, 1 H), 2.93 (d, J = 7.0 Hz, 2 H) ppm.
E2.22.2 (1-Acetyl-azetidin-3-y1)-acetonitrile
A mixture of Example E2.22.1 (231 mg) and K2CO3 in THF (5 mL) was cooled to 0
C before acetyl chloride (138 mg)
was added dropwise. The reaction was allowed to warm to RT and was stirred for
1 h. The mixture was filtered, the
residue was washed with THF, suspended in Me0H, sonicated and filtered. The
filtrate was concentrated under vacuo
to give the desired product as an orange sticky solid (226 mg). It was used
for the next step without further purification.
LC-MS (A): tR = 0.36 min; [M+H]-: 139.14.
E2.22.3 2-(1-Acetyl-azetidin-3-y1)-N-hydroxy-acetamidine
The title compound was synthesized from Example E2.22.2 according to the
procedure described for Example E2.16.
A yellow sticky solid was obtained. LC-MS (A): tR = 0.21 min; [M+H]t 171.87.
Example F1.1: (5-Bromo-pyridin-3-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-isopropyl-
pheny1)-methanol
F1.1 .1 (5-Bromo-pyridin-3-y1)-(4-isopropyl-pheny1)-(3-methyl-azetidin-3-y1)-
methanol
A pale yellow solution of Example 01.1.1 (2.15g) in HCI in dioxane (4M, 10mL)
was stirred for 2h at RT. The reaction
mixture was cooled at 0 C, slowly basified with aq. sat. NaHCO3 solution and
extracted with DCM. The org. phases
were dried (MgSO4), filtrated off and evaporated to dryness to give the title
compound (1.76 g). LC-MS (A): tR = 0.74min;
[M+H]: 375.19.
F1.1.2. (5-Bromo-pyridin-3-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-isopropyl-
pheny1)-methanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
180
To a light yellow solution of Example F1.1.1 (1.75g) in THF (40mL), AcOH
(0.4mL) was added at RT, followed
successively by formaldehyde (37% in water, 0.695 mL) and NaBH(OAc)3 (1.53g).
The resulting solution was stirred
for 1h10 at RT, was basified with aq. sat. NaHCO3 solution, diluted with
water, and extracted with EA. The org. layers
were dried (MgSO4), filtered off, and evaporated to dryness to afford 1.91g of
the title compound as beige solid. LC-MS
(A): tR = 0.76min; [M+H]': 389.22.
Example F1.2: (R)-(5-Bromo-pyridin-3-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-
isopropyl-phenyl)-methanol
F1.2.1. 3-[(R)-(5-Bromo-pyridin-3-0)-hydroxy-(4-isopropyl-pheny1)-methylf-3-
methyl-azetidine-1-carboxylic acid tert-
butyl ester
The title compound was obtained by chiral separation of Example 01.1.1 using
Prep chiral SFC (111). LC-MS (A): tR =
1.12min; [M-F1-1]': 475.20; Chiral SFC (C): 1.4min.
F1.2.2. (R)-(5-Bromo-pyridin-3-y1)-(4-isopropyl-pheny1)-(3-methyl-azetidin-3-
y1)-methanol, dihydrocloride salt
A pale yellow solution of Example F1.2.1 (3.7g) in HCI in dioxane (4M, 27mL)
was stirred 2h at RT and evaporated to
dryness to give the title compound (3.98g) as white solid. LC-MS (A): tR =
0.73min; [M+H]: 375.13
Fl .2.3. (R)-(5-Bromo-pyridi n-3-y1)-(1 ,3-dimethyl-azetidi n-3-y1)-(4-
isopropyl-pheny1)-methanol
The title compound was synthesized starting from Example F1.2.2 and following
the synthesis procedure described in
Example F1.1 step F1.1.2, additional equivalents of formaldehyde and
NaBH(OAc)3 were added until completion of the
reaction. LC-MS (A): tR = 0.74min; [WEN': 389.18.
Example F1.3: (2-Chloro-pyridin-4-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-
isopropyl-pheny1)-methanol
F1.3.1. 342-Chloro-pyridin-4-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-3-methyl-
azetidine-1-carboxylic acid tert-butyl
ester
The title compound (516mg, colorless foam) was synthesized starting from
Example A4.2 (415mg) and 4-bromo-2-
chloropyridine (200pL), and following the procedure described in Example A7.1
step A7.1.1. The crude material was
purified by CC (Biotage, SNAP 40g, solvent A: Hep; solvent B: EA; gradient in
%B: 0 to 50 over 20min). LC-MS (A): tR
= 1.12min; [M-FH]-: 431.31.
F1.3.2. (2-Chloro-pyridin-4-y1)-(4-isopropyl-pheny1)-(3-methyl-azetidin-3-y1)-
methanol, hydrochloride salt
A solution of Example F1.3.1 (644mg) in HCI in dioxane (4M, 5mL) and Me0H
(2.5mL) was stirred at RT for 15min and
evaporated in vacuo. The resulting residue was triturated in Et20 and the
solvent was evaporated in vacuo to afford the
title compound (650mg) as yellow foam. LC-MS (A): tR = 0.72min; [M-FH]:
331.27.
F1.3.3. (2-Chloro-pyridin-4-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-isopropyl-
pheny1)-methanol
The title compound (483mg, beige foam) was synthesized starting from Example
F1.3.2 (650mg), and following the
procedure described in Example F1.1 step F1.1.2. LC-MS (A): tR = 0.75min;
[M+H]-: 345.27.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
181
Example F1.4: 3-[(S)-(2-Chloro-pyridin-4-y1)-hydroxy-(4-isopropyl-pheny1)-
methy11-3-methyl-azetidine-1-carboxylic
acid tert-butyl ester
The title compound was obtained by chiral separation of Example F1.3.1 using
Prep Chiral SFC (XVII). LC-MS (A): tR
= 1.12min; [M-FH]': 431.36; Chiral SFC (Q): 1.31min.
Example F1.5: (S)-(2-Chloro-pyridin-4-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-
isopropyl-pheny1)-methanol
The title compound (852mg, white solid) was synthesized starting from Example
F1.4 (1.1g), and following the
procedure described in Example F1.3 step F1.3.2 and F1.3.3. The crude material
was purified by Prep LC-MS (XIII).
LC-MS (A): tR = 0.74min; [M-FH]-: 345.16.
Example F1.6: (R)-(6-Chloro-pyridazin-4-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-
isopropyl-pheny1)-methanol
F1.6.1. 3-[(6-Chloro-pyridazin-4-y1)-hydroxy-(4-isopropyl-phenyl)-methyll-3-
methyl-azetidine-1 -carboxylic acid tert-
butyl ester
The title compound (4.34g, brown solid) was synthesized starting from Example
A4.2 (8.5g) and 5-bromo-3-
chloropyridazine (6g), and following the procedure described in Example A7.1
step A7.1.1, however using toluene
instead of THF as solvent. The crude material was purified by CC (Biotage,
SNAP 330g, solvent A: Hep; solvent B: EA;
gradient in %B: 0 to 50 over 40 min). LC-MS (A): tR = 1.06min; [M+H]: 432.22.
F1.6.2. 3-[(R)-(6-Chloro-pyridazin-4-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
3-methyl-azetidine-1-carboxylic acid tert-
butyl ester
The title compound was obtained by chiral separation of Example F1.6.1 using
Prep Chiral SFC (XVIII). LC-MS (A). tR
= 1.05min; [M+H]-: 432.22; Chiral SFC (R): 1.26min.
F1.6.3. (R)-(6-Chloro-pyridazin-4-0)-(4-isopropyl-phenyl)-(3-methyl-azetidin-3-
y0-methanol, hydrochloric salt
A solution of Example F1.6.2 (377mg) in HCI in dioxane (4M, 7mL) was stirred
at RT for 2h and evaporated in vacuo.
The crude material was evaporated to dryness to give 351mg of the title
compound as yellow solid. LC-MS (A): tR =
0.67min; [M+H]-: 332.13.
F1.6.4. (R)-(6-Chloro-pyridazin-4-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-
isopropyl-phenyl)-methanol
The title compound (215mg, beige foam) was synthesized starting from Example
F1.6.3, and following the procedure
described in Example F1.1 step F1.1.2, however additional equivalents of
formaldehyde and NaBH(OAc)3 were added
until completion of the reaction. LC-MS (A): tR = 0.67min; [M-F1-1]: 346.10.
Example F1.7 to Example F1.10 were synthesized starting from appropriate
compound of Formula (A4) and 3,5-
dibromopyridine, following the four-step procedure described in Example F1.6.
Chiral Prep SFC conditions used in step
2, Prep LC-MS conditions used in step 4 and LC-MS data of Example F1.7 to
Example F1.10 are listed in the table
below. The LC-MS conditions used were LC-MS (A).
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
182
Example Prep LC-
Chiral Prep
Name tR [M-FI-I]- (A4)
N MS step 4
SFC step 2
(R)-(5-Bromo-pyridin-3-yI)-(1,3-dimethyl-
F1.7 azetidin-3-y1)-[4-(1-methyl-cyclopropy1)- 0.77 401.13
A4.6 none (111)
phenyl]-methanol
(R)-(5-Bromo-pyridin-3-yI)-(1,3-dimethyl-
F1.8 azetidin-3-yI)-[4-(1-trifluoromethyl- 0.79 455.16
A4.7 none (XO()
cyclopropyI)-phenyl]-methanol
(R)-(5-Bromo-pyridin-3-yI)-(4-tert-butyl-
F1.9 pheny1)-(1,3-dimethyl-azetidin-3-y1)- 0.8 403.23
A4.3 (VII) (XIX)
methanol
(R)-(5-Bromo-pyridin-3-yI)-(1,3-dimethyl-
F1.10 0.72 377.19 A4.4 none (XIX)
azetidin-3-y1)-(4-ethyl-pheny1)-methanol
Example F1.11: 3-[(R)-(5-Bromo-pyridin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-3-methyl-azetidine-1-carboxylic
acid tert-butyl ester
Example D1.1.1 (9g) was purified by Prep chiral SFC (111) to afford the title
compound as pure enantiomer (4.31g).
Chiral SFC (C): tR = 1.377min. LC-MS (A): tR = 1.12min; [M-FH]-: 475.20.
Example F1.12 to Example F1.14 were synthesized starting from appropriate
compound of Formula (A7) following the
procedure described in Example 01.1 step 01.1.5. The LC-MS data are listed in
the table below. The LC-MS conditions
used were LC-MS (A).
Example N Name tR [M-FH]-
(A7)
(R)-(5-Bromo-pyridin-3-yI)-(1,3-
F1.12 dimethyl-azetidin-3-yI)-[4-(2,2,2- 0.71
428.93 A7.36
trifluoro-ethyl)-phenyl]-methanol
(R)-(5-Bromo-pyridin-3-y1)-(3-ethy1-1-
F1.13 methyl-azetidin-3-yI)-(4- 0.76
447.02 A7.35
trifluoromethoxy-phenyI)-methanol
(S)-(5-Bromo-pyridin-3-yI)-(3-fluoro-1-
F1.14 methyl-azetidin-3-yI)-(4-isopropyl- 0.74
393.03 A7.37
phenyI)-methanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
183
Example F1.15 (R)-(5-Bromo-pyridin-3-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-
trifluoromethyl-pheny1)-methanol was
synthesized starting from Example A7.38 following the procedure described in
Example 01.1 step 01.1.5 and purified
by prep LC-MS (VI). LC-MS (A) tR = 0.72min; [M-FH]': 415.22.
Example F3.1 (for synthesis, see F4.10): 4-(2-Methoxy-ethyl)-pyrrolidin-2-one
Example F3.2: 4-(2-lsopropyl-pyrimidin-4-y1)-pyrrolidin-2-one
F3.2.1. (E)-3-(2-lsopropyl-pyrimidin-4-y1)-acrylic acid ethyl ester
To an ice-cold suspension of NaH (60% in mineral oil, 250mg) in DMF (5.5L) was
added triethyl phosphonoacetate
(0.36mL) and the resulting mixture was stirred for 30 min at 0 C A solution of
2-isopropyl-4-pyrimidinecarbaldehyde
(250mg) in DMF (0.5mL) was added and the resulting suspension was stirred for
1h30 at RT. The reaction mixture was
quenched and diluted with water and extracted with EA (3x). The combined org.
layers were washed with brine, dried
(MgSO4), filtered off and evaporated to dryness. The resulting crude material
(417mg) was used without further
purification. LC-MS (A): tR = 0.89min; [M-F1-1]': 221.11.
F3.2.2. 3-(2-lsopropyl-pyrimidin-4-y1)-4-nitro-butyric acid ethyl ester
To a solution of Example F.3.2.1 (232mg) in nitromethane (0.2mL) was added
tetramethylguanidine (25 mg) at 0 C and
the reaction mixture was allowed to reach RT. After stirring for 27h, the
reaction mixture was diluted with EA and water,
and extracted with EA. The combined org. layers were washed with brine, dried
(MgSO4), filtered off and evaporated to
dryness. The resulting crude material was purified by CC (Biotage, SNAP 10g,
solvent A: DCM; solvent B: DCM/Me0H
8/2; gradient in %B: 0 over 3CV, 0 to 25 over 8CV, 25 for 2CV, 25 to 50 over
3CV, 50 for 2CV) to afford 65mg of the
title compound as yellow oil. LC-MS (A): tR = 0.88min; [M+H]: 282.1.
F3.2.3. 4-(2-lsopropyl-pyrimidin-4-y1)-pyrrolidin-2-one
To a flask charged with Pd/C (30mg) were added a solution of Example F3.2.2
(65mg) in Et0H (0.5mL) and ammonium
formate (94mg) at RT under argon and the resulting suspension was stirred at
80 C for 1h30. The reaction mixture was
allowed to cool down, filtrated through a syringe filter, and the filtrate
evaporated to dryness. The resulting crude material
was purified by CC (Biotage, SNAP 10g, solvent A: DCM; solvent B: DCM/Me0H
8/2; gradient in %B: Dover 3CV, 0 to
25 over 7CV, 25 for 2CV) to afford 16mg of the title compound as colorless
oil. LC-MS (A): tR = 0.52min; [M-FH]-: 206.17.
Example F3.3: 4-(2-Methyl-thiazol-5-y1)-pyrrolidin-2-one
F3.3.1. (E)-3-(2-Methyl-thiazol-5-y1)-acrylic acid octyl ester
To a solution of 5-bromo-2-methylthiazole (250mg) in DMF (7.5mL) were added
successively n-octyl acrylate (388mg),
DABCO (6.6mg), K2003 (194mg), Pd(OAc) 2 (6.4mg). The resulting brown
suspension was stirred at 120 C for 21h.
After cooling to RT, the reaction mixture was diluted with water, and
extracted with DCM (3x), and the filtrate evaporated
to dryness. The resulting crude material was purified by CC (Biotage, SNAP
10g, solvent A: EA; solvent B: Hept;
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
184
gradient in %B: 0 over 3CV, 0 to 15 over 3CV, 15 to 30 over 3CV, 30 over 3CV)
to afford 57mg of the title compound
as orange resin. LC-MS (A): tR = 1.22min; [M-F1-1]': 282.16.
F332. 3-(2-Methyl-thiazol-5-y1)-4-nitro-butyric acid octyl ester.
The title compound (27mg, orange resin) was synthesized starting from Example
F3.3.1, and following the procedure
described in Example F3.2 step 2, however running the reaction under higher
dilution (1.5 mL nitromethane). LC-MS
(A): tR = 1.12min; [M+H]: 343.05.
F3.3.3. 4-(2-Methyl-thiazol-5-y1)-pyrrolidin-2-one
The title compound (6mg, beige solid) was synthesized starting from Example
F3.3.2, and following the procedure
described in Example F3.2 step 3. Further addition of 3eq ammonium formate and
0.03eq Pd/C were required to
advance the reaction conversion. LC-MS (A): tR = 0.4min; [M+H]: 183.14.
Example F3.4 to Example F3.10 were synthesized starting from the appropriate
heteroaryl bromide derivative,
following the three-step procedure described in Example F3.3, omitting the
addition of ammonium formate and Pd/C in
step 3. If the cyclization was sluggish, 10 eq of TEA was added. The
purification (CC, Biotage, (solumn size) SNAP
solvent A: DCM, solvent B: DCM/Me0H 8/2), gradients in %B and the LC-MS (A)
data can be found in the table below.
Example
Name tR [M+H]
Purification
N
F3.4
4-(6-Methyl-pyridin-3-y 0.24 177.24
I)-pyrrolidin-2-one (25g) 0 for 3CV, 0
to 15 over 5CV, 15
for 5CV, 15 to 30 over 5CV
F3.5
4-(6-lsopropyl-pyridin-2-y 0.37 205.18
1)-pyrrolidin-2- (25g) 25 for 3CV,
25 to 50 over 3CV, 50
one for 1CV
4-(6-Trifl uoromethyl-pyridi n-3-yI)-
F3.6 0.6 231.05 (25g) 25 for
6CV
pyrrolidin-2-one
F3.7
4-(1-Methyl-1H-pyrazol-4-y1)-pyrrolidin-2- 0.43 166.05 (10g) 0 for
3CV, 0 to 15 over 5CV, 15
one for 5CV, 100 for
5CV
4-(1, 3-Di methy1-1H-pyrazol-4-y1)- (10g) 15 for 3CV,
15 to 30 in 5CV, 30
F3.8 0.43 180.25
pyrrolidin-2-one for 5CV
F3.9
4-(1-Difluoromethy1-1H-pyrazol-4-y 0.48 202.13
1)- (10g) 0 to 15 over
3CV, 15 for 5CV, 15
pyrrolidin-2-one to 30 over 5CV
4-(2-Methy1-2H-[1,2,3]triazol-4-y1)-
F3.10 0.41 167.06 -
pyrrolidin-2-one
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
185
Example F4.1: 5-Benzyl-oxazolidin-2-one
1-Amino-3-phenylpropan-2-ol (150mg) and CDI (268mg) were dissolved in THF
(30mL). The resulting mixture was
stirred for 16h30 at RT and evaporated to dryness. The resulting crude
material was purified by CC (Biotage, SNAP
10g, solvent A: Hep; solvent B: EA; gradient in %B: 50 over 4CV, 50 to 100
over 5CV, 100 over 1CV) to afford 57mg of
the title compound as yellow solid. LC-MS (A): tR = 0.65min; [M+H] : 178.40.
Example F4.2: 5-lsopropyl-oxazolidin-2-one
To a solution of 1-amino-3-methyl-2-butanol hydrochloride (150mg) and DIPEA
(69.9pL) in THF (3mL) cooled at 0 C
was added a solution of bis(trichloromethyl)carbonate (101mg) in THF (2mL) and
the resulting suspension was stirred
for 40min at 0 C. The reaction mixture was diluted with EA, washed with aq.
sat. NaHCO3 solution, water and brine.
The org. layers were dried (MgSO4), filtrated off and evaporate to dryness.
The resulting crude material was purified by
CC (Biotage, SNAP 10g, solvent A: Hep; solvent B: EA; gradient in %B: 0 over
2CV, 0 to 100 over 10CV, 100 over
2CV) to afford 16mg of the title compound as yellow solid. LC-MS (A): tR =
0.49min; [M-FH]': 130.30.
Example F4.3 to Example F4.5 were synthesized starting from the appropriate
amine and carbonate reagents,
following the procedure described in Example F4.2 and using solvents and bases
listed in the table below. Prep LC-
MS conditions and LC-MS data of Example F4.3 to Example F4.5 are listed in the
table below. The LC-MS conditions
used were LC-MS (A).
Example
Solvent
Name tR [M+1-1]*
Base
THF
F4.3 4-Oxa-6-aza-spiro[2.4]heptan-5-one 0.36 114.15
Dl PEA
DCM
F4.4 5-(Tetrahydro-pyran-4-yI)-oxazolidin-2-one 0.42
172.14
TEA
TH F
F4.5 5-tert-Butyl-oxazolidin-2-one 0.58 144.28
Dl PEA
Example F4.6: 8,8-D ifl uoro-1-oxa-3-aza-spiro[4.5]decan-2-one
F4.6.1. 4,4-Difluoro-1-trimethylsilanyloxy-cyclohexanecarbonitrile
To an ice-cold solution of 4,4-difluorocyclohexanone (5g) in DCM (100mL) were
added TMSCN (5.71mL) followed by
ZnI (119mg). The reaction mixture was stirred for 2h30 at 0 C, was quenched
with Na2CO3solution (10%) and extracted
with DCM. The org. layers were dried (MgSO4), filtrated off and evaporated to
dryness to give 8.26g of yellow oil.
F4.6.2. 1-Aminomethy1-4,4-difluoro-cyclohexanol, hydrochloride salt
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
186
To an ice-cold solution of LiAll-14 in THF (1M, 53.1mL) in Et20 (28mL) was
added dropwise a solution of Example F4.6.1
(8.26g) in Et20 (14mL). The reaction mixture was stirred for 45min at RT,
cooled down to 0 C and ice-cold water
(4.25mL) followed by NaOH (1M, 4.25mL) were added. The resulting mixture was
stirred for 30min, diluted with Et20,
filtered off and evaporated to dryness. The residue was dissolved in Et20
(67mL) and HCI in dioxane (4M, 34mL) was
added dropwise at 0 C. The mixture was stirred for 1 h at 0 C and filtered
off. The resulting precipitate was dried under
HV to give 5.5g of the title compound as white solid. LC-MS (A): tR = 0.27min;
[M+H]:207.27.
F4.6.3. 8,8-Di f luoro-1-oxa-3-aza-spiro[4.5]decan-2-one
A solution of Example F4.6.2 (250mg) and TEA (174pL) in DCM (7mL) was stirred
for 15min at RT. Carbonic acid di-
2-pyridyl ester (274mg) was added and the mixture was stirred overnight at RT,
diluted with water and extracted with
DCM. The org. layers were dried (MgSO4), filtrated off, and evaporated to
dryness. The resulting crude material was
purified by CC (Biotage, SNAP 10g, solvent A: DCM; solvent B: Me0H; gradient
in %B: 3 over 5CV, 3 to 5 over 2CV, 5
over 2CV) to afford 208mg of the title compound as white solid. LC-MS (A): tR
= 0.57min; [M+H]-: 192.36.
Example F4.7 to Example F4.9 were synthesized starting from the appropriate
ketone derivative, and following the
three-step procedure described in Example F4.6. LC-MS data of Example F4.7 to
Example F4.9 are listed in the table
below. The LC-MS conditions used were LC-MS (A).
Example N Name tR
[M-FH]'
F4.7 7-Oxa-9-aza-dispiro[3.1.4.1]undecan-8-one 0.71 168.17
F4.8 2-Cyclopropy1-5-oxa-7-aza-spiro[3.4]octan-6-one 0.69 168.21
F4.9 2, 2-Dimethy1-5-oxa-7-aza-spi ro[3. 4]octan-6-one 0.65 156.25
Example F4.10: 4-(2-Methoxy-ethyl)-pyrrolidin-2-one
F4.10.1. (E)-5-Methoxy-pent-2-enoic acid ethyl ester
To an ice-cold suspension of NaH (60% in mineral oil, 440mg) in THF (24mL) was
added triethyl phosphonoacetate
(2.26mL) and the resulting mixture was stirred for 20 min at 0 C. A solution
of 3-methoxy-propionaldehyde (1g) in THF
(14mL) was added dropwise over 15min and the resulting solution was stirred
for 1h15 at 0 C. The reaction mixture
was diluted with Et20 and water and extracted with Et20. The org. phases were
washed with brine, dried (MgSO4),
filtered off and evaporated to dryness. The resulting crude material was
purified by CC (Biotage, SNAP 50g, solvent A:
Hep; solvent B: EA; gradient in %B: 0 over 1CV, 0 to 10 over 2CV, 10 over 2CV,
10 to 30 over 2CV, 30 over 1CV, 30
to 50 over 1CV, 50 to 70 over 1CV) to afford 1.02g of the title compound as
colourless oil. LC-MS (A): tR = 0.72min;
[M-F1-1]': 159.18.
F4.10.2. 5-Methoxy-3-nitromethyl-pentanoic acid ethyl ester
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
187
To an ice-cold solution of Example F4.10.1 (564mg) and nitromethane (274pL)
was added DBU (426pL). The reaction
mixture was stirred for 17h at RT, cooled down to 0 C, quenched with water and
extracted with DCM. The org. layers
were washed with aq. HCI solution (1M) and brine, dried (MgSO4), filtrated off
and evaporated to dryness to give 377mg
of the title compound as yellow oil. LC-MS (A): tR = 0.79min; [M+H]: 220.22.
F4.10.3. 4-(2-Methoxy-ethyl)-pyrrolidin-2-one
To a solution of Example F4.10.2 (50mg) and TEA (31.7pL) in Et0H (0.5mL) was
added Pd(OH)2/C (20%, 8.01mg)
and the resulting mixture was stirred for 23h at RT under hydrogen atmosphere.
The reaction mixture was filtered off
and evaporated to dryness. The resulting crude material was dissolved in Et0H
(1mL) and stirred for 22h at 4000 then
for 7 days at RT. The reaction mixture was evaporated to dryness to give 24mg
of the title compound as yellow oil. LC-
MS (A): tR = 0.41min; [M+H]-: 144.21.
Example F5.1: 1-(Tetrahydro-pyran-4-yI)-prop-2-yn-1-ol
F5.1.1. 1-(Tetrahydro-pyran-4-yI)-3-trimethylsilanyl-prop-2-yn-1-ol
To an ice-cold solution of trimethylsilylacetylene (637pL) in THF (10mL) was
added dropwise n-BuLi in hexane (2.5M,
2mL). The resulting mixture was stirred for lh at 0 C and a solution of 4-
formyltetrahydropyran (500mg) in THF (2mL)
was added. The reaction mixture was stirred for 30min at 0 C and for 1h at RT.
Water was added and the mixture was
extracted with Et20. The org. layer was evaporated to dryness to give 875mg of
the title compound as white solid. LC-
MS (A): tR = 0.83min; [M-FH]': 213.33.
F5.1.2. 1-(Tetrahydro-pyran-4-y1)-prop-2-yn-1-01
To a solution of Example F5.1.1 (875mg) in Me0H (30mL) was added K2003
(1.14g). The resulting mixture was stirred
overnight at RT, concentrated, diluted with Et20, and washed successively with
1M HCI solution, 1M NaHCO3 solution
and brine. The org. phases were dried (MgSO4), filtrated off and evaporated to
dryness to afford 384mg of the title
compound as white solid. 1H-NMR (DMSO) :5.36 (d, 1H); 4.00 (m, 1H); 3.86 (m,
2H); 3.28 (d, 1H); 3.25 (m, 2H); 1.63
(m,3H); 1.29 (m,2H).
Example F5.2 to Example F5.17
Example F5.2 to Example F5.17 were synthesized starting from the appropriate
ketone or aldehyde, and following the
procedure described in Example F5.1. Prep LC-MS conditions and LC-MS data of
Example F5.2 to Example F5.17 are
listed in the tables below. The LC-MS conditions used were LC-MS (A).
Prep
Example
Name tR [M+1-]-
LC-
N
MS
F5.2 1-(1,3-Dimethy1-1H-pyrazol-4-y1)-prop-2-yn-1-ol 0.44 151.18
none
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
188
F5.3 1-(2-Methyl-thiazol-4-y1)-prop-2-yn-1-ol 0.45 154.11
(IX)
[M-FMeCN-FH]-
F5.4 2-(3-Fluoro-phenyI)-but-3-yn-2-ol 0.75
none
= 206.08
F5.7 2-0 -Methyl-1H-pyrazol-3-y1)-but-3-yn-2-ol 0.43 151.18
none
F5.8 2-(2-Methyl-thiazol-4-y1)-but-3-yn-2-ol 0.5 168.08
none
F5.9 2-(6-Methoxy-pyridin-2-yI)-but-3-yn-2-ol 0.69 178.25
none
F5.10 2-Pyrimidin-2-yl-but-3-yn-2-ol 0.39 149.18
none
F5.11 2-(1,5-Dimethy1-1H-pyrazol-3-y1)-but-3-yn-2-ol 0.5
165.1 none
F5.12 2-(6-Methyl-pyrimidin-4-yI)-but-3-yn-2-ol 0.49 163.1
none
F5.13 1-(1,5-Dimethy1-1H-pyrazol-3-y1)-prop-2-yn-1-ol 0.45 151.18
(IX)
F5.14 8-Ethyny1-5,6,7,8-tetrahydro-quinolin-8-ol 0.41 174.13
(XV)
F5.15 7-Ethyny1-6,7-dihydro-5H-Npyrindin-7-ol 0.42 160.08
(XV)
F5.16 3-Pyridin-2-yl-pent-1-yn-3-ol 0.4 162.1
(XV)
F5.17 3-(6-Methoxy-pyridin-2-yI)-pent-1-yn-3-ol 0.79 192.21
(XV)
Example
Prep
Name tR H-NMR (CDCI3)
N
LC-MS
2-(4-Methoxy-phenyI)- 7.61 (d, 2H); 6.91 (d, 2H); 3.84 (s,
3H);
F5.5 0.73
none
but-3-yn-2-ol 2.69 (s, 1H); 2.34 (s, 1H); 1.80 (s,
3H)
2-(2-Methoxy-phenyI)- 7.57 (d, 1H); 7.32 (t, 1H); 7.00 (m,
2H);
F5.6 0.76
none
but-3-yn-2-ol 4.58 (s, 1H); 3.97 (s, 1H); 2.59 (s,
1H); 1.92 (s, 3H)
Example F5.18: 1-(3-Ethyny1-3-hydroxy-azetidin-1-y1)-2-methyl-propan-1-one
To a solution of 3-ethyny1-3-hydroxyazetidine trifluoroacetate (100mg) and TEA
(I32pL) in DCM (2mL) was added
dropwise isobutyryl chloride (48.IpL) at RT. The resulting mixture was stirred
for 25min at RT, diluted with water and
basified with aq. sat. NaHCO3 solution. The org. layers were dried (MgSO4),
filtrated off and evaporated to dryness.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
189
The residue was purified by CC (Biotage SNAP 10g, solvent A: DCM; solvent B:
Me0H; gradient in %B: 3 over 2CV, 3
to 5 over 2CV, 5 over 3CV) to afford 37mg of the title compound as yellow oil.
LC-MS (A): tR = 0.48min; [M-F1-1]': 168.05.
Example F5.19: 3-(6-Methyl-pyrimidin-4-yI)-pent-1-yn-3-ol
F5.19.1 6-Methyl-pyrimidine-4-carboxylic acid methoxy-methyl-amide
To a solution of 6-methylpyrimidine-4-carboxylic acid (500mg) in DCM (50mL)
were added N,0-dimethylhydroxylamine
hydrochloride (342mg), DIPEA (2.06mL) and propylphosphonic anhydride solution
in DCM (50% w/w, 2.54mL). The
resulting mixture was stirred 2h at RT and quenched with aq. sat. NaHCO3
solution. The org. layer was washed with
citric acid (10%) and water. The combined org. layers were dried (MgSO4),
filtered off and evaporated to dryness to
afford 453mg of the title compound as brown oil which was used without further
purification. LC-MS (A): tR = 0.48min;
[M-FH]*: 182.22.
F5.19.2 1-(6-Methyl-pyrimidin-4-yI)-propan-1-one
To a yellow solution of Example F5.19.1 (445mg) cooled at -78 C under argon
was added ethylmagnesium bromide in
THF (1M, 4.91mL) and the resulting suspension was stirred 1h. The resulting
crude was quenched with aq. sat. NI-14C1
solution, diluted with water and extracted with DCM. The combined org. layers
were dried (MgSO4), filtrated off and
evaporated to dryness. The residue was purified by CC (Biotage, snap10g,
solvent A: Hep; solvent B: EA; gradient in
%B: 30 over 3CV, 30 to 70 over 6CV, 70 over 2CV to afford 77mg of the title
compound as yellow solid. LC-MS (A): tR
= 0.63min; [M+H]-: 151.16.
F5.19.3 3-(6-Methyl-pyrimidin-4-yI)-pent-1-yn-3-ol
The title compound (45mg, yellow oil) was synthesized from Example F5.19.2
(73mg) and trimethylsilylacetylene
(74.4pL) and following the two-step procedure described in Example F5.1. LC-MS
(A): tR = 0.56min; [M+H]*: 177.27.
Example F5.20 to Example F5.30 were synthesized starting from the appropriate
commercially available ketone or
aldehyde reagent, and following the two-step procedure described in Example
F5.1. Prep LC-MS and Chiral Prep (SFC
& HPLC) conditions, Chiral HPLC & SFC and LC-MS data are listed in the table
below. The LC-MS conditions used
were LC-MS (A).
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
190
Chiral
LC- Prep
Example
Chiral HPLC/
Name MS [M+H]- LC-
N
Prep /SFC IR
tR MS
(method)
7.0
F5.20 (S)-2-(6-Methyl-pyrimidin-4-yI)-but-3-yn-2-ol 0.49
163.08 - XXI
(5)
8.7
F5.21 (R)-2- (6-Methyl-pyri midi n-4-yI)-but-3-yn-2-ol 0.49
163.08 - XXI
(S)
1.56
F5.22 (S)- or (R)-2-(1,5-Dimethy1-1H-pyrazol-3-y1)-but-3-yn-2-ol
0.5 165.12 - XXII
(T)
1.94
F5.23 (R)- or (S)-2-(1,5-Dimethy1-1H-pyrazol-3-y1)-but-3-yn-2-ol
0.5 165.11 - XXII
(T)
1.17
F5.24 (S)- or (R)-2-(1-Methyl-1H-pyrazol-3-y1)-but-3-yn-2-ol
0.43 151.2 (IX) )0(111
(U)
1.67
F5.25 (R)- or (S)-2-(1-Methyl-1H-pyrazol-3-y1)-but-3-yn-2-ol
0.43 151.13 (IX) XXIII
(U)
F5.26 2-(6-Methoxy-pyrimidin-4-yI)-but-3-yn-2-ol 0.53
179.26 -
F5.27 2-(6-Methyl-pyridin-2-yI)-but-3-yn-2-ol 0.36
162.09
F5.28 2-Pyridin-2-yl-but-3-yn-2-ol 0.33 148.17 -
F5.29 1-Cyclopropy1-1-pyridin-2-yl-prop-2-yn-1-ol 0.42
174.13 (XV) -
F5.30 2-(5-Methyl-pyridin-3-yI)-but-3-yn-2-ol 0.36
162.08 -
The absolute configuration for the Example F5.20 was assessed by single
crystal X-ray diffraction (suitable crystal
obtained through diffusion of heptane into a solution of the compound in EA)
and proved to be in absolute (S)-
configuration. Consequently, the absolute configuration for the Example F5.21
was assigned (R).
Example F5.31 to Example F5.42 were synthesized using the commercially
available ketone following the procedure
described in Example F5.1. CO (Biotage) gradients and column size, if CC was
made, and LC-MS data are listed in the
table below. The LC-MS conditions used were LC-MS (A).
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
191
Example Name tR [M-FH]-
solvents
N
(column size, SNAP) CC gradient (in
%B)
F5.31 1-(4-Ethyny1-4-hydroxy-piperidin-1-y1)- 0.42
168.09 A:DCM B: DCM/Me0H 8/2 (10g) 15 for
ethanone
3CV, 15 to 50 over 5CV, 50 for 2CV
F5.32 rac-1-[4-(1-Hydroxy-1-methyl-prop-2- 0.52
196.23 A:DCM B: DCM/Me0H (10g) 15 for
yny1)-piperidin-1-y1Fethanone
2CV, 15 to 25 over 2CV, 25for 2CV, 25
to 50 over 2CV, 50 for 2CV
F5.33 rac-144-(1 -Hydroxy-prop-2-ynyI)- 0.49
182.21 A:DCM B: DCM/Me0H (10g) 15 for
piperidin-1-yI]-ethanone
3CV, 15 to 25 over 3CV, 25 for 2CV
F5.34 rac-4-(1-Hydroxy-prop-2-ynyI)-4- 0.86
254.15 A:DCM B: DCM/Me0H (10g) 30 for
methyl-piperidine-1-carboxylic acid tert-
2CV, 30 to 50 over 2CV, 50 for 3CV
butyl ester
F5.35 rac-4-Ethyny1-3,4-dihydro-2H- 0.43 176.24
pyrano[3,2-b]pyridin-4-ol
F5.36 rac-3-Ethyny1-3-hydroxy-1-methyl-1,3- 0.63
188.17 A:DCM B: DCM/Me0H (10g) 0 for 3CV,
dihydro-indo1-2-one
0 to 15 over 5CV, 15 for 5CV, 15 to 30
over 5CV, 30 for 5CV
F5.37 rac-2-(1H-Indazol-3-y1)-but-3-yn-2-ol 0.62
187.26 A:DCM B: DCM/Me0H (10g) 30 for
2CV, 30 to 50 over 2CV, 50 for 3CV, 50
to 100 over 3CV, 100 for 5CV
F5.38 rac-2-(1-Methyl-1H-indazol-3-y1)-but-3- 0.70
201.18 A:DCM B: DCM/Me0H (10g) 30 for
yn-2-ol
2CV, 30 to 50 over 2CV, 50 for 3CV, 50
to 100 over 30V, 100 for 5CV
F5.39 rac-2-(2-Methyl-pyrimidin-4-yI)-but-3- 0.48
163.06 A:DCM B: DCM/Me0H (10g) 15 for
yn-2-ol
4CV, 15 to 25 over 2CV, 25 for 2CV
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
192
F5.40 rac-2-(5-Methyl-pyrazin-2-yI)-but-3-yn- 0.49 163.11
A:DCM B: DCM/Me0H (10g) 15 for
2-ol 3CV, 15 to 25
over 4CV, 25 for 2CV
F5.41 rac-2-(2-Methyl-thiazol-5-y1)-but-3-yn- 0.53 168.04
A:DCM B: DCM/Me0H (10g) 15 for
2-ol 4CV, 15 to 50
over 6CV, 50 for 2CV
Example F5.42 rac-2-(6-Cyclopropyl-pyrimidin-4-yI)-but-3-yn-2-ol
F5.42.1 1-(6-Cyclopropyl-pyrimidin-4-y/)-ethanone
A vial was charged with Bis(triphenylphosphine)palladium(11) dichloride
(34.2mg), 4-bromo-6-cyclopropylpyrimidine
(100mg), toluene (200pmL) and tributy1(1-ethoxyvinyptin (216pL) at RT, sealed
and shaken at 95 C overnight. After
evaporation to dryness the residue was taken up in dioxane (900pL) and HCI 2N
(169pL) to be stirred for 6h at RT.
The reaction mixture was diluted with EA and washed 2x with water and lx with
brine. Afterwards the aq. layers were
re-extracted with 2x EA. The combined org. layers were dried over MgSO4,
filtrated off, evaporated and purified by
CC (Biotage, 10g snap, solvent A: heptane, solvent B: EA, gradient (in %B): 10
for 3CV, 10 to 100 over 10CV, 100 for
2CV) to give 82mg light yellow oil. LC-MS (A) tR = 0.69min; [M-FH]- 163.10.
F5.42.2 rac-2-(6-Cyclopropyl-pyrimidin-4-yI)-but-3-yn-2-o/
The title compound was synthesized starting from Example F5.42.1 following the
two-step procedure described in
Example F5.1, EA was used instead of Et20 for extraction. Furthermore, instead
of quenching in step 2 the work-up
was skipped and the mixture was filtered and evaporated to dryness and the
crude purified by CC (Biotage, 10 g,
gradients: A:DCM B: DCM/Me0H 0 for 2CV, 0 to 25 for 6CV, 25 for 2CV, 25 to 50
over 3CV, 50 for 2CV. LC-MS (A)
tR = 0.59min; [M--H] 189.20.
Example F5.43 to Example F5.53 were synthesized using the appropriate ketone
following the procedure described
in Example F5.1. CC (Biotage) gradients and column size, if CC was made, and
LC-MS data are listed in the table
below. The LC-MS conditions used were LC-MS (A). For chiral separated
compounds the prep LC-MS and chiral
separation method is listed in the table below. The ketones are listed in the
table below unless commercially available.
Example Name tR [M--H] ' solvents Prep
Chiral Chiral tR Ketone
N (column size, LC-
Separatio (analytic
SNAP) CC MS
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
193
gradient (in al
chiral
%B)
Method)
F5.43 (R)- or (S)-2-(6- 0.53 179.20 A:DCM B: (V)
(XXV) 2.50 F5.K1
Methoxy-pyrimidin- DCM/Me0H (AJ)
4-yI)-but-3-yn-2-ol (10g) 30 for
2CV, 30 to 50
over 2CV, 50
for 3CV
F5.44 (S)- or (R)-2-(6- 0.53 179.21 A:DCM B: (V)
(XXV) 3.17 F5.K1
Methoxy-pyrimidin- DCM/Me0H (AJ)
4-yI)-but-3-yn-2-ol (10g) 30 for
2CV, 30 to 50
over 2CV, 50
for 3CV
F5.45 (R)- or (S)-2-(2- 0.55 179.21 A:DCM B: (V)
(XXXVI I) 1.38 F5. K2
Methoxy-pyrimidin- DCM/Me0H (AH)
4-yI)-but-3-yn-2-ol (10g) 0 to 30
over 3CV, 30
for 5CV, 30 to
over 4CV
F5.46 (S)- or (R)-2-(2- 0.55 179.21 A:DCM B: (V)
(XXXVI I) 1.66 F5. K2
Methoxy-pyrimidin- DCM/Me0H (AH)
4-yI)-but-3-yn-2-ol (10g) 0 to 30
over 3CV, 30
for 5CV, 30 to
5 over 4CV
F5.47 (R)- or (S)-2-(2,6- 0.46 177.26 A:DCM B: (V)
(XXIX) 1.39 (Al) F5.K3
Dimethyl-pyrimidin- DCM/Me0H
4-yI)-but-3-yn-2-ol (25g) 0 to 30
over 3CV, 30
for 5CV, 30 to
50 in 5CV
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
194
F5.48 (S)- or (R)-2-(2,6- 0.46 177.25 A:DCM B: (V)
(XXIX) 1.72 (Al) F5.K3
Dimethyl-pyrimidin- DCM/Me0H
4-yI)-but-3-yn-2-ol (25g) 0 to 30
over 3CV, 30
for 5CV, 30 to
50 in 5CV
F5.49 (R)- or (S)-2-(2,6- 0.68 209.13 A: Hp B:EA
(XVI) (XXXIX) 1.60 F5.K4
Dimethoxy- (10g) 10 for (AG)
pyrimidin-4-yI)-but- 3CV, 10 to 30
3-yn-2-ol over 2CV, 30
for 3CV
Then 10 for
3CV, 10 to 30
over 6CV, 30
for 2CV
F5.50 (S)- or (R)-2-(2,6- 0.68 209.12 A: Hp B:EA
(XVI) (XXXIX) 2.38 F5.K4
Dimethoxy- (10g) 10 for (AG)
pyrimidin-4-yI)-but- 3CV, 10 to 30
3-yn-2-ol over 2CV, 30
for 3CV
Then 10 for
3CV, 10 to 30
over 6CV, 30
for 2CV
F5.51 (S)- or (R)-2-(2- 0.62 193.19 (XVI) 7.36
F5.K5
Methoxy-6-methyl- (AK)
pyrimidin-4-yI)-but-
3-yn-2-ol
F5.52 (R)- or (S)-2-(6- 0.52 193.20 (XVI)
(XLI) 8.4 (AL) F5.K6
Methoxy-2-methyl-
pyrimidin-4-yI)-but-
3-yn-2-ol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
195
F5.53 (S)- or (R)-2-(6- 0.52 193.19 (XVI) (XLI)
9.93 F5.K6
Methoxy-2-methyl- (AL)
pyrimidin-4-yI)-but-
3-yn-2-ol
Precursors ketones for alkynes Examples F5.43 to F5.53
Example F5.K1 1-(6-Methoxy-pyrimidin-4-yI)-ethanone
F5.K1.1 6-Methoxy-pyrimidine-4-carboxylic acid methoxy-methyl-amide
The title compound was synthesized following the procedure described in
Example F5.19.1, except the washing with
citric acid (10%), which was left out, starting from 6-methoxypyrimidine-4-
carboxylic acid. LC-MS (A) tR = 0.53min,
[M+H]': 198.16
F5.K1.2 1-(6-Methoxy-pyrimidin-4-y/)-ethanone
A solution of F5.K1.1 (600mg) in THE abs. (10mL) was 3x evacuated and
backfilled with argon, then cooled to -78 C.
Then a methylmagnesium bromide solution (230pL, 3.0M in diethyl ether) was
added dropwise under argon at -78 C.
After stirring for 15min at RT, the mixture was quenched with aq. sat. NH4CI,
diluted with water and extracted with 3x
DCM (phase separator). The combined org. layers were evaporated to dryness and
purified by CC (Biotage, SNAP
25g, solvent A: heptane, solvent B: EA 0 to 15 over 2CV, 15 for 2CV, 15 to 30
over 2CV, 30 for 2CV, 30 to 70 over
3CV, 70 for 3CV) to give 290mg yellowish solid. LC-MS (A) tR = 0.62min; [M-F1-
1]': 153.10.
Example F5.K2 1-(2-Methoxy-pyrimidin-4-yI)-ethanone
F5.K2.1 2-Methoxy-pyrimidine-4-carboxylic acid methoxy-methyl-amide
The title compound was synthesized following the procedure described in
Example F5.K1, step F5.K1.1, using 2-
methoxypyrimidine-4-carboxylic acid. LC-MS (A) tR = 0.53min, [M+H]: 198.16
F5.K2.2 1-(2-Methoxy-pyrimidin-4-yI)-ethanone
The title compound was synthesized following the procedure described in
Example F5.K1, step F5.K1.2, using
F5.K2.1, purified by CC (Biotage, 25g SNAP, solvent A: Hept, solvent B: EA,
gradient (in %B): 0 to 15 over 2CV, 15
for 2CV, 15 to 30 over 2CV, 30 for 2CV, 30 to 70 over 3CV, 70 for 3CV). LC-MS
(A) tR = 0.61min, [M+H]-: 153.12
Example F5.K3 1-(2,6-Dimethyl-pyrimidin-4-yI)-ethanone
F5.K3.1 2,6-Dimethyl-pyrimidine-4-carboxylic acid methoxy-methyl-amide
The title compound was synthesized following the procedure described in
Example F5.K1, step F5.K1.1, starting from
2,6-dimethylpyrimidine-4-carboxylic acid. LC-MS (A) tR = 0.49min, [M-FH]:
196.19
F5.K3.2 1-(2,6-Dimethyl-pyrimidin-4-yI)-ethanone
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
196
The title compound was synthesized following the procedure described in
Example F5.K1, step F5.K1.2, using
F5.K3.1, purified by CC (Biotage, 109 SNAP, solvent A. Hept, solvent B: EA,
gradient (in %B): 15 for 3CV, 15 to 30
over 5CV, 30 for 5CV. LC-MS (A) tR = 0.58min, [M+H]: 151.15
Example F5.K4 1-(2-Chloro-6-methoxy-pyrimidin-4-yI)-ethanone
F5.K4.1 2-Chloro-6-methoxy-pyrimidine-4-carboxylic acid methyl ester
To a suspension of methyl 2,4-dichloropyrimidine-6-carboxylate (1000mg) in
Me0H abs. (20m1) was added sodium
methoxide solution 0.5M in Me0H (9.46mL) slowly at 0 C under argon. The
resulting suspension was stirred at 0 C
under argon overnight, allowed to reach RT. AcOH was added to the reaction
mixture and stirred for 10min, then the
mixture was evaporated to dryness. The residue was taken up in EA and washed
with lx aq. sat. NaHCO3 and lx
brine. Afterwards the aq. layers were 2x re-extracted with EA. The combined
org. layers were dried over MgSO4,
filtrated off, evaporated and dried at HV to 826mg off-white solid. LC-MS (A)
tR = 0.72; [M+H]*: 203.09
F5.K4.2 2-chloro-6-methoxy-pyrimidine-4-carboxylic acid, as sodium salt
To solution of Example F5.K4.1 (803mg) in Me0H (4mL), THE (2mL) and DCM
(500pL) was added NaOH 1N
(3.96mL) at RT and the solution was stirred for 3h at RT. The reaction mixture
was evaporated and dried at HV to
give 865mg beige solid. LC-MS (A) tR = 0.54min, [M+1-1]+: 189.09.
F5.K4.3 2-Chloro-6-methoxy-pyrimidine-4-carboxylic acid methoxy-methyl-amide
The title compound was synthesized following the procedure described in
Example F5.K1, step F5.K1.1 using
Example F5.K4.2. LC-MS (A) tR = 0.49min, [M-'-H]: 196.19
F5.K4.4 1-(2-Chloro-6-methoxy-pyrimidin-4-yI)-ethanone
The title compound was synthesized following the procedure described in
Example F5.K1, step F5.K1.2, using
F5.K4.3. LC-MS (A) tR = 0.79min, [M-FH]-: 187.13
Example F5.K5 1-(2-Methoxy-6-methyl-pyrimidin-4-yI)-ethanone
F5.K5.1 2-Methoxy-6-methyl-pyrimidine-4-carboxylic acid
Methyl 2-chloro-methylpyrimidine-4-carboxylate (5.0g) was suspended in Me0H
(67mL) and NaOH 1N (67mL) was
added. The mixture was stirred for 1h at RT, then Me0H was evaporated off. At
0 C the mixture was acidified to
pH=2 with HCI (25%). The crystals were filtrated, washed with water and
heptane and dried at HV at 35 C overnight
to give 3.0g beige crystals 1H NMR (400 MHz, DMSO) 6: 13.67-13.76 (m, 1 H),
7.52 (s, 1 H), 3.94 (s, 3 H), 2.50 (s, 3
H)
F5.K5.2 2-Methoxy-6-methyl-pyrimidine-4-carboxylic acid methoxy-methyl-amide
The title compound was synthesized following the procedure described in
Example F5.K1, step F5.K1.1 using
Example F5.K5.1. LC-MS (A) tR = 0.57min, [M+H]-: 212.13
F5.K5.3 1-(2-Methoxy-6-methyl-pyrimidin-4-yI)-ethanone
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
197
The title compound was synthesized following the procedure described in
Example F5.K1, step F5.K1.2, using
Example F5.K5.2. LC-MS (A) tR = 0.68min, [M-F1-1]': 167.06
Example F5.K6 1-(6-Methoxy-2-methyl-pyrimidin-4-yI)-ethanone
F5.K6.1 6-Methoxy-2-methyl-pyrimidine-4-carboxylic acid methyl ester
A flask was charged with Example F5.K4.1 (787mg), Pd(PPh3)4 (227mg) and THF
abs. (60mL) at RT under argon. To
this solution trimethylaluminum solution 2M in toluene (3.89mL) was added in
one portion at RT. The resulting
solution was stirred at 75 C ET under argon over weekend. The mixture was
poured slowly into 1M NaH2PO4 and
extracted with 3x DCM. The combined org. layers were dried over Mg SO4,
filtrated, evaporated and purified by CC
(Biotage, 10g snap, solvent A: heptane , B: EA, Gradient (in %B): 50 for 3CV,
50 to 70 over 2CV, 70 for 1CV) to give
507mg brown solid. LC-MS (A) tR = 0.62min; [M-FH]*: 183.17
F5.K6.2 6-methoxy-2-methyl-pyrimidine-4-carboxylic acid, sodium salt
The title compound was synthesized following the procedure described in
Example F5.K4, step F5.K4.2 using
Example F5.K6.1. LC-MS (A) tR = 0.33min, [M-'-H]: 169.01
F5.K6.3 6-Methoxy-2-methyl-pyrimidine-4-carboxylic acid methoxy-methyl-amide
The title compound was synthesized following the procedure described in
Example F5.K1, step F5.K1.1 using
Example F5.K6.2. LC-MS (A) tR = 0.56min, [M-F1-1]+: 212.13
F5.K6.4 1-(6-Methoxy-2-methyl-pyrimidin-4-yI)-ethanone
The title compound was synthesized following the procedure described in
Example F5.K1, step F5.K1.2, using
F5.K6.3. LC-MS (A) tR = 0.64min, [M-FH]: 167.05
Example F5.54 1-(4-Ethynyl-piperidin-1-yI)-ethanone
To a suspension of the 4-ethynylpiperidine hydrochloride (125mg) in EA (2.5m1)
and aq. sat. NaHCO3 (2.5m1) was
added acetic anhydride (157pL) at RT and the mixture was stirred for 1h. Then
the phases were separated. The aq.
layer was extracted with 'Ix EA and the aq. layers were washed with lx brine.
The combined org. layers were dried
over MgSO4, filtrated off, evaporated and dried at HV to give 136mg colourless
oil. LC-MS (A) tR = 0.61min; [M+1-1]':
152.16.
Example F5.55 1-((S)-2-Ethyny1-2-methyl-pyrrolidin-1-y1)-ethanone
The title compound was synthesized following the procedure described in
Example F5.54 using (2S)-2-ethyny1-2-
methylpyrrolidine hydrochloride as amine. LC-MS (A) tR = 0.59min; [M+H]:
152.15.
Example F5.56 1-(4-Prop-2-ynyl-piperidin-1-yI)-ethanone
F5.55 4-Prop-2-ynyl-piperidine-1-carboxylic acid tert-butyl ester
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
198
To a suspension N-B0C-4-piperidineacetaldehyde (250mg) in Me0H (5mL) were
added K2003 (456mg) and dimethyl
(diazomethyl)phosphonate (191mg) at RT. The resulting suspension was stirred
at RT under argon. After 1h45 the
mixture was diluted with DCM, filtrated over celite and evaporated to dryness.
The crude was purified by CC (Biotage),
25g SNAP, A: Hep, B: EA Gradient (%B) 0 for 3CV, 0 to 15 over 30V, 15 for 3CV,
15 to 30 over 3CV, 30 for 3CV to
give 158mg colorless resin. LC-MS (A) tR = 0.99min; [M+H]': 224.12.
F5.56.2 4-(prop-2-yn-1-Apiperidine, hydrochloride salt
A colorless solution of Example F5.56.1 (155mg) in 4M HCI in dioxane (1.5mL)
was stirred at RT for 2h30. Then the
reaction mixture was evaporated to dryness to give 130mg off-white solid. 1H-
NMR (400 MHz, 0D0I3) 6: 9.58-9.80 (m,
1 H), 9.31-9.46 (m, 1 H), 3.47-3.60 (m, 2 H), 2.81-2.96 (m, 2 H), 2.16-2.34
(m, 2 H), 1.97-2.12 (m, 3 H), 1.74-1.90 (m,
4H)
F5.56.3 1-(4-Prop-2-ynyl-piperidin-1-yI)-ethanone
The title compound was synthesized following the procedure described in
Example F5.54 using F5.56.2 as amine. LC-
MS (A) tR = 0.69min; [M-FH]-: 166.10.
Example F5.57 rac-1-[4-(1-Hydroxy-prop-2-yny1)-4-methyl-piperidin-1-y1]-
ethanone
F5. 57.1 rac-1-(4-Methyl-piperidin-4-y1)-prop-2-yn-1-01, hydrochloride salt
The title compound was synthesized following the procedure described in
Example F5.56, step 2 using F5.34 as Boc-
protected amine. 1H-NMR (400 MHz, Me0D) 6: 1.16 (s, 3 H), 1.64 (d, J = 15.4
Hz, 1 H), 1.69-1.74 (m, 1 H), 1.88-1.98
(m, 2 H), 2.94 (d, J = 1.4 Hz, 1 H), 3.11-3.19 (m, 2 H), 3.30 (m, 2 H), 4.14
(d, J = 1.3 Hz, 1 H)
F5.57.2 rac-144-(1-Hydroxy-prop-2-yny1)-4-methyl-piperidin-1-ylrethanone
The title compound was synthesized following the procedure described in
Example F5.54 using F5.57.1 as amine. LC-
MS (A) tR = 0.56min; [M+H]: 196.20.
Example F5.58 1-(4-Ethyny1-4-methyl-piperidin-1-y1)-ethanone
F5.58.1 4-Ethyny1-4-methyl-piperidine, hydrochloride salt
The title compound was synthesized following the procedure described in
Example F5.56, step 2 using tert-butyl 4-
ethyny1-4-methylpiperdidine-1-carboxylate. LC-MS (A) tR = 0.39min; [M+H]:
124.21
F5. 58.2 1-(4-Ethyny1-4-methyl-piperidin-1-y1)-ethanone
The title compound was synthesized following the procedure described in
Example F5.54 using F5.58.1 as amine. LC-
MS (A) tR = 0.71min; [M+H]-: 166.11.
Example F5.59 rac-1,1,1-Trifluoro-2-methyl-but-3-yn-2-ol
To a solution of ethyl magnesium bromide solution 0.5M in THF (863p1) at 0 C
was added 1,1,1-trifluoroacetone
(399p1). After stirring for 1h, the mixture was quenched with HCI 1N, diluted
with water and extracted with 3x diethyl
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
199
ether. The combined org. layers were dried over MgSO4, filtrated off and
evaporated to dryness to afford 520mg
yellowish resin. 1H NMR (500 MHz, CDCI3) 6: 2.63 (s, 1 H), 1.67 (m, 4 H).
Example F5.60 to F5.62 were synthesized following the procedure described in
Example F5.18 using 2-methy1-3-butyn-
2-amine and the appropriate commercially available acid chloride. The CC
(Biotage, SNAP, 10g, solvent A: heptane,
solvent B: EA) gradients can be found in the table below, if a CC was
necessary. The LC-MS conditions used were LC-
MS (A).
Example Name tR [M+H] CC
gradient (in %B)
F5.60 Cyclopropanecarboxylic acid (1,1-dimethyl-prop-2- 0.60 152.14
30 for 3CV, 30 to 50 over 3CV,
ynyI)-amide 50
for 2CV
F5.61 N-(1,1-Dimethyl-prop-2-ynyI)-isobutyramide 0.62 154.17 30
for 4CV, 30 to 50 over 2CV,
50 for 2CV
F5.62 N-(1,1-Dimethyl-prop-2-ynyI)-2-methoxy-acetamide 0.54 156.17
Example F5.63 3-(1,1-Dimethyl-prop-2-ynyI)-oxazolidin-2-one
F5.63.1 (1,1-Dimethyl-prop-2-yny0-carbamic acid 2-chloro-ethyl ester
K2003 was added to a solution 2-methyl-3-butyn-2-amine (250mg) in MeCN (10m1)
at 0 C. Then a solution of 2-
chloroethyl chloroformate (336pL) in MeCN (5m1) was added dropwise within 5min
at 0 C. The resulting white susp
was stirred at 0 C and allowed to warm up to RT for 2h. The reaction mixture
was evaporated and the residue was
extracted with EA/water. The org. layer was washed one with brine. The aq
layers were back extracted with twice
EA. The combined org. layers were dried over MgSO4, filtrated off and
evaporated to dryness to give 594mg of a
colorless oil.
LC-MS (A) tR = 0.75min; [M+H]-: 190.13
F5.63.2 3-(1,1-Dimethyl-prop-2-yny0-oxazolidin-2-one
To a solution of Example F5.63.1 in THF (12m1) and treated with NaH (60%
dispersion in mineral oil, 357mg) at RT for
1h. The reaction mixture was quenched with HCI 1N (approx. 1m1), diluted with
water and with 3x EA. The org. phases
were washed with lx brine, combined, dried over MgSO4, filtrated off and
evaporated to dryness. The crude was
purified by CC (Biotage, 10g snap, solvent A: Heptane, solvent B: EA, gradient
in %B: 10 for 3CV, 10 to 30 over 2CV,
30 for 3CV, 30 to 50 over 1CV, 50 for 1CV ) to give 528mg pale grey oil. LC-MS
(A) tR = 0.58min; [M-F1-1]+: 154.12
Example F5.64 1-(1,1-Dimethyl-prop-2-ynyI)-pyrrolidin-2-one
F5.64.1 4-Chloro-N-(1,1-dimethyl-prop-2-ynyh-butyramide
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
200
The title compound was synthesized following the procedure described in
Example F5.63.1 using 2-methy1-3-butyn-2-
amine. LC-MS (A) tR = 0.69min; [M-F1-1]': 188.20.
F5.64.2 1-(1,1-Dimethyl-prop-2-ynyl)-pyrrolidin-2-one
The title compound was synthesized following the procedure described in
Example F5.63.2 using the Example F5.64.1,
while the purification by CC was omitted. LC-MS (A) tR = 0.62min; [M-F1-1]+:
152.13
Example F5.65 1-(1,1-Dimethyl-prop-2-ynyI)-imidazolidin-2-one
Example F5.65.1 1-(2-Chloro-ethyl)-3-(1,1-dimethyl-prop-2-yny1)-urea
The title compound was synthesized using the procedure described in F5.55.1
using 2-methyl-3-butyn-2-amine and
purified by CC (Biotage, 10g SNAP, A: DCM, B: DCM/Me0H 8/2, Gradient (in %B):
0 for 3CV, 0 to 15 over 6CV, 15
for 1CV). LC-MS (A) tR = 0.63min; [M+H]:189.18.
F5.65.2 1-(1,1-Dimethyl-prop-2-ynyI)-imidazolidin-2-one
The title compound was synthesized following the procedure described in
Example F5.63.2 using Example F5.65.1,
while the purification by CC was omitted. LC-MS (A) tR = 0.56min; [M-FH]-:
153.13
Example F5.66 1-(1,1-Dimethyl-prop-2-ynyI)-3-methyl-imidazolidin-2-one
Example F5.65 (70mg) was dissolved in THF abs. (1mL) and cooled to 0 C, then
NaH (60% dispersion in mineral oil,
22.1mg) was added under argon and the susp was stirred for 5min at 0 C under
argon, then iodomethane(28.9pL) was
added and the susp was stirred at 0 C under argon, for 1h30.The reaction
mixture was quenched with aq. sat. NH40I,
diluted with water and extracted with 3x DCM (phase separator). The combined
org. layers were evaporated and dried
at HV. 75mg yellow oil. LC-MS (A) tR = 0.64min; [M-FH]+:167.08
Example F5.67 3-(1-Ethynyl-cyclopropyI)-oxazolidin-2-one
F5.67.1 (1-Ethynyl-cyclopropy1)-carbamic acid 2-chloro-ethyl ester
The title compound was synthesized following the procedure described in
Example F5.63, step 1 using 1-ethynyl-
cyclopropylamine hydrochloride as amine. LC-MS (A) tR = 0.68min; [M+H]-:
188.13
F5.67.2 3-(1-Ethynyl-cyclopropyI)-oxazolidin-2-one
The title compound was synthesized following the procedure described in
Example F5.66, step 2 using Example F5.69.1
. The used gradient (in %B) is: 0 for 3CV, 0 to 10 over 8CV, 10 for 3CV, 10 to
100 over 100V, 100 for 2CV LC-MS (A)
tR = 0.53min; [M-FH]t 152.09
Example F5.68 2-(1-Ethynyl-cyclopropyI)-pyridine
F5.68.1 (1-Pyridin-2-yl-cyclopropyI)-methanol
A suspension of the 1-(pyridine-2-yl)cyclopropanecarboxylic acid (300mg) in
diethyl ether abs. (3.6mL) was cooled to
0 C under argon, then LiA11-14 1M in THF (2.14mL) was added slowly. The
resulting suspension was stirred at RT under
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
201
argon overnight. The reaction mixture was quenched by adding 89pL water, 89 pL
NaOH (15%) and 267 pL water.
The mixture was stirred for 20min, then filtrated off and evaporated to
dryness. 218mg yellow oil. LC-MS (A) tR =
0.21min; [M+H]: 150.14
F5.68.2 1-Pyridin-2-yl-cyclopropanecarbaldehyde
To a solution of F5.68.1 (100mg) and DIPEA (950pL) in DCM (2mL) was added a
solution of sulfur trioxide pyridine
complex 45% (308mg) in DMSO (2mL) at 0 C dropwise. The resulting solution was
stirred for 30min at 0 C. After 2h
the reaction mixture was quenched with 5m1 cold water.The two layers were
separated (phase separator) and the aq.
layer was extracted twice with DCM. The combined org. layers were evaporated
to dryness. 273mg orange liquid. LC-
MS (A) tR = 0.28min; [M+1-1]': 148.18
F5.68.3 2-(1-Ethynyl-cyclopropyI)-pyridine
The title compound was synthesized following the procedure described in
Example F5.56, step 1 using F5.68.2
aldehyde. Filtration over plug of celite with DCM/Me0H 9 was carried out
instead of CC. LC-MS (A) tR = 0.47min;
[M-FH]': 144.20.
Example F5.69 4-(1-Ethynyl-cyclopropyI)-6-methyl-pyrimidine
F5.69.1 (6-Methyl-pyrimidin-4-yI)-acetic acid methyl ester
To a solution of methyl 2-(6-chloropyrimidin-4-yl)acetate (600mg) and bis(tri-
tert-butylphosphine)palladium(0) (82.2mg)
in THF abs. (10mL) was added dropwise at 0 00 a dimethylzinc solution (345pL,
2.0 M in toluene) under argon. The
reaction mixture was further stirred at RT overnight. The reaction mixture was
quenched with water, extracted 3x with
DCM and purified by CC (Biotage, 25g SNAP, solvent A: DCM, solvent B: DCM/Me0H
8/2, gradient (in %B) 0 for 3CV,
0 to 15 over 5CV, 15 for 5CV) to give 485mg orange resin. LC-MS (A) tR =
0.52min; [M+H]: 167.04
F5.69.2 1-(6-Methyl-pyrimidin-4-yI)-cyclopropanecarboxylic acid methyl ester
To a solution of F5.69.1 (485mg) in DMF abs. (20mL) was added at 0 C NaH (60%
dispersion in mineral oil) (70mg)
and the mixture stirred at 0 C for 10min. Subsequently, 1,2-dibromoethane
(267pL) was added and the mixture stirred
for additional 5min. Again, NaH (60% dispersion in mineral oil) (70mg) was
added and stirring continued at 0 C for 4h.
The reaction was quenched by the addition of NH40I aq. sat, and extracted with
DCM 3x, dried over MgSO4,
concentrated in vacua, and purified by CC (Biotage, 25g SNAP, solvent A: DCM,
solvent B: DCM/Me0H 8/2, gradient
(in %B): 0 for 30V, 0 to 15 over 100V, 15 for 5CV) to give 280mg yellowish
resin. LC-MS (A) tR = 0.64min, [M-F1-1]-:
193.13
F5.69.2 3 [1-(6-Methyl-pyrimidin-4-y1)-cyclopropylrmethanol
A lithium borohydride solution (10.4mL, 2.0 M in THF) was added dropwise at RI
under argon to a solution of F5.69.2
(180mg) in Me0H abs. (3.5mL) and stirred overnight. The mixture was cooled to
0 C and carefully quenched by the
dropwise addition of HCI 2N to adjust the pH to 7, and the aq. phase was
extracted with DCM 3x.The combined org.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
202
phases were evaporated to dryness and the crude purified by CC (Biotage, 10g
SNAP, solvent A: DCM, solvent B:
DCM/Me0H 8/2, gradient (%B) 0 for 3CV, 0 to 15 over 5CV, 15 for 5CV) to give
68mg yellowish resin. LC-MS (A) tR =
0.42min; [M-FH]-: 165.09
F5. 69.3 1-(6-Methyl-pyrimidin-4-yI)-cyclopropanecarbaldehyde
To solution of F5.69.2 (48mg) in DCM abs. (2.5mL) were added molecular sieves
(3A) and Dess-Martin periodinane
(248mg) at RT under argon. The resulting suspension was stirred at RT for
30min. The mixture was filtrated over silica,
evaporated and purified by CC (Biotage, lOg SNAP, solvent A: DCM, solvent B:
DCM/Me0H 8/2, gradient (%B): 0 for
3CV, 0 to 15 over 5CV, 15 for 5CV to give 110mg yellowish wax. LC-MS (A) tR =
0.54min, [M+H]*:163.12
F5.69.4 4-(1-Ethynyl-cyclopropyI)-6-methyl-pyrimidine
The title compound was synthesized following the procedure described in
Example F5.56.1 using F5.69.3 as
aldehyde. CC (Biotage), 10g SNAP, A: DCM ,B: DCM/Me0H 8/2 0 for 3CV, 0 to 15
over 5CV, 15 for 5CV, 15 to 50
over 3CV, 50 for 5CV. LC-MS (A) tR = 0.74min; [M+H]-: 159.15.
Example F5.70 4-Ethyny1-1-methanesulfonyl-piperidine
To a suspension of the 4-ethynylpiperidine hydrochloride (70mg) and TEA
(324pL) in DCM (1.5mL) was added slowly
methanesulfonyl chloride (54.7pL) at 0 C and the suspension was stirred under
argon overnight. The reaction mixture
was quenched with water and the phases were separated with a phase separator.
The aq. layer was lx re-extracted
with DCM. The combined org. layers were evaporated to dryness and purified by
CC (Biotage, 10g SNAP, solvent A:
heptane, solvent B: EA; 30 for 3CV, 30 to 50 over 2CV, 50 for 1CV) to give
68mg white powder. 1H-NMR (500 MHz,
000I3) 6: 3.41 (m, 2 H), 3.23 (m, 2 H), 2.81 (s, 3 H), 2.69 (m, 1 H), 2.17 (d,
J = 2.5 Hz, 1 H), 1.95 (m, 2 H), 1.82 (m, 2
H).
Example F5.71 4-Ethyny1-4-methyl-piperidine-1-sulfonic acid methylamide
The title compound was synthesized following the procedure described in
Example F5.70 using F5.58.1. CC (Biotage,
10g SNAP, solvent A: heptane, solvent B: EA; 30 for 3CV, 30 to 50 over 3CV, 50
for 2CV). LC-MS (A) tR = 0.76min;
[M+H]: 217.11.
Example F5.72 4 (2-Methyl-but-3-yne-2-sulfonyI)-cyclopropane
To a suspension of sodium cyclopropanesulfinate (150mg), CuCI (9.08mg), 3-
chloro-3-methy1-1-butyne in DMF (500pL)
was stirred at 40 C overnight. The mixture was allowed to cool to RT, diluted
with EA/water and extracted with EA. The
combined org. layers were washed with brine, dried over MgSO4, filtrated off,
evaporated to dryness. The crude was
purified by CC (Biotage, 10g snap, solvent A: Hp, solvent B: EA, Gradient (in
%B): 30 for 3CV, 30 to 100 over 10CV,
100 for 2CV) to give 57mg pale yellow oil. LC-MS (A) tR = 0.85min; [M+H].:
214.09
Example F5.73 rac-2-(1-Cyclopropy1-1H-pyrazol-3-y1)-but-3-yn-2-ol
F5.73.1 1-(1-Cyclopropy1-1H-pyrazoi-3-y1)-ethanone
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
203
A mixture of 1-(1H-pyrazol-3-ypethan-1-one (200mg), cyclopropylboronic acid
(306mg), 2,2'-bipyridyl (550mg), Na2CO3
(185mg), copper(II) acetate (313mg) and toluene (16mL) was stirred at 100 C
under argon overnight.
The reaction mixture was diluted with water and extracted with 3x DCM. The
org. layers were washed with lx brine,
combined, dried over MgSO4, filtrated off, evaporated. The crude was purified
by CC (Biotage, 25g snap, solvent A:
heptane, solvent B: EA, Gradient (in %B): 0 for 3CV, 0 to 30 over 7CV, 30 for
2CV), followed by a second CC (Biotage,
10g snap, solvent A: heptane, solvent B: EA, gradient (in %B): 10 for 3CV, 10
to 30 over 4CV, 30 for 20V) to give 50mg
pale yellow oil. LC-MS (A) tR = 0.64min, [M-F1-1]+: 151.16
F5.73.2 rac-2-(1-Cyclopropy1-1H-pyrazol-3-y1)-but-3-yn-2-ol
To a solution of ethynyl magnesium bromide (918pL, 0.5M in THF) at 0 C was
added dropwise a solution of F5.73.1
(46mg) in THF (800pL) under argon and reaction mixture stirred at 50 C
overnight. The mixture was allowed to cool
down to RT, quenched with aq. sat. NI-14C1, diluted with water and extracted
with 3x DCM. The combined org. layers
were dried over MgSO4, filtrated off, evaporated and purified by CC (Biotage,
10g snap, solvent A: heptane, B: EA,
Gradient (in %B): 30 for 3CV, 30 to 50 for 2CV, 50 for 2CV, 50 to 70 over 2CV,
70 for 2CV) to give 22mg pale yellow
oil. LC-MS (A) tR = 0.57min; [M+H]-: 177.24
Example F5.74 rac-2-(6-Trifluoromethyl-pyrimidin-4-yI)-but-3-yn-2-ol
F5.74.1 6-Trifluoromethyl-pyrimidine-4-carboxylic acid methoxy-methyl-amide
The title compound was synthesized following the procedure described Example
F5.K1 step 1 using 6-
trifluoromethyl)pyrimidine-4-carboxylic acid. LC-MS (A) tR = 0.70min, [M-FH]-:
235.97
F5.74.2 1-(6-Trifluoromethyl-pyrimidin-4-yI)-3-triisopropylsilanyl-propynone
To a solution of the Weinreb amide F5.74.1 (452mg) in THF abs. (9mL) and
(triisopropylsilypacetylene (578pL) in a
heated-out flask was added LiHMDS 1M (2.5mL) dropwise at -78 C under argon.
The resulting solution was stirred at
-78 C under argon for 45min, before it was allowed to warm up to RT, to be
quenched with aq. sat. NaHCO3 and
extracted with 3x EA. Afterwards the org. layers were washed with lx brine,
combined, dried over MgSO4, filtrated off,
evaporated and dried at HV to give 712mg dark brown oil. LC-MS (A) tR =
1.29min, [M+H]: 357.01.
F5.74.3 rac-2-(6-Trifluoromethyl-pyrimidin-4-y1)-4-triisopropylsilanyl-but-3-
yn-2-ol
To a solution of F5.74.2 (723mg) in THF abs. (7mL) was added dropwise
methylmagnesium bromide solution (810pL,
3.0M in diethyl ether) at 0 C under argon. The resulting solution was stirred
at 0 C under argon for 1h30.The reaction
mixture was quenched with aq. sat. NH4CI at 0 C, diluted with water and
extracted with 3x DCM. The combined org.
layers were dried over MgSO4, filtrated off, purified by CC (Biotage, 25g
sphere, A: Hp, B: EA, 10 for 3CV, 10 to 30 over
3CV, 30 for 2CV) to give 507mg brown oil. LC-MS (A) tR = 1.24min, [M+H]t
373.10
F5.74.4 rac-2-(6-Trifluoromethyl-pyrimidin-4-yI)-but-3-yn-2-ol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
204
To a solution of the F5.74.3 (499mg) in THF abs. (5mL) was added TBAF (1.47mL)
at 0 C. After stirring for 30min at
0 C under argon the reaction mixture was quenched and diluted with aq. sat. NI-
14C1 and extracted with 2x DCM.
Afterwards the aq. layers were washed with lx aq. sat. NI-14C1 and lx brine.
The combined org layers were dried over
MgSO4, filtrated, evaporated and purified by Prep LC-MS (XVI, then IX) to give
99mg pale brown oil. LC-MS (A) tR =
0.70min, [M+H]-: 217.07
Example F5.75 rac-2-(6-Difluoromethyl-pyrimidin-4-yI)-but-3-yn-2-ol
F5. 75./ 6-Difluoromethyl-pyrimidine-4-carboxylic acid methoxy-methyl-amide
The title compound was synthesized following the procedure described Example
F5.K1 step F5.K1.1 using 6-
(difluoromethyl)pyrimidine-4-carboxylic acid. LC-MS (A) tR = 0.58min, [M-FH]-:
218.09.
F5. 75.2 1-(6-Difluoromethyl-pyrimidin-4-y0-3-triisopropylsilanyl-propynone
The title compound was synthesized following the procedure described Example
F5.75 step F5.75.2 using F5.75.1. LC-
MS (A) tR = 1.24min, [M-F1-1]': 339.10.
F5. 75.3 rac-2-(6-Difluoromethyl-pyrimidin-4-yI)-4-triisopropylsilanyl-but-3-
yn-2-ol
The title compound was synthesized following the procedure described Example
F5.75 step F5.75.3 using F5.75.2. The
compound was purified by CC (Biotage, 25g SPHERE, 10 for 4CV, 10 to 30 over
2CV, 30 for 1CV ) LC-MS (A) tR =
1.17min, [M-F1-1]+: 355.06.
F5.75.4 rac-2-(6-Difluoromethyl-pyrimidin-4-yI)-but-3-yn-2-ol
The title compound was synthesized following the procedure described Example
F5.75 step F5.75.4 starting from
Example F5.75.3. The compound was purified by CC (Biotage, 25g SPHERE, A:
heptane, B: EA), gradient (in %B): 10
for 3CV, 10 to 30 over 3CV, 30 for 3CV). LC-MS (A) tR = 0.57min, [M+H]:
199.16.
Example F5.76 (R)- or (S)-2-(2-Trifluoromethyl-pyrimidin-4-yI)-but-3-yn-2-ol
F5.76.1 N-methoxy-N-methyl-2-(trifluoromethyOpyrimidine-4-carboxamide
The title compound was synthesized following the procedure described Example
F5.K1 step F5.K1.1 using 2-
(trifluoromethyl)pyrimidine-4-carboxylic acid. LC-MS (A) tR = 0.72min, [M+H]:
235.82.
F5.76.2 1-(2-Trifluoromethyl-pyrimidin-4-yI)-3-triisopropylsilanyl-propynone
The title compound was synthesized following the procedure described Example
F5.75 step F5.75.2 starting from
Example F5.76.1. LC-MS (A) tR = 1.25min, [M-FH]t 357.75.
F5.76.3 rac-2-(2-Trifluoromethyl-pyrimidin-4-y0-4-triisopropylsilanyl-but-3-yn-
2-ol
The title compound was synthesized following the procedure described Example
F5.75 step F5.75.3 using F5.76.2. The
compound was purified by CC (Biotage, 25g SPHERE, 0 for 2CV, 0 to 10 over 30V,
10 for 3CV, 10 to 30 over 3CV) L-
C-MS (A): tR = 1.20min, [M-FH]-: 373.10.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
205
F5.76.4 (R)- or (S)-2-(2-Trifluoromethyl-pyrimidin-4-14)-but-3-yn-2-ol
The title compound was synthesized following the procedure described Example
F5.75 step F5.75.4 using F5.76.3. The
compound was purified by Chiral prep (XLIII) / Analytical (X): 7.0 min. LC-MS
(A): tR = 0.72min. 1H-NMR (400 MHz,
Me0D) 5: 9.00 (d, J = 5.2 Hz, 1 H), 8.04 (d, J = 5.2 Hz, 1 H), 3.07 (s, 1 H),
1.83 (s, 3 H).
Example F5.77 (S)- or (R)-2-(2-Trifluoromethyl-pyrimidin-4-yI)-but-3-yn-2-ol
The title compound was synthesized following the procedure described Example
F5.75 step F5.75.4 using F5.76.3. The
compound was purified by Chiral prep (XLIII) / Analytical (X): 8.9 min. LC-MS
(A): tR = 0.72min. 1H-NMR (400 MHz,
Me0D) 5: 9.00 (d, J = 5.2 Hz, 1 H), 8.04 (d, J = 5.2 Hz, 1 H), 3.07 (s, 1 H),
1.83 (s, 3 H).
Example F5.78 2-(3-Methyl-isoxazol-5-y1)-but-3-yn-2-ol
A heated-out flask was charged with a solution of trimethylsilylacetylene
(361pL) in THF abs. (4mL) and cooled to 0 C,
then n-BuLi 2.5 M in hexanes (11.14mL) was added slowly under argon. The
solution was stirred for 1h at 0 C under
argon, then a solution of 1-(3-methyl-5-isoxazolyl)ethanone (250mg) in THF
abs. (5mL) was added slowly at 0 C under
argon. The resulting solution was further stirred at 0 C under argon for
45min. The reaction mixture was quenched by
dropwise addition of Me0H (5mL), allowed to warm up to RT, then K2003 (262mg)
was added. The resulting suspension
was stirred at RT for 20min, before filtrated off, evaporated to dryness and
purified by CC (Biotage, 10g Snap, A: Hep,
B: EA, gradient (in %B): 30 for 3CV, 30 to 50 over 2CV, 50 for 2CV) to give
200mg yellow oil. LC-MS (A): tR = 0.57min,
[M+H]-:152.14.
Example F5.79 to Example F5.81 were synthesized following the procedure
described in Example F5.78. The LC-
MS data and the CC gradients (in %B) are listed in the table below. The LC-MS
conditions used were LC-MS (A).
CC conditions
Example N Name tR [M+H]-
Column, solvent NB gradient
(in %B)
25g SPHERE, DCM/Me0H 8:2
rac-2-(1-Methy1-1H-imidazol-2-
F5.79 0.29 151.16 25 for
3Cv, 25 to 50 over 3CV,
yI)-but-3-yn-2-ol
50 for 1CV
25g SPHERE, Hep/EA
rac-2-(5-Methyl-thiophen-2-yI)-
F5.80 0.77 149.12 10 for
3CV, 10 to 30 over 2CV,
but-3-yn-2-ol
30 for 1CV
rac-2-(1-Methy1-1H-pyrrol-2-y1)-
F5.81 0.65 150.24 -
but-3-yn-2-ol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
206
Example G1.1: 5-[(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-ol
G1.1.1. 3-Nydroxy-(4-isopropyl-pheny1)-(5-methoxy-pyridin-3-y1)-methylk3-
methyl-azetidine-1-carboxylic acid tert-
butyl ester
A flask was charged with 3-bromo-5-methoxypyridine (5.35g), THF (20mL) and
iPrMgCl.LiCI (1.3M in THF, 24mL) under
argon at RT and the resulting brown solution was stirred at 60 C for 30min. A
solution of Example A4.2 (3.98g) in THF
(20mL) was added dropwise and the resulting mixture was stirred at RT for
3h15, quenched with aq. sat. NI-14C1solution
and water, and extracted with DCM. The org. layers were evaporated in vacuo to
afford 6.45g of dark-orange resin. LC-
MS (A): tR = 0.90min; [M+H]': 427.33.
G1.1.2. (4-lsopropyl-pheny1)-(5-methoxy-pyridin-3-y1)-(3-methyl-azetidin-3-0)-
methanol
A solution of Example G1.1.1 (6.45g) in HCI in dioxane (4M, 30mL) was stirred
for 40min, diluted with water and
extracted with EA. The aq. layer was basified to pH12 with aq. NaOH solution
(1M) and extracted with EA. The org.
layers were dried (MgSO4) and evaporated to dryness to give 5.4g of the title
compound as orange solid. LC-MS (A):
tR = 0.59min; [M+H]: 327.25.
G1.1.3. (1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(5-methoxy-pyridin-3-
y1)-methanol
Example G1.1.3 was synthesized starting from Example G1.1.2 (5.4g), and
following the procedure described in
Example B2.1 step B2.1.2. The resulting crude material was purified by CC
(Biotage, SNAP 100g, solvent A: DCM;
solvent B: Me0H; gradient in %B: 0 over 3CV, 0 to 3 over 5CV, 3 over 5CV, 3 to
6 over 5CV, 6 to 10 over 3CV, 10 over
5CV, 10 to 20 over 2CV, 20% over 5CV) to afford 1.98g of the title compound as
orange solid. LC-MS (A): tR = 0.61min;
[M-FH]': 341.25.
G1.1.4. 511,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyil-
pyridin-3-ol
To a solution of Example G1.1.3 (50mg) in DMF (0.5mL) were added 2-
diethylamino-ethanethiol hydrochloride (31.2mg)
and KOtBu (43.4mg) and the resulting mixture was stirred at reflux for lh. Aq.
sat. NaHCO3 solution was added dropwise
at RT, the resulting mixture was diluted with water and extracted with EA. The
org. layers were dried (MgSO4) and
evaporated to dryness. The resulting crude material was purified by CC
(Biotage, SNAP 10g, solvent A: Me0H; gradient
in %A: 100 over 15CV). The isolated fractions were evaporated off and the
residue was dissolved in THF. The resulting
mixture was filtrated off and the solvent was evaporated to dryness to afford
15mg of the title compound as yellow resin.
LC-MS (A): tR = 0.52 min; [M-FH]': 327.25.
Example G1.2: 5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-ol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
207
G1.2.1. 345-Benzyloxy-pyridin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyll-3-
methyl-azetidine-1-carboxylic acid tert-
butyl ester
The title compound was synthesized starting from Example A4.2 (3.9g) and 3-
(benzyloxy)-5-bromopyridine (4.39g), and
following the procedure described in Example A7.1 step A7.1.1. The crude
material was purified by Prep LC-MS (II) to
afford 4.79g of the title compound as white solid. LC-MS (A): tR = 1.01min;
[M+H]: 503.30.
G1.2.2. 3-[(R)-(5-Benzyloxy-pyridin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
3-methyl-azetidine-1-carboxylic acid
tert-butyl ester
The title compound was obtained by chiral separation of Example G1.2.1 (4.79g)
by Prep chiral SFC (VII). LC-MS (A):
tR = 1.01min; [M-FH]-: 503.16; chiral SFC (G): 2.7min.
G1.2.3. (R)-(5-Benzyloxy-pyridin-3-0)-(4-isopropyl-pheny1)-(3-methyl-azetidin-
3-0)-methanol, hydrochloride salt
A solution of Example G1.2.2 (1.99g) in HCI in dioxane (4M, 15mL) was stirred
for 1h and evaporated to dryness to
give 2.05g of the title compound as off-white solid. LC-MS (A): tR = 0.73min;
[M-F1-1]': 403.12.
G1 .2.4. (R)-(5-Benzyloxy-pyridin-3-y1)-(1 3-dim ethyl-azetidi n-3-y1)-(4-
isopropyl-pheny1)-methanol
The title compound was synthesized starting from Example G1.2.3, and following
the procedure described in Example
B2.1 step B2.1.2. LC-MS (B): tR = 0.783min; [M+H]: 417.4.
G1.2.5. 5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-
methyll-pyridin-3-ol
A mixture of Example G1.2.4 (1.62g) and Pd/C (10% w/w, 50% water, 206mg) in
Et0H (40mL) was stirred at RT under
hydrogen atmosphere for 2h and filtered off over a celite plug. The resulting
solution was evaporated to dryness to
provide the title compound (1.04g) as pale yellow solid. LC-MS (A): tR =
0.52min; [M+H]-: 327.16.
Example J3.1: 2-[3-(5-{(R)-(1,3-Dimethyl-azetidi n-3-yI)-hydroxy-[4-
(4,4, 5, 5-tetramethyl-[1,3,2]clioxaborolan-2-y1)-
phenyl]-methyll-pyridin-3-y1)-[1,2,4]oxadiazol-511]-propan-2-ol
A mixture of Example 532 (1 eq), bis(pinacolato)diboron (1.1 eq),
Pd(dppf)C12.DCM (0.03 eq) and KOAc (3 eq) in
dioxane (5 mL/mmol) was flushed with argon, heated at 80 C in a sealed vial
and stirred for 18h. It was filtered over
Celite, the cake was washed with EA and the filtrate was concentrated in
vacuo. The crude was purified by Prep LC-
MS (XV) to afford the title compound as brown oil. LC-MS (A): tR = 0.70 min;
[M+H]: 521.08
Example J5.1: 4-((R)-(1, methyl-azetidi n-3-y1)-hydroxy-{545-
(1-hydroxy-1-methykethyl)-[1, 2, 4]oxadiazol-3-y1]-
pyridi n-3-y1}-methyl)-phenol
To a solution of boronic ester intermediate (J3.1) (1 eq) in THF (31 mL/mmol)
was added at 0 C, NaOH (2.5 eq) and a
30% aq. solution of hydrogen peroxide (2.5 eq). The mixture was stirred at RT
for 1h and quenched with a half sat.
solution of NH401. It was acidified to pH 0 with 1M HCI and washed with EA.
The aq. phase was basified to pH 10 with
a 1M NaOH and extracted with EA/Me0H 99/1 then EA/Me0H 95/5. The combined org.
phases were dried over MgSO4
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
208
and concentrated in vacuo. The crude was purified by Prep LC-MS (XV) to afford
the title compound as white solid. LC-
MS (A): tR = 0.52 min; [M-F1-1]': 411.08
Depending on the purification conditions, the title compounds/intermediates in
Intermediate Examples of Formula (A3),
(A4), (A5), (A6), (A7), (B2), (Cl), (03), (D1), (02), (D3), (04), (05), (El),
(E2), (F1), (F3), (F4), (F5), (G1), (J3) and (J5)
may be isolated as free bases or as salts such as formate salts, or
hydrochloride salts, or sodium salts. Whenever
isolating a title compound/intermediate as a salt, formate salt or
hydrochloride salt or sodium salt is indicated at the end
of the chemical name and can refer to a mono-, di- or tri-formate salt; or a
mono-, di-, or tri-hydrochloride salt; or a
mono-, or di-sodium salt.
Preparation of Examples of Formula (I)
Example 1: (3-Fluoro-1-methyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-y1)-
(4-trifluoromethoxy-pheny1)-methanol
To a light-yellow solution of Example A7.3 (45mg) in DCM (1mL) was added
formaldehyde (26.2pL, 37% in water)
followed by NaBH(OAc)3 (33.6mg). The resulting solution was stirred for 30min
at RT and was basified with aq. sat.
NaHCO3 solution. The resulting mixture was extracted with DCM and the org.
layers were dried (MgSO4), filtered off
and evaporated to dryness. The resulting crude material was purified by Prep
LC-MS (VI) to afford 17mg of the title
compound as white solid. LC-MS (A): tR = 0.63min; [M+H]': 426.21.
Example 2: 3-[Hydroxy-(5-pyrrolidin-1-yl-pyridin-3-y1)-(4-trifluoromethoxy-
pheny1)-methyl]-1-methyl-azetidine-3-
carbonitrile
Example 2 was synthesized starting from Example A7.2 and following the
procedure described in Example B2.1 step
B2.1.2. The material was purified by Prep LC-MS (V). LC-MS (A): tR = 0.63min;
[M+H]: 433.15.
Example 3: (R)-(1-Ethy1-3-methyl-azetidin-3-y1)-(5-pyrrolidin-l-yl-pyridin-3-
y1)-(4-trifluoromethoxy-phenylymethanol
To a light-yellow solution of Example A7.1 (20mg) in Me0H (0.5mL), AcOH
(0.05mL) was added at RT, followed
successively by acetaldehyde (15.2pL) and NaBH(OAc)3 (19.7mg). The resulting
solution was stirred 17h at RT.
Acetaldehyde (5pL) and NaBH(OAc)3 (9.85mg) were added again and the reaction
mixture was stirred for 4 days. The
mixture was quenched with water, filtered off and evaporated to dryness. The
resulting crude material was purified by
Prep LC-MS (VII) to afford 6mg of the title compound as white solid. LC-MS
(A): tR = 0.64min; [M-F1-1]': 436.29.
Example 4 to Example 8 were synthesized starting from the appropriate aldehyde
or ketone, following the procedure
described in Example 3. LC-MS data of Example 4 to Example 9 are listed in the
table below. The LC-MS conditions
used were LC-MS (A).
Example N Name tR
[M+I-1]+
(R)-(3-Methyl-1-propyl-azetidi n-3-yI)-(5-pyrrolid in-1-yl-pyridin-3-yI)-(4-
0.66
450.05
4 trifluoromethoxy-phenyl)-methanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
209
(R)-(1-lsopropy1-3-methyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-yI)-
0.65 450.04
(4-trifluoromethoxy-phenyI)-methanol
(R)-(1-Cyclopropylmethy1-3-methyl-azetidin-3-y1)-(5-pyrrolidin 1 yl
0.67 462.27
6 pyridi n-3-y1)-(4-trifluoromethoxy-pheny1)-methanol
(R)-(1-Cyclobuty1-3-methyl-azetidin-3-y1)-(5-pyrrolidin 1 yl pyridin-3-
0.67 462.27
7 yI)-(4-trifluoromethoxy-pheny1)-methanol
(R)-(1-lsobuty1-3-methyl-azetidin-3-y1)-(5-pyrrolidin yl pyridin-3-yI)-
0.69 464.3
8 (4-trifluoromethoxy-phenyI)-methanol
Example 9: (R)-(1-Cyclopropy1-3-methyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-
pyridin-3-y1)-(4-trifluoromethoxy-pheny1)-
methanol
To a light yellow solution of Example A7.1 (20mg) in Et0H (0.5mL), AcOH
(0.05mL) was added at RT, followed
successively by (1-ethoxycyclopropoxy)trimethysilane (91.5 pL) and sodium
cyanoborohydride (28.3mg). The resulting
5 solution was stirred for 17h at RT. 1-Ethoxycyclopropoxy)trimethysilane
(45.7pL) and sodium cyanoborohydride
(14.1mg) were added again and the reaction mixture was stirred for 4 days. The
mixture was quenched with water and
evaporated to dryness. The resulting crude material was purified by Prep LC-MS
(VII) to afford 4mg of the title
compound as white solid. LC-MS (A): tR = 0.65min; [M+H]': 448.28.
Example 10: (R)41-(2-Fluoro-ethyl)-3-methyl-azetidin-3-y1]-(5-pyrrolidin-1-yl-
pyridin-3-y1)-(4-trifluoromethoxy-pheny1)-
methanol
To a light yellow solution of Example A7.1 (20mg) in Me0H (0.5mL), TEA
(13.8pL) was added, followed by 1-fluoro-2-
iodo-ethane (17.4pL). The reaction mixture was stirred at reflux for 22h. 1-
Fluoro-2-iodo-ethane (3.95pL) and TEA
(3.14pL) were added and the mixture was stirred at reflux for 48h. The
resulting crude material was filtered off and
purified by Prep LC-MS (V) to afford 5mg of the title compound as white solid.
LC-MS (A): tR = 0.63min; [M-FH]-: 454.24.
Example 11: (R)- -(2,2-Difluoro-ethyl)-3-methyl-azetidin-3-y1]-(5-pyrrolidin-1-
yl-pyridin-3-y1)-(4-trifluoromethoxy-
pheny1)-methanol
The title compound was synthesized starting from Example A7.1 and 1,1-difluoro-
2-iodoethane, following the synthesis
procedure described in Example 10. LC-MS (A): tR = 0.65min; [M+H]: 472.2.
Example 12: (R)-(1-tert-Buty1-3-methyl-azetidi n-3-yI)-(5-pyrrol
uoromethoxy-pheny1)-
methanol
12.1. (R)-(1-lsopropeny1-3-methyl-azetidin-3-0-(5-pyrrolidin-l-yl-pyridin-3-
y1)-(4-trifluoromethoxy-pheny0-methanol
To a light yellow solution of Example A7.1 (20mg) and acetone (19.9pL) in
water (0.5mL) was added dropwise at 0 C
a solution of KCN (18.1mg) in water (0.5mL). The resulting mixture was stirred
for 69h at RT, was diluted with water
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
210
and extracted with EA. The org. layers were washed with brine, dried (MgSO4),
filtrated off and evaporated to dryness
to afford 80mg of the title compound. LC-MS (A): tR = 0.62min; [M-F1-1]':
448.26.
12.2 (R)-(1-tert-Buty1-3-methyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-
y1)-(4-trifluoromethoxy-pheny1)-methanol
To a solution of Example 12.1 (77mg) in THF (1.5mL) was added dropwise
methylmagnesium bromide (3M in diethyl
ether, 573pL) at 0 C. The resulting mixture was stirred for 10min at 0 C then
at 6000 overnight. The reaction mixture
was cooled down, quenched by addition of aq. sat. NH4CI solution, diluted with
water and extracted with EA. The org.
layers were washed with water and brine, dried (MgSO4), filtrated off and
evaporated to dryness. The resulting crude
material was first purified by Prep LC-MS (VIII) then by Prep LC-MS (IX) to
afford 9mg of the title compound as white
powder. LC-MS (A): tR = 0.65min; [M-FH]-: 464.29.
Example 13: (1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(3-methoxy-
prop-1-yny1)-pyridin-3-y1]-methanol
To a solution of Example F1.1 (100mg) in DMSO (2mL) and toluene (0.3mL),
methyl propargyl ether (33.5mg) was
added at RT followed by palladium(II) acetate (2.89mg), triphenylphosphine
(83.7mg) and K3PO4 (65.4mg). The
resulting solution was stirred for 66h at 80 C, was allowed to cool down, was
diluted with Me0H and water, filtered off
and purified by Prep LC-MS (VII) to afford 32mg of the title compound as
purple powder. LC-MS (A): tR = 0.75min;
[M+1-1]+: 379.34.
Example 14: 3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridin-3-yll-prop-1-yn-1-ol
Example 14 was synthesized starting from Example F1.2 and propargyl alcohol
following the procedure described in
Example 13. The crude material was purified by Prep LC-MS (VI). LC-MS (A): tR
= 0.65min; [M+H]: 365.25.
Example 15: 4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridin-3-y11-2-methyl-but-3-
yn-2-ol
To a solution of Example F1.2 (70mg) in THF (2.5mL) was added 2-methyl-3-butyn-
2-ol (24pL) followed by Cul
(0.86mg), tetrakis(triphenylphosphine)palladium(0) (83.2mg) and piperidine (93
pL). The resulting reaction mixture was
stirred for 2h at 80 C, cooled down, diluted with Me0H and water, filtrated
through a syringe filter and purified by Prep
LC-MS (VI) then by Prep LC-MS (XIII) to afford 38mg of the title compound as
white powder. LC-MS (A): tR = 0.69min;
[M+H]-: 393.33.
Example 16 to Example 46 were synthesized starting from Example F1.2 and the
appropriate alkyne of Formula (F5),
and following the procedure described in Example 15. The alkyne precursors of
Formula (F5) are indicated in the table
below unless commercially available. Prep LC-MS conditions and LC-MS data are
listed in the table below. The LC-MS
conditions used were LC-MS (A).
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
211
Example
Prep
Name tR [M+H] (F5)
11
LC-MS
16
(R)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y 0.66 I)-hydroxy-(4-
(V) then
379.3
isopropyl-phenyI)-methy1]-pyridin-3-yll-but-3-yn-2-ol
(XIII)
(S)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(V) then
17 0.67 379.27 -
isopropyl-pheny1)-methy1]-pyridin-3-yll-but-3-yn-2-ol
(XIII)
1-{5-[(R)-(1,3-01methyl-azetidin-3-yI)-hydroxy-(4-
18 isopropyl-pheny1)-methylFpyridin-3-ylethynyll- 0.74
419.33 - (IX)
cyclopentanol
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
19 isopropyl-pheny1)-methy1]-pyridin-3-ylethynyll-
0.69 391.3 (IX)
cyclopropanol
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
20 isopropyl-phenylymethyl]-pyridin-3-ylethyny1}-3- 0.78
506.32 - (IX)
hydroxy-azetidine-1-carboxylic acid tert-butyl ester
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
21 isopropyl-pheny1)-methyl]-pyridin-3-ylethynyll- 0.71
405.14 - (IX)
cyclobutanol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
22 0.64 379.29 - (IX)
isopropyl-pheny1)-methyll-pyridin-3-yll-but-3-yn-1-ol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-phenyly
(IX) then
23 [5-(1-methy1-1H-pyrazol-4-ylethyny1)-pyridin-3-y1]-
0.76 415.31
(VI)
methanol
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(IX) then
24 isopropyl-pheny1)-methy1]-pyridin-3-y11-1-phenyl-prop- 0.78 441.25 -
(VI)
2-yn-1-ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
25 isopropyl-phenylymethy1]-pyridin-3-y1}-2-phenyl-but-
0.81 455.26 - (XIV)
3-yn-2-ol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
212
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
26 isopropyl-pheny1)-methy1]-pyridin-3-y11-4-methyl-pent- 0.75 407.26 -
(VI)
1-yn-3-ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
27 isopropyl-pheny1)-methy1]-pyridin-3-ylethynyll- 0.67
435.24 - (V)
tetrahydro-pyran-4-ol
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
28 isopropyl-pheny1)-methy1]-pyridin-3-y11-1-(tetrahydro-
0.69 449.13 F5.1 (IX)
pyran-4-yI)-prop-2-yn-1-ol
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
29 isopropyl-pheny1)-methyll-pyridin-3-y11-1-(1,3- 0.75
459.24 F5.2 (IX)
dimethy1-1H-pyrazol-4-y1)-prop-2-yn-1-ol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-phenyly
30 [5-(tetrahydro-pyran-4-ylethyny1)-pyridin-3-y1]-
0.77 419.2 (V)
methanol
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
31 isopropyl-phenylymethy1]-pyridin-3-y11-1-(2-methyl- 0.71
462.18 F5.3 (IX)
thiazol-4-y1)-prop-2-yn-1-ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(VIII)
32 isopropyl-pheny1)-methy1]-pyridin-3-y11-2-(3-fluoro-
0.82 473.19 F5.4 then
phenyI)-but-3-yn-2-ol
(XIV)
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(VIII)
33 isopropyl-pheny1)-methyll-pyridin-3-y11-2-(4-methoxy-
0.80 485.07 F5.5 then
phenyI)-but-3-yn-2-ol
(XIV)
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(VIII)
34 isopropyl-pheny1)-methy1]-pyridin-3-y11-2-(2-methoxy-
0.81 485.17 F5.6 then
phenyI)-but-3-yn-2-ol
(XIV)
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(XV)
35 isopropyl-pheny1)-methy1]-pyridin-3-y11-2-(1-methyl-
0.68 459.11 F5.7 then
1H-pyrazol-3-y1)-but-3-yn-2-ol
(XIV)
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
213
4-15-[(R)-(1, 3-Di methyl-azetidi n-3-yI)-hydroxy-(4-
(VII)
36 isopropyl-phenyI)-methy1]-pyridi n-3-yI}-2-(2-methyl-
0.72 476.05 F5.8
then (IX)
thiazol-4-y1)-but-3-yn-2-ol
4-{5-[(R)-(1, 3-Di methyl-azetidi n-3-yI)-hydroxy-(4-
(XV)
37 isopropyl-phenyI)-methy1]-pyridi n-3-yI}-2-(6-methoxy-
0.81 486.17 F5.9
then (IX)
pyridin-2-y1)-but-3-yn-2-ol
4-{5-[(R)-(1, 3-Di methyl-azetidi n-3-yI)-hydroxy-(4-
(XV)
38 isopropyl-pheny1)-methy1]-pyridin-3-y11-2-pyrimidin-2-
0.69 457.17 F5.10
then (IX)
yl-but-3-yn-2-ol
4-{5-[(R)-(1, 3-Di methyl-azetidi n-3-yI)-hydroxy-(4-
(XV)
39 isopropyl-pheny1)-methyll-pyridin-3-y11-2-(1,5- 0.71
473.2 F5.11
then (IX)
dimethy1-1H-pyrazol-3-y1)-but-3-yn-2-ol
4-{5-[(R)-(1, 3-Di methyl-azetidi n-3-yI)-hydroxy-(4-
(XV)
40 isopropyl-phenyI)-methy1]-pyridi n-3-yI}-2-(6-methyl-
0.73 471.17 F5.12
then (IX)
pyrimidin-4-yI)-but-3-yn-2-ol
3-{5-[(R)-(1, 3-Di methyl-azetidi n-3-yI)-hydroxy-(4-
41 isopropyl-phenylymethy1]-pyridin-3-y11-1-(1,5- 0.69
459.16 F5.13 (IX)
dimethyll H-pyrazol-3-y1)-prop-2-yn-1-ol
8-{5-[(R)-(1, 3-Di methyl-azetidi n-3-yI)-hydroxy-(4-
42 isopropyl-phenyI)-methy1]-pyridi n-3-ylethynyI}-5,6,7, 8-
0.65 481.85 F5.14 (IX)
tetrahydro-quinolin-8-ol
7-{5-[(R)-(1, 3-Di methyl-azetidi n-3-yI)-hydroxy-(4-
43 isopropyl-pheny1)-methyll-pyridin-3-ylethyny11-6,7- 0.7
468.14 F5.15 (IX)
dihydro-5H-[1]pyrindin-7-ol
1-{5-[(R)-(1, 3-Di methyl-azetidi n-3-yI)-hydroxy-(4-
44 isopropyl-pheny1)-methy1]-pyridin-3-y11-3-pyridin-2-yl-
0.67 470.18 F5.16 (IX)
pent-1-yn-3-ol
1-{5-[(R)-(1, 3-Di methyl-azetidi n-3-yI)-hydroxy-(4-
45 isopropyl-phenyI)-methy1]-pyridi n-3-yI}-3-(6-methoxy-
0.84 500.14 F5.17 (IX)
pyridin-2-yI)-pent-1-yn-3-ol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
214
1-(3-15-KR)-(1, 3-D imethyl-azetidi n-3-yI)-hydroxy-(4-
46 isopropyl-pheny1)-methyl]-pyridin-3-ylethyny11-3- 0.69 476.17
F5.18 (IX)
hydroxy-azetidin-1-yI)-2-methyl-propan-1-one
Example 47: (R)-(1, 3-Di methyl-azetidin-3-yI)-[5-(1H-i ndo1-2-ylethyny1)-
pyridi n-3-y1]-(4-isopropyl-pheny1)-methanol
47.1. (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(5-
trimethylsilanylethynyl-pyridin-3-A-methanol
Example 47.1 was synthesized starting from Example F1.2 and
trimethylsilylacetylene and following the procedure
described in Example 15. The crude material was purified by Prep LC-MS (V). LC-
MS (A): tR = 0.89min; [M+H]-: 407.22.
47.2. (R)-(1,3-Dimethyl-azetidin-3-y1)-(5-ethynyl-pyridin-3-y1)-(4-isopropyl-
pheny1)-methanol
To a solution of Example 47.1 (24mg) in Me0H (0.5mL) was added K2003 (8.16mg).
The resulting mixture was stirred
for 2h30 at RT, diluted with EA, washed with water and brine. The org. layers
were dried (MgSO4), filtrated off, and
evaporated to dryness to afford 21mg of the title compound as brown solid. LC-
MS (A): tR = 0.73min; [M-FH]-: 335.19.
47.3. (R)-(1,3-Dimethyl-azetidin-3-y1)45-(1H-indol-2-ylethyny1)-pyridin-3-y1]-
(4-isopropyl-pheny1)-methanol
The title compound was synthesized starting from Example 47.2 and 2-iodo-1H-
indole, following the procedure
described in Example 15. The crude material was purified by Prep LC-MS (VII).
LC-MS (A): tR = 0.88min; [M-FI-1]':
449.82.
Example 48: (1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(3-methoxy-
propy1)-pyridin-3-y1]-methanol
A solution of Example 13 (29mg) was dissolved in Et0H (2mL), Pd/C (10% w/w,
50% water, 8.12mg) was added and
resulting mixture was stirred for 41h under hydrogen. The reaction mixture was
filtered off and evaporated to dryness
to afford 21mg of the title compound as brown resin. LC-MS (A): tR = 0.6min;
[M-FH]-: 383.36.
Example 49 to Example 81 were synthesized starting from the appropriate alkyne-
containing Example and following
the procedure described in Example 48. Precursor alkyne-containing Examples,
Prep LC-MS conditions and LC-MS
data are listed in the table below. The LC-MS conditions used were LC-MS (A).
Example Prep LC-
Precursor
Name tR [M+H]-
N MS
Example
3-{5-[(R)-(1, 3-Di methyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
49 0.51 369.18 none 14
pheny1)-methyl]-pyridin-3-y11-propan-1-ol
4-{5-[(R)-(1, 3-Di methyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
50 0.56 397.37 none 15
pheny1)-methyl]-pyridin-3-y11-2-methyl-butan-2-ol
(S)-4-{54(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
51 0.54 383.35 none 16
isopropyl-pheny1)-methyl]-pyridin-3-y1}-butan-2-ol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
215
(R)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
52 0.54 383.36 none 17
isopropyl-pheny1)-methyl]-pyridin-3-y1}-butan-2-ol
1-(2-{5-[(R)-(1,3-0imethyl-azetidin-3-yI)-hydroxy-(4-
53 0.62 423.1 none 18
isopropyl-pheny1)-methyl]-pyridin-3-y1}-ethyl)-cyclopentanol
1-(2-{5-[(R)-(1,3-0imethyl-azetidin-3-y1)-hydroxy-(4-
54 isopropyl-pheny1)-methyl]pyridin-3-y1}-ethyl)- 0.56
395.16 (IX) 19
cyclopropanol
3-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
55 isopropyl-phenyl)-methyl]-pyridin-3-y1}-ethyl)-3-hydroxy-
0.65 510.38 none 20
azetidine-1-carboxylic acid tert-butyl ester
1-(2-{5-[(R)-(1,3-0imethyl-azetidin-3-yI)-hydroxy-(4-
56 0.59 409.37 none 21
isopropyl-pheny1)-methy1]-pyridin-3-ylyethylycyclobutanol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
57 0.54 383.33 none 22
pheny1)-methyl]-pyridin-3-yll-butan-1-ol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{542-
58 0.59 419.32 (IX) 23
(1-methyl-1H-pyrazol-4-y1)-ethyl]-pyridin-3-yll-methanol
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
59 0.64 445.26 (VI) 24
pheny1)-methyl]-pyridin-3-y11-1-phenyl-propan-l-ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
60 0.66 459.26 (VI) 25
pheny1)-methyl]-pyridin-3-y11-2-phenyl-butan-2-ol
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
61 0.62 411.29 (VI) 26
pheny1)-methyll-pyridin-3-y11-4-methyl-pentan-3-ol
4-(2-{5-[(R)-(1,3-0imethyl-azetidin-3-y1)-hydroxy-(4-
62 isopropyl-phenyl)-methyl]-pyridin-3-y1}-ethyl)-tetrahydro-
0.54 439.26 (V) 27
pyran-4-ol
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
63 pheny1)-methyl]-pyridin-3-y11-1-(tetrahydro-pyran-4-y1)-
0.57 453.24 (V) 28
propan-1-ol
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
64 pheny1)-methyl]-pyridin-3-y11-1-(1,3-dimethyl-1H-pyrazol-4- 0.55 463.25 (V)
29
yI)-propan-1-ol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
216
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{542-[2
65 0.62 423.09 (V) 30
(tetrahydro-pyran-4-y1)-ethyl]-pyridin-3-yll-methanol
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
66 phenyl)-methyl]-pyridin-3-y11-1-(2-methyl-thiazol-4-y1)-
0.58 465.97 (V) 31
propan-1-ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
67 0.69 477.23 none 32
pheny1)-methy1]-pyridin-3-y11-2-(3-fluoro-pheny1)-butan-2-ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
68 phenyl)-methyl]-pyridin-3-y11-2-(4-methoxy-phenyl)-butan- 0.66 489.05 none
33
2-ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
69 phenylymethyl]-pyridin-3-y1}-2-(2-methoxy-phenyl)-butan- 0.69 489.24 none
34
2-ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
70 phenyl)-methyl]-pyridin-3-y11-2-(1-methyl-1H-pyrazol-3-y1)-
0.56 463.08 none 35
butan-2-ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
71 pheny1)-methy1]-pyridin-3-y11-2-(2-methyl-thiazol-4-y1)-
0.60 480.08 none 36
butan-2-ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
72 pheny1)-methy1]-pyridin-3-y11-2-(6-methoxy-pyridin-2-y1)-
0.68 490.2 (IX) 37
butan-2-ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
73 0.59 461.19 (IX) 38
pheny1)-methy1]-pyridin-3-y11-2-pyrimidin-2-yl-butan-2-ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
74 pheny1)-methy1]-pyridin-3-y11-2-(1,5-dimethy1-1H-pyrazol-3- 0.59 477.22
none 39
yI)-butan-2-ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
75 phenyl)-methyl]-pyridin-3-y11-2-(6-methyl-pyrimidin-4-y1)-
0.60 475.19 none 40
butan-2-ol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
217
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
76 pheny1)-methy1]-pyridin-3-y11-1-(1,5-dimethy1-1H-pyrazol-3- 0.57 463.20 (V)
41
y1)-propan-1-01
8-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
77 isopropyl-pheny1)-methy1]-pyridin-3-ylyethyl)-5,6,78-
0.53 486.18 (IX) 42
tetrahydro-quinolin-8-ol
7-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
78 isopropyl-pheny1)-methyl]-pyridin-3-ylyethyl)-6,7-dihydro-
0.55 472.17 (IX) 43
5H-[1]pyrindin-7-ol
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
79 0.54 474.19 (IX) 44
pheny1)-methyl]-pyridin-3-y11-3-pyridin-2-yl-pentan-3-ol
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
80 pheny1)-methyl]-pyridin-3-y11-3-(6-methoxy-pyridin-2-y1)-
0.72 504.15 (IX) 45
pentan-3-ol
(R)-(1,3-Dimethyl-azetidin-3-y1)-{542-(1H-indo1-2-y1)-ethyl]-
81 0.71 453.94 none 46
pyridin-3-yIH4-isopropyl-pheny1)-methanol
Example 82: (R)-(4-Cyclopropyl-pheny1)-(1,3-dimethyl-azetidin-3-y1)-{513-
(tetrahydro-pyran-4-y1)41,2,4]oxadiazol-5-
y1]-pyridin-3-ylymethanol
82.1. 3-((R)-(4-Cyclopropyl-phenyl)-hydroxy-{513-(tetrahydro-pyran-4-
y1)11,2,41oxadiazol-5-yll-pyridin-3-y1}-methy0-3-
methyl-azetidine-1-carboxylic acid tert-butyl ester
A vial was charged with Example E1.2 (50mg), Example E2.1 (24.7mg), and
HOBt.H20 (23.4mg). Dioxane (1.1mL) and
DIPEA (0.039mL) were added followed by EDC.HCI (28.7mg). The reaction mixture
was heated at 90 C until completion
of the reaction. The reaction mixture was cooled down to RT and partitioned
between DCM and sat. aq. NaHCO3. The
org. layer was filtered over a phase separator and concentrated in vacuo. The
residue (105mg) was purified by Prep
LC-MS (XII) to afford the title product as a white solid (27.5mg). LC-MS (A):
tR = 1.09min; [M-+I]+: 547.25.
82.2. (R)-(4-Cyclopropyl-phenyl)-(3-methyl-azetidin-3-y1)-{513-(tetrahydro-
pyran-4-y1)11,2,41oxadiazol-5-yil-pyridin-3-
A-methanol, hydrochloride salt
Example 82.1 (27.5mg) was dissolved with HCI in dioxane (4M, 0.13mL). The
reaction mixture was stirred at RT until
completion, was concentrated in vacuo, dried under HV and used without further
purification. LC-MS (A): tR = 0.74min;
[M-FI-1]+: 447.33.
82.3. (R)-(4-CYcloProPYI-phenyl)-(1,3-dimethyl_azetidin-3-0)-{5-13-(tetrahydro-
pyran-4-y1)41,2,4]oxadiazol-5-0.1-
pyridin-3-yll-methanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
218
The title compound was prepared starting from Example 82.2, and following the
procedure described in Example D1.1
step D1.1.5. LC-MS (B): tR = 0.68min; [M-FI-1]-F: 461.40.
Example 83 to Example 93 were synthesized starting from the appropriate
compounds of Formula (E1) and (E2) and
following the three-step procedure described in Example 82, and were purified
by Prep LC-MS at the end of the
sequence if needed. The precursors of Formula (El) and (E2) are indicated in
the table below unless commercially
available. The Prep LC-MS methods and the LC-MS data are listed in the table
below. The LC-MS conditions used
were LC-MS (B) except for Example 92 where the LC-MS conditions used were LC-
MS (A).
Example (El)/
Prep
Name tR [M+H]
N (E2)
LC-MS
(R)-(4-Cyclopropyl-phenyI)-(1,3-dimethyl-azetidin-3-
E1.2
83 y1)-{5-[3-(2-methoxy-1,1-dimethyl-ethyly 0.780
463.3 none
E2.2
[1,2,4]oxadiazol-5-ylypyridin-3-ylymethanol
(R)45-(3-Cyclobutoxymethyl-[1,2,4]oxadiazol-5-yly
El .2
84 pyridin-3-y1]-(4-cyclopropyl-pheny1)-(1,3-dimethyl-
0.786 461.3 (XIV)
azetidin-3-yl)-methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-propyl-pheny1)-{5-
El .3
85 [3-(tetrahydro-pyran-4-yloxymethy1)[l,2,4]oxadiazol-
0.734 493.3 (IX)
5-A-pyridin-3-ylYmethanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-propyl-pheny1)-{5-
El .3
86 [3-(tetrahydro-pyran-4-yl)-[1,2,4]oxadiazol-5-yly
0.743 463.3 none
pyridin-3-ylymethanol
(R)45-(3-Cyclobutoxymethyl-[1,2,4]oxadiazol-5-y1)-
E1.3
87 pyridin-3-y1]-(1,3-dimethyl-azetidin-3-y1)-(4-propyl-
0.840 463.3 none
phenyI)-methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-phenyl)-
E1.1
88 15-[3-(tetrahydro-pyran-4-y1)-[1,2,4]oxadiazol-5-yly
0.718 463.4 (IX)
pyridin-3-ylymethanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-phenyl)-
E1.1
89 [5-(3-morpholin-4-ylmethyl-[1,2,4]oxadiazol-5-y1)-
0.588 478.3 (VI)
E2.5
pyridin-3-ylYmethanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
219
(R)-(1,3-Dimethyl-azetidin-3-yI)-{5-[3-(2,6-dimethyl-
E1.1
90 morpholin-4-ylmethyly[1,2,4]oxadiazol-511]-pyridin-
0.669 506.4 (VI)
3-y11-(4-isopropyl-pheny1)-methanol E2.6
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
E1.1
91 {5-[3-{4-methyl-tetrahydro-pyran-4-yI)- 0.777 477.3
(IX)
E2.7
[1,2,4]oxadiazol-5-y11-pyridin-3-yll-methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-phenyl)-
E1.1
92 {5-[(1S,2S,4R)-3-(7-oxa-bicyclo[2.2.1]hept-2-yI)- 0.78
475.29 (IX)
E2.8
[1,2,4]oxadiazol-511]-pyridin-3-yll-methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-phenyly
E1.1
93 {5[3-{tetrahydro-pyran-4-yloxymethyl)- 0.720 493.3
(IX)
[1,2,4]oxadiazol-511]-pyridin-3-yll-methanol
Example 94: (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[5-(3-
morpholin-4-y141,2,4]oxadiazol-5-y1)-pyridin-3-
A-methanol
94.1. 3-[(R)15-(3-Amino-[1,2,4]oxadiazol-5-A-pyridin-3-ylrhydroxy-(4-isopropyl-
pheny1)-methyl]-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
The title compound was prepared starting from Example E1.1 and N-
hydroxyguanidine sulfate and using the conditions
described in Example 82 step 82.1. The material was purified by Prep LC-MS
(VI). LC-MS (A): tR = 1.00min; [M+H]+:
480.39.
94.2. (R)-(5-(3-Chloro-[1, 2, 4]oxadiazol-5-y1)-pyridin-3-y1]-(4-isopropyl-
pheny1)-(3-methyl-azetidin-3-y1)-methanol
To an ice-cold solution of Example 94.1 (9mg) in conc. HCI (37% fuming, 0.1mL)
was added a solution of sodium nitrite
(1.31mg) in water (0.05mL). The reaction mixture was stirred at 0 C for 1h,
diluted with water and quenched with solid
NaHCO3. The mixture was extracted with DCM and the combined org. layers were
filtered over a phase separator,
concentrated in vacuo and dried under HV to afford the title compound (7.5mg)
that was used without further purification.
LC-MS (A): tR = 0.77min; [M-FH]-F: 399.28.
94.3. (R)-15-(3-Chloro-[1,2,4]oxadiazol-5-y1)-pyridin-3-y1]-(1,3-dimethyl-
azetidin-3-0)-(4-isopropyl-pheny1)-methanol
The title compound was synthesized starting from Example 94.3 and using the
conditions described in Example F1.1
step F1.1.2. LC-MS (A): tR = 0.79min; [M-FH]+: 413.3.
94.4. (R)-(1, 3-Dim ethyl-azetidin-3-y1)-(4-isopropyl-pheny1)15-(3-morpholin-4-
y141, 2, 4]oxadiazol-5-4-pyridi
methanol
Example 94.3 (7.7mg) was dissolved in Et0H (0.1mL) and transferred in a sealed
vial. Morpholine (33.2pL) and DIPEA
(64.4pL) were added and the reaction mixture was heated at 90 C for 4h. The
mixture was cooled down to RT, diluted
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
220
in MeCN and purified by Prep LC-MS (IX) to afford the title compound as white
solid (0.5mg). LC-MS (A): tR = 0.76min;
[M-FH]F: 464.4.
Example 95: (R)-(4-Cyclopropyl-pheny1)-(1,3-dimethyl-azetidin-3-y1)45-[3-(4-
methoxy-tetrahydro-pyran-4-y1)-
[1,2,4]oxadiazol-5-y1]-pyridin-3-yll-methanol
95,1, 3-((R)-(4-Cyclopropyl-phenyl)-hydroxy-{5-13-(4-methoxy-tetrahydro-pyran-
4-y1)41,2,4]oxadiazol-5-ylppyridin-3-
0-methyl)-3-methyl-azetidine-1-carboxylic acid tert-butyl ester
A sealed vial was charged with Example E1.2 (100mg) and DMF (2.2mL) was added,
followed by DIPEA (0.12mL) and
PyBOP (182mg). After stirring at RT for 15min, Example E2.3 (79.4mg) and K3PO4
(198mg) were added. The reaction
mixture was heated overnight at 100 C. The mixture was cooled down to RT,
partitioned between DCM and water/sat.
aq. NaHCO3. The phases were separated, and the aq layer was extracted twice
with DCM. The org. layers were filtered
over a phase separator and concentrated to dryness. The residue was purified
by Prep LC-MS (XII) to afford the desired
product as white solid (94.7mg). LC-MS (A): tR = 1.08min; [M+I-1]+: 577.21.
95.2. (R)-(4-Cyclopropyl-phenyl)-{543-(4-methoxy-tetrahydro-pyran-4-y1)41
,2,4]oxadiazol-5-ylkpyridin-3-4-(3-methyl-
azetidin-3-y1)-methanol, hydrochloride salt
Example 95.2 was prepared starting from Example 95.1 and following the
procedure described in Example 82 step
82.2. LC-MS (A): tR = 0.72min; [M+I-1]+: 477.28.
95.3. (R)-(4-Cyclopropyl-phenyl)-(1 , 3-dim ethyl-azetidi n-3-y1)-{543-(4-
methoxy-tetra hydro-pyran-4-yI)-fl ,2, 4]oxadiazol-
5-1/1.1-Pyridin-3A-methanol
Example 95.3 was prepared starting from Example 95.2 and following the
procedure described in Example D1.1 step
D1.1.5. The material was purified by Prep LC-MS (IX). LC-MS (B): tR =
0.693min; [M+H]+: 491.3.
Example 96 to Example 102 were synthesized starting from the appropriate
compounds of Formula (El) and (E2) and
following the three-step procedure described in Example 95, and were purified
by Prep LC-MS at the end of the
sequence if needed. The precursors of Formula (El) and (E2) are indicated in
the table below unless commercially
available. The Prep LC-MS methods and the LC-MS data are listed in the table
below. The LC-MS conditions used
were LC-MS (B).
Example (El)/
Prep LC-
Name tR [M-FH]
N (E2)
MS
(R)-(4-Cyclopropyl-phenyI)-(1,3-dimethyl-azetidi n-3-
El .2
96 )11)-{543-(3-hydroxymethyl-bicyclo[1.1.1]pent-1-y1)-
0.646 473.4 (IX)
E2.4
[1,2,4]oxadiazol-5-y1Fpyridin-3-yll-methanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
221
(R)-(1,3-Dimethyl-azetidin-3-y1)-{543-(3-
hydroxymethyl-bicyclo[1.1.11pent-1-y1)- E1.1
97 0.695 475.0
(IX)
[1,2,4]oxadiazol-511]-pyridin-3-y11-(4-isopropyl- E2.4
phenyI)-methanol
2-(5-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
E1.1
98 isopropyl-pheny1)-methyl]-pyridin-3-yly 0.709
451.3 (VI)
E2.9
[1,2,4]oxadiazol-3-y1)-2-methyl-propan-1-01
2-(5-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
E1.1
99 isopropyl-phenyI)-methyl]-pyridin-3-yll- 0.671
437.3 (V)
E2.10
[1,2,4]oxadiazol-3-y1)-propan-2-ol
(R)-(1,3-Dimethyl-azetidin-3-yI)-(4-isopropyl-
E1.1
100 pheny1)-{543-(l-methoxy-l-methyl-ethyl)- 0.767
451.3 (VI)
E2.11
[1,2,4]oxadiazol-5-A-pyridin-3-yll-methanol
(R)-(1,3-Dimethyl-azetidin-3-yI)-(4-isopropyl-
E1.1
101 pheny1)-{543-(l-methoxy-cyclobuty1)- 0.808 463.3
(VII)
[1,2,4]oxadiazol-5-y1]-pyridin-3-y1}-methanol E2.12
1-(5-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
E1.1
102 isopropyl-pheny1)-methyl]-pyridin-3-y11- 0.684
451.3 (V)
E2.13
[1,2,4]oxadiazol-3-y1)-2-methyl-propan-2-ol
Example 103: (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(5-
methanesulfonylmethyl-[1,2,4]oxadiazol-3-
y1)-pyridin-3-y1Fmethanol
A mixture of Example D2.1 (25.4mg), 2-(methylsulfonyl)acetic acid (10.7mg),
PyBOP (53.9mg), K3PO4(29.8mg) and
DIPEA (18pL) in DMF (0.8mL) was heated at 85 C for 16h, cooled down to RT, and
water (200pL) was added. The
resulting solution was purified by Prep LC-MS (IX) then (V) to afford the
desired product. LC-MS (B): tR = 0.646min;
[M-Fl-l]*: 471.3.
Example 104 to Example 133 were synthesized starting from Example D2.1 and the
appropriate carboxylic acid of
Formula (D3), and following the procedure described in Example 103. The Prep
LC-MS methods used are indicated in
the table below. LC-MS data of Example 104 to 133 are listed in the table
below. The LC-MS conditions used were LC-
MS (B).
Example
Prep
Name tR [M+1-1]'
LC-MS
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
222
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{545-
104 (2-methoxy-ethyl)-[1,2,4]oxadiazol-311]-pyridin-3-yly
0.702 437.4 (IX)+(VI)
methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(5-
105 0.703 423.3 (IX)+(VI)
methoxymethyl-[1,2,4]oxadiazol-3-y1)-pyridin-3-y1]-methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{545-
106 (tetrahydro-furan-3-y1)41,2,4]oxadiazol-311]-pyridin-3-yll-
0.71 449.3 (IX)+(VI)
methanol
(R)-(1,3-Dimethyl-azetidin-3-yI)-(4-isopropyl-pheny1)-{5-[5-
107 (tetrahydro-pyran-411)41,2,4]oxadiazol-311]-pyridin-3-yly
0.731 463.3 (IX)+(VI)
methanol
1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
108 isopropyl-pheny1)-methyl]-pyridin-3-y1141,2,4]oxadiazol-5-
0.806 477.3 (IX)+(VII)
yI)-cyclohexanol
(R)-[5-(5-tert-Butoxymethyl-[1,2,4]oxadiazol-3-y1)-pyridin-3-
109 y1]-(1,3-dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
0.841 465.3 (XIV)+(VIII)
methanol
(R)-(1,3-Dimethyl-azetidin-3-yI)-(4-isopropyl-pheny1)-{5-[5-
110 (tetrahydro-pyran-4-ylmethy1)41,2,4]oxadiazol-3-4-pyridin-
0.751 477.3 (IX)+(VI)
3-y11-methanol
4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
111 isopropyl-pheny1)-methy1]-pyridin-3-y1141,2,4]oxadiazol-5-
0.69 477.3 (IX)+(VI)
yI)-cyclohexanol
1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
112 isopropyl-pheny1)-methyll-pyridin-3-01-[1,2,4]oxadiazol-5-
0.806 491.4 (IX)+(VII)
ylmethylycyclohexanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-15-[5-
113 (1-methoxy-cyclobuty1)41,2,4]oxadiazol-311]-pyridin-3-y1)-
0.824 463.3 (XIV)+(VIII)
methanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
223
(R)-(1,3-Dimethyl-azetidin-3-yl)-(4-isopropyl-phenyl)-{5-[5-
114 (6-oxa-spiro[2.5]oct-1-y1)41,2,4]oxadiazol-311]-pyridin-3-
y11- 0.779 489.4 (XIV)+(V11)
methanol
(R)-(1,3-Dimethyl-azetidin-3-yl)-(4-isopropyl-phenyl)-{545-
115 (tetrahydro-pyran-3-y1)41,2,4]oxadiazol-311]-pyridin-3-y11-
0.759 463.3 (IX)+(VII)
methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-phenyl)-(5-{5-
116 [1-(tetrahydro-furan-2-yl)methyl]-[1,2,4]oxadiazol-3-y11-
0.741 463.4 (IX)-F(VII)
pyridin-3-yI)-methanol
(R)-2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
117 isopropyl-pheny1)-methyll-pyridin-3-y1141,2,4]oxadiazol-5-
0.778 491.2 (IX)+(VI)
YD-1,1,1-trifluoro-propan-2-ol
(R)-(1,3-0imethyl-azetidin-3-yl)-(4-isopropyl-phenyl)-{5-[5-
118 (1-methoxy-1-methyl-ethyly[1,2,4]oxadiazol-311]-pyridin-3-
0.785 451.3 (IX)-F(VII)
ylymethanol
(R)-{5454(R)-Cyclohexyl-hydroxy-methy1)41,2,4]oxadiazol-
119 311]-pyridin-3-y11-(1,3-dimethyl-azetidin-3-y1)-(4-
isopropyl- 0.847 491.5 (XIV)-F(V111)
phenyI)-methanol
1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
120 isopropyl-pheny1)-methyl]-pyridin-3-y1141,2,4]oxadiazol-5-
0.675 435.2 (IX)-F(XV)
yI)-cyclopropanol
2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
121 isopropyl-pheny1)-methyl]-pyridin-3-y1141,2,4]oxadiazol-5-
0.685 437.3 (IX)+(VI)
yI)-propan-2-ol
1-(3-{5-[(R)-(1,3-0imethyl-azetidin-3-y1)-hydroxy-(4-
122 isopropyl-pheny1)-methyl]-pyridin-3-y11-[1,2,4]oxadiazol-5-
0.753 463.3 (IX)-F(VI)
yI)-cyclopentanol
3-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
123 isopropyl-pheny1)-methyl]-pyridin-3-y1141,2,4]oxadiazol-5-
0.652 449.4 (IX)+(V)
yI)-cyclobutanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
224
(R)-(1,3-D imethyl-azetidi n-3-y I)-{5-[5-(4-fluoro-tetrahydro-
124 pyran-4-y1)-[1,2,4]oxadiazol-3-y1]-pyridin-3-y1}-(4-
isopropyl- 0.772 481.3 (IX)+(VI I)
phenyI)-methanol
(R)-(1, 3-Di methyl-azetidin-3-y1)-{5454(2R,4R,6S)-2,6-
125 dimethyl-tetrahydro-pyran-4-y1)41,2,4]oxadiazol-3-y1]-
0.827 491.3 (XIV)+(VI II)
pyridin-3-y11-(4-isopropyl-phenylymethanol
(R)-(1,3-D imethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-{545-
126 (tetrahydro-pyran-4-yloxymethy1)11,2,4]oxadi azol-3-y1]-
0.728 493.3 (IX)+(VI)
pyridin-3-y1}-methanol
(R)-(1,3-D imethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-(5-{5-
127 [1-methyl-1-(tetrahydro-pyran-4-y1)-ethy1141,2,41oxadiazol-
3- 0.84 505.4 (XIV)+(V)
yll-pyridin-3-y1)-methanol
(R)-(1,3-D imethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-(5-{5-
128 [2-(tetrahydro-pyran-4-y1)-ethyl]-[1,2,4]oxadiazol-3-y1}-
0.792 491.3 (X1V)-F(VI I)
pyridin-3-y1)-methanol
1-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-yI)-hydroxy-(4-
129 isopropyl-phenylymethyl]-pyridin-3-y11-[1,2,4]oxadiazol-5-
0.716 449.3 (I X)-F(VI )
yI)-cycl obutanol
1-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-yI)-hydroxy-(4-
130 isopropyl-pheny1)-methyl]-pyridin-3-y1141,2,4]oxadiazol-5-
0.69 451.3 (IX)+(VI)
yI)-2-methyl-propan-2-ol
(R)-(1 ,3-D imethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-{545-
131 (7-oxa-bicyclo[2.2.1]hept-2-y1)41,2,41oxadiazol-3-y11-
pyridin- 0.76 475.3 (IX)+(VI I)
3-ylymethanol
(R)-(1, 3-0 imethyl-azetidi n-3-yI)-(4-isopropyl-pheny1)-{5-[5-
132 (4-methyl-tetrahydro-pyran-4-yloxymethyl)-[1,2,4]oxadiazol-
0.77 507.3 (IX)-F(VI)
3-yI]-pyridin-3-ylymethanol
(R)-(1,3-D imethyl-azetidi n-3-y1)-(4-isopropyl-pheny1)45-(5-
133 0.667 435.3 (IX)+(VI I)
oxetan-3-y141,2,4]oxadiazol-3-y1)-pyridin-311]-methanol
Example 134: (R)-(1, 3-Di methyl-azetidi n-3-y1)-(4-isopropyl-pheny1)-{5-[5-(2-
methoxy-1, 1-d imethyl-ethyl)-
[1,2, 4]oxadiazol-311]-pyridi n-3-ylymethanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
225
A mixture of Example D2.1 (40mg), 3-methoxy-2,2-dimethylpropanoic acid
(15.8mg), PyBOP (86.8mg), K3PO4(92.2mg)
and DIPEA (55.8pL) in DMF (1mL) was heated at 80 C for 20h, cooled down to RT,
quenched with water and extracted
with DCM. The combined organic layers were dried (MgSO4), filtered off and
concentrated in vacuo. The residue was
purified by Prep LC-MS (XVIII) and (VIII) to afford 25mg of the desired
product. LC-MS (B): tR = 0.834min; [M+H]:
465.3.
Example 135 to 138 were synthesized starting from Example D2.1 and the
appropriate carboxylic acid of Formula (D3),
and following the procedure described in Example 134. LC-MS data of Example
135 to 138 are listed in the table below.
The LC-MS conditions used were LC-MS (B). The Prep LC-MS methods used are
indicated in the table below.
Example
Prep
Name tR [M+H]
N
LC-MS
2-(3-{5-[(R)-(1, 3-0 imethyl-azetidi n-3-yI)-hydroxy- (4-
135 isopropyl-pheny1)-methy1]-pyridin-3-y1141,2,4]oxadiazol-5-
0.179 451.3 (XI I l)+(VI)
yI)-2-methyl-propan-1-ol
(R)-(1, 3-Di methyl-azetidin-3-y1)-(4-isopropyl-pheny1)-15-
(XVIII)+
136 [5-(2-methoxy-2-methyl-propy1)41,2,4]oxadiazol-3-y1]-
0.786 465.3
pyridin-3-ylymethanol
(VII)
1-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-yI)-hydroxy- (4-
(XIII)+(VI)+S
137 isopropyl-pheny1)-methy1]-pyridin-3-y1141,2,4]oxadiazol-5-
0.727 463.3 FC(X111)
ylmethyl)-cyclobutanol
-F(VI)
(R)-(1, 3-Di methyl-azetidin-3-y1)-(4-isopropyl-pheny1)-15-
138 [5-(1-methoxymethyl-cyclopropylmethy1)41,2,4]oxadiazol-
0.801 477.3 (XIII)
3-y1]-pyridin-3-yll-methanol
Example 139: (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{545-(2-
pyrazol-1-yl-ethy1)41,2,41oxadiazol-3-y11-
pyridin-3-yI}-methanol
The title compound was obtained starting from Example D2.1 and 3-(1H-pyrazol-1-
yl)propanoic acid, and following the
procedure described in Example 82 step 82.1, but heating the reaction mixture
at 100 C. The crude material was
purified by Prep LC-MS (IX). LC-MS (A): tR = 0.75min; [M+H]: 473.3.
Example 140: (R)-N-(2-(3-(54(1,3-dimethylazetidin-3-y1)(hydroxy)(4-
isopropylphenyl)methyppyridin-3-y1)-1,2,4-
oxadiazol-5-ypethypacetamide-2,2,2-d3
140.1. 3-[(R)-{5-15-(2-Benzyloxycarbonylamino-ethy1)11,2,41oxadiazol-3111-
pyridin-3-4-hydroxy-(4-isopropyl-pheny1)-
methyl]-3-methyl-azetidine-1-carboxylic acid tert-butyl ester
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
226
The title compound was obtained starting from Example D2.2 and Cbz-beta-
Alanine, and following the procedure
described in Example 134. LC-MS (A): tR = 1.12nnin; [M+1-1]+: 642.18.
140.2. 3-[(R)-{515-(2-Amino-ethyl)41,2,47oxadiazol-3-ylrpyridin-3-4-hydroxy-(4-
isopropyl-phenyl)-methyl]-3-methyl-
azetidine-1-carboxylic acid tert-butyl ester
A mixture of Example 140.1 (76.4mg) and Pd/C (9.88mg) in Et0H (2mL) under H2
atmosphere was stirred at RT for 6h,
filtered over a glass paper fiber filter and the filtrate was concentrated in
vacuo and dried under HV to afford 30.2 mg
of the title compound. LC-MS (A): tR = 0.81min; [M+H]: 508.01.
140.3. tert-Butyl (R)-345-(5-(2-(acetamido-2,2,2-d3)ethyl)-1,2,4-oxadiazol-3-
Apyridin-3-y1)(hydroxy)(4-
isopropylphenyl)methyl)-3-methylazetidine-1-carboxylate
A mixture of Example 140.2 (30.2mg), acetic acid-2,2,2-d3 (99 atom % D,
10.2pL), HOBt (9.65mg), DIPEA (30.6pL)
and EDC.HCI (13.7mg) in DCM (1mL) was stirred overnight at RT, quenched with
aq. sat. NaHCO3 and extracted with
DCM. The combined org. layers were dried (MgS0.4), filtered off and
concentrated in vacuo to afford 32.9mg of the title
compound. LC-MS (A): tR = 0.99min; [M-FH]-: 533.25.
140.4. (R)-N-(2-(3-(5-(hyd roxy(4-isopropylp henyl)(3-m ethylazetidin-3-yOrn
ethyl)pyridin-3-yI)-1, 2, 4-oxadiazol-5-
yOethyl)acetamide-2,2,2-d3, hydrochloride salt
A solution of Example 140.3 (32.9mg) in HCI in dioxane (4M, 2mL) was stirred
at RT for 30min, concentrated in vacuo
and dried under HV to afford 29.1 mg of the title compound. LC-MS (A): tR =
0.66min; [M-FH]': 453.06.
140.5. (R)-N-(2-(3-(54(1,3-dimethylazetidin-3-y1)(hydroxy)(4-
isopropylphenyOmethyl)pyridin-3-y1)-1,2,4-oxadiazol-5-
yOethyl)acetamide-2,2,2-d3
The title compound was obtained starting from Example 140.4, and following the
procedure described in Example D1.1
step D1.1.5. The reaction mixture was filtered off and the filtrate was
purified by Prep LC-MS (IX) and (V) to afford the
desired compound (6.9mg) as a white powder. LC-MS (B): tR = 0.617min; [M-FH]-:
467.3.
Example 141 to Example 143 were synthesized starting from Example D2.2, and
following the five-step procedure
described in Example 140, using the appropriate carboxylic acid derivative of
Formula (D3) in the first step. The Prep
LC-MS methods and the LC-MS data are listed in the table below. The LC-MS
conditions used were LC-MS (B).
Example
Prep LC-
Name tR [M-
FH]-
N MS
(R)-N-(1-(3-(5-((1,3-dimethyl azetidin-3-yI)(hydroxy)(4-
141 isopropylphenyl)methyl)pyridin-3-y1)-1,2,4-oxadiazol-5-y1)-
2- 0.696 495.4 (IX)+(VI)
methylpropan-2-yl)acetamide-2,2,2-d3
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
227
144-(345-[(R)-(1, 3-Dimethyl-azetidi n-3-yI)-hydroxy-(4-
142 isopropyl-pheny1)-methy1]-pyridin-3-y1141,2,4]oxadiazol-
511)- 0.655 520.3 (IX)-F(VI)
piperidin-1-yI]-2-hydroxy-ethanone
(R)-1-(4-(3-(5-((1, 3-di methylazetidi n-3-yI)(hydroxy)(4-
143 isopropylphenyl)methyppyridin-3-y1)-1,2,4-oxadiazol-5-
0.671 507.5 (IX)-F(VI)
yl)piperidin-1-yl)ethan-1-one-2,2,2-d3
Example 144: N42-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridin-3-yll-
[1,2, 4]oxadi azol-5-y1)-ethyl]-2-hydroxy-N-methyl-acetamide
144,1, 3-(tert-Butoxycarbonyl-methyl-amino)-propionic acid benzyl ester
To a solution of 34(tert-butoxycarbonyl)(methypamino]propanoic acid (100mg) in
DMF (1mL) were added benzyl
bromide (58.4pL) and K2CO3 (66.6mg). The reaction mixture was stirred at RT
for 2h, quenched with water and
extracted with EA. The org. layer was dried (MgSO4), filtered off,
concentrated in vacuo and dried under HV to afford
141mg of the title compound. LC-MS (A): tR = 0.99min; [M-FH]-: 294.1.
144,2 3-Methylamino-propionic acid benzyl ester, hydrochloride salt
A solution of Example 144.1 (141mg) in HCI in dioxane (4M, 3mL) was stirred at
RT for 30min, concentrated in vacuo
and dried under HV to afford 46.6mg of the title compound. LC-MS (A): tR =
0.51min; [M-FH]-: 194.21.
144,3 3[(2-Hydroxy-acetyl)-methyl-amino]-propionic acid benzyl ester
The title compound was obtained starting from Example 144.2 and glycolic acid,
and following the procedure described
in Example 140 step 140.3. LC-MS (A): tR = 0.67min; [M-FH]': 252.07.
144,4, 3[(2-Hydroxy-acetyl)-methyl-amino]-propionic acid
A mixture of Example 144.3 (60.6mg) and Pd/C (19.9mg) in EA (1mL) under H2
atmosphere was stirred at RT for 3h.
Additional Pd/C was added and the reaction mixture was stirred overnight at
RT, filtered over a glass paper fiber filter.
The filtrate was concentrated in vacuo and dried under HV to afford 19.4mg of
the desired compound. LC-MS (A): tR =
0.26min; [M-FH]t 162.2.
144.5. 34(R)-Hydroxy-15-(5-{24(2-hydroxy-acetyl)-methyl-
aminoPethyl)41,2,4]oxadiazol-3-y1)-pyridin-3-y11-(4-
isopropyl-phenyl)-methyl]-3-methyl-azetidine-1-carboxylic acid tert-butyl
ester
The title compound was obtained starting from Example D2.2 and Example 144.4,
and following the procedure
described in Example 134, but with a second addition of reagents after the
night and an additional night of stirring. LC-
MS (A): tR = 0.97min; [M+H]: 580.01.
144,6, 2-Hydroxy_N_[243_154(R)_hydroxy-(4-isoProPYI-Pheny1)-(3-methyl-azetidin-
3-y1)-methylrpyridin-3-Y0-
[1 ,2,4]oxadiazol-5-y1)-ethyl]-N-methyl-acetamide, hydrochloride salt
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
228
A solution of Example 144.5 (68.4mg) in HCI in dioxane (4M, 2mL) was stirred
at RT for 1h, concentrated in vacuo and
dried under HV to afford 60.9nng of the title compound. LC-MS (A): tR =
0.65min; [M+1-1]': 480Ø
144.7. N-12-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyll-pyridin-3-y1}41,2,4]oxadiazol-
5-y1)-ethyl]-2-hydroxy-N-methyl-acetamide
The title compound was obtained starting from Example 144.6, and following the
procedure described in Example 82
step 82.3, with a direct filtration of the reaction mixture and purification
by Prep LC-MS (IX) and (VI) to afford 6.2mg of
the desired compound. LC-MS (B): tR = 0.616min; [M+H]: 494.4.
Example 145: (R)-N-(2-(3-(5-((1,3-dimethylazetidin-3-y1)(hydroxy)(4-
isopropylphenyl)methyppyridin-3-y1)-1,2,4-
oxadiazol-5-ypethyl)-N-methylacetamide-d3
The title compound was obtained starting from Example 144.2, and following the
procedure described in Example 144
step 144.3 to 144.7, using acetic acid-2,2,2-d3 instead of glycolic acid in
step 144.3. The crude material was purified by
Prep LC-MS (IX) and (VI). LC-MS (B): tR = 0.651min; [M+H]-: 481.3.
Example 146: (1,3-Dimethyl-azetidin-3-y1)-(6-methoxy-pyridin-3-y1)-(4-
trifluoromethoxy-phenyl)-methanol
5-Bromo-2-methoxypyridine (110pL) and iPrMgCl.LiCI (1.3M in THF, 704pL) were
dissolved in THF (0.5mL) and the
resulting solution was stirred for 2h at 60 C. A solution of Example B2.1
(100mg) in THF (0.5mL) was added, the
resulting mixture was stirred for 4h at RT and was quenched with aq. sat.
NH40I, diluted with water and extracted with
DCM. The org. layers were evaporated to dryness. The resulting crude material
was purified by Prep LC-MS (VI) to
afford 50mg of the title compound as brown solid. LC-MS (A): tR = 0.74min; [M-
FH-F: 383.20.
Example 147 to Example 149 were synthesized starting from the appropriate
commercially available bromo derivative
and following the procedure described in Example 146. Prep LC-MS conditions
and LC-MS data of Example 147 to
Example 149 are listed in the table below. The LC-MS conditions used were LC-
MS (A). An additional purification step
was performed with Example 147 (Preparative TLC, 0.5mm, 254nm, eluent DCM/Me0H
95/5 +0.1% TEA).
Example
Prep LC-
Name tR [M+H]
N
MS
(1,3-Dimethyl-azetidin-3-y1)-(6-phenoxy-pyridin-3-y1)-(4-
147 0.82 445.2 (VII)
trifluoromethoxy-phenyl)-methanol
(1,3-Dimethyl-azetidin-3-y1)-(6-ethoxy-pyridin-3-y1)-(4-
148 0.53 367.23 (V)
trifluoromethoxy-phenyl)-methanol
(1,3-Dimethyl-azetidin-3-y1)-(5-methyl-pyridin-3-y1)-(4-
149 0.75 397.25 (VII)
trifluoromethoxy-phenyl)-methanol
Example 150: (1,3-Dimethyl-azetidin-3-y1)-(4-propyl-pheny1)-(5-pyrrolidin-1-yl-
pyridin-3-y1)-methanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
229
1501, 3-11-lydroxy-(4-propyl-pheny1)-(5-pyrrolidin-1-yl-pyridin-3-y1)-methyil-
3-methyl-azetidine-1-carboxylic acid tert-
butyl ester
The title compound was synthesized starting from Example 01.1 and 1-bromo-4-
propylbenzene, following the
procedure described in Example 146. The resulting crude material was purified
by Prep LC-MS (VII). LC-MS (A): tR =
0.92min; [M+H]: 466.13.
150.2. (3-Methyl-azetidin-3-y1)-(4-propyl-pheny1)-(5-pyrrolidin-1-yl-pyridin-3-
y1)-methanol
A solution of Example 150.1 (11mg) in Me0H (0.1mL) and HCI in dioxane (4M,
0.5mL) was stirred for 4h, was basified
with 1M NaOH solution, diluted with water and extracted with DCM. The org.
layers were evaporated to dryness to give
14mg of the title compound as white solid. LC-MS (A): tR = 0.62min; [M-FH]-:
366.35.
150.3. (1,3-Dimethyl-azetidin-3-y1)-(4-propyl-phenyi)-(5-pyrrolidin-1-yl-
pyridin-3-y1)-methanol
The title compound was synthesized starting from Example 150.2 and following
the procedure described in Example
F1.1 step F1.1.2. LC-MS (A): tR = 0.62min; [M-FI-1]': 380.41.
Example 151 to Example 157 were synthesized starting from the appropriate
commercially available bromo derivative
and following the three-step procedure described in Example 150. Prep LC-MS
conditions and LC-MS data of Example
151 to Example 157 are listed in the table below. The LC-MS conditions used
were LC-MS (A).
Example
Prep LC-
Name tR [M+H]
MS
(1,3-Dimethyl-azetidin-3-yI)-(4-methoxy-phenyl)-(5-pyrrolidin-1-yl-
151 0.53 368.36 (XIII)
pyridin-3-yI)-methanol
(1,3-Dimethyl-azetidin-3-y1)-(4-ethyl-pheny1)-(5-pyrrolidin-1-yl-
152 0.58 366.36 (XIII)
pyridin-3-y1)-methanol
(1,3-Dimethyl-azetidin-3-yI)-phenyl-(5-pyrrolidin 1 yl pyridin-3-yI)-
153 0.50 338.06 (XIII)
methanol
(4-Cyclobutyl-pheny1)-(1,3-dimethyl-azetidin-3-y1)-(5-pyrrolidin-1-
154 0.65 392.38 (XIII)
yl-pyridin-3-yI)-methanol
(4-Cyclobutoxy-pheny1)-(1,3-dimethyl-azetidin-3-y1)-(5-pyrrolidin-
155 0.61 408.36 (XIII)
1-yl-pyridin-3-yI)-methanol
(1,3-Dimethyl-azetidin-3-y1)-(4-ethoxy-pheny1)-(5-pyrrolidin-1-yl-
156 0.55 382.33 (XVIII)
pyridin-3-yI)-methanol
(4-tert-Butyl-pheny1)-(1,3-dimethyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-
157 0.85 394.44 (IX)
pyridin-3-yI)-methanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
230
Example 158 to Example 161 were synthesized starting from Example 03.1 and the
appropriate bromo derivative,
following the procedure described in Example A4.1 step A4.1.2. Prep LC-MS
conditions and LC-MS data of Example
158 to Example 161 are listed in the table below. The LC-MS conditions used
were LC-MS (A).
Example
Prep LC-
Name tR [M+H]-
N
MS
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropoxy-pheny1)-(5-pyrrolidin-
158 0.58 396.41 (IX)
1-yl-pyridin-3-yI)-methanol
(1,3-Dimethyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-y1)-[4-(1-
159 0.65 446.31 (IX)
trifluoromethyl-cyclopropylypheny1]-methanol
(1,3-Dimethyl-azetidin-3-y1)-[4-(1-methyl-cyclopropylyphenyll-
160 0.63 392.42 (IX)
(5-pyrrolidin-1-yl-pyridin-3-yI)-methanol
(4-Cyclopropoxy-phenyl)-(1,3-dimethyl-azetidi n-3-yI)-(5-
161 0.58 394.36 (IX)
pyrrolidin-1-yl-pyridin-3-yI)-methanol
Example 162: (S)2-(3,3-Difluoro-pyrrolidin-1-y1)-pyridin-4-y1]-
(1,3-dimethyl-azetidin-3-y1)-(4-isopropyl-phenyly
methanol
1621, 3-[(S)42-(3,3-Difluoro-pyrrolidin-1-y1)-pyridin-4-41-hydroxy-(4-
isopropyl-phenyl)-methyll-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
Example F1.4 (35mg) and 3,3-difluoropyrrolidine (18.3mg) were suspended in
toluene (1mL), and BINAP (3.03mg),
Pd2(dba)3 (1.53mg) and NaOtBu (24.1mg) were added. The reaction mixture was
stirred for 4h at 100 C, cooled down
to RT, filtered off and evaporated to dryness. The residue was purified by
Prep LC-MS (XIV) to afford 33.5mg of the
title compound as white solid. LC-MS (A): tR = 0.89min; [M-FH]-: 502.31.
162.2. (S)42-(3,3-Difluoro-pyrrolidin-1-y1)-pyridin-4-y1]-(4-isopropyl-phenyl)-
(3-methyl-azetidin-3-y1)-methanol,
hydrochloride salt
Example 162.1 (32.5mg) was dissolved in HCI in dioxane (4M, 0.5mL) and the
reaction mixture was stirred for 4h at RT
and evaporated to dryness to give 42.7mg of the title compound as colorless
oil which was used without purification.
LC-MS (A): tR = 0.59min; [M+1-1]-: 402.00.
1623, (S)-[2-(3,3-Difluoro-pyrrolidin-1-y1)-pyridin-4-y1]-(1,3-dimethyl-
azetidin-3-0)-(4-isopropyl-phenyl)-methanol
The title compound was synthesized starting from Example 162.2, and following
the procedure described in Example
F1.1 step F1.1.2. The crude material was purified by Prep LC-MS (IX). LC-MS
(A): tR = 0.61min; [M-FH]-: 416.25.
Example 163: (S)-[2-((3R,4S)-3,4-Difluoro-pyrrolidin-1-y1)-pyridin-4-y1]-(1,3-
dimethyl-azetidin-3-y1)-(4-isopropyl-
phenyI)-methanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
231
163.1. 3-[(S)-1243R,4S)-3,4-Difluoro-pyrrolidin-1-y1)-pyridin-4-ylphydroxy-(4-
isopropyl-phenyl)-methyll-3-methyl-
azetidine-1-carboxylic acid tert-butyl ester
Example F1.7 (40mg), cis-3,4-difluoropyrrolidine hydrochloride (68.7mg) and
DIPEA (159pL) were dissolved in NMP
(2mL) and the mixture was stirred for 22h at 150 C. DIPEA (159pL) and cis-3,4-
difluoropyrrolidine hydrochloride
(68.7mg) were added, the mixture was further stirred for 2h at 150 C, and the
solvent was evaporated in vacuo. The
residue was purified by Prep LC-MS (IX) to afford 11.7mg of the title compound
as beige solid. LC-MS (A): tR = 0.89min;
[M-F1-1]+: 502.32.
163.2. (S)-(243R, 4S)-3, 4-Difluoro-pyrrolidin-1-y1)-pyridi n-4-y1]-(4-
isopropyl-phenyl)-(3-methyl-azetidin-3-y0-methanol,
hydrochloride salt
Example 163.1 (11.7mg) was dissolved in HCI in dioxane (4M, 0.2mL) and the
mixture was stirred for 1h at RT and
evaporated to dryness to give 13mg of the title compound as colorless oil,
which was used without further purification.
LC-MS (A): tR = 0.58min; [M+H]: 402.01.
163.3. (S)-(2-0R, 4S)-3, 4-Di f luoro-pyrrolidi n-1-yI)-pyridi
3-dimethyl-azetidin-3-yI)-(4-isopropyl-phenyl)-
methanol
The title compound was synthesized starting from Example 163.2 and following
the procedure described in Example
F1.1 step F1.1.2. The resulting crude material was purified by Prep LC-MS
(IX). LC-MS (A): tR = 0.61min: [M+H]:
416.25.
Example 164: (S)-(1,3-Dimethyl-azetidin-3-y1)-(2-isobutoxy-pyridin-4-y1)-(4-
isopropyl-pheny1)-methanol
164.1. (S)-(2-lsobutoxy-pyridin-4-0)-(4-isopropyl-phenyl)-(3-methyl-azetidin-3-
y1)-methanol
To a solution of Example F1.4 (40mg) in 2-methyl-1-propanol (1mL) was added
NaH (60% in mineral oil, 26.7mg) and
the resulting mixture was stirred for 70h at 110 C. After cooling down, the
mixture was diluted with MeCN/water and
purified by Prep LC-MS (XIV) to afford 25.3mg of the title compound as white
solid. LC-MS (A): tR = 0.82min; [M-FI-1]':
369.14.
164.2. (S)-(1,3-Dimethyl-azetidi n-3-yI)-( 2-isobutoxy-pyridi n-4-yI)-(4-
isopropyl-phenyl)-methanol
The title compound was synthesized starting from Example 164.1 (25.3mg) and
following the procedure described in
Example F1.1 step F1.1.2. The resulting crude material was purified by Prep LC-
MS (VIII). LC-MS (A): tR = 0.84min;
[M+H]: 383.23.
Example 165: 4-14-[(S)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridin-2-y11-2-methyl-
butan-2-ol
165.1. 3-[(S)-Hydroxy-12-(3-hydroxy-3-methyl-but-1-yny1)-pyridin-4-yll-(4-
isopropyl-pheny1)-methyll-3-methyl-azetidine-
1-carboxylic acid led-butyl ester
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
232
Example F1.4 (40mg) and 2-methyl-3-butyn-2-ol (13.8pL) were dissolved in DMF
(1mL). Cul (0.884mg), PPh3(5.13mg),
Pd(PPh)30I2 (3.29mg), and Et2NH (150pL) were added and the reaction mixture
was stirred for 17h at 60 C. After
cooling down, DCM was added and the resulting mixture was washed with water
and brine, dried (MgSO4), filtered off
and evaporated to dryness. The residue was purified by Prep LC-MS WO to afford
38.2mg of the title compound as
white solid. LC-MS (A): tR = 0.82min; [M+H]-: 479.32.
165.2. 34(S)-Hydroxy-12-(3-hydroxy-3-methyl-buty1)-pyridin-4-y1]-(4-isopropyl-
pheny1)-methylk3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
The title compound was synthesized starting from Example 165.1, and following
the procedure described in Example
48. LC-MS (A): tR = 0.85min; [M+H]': 483.34.
165.3. 4-{4-[(S)-Hydroxy-(4-isopropyl-pheny1)-(3-methyl-azetidin-3-y1)-
methylppyridin-2-y1}-2-methyl-butan-2-ol,
hydrochloride salt
Example 165.2 (34.4mg) was dissolved in HCI in dioxane (4M, 0.6mL), the
mixture was stirred for 1h at RT and was
evaporated to dryness to give the title compound (43.6mg) as yellow oil. LC-MS
(A): tR = 0.54min; [M-FH]-: 383.24.
165.4. 4-{4-[(S)-(1, 3-Dim ethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
m ethylkpyridi n-2-y1}-2-m ethyl-b uta n-2-ol
The title compound was synthesized starting from Example 165.3, and following
the procedure described in Example
F1.1 step F1.1.2. The crude material was purified by Prep LC-MS (XIV). LC-MS
(A): tR = 0.55min; [M+H]-: 397.28.
Example 166: (R)-[6-(3, 3-Difluoro-pyrrol idin-1-y1)-pyridazin-4-
y1]-(1, 3-dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
methanol
Example F1.6 (35mg) was dissolved in dioxane (1mL), and DIPEA (55.6pL) and 3,3-
difluoropyrrolidine (22mg) were
added. The reaction mixture was stirred for 48h at 100 C. After cooling down,
MeCN/water was added and the resulting
material was first purified by Prep LC-MS (VI) then by Prep LC-MS (IX) to
afford the title compound (5.1mg) as white
solid. LC-MS (A): tR = 0.65min; [M-FH]-: 417.10.
Example 167: (S)-5-tert-Buty1-3-{5-[(R)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-
(4-isopropyl-pheny1)-methyl]-pyridin-3-yll-
oxazolidin-2-one
167.1. 5-tert-Buty1-3-{5-[(R)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-pheny1)-methylTpyridin-3-y1}-oxazolidin-
2-one
Example F1.2 (50mg) and Example F4.5 (18.4mg) were dissolved in dioxane
(2.5mL), K2CO3 (35.5mg), N,N-
dimethylethylenediamine (14pL) and Cul (24.6mg) were added and the reaction
mixture was stirred for 44h at 110 C.
The reaction mixture was allowed to cool down to RT, was filtrated off and
evaporated to dryness. The residue was
purified by Prep LC-MS (VII) to afford 33mg of the title compound as white
solid. LC-MS (A): tR = 0.77min; [M-F1-1]':
452.31.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
233
167.2. (S)-5-tert-Buty1-3-{5-[(R)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-pheny1)-methylPpyridin-3-4-
oxazolidin-2-one
The title compound (3mg) was obtained by Prep chiral SFC (XIV) of Example
167.1 (20.9mg) followed by Prep LC-MS
(VI). The stereochemistry at the oxazolidinone ring was arbitrarily assigned.
LC-MS (B): tR = 0.767min; [M-FFI]': 452.5;
Chiral SFC (N): 4.0min.
Example 168: (R)-1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methylFpyridin-3-yll-pyrrolidin-3-
01
168.1. (3R)-1-(54(1,3-dimethylazetidin-3-y1)(hydroxy)(4-
isopropylphenyl)methyl)pyridin-3-Apyrrolidin-3-ol
The title compound was synthesized starting from Example F1.1 and (R)-3-
pyrrolidinol, following the procedure
described in Example 162 step 162.1. The crude material was purified by Prep
LC-MS (V) to afford 40mg of the title
compound as white solid. LC-MS (A): tR = 0.54min; [M-FH]': 396.40.
168.2. (R)-1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylppyridin-3-0-pyrrolidin-3-ol
The title compound was obtained by Prep chiral SFC (X) of Example 168.1. LC-MS
(A): tR = 0.54min; [M+H]: 396.41.
Chiral SFC (K): tR = 1.3min.
Example 169 and Example 170 were synthesized starting from Example F1.1 and
the appropriate amine reagent, and
following the two-step procedure described in Example 168. Chiral Prep SFC
conditions, Chiral SFC and LC-MS data
are listed in the table below. The LC-MS conditions used were LC-MS (B).
Chiral
Chiral
Example LC-MS
Name [M-Fl-l]
Prep SFC tR
N tR
SFC
(method)
(S)-1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-
1.3
169 (4-isopropyl-phenyl)methyl]-pyridin-3-yll-pyrrolidin-
0.411 396.4 (XI)
3-ol
(K)
(R)-(1,3-Dimethyl-azetidin-3-yI)-[5-((S)-3-
3.3
170 hydroxymethyl-pyrrolidin-1-y1)-pyridin-3-y1]-(4- 0.433
410.5 (XII)
isopropyl-phenyI)-methanol
(L)
Example 171 to Example 188 were synthesized starting from the appropriate
compound of Formula (F1) and amine
reactant, and following the procedure described in Example 162 step 162.1.
Prep LC-MS conditions, compounds of
Formula (F1) and LC-MS data are listed in the table below. The LC-MS
conditions used were LC-MS (B).
Example Prep LC-
Name tR [M-FI-1]'
N MS
(F1)
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
234
(R)-(1,3-Dimethyl-azetidin-3-yI)-(4-isopropyl-
171 0.508 380.4 (V) F1.2
phenyI)-(5-pyrrolidin-l-yl-pyridin-3-y1)-methanol
(S)-1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-
172 hydroxy-(4-isopropyl-phenyI)-methy1]-pyridin-3- 0.447 410.4 (V) F1.2
yI}-3-methyl-pyrrolidin-3-ol
3-Cyclopropy1-1-{5-[(R)-(1,3-dimethyl-azetidin-
173 3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]- 0.512
436.5 (V) F1.2
pyridin-3-yI}-pyrrolidin-3-ol
2-((S)-1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-
174 hydroxy-(4-isopropyl-phenylymethy1]-pyridin-3- 0.504 438.2 (V) F1.2
yll-pyrrolidin-3-y1)-propan-2-ol
(R)-(1,3-Dimethyl-azetidin-3-yI)-(4-isopropyl-
175 phenyl)-(5-morpholin-4-yl-pyridin-3-y1)- 0.492 396.4
(V) F1.2
methanol
(R)-(1,3-Dimethyl-azetidin-3-yI)-(4-isopropyl-
176 pheny1)-(3,4,5,6-tetrahydro-2H-[l,3]bipyridinyl- 0.582 394.4 (V) F1.2
5'-yI)-methanol
(R)45-(7-Aza-bicyclo[2.2.1]hept-7-y1)-pyridin-3-
177 yI]-(1,3-dimethyl-azetidin-3-y1)-(4-isopropyl- 0.612
406.4 (V) F1.2
phenyI)-methanol
(R)-(1,3-Dimethyl-azetidin-3-yI)-(4-isopropyl-
178 pheny1)45-((S)-2-methyl-pyrrolidin-l-y1)- 0.549
394.4 (V) F1.2
pyridin-3-yI]-methanol
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-
179 (4-isopropyl-pheny1)-methyl]-pyridin-3-y11-3- 0.552
464.4 (V) F1.2
trifluoromethyl-pyrrolidin-3-ol
(R)-[5-(3,3-Difluoro-pyrrolidin-l-yI)-pyridin-3-
180 yI]-(1,3-dimethyl-azetidin-3-y1)-(4-isopropyl- 0.576
416.4 (VII) F1.2
phenyI)-methanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
235
5'-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
181 isopropyl-phenyI)-methy1]-3,4,5,6-tetrahydro- 0.437
410.4 (V) F1.2
2H-[l,3]bipyridiny1-4-ol
5'-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
182 isopropyl-phenylymethy1]-3,4,5,6-tetrahydro- 0.462
410.4 (V) F1.2
2H-[l,3Thipyridiny1-3-ol
(R)-{5-[(2-Benzyloxy-ethy1)-methyl-amino]-
183 pyridin-3-y1)-(1,3-dimethyl-azetidin-3-y1)-(4- 0.624
474.5 (IX) F1.2
isopropyl-phenyl)-methanol
(R)-(1,3-Dimethyl-azetidin-3-yI)-[4-(1-methyl-
184 cyclopropyI)-phenyl]-(5-pyrrolidin-l-yl-pyridin- 0.522
392.4 (V) F1.3
3-yI)-methanol
(R)45-((3R,4S)-3,4-Difluoro-pyrrolidin-l-y1)-
185 pyridin-3-y1]-(1,3-dimethyl-azetidin-3-y1)-[4-(1- 0.582
482.4 (V) F1.4
trifluoromethyl-cyclopropy1)-phenyl]-methanol
2-[(S)-1-(5-{(R)-(1,3-Dimethyl-azetidin-3-yI)-
hydroxy-[4-(1-trifluoromethyl-cyclopropy1)-
186 0.558 504.25 (V) F1.4
phenyll-methyll-pyridin-3-0-pyrrolidin-3-4-
propan-2-ol
(R)-(1,3-Dimethyl-azetidin-3-yI)-(5-pyrrolidin-l-
187 yl-pyridin-3-yI)-[4-(1-trifluoromethyl- 0.55 446.4
(V) F1.4
cyclopropyI)-pheny1]-methanol
(R)-(4-tert-Butyl-pheny1)-(1,3-dimethyl-azetidin-
188 3-y1)-(3,4,5,6-tetrahydro-2H-[l,3]bipyridiny1-5'- 0.621
408.5 (VII) F1.9
yI)-methanol
Example 189: (R)-(1,3-Dimethyl-azetidin-3-y1)-(5-pyrrolidin-l-yl-pyridin-3-y1)-
(4-trifluoromethoxy-phenyl)-methanol
The title compound (192mg, white powder) was synthesized starting from Example
A7.1 (339mg), and following the
procedure described in Example F1.1 step F1.1.2. The crude material was
purified by Prep LC-MS (VI). LC-MS (A). tR
= 0.61min; [M+H]: 422.30.
Example 190: (R)-{5-[5-(1-Cyclopropanesulfonyl-piperidin-4-y1)-
[1,2,4]oxadiazol-3-y1]-pyridin-3-y11-(1,3-dimethyl-
azetidin-3-y1)-(4-isopropyl-phenylymethanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
236
190.1. 4-(3-{5-1(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyil-pyridin-3-441,2,41oxadiazol-5-
y1)-piperidine-1-carboxylic acid tert-butyl ester
The title compound (750mg, white powder) was obtained starting from Example
D2.1 (1.01g) and N-Boc-isonipecotic
acid (1.02g), and following the procedure described in Example 134. The crude
material was however purified by prep
LC-MS (XXIV) and (XXV). LC-MS (A): tR = 0.88min; [M+H]: 561.90.
190.2. (R)-(1,3-dimethylazetidin-3-y1)(4-isopropylphenyl)(5-(5-(piperidin-4-
y1)-1,2,4-oxadiazol-3-Apyridin-3-
yOrnethanol, hydrochloride salt
A solution of Example 190.1 (750mg) in HCI in dioxane (4M, 15mL) was stirred
at RT for 2h, concentrated in vacuo and
dried under HV to afford 714mg of the title compound. LC-MS (A): tR = 0.57min;
[M-'-H]: 462.12.
190.3. (R)-{545-(1-Cyclopropanesulfonyl-piperidin-4-y1)41,2,4]oxadiazol-3-01-
pyridin-3111-(1,3-dimethyl-azetidin-3-y1)-
(4-isopropyl-pheny1)-methanol
A solution of Example 190.2 (29.8mg) and DIPEA (38pL) in DCM (1mL) was stirred
at -20 C for 1h.
Cyclopropanesulfonyl chloride (5.7p L) was added, the resulting mixture was
stirred overnight at RT and evaporated in
vacuo. The crude material was purified by Prep LC-MS (XIV) followed by Prep LC-
MS (VI) to afford the title compound
(12mg) as white powder. LC-MS (A): tR = 0.80min; [M+H]+: 566.03.
Example 191: 2-({5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridin-3-yll-methyl-
aminoyethanol
Example 183 (25mg) was dissolved in Et0H (1mL), and TEA (13.4pL) and Pd/C (10%
w/w, 50% water, 2.55mg) were
added and the reaction mixture was stirred at RT under hydrogen for 18h. The
same amounts of TEA and Pd/C were
added again and the mixture was further stirred at RT under hydrogen for 24h.
The mixture was filtered off and the
same amount of Pd/C was added to the resulting solution. The mixture was
stirred under hydrogen for 17h. The same
procedure was repeated 3 times and the mixture was finally filtered off and
evaporated in vacuo. The residue was
purified by Prep LC-MS (IX) to afford 4mg of the title compound as white
solid. LC-MS (B): tR = 0.396min; [M+H]:
384.40.
Example 192: (R)-14(S)-1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-phenyl)-methyll-pyridin-3-yll-
pyrrolidin-3-y1)-ethanol
192.1. (S)-1-Pyrrolidin-3-yl-ethanone, hydrochloride salt
Tert-butyl-(3S)-3-acetylpyrrolidine-1-carboxylate (100mg) was dissolved in HCI
in dioxane (4M, 1mL), the reaction
mixture was stirred for 1h30 at RT and evaporated to dryness to give 69mg of
the title compound as brown oil which
was used without further purification. LC-MS (A): tR = 0.2min; [M+H]-: 114.24.
192,2,,3_Dimetho_azetidin-3-y1)-hydroxy-(4-isopropyl-phenyo_methyil-pyridin-3-
4-pyrrolidin-3-Y0-
ethanone
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
237
The title compound was synthesized starting from Example F1.2 and Example
192.1, and following the procedure
described in Example 162 step 162.1. The crude material was purified by Prep
LC-MS (VI) to afford. LC-MS (A): tR =
0.6min; [M-FH]': 422.38.
/92.3. (R)-14(S)-1-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridin-3-4-pyrrolidin-3-
yI)-ethanol
Example 192.2 (41mg) was dissolved in Me0H (0.5mL) and NaBH4 (3.86mg) was
added at 0 C. The reaction mixture
was stirred for 3h at 0 C then for 70h at RT. The reaction mixture was diluted
with water, extracted with DCM, and the
org. layers were evaporated to dryness. The residue was purified by Prep LC-MS
(V) to afford the title compound as
first eluting peak (11mg) as white solid. The stereochemistry at the a-carbon
to the secondary hydroxy group was
arbitrarily assigned to (R). LC-MS (B): tR = 0.458min; [MA-H]:424.4.
Example 193 to Example 221 were synthesized from Example F1.2 and the
appropriate compound of Formula (F3) or
Formula (F4), following the procedure described in Example 167 step 167.1.
Compounds of Formula (F3) or Formula
(F4), unless commercially available, Prep LC-MS conditions and LC-MS data are
listed in the table below. The LC-MS
conditions used were LC-MS (B).
Example Prep
LC-
Name tR [M+H]
N MS
(F4)
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
193 0.581 396.40 (V)
isopropyl-phenyl)methyl]-pyridin-3-ylyoxazolidin-2-one
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
194 isopropyl-pheny1)-methyl]-pyridin-3-y11-5-phenyl- 0.765
472.4 (VII)
oxazolidin-2-one
5-Benzy1-3-{5-[(R)-(1,3-di methyl-azetidi n-3-yI)-hydroxy-
195 (4-isopropyl-pheny1)-methyl]pyridin-3-y1}-oxazolidin-2-
0.776 486.4 (VII) F4.1
one
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
196 isopropyl-phenylymethyl]-pyridin-3-y11-5-isopropyl-
0.727 438.4 (VI) F4.2
oxazolidin-2-one
6-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
197 isopropyl-pheny1)-methyl]-pyridin-3-y11-4-oxa-6-aza-
0.672 422.4 (VI) F4.3
spiro[2.4]heptan-5-one
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
238
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
198 isopropyl-phenyl)-methyl]-pyridin-3-y11-1-oxa-3-aza-
0.737 450.4 (VI)
spiro[4.4]nonan-2-one
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(V) then
199 isopropyl-phenylymethy1]-pyridin-3-y1}-5-(tetrahydro-
0.651 480.4 IX F4.4
(
pyran-4-yI)-oxazolidin-2-one )
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
200 isopropyl-phenyl)-methyl]-pyridin-3-y11-5,5-dimethyl-
0.664 424.4 (VI)
oxazolidin-2-one
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(VI) then
201 isopropyl-pheny1)-methyll-pyridin-3-y11-8,8-difluoro-1-
0.756 500.4 IX) F4.6
(
oxa-3-aza-spiro[4.5]decan-2-one
9-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
202 isopropyl-phenyl)-methyl]-pyridin-3-y11-7-oxa-9-aza-
0.832 476.4 (VII) F4.7
dispiro[3.1.4.1]undecan-8-one
2-Cyclopropy1-7-{5-[(R)-(1,3-dimethyl-azetidin-3-y1)-
(VII) then
203 hydroxy-(4-isopropyl-phenyl)-methyl]-pyridin-3-y11-5-oxa-
0.822 476.4 F4.8
(IX)
7-aza-spiro[3.4]octan-6-one
7-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
204 isopropyl-phenyl)-methyl]-pyridin-3-y11-2,2-dimethyl-5-
0.807 464.4 (VII) F4.9
oxa-7-aza-spiro[3.4]octan-6-one
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
205 isopropyl-pheny1)-methy1]-pyridin-3-y1}-4-phenyl- 0.771
470.4 (VII)
pyrrolidin-2-one
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4- (V)
then
206 0.595
394.4
isopropyl-phenyl)methyl]-pyridin-3-yll-pyrrolidin-2-one (XIV)
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
207 isopropyl-pheny1)-methy1]-pyridin-3-y1}-4-isopropyl-
0.749 436.5 (VII)
pyrrolidin-2-one
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
239
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
208 isopropyl-pheny1)-methy1]-pyridin-3-y11-3-isopropyl-
0.749 436.5 (VII)
pyrrolidin-2-one
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
209 isopropyl-phenylymethy1]-pyridin-3-y1}-4,4-dimethyl-
0.689 422.5 (VII)
pyrrolidin-2-one
5-(54(R)-(1,3-Dimethyl-azetidin-3-y1)(hydroxy)(4-
210 isopropyl-phenyl)methyl)pyridin-3-yl)hexahydro-4H- 0.594 436.4 (VII)
furo[2,3-c]pyrrol-4-one (mixture of diastereomers)
2-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
211 isopropyl-pheny1)-methyl]-pyridin-3-y11-2-aza- 0.758
448.5 (VII)
spiro[4.4]nonan-3-one
6-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
212 isopropyl-phenylymethy1]-pyridin-3-y11-6-aza- 0.721
434.4 (VII)
spiro[3.4]octan-5-one
2-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
213 isopropyl-pheny1)-methy1]-pyridin-3-y11-8-oxa-2-aza-
0.614 464.4 (VII)
spiro[4.5]decan-3-one
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(VII) then
214 isopropyl-pheny1)-methyl]-pyridin-3-y11-3-{tetrahydro-
0.66 478.5
(IX)
pyran-4-yI)-pyrrolidin-2-one
2-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(VII) then
215 isopropyl-phenylymethy1]-pyridin-3-y1}-2-aza- 0.801
462.5
(XIV)
spiro[4.5]decan-1-one
2-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
((VII)
216 isopropyl-pheny1)-methy1]-pyridin-3-y1}-8-oxa-2-aza-
0.631 464.4
then (IX)
spiro[4.5]decan-1-one
(S)-1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
((VII)
217 isopropyl-pheny1)-methy1]-pyridin-3-y11-4-isobutyl-
0.811 450.5 then
pyrrolidin-2-one (XIV)
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
240
4-Cyclopropy1-1-{5-[(R)-(1,3-dimethyl-azetidin-3-y1)-
(VI) then
218 hydroxy-(4-isopropyl-phenylymethy1]-pyridin-3-y1}- 0.72
434.4
(IX)
pyrrolidin-2-one
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(VI) then
219 isopropyl-phenylymethy1]-pyridin-3-y1}-4-trifluoromethyl- 0.703 462.4
(IX)
pyrrolidin-2-one
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(VI) then
220 isopropyl-pheny1)-methyl]-pyridin 3 yll 3 (2 methoxy-
0.656 452.4
(IX)
ethyl)-pyrrolidin-2-one
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(VI) then F3.1 =
221 isopropyl-pheny1)-methyll-pyridin-3-y11-4-(2-methoxy-
0.646 452.5
(IX)
F4.10
ethyl)-pyrrolidin-2-one
Example 222: 1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyll-pyridazin-3-yll-pyrrolidin-2-
one
The title compound was synthesized starting from Example F1.6 and 2-
pyrrolidone, and following the procedure
described in Example 167 step 167.1. The crude material was purified by Prep
LC-MS (IX). LC-MS (A): tR = 0.68min;
[M+H]: 395.19.
Example 223: (1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-((R)-3-
isopropyl-pyrrolidin-1-y1)-pyridin-3-y1]-
methanol
223.1. 3-Hydroxy-3-isopropenyl-pyrrolidine-1-carboxylic acid tert-butyl ester
To a solution of N-Boc-3-pyrrolidinone (1g) in THF (7mL) was added at RT
isopropenylmagnesium bromide in THF
(0.5M, 11.7mL). The resulting mixture was stirred for 15min at 70 C, allowed
to cool down to RT, poured into aq. sat.
NI-14C1 solution and extracted with EA. The org. layers were dried (MgSO4),
filtrated off and evaporated to dryness. The
resulting crude material was purified by CC (Biotage, SNAP 50g, solvent A:
Hep; solvent B: EA; gradient in %B: 30 over
3CV, 30 to 50 over 3CV, 50 over 2CV) to afford 249mg of the title compound as
yellow oil. LC-MS (A): tR = 0.78min;
[M+H]-: 228.20
223.2. 3-lsopropyl-pyrrolidine-1-carboxylic acid tert-butyl ester
A mixture of Example 223.1 (243mg) and Pd/C (10% w/w, 50% water, 113mg) in
Me0H (3.5mL) was stirred for 1h30
at RT under hydrogen atmosphere, filtrated off and evaporated to dryness. The
resulting crude material was purified by
CC (Biotage, SNAP 10g, solvent A: Hep; solvent B: EA; gradient in %B: 10 over
20V, 10 to 50 over 10CV, 50 over
2CV) to afford 50mg of the title compound as yellow oil. LC-MS (A): tR =
1.02min; [M-F1-1]': 214.36.
223.3. 3-Isopropyl-pyrrolidine, hydrochloride salt
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
241
A mixture of Example 223.2 (47mg) in HCI in dioxane (4M, 0.5mL) was stirred
for 1h at RT and evaporated to dryness
under HV to give 38mg of the title compound as brown solid. LC-MS (A): tR =
0.42min; [M-F1-1]': 114.28.
223.4. (1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-((R)-3-isopropyl-
pyrrolidin-l-y1)-pyridin-3-07-methanol
Example F1.2 (50mg) and Example 223.3 (20.2mg) were dissolved in toluene
(1mL), XantPhos (3.83mg), Pd2(dba)3
(6.06mg) and NaOtBu (27.4mg) were added and the resulting mixture was stirred
for 18h at 100 C, The reaction mixture
was allowed to cool down to RT, filtrated off and evaporated to dryness. The
residue was purified by Prep LC-MS (VII)
then by Prep LC-MS (IX) to afford 6mg of the title compound as white solid. LC-
MS (B): tR = 0.665min; [M+H]*: 422.5.
Example 224 to Example 244 were synthesized from the appropriate amine
reactant and compound of Formula (F1),
and following the procedure described in Example 223 step 223.4. Compounds of
Formula (F1), Prep LC-MS conditions
and LC-MS data are listed in the table below. The LC-MS conditions used were
LC-MS (B).
Example Prep
LC-
Name tR [M+H]
(F1)
N MS
(R)-(1,3-Dimethyl-azetidin-3-yI)-[5-((2R,6S)-2,6-
224 dimethyl-morpholin-4-y1)-pyridin-3-y1]-(4-isopropyl-
0.577 424.4 (VI) F1.2
phenyI)-methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
225 [5-(6-oxa-3-aza-bicyclo[3.1.1]hept-3-y1)-pyridin-3-y1]-
0.439 408.4 (V) F1.2
methanol
(R)-[5-((3R,4S)-3,4-Difluoro-pyrrolidin-1-yI)-pyridin-3-
226 y1]-(1,3-dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
0.526 416.4 (VII) F1.2
methanol
(R)-[5-((3S,4S)-3,4-Difluoro-pyrrolidin-1-yI)-pyridin-3-
227 y1]-(1,3-dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
0.522 416.4 (VII) F1.2
methanol
(R)-(1,3-Dimethyl-azetidin-3-yI)-[5-((2S,6S)-2,6-
(IX) then
228 dimethyl-morpholin-4-y1)-pyridin-3-y1]-(4-isopropyl-
0.556 424.4 (V F1.2
)
phenyI)-methanol
(R)-(1,3-Dimethyl-azetidin-3-yI)-[5-((2R,6R)-2,6-
(IX) then
229 dimethyl-morpholin-4-y1)-pyridin-3-y1]-(4-isopropyl-
0.556 424.4 F1.2
phenyI)-methanol (V)
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
242
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
(IX) then
230 {5-[3-(3-methyl-[1,2,4]oxadiazol-5-y1)-pyrrolidin-1-y1]-
0.531 462.4 F1.2
(V)
pyridin-3-ylymethanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
231 {5-[3-(4-methyl-thiazol-2-y1)-pyrrolidin-l-y1]-pyridin-3- 0.589 477.4 (IX)
F1.2
ylymethanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
232 0.68 456.5 (XIV) F1.2
[543-phenyl-pyrrolidin-l-y1)-pyridin-3-0]-methanol
(R)-(1,3-Dimethyl-azetidin-3-yI)-[5-(3,3-dimethyl-
233 pyrrolidin-1-y1)-pyridin-3-y1]-(4-isopropyl-phenyly
0.6 408.5 (XIV) F1.2
methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
234 [(1S,4S)-5-(2-oxa-5-aza-bicyclo[2.2.1]hept-5-y1)-
0.454 408.4 (VI) F1.2
pyridin-3-yI]-methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
235 [5-(2,2,6,6-tetrafluoro-morpholin-4-y1)-pyridin-3-y1]-
0.752 468.4 (XV) F1.2
methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
(VI) then
236 [5-((R)-2-methoxymethyl-morpholin-4-yI)-pyridin-3-
0.527 440.5 (IX ) F1.2
yI]-methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
(VI) then
237 [5-((S)-2-methoxymethyl-morpholin-4-yI)-pyridin-3-
0.528 440.5 (IX ) F1.2
yI]-methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
(XV) then
238 [543-trifluoromethyl-pyrrolidin-1-y1)-pyridin-3-0]-
0.613 448 (IX) F1.2
methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
(VI) then
239 [(1R,4R)-5-(2-oxa-5-aza-bicyclo[2.2.1]hept-5-y1)-
0.451 408.4 (IX) F1.2
pyridin-3-yI]-methanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
243
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
(VI) then
240 [(1S,5R)-5-(8-oxa-3-aza-bicyclo[3.2.1]oct-3-yI)-
0.515 422.4 F1.2
(IX)
pyridin-3-yI]-methanol
(R)-(1,3-Dimethyl-azetidin-3-yI)-[(1S,4S)-5-(2-oxa-5-
241 aza-bicyclo[2.2.1]hept-5-y1)-pyridin-3-y1H4-(1- 0.504
474.4 (VI) F1.8
trifluoromethyl-cyclopropy1)-phenyl]-methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-ethyl-pheny1)-(5-
242 0.462 366.4 (VI) F1.10
pyrrolidin 1 yl pyridin-3-yI)-methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-ethyl-pheny1)45-
243 (6-oxa-3-aza-bicyclo[3.1.1]hept-3-y1)-pyridin-3-y1]-
0.392 394.4 (V) F1.10
methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-ethyl-phenyl)-
244 [(1S,4S)-5-(2-oxa-5-aza-bicyclo[2.2.1]hept-5-y1)-
0.405 394.4 (V) F1.10
pyridin-3-yI]-methanol
Example 245: (R)-(5-Benzyloxy-pyridin-3-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-
isopropyl-pheny1)-methanol
The title compound was synthesized following the four-step procedure described
in Example G1.2 step G1.2.1 to step
G1.2.4. LC-MS (B): tR = 0.783min; [M-FH]-: 417.40.
Example 246: (1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(2-pyridin-2-
yl-ethoxy)-pyridin-3-y1]-methanol
To a solution of Example G1.1 (30mg) and 2-(2-hydroxyethyl)pyridine (19.3pL)
in toluene (1mL) was added
cyanomethyltributylphosphorane (44.4mg). The reaction mixture was stirred for
23h at 110 C and evaporated to
dryness. The residue was purified by Prep LC-MS (VI) then by Prep LC-MS (IX)
to afford 8mg of the title compound as
off-white solid. LC-MS (B): tR = 0.532min; [M+H]: 432.40.
Example 247 to Example 259 were synthesized from the appropriate compounds of
Formula (G1) and (G2), and
following the procedure described in Example 246. Compounds of Formula (G1),
Prep LC-MS conditions, and LC-MS
data are listed in the table below. The LC-MS conditions used were LC-MS (B).
Example Prep LC-
Name tR [M--H]'
N MS
(G1)
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-phenyl)- (VI)
then
247 0.606 385.2
G1.1
[5-(2-methoxy-ethoxy)-pyridin-3-yI]-methanol (IX)
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-phenyl)- (VI)
then
248 0.585 397.2
G1.1
[5-(oxetan-3-ylmethoxy)-pyridin-3-yI]-methanol (IX)
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
244
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
249 isopropyl-phenyl)-methyl]-pyridin-3-yloxy}- 0.546
385.4 (XIII) G1.2
propan-1-ol
(R)-(1,3-Dimethyl-azetidin-3-yI)-(5-isopropoxy-
250 0.685 369.4 (XIII) G1.2
pyridin-3-y1)-(4-isopropyl-phenyl)methanol
(R)-(5-Cyclohexyloxy-pyridin-3-yI)-(1,3-dimethyl-
251 0.813 409.4 (XIII) G1.2
azetidin-3-y1)-(4-isopropyl-pheny1)-methanol
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
252 isopropyl-phenyl)-methyl]-pyridin-3-yloxy}-2- 0.386
399.3 (XIII) G1.2
methyl-propan-2-ol
(R)-(1,3-Dimethyl-azetidin-3-yI)-(4-isopropyl-
253 phenyl)[5-(3-methoxy-cyclopentyloxy)-pyridin-3- 0.698 425.5 (XIII)
G1.2
yll-methanol
(R)45-(3-Cyclopropy141,2,4]oxadiazol-5-
254 ylmethoxy)-pyridin-3-yI]-(1,3-dimethyl-azetidin-3- 0.726 449.3 (XIII)
G1.2
yI)-(4-isopropyl-pheny1)-methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-{512-(3,5-
255 dimethyl-[1,2,4]triazol-1-y1)-ethoxy]-pyridin-3-yll-
0.553 450.5 (V) G1.2
(4-isopropyl-phenyI)-methanol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
256 isopropyl-phenyl)-methyl]-pyridin-3-yloxy}-2- 0.622
413.4 (XIII) G1.2
methyl-butan-2-ol
(R)-(1,3-Dimethyl-azetidin-3-yI)-(4-isopropyl-
257 0.584 341.3 (XIII) G1.2
pheny1)-(5-methoxy-pyridin-3-y1)-methanol
(R)-[5-(2-Benzyloxy-ethoxy)-pyridin-3-yI]-(1,3-
258 dimethyl-azetidin-3-y1)-(4-isopropyl-phenyl)- 0.792
461.5 (VIII) G1.2
methanol
(R)-(1,3-Dimethyl-azetidin-3-yI)-(4-isopropyl-
259 phenyI)-[5-(tetrahydro-pyran-4-yloxy)-pyridin-3-
0.630 411.2 (IX) G1.2
yI]-methanol
Example 260: 2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridin-3-yloxy}-ethanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
245
A mixture of Example 258 (9mg) and Pd(OH)2/C (20%, 0.686mg) in Me0H (0.5mL)
was stirred for 55h at RT under
hydrogen atmosphere, was filtered off and evaporated to dryness. The resulting
crude material was redissolved in
Me0H (0.5mL), the same amount of Pd(OH)2/C was added, and the resulting
mixture was stirred for 21h at RT under
hydrogen atmosphere, filtered off, evaporated to dryness and purified by Prep
LC-MS (V) to afford 1mg of the title
compound as white solid. LC-MS (B): tR = 0.512min; [M+H]': 371.40.
Example 261: 4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyl]-pyridin-3-yloxy}-
cyclohexanol
261.1. (R)-(1,3-Dimethyl-azetidin-3-y045-(1,4-dioxa-sp1ro[4.5]dec-8-yloxy)-
pyridin-3-y1]-(4-isopropyl-pheny1)-methanol
The title compound was synthesized from 1,4-dioxaspiro[4,5]clecan-8-ol and
Example G1.2, and following the procedure
described in Example 246. The crude material was purified by Prep LC-MS (VI).
LC-MS (A): tR = 0.69min; [M-FI-1]-:
467.18.
261.2 4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y0-hydroxy-(4-isopropyl-phenyl)-
methylppyridin-3-yloxyl-cyclohexanone
To an ice-cold solution of Example 261.1 (58mg) in dioxane (0.6mL) were added
H2SO4 (0.12mL) and water (0.12mL).
The resulting mixture was stirred for 4h at 0 C, basified with aq. sat. NaHCO3
solution, diluted with water and extracted
with DCM. The org. phases were dried (MgSO4), filtrated off and evaporated to
dryness to give 31mg of the title
compound as white solid. LC-MS (A): tR = 0.65min; [M+H]: 423.19.
261.3 4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y0-hydroxy-(4-isopropyl-phenyl)-
methylppyridin-3-yloxy}-cyclohexanol
To an ice-cold solution of Example 261.2 (10mg) in Me0H (0.3mL) was added
NaBH4 (0.94mg). The resulting mixture
was stirred for 1h at 0 C, quenched with water and extracted with DCM. The
org. phases were dried (MgSO4), filtrated
off, evaporated to dryness and purified by Prep LC-MS (V) to give 5mg of the
title compound as white solid. LC-MS (A):
tR = 0.61min; [M+H]t 425.22.
Example 262: 4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyl]-pyridin-3-yloxy}-1-methyl-
cyclohexanol
To a solution of Example 261.2 (16mg) in THF (0.5mL) was added dropwise
methylmagnesium bromide in Et20 (3M,
44.3pL). The resulting mixture was stirred for 1h25 at RT, quenched with aq.
sat. NH401 solution and extracted with
DCM. The org. layers were dried (MgSO4), filtrated off and evaporated to
dryness. The resulting crude material was
purified by Prep LC-MS (VI) to afford 7mg of the title compound as white
powder. LC-MS (A): tR = 0.63min; [M-F1-1]-:
439.22.
Example 263 to Example 265 were synthesized starting from Example B2.1 and the
appropriate bromoderivative of
Formula (A5), and following the procedure described in Example A7.1 step
A7.1.1. Prep LC-MS conditions and LC-MS
data of Example 263 to Example 265 are listed in the table below. The LC-MS
conditions used were LC-MS (A).
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
246
Example
Prep LC-
Name tR [M-FH]-
N MS
(1,3-Dimethyl-azetidin-3-y1)-(2-phenoxy-pyrimidin-5-y1)-(4-
263 0.77 446.21
(VI)
trifluoromethoxy-phenyl)-methanol
(6-Benzyloxy-pyridin-3-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-
264 0.87 459.13 (VIII)
trifluoromethoxy-phenyI)-methanol
265
(1,3-Dimethyl-azetidin-3-y 0.73 419.19
1)-(5-pyrazol-1-yl-(5-3-y1)-(4-
(VI) then
trifluoromethoxy-phenyI)-methanol
(V)
Example 266: (1,3-Dimethyl-azetidin-3-y1)-(6-fluoro-5-pyrrolidin-1-yl-pyridin-
3-y1)-(4-trifluoromethoxy-pheny1)-
methanol
266.1. 5-Bromo-2-fluoro-3-pyrrolidin-1-yl-pyridine
To an ice-cold solution of 3-amino-5-bromo-2-fluoropyridne (200mg) in THF
(4mL) and Me0H (4mL) was added H2SO4
(222pL) in water (1.78mL), followed by 2,5-dimethoxytetrahydrofur2n (403pL)
and finally NaBH4 portionwise (115mg).
The resulting mixture was stirred for 21h at RT, diluted with water and
basified with aq. sat. NaHCO3 solution. The org.
layer was washed with water and brine, dried (MgSO4), filtrated off and
evaporated to dryness. The residue was purified
by CC (Biotage, SNAP 10g, solvent A: Hep; solvent B: EA; gradient in %B: 0
over 1CV, 0 to 10 over 6CV, 10 over 2CV),
followed by Prep LC-MS (VII) to afford 31mg of the title compound as white
solid. LC-MS (A): tR = 0.99min; [M-FH]-:
245.21.
266.2. (1,3-Dimethyl-azetidin-3-yI)-(6-fluoro-5-pyrrolidin 1 yl pyridin-3-y1)-
(4-trifluoromethoxy-phenyfl-methanol
The title compound was synthesized starting from Example B2.1 (34mg) and
Example 266.1 (30.5mg), and following
the procedure described in Example A7.1 step A7.1.1. The crude material was
purified by Prep LC-MS (VII) then by
Prep LC-MS (XIV) to afford 4mg of the title compound as white solid. LC-MS
(B): tR = 0.803min; [M+H]: 440.40.
Example 267: 5-[(1, 3-Di methyl-azetidi n-3-y1)-hydroxy-(4-trifluoromethoxy-
pheny1)-methyl]-3-pyrrol idin-1-yl-pyridine-2-
carbonitrile
267.1. 5-Bromo-3-pyrrolidin 1 yl pyridine-2-carbonitrile
To a solution of 5-bromo-3-fluoropicolinonitrile (186mg) in THF (4.5m1) was
added DIPEA (317pL) and pyrrolidine
(77.6pL). The resulting solution was stirred overnight at RT, diluted with EA
and washed with water and brine. The org.
phases were dried (MgSO4), filtrated off and evaporated to dryness. The
residue was purified by CC (Biotage, snap10g,
solvent A: Hep; solvent B: EA; gradient in %B: 10 over 2CV, 10 to 30 over 3CV,
30 over 2CV) to afford 223mg of the
title compound as pale yellow solid. LC-MS (A): tR = 0.93min; [M+H]: 252.19.
267.2. 541,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-trifluoromethoxy-pheny0-
methylk3-pyrrolidin-1-yl-pyridine-2-
carbonitrile
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
247
The title compound was synthesized starting from Example B2.1 (50mg) and
Example 267.1 (46.1mg), following the
procedure described in Example A7.1 step A7.1.1. The crude material was
purified by Prep LC-MS (VI) to afford 2mg
of the title compound as pale yellow solid. LC-MS (A): tR = 0.82min; [WEN':
447.26.
Example 268: (1,3-Dimethyl-azetidin-3-y1)46-(tetrahydro-pyran-4-yloxy)-pyridin-
3-y1]-(4-trifluoromethoxy-pheny1)-
methanol
268.1. 5-Bromo-2-(tetrahydro-pyran-4-yloxy)-pyridine
To a solution of 5-bromo-2-chloropyridine (250mg) in THF (1.5mL) was added
KOtBu(152mg) and tetrahydro-4-pyranol
(132pL) and the resulting solution was stirred under microwave conditions for
30min at 120 C. The reaction mixture
was diluted with EA and washed with water and brine. The org. phases were
dried (MgSO4), filtrated off and evaporated
to dryness. The residue was purified by CC (Biotage, SNAP10g, solvent A: Hop;
solvent B: EA; gradient in %B: 5 over
2CV, 5 to 10 over 2CV, 10 over 2CV) to afford 240mg of the title compound as
white solid. LC-MS (A): tR = 0.89min;
[M+H]: 260.16.
268.2. (1,3-Dimethyl-azetidin-3-y046-(tetrahydro-pyran-4-yloxy)-pyridin-3-y1]-
(4-trifluoromethoxy-pheny1)-methanol
The title compound was synthesized starting from Example B2.1 (50mg) and
Example 268.1 (104mg), and following
the procedure described in Example A7.1 step A7.1.1. The crude material was
purified by Prep LC-MS (VI) then Prep
LC-MS (IX) to afford 7mg of the title compound as white solid. LC-MS (A): tR =
0.77min; [M+H]*: 453.14.
Example 269: (1,3-Dimethyl-azetidin-3-y1)-[6-(oxetan-3-ylmethoxy)-pyridin-3-
y1]-(4-trifluoromethoxy-phenylymethanol
The title compound (11mg, white solid) was synthesized following the two-step
procedure described in Example 268,
using oxetan-3-yl-methanol instead of tetrahydro-4-pyranol. The crude material
was purified by Prep LC-MS (VI) to
afford 11mg of the title compound as white solid. LC-MS (A): tR = 0.73min;
[M+H]: 439.16.
Example 270: (1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(4-methyl-
thiazol-2-y1)-pyridin-311]-methanol
2701, 3-Bromo-5-(4-methyl-thiazol-2-y1)-pyridine
A suspension of 5-bromopyridine-3-carbothioamide (150mg) and chloroacetone
(66pL) in Et0H (5mL) was stirred for
5 days at 80 C and evaporated to dryness. The resulting residue was purified
by CC (Biotage, SNAP10g, solvent A:
Hep; solvent B: EA; gradient in %B: 10 over 2CV, 10 to 30 over 2CV, 30 over
2CV) to afford 121mg of the title compound
as brown solid. LC-MS (A): tR = 0.89min; [M+H]: 257.13.
270.2. (1, 3-Dim ethyl-azetid n-3-y1)-(4-isopropyl-pheny1)45-(4-m ethyl-
thiazol-2-y1)-pyrid in-3-ylkmetha nol
The title compound was synthesized starting from Example B2.2 (50mg) and
Example 270.1 (71.7mg), and following
the procedure described in Example A7.1 step A7.1.1. The crude material was
purified by Prep LC-MS (VII) then Prep
LC-MS (IX) to afford 5mg of the title compound as white solid. LC-MS (A): tR =
0.79min; [M+H]*: 408.32.
Example 271: (1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(5-methyl-
thiazol-2-y1)-pyridi n-3-yI]-methanol
271,1, 3-Bromo-5-(5-methyl-thiazol-2-y1)-pyridine
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
248
A suspension of 5-bromopyridine-3-carbothioamide (150mg) and 2-bromo-1,1-
diethoxypropane (139mg) in AcOH
(1.5mL) was stirred for 4h at 120 C and was evaporated to dryness. The
resulting residue was taken up in aq. sat.
NaHCO3 solution. The org. layers were washed with water and brine, dried
(MgSO4), filtrated off and evaporated to
dryness. The resulting crude material was purified by CC (Biotage, SNAP 10g,
solvent A: Hep; solvent B: EA; gradient
in %B: 10 over 2CV, 10 to 30 over 2CV, 30 over 2CV) to afford 82mg of the
title compound as brown solid. LC-MS (A):
tR = 0.92min; [M+H]: 257.13.
271.2. (1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(5-methyl-thiazol-2-
4-pyridin-3-y1Tmethanol
The title compound was synthesized starting from Example B2.2 (50mg) and
Example 271.1 (71.7mg), and following
the procedure described in Example A7.1 step A7.1.1. The crude material was
purified by Prep LC-MS (VII) then Prep
LC-MS (XIV) to afford 5mg of the title compound as white solid. LC-MS (B): tR
= 0.734min; [M+H]-: 408.4.
Example 272: (1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(2-nnethoxy-
pyrimidin-5-y1)-methanol
The title compound was synthesized starting from Example B2.2 (50mg) and 5-
bromo-2-methoxypyrimidine (41.7mg),
and following the procedure described in Example A7.1 step A7.1.1. The crude
material was purified by Prep LC-MS
(VI) then Prep LC-MS (IX) to afford 2mg of the title compound as white solid.
LC-MS (A): tR = 0.80min; [M+H]-: 342.19.
Example 273: (S)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(2-
pyrrolidin-1-yl-pyridin-4-y1)-methanol
273.1. (1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(2-pyrrolidin-1-yl-
pyridin-4-y1)-methanol
Example F1.5 (75mg) and pyrrolidine (100pL) were dissolved in Dioxane (1mL)
and the reaction mixture was stirred
for 20h at 110 C. Pyrrolidine (100pL) was added and the mixture was stirred
for 70h at 11000. The mixture was allowed
to cool down, was diluted with MeCN and purified by Prep LC-MS (XIV). The
solvent of collected fractions was
evaporated and the resulting aq. layer was basified with 1M NaOH solution and
extracted with EA. The org. layers were
washed with brine, dried (MgSO4), filtered off and evaporated to dryness. The
residue was purified by Prep LC-MS (VII)
to afford 17.7mg of the title compound as yellow oil. LC-MS (B): tR = 0.58min;
[M-FH]': 380.27.
273.2 (S)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(2-pyrrolidin-1-yl-
pyridin-4-A-methanol
The title compound (9mg) was obtained by Prep chiral SFC (IX) of Example 273.1
(17.7mg). LC-MS (A): tR = 0.58min;
[M+H]-: 380.22; Chiral SFC (J): 2.8min.
Example 274 to Example 276 were synthesized starting from the appropriate
amine reagent of Formula (F2), and
following the procedure described in Example 273 step 273.1, but using
different solvents and bases listed in the table
below. Prep LC-MS conditions and LC-MS data of Example 274 to Example 276 are
listed in the table below. The LC-
MS conditions used were LC-MS (A).
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
249
Example Prep LC-
Solvent
Name tR
N MS
Base
(1,3-Dimethyl-azetidin-3-yI)-[2-((R)-2-hydroxymethyl-
(XV) then NMP
274 pyrrolidin-1-y1)-pyridin-4-y1]-(4-isopropyl-pheny1)-
0.56 410.33
(IX)
DIPEA
methanol
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
n-Butanol
275 0.64 394.32 (XV)
(3,4,5,6-tetrahydro-2H-0 ,Thipyridiny1-4'-y1)-methanol
DIPEA
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(2-
N MP
276 0.59 396.32 (XV)
morpholin-4-yl-pyridin-4-yI)-methanol
none
Example 277: (S)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[2-
(tetrahydro-pyran-4-ylmethoxy)-pyridin-4-y1]-
methanol
Example F1.5 (35mg) and tetrahydro-2H-pyran-4-yl-methanol (52mg) were
dissolved in dioxane (1mL) and NaH (60%
in mineral oil, 17.1mg) was added. The reaction mixture was stirred for 7h at
100 C. DMF (1mL) was added and the
mixture was stirred for 72h at 130 C. After cooling down to RT, the mixture
was diluted with MeCN/water and purified
by Prep LC-MS (IX) to afford the title compound (4.5mg) as white solid. LC-MS
(A): tR = 0.78min; [M+H]': 425.31.
Example 278: (S)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[2-(2-
methoxy-ethoxy)-pyridin-4-y1]-methanol
The title compound (7.4mg, white solid) was prepared following the procedure
described in Example 277, and using 2-
methoxy-eth2n01 instead of tetr2hydro-2H-pyr2n-4-yl-meth2n01. LC-MS (A): tR =
0.74min; [M+H]': 385.14.
Example 279: (S)-(1,3-Dimethyl-azetidin-3-y1)-(2-ethyl-pyridin-4-y1)-(4-
isopropyl-phenyl)-methanol
To a solution of Example F1.5 (30mg) in dioxane (0.5mL) was added diethylzinc
solution in toluene (15%, 104pL) and
Pd(dppf)Cl2 (1.12mg) and the mixture was stirred for 5h30 at 70 C. Diethylzinc
solution in toluene (15%, 104pL) was
added again and the mixture was further stirred for 6h30 at 70 C. The reaction
mixture was quenched by careful addition
of water, diluted with Me0H and filtered off. The resulting crude material was
purified by Prep LC-MS (V) then by Prep
LC-MS (XIV) to afford 1.3mg of the title compound as white solid. LC-MS (A):
tR = 0.54min; [M+H]t 339.25.
Example 280: (S)-(2-Cyclopentyl-pyridin-4-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-
isopropyl-pheny1)-methanol
280.1. (S)-(2-Cyclopent-1-enyi-pyridin-4-y1)-(1,3-dimethyl-azetidin-314)-(4-
isopropyl-pheny1)-methanol
To a solution of Example F1.5 (50mg) and cyclopentene-1-yl-boronic acid
(16.1mg) in MeCN (0.5mL) were added
Na2CO3 (1M, 0.5mL) and Pd(PPh)3Cl2 (4.49mg), and the mixture was stirred for
3h30 at 80 C. The reaction mixture
was cooled down to RT, diluted with Me0H, filtered off and purified by Prep LC-
MS (V) to afford 30mg of the title
compound as white solid. LC-MS (A): tR = 0.63min; [M+H]': 377.33.
280.2. (S)-(2-Cyclopentyl-pyridin-4-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-
isopropyl-pheny1)-methanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
250
The title compound (23mg, white solid) was synthetized starting from Example
280.1 (27mg), and following the
procedure described in Example 48. LC-MS (A): tR = 0.62min; [M-F1-1]': 379.35.
Example 281: (R)-(1,3-Dimethyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-y1)-
(4-trifluoromethyl-pheny1)-methanol
281,1, 3-1-Hydroxy-(5-pyrrolidin-1-yl-pyridin-3-y1)-(4-trifluoromethyl-pheny1)-
methyl]-3-methyl-azetidine-1-carboxylic
acid tert-butyl ester
The title compound was synthesized starting from Example A4.5 (1.13g) and 3-
bromo-5-pyrrolidinopyridine (990mg),
and following the procedure described in Example A7.1 step A7.1.1. The crude
material was purified by Prep LC-MS
(IV) to afford 480mg of the title compound as light yellow solid. LC-MS (A):
tR = 0.89min; [M-+I]': 492.32.
281,2, 34(R)-Hydroxy-(5-pyrrolidin-1-yl-pyridin-3-y1)-(4-trifluoromethyl-
pheny1)-methyl]-3-methyl-azetidine-1-carboxylic
acid tert-butyl ester
The title compound was obtained by Prep chiral SFC (II) of Example 281.1. LC-
MS (A): tR = 0.87min; [M+H]: 492.29;
Chiral SFC (H): 3.0min.
281,3, (R)-(3-Methyl-azetidin-3-y1)-(5-pyrrolidin-1-0-pyridin-3-y1)-(4-
trifluoromethyl-pheny1)-methanol, hydrochloride
salt
A solution of Example 281.2 (215mg) in HCI in dioxane (4M, 2mL) was stirred
for 1h45. The mixture was evaporated to
dryness to give 210mg of the title compound as orange solid. LC-MS (A): tR =
0.60min; [M-F1-1]+: 392.33.
281,4, ((R)-(1,3-Dimethyl-azetidin-3-y1)-(5-pyrrolidi n-1-yl-pyridin-3-y1)-(4-
trif 1 uoromethyl-pheny1)-methanol
The title compound was synthesized starting from Example 281.3 (50mg), and
following the procedure described in
Example B2.1 step B2.1.2 (16mg, colorless resin). LC-MS (B): tR = 0.488min; [M-
FH]: 406.4.
Example 282: (R)-(1, 3-Di methyl-azetidin-3-y1)454(R)-3-hydroxymethy1-3-methyl-
pyrrolidin-1-y1)-pyridi
isopropyl-phenyI)-methanol
282,1, (R)-3-Methyl-pyrrolidine-3-carboxylic acid methyl ester, hydrochloride
salt
To an ice-cold Me0H solution (20mL) was added dropwise SOCl2 (1mL), followed
30min later by (R)-3-methyl-
pyrrolidine-3-carboxylic acid (500mg) and the resulting suspension was stirred
for 65h at RT. The reaction mixture was
evaporated to dryness to afford 784 mg of the title compound as brown solid.
LC-MS (A): tR = 0.31min; [M-+I]: 144.27.
282,2, (R)-3-Methyl-pyrrolidine-1,3-dicarboxylic acid 1-tert-butyl ester 3-
methyl ester
To a solution of Example 282.1 (768mg) in DCM (30mL) were successively added
TEA (1.79mL) and Boc20 (952mg).
The resulting mixture was stirred for 1h10 at RT, diluted with water and
extracted with DCM. The org. layers were dried
(MgSO4), filtrated off, and evaporated to dryness to afford 832mg of the title
compound as brown oil. LC-MS (A): tR =
0.87min; [M-F1-1]+: 244.34.
2823, (R)-3-Hydroxymethy1-3-methyl-pyrrolidine-1-carboxylic acid tert-butyl
ester
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
251
To an ice-cold solution of Example 282.2 (820mg) in THF (30mL) was LiA11-14 in
THF (2M, 1.01mL). The resulting mixture
was stirred for lh at 0 C, carefully quenched with ice-water then with aq.
sat. NaHCO3 solution, and extracted with EA.
The org. layers were washed with water and brine, dried (MgSO4), filtrated
off, and evaporated to dryness to afford
716mg of the title compound as yellow oil. LC-MS (A): tR = 0.72min; [M+H]:
216.22.
282.4. ((R)-3-Methyl-pyrrolidin-3-y1)-methanol, hydrochloride salt
A solution of Example 282.4 (715mg) in HCI in dioxane (4M, 7mL) was stirred
for 1h50 at RT and evaporated to dryness
to give 377mg of the title compound as brown solid. LC-MS (A): tR = 0.2min;
[M+H]: 116.30.
282.5. (R)-(1,
phenyl)-methanol
To a solution of Example F1.2 (50mg) and Example 282.4 (97.4mg) in DMSO (1mL),
were added K2003 (54.1mg), L-
proline (16.3mg) and Cul (13.2mg) and the resulting mixture was stirred for
44h at 100 C, cooled down to RT, filtrated
off and evaporated to dryness. The residue was purified by Prep LC-MS (IX)
then by Prep LC-MS (VI) to afford 14mg
of the title compound as white solid. LC-MS (B): tR = 0.475min; [M-FH]':
424.4.
Example 283: (R)-(1,3-Dimethyl-azetidin-3-y1)-[5-((S)-3-fluoro-pyrrolidin-1-
y1)-pyridin-3-y1]-(4-isopropyl-phenyl)-
methanol
To a solution of Example F1.2 (50mg) and (S)-(+)-3-fluoropyrrolidne
hydrochloride (41.5mg) in toluene (1mL) was added
RuPhos (6.29mg), Pd2(dba)3 (6.05mg) and NaOtBu (57.1mg). The reaction mixture
was stirred for 1h at 110 C, cooled
down to RT, diluted with water and extracted with DCM. The org. layers were
dried (MgSO4) and evaporated to dryness.
The residue was purified by Prep LC-MS (VI) to afford 71mg of the title
compound as white solid. LC-MS (B): tR =
0.493min; [M+H]: 398.40.
Example 284: (R)-(1, 3-Di methyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{544-
(tetrahyd ro-pyran-4-y1)41,2,3]triazol-1-y1]-
pyridi n-3-yI}-methanol
To a solution of Example F1.2 (50mg) in Et0H (0.7mL) were added 4-ethynyloxane
(70.7mg), sodium azide (16.9mg),
(+)-Sodium L-ascorbate (2.57mg), N,N-dimethylethylenediamine (4.23pL) and Cul
(4.92mg). The resulting mixture was
stirred for 20h20 at 90 C. 4-Ethynyloxane (70.7mg) was added again and the
mixture was stirred for 5h30 at 90 C,
diluted with water, Me0H and DMF, filtrated off and purified by Prep LC-MS
(VI) to afford 1mg of the title compound as
white solid. LC-MS (B): tR = 0.66min; [M+H]: 462.40.
Example 285 to Example 288 were synthesized starting from Example F1.1 and the
appropriate boronic acid reagent
of Formula (F6), following the procedure described in Example 280 step 280.1.
Prep LC-MS conditions and LC-MS
data are listed in the table below. The LC-MS conditions used were LC-MS (A).
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
252
Example
Prep
Name tR [M-
FH]-
N
LC-MS
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(5-methyl-pyridin-
0.55 325.31 (V)
285 3-yI)-methanol
(1,3-Dimethyl-azetidin-3-y1)-(5-isopropenyl-pyridin-3-y1)-(4-
0.66 351.31 (VII)
286 isopropyl-phenyI)-methanol
(5-Cyclopropyl-pyridin-3-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-
0.62 351.28 (VII)
287 isopropyl-phenyI)-methanol
(5-Cyclopent-1-enyl-pyridin-3-y1)-(1,3-dimethyl-azetidin-3-y1)-(4-
0.71 377.36 (VII)
288 isopropyl-phenyI)-methanol
Example 289: 3-{5-[(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-yll-cyclopent-2-enol
289.1. 3-{51(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylTpyridin-3-yll-cyclopent-2-enone
The title compound was synthesized starting from Example F1.1 (250mg) and 3-
(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-yl)cyclopent-2-en-1-one (140mg), following the procedure
described in Example 280 step 280.1. The
crude material was purified by Prep LC-MS (XIV) to afford 122mg of the title
compound as off-white powder. LC-MS
(A): tR = 0.7min; [M-FH]-: 391.32.
289.2 3-{54(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylppyridin-3-y1}-cyclopent-2-enol
To an ice-cold suspension of Example 289.1 (122mg) in THF (18mL) and Me0H
(1.8mL) was added NaBH4 (47.3mg)
portionwise and the resulting mixture was stirred for 19h30 at RT. NaBH4
(47.3mg) was added and the mixture was
stirred for 3h30 at RT then for 45h at 40 C and for 6h at 65 C. NaBH4 (23.6mg)
was added and the mixture was stirred
for 18h at 65 C. The reaction mixture was allowed to cool down to RT, quenched
with aq. sat. NH40I and extracted with
EA. The org. layers were washed with aq. sat. NH4CI and brine, dried (MgSO4),
filtrated off, and evaporated to dryness.
The residue was purified by Prep LC-MS (IX) to afford 5mg of the title
compound as white solid. LC-MS (B): tR =
0.576min; [M-FH]-: 393.40.
Example 290 to Example 293 were synthesized from the appropriate precursor,
following the procedure described in
Example 48 and using the solvents listed in the table below. Precursors and LC-
MS data are listed in the table below.
The LC-MS conditions used were LC-MS (B).
Example
Name tR [M--H] -
Precursor Solvent
N
(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-phenyl)-
290 0.609 353.4 286 Me0H
(5-isopropyl-pyridin-3-yI)-methanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
253
(5-Cyclopentyl-pyridin-3-yI)-(1,3-dimethyl-azetidin-
291 0.691 379.4 288 Et0H
3-y1)-(4-isopropyl-phenylymethanol
3-{5-[(1,3-Dimethyl-azetid in-3-yI)-hydroxy-(4-
292 isopropyl-phenyI)-methy1]-pyridin-3-yll- 0.509
395.4 289 Et0H
cyclopentanol
(R)-(1, 3-Di methyl-azetid in-3-yI)- (4-isopropyl-
293 0.492 311.3 F1.2 Et0H
phenyI)-pyridin-3-yl-methanol
Example 294: 3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyl]-pyridin-3-y1}-cyclopent-2-
enone
The title compound (182mg, off-white solid) was synthesized following the
procedure described in Example 289 step
289.1, but starting from Example F1.2 (250mg). The crude material was purified
by Prep LC-MS (V) then by Prep LC-
MS (IX). LC-MS (B): tR = 0.612min; [M+H]: 391.32.
Example 295: 3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyl]-pyridin-3-y11-1-methyl-
cyclopent-2-enol
The title compound (18mg, yellow powder) was synthesized starting from Example
294 (50mg), and following the
procedure described in Example 262. The crude material was purified by Prep LC-
MS (V). LC-MS (B): tR = 0.614min;
[M-FH]*: 407.40.
Example 296: (38)-3-(54(R)-(1,3-dimethylazetidin-3-y1)(hydroxy)(4-
isopropylphenyOmethyppyridin-3-y1)-1-
methylcyclopentan-1-ol
The title compound (mixture of two diastereomers, 8mg, white powder) was
synthesized starting from Example 295
(22mg), and following the procedure described in Example 48. The crude
material was purified by Prep LC-MS (VI).
The chirality at carbon atom 3 of the cyclopentyl ring was arbitrarily
assigned to (S). The chirality at carbon atom 1 of
the cyclopentyl ring is undefined. LC-MS (B): tR = 0.531min; [M+H]: 409.5.
Example 297: (3R)-3-(5-((R)-(1,3-dimethylazetidin-3-y1)(hydroxy)(4-
isopropylphenyl)methyppyridin-3-y1)-1-
methylcyclopentan-1-ol
The title compound (mixture of two diastereomers, 5mg, white powder) was
isolated as second eluting compound from
Prep LC-MS (VI) of Example 296. The chirality at carbon atom 3 of the
cyclopentyl ring was arbitrarily assigned to (R).
The chirality at carbon atom 1 of the cyclopentyl ring is undefined. LC-MS
(B): tR = 0.558min; [M+H]-: 409.5.
Example 298: 3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyl]-pyridin-3-y11-1-ethyl-
cyclopentanol
298.1. 3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylppyridin-3-4-1-ethyl-cyclopent-2-
enol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
254
The title compound (as mixture of diastereomers, 10mg, white powder) was
synthesized starting from Example 294
(64mg) and ethylmagnesium bromide solution in THF (1M, 513pL), following the
procedure described in Example 262.
The crude material was purified by Prep LC-MS (VI). LC-MS (A): tR = 0.64min;
[WEN': 421.34.
298.2 3-{54(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylkpyridin-3-y0-1-ethyl-cyclopentanol
The title compound (10 mg, white powder) was synthesized from Example 298.1
(10mg), and following the procedure
described in Example 48. The crude material was purified by Prep LC-MS (VI).
LC-MS (B): tR = 0.584min; [M+H]:
423.50.
Example 299: 3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridin-3-y11-1-isopropyl-
cyclopentanol
The title compound was synthesized starting from isopropylmagnesiumbromide
solution in THF, and following the two-
step procedure described in Example 298. LC-MS (A): tR = 0.67min; [M-FH]-:
437.40.
Example 300: (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(4-methyl-
oxazol-2-y1)-pyridin-3-y1]-methanol
To a solution of 4-methyloxazole (25mg) in THF (0.5mL) cooled down to -78 C
was added dropwise n-BuLi in hexane
(2.5M, 143pL), followed by ZnCl2 in 2-methyltetrahydrofuran (1.9M, 235pL). The
resulting solution was stirred for 50min
at RT. Example F1.2 (116mg) and Pd(PPh3)4 (35.1mg) were added and the mixture
was stirred for 18h at 60 C, cooled
down to RT, diluted with EA and washed with water and brine. The org. layers
were dried (MgSO4), filtrated off and
evaporated to dryness. The residue was purified by Prep LC-MS (VI) to afford
14mg of the title compound as white
powder. LC-MS (B): tR = 0.692min; [M-FH]-: 392.40.
Example 301: (R)-(1,3-Dimethyl-azetidin-3-y1)-(5-ethyl-pyridin-3-y1)-(4-
isopropyl-phenylymethanol
The title compound (8mg, brown solid) was synthesized starting from Example
F1.2 (50mg), following the procedure
described in Example 279. The crude material was purified by Prep LC-MS (VI).
LC-MS (A): tR = 0.58min; [M-FI-1]':
339.30.
Example 302: (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(5-methyl-
pyridin-3-y1)-methanol
The title compound (14mg, white solid) was synthesized starting from Example
F1.2 (50mg) and methylboronic acid
(8.72mg), following the procedure described in Example 280 step 280.1. The
crude material was purified by Prep LC-
MS (VI) then by Prep LC-MS (XXI). LC-MS (A): tR = 0.54min; [M-F1-1] : 325.33.
Example 303: (R)45-(4,5-Dihydro-furan-3-y1)-pyridin-3-y1]-(1,3-dimethyl-
azetidin-3-y1)-(4-isopropyl-pheny1)-methanol
To a solution of Example F1.2 (50mg) and 4,5-dihydrofuran-3-boronic acid
pinacol ester (52.5mg) in dioxane (1mL) and
water (0.25mL) were added K3PO4 (81.8mg) and [1,1'-
bis(diphenylphosphino)ferrocene]dichloropalladium (II) complex
with DCM (21mg), and the resulting mixture was stirred for lh at 80 C. It was
then allowed to cool down to RT, diluted
with MeCN and water and filtrated off. The resulting solution was purified by
Prep LC-MS (VI) then by Prep LC-MS (IX)
to afford 25mg of the title compound as white solid. LC-MS (B): tR = 0.604min;
[M+H]': 379.40.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
255
Example 304: (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-
(tetrahydro-furan-3-y1)-pyridin-3-y11-methanol
Example 303 (21mg) was dissolved in Et0H with 1% toluene (10mL) and the
reaction was conducted in a HCube -
Pro equipped with a 10% (w/w) Pd/C cartridge (7 cm long) under a flow of
1.0mL/min, a hydrogen pressure of 3 bar
and a temperature of 30 C. After reaction completion, the solvent was
evaporated and the residue was purified by Prep
LC-MS (V) to afford 5.5mg of the title compound as colorless solid. LC-MS (B):
tR = 0.551min; [M+H]: 381.40.
Example 305: 3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyl]-pyridin-3-yll-but-3-en-1-ol
The title compound (35mg, off-white solid) was synthesized starting from
Example F1.2 (50mg) and 3-buten-1-o1-3-
boronic acid pinacol ester (50.9mg), and following the procedure described in
Example 303. The crude material was
purified by Prep LC-MS (VI). LC-MS (B): tR = 0.561min; [M-'-H]: 381.40.
Example 306: N-Cyclopenty1-5-[(R)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-phenyl)-methyTnicotinamide
306.1. 3-KR)-(5-Cyclopentylcarbamoyl-pyridin-3-yh-hydroxy-(4-isopropyl-pheny1)-
methyll-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
A mixture of Example E1.1 (50mg), HATU (56.1mg) and DIPEA (58.3pL) in DCM
(0.6mL) was stirred at RT for 15 min
and cyclopentylamine (14.7pL) was added. The reaction mixture was stirred at
RT for 2h, quenched with aq. sat.
NaHCO3 and extracted with DCM. The combined organic extracts were dried
(MgSO4), filtered off and concentrated in
vacuo. The crude was purified by Prep LC-MS (VII) to afford the title compound
as white solid (45.5mg). LC-MS (A): tR
= 1.06min; [M-FH]': 508.43.
306.2. N-Cyclopenty1-5-[(R)-hydroxy-(4-isopropyl-pheny0-(3-methyl-azetidin-310-
methyli-nicotinamide, hydrochloride
salt
A solution of Example 306.1 (43mg) in HCI in dioxane (4M, 0.21mL) was stirred
at RT for 2h30, concentrated in vacuo
and dried under HV to afford 37.6mg of the title compound. LC-MS (A): tR =
0.72min; [M+H]-: 408.26.
306.3. N-Cyclopenty1-5-1(R)-(1,3-ditnethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methylphicorinamide
Example 306.3 was obtained starting from Example 306.2 (37.6mg) and following
the procedure described in Example
01.1 step D1.1.5, with a direct filtration of the reaction mixture through a
syringue filter and purification by Prep LC-MS
(IX) to afford the title product as a white solid (29.2mg). LC-MS (B): tR =
0.701min; [M-F11-: 422.5.
Example 307 and Example 308 were synthesized starting from Example E1.1 and
the appropriate amine reagent, and
following the three-step procedure described in Example 306. The Prep LC-MS
methods and the LC-MS data are listed
in the table below. The LC-MS conditions used were LC-MS (B).
Example
Prep LC-
Name tR [M+1-1]-
N
MS
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
256
{5-[(R)-(1,3-Dimethyl-azetidi n-3-yI)-hydroxy-(4-isopropyl-
307
0.622 408.4 (IX)
phenyI)-methyl]-pyridin-3-yll-pyrrolidin-1-yl-methanone
5-[(R)-(1, 3-Di methyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
308 0.591
438.4 (IX)
pheny1)-methy1]-N-(tetrahydro-pyran-4-y1)-nicotinamide
Example 309: (1,3-Dimethyl-azetidin-3-y1)44-(3-methoxy-propylyphenyl]-(5-
pyrrolidin 1 yl pyridin-3-yI)-methanol
309.1. 344-Bromo-pheny1)-hydroxy-(5-pyrrolidin-1-yl-pyridin-3-y1)-methyl]-3-
methyl-azetidine-l-carboxylic acid tert-
butyl ester
The title compound (180mg) was prepared starting from Example 01.1 (150mg) and
1,4-dibromobenzene (73.8pL),
and following the procedure described in Example A4.1 step A4.1.2. LC-MS (A):
tR = 0.88min; [M-FH]*: 502.28.
309.2. (4-Bromo-pheny1)-(3-methyl-azetidin-3-y1)-(5-pyrrolidin-1-yl-pyridin-3-
y1)-methanol; hydrochloride salt
A solution of Example 309.1 (175mg) in HCI in dioxane (4M, 5mL) was stirred
for 30min at RT and evaporated to
dryness to afford 170mg of the title compound as yellow solid. LC-MS (A): tR =
0.57min; [M+H]:402.03.
309.3. (4-Bromo-phenyl)-(1,3-dimethyl-azetidin-3-y1)-(5-pyrrolidin 1 yl
pyridin-3-y1)-methanol
The title compound (138mg, beige solid) was synthesized starting from Example
309.2 (170mg), and following the
procedure described in Example B2.1 step B2.1.2. LC-MS (A): tR = 0.57min;
[M+H]: 416.29.
309.4. (1, 3-Di m ethyl-azeti d n-3-y1)-(4-((E)-3-m ethoxy-p ropeny1)-phe ny11-
(5-pyrrolid n-1-yl-pyri d n-3-y1)-metha nol
The title compound (30mg, white solid) was synthesized starting from Example
309.3 (50mg) and trans-3-methoxy-1-
propenylboronic acid pinacol ester (53.7pL), following the procedure described
in Example 303. The crude material
was purified by Prep LC-MS (IX). LC-MS (A): tR = 0.57min; [M+H]: 408.43.
309.5. (1,3-Dimethyl-azetidin-3-y1)-14-(3-methoxy-propy1)-phenyll-(5-
pyrrolidin-1-yl-pyridin-3-y1)-methanol
The title compound (11mg, yellow solid) was synthesized starting from Example
309.4 (28mg), and following the
procedure described in Example 48. The crude material was purified by Prep LC-
MS (XIV). LC-MS (B): tR = 0.445min;
[M+H]: 410.5.
Example 310 and Example 311 were synthesized starting from Example 309.3 and
the appropriate boronic acid
reagent, and following the procedure described in Example 303. Prep LC-MS
conditions and LC-MS data are listed in
the table below. The LC-MS conditions used were LC-MS (B).
CA 03177335 2022- 10- 28
WO 2021/219849 PCT/EP2021/061401
257
Example
N Name tR [M-FH]-
Prep LC-MS
(R)-(4-Cyclopropyl-pheny1)-(1,3-dimethyl-azetidin-3-y1)-(5-
310 0.468 378.2 (VII) then (IX)
pyrrolidin-1-yl-pyridin-3-yI)-methanol
(R)-(1,3-Dimethyl-azetidin-3-yI)-(5-pyrrolidin-1-yl-pyridin-
311 0.408 352.4 (VI) then (IX)
3-yI)-p-tolyl-methanol
Example 312: 5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-31,41,51,61-tetrahydro-21H-
[3,4Thipyridiny1-11-carboxylic acid tert-butyl ester
312.1. 54(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-methyl]-
31,61-dihydro-27-143,47bipyridiny1-1'-
carboxylic acid tert-butyl ester
The title compound was synthesized starting from Example F1.2 (50mg) and 3,6-
dihydro-2H-pyridine-1-N-Boc-4-
boronic acid, pinacol ester (81mg), and following the procedure described in
Example 303. The crude material was
purified by Prep LC-MS (V) to give 33mg of the title compound as white solid.
LC-MS (A): tR = 0.76min; [M-Fl-l]: 492.34.
312.2. 51(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-methyl]-
3',45',6'-tetrahydro-2'H-
13,47bipyridiny1-1'-carboxylic acid tert-butyl ester
The title compound was synthesized from Example 312.1 (30mg) and following the
procedure described in Example
48. The crude material was purified by Prep LC-MS (XV) to give 11mg of the
title compound as white solid. LC-MS (B):
tR = 0.77min; [M-FH]-: 494.50.
Example 313 and Example 314 were synthesized from Example F1.2 and the
appropriate boronic ester following the
two-step procedure described in Example 312. Prep LC-MS conditions and LC-MS
data are listed in the table below.
The LC-MS conditions used were LC-MS (B).
Example
N Name tR [M+H]
Prep LC-MS
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
313 pheny1)-methyl]-pyridin-3-y11-pyrrolidine-1-carboxylic acid 0.76 480.50
(XV)
tert-butyl ester
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-phenyl)-[5-
314 0.556 395.40 none
(tetrahydro-pyran-4-0-pyridin-3-y11-methanol
Example 315: (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
(31,41,51,61-tetrahydro-21H-[2,11;41,3"]terpyridin-5"-
y1)-methanol
315.1. (R)-(3',6'-Dihydro-2'H-12,1';4',3'fterpyridine-5"-y1)-(1,3-dimethyl-
azetidin-3-y1)-(4-isopropyl-phenyl)-methanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
258
The title compound was synthesized starting from Example F1.2 (43mg) and 4-
(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-
2-yI)-3,6-dihydro-2H-1,2'-bipyridine (32.6mg), and following the procedure
described in Example 303. The crude
material was purified by Prep LC-MS (XIII) to afford 35mg of the title
compound as white powder. LC-MS (A): tR =
0.56min; [M+H]: 469.21.
315.2. (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(3',4',5',6'-
tetrahydro-2'H-[2,1';4',3"]terpyridin-5"-y1)-
methanol
The title compound was synthesized starting from Example 315.1 (33mg), and
following the procedure described in
Example 48 but using Me0H as solvent. The crude material was purified by Prep
LC-MS (XXI) to afford 6mg of the title
compound as white solid. LC-MS (B): tR = 0.466min; [M+H]': 471.5.
Example 316 and Example 317 were synthesized starting from Example F1.2 and
the appropriate boronic ester, and
following the two-step procedure described in Example 315. Prep LC-MS
conditions and LC-MS data are listed in the
table below. The LC-MS conditions used were LC-MS (A).
Example
N Name tR [M+H]
Prep LC-MS
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(1'-
316 pheny1-1',2',3',4',5',6'-hexahydro-[3,4Thipyridiny1-5-y1)-
0.61 470.11 (IX) then (VIII)
methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[1'-
317 (toluene-4-sulfony1)-1,2',34,5,6-hexahydro- 0.76
547.9 none
[3,4]bipyridiny1-5-y1]-methanol
Example 318: (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-
(tetrahydro-furan-2-y1)-pyridin-3-y1]-methanol
318.1. 3-{(R)-Hydroxy-(4-isopropyi-pheny1)45-(tetrahydro-furan-2-y1)-pyridin-3-
01-methy0-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
To a vial equipped with a magnetic stirring bar, Example F1.11 (100mg), [4,4'-
bis(1,1-dimethylethyl)-2,2'-bipyridine-
N1, N 1This[3, 5-difluoro-245-(trifluoromethyl)-2-pyridinyl-N]phenyl-
C]iridium(111) hexafluorophosphate .. (2.36mg),
NiCl2-glyme (4.72mg), 4,4'-di-tert-butyl-2,2'-dipyridyl (8.64mg) and Cs2CO3
(208mg) were added. The vial was placed
under nitrogen and dry DMF (10mL) was added followed by tetrahydro-2-furoic
acid (60.6pL). The solution was
degassed for 15min by sparging with nitrogen and was irradiated with a 34W
blue LED placed approximately 8 cm away
from the vial. The reaction mixture was stirred for 17h under irradiation,
,was diluted with aq. sat. NaHCO3 and extracted
with Et20 (3 times). The combined org. layers were washed with water and
brine, dried (MgSO4) and concentrated in
vacuo. The residue was purified by CC (Biotage, SNAP 4g, solvent A: Hep;
solvent B: EA; gradient in %B: 0 over 1min,
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
259
0 to 100 over 12min, 100 over 8min) and by Prep LC-MS (VII) to afford the
title product as white solid (7.2mg). LC-MS
(A): tR = 0.90min; [M+1-1]': 467.36.
318.2. (R)-(4-lsopropyl-pheny1)-(3-methyl-azetidin-3-y1)45-(tetrahydro-furan-2-
y1)-pyridin-3-ylrmethanol, hydrochloride
salt
The title compound was obtained starting from Example 318.1, and following the
protocol described in Example 309
step 309.2. LC-MS (A): tR = 0.59min; [M+H]: 367.27.
318.3. (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(tetrahydro-
furan-2-y1)-pyridin-3-y11-methanol
The title compound was obtained starting from Example 318.2 (9.3mg), and
following the procedure described in
Example F1.1 step F1.1.2. Prep LC-MS (XIV) afforded 2.4mg of colorless oil. LC-
MS (B): tR = 0.60min; [M+H]: 381.4.
Example 319 to Example 320 were synthesized starting from Example F1.11 and
the appropriate carboxylic acid, and
following the three-step procedure described in Example 318. The Prep LC-MS
methods and the LC-MS data are listed
in the table below. The LC-MS conditions used were LC-MS (B).
Example
N Name tR [M+H]
Prep LC-MS
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[5-
319 0.647 395.4 (V)
(5-methyl-tetrahydro-furan-2-y1)-pyridin-3-y1]-methanol
(R)-(1,3-Dimethyl-azetidin-3-yI)-[5-(5,5-dimethyl-
320 tetrahydro-furan-2-y1)-pyridin-3-y1]-(4-isopropyl-pheny1)-
0.689 409.5 (V)+ (XIV)
methanol
Example 321: 3-{5-[(R)-(1,3-Dimethyl-azetidin-3-0-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridin-3-y11-2,2-difluoro-
propan-1-ol
321.1. 3-[(R)-15-(2-Ethoxycarbonyl-2,2-difluoro-ethyl)-pyridin-3-yll-hydroxy-
(4-isopropyl-phenyl)-methyll-3-methyl-
azetidine-1-carboxylic acid tert-butyl ester
To a vial equipped with a stirring bar were added (4,4'-di-tert-buty1-2,2'-
bipyridine)bis[3,5-difluoro-245-trifluoromethy1-2-
pyridinyl-N)phenyl-C]iridium(111) hexafluorophosphate (2.36mg), Example F1.11
(100mg), ethyl 3-bromo-2,2-
difluoropropionate (96.1mg), tris(trimethylsilyl)silane (0.1mL) and LiOH
(10.3mg). The vial was sealed, purged with
nitrogen, and MeCN (1mL) was added. To a separate vial were added nickel(11)
chloride ethylene glycol dimethyl ether
complex (2.36mg) and 4,4'-di-tert-butyl-2,2'-dipyridyl (2.82mg). The vial was
sealed, purged with nitrogen, MeCN (1mL)
was added and after sonicating the resulting solution for 5 minutes, 0.1mL
were added to the first vial. The resulting
mixture was degassed by sparging with nitrogen and irradiated in a
photoreactor device (Penn PhD M2, 100%
irradiation) at RT for 4h. The mixture was purified by CC (Biotage, SNAP 12g,
solvent A: Hep; solvent B: EA; gradient
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
260
in %B: 0 over 1min, 0 to 100 over 12min, 100 over 20min) and by Prep LC-MS ON
to afford the title compound as a
beige solid (12.5mg). LC-MS (A): 'R= 1.01min; [M-F1-1]': 533.24.
321.2. 31(R)15-(2,2-Difluoro-3-hydroxy-propy1)-pyridin-3-yll-hydroxy-(4-
isopropyl-pheny1)-methyl]-3-methyl-azetidine-
1-carboxylic acid tert-butyl ester
To a suspension of NaB1-14(0.66mg) in Et0H (0.5mL) was added dropwise a
solution of Example 321.1 (12.5mg) in
Et0H (1mL). The reaction mixture was stirred at RT for 3h, concentrated in
vacuo and aq. sat. NI-14C1 was added. The
mixture was extracted with EA and the org. layer was dried (MgSO4), filtered
off, and concentrated in vacuo to afford
mg as white solid. LC-MS (A): tR = 0.85min; [M+H]: 491.27.
321.3. 2,2-Difluoro-3-{5-[(R)-hydroxy-(4-isopropyl-pheny1)-(3-methyl-azetidin-
3-y1)-methyll-pyridin-3-y1}-propan-l-ol,
10 hydrochloride salt
A solution of Example 321.2 (10mg) in HCI in dioxane (4M, 0.2mL) was stirred
at RT for lh, and concentrated in vacuo
to afford 11 mg of title compound as oil. LC-MS (A): tR = 0.57min; [M+H]-:
391.22.
321.4. 3451( R)-(1,3-Dim ethyl-azetidin-3-y1)-hyd roxy-(4-isopropyl-pheny1)-m
ethylppyridin-3-y1)-2, 2-difluoro-propa n-1-ol
The title compound (1.2 mg, white solid) was obtained starting from Example
321.3 (11.1mg), and following the
procedure described in Example F1.1 step F1.1.2. The crude was purified by
Prep LC-MS (IX). LC-MS (B): tR =
0.566min; [M+H]: 405.4.
Example 322: (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[5-(5-
methyl-oxazol-2-y1)-pyridin-3-y1]-methanol
322.1. 5-Bromo-N-prop-2-ynyl-nicotinamide
To a solution of 5-bromopyridine-3-carboxylic acid (500mg) in THF (12mL) were
added HATU (1.66g), DIPEA
(0.831mL) and propargylamine (0.19mL) at RT. The resulting mixture was stirred
at 50 C for 1h40, cooled down to RT,
diluted with EA and washed with water and brine. The org. layer was dried
(MgSO4), filtered off and concentrated in
vacuo. The crude material was purified by CC (Biotage, SNAP 50g, solvent A:
Hep; solvent B: EA; gradient in %B: 50
over 4CV, 50 to 70 over 2CV, 70 over 2CV) to afford the title compound as
white solid (513mg). LC-MS (A): tR = 0.65min;
[M+H]-: 239.15.
322.2. 3-Brom o-5-(5-m ethylene-4, 5-di hydro-oxazol-2-y1)-pyridine
To a white suspension of Example 322.1 (488mg) in DCM (20mL) was added
AuCI3(61.9mg). The resulting light yellow
suspension was stirred at RT for 1h30, diluted with DCM and washed with aq.
sat. NaHCO3. The aq. layer was extracted
with DCM. The combined org. layers were dried (MgSO4), filtered off, and
concentrated in vacuo. The crude material
was purified by CC (Biotage, SNAP 25g, solvent A: Hep; solvent B: EA; gradient
in %B: 10 over 3CV, 10 to 30 over
2CV, 30 over 2CV) to afford the title compound as white solid (340mg). LC-MS
(A): tR = 0.82min; [M-FH]-: 239.01.
322.3. 3-Bromo-5-(5-methyl-oxazol-2-y1)-pyridine
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
261
To a solution of Example 322.2 (340mg) in toluene (14mL) was added DBU
(0.263mL). The resulting mixture was
heated at 80 C for 2h, cooled down to RT and quenched with aq. sat. NI-14C1.
The org. layer was washed with aq. sat.
NI-1401, dried (MgSO4), filtered off, and concentrated in vacuo to afford the
title compound as yellow solid (222mg). LC-
MS (A): tR = 0.85min; [M+H]: 241.16.
322.4. 3-{Hydroxy-(4-isopropyl-pheny1)45-(5-methyl-oxazol-2-y1)-pyridin-3-
ylpmethyl}-3-methyl-azetidine-1-carboxylic
acid tert-butyl ester
The title compound was obtained starting from Example A4.2 (125mg) and Example
322.3 (122mg), and following the
procedure described in Example A7.1 step A7.1.1. The crude material was
purified by Prep LC-MS (VI) and (XII). The
resulting material was dissolved in DCM and the solution was washed with aq.
sat. N2HCO3. The aq. layer was extracted
twice with DCM and the combined org. extracts were dried (MgSO4), filtered
off, and concentrated in vacuo to afford
the title compound as yellow solid (39mg). LC-MS (A): tR = 1.13min; [M-FH]-:
478.32.
322.5. 3-{(R)-Hydroxy-(4-isopropyl-pheny0-15-(5-methyl-oxazol-2-y0-pyridin-3-
ylpmethyl)-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
Example 322.4 (39mg) was purified by Prep chiral SFC (XVI) to afford the title
compound as pure enantiomer (14mg).
Chiral SFC (P): tR = 3.217 min. LC-MS (A): tR = 1.13min; [M+H]t 478.33.
322.6. (R)-(4-lsopropyl-pheny1)-(3-methyl-azetidin-3-y045-(5-methyl-oxazol-2-
4)-pyridin-3-4-methanol
A solution of Example 322.5 (13mg) in HCI in dioxane (4M, 0.4mL) was stirred
at RT for 2h, cooled down to 0 C, basified
with aq. sat. NaHCO3 and extracted with DCM. The combined org. extracts were
dried (MgSO4), filtered off, and
concentrated in vacuo to afford 7 mg of the title compound as yellow solid. LC-
MS (A): tR = 0.74min; [M-FI-1].: 378.35.
322.7. (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)15-(5-methyl-
oxazol-2-y1)-pyridin-3-y1Tmethanol
The title compound (2mg, white solid) was obtained starting from Example 322.6
(7mg), and following the procedure
described in Example F1.1 step F1.1.2. However, the reaction mixture was
basified with aq. sat. NaHCO3, filtered
through a syringue filter and purified by Prep LC-MS (VI). LC-MS (B): tR =
0.69min; [M+1-1]+: 392.4.
Example 323: (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{545-
(tetrahydro-pyran-4-y1)-oxazol-2-y1]-pyridin-
3-yll-methanol
323.1. 3-((R)-Hydroxy-(4-isopropyl-pheny1)-{542-oxo-2-(tetrahydro-pyran-4-y0-
ethylcarbamoylppyridin-3-y1}-methyl)-3-
methyl-azetidine-1-carboxylic acid tert-butyl ester
To a solution of Example E1.1 (75mg) and HATU (86.8mg) in DCM (0.85mL) was
added DIPEA (87.4pL). The resulting
mixture was stirred at RT for 15 min and 2-amino-1-(oxan-4-yl)ethan-1-one
hydrochloride (41.9mg) was added. The
reaction mixture was stirred at RT, quenched with aq. sat. NaHCO3 and
extracted with DCM. The org. layers were dried
(MgSO4), filtered off and concentrated in vacuo. The crude material was
purified by CC (Biotage, SNAP 24g, solvent A:
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
262
DCM; solvent B: 9:1 DCM/Me0H; gradient in %B: 0 over 1min, 0 to 10 over 3min,
10 over 5min, 10 to 100 over 20 min,
100 over 5min) to afford the title compound as a white glass (99.8mg). LC-MS
(A): tR = 0.96nnin; [M-F1-1]+: 566.26.
323.2. 34(R)-Hydroxy-(4-isopropyl-pheny1)-{545-(tetrahydro-pyran-4-y1)-oxazol-
2-ylrpyridin-3-0-methyl)-3-methyl-
azetidine-1-carboxylic acid tert-butyl ester
A solution of Example 323.1 (99.8mg) in pyridine (0.466mL) containing
molecular sieves (3A powder, 170mg) was
treated with phosphorus(V) oxychloride (81.6pL). The reaction mixture was
stirred at RT for 5h30, diluted with EA and
poured into an ice-chilled solution of aq. sat. NaHCO3.The two layers were
separated and the aq. phase was extracted
twice with EA. The combined org. layers were dried (MgSO4), filtered off and
concentrated in vacuo. The crude was
purified by Prep LC-MS (XXII) to afford the title compound as beige solid
(9.7mg). LC-MS (A): tR = 1.16min; [M+1-1]':
548.13.
323.3. (R)-(4-lsopropyl-pheny1)-(3-methyl-azetidin-3-y1)-{5-15-(tetrahydro-
pyran-4-y1)-oxazol-2-yll-pyridin-3-y1}-
methanol, hydrochloride salt
A solution of Example 323.2 (9.7mg) in HCI (4M in dioxane, 0.1mL) was stirred
at RT for 2h, concentrated in vacuo and
dried under HV to afford the title compound (9.2mg) as beige solid. LC-MS (A):
tR = 0.76min; [M-+I]': 448.35.
323.4. (R)-(1,
methanol
The title compound (3mg, white solid) was obtained starting from Example 323.3
(9.2mg), and following the procedure
described in Example 01.1 step 01.1.5. However, the reaction mixture was
filtered through a syringue filter and purified
by Prep LC-MS (IX). LC-MS (B): tR = 0.712min; [M-F1-1]': 462.4.
Example 324: (R)-(1,3-Dimethyl-azetidin-3-y1)-[5-(4-fluoro-phenoxymethyl)-
pyridin-3-y1]-(4-isopropyl-phenyl)-methanol
324.1. 3-{(R)-Hydroxy-(4-isopropyl-pheny1)-15-(methoxy-methyl-carbamoy1)-
pyridin-3-ylpmethyl}-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
To a suspension of Example E1.1 (500mg) in DCM (6mL) were added N,0-
dimethylhydroxylamine hydrochloride
(128mg) and DIPEA (0.68mL). The mixture was cooled down to 0 C and
propylphosphonic anhydride (50% w/w in EA,
0.88mL) was slowly added. The resulting solution was stirred 1h at RT and
quenched with aq. sat. NaHCO3 solution.
The aq. layer was extracted twice with DCM and the combined org. layers were
dried (MgSO4), filtered off and
evaporated to dryness to afford 596mg of the title compound as white foam. LC-
MS (A): tR = 0.98min; [M-FH]: 483.99.
324.2. 3-[(R)-(5-Fortnyl-pyridin-3-y1)-hydroxy-(4-isopropyl-phenyl)-methyl]-3-
methyl-azetidine-1-carboxylic acid tert-
butyl ester
To an ice-cold solution of Example 324.1 (596mg) in dry THF (6mL) was added
dropwise LiAIH4 (2M in THF, 1.9mL).
The reaction mixture was stirred for 1h at -78 C and quenched by dropwise
addition of aq. sat. NI-141 solution. The
mixture was allowed to warm to RT and EA was added. The solids were filtered
off, washed with EA and water, and the
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
263
filtrate was transferred in a separatory funnel. The layers were separated and
the aq. phase was extracted twice with
EA. The combined org. layers were dried (MgSO4), filtered off and concentrated
to dryness to afford the title compound
as light yellow foam (494 mg). LC-MS (A): tR = 1.04min; [M-FH]-: 425.19.
324.3. 3-[(R)-Hydroxy-(5-hydroxymethyl-pyridin-3-y1)-(4-isopropyl-pheny1)-
methyll-3-methyl-azetidine-1 -carboxylic
acid tert-butyl ester
A solution of Example 324.2 (494mg) in dry Me0H (5.8mL) under nitrogen
atmosphere was cooled down to 0 C and
treated with NaB1-14(53.9mg). The reaction mixture was stirred at 0 C for 5min
and at RT for 30min, quenched with aq.
sat. NaHCO3 solution. and extracted with EA. The combined org. layers were
dried (MgSO4), filtered off, and
concentrated in vacuo to afford 516.6mg of the title compound as light yellow
foam. LC-MS (A): tR = 0.79min; [M-FH]':
427.19.
324.4. 3-[(R)-15-(4-Fluoro-phenoxymethyl)-pyridin-3-yll-hydroxy-(4-isopropyl-
pheny1)-methyll-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
To an ice-chilled mixture of Example 324.3 (100mg), 4-fluorophenol (58.4mg)
and triphenylphosphine polymer bound
(3mm01/g, 313mg) in THF (1.2mL) under nitrogen atmosphere was added
diisopropyl azodicarboxylate (0.188mL). The
reaction mixture was stirred at RT for 17h, filtered off, and concentrated.
The resulting solution was filtered through a
syringue filter and purified by Prep LC-MS ()OK) to afford 11.5mg of the title
compound as white solid. LC-MS (A): tR =
1.01min; [M+H]: 521.17.
324.5. (R)45-(4-Fluoro-phenoxymethyl)-pyridin-3-y11-(4-isopropyl-pheny1)-(3-
methyl-azetidin-3-y1)-methanol,
hydrochloride salt
A solution of Example 324.4 (11.5mg) in HCI (4M in Dioxane, 0.1mL) was stirred
at RT for 1h and concentrated in vacuo
to afford the title compound (10.5mg) as oil. LC-MS (A): tR = 0.74min; [M+H]:
421.20.
324.6. (R)-(1, 3-Dim ethyl-azetidi n-3-y1)45-(4-fluoro-phenoxymethyl)-pyridi n-
3-y1]-(4-isopropyl-pheny1)-methanol
The title compound (4.5mg, white solid) was obtained starting from Example
324.5 (10.9mg), and following the
procedure described in Example D1.1 step D1.1.5. The reaction mixture was
however diluted with MeCN, filtered
through a syringue filter and purified by Prep LC-MS (VI). LC-MS (B): tR =
0.782min; [M+H]: 435.4.
Example 325: Isopropyl-carbamic acid 5-[(R)-(1,3-dimethyl-azetidin-3-y1)-
hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridin-3-ylmethyl ester
325.1. 3-[(R)-Hydroxy-(5-isopropylcarbamoyloxymethyl-pyridin-3-y1)-(4-
isopropyl-pheny1)-methyl]-3-methyl-azetidine-
1-carboxylic acid tert-butyl ester
To an ice-cold solution of Example 324.3 (100mg) and 4-dimethylaminopyridine
(63mg) in DCM (1.2mL) was added
isopropyl isocyanate (51.7pL). The reaction mixture was stirred at 45 C for
17h, cooled down to RT, quenched with aq.
sat. NaHCO3 solution and extracted with DCM. The combined org. extracts were
dried (MgSO4), filtered off,
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
264
concentrated in vacuo and the residue was purified by Prep LC-MS (XIV) to
afford 74mg of the title compound as white
solid. LC-MS (A): tR = 0.93min; [M-F1-1]': 512.22.
325.2. Isopropyl-carbamic acid 5-[(R)-hydroxy-(4-isopropyl-phenyI)-(3-methyl-
azetidin-3-y1)-methylrpyridin-3-ylmethyl
ester
A solution of Example 325.1 (73.6mg) in HCI (4M in Dioxane, 0.36mL) was
stirred at RT for 1 h, concentrated in vacuo
and dried under HV to afford 70mg of the title compound as oil. LC-MS (A): tR
= 0.64min; [M+H]: 412.22.
325.3. Isopropyl-carbamic acid 5-[(R)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-pheny1)-methylppyridin-3-
ylmethyl ester
The title compound (47.2mg, white solid) was obtained starting from Example
325.2 (69.7mg), and following the
procedure described in Example D1.1 step D1.1.5. The reaction mixture was
however diluted with MeCN, filtered
through a syringe filter and purified by Prep LC-MS (V). LC-MS (B): tR =
0.656min; [M-FH]': 426.4.
Example 326: (R)45-(2-Benzyloxy-ethyl)-pyridin-3-y1]-(1,3-dimethyl-azetidin-3-
y1)-(4-isopropyl-phenyl)-methanol
To a solution of Example F1.2 (50mg) and potassium (2-
benzyloxyethyl)trifluoroborate (32.6mg) in toluene (1.5mL) and
water (0.5mL) were added Cs2CO3 (126mg), Pd(OAc)2 (1.44mg) and RuPhos
(6.31mg). The resulting mixture was
stirred for 23h at 95 C, allowed to cool down to RT, diluted with water and
extracted with EA. The org. layers were dried
(MgSO4), filtrated off and evaporated to dryness. The crude material was
purified by Prep LC-MS (VII) to give 30mg of
the title compound as white solid. LC-MS (B): tR = 0.728min; [M-FH]': 445.50.
Example 327: 4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyl]-pyridin-3-y11-1-methyl-
cyclohexanol
327.1. (R)-(1,3-Dimethyl-azetidin-3-y1)45-(1,4-dioxa-spiro[4.5klec-7-en-8-y1)-
pyridi n-3-y1]-(4-isopropyl-pheny1)-
methanol
The title compound (174mg, white solid) was synthesized starting from Example
F1.2 (400mg) and 1,4-dioxa-
spiro[4,5]clec-7-en-8-boronic acid pinacol ester (558mg), and following the
procedure described in Example 303. The
crude material was however purified by CC (Biotage, SNAP 25g, solvent A: DCM;
solvent B: Me0H; gradient in %B: 0
over 2CV, 0 to 5 over 5CV, 5 over 2CV, 5 to 10 over 3CV; 10 over 2CV, 10 to 20
over 2CV, 20 over 2CV, 100 over 5CV
and 100% Me0H +0.1%TEA over 10CV). LC-MS (A): tR = 0.65min; [M-FH]-: 449.1.
327.2. (R)-(1,3-Dimethyl-azetidin-3-y045-(1,4-dioxa-spirof4.5]dec-8-y0-pyridin-
3-y11-(4-isopropyl-phenyl)-methanol
The title compound (116mg, brown solid) was synthesized starting from Example
327.1 (172mg), and following the
procedure described in Example 48. LC-MS (A): tR = 0.62min; [M+H]: 451.08.
327.3. 4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylPpyridin-3-y1}-cyclohexanone
To an ice-cold solution of Example 327.2 (89mg) in dioxane (1mL) were added
water (0.2mL) and H2SO4 (0.2mL). The
resulting solution was stirred for 3h at 0 C, basified with aq. sat. NaHCO3
solution, diluted with water and extracted with
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
265
DCM. The org. layers were dried (MgSO4), filtrated off, and evaporated to
dryness to give 72mg of the title compound
as brown solid. LC-MS (A): tR = 0.58min; [M-F1-1]': 407.14.
327.4. 4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y0-hydroxy-(4-isopropyl-pheny0-
methylrpyridin-3-y11-1-methyl-cyclohexanol
The title compound (9mg, white powder) was synthesized starting from Example
327.3 (30mg), and following the
procedure described in Example 262. The crude material was purified by Prep LC-
MS (VII). LC-MS (B): tR = 0.591min;
[M+H]: 423.5.
Example 328: 2-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridin-3-y1}-cyclopropyl)-
propan-2-ol
328.1. 2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y0-hydroxy-(4-isopropyl-pheny0-
methylppyridin-3-y1}-cyclopropanecarboxylic
acid ethyl ester
The title compound (10mg, yellow solid) was synthesized starting from Example
F1.2 (250mg) and (2-
(ethoxycarbonyl)cyclopropyl)boronic acid pinacol ester, and following the
procedure described in Example 280 step
280.1. The crude material was purified by Prep LC-MS (XV). LC-MS (A): tR =
0.69min; [M+H]: 423.22.
328.2. 2-(2-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny0-
methylTpyridin-3-yll-cyclopropy1)-propan-
2-ol
The title compound (3.4mg, white solid) was synthesized starting from Example
328.1 (10mg), and following the
procedure described in Example 262. The crude material was purified by Prep LC-
MS (IX). LC-MS (B): tR = 0.566min;
[M+H]: 409.5.
Example 329: (S)-(1, 3-Di methyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{245-
(tetrahydro-pyran-4-y1)-[1,2,4]oxadi azol-3-y1]-
pyridin-4-yI}-methanol
329.1. 342-Cyano-pyridin-4-y0-hydroxy-(4-isopropyl-phenyl)-methyl]-3-methyl-
azetidine-1-carboxylic acid tert-butyl
ester
The title compound was synthesized starting from Example A4.2 and 4-
bromopyridine-2-carbonitrile, and following the
procedure described in Example A7.1 step A7.1.1. LC-MS (A): tR = 1.07min; [M-
FH]-: 422.31.
329.2. 4-11-lydroxy-(4-isopropyl-phenyl)-(3-methyl-azetidin-3-y0-methyll-
pyridine-2-carbonitrile, hydrochloride salt
A solution of Example 329.1 (4.5g) in dioxane (25mL) and HCI in dioxane (4M,
25mL) was stirred for 2h. The solution
was lyophilized to give 3.8g of the title compound as light brown solid. LC-MS
(A): tR = 0.70min; [M+H]': 322.00
329.3. 41(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny0-
methylrpyridine-2-carbonitrile
The title compound (1.92g, off-white solid) was synthesized starting from
Example 329.2, and following the procedure
described in Example D1.1 step D1.1.5. The resulting crude material was
purified by Prep LC-MS (XVI). LC-MS (A): tR
= 0.72min; [M-FH]: 336.23.
329.4. 4-[(S)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-
methylkpyridine-2-carbonitrile
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
266
The title compound (0.8g, off-white powder) was obtained by chiral separation
of Example 329.3 (1.92g) using Prep
chiral SFC (V) method. LC-MS (A): tR = 0.72min; [M+1-1]': 336.12, Chiral SFC
(E): 2.1min.
329.5. 44(S)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylFN-hydroxy-pyridine-2-carboxamidine
The title compound (27mg, beige solid) was synthesized starting from Example
329.4 (25mg), and following the
procedure described in Example E2.1. LC-MS (A): tR = 0.59min, [M+H]': 369.18.
329.6. (S)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{245-(tetrahydro-
pyran-4-y1)41,2,4]oxadiazol-3-ylrpyridin-
4-y11-methanol
The title compound (6mg, white solid) was synthesized starting from Example
329.5 (27mg), and following the
procedure described in Example 95 step 95.1. The crude material was purified
by Prep LC-MS (IX). LC-MS (A): tR =
0.75min; [M-F1-1]+: 463.29.
Example 330: (1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(2-pyrrolidin-1-
yl-pyrimidin-5-y1)-methanol
330.1. 3-Nydroxy-(4-isopropyl-pheny1)-(2-pyrrolidin-1-yl-pyrimidin-5-y1)-
methyl]-3-methyl-azetidine-1-carboxylic acid
tert-butyl ester
The title compound was synthesized starting from Example A4.2 and 5-bromo-2-
(pyrrolidin-1-yl)pyrimidine, and
following the procedure described in Example A4.1 step A4.1.2. LC-MS (A): tR =
0.97min; [M-FH]-: 467.11.
330.2. (4-lsopropylphenyl)(3-methylazetidin-3-y1)(2-(pyrrolidin-1-yOpyrimidin-
5-yl)methanol, hydrochloride salt
The title compound was synthesized starting from Example 330.1, and following
the procedure described in Example
309. LC-MS (A): tR = 0.67min; [M+H]: 367.18.
330.3. (1, 3-Di m ethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(2-pyrrolidi n-1 -
yl-pyri m idin-5-y1)-metha no!
The title compound (54mg, white powder) was synthesized starting from Example
330.2 (110mg), and following the
procedure described in Example 01.1 step 01.1.5. The crude material was
purified by Prep LC-MS (IX) and Prep LC-
MS (VII). LC-MS (A): tR = 0.69min; [M+H]t 381.2.
Example 331: (1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(6-pyrrolidin 1
yl pyrazin-2-yI)-methanol
331.1. 2-Bromo-6-pyrrolidin-1-yl-pyrazine
To a solution of 2,6-dibromopyrazine (500mg) in Me0H (5mL) was added
pyrrolidine ( 0.52mL). The reaction mixture
was stirred at RI overnight, quenched with water and extracted with DCM. The
organic layer was filtered over a phase
separator and concentrated under reduced pressure. The resulting residue was
purified Prep-LC-MS (V) affording the
title compound (406mg) as yellow solid. LC-MS (A): tR = 0.88min; [M-F1-1]':
228.1.
331.2. (1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(6-pyrrolidin-1-yl-
pyrazin-2-y1)-methanol
The title compound was synthesized starting from Example A4.2 and Example
331.1, and following the procedure
described in Example 330 steps 330.1 to 330.3. The crude material was purified
twice by Prep LC-MS (IX). LC-MS (A):
tR = 0.80min; [M+H]: 381.22.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
267
Depending on the purification conditions, the title compounds/intermediates in
Example 1 to 331 may be isolated as
free bases or as salts such as formate salts, or hydrochloride salts. Whenever
isolating a title compound/intermediate
as a salt, formate salt or hydrochloride salt is indicated at the end of the
chemical name and can refer to a mono-, di-
or tri-formate salt, or mono-, di-, or tri-hydrochloride salt.
Example 332: (R)-N-(1-(3-(54(1,3-dimethylazetidin-3-y1)(hydroxy)(4-
isopropylphenyl)methyppyridin-3-y1)-1,2,4-
oxadiazol-5-yl)cyclopropypacetamide-2,2,2-d3
332.1 tert-butyl
(R)-34(5-(5-(1-(acetamido-2,2,2-d3)cyclopropy1)-1,2,4-oxadiazol-3-
yOpyridin-3-0)(hydroxy)(4-
isopropylphenyl)methyl)-3-methylazetidine-1-carboxylate
The title compound (80.2mg) was synthesized starting from Example 02.2
(64.5mg) and Example D3.1 (22.8mg), and
following the procedure described in Example 134. Crude was extracted with
water and DCM over phase separator,
resulting solution was evaporated to dryness. LC-MS (A): tR = 0.98min; [M+H]*:
565.01.
332.2
(R)-N-(1-(3-(5-(hydroxy(4-isopropylphenyl)(3-methylazetidin-3-
yOmethyl)pyridin-3-y1)-1, 2, 4-oxadiazol-5-
Acyclopropyl)acetarnide-2,2,2-d3, hydrochloride salt
A solution of Example 332.1 (80.2mg) in HCI in dioxane (4M, 4mL) was stirred
lh at RT. The crude was evaporated to
dryness and used in the next step without further purification. LC-MS (A): tR
= 0.67min; [M+11]+: 464.99.
332.3 (R)-N-(1-(3-(541,3-dimethylazetidin-3-y1)(hydroxy)(4-
isopropylphenyOmethyl)pyridin-3-y1)-1,2,4-oxadiazol-5-
y0cyclopropyl)acetamide-2,2,2-d3
The title compound (0.2mg, white powder) was synthesized from Example 332.2
(66mg), and following the procedure
described in Example D1.1 step D.1.1.5. The crude was filtered off, and the
filtrate was purified by Prep LC-MS (IX)
and (V). LC-MS (A): tR = 0.69min; [M+H]: 479.18.
Example 333 to Example 336 were synthesized starting from Example D2.2 and the
appropriate acid of formula (D3),
following the three-step procedure described in Example 332. The acid
precursors of formula (03) are indicated in the
table below. Prep LC-MS conditions and LC-MS data are listed in the table
below. The LC-MS conditions used were
LC-MS (A).
Example
Prep
Name tR [M+11-
(D3)
1\1
LC-MS
(R)-N-(1-(3-(5-((1,3-dimethyl azetidi n-3-
yl)(hydroxy)(4-isopropylphenyl)methyl)pyridin-3-y1)-
(IX)
333 0.75 493.19 03.2
1,2,4-oxadiazol-5-yl)cyclopropy1)-N-
then (V)
methylacetamide-d3
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
268
(R)-N-((3-(5-((1, 3-dimethylazetidi n-3-y1) (hydroxy)(4-
(IX)
334 isopropylphenyl)methyl)pyridin-3-y1)-1,2,4-oxadiazol-
0.7 467.19 03.3
then (V)
5-yl)methyl)-N-methylacetamide-d3
N-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
(IX)
335 isopropyl-phenyI)-methy1]-pyridin-3-yll- 0.63
465.84 03.4
then (V)
[1,2,4]oxadiazol-5-ylmethyl)-2-hydroxy-acetamide
(R)-N-((3-(54(1,3-dimethylazetidin-3-y1)(hydroxy)(4-
(IX)
336 isopropylphenyl)methyl)pyridin-3-y1)-1,2,4-oxadiazol-
0.66 453.21 03.5
then (V)
5-yl)methyl)acetamide-2,2,2-d3
Example 337: 1-(3-{5-[(R)-Hydroxy-(1-isopropy1-3-methyl-azetidin-3-y1)-(4-
isopropyl-pheny1)-methyl]-pyridin-3-yll-
[1 ,2,4]oxadiazol-5-y1)-2-methyl-propan-2-ol
337.1 31(R)-Hydroxy-{54542-hydroxy-2-methyl-
propy1)41,2,4]oxadiazol-3-ylppyridin-3-y1}44-isopropyl-phenyl)-
methyll-3-methyl-azetidine-1-carboxylic acid tert-butyl ester
The title compound was synthesized starting from Example 02.2 (1.023g) and 3-
hydroxy-3-methylbutanoic acid
(348mg) following the procedure described in Example 134. The crude material
was purified by Prep LC-MS (XXVI) to
afford 560mg of a yellow solid. LC-MS (A): tR = 1.03min; [M-F1-1]+: 537.29.
337.2 1-(3454(R)-Hydroxy-(4-isopropyl-phenyl)-(3-methyl-azetidin-3-y1)-
methylPpyridin-3-y11-11,2,41oxadiazol-5-y1)-2-
methyl-propan-2-ol, hydrochloride salt
A solution of Example 337.1 (560mg) in HCI in dioxane (4M, 4mL) and dioxane
(4mL) was stirred 1h at RT. HCI in
dioxane (4M, 4mL) was added again and mixture further stirred over weekend.
The crude was evaporated to dryness
to give 450mg of the title compound. LC-MS (A): tR = 0.7min; [M+H]': 437.21.
337.3 1-(3-{54(R)-Hydroxy-(1-isopropy1-3-methyl-azetidin-3-y1)-(4-isopropyl-
pheny1)-methylTpyridin-3-4-
[1,2,4]oxadiazol-5-y1)-2-methyl-propan-2-ol
To a solution of Example 337.2 (47.6mg) in dioxane (2mL) and Et0H (1mL) were
added Et3N (45.5pL) and acetone
(56.1pL), followed by NaBH(OAc)3 (35.7mg). The reaction mixture was stirred at
RT until completion of the reaction,
filtered off, and the filtrate was purified by Prep LC-MS (IX) and (VI) to
afford the title product as off-white solid (14mg).
LC-MS (A): tR = 0.76min; [M+H]: 479.1.
Example 338: 1-(3-{5-[(R)-(1-Ethy1-3-methyl-azetidin-3-0-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridin-3-y11-
[1,2,4]oxadiazol-5-y1)-2-methyl-propan-2-ol
To a solution of Example 337.2 (47.6mg) in dioxane (2mL) and Et0H (1mL) were
added Et3N (45,5pL) and
acetaldehyde (43.1pL), followed by NaBH(OAc)3 (35.7mg). The reaction mixture
was stirred at RT until completion of
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
269
the reaction, filtered off, and the filtrate was purified by Prep LC-MS (IX)
and (VI) to afford the title product as off-white
solid (16mg). LC-MS (A): tR = 0.74min; [M+1-1]': 465.07.
Example 339: 2-(3-15-[(R)-Hydroxy-(1-isopropy1-3-methyl-azetidin-3-y1)-(4-
isopropyl-pheny1)-methyll-pyridin-3-yll-
[1 ,2,4]oxadiazol-5-y1)-propan-2-ol
339.1 3-[(R)-Hydroxy-{5-15-(1-hydroxy-1-methyl-ethy0-11,2,41oxadiazol-3-y11-
pyridin-3-4-(4-isopropyl-phenyl)-methyll-
3-methyl-azetidine-1-carboxylic acid tert-butyl ester
The title compound was synthesized starting from Example 02.2 (1.02 g) and 2-
hydroxy-2-methylpropanoic acid (291
mg) following the procedure described in Example 134. The crude material was
purified by Prep LC-MS (XVII) to afford
580 mg of a yellow solid. LC-MS (A): tR = 1.03min; [M-FH]-: 523.32.
339.2 2-(3-{5-[(R)-Hydroxy-(4-isopropyl-pheny1)-(3-methyl-azetidin-3-y0-
methyll-pyridin-3-441,2,4]oxadiazol-5-y1)-
propan-2-ol
The title compound was synthesized starting from Example 339.1 (580 mg)
following the procedure described in
Example 309 step 309.2. The crude was evaporated to dryness to give 510 mg of
the title compound as white solid as
hydrochloride salt. LC-MS (A): tR = 0.7min; [M+H]: 423.21.
339.3 2-(3-{5-[(R)-Hydroxy-(1-isopropy1-3-methyl-azetidin-3-y1)-(4-isopropyl-
pheny1)-methylppyridin-3-4-
[1,2,4]oxadiazol-5-y1)-propan-2-ol
The title compound was synthesized starting from Example 339.2 following the
procedure described in Example 337
step 337.3. The crude material was purified by Prep LC-MS (IX) and (VI). LC-MS
(A): tR = 0.76min; [M+H]: 465.11.
Example 340: 2-(3-{5-[(R)-(1-Ethyl-3-methyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-pheny1)-methyl]-pyridin-3-yll-
[1,2,4]oxadiazol-5-y1)-propan-2-ol
The title compound was synthesized starting from Example D2.2 and 2-hydroxy-2-
methylpropanoic acid and following
a three-step procedure composed of Example 339 step 339.1 and step 339.2
followed by Example 338. The crude
material was purified by Prep LC-MS (IX) then (VI) and finally by Prep TLC
(Dioxane+2%Et3N 80% / Et0H20%). LC-
MS (A): tR = 0.74min; [M+H]-: 451.11.
Example 341 and Example 342 were synthesized starting from Example E1.1 and
the appropriate hydroxyamidine of
formula (E2), and following the procedure described in Example 332. The
hydroxyamidine precursors of formula (E2)
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
270
are indicated in the table below. Prep LC-MS conditions and LC-MS data are
listed in the table below. The LC-MS
conditions used were LC-MS (A).
Example
Prep
Name tR [M+H] (E2)
N LC-MS
4-(545-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
isopropyl-phenyI)-methy1]-pyridin-3-yll-
341 0.91 596.28 E2.14 (VIII)
[1,2,4]oxadiazol-3-y1)-piperidine-1-carboxylic acid
benzyl ester
144-(5-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-
342 (4-isopropyl-pheny1)-methyl]-pyridin-3-yll- 0.74
504.24 E2.15 (IX)
[1,2,4]oxadiazol-3-y1)-piperidin-1-y1]-ethanone
Example 343 to Example 364 were synthesized starting from Example D2.1 and the
appropriate acid of formula (D3),
and following the procedure described in Example 134. The acid precursors of
formula (03) are indicated in the table
below unless commercially available. Prep LC-MS conditions and LC-MS data are
listed in the table below. The LC-MS
conditions used were LC-MS (A).
Example
Prep
Name tR [M+H]-
(D3)
N LC-MS
(R)-1-(3-(3-(54(1,3-dimethylazetidin-3-y1)(hydroxy)(4-
(IX) then
343 isopropylphenyl)methyppyridin-3-y1)-1,2,4-oxadiazol-5-
0.7 479.07 D3.6
(V)
yl)azetidin-1-yl)ethan-1-one-2,2,2-d3
(R)-1-(3-(3-(5-((1,3-dimethylazetidin-3-yI)(hydroxy)(4-
(IX) then
344 isopropylphenyl)methyppyridin-3-y1)-1,2,4-oxadiazol-5-
0.73 493.09 03.7
(V)
y1)-3-methylazetidin-1-ypethan-1-one-2,2,2-d3
(R)-1-(3-(3-(5-((1,3-dimethylazetidin-3-yI)(hydroxy)(4-
(IX) then
345 isopropylphenyl)methyppyridin-3-y1)-1,2,4-oxadiazol-5-
0.73 496.97 03.8
(V)
y1)-3-fluoroazetidin-1-ypethan-1-one-2,2,2-d3
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-15-
(IX) then
346 [5-(1-methy1-1-morpholin-4-yl-ethyl)-[1,2,4]oxadiazol-3-
0.66 506.1
(VI)
yI]-pyridin-3-y1}-methanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
271
4-(3-15-[(R)-(1,3-Di methyl-azetidin-3-yI)-hydroxy-(4-
(IX) then
347 isopropyl-phenylynnethy1]-pyridin-3-y1}-[1,2,4]oxadiazol-
0.69 490.09
(V)
5-yI)-1-methyl-piperidin-2-one
1-[(S)-3-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-
(IX) then
348 (4-isopropyl-phenylymethy1]-pyridin-3-y1}- 0.72
490.07 03.9
(V)
[1,2,4]oxadiazol-5-y1)-pyrrolidin-1-y11-ethanone
1-[(R)-3-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-
(IX) then
349 (4-isopropyl-phenyI)-methy1]-pyridin-3-yly 0.72
490.06 03.10
(V)
[1,2,4]oxadiazol-5-y1)-pyrrolidin-1-y1]-ethanone
N41-(3-{5-KR)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
(IX) then
350 isopropyl-pheny1)-methyll-pyridin-3-y1141,2,41oxadiazol-
0.69 478.09 D3.11
(V)
5-y1)-1-methyl-ethy1]-acetamide
1-Benzyl 3 (3 {5 [(R) (1,3 dimethyl-azetidin-3-yI)-
(XIV)
351 hydroxy-(4-isopropyl-phenylymethy1]-pyridin-3-yll-
0.81 552.1
then NI)
[1,2,4]oxadiazol-5-y1)-pyrrolidin-2-one
3-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
(IX) then
352 isopropyl-phenyl)methyl]-pyridin-3-y1}-[1,2,4]oxadiazol-
0.71 490.01
(VI)
5-y1)-1,3-dimethyl-pyrrolidin-2-one
4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
(IX) then
353 isopropyl-phenyl)-methyl]-pyridin-3-y1141,2,4]oxadiazol-
0.76 518.09
(VI)
5-yI)-1-isobutyl-pyrrolidin-2-one
4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
(IX) then
354 isopropyl-pheny1)-methyll-pyridin-3-y1141,2,41oxadiazol-
0.75 542.03
(VI)
5-YD-1-furan-2-ylmethyl-pyrrolidin-2-one
4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
(XIV)
355 isopropyl-phenylynnethy1]-pyridin-3-y1}-[1,2,4]oxadiazol-
0.78 538.06
then (VI)
5-yI)-1-phenyl-pyrrolidin-2-one
1-Benzy1-4-(3-{5-[(R)-(1,3-dimethyl-azetidin-3-y1)-
(XIV)
356 hydroxy-(4-isopropyl-phenyl)-methyl]-pyridin-3-yll-
0.78 552.06
then (VI)
[1,2,4]oxadiazol-5-y1)-pyrrolidin-2-one
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
272
5-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(IX) then
357 isopropyl-phenylynnethy1]-pyridin-3-y1}-[1,2,4]oxadiazol-
0.77 538.07
(VI)
5-yI)-1-phenyl-pyrrolidin-2-one
4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(IX) then
358 isopropyl-phenylymethy1]-pyridin-3-y1}-[1,2,4]oxadiazol-
0.64 462.08
(V)
5-yI)-pyrrolidin-2-one
(S)-5-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(IX) then
359 isopropyl-pheny1)-methy1]-pyridin-3-y11-[1,2,4]oxadiazol-
0.65 462.09
(V)
5-yI)-pyrrolidin-2-one
(R)-5-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(IX) then
360 isopropyl-pheny1)-methyll-pyridin-3-y1141,2,41oxadiazol-
0.65 462.1
(V)
5-yI)-pyrrolidin-2-one
(S)-6-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(IX) then
361 isopropyl-phenylymethyl]-pyridin-3-y1}-[1,2,4]oxadiazol-
0.67 476.08
(V)
5-yI)-piperidin-2-one
4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(IX) then
362 isopropyl-phenylymethyl]-pyridin-3-y1}-[1,2,4]oxadiazol-
0.66 476.1
(V)
5-yI)-piperidin-2-one
(S)-4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(IX) then
363 isopropyl-pheny1)-methyl]-pyridin-3-y1141,2,4]oxadiazol-
0.65 448.09
(V)
5-yI)-azetidin-2-one
(S)-3-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(IX) then
364 isopropyl-pheny1)-methyl]-pyridin-3-y1141,2,41oxadiazol-
0.65 448.1
(V)
5-yI)-azetidin-2-one
Example 365 and Example 366 were synthesized starting from Example D2.1 and
1,3-Dimethy1-5-0xopyrrolidine-3-
carboxylic acid, and following the procedure described in Example 134. Prep LC-
MS and Chiral Prep (HPLC)
conditions, Chiral HPLC and LC-MS data are listed in the table below. The LC-
MS conditions used were LC-MS (A).
CA 03177335 2022- 10- 28
WO 2021/219849 PCT/EP2021/061401
273
LC- Prep
Chiral
Example
Chiral
Name MS [M+Hr LC-
HPLC tR
Prep
tR MS
(method)
(S)- or (R)-4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-
6.68
365 hydroxy-(4-isopropyl-phenylymethylypyridin-3-yll- 0.71
490.12 (V) XXVI
(Y)
[1,2,4]oxadiazol-5-y1)-1,4-dimethyl-pyrrolidin-2-one
(R)- or (S)-4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-
8.54
366 hydroxy-(4-isopropyl-pheny1)-methy1]-pyridin-3-y1}- 0.71
490.11 (V) XXVI
(Y)
[1,2,4]oxadiazol-5-y1)-1,4-dimethyl-pyrrolidin-2-one
Example 367 to Example 369 were synthesized starting from Example D2.1 and the
appropriate commercially
available acid following the two-step procedure described in Example 332 steps
332.1 to 332.2. Prep LC-MS conditions
and LC-MS data are listed in the table below. The LC-MS conditions used were
LC-MS (A).
Prep
Example N Name tR [M-F1-1]*
LC-MS
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[5-(5-
367 0.56 462.1 (VI)
piperidin-4-y141,2,4]oxadiazol-3-y1)-pyridin-3-y1]-methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{545-(4-
368 methyl-piperidin-4-y1)41,2,4]oxadiazol-311]-pyridin-3-yll-
0.58 476.03 (VI)
methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-{545-(4-fluoro-pi peridin-4-yI)-
369 [1,2,4]oxadiazol-311]-pyridin-3-y11-(4-isopropyl-pheny1)-
0.58 480.1 (VI)
methanol
Example 370 1-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y11-
[1,2,4]oxadiazol-5-y1)-piperidin-1-y1]-butan-1-one
To a solution of Example 367 (29.8mg) in DCM (1mI) placed at 0 C were added
DIPEA (38,2 pL) and butyryl chloride
(5.84 pL), resulting mixture was left warmed to rt and stirred overnight.
Solvent was evaporated and residue was purified
by Prep LC-MS (IX) and (VI). LC-MS (A): tR = 0.8min; [M-F1-1]': 532.09.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
274
Example 371 to Example 388 were synthesized starting from Example 367 and the
appropriate commercially available
acid chloride, chloroformate or sulfonyl chloride following the procedure
described in Example 370. Prep LC-MS
conditions and LC-MS data are listed in the table below. The LC-MS conditions
used were LC-MS (A).
Example
N Name tR [M+H]
Prep LC-MS
144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-pheny1)-methyl]-pyridin-3-yll-
371 0.83 545.86 (XIV) then (VII)
[1,2,4]oxadiazol-5-y1)-piperidin-1-y1]-2,2-dimethyl-
propan-1-one
1-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-pheny1)-methyll-pyridin-3-yll-
372 0.73 534.07 (IX) then (V)
[1,2,4]oxadiazol-5-y1)-piperidin-1-y1]-2-methoxy-
ethanone
1-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-phenylymethyl]-pyridin-3-yll-
373 0.75 548.07 (IX) then (V)
[1,2,4]oxadiazol-5-y1)-piperidin-1-y1]-3-methoxy-
propan-1-one
Cyclopropy144-(3-15-[(R)-(1,3-dimethyl-azetidin-3-y1)-
374 hydroxy-(4-isopropyl-phenyI)-methyl]-pyridin-3-yll-
0.78 529.83 (IX) then (VI)
[1,2,4]oxadiazol-5-y1)-piperidin-1-y1]-methanone
Cyclopenty144-(3-{5-[(R)-(1,3-dimethyl-azetidin-3-y1)-
375 hydroxy-(4-isopropyl-phenyI)-methyl]-pyridin-3-yll-
0.84 558.11 (XIV) then (VII)
[1,2,4]oxadiazol-5-y1)-piperidin-1-y1]-methanone
[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
isopropyl-phenyI)-methy1]-pyridin-3-yll-
376 0.75 574.1 (IX) then (V)
[1,2,4]oxadiazol-5-y1)-piperidin-1-y1]-(tetrahydro-pyran-
4-y1)-methanone
[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
377 isopropyl-phenylymethyl]-pyridin-3-yll- 0.82
566.06 (XIV) then (VI)
[1,2,4]oxadiazol-5-y1)-piperidin-1-y1]-phenyl-methanone
4-(3-{5-[(R)-(1,3-0imethyl-azetidin-3-yI)-hydroxy-(4-
378 0.8 520.04 (XIV) then (VI)
isopropyl-phenylymethy1]-pyridin-3-yll-
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
275
[1,2,4]oxadiazol-5-y1)-piperidine-1-carboxylic acid
methyl ester
4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
isopropyl-phenyI)-methy1]-pyridin-3-yll-
379 0.83 534.07 (XIV) then (VII)
[1,2,4]oxadiazol-5-y1)-piperidine-1-carboxylic acid ethyl
ester
4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-phenylymethyll-pyridin-3-yll-
380 0.79 564.09 (XIV) then (VI)
[1,2,4]oxadiazol-5-y1)-piperidine-1-carboxylic acid 2-
methoxy-ethyl ester
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
381 {545-(1-methanesulfonyl-piperidin-4-y1)- 0.76
540.02 (IX) then (VI)
[1,2,4]oxadiazol-3-A-pyridin-3-yll-methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
382 (5-{5-[1-(propane-2-sulfony1)-piperidin-4-y1]- 0.82
568.06 (XIV) then (VI)
[1,2,4]oxadiazol-3-y1}-pyridin-3-y1)-methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
383 (5-1541-(propane-1-sulfony1)-piperidin-4-y11- 0.82
568.07 (XIV) then (VI)
[1,2,4]oxadiazol-3-yll-pyridin-3-y1)-methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
384 (5-{541-(2-methoxy-ethanesulfony1)-piperidin-4-y1]-
0.78 584.04 (IX) then (VI)
[1,2,4]oxadiazol-3-yll-pyridin-3-y1)-methanol
2-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
385 isopropyl-phenylymethylFpyridin-3-yll- 0.72 570.02
(IX) then (V)
[1,2,4]oxadiazol-5-y1)-piperidine-1-sulfony1]-ethanol
(R)-{545-(1-Cyclopentanesulfonyl-piperidin-4-y1)-
386 [1,2,4]oxadiazol-311]-pyridin-3-y11-(1,3-dimethyl-
0.86 593.88 (XIV) then (VII)
azetidin-3-y1)-(4-isopropyl-pheny1)-methanol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
387 (5-{5-[1 -(tetrahydro-pyran-4-sulfonyl)-piperidin-4-y1]-
0.79 609.89 (XIV) then (VI)
[1,2,4]oxadiazol-3-y1}-pyridin-3-y1)-methanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
276
4-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-yI)-hydroxy-(4-
isopropyl-phenylymethy1]-pyridin-3-yll-
388 0.76 555.02
(IX) then (VI)
[1,2,4]oxadiazol-5-y1)-piperidine-1-sulfonic acid
methylamide
Example 389: (R)-(1, 3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{5-[5-(1-
methylami no-cyclopropyI)-
[1, 2, 4]oxadiazol-3-yli-pyridi n-3-yll-methanol
A solution of Example 333 (220mg) in MeCN (2m1) and HCI (1M, 2mL) was stirred
48h at 100 C. The crude was
evaporated and placed under HV. The residue was purified by Prep LC-MS (IX)
and (VI). LC-MS (A): tR = 0.59min;
[M-FH]: 448.25.
Example 390 and Example 391 were synthesized starting from Example 389 and the
appropriate commercially
available acid chloride or sulfonyl chloride following the procedure described
in Example 370. Prep LC-MS conditions
and LC-MS data are listed in the table below. The LC-MS conditions used were
LC-MS (A).
Example
Prep
Name tR [M+H]-
N
LC-MS
[1-(3-{5-[(R)-(1, 3-D imethyl-azetidi n-3-yI)-hydroxy-(4-isopropyl-
390 phenyl)-methyl]-pyridin-3-y1141,2,41oxadiazol-5-y1)-
0.65 520.24 (IX) then (VI)
cyclopropyI]-methyl-carbamic acid ethyl ester
N-[1-(3-{5-[(R)-(1, 3-Di methyl-azetidin-3-yI)-hydroxy-(4-
391 isopropyl-pheny1)-methyl]-pyridin-3-y11-[1,2,4]oxadiazol-5-
y1)- 0.78 526.2 (IX) then (VI)
cyclopropyll-N-methyl-methanesulfonamide
Example 392: (R)-(1,3-Dimethyl-azetidin-3-y1)-[6-(2,2-dimethyl-cyclopentyloxy)-
pyridazin-4-y1]-(4-isopropyl-phenyl)-
methanol
392.1 3-[(R)46-(2,2-Dimethyl-cyclopentyloxy)-pyridazin-4-ylkhydroxy-(4-
isopropyl-pheny0-methyl]-3-methyl-azetidine-
1-carboxylic acid tert-butyl ester
To a solution of Example F1.6.2 (35mg) in dioxane (1mL) were added 2,2-
dimethylcyclopentanol (27.8mg) and NaH
(60% in mineral oil, 12.4mg), resulting mixture was stirred 22h at 65 C. The
resulting crude was cooled down to rt,
diluted with MeCN, filtered through a syringe filter and purified by Prep LC-
MS (VIII) to afford 18.1mg of the title
compound as white powder. LC-MS (A): tR = 1.16min; [M+H]t 510.16.
392.2 (R)46-(2,2-Dimethyl-cyclopentylox0-pyridazin-4-yil-(4-isopropyl-pheny1)-
(3-methyl-azetidin-3-0)-methanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
277
A solution of Example 392.1 (18.1mg) solved in HCI in dioxane (4M, 0.5mL) was
stirred 2h at RT. The reaction mixture
was cooled at 0 C, basified to pH=8 with aq. sat. NaHCO3, diluted with water
and extracted with DCM. The combined
org. layers were dried (MgSO4), filtrated off, evaporated and dried under HV
to afford 13.8mg of the title compound as
beige solid. LC-MS (A): tR = 0.84min; [M+H]: 410.19.
392.3 (R)-(1, 3-Di methyl-azetidin-3-y1)46-(2, 2-dimethyl-cyclopentyloxy)-
pyridazi n-4-yil-(4-isopropyl-pheny1)-methanol
The title compound (6.3mg, white powder) was synthesized starting from Example
392.2 (13.8mg) and following the
procedure described in Example F1.1 step F1.1.2. Resulting crude was purified
by Prep LC-MS (V). LC-MS (A): tR =
0.85min; [M+H]: 424.20.
Example 393: (R)46-(3,3-Difluoro-cyclopentyloxy)-pyridazin-4-y1]-(1,3-dimethyl-
azetidin-3-y1)-(4-isopropyl-pheny1)-
methanol
The title compound (7mg, white solid) was synthesized starting from Example
F1.6.2 (35mg) and 3,3-
difluorocyclopentan-1-ol (30.6mg) following the three-step procedure described
in Example 392. Resulting crude was
purified by Prep LC-MS (VII). LC-MS (A): tR = 0.78min; [M-FH]-: 432.12.
Example 394: 2-(2-{5-[(R)-(1,3-Dimethyl-azetidi n-3-y1)-hydroxy-
(4-isopropyl-pheny1)-methyl]-pyridazin-3-yloxy}-
phenyI)-ethanol
394.1 3-[(R)-Hydroxy-{642-(2-hydroxy-ethyl)-phenoxyppyridazin-4-4-(4-isopropyl-
pheny1)-methyl]-3-methyl-azetidine-
1-carboxylic acid tert-butyl ester
To a solution of Example F1.6.2 (200mg) in NMP (2.3mL) were added 2-
hydroxyphenethyl alcohol (67 pL), molecular
sieves (3A powder, 200mg) and Cs2CO3 (366mg). The resulting mixture was
stirred at 65 C for 2.5 days, cooled down
to RT, diluted with MeCN, filtered through a syringe filter and purified by
Prep LC-MS POO to afford 53mg of the title
compound as white solid. LC-MS (A): tR = 1.03min; [M+H]': 534.14.
394.2 2-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyil-pyridazin-3-yloxy}-phenyl)-ethanol
The title compound (13.8mg, white solid) was synthesized starting from Example
394.1 (47.5mg) and following the
procedure described in Example 82 step 82.2 and 82.3. Resulting crude was
purified by Prep LC-MS (V). LC-MS (A):
tR = 0.71min; [M+H]-: 448.18.
Example 395 to Example 397 were synthesized starting from Example F1.6.2 and
the appropriate commercially
available alcohol following the procedure described in Example 394. Prep LC-MS
conditions and LC-MS data are listed
in the table below. The LC-MS conditions used were LC-MS (A).
CA 03177335 2022- 10- 28
WO 2021/219849 PCT/EP2021/061401
278
Example
N Name tR [M-FI-I]-
Prep LC-MS
2-(4-{5-[(R)-(1,3-0imethyl-azetidin-3-yI)-hydroxy-(4-
395 0.71 448.23 (V)
isopropyl-pheny1)-methyl]-pyridazin-3-yloxyl-pheny1)-ethanol
(R)46-(Chroman-6-yloxy)-pyridazin-4-y1]-(1,3-dimethyl-
396 0.81 460.21 (VII)
azetidin-3-y1)-(4-isopropyl-pheny1)-methanol
(XV)
6-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
397 0.69 461.17 then
pheny1)-methyl]-pyridazin-3-yloxy}-3H-benzooxazol-2-one
(IX)
Example 398 to 407 were synthesized starting from the corresponding Example of
formula (F1) and the appropriate
alkyne of formula (F5), and following the procedure described in Example 15.
The precursors of formula (F1) and (F5)
are indicated in the table below unless commercially available. Prep LC-MS
conditions and LC-MS data are listed in
the table below. The LC-MS conditions used were LC-MS (A).
Prep
Example N Name tR [M+H]- (F1)
(F5)
LC-MS
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-
hydroxy-(4-isopropyl-pheny1)-methyl]-
(VI) then
398 0.76
485.21 F1.2 F5.19
pyridin-3-yI}-3-(6-methyl-pyrimidin-4-
(IX)
ylypent-1-yn-3-ol
(S)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-
yI)-hydroxy-(4-isopropyl-pheny1)-
(IX) then
399 0.72
471.27 F1.2 F5.20
methyl]-pyridin-3-y11-2-(6-methyl-
(V)
pyrimidin-4-yI)-but-3-yn-2-ol
(R)-4-{5-[(R)-(1,3-01methyl-azetidin-3-
y1)-hydroxy-(4-isopropyl-pheny1)-
(V) then
400 0.71 471.35 F1.2
F5.21
methyl]-pyridin-3-y11-2-(6-methyl-
(IX)
pyrimidin-4-yI)-but-3-yn-2-ol
(S)- or (R)-4-{5-[(R)-(1,3-Dimethyl-
(IX) then
401 0.7 473.28 F1.2
F5.22
azetidin-3-yI)-hydroxy-(4-isopropyl-
(V)
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
279
phenylymethyll-pyridin-3-y11-2-(1,5-
dinnethy1-1H-pyrazol-3-y1)-but-3-yn-2-ol
(R)- or (S)-4-{5-[(R)-(1,3-Dimethyl-
azetidin-3-y1)-hydroxy-(4-isopropyl-
(IX) then
402 0.7 473.28 F1.2
F5.23
pheny1)-methyl]-pyridin-3-y11-2-(1,5-
(V)
dimethy1-1H-pyrazol-3-y1)-but-3-yn-2-ol
(R)-(1,3-Dimethyl-azetidin-311)-{541-
403
(4-fluoro-pheny 0.91 469.27 F1.2
1)-cyclopropylethyny1]-
(VIII) then
pyridin-3-y11-(4-isopropyl-pheny1)-
(XIV)
methanol
(S)- or (R)-4-{5-[(R)-(1,3-Dimethyl-
azetidin-3-y1)-hydroxy-(4-isopropyl-
(V) then
404 0.68 459.26 F1.2 F5.24
pheny1)-methyl]-pyridin-3-01-2-(1-
(IX)
methyl-1H-pyrazol-3-y1)-but-3-yn-2-ol
(R)- or (S)-4-{5-[(R)-(1,3-Dimethyl-
azetidin-3-y1)-hydroxy-(4-isopropyl-
(V) then
405 0.68 459.26 F1.2 F5.25
pheny1)-methy1]-pyridin-3-01-2-(1-
(IX)
methyl-1H-pyrazol-3-y1)-but-3-yn-2-ol
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-
hydroxy-(4-isopropyl-pheny1)-methyl]-
(VI) then
406 pyridin-3-ylethyny11-3-hydroxy- 0.79 520.15
F1.2
(IX)
pyrrolidine-1-carboxylic acid tert-butyl
ester
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-
hydroxy-(4-isopropyl-pheny1)-methyl]-
407 0.86 518.15 F1.2
pyridin-3-ylethynyll-piperidine-1-
carboxylic acid tert-butyl ester
Example 408: 1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyll-pyridin-3-y1}-3-(6-methyl-
pyrimidin-4-y1)-pentan-3-ol
CA 03177335 2022- 10- 28
WO 2021/219849 PCT/EP2021/061401
280
A solution of Example 398 (15mg) was dissolved in Me0H (0.3mL), Pd/C (10% w/w,
50% water, 3mg) was added and
resulting mixture was stirred for 17h under hydrogen. The reaction mixture was
filtered off and evaporated to dryness
to afford 12mg of the title compound as white solid. LC-MS (A): tR = 0.64min;
[M-FH]-: 489.21.
Example 409 to Example 412 were synthesized starting from Example F1.2 and the
appropriate alkyne of formula
(F5), according to a two-step procedure described in Example 15 (Prep LC-MS
conditions for the purification described
in the table below) followed by Example 408 (no purification performed). The
alkyne precursors of formula (F5) are
indicated in the table below unless commercially available. LC-MS data are
listed in the table below. The LC-MS
conditions used were LC-MS (A).
Prep
Example N Name tR [M+H]- (F5)
LC-MS step 1
2-(2-{54(R)-(1, 3-Di methyl-azetidin-3-yI)-hydroxy-(4-
409 isopropyl-pheny1)-methyl]-pyridin-3-yll-ethyl)-benzoic
0.73 473.21 (VIII)
acid methyl ester
(R)-(1, 3-Di methyl-azetidin-3-y1)-{542-(2-
(VII)
410 hydroxymethyl-phenyI)-ethyl]-pyrid 0.65
445.21
then (IX)
isopropyl-phenyI)-methanol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
411 isopropyl-pheny1)-methy1]-pyridin-3-y11-2-(6-methoxy-
0.62 491.2 F5.26 (VI) then (IX)
pyrimidin-4-yI)-butan-2-ol
3-(2-{54(R)-(1, 3-Di methyl-azetidin-3-yI)-hydroxy-(4-
412 isopropyl-phenyl)methyl]-pyridin-3-ylyethyl)-3- 0.67
524.29 - (VI) then (IX)
hydroxy-pyrrolidine-1-carboxylic acid tert-butyl ester
Example 413: (R)-(1, 3-Di methyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{5-[2-(1H-
pyrrolo[2,3-b]pyridin-2-y1)-ethyl]-pyridin-
3-yll-methanol
413.1 (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(5-
trimethylsilanylethynyl-pyridin-3-y1)-methanol
The title compound (21mg, white powder) was synthesized from Example F1.2
(200mg) and trimethyl acetylene
(97.3pL), and following the procedure described in Example 15. Resulting crude
was purified by Prep LC-MS (VII) then
(XIV). LC-MS (A): tR = 0.88min; [M+1-1]+: 407.20.
413.2 (R)-(1,3-Dimethyl-azetidin-3-y1)-(5-ethynyl-pyridin-3-y1)-(4-isopropyl-
pheny1)-methanol
The title compound (18mg, yellow oil) was synthesized from Example 413.1
(20mg) following the procedure described
in Example F5.1 step F5.1.2. LC-MS (A): tR = 0.73min; [M+H]: 335.17.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
281
413.3 (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-15-(1 H-
pyrrolo[2,3-4]pyridin-2-ylethyny1)-pyridin-3-yIT
methanol
The title compound (12mg, yellow powder) was synthesized from Example 413.2
(18mg) and 2-lodo-1H-pyrrolo[2,3-
B]pyridine (18mg), and following the procedure described in Example 15.
Resulting crude was purified by Prep LC-MS
(VII). LC-MS (A): tR = 0.77min; [M+H]: 451.03.
413.4 (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{5-12-(1H-
pyrrolop,3-b]pyridin-2-y1)-ethylppyridin-3-y1}-
methanol
The title compound (8mg, light yellow solid) was synthesized from Example
413.3 (11mg) following the procedure
described in Example 1119-7775. LC-MS (A): tR = 0.57min; [M-FH]-: 455.19.
Example 414: 4-(2-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-pheny1)-methyl]-pyridin-3-yll-ethyly
piperidine-1-carboxyl ic acid tert-butyl ester
The title compound (28mg, rose resin) was synthesized from Example 407 (52mg)
following the procedure described
in Example 48. Resulting crude was basified with aq. sat. NaHCO3 solution and
extracted with DCM over phase
separator and solvent was evaporated to dryness. LC-MS (A): tR = 0.73min;
[M+H]: 522.18.
Example 415 to Example 417 were synthesized starting from Example F1.2 and the
appropriate alkyne of formula
(F5), according to a two-step procedure described in Example 15 followed by
Example 48. The alkyne precursors of
formula (F5) are indicated in the table below. Prep LC-MS conditions from both
steps and LC-MS data are listed in the
table below. The LC-MS conditions used were LC-MS (A).
Prep
Example Prep
Name tR [M-FH]-
(F5) LC-MS
N LC-MS
step 1
step 2
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-
415 hydroxy-(4-isopropyl-pheny1)-methyl]-pyridin- 0.51
460.21 F5.28 (XV) then (IX) (IX)
3-y11-2-pyridin-2-yl-butan-2-ol
1-Cyclopropy1-3-{5-[(R)-(1,3-dimethyl-
azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
416 0.56 486.17 F5.29 (IX) (IX)
methy1]-pyridin-3-y11-1-pyridin-2-yl-propan-1-
ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-
417 hydroxy-(4-isopropyl-pheny1)-methyl]-pyridin- 0.48
474.17 F5.30 (V) then (XIV)
3-y11-2-(5-methyl-pyridin-3-y1)-butan-2-ol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
282
Example 418: 8-(2-{5-[(R)-(1, 3-Di methyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyll-pyridazin-3-ylyethyl)-
5,6,7,8-tetrahydro-gui nolin-8-ol
418.1 8-{5-KR)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyli-pyridazin-3-ylethyny0-5,6,7,8-
tetrahydro-quinolin-8-ol
The title compound (27mg, brown solid) was synthesized from Example F1.6
(50mg) and Example F5.14 (33.8mg), and
following the procedure described in Example 15. Resulting crude was purified
by Prep LC-MS (IX) LC-MS (A): tR =
0.63min; [M-FH]-: 483.16.
418.2 8-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyo-
methylkpyridazi n-3-y1}-ethyl)-5, 6,7,8-
tetrahydro-quinolin-8-ol
The title compound (2mg, white solid) was synthesized from Example 418.1
(27mg) following the procedure described
in Example 48. Resulting crude was purified by Prep LC-M (IX) LC-MS (A): tR =
0.58min; [WEN': 487.2.
Example 419: 4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyl]-pyridazin-3-y1}-2-(6-
methoxy-pyridin-2-y1)-butan-2-ol
419.1 3-[Hydroxy-{6-[(R)-3-hydroxy-3-(6-methoxy-pyridin-2-y1)-but-1-
ynylPpyridazin-4-4-(4-isopropyl-pheny1)-methy11-
3-methyl-azetidine-1-carboxylic acid tert-butyl ester
The title compound (154mg, pale rose powder) was synthesized from Example
F1.6.2 (200mg) and Example F5.9
(89mg), and following the procedure described in Example 15. Resulting crude
was purified by Prep LC-MS (VII). LC-
MS (A): tR = 1.07min; [M+H]: 573.09.
419.2 3-[Hydroxy-{6-[(R)-3-hydroxy-3-(6-methoxy-pyridin-2-y1)-
butylTpyridazin-4-4-(4-isopropyl-phenyl)-methy11-3-
methyl-azetidine-1-carboxylic acid tert-butyl ester
The title compound (126mg, yellow solid) was synthesized from Example 419.1
(152mg) following the procedure
described in Example 408. LC-MS (A): tR = 1.04min; [M+H]: 578.05.
419,3 4-{5-p)-Hydroxy-(4-isopropyl-pheny1)-(3-methyl-azetidin-3-y1)-
methylppyridazin-3-01-2-(6-methoxy-pyridin-2-
y1)-butan-2-ol
The title compound (25mg, brown solid) was synthesized from Example 419.2
(54mg) following the procedure described
in Example 309 step 309.2. Resulting crude was purified by Prep LC-MS (VII).
LC-MS (A): tR = 0.72min; [M+H]-: 477.03.
419,4 4-{5-11R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylkpyridazin-3-y1}-2-(6-methoxy-pyridin-
2-y1)-butan-2-ol
The title compound (9mg, white powder) was synthesized from Example 419.3
(23mg) following the procedure
described in Example F1.1 step F1.1.2. Resulting crude was purified by Prep LC-
MS (VI). LC-MS (A): tR = 0.74min;
[M--H]: 491.01.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
283
Example 420: 4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyll-pyridazin-3-y11-2-(6-methyl-
pyridin-2-y1)-butan-2-ol
The title compound (10mg, white powder) was synthesized from Example F1.6.2
and Example F5.27, and following the
four-step procedure described in Example 419. Resulting crude was purified by
Prep LC-MS (VI). LC-MS (A): tR =
0.57min; [M+H]: 475.21.
Example 421: (1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(6-pyrrolidin-1-
yl-pyridin-2-y1)-methanol
The title compound (0.7mg, brown solid) was synthesized starting from Example
A4.2 and 2-bromo-6-(pyrrolidine-1-
yl)pyridine, following the procedure described in Example D1.1 but omitting
the steps D1.1.2 and D1.1.3. Resulting
crude was purified by Prep LC-MS (XIV) then (XVIII). LC-MS (A): tR = 0.85min;
[M-FH]-: 380.22.
Example 422 and Example 423 were synthesized starting from AcCI and the
appropriate amine precursor, and
following the procedure described in Example 370. The amine precursors are
indicated in the table below. Prep LC-MS
conditions and LC-MS data are listed in the table below. The LC-MS conditions
used were LC-MS (A).
Example
Prep
Name
tR [M+Hr Precursor
N
LC-MS
1-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
Example (IX) then
422 isopropyl-phenylymethyl]-pyridin-3-y11-[1,2,4]oxadiazol-5-
0.74 522.09
369
(VI)
yI)-4-fluoro-piperidin-1-y1]-ethanone
144-(3-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
Example (IX) then
423 isopropyl-phenylymethyl]-pyridin-3-y11-[1,2,4]oxadiazol-5-
0.75 518.11
368
(VI)
yI)-4-methyl-piperidin-1-y1]-ethanone
Example 424: 4-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyll-pyridin-3-yloxy}-
cyclohexanone
The title compound is Example 261.2 and was synthesized as described exactly
in Example 261 steps 261.1 and 261.2.
LC-MS (A): tR = 0.65min; [M+H]t 423.23.
Example 425: 4-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyll-pyridin-3-yll-cyclohexanol
Title compound (7mg, white powder) was synthesized from Example 327 step 327.3
(41mg) and following the procedure
described in Example 261 step 261.3. Resulting crude was purified by Prep LC-
MS (VI). LC-MS (A): tR = 0.55min;
[M-FH]-: 409.09.
Example 426 (R)-(5-Cyclopentyloxymethyl-pyridin-3-y1)-(1,3-dimethyl-azetidin-3-
y1)-(4-isopropyl-phenyl)-methanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
284
To a solution of Example F1.2 (30mg) and potassium
cyclopentoxymethyltrifluoroborate (15.9mg) in a dioxane/water
mixture (10:1, 1mL) were added Cs2003 (75.4mg), Pd(OAc)2 (0.519mg) and RuPhos
(2.27mg). The resulting mixture
was stirred for 45h at 95 C, allowed to cool down to RT, diluted with Me0H and
water, filtered through a syringe filter
and purified by Prep LC-MS (XIV) to give 6mg of the title compound as
colorless resin. LC-MS (A): tR = 0.69min; [M+H]:
409.16.
Example 427 (1-(4-{5-[(R)-(1, 3-D imethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridi n-3-ylmethoxyy
piperidin-1-yI)-ethanone
427.1 (4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y0-hydroxy-(4-isopropyl-pheny0-
methylPpyridin-3-ylmethoxyl-piperidine-1-
carboxylic acid tert-butyl ester
The title compound (29mg, off-white solid) was synthesized from Example F1.2
(100mg) and potassium (1-Boc-4-
piperidinyloxy)methyl trifluoroborate (82.5mg), and following the procedure
described in Example 426. The resulting
crude was purified by Prep LC-MS (VII). LC-MS (A): tR = 0.79min; [M+H]:
524.15.
427.2 ( R)-(1 , 3-dim ethylazetidi n-3-y1) (4-isopropylphenyl)(5-((piperidin-4-
yloxy)m ethyOpyridi n-3-yOmethanol,
hydrochloride salt
The title compound (6mg, yellow resin) was synthesized from Example 427.1
(9mg) following the procedure described
in Example 309 step 309.2. LC-MS (A): tR = 0.47min; [M+H]: 424.28.
427.3 (1-(4454(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylppyridin-3-ylmethoxy}-piperidin-1-
y0-ethanone
The title compound (0.7mg, colorless resin) was synthesized from Example 427.2
(6mg) and AcOH (2.07pL), and
following the procedure described in Example 140 step 140.3. The resulting
crude was purified by Prep LC-MS (IX).
LC-MS (A): tR = 0.58min; [M+H]t 465.88.
Example 428: (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-phenyly[6-
(tetrahydro-pyran-4-y1)-pyridazin-4-ylymethanol
428.1 (R)46-(3,6-Dihydro-2H-pyran-4-y0-pyridazin-411]-(1,3-dimethyl-azetidin-3-
y1)-(4-isopropyl-phenyl)-methanol
The title compound (27mg, yellow solid) was synthesized from Example F1.6
(50mg) and 3,6-dihydro-2H-pyran-4-
boronic acid pinacol ester (62.6mg), and following the procedure described in
Example 303. The resulting crude was
purified by Prep LC-MS (IX). LC-MS (A): tR = 0.69min; [M-FH]: 394.22.
428.2 (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-phenyi)46-(tetrahydro-
pyran-4-y0-pyridazin-4-ylkmethanol
The title compound (7mg, white solid) was synthesized from Example 428.1
(27mg) following the procedure described
in Example 48. Resulting crude was purified by Prep LC-M (IX). LO-MS (A): tR =
0.68min; [M+1-1]-: 396.24.
Example 429: 4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyl]-pyridazin-3-01-2-phenyl-
butan-2-ol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
285
429.1 3-KR)-Hydroxy-f6-(3-hydroxy-3-phenyl-but-1-yny0-pyridazin-4-y11-(4-
isopropyl-pheny0-methyll-3-methyl-
azetidine-1-carboxylic acid tert-butyl ester
The title compound (52.5mg, white powder) was synthesized from Example F1.6.2
(80mg) and DL-2-phenyl-3-butyn-2-
ol (41.4mg), and following the procedure described in Example 165 step 165.1.
The resulting crude was purified by
Prep LC-MS (XII). LC-MS (A): tR = 1.07min; [M+Fl]: 542.08.
429.2 3-chloro-4-(54(R)-hydroxy(4-isopropylphenyl)(3-methylazetidin-3-
yhmethyl)pyridazin-3-y1)-2-phenylbut-3-en-2-
ol, hydrochloride salt
The title compound (69mg, yellow solid) was synthesized from Example 429.1
(52.5mg) following the procedure
described in Example 309 step 309.2. LC-MS (A): tR = 0.77min; [M-'-H]: 478.10.
429.3. 3-Chloro-4-{5-[(R)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny0-methyll-pyridazin-3-y11-2-phenyl-
but-3-en-2-ol
The title compound (20.4mg, beige solid) was synthesized starting from Example
429.2 (69mg), and following the
procedure described in Example F1.1 step F1.1.2. Resulting crude was purified
by Prep LC-MS (IX). LC-MS (A): tR =
0.79min; [M+H]: 492.13.
429.4. 4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylppyridazin-3-y1}-2-phenyl-butan-2-ol
The title compound (5mg, white powder) was synthesized from Example 429.3
(20.4mg) following the procedure
described in Example 48. Resulting crude was purified by Prep LC-MS (IX). LC-
MS (A): tR = 0.74min; [11/1-FH]-: 460.18.
Example 430 (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{6-[(S)-
(tetrahydro-furan-3-yl)oxy]-pyridazin-4-yll-
methanol
430.1. 34(R)-Hydroxy-(4-isopropyl-pheny0-{6-[(S)-(tetrahydro-furan-3-y0oxyl-
pyridazin-4-y1}-methyl)-3-methyl-
azetidine-1-carboxylic acid tert-butyl ester
The title compound (25.3mg, white powder) was synthesized from Example F1.6.2
(35mg) and (S)-(+)-
hydroxytetrahydrofuran (19.6pL), and following the procedure described in
Example 277 but heating the mixture at
65 C for 1h. The resulting crude was purified by Prep LC-MS (VI). LC-MS (A):
tR = 1.03min; [M-FI-1]*: 484.15.
430.2. (R)-(4-lsopropyl-pheny1)-(3-methyl-azetidin-3-y1)-{6-1(S)-(tetrahydro-
furan-3-y0oxyl-pyridazin-4-4-methanol
The title compound (21.4mg, beige solid) was synthesized from Example 430.1
(25.3mg) following the procedure
described in Example 309 step 309.2. LC-MS (A): tR = 0.66min; [M-F1-1]:
384.11.
430.3. (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{6-[(S)-
(tetrahydro-furan-3-yl)oxyl-pyridazin-4-4-methanol
The title compound (11.3mg, white powder) was synthesized starting from
Example 430.2 (21.4mg), and following the
procedure described in Example F1.1 step F1.1.2. Resulting crude was purified
by Prep LC-MS (V). LC-MS (A): tR =
0.67min; [M-F1-1]-: 398.16.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
286
Example 431 (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{6-[(R)-
(tetrahydro-furan-3-yl)oxyl-pyridazin-4-yly
methanol
The title compound (5.6mg, white powder) was synthesized from Example F1.6.2
(35mg) and (R)-(+)-
hydroxytetrahydrofuran (19.6pL), and following the three-step procedure
described in Example 430. The resulting crude
was purified by Prep LC-MS (V). LC-MS (A): tR = 0.67min; [M+H]-: 398.17.
Example 432 to 444 were synthesized starting from Example F1.6 and the
appropriate commercially available
carbamate (F4) or amide-containing reagent of Formula (F3), and following the
procedure described in Example 167
step 167.1. Prep LC-MS conditions and LC-MS data are listed in the table
below. The LC-MS conditions used were LC-
MS (A).
Prep
Example N Name tR [M+H]
LC-MS
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
432 0.72 409.18 (IX)
pheny1)-methyl]-pyridazin-3-y11-4-methyl-pyrrolidin-2-one
1-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
433 0.80 437.19 (XIV)
pheny1)-methyl]-pyridazin-3-y11-3-isopropyl-pyrrolidin-2-one
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
434 0.75 423.18 (IX)
pheny1)-methyl]-pyridazin-3-y11-3,3-dimethyl-pyrrolidin-2-one
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
435 0.75 423.15 (IX)
pheny1)-methyl]-pyridazin-3-y11-4,4-dimethyl-pyrrolidin-2-one
5-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
436 0.74 421.17 (IX)
pheny1)-methyl]-pyridazin-3-y11-5-aza-spiro[2.4]heptan-6-one
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
437 phenylymethyl]-pyridazin-3-y11-4-trifluoromethyl-
pyrrolidin-2- 0.76 463.10 (IX)
one
4-Cyclopropy1-1-{5-[(R)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-
438 0.79 435.18 (IX)
(4-isopropyl-phenylymethyl]-pyridazin-3-y11-pyrrolidin-2-one
2-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
439 phenyl)-methyl]-pyridazin-3-y11-8-oxa-2-aza-
spiro[4.5]decan- 0.69 465.09 (IX)
3-one
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
287
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
440 pheny1)-methyl]-pyridazin-3-y11-1-oxa-3-aza-
spiro[4.5]decan- 0.82 465.04 (XI V)
2-one
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
441 0.62 472.13 (IX)
phenylymethy1]-pyridazin-3-y1}-4-pyridin-2-yl-pyrrolidin-2-one
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
(IX) then
442 pheny1)-methyl]-pyridazin-3-y11-3(2-methoxy-ethyl)-
0.73 453.16
(VI)
pyrrolidin-2-one
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl- 0.82
471.15 (XIV) then
443
phenylymethyl]-pyridazin-3-y11-4-phenyl-pyrrolidin-2-one
(VII)
2-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
(IX) then
444 0.79
443.14
phenylymethyl]-pyridazin-3-y1}-2,3-dihydro-isoindo1-1-one
(VI)
Example 445:
2-(3-{5-[(S)-(3-Fluoro-1-methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridi
[1,2,4]oxadiazol-5-y1)-propan-2-ol
445.1 345-Cyano-pyridin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-3-fluoro-
azetidine-1-carboxylic acid tert-butyl
ester
To a solution of Example A4.13 (1.50 g) and 5-bromonicotinonitrile (1.08 g) in
THF (30 mL) at -78 C, a solution of
HexLi in THF (2.3 M, 1.3 mL) was added dropwise during 20min. The reaction
mixture was stirred at -78 C for 20min,
before the cooling bath was removed and the reaction was quenched with aq. NI-
14C1. The mixture was extracted with
EA twice, the combined organic layers were washed with brine, dried over
Na2SO4, filtered and concentrated under
vacuo. The residue was purified by CC (CombiFlash, RediSep 40 g, gradient n-
Heptane/EA 90:10 to 60:40 over 25 min
at 40 mL/min) to give the desired product as a yellow foam (1.49 g). It was
further purified by prep LC-MS (XVII then
XII) to give the title compound as white solid (1.04 g). LC-MS (A): tR = 1.09
min; [M+H]0: 426.24.
445.2 3-[(S)-(5-Cyano-pyridin-3-y1)-hydroxy-(4-isopropyl-pheny1)-methytl-3-
fluoro-azetidine-1-carboxylic acid tert-butyl
ester
Example 445.1 (1.04 g) was purified by prep chiral HPLC (XL) to give the title
compound as pure enantiomer (450 mg).
Chiral SFC (F): tR = 1.86 min.
445.3 3-Fluoro-3-[(S)-hydroxy-[5-(N-hydroxycarbamimidoy1)-pyridin-3-y11-(4-
isopropyl-pheny1)-methyikazetidine-1-
carboxylic acid tert-butyl ester
Example 445.2 (150 mg) together with hydroxylamine hydrochloride (74 mg) and
K2003 (195 mg) were suspended in
Et0H (2.3 mL). The mixture was heated to 85 C and stirred for 15 h. The
reaction mixture was filtered, the residue
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
288
was washed with more Et0H and the filtrate was concentrated under vacuo to
give the title compound as off-white solid
(149 mg), which was used in subsequent steps without further purification. LC-
MS (A): tR = 0.80 min; [M+1-1]': 459.28.
445.4 3-Flu oro-3-[(S)-hydroxy-{515-(1-hydroxy-1-m ethyl-ethy1)41, 2, 47oxadi
n-3-y1}-(4-isopropyl-
pheny0-methyll-azetidine-1-carboxylic acid tert-butyl ester
Example 445.3 (120 mg), alpha-hydroxyisobutyric acid (37 mg), PyBOP (423 mg)
and K3PO4 (227 mg) were treated
with DMF (2 mL) and NEt3 (0.14 mL). The mixture was heated to 85 C and
stirred for 15 h. The reaction was cooled
to room temperature, diluted with EA, washed with aq. NaHCO3, aq. NI-14C1 and
brine, dried over Na2SO4, filtered and
concentrated under vacuo to give an orange oil. The residue was purified by CC
(CombiFlash, RediSep 12 g, gradient
n-Heptane/EA 90:10 to 50:50 over 20 min at 30 mL/min) to afford the title
compound as a colorless amorphous solid
(90 mg). LC-MS (A): tR = 1.06 min; [M+H]: 527.28.
445.5 2-(3-{5-[(S)-(3-FI uoro-azetidi n-3-y1)-hydroxy-(4-isopropyl-p henyl)-m
ethyll-pyri di n-3-441, 2, 41ox adiazol-5-y0-
propan-2-ol, hydrochloride salt
Example 445.4 (90 mg) was dissolved in a solution of HCI in dioxane (4 M, 0.43
mL) and stirred at RT for 1.5 h. A thick
slurry resulted and to improve stirring, dioxane (0.5 mL) and little Me0H
(0.15 mL) were added. After another 1.5 h at
RT full conversion was achieved and the mixture was concentrated under vacuo
to give the title compound as white
solid (76 mg). It was used in the next steps without further purification. LC-
MS (A): tR = 0.69 min; [M+1-1]': 427.26.
445.6 2-(3-{5-[(S)-(3-Fluoro-1 -m ethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-
pheny1)-m ethyll-pyri di n-3-y1141, 2, 41oxad iazol-
5-y1)-propa n-2-ol
To a solution of Example 445.5 (35 mg) in DCM (0.5 mL) at 0 C was added a
solution of formaldehyde in H20 (37 wt.
%, 14 uL). After stirring for 5 min, NaBH(OAc)3 (23 mg) was added, the
suspension was stirred at 0 C for 1 h. The
mixture was treated with NEt3 (0.01 mL) and additional aq. formaldehyde
solution (37 wt %, 0.02 mL) and NaBH(OAc)3
(18 mg). After stirring at 4 C for 16 h, the reaction mixture was
concentrated under vacuo, filtered through a syringe
filter and purified by prep LC-MS (V) to give the title compound as a white
solid (21 mg). LC-MS (A): tR = 0.70 min;
[M+H]: 441.25.
Example 446: 2-(3-{5-[(S)-(1-Cyclopropy1-3-fluoro-azetidin-3-y1)-hydroxy-(4-
isopropyl-pheny1)-methyl]-pyridin-3-y11-
[1,2,4]oxadiazol-5-y1)-propan-2-ol
A solution of Example 445.5 (42 mg) in DCM (0.5 mL) and NEt3 (0.01 mL) was
cooled to 0 C, before (1-
ethoxycyclopropoxy)trimethylsilane (0.05 mL) and AcOH (0.02 mL) were added.
After stirring for 5 min, NaBH(OAc)3
(28 mg) was added and the suspension was stirred at 0 C for 1 h. Acetic acid
(0.03 mL) was added and the mixture
was allowed to warm to RT. After stirring for 2.5 h, the reaction mixture was
heated to 40 C and stirred for 2 h. The
mixture was cooled to RT, quenched with sat. aq. NaHCO3 and extracted with EA
three times. The combined organic
layers were dried over Na2SO4, filtered and concentrated under vacuo to give a
yellow oil (being mostly reaction
intermediate. The residue was taken up in Me0H (0.5 mL) and thereto was added
NaBH3CN (18 mg). The mixture was
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
289
heated to 65 C and stirred for 1 h. The reaction mixture was concentrated
under vacuo, redissolved in MeCN, filtered
through a syringe filter and subjected to prep LC-MS (VI then IX) to give the
title compound as a white solid (11 mg).
LC-MS (A): tR = 0.73 min; [M-FH]-: 467.29.
Example 447: 144-(3-{5-[(S)-(3-Fluoro-1-methyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-pheny1)-methyl]-pyridi
[1,2,4]oxadiazol-5-y1)-piperidin-1-y1]-ethanone
447.1 3-[(S)-{545-(1-Acetyl-piperidin-4-y041,2,4]oxadiazol-3-ylppyridin-3-4-
hydroxy-(4-isopropyl-phenyl)-methyll-3-
fluoro-azetidine-1-carboxylic acid tert-butyl ester
A mixture of Example 445.3 (179 mg), 1-acetylpiperidine-4-carboxylic acid (84
mg), PyBOP (568 mg), K3PO4 (304 mg)
and DIPEA (0.18 mL) in DMF (2.5 mL) was stirred at 85 C for 15 h. The
reaction mixture was cooled to RT, diluted
with EA, washed with aq. NaHCO3, aq. NI-1401 and brine, dried over Na2SO4,
filtered and concentrated under vacuo to
give an orange oil, which was purified by CC (CombiFlash, RediSep 12 g,
gradient DCM to DCM/Me0H 90:10 over 20
min at 30 mL/min) to give a colorless amorphous solid (149 mg). LC-MS (A): tR
= 1.10 min; [M+H]: 594.12.
447.2 144-(3-15-[(S)-(3-Fluoro-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-
methylkpyridin-3-4-11,2,4]oxadiazol-5-y1)-
piperidin-1-ylrethanone, hydrochloride salt
Example 447.1 (145 mg) was treated with a solution of HCI in dioxane (4 M,
0.43 mL) and stirred for 1 h. The reaction
mixture was concentrated under vacuo to give the desired product as slightly
yellow solid (155 mg). The compound
was used in the next step without further purification. LC-MS (A): tR = 0.70
min; [M-FH]-: 494.28.
447.3 144-(3-{5-[(S)-(3-Fluoro-1-methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyll-pyridin-3-y1}-
[1 , 2, 4]oxad lazol-5-y1)-piperidi n-1-yll-ethanone
A suspension of Example 447.2 in DCM (1.0 mL) was cooled to 0 C. Thereto was
added a solution of formaldehyde
in water (37 wt. %, 14 uL) and NaBH(OAc)3 (68 mg). The mixture was stirred for
5 h at 0 C and 1 h at RT. More
formaldehyde solution (37 wt. %, 0.05 mL) and NaBH(OAc)3 (65 mg) were added.
The mixture was stirred at RT for 1
h. The reaction mixture was poured into aq. NaHCO3 and extracted with DCM/Me0H
(9:1) three times. The combined
organic layers were passed through a phase separator and concentrated under
vacuo to give a colourless oil, which
was purified by prep LC-MS (V) to give a white solid (64 mg). LC-MS (A): tR =
0.71 min; [M-F1-1]': 508.29.
Example 448: 144-(3-{5-[(S)-(1-Cyclopropy1-3-fluoro-azetidin-3-y1)-hydroxy-(4-
isopropyl-pheny1)-methyl]-pyridin-3-y11-
[1,2,4]oxadiazol-5-y1)-piperidin-111]-ethanone
448.1 5-[(S)-(3-Fluoro-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-
methylknicotitionitrile
Treatment of Example 445.2 (450 mg) with a solution of HCI in dioxane (4 M,
2.65 mL) led to a colourless solution that
quickly turned into a thick suspension. To help stirring, Me0H (1.0 mL) was
added and the mixture was stirred at RT
for 1 h. The mixture was concentrated under vacuo, then purified by prep LC-MS
(V) to give a white solid (160 mg). LC-
MS (A): tR = 0.67 min; [M+H]': 326.20.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
290
448.2 5-[(S)-(1-Cyclopropy1-3-fluoro-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methylpnicotinonitrile
To a solution of Example 448.1 (100 mg) in Me0H (5 mL) was added (1-
ethoxycyclopropoxy)trimethylsilane (0.19 mL),
acetic acid (0.04 mL) and NaBH3CN (41 mg). The suspension was heated to 55 C
and stirred for 90 min, then cooled
to RT and stirred for 17 h. The reaction mixture was diluted with DCM and
washed with aq. NaHCO3. The aqueous
phase was extracted once with DCM/Me0H (9:1). The combined organic layers were
passed through a phase separator
and concentrated under vacuo to give a colorless oil, which was purified by
prep LC-MS (VI) to give the product as a
white foam (74 mg). LC-MS (A): tR = 0.72 min; [M-F1-1]+: 366.24.
448.3 5-[(S)-(1-Cyclopropy1-3-fluoro-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methylkN-hydroxy-nicotinamidine
A suspension of Example 448.2 (74 mg), hydroxylamine hydrochloride (43 mg) and
K2003 (112 mg) in Et0H (1.5 mL)
was heated to 90 C and stirred for 3 h. The reaction mixture was cooled to
RT, filtered and concentrated under vacuo
to give the desired product as a white solid (109 mg). The crude was used in
the next step without further purification.
LC-MS (A): tR = 0.55 min; [M+H]: 399.22.
448.4 144-(3-{5-[(S)-(1-Cyclopropy1-3-fluoro-azetidin-3-y1)-hydroxy-(4-
isopropyl-pheny1)-methylkpyridin-3-y11-
[1,2,4]oxadiazol-5-0)-piperidin-1-ylrethanone
A reaction vessel was charged with Example 448.3 (109 mg), PyBOP (326 mg),
K3PO4 (175 mg) and 1-acetylpiperidine-
4-carboxylic acid (48 mg), followed by DMF (1.0 mL) and DIPEA (0.11 mL). The
mixture was stirred for 17 h at 80 C
and for 7 h at 95 C. More PyBOP (200 mg) was added and the reaction was
stirred at 100 C for 20 h. More 1-
acetylpiperidine-4-carboxylic acid (30 mg), PyBOP (200 mg) and K3PO4 (100 mg)
were added and the mixture was
stirred at 100 C for another 18 h. The reaction mixture was cooled to RT,
diluted with EA, washed with aq. NaHCO3
and with brine, dried over Na2SO4, filtered and concentrated under vacuo to
give a yellow oil, which was purified with
prep LC-MS (VI then IX) and CC (CombiFlash, RediSep 4 g, gradient DCM to
DCM/Me0H/NH4OH 94.5:5.0:0.5 over
20 min at 18 mL/min) to give a white solid (5 mg). LC-MS (A): tR = 0.74 min;
[M-FH]-: 534.31.
Example 449: 2-(3-{5-[(R)-(1-Cyclopropy1-3-methyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-pheny1)-methyl]-pyridin-3-y11-
[1,2,4]oxadiazol-5-y1)-propan-2-ol
449.1 5-[(R)-(1-Cyclopropy1-3-methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methylknicotinonitrile
To a solution of Example D1.1.4 (541 mg) in Me0H (6 mL) was added (1-
ethoxypropoxy)trimethylsilane (0.73 mL),
acetic acid (0.14 mL) and NaBH3CN (158 mg). The suspension was heated to 65 C
and stirred for 16 h. The reaction
mixture was filtered, diluted with DCM and washed with aq. NaHCO3. The aqueous
phase was re-extracted with
DCM/Me0H (9:1). The combined organic layers were passed through a phase
separator and were concentrated under
vacuo to give a colourless oil, which was purified by prep LC-MS (VI) to give
the desired product as a white solid (174
mg). LC-MS (A): tR = 0.71 min, [M-F1-1]+: 362.20.
449.2 5-[(R)-(1-Cyclopropy1-3-methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methylpN-hydroxy-nicotinamidine
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
291
A mixture of Example 449.1 (87 mg), hydroxylamine hydrochloride (49 mg) and
K2003 (127 mg) in Et0H (1.5 mL) was
heated to 85 C and stirred for 1.5 h. The reaction mixture was cooled to RT,
filtered, concentrated and dried under HV
to give a white solid (101 mg), which was used in the next step without
further purification. LC-MS (A): tR = 0.55 min;
[M+H]: 395.26.
449.3 2-(3-{5-[(R)-(1-Cyclopropy1-3-methyl-azetidin-3-y0-hydroxy-(4-isopropyl-
pheny0-methylppyridin-3-y1}-
[1,2,4]oxadiazol-5-y1)-propan-2-ol
A mixture of Example 449.2 (43 mg), alpha-hydroxyisobutyric acid (7 mg), PyBOP
(35 mg) and NEt3 (10 mg) in DMF
(0.5 mL) was heated to 80 C and stirred for 16 h. The reaction mixture was
cooled to RT, diluted with MeCN/H20,
filtered through a syringe filter and purified by prep LC-MS (2 x VI) to give
the title compound as a slightly yellow solid.
LC-MS (A): tR = 0.72 min; [M+H]: 463.31.
Example 450 to Example 453 were prepared from Example 449.2 and the
corresponding carboxylic acid according to
the procedure described for Example 449.3. Prep LC-MS conditions and LC-MS
data are listed in the table below. The
LC-MS conditions used were LC-MS (A).
tR
Prep LC-
Example N Name [M-F1-
1]-
(min)
MS
1-[4-(3-{5-[(R)-(1-Cyclopropy1-3-methyl-azetidin-3-y1)-
450 hydroxy-(4-isopropyl-pheny1)-methyl]-pyridin-3-y1}-
0.74 530.15 (VI)
[1,2,4]oxadiazol-5-y1)-piperidin-1-y1]-ethanone
(R)-(1-Cyclopropy1-3-methyl-azetidin-3-y1)-(4-isopropyl-
451 phenyl)-{5[5-(tetrahydro-pyran-4-y1)41,2,4]oxadiazol-3-
y1]- 0.77 489.34 (VI)
pyridin-3-yll-methanol
trans-4-(3-{5-[(R)-(1-Cyclopropy1-3-methyl-azetidin-3-y1)-
452 hydroxy-(4-isopropyl-pheng-methyl]-pyridin-3-y1}-
0.73 503.35 (VI)
[1,2,4]oxadiazol-5-y1)-cyclohexanol
(R)-(1-Cyclopropy1-3-methyl-azetidin-3-y1)-(4-isopropyl-
453 pheny1)-[5-(5-oxetan-3-y111 ,2,41oxadiazol-3-y1)-pyridin-
3-y11- 0.73 461.27 (VI)
methanol
Example 454: 1-[(R)-3-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-pheny1)-methyl]-pyridin-3-y11-
[1,2,4]oxadiazol-5-ylmethyl)-pyrrolidin-111]-ethanone
454.1 (R)-3-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny0-methylppyridin-3-y1}41, 2,4]oxadiazol-
5-ylmethyl)-pyrrolidine-1-carboxylic acid tert-butyl ester
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
292
A mixture of Example D2.1 (39 mg), (R)-(1-Boc-pyrrolidin-3-yI)-acetic acid (36
mg), PyBOP (128 mg), K3PO4 (89 mg)
and DIPEA (41 mg) in DMF (2 mL) was stirred at 85 C for 64 h. The mixture was
cooled to RT, diluted with H20/MeCN,
passed through a syringe filter and purified by prep LC-MS (XIV) to give the
desired product as a white solid. LC-MS
(A): tR = 0.85 min; [M+H]: 562.21.
454.2 (R)-(1,3-dimethylazetidin-3-y1)(4-isopropylphenyl)(5-(54(R)-pyrrolidin-3-
yOmethyl)-1,2,4-oxadiazol-3-Apyridin-
3-yOmethanol hydrochloride salt
Example 454.1 (76 mg) was treated with a solution of HCI in dioxane (4 M, 2
mL) and stirred at RI for 2 h. The reaction
mixture was concentrated and dried under HV. The resulting white solid was
used in the next step without further
purification. LC-MS (A): tR = 0.55 min; [M+H]+: 462.24.
454.3 1-[(R)-3-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methylkpyridin-3-y11-
[1,2,4]oxadiazol-5-ylmethyl)-pyrrolidin-1-ylkethanone
To a solution of Example 454.2 (15 mg) in THF (1 mL) was added DIPEA (19 mg)
and acetyl chloride (2.4 mg) at 0 C.
The mixture was stirred at 0 C for 45 min, before it was concentrated and
dried under HV. The crude was purified by
prep HPLC (IX) to give the title compound as a white solid (3 mg). LC-MS (A):
tR = 0.71 min; [M+H]-: 504.24.
Example 455 to Example 459 were synthesized according to the 3-step procedure
described for Example 454, using
the corresponding acids in the first step. Prep LC-MS conditions (first and
third steps) and LC-MS data are listed in the
table below. The LC-MS conditions used were LC-MS (A).
tR Prep LC-MS
Prep LC-MS
Example N Name [M-F1-1]*
(min) (Step 1)
(Step 3)
143-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-
y1)-hydroxy-(4-isopropyl-pheny1)-
455 0.73 504.26 (XIV) (IX)
methyl]-pyridin-3-y11-[1,2,4]oxadiazol-5-
yI)-piperidin-1-y1]-ethanone
143-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-
y1)-hydroxy-(4-isopropyl-pheny1)-
456 0.67 520.27 (IX) (IX)
methyll-pyridin-3-y1141,2,41oxadiazol-5-
y1)-3-hydroxy-piperidin-1-y11-ethanone
143-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-
y1)-hydroxy-(4-isopropyl-phenyl)-
457 0.75 518.28 (XIV) (IX)
methyl]-pyridin-3-y1141,2,4]oxadiazol-5-
y1)-3-methyl-piperidin-1-y1Fethanone
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
293
143-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-
y1)-hydroxy-(4-isopropyl-pheny1)-
458 0.74 518.30 (XIV) (IX)
methyll-pyridin-3-y1H1,2,41oxadiazol-5-
yl methyl)-piperidin-1-y11-ethanone
114-(3-15-[(R)-(1,3-Dimethyl-azetidin-3-
y1)-hydroxy-(4-isopropyl-pheny1)-
459 methyl]-pyridin-3-yly[1,2,4]oxadiazol-5- 0.66
534.27 (IX) (IX)
ylmethyl)-4-hydroxy-piperidin-1-y1]-
ethanone
Example 460:
N41-(3-{5-[(R)-(1, 3-Di methyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyl]-pyridi
[1 ,2,4]oxadiazol-5-ylmethylycyclopropyl]-acetamide
460.1 [1 -(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-0)-hydroxy-(4-isopropyl-phenyl)-
methyll-pyridin-3-y1}41,2,4]oxadiazol-5-
ylmethyl)-cyclopropylkcarbamic acid tert-butyl ester
A mixture of Example D2.1 (30 mg), 2-[1-({tert-
butoxycarbonyl}amino)cyclopropyl]acetic acid (15 mg), PyBOP (60 mg),
K3PO4 (59 mg) and DIPEA (27 mg) in DMF (0.75 mL) was heated to 90 C and
stirred for 3 h. After cooling to RT, the
reaction mixture was filtered and directly purified by prep LC-MS (XIV) to
give the desired product as a white solid (4
mg). LC-MS (A): tR = 0.82 min; [M+H]: 548.18.
460.2 (R)-{515-(1-Amino-cyclopropylmethy011,2,4]oxadiazol-3-ylppyridin-3-4-
(1,3-dimethyl-azetidin-3-y1)-(4-
isopropyl-phenyl)-methanol, hydrochloride salt
Example 460.1 (4 mg) was treated with a solution of HCI in dioxane (4 M, 1 mL)
and stirred at RT for 1 h. The reaction
mixture was concentrated under vacuo to give the desired product as a white
solid (3 mg). LC-MS (A): tR = 0.57 min;
[M-FH]-: 448.17.
460.3 NI1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-y1}11,2,41oxadiazol-5-
ylmethyl)-cyclopropylFacetamide
To a suspension of Example 460.2 (2 mg) and acetic acid (0.3 mg) in DMF (1 mL)
was added HATU (1.9 mg) and
DIPEA (1.7 mg). The reaction mixture was stirred at RT for 20 h, filtered and
then directly purified by prep LC-MS (IX)
to give the title compound as a white solid (0.5 mg). LC-MS (A): tR = 0.69
min; [M+H]: 490.04.
Examples 461 and 462 were prepared from Example 460.2, as described for
Example 460, but using the corresponding
acids. Prep LC-MS conditions and LC-MS data are listed in the table below. The
LC-MS conditions used were LC-MS
(A).
Example N Name tR [M+1-1]
Prep LC-MS
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
294
Tetrahydro-pyran-4-carboxylic acid [1-(3-{5-[(R)-(1,3-
di methyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-pheny1)-
461 0.71 560.12 (IX)
methyll-pyridin-3-y1141,2,41oxadiazol-5-ylmethyl)-
cyclopropyI]-amide
N41-(3-15-[(R)-(1, 3-Dimethyl-azetidi n-3-yI)-hydroxy-(4-
462 isopropyl-pheny1)-methyl]-pyridin-3-y1141,2,4]oxadiazol-
5- 0.71 520.12 (IX)
ylmethylycyclopropy1]-2-methoxy-acetamide
Example 463: N41-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridin-3-yll-
[1 ,2,4]oxadiazol-5-ylmethyl)-cyclopropyll-N-methyl-acetamide
The title compound was prepared from Example 02.1, according to the 3-step
procedure described for Example 460,
but using the corresponding carboxylic acid in the first step. Prep LC-MS was
used to purify the intermediate after step
1 (VII) and the final product (IX). LC-MS (A): tR = 0.72 min; [M+H]: 504.29.
Example 464: N-[2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y11-
[1,2,4]oxadiazol-5-y1)-1,1-dimethyl-ethyl]-propionamide
hydrochloride464.1 [2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-
(4-isopropyl-phenyl)-methyll-pyridin-314}-
[1,2,4]oxadiazol-5-y0-1,1-dimethyl-ethylkcarbamic acid tert-butyl ester
A mixture of Example D2.1 (789 mg), N-Boc-3-amino-3-methylbutanoic acid (633
mg), PyBOP (3.00 g), K3PO4 (1.63 g)
and DIPEA (738 mg) in DMF (12 mL) was heated to 80 C and stirred for 16 h.
The reaction mixture was cooled to RT,
diluted with H20/MeCN, filtered through a syringe filter and purified by prep
LC-MS (VII) to give a lightly yellow solid
(516 mg). LC-MS (A): tR = 0.88 min; [M+H]: 550.31.
464.2 (R)-{545-(2-Amino-2-methyl-propy1)41,2,41oxadiazol-3-ylkpyridin-3-y0-
(1,3-dimethyl-ezetidin-3-y1)-(4-isopropyl-
phenyl)-methanol, hydrochloride salt
Example 464.1 (516 mg) was treated with a solution of HCI in dioxane (4 M, 4.7
mL) and stirred at RT for 1.5 h. The
reaction mixture was concentrated under vacuo to give the desired product as a
white solid (577 mg). LC-MS (A): tR =
0.59 min; [M+H]: 450.08.
464.3 N-12-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyll-pyridin-3-y1}41,2,41oxadiazol-5-
y1)-1,1-dimethyl-ethyll-propionamide
To a solution of propionic acid (5 mg) in DMF (0.15 mL) were added DBU (32 mg)
and HATU (32 mg). The solution
was stirred at RT for 10 min and was then added to a solution of Example 464.2
(30 mg) and DBU (25 mg) in DMF
(0.15 mL) at 0 C. The reaction mixture was stirred at 0 C for 1 h, before
more premixed (for 10 min) propionic acid (5
mg), DBU (32 mg) and HATU (32 mg) in DMF (0.15 mL) was added. The mixture was
stirred for 1 h at 0 C, before it
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
295
was diluted with MeCN/H20, filtered through a syringe filter and purified by
prep LC-MS (IX) to give the title compound
as white solid (5.5 mg). LC-MS (A): tR = 0.78 min; [M+1-1]': 506.31.
Examples 465 to Example 468 were synthesized starting from Example 464.2
according to the procedure described
for Example 464, with the difference that the corresponding preactivated acid
(1.2 equiv.) was only added once (since
the conversion was sufficient). Prep LC-MS conditions and LC-MS data are
listed in the table below. The LC-MS
conditions used were LC-MS (A).
Example tR
Prep LC-MS
Name [M-FH]-
N (min)
Method
N-[2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
465 isopropyl-phenyl)-
methyll-pyridin-3-y1H1,2,41oxadiazol- 0.77 522.31 (IX)
5-y1)-1,1-dimethyl-ethyl]-2-methoxy-acetamide
Tetrahydro-pyran-4-carboxylic acid [2-(3-{5-[(R)-(1,3-
dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyly
466 0.74 562.14 (IX)-FOX)
methyl]-pyridin-3-y1}-[1,2,4]oxadiazol-5-y1)-1,1-dimethyl-
ethyl]-amide
N-[2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
467 isopropyl-
phenylymethyl]-pyridin-3-y1141,2,4]oxadiazol- 0.80 520.32 (IX)+(lX)
5-y1)-1,1-dimethyl-ethylPsobutyramide
Cyclopropanecarboxylic acid [2-(3-{5-[(R)-(1,3-dimethyl-
azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyll-
468 0.76 518.33 (IX)
pyridin-3-y1}-[1,2,4]oxadiazol-5-y1)-1,1-dimethyl-ethyl]-
amide
Examples 469 to Example 472 were synthesized from Example D2.1 and the
corresponding substituted 1-Boc-
piperidine-4-carboxylic acids according to the 3-step procedure described for
Example 464. In the last step,
correspondingly different carboxylic acids were used. Prep LC-MS conditions
(first and third steps) and LC-MS data are
listed in the table below. The LC-MS conditions used were LC-MS (A).
Prep LC-MS Prep LC-MS
Example tR
Name [M-FH] Method
Method
N (min)
(Step 1)
(Step 3)
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
296
1-[cis-4-(3-{5-[(R)-(1,3-0imethyl-azetidin-3-yI)-
hydroxy-(4-isopropyl-pheny1)-methy1]-pyridin-3-y11-
469 0.73 518.20 (XIV) (IX)+(VI)
[1,2,4]oxadiazol-5-y1)-2-methyl-piperidin-1-y11-
ethanone
144-(3-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-
hydroxy-(4-isopropyl-pheny1)-methy1]-pyridin-3-y11-
470 0.71 548.36 (VII) (IX)+(lX)
[1,2,4]oxadiazol-5-y1)-4-hydroxy-piperidin-1-y1]-2-
methyl-propan-1-one
1-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-
hydroxy-(4-isopropyl-pheny1)-methyli-pyridin-3-yll-
471 0.68 534.37 (VII) (IX)+(V)
[1,2,4]oxadiazol-5-y1)-4-hydroxy-piperidin-1-y1]-
propan-1-one
144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-
hydroxy-(4-isopropyl-phenylymethy1]-pyridin-3-y11-
472 0.73 534.37 (XIV) (1X)-FOX)
[1,2,4]oxadiazol-5-y1)-4-methoxy-piperidin-1-y1]-
ethanone
Example 473: 144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyl]-pyridin-3-y11-
[1,2,4]oxadiazol-5-y1)-piperidin-1-y1]-3,3,3-trifluoro-propan-1-one
473.1 4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-0)-hydroxy-(4-isopropyl-pheny1)-
methyil-pyridin-3-4-11,2,41oxadiazol-5-
yl)-piperidine-1-carboxylic acid tert-butyl ester
To a solution of N-Boc-piperidine-4-carboxylic acid (69 mg) in DMSO (0.25 mL)
was added CDI (52 mg). The mixture
was stirred at RT for 30 min. Thereto was added a solution of Example 02.1
(100 mg) in DMSO (0.25 mL). The resulting
solution was stirred at 85 00 for 30 min and directly purified by prep LC-MS
(IX) to give the title compound as white
solid (83 mg). LC-MS (A): tR = 0.87 min; [M+H]: 562.43.
473.2 (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-15-(5-piperidin-4-
y141,2,4]oxadiazol-3-y1)-pyridin-3-y11-
methanol
Example 473.1 (83 mg) was treated with a solution of HCI in dioxane (4 M, 1.11
mL) and stirred at RT for 2 h. The
reaction mixture was concentrated and dried under HV. The residue was purified
by prep LC-MS (VII) to give the desired
product as white solid (31 mg). LC-MS (A): tR = 0.57 min; [M+H]: 462.38.
473.3 144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyil-pyridin-3-y1}41,2,4Joxadiazol-5-
y1)-piperidin-1-y1]-3,3,3-trifluoro-propan-1-one
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
297
To a solution of 3,3,3-trifluoropropionic acid (3.4 mg) and HATU (10 mg) in
DMF (0.5 mL) was added DIPEA (10 mg)
and the mixture was stirred for 5 min before Example 473.2 (10 mg) in DMF (0.5
mL) was added. The reaction mixture
was stirred for 2 h at RT, then it was diluted with MeCN/H20, filtered through
a syringe filter and purified by prep LC-
MS (VI) to give the title compound as white solid (2.5 mg). LC-MS (A): tR =
0.79 min; [M+H]: 572.37.
Example 474: 144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyl]-pyridin-3-y11-
[1,2,4]oxadiazol-5-y1)-piperidin-1-y1]-2-oxetan 3 yl ethanone
474.1 Oxetan-3-yl-acetic acid (R)-(1,3-dimethyl-azetidin-3-y1)-(4-isopropyl-
phenyl)-(5-{5-11-(2-oxetan-3-yl-acely0-
piperidin-4-y1H1,2,4]oxadiazol-3-0J-pyridin-3-y1)-methyl ester
The compound was synthesized from Example D2.1 (100 mg) and N-Boc-piperidine-4-
carboxylic acid (86 mg)
according to the 3-step procedure described for Example 464. In the third
step, the corresponding acid (3-oxetanacetic
acid) was used. Prep LC-MS purification was used after the first step (XIV)
and the third step (V). LC-MS (A): tR = 0.73
min; [M-FH]': 658.39.
474.2 1 44-(3-{5-[(R)-(1 ,3-Dimethyl-azetidi n-3-yI)-hyd roxy-(4-isopropyl-
phenyI)-m ethyil-pyrid n-3-y1}41 , 2,4]oxa d iazol-5-
yO-piperidin-1-y1]-2-oxetan-3-yl-ethanone
To a solution of Example 474.1 (11 mg) in Me0H (1 mL) was added K2003 (22 mg)
and the mixture was stirred at 45
C for 5 h. The reaction was cooled to RT, diluted with EA and washed with
water. The aqueous phase was reextracted
with EA twice, the combined organic layers were dried over Na2SO4, filtered
and concentrated under vacuo. The crude
was purified by prep LC-MS (V) to give the title compound as a white solid
(4.4 mg). LC-MS (A): tR = 0.72 min; [M-FI-1]-:
560.31.
Examples 475, 476 and 477 were synthesized from Example 02.1 and the
corresponding piperidine carboxylic acids,
according to the 4-step procedure described for Example 474 (in the first step
of Example 475, molecular sieves instead
of K3PO4 were used). In the third step the corresponding carboxylic acids were
used. For Example 477, the reaction
mixture in the first step was stirred at 100 C for 41 h to reach full
conversion. Prep LC-MS conditions (steps 1, 3 and
4) and LC-MS data are listed in the table below. The LC-MS conditions used
were LC-MS (A).
LC-MS LC-MS
LC-MS
Example tR
Name [M-+I] Method
Method Method
N (min)
(Step 1) (Step
3) (Step 4)
1-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-
y1)-hydroxy-(4-isopropyl-phenylymethyl]-
475 0.80 532.43 (XIV) (VII) (VI)
pyridin-3-y11-[1,2,4]oxadiazol-5-y1)-4-
ethyl-pi peridi n-1-yll-ethanone
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
298
[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-
hydroxy-(4-isopropyl-phenyI)-methy1]-
476 0.71 546.33 (XIV) (V) (V)
pyridin-3-y1141,2,41oxadiazol-5-y1)-
piperidin-1-yll-oxetan-3-yl-methanone
144-(3-15-[(R)-(1,3-Dimethyl-azetidin-3-
y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
(VI I )+
477 pyridin-3-y1141,2,4]oxadiazol-5-y1)-4- 0.78
576.41 (VI) (VII)
(IX)
isopropyl-pi peridi n-1-yI]-2-methoxy-
ethanone
Example 478:
1-[cis-4-(3-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridi
[1 ,2,4]oxadiazol-5-y1)-3-methyl-piperidin-1-y1]-ethanone
478.1 cis-4-({{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyll-pyridin-3-y1}-[(E)-hydroxyitnino]-
methyll-carbamoy0-3-methyl-piperidine-1-carboxylic acid tert-butyl ester
A mixture of Example D2.1 (65 mg), cis-N-Boc-3-methylpiperidine-4-carboxylic
acid (56 mg), PyBOP, molecular sieves
(3A, 50 mg) and DIPEA (67 mg) in DMF (3 mL) was stirred at RT for 16 h. The
reaction mixture was filtered, diluted
with MeCN/H20, passed through a syringe filter and directly purified by prep
LC-MS (IX) to give the desired compound
as yellow oil (143 mg). LC-MS (A): tR = 0.78 min; [M+H]: 594.44.
478.2 4-(3-151(R)-(1,3-Dimethyl-azetidin-3-y0-hydroxy-(4-isopropyl-phenyl)-
methylrpyridin-3-411,2, 41oxadiazol-5-y1)-
cis-3-m ethyl-pi peridi ne-1 -carboxylic acid tert-butyl ester
A mixture of Example 478.1 (143 mg), molecular sieves (3A, 150 mg) and DIPEA
(32 mg) in DMF (1 mL) was heated
to 100 C and stirred for 16 h. The reaction mixture was cooled to RI, diluted
with MeCN/H20, passed through a syringe
filter and purified by prep LC-MS (Method XIV) to give the desired product as
brown powder (40 mg). LC-MS (A): tR =
0.90 min; [M+H]: 576.43.
478.3 (R)-(1,3-Dimethyl-azetidin-3-yl)-(4-isopropyl-pheny1)-{5-15-(cis-3-
methyl-piperidin-4-y041,2,4.1oxadiazol-3-yli-
pyridin-3-y1}-methanol, hydrochloride salt
Example 478.2 (40 mg) was treated with HCI in dioxane (4 M, 0.5 mL) and the
mixture was stirred at RI for 30 min.
The reaction was concentrated and dried under HV to give the desired product
as a sticky solid (31 mg). LC-MS (A): tR
= 0.59 min; [M+H]-: 476.37.
478.4 Acetic acid (R)-{5-15-(cis-1-acelyl-3-methyl-piperidin-4-
y1)41,2,41oxadiazol-3-yil-pyridin-3-0}-(1,3-dimethyl-
azetidin-3-y1)-(4-isopropyl-phenyl)-methyl ester
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
299
To a solution of Example 478.3 (31 mg) in DMF (0.5 mL) was added DBU (27 mg),
before it was cooled to 0 C. Thereto
was added a premixed (10 min) solution of acetic acid (5.1 mg), DBU (11 mg)
and HATU (38 mg) in DMF (0.5 mL). The
mixture was stirred at 0 C for 1 h and 1 h at RT. The reaction was diluted
with MeCN/H20, passed through a syringe
filter and purified by prep LC-MS (IX) to give the bis-acylated product as a
white solid (11 mg). LC-MS (A): tR = 0.79
min; [M+H]': 560.43.
478.5 1Icis-4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y0-hydroxy-(4-isopropyl-
phenyo-methylppyridin-3-y1)-
[1,2,4]oxadiazol-5-y1)-3-methyl-piperidin-1-ylPethanone
To a solution of Example 478.4 (11 mg) in Me0H (0.5 mL) was added K2003 (26
mg) and the mixture was stirred at
RT for 16 h. The reaction was diluted with MeCN/H20 passed through a syringe
filter and purified by prep LC-MS
(Method VI) to give the title compound as white solid (6 mg). LC-MS (A): tR =
0.77 min; [M+H]-: 518.24.
Example 479: 5-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y11-
[1,2,4]oxadiazol-5-y1)-1-methyl-piperidin-2-one
A mixture of Example D2.1 (25 mg), 1-methyl-6-oxo-piperidine-3-carboxylic acid
(16 mg), PyBOP (83 mg), K3PO4 (58
mg) and DIPEA (26 mg) in DMF (1 mL) was heated to 85 C and stirred for 16 h.
The reaction mixture was diluted with
MeCN/H20, passed through a syringe filter and purified by prep LC-MS (IX then
V) to give the title compound as white
solid (7.6 mg). LC-MS (A): tR = 0.69 min; [M-F1-1]': 490.11.
Example 480 to Example 497 were synthesized from Example D2.1 and the
corresponding carboxylic acids according
to the procedure described for Example 479. If necessary, to achieve full
conversion the reaction mixture can be stirred
up to 20 h, at slightly higher temperature (90 C). Prep LC-MS conditions and
LC-MS data are listed in the table below.
The LC-MS conditions used were LC-MS (A).
prep LC-
Example tR
Name [M+H]-
MS
N (min)
Method
5-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
480 phenylymethyl]-pyridin-3-y11-0,2,41oxadiazol-5-y1)-5-
methyl- 0.69 490.15 (IX)
piperidin-2-one
5-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
481 0.66 476.16 (IX)
pheny1)-methy1]-pyridin-3-y11-[1,2,4]oxadiazol-5-y1)-piperidin-2-one
4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
482 pheny1)-methyl]-pyridin-3-y1141,2,4]oxadiazol-5-y1)-4-
methyl- 0.68 476.15 (IX)
pyrrolidin-2-one
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
300
1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
483 phenylymethy1]-pyridin-3-y1H1,2,4]oxadiazol-5-ylmethyl)-
3,3- 0.76 518.10 (IX)
dimethyl-pyrrolidine-2,5-dione
1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
484 pheny1)-methyll-pyridin-3-y11-[1,2,4]oxadiazol-5-
ylmethyl)- 0.65 491.09 (IX)
imidazolidine-2,4-dione
1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
485 phenylymethyll-pyridin-3-yly[1,2,4]oxadiazol-5-ylmethyly
0.70 476.13 (IX)
pyrrolidin-2-one
3-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
486 pheny1)-methy1]-pyridin-3-yly[1,2,4]oxadiazol-5-ylmethyly
0.66 491.09 (IX)
imidazolidine-2,4-dione
3-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
487 pheny1)-methy1]-pyridin-3-yIH1,2,4]oxadiazol-5-ylmethyl)-
1- 0.69 505.13 (IX)
methyl-imidazolidine-2,4-dione
1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
488 pheny1)-methy1]-pyridin-3-yIH1,2,4]oxadiazol-5-ylmethyl)-
3- 0.69 505.10 (IX)
methyl-imidazolidine-2,4-dione
3-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
489 pheny1)-methy1]-pyridin-3-y1}-[1,2,4]oxadiazol-5-
ylmethyl)- 0.69 478.12 (IX)
oxazolidin-2-one
1-Cyclopropy1-3-(3-{5-[(R)-(1,3-dimethyl-azetidin-3-y1)-hydroxy-(4-
490 isopropyl-phenylymethy1]-pyridin-3-y1}41,2,4]oxadiazol-5-
0.73 517.15 (IX)
ylmethyl)-imidazolidin-2-one
1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
491 pheny1)-methyll-pyridin-3-y11-[1,2,4]oxadiazol-5-
ylmethyl)- 0.70 490.13 (IX)
pyrrolidine-2,5-dione
1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
492 pheny1)-methyll-pyridin-3-y1H1,2,41oxadiazol-5-ylmethyl)-
3- 0.70 491.13 (IX)
methyl-imidazolidin-2-one
1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
493 pheny1)-methyl]-pyridin-3-y1141,2,4]oxadiazol-5-ylmethyly
0.67 477.14 (V)
imidazolidin-2-one
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
301
2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
494 phenylymethy1]-pyridin-3-y11-[1,2,4]oxadiazol-5-y1)-propane-
1,2- 0.62 453.27 (V)
diol
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(5-pyridin-
495 0.76 456.32 (IX)
3-y1-[l,2,4]oxadiazol-3-y1)-pyridin-3-y11-methanol
144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
496 pheny1)-methyl]-pyridin-3-y1141,2,4]oxadiazol-5-ylmethyl)-
0.74 518.12 (IX)-F(VI)
piperidin-1-yll-ethanone
(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
497 0.71 418.33 (V1)-FOX)
pheny1)-methy1]-pyridin-3-y1141,2,4]oxadiazol-5-y1)-acetonitrile
Example 498: 144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyl]-pyridin-3-y11-
[1,2,4]oxadiazol-5-y1)-4-hydroxy-piperidin-1-y1]-ethanone
The title compound was synthesized from Example D2.1 and Example D3.13
according to the procedure described for
Example 479. The product was purified by prep LC-MS (IX then V) and prep
chiral SFC (00(11) to give the title
compound as a yellow, sticky solid. LC-MS (A): tR = 0.66 min; [M+H]: 520.36.
Example 499: 3-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y1}-
[1,2,4]oxadiazol-5-y1)-propionitrile
To a solution of 3-cyanopropionic acid (33 mg) in DMSO (0.25 mL) was added CDI
(55 mg) and the mixture was stirred
at RT for 30 min. Thereto was added a solution of Example D2.1 (65 mg) in DMSO
(0.25 mL). The mixture was stirred
at 85 00 for 1 h. The reaction was cooled to RT, diluted with MeCN/H20, passed
through a syringe filter and purified by
prep LC-MS (XIV) to give the title compound as a white solid (25 mg). LC-MS
(A): tR = 0.73 min; [M+H]*: 432.35.
Example 500: 144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyl]-pyridin-3-yll-
[1 ,2,4]oxadiazol-5-y1)-3-methoxy-piperidin-1-y1]-ethanone
The title compound was synthesized from Example 02.1 and Example 03.14,
according to the procedure described for
Example 499. The product was purified by prep LC-MS (V+IX) to yield to title
compound as a white solid. LC-MS (A):
tR = 0.73 min; [M+I-1]+: 534.41.
Example 501: 1-[(R)-3-(3-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-pheny1)-methyl]-pyridin-3-y11-
0,2,41oxadiazol-5-y1)-piperidin-1-y11-ethanone
501.1 3-[(R)-{5-15-((R)-1-Acetyl-piperidin-3-y1)-(1,2,4]oxadiazol-3-y1J-
pyridin-3-y11-hydroxy-(4-isopropyl-pheny1)-methyl]-
3-methyl-azetidine-1-carboxylic acid tert-butyl ester
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
302
A mixture of Example D2.2 (125 mg), (3R)-1-acetylpiperidine-3-carboxylic acid
(74 mg), PyBOP (244 mg), K3PO4 (238
mg) and DIPEA (109 mg) in DMF (3 mL) was heated to 90 C and stirred for 20 h.
Due to incomplete conversion the
same amounts of carboxylic acid and PyBOP were added again and the mixture was
stirred for another 6 h at 90 'C.
The reaction mixture was cooled to RT, diluted with MeCN/H20, passed through a
syringe filter and purified by prep
LC-MS (XII) to give the desired product as an off-white solid (120 mg). LC-MS
(A): tR = 1.08 min; [M+H]-: 590.24.
501.2 1-[(R)-3-(3-{54(R)-Hydroxy-(4-isopropyl-pheny1)-(3-methyl-azetidin-3-y1)-
methylppyridin-3-y1}41,2,4]oxadiazol-5-
y1)-piperidin-1-ylFethanone hydrochloride salt
Example 501.1 (113 mg) was treated with a solution of HCI in dioxane (4 M, 1.5
mL) and stirred at RT for 1 h. The
reaction mixture was concentrated under vacuo to give the desired product as
an off-white solid (106 mg). LC-MS (A):
tR = 0.72 min; [M+H]-: 490.16.
501.3 1-[(R)-3-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methylkpyridin-3-y1}-
[1,2,4]oxadiazol-5-y1)-piperidin-1-ylPethanone
To a solution of Example 501.2 (89 mg) in dioxane (4.7 mL) was added NEt3 (55
mg), a solution of formaldehyde in
H20 (37 wt. %, 52 mg) and NaBH(OAc)3 (60 mg). The mixture was stirred at RT
for 3 h, before it was diluted with
MeCN/H20, filtered and purified by prep LC-MS (IX) to give the desired product
as an off-white solid (49 mg). LC-MS
(A): tR = 0.73 min; [M-FI-1]': 504.14.
Examples 502 to Example 508 were prepared from D2.2 and the corresponding
carboxylic acids, according to the 3-
step procedure described for Example 501. In case of incomplete conversion in
the first step, more carboxylic and
PyBOP was added, or the reaction mixture was stirred at slightly higher
temperatures (up to 100 C). For Examples
507 and 508, K3PO4 was replaced by molecular sieves (3A). Example 504 was
additionally purified by CC (CombiFlash,
RediSep 4 g, gradient DCM to DCM/Me0H/NH4OH 90:10:1 over 20 min at 18 mL/min)
after the prep LC-MS. Prep LC-
MS conditions (steps 1, 3) and LC-MS data are listed in the table below. The
LC-MS conditions used were LC-MS (A).
Example tR prep LC-MS
prep LC-MS
Name [M+H]-
N (min) (Step 1)
(Step 3)
4-(3-{5-[(R)-(1, 3-Di methyl-azetidin-3-y1)-hydroxy-
502 (4-isopropyl-pheny1)-methyl]-pyridin-3-yly 0.72
479.30 (VI) (VI)
[1,2,4] oxadi azol-5-y1)-tetrahydro-pyran-4-ol
(R)-(1, 3-Di methyl-azetidi n-3-yI)-{5-[5-(3-
hydroxymethyl-bicyclo[1.1.1 ]pent-1-y-
503 0.76 475.29 (VI) (VI)
[1,2,4]oxadiazol-3-4-pyridin-3-y1)-(4-isopropyl-
phenyI)-methanol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
303
4-(3-{54(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-
(4-isopropyl-pheny1)-methyl]-pyridin-3-yly
504 0.72 504.30 (VI) (VD-'-(CC)
[1,2,4]oxadiazol-5-y1)-1-methyl-piperidine-2,6-
dione
2-(3-{5-KR)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-
505 (4-isopropyl-phenyI)-methyl]-pyridin-3-yll- 0.72
459.32 (VI) (VI)
[1,2,4]oxadiazol-5-y1)-2,2-difluoro-ethanol
(R)-(1,3-Dimethyl-azetidin-3-yI)-(4-isopropyl-
506 phenyI)-{5-[5-(3-methoxy-pheny1)-[1,2,4]oxadiazol- 0.85 485.20 (VIII)
(VI)
(R)-(1,3-Dimethyl-azetidin-3-yI)-[5-(5-isopropyl-
507 [1,2,4]oxadiazol-3-y1)-pyridin-3-y1]-(4-isopropyl- 0.80
421.26 (VIII) (VIII)
phenyI)-methanol
(R)-(1,3-Dimethyl-azetidin-3-yI)-(4-isopropyl-
508 pheny1)-{5-[5-(6-methyl-pyrimidin-4-y1)- 0.76
471.35 (XI I) (VI)
[1,2,4]oxadiazol-311]-pyridin-3-yll-methanol
Examples 509 to Example 514 were prepared according to the 3-step procedure
described for Example 501, using
Example D2.2 and the corresponding carboxylic acids indicated in the table
below. Examples 510 and 511 were derived
from the same carboxylic acid (Example 03.16), the corresponding epimeric
mixture after step 1 was separated by prep
chiral SFC (=111). Prep LC-MS conditions (steps 1, 3) and LC-MS data are
listed in the table below. The LC-MS
conditions used were LC-MS (A).
carboxylic prep
LC- prep LC-
Example tR
Name acid [M+1-1]* MS
MS
1\1 (min)
(Step 1) (Step
1) (Step 3)
(R)- or (S)-4-(3-{5-[(R)-(1,3-Dimethyl-
azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
509 03.15 0.68 476.11 (XX) (IX)
methy1]-pyridin-3-y11-[1,2,4]oxadiazol-5-y1)-
1-methyl-pyrrolidin-2-one
(S)- or (R)-4-(3-{5-[(R)-(1,3-Dimethyl- (VD+
azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
chiral
510 D3.16 0.72
490.30 (VI)
methy1]-pyridin-3-y1141,2,41oxadiazol-5-y1)- SFC
(2nd
1-ethyl-pyrrolidin-2-one
eluting)
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
304
(R)- or (S)-4-(3-{5-[(R)-(1,3-Dimethyl-
(VD+
azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
chiral
511 03.16 0.72 490.32
(VI)
methyll-pyridin-3-y1141,2,41oxadiazol-5-y1)- SFC
(1st
1-ethyl-pyrrolidin-2-one
eluting)
(S)- or (R)-4-(3-15-[(R)-(1,3-Dimethyl-
azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
512 D3.17 0.73 504.12 (XX) (IX)
methy1]-pyridin-3-y11-[1,2,4]oxadiazol-5-y1)-
1-isopropyl-pyrrolidin-2-one
(R)- or (S)-4-(3-{5-[(R)-(1,3-Dimethyl-
azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
513 03.18 0.73 504.13 (XX) (IX)
methyll-pyridin-3-y1H1,2,41oxadiazol-5-y1)-
1-isopropyl-pyrrolidin-2-one
1-[(S)-3-(3-{5-[(R)-(1,3-0imethyl-azetidin-3-
ylyhydroxy-(4-isopropyl-pheny1)-methyl]-
514 03.19 0.73 504.34 (VI) (V)
pyridin-3-y1141,2,4]oxadiazol-5-ylmethyl)-
pyrrolidin-1-yI]-ethanone
Example 515 and Example 516 were synthesized according to the 3-step procedure
described for Example 501, using
Example D2.2 and 2-oxopiperidine-4-carboxylic acid. The epimeric mixture after
step 1 was separated by prep chiral
HPLC (XLIII). Prep LC-MS conditions (steps 1, 3) and LC-MS data are listed in
the table below. The LC-MS conditions
used were LC-MS (A).
Example tR prep LC-MS
prep LC-MS
Name [M-FH]+
N (min) (Step 1)
(Step 3)
(S)- or (R)-4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3- 0.67 476.16 (XX)-F
(IX)
515 y1)-hydroxy-(4-isopropyl-phenyl)methyl]-pyridin- chiral
HPLC
3-y1141,2,41oxadiazol-5-y1)-piperidin-2-one (1st
eluting)
(R)- or (S)-4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3- 0.67 476.14 (XX)+
(IX) + (IX)
516 y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-pyridin- chiral
HPLC
3-y1}-[l,2,4]oxadiazol-5-y-piperidin-2-one (2nd
eluting)
Example 517: (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(5-methyl-
[1,2,4]oxadiazol-3-y1)-pyridin-3-y1]-
meth2no1
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
305
517.1 3-{(R)-Hydroxy-(4-isopropyl-phenyl)-15-(5-methyl-[1,2,4]oxadiazol-3-y1)-
pyridin-3-yil-methyl}-3-methyl-azetidine-
1-carboxylic acid tert-butyl ester
To a solution of acetic acid (9.5 mg) in DMS0 (0.25 mL) was added CDI (28 mg).
The mixture was stirred at RT before
a solution of Example D2.2 (60 mg) in DMS0 (0.25 mL) was added. The reaction
was stirred at 85 C for 16 h. It was
then purified by prep LC-MS (VII) to give the desired product as a white solid
(43 mg). LC-MS (A): tR = 1.08 min; [M+H]-:
479.38.
517.2 (R)-(4-lsopropyl-phenyl)-(3-methyl-azetidin-3-y1)45-(5-methyl-[1,
2,4]oxadiazol-3-y0-pyridi n-3-1/1]-m ethanol,
hydrochloride salt
Example 517.1 (43 mg) was treated with a solution of HCI in dioxane (4 M, 0.6
mL) and stirred at RT for 0.5 h. The
reaction mixture was concentrated under vacuo to give the desired product as
yellow amorphous solid (46 mg). LC-MS
(A): tR = 0.71 min; [M+1-1]+: 379.27.
5/7.3 (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-phenyl)-(5-(5-methyl-
[1,2,4]oxadiazol-3-0)-pyridi n-3-ylkmethanol
To a solution of Example 517.2 (46 mg) in dioxane (1 mL) was added NEt3 (55
mg), a solution of formaldehyde in H20
(37 wt. %, 17 mg) and NaBH(OAc)3 (35 mg). The mixture was stirred at RT for 1
h, before it was diluted with MeCN/H20,
filtered and purified by prep LC-MS (VI) to give the desired product as a
white solid (20 mg). LC-MS (A): tR = 0.73 min;
[M+H]: 393.31.
Example 518: (R)-{5-[5-(1,1-Difluoro-ethy1)41,2,4]oxadiazol-3-y1]-pyridin-3-
y11-(1,3-dimethyl-azetidin-3-y1)-(4-
isopropyl-phenylymethanol
The title compound was synthesized from Example D2.2 and 2,2-difluoropropionic
acid, according to the 3-step
procedure described for Example 517. The crude material was purified by Prep
LC-MS (VII) (step 1) and (VI) (step 3).
LC-MS (A): tR = 0.81 min; [M-F1-1]+: 443.33.
Example 519: 4-(5-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y11-
[1,2,4]oxadiazol-3-y1)-tetrahydro-pyran-4-ol
519.1 3-[(R)-Hydroxy-{543-(4-hydroxy-tetra hydro-pyran-4-y1)-[1, 2, il]oxadi
n-3-y1}-(4-isopropyl-pheny0-
methyll-3-methyl-azetidine-1-carboxylic acid tert-butyl ester
To a solution of Example E1.1 (50 mg) in DMF (1 mL) was added CDI (23 mg). The
mixture was stirred for 30 min
before DIPEA (30 mg) was added. Then it was stirred at RT for 16 h. Example
E2.16 (27 mg) was added and the
reaction was heated to 90 C for 3 h, after which molecular sieves (3A, 60 mg)
were added and the mixture was heated
again at 90 C for 14 h. The reaction was cooled to RT, diluted with MeCN/H20,
passed through a syringe filter and
purified by prep LC-MS (VI) to give the desired compound as a white solid (12
mg). LC-MS (A): tR = 1.01 min; [M-F1-1]':
565.40.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
306
519.2 4-(5-{5-[(R)-Hydroxy-(4-isopropyl-phenyl)-(3-methyl-azetidin-3-y1)-
tnethyll-pyridin-3-0-[1,2,4]oxadiazol-3-y1)-
tetrahydro-pyran-4-ol hydrochloride salt
Example 519.1 (12 mg) was treated with a solution of HCI in dioxane (4 M, 1
mL), stirred at RT for 90 min, then
concentrated under vacuo to give the desired product as a white solid (14 mg).
LC-MS (A): tR = 0.67 min; [M-FH]':
465.13.
519.3 4-(5-{5-[(R)-(1,3-Dimethyl-azetidin-3-y0-hydroxy-(4-isopropyl-pheny1)-
methylppyridin-3-441,2,4Joxadiazol-3-
y1)-tetrahydro-pyran-4-ol
To a suspension of Example 519.2 (14 mg) in dioxane (0.2 mL) was added NEt3 (9
mg), a solution of formaldehyde in
H20 (37 wt. %, 9 mg) and NaBH(OAc)3 (10 mg). The mixture was stirred at RT for
90 min. The reaction was diluted
with MeCN/H20, passed through a syringe filter and purified by prep LC-MS (IX
then V) to give the title compound as
a white solid (3 mg). LC-MS (A): tR = 0.67 min; [M+1-1]': 479.38.
Examples 520 to Example 523 were prepared from Example E1.1 and the
corresponding hydroxyamidines (Examples
indicated in the table below) according to the 3-step procedure described for
Example 501, but using one equivalent of
acid (Example E1.1) and 1.5-2.0 equivalents of hydroxyamidine. The applied
hydroxyamidine of formula E2, the LC-
MS retention times and observed masses, as well as the purification methods
used after step 1 and 3 are shown in the
table below. Examples 520 and 521 were generated from the same reaction
sequence. Example 523 was prepared
from Example E1.1 and the corresponding hydroxyamidine (indicated in the
table) according to the procedure described
for Example 519. The LC-MS conditions used were LC-MS (A).
prep LC-MS prep LC-MS
Example hydroxy-
Name tR [M-FH]F Method
Method
N amidine
(Step 1)
(Step 3)
(R)-[5-(3-tert-Butoxymethyl-
[1,2,4]oxadiazol-5-y1)-pyridin-3-y1]-
520 E2.17 0.82 465.22 (VII) (VII)
(1,3-dimethyl-azetidin-3-yI)-(4-
isopropyl-phenyI)-methanol
(R)-(1, 3-Dimethyl-azetid in-3-yI)-[5- (3-
hydroxymethyl-[1, 2, 4]oxadiazol-5-y1)-
521 E2.17 0.65 409.32 (VII) (VII)
pyridin-3-y1]-(4-isopropyl-pheny1)-
methanol
1-[4-(5-{5-[(R)-(1, 3-Di methyl-azetidin-
522 E2.18 0.73 505.42 (VI) (IX)
3-y1)-hydroxy-(4-isopropyl-pheny1)-
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
307
methy1]-pyridin-3-y1141,2,4]oxadiazol-
3-y1)-piperazin-1-y1]-ethanone
143-(5-{5-[(R)-(1, 3-Di methyl-azetidin-
3-y1)-hydroxy-(4-isopropyl-pheny1)-
523 E2.22 0.71 490.40 (XV) (V)
methyll-pyridin-3-y11-[1,2,4]oxadiazol-
3-ylmethyl)-azetidin-1-y1]-ethanone
Example 524: 4-(5-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridin-3-yll-
[1,2, 4]ox2di 2zol-3-ylmethyl)-tetr2hydro-pyran-4-ol
The title compound was synthesized from Example E1.1 and the corresponding
hydroxyamidine (Example E2.20)
according to the 3-step procedure described for 517, but using 1 equivalent of
acid and 1.5 equivalents of
hydroxyamidine. Prep LC-MS was used to purify the intermediate after step 1
(Method VI) and the final product after
step 3 (Method V). LC-MS (A): tR = 0.68 min; [M+1-1]': 493.39.
Example 525: [4-(5-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethylLpyridin-3-y11-
[1,2,4]oxadiazol-3-y1)-piperidin-1-y1]-[1,4]dioxan-2-yl-methanone
525.1 5-[(R)-Hydroxy-(4-isopropyl-phenyl)-(3-methyl-azetidin-3-yI)-methyll-
nicotinic acid, hydrochloride salt
Example E1.1 (1.20 g) was treated with a solution of HCI in dioxane (4 M, 14
mL). The mixture was stirred at RT for 1
h. The suspension was filtered, the residue was washed with dioxane, then
dried under HV to give the desired
compound as a white solid (1.13g). LC-MS (A): tR = 0.67 min; [M-F11-: 341.22.
525.2 5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-phenyl)-
methylrnicotinic acid, formic acid salt
To a suspension of Example 525.1 (1.13 g) in dioxane (27 mL) were added NEt3
(1.14 mL), a solution of formaldehyde
in H20 (37 wt. %, 0.32 mL) and NaBH(OAc)3 (895 mg). The mixture was stirred at
RT for 90 min, filtered and directly
purified by prep LC-MS (IX) to give the title compound as a white solid (142
mg). LC-MS (A): tR = 0.59 min; [M+I-1]-:
355.25.
525.3 4-(5-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylkpyridin-3-4-[1,2,4]oxadiazol-3-
yl)-piperidine-1-carboxylic acid tert-butyl ester
To a solution of Example 525.2 (140 mg) in DMF (3.2 mL) were added DIPEA (0.17
mL) and PyBOP (236 mg). After
stirring at RT for 15 min, a solution of E2.19 (170 mg) in DMF (0.9 mL) and
K3PO4 (282 mg) were added. The mixture
was stirred at 85 C for 16 h. After cooling to RT, more PyBOP (259 mg), K3PO4
(282 mg) and DIPEA (0.17 mL) were
added and the reaction was stirred at 85 C for another 4 h. The mixture was
cooled to RT, diluted with MeCN/H20,
passed through a syringe filter and purified by prep LC-MS (Method VIII) to
give the desired product as a white solid
(88 mg). LC-MS (A): tR = 0.88 min; [WEN': 562.35.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
308
525.4 (R)-(1,
methanol
Example 525.3 (88 mg) was treated with a solution of HCI in dioxane (4 M, 1.2
mL) and stirred for 1 h. The reaction
mixture was concentrated under vacuo to give the title compound as a pale
yellow solid (102 mg). LC-MS (A): tR = 0.56
min; m/z [M+2H+MeCN]2: 252.35.
525.5 [4-(5-{5-[(R)-(1, 3-Dim ethyl-azetidi n-3-y0-hydroxy-(4-isopropyl-pheny0-
methylppyridi n-3-yI}-[1, 2, il]oxadiazol-3-
y1)-pPeridin-1-y1H1,41dioxan-2-yl-methanone
To a solution of Example 525.4 (50 mg) in DMF (0.36 mL) was added DBU (51 mg)
and a premixed (10 min) solution
of 1,4-dioxane-2-carboxylic acid (23 mg), HATU (72 mg) and DBU (20 mg) in DMF
(0.36 mL) at 0 C. The mixture was
stirred at 0 C for 1 h. The reaction was then diluted with MeCN/H20, passed
through a syringe filter and purified by
prep LC-MS (V then IX) to give the title compound as a white solid (25 mg). LC-
MS (A): tR = 0.73 min; [M+H]*: 576.41.
Example 526: 144-(5-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyl]-pyridin-3-y11-
[1,2,4]oxadiazol-3-y1)-piperidin-1-y1]-2-methoxy-ethanone
526.1 Methoxy-acetic acid (R)-(1,3-dimethyl-azetidin-3-y1)-(4-isopropyl-
phenyl)-(5-{341-(2-methoxy-acety1)-piperidin-4-
y1H1,2,4]oxadiazol-5-4-pyridin-3-y0-methyl ester
The title compound was synthesized from Example 525.4 and methoxyacetic acid,
according to the procedure described
for Example 525 (step 5). LC-MS (A): tR = 0.74 min; [M-FH]+: 606.44.
526.2 1-14-(5-{5-[(R)-(1,3-Dimethyl-azetidin-3-y0-hydroxy-(4-isopropyl-pheny0-
methylppyridin-3-y1}41,2,4i0xadiaz01-3-
y1)-piperidin-1-y!]-2-methoxy-ethanone, formic acid salt
To a solution of Example 526.1 (59 mg) in Me0H was added K2003 (128 mg) and
the mixture was stirred at 4500 for
2 h. The reaction was cooled to RT, filtered, diluted with MeCN/H20, passed
through a syringe filter and purified by
prep LC-MS (V then IX) to give the title compound as a white solid (30 mg). LC-
MS (A): tR = 0.72 min; [M-FH]-: 534.45.
Example 527: (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{543-(1-
methanesulfonyl-piperidin-4-y1)-
[1,2,4]oxadiazol-5-y1]-pyridin-3-yll-methanol
To a solution of Example 525.4 (51 mg) and DIPEA (46 mg) in DCM (1.5 mL) at 0
C was added methanesulfonyl
chloride (10 mg). The mixture was stirred at 0 00 for 90 min. The solvent was
then removed under vacuo, the residue
was purified by prep LC-MS (IX) to give the desired product as a white solid
(22 mg). LC-MS (A): tR = 0.75 min; [M+H]:
540.36.
Example 528: (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(5-{3-[1-(2-
methoxy-ethanesulfony1)-piperidin-4-
y1]-[1,2,4]oxadiazol-5-yll-pyridin-3-y1)-methanol
Example 528 was synthesized from Example 525.4 and 2-methoxyethane-1-sulfonyl
chloride according to the
procedure described for Example 527. LC-MS (A): tR = 0.78 min; [M+H]+: 584.38.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
309
Example 529: 144-(5-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyll-pyridin-3-y11-
[1,2,4]oxadiazol-3-y1)-piperidin-1-y1]-2-hydroxy-ethanone
529.1 2-(tert-Butyl-diphenyl-silanyloxy)-144-(5-{5-[(R)-(1,3-dimethyl-azetidin-
3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyil-pyridi n-3-y1141 , 2, 4]oxadiazol-3-y1)-piperidi n-1 -y1]-ethanone
To a solution of Example 525.2 (45 mg) in DMF (0.56 mL) were added DIPEA (44
mg) and PyBOP (182 mg). After
stirring at RT for 15 min, a solution of E2.21 (74 mg) in DMF (0.5 mL) and
K3PO4 (97 mg) were added and the reaction
was stirred at 85 C for 16 h. More PyBOP (91 mg), K3PO4 (50 mg) and DIPEA (44
mg) were added and the mixture
was stirred at 100 C for 5 h. The reaction was cooled to RT, diluted with
MeCN/H20, passed through a syringe filter
and purified by prep LC-MS (2x Method XX) to give the desired product as a
white solid (10 mg). LC-MS (A): tR = 1.02
min; [M+H]: 758.52.
529.2 144-(5-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyil-pyridin-3-y1}41,2,4Joxadiazol-3-
y0-piperidin-1-yil-2-hydroxy-ethanone
To a solution of Example 529.1 (10 mg) in THE (0.2 mL) was added a solution of
TBAF in THE (1.0 M, 0.02 mL). The
reaction mixture was stirred at RT for 90 min, then diluted with MeCN/H20,
passed through a syringe filter and purified
by prep LC-MS (V then IX) to give the title compound as a white solid (1.5
mg). LC-MS (A): tR = 0.69 min; [M-+I]':
520.39.
Example 530: (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-
(541,2,4]oxadiazol-3-yl-pyridin-3-y1)-methanol
530.1 3-[(R)-Hydroxy-(4-isopropyl-pheny1)-(511,2,4]oxadiazol 3 yi pyridin-3-y0-
methy1]-3-methyl-azetidine-1-
carboxylic acid tert-butyl ester
To a solution of Example D2.2 (50 mg) in DMA (0.5 mL) was added trimethyl
orthoformate (0.11 mL) and boron
trifluoride diethyl etherate (4.6 mg). The reaction mixture was stirred at 50
00 for 2.5 h. MeCN was added and the
mixture was passed through a syringe filter and purified by prep LC-MS (VII)
to give the desired product as a white solid
(32 mg). LC-MS (A): tR = 1.06 min; [M-F1-1]': 465.34.
530.2 (R)-(4-lsopropyl-phenyl)-(3-methyl-azetidin-3-y0-(541,2,4]oxadiazol-3-yl-
pyridin-3-y1)-methanol
Example 530.1 (32 mg) was treated with a solution of HCI in dioxane (4 M, 0.3
mL) and stirred at RT for 30 min. The
solvent was evaporated and the residue dried under HV to give the desired
product as a white solid (39 mg). LC-MS
(A): tR = 0.69 min; [M-F1-1]+: 365.18.
530.3 (R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-(541,2,4]oxadiazol-
3-yl-pyridin-3-y1)-methanol
To a solution of Example 530.2 (39 mg) in dioxane (1 mL) was added NEt3 (10
mg), a solution of formaldehyde in H20
(37 wt. %, 16 mg) and NaBH(OAc)3 (32 mg). The reaction was stirred at RT for
1.5 h. The mixture was filtered and
concentrated under vacuo, the crude was purified by prep LC-MS (VI) to give
the title compound as a white solid (29
mg). LC-MS (A): tR = 0.71 min; [M+H]: 379.26.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
310
Example 531: 144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyll-pyridin-3-y11-
[1,2,4]oxadiazol-5-y1)-piperidin-1-y1]-ethanone
531.1 31(R)-{515-(1-Acetyl-piperidin-4-y1)41,2,4]oxadiazol-3-yil-pyridin-3-01-
hydroxy-(4-isopropyl-pheny1)-methyl]-3-
methyl-azetidine-1-carboxylic acid tert-butyl ester
To a solution of Example D2.2 (1.7 g) in DMF (34mL) were added 1-
acetylpiperidine-4-carboxylic acid (967 mg),
followed by PyBOP (4.57 g), DIPEA (1.96 mL) and K3PO4 (3.23 g). The resulting
solution was stirred at 85 C overnight.
Additional 0.5 equivalent of reagents were added and the resulting mixture was
stirred at 85 C overnight. The reaction
mixture was allowed to cool down to RT, filtered off, and evaporated in vacuo.
The crude material was purified by Prep
LC-MS (XXXV) to give the title compound as a white solid (1.76 g). LC-MS (A)
tR = 1.08 min; [M+H]': 590.13.
531.2 144-(3-{5-[(R)-Hydroxy-(4-isopropyl-pheny1)-(3-methyl-azetidin-3-0)-
methyil-pyridin-3-411,2,41oxadiazol-5-y1)-
piperidin-1-ylkethanone
A solution of Example 531.1 (1.76 g) in HCI 4M in dioxane (35 mL) was stirred
at RT for lh. The reaction mixture was
evaporated in vacuo and dried in HV to afford 1.57 g as yellow solid. LC-MS
(A) tR = 0.72min; [M+H]': 490.2.
53/.3 1-14-(3-{5-[(R)-(1 ,3-Dimethyl-azetidi n-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methylkpyridin-3-y11-11,2,4Joxadiazol-5-
y1)-piperidin-1-ylrethanone
The title compound was prepared starting from Example 531.2 (1.57 g), and
following the procedure described in
Example D1.1 step D1.1.5. The crude material was purified by Prep LC-MS
(XXXVI) followed by Prep LC-MS (XXXVII)
to give the title compound as a white solid (900 mg). LC-MS (A): tR = 0.72
min; [M+H]: 504.12.
Example 532 to Example 566 were synthesized starting from the appropriate
precursor of Formula (A7) or (05) and
following the procedure described in Example 1, except that TEA was used to
neutralize the hydrochloride salt and
DCM was replaced by dioxane. Prep LC-MS conditions and LC-MS data of Example
532 to Example 566 are listed in
the table below. The LC-MS conditions used were LC-MS (A).
Example Prep LC-
Precursor
Name tR [M+H]-
N MS
A7 or D5
2-(3-{5-[(R)-(4-Bromo-pheny1)-(1,3-dimethyl-azetidin-3-
532 y1)-hydroxy-methyl]-pyridin-3-y1)-[1, 2, 4]oxadi azol-5-y1)-
0.65 472.96 V 05.1
propan-2-ol
2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-
533 naphthalen-2-yl-methy1]-pyridin-3-y1141,2,4]oxadiazol-5-
0.67 445.10 A7.4
yI)-propan-2-ol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
311
243-(5-{(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-[4-(1-
534 trifluoromethyl-cyclopropy1)-phenyl]-methyll-pyridin-3-y1)- 0.72 503.04 VI
A7.5
[1,2,4]oxadiazol-5-y1]-propan-2-ol
243-(5-{(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-[4-
535 (2,2,2-trifluoro-1,1-dimethyl-ethyl)-pheny1]-methyly 0.72
505.01 VI A7.6
pyridin-3-041,2,4]oxadiazol-511]-propan-2-ol
2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
536 isopropoxy-pheny1)-methyll-pyridin-3-yll- 0.66 453.28
V A7.7
[1,2,4]oxadiazol-5-y1)-propan-2-ol
243-(5-{(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-[4-
537 (1,2,2,2-tetrafluoro-1-trifluoromethyl-ethyl)-phenyl]-
0.76 563.00 VI D5.2
methyll-pyridin-3-041,2,4]oxadiazol-511]-propan-2-ol
(R)-2-(3-(5-((1,3-dimethylazetidin-3-yI)(hydroxy)(4-
538 (pentafluoro-2c6-sulfaneyl)phenyl) methyl)pyridin-3-yI)- 0.69 521.00 V
D5.3
1,2,4-oxadiazol-5-yl)propan-2-ol
2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-(3-fluoro-4-
539 isopropyl-phenyI)-hydroxy-methy1]-pyridin-3-yll- 0.71
455.00 VI A7.8
[1,2,4]oxadiazol-5-y1)-propan-2-ol
2-(3-{5-[(R)-(4-tert-Butyl-pheny1)-(1,3-dimethyl-azetidin-
540 3-y1)-hydroxy-methy1]-pyridin-3-y1}-[1,2,4]oxadiazol-5-y1)-
0.70 451.17 VI A7.9
propan-2-ol
2-(3-{5-[(R)-Benzo[b]thiophen-5-y1-(1,3-dimethyl-
541 azetidin-3-y1)-hydroxy-methy1]-pyridin-3-yll- 0.64
451.09 V A7.10
[1,2,4]oxadiazol-5-y1)-propan-2-ol
2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
542 pentafluoroethyl-phenyl)-methyl]-pyridin-3-yll- 0.71
512.91 VI A7.11
[1,2,4]oxadiazol-5-y1)-propan-2-ol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
312
243-(5-{(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-[4-(1-
543 methyl-cyclopropy1)-phenylFmethyll-pyridin-3-y1)- 0.69
449.04 V A7.12
[1,2,4]oxadiazol-5-y1]-propan-2-ol
2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
544 isopropy1-3-methyl-pheny1)-methyl]-pyridin-3-yll- 0.71
451.19 VI A7.13
[1,2,4]oxadiazol-5-y1)-propan-2-ol
2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
545 trifluoromethoxy-pheny1)-methyl]-pyridin-3-y11- 0.67
479.10 V A7.14
[1,2,4]oxadiazol-5-y1)-propan-2-ol
144-(3-{5-[(R)-(4-Bromo-pheny1)-(1,3-dimethyl-azetidin-
546 3-y1)-hydroxy-methy1]-pyridin-3-y1)-[1,2,4]oxadiazol-5-y1)-
0.68 539.97 V D5.4
piperidin-1-yI]-ethanone
243-(5-{(R)-(1,3-Dimethyl-azetidin-3-y1)-[4-(1-fluoro-1-
547 methyl-ethyl)-pheny1]-hydroxy-methyl}-pyridin-3-y1)- 0.65
455.17 V A7.15
[1,2,4]oxadiazol-5-y1]-propan-2-ol
2-(3-{5-[(R)44-(1,1-Difluoro-ethyl)-phenyl]-(1,3-dimethyl-
548 azetidin-3-y1)-hydroxy-methyl]-pyridin-3-y1}- 0.64
459.15 V A7.16
[1,2,4]oxadiazol-5-y1)-propan-2-ol
2-(3-{5-[(R)-(4-Bicyclo[1.1.1]pent-1-yl-pheny1)-(1,3-
549 dimethyl-azetidin-3-0-hydroxy-methyl]-pyridin-3-y11- 0.73
461.21 VI A7.17
[1,2,4]oxadiazol-5-y1)-propan-2-ol
(R)-(1,3-Dimethyl-azetidin-3-y1)-{545-(tetrahydro-pyran-
550 4-y1)41,2,4]oxadiazol-311]-pyridin-3-y1H4-(2,2,2- 0.71
503.14 V D5.5
trifluoro-ethyp-phenyl]-methanol
243-(5-{(R)-(1,3-Dimethyl-azetidin-3-y1)44-(1,1-dimethyl-
551 propy1)-phenyl]-hydroxy-methyl}-pyridin-3-y1)- 0.77
465.07 VII A7.18
[1,2,4]oxadiazol-5-y1]-propan-2-ol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
313
144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-(3-fluoro-4-
552 isopropyl-phenyI)-hydroxy-methy1]-pyridin-3-yll- 0.74
522.11 VI A7.19
[1,2,4]oxadiazol-5-y1)-piperidin-1-y1]-ethanone
1-{4-[3-(5-{(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-[4-
553 (1-trifluoromethyl-cyclopropy1)-phenyTmethylypyridin-3- 0.75 570.12 V
A7.20
y1)41,2,4]oxadiazol-5-y1]-piperidin-1-yll-ethanone
1-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
554 trifluoromethoxy-pheny1)-methyll-pyridin-3-yll- 0.72
545.82 V A7.21
[1,2,4]oxadiazol-5-y1)-piperidin-1-y1]-ethanone
144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
555 isopropoxy-phenylymethy1]-pyridin-3-yll- 0.70 520.10
V A7.22
[1,2,4]oxadiazol-5-y1)-piperidin-1-y1]-ethanone
2-(3-{5-[(R)-(3-Chloro-4-isopropyl-pheny1)-(1,3-dimethyl-
556 azetidin-3-y1)-hydroxy-methy1]-pyridin-3-yll- 0.75
471.16 VI A7.23
[1,2,4]oxadiazol-5-y1)-propan-2-ol
2-(3-{5-[(R)-(4-Cyclobutyl-pheny1)-(1,3-dimethyl-azetidin-
557 3-y1)-hydroxy-methy1]-pyridin-3-y1}-[1,2,4]oxadiazol-5-y1)-
0.73 449.07 VI A7.24
propan-2-ol
144-(3-15-[(R)-(4-Cyclobutyl-pheny1)-(1,3-dimethyl-
558 azetidin-3-y1)-hydroxy-methyl]-pyridin-3-yll- 0.76
516.20 VI A7.25
[1,2,4]oxadiazol-5-y1)-piperidin-1-y1Fethanone
144-(3-{5-[(R)-(3,5-Difluoro-4-isopropyl-pheny1)-(1,3-
559 dimethyl-2zetidin-3-y1)-hydroxy-methyl]-pyridin-3-yll-
0.76 540.18 VI A7.26
[1,2,4]oxadiazol-5-y1)-piperidin-1-y1]-ethanone
2-(3-{5-[(R)-(3,5-Difluoro-4-isopropyl-phenyI)-(1,3-
560 dimethyl-azetidin-3-y1)-hydroxy-methyl]-pyridin-3-yly
0.74 473.18 VI A7.27
[1,2,4]oxadiazol-5-y1)-propan-2-ol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
314
144-(3-{5-[(R)-(4-Cyclobutoxy-pheny1)-(1,3-dimethyl-
561 azetidin-3-y1)-hydroxy-methyl]-pyridin-3-yll- 0.72
532.12 VI A7.28
[1,2,4]oxadiazol-5-y1)-piperidin-1-y1]-ethanone
2-[3-(5-{(1,3-Dimethyl-azetidin-3-y1)-[4-(1-ethyl-propy1)-
562 pheny1]-hydroxy-methyll-pyridin-3-y1)-[1 ,2,4]oxadiazol-5-
0.78 465.07 VI A7.29
yq-propan-2-ol
1-{443-(5-{(1,3-Dimethyl-azetidin-3-y1)44-(1-ethyl-
563 propy1)-phenyll-hydroxy-methyll-pyridin-3-y1)- 0.81
532.23 VI A7.30
[1,2,4]oxadiazol-5-y1]-piperidin-1-ylyethanone
243-(5-{(1,3-Dimethyl-azetidin-3-y1)44-(2,2-dimethyl-
564 propy1)-pheny1]-hydroxy-methyll-pyridin-3-y1)- 0.77
465.04 VI A7.31
[1,2,4]oxadiazol-5-y1]-propan-2-ol
1-14-[3-(5-{(1,3-Dimethyl-azetidin-3-y1)-[4-(2,2-dimethyl-
565 propy1)-phenylFhydroxy-methyll-pyridin-3-y1)- 0.78
532.16 VII A7.32
[1,2,4]oxadiazol-511]-piperidin-1-yll-ethanone
1-{443-(5-{(1,3-Dimethyl-azetidin-3-y1)-hydroxy-[4-(1-
566 methyl-cyclopropy1)-pheny1]-methyl}-pyridin-3-y1)- 0.74
516.22 VI A7.33
[1,2,4]oxadiazol-5-y1]-piperidin-1-ylyethanone
Example 567 to Example 577 were synthesized starting from the appropriate
precursor of Formula (J1), (J3) or (J5)
and the appropriate precursor of Formula (J2), (J4) or (J6), respectively,
following the method as indicated in the table
below. Prep LC-MS conditions and LC-MS data of Example 567 to Example 577 are
listed in the table below. The LC-
MS conditions used were LC-MS (A).
Method A
A mixture of compound (J1) (1 eq), boron species (J2) (1.3 to 1.6 eq),
cataCXium A Pd G3 (0.05 to 0.1 eq) and Cs2CO3
(3 eq) in a mixture of toluene (5 mL/mmol) and water (0.5 mL/mmol) was flushed
with argon, heated at 100 C in a
sealed vial and stirred for 18h. It was filtered over Celite, the cake was
washed with EA and the filtrate was concentrated
in vacuo. The crude was purified by Prep LC-MS (see method in the Table below)
and/or CC using SNAP KPNHTM
prepacked cartridges from Biotage and eluting with EA/Me0H or DCM/Me0H.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
315
Method B
A mixture of boronic ester (J3) (1 eq), copper(I) reagent (J4) (1.2 eq) and KF
(1 eq) in DMF (10 mUmmol) was heated
at 50 C in a sealed vial and stirred for 18h. It was filtered over Celite, the
cake was washed with EA and the filtrate was
concentrated in vacuo. The crude was purified by Prep LC-MS (see method in the
Table below) and/or CC using SNAP
KP-NHTm prepacked cartridges from Biotage and eluting with EA/Me0H or
DCM/Me0H.
Method C
To a solution of phenol (J5) (1 eq) in DMF (20 mL/mmol) was added at RT,
halide (J6) (5 eq) and K2003 (2.4 eq). The
mixture was heated at 100 C and stirred for 40h. It was partitioned between EA
and half sat. NaHCO3. The aq. phase
was extracted with EA/Me0H 95/5 and the combined org. phases were dried over
MgSO4 and concentrated in vacuo.
The crude was purified by Prep LC-MS (see method in the Table below) and/or CC
using SNAP KPNHTM prepacked
cartridges from Biotage and eluting with EA/Me0H or DCM/Me0H.
Prep Precursor
Example
Precursor
Name tR [M+H] LC-
(J1), (J3) Method
N (J2),
(J4) or (J6)
MS or (J5)
2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-
3-y1)-hydroxy-(4-propyl-pheny1)- Example Potassium
567 0.70 437.09 V propyl
A
methyl]-pyridin-3-yll- 532
trifluoro borate
[1,2,4]oxadiazol-5-y1)-propan-2-ol
2-(3-{5-[(R)-(4-Cyclopropyl-
Potassiurn
phenyI)-(1,3-dimethyl-azetidin-3- Example
568 0.66 435.10 V
cyclopropyl A
yI)-hydroxy-methyl]-pyridin-3-yll- 532
trifluoro borate
[1,2,4]oxadiazol-5-y1)-propan-2-ol
2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-
3-y1)-(4-ethyl-pheny1)-hydroxy- Example Ethyl
boronic
569 0.66 423.11 V
A
methyll-pyridin-3-y11- 532 acid
[1,2,4]oxadiazol-5-y1)-propan-2-ol
2-(3-{5-[(R)-(4-Butyl-pheny1)-(1,3-
dirnethyl-azetidin-3-yI)-hydroxy- Example Butyl
boronic
570 0.74 451.13 VI
A
methyl]-pyridin-3-yll- 532 acid
[1,2,4]oxadiazol-5-y1)-propan-2-ol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
316
2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-
3-yI)-hydroxy-(4-trifluoromethyl-
571 0.66 463.05 V J3.1 (Phen)CuCF3
phenyI)-methy1]-pyridin-3-yll-
[1,2,4]oxadiazol-5-y1)-propan-2-ol
4,4,5,5-
2-[3-(5-{(R)-(1,3-Dimethyl-azetidin-
tetramethy1-2-
3-yI)-hydroxy-[4-(2,2,2-trifluoro- Example
572 0.67 477.06 V (2,2,2-
trifluoro A
ethyl)-phenyll-methy1}-pyridin-3-y1)- 532
ethyl)-1,3,2-
[1,2,4]oxadiazol-5-y11-propan-2-ol
dioxaborolane
2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-
3-y1)-hydroxy-(4-isobutyl-pheny1)- Example
Isobutyl boronic
573 0.73 451.14 VI
A
532 acid
[1,2,4]oxadiazol-5-y1)-propan-2-ol
2-(3-{5-[(R)-(4-Cyclobutoxy-
phenyI)-(1,3-dimethyl-azetidin-3-
Bromo
574 0.69 464.99 VI J5.1
y1)-hydroxy-methyl]-pyridin-3-yll-
cyclobutane
[1,2,4]oxadiazol-5-y1)-propan-2-ol
2-(3-{5-[(R)-(1,3-Dimethyl-azetidin-
3-y1)-hydroxy-(4-isopropenyl- Example (Prop-1-
en- 2-y1)
575 0.67 435.16 V
A
phenyI)-methy1]-pyridin-3-yll- 532 boronic
acid
[1,2,4]oxadiazol-5-y1)-propan-2-ol
1-{4-[3-(5-{(R)-(1,3-Dimethyl-
4,4,5,5-
azetidin-3-yI)-hydroxy-[4-(2,2,2- Example
tetramethy1-2-
576 trifluoro-ethyl)-phenyl]-methyl}- 0.71 544.02 V (2,2,2-
trifluoro A
546
pyridin-3-y1)41,2,4]oxadiazol-511]- ethyl)-
1,3,2-
piperidin-1-ylyethanone
dioxaborolane
144-(3-{5-[(R)-(1,3-Dimethyl-
azetidin-3-yI)-hydroxy-(4-propyl-
Propyl boronic
577 pheny1)-methyll-pyridin-3-yll- 0.73 504.21 VI
546 A
acid
[1,2,4]oxadiazol-5-y1)-piperidin-1-
yI]-ethanone
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
317
Example 578 144-(3-{5-[(R)-Hydroxy-(1-isopropy1-3-methyl-azetidin-3-y1)-(4-
isopropyl-pheny1)-methyll-pyridin-3-yll-
[1 ,2,4]oxadiazol-5-y1)-piperidin-111]-ethanone
Example 531.2 (40mg) was dissolved in dioxane (881pL), then acetone (23.5pL)
and sodium triacetoxyborohydride
(35.7mg) were added and the mixture was stirred at RT for 15min. The reaction
mixture was taken up in DCM, quenched
with aq. sat. NaHCO3. The phases were separated with a phase separator,
evaporated and purified by prep LC-MS
(VI), then by CC (Biotage, 11g KP-NH, solvent A: Hept, solvent B: EA, gradient
(in %B): 25 for 1CV, 15 to 100 over
4CV, 100 for 9CV) to give 5mg colorless glassy oil. LC-MS (A) tR = 0.78min; [M-
FH]t 532.22.
Example 579 144-(3-15-[(R)41-(2,2-Difluoro-ethyl)-3-methyl-azetidin-3-y1]-
hydroxy-(4-isopropyl-phenylymethyl]-
pyridin-3-y1141,2,41oxadiazol-5-y1)-piperidin-1-y11-ethanone
Example 531.2 (32mg) was dissolved in Me0H (0.256pL), then TEA (44.6pL) and 2-
lodo-1,1-difluoroethane (11.5pL)
were added. The mixture was shaken at 60 C for 6 days, while 2x 2-iodo-1,1-
difluoroethane (11.5pL, 23pL) were
added after 4h and 22h. The mixture was filtrated through a syringe filter,
diluted with Me0H and purified by prep LC-
MS (VI) to give 20mg off-white foam. LC-MS (A) tR = 0.76min; [M+H]: 554.10
Example 580 144-(3-{5-[(R)-Hydroxy-[1-(2-hydroxy-ethyl)-3-methyl-azetidin-3-
y1]-(4-isopropyl-pheny1)-methyl]-pyridin-
3-y1}-[l,2,4]oxadiazol-5-y-piperidin-111]-ethanone
580.1 144-(3-{5-[(R)-{1-12-(tert-Butyl-dimethyl-silanyloxy)-ethy1]-3-methyl-
azetidin-3-4-hydroxy-(4-isopropyl-pheny1)-
methylTpyridin-3-0111,2,41oxadiazol-5-y1)-piperidin-1-ylrethanone
Example 531.2 (50mg) and Cs2003 (65.2g) were taken up in DMF (500pL), then (2-
bromoethoxy)-tert-
butyldimethylsilane (33.1pL) was added. The mixture was shaken at 70 C for
1h40, filtrated through a syringe filter,
diluted with DMF and purified by prep LC-MS (VIII) to give 29mg off-white
foam. LC-MS (A) tR = 0.97min; [M+1-1]-:
648.34.
580.2 1-14-(3-{5-[(R)-Hydroxy41-(2-hydroxy-ethy0-3-methyl-azetidin-3-
y1]-(4-isopropyl-pheny0-methyll-pyridin-3-34}-
[7,2,4]oxadiazol-5-0)-piperidin-1-ylPethanone
Example 580.1 (29mg) was dissolved in THF (500pL), cooled to 0 C and then TBAF
1M in THF (102pL) was added,
the mixture was stirred for 1h at RT. The mixture was taken up in EA, washed
with aq. sat. NaHCO3 and water. The
org. layer was washed with brine, dried over MgSO4, filtrated, concentrated
and purified by prep LC-MS (V) to give
11mg beige glassy oil. LC-MS (A) tR 0.72min; [M-FI-1]': 534.13.
Example 581 to Example 585 were synthesized following the procedure described
in Example F1.1 step F1.1.2 starting
from Example 531.2 and using the appropriate commercially available aldehyde
and purified by prep LC-MS and CC
(Biotage, 11g SPHERE Amino, solvent A: Hept, solvent B: EA), if necessary. The
purification conditions and the LC-
MS (A) data are listed in the table below.
Example Prep CC gradient (in
Name tR
[M+H]
N LC-MS %B)
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
318
1-[4-(3-{5-[(R)-(1-Cyclopropylmethy1-3-methyl-
azetidin-3-y1)-hydroxy-(4-isopropyl-phenyly
581 (VI) 0.79 544.15
methy1]-pyridin-3-yly[1,2,4]oxadiazol-5-y1)-
piperidin-1-yI]-ethanone
1-{443-(5-{(R)-Hydroxy-(4-isopropyl-pheny1)41-(2-
methoxy-ethyl)-3-methyl-azetidin-3-y1]-methyll-
582 (VI) 0.76 548.13
pyridin-3-y1)-[1,2,4]oxadiazol-5-y11-piperidin-1-yll-
ethanone
1-{443-(5-{(R)-Hydroxy-(4-isopropyl-pheny1)43-
methyl-143, 3,3-trifluoro-propy1)-azetidin-3-y11-
583 (VI) 0.82 586.24
methyll-pyridin-3-y1)41,2,41oxadiazol-5-y11-
piperidin-1-yll-ethanone
144-(3-{5-[(R)-Hydroxy-(4-isopropyl-pheny1)-(3- 25 for 1CV, 25 to
584 methyl-1-propyl-azetidin-3-y1)-methyl]-pyridin-3-
(VI) 100 over 4CV, 100 0.79 532.23
yll-[l,2,4]oxadiazol-5-y1)-piperidin-111]-ethanone for 4CV
1-[4-(3-{5-[(R)-(1-Ethy1-3-methyl-azetidin-3-y1)- 25 for 1CV, 25 to
585 hydroxy-(4-isopropyl-phenylymethyl]-pyridin-3-yll-
(VI) -- 100 over 4CV, 100 0.77 -- 518.17
[1,2,4]oxadiazol-5-y1)-piperidin-1-y1]-ethanone for 19CV
Example 586 to Example 635 were synthesized starting from the corresponding
Example F1.2 and the appropriate
alkyne, and following the procedure described in Example 15. The precursor
alkyne is indicated in the table below
unless commercially available. Prep LC-MS conditions and LC-MS data are listed
in the table below. The LC-MS
conditions used were LC-MS (A).
Prep
Example N Name tR [M+H]
Alkyne
LC-MS
(VI)
1-(4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
586
0.72 460.18 F5.54 then
pheny1)-methyl]-pyridin-3-ylethynyll-piperidin-1-y1)-ethanone
(IX)
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
587 pheny1)-methy1]-pyridin-3-y11-1,1,1-trifluoro-2-methyl-but-3-yn-
0.72 447.13 F5.59 (XIV)
2-01
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
319
Cyclopropanecarboxylic acid (3-15-[(R)-(1, 3-dimethyl-azetidin-
(XIV)
588 3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-pyridin-3-y11-1,
1- 0.71 460.16 F5.60 then
dimethyl-prop-2-ynyI)-amide
(VII)
N-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
(IX)
589 pheny1)-methy1]-pyridin-3-y11-1,1-dimethyl-prop-2-yny1)-
0.73 462.18 F5.61 then
isobutyramide
(VII)
N-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
(XIV)
590 pheny1)-methy1]-pyridin-3-01-1,1-dimethyl-prop-2-ynyl)-2-
0.69 464.14 F5.62 then
methoxy-acetamide
(VI)
3-(3-{5-[(R)-(1, 3-Di methyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
(VI)
591 pheny1)-methyll-pyridin-3-y11-1,1-dimethyl-prop-2-yny1)-
0.72 462.17 F5.63 then
oxazolidin-2-one
(VII)
1-(3-{5-[(R)-(1, 3-Di methyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
(VI)
592 pheny1)-methy1]-pyridin-3-01-1,1-dimethyl-prop-2-ynyly
0.73 460.18 F5.64 then
pyrrolidin-2-one
(IX)
1-(3-{5-[(R)-(1, 3-Di methyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
(XIV)
593 phenylymethy1]-pyridin-3-y11-1,1-dimethyl-prop-2-yny1)-3-
0.72 475.17 F5.66 then
methyl-imidazolidin-2-one
(VI)
1-(3-{5-[(R)-(1, 3-Di methyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
(XIV)
594 pheny1)-methy1]-pyridin-3-01-1,1-dimethyl-prop-2-ynyl)-
0.69 461.18 F5.65 then
imidazolidin-2-one
(VI)
3-(1-{5-[(R)-(1, 3-Di methyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
(XIV)
595 pheny1)-methyll-pyridi n-3-ylethynyll-cyclopropyI)-oxazol
idi n-2- 0.69 460.14 F5.67 then
one
(VI)
1-((R)-2-{5-[(R)-(1, 3-Di methyl-azetidin-3-yI)-hydroxy-(4-
(IX)
596 isopropyl-pheny1)-methy1]-pyridin-3-ylethyny11-2-methyl-
0.73 460.17 F5.55 then
pyrrolidin-1-yI)-ethanone
(VI)
1-(4-{5-[(R)-(1, 3-Di methyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
(V) then
597 pheny1)-methy1]-pyridin-3-ylethyny11-4-hydroxy-piperidin-1-
y1)- 0.65 476.16 F5.31
(IX)
ethanone
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
320
1-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
(IX)
598 isopropyl-pheny1)-methy1]-pyridin-3-yII-1-hydroxy-1-methyl-
-- 0.69 504.15 F5.32
then (V)
prop-2-yny1)-piperidin-1-y1]-ethanone
144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
(IX)
599 isopropyl-pheny1)-methy1]-pyridin-3-y11-1-hydroxy-prop-2-
yny1)- 0.67 490.16 F5.33
then (V)
piperidin-1-yll-ethanone
144-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
600 isopropyl-pheny1)-methy1]-pyridin-3-yll-prop-2-yny1)-
piperidin-1- 0.73 474.18 F5.56 (XIV)
yI]-ethanone
1-[4-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
601 isopropyl-pheny1)-methyll-pyridin-3-y11-1-hydroxy-prop-2-
yny1)- 0.69 504.16 F5.57 (XIV)
4-methyl-piperidin-1-yI]-ethanone
1-(4-{5- [(R)-(1, 3-Di methyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
602 pheny1)-methy1]-pyridin-3-ylethyny1}-4-methyl-piperidin-1-
y1)- 0.75 474.06 F5.58 (XIV)
ethanone
(XIV)
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[5-(1-
603
0.73 496.09 F5.70 then
methanesulfonyl-piperidin-4-ylethyny1)-pyridin-3-y1]-methanol
NI)
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
(XIV)
604 phenyI)-methy1]-pyridin-3-ylethynyll-4-methyl-piperidine-1-
0.76 525.12 F5.71 then
sulfonic acid methylamide
(VI)
(XIV)
(R)-(1, 3-Di methyl-azetidin-3-y1)-(4-isopropyl-pheny1)-[5-(3-
605 0.70 455.12 -
then
methanesulfony1-3-methyl-but-1-yny1)-pyridin-3-y11-methanol
NI)
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
606 phenyI)-methy1]-pyridin-3-ylethyny1}-cyclopropanesulfonic
acid 0.74 481.90 - (XIV)
dimethylamide
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
(XIV)
607 phenyl)-methyl]-pyridin-3-ylethynyll-cyclopropanesulfonic
acid 0.66 454.10 -- -
then (V)
amide
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
321
(XIV)
(R)-[5-(3-Cyclopropanesulfony1-3-methyl-but-1-yny1)-pyridin-3-
608
0.73 480.98 F5.72 then
y1]-(1,3-dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-methanol
NI)
(R)-3-{5-[(R)-(1,3-D imethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
609 phenyI)-methy1]-pyridin-3-ylethynyll-3-hydroxy-1-methyl-
0.63 448.17 - (XIV)
pyrrolidin-2-one
(S)-3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
610 phenyI)-methy1]-pyridin-3-ylethynyll-3-hydroxy-1-methyl-
0.63 448.15 - (XIV)
pyrrolidin-2-one
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
611 pheny1)-methyll-pyridin-3-ylethyny11-3,4-dihydro-2H-
0.68 484.06 F5.35 (XIV)
pyrano[3,2-b]pyridin-4-ol
3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
(IX)
612
phenylymethy1]-pyridin-3-ylethynyll-3-hydroxy-1-methyl-1,3- 0.72 496.05
F5.36 then
di hydro-indo1-2-one
(VI)
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
613 0.72 495.10 F5.37 (VI)
phenylymethy1]-pyridin-3-y11-2-(1H-indazol-3-y1)-but-3-yn-2-ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
614 phenyl)-methyl]-pyridin-3-yII-2-(1-methyl-1 H-indazol-3-
y1)-but- 0.76 509.06 F5.38 (VI)
3-yn-2-ol
2-(1-Cyclopropy1-1H-pyrazol-3-y1)-4-{5-[(R)-(1,3-dimethyl-
(VI)
615 azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-methyl]-pyridi
n-3- 0.89 585.16 F5.73 then
(IX)
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
(XIV)
616 phenylymethy1]-pyridi n-3-yI}-2-(2-methyl-pyri midi n-4-
yI)-but-3- 0.68 471.15 F5.39
then (V)
yn-2-ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
(XIV)
617
pheny1)-methy1]-pyridin-3-01-2-(5-methyl-pyrazin-2-y1)-but-3- 0.68
471.14 F5.40 then
yn-2-ol
(VI)
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
322
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
(XIV)
618 pheny1)-methyl]-pyridin-3-y11-2-(2-methyl-thiazol-5-y1)-
but-3-yn- 0.71 476.09 F5.41 then
2-01
(VI)
2-(6-Cyclopropyl-pyrimidin-4-y1)-4-{5-[(R)-(1,3-dimethyl-
(XIV)
619 azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-methyl]-
pyridin-3- 0.73 497.04 F5.42 then
(VI)
(R)- or (S)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(IX)
620 isopropyl-phenyI)-methy1]-pyridi n-3-y11-2-(6-methoxy-pyri
midi n- 0.70 487.13 F5.43
then (V)
4-yI)-but-3-yn-2-ol
N-(3-{5-[(R)-(1, 3-Di methyl-azetidi n-3-yI)-hydroxy- (4-isopropyl-
621 pheny1)-methyll-pyridin-3-y11-1,1-dimethyl-prop-2-yny1)-
0.62 433.81 - (IX)
acetamide
(S)- or (R)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(IX)
622 isopropyl-phenyly methyI]-pyridi n-3-yI}-2-(6-methoxy-pyri
midi n- 0.70 487.13 F5.44
then (V)
4-yI)-but-3-yn-2-ol
(R)- or (S)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(IX)
623 isopropyl-phenyly methyI]-pyridi n-3-y11-2-(2-methoxy-pyri
midi n- 0.70 487.13 F5.45
then (V)
(S)- or (R)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(IX)
624 isopropyl-phenylymethy1]-pyridin-3-y11-2-(2-methoxy-
pyrimidin- 0.70 487.13 F5.46
then (V)
(R)- or (S)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(XIV)
625 isopropyl-pheny1)-methyll-pyridin-3-y11-2-(2,6-dimethyl-
0.67 485.18 F5.47
then (V)
pyri midi n-4-yI)-but-3-yn-2-ol
(S)- or (R)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(XIV)
626 isopropyl-pheny1)-methy1]-pyridin-3-y11-2-(2,6-dimethyl-
0.67 485.16 F5.48
then (V)
pyri midi n-4-yI)-but-3-yn-2-ol
(R)- or (S)-2-(2,6-Dimethoxy-pyrimidin-4-yI)-4-{5-[(R)-(1,3-
(XIV)
627 dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-
methyl] 0.75 517.13 F5.49 then
(VI)
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
323
(S)- or (R)-2-(2,6-Dimethoxy-pyrimidin-4-y1)-4-15-KR)-(1,3-
(XIV)
628
dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-rnethyl]- 0.75
517.13 F5.50 then
(VI)
(S)- or (R)-4-{54(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
(XIV)
629 isopropyl-pheny1)-methyl]-pyridin-3-y11-2-(2-methoxy-6-
methyl- 0.74 501.15 F5.51 then
pyrimidin-4-yI)-but-3-yn-2-ol
(VI)
(R)- or (S)-4-{54(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
630 isopropyl-pheny1)-methy1]-pyridin-3-y11-2-(6-methoxy-2-
methyl- 0.74 501.22 F5.52 (VI)
pyri midi n-4-yI)-but-3-yn-2-ol
(S)- or (R)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
631 isopropyl-pheny1)-methyll-pyridin-3-y11-2-(6-methoxy-2-
methyl- 0.74 501.19 F5.53 (VI)
pyri midi n-4-yI)-but-3-yn-2-ol
4-{54(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
(XIV)
632 phenyI)-methyl]-pyridi
uoromethyl-pyrimidin-4- 0.77 525.17 F5.74 then
yI)-but-3-yn-2-ol
(VI)
2-(6-Difluoromethyl-pyrimidin-4-yI)-4-{5-[(R)-(1,3-dimethyl-
(XIV)
633
azetidin-3-y1)-hydroxy-(4-isopropyl-phenylymethyl]-pyridin-3- 0.75
507.17 F5.75 then
(VI)
(VIII)
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)45-(1-
634
0.74 452.18 F5.68 then
pyridin-2-yl-cyclopropylethyny1)-pyridin-3-y1]-methanol
(XIV)
(R)-(1,3-Dimethyl-azetidin-3-y1)-(4-isopropyl-pheny1)-{5-[1-(6-
(XIV)
635 methyl-pyrimidin-4-y1)-cyclopropylethynyll-pyridin-3-yly
0.80 467.19 F5.69 then
methanol
(VI)
Example 636 to Example 647 were synthesized from the appropriate alkyne
following the procedure described in
Example 408. LC-MS and prep LC-MS data are listed in the table below. The LC-
MS conditions used were LC-MS (A).
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
324
Alkyne
Example Prep
N
Name
tR [M+Hr LC-M8 Example
No
1-[4-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
636 isopropyl-phenylymethyl]-pyridin-3-ylyethylypiperidin-1-y1]-
0.61 464.2 586
ethanone
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
637 pheny1)-methyl]pyridin-3-y11-1,1,1-trifluoro-2-methyl-butan-2- 0.62 451.07
(IX) 587
ol
3-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
638 pheny1)-methy1]-pyridin-3-01-1,1-dirnethyl-propyl)-oxazolidin- 0.62 465.99
- 591
2-one
1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
639 pheny1)-methy1]-pyridin-3-01-1,1-dimethyl-propyl)-pyrrolidin-2- 0.62
464.12 - 592
one
1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
640 phenylymethyl]-pyridin-3-y11-1,1-dimethyl-propy1)-3-methyl- 0.62 479.16
- 593
imidazolidin-2-one
1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
641 pheny1)-methyll-pyridin-3-y11-1,1-dimethyl-propy1)-imidazolidin- 0.60
465.08 (VII) 594
2-one
1-[(S)-2-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
642 isopropyl-phenyl)-methyl]-pyridin-3-y11-ethyl)-2-methyl- 0.63 464.19
- 596
pyrrolidin-1-yI]-ethanone
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
643 0.60 499.10 (XIV) 613
pheny1)-methy1]-pyridin-3-01-2-(1H-indazol-3-y1)-butan-2-ol
2-(1-Cyclopropy1-1H-pyrazol-3-y1)-4-{5-[(R)-(1,3-dimethyl-
644 azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-methyl]-pyridi n-
3- 0.59 489.18 - 615
ylybutan-2-ol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
325
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
645 pheny1)-methy1]-pyridin-3-y11-2-(2-methyl-pyrimidin-4-y1)-
0.57 475.15 - 616
butan-2-ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
646 pheny1)-methy1]-pyridin-3-y11-2-(5-methyl-pyrazin-2-y1)-butan- 0.58 475.16
- .. 617
2-ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-isopropyl-
647 pheny1)-methy1]-pyridin-3-01-2-(2-methyl-thiazol-5-y1)-butan-2- 0.57
480.11 - 618
ol
Example 648 to Example 653 were synthesized starting from the corresponding
Example F1.2 and the appropriate
alkyne, and following the procedure described in Example 15, where the amount
of Pd(PPh3)4 was adjusted to 0.1eq
and the base was changed to pyrrolidine (3.5eq). The precursor alkyne is
indicated in the table below unless
commercially available. Prep LC-MS conditions and LC-MS data are listed in the
table below. The LC-MS conditions
used were LC-MS (A).
Prep
Example N Name tR [M+1-1]'
Alkyne
LC-MS
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
648 isopropyl-phenylymethyl]-pyridin-3-y11-2-(3-methyl-
0.73 460.21 F5.78 (VI)
isoxazol-5-y1)-but-3-yn-2-ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
649 isopropyl-phenyl)methyl]-pyridin-3-y11-2-(1-methyl-1H-
0.56 459.21 F5.79 (V)
imidazol-2-y1)-but-3-yn-2-ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(V)
650 isopropyl-pheny1)-methyl]-pyridin-3-y11-2-(5-methyl-
0.81 475.20 F5.80 then
thiophen-2-yI)-but-3-yn-2-ol
(XIV)
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
651 isopropyl-pheny1)-methyl]-pyridin-3-y11-2-(1-methyl-1H-
0.76 458.24 F5.81 (VI)
pyrrol-2-y1)-but-3-yn-2-ol
(R)- or (S)-4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-
(V)
652 (4-isopropyl-pheny1)-methyl]-pyridin-3-y1}-2-(2-
0.79 525.16 F5.76 then
trifluoromethyl-pyrimidin-4-yI)-but-3-yn-2-ol
(XIV)
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
326
(S)- or (R)-4-15-KR)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy- (V)
653 (4-isopropyl-pheny1)-methy1]-pyridin-3-y11-2-(2- 0.79
525.17 F5.77 then
trifluoromethyl-pyrimidin-4-yI)-but-3-yn-2-ol
(XIV)
Example 654 to Example 661 were synthesized starting from the corresponding
bromopyridine of structure Fl using
Example F5.27, and following the procedure described in Example 15, where the
amount of Pd(PPh3)4 was adjusted to
0.1eq and the base was changed to pyrrolidine (3.5eq). The precursor
bromopyridine is indicated in the table below.
Prep LC-MS conditions and LC-MS data are listed in the table below. The LC-MS
conditions used were LC-MS (A).
Example Prep
Name tR [M+H]-
Bromopyridine
N
LC-MS
4-(5-{(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-[4-(1-
(VI) then
654 trifluoromethyl-cyclopropyI)-phenyl]-methyll-pyridin-3- 0.66 536.15
F1.8
(XIV)
yI)-2-(6-methyl-pyridin-2-y1)-but-3-yn-2-ol
4-(5-{(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy44-(1-
655 methyl-cyclopropyI)-phenyl]-methyl}-pyridin-3-y1)-2- 0.63
481.99 F1.7 (VI)
(6-methyl-pyridin-2-yI)-but-3-yn-2-ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(VI) then
656 trifluoromethyl-phenyl)methyl]-pyridin-3-y11-2-(6- 0.60
496.14 F1.15
(XIV)
methyl-pyridin-2-yI)-but-3-yn-2-ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-(4-ethyl-
(VI) then
657 pheny1)-hydroxy-methyl]-pyridin-3-y11-2-(6-methyl- 0.59
456.21 F1.10
(XIV)
pyridin-2-yI)-but-3-yn-2-ol
4-{5-KR)-(4-tert-Butyl-pheny1)-(1,3-dimethyl-azetidin-
658 3-y1)-hydroxy-methyl]-pyridin-3-y11-2-(6-methyl- 0.65
484.26 F1.9 (XIV)
pyridin-2-yI)-but-3-yn-2-ol
4-{54(R)-(3-Ethy1-1-methyl-azetidin-3-y1)-hydroxy-(4-
659 trifluoromethoxy-phenylymethylFpyridin-3-y11-2-(6- 0.65
526.13 F1.13 (VII)
methyl-pyridin-2-yI)-but-3-yn-2-ol
4-(5-{(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-[4-
(VI) then
660 (2,2,2-trifluoro-ethyl)-phenyl]-methyl}-pyridin-3-0-2-
0.60 510.14 F1.12
(XIV)
(6-methyl-pyridin-2-yI)-but-3-yn-2-ol
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
327
4-{5-[(S)-(3-Fluoro-1-methyl-azetidin-3-yI)-hydroxy-
661 (4-isopropyl-phenylymethyl]-pyridin-3-y1}-2-(6- 0.63
474.22 F1.14 (VI)
methyl-pyridin-2-yI)-but-3-yn-2-ol
Example 662 to Example 664 were synthesized starting from Example F1.5 the
appropriate alkyne, and following the
procedure described in Example 15. The precursor alkyne is indicated in the
table below unless commercially available.
Prep LC-MS conditions and LC-MS data are listed in the table below. The LC-MS
conditions used were LC-MS (A).
Example
Prep
Name tR [M+H]-
Alkyne
N
LC-MS
1-(4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
(XIV)
662 isopropyl-pheny1)-methyl]-pyridazin-3-ylethynyll-
0.66 461.18 F5.54
then (V)
piperidin-1-yI)-ethanone
N-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
(XIV)
663 isopropyl-pheny1)-methyll-pyridazin-3-y11-1,1-dimethyl-
0.58 435.18
then (V)
prop-2-ynyI)-acetamide
1-(3-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
(XIV)
664 isopropyl-phenylymethy1]-pyridazin-3-y11-1,1-dimethyl-
0.68 461.19 F5.64
then (VI)
prop-2-ynyI)-pyrrolidin-2-one
Example 665 and Example 666 were synthesized following the procedure described
in Example 01.1 step 01.1.5
using the corresponding free azetidine A7.34 and the appropriate carbonyl
reagent, purified (if necessary) by prep
LC-MS. The LC-MS (A) data are listed in the table below.
Example
Prep
Name tR [M-FH]
Ketone/formaldehyde
N
LC-MS
4-15-[(R)-Hydroxy-(1-isopropy1-3-methyl-azetidin-
665 3-y1)-(4-isopropyl-phenyl)-methyl]-pyridin-3-y11-2- 0.67 497.97 acetone
(V)
(6-methyl-pyridin-2-yI)-but-3-yn-2-ol
4-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
666 isopropyl-phenylymethyl]-pyridin-3-y11-2-(6- 0.70 511.15
formaldehyde (XIV)
methyl-pyridin-2-yI)-but-3-yn-2-ol
Example 667 and Example 668 To a solution of A7.34 (30mg) in Me0H (1mL) were
added at RT TEA (20.2pL) followed
by commercial available 2-iodoethanol (57.3mg) for Example 667 and 1,1-
difluoro-2-iodoethane (57mg) for Example
668, respectively. The resulting mixture was shaken at reflux over weekend.
The mixture was purified by prep LC-MS.
Prep LC-MS data and LC-MS (A) data are listed in the table below.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
328
Example Prep LC-
Name tR
[M+H]
N MS
4-{5-[(R)-Hydroxy-[1-(2-hydroxy-ethyl)-3-methyl-azetidin-3-y1]-(4-
(V) then
667 isopropyl-pheny1)-methy1]-pyridin-3-y11-2-(6-methyl-pyridin-
2-0-but- 0.61 500.24
(VI)
3-yn-2-ol
4-{5-[(R)-[1-(2, 2-0 ifl uoro-ethyl)-3-methyl-azetidin-311]-hydroxy-(4-
668 isopropyl-phenylymethy1]-pyridin-3-y11-2-(6-methyl-pyridin-
2-y1)-but- (V) 0.66 520.15
3-yn-2-ol
Example 669 1-(4-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridin-3-yloxymethyll-
piperidin-1-ylyethanone
669.1 4-{5-f(R)-(1,3-Dimethyl-azetidin-3-4-hydroxy-(4-isopropyl-pheny1)-
methyli-pyridin-3-yloxymethyl)-piperidine-1-
carboxylic acid tert-butyl ester
A flask was charged with G1.2 (11mg), N-Boc-4-piperidinemethanol (22.4mg),
toluene abs. (300pL) and
cyanomethylenetributylphosphane (19.6pL) at RT. The resulting suspension was
stirred at 110 C under argon
overnight, then evaporated and purified by prep LC-MS (VIII) to give 25mg off-
white powder. LC-MS (A) tR = 0.77min;
[M+H]-: 524.15.
669.2 (R)-(1,3-dimethylazetidin-3-0)(4-isopropylpheny0(5-(piperidin-4-
ylmethoxy)pyridin-3-yhmethanol, hydrochloride
salt
The title compound was synthesized from Example 669.1 and following the
procedure described in Example 309 step
309.2. LC-MS (A) tR = 0.49; [M+H]: 424.22.
669.3 1-(4-{5-[(R)-(1,3-Dimethyl-azetidin-3-0)-hydroxy-(4-isopropyl-phenyl)-
methylkpyridin-3-yloxymethyll-piperidin-1-
0)-ethanone
To a suspension of Example 669.2 (28mg) in DCM (1mL) were added DIPEA
(48.3pL), AcOH (9.7pL), EDC.HCI (13mg)
and HOBt (9mg) at RT. The resulting solution was stirred at RT for lh, then
the mixture was evaporated and purified
by prep LC-MS (IX) to give 1mg white powder. LC-MS (A) tR = 0.63min; [M+Hr
465.98.
Example 670 4-{5-[(R)-(1-Cyclopropy1-3-methyl-azetidin-3-y1)-hydroxy-(4-
isopropyl-pheny1)-methyl]-pyridin-3-y1}-2-(6-
methyl-pyridin-2-y1)-but-3-yn-2-ol
The title compound was synthesized following the procedure described in
Example B2.1, step 2 using (1-
ethoxycyclopropoxy)trimethylsilane instead of formaldehyde and using sodium
cyanoborohydride instead of
NaBH(OAc)3. The crude was purified by prep LC-MS (V). LC-MS (A) tR = 0.66min;
[M+H]+: 496.21.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
329
Example 671 to Example 679 were synthesized starting from the appropriate
Example F3.2 to F3.10, and following
procedure described in Example 167.1. LC-MS (A) data, prep LC-MS methods and
the precursors are listed in the table
below.
Prep
Example Prep
Name tR [M+H]-
LC- Precursor
N LC-MS 1
MS 2
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
671 isopropyl-pheny1)-methyl-pyridin-3-y11-4-(2- 0.71
513.7 (VII) (XIV) F3.2
isopropyl-pyrimidin-4-yI)-pyrrolidin-2-one
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
672 isopropyl-phenylymethyl]-pyridin-3-y11-4-(2- 0.57
491.1 (VII) F3.3
methyl-thiazol-5-y1)-pyrrolidin-2-one
1-15-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-
673 isopropyl-pheny1)-methyl]-pyridin-3-0-4-(6- 0.52
485.13 (VI) (XIV) F3.4
methyl-pyridin-3-yI)-pyrrolidin-2-one
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
674 isopropyl-pheny1)-methyl]-pyridin-3-y11-4-(6- 0.64
512.99 (VIII) (IX) F3.5
isopropyl-pyridin-2-yI)-pyrrolidin-2-one
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
675 isopropyl-pheny1)-methyl]-pyridin-3-y11-4-(6- 0.72
539.02 (VII) F3.6
trifluoromethyl-pyridin-3-yI)-pyrrolidin-2-one
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
676 isopropyl-phenyl)-methyl]-pyridin-3-y11-4-(1- 0.63
474.12 (IX) F3.7
methyl-1H-pyrazol-4-y1)-pyrrolidin-2-one
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
677 isopropyl-phenylymethyl]-pyridin-3-y11-4-(1,3- 0.64
488.15 (IX) F3.8
dimethy1-1H-pyrazol-4-y1)-pyrrolidin-2-one
4-(1-Difluoromethy1-1H-pyrazol-4-y1)-1-{5-[(R)-
678 (1,3-dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl- 0.67
510.12 (V) F3.9
pheny1)-methyl]-pyridin-3-yll-pyrrolidin-2-one
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
330
1-{5-[(R)-(1,3-Dimethyl-azetidin-3-yI)-hydroxy-(4-
679 isopropyl-phenylymethy1]-pyridin-3-y11-4-(2- 0.63
475.15 (IX) F3.10
methy1-2H-[1,2,3]triazol-4-y1)-pyrrolidin-2-one
Example 680: 4-(1-Acetyl-piperidin-4-y1)-1-15-[(R)-(1,3-dimethyl-azetidin-3-
y1)-hydroxy-(4-isopropyl-pheny1)-methyl]-
pyridazin-3-yll-pyrrolidin-2-one
680.1. 4-(1-Acetyl-piperidin-4-y0-pyrrolidin-2-one
To a suspension of 4-(piperidin-4-yl)pyrrolidin-2-one (100mg) in THF (2mL) was
added DIPEA (0.12mL) followed by
acetic anhydride (0.053mL) at RT and the resulting suspension was stirred at
RT for 3h. The reaction mixture was
diluted with water and extracted with DCM (3x). The combined org. layers were
dried over MgSO4 and concentrated to
dryness to give the desired product (98mg) as white solid. LC-MS (A): tR =
0.47min; [M+H]: 211.2.
680.1. 4-(1-Acetyl-piperidin-4-y0-145-[(R)-(1,3-dimethyl-azetidin-310-hydroxy-
(4-isopropyl-pheny0-methylppyridazin-
3-y1}-pyrrolidin-2-one
The title compound was synthesized starting from the Example F1.6 and Example
680.1, and following the procedure
described in Example 167.1. The crude product was purified by described Prep
LC-MS (IX)). LC-MS (A): tR = 0.68min;
[M-F1-1]+: 520.13.
Example 681: 5-{5-[(R)-(1,3-Dimethyl-azetidin-311)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridazin-3-y11-hexahydro-
furo[2,3-c]pyrrol-4-one
The title compound was synthesized starting from the Example F1.6 and rac-
(3aR,6aS)-hexahydro-2H-fure[2,3-c]pyrrol-
4-one, and following the procedure described in Example 167, step 1. The crude
product was purified by described
Prep LC-MS (IX). LC-MS (A): tR = 0.66min; [M+1-1]+: 437.1.
Example 682: 1-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridazin-3-y11-4-{6-
isopropyl-pyridin-2-y1)-pyrrolidin-2-one
The title compound was synthesized starting from the Example F1.6 and Example
F3.2, and following the procedure
described in Example 167, step 1. The crude product was purified by described
Prep LC-MS (V). LC-MS (A): tR =
0.63min; [M+1-1]': 513.88.
Example 683: 1-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyl]-pyridin-3-y11-7,8-dihydro-
5H-pyrido[4,3-d]pyrimidin-6-y1)-ethanone
683.1 1-(2-Chloro-7 8-dihydro-5H-pyrido[4,3-d]pyrimidin-6-y0-ethanone
The title compound was synthesized from 2-chloro-5H,6H,7H,8H-pyrido[4,3-
d]pyrimidine hydrochloride following the
procedure in Example F5.54. LC-MS (A) tR = 0.53; [M+H]-: 212.03
683.2 (R)-(54(1,3-dimethylazetidin-3-y0(hydroxy)(4-
isopropylphenyOmethyOpyridin-3-yOboronic acid
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
331
A heated-out vial was charged with F1.2 (200mg), Bis(pinacolato)diboron
(200mg), [1,1'-
Bis(diphenylphosphino)ferrocene]dichloropalladium(II) complex with
dichloromethane (21mg), potassium acetate
(153mg) and DMF (4mL) at RT, sealed, 3x evacuated and backfilled with argon
and shaken at 8000 overnight. The
reaction mixture was diluted with water and Me0H, filtrated off and purified
by prep LC-MS, method (VIII) to give
107mg pale purple powder. LC-MS (A) tR = 0.49min; [M+H]': 355.07
683.3 1-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-phenyl)-
methylppyridin-311}-7,8-dihydro-5H-
pyrido[4,3-d]pyrimidin-6-y1)-ethanone
A vial was charged with the Example 683.1 (22mg), Example 683.2 (44.4mg),
Pd(PPh3)4 (18.3mg), K2003 1M (1mL)
and dioxane (1mL) at RT, sealed degassed with argon in ultrasonic bath for
5min and shaken at 100 C for 1h30 The
resulting light brown susp was shaken at 100 C. The reaction mixture was
allowed to cool down, diluted with water
and Me0H, filtrated off and purified by prep LC-MS (IX) to give 22mg white
powder after lyophilization. LC-MS (A) tR =
0.65min; [M+H]-: 486.12.
Example 684: 1-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-y1}-7,8-dihydro-
5H-[1,6]naphthyridin-6-y1)-ethanone
684.1 -(2-Chloro-7,8-dihydro-5H41,6]naphthyridin-6-y1)-ethanone
The title compound was synthesized from 2-chloro-5,6,7,8-tetrahydro-1,6-
naphthyridine hydrochloride following the
procedure in Example F5.54. LC-MS (A) tR = 0.63min; [M-FH]-: 211.09
684.2 1-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylTpyridin-3-yl)-7,8-dihydro-5H-
[1,6]naphthyridin-6-y1)-ethanone
The title compound was synthesized following the procedure in Example 683,
step 3, Example 684.1 replacing
Example 683.1. The crude product was purified by Prep LC-MS (XIV). LC-MS (A)
tR = 0.64min; [M+H]t 485.13
Example 685: 144-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenyl)-methyl]-pyridin-3-yll-pyrimidin-
5-y1)-piperidin-1-y1]-ethanone
685.1 4-(2-Chloro-pyrimidin-5-yI)-3,6-dihydro-2H-pyridine-1-carboxylic acid
tert-butyl ester
The title compound was synthesized following the procedure described in
Example 280, step 1 using 5-bromo-2-
chloropyrimidne and 3,6-dihydro-1H-pyridine 1 N Boc 4 boronic acid, pinacol
ester. Instead of direct purification via
prep LC-MS the reaction mixture was extracted with 3x DCM. The combined org.
layers were dried over MgSO4,
filtrated off, evaporated and purified by CC (Biotage, 10g SNAP, solvent A:
Hept, solvent B: EA, gradient (in %B): 10
for 2CV, 10 to 30 over 50V, 30 for 2CV) and by a second CC Biotage, 10g SNAP,
solvent A: Hept, solvent B: EA,
gradient (in %B): 10 for 3CV, 10 to 30 over 3CV, 30 for 2CV. LC-MS (A): tR =
0.94min; [M+H]t 296.03
685.2 2-chloro-5-(1,2,3,6-tetrahydropyridin-4-Apyrimidine, hydrochloride salt
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
332
The title compound was synthesized following the procedure described in
Example F5.56.2 using Example 658.1. LC-
MS (A) tR = 0.36min ; [M-FH]t196.12
685.3 144-(2-Chloro-pyrimidin-5-0)-3,6-dihydro-2H-pyridin-1-ylrethanone
The title compound was synthesized following the procedure described in
Example F5.54 using Example 685.2, LC-
MS (A) tR = 0.63; [M-FH] 138.04
685.4 144-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylppyridin-3-y1}-pyrimidin-5-y1)-3,6-
dihydro-2H-pyridin-1-ylkethanone
The title compound was synthesized like described in Example 683.3 using
Example 685.3 and purified by prep LC-
MS (VI) then (XX). LC-MS (A) tR= 70min; [M+H] 512.11
685.5 144-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methyll-pyridin-3-y1}-pyrimidin-5-y1)-
piperidin-1-ylrethanone
To a flask already containing Example 685.4 (8mg) were added Pd/C (1.66mg) and
Me0H (300pL) at RT under
argon. The flask was 3x evacuated and backfilled with argon, then 3x evacuated
and backfilled with H2. The resulting
mixture was stirred at RT under H2 overnight. The flask was 3x evacuated and
backfilled with argon. The reaction
mixture was filtrated through a syringe filter, the filter was washed with
Me0H and the filtrate was evaporated and
dried at HV to 6mg white solid. LC-MS (A) tR = 0.69min; [M+1-1]+: 513.72
Example 686: 144-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
phenylymethyl]-pyridin-3-yll-pyrimidin-
4-y1)-piperidin-1-y1]-ethanone
686.1 4-(2-Chloro-pyrimidin-4-yI)-3,6-dihydro-2H-pyridine-1-carboxylic acid
tert-butyl ester
The title compound was synthesized following the procedure described in 280,
step 1 using 4-bromo-2-
chloropyrimidine and 3,6-dihydro-1H-pyridine-1-N-Boc-4-boronic acid, pinacol
ester and purified with the adapted
gradient (in %B): 30 for 3CV, 30 to 50 over 3CV, 50 for 2CV, 50 to 70 over
2CV, 70 for 1CV. LC-MS (A) tR = 0.98min;
[M+I-1]': 296.06
686.2 2-chloro-4-(1,2,3,6-tetrahydropyridin-4-Apyrimidine hydrochloride salt
The title compound was synthesized following the procedure described in
F5.56.2 using Example 686.1. LC-MS (A) tR
= 0.38min; [M+H]-: 193.14
686.3 1-14-(2-Chloro-pyrimidin-4-y0-3,6-dihydro-2H-pyridin-1-yil-ethanone
The title compound was synthesized following the procedure described in
Example F5.54 using Example 686.2. LC-
MS (A) tR = 0.67min; [M-FH]-: 238.08
686.4 1-14-(2-{5-[(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-
pheny1)-methyll-pyridin-3-y1}-pyrimidin-4-y1)-3,6-
dihydro-2H-pyridin-1-yll-ethanone
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
333
The title compound was synthesized like described in Example 683.3 using
Example 686.3 and purified by prep LC-
MS method (VI). LC-MS (A) tR = 0.72min; [M-F1-1]-: 512.05
686.5 114-(2-{54(R)-(1,3-Dimethyl-azetidin-3-y1)-hydroxy-(4-isopropyl-pheny1)-
methylrpyridin-3-yll-pyrimidin-4-y1)-
piperidin-1-ylkethanone
The title compound was synthesized following the procedure described in
Example 685.5 using 686.4. LC-MS (A) tR =
0.71min; [M+H]: 513.77.
Depending on the purification conditions, the title compounds/intermediates in
Example 332 to 686 may be isolated as
free bases or as salts such as formate salts, or hydrochloride salts. Whenever
isolating a title compound/intermediate
as a salt, formate salt or hydrochloride salt is indicated at the end of the
chemical name and can refer to a mono-, di-
or tri-formate salt, or mono-, di-, or tri-hydrochloride salt.
II. BIOLOGICAL ASSAYS
FLIPR assay: The bioactivity of compounds is tested in a fluorometric imaging
plate reader (FLIPR: Molecular Devices)
using engineered HEK-293 cells expressing the human CCR6 (Gen Bank: AY242126).
Frozen cells are plated on Poly-
L-Lysine precoated 384-well plates 2 days prior to bioassay in DMEM medium
supplemented with 10% FCS and 1%
Penicillin-Streptomycin. At the day of bioassay, cell supernatant is discarded
and cells are dye loaded for 30 minutes
at room temperature in the dark with Fluo-8-AM (Focus Biomolecules) in Hanks
Balanced Salt Solution (Gibco),
buffered with 20 mM Hepes at pH 6.75 and supplemented with 0.05 % BSA. This
buffer, but lacking the dye, is also
used for washing and compound dilution steps (assay buffer). Cells are washed
free of excess dye with a wash-station
(Biotek), leaving 40 microliter of assay buffer at the end. Cells were
incubated for 15 minutes at room temperature in
the dark, before adding compounds. Stock solutions of test compounds are made
up at a concentration of 10 mM in
DMSO, and serially diluted first in DMSO and then transferred in assay buffer
to concentrations required for inhibition
dose response curves. After a 45-minute incubation period in assay buffer at
room temperature, 10 microliters of each
compound dilution are transferred from a compound plate to the plate
containing the recombinant cells in the FLIPR
instrument according to the manufacturer's instructions. After cells and
compounds were preincubated for 30 minutes
at room temperature in the dark, 10 microliter agonist CCL20 (Peprotech) at a
final concentration of 10 nM is added,
again using the FLIPR instrument. Changes in fluorescence are monitored before
and after addition of the test
compounds and agonist. Emission peak values above base level after CCL20
addition are exported after base line
subtraction.
The calculated IC50 values may fluctuate depending on the daily assay
performance. Fluctuations of this kind are known
to those skilled in the art. In the case where 1050 values have been
determined several times for the same compound,
mean values are given. Data are shown in Table 1.
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
334
Example FLIPR Example FLIPR Example FLIPR Example
FLIPR
N 1050 (nM) N 1050 (nM) N 1050 (nM) N
1050 (nM)
1 114 173 77.9 345 252 516
104
2 147 174 75.2 346 62.5 517
63.5
3 28 175 183 347 120 518
41.2
4 36.2 176 39.2 348 135 519
168
33.3 177 284 349 113 520 62.6
6 47.2 178 47.5 350 242 521
153
7 54.3 179 64.5 351 27.6 522
119
8 110 180 55.7 352 110 523
297
9 74.7 181 295 353 40.3 524
178
64.1 182 86.5 354 29.0 525 187
11 130 183 31.7 355 24.8 526
171
12 73.5 184 16.5 356 12.7 527
74.3
13 43.5 185 27.9 357 26.1 528
66.9
14 70.6 186 43.5 358 131 529
134
80.8 187 15.8 359 234 530 58.7
16 52 188 23.4 360 292 531
90
17 53.5 189 19.1 361 164 532
718
18 33.5 190 38 362 90.0 533
1020
19 44.9 191 317 363 178 534
47.0
23 192 85.4 364 142 535 331
21 47.9 193 184 365 147 536
2450
22 126 194 20.5 366 165 537
152
23 46.8 195 29.3 367 321 538
144.8
24 6.44 196 67.6 368 271 539
56.0
7.69 197 106 369 187 540 122
26 13 198 70.8 370 65.9 541
452
27 43.7 199 487 371 49.7 542
34.8
28 44.6 200 407 372 102.8 543
22.8
29 85.5 201 159 373 88.9 544
257
33.9 202 29.9 374 39.0 545 61.0
31 81.2 203 64.9 375 39.7 546
776
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
335
32 18.7 204 48.2 376 102 547
797
33 20.8 205 26.9 377 39.5 548
742
34 21.1 206 89 378 54.9 549
58.2
35 141 207 52.5 379 41.2 550
70.8
36 61.7 208 38.4 380 71.5 551
135
37 20.7 209 205 381 53.4 552
76.8
38 130 210 326 382 43.7 553
62.3
39 76.5 211 50.1 383 53.8 554
117
40 92.5 212 70.8 384 42.1 555
986
41 133 213 459 385 117 556
615
42 41.5 214 184 386 41.0 557
46.3
43 55.9 215 50.9 387 55.1 558
84.5
44 63.3 216 476 388 28.2 559
85.6
45 32.8 217 43.3 389 58.1 560
74.6
46 145 218 60.4 390 175 561
766
47 35 219 135 391 58.8 562
44.7
48 100 220 214 392 24.2 563
45.2
49 219 221 350 393 25.1 564
91.9
50 90.2 222 164 394 1040 565
116
51 134 223 29 395 60.1 566
209
52 200 224 48.5 396 34.4 567
59.1
53 46.3 225 83.3 397 29.1 568
97.6
54 66.9 226 26.6 398 51.8 569
118
55 65.8 227 26.1 399 106.6 570
65.8
56 61 228 44.6 400 160 571
1190
57 349 229 59.1 401 117 572
149
58 440 230 36.7 402 88.3 573
75.9
59 7.1 231 14.9 403 40.4 574
1340
60 9.06 232 46.7 404 164 575
103
61 19.4 233 29.7 405 102 576
267
62 242 234 123 406 37.6 577
66.1
63 601 235 23.4 407 51.8 578
97.2
64 441 236 210 408 138 579
411
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
336
65 44.7 237 217 409 48.8 580
294
66 223 238 22.4 410 84.9 581
113
67 11.8 239 283 411 86.3 582
485
68 17.9 240 82.9 412 90.0 583
239
69 16.1 241 128 413 36.7 584
130
70 434 242 22.1 414 34.7 585
116
71 147 243 211 415 61.9 586
67.0
72 15 244 274 416 14.7 587
14.8
73 112 245 26 417 118 588
119
74 154 246 33.3 418 190 589
154
75 184 247 250 419 155 590
235
76 627 248 247 420 277 591
71.6
77 52.1 249 78.4 421 712 592
44.8
78 80.6 250 10.9 422 66.7 593
51.6
79 30 251 8.25 423 61.8 594
45.4
80 10.5 252 238 424 107 595
93.2
81 19.1 253 13.1 425 1730 596
117
82 185 254 19.3 426 17.7 597
395
83 128 255 774 427 292 598
94.8
84 52.8 256 57.8 428 598 599
149
85 89.9 257 87.4 429 103 600
45.7
86 75.3 258 15.5 430 209 601
75.5
87 61.5 259 56.7 431 197 602
39.5
88 98.4 260 324 432 146 603
31.2
89 231 261 206 433 58.5 604
18.9
90 163 262 72.6 434 147 605
78.6
91 46.2 263 162 435 175 606
22.6
92 81.5 264 167 436 114.7 607
104
93 86.8 265 90.6 437 95.2 608
43.5
94 71.1 266 79.1 438 53.6 609
656
95 196 267 103 439 265 610
426
96 130 268 99.3 440 50.7 611
74.9
97 71.3 269 224 441 118 612
58.5
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
337
98 60 270 27.4 442 177 613
11.1
99 111 271 36.5 443 35.3 614
12.6
100 69.9 272 485 444 33.4 615
39.4
101 41.8 273 32 445 44.8 616
102
102 103 274 252 446 48.4 617
53.5
103 110 275 67.1 447 43.2 618
23.3
104 42.2 276 413 448 35.2 619
68.8
105 43.4 277 42.2 449 40.8 620
55.3
106 46.7 278 48.9 450 57.8 621
235
107 54.3 279 166 451 43.5 622
105
108 21 280 37.4 452 33.6 623
44.9
109 21.2 281 82.2 453 58.1 624
81.8
110 39.1 282 44.3 454 103 625
107
111 43 283 61.6 455 83.1 626
111
112 38.5 284 222 456 201 627
11.5
113 29.8 285 78 457 56.4 628
20.2
114 38 286 36.3 458 99.0 629
60.9
115 41 287 42.8 459 440 630
61.1
116 40.8 288 33.9 460 469 631
79.5
117 20.1 289 82.4 461 384 632
14.1
118 43.1 290 100 462 286 633
42.5
119 20.5 291 24.8 463 122 634
40.6
120 59.2 292 748 464 129 635
23.2
121 57.7 293 246 465 87.5 636
106.7
122 42.1 294 49.4 466 143 637
46.3
123 46.9 295 61.1 467 129 638
509
124 35.9 296 118 468 62.3 639
96.6
125 46.9 297 93.5 469 137 640
169
126 84.9 298 65.2 470 131 641
67.0
127 32.4 299 80.6 471 125 642
277
128 40.3 300 40.8 472 79.8 643
16.9
129 35.9 301 60 473 77.6 644
147
130 74.7 302 44.5 474 121 645
110
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
338
131 60.8 303 30.7 475 54.7 646
129
132 50.1 304 385 476 112 647
76.3
133 74.5 305 121 477 33.9 648
46.6
134 62 306 101 478 88.9 649
185
135 74 307 567 479 111.8 650
15.9
136 123 308 515 480 128 651
16.1
137 60.3 309 58.6 481 184 652
10.9
138 45.8 310 48.6 482 92.3 653
10.1
139 66.6 311 140 483 73.1 654
46.2
140 681 312 23.9 484 593 655
55.0
141 211 313 158 485 259 656
208
142 274 314 111 486 287 657
86.6
143 188 315 23.6 487 248 658
86.6
144 376 316 23.4 488 382 659
344
145 274 317 15.2 489 135 660
82.4
146 309 318 154 490 143 661
54.3
147 63.5 319 94.4 491 172 662
331
148 138 320 86 492 267 663
992
149 149 321 273 493 185 664
211
150 35.8 322 49.9 494 240 665
40.4
151 336 323 67.8 495 20.9 666
26.5
152 60.2 324 15 496 69.7 667
52.4
153 214 325 595 497 74.3 668
101
154 36.8 326 83.3 498 234 669
62.1
155 28 327 168 499 53.8 670
42.8
156 147 328 187 500 149.5 671
50.5
157 27.7 329 485 501 108 672
172
158 222 330 143 502 65.8 673
199
159 91.6 331 50.1 503 51.1 674
25.9
160 82.9 332 290 504 143 675
227
161 202 333 112.8 505 35.9 676
108
162 43.4 334 216 506 24.0 677
105
163 24.6 335 337 507 32.7 678
156
CA 03177335 2022- 10- 28
WO 2021/219849
PCT/EP2021/061401
339
164 18.9 336 207 508 49.1 679
222
165 677 337 95.1 509 208 680
428
166 85.3 338 93.8 510 184.5 681
281
167 66.9 339 100.4 511 133 682
7.98
168 109 340 79.4 512 61.1 683
105
169 101 341 66.6 513 101 684
125
170 133 342 154 514 142.5 685
242
171 34.7 343 532 515 181 686
145
172 205 344 441 -
CA 03177335 2022- 10- 28