Note: Descriptions are shown in the official language in which they were submitted.
THERAPEUTIC COMPOSITIONS COMPRISING DEUTERATED OR PARTIALLY
DEUTERATED N,N-DIMETHYLTRYPTAMINE COMPOUNDS
FIELD OF THE INVENTION
The present invention provides a deuterated N,N-dimethyltryptamine compound or
a
plurality of deuterated N,N-dimethyltryptamine compounds for use in therapy,
selected from
N,N-dimethyltryptamine compounds, a-protio, a-deutero-N,N-dimethyltryptamine
compounds, a,a-dideutero- N,N-dimethyltryptamine compounds, and
pharmaceutically
acceptable salts of these compounds, preferably wherein the deuterated N,N-
dimethyltryptamine compound has an increased half-life when compared with the
half-life of
undeuterated N,N-dimethyltryptamine.
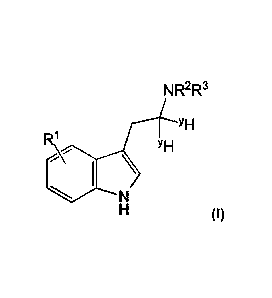
In particular the invention provides a compound of Formula I, or a
pharmaceutically
acceptable salt thereof;
NR2R3
R\ IYH
YH
N
(10
wherein the ratio of deuteriunrprotium in the compound is greater than that
found naturally in
hydrogen;
each 10- is independently selected from H and D;
R2 is selected from CH3 and CD3;
R3 is selected from CH3 and CD3; and
each YH is independently selected from H and D.
Methods of synthesising compounds of the present invention, and methods of use
of
such compositions in treating psychiatric or neurological disorders, such as
major depressive
disorder, are also provided.
BACKGROUND OF THE INVENTION
Classical psychedelics have shown preclinical and clinical promise in treating
psychiatric disorders (Carhart-Harris and Goodwin (2017), The Therapeutic
Potential of
Psychedelic Drugs: Past, Present and Future, Neuropsychopharmacology 42, 2105-
2113). In
particular, psilocybin has demonstrated significant improvement in a range of
1
Date Recue/Date Received 2022-12-13
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
depression and anxiety rating scales in randomised double blind studies
(Griffiths et a/.
(2016), Psilocybin produces substantial and sustained decreases in depression
and
anxiety in patients with life-threatening cancer a randomised double-blind
trial, Journal
of Psychopharmacology 30(12), 1181-1197).
N,N-dimethyltryptamine (DMT) is also understood to hold therapeutic value as a
short-acting psychedelic, however its duration of action (under 20 minutes) is
so short
as to limit effective therapy. Administration protocols have been developed to
extend
the immersive psychedelic experience of DMT (Gallimore and Strassman (2016), A
model for the application of target-controlled intravenous infusion for a
prolonged
immersive DMT psychedelic experience, Frontiers in Pharmacology, 7:211).
However,
these protocols carry risk of toxic buildup in patients who are poor
metabolisers of DMT
(for further discussion see Strassman et al (1994), Dose response study of N,N-
dimethyltryptamine in humans, Arch Gen Psychiatry 51, 85).
a,a,8,0-Tetradeutero-NN-dimethyltryptamine is known to exhibit a kinetic
isotope effect which bestows a significant difference on its in vivo
pharmacokinetic profile
as compared with N,N-dimethyltryptamine. Substitution of hydrogen with a
deuterium at
an sp3 carbon centre is known to give rise to a 'kinetic isotope effect' by
virtue of the
difference in bond strength between a CH and a CD bond. First demonstrated in
1982
(Barker et a/. (1982), Comparison of the brain levels of N,N-
dimethylttyptamine and
a,a,p,p-tetradeutero-N,N-dimethylttyptamine following intraperitoneal
injection,
Biochemical Pharmacology, 31(15), 2513-2516), the half-life of a,a,13,8-
tetradeutero-
N,N-dimethyltryptamine in the rodent brain is suggestive that administration
of a,a,I3,13-
tetradeutero-N,N-dimethyltryptamine alone would maintain a patient in DMT
space for
longer than therapeutically essential.
SUMMARY OF THE INVENTION
The present invention is based, in part, upon the ability to apply knowledge
of the
kinetic isotope effect exhibited by a,a,13,13-tetradeutero-N,N-
dimethyltryptamine in order
to modify, controllably, the pharmacokinetic profile of N,N-
dimethyltryptamine, thereby
permitting more flexible therapeutic application. In particular, by providing
individual drug
substance compositions comprising deuterated N,N-dimethyltryptamine analogues,
in
particular N,N-dimethyltryptamine comprising at least one deuterium atom at
the alpha
position (i.e. attached to the carbon atom to which the dimethylamino moiety
is attached),
the present invention provides compositions and methods which enable a finely
tuned
single dose to maintain a patient in full dissociation from the external
world, referred to
herein as 'DMT space', for a therapeutically optimised duration without
relying on infusion
protocols or combination therapy with monoamine oxidase inhibitors in the
clinic. The
2
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
present invention provides a clinically applicable solution which reduces
clinical
complexity and increases clinical flexibility in the administration of DMT-
assisted therapy.
Moreover, we have observed a quantifiable relationship between the extent of
deuteration, and by proxy the H:D ratio of input reducing agent in synthetic
methods
disclosed herein, and the effect on potentiation (i.e. increase) of the
metabolic half-life of
the parent compound. Such technical effect may be used to quantifiably
increase the
precision with which deuterated N,N-dimethyltryptamine compositions (that is
to say
isolated deuterium-containing N,N-dimethyltryptamine compounds or compositions
comprising more than one type of compound selected from N,N-dimethyltryptamine
and
its deuterated analogues, in particular those deuterated at the alpha
positions and/or
N,N-dimethyl positions, or pharmaceutically acceptable salts of these) may be
prepared.
Viewed from a first aspect, therefore, the invention provides a deuterated N,N-
dimethyltryptamine compound or a plurality of deuterated N,N-
dimethyltryptamine
compounds for use in therapy, selected from N,N-dimethyltryptamine compounds,
a-
protio, a-deutero-N,N-dimethyltryptannine
compounds, a,a-dideutero-N,N-
dimethyltryptamine compounds, and pharmaceutically acceptable salts of these
compounds, preferably wherein the deuterated N,N-dimethyltryptamine compound
has
an increased half-life compared with the half-life of undeuterated N,N-
dimethyltryptamine.
Viewed from a second aspect, the invention provides a deuterated N,N-
dimethyltryptamine compound of Formula (I) for use in therapy:
NR2R3
YH
R\1 YH
.<
(I),
wherein the ratio of deuterium:protium in the compound is greater than that
found
naturally in hydrogen:
each R1 is independently selected from H and D;
R2 is selected from CH3 and CD3;
R3 is selected from CH3 and CD3;
each Y H is independently selected from H and D,
or a pharmaceutically acceptable salt thereof.
3
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
In preferred embodiments of the second aspect each R1 is H. In primary
embodiments of the second aspect both YH are D. In secondary embodiments of
the
second aspect both R2 and R3 are CD3.
Viewed from a third aspect, the invention provides a compound of formula (I)
or
a pharmaceutically acceptable salt thereof, obtainable by a method of
synthesis
comprising reacting a compound of formula (II) with LiA11-14 and/or LiAID4,
NR2R3 NR2R3
YH
Rµl W 0
YH
(I) H (II)
wherein R1 is selected from H and D,
R2 is selected from CH3 and CD3;
R3 is selected from CH3 and CD3;
and each YH is independently selected from H and D.
Viewed from a fourth aspect, the invention provides a pharmaceutical
composition comprising a compound or composition as defined according to any
one of
the first to third aspects in combination with a pharmaceutically acceptable
excipient.
Viewed from a fifth aspect, the invention provides a compound or composition
as
defined according to any one of the first to fourth aspects for use in a
method of
psychedelic-assisted psychotherapy.
Viewed from a sixth aspect, the invention provides a compound or composition
as defined according to any one of the first to fifth aspects for use in a
method of treating
a neurological disorder or a psychological disorder in a patient.
Viewed from a seventh aspect, the invention provides a method of treating a
neurological disorder or a psychological disorder comprising administering to
a patient
in need thereof a compound or composition as defined according to any one of
the first
to fourth aspects.
Viewed from a eighth aspect, the invention provides the use of a compound or
composition as defined according to any one of the first to fourth aspects in
the
manufacture of a medicament for use in a method of treating a a neurological
disorder
or a psychological disorder in a patient.
Viewed from a ninth aspect, the invention provides a method of preparing a
compound in accordance with any of the first to third aspects of the invention
comprising
4
contacting deuterated or undeuterated 2-(3-indoly1)-N,N-dimethylacetamide with
a reducing
agent consisting essentially of lithium aluminium hydride and/ or lithium
aluminium deuteride.
Viewed from a tenth aspect, the invention provides a compound selected from
Compounds 1 - 5, or a pharmaceutically acceptable salt thereof.
Inca
+0
cri7.11H
ists
3
CHO
4
According to an aspect of the invention is a therapeutic composition
comprising:
a deuterated N,N-dimethyltryptamine compound of Formula (I) or a
pharmaceutically
acceptable salt thereof:
NR2R3
YH
YH
wherein:
each R1 is H;
R2 is CD3;
R3 is CD3;
each YH is independently selected from H and D; and
a pharmaceutically acceptable excipient.
Further aspects and embodiments of the present invention will be evident from
the
discussion that follows below.
5
Date Recue/Date Received 2023-05-10
BRIEF DESCRIPTION OF THE FIGURES
Fig. 1. depicts the predicted phannacokinetic profile of partially deuterated
DMT
compared to undeuterated DMT and fully deuterated DMT. Predicted A) plasma
concentration and B) brain tissue concentration, showing the extended half-
life of partially
deuterated DMT. Hashed area depicts effect site concentrations (>60 ng/mL)
that are
experienced as full dissociation from the external world, referred to as 'DMT
space'.
Fig. 2 plots calculated in vitro half-life for DMT and 6 deuterated-containing
compositions described in Example 1. A) Linear regression analysis. The r2
value for half-
life is 0.754; where the slope was found to be significantly different to
zero, p=0.01. B) Half-
life of deuterated analogues as a percent change from (undeuterated) DMT
(dashed line).
Fig. 3 In vitro intrinsic clearance for DMT and 6 deuterium-containing
compositions
described in Example 1. A) Linear regression analysis. The r2 value for
intrinsic clearance is
0.7648; where the slope was found to be significantly different to
5a
Date Recue/Date Received 2022-12-13
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
zero, p=0.01. B) Intrinsic clearance of deuterated analogues as a percent
change from
(undeuterated) DMT (dashed line).
Fig. 4 In vitro intrinsic clearance (A) and half-life (B) of DMT (SPL026) and
6
different D2-deuterated SPL028 analogue blends in human hepatocytes with and
without
MAO-A/B inhibitor combination, as described in the Example section, below.
DETAILED DESCRIPTION OF THE INVENTION
Throughout this specification, one or more aspect of the invention may be
combined with one or more features described in the specification to define
distinct
embodiments of the invention.
References herein to a singular of a noun encompass the plural of the noun,
and
vice-versa, unless the context implies otherwise.
Throughout this specification the word "comprise", or variations such as
"comprises" or "comprising", will be understood to imply the inclusion of a
stated element,
integer or step, or group of elements, integers or steps, but not the
exclusion of any other
element, integer or step, or group of elements, integers or steps.
The present invention provides a deuterated N,N-dimethyltryptamine compound
selected from N,N-dimethyltryptamine compounds, a-protio, a-deutero-N,N-
dimethyltryptamine compounds a,a-dideutero-N,N-dimethyltryptamine compounds,
and
pharmaceutically acceptable salts of these compounds.
As used herein, the term deuterated N,N-dimethyltryptamine compound means
an N,N-dimethyltryptamine compound having a deuterium composition greater than
found naturally occurring in hydrogen (approx. 1.6%). As used herein the term
undeuterated N,N-dimethyltryptamine compound means an N,N-dinnethyltryptamine
compound having a deuterium composition equal to or less than found naturally
occurring in hydrogen.
As used herein, the term N,N-dimethyltryptamine compounds means a
compound of Formula la wherein each xH is independently selected from protium
(H)
and deuterium (D). For example, N,N-dimethyltryptamine compounds may comprise
0,
1 or 2 deuterium atoms at the [3 position. For the avoidance of doubt, the
invention does
not encompass a compound of Formula la where each xH is H.
As used herein, the term a-protio, a-deutero-N,N-dimethyltryptamine compounds
means a compound of Formula lb wherein each xH is independently selected from
protium (H) and deuterium (D).
For example, a-protio, a-deutero-N,N-
dimethyltryptamine compounds may comprise 0, 1 or 2 deuterium atoms at the 13
position.
6
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
As used herein, the term a,a-dideutero-N,N-dimethyltryptamine compounds
means a compound of Formula lc wherein each xH is independently selected from
protium (H) and deuterium (D). For example, a,a-dideutero-N,N-
dimethyltryptamine
compounds may comprise 0, 1 or 2 deuterium atoms at the 13 position.
A protium atom (H) is a hydrogen atom with zero neutrons. A deuterium atom (D)
is a hydrogen atom with one neutron.
xH xH
xH xH xH xH
xH N xH
xH xH xH
xH xH N xH
xH xH
1401 \ X H \ xH
xj II IN( xH
xH
xH xH xH
Ia lb
xH
xH
xH
xu N
xH
xH
xH
xH
xH 111
xH
xH
Ic
The inventors have discovered that compounds of the present invention exhibit
a primary kinetic isotope effect when one or two deuterium atoms are
positioned on the
alpha carbon of an N,N-dimethyltryptamine compound. This primary kinetic
isotope
effect is exhibited to its fullest extent by a,a-dideutero-N,N-
dimethyltryptamine
compounds and to a lesser extent by a-protio, a-deutero-N,N-dimethyltryptamine
compounds, such that the fold-change in half-life of an a-protio, a-deutero-
N,N-
7
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
dimethyltryptamine compound compared with the analogous N,N-dimethyltryptamine
compound is about half that of the analogous a,a-dideutero-N,N-
dimethyltryptamine
compound.
Compositions comprising mixtures of two or more compounds selected from N, N-
dimethyltryptamine, a,a-dideutero-N,N-dimethyltryptamine compounds and a-
protio, a-
deutero-N,N-dimethyltryptamine compounds can be used to apply the therapeutic
benefits of the primary kinetic isotope effect to a variable degree.
Accordingly, the invention provides a composition comprising two or more
compounds selected from N,N-dimethyltryptamine compounds, a,a-dideutero-N,N-
dimethyltryptamine compounds and a-protio, a-deutero-N,N-dimethyltryptamine
compounds.
The inventors have also discovered that compounds of the present invention
exhibit a secondary kinetic isotope effect when the N,N-dimethyl groups are
deuterated.
When such N,N-dimethyl groups comprise one or more deuterium and the alpha
position
is also mono- or di-deuterated, the secondary kinetic isotope is synergistic
with the
primary kinetic isotope effect, producing greater than a 14-fold increase in
half-life
compared with undeuterated N,N-dimethyltryptamine (see Example 3).
N,N-dimethyltryptamine and all its deuterated analogues freely form addition
salts with anionic counterions. Throughout the specification, an N,N-
dimethyltryptamine
compound (in particular N,N-dimethyltryptamine, a, a-dideutero-N,N-di
nnethyltryptamine
compounds and a-protio, a-deutero-N,N-dimethyltryptamine compounds) refers
equally
to any pharmaceutically acceptable salt, e.g. the fumarate salt.
Typically, acidic reagents may be used to prepare salts, in particular
pharmaceutically acceptable salts, of N,N-dimethyltryptannine compounds.
Examples of
suitable acidic reagents are selected from the group consisting of fumaric
acid,
hydrochloric acid, tartaric acid, citric acid, hydrobromic acid, sulfuric
acid, phosphoric
acid, acetic acid, maleic acid, lactic acid, tartaric acid and gluconic acid.
Often, where in
the form of salts, N,N-dimethyltryptamine compounds, in particular as the
compounds of
the invention, in the compositions of the invention or otherwise used
according to the
various aspects of the present invention, and embodiments thereof, are
fumarate,
hydrochloride, tartrate or citrate salts, in particular fumarate salts.
The compounds of the first aspect of the invention, and indeed those of the
second and third (and other, as appropriate) aspects of the invention, may
thus be
present in free base or salt form (such as the salts described herein),
optionally as
solvates (e.g. hydrates) thereof.
Embodiments of the first aspect provide a composition comprising 2% or more
by weight of one or more deuterated N,N-dimethyltryptamine compound. In
preferred
8
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
embodiments of the first aspect, the composition comprises 5% or more by
weight of the
one or more deuterated N,N-dimethyltryptamine compound. In preferred
embodiments
of the first aspect, the composition comprises 10% or more by weight of the
one or more
deuterated N,N-dimethyltryptamine compound. In preferred embodiments of the
first
aspect, the composition comprises 15% or more by weight of the one or more
deuterated
N,N-dimethyltryptamine compound. In preferred embodiments of the first aspect,
the
composition comprises 20% or more by weight of the one or more deuterated N,N-
dimethyltryptamine compound. In preferred embodiments of the first aspect, the
composition comprises 25% or more by weight of the one or more deuterated N,N-
dimethyltryptamine compound. In preferred embodiments of the first aspect, the
composition comprises 30% or more by weight of the one or more deuterated N,N-
dimethyltryptamine compound. In preferred embodiments of the first aspect, the
composition comprises 50% or more by weight of the one or more deuterated N,N-
dimethyltryptamine compound. In preferred embodiments of the first aspect, the
composition comprises 60% or more by weight of the one or more deuterated N,N-
dimethyltryptamine compound. In preferred embodiments of the first aspect, the
composition comprises 75% or more by weight of the one or more deuterated N,N-
dimethyltryptamine compound. In preferred embodiments of the first aspect, the
composition comprises up to 90% by weight of the one or more deuterated N,N-
dimethyltryptamine compounds. In preferred embodiments of the first aspect,
the
composition comprises up to 95% by weight of the one or more deuterated N,N-
dimethyltryptamine compound. In preferred embodiments of the first aspect, the
composition comprises up to 96% by weight of the one or more deuterated N,N-
dimethyltryptamine compound. In preferred embodiments of the first aspect, the
composition comprises up to 97% by weight of the one or more deuterated N,N-
dimethyltryptamine compound. In preferred embodiments of the first aspect, the
composition comprises up to 98% by weight of the one or more deuterated N,N-
dimethyltryptamine compound. In preferred embodiments of the first aspect, the
composition comprises up to 99% by weight of the one or more deuterated N,N-
dimethyltryptamine compound. In preferred embodiments of the first aspect, the
composition comprises up to 99.5% by weight of the one or more deuterated N,N-
dimethyltryptamine compound. In preferred embodiments of the first aspect, the
composition comprises up to 99.9% by weight of the one or more deuterated N,N-
dimethyltryptamine compound.
Accordingly, it will be understood from the foregoing that, according to
particular
embodiments of the first aspect of the invention, in particular those
embodiments
discussed in the following eight paragraphs, the composition comprises between
2% and
9
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
90%, 2% and 95%, 2% and 96%, 2% and 97%, 2% and 98%, for example between 5%
and 90%, 5% and 95%, 5% and 96%, 5% and 97%, 5% and 98%; 10% and 90%, 10%
and 95%, 10% and 96%, 10% and 97%, 10% and 98%; 15% and 90%, 15% and 95%,
15% and 96%, 15% and 97%, 15% and 98%; 20% and 90%, 20% and 95%, 20% and
96%, 20% and 97%, 20% and 98%; 25% and 90%, 25% and 95%, 25% and 96%, 25%
and 97%, 25% and 98%; 30% and 90%, 30% and 95%, 30% and 96%, 30% and 97%,
30% and 98%; 50% and 90%, 50% and 95%, 50% and 96%, 50% and 97%, 50% and
98%; 60% and 90%, 60% and 95%, 60% and 96%, 60% and 97%, 60% and 98%; or
75% and 90%, 75% and 95%, 75% and 96%, 75% and 97%, 75% and 98%, 75% and
99%, 90% and 99%, 90% and 99.9%, 99% and 99.9%, by weight of one or more
deuterated N,N-dimethyltryptamine compound.
It will be understood that, wherever a composition comprises 2% or more by
weight of one or more deuterated N,N-dimethyltryptamine compounds, that such
compositions may comprise up to 95% by weight of one or more deuterated N,N-
dimethyltryptamine compounds, or up to 96% by weight, up to 97% by weight or
up to
98% by weight.
In preferred embodiments, the one or more partially deuterated N,N-
dimethyltryptamine compound comprises up to 50% by weight of the total
composition.
According to other preferred embodiments of the first aspect of the invention,
the
composition comprises up to 50% by weight, based on the total weight of the
composition, of one or more compounds selected from a,a-dideutero-N,N-
dimethyltryptamine compounds, a-protio, a-deutero-N,N-dimethyltryptamine
compounds
and pharmaceutically acceptable salts thereof. It will be understood that, in
such
embodiments, such compositions may comprise 2% or more by weight, for example
5%
or more, 10% more, 15% more, 20% or more, 25% or more or 30% or more, based on
the total composition, of the said one or more compounds.
According to specific embodiments, compositions of the present invention,
including all of the embodiments described herein, including but not limited
to those
embodiments comprising N,N-dimethyltryptamine, consist essentially of one or
more
compounds selected from N,N-dimethyltryptamine and its deuterated analogues,
in
particular those deuterated at the alpha position, or pharmaceutically
acceptable salts of
these. By the composition consisting essentially of one or more compounds
selected
from N,N-dimethyltryptamine and its deuterated analogues is meant that the
composition
may comprise additional components (other than N,N-dimethyltryptamine
compounds)
but that the presence of these additional components will not materially
affect the
essential characteristics of the composition. In particular, cornpositions
consisting
essentially of N,N-dimethyltryptamine compounds will not comprise material
amounts of
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
other pharmaceutically active substances (i.e. material amounts of other drug
substances).
The composition of the present invention may comprise from 2% to 98% by
weight of N,N-dimethyltryptamine, and preferably comprises from 5% to 95% by
weight
of N,N-dimethyltryptamine. Preferred compositions of the present invention
comprise
from 10% to 90% by weight of N,N-dimethyltryptamine, or from 15% to 85% by
weight of
N,N-dimethyltryptamine, or from 20% to 80% by weight of N,N-
dimethyltryptamine, or
from 25% to 75% by weight of N,N-dimethyltryptamine, or from 30% to 70% by
weight of
N,N-dimethyltryptamine, or from 40% to 60% by weight of N,N-
dimethyltryptamine.
The composition of the present invention preferably comprise from 5% to 99.9%
by weight of a deuterated N,N-dimethyltryptamine compound selected from a,a-
dideutero-N,N-dimethyltryptamine and a,a,8,8-tetradeutero-N,N-
dimethyltryptamine.
An aspect of the invention provides a composition obtainable by the reduction
of
a composition obtainable by the reduction of 2-(3-indolyI)-N,N-
dimethylacetamide with a
reducing agent consisting essentially of lithium aluminium hydride and/or
lithium
aluminium deuteride. In both aspects, the reducing agent may be dissolved or
suspended in a liquid medium. Typically, owing to strong reactivity with water
and protect
solvents such as alcohols, although available in solid (powdered) form,
lithium aluminium
hydride (or deuteride) are often manipulated in dried, aprotic solvents such
as ethers,
often under an inert atmosphere. The skilled person is well aware of such
precautions
and appropriate protocols.
It will be understood that the invention thus provides a composition
obtainable by
the reduction of a composition comprising 2-(3-indolyI)-N,N-dimethylacetamide
with a
reducing agent consisting essentially of lithium aluminium hydride and/or
lithium
aluminium deuteride, optionally dissolved or suspended in a liquid medium. The
invention also provides a composition obtained by such reduction or, more
generally,
obtained by a reduction in accordance with the second or third aspect of the
invention.
It is also to be understood that the amounts of N,N-dimethyltryptamine
compounds described herein with specific reference to the composition of the
first aspect
of the invention may be applied mutatis mutandis to the compositions of the
second and
third aspects of the invention.
According to particular embodiments, by reciting that the reducing agent
consists
essentially of lithium aluminium hydride and/or lithium aluminium deuteride is
meant that
the reducing agent may comprise additional components but that the presence of
these
components will not materially affect the essential characteristics of the
reducing agent
(in particular stability and reductive propensity).
11
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
According to the fourth aspect of the invention, there is provided a
pharmaceutical
composition comprising a composition as defined in accordance with the first
to third
aspects of the invention, in combination with a pharmaceutically acceptable
excipient.
The pharmaceutical composition of the invention comprises a composition of the
invention (according to any one of its first to third aspects) in combination
with one or
more pharmaceutically acceptable excipients. Suitable pharmaceutical
compositions
can be prepared by the skilled person, with examples of pharmaceutically
acceptable
excipients including but not being limited to those described in Gennaro et.
al.,
Remmington: The Science and Practice of Pharmacy, 20th Edition, Lippincott,
Williams
and Wilkins, 2000 (specifically part 5: pharmaceutical manufacturing).
Suitable
excipients are also described in the Handbook of Pharmaceutical Excipients,
2nd Edition;
Editors A. Wade and P. J.Weller, American Pharmaceutical Association,
Washington,
The Pharmaceutical Press, London, 1994.
Pharmaceutical compositions of the invention are expected to display superior
oral bioavailability compared with undeuterated N,N-dimethyltryptamine.
Accordingly a
compound or composition of the present invention may be compressed or
otherwise
formulated into solid dosage units, such as tablets, capsule, orally
disintegrating tablets,
thin films, buccal patches and buccal tablets, or be processed into capsules
or
suppositories. When formulated as an orally disintegrating tablet, a compound
or
composition of the present invention is compatible with the Zydis platform. A
Zydis
tablet is produced by lyophilizing or freeze-drying a freebase compound or
composition
of the present invention in a matrix. The resulting product is very
lightweight. Such
embodiments of a formulation comprise particles, preferably with a particle
size of less
than 50 m, of a compound or composition of the present invention physically
suspended
in a water-soluble matrix which is then lyophilised. An orally disintegrating
tablet
formulated this way dissolves rapidly when placed in mouth.
By means of pharmaceutically suitable liquids the compounds can also be
prepared in the form of a solution, suspension, emulsion, or as a spray. For
making
dosage units, including tablets, the use of conventional additives such as
fillers,
colorants, polymeric binders and the like is contemplated. In
general, any
pharmaceutically acceptable additive can be used.
Suitable fillers with which the pharmaceutical compositions can be prepared
and
administered include lactose, starch, cellulose and derivatives thereof, and
the like, or
mixtures thereof used in suitable amounts. For parenteral administration,
aqueous
suspensions, isotonic saline solutions and sterile injectable solutions may be
used,
containing pharmaceutically acceptable dispersing agents and/or wetting
agents, such
as propylene glycol or butylene glycol.
12
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
For parenteral administration, aqueous solutions, isotonic saline solutions
and
sterile injectable solutions may be used, containing pharmaceutically
acceptable dis-
persing agents and/or wetting agents, such as propylene glycol or butylene
glycol.
Formulations suitable for inhalation, transdermal, mucosal or transmembrane
administration comprise a freebase of a deuterated N,N-dimethyltryptamine
compound,
typically with one or more biocompatible excipient. Such formulations achieve
a longer
lasting therapeutic effect than equivalent formulations of undeuterated N,N-
dimethyltryptamine.
Thus, an aspect of the invention provides a parenteral formulation comprising
a
freebase of one or more deuterated N,N-dimethyltryptamine compound selected
from
N,N-dimethyltryptamine compounds,
a, a-dideutero-N,N-di methyltryptami ne
compounds, a-protio, a-deutero-N,N-dimethyltryptamine compounds, together with
a
biocompatible excipient. In preferred embodiments the deuterated N,N-
dimethyltryptamine compound is a compound of Formula I, wherein the ratio of
deuterium:protium in the compound is greater than that found naturally in
hydrogen; each
R1 is independently selected from H and D; R2 is selected from CH3 and CD3; R3
is
selected from CH3 and CD; and each YH is independently selected from H and D.
Typically the biocompatible excipient comprises a solvent. Preferably the
solvent
is selected from any one or a combination of two or more of propylene glycol,
glycerine,
polyethylene glycol, water, ethanol and triacetin. For preferred inhalable
formulations the
solvent is selected from propylene glycol, glycerine and polyethylene glycol,
or a mixture
thereof. Preferably the solvent is a mixture of propylene glycol and glycerine
in a ratio of
from about 50:50 to about 30:70 by weight The concentration of the freebase is
from
about 1 mg/mL to about 1000 nng/mL. Preferably the biocompatible excipient
comprises
a taste-masking agent.
In preferred embodiments the formulation has an oxygen content of less than 2
ppm. In embodiments the formulation is stored in a container adapted to
prevent
penetration of ultraviolet light.
The invention also provides a pharmaceutical composition of the invention, in
combination with packaging material suitable for the composition, the
packaging material
including instructions for the use of the pharmaceutical composition.
The compositions of the invention are useful in therapy and may be
administered
to a patient in need thereof. As used herein, the term 'patient' preferably
refers to a
human patient, but may also refer to a domestic mammal. The term does not
encompass
laboratory mammals.
In accordance with the sixth aspect of the invention, there is provided a
composition as defined according to any one of the first to fourth aspects for
use in a
13
method of treating a psychiatric disorder or a neurological disorder in a
patient. The seventh
aspect of the invention provides a method of treating a psychiatric disorder
or a neurological
disorder comprising admin. istering to a patient in need thereof a composition
as defined
according to any one of the first to fourth aspects and the eighth aspect
provides the use of a
composition as defined according to any one of the first to fourth aspects in
the manufacture
of a medicament for use in a method of treating a psychiatric disorder or a
neurological
disorder in a patient. In embodiments of the sixth to eight aspects of the
present invention the
psychiatric or neurological disorder is selected from (i) an obsessive
compulsive disorder, (ii)
a depressive disorder, (iii) a schizophrenia disorder, (iv) a schizotypal
disorder, (v) an anxiety
disorder, (vi) substance abuse, (vii) an avolition disorder, and (viii) a
brain injury disorder.
As used herein the term 'psychiatric disorder' is a clinically significant
behavioural or
psychological syndrome or pattern that occurs in an individual and that is
associated with
present distress (e.g., a painful symptom) or disability (Le., impairment in
one or more
important areas of functioning) or with a significantly increased risk of
suffering death, pain,
disability, or an important loss of freedom.
As used herein the term 'neurological disorder' means a disease of the central
and
peripheral nervous system.
Diagnostic criteria for psychiatric and neurological disorders referred to
herein are
provided in, for example, the Diagnostic and Statistical Manual of Mental
Disorders, Fifth
Edition, (DSM-5)
As used herein the term 'obsessive-compulsive disorder' is defined by the
presence of
either obsessions or compulsions, but commonly both. The symptoms can cause
significant
functional impairment and/or distress. An obsession is defined as an unwanted
intrusive
thought, image or urge that repeatedly enters the person's mind. Compulsions
are repetitive
behaviours or mental acts that the person feels driven to perform. Typically
obsessive-
compulsive disorder (OCD) manifests as one or more obsession which drives
adoption of a
compulsion. For example, an obsession with germs may drive a compulsion to
clean. A
compulsion can either be overt and observable by others, such as checking that
a door is
locked, or a covert mental act that cannot be observed, such as repeating a
certain phrase in
one's mind.
As used herein the term 'depressive disorder' includes major depressive
disorder,
persistent depressive disorder, bipolar disorder, bipolar depression, and
depression in
terminally ill patients.
As used herein the term 'major depressive disorder' (MDD, also referred to as
major
depression or clinical depression) is defined as the presence of five or more
of the
14
Date Recue/Date Received 2022-12-13
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
following symptoms over a period of two-weeks or more (also referred to herein
as a
'major depressive episode'), most of the day, nearly every day:
= depressed mood, such as feeling sad, empty or tearful (in children and
teens,
depressed mood can appear as constant irritability);
= significantly reduced interest or feeling no pleasure in all or most
activities;
= significant weight loss when not dieting, weight gain, or decrease or
increase in
appetite (in children, failure to gain weight as expected);
= insomnia or increased desire to sleep;
= either restlessness or slowed behaviour that can be observed by others;
= fatigue or loss of energy;
= feelings of worthlessness, or excessive or inappropriate guilt;
= trouble making decisions, or trouble thinking or concentrating;
= recurrent thoughts of death or suicide, or a suicide attempt.
At least one of the symptoms must be either a depressed mood or a loss of
interest or pleasure.
Persistent depressive disorder, also known as dysthymia, is defined as a
patient
exhibiting the following two features:
A. has depressed mood for most the time almost every day for at least two
years. Children and adolescents may have irritable mood, and the time
frame is at least one year.
B. While depressed, a person experiences at least two of the following
symptoms:
= Either overeating or lack of appetite.
= Sleeping too much or having difficulty sleeping.
= Fatigue, lack of energy.
= Poor self-esteem.
= Difficulty with concentration or decision making.
As used herein the term 'treatment resistant depression' describes MDD which
fails to achieve an adequate response to an adequate treatment with standard
of care
therapy.
As used herein 'bipolar disorder' also known as manic-depressive illness, is a
disorder that causes unusual shifts in mood, energy, activity levels, and the
ability to
carry out day-to-day tasks.
There are two defined sub-categories of bipolar disorder; all of them involve
clear
changes in mood, energy, and activity levels. These moods range from periods
of
extremely "up," elated, and energised behaviour (known as manic episodes, and
defined
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
further below) to very sad, "down," or hopeless periods (known as depressive
episodes).
Less severe manic periods are known as hypomanic episodes.
Bipolar I Disorder¨ defined by manic episodes that last at least 7 days, or by
manic symptoms that are so severe that the person needs immediate hospital
care.
Usually, depressive episodes occur as well, typically lasting at least 2
weeks. Episodes
of depression with mixed features (having depression and manic symptoms at the
same
time) are also possible.
Bipolar ll Disorder¨ defined by a pattern of depressive episodes and hypomanic
episodes, but not the full-blown manic episodes described above.
As used herein 'bipolar depression' is defined as an individual who is
experiencing depressive symptoms with a previous or coexisting episode of
manic
symptoms, but does not fit the clinical criteria for bipolar disorder.
As used herein the term 'anxiety disorder' includes generalised anxiety
disorder,
phobia, panic disorder, social anxiety disorder, and post-traumatic stress
disorder.
'Generalised anxiety disorder' (GAD) as used herein means a chronic disorder
characterised by long-lasting anxiety that is not focused on any one object or
situation.
Those suffering from GAD experience non-specific persistent fear and worry,
and
become overly concerned with everyday matters. GAD is characterised by chronic
excessive worry accompanied by three or more of the following symptoms:
restlessness,
fatigue, concentration problems, irritability, muscle tension, and sleep
disturbance.
'Phobia' is defined as a persistent fear of an object or situation the
affected
person will go to great lengths to avoid, typically disproportional to the
actual danger
posed. If the feared object or situation cannot be avoided entirely, the
affected person
will endure it with marked distress and significant interference in social or
occupational
activities.
A patient suffering from a 'panic disorder' is defined as one who experiences
one
or more brief attack (also referred to as a panic attack) of intense terror
and
apprehension, often marked by trembling, shaking, confusion, dizziness,
nausea, and/or
difficulty breathing. A panic attack is defined as a fear or discomfort that
abruptly arises
and peaks in less than ten minutes.
'Social anxiety disorder' is defined as an intense fear and avoidance of
negative
public scrutiny, public embarrassment, humiliation, or social interaction.
Social anxiety
often manifests specific physical symptoms, including blushing, sweating, and
difficulty
speaking.
'Post-traumatic stress disorder' (PTSD) is an anxiety disorder that results
from a
traumatic experience. Post-traumatic stress can result from an extreme
situation, such
as combat, natural disaster, rape, hostage situations, child abuse, bullying,
or even a
16
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
serious accident. Common symptoms include hypervigilance, flashbacks, avoidant
behaviours, anxiety, anger and depression.
As used herein the term 'substance abuse' means a patterned use of a drug in
which the user consumes the substance in amounts or with methods which are
harmful
to themselves or others.
As used herein the term 'an avolition disorder' refers to a disorder which
includes
as a symptom the decrease in motivation to initiate and perform self-directed
purposeful
activities.
As used herein the term 'brain injury disorder' refers to an injury to the
brain that
occurs after birth and is not congenital, degenerative or hereditary. The term
encompasses traumatic brain injury, for example from a car accident or a
sports injury,
and acquired brain injury, such as ischaemic stroke, transient ischaemic
stroke,
haemorrhagic stroke, brain tumour, meningitis or encephalitis.
In preferred embodiments of the sixth to eighth aspects of the present
invention,
the psychiatric or neurological disorder is selected from (i) an obsessive
compulsive
disorder, (ii) a depressive disorder, (iii) an anxiety disorder, (iv)
substance abuse, (v) an
avolition disorder, and (vi) a brain injury disorder.
According to particular embodiments of the sixth to eighth aspects of the
present
invention, the depressive disorder is major depressive disorder. According to
still more
particular embodiments, the major depressive disorder is treatment-resistant
major
depressive disorder.
Compositions comprising deuterated N,N-dimethyltryptamine compounds of the
present invention can be synthesised at gram scale up to multi-kg scale
following the
reaction scheme (synthetic scheme) provided in Scheme 1.
The relative proportions of N,N-dimethyltryptamine compounds against
deuterated N,N-dimethyltryptamine compounds and partially deuterated N,N-
dimethyltryptamine compounds may be controlled by varying the ratio of lithium
aluminium hydride and lithium aluminium deuteride in the reducing agent.
Relative
proportions may further be varied by adding one or more of N,N-
dimethyltryptamine, 0,0-
dideutero-N,N-dimethyltryptarnine and a,a,13,[3-tetradeutero-N,N-
dimethyltryptarnine to
the compositions described hereinabove.
A particular advantage of the present invention, in particular but not limited
to the
compositions obtainable in accordance with its third aspect and the method of
its ninth
aspect, is that the reductions described in accordance with these aspects of
the invention
allow particularly high purities to be obtained, without the necessity for
subsequent
chromatographic purification (e.g. column chromatography), thereby increasing
the
efficiency through which compositions of the invention may be prepared.
Moreover, the
17
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
ability to avoid the use of chromatography in order to achieve high purities
makes scale
up more efficient and therefore cost-effective.
Identification of the compositions obtained by methods of the present
invention
may be achieved, if desired, by chromatographic separation of the components
of the
mixtures by conventional means at the disposal of the skilled person in
combination with
spectroscopic and/or mass spectrometric analysis.
Alternative compositions are obtainable by mixing undeuterated N,N-
dimethyltryptamine, obtainable by Scheme 1 when the reducing agent is
exclusively
lithium aluminium hydride, with a deuterated N,N-dimethyltryptamine compound
obtainable from Scheme 1 when the reducing agent is exclusively lithium
aluminium
deuteride.
The compositions described hereinabove may be further modified by adding one
or more deuterated N,N-dimethyltryptamine compounds. Stocks of such deuterated
N,N-dimethyltryptamine compounds may be obtained, for example, from the
chromatographic separation described above. In this way, for example, the
compounds
of the tenth aspect of the invention may be obtained.
Whilst identification of the compositions resultant from the reduction
described
herein may be achieved by chromatographic separation of the components of the
mixtures, in combination with spectroscopic and/or mass spectrometric
analysis, a
particular benefit of the present invention is that, according to particular
embodiments,
there may be no necessity to do so. This is because, over and above the
purities
achievable in accordance with the present invention, we have as alluded to
above
recognised that there is a quantifiable relationship between the extent of
deuteration (or
in other words the quantity or proportion of deuterium in the N,N-
dimethyltryptamine
compounds in the compositions of the present invention) and the metabolic half-
life of
the resultant composition. The extent of deuteration may be controlled through
the
amount of deuterium-containing reducing agent used in the method of the
invention,
through which (according to particular embodiments) the compositions of the
invention
may be obtained, and thus control exercised, in a predictable way, over
potentiation of
the metabolic half-life of the parent compound (undeuterated N,N-
dimethyltryptamine).
In particular, as detailed in Example 1 and related Figures 2 and 3, the
inventors
have demonstrated that increasing deuterium enrichment at the a-carbon of N,N-
dimethyltryptamine increases metabolic stability, leading to a decrease in
clearance and
longer half-life, wherein a linear relationship exists between molecular
weight and half-
life between 188.3 and 190.3 grams per mole, and synergistic primary and
secondary
kinetic isotope effects provide a predictable relationship between molecular
weight and
18
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
half-life for compounds and compositions of Formula I wherein R1 is H between
188.3
and 196.3 grams per mole.
Such types of composition constitute specific embodiments of the first aspect
of
the invention. According to these specific embodiments, the composition
consists
essentially of two or three compounds selected from N,N-dimethyltryptamine, a-
protio,
a-deutero-N, N-dimethyltryptamine and a, a-dideutero-N,N-di methyltryptamine,
the
composition optionally being in the form of a pharmaceutically acceptable
salt, wherein
the mean molecular weight of N,N-dimethyltryptamine, a-protio, a-deutero-N,N-
dimethyltryptamine and a, a-dideutero-N, N-dimethyltryptamine present in the
composition is from 188.28 to 190.28.
According to additional specific aspects, the composition consists essentially
of
one, two or three compounds selected from N,N-
bis(trideutero)dinnethyltryptamine
(Compound 5), a-protio, a-deutero-N,N-bis(trideutero)dimethyltryptamine
(Compound 2)
and a,a-dideutero-N,N-bis(trideutero)dimethyltryptannine (Compound 1), said
compounds optionally being in the form of a pharmaceutically acceptable salt,
wherein
the molecular weight or mean molecular weight of N,N-
bis(trideutero)dimethyltryptamine,
a-protio, a-deutero-N,N-bis(trideutero)dimethyltryptannine, and a,a-dideutero-
N,N-
bis(trideutero)dimethyltryptannine, present in the composition is from 188.9
to 196.3. In
preferred embodiments of this aspect, the composition consists essentially of
one
compound selected from N,N-bis(trideutero)dimethyltryptamine (Compound 5),
preferably a-protio, a-deutero-N,N-bis(trideutero)dimethyltryptamine (Compound
2) and
more preferably a,a-dideutero-N,N-bis(trideutero)dimethyltryptamine (Compound
1) in
order of increasing metabolic stability.
As used herein, mean molecular weight means the weighted average of
molecular weights of the of the N,N-dimethyltryptamine compounds, a-protio, a-
deutero-
N,N-dimethyltryptamine compounds and a,a-dideutero-N,N-dinnethyltryptamine
compounds, as measured by an appropriate mass spectroscopic technique, for
example
LC-MS SIM (selected-ion monitoring), ignoring any weight contribution by
formation of
pharmaceutically acceptable salts, where applicable.
It will be understood that providing compositions with such specific mean
molecular weights can be achieved by those skilled in the art through the
teachings
herein, in particular by adjusting the relative proportions of lithium
aluminium hydride:
lithium aluminium deuteride used in stage 2 when varying the level of
deuteration at the
alpha position, and by adjusting the relative proportions of
dimethylamine:deuterated
dimethylamine used in stage 1 when varying the level of deuteration at the N,N-
dimethyl
position.
19
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
In this context, by reciting that the composition consists essentially of the
mixture
of N,N-dimethyltryptamine and one or both of a-protio, a-deutero-N,N-
dimethyltryptamine and a,a-dideutero-N,N-dimethyltryptamine means that the
composition may comprise additional components to these but that the presence
of such
additional components will not materially affect the essential characteristics
of the
composition. In particular, the composition will not comprise material
quantities of other
pharmaceutically active compounds, including other N,N-dimethyltryptamine
compounds. Thus material quantities of other deuterated N,N-dimethyltryptamine
compounds, in particular p-protio, p-deutero-N,N-dimethyltryptamine compounds
and
13j3-dideutero-N,N-dimethyltryptamine compounds, such as p-protio, p-deutero-
N,N-
dimethyltryptamine and p,p-dideutero-N,N-dimethyltryptamine and p-protio, p-
deutero-
N,N-dimethyltryptamine compounds and
13, p-dideutero-N,N-dinnethyltryptamine
compounds having respectively one or two deuterium atoms in place of hydrogen
atoms
at the a position are not present in compositions of such embodiments.
In other words, and alternatively put, the compositions according to one
specific
embodiment constitute a drug substance comprising a biologically active
ingredient
consisting essentially of one or more of N,N-dimethyltryptamine, a-protio, a-
deutero-N,N-
dimethyltryptamine and a,a-dideutero-N,N-dimethyltryptamine, wherein the
biologically
active ingredient has a mean molecular weight from 188.3 to 190.3 and wherein
the
compounds comprised in the drug substance are optionally in the form of a
pharmaceutically acceptable salt. The compositions according to a second
specific
embodiment constitute a biologically active ingredient consisting essentially
of one or
more of N,N-bis(trideutero)dimethyltryptamine (Compound 5), a-protio, a-
deutero- N,N-
bis(trideutero)dimethyltryptannine (Compound 2) and
a, a-d ideutero-N, N-
bis(trideutero)dimethyltryptamine (Compound 1), wherein the biologically
active
ingredient has a mean molecular weight from 188.9 to 196.3 and wherein the
drug
substance is optionally in the form of a pharmaceutically acceptable salt.
It will be understood that the compositions according to these specific
embodiments comprise one or more of a-protio, a-deutero-N,N-
dinnethyltryptamine
compounds and a,a-dideutero-N,N-dimethyltryptamine compounds in amounts
greater
than found in isotopically unenriched N,N-dimethyltryptamine. It will also be
understood
that the greater the proportion of a-protio, a-deutero-N,N-dimethyltryptamine
compounds
and a,a-dideutero-N,N-dimethyltryptamine compounds in these specific
embodiments,
the higher the mean molecular weight of the composition.
According to more specific embodiments, the mean molecular weight of N,N-
dimethyltryptamine, a-protio, a-deutero-N,N-dimethyltryptamine and a,a-
dideutero-N,N-
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
dimethyltryptamine present in the composition is from 188.9 to 189.7, for
example
188.90 to 189.70.
According to still more specific embodiments of the specific embodiments
described herein, including compositions in which the mean molecular weight of
N,N-
dimethyltryptamine, a-protio, a-deutero-N,N-dimethyltryptamine and a,a-
dideutero-N,N-
dimethyltryptamine present in the composition is from 188.9 to 189.7, the
compounds
comprised in the composition optionally are in the form of a pharmaceutically
acceptable
salt, by which it will be understood that the N,N-dimethyltryptamine, a-
protio, a-deutero-
N,N-dimethyltryptamine and a,a-dideutero-N,N-dimethyltryptamine present in the
composition are present in pharmaceutically acceptable salt form. Such salts
may be as
described elsewhere herein and, according to yet more specific embodiments,
the
composition is in the form of a fumarate salt.
Methods by which the compounds of formula I may be produced are described
below and are suitable for the production of high purity compounds of formula
I. In some
embodiments, the compound of formula I, or a pharmaceutically acceptable salt
thereof,
is of a purity of between 99% and 100% by HPLC, such as a purity of between
99.5%
and 100% by HPLC. In some embodiments, the compound of formula I, or a
pharmaceutically acceptable salt thereof, is of a purity of between 99.9% and
100% by
HPLC, such as a purity of between 99.95% and 100% by HPLC.
In some embodiments, the compound of formula I, or a pharmaceutically
acceptable salt thereof, produces two or fewer impurity peaks by HPLC. In some
embodiments, where the compound of formula I, or a pharmaceutically acceptable
salt
thereof, produces impurity peaks by HPLC, no impurity peak is greater than
0.2%. In
some embodiments, no impurity peak by HPLC is greater than 0.1%.
In some embodiments, the compound of formula I is in the form of a
pharmaceutically acceptable salt. The pharmaceutically acceptable salt often
comprises
a compound of formula I and a suitable acid. The compound of formula I is
typically
protonated at ¨N(R2R3)2, forming ¨[NHR2R3], and the resultant positive charge
is
countered by an anion.
P. H. Stahl and C. G. Wermuth provide an overview of pharmaceutical salts and
the acids comprised therein in Handbook of Pharmaceutical Salts: Properties,
Selection
and Use, Weinheim/Zurich:Wiley-VCHNHCA, 2002. The acids described in this
review
are suitable components of the pharmaceutically acceptable salt of formula I.
In some embodiments, the acid is any one selected from the group consisting of
fumaric acid, tartaric acid, citric acid, hydrochloric acid, acetic acid,
lactic acid, gluconic
acid, 1-hydroxy-2-naphthoic acid, 2,2-dichloroacetic acid, 2-
hydroxyethanesulfonic acid,
2-oxoglutaric acid, 4-acetamidobenzoic acid, 4-aminosalicylic acid, adipic
acid, ascorbic
21
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
acid, aspartic acid, benzenesulfonic acid, benzoic acid, camphoric acid,
camphor-10-
sulfonic acid, decanoic acid, hexanoic acid, octanoic acid, carbonic acid,
cinnamic acid,
cyclamic acid, dodecylsulfuric acid, ethane-1,2-disulfonic acid,
ethanesulfonic acid,
formic acid, galactaric acid, gentisic acid, glucoheptonic acid, glucuronic
acid, glutamic
acid, glutaric acid, glycerophosphoric acid, glycolic acid, hippuric acid,
hydrobromic acid,
isobutyric acid, lactobionic acid, lauric acid, maleic acid, malic acid,
malonic acid,
mandelic acid, methanesulfonic acid, naphthalene-1,5-disulfonic acid,
naphthalene-2-
sulfonic acid, nicotinic acid, nitric acid, oleic acid, oxalic acid, palmitic
acid, pamoic acid,
phosphoric acid, proprionic acid, pyroglutamic acid (- L), salicylic acid,
sebacic acid,
stearic acid, succinic acid, sulfuric acid, thiocyanic acid, toluenesulfonic
acid and
undecylenic acid .
Often, the acid is any one selected from fumaric acid, tartaric acid, citric
acid and
hydrochloric acid. In some embodiments,
the acid is fumaric acid, i.e. the
pharmaceutically acceptable salt is a fumarate salt.
Also disclosed herein is a synthetic method for making a compound of formula I
or a pharmaceutically acceptable salt thereof. The method comprises stage 2
and
optionally stage 1, wherein stage 1 comprises:
(i) reacting a compound of formula III with two or more coupling agents to
produce
an activated compound;
(ii) reacting the activated compound with an amine having the formula (R2)2NH
to
produce a compound of formula II;
and wherein stage 2 comprises reacting the compound of formula II with LiAlat
or LiAIH4 and LiAlat,
OH NR23 NR2R3
11.! yH
I \
.0" II ..=" N 44
111 11
wherein:
each R1 is independently selected from H and D;
R2 is selected from CH3 and CD3;
R3 is selected from CH3 and CD3;
each YH is independently selected from H and D.
22
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
For the avoidance of doubt, embodiments related to the compound of formula I,
or a pharmaceutically acceptable salt thereof, of the first aspect of the
invention also
apply mutatis mutandis to the compound of formula I (and thus compounds of
formulae
III and II) of the synthetic method.
The synthetic method avoids the use of problematic oxalyl chloride and employs
compounds of formula III, which may be derived from auxin derivatives. High
quality and
purity auxins of formula III are commercially available at scale and/or can be
readily
synthesised via the Fischer synthesis, Bartoli synthesis, Japp-Klingemann
synthesis or
Larock synthesis (see, for example, M. B. Smith and J. March, 2020, March's
Advanced
Organic Chemistry, 81h edition, Wiley, New Jersey). The method is efficient,
scalable,
compatible with Current Good Manufacturing Practices (cGMP), and is suitable
for the
production of high purity compounds of formula I. For example, the method is
suitable
for the production of compounds of formula I in batch scales ranging from 1 g
to 100 kg
and is suitable for the production of compounds of formula I with a purity of
>99.9% and
overall yield of 65% or more.
The compound of formula II is produced on reacting a compound of formula III
with two or more coupling agents to produce an activated compound, and
reacting the
activated compound with an amine having the formula R2R3NH. Without wishing to
be
bound by theory, it is understood that the nitrogen atom of the amine binds to
the carbon
atom of the carbonyl of formula III, resulting in the formation of the
compound of formula
II. For the avoidance of doubt, the R2 and R3 groups of formulae II and I are
derived from
the R2 and R3 groups of the amine. Thus, as described above, R2 and R3 of
formulae II
and I are independently selected from CH3 and CD3.
The compound of formula I is produced on reacting the compound of formula ll
with LiAID4or L1A11-14 and LiAlat. Without wishing to be bound by theory, it
is understood
that the hydride or deuteride ions provided by LiAID4 or LiA11-14 and LiAlat
bind to the
carbon atom of the carbonyl of formula II, resulting in the formation of the
compound of
formula I. For the avoidance of doubt, the xH groups of formula I are derived
from the
hydride or deuteride ions provided by LiAID4 or LiAIH4 and LiAID4.
As described above, the method comprises stage 1 and stage 2. Stage 1
comprises:
(i) reacting a compound of formula III with two or more coupling agents to
produce
an activated compound; and
(ii) reacting the activated compound with an amine having the formula R2RNH
to produce a compound of formula II.
The term "coupling agent" refers to an agent which facilitates the chemical
reaction between an amine and a carboxylic acid. The two or more coupling
agents may
23
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
comprise a carboxylic acid activating agent, i.e. an agent which reacts with
the carboxylic
acid moiety of formula III to produce a compound comprising an activated
moiety derived
from the original carboxylic acid moiety that is more likely to react with an
amine than the
original carboxylic acid moiety.
The activated compound is the product of the reaction between the compound of
formula III and the two or more coupling agents. Where the two or more
coupling agents
comprise carboxylic acid activating agents, the activated compound comprises
an
activated moiety, derived from the original carboxylic acid moiety of formula
III, which is
more likely to react with an amine than the original carboxylic acid moiety.
The two or more coupling agents may comprise a carboxylic acid activating
agent. The two or more coupling agents may comprise an additive coupling
agent.
An additive coupling agent (also referred to herein as an "additive") is an
agent
which enhances the reactivity of a coupling agent. The additive may be a
compound
capable of reacting with the product of the reaction of formula III and the
coupling agent
(the product being a compound comprising an activated moiety) to produce a
compound
comprising an even more activated moiety that is more likely to react with an
amine than
the original activated moiety.
The additive may be capable of reacting with the product of the reaction of
formula III and the coupling agent (the product being a compound comprising an
activated moiety) to produce an activated compound comprising an even more
activated
moiety that is more likely to react with an amine than the original activated
moiety.
Often, the two or more coupling agents comprise a carboxylic acid activating
agent and an additive coupling agent.
At least one of the two or more coupling agents may be selected from the group
consisting of carbodiimide coupling agents, phosphonium coupling agents and 3-
(diethoxy-phosphoryloxy)-1,2,3-benzo[d]triazin-4(3H)-one (DEPBT), such as a
carbodiimide coupling agent or a phosphonium coupling agent. At least one of
the two
or more coupling agents may be a carbodiimide coupling agent.
A carbodiimide coupling agent is a coupling agent which comprises a
carbodiimide group R'-N=C=N-R", wherein R' and R" are hydrocarbyl groups
optionally
substituted with heteroatoms selected from nitrogen, sulfur and oxygen,
typically
nitrogen. Often, R' and R" are independently selected from C1-C6alkyl, C5-
C6cycloalkyl,
Cl-C6alkylamino and morpholinoCi-C6alkyl. Often, C1-C6alkyl is C3alkyl, 05-
C6cycloalkyl
is cyclohexyl, Cl-C6alkylamino is dimethylaminopropyl and/or morpholinoCi-
C6alkyl is
morpholinoethyl.
The carbodiimide coupling agent may be any one selected from the group
consisting of dicyclohexylcarbodiimide (DCC), diisopropylcarbodiimide (DIC),
(N-(3-
24
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
Dimethylaminopropy1)-N'-ethylcarbodiimide (EDC) and
1-cyclohexyl-(2-
morpholinoethyl)carbodiimide metho-p-toluene sulfonate (CMCT). The
carbodiimide
coupling agent may be any one selected from the group consisting of
dicyclohexylcarbodiimide (DCC), di
isopropylcarbodiimide (DI C) and (N-(3-
DimethylaminopropyI)-N'-ethylcarbodiimide (EDC). Often, the carbodiimide
coupling
agent is N-(3-DimethylaminopropyI)-N'-ethylcarbodiinnide (EDC), typically as a
hydrochloride salt (EDC.HCI). EDC or EDC.HCI are particularly preferred as
they are
non-toxic and are highly water soluble, facilitating their virtually complete
removal in
workup and wash steps of stage 1.
A phosphonium coupling agent comprises a phosphonium cation and a
counterion, typically a hexafluorophosphate anion. The phosphonium cation may
be of
formula [PRa3Rb]+ wherein Ra is di(Ci-Ce)alkylamino or pyrrolidinyl and RID is
halo or a
hydrocarbyl group optionally substituted with nitrogen and/or oxygen atoms.
Often, RID
is bromo, benzotriazol-1-yloxy or 7-aza-benzotriazol-1-yloxy.
The phosphonium coupling agent may be any one selected from the group
consisting of
benzotriazol-1-yloxy-tris(dimethylamino)-phosphonium
hexafluorophosphate (BOP), bromo-tripyrrolidino-phosphonium
hexafluorophosphate
(PyBrOP), benzotriazol-1-yloxy-
tripyrrolidino-phosphonium -- hexafluorophosphate
(PyBOP), 7-aza-benzotriazol-1-yloxy-tripyrrolidinophosphonium
hexafluorophosphate
(PyA0P) and ethyl cyano(hydroxyinnino)acetato-02) tri-(1-pyrrolidinyI)-
phosphonium
hexafluorophosphate (PyOxim).
At least one of the two or more coupling agents may be an additive coupling
agent selected from the group consisting of 1-hydroxybenzotriazole (HOBt),
hydroxy-
3,4-dihydro-4-oxo-1,2,3-benzotriazine (HOOBt), N-hydroxysuccinimide (HOSu), 1-
hydroxy-7-azabenzotriazole (HOAt), ethyl 2-cyano-2-(hydroximino)acetate (Oxyma
Pure), 4-(N,N-Dinnethylamino)pyridine (DMAP), N-hydroxy-5-norbornene-2,3-
dicarboxinnide (HONB), 6-chloro-1-hydroxybenzotriazole (6-CI-HOBt), 3-hydroxy-
4-oxo-
3,4-dihydro-1,2,3-benzotriazine (HODhbt), 3-hydroxy-4-oxo-3,4-dihydro-5-
azabenzo-
1,2,3-triazene (HODhat) and 3-hydroxyl-4-oxo-3,4-dihydro-5-azepine benzo-1,3-
diazines (HODhad).
At least one of the two or more coupling agents may be an additive coupling
agent selected from the group consisting of 1-hydroxybenzotriazole (HOBt),
hydroxy-
3,4-dihydro-4-oxo-1,2,3-benzotriazine (HOOBt), N-hydroxysuccinimide (HOSu), 1-
hydroxy-7-azabenzotriazole (HOAt), ethyl 2-cyano-2-(hydroximino)acetate (Oxyma
Pure) and 4-(N,N-Dimethylamino)pyridine (DMAP).
At least one of the two or more coupling agents may be an additive coupling
agent which is 1-hydroxybenzotriazole.
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
The two or more coupling agents may consist of a coupling agent and an
additive
coupling agent wherein the coupling agent and additive coupling agent may be
as
described in the above embodiments.
A benefit of using both a coupling agent and an additive coupling agent is an
increased rate of formation of compounds of formula II from compounds of
formula III
and an amine having the formula (R2)2NH. In addition, when an additive
coupling agent
is used together with a carbodiimide coupling agent, the likelihood of
unwanted side
reactions may be reduced. For example, reaction of a compound of formula III
with a
carbodiimide coupling reagent is likely to form an 0-acylisourea. This may
undergo a
rearrangement to form an N-acylurea, which is a stable compound unlikely to
react with
an amine. Additive coupling reagents may react with 0-acylureas before
rearrangement
to N-acylureas, and produce compounds that go on to react with an amine,
rather than
inactive N-acylureas.
Therefore, the two or more coupling agents may consist of a carbodiimide
coupling agent and an additive coupling agent.
The two or more coupling agents may consist of N-(3-DimethylaminopropyI)-N'-
ethylcarbodiimide (EDC), typically as a hydrochloride salt (EDC.HCI), and 1-
hydroxybenzotriazole (HOBO.
Often, an excess of coupling agent with respect to compound of formula III is
used. The ratio of coupling agent:compound of formula III may be about 1:1 to
about
3:1, typically about 1:1 to about 2:1 and most typically about 1:1 to about
1.5:1.
Often, an excess of additive coupling agent with respect to compound of
formula
III is used. Sometimes, the ratio of additive coupling agent:compound of
formula III is
about 1:1 to about 3:1, typically about 1:1 to about 2:1 and most typically
about 1:1 to
about 1.5:1.
Where the two or more coupling agents comprise a coupling agent and an
additive coupling agent, a ratio of coupling agent:compound of formula III and
additive
coupling agent:compound of formula III of about 1:1 to about 1.5:1 may be
used.
As described above, stage 1 comprises reacting the activated compound (the
product of reacting a compound of formula III with two or more coupling
agents) with an
amine having the formula R2R3NH to produce a compound of formula II. R2 and R3
of
formulae ll and I are independently selected from CH3 and CD3.
The ratio of amine:compound of formula III employed in the method is often
about
1:1. Sometimes, the ratio of amine:compound of formula III is about 1:1 to
about 3:1,
typically about 1:1 to about 2:1.
Sometimes, stage 1 further comprises isolating the compound of formula II. The
skilled person is aware of techniques in the art suitable for isolation of a
compound of
26
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
formula IL For example, a compound of formula II may be extracted into an
organic
solvent such as dichloromethane or ethyl acetate, washed with an aqueous
solution such
as an aqueous basic solution, and concentrated. To increase purity, the
isolated
compound of formula ll may be recrystallized. The skilled person is aware of
techniques
that are suitable for recrystallisation of compounds of formula II. For
example, the
compound of formula II may be dissolved in the minimum amount of solvent at a
particular temperature (e.g. at ambient temperature (e.g. 15 to 25 C) or at
elevated
temperatures where heat is applied to the solution) and the resultant solution
cooled to
encourage precipitation. Alternatively, or in addition, the volume of the
solution may be
reduced to encourage precipitation, e.g. by simple evaporation at ambient
temperature
and pressure. Alternatively, or in addition, an anti-solvent may be used (in
which the
compound of formula II is less soluble than the solvent already present).
Isolated compounds of formula II are stable and may be stored as solids at
ambient temperature, e.g. at about 20 C, in the air. They may, but need not
be, stored
under inert conditions, e.g. under nitrogen or argon, or at reduced
temperatures, e.g. in
a refrigerator or freezer.
Typically, steps (i) and (ii) of stage 1 are carried out in a suitable
solvent. The
skilled person is able to assess which solvents are suitable for these steps.
Examples
of suitable solvents include dichloromethane (DCM), acetone, isopropyl alcohol
(IPA),
isopropyl acetate (iPrOAc), tert-butyl methyl ether (TBME), 2-methyl
tetrahydrofuran (2-
MeTHF) and ethyl acetate (Et0Ac). In some embodiments, steps (i) and (ii) of
stage 1
are carried out in dichloromethane.
Steps (i) and (ii) of stage 1 are carried out at a suitable temperature and
the
skilled person is able to assess which temperatures are suitable for these
steps. Often,
steps (i) and (ii) of stage 1 are carried out at temperatures of about 10 C
to about 30 C.
In some embodiments, steps (i) and (ii) of stage 1 are carried out at room
temperature
(about 20 C).
Sometimes, stage 1 of the method comprises the steps of:
(1) contacting a compound of formula III and between 1 and 1.5 equivalents
of
an additive coupling agent, and between 1 and 1.5 equivalents of a
carbodiimide coupling agent to produce a first composition; and
(2) contacting the first composition with between 1 and 2 equivalents of an
amine
having the formula R2R3NH to produce a second composition.
Often, 1 g or more, such as 1 g to 100 kg or 1 g to 1 kg of a compound of
formula III
is employed in the method.
The contacting of steps i. and ii. is often carried out in the presence of a
first solvent,
such as between 5 and 20 volumes of a first solvent. The first solvent may be
selected
27
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
from any one of dichloromethane (DCM), acetone, isopropyl alcohol (IPA),
isopropyl
acetate (iPrOAc), tert-butyl methyl ether (TBME), 2-methyl tetrahydrofuran (2-
MeTHF)
and ethyl acetate (Et0Ac). Typically, the first solvent is DCM.
Often, step i. further comprises stirring or agitating the first composition.
The first
composition may be stirred or agitated for at least 30 minutes, such as 30
minutes to 3
hours or 30 minutes to 2 hours, preferably at least 1 hour, for example 1 to 3
hours or 1
to 2 hours. The first composition may be maintained at a temperature of
between 10 C
and 30 C.
The amine of step ii. is often dissolved in a solvent, such as tetrahydrofuran
(THE) or
ether, prior to contacting. The amine may be present in the solvent at a
concentration of
about 2 M. Typically, the amine of step ii. is dissolved in THE.
Sometimes, step ii. further comprises stirring or agitating the second
composition.
The second composition may be stirred or agitated for at least 30 minutes,
such as 30
minutes to 3 hours or 30 minutes to 2 hours, preferably at least 1 hour, for
example 1 to
3 hours or 1 to 2 hours. The second composition may be maintained at a
temperature
of between 10 C and 30 C.
Step ii. may further comprise contacting the second composition with an
aqueous
basic solution to produce a third composition, for example contacting the
second
composition with between 2 and 10 volumes of an aqueous basic solution such as
an
aqueous solution comprising potassium carbonate.
Sometimes, step ii. further comprises stirring or agitating the third
composition. The
third composition may be stirred or agitated for at least 1 minute, such as 1
to 15 minutes
or Ito 10 minutes, preferably at least 5 minutes, for example 5 to 15 minutes
or 5 to 10
minutes. The third composition may be maintained at a temperature of between
10 C
and 30 C.
Where the third composition comprises an organic and an aqueous component,
step
ii. may further comprise separating the organic component from the aqueous
component.
The organic component may be separated from the aqueous component within 8
hours
of the contacting of step i.
Sometimes, stage 1 of the method comprises the steps of:
I. adding to a first vessel 1 g or more of a compound of formula
III and between
1 and 1.5 equivalents of an additive coupling agent,
adding to the first vessel between 5 and 20 volumes of a first solvent
selected
from DCM, acetone, IPA, iPrOAc, TBME, 2-MeTHF and Et0Ac,
iii. adding to the first vessel between 1 and 1.5 equivalents of a
carbodiimide
coupling agent,
28
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
iv. stirring the contents of the first vessel for at least 30 minutes,
preferably at
least 1 hour (such as 1 to 2 hours), at between 10 C and 30 C,
v. adding to the first vessel between 1 and 2 equivalents of an amine
having the
formula R2R3NH, wherein the amine is preferably dissolved in an ether
solvent,
vi. further stirring the contents of the first vessel for at least 30
minutes,
preferably at least 1 hour (such as 1 to 2 hours), at between 10 C and 30 C,
vii. adding to the first vessel between 2 and 10 volumes of an aqueous
basic
solution,
viii. further stirring the contents of the first vessel for at least 1
minute, preferably
at least 5 minutes (such as 5 to 10 minutes), at between 10 C and 30 C,
ix. allowing an immiscible organic fraction to separate from an aqueous
fraction,
wherein the organic fraction comprises the compound of formula II, and
x. removing the organic fraction comprising the compound of formula II,
wherein steps i. to x. are carried out within a single 8 hour period.
Often, the first solvent is DCM.
Often, the amine is dinnethylamine. The amine may be dissolved in THE, for
example at a concentration of 2 M.
Often, the aqueous basic solution comprises potassium carbonate.
Sometimes, stage 1 of the method further comprises the steps of:
xi. drying the organic fraction with a drying agent, for example a drying
agent
selected from calcium chloride, magnesium sulphate, and sodium sulphate,
xii. filtering the organic fraction,
xiii. concentrating the organic fraction, for example under vacuum such as
under
a pressure of less than 1 atmosphere,
xiv. adding the concentrated organic fraction to a second vessel,
xv. adding between 2 and 10 volumes of a second solvent to the second
vessel,
wherein the second solvent is selected from IPA, Et0Ac, IPrOAc, acetonitrile
(MeCN), TBME, THF, 2-MeTHF and toluene,
xvi. stirring the contents of the second vessel for at least 1 hour,
preferably at
least 2 hours (such as 2 to 3 hours), at temperatures of between 45 C and
55 C,
xvii. cooling the contents of the second vessel to temperatures of
between 15 C
and 25 C,
xviii. filtering contents of the second vessel to obtain a filtrate,
wherein the filtrate
comprises the compound of formula II, and
xix. drying the filtrate.
29
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
The drying agent of step xi. is typically magnesium sulphate. Often, the
solvent
of step xv. is selected from TBME and IPA.
Stage 2 of the method comprises reacting the compound of formula II with
LiAlat
or LiA11-14 and LiAlas to produce a compound of formula I. LiAlat or mixtures
of LiA11-14
and LiAlat may be reacted with the compound of formula II. In preferred
embodiments,
stage 2 of the method comprises reacting the compound of formula ll with a
mixture of
LiAlF14 and LiAlai. Such mixtures comprise LiAID4 and comprise between 0.1 and
99.9%
hydride. Mixtures of between 2% and 98% lithium aluminium hydride or between
2%
and 98% lithium aluminium deuteride may be employed. Sometimes, mixtures of
LiA11-14
and LiAID4 consist essentially of 98% LiAlai / 2% LiA11-14. Sometimes, such
mixtures
consist essentially of 95% LiAID4/ 5% LiAlF14, 95% LiAlat/ 5% LiAlF14, 85%
LiAlat/ 15%
LiA11-14, 80% LiAID4/ 20% LiA11-14, 75% LiAID4/ 25% LiA11-14, 70% LiAID4/ 30%
LiAIH4, 65%
LiAlat/ 35% LiA11-14, 60% LiAlat/ 40% LiAlF14, 55% LiAID4/ 45% LiAlF14, 50%
LiAID4/
50% LiAlF14, 45% LiAlat/ 55% LiA11-14, 40% LiAllia 60% LiAlF14, 35% UAID¶ 65%
LiA11-14,
30% LiAID4/ 70% LiA11-14, 25% LiAlaa 75% LiA11-14, 20% LiAID4/ 80% LiA11-14,
15% LiAID4
/ 85% LiA11-14, 10% LiAlat/ 90% LiAlF14, 5% LiAlat/ 95% LiA11-14, or 2% LiAID4
/ 98%
LiAlF14.
By the mixtures of LiA11-14 and LiAlat consisting essentially of specified
percentages of L1A1F14 and LiAID4 is meant that the mixture may comprise
additional
components (other than LiAlF14 and LiAlat) but that the presence of these
additional
components will not materially affect the essential characteristics of the
mixture. In
particular, mixtures consisting essentially of LiAlF14 and LiAlat will not
comprise material
amounts of agents that are detrimental to the reduction of compounds of
formula ll to
produce compounds of formula I (e.g. material amounts of agents that react
with LiAl H4
and LiAlat, compounds of formula ll and/or compounds of formula I in a way
that inhibits
the reduction of compounds of formula ll to produce compounds of formula l).
The amount of LiA11-14 or LiAlai comprised in mixtures of the two depends on
the
degree of deuteration sought in the compound of formula I. For example, where
compounds of formula I are sought in which one YH is protium and the other is
deuterium,
a mixture of 50% LiAlF14 and 50% LiAID4 may be preferred. Alternatively, where
a mixture
of compounds of formula I are sought, in which approximately half of the
compounds
comprise two deuterium atoms at the a-position (i.e. both YH are deuterium)
and
approximately half of the compounds comprise one deuterium atom and one
protium
atom at the a-position (i.e. one YH is deuterium and the other is protium), a
mixture of
25% LiA11-14 and 75% LiAID4 may be preferred.
The amount of LiAlat or LiA11-14 and LiAID4 employed relative to compound of
formula II
is often 51:1. For the avoidance of doubt, the ratios of LiAlator LiAlF14 and
LiAlat relative
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
to compound of formula ll refer to the total amount of LiAlat or LiA11-14 and
LiAlat used
with respect to the amount of compound II. Sometimes, the ratio of LiAlat or
LiA11-14 and
LiAlas:compound of formula Ills 0.5:1 to 1:1, such as 0.8:1 to 1:1. Typically,
the ratio of
LiAIH4 and/or LiAlas:compound of formula II is 0.9:1.
Typically, stage 2 of the method is carried out in a suitable solvent. The
skilled
person is able to assess which solvents are suitable for stage 2. Examples of
suitable
solvents include ethers such as THF and diethyl ether. Often, stage 2 is
carried out in
THF.
Often, the LiAID4or LiAlF14 and LiAlat is provided as a solution or suspension
of
LiAlat or LiA11-14 and LiAlas in a suitable solvent such as an ether, for
example THE or
diethyl ether, typically THF.
Stage 2 of the method is carried out at a suitable temperature and the skilled
person is able to assess which temperatures are suitable for these steps.
Often, stage
2 is carried out at temperatures of about -5 C to about 65 C.
Typically, stage 2 further comprises isolating the compound of formula I. The
skilled person is aware of techniques in the art suitable for isolation of a
compound of
formula I. For example, on quenching the reaction (e.g. with an aqueous
solution of a
tartrate salt such as Rochelle's salts), a compound of formula I may be
extracted into an
organic solvent such as an ether, e.g. THF or diethyl ether, washed with an
aqueous
solution such as an aqueous basic solution, and concentrated. The isolated
compound
of formula I may be recrystallized. The skilled person is aware of techniques
that are
suitable for recrystallisation of a compound of formula I.
The examples of
recrystallisation techniques described with respect to recrystallisation of a
compound of
formula II apply mutatis mutandis to recrystallisation of a compound of
formula I.
Often, about 1 g or more, such as about 1 g to about 100 kg or about 1 g to
about
1 kg of a compound of formula II is employed in the method.
Typically, stage 2 of the method comprises contacting a compound of formula II
and between about 0.8 and about 1 equivalents, such as about 0.9 equivalents
of LiAlat
or LiA11-14 and LiAlat to produce a first composition.
The contacting is typically carried out in the presence of a solvent such as
an
ether, e.g. THF or diethyl ether, typically THF.
Often, the contacting comprises dropwise addition of LiAID4or LiAIH4 and
LiAlat
to a compound of formula II, wherein LiAlator LiAIH4 and LiAlat is provided as
a solution
or suspension of LiAID4 or LiAIH4 and LiAID4 in a suitable solvent, such as an
ether, e.g.
THF or diethyl ether. The LiAlas or LiAIH4 and LiAlat may be provided as a 2.4
M or 2
M solution or suspension of LiAID4 or LiAIH4 and LiAID4 in THF. Sometimes, the
LiAlID4
31
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
or LiAIH4 and LiAlat is provided as a 2 M solution or suspension of LiAlat or
LiA11-14 and
LiAlat in THF.
The contacting is often carried out at temperatures of about -5 C to about 65
'C.
Often, stage 2 further comprises stirring or agitating the first composition.
The
first composition may be stirred or agitated for about 1 hour to about 6
hours, typically
for about 2 hours. The first composition may be stirred or agitated at a
temperature of
about 55 C to about 65 C. Often, the first composition is stirred or
agitated at a
temperature of about 55 C to about 65 C and then cooled to temperatures of
about 10
C to about 30 C.
Typically, the compound of formula II is contacted with about 0.9 equivalents
of
LiAlat or LiA11-14 and LiAID.s.
Stage 2 of the method of the invention may comprise the steps of:
I. adding to a third vessel 1 g or more (such as 1 g to 1 kg) of a
compound of
formula II,
ii. adding to the third vessel between 5 and 20 volumes of an ether
solvent,
adding to the third vessel, dropwise over at least 15 minutes (e.g. 15 to 30
minutes), a solution of between 0.8 and 1 equivalents of LiAID4 or LiA11-14
and
LiAlat in the ether solvent at a temperature of between -5 C and 65 C,
iv. stirring the contents of the third vessel at between 55 C and 65 C
for
between 1 hour and 6 hours, preferably 2 hours, and
v. cooling the contents of the third vessel to between 10 C and 30 C,
wherein the contents of the third vessel comprise a compound of formula I.
Often, the ether solvent is THF. Typically, 0.9 equivalents of LiAID4or LiA11-
14 and
LiAlat are added to the third vessel in step iii. The LiAlat or LiAIH4 and
LiAlat is typically
added to the third vessel as a 2.4 M or 2 M solution in THE. Sometimes, the
LiAID4 or
LiAIH4 and LiAID4 is added to the third vessel as a 2 M solution in THF.
Sometimes, stage 2 of the method comprises a workup comprising the steps of:
vi. adding between 5 and 20 volumes of an aqueous solution of a tartrate
salt
(such as Rochelle's salts) to a fourth vessel,
vii. adding a composition comprising crude compound of formula I, over at
least
15 minutes (such as 15 minutes to 1 hour), preferably at least 30 minutes
(such as 30 minutes to 1 hour), to the fourth vessel at between 15 C and 25
C, and
viii. stirring the contents of the fourth vessel at between 15 C and
25 C for at
least 30 minutes (such as 30 minutes to 1 hour).
32
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
For the avoidance of doubt, the composition comprising crude compound of
formula I refers to the contents of the third vessel on completion of step v.
of stage 2,
described above.
Stage 2 of the method may further comprise the steps of:
ix. allowing
an organic fraction to separate from an aqueous fraction, wherein
the organic fraction comprises the compound of formula I,
x. removing the aqueous fraction from the fourth vessel,
xi. adding between 5 and 20 volumes of a brine solution to the fourth
vessel,
xii. stirring the contents of the fourth vessel at a temperature between 15
C and
25 C for at least 5 minutes (such as 5 to 15 minutes),
xiii. removing the organic fraction comprising the compound of formula I as
a
freebase,
xiv. drying the organic fraction using a drying agent, such as a drying
agent
selected from calcium chloride, magnesium sulphate, and sodium sulphate,
xv. filtering the organic fraction, and
xvi. concentrating the organic fraction, for example under vacuum such
as under
a pressure of less than 1 atmosphere.
Isolated compounds of formula I (produced via stage 2) are stable and may be
stored as solids at ambient temperature, e.g. at about 20 C, in the air. They
may, but
need not be, stored under inert conditions, e.g. under nitrogen or argon, or
at reduced
temperatures, e.g. in a refrigerator or freezer. Sometimes, the compound of
formula I is
stored in a solvent, for example dissolved in ethanol. Sometimes, the compound
of
formula I is stored in a solvent for more than 8 hours, typically more than 12
hours.
As described above, the compound of formula I may be in the form of a
pharmaceutically acceptable salt. A pharmaceutically acceptable salt may be
formed
from a compound of formula I by reaction with a suitable acid. Thus, the
method may
further comprise a stage 3, in which the compound of formula I is reacted with
an acidic
reagent to produce a pharmaceutically acceptable salt of the compound of
formula I.
The acidic reagent may be suitable for crystallising a pharmaceutically
acceptable salt
of the compound of formula I.
For the avoidance of doubt, where a reagent is expressed herein as a number of
equivalents, this is with respect to the molar equivalents of the compound of
formula Ill,
formula II or formula I for reagents in stage 1, stage 2 or stage 3
respectively.
A method of synthesising a compound of formula I, or a pharmaceutically
acceptable salt thereof often comprises stage 1, stage 2 and stage 3, wherein
stage 1
comprises:
33
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
(i) reacting a compound of formula III with two or more coupling agents to
produce
an activated compound;
(ii) reacting the activated compound with an amine having the formula R2R3NH
to produce a compound of formula II; and
(iii) isolating the compound of formula II;
stage 2 comprises reacting the compound of formula II with LiAlas or LiA11-14
and
LiA104; and
stage 3 comprises the step of reacting the compound of formula I with an
acidic
reagent suitable for crystallising a pharmaceutically acceptable salt of the
compound of
formula I.
Sometimes, a ratio of acidic reagent:compound of formula I of
:1 is used.
Often, the ratio of acidic reagent:compound of formula I is 1:1.
Typically, stage 3 of the method is carried out in a suitable solvent. The
skilled
person is able to assess which solvents are suitable for stage 3. Examples of
suitable
solvents include ethanol, IPA, iPrOAc and MeCN. Stage 3 is often carried out
in ethanol.
Stage 3 of the method of the invention is carried out at a suitable
temperature
and the skilled person is able to assess which temperatures are suitable for
these steps.
Stage 3 of the method often comprises contacting a compound of formula I and
an acidic reagent to produce a first composition. Often, the contacting of
stage 3 is
carried out at temperatures of 70 to 100 C, for example 70 to 90 C or 70 to
80 C.
Sometimes, the contacting of stage 3 is carried out at temperatures of about
75 C.
Often, stage 3 further comprises isolating the pharmaceutically acceptable
salt of
formula I. The skilled person is aware of techniques in the art suitable for
isolation of
such a compound. For example, where the compound is dissolved within a
suspension,
it may be separated from some of the other components of the suspension via
filtration,
such as hot filtration. The pharmaceutically acceptable salt of formula I may
precipitate
from the filtrate. The skilled person is aware of methods to encourage
precipitation of a
compound from a solution, such as cooling the solution, concentrating the
solution and/or
adding into the solution a crystalline form of the compound to encourage
nucleation and
the growth of further crystals of the compound from the solution (i.e.
seeding). The
pharmaceutically acceptable salt of formula I may be recrystallized. The
skilled person
is aware of techniques that are suitable for recrystallisation of a
pharmaceutically
acceptable salt of formula I. The examples of recrystallisation techniques
described with
respect to recrystallisation of a compound of formula II apply mutatis
mutandis to
recrystallisation of a pharmaceutically acceptable salt of formula I.
Stage 3 of the method may comprise the steps of:
34
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
i. adding to a fifth vessel at least one equivalent of an acidic reagent
suitable
for crystallising a pharmaceutically acceptable salt of a compound of formula
I,
ii. dissolving a compound of formula I as a freebase in between 5 and 20
volumes of a solvent such as a solvent selected from ethanol, IPA, iPrOAc
and MeCN and adding the solution to the fifth reaction vessel,
iii. stirring the contents of the fifth vessel at a temperature of above 72
C (such
as 72 to 90 C),
iv. filtering the contents of the fifth vessel,
v. adding the
filtrate to a sixth vessel and cooling the contents to a temperature
of 67 C t073 C,
vi. optionally seeding the sixth vessel with a crystalline form of the
pharmaceutically acceptable salt of the compound of formula I,
vii. stirring the contents of the sixth vessel at a temperature of 67 C to
73 C for
at least 30 minutes (such as 30 minutes to 1 hour),
viii. cooling the contents of the sixth vessel to a temperature of -5 C to
5 C at a
rate of 2 to 8 C per hour, and
ix. filtering the contents of the sixth vessel to produce a filter cake
comprising a
pharmaceutically acceptable salt of the compound of formula I.
Often, the solvent of step ii. is ethanol. Often, the rate of cooling in step
viii. is 5
C per hour.
As described above, the pharmaceutically acceptable salt often comprises a
compound of formula I and a suitable acid. The acids listed above as suitable
components of the pharmaceutically acceptable salts of the invention apply
mutatis
mutandis to the acidic reagents of stage 3 of the method
Often, the acidic reagent is any one selected from fumaric acid, tartaric
acid, citric
acid and hydrochloric acid, such as fumaric acid.
The synthetic method disclosed herein is particularly useful for producing
therapeutic deuterated substituted dialkyl tryptamines, as the method employs
significantly less LiAID4 than other syntheses known in the art since the
method
substitutes deuterium at the alpha position but not the beta position. LiAID4
is among
the most expensive and difficult to manufacture reagents in this synthesis.
Moreover,
optimised methods disclosed herein reduce LiAlat or LiAll-hand LiAlID4
requirements, for
example from 2 equivalents to 0.9 equivalents which increases economic
efficiency in
manufacturing deuterated compounds of formula I. In view of this, compounds of
formula
I are cheaper to make, via the synthetic method disclosed herein, than known
deuterated
analogues which are typically deuterated at both the alpha and beta position.
The synthetic method disclosed herein is efficient; compounds of formula I may
be
produced with an overall yield of between 50% and 100%, such as between 60%
and 100% or
between 65% and 100%.
The invention may be further understood with reference to the following non
limiting
examples:
Example 1
In the first example, the inventors demonstrate that the primary kinetic
isotope effect
bestowed upon N,N-dimethyltryptamine when enriched by one or two deuteriums at
the alpha
position exhibits a linear relationship between mean molecular weight and half-
life in human
hepatocyte assays.
Use of human hepatocytes to assess the in vitro intrinsic clearance of
deuterated DMT
analogue blends relative to DMT
In vitro determination of intrinsic clearance is a valuable model for
predicting in vivo
hepatic clearance. The liver is the main organ of drug metabolism in the body,
containing both
phase I and phase II drug metabolising enzymes, which are present in the
intact cell.
Synthesis of samples
N,N-DMT 220.9 g (as free base) was prepared as N,N-DMT fumarate, using the
chemistry
depicted in Scheme 1. An additional 4-6 g of six partially deuterated mixtures
were also
produced using modified conditions.
36
Date Recue/Date Received 2022-12-13
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
OH
1) DCM/HOBt/EDC
2) 2M Me2NH in THF 0 THF, LiAll-14
0
___________________________________ 40 \ _______________
101 r.'; Stage 1 N Stage 2 N
CioH9NO2 C121-114N20 C12H16N2
Mol. Wt.: 175.18 Mol. VVI: 202.25 Mol. VVt.: 188.27
Et0H
Stage 3
Fumaric acid
V
0
.H0)-r"OH
0
Ci6H2oN204
Mol. \M.: 304.34
Scheme 1
Synthesis of DMT
Stage 1: coupling of indole-3-acetic acid and dimethylamine
To a 5 L vessel under N2 was charged indole-3-acetic acid (257.0 g, 1.467
mol),
hydroxybenzotriazole (HOBt, -20% wet) (297.3 g, 1.760 mol) and dichloromethane
(2313mL) to give a milky white suspension. 1-Ethyl-3-(3-dimethylaminopropyl)
carbodiimide hydrochloride (EDC.HCI, 337.5 g, 1.760 mol) was then charged
portion-wise
over 5 minutes at 16-22 C. The reaction mixture was stirred for 2 hours at
ambient
temperature before 2M dimethylamine in THF (1100 mL, 2.200 mol) was charged
dropwise over 20 minutes at 20-30 C. The resultant solution was stirred at
ambient
temperature for 1 hour where HPLC indicated 1.1% indole-3-acetic acid and
98.1%
target product referred to as Stage 1). The reaction mixture was then charged
with 10%
K2CO3 (1285 mL) and stirred for 5 minutes. The layers were separated, and the
upper
aqueous layer extracted with dichloromethane (643 mL x 2). The organic
extracts were
combined and washed with saturated brine (643 mL). The organic extracts were
then
dried over MgSO4, filtered and concentrated in vacuo at 45 C. This provided
303.1 g of
crude Stage 1 as an off-white sticky solid. The crude material was then
subjected to a
slurry in tert-butyl methyl ether (TBME, 2570 mL) at 50 C for 2 hours before
being cooled
to ambient temperature, filtered and washed with TBME (514 mL x 2). The filter-
cake
was then dried in vacuo at 50 C to afford Stage 1 266.2 g (yield=90%) as an
off-white
solid in a purity of 98.5 % by HPLC and >95 % by NMR.
37
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
Stage 2: preparation of DMT
To a 5 L vessel under N2 was charged Stage 1 (272.5 g, 1.347 mol) and
tetrahydrofuran (THF, 1363nnL) to give an off-white suspension. 2.4 M LiAIH4
in THF
(505.3 mL, 1.213m01) was then charged dropwise over 35 minutes at 20-56 C to
give
an amber solution. The solution was heated to 60 C for 2 hours where HPLC
indicated
Stage 1 ND, target product bracket referred to as Stage 2, 92.5%), Impurity 1
(2.6%),
Impurity 2 (1.9%). The complete reaction mixture was cooled to ambient
temperature
and then charged to a solution of 25% Rochelle's salts (aq) (2725 mL) dropwise
over 30
minutes at 20-30 C. The resultant milky white suspension was allowed to stir
at 20-
25 C for 1 hour after which the layers were separated and the upper organic
layer
washed with saturated brine (681 mL). The organic layer was then dried over
MgSO4,
filtered and concentrated in vacuo at 45 C. The resultant crude oil was
subjected to an
azeotrope from ethanol (545mL x 2). This provided 234.6g (yield=92%) of Stage
2 in a
purity of 95.0% by HPLC and >95% by NMR.
Stage 3a (i)-(iii): preparation of seed crystals of DMT fumarate
(i) Stage 2 (100 mg) was taken up in 8 volumes of isopropyl acetate and
warmed to 50 C before charging funnaric acid (1 equivalent) as a solution in
ethanol.
The flask was then allowed to mature at 50 C for 1 hour before cooling to
room
temperature and stirring overnight, resulting in a white suspension. The
solids were
isolated by filtration and dried for 4 hours at 50 C to provide 161 mg of
product (> 99%
yield). Purity by HPLC was determined to be 99.5% and by NMR to be > 95%.
(ii) Substitution of isopropyl acetate for isopropyl alcohol in method (i)
afforded a white suspension after stirring overnight. The solids were isolated
by filtration
and dried for 4 hours at 50 C to provide 168 mg of product (> 99% yield).
Purity by
HPLC was determined to be 99.8% and by NMR to be > 95%.
Substitution of isopropyl acetate for tetrahydrofuran in method (i) afforded a
white
suspension after stirring overnight. The solids were isolated by filtration
and dried for 4
hours at 50 C to provide 161 mg of product (> 99% yield). Purity by HPLC was
determined to be 99.4% and by NMR to be > 95%.
Analysis by x-ray powder diffraction, showed the products of each of methods
9i)
to (iii) to be the same, which was labelled Pattern A.
Stage 3b: preparation of DMT fumarate
To a 5 L flange flask under N2 was charged fumaric acid (152.7 g, 1.315 mol)
and
Stage 2 (248.2 g,1.315 mol) as a solution in ethanol (2928 mL). The mixture
was heated
38
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
to 75 C to give a dark brown solution. The solution was polish filtered into
a preheated
(80 C) 5 L jacketed vessel. The solution was then cooled to 70 C and seeded
with
Pattern A (0.1 wt%), the seed was allowed to mature for 30 minutes before
cooling to
0 C at a rate of 5 C/hour. After stirring for an additional 4 hours at 0 C,
the batch was
filtered and washed with cold ethanol (496 mL x 2) and then dried at 50 C
overnight.
This provided 312.4 g (yield=78%) of Stage 3 in a purity of 99.9% by HPLC and
>95%
by NMR. XRPD: Pattern A.
Synthesis of deuterated mixtures of DMT compounds
A modified synthesis at stage 2 using solid LiA11-14/LiAID4 mixtures was
adopted,
using 1.8 equivalents of LiA11-14/LiAID4 versus 0.9 equivalents using the
process
described above for undeuterated DMT.
Representative synthesis of a deuterated (1:1 LiA11-14 : LiAID4) DMT
composition: To a
250 mL 3-neck flask under N2 was charged LiAIH4 (1.013 g, 26.7 mmol), LiAID4
(1.120
g, 26.7 mmol) and THF (100 mL). The resultant suspension was stirred for 30
minutes
before stage 1 (6 g, 29.666 mmol) was charged portion-wise over 15 minutes at
20-40
C. The reaction mixture was then heated to reflux (66 C) for 2 hours where
HPLC
indicated no stage 1 remained. The mixture was cooled to 0 C and quenched
with 25%
Rochelle's salts (aq) (120 mL) over 30 minutes at <30 C. The resultant milky
suspension
was stirred for 1 hour and then allowed to separate. The lower aqueous layer
was
removed and the upper organic layer washed with saturated brine (30mL). The
organics
were then dried over MgSO4, filtered and concentrated in vacuo. This provided
4.3 g of
crude material. The crude was then taken up in ethanol (52 mL) and charged
with fumaric
acid (2.66 g, 22.917 mmol) before heating to 75 C. The resultant solution was
allowed
to cool to ambient temperature overnight before further cooling to 0-5 C for
1 hour. The
solids were isolated by filtration and washed with cold ethanol (6.5 mL x 2).
The filtercake
was dried at 50 C overnight to provided 5.7 g (yield=63%) of product in a
purity of 99.9%
by HPLC and >95% by NMR.
No. Input Output Purity Purity by Deuteration
%
(LiA11-14:LiAID4) (yield) HPLC NMR Do Di D2
SPL028i (0:1) 5= g 5.3g (65%) 99.7%
>95% - 0= .7% 2.7% 96.6%
SPL028ii (1:1) 6= g 5= .699g (63%) 99.9% >95%
3= 0.0% 48.3% 21.7%
SPL028iii (1:2) 5g 4.206g (52%) 99.9% >95%
16.5% 46.8% 36.8%
SPL028iv (1:3) - 5= g 5.558g (68% 99.8%
>95% - 9= .3% 41.5% 49.2%
P5L028v (2:1) 5= g 4= .218g (52%) 99.9% >95%
4= 7.5% 41.3% 11.2%
SPL028vi (3:1) 5g 5.0g (62%) 99.4% >95% 57.5% 35.3%
7.4%
Table 1
39
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
In vitro intrinsic clearance of deuterated DMT compounds and compositions
Human hepatocyte intrinsic clearance
In vitro determination of intrinsic clearance (CLint) is a valuable model for
predicting in vivo clearance. The liver contains both phase I and phase II
drug
metabolising enzymes, which are present in the intact cell and thereby
provides a
valuable model for the study of drug metabolism. In particular CLint in
hepatocytes is a
measure of the potential of a compound to undergo metabolism and can be
related to
hepatic clearance in vivo by also taking into consideration plasma protein
binding and
liver blood flow. Therefore, CLint may be used as an index of the relative
metabolic
stability of compounds and compared with other external probe substrates.
Furthermore,
the measurement of CLint in vitro, where hepatic metabolic clearance is known
to be an
issue, may be a useful means of understanding the different pharmacokinetic
behaviour
of compounds in vivo.
Assay method
Human (mixed gender) hepatocytes pooled from 10 donors were used to
investigate the in vitro intrinsic clearance of SPL026 and SL028 analogues in
three
separate experiments:
= First experiment - Human (Mixed Gender) Hepatocytes; 0.545 million cells/mL.
Final organic concentration 1.05% consisting of 80.74% of MeCN and 19.26%
DMSO
= Second experiment - Human (Mixed Gender) Hepatocytes; 0.427 million
cells/mL. Final organic concentration 1% consisting of 84.7% of MeCN and
15.3% DMSO.
= Third experiment - Human (Mixed Gender) Hepatocytes; 0.362 million
cells/mL
Mouse CD-1 (Male) Hepatocytes
= Final organic concentration 1% consisting of 84.7% of MeCN and 15.3% DMSO
Assay preparation
= Hepatocyte buffer is prepared as 26.2 mM NaHCO3, 9 mM Na HEPES, 2.2 mM
D-Fructose and DMEM in MilliQ water.
= Compound and marker stocks are prepared at 10 mM in DMSO and further
diluted to 100 x the assay concentration in 91:9 acetonitrile: DMSO.
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
= Hepatocytes are thawed rapidly in a water bath at 37 C and, once just
thawed,
decanted into hepatocyte buffer. Cells are centrifuged and the supernatant
removed before counting and resuspension at the final assay concentration.
Assay procedure
A concentration of 5 pM was used for all test compounds, as well as
sumatriptan,
serotonin, benzylamine controls with 2 replicate incubations per compound in
each
experiment. This concentration was chosen in order to maximise the signal-to-
noise
ratio, while remaining under the Michaelis constant (Km) for the monoamine
oxidase
enzyme (MAO). Diltiazem and diclofenac controls were used at a laboratory-
validated
concentration of 1 pM.
Hepatocytes are added to pre-warmed incubation tubes (37 C). Pre-prepared
100 x assay compound stocks are then added to the incubation tubes and mixed
carefully. Samples are taken at 7 time points (2, 4, 8, 15, 30,45 and 60
minutes). At each
tirnepoint, small aliquots were withdrawn from the incubation and quenched 1:4
with ice-
cold acidified methanol or acetonitrile containing internal standard.
Incubation tubes are orbitally shaken at 37 C throughout the experiment.
Standard final incubation conditions are 1 pM compound in buffer containing
nominally -0.5 million viable cells/nnL, -0.9% (v/v) acetonitrile (MeCN) and -
0.1% (v/v)
DMSO (specific assay concentrations outlined above, section 2).
Quenched samples are mixed thoroughly, and protein precipitated at -20 oc for
a minimum of 12 hours. Samples are then centrifuged at 4 oc. Supernatants are
transferred to a fresh 96-well plate for analysis,
Liquid Chromatography-mass spectrometry (LC-MS/MS)
The following LC-MS/MS conditions were used for the analysis:
Instrument: Thermo TSQ Quantiva with Thermo Vanquish UPLC system
Column: Luna Omega 2.1x50 mm 2.6pm
Solvent A: H20 + 0.1% formic acid
Solvent B: Acetonitrile + 0.1% formic acid
Flow rate: 0.8 ml/min
Injection vol: 1 pl
Column temp: 65 C
41
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
Gradient:
Time (mins) % Solvent B
0.00 5.0
0.90 75.0
1.36 99.0
1.36 5.0
1.80 5.0
MS parameters:
Positive ion spray voltage: 4000 V
Vaporiser temperature: 450 C
Ion transfer tube temp: 365 C
Sheath gas: 54
Aux gas: 17
Sweep gas: 1
Dwell time 8 ms
MRM transitions:
= DO = mass to charge ratio 189.136> 144.179 (method determined from SPL026
analysis)
= D1 = mass to charge ratio 190.136 > 59.17 (method determined from
SPL028ii
analysis)
= D2 = mass to charge ratio 191.137 > 60.169 (method determined from
SPL028i
analysis)
= D6 = mass to charge ratio 195.17 > 64.127
= D8 = mass to charge ratio 197.2> 146.17
The MRM transitions were determined from a preliminary analysis of DMT
samples containing either no deuterium (for DO transition), or high levels of
either D1,
D2, D6 or D8 deuteration (for the D1, D2, D6 and D8 transitions respectively).
The resulting concentration-time profile was then used to calculate intrinsic
clearance (CLint) and half-life (t1/2). To do this, the MS peak area or MS
peak area/IS
response of each analyte is plotted on a natural log scale on the y axis
versus time (min)
of sampling on the X axis. The slope of this line is the elimination rate
constant. This is
converted to a half-life by -In(2)/slope. Intrinsic clearance is calculated
from the
slope/elimination rate constant and the formula is CLint = (-1000*slope)/cell
denisty in
1E6 cells/ml, to give units of microlitre/min/million cells.
42
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
Clearance of six different D2DMT analogue blends (SPL028i - SPL028vi) with and
without MAO inhibitors
The contribution of MAO of six different a,a,-dideutero-N,N-dimethyltryptamine
(D2DMT) compounds was examined using an irreversible, combined MAO-A/B
inhibitor
(100nM clorgyline and 100nM Deprenyl/Selegiline added as a cassette) via the
measurement of in vitro intrinsic clearance using human (mixed gender)
hepatocytes
from 10 donors (0.545 million cell s/mL; final organic concentration 1.05%
consisting of
80.74% of MeCN and 19.26% DMSO).
Effect of deuteration
Data were fitted with two separate linear models using linear regression
analyses
(one-way ANOVA), which revealed that deuterium enrichment at the a-carbon of
DMT
decreases intrinsic clearance linearly with increasing percentage of D2-
deuteration using
the formula: y = D2 * -6.04 + 12.9, r2=0.748 and molecular weight (MVV) using
the
formula: y = MW * 79.5 + 98.8, r2=0.811.
96.6% 02-DMT (SPL028i) saw the biggest change in metabolic stability, - 2-fold
change in intrinsic clearance and half-life compared to SPL026 in initial
hepatocyte
studies (Table 3 and Table 4). The metabolic stability of intermediate blends
of
deuteration (SPL028ii - SPL028vi) increased in a manner which correlated with
increasing level of deuteration and molecular weight (Table 3 and Table 4).
Intrinsic clearance (uUminimillion cells)
Fold
Fold
Ratio of
Cpd Molecular Without change With change
deuteration
name weight inhibitors from inhibitors
from
(Do:Di: 02)
SPL026
SPL026
SPL026 188.27 100:0:0 13.77 1.00 13.24
1.00
SPL028v 188.9098 48:41:11 10.99 1.25
9.51 1.39
SPL028vi 188.9613 57:35:7 13.64 1.01 10.79
1.23
SPL028ii 189.1915 30:48:22 10.46 1.32
8.78 .. 1.51
SPL028iii 189.6685 17:47:37 9.36 1.47
6.90 1.92
SPL028iv 189.6764 9:42:49 11.14 1.24 7.46
1.77
SPL028i 190.2398 1:3:97 - 7.15 1.93 7.50 1.77
Benzylamine 16.70 <3.0
Serotonin 38.60 10.10
43
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
Table 3 In vitro Intrinsic clearance of SPL026 and 6 different D2-deuterated
SPL028
analogue blends in in human hepatocytes, highlighting the fold change in
intrinsic
clearance from SPL026 for each deuterated compound, with and without
inhibitors.
Compounds ordered via molecular weight.
Fold
Fold
Ratio of
Molecular Without change With change
Cpd name deuteration
weight inhibitors from inhibitors from
(Do:Di: D2)
SPL026 SPL026
SPL026 188.27 100:0:0 92.39 1.00 96.06
1.00
SPL028v 188.9098 48:41:11 119.61 1.29 135.10
1.41
SPL028vi 188.9613 57:35:7 95.04 1.03
119.62 1.25
SPL028ii 189.1915 30:48:22 125.80 1.36 147.47
1.54
SPL028iii 189.6685 17:47:37 140.43 1.52 189.60
1.97
SPL028iv 189.6764 9:42:49 116.84 1.26
171.17 1.78
SPL028i 190.2398 1:3:97 178.79 1.94 169.75
1.77
Benzylamine 76.30 460.00
Serotonin 33.00 125.70
Table 4 In vitro half-life of SPL026 and 6 different D2-deuterated SPL028
analogue
blends in in human hepatocytes, highlighting the fold change in intrinsic
clearance from
SPL026 for each deuterated compound, with and without inhibitors. Compounds
ordered
via molecular weight.
Contribution of MAO (see also Fig. 4)
Two-way ANOVA was carried out to determine the influence of MAO inhibitors
and compound deuteration on intrinsic clearance. There was a significant
effect of MAO
inhibitors on intrinsic clearance F(1, 6) = 11.42, p=0.0149, and deuteration
on intrinsic
clearance F(1,6)=9.996, p=0.006.
The inclusion of MAO inhibitors was shown to have minimal effect on the
metabolism of SPL026 (DMT) resulting in -4% slower intrinsic clearance (Table
5). MAO
inhibitors were also shown to have a small effect on the 96.6% D2_deuterated
analgoue
(SPL028i) which saw a -5% quicker intrinsic clearance in the presence of MAO
inhibitors
(Table 5). These results indicate that MAO enzymes do not significantly
contribute to the
metabolism of SPL026 and SPL028i in human liver hepatocytes.
MAO inhibitors were shown to have a greater inhibitory effect on intrinsic
clearance for the remaining five D2.deuterated analogue blends (SPL028ii -
SPL028vi).
For these five compounds, the inhibitory action of MAO inhibitors was shown to
increase
44
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
linearly with increasing level of deuteration and molecular weight, with the
exception of
SPL028vi (Table 3). 49% D2_deuterated SPL028iv saw the largest change in
intrinsic
clearance (49%) with the inclusion of MAO inhibitors (Table 5), whereas 36.8%
D2-
deuterated SPL028iii saw the largest change (-2 fold) in metabolic stability
relative to
SPL026 in cellular fractions with inhibitors (Table 3 and 4).
Intrinsic clearance
Half-life (min)
(IL/min/million cells)
Ratio of
Cpd Mol. Without With Without With
deuteration
Name weight inhibitors inhibitors change inhibitors
inhibitors change
(Do:Di:D2)
SPL026 188.27 100:0:0 13.77 13.24 -4.00 92.39
96.06 3.82
SPL028v 188.9098 48:41:11 10.99 9.51 -15.56
119.61 135.1 11.47
SPL028vi 188.9613 57:35:7 13.64 10.79 -26.41 95.04
119.62 20.55
SPL02811 189.1915 30:48:22 10.46 8.78 -19.13
125.8 147.47 14.69
SPL028111 189.6685 17:47:37 9.36 6.9 -35.65 140.43
189.6 25.93
SPL0281v 189.6764 9:42:49 11.14 7.46 -49.33 116.84
171.17 31.74
SPL0281 190.2398 1:3:97 7.15 7.5 4.67 178.79
169.75 -5.33
Benzylamine 16.7 <3.0 <-450 76.3
460 >83.41
Serotonin 38.6 10.1 -282.18 33 125.7
73.75
Table 5/n vitro Intrinsic clearance and half-life of SPL026 and 6 different D2-
deuterated
SPL028 analogue blends in in human hepatocytes with and without MAO-A/B
inhibitor
combination. Percentage change (h)) values represent the % change in metabolic
stability with the inclusion of MAO inhibitors vs no inhibitors, measured by
intrinsic
clearance and half-life separately. Compounds are ordered by increasing
molecular
weight.
These results indicate that increasing the level of deuteration at the a-
carbon of
DMT decreases the MAO enzyme metabolism of the compound.
Clearance of six D2DMT analogue blends (SPL0281 - SPL028vi) and one D6-DMT
(SPL028vii) analogue blends
Synthesis of d6-DMT: 028vii (Compound 5)
Stage 1
DCM
0 0
HOBt
OH EDC.HCI N--CD3
NH(CD3)2.HCI \ 3d
DIPEA
Molecular Weight: 175.18 Molecular Weight: 208.29
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
EDC.HCI (15.7 g, 81.90 mmol) was added to 3-indoleacetic acid (12.0 g, 68.50
mmol) and HOBt.H20 (1.16 g, 75.75 mmol) in DCM (108 mL) at room temperature.
The
reaction was stirred for 1 hour after which N,N-diisopropylethylamine (DIPEA)
(35.6 mL,
205.75 mmol) and do-dimethylamine.HCI (9.0 g, 102.76 mmol) were added
(temperature
maintained below 30 C). The reaction was stirred for 1 hour at room
temperature after
which analysis by HPLC indicated 65.6% product with 28.9% 3-indoleacetic acid
remaining. DI PEA (11.9 mL, 68.78 mmol) was added and the reaction was stirred
for 1
hour at room temperature. HPLC indicated no change in conversion. Aqueous
potassium
carbonate (6.0 g in 54 mL water) was added and the phases were separated. The
aqueous phase was extracted with DCM (2 x 30 mL). The combined organics were
washed with brine (2 x 30mL) then aqueous citric acid (20 w/w%, 50 mL), dried
over
MgS0.4 and filtered. The filtrate was stripped and the resulting solids were
slurried in
TBME (120 mL) and isolated by filtration. Purification by flash column
chromatography
yielded 8.34 g of the desired product (58% yield). 1H NMR confirmed the
identity of the
product.
Stage 2
0
N-CD3
N -CD3 THF
\ 36
\ = 36 LiAIH4
Molecular Weight: 194.31
Molecular Weight: 208.29
LiA11-14 (1 M in THF, 17.3mL, 17.28 mmol) was added to a suspension of stage 1
(4.0 g, 19.20 mmol) in THE (10 mL) at <30 C. The resulting reaction was
heated to 60-
65 C and stirred for 2 hours. HPLC analysis indicated complete consumption of
stage 1
with 97.3% product formed. The reaction was cooled to room temperature and
quenched
into aqueous Rochelle's salts (10 g in 30 mL water) at <30 C. After stirring
for 1 hour,
the phases were separated. The aqueous phase was extracted with THF (20 mL).
The
combined organics were washed with brine (20 mL), dried over MgSO4, filtered
and
stripped (azeotroped with ethanol, 20 mL) to give the desired product as an
amber oil
(3.97 g). 1H NMR confirmed the identity of the product and indicated 8.5%
ethanol was
present (no THF) giving an active yield of 3.63 g, 97%.
46
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
Stage 3
,N-CD3 Et0H N--CD3 0
D3C
=\ D36 H0)1'OH
Fumaric acid 0
Molecular Weight: 194.31 Molecular Weight: 310.38
d6-DMT free base (3.6 g active, 18.53 mmol) was dissolved in ethanol (43 mL)
at
room temperature. Fumaric acid (2.15 g, 18.53 mmol) was added and the solution
was
heated to 75 C (solids crystallised during heating and did not re-dissolve).
The resulting
suspension was cooled to 0-5 C and stirred for 1 hour. The solids were
isolated by
filtration, washed with ethanol (2 x 7 mL) and pulled dry. Further drying in a
vacuum oven
at 50 C yielded the desired d6-DMT fumaric acid salt (4.98 g, 87%).
Synthesis of d8-DMT: 028viii (Compound 1)
For stage 1 (coupling of 3-indoleacetic acid and d6-dimethylamine), see above
Stage 2
0 DD
N-CD3 THF N--CD3
\ .36
LiAID4 13c'
Molecular Weight: 208.29 Molecular Weight: 196.32
LiAlat (1 M in THF, 17.3 mL, 17.28 mmol) was added to a suspension of stage 1
(4.0 g, 19.20 mmol) in THF (10 mL) at <30 C. The resulting reaction was
heated to 60-
65 C and stirred for 2 hours. HPLC analysis indicated complete consumption of
the
stage 1 with 97.3% product formed. The reaction was cooled to room temperature
and
quenched into aqueous Rochelle's salts (10 g in 30 mL water) at <30 C. After
stirring
for 1 hour, the phases were separated. The aqueous phase was extracted with
THE (20
mL). The combined organics were washed with brine (20 mL), dried over MgSO4,
filtered
and stripped (azeotroped with ethanol, 20 mL) to give the desired product as
an amber
oil (4.01 g). 1H NMR confirmed the identity of the product and indicated 8.6%
ethanol
was present (no THF) giving an active yield of 3.66 g, 97%.
47
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
Stage 3
D D D D
0
N--CD3 Et0H N --CD3
Fumaric acid 0
N N
H H
Molecular Weight: 196.32 Molecular Weight: 312.39
Compound 1 free base (3.6 g active, 18.53 mmol) was dissolved in ethanol (43
mL) at room temperature. Fumaric acid (2.15 g, 18.53 mmol) was added and the
solution
was heated to 75 C (solids crystallised during heating and did not re-
dissolve). The
resulting suspension was cooled to 0-5 C and stirred for 1 hour. The solids
were isolated
by filtration, washed with ethanol (2 x 7 mL) and pulled dry. Further drying
in a vacuum
oven at 50 C yielded the desired Compound 1 as a fumaric acid salt (4.62 g,
81%).
Assessment of extents of deuteration
This was achieved by LCMS-SIM (SIM = single ion monitoring), the analysis
giving a separate ion count for each mass for the three deuterated N,N-
dimethyltryptamine cornpounds (N,N-dimethyltryptamine (DO), a-protio, a-
deutero-N,N-
dimethyltryptamine (D1) and a,a-dideutero-N,N-dimethyltryptamine (D2)) at the
retention
time for N,N-dimethyltryptamine. The percentage of each component was then
calculated from these ion counts.
For example, %DO = [D0/(DO + D1 + D2)] x 100.
HPLC Parameters
System: Agilent 1100/1200 series liquid chromatograph or
equivalent
Column: Triart Phenyl; 150 x 4.6mm, 3.0pm particle size (Ex:
YMC, Part
number: TPH12S03-1546PTH)
Mobile phase A: Water: Trifluoroacetic acid (100:0.05%)
Mobile phase B: Acetonitrile : Trifluoroacetic acid (100:0.05%)
Gradient: Time Okik %B
0 95 5
13 62 38
26 5 95
30.5 5 95
31 95 5
Flow rate: 1.0 mL/min
Stop time: 31 minutes Post runtime: 4
minutes
48
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
Injection volume: 5 pL Wash vial: N/A
Column temperature: 30 C combined
Wavelength: 200 nm, (4 nm) Reference: N/A
Mass spectrometry parameters
System: Agilent 6100 series Quadrupole LC-MS or equivalent
Drying gas flow: 12.0 Umin Drying gas temp.: 350 C
Nebuliser pressure: 35 psig
Fragmentor: 110 Gain: 1.00
Cpd RT RRT Conc Diluent Detection Mass
DO 10.64 1.00 0.30 mg/ml CH3CN:H20 (50:50) (+) SIM 189.10
m/z
D1 10.64 1.00 0.30 mg/ml CH3CN:H20 (50:50) (+) SIM 190.10
m/z
D2 10.64 1.00 0.30 mg/ml CH3CN:H20 (50:50) (+) SIM 191.10
m/z
MS-SIM range is the target mass 0.1 m/z
In vitro human hepatic intrinsic clearance of the six different a,a,-dideutero-
N,N-
dimethyltryptamine (D2DMT) compounds and one N,N-bis(trideutero-
dimethyl)tryptamine (D6DMT, SPL028vii) were measured to investigate the
effects of
methyl group deuteration vs a-carbon deuteration on metabolic stability in
using human
(mixed gender) hepatocytes from 10 donors (0.427 million cells/mL; final
organic
concentration 1% consisting of 84.7% of MeCN and 15.3% DMSO).
Compound Name Intrinsic clearance Half-life (min)
(pL/min/million cells)
SPL028v 14.1 119
SPL028vi 13.4 126.8
SPL028ii 9.1 191.1
SPL028iii 8.2 213.9
SPL028iv 7.7 223.9
SPL028i 6.3 258.3
SPL028vii (DO 13.3 122.2
Diltiazem (A) 15.3 15.0
Diltiazem (B) 17.2 18.2
Diclofenac (A) 155.0 154.0
49
CA 03179161 2022-09-30
WO 2021/116503
PCT/EP2021/060750
Diclofenac (B) 150.1 154.3
Table 6. In vitro intrinsic clearance and half-life of 6 different D2-
deuterated DMT and D8-
deuterated DMT analogue blends in human hepatocytes, ordered by increasing
level of
molecular weight
Data fitted with a linear regression model on the six different D2-deuterated
confirmed previous findings that deuterium enrichment at the a-carbon of DMT
decreases intrinsic clearance linearly with increasing level of D2-
deuteration,
y = D2 * ¨8.07 + 12.9, r2=0.690 and molecular weight. A linear regression
model was
also fitted by molecular weight using formula: y = MW * 13.9 + 6.06, r2=0.923
revealing
molecular weight is a strong predictor of intrinsic clearance for the 6
different D2-
deuterated SPL028 blends.
Initial hepatocyte data did not suggest a relationship between molecular
weight
and intrinsic clearance of D2-deuterated and De-deuterated SPL028 blends,
r2=0.0395.
Example 2
In the second example, the inventors detect a possible increase in half-life
when
N,N-dimethyltryptamine is deuterated at the N,N-dimethyl position.
Clearance of two D2DMT (SPL028i and SPL028ii) blends, one D6DMT (SPL028vii,
Compound 5) and one IN-DMT (SPL028viii, Compound 1) analogue
Further human hepatocyte assays were conducted with two D2-deuterated
SLP028 analogue blends and two additional deuterated analogues: D6DMT and
D8DMT
to measure in vitro intrinsic clearance using human (mixed gender) hepatocytes
from 10
donors (0.362 million cells/mL).
Compound Intrinsic Fold change
Half-life I Fold change
Name Clearance from (min) from SPL026
(pL/min/million SPL026
cells)
SPL026 19.4 1.0 98.9 1.0
SPL028ii 11.7 1.7 170.9 1.7
SPL028i 8.3 2.3 233.1 2.4
SPL028vii
(Compound 5) 17.1 1.1 112.1 1.1
SPL028viii
(Compound 1) 9.3 2.1 206.9 2.1
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
Diltiazem 22.0 87.3
Diclofenac 92.5 20.7
1
Table 7. In vitro intrinsic clearance and half-life of two different D2-
deuterated DMT, D6-
deuterated DMT and Da-deuterated DMT analogue blends in human hepatocytes,
ordered by increasing level of molecular weight
The possible presence of a secondary kinetic isotope effect was noted in the
data, however a linear regression model did not support a predictive
relationship
between molecular weight and intrinsic clearance for SPL026, SPL028i,
SPL028ii,
SPL029vii and SPL028viii, r2=0.0445 in the human hepatocyte assay.
Example 3
In the third example, the inventors provide evidence that an additional
protective effect is observed between D2-deuterated SPL028i and D8-deuterated
SPL028viii (Compound 1). The data are supportive of a synergistic effect on
metabolic
stability when deuterium is present at both the alpha position and the N,N-
dimethyl
positions of a compound of Formula I or any other compound or composition of
any
aspect of the present invention.
Use of liver mitochondrial fraction to model human metabolism of deuterated
DMT
Given the predicted 5 minute half-life of DMT in humans, the inventors expect
that DMT
is largely broken down before reaching the human liver. Therefore, a non-
tissue or organ
specific alternative in vitro assay was sought as a more appropriate system to
model
human metabolism of DMT. An analysis of non-tissue or organ specific human
metabolism may be carried out in Human Liver Mitochondrial fractions.
The following assays conducted on Human Liver Mitochondrial (HLMt) fractions
predict enhanced fold-change between SPL026 and D2-deuterated SPL028i compared
with the fold-change predicted in hepatocyte studies.
In vitro human mitochondrial fraction intrinsic clearance of SPL026 (DMT)
with/without MAO-A and MAO-B inhibitors
In vitro determination of the intrinsic clearance of SPL026 with selective and
irreversible MAO-A inhibitor (100nM clorgyline) and MAO-B inhibitor (100nM
Deprenyl/Selegiline) were added separately to 0.5 mg/mL of human liver
mitochondria!
fraction. The MAO-A substrate, Serotonin and MAO-B substrate, Benzylannine
were
added as positive controls which confirmed the presence of MAO-A and MAO-B and
the
action of Clorgyline and Deprenyl inhibitors.
51
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
Compound Inhibitor Intrinsic
Clearance Half-life (min)
Name (pL/min/mg protein)
SPL026 DMSO Vehicle 42.9 33.7
SPL026 Clorgyline <3.9 >373.7
(MAO-A inhibitor)
SPL026 Deprenyl 42.7 32.5
(MAO-B inhibitor)
Serotonin DMSO Vehicle 124.6 11.1
Serotonin Clorgyline <3.3 >420.2
Benzylamine DMSO Vehicle 45.7 30.4
Benzylamine Deprenyl <3.3 >420.2
Table 8 Intrinsic clearance and half-life of SPL026 in human liver
mitochondrial fraction
SPL026 half-life and intrinsic clearance significantly increased with MAO-A
inhibitor (Clorgyline), resulting in a 10-fold increase in intrinsic clearance
compared data
from SPL026 without MAO inhibitors. Deprenyl (MAO-B inhibitor) showed no
difference
in human mitochondrial intrinsic clearance relative to fraction without
inhibitors. These
results suggest that a role of MAO-A but not MAO-B, in the metabolism of
SPL026.
In vitro human mitochondrial fraction intrinsic clearance of SPL026 (DMT),
SPL028i (96.6% D2-DMT), SPL028iii (36.8% D2-DMT), SPL028vii (Compound 5) and
SPL028viii (Compound 1)
In vitro determination of the intrinsic clearance of SPL026, SPL028i,
SPL028iii
and SPL028viii were added separately to 0.5 mg/mL of human liver mitochondrial
fraction. The MAO-A substrate 'Serotonin' and MAO-B substrate 'Benzylamine'
were
added as positive controls and confirmed the presence of MAO-A and MAO-B. The
experiment was repeated with the same substances and also with SPL028iii and
SPL028vii.
Compound Intrinsic Clearance 1/Fold Half-life Fold
change
Name (pt./min/mg protein) change (min) from
from SPL026
SPL026
SPL026 161.0 1.0 8.6 1.0
SPL028iii 15.0 3.6 31.1 3.6
SPL028i 44.6 10.7 92.8 10.8
SPL028viii 10.9 14.8 127.7 14.8
Serotonin 151.0 9.2
Benzylamine 60.0 23.2
52
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
Compound Intrinsic Clearance 1/Fold Half-life Fold
Name (pUmin/mg change (min) change
protein) from from
SPL026 SPL026
SPL026 175.0 1.0 7.9 1.0
SPL028vii 137.1 1.3 10.2 1.3
SPL028ii 47.3 3.7 ' 29.4 3.7
SPL028iii 39.8 4.4 34.8 4.4
SPL0281 18.0 9.7 77.3 9.7
SPL028viii 12.7 13.7 112.2 14.1
Serotonin 157.0 - 22.4 -
Benzylamine 62.1 - 8.8 -
Table 9 Intrinsic clearance and half-life of SPL026, SPL028i, SPL028ii,
SPL028iii,
SPL028vii and SPL028viii in human liver mitochondria! fraction
Half-life increased with increasing level of deuteration for the SPL028
compounds, when compared to SPL026. D8-deuterated SPL028viii (Compound 1) saw
the greatest change in half-life (14 fold increase when averaged across both
repeats)
relative to SPL026. 96.6% D2-deuterated SPL0281 also showed a large change in
half-
life (10 fold increase when averaged across both repeats) compared to SPL026.
36.80%
D2-deuterated SPL028iii demonstrated a smaller change (3.6 fold increase) in
clearance
compared to SPL026. An independent Welch's t-test was performed for each
deuterated
compound compared to SPL026, results are provided in Table 10
t-test vs
Compound Name Half-Life (min) SPL026)
' R1 [ R2 [ R3 R4 p value
[ ___________________________________________________________________________
SPL026 8.8 8.4 7.6 8.3
SPL028i 86.1 99.4 82.6 72.1
0.0004267
SPL028ii 28.0 30.7
0.0206256
SPL028iii 31.5 30.7 34.7 35.0
0 0001036
SPL028vii 9.4 10.9
0.1211645
SPL028viii 121.4 133.9 92.9 131.5
. 0.0006452
Benzylamine 24.3 22.1 21.2 23.5
Serotonin 9.3 9.1 9.1 8.5
Table 10 t-test showing significance of half-life extension of SPL028(i-viii)
53
CA 03179161 2022-09-30
WO 2021/116503 PCT/EP2021/060750
Conclusions
Complete deuteration at the alpha position of NN-dimethyltryptamine increases
metabolic stability 10-fold via primary kinetic isotope effect in human
mitochondrial
fraction assays.
N,N-dimethyl deuteration potentially increases metabolic stability via
secondary
kinetic isotope effect in human hepatocyte assays.
Most unexpectedly, the primary and secondary isotope effects of deuteration at
both
the alpha position and the N,N-dimethyl position increase metabolic stability
synergistically in Compound 1, demonstrated with a 14-fold increase metabolic
stability
in human mitochondrial fraction assays.
54