Note: Descriptions are shown in the official language in which they were submitted.
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
1
PROCESS FOR RECOVERY OF LITHIUM FROM BRINE
CROSS-REFERENCE TO RELATED APPLICATION
[0001] This application is a Continuation-In-Part of copending Application No.
16/410,523,
filed on May 13, 2019, which is a Continuation-In-Part of copending
Application No.
16/224,463, filed on December 18, 2018, which claims the benefit of U.S.
Provisional
Application No. 62/610,575, filed December 27, 2017, all of which are hereby
expressly
incorporated by reference into the present application.
BACKGROUND OF THE INVENTION
1. Field of the Invention:
[0002] The present invention generally relates to methods for recovering ions
from brine, and
more particularly, to methods for recovering lithium ions from brine.
2. Description of the Background Art:
[0003] As a result largely of the recent interest in the use of lithium ion
batteries for electric
vehicles and stationary electric power storage associated with renewable
energy systems
including from wind, solar, and tidal sources, the demand for lithium has
increased
substantially and may soon outstrip supply. There is potentially a large
supply of lithium
available in various sources, such as seawater, brines, geothermal fluids, and
continental salt
lakes. As used herein, "brine" and "brines" refer to these various lithium-
containing solutions.
To date, however, there have been few viable ways to recover the lithium from
these sources
without extensive concentration by evaporation, as the lithium concentrations
in these
resources are typically very low. In addition, the much higher concentration
of other metal ions,
such as sodium, potassium, calcium, and magnesium, interferes with recovery of
the lithium.
[0004] Ion exchange is a well-known technology for recovery of low
concentrations of metal
ions from aqueous solutions. However, conventional ion exchange resins, such
as strong acid
cation exchange resins with sulfonic acid functional groups and chelating
resins with
iminodiacetate groups, have a higher preference for multivalent ions, such as
calcium and
magnesium, which may be present. Although the selectivity for lithium over
other monovalent
ions, such as sodium and potassium, may be similar, the presence of these
competitive
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
2
monovalent ions, which normally exist in great excess in brines, makes
recovery of lithium
unfeasible.
[0005] Inorganic ion exchange media, such as ionic sieves, based upon
manganese, titanium,
or other oxides, have been identified as potentially useful for recovery of
lithium from brines
where there exists high concentrations of competitive ions, such as calcium,
magnesium,
sodium, and potassium. These materials can be termed lithium ion sieves (LIS).
LIS exhibit a
high preference for lithium because the LIS exchange sites are so narrow that
Na + (0.102 nm),
IC (0.138 nm), and Ca2+ (0.100 nm), which have ionic radii larger than Li +
(0.074 nm), cannot
enter the exchange sites. Although the ionic radius of the Mg2+ (0.072 nm) ion
is similar to the
ionic radius of Li, a high amount of energy is required for the dehydration of
magnesium ions
to allow it to enter the exchange sites so that selectivity over Mg2+ is
maintained.
[0006] However, LIS have a number of disadvantages. First, they are weakly
acidic in nature
and, as a result, have reduced capacity at lower pH levels. Second, they are
not stable in acid
solutions since some of the components dissolve in acid. As they degrade, they
lose capacity
to take up lithium so that they must be replaced on a frequent basis.
Replacing LIS represents
a significant cost. Moreover, removal and replacement of the degraded LIS,
when it is installed
in a conventional column, is difficult and time consuming. Finally, LIS are
synthesized as fine
powders and, therefore, due to high pressure drop, cannot be used in fixed
beds, as is done with
conventional ion exchange resins. A number of attempts have been made to
improve the form
by, for example, granulation, foaming, membranes, fibers, and magnetization.
However, when
these powders are agglomerated into larger geometries, the kinetics are
severely impaired as a
result of blockage of the pores and active exchange sites by the binding
agents, and, typically,
lower surface area to volume/mass ratio with larger particle sizes.
[0007] For example, the reference, Chitrakar et al., "Lithium Recovery from
Salt Lake Brine
by H21.103," Dalton Transactions, 43(23), pages 8933-8939, June 21, 2014
(hereinafter
referred to as "Chitrakar"), relates to the synthesis, characterization, and
laboratory evaluation
of lithium selective adsorbents based upon metatitanic acid. However,
Chitrakar does not
mention an industrial process and does not discuss issues concerning
solid/liquid separation or
washing the brine and eluent from the adsorbent on an industrial scale. For
example, adsorption
tests in Chitrakar were conducted in beakers at an adsorbent solids
concentration of 20 g/L,
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
3
and elution tests with HC1 were conducted at an adsorbent solids concentration
of 10 g/L.
Chitrakar does not disclose how to use the adsorbent on a continuous
industrial scale.
Specifically, the laboratory filtration used in the tests would not be
applicable on an industrial
scale.
[0008] As such, there is still a need to improve the method for recovering
lithium from brine
using lithium ion sieves that overcome the disadvantages above.
SUMMARY OF THE INVENTION
[0009] In one aspect, the present invention provides a process for recovery of
lithium ions from
a lithium-bearing brine by contacting the lithium-bearing brine with a lithium
ion sieve for less
than about one hour in a first mixed or stirred reactor to form a lithium ion
complex with the
lithium ion sieve and decomplexing the lithium ion from the lithium ion sieve
in a second
mixed or stirred reactor to form the lithium ion sieve and an acidic lithium
salt eluate.
[0010] In one embodiment, a process for recovery of lithium ions from a
lithium-bearing brine
comprises contacting the lithium-bearing brine with a lithium ion sieve for
less than about one
hour in a first mixed or stirred reactor to form a lithium ion complex with
the lithium ion sieve.
Then, the process includes a step of decomplexing lithium ions from the
lithium ion sieve in a
second mixed or stirred reactor to form an acidic lithium salt eluate solution
separated from the
lithium ion sieve. The lithium ion sieve may comprise an oxide of titanium or
niobium (e.g.,
metatitanic acid or lithium niobate).
[0011] The decomplexing may be performed by elution using an acid. A
concentration of the
acid may be maintained at a constant value through additions of said acid. The
concentration
of the acid should be less than 0.1 M and preferably at a pH of greater than 1
and less than 3
and most preferably at pH of about 2. An average contact time of the lithium
ion complex with
the lithium ion sieve and the acid may be less than 1 hour. The acid may be
hydrochloric acid
or sulfuric acid.
[0012] A pH of the first reactor may be maintained at a constant value through
addition of an
alkali. The pH may be maintained at the constant value of greater than 4 and
less than 9 or
greater than 6 and less than 8. The alkali may be sodium hydroxide (NaOH),
ammonium
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
4
hydroxide, potassium hydroxide, sodium carbonate, magnesium hydroxide, calcium
hydroxide, or anhydrous ammonia. For example, the alkali may be sodium
hydroxide at a
concentration of less than 8% w/w.
[0013] More than 90% of the lithium ion sieves may have an average particle
diameter of less
than 40 gm and more than 90% of the lithium ion sieves may have an average
particle diameter
of greater than 0.4 gm. More than 90% by volume of particles of the lithium
ion sieve may be
less than 100 gm in diameter and greater than 0.5 jim in diameter. More than
90% by volume
of particles of the lithium ion sieve may be greater than 0.5 gm in diameter.
The process may
further comprise the step of removing lithium ion sieves having an average
particle diameter
of less than 1 p.m before contacting the lithium-bearing brine with the
lithium ion sieve.
[0014] The process may further comprise the steps of separating the lithium
ion complex with
the lithium ion sieve from the brine with a solid/liquid separation device;
and contacting the
lithium ion complex with the lithium ion sieve with water before decomplexing
in the second
reactor. The process may also further comprise the steps of separating the
lithium ion sieve
from the acidic lithium salt eluate solution with a solid/liquid separation
device; contacting the
lithium ion sieve with water after decomplexing in the second reactor to
obtain a regenerated
lithium ion sieve and a dilute acid water wash; and adding the regenerated
lithium ion sieve to
the first reactor. This process may further comprise the step of dewatering
the lithium ion
complex with the lithium ion sieve to a moisture content of less than 90% by
weight before
decomplexing the lithium ion from the lithium ion sieve in the second reactor.
This process
may also further comprise the step of dewatering the regenerated lithium ion
sieve before being
added to the first reactor. The step of contacting the lithium ion sieve with
water may comprise
contacting the lithium ion sieve with sufficient water such that more than 50%
of the lithium
ion that has been decomplexed from the lithium ion sieve is washed from the
lithium ion sieve
prior to adding the regenerated lithium ion sieve to the first reactor. The
step of contacting the
lithium ion sieve with water may also comprise contacting the lithium ion
sieve with water in
more than one counter-current stage such that more than 50% of the lithium ion
that has been
decomplexed from the lithium ion sieve is washed from the lithium ion sieve
prior to adding
the regenerated lithium ion sieve to the first reactor. The process may also
further comprise
the step of adding the dilute acid water wash and additional concentrated acid
to the second
reactor.
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
[0015] The first reactor may comprise ultrafiltration or microfiltration
membranes. Air or
other gas may be used to agitate contents of the first reactor. A flux rate
through the
ultrafiltration membrane or the microfiltration membrane may be greater than
30 LMH at
transmembrane pressures of less than 30 kPa.
[0016] A concentration of the lithium ion sieve may be greater than 50 g/L or
greater than 100
g/L.
[0017] Further scope of applicability of the present invention will become
apparent from the
detailed description given hereinafter. However, it should be understood that
the detailed
description and specific examples, while indicating preferred embodiments of
the invention,
are given by way of illustration only, since various changes and modifications
within the spirit
and scope of the invention will become apparent to one of ordinary skill in
the art from this
detailed description.
BRIEF DESCRIPTION OF THE DRAWINGS
[0018] The present invention will become more fully understood from the
detailed description
given below and the accompanying drawings that are given by way of
illustration only and are
thus not limitative of the present invention. In the drawings, like reference
numerals are used
to indicate like features in the various views.
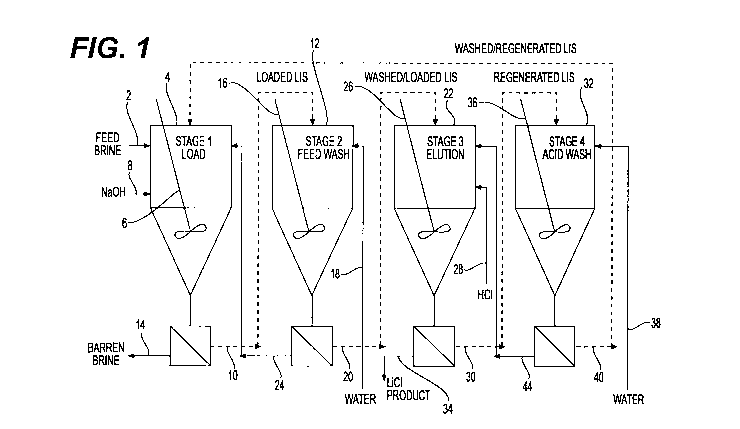
[0019] FIG. 1 is a diagrammatic view of an exemplary lithium extraction system
for the present
process.
[0020] FIG. 2 is a graph showing the amount of metal ion uptake as a function
of pH.
[0021] FIG. 3 is a graph showing the amount of lithium and titanium extracted
as a function of
hydrochloric acid concentration.
[0022] FIG. 4 is a graph showing an exemplary US particle size distribution of
a sample of
metatitanic acid lithium ion sieve taken after a few hours of air agitation in
a slurry.
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
6
[0023] FIG. 5 is a diagrammatic view of an alternative lithium extraction
system for the present
process.
[0024] FIG. 6 is a graph of lithium concentration as a function of time for an
exemplary
extraction trial.
[0025] FIG. 7 is a graph showing the amount of lithium and titanium extracted
as a function of
pH.
[0026] FIG. 8 is a graph showing the effect of contact time on lithium
capacity and calcium
separation.
DETAILED DESCRIPTION OF THE INVENTION
[0027] As a result of the disadvantages discussed above, lithium ion sieves
have not been
applied widely to recovery of lithium from brine on an industrial scale to
date. The present
invention overcomes these disadvantages, making the use of lithium ion sieves
for selective
recovery of lithium from brine more commercially feasible.
[0028] The average particle diameter of conventional ion exchange resins is
typically about
400-1250 micrometers. The RECOFLO short bed ion exchange process utilizes
what is
normally considered the finest particles used in large-scale industrial
applications. These
particles typically have an average particle diameter of 100-200 micrometers.
[0029] By comparison, the lithium ion sieves utilized in the present invention
are preferably in
powder form. The average particle size of the powder does not necessarily have
to be limited.
However, the average particle size is preferably less than about 100 rim, more
preferably 10 to
100 rim, even more preferably 20 to 100 r.im, and yet even more preferably 20
to 95 rim.
Further, the average particle size may be 0.4 to 40 rim. For instance, more
than 90% (by
volume) of the lithium ion sieve particles may be less than 100 rim in
diameter and greater than
0.5 p.m in diameter. In the same or different embodiments, more than 90% (by
volume) of the
lithium ion sieve particles may be greater than 0.5 pin in diameter. Since
these materials are
synthesized as powders, the cost of agglomeration is avoided. Moreover, the
higher surface
area afforded by such a powder significantly improves the kinetics of the ion
exchange process.
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
7
In other words, the lithium ion sieves are not a composite bound together with
a polymer or
other binding agent.
[0030] Various lithium ion sieves are potentially useful for lithium recovery.
Exemplary US
include, but are not limited to, oxides of manganese and titanium.
Specifically, an exemplary
LIS may include an oxide of titanium, preferably metatitanic acid (MTA).
However, the present
invention is equally applicable to other types of lithium ion sieve media such
as manganese
oxide and lithium niobate (i.e., niobic acid). The lithium ion sieve may also
comprise doping
agents in addition to an oxide of titanium, niobium, or manganese. However,
the content of
the lithium ion sieve would be predominately an oxide of titanium, niobium, or
manganese.
[0031] In one embodiment of the present invention, the powdered lithium ion
sieve media may
be contacted with a lithium-containing brine in a stirred tank reactor (STR or
reactor). For
example, the reactor may be a tank containing the liquid to be treated along
with the lithium
ion sieve. The lithium ion sieve may be maintained in suspension by a mixer or
by fluidization
by upward liquid or gas bubble flow, which provides intimate contact between
the lithium ion
sieve and the brine. The pH of the brine in the reactor may be maintained at a
constant level
through additions of an alkali, such as sodium hydroxide (NaOH), ammonium
hydroxide,
potassium hydroxide, sodium carbonate, magnesium hydroxide, and calcium
hydroxide. For
example, the pH of the brine in the reactor may be maintained at greater than
5 and less than
9.
[0032] Many of the brines that can be treated with the present invention
contain appreciable
concentrations of magnesium. Neutralization of the brine with an alkali may
present some
problems for brines that contain high concentrations of magnesium. Although
magnesium
hydroxide does not normally precipitate below a pH of about 8, when the alkali
is added,
localized high pH conditions at the point where the alkali contacts the brine
results in
precipitation of magnesium hydroxide. Despite the fact that the pH of the bulk
brine is below
the theoretical precipitation pH, the precipitate does not quickly dissolve.
The presence of
magnesium hydroxide causes a number of problems. For example, it can adhere to
the surface
of the US, inhibiting uptake of lithium. If a membrane is utilized for
solid/liquid separation,
it can reduce the permeate flux and possibly foul the membrane.
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
8
[0033] When sodium hydroxide is utilized, the magnesium hydroxide
precipitation problem is
particularly acute and more pronounced at higher NaOH concentrations. When 50%
w/w
NaOH is utilized, large quantities of Mg(OH)2 are produced that do not re-
dissolve. If a more
dilute NaOH solution is utilized, the quantity of Mg(OH)2 produced is less and
the Mg(OH)2
re-dissolves more quickly. If 4% w/w NaOH is utilized, only very small
quantities of Mg(OH)2
are produced, which re-dissolve in just a few seconds. As such, if sodium
hydroxide is used,
the sodium hydroxide is preferably at a concentration of less than 8% w/w.
[0034] Use of dilute NaOH is disadvantageous in that it dilutes the barren
brine. In locations
where the barren brine must be re-injected under-ground, the resulting brine
over-volume can
be problematic, as more brine cannot be pumped back into the ground than was
withdrawn.
[0035] This problem can be avoided by utilization of ammonia for
neutralization. The
ammonia can be in the form of anhydrous ammonia gas or liquid ammonium
hydroxide so that
the amount of brine over-volume is negligible. Only small quantities of
Mg(OH)2 precipitate
out at the injection point, even if anhydrous ammonia gas or 30% ammonium
hydroxide are
used, and this precipitate re-dissolves quickly, having no negative impacts on
the process.
[0036] After the ion exchange reaction has been completed, the lithium-
depleted (i.e., barren)
brine may be separated from the lithium ion sieve and removed from the reactor
by various
means. For example, the brine/lithium ion sieve slurry (i.e., loaded lithium
ion sieve) may be
contacted with water in an additional stirred reactor to remove residual brine
before proceeding
to the next step. Where the particle size of the lithium ion sieve is greater
than about 10 microns,
gravity sedimentation can be used. Where the particle size is less than 10
microns, filtration
devices such as a rotary drum vacuum or belt filters can be used. Where the
particles size is
less than 1 micron, membrane filtration may be used. Combinations of these
solid/liquid
separation devices can be advantageously used. One example of a possible
solid/liquid
separation device may be a centrifuge.
[0037] After removal of the barren brine, the lithium ion sieve contained in
the reactor may be
contacted with an eluent. This eluent may be, among other things, an acid,
such as hydrochloric
acid (HC1) or sulfuric acid (H2SO4). For example, the acid may be added in a
concentration in
the order of less than 0.1 M, preferably at a pH of greater than 1 and less
than 3 and most
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
9
preferably at a pH of about 2. Without intending to be bound to any particular
theory, it is
believed that the acid elutes (decomplexes) the lithium from the LIS, thus
producing a
concentrated lithium salt product solution and regenerating the LIS. As used
herein, a
"complex" is a combination of individual atom groups, ions, or molecules that
combine to
create one large ion or molecule. As used herein, "decomplexing" is the act of
separating
individual atom groups, ions, or molecules from such a large ion or molecule.
Because of the
selectivity of the lithium ion sieve for lithium over other metals, the ratio
of lithium to other
metals may be appreciably higher in the product solution than the feed brine.
[0038] After the lithium ion sieve has been regenerated, the lithium ion sieve
can be reused to
treat more brine and extract more lithium.
[0039] In an embodiment of the invention, the process may be conducted
continuously. Two
reactor stages may be needed in such a continuous process. Brine may be fed
continuously to
a loading stage wherein lithium ion sieve is contacted with the brine as a
continuously mixed
slurry. Lithium ions may then be removed from the brine via uptake by the
lithium ion sieve,
resulting in the barren brine and a lithium-loaded LIS. The barren brine may
then be separated
from the lithium-loaded lithium ion sieve and removed from the reactor. The
lithium-loaded
lithium ion sieve, now separated from the brine, may be passed on to an
elution stage.
[0040] Regarding the contact time between the brine and the lithium ion sieve,
it is known that
metatitanic acid lithium ion sieves have very poor kinetic properties. The
lithium ions take a
relatively long time to diffuse through the narrow exchange sites. One would
therefore expect
the amount of lithium taken up by the LIS to increase with increased contact
time in the brine.
In fact, Fig. 4(b) of Chitrakar shows the effect of contact time of LIS with
brine. This data
clearly shows that the amount of lithium taken up by the LIS increases with
time and is what
would normally be expected with any ion exchange sorbent. However, the effect
of contact
time on lithium uptake over time was found to have the opposite effect as
shown in FIG. 8.
Specifically, the lithium capacity decreases over time. As FIG. 8 shows, the
lithium capacity
decreased from 15.5 mg/g at 1 hour to 12.5 mg/g at 2 hours and further
decreased to 12 mg/g
after 71 hours. The Ca separation factor also decreases with contact time.
More calcium and
less lithium are taken up on the LIS as contact time increases. Perhaps given
enough time, the
larger calcium ions slowly diffuse to the narrow exchange sites and displace
lithium. This
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
phenomenon may not have been observed in Chitrakar since the brine of
Chitrakar had a much
higher lithium concentration and lower calcium concentration ([Li] = 1630
mg/L, [Ca] = 230
mg/L) than the brine typically used in the present invention. In FIG. 8, the
brine used in the
experiment had a lithium concentration of 219 mg/L and a calcium concentration
of 34,500
mg/L. Thus, in order to maximize lithium capacity and calcium separation, at
least for brines
containing relatively high calcium and low lithium concentrations (e.g., brine
obtained from
the Smackover formation in southern Arkansas), the contact time between the US
and the brine
should be less than about 1 hour.
[0041] Eluent may be fed continuously to the elution stage, and the lithium-
loaded lithium ion
sieve removed from the loading stage may be contacted with the eluent as a
continuously mixed
slurry. The lithium ion sieve and liquid are separated, and this separated
liquid (i.e., the eluate)
is the lithium salt product solution.
[0042] The lithium content of the lithium ion sieve leaving the elution stage
is appreciably
reduced and, the lithium ion sieve may be recycled back to the loading stage
for reuse. In this
manner, the lithium ion sieve may be reused multiple times, and the process
may be operated
continuously.
[0043] In one embodiment, additional stages may be utilized as shown in FIG.
1. Specifically,
a feed brine flows through a line 2 into a first stirred reactor 4, which
contains lithium ion sieve,
as part of a loading stage. The lithium ion sieve is maintained in suspension
by a mixer 6. The
brine/lithium ion sieve slurry is maintained at a constant pH through the
addition of NaOH via
line 8. The lithium ion sieve loaded with brine flows through line 10 into an
additional stirred
reactor 12 as part of a washing stage. The barren brine is separated from the
loaded lithium
ion sieve and flows through line 14. The lithium ion sieve loaded with brine
is maintained in
suspension by a mixer 16. In the washing stage, the loaded lithium ion sieve
is contacted with
water via line 18 to wash the brine from the lithium ion sieve, which is
believed to reduce cross-
contamination of the lithium salt product with contaminant ions present in the
feed brine. The
washed and loaded lithium ion sieve flows through line 20 into a second
stirred reactor 22 as
part of an elution stage. The wash water is separated from the washed and
loaded lithium ion
sieve and flows through line 24 to return to the first stirred reactor 4. The
washed and loaded
lithium ion sieve is maintained in suspension by a mixer 26. In the elution
stage, the washed
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
11
and loaded lithium ion sieve is contacted with HC1 via line 28 to elute the
lithium ions from
the lithium ion sieve. The concentration of acid in the second stirred reactor
22 is maintained
at a constant value through the addition of HCl via line 28. The regenerated
lithium ion sieve
flows through line 30 into another stirred reactor 32 as part of an acid wash
stage. The lithium
ions, as LiC1 product, are separated from the regenerated lithium ion sieve
and flow through
line 34. The regenerated lithium ion sieve is maintained in suspension by a
mixer 36. In the
acid wash stage, residual acid is washed from the lithium ion sieve through
the addition of
water via line 38 so that the feed brine is not acidified in the loading stage
when the lithium ion
sieve is recycled and recovered lithium is not recycled back to the loading
stage. The washed
and regenerated lithium ion sieve flows through line 40 back to the first
stirred reactor 4 to be
used again in the loading stage. The dilute acid washings are separated from
the washed and
regenerated lithium ion sieve and flow through line 44 to be used along with
the additional
concentrated acid in the elution stage.
[0044] In one embodiment, several loading stages may be utilized in series and
operated
counter-currently. The brine may be initially processed in a first loading
stage. The treated
brine from the first loading stage, still containing some residual lithium,
may be passed to a
second loading stage wherein contact with lithium ion sieve further reduces
the lithium content
of the brine. The lithium ion sieve from the second loading stage, containing
some lithium but
still having further lithium capacity available, may be passed to the first
loading stage. The
loaded lithium ion sieve from the first loading stage may then be passed to an
elution stage. By
this means, the lithium content of the barren brine can be further reduced. To
further reduce
the lithium content of the barren brine, additional loading stages may be
utilized in this manner.
[0045] The loaded lithium ion sieve can similarly be processed in several
elution stages
whereby the lithium ion sieve passes counter-currently to the eluate flow. By
this means, the
lithium content of the lithium ion sieve can be further reduced, and the
lithium concentration
in the eluate (i.e., the lithium product) can be increased.
[0046] The exchange reaction for uptake of the lithium ions onto the lithium
ion sieve from
the brine is shown in equation (1)
LIS.H + Li+ LIS.Li + H+ (1)
where LIS.H represents the lithium ion sieve in the freshly regenerated,
hydrogen form and
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
12
LIS.Li represents the lithium ion sieve in the loaded lithium form.
[0047] As the reaction proceeds, hydrogen ions are released to the brine,
decreasing the pH of
the brine. The active component of the lithium ion sieve may be, for example,
an oxide of
titanium, such as metatitanic acid (MTA). MTA is a weak acid and, therefore,
has a high
affinity for hydrogen ions. As a result, at a low pH, where hydrogen ions are
available, MTA
may not easily exchange hydrogen ions for lithium. The lithium ion sieve may
also further
comprise small amounts of doping agents.
[0048] FIG. 2 shows the amount of metal ion uptake as a function of pH. It can
be seen that
lithium uptake is reduced significantly below a pH of about 6.5 and little
lithium will be taken
up below a pH of about 4. As the lithium loading proceeds, the pH of the brine
drops. When
the pH drops to a pH of about 4, no further uptake of lithium can occur.
[0049] This phenomenon is similar to that which is observed with conventional
polymeric
weak acid cation exchange resins. The conventional approach to dealing with
this issue is to
pre-neutralize the ion exchange resin with sodium hydroxide, which converts
the exchanger to
the sodium form so that, during loading, the pH of the solution remains
constant. However, this
approach will not work with a lithium ion sieve since the sodium ion is too
large to penetrate
the lithium ion sieve.
[0050] In one embodiment, the pH may be adjusted prior to contacting the brine
with the US
by dosing the brine with NaOH or another base, such as sodium carbonate or
ammonium
hydroxide, prior to treatment. Such a pre-treatment will raise the initial pH
so that the final pH
will not be so low as to prevent lithium uptake. The disadvantage of this
approach, however, is
that, as shown in FIG. 2, at increased pH levels the amount of sodium ions
taken up by the
lithium ion sieve increases. In addition, if the pH is raised above 8,
magnesium hydroxide may
precipitate out of solution.
[0051] In one embodiment, the brine/lithium ion sieve slurry in the loading
reactor may be
neutralized with an alkali, such as NaOH, in order to maintain the pH so as to
maximize the
uptake of lithium while minimizing the uptake of sodium. The pH may generally
be greater
than about 5 and less than about 9, preferably greater than 6 and less than 8.
When the lithium
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
13
ion sieve is MTA, the pH is preferably between 6 and 7.
[0052] Lithium is typically eluted from the US with an acid, such as
hydrochloric acid, to
concurrently regenerate the lithium ion sieve and produce a lithium product,
as shown by
equation (2). The lithium ion sieve effectively neutralizes the acid by this
reaction.
LIS.Li + H+ ¨> LIS.H + Li + (2)
[0053] As shown in FIG. 3, the amount of lithium eluted from the lithium ion
sieve increases
as the concentration of HC1 increases. For optimum elution efficiency, the
acid concentration
may be maintained at a concentration of less than 0.1 M (defined as mo1.dm-3
in FIG. 3). As
shown in FIG. 7, for optimum elution efficiency, the acid concentration may
correspond to a
pH of less than 3 and greater than 1 and preferably at a pH of approximately
2.
[0054] However, as also shown in FIG. 3, at acid concentrations of greater
than 0.1 M,
increasing amounts of titanium are extracted from the lithium ion sieve,
thereby degrading the
lithium ion sieve and reducing its useful life. Above an acid concentration of
about 0.1 M,
excessive amounts of titanium are extracted, resulting in a prohibitively
short life.
[0055] One method to minimize such degradation of the lithium ion sieve is to
minimize the
contact time between the US and the acid. Because in one embodiment the
lithium ion sieve
is in powdered form, the kinetics of the ion exchange process are quite rapid
and the exchange
reaction of equation (2), above, is mostly completed in less than one hour. In
an embodiment,
the contact time between the US and the elution acid is less than one hour.
Therefore, lithium
is essentially completely removed from the lithium ion sieve while minimizing
the degradation
of the lithium ion sieve.
[0056] Additionally, the particle size of the lithium ion sieve particles
plays a role in the design
of the system described herein. FIG. 4 shows a typical particle size
distribution of a sample of
metatitanic acid lithium ion sieve taken after a few hours of air agitation in
a slurry. The
effective particle size (dio) is about 0.51.1m and 90% (by volume) of the
material is in the range
of 0.4-40 p.m. The effective size is the diameter of the particle for which 10
percent of the total
grains are smaller, and 90 percent of the total grains are larger, on a weight
or volume basis.
The effective size of this material is about 0.5 p.m. While the coarser
material may settle from
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
14
a water slurry by gravity in less than one hour, the finer particles do not
easily settle even after
a day. Without intending to be bound by any particular theory, it is believed
that larger lithium
ion sieve particles are agglomerates of fine particles produced by sintering
during the synthesis
process. As a result, the large particles are susceptible to mechanical
attrition during mixing
with the process liquids, so that there would be an increasing proportion of
fine particles over
time. Consequently, separation of the lithium ion sieve from the process
liquids by gravity
sedimentation is not ideal.
[0057] Membranes are being increasingly used in bioreactors for wastewater
treatment. In a
typical membrane bioreactor (MBR), microfiltration or ultrafiltration
membranes with pore
sizes of less than 0.1 jam, either in hollow-fiber, tubular or flat sheet
form, are submerged in a
suspension of wastewater and bio-solids. Clear filtered/treated wastewater is
drawn through
the membranes by vacuum. The wastewater/biosolids slurry is typically agitated
by air
sparging. Air agitation promotes oxygen transfer to the bio-solids and
prevents membrane
fouling due to build-up of bio-solids on the membrane surface.
{0058] In membrane bioreactors, the suspended solids concentration is
typically less than 30
g/L and more typically 10-15 g/L. Higher suspended concentrations are not
employed, as
oxygen transfer is impeded due to resulting higher and non-Newtonian fluid
viscosity. In
addition, \higher suspended solids concentrations reduce the membrane flux
rate and/or increase
the trans-membrane pressure. Typical flux rates for submerged membranes in
membrane
bioreactors are 10-30 liters per hour per square meter (which units are
normally abbreviated as
"LMH").
[0059] In one embodiment, the submerged ultrafiltration or microfiltration
membrane process
may be used in the present invention as a means of separating the lithium ion
sieve from the
process liquids. The pore size of the membranes, at typically less than about
1 i.tm, is smaller
than the smallest lithium ion sieve particles, so nearly 100% solids
separation can be achieved.
In the present invention, oxygen transfer is not an issue. However, submerged
aeration (air
agitation) may provide the necessary mixing of the slurry, while the rising
bubbles scour the
membrane surfaces to reduce membrane fouling, and reduce the attrition and
shearing of the
US particles compared with mechanical mixing.
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
[0060] Embodiments described here are a significant departure from typical
immersed
membrane applications such as MBRs. Lithium ion sieve particles allow
treatment of much
higher suspended solids concentrations while achieving appreciable higher
fluxes. Fluxes
obtained in conventional MBR applications are typically less than 30 LMH at
transmembrane
pressures of 10-30 KPa and total suspended solids (TSS) levels of less than 30
g/L. In contrast,
with the present invention, fluxes as high as 300 LMH at transmembrane
pressures of 20 KPa
have been obtained with lithium ion sieves, at TSS levels of more than 100
g/L.
[0061] According to the present invention, the suspended solids concentration
may be greater
than about 50 g/L and preferably greater than 100 g/L. Without intended to be
bound by any
particular theory, it is believed that a higher solids concentration in the
reactor is advantageous
because it reduces the reactor volume required to achieve a given lithium ion
sieve-liquid
contact time.
[0062] In a fixed bed ion exchange system, the acid eluent becomes lower in
acid concentration
as it passes through the bed and is neutralized by the reaction provided by
equation (2) above.
In order to maintain the pH of the acid in contact with the lithium ion sieve
at less than 3, to
maintain elution efficiency, the pH of acid entering the bed may then be
appreciably less than
less than 1. Consequently, if the lithium ion sieve is regenerated in a fixed
bed, the lithium ion
sieve toward the entry end of the bed will be severely degraded by the more
concentrated acid.
[0063] According to the present invention, the lithium ion sieve may be
regenerated as a slurry
in a reactor vessel where the lithium ion sieve is in contact with acid at a
uniform concentration.
The acid concentration may be maintained at a concentration of less than 0.1
M, and preferably
at an acid concentration corresponding to a pH of less than 3 and greater than
1 and preferably
at a pH of approximately 2. This concentration can be maintained by
continuously measuring
the acid concentration of the liquid in the reactor by suitable means and
adding concentrated
acid as required, to maintain the concentration in the desired range (e.g., at
pH= 2).
[0064] To minimize impurities, such as calcium, magnesium, potassium, and
sodium, in the
final lithium salt product produced by acid elution of the lithium ion sieve,
the residual feed
brine may be removed from the lithium ion sieve after loading and prior to
acid elution by
mixing the loaded lithium ion sieve with water and then separating out the
water. In an
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
16
alternative embodiment, the residual feed brine may be removed by directly
filtering the loaded
lithium ion sieve through a suitable filter. According to the present
invention, the preferred
particle size of the lithium ion sieve is in the range of 0.4-40 Elm. Solids
particles in this range
can be filtered and de-watered using conventional solid/liquid separation
devices, employing
filter media, such as woven filter cloths with openings of greater than 10 lam
in lieu of
membranes with pore sizes of less than 1 p.m. Thus, the bulk of the feed brine
will be separated
from the loaded lithium ion sieve. The dewatered lithium ion sieve may then be
washed
directly on the filter to remove the residual brine from the lithium ion sieve
without the
necessity of re-slurrying the lithium ion sieve in water. Exemplary types of
filters include, but
are not limited to, horizontal belt vacuum and pressure filters, rotary drum
vacuum and rotary
disk vacuum and pressure filters, pressure filter presses, and centrifuges.
[0065] As discussed above, elution of lithium from the lithium ion sieve with
acid yields an
acidic lithium salt solution. The lithium ion sieve is preferably separated
from the acidic
lithium salt eluate solution to minimize the return of the recovered lithium
with the regenerated
lithium ion sieve back to the loading reactor. A similar approach may be
utilized as is used for
separating feed brine from the loaded lithium ion sieve. Thus, the regenerated
lithium ion sieve
may be mixed with water and then separating out the water. Alternatively, the
lithium ion sieve
may be filtered through a suitable filter, preferably one with water washing
capabilities.
[0066] Care should be exercised to minimize the moisture content of the
lithium ion sieve
transferred into the regeneration reactor. If excessive amounts of water
accompany the lithium
ion sieve into the regeneration reactor, the recovered lithium salt eluate
solution will be too
dilute. Similarly, the lithium should be recovered with the liquid entrained
on the loaded
lithium ion sieve that is withdrawn from the regeneration reactor.
[0067] As shown in Example 1 below, the working capacity of a metatitanic acid
lithium ion
sieve may be about 0.01 g lithium per gram of lithium ion sieve. The flow of
lithium ion sieve
on a dry basis would then be 100 g lithium ion sieve/g Li recovered. When the
slurry in the
loading reactor contains a suspended solids concentration of 100 g/L (i.e..
about 90% moisture
by weight and about 10% solids weight; 1 liter of water per 100 grams lithium
ion sieve) and
this slurry was transferred directly to the regeneration reactor, it would
bring (1 g lithium ion
sieve/0.01 g Li /100 g/L lithium ion sieve) = 1.0 liter of water per gram of
lithium recovered.
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
17
Ignoring the water in the concentrated acid, the concentration of lithium in
the eluate would
then be 1 g/l.
[0068] If the suspended solids concentration in the regeneration reactor is
also maintained at
100 g/L and withdrawn at this concentration, the amount of lithium entrained
with the
regenerated lithium ion sieve would be (1 liter/g Li xl g/L Li) = 1 g Li/g Li
recovered. In other
words, all of the lithium eluted from the lithium ion sieve would be withdrawn
with the lithium
ion sieve. If this lithium ion sieve was then recycled directly back to the
loading reactor, no
net lithium would be recovered.
[0069] The regenerated lithium ion sieve slurry could be mixed with water in a
washing reactor
to recover the lithium values prior to recycling the lithium ion sieve to the
loading reactor. To
separate 90% of the lithium from the lithium ion sieve would require 9 liters
of water per gram
of recovered lithium. The diluted liquid in the washing reactor could then be
separated by
gravity or a membrane, for example. The concentration of lithium would then be
only 0.1 g/l.
However, this concentration is too low to be of practical use. Thus, the
lithium ion sieve should
be dewatered to a moisture content appreciably less than 90%.
[0070] For example, if the loaded lithium ion sieve slurry is dewatered to 50%
moisture (i.e..
1 liter water/1000 g lithium ion sieve) the lithium ion sieve would carry only
(1 liter water/1000
g lithium ion sieve)/(0.01 g Li/g lithium ion sieve) = 0.1 liter water per
gram of Li recovered.
Ignoring the water in the concentrated acid, the concentration of lithium in
the eluate would
then be 10 g/liter.
[0071] Additionally, the regenerated lithium ion sieve should be dewatered
when it is removed
from the regeneration reactor. Otherwise, a large portion of the recovered
lithium will be
recycled with the lithium ion sieve back to the loading reactor. Even if the
regenerated lithium
ion sieve is dewatered to a high degree, the lithium lost to the moisture
entrained in the lithium
ion sieve may be problematic. For example, if the regenerated lithium ion
sieve is dewatered
to 50% moisture content by weight (i.e., 1 liter water per 1000 g of lithium
ion sieve), the
amount of lithium entrained with the lithium ion sieve would be (1 liter/1000
g lithium ion
sieve) / (0.01 g Li/g lithium ion sieve) x 10 g Li/lL) = 1 g Li/g Li
recovered. In other words,
all of the lithium eluted from the lithium ion sieve would be withdrawn with
the lithium ion
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
18
sieve. If this lithium ion sieve was then recycled back to the loading
reactor, no net lithium
would be recovered.
[0072] Thus, the lithium from the liquid entrained with the dewatered lithium
ion sieve should
be recovered. For instance, the regenerated lithium ion sieve may be washed
with water. The
lithium would then be recovered in the wash water. The amount of wash water
should be
sufficient to recover most of the lithium, but not so much as to excessively
dilute the recovered
lithium salt solution. One method to achieve this would be to re-slurry the
lithium ion sieve in
water and then re-filter the lithium ion sieve from the slurry. To wash 90% of
the lithium from
the lithium ion sieve would require about 9 mL of water per mL of entrained
liquid in the
lithium ion sieve, allowing recovery of a lithium salt solution containing 1
g/L lithium under
these conditions.
[0073] The amount of wash water can be reduced and the lithium concentration
can
concomitantly be increased by utilizing two or more counter-current washes.
Accordingly, the
dewatered lithium ion sieve recovered from the first wash stage is re-slurried
in water once
again in a second wash stage and then dewatered yet again. The wash water
recovered from
the second stage dewatering device is utilized in the first wash stage in lieu
of fresh water.
With two counter-current wash stages, the amount of water required for 90%
lithium recovery
can be reduced from about 9 mL of water per mL of entrained liquid to about 3
mL of water
per mL of entrained liquid, and the concentration of recovered lithium can be
increased from
1 g/L to about 3 g/L.
[0074] In a further embodiment, the slurry may be dewatered by a device such
as a horizontal
vacuum belt filter. The dewatered lithium ion sieve cake may then be washed
directly on the
filter. One or more count-current wash stages can be employed on the filter.
As another option,
a centrifuge may be used. If a centrifuge is used, the solids may be re-
slurried in water and
then dewatered with the centrifuge. If several washing stages are used, the
dewatered solids
from a first centrifuge may be re-slurried with water again and then dewatered
in a second
centrifuge. The centrate from the second centrifuge may be used as the water
to slurry the
solids feeding the first centrifuge. Additional centrifuges can be utilized in
this manner to
effectively achieve a multi-stage countercurrent solids wash.
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
19
[0075] If the particle size of the lithium ion sieve is too small, such
dewatering becomes more
difficult. Indeed, even if the majority of the particles are greater than 10
micrometers in
diameter, the presence of particles much less than 10 micrometers in diameter
makes
dewatering difficult. In particular, if the average particle size of the ion
sieve is 0.1 m or less,
dewatering becomes virtually impossible.
[0076] In another embodiment of the present invention, the dry lithium ion
sieve may be
classified by a suitable device such as an air classifier or the wet lithium
ion sieve may be
classified by elutriation to remove the fine particles with a diameter of less
than 1-10
micrometers. By doing so, separation of the lithium ion sieve from the liquid
to be treated is
facilitated. Removal of the fine particles will significantly improve
filtration rates, avoid
blinding of filtration media, and produce a filter cake with a lower moisture
content. By
removing the fine particles in this manner, conventional solid/liquid
separation devices, such
as horizontal belt vacuum and pressure filters, rotary drum vacuum and rotary
disk vacuum and
pressure filters, pressure filter presses, centrifuges, and the like may be
more effectively
employed.
[0077] To maximize the purity of the recovered lithium salt product, the feed
brine should be
efficiently separated from the loaded lithium ion sieve. Purity requirements
for battery grade
lithium carbonate, for instance, are very stringent. Any residual feed brine
retained with the
loaded lithium ion sieve will contaminate the product with impurities in the
feed brine, such as
calcium, magnesium, sodium, potassium, etc. As the concentration of these
impurities in the
brine is much higher than the lithium, even minimal amounts of brine carry-
over are
problematic. In fact, the impurity contribution from entrained brine on the
loaded lithium ion
sieve is potentially greater than the quantity of impurities actually
exchanged on to the lithium
ion sieve in most cases. While additional processes, such as lime/soda and ion
exchange
softening, can be used to purify the recovered lithium solution, these
additional process steps
involve additional capital and operating expense. However, efficient
dewatering and washing
of the loaded lithium ion sieve prior to passing it onto the regeneration
reactor can minimize
need for these costly processes. As discussed above, efficient dewatering can
be achieved with
conventional solids/liquid separation devices, provided that the lithium ion
sieve does not have
significant quantities of particles less than 1-10 micrometers in diameter. In
addition, the wash
water requirements can be reduced by employing multi-stage counter-current
washing.
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
[0078] The present invention will hereinafter be described with reference to
exemplary
embodiments, which are written to be understood only as examples and are not
intended to
limit the scope of the present application.
EXAMPLE
[0079] A test unit was constructed to demonstrate the process according to one
embodiment of
the invention. A schematic drawing of the test unit is shown in FIG. 5.
[0080] The test unit consisted of six reactors (R1-R6), each one equipped with
air agitation
diffusers and five of which were equipped with immersed membrane modules.
Reactor R4,
utilized for acid regeneration of the lithium ion sieve, was not equipped with
a membrane. The
working volume of each of the reactors was approximately 5 liters with the
exception of the
reactor R4, which had a working volume of approximately 1.1 liter.
[0081] Lithium titanate (LTO) was used as the lithium ion sieve. The LTO was
synthesized by
reacting lithium hydroxide with titanium dioxide at a molar ratio of
approximately 2.2:1 at a
temperature of 700 C for 4 hours. FIG. 4, discussed above, provides the
particle size
distribution of the LTO used in this example. The initial LTO produced from
the synthesis
was converted to metatitanic acid (HTO) by pickling the LTO in 0.2 N HC1 for
16 hours and
then washing the resulting HTO with water. Reactor R1 and reactor R2 were
initially charged
with 100 g/L aqueous slurries of the US, while the remaining reactors were
initially charged
with 500 g/L slurries of the US. The lithium ion sieve was conveyed from
reactor to reactor
as a slurry by peristaltic pumps. The flow rate of the lithium ion sieve
slurries was adjusted so
that the solids transfer rate was approximately 100 g/h on a dry weight basis.
[0082] The membrane modules were lab-scale immersed-type POREFLONTM units,
manufactured by Sumitomo Electric Corporation, each having an effective
membrane area of
0.1 m2. Liquid was drawn through the membranes by vacuum using peristaltic
pumps. The
vacuum was maintained at less than 40 kPa.
[0083] The lithium containing brine was made up from brine obtained from the
Smackover
formation in southern Arkansas and had a composition as shown in Table 1
below. After
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
21
extraction of lithium from the brine in accordance with the process, the brine
was re-fortified
with lithium chloride and recycled to the process. As a result, the lithium
concentration in the
feed brine was somewhat higher than the initial brine as received. The sodium
and potassium
concentrations were estimated based upon published brine assays.
Table 1
TSS [Li] [Ca] [Na] [K] [Mg] Flow
(g/L) (mg/L) (mg/L) (mg/L) (mg/14 (mg/L) (L/h)
Feed Brine 244 22,000 43,000* 1,384* 2,170 4.96
Barren Brine 61 4.15
Product 4,300 1,400 9,770 76 0.54
Dilute slurry 100 1.02
Conc. slurry 500 0.21
* Estimated from published brine assay data.
[0084] Reactor R1, the loading reactor, was equipped with a pH controller that
automatically
controlled the addition of 1 N NaOH such that a pH of 7.8 was maintained.
Thus, the acid
generated by the ion exchange reaction was continually neutralized. Feed brine
was introduced
to reactor R1 and allowed to contact the HTO. The HTO was fed to reactor R1
from reactor
R6 as a 500 g/L slurry. As a result of the mixing of the concentrated slurry
from reactor R6
with the feed brine, the solids concentration of the lithium ion sieve in
reactor R1 was about
100 g/L. As the HTO extracted lithium ions from the brine, the HTO was
partially converted
back to LTO. Lithium-depleted (i.e., barren) brine was drawn through the
membranes by a
pump.
[0085] The loaded lithium ion sieve (i.e., LTO) was withdrawn from the reactor
R1 as a brine
slurry and directed to reactor R2, which was the brine wash reactor. Water was
fed to reactor
R2 so that the residual brine was washed from the LTO. The wash water was
withdrawn from
reactor R2 through another immersed membrane module.
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
22
[0086] Loaded/washed LIS was withdrawn from reactor R2 as a water slurry and
directed to
reactor R3, which was the thickener reactor. Water was withdrawn from reactor
R3 through
another immersed membrane module, thus increasing the solids concentration in
reactor R3 to
approximately 500 g/L.
[0087] The thickened slurry of loaded/washed LIS at a solids concentration of
about 500 g/L
was withdrawn from reactor R3 and directed to reactor R4, which was the
regeneration reactor.
The lithium ion sieve in reactor R4 was contacted with hydrochloric acid at a
concentration of
approximately 0.2 M. The lithium ion sieve solids concentration in reactor R4
was
approximately 500 g/L. The acid concentration was monitored and maintained by
a
conductivity controller at a constant level through the addition of 5 M HC1 to
a conductivity
set point of 150 mS/cm. Contacting the lithium ion sieve with acid converted
it from the LTO
form back to the HTO form and resulted in a lithium ion sieve slurry of about
0.2 M
hydrochloric acid along with lithium chloride. Reactor R4 was not equipped
with a membrane,
and the lithium ion sieve slurry of HC1/lithium chloride was simply allowed to
overflow to
reactor R5. It is recognized that an acid concentration of 0.2 M is not
preferred due to excessive
dissolution of titanium from the LIS, but this example still illustrates the
process of the present
invention.
[0088] Reactor R5 was the first of two counter-currently operated acid wash
reactors. The
majority of the HC1/lithium chloride was washed from the lithium ion sieve in
reactor R5, while
most of the residual HC1/lithium chloride was washed from the lithium ion
sieve in reactor R6.
The lithium ion sieve in reactor R5, at a solids concentration of about 500
g/L, was contacted
with wash-water from reactor R6. The acid wash-water was withdrawn from
reactor R5
through another immersed membrane module. The acid wash-water withdrawn from
reactor
R5 constituted the recovered lithium chloride product from the process. A
slurry of lithium
ion sieve at a concentration of about 500 g/L was withdrawn from reactor R5
and directed to
reactor R6.
[0089] Fresh water added to reactor R6 washed most of the remaining
HC1/lithium chloride
from the lithium ion sieve. The wash-water was withdrawn from reactor R6
through another
immersed membrane module and directed to reactor R5. The concentration of
lithium chloride
in the wash-water in reactor R6 was thereby reduced to less than 10% of the
lithium
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
23
concentration in reactor R4. The lithium ion sieve/wash-water slurry was
withdrawn from
reactor R6 and directed back to reactor R1 wherein it was reused to extract
lithium from the
feed brine.
[0090] A continuous 12 hour test run was conducted. Aliquots of barren brine
and product
were sampled and assayed hourly. A graph showing the barren and product
concentrations over
the course of the run is shown in FIG. 6. The results summarized in Table 1
were from 1-hour
composite samples taken after 10 hours of operation. The lithium concentration
was reduced
from 244 mg/L to 61 mg/L, a 75% recovery rate. The liquid residence time in
the loading
reactor was about 1 hour.
[0091] The lithium product contained a lithium concentration of 4,300 mg/L.
More lithium
was removed from the product (2,322 mg/h) than was actually extracted from the
brine (957
mg/h). Without intending to be bound to any particular theory, it is believed
that the difference
(1,365 mg/h) was likely residual lithium on the lithium ion sieve that had not
been completely
removed from the LTO during the initial pickling in HC1. Based upon the
lithium that was
actually extracted from the brine, the lithium ion sieve capacity was 9.6
mg/g. The liquid
residence time in the strip reactor was 2.2 hours. Based upon the lithium that
was loaded and
recovered, the lithium concentration factor was about 10 times.
[0092] The feed brine contained a calcium concentration of 22,000 mg/L while
the product
contained a calcium concentration of only 1,400 mg/L. The ratio of calcium to
lithium in the
feed was 90. The ratio in the product was 0.33. However, only about half of
the lithium in the
product was actually extracted from the brine. If only the lithium in the
product that was
extracted from the brine is considered, the ratio of Ca to Li in the product
was 0.62, which
represents an enrichment factor of 90/0.62= 145.
[0093] The feed brine contained an estimated sodium concentration of 43,000
mg/L while the
product contained a sodium concentration of only 9,770 mg/L. The ratio of
sodium to lithium
in the feed was 176. The ratio in the product was 2.3. If only the lithium in
the product that
was extracted from the brine is considered, the ratio of Na to Li in the
product was 4.3, which
represents an enrichment factor of 176/4.3= 41.
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
24
[0094] The feed brine contained a magnesium concentration of 2,170 mg/L while
the product
contained a magnesium concentration of only 76 mg/L. The ratio of magnesium to
lithium in
the feed was 8.9. The ratio in the product was 0.018. If only the lithium in
the product that was
extracted from the brine is considered, the ratio of Mg to Li in the product
was 0.034, which
represents an enrichment factor of 8.9/.034= 262.
[0095] Thus, the system and method described herein have the ability to
selectively recover
lithium from brines containing high concentrations of calcium, sodium, and
magnesium.
[0096] In this example, only one brine wash reactor was used, so some brine
would have passed
into the regeneration reactor on the loaded lithium ion sieve, thus carrying
some calcium,
sodium, and/or magnesium into the regeneration reactor on the loaded lithium
ion sieve.
Without intending to be bound to any particular theory, it is believed that
the results could be
improved by including a second brine washing reactor. In addition, as
discussed above, by
lowering the loading pH to 6-7, the amount of sodium loaded onto the lithium
ion sieve could
be reduced without appreciably decreasing the lithium capacity.
COMPARATIVE EXAMPLE
[0097] A key test was done in Chitrakar to evaluate the effect of HCl
concentration on initial
extraction of lithium and titanium from the adsorbent, which is shown in
Figure 4a of Chitrakar.
Figure 4a of Chitrakar shows the amount of lithium and titanium extracted as a
function of the
HC1 concentration. The data in Chitrakar shows that the HCl concentration
should be 0.2 M
or more. In fact, no data is shown in Figure 4a of Chitrakar for lithium
extraction from the
adsorbent below an acid concentration of 0.1 M, which is the preferred acid
concentration in
which the present invention operates. In the present invention, the lithium
and titanium
components of the LTO adsorbent are extracted at much lower acid
concentrations than
predicted by Chitrakar.
[0098] References herein to terms such as "vertical," "horizontal," etc. are
made by way of
example, and not by way of limitation, to establish a frame of reference. It
is understood that
various other frames of reference may be employed for describing the invention
without
departing from the spirit and scope of the invention. It is also understood
that features of the
invention are not necessarily shown to scale in the drawings. Furthermore, to
the extent that
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
the terms "composed of," "includes," "having," "has," "with," or variants
thereof are used in
either the detailed description or the claims, such terms are intended to be
inclusive and open-
ended in a manner similar to the term "comprising."
[0099] References herein to terms modified by language of approximation, such
as "about,"
"approximately," and "substantially," are not to be limited to the precise
value specified. The
language of approximation may correspond to the precision of an instrument
used to measure
the value and, unless otherwise dependent on the precision of the instrument,
may indicate +/-
10% of the stated value(s).
[00100] A feature "connected" or "coupled" to or with another feature may
be directly
connected or coupled to or with the other feature or, instead, one or more
intervening features
may be present. A feature may be "directly connected" or "directly coupled" to
or with another
feature if intervening features are absent. A feature may be "indirectly
connected" or
"indirectly coupled" to or with another feature if at least one intervening
feature is present. A
feature "on" or "contacting" another feature may be directly on or in direct
contact with the
other feature or, instead, one or more intervening features may be present. A
feature may be
"directly on" or in "direct contact" with another feature if intervening
features are absent. A
feature may be "indirectly on" or in "indirect contact" with another feature
if at least one
intervening feature is present.
[00101] The terminology used herein is for the purpose of describing
particular
embodiments only and is not intended to be limiting of the invention. As used
herein, the
singular forms "a," "an," and "the" are intended to include the plural forms
as well, unless the
context clearly indicates otherwise. It will be further understood that the
terms "comprises"
and/or "comprising," when used in this specification, specify the presence of
stated features,
integers, steps, operations, elements, and/or components, but do not preclude
the presence or
addition of one or more other features, integers, steps, operations, elements,
components,
and/or groups thereof.
[00102] While the invention has been illustrated by a description of
various
embodiments and while these embodiments have been described in considerable
detail, it is
not the intention of the applicant to restrict or in any way limit the scope
of the appended claims
CA 03184777 2022-11-24
WO 2021/248221 PCT/CA2020/000069
26
to such detail. Additional advantages and modifications will readily appear to
those skilled in
the art. Thus, the invention in its broader aspects is therefore not limited
to the specific details,
representative apparatus and method, and illustrative example shown and
described. In the
interest of fully enabling persons ordinarily skilled in the art to make and
use the claimed
invention, the applicant has provided information as to both advantages and
disadvantages of
various detailed embodiments. Persons of ordinary skill will understand that,
in some
applications, the disadvantages of a specific embodiment as detailed above may
be avoided
altogether or outweighed by the overall advantages provided by the invention
as claimed.
Accordingly, departures may be made from detailed teachings above without
departing from
the spirit or scope of applicant's general inventive concept.
=