Note: Descriptions are shown in the official language in which they were submitted.
WO 2022/031362
PCT/US2021/036128
SYSTEM AND METHOD FOR PREVENTING OR TREATING ALZHEIMER'S AND
OTHER NEURODEGENEFtATIVE DISEASES
DESCRIPTION
BACKGROUND OF THE INVENTION
[Para 1] The present invention generally relates to a system and process for
treating biological tissues. More particularly, the present invention relates
to a
system and process for preventing or treating Alzheimer's and other
neurodegenerative diseases.
[Para 2] Chronic progressive diseases (CPDs) currently, and increasingly in
the
future, are healthcare challenges. There are many such CPDs, including Type II
Diabetes, Alzheimer's Disease, Idiopathic Pulmonary Fibrosis (IPF), heart
disease
and the like. There are many diseases for which the underlying cause is
unknown, and which either have no treatment or suboptimal treatment. Some
of these diseases are either uniformly terminal in short-order, or constitute
major public health problems due to increasing at-risk populations and
chronicity leading to epidemic increase in prevalence.
[Para 3] These diseases are both chronic and progressive. Chronic progressive
diseases may have any number of underlying causes, including age, infectious,
genetic, multi-factorial and immune. The progressive nature of these disorders
implies that all worsen with age. While there are many different causes of
CPDs,
they share fundamental commonalities. A unifying feature of all CPDs is the
1
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
accumulation of abnormal intracellular proteins. Another common feature of all
CPDs is increasing cellular and organ dysfunction, leading to failure. Yet
another common and unifying feature of CPDs is cellular and organ dysfunction
that causes and promotes chronic inflammation. These features of all CPDs
create a vicious cycle leading to the disease worsening over time.
[Para 4] Thus, interruption of the cycle is essential to ameliorate the course
of
the disease. One approach to treatment of CPDs is gene therapy, which requires
identification and repair or replacement of the defective gene that is the
cause
of the disease. However, for some CPDs, the gene defect is unknown. For
others, there may be many potential gene defects which lead to the same
disease. For example, retinitis pigmentosa can be caused by any of over 150
different genetic defects. This potential multiplicity of underlying defects
makes
gene therapy difficult.
[Para 5] Another approach to treatment of CPDs is drug therapy which typically
attempts to target specific cellular proteins thought to be critical to the
disease
process to either inhibit or enhance their action. However, as there are an
estimated 2,000 different protein types in the typical cell having 10680
potential
interactions, finding successful, safe and clinically effective targeted drugs
therapies without unacceptable side effects is difficult.
[Para 61 Another approach to treatment of CPDs is use of non-specific and
anti-inflammatory treatments. These include various steroidal and non-
steroidal anti-inflammatory agents and immunosuppressive drugs. However,
anti-inflammatory drugs have many drawbacks in CPDs. As they do not address
2
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
the underlying cause of the disease, they must be used long-term and have
limited effectiveness. Because of their modes of action and necessity of long-
term use, the side effects and complications of treatment limit their
usefulness.
Immunosuppressive drugs have the same limitations as anti-inflammatory
drugs. However, as they alter the normal function of the immune system apart
from the disease process, they can cause further complications including other
disease syndromes and neoplasia. Radiation therapy, such as using x-ray
radiation, is another treatment for CPDs. It has effects similar to using anti-
inflammatory and immunosuppressive drugs. However, it can also present more
problematic side effects that worsen with time even after cessation of
treatment, often making it unacceptable if long-term survival is anticipated.
[Para 7] Yet another, newer, approach to treatment of CPDs is identification
and inhibitor of manager proteins. Such manager protein therapy attempts to
address the problems presented by gene, drug, and anti-
inflammatory/immunosuppression therapy by finding proteins or enzymes
which are both key and common to several disease states, regardless of the
underlying cause, and inhibiting them in various ways. As a single manager
protein may be central to the development of a number of disease conditions,
such as various and otherwise unrelated cancers, blocking this key protein
could have wider therapeutic application than more disease-targeted therapies.
However, manager protein therapy shares the general limitation of targeted
drug therapy if the protein itself is targeted, with the additional problem
common to targeted therapies of triggering compensatory mechanisms such as
3
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
up-regulation leading to permanent insensitivity to a drug action. Moreover,
manager protein therapy shares the general limitations of gene therapy if the
transcriptional and translational mechanisms that produce the protein are
targeted. Although implicated in the disease process, such proteins virtually
always have key roles in normal physiology which may lead to problems if
inhibited generally or indiscriminately. Thus, such manager protein therapy
also
shares the problems targeted drug therapy has, as mentioned above.
[Para 81 Stem cell transplantation (SCT) is yet another approach to treatment
of
CPDs. SCT attempts to replace dead or dysfunctional tissue with new functional
tissue by transplanting stem cells into the tissue or area surrounding the
tissue.
SCT is highly complex and expensive, with significant risks and adverse
treatment effects. Despite much public interest, SCT has been thus far largely
ineffective.
[Para 9] The current approaches described above for treatment of
CPDs are
of limited success and usefulness and thus most CPDs have either no treatment
currently or only supportive, symptomatic, palliative or ineffectual
treatment.
These treatments are of limited success and usefulness by virtue of practical
limitations, including unknown or multiple causes, cost, time, as well as non-
physiologic (being unnatural and artificial) modes of action, which by
definition,
superimpose a new drug/intervention-induced disease state overlying the CPD.
In light of this, the ideal treatment for CPDs would be independent of the
underlying cause, physiologic, and thus both effective and well tolerated
without side-effects, and able to break the vicious CPD cycle by intervening
at
4
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
multiple points in the cycle including immediately distal to the primary
defect
for maximum effectiveness.
[Para 1 01 As mentioned above, Alzheimer's disease, and other
neurodegenerative diseases, are chronic progressive diseases. For several
decades, researchers have been attempting to find treatments or cures to
Alzheimer's and other degenerative diseases, but with little success. It is
believed that the potentially disease-modifying drugs which could arrest or
reverse severe memory impairment and other such aspects of Alzheimer's and
other degenerative diseases may not be effective as they have difficulty in
crossing the blood-brain barrier and entering the brain's neurons.
[Para 11] In view of the inability of drugs to slow or reverse the
cognitive
impairment of Alzheimer's and other degenerative diseases thus far, other non-
pharmaceutic interventions are warranted. Transcranial stimulation, such as
using electromagnetic energy sources, including radiofrequency, has been
found to enable treatment of tissue and fluids at the blood-brain barrier and
beyond the barrier and into the tissues of the brain.
SUMMARY OF THE INVENTION
[Para 1 21 The present invention is directed to a system and method
for
preventing and treating chronic progressive diseases, including Alzheimer's
and other degenerative diseases. In accordance with the present invention, it
is
determined that an individual has Alzheimer's or other degenerative disease or
is at a risk of developing such a neurodegenerative disease. A pulsed
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
electromagnetic energy source comprising radiofrequency or microwave having
selected energy parameters, including wavelength or frequency, duty cycle and
pulse train duration is applied to the brain of the individual so as to
prevent or
treat the Alzheimer's or other degenerative disease. The pulsed
electromagnetic energy may be directed to one or more of the leaky blood-
brain barrier, inflamed portions of the brain, junk proteins of the brain,
beta
amyloid proteins of the brain, and/or tangled tau proteins of the brain.
[Para 13] The pulsed energy source parameters may be selected so as
to
raise a temperature of the treated tissue sufficiently to stimulate heat shock
protein activation in the treated tissue or fluid. The energy parameters are
selected so as to raise the target tissue or bodily target fluid temperature
up to
11 C., typically between 6 C. to 11 C. at least during the application of
the
pulsed energy source to the target tissue or target fluid, to achieve a
therapeutic or prophylactic effect. The average temperature rise of the tissue
or
target fluid over several minutes is maintained at or below a predetermined
level so as to not permanently damage the target tissue or target fluid. For
example, the average temperature rise of the target tissue or target fluid
over
several minutes may be maintained at 6 C. or less. More often, the average
temperature rise of the target tissue or target fluid is maintained at
approximately 1 C. or less over several minutes, such as over a six-minute
period of time.
[Para 14] The radiofrequency may be between 3-6 megahertz (MHz), and
has
a duty cycle of between 2.5% to 5%, and a pulse train duration between 0.2 to
6
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
0.4 seconds. The radiofrequency may be generated with a device having a coil
radii between 2 and 6 mm and between 13 and 57 amp turns.
[Para 1 5] The pulsed electromagnetic energy parameters may be
selected
and applied to the brain to cause resonant interactions within biomolecules
within and around brain tissue. The pulsed energy parameters may be selected
and applied to the brain so as to disrupt the structural integrity of the beta
amyloid molecules. More particularly, the pulsed energy parameters may be
selected so as to interact resonantly with the pi electron stacks in the beta
amyloid and other biomolecules.
[Para 1 6] A plurality of spaced-apart radiofrequency emitters may be
disposed adjacent to a head of an individual to be treated. The radiofrequency
fields of the spaced-apart radiofrequency emitters preferably do not overlap.
The power level of each emitter may be set so that a specific absorption rate
in
the brain is between 1.0 W/kg and 2.0 W/kg. Each emitter may transmit a
radiofrequency field at 850-950 megahertz every 4 to 5 milliseconds.
[Para 17] The radiofrequency energy source may be applied to the
brain at a
given interval over a given period of time. For example, the radiofrequency
may be applied to the brain for two spaced-apart one-hour treatment periods
each day. This may occur over several days, weeks or even months.
[Para 1 81 Other features and advantages of the present invention
will become
apparent from the following more detailed description, taken in conjunction
with the accompanying drawings, which illustrate, by way of example, the
principles of the invention.
7
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
BRIEF DESCRIPTION OF THE DRAWINGS
[Para 19] The accompanying drawings illustrate the invention. In
such
drawings:
[Para 20] FIGURE 1 is a diagrammatic view illustrating a system
used to
generate a pulsed energy source in the form of a laser light beam, in
accordance with the present invention;
[Para 21] FIGURE 2 is a diagrammatic view of optics used to generate
a laser
light geometric pattern, in accordance with the present invention;
[Para 22] FIGURE 3 is a diagrammatic view illustrating an alternate
embodiment of the system to use to generate laser light beams for treating
tissue and fluid, in accordance with the present invention;
[Para 23] FIGURE 4 is a diagrammatic view illustrating yet another
embodiment of a system used to generate laser light beams to treat tissue in
accordance with the present invention;
[Para 24] FIGURE 5 is a top plan view of an optical scanning
mechanism, used
in accordance with the present invention;
[Para 25] FIGURE 6 is a partially exploded view of the optical
scanning
mechanism of FIG. 5, illustrating various component parts thereof;
[Para 261 FIGURE 7 illustrates controlled offset of exposure of an
exemplary
geometric pattern grid of laser spots to treat a target tissue, in accordance
with
the present invention;
8
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 27] FIGURE 8 is a diagrammatic view illustrating a geometric
object in
the form of a line controllably scanned to treat a target tissue, in
accordance
with the present invention;
[Para 28] FIGURE 9 is a diagrammatic view similar to FIG. 8, but
illustrating
the geometric line or bar rotated to treat an area, in accordance with the
present invention;
[Para 291 FIGURES 10 and 11 are graphs illustrating the average
power of a
laser source compared to a source radius and pulse train duration of the
laser;
[Para 30] FIGURES 12 and 13 are graphs illustrating the time for the
temperature for decay depending upon the laser source radius and wavelength;
[Para 31] FIGURES 14-17 are graphs illustrating peak ampere turns
for
various radiofrequencies, duty cycles and coil radii;
[Para 32] FIGURE 18 is a graph depicting the time for temperature
rise to
decay compared to radiofrequency coil radius;
[Para 33] FIGURES 19 and 20 and graphs depicting the average
microwave
power compared to microwave frequency and pulse train duration;
[Para 34] FIGURE 21 is a graph depicting the time for the
temperature to
decay for various microwave frequencies;
[Para 35] FIGURE 22 is a graph depicting the average ultrasound
source
power compared to frequency and pulse train duration;
[Para 36] FIGURES 23 and 24 are graphs depicting the time for
temperature
decay for various ultrasound frequencies;
9
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 37] FIGURE 25 is a graph depicting the volume of focal heated
region
compared to ultrasound frequency;
[Para 38] FIGURE 26 is a graph comparing equations for temperature
over
pulse durations for an ultrasound energy source;
[Para 39] FIGURES 27 and 28 are graphs illustrating the magnitude of
the
logarithm of damage and HSP activation Arrhenius integrals as a function of
temperature and pulse duration;
[Para 40] FIGURE 29 is a diagrammatic view of a light generating
unit that
produces timed series of pulses, having a light pipe extending therefrom, in
accordance with the present invention;
[Para 41] FIGURE 30 is a cross-sectional view of a photostimulation
delivery
device delivering electromagnetic energy to target tissue, in accordance with
the present invention;
[Para 42] FIGURE 31 is a cross-sectional and diagrammatic view of an
end of
an endoscope inserted into the nasal cavity and treating tissue therein, in
accordance with the present invention;
[Para 43] FIGURE 32 is a diagrammatic and partially cross-sectioned
view of
a bronchoscope extending through the trachea and into the bronchus of a lung
and providing treatment thereto, in accordance with the present invention;
[Para 44] FIGURE 33 is a diagrammatic view of a colonoscope
providing
photostimulation to an intestinal or colon area of the body, in accordance
with
the present invention;
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 45] FIGURE 34 is a diagrammatic view of an endoscope inserted
into a
stomach and providing treatment thereto, in accordance with the present
invention;
[Para 46] FIGURE 35 is a partially sectioned perspective view of a
capsule
endoscope, used in accordance with the present invention;
[Para 47] FIGURE 36 is a diagrammatic view of a pulsed high
intensity
focused ultrasound for treating tissue internal the body, in accordance with
the
present invention;
[Para 48] FIGURE 37 is a diagrammatic view for delivering therapy to
the
bloodstream of a patient, through an earlobe, in accordance with the present
invention;
[Para 49] FIGURE 38 is a cross-sectional view of a stimulating
therapy device
of the present invention used in delivering photostimulation to the blood, via
an
earlobe, in accordance with the present invention;
[Para 50] FIGURE 39 is a diagrammatic and perspective view of a
device for
treating multiple areas or an entire body of an individual, in accordance with
the present invention;
[Para 51] FIGURE 40 is a diagrammatic perspective view of a
plurality of
spaced-apart radiofrequency emitters disposed adjacent to a head of an
individual to be treated; and
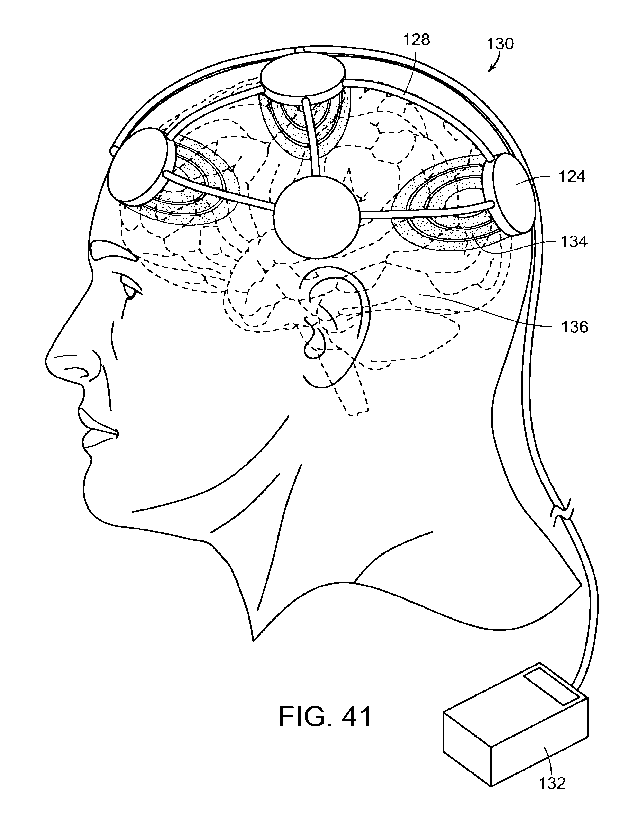
[Para 52] FIGURE 41 is a diagrammatic view illustrating the emitters
emitting
electromagnetic energy into the head and brain of the individual, in
accordance
with the present invention.
11
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
[Para 531 The present invention, as more fully described and
illustrated
herein, resides in processes and systems that provides protective therapy for
biological tissues or fluids having a chronic progressive disease or at a risk
of
having a chronic progressive disease. More particularly, the present invention
is directed to a system and method for preventing or treating Alzheimer's
disease or other neurodegenerative diseases.
[Para 54] In accordance with an embodiment of the invention, a
pulsed
energy source having energy parameters including wavelength or frequency,
duty cycle and pulse train duration selected so as to raise a target tissue or
bodily target fluid temperature up to eleven degrees Celsius for a short
period
of time of seconds or less, while maintaining an average temperature rise of
the
tissue or target fluid over several minutes at or below a predetermined level
so
as not to permanently damage the target tissue or target fluid. The pulsed
energy source is applied to the target tissue or target fluid which is either
determined to have a chronic progressive disease or at a risk of having a
chronic progressive disease. This determination may be made before imaging,
serologic, immunologic, or other abnormalities are detectable and may be done
prophylactically. The determination may be accomplished by ascertaining if the
patient is at risk for the chronic progressive disease. Alternatively, or
additionally, results of an examination or test of the patient may be
abnormal.
A specific test, such as a genetic test, may be conducted to establish that
the
patient has a risk for the chronic progressive disease. In the case of
Alzheimer's
12
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
or other neurodegenerative diseases, MRI or CAT scans of the brain may be
performed, cognitive or memory tests may be administered, inheritance factors
or genetics, such as a genetic test, may be utilized, or any other test which
can
determine that the individual is at a risk of acquiring or has Alzheimer's
disease
or another neurodegenerative disease.
[Para 551 It is believed that a mechanism by which the invention is
able to
therapeutically or prophylactically treat the biological tissue or fluid is by
stimulating heat shock protein activation in a target tissue or target fluid.
Heat
shock proteins (HSPs) are ubiquitous in highly conserved families of enzymes
present in all cells of all creatures. This may account for as much as 40% of
all
proteins present in a given cell. HSPs are active and essential in maintenance
of
normal cell function and homeostasis. HSPs have many critical functions, one
of
which is to protect the cell from lethal injury of any kind and repair
sublethal
injuries.
[Para 561 While chronic inflammation is pathologic and destructive,
acute
inflammation can be reparative. Acute inflammation may occur in response to
an acute injury. Common injuries requiring repair are typically associated
with
cellular and tissue damage, such as a wound or infection. Depending upon the
severity of injury and the functional sensitivity of the tissue, loss of key
functions may result despite wound repair. Incomplete repair or continued or
repeated injury may lead to chronic inflammation, as in CPDs.
[Para 57] The normal state of health of maintained by complex
physiologic
processes of constant surveillance for and repair of defective proteins and
13
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
potential threats, such as bacteria, viruses and neoplasia. These normal
physiologic processes and their actions are ideal as good health and function
is
the result of their normal function. While the normal function of these
physiological processes are ideal, such homeostatic processes themselves are
not always perfectively effective. Potential threats and abnormalities may
either
escape detection or exceed the ability for repair. Failure of surveillance and
response may result from any number of reasons, including disease causing
immunosuppression, evasion of detection by hiding of antigenic stimuli, such
as occurring in certain cancers and retroviruses, and the onset and
progression
below the level of symptoms recognition and activation.
[Para 581 HSPs are a first step in the acute inflammatory process.
Activation
of HSPs by a threat initiates a cascade of subsequent events leading to
improved cell function, reduced chronic inflammation, and reparative
immunomodulation locally and systemically. The effective HSP activations
preserve the life of the cell and normalize cell function, also referred to as
homeotrophy. Sudden and severe yet sublethal (to the cell) stimuli are the
most
potent stimulators of homeotrophic HSP activation. Slowly progressive and
chronic stimuli are not effective activators HSP response. Thus, insidiously
developing and progressing CPDs do not stimulate a reparative response of the
HSP activation. In some CPDs, like diabetes and Alzheimer disease, HSP
function
itself can become abnormal to the point of failure.
[Para 59] Typically, however, HSPs normalize cell function
independent of the
cause of abnormality by identifying and repairing abnormal cell proteins
14
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
without regard to what made them abnormal, thus normalizing cell function.
HSPs have an ability to restore every protein to its correct state or
eliminate the
irreparable, leading to replacement. As the HSP response is physiologic and
thus perfect and without adverse effects, fixing what is broken without regard
to the cause of the breakage, the repair response of HSPs is exactly tailored
to
the disease process. Agnostic to the cause of protein misfolding and
consequent cellular dysfunction, homeotrophic HSP activation is thus a non-
specific trigger of disease-specific repair.
[Para 601 The inventors have discovered that it is possible to
stimulate HSP
activation without cell or tissue damage by electromagnetic radiation-induced
acute, but sublethal, cellular hyperthermy. In the absence of cell death or
tissue
damage, the cascade of physiologic repair and homeotrophy of the acute
inflammatory response can thus be triggered without any adverse treatment
effects. Acute inflammation incited without tissue damage may be thought of as
"as if" acute inflammation. That is to say that homeotrophic cellular
hyperthermy is able to elicit the acute inflammatory response that is entirely
and only beneficial, "as if" it were caused by tissue damage, but in the
absence
of tissue damage. It has been found that the safest and most efficient
stimulus
of homeotrophic HSP activation is by pulsed electromagnetic radiation (PEMR).
Pulsing allows significant increases in the abruptness and severity of the
threat
stimulus without killing the target cell to maximize HSP activation in the
homeotrophic healing response. The various types of PEMR are best suited to
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
different biological applications include light, laser, radio wave and
microwave
and ultrasound.
[Para 611 The eye is the most functionally sensitive organ in the
body. There
are a number of CPDs that effect the retina that share the typical
characteristics
of CPDs in general, and neurodegenerative diseases in particular. Accordingly,
CPDs of the retina may serve as a model for CPDs elsewhere in the body. Over
many years of clinical experience in a large number of patients, it has been
found that PEMR in the form of low-intensity/high-density subthreshold diode
micropulsed laser treatment (SDM) has been shown to effectively treat,
prevent,
slow, reverse or stop the progression of every major chronic progressive
disease of the retina, without regard to the cause. These include age-related,
genetic, metabolic and diseases of unknown etiology of widely varying
genotypes and phenotypes. Despite the thermal sensitivity of the retina, SDM
does this without any known adverse treatment effects due to the selection of
the operating parameters of the PEMR and thus is performed in complete
safety.
[Para 62] With respect to conventional retinal photocoagulation, the
physician must intentionally create retinal damage as a prerequisite to
therapeutically effective treatment. However, the inventors surmised that the
therapeutic alterations in the retinal pigment epithelium (RPE) cytokine
production elicited by conventional photocoagulation comes from cells at the
margins of traditional laser burns which were affected but not killed by the
laser exposure. The inventors created energy parameters which created "true
16
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
subthreshold photocoagulation", which is invisible and includes laser
treatment
non-discernable by any known means such as FFA, FAF, retrograde FAF, or even
SD-OCT and produces absolutely no retinal damage detectible by any means at
the time of treatment or any time thereafter by any known means of detection,
but still yields the benefits of conventional retinal photocoagulation. This
is
discussed in U.S. Publication No. 201 6/03461 26 Al, the contents of which are
hereby incorporated by reference.
[Para 631 Various parameters have been determined to achieve true
subthreshold effective photocoagulation, including providing sufficient power
to produce effective treatment but not too high to create tissue damage or
destruction. It has been found that the intensity or power of a low duty cycle
810 nm laser beam between 100 watts to 590 watts per square centimeter is
effective yet safe. A particularly preferred intensity or power of the laser
light
beam is approximately 250-350 watts per square centimeter for an 810 nm
micropulsed diode laser.
[Para 64] Power limitations in current micropulsed diode lasers
require fairly
long exposure duration, although it is important that the generated heat be
able to dissipate toward the unexposed tissue at the margins of the laser spot
so as not to damage or destroy the cells or tissue. It has been found that a
radiant beam of an 810 nm diode laser should have an exposure envelope of
500 milliseconds or less, and preferably approximately 100-300 milliseconds.
If micropulsed diode lasers become more powerful, the exposure duration can
be lessened accordingly. It has been found that invisible phototherapy or true
17
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
subthreshold photocoagulation in accordance with the present invention can be
performed at various laser light wavelengths, such as from a range of 532 nm
to 1300 nm. Use of a different wavelength can impact the preferred intensity
or
power of the laser light beam and the exposure envelope duration in order that
the retinal tissue is not damaged, yet therapeutic effect is achieved.
Typically,
the laser light pulse is less than a millisecond in duration, and typically
between
50 microseconds to 100 microseconds in duration.
[Para 651 Another parameter of the present invention when utilizing
laser
light is the duty cycle, or the frequency of the train of micropulses or the
length
of the thermal relaxation time in between consecutive pulses. It has been
found
that the use of a 10% duty cycle or higher can increase the risk of lethal
cell
injury in the retina. Thus, duty cycles less than 10%, and preferably
approximately 5% duty cycle or less are used as this parameter has been
demonstrated to provide adequate thermal rise in treatment that remains below
the level expected to produce lethal cell injury. The lower the duty cycle,
the
longer the exposure envelope duration can be. For example, if the duty cycle
is
less than 5%, the exposure envelope duration in some instances can exceed
500 milliseconds.
[Para 661 Thus, the following key parameters have been found in
order to
create harmless, true (sublethal to the retina) subthreshold photocoagulation
in
retinal tissue in accordance with the present invention:
[Para 67] a) light beam having a wavelength of at least 532 nm, and
preferably between 532 nm to 1 300 nm;
18
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 681 b) low duty cycle, such as less than 10% and preferably 5%
or less;
[Para 691 c) a sufficiently small spot size to minimize heat
accumulation and
assure uniform heat distribution within a given laser spot so as to maximize
heat dissipation; and
[Para 70] d) sufficient power to produce retinal laser exposures
between 18-
55 times MPE producing an RPE temperature rise of 7 C - 14 C and retinal
irradiance of between 100-590 W/CM2.
[Para 71] Using these foregoing parameters, harmless yet
therapeutically
effective true subthreshold or invisible photocoagulation phototherapy
treatment can be obtained which can be attained which has been found to
produce benefits of conventional photocoagulation phototherapy but avoid
drawbacks and complications of conventional phototherapy. Adverse treatment
effects are completely eliminated and functional retina preserved rather than
sacrificed. Moreover, the entire retina can be exposed to the pulsed energy
source of the present invention, allowing both preventative and therapeutic
treatment of eyes with retinal disease completely rather than locally or
subtotally.
[Para 72] In the retina, the clinical benefits of SDM are produced
by
photothermal RPE HSP activation sublethal to the RPE. In dysfunctional RPE
cells,
HSP stimulation by SDM results in normalized cytokine expression and
consequently improved retinal structure and function. As normally functioning
cells are not in need of repair, HSP stimulation in normal cells would tend to
have no notable clinical effect. The "patho-selectivity" of near infrared
laser
19
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
effects, such as SDM affecting sick cells but not affecting normal ones on
various cell types, is consistent with clinical observations of SDM. This
facility is
key to the suitability of SDM for early and preventative treatment of eyes
with
chronic progressive disease and eyes with minimal retinal abnormality and
minimal dysfunction. Despite the safety of SDM, the clinical effects of SDM
are
marked and profound. For instance, SDM reduces the rate of progression of
diabetic retinopathy by 85% (P=0.0001) and age-related macular degeneration
by at least 95% (P<0.0001), improves optic nerve function in glaucoma
(P=0.001) and visual fields in glaucoma and all retinal diseases including
retinitis pigmentosa (P<0.0001).
[Para 73]
With reference now to FIG. 1, a schematic diagram is shown of a
system for realizing the process of the present invention. The system,
generally
referred to by the reference number 10, includes a laser console 12, such as
for
example the 810 nm near infrared micropulsed diode laser in the preferred
embodiment. The laser generates a laser light beam which is passed through
optics, such as an optical lens or mask, or a plurality of optical lenses
and/or
masks 14 as needed. The laser projector optics 14 pass the shaped light beam
to a coaxial wide-field non-contact digital optical viewing system/camera 16
for projecting the laser beam light onto the eye 18 of the patient, or other
biological target tissue or bodily fluid as more fully discussed herein. It
will be
understood that the box labeled 16 can represent both the laser beam projector
as well as a viewing system/camera, which might in reality comprise two
different components in use. The viewing system/camera 16 provides feedback
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
to a display monitor 20, which may also include the necessary computerized
hardware, data input and controls, etc. for manipulating the laser 12, the
optics
14, and/or the projection/viewing components 16.
[Para 74] With reference now to FIG. 2, in one embodiment, the laser
light
beam 22 is passed through a collimator lens 24 and then through a mask 26. In
a particularly preferred embodiment, the mask 26 comprises a diffraction
grating. The mask/diffraction grating 26 produces a geometric object, or more
typically a geometric pattern of simultaneously produced multiple laser spots
or
other geometric objects. This is represented by the multiple laser light beams
labeled with reference number 28. Alternatively, the multiple laser spots may
be
generated by a plurality of fiber optic wires. Either method of generating
laser
spots allows for the creation of a very large number of laser spots
simultaneously over a very wide treatment field, such as consisting of the
entire
retina. In fact, a very high number of laser spots, perhaps numbering in the
hundreds even thousands or more could cover the entire ocular fundus and
entire retina, including the macula and fovea, retinal blood vessels and optic
nerve. The intent of the process in the present invention is to better ensure
complete and total coverage and treatment of the target area, which may
comprise a retina, and sparing none of the retina by the laser so as to
improve
vision.
[Para 75] Using optical features with a feature size on par with the
wavelength of the laser employed, for example using a diffraction grating, it
is
possible to take advantage of quantum mechanical effects which permits
21
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
simultaneous application of a very large number of laser spots for a very
large
target area. The individual spots produced by such diffraction gratings are
all of
a similar optical geometry to the input beam, with minimal power variation for
each spot. The result is a plurality of laser spots with adequate irradiance
to
produce harmless yet effective treatment application, simultaneously over a
large target area. The present invention also contemplates the use of other
geometric objects and patterns generated by other diffractive optical
elements.
[Para 76] The laser light passing through the mask 26 diffracts,
producing a
periodic pattern a distance away from the mask 26, shown by the laser beams
labeled 28 in FIG. 2. The single laser beam 22 has thus been formed into
multiple, up to hundreds or even thousands, of individual laser beams 28 so as
to create the desired pattern of spots or other geometric objects. These laser
beams 28 may be passed through additional lenses, collimators, etc. 30 and 32
in order to convey the laser beams and form the desired pattern on the
patient's retina. Such additional lenses, collimators, etc. 30 and 32 can
further
transform and redirect the laser beams 28 as needed.
[Para 77] Arbitrary patterns can be constructed by controlling the
shape,
spacing and pattern of the optical mask 26. The pattern and exposure spots
can be created and modified arbitrarily as desired according to application
requirements by experts in the field of optical engineering. Photolithographic
techniques, especially those developed in the field of semiconductor
manufacturing, can be used to create the simultaneous geometric pattern of
spots or other objects.
22
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 78] Although hundreds or even thousands of simultaneous laser
spots
could be generated and created and formed into patterns to be applied to the
tissue, due to the requirements of not overheating the tissue, there are
constraints on the number of treatment spots or beams which can be
simultaneously used in accordance with the present invention. Each individual
laser beam or spot requires a minimum average power over a train duration to
be effective. However, at the same time, tissue cannot exceed certain
temperature rises without becoming damaged. For example, using an 810 nm
wavelength laser, the number of simultaneous spots generated and used could
number from as few as 1 and up to approximately 100 when a 0.04 (4%) duty
cycle and a total train duration of 0.3 seconds (300 milliseconds) is used.
[Para 79] Absorption by water increases as the wavelength is
increased,
resulting in heating over the long path length through the vitreous humor in
front of the retina. For shorter wavelengths, e.g., 577 nm, the absorption
coefficient in the RPE's melanin can be higher, and therefore the laser power
can be lower. For example, at 577 nm, the power can be lowered by a factor of
4 for the invention to be effective. Accordingly, there can be as few as a
single
laser spot or up to approximately 400 laser spots when using the 577 nm
wavelength laser light, while still not harming or damaging the eye or other
tissue. The present invention can use a multitude of simultaneously generated
therapeutic light beams or spots, such as numbering in the dozens or even
hundreds, as the parameters and methodology of the present invention create
23
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
therapeutically effective yet non-destructive and non-permanently damaging
treatment.
[Para 801 FIG. 3 illustrates diagrammatically a system which couples
multiple
light sources into the pattern-generating optical subassembly described above.
Specifically, this system 10' is similar to the system 10 described in FIG. 1
above. The primary differences between the alternate system 10' and the
earlier
described system 10 is the inclusion of a plurality of laser consoles 12, the
outputs of which are each fed into a fiber coupler 34. The fiber coupler
produces a single output that is passed into the laser projector optics 14 as
described in the earlier system. The coupling of the plurality of laser
consoles
12 into a single optical fiber is achieved with a fiber coupler 34 as is known
in
the art. Other known mechanisms for combining multiple light sources are
available and may be used to replace the fiber coupler described herein.
[Para 81] In this system 10' the multiple light sources 12 follow a
similar
path as described in the earlier system 10, i.e., collimated, diffracted,
recollimated, and directed into the retina with a steering mechanism. In this
alternate system 10' the diffractive element functions differently than
described
earlier depending upon the wavelength of light passing through, which results
in a slightly varying pattern. The variation is linear with the wavelength of
the
light source being diffracted. In general, the difference in the diffraction
angles
is small enough that the different, overlapping patterns may be directed along
the same optical path through the steering mechanism 16 to the retina 18 for
24
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
treatment. The slight difference in the diffraction angles will affect how the
steering pattern achieves coverage of the retina.
[Para 821 Since the resulting pattern will vary slightly for each
wavelength, a
sequential offsetting to achieve complete coverage will be different for each
wavelength. This sequential offsetting can be accomplished in two modes. In
the first mode, all wavelengths of light are applied simultaneously without
identical coverage. An offsetting steering pattern to achieve complete
coverage
for one of the multiple wavelengths is used. Thus, while the light of the
selected wavelength achieves complete coverage of the tissue area to be
treated, the application of the other wavelengths achieves either incomplete
or
overlapping coverage of the tissue. The second mode sequentially applies each
light source of a varying or different wavelength with the proper steering
pattern to achieve complete coverage of the tissue for that particular
wavelength. This mode excludes the possibility of simultaneous treatment
using multiple wavelengths, but allows the optical method to achieve identical
coverage for each wavelength. This avoids either incomplete or overlapping
coverage for any of the optical wavelengths.
[Para 831 These modes may also be mixed and matched. For example,
two
wavelengths may be applied simultaneously with one wavelength achieving
complete coverage and the other achieving incomplete or overlapping coverage,
followed by a third wavelength applied sequentially and achieving complete
coverage.
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 84] FIGURE 4 illustrates diagrammatically yet another
alternate
embodiment of the inventive system 10". This system 10" is configured
generally the same as the system 10 depicted in FIG. 1. The main difference
resides in the inclusion of multiple pattern-generating subassembly channels
tuned to a specific wavelength of the light source. Multiple laser consoles 12
are arranged in parallel with each one leading directly into its own laser
projector optics 14. The laser projector optics of each channel 38a, 38b, 38c
comprise a collimator 24, mask or diffraction grating 28 and recollimators 30,
32 as described in connection with FIG. 2 above--the entire set of optics
tuned
for the specific wavelength generated by the corresponding laser console 12.
The output from each set of optics 14 is then directed to a beam splitter 36
for
combination with the other wavelengths. It is known by those skilled in the
art
that a beam splitter used in reverse can be used to combine multiple beams of
light into a single output.
[Para 851 The combined channel output from the final beam splitter
36c is
then directed through the camera 16 which applies a steering mechanism to
allow for complete coverage of the retina 18.
[Para 861 In this system 10" the optical elements for each channel
are tuned
to produce the exact specified pattern for that channel's wavelength.
Consequently, when all channels are combined and properly aligned a single
steering pattern may be used to achieve complete coverage of the retina for
all
wavelengths.
26
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 87] The system 10" may use as many channels 38a, 38b, 38c,
etc. and
beam splitters 36a, 36b, 36c, etc. as there are wavelengths of light being
used
in the treatment.
[Para 88] Implementation of the system 10" may take advantage of
different
symmetries to reduce the number of alignment constraints. For example, the
proposed grid patterns are periodic in two dimensions and steered in two
dimensions to achieve complete coverage. As a result, if the patterns for each
channel are identical as specified, the actual pattern of each channel would
not
need to be aligned for the same steering pattern to achieve complete coverage
for all wavelengths. Each channel would only need to be aligned optically to
achieve an efficient combination.
[Para 89] In system 10", each channel begins with a light source 12,
which
could be from an optical fiber as in other embodiments of the pattern-
generating subassembly. This light source 12 is directed to the optical
assembly 14 for collimation, diffraction, recollimation and directed into the
beam splitter which combines the channel with the main output.
[Para 90] The field of photobiology reveals that different biologic
effects may
be achieved by exposing target tissues to lasers of different wavelengths. The
same may also be achieved by consecutively applying multiple lasers of either
different or the same wavelength in various sequences with variable time
periods of separation and/or with different irradiant energies. The present
invention anticipates the use of multiple laser, light or radiant wavelengths
(or
modes) applied simultaneously or in sequence to maximize or customize the
27
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
desired treatment effects. This method also minimizes potential detrimental
effects. The optical methods and systems illustrated and described above
provide simultaneous or sequential application of multiple wavelengths.
[Para 91] Typically, the system of the present invention
incorporates a
guidance system to ensure complete and total treatment with photostimulation.
Fixation/tracking/registration systems consisting of a fixation target,
tracking
mechanism, and linked to system operation can be incorporated into the
present invention.
[Para 92] In a particularly preferred embodiment, the geometric
pattern of
simultaneous laser spots is sequentially offset so as to achieve confluent and
complete treatment of the target tissue. This is done in a time-saving manner
by placing a plurality of spots over the target tissue at once. This pattern
of
simultaneous spots is scanned, shifted, or redirected as an entire array
sequentially, so as to cover the entire target tissue in a single treatment
session.
[Para 931 This can be done in a controlled manner using an optical
scanning
mechanism 40. FIGS. 5 and 6 illustrate an optical scanning mechanism 40
which may be used in the form of a MEMS mirror, having a base 42 with
electronically actuated controllers 44 and 46 which serve to tilt and pan the
mirror 48 as electricity is applied and removed thereto. Applying electricity
to
the controller 44 and 46 causes the mirror 48 to move, and thus the
simultaneous pattern of laser spots or other geometric objects reflected
thereon to move accordingly on the target tissue of the patient. This can be
28
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
done, for example, in an automated fashion using an electronic software
program to adjust the optical scanning mechanism 40 until complete coverage
of the target tissue, or at least the portion of the target tissue desired to
be
treated, is exposed to the phototherapy. The optical scanning mechanism may
also be a small beam diameter scanning galvo mirror system, or similar system,
such as that distributed by Thorlabs. Such a system is capable of scanning the
lasers in the desired offsetting pattern.
[Para 94] Since the parameters of the present invention dictate that
the
applied radiant energy or laser light is not destructive or damaging, the
geometric pattern of laser spots, for example, can be overlapped without
destroying the tissue or creating any permanent damage. However, in a
particularly preferred embodiment, as illustrated in FIG. 7, the pattern of
spots
are offset at each exposure so as to create space between the immediately
previous exposure to allow heat dissipation and prevent the possibility of
heat
damage or tissue destruction. Thus, as illustrated in FIG. 7, the pattern,
illustrated for exemplary purposes as a grid of sixteen spots, is offset each
exposure such that the laser spots occupy a different space than previous
exposures. It will be understood that the diagrammatic use of circles or empty
dots as well as filled dots are for diagrammatic purposes only to illustrate
previous and subsequent exposures of the pattern of spots to the area, in
accordance with the present invention. The spacing of the laser spots prevents
overheating and damage to the tissue. It will be understood that this occurs
until the entire target tissue has received phototherapy, or until the desired
29
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
effect is attained. This can be done, for example, by a scanning mechanism,
such as by applying electrostatic torque to a micromachined mirror, as
illustrated in FIGS. 5 and 6. By combining the use of small laser spots
separated
by exposure free areas, prevents heat accumulation, and grids with a large
number of spots per side, it is possible to atraumatically and invisibly treat
large target areas with short exposure durations very rapidly.
[Para 951 By rapidly and sequentially repeating redirection or
offsetting of
the entire simultaneously applied grid array of spots or geometric objects,
complete coverage of the target tissue, such as a human retina, can be
achieved
rapidly without thermal tissue injury. This offsetting can be determined
algorithmically to ensure the fastest treatment time and least risk of damage
due to thermal tissue, depending on laser parameters and desired application.
[Para 96] For example, the following has been modeled using the
Fraunhoffer Approximation. With a mask having a nine by nine square lattice,
with an aperture radius 9 µm, an aperture spacing of 600 µm, using a
890 nm wavelength laser, with a mask-lens separation of 75 mm, and
secondary mask size of 2.5 mm by 2.5 mm, the following parameters will yield
a grid having nineteen spots per side separated by 133 µm with a spot size
radius of 6 µm. The number of exposures "m" required to treat (cover
confluently with small spot applications) given desired area side-length "A",
given output pattern spots per square side "n", separation between spots "R",
spot radius "r" and desired square side length to treat area "A", can be given
by
the following formula:
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
A
in = ¨
floor (-12
2?r)
[Para 97] With the foregoing setup, one can calculate the number of
operations m needed to treat different field areas of exposure. For example, a
3
mm times 3 mm area, which is useful for treatments, would require 98
offsetting operations, requiring a treatment time of approximately thirty
seconds. Another example would be a 3 cm times 3 cm area. For such a large
treatment area, a much larger secondary mask size of 25 mm by 25 mm could
be used, yielding a treatment grid of 190 spots per side separated by 133 pm
with a spot size radius of 6 pm. Since the secondary mask size was increased
by the same factor as the desired treatment area, the number of offsetting
operations of approximately 98, and thus treatment time of approximately
thirty seconds, is constant. Field sizes of 3 mm would, for example, allow
treatment of the entire human macula in a single exposure, useful for
treatment
of common blinding conditions such as diabetic macular edema and age-
related macular degeneration. Performing the entire 98 sequential offsettings
would ensure entire coverage of the macula.
[Para 981 Of course, the number and size of spots produced in a
simultaneous pattern array can be easily and highly varied such that the
number of sequential offsetting operations required to complete treatment can
be easily adjusted depending on the therapeutic requirements of the given
application.
[Para 991 Furthermore, by virtue of the small apertures employed in the
diffraction grating or mask, quantum mechanical behavior may be observed
31
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
which allows for arbitrary distribution of the laser input energy. This would
allow for the generation of any arbitrary geometric shapes or patterns, such
as
a plurality of spots in grid pattern, lines, or any other desired pattern.
Other
methods of generating geometric shapes or patterns, such as using multiple
fiber optical fibers or microlenses, could also be used in the present
invention.
[Para 100] With reference now to FIGS. 8 and 9, instead of a geometric pattern
of small laser spots, the present invention contemplates use of other
geometric
objects or patterns. For example, a single line 50 of laser light, formed
continuously or by means of a series of closely spaced spots, can be created.
An offsetting optical scanning mechanism can be used to sequentially scan the
line over an area, illustrated by the downward arrow in FIG. 8. With reference
now to FIG. 9, the same geometric object of a line 50 can be rotated, as
illustrated by the arrows, so as to create a circular field of phototherapy.
The
potential negative of this approach, however, is that the central area will be
repeatedly exposed, and could reach unacceptable temperatures. This could be
overcome, however, by increasing the time between exposures, or creating a
gap in the line such that the central area is not exposed.
[Para 101] Power limitations in current micropulsed diode lasers require
fairly
long exposure duration. The longer the exposure, the more important the
center-spot heat dissipating ability toward the unexposed tissue at the
margins
of the laser spot. Thus, the micropulsed laser light beam of an 810 nm diode
laser should have an exposure envelope duration of SOO milliseconds or less,
and preferably approximately 300 milliseconds. Of course, if micropulsed diode
32
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
lasers become more powerful, the exposure duration should be lessened
accordingly.
[Para 102] Aside from power limitations, another parameter of the present
invention is the duty cycle, or the frequency of the train of micropulses, or
the
length of the thermal relaxation time between consecutive pulses. It has been
found that the use of a 10% duty cycle or higher adjusted to deliver
micropulsed
laser at similar irradiance at similar MPE levels significantly increase the
risk of
lethal cell injury. However, duty cycles of less than 10%, and preferably 5%
or
less demonstrate adequate thermal rise and treatment at the level of the MPE
cell to stimulate a biological response, but remain below the level expected
to
produce lethal cell injury. The lower the duty cycle, however, the exposure
envelope duration increases, and in some instances can exceed 500
milliseconds.
[Para 103] Each micropulse lasts a fraction of a millisecond, typically
between
50 microseconds to 100 microseconds in duration. Thus, for the exposure
envelope duration of 300-500 milliseconds, and at a duty cycle of less than
5%,
there is a significant amount of time between micropulses to allow the thermal
relaxation time between consecutive pulses. Typically, a delay of between 1
and
3 milliseconds, and preferably approximately 2 milliseconds, of thermal
relaxation time is needed between consecutive pulses. For adequate treatment,
the cells are typically exposed or hit by the laser light between 50-200
times,
and preferably between 75-150 at each location. With the 1-3 milliseconds of
relaxation or interval time, the total time in accordance with the embodiments
33
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
described above to treat a given area, or more particularly the locations of
the
target tissue which are being exposed to the laser spots is between 200
milliseconds and 500 milliseconds on average. The thermal relaxation time is
required so as not to overheat the cells within that location or spot and so
as to
prevent the cells from being damaged or destroyed.
[Para 104] The inventors have found that treatment in accordance with the
invention of patients suffering from age-related macular degeneration (AMD)
can slow the progress or even stop the progression of AMD. Further evidence of
this restorative treatment effect is the inventor's finding that treatment can
uniquely reduce the risk of vision loss in AMD due to choroidal
neovascularization by as much as 90%. Most of the patients have seen
significant improvement in dynamic functional mesopic logMAR visual acuity
and contrast visual acuity after the treatment in accordance with the
invention,
with some experiencing better vision. It is believed that this works by
targeting,
preserving, and "normalizing" (moving toward normal) function of the retinal
pigment epithelium (RPE).
[Para 105] Treatment in accordance with the invention has also been shown to
stop or reverse the manifestations of the diabetic retinopathy disease state
without treatment-associated damage or adverse effects, despite the
persistence of systemic diabetes mellitus. Studies by the inventor have shown
that the restorative effect of treatment can uniquely reduce the risk of
progression of diabetic retinopathy by 85%. On this basis it is hypothesized
that
the invention might work by inducing a return to more normal cell function and
34
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
cytokine expression in diabetes-affected RPE cells, analogous to hitting the
"reset" button of an electronic device to restore the factory default
settings.
[Para 106] Based on the above information and studies, SDM treatment may
directly affect cytokine expression and heat shock protein (HSP) activation in
the targeted tissue, particularly the retinal pigment epithelium (RPE) layer.
Panretinal and panmacular SDM has been noted by the inventors to reduce the
rate of progression of many retinal diseases, including severe non-
proliferative
and proliferative diabetic retinopathy, AMD, DME, etc. The known therapeutic
treatment benefits of individuals having these retinal diseases, coupled with
the
absence of known adverse treatment effects, allows for consideration of early
and preventative treatment, liberal application and retreatment as necessary.
The reset theory also suggests that the invention may have application to many
different types of RPE-mediated retinal disorders. In fact, the inventor has
recently shown that panmacular treatment can significantly improve retinal
function and health, retinal sensitivity, and dynamic logMAR visual acuity and
contrast visual acuity in dry age-related macular degeneration, retinitis
pigmentosa, cone-rod retinal degenerations, and Stargardt's disease where no
other treatment has previously been found to do so.
[Para 107] Currently, retinal imaging and visual acuity testing guide
management of chronic, progressive retinal diseases. As tissue and/or organ
structural damage and vision loss are late disease manifestations, treatment
instituted at this point must be intensive, often prolonged and expensive, and
frequently fails to improve visual acuity and rarely restores normal vision.
As
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
the invention has been shown to be an effective treatment for a number of
retinal disorders without adverse treatment effects, and by virtue of its
safety
and effectiveness, it can also be used to treat an eye to stop or delay the
onset
or symptoms of retinal diseases prophylactically or as a preventative
treatment
for such retinal diseases. Any treatment that improves retinal function, and
thus
health, should also reduce disease severity, progression, untoward events and
visual loss. By beginning treatment early, prior to pathologic structural
change,
and maintaining the treatment benefit by regular functionally-guided re-
treatment, structural degeneration and visual loss might thus be delayed if
not
prevented. Even modest early reductions in the rate of disease progression may
lead to significant long-term reductions and complications in visual loss. By
mitigating the consequences of the primary defect, the course of disease may
be muted, progression slowed, and complications and visual loss reduced. This
is reflected in the inventor's studies, finding that treatment reduces the
risk of
progression and visual loss in diabetic retinopathy by 85% and AMD by 80%.
[Para 1 081 In accordance with an embodiment of the present invention, it is
determined that a patient, such as an eye of the patient, has a risk for a
disease. This may be before imaging abnormalities are detectable. Such a
determination may be accomplished by ascertaining if the patient is at risk
for a
chronic progressive disease, such as retinopathy, including diabetes, a risk
for
age-related macular degeneration or retinitis pigmentosa. Alternatively, or
additionally, results of an examination or test of the patient may be
abnormal.
36
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
A specific test, such as a physiology test or a genetic test, may be conducted
to
establish that the patient has a risk for a disease.
[Para 1 091 When treating or prophylactically protecting retinal or other eye
tissue having a chronic progressive disease or a risk of a chronic progressive
disease, a laser light beam, that is sublethal and creates true subthreshold
photocoagulation and retinal tissue, is generated and at least a portion of
the
retinal tissue is exposed to the generated laser light beam without damaging
the exposed retinal or foveal tissue, so as to provide preventative and
protective treatment of the retinal tissue of the eye. The treated retina may
comprise the fovea, foveola, retinal pigment epithelium (RPE), choroid,
choroidal neovascular membrane, subretinal fluid, macula, macular edema,
parafovea, and/or perifovea. The laser light beam may be exposed to only a
portion of the retina, or substantially the entire retina and fovea.
[Para 11 0] While many treatment effects appear to be long-lasting, if not
permanent, clinical observations and laboratory studies suggest that others
can
wear off. Accordingly, the tissue is periodically retreated to maintain
maximum
effects and treatment benefits. This may be done according to a set schedule
or
when it is determined that the tissue of the patient is to be retreated, such
as
by periodically monitoring visual and/or retinal function or condition of the
patient.
[Para 1 1 1 1 Although the present invention is particularly suited for
treatment
of retinal diseases, such as diabetic retinopathy and macular edema, it has
been
found that it can be used for other diseases as well. The system and process
of
37
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
the present invention could target the trabecular mesh work as treatment for
glaucoma, accomplished by another customized treatment field template.
Moreover, treatment of retinal tissue using SDM, as explained above, in eyes
with advanced open-angle glaucoma have shown improved key measures of
optic nerve and ganglion cell function, indicating a significant
neuroprotective
effect of this treatment. Visual fields also improved, and there was no
adverse
treatment effects. Thus, it is believed that SDM, in accordance with the
present
invention, may aid in the clinical management of glaucoma by reducing the risk
of visual loss, independent of intraocular pressure (10P) lowering.
[Para 11 2] Low-intensity/high density subthreshold (sublethal) diode
micropulsed laser (SDM), as explained in detail above, has been shown to be
effective in the treatment of traditional retinal laser indications such as
diabetic
macular edema, proliferative diabetic retinopathy, central serious
chorioretinopathy, and branch retinal vein occlusion, without adverse
treatment
effects. As described above, the mechanism of the retinal laser treatment is
sometimes referred to herein as "reset to default" theory, which postulates
that
the primary mode of retinal laser action is sublethal activation of the
retinal
pigment epithelial (RPE) heat shock proteins.
[Para 11 3] A study recently conducted by the inventors also shows that SDM
should be neuroprotective in open-angle glaucoma. Linear regression analysis
demonstrated that the most abnormal values prior to treatment improved the
most following treatment for nearly all measures. Panmacular SDM treatment,
in accordance with the present invention, in eyes with advanced open-angle
38
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
glaucoma (OAG) improved key measures of optic nerve and ganglion cell
function, indicating a significant neuroprotective effective treatment. The
visual
fields also improved, and there were no adverse treatment effects. Thus,
generating a micropulsed laser light beam having characteristics and
parameters discussed above and applying the laser light beam to the retinal
and/or foveal tissue of an eye having glaucoma or a risk of glaucoma creates a
therapeutic effect to the retinal and/or foveal tissue exposed to the laser
light
beam without destroying or permanently damaging the retinal and/or foveal
tissue and also improves function or condition of an optic nerve and/or
retinal
ganglion cells of the eye.
[Para 114] Retinal ganglion cells and the optic nerve are subject to the
health
and function of the retinal pigment epithelium (RPE). Retinal homeostasis is
principally maintained by the RPE via still the poorly understood but
exquisitely
complex interplay of small proteins excreted by the RPE into the intercellular
space called "cytokines". Some RPE-derived cytokines, like pigment epithelial
derived factor (PEDF) are neuroprotective. Retinal laser treatment may alter
RPE
cytokine expression, including, but not limited to, increasing expression of
PEDF. Absent retinal damage, the effect of SDM, in accordance with the present
invention, is "homeotrophic", moving retinal function toward normal. By
normalizing RPE function, it follows that retinal autoregulation and cytokine
expression is also normalized. This suggests the normalization of retinal
cytokine expression may be the source of the neuroprotective effects from SDM
in OAG.
39
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 11 5] Despite the markedly beneficial effects of SDM in chronic
progressive retinal diseases, there are no other treatments for most of these
diseases that have any benefit at all. In this respect, retinal CPDs are also
like
CPDs elsewhere. In all CPDs including type ll diabetes, Alzheimer disease,
idiopathic pulmonary fibrosis (IPF) and ischemic heart disease and various
cardiomyopathies, abnormalities of the HSP system has been recognized.
Currently, outside of the present invention, there is no non-physical therapy
to
stimulate HSP homeotrophic effects in systemic CPDs. Experience with SDM in
connection with eye diseases suggests that appropriately designed PEMR should
effectively and safely treat any CPDs affecting any other part of the body.
Moreover, experience with SDM in otherwise untreatable retinal diseases
suggests that the beneficial effects of PEMR elsewhere should be major and not
minor, robust, significant and safe. As with SDM, the effect of PEMR for CPDs
elsewhere in the body would most likely not cure the primary cause of disease
(age, diabetes, genetic defect, etc.), but instead the effect would be to
slow,
stop or reverse the disease process by repair of the abnormalities that
develop
as a consequence of the primary disease defect. By maintenance of the
treatment benefits via periodic retreatment, the course of the disease process
should be attenuated, reducing the risks of death and disability.
[Para 1161 As heat shock proteins play a role in responding to a large number
of abnormal conditions in body tissue other than eye tissue, it is believed
that
similar systems and methodologies can be advantageously used in treating
such abnormal conditions, infections, etc, outside the eye. As such, the
present
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
invention is also directed to the controlled application of pulsed ultrasound
or
electromagnetic radiation to treat abnormal conditions including
inflammations, autoimmune conditions, and cancers that are accessible by
means of fiber optics of endoscopes or surface probes as well as focused
electromagnetic/sound waves. For example, cancers on the surface of the
prostate that have the largest threat of metastasizing can be accessed by
means of fiber optics in a proctoscope. Colon tumors can be accessed by an
optical fiber system, like those used in colonoscopy.
[Para 11 7] As indicated above, subthreshold diode micropulsed laser (SDM)
photostimulation has been effective in stimulating direct repair of slightly
misfolded proteins in eye tissue. Besides HSP activation, another way this may
occur is because the spikes in temperature caused by the micropulses in the
form of a thermal time-course allows diffusion of water inside proteins, and
this allows breakage of the peptide-peptide hydrogen bonds that prevent the
protein from returning to its native state. The diffusion of water into
proteins
results in an increase in the number of restraining hydrogen bonds by a factor
on the order of a thousand. Thus, it is believed that this process could be
applied to other diseases advantageously as well.
[Para 11 81 Laser treatment can induce HSP production or activation and alter
cytokine expression. The more sudden and severe the non-lethal cellular stress
(such as laser irradiation), the more rapid and robust HSP activation. Thus, a
burst of repetitive low temperature thermal spikes at a very steep rate of
change (¨ 7 C elevation with each 100ps micropulse, or 70,000 C/sec)
41
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
produced by each SDM exposure is especially effective in stimulating
activation
of HSPs, particularly compared to non-lethal exposure to subthreshold
treatment with continuous wave lasers, which can duplicate only the low
average tissue temperature rise.
[Para 119] In accordance with the system and method of the present
invention, a pulsed energy source, such as laser, ultrasound, ultraviolet,
radiofrequency, microwave radiofrequency and the like, having energy
parameters selected to cause a thermal time-course in tissue or bodily fluid
to
raise the target tissue or bodily fluid temperature over a short period of
time to
a sufficient level to achieve a therapeutic effect while maintaining average
tissue temperature over a prolonged period of time below a predetermined level
so as to avoid permanent tissue damage. It is believed that the creation of
the
thermal time-course stimulates heat shock protein activation or production and
facilitates protein repair without causing any cellular damage. The parameters
of the pulsed energy source and its application to the target tissue or target
bodily fluid is important in creating the thermal time-course so as to have a
therapeutic effect without causing damage.
[Para 120] The selection of these parameters may be determined by requiring
that the Arrhenius integral for HSP activation be greater than 1 or unity.
Arrhenius integrals are used for analyzing the impacts of actions on
biological
tissue. See, for instance, The CRC Handbook of Thermal Engineering, ed. Frank
Kreith, Springer Science and Business Media (2000). At the same time, the
selected parameters must not permanently damage the tissue. Thus, the
42
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
Arrhenius integral for damage may also be used, wherein the solved Arrhenius
integral is less than 1 or unity.
[Para 1211 Alternatively, the FDA/FCC constraints on energy deposition per
unit gram of tissue and temperature rise as measured over periods of minutes
be satisfied so as to avoid permanent tissue damage. The FDA/FCC
requirements on energy deposition and temperature rise are widely used and
can be referenced, for example, at
www.fda.gov/medicaldevices/deviceregulationandguidance/guidancedocument
s/ucm073817.htm#attacha for electromagnetic sources, and Anastosio and P.
LaRivero, ed., Emerging Imaging Technologies. CRC Press (2012), for
ultrasound sources.
[Para 122] Generally speaking, tissue temperature rises of between 6 C and
11 C for a short period of time, such as seconds or fractions of a second, can
create therapeutic effect, such as by activating heat shock proteins, whereas
maintaining the average tissue temperature over a prolonged period of time,
such as over several minutes, such as six minutes, below a predetermined
temperature, such as 6 C and even 1 C or less in certain circumstances, will
not
permanently damage the tissue.
[Para 123] As explained above, the energy source to be applied to the target
tissue will have energy and operating parameters which must be determined
and selected so as to achieve the therapeutic effect while not permanently
damaging the tissue. Using a light beam energy source, such as a laser light
beam, as an example, the laser wavelength, duty cycle and total pulse train
43
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
duration parameters must be taken into account. Other parameters which can
be considered include the radius of the laser source as well as the average
laser
power. Adjusting or selecting one of these parameters can have an effect on at
least one other parameter.
[Para 124] FIGURES 10 and 11 illustrate graphs showing the average power in
watts as compared to the laser source radius (between 0.1 cm and 0.4 cm) and
pulse train duration (between 0.1 and 0.6 seconds). FIG. 10 shows a wavelength
of 880 nm, whereas FIG. 11 has a wavelength of 1000 nm. It can be seen in
these figures that the required power decreases monotonically as the radius of
the source decreases, as the total train duration increases, and as the
wavelength decreases. The preferred parameters for the radius of the laser
source is 1 mm-4 mm. For a wavelength of 880 nm, the minimum value of
power is 0.55 watts, with a radius of the laser source being 1 mm, and the
total
pulse train duration being 600 milliseconds. The maximum value of power for
the 880 nm wavelength is 52.6 watts when the laser source radius is 4 mm and
the total pulse drain duration is 100 milliseconds. However, when selecting a
laser having a wavelength of 1000 nm, the minimum power value is 0.77 watts
with a laser source radius of 1 mm and a total pulse train duration of 600
milliseconds, and a maximum power value of 73.6 watts when the laser source
radius is 4 mm and the total pulse duration is 100 milliseconds. The
corresponding peak powers, during an individual pulse, are obtained from the
average powers by dividing by the duty cycle.
44
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 125] The volume of the tissue region to be heated is determined by the
wavelength, the absorption length in the relevant tissue, and by the beam
width. The total pulse duration and the average laser power determine the
total
energy delivered to heat up the tissue, and the duty cycle of the pulse train
gives the associated spike, or peak, power associated with the average laser
power. Preferably, the pulsed energy source energy parameters are selected so
that approximately 20 to 40 joules of energy is absorbed by each cubic
centimeter of the target tissue.
[Para 126] The absorption length is very small in the thin melanin layer in
the
retinal pigmented epithelium. In other parts of the body, the absorption
length
is not generally that small. In wavelengths ranging from 400 nm to 2000 nm,
the penetration depth and skin is in the range of 0.5 mm to 3.5 mm. The
penetration depth into human mucous tissues in the range of 0.5 mm to 6.8
mm. Accordingly, the heated volume will be limited to the exterior or interior
surface where the radiation source is placed, with a depth equal to the
penetration depth, and a transverse dimension equal to the transverse
dimension of the radiation source. Since the light beam energy source is used
to treat diseased tissues near external surfaces or near internal accessible
surfaces, a source radii of between 1 mm to 4 mm and operating a wavelength
of 880 nm yields a penetration depth of approximately 2.5 mm and a
wavelength of 1000 nm yields a penetration depth of approximately 3.5 mm.
[Para 127] It has been determined that the target tissue can be heated to up
to
approximately 11 C for a short period of time, such as less than one second,
to
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
create the therapeutic effect of the invention while maintaining the target
tissue
average temperature to a lower temperature range, such as less than 6 C or
even 1 C or less over a prolonged period of time, such as several minutes. The
selection of the duty cycle and the total pulse train duration provide time
intervals in which the heat can dissipate. A duty cycle of less than 10%, and
preferably between 2.5% and 5%, with a total pulse duration of between 100
milliseconds and 600 milliseconds has been found to be effective. FIGS. 12 and
13 illustrate the time to decay from 10 C to 1 C for a laser source having a
radius of between 0.1 cm and 0.4 cm with the wavelength being 880 nm in FIG.
12 and 1000 nm in FIG. 13. It can be seen that the time to decay is less when
using a wavelength of 880 nm, but either wavelength falls within the
acceptable
requirements and operating parameters to achieve the benefits of the present
invention while not causing permanent tissue damage.
[Para 1 28] It has been found that the average temperature rise of the desired
target region increasing at least 6 C and up to 11 C, and preferably
approximately 10 C, during the total irradiation period results in HSP
activation.
The control of the target tissue temperature is determined by choosing source
and target parameters such that the Arrhenius integral for HSP activation is
larger than 1, while at the same time assuring compliance with the
conservative
FDA/FCC requirements for avoiding damage or a damage Arrhenius integral
being less than 1.
[Para 129] In order to meet the conservative FDA/FCC constraints to avoid
permanent tissue damage, for light beams, and other electromagnetic radiation
46
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
sources, the average temperature rise of the target tissue over any six-minute
period is 1 C or less. FIGS. 12 and 13 above illustrate the typical decay
times
required for the temperature in the heated target region to decrease by
thermal
diffusion from a temperature rise of approximately 10 C to 1 C as can be seen
in FIG. 12 when the wavelength is 880 nm and the source diameter is 1
millimeter, the temperature decay time is 16 seconds. The temperature decay
time is 107 seconds when the source diameter is 4 mm. As shown in FIG. 13,
when the wavelength is 1000 nm, the temperature decay time is 18 seconds
when the source diameter is 1 mm and 136 seconds when the source diameter
is 4 mm. This is well within the time of the average temperature rise being
maintained over the course of several minutes, such as 6 minutes or less.
While
the target tissue's temperature is raised, such as to approximately 10 C, very
quickly, such as in a fraction of a second during the application of the
energy
source to the tissue, the relatively low duty cycle provides relatively long
periods of time between the pulses of energy applied to the tissue and the
relatively short pulse train duration ensure sufficient temperature diffusion
and
decay within a relatively short period of time comprising several minutes,
such
as 6 minutes or less, that there is no permanent tissue damage.
[Para 1 301 The parameters differ for the individual energy sources, including
microwave, infrared lasers, radiofrequency and ultrasound, because the
absorption properties of tissues differ for these different types of energy
sources. The tissue water content can vary from one tissue type to another,
however, there is an observed uniformity of the properties of tissues at
normal
47
CA 03187662 2023- 1-30
WO 2022/031362 PCT/US2021/036128
or near normal conditions which has allowed publication of tissue parameters
that are widely used by clinicians in designing treatments. Below are tables
illustrating the properties of electromagnetic waves in biological media, with
Table 1 relating to muscle, skin and tissues with high water content, and
Table
2 relating to fat, bone and tissues with low water content.
[Para 131] Table 1. Properties of Electromagnetic Waves in Biological Media:
Muscle, Skin, and Tissues with High Water Content
Reflection Coefficient
Wavelength Dielectric Conductivity Wavelength Depth of
Air-Muscle Interface Muscle-Fat Interface
Frequency in Air Constant o-H _________________________________
XH Penetration
(MHz) (cm) EH (mho/m) (cm) (cm) r 0 r 0
1 30000 2000 0.400 436 91.3 0.982 +179
3000 160 0.625 118 21.6 0.956 +178
27.12 1106 113 0.612 68.1 14.3 0.925 i
177 0.651 -11.13
40.68 738 97.3 0.693 51.3 11.2 0.913 i
176 0.652 -10.21
100 300 71.7 0.889 27 6.66 0.881 +175
0.650 -7.96
200 150 56.5 1.28 16.6 4.79 0.844 +175
0.612 -8.06
300 100 54 1.37 11.9 3.89 0.825 +175
0.592 -8.14
433 69.3 53 1.43 8.76 3.57 0.803 +175
0.562 -7.06
750 40 52 1.54 5.34 3.18 0.779 +176
0.532 -5.69
915 32.8 51 1.60 4.46 3.04 0.772 +177
0.519 -4.32
1500 20 49 1.77 2.81 2.42 0.761 +177
0.506 -3.66
2450 12.2 47 2.21 1.76 1.70 0.754 +177
0.500 -3.88
3000 10 46 2.26 1.45 1.61 0.751 +178
0.495 -3.20
5000 6 44 3.92 0,89 0.788 0.749 +177
0.502 -4.95
5800 5.1 7 43.3 4.73 0.775 0.720 0.746 +177
0.502 -4.29
8000 3,75 40 7.65 0.578 0.413 0.744 +176
0.513 -6.65
10000 3 39.9 10.3 0.464 0.343 0.743 +176
0.518 -5.95
[Para 1 32] Table 2. Properties of Electromagnetic Waves in Biological Media:
Fat, Bone, and Tissues with Low Water Content
Reflection Coefficient
Depth of
Wavelength Dielectric
Conductivity Wavelength - renetration Air-Fat Interface Fat-Muscle Interface
Frequency in Air Constant o-L, XL
(MHz) (cm) EL (mmho/m) (cm (cm) r 0 r 0
1 30000
10 3000
27.12 1106 20 10.9-43.2 241 159 0.660 +174
0.651 +169
40.68 738 14.6 12.6-52.8 187 118 0.617 i 1 7 3
0.652 i 1 70
100 300 7.45 19.1-75.9 106 60.4 0.511 +168
0.650 +172
200 150 5.95 25.8-94.2 59.7 39.2 0.458 +168
0.612 +172
300 100 5.7 31.6-107 41 32.1 0.438
+169 0.592 +172
433 69.3 5.6 37.9-118 28.8 26.2 0.427
+170 0.562 +173
750 40 5.6 49.8-138 16.8 23 0.415 +173
0.532 +174
915 32.8 5.6 55.6-147 13.7 17.7 0.417
+173 0.519 +176
1500 20 5.6 70.8-171 8.41 13.9 0.412 +174
0.506 +176
2450 12.2 5.5 96.4-213 5.21 11.2 0.406
+1 76 0.500 +1 76
3000 10 5.5 110-234 4.25 9.74 0.406
+176 0.495 +177
5000 6 5.5 162-309 2.63 6.67 0.393 +176
0.502 +1 75
5900 5.17 5.05 186-338 2.29 5.24 0.388
+176 0.502 +176
8000 3.75 4.7 255-431 1.73 4.61 0.371 +176
0.513 +173 -
10000 3 4.5 324-549 1.41 3.39 0.363 +175
0.518 +174,-
48
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 133] The absorption lengths of radiofrequency in body tissue are long
compared to body dimensions. Consequently, the heated region is determined
by the dimensions of the coil that is the source of the radiofrequency energy
rather than by absorption lengths. Long distances r from a coil the magnetic
(near) field from a coil drops off as 1/r3. At smaller distances, the electric
and
magnetic fields can be expressed in terms of the vector magnetic potential,
which in turn can be expressed in closed form in terms of elliptic integrals
of
the first and second kind. The heating occurs only in a region that is
comparable in size to the dimensions of the coil source itself. Accordingly,
if it
is desired to preferentially heat a region characterized by a radius, the
source
coil will be chosen to have a similar radius. The heating drops off very
rapidly
outside of a hemispherical region of radius because of the 1/r3 drop off of
the
magnetic field. Since it is proposed to use the radiofrequency the diseased
tissue accessible only externally or from inner cavities, it is reasonable to
consider a coil radii of between approximately 2 to 6 mm.
[Para 134] The radius of the source coil(s) as well as the number of ampere
turns (NI) in the source coils give the magnitude and spatial extent of the
magnetic field, and the radiofrequency is a factor that relates the magnitude
of
the electric field to the magnitude of the magnetic field. The heating is
proportional to the product of the conductivity and the square of the electric
field. For target tissues of interest that are near external or internal
surfaces,
the conductivity is that of skin and mucous tissue. The duty cycle of the
pulse
49
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
train as well as the total train duration of a pulse train are factors which
affect
how much total energy is delivered to the tissue.
[Para 1 351 Preferred parameters for a radiofrequency energy source have been
determined to be a coil radii between 2 and 6 mm, radiofrequencies in the
range of 3-6 MHz, total pulse train durations of 0.2 to 0.4 seconds, and a
duty
cycle of between 2.5% and 5%. FIGS. 14-17 show how the number of ampere
turns varies as these parameters are varied in order to give a temperature
rise
that produces an Arrhenius integral of approximately one or unity for HSP
activation. With reference to FIG. 14, for an RF frequency of 6 MHz, a pulse
train
duration of between 0.2 and 0.4 seconds, a coil radius between 0.2 and 0.6 cm,
and a duty cycle of 5%, the peak ampere turns (NI) is 13 at the 0.6 cm coil
radius and 20 at the 0.2 cm coil radius. For a 3 MHz frequency, as illustrated
in
FIG. 15, the peak ampere turns is 26 when the pulse train duration is 0.4
seconds and the coil radius is 0.6 cm and the duty cycle is 5%. However, with
the same 5% duty cycle, the peak ampere turns is 40 when the coil radius is
0.2
cm and the pulse train duration is 0.2 seconds. A duty cycle of 2.5% is used
in
FIGS. 16 and 17. This yields, as illustrated in FIG. 16, 18 amp turns for a 6
MHz
radiofrequency having a coil radius of 0.6 cm and a pulse train duration of
0.4
seconds, and 29 amp turns when the coil radius is only 0.2 cm and the pulse
train duration is 0.2 seconds. With reference to FIG. 17, with a duty cycle of
2.5% and a radiofrequency of 3 MHz, the peak ampere turns is 36 when the
pulse train duration is 0.4 seconds and the coil radius is 0.6 cm, and 57 amp
turns when the pulse train duration is 0.2 seconds and the coil radius is 0.2
cm.
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 136] The time, in seconds, for the temperature rise to decay from
approximately 10 C to approximately 1 C for coil radii between 0.2 cm and 0.6
cm is illustrated for a radiofrequency energy source in FIG. 18. The
temperature
decay time is approximately 37 seconds when the radiofrequency coil radius is
0.2 cm, and approximately 233 seconds when the radiofrequency coil radius is
0.5 cm. When the radiofrequency coil radius is 0.6 cm, the decay time is
approximately 336 seconds, which is still within the acceptable range of decay
time, but at an upper range thereof.
[Para 1 37] Microwaves are another electromagnetic energy source which can
be utilized in accordance with the present invention. The frequency of the
microwave determines the tissue penetration distance. The gain of a conical
microwave horn is large compared to the microwave wavelength, indicating
under those circumstances that the energy is radiated mostly in a narrow
forward load. Typically, a microwave source used in accordance with the
present invention has a linear dimension on the order of a centimeter or less,
thus the source is smaller than the wavelength, in which case the microwave
source can be approximated as a dipole antenna. Such small microwave sources
are easier to insert into internal body cavities and can also be used to
radiate
external surfaces. In that case, the heated region can be approximated by a
hemisphere with a radius equal to the absorption length of the microwave in
the body tissue being treated. As the microwaves are used to treat tissue near
external surfaces or surfaces accessible from internal cavities, frequencies
in
51
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
the 10-20 GHz range are used, wherein the corresponding penetration
distances are only between approximately 2 and 4 mm.
[Para 138] The temperature rise of the tissue using a microwave energy source
is determined by the average power of the microwave and the total pulse train
duration. The duty cycle of the pulse train determines the peak power in a
single pulse in a train of pulses. As the radius of the source is taken to be
less
than approximately 1 centimeter, and frequencies between 10 and 20 GHz are
typically used, a resulting pulse train duration of 0.2 and 0.6 seconds is
preferred.
[Para 139] The required power decreases monotonically as the train duration
increases and as the microwave frequency increases. For a frequency of 10
GHz, the average power is 18 watts when the pulse train duration is 0.6
seconds, and 52 watts when the pulse train duration is 0.2 seconds. For a 20
GHz microwave frequency, an average power of 8 watts is used when the pulse
train is 0.6 seconds, and can be 26 watts when the pulse train duration is
only
0.2 seconds. The corresponding peak power are obtained from the average
power simply by dividing by the duty cycle.
[Para 140] With reference now to FIG. 1 9, a graph depicts the average
microwave power in watts of a microwave having a frequency of 10 GHz and a
pulse train duration from between 0.2 seconds and 0.6 seconds. FIG. 20 is a
similar graph, but showing the average microwave power for a microwave
having a frequency of 20 GHz. Thus, it will be seen that the average microwave
source power varies as the total train duration and microwave frequency vary.
52
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
The governing condition, however, is that the Arrhenius integral for HSP
activation in the heated region is approximately 1.
[Para 141] With reference to FIG. 21, a graph illustrates the time, in
seconds,
for the temperature to decay from approximately 10 C to 1 C compared to
microwave frequencies between 58 MHz and 20000 MHz. The minimum and
maximum temperature decay for the preferred range of microwave frequencies
are 8 seconds when the microwave frequency is 20 GHz, and 16 seconds when
the microwave frequency is 10 GHz.
[Para 142] Utilizing ultrasound as an energy source enables heating of surface
tissue, and tissues of varying depths in the body, including rather deep
tissue.
The absorption length of ultrasound in the body is rather long, as evidenced
by
its widespread use for imaging. Accordingly, ultrasound can be focused on
target regions deep within the body, with the heating of a focused ultrasound
beam concentrated mainly in the approximately cylindrical focal region of the
beam. The heated region has a volume determined by the focal waist of the airy
disc and the length of the focal waist region, that is the confocal parameter.
Multiple beams from sources at different angles can also be used, the heating
occurring at the overlapping focal regions.
[Para 143] For ultrasound, the relevant parameters for determining tissue
temperature are frequency of the ultrasound, total train duration, and
transducer power when the focal length and diameter of the ultrasound
transducer is given. The frequency, focal length, and diameter determine the
volume of the focal region where the ultrasound energy is concentrated. It is
53
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
the focal volume that comprises the target volume of tissue for treatment.
Transducers having a diameter of approximately 5 cm and having a focal length
of approximately 10 cm are readily available. Favorable focal dimensions are
achieved when the ultrasound frequency is between 1 and 5 MHz, and the total
train duration is 0.1 to 0.5 seconds. For example, for a focal length of 10 cm
and the transducer diameter of 5 cm, the focal volumes are 0.02 cc at 5 MHz
and 2.36 cc at 1 MHz.
[Para 144] With reference now to FIG. 22, a graph illustrates the average
source power in watts compared to the frequency (between 1 MHz and 5 MHz),
and the pulse train duration (between 0.1 and 0.5 seconds). A transducer focal
length of 10 cm and a source diameter of 5 cm have been assumed. The
required power to give the Arrhenius integral for HSP activation of
approximately 1 decreases monotonically as the frequency increases and as the
total train duration increases. Given the preferred parameters, the minimum
power for a frequency of 1 GHz and a pulse train duration of 0.5 seconds is
5.72 watts, whereas for the 1 GHz frequency and a pulse train duration of 0.1
seconds the maximum power is 28.6 watts. For a 5 GHz frequency, 0.046 watts
is required for a pulse train duration of 0.5 seconds, wherein 0.23 watts is
required for a pulse train duration of 0.1 seconds. The corresponding peak
power during an individual pulse is obtained simply by dividing by the duty
cycle.
[Para 145] FIGURE 23 illustrates the time, in seconds, for the temperature to
diffuse or decay from 10 C to 6 C when the ultrasound frequency is between 1
54
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
and 5 MHz. FIG. 24 illustrates the time, in seconds, to decay from
approximately 10 C to approximately 1 C for ultrasound frequencies from 1 to
MHz. For the preferred focal length of 10 cm and the transducer diameter of
5 cm, the maximum time for temperature decay is 366 seconds when the
ultrasound frequency is 1 MHz, and the minimum temperature decay is 15
seconds when the microwave frequency is 5 MHz. As the FDA only requires the
temperature rise be less than 6 C for test times of minutes, the 366 second
decay time at 1 MHz to get to a rise of 1 C over the several minutes is
allowable. As can be seen in FIGS. 23 and 24, the decay times to a rise of 6 C
are much smaller, by a factor of approximately 70, than that of 1 C.
[Para 146] FIGURE 25 illustrates the volume of focal heated region, in cubic
centimeters, as compared to ultrasound frequencies from between 1 and 5
MHz. Considering ultrasound frequencies in the range of 1 to 5 MHz, the
corresponding focal sizes for these frequencies range from 3.7 mm to 0.6 mm,
and the length of the focal region ranges from 5.6 cm to 1.2 cm. The
corresponding treatment volumes range from between approximately 2.4 cc
and 0.02 cc.
[Para 147] Examples of parameters giving a desired HSP activation Arrhenius
integral greater than 1 and damage Arrhenius integral less than 1 is a total
ultrasound power between 5.8-17 watts, a pulse duration of 0.5 seconds, an
interval between pulses of 5 seconds, with total number of pulses 10 within
the
total pulse stream time of 50 seconds. The target treatment volume would be
approximately 1 mm on a side. Larger treatment volumes could be treatable by
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
an ultrasound system similar to a laser diffracted optical system, by applying
ultrasound in multiple simultaneously applied adjacent but separated and
spaced columns. The multiple focused ultrasound beams converge on a very
small treatment target within the body, the convergence allowing for a minimal
heating except at the overlapping beams at the target. This area would be
heated and stimulate the activation of HSPs and facilitate protein repair by
transient high temperature spikes. However, given the pulsating aspect of the
invention as well as the relatively small area being treated at any given
time, the
treatment is in compliance with FDA/FCC requirements for long term (minutes)
average temperature rise <1K. An important distinction of the invention from
existing therapeutic heating treatments for pain and muscle strain is that
there
are no high T spikes in existing techniques, and these are required for
efficiently activating HSPs and facilitating protein repair to provide healing
at
the cellular level.
[Para 148] The pulse train mode of energy delivery has a distinct advantage
over a single pulse or gradual mode of energy delivery, as far as the
activation
of remedial HSPs and the facilitation of protein repair is concerned. There
are
two considerations that enter into this advantage:
[Para 149] First, a big advantage for HSP activation and protein repair in a
PEMR energy delivery mode comes from producing a spike temperature of the
order of 10 C. This large rise in temperature has a big impact on the
Arrhenius
integrals that describe quantitatively the number of HSPs that are activated
and
the rate of water diffusion into the proteins that facilitates protein repair.
This
56
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
is because the temperature enters into an exponential that has a big
amplification effect.
[Para 150] It is important that the temperature rise not remain at the high
value (10 C or more) for long, because then it would violate the FDA and FCC
requirements that over periods of minutes the average temperature rise must
be less than 1 C (or in the case of ultrasound 6').
[Para 1511 An SDM or other PEMR mode of energy delivery uniquely satisfies
both of these foregoing considerations by judicious choice of the power, pulse
time, pulse interval, and the volume of the target region to be treated. The
volume of the treatment region enters because the temperature must decay
from its high value of the order of 10 C fairly rapidly in order for the long
term
average temperature rise not to exceed the long term FDA/FCC limit of 6 C for
ultrasound frequencies and 1 C or less for electromagnetic radiation energy
sources.
[Para 152] For a region of linear dimension L, the time that it takes the peak
temperature to e-fold in tissue is roughly L2/16D, where D = 0.00143 cm2/sec
is the typical heat diffusion coefficient. For example, if L=1 mm, the decay
time
is roughly 0.4 sec. Accordingly, for a region 1 mm on a side, a train
consisting
of 10 pulses each of duration 0.5 seconds, with an interval between pulses of
5
second can achieve the desired momentary high rise in temperature while still
not exceeding an average long term temperature rise of 1 C. This is
demonstrated further below.
57
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 153] The limitation of heated volume is the reason why RE
electromagnetic radiation is not as good of a choice for treatment of regions
deep with the body as ultrasound. The long skin depths (penetration distances)
and Ohmic heating all along the skin depth results in a large heated volume
whose thermal inertia does not allow both the attainment of a high spike
temperature that activates HSPs and facilitates protein repair, and the rapid
temperature decay that satisfies the long term FDA and FCC limit on average
temperature rise.
[Para 154] Ultrasound has already been used to therapeutically heat regions of
the body to ease pain and muscle strain. However, the heating has not followed
the protocol of the invention and does not have the temperature spikes that
are
responsible for the excitation of HSPs.
[Para 155] Consider, then, a group of focused ultrasound beams that are
directed at a target region deep within the body. To simplify the mathematics,
suppose that the beams are replaced by a single source with a spherical
surface
shape that is focused on the center of the sphere. The absorption lengths of
ultrasound can be fairly long. Table 3 below shows typical absorption
coefficients for ultrasound at 1 MHz. The absorption coefficients are roughly
proportional to the frequency.
58
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 156] Table 3. Typical absorption coefficients for 1 MHz ultrasound in
body tissue:
Body Tissue Attenuation Coefficient at 1 MHz (cm-1)
Water 0.00046
Blood 0.0415
Fat 0.145
Liver 0.115-0.217
Kidney 0.23
Muscle 0.3-0.76
Bone 1.15
[Para 157] Assuming that the geometric variation of the incoming radiation
due to the focusing dominates any variation due to attenuation, the intensity
of
the incoming ultrasound at a distance r from the focus can be written
approximately as:
l(r) = P/(4-rrr2)
[1]
where P denotes the total ultrasound power.
The temperature rise at the end of a short pulse of duration tp at r is then
dT(tp) = Pot p / (4-rrCvr2)
[2]
where oc is the absorption coefficient and Cv is the specific volume heat
capacity. This will be the case until the r is reached at which the heat
diffusion
length at tp becomes comparable to r, or the diffraction limit of the focused
beam is reached. For smaller r, the temperature rise is essentially
independent
of r. As an example, suppose the diffraction limit is reached at a radial
distance
that is smaller than that determined by heat diffusion. Then
59
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
rdif = (4Dtp)1/2
[3]
where D is the heat diffusion coefficient, and for r<rdif, the temperature
rise at
tp is
dT(rchf, tp) = 3Poc/(81rrCvD) when r< rdir
[4]
Thus, at the end of the pulse, we can write for the temperature rise:
dTp(r) = {Patp/(47Cvl[(6/rdir2)Ufrchf-r) /r2)U(r-rdir)]
[5]
On applying the Green's function for the heat diffusion equation,
G(r,t) = (4f1D0-3/2 exp[-r2/(4Dt)]
[6]
to this initial temperature distribution, we find that the temperature dT(t)
at the
focal point r=0 at a time t is
dT(t) = [dT0/{(1/2)+(rr1/2/6)}][(1/2)(tp/t)3/2 + (Tr' /2/6)(tp/t)]
[7]
with
dTo = 3Poz/(8-rrCvD)
[8]
[Para 158] A good approximation to eq. [7] is provided by:
dT(t) dTo(tp/t)3/2
[9]
as can be seen in FIG. 26, which is a comparison of eqs. [7] and [9] for
dT(t)/
dTo at the target treatment zone. The bottom curve is the approximate
expression of eq [9].
The Arrhenius integral for a train of N pulses can now be evaluated with the
temperature rise given by eq. [9]. In this expression,
dTN(t) = E dT(t-nti)
[11]
where dT(t-nti) is the expression of eq. [9] with t replaced by t-ntrand with
ti
designating the interval between pulses.
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 1 59] The Arrhenius integral can be evaluated approximately by dividing
the integration interval into the portion where the temperature spikes occur
and
the portion where the temperature spike is absent. The summation over the
temperature spike contribution can be simplified by applying Laplace's end
point formula to the integral over the temperature spike. In addition, the
integral over the portion when the spikes are absent can be simplified by
noting
that the non-spike temperature rise very rapidly reaches an asymptotic value,
so that a good approximation is obtained by replacing the varying time rise by
its asymptotic value. When these approximations are made, eq. [10] becomes:
0 = AN[{tp(2kBT02/(3EdT0)lexp[-(E/kB)1 /(To + dTo+ dTN(N0)]
+exp[-(E/kB)1 /(To + dTN(N0)]l
[1 2]
where
dTN(Nti) -,-,-, 2.5 dTo (t/03I2
[1 3]
(The 2.5 in eq. [13] arises from the summation over n of (N-n)-3/2 and is the
magnitude of the harmonic number (N,3/2) for typical N of interest).
[Para 1 601 It is interesting to compare this expression with that for SDM
applied to the retina. The first term is very similar to that from the spike
contribution in the retina case, except that the effective spike interval is
reduced by a factor of 3 for this 3D converging beam case. The second term,
involving dTN(Nti) is much smaller than in the retina case. There the
background temperature rise was comparable in magnitude to the spike
temperature rise. But here in the converging beam case, the background
temperature rise is much smaller by the ratio (tp/03/2. This points up the
61
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
importance of the spike contribution to the activation or production of HSP's
and the facilitation of protein repair, as the background temperature rise
which
is similar to the rise in a continuous ultrasound heating case is
insignificant
compared to the spike contribution. At the end of the pulse train, even this
low
background temperature rise rapidly disappears by heat diffusion.
[Para 1611 FIGURES 27 and 28 show the magnitude of the logarithm of the
Arrhenius integrals for damage and for HSP activation or production as a
function of dTo for a pulse duration tp = 0.5 sec, pulse interval ti = 10 sec,
and
total number of pulses N = 10. Logarithm of Arrhenius integrals [eq. 12] for
damage and for HSP activation as a function of the temperature rise in degrees
Kelvin from a single pulse dTo, for a pulse duration tp = 0.5 sec., pulse
interval
ti = 10 sec., and a total number of ultrasound pulses N = 10. FIG. 27 shows
the logarithm of the damage integral with the Arrhenius constants A =
8.71x1 033 5ec-1 and E = 3.55x10-12 ergs. FIG. 28 shows the logarithm of the
HSP activation integral with the Arrhenius constants A = 1.24x1027 sec-, and E
= 2.66x10-12 ergs. The graphs in FIGS. 27 and 28 show that damage does not
exceed 1 until dTo exceeds 11.3 K, whereas nhsp is greater than 1 over the
whole interval shown, the desired condition for cellular repair without
damage.
[Para 1621 Equation [8] shows that when a = 0.1 cm-1, a dT0 of 11.5 K can be
achieved with a total ultrasound power of 5.8 watts. This is easily
achievable.
If a is increased by a factor of 2 or 3, the resulting power is still easily
achievable. The volume of the region where the temperature rise is constant
62
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
(i.e. the volume corresponding to r=rd = (4Dtp)1/2 ) is 0.00064 cc. This
corresponds to a cube that is 0.86 mm on a side.
[Para 1 631 This simple example demonstrates that focused ultrasound should
be usable to stimulate reparative HSP's deep in the body with easily
attainable
equipment:
Total ultrasound power: 5.8 watts - 17 watts
Pulse time 0.5 sec
Pulse interval 5 sec
Total train duration (N=10) 50 sec
To expedite the treatment of larger internal volumes, a SAPRA system can be
used.
[Para 1 64] The pulsed energy source may be directed to an exterior of a body
which is adjacent to the target tissue or has a blood supply close to the
surface
of the exterior of the body. Alternatively, a device may be inserted into a
cavity
of a body to apply the pulsed energy source to the target tissue. Whether the
energy source is applied outside of the body or inside of the body and what
type of device is utilized depends upon the energy source selected and used to
treat the target tissue.
[Para 1651 Photostimulation, in accordance with the present invention, can be
effectively transmitted to an internal surface area or tissue of the body
utilizing
an endoscope, such as a bronchoscope, proctoscope, colonoscope or the like.
Each of these consist essentially of a flexible tube that itself contains one
or
more internal tubes. Typically, one of the internal tubes comprises a light
pipe
63
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
or multi-mode optical fiber which conducts light down the scope to illuminate
the region of interest and enable the doctor to see what is at the illuminated
end. Another internal tube could consist of wires that carry an electrical
current
to enable the doctor to cauterize the illuminated tissue. Yet another internal
tube might consist of a biopsy tool that would enable the doctor to snip off
and
hold on to any of the illuminated tissue.
[Para 1661 In the present invention, one of these internal tubes is used as an
electromagnetic radiation pipe, such as a multi-mode optical fiber, to
transmit
the SDM or other electromagnetic radiation pulses that are fed into the scope
at
the end that the doctor holds. With reference now to FIG. 29, a light
generating
unit 10, such as a laser having a desired wavelength and/or frequency is used
to generate electromagnetic radiation, such as laser light, in a controlled,
pulsed manner to be delivered through a light tube or pipe 52 to a distal end
of
the scope 54, illustrated in FIG. 30, which is inserted into the body and the
laser light or other radiation 56 delivered to the target tissue 58 to be
treated.
[Para 167] The light generator unit 10 of FIG. 29 could comprise the light
generator units discussed above with respect to FIGS. 1-6. The delivery device
or component, however could comprise an endoscope, bronchoscope, with the
generated laser light beam passed through a light tube or pipe 52. The system
could include both a laser beam projector or delivery device, such as a scope,
as well as a viewing system/camera will comprise two different components in
use. The viewing system/camera could provide feedback to a display monitor
which may also include the necessary computerized hardware, data input and
64
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
controls, for manipulating the optics, delivered laser light or other pulsed
energy source and/or the projection/viewing components. Moreover, patterns
can be generated which may be offset, as described above. Of course, the laser
light generating systems of FIGS. 1-6 are exemplary, and other devices and
systems can be utilized to generate a source of laser light or other pulsed
electromagnetic radiation which can be operably passed through a projector
device, such as the endoscope or light pipe or the like illustrated in FIGS.
29
and 30.
[Para 168] Other forms of electromagnetic radiation may also be generated
and used, including ultraviolet waves, microwaves, other radiofrequency waves,
and laser light at predetermined wavelengths. Moreover, ultrasound waves may
also be generated and used to create a thermal time-course temperature spike
in the target tissue sufficient to activate or produce heat shock proteins in
the
cells of the target tissue without damaging the target tissue itself. In order
to
do so, typically, a pulsed source of ultrasound or electromagnetic radiation
energy is provided and applied to the target tissue in a manner which raises
the
target tissue temperature, such as between 6 C and 11 C, transiently while
only
6 C or 1 C or less for the long term, such as over several minutes.
[Para 169] For deep tissue that is not near an internal orifice, a light pipe
is
not an effective means of delivering the pulsed energy. In that case, pulsed
low
frequency electromagnetic energy or preferably pulsed ultrasound can be used
to cause a series of temperature spikes in the target tissue.
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 170] Thus, in accordance with the present invention, a source of pulsed
ultrasound or electromagnetic radiation is applied to the target tissue or
fluid in
order to stimulate HSP production or activation and to facilitate protein
repair in
the living animal tissue. In general, electromagnetic radiation may be
ultraviolet
waves, microwaves, other radiofrequency waves, laser light at predetermined
wavelengths, etc. On the other hand, if electromagnetic energy is to be used
for
deep tissue targets away from natural orifices, absorption lengths restrict
the
wavelengths to those of microwaves or radiofrequency waves, depending on the
depth of the target tissue. However, ultrasound is to be preferred to long
wavelength electromagnetic radiation for deep tissue targets away from natural
orifices.
[Para 171] The ultrasound or electromagnetic radiation is pulsed so as to
create a thermal time-course in the tissue that stimulates HSP production or
activation and facilitates protein repair without causing damage to the cells
and
tissue being treated. The area and/or volume of the treated tissue is also
controlled and minimized so that the temperature spikes are on the order of
several degrees, e.g. approximately 10 C, while maintaining the long-term rise
in temperature to be less than the FDA mandated limit, such as 1 C. It has
been
found that if too large of an area or volume of tissue is treated, the
increased
temperature of the tissue cannot be diffused sufficiently quickly enough to
meet the FDA requirements. However, limiting the area and/or volume of the
treated tissue as well as creating a pulsed source of energy accomplishes the
goals of the present invention of stimulating HSP activation or production by
66
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
heating or otherwise stressing the cells and tissue, while allowing the
treated
cells and tissues to dissipate any excess heat generated to within acceptable
limits.
[Para 172] It is believed that stimulating HSP production in accordance with
the present invention can be effectively utilized in treating a wide array of
tissue abnormalities, ailments, and even infections. For example, the viruses
that cause colds primarily affect a small port of the respiratory epithelium
in the
nasal passages and nasopharynx. Similar to the retina, the respiratory
epithelium is a thin and clear tissue. With reference to FIG. 31, a cross-
sectional
view of a human head 60 is shown with an endoscope 54 inserted into the nasal
cavity 62 and energy 56, such as laser light or the like, being directed to
tissue
58 to be treated within the nasal cavity 62. The tissue 58 to be treated could
be
within the nasal cavity 62, including the nasal passages, and nasopharynx.
[Para 173] To assure absorption of the laser energy, or other energy source,
the wavelength can be adjusted to an infrared (IR) absorption peak of water,
or
an adjuvant dye can be used to serve as a photosensitizer. In such a case,
treatment would then consist of drinking, or topically applying, the adjuvant,
waiting a few minutes for the adjuvant to permeate the surface tissue, and
then
administering the laser light or other energy source 56 to the target tissue
58
for a few seconds, such as via optical fibers in an endoscope 54, as
illustrated
in FIG. 31. To provide comfort of the patient, the endoscope 54 could be
inserted after application of a topical anesthetic. If necessary, the
procedure
could be repeated periodically, such as in a day or so.
67
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 174] The treatment would elevate intracellular temperatures, and this
temperature elevation in itself would be anti-viral in the same way as the
fever
response to viral infections is anti-viral. In addition, treatment would
thermally
stimulate the activation or production of heat shock proteins and facilitate
protein repair without damaging the cells and tissues being treated. As
discussed above, certain heat shock proteins have been found to play an
important role in the immune response as well as the well-being of the
targeted
cells and tissue. The source of energy could be monochromatic laser light,
such
as 810 nm wavelength laser light, administered in a manner similar to that
described in the above-referenced patent applications, but administered
through an endoscope or the like, as illustrated in FIG. 31. The adjuvant dye
would be selected so as to increase the laser light absorption. While this
comprises a particularly preferred method and embodiment of performing the
invention, it will be appreciated that other types of energy and delivery
means
could be used to achieve the same objectives in accordance with the present
invention.
[Para 175] With reference now to FIG. 32, a similar situation exists for other
illnesses or diseases, where the primary target is the epithelium of the upper
respiratory tree, in segments that have diameters greater than about 3.3 mm,
namely, the upper six generations of the upper respiratory tree. A thin layer
of
mucous separates the targeted epithelial cells from the airway lumen, and it
is
in this layer that the antigen-antibody interactions occur that result in
inactivation of viruses, such as cold and flu viruses.
68
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 176] With continuing reference to FIG. 32, the flexible light tube 52 of
a
bronchoscope 54 is inserted through the individual's mouth 64 through the
throat and trachea 66 and into a bronchus 68 of the respiratory tree. There
the
laser light or other energy source 56 is administered and delivered to the
tissue
in this area of the uppermost segments to treat the tissue and area in the
same
manner described above with respect to FIG. 32. It is contemplated that a
wavelength of laser or other energy would be selected so as to match an IR
absorption peak of the water resident in the mucous to heat the tissue and
stimulate HSP activation or production and facilitate protein repair, with its
attendant benefits.
[Para 177] With reference now to FIG. 33, a colonoscope 54 could have
flexible optical tube 52 thereof inserted into the anus and rectum 70 and into
either the large intestine 72 or small intestine 74 so as to deliver the
selected
laser light or other energy source 56 to the area and tissue to be treated, as
illustrated. This could be used to assist in treating colon cancer as well as
other
gastrointestinal issues.
[Para 178] Typically, the procedure could be performed similar to a
colonoscopy in that the bowel would be cleared of all stool, and the patient
would lie on his/her side and the physician would insert the long, thin light
tube portion 52 of the colonoscope 54 into the rectum and move it into the
area of the colon, large intestine 72 or small intestine 74 to the area to be
treated. The physician could view through a monitor the pathway of the
inserted flexible member 52 and even view the tissue at the tip of the
69
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
colonoscope 54 within the intestine, so as to view the area to be treated.
Using
one of the other fiber optic or light tubes, the tip 76 of the scope would be
directed to the tissue to be treated and the source of laser light or other
radiation 56 would be delivered through one of the light tubes of the
colonoscope 54 to treat the area of tissue to be treated, as described above,
in
order to stimulate HSP activation or production in that tissue 58.
[Para 179] With reference now to FIG. 34, another example in which the
present invention can be advantageously used in the GI tract, for example what
is frequently referred to as "leaky gut" syndrome, a condition of the
gastrointestinal (Cl) tract marked by inflammation and other metabolic
dysfunction. Since the Cl tract is susceptible to metabolic dysfunction
similar to
the retina, it is anticipated that it will respond well to the treatment of
the
present invention. This could be done by means of subthreshold, diode
micropulsed laser (SDM) treatment, as discussed above, or by other energy
sources and means as discussed herein and known in the art.
[Para 1 801 With continuing reference to FIG. 34, the flexible light tube 52
of
an endoscope or the like is inserted through the patient's mouth 64 through
the throat and trachea area 66 and into the stomach 78, where the tip or end
64 thereof is directed towards the tissue 58 to be treated, and the laser
light or
other energy source 56 is directed to the tissue 58. It will be appreciated by
those skilled in the art that a colonoscope could also be used and inserted
through the rectum 70 and into the stomach 78 or any tissue between the
stomach and the rectum.
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 181] If necessary, a chromophore pigment or other light-absorbing
material such as metallic nanoparticles could be delivered to the Cl tissue
orally
to enable absorption of the radiation. If, for instance, unfocused 810 nm
radiation from a laser diode or LED were to be used, the pigment would have an
absorption peak at or near 810 nm. Alternatively, the wavelength of the energy
source could be adjusted to a slightly longer wavelength at an absorption peak
of water, so that no externally applied chromophore would be required.
[Para 182] It is also contemplated by the present invention that a capsule
endoscope 80, such as that illustrated in FIG. 35, could be used to administer
the radiation and energy source in accordance with the present invention. Such
capsules are relatively small in size, such as approximately one inch in
length,
so as to be swallowed by the patient. As the capsule or pill 80 is swallowed
and
enters into the stomach and passes through the Cl tract, when at the
appropriate location, the capsule or pill 80 could receive power and signals,
such as via antenna 82, so as to activate the source of energy 84, such as a
laser diode and related circuitry, with an appropriate lens 86 focusing the
generated laser light or radiation through a radiation-transparent cover 88
and
onto the tissue to be treated. It will be understood that the location of the
capsule endoscope 80 could be determined by a variety of means such as
external imaging, signal tracking, or even by means of a miniature camera with
lights through which the doctor would view images of the Cl tract through
which the pill or capsule 80 was passing through at the time. The capsule or
pill
80 could be supplied with its own power source, such as by virtue of a
battery,
71
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
or could be powered externally via an antenna, such that the laser diode 84 or
other energy generating source create the desired wavelength and pulsed
energy source to treat the tissue and area to be treated.
[Para 183] As in the treatment of the retina in previous applications, the
radiation would be pulsed to take advantage of the micropulse temperature
spikes and associated safety, and the power could be adjusted so that the
treatment would be completely harmless to the tissue. This could involve
adjusting the peak power, pulse times, and repetition rate to give spike
temperature rises on the order of 10 C, while maintaining the long term rise
in
temperature to be less than the FDA mandated limit of 1 C. If the pill form 80
of
delivery is used, the device could be powered by a small rechargeable battery
or
over wireless inductive excitation or the like. The heated/stressed tissue
would
stimulate activation or production of HSP and facilitate protein repair, and
the
attendant benefits thereof.
[Para 184] From the foregoing examples, the technique of the present
invention is limited to the treatment of conditions at near body surfaces or
at
internal surfaces easily accessible by means of fiber optics or other optical
delivery means. The reason that the application of SDM or PEMR to activate HSP
activity is limited to near surface or optically accessibly regions of the
body is
that the absorption length of IR or visible radiation in the body is very
short.
However, there are conditions deeper within tissue or the body which could
benefit from the present invention. Thus, the present invention contemplates
the use of ultrasound and/or radio frequency (RE) and even shorter wavelength
72
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
electromagnetic (EM) radiation such as microwave which have relatively long
absorption lengths in body tissue. The use of pulsed ultrasound is often
preferable to RF electromagnetic radiation to activate remedial HSP activity
in
abnormal tissue that is inaccessible to surface SDM or the like. Pulsed
ultrasound sources can also be used for abnormalities at or near surfaces as
well.
[Para 185] With reference now to FIG. 36, with ultrasound, microwave or RE, a
specific region deep in the body can be specifically targeted by using one or
more beams that are each focused on the target site. The pulsating heating
will
then be largely only in the targeted region where the beams are focused and
overlap.
[Para 186] As illustrated in FIG. 36, an ultrasound transducer 90 or the like
generates a plurality of ultrasound beams 92 which are coupled to the skin via
an acoustic-impedance-matching gel, and penetrate through the skin 94 and
through undamaged tissue in front of the focus of the beams 92 to a target
organ 96, such as the illustrated liver, and specifically to a target tissue
98 to
be treated where the ultrasound beams 92 are focused. As mentioned above,
the pulsating heating will then only be at the targeted, focused region 98
where
the focused beams 92 overlap. The tissue in front of and behind the focused
region 98 will not be heated or affected appreciably.
[Para 187] The present invention contemplates not only the treatment of
surface or near surface tissue, such as using the laser light or the like,
deep
tissue using, for example, focused ultrasound, RE, or microwave beams or the
73
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
like, but also treatment of blood diseases, and other bodily fluid diseases,
such
as sepsis. As indicated above, focused ultrasound treatment could be used both
at surface as well as deep body tissue, and could also be applied in this case
in
treating blood. However, it is also contemplated that the SDM and similar PEMR
treatment options which are typically limited to surface or near surface
treatment of epithelial cells and the like be used in treating blood or fluid
diseases at areas where the blood or fluid is accessible through a relatively
thin
layer of tissue, such as the earlobe.
[Para 188] With reference now to FIGS. 37 and 38, treatment of blood
disorders simply requires the transmission of SDM or other electromagnetic
radiation or ultrasound pulses to the earlobe 100, where the SDM or other
radiation source of energy could pass through the earlobe tissue and into the
blood which passes through the earlobe. It would be appreciated that this
approach could also take place at other areas of the body where the blood flow
is relatively high and/or near the tissue surface, such as fingertips, inside
of the
mouth or throat, etc.
[Para 189] With reference again to FIGS. 37 and 38, an earlobe 100 is shown
adjacent to a clamp device 102 configured to transmit SDM radiation or the
like. This could be, for example, by means of one or more laser diodes 104
which would transmit the desired frequency at the desired pulse and pulse
train
to the earlobe 100. Power could be provided, for example, by means of a lamp
drive 106. Alternatively, the lamp drive 106 could be the actual source of
laser
light, which would be transmitted through the appropriate optics and
74
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
electronics to the earlobe 100. The clamp device 102 would merely be used to
clamp onto the patient's earlobe and cause that the radiation be constrained
to
the patient's earlobe 100. This may be by means of mirrors, reflectors,
diffusers, etc. This could be controlled by a control computer 108, which
would
be operated by a keyboard 110 or the like. The system may also include a
display and speakers 112, if needed, for example if the procedure were to be
performed by an operator at a distance from the patient.
[Para 190] As mentioned above, although FIGS. 37 and 38 illustrate, for
exemplary purposes, the treatment of a bodily fluid, namely blood, through a
readily accessible external earlobe 100, it will be appreciated that the
pulsed
energy source of the present invention can be applied to other external areas
of
the body, internal areas of the body, and utilize a wide variety of energy
sources, including laser light, radiofrequency, microwave, and ultrasound.
Moreover, the present invention is not only limited to the treatment of blood
and blood diseases, but can also be applied to other bodily fluids, such as
lymph fluid, etc. The type of bodily fluid treated may dictate the area where
the
treatment occurs, such as applying the energy source in an armpit, tonsil,
etc.
when treating lymph fluid.
[Para 1911 Although not specifically described above, it will be appreciated
that various diseases or potential diseases could be treated in various areas
of
the body, depending upon the disease and the target tissue to be treated
either
for treatment purposes or for prophylactic or protective therapy. For example,
IPF may be treated by PEMR infrared laser locally via bronchoscopic
application.
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
Heart disease, due to the heart being located near the bronchial tree and
lungs,
could also be treated via bronchoscopy. Alternatively, as infrared absorption
lengths are small, as indicated above, PEMR radiofrequency, ultrasound or
microwave may be used to treat the heart, lungs, etc. An additional advantage
would be not requiring the discomfort of a bronchoscope being inserted into
the lungs of the patient.
[Para 1 921 Once again, the selected treatment type and operating procedure
and parameters could change depending upon the location of the chronic
progressive disease. For example, Alzheimer disease may be treated by RF or
microwave application to the brain. A person having cancer, or a risk for
cancer, could have the energy source in accordance with the present invention
applied to the organ(s) or area of the body in question, whether it be a
tissue or
blood (generally not the cancer itself, as activation of HSPs in cancer cells
may
enhance the survival and growth of the cancer; but to treat components of the
immune system to enhance their effectiveness against the cancer). Even mental
conditions, such as depression, could potentially be treated in accordance
with
the present invention.
[Para 1 931 The present invention also contemplates that the time course, and
possibly powers, and other energy and operating parameters may need to be
changed depending upon the tissue, organ, or area of the body to be treated.
For example, for idiopathic pulmonary fibrosis and other lung diseases, such
parameters may need to be changed due to the convective air flow which can
cool the lung tissue. Having the individual exhale and hold his or her breath
for
76
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
a couple of seconds can also alter these energy parameters as an inflated lung
has a conductivity of 0.2 S/m while a deflated lung has a conductivity twice
as
large, 0.41 S/m, and the absorption length is inversely proportional to the
square root of the conductivity. The important aspect is that the tissue or
bodily fluid is heated very quickly up to approximately 11 C while maintaining
a
much lower temperature, such as below 6 C or even 1 C over several minutes,
such as 6 minutes. This will provide the therapeutic benefit, such as
activating
HSPs, while not damaging the bodily fluid, cells and tissue.
[Para 1 94] With reference now to FIG. 39, it is contemplated by the present
invention that some diseases or risk of diseases may require treatment of
multiple areas of the body. For example, diabetes may be treated by
microwave, RF application or the like to many areas of the body, and
potentially
the entire body. Also, the individual may either have multiple chronic
progressive diseases or may be at a risk of having multiple chronic
progressive
diseases which could require treatment of various areas of the body.
Furthermore, since the process of treatment in accordance with the present
invention appears to have only beneficial therapeutic and protective
consequences, without permanently damaging or destroying cells or tissue, the
entire body could be treated as healthy cells and tissue would not be
negatively
impacted by the application of the pulsed energy source applied in accordance
with the present invention while those that are damaged would be benefitted.
[Para 1 95] Accordingly, with continuing reference to FIG. 39, a device 114 is
contemplated by the present invention which can hold and/or support an entire
77
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
body 116, such as by means of a platform 118 upon which the individual lies.
It will be understood, however, that the individual could be in different
positions, such as standing, and not necessarily need to lie down. The device
114 would include a pulsed energy emitter 120 which could emit a pulsed
energy source having the parameters discussed above so as to treat various
types of tissue, organs, bodily fluids, etc. of the individual. This could be,
for
example, by means of microwave, radiofrequency (RE) and/or ultrasound, or
even light sources used to treat external portions of the individual's body or
bodily fluids passing adjacent to such surfaces. The fluid, organs in question
or other tissue could be treated accordingly. In fact, as mentioned above, the
entire body could be treated as the emitter 120 is moved, such as along track
122, to different areas of the body, either progressively or in a
predetermined
pattern, in such a manner so as to fairly quickly treat the desired areas of
target
tissue or target bodily fluid and/or the entire body by heating up the areas
to
the predetermined temperature while maintaining the predetermined lower
temperature over a more prolonged period of time. The whole body treatment
could be a sum of the localized treatments. This could be a way, for example,
to treat diabetes and other similar diseases which affect the entire body or
multiple areas of the body. This could also be, for example, a system and
method for protectively and prophylactically treating the whole body of an
individual, such as on a period basis.
[Para 196] The proposed treatment with a train of electromagnetic or
ultrasound pulses has two major advantages over earlier treatments that
78
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
incorporate a single short or sustained (long) pulse. First, the short
(preferably
subsecond) individual pulses in the train activate cellular reset mechanisms
like
HSP activation with larger reaction rate constants than those operating at
longer
(minute or hour) time scales. Secondly, the repeated pulses in the treatment
provide large thermal spikes (on the order of 10,000) that allow the cell's
repair
system to more rapidly surmount the activation energy barrier that separates a
dysfunctional cellular state from the desired functional state. The net result
is a
"lowered therapeutic threshold" in the sense that a lower applied average
power
and total applied energy can be used to achieve the desired treatment goal.
[Para 197] The present invention has been found to also prevent or treat
neurodegenerative diseases, including Alzheimer's disease. It is determined
that an individual has a neurodegenerative disease, such as Alzheimer's, or is
at
a risk of developing a neurodegenerative disease. This could be determined,
for example, by genetic testing, cognitive testing, blood or cerebral spinal
fluid
testing, inheritance determinations, or any other available test which could
lead
a medical professional to determine that the individual either has a
neurodegenerative disease or is at risk of developing a neurodegenerative
disease.
[Para 198] A pulsed electromagnetic energy, typically either radiofrequency or
microwave, having selected energy parameters, including wavelength or
frequency, duty cycle and pulse train duration, is provided and applied to the
brain of the individual so as to prevent or treat the neurodegenerative
disease.
The pulsed electromagnetic energy may be directed to one or more of a leaky
79
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
blood-brain barrier, inflamed portions of the brain, junk proteins of the
brain,
beta amyloid proteins of the brain, or tangled tau proteins of the brain, or
any
other portion of the brain, brain tissue, or cerebral spinal fluid or the like
to
provide treatment. The energy parameters and application parameters may be
selected to either create thermal interactions with such tissue, proteins or
other
molecules, or resonant interactions.
[Para 199] The pulsed energy could be applied to the individual's brain tissue
by means of the device 114 illustrated in FIG. 39, which would selectively
apply
the pulsed energy just to the brain or area of interest of the patient 116.
Other
devices or means of applying the pulsed energy are also contemplated by the
present invention, such as disposing a plurality of spaced-apart transmitters
124 disposed adjacent to a head 126 of the individual to be treated, as
illustrated in FIGS. 40 and 41. The invention contemplates using a single
electromagnetic emitter which would emit the electromagnetic energy, such as
radiofrequency or microwave energy, to the individual's brain, through his or
her head, and moving the emitter, as necessary.
[Para 200] However, the "head cap" 130 having an array of emitters 124
interconnected by electrical leads 128 which can be placed over or on the head
of the individual is particularly convenient as it is easily worn and places
the
emitters 124 in close proximity to the brain 136. The head cap 130 illustrated
in FIG. 40 has eight emitters 124, although the number, size, and
configuration
can be adjusted as needed. Preferably, the emitters 124 are sufficiently
spaced
apart from one another such that the electromagnetic energy 134 emitted by
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
the emitters 124 do not overlap. The configuration of the emitters 124 in the
head cap 130 illustrated in FIGS. 40 and 41 could be used to treat
substantially
the entire brain of the individual. However, it may be more desirable to treat
only a portion of the brain, and thus a head cap of a different configuration
with a different number of emitters could be utilized, or some emitters 124
could be deactivated, as deemed necessary.
[Para 2011 A power and control device 132 could be operably connected to the
head cap 130 and/or emitters 124. The control box 132 could provide the
power necessary for the emitters 124 to emit their electromagnetic waves, and
could also include electronics so as to control the intensity, timing, and the
like
of the emitters 124. It will be understood that the control box 132 could vary
in size depending upon power and control requirements. When the emitters
124 are to emit relatively large frequencies and/or power, the control and
power device 132 may be somewhat large and substantially non-portable.
However, in other cases the power and control device 132 could be quite small
and be carried by the user to allow the user to be mobile during treatment.
[Para 202] In accordance with an embodiment of the invention, the pulsed
energy parameters are selected so as to raise a temperature of treated tissue
sufficiently to stimulate heat shock protein activation in the treated tissue
or
fluid. For example, the pulsed energy may comprise a radiofrequency between
3 to 6 megahertz, a duty cycle between 2.5% and 5.0% and a pulse train
duration between 0.2 and 0.4 seconds. The radiofrequency may be generated
with a coil having a radii between 2 mm and 6 mm. The coil may have between
81
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
13 and 45 amp turns. With such parameters, the pulsed energy would raise the
temperature of the treated tissue sufficiently to stimulate heat shock protein
activation in the treated tissue or fluid, which would result in protein or
cellular
repair so as to provide effective treatment.
[Para 203] Recently, a non-invasive electromagnetic treatment, more
particularly a transcranial electromagnetic treatment (TEMT) has been found to
provide statistically significant improvement in the individual's cognitive
enhancement, changes to cerebral spinal fluid and blood markers for
Alzheimer's disease, and evidence of enhanced brain connectivity.
[Para 2041 In accordance with the treatment, a head cap 130 having a plurality
of electromagnetic energy coils 124, such as radiofrequency coils, is placed
on
the individual's head and each transmitter transmits a radiofrequency field
between 850-950 megahertz, and more particularly 915 megahertz, every 4 to
milliseconds, such as every 4.6 milliseconds, providing a pulse repetition
frequency of 217 Hz. As illustrated in FIG. 41, the emitter coils 124 are
spaced
sufficiently far apart that their fields 134 extend through the individual's
skull
and into the brain tissue 136, but the fields do not substantially overlap or
more preferably do not overlap. The power level of each emitter is set so that
the specific absorption rate (SAR) in the brain is between 1.0 and 2.0 W/kg.
The pulsed energy is applied to the individual's brain for multiple, spaced-
apart
treatments each day. For example, the patient could be treated for one hour
period in the morning and another hour period in the afternoon. Such
treatment is applied in such a manner over a prolonged period of time,
82
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
comprising weeks or even months. For example, the treatment could occur for
a period of sixty days.
[Para 2051 Various tests performed before and after treatment have indicated
a statistically significant improvement to the individual. These tests include
cognitive tests, human phosphor-tau (p-tau) and total tau determination,
human amyloid beta determination, and PET and functional MRI scanning of the
brain. Treatment shows statistically significant improvements in ADAS-COG,
increases in cerebral spinal fluid levels of amyloid beta and decreased levels
of
CSF p-tau protein/amyloid beta ratio, and reduced levels of oligometric
amyloid
beta in plasma. Enhanced glucose utilization and increased functional
connectivity in the brain has also been found.
[Para 206] The amount of power delivered to the brain by the radiofrequency
emitters is quite low, and would probably not significantly increase the brain
tissue temperature. As a rough estimate of the expected temperature rise,
consider the balance between the SAR of 1.0 W/kg delivered to the brain tissue
by the electromagnetic fields to the heat removed by blood flow to the brain.
About 15% of the cardiac output is supplied to the brain: in the adult this
amounts to 750 milliliters per minute. This amounts to a cerebral blood flow
of
50 ml per 100 g per min. The blood flow of 50 ml per 100 g per minute is
tantamount to a residence time of the blood of about 2 minutes. This is the
time At over which the SAR has the opportunity to raise the temperature.
83
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 207] The temperature rise AT during that time will be given roughly by:
CvdT/dt = SARp
[14]
i.e.
AT = SARp(At/Cv)
[15]
where p denotes the density.
[Para 208] The specific heat capacity of brain tissue is 3630 joules/kg/degC ,
compared to 41 78 joules/kg/degC for water and p is about 1 gm/cc.
[Para 209] Equation [15] then gives:
AT 0.03 0C
[1 6]
[Para 210] The temperature rise is very small indeed, much less than the rise
in temperatures required for heat shock protein activation in the embodiments
described above.
[Para 211] Notwithstanding the small field intensities and power, the pulsed
electromagnetic fields are able to penetrate into the brain, as shown below.
[Para 212] The skin depth 5 of penetration of an electromagnetic field of
angular frequency co into a medium of conductivity +a and magnetic
permeability
1.t is:
[17]
[Para 213] The conductivity of brain tissue is:
G =0.3300 S/m
[18]
[Para 214] For 915 MHz and 1.t=47c10-7MKS, this gives:
8 = 2.9 cm
[19]
84
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 215] Thus, the top layer of the cerebral cortex is subjected to the TEMT
fields.
[Para 2161 The wavelength of a 915 Hz field is:
X = 3x1010/915x106= 32.8 cm
[20]
[Para 217] Hence, since the wavelength is much larger than the penetration
depth into the brain, the fields in the cortex from the coils are induction
(near)
fields.
[Para 218] The induction electric field from a coil can be obtained from the
azimuthal vector potential A:
E= -icoA
[21]
[Para 2191 An integral expression for A from a coil of radius a carrying a
current I is given by:
A[r,z] = ( 1/2)facoscpckp[a2 +r2+z2-2arcoscp]-1 /2
[22]
where the integral is from 0 to TC, and r and z are the radius and axial
distance
in cylindrical coordinates.
[Para 220] When the loop is small compared to the distance to the field point,
this expression reduces to:
A[r,z] = (ialra2/(r2 z2)3/2
[23]
[Para 221] From eq. [23], it is easy to see that the induction E field from
the
coil will penetrate along the axis about a distance equal to the radius of the
coil
and will extend in the radial direction a distance comparable to the coil
radius.
Thus, it can be seen that the induction electric fields penetrate into the
brain as
well as the electromagnetic waves.
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 222] The E field can be estimated and the mechanical effect of E can
also
be calculated. In a medium of electrical conductivity G, the SAR is given by
the
expression:
SAR p = 0 E2
[24]
where p is the density. For p = 1000 kg/m3, this gives an electric field of:
E = 55 V/m
[25]
[Para 2231 The stress energy tensor corresponding to the electric field is:
T = (E2/2)[e +(silo)] Newtons/m2
[26]
[Para 224] For 915 MHz, cy=0.33 S/m, and E = 80 /(36n109),
-F,-', 10-6 Nim2
[27]
[Para 2251 Notably, this is much smaller than any molecular forces.
[Para 226] The maximum induced charge densities, as well as the effects of E
fields on membranes can be calculated or estimated according to the following.
[Para 227] The current density j from the induced electric field E is:
i = uE
[28]
[Para 228] Thus, the maximum possible surface charge density that it can
deliver to a surface is:
E = (ico)-1 csE
[29]
[Para 229] At 915 MHz, and with 6= 0.33 S/m and E = 55 V/m, this gives:
E = 3.16 x10-9cou1ombs/m2
[30]
[Para 230] The nominal surface density of charges in a solid is 16
coulombs/m2, so the induced charge densities are much less than the naturally
occurring densities.
86
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 231] The capacitance of a cellular membrane is of the order of:
C = 10 mF/m2
[31]
[Para 232] We can estimate the voltage v across the membrane due to the
current density of eq. [28] from the equation:
icoCv = ciE
[32]
[Para 233] For 915 MHz and C = 10 mF/m2, 6= 0.22 S/m, and E = 55 V/m. we
find:
v = 1.3x10-6 volts
[33]
[Para 234] Notably, this is much less than the nominal naturally occurring
(10's - 100) mV membrane potentials.
[Para 235] Notwithstanding the relatively low frequency and power level, the
emitters 124, as illustrated in FIGS. 40 and 41 collectively provide both
global
and penetrating TEMT to the human forebrain, including the cerebral cortex
and underlying structures of the brain. The success of the treatment, in this
embodiment, is not due to a thermal effect. The successful treatment also does
not appear to be due to large induced electric fields, induced charge or
current
densities, mechanical stresses, or appreciable changes in membrane potentials.
Instead, it appears that the radiofrequency, or other pulsed energy, is
directly
acting on the biomolecules in the cells. From the magnitudes of the fields in
the induced charge and current densities, it appears that the effect would
most
likely involve a resonance interaction with a collective mode. The
calculations
suggest that the very low intensity electric fields of appropriate frequencies
can
be considerably amplified by pi electrons in biomolecular complexes. The
87
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
TEMT can be used to prevent or even reverse both oligomeric and insoluble
amyloid-beta aggregation, both inside and outside neurons. The TEMT cannot
only disaggregate amyloid-beta oligomers, but also disaggregate tau and oc-
synuclein oligomers. It is believed that this is due to the excitation of
resonant
cooperative oscillations within the brain cells.
[Para 2361 To interact with the induced electric fields of TEMT, the resonant
cooperative oscillations would have to involve charges. Research and
calculations suggest that the charges are electrons in the biomolecules, and
not
ions. Radiofrequencies fall within 20 KHz - 300 GHz and 300 MHz - 300 GHz
for microwaves. A rough description of the central frequencies for RE and
microwaves is 5 MHz - 300 GHz. There are resonances in the beta amyloid in
the 1 GHz -30 GHz range of frequencies, which lower range is close to the 850
MHz -950 MHz considered herein. Resonances have been observed in these
radiofrequency/microwave frequency ranges.
[Para 237] From the foregoing, however, it has been found that the induced
electric fields appear to be very small compared to the naturally occurring
cellular fields. The associated current densities appear to be much less than
normal occurrence. Induced mechanical stresses seem much less than those
associated with molecular forces. The interfacial charges induced appear to be
very small compared to naturally occurring charges. However, as indicated
above, and as more fully shown below, the resonant frequencies of relevant
biomolecules depend on: electron density, whether the electrons are
conducting or insulating, the shape of the region containing the electrons,
and
88
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
the surrounding dielectric of the relevant biomolecules that are present in
the
brains of people that have Alzheimer's or a risk of developing Alzheimer's.
[Para 2381 A crude Drude model, as more detailed below, has led to the
conclusion that the charges involved are electrons within the biomolecules in
and around the brain, rather than ions in the electrolytes internal or
external to
the brain cells. The Drude model, as indicated below, shows that resonances
exist at frequencies where considerable amplification of the applied fields
can
occur. The large amplification can occur only for electrons since the ions
have
too much viscous drag. It is believed that the electrons are pi electrons
associated with conjugated bonds, such as those present in Alzheimer's beta
amyloids, since valence electrons appear to have resonant frequencies that are
much higher than the GHz frequencies that the TEMT uses. The resonant
interactions have disruptive effects on the molecular complexes rather than
thermal activation of heat shock proteins at these parameters.
[Para 239] The interaction of the GHz electric fields with the biomolecules in
the brain will be described by a simple Drude model applied to a finite
conductor, and a distribution of parameters in order to obtain the Cole-Cole
plot characteristic of tissue.
[Para 2401 The Drude model of the interacting charge is obtained by writing
Newton's second law F=MA for a particle of mass m and charge e subjected to
a perturbing electric field E. Denoting the velocity of the particle by v, and
assuming a frictional drag force on the particle of the form - mvv, where v is
the
89
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
mdv(t)/dt = - mvv(t) +eE(t)
[34]
[Para 2411 In the TEMT treatment, an oscillating electric field is applied.
Accordingly, assume that the electric field has the time dependence
E(t) = E exp[icot]
[35]
[Para 2421 In that case,
v(t) = v exp[icot]
[36]
and the equation for v is simply
v = {e/m(ico+v)}E
[37]
[Para 243] If there is a number density N of these charges present, then the
current density j is given by
j = Nev
[38]
i.e.
j = {Ne2/m(ico+v)}E
[39]
[Para 244] In terms of the angular plasma frequency
cop2 = 47c Ne2/m
[40]
this can be written
j = (1/42-c){cop2/(ico+v)}E
[41]
[Para 245] From the foregoing, we can see that the current in a conductor
resulting from an electric field is larger than the number of conducting
charges
present (as indicated by the square of the plasma frequency 0)p2), and is
smaller
the larger the collision frequency v.
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 246] To determine the net field and the conductor, suppose the
conductor described by eq. [41] is subjected to an externally applied electric
displacement field in the z-direction:
Dapp = Dapp iz = Eext Eapp iz
[42]
and that the conductor has an extent w in the z-direction about z=0 and a
large area A in the xy plane. Here Eapp is the applied electric field at the
large
area A and Eext is the dielectric constant external to the conductor.
[Para 247] The current density will cause a surface charge density
[43]
to deposit on the surface at z = w/2, with an equal surface charge density of
the opposite sign to deposit on the surface at z = -w/2, where the magnitude
of the electric field in eq. [41] is given by
E = Dapp - 4TCE
[44]
since it is the "net" field resulting from the applied field minus the "back"
field
due to the induced surface charge density. (Here we assume no contribution to
the surface charge density from any external currents besides those described
by the displacement Dapp .)
E = R-co2+ icov)/{-o2+ 01p2 + iCOV}] Dapp
[45]
[Para 248] The interesting feature about eq. [45] is that it has a resonance
denominator{-w2+ cop2+ icov}.
[Para 249] We note that this result could also have been obtained by requiring
continuity of the displacement vector Dapp perpendicular to the area A, using
as
91
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
the internal dielectric s = 1- Wp2 / (CO2 - low), the expression for a
collisional
plasma.
[Para 2501 Thus, for a conductor, if COp 2 > > i OW and ct)2,---- cop2, then
the
magnitude of the resonance denominator can be very small and the magnitude
of the net electric field E acting on the conducting charges can be very
large.
[Para 2511 At resonance, the numerator in eq. [45] is approximately cop2 and
the denominator has the approximate value of copy.
[Para 2521 Thus, for a conductor, if cop2>> low and o)2,-,_' cop2, then at
this
resonance the net electric field E acting on the conducting charges is larger
than the applied field Eapp by a factor Eext COp2 ROpV = Eext (Op /V , i.e.
the
amplification is
Amplification = cext cop/v
[46]
[Para 253] When this ratio is large, the resulting field can be quite
disruptive.
[Para 254] To modify the Drude models to describe an insulator rather than a
conductor, it is only necessary to add a single term to the Drude treatment of
eq. [34]. Thus, eq. [34] describes a charge that is free to move anywhere
under
the influence of an electric field, subject only to a drag due to collisions.
In an
insulator, the charges are not free to move anywhere, but are constrained.
Accordingly, if we denote the charge displacement at a position r by (r) ,
then
eq. [1] will describe an insulator if a term -I<(r) is added to the right hand
side
to describe the restraining force:
mdv(t)/dt = - mvv(t) +eE(t) - K(r)
[47]
92
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 255] For a disturbance of the form E(t) = E exp[icot], eq. [8] for the
current density would become
j = (1/47c)ficocop2/(-co2 + 0)02 +icov)IE
[48]
where (002=K/m, since 4 = /Jo* .
And eq. [12] relating the net field to the applied field becomes
E = R-co2+ 0)02+ iow)/{-w2+ co02 + cop2+ low}] Eext Eapp
[49]
[Para 2561 Thus, for an insulator, if cop2 + co02>> icov and 02 c0p2+
(0025
then the magnitude of the resonance denominator can be very small and the
magnitude of the net electric field E acting on the constrained charges can be
very large. The amplification of the applied field at resonance is
approximately
Amplification = Eext [ cop2 (002]1/2/v
[50]
[Para 257] When the charge is moving in a viscous medium, it is sometimes
revealing to express the collision frequency in terms of the viscosity i of
the
medium.
[Para 258] This can be done through the Stokes law relation that gives the
viscous force Fvisc acting on a spherical object of radius a moving at a
velocity v
through the medium:
Fvisc - 67ciav
[51]
[Para 259] On comparing this with the drag force my in eq. [34], we see that
in a viscous medium,
v = 67-ma/ m
[52]
[Para 260] Thus, in a viscous medium, the collision frequency is proportional
to the viscosity, and since the mass of the charge is proportional to the cube
of
93
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
the radius of the mass, the collision frequency is inversely proportional to
the
square of the mass's radius.
[Para 2611 In the RF range of frequencies, the complex dielectric "constant" e
of biological tissues approximately satisfies a Cole-Cole distribution:
e(o)) = e- + {e. - e-}/{1 + (ion)(1-a)}
[53]
[Para 2621 This equation can be separated into its real c' and imaginary c"
parts as follows:
e' = e- +(1 /2) fe. - - sinh((1 -a)x)/{cosh((1 -
a)x)+cosfsarc/2)}] [54]
E" = (1/2) {E. - e-}[ cos{arr/2)/{cosh((1 -a)x)+sinfan/2)}]
[SS]
where
x = In(orc)
[56]
[Para 263] Moreover, the conductivity can be written in terms of the imaginary
part of the dielectric constant:
= icoe"
[57]
[Para 2641 This type of distribution can be obtained from the above Drude
expressions by allowing a distribution of parameters in those expressions.
Thus, the distribution of parameters in the Drude expressions means that in
biological tissues it is possible to observe the desired resonances at several
frequencies rather than at just one frequency.
[Para 2651 It was established above that the electric field applied by a TEMT
coil is very small compared to naturally occurring cellular fields, on the
order of
SS V/m. It was concluded that in order to have an appreciable effect, some
94
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
resonance phenomena must have been involved in the brain cells in which the
applied field was amplified at resonance.
[Para 2661 The development of a simple framework using the Drude model
helps to identify which type of cellular components might be involved, and has
led to the following conclusions:
[Para 2671 If conducting (mobile) charges are involved, then the applied field
can be amplified by a factor
Amplification = text cop/v
[Para 2681 At the resonance (02,-- COp2 when COp2 > > iCOV. Here, 0) is the
(angular) frequency of the TEMT field, cop is the plasma frequency defined by
eq.
[40], v is the collision frequency of the charges, and text is the dielectrtic
constant of the region surrounding the conductor
[Para 269] A similar amplification can occur for insulating (constrained)
charges, and is given in eq. [50]
[Para 2701 In biological tissues, there is a distribution in the Drude
parameters, so that resonances can occur over a relatively broad range of
frequencies rather than at just a single frequency.
[Para 271] The collision frequency v can be related to viscosity ri [Cf. eq.
52]
when the charge moves through a viscous medium (such as the cell electrolyte).
[Para 272] The last observation leads us to suggest that the important charges
involved in TEMT are electrons within cellular biomolecules, rather than the
ions
in either the intra- or extra-cellular electrolyte. Thus, a typical ion in the
electrolyte is Nat It has a hydrated radius of close to 1 Angstrom. The
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
molecular weight of Na is 23 and the hydrated ion has 6 water molecules in its
innermost hydration shell. So we can assign a molecular to the hydrated ion of
approximately (23+6x18) =131. The corresponding mass is approximately 1.3
x 10-22 gm. With a viscosity of water of about 0.01 poise, eq. [52] then gives
for a typical ion collision frequency in the electrolyte:
v = &ma/ m = 67cx10-210- 8 /1 .3x1 0-22 1.4 x 1013 5ec-1
[Para 2731 This typical ion collision frequency is four orders of magnitude
larger than the 1 GHz (resonant) frequencies of TEMT, and suggests that ions
cannot be the charges involved in any resonant amplification of the applied
fields. It is believed that the biomolecular electrons involved are those that
are
conducting - most likely pi electrons from conjugated bonds. The reason is
that the bound electrons (valence) electrons have resonant frequencies that
are
much higher than the GHz type frequencies that are effective in TEMT. Thus,
TEMT is effective in treating Alzheimer's because its applied GHz fields are
interacting resonantly with internal biomolecular electrons.
[Para 274] The aforementioned calculations assumed a simple wide block
geometry of the treated biomolecular complexes. However, the resonant
amplification of the pulsed energy fields, such as the radiofrequency and
microwave fields, is not limited to block-like geometries, although the
resonant
frequencies can depend on the shape of the complexes. The biomolecule
complexes surrounding and in the brain tissue have different geometric shapes,
pi electrons are present not just in beta amyloid complexes, but also in the
96
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
ubiquitous microtubules. Thus, the following takes in to account the different
shapes of the conduction pi electron complexes using the Drude model.
[Para 275] The electrons that occupy these geometrical shapes will be
characterized by a simple frequency-dependent dielectric constant e(w). This
dielectric constant was derived with a simple Drude model, as indicated above
which leads to the expression for the frequency-dependent dielectric constant:
E(0)) _ 1 _ cop2 {(02 _ (002 _ imp
[58]
[Para 2761 Here co is the (angular) frequency and v is the electron collision
frequency.
[Para 277] The quantity
cop2= 47c Ne2/m
[59]
is the square of the (angular) plasma frequency, where N is the electron
number
density, e is the electron charge, and m is the electron mass.
[Para 278] The quantity
coo2= K/m
[60]
is the square of the (angular) restoration frequency, where K is a restoring
force
constant;
and v is the collisional frequency of the electrons. For an insulator, (002 is
non-
zero. For a conductor, coo2 = 0.
[Para 279] When the Drude calculation model is applied to arbitrary
geometries, such as a long cylinder, a disk, an ellipsoid, an infinitely thin
prolate ellipsoid (thin needle), an infinitely thin oblate ellipsoid (thin
disk), the
result is that both the resonant frequencies and the field amplifications
change.
97
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
The complexes have been shown to experience amplified internal electric fields
at particular frequencies.
[Para 2801 For convenience, the resonant frequencies are summarized in Table
4 for each of the five shapes, and the approximate amplification (ratio of the
internal field to the applied field) factors at these resonant frequencies are
summarized in Table 5. In both Tables 4 and 5, it has been assumed that:
[Para 2811 The complexes are good electron conductors, i.e. the restoration
frequency co0 characterizing binding forces is assumed to be close to zero and
is ignored (c). -> 0 );
[Para 2821 The electron collision frequency v is assumed to be much less than
the (angular) frequency co of the applied field (v<<co);
[Para 2831 The dielectric constant cext of the external medium is assumed to
be large (Eext >>1) as will be the case for intra- and extra-cellular
electrolytes
where Eext 80.
[Para 2841 Table 4. Approximate (angular) resonant frequencies for
frequency-dependent dielectric of eq. [58] with approximations enumerated
above.
Long thin cylinder (radius a<<length L), co2 cop2(a/L)( 2/C2
/EeXt)
[app along axis
Thin plate (thickness 2a<<width wand
cop2(4/7c)(a/L)In(L/2a)(1 /Eext)
length L), Eapp along plate along L
Collection of spheres 0)2 p2
co /(2eext)
98
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
Collection of randomly oriented thin (02
w /Cext
prolate ellipsoids (needles)
Collection of randomly oriented thin co2 CO p2
oblate ellipsoids (discs)
[Para 2851 Table 5. Approximate amplification (Ei
nt Eapp ) at the resonant
frequencies of Table 4, for frequency-dependent dielectric of eq. [58] with
approximations enumerated above before Table 4.
Long thin cylinder (radius a<<length Eint /E app (00p/V)(
27c2a/cextL)1 /2
L), Eapp along axis
Thin plate (thickness 2a<<width w and Eint /E appl
length L), Eapp along plate along L (0)p/V)[(4a/TCEextL)111(L/
2a)]1 /2
Collection of spheres Eint /E app l ( 3
\12/4)(03p/v)(1/gext1/2)
Collection of randomly oriented thin Eint /E app l ( 4/3)
(C0p/V) (1 /Fext' /2)
prolate ellipsoids (needles )
Collection of randomly oriented thin Eint /E app (C0p/V)Cext
oblate ellipsoids (discs)
[Para 286] Tables 4 and 5 show that biomolecular complexes of different
shapes can amplify externally applied electric fields at particular resonant
frequencies. Both the resonant frequencies and the amplifications at the
resonant frequencies depend on shapes of the complexes. For the first four out
of the five biomolecular shapes, the frequencies at which resonance (and field
amplification) occur are reduced from what would be expected for bulk electron
conductors. Only in the fifth case of randomly oriented thin oblate ellipsoids
99
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
(discs) does the resonance occur at the plasma frequency of the internal
conducting electrons. In the other four cases, the dielectric of the external
medium decreases the resonance by a factor (1 ieexti/2). In addition, for the
thin
conducting cylinder and thin conducting disc, additional reductions in the
resonant frequency occur that depend on the ratio of the thickness of the
conductors to their large dimensions.
[Para 2871 Tables 4 and 5 also show that when the shapes of the complexes
cause the resonant frequencies to decrease from the plasma frequency (at
which resonance occurs for bulk dielectrics), the magnitude of the field
amplification also decreases. For the first four cases, the decrease in
amplification is proportional to 1 icext1/2. In addition, for the first two
cases of a
thin cylinder and thin plate, a reduction depending on the ratio of the
thickness
to the large dimensions also enters. The largest amplification occurs for the
case of randomly oriented thin oblate ellipsoids (discs): there the
amplification
is directly proportional to the large text.
Large amplification is all cases also
depends on the conduction electron collision frequency v being small.
[Para 288] It has been shown above that the low magnitudes of the pulsed
electromagnetic energy fields and the large viscous damping of the ions in the
brain cells point to interactions of the electromagnetic fields with
intramolecular electrons. It is also shown above that in a simple wide block
geometry, conducting electrons could amplify the low intensity fields at
certain
resonant frequencies. The broad distribution of parameters in tissues is
evidenced by the Cole-Cole distribution of dielectric properties that enable
100
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
these resonances to occur over a wide range of frequencies. It was also shown
above that these results also apply to biomolecular clusters of different
shapes
and the resonant amplification of the electromagnetic fields is not limited to
block-like geometries but that the resonant frequency tuning could be obtained
by varying the shape of the complexes. Given the foregoing, it is believed
that
the successful treatment of Alzheimer's disease and other neurodegenerative
diseases in accordance with the invention is due to interaction with pi-
conjugated systems, such as the typical pi-conjugated biomolecules expected
to exist in the brain.
[Para 2891 Recent research has indicated that vibration modes of proteins are
in the THz range of frequencies rather than the much lower 3 MHz - 300 GHz
range of radiofrequencies of interest. Cytoskeletal filamentary structures
could
have lower vibration frequencies, but the viscous damping of these modes
would be very large. To counter the damping, energy could be supplied by ATP
or GTP hydrolysis.
[Para 2901 The state of conducting polymers has been researched recently and
remarkable progress has been made in organic conductors. It has been found
that conductivities range from those of insulators (10-16 S/cm) through those
of
semiconductors 10-7. 102 S/cm) to those of good conductors (104 S/cm -108
S/cm). The conductivities depend strongly on the doping of the doping of the
polymer: e.g. polymers in a NaCI electrolyte can have a very respectable 10
S/cm conductivity). Band gap theory is not adequate to understand the
conductivity mechanism, since doping plays such an important role: the results
101
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
in a variety of charge carriers, such as polarons, bipolarons, and solitons.
The
mobility of these charge carriers is not determined by the usual viscosity
mechanism, but can be much less, depending on dopant, temperature, and
inherent structure.
[Para 291] Amyloid fibril formation is a common characteristic of a variety of
unrelated diseases, including Alzheimer's disease, diabetes mellitus, prion
diseases, and familial amyloidosis. It is believed that pi-stacking may play
an
important role in amyloid fibril formation. The attractive non-bonded
conjugated pi electron systems tend to hold the fibrils together in different,
typically four, configurations. Three aromatic residues are the most frequent
ones present, namely, tryptophan, tyrosine, and phenylalanine.
[Para 292] The presence of conjugated pi-electron systems in the amyloid
fibrils and the demonstrated amplified radiofrequency interactions of similar
systems doped by immersion in electrolytes suggests that the successful
treatment of Alzheimer's disease with radiofrequency electromagnetic radiation
may involve radiofrequency, or other pulsed electromagnetic field, interaction
with these pi-electron systems. Disruption of the systems could very well
result in destruction of the amyloid fibrils. Binding of conducting chains has
been shown to differ drastically in magnitude from two chains in which the
conduction is prevented. Moreover, the interaction between conducting
systems in which the conductivity is anisotropic, as in pi-electron systems,
has
been shown to be strongly dependent on the density of participating electrons.
102
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
[Para 293] Thus, the successful treatment of Alzheimer's disease, and other
neurodegenerative diseases, with low power electromagnetic fields, such as in
the radiofrequency range of frequencies, is believed to involve resonant
interaction with conjugated pi electron systems in the biomolecules
surrounding or within the brain tissue, such as in the beta amyloid protein
present in brains of Alzheimer's patients. Other targets include tau proteins,
particularly tangled tau proteins, which are present in the brain cells of
Alzheimer's patients. Other areas which could be targeted include a leaky
blood-brain barrier, inflamed portions of the brain, and junk proteins of the
brain.
[Para 294] It is believed that in particular the leaky blood-brain barrier,
which
has been found to be compromised in patients with Alzheimer's to allow
neurotoxic plasma-derived components to enter the brain, could be treated
with the non-resonant thermally activated heat shock proteins. It has also
been
found that there is a correlation between brain inflammation and the junk
proteins present in inflamed areas in Alzheimer's disease, along with other
forms of dementia. As activated heat shock proteins repair or destroy
malformed proteins, inflamed portions of the brain are a natural target for
heat
shock protein treatment which are thermally activated by electromagnetic
fields, in accordance with the present invention. The low power resonant
treatment of Alzheimer's and other neurodegenerative diseases, could be for
molecular and tissue targets in which the electromagnetic fields which are
applied interact resonantly with the pi electron stacks in these target
103
CA 03187662 2023- 1-30
WO 2022/031362
PCT/US2021/036128
biomolecular complexes and tissues, including beta amyloids, which are a
characteristic of Alzheimer's brains. This interaction disrupts the structural
integrity of the beta amyloid, or other molecular complexes. The resonant
frequency for the interaction is shown to depend on several factors, including
the number density of electrons, whether the electrons are conducting or
insulating, the shape of the region containing the electrons, and the
surrounding dielectric. The width of the resonant frequency is shown to
depend strongly on the collision frequency of the electrons. The usable
electromagnetic fields have been found to be in the frequency range of both
radiofrequencies and microwaves as mentioned above.
[Para 295] Although several embodiments have been described in detail for
purposes of illustration, various modifications may be made without departing
from the scope and spirit of the invention. Accordingly, the invention is not
to
be limited, except as by the appended claims.
104
CA 03187662 2023- 1-30