Note: Descriptions are shown in the official language in which they were submitted.
WO 2022/031726
PCT/US2021/044378
SYSTEM AND METHOD FOR CARBON DIOXIDE
REACTOR CONTROL
INCORPORATION BY REFERENCE
[0001] A PCT Request Form is filed concurrently with this specification as
part of the present
application. Each application that the present application claims benefit of
or priority to as
identified in the concurrently filed PCT Request Form is incorporated by
reference herein in
their entireties and for all purposes.
STATEMENT OF GOVERNMENT SUPPORT
[0002] This invention was made with Government support under Award Number
1738554
awarded by the National Science Foundation, under Award Numbers DE-SC0015872,
DE-
SC0017725, DE-SC0018549, and DE-SC0018549 awarded by the Department of Energy
Office of Science, under Agreement Numbers FA864920P0616 and FA8649-19-9-9026
awarded by the United States Air Force. The Government has certain rights in
the invention.
TECHNICAL FIELD
[0003] This disclosure relates generally to the carbon oxide reactor field,
and more specifically
to a new and useful system and method for reactor control in the carbon oxide
reactor field.
BACKGROUND
[0004] Typical systems and methods for carbon dioxide reactor control focus on
maximization
of aspects relating to production of carbon monoxide (CO) and/or other carbon-
containing
products (CCPs), such as maximizing or adjusting ratios of CO to other reactor
products (e.g.,
CO:H2 ratio), CO concentration, and/or total CO output or output rate.
100051 Thus, there is a need in the carbon oxide reactor field to create a new
and useful system
and method for reactor control.
SUMMARY
[0006] Some aspects of this disclosure pertain to systems for producing a
polycarbonate
polymer. Such systems may be characterized by the following features: (a) a
carbon dioxide
reduction electrolyzer comprising a membrane electrode assembly, which
comprises one or
more ion conductive polymer layers and a cathode catalyst for facilitating
chemical reduction
of carbon dioxide to carbon monoxide; (b) a plurality of intermediate reactors
collectively
1
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
configured to receive carbon monoxide produced by the carbon dioxide reduction
electrolyzer
and produce one or more intermediate chemicals; and (c) a polycarbonate
synthesis reactor
configured to receive the one or more intermediate chemicals or one or more
derivatives thereof
and synthesize polycarbonate polymer.
[0007] Certain aspects of this disclosure pertain to methods for producing a
polycarbonate
polymer. Such methods may be characterized by the following operations: (a)
reducing carbon
dioxide to carbon monoxide in a carbon dioxide reduction electrolyzer
comprising a membrane
electrode assembly, which comprises one or more ion conductive polymer lavers
and a cathode
catalyst for facilitating chemical reduction of carbon dioxide to carbon
monoxide; (b) reacting
carbon monoxide produced by the carbon dioxide reduction electrolyzer in one
or more of a
plurality of intermediate reactions to produce one or more intermediate
chemicals; and (c)
synthesizing polycarbonate polymer from the one or more intermediate chemicals
or one or
more derivatives thereof
[0008] Certain aspects of this disclosure pertain to systems for producing a
metal formate.
Such systems may be characterized by the following features: (a) a carbon
dioxide reduction
electrolyzer comprising a membrane electrode assembly, which comprises one or
more ion
conductive polymer layers and a cathode catalyst for facilitating chemical
reduction of carbon
dioxide to carbon monoxide; (b) a formate synthesis reactor configured to
receive carbon
monoxide produced by the carbon dioxide reduction electrolyzer and produce a
metal formate;
and (c) one or more units configured to separate and/or purify the metal
formate produced by
the formate synthesis reactor.
[0009] Certain aspects of this disclosure pertain to methods for producing a
metal formate.
Such methods may be characterized by the following operations: (a) reducing
carbon dioxide
to carbon monoxide in a carbon dioxide reduction electrolyzer comprising a
membrane
electrode assembly, which comprises one or more ion conductive polymer layers
and a cathode
catalyst for facilitating chemical reduction of carbon dioxide to carbon
monoxide; (b) reacting
carbon monoxide produced by the carbon dioxide reduction electrolyzer with a
metal
hydroxide to produce a metal formate; and (c) separating and/or purifying the
metal formate
produced in (b).
100101 Certain aspects of this disclosure pertain to systems for producing one
or more chemical
compounds. Such systems may be characterized by the following features: (a)
carbon dioxide
capture unit configured to capture carbon dioxide from air and output carbon
dioxide at a
concentration greater than the concentration of carbon dioxide in air; and (b)
a carbon dioxide
reduction electrolyzer comprising a membrane electrode assembly, which
comprises one or
2
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
more ion conductive polymer layers and a cathode catalyst for facilitating
chemical reduction
of the carbon dioxide to a carbon-containing reaction product. The system may
be configured
to provide carbon dioxide from the carbon dioxide capture unit to the carbon
dioxide reduction
el ectrolyzer.
[0011] Certain aspects of this disclosure pertain to systems for producing
liquid hydrocarbons.
Such systems may be characterized by the following features: (a) a carbon
dioxide reduction
electrolyzer comprising a membrane electrode assembly, which comprises one or
more ion
conductive polymer layers and a cathode catalyst for facilitating chemical
reduction of carbon
dioxide to carbon monoxide; and (b) a Fischer Trosch reactor configured to
produce a liquid
hydrocarbon mixture from carbon monoxide and hydrogen, wherein the system is
configured
to transport carbon monoxide and hydrogen from the carbon dioxide reduction
electrolyzer to
the Fischer Tropsch reactor.
100121 Certain aspects of this disclosure pertain to systems for producing one
or more chemical
compounds. Such systems may be characterized by the following features: (a) a
carbon oxide
reduction electrolyzer comprising a membrane electrode assembly, which
comprises one or
more ion conductive polymer layers and a cathode catalyst for facilitating
chemical reduction
of the carbon oxide to a carbon-containing reaction product; and (b) a gas
fermentation reactor
configured to receive the carbon-containing reaction product produced by the
carbon dioxide
reduction electrolyzer and produce the one or more chemical compounds.
[0013] These and other features of the disclosure will be described in detail
below with
reference to associated figures.
BRIEF DESCRIPTION OF THE FIGURES
[0014] Figure 1 is a flow chart representation of an embodiment of the method.
[0015] Figures 2A-2B are a schematic representation of an embodiment of the
system and a
variation of the embodiment, respectively.
[0016] Figures 2C-2D are schematic representations of a first and second
example,
respectively, of the embodiment of the system.
[0017] Figures 3A-3B are examples of idealized and non-idealized dependence of
reactor
outputs on current density, respectively.
100181 Figure 4 depicts a gas fermentation system comprising a carbon oxide
reduction
el ectrolyzer upstream from a gas fermentation bioreactor.
[0019] Figure 5 depicts a system including a carbon dioxide electrolyzer
configured to produce
syngas.
3
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
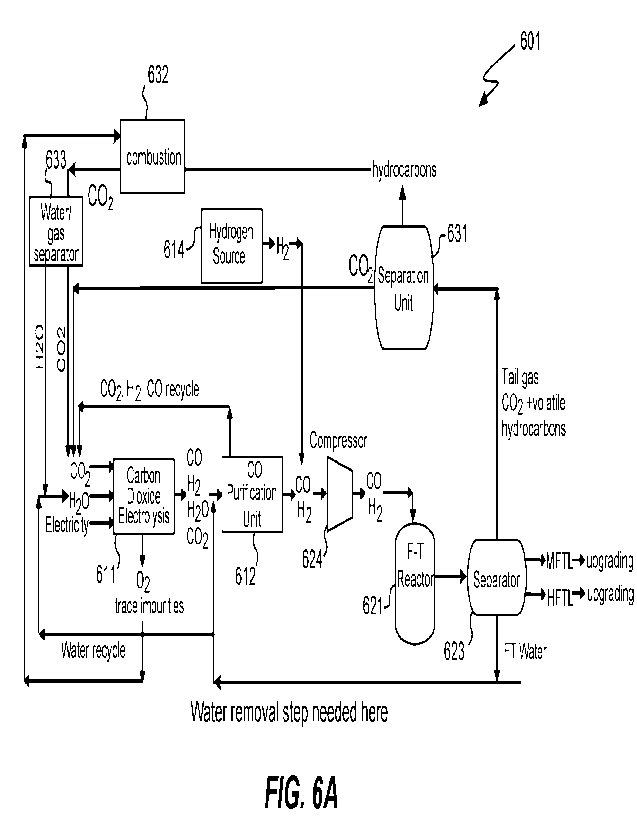
[0020] Figure 6A depicts a Fischer Tropsch system configured to produce liquid
hydrocarbons
in which a source of carbon is a carbon oxide feedstock such as one containing
carbon dioxide
and/or carbon monoxide.
[0021] Figure 6B depicts a Fischer Tropsch system configured to produce liquid
hydrocarbons
in which a source of carbon is a carbon oxide feedstock and tail gas from the
system reformed
to produce addition carbon monoxide and hydrogen.
[0022] Figure 7A illustrates an air capture CO2 electrolyzer system comprising
a direct air CO2
capture subsystem and an CO2 reduction electrolyzer subsystem.
[0023] Figure 7B illustrates an air capture CO2 electrolyzer system comprising
a direct air CO2
capture subsystem on a vehicle or vessel.
[0024] Figure 8A presents a general representation of a system for producing a
polycarbonate
polymer using a carbon dioxide reduction electrolyzer.
100251 Figure 8B presents an example of a polycarbonate synthesis system
having a carbon
dioxide reduction electrolyzer configured to receive carbon dioxide and water
as reactant inputs
and electricity to drive the electrolysis reactions at the anode and cathode.
[0026] Figure 8C depicts a polycarbonate production system.
[0027] Figure 8D illustrates a polycarbonate synthesis system, which has a
parallel path from
a carbon dioxide electrolyzer to a bisphenol A synthesis reactor to deliver
acetone input to a
reactor.
[0028] Figure 8E depicts a polycarbonate production system including a
polycarbonate
synthesis reactor configured to receive phosgene from electrolyzer-produced
carbon monoxide
and to receive bisphenol A from a reactor that receives fermentation-produced
acetone.
[0029] Figure 8F depicts a polycarbonate production system that includes an
electrolysis
subsystem comprising a carbon dioxide reduction electrolyzer and a chlor-
alkali system.
[0030] Figure 8G depicts a polycarbonate production system that employs three
separate
pathways from a carbon dioxide reduction electrolyzer subsystem.
[0031] Figure 8H depicts a polycarbonate polymer production system including a
carbon
dioxide reduction electrolyzer and components for conveying carbon monoxide
and hydrogen
gas to a gas fermentation and conversion reactor or subsystem, which is
configured to directly
produce phenol and excess carbon dioxide.
100321 Figure 9 illustrates an example formate production system comprising a
carbon dioxide
reduction electrolyzer, a formate production reactor, and various downstream
formate recovery
units.
[0033] Figure 10A depicts a monoethylene glycol (MEG) production system
including a
4
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
carbon oxide electrolyzer, an ethylene oxide production reactor, and a MEG
production reactor.
[0034] Figure 10B depicts a MEG production system including a carbon dioxide
electrolyzer
and an MEG production reactor.
[0035] Figure 11 provides a schematic illustration of systems that may be
employed to produce
polyethylene terephthalate.
[0036] Figure 12 schematically illustrates a system for producing acetic acid
from carbon
monoxide and hydrogen produced by a carbon dioxide electrolyzer.
[0037] Figure 13 illustrates schematically a system configured to produce a
diisocyanate from
electrolytically generated carbon monoxide.
[0038] Figure 14 provides a schematic illustration of systems that may be
employed to produce
polyurethane.
[0039] Figure 15 depicts a system comprising a carbon dioxide electrolyzer
configured to
produce carbon monoxide and hydrogen for use in producing oxalic acid.
[0040] Figure 16 illustrates a system configured to produce oxalic acid from
carbon monoxide
produced by an electrolyzer.
[0041] Figure 17A depicts a system comprising a carbon dioxide electrolyzer, a
metal formate
production reactor, and an oxalic acid formation reactor.
[0042] Figure 17B depicts a process and some associated components for
producing oxalic
acid using carbon monoxide from a carbon dioxide electrolyzer.
[0043] Figure 18 depicts a system comprising a carbon dioxide electrolyzer
configured to
produce carbon monoxide and hydrogen for use in producing oxalic acid.
[0044] Figure 19 depicts a system for verifying a carbon monoxide stream
containing carbon
dioxide and possibly other components such as hydrogen.
[0045] Figure 20 illustrates a hybrid carbon monoxide purification system
having a cryogenic
preprocessing subsystem and a sorbent postprocessing subsystem.
100461 Figure 21A illustrates a system having an upstream DAC unit configured
concentrate
carbon dioxide from air, and a downstream DAC unit configured to remove
unreacted CO2
from product gas of a carbon dioxide electrolyzer.
[0047] Figure 21B illustrates a system having a DAC unit configured to capture
carbon dioxide
from air and separate unreacted carbon dioxide from product gas of an
electrolyzer.
100481 Figure 22A depicts a system that couples a carbon oxide reduction
electrolyzer to an
electrical grid or other source of electrical energy.
[0049] Figures 22B and 22C depict further examples of grid management systems
employing
carbon oxide electrolyzers.
5
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
[0050] Figure 23A depicts a system for producing a mixture of hydrogen and
carbon monoxide
by processing carbon dioxide electrolyzer output to first remove carbon
monoxide and then
remove hydrogen from a carbon dioxide-containing stream.
[0051] Figure 23B depicts a system for producing a mixture of hydrogen and
carbon monoxide
by processing carbon dioxide electrolyzer output to directly remove carbon
dioxide.
[0052] Figure 23C depicts a system for producing a mixture of hydrogen and
carbon monoxide
by processing carbon dioxide electrolyzer output to first remove carbon
monoxide and then
remove hydrogen from a carbon dioxide-containing stream.
[0053] Figure 23D depicts a system for producing a mixture of hydrogen and
carbon monoxide
by processing carbon dioxide electrolyzer output to directly remove carbon
dioxide.
DESCRIPTION
100541 The following description of the preferred embodiments is not intended
to limit the
disclosure to these embodiments, but rather to enable any person skilled in
the art to make and
use this disclosure.
Overview
[0055] A system and/or method for carbon dioxide reactor control may be
configured to control
aspects of reactor production, such as aspects relating to quantity,
concentration, and/or ratios
of reactor products. Electrochemical carbon oxide reduction cells may be
integrated with any
of various other chemical processing systems such as chemical reactors,
chemical separation
units, purification units, and the like, along with associated sensing and/or
control systems.
Integrated systems may employ an electrochemical carbon oxide reduction cell
and another
chemical processing system disposed upstream, downstream, or in parallel with
the
electrochemical carbon oxide reduction cell.
[0056] Examples of carbon oxide reactants include carbon dioxide and carbon
monoxide,
typically though not necessarily in gaseous form. Other examples of carbon
oxide reactant
include carbonate ions and compound, and bicarbonate ions and compounds.
[0057] Typical systems and methods for carbon dioxide reactor control have
focused on
maximization of aspects relating to production of carbon monoxide (CO) and/or
other carbon-
containing products (CCPs) (e.g., carbon-containing species (CCSs)), such as
maximizing
ratios of CO to other reactor products (e.g., CO:H2 ratio), CO concentration,
and/or total CO
output or output rate.
[0058] However, for some applications, simply maximizing aspect values can be
undesirable,
and that arbitrary control of such aspects (e.g., dynamic or selective aspect
control to meet a
6
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
value within a range of target aspect values), rather than simple
maximization, can be
beneficial. For example, it can be desirable to selectively control the CO:H2
ratio of the reactor
products (e.g., enabling arbitrary control within a spectrum from the highest
CO:H2 ratio
possible for a given system and/or process, down to approximately 1:3 CO:H2 or
lower). With
such control, the reactor output can be more effectively used (e.g., wherein
the reactor outputs
are directly fed to a subsequent input) for applications such as liquid
hydrocarbon production
via the Fischer¨Tropsch process (e.g., controlling the reactor to produce an
approximately 1:2
CO:H2 output ratio), chemical synthesis processes, and/or gas (e.g., syngas)
fermentation
processes (e.g., bioreactors).
System
[0059] The system can include a carbon dioxide reactor, such as a reactor that
generates
carbon-containing products (e.g., CO, alkanes, alcohols, etc.) and/or hydrogen
from an input
(e.g., an input stream, such as a fluid stream) that includes carbon dioxide.
Example carbon
oxide electrolyzers are illustrated in Figures 2A-2D. The reactor may be
configured to accept
a gas-phase carbon dioxide input and/or performs the reaction(s) using gas-
phase carbon
dioxide (e.g., is a gas-phase reactor), but can additionally or alternatively
accept liquid-phase
carbon dioxide, supercritical fluid-phase carbon dioxide, solid-phase carbon
dioxide, and/or
any other suitable carbon dioxide input. While the discussion herein focuses
on carbon dioxide
reactors, in many cases the discussion applies equally to carbon monoxide
reactors (e.g.,
electrochemical carbon monoxide reduction reactors), and carbonate and/or
bicarbonate
reduction reactors. So, unless otherwise specified or clear from context,
reference to carbon
dioxide reactors is understood to more generally reference carbon oxide
reactors. As indicated,
the reactor may an electrolyzer (e.g., electrochemical reactor) such as a gas-
phase polymer-
electrolyte membrane electrolyzer, but can additionally or alternatively
include any other
suitable reactors.
100601 The reactor may include one or more: electrodes (e.g., anode, cathode),
catalysts (e.g.,
within and/or adjacent the cathode and/or anode), gas diffusion layers (e.g.,
adjacent the
cathode and/or anode), and/or flow fields (e.g., defined within and/or
adjacent the electrodes
and/or gas diffusion layers, such as one or more channels defined opposing the
cathode across
the gas diffusion layer). In some embodiments, the reactor includes a membrane
stack or
membrane electrode assembly (MEA) having one or more polymer electrolyte
membranes
(PEMs), providing ionic communication between the anode and cathode of the
reactor. In
certain embodiments, the reactor includes a membrane stack including: a
cathode layer
including a reduction catalyst and an ion-conducting polymer; a PEM membrane
(e.g., bipolar
7
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
membrane, monopolar membrane, etc.; membrane including one or more anion
conductors
such as anion exchange membranes (AEMs), proton and/or cation conductors such
as proton
exchange membranes, and/or any other suitable ion-conducting polymers;
membrane including
one or more buffer layers; etc.); and an anode layer including an oxidation
catalyst and an ion-
conducting polymer. The ion-conducting polymers of each layer can be the same
or different
ion-conducting polymers.
[0061] In some embodiments, one or more of the catalysts (e.g., reduction
catalyst, oxidation
catalyst) can include catalyst particles (e.g., defining a porous network of
particles), such as
nanoparticles. One or more of the catalysts can additionally or alternatively
include one or more
polymer electrolytes, optionally wherein the polymer electrolyte is mixed with
the catalyst
nanoparticles (e.g., arranged within the porous network, such as loaded into
the open regions
defined by the porous network). The catalyst nanoparticles can define one or
more
characteristic sizes (e.g., mean size, median size, minimum size, maximum
size, size at a
particular percentile of the particle size distribution, etc.), and/or the
porous network can define
a porosity (e.g., fraction of empty space within the network), density,
circuitousness (e.g.,
characteristic path length per layer thickness, area, and/or volume, such as
path through the
empty spaces or path along interconnected particles, etc.), and/or any other
suitable porous
network metrics.
[0062] In some configurations, a bipolar MEA has the following stacked
arrangement: cathode
layer/cathode buffer layer (an anion conducting layer)/cation conductive layer
(with may be a
PEM)/anode layer. In some implementations, the bipolar MEA has a cathode layer
containing
an anion conductive polymer and/or an anode layer containing a cation
conductive layer. In
some implementations, the bipolar MEA has an anode buffer layer, which may
contain a cation
conductive material, between the cation conductive layer and the anode layer.
[0063] In some configurations, a bipolar MEA has the following stacked
arrangement: cathode
layer/cation conducting layer (with may be a PEM)/anion conductive layer/anode
layer. In
some applications, a bipolar MEA having this arrangement is configured in a
system for
reducing a carbonate and/or bicarbonate feedstock such as an aqueous solution
of carbonate
and/or bicarbonate.
100641 In some configurations, an MEA has the following stacked arrangement:
cathode
layer/anion conducting layer/anode layer. In some implementations, this MEA
has no cation
conductive layers between the cathode layer and the anode layer. In some
applications, an
MEA containing only anion conductive material between the cathode and anode is
configured
in a system for reducing carbon monoxide feedstock.
8
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
[0065] In one example ("reactor configuration A"), the system includes: a
carbon fiber paper
gas diffusion layer (e.g., Sigracet 39BC); a catalyst layer including
approximately 20% by
weight of approximately 4 nm gold particles on Vulcan carbon and an anion-
conducting
polymer (e.g., Fumasep FAA-3); a bipolar PEM; and a flow field such as a
single, double,
triple, or quadruple serpentine flow field or an interdigitated flow field. In
a specific example,
the electrodes define an area of approximately 25 cm', but can additionally or
alternatively
define any other suitable area.
[0066] In some embodiments, the reactor includes one or more elements such as
described in
U.S. Patent Application serial number 15/586,182, filed 03-MAY-2017 and titled
"Reactor
with Advanced Architecture for the Electrochemical Reaction of CO2, CO and
Other Chemical
Compounds", which is hereby incorporated in its entirely by this reference.
However, the
reactor can additionally or alternatively include any other suitable elements
in any suitable
arrangement.
[0067] Additional information regarding optional embodiments and/or elements
of the system
and/or method are provided below, in US Patent Application Publication No.
2017/0321334,
filed May 3, 2017, and in US Provisional Patent Application No. 62/939,960,
filed November
25, 2019, which are incorporated herein by reference in their entireties.
[0068] A carbon oxide reduction reactor may comprise more than one cells or
MEAs. The
multiple cells or MEAs may be arranged in a stack, electrically connected to
one another in
series and/or parallel. Unless otherwise indicated, all references herein to a
carbon oxide
reduction reactor, a carbon oxide electrolyzer, and the like embody single
cell electrolyzers and
multicell stacks of electrolyzers.
[0069] A carbon oxide reduction reactor may obtain carbon oxides from various
sources. As
mentioned, examples of carbon oxide reactants include carbon dioxide, carbon
monoxide,
carbonate, and/or bicarbonate. In certain embodiments, a carbonate or
bicarbonate is provided
in the form of an aqueous solution (e.g., an aqueous solution of potassium
bicarbonate) that
can be delivered to the cathode of a reduction cell. Carbonates and
bicarbonates may be
obtained from various sources (e.g., minerals) and/or by various reactions
(e.g., reacting carbon
dioxide with hydroxide).
100701 A system may optionally include an upstream source of carbon dioxide
input, connected
to an input of a carbon dioxide reactor of the disclosure, including one or
more of: a biogas
production system; an ethanol fermentation system such as corn ethanol
production system, a
beer production system, a wine production system; a natural gas processing
system; a cement
production system; a blast furnace system, for example a steel blast furnace
system, capable of
9
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
producing blast furnace gas; a coke gas production system; power plant
systems, such as
petroleum power plant systems, natural gas power plant systems, coal power
plant systems;
petroleum refinery systems; ethylene production systems; ammonia production
systems;
hydrogen production systems, such as water-gas shift systems; natural gas
processing plants
(e.g., Benfield processing); ethylene oxide production systems; aluminum
smelting systems;
liquified natural gas (LNG) production systems; solid feedstock gasifiers
(e.g., municipal solid
waste, biomass, or coal feedstocks); reformers (e.g., steam methane reformers,
autothermal
reformers); systems performing Boudouard reactions; direct air capture (DAC)
of carbon
dioxide process; atmospheres of planets or moons (e.g., the Martian
atmosphere), soil of moons
(e.g., the soil of the earth's moon), and/or any other system capable of
producing carbon
dioxide. An upstream source of carbon dioxide may be connected directly to an
input of a
carbon dioxide reactor of the disclosure (e.g., serves as the input, such as
connected to the
reduction catalyst via the cathode flow field and/or gas diffusion layer,
etc.) or alternatively the
upstream source may be connected to a purification system; a gas compression
system; or both
a purification system and a gas compression system, in either order; which
then connect to an
input of a carbon dioxide system of the disclosure. Multiple purification
and/or gas
compression systems (e.g., scrubbers, etc.) may be employed.
[0071] The carbon dioxide, carbon monoxide, or carbonate provided as input to
a carbon oxide
reduction reactor may, depending on the construction and operating conditions
of the reactor,
have a range of concentrations. In certain embodiments, carbon dioxide
provided to a carbon
dioxide reduction reactor has a concentration of at least about 20 mole
percent, or at least about
40 mole percent, or at least about 75 mole percent, or at least about 90 mole
percent. In certain
embodiments, carbon dioxide provided to a carbon dioxide reduction reactor has
a
concentration of about 40 to 60 mole percent.
[0072] An upstream source of water for an electrolytic carbon oxide reduction
reactor may
come from any of various source and in various forms such as purified tap
water, purified sea
water, a byproduct of direct air capture of water, optionally with capture of
carbon dioxide,
combustion processes that may also produce carbon dioxide feedstock, fuel cell
byproduct, and
the like.
100731 A system may include an input of a downstream system, capable of
transforming
chemical outputs from a carbon dioxide reactor of the disclosure, connected to
an output of a
carbon dioxide reactor of the disclosure. As examples, a downstream system of
the disclosure
may include one or more of: a bioreactor system; a Fischer-Tropsch system; an
anaerobic
fermentation system; an aerobic fermentation system, a syngas fermentation
system; a ketone
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
and/or polyketone production system; a formate production system; a formate
ester production
system; a formamide production system; a hydroformylation system; a methanol
synthesis
system; an ethylene polymerization system; a phosgene production system, an
isocyanate
production system, a polymer (e.g., a polycarbonate, polyethylene
terephthalate, or
polyurethane) production system, a monoethylene glycol production system, a
polyethylene
glycol production system, and oxalic acid production system, and/or any other
system capable
of transforming chemical outputs from a carbon oxide reduction reactor. A
carbon dioxide
reactor output of the disclosure may be directly connected (e.g., via the
cathode flow field
and/or gas diffusion layer) to a downstream system, and/or the carbon dioxide
reactor output
may be connected to a purification system; a gas compression system; or both a
purification
system and a gas compression system, in either order; which then optionally
connect to an input
of a downstream system. Multiple purification systems and/or gas compression
systems may
be employed.
[0074] A downstream system may produce carbon dioxide output in addition to
other product
outputs. A system may further include a connection between a carbon dioxide
containing
output of a downstream system and an input of a carbon dioxide reactor. The
carbon dioxide
containing output of a downstream system may be directly connected to an input
of a carbon
dioxide reactor or alternatively the downstream carbon dioxide containing
output may be
connected to a purification system; a gas compression system; or both a
purification system
and a gas compression system, in either order; which then connect to an input
of a carbon
dioxide reactor of the disclosure. Multiple purification systems and/or gas
compression systems
may be employed.
[0075] A carbon dioxide reactor can make a range of products (for example,
methane, ethylene,
carbon monoxide (CO), molecular hydrogen (H2), ethanol, formate, formic acid,
acetate, acetic
acid, propanol, butanol, ethane, methanol) that can be used in downstream
systems and
processes. Different carbon dioxide reactors (e.g., including different layer
stacks, catalysts
and/or catalyst layers, PEMs, flow fields, gas diffusion layers, cell
compression configurations,
and/or any other suitable aspects, etc.) can be used to achieve different
reduction products (e.g.,
product compositions such as HCR); however, different reduction products can
additionally or
alternatively be achieved by adjusting the operation parameters, and/or be
otherwise achieved.
Many possible downstream systems and processes release CO2 (examples include
bio-
utilization of methane, bio-utilization of formic acid or formate, bio-
utilization of acetic acid
or acetate, Fischer-Tropsch processes, and methanol synthesis). A carbon
dioxide recycling
system sized appropriately for the specific application can be used in many of
these cases to
11
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
return CO2 from the downstream system output to an input of a carbon dioxide
reactor of the
disclosure to increase the carbon efficiency of the overall process.
[0076] A system may further include a source of electrical energy connected to
a carbon
dioxide reactor, the source of electrical energy comprising one or more of: a
solar electrical
energy production system; a wind electrical energy production system; a
geothermal electrical
energy production system; a fossil fuel electrical energy production system;
or any other system
capable of electrical energy production.
[0077] A system may be employed to store electrical energy in the form of
chemical energy.
For example, power producers may produce excess power during off-peak usage
periods.
Systems containing carbon oxide reduction reactors are able to respond quickly
to a need to
consume excess power. They do not need to warm up to operate, and they can be
cycled
between power on and power off states without deterioration of carbon dioxide
reactors. The
ability to respond quickly to power utilization needs allows systems to work
well with
intermittent sources of power such as solar electrical energy production
systems, and wind
electrical energy pro ducti on systems.
[0078] An embodiment of a system may include an upstream bioreactor, a carbon
dioxide
reactor, and an intermittent source of electrical energy. When electrical
power is available from
solar, or wind, or low off-peak demand, or other sources, a power availability
detector may be
used to start the carbon dioxide reactor. In addition, the system may boost
the output of the
upstream bioreactor by, for example, raising the temperature of the upstream
bioreactor and
increasing the flow of nutrients to the upstream bioreactor. For other
upstream carbon dioxide
sources, other means may be used as necessary to increase the flow of carbon
dioxide to an
input of a carbon dioxide reactor of the disclosure.
[0079] Any of the systems disclosed herein may include components (e.g.,
sensors, systems,
etc.) to measure conditions, outputs, and inputs in the systems connected to a
carbon dioxide
reactor. Such components may include chemical property measurement systems
such as gas
chromatographs, mass spectrometers, infrared spectrometers, visible light
spectrometers,
and/or ultraviolet light spectrometers; temperature detectors; flow rate
measurement sensors;
electrical power availability detectors; and/or any other monitoring systems.
The monitoring
systems can monitor the parameters of the input and/or output streams, the
parameters of a
component of the input and/or output streams (e.g., the impurity
concentration, the carbon
dioxide concentration, the product concentration, etc.), and/or monitor any
other suitable
parameter(s) of the stream.
[0080] Any of the systems disclosed herein may include components for
responding to
12
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
conditions measured in systems connected to a carbon dioxide reactor. Such
components may
include systems for adjusting flow rates, temperatures, power consumption or
other system
parameters. A system may include one or more carbon dioxide reactors. However,
the system
can additionally or alternatively include any other suitable elements in any
suitable
arrangement. In various embodiments, one or more monitoring or sensing
components is used
in conjunction with a control system including a controller with appropriately
programmed or
constructed logic (e.g., processors and memory) for determining that one or
more operating
conditions should be modified and causing such operating condition(s) to be
modified.
Feedforward and/or feedback control systems may be employed.
Method
[0081] The method may be implemented using any of the components described
above
including an electrochemical carbon oxide reduction reactor but can
additionally or
alternatively be implemented using any other suitable system(s). The method
optionally
includes running the reactor under controlled process conditions (e.g., as
described below in
further detail) to produce the desired outputs (e.g., CO, Hz, etc.) in the
desired ratios (e.g.,
molecular hydrogen-to-CCP ratio (HCR) and/or CCP-to-molecular hydrogen ratio),
and/or
altering the process conditions to alter the outputs and/or output ratios
(e.g., as shown in Figure
1).
[0082] Running the reactor can include: providing one or more inputs (e.g.,
gasses, liquids,
solids, etc.), such as carbon dioxide, carbon monoxide, a carbon oxide source
(e.g., waste gas),
and/or water; causing all or some of the inputs to undergo reactions (e.g., by
applying a voltage
across the device electrodes), thereby generating products; and/or removing
the products from
the reactor (e.g., as an output gas stream). Such reactions can include, for
example, reducing
carbon dioxide and/or water to generate products such as CO (and/or other
CCPs, such as
formic acid, methanol, glyoxal, methane, acetic acid, glycolaldehyde, ethylene
glycol,
acetaldehyde, ethanol, ethylene, hydroxyacetone, acetone, allyl alcohol,
propionaldehyde, n-
propanol, etc.), and/or H2.. However, running the reactor can additionally or
alternatively
include causing any other suitable reactions to occur, and/or can additionally
or alternatively
include any other suitable elements performed in any suitable manner.
100831 The method can include controlling the system to achieve a desired set
of process
conditions (e.g., aspects), such as process conditions known to result in a
desired output metric
value (e.g., a desired CCP:Hz ratio, such as a CO:Hz ratio). The method can
additionally or
alternatively include altering process conditions, such as based on a
difference between actual
and desired outputs (e.g., to reduce or eliminate the difference). For
example, the method can
13
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
include: imposing an initial set of process conditions; monitoring one or more
output metrics
(e.g., CCP:H2 ratio); determining that an output metric differs from a target
output metric (e.g.,
is greater than or less than the target); altering one or more process
conditions to reduce the
output metric difference (e.g., reducing or increasing a process condition
value, such as a
condition for which the output metric tends to increase or decrease along with
an increasing
process condition value); and optionally continuing to monitor the output
metrics and/or alter
the process conditions (e.g., implementing a closed-loop control of the
process conditions
based on the output metrics).
[0084] The method can optionally include determining the target output
metric(s), which
functions to determine which parameter(s) or aspect(s) to target (e.g., key
parameter for a given
application or downstream system). One or more target output metrics can be
selected for a
given process. The target output metric can be: the output metric associated
with (e.g.,
predetermined for, dictated by, etc.) an application (e.g., applications
described above, such as
Fischer-Tropsch); randomly selected; empirically determined (e.g., through
iterative testing
and
monitoring of downstream application p erform an ce); optimized (e.g. ,
based on
downstream application operation parameters, reactor operation parameters,
etc.); specified by
a user; and/or otherwise determined.
[0085] The method can optionally include determining the target value for the
target output
metric, which functions to identify a value (from a range of values) to
target. In some variations,
the target value can be a maximum or minimum value (e.g., maximum or minimum
practically
achievable value, theoretical maximum or minimum, etc.). However, the target
value can
additionally or alternatively not be an extremal value (e.g., can be an
intermediate value or
range of values between the maximum and minimum). The target value can be: a
value
associated with the application (e.g., predetermined, pre-associated);
randomly selected;
empirically determined (e.g., through iterative target value selection,
monitoring of
downstream application performance, and target value adjustment based on the
application
performance); optimized (e.g., based on downstream application operation
parameters, reactor
operation parameters, etc.); or otherwise determined. However, the target
value can be any
other suitable value and can be determined in any suitable manner.
100861 Under some conditions, the method may achieve carbon dioxide conversion
(e.g., CO
fractional yield) greater than 95% (e.g., up to 100%), such as wherein the
system, run under
such conditions, can achieve at least the threshold conversion metric.
However, the method can
additionally or alternatively include achieving carbon dioxide conversion
greater than 50%,
60%, 70%, 80%, 90%; between 10%-100%, such as 10-40, 30-50, 40-60, 50-70, 60-
75, 70-
14
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
85, 80-95, 90-95, 92-98, and/or 95-100%; and/or any other suitable carbon
dioxide
conversion.
[0087] The method optionally includes providing the reactor products (or a
subset thereof) to
a downstream consumer of the products (e.g., as described above, such as
regarding
applications of the reactor output; as described below, such as in the example
section; etc.).
The method can optionally include altering the reactor products after they are
produced (e.g.,
before feeding the altered products to a downstream consumer, etc.). Altering
the reactor
products can optionally include purifying the products (e.g., removing
impurities, such as SOx
and/or NOR, from a reactor output stream). Altering the reactor products can
additionally or
alternatively include mixing additional gasses (and/or other substances) into
a reactor output
stream (and/or input stream), such as to achieve a desired output metric. In
one variation, if the
CO:Hz ratio of the reactor output differs from a desired value, the ratio can
be adjusted by
mixing the reactor output with other gasses (e.g., substantially pure CO
and/or Hz; another
mixture of CO and Hz, such as previously produced and stored outputs of the
reactor, the output
of a second reactor, outputs and/or waste gasses of other systems, etc.). For
example, the CO.H2
ratio of the output stream (and/or gasses in any other portion of the reactor)
can be monitored
(e.g., continuously during reactor production), and deviations from the
desired value can be
compensated for by mixing in other gasses (e.g., adding CO and/or a CO-rich
mixture to
increase the ratio, adding H2 and/or an Hz-rich mixture to decrease the
ratio). This example
may also include altering the process conditions in order to correct the
reactor outputs (e.g., as
described above regarding closed-loop control). In a second variation, in
which an external gas
supply (e.g., the outputs and/or waste gasses of one or more other system,
such as a steel mill)
is fed to a downstream consumer (e.g., a gas fermenter), the reactor products
are used to alter
the CCP:H2 ratio (e.g., CO:f12 ratio) of the external gas supply (e.g., if the
CCP:f12 ratio of the
external gas supply differs from a desired value, mixing in the reactor
products to achieve the
desired value). For example, based on the deviation of the external gas supply
from the desired
value, the process conditions can be controlled to alter the CO:Hz ratio of
the reactor products
(e.g., increasing the ratio in response to a CO-poor external gas supply,
decreasing the ratio in
response to a CO-rich external gas supply), and/or the quantity of reactor
product mixed into
the external gas supply can be controlled (e.g., to achieve the desired
value). However, the
reactor output stream can additionally or alternatively be altered in any
other suitable manner
or can be used without alteration.
[0088] In some examples, the method includes determining one or more metrics
(e.g.,
operation metrics) associated with the one or more upstream and/or downstream
elements of
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
the system (e.g., downstream reactors, upstream inputs, etc.). Such operation
metrics can
include, for example: reactor conditions such as temperature, pressure, etc.;
downstream
reactor and/or upstream source output metrics such as output quantity,
composition, purity,
etc.; metrics associated with other inputs for the downstream reactor(s), such
as input quantity,
composition, purity, etc.; reactor efficiency metrics; and/or any other
suitable metrics. In such
examples, the method may include altering carbon dioxide reactor operation
based on the
metrics (e.g., to improve and/or maintain operation of the downstream reactor;
to improve
and/or maintain operation of the carbon dioxide reactor, such as to
accommodate changes in
the upstream source; to improve and/or maintain a target output metric, such
as HCR or
reduction product concentration, such as given a varying carbon dioxide
source; etc.), such as
by altering the HCR of the carbon dioxide reactor output. However, the method
can
additionally or alternatively include determining any other suitable metrics
and/or acting (e.g.,
based on the metrics) in any other suitable manner.
Process conditions
[0089] The process conditions can include, e.g., input carbon dioxide flow
rate and/or pressure,
input gas hydration, current density, voltage (e.g., maintained between about
1.5 V and 3 V,
additionally or alternatively operated at less than about 1.5 V. between about
2 V-2.5 V.
between about 2 V-4 V, greater than about 4 V, and/or at any other suitable
voltage(s)), and/or
temperature. The process conditions can additionally or alternatively include
system
configurations, such as gas diffusion layer aspects, catalyst aspects, flow
field aspects, and/or
PEM aspects. However, any other suitable process condition can be controlled
or targeted. The
process condition can be uncontrolled (e.g., dictated by an upstream system),
controlled to meet
a target value (e.g., wherein the target value can be determined based on the
application
receiving the reactor output, the instantaneous or anticipated reactor
operation parameters, or
otherwise determined), or otherwise determined.
100901 The process conditions may include a pressure (e.g., input gas
pressure, reactor
pressure, etc.) greater than atmospheric pressure (e.g., within and/or greater
than a threshold
pressure range, such as about 1-5, about 5-10, about 10-20, about 20-50, about
50-100, about
100-300, about 300-1000, about 1-10, about 5-50, about 10-100, about 20-500,
and/or
greater than about 1000 atm, about 14-50, about 50-150, about 100-300, about
200-500, about
500-1000, about 750-1500, about 1000-3000, about 3000-10,000, about 10,000-
20,000,
and/or greater than about 20,000 psi, etc.) and/or greater than pressures
typically feasible in
electrolyzers other than gas-phase electrolyzers, but can additionally or
alternatively include
pressures substantially equal to 1 atmosphere, less than about 1 atmosphere,
and/or any other
16
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
suitable pressures. The process conditions may include a temperature (e.g.,
reactor
temperature) greater than typical room temperature (e.g., within and/or
greater than a threshold
temperature range, such as about 25-50, about 40-60, about 50-100, about 50-
75, about 70-
100, and/or greater than about 100 C, etc.) and/or greater than temperatures
typically feasible
in electrolyzers other than gas-phase electrolyzers, but can additionally or
alternatively include
temperatures substantially equal to room temperature (e.g., about 20-30 C),
less than room
temperature, and/or any other suitable temperatures. However, the process
conditions can
additionally or alternatively include any other suitable process conditions.
[0091] A higher carbon dioxide flow rate can lead to increased production of
CCPs such as
CO (e.g., due to greater availability of carbon dioxide for reduction), and
thus an increased
CCP:H2 ratio (and correspondingly, lower carbon dioxide flow rate can lead to
decreased CCP
production and CCP:H2 ratio). In some embodiments, higher carbon dioxide flow
rate can also
result in reduced carbon dioxide conversion efficiency, thereby diluting the
output stream (e.g.,
syngas output) with unreacted carbon dioxide. For example, carbon dioxide flow
rate (e.g.,
measured at the reactor inlet) can be maintained at one or more values in the
range of about
0.1-1000 sccm/cm2 (e.g., about 0.1-1, about 1-10, about 10-100, and/or about
100-1000
sccm/cm2).
[0092] In a first specific example of control based on input gas flow rate,
reactor configuration
A with a triple serpentine flow field is used, reactor pressure is
substantially maintained at 120
psi, current density is substantially maintained at 500 mA/cm2, and reactor
temperature is
substantially maintained at 30 C. In this specific example, substantially
pure carbon dioxide
gas is input at various flow rates, wherein input flow rates (e.g., measured
at the reactor inlet)
of 12 sccm/cm2, 20 sccm/cm2, and 40 sccm/cm2 result in CO:H2 ratios of
approximately 1:1,
2:1.1, and 4:1, respectively.
[0093] In a second specific example of control based on input gas flow rate,
reactor
configuration A with a serpentine flow field is used, reactor pressure is
substantially maintained
at 130 psi, and current density is substantially maintained at 500 mA/cm2. In
this specific
example, substantially pure carbon dioxide gas input at a 40 sccm/cm2 flow
rate results in a
CO:H2 ratio of approximately 8:2, whereas a 12 sccm/cm2 flow rate results in
an approximately
1:1 ratio.
100941 Higher carbon dioxide pressure can lead to increased CCP fractional
yield and/or
CCP:H2 ratio (and correspondingly, lower carbon dioxide pressure can lead to
decreased CCP
fractional yield and/or CCP:H2 ratio). First, increased carbon dioxide
pressure can result in
greater availability of carbon dioxide for reduction, thereby increasing the
total production of
17
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
CCPs. Second, higher pressure at the catalyst can reduce water ingress to the
catalyst (e.g.,
from the cathode), thereby lowering the amount of water available for
reduction, which can
directly increase the CCP:H2 ratio and/or can reduce competition for catalyst
reaction sites
and/or reaction energy (e.g., thereby favoring reduction of carbon dioxide).
Thus, in some
embodiments (e.g., in which high CCP fractional yield and/or CCP:H2 ratio is
desired), high
reactor pressure (e.g., greater than 100 psi, up to but no greater than a
carbon dioxide phase
transition pressure, such as a critical pressure of 1070 psi, etc.) may be
employed. For example,
reactor pressure can be maintained at one or more values in the range of about
1-1100 psi (e.g.,
about 1-10, about 10-100, about 100-300, about 200-600, and/or about 500-1100
psi), and/or
at any other suitable pressure.
[0095] In a specific example of control based on reactor pressure, reactor
configuration A with
a single serpentine flow field is used, substantially pure carbon dioxide gas
is input at about
100 sccm/cm2, current density is substantially maintained at about 150 mA/cm2,
and reactor
temperature is substantially maintained at about 20 C. In this specific
example, reactor
pressure is substantially maintained at various pressures, wherein reactor
pressures of 25, 50,
75, and 100 psi result in CO:H2 ratios of approximately 3:2, 2.4:1, 3:1, and
5:1 and CO
fractional yields of approximately 59%, 69%, 75%, and 84%, respectively.
[0096] Increasing input gas hydration can lead to increased water reduction
(e.g., due to greater
availability of water for reduction), and thus to a decreased CCP:H2 ratio.
For a substantially
pure carbon dioxide input, only small amounts of water reach the catalyst
(coming almost
exclusively from the cathode side of the reactor), leading to a higher CCP:H2
ratio. In contrast,
when hydrated input gas is used, significant amounts of water from the input
gas can reach the
catalyst and react. For example, input gas hydration (e.g., proportion of
water vapor in the input
gas) can be maintained at one or more values in the range of 0% (e.g.,
substantially pure carbon
dioxide, substantially unhydrated input gas) to 100% (e.g., 0-1, 1-3, 3-5, 5-
7, 7-10, 10-15,
15-25, 25-50, 50-75, and/or 75-100 percent).
[0097] In a specific example of control based on input gas hydration, reactor
configuration A
with a single serpentine flow field is used, current density is substantially
maintained at 50
mA/cm2, reactor pressure is substantially maintained at 12 psi, and reactor
temperature is
substantially maintained at 20 C. In this specific example, carbon dioxide
gas with varying
amounts of hydration is input at 100 sccm/cm2, wherein pure carbon dioxide
input gas results
in a CO:H2 ratio of approximately 3:2, input gas with 12.2% hydration results
in a CO:H2 ratio
of approximately 1:5.67, and intermediate hydration amounts result in CO:H2
ratios between
these two values.
18
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
100981 Reactors can exhibit different regimes of CCP and H2 production with
respect to current
density. In an idealized reactor, at low current densities, no water reduction
occurs and all
current goes to reducing carbon dioxide, resulting in a substantially linear
dependence of CO
production on current and substantially no H2 production; whereas at higher
current densities,
additional current (e.g., above a threshold current at which substantially all
carbon dioxide is
already being consumed) is used to reduce water, resulting in a substantially
linear dependence
of H2 production on the additional current and substantially constant CO
production (e.g., as
shown in Figure 3A). In many typical reactors, these idealities are loosened,
but the two general
regimes are still exhibited: CO production increases much faster than H2
production in the low
current density regime, then approaches a plateau in the higher current
density regime while
H2 production increases more rapidly (e.g., as shown in Figure 3B). The method
can include
controlling CO and/or H2 production (e.g., controlling CO:H2 ratio) by
operating at any or all
of a wide range of current densities (e.g., controlling the reactor operation
within the low and/or
high current density regime, etc.). In some embodiments, the use of gas phase
input carbon
dioxide can enable relatively high current densities (whereas reactors using
aqueous carbon
dioxide may be limited to current densities of tens of mA/cm2 or less). For
example, the method
can include operating at current densities between about 1 mA/cm2 and 100
A/cm2 (e.g., about
1-75 mA/cm2, about 50-100 mA/cm2, about 100-200 mA/cm2, about 200-500 mA/cm2,
about
500-1000 mA/cm2, about 50-1000 mA/cm2, about 0.5-10 A/cm2, about 1-2 A/cm2,
about 2-
5 A/cm2, about 5-10 A/cm2, about 5-100 A/cm2, about 10-20 A/cm2, about 20-50
A/cm2,
about 50-100 A/cm2, etc.; at, above, or below a threshold value such as about
50 mA/cm2,
about 65 mA/cm2, about 80 mA/cm2, about 90 mA/cm2, about 100 mA/cm2, about 110
mA/cm2, about 120 mA/cm2, about 130 mA/cm2, about 140 mA/cm2, about 150
mA/cm2, about
200 mA/cm2, about 300 mA/cm2, about 500 mA/cm2, about 700 mA/cm2, about 1000
mA/cm2,
about 1500 mA/cm2, etc.) and/or at any other suitable current densities.
100991 In some embodiments, increased reactor temperature can result in a
reduced CO:H2
ratio (e.g., due to increased ingress of water from the cathode, increased
reactivity of water,
etc.). The method can include controlling reactor temperature within an
operation range, such
as a range between a minimum temperature (e.g., a water freezing temperature
such as 0 C)
and a maximum temperature (e.g., about 40 C, about 50 C, about 60 C, about
75 C, etc.; a
water boiling temperature such as 100 C), in order to control CO:H2 ratio
and/or any other
suitable output metrics.
[0100] In a specific example of control based on reactor temperature, reactor
configuration A
with a quadruple serpentine flow field is used, substantially pure carbon
dioxide gas is input at
19
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
70 sccm/cm2, current density is substantially maintained at 150 mA/cm2, and
reactor pressure
is substantially maintained at 100 psi. In this specific example, reactor
temperature is
substantially maintained at various temperatures, wherein reactor temperatures
of 26.7, 35,
38.7, and 41.9 C result in CO:H2 ratios of approximately 1:0.4, 2:1, 1:1.8,
and 1:3,
respectively.
[0101] Characteristics of the gas diffusion layer (GDL) can additionally or
alternatively be
used to affect CCP and/or H2 production. For example, the GDL hydrophobicity
can alter H2
production (e.g., by affecting water transport), wherein a more hydrophilic
GDL favors H2
production (thereby reducing the CCP:H2 ratio) and a more hydrophobic GDL
inhibits H2
production (thereby increasing the CCP:H2 ratio). Other GDL characteristics,
such as thickness
and/or pore size, can also be used to alter the reactor output.
[0102] Characteristics of the membrane (e.g., polymer electrolyte membrane)
can additionally
or alternatively be used to affect CCP and/or H2 production. In examples, an
anion exchange
membrane, which favors CCP production, can be used to achieve high CCP:H2
ratios, a cation
exchange membrane, which favors H2 production, can be used to achieve low
CCP:H2 ratios,
and hybrid membranes (e.g., enabling both anion and cation transport)
exhibiting various anion
and cation transport characteristics (e.g., mobilities) can be used to achieve
various
intermediate ratios (e.g., membranes favoring anion transport for higher
ratios, membranes
favoring cation transport for lower ratios).
[0103] Characteristics of the catalysts (e.g., particle size, catalyst
species, etc.) can additionally
or alternatively be used to affect CCP and/or H2 production. For example,
larger catalyst
particles can result in poor carbon dioxide transport, thereby inhibiting CCP
production and
reducing the CCP:H2 ratio, whereas smaller catalyst particles can favor CCP
production,
thereby increasing the ratio. The relative number of active sites with high
turnover frequency
for hydrogen evolution (-hydrogen sites") and those with high turnover
frequency for carbon
dioxide reduction ("carbon dioxide sites") can additionally or alternatively
be dependent on
catalyst particle size: larger catalyst particles typically have a higher
ratio of hydrogen sites to
carbon dioxide sites, favoring H2 production, whereas smaller catalyst
particles typically have
a lower ratio, favoring CO production. The catalyst type (e.g., catalyst
species) can additionally
or alternatively be used to control the reactor output, such as by employing a
mixture of one or
more catalyst materials, wherein a first set of catalyst materials (e.g.,
gold) favor carbon dioxide
reduction and a second set of catalyst materials (e.g., platinum) favor water
reduction. In
examples, a substantially pure gold catalyst can be used to achieve high
CCP:H2 ratios, a
substantially pure platinum catalyst can be used to achieve low CCP:H2 ratios,
and gold-
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
platinum mixtures (e.g., alloyed particles, mixtures of gold particles and
platinum particles,
etc.) of varying composition can be used to achieve various intermediate
ratios (e.g., more gold
for higher ratios, more platinum for lower ratios). The catalyst can
additionally or alternatively
include V. Cr, Mn, Fe, Co, Ni, Cu, Sn, Zr, Nb, Mo, Ru, Rh, Pd, Ag, Cd, Hf, Ta,
W, Re, Ir, Hg,
Al, Si, In, Ga, Tl, Pb, Bi, Sb, Te, Sm, Tb, Ce, Nd, and/or combinations
thereof The catalyst
can additionally or alternatively be associated with (e.g., attached to,
supported by, embedded
in, adjacent, in contact with, etc.) one or more support structures (e.g.,
support particles, support
matrix, etc.), which may be conductive support structures such as carbon,
boron-doped
diamond, and/or fluorine-doped tin oxide. However, the catalyst can
additionally or
alternatively include any other suitable materials.
[0104] In a specific example of control based on catalyst particle size,
variations of reactor
configuration A with two catalyst particle sizes are used, both with reactor
temperature
substantially maintained at 30 C, reactor pressure substantially maintained
at 100 psi, an
interdigitated flow field, substantially pure carbon dioxide gas input at 10
sccm/cm2, and
current density substantially maintained at 500 mA/cm2. The first set of
catalyst particles have
a characteristic size of 4 nm (as in the standard reactor configuration A),
resulting in an HCR
of 1:1.6 and a voltage of 3.8 V. The second set of catalyst particles have a
characteristic size
of 20 nm, resulting in an HCR of 1:2.8 and a voltage of 4.2 V.
[0105] Characteristics of reactor cell compression can additionally or
alternatively be used to
affect CCP and/or H2 production. In a specific example of control based on
reactor cell
compression, reactor configuration A is used with two different gasket
thicknesses (resulting
in greater compression for a larger gasket thickness), both with reactor
temperature
substantially maintained at 30 C, reactor pressure substantially maintained
at 100 psi, a triple
serpentine flow field, substantially pure carbon dioxide gas input at 40
sccm/cm2, and current
density substantially maintained at 500 mA/cm2. The first gasket is 0.012
inches thick, resulting
in an HCR of 1:4 and a voltage of 3.6 V. The second gasket is 0.010 inches
thick, resulting in
an HCR of 1:10.1 and a voltage of 3.8 V.
[0106] Characteristics of the flow field can additionally or alternatively be
used to affect CCP
and/or H2 production. In a first specific example of control based on flow
field characteristics,
reactor configuration A is used under two different sets of process
conditions, both with reactor
temperature substantially maintained at 30 C and reactor pressure
substantially maintained at
120 psi. In the first set of conditions, an interdigitated flow field is used,
substantially pure
carbon dioxide gas is input at 10 sccm/cm2, and current density is
substantially maintained at
160 mA/cm2, resulting in a CO:H2 ratio of 1.6:1. In the second set of
conditions, a quadruple
21
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
serpentine flow field is used, substantially pure carbon dioxide gas is input
at 40 sccm/cm2, and
current density is substantially maintained at 120 mA/cm2, resulting in a
CO:H2 ratio of 18.5:1.
[0107] In a second specific example of control based on flow field
characteristics, reactor
configuration A is used under two different sets of process conditions, both
with reactor
temperature substantially maintained at 30 C, reactor pressure substantially
maintained at 100
psi, substantially pure carbon dioxide gas input at 40 sccm/cm2, and current
density is
substantially maintained at 500 mA/cm2. In the first set of conditions, an
interdigitated flow
field is used and a voltage of 3.6 V is substantially maintained, resulting in
a CO:H2 ratio of
1.6:1. In the second set of conditions, a triple serpentine flow field is used
and a voltage of 3.8
V is substantially maintained, resulting in a CO:H2 ratio of 10.1:1.
[0108] However, any other suitable flow field can additionally or
alternatively be employed to
control the reactor outputs, the process conditions can additionally or
alternatively include any
other suitable reactor conditions, and the method can additionally or
alternatively include
controlling the reactor output in any suitable manner.
Impurity tolerance
[0109] In some embodiments, such as embodiments in which the reactor is run at
a high
pressure and/or the catalyst is held at low voltage (e.g., negative voltage
relative to the anode),
the system and/or method may achieve high tolerance to impurities and/or
dilute carbon dioxide
inputs (e.g., as compared to other carbon dioxide reactors), such as tolerance
to poisoning by
impurities in the reactor input(s) and/or to inputs diluted by species such as
methane, CO, 02,
and/or N2. For example, the method can include determining target process
conditions (e.g.,
reactor configuration such as PEM type, high target reactor pressure, etc.) to
achieve impurity
and/or dilute input tolerance (e.g., always selecting such process conditions;
selecting such
process conditions in response to a current and/or anticipated state of the
reactor input, such as
an impure and/or dilute state; etc.). These impurities can include species
typically present in
reactor input streams (e.g., products of coal and/or natural gas combustion,
such as outputs
from coal- or natural gas-fired power plants), such as SO x and/or NOR, and/or
can include any
other impurities such as ammonia, hydrogen sulfide, and mercury. In one
example, the system
and/or method are capable of functioning effectively using input streams
including up to 4%
CO, 6% 02, 10% N2, 800 ppm NOx, and/or 100 ppm SO,, with a sum of CO, 02, and
N2
impurities, e.g., no greater than 10%.
[0110] In a specific example of dilute input tolerance, reactor configuration
A with a single
serpentine flow field is used, current density is substantially maintained at
160 mA/cm2, reactor
pressure is substantially maintained at 110 psi, reactor temperature is
substantially maintained
22
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
at 20 C, and carbon dioxide-containing gasses with various levels of dilution
in methane or
nitrogen are input at 200 sccm/cm2. In this specific example, reactor
performance is highly
tolerant of methane dilution up to at least 50% methane, wherein methane
concentrations of
0%, 25%, and 50% result in CO:H2 ratios between 9.5:1 and 8.5:1 and CO
fractional yields
greater than 90%. More significant performance reduction is observed using 75%
methane,
with a reduction in CO fractional yield to approximately 84%. In this specific
example, similar
tolerance to nitrogen dilution is observed, wherein nitrogen concentrations of
0%, 25%, 50%,
and 75% result in CO:H2 ratios between 9:1 and 8:1, and nitrogen
concentrations up to 50%
result in CO fractional yields greater than 85% (with 75% nitrogen
concentration resulting in
a CO fractional yield of approximately 81%).
[0111] In a specific example of impurity tolerance, reactor configuration A
with a single
serpentine flow field is used, current density is substantially maintained at
150 mA/cm2, reactor
pressure is substantially maintained at 100 psi, reactor temperature is
substantially maintained
between 20 C and 25 C, and carbon dioxide-containing gasses with various
impurities are
input at 100 sccm/cm2. In this specific example, reactor output metrics (e.g ,
CO fractional
yield) under the various impurity conditions are compared to baseline reactor
performance
under the same conditions but using a substantially impurity-free carbon
dioxide input. In this
specific example, reactor performance was shown not to deviate significantly
from the baseline
performance for CO concentrations of 4% or less, for NO concentrations of 800
ppm or less,
for SO, concentrations of 120 ppm or less, or for oxygen concentrations of 6%
or less.
[0112] However, the system and/or method can additionally or alternatively
exhibit any
suitable tolerance to impure and/or dilute inputs or exhibit no such
tolerance.
[0113] In certain embodiments, an impurity or multiple impurities pass through
the carbon
oxide reduction reactor to an output stream where they are (a) separated
upstream of another
chemical reactor, and/or (b) passed into another chemical reactor. In
embodiments where
impurities in an output stream are passed to another chemical reactor,
impurities may be used
by the other reactor in the chemical manipulation of that process. For
example, hydrogen
sulfide or other sulfur-containing impurity may be employed by microbial
species in a
downstream bioreactor.
System configuration selection
101141 One or more system configurations may be employed based on output HCR
considerations, such as based on a desired output HCR (e.g., given a
particular set of process
conditions and/or a range of acceptable process conditions) and/or HCR range.
[0115] In some embodiments, this includes: at a first reactor (e.g.,
electrolyzer, such as a gas-
23
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
phase electrolyzer), accepting an input including a carbon oxide and
electrochemically
producing a first reduction product (e.g., including molecular hydrogen and/or
one or more
CCPs other than the carbon oxide input at a first HCR) from the input (e.g.,
under a first set of
process conditions). The choice of the first reactor design and its operating
conditions may
include determining a desired HCR and/or HCR range (e a based on downstream
reactor
metrics, market price metrics, efficiency metrics, and/or any other suitable
metrics) and
selecting a system configuration (e.g., for a second reactor) based on the
first HCR and/or the
desired HCR (e.g., such that the second reactor will or can output a reduction
product with an
HCR closer to the desired HCR relative to the first HCR, optionally
substantially under the
first set of process conditions but additionally or alternatively under any
other suitable process
conditions). For example, the configuration for the second reactor can be
selected such that the
second reactor would, under conditions substantially identical to those of the
first reactor (e.g.,
while accepting the input under the first set of process conditions), produce
a second reduction
product from the input, wherein the second reduction product includes
molecular hydrogen and
the same CCSs as the first reduction product (e.g., includes substantially all
species present in
the first reduction product), wherein the second reduction product defines a
second HCR
substantially different from the first HCR, wherein the second HCR may be
closer to the
desired HCR than the first HCR. Substantial difference between the first HCR
and second
HCR, for this example and/or any other embodiment described herein, can
include the second
HCR: being closer to the desired HCR than the first HCR; differing from the
first HCR (e.g.,
being greater or lesser than the first HCR) by at least 1%, 5%, 10%, 30%, 40%,
50%, 60%,
70%, 80%, 90%, 0.5-5%, 2-10%, 5-25%, 20-50%, 40-80%, and/or 75-100%; and/or
otherwise differing from the first HCR.
[0116] In some embodiments, selecting system configurations can include
selecting one or
more aspects of a PEM, such as to alter the output HCR. Such selection can
include selecting
membrane compositions (e.g., different polymer species) and/or
microstructures, selecting
membrane layer thicknesses, and/or selecting any other suitable aspects of the
PEM. In some
examples, such selection includes selecting a thickness of an anion exchange
membrane and/or
proton exchange membrane (e.g., wherein a bipolar PEM with more AEM will tend
to produce
a lower output HCR than one with more proton exchange membrane). In a first
specific
example, selecting a thinner AEM (e.g., thinner than a reference AEM thickness
such as a
thickness of the first reactor AEM, thinner than an optimized AEM thickness
substantially
corresponding to optimal CCP production, etc.) can result in a reactor
configured to produce a
higher output HCR, whereas selecting a thicker AEM (e.g., thicker than the
reference AEM
24
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
thickness but optionally no thicker than the optimized AEM thickness) can
result in a reactor
configured to produce a lower output HCR.
[0117] Selecting system configurations can additionally or alternatively
include selecting one
or more aspects of reactor catalyst(s) (e.g., reduction catalyst, oxidation
catalyst), such as to
alter the output HCR. In some variations, selecting reactor catalyst aspects
can include selecting
a catalyst layer thickness (e.g., wherein a thicker reduction catalyst will
tend to produce a higher
HCR). In one example, selecting a thicker reduction catalyst layer (e.g.,
thicker than a reference
reduction catalyst layer thickness such as a thickness of the first reactor
reduction catalyst layer,
thicker than an optimized reduction catalyst layer thickness substantially
corresponding to
optimal CCP production, etc.) can result in a reactor configured to produce a
higher output
HCR, whereas selecting a thinner reduction catalyst layer (e.g., thinner than
the reference
reduction catalyst layer thickness but optionally no thinner than the
optimized reduction
catalyst layer thickness) can result in a reactor configured to produce a
lower output HCR.
[0118] Selecting reactor catalyst aspects can additionally or alternatively
include (e.g., in
embodiments in which a catalyst layer includes catalyst particles, such as
nanoparticles,
defining a porous network) selecting a catalyst porosity (e.g., wherein a more
porous reduction
catalyst network will tend to produce a lower HCR). In one example, selecting
a less porous
reduction catalyst network (e.g., less porous than a reference reduction
catalyst such as a
porosity of the first reactor reduction catalyst network, less porous than an
optimized reduction
catalyst substantially corresponding to optimal CCP production, etc.) can
result in a reactor
configured to produce a higher output HCR, whereas selecting a more porous
reduction catalyst
(e.g., more porous than the reference reduction catalyst but optionally no
more porous than the
optimized reduction catalyst) can result in a reactor configured to produce a
lower output HCR.
[0119] Selecting reactor catalyst aspects can additionally or alternatively
include (e.g., in
embodiments in which a catalyst layer includes catalyst particles, such as
nanoparticles, and
one or more polymer electrolytes, such as wherein the catalyst particles
define a porous
network that contains the polymer electrolyte and/or are mixed into a medium
including the
polymer electrolyte) selecting a catalyst-to-polymer electrolyte ratio (CPR)
(e.g., wherein a
higher reduction catalyst CPR will tend to produce a higher HCR), such as by
selecting a degree
of polymer electrolyte loading into a porous reduction catalyst network. In
one example,
selecting a higher reduction catalyst CPR (e.g., higher CPR than a reference
reduction catalyst
CPR such as a CPR of the first reactor reduction catalyst network, higher CPR
than an
optimized reduction catalyst substantially corresponding to optimal CCP
production, etc.) can
result in a reactor configured to produce a higher output HCR, whereas
selecting a lower CPR
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
reduction catalyst (e.g., lower CPR than the reference reduction catalyst but
optionally no lower
than the optimized reduction catalyst CPR) can result in a reactor configured
to produce a lower
output HCR.
[0120] Selecting reactor catalyst aspects can additionally or alternatively
include (e.g., in
embodiments in which a catalyst layer includes catalyst particles, such as
nanoparticles)
selecting a characteristic catalyst particle size (e.g., wherein a larger
particle size will tend to
produce a higher HCR). In one example, selecting a larger reduction catalyst
particle size (e.g.,
larger than the particles of a reference reduction catalyst such as the first
reactor reduction
catalyst, larger than an optimized reduction catalyst substantially
corresponding to optimal
CCP production, etc.) can result in a reactor configured to produce a higher
output HCR,
whereas selecting a smaller reduction catalyst particle size (e.g., smaller
than the particles of
the reference reduction catalyst but, e.g., no smaller than the particles of
the optimized
reduction catalyst) can result in a reactor configured to produce a lower
output HCR. However,
the method can additionally or alternatively include selecting any other
suitable reactor catalyst
aspects.
[0121] The method can additionally or alternatively include selecting a
reactor cell
compression (e.g., wherein lower compression will tend to result in higher HCR
and higher
compression will tend to result in lower HCR), a flow field, and/or any other
suitable aspects
of the system.
[0122] U.S. Provisional Application serial number 62/619,996, filed on 22-JAN-
2018, U.S.
Provisional Application serial number 62/620,109, filed on 22-JAN-2018, and
U.S. Provisional
Application serial number 62/685,771, filed on 15-JUN-2018, are each
incorporated herein by
reference in its entirety.
[0123] The electrolyzer design and operating conditions can be tuned for
particular
applications, and for producing a cathode output having specified
compositions. In some
implementations, one or more general principles may be applied to operate in a
way that
produces a required output stream composition.
[0124] 1. Restrict carbon dioxide reactant availability at the cathode active
sites and/or increase
current density at the cathode. These operating condition ranges tend to
produce the following
results: (a) initially, upon decreasing the carbon dioxide reactant
availability and/or increasing
the current density, the fraction of CO2 converted to CO increases (i.e.,
CO:CO2 in the output
stream increases); (b) at some point, upon further decreasing the carbon
dioxide reactant
availability and/or increasing the current density, the hydrogen ion reduction
reaction becomes
more pronounced (i.e., H2:CO increases). Electrolyzers that can operate with
relatively little
26
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
carbon dioxide input/availability may have flow fields or gas diffusion
components that restrict
carbon dioxide from reaching active sites on the electrolyzer cathode. In
certain embodiments,
flow field designs that are not interdigitated, and such flow field designs
that have long paths
such as serpentine paths between the source of CO2 and the cathode result in
higher ratios of
CO:H2. Interdigitated flow field forces input gas (carbon oxide) to flow
through the gas
diffusion layer before exiting at a different location on the flow field. Non-
interdigitated
designs have long continuous paths for the carbon oxide feed gas to flow into
and out of the
cathode. Channels on the inlet side are spaced from the channels on the outlet
side. In certain
embodiments, gas diffusion electrodes that are relatively thick restrict CO2
mass transport to
the cathode active sites and therefor tend to increase the ratio of CO:C 02
and/or H2: CO.
[0125] 2. Make hydrogen ions relatively more available at the cathode. Making
hydrogen ions
relatively more available at the cathode may produce a cathode product stream
with a relatively
high ratio of H2:CO. Electrolyzers configured in a way that provide a
relatively hydrogen rich
product may employ designs that (a) starve the cathode of carbon dioxide
reactant (as described
in 1), (b) permit a relatively high flux of hydrogen ions to be transported
from the anode, where
they are generated, to the cathode, and/or (c) operate at a relatively high
cell temperature.
Electrolyzers that can operate with a relatively high flux of hydrogen ions to
the cathode may
have MEAs with cation conducting polymers and/or mixed ion conducting polymers
at the
cathode. Alternatively or additionally, in MEAs including a cathode buffer
layer, the layer is
designed to be relatively thin and/or have a relatively high hydrogen ion
transference number.
[0126] 3. Make hydrogen ions less available at the cathode. Making hydrogen
ions relatively
more less at the cathode may produce a cathode product stream with relatively
high ratios of
CO:H2. Electrolyzers configured in a way that provides a relatively hydrogen
poor product
may employ designs that (a) provide the cathode with surplus carbon dioxide
reactant for a
given current density, (b) contain MEA designs that prevent hydrogen ions from
reaching the
cathode, and/or (c) operate at a relatively low cell temperature.
High CO2 reduction product to CO2 ratio operating parameter regime
[0127] In certain embodiments, an electrolyzer is configured to produce, and
when operating
actually produces, an output stream having a CO:CO2 molar ratio of at least
about 1:1 or at
least about 1:2 or at least about 1:3.
A high CO output stream may alternatively be
characterized as having a CO concentration of at least about 25 mole %, or at
least about 33
mole %, or at least about 50 mole %.
[0128] In certain embodiments, this high carbon monoxide output concentration
is obtained by
operating a carbon dioxide electrolyzer in a manner that produces any one of
or any
27
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
combination of the following operating conditions:
a current density of at least about 300 mA/cm2, at the cathode,
a CO2 stoichiometric flow rate (as described elsewhere herein) of at most
about 4, or at most
about 2.5, or at most about 1.5
a temperature of at most about 80 C or at most about 65,
a pressure range of about 75 to 400 psig,
an anode water composition of about 0.1 to 50mM bicarbonate salt, and
an anode water pH of at least about 1.
[0129] In certain embodiments, the electrolyzer may be built to favor high
CO:CO2 molar
ratios or concentrations, as defined here, by using a carbon dioxide
electrolyzer having any one
of or any combination of the following properties:
relatively small nanoparticle cathode catalysts (e.g., having largest
dimensions of, on average,
about 0.1-15nm),
gold as the cathode catalyst material,
a cathode catalyst layer thickness of about S-20um,
a cathode gas diffusion layer (GDL) with a microporous layer (MPL),
a cathode GDL with PTFE present at about 1-20 wt%, or about 1-10 wt%, or about
1-5 wt%,
a GDL that has a thickness of at least about 200um
I bipolar MEA having an anion-exchange cathode buffer layer having a thickness
of at least
about Sum, and
a cathode flow field having parallel and/or serpentine flow paths.
High reduction product (H2+CO) to CO2 ratio operating parameter regime
[0130] In certain embodiments, an electrolyzer is configured to produce, and
in operation
actually produces, an output stream having a (H2+CO):CO2 molar ratio of at
least about 2:1 or
at least about 1:2 or at least about 1:3.
101311 In certain embodiments, this high reduction product output
concentration is obtained
by operating a carbon dioxide electrolyzer in a manner that produces any one
of or any
combination of the following operating conditions:
a current density of at least about 300 mA/cm2,
a CO2 stoichiometric flow rate of at most about 4, or at most about 2.5, or at
most about 1.5
a temperature of at most about 125 C,
a pressure of at most about 800 psi,
anode water composition of 0 to about 500mM bicarbonate salt, and
an anode water pH of about 0-15.
28
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
[0132] In certain embodiments, the electrolyzer may be built to favor high (CO-
F1-12):CO2 molar
ratios or concentrations, as defined here, by using a carbon dioxide
electrolyzer having any one
of or any combination of the following properties:
nanoparticle cathode catalysts (e.g., having a largest dimension, on average,
of about 0.1-
1000nm),
a transition metal as a cathode catalyst material,
a cathode catalyst layer thickness of about 0.1-100um,
a cathode gas diffusion layer with or without a microporous layer (MPL),
a GDL with about 0-70wt% PTFE,
a GDL that is about 10-1000 urn thick, and
a bipolar MEA having an anion-exchange cathode buffer layer that is about 0-
100um thick.
Hydrogen rich product stream operating parameter regime
101331 In certain embodiments, a carbon dioxide electrolyzer is configured to
produce, and
when operating actually produces, an output stream having Hz:CO in a molar
ratio of at least
about 1:1.
[0134] In certain embodiments, such hydrogen rich output concentration is
obtained by
operating a carbon dioxide electrolyzer in a manner that produces any one of
or any
combination of the following operating conditions:
a current density of at least about 300 mA/cm2,
a CO2 mass transfer stoichiometric flow rate to the cathode of up to about 2,
a temperature of at least about 65 C or at least about 80 C,
a pressure range of about 75 to 500 psig,
an anode water composition of pure water or at least about50 mM bicarbonate
salt, and
an anode water pH of at most about 1.
[0135] In certain embodiments, the electrolyzer may be built to favor hydrogen
rich molar
ratios or concentrations, as defined here, by using a carbon dioxide
electrolyzer having any one
of or any combination of the following properties:
relatively large nanoparticle cathode catalysts (e.g., having a largest
dimension of, on average,
at least about 80 nm)
silver, palladium, or zinc as the cathode catalyst material,
a cathode catalyst layer thickness of at most about 5 urn or a thickness of at
least about 25um,
a cathode gas diffusion layer with no microporous layer (MPL),
a cathode GDL with no PTFE present or at least about20wt% PTFE,
a cathode GDL having a thickness that is at most about 200um or at least
about500um, and
29
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
a bipolar MEA having an anion-exchange cathode buffer layer with a thickness
that is about 0-
5um.
High reduction product to hydrogen product stream operating parameter regime
[0136] In certain embodiments, a carbon dioxide electrolyzer is configured to
produce, and
when operating actually produces, an output stream having CO:H2 in a molar
ratio of at least
about 2:1.
[0137] In certain embodiments, such product rich output concentration is
obtained by operating
a carbon dioxide electrolyzer in a manner that produces any one of or any
combination of the
following operating conditions:
a current density at the cathode of at least about 300 mA/cm2,
a CO2 mass transfer stoichiometric flow rate to the cathode of at least about
1.5, or at least
about 2.5, or at least about 4,
a temperature of at most about 80 C,
a pressure in the range of about 75 to 400 psig,
an anode water composition of about 0.1 mM to 50mM bicarbonate salt, and
an anode water pH of greater than about 1.
[0138] In certain embodiments, the electrolyzer may be built to favor product-
rich molar ratios
or concentrations, as defined here, by using a carbon dioxide electrolyzer
having any one of or
any combination of the following properties:
relatively small nanoparticle catalysts (e.g., having largest dimensions of,
on average, about
0.1-15nm),
gold as the cathode catalyst material,
a cathode catalyst layer thickness of about 5-20um,
a cathode gas diffusion layer with a microporous layer (MPL),
a cathode GDL with PTFE present at about 1-20 wt%, or about 1-10 wt%, or about
1-5 wt%,
a cathode GDL that has a thickness of at least about 200um, and
a bipolar MEA having an anion-exchange layer with a thickness of at least
about 5um.
Stoichiometric flow rate
[0139] Given that a molar flow rate may be determined, at least in part, by
the electrical current
delivered to the cell, the molar flow rate may be tied to the current. As an
example, the molar
flow rate of carbon oxide in the input stream may be defined in terms of flow
rate per unit of
reaction expected for a given current. Herein, the term "stoichiometric" flow
rate refers to a
fraction or multiple of the flow rate of reactant carbon oxide required to
fully utilize all current
at the cathode, assuming that the reduction reaction of carbon oxide is 100%
efficient at the
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
cathode to a given reaction. A flow rate of carbon oxide having a
stoichiometric value of "1"
is the flow rate required to consume all electrons provided at the cathode,
and no more than
that, in the given reduction reaction at the cathode. Stated another way, the
stoichiometric flow
rate is the amount of excess (or shortfall) reactant that is present beyond
(or below) what could
be theoretically reacted if the current efficiency for a given reaction were
100%.
[0140] For the carbon dioxide reduction reaction that produces carbon monoxide
in an acidic
environment (CO2+ 2H+ + 2e¨ CO + H20), a carbon dioxide flow rate with a
stoichiometric
value of 1 provides one mole of carbon dioxide for every two moles of
electrons provided by
the cell. Stated another way, a cell having a current providing 2 moles of
electrons/second and
a carbon dioxide flow rate providing 1 mole of carbon dioxide molecules/second
would have
a stoichiometric flow rate of 1. For the same current and a flow rate of 0.5
carbon dioxide
moles/second, the cell would have a stoichiometric flow rate of 0.5. And,
again for the same
current but with a flow rate of 1.5 carbon dioxide moles/second, the cell
would have a
stoichiometric flow rate of 1.5. The molar flow rate needed to achieve a
stoichiometric flow
rate of 1 can he calculated:
Stoichiometric Flow Rate (sccm) = 1160 (s/min) * Molar gas volume at STP
(mL/mo1)1 /
[Faraday's constant (C/mol e-) * #e-'s/mole CO21 * Amps of current fed to the
electrolyzer
Total amps of current can be calculated from the current density, the area of
the electrolyzer
cell and the number of cells in the electrolyzer:
Amps of current = current density * area of the electrolyzer cell * number of
cells
[0141] In an example, a 100cm2 electrolyzer with a current density of
500mA/cm2 performing
the electrochemical reduction of CO2 to CO has a total current of 50A and the
reaction requires
2 moles of e-/mole CO produced, so the stoichiometric flow rate of 1 is:
[60*22,413] / 119,6485 * 21 * 50 = 348.4 sccm
In this example a stoichiometric flow rate of 0.5 would be:
0.5 * 348.4 = 174.2 sccm
And a stoichiometric flow rate of 2 is:
2 * 348.4 = 696.8 sccm
[0142] In another example of a cell producing ethylene from carbon dioxide, 12
moles of
electrons are needed to reduce 2 moles of carbon dioxide to 1 mole of
ethylene. The
stoichiometric flow rate for a 3 cell 1500cm2 electrolyzer with a current
density of 300mA/cm2
is:
[60*22,413] / [96,485 * 6] * 1350 = 3,136 seem.
[0143] The following examples were conducted and illustrate the effects of
certain electrolyzer
31
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
design and operating parameters on the molar ratios of gases in a cathode
output stream.
[0144] All examples used 20 wt% Au/Vulcan XC-72R with 4nm Au particles in a
cathode
catalyst layer. All examples used a 100% CO2 input with no humidification as
the input to the
cathode of the electrolyzer. All examples used a bipolar MEA with an anion-
exchange
polymer-electrolyte adjacent to the cathode layer. The anion-exchange polymer
had a
backbone repeat unit comprised of three aryl groups and a methylene carbon
having a CF3
pendant group and an alkyl quaternary ammonium pendant group. The polymer used
bicarbonate, carbonate, hydroxide, and/or bromide as the counter ion to the
quaternary
ammonium group (Orion Polymers and Membrane, Cohoes, NY).
All use IrOx catalyst at the anode for water oxidation, could also use IrRuOx
Flow rate of water to the anode ranges from 4L/min to 40mL/min
All are single cells
101451 Example 1.
Composition of output stream: 30% CO, 20%H2, 50% CO2 (3:2 CO:H2 ratio)
Current Density: 300 mA/cm2
CO2 input flow rate: 400sccm
Cell temperature: 50C
Cell Area: 100cm2
Flow field type: Interdigitated
GDL type: Sigracet 29BC
Au metal loading: 0.3 mg/cm2
Catalyst layer thickness: 15um
AEM layer thickness: 12um
Membrane type and thickness: Nafion 117, 183um thick
[0146] Applications:
1. Feed
directly to a gas fermentation reactor, or CO2 may be removed to increase
CO+H2
concentration, or H2 removed or added to change CO:H2 ratio and affect
products or
combination of these.
2.
H2 may be added to make feedstock for F-T, not necessary to remove CO2 for
all reactor
designs, but could remove CO2 to higher activity in some reactors.
[0147] Example 2.
Composition of output stream: 50% CO, 5%H2, 45% CO2 (10:1 CO:H2 ratio)
Current Denstity: 400 mA/cm2
CO2 input flow rate: 110sccm
32
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
Cell temperature: 45C
Cell Area: 25cm2
Flow field type: serpentine
GDL type: Sigracet 39BC
Au metal loading: 0.27 mg/cm2
Catalyst layer thickness: 14um
AEM layer thickness: 14um
Membrane type and thickness: Nafion 115, 127um thick
[0148] Applications:
1. Feed directly to a gas fermentation reactor, or CO2 removed to increase
CO+H2
concentration, or H2 removed or added to change CO:H2 ratio and affect
products or
combination of these.
2. H2 may be added to make feedstock for F-T, not necessary to remove CO2
for all reactor
designs, but could remove CO2 to higher activity in some reactors.
For formate production, may need to remove CO2, but not H2
4. For
polycarbonate production, may need to remove CO2+H2 to less than about 2%
combined concentration
[0149] Example 3.
Composition of output stream: 20% CO, 20%H2, 60% CO2 (1:1 CO:H2 ratio)
Current Denstity: 300 mA/cm2
CO2 input flow rate: 400sccm
Cell temperature: 50C
Cell Area: 100cm2
Flow field type: interdigitated
GDL type: Sigracet 29BC
Au metal loading: 0.3 mg/cm2
Catalyst layer thickness: 15um
AEM layer thickness: 12um
Membrane type and thickness: Nafion 117, 183um thick
101501 Applications:
1. H2 may be
added to make feedstock for F-T, not necessary to remove CO2 for all reactor
designs, but could remove CO2 to higher activity in some reactors.
[0151] Example 4.
Composition of output stream: 35% CO, 35%H2, 30% CO2 (1:1 CO:H2 ratio)
33
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
Current Denstity: 300 mA/cm2
CO2 input flow rate: 60sccm
Cell temperature: 50C
Cell Area: 25cm2
Flow field type: interdigitated
GDL type: Sigracet 29BC
Au metal loading: 0.32 mg/cm2
Catalyst layer thickness: 15.6um
AEM layer thickness: 14um
Membrane type and thickness: Nafion 115, 127um thick
[0152] Applications:
1. Feed directly to a gas fermentation reactor, or CO2 removed to increase
CO+H2
concentration, or Hz removed or added to change CO:Hz ratio and affect
products or
combination of these.
2. H2 may be added to make feedstock for F-T, not necessary to remove CO2
for all reactor
designs, but could remove CO2 to higher activity in some reactors.
[0153] Example 5.
Composition of output stream: 55% CO, 10%H2, 35% CO2 (5.5:1 CO:H2 ratio)
Current Density: 300 mA/cm2
CO2 input flow rate: 60sccm
Cell temperature: 50C
Cell Area: 25cm2
Flow field type: Serpentine
GDL type: Sigracet 39BC
Au metal loading: 0.24-0.3 mg/cm2
Catalyst layer thickness: 13-15um
AEM layer thickness: 12-14um
Membrane type and thickness: Nafion 117, 183urn thick
[0154] Applications:
1. May be fed directly to a gas fermentation reactor, or CO2 removed to
increase C0+1-12
concentration, or Hz removed or added to change CO:Hz ratio and affect
products or
combination of these.
2. H2 may be added to make feedstock for F-T, not necessary to remove CO2
for all reactor
designs, but could remove CO2 to higher activity in some reactors.
34
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
3. for formate production, need to remove CO2, but not H2
4. For polycarbonate production, need to remove CO2+H2 to less
than about 2% combined
concentration
[0155] Example 6.
Composition of output stream: 15% CO, 1%H2, 84% CO2 (15:1 CO:Hz ratio)
Current Denstity: 600 mA/cm2
CO2 input flow rate: 500sccm
Cell temperature: 50C
Cell Area: 25cm2
Flow field type: Serpentine
GDL type: Sigracet 39BC
Au metal loading: 0.24-0.3 mg/cm2
Catalyst layer thickness: 13-15um
AEM layer thickness: 12-14um
Membrane type and thickness: Nation 117, 183um thick
[0156] Applications:
1. for formate production, may need to remove CO2, but not Hz
2. For polycarbonate production, may need to remove CO2 1-12 to less than
about 2%
combined concentration
[0157] In various embodiments, oxygen produced at the anode of a carbon oxide
electrolyzer
is used in an integrated process. As examples, the electrolyzer-produced
oxygen may be used
in partial oxidation gasification processes, aerobic fermentation processes,
electrolysis
processes employing oxygen depolarization electrodes, etc. In one example of
an integration
scheme, a system having a Fischer Tropsch reactor may employ a carbon dioxide
electrolyzer
configured to produce syngas as an input to the Fischer Tropsch reactor and to
produce oxygen
as an input to reactor for gasification of biomass, which also produces syngas
for input to the
Fischer Tropsch reactor.
Integration Schemes
[0158] Additional information regarding optional embodiments and/or elements
of the system
and/or method are provided below.
101591 A product gas from a carbon dioxide reactor of the disclosure can be
used in one or
more downstream processes. For example, a carbon dioxide reactor of the
disclosure
configured for syngas production can output a stream of CO, H2, and/or CO2.
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
Aerobic and Anaerobic Fermentation
[0160] This output stream can be fed to an input of a bioreactor where
microbes (e.g.,
clostridium autoethanogenum, Clostridium carboxidovorans, Clostridium
Ijungdahlii,
Clostridium ragsdalei, Clostridium thermoaceticum, Clostridium
thermoautotrophicum,
Eubacterium limosum, Peptostreptococcus productus, Butyribacterium
methylotrophicum,
acetogens, E. coli, etc.) use the energy of CO, H2, and/or some of the carbon
contained in CO
and CO2 to make one or more bioproducts (e.g., ethanol, acetic acid, butanol,
butyric acid,
methane, etc.). Unutilized carbon can be released from an output of the
downstream bioreactor
(e.g., as CO2, optionally along with water vapor and/or other volatile
compounds).
[0161] CO2 released as an output of a downstream bioreactor can optionally be
recycled back
to an input of a carbon dioxide reactor of the disclosure (e.g., to increase
the carbon efficiency
of bioproduct production, to control carbon dioxide reactor operation, etc.).
In some
embodiments, it may be desirable to process this CO2 before it enters (e.g.,
re-enters) a carbon
dioxide reactor of the disclosure. For example, the water vapor may be
removed, any volatile
products that will inhibit carbon dioxide reactor function may be removed,
and/or the CO2 may
be pressurized to the level desired for operation of a carbon dioxide reactor
of the disclosure.
Carbon dioxide leaving the bioreactor may be near atmospheric pressure and/or
have any other
suitable pressure, and typical carbon dioxide reactor pressures may be 20 psi
to 800 psi, 50psi
to 400p5i, 100psi to 500 psi, and/or any other suitable range. In some
examples, water vapor is
removed by a phase separator and/or a desiccant (e.g., a phase separator
followed by a
desiccant). In some examples, volatile products are removed by oxidation,
adsorption onto a
suitable adsorbent, and/or condensation. A CO2 compressor can be used to raise
the pressure
of the CO2 to the pressure suitable for a carbon dioxide reactor. If the
carbon dioxide reactor is
capable of running on low pressure CO2 and is not inhibited by water vapor or
any volatile
compounds found in the CO2 stream output from the downstream bioreactor, then
the system
can be simplified to remove unnecessary purification and compression systems
and processes.
[0162] For each liter of culture media in the downstream bioreactor, a flow
rate in the range of
about 1 sccm to 1000sccm or about 1 sccm to 2000 sccm or about 10 sccm to 500
sccm or any
other suitable range of gas from an output of a carbon dioxide reactor can be
desirable. For
each liter of culture media in the downstream bioreactor, CO2 released can be
in the range of
about 1 sccm to 2000 sccm or about 10 sccm to 1000 sccm or about 10 sccm to
500 sccm or
any other suitable range. For each liter of culture media in the downstream
bioreactor, water
vapor in an output gas stream exiting the bioreactor may be about 1%-2% of the
stream by
volume, about 2%-5% of the stream by volume, about 5%-10% of the stream by
volume, about
36
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
10%-25% of the stream by volume, about 25% to 50% of the stream by volume,
about 50% to
90% of the stream by volume, and/or any other suitable amount. Volatile
products leaving the
downstream bioreactor may make up less than about 0.1%, less than about 0.5%
of the stream
by volume, less than about 1% of the stream by volume, less than about 4% of
the stream by
volume, and/or any other suitable amount of the stream.
[0163] Some microbial processes can use syngas produced by a carbon dioxide
reactor of the
disclosure. A syngas output stream of CO, H2, and optionally CO2 may be used
as a feedstock
for a downstream bioreactor where microbial processes take place to make a
range of useful
compounds (examples include ethanol, acetic acid, butanol, butyric acid,
acetone, methane).
The syngas stream itself may not contain all the nutrients needed for the
microbes in the
downstream bioreactor to grow. The addition of other nutrients to the
bioreactor may be
required for the microbes to grow and produce products. Examples of suitable
microbes include
clostridium autoethanogenum, Clostridium carboxidovorans, Clostridium
hungdahlii,
Clostridium ragsdalei, Clostridium thermoaceticum, Clostridium
thermoautotrophicum,
Eubacterium limosum, Peptostreptococcus productus, Butyribacterium methyl
otrophi cum,
acetogens, and/or E. coli.
[0164] One nutrient that can be particularly difficult to introduce to a
downstream bioreactor
is sulfur. Many microbes require sulfur for certain amino acid syntheses and
enzymatic
processes. A carbon dioxide reactor of the disclosure that is tolerant to
sulfur may simplify the
addition of sulfur to a downstream bioreactor (e.g., in addition to providing
syngas to the
downstream bioreactor). Sulfur in the form of one or more sulfur-containing
species (SCSs)
such as H2S, SO2, and/or other sulfur oxides (SON) can be present in the CO2
gas fed to an input
of a carbon dioxide reactor of the disclosure. F125 may pass through a carbon
dioxide reactor
of the disclosure unchanged and exit with the syngas output stream. The SCSs
(e.g., SO2 and/or
SON) may pass through unchanged and/or they may be converted to one or more
other SCSs
(e.g., H2S), and may be output with the syngas output stream. The syngas
further comprising
sulfur species (e.g., H2S, 502, and/or SON) can then be fed to an input of a
downstream
bioreactor (e.g., without the need for additional sulfur nutrients). Sulfur
species concentration
can be in the range of about 'ppm- lOppm, about 5ppm-50ppm, about 5ppm-100ppm,
about
lOppm to 200ppm, about 2Oppm to 1000ppm, and/or any other suitable range.
101651 In some embodiments, the carbon dioxide reactor can be coupled to one
or more gas
fermentation reactors (e.g., downstream of the carbon dioxide reactor, such as
accepting one
or more products of the carbon dioxide reactor). The method can optionally
include controlling
reactor operation based on this coupling, such as to optimize for carbon
efficiency and/or
37
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
energy efficiency. Acetogens are most energy efficient with pure CO as the
input, as seen in
the energy balances shown in Table 1, and in many cases, exhibit the highest
selectivity toward
the desired end product as well. However, there are cases where an integrated
electrochemical-
gas fermentation system may be designed to utilize hydrogen-containing syngas
for a number
of reasons.
Table 1
6 CO + 314.40 (41-15.01-1 4. 4 Cl.h ¨217
klinA. (1.1
3 CO + 3 tt, Cii=60.1.1 ,+. (r),,; AG"'
az:- --136.9k)/inol (2)
2 CO 4 CkkOH
C.;Ø 4, 6 C:WOH + 3 /.0:) = ¨96,7
10.S.m1 (4)
[0166] Using CO for most or all of the electron transfer chemistry in a
downstream bioreactor
typically results in the production of CO2, which can then be vented in an
output stream of the
bioreactor. Typically, as the ratio of hydrogen in the syngas is increased,
less CO2 is produced,
and CO2 byproduct can be eliminated stoichiometrically above a certain ratio
of hydrogen to
carbon monoxide. In the case of gas fermentation to ethanol, for example, a
CO:H2 ratio less
than about 1:2 will typically result in the incorporation of all input carbon
into the ethanol end
product. Hence, tuning the CO:H2 ratio in the output stream of a carbon
dioxide reactor of the
disclosure could enable an operator to optimize for carbon efficiency (e.g.,
to minimize CO2
emissions) by shifting toward more H2 production and/or to optimize for energy
efficiency by
shifting toward higher CO production. Monitoring input costs, such as time of
day electricity
prices or incentives for carbon utilization, could inform the optimal
operating parameters at
any time. Tuning production in this manner could also change the outputs, for
example by
driving toward greater ethanol production (e.g., higher CO) or greater acetate
production (e.g.,
higher H2). Monitoring market prices of outputs could inform the optimal
operating parameters
at any given time (e.g., wherein the operating parameters are determined based
on the market
prices, such as to optimize the market price of the products or to optimize
total profit from
reactor operation).
101671 However, the system and/or method can additionally or alternatively
include any other
suitable elements.
[0168] A system comprised of a CO2 electrolyzer and an aerobic fermentation
reactor can be
used to generate products such as protein, polyhydroxyalkanoates, acetone,
isopropanol,
ethanol, and other products. The CO2 electrolyzer takes inputs CO2, water, and
electricity and
outputs a stream of oxygen and a separate stream containing one or more carbon-
based
product(s) derived from CO2, hydrogen, unreacted CO2, and water. The carbon-
based product
38
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
can be CO, methane, ethylene, or mixtures of these compounds along with other
carbonaceous
compounds.
[0169] The oxygen and carbon product stream can be fed to an aerobic gas
fermentation system
containing yeast, E. coli, or other microorganisms that can metabolize the
compounds in the
0-as stream to make desired products. Output of the aerobic gas fermentation
reactor include a
stream containing at least one bioproduct which is typically in the liquid
phase and a gas stream
containing CO2, water vapor, un-metabolized components of the gas feedstock,
and other
volatile compounds generated during fermentation such as trace hydrocarbons or
H2S. CO2 in
this gas stream can be recycled back to the inlet of the CO2 electrolyzer in
the same way as
anaerobic fermentation processes, but 02 removal may be necessary if the 02
concentration in
the stream is greater than about 5%, 1% or 0.25%.
[0170] Various microorganism metabolic pathways may be utilized for a gas
fermentation
reactor configured to receive products of a carbon oxide electrolyzer. The
design and operation
of the elecrolyzer will match the metabolic pathway(s), and hence the required
inputs of the
pathway(s). One example pathway is the Wood-Ljungdahl Pathway (WLP), which has
a set
of biochemical reactions that can utilize CO, CO2, formate, methanol, H2,
and/or other single-
carbon compounds to make acetyl coenzyme A (Acetyl-CoA). Acetyl-CoA is a
molecule used
as a carbon and energy source for microbes. The Wood-Ljungdahl Pathway is a
native
metabolic pathway found in acetogenic microbes such as Clostridium
Ljungdahlii, but other
microbes, such as E. coli, can be genetically engineered to have this pathway.
Acetyl-CoA can
be utilized by microbes to build cell mass and/or it can be used as a starting
molecule for other
biochemical pathways to make other bioproducts such as acetone, ethanol, etc.
Biochemical
pathways utilizing Acetyl-CoA to make products can be native to an organism or
they may be
added by genetically engineering so the microbe will make a desired product.
[0171] Microbes may utilize multiple metabolic pathways at the same time. For
example,
sugars (e.g., glucose) may be metabolized by microbes through the glycolysis
or nonoxidative
glycolysis pathway concurrently with inputs to the WLP. Biomolecules (e.g.
ATP/ADP,
NADH/NAD+) maybe be generated in one metabolic pathway and used in another
metabolic
pathway.
101721 WLP does not produce ATP. If cells are ATP starved, they will favor
acetate
production from Acetyl-CoA. To generate ATP, cells can directly utilize H2
through the
Flavin-Based Electron Bifurcation Pathway to generate a proton concentration
gradient across
their cell membranes. This gradient can drive ATP production through ATP
Synthase. When
cells have ATP available, then Acetyl-CoA can be converted into a range of
desired products
39
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
through either native or genetically engineered biochemical pathways. Ratio of
I-12:CO in gas
stream can determine availability of ATP and determine the carbon efficiency
of the
bioprocess.
[0173] In some embodiments, an aerobic fermentation process employing methane
and carbon
dioxide inputs produces 3-hydroxy propanoic acid. In some example systems, a
carbon oxide
electrolyzer is configured to provide methane and carbon dioxide to a gas
fermentation reactor
that produces, among other products, 3-hydroxy propanoic acid.
[0174] In another example, a carbon dioxide electrolyzer is configured to
produce an output of
carbon monoxide, carbon dioxide, and hydrogen, which may be processed to have
the ratios of
these gases adjusted before delivery to a gas fermentation reactor that
additionally receives a
sugar (e.g., glucose) as an input and reacts these components to produce
acetone.
[0175] Figure 4 depicts a gas fermentation system 401 comprising a carbon
oxide reduction
electrolyzer 403 upstream from a gas fermentation bioreactor 405, and a
recycle unit 413
configured to recycle output of bioreactor 405 to the input of electrolyzer
403.
[0176] As shown, electrolyzer 403 is configured to receive water and a carbon
oxide (carbon
dioxide in this example) as reactants and electricity to drive the anodic and
cathodic reactions.
The inputs to electrolyzer 403 are provided at a relatively high pressure (at
least above
atmospheric pressure). The electrolyzer's anodic reaction produces oxygen,
which is used as
an input to bioreactor 405 only when aerobic fermentation is employed. Outputs
from the
cathode side of electrolyzer 403 include unreacted carbon dioxide along with
hydrogen gas and
one or more carbon containing products such as carbon monoxide and/or methane.
These
outputs are provided at a relatively high pressure.
[0177] System 401 is configured to transport the cathode side outputs of
electrolyzer 403 along
with, optionally, anodically-produced oxygen to bioreactor 405. System 401 is
configured to
reduce the pressure of some or all the electrolyzer products before or during
delivery to
bioreactor 405. In certain embodiments, system 401 includes as gas
purification unit (not
shown) between electrolyzer 403 and bioreactor 405. A gas purification unit is
configured to
adjust the concentration of the electrolyzer products before they enter
bioreactor 405. In some
cases, a purification unit reduces the concentration of carbon dioxide in the
gas stream. In
some cases, a purification unit increases the concentration of carbon monoxide
in the gas
stream.
[0178] Bioreactor 405 is configured to covert the inputs, optionally along
with other inputs
such as sugar, to desired bioproduct and byproducts. In the depicted example,
the biproducts
are relatively volatile or otherwise separable from the desired product and
can therefore be
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
passed to recycle unit 413. In the depicted embodiment, system 401 is
configured to convey
carbon dioxide, water vapor, and possibly other volatile compounds from the
bioreactor to
recycle unit 413 at a low pressure.
[0179] Recycle unit 413 comprises one or more separation or removal units and
a carbon
dioxide compressor. In the depicted embodiment, a recycle unit 413 is
configured to initially
receive the bioreactor vapor outputs and pass them to a water and optionally
oxygen separation
unit 407. The output of separation unit 407 may be a mixture of low-pressure
carbon dioxide
and optionally volatile compounds. Recycle unit 413 is further configured to
pass the carbon
dioxide and volatiles to a volatile compounds removal unit 409, which removes
the volatiles
and outputs low pressure carbon dioxide. Recycle unit 413 also includes a
carbon dioxide
compressor 411 configured to receive the low-pressure carbon dioxide from
removal unit 409
and pressurize it a level suitable for input to electrolyzer 403.
101801 In certain embodiments, recycle unit 413 comprises one or more carbon
dioxide capture
units containing a sorbent for capturing carbon dioxide during a first phase
and releasing carbon
dioxide during a second phase. Separation unit 409 may he configured to
include or work in
conjunction with such carbon dioxide capture unit. Examples of such capture
units are
provided in the description of direct air capture units described herein.
[0181] In the depicted embodiment, system 401 is configured to transport
pressurized carbon
dioxide from recycle unit 413 to a point upstream from a cathode side inlet to
electrolyzer 403,
where the carbon dioxide mixes with pressurized feedstock carbon dioxide.
[0182] In certain embodiments, depending on the bioreaction undertaken, a
carbon dioxide
electrolyzer located upstream from the bioreactor is configured to operate in
(a) a hydrogen
rich product stream operating parameter regime as described herein, or (b) a
high reduction
product to hydrogen product stream operating parameter regime as described
herein.
[0183] In various embodiments, oxygen produced by the electrolyzer is used in
an integrated
process such as in gasification processes. For example, a system having a
Fischer Tropsch
reactor may employ an electrolyzer configured to receive carbon dioxide input
from partial
oxidation of hydrocarbons or from gasification of biomass, which consume
oxygen. The
oxygen may come from the anode side of a carbon dioxide electrolyzer and/or
from an air
separation unit.
101841 Figure 5 depicts a system 501 including a carbon dioxide electrolyzer
503 configured
to produce syngas. System 501 also includes an aerobic fermentation reactor
505 configured
to produce syngas. System 501 is also configured to deliver oxygen produced at
the anode of
electrolyzer 503 to reactor 505. This oxygen may replace some of the oxygen
normally
41
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
provided from alternative sources such as air separation and therefore
reducing the energy duty
and scale of the air separation unit.
[0185] System 501 is configured to deliver the syngas produced by electrolyzer
503, the
fermentation reactor 505, and potentially other sources to a downstream
gasification unit or
partial oxidation unit 507. System 501 may be configured to deliver some waste
carbon dioxide
from unit 507 to the cathode input stream of electrolyzer 503.
Napthas and Fuels
[0186] As indicated, Fischer Tropch reactions may be characterized by the
following general
expression:
(2n+1)H2 + nC0 -> Cn1-12n + n1-120
[0187] While the following discussion focuses on Fisher Tropsch reactions,
those of skill in
the art appreciate that a class of related reactions may be employed to
produce liquid
hydrocarbons and mixtures thereof (often generally referred to as naphthas)
from input streams
that include hydrogen and carbon monoxide. The class of reactions produce
various
compositions of liquid hydrocarbon mixtures dependent on the composition of
the input stream
and the reaction conditions. While the term Fischer Tropsch is used herein, it
should be
understood to cover any of a class of reactions that produce naphtha from a
mixture including
carbon monoxide and hydrogen. Generally, such reactions or exothermic.
[0188] In various embodiments, the input stream to a Fischer Tropsch reactor
is about 1:2
molar ratio of CO:Hz. To use CO2 as starting point for producing CO/H2 mixture
(or other
Fischer Tropsch input), some conventional, non-electrolytic processes require
two steps. For
example, a conventional process employs a first process to produce CO2 + H2
(step 1) and then
a reverse water gas shift (RWSG) reaction (step 2) to react CO2 + H2 and
produce CO and
water to result in a gas having a ratio close to the required 2:1 CO:Hz. Thus,
in a conventional
process, only after obtaining the CO and hydrogen in the correct ratio can a
Fischer Tropsch
reaction be employed to produce liquid hydrocarbons. Water shift (WSG)
reaction and reverse
water shift reaction catalysts can produce metal dust that is detrimental to
downstream
processes. Further, the water shift reactions require a feed of carbon
monoxide and/or
hydrogen.
101891 Note that a conventional syngas process is sometimes used to directly
produce CO +
Hz mixture (rather than using a WSG and/or RWSG reaction or a carbon dioxide
electrolyzer
which my emphasize production of CO). However, syngas production often uses
coal.
[0190] A Fischer Tropsh system that employs a carbon dioxide electrolyzer as a
source of
carbon monoxide has various advantages over the WSG or syngas routes. For
example, unlike
42
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
a RWSG reaction, a carbon dioxide electrolyzer does not produce metal dust.
Additionally, in
comparison to the RWGS reaction, a carbon dioxide electrolyzer provides a
higher conversion
of CO2 to CO.
[0191] However, a carbon dioxide electrolzer may not produce gas having the
required
approximately 1:2 molar ratio of CO:H2 for a Fischer Tropsch feed. In some
cases, a carbon
dioxide electrolyzer produces a CO-rich stream. Therefore, in some
embodiments, a Fischer
Tropsch system, or any other system that requires a carbon monoxide and
hydrogen mixture,
may employ a water electrolyzer or other source of hydrogen that optionally
works in
conjunction with carbon dioxide electrolyzer. The water electrolyzer is
configured to make
gaseous hydrogen to supplement the CO-rich output of the carbon dioxide
electrolyzer. In
some embodiments, syngas that is relatively rich in hydrogen can be produced
as part of co-
electrolysis of carbon dioxide and water. To achieve an approximately1:2 CO:H2
feed
concentration for a F-T reaction, the system may include sensors configured to
determine the
concentration of CO and H2 coming through the gas separation unit from the CO2
electrolyzer.
IJsing the sensed information as feedback, the operating conditions of a water
electrolyzer may
be adjusted to deliver a hydrogen stream with the quantity of H2 needed to
bring the total stream
to approximately 1:2 CO:H2 concentration.
[0192] Alternatively, a single CO2 electrolyzer can be used to produce a
suitable Fischer
Tropsch CO and H2 feed blend. This can be accomplished by operating the
electrolyzer in a
way that biases the output toward hydrogen production and/or by processing the
electrolyzer
output to adjust its composition prior to delivery to the Fischer Tropsch
reactor. In certain
embodiments, a carbon dioxide electrolyzer includes an MEA that allows a
relatively high
proportion of H+ to reach the cathode. One way to promote a relatively high
flux of H+ at the
cathode is for a bipolar MEA to employ a relatively thin cathode buffer layer
and/or to employ
cathode and cathode buffer layers having polymers with a relatively high H+
transference
number. In another approach, the carbon dioxide electrolyzer is constructed or
operated in a
way that starves it of carbon dioxide. In certain embodiments, the
electrolyzer is operated at a
relatively high current density, which tends to produce a higher ratio of
hydrogen to carbon
monoxide ratio. In some implementations, the electrolyzer employs both a
relatively high
current density and relatively low carbon dioxide feed to the electrolyzer.
Operating at a
relatively high current density has the advantage of producing employing a
relatively
inexpensive electrolyzer for the cost of the equipment.
[0193] The output of a CO2 electrolyzer contains product CO, byproduct H2,
unreacted CO2,
and water vapor. The system may be configured to remove the water vapor and
separate the
43
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
unreacted carbon dioxide. A gas separation unit may be used to separate the
CO2 from the CO
and H2 and/or otherwise concentrate the CO and H2. The system may include a
recycle loop to
recycle water to a water inlet of a CO2 or water electrolyzer. The unreacted
and separated CO2
is then compressed and returned to the inlet of the CO2 electrolyzer. Examples
of gas
separation units are presented in Figures 19, 20, 23A-D, and the associated
description.
[0194] A F-T reactor may operate above about 300 psi and between about 150-300
C. If the
output of a carbon dioxide electrolyzer and optional water electrolyzer is not
at the required
pressure, the system may employ a compressor to bring up the feed gas pressure
before entering
the F-T reactor. In the F-T reactor, the CO-H2 mixture is converted into raw F-
T liquid and
waxes. A system may include a separator following the F-T reactor to separate
water, high
melting point F-T liquid, medium melting point F-T liquid, and tail gas, a
mixture of volatile
hydrocarbons, CO2, CO, and H2. The F-T liquid may be further upgraded via
hydrocracking.
Distillation and separation of different fractions of the F-T liquid may
result in jet fuel, diesel,
and gasoline. Water from the F-T reactor can be filtered to remove impurities
and fed to a water
input of the CO2 and/or optional water electrolyzers.
[0195] A F-T system may be designed so that tail gas and/or volatile
hydrocarbons (e.g.
including methane) are recycled back to the CO2 electrolyzer. The system may
be configured
to separate the tail gas into CO2, which may be compressed and fed directly to
the electrolyzer
inlet and volatile hydrocarbons and unreacted CO and H2. The system may be
designed or
configured such that these products are fed to a combustion reactor to
generate heat, energy,
and CO2. The CO2 is then fed to the CO2 electrolyzer inlet. The 02 from the
electrolyzer may
be used as the oxygen source for combustion, resulting in a pure CO2 output
stream. The
combustion reactor may be run in "rich burn" mode utilizing an excess of fuel
to oxygen to
minimize the concentration of oxygen in the outlet stream. Water from the
combustion reaction
may be separated from the gas output and can be fed to the water input of the
CO2 electrolyzer
or water electrolyzer.
[0196] Because a Fischer Tropsch reaction is exothermic, it produces heat that
may be used
for other purposes in a system. Examples of such other uses include
separations (e.g.,
distillation of light hydrocarbons) and reactions. In conventional systems,
such reactions are
endothermic reactions for production of syngas such as reforming of fossil
fuels, gasification
of biomass, or production from carbon dioxide and hydrogen via reverse water
gas shift.
Hence, in conventional processing, all or a significant portion of the excess
heat from a Fischer
Tropsch reaction is typically directed to the syngas production. In the
present case, however,
which produces syngas at a low temperature (e.g. less than about 100 C) by
processes such as
44
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
carbon dioxide electrolysis optionally along with low temperature water
electrolysis, there is
more excess heat from the Fischer Tropsch reaction available for other
processes such as
carbon dioxide capture, thereby reducing the overall external heat requirement
of the system
and improving carbon and energy efficiency of the carbon dioxide to fuel
synthesis pathway.
[0197] In some embodiments, tail gas is fed to a reformer where methane or
other gaseous
hydrocarbon react with water to produce a mixture of hydrogen and carbon
monoxide, a form
of syngas. This may increase the yield of carbon from carbon dioxide in liquid
hydrocarbon
product. Depending on the composition of tail gas, the ratio of hydrogen to
carbon monoxide
may vary. In some embodiments, some amount of carbon dioxide and/or oxygen is
present in
reformer. In many cases, the reforming reaction is endothermic. In some
embodiments, heat
to drive the endothermic reaction is provided, at least in part, from excess
heat generated during
the Fischer Tropsch reaction. In some cases, some heat may be provided by
combustion or
direct electrical heat. For combustion-derived heat, oxygen (optionally from
an electrolyzer)
may be fed to the furnace to improve efficiency, and carbon dioxide emissions
could be
captured and fed to the electrolyzer.
101981 Figure 6A depicts a system 601 configured to produce liquid
hydrocarbons in which a
primary or exclusive source of carbon is a carbon oxide feedstock such as one
containing
carbon dioxide and/or carbon monoxide. The system includes two primary
reactors: an
electrolytic carbon oxide reduction cell or electrolyzer 611 and a Fischer
Tropsch reactor 621.
[0199] The electrolyzer 611 is connected to a source of electricity and has
one or more inlets
for receiving reactants such as carbon dioxide and water. The electrolyzer 611
has one or more
outlets on the anode side for removing oxygen and possibly trace impurities
and one or more
outlets on the cathode side for removing reduction products including at least
carbon monoxide.
Other compounds leaving the cathode side may include hydrogen, water, and
carbon dioxide.
[0200] The cathode side outlet is connected to a purification unit such as a
carbon monoxide
purification unit 612 which is designed to separate or purify carbon monoxide
from other
components. In the depicted embodiment, purification unit 612 has one outlet
for providing
carbon monoxide and another outlet for providing carbon dioxide, hydrogen, and
possibly
some carbon monoxide. In certain embodiments, the carbon monoxide purification
unit 612
may be a sorbent-based unit such as presented in Figures 19, 20, and/or 23A-D
and the
associated description.
[0201] In the system 601, carbon dioxide, possibly along with some hydrogen
and carbon
monoxide, are recycled from outlet of the CO purification unit 612 back to the
inlet streams
for the cathode side of electrolyzer 611.
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
[0202] The Fischer Tropsch reactor 621 is configured to receive carbon
monoxide and
hydrogen in a pressurized feed stream and at a specified composition. In
system 601, a
compressor 624 compresses the carbon monoxide from the electrolyzer 611 along
with
hydrogen to an appropriate pressure for the Fischer-Tropsch reaction. A
Fischer-Tropsch
reaction may take place at a temperature of about 150-300 C and at a pressure
of about one to
several tens of atmospheres. The reaction is exothermic, so little or no heat
is provided to the
reactor 621.
[0203] As mentioned, the input to a Fischer Tropsch reactor may have a CO:H
ratio of about
n:(2n+1), where n is the length in carbon atoms of the desired alkane product
of the reaction.
Thus, in various embodiments, the molar ratio of hydrogen to carbon monoxide
provided to
reactor 621 is about (2n+1) to n. To provide the desired inlet composition
ratio of hydrogen to
carbon monoxide for the Fischer Tropsch reaction, a hydrogen source 614 may be
coupled to
the outlet of CO purification unit 612 or to the inlet of compressor 624.
Alternatively, or in
addition, the electrolyzer 611 may be designed or operated in a manner that
produces a
relatively high ratio of hydrogen to carbon dioxide. Reactor designs and
operating conditions
for accomplishing this ratio are described elsewhere herein. In some cases, a
gas having a
relatively high ratio of hydrogen to carbon monoxide is produced from
reforming reaction,
such as reaction using FT tail gas as an input.
[0204] As depicted, system is 601 is configured to provide the output of
Fischer Tropsch
reactor 621 to a separator 623 configured to separate MFTL and HFTL Fischer
Tropsch liquids
from water and tail gas. As depicted the Fischer Tropsch water may be recycled
back to the
input of the CO purification unit 612 and/or the input of electrolyzer 611.
[0205] System 601 comprises a main recycle loop having a separation unit 631,
a combustion
chamber 632, and a water/gas separator 633. Separation unit 631 is configured
to receive tail
gas from separator 623 and remove carbon dioxide from volatile hydrocarbons.
System 601 is
configured to recycle carbon dioxide from unit 631 to a carbon dioxide feed
stream to
electrolyzer 611.
[0206] System 601 is configured to transport the volatile hydrocarbons from
separation unit
631 to combustion unit 632, which is configured to burn the hydrocarbons using
a source of
oxygen from electrolyzer 611. System 601 is configured to transport the
combustion products
from combustion unit 632 to gas/water separator unit 633, which is configured
to separate
carbon dioxide and water combustion products. System 601 is configured to
transport the water
to an anode inlet of electrolyzer 611 and transport the carbon dioxide to a
cathode inlet of
electrolyzer 611.
46
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
[0207] In certain embodiments, a carbon dioxide electrolyzer located upstream
from a Fischer
Tropsch reactor is configured to operate in (a) a hydrogen rich product stream
operating
parameter regime as described herein, and/or (b) a high reduction product to
CO2 ratio
operating parameter regime as described herein.
[0208] In certain embodiments, system 601 comprises one or more carbon dioxide
capture
units containing a sorbent for capturing carbon dioxide during a first phase
and releasing carbon
dioxide during a second phase. Separation unit 631 and/or gas/water separator
unit 633 may
be configured to include or work in conjunction with such carbon dioxide
capture unit.
Examples Some principles of operation are provided in the description of
direct air capture
units described herein. In some embodiments, a Fischer Tropsch system is
configured to
provide waste heat produced from an exothermic Fischer Tropsch reaction to a
carbon dioxide
capture unit.
102091 Figure 6B presents an example system 634 for producing a liquid
hydrocarbon mixture
from a carbon dioxide input stream 635 by using (a) a carbon dioxide
electrolyzer 636 to
produce carbon monoxide and hydrogen 637 and (11) a Fischer Tropsch reactor
638 configured
to receive carbon monoxide and hydrogen and produce liquid hydrocarbons.
Carbon monoxide
and hydrogen, at least some produced by electrolyzer 636 is preprocessed in
syngas processing
element 640 which may purify or otherwise modify the syngas (e.g., removal of
unreacted CO2
from the electrolyzer as well as compression and/or heating or cooling of the
syngas stream)
prior to delivery prior to entering the Fischer Tropsch reactor. System 634 is
further configured
to provide processed gas from element 640 to Fischer Tropsch reactor 638,
which can produce
a mixture light hydrocarbons and other components 642, which the system makes
available to
a product separation subsystem 643, which may include a feature for separating
tail gas 641
from one or more liquid hydrocarbon streams 644. In the depicted embodiment,
system 634
includes a reformer 645 and is configured to provide tail gas 641 to the
reformer. The tail gas
contains methane that can react with water (optionally also included in tail
gas 641) by a
methane reforming reaction to produce a hydrogen-rich mixture 647 of carbon
monoxide and
hydrogen. System 634 is also configured to deliver mixture 647 to syngas
processing element
640, which prepares the gas for introduction to the Fischer Tropsch reactor
638. The methane
reforming reaction is endothermic. In some embodiments, excess heat from the
reaction in
Fischer Tropsch reactor 638 is provided reformer 645.
[0210] In the embodiment depicted in Figure 6B, system 634 is optionally
configured to
provide oxygen 649 from electrolyzer 636 to a furnace 651, which is configured
to burn fuel
and produce additional heat for use with system 634 or elsewhere.
47
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
Direct Air Capture of CO2
[0211] In certain embodiments, an electrolytic carbon dioxide reduction system
uses carbon
dioxide received directly from air. A system for such embodiments includes a
direct air CO2
capture subsystem and a carbon dioxide reduction electrolyzer subsystem. The
system is
configured so that CO2 from the capture subsystem supplies CO2, directly or
indirectly, to the
cathode side of the electrolyzer subsystem.
[0212] Because air is often the only significant feedstock, an air capture CO2
electrolysis
system may be deployed at any location where there is space for the system
components. In
some deployments, the system occupies a relatively unpopulated area. In some
deployments,
the system occupies a populated area. In some embodiments, the system is
deployed, at least
partially, on a vehicle or vessel. For example, an air capture unit may be
provided on a vehicle
or vessel while a carbon dioxide electrolyzer may be provided at a port or
offshore platform.
In some cases, the deployment location has a ready supply of energy, e.g., a
location where
solar and/or wind power is plentiful. In some cases, the deployment location
is a desert. In
some embodiments, the system is deployed in an extraterrestrial environment
having a CO2-
containing atmosphere. In some embodiments, the system is deployed on large
vessel such as
a cargo ship or military vessel such as an aircraft carrier. In some
embodiments, the energy
source is provided by a solar or windfarm associated with an offshore platform
or port, while
carbon dioxide capture unit is provided on a ship or other watercraft. A
carbon dioxide
electrolyzer may be provided on the offshore platform or port.
[0213] The system may be designed so that air or other gas is provided under
specified
conditions to the CO2 capture subsystem. In certain embodiments, fans, vacuum
pumps, or
simply wind are used to deliver air to a CO2 capture subsystem.
[0214] In certain embodiments, the CO2 capture subsystem comprises two stages:
a first stage
in which air is contacted with a sorbent that removes CO2 from air (phase 1),
and second stage
in which heat, electricity, pressure, and/or humidity is applied to the
sorbent to release CO2
and/or water (phase 2).
[0215] In some implementations, the CO2 capture subsystem employs a solid or
liquid
absorbent or adsorbent to capture the CO2 in phase 1. In various
implementations, phase 1 is
performed at ambient conditions or near ambient conditions. In phase 2, a
temperature,
electrical, pressure, and/or moisture swing is applied, causing the absorbed
or adsorbed CO2,
and optionally water, to be released.
[0216] In certain embodiments, the absorbent is heated to release the CO2. As
an example, the
sorbent is heated from, e.g., ambient temperature (e.g., about 20-40 C) to a
temperature of at
48
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
least about 75 C to release CO2 and optionally water. In some cases, the
temperature swing
is from ambient to about 50 to 10000 C or from ambient to about 75-2000 C or
from ambient
to about 600 to 1000 C. As an example, the sorbent is heated for a duration
of time sufficient
remove a desired fraction of CO2 and optionally water. The duration is a
function of the amount
of the sorbent to be treated, the fraction of CO2 and/or water to be removed,
and the heat transfer
to the sorbent.
[0217] In certain embodiments, the absorbent is exposed to humidity to release
the CO2. As
an example, the sorbent is initially exposed to dry air (e.g., air having at
most about 50 mole%
water, or at most about 30 mole% water, or at most about 5 mole% water) and
subsequently
exposed to humid vapor (e.g., air having at least about 75 mole% water, or at
least about 90
mole% water, or about 100 mole% water.
[0218] In some embodiments, a CO2 capture unit employs an electro-swing
mechanism for
capturing and later releasing CO2. In certain cases, an electro-swing carbon
dioxide unit
comprises a faradaic adsorption system comprising an electrochemical cell that
exploits the
reductive addition of CO2 to a redox species such as a quinone (e.g., 2,6-di-
tert-buty1-1,4-
benzoquinone), 4,4'-bipyridine, or a thiolate, for carbon dioxide capture.
These redox agents
may be provided in an organic electrolyte. In some cases, an electro-swing
adsorption system
provides carbon dioxide capture materials on a solid support such as a carbon
nanotube support
and/or a zeolite support. In some cases, an electro-swing CO2 capture unit
releases CO2 by
providing heat (e.g., by Joule heating) to an absorbent and/or electrode
holding the captured
C07.
[0219] Depending on the configuration of the CO2 capture subsystem and its
operating
conditions, it can produce CO2 from air at a high concentration of, e.g.,
about 90 mole% or
greater. In some cases, the CO2 capture subsystem is configured to produce CO2
at a relatively
lower concentration, which is still sufficient for CO2 reduction electrolyzers
to operate.
102201 As examples, CO2 capture sorbents and associated subsystem components
are available
from Climeworks AG of Zurich, Switzerland, Global Thermostat of New York, NY,
Carbon
Engineering Ltd. of Squamish, B.C., Canada, and Silicon Kingdom Holdings of
Dublin,
Ireland.
102211 As indicated, captured and subsequently released CO2 is feedstock that
is delivered
directly or indirectly to the cathode side of the CO2 reduction electrolyzer.
In certain
embodiments, water captured from the air is also used in the feedstock of the
CO2 electrolyzer.
[0222] In certain embodiments, an air capture CO2 electrolysis system is
configured to operate
in a manner that delivers CO2 from direct air capture subsystem in a
substantially pure stream
49
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
of, e.g., about 99 mole% CO2 or greater. In certain embodiments, the system is
configured to
operate using a lower concentration of CO2 to the electrolyzer, e.g., about 98
mole% CO2 or
greater, or about 90 mole% CO2 or greater, or even about50 mole% CO2 or
greater. In some
cases, quite low CO2 concentrations are used as the feedstock. Such
concentrations are still
substantially greater than the atmospheric concentration of carbon dioxide,
which is about
0.035 mole%. In certain embodiments, the system is configured to operate using
a CO2
concentration of about 5-15 mole %, which is mixed with air or another gas
such as nitrogen.
[0223] Depending on the type of sorbent used in the process, water may also be
captured along
with CO2 and released with it. In certain embodiments, the output of the CO2
capture subsystem
is humidified CO2 having a water concentration about 0 to 20 mole% water.
[0224] In certain embodiments, the output of the CO2 capture subsystem
contains only CO2
and other components in air such as nitrogen, oxygen, water, argon, or any
combination. In all
cases, the CO2 is present at a concentration that is greater than its
concentration in air. In
certain embodiments, the output of the CO2 capture subsystem contains no
sulfur.
[0225] A direct air capture unit and CO2 electrolyzer can be integrated in
several ways
depending on the type of air capture technology. Heat and mass transfer
components may be
integrated in the overall air capture CO2 electrolysis system.
[0226] For example, in some designs, CO2 reduction electrolyzer is configured
to receive CO2
from and provide heat and/or humidity to the direct air capture subsystem. The
provided heat
may release captured CO2 during phase 2 of a direct air capture subsystem
employing a
temperature swing desorption mechanism. Humidified electrolyzer product gas
can be used to
release captured CO2 during phase 2 of a direct air capture subsystem
employing a moisture
swing desorption mechanism.
[0227] In certain embodiments, the CO2 electrolyzer is designed or configured
to receive dilute
CO2 (e.g., no greater than about 50 mole% CO2) as an input.
102281 Direct air capture units can be designed with multiple sorbent vessels.
To receive a
continuous stream of CO2 (and optionally water) from the air capture
subsystem, at least two
different vessels are operated to be at a different stage of
sorption/desorption during operation
of the overall air capture CO2 electrolysis system. For instance, while one
sorbent vessel is
taking in air to capture CO2, another may be heated to release CO2; as each
vessel continues
through the sorption/desorption cycle, the sorption vessel that was taking in
CO2 will vent CO2
and vice versa. The addition of many vessels at different points in the cycle
can deliver a
continuous stream of inputs to the CO2 electrolyzer and accept a continuous
stream of air
containing CO2 and moisture and/or heat and/or vacuum.
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
[0229] Direct air capture units can be sized to deliver the desired volume of
CO2 flow for a
downstream process, such as a CO2 electrolyzer. This may involve employing
multiple sorbent-
containing vessels. For example, a direct air capture subsystem may be
configured to deliver
750 slpm CO2. Such subsystem may couple to a 200-cell electrochemical stack
composed of
1000cm2 membrane-electrode assemblies operated at 300mA/cm2 and 3 V/cell to
produce 378
slpm CO and 42 slpm hydrogen given 90% CO2 to CO current efficiency of the
process.
Unreacted CO2 at the outlet of the electrolyzer may be recycled to the inlet
to increase carbon
efficiency. Operated continuously, the combined air capture and electrolyzer
unit may produce
approximately 675 kg/day CO. In general, in some designs, an air capture CO2
electrolyzer
system is configured to output at least about 100 kg/day CO and/or other CO2
reduction
product(s). in some designs, an air capture CO2 electrolyzer system is
configured to output at
least about 500 kg/day CO and/or other CO2 reduction product(s).
102301 In certain embodiments, systems employing a carbon oxide electrolyzer
and optionally
a direct air capture of carbon dioxide unit also include a module configured
to capture water
from air or an atmosphere. In some embodiments, the module configured to
capture water
form air utilize solar energy from photovoltaics and/or thermal solar along
with hygroscopic
material. In certain embodiments, the module configured to capture water is an
ambient
dehumidifier such as a hydropanel (available from, e.g., Zero Mass Water, Inc.
of Scottsdale,
AZ).
[0231] Figure 7A illustrates an air capture CO2 electrolyzer system 701
comprising a direct air
CO2 capture subsystem 703 and an CO2 reduction electrolyzer subsystem 705. As
illustrated
direct air CO2 capture subsystem 703 is configured to receive, during sorption
phase 1, air
containing CO2 under, e.g., atmospheric conditions (about 0.035 mole % CO2)
optionally with
humidity, and release air with most CO2 removed and optionally with much
humidity removed.
[0232] Direct air CO2 capture subsystem 703 is configured to release, during
phase 2, CO2 and
optionally water. At least the CO2, and optionally the water, are provided as
inputs to the CO2
electrolyzer 705. The CO2 released from direct air capture subsystem 703
during phase 2 is
provided to the cathode side of electrolyzer 705. As depicted, an optional CO2
purification
unit 707 is interposed between direct air CO2 capture subsystem 703 and
electrolyzer 705. The
water optionally provided by direct air CO2 capture subsystem 703 may be
directed to the
cathode side (as humidity in the CO2 feedstock) or anode side (as reactant) of
electrolyzer 705.
[0233] In the depicted embodiment, electrolyzer 705 is configured to receive
electricity (to
drive the CO2 reduction reaction and the anode oxidation reaction). Also,
electrolyzer 705 is
configured to provide excess heat from the electrolysis reaction to direct air
CO2 capture
51
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
subsystem 703 and drive phase 2 (CO2 release from the sorbent).
[0234] CO2 electrolyzer 705 is configured to output oxygen (the anode reaction
product when
water is the reactant) and one or more CO2 reduction products, which may
include CO and/or
other carbon-based products as described elsewhere herein. The product
stream(s) of CO2
electrolyzer 705 may contain hydrogen, CO2, and/or water. As depicted, system
701 is
configured to provide the electrolyzer output to a separations unit 709,
configured to separate
CO and/or other carbon-based electrolysis products from hydrogen, CO2, water,
and/or other
components. In the depicted embodiment, system 701 is configured to deliver
humidified CO2
from separations unit 709 to direct air CO2 capture subsystem 703.
[0235] In certain embodiments, a carbon oxide is captured onboard a vessel or
vehicle as fuel
is combusted. The fuel may be used, for example, in an internal combustion
engine to propel
the vessel or vehicle. The fuel may be used for other purposes such as
heating, electricity
generation etc. The captured carbon oxide is provided to the cathode of a
carbon oxide
electrolyzer, which produces a reduced product that is directly used, stored,
or converted to a
different product (e.g., a chemical, polymer, or fuel) by downstream
processing. In
embodiments, where the electrolzyer and associated downstream components are
configured
to produce fuel, the resulting fuel may be employed in the original vessel or
vehicle, or in one
or more other vessels or vehicles. In some implementations, both the carbon
oxide capture
subsystem and the electrolyzer, as well as an optional downstream processing
subsystem, are
provided on board, on the vehicle or vessel. In some implementations, only the
carbon oxide
capture subsystem is provided to the vehicle or vessel. In some cases, the
captured carbon
oxide is temporarily stored on the vehicle or vessel. For example, carbon
dioxide may be stored
in one or more tanks, pressurized containers, tanker ships, and the like. In
other cases, the
captured carbon oxide is stored off the vehicle or vessel such as in
underground reservoirs,
tanker ships, offshore platforms, and the like. In some cases, stored carbon
oxide is offloaded
from the vessel or vehicle where it is provided to a carbon oxide
electrolyzer. Examples of
locations where stored carbon oxide may be offloaded and/or where the
electrolyzer is located
include chemical plants, ports, and offshore platforms, including some located
proximate a
source of green energy such as a wind energy or solar energy. Examples of
vessels and vehicles
include ships, trucks, buses, passenger vehicles, aircraft, and other craft.
102361 Figure 7B depicts an example in which carbon dioxide is captured from
fuel combustion
products onboard a vessel or vehicle. As illustrated, a vessel 720 produces a
carbon oxide 721,
which is stored in a storage medium 722 prior to being supplied to a carbon
oxide electrolyzer
723. The electrolyzer and associated downstream chemical processing apparatus
may produce
52
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
a chemical product 725 and/or a fuel 726. In the case of a fuel, the fuel may
be utilized by the
vehicle or vessel.
[0237] In certain embodiments, depending on the needs of the system, a carbon
dioxide
electrolyzer located downstream from a direct air CO2 capture subsystem is
configured to
operate in (a) a high reduction product to CO2 ratio operating parameter
regime as described
herein, (b) a hydrogen rich product stream operating parameter regime as
described herein, or
(c) a high reduction product to hydrogen product stream operating parameter
regime as
described herein.
Polycarbonates
[0238] Certain aspects of this disclosure pertain to polycarbonate production
systems that
include (a) one or more carbon oxide electrolyzers configured to produce one
or more carbon-
containing products and (b) one or more polycarbonate synthesis reactors
configured to
produce polycarbonate polymer from carbon containing compounds derived,
directly or
indirectly, from the products of the one or more electrolyzers.
[0239] In certain embodiments, at least one electrolyzer in a polycarbonate
production system
includes a membrane electrode assembly (MEA), optionally including a polymer
electrolyte
membrane (PEM) such as a cation exchange polymer membrane. Unless otherwise
specified
or clear from context references here to a carbon oxide electrolyzer,
including carbon dioxide
reduction electrolyzers, encompass MEA-based electrolyzers, of which certain
embodiments
are described elsewhere herein.
[0240] In certain embodiments, a polycarbonate production system includes a
carbon dioxide
reduction electrolyzer configured to produce carbon monoxide and one or more
other
subsystems that convert carbon monoxide into one or more intermediates for
subsequent
reaction to produce a polycarbonate polymer.
[0241] In various embodiments, the direct output of the carbon oxide
electrolyzer is converted
to one or more intermediate compounds, such as a phenol, a ketone, and/or an
organic
carbonate, which is or are reacted to produce polycarbonate. The conversion to
such
intermediate compounds may take place by any of various processes. Examples
include
Fischer Tropsch reactions, gas fermentation reactions, and cracking reactions.
102421 In gas fermentation subsystem embodiments, a carbon dioxide
electrolyzer is used to
produce carbon monoxide, and optionally hydrogen, that is subsequently used in
a downstream
gas fermentation process to produce one or more intermediate compounds for
producing a
polycarbonate polymer. Examples of these intermediate compounds include
ketones (e.g.,
acetone), light hydrocarbons, and phenol.
53
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
[0243] In Fischer Tropsch subsystems, which may correspond with other
embodiments
described herein, carbon monoxide and hydrogen from a carbon dioxide
electrolyzer are
reacted to form naphtha or other light hydrocarbon product.
[0244] In certain embodiments, one or more subsystems for producing
intermediates produce
or are configured to produce a diol compound such as bisphenol A. Other diol
polycarbonate
intermediates may be produced in alternative embodiments, these include
bisphenols other than
bisphenol A. In certain embodiments, a subsystem comprises a reactor to
produce phosgene
from carbon monoxide and chlorine. The carbon monoxide may be produced from
the carbon
dioxide reduction electrolyzer.
[0245] In certain embodiments, a polycarbonate synthesis system employs at
least two distinct
electrolytic modules that may share some common electrical infrastructure such
as a common
electrical bus. In certain embodiments, the two distinct electrolytic modules
are two distinct
electrolytic carbon oxide reduction electrolyzers. As an example, a first
carbon oxide reduction
electrolyzer is a carbon dioxide electrolyzer designed, configured, or
operated in a manner that
produces carbon monoxide and optionally hydrogen gas, and a second carbon
oxide reduction
electrolyzer is a carbon dioxide electrolyzer designed, configured, or
operated in a manner that
produces at least one product compound having at least two carbon atoms such
as ethylene or
a ketone such as acetone. In some implementations, the first electrolzyer
comprises a cathode
with a noble metal catalyst such as gold and the second electrolyzer comprises
a cathode with
a transition metal catalyst such as copper. As disclosed elsewhere herein, an
MEA-based
carbon oxide electrolyzer may have various designs or configurations that
allow production of
distinct products (e.g., CO versus C2 compounds).
[0246] In certain embodiments, a first electrolytic module is a carbon oxide
reduction module
and a second electrolytic module is a chlorine generation module such as a
chlor-alkali cell.
These two modules may share common electrical infrastructure. In some
embodiments, the
chlorine generating module is a conventional chlor-alkali module configured to
receive a
chloride salt and water as inputs and produce chlorine gas and hydrogen gas as
outputs. In
some embodiments, the chlorine generating module comprises an oxygen reduction
chlor-
alkali cell configured to receive a chloride salt in the electrolyte and
receive oxygen gas at the
cathode (an oxygen depolarized cathode) and produce chlorine gas at the anode
and water at
the cathode. Such oxygen reduction chlor-alkali cells run for efficiently and
consume less
electrical energy than conventional chlor-alkali cells. However, they require
a source of
oxygen. In some implementations, the polycarbonate production system is
configured so that
oxygen gas produced as the anode of a carbon oxide reduction cell is provided
to the cathode
54
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
of a chlorine generating cell configured to reduce oxygen. In some
implementations, the
polycarbonate production system is configured to provide water produced by an
oxygen
reduction chlor-alkali cell to the carbon oxide rejection cell, either as
anode water or
humidification for a carbon oxide feed stream.
[0247] In certain embodiments as depicted in Figure 8A, a system 801 includes
an electrolytic
cell such as chlor-alkali cell for producing chlorine. In some
implementations, system 801 is
configured to feed the oxygen from the electrolyzer 803 to a cathode of a
chlor-alkali cell
comprising an oxygen depolarized cathode. In some implementations, system 801
is
configured to feed the oxygen from the water electrolyzer to an oxygen
depolarized cathode of
a chlor-alkali cell. System 801 may be configured to provide hydrogen from the
water
electrolyzer to a Fischer Tropsch reactor, a gas fermentation reactor, or
other reactor used in
production of a polyol precursor. Oxygen from electrolyzer 803 or other
electrolyzer may be
used in lieu of oxygen from other sources such as air separation.
[0248] In certain embodiments, the alkaline biproduct of a chlor-alkali cell
(e.g., sodium
hydroxide) is provided as a feedstock to a complementary chemical production
system such as
formate production system, including as an example, a formate production
system employing
a carbon oxide electrolyzer.
[0249] In certain embodiments, the polycarbonate employs a bisphenol A linkage
in the
polymer backbone, and, in fact, most system and method examples presented
herein describe
bisphenol A as the polycarbonate precursor, along with phosgene. However, for
some
applications, other diols are used in place of bisphenol A. Examples include
other linear and
ring unsaturated diols, as well as diphenols and other bisphenols. It should
be understood that,
in the examples described herein, when reference is made to bisphenol A, it is
intended that
other biphenols may be employed as appropriate for the desired poly carbonate
end product. It
should also be understood that appropriate system and method modifications may
be employed
to replace phenol production modules with modules configured to produce phenol
derivatives
or analogs, and/or replace acetone production modules with modules configured
to produce
other ketones.
[0250] A polycarbonate synthesis reaction may involve treatment of bisphenol A
with sodium
hydroxide, which deprotonates the hydroxyl groups of the bisphenol A.
(HOC6H4)2CMe2 +2 NaOH ¨> Na2(0C6H4)2CMe2 + 2 H20
[0251] The diphenoxide (Na2(0C6H4)2C,Me2) reacts with phosgene to give a
chloroformate,
which subsequently is attacked by another phenoxide. The net reaction from the
diphenoxide
is:
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
Na2(0C6H4)2CMe2 + COC12 ¨> 1/n [OC(0C6H4)2CMe21n 2 NaC1
[0252] In various embodiments, at least one carbon dioxide reduction
electrolyzer used in a
polycarbonate production system is designed or configured to operate in a
manner that
produces a significant fraction of hydrogen in addition to carbon monoxide. In
certain
embodiments, a carbon dioxide electrolyzer located upstream of a diol
production reactor is
configured to operate in (a) a hydrogen rich product stream operating
parameter regime as
described herein, and/or (b) a high reduction product to CO2 ratio operating
parameter regime
as described herein.
[0253] Electrolyzers for producing carbon monoxide often employ a cathode
catalyst
comprising a noble metal such as gold. Such catalysts favor production of
carbon monoxide
over hydrogen-containing compounds such as methane, ethylene, formic acid,
etc.
Electrolyzers configured in a way that provides a hydrogen rich product may
employ designs
that (a) starve the cathode of carbon dioxide reactant, and/or (b) permit a
relatively high flux
of hydrogen ions to be transported from the anode, where they are generated,
to the cathode.
Electrolyzers that can operate with relatively little carbon dioxide may input
may have flow
fields or gas diffusion components that restrict carbon dioxide from reaching
active sites on the
electrolyzer cathode. Electrolyzers that can operate with a relatively high
flux of hydrogen
ions to the cathode may have MEAs with cation conducting polymers and/or mixed
ion
conducting polymers at the cathode and/or a cathode buffer layer, if one is
used. In some cases,
in which an MEA includes an anion conductive cathode buffer layer, the layer
is designed to
be relatively thin and/or have a relatively high hydrogen ion transference
number.
[0254] Figure 8A depicts a general representation of a system for producing a
polycarbonate
polymer using a carbon dioxide reduction electrolyzer. As depicted, a
polycarbonate
production system 801 includes a carbon dioxide reduction electrolyzer 803
configured to
receive carbon dioxide and water as reactants and electricity to drive the
anodic and cathodic
reactions that produce oxygen and one or more carbon dioxide reduction
products. In the
depicted embodiment, the carbon dioxide reduction reactor 803 is configured to
produce at
least carbon monoxide as one reduction product. System 801 is specifically
configured to
deliver the carbon monoxide from electrolyzer 803 to a phosgene production
reactor 805.
Reactor 805 additionally includes an input for receiving chlorine gas. The
chlorine gas and
carbon monoxide react in phosgene reactor 805 to produce phosgene as an
output. System 801
is further configured to deliver phosgene from phosgene reactor 805 to a
polycarbonate
synthesis reactor. 807.
[0255] In certain embodiments, a carbon dioxide electrolyzer located upstream
from a
56
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
phosgene production reactor is configured to operate in (a) a high reduction
product to
hydrogen product stream operating parameter regime as described herein, and/or
(b) a high
reduction product to CO2 ratio operating parameter regime as described herein.
[0256] As depicted, polycarbonate synthesis reactor 807 also has an input for
receiving a diol
input material. The diol input may be produced by a variety of methods
including via a reactor
not shown in this figure. Alternatively, as depicted here, system 801 includes
a diol synthesis
reactor or subsystem 809 that is configured to receive carbon dioxide
reduction products from
electrolyzer 803. In various embodiments, these electrolyzer reaction products
include carbon
monoxide and hydrogen. In some cases, these electrolyzer products include a C2
or higher
product such as acetone or formaldehyde. In certain embodiments, reactor or
subsystem 809
is configured to receive and react inputs from sources other than electrolyzer
803. These other
inputs may include, for example, phenolic compounds such as bisphenols. System
801 is also
configured to transport the diol produced by diol synthesis reactor or
subsystem 809 to
polycarbonate synthesis reactor 807. Within polycarbonate synthesis reactor
807, the diol and
phosgene react to produce polycarbonate polymer. In the depicted embodiment,
the
polycarbonate final product is available via an outlet from the polycarbonate
synthesis reactor.
807.
[0257] It should be understood that a polycarbonate production system such as
depicted in
Figure RA may include additional or alternative types of modules not shown in
the figure.
These include, for example, one or more purification units, such as a carbon
monoxide
purification modules, heaters, compressors, condensers, and other chemical
reactors.
Examples of gas purification units for use in the system of 8A or any other
polycarbonate
production system described herein are presented in Figures 19 and 20 and the
associated
description.
[0258] Among the types of reactors that can be used to generate phenolic,
ketone, and organic
carbonate intermediates include gas fermentation reactors, Fischer Tropsch
reactors, and
oxidative carbonylation reactors. In some cases, particularly for reaction
paths used to form by
phenol compounds, the system 801 may include multiple intermediate modules or
reactors. In
one example, system 801 includes one module for producing simple liquid
hydrocarbons,
another module for cracking those hydrocarbons to produce aromatics and other
unsaturated
carbon-containing compounds, and/or one or more additional intermediate
reactors for
producing for ketones, organic carbonates, and/or phenol derivatives. In
various embodiments,
these intermediate modules for producing ketones and/or phenols employ a
combination of
carbon monoxide and excess hydrogen produced by the carbon dioxide reduction
electrolyzer
57
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
803.
[0259] Figure 8B presents an example of a poly carbonate synthesis system 811
having a carbon
dioxide reduction electrolyzer 813 configured to receive carbon dioxide and
water as reactant
inputs and electricity to drive the electrolysis reactions at the anode and
cathode. Carbon
dioxide electrolyzer 813 is configured to output carbon monoxide. As depicted,
system 811 is
configured to deliver carbon monoxide output by electrolyzer 813 to a carbon
monoxide
purification module 812.
[0260] In certain embodiments, a carbon monoxide purification unit such as 812
is configured
as described above such as in any one of the embodiments described in
connection with Fischer
Tropsch processes. See for example, the CO purification units in Figures 19
and 20.
[0261] In certain embodiments, system 811 is configured to provide waste heat
from
electrolyzer 813 to carbon monoxide purification unit 812 in order to
facilitate purification of
the carbon monoxide.
[0262] System 811 is further configured to deliver purified carbon monoxide
from carbon
monoxide purification unit 812 to a phosgene production reactor 815. As
depicted, phosgene
production reactor 815 is configured to receive, in addition to the purified
carbon monoxide,
chlorine gas. Phosgene production reactor 815 is configured to generate
phosgene, which,
during operation, is provided via an appropriate transport component to a
polycarbonate
production reactor 819.
[0263] In the depicted embodiment, system 811 includes a gas fermentation
subsystem 817
configured to receive carbon monoxide and hydrogen gas, as reactants, from the
carbon dioxide
electrolyzer 813. In the depicted embodiment, gas fermentation subsystem 817
is configured
to react carbon monoxide and hydrogen, and to produce acetone.
[0264] Gas fermentation subsystem 817 is also configured to produce carbon
dioxide as an
output. In certain configurations, system 811 is configured to provide excess
carbon dioxide
directly from the output of subsystem 817 to electrolyzer 813. In some
implementations, system
811 is configured to provide carbon dioxide directly to the feedstock for
electrolyzer 813.
[0265] As depicted, system 811 is configured to transport acetone from gas
fermentation
subsystem 817 to a bisphenol A production unit 814. Bisphenol A production
unit 814 is
configured to react acetone and phenol to produce bisphenol A. The acetone
comes, as
mentioned, from gas fermentation subsystem 817. The phenol may be provided
from any of a
variety of sources, including some that use carbon monoxide or other output
from electrolyzer
813.
[0266] In the depicted embodiment, bisphenol A from reactor 814 is provided,
during
58
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
operation, via an appropriate conveyance component to a polycarbonate
production reactor
819. Additionally, phosgene from reactor 815 is provided, during operation,
via a conveyance
component to reactor 819, which is configured to react phosgene and bisphenol
A to produce
a polycarbonate as a final output.
[0267] In the depicted embodiment, system 811 is configured with heat
exchangers and/or
other heat transfer components to provide heat, as needed, among the various
intermediate
reactors and subsystems. For example, bisphenol a synthesis reactor 814 and
phosgene reactor
815 may be configured to transfer heat therebetween as necessary during
various reaction
phases.
[0268] Figure 8C depicts a polycarbonate production system 821. The depicted
system
includes a carbon dioxide reduction electrolyzer 823 configured to provide
carbon monoxide
output to a carbon monoxide purification unit 822, which is, in turn,
configured to provide
purified carbon monoxide to a phosgene production reactor 825. Phosgene
production reactor
825 is configured to produce and output phosgene to a polycarbonate synthesis
reactor 829,
which produces final polycarbonate polymer. The components of this phosgene
production
pathway may be generally constructed and operated as described in conjunction
with other
polycarbonate production embodiments described herein.
[0269] In the embodiment depicted in Figure 8C, bisphenol A is provided via a
pathway that
receives carbon monoxide and hydrogen gas from electrolyzer 823 and converts
these input
gases to phenol and acetone via a naphtha production reaction.
[0270] In the depicted embodiment, system 821 is configured to transport some
fraction of the
carbon monoxide produced by electrolyzer 823 along with hydrogen gas produced
by
electrolyzer 823 to a reactor 827 configured to produce naphtha. Reactor 827
is, in certain
embodiments, a Fischer Tropsch reactor. In other embodiments, reactor. 827 is
a gas
fermentation reactor configured to produce naphtha from carbon monoxide and
hydrogen
input. Regardless of which choice reactor is used, the output is naphtha.
Naphtha is a mixture
of various hydrocarbons that may contain straight, branched, and/or cyclic
aliphatic
hydrocarbons having, e.g., about five to ten carbon atoms. System 821 is
further configured to
provide excess carbon dioxide optionally produced by reactor 827 to carbon
dioxide
electrolyzer 823 to combine with input carbon dioxide feedstock to the
electrolyzer cathode.
102711 System 821 is configured to deliver naphtha from reactor 827 to a
naphtha cracking
unit 826 configured to operate in a mode that converts reactant naphtha to
various unsaturated
hydrocarbons such as toluene, benzene, and propylene. In the depicted
embodiment, waste
products of the naphtha cracking reaction performed at reactor 826 include
hydrogen and waste
59
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
heat. In the depicted embodiment, system 821 is configured to provide waste
heat and hydrogen
gas from naphtha cracking unit 826 to Fischer Tropsch or gas fermentation
reactor 827.
[0272] System 821 is configured to provide the unsaturated hydrocarbon outputs
of cracking
unit 826 to one or more reactors for converting these unsaturated hydrocarbons
to phenol and/or
acetone. In the depicted embodiment, system 821includes a phenol synthesis
reactor 828
configured to receive toluene from naphtha cracking reactor 826 and convert
the toluene to
phenol.
[0273] Various processes may be employed to convert toluene to phenol. One of
these
involves oxidation of toluene with atmospheric oxygen to benzoic acid, which
is carried out in
the liquid phase at temperatures of about 100-150 C and an absolute pressure
of about 3 bar.
A cobalt naphthenate is used as a soluble catalyst at concentrations of 0.1-
0.3%. In a second
step, the oxidation of benzoic acid with atmospheric oxygen and steam uses
molten benzoic
acid as the reactant and solvent at a temperature of about 230-240 C and
atmospheric pressure.
Copper(II) benzoate is used as a soluble catalyst. Magnesium salts may be
added to act as a
promoter. In this reaction, copper(TT)benzoate decomposes to copper(I)
benzoate and
benzoylsalicylic acid (2-(benzoyloxy)benzoic acid). The copper(I) benzoate is
regenerated to
copper(II)benzoate with atmospheric oxygen. The benzoylsalicylic acid is
hydrolyzed with
steam to benzoic acid and salicylic acid (2-hydroxybenzoic acid). The
salicylic acid is
decarboxylated rapidly to phenol and carbon dioxide.
[0274] Additionally, system 821 is configured with components to transport
benzene and
propylene from naphtha cracking reactor 826 to a cumene process reactor or
subsystem 828'
that is configured to react the benzene and propylene to produce phenol and
acetone. In some
implementations, subsystem 828' is configured to react benzene and propylene,
via an
alkylation reaction, in the presence of phosphoric acid and catalyst to
produce cumene, which
may then react in the presence of oxygen and sulfuric acid to produce phenol
and acetone (Hock
rearrangement).
[0275] System 821 further comprises a bisphenol A production reactor 824
configured to
receive phenol and acetone from the phenol production reactor 828 and the
cumene process
reactor/subsystem 828'. Bisphenol A synthesis reactor 824 is configured to
produce bisphenol
A from the phenol and acetone reactants.
102761 System 821 further comprises the polycarbonate synthesis reactor 829,
as previously
mentioned. Reactor 829 is configured to receive bisphenol A from reactor 824
and, as
mentioned, phosgene from reactor 825 to produce polycarbonate output.
[0277] As illustrated, system 821 is configured to transfer heat as needed
between various
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
components such as between the cumene process reactor/subsystem 828' and
phosgene reactor.
825.
[0278] Figure 8D illustrates a polycarbonate synthesis system 831, which in
some
implementations is similar to system 821 of Figure 8C but with a parallel path
from electrolyzer
823 to bisphenol A synthesis reactor 824 to deliver acetone input to reactor
824.
[0279] In the embodiment of Figure 8D, system 831 includes a gas fermentation
reactor 837'
configured to receive carbon monoxide and hydrogen gas from electrolyzer 833
and, via a
biological fermentation reaction, produce acetone. In the depicted embodiment,
system 831 is
configured to convey acetone from fermentation reactor 837' to a bisphenol A
synthesis reactor
834.
[0280] System 831 additionally includes a phosgene path configured to receive
carbon
monoxide produced by a carbon dioxide electrolyzer 833 and output phosgene to
a
polycarbonate production reactor 839. The phosgene path includes, as depicted,
a carbon
monoxide purification unit 832 configured to receive the carbon monoxide from
electrolyzer
832 and a phosgene production reactor 835 configured to receive purified
carbon monoxide
from unit 832.
[0281] System 831 additionally includes a phenol production path configured to
receive carbon
monoxide and hydrogen gas produced by carbon dioxide electrolyzer 833 and
output phenol to
bisphenol A production reactor 834.
[0282] In the phenol path, system 831 comprises a reactor gas fermentation or
Fischer Tropsch
reactor 837 configured to produce naphtha from electrolyzer produced carbon
monoxide and
hydrogen. System 831 is further configured to provide excess carbon dioxide
optionally
produced by reactor 837 to carbon dioxide electrolyzer 833.
[0283] System 831 further comprises a naphtha cracking unit 836 configured to
convert
naphtha from reactor 837 to various unsaturated hydrocarbons such as toluene,
benzene, and
propylene. System 831 is configured to deliver hydrogen and heat produced by
the naphtha
cracking reaction in reactor 836 to Fischer Tropsch or gas fermentation
reactor 837.
[0284] System 831 is configured to provide the unsaturated hydrocarbon outputs
of cracking
unit 836 to one or more reactors configured to convert these unsaturated
hydrocarbons to
phenol and/or acetone. In the depicted embodiment, system 831 includes a
phenol synthesis
reactor 838 configured to receive toluene from naphtha cracking reactor 836
and convert the
toluene to phenol. Additionally, system 831 comprises a cumene process reactor
or subsystem
838' configured to react the benzene a propylene from reactor 836 and produce
phenol and
acetone.
61
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
[0285] Phenol and acetone from reactor 838', acetone from gas fermentation
reactor 837',
phenol from phenol synthesis reactor 838 are provided to bisphenol A synthesis
reactor 834.
System 831 is configured to provide biphenol A from reactor 834 and phosgene
from reactor
835 to polycarbonate production reactor 839, which is configured to act on
these inputs and
produce polycarbonate polymer.
[0286] Figure 8E depicts a polycarbonate production system 841 including a
polycarbonate
synthesis reactor 849 configured to receive phosgene from electrolyzer-
produced carbon
monoxide and to receive bisphenol A from a reactor 844 that receives
fermentation-produced
acetone. Additionally, system 841 includes a chlorine production pathway for
providing
electrolytically produced chlorine to a phosgene production reactor 845.
[0287] In the depicted embodiment, system 841 includes a carbon dioxide
reduction
electrolyzer, 843 that may operate and be configured in a manner similar to
that of the
electrolyzers described in other polycarbonate production systems herein. As
depicted, system
841 is configured to transport carbon monoxide produced by electrolyzer 843
directly to a
carbon monoxide purification unit 842. System 841 is also configured to
transport purified
carbon monoxide from carbon monoxide purification unit 842 to phosgene
production reactor
845. The phosgene production pathway may be configured to operate in a manner
similar to
that of and employ components similar to that of other polycarbonate
production systems
described herein. However, in the depicted embodiment, the chlorine used in
phosgene
production reactor 845 is produced electrolytically in conjunction with
operation of the carbon
dioxide reduction electrolyzer 842. In certain embodiments, the chlorine is
produced by a chlor-
alkali cell employing a chloride salt (e.g., NaCl) as a source of chloride
ions for electrolytic
oxidation to produce chlorine gas.
[0288] In the depicted embodiment, system 841 is further configured to provide
carbon
monoxide and hydrogen gas from electrolyzer 843 to a gas fermentation reactor
847 configured
to convert the carbon monoxide, hydrogen gas, via a biological fermentation
reaction, to
acetone. System 841 is further configured to convey acetone from fermentation
reactor 847 to
bisphenol A production reactor 844. As illustrated, bisphenol A production
reactor 844 is
configured to receive phenol in addition to the acetone as inputs, and react
them to produce
bisphenol A. As illustrated, system 841 is configured to deliver bisphenol A
from reactor 844
to polycarbonate production reactor 849. Additionally, system 841 is
configured to transfer
heat, as appropriate during the course of the polycarbonate production
process, between
bisphenol A production reactor 844 and phosgene production reactor 845.
[0289] Figure 8F depicts a polycarbonate production system 851 that includes
an electrolysis
62
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
subsystem comprising a carbon dioxide reduction electrolyzer 853 and a chlor-
alkali system
853'. Chlor-alkali system 853' is configured to receive as inputs water and
sodium chloride
and produce as outputs chlorine gas and hydrogen gas. System 851 is configured
to deliver
chlorine produced by chlor-alkali system 53' to a phosgene production reactor
855. System
851 is also configured to deliver hydrogen gas optionally produced by chlor-
alkali system 853'
to a gas fermentation reactor 857. In certain embodiments, system 851 is
configured to feed
the oxygen from the electrolyzer 853 to a cathode of chlor-alkali system 853',
which comprises
an oxygen depolarized cathode. In certain embodiments, system 851 includes a
water
electrolyzer configured to produce oxygen which may be delivered to an oxygen
depolarized
cathode of a chlor-alkali cell. System 851 may also be configured to provide
hydrogen from
the water electrolyzer to a gas fermentation reactor or other reactor used to
produce diols.
[0290] System 851 is further configured to deliver carbon monoxide and
hydrogen gas from
carbon dioxide reduction electrolyzer 853 to gas fermentation reactor 857.
Thus, gas
fermentation reactor 857 is configured to receive hydrogen from both carbon
dioxide reduction
electrolyzer 853 and from chl or-al kali system 853' Gas fermentation reactor
857 is configured
to conduct biological fermentation on the carbon monoxide and hydrogen gas
inputs and
produce acetone as an output. Gas fermentation reactor 857 is also configured
to produce
carbon dioxide as a byproduct. System 851, in the depicted embodiment, is
configured to
deliver excess carbon dioxide produced by reactor 857 to electrolyzer 853.
[0291] System 851 is further configured to transport acetone produced by gas
fermentation
reactor 857 to a bisphenol A production reactor 854. Bisphenol A production
reactor 854 is
also configured with an input to receive phenol. The bisphenol A production
reactor 854 is
configured to react acetone and phenol and produce bisphenol A.
[0292] Another pathway in system 851 is a phosgene production pathway that
includes a
carbon monoxide purification unit 852 configured to receive and purify carbon
monoxide
produced by carbon dioxide reduction electrolyzer 853. System 851 is further
configured to
provide purified carbon monoxide from purification unit 852 to phosgene
production reactor
855. As mentioned, phosgene production reactor 855 is also configured to
receive chlorine
from chlor-alkali system 855'.
102931 As depicted, polycarbonate production system 851 additionally comprises
a
polycarbonate production reactor 859 as well as components for conveying
bisphenol A from
bisphenol A production reactor 854 and for conveying phosgene from phosgene
production
reactor 855 to polycarbonate production reactor 859. Reactor 859 is configured
to react the
bisphenol A and phosgene to produce polycarbonate polymer.
63
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
[0294] Figure 8G depicts a polycarbonate production system 861 that employs
three separate
pathways from a carbon dioxide reduction electrolyzer subsystem. A first
carbon dioxide
reduction electrolyzer 863 is configured to (a) produce carbon monoxide and
hydrogen for a
naphtha path, and (b) produce a carbon monoxide for a phosgene path. A
separate carbon
dioxide reduction electrolyzer 863' is configured to produce acetone for an
acetone path.
[0295] The phosgene production path may be similar to that described in other
systems for
producing polycarbonate. It includes a carbon monoxide purification unit 862
and a phosgene
production reactor 865. Carbon monoxide purification unit 862 is configured to
receive carbon
monoxide and waste heat from electrolyzer 863. It is also configured to
provide purified carbon
monoxide to phosgene production reactor 865, which has inputs for receiving
chlorine gas as
well as phosgene.
[0296] The acetone pathway includes, as mentioned, a separate carbon dioxide
reduction
electrolyzer, 863', which is designed and/or operated in a manner distinct
from electrolyzer
863. As depicted, system 861 is configured to deliver acetone directly from
electrolyzer 863'
to a bisphenol A synthesis reactor 864.
[0297] Similar to polycarbonate production system 831 depicted in Figure 8D,
the naphtha
pathway includes a Fisher Tropsch or gas fermentation reactor 867, a naphtha
cracking
subsystem 866, a phenol production reactor 868, and a cumene process reactor
868'. The Fisher
Tropsch or gas fermentation reactor is configured to receive carbon monoxide
and hydrogen
gas from carbon dioxide electrolyzer 863 and output naphtha. The Fisher
Tropsch or gas
fermentation reactor 867 is also configured to receive hydrogen gas from
naphtha cracking
reactor 866 and to deliver excess carbon dioxide back to electrolyzer 863.
[0298] Naphtha cracking subsystem 866 is configured to produce at least
propylene, benzene,
and toluene. System 861 is configured with conveyance components to deliver
the benzene and
propylene from the naphtha cracking subsystem 866 to cumene process reactor
868, which is
configured to produce phenol and acetone as outputs. System 861 is also
configured to convey
toluene from naphtha cracking subsystem 866 to phenol production reactor 868
which is
configured to produce phenol. System 861 is further configured to deliver the
acetone and
phenol from cumene process reactor 868' along with phenol produced by phenol
production
reactor 868 to bisphenol A synthesis reactor 864. As mentioned, system 861 is
also configured
to deliver acetone from electrolyzer 863' to bisphenol A synthesis reactor
864.
[0299] System 861 is additionally configured to deliver bisphenol A produced
by reactor 868
along with phosgene produced by reactor 865 to a polycarbonate synthesis
reactor 869.
[0300] Figure 8H depicts another implementation of a polycarbonate polymer
production
64
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
system. This system is denoted as 871. It includes a single carbon dioxide
reduction
electrolyzer 873 and components for conveying carbon monoxide and hydrogen gas
from
electrolyzer 873 to a gas fermentation and conversion reactor or subsystem
877, which is
configured to directly produce phenol and excess carbon dioxide. System 871 is
configured to
deliver excess carbon dioxide produced by reactor or subsystem 877 back to the
inlet on a
cathode side of electrolyzer 873.
[0301] Reactor or subsystem 877 may be configured to produce phenol from gas
input, alone
or in combination with sugar and/or biomass. In some implementations, the gas
fermentation
reactor produces mevalonic acid or mevalonolactone, which may be converted to
phenol by
heating in the precess a silica catalyst. In certain embodiments, a
microorganism used to
produce mevlonic acid is a naturally occurring microorganism such as E. Coli
modified to
express a MVL pathway.
103021 System 871 is further configured to deliver phenol from reactor 877 to
a bisphenol A
synthesis reactor 874, which is also configured to receive acetone and phenol
as inputs and
produce bisphenol A as an output
[0303] System 871 is also configured to transport carbon monoxide produced by
electrolyzer
873 to a carbon monoxide purification unit 872 and to transport purified
carbon monoxide from
unit 872 to a phosgene production reactor 875, which is configured to receive
the purified
carbon monoxide along with chlorine and to produce phosgene.
[0304] System 871 is further configured to transport phosgene from reactor 875
and bisphenol
A from reactor 874 to a polycarbonate synthesis reactor 879, which reacts the
bisphenol A and
phosgene to produce and output polycarbonate polymer.
[0305] In certain embodiments, polycarbonate synthesis is conducted without
phosgene but
nevertheless using carbon monoxide produced from a carbon dioxide
electrolyzer.
Polycarbonate synthesis systems may be configured to for various non- phosgene
routes to
polycarbonate. In some phosgene-free routes, polymerisation relies on the
transesterification
of DPC (diphenyl carbonate) with bisphenol A. In certain embodiments, non-
phosgene
systems are configured to produce an intermediate dialkyl carbonate, such as
dimethyl
carbonate (DMC), as the source of carbonate functionality. These systems may
be configured
to react phenol with dimethyl carbonate to make, e.g., phenyl methyl
carbonate. Various non-
phosgene routes employ a method to make the dialkyl carbonates. In certain
embodiments,
these are made using carbon monoxide from a carbon dioxide electrolyzer. As an
example,
DMC may be produced using oxidative carbonylation:
CO + 1/2 02 + 2 CH3OH (CH30)2C0 + H20
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
Formates
[0306] Alkali metal formates have many uses including as enzyme stabilizers in
liquid
detergents. The enzymes may be lipases, amylases, proteases, etc. Other
formates such as
alkali earth metal formates also have many uses. In certain embodiments, a
formate production
system employs a carbon dioxide reduction electrolyzer to convert carbon
dioxide to carbon
monoxide, which is processed to produce alkali metal formates. In various
embodiments, a
metal formate is produced by contacting a metal hydroxide with carbon
monoxide. The contact
may occur in a liquid (e.g., aqueous) or solid medium.
103071 Figure 9 illustrates an example formate production system 901
comprising a carbon
dioxide reduction electrolyzer 903, a formate production reactor 905, and
various downstream
formate recovery units. Electrolyzer 903 is configured to receive oxygen and
carbon dioxide
as reactants and to receive electricity to drive reduction of carbon dioxide
to produce carbon
monoxide. System 901 is configured to transport carbon monoxide from
electrolyzer 903 to
formate production reactor 905 where the carbon monoxide reacts with a
hydroxide (e.g.,
sodium hydroxide, potassium hydroxide, cesium hydroxide, or calcium hydroxide)
to produce
dissolved metal formate. Reactor 905 is configured to receive not only the
carbon monoxide
from electrolyzer 903 but metal hydroxide, solvent, and catalyst. Reactor 905
may be a stirred
tank reactor.
[0308] In certain embodiments, a carbon dioxide electrolyzer located upstream
from a metal
formate production reactor is configured to operate in (a) a high reduction
product to hydrogen
product stream operating parameter regime as described herein, and/or (b) a
high reduction
product to CO2 ratio operating parameter regime as described herein.
[0309] System 901 is configured to transport formate-containing solution from
reactor 905 to
a degassing unit 907, which, during operation, removes gases from the formate
solution. Such
gases include unreacted carbon monoxide. System 901 is further configured to
transport
degassed formate solution from unit 907 to an evaporator 909 configured to at
least partially
evaporate solvent from the formate solution and produce a slurry or other
liquid-solid mixture
containing precipitated metal formate. System 901 additionally includes a
filtration unit 911
configured to receive and filter the output of evaporator 909. The output of
filtration unit 911
includes concentrated solid metal formate salt. System 901 additionally
comprises a solvent
wash unit 913 configured to wash the solid formate-containing output of unit
911 by contacting
the formate material with a solvent. System 901 is further configured to
transport the filtered
and washed solid formate from unit 913 to a drier 915 configured to dry the
solid formate and
produce the solid metal formate in final form. Drier 915 is configured to
receive a drying gas
66
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
such as nitrogen or carbon dioxide along with heat. In certain embodiments,
drier 915 is
configured to receive waste heat from electrolyzer 903. In certain
embodiments, drier 915 is
configured to receive carbon dioxide from electrolyzer 903 or from the inlet
stream to
electrolyzer 903. In some implementations, externally provided drying carbon
dioxide is
passed from drier 915 to an input stream for electrolyzer 903.
[0310] In some implementations, the formate produced by system 901 is an
alkali metal
formate such as sodium, potassium, or cesium formate, or an alkali earth
formate such as
calcium or barium formate. In some cases, system 901 is configured to produce
formic acid
from metal formate by using a reactor configured to contact the metal formate
with an acid
such hydrochloric acid.
[0311] In some embodiments, a metal formate is produced by contacting carbon
monoxide
produced by a carbon dioxide electrolyzer with solid or slurry-form metal
hydroxide. For
example, sodium formate may be produced by contacting solid sodium hydroxide
with a carbon
monoxide stream. The reaction may be represented as NaOH(s) + CO (g) -> NaCOOH
(s).
Solid hydroxide may be provided in various forms such as a powder. In some
cases, to increase
surface area of the hydroxide available for reaction, it is milled,
pulverized, or otherwise
reduced in particle size, optionally during reaction with carbon monoxide. For
example, solid
hydroxide may be ground in a ball mill autoclave during contact with carbon
monoxide. In
some cases, optionally during reaction in an autoclave, the solid hydroxide is
contacted with
carbon monoxide at a temperature of at least about 200 C (e.g., about 230 to
300 C) and/or
at a pressure of at least about 2 bar (e.g., about 5 to 10 bar). In some
embodiments, a formate
production reaction in an autoclave has a residence time of at least about 15
to 60 minutes or
about 20 to 40 minutes.
[0312] In some metal formate syntheses, the carbon monoxide is provided to a
reactor (e.g., an
autoclave with solid metal hydroxide) in a concentration of at least about 0.5
mole fraction, or
at least about 0.8 mole fraction, or at least about 0.9 mole fraction.
Ethylene Glycol
[0313] Figures 10A and 10B illustrate processes for preparing ethylene glycol
(monoethylene
glycol or MEG). As depicted in Figure 10A, an MEG production system 1001
includes a
carbon oxide electrolyzer 1003, an ethylene oxide production reactor 1005, and
a MEG
production reactor 1007. Electrolyzer 1003 is configured to produce ethylene.
System 1001
is configured to deliver the ethylene from electrolyzer 1003 to ethylene oxide
production
reactor 1005. Optionally, system 1001 is additionally configured to deliver
oxygen from
electrolyzer 1003 to reactor 1005. Regardless of the source of oxygen, reactor
1005 is
67
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
configured to react ethylene and oxygen to produce ethylene oxide.
[0314] In certain embodiments, reactor 1005 is a direct ethylene oxide reactor
designed or
configured to produce ethylene oxide directly from ethylene and oxygen. In
this approach,
ethylene and compressed oxygen may be fed to a multi-tubular catalytic reactor
(an example
of reactor 1005). During operation of such reactor, the mixture is passed over
a silver oxide
catalyst supported on a porous carrier at about 200-300 C and about 10-30 bar.
The reaction is
exothermic, and heat removed can be used elsewhere in the system. System 1001
may be
configured to cool gases from the reactor 1005 and pass them through a
scrubber where the
ethylene oxide is absorbed as a dilute aqueous solution.
[0315] The system 1001 may be configured to deliver ethylene oxide from
reactor 1005 to
ethylene glycol production reactor 1007. Reactor 1007 may be configured to
react the ethylene
oxide and water to produce ethylene glycol. The reaction may be catalyzed by
acid or base, or
performed at neutral pH and at elevated temperature. In certain embodiments,
system 1001 is
configured to provide heat from electrolyzer 1003 and/or ethylene oxide
production reactor
1005 to MEG reactor 1007.
[0316] As depicted in Figure 10B, an MEG production system 1011 includes a
carbon dioxide
electrolyzer 1013 and an MEG production reactor 1017. Electrolyzer 1013 is
designed or
configured to produce carbon monoxide and hydrogen. System 1011 is configured
to deliver
these outputs to reactor 1017, along with oxygen (optionally from electrolyzer
1013) where the
reactants react to produce ethylene glycol. Reactor 1017 may be configured to
produce
ethylene glycol from these reactants via a two-step process that produces
dimethyl oxalate as
intermediate from a reaction pathway involving methanol, dinitrogen trioxide,
and carbon
monoxide. The production of dimethyl oxalate may employ a palladium catalyst.
Reactor
1017 may be configured to perform the second step by reacting the dimethyl
oxalate with
hydrogen gas using a copper catalyst to produce the ethylene glycol. In this
process, only
carbon monoxide, hydrogen, and oxygen are consumed. Hydrogen for the reaction
may come
from any suitable source. A generic hydrogen source is depicted as hydrogen
producer 1019
in system 1011. In certain embodiments, hydrogen producer 1019 is a water
electrolyzer. In
certain embodiments, hydrogen producer 1019 is a reactor configured to perform
a water shift
reaction. In certain embodiments, hydrogen is produced from a fossil fuel, and
carbon dioxide
product is optionally recycled to electrolyzer 1013. System 1011 may be
configured to provide
excess oxygen from electrolyzer 1013 to a combustion reactor.
[0317] In certain embodiments, a carbon dioxide electrolyzer located upstream
from a MEG
production reactor is configured to operate in (a) a hydrogen rich product
stream operating
68
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
parameter regime as described herein, and/or (b) a high reduction product to
CO2 ratio
operating parameter regime as described herein.
Polyethylene terephthalate
[0318] Figure 11 provides a schematic illustration of systems 1101 that may be
employed to
produce the polymer polyethylene terephthalate. One process or group of
processes employs
a carbon oxide electrolyzer 1103 configured to produce ethylene. Another
process or group of
processes employs a carbon dioxide electrolyzer 1113 configured to produce
carbon monoxide
and hydrogen.
[0319] A system 1101 is configured to implement a PET production pathway
including
electrolyzer 1103. As depicted, system 1101 additionally includes an ethylene
oxide
production reactor 1105 and an ethylene glycol production reactor 1107, which
may be
configured and arranged as in system 1001 of Figure 10A. System 1101 is
configured to deliver
ethylene glycol to a PET production reactor 1109, which is configured to react
with ethylene
glycol with phthalic acid to produce PET polymer.
[0320] A version of system 1101 for producing both ethylene glycol and
phthalic acid includes
an electrolyzer 1113 configured to produce carbon monoxide and hydrogen. The
ethylene
glycol production pathway is configured to react these products, possibly with
the addition of
extra hydrogen from a source 1119 in an MEG production reactor 1117. System
1101 is
configured to provide MEG from reactor 1117 to PET production reactor 1109.
The version
of system 1101 employing electrolyzer 1113 optionally does not include
components for the
MEG pathway employing electrolyzer 1103, ethylene oxide production reactor
1105, and MEG
synthesis reactor 1107.
[0321] The version of system 1101 employing electrolyzer 1113 may also include
reactors for
producing terephthalic acid from carbon monoxide and hydrogen produced by
electrolyzer
1113. The reactors may produce naptha and p-xylene as intermediates. In the
depicted
embodiment, A Fischer Tropsch or gas fermentation reactor 1121 is configured
to produce
naptha from the carbon monoxide and hydrogen output by electrolyzer 1113.
Reactor 1121
may be designed or configured as described elsewhere herein. A naptha cracking
reactor 1123
is configured to crack the naptha and produce p-xylene. In certain
embodiments, system 1101
is configured to supply excess heat and/or hydrogen produced by cracker 1123
to reactor 1121.
A PTA reactor 1125 is configured to convert the p-xylene to terephthalic acid.
[0322] In certain embodiments, a carbon dioxide electrolyzer located upstream
from a Fischer
Tropsch reactor is configured to operate in (a) a hydrogen rich product stream
operating
parameter regime as described herein, and/or (b) a high reduction product to
CO2 ratio
69
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
operating parameter regime as described herein.
[0323] In some versions of system 1101, a carbon oxide reduction electrolyzer
is configured
to produce ethylene glycol (MEG) directly, by electrolysis. In such versions,
the electrolyzer
would replace or supplement another MEG production pathway, such as one
employing reactor
1107 or reaction 1117. Some versions of system 1101 employ a reactor
configured to directly
convert carbon monoxide from reactor 1113 along with hydrogen to p-xylene.
This version of
system 1101 is configured to transport such p-xylene to PTA reactor 1125.
Acetic Acid
[0324] Figure 12 illustrates schematically a system 1201 for producing acetic
acid from carbon
monoxide and hydrogen produced by a carbon dioxide electrolyzer 1203. System
1201
comprises a methanol production reactor 1205 configured to react carbon
monoxide and
hydrogen to produce methanol. Reactor 1205 may be configured in the manner of
conventional
methanol synthesis reactors that employ syngas.
[0325] In certain embodiments, a carbon dioxide electrolyzer located upstream
from a
methanol production reactor is configured to operate in (a) a hydrogen rich
product stream
operating parameter regime as described herein, and/or (b) a high reduction
product to CO2
ratio operating parameter regime as described herein.
[0326] System 1201 also includes an acetic acid production reactor 1207
configured to react
the methanol and purified carbon monoxide to produce acetic acid. Reactor 1207
may be
configured to perform methanol carbonylation using, e.g., a metal carbonyl
catalyst. In certain
embodiments, system 1201 includes a carbon monoxide purification unit 1209
configured to
produce the purified carbon monoxide. The carbon dioxide purification unit may
be designed
in a manner described elsewhere herein (e.g., in the manner of the units in
Figures 19 and 20.
Isocyclitates
[0327] Figure 13 illustrates schematically a system 1301 configured to produce
a diisocyanate
from electrolytically generated carbon monoxide.
[0328] System 1301 may be configured to transport carbon monoxide produced by
a carbon
dioxide electrolyzer 1313 to a carbon monoxide purification unit 1317. System
1301 may also
be configured to transport purified carbon monoxide from unit 1317 to a
phosgene production
reactor 1319 configured to react the purified carbon monoxide with chlorine to
produce
phosgene. In certain embodiments, phosgene production reactor 1319 is designed
or
configured to operate in a manner similar to other phosgene production
reactors described
herein, such as in connection with polycarbonate production systems. Examples
of carbon
monoxide purification units are presented in Figures 19 and 20 and the
associated description.
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
[0329] In certain embodiments, a carbon dioxide electrolyzer located upstream
from a
phosgene production reactor is configured to operate in (a) a high reduction
product to
hydrogen product stream operating parameter regime as described herein, and/or
(b) a high
reduction product to CO2 ratio operating parameter regime as described herein.
[0330] In certain embodiments, system 1301 includes an electrolytic cell such
as chlor-alkali
cell for producing chlorine. System 1301 may be configured to provide chlorine
to phosgene
production reactor 1319. In some implementations, system 1301 is configured to
feed the
oxygen byproduct of electrolyzer 1313 to a cathode of a chlor-alkali cell
comprising an oxygen
depolarized cathode. Oxygen from electrolyzer 1313 may be used in lieu of
oxygen from other
sources such as air separation. In some implementations, system 1301 includes
a water
electrolyzer and system 1301 is configured to feed oxygen produced by the
water electrolyzer
to an oxygen depolarized cathode of a chlor-alkali cell. System 1301 may be
configured to
provide hydrogen from a water electrolyzer to an amine production reactor.
[0331] In the depicted embodiment, system 1301 is configured to transport
phosgene from
phosgene production reactor 1319 to an isocyanate production reactor 1321 that
is configured
to react phosgene and an amine to produce a polyisocyanate, e.g., a
diisocyante such as toluene
diisocyanate (TDI) or methylene diisocyanate (MDI), depending on the structure
of the
supplied amine. In some implementations, reactor 1321 is configured to react
phosgene and
free amine in an inert organic solvent at low temperature. The resulting
mixture of carbamoyl
chlorides and amine hydrochloride is then reacted at higher temperature to
produce the desired
polyisocyanate.
[0332] In certain embodiments, the amine reactant is produced by a reactor or
reaction
employing one or more carbon oxide reduction products produced by a carbon
oxide
electrolyzer as described herein, e.g., via Fischer Tropsch and cracking
reactions. In certain
embodiments, the amine reactant is produced by a bioreactor such as a gas
fermentation reactor.
In certain embodiments, system 1301 is configured to provide electrolytically
generated
hydrogen (optionally from electrolyzer 1313 or a water electrolyzer) to a gas
fermentation
reactor configured to produce an amine product or an intermediate used in
amine production.
In some implementations, hydrogen for amine production is provided by a
separate source.
103331 Regardless of the amine source, reactor 1321 may be configured to react
a polyamine
with phosgene by a phosgenation reaction to produce the polyisocyanate such as
a diisocyanate.
In certain embodiments, the diisocyanate is 2,4-toluene diisocyanate and/or
2,6-toluene
diisocyanate. In certain embodiments, the diisocyanate is 4,4'-diphenylmethane
diisocyanate.
[0334] In various embodiments, substantially pure carbon monoxide is used to
produce
71
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
phosgene, which is then reacted with various amines to produce isocyanates.
Some amines may
be produced using hydrogen, thus providing an application for byproduct
hydrogen from a
carbon dioxide electrolyzer or from a co-located water electrolyzer.
[0335] As an example, for systems configured to produce toluene diisocyanate
(TDI), a carbon
dioxide electrolyzer may be configured or operated to produce a hydrogen rich
output stream.
A high hydrogen content stream (e.g., an approximately 1:1 H2: CO ratio) may
be used for the
production of the precursors toluene diamine (TDA) and phosgene. The TDA may
be produced
by hydrogenation of dinitrotoluene. For example, hydrogen may be used to
produce nitric acid
(described elsewhere), which is used for the nitration of toluene to produce
dinitrotoluene.
[0336] In certain embodiments, a system comprising a carbon dioxide
electrolyzer is
configured to produce methylene diisocyanate (MDI) from aniline and phosgene.
Aniline may
be produced by hydrogenation of nitrobenzene. In some embodiments, a system
for producing
MDI via aniline is configured to produce a feed gas having a relatively high
ratio of hydrogen
to carbon monoxide (e.g., in the neighborhood of about 3:1 hydrogen:CO). In
some
embodiments, a system is configured to produce nitric acid (described
elsewhere), which is
used for the nitration of benzene to produce nitrobenzene. A system may be
configured to
employ a separate gas stream containing a relatively lower concentration of
hydrogen (e.g., a
ratio in the neighborhood about 1:1 hydrogen and CO), which may be used to
produce
formaldehyde, which is in turn reacted with aniline to produce diamines, which
are
subsequently phosgenated to produce MDI.
[0337] In certain embodiments, a system comprising a carbon dioxide
electrolyzer is
configured to produce hexamethylene diisocyanate (HDI) using a hydrogen rich
gas stream
(e.g., a gas stream having a H2:CO ratio of about 4:1) The system may be
configured to
hydrogenate adiponitrile to produce hexamethylenediamine, which is
subsequently
phosgenated to produce HDI.
Polyurethane
[0338] Figure 14 provides a schematic illustration of systems 1401 that may be
employed to
produce the polymer polyurethane. One group of reactors employs a carbon oxide
electrolyzer
1403 configured to produce ethylene. Another group of reactors employs a
carbon dioxide
electrolyzer 1413 configured to produce carbon monoxide and hydrogen. In
alternative
embodiments, only one of these two groups of reactors is employed, and an
alternative source
is used to provide the intermediate chemicals that would otherwise be produced
by the other of
the group of reactors.
[0339] In some implementations, system 1401 is configured to transport
ethylene and
72
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
optionally oxygen from electrolyzer 1403 to an ethylene oxide production
reactor 1405.
System 1401 is also configured to provide ethylene oxide from reactor 1405 to
an ethylene
glycol production reactor 1407 and/or to a polyethylene glycol production
reactor 1409. In
certain embodiments, ethylene oxide production reactor 1405 and/or ethylene
glycol
production reactor 1407 are designed or configured to operate in a manner
similar to that of
reactors 1005 and 1007 of system 1001.
[0340] Polyethylene glycol production reactor 1409 may be configured to
produce
polyethylene glycol from the interaction of ethylene oxide with water,
ethylene glycol, and/or
ethylene glycol oligomers. The length of the PEG chain and polydispersity of
the product are
affected by the choice and ratio of reactants. System 1401 may be configured
to transport heat
generated by the exothermic PEG production reaction from reactor 1409 to a
carbon monoxide
purification process (e.g., for phosgene production) or other energy-requiring
process.
Examples of carbon monoxide purification units are presented in Figures 19 and
20 and the
associated description.
[0341] In some implementations, system 1401 is configured to transport carbon
monoxide and
optionally hydrogen from electrolyzer 1413 to a reactor or group of reactors
1415 configured
to produce one or more polyol (e.g., a polyethylene glycol). Reactor or group
of reactors 1415
may be a bioreactor configured to produce polyols by a gas fermentation
reaction. In some
embodiments, reactor(s) 1415 are configured to produce a polyol using an algae-
based reaction.
In certain embodiments, reactor or group of reactors 1415 includes Fischer
Tropsch reactor
and/or a naphtha cracking reactor used to produce hydrocarbons that can be
converted to
polyols.
[0342] In certain embodiments, a carbon dioxide electrolyzer located upstream
from a naphtha
generating and cracking subsystem is configured to operate in (a) a hydrogen
rich product
stream operating parameter regime as described herein, and/or (b) a high
reduction product to
CO2 ratio operating parameter regime as described herein.
[0343] System 1401 may be configured to utilize carbon monoxide and optionally
hydrogen
produced by electrolyzer 1413 to produce a diisocyanate such as MDI or TDI. In
certain
embodiments, system 1401 is configured to implement diisocyanate production
using as a
subsystem the system 1301 depicted in Figure 13. Such subsystem may include a
carbon
monoxide purification unit and a phosgene production reactor. Regardless of
how the
diisocyanate precursors such as phosgene and free amines are produced, system
1401 is
configured to react them in a diisocyanate production reactor 1421. Depending
on the
polyurethane to be produced, different types of diisocyanates may be employed.
Examples
73
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
include 2,4-toluene diisocyanate and/or 2,6-toluene diisocyanate. In certain
embodiments, the
diisocyanate is 4,4'-diphenylmethane diisocyanate.
[0344] System 1401 is optionally configured to provide excess carbon monoxide
in the outlet
stream of ethylene-production electrolyzer 1403 to an phosgene/isocyanate
production
pathway.
[0345] System 1401 includes a polyurethane production reactor 1411 configured
to receive a
polyol and a diisocyanate, and to react them to produce polyurethane polymer.
In certain
implementations, the polyol is produced by, e.g., reactor or reactor group
1415 and/or
polyethylene glycol production reactor 1409. In some implementation, system
1401 is
configured to transport the polyol from one or both of these reactors and/or
to transport
cliisocyanate from reactor 1421 to polyurethane production reactor 1411. In
certain
embodiments, reactor 1411 is designed or configured to contact and react
steams of polyol and
diisocyanate. In certain embodiments, the polyol stream includes a catalyst
(e.g., and acidic or
basic amine), a surfactant, and/or a blowing agent.
Oxalic Acid
[0346] In certain embodiments, oxalic acid is produced from carbon monoxide
generated by a
carbon dioxide electrolyzer. Various pathways may be employed to produce
oxalic acid from
carbon monoxide. Examples of systems incorporating these pathways are depicted
in Figures
15 through 18.
[0347] In certain embodiments, a carbon dioxide electrolyzer and associated
oxalic acid
production units are deployed at or near a plant for producing cement. Carbon
dioxide produced
by the cement plant may be used to feedstock for the carbon dioxide
electrolyzer. The oxalic
acid produced by the system may be used for curing cement. In certain
embodiments, oxalic
acid used in cement produces calcium oxalic, which has a very low solubility.
Cements
produced with oxalic acid may resist degradation due to contact with acids
while in use (e.g.,
after installation or construction).
[0348] Figure 15 depicts a system 1501 comprising a carbon dioxide
electrolyzer 1503
configured to produce carbon monoxide and hydrogen. System 1501 is configured
to transport
some of the carbon monoxide and hydrogen, optionally along with some oxygen,
produced by
electrolyzer 1503 to an alcohol production reactor 1505. Reactor 1505 is
configured produce,
in certain embodiments, methanol or butanol.
[0349] In some implementations, reactor 1505 is a bioreactor employing an
organism having
a metabolic pathway for converting carbon monoxide to an alcohol such as
butanol. Examples
of such organisms include autotrophic acetogens such as Clostridium
carboxidivorans.
74
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
Butyribacterium methylotrophicum. In some embodiments, reactor 1505 is
configured to
produce methanol by a catalytic reaction of carbon monoxide and hydrogen at
elevated
temperature and pressure. In some embodiments, the catalyst is a mixture of
copper and zinc
oxides supported on alumina. In some embodiments, system 1501 is configured to
provide
additional hydrogen, beyond that produced by electrolyzer 1503, to alcohol
reactor 1505.
[0350] In certain embodiments, a carbon dioxide electrolyzer located upstream
from an alcohol
production reactor is configured to operate in (a) a hydrogen rich product
stream operating
parameter regime as described herein, and/or (b) a high reduction product to
CO2 ratio
operating parameter regime as described herein.
[0351] System 1501 is further configured to transport alcohol produced by
reactor 1505 to an
oxalic acid production reactor 1507. In certain embodiments, reactor 1507 is
configured to
react the alcohol with carbon monoxide and oxygen to produce an oxalic acid
diester, which is
subsequently hydrolyzed to produce free oxalic acid.
4 ROH +4 CO +02 ¨*2 (CO2R)2 +2 H20
[0352] The carbon monoxide provided to reactor 1507 may be provided directly
from
electrolyzer 1503. In some embodiments, the carbon monoxide from electrolyzer
1503 is
purified before delivery to reactor 1507.
[0353] Reactor 1507 may produce oxalic acid in an impure form. Therefore,
system 1501 may
be further configured to provide the oxalic acid product to a separator 1509,
which may be
configured to purify the oxalic acid and return unreacted alcohol to reactor
1507. In certain
embodiments, separator 1509 is configured to perform an azeotropic
distillation on the oxalic
acid product from reactor 1507.
[0354] Figure 16 illustrates a system 1601 configured to produce oxalic acid
from carbon
monoxide produced by an electrolyzer 1603. As depicted, system 1601 is
configured to
transport carbon monoxide and hydrogen produced by electrolyzer 1603 to an
ethylene glycol
production reactor 1605. Reactor 1605 may be designed or configured to produce
ethylene
glycol in a fashion similar to that described above with reference to system
1011 (see reactor
1017) of Figure 10A.
[0355] In certain embodiments, a carbon dioxide electrolyzer located upstream
from an MEG
production reactor is configured to operate in (a) a hydrogen rich product
stream operating
parameter regime as described herein, and/or (b) a high reduction product to
CO2 ratio
operating parameter regime as described herein.
[0356] System 1601 is configured to transport ethylene glycol produced by
reactor 1605 to an
oxalic acid production reactor 1607 configured to oxidize ethylene glycol and
produce oxalic
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
acid. In certain embodiments, reactor 1607 is configured to utilize oxidants
such as nitric acid
and/or air to produce the oxalic acid from ethylene glycol. In certain
embodiments, reactor
1607 is configured to react an alcohol (MEG) in the presence of an oxidizing
agent such as air
or nitric acid using a catalyst such as vanadium pentoxide to produce oxalic
acid.
[0357] In certain embodiments, system 1601 includes a reactor 1609 for
producing nitric acid.
In certain embodiments, reactor 1609 is designed of configured to implement
the Ostwald
process. In certain embodiments, system 1601 additionally includes a reactor
for implementing
the Haber process to produce ammonia. System 1601 may be configured to provide
the
ammonia to Ostwald reactor 1609. In some implementations, a Haber reactor and
Ostwald
reactor 1609 are provided as a subsystem that takes hydrogen and nitrogen as
reactants and
produces nitric acid as a product. System 1601 may be configured to direct
hydrogen produced
by electrolyzer 1603 to a subsystem for producing nitric acid (e.g., a
subsystem that first
generates ammonia from hydrogen and nitrogen). In some embodiments, nitric
acid is supplied
from an external source. It should be understood that in other embodiments
that require nitric
acid, e.g., other systems for producing oxalic acid, nitric acid can be
produced from a Haber
process subsystem that receives hydrogen from a carbon oxide electrolyzer.
[0358] In the depicted embodiment, reactor 1607 receives nitric acid from
reactor 1609 and
produces oxalic acid. In certain embodiments, reactor 1609 produces relatively
impure oxalic
acid, such as oxalic acid that contains some amount of nitric acid. In the
depicted embodiment,
system 1601 is configured to deliver impure oxalic acid to a crystallizer and
separator unit 1611
that is configured to purify the oxalic acid and return nitric acid to reactor
1607.
[0359] Figure 17A depicts a system 1701 comprising a carbon dioxide
electrolyzer 1703, a
metal formate production reactor 1705, and an oxalic acid formation reactor
1707. System
1701 is configured to deliver carbon monoxide produced by electrolyzer 1703 to
formate
production reactor 1705. During operation, a metal hydroxide along with heat
_____ optionally,
waste heat from electrolyzer 1703¨is provided to reactor 1705 which produces
the metal
formate. The production and purification and/or extraction of the formate may
proceed as
described above with reference to Figure 9. In some implementations, the
formate is an alkali
metal formate such as sodium, potassium, or cesium formate, or an alkali earth
formate such
as calcium or barium formate. In certain embodiments, the formate is sodium
formate. In
certain embodiments, the formate is potassium formate.
[0360] Regardless of how the metal formate is produced and optionally
extracted, system 1701
is configured to transport the formate to oxalic acid production reactor 1707.
Reactor 1707 may
be configured to convert formate to oxalate via a pyrolysis reaction. Reactor
1707 may also be
76
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
configured to convert metal oxalate to oxalic acid through contact with acid.
In certain
embodiments, oxalic acid formation reactor 1707 is configured to receive an
acid, such as
hydrochloric acid to, for example, to drive the reaction. In certain
embodiments, a halide
byproduct (e.g., NaCl) of the oxalic production reaction (in reactor 1707) is
provided to a chlor-
alkali electrolyzer or other system 1709 configured to produce chlorine gas
and metal
hydroxide. In some implementations, system 1701 is configured to utilize the
chlorine to
produce hydrogen chloride or hydrochloric acid, which may be delivered to
oxalic acid
production reactor 1707. In some implementations, system 1701 is configured to
utilize
hydroxide from reactor 1709 in formate production reactor 1705.
[0361] As indicated, oxalic acid may be produced from a metal formate by
conversion to a
metal oxalate and subsequent acidification. In some implementations, a process
may include
the following operations: (1) produce a metal formate from carbon monoxide
produced by a
carbon dioxide electrolyzer (2) produce a metal oxalate from the metal formate
by, e.g.,
pyrolysis, and (3) produce oxalic acid by exposing the metal oxalate to acid.
The overall
process can be conducted as a batch process. In some cases, at least the metal
formate
production operation and the metal oxalate production operation are performed
in the same
vessel. In some examples, a formate and/or oxalate production vessel may be a
pressure vessel
such as an autoclave. In some cases, a metal formate production vessel and/or
a metal oxlate
production vessel includes mechanism for reducing the particle size of a solid
reactant such as
a metal hydroxide. In some examples, the production vessel includes a ball
mill.
[0362] In some embodiments, optionally during reaction in an autoclave, a
formate production
reaction (e.g., a reaction between carbon monoxide and solid sodium hydroxide)
has a
residence time of at least about 15 to 60 minutes or about 20 to 40 minutes.
In some casesthe
solid hydroxide is contacted with carbon monoxide at a temperature of at least
about 200 C
(e.g., about 230 to 3000 C) and/or at a pressure of at least about 2 bar
(e.g., about 5 to 10 bar).
103631 The operation of converting a metal formate to a metal oxalate may be
accomplished
by pyrolysis, optionally in the same reaction in which metal formate was
produced. In certain
embodiments, the chemical reaction is NaCOOH + CO -> Na2C204. The reaction may
be
conducted in the presence of heat and sodium carbonate. In batch processes in
which a single
reactor is used to produce metal formate and metal oxalate, a metal carbonate
(e.g., sodium
carbonate) may be the only input added to the reactor prior to the oxalate
reaction.
[0364] In some embodiments, the metal oxalate production reaction is conducted
at a pressure
of about 0.5 to 5 bar (e.g., substantially atmospheric pressure). In some
embodiments, the
metal oxalate production reaction is conducted at a temperature of at least
about 200 C, or at
77
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
least about 3000 C, or about 300 to 400 C. In some embodiments the residence
time of the
metal oxylate production reaction about 10 to 100 minutes, or about 20 to 40
minutes.
[0365] In some implementations, at the beginning the metal oxalate production
reaction, the
reactor pressure is reduced to a low pressure (e.g., about 1 bar), while the
reactor heats until
reaching a temperature of at least about 300 C (e.g., about 360 C). During the
pyrolysis
operation, the metal hydroxide residue may continue to react with carbon
monoxide, and this
increases the overall conversion of this reaction.
[0366] A solid metal carbonate may serve as a catalyst. Further, it may
inhibit the thermal
decomposition of metal oxalate to metal carbonate and carbon monoxide. In some
implementations, the reactor pressure is reduced to a low pressure (e.g.,
about 1 bar), while the
reactor heats until reaching a temperature of at least about 300 C (e.g.,
about 360 C. During
the pyrolysis operation, the metal hydroxide residue may continue to react
with carbon
monoxide, and this increases the overall conversion of this reaction.
[0367] In certain embodiments, oxalic acid is formed from metal oxalate and an
acid such as a
hydrohalic acid_ In certain embodiments, the acid is hydrochloric acid having
a concentration
of about 0.05 to 0.2 M. In some implementations, an oxalic acid formation
reaction is
conducted in a crystallizer such as a batch crystallizer. In some embodiments,
the reactor is
configured to produce a recirculation stream to increase the efficiency of
oxalic acid crystals
separation.
[0368] In some embodiments, oxalic acid is produced at a temperature of about
20 to 100 C,
or about 50 to 100 C. In some embodiments, the newly produced oxalic acid is
cooled (e.g.,
to about 30 C or lower) for about 10 to 60 minutes. In some embodiments, the
pressure
employed during the oxalic acid formation reaction is about 0.5 to 2 bar
(e.g., approximately
atmospheric pressure). In certain embodiments, a reaction solution containing
metal oxalate is
brought to a low pH, e.g., about 1 to 3 or simply about 1.
103691 In some implementations, the mass of water added to metal oxalate is
sufficient to
complete dissolution of the metal oxalate at an initial temperature. The
dissolution process may
occur while the temperature in the batch is about 80C, for example. The
crystallization process
may occur when the temperature of the solution in the batch decreased, e.g.,
rapidly decreases.
This operation may be enabled by the using a by-pass stream of water that was
initially used to
dissolve the metal oxalate. Namely, when oxalic acid is completely dissolved,
the water stream
may avoid passage through a heater and flow directly to a heat exchanger
placed in the batch.
A process for forming and crystallizing oxalic acid may be accomplished using
a crystallizer
such as crystallizer 1731 depicted in Figure 17B.
78
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
[0370] Crystallized oxalic acid is optionally filtered and/or dried. In some
embodiments, oxalic
acid is filtered using a pusher centrifuge. In some embodiments, oxalic acid
is dried using a
fluidized bed drier. In some implementation, filtered oxalic acid has a
moisture content of
about 20-30% by mass.
[0371] Figure 17B depicts a process 1720 of forming oxalic acid from solid
metal formate. As
shown, process 1720 begins with formation of a metal formate at an operation
1721. This
process involves reaction of carbon monoxide from a carbon dioxide
electrolyzer with a metal
hydroxide. Any suitable process of creating metal formate may be employed.
Examples
include all processes disclosed herein for producing metal formate, including
those processes
described in association with Figure 9. After the metal formate is produced,
it is converted to
a metal oxalate in an operation 1723. In the depicted operation, the formate
to oxalate
conversion reaction is performed in the presence of a metal carbonate such as
sodium
carbonate. In certain embodiments, one or both of operations 1721 and 1723 are
performed in
a ball mill autoclave such as autoclave 1729 depicted in the figure.
[0372] After the metal oxalate is formed in operation 1723, the metal oxalate
is reacted with
an acid such as a hydrohalic acid (e.g., hydrochloric acid) to form oxalic
acid. See operation
1725. In some embodiments, the reaction may take place in a crystallizer such
as a batch
cooling crystallizer 1731 depicted in the figure. The batch cooling
crystallizer includes (1) an
agitator, (2) baffles, (3) a cooling jacket, (4) a jacket fluid inlet, (5) a
jacket fluid outlet, and
(6) an outlet valve. This arrangement facilitates both formation and
crystallization of oxalic
acid.
[0373] After the oxalic acid is formed and optionally crystallized in
operation 1723, it is
optionally purified and/or dried. In one example, purification is accomplished
by filtering. In
one example, drying is accomplished in a fluidized bed.
[0374] Figure 18 depicts a system 1801 comprising a carbon dioxide
electrolyzer 1803
configured to produce carbon monoxide and hydrogen. System 1801 is configured
to provide
the carbon monoxide and hydrogen produced by electrolyzer 1803 to a Fischer
Tropsch reactor
or a gas fermentation reactor, either generically illustrated by block 1805.
Depending upon the
content of the products produced by electrolyzer 1803 and the reaction taking
place in reactor
1805, additional hydrogen may be needed to promote the reaction of reactor
1805. To this end,
reactor 1805 may be configured with an inlet for externally produced hydrogen
gas. Reactor
1805 is configured to produce naphtha, and system 1 801 is configured to
transfer the naphtha
from reactor 1805 to a naphtha cracking reactor 1807. Naphtha cracking reactor
1807 is
configured to crack naphtha in a manner that produces at least some propylene.
79
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
103751 In certain embodiments, a carbon dioxide electrolyzer located upstream
from an
propylene production reactor is configured to operate in (a) a hydrogen rich
product stream
operating parameter regime as described herein, and/or (b) a high reduction
product to CO2
ratio operating parameter regime as described herein.
103761 System 1801 is configured to deliver the propylene to an oxalic acid
production reactor
1809 that is configured to receive nitric acid and optionally oxygen, in
addition to the
propylene. In some implementations, system 1801 is configured to deliver
excess oxygen from
electrolyzer 1803 to reactor 1809. In certain embodiments, reactor 1809 is
configured to absorb
propylene into nitric acid and to heat the resulting mixture while adding
oxygen and removing
nitrogen oxides. The resulting process produces oxalic acid, which system 1801
is configured
to deliver to a separation unit such as a crystallizer and separator unit
1811. In certain
embodiments, unit 1811 is configured to produce pure oxalic acid. System 1801
may be
configured to return nitric acid from unit 1811 to reactor 1809.
103771 In a version of system 1801, propylene is generated from electrolyzer-
produced carbon
monoxide and hydrogen by a different route. In this version, system 1 801 is
configured to
deliver carbon monoxide and hydrogen from carbon dioxide electrolyzer 1803 to
an alcohol
synthesis reactor 1813 that is configured to produce methanol or other
alcohol. In certain
embodiments, reactor 1813 is configured to receive additional hydrogen from a
source apart
from electrolyzer 1803. In some implementations, reactor 1813 is configured to
produce
alcohol in a manner similar to that of the methanol synthesis reactor 1205 in
Figure 12.
103781 Reactor 1801 is configured to transport alcohol produced by alcohol
synthesis reactor
1813 to a methanol to olefins reactor 1815 configured to convert the alcohol
to one or more
olefins including propylene. System 1801 is configured to transport propylene
produced by
reactor 1815 to oxalic acid synthesis reactor 1809. Methanol to olefins
reactor 1815 may be
configured to convert alcohol (e.g., methanol) to olefins by a reaction
involving a network of
chemical reactions in the presence of an acidic zeolite catalyst such as H-
SAP0-34. The
temperature and other parameters of the reactions may be tuned to produce a
desired product,
which is propylene in system 1801. In certain embodiments, system 1801 is
configured to
operate reactor 1815 at a temperature of about 600 to 650 C.
103791 In various embodiments, a system employing a carbon dioxide
electrolyzer to produce
carbon monoxide and hydrogen is configured to produce methanol from the carbon
monoxide
and hydrogen by a method such as the methanol synthesis reactor 1205 in Figure
12. The
associated system is configured to provide the resulting methanol to a formate
synthesis
reactor, which may be configured to perform the BASF and/or Kemira-Leonard
processes to
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
produce methyl formate.
Purification Units
[0380] Various types of purification units may be employed for purifying or
otherwise
concentrating carbon monoxide produced by a carbon dioxide electrolyzer.
Examples include
amine absorption units (used with, e.g., gas streams having about CO2
concentrations of about
20% by volume or lower), CO2 adsorption units that take advantage of CO2's
acidity, CO
adsorption units (using, e.g., copper compounds), CO/CO2 separation
compositions such as
molecular sieves and metal organic frameworks, cryogenic system (e.g., flash
distillation
systems), and membrane permeation units. In certain embodiments, a CO
purification unit is
configured to operate at a pressure of about 100 to 400 psia. An amine-based
CO2 absorption
unit may employ an aqueous solution of an ethanol amine such as methyl
diethanolamine
MDEA, optionally with piperazine to enhance absorption kinetics. The amine
absorbent may
be regenerated by application of heat. An example unit employs a water
solution of about 30%
(weight) MDEA and 1% (weight) piperazine.
[0381] Cryogenic systems work by cooling the gas mixture and then passing
through a
fractionation column to separate gases by boiling point. Multiple
fractionation columns may
be used in a single process to separate and deliver purified components of gas
mixtures.
[0382] A membrane purification process uses membranes that retain the desired
product gas
but are highly permeable to the impurities in the gas stream. Membranes are
packaged into
modules where a high-pressure gas mixture is input at the inlet. The membrane
retains the
desired product gas at high pressure and allows the undesired impurity gases
to leave in a
separate low-pressure stream. For CO purification, the membrane retains CO,
but allows H2
and CO2 to pass through. Pure CO will exit in the product stream. H2, CO2, and
a small volume
of CO will leave in the waste stream. The low-pressure waste stream can be
repressurized by
a compressor and passed through another membrane stage to increase the
recovery of CO
product. Greater than 99% pure CO can result from a membrane-based separation
process.
[0383] A sorption process for removing CO may be employed. Sorption processes
may
employ pressure swing, temperature swing, vacuum swing, or changes in other
operating
conditions (e.g. humidity swing). Sorbents are typically solids or liquids
with a high affinity
for the desired gas molecule under one operating condition extreme and a low
affinity for the
gas molecule under the other extreme of operating condition. For example,
sorbents for CO
under high pressure conditions (e.g., about 300 kPa, and about 40-60C) can
capture CO (about
60-70m01%) from a mixture of CO2 and Hz. A system with 4 adsorption towers
containing 8L
each of sorbent results in a flow rate of about 5-10 Nm3 of 99-99.9% pure
product CO. Use
81
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
of larger towers or additional stages allows for about 20-30% CO-containing
gas mixtures to
be upgraded to about 99% or greater purity. Sorbents for CO adsorption have
been developed
by Kobe steel using copper dopants in a solid matrix such as carbon, alumina,
or silica.
103841 Figure 19 depicts a system 1901 for verifying a carbon monoxide stream
containing
carbon dioxide and possibly other components such as hydrogen. As depicted,
system 1901
includes an absorber 1903 configured to selectively remove carbon dioxide by
contacting the
carbon dioxide containing gas with absorbent material such as an ethanolamine.
Absorber 1903
includes a gas inlet 1905 for receiving an inlet gas stream such as a gas
stream from a carbon
dioxide electrolyzer (not shown). Absorber 1903 also includes a gas outlet
1907 for releasing
purified carbon monoxide.
[0385] Also, as depicted, absorber 1903 includes an inlet 1911 for receiving
purified sorbent
and an outlet 1909 for expelling loaded sorbent, e.g., sorbent containing
higher concentrations
of carbon dioxide than the sorbent entering the absorber 1903.
[0386] System 1901 also includes a regenerator 1913 configured to remove
carbon dioxide
expelled by absorber 1903 and thereby produce a regenerated sorbent material
for reuse in the
absorber 1903. In the depicted embodiment, the sorbent is regenerated by
heating, which
releases carbon dioxide. Heating occurs using a pre-heater 1915, and a
reboiler 1917. Preheater
1915 is configured to receive loaded sorbent from the outlet 1909 of absorber
1903 and deliver
the preheated sorbent to an inlet 1919 of regenerator 1913. Preheater 1915
receives some heat
from lean sorbent on its way to absorber 1903 from regenerator 1913.
[0387] System 1901 is configured to transport lean sorbent from an outlet 1923
of regenerator
1913, using a lean solvent pump 1921, to preheater 1915, where the sorbent
loses some of its
heat. System 1901 is also configured to transport lean sorbent from preheater
1915 to absorber
inlet 1911 via a trim cooler 1925. Trim cooler 1925 is configured to further
cool the sorbent
to a temperature where it can effectively do its job of removing CO2 in
absorber 1903. The trim
cooler may be a water-cooled module.
[0388] As indicated, reboiler 1917 is configured to provide heat for
regenerator 1913 to release
carbon dioxide from loaded sorbent. As illustrated, reboiler 1917 is included
in a recirculation
loop that receives a fraction of the lean sorbent from regenerator outlet 1923
and returns heated
lean sorbent to regenerator 1913 via an inlet 1927.
103891 Additionally, system 1901 includes a recycle compressor 1931 configured
to compress
the carbon dioxide to, e.g., a pressure suitable for entering the carbon
dioxide electrolyzer that
produces the inlet carbon monoxide-containing stream.
[0390] System 1901 also includes a subsystem associated with regenerator 1913
that employs
82
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
a condenser 1933 to condense some of the sorbent that may be included in the
released carbon
dioxide that exits regenerator 1913 through a gas outlet 1935. Note that
condenser 1933 is
configured to condense the sorbent and deliver it back to regenerator 1913 at
a sorbent inlet
1937.
103911 In some cases, a CO purifier is a hybrid system having two different
purification
subsystems connected in series. For example, a CO purifier may have a
cryogenic subsystem
upstream from sorbent subsystem. Hybrid systems may be used, for example, in
systems where
the input CO stream has a relatively low concentration carbon monoxide such as
below about
70% molar.
103921 Figure 20 illustrates a hybrid carbon monoxide purification system 2001
having a
cryogenic preprocessing subsystem 2003 and a sorbent postprocessing subsystem
2005. The
first phase of the system 2001 is the cryogenic subsystem 2003, which is
configured to partially
concentrate the carbon monoxide. In certain embodiments, the cryogenic
subsystem is
configured to concentrate carbon monoxide to a level of at least about 70% by
volume.
103931 As depicted, cryogenic preprocessing subsystem 2003 is configured to
feed product gas
from, e.g., a carbon dioxide electrolyzer (not shown) to a compressor 2007
which is configured
to compress the gas to a defined pressure or density. System 2001 is
configured to transport
the compressed gas from compressor 2007 to a chiller 2011 configured to reduce
the
temperature of the compressed gas. Chiller 2011 is coupled to a refrigeration
system 2009,
configured to remove sufficient heat from Chiller 2011 to maintain the
compressed gas at or
below a desired temperature.
103941 Chiller 2011 is configured to chill the compressed gas to a reduced
temperature. Chiller
2011 is also configured to receive a CO2 slurry (which provides a reduced
temperature) from a
separator 2019 and release the CO2 to a trim vaporizer 2013 configured to
release vaporized
carbon dioxide. The vaporized carbon dioxide from trim vaporizer 2013 may be
transported by
system 2001 to a recycle compressor 2015, configured to provide pressurized
carbon dioxide
suitable for feed to the cathode side of the carbon dioxide electrolyzer.
103951 In the depicted embodiment, cryogenic subsystem 2003 is configured to
provide chilled
and compressed from an output of chiller 2011 to a Joule Thompson valve 2017
configured to
rapidly expand the compressed gas and thereby further cool the gas. This
action may
sufficiently cool the gas to convert some of the gaseous CO2 into liquid or
solid or slurry.
Regardless, the cold gas is provided to separator 2019 connected to the Joule
Thompson valve
2017 and having a carbon dioxide slurry outlet 2021 and a partially purified
carbon monoxide
gas stream outlet 2023. In certain embodiments, the partially purified carbon
monoxide stream
83
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
has a concentration of at least about 50% molar or at least about 70% molar.
[0396] In the depicted embodiment, system 2001 is configured to provide the
partially purified
carbon monoxide gas stream from outlet 2023 to the sorbent postprocessing
subsystem 2005,
which in the depicted embodiment is configured similarly to the entire sorbent
system 1901.
As depicted, subsystem 2005 has an absorber 2033 configured to absorb carbon
dioxide from
the partially purified CO stream and output purified CO gas. Subsystem 2005
also has sorbent
regenerator 2043. Other components of subsystem 2005 include a sorbent trim
cooler, a
sorbent preheater, a lean sorbent pump, a sorbent reboiler, and a sorbent
condenser.
[0397] While the discussion of Figures 19 and 20, as well as the other
discussion of carbon
monoxide purifiers describes cases that involve removal of carbon dioxide,
carbon monoxide
purifiers may additionally or alternatively be configured to remove other
impurity gases such
as sulfur containing gases (e.g., sulfur oxides).
Supplemental Hydrogen Sources
[0398] In certain embodiments, an integrated system employing a carbon oxide
electrolyzer
includes an additional source of hydrogen (beyond that generated from the
electrolyzer) or are
configured to receive additional hydrogen from an external source. Examples of
integrated
systems that may employ additional sources of hydrogen include Fischer Tropsch
systems,
polycarbonate production systems, ethylene glycol production systems,
polyethylene
terephthalate production systems, methanol, butanol, and/or other alcohol
production systems,
acetic acid production systems, isocyanate production systems, polyurethane
production
systems, and oxalic production systems. In certain embodiments, the additional
source of
hydrogen is a water electrolyzer such a proton exchange membrane water
electrolyzer. In some
implementation, a water electrolyzer shares electrical infrastructure with a
carbon oxide
reduction electrolyzer. In certain embodiments, the additional source of
hydrogen is or
comprises units configured to perform (a) steam reforming, thermal cracking,
and/or partial
oxidation of methane, fuel oil, petroleum coke, and/or other fossil fuels,
coal gasification, (b)
steam methane reforming, (c) gasification, pyrolysis, and/or other high
temperature conversion
of biomass, municipal solid waste, and/or other waste sources, (d) pressure
swing adsorption
of refinery waste streams, (e) separation of hydrogen byproduct from
industrial reactions such
as molten salt chlorine production, and/or (f) dissociation of water by, e.g.,
solar/thermal
energy. In certain embodiments, methane or other simple hydrocarbon used in
one or more
these units is derived from biogas.
84
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
Recovery of Carbon Dioxide from Electrolyzer Output
[0399] In many implementations, the product gas exiting the cathode of a
carbon dioxide
electrolyzer includes a significant fraction of unreacted carbon dioxide. For
example, the
product gas may contain between about 10 and 70% molar carbon dioxide. In
certain
embodiments, a system includes carbon dioxide recovery unit arranged to
receive product gas
from a carbon dioxide electrolyzer and produce a concentrated carbon dioxide
product, which
may optionally be recycled to the electrolyzer. In one example, carbon dioxide
recovery unit
comprises a direct air carbon dioxide recovery module such as described
elsewhere herein. It
should be understood that the product gas from a carbon dioxide electrolyzer
may contain a
much higher concentration of carbon dioxide than air. Therefore, a direct air
capture unit used
with an electrolyzer may have a modified configuration compared to a
corresponding unit used
for direct air capture. As examples, the direct air capture unit may employ
temperature swing
absorption, pressure swing absorption, or electro-swing absorption.
[0400] Figure 21A illustrates a system 2101 having an upstream DAC unit 2105
configured
concentrate carbon dioxide from air, and a downstream DAC unit 2107 configured
to remove
unreacted CO2 from product gas of a carbon dioxide electrolyzer 2103. System
2101 is
configured to combine the purified unreacted carbon dioxide with fresh carbon
dioxide from
the upstream DAC unit 2105 and introduce it to the electrolyzer 2103. The
downstream DAC
unit 2107 may be designed differently from the upstream DAC unit 2105 (e.g.,
amount of
solvent, dimensions of contactor) in order to address the significantly
different concentrations
of carbon dioxide in air versus in the product gas.
[0401] Figure 21B illustrates a system 2111 having a DAC unit 2115 configured
to capture
carbon dioxide from air and separate unreacted carbon dioxide from product gas
of an
electrolyzer 2113. System 2111 is configured to feed the separated carbon
dioxide into the
electrolyzer 2113. The carbon dioxide-lean products and carbon dioxide-lean
air leave DAC
unit 2115 for downstream processing.
Integration with the electrical grid
[0402] When the source of electrical energy for a grid is not directly
controllable in response
to demand, various problems may arise. Solar, wind, and certain other non-
combustion-based
sources of electrical energy are examples of sources where the energy
generation is decoupled
from energy demand.
[0403] When a renewable energy source is connected to an electrical grid,
fluctuations in wind
speed or sunlight intensity may lower the amount of power available on the
grid to a point
where the demand exceeds supply. This may lower the frequency of the grid,
which can
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
damage some electrical equipment and/or cause a brown out or black out.
[0404] To prevent this, a flexible power generator and consumer may be used in
conjunction
with a renewable power source. Such component may facilitate electrical grid
load leveling
system and be configured to draw a flexible load from the grid, reducing power
demand when
necessary to prevent demand from approaching the supply. This can provide
frequency
stabilization to the grid to allow it to operate with high amounts of
renewable electricity.
[0405] Various approaches have been proposed to store the excess energy
generated when
supply outstrips demand. Examples include water reservoirs, batteries, and
water electrolyzers.
Using batteries, as an example, to store excess energy on a grid requires a
large number of high
capacity batteries to provide sufficient capacity to store the maximum excess
energy that
energy sources on the grid may produce.
[0406] By comparison, a carbon oxide electrolyzer may store excess energy in
the form of a
liquid or a gas, which is relatively easy to store. And, compared to water
electrolyzers, a carbon
oxide reduction cell can be operated in a manner that produces liquid products
rather than
gaseous products. Liquid products may he easier to store, particularly given
their relatively
higher density.
[0407] The products of a carbon oxide electrolyzer can be used as fuel for
generating electrical
energy to put on the grid in periods where demand may exceed supply. The
electrolyzer
products may be combusted in a turbine or other mechanical source of
electrical power and/or
electrochemically consumed in a fuel cell to directly produce electrical
power. In certain
embodiments, a carbon oxide electrolyzer output such as carbon monoxide or
methanol is
stored for later use in a fuel cell to directly inject electrical energy back
into the grid. In certain
embodiments, the fuel cell is a fuel cell configured to oxidize carbon-
containing reactants (e.g.,
natural gas) such as a solid oxide fuel cell from Bloom Energy of Sunnyvale,
CA.
[0408] CO2 electrolysis products can be gas (e.g. CO, methane, ethylene) or
liquid phase (e.g.
ethanol, methanol, ethylene glycol). Liquid products have the advantage of
being easy to store
for extended periods of time. Gas phase products can be converted to liquid-
phase chemical
compounds through a range of downstream processes, such as gas fermentation or
thermochemical reactions. Gas and liquid phase products can also be used to
make solid
materials. For example, CO is one of the inputs needed to make polycarbonate
or can react
with potassium hydroxide to make potassium formate.
[0409] Figure 22A depicts a system 2201 that couples a carbon oxide reduction
electrolyzer
2203 to an electrical grid 2205 or other source of electrical energy. This
system 2201 may be
configured to operate in a manner that stores excess energy generated by the
electrical system
86
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
when the energy being produced exceeds the demand for electrical energy.
[0410] In system 2201, a variable electrical energy source 2206 generates
electrical energy that
is optionally provided to an electrical grid 2205. In the depicted embodiment
energy source
2206 is coupled to the grid 2205 via an electrical line having a rectifier
2207 and a transformer
2209. These and/or other electrical elements may be employed to provide the
electrical energy
from the variable source 2206 to the grid 2205 at an appropriate voltage and
waveform,
[0411] In the depicted embodiment, the grid 2205 is connected to a demand for
electrical
energy that is approximately constant, at least compared to the variability of
the electrical
energy supply 2206. In the depicted embodiment, the demand is illustrated by
element 2208,
which generically represents one or more electrical energy consumers such as
residential and/or
industrial consumers. For many applications, the energy consumers require
electrical energy at
a voltage and electrical waveform that can be produced by a rectifier 2211 and
a transformer
2213. As shown, these elements are provided between the grid 2205 and the
demand 2208.
[0412] As indicated, the depicted embodiment provides an electrolytic carbon
oxide reduction
cell or stack 2203, which is configured to consume excess electrical energy
from the grid or
other electrical energy system by converting the excess energy to chemical
products of
electrolytic carbon oxide reduction. If this cell or stack 2203 receives
electrical energy directly
from a grid, it may require that the electrical energy be rectified and
transformed such as by a
rectifier 2217, a transformer 2219, and/or other electrical components.
[0413] A cathode side of the electrolyzer 2203 receives a carbon oxide
reactant (carbon dioxide
and/or carbon monoxide) via an inlet line. The carbon oxide may be provided by
any one or
more of many possible sources or feedstocks 2222 such as those described
elsewhere herein.
[0414] The cathode is configured to produce a product such as a gaseous or
liquid Cl
compound (e.g., carbon monoxide, methane, formaldehyde, or formic acid) or a
gaseous or
liquid higher carbon compound such as ethylene. Such product along with other
components
are removed from the electrolyzer 2203 via an outlet line. In certain
embodiments, the outlet
gas, which may be humidified, is provided to a gas separator 2225. Which
condenses water
and/or one or more liquid products. Unreacted carbon oxide and/or water may be
provided
back to the carbon oxide electrolyzer 2203 via a line 2221. In certain
embodiments, carbon
oxide supplied to the electrolyzer is humidified to facilitate the reduction
reaction.
104151 In the depicted embodiment, various optional components are provided
downstream
from the cathode side of the electrolyzer 2203. These include a demister 2227,
a mass flow
meter or controller 2229, a condensate trap 2231, a valve 2233, a gas
reservoir 2235, a
purification module 2237, and a product conversion system/reactor 2239. A
resulting fuel,
87
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
chemical, or other material 2241 is appropriately stored or used. As
mentioned, the chemical
products of electrolyzer 2203 store the excess energy produced by the variable
energy source.
That energy may be recovered by converting the chemical energy stored in the
products to
thermal or electrical energy by combustion, fuel cell operation, etc.
Alternatively, the energy
is not recovered, at least not in the near term, and the electrochemical
reduction products are
converted to another useful commodity such as a plastic.
[0416] The electrolyzer 2203 also contains an anode that receives an anode
reactant such as
water that is electrolytically oxidized at the anode. In the depicted
embodiment, a source of
water 2243 is provided to the anode of cell 2203 via one or more optional
components such as
a feed water pump 2245, a demister 2247, a gas separation unit 2249¨which also
has an inlet
for receiving product from the anode, which product may include water vapor
and oxygen a
circulation pump 2251, a mass flow meter or controller, and an ion exchanger
configured to
remove ions or other components of the anode water that could be deleterious
to the operation
of the electrolyzer 2203. In certain embodiments system 2201 contains a line
connecting the
anode water recirculation loop to the carbon oxide inlet line 2221 to humidify
the carbon oxide
delivered to the cathode side of electrolyzer 2203.
[0417] The system 2201 may also have one or more components for removing
oxygen or other
product of the electrolyzer 2203. The anode side of electrolyzer 2203 includes
an outlet line
22057 configured to remove oxygen or other product from the electrolyzer.
Outlet line 2207
connects with separation unit 2249. The oxygen and water in the anode product
stream may
be separated from one another in unit 2249, so that the water can be recycle
back to the anode
via the circulation pump 2251.
[0418] System 2201 may be configured with components for removing oxygen or
other
gaseous product of the anode. In the depicted embodiment, these components are
a demister
2259 and a control valve 2261.
104191 In these examples and throughout the disclosure, the described systems
may be
provided in a facility, a plant, or a complex of buildings. In some
embodiments, all or many
reactors and units and/or modules of a system are provided in a common
factory, plant, or
complex. For example, a system for producing a particular material such as a
polycarbonate
polymer or transportation fuel may comprise a carbon oxide electrolyzer and
one or more other
reactors that utilize a product of the electrolyzer and/or provide a reactant
to the electrolyzer,
and the electrolyzer and other reactors are provided in a single building or
plant. In some cases,
one or more system components is provided in the exterior environment. For
example, a direct
air capture unit may be provided outside, while a carbon dioxide electrolyzer
is located inside
88
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
a building, even though the system is configured to provide carbon dioxide
from the direct air
capture unit to the electrolyzer.
[0420] Further examples of grid management systems employing electrolyzers are
depicted in
Figures 22B and 22C. As depicted, systems 2258 and 2259 employ fuel cells
configured to
oxidize carbon containing reactants (e.g., methane or natural gas) such as
solid oxide fuel cells
from Bloom Energy as mentioned above. In the depicted embodiments, a carbon
oxide
electrolyzer 2261 is coupled to a grid or other source of electrical energy
2263 such as a device
configured to generate electricity from wind, solar, or other renewable energy
source. In
addition to electrical energy, electrolyzer 2261 receives carbon dioxide and
water as inputs. In
certain embodiments, the carbon dioxide input is received, at least in part,
from output of a fuel
cell 2265. A compressor 2267 may be provided to compress such carbon dioxide
before
delivery to electrolyzer 2261.
104211 As depicted in the embodiment of Figure 22B, electrrolyzer 2261 is
configured to
output carbon monoxide, and system 2258 is configured to deliver the carbon
monoxide to fuel
cell 2265. System 2258 may be further configured to provide natural gas or
other input to fuel
cell 2265, which is in turn configured to generate electricity that may be
provide to grid 2263.
In some implementations, system 2258 includes a steam methane reformer unit
2271
configured to produce hydrogen for input to the fuel cell 2265.
[0422] As depicted in the embodiment of Figure 22C, electrrolyzer 2261 is
configured to
output any one or more of various compounds including carbon monoxide,
ethylene, methane,
and the like. In certain embodiments, one or more of these compounds is
removed from system
2259 for purposes potentially unrelated to electrical load leveling. For
example, one or more
of the compounds may be used as a feed stock for synthesizing a compound or
polymer such
as described elsewhere herein.
[0423] In the depicted embodiment, system 2259 is configured to deliver at
least some of the
output of electrolyzer 2261 to fuel cell 2265, optionally together with
natural gas or other fuel
from an external source. In certain embodiments, output from electrolyzer 2261
is provided as
a synthetic natural gas or is converted to form such gas. System 2259 is
configured to supply
the synthetic natural gas alone or with other natural gas to fuel cell 2265.
In certain
embodiments, system 2259 is configured to provide carbon monoxide and/or other
output of
electrolyzer 2261 directly to fuel cell 2265. In some implementations, system
2259 includes a
steam methane reformer and/or a pressure swing absorption unit 2273 or other
purification unit
configured to process gas before it is input fuel cell 2265. A steam methane
reformer may
convert methane from a natural gas source and/or the electrolyzer to hydrogen
for delivery to
89
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
the fuel cell 2265. A pressure swing absorption unit may be used to remove
some carbon
dioxide or other impurity from the inlet stream to the fuel cell 2265. In some
embodiments,
another type of impurity removal unit is employed.
[0424] In certain embodiments such as those depicted in Figure 22B and/or
Figure 22C, a
pressure swing absorber or other gas purification unit is configured to
separate a pure hydrogen
stream from the gas mixture exiting a steam methane reformer. The tail gas
from the reformer
may include CO, CO2, unreacted CH4, and some H2. The tail gas is fed to the
fuel cell, which,
in turn, produces a stream of relatively pure CO2 (and water) which is then
fed to the CO2
electrolyzer. Some steam methane reformers are configured to make a product
having a
relatively high concentration of hydrogen by employing a water gas shift
reaction after the
reforming reaction. The water gas shift reaction converts carbon monoxide and
water
(reactants) into carbon dioxide and hydrogen. In some implementations, steam
methane
reformers are configured to make a syngas mixture and may not implement the
water gas shift
step.
[0425] In some implementations such as those depicted in Figure 22B and/or
Figure 22C, a
steam methane reformer produces two CO2-containing streams: (1) the tail gas,
as described
above, which comes from the reactor, and (2) flue gas, which comes from the
furnaces that are
used to heat the reactor tubes and generate steam for the reaction. The tail
gas may contain
fairly high concentrations of carbon dioxide (e.g., about 15% before
separation and then about
50% after separation). The flue gas has a relatively lower concentration of
carbon dioxide (e.g.,
only about 3-5% CO2 concentration). In various implementations, about two
thirds of the
carbon dioxide emissions are from the tail gas, and about one-third are from
the flue gas. In
certain embodiments, the system is configured to capture emissions from (a)
the tail gas before
the purification (e.g., with a pressure swing absorber), (b) the tail gas
after the purification, (c)
the flue gas, or (d) a combined stream from flue gas and the tail gas. In some
implementations,
a system is configured to feed oxygen from a carbon oxide electrolyzer into
one or more
furnaces of a steam methane reformer. In some implementations, this provides
higher
efficiency and/or yields a higher carbon dioxide concentration in the flue
gas, thereby
simplifying carbon dioxide capture.
104261 Fuel cell 2265 is configured to output electricity that may be
delivered to grid 2263. In
certain embodiments, system 2259 comprises a carbon dioxide storage unit 2269
configured to
store carbon dioxide output from fuel cell 2265 prior to use by electrolyzer
2261.
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
Syngas Preparation
[0427] Embodiments described in this section and/or illustrated in Figures 23A-
D pertain to
making mixtures of carbon monoxide and hydrogen. Some of these mixtures may be
referred
to as syngas. The embodiments described here concern methods and systems
configured to
receive a mixture of carbon monoxide, carbon dioxide, and hydrogen and modify
the mixture
to produce a mixture of carbon monoxide and hydrogen having a particular
composition. In
some cases, the input is a gaseous mixture obtained from a carbon oxide
electrolyzer such as
one of the carbon oxide electrolyzers described herein.
[0428] A carbon monoxide and hydrogen mixture produced as described here may
have
various applications. It can be used to produce a naphtha or other liquid
hydrocarbon
composition such as may be produced by a Fischer Tropsch process (see e.g.,
the discussion of
Figures 6A and 6B). It can also be used as an input to a gas fermentation
reactor (see e.g.,
Figures 4 and 5). It can also be sued to produce any of various chemicals such
as alcohols (see
e.g., Figures 15 and 18) and/or polyols (see e.g., Figures 14 and 16).
[0429] Various embodiments for producing a mixture of carbon monoxide and
hydrogen may
employ carbon oxide separator system such as described in relation to Figures
19 and 20.
[0430] A mixture of carbon monoxide and hydrogen may be produced by directly
removing
either CO or CO2 from an input stream. In embodiments that produce the mixture
by directly
separating CO from the input stream, hydrogen may be added to a purified CO
stream
downstream from a CO purification operation. For example, purified hydrogen
may be
prepared by separating it from CO2 in an operation that is downstream from the
CO separation
operation.
[0431] Figure 23A depicts a general scheme for producing a mixture of carbon
monoxide and
hydrogen in a process that separates carbon monoxide directly from the input
stream. The
input stream, which may be provided from the cathode outlet of a carbon
dioxide electrolyzer,
contains carbon dioxide, carbon monoxide, hydrogen, and optionally other
components such
as small amounts of water and/or hydrocarbon(s). The input stream is fed to
one or more
separation elements 2303 configured to produce one stream 2305 containing
purified carbon
monoxide and another stream 2307 containing a mixture of carbon dioxide and
hydrogen.
Element(s) 2303 may include, for example, a CO absorption element and/or a CO
absorption
and stripping subsystem such as a pressure swing or temperature swing
subsystem. Steam
2307 is fed to one or more elements 2309 configured to separate hydrogen from
carbon dioxide.
In some embodiments, element(s) 2309 contain a membrane separator that, e.g.,
blocks passage
of carbon dioxide while allowing passage of hydrogen. In operation, element(s)
2309 produce
91
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
a hydrogen stream 2311 and a carbon dioxide stream 2313. Hydrogen stream 2311
may be
combined with carbon monoxide stream 2305 to produce a stream 2315 containing
a mixture
of carbon monoxide and hydrogen. Carbon dioxide stream 2313 may be,
optionally, recycled
to a carbon oxide electrolyzer.
[0432] In some embodiments, a process of making a mixture of hydrogen and
carbon
monoxide may be characterized by the following operations:
1. Separation of CO from the mixture by, e.g., ionic liquid absorption in a
pressure swing
absorption process
2. Separate H2 from CO2 via, e.g., a membrane
3. Mix H2 and CO
[0433] In certain embodiments for producing purified carbon monoxide directly
from the
input stream, an ionic liquid is used to strip the carbon monoxide from an
input gas stream.
During the separation, the ionic liquid contacts the input gas and selectively
absorbs the carbon
monoxide while allowing most of the hydrogen and carbon dioxide to pass
(undissolved or
unabsorbed). In some embodiments, the input stream contacts ionic liquid in an
absorption
column. After contacting, the input stream, a carbon monoxide rich stream of
ionic liquid is
fed to a stripper which operates under conditions that strip carbon monoxide
from the ionic
liquid. A resulting lean stream of ionic liquid may be recycled back to the
component(s) that
selectively absorb carbon monoxide.
[0434] Suitable ionic liquids for separating carbon monoxide preferentially
absorb carbon
monoxide without substantially absorbing carbon dioxide and/or hydrogen.
Other
characteristics may include low cost, low vapor pressure (e.g., no production
of volatile organic
compounds during use), low kinematic viscosity (e.g., below about 150 cSt),
moderate
absorption conditions (e.g., temperature of about 0 C to 20 C; pressure of
about 25 bar or lower
(e.g., about 17 bar)), moderate stripping conditions (e.g., temperature of
about 0-100 C or
lower; pressure of about 5 bar or lower),and/or low toxicity. Examples of such
ionic liquids
include 1-hexy1-3-methylimidazolium chloride (with cuprous chloride).
[0435] In some embodiments, a mixture of carbon monoxide and hydrogen is
produced by
directly removing carbon dioxide from an input stream containing carbon
monoxide, hydrogen,
and carbon dioxide. In such embodiments, one output stream of the separation
includes a
desired mixture of hydrogen and carbon monoxide.
[0436] Figure 23B depicts a general scheme for producing a mixture of carbon
monoxide and
hydrogen in a process that separates carbon dioxide directly from the input
stream. The input
stream, which may be provided from the cathode outlet of a carbon dioxide
electrolyzer,
92
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
contains carbon dioxide, carbon monoxide, hydrogen, and optionally other
components such
as small amounts of water and/or hydrocarbon(s). The input stream is fed to
one or more
elements 2302 configured to produce a carbon dioxide stream 2304 containing
purified carbon
dioxide and another stream 2306 containing a mixture of carbon monoxide and
hydrogen.
Element(s) 2302 may include, for example, a carbon dioxide absorption element
or a subsystem
containing carbon dioxide absorption and stripping elements such as a pressure
swing or
temperature swing subsystem. Carbon dioxide stream may be, optionally,
recycled to a carbon
oxide electrolyzer.
[0437] In certain embodiments for removing carbon dioxide directly from the
input stream,
an ionic liquid is used to strip the carbon dioxide from an input gas stream.
The ionic liquid
contacts the input gas and selectively absorbs the carbon dioxide while
allowing most of the
hydrogen and carbon monoxide to pass. In some embodiments, the input stream
contacts ionic
liquid in an absorption column. After contacting, the input stream, a carbon
dioxide rich stream
of ionic liquid is fed to a stripper which operates under conditions that
strip carbon dioxide
from the ionic liquid. A resulting lean stream of ionic liquid may he recycled
back to the
component(s) that selectively absorb carbon dioxide.
[0438] Suitable ionic liquids for separating carbon dioxide preferentially
absorb carbon
dioxide without substantially absorbing carbon monoxide and/or hydrogen.
Other
characteristics may include low cost, low vapor pressure (e.g., no production
of volatile organic
compounds during use), low kinematic viscosity (e.g., below about 150 cSt),
moderate
absorption conditions (e.g., temperature of about 15 C or higher; pressure of
about 50 bar or
lower), moderate stripping conditions (e.g., temperature of about 0-100 C or
lower; pressure of
about 5 bar or lower),and/or low toxicity. Examples of such ionic liquids
include 1-Buty1-3-
methylimidazolium hexafluorophosphate [bmim][PF6].
[0439] In some implementations, a system configured to produce a mixture of
carbon
monoxide and hydrogen contains no components configured to cool an input or
outstream
below about 30 C or below about 20 C or below about 10 C. For example, the
system does
not include a compressor to chill the inlet gas below about 20 C
CO separation example
104401 As explained, carbon monoxide and hydrogen are included in output from
a carbon
dioxide electrolzyer. This electrolyzer output gas serves as an input to
separation system that
produces a mixture of carbon monoxide and hydrogen. In some embodiments, the
carbon
monoxide is recovered from the gas stream by a pressure swing absorption
process using an
ionic liquid such as 1-hexy1-3-methylimidazolium chloride (CuC1).
93
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
[0441] A pressurized inlet gas stream enters at the bottom of the absorption
column, while
absorbent is sprayed from the column's top. A packed bed may enhance the
contact between
the gas and liquid phase and facilitate carbon monoxide absorption. The
remaining gas
compounds (CO2, H2, and minor leftover CO) leave from the column's top while
CO enriched
liquid phase leaves from the column's bottom.
[0442] In certain embodiments, the absorbent includes a mixture of CuCl and
the ionic liquid
(e.g., about 50% mol CuCl and 50% mol ionic liquid 1-hexy1-3-methylimidazolium
chloride).
The inlet molar amount of CuCl may be about 1.5 to 2.5 times (e.g., about 1.9
times) larger
than the amount of carbon monoxide to be absorbed.
[0443] The CO enriched liquid phase is heated in a heat exchanger and then
enters a stripping
column. The stripping column's pressure may be less than 2 bar (e.g.,
approximately
atmospheric), while temperature may be relatively low at the top (e.g., about
30 C) to relatively
high at the bottom (e.g., about 60 C). Under these conditions, most of the CO
may evaporate.
The liquid phase flows to the bottom of the column, where some fraction (e.g.,
about 15%) is
recirculated back to the column using, e.g., a total reboiler, while the
remaining (e.g., about
85%) flows back to the absorption column. In certain embodiments, boiled
liquid coming from
a reboiler transfers heat to the incoming stream.
[0444] See the following CO absorption-stripping components of system 2321
shown in
Figure 23C: An inlet stream 2322 is compressed and chilled by a compressor
2323 working in
conjunction with a chiller 2324. Compressed and chilled inlet gas enters the
bottom of an
absorption column 2325 where CO in the inlet stream is selectively absorbed by
a liquid. A
CO-enriched liquid phase is heated in the heat exchanger 2326 (stream 5) and
then enters the
stripping column 2327 (stream 6). The stripping column's pressure may be
atmospheric, while
temperature may, for example, range from 30 C at the top to 60 C at the
bottom. The CO's
solubility in the absorbent at these operating parameters is low, causing most
CO to evaporate.
The liquid phase flows to column's 2327 bottom (stream 8), where around, e.g.,
15% is
recirculated back to column 2327 using a reboiler 2328 (stream 9/10), while
the remaining,
e.g., 85% flows back to absorption column 2325 (stream 11-16).
[0445] A gas stream of CO2 and Hz exits the top of absorption column 2325. The
CO2 and
Hz may be separated by various techniques. In some embodiments, they are
separated using a
membrane filter 2330. In some embodiments, the membrane filter is a
Polaris11`4 filter from
Membrane Technology and Research Corporation Inc. of Newark, CA. Such membrane
may
have a high permeability of CO2 compared to Hz, which is obtained as a
retentate. In some
example, a membrane filter operates at a pressure of about 5 to 15 bar (e.g.,
about 9 bar
94
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
pressure) and/or at a temperature of about 0-20 C (e.g., about 5 C).
CO2 separation example
[0446] As in the CO absorption embodiments, carbon monoxide and hydrogen are
included
in output from a carbon dioxide electrolzyer. This electrolyzer output gas
serves as an input to
a separation system that produces a mixture of carbon monoxide and hydrogen.
In certain
implementations, CO2 is absorbed directly from the input stream using an ionic
liquid such as
[bmina] [PF6J. The absorption may be conducted at any of various pressures
(e.g., about 10-60
bar). In some implementations, the inlet gas stream's pressure may be about 8
to 16 bar. To
this end, the system may employ additional compression by a compressor.
[0447] In some embodiments, a CO2 absorption column is operated at a
relatively high
pressure such as about 20 to 60 bar or about 40-50 bar (e.g., about 44 bar).
At this pressure and
C, the solubility of CO2 is about 0.2 moles in 1 mole of ionic liquid. In some
embodiments,
a CO2 absorption column is operated at a relatively low pressure such as about
1 to 20 bar. In
certain embodiments, the absorption column is operated at temperature of about
20 to 80 C, or
about 20-30 C, or about 40 to 60 C.
20 [0448] Figure 23D illustrates an example CO2 absorption-stripping system
2351 for
processing a carbon oxide electrolyzer's output to produce a mixture of carbon
monoxide and
hydrogen. An inlet stream is compressed by a compressor 2353. Compressed inlet
gas (stream
(2)) enters the bottom of an absorption column 2355 where CO2 in the inlet
stream is selectively
absorbed by a liquid (e.g., an ionic liquid). The flow rate of absorbent
liquid may be adjusted
25 or controlled by monitoring the molar flow rate of carbon dioxide in
inlet stream. The
absorbent liquid in the column may be maintained at particular level. Because
the absorbent
recirculates through the system, it may not accumulate in the column's bottom.
A controller
may be employed to sense the amount of absorbent in the column bottom and
adjust or maintain
the absorbent level to a desired level within the column.
104491 In certain embodiments, a CO2 absorption column operates under the
following
conditions:
The partial pressure of CO2: 13 bars
Temperature: 28.1 C
Liquid phase viscosity: 110 cP
Gaseous phase viscosity: 0.017 cP
Diffusivity of the CO2 in the absorbent: 500 1nni2/s
Column diameter: 0.5 m
Column height: 12.4 m
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
Pressure drop: 0.012 bar
Cf (packing constant): 170
Packing total specific area: 108 m2/m3
Nominal packing diameter: 0.005 m
[0450] A gas stream of CO and H2 (stream (3)) exits the top of absorption
column 2355. This
stream may be employed as a syngas.
[0451] A CO2-enriched liquid phase from the absorption column 2355 is heated
in a heat
exchanger 2356 (stream 5) and then enters a stripping column 2357 (stream 6).
The CO2's
solubility in the absorbent at operating parameters in the stripper is low,
causing most CO2 to
evaporate. A liquid phase flows to stripping column's 2357 bottom (stream 8),
where a fraction
of it is recirculated back to column 2357 using a reboiler 2358 (stream 9/10),
while the
remaining liquid flows back to absorption column 2355 (stream 11-16). In
certain
embodiments, around 4 to 5% of the bottom liquid at the bottom of the
absorption column is
recirculated back to the column using, e.g., a reboiler.
[0452] In certain embodiments, a CO2 stripping column is operated at a
pressure of
approximately 0.5 to 5 bar (e.g., about 1 bar), with the inlet CO2-rich
absorbent stream having
a temperature of about 40 to 60 C, and the column's bottom having a
temperature of about 60
to 80 'C.
Ethylene Purification
[0453] Embodiments described in this section pertain to making ethylene. The
embodiments
described here concern methods and systems configured to receive a mixture
containing
ethylene and modifying the mixture to produce purified ethylene. In some
cases, the input is a
gaseous mixture obtained from a carbon oxide electrolyzer such as one of the
carbon oxide
el ectrolyzers described herein.
[0454] In some implementations, an ethylene purification system is configured
to produce
relatively pure ethylene, without necessarily producing a relatively pure
stream of any other
components produced by the electrolyzer. In some implementations, an ethylene
purification
system is configured to produce relatively pure ethylene along with a
relatively pure stream of
one or more other components such as hydrogen, carbon monoxide, carbon
dioxide, methane,
ethanol, or any combination thereof
104551 In various embodiments, an ethylene purification system includes one or
more
components or subsystems for (a) absorbing and separating carbon dioxide, (b)
separating
ethylene from one or more other components by membrane filtration, (c)
fractional distillation
to separate ethylene and methane, (d) chemically converting methane to
ethylene, and (e) any
96
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
combination of (a)-(d). In some embodiments, an amine or ionic liquid is used
to absorb carbon
dioxide. In some embodiments, a membrane filtration component is configured to
separate
carbon monoxide and hydrogen from ethylene (optionally along with methane).
[0456] Ethylene produced as described here may have various applications. For
example, it
can be used to produce ethylene oxide (see e.g., the discussion of Figures
10A, 11, and 14) and,
in some cases, reaction products of ethylene oxide such as monoethylene glycol
and
polyethylene glycol.
[0457] In certain embodiments described in this section, the input gas
includes ethylene and
typically some methane and unreacted carbon dioxide. Other components that may
be present
include hydrogen, carbon monoxide water, ethanol, and any combination thereof
[0458] As an example, an inlet stream to an ethylene purification system may
have a
composition of the follow mol%: hydrogen (4.75%), methane (23.72%), carbon
monoxide
(0%), carbon dioxide (50.73%), ethylene ( 9.49%), ethyl alcohol (4.75%), and
water ( 6.57%).
A composition such as this may be produced by a carbon dioxide electrolyzer.
Pathway 1: Cryogenic distillation for ethylene separation
[0459] In some embodiments, ethylene is separated from other components by a
pathway
including absorption of carbon dioxide and subsequent fractional distillation
to remove
hydrogen, carbon monoxide, and/or methane to produce purified ethylene. As an
example, the
process may include the following sequence of operations:
Operation 1: Condensation of liquid products to remove ethanol and water
Operation 2: CO2 removal
Operation 3: Distillation ¨ removal of hydrogen, CO and methane
Operation 3 (alternate or optional): Methane conversion to ethylene by
oxidative coupling of
methane
[0460] The separation process to remove water and ethanol from an input stream
may be
implemented in various ways. In some embodiments, it is implemented in a two-
step process
of condensation and then a molecular sieve absorption process to further
remove the water and
ethanol. In some embodiments, the condensation of water and ethyl alcohol is
accomplished
using compression of the input stream. In some embodiments, the condensation
of water and
ethyl alcohol is accomplished using an absorption column, e.g., a
countercurrent column with
water as on stream. The column optionally includes a catalyst. In some
implementations,
thermal equilibrium is reached in the column and both outlet streams have the
same
temperature (e.g., about 25 to 50 C).
[0461] In some cases, carbon dioxide is removed from a gas stream using an
amine such as
97
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
diethanolamine, monoethanolamine, dimethylamine, piperazine, 2-aminopropanol,
diisopropanolamine, aminoethoxy ethanol, and/or methyl diethanolamine, or by
an ionic liquid.
In some implementations, the concentration of a chosen amine is at least about
10 times greater
than the concentration of carbon dioxide. In some embodiments, the amine-
containing liquid
has about 50-80 mole percent amine in an aqueous solution.
[0462] Example working conditions for an amine-based carbon dioxide removal
process:
Absorber: about 35 to 50 C and about 5 to 205 atm of absolute pressure;
Regenerator: about 100 to 126 C and about 1.4 to 1.7 atm of absolute pressure
at a tower
bottom.
[0463] In some implementations, a temperature difference of about 5 C or more
is maintained
between the lean amine and sour gas. If the temperature difference is closer,
the condensation
of hydrocarbons may occur.
[0464] Various approaches may be employed to demethanizing an ethylene and
methane
containing stream. In some embodiments, a cryogenic distillation process is
performed. See
e.g., US Patent No. 3,902,329 (King III, et al.), which is incorporated herein
by reference in its
entirety. In some embodiments, cryogenic distillation is conducted at a
temperature of about -
90 C or lower.
104651 In some implementations, an ethylene/methane gas mixture is pressurized
in the
compressor (e.g., to a pressure of about 100 bar and an outlet temperature of
about 15 C). The
gas mixture is cooled with the chilled water. Then, by, e.g., throttling the
compressed gas
mixture with a throttle valve, the outlet gas may be substantially cooled
(e.g., to a temperature
of about -100 C).
[0466] Below is presented an example process for separation of methane from
the ethylene
by cryogenic distillation:
20-30 plates or more
Temperature: -90 C to -105 C on the plate at which the condensate is returned
to the column.
Pressure: 25 to 40 bar or higher
98% Efficiency
[0467] In some examples, a cryogenic distillation column has the following
design
parameters:
Diameter:0.085m
Height: 6.8m
Stages:17
Stage efficiency:80%
98
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
Reflux ratio:1.129
Vapor linear velocity:3 m/s
Separation efficiency: 98%
[0468] In some embodiments, the process removes hydrogen from ethylene (and
optionally
other components) via membrane separation.
[0469] In some embodiments, the process employs oxidative coupling of methane
(OCM) to
ethylene. OCM may be performed on a methane-containing steam after the
separation of
methane and ethylene. This process can produce ethane, CO, Hz, and CO2 as
unwanted
byproducts. Besides the temperature of the reaction, an important parameter is
the amount of
oxygen that reacts with the methane.
[0470] OCM may include some of or all the below-presented reactions. See e.g.,
Bhatia,
Subhash & Thien, Chua & Mohamed, Abdul. (2009). Oxidative coupling of methane
(OCM)
in a catalytic membrane reactor and comparison of its performance with other
catalytic
reactors. Chemical Engineering Journal - CHEM ENG J. 148. 525-532.
10.1016/j.cej.2009.01.008, which is incorporated herein by reference in its
entire-Ey.
Step 1: CH4 + 202 CO2 + 21-yD
Step 2: 2CH4 + 0:502 C21.1 + H20
Step 3: CH4 + 02 CO + H20 + H2
Step 4: CO + 0.502 CO2
Step 5: C2K,,, 4- 0.502 --, C2H4 + H:20
Step 6: C2H$ + 202 2C0 2H20
Step 7: 021-1t, C2H4 + H2
Step 6: C2H4 2C0 + 4H2
Step 3: CO + H20 H2
Step 10: COz + H2 CO + H20
[0471] The yield of this process is dependent on reaction conditions and the
oxygen ratio.
The amounts of methane and oxygen may be chosen to promote reactions in, e.g.,
steps 2 and
5. In certain embodiments, this process occurs in the catalyst membrane
reactor consisting of
disk-shaped planar BSCF membranes.
[0472] Examples of OCM reactor designs and operation are presented in the
following table
taken from X. Tan, K. Li, in Handbook of Membrane Reactors: Reactor Types and
Industrial
Applications, 2013, which is incorporated herein by reference in its entirety.
99
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
%Material 7 CC) Geometry Catalyst SCZ (54) Ycg (%)
Fletereraxas
Eic1.e0.8Stme.203-3 Totsier--
GE) Doi< Le -St, liCe0 ft6 148
E3CF:1514:3lDsv .13.8 153
2r.r>3
18,1 , .978 i-kAkMailt,er
EkIkm31.la3Ck., 21
ISCP Holloo..11bsa
E31.SYS..Sna a05 a `a::r15
BCH fkk 1+41ke-av Mr:43a?.W3:).3,a0:so,
850 -COMM NM-W-Mo eT 114.7
1.1tWitt =-e
[0473] In various embodiments, the OCM temperature is in a range for the steam
cracking of
ethane to produce ethylene. The analysis shows that operating at a temperature
range of 850 C
¨ 950 C and steam to hydrocarbon ratio of 0.3-0.5 produces good ethylene yield
while
minimizing byproducts. In some embodiments, the OCM-cracking reaction is
conducted in a
tubular reactor and at elevated pressure (e.g., about 2 to 2.5 bar).
[0474] In certain embodiments, about 0.3 of the methane is converted to
ethylene (molar),
which may be about the amount of ethylene present in a typical inlet feed.
Therefore, by
utilization of the above process, the produced amount of ethylene is almost
doubled.
[0475] In some embodiments, a process includes an operation of separating
steam and
hydrogen from the formed ethylene. An absorption counter flow column may be
employed for
this operation. As an example, process conditions may include a pressure of
about 5 to 50 bar
(e.g., about 10 bar) and a temperature of about 150 to 500C (e.g., about
300C). In some
embodiments, an absorption column with an adequate volume flow of the water
separates
nearly 100% of water and hydrogen from the gas.
Pathway 2: Usage of the membranes for ethylene separation
104761 In some embodiments, ethylene is separated from other components by a
pathway
including membrane separation of gas streams to produce an ethylene-rich
stream. As an
example, such process may include the following sequence of operations:
Operation 1: Condensation of liquid products to remove ethanol and water
Operation 2: CO2 removal with amine treatment
Operation 3: CO + H2 Removal with membrane separation
Operation 4: Ethylene separation from methane with membrane separation.
[0477] In some implementations, operations I and 2 are performed in the same
manner as in
the above-described pathway that employed cryogenic distillation. Operations 3
and 4 are
performed using membranes designed or configured to separate gaseous
components from one
another. In certain embodiments, suitable membranes are provided by Membrane
Technology
& Research, Inc. of Newark, CA.
[0478] The design may include a compression stage that removes ethanol and
water prior to
100
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
membrane separation.
Membrane Stage to Remove Non-Hydrocarbons
[0479] A first membrane may separate nearly 100% of H2, CO2, ethanol, and
water. It may
not significantly separate CH4 from C2H4.
[0480] As an example, starting from an initial gas mixture of 50.7 mol% CO2,
23.7 mol%
CH4, and 9.5 mol% C2H4, the resulting product stream contains 64.7 mol% CH4
and 30.5 mol%
C2H4, with most of the remaining gas being CO2 (3.7%). To reduce loss of the
CH4/C2H4
mixture in a permeate stream, a two-stage separation design may be employed.
[0481] The pressure is 10 bar, and the inlet stream is at 30 C temperature.
The cooling process
occurs in the membrane, and the temperature of the residue is -2.1 C. The 10%
percent loss is
satisfying from my point of view.
[0482] In some embodiments, the membrane is a hollow fiber membrane comprising
polypropylene (PP), polyethylene (PE), polytetrafluoroethylene (PTFE), PVDF,
polysulfone
(PS), polyetherimide (PEI), or any combination thereof In some embodiments,
the membrane
has a porosity of about 50-70% (e.g., about 60%). In some embodiments, the
membrane has a
mean pore size of about 2 to 3 nm.
Ethylene/methane separation
104831 In certain embodiments, a methane-ethylene separation membrane includes
a metal-
organic membrane for separation at the room temperature. In certain
embodiments, a methane-
ethylene membrane separation, has an adsorption selectivity of 12 to 20 at 296
K. An
adsorption selectivity=20 represents the 95% separation process efficiency.
[0484] The membrane may include a microporous metal¨organic framework
Zn4L(DMA)4
(UTSA-33, H8L=1,2,4,5-tetra (5-isophthalic acid)benzene,DMA=N,N'-
dimethylacetamide)
with small pores of about 4.8 to 6.5 A (He, Yabing, et al. "A microporous
metal¨organic
framework for highly selective separation of acetylene, ethylene, and ethane
from methane at
room temperature." Chemi stry -A European Journal 18.2 (2012): 613, which is
incorporated
herein by reference in its entirety).
[0485] In some implementations, after the methane separation, the methane is
subjected to
OCM to increase the yield of ethylene.
[0486] Pathway 3: Usage of filtration membranes and cryogenic distillation for
ethylene
separation
[0487] In certain embodiments, a membrane filter is employed to separate
methane and
ethylene from other components such as hydrogen, carbon monoxide, and carbon
dioxide. In
some embodiments, a methane ¨ ethylene mixture is subsequently separated by
cryogenic
101
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
distillation into relatively pure streams of ethylene and methane. In some
implementations, a
separate membrane filter is employed to separate hydrogen from carbon
monoxide, carbon
dioxide, and optionally other components.
[0488] In some implementations, a process may include the following
operations:
Operation 1: Compression of gas stream to enable condensation of ethyl alcohol
and water
Operation 2: Removal of water and ethyl alcohol in countercurrent absorption
column with
catalyst
Operation 3: Membrane filtration to separate methane and ethylene from other
gases such as
carbon dioxide, carbon monoxide, and hydrogen
Operation 4 (optional): Membrane filtration to separation hydrogen from carbon
monoxide and
carbon dioxide.
Operation 5: Cryogenic distillation to separate methane and ethane (optionally
use the cooled
methane output stream as a cooling utility)
Oxygen Production
[0489] A carbon oxide electrolyzer anode may produce oxygen from water. The
oxygen may
be employed in any of various integration schemes for the electrolyzer. In
some cases, oxygen
can be used in a combustion reaction with a fuel. In some cases, oxygen can be
compressed
and stored for later use. In certain embodiments, compressed oxygen is cooled
and then passed
through a throttle valve causing the oxygen to liquify. Cooling may be
accomplished using a
Freon-type cooler. In some cases, an oxygen stream is first cooled using a
brine cooler (e.g.,
employing CaCl2 brine). For example, at 40 bars and -120 C, oxygen becomes
liquid. In some
implementations, the oxygen stream is cooled to about -70C or lower.
Controller Embodiments
[0490] In embodiments employing a controller or other logic for controlling
operation of one
or more reactors, pumps, separators, and/or other components of a system the
controller or
logic may employ program instructions such as executable instructions on
computer-readable
medium. The instructions may be executed by computer-executable components
such as those
integrated with a communication system. The computer-readable medium may be
stored on
any suitable computer readable media such as RAMs, ROMs, flash memory,
EEPROMs,
optical devices (CD or DVD), hard drives, floppy drives, or any suitable
device. The computer-
executable component is optionally a processor, but the instructions may
alternatively or
additionally be executed by any suitable dedicated hardware device.
[0491] Although omitted for conciseness, embodiments of the system and/or
method can
include every combination and permutation of the various system components and
the various
102
CA 03187895 2023- 1- 31
WO 2022/031726
PCT/US2021/044378
method processes, wherein one or more instances of the method and/or processes
described
herein can be performed asynchronously (e.g., sequentially), concurrently
(e.g., in parallel), or
in any other suitable order by and/or using one or more instances of the
systems, elements,
and/or entities described herein.
[0492] The Figures illustrate the architecture, functionality and operation of
possible
implementations of systems, methods and computer program products according to
disclosed
embodiments, example configurations, and variations thereof. In this regard,
each block in the
flowchart or block diagrams may represent a module, segment, step, or portion
of code, which
comprises one or more executable instructions for implementing the specified
logical
function(s). It should also be noted that, in some alternative
implementations, the functions
noted in the block can occur out of the order noted in the FIGURES. For
example, two blocks
shown in succession may, in fact, be executed substantially concurrently, or
the blocks may
sometimes be executed in the reverse order, depending upon the functionality
involved. It will
also be noted that each block of the block diagrams and/or flowchart
illustration, and
combinations of blocks in the block diagrams and/or flowchart illustration,
can be implemented
by special purpose hardware-based systems that perform the specified functions
or acts, or
combinations of special purpose hardware and computer instructions.
[0493] As a person skilled in the art will recognize from the previous
detailed description and
from the figures and claims, modifications and changes can be made to the
disclosed
embodiments of the disclosure without departing from the scope of this
disclosure defined in
the following claims.
103
CA 03187895 2023- 1- 31