Note: Descriptions are shown in the official language in which they were submitted.
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
CRYSTAL FORMS OF 244-[(2,3,4-TRIMETHOXYPHENYL)METHYL]PIPERAZIN-1-
YLJETHYL PYRIDINE-3-CARBOXYLATE AND METHODS OF SYNTHESIS
Cross-Reference to Related Applications
This application claims the benefit of, and priority to, U.S. Provisional
Patent Application
No. 63/046,120, filed June 30, 2020, and U.S. Provisional Patent Application
No. 63/046,123,
filed June 30, 2020, the contents of each of which are incorporated by
reference.
Field of the Invention
The invention relates to crystallographic forms of 2-[4-[(2,3,4-
trimethoxyphenyl)methyl]piperazin-1-yl]ethyl pyridine-3-carboxylate and method
of chemical
synthesis of that compound.
Background
Heart disease is the leading cause of death worldwide, accounting for 15
million deaths
across the globe in 2015. In many forms of heart disease, decreased cardiac
efficiency stems
from changes in mitochondrial energy metabolism. Mitochondria are sub-cellular
compartments
in which metabolites derived from glucose and fatty acids are oxidized to
produce high-energy
molecules. Increasing fatty acid oxidation in the heart decreases glucose
oxidation, and vice
versa. Glucose oxidation is a more efficient source of energy, but in certain
types of heart
disease, such as heart failure, ischemic heart disease, and diabetic
cardiomyopathies, fatty acid
oxidation predominates in cardiac mitochondria. As a result, the pumping
capacity of the heart
is reduced.
CV-8972, which has the IUPAC name 244-[(2,3,4-
trimethoxyphenyl)methyl]piperazin-
1-yl]ethyl pyridine-3-carboxylate and the following structure:
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
CH
H-CO
0
I
N
1-13C0 '
was recently identified as a promising therapeutic candidate for treating or
preventing cardiac
conditions due to its pharmacokinetic profile.
Summary
Provided herein are crystallographic forms of CV-8972 and compositions
containing
them. The invention recognizes that crystals of CV-8972 exist in multiple
polymorphic forms
and that one polymorph, Form A, is the most stable under conditions of ambient
temperature and
relative humidity. Therefore, Form A crystals of CV-8972 are useful for the
manufacture of
pharmaceutical compositions. For example, pharmaceutical compositions that
contain the Form
A polymorph do not require special handling during storage or distribution. In
addition, such
compositions may retain their efficacy better than compositions containing
other polymorphs or
mixtures of polymorphs. The invention also provides methods of treating
cardiac conditions in
subject using CV-8972 polymorphs, such as Form A.
The invention also provides methods of synthesis of CV-8972. Prior schemes for
synthesis of CV-8972 require formation of a free base form of 2444(2,3,4-
trimethoxyphenyl)methyl]piperazin-1-yl]ethanol, also called CV-8814, and
conversion of the
free base form of CV-8814 to a hydrochloride salt. In such schemes, CV-8814
must then be
converted back to its free base form for coupling to nicotinic acid to form
the free base form of
CV-8972. The invention provides a CV-8972 synthesis scheme that bypasses the
reversible
conversion of CV-8814 between the free base and HC1 salt forms. In the schemes
provided
herein, the free base form of CV-8814 is formed in a reductive amination
reaction, and the free
base product is used directly as a substrate for coupling to nicotinic acid to
form CV-8972.
Because fewer steps are required, the synthesis schemes of the invention are
simpler, faster, and
provide better yields than prior methods of making CV-8972.
2
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
In an aspect, the invention provides crystals comprising a polymorph of a
compound of
Formula (X):
OCH3
H,C0
0
is, Kt It,
ii3co
'N'
00.
The polymorph may be Form A, Form B, Form C, Form D, or Form E.
The crystal may be substantially free of one or more other polymorphs. For
example, the
crystal may include a Form A polymorph and be substantially free of polymorphs
of Form B,
Form C, Form D, and Form E.
The crystal may include a hydrochloride salt of the compound of Formula (X).
The
crystal may include the compound of Formula (X) and the hydrochloride ion in a
defined
stoichiometric ratio. The crystal may include the compound and the
hydrochloride ion in a 1:3
stoichiometric ratio.
The crystal may include a hydrated form of the compound of Formula (X). The
crystal
may include a monohydrate form of the compound. The crystal may include an
anhydrous form
of the compound.
In another aspect, the invention provides pharmaceutical compositions that
include a
polymorph of the compound of Formula (X).
The polymorph may be Form A, Form B, Form C, Form D, or Form E.
The composition may be substantially free of one or more other polymorphs. For
example, the composition may include a Form A polymorph and be substantially
free of
polymorphs of Form B, Form C, Form D, and Form E.
The composition may include a hydrochloride salt of the compound of Formula
(X). The
composition may include the compound of Formula (X) and the hydrochloride ion
in a defined
stoichiometric ratio. The composition may include the compound and the
hydrochloride ion in a
1:3 stoichiometric ratio.
3
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
The composition may include a hydrated form of the compound of Formula (X).
The
composition may include a monohydrate form of the compound. The composition
may include
an anhydrous form of the compound.
The composition may be formulated for any route or mode of administration. The
composition may be formulated for buccal, dermal, enteral, intraarterial,
intramuscular,
intraocular, intravenous, nasal, oral, parenteral, pulmonary, rectal,
subcutaneous, topical, or
transdermal administration. The composition may be formulated for
administration by injection
or with or on an implantable medical device (e.g., stent or drug-eluting stent
or balloon
equivalents).
The composition may be formulated as a single unit dosage. The composition may
be
formulated as divided dosages.
The composition may contain a defined dose of the compound. The dose may
contain
from about 10 mg to about 2000 mg, from about 10 mg to about 1000 mg, from
about 10 mg to
about 800 mg, from about 10 mg to about 600 mg, from about 10 mg to about 400
mg, from
about 10 mg to about 300 mg, from about 10 mg to about 200 mg, from about 25
mg to about
2000 mg, from about 25 mg to about 1000 mg, from about 25 mg to about 800 mg,
from about
mg to about 600 mg, from about 25 mg to about 400 mg, from about 25 mg to
about 300 mg,
about 25 mg to about 200 mg, from about 50 mg to about 2000 mg, from about 50
mg to about
1000 mg, from about 50 mg to about 800 mg, from about 50 mg to about 600 mg,
from about 50
20 mg to about 400 mg, from about 50 mg to about 300 mg, about 50 mg to
about 200 mg, from
about 100 mg to about 2000 mg, from about 100 mg to about 1000 mg, from about
100 mg to
about 800 mg, from about 100 mg to about 600 mg, from about 100 mg to about
400 mg, from
about 100 mg to about 300 mg, about 100 mg to about 200 mg, from about 200 mg
to about 2000
mg, from about 200 mg to about 1000 mg, from about 200 mg to about 800 mg,
from about 200
25 mg to about 600 mg, from about 200 mg to about 400 mg, from about 200 mg
to about 300 mg,
from about 300 mg to about 2000 mg, from about 300 mg to about 1000 mg, from
about 300 mg
to about 800 mg, from about 300 mg to about 600 mg, or from about 300 mg to
about 400 mg of
the compound. The dose may contain about 10 mg, about 25 mg, about 50 mg,
about 100 mg,
about 200 mg, about 300 mg, or about 400 mg of the compound.
The composition may contain a crystal of the compound of Formula (X). The
crystal
may have any of the properties described above in relation to crystals of the
compound.
4
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
In another aspect, the invention provides methods of treating a condition in a
subject by
providing to a subject having, or at risk of developing, a condition a
composition containing a
therapeutically effective amount of a polymorph of a compound of Formula (X).
The polymorph may be Form A, Form B, Form C, Form D, or Form E.
The composition may have any of the properties described above in relation to
compositions that include the compound of Formula (X), including crystals of
the compound.
The composition may be provided by any suitable route or mode of
administration. The
composition may be provided buccally, dermally, enterally, intraarterially,
intramuscularly,
intraocularly, intravenously, nasally, orally, parenterally, pulmonarily,
rectally, subcutaneously,
topically, transdermally, by injection, or with or on an implantable medical
device (e.g., stent or
drug-eluting stent or balloon equivalents).
The composition may be provided as a single unit dosage. The composition may
be
provided as divided dosages.
The composition may be provided in one dose per day. The composition may be
provided in multiple doses per day. The composition may be provided in two,
three, four, five,
six, eight, or more doses per day.
The composition may contain a defined dose of the compound, such as any of the
doses
described above.
The dose or doses may be provided for a defined period. One or more doses may
be
provided daily for at least one week, at least two weeks, at least three
weeks, at least four weeks,
at least six weeks, at least eight weeks, at least ten weeks, at least twelve
weeks or more.
The condition may be a cardiovascular condition. The cardiovascular condition
may be
aneurysm, angina, atherosclerosis, cardiomyopathy, cerebral vascular disease,
congenital heart
disease, coronary artery disease, coronary heart disease, diabetic
cardiomyopathy, heart attack,
heart disease, heart failure, hypertension, ischemic heart disease,
pericardial disease, peripheral
arterial disease, rheumatic heart disease, stroke, transient ischemic attacks,
or valvular heart
disease. The angina may be refractory to other medical interventions.
The condition may be a rheumatic condition. The rheumatic condition may be
acute
kidney injury, alcoholic cardiomyopathy, angina (e.g., refractory angina and
angina associated
with heart failure), ankylosing spondylitis, autoimmune-related lung disease,
Behcet's Disease,
bursitis, cachexia, cardiac fibrosis, chemotherapy chronic fatigue syndrome,
claudication (e.g.,
5
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
peripheral claudication), contrast nephropathy, cyanotic heart disease,
dermatomyositis, dilated
cardiomyopathy, disequilibrium, fibromyalgia, frailty, gout, Gulf War
syndrome, heart failure,
hypertrophic cardiomyopathy, induced nephropathy, infectious arthritis,
inflammatory arthritis,
inflammatory eye disease, inflammatory myositis, ischemic cardiomyopathy,
juvenile idiopathic
arthritis, left ventricular dysfunction, lupus, muscle myopathy, myofascial
pain syndrome,
myositis, osteoarthritis, osteonecrosis of the jaw, osteoporosis, polymyalgia
rheumatica,
polymyositis, psoriatic arthritis, pulmonary arterial hypertension, pulmonary
fibrosis, a rare
muscle disease, rheumatoid arthritis, sarcoidosis, sarcopenia, scleroderma,
Sjogren's syndrome,
tendinitis, tinnitus, vasculitis, or vertigo.
The condition may fibrosis. The fibrosis may be associated with another
disease,
disorder, or condition. For example, the fibrosis may include or be associated
with adhesive
capsulitis, aneurysm, angina, arterial stiffness, arthrofibrosis,
atherosclerosis, atrial fibrosis,
cardiomyopathy, cerebral vascular disease, cirrhosis, congenital heart
disease. coronary artery
disease, coronary heart disease, Crohn's disease, cystic fibrosis, diabetic
cardiomyopathy,
Dupuytren's contracture, endomyocardial fibrosis, glial scar, heart attack,
heart failure, high
blood pressure (hypertension), idiopathic pulmonary fibrosis, ischemic heart
disease, keloid,
mediastinal fibrosis, myelofibrosis, nephrogenic systemic fibrosis, old
myocardial infarction,
pericardial disease, peripheral arterial disease, Peyronie's disease,
progressive massive fibrosis,
pulmonary fibrosis, radiation-induced lung injury, retroperitoneal fibrosis,
rheumatic heart
disease, scleroderma, stroke, systemic sclerosis transient ischemic attacks,
or valvular heart
disease.
The condition may be cancer. The cancer may be bladder cancer, brain cancer,
breast
cancer, carcinoma, cervical cancer, colon cancer, colorectal cancer, gastric
cancer, glioblastoma,
glioma, head and neck cancer, kidney cancer, leukemia, liposarcoma, liver
cancer, lung cancer,
lymphoma, medullablastoma, melanoma, muscle cancer, neuroblastoma,
oligoastrocytoma,
oligodendroglioma, osteosarcoma, ovarian cancer, pancreatic cancer,
paraganglioma, prostate
cancer, sarcoma, or thyroid cancer.
In another aspect, the invention provides methods of altering cardiac
remodeling by
providing to a subject that has developed, or is at risk of developing,
cardiac remodeling a
composition containing a therapeutically effective amount of a polymorph of a
compound of
Formula (X).
6
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
The polymorph may be Form A, Form B, Form C, Form D, or Form E.
The composition may have any of the properties described above in relation to
compositions that include the compound of Formula (X), including crystals of
the compound.
The composition may be provided by any suitable route or mode of
administration. The
composition may be provided buccally, dermally, enterally, intraocular
intravenously, nasally,
orally, parenterally, pulmonarily, subcutaneously, topically, transdermally,
by injection, or with
or on an implantable medical device (e.g., stent or drug-eluting stent or
balloon equivalents).
The composition may be provided as a single unit dosage. The composition may
be
provided as divided dosages.
The composition may be provided in one dose per day. The composition may be
provided in multiple doses per day. The composition may be provided in two,
three, four, five,
six, eight, or more doses per day.
The composition may contain a defined dose of the compound, such as any of the
doses
described above.
The dose or doses may be provided for a defined period. One or more doses may
be
provided daily for at least one week, at least two weeks, at least three
weeks, at least four weeks,
at least six weeks, at least eight weeks, at least ten weeks, at least twelve
weeks or more.
The cardiac remodeling may be associated with a disease, disorder, or
condition. The
cardiac remodeling may be associated with a cardiovascular disease. For
example, the cardiac
remodeling may be associated with aberrant subclavian artery, aortic
regurgitation, aortic
stenosis, arteriovenous malformation and fistula, atrial septal defect,
atrioventricular septal
defect, bicuspid aortic valve, cardiomegaly, cardiomyopathy, coarctation of
the aorta, complete
heart block, concentric hypertrophy, congenital heart defects, congenital
heart disease, coronary
artery disease, dextrocardia, dextro-transposition of the great arteries,
diabetes, diet, double
aortic arch, double inlet left ventricle, double outlet right ventricle,
Ebstein's anomaly, giant
hepatic hemangioma, heart failure, high cholesterol, high-output hemodialysis
fistula,
hypertension, hypertension, hypoplastic left heart syndrome, hypoplastic right
heart syndrome,
interrupted aortic arch, levo-transposition of the great arteries, mitral
regurgitation, also causing
left atrial volume overload, mitral stenosis, myocardial ischemia, obesity,
outflow obstruction.,
partial anomalous pulmonary venous connection, patent ductus arteriosus,
pentalogy of Cantrell,
persistent truncus arteriosus, pressure overload, pulmonary atresia, pulmonary
hypertension,
7
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
pulmonary regurgitation, pulmonary stenosis, rhabdomyomas, right ventricular
volume overload,
scimitar syndrome, Shone's syndrome, tetralogy of Fallot, total anomalous
pulmonary venous
connection, transposition of the great vessels, tricuspid atresia, tricuspid
regurgitation, use of
tobacco, alcohol, or other drugs, valvular heart disease, ventricular
dilation, ventricular
hypertrophy, ventricular septal defect, volume overload, and Wolff-Parkinson-
White syndrome.
In another aspect, the invention provides uses of crystals containing a
polymorph of a
compound of Formula (X) for making a medicament.
In embodiments of the use, the polymorph is Form A, Form B, Form C, Form D, or
Form
E.
In embodiments of the use, the crystal is substantially free of one or more
other
polymorphs. In embodiments of the use, the crystal includes a Form A polymorph
and is
substantially free of polymorphs of Form B, Form C, Form D, and Form E.
In embodiments of the use, the crystal includes a hydrochloride salt of the
compound of
Formula (X). In embodiments of the use, the crystal includes the compound of
Formula (X) and
the chloride ion in a defined stoichiometric ratio. In embodiments of the use,
the crystal includes
the compound and the chloride ion in a 1:3 stoichiometric ratio.
In embodiments of the use, the medicament includes a hydrated form of the
compound of
Formula (X). In embodiments of the use, the medicament includes a monohydrate
form of the
compound. In embodiments of the use, the medicament includes an anhydrous form
of the
compound.
In embodiments of the use, the medicament is formulated for buccal, dermal,
enteral,
intraarterial, intramuscular, intraocular, intravenous, nasal, oral,
parenteral, pulmonary, rectal,
subcutaneous, topical, or transdermal administration. In embodiments of the
use, the
medicament is formulated for administration by injection or with or on an
implantable medical
device (e.g., stent or drug-eluting stent or balloon equivalents).
In embodiments of the use, the medicament is formulated as a single unit
dosage. In
embodiments of the use, the medicament is formulated as divided dosages.
In embodiments of the use, the medicament contains from about 10 mg to about
2000
mg, from about 10 mg to about 1000 mg, from about 10 mg to about 800 mg, from
about 10 mg
to about 600 mg, from about 10 mg to about 400 mg, from about 10 mg to about
300 mg, from
about 10 mg to about 200 mg, from about 25 mg to about 2000 mg, from about 25
mg to about
8
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
1000 mg, from about 25 mg to about 800 mg, from about 25 mg to about 600 mg,
from about 25
mg to about 400 mg, from about 25 mg to about 300 mg, about 25 mg to about 200
mg, from
about 50 mg to about 2000 mg, from about 50 mg to about 1000 mg, from about 50
mg to about
800 mg, from about 50 mg to about 600 mg, from about 50 mg to about 400 mg,
from about 50
mg to about 300 mg, about 50 mg to about 200 mg, from about 100 mg to about
2000 mg, from
about 100 mg to about 1000 mg, from about 100 mg to about 800 mg, from about
100 mg to
about 600 mg, from about 100 mg to about 400 mg, from about 100 mg to about
300 mg, about
100 mg to about 200 mg, from about 200 mg to about 2000 mg, from about 200 mg
to about
1000 mg, from about 200 mg to about 800 mg, from about 200 mg to about 600 mg,
from about
200 mg to about 400 mg, from about 200 mg to about 300 mg, from about 300 mg
to about 2000
mg, from about 300 mg to about 1000 mg, from about 300 mg to about 800 mg,
from about 300
mg to about 600 mg, or from about 300 mg to about 400 mg of the compound. In
embodiments
of the use, the medicament contains about 10 mg, about 25 mg, about 50 mg,
about 100 mg,
about 200 mg, about 300 mg, or about 400 mg of the compound.
In another aspect, the invention provides methods for preparing a compound of
Formula
(X):
OCH3
'1 3
HCO
0
'N'
(X),
by performing the steps of:
reacting 2,3,4-trimethoxybenzaldehyde and 2-(piperazin-1-yl)ethan-1-ol to
produce a free
base form of a compound of Formula (IX):
9
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
OC HI
Nif. .
1 I
N ,----
-----,, .,--7'
1-18C0'
(Do; and
reacting the free base form of the compound of Formula (IX) with nicotinic
acid to
produce the compound of Formula (X),
wherein the method does not comprise producing a salt form of the compound of
Formula (IX).
The step of reacting 2,3,4-trimethoxybenzaldehyde and 2-(piperazin-1-yl)ethan-
1-ol may
include one or more solvents, catalysts, or other chemicals. The step of
reacting 2,3,4-
trimethoxybenzaldehyde and 2-(piperazin-1-yl)ethan-1-ol may include one or
more of sodium
triacetoxyborohydride, acetic acid, and 2-methyltetrahydrofuran.
The step of reacting 2,3,4-trimethoxybenzaldehyde and 2-(piperazin-1-yl)ethan-
1-ol may
be performed at a defined temperature. The step of reacting 2,3,4-
trimethoxybenzaldehyde and
2-(piperazin-1-yl)ethan-1-ol may be performed at from about 10 C to about 30
C, from about
C to about 30 C, from about 20 C to about 30 C, from about 25 C to about 30 C,
from about
10 C to about 25 C, from about 15 C to about 25 C, from about 20 C to about 25
C, from about
15 10 C to about 20 C, or from about 15 C to about 20 C.
The step of reacting 2,3,4-trimethoxybenzaldehyde and 2-(piperazin-1-yl)ethan-
1-ol may
not include a specific solvent, catalyst, or other chemical. The step of
reacting 2,3,4-
trimethoxybenzaldehyde and 2-(piperazin-1-ypethan-1-ol may not include
dichloromethane.
The step of reacting the free base form of the compound of Formula (IX) with
nicotinic
acid may produce a free base form of the compound of Formula (X).
The step of reacting the free base form of the compound of Formula (IX) with
nicotinic
acid may include one or more solvents, catalysts, or other chemicals. The step
of reacting the
free base form of the compound of Formula (IX) with nicotinic acid may include
one or more of
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide, 4-(dimethylamino)pyridine, and
dichloromethane.
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
The step of reacting the free base form of the compound of Formula (IX) with
nicotinic
acid may be performed at a defined temperature. The step of reacting the free
base form of the
compound of Formula (IX) with nicotinic acid may be performed at from about 15
C to about
30 C, from about 20 C to about 30 C, from about 25 C to about 30 C, from about
15 C to about
25 C, from about 20 C to about 25 C, or from about 15 C to about 20 C.
The method may include converting the free base form of the compound of
Formula (X)
to a salt form of the compound of Formula (X). The salt form of the compound
of Formula (X)
may be a HC1 salt. The salt form of the compound of Formula (X) may be
monohydrate.
The step of converting the free base form of the compound of Formula (X) to
the salt
form of the compound of Formula (X) may include one or more solvents,
catalysts, or other
chemicals. The step of converting the free base form of the compound of
Formula (X) to the salt
form of the compound of Formula (X) may include one or more of HC1 and methyl
ethyl ketone.
The step of converting the free base form of the compound of Formula (X) to
the salt
form of the compound of Formula (X) may be performed at a defined temperature.
The step of
.. converting the free base form of the compound of Formula (X) to the salt
form of the compound
of Formula (X) may be performed at from about 40 C to about 60 C, from about
45 C to about
60 C, from about 50 C to about 60 C, from about 55 C to about 60 C, from about
40 C to about
55 C, from about 45 C to about 55 C, from about 50 C to about 55 C, from about
40 C to about
50 C, from about 45 C to about 50 C, from about 40 C to about 50 C, about 40
C, about 45 C,
about 50 C, about 55 C, or about 60 C.
The method may include converting the salt form of the compound of Formula (X)
from
a first crystal form to a second crystal form. Each of the first and second
crystal forms may
independently be Form A, Form B, Form C, Form D, or Form E.
The step of converting the salt form of the compound of Formula (X) from a
first crystal
form to a second crystal form may include one or more of changing the solvent
of the salt form
of the compound of Formula (X) and incubating the salt form of the compound of
Formula (X),
at about 60 C.
The method may be performed without the use of one or more solvents,
catalysts, or
other chemicals. The method may be performed without the use of one or more of
dioxane,
ethylacetate, or potassium carbonate.
11
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
The method may include purifying the free base form of the compound of Formula
(IX)
The method may include crystallizing the free base form of the compound of
Formula (IX).
In another aspect, the invention provides methods of preparing a compound of
Formula
(X) by performing the steps of:
reacting a compound of Formula (1):
µN-0
(1)
with a compound of Formula (2):
H N
0 H
(2)
to produce a free base form of a compound of Formula (IX):
0 C H 3
H C
N
= N
H OH
CO
(IX);
reacting the free base form of a compound of Formula (IX) with a compound of
Formula
(3):
12
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
0
(3)
to produce a free base form of the compound of Formula (X); and
converting the free base form of the compound of Formula (X) to a HC1 salt of
the
compound of Formula (X),
wherein the method does not comprise producing a salt form of the compound of
Formula (IX).
The method may include purifying the free base form of the compound of Formula
(IX).
The method may include crystallizing the free base form of the compound of
Formula (IX).
Brief Description of the Drawings
FIG. 1 is a space-filling three-dimensional model of the crystal structure of
the Form D
polymorph of CV-8972.
FIG. 2 is a space-filling three-dimensional model of the crystal structure of
the Form D
polymorph of CV-8972 at room temperature.
FIG. 3 is a space-filling three-dimensional model of the crystal structure of
the Form A
polymorph of CV-8972.
FIG. 4 is an XRPD diffractogram of the CV-8972 starting material.
FIG. 5 shows TGA and DSC thermograms of the CV-8972 starting material.
FIG. 6 shows XRPD diffractograms of various forms of CV-8972.
FIG. 7 is a polarized microscopic image of CV-8972 starting material.
FIG. 8 is a dynamic vapor sorption isotherm plot.
FIG. 9 shows XRPD diffractograms of CV-8972 before and after dynamic vapor
sorption.
FIG. 10 shows XRPD diffractograms of CV-8972 in its dehydrated and rehydrated
forms.
FIG. 11 shows XRPD diffractograms of various polymorphs of CV-8972.
13
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
FIG. 12 is a PLM image of a batch of single crystals of C22H34C13N306 (CV-
8972).
FIG. 13 shows PLM images of a crystal used for single-crystal diffractometer.
FIG. 14 shows images of a crystal mounted on a 100 micro Mitegen loop on the
diffractometer.
FIG. 15 is an Ortep diagram of an asymmetric unit of the C22H34C13N306
crystal.
FIG. 16 shows one unit cell of the C22H34C13N306 crystal.
FIG. 17 is a diagram of hydrogen bonds networks and counter-ion pairs in the
C22H34C13N306 crystal.
FIG. 18 shows calculated and measured XRPD diagrams of the C22H34C13N306
crystal.
FIG. 19 shows PLM images of single anhydrous crystals from recrystallized CV-
8972.
FIG. 20 is an image of a single anhydrous crystal from recrystallized CV-8972
mounted
on a tip of a glass fiber.
FIG. 21 is a thermal ellipsoid diagram of an asymmetric unit of the
C22H32C13N305
crystal.
FIG. 22 shows one unit cell of the C22H32C13N305 crystal.
FIG. 23 is a diagram of hydrogen bonds networks and counter-ion pairs in the
C22H32C13N305 crystal.
FIG. 24 shows calculated and measured XRPD diagrams of the C22H34C13N306
crystal.
Detailed Description
The recently-identified compound CV-8972 holds promise as a therapeutic agent
for
treating a variety of conditions, including cardiovascular conditions,
rheumatic diseases, fibrosis,
and cancer. CV-8972, which has the IUPAC name 2444(2,3,4-
trimethoxyphenyl)methyl]piperazin-1-yl]ethyl pyridine-3-carboxylate and the
following
structure:
14
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
OC F+,
H3:00..,.õ-cõ.,.."N.,,,N,,,
0
õ, N
113C0 '
1 ,)
,
,,,-L,,,11
is metabolized in the body into two sets of products that increase
mitochondrial energy
production in different ways. In an initial reaction, the molecule is split
into CV-8814, which
has the following structure:
0 C H3
Ø..
j
,
and nicotinic acid. Over time, CV-8814 converted in the body to trimetazidine.
Both CV-8814
.. and trimetazidine inhibit beta-oxidation of fatty acids and therefore shift
mitochondrial
metabolism toward oxidation of glucose, a more oxygen-efficient source of
energy. Nicotinic
acid serves as precursor for synthesis of nicotinamide adenine dinucleotide
(NAD). NAD+
promotes mitochondrial respiration to drive ATP synthesis, regardless of
whether glucose or
fatty acids are used as the carbon source. Thus, the two sets of products that
result from
breakdown of CV-8972 in vivo act synergistically to stimulate energy
production in
mitochondria in cardiac tissue and other cell types. CV-8972 and its mechanism
of action are
described in U.S. Patent No. 10,556,013, the contents of which are
incorporated herein by
reference.
U.S. Patent No. 10,556,013 also provides a scheme for synthesis of CV-8972.
The
scheme entails formation of a free base form of CV-8814 by reductive amination
of 2,3,4-
trimethoxybenzaldehyde and 2-(piperazin-1-ypethan-1-ol. Due to the difficulty
of isolating CV-
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
8814 in a solid form in this prior method, the product of this reaction is
then converted to a
hydrochloride salt of CV-8814. However, CV-8814 must be converted back to its
free base form
for use in the esterification reaction with nicotinic acid that produces CV-
8972.
The present invention recognizes that crystals of CV-8972 exist in multiple
polymorphic
forms. One polymorph, Form A, is most stable under conditions of ambient
temperature and
relative humidity and therefore has particular utility for the manufacture of
pharmaceutical
compositions. Due to the stability of Form A, compositions containing this
polymorph can
readily be stored and distributed without loss of therapeutic efficacy. Thus,
the invention
provides compositions containing polymorphs of crystalline CV-8972, methods of
making such
compositions, and methods of using them to treat various conditions in a
subject.
Polymorphs of CV-8972
As described in the examples below, crystals of CV-8972 may exist in at least
five
polymorphic forms: Form A, Form B, Form C, Form D, and Form E. Form A is
monohydrate,
and Forms B, D, and E are anhydrous. Form C was not obtained in purified form,
so its
hydration state could not be determined.
Crystals may be formed as salts of CV-8972. For example, crystals may be
formed as
hydrochloride salts of CV-8972.
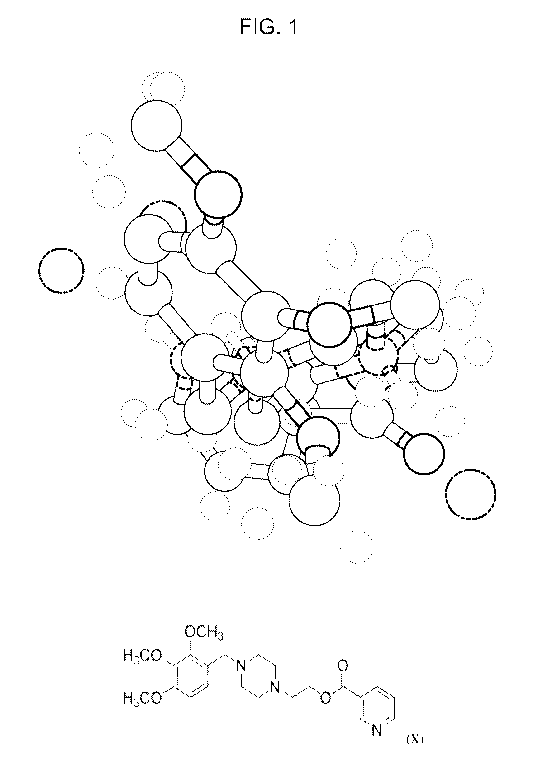
FIG. 1 is a space-filling three-dimensional model of the crystal structure of
the Form D
polymorph of CV-8972. The polymorph is a trihydrochloride salt, and chloride
ions are shown
in green.
FIG. 2 is a space-filling three-dimensional model of the crystal structure of
the Form D
polymorph of CV-8972 at room temperature. The polymorph is a trihydrochloride
salt, and
chloride ions are shown in green.
FIG. 3 is a space-filling three-dimensional model of the crystal structure of
the Form A
polymorph of CV-8972. The polymorph is a trihydrochloride salt, and chloride
ions are shown
in green.
Pharmaceutical compositions
The invention provides pharmaceutical compositions that contain crystals of a
polymorph
of CV-8972. For example, the composition may contain CV-8972 crystals in Form
A, Form B,
16
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
Form C, Form D, or Form E. The composition may be substantially free of one or
more other
polymorphs. For example, the composition may include a Form A polymorph and be
substantially free of polymorphs of Form B, Form C, Form D, and Form E.
A composition containing a polymorph of CV-8972 may be substantially free of
one or
more other polymorphic forms of CV-8972 if the composition contains the
predominant
polymorph at a defined level of purity. Purity may be expressed as the amount
of predominant
polymorph as a percentage of the total weight of two of more polymorphs of CV-
8972.
In certain embodiments, the total weight is the weight of all polymorphs of CV-
8972 in
the composition. For example, a composition that contains the Form A polymorph
and is
substantially free of other polymorphs may contain Form A at a defined weight
percentage of all
polymorphs of CV-8972 in the composition. For example, the composition may
contain Form A
at at least 95% by weight, at least 96% by weight, at least 97% by weight, at
least 98% by
weight, at least 99% by weight, at least 99.5% by weight, at least 99.6% by
weight, at least
99.7% by weight, at least 99.8% by weight, or at least 99.9% by weight of all
polymorphs of
CV-8972 in the composition.
In certain embodiments, the total weight is the weight of selected polymorphs
of CV-
8972 in the composition. For example, a composition that contains the Form A
polymorph and
is substantially free of the Form B polymorph may contain Form A at a defined
weight
percentage of Forms A and B. For example, the composition may contain Form A
at at least
95% by weight, at least 96% by weight, at least 97% by weight, at least 98% by
weight, at least
99% by weight, at least 99.5% by weight, at least 99.6% by weight, at least
99.7% by weight, at
least 99.8% by weight, or at least 99.9% by weight of Forms A and B of CV-8972
in the
composition. Similarly, a composition that contains the Form A polymorph and
is substantially
free of the Form B and C polymorphs may contain Form A at a defined weight
percentage of
Forms A, B, and C. For example, the composition may contain Form A at at least
95% by
weight, at least 96% by weight, at least 97% by weight, at least 98% by
weight, at least 99% by
weight, at least 99.5% by weight, at least 99.6% by weight, at least 99.7% by
weight, at least
99.8% by weight, or at least 99.9% by weight of Forms A, B, and C of CV-8972
in the
composition.
Alternatively or additionally, a composition containing a polymorph of CV-8972
may be
substantially free of one or more other polymorphic forms of CV-8972 if the
composition
17
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
contains the secondary polymorphs at levels below a defined level. Presence of
a secondary
polymorphs may be defined as the amount of one or more secondary polymorphs as
a percentage
of the total weight of two of more polymorphs of CV-8972.
In certain embodiments, the total weight is the weight of all polymorphs of CV-
8972 in
the composition. For example, a composition that contains the Form A polymorph
and is
substantially free of other polymorphs may contain all polymorphs other than
Form A at a
defined weight percentage of all polymorphs of CV-8972 in the composition. For
example, the
composition may contain all polymorphs other than Form A at below 5% by
weight, below 4%
by weight, below 3% by weight, below 2% by weight, below 1% by weight, below
0.5% by
weight, below 0.4% by weight, below 0.3% by weight, below 0.2% by weight, or
below 0.1% by
weight of all polymorphs of CV-8972 in the composition.
In certain embodiments, the total weight is the weight of selected polymorphs
of CV-
8972 in the composition. For example, a composition that contains the Form A
polymorph and
is substantially free of the Form B polymorph may contain Form B at a defined
weight
percentage of Forms A and B. For example, the composition may contain Form B
at below 5%
by weight, below 4% by weight, below 3% by weight, below 2% by weight, below
1% by
weight, below 0.5% by weight, below 0.4% by weight, below 0.3% by weight,
below 0.2% by
weight, or below 0.1% by weight of Forms A and B of CV-8972 in the
composition. Similarly, a
composition that contains the Form A polymorph and is substantially free of
the Form B and
Form C polymorphs may contain Forms B and C at a defined weight percentage of
Forms A, B,
and C. For example, the composition may contain Forms B and C at below 5% by
weight, below
4% by weight, below 3% by weight, below 2% by weight, below 1% by weight,
below 0.5% by
weight, below 0.4% by weight, below 0.3% by weight, below 0.2% by weight, or
below 0.1% by
weight of Forms A, B, and C of CV-8972 in the composition.
The composition may include a hydrochloride salt of a CV-8972 polymorph. The
composition may include CV-8972 and the chloride ion a defined stoichiometric
ratio. The
composition may include CV-8972 and the chloride ion in a 1:3 stoichiometric
ratio.
The composition may include a hydrated form of CV-8972. The composition may
include a monohydrate form of CV-8972, such as the Form A polymorph. The
composition may
include an anhydrous form of CV-8972, such as a Form B, Form D, or Form E
polymorph.
18
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
The composition may be formulated for any route or mode of administration. The
composition may be formulated for buccal, dermal, enteral, intraarterial,
intramuscular,
intraocular, intravenous, nasal, oral, parenteral, pulmonary, rectal,
subcutaneous, topical, or
transdermal administration. The composition may be formulated for
administration by injection
or with or on an implantable medical device (e.g., stent or drug-eluting stent
or balloon
equivalents).
The composition may be formulated as a single unit dosage. The composition may
be
formulated as divided dosages.
The composition may contain a defined dose of CV-8972. The dose may contain
from
about 10 mg to about 2000 mg, from about 10 mg to about 1000 mg, from about 10
mg to about
800 mg, from about 10 mg to about 600 mg, from about 10 mg to about 400 mg,
from about 10
mg to about 300 mg, from about 10 mg to about 200 mg, from about 25 mg to
about 2000 mg,
from about 25 mg to about 1000 mg, from about 25 mg to about 800 mg, from
about 25 mg to
about 600 mg, from about 25 mg to about 400 mg, from about 25 mg to about 300
mg, about 25
mg to about 200 mg, from about 50 mg to about 2000 mg, from about 50 mg to
about 1000 mg,
from about 50 mg to about 800 mg, from about 50 mg to about 600 mg, from about
50 mg to
about 400 mg, from about 50 mg to about 300 mg, about 50 mg to about 200 mg,
from about 100
mg to about 2000 mg, from about 100 mg to about 1000 mg, from about 100 mg to
about 800
mg, from about 100 mg to about 600 mg, from about 100 mg to about 400 mg, from
about 100
.. mg to about 300 mg, about 100 mg to about 200 mg, from about 200 mg to
about 2000 mg, from
about 200 mg to about 1000 mg, from about 200 mg to about 800 mg, from about
200 mg to
about 600 mg, from about 200 mg to about 400 mg, from about 200 mg to about
300 mg, from
about 300 mg to about 2000 mg, from about 300 mg to about 1000 mg, from about
300 mg to
about 800 mg, from about 300 mg to about 600 mg, or from about 300 mg to about
400 mg of
CV-8972. The dose may contain about 10 mg, about 25 mg, about 50 mg, about 100
mg, about
200 mg, about 300 mg, or about 400 mg of CV-8972.
A pharmaceutical composition containing a polymorph of CV-8972 may be in a
form
suitable for oral use, such as tablets, troches, lozenges, fast-melts,
dispersible powders or
granules, or capsules. Compositions intended for oral use may be prepared
according to any
method known in the art for the manufacture of pharmaceutical compositions and
such
compositions may contain one or more agents selected from sweetening agents,
flavoring agents,
19
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
coloring agents and preserving agents, in order to provide pharmaceutically
elegant and palatable
preparations. Tablets contain the polymorph in admixture with non-toxic
pharmaceutically
acceptable excipients. These excipients may be for example, inert diluents,
such as calcium
carbonate, sodium carbonate, lactose, calcium phosphate or sodium phosphate;
granulating and
disintegrating agents, for example corn starch, or alginic acid; binding
agents, for example
starch, gelatin or acacia, and lubricating agents, for example magnesium
stearate, stearic acid, or
talc. Preparation and administration of pharmaceutical compositions is
discussed in U.S. Patent
No. 6,214,841 and U.S. Patent Publication No. 2003/0232877, the contents of
each of which are
incorporated by reference herein. Formulations for oral use may also be
presented as hard
gelatin capsules in which the compounds are mixed with an inert solid diluent,
such as calcium
carbonate, calcium phosphate or kaolin. The formulation may allow controlled
release of the
polymorph of CV-8972 in the gastrointestinal tract by encapsulating the
polymorph in an enteric
coating.
Dispersible powders and granules provide the compounds in admixture with a
dispersing
.. or wetting agent, suspending agent and one or more preservatives. Suitable
dispersing or wetting
agents and suspending agents are exemplified, for example sweetening,
flavoring and coloring
agents, may also be present.
Pharmaceutical compositions may contain mixtures that include erodible
polymers that
promote swelling of the mixture in an aqueous environment. An erodible polymer
is any
.. polymer that breaks down inside the body within a physiologically relevant
time frame. The
erodible polymer may have other characteristics that promote the gradual
release of the
polymorphic form of CV-8972 from the mixture. For example and without
limitation, the
polymer may be one or more of the following: biocompatible, i.e., not harmful
to living tissue;
hydrophilic; hygroscopic; tending to form a hydrogel.
Without wishing to be bound by theory, the polymer-containing mixtures may
promote
gradual release by one or more mechanisms. For example, swelling of the
mixture by absorption
of water may facilitate diffusion of the polymorphic form of CV-8972 from the
mixture.
Degradation of the polymer may also allow the polymorphic form of CV-8972 to
be released
from the mixture. Osmotic pressure due the high concentration gradient of
compound between
the inside and outside of the mixture may also contribute to diffusion of the
polymorphic form of
CV-8972 from the mixture.
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
For example and without limitation, the polymer may be a cellulose derivative,
a gelatin
derivative, e.g., a cross-linked gelatin derivative, or a polyester
derivative.
Derivatives of cellulose, is a linear chain 13(1-4) linked D-glucose units,
include
polymers that contain substitutions on one of more of the hydroxyl groups of
each glucose unit.
Substituents may be organic or inorganic and are typically attached via ester
or ether linkages.
Cellulose ester derivatives include carboxymethyl cellulose (CMC), e.g.,
sodium carboxymethyl
cellulose, ethyl cellulose, ethyl hydroxyethyl cellulose, ethyl methyl
cellulose, hydroxyethyl
cellulose, hydroxyethyl methyl cellulose, hydroxypropyl cellulose (HPC),
hydroxypropyl
methylcellulose (HPMC), and methylcellulose. Cellulose ether derivatives
include cellulose
acetate, cellulose acetate butyrate, cellulose acetate propionate, cellulose
propionate, cellulose
sulfate, cellulose triacetate, and nitrocellulose. The use of cellulose-based
polymers to form
biodegradable hydrogels is known in the art and described in, for example,
Sannino, et al.,
Biodegradable Cellulose-based Hydrogels: Design and Applications, Materials
2009, 2, 353-373;
doi:10.3390/ma2020353, the contents of which are incorporated herein by
reference.
The mixture may contain multiple polymers or multiple polymeric forms of the
same
polymer. For example, HPMC polymeric forms may differ in a variety of physical
properties,
including viscosity, degree of methoxyl substitution, degree of
hydroxypropoxyl substitution, or
average molecule weight.
The viscosity of a HMPC polymeric form may be determined by testing under
standard
conditions, including the concentration of EIMPC in the solution and the
temperature of the
solution. For example and without limitation, the HPMC concentration may be
1%, 1.5%, 2%,
2.5%, or 3%. For example and without limitation, the temperature of the
solution may be 15 C,
16 C, 17 C, 18 C, 19 C, 20 C, 21 C, 22 C, 23 C, 24 C, or 25 C.
A polymeric form of a cellulose derivative, such as HPMC, may have a defined
viscosity.
For example and without limitation, a polymeric form of HPMC may have a
viscosity of from
about 2 cP to about 4 cP, from about 4 cP to about 6 cP, from about 5 cP to
about 8 cP, from
about 12 cP to about 18 cP, from about 40 cP to about 60 cP, from about 80 cP
to about 120 cP,
from about 300 cP to about 500 cP, from about 1200 cP to about 2400 cP, from
about 2500 cP to
about 5000 cP, from about 9000 cP to about 18,000 cP, from about 12,000 cP to
about 24,000
cP, from about 12,000 cP to about 24,000 cP, from about 75,000 cP to about
150,000 cP, at least
about 2 cP at least about 4 cP at least about 5 cP at least about 12 cP at
least about 40 cP at least
21
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
about 80 cP at least about 300 cP at least about 1200 cP at least about 2500
cP at least about
9000 cP at least about 12,000 cP at least about 12,000 cP at least about
75,000 cP less than about
4 cP, less than about 6 cP, less than about 8 cP, less than about 18 cP, less
than about 60 cP, less
than about 120 cP, less than about 500 cP, less than about 2400 cP, less than
about 5000 cP, less
than about 18,000 cP, less than about 24,000 cP, less than about 24,000 cP, or
less than about
150,000 cP for a 2% aqueous solution of the polymeric form at 20 C.
Polymeric forms of cellulose derivatives, such as HPMC, may vary in their
degree of
substitution of the glucose units. The degree of substitution may be expressed
as a weight
percentage of the substituent or as a molar ratio of substituent to glucose
unit. For a cellulose
derivative that has two different substituents, such as HPMC, the polymeric
form may be
described by the degree of substitution for each substituent.
Each polymeric form of HPMC may independently have a defined degree of
methoxyl
substitution. For example and without limitation, the degree of methoxyl
substitution may be
from about 19% to about 24%, from about 22% to about 24%, from about 27% to
about 30%,
from about 27% to about 30%, or from about 28% to about 32%.
Each polymeric form of HPMC may independently have a defined degree of
hydroxypropoxyl substitution. For example and without limitation, the degree
of
hydroxypropoxyl substitution may be from about 4% to about 8%, from about 7%
to about 10%,
from about 7% to about 12%, from about 8% to about 10%, from about 8% to about
11%, or
from about 9% to about 12%.
Each polymeric form of HPMC may independently have a defined average molecular
weight. The average molecular weight may be about 10 kDa, about 13 kDa, about
20 kDa, about
26 kDa, about 41 kDa, about 63 kDa, about 86 kDa, about 110 kDa, about 120
kDa, about 140
kDa, about 180 kDa, or about 220 kDa.
When multiple forms of a polymer, such as HPMC, are present, one or more
polymeric
forms may be present in a defined amount. For example and without limitation,
a polymer, such
as HPMC, may contain about 50%, about 60%, about 70%, about 80%, about 90%,
about 95%,
about 96%, about 97%, about 98%, about 99%, at least 50%, at least 60%, at
least 70%, at least
80%, at least 90%, at least 95%, at least 96%, at least 97%, at least 98%, or
at least 99% by
weight of one polymeric form.
22
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
Pharmaceutical compositions may include modified-release formulations that
contain one
or more polymorphic forms of CV-8972. The formulations contain mixtures that
include one or
more polymorphic forms of CV-8972 and one or more erodible polymers that
promote swelling
of the mixture in an aqueous environment. The hygroscopic and erodible
properties of the
polymers may allow the mixture to form a hydrogel that slowly breaks down in
the digestive
tract of the subject. Consequently, the mixture promotes the steady release of
the polymorphic
form of CV-8972 and metabolic products thereof into circulation.
The mixture may contain a defined amount of the polymorphic form of CV-8972.
The
mixture may contain at least 5%, at least 10%, at least 20%, at least 30%, at
least 40%, at least
50%, at least 60%, at least 70%, or at least 80% by weight of the polymorphic
form of CV-8972.
The mixture may contain the polymorphic form of CV-8972 and the polymer in a
defined
weight ratio. For example and without limitation, the mixture may contain the
polymorphic form
of CV-8972 and the polymer in a weight ratio of about 1:5, about 1:4, about
1:3, about 1:2, about
1:1, about 3:2, about 2:1, about 3:1, about 4:1, about 5:1, from about 1:100
to about 100:1, from
about 1:100 to about 50:1, from about 1:100 to about 20:1, from about 1:100 to
about 10:1, from
about 1:100 to about 5:1, from about 1:100 to about 2:1, from about 1:50 to
about 100:1, from
about 1:50 to about 50:1, from about 1:50 to about 20:1, from about 1:50 to
about 10:1, from
about 1:50 to about 5:1, from about 1:50 to about 2:1, from about 1:20 to
about 100:1, from
about 1:20 to about 50:1, from about 1:20 to about 20:1, from about 1:20 to
about 10:1, from
about 1:20 to about 5:1, from about 1:20 to about 2:1, from about 1:10 to
about 100:1, from
about 1:10 to about 50:1, from about 1:10 to about 20:1, from about 1:10 to
about 10:1, from
about 1:10 to about 5:1, from about 1:10 to about 2:1, from about 1:5 to about
100:1, from about
1:5 to about 50:1, from about 1:5 to about 20:1, from about 1:5 to about 10:1,
from about 1:5 to
about 5:1, from about 1:5 to about 2:1, from about 1:3 to about 100:1, from
about 1:3 to about
50:1, from about 1:3 to about 20:1, from about 1:3 to about 10:1, from about
1:3 to about 5:1, or
from about 1:3 to about 2:1.
The pharmaceutical composition may be formulated for a particular route of
administration. The pharmaceutical may be formulated for oral, enteral,
intravenous, or rectal
administration.
The pharmaceutical composition may be formulated as a unit dosage containing a
defined
amount of the polymorphic form of CV-8972. The unit dosage may contain about 5
mg, about
23
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
mg, about 20 mg, about 50 mg, about 100 mg, about 200 mg, about 500 mg, from
about 5 mg
to about 10 mg, from about 5 mg to about 20 mg, from about 5 mg to about 50
mg, from about 5
mg to about 100 mg, from about 5 mg to about 200 mg, from about 5 mg to about
500 mg, from
about 10 mg to about 20 mg, from about 10 mg to about 50 mg, from about 10 mg
to about 100
5 mg, from about 10 mg to about 200 mg, from about 10 mg to about 500 mg,
from about 20 mg to
about 50 mg, from about 20 mg to about 100 mg, from about 20 mg to about 200
mg, from about
mg to about 500 mg, from about 50 mg to about 100 mg, from about 50 mg to
about 200 mg,
from about 50 mg to about 500 mg, from about 100 mg to about 200 mg, from
about 100 mg to
about 500 mg, or from about 200 mg to about 500 mg of the polymorphic form of
CV-8972.
10 The pharmaceutical composition may be formulated such that it produces a
defined value
for one or more parameters, as described below in relation to methods of the
invention. For
example and without limitation, the parameter may be Cmax, the interval
between administration
and achieving Cmax, T1/27 or AUC.
Pharmaceutical compositions of the invention may contain excipients. For
example and
15 without limitation, the composition may contain sweetening agents,
flavoring agents, coloring
agents, or preserving agents. The compositions may contain one or more of
mannitol, starch,
and magnesium stearate.
Providing a polymorph of CV-8972 to a subject
20 The invention provides methods of treating a condition in a subject by
providing a
polymorph of CV-8972. The polymorph may be Form A, Form B, Form C, Form D, or
Form E.
The polymorph of CV-8972 may be provided in a pharmaceutical composition, as
described
above. In certain embodiments of the methods, only a polymorph of Form A is
provided.
The polymorph of CV-8972 may be provided by any suitable route or mode of
administration. For example and without limitation, the polymorph of CV-8972
may be
provided buccally, dermally, enterally, intraarterially, intramuscularly,
intraocularly,
intravenously, nasally, orally, parenterally, pulmonarily, rectally,
subcutaneously, topically,
transdermally, by injection, or with or on an implantable medical device
(e.g., stent or drug-
eluting stent or balloon equivalents).
The polymorph of CV-8972 may be provided according to a dosing regimen. A
dosing
regimen may include a dosage, a dosing frequency, or both.
24
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
Doses may be provided at any suitable interval. For example and without
limitation,
doses may be provided once per day, twice per day, three times per day, four
times per day, five
times per day, six times per day, eight times per day, once every 48 hours,
once every 36 hours,
once every 24 hours, once every 12 hours, once every 8 hours, once every 6
hours, once every 4
hours, once every 3 hours, once every two days, once every three days, once
every four days,
once every five days, once every week, twice per week, three times per week,
four times per
week, or five times per week.
The dose may contain a defined amount of CV-8972 that improves cardiac
mitochondrial
function, such as any of the doses described above in relation to
pharmaceutical compositions
containing a polymorph of CV-8972.
The dose may be provided in a single dosage, i.e., the dose may be provided as
a single
tablet, capsule, pill, etc. Alternatively, the dose may be provided in a
divided dosage, i.e., the
dose may be provided as multiple tablets, capsules, pills, etc.
The dosing may continue for a defined period. For example and without
limitation, doses
.. may be provided for at least one week, at least two weeks, at least three
weeks, at least four
weeks, at least six weeks, at least eight weeks, at least ten weeks, at least
twelve weeks or more.
The subject may be a human. The subject may be a human that has a
cardiovascular
condition, rheumatic condition, fibrosis, or cancer. The subject may be a
human that is at risk of
developing a cardiovascular condition, rheumatic condition, fibrosis, or
cancer. A subject may
.. be at risk of developing a condition if the subject does not meet
established criteria for diagnosis
of the condition but has one or more symptoms, markers, or other factors that
indicate the subject
is likely to meet the diagnostic criteria for the condition in the future. The
subject may be a
pediatric, a newborn, a neonate, an infant, a child, an adolescent, a pre-
teen, a teenager, an adult,
or an elderly subject. The subject may be in critical care, intensive care,
neonatal intensive care,
.. pediatric intensive care, coronary care, cardiothoracic care, surgical
intensive care, medical
intensive care, long-term intensive care, an operating room, an ambulance, a
field hospital, or an
out-of-hospital field setting.
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
Conditions that may be treated with a polymorph of CV-8972
The invention provides methods of treating a condition in a subject by
providing a
polymorph of CV-8972. The condition may be any disease, disorder, or condition
for which
increasing mitochondrial energy production provides a therapeutic benefit.
The condition may be a cardiac condition. For example and without limitation,
the
cardiac condition may be aneurysm, angina, atherosclerosis, cardiomyopathy,
cerebral vascular
disease, congenital heart disease, coronary artery disease (CAD), coronary
heart disease, diabetic
cardiomyopathy, heart attack, heart disease, heart failure, high blood
pressure (hypertension),
ischemic heart disease, pericardial disease, peripheral arterial disease,
refractory angina,
rheumatic heart disease, stable angina, stroke, transient ischemic attack,
unstable angina, or
valvular heart disease.
Angina pectoris (angina) is chest pain or pressure that is typically due to
insufficient
blood flow to the heart muscle. The pain or discomfort is retrosternal or left-
sided and may
radiate to the left arm, neck, jaw, or back. Several classifications of angina
are known.
Stable angina, also called effort angina, is related to myocardial ischemia.
In stable
angina, chest discomfort and associated symptoms are usually triggered by some
physical
activity, such as running or walking, but symptoms are minimal or non-existent
when the patient
is at rest or has taken sublingual nitroglycerin. Symptoms typically abate
several minutes after
activity and recur when activity resumes. Symptoms may also be induced by cold
weather,
heavy meals, and emotional stress.
Unstable angina is angina that changes or worsens. Unstable angina has at
least one of
the following features: (1) it occurs at rest or with minimal exertion,
usually lasting more than
10 minutes, (2) it is severe and of new onset, i.e., within the prior 4-6
weeks, and (3) it occurs
with a crescendo pattern, i.e., distinctly more severe, prolonged, or frequent
than before.
Cardiac syndrome X, also called microvascular angina, is angina-like chest
pain, in the
context of normal epicardial coronary arteries on angiography. Its primary
cause is unknown,
but factors apparently involved are endothelial dysfunction and reduced flow
in the tiny
resistance blood vessels of the heart. Microvascular angina may be part of the
pathophysiology
of ischemic heart disease.
Refractory angina is a chronic condition (> 3 months in duration) in which
angina (1)
occurs in the context of coronary artery disease (CAD), (2) cannot be
controlled by a
26
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
combination of optimal medical therapy, angioplasty, or bypass surgery, and
(3) in which
reversible myocardial ischemia has been clinically established to be the cause
of the symptoms.
Providing a polymorph of CV-8972 may improve cardiac efficiency in the
subject. A
variety of definitions of cardiac efficiency exist in the medical literature.
See, e.g., Schipke, J.D.
.. Cardiac efficiency, Basic Res. Cardiol. 89:207-40 (1994); and Gibbs, C.L.
and Barclay, C.J.
Cardiac efficiency, Cardiovasc. Res. 30:627-634 (1995), incorporated herein by
reference. One
definition of cardiac mechanical efficiency is the ratio of external cardiac
power to cardiac
energy expenditure by the left ventricle. See Lopaschuk G.D., et al.,
Myocardial Fatty Acid
Metabolism in Health and Disease, Phys. Rev. 90:207-258 (2010), incorporated
herein by
reference. Another definition is the ratio between stroke work and oxygen
consumption, which
ranges from 20-25% in the normal human heart. Visser, F., Measuring cardiac
efficiency: is it
useful? Hear Metab. 39:3-4 (2008), incorporated herein by reference. Another
definition is the
ratio of the stroke volume to mean arterial blood pressure. Any suitable
definition of cardiac
efficiency may be used to measure the effects of compounds of the invention
A polymorph of CV-8972 may be used to treat a rheumatic disease, disorder, or
condition. As used herein, a rheumatic disease, disorder, or condition is any
condition that
affects the joints, tendons, ligaments, bones, muscles, or connective tissue
or is associated with
pain in or more of such tissues. The rheumatic disease, disorder, or condition
may primarily
affect the joints, tendons, ligaments, bones, muscles, or connective tissue.
Examples of such
conditions include ankylosing spondylitis, autoimmune-related lung disease,
Behcet's Disease,
bursitis, chronic fatigue syndrome, dermatomyositis, fibromyalgia, gout, Gulf
War syndrome,
infectious arthritis, inflammatory arthritis, inflammatory eye disease,
inflammatory myositis,
juvenile idiopathic arthritis, lupus, myofascial pain syndrome,
osteoarthritis, osteonecrosis of the
jaw, osteoporosis, polymyalgia rheumatica, polymyositis, psoriatic arthritis,
rheumatoid arthritis,
.. sarcoidosis, scleroderma, Sjogren's syndrome, tendinitis, and vasculitis.
The rheumatic disease, disorder, or condition may primarily affect the
cardiovascular
system and have secondary effects on the joints, tendons, ligaments, bones,
muscles, or
connective tissue. For example and without limitation, the condition may be
alcoholic
cardiomyopathy, aneurysm, angina (including refractory angina and angina in
the context of
heart failure), atherosclerosis, cardiac fibrosis, cardiomyopathy, cerebral
vascular disease,
claudication (e.g., peripheral claudication) congenital heart disease,
coronary artery disease,
27
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
coronary heart disease, cyanotic heart disease, diabetic cardiomyopathy,
dilated cardiomyopathy,
heart attack, heart failure, high blood pressure (hypertension), hypertrophic
cardiomyopathy,
ischemic cardiomyopathy, ischemic heart disease, left ventricular dysfunction,
pericardial
disease, peripheral arterial disease, rheumatic heart disease, stroke,
transient ischemic attacks, or
valvular heart disease.
The rheumatic disease, disorder, or condition may be a rare muscle disease.
For example
and without limitation, the condition may be CAV3-related distal myopathy,
Duchenne Muscular
Dystrophy, hypertrophic cardiomyopathy, isolated hyperCKemia, limb-girdle
muscular
dystrophy 1C, muscle myopathy, myositis, or rippling muscle disease. The rare
muscle disease
may be associated with a mutation in BICD2, CAV3, or DMD.
The rheumatic disease, disorder, or condition may be a glycogen storage
disease. For
example and without limitation, the glycogen storage disease may be aldolase A
deficiency,
Andersen disease, Con's disease, Fanconi-Bickel syndrome, Hers' disease,
Lafora disease,
McArdle disease, Pompe's disease, Tarui's disease, or von Gierke's disease.
The glycogen
storage disease may be associated with a deficiency in an enzyme or protein,
such as
acid alpha-glucosidase, aldolase A, P-enolase, glucose transporter, glucose-6-
phosphatase,
glycogen branching enzyme, glycogen debranching enzyme, glycogen synthase,
glycogenin-1,
liver glycogen phosphorylase, muscle glycogen phosphorylase, muscle lactate
dehydrogenase,
muscle phosphofructokinase, muscle phosphoglycerate mutase, phosphoglycerate
mutase, or
phosphorylase kinase. The glycogen storage disease may be associated with a
mutation in a
gene, such as AGL, ALDOA, EN03, G6PC, GAA, GBE1, GLUT2, GYG1, GYS2, LDHA,
PGAM2, PGAM2, PHKA1, PHKA2, PHKB, PHKG2, PKFM, PYGL, PYGM, or SLC37A4.
The rheumatic disease, disorder, or condition may be another condition that
affects the
joints, tendons, ligaments, bones, muscles, or connective tissue, such as
acute kidney injury,
cachexia, chemotherapy induced nephropathy, contrast nephropathy,
disequilibrium, frailty,
pulmonary arterial hypertension, pulmonary fibrosis, sarcopenia, tinnitus, or
vertigo.
A polymorph of CV-8972 may be used to treat fibrosis or a disease, disorder,
or
condition associated with fibrosis. In particular, the methods are useful for
treating diseases,
disorders, or conditions in which fibrosis in an organ or tissue is associated
with reduced energy
production by that organ or tissue. The fibrosis may affect any organ or
tissue, such as the heart,
28
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
lungs, liver, brain, cardiovascular system, joints, gastrointestinal system,
limbs, digits, skin, bone
marrow, or penis.
The fibrosis may be associated with another condition, e.g., it may be
secondary to
another condition, or it may lead to the other condition. For example and
without limitation, the
fibrosis may include or be associated with adhesive capsulitis, aneurysm,
angina, arterial
stiffness, arthrofibrosis, atherosclerosis, atrial fibrosis, cardiomyopathy,
cerebral vascular
disease, cirrhosis, congenital heart disease, coronary artery disease,
coronary heart disease,
Crohn's disease, cystic fibrosis, diabetic cardiomyopathy, Dupuytren's
contracture,
endomyocardial fibrosis, glial scar, heart attack, heart failure, high blood
pressure
(hypertension), idiopathic pulmonary fibrosis, ischemic heart disease, keloid,
mediastinal
fibrosis, myelofibrosis, nephrogenic systemic fibrosis, old myocardial
infarction, pericardial
disease, peripheral arterial disease, Peyronie's disease, progressive massive
fibrosis, pulmonary
fibrosis, radiation-induced lung injury, retroperitoneal fibrosis, rheumatic
heart disease,
scleroderma, stroke, systemic sclerosis transient ischemic attacks, or
valvular heart disease.
A polymorph of CV-8972 may be used to treat cancer. For example and without
limitation, the cancer may be bladder cancer, brain cancer, breast cancer,
carcinoma, cervical
cancer, colon cancer, colorectal cancer, gastric cancer, glioblastoma, glioma,
head and neck
cancer, kidney cancer, leukemia, liposarcoma, liver cancer, lung cancer,
lymphoma,
medullablastoma, melanoma, muscle cancer, neuroblastoma, oligoastrocytoma,
oligodendroglioma, osteosarcoma, ovarian cancer, pancreatic cancer,
paraganglioma, prostate
cancer, sarcoma, or thyroid cancer.
Synthesis of CV-8972
The invention also provides CV-8972 synthesis schemes in which the free base
form of
CV-8814 formed as a product in the reductive amination reaction can be used
directly as a
substrate in the esterification reaction. The invention is based in part on
the identification of
conditions that improve the stability of CV-8814 free base and allow the free
base form to be
crystallized. Thus, the schemes provided herein obviate the need to convert CV-
8814 from its
free base form to a HCl salt and then back to the free base form.
Consequently, the invention
provides simpler, quicker, and higher-yield methods for making CV-8972.
The invention provides methods for preparing a compound of Formula (X):
29
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
OCH3
4CO, =1"\-3 -,^ ,- , ,,-"N.,
LT,,.
N
1 0
c0- - - .--- ------ -0- iii N-
' N'
(X),
by performing the steps of:
reacting 2,3,4-trimethoxybenzaldehyde and 2-(piperazin-1-yl)ethan-1-ol to
produce a free
base form of a compound of Formula (IX):
OHa
-c--*-
N
1
(IX); and
\I
reacting the free base form of the compound of Formula (IX) with nicotinic
acid to
produce the compound of Formula (X),
wherein the method does not comprise producing a salt form of the compound of
Formula (IX).
2,3,4-trimethoxybenzaldehyde has the following structure:
N....,
0
,-
J,,,,:r ......,.....,
-u
--,,0----------:
.
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
2-(piperazin-1-yl)ethan-1-01 has the following structure:
HNF
=
Nicotinic acid has the following structure:
0
1
.,,,\ ..--""'"
I [ N's0H
N .
The step of reacting 2,3,4-trimethoxybenzaldehyde and 2-(piperazin-1-yl)ethan-
1-ol may
include one or more solvents, catalysts, or other chemicals. The step of
reacting 2,3,4-
trimethoxybenzaldehyde and 2-(piperazin-1-yl)ethan-1-ol may include one or
more of sodium
triacetoxyborohydride, acetic acid, and 2-methyltetrahydrofuran.
The step of reacting 2,3,4-trimethoxybenzaldehyde and 2-(piperazin-1-yl)ethan-
1-ol may
be performed at a defined temperature. The step of reacting 2,3,4-
trimethoxybenzaldehyde and
2-(piperazin-1-yl)ethan-1-ol may be performed at from about 10 C to about 30
C, from about
15 C to about 30 C, from about 20 C to about 30 C, from about 25 C to about 30
C, from about
10 C to about 25 C, from about 15 C to about 25 C, from about 20 C to about 25
C, from about
10 C to about 20 C, or from about 15 C to about 20 C.
The step of reacting 2,3,4-trimethoxybenzaldehyde and 2-(piperazin-1-yl)ethan-
1-ol may
not include a specific solvent, catalyst, or other chemical. The step of
reacting 2,3,4-
trimethoxybenzaldehyde and 2-(piperazin-1-yl)ethan-1-ol may not include
dichloromethane.
The step of reacting the free base form of the compound of Formula (IX) with
nicotinic
acid may produce a free base form of the compound of Formula (X).
31
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
The step of reacting the free base form of the compound of Formula (IX) with
nicotinic
acid may include one or more solvents, catalysts, or other chemicals. The step
of reacting the
free base form of the compound of Formula (IX) with nicotinic acid may include
one or more of
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide, 4-(dimethylamino)pyridine, and
dichloromethane.
The step of reacting the free base form of the compound of Formula (IX) with
nicotinic
acid may be performed at a defined temperature. The step of reacting the free
base form of the
compound of Formula (IX) with nicotinic acid may be performed at from about 15
C to about
30 C, from about 20 C to about 30 C, from about 25 C to about 30 C, from about
15 C to about
25 C, from about 20 C to about 25 C, or from about 15 C to about 20 C.
The method may include converting the free base form of the compound of
Formula (X)
to a salt form of the compound of Formula (X). The salt form of the compound
of Formula (X)
may be a HC1 salt. The salt form of the compound of Formula (X) may be
monohydrate.
The step of converting the free base form of the compound of Formula (X) to
the salt
form of the compound of Formula (X) may include one or more solvents,
catalysts, or other
chemicals. The step of converting the free base form of the compound of
Formula (X) to the salt
form of the compound of Formula (X) may include one or more of HC1 and methyl
ethyl ketone.
The step of converting the free base form of the compound of Formula (X) to
the salt
form of the compound of Formula (X) may be performed at a defined temperature.
The step of
converting the free base form of the compound of Formula (X) to the salt form
of the compound
of Formula (X) may be performed at from about 40 C to about 60 C, from about
45 C to about
60 C, from about 50 C to about 60 C, from about 55 C to about 60 C, from about
40 C to about
55 C, from about 45 C to about 55 C, from about 50 C to about 55 C, from about
40 C to about
50 C, from about 45 C to about 50 C, from about 40 C to about 50 C, about 40
C, about 45 C,
about 50 C, about 55 C, or about 60 C.
The compound of Formula (X) may exist in at least five crystal forms: Form A,
Form B,
Form C, Form D, and Form E. Form A is monohydrate, and Forms B, D, and E are
anhydrous.
The method may include converting the compound of Formula (X) from a first
crystal form to a
second crystal form. Each of the first and second crystal forms may
independently be Form A,
.. Form B, Form C, Form D, or Form E. The method may include one or more of
the following
conversions of the compound of Formula (X): from an anhydrous form to a
hydrated form; from
32
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
a hydrated form to an anhydrous form; from one anhydrous form to another; and
from one
hydrated form to another.
The step of converting the salt form of the compound of Formula (X) from a
first crystal
form to a second crystal form may include one or more of changing the solvent
of the salt form
of the compound of Formula (X) and incubating the salt form of the compound of
Formula (X),
at about 60 C.
The method may be performed without the use of one or more solvents,
catalysts, or
other chemicals. The method may be performed without the use of one or more of
dioxane,
ethylacetate, or potassium carbonate.
The method may include purifying the free base form of the compound of Formula
(IX).
The method may include crystallizing the free base form of the compound of
Formula (IX).
Examples
Example 1
Summary
A comprehensive polymorph screening for CV-8972, which has the structure of
Formula
(X), was undertaken. The CV-8972 starting material was characterized by X-ray
powder
diffraction (XRPD), thermogravimetric analysis (TGA), differential scanning
calorimetry (DSC),
dynamic vapor sorption (DVS), and polarized light microscopy (PLM). The data
showed that
the material is crystalline in nature and has similar XRPD pattern to that of
the Form A. Starting
with Form A, polymorph/single crystal screening experiments were set up under
34 conditions
using methods of vapor diffusion, slow evaporation, and cooling
crystallization. Five unique
XRPD patterns were observed, which include Form A, Form B, Forms A + C, Form
D, and
Form E. Form A is monohydrate form as confirmed by single crystal structure.
Form D is
anhydrous, and it was also confirmed by single crystal structure. Form E is an
anhydrous form
produced through dehydration of Form A at ¨90 C. Form B is a known anhydrate
from a
separate study. Form C was not obtained in the pure form during the study but
rather appeared
as a mixture of Forms A + C. Water activity analysis indicated that Form E
converts to Form A
under all conditions tested. In addition, when Form E was exposed to ambient
temperature and
humidity, it showed partial conversion to Form A. Further, the results from
slurry competition
between both anhydrous Forms D and E also indicated that both forms converted
to Form A
33
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
during the experiments. These results suggest that the Form A is the most
stable form at the
ambient temperature and humidity.
Characterization of Form A
The starting material of CV-8972 was characterized using X-ray powder
diffraction
(XRPD), thermogravimetric analysis (TGA), differential scanning calorimetry
(DSC), and
polarized light microscopy (PLM).
FIG. 4 is an )(RFD diffractogram of the CV-8972 starting material. The XRPD
results
suggested high crystallinity of the starting material. Comparison of the XRPD
of the starting
material with previously known polymorphs indicated that it is Form A.
FIG. 5 shows TGA and DSC thermograms of the CV-8972 starting material. TGA
thermogram is shown in green, and DSC thermogram is shown in blue. As shown by
the TGA
and DSC data, about 3.46% weight loss was observed up to 150 C before
decomposition. DSC
showed a small endotherm at 85.3 C (peak) and a possible melting endotherm at
214.6 C
(onset), followed by decomposition. and a melting point at 131.7 C (peak) was
observed.
FIG. 6 shows )(RFD diffractograms of various forms of CV-8972. CV-8972
starting
material is shown in blue; CV-8972 following incubation at 90 C for 8 hours
is shown in red;
and CV-8972 following incubation at 65 C in a vacuum for 2 hours is shown in
purple. To see if
the small DSC endotherm at 85.3 C corresponds to polymorphic phase transition
or dehydration,
)(RFD was performed on Form A after storing it in an oven at 90 C for 8
hours. The data
showed that the Form A converts to Form E.
FIG. 7 is a polarized microscopic image of CV-8972 starting material. Very
platy "mica-
like" morphology of the crystals was observed by PLM.
FIG. 8 is a dynamic vapor sorption isotherm plot. Cycle sorption is shown in
red; cycle 1
desorption is shown in blue; and cycle 2 sorption is shown in green. DVS
results showed that
water uptake of CV-8972 is <0.2% at 25 C and 80% relative humidity (RH)
indicated that
starting material was non hygroscopic. However, there is a drastic increase in
the mass change
beyond 80% RH, which indicates there could be deliquescence.
FIG. 9 shows XRPD diffractograms of CV-8972 before and after dynamic vapor
sorption. Pre-DVS data is shown in red; and post-DVS data is shown in blue.
The XRPD of the
.. sample after DVS indicated weak crystalline peaks but was mostly similar to
the starting
material.
34
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
FIG. 10 shows XRF'D diffractograms of CV-8972 in its dehydrated and rehydrated
forms.
Data from starting material is shown in blue; data following incubation for 2
hours in vacuum
oven are shown in red; and data from heated material that was exposed to
ambient relative
humidity is shown in green. To monitor Form A in its dehydrated state, it was
placed in a
vacuum oven at 65 C for 2 hours followed by its 'MD analysis. The XRPD results
showed that
this process created a new anhydrous form of the material and assigned as Form
E. Rehydration
of Form E when exposed to ambient RH resulted in its partial conversion to
Form A.
X-ray powder diffraction data has been challenging to interpret due to the
extreme
preferred orientation, which results in large variations in the peak
intensities from one sample
preparation to the next. To minimize this effect, single crystal X-ray
diffraction was used to
acquire the crystallographic structure, and the X-ray powder diffraction that
should be observed
in an ideal sample that is absent of preferred orientation was calculated.
Polymorph/single crystal screening
Starting with Form A, polymorph screening experiments were set up under 34
conditions
using methods of slurry conversion, liquid vapor diffusion, slow evaporation,
and slow cooling.
The approximate solubility of starting material was determined at room
temperature (RT).
Accurately weighed samples of approximately 2 mg weight were added into a 3-mL
glass vial.
Solvents were then added step wise (50/50/200/700 L) into the vials until the
solids were
dissolved or a total volume of 1 mL was reached. The solubility of the
starting material in
various solvents is shown in Table 1.
Table 1. Approximate solubility of starting material (6010242-01-A) at RT
Experiment ID Solvent (v:v) Solubility (mg/mL)
6010242-02-Al n-Heptane S<1.9
6010242-02-A2 ACN S<1.5
6010242-02-A3 MIBK S<2.0
6010242-02-A4 Et0Ac S<1.8
6010242-02-A5 THF S<2.2
6010242-02-A6 Et0H S<1.7
6010242-02-A7 Acetone S<2.0
6010242-02-A8 MEK S<2.0
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
Experiment ID Solvent (v:v) Solubility (mg/mL)
6010242-02-A9 IPA S<2.3
6010242-02-A10 CHCI3 S<1.8
6010242-02-All IPAc S<1.7
6010242-02-Al2 1,4-Dioxane S<1.7
6010242-02-A13 CPME S<1.5
6010242-02-A14 DCM S<2.2
6010242-02-A15 Toluene S<2.3
6010242-02-A16 DMSO 5.0<S<15
6010242-02-A17 DMF 1.8<S<6.0
6010242-02-A18 NMP 6.7<S<20
6010242-02-A19 H20 S>44.0
6010242-02-A20 Me0H S>46.0
Results from solubility analysis were used to guide the solvent selection in
polymorph
screening. Polymorph screening experiments were performed using different
crystallization or
solid transition methods. Polymorph screening experiments are summarized in
Table 2.
Table 2,
Method No. of Experiments Crystal Type
Liquid vapor diffusion 24 Form A, B, C, D, and
E
Slow evaporation 2 Gel
Slow cooling 2 No precipitation
Slurry conversion 6 Form A
Total 34 Form A, B, C, D, and
E
FIG. 11 shows XRF'D diffractograms of various polymorphs of CV-8972. Form A is
shown in blue; Form B is shown in green; a mixture of Forms A and C is shown
in navy; Form D
is shown in orange; and Form E is shown in purple.
The various crystal forms of CV-8972 are summarized in Table 3.
36
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
Table 3.
Crystal Form Method of obtaining
Form Identification
Form A Starting material Monohydrate*
Form B Liquid vapor diffusion of MTBE
Hydrate of unknown
in Me0H stoichiometry#
Form C Multiple methods as listed below Solvate/hydrate
(mixed with Form A)
Form D Liquid vapor diffusion of IPA in Anhydrate*
Me0H
Form E 65 C Vacuum for 2 hours Anhydrate
*Single crystal structures for these forms are available and are provided as
separate reports
Information obtained from separate report
Liquid vapor diffusion experiments were conducted in different solvent
conditions.
Approximately 15-25 mg of starting material was dissolved in an appropriate
solvent to obtain a
clear solution in a 3-mL vial. This solution was then placed into a 20-mL vial
with 3 mL of
volatile solvents. The 20-mL vial was sealed with a cap and kept at RT
allowing enough time for
organic vapor to interact with the solution. The precipitates were isolated
for XRPD analysis.
Results from liquid vapor diffusion experiments are summarized in Table 4.
Table 4.
Experiment ID Solvent Anti-solvent Observation
6010242-03-Al H20 MEK Amorphous
6010242-03-A2 1,4-Dioxane Gel
6010242-03-A3 THF Form A + C
6010242-03-A4 Acetone Form A + C
6010242-03-A5 ACN Form A + C
6010242-03-A6 Et0H Form A + C
6010242-03-A7 IPA Clear
37
CA 03188286 2022-12-23
WO 2022/005928 PCT/US2021/039305
Experiment ID Solvent Anti-solvent Observation
6010242-03-A8 Me0H MEK Form A + C
6010242-03-A9 MTBE Single crystal (Form B)
6010242-03-A10 THF Gel
6010242-03-All Acetone Gel
6010242-03-Al2 ACN Gel
6010242-03-A13 Et0H FormA+C
6010242-03-A14 IPA Single crystal (Form D)
6010242-03-A15 Et0Ac Amorphous solid
6010242-07-Al Me0H IPA Form E
6010242-07-A2 IPA Form D
6010242-07-A3 IPA Form D
6010242-07-A4 Toluene Form A + C
6010242-07-A5 Dioxane Small single
crystal on the wall
(Form A + C)
6010242-07-A6 MIBK Small single
crystal on the wall
(Form A + C)
6010242-04-Al H20 MTBE Gel
6010242-04-A2 H20 IPA Gel
6010242-04-A3 Me0H DCM Small
single crystal on the wall
(Form A + C)
Slow evaporation experiments were performed under various conditions. Briefly,
a
saturated solution of starting material prepared in different solvents was
added to a HPLC vial.
The visually clear solutions were covered by Parafilm with 5-10 pinholes and
subjected to
evaporation at RT. The solids were isolated for XRPD analysis. Results from
slow evaporation
experiments are summarized in Table 5.
38
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
Table 5.
Experiment ID Solvent (v:v) Solid Form
6010242-06-A1 Me0H White solid poor crystalline
6010242-06-A2 H20 Gel
Slow cooling experiments were conducted in two different solvent systems.
About 10-15
mg of starting material was suspended in appropriate solvent in a 2-mL glass
vial at RT. The
suspension was then heated to 50 C, equilibrated for about two hours and
filtered using a nylon
membrane (pore size of 0.22 m). Each filtrate was slowly cooled down to 5 C
at a rate of 0.1
C/min. Results from slow cooling experiments are summarized in Table 6,
Table 6.
Experiment ID Solvent (v:v) Solid Form
6010242-05-Al DMSO No
precipitation
6010242-05-A2 NMP No
precipitation
Slurry conversion experiments were conducted at RT in different solvent
systems.
Approximately 20 mg of starting material was suspended in 0.1 mL of solvent in
HPLC vials.
After the suspension was stirred magnetically for 48 hours at RT, the
remaining solids were
isolated for XRPD analysis. Results from slurry conversion experiments are
summarized in
Table 7.
Table 7.
Experiment ID Solvent (v:v) Solid Form
6010242-15-Al Acetone Form A
6010242-15-A2 Acetone/H20 (aw=0.2, 941/59) Form A
6010242-15-A3 Acetone/H20 (aw=0.4, 857/143) Form A
6010242-15-A4 Acetone/H20 (aw=0.6, 726/274) Form A
6010242-15-A5 Acetone/H20 (aw=0.8, 492/508) Form A
6010242-15-A6 H20 Form A
39
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
Experiment ID Solvent (v:v) Solid Form
6010242-15-A7 Form D + E in acetone Form A
Conclusions
The Form A was successfully characterized to understand its form behavior. A
comprehensive polymorph screening in 34 different conditions was performed.
Five polymorph
of the CV-8972 were identified during the screening, including Form A, Form B,
a mixture of
Forms A + C, Form D, and Form E. Form D and E are anhydrous, Form A is a
monohydrate, and
Form B is a hydrate with unknown stoichiometry. Phase origin of Form C is not
known since it
was not obtained it in pure form; it always crystallized as mixture with Form
A. Based on
polymorph screening it is apparent that CV-8972 has a tendency to form
multiple polymorphs.
Current studies have concluded that Form A is the best form for development of
CV-8972 and is
a stable monohydrate form, and is the most stable form under conditions of
ambient temperature
and humidity.
Instruments and Methods
Starting material was used to analyze Form A and screen for other polymorphs.
XRF'D was performed with a Panalytical X'Pert3 Powder XRPD on a Si zero-
background
holder. The 20 position was calibrated against a Panalytical Si reference
standard disc.
Instrumental parameters used for XPRD are listed in Table 8.
Table 8.
Parameters Reflection Mode
X-Ray wavelength Cu, ka
Ka1 (A): 1.540598,
Ka2 (A): 1.544426,
Ka2/Ka1 intensity ratio: 0.50
X-Ray tube setting 45 kV, 40 mA
Divergence slit Fixed 1/8
Scan mode Continuous
Scan range 3-40
( 2TH)
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
Parameters Reflection Mode
Scan step time [s] 18.87
Step size ( 2TH) 0.0131
Test Time 4 min 15 s
TGA data was collected using a TA Discovery 550 TGA from TA Instrument. DSC
was
performed using a TA Q2000 DSC from TA Instrument. DSC was calibrated with
Indium
reference standard and the TGA was calibrated using nickel reference standard.
Detailed
parameters used for TGA and DSC are listed in Table 9.
Table 9.
Parameters TGA DSC
Method Ramp Ramp
Sample pan Platinum, open Aluminum, crimped
Temperature RT ¨ 300 C
Heating rate 10 C/min
Purge gas N2
Polarized light microscopic (PLM) pictures were captured on a Nikon DS-Fi2
upright
microscope at room temperature. Low viscosity microscope immersion oil
(Resolve ) was used
to disperse powder crystals.
Example 2
Summary
To determine the crystal structure of CV-8972, a single monohydrate crystal
was grown,
and a suitable single crystal was used for a full crystal X-ray diffraction
(SCXRD) data
collection at 199 K. A crystal structure with a Ri value of 0.0303 (I>2G(I))
was obtained. The
structure showed that this crystal form is a monohydrate, tri-HC1 salt.
41
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
Crystal Growth and SCXRD preparation
Single crystals of C22H34C13N306 (CV-8972) were obtained via slow cooling:
163.2 mg
starting material was weighed into a 2-mL glass vial, and 0.100 mL water was
added to dissolve
the solids at 50 C, then the solution was slowly cooled to 10 C over 12
hours before harvesting.
FIG. 12 is a PLM image of a batch of single crystals of C22H34C13N306 (CV-
8972). Bar
represents 100 p.m.
FIG. 13 shows PLM images of a crystal used for single-crystal diffractometer.
Bars
represent 100 p.m. A thick needle was picked out and trimmed down to a size of
200 x 160 x 100
um uniform block. This sample was mounted on a 100 mm MiTeGen MicroLoopTm with
low
viscosity cryo-oil (MiTeGen LV Cryo0i1Tm).
FIG. 14 shows images of a crystal mounted on a 100 micro Mitegen loop on the
diffractometer.
Single Crystal Structure Determination
A total of 9576 frames were collected using Bruker Apex3 v2018-7.2. The total
exposure
time was 18 hours (exposure times were adjusted based on 20). The frames were
integrated with
the Bruker SAINT software package using a narrow-frame algorithm. The
integration of the data
using an orthorhombic unit cell yielded a total of 157237 reflections to a
maximum 0 angle of
81.010 (0.78 A resolution), of which 5641 were independent (average redundancy
27.874,
completeness = 99.8%, Rim = 4.41%, Rsig = 1.34%) and 5388(95.51%) were greater
than 2a(F2).
The final cell constants of a = 7.8826(2) A, b = 12.4776(3) A, c = 52.3580(13)
A, volume =
5149.7(2) A3, are based upon the refinement of the XYZ-centroids of 1406
reflections above 20
G(I) with 11.750 <20 < 100.6 . Data were corrected for absorption effects
using the Multi-Scan
method (SADABS). The ratio of minimum to maximum apparent transmission was
0.788. The
calculated minimum and maximum transmission coefficients (based on crystal
size) are 0.5340
and 0.7160.
The structure was solved and refined using the 01ex2 incorporating SEIELXTL
Software
Package using the orthorhombic space group Pbca, with Z = 8 for the formula
unit,
C22H34C13N306. One asymmetric unit contains one whole API molecule. The final
anisotropic
full-matrix least-squares refinement on F2 with 330 variables (0 restraints)
converged at Ri =
3.03%, for the observed data and wR2 = 7.98% for all data. The goodness-of-fit
was 1.041. The
largest peak in the final difference electron density synthesis was 0.358 e-
/A3 (0.81 A from Cli)
42
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
and the largest hole was -0.438 e-/A3 (0.66 A from Cli). Most of the positions
and thermal
ellipsoids of hydrogens were treated as riding models (AFIX 23, AFIX 43, and
AFIX 137 used).
However, the crucial hydrogen atoms involving hydrogen bonding and salt
formation were
refined freely without any constraints. On the basis of the final model, the
calculated density was
1.400 g/cm3 and F(000), 2288 e-. Crystallographic parameters of the
C22H34C13N306 crystal are
summarized in Table 10.
Table 10.
Identification code 6010242 13
Chemical formula C22H34C13N306
Formula weight 542.87 g/mol
Wavelength 1.54178 A
Temperature 199.0 K
Crystal size 0.10 x 0.16 x 0.20 mm
Crystal habit colorless trimmed needle
Crystal system orthorhombic
Space group Pbca
Unit cell dimensions a = 7.8826(2) A a = 900
b = 12.4776(3) A 13 = 90
c = 52.3580(13) A 7 = 90
Volume 5149.7(2) A3
8
Density (calculated) 1.400 g/cm3
Absorption coefficient( (CuKa)) 3.583 mm-1
F(000) 2288
Theta range for data collection 3.38 to 81.01
Index ranges -10<=h<=10, -15<=k<=15, -66<=1<=66
Reflections collected 157237
Independent reflections 5641 [R(int) = 0.0441]
Coverage of independent reflections 99.8%
43
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
Absorption correction Multi-Scan
Max. and min. transmission 0.7160 and 0.5340
Structure solution technique direct methods
Structure solution program XS (Sheldrick, 2008)
Refinement method Full-matrix least-squares on F2
Refinement program XL (Sheldrick, 2008)
Function minimized w(F02 _ Fc2)2
Data/restraints /parameters 5641/0/330
Goodness-of-fit on F2 1.041
A/amax 0.001
Final R indices 5388 data; I>2(I)
Ri = 0.0303, wR2 = 0.0783
all data
Ri = 0.0318, wR2 = 0.0798
Weighting scheme w=1/[a2(F02)+(0.0364P)2+2.762713]
where P=(F02+2F02)/3
Largest cliff, peak and hole 0.358 and -0.438 eA-3
R.M.S. deviation from mean 0.045 eA-3
FIG. 15 is an Ortep diagram of an asymmetric unit of the C22H34C13N306
crystal. The
Ortep diagram of an asymmetric unit of the C22H34C13N306 crystal demonstrates
that this API is
a monohydrate tris-HC1 salt, as a ratio of 1:3:1 (API:HC1:H20) was observed.
FIG. 16 shows one unit cell of the C22H34C13N306 crystal.
FIG. 17 is a diagram of hydrogen bonds networks and counter-ion pairs in the
C22H34C13N306 crystal. The diagram shows that the three hydrochloride
molecules are
deprotonated, whereas the three nitrogens are protonated. The water molecule
serves as a
hydrogen bond donor to bridging two chlorine anions. The crystallographic
measurements of the
hydrogen bonds and counter-ion pairs in the C22H34C13N306 crystal are
summarized in Table 11.
Table 11.
44
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
D-H¨A d(DA)/A (D-I-1===A)/ d(D... A) ADHA)
06-H6A-C13#1 0.85(3) 2.37(3) 3.1997(18) 167.(2)
06-H6B-C12#2 0.91(3) 2.27(3) 3.1815(16) 176.(2)
N2-H2-C11#3 0.932(18) 2.119(18) 3.0499(11)
177.1(16)
0.919(17) 2.127(18) 3.0462(11)
179.2(16)
N3-H3-C13 0.95(2) 2.07(2) 2.9891(13)
163.9(19)
Symmetry transformations used to generate equivalent atoms: #1: 2-X,0.5+Y,0.5-
Z;#2:
0.5+X,+Y,0.5-Z;#3: 1+X,+Y,+Z;$4: 1.5-X,0.5+Y,+Z
The final cif file was checked with Platon using 01ex2 locally, only one level
C alert was
found (missing three reflections), together with eight level G alerts. An
extensive data collection
strategy was implemented to prevent this issue, and the completeness and
redundancy of this
dataset were 99.8% and 27.87, respectively.
FIG. 18 shows calculated and measured XRPD diagrams of the C22H34C13N306
crystal.
Calculated XRPD diffractogram is shown in red; and measured XRPD diffractogram
is shown in
blue. Powder x-ray diffraction of this batch was obtained and compared with a
calculated pattern
based on this crystal structure using Mercury. The experimental peak positions
and intensities fit
well with the calculated pattern.
Instruments and Methods
The X-ray intensity data were measured at 199.0 K (controlled by Oxford
Cryostream
800) on a Bruker Venture X-ray diffractometer. Incoatec Microfocus Source
(41.S 3.0)
monochromated Cu Ka radiation (X= 1.54178 A, voltage= 50 kV, current=1.1mA)
was used as
the x-ray source. The intensity data was collected by a Photon II detector.
Polarized light microscopic picture was captured on Nikon DS-Fi2 upright
microscope at
room temperature.
XRPD was performed with a Panalytical X'Pert3 Powder XRPD on a Si zero-
background
holder. The 20 position was calibrated against a Panalytical Si reference
standard disc.
Example 3
Summary
To determine the crystal structure of CV-8972, a single anhydrate crystal of
CV-8972
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
was grown, and a suitable single crystal was used for a full Seal) data
collection at 102 K. A
crystal structure with a Ri value of 0.0328 (I>2a(I)) was obtained. The
structure showed that this
crystal form is an anhydrous tri-HC1 salt.
Crystal Growth and SCXRD preparation
Single crystals of anhydrous tri-HC1 salt of CV-8972 were obtained via liquid
vapor
diffusion of MTBE in Me0H solution. Briefly, a saturated solution of CV-8972
in Me0H was
obtained at RT and filled into 2-mL glass vial, which was then kept inside a
bigger 20 mL vial
having 2 mL of MTBE, The vial was taken out when it showed presence of white
crystalline
material.
FIG. 19 shows PLM images of single anhydrous crystals from recrystallized CV-
8972.
FIG. 20 is an image of a single anhydrous crystal from recrystallized CV-8972
mounted
on a tip of a glass fiber. The colorless crystal was subsequently set up on
the SCXRD instrument.
Single Crystal Structure Determination
A colorless crystal was mounted on a tip of a glass fiber. The X-ray intensity
data were
measured at 102K temperature on a Bruker D8 Quest PHOTON 100 CMOS X-ray
diffractometer
system with Incoatec Microfocus Source (I S) monochromated Mo Ka radiation (X,
= 0.71073
A, sealed tube) using omega/phi-scan technique. The data were collected in
1660 frames with 10
second exposure times. Crystallographic data: C22H3205N3C13: a = 6.9940(6) A,
b = 10.5742(9)
A, c = 17.5786(14) A, a= 78.252(2)0, ,8 = 82.823(2) 0, y = 82.476(2)0, V=
1255.37(18) A3, Z =
2, F.W. = 524.85, t = 0.403 mm-1, d = 1.389 g/cm3, F(000) = 552.
Crystal data and structure refinement for j l_a are provided in Table 12.
Table 12.
jl a
Crystal data
Chemical formula C22H32C13N305
Mr 524.85
Crystal system, space group Triclinic, Pi
Temperature (K) 102
a, b, c (A) 6.9940 (6), 10.5742 (9), 17.5786 (14)
46
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
ct, (3, y ( ) 78.252 (2), 82.823 (2), 82.476 (2)
V (A3) 1255.37 (18)
2
Radiation type Mo Ka
(mm-i) 0.40
Crystal size (mm) 0.29 x 0.23 x 0.06
Data collection
Diffractometer Bruker D8 Quest PHOTON 100 CMOS
Absorption correction Multi-scan
BRUKER SADABS
Tmax 0.687, 0.747
No. of measured, independent 30843, 8732, 7553
and
observed [I> 2G(/)]
reflections
Rint 0.027
(sin 0/X)max (A-1) 0.746
Refinement
R[F2 > 2G(F2)], .wR(F2), s 0.033, 0.098, 1.01
No. of reflections 8732
No. of parameters 310
No. of restraints 3
H-atom treatment H atoms treated by a mixture of independent and
constrained
refinement
A)max, Amin (e k3) 0.55, -0.46
47
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
Atomic coordinates ( x 104) and equivalent isotropic displacement parameters
(A2 x 103)
for] 1_a are provided in Table 13. U(eq) is defined as one third of the trace
of the orthogonalized
Uij tensor
Table 13.
z U(eq)
C1(1) 3641(1) 9477(1) 6048(1) 14(1)
C1(2) 11992(1) 5170(1) 5985(1) 13(1)
C1(3) 1821(1) 10077(1) 2138(1) 17(1)
0(1) 11846(1) 6620(1) 2868(1) 16(1)
0(2) 9860(1) 7793(1) 3643(1) 12(1)
0(3) 7371(1) 5399(1) 8524(1) 14(1)
0(4) 6912(1) 6708(1) 9782(1) 14(1)
0(5) 4066(1) 8571(1) 9907(1) 16(1)
N(1) 5606(1) 8451(1) 2202(1) 15(1)
N(2) 9414(1) 7581(1) 5446(1) 9(1)
N(3) 6144(1) 6997(1) 6601(1) 9(1)
C(1) 5518(2) 7647(1) 1711(1)
18(1)
C(2) 6990(2) 6666(1) 1628(1)
18(1)
C(3) 8550(2) 6526(1) 2067(1)
15(1)
C(4) 8618(2) 7383(1) 2564(1) 12(1)
C(5) 7105(2) 8356(1) 2629(1)
14(1)
C(6) 10306(2) 7218(1) 3030(1)
12(1)
C(7) 11386(2) 7722(1) 4138(1)
13(1)
C(8) 10548(2) 8407(1) 4797(1)
11(1)
C(9) 8935(2) 8244(1) 6132(1) 11(1)
C(10) 7968(2) 7358(1) 6820(1) 11(1)
48
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
C(11) 6595(2) 6347(1) 5908(1) 11(1)
C(12) 7583(1) 7222(1) 5220(1) 10(1)
C(13) 5051(2) 6149(1) 7265(1) 12(1)
C(14) 4672(2) 6764(1) 7977(1) 11(1)
C(15) 5894(1) 6385(1) 8580(1) 11(1)
C(16) 5670(1) 7028(1) 9206(1) 11(1)
C(17) 4153(2) 8022(1) 9262(1) 12(1)
C(18) 2891(2) 8375(1) 8683(1) 13(1)
C(19) 3177(2) 7754(1) 8045(1) 12(1)
C(20) 7254(2) 4307(1) 9160(1) 17(1)
C(21) 8819(2) 7087(1) 9516(1) 18(1)
C(22) 2481(2) 9539(1) 10007(1) 21(1)
Bond lengths [A] for j la are provided in Table 14.
Table 14.
0(1)-C(6) 1.2075(13)
0(2)-C(6) 1.3282(12)
0(2)-C(7) 1.4443(13)
0(3)-C(15) 1.3774(12)
0(3)-C(20) 1.4371(14)
0(4)-C(16) 1.3774(13)
0(4)-C(21) 1.4372(14)
0(5)-C(17) 1.3658(13)
0(5)-C(22) 1.4268(14)
N(1)-H(1) 0.868(14)
N(1)-C(1) 1.3404(15)
N(1)-C(5) 1.3460(14)
N(2)-H(2) 0.881(13)
49
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
N(2)-C(8) 1.4972(13)
N(2)-C(9) 1.4964(13)
N(2)-C(12) 1.5025(13)
N(3)-H(3) 0.868(13)
N(3)-C(10) 1.4937(13)
N(3)-C(11) 1.4993(13)
N(3)-C(13) 1.5099(13)
C(1)-C(2) 1.3793(17)
C(2)-C(3) 1.3893(16)
C(3)-C(4) 1.3898(15)
C(4)-C(5) 1.3874(15)
C(4)-C(6) 1.4909(15)
C(7)-C(8) 1.5071(14)
C(9)-C(10) 1.5162(14)
C(11)-C(12) 1.5160(14)
C(13)-C(14) 1.5052(14)
C(14)-C(19) 1.3915(14)
C(14)-C(15) 1.4062(14)
C(15)-C(16) 1.3903(14)
C(16)-C(17) 1.4014(14)
C(17)-C(18) 1.3922(15)
C(18)-C(19) 1.3926(15)
Angles [deg] for j la are provided in Table 15.
Table 15.
C(6)-0(2)-C(7) 116.27(8)
C(15)-0(3)-C(20) 113.71(8)
C(16)-0(4)-C(21) 112.87(8)
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
C(17)-0(5)-C(22) 116.45(9)
H(1)-N(1)-C(1) 115.0(12)
H(1)-N(1)-C(5) 122.2(12)
C(1)-N(1)-C(5) 122.73(10)
H(2)-N(2)-C(8) 108.6(11)
H(2)-N(2)-C(9) 105.1(11)
C(8)-N(2)-C(9) 110.24(8)
H(2)-N(2)-C(12) 109.0(11)
C(8)-N(2)-C(12) 114.11(8)
C(9)-N(2)-C(12) 109.30(8)
H(3)-N(3)-C(10) 107.1(11)
H(3)-N(3)-C(11) 107.3(11)
C(10)-N(3)-C(11) 109.71(8)
H(3)-N(3)-C(13) 108.8(11)
C(10)-N(3)-C(13) 113.17(8)
C(11)-N(3)-C(13) 110.51(8)
N(1)-C(1)-C(2) 120.40(11)
C(1)-C(2)-C(3) 118.57(10)
C(2)-C(3)-C(4) 119.88(10)
C(5)-C(4)-C(3) 119.57(10)
C(5)-C(4)-C(6) 121.37(9)
C(3)-C(4)-C(6) 119.05(9)
N(1)-C(5)-C(4) 118.85(10)
0(1)-C(6)-0(2) 125.57(10)
0(1)-C(6)-C(4) 123.74(10)
0(2)-C(6)-C(4) 110.67(9)
0(2)-C(7)-C(8) 107.00(8)
N(2)-C(8)-C(7) 113.78(8)
N(2)-C(9)-C(10) 110.57(8)
N(3)-C(10)-C(9) 109.72(8)
N(3)-C(11)-C(12) 111.10(8)
51
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
N(2)-C(12)-C(11) 109.69(8)
C(14)-C(13)-N(3) 110.97(8)
C(19)-C(14)-C(15) 118.22(9)
C(19)-C(14)-C(13) 121.58(9)
C(15)-C(14)-C(13) 120.14(9)
0(3)-C(15)-C(16) 120.40(9)
0(3)-C(15)-C(14) 118.90(9)
C(16)-C(15)-C(14) 120.65(9)
0(4)-C(16)-C(15) 121.32(9)
0(4)-C(16)-C(17) 118.62(9)
C(15)-C(16)-C(17) 120.07(9)
0(5)-C(17)-C(18) 125.51(9)
0(5)-C(17)-C(16) 114.73(9)
C(18)-C(17)-C(16) 119.75(9)
C(17)-C(18)-C(19) 119.51(10)
C(18)-C(19)-C(14) 121.71(10)
Of the 8732 unique reflections collected to a maximum theta angle of 32.02
(0.67 A
resolution), 7553 were observed (I> 2 GO). The linear absorption coefficient
for Mo Ka
radiation is 0.403 mm-1. The data were integrated with the manufacturer's
SAINT software and
corrected for absorption effects using the Multi-Scan method (SADABS).
Subsequent solution and refinement were performed using the SHELXTL-2014
solution
package operating on a Pentium computer. The structure was solved by direct
method using
SHELXTL-2014 Software Package. Non-hydrogen atomic scattering factors were
taken from the
literature tabulations. Non-hydrogen atoms were located from successive
difference Fourier map
calculations. In the final cycles of each refinement, all the non-hydrogen
atoms were refined in
anisotropic displacement parameters. Except for H(1), H(2), H(3) on N(1),
N(2), N(3) atoms of
the molecule that were located from difference Fourier map and refined with
proper restraints,
the rest of hydrogen atom positions were calculated and allowed to ride on the
carbon to which
they are bonded, assuming a C¨H bond length of m A (m = 0.990 for CH2 groups,
m = 0.980 for
52
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
CH3 groups, m = 0.950 for Ph-H groups). Hydrogen atom temperature factors were
fixed at n (n
= 1.2 for CH2, Ph-H groups, n = 1.5 for CH3) times the isotropic temperature
factor of the C-
atom to which they are bonded. The crystal system of compound is triclinic,
space group P-1
(No. 2) and the final residual values based on 310 variable parameters and
7553 observed
reflections (I> 2 (7(1)) are Ri = 0.0328, wR2 = 0.0926, and those for all
unique reflections are Ri
= 0.0408, w R2 = 0.0975. The goodness-of-fit indicator for all data is 1.014.
Peaks on the final
difference map, ranging from 0.549 to -0.459 e/A3, are of no chemical
significance. The efforts
have been made to resolve as many alerts as possible generated by CheckCIF
program. The
current highest alerts are at level G.
FIG. 21 is a thermal ellipsoid diagram of an asymmetric unit of the
C22H32C13N305
crystal. The diagram demonstrates that this form is an anhydrate, tris-HCl
salt form.
FIG. 22 shows one unit cell of the C22H32C13N305 crystal.
FIG. 23 is a diagram of hydrogen bonds networks and counter-ion pairs in the
C22H32C13N305 crystal.
FIG. 24 shows calculated and measured XRPD diagrams of the C22H34C13N306
crystal.
Calculated XRPD diffractogram is shown in red; and measured MUD diffractogram
is shown in
green.
The compound crystallizes in triclinic, space group P-1 (No. 2). The
asymmetric unit
contains one molecule in the form of cation/anion salt (an anhydrate tri-HCI
salt) with formula of
C22H3205N3C13. There might be some intra-molecular H-bonding between N(1)-
H(1)...C1(3)
(with distance of 2.9634(10)), N(2)-H(2)...C1(2) (with distance of 2.9822(9)),
N(3)-H(3)...C1(1)
(with distance of 3.0120(9)). Structure solution, refinement and the
calculation of derived results
were performed using the SHELXTL-2014 package of computer programs. Neutral
atom
scattering factors were those of Cromer and Waber, and the real and imaginary
anomalous
dispersion corrections were those of Cromer.
Example 4
Introduction
CV-8972 was synthesized according to Scheme 1.
Scheme 1:
53
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
,O, HN7N-i
\\in \NnO
2134-trimethoxybenzaidehyde 2-(piperazin-1 -yi)ethan-1 -of
54
CA 03188286 2022-12-23
WO 2022/005928 PCT/US2021/039305
\\)0
NaBH(0A0).3 Ac OH 0 . .
= _4* = = N =
2-MeTHF8 15 *(.; to RT
step 1 01881.4 Free Base
0
ovi
N Q
EDC, DMAP', DM
RT
=
step 2a CV8972 Free Base
0
Ha =
corm, HC 'N)
MEK,. 50 'T N,,,No" =
H20 Ha
N'
step 2b CV8912 MonohydrateHa
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
NI+)
HC1
H20, Me0H, MEK 0
N". 0
60 to RI N'OFL HO Ncr ,N os -
step 3 HC
CV-8972
Step 1 is a reductive amination using 2,3,4-trimethoxybenzaldehyde and 2-
(piperazin-1-
yl)ethan-l-ol starting materials, with sodium triacetoxyborohydride (STAB) as
the reductant, in
the presence of catalytic acetic acid (AcOH), and 2-methyltetrahydrofuran (2-
MeTHF) as
solvent. After the reaction is completed, an aqueous workup, solvent exchange
to MTBE, and
recrystallization from MTBE/n-heptane forms the intermediate CV-8814 Free Base
(CV8814
Free Base).
In step 2, CV-8814 Free Base (CV8814 Free Base) undergoes acid coupling with
nicotinic acid in the presence of 1-ethyl-3-(3-
dimethylaminopropyl)carbodiimide (EDC) and
catalytic 4-(dimethylamino)pyridine (DMAP) in dichloromethane (DCM) solvent.
After aqueous
workup, CV8972 Free Base is formed. Solvent exchange to 2-butanone (MEK)
followed by
addition into concentrated HC1 in MEK forms CV8972 Monohydrate intermediate.
The final step 3 is a form conversion in a mixture of water, methanol, and MEK
at 60 C
+ 5 C followed by a precipitation by the addition of MEK to obtain the desired
form A of final
product CV-8972 by )(RFD analysis.
Manufacturing details
Manufacturing details are provided in Table 16.
Table 16.
Step # BOP#
Amount Started (Kg) Amount Produced (Kg) Yield (%) HPLC Purity (%)
1 2493-1903-00484 27.5 kg 34.0 kg 76.0%
100.0%
2 2479-1903-00489 28.8 kg 48.6 kg 96.4%
99.1%
3 2479-1904-00494 48.5 kg 41.7 kg 86.0 %
99.9%
56
CA 03188286 2022-12-23
WO 2022/005928 PCT/US2021/039305
Production details
Step 1, formation of CV8814 Free Base (2493-1903-00487), was performed
according to
Scheme 2.
Scheme 2:
'µ'NO
-Li liN"'N,
0. ,=-=N 1 i
+
,N.0õ,, ...c....=5
2,3,4-trimethoxybenzaidehyde 2-(piperazirt-111)ethan-l-ol
\'µO
NaBH(0A03, MOH
2-MeTHF, 15 T to RT 0 /L .11,
N
NCr -', L..õ '-e =
step I cvasu Free Base
Production details for step 1 are provided in Table 17.
Table 17.
Identity Vendor / Grade / MW Equiv. Moles Expected
Actual
Quantity
Quantity
Used
Name Catalog # g/mol Moles! moles Units
Units
mol-LR
2,3,4-Trimethoxy- Oakwood / 98% / 196.20 1.00 140.16 27.5 kg
27.5 kg
benzaldehyde 078721; Chem-Impex
/ NA / 26868; Chem-
Impex / NA / 26868
57
CA 03188286 2022-12-23
WO 2022/005928 PCT/US2021/039305
Identity Vendor / Grade / MW Equiv. Moles Expected Actual
Quantity
Quantity
Used
Name Catalog # g/mol Moles! moles Units Units
mol-LR
1-(2-hydroxyethyppiperazine Aldrich / 98% / 130.19 1.1
154.18 20.1 kg 20.0 kg
H28807
2-Methyltetrahydrofuran (2- Aldrich 1>99.5% / N/A N/A N/A
309.5 kg 338.3 kg
MeTHF) 155810 Plus As
Needed
Acetic Acid (AcOH) Aldrich /?99%1 N/A N/A N/A 1.40 kg 1.4101
kg
A6283
Sodium Triacetoxy Oakwood / 95% / 211.94 2.00 280.32 59.4 kg
Plus 60.3 kg
Borohydride (STAB) 044864 As Needed
Sodium Hydroxide solution, Aldrich / 50% in H20 40 6.09
853.57 68.3 kg 68.3 kg
50% in H20 (50% NaOH in /415413
H20)
Sodium Chloride Aldrich / ACS / N/A N/A N/A 19.0 kg 19.0
kg
(NaC1) S9888
Methyl tert-Butyl Ether Aldrich/ACS / N/A N/A N/A
417.3 Plus 552.1 kg
(MTBE) 443808; Oakwood / As Needed
ACS / 099538
Water Mediatech / WFI / 25- N/A N/A N/A 323.4 kg
323.4 kg
055-Xl; RMBI/ USP /
WPW-USN-2XL
Heptane Aldrich / 99% / N/A N/A N/A 159.8 kg 162.1
kg
H2198, Oakwood /
ACS / 044743; BDH /
ACS / BDH1127
Nitrogen Gas Airgas N/A N/A N/A Quantity
Quantity
(house system) >99% Sufficient
Sufficient
(QS)
58
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
1) Sodium Triacetoxy Borohydride (STAB; 60.3 kg; CHP Lot#: 181-190220) and
2-MeTHF
(189.2 kg; CHP Lot#: 234-190227) was charged to reactor R-401.
2) The contents of R-401 were agitated and the temperature was adjusted to
15 C + 5 C
3) 2,3,4-trimethoxybenzaldehyde (27.5 kg; CHP Lot#: 275-190311, 142-190212)
and 2-
MeTHIF (71.1 kg: CHP Lot#: 234-190227) was charged to reactor R-402.
4) Agitation of reactor R-402 was started.
5) 2-(piperazin-1-yl)ethan-1-ol (20.0 kg; CHP Lot#: 173-190220) and 2-MeTHF
(47.3 kg;
CHP Lot#: 234-190227) was charged to reactor R-402.
6) The contents of reactor R-402 were agitated for at least 5 minutes.
7) Acetic Acid (1.41 kg: CHP Lot#: 151-190214) was charged to reactor R-401
while
keeping the temperature of the mixture below 25 C
8) Tmax = 14.4 C
9) The contents of reactor R-402 were transferred over 1 h 19 min while
keeping the
temperature of the mixture below 25 C.
10) Tmax = 29.2 C (the temperature went out of range during the addition)
11) The reaction was approved to proceed forward
12) The temperature of the contents of R-401 were adjusted to 20 C 5 C
and agitated for at
least 6 hours at 20 C + 5 C.
13) The contents of R-401 were sampled after approximately 19 hours.
14) Analysis of the sample by QC indicated no peak of 2,3,4-
trimethoxybenzaldehyde was
detected, (specification < 1.5 area %)
15) The temperature of the contents of R-401 were adjusted to 15 C + 5 C
16) Water (247.5 kg; CHP Lot#: 232-190227) was charged to R-401 while
keeping the
temperature below 25 C.
17) Tmax = 14.6 C
18) The temperature of the contents of R-401 was adjusted to 20 C + 5 C and
the contents
were agitated for 30 minutes.
19) The contents of R-401 were allowed to settle for 30 minutes.
20) Phase cut was performed with the aqueous layer being transferred to R-
402.
21) MTBE (203.8 kg; CHP Lot#: 207-190222) was charged to R-402.
22) The temperature of the contents of R-402 was adjusted to 0 C + 5 C.
59
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
23) 50% NaOH (68.3 kg; CHIP Lot#: 145-190213, 150-190214) was charged to R-
402 while
maintaining a temperature below 25 C.
24) Tmax = 11.5 C
25) After complete addition, the temperature of the contents of R-402 was
adjusted to 20 C +
5 C.
26) The contents in R-402 were agitated for at least 30 min, followed by
allowing the
contents to settle for at least 30 min.
27) A phase cut was performed with the aqueous layer being transferred to
reactor R-401 and
the organic layer remaining in reactor R-402.
28) MTBE (61.0 kg; CHP Lot#: 207-190222) was charged to R-401.
29) The temperature of the contents of R-401 was adjusted to 20 C + 5 C.
30) The contents in R-401 were agitated for at least 15 min, followed by
allowing the
contents to settle for at least 15 min.
31) A phase cut was performed with the aqueous layer being transferred to a
drum and the
organic later remaining in reactor R-401.
32) FIO pH check of drummed aqueous layer pH = 13.20
33) The contents in R-402 were transferred to R-401.
34) 20% NaCl solution (94.4 kg; NaCl: 19.0 kg, CHIP Lot#: 156-190214; Water
(75.9 kg;
CUP Lot#: 232-190227) was charged to R-401, agitated for at least 15 minutes
and allowed to
settle for at least 30 minutes.
35) A phase cut was performed with the aqueous layer being transferred to a
drum.
36) The solution in R-401 was distilled under reduced pressure while
maintaining a
temperature <45 C to approximately ¨55 L total volume.
37) MTBE (61.1 kg; CHIP Lot#: 207-190222) was charged to R-401.
38) The contents in R-401 were distilled under reduced pressure while
maintaining a
temperature <45 C to approximately ¨82 L total volume.
39) MTBE (61.1 kg; CHIP Lot#: 207-190222) was charged to R-401.
40) The contents in R-101 were distilled under reduced pressure while
maintaining a
temperature <45 C to approximately ¨82 L total volume.
41) The contents of R-401 were sampled (IPC sample: 2493-1903-00484-85-01)
to check the
water content of the solution by KF analysis.
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
42) KF = 1.6% (specification < 0.5%)
43) MTBE (61.1 kg; CHP Lot#: 207-190222) was charged to R-401.
44) The contents in R-101 were distilled under reduced pressure while
maintaining a
temperature <45 C to approximately ¨82 L total volume.
45) The contents of R-401 were sampled (IPC sample: 2493-1903-00484-88-01)
to check the
water content of the solution by KF analysis.
46) KF = 0.8% (specification < 0.5%)
47) MTBE (61.1 kg; CHP Lot#: 207-190222) was charged to R-401.
48) The contents in R-101 were distilled under reduced pressure while
maintaining a
temperature <45 C to approximately ¨82 L total volume.
49) The contents of R-401 were sampled (IPC sample: 2493-1903-00484-91-01)
to check the
water content of the solution by KF analysis.
50) KF = 0.4% (specification < 0.5%)
51) MTBE (30.9 kg; CHP Lot#: 207-190222) was charged to R-401.
52) The temperature of the contents of R-401 was adjusted to 40 C + 5 C.
53) Heptane (56.3 kg; CHP Lot#: 233-190227) was charged over 8 min to R-401
while
maintaining the temperature at 40 C 5 C.
54) = 38. PC
55) A FIO sample (FIO sample: 2493-1903-00484-100-01) was taken to observe
the ratio of
MTBE : Heptane of the contents in R-401. The ratio was 1.5 : 6Ø
56) The temperature of the contents of R-401 were adjusted to 28 C + 5 C
(Target 26 C to
29 C ) over at least 30 min and agitated at that temperature for at least 30
min.
57) Solid formation was observed.
58) The temperature of the contents of R-401 were adjusted to 30 C 3 C and
agitated for at
least 30 min.
59) Heptane (56.4 kg; CHP Lot#: 233-190227) was charged over 24 min to R-
401 while
maintaining the temperature at 30 C + 5 C.
60) Tinin = 30.2 C.
61) The temperature of the contents of R-401 were adjusted to 30 C 3 C
and agitated for at
least 20 min.
61
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
62) The temperature of the contents of R-401 were adjusted to 20 C + 5 C
over at least 30
min and agitated for at least 20 min.
63) The temperature of the contents of R-401 were adjusted to 5 C + 5 C
over at least 30 min
and agitated for at least 30 min.
64) The solid was collected on Filter-FD-400.
65) The contents of Filter-FD-400 were washed with cold heptane (49.4 kg;
CHP Lot#: 233-
190227).
66) The contents of Filter-FD-400 were dried under vacuum at <25 C with a
stream of N2
for at least 16 hours.
67) An IPC sample (IPC sample: 2493-1903-00484-122-01) was submitted to QC
for LOD.
= LOD = 0.20 % (specification < 0.5 %)
68) The product CV-8814 Free Base (CV8814 Free Base) was double bagged,
goose necked,
and weighed.
69) Analysis of dried material (IPC sample: 2493-1903-00484-122-01):
= Appearance: White to off white solid
= Weight: 34.0 kg (78.0 % yield)
= HPLC Purity = 100.0 %
= 1H NMR: conforms to structure
70) AS kg portion of CV-8814 Free Base (CV8814 Free Base) was removed
from the bulk
material double bagged, goose necked, and set aside for release under the Lot#
2493-1903-
00484.
Step 2, formation of CV8972 Monohydrate (2479-1903-00489), was performed
according to Scheme 3.
Scheme 3:
62
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
0
:
s'N = :
1r, = ,ez
MC, MAP. MM
C'VW4 Free mizso stw: PA
õ -$
NO
õ0 01,,,,e,", ..") 0
..,,- -y -...., tsi
N.VA) =Nµsee'\\D'An
$
Nr
õ cvfasn Fill* elamo
N'O
s.:n kJ J1CH
am. RC ,.-= ,...,ii,..,, .,---. Q
NoAN,
1120 HO 1
4
Ap Pt W12 Monohydnite HO
Production details for steps 2a and 2b are provided in Table 18.
Table 18.
Identity Vendor / Grade / MW Equiv. Moles
Expected Actual
Quantity
Quantity
Used
Name Catalog # g/mol Moles / moles Units
Units
mol-LR
CV8814 Free Base (IMB- CHP / NA / CHP Lot 310.39 1.00 92.79
28.8 kg 28.8 kg
1028814) #
2493-1903-00484
>
Nicotinic Acid Aldrich /98% / 123.11 1.5 139.19 17.1 kg 17 1
kg
N4126; Alfa Aesar/
99% / A12683
63
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
Identity Vendor! Grade! MW Equiv. Moles Expected
Actual
Quantity
Quantity
Used
Name Catalog # g/mol Moles / moles Units
Units
mol-LR
CV8814 Free Base (IMB- CHP / NA / CHP Lot 310.39 1,00 92.79
28.8 kg 28,8 kg
1028814)
2493-1903-00484
>
Nicotinic Acid Aldrich /98% / 123.11 1.5 139.19 17.1 kg 17.1
kg
N4126; Alfa Aesar/
99% / A12683
Dichloromethane (DCM) Aldrich / ACS / N/A N/A N/A 536.2 kg
.. 533.4 kg
D65100
EDC Oakwood / 99% / 191.71 1.5 139.19 26.7 kg
26.7 kg
024810; Chem-Impex
/ >99% / 00050
4-(Dimethylamino)pyridine Aldrich / >99% / 122.17 0.15 13.92
1.70 kg 1.70 kg
(DMAP) 107700
Hydrochloric Acid (Conc Avantor / ACS / 36.46 3.3 306.21
30.2 kg 30.2 kg
HC1) 2612-X'
Sodium Bicarbonate Aldrich / ACS / N/A N/A N/A 4,0 kg 4.00
kg
(NaHCO3) S6014
Water Mediatech / WFI / N/A N/A N/A 140.9 kg
.. 140.4 kg
25-055-X'; RMBI/
USP /
WPW-USN-2XL
Methyl Ethyl Ketone Oakwood / 99.9% / N/A N/A N/A 858.0 kg
857.8 kg
(MEK) 075238
Nitrogen Gas Airgas N/A N/A N/A Quantity
Quantity
(house system) >99% Sufficient
Sufficient
(QS)
1) Nicotinic Acid (17.1 kg, CHP Lot# 201-190222) and DCM (153.0 kg, CHP
Lot# 328-
190326) were charged to reactor R-401.
2) The contents of R-401 was agitated and the temperature was adjusted to
15 5 C.
3) CV8814 Free Base (28.8 kg, CHIP Lot# 2493-1903-00484), EDC (26.7 kg,
CHIP Lot#
147-190213), DMAP (1.70 kg, CHIP Lot# 152-190214), and DCM (306.6 kg, CHP Lot#
328-
190326) were charged to reactor R-402.
64
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
4) The contents of the R-402 were agitated for at least 20 minutes.
5) The contents of R-402 were transferred to R-401 over at least 30 minutes
while keeping
the temperature below 25 C.
6) Tmax = 20.0 C
7) The temperature of the contents of R-401were adjusted to 20 + 5 C and
was agitated for
at least 16 hours.
8) The contents of R-401 were sampled after approximately 16 hours.
9) Analysis of the sample by QC indicated 0 % (0.05%) of CV-8814 Free Base
(CV8814
Free Base) with respect to CV8972 was detected (Specification: < 1% CV8814
Free Base).
10) The contents of the R-401 were adjusted to 10 + 5 C.
11) Water (29.2 kg, CHP Lot# 329-190326) was slowly added while keeping the
temperature
below 25 C.
12) Tmax = 12.3 C
13) The temperature of the contents of R-401 was adjusted to 20 + 5 C,
agitated for at least
15 min and allowed to settle for at least 15 min.
14) Phases were separated.
15) The lower organic layer containing product was transferred to reactor R-
402. The
aqueous layer was sent to a drum.
16) Water (29.0 kg, CHP Lot# 329-190326) was charged to the reactor.
17) The biphasic mixture was agitated for 15 min and allowed to settle for
15 min.
18) Phases were separated.
19) The organic layer containing product was transferred to reactor R-401.
The aqueous layer
was sent to a drum.
20) 8% NaHCO3 aqueous solution (Sodium Bicarbonate, 4.0 kg, CHP Lot# 192-
190221;
Water, 53.2 kg, CHP Lot# 329-190326) was added to the reactor.
21) The mixture was agitated for at least 15 min and allowed to settle for
at least 15 min.
22) Phases were separated.
23) The lower organic layer containing product was transferred to reactor R-
402. The
aqueous layer was sent to a drum.
24) Water (29.0 kg, CHP Lot# 329-190326) was added to the reactor.
25) The mixture was agitated for at least 15 min and allowed to settle
for at least 15 min.
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
26) R-401 was cleaned with Water (17.6 kg, CHP Lot# 329-190326) and 1ViEK
(5.9 kg, CHP
Lot# 330-190326) and dried with a stream of N2.
27) Phases were separated.
28) The lower organic layer containing product was transferred to reactor R-
401. The
aqueous layer was sent to a drum.
29) The contents of R-401 were concentrated under reduced pressure to
approx. 72 L keeping
the temperature below 45 C.
30) Tmax = 32.0 C
31) MEK (139.0 kg, CHP Lot# 330-190326) was charged to R-401.
32) The contents of R-401 were concentrated under reduced pressure to
approx. 72 L keeping
the temperature below 45 C.
33) Tmax = 32.0 C
34) MEK (139.1 kg, CHP Lot# 330-190326) was charged to R-401.
35) The contents of R-401 were concentrated under reduced pressure to
approx. 72 L keeping
the temperature below 45 C.
36) Tmax = 29.2 C.
37) FIO 1H NMR to determine the DCM : MEK ratio was taken. DCM : MEK = 1 :
214.9
38) MEK (185.5 kg, CHP Lot# 330-190326) was charged to R-401.
39) MEK (208.7 kg, CHP Lot# 330-190326) and conc. HC1 (30.2 kg, CHP Lot#
274-190311)
were charged to a cleaned R-402.
40) The temperature of the contents of reactor was adjusted to 25 + 5 C.
41) The contents of R-401 were transferred to R-402 over approx. 1 hour
while maintaining a
temperature below 35 C.
42) Tmax = 27.2 C
43) The temperature of the contents of R-402 was heated to 50 + 5 C and was
agitated for at
least 1 hour.
44) The temperature of the contents of the reactor was cooled to 20 + 5 C
over 2 hours.
45) The contents of the reactor were agitated at 20 + 5 C for 15 hours.
46) The solid was filtered.
47) The filter cake was rinsed with MEK (58.0 kg, CHP Lot# 330-190326).
48) The filter cake was rinsed with MEK (58.0 kg, CHP Lot# 330-190326).
66
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
49) The wet cake was dried in tray dryer 20 ¨ 25 C without nitrogen bleed
for at least 16
hours.
50) An IPC sample (IPC sample: 2479-1903-00489-87-01) was submitted to QC
for KF.
= Water content (Specification: < 4% by cKF): 3.3%
51) The product CV8972 Monohydrate was doubled bagged, goose necked, and
weighed.
52) Analysis of the dried material (IPC sample: 2479-1903-00489-87-01):
Appearance: White to off-white solid
= Weight: 48.6 kg (96.4% yield)
= 1H NMR: conforms to structure
= HPLC purity (area %): 99.1%
= FIO Residual Solvents GC Analysis:
2-MeTHF: No Peak
DCM: No Peak
MTBE: No Peak
Heptane: No Peak
MEK: 343 ppm
Acetic Acid: 874 ppm
Step 3, formation of CV-8972 (2479-1904-00494), was performed according to
Scheme
4.
Scheme 4:
N.,
0
HCI
0 HP, Me0H, MEK
I
w 0
1120 HO :k
e
CV8972 Monohydrate HC I step 3
67
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
\O
0
,
,
0 H20 --Hcir 0
HCr
CV-8972
Production details for step 3 are provided in Table 19.
Table 19.
Identity Vendor! Grade / MW Equiv. Moles Expected
Actual
Quantity
Quantity
Used
Name Catalog # g/mol Moles / moles Units
Units
mol-LR
CV8972 CHIP / NA / CHP Lot 2479- 542.88 1 89.34
48.5 kg 48.5 kg
monohydrate 1903-00489
Methanol Aldrich / ACS / 179337; N/A N/A N/A 230.5 kg
230.5 kg
Pharmco / WORLD /
339WORLD-X'; BDH / ACS /
BDH1135
Methyl Ethyl Oakwood / 99.9% / 075238 N/A N/A N/A
1147.8 kg 1149.4 kg
Ketone (MEK)
Water Mediatech / WEI / 25-055-X1; N/A N/A N/A 72.8 kg
72,5 kg
RMBI/ USP / WPW-USN-2XL
Quantity
Nitrogen Gas Airgas Quantity
N/A N/A N/A Sufficient
(house system) 99% (QS)
Sufficient
1) CV8972 monohydrate (48.5 kg, CHP Lot# 2479-1903-00489), Water (48.5
kg, CHP Lot#
329-190326), Methanol (19.2 kg, CHP Lot# 379-190404), and MEK (39.0 kg, CHP
Lot# 330-
190326) were charged respectively to a reactor R-402.
68
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
2) The contents of the R-402 was adjusted to 20 C + 5 C and agitated until
a solution was
obtained.
3) The contents of R-402 were transferred to R-401 through a 0.45 micron in-
line filter.
4) Water (24.0 kg, CHP Lot# 329-190326) and Methanol (19.2 kg, CHP Lot# 379-
190404)
were charged to R-402 and transferred to R-401 through a 0.45 micron in-line
filter.
5) Methanol (192.1 kg, CHP Lot# 379-190404) was charged to R-401 through a
0.45
micron in-line filter.
6) The temperature of the contents of R-401 was adjusted to 60 + 5 C.
7) MEK (899.6 kg, CHP Lot#330-190326; 380-190404) was charged to R-401
through a
0.45 micron in-line filter over approx. 2 hours while maintaining a
temperature of 60 + 5 C.
8) The contents of R-401 were agitated at 60 + 5 C for at least 4 hours.
9) The temperature of R-401 was adjusted to 20 5 C over at least 3 hours.
10) The contents of R-401 were agitated at 20 + 5 C for approx. 9 hours.
11) The contents of R-401 were sent to a filter.
12) The filter cake was rinsed with MEK (105.4 kg, CHP Lot # 380-190404).
13) The filter cake was rinsed with MEK (105.4 kg, CHP Lot# 380-190404).
14) The wet cake were dried on filter with vacuum for at least 30 minutes.
15) The wet cake was packaged into a filer dryer and dried under reduced
pressure at < 30 C
for at least 12 hours.
16) An IPC sample (IPC sample: 2479-1904-00494-32-01) was submitted to QC
for KF and
Residual Solvent GC.
= Water content (Specification: 2.8 ¨ 3.8% by cKF) = 3.5%
= Residual Solvent GC Analysis (Specification: Me0H < 3000 ppm; MEK < 5000
ppm)
Me0H = 215 ppm; MEK = 185 ppm
17) The product CV-8972 was double bagged, goose necked, and weighed.
18) Analysis of the dried material (IPC sample: 2479-1904-00494-32-01)
= Appearance: White to off-white solid
= Weight = 41.7 kg (86.0% yield)
= HPLC purity (area %) = 99.9%
Known Impurities:
Nicotinic Acid: No Peak
69
CA 03188286 2022-12-23
WO 2022/005928 PCT/US2021/039305
DMAP: No Peak
CV8814: 0.1%
2,3,4-Trimethoxy Benzaldehyde: No Peak
Trimetazidine: No Peak
CV-10099: No Peak
CV-10046: No Peak
= XRPD: Conforms to Form A
= Chloride Ion content: 19.4%
= 1H NMR: conforms to structure
Conclusion
The results provided above show that CV-8972 can be synthesized using Scheme
1. The
reductive amination in step 1 using 2,3,4-trimethoxybenzaldehyde and 2-
(piperazin-1-yl)ethan-1-
ol starting materials, sodium triacetoxyborohydride (STAB) as the reductant,
catalytic acetic acid
(AcOH), and 2-methyltetrahydrofuran (2-MeTHF) gave a 78.0% yield of CV-8814
Free Base
(CV8814 Free Base) with 100.0% purity by HPLC after aqueous workup, solvent
exchange, and
crystallization. A 5 kg portion of CV-8814 Free Base (CV8814 Free Base) was
diverted from the
synthesis for release. The step 2 coupling of CV8814 Free Base with nicotinic
acid in the
presence of EDC and catalytic DMAP in DCM went to complete conversion to
CV8972 Free
Base by HPLC IPC. Solvent exchange to MEK and addition into concentrated HC1
in MEK
afforded CV8972 Monohydrate in a 96.4% yield with 99.1% purity by HPLC. The
final form
conversion in step 3 was completed by heating CV8972 Monohydrate to 60 C + 5 C
in a
mixture of water, methanol, and MEK and precipitating out with the addition of
MEK. The white
solid CV-8972 was obtained as form A confirmed by XRF'D analysis, in an 86.0%
yield with
99.9% purity by HPLC. The overall yield of the GlVIP synthesis of CV-8972 was
64.7%. The
final amount of CV-8972 produced was 41.7 kg.
Incorporation by Reference
References and citations to other documents, such as patents, patent
applications, patent
publications, journals, books, papers, web contents, have been made throughout
this disclosure.
All such documents are hereby incorporated herein by reference in their
entirety for all purposes.
CA 03188286 2022-12-23
WO 2022/005928
PCT/US2021/039305
Equivalents
Various modifications of the invention and many further embodiments thereof,
in
addition to those shown and described herein, will become apparent to those
skilled in the art
from the full contents of this document, including references to the
scientific and patent literature
cited herein. The subject matter herein contains important information,
exemplification, and
guidance that can be adapted to the practice of this invention in its various
embodiments and
equivalents thereof.
71