Note: Descriptions are shown in the official language in which they were submitted.
[Description]
[Title of Invention]
PHARMACEUTICAL COMPOSITION FOR TREATMENT OF
NONALCOHOLIC STEATOHEPATITIS AND LIVER FIBROSIS
[Technical Field]
The present invention relates to a pharmaceutical
composition for the treatment of nonalcoholic
steatohepatitis (NASH) and liver fibrosis, particularly
to a pharmaceutical composition comprising sodium
taurodeoxycholate (Na-TDC), a solvate thereof, or a
pharmaceutically acceptable salt thereof.
[Background Art]
Nonalcoholic fatty liver disease (NAFLD) is the most
common chronic liver disease and is a disease closely
related to obesity, diabetes, and metabolic syndrome.
This refers to liver disease in a broad sense, ranging
from simple steatosis, in which triglycerides are
excessively accumulated in the liver, to cirrhosis, which
is the aggravated form of the disease.
Nonalcoholic steatohepatitis (NASH) is a type of
nonalcoholic fatty liver disease, and refers to a disease
that exhibits pathological phenomena such as expansive
degeneration, apoptosis, and inflammatory infiltration,
and furthermore, fibrosis. Not all nonalcoholic fatty
liver disease progresses to nonalcoholic steatohepatitis.
It takes about 7 years to progress from nonalcoholic
fatty liver to nonalcoholic steatohepatitis and more than
50% of patients with nonalcoholic steatohepatitis are
asymptomatic, making it difficult to diagnose
nonalcoholic steatohepatitis. In this regard, the
accuracy of liver damage indicators such as ALT and AST
CA 03192690 2023- 3- 14
1
and the measurement by imaging equipment and the like is
not high, so the possibility of death from complications
is high. It is reported that 10% to 29% of patients with
nonalcoholic steatohepatitis undergo cirrhosis within 10
years, and 4% to 27% of patients with cirrhosis further
undergo liver cancer.
Although the cause of nonalcoholic steatohepatitis
is not known directly, it is presumed that nonalcoholic
steatohepatitis is related to metabolic imbalance such as
oxidative stress, lipid peroxidation, cytokine damage,
lipid metabolism abnormality, and insulin resistance.
Efforts to treat nonalcoholic steatohepatitis are being
made in various fields at home and abroad. In the United
States, the FDA have recently issued guidelines to
promote the development of new drugs for NASH, and
multinational pharmaceutical companies such as Intercept
Pharmaceuticals, Gilead, Allergan, and NGM
Biopharmaceuticals are making efforts to treat
nonalcoholic steatohepatitis through clinical trials. In
this regard, fibrates that activate PPARa and PPARy
glitazones have been known as drugs for improving lipid
metabolism abnormalities. Korean Patent Publication No.
10-2018-0124123 discloses a therapeutic agent for NASH
comprising obeticholic acid or a pharmaceutically
acceptable salt thereof, and US Patent No. 10457703
discloses a use of a pharmaceutical composition
comprising a bile acid derivative to treat FXR or TGR5-
mediated diseases.
However, there is an urgent need to develop a
therapeutic agent for nonalcoholic fatty liver disease
since no therapeutic agent exerting a significant effect
has been developed so far although the use of various
substances to treat nonalcoholic fatty liver disease is
known. The use of sodium taurodeoxycholate for the
CA 03192690 2023- 3- 14
2
treatment of nonalcoholic fatty liver disease is not
known. Accordingly, the present inventors have attempted
to reveal the use of sodium taurodeoxycholate to treat
nonalcoholic fatty liver disease and liver fibrosis, and
as a result, completed the present invention by finding
the therapeutic use of a pharmaceutical composition
comprising sodium taurodeoxycholate, a solvate thereof,
or a pharmaceutically acceptable salt thereof.
[Summary of Invention]
[Technical Problem]
An object of the present invention is to provide a
pharmaceutical composition comprising sodium
taurodeoxycholate (Na-TDC), a solvate thereof, or a
pharmaceutically acceptable salt thereof for the
prevention and treatment of metabolic disease, more
particularly the prevention and treatment of nonalcoholic
steatohepatitis (NASH) and liver fibrosis.
[Solution to Problem]
In order to achieve the object, the present
invention provides a pharmaceutical composition for
prevention and treatment of metabolic disease comprising
sodium taurodeoxycholate, a solvate thereof, or a
pharmaceutically acceptable salt thereof as an active
ingredient.
In an aspect of the present invention, the metabolic
disease is one or more selected from nonalcoholic fatty
liver disease (NAFLD), nonalcoholic steatohepatitis
(NASH), liver fibrosis, obesity, diabetes or
hyperlipidemia.
In an aspect of the present invention, the metabolic
disease is nonalcoholic steatohepatitis (NASH).
In an aspect of the present invention, the metabolic
CA 03192690 2023- 3- 14
3
disease is liver fibrosis.
In an aspect of the present invention, the
composition is to reduce steatosis, inflammation or
ballooning.
In an aspect of the present invention, the
composition is to reduce fibrosis or cirrhosis.
The present invention also provides an oral
preparation comprising the pharmaceutical composition for
prevention and treatment of metabolic disease. In a
specific aspect of the present invention, the oral
preparation is selected from the group consisting of
tablets, granules, pills, powders, capsules and liquids.
The present invention also provides an injectable
preparation comprising the pharmaceutical composition for
prevention or treatment of metabolic disease.
The composition of the present invention may be
administered in combination with a therapeutic agent for
nonalcoholic fatty liver disease (NAFLD). The composition
of the present invention may be administered in
combination with a therapeutic agent for nonalcoholic
steatohepatitis (NASH).
In an aspect of the present invention, the
therapeutic agent for nonalcoholic fatty liver disease is
one or more selected from the group consisting of OCA
(obeticholic acid), thiazolidinediones, vitamin E,
metformin, statins, ursodeoxycholic acid, unsaturated
fatty acids, angiotensin receptor blockers,
pentoxifylline, GLP-1 receptor agonists (glucagon-like
peptide 1 receptor agonists), DPP-4 inhibitors
(dipeptidyl peptidase-4 inhibitors), SGLT2 inhibitors
(sodium/glucose cotransporter 2 inhibitors), elafibranor,
and telmisartan.
The present invention also provides a food
composition for prevention or improvement of metabolic
CA 03192690 2023- 3- 14
4
disease comprising sodium taurodeoxycholate, a solvate
thereof, or a sitologically acceptable salt thereof.
The present invention also provides a method for
treating metabolic disease, the method comprising sodium
taurodeoxycholate, a solvate thereof, or a
pharmaceutically acceptable salt thereof as an active
ingredient.
[Advantageous Effects of Invention]
In the present invention, nonalcoholic
steatohepatitis and liver fibrosis can be treated using a
pharmaceutical composition comprising sodium
taurodeoxycholate (Na-TDC), a solvate thereof, or a
pharmaceutically acceptable salt thereof, and the
pharmaceutical composition has few side effects and thus
can be used as a pharmaceutical.
[Brief Description of Drawings]
FIGS. lA to 10 are diagrams illustrating the effect
of TDCA in the STAN model.
FIGS. 2A and 2B are diagrams confirming the effect
of TDCA through histological analysis of liver.
FIGS. 3A and 3B are diagrams confirming the
therapeutic effect of TDCA on fibrosis.
FIGS. 4A and 4B are diagrams confirming the anti-
inflammatory effect of TDCA.
[Description of Embodiments]
The present invention relates to a pharmaceutical
composition for prevention or treatment of metabolic
disease, more particularly nonalcoholic steatohepatitis
(NASH), comprising sodium taurodeoxycholate, a solvate
thereof, or a pharmaceutically acceptable salt thereof as
an active ingredient.
CA 03192690 2023- 3- 14
[Embodiments]
Hereinafter, the present invention will be described
in detail.
Throughout the specification of the present
invention, when a part "includes" a certain component,
this means that the part does not exclude other
components but may further include other components
unless otherwise stated.
As used herein, the term "sodium taurodeoxycholate"
refers to a compound having the following chemical
structure.
0
11 5 , 0....
J.--- "
0
Na+
HN
H C
3 .e,õ 0
OH .....,
,-.
CH3
1
CH3 11111011111,
HO"' H
=
H
Sodium taurodeoxycholate may be named the same as 2-
([3a,12a-dihydroxy-24-oxo-53-cholan-24-
yl]amino)ethanesulfonic acid, taurodeoxycholic acid
sodium salt hydrate, and CAS number 207737-97-1.
In the present invention, sodium taurodeoxycholate
may exist in all forms, and may exist, for example, in a
crystalline form, an amorphous form, and the form of a
CA 03192690 2023- 3- 14
6
solvate.
As used herein, the term "solvate" refers to a form
formed by adding a solvent to a compound, and includes
monohydrate, dihydrate, and the like.
As used herein, the term "pharmaceutically
acceptable" means that the contained ingredients do not
significantly stimulate living organisms and do not
impair biological activity and properties.
As used herein, the term "pharmaceutically
acceptable salt" refers to a salt having desirable
biological activity, and includes, but is not limited to,
salts of inorganic acids (hydrochloric acid, sulfuric
acid, phosphoric acid, and nitric acid), salts of organic
acids (acetic acid, oxalic acid, maleic acid, fumaric
acid, succinic acid, benzoic acid, ascorbic acid, tannic
acid, pamoic acid, alginic acid, triethylamine,
cyclohexylamine, and pyridine), alkali metal salts
(sodium salt, potassium salt), alkaline earth metal salts
(calcium salt), ammonium salt, or addition salts thereof.
As used herein, the term "prevention" refers to any
action that suppresses the symptoms or delays the
progression of a specific disease (for example,
nonalcoholic steatohepatitis) by injecting the
composition of the present invention into the body.
As used herein, the term "treatment" refers to any
action that improves or beneficially changes the symptoms
of a specific disease (for example, nonalcoholic
steatohepatitis) by injecting the composition of the
present invention into the body.
Compounds described herein refer to commonly
recognized compounds unless otherwise defined.
The present invention relates to a pharmaceutical
composition for prevention or treatment of metabolic
CA 03192690 2023- 3- 14
7
disease comprising sodium taurodeoxycholate, a solvate
thereof, or a pharmaceutically acceptable salt thereof as
an active ingredient.
In the present invention, metabolic disease refers
to any disease that occurs by an abnormality in the
pathway of chemical reactions taking place in the body,
and non-limiting examples of metabolic disease include
the following diseases. Metabolic disease includes, but
is not limited to, for example, type 1 diabetes, type 2
diabetes, hyperlipidemia, hypertension, insulin
resistance, obesity, abnormal glucose metabolism,
diabetic retinopathy, diabetic nephritis, diabetic
neuropathy, increase in body mass, hypercholesterolemia,
nonalcoholic fatty liver disease (NAFLD), nonalcoholic
steatohepatitis (NASH), liver fibrosis, polycystic ovary
syndrome (PCOS), cirrhosis, hepatitis C, alcoholic liver
disease, primary sclerosing cholangitis, and primary
biliary cholangitis.
In an aspect of the present invention, the
pharmaceutical composition of the present invention
exhibits the effect of preventing or treating one or more
selected from the group consisting of nonalcoholic fatty
liver disease (NAFLD), nonalcoholic steatohepatitis
(NASH), liver fibrosis, obesity, diabetes and
hyperlipidemia.
In a specific aspect of the present invention, the
pharmaceutical composition of the present invention
exhibits the effect of preventing or treating
nonalcoholic steatohepatitis (NASH).
In a specific aspect of the present invention, the
pharmaceutical composition of the present invention
exhibits the effect of preventing or treating liver
fibrosis.
In an aspect of the present invention, the
CA 03192690 2023- 3- 14
8
composition is to reduce steatosis, inflammation or
ballooning.
In an aspect of the present invention, the
composition is to reduce fibrosis or cirrhosis.
The pharmaceutical composition of the present
invention may be administered orally or parenterally
according to the desired method, and the dosage thereof
may vary depending on the patient's weight, age, health
status, and diet, time of administration, method of
administration, type of disease, severity of disease and
the like. When the pharmaceutical composition of the
present invention is administered, the daily dosage is
about 0.01 to 1000 mg/kg, more specifically 0.1 to 100
mg/kg, and the pharmaceutical composition may be
administered one time to several times a day.
In an aspect of the present invention, there is also
provided an oral preparation comprising the
pharmaceutical composition for prevention and treatment
of metabolic disease described above. In a specific
aspect of the present invention, the oral preparation is
selected from the group consisting of tablets, granules,
pills, powders, capsules and liquids.
The present invention also provides an injectable
preparation comprising the pharmaceutical composition for
prevention or treatment of metabolic disease described
above.
In an aspect of the present invention, the
pharmaceutical preparation for oral administration may
exist, for example, in the form of dosage units such as
tablets, granules, pills, powders, capsules or liquids,
and in the form of ampoules. These are prepared by
methods known per se, for example by a common mixing,
granulation, conserve preparation, dissolution or
CA 03192690 2023- 3- 14
9
lyophilization method.
Other orally administrable pharmaceutical
preparations include capsules, and capsules include dry-
filled capsules made of gelatin, and sealed soft capsules
made of gelatin and a plasticizer such as glycerol or
sorbitol. Dry-filled capsules may contain the active
ingredient in the form of particles, for example, in the
form of a mixture with a filler such as lactose, a binder
such as starch, and/or a glidant such as talc or
magnesium stearate, and if appropriate, a stabilizing
agent. In soft capsules, the active ingredient is
dissolved or suspended in a suitable liquid such as fatty
oil, paraffin oil or liquid polyethylene glycol, to which
a stabilizing agent may be added.
When the formulation of the present invention is a
liquid formulation, a solvent, solubilizer, or emulsifier
is used as a carrier ingredient, and examples thereof
include water, ethanol, isopropanol, ethyl carbonate,
ethyl acetate, benzyl alcohol, benzyl benzoate, propylene
glycol, 1,3-butyl glycol oil, glycerol-based aliphatic
esters, fatty acid esters with polyethylene glycol or
sorbitan.
In an aspect of the present invention, parenteral
administration may be applied, for example, but not
limited to, intravenously, subcutaneously, intradermally,
intraperitoneally or topically. Pharmaceutical
preparations for parenteral administration may be
sterilized and/or contain additives such as
preservatives, stabilizing agents, wetting agents and/or
emulsifying agents, solubilizing agents, salts for
adjusting the osmotic pressure and/or buffers.
The pharmaceutical composition of the present
invention may be administered in combination with a
CA 03192690 2023- 3- 14
therapeutic agent for nonalcoholic fatty liver disease
(NAFLD).
The pharmaceutical composition of the present
invention may be administered in combination with a
therapeutic agent for nonalcoholic steatohepatitis
(NASH).
In an aspect of the present invention, the
therapeutic agent for nonalcoholic fatty liver disease is
one or more selected from the group consisting of OCA
(obeticholic acid), thiazolidinediones, vitamin E,
metformin, statins, ursodeoxycholic acid, unsaturated
fatty acids, angiotensin receptor blockers,
pentoxifylline, GLP-1 receptor agonists (glucagon-like
peptide 1 receptor agonists), DPP-4 inhibitors
(dipeptidyl peptidase-4 inhibitors), SGLT2 inhibitors
(sodium/glucose cotransporter 2 inhibitors), elafibranor,
and telmisartan.
The present invention also relates to a food
composition for prevention or improvement of metabolic
disease comprising sodium taurodeoxycholate, a solvate
thereof, or a sitologically acceptable salt thereof.
The present invention also provides a method for
treating metabolic disease, the method comprising sodium
taurodeoxycholate, a solvate thereof, or a
pharmaceutically acceptable salt thereof as an active
ingredient.
[Examples]
Hereinafter, the present invention will be described
in detail by way of Examples and Experimental Examples.
However, the following Examples and Experimental
Examples are merely illustrative of the present
invention, and the contents of the present invention are
not limited to the following Examples and Experimental
CA 03192690 2023- 3- 14
11
Examples.
<Example 1> Preparation of sample
Sodium taurodeoxycholate was purchased from New
Zealand Pharmaceuticals Ltd. (Palmerston North, New
Zealand). Telmisartan (Micardis10) was purchased from
Boehringer Ingelheim GmbH (Germany) and dissolved in
purified water. DPBS (Dulbecco's phosphate-buffered
saline) was purchased from WelGene Inc. (LB001-02, Korea)
and used as a vehicle compound.
<Experimental Example 1> Animal model of
nonalcoholic steatohepatitis and test design
<1-1> Animal model of nonalcoholic steatohepatitis
In order to confirm the therapeutic effect of sodium
taurodeoxycholate on nonalcoholic steatohepatitis, a
mouse STAN model was prepared.
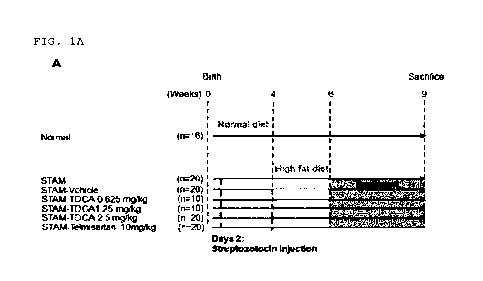
The STAN model was induced using C57BL/6 mice
through the combination of a high-fat diet and a
chemical, streptozotocin. C57BL/6 mice (14-day pregnant
female) were procured from Japan SLC, Inc. (Japan), and
the STAN model was prepared at SMC Laboratories, Inc.
(Tokyo, Japan) using 136 C57BL/6 male mice. All animals
used in the study were housed and managed in conformity
with the Animal Use Guidelines of the Japanese
Pharmacological Association. Specifically, nonalcoholic
steatohepatitis (NASH) was induced by giving single
subcutaneous injection of 200 pg streptozotocin (STZ,
Sigma-Aldrich, USA) solution 2 days after birth and
providing ad libitum feeding of a high-fat diet (HFD, 57
kcal% fat, CLEA Japan, Cat# HFD32, Japan) after 4 weeks
of age. Mice were randomly assigned, and the specific
study design is illustrated in FIG. 1A.
At 6 weeks of age, vehicle compound DPBS,
CA 03192690 2023- 3- 14
12
taurodeoxycholic acid (TDCA) and telmisartan were each
orally administered every day, and the treatment was
performed until 9 weeks of age. Body weight was measured
prior to each treatment. Then, 9-week-old mice were
euthanized by exsanguination by direct cardiac puncture
under isoflurane (Pfizer Inc., USA) anesthesia.
<1-2> Histological analysis
1-2-1. For H&E staining, sections were cut from
paraffin blocks of liver tissue fixed with Bouin's
solution, and stained with Lillie-Mayer's Hematoxylin
(Muto Pure Chemicals Co., Ltd., Japan) and eosin solution
(FUJIFILM Wako Pure Chemical Corporation, Japan).
1-2-2. NAFLD activity score (NAS) was calculated
according to the Kleiner criteria.
1-2-3. For visualization of collagen deposition,
liver sections fixed with Bouin's solution were stained
using picro-Sirius Red solution (Waldeck, Germany).
1-2-4. For quantitative analysis of fibrosis
regions, sections stained with Sirius Red were captured
around the central vein using a 200x magnification
digital camera (DFC295; Leica, Germany). Positive regions
in 5 fields/section were measured using Image J software
(National Institute of Health, USA).
1-2-5. For immunohistochemistry, sections were cut
from frozen liver tissue fixed in Tissue-Tek 0.C.T
compound and fixed in acetone. Endogenous peroxidase
activity was blocked using 0.03% H202 for 10 minutes, and
then the sections were incubated with Block Ace (DS
pharma Biomedical Co., Ltd., Japan) for 10 minutes. The
sections were incubated with reticular fibroblast
antibodies (BMA Biomedicals Inc., Cat#: T-2109, Clone:
ER-TR7, Switzerland) at room temperature for 1 hour.
Subsequently, the sections were incubated with a biotin-
CA 03192690 2023 3 14
13
conjugated secondary antibody (VECTASTAIN Elite ABC kit,
Vector Laboratories, Cat#: PK-4004, USA), and then
incubated with ABC reagent at room temperature for 30
minutes.
The enzyme substrate reaction was conducted using a
3,3'-diaminobenzidine/H202 solution (Nichirei Bioscience
inc., Japan).
1-2-6. For quantitative analysis of ER-TR7 positive
regions, bright field images of ER-TR7 immunostained
sections were captured around the central vein using a
200x magnification digital camera (DFC295; Leica).
Positive regions in 5 fields/section were measured using
Image J software (National Institute of Health, USA).
<1-3> Quantitative RT-PCR (real time polymerase
chain reaction)
Total RNA was extracted from liver samples using
RNAiso (Takara Bio, Japan) according to the
manufacturer's instructions. Specifically, 1 pg of RNA
was reverse transcribed using 20 pL of a reaction
mixture. The reaction mixture contains 4.4 mM MgCl2 (F.
Hoffmann-La Roche, Switzerland), 40 U RNase inhibitor
(Toyobo, Japan), 0.5 mM dNTP (Proemga, USA), 6.28 pM
random hexamer (Promega, USA), 5x first strand buffer
(Promega, USA), 10 mM dithiothreitol (Invitrogen, USA),
and 200 U MMLV-RT (Invitrogen, USA). The reaction was
conducted at 37 C for 1 hour and then at 99 C for 5
minutes.
Specific gene expression was quantified through
real-time PCR using PowerUpTM SYBRTM Green Master Mix
(Applied Biosystems Inc., USA, Cat# A25779), and the
real-time PCR was performed using the QuantStudio 6 Flex
System Instrument (Applied Biosystems Inc., USA).
60S acidic ribosomal protein PO (RPLPO) RNA
CA 03192690 2023- 3- 14
14
expression was measured as an internal control.
Relative expression levels of target genes were
normalized to RPLPO and compared. Relative quantification
was performed using the ACt method.
The primer sequence is as follows: CCL2(MCP1)
(Forward: 5'- AAACTGCATCTGCCCTAAGG-35 / Reverse: 55-
AGAAGTGCTTGAGGTGGTTG-35).
<1-4> ELISA
TNF-a in the mouse plasma was analyzed using a mouse
TNF-a uncoated ELISA Kit (Invitrogen, Cat# 88-7324, USA)
and TIMP1 was analyzed using a mouse TIMP1 ELSIA Kit
(abcam, Cat# ab196265, Cambridge, UK), and the kits were
used according to the manufacturer's instructions.
<1-5> Data and statistical analysis
Data are presented as mean SD, all data including
two or more groups were analyzed by one-way ANOVA, then
analyzed by Dunnett or Tukey multiple comparison test, or
Kruskal Wallis test, and then analyzed by Dunn multiple
comparison test. Asterisks in the figures mean *: p <
0.05, **: p < 0.01, ***: p < 0.001 and ****: p < 0.0001,
respectively. Analysis was performed using GraphPad Prism
8.3.1 (San Diego, CA, USA).
<Experimental Example 2> Confirmation of effect of
treating nonalcoholic steatohepatitis
<2-1> Confirmation of changes in body weight and
liver weight
The effect of TDCA in the STAN model of nonalcoholic
steatohepatitis by the experimental method according to
<Experimental Example 1> was investigated. Telmisartan,
an angiotensin II receptor antagonist and peroxisome
proliferator-activated receptor-y agonist, has the
CA 03192690 2023 3 14
property of improving NASH by reducing fibrosis and
inflammation. In the experiment of the present invention,
telmisartan was used as a reference drug.
It has been confirmed that TDCA and telmisartan have
therapeutic effects in the STAN model (FIG. 1A).
A significant difference in the body weight between
the treatment group (TDCA) and the vehicle group (DPBS)
has not been observed, and it has been thus confirmed
that there is no weight loss due to general drug
toxicity. However, it has been confirmed that a
remarkable decrease occurs in the telmisartan group
compared to the vehicle group (FIG. 1B).
A significant difference in the liver to body weight
ratio between the treatment group (TDCA) and the vehicle
group (DPBS) has not been observed. However, it has been
confirmed that a remarkable decrease occurs in the
telmisartan group compared to the vehicle group (FIG.
10). The telmisartan group decreased the liver to body
weight ratio in the animal group as previously reported.
<2-2> Histological analysis of liver
Compared to the normal group, hepatocyte steatosis,
ballooning, and inflammatory cell infiltration occurred
in the STAN model group (FIG. 2A).
Compared to the vehicle group (DPBS), a significant
decrease in total NAS has been confirmed in the TDCA 1.25
mg/kg treatment group and the telmisartan group, and the
improvement in NAS lesion index, an important clinical
symptom measure of NASH, has been thus confirmed (FIG.
2B).
<Experimental Example 3> Confirmation of therapeutic
effect on fibrosis
Compared to normal mice, an increase in collagen
CA 03192690 2023- 3- 14
16
deposition in the hepatic lobule region has been observed
in the liver sections stained with Sirius Red and liver
sections stained with anti-fibroblast antibody ER-TR7 in
the STAN mice (FIG. 3A).
Compared to the vehicle group (DPBS), a significant
decrease in the area of fibrosis in the seropositive
region has been observed in the TDCA 2.5 mg/kg treatment
group and the telmisartan group, and the therapeutic
effect on fibrosis, an important clinical treatment
measure of NASH drugs, has been thus confirmed (FIG. 3B).
Compared to the vehicle group (DPBS), a significant
decrease in the ER-TR7 positive region has been confirmed
in the TDCA 10 mpk group, and it has been thus confirmed
that the improvement of fibrosis is correlated with the
effect of inhibiting fibroblasts in the liver tissue
(FIG. 3B).
<Experimental Example 4> Gene and protein expression
of inflammatory collagen/fibrosis marker
The mRNA expression levels of mouse liver tissue
genes related to inflammation and fibrosis in NASH were
analyzed according to the experimental example described
above. The inflammatory marker analyzed was MCP-1
(monocyte chemoattractant protein-1). As a result of the
analysis, a remarkable decrease in MCP-1 was observed in
the TDCA 2.5 mg/kg treatment group compared to the
vehicle group (DPBS), and the anti-inflammatory effect
has been thus confirmed (FIG. 4A).
Protein expression levels of the inflammatory
cytokine TNF-a and the fibrosis marker TIMP1 in mouse
plasma were analyzed by ELISA. As a result of the
analysis, a remarkable decrease in TNF-a was observed in
the TDCA 2.5 mg/kg treatment group compared to the
vehicle group (DPBS), and it has been thus confirmed that
CA 03192690 2023- 3- 14
17
there is an anti-inflammatory effect of inhibiting
important inflammatory cytokines (FIG. 4B).
[Industrial Applicability]
The present invention can be usefully utilized for
the prevention or treatment of metabolic disease, more
specifically the prevention or treatment of nonalcoholic
steatohepatitis (NASH).
CA 03192690 2023- 3- 14
18