Note: Descriptions are shown in the official language in which they were submitted.
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
ANTI-ABCC1 ANTIBODIES AND USES THEREOF
CROSS-REFERENCE TO RELATED APPLICATiONS
This application cialms the benefit of priority to U.S. Provisional Patent
Application No.
63/073826 filed on September 2, 2020, which application is incorporated herein
by reference in
its entirety.
INCORPORATION BY REFERENCE OF SEQUENCE LISTING PROVIDED AS TEXT FILE
A Sequence Listing is provided herewith as a text file, 1KNJY-008W0 SEQ
LIST_ST25.txt," created on August 24, 2021 and having a size of 151 KB. The
contents of the
text file are incorporated by reference herein in their entirety.
INTRODUCTION
Drug resistance, a well-known phenomenon that results when diseases become
tolerant
to pharmaceutical treatments, is a major and increasing challenge in various
fields of medicine,
including oncology. Although many types of cancers are initially susceptible
to chemotherapy,
over time they can develop resistance through these and other mechanisms,
including DNA
mutations and metabolic changes that promote drug inhibition, degradation and
enhanced efflux.
Efflux pumps (EP) are proteins expressed by living cells and have evolved to
naturally
expel various compounds from the cells. Members of the ATP-binding cassette
(ABC) transporter
family proteins are examples of EPs that enable drug efflux. Though a
transporters structure
varies from protein to protein (e.g., there are 49 known members of the ABC
family in humans),
they are all classified by the presence of two distinct domains¨a highly
conserved nucleotide
binding domain and a more variable transmernbrane domain. Multidrug resistance
protein 1
(MDR1), encoded by the ATP Binding Cassette Subfamily B Member 1 (ABCB1) gene,
was the
first of these to be identified and has been studied extensively. ATP Binding
Cassette Subfamily
C Member 1 (ABCC1) expression is increased in response to treatment with
certain
oh emot h e ra pe utics
EPs enable tumors to develop resistance to chemotherapeutic agents. Such
resistance is
frequently associated with enhanced efflux of the chemotherapeutic agent from
the drug resistant
1
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
cells, This chemotherapy resistance is termed multi drug resistance (MDR) when
it applies to
more than one chemotherapeutic agent.
As such there is a need to develop reagents that may be used for assaying for
expression
of EPs and/or inhibiting EPs.
SUMMARY
Provided are antibodies that target the cellular efflux pump ATP Binding
Cassette
Subfamily C Member 1 (ABCC1.), Also provided are pharmaceutical compositions,
nucleic acids,
recombinant expression vectors, cells, and kits that include or encode such
antibodies. Methods
of using the antibodies for detecting presence or absence of ABCC1 expression
in cells, e..-g..,
tumor cells, level of ABCC1 expression, and/or inhibiting ABCC1 function are
also disclosed.
Also provided are methods for treating a subject for a cancer that include
administering to the
subject an anti-ABCC1 antibody disclosed herein,
BRIEF DESCRIPTION OF THE FIGURES
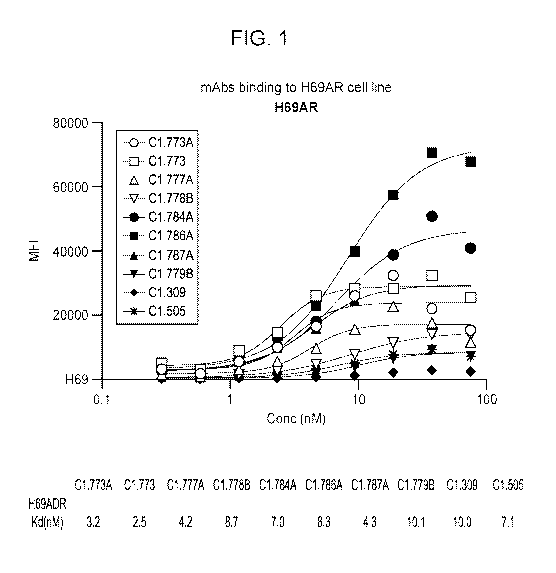
FIG. 1 depicts the results of titration binding of the indicated anti-ABCC1
monoclonal
antibodies to the doxorubicin-resistant lung carcinoma cell line H69AR (ATCC
CRL-11351) that
endogenously expresses ABCC1.. H69AR (ATCC CRL-11351) was established from NCI-
H69
cells that were grown in the presence of increasing concentrations of
Adriamycin (doxorubicin)
over a total of 14 months,.
FIGS. 2A-28 depict the results of titration binding of the indicated anti-
ABCC1 monoclonal
antibodies to human and cynomolgus ABCC1 -overexpressing rat C6 glioma cell
lines.
FIGS, 3A-3C, 4, 5A-5B, and 6A-613 show the results of titration binding of
further anti-
ABCC1 monoclonal antibodies to human and .cynomolgus ABCC1-overexpressing rat
CS glioma
cell lines.
Ab only refers to not adding a primary antibody before binding with
secondary
-- antibody (i.e., 2n4 Ab) to provide a negative control.
FIGS, 7A-78 show the results of ABCC1 efflux assay performed using HEK293T
cells
expressing human ABCC1.
FIGS, 8A-8C present titration binding and efflux assay characterization of
humanized anti-
ABCC1 monoclonal antibodies,
FIGS. 9A-9C show the binding of humanized anti-ABCC1 monoclonal antibodies to
human.
and cynomolgus ABCC1 oyerexpressing rat CC glioma cell lines,
2
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
FIGS. .10A-10B show the binding of various humanized C1.851 anti-A8001
antibodies to
human and cynomolgus ABCC1 overexpressing rat C6 .glioma cell lines.
FIGS, 11A-11C present the binding of four humanized Cl/KT9 bispecific
antibodies to
human and cynomolgus 06 cell lines, overexpressing AB-CC1 and KT9,
respectively. The
schematic bispecific antibody structure is also shown. KT9 stands for
atezolizumab, an anti-PD-
L1 monoclonal antibody. The bispecific antibodies include the heavy and light
chains from the
indicated ABCC1 antibodies and a .scFv region formed from the KT9 antibody.
FIGS. 12A-12C present the binding of four humanized Cl/KT1 bispecific
antibodies to
293.T cells expressing human ABCC1 and 293T cells expressing human or
cynomolgus KT1,
respectively. KT1 stands for trastuzumab, an anti-E.rbB2 (anti-HER2)
monoclonal antibody. The
bispecific antibodies include the heavy and light chains from the indicated
anti-ABCC1 antibodies
and a s.c.Fy region formed from the KT1 antibody.
FIGS. 13A-13B, 14A-14B, and 15 show the effect of the tested anti-ABCC1
monoclonal
antibodies on vincristine cytotoxicity in the 293T cytotoxicity assay.
FIG. 16 shows that the three anti-ABCC1 monoclonal antibodies tested inhibit
tumor
growth in vivo in the H69AR cytotoxicity assay. The assay evaluates the effect
of the tested
antibodies on Adriamycin cytotoxicity on the H69AR cell line, which is an
Adriamycin-selected,
CI-positive variant of the human small cell lung cancer cell line, NCI-H69.
Tull101 formed from
H89AR cell line is resistant to Adriamycin (Doxorubicin). All three of the
anti-ABCC1 monoclonal
antibodies tested sensitized the tumor to Adriamycin.
FIG. 17 shows that anti-ABCC1 monoclonal antibodies C1-831 and C1-737A inhibit
tumor
growth in vivo in the 0126 syngeneic mouse tumor model. The inhibition of
tumor growth by these
antibodies is enhanced by ilioxorubicin,
DEFINITIONS
The terms "antibody" and "immunoglobulin" include antibodies or
immunoglobulins of any
isotype, fragments of antibodies which retain specific binding to antigen,
including, but not limited
to, Fab, Fv, soFv, Fd, Fab', Fv, F(ab)2, chimeric antibodies, humanized
antibodies, monoclonal
antibodies, single-chain antibodies, including antibodies comprising only
heavy chains (e..g. VHH
camelid antibodies), bispecific antibodies, and fusion proteins comprising an
antigen-binding
portion of an antibody and a non-antibody protein. The antibodies may be
detectably labeled, e.g.,
with a radioisotope, an enzyme which generates a detectable product, a
fluorescent protein, and
the like. The antibodies may be further conjugated to other moieties, such as
members of specific
binding pairs, e.g., biotin (member of biotin-avidin specific binding pair),
and the like. The
3
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
antibodies may also be bound to a solid support, including, but not limited
to, polystyrene plates
or beads, and the like. An antibody may be monovalent or bivalent. An antibody
may be
conjugated to a toxic moiety, such as, a chemotherapeutic agent.
"Antibody fragments" comprise a portion of an intact antibody, for example,
the antigen
binding or variable region of the intact antibody. Examples of antibody
fragments include Fab,
Fab', F(ab')2, and Fv fragments; diaborlies: linear antibodies (Zapata et al.,
Protein Eng. 8(10):
1057-1062 (1995)); single-chain antibody molecules, including antibodies
comprising only heavy
chains (e.g. VHH oarnelid antibodies); and multispecific antibodies formed
from antibody
fragments. Papain digestion of antibodies produces two identical antigen-
binding fragments,
called "Fab" fragments, each with a single antigen-binding site, and a
residual 'To" fragment, a
designation reflecting the ability to crystallize readily. Pepsin treatment
yields an F(ab')2fragment
that has two antigen combining sites and is still capable of cross-linking
antigen.
is the minimum antibody fragment which contains a complete antigen-recognition
and
-binding site, This region consists of a dimer of one heavy- and one light-
chain variable domain
in tight, non-covalent association. It is in this configuration that the three
CDRs of each variable
domain interact to define an antigen-binding site on the surface of the VH-
V1,. dimer, Collectively,
the six CDRs confer antigen-binding specificity to the antibody. However, even
a single variable
domain (or half of an Fv comprising only three CDRs specific for an antigen)
has the ability to
recognize and bind antigen, although at a lower affinity than the entire
binding site comprising the
three CDRs of each variable domain.
The "Fab' fragment also contains the constant domain of the light chain and
the first
constant domain (CH) of the heavy chain. Feb fragments differ from Fab'
fragments by the
addition of a few residues at the carboxyl terminus of the heavy chain CHI
domain including one
or more cysteines from the antibody hinge region, Fab'-SH is the designation
herein for Fab' in
which the cysteine residue(s) of the constant domains bear a free thiol group.
F(ab')2 antibody
fragments originally were produced as pairs of Fab' fragments which have hinge
oysteines
between them. Other chemical couplings of antibody fragments are also known,
The "light chains" of antibodies (immunogiobulins) from any vertebrate species
can be
assigned to one of two clearly distinct types, called kappa and lambda, based
on the amino acid
sequences of their constant domains. Depending on the amino acid sequence of
the constant
domain of their heavy chains, immunoglobulins can be assigned to different
classes. There are
five major classes of immunoglobulins: IgA, IgD, IgE, igG, and IgM, and
several of these may be
further divided into subclasses (isotypes), e.g., igGI, IgG2, IgG3, IgG4, igA,
and IgA2.
4
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
"Single-chain Fv", "sfv" or "scFv" antibody fragments comprise the VH and VI,
domains of
antibody, wherein these domains are present in a single polypeptide chain. in
some
embodiments, the Ev polypeptide further comprises a polypeptide linker between
the VH and VL
domains, which enables the sFy to form the desired structure for antigen
binding. For a review of
sfy, see Pluckthun in The Pharmacology of Monoclonal Antibodies, vol, 11.3,
Rosenburg and
Moore eds., Springer-Verlag, New York, pp. 269-315 (1994).
The term "diabodies" refers to small antibody fragments with two antigen-
binding sites,
which fragments comprise a heavy-chain variable domain (VH) connected to a
light-chain variable
domain (VL.) in the same polypeptide chain (VH-VL.). By using a linker that is
too short to allow
pairing between the two domains on the same chain, the domains are forced to
pair with the
complementary domains of another chain and create two antigen-binding sites.
Diabodies are
described more fully in, for example, EP 404,097; WO 93111161; and Hollinger
at al., Proc, Nati,
Acad. Sci. USA, 90:6444-6448 (1993)õ
As used herein, the term "affinity" refers to the equilibrium, constant for
the reversible
binding of two agents and is expressed as a dissociation constant (Kd).
Affinity can be at least
1-fold greater, at least 2-fold greater, at least 3-fold greater, at least 4-
fold greater, at least 5-fold
greater., at least 6-fold greater, at least 7-fold greater, at least 8-fold
greater, .at least 9-fold greater:,
at least 10-fold greater:. at east 20-fold greater, at least 30-fold greater,
at least 40-fold greater,
at least 50-fold greeter, at ieast 60-fold greater., at least 70-fold greater,
at least 80-fold greater,
at least 90-fold greater, at least 100-fold greater, or at least 1000-fold
greater, or more, than the
affinity of an antibody for unrelated amino acid sequences. Affinity of an
antibody to a target
protein can be, for example, from about 100 n.anomolar (nM) to about 0.1 nM,
from about 100 nM
to about 1 picomolar (pM), or from about 100 nM to about 1 femtomolar (fM) or
more. As used
herein, the term "avidity" refers to the resistance of a complex of two or
more agents to
dissociation after dilution. The terms Immunoreactive" and "preferentially
binds" are used
interchangeably herein with respect to antibodies and/or antigen-binding
fragments..
The term "binding" refers to a direct association between two molecules, due
to, for
example, covalent, electrostatic, hydrophobic, and ionic and/or hydrogen-bond
interactions,
including interactions such as salt bridges and water bridges.. An ABCCI -
specific antibody binds
specifically to an epitope within a ABCCI polypeptide. The epitope may be a
linear epitope formed
by a contiguous stretch of amino acids or a non-linear or a conformational
epitope formed by non-
contiguous stretches of amino acids. Non-specific binding would refer to
binding with an affinity
of less than about 10-1 M, e.g., binding with an affinity of 10-6 M, i0 M, 104
M. etc.
CA 03193588 2023-03-01
WO 2022/051390 PCT/US2021/048701
As used herein, the term 'CDR" or "complementarily determining region" is
intended to
mean the non-contiguous antigen combining sites found within the variable
region of both heavy
and light chain polypeptides. CDRs are hypervariable regions and are
interspersed with regions
that are more conserved, termed "framework regions (FR)'. CDRs have been
described by Kabat
et al., J. Bid, Chem, 2526609-6616 (1977); Kabat et at, U.S, Dept. of Health
and Human
Services, 'Sequences of proteins of immunological interest" (1991); by Chothia
et at, J. Mol. Biot
196:901-917 (1987); and M.acCallum et al., j. Mot Biol. 262:732-745 (1996),
where the
definitions include overlapping or subsets of amino acid residues when
compared against each
other. Nevertheless, application of either definition to refer to a CDR of an
antibody or grafted
antibodies or variants thereof is intended to be within the scope of the term
as defined and used
herein. The amino acid residues which encompass the CDRs as defined by each of
the above
cited references are set forth below in Table 1 as a comparison,.
Table CDR Definitions
Kobe Chothia2 MaoCailum3.
VH CDR1 31-35 26-32 30-35
VH. CDR2 50-65 53-55 47-58
VH CDR3 95-102 96-101 .93-101
...... ...... ..... ..... ......
.CDR1 24-34 26-32. 30-36
VE. CDR2 50-56 50-5.2 46-55
CDR3 89-97 91-96. 89-96
Residue numbering follows the nomenclature of Kabat et at, supra
2 Residue numbering follows the nomenclature of .Chothia et at , supra
Residue numbering follows the nomenclature of MacCallum et at, supra
As used herein, the term "framework" when used in reference to an antibody
variable
region is intended to mean all amino acid residues outside the CDR regions
within the variable
region of an antibody. A variable region framework is generally a
discontinuous amino acid.
sequence between about 100-120 amino acids in length but is intended to
reference only those
amino acids outside of the .CDRs. As used herein, .the term "framework region"
is intended to
mean each domain of .the framework that is separated by the CDRs. A VH chain
can comprise
three CDRs and four FRs arranged from N-terminus to C-terminus in the
following order; FR1,
CDR1, FR2, CDR2, FR3õ CDR3, FR4, Similarly, a .VL chain can comprise three
CDRs and four
FI:Rs arranged from N-terminus to C-terminus in the following order: FR1,
CDR1, FR2, CDR2.,
FR3, CDR3, FR4, The terms VH chain and .VH region are used interchangeably
herein. The terms
VI,. chain and VL region are used interchangeably herein.
As used herein, the term antibody encompasses a tetramer of two heavy and two
light
chains, wherein the heavy and light chains are interconnected by, for example,
disulphide bonds.
6
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
The heavy chain constant region is comprised of three domains, CH-I. CH2 and
CH3.. The light
chain constant region is comprised of one domain, CL. The variable regions of
the heavy and light
chains comprise binding regions that interact with antigen. The constant
regions of the antibodies
typically mediate the binding of the antibody to host tissues and factors,
including various cells of
the immune system and the first component of the complement system. The term
"antibody"
includes immunoglo-bulins of types Ig.A, IgG, IgE, IgD, IgM and subtypes
thereof. In some
embodiments, a subject antibody is an IgG isoty-pe, e.g.., IgG.1.
As used herein the term "im.munogiobulin" refers to a protein including one or
more
polypeptides substantially encoded by immunoglobulin genes. The recognized
human
immunoglobulin genes include the kappa, lambda, alpha (lgAl and IgA2), gamma
(IgG-1, IgG2õ
IgG3, IgG4), delta, epsilon and mu constant region genes; and numerous
immunoglobulin
variable region genes,. Full-length immunoglobulin light chains (about 25 kD
or 214 amino acids)
are encoded by a variable region gene at the N-terminus (about 110 amino
acids) and a kappa
or lambda constant region at the C-terminus, Full-length immunoglobulin heavy
chains (about 50
kD or 446 amino acids) are encoded by a variable region gene at the N-terminus
(about 116
amino acids) and one of the other aforementioned constant region genes at the
C-terminus, e.g..
gamma (encoding about 330 amino acids). In some embodiments, a subject
antibody comprises
full-length immunoglobulin heavy chain and a full-length immunoglobulin light
chain.
The term "antigen-binding fragment" refers to one or more fragments of a full-
length,
antibody that are capable of specifically binding to an antigen. Examples of
binding fragments
include (i) a Fab fragment (a monovalent fragment including, e.g.., consisting
of, the VL, VH, CL
and CHI domains; (ii) a .F(ab')2 fragment (a bivalent fragment comprising two
Fab fragments
linked by a disulfide bridge at the hinge region; (iii) a Fd fragment
(including, e.g., consisting of,
the VH and CHI domains); (iv) a Fv fragment (including, e.g,, consisting of,
the VH and VL
domains of a single arm of an antibody); (v) a dAb fragment (including, e.g..
consisting .of, the VH
domain); (vi) an isolated CDR; (vii) a single chain Fv (scFv) (including,
e.g., consisting of, the VH
and .VL domains of a single arm of an antibody joined by a synthetic linker
using recombinant
means such that the VH and VL domains pair to form a monovalent molecule);
(viii) diabodies
(including, e.g., consisting of, two soFvs in which the VH and VL domains are
joined such that
they do not pair to form a monovatent molecule; the VH of each one of the
.scFv pairs with the VL
domain of the other sc-F-v to form a bivalent molecule).
The term chimeric" antibody refers to an antibody in which a portion of the
heavy and/or
light chain is derived from a particular source or species, while the
remainder of the heavy and/or
light chain is derived from a different source or species,
7
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
A "human antibody" is one which possesses an amino acid sequence which
corresponds
to that of an antibody produced by a human or a human cell or derived from a
non-human source
that utilizes human antibody repertoires or other human antibody- encoding
sequences. This
definition of a human antibody specifically excludes a humanized antibody
comprising non-human
antigen-binding residues.
A 'human consensus framework". is a framework (FR) which represents the most
commonly occurring amino acid residues in a selection of human immunoglobulin
variable light
chain (VL) or variable heavy chain (VH) framework sequences. Generally: the
selection of human
immunoglobulin VL or VH sequences is from a subgroup of variable domain
sequences,
Generally, the subgroup of sequences is a subgroup as in Kabat et al.,
Sequences of Proteins of
Immunological Interest, Fifth Edition, NH Publication 91-3242, Bethesda Md.
(1991), vols. 1-3.1n
one embodiment, for the VL, the subgroup is subgroup kappa I as in Kabat et
al., supra. In one
embodiment, for the VH, the subgroup is subgroup III as in Kabat et al.,
supra.
A "humanized" antibody refers to a chimeric antibody comprising amino acid
residues from
non-human CDRs and amino acid residues from human frameworks (FRs). At least a
portion of
a humanized antibody constant region is derived from a human antibody, e.g,, a
human IgG1
antibody. in preferred embodiments, the antibody molecules disclosed herein
include a heavy
chain comprising a variable heavy chain region as provided herein and a human
IgG1 constant
region having the amino acid sequence sequence set forth in UniProt: P01857-1,
version 1. In
preferred embodiments, the antibody molecules disclosed herein include a light
chain comprising
a variable light chain region as provided herein and a human light chain
constant region. In
preferred embodiments, the human light chain constant region is a human kappa
light chain
constant region having the amino acid set forth in UniProtKB/Swiss-Prot:
P018342, In certain
embodiments, the human IgG1 heavy chain constant region present in the subject
antibodies may
include mutations, e.g., substitutions to modulate Fc function. For example,
the LALAPG effector
function mutations (L234A, L235A, and P329G) or the N297A mutation may be
introduced to
reduce antibody dependent cellular cytotoxicity (ADGC). The numbering of the
substitutions is
based on the EU numbering system, The "EU numbering system' or "EU index" is
generally used
when referring to a residue in an immunoglobulin heavy chain constant region
(e.g,, the EU index
reported in Kabat at ai,, Sequences of Proteins of immunological Interest, 5th
Ed. Public Health
Service, National Institutes of Health, Bethesda, MD. (1991)), The "EU index
as in Kabat" refers
to the residue numbering of the human IgG 1 EU antibody.
A "humanized form" of an antibody, e.g., a non-human antibody, refers to an
antibody that
has undergone humanization.
8
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
The term "epitope refers to a region of an antigen that is recognized by the
immune
system, for example by antibodies, B cells, or T cells.. For example, the
epitope is the specific
region of the antigen to which an antibody binds.
An "isolated" antibody is one that has been identified and separated and/or
recovered from
a component of its natural .environment Contaminant components of its natural
environment are
materials that would interfere with diagnostic or therapeutic uses for the
antibody, and may include
enzymes, hormones, and other proteinaceous or nonproteinaceous solutes, in
some
embodiments, the antibody will be purified (1) to greater than 90%, greater
than 95%, or greater
than 98%, by weight of antibody as determined by the Lowry method, for
example, more than
99% by weight, (2) to a degree sufficient to obtain at least 15 residues of N-
terminal or internal
amino acid sequence by use of a spinning cup sequenator, or (3) to homogeneity
by sodium
dociecyi sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing
or nonreducing
conditions using Coomassie blue or silver stain. Isolated antibody includes
the antibody in situ
within recombinant cells since at least one component of the antibody's
natural environment will
not be present. In some instances, isolated antibody will be prepared by at
least one purification
step.
The term "cytotoxic agent" as used herein refers to a substance that inhibits
or prevents a
cellular function and/or causes cell death or destruction, A "chemotherapeutic
agent,' also
referred to an "antineoplastic agent," can be a cytotoxic agent which is used
for treating a cancer
or other disease or disorder.
As used herein, the terms "treatment," "treating,.' and the like, refer to
obtaining a desired
pharmacologic and/or physiologic effect. The effect may be prophylactic in
terms of completely or
partially preventing a disease or symptom thereof and/or may be therapeutic in
terms of a partial
or complete cure for a disease and/or adverse effect attributable to the
disease. "Treatment!' as
used herein, covers any treatment of a disease in a mammal, including in a
human, and includes:
(a) preventing the disease from occurring in a subject which may be
predisposed to the disease
but has not yet been diagnosed as having
(b) inhibiting the disease, i.e., arresting its
development; and (c) relieving the disease, i.e., causing regression of the
disease.
The terms "individual," "subject," 'host," and 'patient" used interchangeably
herein, refer
to a mammal, including, but not limited to, murines (rats, mice), non-human
primates, humans,
canines, felines, ungulates equines, bovines, vines, porcine.s, caprines),
etc.,
A "therapeutically effective amount" or 'efficacious amount" refers to the
amount of a
target-specific antibody that, when administered to a mammal or other subject
for treating a
disease, is sufficient to affect such treatment for the disease,. The
"therapeuticaliy effective
9
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
amount" will vary depending on the antibody, the disease and its severity and
the age, weight,
etc., of the subject to be treated.
The term "refractory", used herein, refers to a disease or condition that does
not respond
to treatment, With regard to cancer, "refractory cancer", as used herein,
refers to cancer that does.
not respond to treatment. A refractory cancer may be resistant at the
beginning of treatment or it
may become resistant during treatment. Refractory cancer may also be called
resistant cancer.
A "biological sample" encompasses a variety of sample types obtained from an
individual
and .can be used in a diagnostic or monitoring assay. The definition
encompasses blood and other
liquid samples of biological origin, solid tissue samples such as a biopsy
specimen Of tissue
cultures or cells derived therefrom and the progeny thereof. The definition
also includes samples
that have been manipulated in any way after their procurement, such as by
treatment with:
reagents, solubilizationõ or enrichment for certain components, such as
polynucleotides. The term
"biological sample" encompasses a clinical sample, and also includes cells in
culture, cell
supernatants, cell lysates, serum, plasma, biological fluid, and tissue
samples.
Percent identity between a pair of sequences may be calculated by multiplying
the number
of matches in the pair by 100 and dividing by the length of the aligned
region, including gaps.
identity scoring only counts perfect matches and does not consider the degree
of similarity of
amino acids to one another. Only internal gaps are included in the length, not
gaps at the
sequence ends, Percent Identity (Matches x 1.00)/Length of aligned region
(with gaps)
The phrase "conservative amino acid substitution" refers to substitution of
amino acid
residues within the following groups: 1) L., I, M. V, F; 2) R, K; 3) F, Y. H.
W, R; 4) G. A. T, S; 5) Q,
N; and 6) D, E. Conservative amino acid substitutions may preserve the
activity of the protein by
replacing an amino acid(s) in the protein with an amino acid with a side chain
of similar acidity,
basicity, charge, polarity, or size of the side chain.
Guidance for substitutions, insertions, or deletions may be based on
alignments of amino
acid sequences of proteins from different species or from a consensus sequence
based on a
plurality of proteins having the same or similar function.
The term "vector" means any molecule or entity (e.g., nucleic acid, plasmid,
bacteriophag:e
or virus) used to transfer protein coding information into a host cell.
The term "expression vector" or "expression construct" refers to a vector that
is suitable
for transformation of a host cell and .contains nucleic acid sequences that
direct and/or control (in.
conjunction with the host cell) expression of one or more heterolog.ous coding
regions operatively
linked thereto. An expression construct may include, but is not limited to,
sequences that affect
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
or control transcription, translation, and, if introns are present, affect RNA
splicing of a coding
region operably linked thereto.
The term 'stimulation,' refers to a primary response induced by binding of a
stimulatory
molecule (e.g., a TCRICD3 complex or CAR) with its cognate ligand (or tumor
antigen in the case
of a CAR) thereby mediating a signal transduction event, such as, but not
limited to, signal
transduction via the TCRICD3 complex or signal transduction via the
appropriate NK receptor or
signaling domains of the CAR. Stimulation can mediate altered expression of
certain molecules.
The term "stimulatory molecule,' refers to a molecule expressed by an immune
cell (e.g.....
T cell, NK cell, B cell) that provides the cytoplasmic signaling sequence(s)
that regulate activation
of the immune cell in a stimulatory way for at least some aspect of the immune
cell signaling
pathway. in one embodiment, the signal is a primary signal that is initiated
by, for instance, binding
of a TCR/CD3 complex with an MHC molecule loaded with peptide, and which leads
to mediation
of a T cell response, including: but not limited to, proliferation,
activation, differentiation, and the
like. A primary cytoplasmic signaling sequence (also referred to as a "primary
signaling domain")
that acts in a stimulatory manner may contain a signaling motif which is known
as immunoreceptor
tyrosine-based activation motif or ITAM. Examples of an lTAM containing
cytoplasmic signaling
sequence that is of particular use in the invention includes, but is not
limited to, those derived
from CD3 zeta, common FcR gamma .(FCER1G): Fc gamma Rile, FcR beta (Fc Epsilon
Rib),
CD3 gamma, CD3 delta,. CD3 epsilon, CD79a.õ CD79bõ DAP10, and DAP12.
The term a "costimulatory molecule" refers to a cognate binding partner on a T
cell that
specifically binds with a .costimulatory ligand, thereby mediating a
co.stimulatory response by the
T cell, such as, but not limited to, proliferation. Costimulatory molecules
are cell surface molecules
other than antigen receptors or their ligands that are contribute to an
efficient immune response.
Costimulatory molecules include, but are not limited to an MHC class I
molecule, BTLA and a Toll
ligand receptor, as well as 0X40õ .CD27, CD28, CDS, ICAM-1, LFA-1 (CD11
a/CD18), ICOS
(CD278)., and 4-1BB .(CD137).
The term "autologous' refers to any material derived from the same individual
to whom it
is later to be re-introduced into the individual,
An "intracellular signaling domain," as the term is used herein, refers to an
intracellular
portion of a molecule. The intracellular signaling domain generates a signal
that promotes an
immune effector function of the CAR containing cell, e.g.,, a CART cell..
Examples of immune
effector function: e.g., in a CAR-T cell, include cytolytic activity and
helper activity, including the
secretion of cytokines.
11
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
"Immune effector
as that term is used herein, refers to a cell that is involved in an
immune response, e.g, in the promotion of an immune effector response.
Examples of immune
effector cells include T celis, e.g., alpha/beta T cells and gamma/delta T
cells, B cells, natural
killer (Nk) cells, natural killer T (NKT) cells, mast cells, and myeloid-
derived phagocytes.
DETAILED DESCRIPTION
Provided are antibodies that bind to the cellular efflux pump ABCC1. Also
provided are
pharmaceutical compositions, nucleic acids, recombinant expression vectors,
cells, and kits that
include or encode such antibodies. Methods of using the antibodies for
detecting presence or
absence of ABCC1 expression in cells, e.g., tumor cells, level of ABCC1
expression, and/or
inhibiting ABCC1 function ere also disclosed. Also provided are methods for
treating a subject for
a cancer that include administering to the subject an anti-ABCC1 antibody as
disclosed herein.
Before the present invention is described in greater detail, it is to be
understood that this
invention is not limited to particular embodiments described, as such may, of
course, vary. It is
also to be understood that the terminology used herein is for the purpose of
describing particular
embodiments only, and is not intended to be limiting, since the scope of the
present invention will
be limited only by the appended claims.
Where a range of values is provided, it is understood that each intervening
value, to the
tenth of the unit of the lower limit unless the context clearly dictates
otherwise, between the upper
and lower limit of that range and any other stated or intervening value in
that stated range, is
encompassed within the invention. The upper and lower limits of these smaller
ranges may
independently be included In the smaller ranges and are also encompassed
within the invention,
subject to any specifically excluded limit in the stated range. Where the
stated range includes one
or both of the limits, ranges excluding either or both of those included
limits are also included in
the invention.
Certain ranges are presented herein with numerical values being preceded by
the term
"about." The term "about" is used herein to provide literal support for the
exact number that it
precedes, as well as a number that is near to or approximately the number that
the term precedes.
In determining whether a number is near to or approximately a specifically
recited number, the
near or approximating unrecited number may be a number which, in the context
in which it is
presented, provides the substantial equivalent of the specifically recited
number.
12
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
Unless defined otherwise, all technical and scientific terms used herein have
the same
meaning as commonly understood by one of ordinary skill in the art to which
this invention
belongs. Although any methods and materials similar or equivalent to those
described herein can
also be used in the practice or testing of the present invention,
representative illustrative methods.
and materials are now described.
All publications and patents cited in this specification are herein
incorporated by reference
as if each individual publication or patent were specifically and individually
indicated to be
incorporated by reference and are incorporated herein by reference to disclose
and describe the
methods and/or materials in connection with which the publications are cited.
The citation of any
publication is for its disclosure prior to the filing date and should not be
construed as an admission
that the present invention is not entitled to antedate such publication.
Further, the dates of
publication provided may be different from the actual publication dates which
may need to be
independently .confirmed.
It is noted that, as used herein and in the appended claims, the singular
forms 'a", "an".
and "the" include plural referents unless the context clearly dictates
otherwise_ It is further noted
that the claims may be drafted to exclude any optional element. As such, this
statement is
intended to serve as antecedent basis for use of such exclusive terminology as
"solely," "only"
and the like in connection with the recitation of claim elements, or use of a
"negative" limitation,.
As will be apparent to those of skill in the art upon reading this disclosure,
each of the
individual embodiments described and illustrated herein has discrete
components and features
which may be readily separated from or combined with the features of any of
the other several
embodiments without departing from the scope or spirit of the present
invention. Any recited
method can be carried out in the order of events recited or in any other order
which is logically
possible.
While the methods and compositions have or will be. described for the sake of
grammatical
.fluidity with functional explanations, it is to be expressly understood that
the claims, unless
expressly formulated under 35 U.S.C, 112(f)õ are not to be construed as
necessarily limited in
any way by the construction of "means" or "steps" limitations, but are to be
accorded the full scope
of the meaning and equivalents of the definition provided by the claims under
the judicial doctrine
of equivalents, and in the case where the claims are expressly formulated
under 35 U.S.C, .112(f)
are to be accorded full statutory equivalents under 35 U.S.C. 112(f).
13
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
ANTIBODIES
As summarized above, the present disclosure provides antibodies that bind a
cellular
efflux pump ABCC1 expressed on surface of a mammalian cell, e.g., a human
cell. ABCC1, also
known as Glutathione-S-Conjugate-Translocating ATPase ABCC1 or MUltidrug
Resistance-
Associated Protein 1 (MRP1), is an energy-dependent pump that effluxes drugs
and organic
anions across the plasma membrane, It includes 17 transmembrane hetice.s
linked by
extracellular and cytoplasmic loops. .ABCC1 mediates resistance to
doxorubicin, etoposide, and
vincristine among others.
In some embodiments, the antibodies disclosed herein bind to one or more sites
on an.
extra.cellular region of ABCC1. In certain embodiments, the anti-ABCC1
antibodies of the present
disclosure bind to human ABCC1. In certain embodiments, the anti-.ABCC1
antibodies of the
present disclosure bind to human ABCC1 expressed on the cell surface of a
human cell, eg., a
cancer cell.
Antibodies of the present disclosure may have one or more of the following
properties;
i) inhibits efflux from ABCC1:
ii) increases sensitivity of cancer cell to treatment with a
chemotherapeutic agent
thereby lowering the IC.50 of the chemotherapeutic agent by at least a factor
of 2;
iii) binds to human and cynomolgus ABCC1 on tell surface;
iv) is effective in in vitro cell killing assays;
:v) is effective in inhibiting tumor growth even in absence of
chemotherapy; and
vi) has an affinity for ABCC1 in a lower range such that it binds to
cancer cells that
express ABC-C1 at a higher level as compared to non-cancer cells and binds
significantly less to non-cancer cells.
As used herein, ECK- refers to the concentration of an antibody that provides
half maximal
response (e.g., half of the maximum fluorescence intensity). The antibodies of
the present
disclosure may have an EC-50 of 100 nIV1 or lower, e.g., 100 nM 4nMõ 80 nM
4nM, SO nM
4nM, 40 nM 4nM, 30 nM 4nM, . 20 nM 4nM, 15 nM 4nM, or 10 ni\I 4nM. EC50 of a
test
antibody many be determined by flow cytometry or ELISA. For example, flow
cytom-etry may
involve contacting a cell expressing ABCC1 (e.g. human .ABCC1) with the
antibody in a flow
cytometry buffer., where the antibody iS serially diluted, and incubating at
room temperature or
4.T. for a period of time sufficient for the antibody to bind to the cells
(e.g. .10 min-1 hr). After
incubating, the cells may optionally be washed to remove and non-specifically
bound antibody
and/or the cells may be contacted with a fiuorescently labeled secondary
antibody that specifically
binds to the test antibody. After incubation, the fluorescently labeled
secondary antibody may be
14
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
removed and the cells washed. The washed cells may be sorted by flow oytometiy
and the
number of cells bound to the fluorescently labeled secondary antibody counted.
The concentration
that provides half maximal response (e.g., half of the maximum fluorescence
intensity) is
measured as the EC50. In variations of the flow cytometry assay, the cell may
be a 293T cell
overexpressing ABCC1.
The I050 of a test antibody may be determined by measuring inhibition of cell
growth.
IC50 may be measured by using the test antibody alone to determine the
concentration of the
antibody that produced half maximal response. The 1050 of a chemotherapeutic
agent may be
measured in the absence and in the presence of the test antibody to determine
the effect of the
antibody on the 1050 chemotherapeutic agent. The chemotherapeutic agent may be
doxorubicin,
daunorubicin, etoposide, vincristine etc. The cell may be a cancer cell line.
The cancer cell line
may be H69AR, a doxorubicin-selected, Cl-positive variant of the lung cancer
cell line, H69. Cells
may be contacted with antibody alone if determining the 1050 of the antibody,
wherein the
antibody is tested at serial dilutions. Cells may be contacted with antibody
and the
chemotherapeutic agent to determine the effect of the antibody on the 1050 of
the agent, where
the agent is tested at serial dilutions. The cells may be incubated at 37 C
for a period of time (e.g.
24 hr-84hr) and cell viability assessed using standard reagents and methods,
The antibodies
disclosed herein may increase sensitivity of cancer cell to treatment with a
chemotherapeutic
agent thereby lowering the 1050 of the chemotherapeutic agent by at least a
factor of 5. In certain
embodiments, the antibodies of the present disclosure may lower the I050 of
the
chemotherapeutic agent by factor of 5 or more, e.g., factor of 6 or more,
factor of 7 or more, factor
of 8 or more, factor of 9 or more, or factor of 10 or more, e.g., by a factor
of 5 to 10,
In certain embodiments, one or more of the anti-ABCC1 antibodies disclosed
herein bind
to both human and cynomolgus ABCC1. This property may be utilized in
determining safety of
the antibody in an animal model.
In certain embodiments, the anti-ABCC1 antibodies disclosed herein are
specific for
ABCC1 and do not show significant binding to other antigens,
In some embodiments, one or more of the subject antibodies may, when bound to
a cell
expressing ABCC1, prevent the functioning of the cellular ABCC1 protein,
Accordingly, one or
more antibodies of the present disclosure may inhibit efflux by the ABCC1
protein, including e.g.,
where efflux is reduced by 5% or more, including e.g,, 10% or more, 15% or
more. 20% or more,
25% or more, 30% or more, 40% or more, 50% or more, 60% or more, 70% or more,
60% or
more, or 90% or more, as compared to efflux by ABCC1 in the absence of the
subject antibody.
In some embodiments, the subject antibodies may, when bound to a cell
expressing ABCC1 may
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
otherwise impede the action of ABCC1 by other mechanisms, e.g., rendering
ABCC1 leaky which
in turn may enhance uptake of a chemotherapeutic agent and/or decrease
viability of the cell.
In certain embodiments, an anti-ABCC1 antibody that competes for binding to
ABCC1 with
an antibody comprising heavy chain complementarity determining regions (HCDRs)
and light
chain CDRs (LCDRs) of the variable heavy chain (VH) region and the variable
light chain (VL)
region pair, respectively, of an antibody listed in Table 2 is provided. For
example, in one
embodiment, an anti-ABCC1 antibody of the present disclosure competes for
binding to ABCC1
with the C1.309 antibody listed in Table 2, in certain embodiments, HCDRs 1-3
and LCDRs 1-3
are defined as per Kabat nomenclature.
In certain embodiments, the anti-ABCC1 antibody comprises the HCDR1, HCDR2,
and
HCDR3 of the VH region of the antibody listed in Table 2, in certain
embodiments, the HCDR1,
HCDR2, and HCDR3 are defined as per Kabat nomenclature, For example, in one
embodiment,
the anti-ABCC1 antibody of the present disclosure that competes for binding to
ABCC1 with the
C1.309 antibody listed in Table 2 comprises the HCDR1, HCDR2, and HCDR3 of the
VH region
of the C1.309 antibody.
Any suitable approach for determining whether a first antibody competes with a
second
antibody for binding to ABCC1 may be employed. Whether a first antibody
"competes with" a
second antibody for binding to an antigen may be readily determined using
competitive binding
assays known in the art. Competing antibodies may be identified, for example,
via an antibody
competition assay. For example, a sample of a first antibody can be bound to a
solid support.
Then, a sample of a second antibody suspected of being able to compete with
such first antibody
is added. One of the two antibodies is labelled. If the labeled antibody and
the unlabeled antibody
bind to separate and discrete sites on the antigen, the labeled antibody will
bind to the same level
whether or not the suspected competing antibody is present. However, if the
sites of interaction
are identical or overlapping, the unlabeled antibody will compete, and the
amount of labeled
antibody bound to the antigen will be lowered. if the unlabeled antibody is
present in excess, very
little, if any, labeled antibody will bind.
For purposes of the present disclosure, competing antibodies are those that
decrease the
binding of an antibody to the antigen by about 30% or more, about 40% or more,
about 50% or
more, about 60% or more, about 70% or more, about 80% or more, about 85% or
more, about
90% or more, about 95% or more, or about 99% or more. Details of procedures
for carrying out
such competition assays are well known in the art and can be found, for
example, in Harlow and
Lane, Antibodies, A Laboratory Manual, Cold Spring Harbor Laboratory Press,
Cold Spring
Harbor, New York, 1988, 567-569, 1988, ISBN 0-87969-314-2. Such assays can be
made
16
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
quantitative by using purified antibodies. A standard curve may be established
by titrating one
antibody against itself, i.e., the same antibody is used for both the label
and the competitor. The
capacity of an unlabeled competing antibody to inhibit the binding of the
labeled antibody to the
antigen may be titrated. The results may be plotted, and the concentrations
necessary to achieve
the desired degree of binding inhibition may be compared.
In certain embodiments, an antibody that specifically binds to ABCC1 comprises
(i)
HCDRs 1-3 and light chain CDRs (LCDRs 1-3) of a pair of variable heavy chain
(VH) region and
variable light chain (VL) region of an antibody listed in Table 2; (ii) HCDRs
1-3 of a VH region of
an antibody fisted in Table 2; (iii) LCDRs 1-3 of a VH region of an antibody
listed in Table 2; or
(iv) HCDRs 1-3 of a VH region of a first antibody listed in Table 2 and LCDRs
1-3 of a VL region
of second antibody listed in Table 2. The HCDRs and the LCDRs may be defined
based on the
Kabat nomenclature.
In certain embodiments, an antibody of the present disclosure that binds
specifically to
human ABCC1 comprises the HCDR1, HCDR2, and HCDR3 sequences and the LCDR1.
LCDR2,
and LCDR3 sequences of an antibody listed in Table 2. In addition to binding
to human ABCC1
one or more of the antibodies provided herein may bind to ABCC1 from other
mammalian species,
such as, mouse, monkey, chimpanzee, etc. The antibodies may be raised in mouse
or rat. In
Table 2, the animal in which the antibody was generated is indicated.
Table 2: From left to right, 1st column: Anti-ABCC1 antibody name, 2" column:
VH region,
3m column: HCDR1, 4 column: HCDR2, 5th column: HCDR3, 6th column: VL region,
7th column:
LCDR1, 8th column: LCDR2, 9th column: LCDR3.
17
Antibody T Heavy Chain Variable Region r----CoRHI CDRH2 CDRH3
UghtChan Variable Regiorr CDRLI CDRL2 ¨6DRL3
C1.309 QvQLKESGPGLVAPSOSISITCTV SYDIT (SEQ VIWIGGGINY DLRYGYDOF
DI/VMTOTPLSLPVSLGDOASISCRS RSSQSIVHS KVSNRES FQGSHVPRT
0
SGFSITSYDITWIROPPGKGLEW ID NO:37) NSAFMS (SEQ MEM/ (SEQ
SQSIVHSNGNP/LEWYLQKPGQSP NGNTYLE (SEQ ID (SEQ ID
LGVIWIGGGINYNSAFMSRLSIS ID NO:47) ID NO:64)
KWYKVSNRFSGVPDRFSGSGIGT (SEQ ID NO:124) NO:13S)
KDSSKSQVFLKMNSLRIDDTAIY
DFTLKISRVEAEDLGWYCFQGSHV N0:111)
YCVROLRYGYDGFINYFOVWGA
PRIFGGGTKLEIK (5E0 ID NO:76)
GTIVRiSS (SEQ ID N0:1)
C1.310 OVUM KQSGPG LVAPSCISISFIC SYDIT (SEQ VIWIGGGTNY
RYGYDGF DVVIVITQAPScLSASLGEWSLTCRA RASQ03YGS 0155I0$
LQYASYPYT
TVSGFSLTSYDITWIRQPPGKGLE ID N)37) NSAFMS (SEQ WYEDV (SEQ
SQDIYGSINWFQQKPDGIIKWYG LN (SEQ ID (SEQ ID (SEQ ID
WLGVEWIGGGTNYNSAFIVSRL ID NO:47) ID NO:64)
ISSIDSGVPK8FSGSRSGSDYSITIS NO:112) NO:125)
N0:136)
SISKDSSKSQVF LKmNst.RT DDT
SLESEDFADYYCLQYASSPYTFeGG
AlYYCVRDLRYGYDGFWYFDVW TKLEIK (HO
ID NO:77)
GIGTTVIVSS (SEQ ID Na2)
C1.499 EVELKESG PG LVQPSCITISLICTV SNGVS AISSGGNIYYN
HRGYYGYN DICWITQSPSLLSASVGDRVILSCKG KGSQNINN KINSLQT VQYNNWYT
Co
SGFSLTSNGVSWVRO.PPGKGLE (SEQ ID SAFKS (SEQ. WGYFDY
SQNINNYLAWYQQKLGGAPK WY VIA (SEQ ID (SEQ ID (SEQ
ID
WEAAISSGGNIYYNSAFKSRLSISR N0:38) ID NO:48) (SEQ ID
KTNSLQTGIPSRFSGSGSGTDYILTI NO:113) NO:126) N0:=137)
HTSKSQVIIKMNSLQTEOTAMY NO:65)
SSLHSEDLATYYCYQYNNWYTFGP
FCVRHRGYYGYNWGYFDYWGQ GTKLELK MCI
ID N0:78)
GAS VIVSS (SEQ ID NO:3)
C1.505 EVOLTESGPGLVQRSQTLSLICT SNGVS A1SSGGNIYYN ERGWNYPGI
DIVLMSPSLLSASVGDRVILSCKAG KAGQKINN NAN51QT QQYNSWYT
VSGFSLISNGVSWVRIPPGKGLE (SEQ ID SALKS (SEQ TNGYYFDY
QKINNYLAWYOQKLGEAPKWYP,I VIA (SEQ ID (SEQ ID
(SEQ ID
WIAAISSGGNIYYNSALKSR1SISR NO:38) ID NO:49) (SEQ ID
ANSLCITGIPSRFSGSGSGTDYTLTIS N0:114} NO:127) N0:138)
DTSKSQVFLKNINSLQTEDTAIYF N0:66)
SLOPEDVATYFCQQYNSWYTF-GA
CTRERGWNYPGITNGYYFDYW GTKLEIK (SEQ
ID N0:79)
oe
GOGASVIVSS (SEQ ID NO:4)
Al
C1,773 EIOLAQS6PELVKPGASVKMSCK GYFMN RINRYNGDTF SGWGYDVYS
DIOMMTPSSLSASIGDRVTISCRA RASOLDISNY YTSRLFIS QQGNTLPW
ASGYSFIGYFMNWVKOSFIGKSL (SEQ ID YNQKFKG F DY (SEQ ID
SQDISNYLNWYQCIKPDGTVKLLIYY LN (SEQ ID (SEQ ID T (SEQ ID
0
EWIGRINPYNGDIFYNQKFKGK N0:39) (SEQ ID NØ67)
TSRLHSGVPSRFSGSGSGTDYSITIS NO:115) NO:128) N0139) w
o
w
AILTVDKSSSTAHMELRSLTSED N0:$0)
INLEQEDIATYFCQQGNTLPWIFGG w
CB
un
SALYYCTRSGWGYDVYSFDYWG ETKLEIK (SEQ
ID N0:80)
OGTLVTVSS (SEQ ID N0:5)
o
1 C1,773a EVQLKESGPGLVQRSQTLSLTCT SNGVS
AISSGGSIYYN HRGYYGYN NIQLMSPSILSASVGDRVTLSCKGS KGSQNINN KTNSLO,T SQYNNGYT
1 VSGFSLTSNGVSVVVRQPPGK:GL (SEQ. ID SAFKS (SEQ WGYFDY
QNINNYLAWYQQKLGEAPKWYK YLA (SEQ, ID (SEQ ID (SEQ ID
1
1
1
1 EWIAALSSGGSIYYNSAFKSRLS IS N0:38) ID NO:51) (SEQ ID
INSLQTGIPSRFSGSGSGTDYILTIS N0;113) Na126) N0:140)
1
1
,
1
1 RHTSKSQVLIKMNSLQTEDTANI N.065)
SLHSEDLATYYCSQYNNUTFGAG
1
1
1
1 YFCARHRGYYGYNWGYFDYWG TKLEIK(SEQ ID
N0:81)
1
,
1
P
1
,
QGASVTVSS (SECI ID c10:6)
0 , L.
1
_______________________________________________________________________________
_______________________________
C1377a EVQLVESGPGLVQPSQTLSLTCT NNOVS AISSGGSIYYN = HRGYYGFN
NIQLTQSPSLLSASVGDRVTLSCKGS
KG$QN1NN KTNSLQT YQYNDGYT L.
u,
0
0
, VSGFSLINNGVSWVRQPPGKGL (SEQ ID LAFKS (SEQ WGYFDY
CZNINNYLAWYQQKLGEARKLLIYK ?LA (SEQ ID (HQ ID (SEQ ID
"
¨.a
0
n,
CD 1
L.
1
EWIAAISSGGSIYYNLAFKSRLSIS N0:40) ID NO:52) (SEQ ID
TNSLQTGIPSRFSGSGSGTDYTLTIS NO:113) t,10126) N0:141) 0
L.
1
0
1
1 RNISKGFIVIAKMNSLQTEDTAM NØ68)
SIHSEDLAINYCYQYNDG'{TFGAG
,
1
1
,
1 YFCVRHRGYYGFNWGYFDYWG TKLELK (SEQ. ID
NO:82)
1 ,
,
1
1
1 QGVIVIVIVSS (SEQ ID NO:7)
1
C1,778b EVQRKESGPGLVQRSOILSITCT NNGVS
' AISSGGSIYYN FIRG-CiYGY-
N¨NTQ¨LTQSPSLISASVGDRVTISCKGS KGSQNIDN KTN-SLTir¨Y7-Q-YNNGYT¨
VSGESLINNGVSWVRQPPGKGL (SEQ ID SAFKS (SEQ VVGYFDY
QMONYLAWYQQKLGEPPKWYKT Y LA (SEQ ID (HQ ID (SEQ ID
EWIAAISSGGSIYYNSAFKSRLSIS NO:40) 10 N0:51) (5E0 U)
NSLQTGIPSRFSGSGSGTDYTLTÃSS N0116) NO:126) N0:142) IV
n
,-i
RITSKSQVILKNINSLQTEDTAMY NO:65) LI-
ESEDUATYYCYQYNNGYTFGAGT
ci)
w
FCARFIRGYYGYNWGYFDYWG KLELKRADA
(SEC), ID NOM) o
w
1-,
QGVNIVIVSS (SEQ ID N0:8)
CB
.6.
................................ ,
...............................................................................
.... oe
--.1
o
1-,
C1.784a EVKLEESGPGLVQPSQRSILTCTV NNUS AIVISSI3GMYY HRGYYWYN
NICILMSPSLISASVGDRVIL5CKGS KGSQNINN KTNSLOT CO,YNNGYT
SGESITNNGVSWVRQPPGKGLE (SEQ ID NSA EKS (SEQ WGFEDY
QNINNYLAWYQM,GEAPKI_LIYK YLA (SEQ ID (SEQ ID (SEQ ID
0
WIAAMSSGGNIYYNSARSR LSI N0:40) ID NO:53) (HQ ID
TNSLQTGIPSRESGSGSGIDETLTIS NO:113) NO:126) N0:13.3) w
o
w
SRIDTSKSQVIII(MSSLCITEDTA NO:69)
SIHSEDLATYYCCQYNNGYTEGAG w
Ci5
un
MYFCG RH RGYYWYN WGF F DY 'MEV (SEQ 10
N0:84)
w
o
WGQGASVIVSS (SEC) ID NO:9)
o
/ C1.786a EVQLTESGLGLVQPSQILSacsv SYNVII V IWTGGYTDS ERHTIAGITK
DVQMTQSPASLSASLGETISIECLAS LASEGIESYL GANT LQA QQ.SY KEPVT
1 SGESITSYNVEIWVROPTGKGLE (SEQ ID NSPLKS (SEQ SWYPDY
EGIFSYLAWYQQKPGKSPQLLEYGA A (SEQ. ID (SEQ ID (SEQ ID
,
1
1
1 W MGVIWIGGYTOSNSPLKSRIS N0:41) ID NO.541) (SEQ ID
NTLQAGVPSRESGSGSGTOYSIAIS N0;117) NO:129) N0:144)
1 ,
1
1
,
1 EIRDISKSQVFLIKMNSLCISEDIAT Na70) SM QPEDEG
DYFCQQSYKEPV TUGS
,
1
1
1
1 YYCARERHTMGIIKSWYEDYW GIKIELIC (SEQ
ID N0:85)
, ,
1
P
1
,
1 GQGVMVIVSS WO. ID NO:10)
0
1
L.
1
_______________________________________________________________________________
________________________________________
0
Cl 387a EVQLTESGPGLVQPSQTLSLTCT SNGVS AISSGGSTYYN ' HRGYYWSN
NIQLIQSPSLISASVGDRvmsCKGS KG$QN1NN KTNSE.QT CQYNDGYT L.
u,
0
0
VSGESLISNGVSWVRQPPGKGL (SEQ ID SAEKS (SEQ WC-5'1E0Y
QNINNYLAWYQQKLGEARKWYK YU\ (SEQ ID (HQ ID (HQ ID
"
0
L.
EWIAAISSGGSTYY NSAFKSRISIS N0:38) ID NO:55 (SEQ ID
TNSEQTGIPSRFSGSGSGTDYTUELS NO:113) NO:126) NO:14S) ,
0
L.
,
1
0
1
1 RHTSKSQVIINMNSLQTEDTAM NO:71)
SIHSEDLAINYCCQYNDGYTEGAG
, ,
1
1
1 YFCARHRGYYWSNWGYFDYW 'MILK (SEQ. ID
NO:86)
1
,
1
1
1
1 GM-WW1-MS {SEQ ID NO:II)
,
1
,
C.779b EVKILESGPGIVQPSQTLSncry -5-NGVE iiÃ7.C.k AISSGGNIYYN1 ARGTTTAYF ETV
MTQSPSSIAVSAGETVTMNCK I(SSQSIT.CIS WA¨ST-RQS QQYY¨DTIWIK
SGFSITSNGVIWVROPPGKGLE ID N0:42) SGLKS (SEQ MDA (SEQ
SSQSLLYSE,,NQKNYLAWYCIO.KPG GNQI<NYLA (HQ ID M MO
ID
WIAAISSGGNIYYNSGLKSRLGIS ID NO:S61 ID N032)
0.SPKILIMASTRQSGVPDE(FIGS6 (SEQ ID NO:130) NO:146) IV
n
,-i
fiDTSKSCIVELKMNSIQTEDIAIY
SGIDETUTISSVQAEDLAIYYCQQrf N0:118)
ci)
w
ECTRARGMAYEMDAWGQGA DTI.MKMEGAGTE4
OK (SEQ ID o
w
1-,
SVIVSS (SEQ ID N01.2) N0:87)
Ci5
.6.
............................. .,
oe
--.1
o
1-,
C1.505 EVQLTESGPGIVQPSQTLSLICT SNGVS AISSGGNIYYN ERGWNYPGI
DIVLIQSPSLISASVGDRVILSCKAG KAGQKINN NANSLQT QQYNSWYT
VSGFSLTSNGVSWVR1PPGKGLE (SEQ D SALKS (SEQ TNGYYFDY
QKINNYLAWYQQKLGEAPKWYN YLA (SEQ ID (SEQ ID (SEQ ID
0
WIAAISSGGN(YYNSALKSRLS(SR NO:38) ID NO:49) (SEQ 0
ANSLQTGIPSRFSGS(SGTOYILTIS NO:114) NO:127) NO:138)
DISKSQVFLKMNSLQTEDTA(YE NO:66)
SLOPEDVATYFCQQYNSWYTFGA
8
CTRERGWNYPGITNGYYFDYW GTKLEIK (SEQ
ID NO:79)
t.J
GQGTSVTVSS (SEQ ID NO:13)
C1.827 EVQLVESGPGLVQPSCITISLICT NNGVS AISNGGNIYH HRGYYGYN
NIQVTQSPSLLSASVGDRVILSCKG KGSQNIYNY KTNSLQT CQYNNGYT
VSGFSLINNGVSWVRCIPPGKGL (SEQ ID NSALKS (SEQ WGYFDY
SQN1YNYLAWYQCtKLGEAPKWYK LA (SEQ ID (SEQ ID (SEQ ID
EWIAAISNGGNIYHNSALKSRLSI NO:40) ID NO:57) (SEQ ID
INSLQTGIPSRFSGSGSGTDYTITIS NO:119) NO:126) NO:143)
SRNTAKSQVILKIVINSLQTEDTA NO:65)
SLHSEDLATYYCCQYNNGYTFGAG
MYFCARHRGYYGYNWGYFDY TKLELK (SEQ
ID NO:88)
WGQGASVTVSS (SEQ ID
NO:14)
co
co
1,..) C1.8308 EVQLKESGPGIVRPSQ11.5LICSV NYNVH I IWIGGYT DY ERHTIVIGITK
DI LIVITQSPASISASIGETISIECLAS E LASEG I FSYL GA NSLQA QQSYKFPVT
=
SGFSLTNYNVHWVROPTGKGLE (SEQ ID NSDLKS (SEQ SWYFDY
GIFSYLGWYQQKPGKSPQWYGA G (SEQ ID (SEQ ID (SEQ ID
=
WMGI(IIVIGGYTDYNSOLKSRLSI NO:43) ID N0:58) (SEQ ID
NSLOAGVPSRFSGSGSGTQYSLKIS NO:120) NO:131) NO:144)
TRDTSKSQVFLKMNGLCITEDVA NO:70)
SMQPEDEGDYFCQQSYKFPVTFGS
TYYCARERHTMGITKSWYFDYW GTKLELK (SEQ
ID NO:89)
GQGASVTVSS (SEQ ID NO:15)
C1.831 EVQLTESGPGLVQPSQTLSLTCT SNGVS AISSGGNIYYN ERGWNYPFI
EIMITO.SPSLLSASVGDRVTLSCKA KAGQNINN NANSLQT QQYNSWYT
VSGFSLTSNGVSWVROPPGKGL (SEQ (0 SVLKS (SEQ ID INGYYFOY
GQNINNYLAWYQQKLGEAPKWY VIA (SEQ ID (SEQ ID (SEQ ID
A
EWIAAISSGGNIYYNSVLKSRLSIS NO:38) NO:59) (SEQ ID
NANSLQTG(PSRFSGSGSGTDYTLT1 NO:121) NO:127) N0:138)
r.>
RDTSKSQVFLKIVISSVQTEDTA1Y NO:73)
SSLQPEDVATYFCQQYNSWYTFGA
r.>
FCTRERGWNYPFITNGYYFDYW GTKLEIK (SEQ
ID NO:90)
00
GQGVMVTVSS (SEQ ID NO:16)
C1,835 EVQIIESGPGWQRSQIISLTC1 NNUVS AMSSUGNIYY HRGYYGYN
NIQLMSP5LISASVG0RVIL5CKGS KGSQNIYNY KTNSLO,T COYNNGYT
VSGFSLTNNGV$WVROPPGKG1_ (SEQ ID NSA FKS (SEQ WGYFDY
QMYNYLAWYQQKLGEAPKWYRT LA (SEQ ID (SEQ ID {SEQ ID
0
EWVAAEVISSGGNIYYNSAFKSRL N0:40) ID NO:53) (SEQ ID
NSLCITDIPSRFSGSGSGIDYTLTISS NO:119) NO:126) N0:143)
SISRHTSKSQVLIKMNSIQTEDT NO:65)
IHSEDIATYYC,CQYNNGYTFGAGT
AMYFCARHRGYYGYNWGYFDY MILK (SEQ ID
NO:91)
WGQ6VMVTV5S (SEQ ID
NO:17)
C-1,841 EVQLLESGPGWQRSOTISITCT NNGVS AMSSGGNITY HRGYYGYN
NICILIQSPSLLSASVGDSVSLRCKGS KGSQNIYNY KTNNLO3 CQYNNGYT
VSGFSLTNNGVSWVRO.PPUKUL (SEQ 10 NSAFKS (HO WGYFDY
QMYNYLAWYQQKLGEAPKWYKT LA (SEQ. I(3 (SEQ 0 (SEQ ID
EWVAAMSSGGNIYYNSAFKSRL NO:40) ID NO:53) (SEQ ID
NNIQTGIPSRFSGSGSGTDYILTISS NO:119) NO:132) N0;143)
SISRHTSKSQVLLKMNSLQTEDT NO:65)
INSEDLATYYCCQYNNGYIFGAGT
A MYFCAR H RGYYGYNWG YFD Y KIRK (HQ ID
NO:92)
WGQGVIVIVTVSS (SEQ ID
NO:17)
N.7
_______________________________________________________________________________
_______________________________
r") C.1.844 EVQLKESGPGLVQPSQIISLICT SNGVI (SEQ. AISSUGNIYYN ARG AYV
DIVNATO.SPSSLAVSAGETVNINCKS KSSQSLLYS WASTRQS QQYYOTLNIK
0
VSGFSITSNGVI,ANROPPGKGL ID NO:42) SULKS (SEQ MDA (SEQ
SQSLLYSVNQKNYIAWYOQKPGQ VNQKNYLA (SEQ ID M (SEQ
EWIAAISSGGNIYYNSGLKSRLGI ID NO:56) ID NO:74)
SPKILIYWASTRQSGVPDRFIGSGS (SEQ ID NO:130) NO:146)
SRDISKSQVFLIWINSIOJEDTAI
ETDFTITISSVOAEDLAIYYCQQYY N0:122)
Y F CT RARG TTTAYVM DAWC5QG DTLM KM
FUAGTKLELK (SEQ. ID
ASVIVS5 (SEQ ID NO:18) N0:93)
C1.845 EVQLVESGPGINCIPSOTLSLICT NNGVS AISSGGSIYYN HRGYYGYN
NIQLTO,SPSLLSASVGDRVILSCKGS KGSQNINN KINSLQT YQVNDOVT
VSGFSLTNNGVSWVROPPUKUL (SEQ ID WKS (SEQ WGYEDY NINNY
LAWYQQKLGEAPKWYK YLA (SEQ ID (SEQ ID (SEQ I0
EWIAAISSUGSIYYNLAFKSRLSIS N0:40) ID NO:S2) {SEQ ID
TNSLOTGIPSRFSGSGS(SDVILTIS NO:113) NO:126) NO:141)
RNTSKGQVLLKMNSLQTEDTA NO:65)
SLHSEDLAINYCYQYNDGYTEGAG
oe
MYFCARHRGYYGYNWGYFDY TKLELK (SEQ
ID N0:94)
WGQGASVTVSS (SEQ ID
NO:19)
0
C1.847 EVQLKESGPGLVQPSQTLSITCT NNGVS AISSGGNIYHN HRGYYGYN
NIQLTQSPSLLSASVGDRVTLSCKGS KGSQNIYNY KINSLCIT CQYNNGYT
VSGFSLINNGVSWVRCLPPGKGL (SEQ10 SALKS (SEQ WGYFDY
QNIYNYLAWYQQKLGEAPKWYKT LA (SEQ 10 (SEQ ID (SEQ ID
8
EWIAAISSGGNIYHNSALKSRLSI NO:40) ID NO:60) (SEQ ID
NSLQTGIPSRFSGSGSGMYTITISS NO:119) NO:126) NO:143)
t.J
SRNTAQSQVLL.KMNSLQTEDTA NO:65)
LFISEDLATYYCCQYNNGYTFGAGT
MYFCARFIRGYYGYNWGYFDY MILK (SEQ ID
N0:95)
WGQGTINTVSS (SEQ ID
NO:20)
C1.851 EVQLKESGPGLVQPSCITLSLICT NNGVS AISSGGNIYYN HRGYYGYN
NIQLTQSPSLISASVGDRVTLSCKGS KGSQNINN KTNSLQT COYNNGYT
VSGFSLINNGVSWIRCIPPGKGL (SEQ10 SAFKS (SEQ WGYFDY
QNINNYLAWYQQKLGEAPKWYK VIA (SEQ ID (SEQ ID (SEQ ID
EWIAAISSGGN1YYNSAFKSRLSIS N0:40) ID NO:4S) (SEQ ID
TNSLQTGIPSRFSGSGSGTDYTLTIS NO:113) NO:126) NO:143)
RNTSKRQVILKMNSLOTEDTAM NO:65)
SLHSEDLATYYCCQYNNGYTFGAG
YFCARHRGYYGYNWGYFDYWG TKLELKR (SEQ
ID NO:96)
=
(.4 0GASVIVSS (SEQ NO:21)
=
0
C1.855 EVQLKESGPGLVQPSCITLSLICT SNGVS AISNGGNIYYN HRGYYGYN
NICILTQSPSLLSASVGDRVTLSCKGS KGSQNINN KTNSLQT YQYNNGYT
VSGFSLISNGVSWVRCLPPGKGL (SEQ10 SAFK5 (5E0 WGYFDY
CLNINNYLGWYQQKLGEAPRILIYK YLG (SEQ (SEQ ID (SEQ ID
EWIAAISNGGNIYYNSAFKSRLS1 N0:38) ID NO:61) (SEQ ID
INSLQTGIPSRFSGSGSGTDYTITIS ID NO:123) NO:126) NO:142)
SRFITSKSQVILKIVINSIQTEDTA NO:65)
St.HSEDLATYYCYQYNNGYTFGAG
MYFCARHRGYYGYNWGYFDY TKLELK (SEQ
ID NO:97)
WGQGASVINSS (SKI ID
A
NO:22)
r.>
C1861 EVQLKESGPGLVQPSLITISLICT SNGVT AISSGGNIYYN ARGMAYV
OVVITQSPSSLAVSAGETVTINCKSS KSSCISLLYS WASTRQS QQYYDVLM
r.>
VSGESLTSNGVTWVROPPGKG1 (SEQ10 SAVKS (SEQ MDA (SEQ
OSLLYSGNQKNYLAWYCIQKPGQS GNQKNYLA (SEQ ID NM
(SECIID
00
EWIAAISSGGNIYYNSAVKSRLS1 N0:44) ID NO:62) ID NO:74)
PKWYWASTRQSGVPDRFIGSGSG (SEQ ID NO:130) NO:147)
SRDTSKSQVFLKMNSICITEDTAI
TDFTLTISSVQAEDLAIYYCQQYYDV NO:118)
YFCTRARGTTTAYVMDAWGQG
LIsANNIFGAGTKLEIK (SEQ ID
ASVTVSS (SEQ ID NO:23) NO:98)
0
C1.863 EVQRKESGPGLVQPSQTLSLTCT NNGVS AISSGGSIYYN HRGYYGYN
NIQNITQSPSILSASVGDRVILSCKG KGSCINIYNY KTSSLOA CQYNNGYT
VSGFSLINNGV5WVRQPPGKGL (SEOID SAFKS MCI WGYFDY
SLINIYNYLAWYCKKLGEAPKWYK LA (SEQ ID (SEQ ID (SW ID
8
EWIAAISSGGSIYYNSAFICSRLSIS NO:40) ID NO:511 (SEQ ID
TS5LQAGIPSRFSGSGSDTDYTITIS NO:119) NO:133) NO:143)
t.J
RITSKSQVILKIVINSIQTEDTAMY NO:65)
SLHSEDLATYYCCQYNNGYTFGAG
FCARHRGYYGYNWGYFDYWG TKLEIK (SEQ ID
NO:99)
QGvivivrvss (SEQ ID NO:8)
C1.876 QVQLKESGPGLVQPSQTLSLTCT NNGVS AMSSGGSIYY HRGYYGYN
DIQLTQSPSLLSASVGDSVSLRCKGS KGSQNIYNY KTNNICIT CQYNNGYT
VSGFSLINNGVSWVREIPPGKG1 (SEQ ID NSAFKS (SEQ WGYFDY
QNWNYLAWYQQKLGEAPKWYKT LA (SEC( ID (SEQ ID (SEQ ID
EWIAANISSGGSIYYNSAFICSRLSI NO:40) ID NO:63) (SEQ ID
NNIQTGIPSRFSGSGSGTDYTLTISS NO:119) NO:132) N0:143)
SRHTSKSQVILQIVINSLQTEDTA NO:65)
LNSEDLATYYCCQYNNGYTFGAGT
MYFCTRHRGYYGYNWGYFDYW KVELK (SEQ ID
NO:100)
co
co
GQGVNIVIVSS (SEQ ID NO:24)
=
C1.877 EVQLTESGPGLVQPSQTLSLICT SNGVS A1SSGGSTYYN HRGYYWSN
DIVMTQSPSLLSASVGDRVTLSCKG KGSQNINN EINSLQT YQYNDGYT
=
VSGFSLTSNGVSWVRQPPGKGL (SEQ ID SAFKS (SEQ WGYFDY
SQNINNYLAWYQQKLGEAPKWYE VIA (SEQ ID (SEQ ID (SEQ ID
EWIAAISSGGSTYYNSAFKSRLSIS NO:38) ID NO:55) (SEQ ID
TNSLQTGIPSRFSGSGSGTDYTITLS NO:113) NO:134) NO:141)
RHTSKSQVL1NMNSLQTEDTAM NO:71)
SLHSEDLATYYCYQYNDGYTFGAG
YFCARFIRGYYWSNWGYFDYW TKLEIK (SEQ ID
NO:101)
GQGVNIVIVSS (SEQ ID NO:11)
C1.879A EVQLTESGPGLVQPSCITISLICT SNGVS A1SSGGSTYYN HRGYYWSN
DIQLTQSPSLISASVGDRVILSCKGS KGSQNINN KTNSLQT YQYNNGYT
(-5
VSGFSLISNGVSWVRQPPGKGL (SEQ ID SAFKS (SEQ WGYFDY
QNINNYLAWYQQKVGEAPKWYK VIA (SEQ ID (SEQ $0 (SEQ ID
t=.>
EWIAAISSGGSTYYNSAFKSRLSIS NO:38) ID NO:55) (SEQ ID
INSLQTGIPSRFSGSGSGTDYTITIS NO:113) NO:126) NO;142)
t=.>
RHTSKSQVILNIVINSLQTEDTANI NO:71) SLH SE
DLATYYCYQYNNGYTFGAG
00
YFCARHRGYYWSNWGYFDYW TKLELKRADAAPTV
(SEQ ID
GQGVIVIVTVSS (SEQ ID NO:11) NO:102)
C1,8308.hul EVQ,LOESGPGINKRSQTISLTCA NYNVH liWTGGYTDY ERHTIVIGITK MOM
TQSPSSLSASVGDRVT ITCLA LASEG1F5YL GA NSW/A OQSY KENT
i VYGFSLTNYWHWVRQPPGKGL (SEQ )0 NSDLKS (SEQ SWYFDY
SEGEESYLGWYQQKPGKSPKLLIYG G (SEQ ID (2) MO iD
0
Humanized EWMGIIWIGGYTDYNSDLKSRL N0:43) 10 NO:58) (SEQ ID
ANSLQAGVPSRFSGSGSGMYTIT I NO:120) N0:144) w
o
w
TISRDISKNOVSIKL.SSVTAADTA NO:70)
SSIOPEDFATYY010.SYKE PVTFGO w
.1
un
vYYCARERHTIAGITKSWYFOYW MUD< (SEQ ID
NO:103)
w
GEIGTLVIVSS (SEQ ID NO:26)
o
C1,851.hul1 EVQLOESGPGINKPSETLSLICT,,, NNGVS AISSGGNIYYN HRGYYGYN
DIQLTQSPSSISAWGDRVTFICKGS KGSQNINN KINSLQT CQYNNGYT
Humanized SGESLTNNGVSWIROPPGKGLE (SEQ JO SAM (SEQ VVGYFDY
QNINNYLAWYQQKPGKAPKL LIYK 'a-Ps (SEQ ID (SEQ ID (SEQ iD
WIAAISSGGNIYYNSAFXSRLTIS NO:40) 10 NO:48) (SEQ ID
TNSLQT6IESRFSGSGS(51-DYTITIS NO:113) NO:126) N0:143)
80TSKNOVSLKESSV1AAD1AVY NO:65) SLOPE
DEATYYCCOY NNGYTP GQG
P
YCARHRGYYGYNWGYFDYWG MEW (SEQ ID
NO:1.04) 0
L.
,
QGTINTVSS MO, ID NO 2i'
L.
u,
0
0
NI C1,851,hul2 EVQLQESGPOLVKPSETLSITCTV NNGV5 ALS5GGNIYYN
HRGYYGYN DiaLTQSPSSISASVGDRVTITCKGS ¨
KGSQNINN KTNSLQT YQYNNGYT "
0
01
L.
Humanized SGFRTNNGV.SW)P.C1PPGKGLE (SEQ 10 SAFKS (SEQ WGYFDY
QNINNYLAWYQQKPGKAPKWYK YLA (SEQ ID (SEQ. ID MCI
it) ,
0
L.
,
0
WEAAISSGGNIYYN5AFI<S8LTIS NO:40) 10 NO:48) (SEQ ID
TNSLQTGIPSRFSGSGSGWYTITIS NO:113) NO:126) N0:142) ,
80TSKNOVSLI(LSSVTAADIAVY NO:65) SLOPE DE
ATYYCYOYN NGYTFGQG
YCARHRGYYGYNWGYEDYWG TKLEIK (SEQ
ID NO:105)
QGTINTVSS (HQ ID NO:27)
C1,851,hu13 EVQLOESGPGLVKPSEILSLICTV NNGVS AISSGGNIYYN HRGYYGYN
DiQLTQSPSSLSASVGDRVTITCKGS KGSONMIN KTNSLQT SQYNNGYT
Humanized SGESLTNNGVSWIRQPPGKGLE (SEQ )0 SAFKS (SEQ WGYFDY
QNINNYLAWYQQK PGKAPKL LI YK YLA (SEQ ID (SEQ ID
(SEQ 1D IV
n
,-i
WIAAISSGGNIYYNSAF(SRLTIS NO:40) ID NO:48) (5E0, fD
TNSLOTGIPSRESGSGS(MYTLT15 NO:113) NO:126) N01.40)
cp
w
8DTSKN QVSIK ISM/ TAA DTAVY NO:65)
SLQPEDFATYYCSQYNNGYTFGQCi o
w
1¨,
YCARFIRGYYGYNWGYFENWG TKLEIK (SEQ
ID NO:106) .1
.6.
oe
OGILVTVSS. (SEQ a NO:2 7)
--.1
o
1¨,
,
_______________________________________________________________________________
_____
C1.787ashul EVQLQE5GPGINKRSETLSLICTV SNGVS AISSGGSTYYN HRGYYWSN
DIQLTOSP5SISA5VG0RVTITCKG5 KG5QNINN KTNSLOT COYNOGYT
1 5GFS1TSNGVSWVROPPGI<GLE (SEQ ID SAFKS (SEQ WGYFDY
QNINNYLAWYQQRPGKAPKWYK YLA (SEQ ID (SEQ ID (SEQ
ID
0
Humanized WIAAISSGGSTYYNSAFKSRLTIS NO:38) ID NO:55) (SEQ ID
TNSLQTGIPSRFSGSGSGTDYILTIS NO:113) NO:126) NO135)
w
o
w
ROTSKNQVSLKISSVTAADTAVY NO:71)
SLQPEDFATYYCCQYNDGYTFGQG w
-1
un
YCARHRGYYWSNWGYFDYWG TKLEIK (Sal
ID NO:107)
w
OGTLVTVSS (SEQ ID NO:28)
o
1 C1,831.hull EVQLQESGPOLVITSETLSLTOTV SNGVS AISSGGNIYYN ERGWNYPF1
DIQLTQSPSSISASVGDRVTITCKAG KAGQNINN NANSLQT QQYN5WYT
I Humanized SGFSLTSNGVSWVRQPPGI<GLE (SEQ ID SVLKS (SEQ
#0 INGYYFOY QNINNYLAWYQQI<PGKAPKILIYN YLA (SEQ ID (SEQ ID
(SEQ ID
, WIAAISSGGNIYYNSVLKSRLTISR NO3) NO:59) (SEW()
ANSLQTGIPSRFSGSGSGTDYTITIS NO:121) NO:127) NO:138)
1
,
,
,
,
,
1
, OTSKNEWSLKL5SVIAADTAVYY NO:73)
SLQPEDFATYYCQQYNSWYTEGQ
1
,
,
,
,
, cr R E R GW N 'JP HT N G Y Y F 0 Y W G GTKLEIK
(5E010 P40:108)
,
1
,
P
,
,
,
,
1 QGTLV1VSS (SEQ ID NO;29)
0 , L.
1
_______________________________________________________________________________
_________________________________________ ,
w
C1.831..hu41 EVQLQESGPOLVICPSETLSITCTV SQGVS AISSGGNIYYN ' ERGWNYPFI
DIQLMSPSSISASVGDRVIITCKAG KAGO,NINN NANSLQT QQYNSWYT L.
u,
03
03
po I Humanized SGESLISQGVSWVRQPPGRGLE (SEQ I0
SVIKS (SEQ 10 TSGYVEDY CZNINNYLAWYQQKPGKAPKLIAN
'YLA, (SEQ ID (HO ID (SEQ ID ^,
C) 1
0,
L.
WIAAISSGGNIVYNSVLKSRLTISR NO:45) NO:59) (SEQ. ID
ANSLQTGIPSRFSGSGSGTDYILTIS NO:121) NO:127) NO:138) ,
0
L.
,
,
0
,
1 OTSKNEWSLKLSSVTAADIAVYY NO:75)
SLOPEDFATYYCQQYNSWYTFGQ ,
,
1
,
,
,
,
,
CTRERGWNYPFETSGYYFDYWG GIKLEIK (SEQ
10 NO:108)
1
1
,
,
,
,
,
1 QGTLVIVSS (SEQ ID NO;30)
,
C1.844.11u1.1 EVQLQESGPOLVKPSETLSITCTV --S-NOVI iiÃ7.C.k AISSGGNIYYNI
ARGITTAYV DIVIVITOSPDSLiiVSLGERATINCICS¨ I(SSQS171)7S WA¨ST-RQS OQYY--DT-LIVIK
Humanized SGFSLTSNOVIWVROPPGI<GLE ID NO:42) SGLKS (SEQ MDA (SEQ
SQ5LLYSVNQKNYLAWYQQKPGQ VNQKNYLA (HQ ID M (SEQ.
ID
WEAAISSGGNIVYNSGLKSRLTIS 10 NO:S6) ID NO:74)
SPKLISYWASTRQSGVPDRFSGSGS (HQ ID NO:130) NO:146)
IV
n
,-i
RDTSKNQVSLKLSSVIAADTAVY
GTDETLTISSLOAEDVAVYYCQQYY NO:122)
cp
w
YCTRARGITTAYVMDAWGQGT
DTINIKMFGAGUL ELK (SEQ ID o
w
1¨
LVTVSS (SEQ10 NO:31) NO:109)
-1
.6.
................................ .,
oe
--.1
o
1¨
C1.844 .hu21 EVQIN ESGG6 LI QP GGSL RLSCA SN G VI (SEQ AISSGGN IY Y N AR
GTTTA.YV DIV MTQSPDSLAVSLGERATI NCKS K5SQ5LLY 5 WASTRQS QQY YOT WU(
H umanized VSGFSLTSNGVI W VRQP PG K GL I D NO42) SG LK S (SEQ
M DA (SEC( SQSLI.YSVNQKN YtAWY QQKPGQ V NQK
NYLA (SEQ ID M MO E D
0
EWIAAISSGGNIYYNSGLKSRLTIS ID NO:56) ID NO:74)
SPKWYWASTRQSGVPDRFSGSGS ( SEQ ED NO:130) NO 136)
w
o
w
R. DTSKNTVY LWOW RA E DTAV GIN-MI SSLQA
EDVAVYYCQQYY N 0 122) w
-1
un
YYCTRARGTTTAYVMDAWGQG DTLM KM
FGAGTKL E LK (SEQ E D
w
TLVTVSS (SEQ. ID NO32) NO:109)
o
1 C1 .861 . hul 1 EVQLQE SG P GM PSE TLSL TCTV SN G VT A ISSGGN IYY N
ARGTTTAXV DVQLTQS PSSLSASVG DRV TI TCKS5 K5SQ5LLYS WASTRQS QQYYDVL M
1 H umani zed SGFSLTSNG vTWVROP PG KGLI-..: (5E0 ID SAVKS (SEQ
M DA (SEQ. Q.SLLYSGNQI<NYLAWYCIQKPGKS G MIKA
YLA ( SEQ ID NM (SEQ. I D
, W I AAI SSGG NEYYNSAVKSRLTES NO:44) ID NO(Q) 1 D NO 74)
PKW YWASTRQSGV PSRFSGSGSG (SEQ I D NO:130) N0.:147)
1
,
,
,
,
,
1
, RDTSKN QVSIKLSSNITAADTAVY T DUO-
IS:SLOP EDVATYYCQQYYD NO:118 )
1
,
,
,
,
1 YCTRARGITTAYVMDAWGQGT VLM NM
FGGGTK LE IK (SEQ, ID
,
1
,
P
,
,
,
,
1 TVTVSS (,SEQ 10 NO:33) NO:110)
0 , L.
1
_______________________________________________________________________________
__________________________________________ ,
w
C1.861.11021 EVQI.V ESGGGLI QP GG SLR LSCA SNGVI
A ISSG G N IYYN ' ARGTTTAYV
DVOLIOSPSSLSASVGDRVIETCKSS KSSQS1.1YS WASTRQS QQYY DV LM L.
u,
0
0
/No I H umani zed VSGFSLISNGVTVVVRQP PGKG I (SEQ ID
SAVKS (SEQ M DA MO. Q51, LYSE
NQKNYLAWYQQKPGKS GNQKNYLA ( SEQ ID NM (SEQ I 0 "
0
L.
EW IA AESSGGN IYYNSAVKSR LTI NO:44) ID NO:62) ID N(174)
PK ILI YWASTRQSGV PSRFSGSGSG (SEQ I 0 NO:130)
NO:147) ,
0
L.
,
,
0
,
1
, SRDT5KNTVYLON MLR AE DTA T INT
LTISSLQP E DVATYYCQQYYD NO:118 ) ,
1
,
,
,
,
1
, VYFCTRARG i I I
AYVIVIDAWGQ VLM NM FGGGTK LE If< ( SEQ. ED
1
,
,
,
,
,
1 (iITVIVSS (SEQ ID NO:34) NO:110)
,
Ct861.hu41 FiVaLQ ESG P GINK PS EIL SITC:TV -T.3SGVT (SEQ AISSGGN IYY NI
ARGTTTAYV DVOITOSPS¨SE:SASVGDRVIETC¨KS-S KSSIDSIT.CIS WASTRQS OQYYDVIAT¨
I1 urn a n i zed SGFSITSSGVFWVROPPGKGLE ID NO:46) SAM (SEQ MDA (SEQ.
OSILYSGNCIKNYLAWYQQKPGKS GNQKNYLA MO ID NM (SEQ
ID
W EAAISSGG NEYYNSAVKSRLTIS ID NO:62) ID N 0 74 )
PKILEYWASTRQSGVPS RFSGSGSG (HO; ID NO:130) NO:147)
IV
n
,-i
RDTSKN QVSLKISSVTAADTAVY TDFT
LTISSLQP EDVATYYCQQYYD N0:118 ):
cp
w
YCTRARGITTAYVMDAWGQGT VLM NM
FGGGTK LE IK (SEQ ED o
w
1¨,
TVTVSS (SEQ ID NO:35) NO:110)
-1
.6.
................................ .,
oe
--.1
o
1¨,
C1.861.hu61 EVQINESGGGLIQPGGSLRLSCA SSC5VT (SEQ AISSGGNIYYN AR GITTAYV
DVQLTO5P55LSASVGDRVT(TCKS5 K55(51135 WASTROS OQYYDVLNI
Humanized VSGFSLTS5GVIWVRQPPGI<G1 ID NO:46) SAVKS (SEQ.
MDA (SEQ. Q.SLLYSGNOKNYLAWYOQKPGKS GNOKNYLA (SEQ ID
NM (SEQ ID
0
EWIAAISSGGNIYYNSAVKSR LT( ID NO:62) ID NO:74)
PKWYWASTROSGVPSRFSGSGSG (SEQ ID NO:130) N0:137)
DISKNTVYLOMNSIRAEDTA
TDFTLTISSLOPEDVATYYMYYD NO:118)
VYFCTRARGTITAYVMDAWGQ
VLMNIVIEGGGTKLEIK (SEQ ID
Gn ________ VIVS5 (5EO, ID NO:36) NO:110)
C1.851.hul4 EVQLQESGPOLVITSETISLTCTV NNGV5 AISSGGNIYYN
HRGYYGYN .. DVVMMTP LSLPVSLGDOKSISCRS KGSONINN KTNSI-03 YQYNO.GYT
Humanized SGFSITNNGVSWIROPPGKGLE (SEQ ID SAFKS (SEQ WGYFDY
SQSIVHSNGNTYLEWYLO,KKOSP YLA (SEQ ID (SEQ (0
(SEQ ID
WIAAISSGGNIYYNSAFKSRLT(S N0:40) ID NO:48) (SR/ ID
KWYKVSNRFSGVPDRFSGSGTGT N0:113) NO:126) N0:148)
RDTSKNQVSIKLSSVTAADTAVY NO:65) DFT LKISRV
EAEDL(3ANYCFC(GSH V
YCARHRGYYGYNWGYFDYWG PRTEGGGTKLEIK
(SEQ, ID NO:76)
QGTLVIVSS OM ID NO27)
C1.851.huiS EVQLQESGPGINICPSETLSITCTV NNGVS AISSGGNIYYN HRGYYGYN
DWMTQAPSSLSASIGERISITCRA KGSQNINN KTNSL.QT YQYNSGYT
Humanized SGESITNNGVSWIROPPGKGLE MO ID SAES (SEQ WGYFDY
SQD1Y6SINWFQQKP0GTIKAING ?LA (SEQ ID (SEQ
(SEQ ID
WIAAISSGGNIYYNSAFKSRLT(S N0:40) ID NO:48 (SEQ. ID
TSSLDSGVPKRFSGSRSGSDYSLTIS NO:113) NO:126) NO:149) 0
RDTSKNQV$LKLSSVTAADTAVY NO:65)
SLESEDFADYYCLQYASSPYTFGGG
YCARIARGYYGYNINGYFDYWG TKLEIK (SEQ ID
NO:77)
QGTINIVSS (SEQ ID NO:27)
oe
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
The anti-ABCC1 antibodies listed in Table 2 are also referred to as anti-KPC1
antibodies
or anti-C1 antibodies and can be referred to by the antibody number listed in
Table 2.
In some embodiments, the antibody comprises a VL region and a VH region that
are
present in separate potypeptide.s; in other embodiments, the VL region and a
VH region are
contained within a single polypeptide.
The antibody of the present disclosure may be selected from the group
consisting of an Ig
monomer, a Fab fragment. a F(ab)2 fragment, a Ed fragment a .scFv., a scAb, a
dAb, and a Fv.
In some embodiments, a subject antibody is a recombinant or modified antibody,
a
chimeric, humanized, deimmunized or an in vitro generated antibody. The term
"recombinant" Of
"modified" antibody as used herein is intended to include all antibodies that
are prepared,
expressed, created, or isolated by recombinant means, such as (i). antibodies
expressed using a
recombinant expression vector transfected into a host cell; (ii) antibodies
isolated from a
recombinant, combinatorial antibody library; (iii) antibodies isolated from an
animal (e.g, a mouse)
that is transgenic for human immunoglobulin genes; or (iv) antibodies
prepared, expressed,
created, or isolated by any other means that involves splicing of human
immunoglobulin gene
sequences to other DNA sequences, Such recombinant antibodies include
humanized, CDR
grafted, chimeric, deimmunized, and in vitro generated antibodies; and can
optionally include
constant regions derived from human germline immunoglobulin sequences.
As noted above, the subject anti-ABCC1 antibody specifically binds one or more
epitopes
of .ABCC1.. Thus, the epitope is an ABCC1 epitope. The size of a ABCC1 epitope
bound by anti-
ABCC1 antibody may vary, including where the ABCC1 epitope is formed by a
polypeptide having
a contiguous stretch of an ABCC1 sequence that may range from 3 aa or less to
12 aa or more,
including but not limited to .e.g.õ 4 aa, 5 aa, 6 aa, 7 aa, 8 aa, 9 aa, 10 aa,
11 aa, 12 aa, 4 aa to 10
aa, 5 aa to 10 ea, 6 aa to 10 aa, 4 aa to 8 ea, 5 aa to 8 aa, 6 .aa to 8 aa,
etc.
In some embodiments., the ABCC1 epitope can be formed by a polypeptide having
at least
about 75%, at least about 80%, at least about 85%, at least about 90%, at
least about 95%, at
least about 98%, at least about 99%, or 100%, amino acid sequence identity to
a contiguous
stretch of a ABCC1 sequence, including but not limited to e.g., the human
ABCC1 sequence:
NIALRGFCSADGSDPLWDWNVTWNTSNPDFTKCFQNTVLVWVPCFYLWACFPFYFLYLSRHD
RGYIQIVITPLNKTK.TALGFLLWIVCWADLFYSFWERSRGiFLAPVFLVSPTLLGITMLLATFLIQLE
RRKGVQS.SG.IMLTFWLVALVC.A.LAILR.SKIMTALKEDAQVDLFRDITFYVYF.SLLLIQLVLSCFSD
RSPLFSETIHDPNPCPESSASELSRITFWWITGLIVRGYRQPLEGSDLW.S.LNKEDTSEQVVPVL
VKNWKKE.CAKTRKQPVKVVYSSKDPAQPKESSKVDANEEVEALIVKSPQKEWNPSLFKVLYKT
FGPYFLNISFFFKAIHDLMMFSGPQI,LKLLIKFVN.DTKAPDWQGYFYIVLIFVTACLQTLVLHQYF
29
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
HmsGmRIKTAAGAvYRKALVITNSARKSSTVGEIVNLMSVDAQRFINADLATYINMIWSAPLQ
VI LALYLLWLNLGPSVLAGVAVNIVL MVPVNAVMAMKTKTYQVAHMKSKDN RI KLMWEILNGI KV
LKLYAWELAFKDKVLAIRQEELKVLKKSAYLSAVGTFTWVCTPFLVALCTFAVYVTIDENNILDA
QTAFVSLALFNILRFPLNILPMVISSIVQASVSLKRLRIFLSHEELEPDSIERRPVKOGGGTNSITV
RNATFTWARSDPPTLNGITFSIPEGALVAVVGQVGCGKSSLLSALLAEMDKVEGFIVAIKGSVAY
VPQQAWIQNDSLRENILFGCOLEEPYYRSVIQACALLPDLEILPSGDRTEIGEKGVNLSGGOKQ
RVSLARAVYSNADIYLFDDPLSAVDAHVGKHIFENVIGPKGMLKNKTRILVTHSMSYLPQVDVIIV
MSGGKISEMGMELLARDGAFAEFLRTYASTEQEQDAEENGVTGVSGPGKEAKQMENGIAL
VTDSAGKQLQRQLSSSSSYSODISRHHNSTAELQKAEAKKEETWKLMEADKAQTGQVKLSW
WDYMKAIGLFISFLSIFLFMCNHVSALASNYWLSLWTDDPIVNGTQEHTKVRLSVYGALGISQGI
AVFGYSMAVSIGGILASRCLHVDLLIASILRSPMSFFERTPSGNLVNRFSKELDTVDSMIPEVIKM
FIVIGSLFNVIGACIVILLATPIAAIIIPPLGLIYFFVQRFYVASSRQLKRLESVSRSPVYSHFNETLLG
VSVIRAFEEQERFINQSDLKVDENOKAYYPSIVANRWLAVRLECVGNGIVLFAALFAVISRHSLS
AGLVGLSVSYSLQVITYLNWLVRMSSEMETNIVAVERLKEYSETEKEAPWQIQETAPPSSWPQ
VGRVEFRNYCLRYREDLDFVLRHINVTINGGEKVGIVGRTGAGKSSLTLGLFRINESAEGEIIIDG
INIAKIGLHDLRFKITIIPQDPVLFSGSLRNINLDPFSONSDEEWITSLELAHLKOFVSALPDKLDH
ECAEGGENLSVGQRQLVOLARALLRKTKILVLDEATAAVDLETDDLIQSTIRTQFEDCTVLTIAHR
LNTIMDYTRVIVLDKGEIQEYGAPSDLLQQRGLFYSMAKDAGLV (SEQ ID NO:150). or an
extracellular region thereof, e.g., Loop 1 (amino
acids 1 ¨ 33):
MALRGFCSADGSDPLWDWNVIWNTSNPDFTKCF (SEQ ID NO:151); Loop 2 (amino acids 96-
100)1 RSRGI (SEQ ID NO1152); Loop 3 (amino acids 155-172)1
SKIIVITALKEDAQVDLFRD (SEQ
ID NO:153); Loop 4 (amino acids 338-363): MNIFSGPOILKLLIKFVNDTKAPDWQG (SEQ ID
NO;154); Loop 5 (amino acids 462-464): LGP; Loop 6 (amino acids 569-690):
VTIDENNILDAQTAFVSLALFN (SEQ ID NO:155); Loop 7 (amino acids 989-1025):
ALASNYWLSLWIDDPIVNGTOEHTKVRLSVYGALGIS (SEQ ID NO:156); Loop 8 (amino acid
1111): A; Loop 9 (amino acids 1225-1226): LS; or a fragment of human ABCC1,
e.g., a fragment
comprising amino acid residues
204-1531:
MDPNPCPESSASFLSRITFWWITGLIVRGYRQPLEGSDLWSLNKEDTSEOVVPVLVKNWKKEC
AKTRKQPVKWYSSKDPAQPKESSKVDANEEVEALIVKSPQKEWNPSLFKVLYKTFGPYFLMS
FFF KAIHDLNIMF SGPQILKLL I KFVNDTKARDWQGY FYIVLLFVTACLQTLVLHQYFHICR/SGM
RIKTAVIGAVYRKALViTNSARKSSTVGEIVNLMSVDAQRFMDLATYINMIWSAPLQVILALYLUN
LNLGPSVLAGVAVMVLIVIVPVNAVMAMKTKTYQVAHMKSKDNRIKLMNDLNGIKVLKLYAWEL
AFKDKVLAIRQEELKVLKKSAYLSAVGTFTWVCTPFLVALCTFAVWFIDENNILDAQTAFVSLAL
FNILRFPLNILPMVISSIVQASVSLKRLRIFLSHEELEPDSIERRPVKDGGGINSITVRNATFTWAR
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
SDPPTLNGITFSIPEGALVAVVGQVGCGKSSLLSALLAEMDKVEGHVAIKGSVAYVPQQAWIQN
DSLRENILFGCQLEEPYYRSVIQACALLPDLEILPSGDRTEIGEKGVNLSGGQKQRVSLARAVYS
NADiYLFDDPLSAVDAHVGKHIFENVIGPKGMLKNKTRILVTHSMSYLPQVDVIIVMSGGKISEM
GSYQELLAROGAFAEFLRTYASTEQEQDAEENGVTGVSGPGKEAKOMENGMLVTDSAGKQL
QRQLSSSSSYSGDISRHI-NSTAELQKAEAKKEETWKLMEADKAQTGQVKLSVYWDYMKAIGL
,FISFLSIFLFMCNHVSALASNYWLSLVVTDDPIVNGTQEHTKVRLSVYGALGISQGIAVFGYSMAV
SIGGILASRCLHVDLLHSILRSPMSFFERTPSGNLVNRFSKELDTVDSMIPEVIKMFMGSLFNVIG
ACIVILLATPIAAIIIPPLGLIYFFVQRFYVASSRQLKRLESVSRSPVYSHFNETLLGVSVIRAFEEQ
ERFIHQSDLKVDENQKAYYPSIVANRWLAVRLECVGNCIVLFAALFAVISRHSLSAGLVGLSVSY
SLQVTTYLNWLVRMSSEMETNIVAVERLKEYSETEKEAPWQIQETAPPSSWPQVGRVEFRNY
CLRYREDLDFVLRHINVTINGGEKVGIVGRTGAGKSSLTLGLFRINESAEGEIIIDGINIAKIGLHDL
RFKMIPQDPVLFSGSLRMNLDPFSQYSDEEVWTSLELAHLKDFVSALPDKLDHECAEGGENL
SVGQRQLVCLARALLRKTKILVLDEATAAVDLETDDLIQSTIRTQFEDCTVLTIAHRLNTIMDYTR
VIVLDKGEIQEYGAPSDLLQQRGLFYSMAKDAGLV (SEQ ID NO:157): or a cynomolgus monkey
ABCCI
sequence.:
MALRGFCSADGSDPLWDWNVTWYTSNPDFTKCFQNTVLVWVPCFYLWACFPFYFLYLSRHD
RGYIQMTLLNKTKTALGFLLWWCWADLFYSFWERSRGIFLAPVFLVSPTLLGITMLLATFLIQLE
RRKGVQSSGIMLTFWLVALLCALAILRSKIMTALKEDVQVDLFRDMTFYVYFSLVLIQLVLSCFS
DRSPLFSETIHDPNPCPESSASFLSRITFWWITGLIVRGYRQPLEGSDLWSLNKEDTSEQVVPV
LVKNWKKECAKTRKQPVKVVYSSKDPAQPKDSSKVDANEEVEALIVKSPQKEWNPSLFKVLYK
TFGPYFLMSFFFKAIHDLMMFSGPEfl_KLLINFVNDTKAPDWQGYFYTALLFVAACLQTLVLHQY
FHICFVSGMRIKTAVIGAVYRKALVITNAARKSSTVGEIVNLMSVDAQRFMDLATYINMIWSAPL
QVILALYLLWRNLGPPILAGVAVMVLMVPVNAVMAMKTKTYQVAHMKSKDNRIKLMNEILNGIK
VLKLYAWELAFKDKVLAIRQEELKVLKKSAYLAAVGTFTWVCTPFLVALCTFAVYVTIDKNNVLD
AQKAFVSLALFNILRFPLNILPMVISSIVQASVSLKRLRIFLSHEELEPDSIERRPVKDGGDTNSIT
VRNATFTWARSDPPTLNGITFSIPEGALVAVVGQVGCGKSSLLSALLAEMDKVEGHVALKGSV
AYVPQQAWIQNDSLQENILFGCQLEEPYYRSVIQACALLPDLEILPSGDRTEIGEKGVNLSGGO
KQRVSLARAVYCNADIYLFDDPLSAVDAHVGKHIFENVIGPKGMLKNKTRILVTHSMSYLPQVD
VIIVMSGGKISEMGSYQELLARDGAFAEFLRTYASAEQEQDPEDNGVTGVSGPGKEAKQMEN
GMLVTDSA.GKQLQRQLSSSSSYSGDVSRQHNSTAELOK.DGAKKEETWKLMEADKAQTGQVK
LSVYWDYMKAIGLFISFLSIFLFICNHVAAL,ASNYWLSLWTDDPIVNGTQEHTKVRLSVYGALGIS
QGIAVFGYSMAVSIGGILASRCLHVDLLHSILRSPMSFFERTPSGNLVNRFSKELDTVDSMIPEVI
KMFMGSLFNVIGACIVILLATPIAAIIIPPLGLIYFFVQRFYVASSRQLKRLESVSRSPVYSHFNETL
LGVSVIRAFEEQERFIHOSDLKVDENOKAYYPSIVANRWLAVRLECVGNCIVLFAALFAVISRHS
31
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
LSAGLVGLSVSYSLQVTTYLNWLVRMSSEMETNIVAVERLKEYSETEKEAPWQIQETAPPSNW
PQVGRVEFRNYCLRYREDLOFVLRHINVTINGGEKVGIVGRIGAGKSSLTLGLFRINESAEGEIII
DGiNIARIGLHDLRFKITIIPCDPVLFSGSLRMNLDPFSQYSDEEVWTSLELAHLKGFVSALPDKL
DHECAEGGENLSVGQRQLVCLARALLRKTKILVLDEATAAVDLETDDLIQSTIRTQFEDCTVLTIA
HRLNTIMDYTRVIVLDKGEIQEYGAPSDLLQQRGLFYNMARDAGLV (SEQ ID NO:158): an
extracellular region thereof; or a fragment of cynornolgus monkey ABCC1 ,
e.g., a fragment
comprising amino add residues 204-1531:
MDPNPCPESSASFLSRITFWWITGLIVRGYROPLEGSDLW5LNKEDTSEQ\NPVLVKN
WKKECAKTRKQPVKVVYSSKDPAQPKDSSKVDANEEVEALIVKSPQKEWNPSLFKVLYKTFG
PYFLMSFEFKAIHDLMMFSGPEILKLLINFVNDTKAPDWOGYFYTALLFVAACLQTLVLHQYFHI
CFVSGMRIKTAVIGAVYRKALVITNAARKSSTVGEIVNLMSVDAORFMDLATYINMIWSARLOVI
LALYLLWRNLGPPlLAGVAVMVLMVPVNAVMAMKTKTYQVAHMKSKDNRIKLMNEILNGKVLK
LYAWELAFKDKVLAIRQEELKVLKKSAYLAAVGTFTWVCTPFLVALCTFAVYVTIDKNNVLDAQK
AFVSLALFNILRFPLNILPMVISSIVOASVSLKRLRIFLSHEELEPDSIERRPVKDGGDTNSITVRN
ATFTWARSDPPTLNGITFSIPEGALVAVVGQVGCGKSSLLSALLAEMDKVEGHVALKGSVAYV
PQQAWIQNDSLQENILFGCQLEEPYYRSVIQACALLPDLEILPSGDRTEIGEKGVNLSGGQKQR
VSLARAVYCNADIYLFDDPLSAVDAHVGKIllFENVIGPKGMLKNKTRILVTHSMSYLPQVDVilV
MSGGKISEMGSYQELLARDGAFAEFLRTYASAEQEQDPEDNGVTGVSGPGKEAKQMENGML
VTDSAGKQLQRQLSSSSSYSGDVSRQHNSTAELQKDGAKKEETWKLMEADKAQTGQVKLSV
YWDYMKAIGLFISFLSIFLFICNHVAALASNYWLSLWTDDPIVNGTQEHTKVRLSVYGALGISQGI
AVFGYSMAVSIGGILASRCLHVDLLHSILRSPMSFFERTPSGNLVNRFSKELDTVDSMIPEVIKM
FMGSLFNVIGACIVILLATPIAAIIIPPLGLIYFFVORFYVASSRQLKRLESVSRSPVYSHFNETLLG
VSVlRAFEEQERFIHQSDLKVDENQKAYYPSIVANRWLAVRLECVGNCIVLFAALFAVISRHSLS
AGLVGLSVSYSLQVTTYLNWLVRMSSEMETNIVAVERLKEYSETEKEAPWQIQETAPPSNWPQ
VGRVEFRNYCLRYREDLDFVLRHINVTINGGEKVGIVGRTGAGKSSLTLGLFRINESAEGEIIIDG
INIARIGLHDLRFKlTlIPQDPVLFSGSLRMNLDPFSQYSDEEVWTSLELAHLKGFVSALPDKLDFI
ECAEGGENLSVGQROLVCLARALLRKTKILVLDEATAAVDLEIDDLIQSTIRTQFEDCTVLTIAH
RLNTINIDYTRVIVLDKGEUEYGAPSDLLQQRGLFYNMARDAGLV (SEC) ID NO:159),
A subject anti-ABCC1 antibody exhibits high affinity binding to ABCC1 For
example, a
subject anti-ABCC1 antibody binds to a human ABCC1 with an affinity of at
least about 104 M, at
least about 10-8 M. at least about 10 M, at least about 10-I(' M, at least
about 10-11 M, or at least
about 10-12 M, or greater than 10-'2 M. A subject anti-ABCC1 antibody binds to
an epitope present
on ABCC1 with an affinity of from about 10-7 M to about 104 M, from about 10 M
to about 10-9
32
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
M, from about 10-9 M to about 10-'" M, from about 10-''' M to about 10'11 M,
or from about 10-" M
to about 10-12 M, or greater than 10-12 M.
A subject anti-ABCC1 antibody exhibits substantially no binding to any
epitopes formed
by amino acids within other related, but sequence dissimilar, proteins such as
related but
sequence dissimilar EPs. Any binding of a subject anti-ABCC1 antibody to an
epitope formed by
amino acids within a related, but sequence dissimilar: protein is generally
non-specific binding of
a substantially lower affinity than the specific binding of the anti-ABCC1
antibody to the epitope
on ABCC1. A substantially lower affinity is generally at least a 2 fold, 3
fold, 5 fold, 10 fold, 50
fold, 100 fold, 500 fold, or 1000 fold lower affinity,
A subject anti-ABCC1 antibody can reduce transport of molecules through an
ABCC1
transporter, e.g., a human ABCC1. For example, a subject anti-ABCC1 antibody
can reduce
transport by at least about 5%, at least about 10%, at least about 15%, at
least about 20%, at
least about 25%, at least about 30%, at least about 40%, at least about 50%,
at least about 60%,
at least about 70%, at least about 80%, at least about 90%, or more, compared
to the degree of
transport in the absence of the anti-ABCC1 antibody.
In some embodiments, a subject antibody comprises FR regions that are
mammalian
sequences: including e.g.: rodent, non-human primate, and human sequences
(e.g., encoded by
the respective heavy chain FR-encoding sequences),
A subject antibody can comprise a heavy chain variable (VH) region comprising
an
amino acid sequence that is 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%,
95%,
96%, 97%, 98%, 99%, or more, including 100%, identical to a sequence for a VH
region of a
VH-VL pair of an antibody set forth in Table 2, The subject antibody can
comprise a light chain
variable (VL) region comprising an amino acid sequence that is 85%, 86%, 87%,
88%, 89%,
90%, 91%, 92%, 98%, 94%, 95%, 96%, 97%, 98%, 99%, or more, including 100%,
identical to a
sequence for a VL of the VH-VL region pair of the antibody set forth in Table
2,
In one aspect, the antibody molecule comprises the HCDRs 1-3 and/or LCDRs 1-3
of
one of the C1.844 antibody, the C1.851 antibody: the C1.831 antibody, or the
C1.861 antibody
listed in Table 2.
In one aspect, the antibody molecule comprises the HCDRs 1-3 and/or LCDRs 1-3
of
one of the C1.773 antibody, the C1,773a antibody, the C1.777a antibody, the
C1.784a antibody,
the Cl .786a antibody, the C1 .787a antibody, the C1.827 antibody, the Cl
.8306 antibody, the
C1.831 antibody, the C1.835 antibody, the C1.841 antibody, the C1.844
antibody, the C1.845
antibody, the CI .847 antibody, the C1,851 antibody, the C1,855 antibody, the
C1,861 antibody,
33
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
the C1.863 antibody, the 01.876 antibody, the 01.877 antibody, or the C1,879A
antibody listed
in Table 2.
In certain embodiments, the antibodies of the present disclosure may lower the
IC50 of
the Chemotherapeutic agent (e.g., vincristine) by factor of 5 or more, e.g.,
factor of 6 or more,
factor of 7 or more, factor of 8 or more, factor of 9 or more, factor of 10 or
more, or factor of 20
or more, e.g., by a factor of 5 to 50. in one aspect, the antibody molecule
may lower the 1050 of
the chemotherapeutic agent (e.g., vincristine) by factor of 5 or more and may
comprises the
HCDRs 1-3 and/or LCDRs 1-3 of the C1,851 antibody, the 01...84I antibody, the
01.861
antibody, the C1.831 antibody, C1.786a the antibody, the Cl .787a antibody, or
the C1.777
.. antibody, listed in Table 2.
Regions and/or chains of the subject antibodies may or may not be joined by
one or more
linker regions. Where present: the linker region can be from about 5 amino
acids to about 50
amino acids in length: e.g.., from about 5 aa to about 10 aa, from about 10 aa
to about 15 aa: from
about 15 aa to about 20 aa: from about 20 aa to about 25 aa, from about 25 aa
to about 30 aa,
from about 30 aa to about 35 .aa., from about 35 aa to about 40 aa, from about
40 aa to about 45
aa, or from about 45 aa to about 50 aa in length.
Linkers suitable for use a subject antibody include '''flexible linkers". If
present, the linker
molecules are generally of sufficient length to permit some flexible movement
between linked
regions. The linker molecules are generally about 6-50 atoms long. The linker
molecules may
also be, for example, aryl acetylene, ethylene glycol oligomers containing 2-
10 monomer units,
diarnines, diacid.s, amino acids, or combinations thereof. Other tinker
molecules which can bind.
to polypeptides may be used in light of this disclosure.
Suitable linkers can be readily selected and can be of any of a suitable of
different lengths,
such as from 1 amino acid (e.g., ,Gly) to 20 amino acids, from 2 amino acids
to 15 amino acids,
from 3 amino acids to 12 amino acids, including 4 amino acids to 10 amino
acids, 5 amino acids
to 9 amino acids, 6 amino acids to 8 amino acids, or 7 amino acids to 8 amino
acids, and may be
1, 2, 3: 4, 5, 6, or 7 amino acids.
Exemplary flexible linkers include glycine polymers (G)rõ ,glycine-serine
polymers
(including, for example, (GS)õ, GSGGSõ (SEQ ID NO.:.160) and .GGGSõ (SEQ ID
NO:161), where
n is an integer of at least one), .glycine-alanin-e polymers, alanine-serine
polymers, and other
flexible linkers known in the art. Glycine and giycine-serine polymers are of
interest since both of
these amino acids are reatively unstructured, and therefore may serve as a
neutral tether
between components. Glycine polymers are of particular interest since .glycine
accesses
34
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
significantly more phi-psi space than even alanine, and is much less
restricted than residues with
longer side chains (see Scheraga, Rev. Computational Chem. 11173-142 (1992)).
Exemplary
flexible linkers include, but are not limited to GGSG (SEQ ID 'NO.:162), GGSGG
(SEQ ID NO:163),
GSGSG (SEQ ID NO:164), GSGGG (SEQ ID NO:166)., GG-GSG (SEQ ID NO:166), GSSSG
(SEQ
ID NO:167), and the like. The ordinarily skilled artisan will recognize that
design of a peptide
conjugated to any elements described above can include linkers that are all or
partially flexible,
such that the linker can include a flexible linker as well as one or more
portions that confer less
flexible .structure.
In other instances, the flexibility of the hinge region of an antibody of the
present
disclosure may be reduced by either mutating amino acid C220 to serine or any
other natural
amino acid, by removing C220; by removing the complete hinge, or by replacing
the IgG1 hinge
with an IgG3 hinge, an antibody is formed in which the light chains are
connected via their C-
terminal cysteines., analogous to the situation found in the human isotype
IgA2m. This results in
a reduced flexibility of the .Fabs relative to the Fc and consequently reduced
cross-linking
.. capacity.. Another strategy to reduce the flexibility of an IgG1 molecule
is to replace the IgGi
hinge with the IgG2 hinge or IgG2-like hinge. Alternatively, a variant of the
Ig-G1 hinge that
resembles the I9G2 hinge can be introduced. This mutant (TH7A6-9) contains
mutation T223C
and two deletions (K222 and T225) in order to create a shorter hinge with an
additional
cysteine.
The substitution of mouse CDRs into a human variable domain framework can
result in
retention of their correct spatial orientation where, e.g., the human variable
domain framework
adopts the same or similar conformation to the mouse variable framework from
which the CDRs
originated, This can be achieved by obtaining the human variable domains from
human antibodies
whose framework sequences exhibit a high degree of sequence identity with the
murine variable
framework domains from which the CDRs were derived. The heavy and light chain
variable
framework regions can be derived from the same or different human antibody
sequences. The
human antibody sequences can be the sequences of naturally occurring human
antibodies or can:
be consensus sequences of several human antibodies, See Kettleborough et al.,
Protein
Engineering 4773 (1991); Kolbinger et al., Protein Engineering 6971 (1993).
Having identified the complernentarity determining regions of the murine donor
immunoglo:bulin and appropriate human acceptor immunoglobulins, the next step
is to determine
which, if any, residues from these components should be substituted to
optimize the properties of
the resulting humanized antibody. In general, substitution of human amino acid
residues with
murine should be minimized, because introduction of murine residues increases
the risk of the
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
antibody eliciting a human-anti-mouse-antibody (HAMA) response in humans. Art-
recognized
methods of determining immune response can be performed to monitor a HAMA
response in a
particular patient or during clinical trials. Patients administered humanized
antibodies can be
given an immunogenicity assessment at the beginning and throughout the
administration of said
therapy. The HAMA response is measured, for example, by detecting antibodies
to the humanized
therapeutic reagent, in serum samples from the patient using a method known to
one in the art,
including surface plasm.on resonance technology (BIACORE) and/or solid-phase
ELISA analysis..
In many embodiments, a subject humanized antibody does not substantially
elicit a HAMA
response in a human subject.
Certain amino acids from the human variable region framework residues are
selected for
substitution based on their possible influence on CDR conformation and/or
binding to antigen.
The unnatural juxtaposition of murine CDR regions with human variable
framework region can
result in conformational 'restraints, which, unless corrected by substitution
of certain amino acid
residues, lead to loss of 'binding affinity,
The selection of amino acid residues for substitution can be determined, in
part, by
computer modeling. Computer hardware and software for producing three-
dimensional images of
immunoglobulin molecules are known in the art. In general, molecular models
are produced
starting from solved structures for immunoglobulin chains or domains thereof.
The chains to be
modeled are compared for amino acid sequence similarity with chains or domains
of solved three-
dimensional structures, and the chains or domains showing the greatest
sequence similarity is/are
selected as starting points for construction of the molecular model. Chains or
domains sharing at
least 50% sequence identity are selected for modeling, and preferably those
sharing at least 60%,
70%, 80%, 90% sequence identity or more are selected for modeling. The solved
starting
structures are modified to allow for differences between the actual amino
acids in the
immunoglobulin chains or domains being modeled, and those in the starting
structure. The
modified structures are then assembled into a composite immunoglobulin.
Finally, the model is
refined by energy minimization and by verifying that all atoms are within
appropriate distances
from one another and that bond lengths and angles are within chemically
acceptable limits.
In some embodiments, a subject antibody comprises scFv. :multimers. For
example, in
some embodiments, a subject antibody is an scFv dimer (e.g., comprises two
tandem scFv
(ScFV2)), an scFv trimer (e.g...õ comprises three tandem :scFv (scFv)), an
scFv tetramer
comprises four tandem scFv (scFv4)), or is a multimer of more than four scFv
(e,g.õ in tandem).
The scFv monomers can be linked in tandem via linkers of from about 2 amino
acids to about 15
amino acids in length, e.g., 2 as, 3 as, 4 as, 5 as, 6 aa, 7 aa, 8 aa, 9: as,
10 as, 11 as, 12 as, 13
36
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
aa, 14 as, or 15 as in length. Suitable linkers include, e.g., (Gly), (SEQ ID
NO:168), where x is an
integer from 2 to 15. Other suitable linkers are those discussed above. in
some embodiments,
each of the scFv monomers in a subject scFV multimer is humanized, as
described above. In
certain embodiments, a bispecific antibody may be in any molecular format
known in the literature,
For example, a bispecific antibody of the present disclosure may have a
molecular format
described in Spiess C. et al., Mel Immunol. 2015 Oct;67(2 Pt A).95-106,
In some embodiments, a subject antibody comprises a constant region of an
immunogiobuiin (e.g., an Fe region). The Fe region, if present, can be a human
Fe region. If
constant regions are present, the antibody can contain both light chain and
heavy chain constant
regions, Suitable heavy chain constant region include Cl-i1, hinge, CH2, CH3,
and CH4 regions.
The antibodies described herein include antibodies having all types of
constant regions, including
IgM, IgG, igD, IgA and IgE, and any isotype, including lgGI, igG2, IgG3 and
IgG4. An example
of a suitable heavy chain Fc region is a human isotype IgG1 Fe. Light chain
constant regions can
be lambda or kappa. A subject antibody (e.g.. a subject humanized antibody)
can comprise
sequences from more than one class or isotype. Antibodies can be expressed as
tetramers
containing two light and two heavy chains, as separate heavy chains, fight
chains, as Fab, Fab'
F(ab')2, and Fv, or as single chain antibodies in which heavy and light chain
variable domains are
linked through a spacer.
in some embodiments, a subject antibody comprises a free thiol (-SH) group at
the
carboxyl terminus, where the free thioi group can be used to attach the
antibody to a second
polypeptide (e.g., another antibody, including a subject antibody), a
scaffold, a carrier, etc.
A subject antibody can be covalently linked to a second moiety (e.g., a lipid,
a polypeptide
other than a subject antibody: a synthetic polymer, a carbohydrate, a toxin
and the like) using for
example, glutaraldehyde, a homobifunctional cross-linker, or a
heterobifunctional cross-linker.
Glutaraldehyde cross-links polypeptides via their amino moieties,
Homobifunctional cross-linkers
(e.g., a homobifunctional imidoester, a homobifunctional N-hydroxysuccinimidyl
(NHS) ester, or
a homobifunctional sulfhydryi reactive cross-linker) contain two or more
identical reactive moieties
and can be used in a one-step reaction procedure in which the cross-linker is
added to a solution
containing a mixture of the polypeptides to be linked. Hornobifunctional NHS
ester and imido
esters cross-link amine containing polypeptides. In a mild alkaline pH, irnido
esters react only with
primary amines to form imidoamides, and overall charge of the cross-linked
polypeptides is not
affected. Homobifunctional sulfhydryi reactive cross-linkers includes
bismaleimidohexane (BMH),
1,5-difluoro-2,4-dinitrobenzene (DFDNB), and 1,4-Bist3-(2-
pyridyldithio)propionamidojbutane
(DPDPB),
37
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
Bispecifio Antibodies
In certain aspects, the antibodies provided herein may be bispecific
antibodies
comprising comprising a VH region and a VL region of an anti-ABCC1 antibody as
set forth in
the preceding section, and further comprises a second VH region comprising
HCDRs1-3 of an
antibody that binds to a tumor associated antigen (TAA).
The antibody may include a second VL region and wherein the second VH region
and
the second VL region bind to the TM In certain embodiments, the second VH
region and the
second VL region may be present in a single polypeptide. In certain
embodiments, the second
VH region and the second VL region are present in a scFv.
In certain embodiments, the bispecific antibody comprises a common light
chain,
wherein the common light chain comprises a VL region of an anti-ABCC1 antibody
as set forth
in the preceding section.
The TM may be any antigen that is known to be overexpressed in cancer cells.
For
example, the TAA may be an antigen that is not expressed at detectable levels
in a normal cell
and is expressed in cancer cells, where the normal and cancer cells are the
same cell type, e.g.,
epithelial cells. For example, TAAs may be neoantigens that are a class of
tumor antigens that
arise from a tumor-specific mutation(s) which alters the amino acid sequence
of encoded
proteins as compared to the amino acid sequence of the unmutated protein. In
other aspects, a
TM is an antigen that is expressed in normal cells but is expressed at higher
levels in cancer
cells.
In certain embodiments, the TAA may be PD-L1. PD-L1 is also known as cluster
of
differentiation 274 (CD274) or B7 homolog 1 (B7-H1). In certain embodiments,
the antibody that
binds to PD-Ll may be atezolizumab.
In certain embodiments, the bispecific antibody may include a VH region
comprising
HCDRs1-3 and a VL region comprising LCDRs1-3 of the VH region and VL region,
respectively,
of the C1.844 antibody or the C1.851 antibody as listed in Table 2 and a
second VH region and
a second VL region comprising HCDRs1-3 and LCDRs1-3, respectively, of
atezolizumab. In
certain embodiments, the HCDRs1-3 and LCDRs1-3 are defined as per Kabat
nomenclature,
In certain embodiments, the bispecific antibody comprises: a VH region and a
VL region
of the C1.844 antibody or the C1,851 antibody listed in Table 2 and a scFv
comprising
HCDRs1-3 and LCDRs1-3 of atezolizumab, where the HCDRs1-3 and LCDR61-3 are
defined
as per Kabat nomenclature,
In certain embodiments, the bispecific antibody may include a VH region
comprising
HCDRs1-3 and a VL region comprising LCDRs1-3 of the VH region and VL region,
respectively.
38
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
of the C1,844hu21 antibody or the CI,851hu12 antibody as listed in Table 2 and
a second VH
region and a second VL region comprising HCDRs1-3 and LCDRsI-3, respectively,
of
atezolizumab, in certain embodiments, the HCDRs1-3 and LCDRs1-3 are defined as
per Kabat
nomenclature,
In certain embodiments, the bispecific antibody comprises: a VH region and a
VL region
of the C1.8414hu21 antibody or the C1,851hu12 antibody listed in Table 2 and a
scFv comprising
HCDRs1-3 and LCDRs1-3 of atezolizumab, where the HCDRs1-3 and LCDRs1-3 are
defined
as per Kabat nomenclature,
In certain embodiments, the HCDRs1-3 present in the second VH region or the
scFv
region of the bispecific antibody are the HCDRs1-3 of atezolizumab, where the
HCDR1
comprises the sequence OS WIN (SEQ ID NO.25), the HCDR2 comprises the sequence
WISPYGGSTYYADSVKG (SEQ ID NO.169), and the HCDR3 comprises the sequence
RHWPGGFDY (SEQ ID NO:170). In certain embodiments, the bispecific antibody
that binds to
ABCC1 and PD-Ll may further include a second VL region that includes the
LCDRs1-3 of
atezolizumab, where the LCDR1 comprises the sequence RASODVSTAVA (SEQ ID NO
171),
the LCDR2 comprises the sequence SASFLYS (SEQ ID NO 172) and the LCDR3
comprises
the sequence QQYLYHPAT (SEQ ID NO:173). In certain embodiments, the LCDRs1-3
present
in the scFv region include the LCDRs1-3 of atezolizumab, defined as per Kabat
nomenclature,
In certain embodiments, the bispecific antibody that binds to ABCC1 and PD-Ll
comprises a second VH region comprising an amino acid sequence at least 80%,
at least 90%,
at least 95%, or a 100% identical to the amino acid sequence of the VH region
of atezolizumab
as set forth below:
EVOLVESGGGLV0PGGSLRLSCAASGFTFSDSWIHVVVROAPGKGLEWVAWISPYGG
STYYADSVKGRFTISADTSKNTAYLQMNSLRAEDTAVYYCARRHWPGGFDYWGQGTLVTVSS
(SEQ ID NO:174),
In certain embodiments, the bispecific antibody that binds to ABCCI and PD-L1
comprises a second VL region comprising an amino acid sequence at least 80%,
at least 90%,
at least 95%, or a 100% identical to the amino acid sequence of the VL region
of atezolizumab
as set forth below:
DIQN,ITQSPSSLSASVGDRVTITCRASQDVSTAVAWYQQKPGKAPKLLIYSASFLYSGVP
SRFSGSGSGTDFTLTISSLQPEDFATYYCQQYLYHPATFGQGTKVElk (SEQ ID NO-175)
In certain embodiments, the bispecific antibody that binds to ABCC1 and PD-L1
comprises a scFv region that binds to PD-L1 and comprises an amino acid
sequence at least
39
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
80%, at least 90%, at least 95%, or a 100% identical to the amino acid
sequence set forth
below:
EVOLVESGGGLVQPGGSLRLSCAASGFTFSDSWlHVVVRQAPGKGLEWVAWISPYGG
STYYADSVKGRFTISADTSKNTAYLQMNSLRAEDTAVYYCARRHWPGGFDYWGQGTLVTVSS
GGGGSGGGGSGGGGSDIOMTQSPSSLSASVGDRVTiTCRASQDVSTAVAWYQQKPGKAPKL
LlYSASFLYSGVPSRFSGSGSGTDFTLTISSLOPEDFATYYCQQYLYHPATEGQGTKVEIK (SEQ
ID NO: 176).
The italized sequence is a linker sequence between the VH and VL regions. Any
other
linker sequence may be used to connect the VH and VL regions,
In certain embodiments, the TAA is ErbB2 (HER2). ErbB2 is also known as
Receptor
Tyrosine Kinase 2 or HER2. in certain embodiments, the antibody that binds to
ErbB2 is
trastuzumab.
In certain embodiments, the bispecific antibody comprises: a VH region
comprising
HCDRs1-3 and a VL region comprising LCDRs1-3 of the VH region and VL region,
respectively,
of the C1.844 antibody, the C1,831 antibody, or the C1.851 antibody listed in
Table 2 and and a
second VH region and a second VL region comprising HCDRs1-3 and LCDRs1-3,
respectively,
of trastuzumab. in certain embodiments, the HCDRs1-3 and LCDRs1-3 are defined
as per
Kabat nomenclature,
In certain embodiments, the bispecific antibody comprises: a VH region
comprising
HCDRs1-3 and a VL region comprising LCDRs1-3 of the VH region and VL region,
respectively,
of the CI .844 antibody, the C1.831 antibody, or the C1.851 antibody listed in
Table 2 and a
scFv comprising HCDRs1-3 and LCDRs1-3 of trastuzumab.
In certain embodiments, the bispecific antibody comprises: a VH region and a
VL region
of the C1.844hu21 antibody, the C1,831hul 1 antibody, or the C1,851hul2
antibody and a scFy
comprising HCDRs1-3 and LCDRs1-3 of trastuzumab, where the HCDRs1-3 and LCDRs1-
3 are
defined as per Kabat nomenclature.
In certain embodiments, the HCDRs1-3 present in the second VH region or the
scFy
region of the bispecific antibody are the HCDRs1-3 of trastuzumab, where the
HCDR1
comprises the sequence DTYIH (SEQ ID NO:177), the HCDR2 comprises the sequence
RIYPTNGYTRYADSVKG (SEQ ID NO:178), and the HCDR3 comprises the sequence
WGGDGFYAIADY (SEQ ID NO:179). In certain embodiments, the bispecific antibody
that binds
to ABCC1 and HER2 may further include a second VL region that includes the
LCDRs1-3 of
trastuzumab, where the LCDR1 comprises the sequence RASQDVNTAVA (SEQ ID
NO:180);
the LCDR2 comprises the sequence SASFLYS (SEC) ID NO:172), and the LCDR3
comprises
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
the sequence 00HYTTPPT (SEQ ID NO:181). In certain embodiments, the LCDRs1-3
present
in the scFv region include tne LCDRs1-3 of trastuzumab, defined as per Kabat
nomenclature.
In certain embodiments the bispecific antibody that binds to ABCCI and HER2
comprises a second VH region comprising an amino acid sequence at least 80%,
at least 90%,
at least 95%, or a 100% identical to the amino acid sequence of the VH region
of trastuzumab
as set forth below:
EVQLVESGGGLVQPGGSLRLSCAASGFNlKDTYlHWVRQAPGKGLEWVARlYPINGYT
RYADSVKGRFTlSADTSKNTAYLQMNSLRAEDTAVYYCSRWGGDGFYAMDYWGQGTLVR/S
S (SEQ ID NO:182),
In certain embodiments, the bispecific antibody that binds to ABCC1 and HER2
comprises a second VL region comprising an amino acid sequence at least 80%,
at least 90%,
at least 95%, or a 100% identical to the amino acid sequence of the VL region
of trastuzumab
as set forth below:
DIQMTQSPSSLSASVGDRVTITCRASCIDVNTAVAWYQQKPGKAPKWYSASFLYSGVP
SRFSGSRSGTDFTLAISSLOPEDFATYYCQQHY11PPTFGQGTKVEIK (SEQ ID NO:183),
In certain embodiments; the bispecific antibody that binds to ABCC1 and HER2
comprises a scFv region that binds to HER2 and comprises an amino acid
sequence at least
80%, at least 90%, at least 95%, or a 100% identical to the amino acid
sequence set forth
below:
EVQLVESGGGLVQPGGSLRLSCAASGFNIKDTYlHWVRQAPGKGLEWVARIYPINGYT
RYADSVKGRFTlSADTSKNTAYLQMNSLRAEDTAVYYCSRWGGDGFYANADYWGCGTLVTVS
SGGGGSGGGGSGGGGSMMTQSPSSLSASVGDRVTITGRASQDVNTAVAWYQQKPGKCP
KWYSASFLYSGVPSRFSGSRSGTDFTLTISSLOPEDFATYYCQQHYTTPPTFGOGTKVEIK
(SEQ ID NO:184)
The italized sequence is a linker sequence between the VH and VI_ regions. Any
other
linker sequence may be used to connect the VH and VL regions.
In certain embodiments, the heavy chain of the bispecific antibodies may
include a VH
region as provided herein and a heavy chain constant region, In certain
embodiments, the light
chain of the bispecific antibodies may include a VL region as provided herein
and a light chain
constant region. In certain embodiments, the scFv may be conjugated to a heavy
chain
constant region.
The heavy chain constant region and the light chain constant region may be of
a human
IgG antibody, e.g., a human igG1 antibody. The constant region may have a wild
type sequence
41
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
or a modified sequence. in some embodiments, the bispecific antibody may
include a modified
heavy chain, including a modified Fc domain, including a modified CH2 and/or
modified CH3
domain. In some instances, modified Fc domains may employ electrostatic
steering effects,
including but not limited to e.g., through the use of the procedures described
in Gunasekeran et
al, (2010) Journal of Biological Chemistry 285, 19637-19646; the disclosure of
which is
incorporated herein by reference in its entirety. In some instances, a
bispecific antibody is
assembled through charge pair substitutions at the CH3 domain, including but
not limited to e.g.,
where one heavy chain is modified to contain K392D and K409D substitutions and
the other heavy
chain is modified to contained E356k and D399K substitutions. Charge pair
substituted chains
may preferentially form a heterodimer with one another. The numbering of the
amino acid
substitutions is per EU numbering system for HCs,
In some instances, an antibody of the present disclosure includes charge pair
substitutions. In some instances, an antibody of the present disclosure does
not include charge
pair substitutions. In some instances, an alternative means of promoting
preferential heterodimer
formation of desired chains may be employed.
In some instances, a modified heavy chain may include a knob-into-hole
modification.
"Knobs-into-holes" amino acid modification is a rational design strategy in
antibody engineering,
used for heterodimerization of the heavy chains, in the production of multi-
specific antibodies,
including bispecific igG antibodies. For example, in incorporating the knobs-
into-holes strategy
into a bispecific antibody made from two monoclonal antibodies of different
specificities, amino
acid changes are engineered in order to create a "knob" on the CH3 of the
heavy chain of
monoclonal antibody 1 (mAbl) and a "hole" on the CH3 of the heavy chain of
monoclonal
antibody 2 (mAb2). The knob may be represented by a large amino acid, such as
e.g., a
tyrosine (Y), whereas the hole may be represented by small amino acid, such as
a threonine
(T). For example, a knobs-into-holes pair modification may be created a T22Y
substitution in a
first CH3 domain and Y86T substitution in the partner CH3 domain. Examples of
knobs-into-
holes modifications are described in Carter, J. immunol. Methods, 248(1-2):7-
15 (2001);
Ridgway: J. B. et al. Protein Eng. 9(7):617-2 (1996); and Merchant, A. M. et
al. Nat. Biotechnol.
16(7)1677-81 (1998); the disclosures of which are incorporated herein in their
entirety. In
antibodies generated from paired knob-into-hole modified domains the
bispecific heterodimer
will generally represent the major fraction.
In certain embodiments, the bispecific antibodies provided herein bind to
cancer cells
expressing both ABCC1 and the TAA while showing reduced binding to non-cancer
cells
expressing ABCC1 and/or the IAA. In other words, the bispecific antibodies
provided herein
42
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
bind with low affinity to (1) cells expressing TAA where ABCC1 expression is
low or absent and
(2) cells expressing ABCC1 where TAA expression is low or absent, and with
high affinity to
cancer cells that express at least one or both ABCC1 and TAA at relatively
high levels,
levels higher than normal cells,
COMPOSITIONS AND FORMULATIONS.
The present disclosure provides a composition comprising a subject antibody. A
subject
antibody composition can comprise, in addition to a subject antibody, one or
more of a salt,
e,g., NaCI,
KCIõ Mg304, etc.; a buffering agent, e.g., a Tris buffer, a nistidine buffer.
N-
(2-Hydroxy-ethyl)pipera.zine-N'-(2-ethanesulfonic acid) (HEPES), 2-(N-
Morpholino)ethanesulfonic
acid (MES), 2-(N-Morpholino)ethanesulfonic add sodium salt .(MES),. 3-(N-
Morpholino)propanesulfonic acid (MOPS), N-tris[Hydroxymethylimethyl-3-
aminopropanesulfonic
acid (TAPS), etc.; a solubilizing agent_ a detergent, e.g,, a non-ionic
detergent such as Tween-
20, etc.: a protease inhibitor; glycerol; and the like.
Compositions of the present disclosure also include pharmaceutical
compositions that
include an antibody described herein.. In general, a formulation comprises an
effective amount of
the subject antibody, An "effective amount" means a dosage sufficient to
produce a desired result,
e.g., reduction in a cancer of a subject, reduction in the growth rate of a
cancer in a subject,
amelioration of a symptom of cancer, and the like. Generally, the desired
result is at least a
reduction in a symptom of a cancer, reduction in the growth of a cancer,
reduction in the size of
a cancer, etc.., as compared to a control. A subject antibody can be
delivered, or be formulated,
in such a manner as to avoid the blood-brain barrier.
In some instances, an antibody may include a delivery enhancer, including
where such,
enhancers may facilitate crossing of the blood-brain barrier, increased
permeability, e.g., allowing
for efficient transdermal delivery., and the like.
In some instances, the antibodies of the present disclosure may not be
administered in a
formulation with a delivery enhancer. In some instances., the antibodies of
the present disclosure
may themselves enhance permeability across the blood-brain barrier. In some
instances, the
antibodies of the present disclosure may be used as a delivery enhancer to
facilitate crossing of
the blood-brain barrier by an anti-neoplastic agent, e.g., an
immunotherapeutic agent or a.
chemotherapeutic agent. In some instances, the antibodies of the present
disclosure may be used
as a delivery enhancer to facilitate crossing of the blood-brain barrier,
blood-cerebrospinal fluid
(CSF) barrier, blood-testis barrier, or blood-placenta barrier by an active
agent, such as, another
antibody or a chemotherapeutic agent.
43
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
In the subject methods, a subject antibody can be administered to the host
using any
convenient means capable of resulting in the desired therapeutic effect or
diagnostic effect. Thus,
the agent can be incorporated into a variety of formulations for therapeutic
administration. More
particularly, a subject antibody can be formulated into pharmaceutical
compositions by
combination with appropriate, pharmaceutically acceptable carriers or
diluents, and may be
formulated into preparations in solid, semi-solid, liquid or gaseous forms,
such as tablets,
capsules, powders, granules, ointments, solutions, suppositories, injections,
inhalants and
aerosols.
In pharmaceutical dosage forms,. a subject antibody can be administered in
conjunction
with a pharmaceutically acceptable excipient, or they may also be used alone
or in appropriate
association, as well as in combination, with other pharmaceutically active
compounds. The
following methods and excipients are merely exemplary and are in no way
limiting.
A subject antibody can be formulated into preparations for injection by
dissolving,
suspending or emulsifying them in an aqueous or nonaqueous solvent, such as
vegetable or other
similar oils, synthetic aliphatic acid glycerides, esters of higher aliphatic
acids or propylene glycol;
and if desired, with conventional additives such as solubilizers, isotonic
agents, suspending
agents, emulsifying agents, stabilizers and preservatives.
Pharmaceutical compositions comprising a subject antibody are prepared by
mixing the
antibody having the desired degree of purity with optional physiologically
acceptable carriers,
excipients, stabilizers, surfactants, buffers and/or tonicity agents.
Acceptable carriers, excipients
and/or stabilizers are nontoxic to recipients at the dosages and
concentrations employed, and
include buffers such as phosphate, citrate, and other organic acids;
antioxidants including
ascorbic acid, giutathione, cysteineõ methionine and citric acid;
preservatives (such as ethanol,
benzyl alcohol, phenol, m-cresol, p-ohlor-m-cresolõ methyl or propyl parabens,
benzalkonium
chloride, or combinations thereof); amino acids such as arginine, glycine,
ornithine, :lysine,
histidineõ .glutarnic acid, aspartic acid, isoleucine, ieucine, alanine,
phenylalanine, tyrosine,
try-ptophan, methionine, serine, proline and combinations thereof;
monosaccharides,
disaccharides and other carbohydrates; low molecular weight (less than about
10 residues)
polypeptides; proteins, such as gelatin or serum albumin: &elating:agents such
as EDTA; sugars
such as trehalose, sucrose, lactose, glucose, mannose, maltose, galactose,
fructose, sorboseõ
raffinose, glucos-amine.õ N-tnethytglucosamine, galactosamine, and neuraminic
add; and/or non-
ionic surfactants such as Tween, Brij Piuronics, Triton-X, or polyethylene
glycol (PEG),
44
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
The pharmaceutical composition may be in a liquid form, a lyophilized form or
a liquid form
reconstituted from a lyophilized form, wherein the lyophilized preparation is
to be reconstituted
with a sterile solution prior to administration.
Exemplary antibody concentrations in a subject pharmaceutical composition may
range
from about 1 mg/mL to about 200 rngimi or from about 50 mg/mL to about 200
mg/mL, or from
about 150 mg/mL to about 200 mg/mL.
An aqueous formulation of the antibody may be prepared in a pH-buffered
solution, e.g.,
at pH ranging from about 4.0 to about 7.5 or from about 5.0 to about 6.0, or
alternatively about
5.5. Examples of buffers that are suitable for a pH within this range include
phosphate-, .histidine-
õ citrate-, succinate-, acetate-buffers and other organic acid buffers. The
buffer concentration can
be from about 1 mM to about 100 mM,. or from about 5 mM to about 50 mM.õ
depending, e.g,õ on
the buffer and the desired tonicity of the formulation.
In some embodiments, the aqueous formulation is isotonic, although hypertonic
or
hypotonic solutions may be suitable. The term "isotonic denotes a solution
having the same
tonicity as some other solution with which it is compared, such as
physiological salt solution or
serum. Tonicity agents may be used in an amount of about 5 miVI to about 350
mM, e.g., in an
amount of 100 mM to 350 nM.
A surfactant may also be added to the antibody formulation to reduce
aggregation of the
formulated antibody and/or minimize the formation of particulates in the
formulation and/or reduce
adsorption. Exemplary surfactants include polyoxyethylensorbitan fatty acid
esters (Tween),
polyox.yethylene alkyl ethers (Brij), alkylphenyip-olyoxyethylene ethers
(Triton-X),
polyoxyethylene-potyoxypropylene copolymer (Poloxamer, Pluronic), and sodium
dodecyl sulfate
(SDS). Exemplary concentrations of surfactant may range from about 0,001% to
about 1%w !v.
A lyoprotectant may also be added in order to protect the labile active
ingredient (e.g. a
protein) against destabilizing conditions during the lyophilization process.
For example, known
lyoprotectants include sugars (including glucose and sucrose); polyols
(including mannitol,
sorbitol and glycerol); and amino acids (including alanine, glycine and
giutamic acid).
Lyoprotectants can be included in an amount of about 10 mM to 500 nrvi,
In some embodiments, a subject formulation includes a subject antibody, and
one or more
of the above-identified agents (e.g., a surfactant, a buffer, a stabilizer, a
tonicity agent) and is
essentially free of one or more preservatives, such as ethanol, benzyl
alcohol, phenol, m-cresol,
p-chlor-m-cresol, methyl or propyi parabens, benzaikonium chloride, an'
combinations thereof_
in other embodiments, a preservative is included in the formulation, e.g., at
concentrations
ranging from about 0.001 to about .2% (wIv).
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
For example, a subject formulation can be a liquid or lyophilized formulation
suitable for
parenteral administration, and can comprise: about 1 mg/mt_ to about 200
mg/ml_ of a subject
antibody; about 0,001 % to about 1 % of at least one surfactant; about 1 mM to
about 100 mM of
a buffer; optionally about 10 .rnM to about 500 WO of a stabilizer; and about
5 mM to about 305
.. mM of a tonicity agent; and has a pH of about 4.0 to about 7Ø
A subject antibody can be utilized in aerosol formulation to be administered
via inhalation.
A subject antibody can be formulated into pressurized acceptable propetlants
such as
dichlorodifluorornethane, propane, nitrogen and the like.
The term "unit dosage form,' as used herein, refers to physically discrete
units suitable as
unitary dosages for human and animal subjects, each unit containing a
predetermined quantity of
compounds of the present invention calculated in an amount sufficient to
produce the desired
effect in association with a pharmaceutically acceptable diluent, carrier or
vehicle. The
specifications for a subject antibody may depend on the particular antibody
employed and the
effect to be achieved, and the pharmaroclynamics associated with each antibody
in the host,
A subject antibody can be administered as an injectable formulation,
Typically, injectable
compositions are prepared as liquid solutions or suspensions; solid forms
suitable for solution in,
or suspension in, liquid vehicles prior to injection may also be prepared. The
preparation may also
be emulsified or the antibody encapsulated in liposome vehicles.
Suitable excipient vehicles are, for example, water, saline, dextrose.,
glycerol, ethanol., or
.. the like, and combinations thereof. In addition, if desired, the vehicle
may contain minor amounts
of auxiliary substances such as wetting or emulsifying agents or pH buffering
agents. Actual
methods of preparing such dosage forms are known, or will be apparent, to
those skilled in the
art..
The pharmaceutically acceptable excipients, such as vehicles, adjuvants,
carriers or
diluents, are readily available to the public. Moreover, pharmaceutically
acceptable auxiliary
substances, such as pH adjusting and buffering agents, tonicity adjusting
agents, stabilizers,
wetting agents and the like, are readily available to the public.
In some embodiments, a subject antibody is formulated in a controlled release
formulation.
Sustained-release preparations may be prepared using methods well known in the
art.
Dosages
A suitable dosage can be determined by an attending physician or by other
qualified
medical personnel, based on various clinical factors. As is well known in the
medical arts., dosages
for any one patient depend upon many factors, including the patients size,
body surface area.,
age, the particular compound to be administered, sex of the patient, time, and
route of
46
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
administration, general health, and other drugs being administered
concurrently. A subject
antibody may be administered in amounts between 1 ng/kg body weight and 20
mg/kg body
weight per dose, e.g. between 0.1 mg/kg body weight to 10 mg/kg body weight,
e.g. between 0.5
mg/kg body weight to 5 mg/kg body weight; however, doses below or above this
exemplary range
are envisioned, especially considering the aforementioned factors. If the
regimen is a continuous
infusion, it can also be in the range of 1 ug to 10 mg per kilogram of body
weight per minute.
Those of skill will readily appreciate that dose levels can vary as a function
of the specific
antibody, the severity of the symptoms and the susceptibility of the subject
to side effects.
Preferred dosages for a given compound are readily determinable by those of
skill in the art by a
variety of means.
Routes of administration
A subject antibody is administered to an individual using any available method
and route
suitable for drug delivery, including in vivo and ex vivo methods, as well as
systemic and localized
routes of administration,
Conventional and pharmaceutically acceptable routes of administration include
intranasal,
intramuscular, intratracheal, subcutaneous, intradermal: topical application,
intravenous.
intraarterial: rectal, nasal, oral, and other enteral and parenteral routes of
administration. Routes
of administration may be combined: if desired, or adjusted depending upon the
antibody and/or
the desired effect, A subject antibody composition can be administered in a
single dose or in
multiple doses. In some embodiments, a subject antibody composition is
administered orally. In
some embodiments, a subject antibody composition is administered via an
inhalational route. In
some embodiments, a subject antibody composition is administered intranasally.
hi some
embodiments, a subject antibody composition is administered locally. In some
embodiments, a
subject antibody composition is administered intracranially. In some
embodiments, a subject
antibody composition is administered intravenously,
The agent can be administered to a host using any available conventional
methods and
routes suitable for delivery of conventional drugs, including systemic or
localized routes. In
general, routes of administration contemplated by the invention include, but
are not necessarily
limited to, enteral, parenteral, or inhalational routes,
Parenteral routes of administration other than inhalation administration
include, but are
not necessarily limited to, topical, transdermal, subcutaneous, intramuscular,
intraorbital,
intracapsular: intraspinal, intrasternal, and intravenous routes, i.e., any
route of administration
other than through the alimentary canal. Parenteral administration can be
carried to effect
systemic or local delivery of a subject antibody. Where systemic delivery is
desired, administration
47
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
typically involves invasive or systemically absorbed topical or mucosal
administration of
pharmaceutical preparations.
A subject antibody can also be delivered to the subject by enteral
administration. Enteral
routes of administration include, but are not necessarily limited to, oral and
rectal .(e.g., using a
.. suppository) delivery.
By treatment is meant at least an amelioration of the symptoms associated with
the
pathological condition afflicting the host, where amelioration is used in a
broad sense to refer to
at least a reduction in the magnitude of a parameter, _e.g. symptom,
associated with the
pathological condition being treated, such as cancer and/or the growth of a
cancer and pain
associated therewith. As such, treatment also includes situations where the
pathological
condition, or at least symptoms associated therewith, are completely
inhibited, .e.g. prevented
from happening, or stopped, e.g. terminated, such that the host no longer
suffers from the
pathological condition, or at least the symptoms that characterize the
pathological condition.
A variety of subjects (wherein the term "subject" is used interchangeably
herein with the
terms "individual" and 'patient") are treatable according to the presently
disclosed methods.
Generally, such subjects are "mammals" or "mammalian," where these terms are
used broadly to
describe organisms which are within the class ma.mmalia, including the orders
carnivore (e.g.,
dogs and cats), rodentia (e.g.., mice, guinea pigs, and rats), and primates
(e.g.., humans,
chimpanzees, and monkeys). In some embodiments, the hosts will be humans,
Kits with unit doses of a subject antibody, e.g. in oral or injectable doses,
are provided. In
some embodiments, in addition to the containers containing the unit doses will
be an informational
package insert describing the use and attendant benefits of the antibody in
treating pathological
condition of interest,
NUCLEIC ACIDS
The present disclosure provides nucleic acids comprising nucleotide sequences
encoding
a subject antibody. A nucleotide sequence encoding a subject antibody can be
operably linked to
one or more regulatory elements, such as a promoter and enhancer, that allow
expression of the
nucleotide sequence in the intended target cells (e.g., a cell that is
genetically modified to
synthesize and/or secrete the encoded antibody).
Suitable promoter and enhancer elements are known in the art. For expression
in a
bacterial cell, suitable promoters include, but are not limited to, lad, lea,
T3, T7, .gpt, lambda P
and trc. For expression in a eukaryotic cell, suitable promoters include., but
are not limited to, light
and/or heavy chain immunoglobulin gene promoter and enhancer elements;
.cytomegalovirus
48
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
immediate- early promoter; herpes simplex virus thymidi:ne kinase promoter;
early and late SV40
promoters; promoter present in long terminal repeats from a retrovirus; mouse
metallothionein-I
promoter; and various art-known tissue specific promoters.
A nucleotide sequence encoding a subject antibody can be present in an
expression
vector and/or a cloning vector. Where a subject antibody comprises two or more
separate
polypeptides, nucleotide sequences encoding the two polypeptides can be cloned
in the same or
separate vectors.. Separate polypeptides may be expressed from a single
nucleic acid or single
vector using various strategies, such as separate promoters, one or more
internal ribosomal entry
sites (IRES), one or more self-cleaving sequences (e.g.:, 2A cleavage
sequences, e.g., P2A, T2A,
E2A, and F2A)õ combinations thereof, and the like. An expression vector can
include a selectable
marker, an origin of re:plication, and other features that provide for
replication and/or maintenance
of the vector.
Large numbers of suitable vectors and promoters are known to those of skill in
the art;
many are commercially available for generating a subject recombinant
construct, The following
vectors are provided by way of example. Bacterial: pBs, phagescript, PsiX174,
oBluescript SK,
pBs KS, p:NH8a, p:NH16aõ pNH18a.õ pNH46a (Stratagene, La Jolla. Calif., USA);
pTrc99A,
pKK223-3, pKK233-3, pDR540, and pRIT5 (Pharmaciaõ. Uppsala, Sweden).
Eukaryotic: -pWL:neo,
pSV2catõ .p0G44, PXR1, pSG (Stratagene) pSVK:3, oBPV, pMSG and pSVL
(Pharmacia).
Expression vectors generally have convenient restriction sites located near
the promoter
sequence to provide for the insertion of nucleic acid sequences encoding
heterologous proteins.
A selectable marker operative in the expression host may be present. Suitable
expression vectors
include, but are not limited to, viral vectors (e.g. viral vectors based on
vaccinia virus; poliovirus;
adenovirus; adeno-associated virus; 5V40; herpes simplex virus; human
immunodeficiency virus;
a retroviral vector (e.g., Murine Leukemia Virus, spleen necrosis virus, and
vectors derived from
retroviruses such as Rous Sarcoma Virus, Harvey Sarcoma Virus, avian leukosis
virus, human
immunodeficiency virus, myeloproliferative sarcoma virus, and mammary tumor
virus); and the
like.
Nucleic acids, e.g., as described herein, may, in some instances, be
introduced into a cell,
e.g., by contacting the cell with the nucleic acid. Cells with introduced
nucleic acids will generally
be referred to herein as genetically modified cells. Various methods of
nucleic acid delivery may
be employed including but not limited to e.g., naked nucleic acid delivery,
viral delivery, chemical
transfection, brolistics, and the like.:
49
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
CELLS
The present disclosure provides isolated .genetically modified cells (e.g,, in
vitro cells, et
vivo cells, cultured cells, etc,) that are genetically modified with a subject
nucleic acid. In some
embodiments, a subject isolated genetically modified cell can produce a
subject antibody. In some
instances, a .genetically modified cell can deliver an antibody, e.g.., to a
subject in need thereof.
Suitable cells include eukaryotic cells, such as a mammalian cell, an insect
cell, a yeast
cell; and prokaryotic cells, such as a bacterial cell. Introduction of a
subject nucleic acid into the
host cell can be affected, for example by calcium phosphate .precipitation,
DEAE dextran
mediated transfection, liposome-mediated transfection, electroporation, or
other known method,
Suitable mammalian cells include primary cells and immortalized cell lines.
Suitable
mammalian cell lines include human cell lines, non-human primate cell lines,
rodent (e.g., mouse,
rat) cell lines, and the like. Suitable mammalian cell lines include, but are
not limited to, HeLa
cells, CHO cells, 293 cells, 3T3 cells, Vero cells, Huh-7 cells, BHK cells,
PC12 cells, COS cells,
COS-7 cells, RAT1 cells, mouse L cells, human embryonic kidney (HEK) cells,
HL.,HepG2 cells,
and the like,
In some instances, useful mammalian cells may include cells derived from a
mammalian.
tissue or organ. In some instances, cells employed are kidney cells, including
.e.g., kidney cells of
an established kidney cell :line, such as HEK 293T cells.
In some instances, cells of the present disclosure may be immune cells. As
used herein,
the term "immune cells" generally includes white blood cells (leukocytes)
which are derived from
hematopoietic stem cells (HSC) produced in the bone marrow. "Immune cells"
includes, e.g.,
lymphocytes (T cells, B cells, natural killer (NK) cells) and myeloid-derived
cells (neutrophil,
eosinophil, basophil, monocyte, macrophage, dendritic cells). "T cell"
includes all types of immune
cells expressing CD3 including 1-helper cells (CD4+ cells), cytotoxic 1-cells
(CD8+ cells), 1-
regulatory 'cells (Treg) and gamma-delta T cells, A "cytotoxic cell" includes
CD8+ T cells, natural-
killer (NK) cells, and neutrophils, which cells are capable of mediating
cytotoxicity responses.
In some instances, useful cells expressing an antibody such as a multi-
specific antibody
of the present disclosure may include producer T cells. Producer T cells
engineered to include
nucleic acid sequence encoding an antibody of the present disclosure may, in
some instances,
be employed to deliver the antibody to a subject in need thereof.
In some instances, immune cells of the present disclosure include immune
effector cell
comprising a chimeric antigen receptor (CAR) comprising an ABCC1 binding
domain, a
transmembrane domain, and an intracellular signaling domain, and wherein the
ABCC1 binding
domain comprises heavy chain complementarily determining regions (HCDRs) and
light chain
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
CDRs (LCDRs) of a pair of variable heavy chain (VI-I) region and variable
light chain (VL) region
of an antibody listed in Table 2. In one embodiment, the intraceilular
signaling domain may include
one or more functional signaling domains derived from at least one
costimulatory molecule, e.gõ
4-1BB (i.e., CD137), CD27 and/or CD28. The intracellular signaling domain may
include a
functional signaling domain derived from a costimulatory molecule and a
functional signaling
domain derived from a stimulatory molecule.
The immune effector cell may be a T-cell. The immune effector cell may be an
autologous
cell.
METHODS
As summarized above, methods of the present disclosure include methods of
contacting
a cell with an antibody of the present disclosure, methods of treating a
subject according to a
method that involves administering to the subject an antibody of the present
disclosure, methods
of making elements described in the instant application, including e.g.,
antibodies, compositions
and formulations, nucleic acids, expression vectors, cells, and the like.
As summarized above, methods of the present disclosure include contacting a
cancer cell
with an antibody of the present disclosure, e.g., to detect presence of
expression of ABCC1 on
the cancer cell, measure level of expression of ABCC1 on the cancer cell, or
to facilitate and/or
enhance killing of the cancer cell. In some instances, killing of the cancer
cell is mediated by an
immune response or immune cell acting upon the cancer cell bound by the
antibody. In some
instances, killing of the cancer cell is mediated by inhibition of cellular
efflux of the cancer cell,
e.g., as a result of ABCC1 inhibition by the antibody. In some instances,
killing of the cancer cell
is mediated by a combination of inhibition of cellular efflux of the cancer
cell plus an immune
mediated response (e.g., via Fe region of the antibody). Methods that involve
contacting a cancer
cell with an antibody of the present disclosure may or may not include
contacting the cancer cell
with an additional therapy or active agent, including e.g., a
chemotherapeutic, an immunotherapy,
radiation therapy, or the like.
Treatment Methods
The present disclosure provides methods of treating a cancer, the methods
generally
involving administering to an individual in need thereof (e.g., an individual
having a cancer) an
effective amount of an antibody as provided herein: alone (e.g., in
monotherapy) or in combination
(e.g., in combination therapy) with one or more additional therapeutic agents.
Administration of
51
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
an antibody of the present disclosure may be performed by any convenient and
appropriate route
of delivery.
Accordingly, administration includes but is not limited to e.g., delivery of
the antibody by
injection, delivery of the antibody by infusion, delivery of a. nucleic acid
or expression vector
encoding the antibody, delivery of the antibody by administering to the
subject a cell that
expresses and secretes the antibody, delivery of an immune effector cell
(e.g., a CAR-T cell) that
expresses on the cell surface a chimeric antigen receptor (CAR) comprising a
ABCC1 binding,
domain, a transrnembrane domain, and an intracellular signaling domain, and
wherein the ABCC1
binding domain comprises H.CDRs and LCDRs of a pair of VH region and \IL
region of an antibody
listed in Table 2, and the like. Administration of an agent, a nucleic acid
encoding an agent, a cell
expressing an agent, etc. may include contacting with the agent, contacting
with the nucleic acid,
contacting with the cell, and the like.
In some embodiments, an effective amount of a subject antibody is an amount
that, when
administered alone (e.g., in monotherapy) or in combination (e.g., in
combination therapy) with
one or more additional therapeutic agents, in one or more doses, is effective
to reduce an adverse
symptom of cancer by at least about 5%, at least about 10%, at least about
15%, at least about
20%, at least about 25%, at least about 30%, at least about 40%, at least
about 50%, at least
about 60%, at least about 70%, at least about 80%, at least about 90%, or
more, compared to the
severity of the adverse symptom in the absence of treatment with the antibody.
In some embodiments, an effective amount of a subject antibody is an amount
that, when
administered alone (e.g., in monotherapy) or in combination (e.g.õ in
combination therapy) with
one or more additional therapeutic agents, in one or more doses, is effective
to improve the cancer
0,e,, slow the growth of the cancer, stop the growth of the cancer, reverse
the growth of the
cancer, kill cancer cells (including tumor cells, or the like) in the
individual being treated. For
example, an, effective amount of a subject antibody can reduce a cancer growth
rate or reduce a
cancer size in an individual by at least about 5%, at least about 10%, at
least about 15%, at least
about 20%, at least about 25%, at least about 30%, at least about 40%, at
least about 50%, or
more, compared to in the absence of treatment with an antibody.
In some instances, a subject may be treated systemically, including with the
subject
antibody, with or without one or more additional reagents. By "systemic
treatment", as used
herein, is meant a treatment that is not directed solely to target a specific
tumor (such as e.g.:, a
primary tumor or a defined secondary tumor) or a specific cancer containing
tissue (such as e.g.,
the liver in the case of liver cancer, the blood in the case of a blood
cancer, eto). Systemic
treatments will generally be directed to the subject's body as a whole and may
include but are not
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
limited to .e.g., systemic radiation therapy, systemic chemotherapy; systemic
immunotherapy,
combinations thereof and the like.
In some instances, a subject may be treated locally, including with the
subject antibody,
with or without one or more additional reagents. By "local treatment", as used
herein, is meant a
treatment that is specifically directed to the location of a tumor (such as
e.g., a primary tumor or
a defined secondary tumor) or specifically directed to a cancer containing
tissue (such as e.g..,
the liver in the case of liver cancer, the blood in the case of a blood
cancer, etc.). In some
instances, local treatment may also be administered in such a way as to affect
the environment
surrounding a tumor, such as tissue surrounding the tumor, such as tissue
immediately adjacent
to the tumor. Local treatment will generally not affect or not be targeted to
tissues distant from the
site of cancer including the site of a tumor; such as a primary tumor. Useful
local treatments that
may be administered in addition to or in combination with a subject antibody,
.e.g., include but are
not limited to surgery, local radiation therapy, local cryotherapy, local
laser therapy, local topical
therapy, combinations thereof, and the like.
In some embodiments, a subject treatment method involves administering a
subject
antibody and one or more additional therapeutic agents. Suitable additional
therapeutic agents
include., but are not limited to, chemotherapeutic agents, radiation therapy
reagents,
im-munotherapy reagents, other antibody agents, and the like. Additional
therapies that may be
administered to a subject before, during or after a subject administering an
antibody of the present
disclosure will vary depending on numerous factors including the type of
cancer, the subject's
medical history, general state of health and/or any co-morbidities, and the
like. Useful cancer
therapies include but are not limited to
radiation therapy, chemotherapy, immunotherapy,
and the like.
Radiation therapy includes, but is not limited to, x-rays or gamma rays that
are delivered
from either an externally applied source such as a beam, or by implantation of
small radioactive
sources.
Suitable antibodies for use in cancer treatment include, but are not limited
to, naked
antibodies, e.g., trastuzumab (Herceptin) õ bevacizumab (AvastinTm), cetuximab
(Erbituxml.).,
panitumumab (Ve-ctibix), Ipilimurnab (Yervoyin, rituximab (Rituxan),
alemtuzumab
(Lemtradaim), Ofatumumab (Arzerram,), Oregovomab (OvaRexTm.), Lambroiizumab
(MK-3475),
pertuzumab (Perjetarm), ranibizumab (LucentisTm) etc., and conjugated
antibodies, e.g.,
gemtuzuMab ozogamicin (Mylortargml), Brentuxima. b vedotin (Adcetris)., 90Y-
labelled
ibritumomab tiuxetan (Zevalin I"), 131I-labelled tositumoma (Bexxar ), etc,
53
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
Suitable antibodies for use in cancer treatment also include, but are not
limited to,
antibodies raised against tumor-associated antigens. Such antigens include,
but are not limited
to, CD20, CD30, CD33, CD52, EpCAM, CEA, gpA33, Mucins, TAG-72, CAIX, PSMA,
Folate-
binding protein, Gangliosides (e.g., GD2, GD3, GM2, etc.), Ley, VEGF, VEGFR,
Integrin alpha-
V-beta-3, integrin alpha-5-beta-1, EGFR, ERBB2, ERBB3, MET, IGF1R, EPHA3,
TRAlLR1
TRAILR2, RANKL, FAP, Tenascin, Programmed Death-Ligand 1 (PD-L1), androgen
receptor
(AR), Bruton's Tyrosine Kinase (BTK), BCR-Abl, c-kit, PIK3CA, EML4-ALK, KRAS,
ALK, ROS1..
AKT1, BRAF, tvIEKJ, MEK2, NRAS, RA01, ESR1, CTLA-4, LAG-3 and TIM-3, etc.
These
antibodies may be administered as a combination therapy with an anti-ABCC1
antibody provided
herein,
Conventional cancer therapies also include targeted therapies for cancer
including but not
limited to e.g., Ado-trastuzumab emtansine (Kadcyla) targeting HER2
(ERBB2Ineu) (approved for
use in Breast cancer); Afatinib (Gilotrif) targeting EGFR (HER1/ER8B1), HER2
(ERBB2/neu)
(approved for use in Non-small cell lung cancer); Aldesleukin (Proleukin)
targeting (approved for
use in Renal cell carcinoma, Melanoma); Alectinib (Alecensa) targeting ALK
(approved for use in
Non-small cell lung cancer); Alemtuzurnab (Campath) targeting CD52 (approved
for use in 6-cell
chronic lymphocytic leukemia); Atezolizumab (Tecentriq) targeting PD-L1
(approved for use in
Urothelial carcinoma, Non-small cell lung cancer); Avelumab (Bavencio)
targeting PD-L1
(approved for use in Merkel cell carcinoma); Axitinib (Inlyta) targeting KIT,
PDGFRp, VEGFR1/2/3
(approved for use in Renal cell carcinoma); Belimumab (Benlysta) targeting
GAFF (approved for
use in Lupus erythematosus); Belinostat (Beleodaq) targeting HDAC (approved
for use in
Peripheral T-cell lymphoma); Bevacizumab (Avastin) targeting VEGF ligand
(approved for use in
Cervical cancer, Colorectal cancer, Fallopian tube cancer, Glioblastoma, Non-
small cell lung
cancer, Ovarian cancer, Peritoneal cancer, Renal cell carcinoma); Blinatumomab
(Blincyto)
targeting CD19/CD3 (approved for use in Acute lymphoblastic leukemia
(precursor B-cell));
Bortezomib (Veleade) targeting Proteasome (approved for use in Multiple
myeloma, Mantle cell
lymphoma); Bosutinib (Bosulif) targeting ABL (approved for use in Chronic
myeiogenous
leukemia); Brentuximab vedotin (Adcetris) targeting CD30 (approved for use in
Hodgkin
lymphoma, Anaplastic large cell lymphoma); Brigatinib (Alunbrig) targeting ALK
(approved for use
in Non-small cell lung cancer (ALK+)); Cabozantinib (Cabometyx, Cometrig)
targeting FLT3, KIT.,
MET, RET, VEGFR2 (approved for use in Medullary thyroid cancer, Renal cell
carcinoma);
Carfilzomib (Kyprolis) targeting Proteasome (approved for use in Multiple
myeloma); Ceritinib
(Zykadia) targeting ALK (approved for use in Non-small cell lung cancer);
Cetuximab (Erbitux)
targeting EGFR (HERVERBB1) (approved for use in Colorectal cancer, Squamous
cell cancer of
54
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
the head and neck); Cobimetinib (Cotellic) targeting MEK (approved for use in
Melanoma);
Crizotinib (Xalkori) targeting ALK, MET, ROS1 (approved for use in Non-small
cell lung cancer):
Dabrafenib (Tat nlar) targeting BRAE (approved for use in Melanoma, Non-small
cell lung cancer);
Daratumumab (Darzalex) targeting CD38 (approved for use in Multiple rnyeloma);
Dasatinib
(Sprycel) targeting ABL (approved for use in Chronic myelogenous leukemia,
Acute iymphoblastic
leukemia); Denosurnab (Xgeva) targeting RANKL (approved for use in Giant cell
tumor of the
bone); Dinutuximab (Unituxin) targeting B4GALNT1 (GD2) (approved for use in
Pediatric
neuroblastoma); Durvalumab (Imfinzi) targeting PD-Ll (approved for use in
Urothelial carcinoma);
Elotuzumab (Empliciti) targeting SLAMF7 (CS1/CD319/CRACC) (approved for use in
Multiple
myeloma); Enasidenib (White) targeting IDH2 (approved for use in Acute myeloid
leukemia);
Erlotinib (Tarceva) targeting EGFR (HER1/ERBB1) (approved for use in Non-small
cell lung
cancer, Pancreatic cancer); Everolimus (Afinitar) targeting mTOR (approved for
use in Pancreatic,
gastrointestinal: or lung origin neuroendocrine tumor, Renal cell carcinoma:
Nonresectabie
subependymal giant cell astrocytoma, Breast cancer); Gefitinib (Iressa)
targeting EGFR
(HER1ERBB1) (approved for use in Non-small cell lung cancer); Ibriturnomab
tiuxetan (Zevalin)
targeting CD20 (approved for use in Non-Hodgkin's lymphoma); lbrutinib
(Imbruvica) targeting
BTK (approved for use in Mantle cell lymphoma, Chronic lymphocytic leukemia:
Waldenstrom's
macroglobulinemia); Idelalisib (Zydelig) targeting PI3K6 (approved for use in
Chronic lymphocytic
leukemia, Follicular B-cell non-Hodgkin lymphoma, Small lymphocytic lymphoma):
Irnatinib
(Gleevec) targeting KiT, PDGFR, ABL (approved for use in GI stromal tumor
(KIT+),
Dermatofibrosarcoma protuberans, Multiple hematologic malignancies);
1pilimumab (Yervoy)
targeting CTLA-4 (approved for use in Melanoma); kazonnib (Ninlaro) targeting
Proteasome
(approved for use in Multiple Myeloma); Lapatinib (Tykerb) targeting HER2
(ERBB2ineu): EGFR
(HERVERBB1) (approved for use in Breast cancer (HER2+)); Lenvatinib (Lenvima)
targeting
VEGFR2 (approved for use in Renal cell carcinoma, Thyroid cancer); Midostaurin
(Rydapt)
targeting FLT3 (approved for use in acute myeloid leukemia (FLT3+));
Necitumumab (Portrazza)
targeting EGFR (HERVERBB1) (approved for use in Squamous non-small cell lung
cancer):
Neratinib (Nerlynx) targeting HER2 (ERBB2/neu) (approved for use in Breast
cancer); Nilotinib
(Tasigna) targeting ABL (approved for use in Chronic myelogenous leukemia);
Niraparib (Zejula)
targeting PARP (approved for use in Ovarian cancer, Fallopian tube cancer:
Peritoneal cancer):
Nivolumab (Opdivo) targeting PD-1 (approved for use in Colorectal cancer, Head
and neck
squamous cell carcinoma, Hodgkin lymphoma: Melanoma: Non-small cell lung
cancer, Renal cell
carcinoma, Urothelial carcinoma); Obinutuzumab (Gazyva) targeting CD20
(approved for use in
Chronic lymphocytic leukemia, Follicular lymphoma); Ofatumurnab (Arzerra,
Hurvlax-CD20)
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
targeting CD20 (approved for use in Chronic lymphocytic leukemia); Olaparib
(Lynparza) targeting
PARP (approved for use in Ovarian cancer); Olaratumab (Lartruvo) targeting
PDGFRa (approved
for use in Soft tissue sarcoma); Osimertinib (Tagrisso) targeting EGFR
(approved for use in Non-
small cell lung cancer); Palbociclib (lbrance) targeting CDK4, CDK6 (approved
for use in Breast
cancer); Panitumumab (Vectibix) targeting EGFR (HER VERBB1) (approved for use
in Colorectal
cancer); Panobinostat (Farydak) targeting HDAC (approved for use in Multiple
myeloma);
Pazopanib (Votrient) targeting VEGFR, PDGFR, KIT (approved for use in Renal
cell carcinoma);
Perribrolizumab (Keytruda) targeting PD-1 (approved for use in Ciassicai
Hodgkin lymphoma,
Melanoma, Non-small cell lung cancer (PD-L1+), Head and neck sguamous cell
carcinoma, Solid
tumors (MSI-H)); Pertuzumab (Perjeta) targeting HER2 (ERBB2ineu) (approved for
use in Breast
cancer (HER2+)); Ponatinib (Iclusig) targeting ABL, EGFR1-3, FLT3, VEGFR2
(approved for use
in Chronic myelogenous leukemia, Acute lymphoblastic leukemia); Ramucirumab
(Cyrarnza)
targeting VEGFR2 (approved for use in Colorectal cancer: Gastric cancer or
Gastroesophageal
junction (GEJ) adenocarcinoma, Non-small cell lung cancer); Regorafenib
(Stivarga) targeting
.. Kn., PDGFR, RAF, RET, VEGFR11213 (approved for use in Colorectal cancer,
Gastrointestinal
stromal tumors, Hepatocellular carcinoma); Ribociclib (Kisciali) targeting
CDK4, CDK6 (approved
for use in Breast cancer (HR+, HER2-)); Rituximab (Rituxan, Mabthera)
targeting CD20 (approved
for use in Non-Hodgkin's lymphoma, Chronic lymphocytic leukemia, Rheumatoid
arthritis,
Granuiomatosis with polyangiitis); Rituximablhyaluronidase human (Rituxan
Hycela) targeting
CD20 (approved for use in Chronic lymphocytic leukemia, Diffuse large B-cell
lymphoma;
Follicular lymphoma); Romidepsin (Istodax) targeting HDAC (approved for use in
Cutaneous T-
cell lymphoma, Peripheral 1-cell lymphoma); Rucaparib (Rubraca) targeting PARP
(approved for
use in Ovarian cancer); Ruxolitinib (Jakafi) targeting JAK1/2 (approved for
use in Myelofibrosis);
Siltuximab (Sylvant) targeting 1L-6 (approved for use in Multicentric
Castleman's disease);
Sipuleucel-T (Provenge) targeting (approved for use in Prostate cancer);
Sonidegib (Odomzo)
targeting Smoothened (approved br use in Basal cell carcinoma); Sorafenib
(Nexavar) targeting
VEGFR, PDGFR, KIT, RAF (approved for use in Hepatocellular carcinoma, Renal
cell carcinomas
Thyroid carcinoma); Temsirolimus (Torisel) targeting mTOR (approved for use in
Renal cell
carcinoma); Tositumomab (Bexxar) targeting CD20 (approved for use in Non-
Hodgkin's
lymphoma); Trametinib (Mekinist) targeting MEK (approved for use in Melanoma,
Non-smali cell
lung cancer); Trastuzurnab (Herceptin) targeting HER2 (ERBB2ineu) (approved
for use in Breast
cancer (HER2+), Gastric cancer (HER2+)); Van detanib (Caprelsa) targeting EGFR
(HER1IERBB1), RET, VEGFR2 (approved for use in Medullary thyroid cancer);
Vemurafenib
(Zelboraf) targeting BRAF (approved for use in Melanoma); Venetoclax
(Venclexta) targeting
56
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
BC1..2 (approved for use in Chronic lymphocytic leukemia); Vism-odegib
(Erivedge) targeting
PTCH, Smoothened (approved for use in Basal cell carcinoma); Vorinostat
(Zolinza) targeting
HDAC (approved for use in Cutaneous T-cell lymphoma): Ziv-aflibercept
(Zaltra.p) targeting PIGF,
VEGFA/B (approved for use in Colorectal cancer); and the like. These
antibodies may be
administered as a combination therapy with an anti-ABCC1 antibody Provided
herein,
Biological response modifiers suitable for use in connection with the methods
of the
present disclosure include, but are not limited to, (1) inhibitors of tyrosine
kinase (RTK) activity;
(2) inhibitors of serineithreonine kina.se activity; (3) tumor-associated
antigen antagonists, such
as antibodies that bind specifically to a tumor antigen; ( 4) apoptosis
receptor agonists; (5)
interleukin-2; (6) interfaron-d4 (7) interferon-y; (8) colony-stimulating
factors, (9) inhibitors of
angiogenesis; and (10) antagonists of tumor necrosis factor.
Chemotherapeutic agents or antineoplastic agents are non-peptidic (i.e., non-
proteinaceous) compounds that reduce proliferation of cancer cells, and
encompass cytotoxic
agents and cytostatic agents. Non-limiting examples of chemotherapeutic agents
include
alkylating agents (e.g., nitrosourea:s), -antimetabolites (e.g.,
methotrex.ate), antitumor antibiotics
(e.g., anthracyclins), plant alkaloids
vinca alkaloids, ta.xanes, etc.), toposiomerase inhibitors,
and steroid hormones.
Agents that act to reduce cellular proliferation are known in the art and
widely used. Such
agents include alkylating agents, such as nitrogen mustards, .nitrosoureas,
ethyienimine
derivatives, alkyl sultanates, and triazenes, including, but not limited to,
mechlorethamine,
cyclophosphamide (Cytoxan'"'), melphalan (L-sarcolysin), carmustine (BCNU),
lomustine
(CCNU), semustine (methyl-CCNU), streptozocin, chlorozotocin, uracil mustard,
chlormethine,
ifostamideõ chloram-bucit, pipobroman, triethylenemelamine,
triethylenethiophosphoramine,
busulfan, dacarbazine, and temozolomide,
Antimetabolite agents include folic acid analogs, pyrimidine analogs, purine
analogs, and.
adenosine -deaminase inhibitors, including, but not limited to, cytarabine
(CYTOSAR-U), cytosine
arabi:noside: fluorouracil (5-FU), fioxuridine (FudR), 6-thioguanine, 6-
mercaptoputine (8-MP),
pentostatin, 5-fluorouracil (5-FU), methotrexate, 10-propargy1-5,6-
didea.zafolate (PDDF,
CB3717), 5,8-dideazatetrahydrofolic acid (DDATHF), leucovorin, .fludarabine
phosphate,
pentostatine, and gemcitabine.
Suitable natural products and their derivatives, (e.g., vinca alkaloids:
antitumor antibiotics,
enzymes, lymphokines, and epipodophyllotoxins), include, but are not limited
to, Ara-C, paclitaxel
(Taxole)õ docetaxel (Taxoteree), deoxycoformycin, mitomycin-C. L-asparaginase,
azathioprine;
brequinar; alkaloids, e.g, vincristine, vinblastine, vinorelbine, vindesine,
etc.; podophyllotoxins,
57
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
e.g. etoposide, teniposide, etc.; antibiotics, e.g. anthracycline,
daunorubioin hydrochloride
(daunomycin, wbidomycin, cerubidine), idarubicin, doxorubicin, epirubicin and
morpholino
derivatives, etc.: phenoxizone biscyclopeptides, e.g. dactinomycin; basic
glycopeptides, e.g.
.bleomycin; anthraquinone glycosides, e.g. plicamycin (mithram-ycin);
anthracenediones, e.g.
mitoxantrone; azirinopyrrolo indoiediones, e.g. mitomycin; macrocyclic
immunosuppressants, e.-g...
cyclosporineõ FK-506 (tacrolimusõ prograf), rapamycin, etc.; and the like.
Other anti-proliferative -cytotoxic agents are navelbene, CPT-11, anastrazole,
letrazole,
capecitabine, reloxafine, -cyclophosphamide, ifosamide, and droloxafine.
Microtubule affecting agents that have antiproliferative activity are also
suitable for use
and include, but are not limited to, allocolchicine (NSC 406042), Halichondrin
B (NSC 609395),
colchicine (NSC 757), colchicine derivatives (e.g., NSC 33410), doistatin 10
(NSC 376128),
maytansine (NSC 153858), rhizoxin (NSC 332598), paclitaxel (Taxo10), Ta.xola
derivatives,
docetaxel (Taxotere0), thiocolchloihe (NSC 361792), trityl oysterin,
vinblastine sulfate, vincristine
sulfate, natural and synthetic epothilones including but not limited to,
eopthilone A, epothilone B.
discodermolide; estramustine, nocodazoleõ and the like.
Hormone modulators and steroids (including synthetic analogs) that are
suitable for use
include., but are not limited to, adrenocorticosteroids, e.g.. prednisone,
dexamethasone, etc.;
estrogens and pre-gestins, e.g.. hydroxyprogesterone caproate,
medroxyprogesterone acetate,
megestrol acetate, estradiol, clomiphene, tamoxifen; etc.; and adren.ocortical
suppressants, e.g.
aminoglutethimide; 17a-ethinyle.stradiol, diethylstilbestrol, testosterone,
fluoxymesterone,
dromostanolone propionate, testolactone, methylprednisolone, methyl-
testosterone,
prednisolone, triamcinolone-, chlorotrianisene, hydroxyprogesterone,
aminoglutethimide,
estramustineõ medroxyprcgesterone acetate, leuprolide, Flutamide (Drogertil).
Toremifene
(Fareston), and Zoladex. Estrogens stimulate proliferation and
differentiation, therefore
compounds that bind to the estrogen receptor are used to block this activity.
Corticosteroids may
inhibit I cell proliferation.
Other chemotherapeutic agents include metal complexes, e.g. cisplatin (cis-
DDP),
carboplatin, etc.; ureas, e.g. hydroxyurea; and hydra.zines, e.g. N-
methylhydrazine;
epidophyllotoxin; a topoisomerase inhibitor; procarba.zine; mitoxantro-ne;
leucovorin, tegafur; etc.
Other anti-proliferative agents of interest include immunosuppressants, e.g.
myco,phenolic acid,
thalidomide, deso.xyspergualin, azasporine, leflunomide, mizoribine, aza-
spirane (SKF 105685);
IressaV (ZD 1839, 4-(3-chioro-4-fluorophenylamino)-7-
methoxy-6-(3-(4-
morpholinyl)propoxy)quinazoline), etc
58
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
"Taxanes" include paclitaxel, as well as any active taxane derivative or pro-
drug.
"Paclitaxel" (which should be understood herein to include analogues,
formulations, and
derivatives such as, for example, docetaxel, TAXOLT, TAXOTEREr''' (a
formulation of
docetaxel), 10-desacetyl analogs of paclitaxel and 3'N-desbenzoy1-3'N-t-
butoxycarbonyl analogs
of paclitaxel) may be ready prepared utilizing techniques known to those
skilled in the art (see
also WO 94/07882, WO 94/07881, WO 94/07880, WO 94/07876, WO 93/23555, WO
93/10076;
U.S. Pat. Nos. 5,294,637; 5,283,253; 5,279,949; 5,274,137; 5,202,448;
5,200,534; 5,229,529:
and EP 590,287), or obtained from a variety of commercial sources, including
for example, Sigma
Chemical Co., St. Louis, Mo. (T7402 from Taxus brevifolia; or T-1912 from
Taxus yannanensis).
Paclitaxel should be understood to refer to not only the common chemically
available form
of paclitaxel, but analogs and derivatives (e.g., Taxoterem' docetaxel, as
noted above) and
paclitaxel conjugates (e.g., paclitaxel-PEG, paclitaxel-dextran, paclitaxel-
xylose, or protein bound
paclitaxel such as Abraxanee).
Also included within the term "taxane are a variety of known derivatives,
including both
hydrophilic derivatives, and hydrophobic derivatives. Taxane derivatives
include, but are not
limited to, galactose and mannose derivatives described in International
Patent Application No.
WO 99/18113; piperazino and other derivatives described in WO 99/14209; taxane
derivatives
described in WO 99/09021, WO 98/22451, and U.S. Patent No. 5,869,680; 6-thio
derivatives
described in WO 98/28288; sulfenamide derivatives described in U.S. Patent No
5,821,263; and
taxol derivative described in U.S. Patent Na 5,415,869. It further includes
prodrugs of paclitaxel
including, but not limited to, those described in WO 98/58927; WO 98/13059;
and U.S, Patent No.
5,824,701.
Useful immunotherapies include anti-PD-1/PD-L1 immunotherapies, and/or other
immunotherapy targets, such as e.g., immune check point markers, such as CTLA-
4, LAG-3 and
TIM-3, that may be targeted in treatment methods. Anti-PD-1 /PD-L1
immunotherapies which
include but are not limited to e.g., those therapies that include
administering to a subject an
effective amount of one or more anti-PD-1/PD-L1 therapeutic antagonists where
such antagonists
include but are not limited to e.gõ OPDIVO (nivolumab), KEYTRUDA,1)
(pembrolizumab),
Tecentriom, (atezolizurnab), durvalumab (MEDI4736), avelumab (MSB0010718C),
BMS-936559
(MDX-1105), CA-170, BMS-202, BMS-8, BmS-37, BMS-242 and the like. These
antibodies may
be administered as a combination therapy with an anti-ABCC1 antibody provided
herein.
CTLA-4, also known as CD152, binds to CD80 and CD86. Antibodies against CTLA-4
have been approved for treating some cancer types. The co-inhibitory effect of
CTLA-4 with other
immunotherapies make CTLA-4 a good candidate for use in combination with other
59
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
immunotherapies to treat certain: cancers. TIM-3 may also be targeted for
immunotherapy for
several cancer types.
LAG-3 is in clinical trials for treating cancers. Mb-LAG-3 immunotherapies
include those
that employ antagonist LAG-3 antibodies that can both activate T effector
cells (by downregulating
the LAG-3 inhibiting signal into pre-activated LAG-3+ cells) and inhibit
induced (iõe, antigen-
specific) Treg suppressive activity. Useful LAG-3 antagonistic antibodies
include relatlimab (BMS-
986016: developed by Bristol-Myers Squibb), IMP701 (developed by immutep), TSR-
033 (anti-
LAG-3 mAb: developed by TESARO, Inc.), and the like.
Immunotherapies aiso include T cell-based immunotherapies such as e.g.,
adoptive cell
therapy (ACT) and .chimeric antigen receptor (CAR) T cell therapies. For
example, a subject may
be administered a population of CAR T cells engineered to target an antigen
expressed by the
subject's cancer. A T cell-based therapy may involve, in some instances,
obtaining a cellular.
sample from a subject, such as a blood sample or a tumor biopsy, and culturing
immune cells
from the sample ex vivo, with or without genetic modification of the cultured
immune cells, As an
example, immune cells may be obtained from a subject, cultured ex vivo and
modified with a CAR
specific for an antigen expressed by the cancer to produce a population of CAR
T cells. Then, the
CAR T cells may be reintroduced into the subject to target the cancer. T cell-
based
im-munotherapies may be configured in various ways, e.g.., by targeting
various antigens, by
collecting/culturing various cell types, etc.., depending on a particular
cancer to be treated. In
addition, T cell-based immunotherapies may be administered systemically,
.e.g.., by intravenous
injection, or locally, e.g., by infusion (e.g., intraperitoneal infusion,
pleural catheter infusion, etc.),
direct injection, and the like.
In some instances, a method of treatment described herein may include
administering to
a subject one or more inhibitors of a multidrug resistance transporter,
including but not limited to
e.g., a multidrug resistance transporter other than ABCC1 Useful inhibitors of
multidrug
resistance .transporters include e.g., tyrosine kinase inhibitors, natural
products, microRNAs, and
small molecule inhibitors. Inhibitors of multidrug resistance transporters
Include ABC transporter
inhibitors,
Individuals suitable for treatment using a method of the present disclosure
include an
individual having a cancer; an individual diagnosed as having a cancer; an
individual being treated
for a cancer with chemotherapy, radiation therapy, antibody therapy, surgery,
etc.): an individual
who has been treated for a cancer (e,g.õ with one or more of chemotherapy,
radiation therapy.
antibody therapy, surgery, etc.), and who has failed to respond to the
treatment: an individual who
has been treated for a cancer (e.g,, with one or more of chemotherapy,
radiation therapy, antibody
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
therapy, surgery, etc.), and who initially responded to the treatment but who
subsequently
relapsed, Lea the cancer recurred.
The methods of the present disclosure may be employed to target and treat a
variety of
cancers, including e.g., primary cancer, secondary cancers, re-growing
cancers, recurrent
cancers, refractory cancers and the like, For example, in some instances, the
methods of the
present disclosure may be employed as an initial treatment of a primary cancer
identified in a
subject. In some instances, the methods of the present. disclosure may be
employed as a non-
primary (e.g., secondary or later) treatment, e.g.: in a subject with a cancer
that is refractory to a
prior treatment, in a subject with a cancer that is re-growing following a
prior treatment, in a subject
with a mixed response to a prior treatment (e.g., a positive response to at
least one tumor in the
subject and a negative or neutral response to at least a second tumor in the
subject), and the like.
In some instances,, the methods of the present disclosure may be employed to
treat a
subject with a drug resistant cancer, such as a multi-drug resistant cancer.
Multidrug resistance
(MDR) is the mechanism by which many cancers develop resistance to
chemotherapy drugs,
resulting in minimal cell death and the expansion of drug-resistant tumors,
MDR cancers may
involve one or more resistance mechanisms including but not limited to e.g.,
increased expression.
of efflux pumps, decreased absorption of drug, inhibition of cell death or
apoptosis, modulating
drug metabolism, and the like., In some instances, the methods of the present
disclosure may
prevent, reverse or circumvent MDR.
In some instances, methods of the present disclosure may include treating a
subject
having a cancer that is resistant to a first agent with an effective amount of
a subject antibody
described herein in combination with a second agent that is different from the
first agent. For
example, in some instances, cancer of a subject may be resistant to a first
chemotherapeutic and
the subject may be treated by administering an effective amount of a subject
antibody as
described herein in combination with a second chemotherapeutic that is
different from the first.
Various combinations of first and second chemotherapeutics may be employed
depending on.
e.g., the type of cancer to be treated, the likelihood of developing
resistance, etc.
Numerous cancers are known to develop drug resistance. For this and other
reasons the
methods of the present disclosure may find use in treating various cancers
including but not
limited to, e.g., Acute .Lymphoblastic Leukemia (ALL), Acute Myeloid Leukemia
(AML),
Adrenocortical Carcinoma, AIDS-Related Cancers .(e.g., Kaposi Sarcoma,
Lymphoma, etc.), Anal
Cancer: Appendix Cancer, Astrocytomas, Atypical Teratoid/Rhabdoid Tumor, Basal
Cell
Carcinoma, Bile Duct Cancer (Extrahepatic), Bladder Cancer, Bone Cancer (e.g.,
Ewing
Sarcoma, Osteosarcoma and Malignant Fibrous Histiocytoma, etc.), Brain Stern
Glioma, Brain
61
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
Tumors (e.g., Astrocytomas, Central Nervous System Embryonal Tumors, Central
Nervous
System Germ Cell Tumors, Craniopharyngioma, Ependymoma, etc.), Breast Cancer
(e.g., female
breast cancer, male breast cancer, childhood breast cancer, etc.), Bronchial
Tumors, Burkitt
Lymphoma, Carcinoid Tumor (e.g,, Childhood, Gastrointestinal, etc.), Carcinoma
of Unknown
Primary, Cardiac (Heart) Tumors, Central Nervous System (e.g., Atypical
TeratoldiRhabdold
Tumor, Embryonal Tumors, Germ Cell Tumor, Lymphoma, etc.), Cervical Cancer,
Childhood
Cancers, Chordoma, Chronic Lymphocyte Leukemia (CLL), Chronic Myelogenous
Leukemia
(CML), Chronic Myeloproliferative Neoplasms, Colon Cancer, Colorectal Cancer,
Craniopharyngioma, Cutaneous T-Cell Lymphoma, Duct (e.g., Bile Duct,
Extrahepatic, etc.),
Ductal Carcinoma In Situ (DCIS), Embryonal Tumors, Enclometrial Cancer,
Ependymoma,
Esophageal Cancer, Esthesioneuroblastoma, Ewing Sarcoma, Extracranial Germ
Cell Tumor,
Extragonadal Germ Cell Tumor, Extrahepatic Bile Duct Cancer, Eye Cancer (e.g.,
intraocular
Melanoma, Retinoblastoma, etc.), Fibrous Histiocytoma of Bone (e.g..
Malignant, Osteosarcoma,
ect,), Gallbladder Cancer, Gastric (Stomach) Cancer, Gastrointestinal
Carcinoid Tumor,
Gastrointestinal Strome! Tumors (GIST), Germ Cell Tumor (e.g., Extracranial,
Extragonadal,
Ovarian, Testicular, etc.), Gestational Trophoblastic Disease, Glioma, Hairy
Cell Leukemia, Head
and Neck Cancer, Heart Cancer, Hepatocellular (Liver) Cancer, Histioeytosis
(e.g., Langerhans
Cell, etc.), Hodgkin Lymphoma, Hypopharyngeal Cancer, Intraocular Melanoma,
Islet Cell
Tumors (e.g., Pancreatic Neuroendocrine Tumors, etc.), Kaposi Sarcoma, Kidney
Cancer (e.g.,
Renal Cell, Wilms Tumor, Childhood Kidney Tumors, etc.), Langerhans Cell
Histiocytosis,
Laryngeal Cancer, Leukemia (e.g., Acute Lyrnphoblastic (ALL), Acute Myeloid
(AML), Chronic
Lymphocyte (CLL), Chronic Myelogenous (CML), Hairy Cell, etc.), Lip and Oral
Cavity Cancer,
Liver Cancer (Primary), Lobular Carcinoma In Situ (LCIS), Lung Cancer (e.g.,
Non-Small Cell,
Small Cell, etc.), Lymphoma (e.g., AIDS-Related, Burkitt, Cutaneous T-Cell,
Hodgkin, Non.
Hodgkin, Primary Central Nervous System (CNS), etc.), Macroglobulinemia (e.g.,
Waldenstrom,
etc.), Male Breast Cancer, Malignant Fibrous Histiocytoma of Bone and
Osteosarcoma,
Melanoma, Merkel Cell Carcinoma, Mesothelloma, Metastatic Squamous Neck Cancer
with
Occult Primary, Midline Tract Carcinoma Involving NUT Gene, Mouth Cancer,
Multiple Endocrine
Neoplasia Syndromes, Multiple MyelomaiPlasma Cell Neoplasm, Mycosis Fungoides,
Myelodysplastic Syndromes, MyelodysplasticiMyeloproliferative Neoplasms,
Myelogenous
Leukemia (e.g., Chronic (CML), etc.), Myeloid Leukemia (e.g., Acute (AML),
etc.),
Myeloproliferative Neoplasms (e.g., Chronic, etc.), Nasal Cavity and Paranasal
Sinus Cancer,
Nasopharyngeal Cancer, Neuroblastoma, Non-Hodgkin Lymphoma, Non-Small Cell
Lung
Cancer, Oral Cancer, Oral Cavity Cancer (e.g., Lip, etc.), Oropharyngeal
Cancer, Osteosarcoma
62
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
and Malignant Fibrous Histiocytorna of Bone, Ovarian Cancer (e.g., Epithelial,
Germ Cell Tumor,
Low Malignant Potential Tumor, etc.), Pancreatic Cancer, Pancreatic
Neuroendocrine Tumors
(Islet Cell Tumors), Papillomatosis, Paraganglioma, Paranasal Sinus and Nasal
Cavity Cancer,
Parathyroid Cancer, Penile Cancer, Pharyngeal Cancer, Pheochromocytoma,
Pituitary Tumor,
Pleuropulrnonary Blastoma, Primary Central Nervous System (CNS) Lymphoma,
Prostate
Cancer, Rectal Cancer, Renal Cell (Kidney) Cancer, Renal Pelvis and Ureter,
Transitional Cell
Cancer, Retinoblastoma, Rhabdomyosarcoma, Salivary Gland Cancer, Sarcoma
(e.g., Ewing,
Kaposi, Osteosarcoma, Rhabdornyosarcoma, Soft Tissue, Uterine, etc.), Sezary
Syndrome, Skin
Cancer (e.g., Childhood, Melanoma, Merkel Cell Carcinoma, Nonmelanoma, etc.),
Small Cell
Lung Cancer, Small Intestine Cancer, Soft Tissue Sarcoma, Squamous Cell
Carcinoma,
Squarnous Neck Cancer (e.g., with Occult Primary, Metastatic, etc,), Stomach
(Gastric) Cancer,
T-Cell Lymphoma, Testicular Cancer, Throat Cancer, Thymoma and Thymic
Carcinoma, Thyroid
Cancer, Transitional Cell Cancer of the Renal Pelvis and Ureter, Ureter and
Renal Pelvis Cancer,
Urethral Cancer, Uterine Cancer (e.g., Endometrial, etc.), Uterine Sarcoma,
Vaginal Cancer,
Vulvar Cancer, Waldenstrom Macrogrobulinemia, Wilms Tumor, and the like.
The methods of treating described herein may, in some instances, be performed
in a
subject that has previously undergone one or more conventional treatments. For
example, in the
case of oncology, the methods described herein may, in some instances, be
performed following
a conventional cancer therapy including but not limited to e.g., conventional
chemotherapy,
conventional radiation therapy, conventional immunotheraoy, surgery, etc. In
some instances, the
methods described herein may be used when a subject has not responded to or is
refractory to a
conventional therapy. In some instances, the methods described herein may be
used when a
subject has responded to a conventional therapy.
In some instances, the method of the present disclosure may be employed to
target, treat
or clear a subject for minimal residual disease (MRD) remaining after a prior
cancer therapy,
Targeting, treating and/or clearance or MRD may be pursued using the instant
methods whether
the MRD is or has been determined to be refractory to the prior treatment or
not. In some
instances, a method of the present disclosure may be employed to target, treat
and/or clear a
subject of MRD following a determination that the MRD is refractory to a prior
treatment or one or
more available treatment options other than those employing the herein
described multi-specific
antibodies.
In some instances, the instant methods may be employed prophylactically for
surveillance.
For example. a subject in need thereof may be administered a treatment
involving one or more of
the herein described antibodies when the subject does not have detectable
disease but is at risk
63
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
of developing a recurrent cancer, including e.g., a drug resistant cancer. In
some instances, a
prophylactic approach may be employed when a subject is at particularly high
risk of developing
a primary cancer that would be predicted to be drug resistant or expected to
become drug
resistant. in some instances, a prophylactic approach may be employed when a
subject has been
previously treated for a cancer and is at risk of reoccurrence or development
of drug resistance.
In some instances, methods of the present disclosure may involve analyzing a
cancer for
expression of one or more markers or therapeutic targets. For example, in some
instances,
methods may involve a.nalyzing a sample of a cancer from a subject to
determine whether the
cancer expresses .ABCC1 above a predetermined threshold.
In son-le instances, whether a subject is treated with an antibody of the
present disclosure
may depend on the results of ABCC1 expression assessment For example, in some
instances,
if a cancer expresses ABCC1 at or above a predetermined threshold then the
subject may be
treated with an anti-ABCC1 antibody of the present disclosure and if the
cancer expresses ABCC1
below the predetermined threshold then the subject may not be treated with an
anti-.ABCC1
antibody of the present disclosure.
Any convenient assay may be employed for analyzing ABCC1 levels, including but
not
limited to e.g., flow cytometry, nucleic acid-based assays (e.g..,
amplification, sequencing, etc.),
cell cytometry, immunohistochemistry, and the like. Any convenient biological
sample may be
employed, including but not limited to
cancer biopsy samples. Useful predetermined
thresholds for assessing expression of one or more markers and/or targets may
be determined
by any convenient and appropriate method, including comparison of the measured
level of
expression to a corresponding control. For example, in some instances, a
useful predetermined
threshold for the level of ABCC1 in a sample may correspond to a level of
ABCC1 measured in a
reference cell, such as a healthy/normal cell.
Methods of Making
As summarized above, methods of the present disclosure also include methods or
making
and/or identifying antibodies as described herein. A subject antibody can be
produced by any
known method, .e.g.., conventional synthetic methods for protein synthesis;
recombinant DNA
methods; etc.
Where a subject antibody is a single chain polypeptide, it can be synthesized
using
standard chemical peptide synthesis techniques. Where a polypeptide is
chemically synthesized,
the synthesis May proceed via liquid-phase or solid-phase. Solid phase
polypeptide synthesis
(SPPS), in which the C-terminal amino acid of the sequence is attached to an
insoluble support
followed by sequential addition of the remaining amino acids in the sequence,
is an example of a.
64
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
suitable method for the chemical synthesis of a subject antibody. Various
forms of SPPS, such
as Fmoc and Boc, are available for synthesizing a subject antibody.
Standard recombinant methods can be used for production of a subject antibody.
For
example, nucleic acids encoding light and heavy chain variable regions,
optionally linked to
constant regions, are inserted into expression vectors. The light and heavy
chains can be cloned
in the same or different expression vectors. The DNA segments encoding
immunoglobulin chains
are operably linked to control sequences in the expression vector(s) that
ensure the expression
of immunoglobulin polypeptides. Expression control sequences include, but are
riot limited to,
promoters (e.g., naturally-associated or heterologous promoters), signal
sequences, enhancer
elements, and transcription termination sequences. The expression control
sequences can be
eukaryotic promoter systems in vectors capable of transforming or transfecting
eukaryotic host
cells (e.g., COS or CHO cells). Once the vector has been incorporated into the
appropriate host,
the host is maintained under conditions suitable for high level expression of
the nucleotide
sequences, and the collection and purification of the antibodies.
Because of the degeneracy of the genetic code, a variety of nucleic acid
sequences can
encode each immunoglobulin amino acid sequence. The desired nucleic acid
sequences can be
produced by de novo solid-phase DNA synthesis or by polymerase chain reaction
(PCR)
mutagenesis of an earlier prepared variant of the desired polynucleotide.
Oligonucieotide-
mediated mutagenesis is an example of a suitable method for preparing
substitution, deletion and
insertion variants of target polypeptide DNA. See Adelman at al., DNA 2:183
(1983), Briefly, the
target polypeptide DNA is altered by hybridizing an oligonucleotide encoding
the desired mutation
to a single-stranded DNA template, After hybridization, a DNA polymerase is
used to synthesize
an entire second complementary strand of the template that incorporates the
oligonucleotide
primer, and encodes the selected alteration in the target poiypeptide DNA.
Suitable expression vectors are typically replicable in the host organisms
either as
episomes or as an integral part of the host chromosomal DNA. Commonly,
expression vectors
contain selection markers (e.g., ampicillin-resistance, hygromycin-resistance,
tetracycline
resistance, kanamycin resistance or neomycin resistance) to permit detection
of those cells
transformed with the desired DNA sequences.
Escherichia cofi is an example of a prokaryotic host cell that can be used for
cloning a
subject antibody-encoding polynucleatide. Other microbial hosts suitable for
use include bacilli,
such as Bacillus subtifis, and other enterobactenaceae, such as Salmonella,
Serratia, and various
Pseudomonas species,
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
Other microbes, such as yeast, are also useful for expression. Saccharomyces
(e.g., S.
cerevisiae) and P.ichia are examples of suitable yeast host cells, with
suitable vectors having
expression control sequences (e.g., promoters), an origin of replication,
termination sequences
and the like as desired. Typical promoters include 3-phosphoglycerate kinas-e
and other glycolytic
enzymes. Inducible yeast promoters include, among others, promoters from
alcohol
dehydrogenase, isocytochrome C, and enzymes responsible for maltose and
galactose
utilization.
In addition to microorganisms, mammalian cells (e.g., mammalian cells grown in
in vitro
cell culture) can also be used to express and produce the polypeptides of the
present invention
(e.g., polynucleotides encoding imm.unoglobulins or fragments thereof). See
Winnacker, From
Genes to Clones, VCH Publishers, N.Y., N.Y. (1987). Suitable mammalian host
cells include CHO
cell lines, various Cos cell lines, HeLa. cells, HEK. cells, myeloma cell
lines, and transformed B-
cells or hybridomas. Expression vectors for these cells can include expression
control sequences:
such as an origin of replication, a promoter, and an enhancer (Queen et al.,
Immunol. Rev. 89:49
(1986)), and necessary processing information sites, such as hbosome binding
sites, RNA splice
sites, polyadenylation sites, and transcriptional terminator sequences.
Examples of suitable
expression control sequences are promoters derived from immunoglobtflin genes,
SV40,
adenovirusõ bovine =papillorna virus, cytomegalovirus and the like. See Co et
al.: J. Immunol.
148:1149 (1992).
Once synthesized (either chemically or recornbinantly), the whole antibodies,
their dimersõ
individual light and heavy chains: or other forms of a subject antibody (e.g.,
scFv, etc.) can be
purified according to standard procedures of the art, including ammonium
sulfate precipitation,
affinity columns, column chromatography, high performance liquid
chromatography (HPLC)
purification: gel electrophoresis, and the like (see generally Scopes, Protein
Purification (Springer..
Verlag, N.Y., (1982)). A subject antibody can be substantially pure, e.g., at
least about 80% to
85% pure, at least about 85% to 90% pure, at least about 90% to 95% pure, or
98% to 99%, or
more, pure, e.g.: free from contaminants such as cell debris, macromolecules
other than a subject
antibody, etc.
KfTS
Aspects of the present disclosure also include kits. The kits may include,
e.g.: any
combination of the antibodies, reagents, compositions, formulations, cellsõ
nucleic acids:
expression vectors, or the like, described herein. A subject kit can include
one or more of: a
subject antibody: a nucleic acid encoding the same: or a cell comprising a
subject nucleic acid.
66
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
Kits may be configured for various purposes, including e.g., treatment kits
(e.g., where a kit may
include an anti-ABCC1 antibody and e.g., one or more additional active agents,
such as a
chemotherapeutic), kits for producing antibodies, kits for screening
antibodies, and the like.
Optional components of the kit will vary and may, e.g., include: a buffer: a
protease
inhibitor; etc. Where a subject kit comprises a subject nucleic acid, the
nucleic acid may also have
restrictions sites, multiple cloning sites, primer sites, etc. The various
components of the kit may
be present in separate containers or certain compatible components may be pre-
combined into a
single container, as desired.
In addition to above-mentioned components, a subject kit can include
instructions for using
the components of the kit to practice a subject method. The instructions for
practicing a subject
method are generally recorded on a suitable recording medium For example, the
instructions
may be printed on a substrate, such as paper or plastic, etc. As such, the
instructions may be
present in the kits as a package insert, in the labeling of the container of
the kit or components
thereof (i.e., associated with the packaging or subpackaging) etc. In other
embodiments, the
instructions are present as an electronic storage data file present on a
suitable computer readable
storage medium, e.d. compact disc-read only memory (CD-ROM), digital versatile
disk (DVD).
diskette, etc. In yet other embodiments, the actual instructions are not
present in the kit, but means
for obtaining the instructions from a remote source, e.g. via the internet,
are provided. An example
of this embodiment is a kit that includes a web address where the instructions
can be viewed
and/or from which the instructions can be downloaded. As with the
instructions, this means for
obtaining the instructions is recorded on a suitable substrate.
EXAMPLES
The following examples are put forth so as to provide those of ordinary skill
in the art with
a complete disclosure and description of how to make and use the present
invention, and are not
intended to limit the scope of what the inventors regard as their invention
nor are they intended
to represent that the experiments below are all or the only experiments
performed. Efforts have
been made to ensure accuracy with respect to numbers used (e.g. amounts,
temperature, etc.)
but some experimental errors and deviations should be accounted for. Unless
indicated
otherwise, parts are parts by weight, molecular weight is weight average
molecular weight,
temperature is in degrees Centigrade, and pressure is at or near atmospheric.
General methods in molecular and cellular biochemistry can be found in such
standard
textbooks as Molecular Cloning: A Laboratory Manual, 3rd Ed. (Sambrook at al.,
HaRBor
Laboratory Press 2001); Short Protocols in Molecular Biology, 4th Ed, (Ausubel
at al. eds., John
67
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
Wiley & Sons 1999), Protein Methods (Bollag et al., John Wiley & Sons 1996);
Nonviral Vectors
for Gene Therapy (Wagner et al. eds., Academic Press 1999); Viral Vectors
(Kapiift & Loewy eds.,
Academic Press 1995); Immunology Methods Manual (I. Lefkovits ed., Academic
Press 1997)'
and Cell and Tissue Culture: Laboratory Procedures in Biotechnology (Doyle &
Griffiths, John
Wiley & Sons 1998), the disclosures of which are incorporated herein by
reference, Reagents,
cloning vectors, cells, and kits for methods referred to in, or related to,
this disclosure are available
from commercial vendors such as BioRad, Agilent Technologies, Thermo Fisher
Scientific,
Sigma-Aldrich, New England Biolabs (NEB), Takara Bio USA, inc., and the like,
as well as
repositories such as e.g., Addgene, Inc., American Type Culture Collection
(ATCC), and the like,
Example 1: Generation of antibodies that bind specifically to cells
expressing ABCC1
Materials and Methods
Antibody Generation
Wild type (WT) human and cynolomgus ABCC1, full length and truncated versions,
were
.. used to immunize mice or rats. Spleen and lymph node cells from the
vaccinated animals were
fused with SP2/0 myelorna cells (hybridoma technology). Hybridoma supernatants
were screened
for the presence of anti-ABCC1 antibodies by flow cytometry. CDRs from
selected murine IgGs
were cloned into mammalian 1961 backbone expression vectors for full-length
1961 antibody
expression and production in HEK 293 host cells via transfection using
standard protocols and as
described below.
Expression Vectors
For the generation of the antibody expression vectors, the variable regions of
heavy and
light chain DNA sequences were subcloned in frame with either the human IgG1
constant heavy
chain or the human IgGi kappa constant light chain pre-inserted into the
respective generic
recipient expression vectors optimized for expression in mammalian cell lines.
The genes to be
expressed were cloned into the pCI-neo Mammalian Expression Vector (Promega)
that uses the
full-length human cytomegaiovirus (CMV) immediate- early promoter for high
level gene
expression. The two antibody chains were cloned into two different vectors.
The N-terminal signal sequences from mouse IgG heavy chain and kappa light
chain were
used for the secreted expression of the heavy and light chain, respectively.
The signal peptide
was cleaved during expression, leaving intact N-terminus. In the Fab
constructs, the C-terminus
of the CH1 IgG1 constant region was fused with a ex His tag for purification.
68
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
Production of mAb
Antibody constructs were expressed using polymer-based co-transfection of
Expi293 cells
(A14627, ThermoFisher) cells growing in suspension with the mammalian
expression vectors
following the manufacturer's recommendations.
About six days after transfection the cells were harvested by centrifugation.
In detail, 1 ug
of total encoding DNA per ml of transfected culture was diluted into of Opti-
lVIEMO medium (Life
Technologies), and incubated with Expifectamine reagent (Life Technologies) in
the same
medium for 20 min. The mixture was then added into the Expi293 cells growing
in suspension
in Expi293 Expression medium (Life Technologies) at 2.5 million cells/ml at 37
C with and
overlay of 8% of 002 in air. After 6 days, the medium containing the antibody
construct was
harvested by centrifugation_
Purification of mAbs
To purify antibody formats containing the human Fc, 10 pl of MabSelect7"' SuRe
TM (GE
Healthcare) per1 ml of supernatant were added to the harvested medium and kept
stirring at 40C
overnight. The next day, the protein A resin was applied in a 24 well filter
plate using a vacuum
manifold unit (Pail Lifesciences, USA). The resin was washed with PBS and the
antibody eluted
in 50 mM phosphate pH 3 and neutralized with 10x PBS pH 13.
Analytical test for mAbs (GX11 reduced and non-reduced)
Purity and monomer content of the final protein preparation was determined by
high-
throughput analysis on the Caliper's LabChip GXII using Protein Express
LabChip Kit (Perkin -
Elmer) as described by the manufacturer. The chip was automatically primed on
the instrument
with polymer solution containing 0.2% SDS and fluorescent staining dye_ The
destain channels
were filled with polymer solution free of SOS and dye. Briefly, proteins in
reducing and not
reducing conditions were prepared by mixing a small volume (2-5 pL) of sample
with the caliper
sample buffer with or without DDT. The samples were denatured at 75C for 5
minutes,
centrifuged at 2000g for 3 minutes, and then run. Eleotropherograms were
generated by LabChip
GM Touch software (Perkin Elmer),
69
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
Analytical test for inAbs (HPLC)
Purity and monomer content of the final protein preparation was determined by
high-
throughput analysis on HPLC. Size exclusion chromatography (SEC) was performed
using an
Advancebio SEC 300A 4,6x300mm, 2.7 urn (pin PL1580-5301) (Agilent
Technologies) on an
Infinity 1260 Agilent HPLC system. Injections were made under isocratic
elution conditions using
a mobile phase of PBS, 400 ml\il sodium chloride, pH 7.4, and detected with
absorbance at 280
nm. Quantification is based on the relative area of detected peaks.
A subject antibody can be substantially pure, e.g., at least about 80% to 85%
pure, at least
about 85% to 90% pure, at least about 90% to 96% pure, or 98% to 99%, or more,
pure, e.g., free
from contaminants such as cell debris, macromolecules other than a subject
antibody, etc.
Monoclonal antibody titration binding to KPC I
Binding titration of recombinant antibodies to KPC1 transfectants was
performed by serial
dilution of antibodies from about 666 nM. Diluted antibody in flow cytometry
buffer was incubated
with cells on ice for 30 min. After 2 washes with flow cytometry buffer, bound
antibody was
detected with PE-labeled F(ab`)2 fragment goat anti-human IgG (Jackson
ImmunoResearch)
diluted 1:200 in flow cytorriE.4ry buffer and incubated with cells for 20 min
on ice. After 2 washes
with flow cytometry buffer fluorescence was measured on an Attune NxT flow
cytometer. Data
were analyzed with GraphPad Prism 8.0 software to determine EC50's.
Cell Binding Assays.
Antibody binding to cells was evaluated by flow cytometry. 293T cells stably
transfected
to express human or cynomoigus ABCC1 were washed once in flow cytometry buffer
(PBS + 2%
FBS 0.02% sodium azide), resuspended at 2 x 101\6 cellsimL in flow cytometry
buffer, and
dispensed into 96-well microtiter plates at 0.1 mUwell. Recombinant antibodies
were added to
cells at 5 ugimt_ for initial binding confirmation, or serially diluted from
100 ugimL in flow cytometry
buffer. After incubating cells on ice for 30 min, cells were washed twice with
flow cytometry buffer.
Bound antibody was detected with PE-labeled F(ab').2 fragment goat anti-human
IgG (Jackson
ImmunoResearch) and evaluated on an Attune NxT flow cytorneter. EC50 is
calculated to be the
concentration of antibody that gives half maximal response.
ABCC1 Efflux Assay.
HEK 293T cells expressing human ABCC1 were washed several times and aliquoted
into
98-well plates as 50 ul aliquots/well at a cell density of 1 x 10'3 cells per
ml in phenol red-free
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
DMEMõ Cells were mixed with 50 ul antibodies and incubated for 1.5 h at 37
'DC. The cells were
then washed twice and finally resuspended in 200 ml PBS. Calcein AM
fluorescence was
measured by flow cytometry,
Cyloto.xicity Assay
The effect of anti-ABCC1 antibodies on vincristine cytotoxicity was evaluated
on 293T cells
stably transfected to express ABCC1. Cells were plated in 0,05 mL of Assay
Media (DMEM +10%
FBS) at 5000 .cells/well in white.: flat bottom 96-well tissue culture plates.
Vincristine was prepared
at 2X final assay concentration by serial dilution from 200 uM in assay media
containing test
antibodies or control antibodies at 100 ugimL. (2X final concentration), An
equivalent volume
(0.05 mL) of the vicristinelantibody mixture was added to the 293T_ABCC1 cells
in 96-well plates,.
The plates were then incubated at 37 C, in 5% CO2. After about 72-96 hr
plates were equilibrated
to room temperature and cell viability assessed using Pro.megat CellTiter-Glo
Luminescent Cell
Viability Assay according to the manufacturer's recommended protocol.
Luminescence was
measured on a Molecular Devices FlexStation 3 Multi-Mode Micropiate Reader
and data
analyzed using GraphPad Prism 8.0 software. Half maximal inhibitory
concentration (IC50) is the
concentration of drug (vuncristine or other chemotherapy cytotoxic agent)
where the response
(cell growth) is reduced by 50%,
CT26 syngeneic xenograft mouse model.
Syn.geneic models are allografts immortalized from mouse cancer cell lines,
which are
then grafted back into the same inbred immunocompetent mouse strain. CT26 is
an N-nitroso-N-
methylurethane-(NNMU) induced undifferentiated fibroblastic colon carcinoma
cell line
established from BALBic mice with aggressive colon carcinoma. C26 cells were
purchased from
.. ATCCO (CRL-2638") and maintained in RPMi-1640 medium supplemented with 10%
FE3,S and
1% penicillinõ 1% streptomycin at 37 C, 5 A3C0.2, Cell lines used were
authentic and confirmed to
be mycoplasrna negative. The cells are adherent with a fibroblast morphology
and will form
tumors and metastases post implantation into sy-ngeneic BALBic mice or
immunocompromised
mice.
1 X I05 cells diluted inPBS..:.Matrigei (1.1) were subcutaneously implanted
.u.sing a 27G
insulin syringe into anesthetized 5-6-week old female .Balbic mice under
aseptic conditions All
animal maintenance, handling, surveillance, and animal procedures were
performed in
accordance with an approval from the APLAC protocol,
71
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
Once tumors reached 100-150 mm3, mice were randomized into 6 groups of five
mice
each:
1. Control isotype 3 mg/kg,
2. Control isotype 3 mg/kg Doxorubicin 2 mg/kg
3, KNUY CI -831 3 mg/kg
4. KIVY C1-831 3 mg/kg Doxorubicin
5. KNJY CI-787A 3 mg/kg
6. MN C1-787A 3 mg/kg + Doxorubicin 2 mg/kg,
Doxorubicin is soluble in water. All test articles were dosed
intraperitoneally twice a week
for two consecutive weeks. Antibodies were dosed 4hr prior to Doxorubicin.
Tumors were measured three times a week using a calibrated Verner Caliper and
tumor
volume calculated as per the formula WL*S*S, where L is the long axis and S is
the short axis of
the tumor. Body weights were recorded before treatment started and were
continuously monitored
throughout the study. Animals were euthanized when turning moribund according
to the above-
mentioned predefined criteria rapid weight loss, loss of ability to ambulate,
labored respiration, or
inability to drink or feed to avoid animal suffering.
Results
Table 3 lists the following characteristics of the anti-ABCCI antibodies:
binding to 293T
cells stably transfected to express human ABCCI measured by FACS and binding
to 293T cells
stably transfected to express cynomolgus ABCCI measured by FAGS.
Antibody Binding Cynntrolgus
by ABCC1
FACS binding
C1.309
C1,310
C1,499
C1.773
C1.773a
C1.777a
C1.778b
CL784a
C1.786a
C1,787a
72
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
FIG. I depicts the results of titration binding of ten anti-ABCC1 monoclonal
antibodies to
the doxorubicin-resistant lung carcinoma cell line H69AR (ATCCA CRL-11351 )
that
endogenously expresses ABCCI in the flow cytometry (FACS) assay described
above. All
antibodies tested show significant (>10-folds of background fluorescence mean
intensity (FMI))
binding to the H69AR cells.
FIGS. 2A-2B depict the results of titration binding of the indicated anti-ABCC
I monoclonal
antibodies to human and cynomolgus ABCC1-overexpressing rat CS glioma cell
lines in the FACS
assay described above. All antibodies tested show significant binding to both
C6 cells
overexpressing human ABCCI and CS cells overexpressing cynomolgus ABCC I .
FIGS. 3A-3C, 4, 5A-58, and 6A-68 show the results of titration binding of
further anti-
ABCC1 monoclonal antibodies to human and cynomolgus ABCC1-overexpressing rat
C6 glioma
cell lines in the FACS assay described above, "2" Ab only" refers to a not
primary antibody used
as negative control. All anti-ABCC1 antibodies tested show significant
binding, relative to
negative control, to both CS cells overexpressing human ABCCI and CS cells
overexpressing
cynomolgus ABCCI.
FIGS. 7A-7B show the results of ABCC1 efflux assay performed using HEK293T
cells
expressing human ABCCI All anti-ABCC1 antibodies tested significanly inhibit
the efflux function
of the ABCCI transporter.
FIGS. 8A-8C present titration binding and efflux assay characterization of
humanized anti.
ABCC1 monoclonal antibodies. Humanized anti-ABCC1 antibodies C1.831 .hull,
01.861.hu11,
C1,861 ,hu21: and C1.844.hu21 bind both human and cynomolus ABCC1 in the
titration binding
assay and significantly inhibit the efflux function of the ABCCI transporter
in the efflux assay.
FIGS. 9A-9C show the binding of humanized variants of the anti-ABCCI
monoclonal
antibodies C1.831, C1.861 and C1.844 to human and cynomolgus ABCC1
overexpressing rat CS
glioma cell lines. All humanized antibodies retain the ability to bind both
human and cynomolgus
ABCC1.
FIGS, 10A-10B show the binding of humanized anti-ABCC1 antibodies C1,851,12,
C1.851.14 and C1.851.15 to human and cynomolgus ABCCI overexpressing rat C6
glioma cell
lines. All three antibodies retain the ability to bind both human and
cynomolgus ABCC1.
FIGS. 11A-1 1C present the binding of four humanized ABCC1IKT9 bispecific
antibodies
to human and cynomolgus CS cell lines, overexpressing ABCCI and KT,
respectively, The
schematic bispecific antibody structure is also shown. KT9 stands for
atezolizumab, an anti-PD-
L1 monoclonal antibody. The bispecific antibodies include the heavy and light
chains from the
73
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
indicated ABCC1 antibodies and a scFv region formed from the KT9 antibody. Ail
bispecific
antibodies tested bind both C6 cells. overexpressing ABCCI and C6 cells
overexpressing KT9.
FIGS, 12A-12C present the binding of four humanized CliKT1 bispecific
antibodies to
293T cells expressing human ABCC1 and 293T cells expressing human Of
cynomolgus. KT1,
respectively.. KT1 stands for trastuzumab, an anti-ErbB2 (anti-HER2)
monoclonal antibody. The
bisp-ecific antibodies include the heavy and light chains from the indicated
anti-ABCC1 antibodies
and a .scFv region .formed from the KT1 antibody. The bispecific antibodies
tested bind both 293T
cells expressing human KTi and 293T expressing cynomolgus KT1, and retain the
ability to bind
293.T cells expressing human ABCC1...
FIGS, 13A-138,. 14A-14B, and 15 show the effect of the tested anti-ABCC1
monoclonal
antibodies on vincristine cytotoxicity in the 293T cytotoxicity assay. The
tested anti-.ABCC1
antibodies increase the cytotoxicity of vincristine in this assay. MK571 is a
commercially available
small molecule inhibitor of ABCC1-mediated transport.
FIG, 16 shows that the three anti-.A8CC1 monoclonal antibodies tested inhibit
tumor
growth in vivo in the H69AR cytotoxicity assay. The assay evaluates the effect
of the tested
antibodies on vincristine cytotoxicity on the H.6.9AR cell line, which is an
Adriamycin-selected, C1.
positive variant of the human small cell lung cancer cell line, NCI-H69.
FIG, 17 shows that anti-A.BCC1 monoclonal antibodies C1.-831 and C1 -737A
inhibit tumor
growth in vivo in the C126 synge-neic mouse tumor model,
Although the foregoing invention has been described in some detail by way of
illustration
and example for purposes of clarity of understanding, it is readily apparent
to those of ordinary
skill in the art in light of the teachings of this invention that certain
changes and modifications may
be made thereto without departing from the spirit or scope of the appended
claims,
Accordingly, the preceding merely illustrates the principles of the invention.
It will be
appreciated that those skilled in the art will be able to devise various
arrangements which,
although not explicitly described or shown herein, embody the principles of
the invention and are
included within its spirit and scope. Furthermore, all examples and
conditional language recited
herein are principally intended to aid the reader in understanding the
principles of the invention.
and the concepts contributed by the inventors to furthering the art, and are
to be construed as
being without limitation to such specifically recited examples and conditions.
Moreover, all
statements herein reciting principles, aspects, and embodiments of the
invention as well as
specific examples thereof, are intended to encompass both structural and
functional equivalents
thereof. Additionally, it is intended that such equivalents include both
currently known equivalents
74
CA 03193588 2023-03-01
WO 2022/051390
PCT/US2021/048701
and equivalents developed in the future, Le,: any elements developed that
perform the same
function, regardless of structure. Moreover, nothing disclosed herein is
intended to be dedicated
to the public regardless of whether such disclosure is explicitly recited in
the claims..
The scope of the present invention, therefore, is not intended to be limited
to the exemplary
embodiments shown and described herein. Rather, the scope and spirit of
present invention is
embodied by the appended claims. In the claims, 35 U.S.C. 112(f) or 35 U.S,C.
112(6) is
expressly defined as being invoked for a limitation in the claim only when the
exact phrase "means
for" or the exact phrase "step for" is recited at the beginning of such
limitation in the claim if such
exact phrase is not used in a limitation in the claim, then 36 U.S.C. 112
(f) or .35 11.2(6)
is not invoked.