Note: Descriptions are shown in the official language in which they were submitted.
WO 2022/132853
PCT/US2021/063431
WATER-SOLUBLE FILMS, WATER-SOLUBLE UNIT DOSE ARTICLES, AND METHODS OF
MAKING AND USING THE SAME
FIELD OF THE INVENTION
[0001] Water-soluble films, water-soluble unit dose articles, and
methods of their
manufacture and use.
BACKGROUND OF THE INVENTION
[0002] Water-soluble unit dose articles are liked by consumers as they are
convenient and
efficient to use. Such water-soluble unit dose articles often comprise
detergent compositions.
Without wishing to be bound by theory, when the water-soluble unit dose
article is added to
water, the film dissolves/disintegrates releasing the internal contents into
the surrounding water
to create a wash liquor. The water soluble film used must meet the dual
criteria of providing
sufficient strength such that the film does not rip or tear resulting in
premature rupture of the
water-soluble unit dose article during storage and transport, but also
adequately dissolves
during the wash cycle to minimise unwanted film residues at the end of the
wash operation.
Films comprised of polyvinyl alcohol have been used to meet these needs. A
preferred method
of making such unit dose articles is to deform a first water-soluble film into
a mould to create an
open cavity, fill the open cavity with a detergent composition, then close the
open cavity with a
second water-soluble film and seal the first and second water-soluble films
together to create
the water-soluble unit dose article.
[0003] There is an increasing desire to wash fabrics under more
environmentally friendly
conditions, such as shorter wash cycles and cooler wash temperatures. Under
such conditions,
unit dose articles made using traditional water-soluble films (which primarily
comprise polyvinyl
alcohol homopolymers), can suffer from incomplete dissolution during the wash
cycle, resulting
in undissolved film material remaining and depositing onto articles to be
washed.
[0004] Efforts have previously been made to overcome this issue. U.S. Patent
No.
10,619,042 describes a water-soluble unit dose article comprising a water-
soluble film
comprising polyvinyl alcohol (PVOH) resin blend comprising: a first PVOH
polymer comprising
carboxylated anionic monomer units, vinyl alcohol monomer units and optionally
vinyl acetate
monomer units, wherein the carboxylated anionic monomer unit is present in the
first PVOH
polymer in an amount of from about 3mo1.% to about 6rno1. /0, a second PVOH
polymer
consisting essentially of vinyl alcohol monomer units and optionally vinyl
acetate monomer
units, wherein the first PVOH polymer is present in an amount in a range from
about 10wt. /0 to
about 70wt.% of total PVOH polymers in the film. Such water-soluble unit dose
articles provide
both excellent dissolution yet also acceptable film structural integrity, yet
as described below are
subject to additional improvement.
1
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
SUMMARY
[0005] A first aspect includes water-soluble unit dose article comprising at
least two
compartments and optionally containing a composition housed in at least one of
the
compartments, wherein the unit dose article comprises;
a. a first water-soluble film, wherein the first water-soluble film has a
first
side and a second side, and wherein the first water soluble film comprises
a first PVOH resin wherein the first polyvinyl alcohol resin comprises a
polyvinyl alcohol consisting of a polyvinyl alcohol homopolymer, an
anionic polyvinyl alcohol copolymer, or a blend thereof;
b. a second water-soluble film, wherein the second water-soluble film has a
first side and a second side, and wherein the second water-soluble film
comprises a second polyvinyl alcohol resin wherein the second polyvinyl
alcohol resin comprises;
i. less than 15% by weight of the second polyvinyl alcohol resin of a
polyvinyl alcohol polymer comprising carboxylated anionic monomer
units, vinyl alcohol monomer units and optionally vinyl acetate monomer
units, and wherein the carboxylated anionic monomer unit is derived from
a member selected from the group consisting of maleic acid, monoalkyl
maleate, dialkyl maleate, maleic anhydride, and combinations thereof;
ii.about 85% to 100% by weight of the second polyvinyl alcohol resin of a
polyvinyl alcohol homopolymer or a homopolymer blend, wherein the
homopolymers consist of vinyl alcohol monomer units and optionally vinyl
acetate monomer units;
wherein the second polyvinyl alcohol resin has an average
viscosity of about less than 12 mPa.s measured as a 4% polyvinyl alcohol
polymer solution in deionized water at 20 C;
c. a third water-soluble film wherein the third water-soluble film has a
first
side and a second side, and wherein the third water soluble film
comprises a third polyvinyl alcohol resin, wherein the third polyvinyl
alcohol resin optionally comprises a polyvinyl alcohol consisting of a
polyvinyl alcohol homopolymer, an anionic polyvinyl alcohol copolymer, or
a blend thereof;
wherein the first side of the first water-soluble film is sealed to the second
side of
the second water-soluble film to create a first compartment between the first
water-
soluble film and the second water-soluble film, and the first side of the
second water-
soluble film is sealed to the second side of the third water-soluble film to
create at least a
2
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
second compartment between the second water-soluble film and the third water-
soluble
film, and wherein the second compartment is positioned above the first
compartment;
provided that when the article contains a composition housed within a
compartment,
then the composition is not a laundry composition and is not an automatic dish
washing
composition.
[0006] Another aspect is a process of making a water-soluble unit dose article
according to
the disclosure herein, comprising the steps of;
a. deforming the first water-soluble film to create an open cavity;
b. filling the open cavity with a composition;
c. separately deforming the third water-soluble film to create at least one
open
cavity;
d. filling the at least one open cavity from step (c) with a composition;
e. closing the open filled cavity from step (d) with the second water-
soluble film;
f. sealing the second water-soluble film to the third water-soluble film to
create a
closed intermediate;
9. closing the open filled cavity from step (b) with the
closed intermediate from step
(f); and
h. sealing the first water-soluble film to the second water-
soluble film to create the
water-soluble unit dose article.
[0007] For the compositions and methods described herein, optional
features, including but
not limited to components, compositional ranges thereof, substituents,
conditions, and steps,
are contemplated to be selected from the various aspects, embodiments, and
examples
provided herein.
[0008] Further aspects and advantages will be apparent to those of
ordinary skill in the art
from a review of the following detailed description, taken in conjunction with
the drawings.
While the films, unit dose articles, and methods described herein are
susceptible of
embodiments in various forms, the description hereafter includes specific
embodiments with the
understanding that the disclosure is illustrative, and is not intended to
limit the invention to the
specific embodiments described herein.
BRIEF DESCRIPTION OF THE DRAWINGS
[0009] FIG.1 is a water-soluble unit dose article according to the
present invention.
[0010] FIG. 2 shows a schematic illustration of the basic
configuration of the % pouch
strength pass rate and % seal failure test.
3
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
DETAILED DESCRIPTION
[0011] As mentioned above, U.S. Patent No. 10,619,042 describes a water-
soluble unit dose
article comprising a water-soluble film comprising a PVOH resin blend
comprising: a first PVOH
polymer comprising carboxylated anionic monomer units, vinyl alcohol monomer
units and
optionally vinyl acetate monomer units, wherein the carboxylated anionic
monomer unit is
present in the first PVOH polymer in an amount of from about 3m01.% to about
6m01.%, a
second PVOH polymer consisting essentially of vinyl alcohol monomer units and
optionally vinyl
acetate monomer units, wherein the first PVOH polymer is present in an amount
in a range from
about lOwt.% to about 70wt.% of total PVOH polymers in the film. However, an
issue was
discovered with such unit dose articles, specifically that rather than a
failure in the structural
integrity of the film itself, failures can occur in the seal between the first
and second water-
soluble films. Such seal failure can result in premature rupture of the water-
soluble unit dose
article.
[0012] Therefore, there is a need in the art for a water-soluble unit dose
article that provides
reduced seal failures as compared to U.S. Patent No. 10,619,042 yet also
maintains structural
integrity of the film itself and dissolution profiles comparable with those of
U.S. Patent No.
10,619,042.
[0013] It was surprisingly found that a water-soluble unit dose
article describe below
achieved this. This is even more surprising considering that the second water-
soluble film
comprises a higher degree of polyvinyl alcohol homopolymer, which the skilled
person would
expect to result in reduced dissolution under short and cold wash cycles.
[0014] In addition, wherein the films according to the prior art
are sealed via solvent sealing,
it was discovered there is a tendency for the solvent sealing solution to not
deposit
homogeneously on the water-soluble film prior to sealing. Without wishing to
be bound by
theory, wherein two films are intended to be sealed together, the solvent
sealing solution needs
to be applied to at least one of the films. If the solvent sealing solution
does not provide a
homogeneous layer on the film that it is applied to then this can result in
weaker seals between
the two films leading to seal failure and premature rupture of the water-
soluble unit dose article.
It was surprisingly found that in the unit dose articles described below, a
more
uniform/homogeneous layer of solvent sealing solution was obtained between the
water-soluble
films to be sealed, resulting in reduced seal failures.
[0015] The water-soluble films, unit dose articles, and methods are
contemplated to include
embodiments including any combination of one or more of the elements,
features, and steps
further described below (including those shown in the figures), unless stated
otherwise.
Water-soluble unit dose article
4
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
[0016] A first aspect of the present invention is a water-soluble
unit dose article. The water-
soluble unit dose article comprises at least a first compartment and
optionally a composition
housed in the compartment. Suitable compositions are described in more detail
below. The
water-soluble unit dose article comprises a first water-soluble film and a
second water-soluble
film and optionally a third water-soluble film. The first water-soluble film,
the second water-
soluble film and the optional third water-soluble films are described in more
detail below.
[0017] The final water-soluble unit dose article comprises water-soluble film
shaped such that
the unit-dose article comprises at least one internal compartment surrounded
by the water-
soluble film. An intermediate construct contemplated as an aspect of the
disclosure herein can
include elements of the article or portions of the article in an unsealed
state, e.g. to allow for
provision of a composition into the intermediate construction prior to final
filling. The water-
soluble unit dose article is constructed such that the composition does not
leak out of the
compartment during storage. However, upon contact of the water-soluble unit
dose article with
water, the water-soluble film dissolves and releases the contents of the
internal compartment,
e.g. into a wash liquor, bulk water, or other environment (e.g. onto soil in
the case of an
agricultural composition).
[0018] A compartment of the final unit dose article should be understood as
meaning a
closed internal space within the unit dose article, which holds the
composition when present. In
practice, a compartment can be devoid of a composition, or devoid of a solid
or liquid type
composition disposed therein, e.g. containing only air to provide an article
which has a degree
of buoyancy for a period of time prior to dissolution.
[0019] The first water-soluble film has a first side and a second side. The
second water-
soluble film has a first side and a second side. The optional third water-
soluble film has a first
side and a second side.
[0020] The first side of the first water-soluble film is sealed to the second
side of the second
water-soluble film to create a first compartment between the first water-
soluble film and the
second water-soluble film. Optionally, the first side of the second water-
soluble film is sealed to
the second side of the third water-soluble film to create at least a second
compartment between
the second water-soluble film and the third water-soluble film, and optionally
wherein the
second compartment is positioned above the first compartment.
[0021] The first water-soluble film and the second water-soluble film can be
sealed via any
suitable method, e.g. solvent sealing, heat sealing or a mixture thereof, for
example via solvent
sealing. The solvent sealing solution optionally comprises an aqueous solvent,
a non-aqueous
solvent or a mixture thereof. The solvent sealing solution can comprise water
or consist of
water. The solvent sealing solution optionally comprises at least 95%, or at
least 98%, or at
least 99%, or 100% by weight of the solvent sealing solution of water. The
solvent sealing
solution can be applied to a film by any suitable method, including contact
and/or non-contact
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
methods. For example, the solvent solution can be applied in a contact
transfer process, e.g.
using a contact member comprising a nonabsorbent or substantially impermeable
material, e.g.
using an anilox roller, rubber (e.g. EPDM) roller, or any combination thereof,
optionally in
combination with a doctor blade. The sealing solution can be applied using a
drawdown bar,
Mayer bar, or similar apparatus. In another type of embodiment the sealing
solution can be
applied using a contact member comprising an absorbent material, for example
natural felt,
synthetic felt, porous plastic, foam, sponge, microfiber, cotton, polyester,
extruded polyester
fibers, nonwoven webs and the like, e.g. in pad or roller form. As another
option, the sealing
solution can be applied via a dosing nozzle or a spraying nozzle. Combinations
of any of the
foregoing methods and apparatus are contemplated. In one type of embodiment, a
contact
transfer method using an absorbent material is contemplated; and optionally in
a continuous
process, e.g. using a felt roll applicator. In one type of embodiment, the
solvent sealing solution
is applied to the second side of the second water-soluble film, the second
side of the second
water soluble film facing the first side of the first water-soluble film.
[0022] The second water-soluble film and the optional third water-soluble film
can be sealed
via solvent sealing, heat sealing or a mixture thereof, e.g. via solvent
sealing. The solvent
sealing solution can comprise an aqueous solvent, a non-aqueous solvent or a
mixture thereof.
For example, the solvent sealing solution can comprise water, or can consist
of water. The
solvent sealing solution can comprise at least 95%, or at least 98%, or at
least 99%, or 100% by
weight of the solvent sealing solution of water. The solvent sealing solution
can be applied by
any suitable method, including contact and/or non-contact methods. For
example, the solvent
solution can be applied in a contact transfer process, e.g. using a
nonabsorbent or substantially
impermeable material, e.g. using an anilox roller, rubber (e.g. EPDM) roller,
or any combination
thereof, optionally in combination with a doctor blade. The sealing solution
can be applied using
a drawdown bar, Mayer bar, or similar apparatus. In another type of embodiment
the sealing
solution can be applied using an absorbent material, for example natural felt,
synthetic felt,
porous plastic, foam, sponge, microfiber, cotton, polyester, extruded
polyester fibers, nonwoven
webs and the like, e.g. in pad or roller form. As another option, the sealing
solution can be
applied via a dosing nozzle or a spraying nozzle. Combinations of any of the
foregoing
methods and apparatus are contemplated. In one type of embodiment, a contact
transfer
method using an absorbent material is contemplated; and optionally in a
continuous process,
e.g. using a felt roll applicator. The solvent sealing solution can be applied
to the first side of
the second water-soluble film, the first side of the second water soluble film
facing the second
side of the third water-soluble film.
[0023] Without wishing to be bound by theory, it is believed that addition of
the solvent
sealing solution onto a water-soluble film can create a thin foam layer. It is
believed that this
thin foam layer interferes with optimal sealing of the two films, e.g. as a
result of a non-
homogeneous layer of the solvent sealing solution being present on the water-
soluble film. The
6
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
thus-modified seals are relatively weak in whole or in part, resulting in
premature seal failure,
e.g. under conditions of stress. It was surprisingly found that addition of a
solvent sealing
solution to the second water-soluble film according to the present invention
resulted in reduction
or even absence of the foam layer, and as such reduction in instances of seal
failure.
[0024] Optionally, the unit dose article comprises at least a third
compartment, optionally at
least a third and a fourth compartment between the second water-soluble film
and the third
water-soluble film. The second compartment and the third compartment,
optionally the second
compartment, the third compartment and the fourth compartments can be
positioned side-by-
side to one another, further optionally the second compartment and the third
compartment, e.g.
the second compartment, the third compartment and the fourth compartment can
be positioned
above the first compartment. In one type of embodiment, the second and third
compartments,
or the second, third and fourth compartments can be smaller than the first
compartment. The
second and third compartments, or the second, third and fourth compartments
can be the same
size as one another or can be different sizes. Some of the compartments can be
the same size
and some can be different sizes.
[0025] As mentioned above, one class of embodiments of the water-soluble unit
dose article
includes at least two compartments. This type of embodiment is further
described below. The
water-soluble unit dose article comprises at least two compartments and
optionally a
composition housed in one or more of the compartments. Suitable compositions
are described
in more detail below. The water-soluble unit dose article comprises a first
water-soluble film, a
second water-soluble film and a third water-soluble film. The first water-
soluble film, the second
water-soluble film and the third water-soluble films are described in more
detail below.
[0026] The final water-soluble unit dose article comprises water-soluble film
shaped such that
the unit-dose article comprises at least two internal compartments surrounded
by the water-
soluble film. Intermediate constructions contemplated as aspects of the
disclosure herein can
include elements of the article or portions of the article in an unsealed
state, e.g. to allow for
provision of a composition into the intermediate construction prior to final
filling of each of the
compartments. Thus, for example, an intermediate construction can include a
first sealed
compartment and a second, partially open compartment ready for filling. The
water-soluble unit
dose article is constructed such that the two or more compositions does not
leak out of the two
or more compartments during storage. However, upon addition of the water-
soluble unit dose
article to water, the water-soluble film dissolves and releases the contents
of the internal
compartment, e.g. into a wash liquor, bulk water, or other environment.
[0027] A compartment of the final unit dose article should be understood as
meaning a
closed internal space within the unit dose article, which holds the
composition when present. In
practice, one or more compartments can be devoid of a composition disposed
therein, e.g. to
provide an article which has a degree of buoyancy for a period of time prior
to dissolution.
7
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
[0028] The first water-soluble film has a first side and a second side. The
second water-
soluble film has a first side and a second side. The third water-soluble film
has a first side and a
second side.
[0029] The first side of the first water-soluble film is sealed to the second
side of the second
water-soluble film to create a first compartment between the first water-
soluble film and the
second water-soluble film, and the first side of the second water-soluble film
is sealed to the
second side of the third water-soluble film to create at least a second
compartment between the
second water-soluble film and the third water-soluble film, and wherein the
second compartment
is positioned above the first compartment.
[0030] The first water-soluble film and the second water-soluble film can be
sealed via any
suitable method, e.g. solvent sealing, heat sealing or a mixture thereof, for
example via solvent
sealing. The solvent sealing solution optionally comprises an aqueous solvent,
a non-aqueous
solvent or a mixture thereof. In embodiments, the solvent sealing solution can
comprise water
or consist of water. The solvent sealing solution optionally comprises at
least 95%, or at least
98%, or at least 99%, or 100% by weight of the solvent sealing solution of
water. The solvent
sealing solution can be applied by any suitable method, including contact
and/or non-contact
methods. For example, the solvent solution can be applied in a contact
transfer process, e.g.
using a contact member comprising a nonabsorbent or substantially impermeable
material, e.g.
using an anilox roller, rubber (e.g. EPDM) roller, or any combination thereof,
optionally in
combination with a doctor blade. The sealing solution can be applied using a
drawdown bar,
Mayer bar, or similar apparatus. In another type of embodiment the sealing
solution can be
applied using a contact member comprising an absorbent material, for example
natural felt,
synthetic felt, porous plastic, foam, sponge, microfiber, cotton, polyester,
extruded polyester
fibers, nonwoven webs and the like, e.g. in pad or roller form. As another
option, the sealing
solution can be applied via a dosing nozzle or a spraying nozzle. Combinations
of any of the
foregoing methods and apparatus are contemplated. In one type of embodiment, a
contact
transfer method using an absorbent material is contemplated; and optionally in
a continuous
process, e.g. using a felt roll applicator. The solvent sealing solution can
be applied to the
second side of the second water-soluble film, the second side of the second
water soluble film
facing the first side of the first water-soluble film.
[0031] The second water-soluble film and the third water-soluble film can be
sealed via any
suitable method, e.g. solvent sealing, heat sealing or a mixture thereof, for
example via solvent
sealing. The solvent sealing solution optionally comprises an aqueous solvent,
a non-aqueous
solvent or a mixture thereof. In embodiments, the solvent sealing solution can
comprise water.
The solvent sealing solution optionally comprises at least 95%, or even at
least 98%, or even at
least 99%, or even 100% by weight of the solvent sealing solution of water.
The solvent sealing
solution can be applied by any suitable method, including contact and/or non-
contact methods.
8
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
For example, the solvent solution can be applied in a contact transfer
process, e.g. using a
contact member comprising a nonabsorbent or substantially impermeable
material, e.g. using
an anilox roller, rubber (e.g. EPDM) roller, or any combination thereof,
optionally in combination
with a doctor blade. The sealing solution can be applied using a drawdown bar,
Mayer bar, or
similar apparatus. In another type of embodiment the sealing solution can be
applied using a
contact member comprising an absorbent material, for example natural felt,
synthetic felt,
porous plastic, foam, sponge, microfiber, cotton, polyester, extruded
polyester fibers, nonwoven
webs and the like, e.g. in pad or roller form. As another option, the sealing
solution can be
applied via a dosing nozzle or a spraying nozzle. Combinations of any of the
foregoing
methods and apparatus are contemplated. In one type of embodiment, a contact
transfer
method using an absorbent material is contemplated; and optionally in a
continuous process,
e.g. using a felt roll applicator. The solvent sealing solution can be applied
to the first side of
the second water-soluble film, the first side of the second water soluble film
facing the second
side of the third water-soluble film.
[0032] Optionally, the unit dose article comprises at least a third
compartment, optionally at
least a third and a fourth compartment between the second water-soluble film
and the third
water-soluble film. The second compartment and the third compartment,
optionally the second
compartment, the third compartment and the fourth compartments can be
positioned side-by-
side to one another, further optionally the second compartment and the third
compartment, e.g.
the second compartment, the third compartment and the fourth compartment can
be positioned
above the first compartment. In one type of embodiment, the second and third
compartments,
or the second, third and fourth compartments can be smaller than the first
compartment. The
second and third compartments, or the second, third and fourth compartments
can be the same
size as one another or can be different sizes. Some of the compartments can be
the same size
and some can be different sizes.
[0033] A composition according to the present invention can be comprised in at
least one of
the compartments. It can for example be comprised in just one compartment, or
can be
comprised in two compartments, or even in three compartments, or even in four
compartments.
[0034] Each compartment can comprise the same or different compositions. The
different
compositions could all be in the same form, or they may be in different forms,
e.g. solid, powder,
gel, paste, liquid, etc.
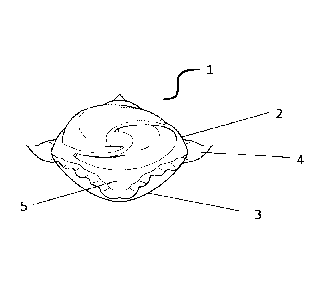
[0035] FIG.1 discloses a water-soluble unit dose article (1)
according to the present
invention. Shown are the first water-soluble film (2) and the third water-
soluble film (3) which
are sealed together at a seal region (4). Not shown is the second water-
soluble film which is
positioned between the first water-soluble (2) and the third water-soluble
film (3). A composition
(5) is comprised within the water-soluble soluble unit dose article (1).
9
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
[0036] The water-soluble unit dose article can be coated with a lubricating
agent. The
lubricating agent can be selected from talc, zinc oxide, silicas, siloxanes,
zeolites, silicic acid,
alumina, sodium sulphate, potassium sulphate, calcium carbonate, magnesium
carbonate,
sodium citrate, sodium tripolyphosphate, potassium citrate, potassium
tripolyphosphate, calcium
stearate, zinc stearate, magnesium stearate, starch, modified starches, clay,
kaolin, gypsum,
cyclodextrins or mixtures of any of the foregoing, for example.
First water-soluble film
[0037] The water-soluble unit dose article comprises a first water-
soluble film. The first
water-soluble film of the present invention is soluble or dispersible in
water. The first water-
soluble film can have an average thickness, prior to any deformation, in a
range of about 20 to
about 150 micron, or about 35 to about 125 micron, or about 50 to about 110
micron, or about
76 micron. The first water-soluble film has a first side and a second side.
[0038] The first water-soluble film can have a water-solubility of at least
50%, or at least 75%
or at least 95%, as measured by the method set out here after using a glass-
filter with a
maximum pore size of 20 microns: 5 grams 0.1 gram of film material is added
in a pre-
weighed 3L beaker and 2L 5m1 of distilled water is added. This is stirred
vigorously on a
magnetic stirrer, Labline model No. 1250 or equivalent and 5 cm magnetic
stirrer, set at 600
rpm, for 30 minutes at 30 C. Then, the mixture is filtered through a folded
qualitative sintered-
glass filter with a pore size as defined above (max. 20 micron). The water is
dried off from the
collected filtrate by any conventional method, and the weight of the remaining
material is
determined (which is the dissolved or dispersed fraction). Then, the
percentage solubility or
dispersability can be calculated.
[0039] The first water-soluble film can be obtained by casting, blow-moulding,
extrusion or
blown extrusion of the polymeric material, as known in the art and described
further below. The
first water-soluble film can be a solvent casted water-soluble film.
[0040] The first water soluble film comprises a first PVOH resin wherein the
first polyvinyl
alcohol resin comprises a polyvinyl alcohol consisting of a polyvinyl alcohol
homopolymer, an
anionic polyvinyl alcohol copolymer, or a blend thereof.
[0041] In one aspect, the first water-soluble film can comprise a
blend of polyvinyl alcohol
homopolymers and/or anionic polyvinyl alcohol copolymers. For example, the
first water-
soluble film can comprise a blend of a polyvinyl alcohol homopolymer and an
anionic polyvinyl
alcohol copolymer, wherein the polyvinyl alcohol homopolymer and the anionic
polyvinyl alcohol
copolymer are present in a relative weight ratio of 90/10 to 10/90, or 80/20
to 20/80, or 70/30 to
50/50.
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
[0042] The first water-soluble film can comprise an anionic polyvinyl alcohol
copolymer
comprising an anionic monomer unit, optionally wherein the anionic monomer
unit is present in
the anionic polyvinyl alcohol copolymer in an average amount in a range of
about 1 mol.% to
about 10 mol.%, or about 2 mol.% to about 5 mol%. The anionic polyvinyl
alcohol copolymer
can be selected from sulphonated and carboxylated anionic polyvinyl alcohol
copolymers, e.g.
carboxylated anionic polyvinyl alcohol copolymers.
[0043] The first water-soluble film can comprise a blend of a polyvinyl
alcohol homopolymer
and a carboxylated anionic polyvinyl alcohol copolymer, optionally wherein the
carboxylate is
selected from an acrylate, a methacrylate, a maleate, or a mixture thereof,
and a maleate is
particularly contemplated. The carboxylated anionic monomer unit in the first
water-soluble film
can be derived from a monoalkyl maleate unit optionally selected from the
group consisting of
monomethyl maleate, salts, e.g. alkali metal salts, thereof, and combinations
thereof. Without
wishing to be bound by theory polyvinyl alcohol polymer comprising
carboxylated anionic
monomer units, vinyl alcohol monomer units and optionally vinyl acetate
monomer units is an
anionic polyvinyl alcohol copolymer. Each carboxylated anionic monomer unit
can be present in
the carboxylated anionic polyvinyl alcohol copolymer in an average amount in a
range of about
3m01.% to about 6m01.%, or about 3m01.% to about 5mo1.%, or about 3.5mo1.% to
about
4.5mo1.%, or about 4mo1. /0 to about 4.5mo1. /0.
[0044] Without wishing to be bound by theory, the term "homopolymer" generally
includes
polymers having a single type of monomeric repeating unit (e.g., a polymeric
chain comprising
or consisting of a single monomeric repeating unit). For the particular case
of polyvinyl alcohol
polymer, the term "homopolymer" further includes copolymers having a
distribution of vinyl
alcohol monomer units and optionally vinyl acetate monomer units, depending on
the degree of
hydrolysis (e.g., a polymeric chain comprising or consisting of vinyl alcohol
and vinyl acetate
monomer units). In the case of 100% hydrolysis, a polyvinyl alcohol
homopolymer can include
only vinyl alcohol units. Without wishing to be bound by theory, the term
"copolymer" generally
includes polymers having two or more types of monomeric repeating units (e.g.,
a polymeric
chain comprising or consisting of two or more different monomeric repeating
units, whether as
random copolymers, block copolymers, etc.). For the particular case of
polyvinyl alcohol
polymer, the term "copolymer" (or "polyvinyl alcohol copolymer") can include
copolymers having
a distribution of vinyl alcohol monomer units and vinyl acetate monomer units,
depending on the
degree of hydrolysis, as well as at least one other type of monomeric
repeating unit (e.g., a ter-
(or higher) polymeric chain comprising or consisting of vinyl alcohol monomer
units, vinyl
acetate monomer units, and one or more other monomer units, for example
anionic monomer
units). In the case of 100% hydrolysis, a polyvinyl alcohol copolymer can
include a copolymer
having vinyl alcohol units and one or more other monomer units, but no vinyl
acetate units.
Without wishing to be bound by theory, the term "anionic copolymer" includes
copolymers
having an anionic monomer unit comprising an anionic moiety. General classes
of anionic
11
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
monomer units include the vinyl polymerization units corresponding to
monocarboxylic acid vinyl
monomers, their esters and anhydrides, dicarboxylic monomers having a
polymerizable double
bond, their esters and anhydrides, vinyl sulfonic acid monomers, and alkali
metal salts of any of
the foregoing. Examples of anionic monomer units include the vinyl
polymerization units
corresponding to vinyl anionic monomers including vinyl acetic acid, maleic
acid, monoalkyl
maleate, dialkyl maleate, monomethyl maleate, dimethyl maleate, maleic
anhydride, fumaric
acid, monoalkyl fumarate, dialkyl fumarate, monomethyl fumarate, dimethyl
fumarate, fumaric
anyhydride, itaconic acid, monomethyl itaconate, dimethyl itaconate, itaconic
anhydride, vinyl
sulfonic acid, allyl sulfonic acid, ethylene sulfonic acid, 2-acrylamido-1-
methylpropanesulfonic
acid, 2-acrylamido-2-methylpropanesulfonic acid, 2-methylacrylamido-2-
methylpropanesulfonic
acid, 2-sufoethyl acrylate, alkali metal salts of the foregoing (e.g., sodium,
potassium, or other
alkali metal salts), esters of the foregoing (e.g., methyl, ethyl, or other C1-
C4 or C6 alkyl esters),
and combinations thereof (e.g., multiple types of anionic monomers or
equivalent forms of the
same anionic monomer). Anionic monomers may include one or more acrylamido
methylpropanesulfonic acids (e.g., 2-acrylamido-1-methylpropanesulfonic acid,
2-acrylamido-2-
methylpropanesulfonic acid, 2-methylacrylamido-2-methylpropanesulfonic acid),
alkali metal
salts thereof (e.g., sodium salts), and combinations thereof.
[0045] The first polyvinyl alcohol resin optionally can be present in a range
of about 50% to
about 95%, or in a range of about 50% to about 80%, or about 60% to about 75%,
by weight of
the first water-soluble film.
[0046] The first polyvinyl alcohol resin optionally can comprise:
I. a first polyvinyl alcohol polymer comprising
carboxylated anionic monomer
units, vinyl alcohol monomer units and optionally vinyl acetate monomer
units, and wherein the carboxylated anionic monomer unit is derived from a
member selected from the group consisting of maleic acid, monoalkyl
maleate, dialkyl maleate, maleic anhydride, and combinations thereof;
ii. a second PVOH polymer wherein the second PVOH
polymer is a
homopolymer wherein the homopolymer consists of vinyl alcohol monomer
units and optionally vinyl acetate monomer units.
[0047] The first polyvinyl alcohol polymer in the first water-
soluble film optionally can be
characterized by;
a. an average 4% aqueous solution viscosity (in
deionized water) at 20 C of
in a range of about lOmPa.s to about 40mPa.s, or in a range of about
lOmPa.s to about 30mPa.s, or in a range of about 12mPa.s to about
25mPa.s, or in a range of 14mPa.s to about 20mPa.s, or
12
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
b. an average degree of hydrolysis of in a range of about 60% to about 99%,
in a range of 80% to about 98%, or in a range of about 83% to about
95%, or in a range of about 85% to about 92%, or
c. a combination thereof.
[0048] The second polyvinyl alcohol polymer in the first water-soluble film
optionally can be
characterized by:
a. an average 4% aqueous solution viscosity (in deionized water) at 20 C in
a range of about 3mPa.s to about 30mPa.s, or in a range of about 7mPa.s to
about 30mPa.s, or in a range of about lOmPa.s to about 30mPa.s, or in a range
of about 12mPa.s to about 25mPa.s; or
b. an average degree of hydrolysis of in a range of about 60% to about 99%,
or in a range of about 80% to about 98%, or in a range of about 85% to about
95%, or in a range of about 87% to about 92%; or
c. a combination thereof.
[0049] The viscosity of a polyvinyl alcohol polymer is determined by measuring
a freshly
made solution using a Brookfield LV type viscometer with UL adapter as
described in British
Standard EN ISO 15023-2:2006 Annex E Brookfield Test method. It is
international practice to
state the viscosity of 4% aqueous polyvinyl alcohol solutions (in deionized
water) at 20 C.
[0050] In the first water-soluble film, the relative weight ratio
of the first PVOH polymer and
second PVOH polymer optionally can be in a range of about 90/10 to about
10/90, or in a range
of about 80/20 to about 20/80, or in a range of about 70/30 to about 50/50.
[0051] The water-soluble films, including the first, second, and
third water-soluble films can
be characterized by or tested for tensile stress according to the Modulus
(MOD) Test as follows.
The procedure includes the determination of modulus at 10% elongation
according to ASTM D
882 ("Standard Test Method for Tensile Properties of Thin Plastic Sheeting")
or equivalent. An
INSTRON tensile testing apparatus (Model 5544 Tensile Tester or equivalent) is
used for the
collection of film data. A minimum of three test specimens, each cut with
reliable cutting tools to
ensure dimensional stability and reproducibility, are tested in the machine
direction (MD) (where
applicable) for each measurement. Tests are conducted in the standard
laboratory atmosphere
of 23 2.0 C and 35 5 % relative humidity. One inch wide (2.54 cm) samples
of a single film
sheet having a thickness of 75 m are prepared. The sample is then transferred
to the
INSTRON tensile testing machine to proceed with testing while minimizing
exposure in the 35%
relative humidity environment. The tensile testing machine is prepared
according to
manufacturer instructions, equipped with a 500 N load cell, and calibrated.
The correct grips
and faces are fitted (INSTRON grips having model number 2702-032 faces, which
are rubber
coated and 25 mm wide, or equivalent). The samples are mounted into the
tensile testing
13
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
machine and analyzed to determine the 100% modulus (i.e., stress required to
achieve 100%
film elongation).
[0052] The first water-soluble film can be characterized by 100% modulus value
of at least
about 20 N/mm2 as measured by the MOD Test at 35% RH. Generally, higher MOD
values are
desirable because they correspond to pouches having a greater stiffness and a
lower likelihood
of deforming and sticking to each other when loaded on top of each other
during production or
in final consumer packaging. Further, MOD values at 10% elongation correspond
to the ability
of the film to maintain stiffness rather than loosen and droop when in contact
with liquid pouch
contents. In particular, films having higher MOD values correspond to pouches
that are less
likely to soften and take on a loose and droopy appearance when in contact
with liquid pouch
contents comprising a low molecular weight polyol. In various embodiments, the
first water-
soluble film has a MOD value of at least about 20, 21, 22, 23, 24, 25, or 27
N/mm2 and/or up to
about 24, 25, 27, 28, 29, or 30 N/mm2 (e.g., about 20 N/mm2 to about 30 N/mm2,
or about
20 N/mm2 to about 28 N/mm2, or about 22 N/mm2 to about 25 N/mm2).
[0053] The first water-soluble film optionally can comprise a surfactant
content in a range of
about 0.1% to about 3.5%, or about 0.1% to about 2.5%, or in a range of about
1% to about 2%,
or in a range of about 0.5% to about 2% by weight of the water-soluble film.
Suitable surfactants
can include the nonionic, cationic, anionic and zwitterionic classes. Suitable
surfactants include,
but are not limited to nonionics, including but not limited to
polyoxyethylenated
polyoxypropylene glycols, alcohol ethoxylates, alkylphenol ethoxylates,
tertiary acetylenic
glycols and alkanolamides; cationics, including but not limited to
polyoxyethylenated amines,
quaternary ammonium salts and quaternized polyoxyethylenated amines; and
zwitterionics,
including but not limited to amine oxides, N-alkylbetaines and sulfobetaines.
For example, a
nonionic surfactant can be selected from alcohol ethoxylates; a cationic
surfactant can be
selected from quaternary ammonium salts; and a zwitterionic surfactant can be
selected from
amine oxides. Other suitable surfactants include dioctyl sodium
sulfosuccinate, lactylated fatty
acid esters of glycerol and propylene glycol, lactylic esters of fatty acids,
sodium alkyl sulfates,
polysorbate 20, polysorbate 60, polysorbate 65, polysorbate 80, lecithin,
acetylated fatty acid
esters of glycerol and propylene glycol, and acetylated esters of fatty acids,
and combinations
thereof.
[0054] The first water-soluble film optionally can have a residual moisture
content of at least
4%, or in a range of about 4% to about 15%, or about 5% to about 10% by weight
of the first
water-soluble film as measured by Karl Fischer titration.
[0055] The first water-soluble film optionally can comprise one or more
components selected
from the group consisting of plasticizers, plasticizer connpatibilizers,
lubricants, release agents,
fillers, extenders, cross-linking agents, antiblocking agents, antioxidants,
detackifying agents,
14
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
antifoams, nanoparticles, bleaching agents, aversive agents, surfactants, and
combinations
thereof.
[0056] The first water-soluble film optionally can comprise one or more
plasticizers in an
amount in a range of about 5% to about 50%, or about 10% to about 40%, or
about 20% to
about 30% by weight of the first water-soluble film. The plasticiser in the
first water-soluble film
optionally can be selected from polyols, sugar alcohols, or a mixture thereof,
e.g. wherein the
polyols include polyols selected from the group consisting of glycerol,
diglycerin, ethylene
glycol, diethylene glycol, triethyleneglycol, tetraethylene glycol,
polyethylene glycols up to 400
MW, neopentyl glycol, 1,2-propylene glycol, 1,3-propanediol, dipropylene
glycol, polypropylene
glycol, 2-methyl-1,3-propanediol, trimethylolpropane and polyether polyols, or
a mixture thereof,
wherein sugar alcohols include sugar alcohols selected from the group
consisting of isomalt,
maltitol, sorbitol, xylitol, erythritol, adonitol, dulcitol, pentaerythritol
and mannitol, or a mixture
thereof. The plasticizer optionally can be selected from the group consisting
of sorbitol, glycerol,
dipropyleneglycol, polyethyleneglycol, trimethylolpropane, and mixtures
thereof.
[0057] The first water-soluble film according to the invention optionally can
comprise
lubricants / release agents. Suitable lubricants/release agents can include,
but are not limited to,
fatty acids and their salts, fatty alcohols, fatty esters, fatty amines, fatty
amine acetates and fatty
amides. Lubricants/release agents can be selected from fatty acids, fatty acid
salts, and fatty
amine acetates. The amount of lubricant/release agent in the first water-
soluble film optionally is
in a range of about 0.02% to about 1.5%, or about 0.1% to about 1% by weight
of the first
water-soluble film.
[0058]
The first water-soluble film optionally can comprise fillers, extenders,
antiblocking
agents, detackifying agents or a mixture thereof. Suitable fillers, extenders,
antiblocking agents,
detackifying agents or a mixture thereof include, but are not limited to,
starches, modified
starches, crosslinked polyvinylpyrrolidone, crosslinked cellulose,
microcrystalline cellulose,
silica, metallic oxides, calcium carbonate, talc and mica. Starches, modified
starches, silica, and
mixtures thereof are particularly contemplated. The amount of filler,
extender, antiblocking
agent, detackifying agent or mixture thereof in the first water-soluble film
optionally can be in a
range of about 0.1% to about 25%, or about 1% to about 10%, or about 2% to
about 8%, or
about 3% to about 5% by weight of the first water-soluble film. In the absence
of starch, a
suitable filler, extender, antiblocking agent, detackifying agent or mixture
thereof optionally can
be present in a range of about 0.1% to about 1%, or about 4%, or about 6%, or
about 1% to
about 4%, or about 1% to about 2.5%, by weight of the first water-soluble
film. The first water-
soluble film can comprise a printed area. The area of print can be achieved
using standard
techniques, e.g. flexographic printing or inkjet printing.
[0059] The first water-soluble film can comprise an aversive agent, for
example a bittering
agent. Suitable bittering agents include, but are not limited to, naringin,
sucrose octaacetate,
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
quinine hydrochloride, denatonium benzoate, and mixtures thereof. Any suitable
level of
aversive agent may be used in the film. Suitable levels include, but are not
limited to, about
1ppm to about 5000ppm, or about 100ppm to about 2500ppm, or about 250ppm to
about
2000ppm.
[0060] The first water-soluble film, and each individual component thereof,
independently can
comprise Oppm to about 20ppm, or Oppm to about 15ppm, or Oppm to about lOppm,
or Oppm to
about 5ppm, or Oppm to about 1ppm, or Oppb to about 100ppb, or Oppb dioxane.
Those skilled
in the art will be aware of known methods and techniques to determine the
dioxane level within
water-soluble films and ingredients thereof.
[0061] A subtype of the general first water-soluble film described above will
now be provided.
This type of first water-soluble film is specifically contemplated for use
with every other water-
soluble film described herein, including the second water-soluble films, and
third water-soluble
films, as well as with each composition described herein and each method
described herein.
[0062] This first water-soluble filmcan have an average thickness, prior to
any deformation, in
a range of about 20 to about 150 micron, or about 35 to about 125 micron, or
about 50 to about
110 micron, or about 76 micron. The first water-soluble film has a first side
and a second side.
[0063] The first water-soluble film can have a water-solubility of at least
50%, or at least 75%
or at least 95%, as measured by the method described above using a glass-
filter with a
maximum pore size of 20 microns.
[0064] The first water-soluble film can be obtained by casting, blow-moulding,
extrusion or
blown extrusion of the polymeric material, as known in the art and described
further below. The
first water-soluble film can be a solvent casted water-soluble film.
[0065] This first water-soluble film comprises a first PVOH resin
wherein the first polyvinyl
alcohol resin comprises;
a first polyvinyl alcohol polymer comprising carboxylated anionic
monomer units, vinyl alcohol monomer units and optionally vinyl
acetate monomer units, and wherein the carboxylated anionic
monomer unit is derived from a member selected from the group
consisting of maleic acid, monoalkyl maleate, dialkyl maleate,
maleic anhydride, and combinations thereof;
a second PVOH polymer wherein the second PVOH polymer is a
homopolymer wherein the homopolymer consists of vinyl alcohol
monomer units and optionally vinyl acetate monomer units.
16
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
[0066] The first polyvinyl alcohol resin optionally can be present in a range
of about 50% to
about 95%, or in a range of about 50% to about 80%, or about 60% to about 75%,
by weight of
this first water-soluble film.
[0067] The carboxylated anionic monomer unit in this first water-soluble film
optionally can be
derived from a monoalkyl maleate unit, e.g. selected from the group consisting
of monomethyl
maleate, salts, e.g. alkali metal salts, thereof, and combinations thereof.
Without wishing to be
bound by theory a polyvinyl alcohol polymer comprising carboxylated anionic
monomer units,
vinyl alcohol monomer units and optionally vinyl acetate monomer units is an
anionic polyvinyl
alcohol copolymer. Each carboxylated anionic monomer unit optionally can be
present in the
first polyvinyl alcohol polymer in an average amount in a range of about
3mo1.% to about
6mol. A, or in a range of about 3mo1.% to about 5mo1. /0, or in a range of
about 3.5mo1.% to
about 4.5m01.%, or in a range of about 4m01.% to about 4.5m01.%.
[0068]
General classes of anionic monomer units include the vinyl
polymerization units
corresponding to monocarboxylic acid vinyl monomers, their esters and
anhydrides, dicarboxylic
monomers having a polymerizable double bond, their esters and anhydrides,
vinyl sulfonic acid
monomers, and alkali metal salts of any of the foregoing. Examples of anionic
monomer units
include the vinyl polymerization units corresponding to vinyl anionic monomers
including vinyl
acetic acid, maleic acid, monoalkyl maleate, dialkyl maleate, monomethyl
maleate, dimethyl
maleate, maleic anyhydride, fumaric acid, monoalkyl fumarate, dialkyl
fumarate, monomethyl
fumarate, dimethyl fumarate, fumaric anyhydride, itaconic acid, monomethyl
itaconate, dimethyl
itaconate, itaconic anhydride, vinyl sulfonic acid, allyl sulfonic acid,
ethylene sulfonic acid, 2-
acrylamido-1-methylpropanesulfonic acid, 2-acrylamido-2-methylpropanesulfonic
acid, 2-
methylacrylamido-2-methylpropanesulfonic acid, 2-sufoethyl acrylate, alkali
metal salts of the
foregoing (e.g., sodium, potassium, or other alkali metal salts), esters of
the foregoing (e.g.,
methyl, ethyl, or other C1-04 or 06 alkyl esters), and combinations thereof
(e.g., multiple types
of anionic monomers or equivalent forms of the same anionic monomer). Anionic
monomers
may include one or more acrylamido methylpropanesulfonic acids (e.g., 2-
acrylamido-1-
methylpropanesulfonic acid, 2-acrylamido-2-methylpropanesulfonic acid, 2-
methylacrylamido-2-
methylpropanesulfonic acid), alkali metal salts thereof (e.g., sodium salts),
and combinations
thereof.
[0069] The first polyvinyl alcohol polymer in the first water-
soluble resin optionally is
characterized by:
a. an average viscosity in a range of about lOmPa.s
to about 40mPa.s, or in
a range of about lOmPa.s to about 30mPa.s, or in a range of about
12mPa.s to about 25mPa.s, or in a range of about 14mPa.s to about
20mPa.s, measured as a 4% polyvinyl alcohol polymer solution in
deionized water at 20 C or
17
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
b. an average degree of hydrolysis of about 60% to about 99%, or about
80% to about 98%, or about 83% to about 95%, or about 85% to about
92%, or
c. a combination thereof.
[0070] The second polyvinyl alcohol polymer in the first water-
soluble resin optionally is
characterized by;
a. an average viscosity in a range of about 3mPa.s to about 30mPa.s, or in
a range of about 7mPa.s to about 30mPa.s, or in a range of about
lOmPa.s to about 30mPa.s, or in a range of about 12mPa.s to about
25mPa.s, measured as a 4% polyvinyl alcohol polymer solution in
deionized water at 20 C, or
b. an average degree of hydrolysis in a range of about 60% to about 99%,
or about 80% to about 98%, or about 85% to about 95%, or about 87% to
about 92%, or
c. a combination thereof.
[0071] In the first water-soluble resin, the relative weight ratio
of the first PVOH polymer and
second PVOH polymer optionally can be in a range of about 90/10 to about
10/90, or about
80/20 to about 20/80, or about 70/30 to about 50/50.
[0072] In one type of embodiment, e.g. when used with laundry and automatic
dishwashing
compositions, or with fabric and homecare compositions generally, a first
polyvinyl alcohol resin
can have at least 65 wt.% of the first polyvinyl alcohol polymer comprising
carboxylated anionic
monomer units, vinyl alcohol monomer units and optionally vinyl acetate
monomer units, and
wherein the carboxylated anionic monomer unit is derived from a member
selected from the
group consisting of maleic acid, nnonoalkyl maleate, dialkyl maleate, maleic
anhydride, and
combinations thereof. Optionally, the amount of such first polyvinyl alcohol
polymer can be in a
range of about 65 wt.% to about 95wt.%, or 65wt.% to about 90wt.%, or in a
range of greater
than 65wt.% to about 95%, or greater than 65wt.% to about 90wt.%, or greater
than 65wt.% to
about 85wt.%, or about 70wt.% to about 90wt.% based on the weight of the first
polyvinyl
alcohol resin.
[0073] The first water-soluble film can be characterized by 100% modulus value
of at least
about 20 N/mm2 as measured by the MOD Test at 35% RH. Generally, higher MOD
values are
desirable because they correspond to pouches having a greater stiffness and a
lower likelihood
of deforming and sticking to each other when loaded on top of each other
during production or
in final consumer packaging. Further, MOD values at 10% elongation correspond
to the ability
of the film to maintain stiffness rather than loosen and droop when in contact
with liquid pouch
contents. In particular, films having higher MOD values correspond to pouches
that are less
18
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
likely to soften and take on a loose and droopy appearance when in contact
with liquid pouch
contents comprising a low molecular weight polyol. In various embodiments, the
first water-
soluble film has a MOD value of at least about 20, 21, 22, 23, 24, 25, or 27
N/mm2 and/or up to
about 24, 25, 27, 28, 29, or 30 N/mm2 (e.g., about 20 N/mm2 to about 30 N/mm2,
or about
20 N/mm2 to about 28 N/mm2, or about 22 N/mm2 to about 25 N/mm2).
[0074] The first water-soluble film optionally can comprise a surfactant
content in a range of
about 0.1% to about 3.5%, or about 0.1% to about 2.5%, or in a range of about
1% to about 2%,
or in a range of about 0.5% to about 2% by weight of the water-soluble film.
Suitable surfactants
can include the nonionic, cationic, anionic and zwitterionic classes. Suitable
surfactants include,
but are not limited to nonionics, including but not limited to
polyoxyethylenated
polyoxypropylene glycols, alcohol ethoxylates, alkylphenol ethoxylates,
tertiary acetylenic
glycols and alkanolamides; cationics, including but not limited to
polyoxyethylenated amines,
quaternary ammonium salts and quaternized polyoxyethylenated amines; and
zwitterionics,
including but not limited to amine oxides, N-alkylbetaines and sulfobetaines.
For example, a
nonionic surfactant can be selected from alcohol ethoxylates; a cationic
surfactant can be
selected from quaternary ammonium salts; and a zwitterionic surfactant can be
selected from
amine oxides. Other suitable surfactants include dioctyl sodium
sulfosuccinate, lactylated fatty
acid esters of glycerol and propylene glycol, lactylic esters of fatty acids,
sodium alkyl sulfates,
polysorbate 20, polysorbate 60, polysorbate 65, polysorbate 80, lecithin,
acetylated fatty acid
esters of glycerol and propylene glycol, and acetylated esters of fatty acids,
and combinations
thereof.
[0075] The first water-soluble film optionally can have a residual moisture
content of at least
4%, or in a range of about 4% to about 15%, or in a range of about 5% to about
10% by weight
of the first water-soluble film as measured by Karl Fischer titration.
[0076] The first water-soluble film can comprise one or more components
selected from the
group consisting of plasticizers, plasticizer compatibilizers, lubricants,
release agents, fillers,
extenders, cross-linking agents, antiblocking agents, antioxidants,
detackifying agents,
antifoams, nanoparticles, bleaching agents, aversive agents, surfactants, and
combinations
thereof.
[0077] The first water-soluble film can comprise one or more plasticizers in
an amount in a
range of about 5% to about 50%, or about 10% to about 40%, or about 20% to
about 30% by
weight of the first water-soluble film. The plasticiser in the first water-
soluble film optionally can
be selected from polyols, sugar alcohols, or a mixture thereof, e.g. wherein
the polyols include
polyols selected from the group consisting of glycerol, diglycerin, ethylene
glycol, diethylene
glycol, triethyleneglycol, tetraethylene glycol, polyethylene glycols up to
400 MW, neopentyl
glycol, 1,2-propylene glycol, 1,3-propanediol, dipropylene glycol,
polypropylene glycol, 2-methyl-
1,3-propanediol, trimethylolpropane and polyether polyols, or a mixture
thereof, wherein sugar
19
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
alcohols can include sugar alcohols selected from the group consisting of
isomalt, maltitol,
sorbitol, xylitol, erythritol, adonitol, dulcitol, pentaerythritol and
mannitol, or a mixture thereof.
The plasticizer can be selected from the group consisting of sorbitol,
glycerol, dipropyleneglycol,
polyethyleneglycol, trimethylolpropane, and mixtures thereof.
[0078] The first water-soluble film optionally can comprise lubricants /
release agents.
Suitable lubricants/release agents can include, but are not limited to, fatty
acids and their salts,
fatty alcohols, fatty esters, fatty amines, fatty amine acetates and fatty
amides.
Lubricants/release agents can be selected from fatty acids, fatty acid salts,
and fatty amine
acetates. The amount of lubricant/release agent in the first water-soluble
film optionally can be
in a range in a range of about 0.02% to about 1.5%, or about 0.1% to about 1%
by weight of the
first water-soluble film.
[0079]
The first water-soluble film optionally can comprise fillers, extenders,
antiblocking
agents, detackifying agents or a mixture thereof. Suitable fillers, extenders,
antiblocking agents,
detackifying agents or a mixture thereof include, but are not limited to,
starches, modified
starches, crosslinked polyvinylpyrrolidone, crosslinked cellulose,
microcrystalline cellulose,
silica, metallic oxides, calcium carbonate, talc and mica. Starches, modified
starches, silica, and
mixtures thereof are particularly contemplated. The amount of filler,
extender, antiblocking
agent, detackifying agent or mixture thereof in the first water-soluble film
optionally can be in a
range of about 0.1% to about 25%, or about 1% to about 10%, or about 2% to
about 8%, or
about 3% to about 5% by weight of the first water-soluble film. In the absence
of starch, a
suitable filler, extender, antiblocking agent, detackifying agent or mixture
thereof optionally can
be present in a range of about 0.1% to about 1%, or about 4%, or about 6%, or
about 1% to
about 4%, or about 1% to about 2.5%, by weight of the first water-soluble
film.
[0080] The first water-soluble film can comprise a printed area. The area of
print can be
achieved using standard techniques, e.g. flexographic printing or inkjet
printing.
[0081] The first water-soluble film can comprise an aversive agent, for
example a bittering
agent. Suitable bittering agents include, but are not limited to, naringin,
sucrose octaacetate,
quinine hydrochloride, denatonium benzoate, and mixtures thereof. Any suitable
level of
aversive agent may be used in the film. Suitable levels include, but are not
limited to, about
1ppm to about 5000ppm, or about 100ppm to about 2500ppm, or about 250ppm to
about
2000ppm.
[0082] The first water-soluble film, and each individual component thereof,
independently can
comprise Oppm to about 20ppm, or Oppm to about 15ppm, or Oppm to about lOppm,
or Oppm to
about 5ppm, or Oppm to about 1ppm, or Oppb to about 100ppb, or Oppb dioxane.
Those skilled
in the art will be aware of known methods and techniques to determine the
dioxane level within
water-soluble films and ingredients thereof.
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
Second water-soluble film
[0083] The water-soluble unit dose article comprises a second water-soluble
film. The
second water-soluble film has a first side and a second side. The second water-
soluble film
comprises a second polyvinyl alcohol resin.
[0084] The second water-soluble film of the present invention is soluble or
dispersible in
water. The second water-soluble film can have an average thickness, prior to
any deformation,
in a range of about 20 to about 150 micron, or about 35 to about 125 micron,
or about 50 to
about 110 micron, or about 76 micron.
[0085] The second water-soluble film can have a water-solubility of at least
50%, or at least
75% or at least 95%, as measured by the method set out here after using a
glass-filter with a
maximum pore size of 20 microns: 5 grams 0.1 gram of film material is added
in a pre-
weighed 3L beaker and 2L 5m1 of distilled water is added. This is stirred
vigorously on a
magnetic stirrer, Labline model No. 1250 or equivalent and 5 cm magnetic
stirrer, set at 600
rpm, for 30 minutes at 30 C. Then, the mixture is filtered through a folded
qualitative sintered-
glass filter with a pore size as defined above (max. 20 micron). The water is
dried off from the
collected filtrate by any conventional method, and the weight of the remaining
material is
determined (which is the dissolved or dispersed fraction). Then, the
percentage solubility or
dispersability can be calculated.
[0086] The second water-soluble film can be obtained by casting, blow-
moulding, extrusion
or blown extrusion of the polymeric material, as known in the art and
described further below.
The second water-soluble film can be a solvent casted water-soluble film.
[0087] The second water-soluble film comprises a polyvinyl alcohol resin. The
polyvinyl
alcohol resin optionally can be present in a range of about 50% to about 95%,
or in a range of
about 50% to about 80%, or about 60% to about 75%, by weight of the second
water-soluble
film...
[0088] The second polyvinyl alcohol resin comprises less than 15% by weight of
the second
polyvinyl alcohol resin of a polyvinyl alcohol polymer comprising carboxylated
anionic monomer
units, vinyl alcohol monomer units and optionally vinyl acetate monomer units,
and wherein the
carboxylated anionic monomer unit is derived from a member selected from the
group
consisting of maleic acid, monoalkyl maleate, dialkyl maleate, maleic
anhydride, and
combinations thereof. The polyvinyl alcohol polymer comprising carboxylated
anionic monomer
units can be present in the second polyvinyl alcohol resin in a range of about
lwt.% to less than
15wt%, or about 1wt% to about lOwt%, or about 1wt% to about 5wt%, or about
5wt% to about
15wt% or about 5wt% to about 10wt 70 of the second polyvinyl alcohol resin,
for example.
Without wishing to be bound by theory, a polyvinyl alcohol polymer comprising
carboxylated
anionic monomer units, vinyl alcohol monomer units and optionally vinyl
acetate monomer units
21
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
is an anionic polyvinyl alcohol copolymer. The second polyvinyl alcohol resin
also can comprise
about 85% to about 100% by weight of the second polyvinyl alcohol resin of a
polyvinyl alcohol
homopolymer or a polyvinyl alcohol homopolymer blend, wherein the polyvinyl
alcohol
homopolymers consist of vinyl alcohol monomer units and optionally vinyl
acetate monomer
units. In one type of embodiment, the second polyvinyl alcohol resin can
comprise the first
polyvinyl alcohol polymer (anionic copolymer) in an amount described herein
and the balance
can be the second polyvinyl alcohol polymer (polyvinyl alcohol hornopolymer or
a polyvinyl
alcohol homopolymer blend).
[0089] If present, the carboxylated anionic monomer unit optionally
can be present in the
polyvinyl alcohol polymer comprising a carboxylated anionic monomer unit in an
average
amount of at least 3mo1.%, or in a range of about 3mo1.% to about 6mo1.%, or
about 3mo1.% to
about 5m01.%, or about 3.5m01.% to about 4.5mo1.%, or about 4m01.% to about
4.5m01.%. The
polyvinyl alcohol polymer comprising carboxylated anionic monomer units, vinyl
alcohol
monomer units and optionally vinyl acetate monomer units in the polyvinyl
alcohol resin of the
second water-soluble film, if present, optionally can be characterized by an
average 4%
aqueous solution viscosity (in deionized water) at 20 C in a range of
about1OmPa.s to about
40mPa.s, or about lOmPa.s to about 30mPa.s, or about 12mPa.s to about 25mPas,
or about
14mPa.s to about 20mPa.s, or by an average degree of hydrolysis in a range of
about 60% to
about 99%, or about BO% to about 98%, or about 83% to about 95%, or about 85%
to about
92%, or a combination of such a viscosity and average degree of hydrolysis.
[0090] The carboxylated anionic unit can be derived from maleic acid,
monoalkyl maleate,
dialkyl maleate, monomethyl maleate, dimethyl maleate, maleic anhydride, or
mixtures thereof.
The maleate unit can be derived from a monoalkyl maleate unit optionally
selected from the
group consisting of monomethyl maleate, salts, e.g. alkali metal salts,
thereof, and combinations
thereof.
[0091] The second polyvinyl alcohol resin also can comprise a polyvinyl
alcohol
homopolymer or a polyvinyl alcohol homopolymer blend in a range of about 85%
to about 100%
by weight of the polyvinyl alcohol resin, wherein the polyvinyl alcohol
homopolymer or polyvinyl
alcohol homopolymer blend consists of vinyl alcohol monomer units and
optionally vinyl acetate
monomer units. The second water-soluble film can comprise the polyvinyl
alcohol
homopolymer or a polyvinyl alcohol homopolymer blend in a range of about 90%
to about
100%, e.g. 100% by weight of the second polyvinyl alcohol resin.
[0092] The polyvinyl alcohol resin of the second water-soluble film can
comprise a polyvinyl
alcohol homopolymer or a blend of a first polyvinyl alcohol homopolymer and a
second polyvinyl
alcohol homopolymer, the homopolymer or blend of the first and second
polyvinyl alcohol
homopolymers having an average viscosity in a range of about 8mPa.s or more
and less than
12mPa.s, or about 8.5mPa.s or more and less than 12mPa.s, or about 9mPa.s or
more and less
22
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
than 12mPa.s, or about 9.5mPa.s or more and less than 12mPa.s, or about
lOmPa.s or more
and less than 12mPa.s, or about 10.5mPa.s or more and less than 12mPa.s,
measured as a
4% polyvinyl alcohol solution in deionized water at 20 C, optionally wherein
blend of first
polyvinyl alcohol homopolymer and second polyvinyl alcohol homopolymer are
present in a
relative weight ratio in a range of about 90/10 to about 10/90, or about 80/20
to about 20/80, or
about 70/30 to about 50/50. In other embodiments, the polyvinyl alcohol
homopolymer or blend
of the first and second polyvinyl alcohol homopolymers can have an average
viscosity in a
range of about 8mPa.s to about 11.5mPa.s, or about 8.5mPa.s to about
11.5mPa.s, or about
9mPa.s to about 11.5mPa.s, or about 9.5mPa.s to about 11.5mPa.s, or about
10mPa.s to about
11.5mPa.s, or about 10.5mPa.s to about 11.5mPa.s, or about 8mPa.s to about
11mPa.s, or
about 8mPa.s to about lOmPa.s, measured as a 4% polyvinyl alcohol solution in
deionized
water at 20 C, optionally wherein the first polyvinyl alcohol homopolymer and
second polyvinyl
alcohol homopolymer are present in a relative weight ratio in a range of about
90/10 to about
10/90, or about 80/20 to about 20/80, or about 70/30 to about 50/50. Herein,
the first polyvinyl
alcohol homopolymer optionally can have an average viscosity in a range of
about 11mPa.s to
about 20mPa.s, of about 11mPa.s to about 18mPa.s, or about 11mPa.s to about
15mPa.s,
measured as a 4% polyvinyl alcohol polymer solution in deionized water at 20
C; and the
second polyvinyl alcohol homopolymer can have an average viscosity in a range
of about
1mPa.s to about lOmPa.s, optionally in a range of about 5mPa.s to about
lOmPa.s, optionally
in a range of about 6mPa.s to about lOmPa.s, optionally in a range of about
7mPa.s to about
lOmPa.s, optionally in a range of about 8mPa.s to about lOmPa.s, measured as a
4% polyvinyl
alcohol polymer solution in deionized water at 20 C. Optionally, the
difference in average
viscosity between the first polyvinyl alcohol polymer and the second polyvinyl
alcohol
homopolymer is at least about 1mPa.s, or in a range of about 2 to about
lOmPa.s, or in a range
of about 3 to about 8mPa.s, measured as a 4% polyvinyl alcohol polymer
solution in deionized
water at 20 C. In any types of embodiments, the first and the second
polyvinyl alcohol
homopolymers independently can have an average degree of hydrolysis in a range
of about
75% to about 99%, or in a range of about 80% to about 95%, or in a range of
about 85% to
about 95%. Optionally, the polyvinyl alcohol resin of the second water soluble
film can have an
average degree of hydrolysis in a range of about 75% to about 99%, or about
80% to about
95%, or about 85% to about 95%. A suitable test method to measure the degree
of hydrolysis is
as according to standard method JIS K6726.
[0093] The second water-soluble film can be characterized 100% modulus values
of less
than 20 N/mm2 as measured by the MOD Test at 35% RH. Generally, higher MOD
(e.g. 20
N/mm2 or greater) values are desirable because they correspond to films having
a greater
stiffness and a lower likelihood of deforming and sticking to each other when
loaded on top of
each other during production or in final consumer packaging. Further, MOD
values at 100%
elongation correspond to the ability of the film to maintain stiffness rather
than loosen and droop
23
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
when in contact with liquid pouch contents. In particular, films having higher
MOD values
correspond to pouches that are less likely to soften and take on a loose and
droopy appearance
when in contact with liquid pouch contents comprising a low molecular weight
polyol. However,
it was determined that the second polyvinyl alcohol film can advantageously
have a lower 100%
modulus as described herein. Furthermore, when used as a middle film in a
superposed pouch
configuration as described herein, a relatively lower MOD value and the
resultant tendency of a
film to droop is negated by the configuration of the pouch, wherein the second
film is essentially
wholly within the pouch product. In various embodiments, the second water-
soluble film can
have a MOD value of less than about 20N/mm2, or less than about 19N/mm2, or
less than
about 18N/mm2, or less than about 17N/mm2, or less than about 16N/mm2, or less
than about
15N/mm2, or less than about 14N/mm2, and optionally at least about 9N/mm2, or
at least about
10N/mm2, or at least about 11N/mm2, at least about 12N/mm2, or at least about
13N/mm2, for
example in a range of about 10N/mm2 to about 16N/mm2, or about 11N/mm2 to
about 15N/mm2,
or about 12N/mm2 to about 14N/mm2. In a related aspect, the second water-
soluble film can be
characterized by having a 100% modulus value that is at least about 1N/mm2, or
at least about
2N/mm2, or at least about 3N/mm2, or at least about 4N/mm2, or at least about
5N/mm2, or at
least about 6N/mm2, or at least about 7N/mm2, or at least about 1 ON/mm2, or
at least about
20N/mm2, or at least about 25N/mm2 different from the 100% modulus value of
the first water-
soluble film, and further optionally at least about 1N/mm2, or at least about
2N/mm2, or at least
about 3N/mm2, or at least about 4N/mm2, or at least about 5N/mm2, or at least
about 6N/mm2,
or at least about 7N/mm2, or at least about 10N/mm2, or at least about 20N/mm2
different from
the 100 /0 modulus value of the third water-soluble film, and still further
optionally at least about
1N/mm2, or at least about 2N/mm2, or at least about 3N/mm2, or at least about
4N/mm2, or at
least about 5N/mm2, or at least about 6N/mm2, or at least about 7N/mm2, or at
least about
10N/nrim2, or at least about 20N/mm2 different from the 100% modulus value of
both the values
of the first water-soluble film and the third water-soluble film,
respectively.
[0094] The second water-soluble film optionally can comprise a surfactant
content in a range
of about 0.1% to about 3.5%, or about 0.1% to about 2.5%, or in a range of
about 1% to about
2%, or in a range of about 0.5% to about 2% by weight of the water-soluble
film. Suitable
surfactants can include the nonionic, cationic, anionic and zwitterionic
classes. Suitable
surfactants include, but are not limited to nonionics, including but not
limited to
polyoxyethylenated polyoxypropylene glycols, alcohol ethoxylates, alkylphenol
ethoxylates,
tertiary acetylenic glycols and alkanolamides; cationics, including but not
limited to
polyoxyethylenated amines, quaternary ammonium salts and quaternized
polyoxyethylenated
amines; and zwitterionics, including but not limited to amine oxides, N-
alkylbetaines and
sulfobetaines. For example, a nonionic surfactant can be selected from alcohol
ethoxylates; a
cationic surfactant can be selected from quaternary ammonium salts; and a
zwitterionic
surfactant can be selected from amine oxides. Other suitable surfactants
include dioctyl sodium
24
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
sulfosuccinate, lactylated fatty acid esters of glycerol and propylene glycol,
lactylic esters of
fatty acids, sodium alkyl sulfates, polysorbate 20, polysorbate 60,
polysorbate 65, polysorbate
80, lecithin, acetylated fatty acid esters of glycerol and propylene glycol,
and acetylated esters
of fatty acids, and combinations thereof.
[0095] The second water-soluble film optionally can have a residual moisture
content of at
least about 4%, or in a range of about 4% to about 15%, or about 5% to about
10% by weight of
the second water-soluble film as measured by Karl Fischer titration.
[0096] The second water-soluble film optionally can comprise one or more
components
selected from the group consisting of plasticizers, plasticizer
compatibilizers, lubricants, release
agents, fillers, extenders, cross-linking agents, antiblocking agents,
antioxidants, detackifying
agents, antifoams, nanoparticles, bleaching agents, aversive agents,
surfactants, and
combinations thereof.
[0097] The second water-soluble film optionally can comprise one or more
plasticizers in an
amount in a range of about 5% to about 50%, or about 10% to about 40%, or
about 20% to
about 30% by weight of the second water-soluble film. The plasticiser in the
second water-
soluble film optionally can be selected from polyols, sugar alcohols, or a
mixture thereof, e.g.
wherein the polyols include polyols selected from the group consisting of
glycerol, diglycerin,
ethylene glycol, diethylene glycol, triethyleneglycol, tetraethylene glycol,
polyethylene glycols up
to 400 MW, neopentyl glycol, 1,2-propylene glycol, 1,3-propanediol,
dipropylene glycol,
polypropylene glycol, 2-methyl-1,3-propanediol, trimethylolpropane and
polyether polyols, or a
mixture thereof, wherein sugar alcohols include sugar alcohols selected from
the group
consisting of isomalt, maltitol, sorbitol, xylitol, erythritol, adonitol,
dulcitol, pentaerythritol and
mannitol, or a mixture thereof. The plasticizer can be selected from the group
consisting of
sorbitol, glycerol, dipropyleneglycol, polyethyleneglycol, trimethylolpropane,
and mixtures
thereof.
[0098] The second water-soluble film according to the invention can comprise
lubricants /
release agents. Suitable lubricants/release agents can include, but are not
limited to, fatty acids
and their salts, fatty alcohols, fatty esters, fatty amines, fatty amine
acetates and fatty amides.
Lubricants/release agents can be selected from fatty acids, fatty acid salts,
and fatty amine
acetates. The amount of lubricant/release agent in the second water-soluble
film optionally can
be in a range of about 0.02% to about 1.5%, or about 0.1% to about 1% by
weight of the second
water-soluble film.
[0099] The second water-soluble film optionally can comprises fillers,
extenders, antiblocking
agents, detackifying agents or a mixture thereof. Suitable fillers, extenders,
antiblocking agents,
detackifying agents or a mixture thereof include, but are not limited to,
starches, modified
starches, crosslinked polyvinylpyrrolidone, crosslinked cellulose,
microcrystalline cellulose,
silica, metallic oxides, calcium carbonate, talc and mica. Starches, modified
starches, silica, and
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
mixtures thereof are particularly contemplated. The amount of filler,
extender, antiblocking
agent, detackifying agent or mixture thereof in the second water-soluble film
optionally can be in
a range of about 0.1% to about 25%, or about 1% to about 10%, or about 2% to
about 8%, or
about 3% to about 5% by weight of the second water-soluble film. In the
absence of starch, a
suitable filler, extender, antiblocking agent, detackifying agent or mixture
thereof optionally can
be present in a range of about 0.1% to about 1%, or about 4%, or about 6%, or
about 1% to
about 4%, or about 1% to about 2.5%, by weight of the second water-soluble
film.
[0100] The second water-soluble film can comprise a printed area. The area of
print can be
achieved using standard techniques, e.g. flexographic printing or inkjet
printing.
[0101] The second water-soluble film can comprise an aversive agent, for
example a bittering
agent. Suitable bittering agents include, but are not limited to, naringin,
sucrose octaacetate,
quinine hydrochloride, denatonium benzoate, and mixtures thereof. Any suitable
level of
aversive agent may be used in the second water-soluble film. Suitable levels
include, but are
not limited to, about 1ppm to about 5000ppm, or about 100ppm to about 2500ppm,
or about
250ppm to about 2000ppm.
[0102] The second water-soluble film, and each individual component thereof,
independently
can comprise Oppm to about 20ppm, or Oppm to about 15ppm, or Oppm to about
lOppm, or
Oppm to about 5ppm, or Oppm to about 1ppm, or Oppb to about 100ppb, or Oppb
dioxane.
Those skilled in the art will be aware of known methods and techniques to
determine the
dioxane level within water-soluble films and ingredients thereof.
Third water-soluble film
[0103] The water-soluble unit dose article can comprise a third water-soluble
film. The third
water-soluble film of the present invention is soluble or dispersible in
water. The third water-
soluble film can have an average thickness, prior to any deformation, in a
range of about 20 to
about 150 micron, or about 35 to about 125 micron, or about 50 to about 110
micron, or about
76 micron. The third water-soluble film has a first side and a second side.
[0104] The third water-soluble film can have a water-solubility of at least
50%, or at least 75%
or at least 95%, as measured by the method described above using a glass-
filter with a
maximum pore size of 20 microns.
[0105] The third water-soluble film can be obtained by casting, blow-moulding,
extrusion or
blown extrusion of the polymeric material, as known in the art and described
further below. The
third water-soluble film can be a solvent casted water-soluble film.
[0106] The third water soluble film comprises a third PVOH resin wherein the
third polyvinyl
alcohol resin comprises a polyvinyl alcohol consisting of a polyvinyl alcohol
homopolymer, an
anionic polyvinyl alcohol copolymer, or a blend thereof.
26
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
[0107] In one aspect, the third water-soluble film can comprise a blend of
polyvinyl alcohol
homopolymers and/or anionic polyvinyl alcohol copolymers. For example, the
third water-
soluble film can comprise a blend of a polyvinyl alcohol homopolymer and an
anionic polyvinyl
alcohol copolymer, wherein the polyvinyl alcohol homopolymer and the anionic
polyvinyl alcohol
copolymer are present in a relative weight ratio of 90/10 to 10/90, or 80/20
to 20/80, or 70/30 to
50/50.
[0108] The third water-soluble film can comprise an anionic polyvinyl alcohol
copolymer
comprising an anionic monomer unit, optionally wherein the anionic monomer
unit is present in
the anionic polyvinyl alcohol copolymer in an average amount in a range of
about 1 mol.% to
about 10 mol.%, or about 2 mol.% to about 5 mor/o. The anionic polyvinyl
alcohol copolymer
can be selected from sulphonated and carboxylated anionic polyvinyl alcohol
copolymers, e.g.
carboxylated anionic polyvinyl alcohol copolymers.
[0109] The third water-soluble film can comprise a blend of a polyvinyl
alcohol homopolymer
and a carboxylated anionic polyvinyl alcohol copolymer, optionally wherein the
carboxylate is
selected from an acrylate, a methacrylate, a maleate, or a mixture thereof,
and a maleate is
particularly contemplated. The carboxylated anionic monomer unit in the third
water-soluble film
can be derived from a monoalkyl maleate unit optionally selected from the
group consisting of
monomethyl maleate, salts, e.g. alkali metal salts, thereof, and combinations
thereof. Without
wishing to be bound by theory polyvinyl alcohol polymer comprising
carboxylated anionic
monomer units, vinyl alcohol monomer units and optionally vinyl acetate
monomer units is an
anionic polyvinyl alcohol copolymer. Each carboxylated anionic monomer unit
can be present in
the carboxylated anionic polyvinyl alcohol copolymer in an average amount in a
range of about
3mo1.% to about 6mo1.%, or about 3mo1.(3/0 to about 5mo1.%, or about 3.5mo1.%
to about
4.5m01.%, or about 4m01.% to about 4.5m01.%.
[0110] General classes of anionic monomer units include the vinyl
polymerization units
corresponding to monocarboxylic acid vinyl monomers, their esters and
anhydrides, dicarboxylic
monomers having a polymerizable double bond, their esters and anhydrides,
vinyl sulfonic acid
monomers, and alkali metal salts of any of the foregoing. Examples of anionic
monomer units
include the vinyl polymerization units corresponding to vinyl anionic monomers
including vinyl
acetic acid, maleic acid, monoalkyl maleate, dialkyl maleate, monomethyl
maleate, dimethyl
maleate, maleic anhydride, fumaric acid, monoalkyl fumarate, dialkyl fumarate,
monomethyl
fumarate, dimethyl fumarate, fumaric anhydride, itaconic acid, monomethyl
itaconate, dimethyl
itaconate, itaconic anhydride, vinyl sulfonic acid, allyl sulfonic acid,
ethylene sulfonic acid, 2-
acrylamido-1-methylpropanesulfonic acid, 2-acrylamido-2-methylpropanesulfonic
acid, 2-
methylacrylamido-2-methylpropanesulfonic acid, 2-sufoethyl acrylate, alkali
metal salts of the
foregoing (e.g., sodium, potassium, or other alkali metal salts), esters of
the foregoing (e.g.,
methyl, ethyl, or other C1-C4 or C6 alkyl esters), and combinations thereof
(e.g., multiple types
27
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
of anionic monomers or equivalent forms of the same anionic monomer). Anionic
monomers
may include one or more acrylamido methylpropanesulfonic acids (e.g., 2-
acrylamido-1-
methylpropanesulfonic acid, 2-acrylamido-2-methylpropanesulfonic acid, 2-
methylacrylamido-2-
methylpropanesulfonic acid), alkali metal salts thereof (e.g., sodium salts),
and combinations
thereof.
[0111] The third polyvinyl alcohol resin optionally can be present in a range
of about 50% to
about 95%, or in a range of about 50% to about 80%, or about 60% to about 75%,
by weight of
the third water-soluble film.
[0112] The third polyvinyl alcohol resin optionally can comprise:
a first polyvinyl alcohol polymer comprising carboxylated anionic
monomer units, vinyl alcohol monomer units and optionally vinyl
acetate monomer units, and wherein the carboxylated anionic
monomer unit is derived from a member selected from the group
consisting of maleic acid, monoalkyl maleate, dialkyl maleate,
maleic anhydride, and combinations thereof;
a second PVOH polymer wherein the second PVOH polymer is a
homopolymer wherein the homopolymer consists of vinyl alcohol
monomer units and optionally vinyl acetate monomer units.
[0113] The first polyvinyl alcohol polymer in the third water-soluble film
optionally can be
characterized by;
a. an average 4% aqueous solution viscosity (in deionized water) at 20 C of in
a
range of about lOmPa.s to about 40mPa.s, or in a range of about lOmPa.s to
about 30mPa.s, or in a range of about 12mPa.s to about 25mPa.s, or in a range
of 14mPa.s to about 20mPa.s, or
b. an average degree of hydrolysis of in a range of about 60% to about 99%, in
a
range of 80% to about 98%, or in a range of about 83% to about 95%, or in a
range of about 85% to about 92%, or
c. a combination thereof.
[0114] The second polyvinyl alcohol polymer in the third water-soluble film
optionally can be
characterized by:
a. an average 4% aqueous solution viscosity (in deionized water) at 20 C in a
range of about 3mPa.s to about 30mPa.s, or in a range of about 7mPa.s to about
30mPa.s, or in a range of about lOmPa.s to about 30mPa.s, or in a range of
about 12mPa.s to about 25mPa.s; or
28
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
b. an average degree of hydrolysis of in a range of about 60% to about 99%, or
in a
range of about 80% to about 98%, or in a range of about 85% to about 95%, or
in
a range of about 87% to about 92%; or
c. a combination thereof.
[0115] In the third water-soluble film, the relative weight ratio of the first
PVOH polymer and
second PVOH polymer optionally can be in a range of about 90/10 to about
10/90, or in a range
of about 80/20 to about 20/80, or in a range of about 70/30 to about 50/50.
[0116] The third water-soluble film can be characterized by 100% modulus value
of at least
about 20 N/mm2 as measured by the MOD Test at 35% RH. Generally, higher MOD
values are
desirable because they correspond to pouches having a greater stiffness and a
lower likelihood
of deforming and sticking to each other when loaded on top of each other
during production or
in final consumer packaging. Further, MOD values at 10% elongation correspond
to the ability
of the film to maintain stiffness rather than loosen and droop when in contact
with liquid pouch
contents. In particular, films having higher MOD values correspond to pouches
that are less
likely to soften and take on a loose and droopy appearance when in contact
with liquid pouch
contents comprising a low molecular weight polyol. In various embodiments, the
third water-
soluble film has a MOD value of at least about 20, 21, 22, 23, 24, 25, or 27
N/mm2 and/or up to
about 24, 25, 27, 28, 29, or 30 N/mm2 (e.g., about 20 N/mm2 to about 30 N/mm2,
or about
20 N/mm2 to about 28 N/mm2, or about 22 N/mm2 to about 25 N/mm2).
[0117] The third water-soluble film optionally can comprise a surfactant
content in a range of
about 0.1% to about 3.5%, or about 0.1% to about 2.5%, or in a range of about
1% to about 2%,
or in a range of about 0.5% to about 2% by weight of the water-soluble film.
Suitable surfactants
can include the nonionic, cationic, anionic and zwitterionic classes. Suitable
surfactants include,
but are not limited to nonionics, including but not limited to
polyoxyethylenated
polyoxypropylene glycols, alcohol ethoxylates, alkylphenol ethoxylates,
tertiary acetylenic
glycols and alkanolamides; cationics, including but not limited to
polyoxyethylenated amines,
quaternary ammonium salts and quaternized polyoxyethylenated amines; and
zwitterionics,
including but not limited to amine oxides, N-alkylbetaines and sulfobetaines.
For example, a
nonionic surfactant can be selected from alcohol ethoxylates; a cationic
surfactant can be
selected from quaternary ammonium salts; and a zwitterionic surfactant can be
selected from
amine oxides. Other suitable surfactants include dioctyl sodium
sulfosuccinate, lactylated fatty
acid esters of glycerol and propylene glycol, lactylic esters of fatty acids,
sodium alkyl sulfates,
polysorbate 20, polysorbate 60, polysorbate 65, polysorbate 80, lecithin,
acetylated fatty acid
esters of glycerol and propylene glycol, and acetylated esters of fatty acids,
and combinations
thereof.
29
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
[0118] The third water-soluble film optionally can have a residual moisture
content of at least
4%, or in a range of about 4% to about 15%, or about 5% to about 10% by weight
of the third
water-soluble film as measured by Karl Fischer titration.
[0119] The third water-soluble film optionally can comprise one or more
components selected
from the group consisting of plasticizers, plasticizer connpatibilizers,
lubricants, release agents,
fillers, extenders, cross-linking agents, antiblocking agents, antioxidants,
detackifying agents,
antifoams, nanoparticles, bleaching agents, aversive agents, surfactants, and
combinations
thereof.
[0120] The third water-soluble film optionally can comprise one or more
plasticizers in an
amount in a range of about 5% to about 50%, or about 10% to about 40%, or
about 20% to
about 30% by weight of the third water-soluble film. The plasticiser in the
third water-soluble
film optionally can be selected from polyols, sugar alcohols, or a mixture
thereof, e.g. wherein
the polyols include polyols selected from the group consisting of glycerol,
diglycerin, ethylene
glycol, diethylene glycol, triethyleneglycol, tetraethylene glycol,
polyethylene glycols up to 400
MW, neopentyl glycol, 1,2-propylene glycol, 1,3-propanediol, dipropylene
glycol, polypropylene
glycol, 2-methyl-1,3-propanediol, trimethylolpropane and polyether polyols, or
a mixture thereof,
wherein sugar alcohols include sugar alcohols selected from the group
consisting of isomalt,
maltitol, sorbitol, xylitol, erythritol, adonitol, dulcitol, pentaerythritol
and mannitol, or a mixture
thereof. The plasticizer optionally can be selected from the group consisting
of sorbitol, glycerol,
dipropyleneglycol, polyethyleneglycol, trimethylolpropane, and mixtures
thereof.
[0121] The third water-soluble film according to the invention optionally can
comprise
lubricants / release agents. Suitable lubricants/release agents can include,
but are not limited to,
fatty acids and their salts, fatty alcohols, fatty esters, fatty amines, fatty
amine acetates and fatty
amides. Lubricants/release agents can be selected from fatty acids, fatty acid
salts, and fatty
amine acetates. The amount of lubricant/release agent in the third water-
soluble film optionally
is in a range of about 0.02% to about 1.5%, or about 0.1% to about 1% by
weight of the third
water-soluble film.
[0122] The third water-soluble film optionally can comprise fillers,
extenders, antiblocking
agents, detackifying agents or a mixture thereof. Suitable fillers, extenders,
antiblocking agents,
detackifying agents or a mixture thereof include, but are not limited to,
starches, modified
starches, crosslinked polyvinylpyrrolidone, crosslinked cellulose,
microcrystalline cellulose,
silica, metallic oxides, calcium carbonate, talc and mica. Starches, modified
starches, silica, and
mixtures thereof are particularly contemplated. The amount of filler,
extender, antiblocking
agent, detackifying agent or mixture thereof in the third water-soluble film
optionally can be in a
range of about 0.1% to about 25%, or about 1% to about 10%, or about 2% to
about 8%, or
about 3% to about 5% by weight of the third water-soluble film. In the absence
of starch, a
suitable filler, extender, antiblocking agent, detackifying agent or mixture
thereof optionally can
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
be present in a range of about 0.1% to about 1%, or about 4%, or about 6%, or
about 1% to
about 4%, or about 1 /0 to about 2.5%, by weight of the third water-soluble
film. The third water-
soluble film can comprise a printed area. The area of print can be achieved
using standard
techniques, e.g. flexographic printing or inkjet printing.
[0123] The third water-soluble film can comprise an aversive agent, for
example a bittering
agent. Suitable bittering agents include, but are not limited to, naringin,
sucrose octaacetate,
quinine hydrochloride, denatonium benzoate, and mixtures thereof. Any suitable
level of
aversive agent may be used in the film. Suitable levels include, but are not
limited to, about
1ppm to about 5000ppm, or about 100ppm to about 2500ppm, or about 250ppm to
about
2000ppm.
[0124] The third water-soluble film, and each individual component thereof,
independently can
comprise Oppm to about 2Oppm, or Oppm to about 15ppm, or Oppm to about lOppm,
or Oppm to
about 5ppm, or Oppm to about 1ppm, or Oppb to about 100ppb, or Oppb dioxane.
Those skilled
in the art will be aware of known methods and techniques to determine the
dioxane level within
water-soluble films and ingredients thereof.
[0125] A subtype of the general third water-soluble film described above will
now be provided.
This type of third water-soluble film is specifically contemplated for use
with every other water-
soluble film described herein, including the second water-soluble films, and
first water-soluble
films, as well as with each composition described herein and each method
described herein.
[0126] This third water-soluble film can have an average thickness, prior to
any deformation,
in a range of about 20 to about 150 micron, or about 35 to about 125 micron,
or about 50 to
about 110 micron, or about 76 micron. The third water-soluble film has a first
side and a second
side.
[0127] The third water-soluble film can have a water-solubility of at least
50%, or at least 75%
or at least 95%, as measured by the method set out here after using a glass-
filter with a
maximum pore size of 20 microns: 5 grams 0.1 gram of film material is added
in a pre-
weighed 3L beaker and 2L 5m1 of distilled water is added. This is stirred
vigorously on a
magnetic stirrer, Labline model No. 1250 or equivalent and 5 cm magnetic
stirrer, set at 600
rpm, for 30 minutes at 30 C. Then, the mixture is filtered through a folded
qualitative sintered-
glass filter with a pore size as defined above (max. 20 micron). The water is
dried off from the
collected filtrate by any conventional method, and the weight of the remaining
material is
determined (which is the dissolved or dispersed fraction). Then, the
percentage solubility or
dispersability can be calculated.
[0128] The third water-soluble film can be obtained by casting, blow-moulding,
extrusion or
blown extrusion of the polymeric material, as known in the art and described
further below. The
third water-soluble film can be a solvent casted water-soluble film.
31
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
[0129] The third water soluble film comprises a third PVOH resin wherein the
third polyvinyl
alcohol resin comprises;
i. a first polyvinyl alcohol polymer comprising carboxylated anionic
monomer units, vinyl alcohol monomer units and optionally vinyl acetate
monomer units, and wherein the carboxylated anionic monomer unit is
derived from a member selected from the group consisting of maleic acid,
monoalkyl maleate, dialkyl maleate, maleic anhydride, and combinations
thereof;
ii. a second PVOH polymer wherein the second PVOH polymer is a
homopolymer wherein the homopolymer consists of vinyl alcohol
monomer units and optionally vinyl acetate monomer units.
[0130] The third polyvinyl alcohol resin optionally can be present in a range
of about 50% to
about 95%, or in a range of about 50% to about 80%, or about 60% to about 75%,
by weight of
the third water-soluble film.
[0131] The carboxylated anionic monomer unit in the third water-soluble film
optionally can be
derived from a monoalkyl maleate unit, e.g. selected from the group consisting
of monomethyl
maleate, salts, e.g. alkali metal salts, thereof, and combinations thereof.
Without wishing to be
bound by theory a polyvinyl alcohol polymer comprising carboxylated anionic
monomer units,
vinyl alcohol monomer units and optionally vinyl acetate monomer units is an
anionic polyvinyl
alcohol copolymer. Each carboxylated anionic monomer unit optionally can be
present in the
first polyvinyl alcohol polymer in an average amount in a range of about
3mo1.% to about
6m01.%, or in a range of about 3nno1.% to about 5mo1.%, or in a range of about
3.5mo1.% to
about 4.5m01.%, or in a range of about 4m01.% to about 4.5m01.%.
[0132] Without wishing to be bound by theory, the term "homopolymer" generally
includes
polymers having a single type of monomeric repeating unit (e.g., a polymeric
chain comprising
or consisting of a single monomeric repeating unit). For the particular case
of polyvinyl alcohol
polymer, the term "homopolymer" further includes copolymers having a
distribution of vinyl
alcohol monomer units and optionally vinyl acetate monomer units, depending on
the degree of
hydrolysis (e.g., a polymeric chain comprising or consisting of vinyl alcohol
and vinyl acetate
monomer units). In the case of 100% hydrolysis, a polyvinyl alcohol
homopolymer can include
only vinyl alcohol units. Without wishing to be bound by theory, the term
"copolymer" generally
includes polymers having two or more types of monomeric repeating units (e.g.,
a polymeric
chain comprising or consisting of two or more different monomeric repeating
units, whether as
random copolymers, block copolymers, etc.). For the particular case of
polyvinyl alcohol
polymer, the term "copolymer" (or "polyvinyl alcohol copolymer") can include
copolymers having
a distribution of vinyl alcohol monomer units and vinyl acetate monomer units,
depending on the
degree of hydrolysis, as well as at least one other type of monomeric
repeating unit (e.g., a ter-
32
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
(or higher) polymeric chain comprising or consisting of vinyl alcohol monomer
units, vinyl
acetate monomer units, and one or more other monomer units, for example
anionic monomer
units). In the case of 100% hydrolysis, a polyvinyl alcohol copolymer can
include a copolymer
having vinyl alcohol units and one or more other monomer units, but no vinyl
acetate units.
Without wishing to be bound by theory, the term "anionic copolymer" includes
copolymers
having an anionic monomer unit comprising an anionic moiety. General classes
of anionic
monomer units include the vinyl polymerization units corresponding to
monocarboxylic acid vinyl
monomers, their esters and anhydrides, dicarboxylic monomers having a
polymerizable double
bond, their esters and anhydrides, vinyl sulfonic acid monomers, and alkali
metal salts of any of
the foregoing. Examples of anionic monomer units include the vinyl
polymerization units
corresponding to vinyl anionic monomers including vinyl acetic acid, maleic
acid, monoalkyl
maleate, dialkyl maleate, monomethyl maleate, dimethyl maleate, maleic
anyhydride, fumaric
acid, monoalkyl fumarate, dialkyl fumarate, monomethyl fumarate, dimethyl
fumarate, fumaric
anyhydride, itaconic acid, monomethyl itaconate, dimethyl itaconate, itaconic
anhydride, vinyl
sulfonic acid, allyl sulfonic acid, ethylene sulfonic acid, 2-acrylamido-1-
methylpropanesulfonic
acid, 2-acrylamido-2-methylpropanesulfonic acid, 2-methylacrylamido-2-
methylpropanesulfonic
acid, 2-sufoethyl acrylate, alkali metal salts of the foregoing (e.g., sodium,
potassium, or other
alkali metal salts), esters of the foregoing (e.g., methyl, ethyl, or other C1-
C4 or C6 alkyl esters),
and combinations thereof (e.g., multiple types of anionic monomers or
equivalent forms of the
same anionic monomer). Anionic monomers may include one or more acrylamido
methylpropanesulfonic acids (e.g., 2-acrylamido-1-methylpropanesulfonic acid,
2-acrylamido-2-
methylpropanesulfonic acid, 2-methylacrylamido-2-methylpropanesulfonic acid),
alkali metal
salts thereof (e.g., sodium salts), and combinations thereof.
[0133] The first polyvinyl alcohol polymer in the third water-soluble resin
optionally is
characterized by:
a. an average 4% aqueous solution viscosity (in deionized water) at 20 C in a
range of about lOmPa.s to about 40mPa.s, or in a range of about lOmPa.s to
about 30mPa.s, or in a range of about 12mPa.s to about 25mPa.s, or in a range
of about 14mPa.s to about 20mPa.s, or
b. an average degree of hydrolysis of about 60% to about 99%, or about 80% to
about 98%, or about 83% to about 95%, or about 85% to about 92%, or
c. a combination thereof.
[0134] The second polyvinyl alcohol polymer in the third water-soluble resin
optionally is
characterized by;
a. an average 4% aqueous solution viscosity (in deionized water) at 20 C in a
range of about 3mPa.s to about 30mPa.s, or in a range of about 7mPa.s to about
33
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
30mPa.s, or in a range of about lOmPa.s to about 30mPa.s, or in a range of
about 12mPa.s to about 25mPa.s; or
b. an average degree of hydrolysis in a range of about 60% to about 99%, or
about
80% to about 98%, or about 85% to about 95%, or about 87% to about 92%; or
c. a combination thereof.
[0135] In the third water-soluble resin, the relative weight ratio of the
first PVOH polymer and
second PVOH polymer optionally can be in a range of about 90/10 to about
10/90, or about
80/20 to about 20/80, or about 70/30 to about 50/50.
[0136] In one type of embodiment, e.g. when used with laundry and automatic
dishwashing
compositions, or with fabric and homecare compositions generally, a third
polyvinyl alcohol
resin can have at least 65 wt.% of the first polyvinyl alcohol polymer
comprising carboxylated
anionic monomer units, vinyl alcohol monomer units and optionally vinyl
acetate monomer units,
and wherein the carboxylated anionic monomer unit is derived from a member
selected from the
group consisting of maleic acid, monoalkyl maleate, dialkyl maleate, maleic
anhydride, and
combinations thereof. Optionally, the amount of such first polyvinyl alcohol
polymer can be in a
range of about 65 wt.% to about 95wt.%, or 65wt.% to about 90wt.%, or in a
range of greater
than 65wt. /0 to about 95%, or greater than 65wt. /0 to about 90wt. /0, or
greater than 65wt.% to
about 85wt.%, or about 70wt.% to about 90wt.% based on the weight of the third
polyvinyl
alcohol resin.
[0137] The third water-soluble film can be characterized by 100% modulus value
of at least
about 20 N/mm2 as measured by the MOD Test at 35% RH. Generally, higher MOD
values are
desirable because they correspond to pouches having a greater stiffness and a
lower likelihood
of deforming and sticking to each other when loaded on top of each other
during production or
in final consumer packaging. Further, MOD values at 10% elongation correspond
to the ability
of the film to maintain stiffness rather than loosen and droop when in contact
with liquid pouch
contents. In particular, films having higher MOD values correspond to pouches
that are less
likely to soften and take on a loose and droopy appearance when in contact
with liquid pouch
contents comprising a low molecular weight polyol. In various embodiments, the
third water-
soluble film has a MOD value of at least about 20, 21, 22, 23, 24, 25, or 27
N/mm2 and/or up to
about 24, 25, 27, 28, 29, or 30 N/mm2 (e.g., about 20 N/mm2 to about 30 N/mm2,
or about
20 N/mm2 to about 28 N/mm2, or about 22 N/mm2 to about 25 N/mm2).
[0138] The third water-soluble film optionally can comprise a surfactant
content in a range of
about 0.1% to about 3.5%, or about 0.1% to about 2.5%, or in a range of about
1% to about 2%,
or in a range of about 0.5% to about 2% by weight of the water-soluble film.
Suitable surfactants
can include the nonionic, cationic, anionic and zwitterionic classes. Suitable
surfactants include,
but are not limited to nonionics, including but not limited to
polyoxyethylenated
34
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
polyoxypropylene glycols, alcohol ethoxylates, alkylphenol ethoxylates,
tertiary acetylenic
glycols and alkanolamides; cationics, including but not limited to
polyoxyethylenated amines,
quaternary ammonium salts and quaternized polyoxyethylenated amines; and
zwitterionics,
including but not limited to amine oxides, N-alkylbetaines and sulfobetaines.
For example, a
nonionic surfactant can be selected from alcohol ethoxylates; a cationic
surfactant can be
selected from quaternary ammonium salts; and a zwitterionic surfactant can be
selected from
amine oxides. Other suitable surfactants include dioctyl sodium
sulfosuccinate, lactylated fatty
acid esters of glycerol and propylene glycol, lactylic esters of fatty acids,
sodium alkyl sulfates,
polysorbate 20, polysorbate 60, polysorbate 65, polysorbate 80, lecithin,
acetylated fatty acid
esters of glycerol and propylene glycol, and acetylated esters of fatty acids,
and combinations
thereof.
[0139] The third water-soluble film optionally can have a residual moisture
content of at least
4%, or in a range of about 4% to about 15%, or in a range of about 5% to about
10% by weight
of the third water-soluble film as measured by Karl Fischer titration.
[0140] The third water-soluble film can comprise one or more components
selected from the
group consisting of plasticizers, plasticizer compatibilizers, lubricants,
release agents, fillers,
extenders, cross-linking agents, antiblocking agents, antioxidants,
detackifying agents,
antifoams, nanoparticles, bleaching agents, aversive agents, surfactants, and
combinations
thereof.
[0141] The third water-soluble film can comprise one or more plasticizers in
an amount in a
range of about 5% to about 50%, or about 10% to about 40%, or about 20% to
about 30% by
weight of the third water-soluble film. The plasticiser in the third water-
soluble film optionally
can be selected from polyols, sugar alcohols, or a mixture thereof, e.g.
wherein the polyols
include polyols selected from the group consisting of glycerol, diglycerin,
ethylene glycol,
diethylene glycol, triethyleneglycol, tetraethylene glycol, polyethylene
glycols up to 400 MW,
neopentyl glycol, 1,2-propylene glycol, 1,3-propanediol, dipropylene glycol,
polypropylene
glycol, 2-methyl-1,3-propanediol, trimethylolpropane and polyether polyols, or
a mixture thereof,
wherein sugar alcohols can include sugar alcohols selected from the group
consisting of
isomalt, maltitol, sorbitol, xylitol, erythritol, adonitol, dulcitol,
pentaerythritol and mannitol, or a
mixture thereof. The plasticizer can be selected from the group consisting of
sorbitol, glycerol,
dipropyleneglycol, polyethyleneglycol, trimethylolpropane, and mixtures
thereof.
[0142] The third water-soluble film according to the invention optionally can
comprise
lubricants / release agents. Suitable lubricants/release agents can include,
but are not limited to,
fatty acids and their salts, fatty alcohols, fatty esters, fatty amines, fatty
amine acetates and fatty
amides. Lubricants/release agents can be selected from fatty acids, fatty acid
salts, and fatty
amine acetates. The amount of lubricant/release agent in the third water-
soluble film optionally
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
can be in a range in a range of about 0.02% to about 1.5%, or about 0.1% to
about 1% by
weight of the third water-soluble film.
[0143] The third water-soluble film optionally can comprise fillers,
extenders, antiblocking
agents, detackifying agents or a mixture thereof. Suitable fillers, extenders,
antiblocking agents,
detackifying agents or a mixture thereof include, but are not limited to,
starches, modified
starches, crosslinked polyvinylpyrrolidone, crosslinked cellulose,
microcrystalline cellulose,
silica, metallic oxides, calcium carbonate, talc and mica. Starches, modified
starches, silica, and
mixtures thereof are particularly contemplated. The amount of filler,
extender, antiblocking
agent, detackifying agent or mixture thereof in the third water-soluble film
optionally can be in a
range of about 0.1% to about 25%, or about 1% to about 10%, or about 2% to
about 8%, or
about 3% to about 5% by weight of the third water-soluble film. In the absence
of starch, a
suitable filler, extender, antiblocking agent, detackifying agent or mixture
thereof optionally can
be present in a range of about 0.1% to about 1%, or about 4%, or about 6%, or
about 1% to
about 4%, or about 1% to about 2.5%, by weight of the third water-soluble
film.
[0144] The third water-soluble film can comprise a printed area. The area of
print can be
achieved using standard techniques, e.g. flexographic printing or inkjet
printing.
[0145] The third water-soluble film can comprise an aversive agent, for
example a bittering
agent. Suitable bittering agents include, but are not limited to, naringin,
sucrose octaacetate,
quinine hydrochloride, denatonium benzoate, and mixtures thereof. Any suitable
level of
aversive agent may be used in the film. Suitable levels include, but are not
limited to, about
1ppm to about 5000ppm, or about 100ppm to about 2500ppm, or about 250ppm to
about
2000ppm.
[0146] The third water-soluble film, and each individual component thereof,
independently can
comprise Oppm to about 20ppm, or Oppm to about 15ppm, or Oppm to about lOppm,
or Oppm to
about 5ppm, or Oppm to about 1ppm, or Oppb to about 100ppb, or Oppb dioxane.
Those skilled
in the art will be aware of known methods and techniques to determine the
dioxane level within
water-soluble films and ingredients thereof.
Method of Making Films
[0147] The water-soluble films used in the water-soluble unit-dose articles of
the disclosure
films can be made by any suitable method. Processes for making water-soluble
films include
solvent casting, blow-molding, extrusion, and blown extrusion, as generally
known in the art.
Processes for solvent casting are well-known in the art. For example, in the
film-forming
process, the resins and secondary additives are dissolved in a solvent,
typically water, metered
onto a surface, allowed to substantially dry (or force-dried) to form a cast
film, and then the
resulting cast film is removed from the casting surface. The process can be
performed
batchwise, and is more efficiently performed in a continuous process.
36
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
[0148] In the formation of continuous films, it is the conventional practice
to meter a solution
of the resin and secondary components onto a moving casting surface, for
example, a
continuously moving metal drum or belt, causing the solvent to be
substantially removed from
the liquid, whereby a self-supporting cast film is formed, and then stripping
the resulting cast
film from the casting surface. The solution can optionally be metered or
coated onto a carrier
film, release liner, or removable backing, whereby after solvent removal, the
resulting cast film
or coating can be separated from the carrier film, release liner, or removable
backing (for
example, immediately upon drying or at a later point in time, e.g., prior to
use) or remain
attached to the carrier film, release liner, or removable backing. A film or
coating prepared on a
carrier film, release liner, or removable backing can be self-supporting or
non-self-supporting.
[0149] In general, the amount of water in the metered solution of polyvinyl
alcohol, additional
resins, and/or secondary components for film casting is selected such that
when the solution is
heated to the casting temperature, the solution has the highest solids level
below the viscosity
inflection point. Methods of determining the amount of solids at the viscosity
inflection point are
known in the art. In general, the water content of the metered solution can
comprise between
60 to 85% water, or 60 to 75% water to provide suitable solutions for casting
at typical casting
solutions. The viscosity of the casting solution can be, for example, at least
about 20,000 cps at
185 F (85 C), at least 30,000 cps at 185 F (85 C), for example about
40,000 cps to about
50,000 cps at 185 F (85 C).
[0150] The solution can be cast at any suitable temperature such that the film
has a
temperature, for example, in a range of about 50 C to about 105 C, during
drying. Without
intending to be bound by theory, it is believed that as the casting solution
and film temperature
decreases below about 50 C, the amount of time required to dry the film
undesirably increases,
and the length of the drying chamber needed to fully dry the cast solution
undesirably increases.
Further, without intending to be bound by theory, it is believed that as the
solution and film
temperature increases above about 105 00, the solvent may rapidly boil out of
the film, resulting
in defects in the film surface such as holes or blisters in the finished films
and/or facilitate
undesirable reactions between adjacent PVOH backbone chain resulting in a film
having
reduced solubility.
[0151] In a continuous or semi-continuous casting process, the moving casting
surface can
have a line speed in a range of about 5 m/min to about 50 m/min. The line
speed can affect the
properties of the resulting film, for example, physical properties, thickness,
residual moisture
content and film quality. In general, as the line speed decreases, the
thickness of the resulting
film will increase and as the line speed increases, the thickness of the
resulting film will
decrease, assuming the delivery rate of solution remains constant. In general,
as the line speed
increases the residence time of the film in the dryer decreases, thereby
requiring an increase in
drying temperatures, which may result in drying defects or sticking at high
enough
37
CA 03196098 2023- 4- 18
temperatures. In contrast, as the line speed decreases, the residence time of
the film in the
dryer increases.
[0152] Any of the first, second, third, or additional films according to the
disclosure herein can
be produced by solvent casting, e.g. using a solvent band casting system. The
system can
include a tank for mixing and/or storing a polymer solution, having optional
secondary additives,
for use with a band casting machine having at least a first and a second
rotating drums about
which a continuous band (e.g. metal band) is tensioned to travel with the
rotation of the drums.
A sheeting die can apply the polymer solution from the tank to the metal band
where a drying
chamber, enclosing at least a portion of the metal band downline of the
sheeting die, is used to
remove solvent from the polymer solution as it travels in a thin sheet on the
metal band. In
addition, a release coating can be used to provide one or more advantages to
the film and/or
the process. For example, the release coating can substantially reduce or
eliminate bubbles in
the produced polymer film, or the release coating can improve the ease of
release of the
produced film from the casting surface. A roll coater release coating
applicator in
communication with a supply of a release coating and a portion of the band can
transfer fluid
release coating to the casting surface prior to application of the polymer
solution to the band. A
suitable solvent band casting system and related materials are further
described in U.S. patent
application publication No. 2006/0081176 Al.
[0153] In general, the casting surface can be any suitable substrate for
producing polymeric
films to one of skill in the art. In embodiments, the substrate can be a
casting roller or drum, a
casting belt, or a combination thereof. As used herein, the substrate is used
for producing a
polymer film from a polymer resin or polymer resin solution. The substrate
comprises a
substrate surface and the substrate surface is coated with a release coating.
The polymer resin
solution can be cast onto a substrate while the substrate is moving, e.g.
rotating. In
embodiments, the substrate is a casting drum. In embodiments, the substrate is
a casting belt.
The substrate can comprise stainless steel, and optionally can have a
stainless steel surface.
The substrate can comprises stainless steel that is optionally plated, e.g.
chrome plated, nickel
plated, zinc plated or a combination thereof.
[0154] In general, the release coating can comprise one or more surfactants
and an optional
carrier, e.g. water. The release coating can comprise one or more surfactants,
e.g. selected
from a fluorosurfactant, a non-fluorinated anionic surfactant, a non-
fluorinated zwitterionic
surfactant, salts thereof, or any combination thereof. In embodiments, the
anionic or
zwitterionic surfactant(s) can be non-fluorinated and comprise a C6-C30
phosphate ester, a C6-
C30 phosphate diester, a C6-C30carboxylate, a C6-C30dicarboxylate, a C6-030
sulfate, a C6-C30
disulfate, or salts thereof. In embodiments, the release coating comprises a
non-fluorinated
zwitterionic surfactant or salts thereof. In embodiments, the release coating
comprises a non-
38
Date Recue/Date Received 2023-09-15
WO 2022/132853
PCT/US2021/063431
fluorinated anionic surfactant or salts thereof. In embodiments, the non-
fluorinated anionic
surfactant comprises a C6-C30 phosphate ester, or a C8-C16 phosphate ester, Cs-
Cso phosphate
diester, C16-C32 phosphate diester, a 06-C30carboxylate, a C6-
C30dicarboxylate, a C6-030 sulfate,
a C6-C30disulfate, or salts thereof. In embodiments, the non-fluorinated
anionic surfactant
comprises a C6-C30 phosphate ester, or a C6-C18 phosphate ester, C6-C60
phosphate diester,
018-032 phosphate diester, or salts thereof. In embodiments, the anionic
surfactant can be
selected from one or more of a Cs-based ammonium fluoroaliphatic phosphate
ester; tridecyl
alcohol ethoxylate phosphate ester, POE-12; tridecyl alcohol ethoxylate
phosphate ester, POE-
3; laureth-11 carboxylic acid; crypto-anionic surfactant - laureth-6
carboxylic acid; or sodium
lauryl ether sulfate, POE-4.
[0155] As used herein, the term "non-fluorinated" refers to a surfactant that
has less than 0.01
wt% fluorine based on the total molecular weight of the compound, or less than
0.001 wt%
fluorine based on the total molecular weight of the compound, or less than
0.0001 wt% fluorine
based on the total molecular weight of the compound.
[0156] In embodiments, the release coating can include a fluorosurfactant,
e.g. a
perfluoroalkyl-containing compound. In embodiments, the fluorosurfactant can
include a solution
of ZONYL FSP surfactant (E.I. du Pont de Nemours and Company). A range of from
about
0.05% by weight to about 5.0% by weight of surfactant in the release coating
is contemplated.
The amount of surfactant required to provide adequate wetting can vary
depending on the film
being coated on the band. Other products may require higher concentrations to
improve release
properties. Hard surface spreading wetting will be more efficient with higher
surfactant
concentrations until the surfactant solution reaches the critical micelle
concentration (CMC).
This concentration represents a threshold beyond which additional surfactant
will not produce
any further efficiency in spreading wetting. However, increasing the
concentration beyond the
CMC may improve wetting by the polymer solution and improve the release
properties of some
film formulations.
[0157] The release coating can be applied to the surface of a substrate and
optionally
subsequently dried prior to casting a polymer resin or polymer resin solution
onto the surface
coated substrate. In embodiments, the release coating can have a pH of about 1
to about 5
when applied to the surface of the substrate, prior to drying the release
coating on the surface
of the substrate. In embodiments wherein the surfactant comprises a non-
fluorinated anionic
surfactant, a non-fluorinated zwitterionic surfactant, salts thereof, and a
combination thereof, the
release coating can have a pH of about 1 to about 8 or a pH of about 1 to
about 5 when applied
to the surface of the substrate, prior to drying the release coating on the
surface of the
substrate. For example, the release coating, when applied to the surface of
the substrate, can
have a pH of about 1, about 1.5, about 2, about 2.5, about 3, about 3.5, about
4, about 5, about
6, about 7, or about 8. In embodiments, the release coating can have a pH of
about 1 to about
39
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
7, or about 1 to about 6, or about 1 to about 4, or about 1 to about 3, or
about 2 to about 7, or
about 2 to about 6, or about 2 to about 5, or about 2 to about 4 , or about 2
to about 3, or about
3 to about 7, or about 3 to about 5, or about 1.5 to about 3.5, or about 4 to
about 7 when applied
to the surface of the substrate, prior to drying the release coating on the
surface of the
substrate.
[0158] In general, the release coating can have a surfactant concentration in
a range of about
0.001 wt% to about 100 wt%, based on the total weight of the release coating.
In embodiments,
the release coating can have a surfactant concentration in a range of about
0.001 wt% to about
20 wt% prior to drying the release coating on the surface of the substrate.
For example, the
release coating can have a surfactant concentration in a range of about 0.001
wt% to about 10
wt%, or about 0.01 wt% to about 5 wt%, or about 0.01 wt% to about 4 wt%, or
about 0.01 wt%
to about 3 wt%, or about 0.01 wt% to about 2 wt%, or about 0.05 wt% to about 2
wt%, or about
0.1 wt% to about 2 wt%, or about 0.5 wt% to about 2 wt%, prior to drying the
release coating on
the surface of the substrate. In embodiments, the release coating can have a
surfactant
concentration in a range of about 0.01 wt% to about 4.00 wt%, based on the
total weight of the
release coating prior to drying the release coating on the surface of the
substrate. In
embodiments, the release coating can have a surfactant concentration in a
range of about 0.05
wt% to about 2.00 wt%, based on the total weight of the release coating prior
to drying the
release coating on the surface of the substrate. In embodiments, the release
coating can have a
surfactant concentration in a range of about 2.5 wt% to about 100 wt%, based
on the total
weight of the release coating, after drying the release coating on the surface
of the substrate.
For example, after drying the release coating on the surface of the substrate,
the release
coating can have a surfactant concentration in a range of about 3 wt% to about
100 wt%, or
about 4 wt% to about 90 wt%, or about 4 wt% to about 80 wt%, or about 4 wt% to
about 70
wt%, or about 4 wt% to about 50 wt%, or about 4 wt% to about 30 wt%, or about
4 wt% to about
20 wt%, or about 4.7 wt% to about 100 wt%, or about 5 wt% to about 90 wt%,
based on the
total weight of the release coating. In embodiments, the release coating can
have a surfactant
concentration in a range of about 4.7 wt% to about 100 wt%, based on the total
weight of the
release coating, after drying the release coating on the surface of the
substrate. For example,
the release coating can include an amount of ZONYL surfactant in a range of
about 0.05% by
weight to about 5.0% by weight, based on the total weight of the release
coating.
[0159] In general, the release coating as described herein can have a
hydrophilic-lipophilic
balance in a range of about 1 to about 30. In embodiments, the release coating
can have a
hydrophilic-lipophilic balance in a range of about 1 to about 20, or about 1
to about 18, or about
1 to about 17, or about 1 to about 16, or about 1 to about 15, or about 2 to
about 17, or about 3
to about 17, or about 4 to about 15, or about 5 to about 12, or about 8 to
about 12. In
embodiments, the release coating can have a hydrophilic-lipophilic balance in
a range of about
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
1 to about 20. In embodiments, the release coating can have a hydrophilic-
lipophilic balance in
a range of about 3 to about 17.
[0160] In general, the release coating has a thickness of about 0.1 nm to
about 100 nm on the
surface of the substrate. In embodiments, the release coating has a thickness
of about 0.1 nm
to about 80 nm, or about 0.1 nm to about 60 nm, or about 0.1 nm to about 40
nm, or about 0.1
nm to about 40 nm, or about 0.1 nm to about 20 nm, or about 0.1 nm to about 10
nm, or about 1
nm to about 10 nm, or about 1 nm to about 5 nm, on the surface of the
substrate. In
embodiments, the release coating has a thickness of about 0.1 nm to about 40
nm on the
surface of the substrate. In embodiments, the release coating has a thickness
of about 0.1 nm
to about 10 nm on the surface of the substrate.
[0161] Methods of making a water-soluble unit dose article, such as a pouch or
a packet will
now be described in more detail. In one aspect, a method can include
optionally deforming a
first water-soluble film as described herein (e.g. thermoforming the film in a
mould) to create an
open cavity, filling the open cavity with a composition (e.g., a detergent
composition), closing
the open filled cavity with a second water-soluble film as described herein,
and solvent sealing
the second water-soluble film to the first water-soluble film to create a
water-soluble unit dose
article.
[0162] The unit dose articles, such as pouches and packets, may be made using
any suitable
equipment. For example, single compartment pouches may be made using vertical
form filling,
horizontal form filling, or rotary drum filling techniques commonly known in
the art. Such
processes may be either continuous or intermittent. Thus, the mould can be in
any desired
orientation with respect to the film; however, in one convenient type of
embodiment the mould is
below the film, to readily facilitate gravity filling of contents into a
deformed film. The film may be
dampened, and/or heated to increase the malleability thereof. The water-
soluble films may be
pre-heated ahead of deformation via a hot plate, an infra-red lamp, or a
combination thereof,
and use of an infra-red lamp is particularly contemplated. The method can also
involve the use
of a vacuum, e.g. to draw the film into a suitable mould. The vacuum, e.g. for
drawing a film
into a mould, can be applied for any suitable time, e.g. about 0.2 to about 5
seconds, or about
0.3 to about 3, or about 0.5 to about 1.5 seconds, e.g. once the film is on
the mould surface.
This vacuum can be such that it provides an under-pressure in a range of 10
mbar to 1000
mbar, or in a range of 100 mbar to 600 mbar, for example.
[0163] The process for making the water-soluble unit dose articles can include
automated
manufacturing process equipment and steps, e.g. a conveyer belt, a series of
conveyer belts, a
drum, a series of drums or a combination thereof. The process for making the
water-soluble
unit dose articles can include or consist of manual manufacturing steps, in
which the one or
more sequences or steps described herein are conducted manually. Optionally,
the process
can include both manual and automated steps. A fully automated process is also
contemplated.
41
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
[0164] The process of making the water-soluble unit dose articles, or portions
of the process,
can be performed as a continuous process. Alternatively, the process of making
the water-
soluble unit dose articles, or portions of the process, can be an intermittent
or batch process. A
process of making the water-soluble unit dose articles in a continuous or
substantially
continuous process is contemplated.
[0165] The moulds, in which the articles may be made, can have any shape,
length, width and
depth, depending on the required dimensions of the pouches. The moulds may
also vary in
size and shape from one to another, if desirable. For example, the volume of
the final unit dose
articles can be about 5 ml to about 300 ml, or about 10 ml to 150 ml, or about
20 ml to about
100 ml, and that the mould sizes are adjusted accordingly.
Thermoforming
[0166] The deforming of a film can include thermoforming, e.g. thermoforming
the first water-
soluble film in a mould to create an open cavity. A thermoformable film is one
that can be
shaped through the application of heat and a force. Thermoforming a film is
the process of
heating the film, shaping it (e.g., in a mould), and then allowing the film to
cool, whereupon the
film will hold its shape, e.g. the shape of the mould. The heat may be applied
using any suitable
means. For example, the film may be heated directly by passing it under a
heating element or
through hot air, prior to feeding it onto a surface or once on a surface.
Alternatively, it may be
heated indirectly, for example by heating the surface or applying a hot item
onto the film. In
embodiments, the film can be heated using an infrared light. The film may be
heated to a
temperature in a range of about 50 C to about 150 C, about 50 C to about
120 C, about 60
C to about 130 C, about 70 C to about 120 C, or about 60 C to about 90 C.
Thermoforming can be performed by any one or more of the following steps and
processes: the
manual draping of a thermally softened film over a mould, or the pressure
induced shaping of a
softened film to a mould (e.g., vacuum forming), or the automatic high-speed
indexing of a
freshly extruded sheet having an accurately known temperature into a forming
and trimming
station, or the automatic placement, plug and/or pneumatic stretching and
pressuring forming of
a film.
[0167] Alternatively, the film can be dampened wetted by any suitable means,
for example
directly by spraying a wetting agent (including water, a solution of the film
composition, a
plasticizer for the film composition, or any combination of the foregoing)
onto the film, prior to
feeding it onto the surface or once on the surface, or indirectly by wetting
the surface or by
applying a wet item onto the film surface, e.g. as described further herein.
For example the film
can be wetted by a contact transfer method in which a wetted absorbent member
is applied to
the film surface.
[0168] Once a film has been heated and/or wetted, it can be drawn into an
appropriate mould,
optionally using a vacuum. The filling of the moulded film can be accomplished
by utilizing any
42
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
suitable means. In embodiments, the choice of method can be guided by the
product form and
required speed of filling. In embodiments, the moulded film can be filled by
in-line filling
techniques. The filled, open packets are then closed, forming the pouches,
using the second
film, by any suitable method. This may be accomplished while in horizontal
position and in
continuous, constant motion, for example. A plurality of packets can be made
simultaneously,
e.g. using a mould that has a plurality of cavities. The closing may be
accomplished by
continuously feeding the second water-soluble film, over and onto the open,
filled packets and
then sealing the first and second film together, typically in the area between
the mould cavities
and thus between the packets.
Sealing the Water-Soluble Unit Dose Articles
[0169] The first water-soluble film and the second water-soluble film can be
sealed via any
suitable method, e.g. solvent sealing, heat sealing or a mixture thereof, for
example via solvent
sealing. The solvent sealing solution optionally comprises an aqueous solvent,
a non-aqueous
solvent or a mixture thereof. In embodiments, the solvent sealing solution can
comprise water.
The solvent sealing solution optionally comprises at least 95%, or even at
least 98%, or even at
least 99%, or even 100% by weight of the solvent sealing solution of water.
The solvent sealing
solution can be applied by any suitable method, including contact and/or non-
contact methods.
For example, the solvent solution can be applied in a contact transfer
process, e.g. using a
contact member comprising a nonabsorbent or substantially impermeable
material, e.g. using
an anilox roller, rubber (e.g. EPDM) roller, or any combination thereof,
optionally in combination
with a doctor blade. The sealing solution can be applied using a drawdown bar,
Mayer bar, or
similar apparatus. In another type of embodiment the sealing solution can be
applied using a
contact member comprising an absorbent material, for example natural felt,
synthetic felt,
porous plastic, foam, sponge, microfiber, cotton, polyester, extruded
polyester fibers, nonwoven
webs and the like, e.g. in pad or roller form. Application of solvent sealing
solution via a felt roll
is particularly contemplated. As another option, the sealing solution can be
applied via a dosing
nozzle or a spraying nozzle. Combinations of any of the foregoing methods and
apparatus are
contemplated. In one type of embodiment, a contact transfer method using an
absorbent
material is contemplated. The solvent sealing solution can be applied to the
second water-
soluble film, for sealing it to a first water soluble film or a third water
soluble film. For example,
the solvent sealing solution can be applied to the second side of the second
water-soluble film,
the second side of the second water soluble film facing the first side of the
first water-soluble
film. In one type of embodiment, the solvent sealing solution is applied on
the water-soluble film
in an amount in a range of about 1g to about 30g of sealing solution per
square meter of film, or
in a range of about 5g to about 20g of sealing solution per square meter of
film.
[0170] As mentioned above, one type of method include solvent sealing to form
the water-
soluble unit dose article. Typically, only the area which is to form the seal
is treated with
43
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
solvent. The solvent can be applied on either film, and in one type of
embodiment is applied on
the closing film, and typically only on the areas which are to form the seal.
In embodiments, it
may be preferred that heat is also applied. Preferred solvent sealing methods
include
selectively applying solvent onto the area between the mould cavities, or on
the closing film, by
for example, spraying, printing, or contact application onto these areas, and
then applying
pressure onto these areas, to form the seal. Sealing rolls and belts
(optionally also providing
heat) can be used, for example, to apply pressure.
[0171] In embodiments, the first water-soluble film is sealed to the second
water-soluble film
by solvent sealing. In embodiments, the sealing solution comprises water and
further can
include one or more diols and/or glycols such as 1 ,2-ethanediol (ethylene
glycol), 1,3-
propanediol, 1,2-propanediol, 1 ,4-butanediol (tetramethylene glycol), 1,5-
pantanediol
(pentamethylene glycol), 1,6-hexanediol (hexamethylene glycol), 2,3-
butanediol, 1 ,3-butanediol,
2-methyl-1,3-propanediol, various polyethylene glycols (e.g., diethylene
glycol, triethylene
glycol), and combinations thereof. In embodiments, the sealing solution
comprises erythritol,
threitol, arabitol, xylitol, ribitol, mannitol, sorbitol, galactitol, fucitol,
iditol, inositol, volemitol,
isomalt, maltitol, lactitol.
[0172] The sealing solution can be applied to the interfacial areas of the
first water-soluble
film in any amount suitable to adhere the first and the second water-soluble
films. As used
herein, the term "coat weight" refers to the amount of sealing solution
applied to the film in
grams of solution per square meter of film. In general, when the coat weight
of the sealing
solvent is too low, the films do not adequately adhere and the risk of pouch
failure at the seams
increases. Further, when the coat weight of the sealing solvent is too high,
the risk of the
solvent migrating from the interfacial areas increases, increasing the
likelihood that etch holes
may form in the sides of the pouches. The coat weight window refers to the
range of coat
weights that can be applied to a given film while maintaining both good
adhesion and avoiding
the formation of etch holes. A broad coat weight window is desirable as a
broader window
provides robust sealing under a broad range of operations. Suitable coat
weight windows
include amounts of at least about 3 g/m2, or at least about 4 g/m2, or at
least about 5 g/m2, or at
least about 6 g/m2.
Cutting the Water-Soluble Unit Dose Articles
[0173] The unit dose articles, e.g., pouches, may be cut by a cutting device.
Cutting can be
accomplished using any known method. It may be preferred that the cutting is
also done in
continuous manner, and preferably with constant line speed and preferably
while in horizontal
position. The cutting device can, for example, be a sharp item, or a hot item,
or a laser,
whereby in the latter cases, the hot item or laser 'burns' through the film/
sealing area. The
cutting can be performed by one or more rotating knives.
44
CA 03196098 2023- 4- 18
Forming and Filling Multi-Compartment Pouches
[0174] The different compartments of a multi-compartment pouches may be made
together in
a side-by-side style or concentric style wherein the resulting, cojoined
pouches may or may not
be separated by cutting. Alternatively, the compartments can be made
separately.
[0175] In embodiments, unit dose articles may be made according to a process
comprising
the steps of (a) forming a first compartment (as described above); (b) forming
a recess within
part or all of the closed compartment formed in step (a), to generate a second
moulded
compartment superposed above the first compartment; (c) filling and closing
the second
compartment by means of a third film; (d) sealing the first, second and third
films; and (e) cutting
the films to produce a multi-compartment pouch. The recess formed in step (b)
may be
achieved by applying a vacuum to the compartment prepared in step (a).
[0176] In embodiments, second, and/or third compartment(s) can be made in a
separate step
and then combined with the first compartment, for example as described in
European Patent
Application Number 08101442.5 or U.S. Patent Application Publication No.
2013/240388 Al or
US Patent No. 7,964,549 B2.
[0177] Unit dose articles can be made according to a process comprising the
steps of: (a)
deforming a first water-soluble film as described herein in a mould to create
an open cavity, the
first water soluble film comprising a first polyvinyl alcohol resin; (b)
filling the open cavity formed
by the first water-soluble film with a composition; (c) closing the open
filled cavity from step (b)
with a second water-soluble film as described herein, e.g. the second water
soluble film
comprising a second polyvinyl alcohol resin wherein the second polyvinyl
alcohol resin
comprises; (1) less than 15% by weight of the second polyvinyl alcohol resin
of a polyvinyl
alcohol polymer comprising carboxylated anionic monomer units, vinyl alcohol
monomer units
and optionally vinyl acetate monomer units, and wherein the carboxylated
anionic monomer unit
is derived from a member selected from the group consisting of maleic acid,
monoalkyl maleate,
dialkyl maleate, maleic anhydride, and combinations thereof; and (2) 85% about
100% by
weight of the second polyvinyl alcohol resin of a polyvinyl alcohol
homopolymer or a
homopolymer blend, wherein the homopolymers consist of vinyl alcohol monomer
units and
optionally vinyl acetate monomer units; and wherein the second polyvinyl
alcohol resin has an
average 4% solution viscosity in deionized water at 20 C in a range of 8mPa.s
to less than
12mPa.s; (d) sealing the second water-soluble film to the first water-soluble
film, optionally via
solvent sealing, to create the water-soluble unit dose article.
[0178] Another method of pouch production can include: (a) deforming a first
water-soluble
film as described herein in a mould to create an open cavity; (b) filling the
open cavity formed by
the first water-soluble film with a composition; (c) separately deforming a
third water-soluble film
as described herein in a mould to create at least one open cavity; (d) filling
the at least one
Date Recue/Date Received 2023-09-15
WO 2022/132853
PCT/US2021/063431
open cavity in the third film with a composition (e.g., that is the same or
different from the
composition of step (b)); (e) closing the open filled cavity or cavities of
step (d) with a second
water-soluble film as described herein; (f) sealing the second water-soluble
film and third water-
soluble film, optionally via solvent sealing, to create a closed intermediate;
(g) closing the open
filled cavity of step (b) with the closed intermediate of step (f); and (h)
sealing the first water-
soluble film to the second water-soluble film of the closed intermediate of
step (g), optionally via
solvent sealing, to create the water-soluble unit dose article. Use of this
method can provide,
for example, a unit-dose article having compartments of compositions that are
superposed, e.g.
one or more compartments superposed with respect to another compartment. In
embodiments,
any one of the sealing steps can comprise solvent sealing, and in one type of
embodiment at
least one of the sealing steps includes solvent sealing. In embodiments, each
of the first and
the second water-soluble film has a first side and a second side, and the
first side of the first
water-soluble film is sealed to the second side of the second water-soluble
film to create a first
compartment between the first water-soluble film and the second water-soluble
film, and the first
side of the second water-soluble film is sealed to the second side of the
third water-soluble film
to create at least a second compartment between the second water-soluble film
and the third
water-soluble film, and wherein the second compartment is positioned above the
first
compartment. In embodiments, the first water-soluble film and the third water-
soluble film can
be identical prior to thermoforming, i.e. physically and chemically identical,
wherein the term
'identical' means within standard processing of making specification
variations. Optionally, the
sealing of the first water soluble film and the second water soluble film can
include a step of
wetting the second water soluble film with a sealing solution as described
herein, e.g. via a
contact wetting method. In addition or in the alternative, the sealing of the
second water
soluble film and the third water soluble film optionally can include a step of
wetting the second
water soluble film with a sealing solution as described herein, e.g. via a
contact wetting method.
[0179] The methods can further include forming at least a third compartment,
optionally at
least a third and a fourth compartment, between the second water-soluble film
and the third
water-soluble film. The second compartment and the third compartment, e.g. the
second
compartment, the third compartment and the fourth compartments, optionally can
be positioned
side-by-side to one another and wherein the second compartment and the third
compartment,
e.g. the second compartment, the third compartment and the fourth compartment
optionally can
be positioned above the first compartment. Optionally, the sealing of the
second water soluble
film and the third water soluble film can include a step of wetting the second
water soluble film
with a sealing solution as described herein, e.g. via a contact wetting
method.
[0180] Unit dose articles can be made according to a process comprising the
steps of: (a)
forming a first compartment, optionally using heat and/or vacuum, using a
first film as described
herein on a first forming machine; (b) filling the first compartment with a
first composition; (c) on
a second forming machine, deforming a third film as described herein,
optionally using heat and
46
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
vacuum, to make a second and optionally third moulded compartment; (d) filling
the second and
optionally third compartments; (e) sealing the second and optionally third
compartment using a
second film as described herein; (f) placing the sealed second and optionally
third
compartments onto the first compartment; (g) sealing the first, second and
optionally third
compartments by sealing the first film to the second film; and (h) cutting the
films to produce a
multi-compartment pouch. Optionally, the sealing of the first water soluble
film and the second
water soluble film can include a step of wetting the second water soluble film
with a sealing
solution as described herein, e.g. via a contact wetting method. In addition
or in the alternative,
the sealing of the second water soluble film and the third water soluble film
optionally can
include a step of wetting the second water soluble film with a sealing
solution as described
herein, e.g. via a contact wetting method.
[0181] The first and second forming machines may be selected based on their
suitability to
perform the above process. The first and second forming machines can be
horizontal forming
machines and/or rotary drum forming machines, for example. In embodiments, the
first forming
machine can be a horizontal forming machine, and the second forming machine
can be a rotary
drum forming machine, e.g. located above the first forming machine.
[0182] The closed intermediate can be made on a rotating drum, or on a
horizontal belt, for
example, and a rotating drum is particularly contemplated. The filled open
cavity in steps (a)
and (b) can be made on a horizontal belt or a rotating drum, and a horizontal
belt is particularly
contemplated. Wherein a rotating drum is used, the water-soluble film is
preferably maintained
in place via vacuum. Wherein a horizontal belt is used, the water-soluble film
is preferably
maintained in place via vacuum.
[0183] Multiple unit dose articles can be formed in such a manner that they
are connected to
one another by flat areas. Without wishing to be bound by theory, such a
process involves
making a plurality of water-soluble unit dose articles joined together by non-
deformed film to
create a water-soluble web of unit dose articles. The non-deformed films are
the flat areas of
the water-soluble web between the unit dose articles. Therefore, the flat
areas may comprise
two or more water-soluble films sealed together as described herein. Thus, a
step of deforming
a film as described herein would be understood by the skilled artisan to
include a process of
deforming a portion of a film article while leaving one or more other portions
non-deformed.
[0184] The resultant web of water-soluble unit dose articles connected via
flat areas is can be
transferred to a cutting station and cut to produce individual unit dose
articles. In one type of
embodiment, the cutting station cuts the flat areas in the web in a machine
direction and cross-
machine direction. Cutting can be achieved using rotating knives.
[0185] It should be understood that by the use of appropriate feed stations,
it may be possible
to manufacture multi-compartment pouches incorporating a number of different
or distinctive
47
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
compositions and/or different or distinctive forms of compositions, e.g. solid
(powder, granule,
tablet, or other), liquid, gel, or paste compositions.
[0186] The film and/or the pouch can be sprayed or dusted with a suitable
material, such as
an active agent, a lubricant, an aversive agent, or mixtures thereof. The film
and/or the pouch
can be printed upon, for example, with an ink and/or an active agent.
Vertical Form, Fill and Seal
[0187] In embodiments, the sealed unit dose article can include a vertical
form, filled, and
sealed article. The vertical form, fill, and seal (VFFS) process is a
conventional automated
process. VFFS includes an apparatus such as an assembly machine that wraps a
single piece
of the film around a vertically oriented feed tube. The machine heat seals or
otherwise secures
the opposing edges of the film together to create the side seal and form a
hollow tube of film.
Subsequently, the machine heat seals or otherwise creates the bottom seal,
thereby defining a
container portion with an open top where the top seal will later be formed.
The machine
introduces a specified amount of flowable product into the container portion
through the open
top end. Once the container includes the desired amount of product, the
machine advances the
film to another heat sealing device, for example, to create the top seal.
Finally, the machine
advances the film to a cutter that cuts the film immediately above the top
seal to provide a filled
package.
[0188] During operation, the assembly machine advances the film from a roll to
form the
package. Accordingly, the film must be able to readily advance through the
machine and not
adhere to the machine assembly or be so brittle as to break during processing.
Such a formed
package can be joined with another film and/or package to make a unit dose
article as
described herein. For example, the VFFS-produced package can be made from a
first film as
described herein, and joined with one or more additional filled cavities made
as described
above from a second film as described herein and a third film as described
herein. As another
example, the VFFS-produced package can be made from a second film as described
herein,
and joined with one or more additional filled cavities made as described above
from a first film
as described herein or a third film as described herein. The other
permutations of combinations
of forming and filling the first, second, and third films described herein are
also contemplated.
Compositions
Household care compositions
[0189] In embodiments, the water-soluble unit dose article can comprise a
household care
composition.
[0190] The household care composition is preferably selected from the group
consisting of
light duty liquid detergent compositions, heavy duty liquid detergent
compositions, hard surface
48
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
cleaning compositions, laundry detergent gels, bleaching compositions, laundry
additives, fabric
enhancer compositions, shampoos, body washes, other personal care
compositions, and
mixtures thereof, preferably a liquid laundry detergent composition.
[0191] In another aspect, the composition can be selected from the group of
laundry and
automatic dishwashing compositions, including liquid laundry detergent
compositions.
[0192] In another aspect, the household care composition can be selected from
non-laundry
and non-automatic dishwashing compositions, e.g. selected from the group
consisting of light
duty liquid detergent compositions, heavy duty liquid detergent compositions,
hard surface
cleaning compositions, bleaching compositions, shampoos, body washes, other
personal care
compositions, and other compositions which are non-laundry and non-automatic
dishwashing
compositions, or mixtures of any of the foregoing.
[0193] The term 'liquid laundry detergent composition' refers to any laundry
detergent
composition comprising a liquid capable of wetting and treating a fabric, and
includes, but is not
limited to, liquids, gels, pastes, dispersions and the like. The liquid
composition can include
solids or gases in suitably subdivided form, but the liquid composition
excludes forms which are
non-fluid overall, such as tablets or granules.
[0194] The liquid detergent composition can be used in a fabric hand wash
operation or may
be used in an automatic machine fabric wash operation.
[0195] Preferably, the liquid laundry detergent composition comprises from 15%
to 55% by
weight of the laundry detergent composition of a non-soap anionic surfactant.
Preferably, the
detergent composition comprises between 20% and 55%, more preferably between
25% and
50% of a non-soap anionic surfactant.
[0196] Preferably, the non-soap anionic surfactant comprises linear
alkylbenzene sulphonate.
Preferably, the linear alkylbenzene sulphonate comprises Clo-C16 alkyl benzene
sulfonate, Ci 1-
C 14 alkyl benzene sulphonate or a mixture thereof. Preferably, the
alkylbenzene sulphonate is
an amine neutralized alkylbenzene sulphonate, an alkali metal neutralized
alkylbenzene
sulphonate or a mixture thereof. The amine is preferably selected from
monoethanolamine,
triethanolamine or mixtures thereof. The alkali metal is preferably selected
from sodium,
potassium, magnesium or a mixture thereof. Preferably, the liquid laundry
detergent
composition comprises between 1% and 40%, preferably between 3% and 40%, more
preferably between 6% and 35% by weight of the liquid laundry detergent
composition of the
linear alkylbenzene sulphonate.
[0197] Preferably, the non-soap anionic surfactant comprises an alkyl sulphate
anionic
surfactant wherein the alkyl sulphate anionic surfactant is selected from
alkyl sulphate, an
alkoxylated alkyl sulphate or a mixture thereof. The alkyl sulphate anionic
surfactant may be a
primary or a secondary alkyl sulphate anionic surfactant, or a mixture
thereof, preferably a
49
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
primary alkyl sulphate anionic surfactant. Preferably, the alkoxylated alkyl
sulphate comprises
ethoxylated alkyl sulphate, propoxylated alkyl sulphate, a mixed
ethoxylated/propoxylated alkyl
sulphate, or a mixture thereof, more preferably an ethoxylated alkyl sulphate.
Preferably, the
ethoxylated alkyl sulphate has an average degree of ethoxylation of between
0.1 to 5,
preferably between 0.5 and 3. Preferably, the ethoxylated alkyl sulphate has
an average alkyl
chain length of between 8 and 18, more preferably between 10 and 16, most
preferably
between 12 and 15. Preferably, the alkyl chain of the alkyl sulphate anionic
surfactant is linear,
branched or a mixture thereof. Preferably, the branched alkyl sulphate anionic
surfactant is a
branched primary alkyl sulphate, a branched secondary alkyl sulphate, or a
mixture thereof,
preferably a branched primary alkyl sulphate, wherein the branching preferably
is in the 2-
position, or alternatively might be present further down the alkyl chain, or
could be multi-
branched with branches spread over the alkyl chain. The weight average degree
of branching
of alkyl sulphate anionic surfactant may be from 0% to 100% preferably from 0%
to 95%, more
preferably from 0% to 60%, most preferably from 0% to 20%. Alternatively, the
weight average
degree of branching of alkyl sulphate anionic surfactant may be from 70% to
100%, preferably
from 80% to 90%. Preferably, the alkyl chain is selected from naturally
derived material,
synthetically derived material or mixtures thereof. Preferably, the
synthetically derived material
comprises oxo-synthesized material, Ziegler-synthesized material, Guerbet-
synthesized
material, Fischer-Tropsch ¨ synthesized material, iso-alkyl synthesized
material, or mixtures
thereof, preferably oxo-synthesized material. Preferably, the liquid laundry
detergent
composition comprises between 1% and 35%, preferably between 3% and 30%, more
preferably between 6% and 20% by weight of the liquid laundry detergent
composition of the
alkyl sulphate anionic surfactant.
[0198] Preferably, the non-soap anionic surfactant comprises linear alkyl
benzene sulphonate
and an alkoxylated alkyl sulphate, more preferably, wherein the weight ratio
of linear
alkylbenzene sulphonate to alkoxylated alkyl sulphate is from 1:2 to 9:1,
preferably from 1:110
7:1, more preferably from 1:1 to 5:1, most preferably from 1:1 to 4:1.
[0199] The liquid laundry detergent composition comprises from 2.5% to 30% by
weight of the
liquid laundry detergent composition of a non-ionic surfactant. The non-ionic
surfactant is
described in more detail below.
[0200] Preferably, the weight ratio of non-soap anionic surfactant to non-
ionic surfactant is
from 1:1 to 13:1, preferably from 1.25:1 to 10:1, more preferably from 1.5:1
to 7.5:1.
[0201] Preferably, the liquid laundry detergent composition comprises a non-
ionic surfactant.
Preferably, the non-ionic surfactant comprises an alkoxylated alcohol, wherein
the alkoxylated
alcohol is derived from a synthetic alcohol, a natural alcohol or a mixture
thereof. The
alkoxylated alcohol can be a primary alkoxylated alcohol, a secondary
alkoxylated alcohol, or a
mixture thereof, preferably a primary alkoxylated alcohol. Preferably, the
alkoxylated alcohol
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
comprises ethoxylated alcohol, propoxylated alcohol, a mixed
ethoxylated/propoxylated alcohol,
or a mixture thereof, more preferably an ethoxylated alcohol. Alternatively,
the alkoxylated
alcohol might also include higher alkoxy groups such as butoxy groups. When
mixed alkoxy
groups, the alkoxy groups can be randomly ordered or present in blocks,
preferably are present
in blocks. For example, mixed ethoxy (E0)/propoxy (PO) groups might be ordered
in EO/PO
blocks, PO/E0 blocks, EO/PO/E0 blocks or PO/E0/P0 blocks. Preferably, the
ethoxylated
alcohol has an average degree of ethoxylation of between 0.1 to 20, preferably
between 5 and
15, most preferably between 6 and 10. If propoxylation is present, preferably
the average
degree of propoxylation is between 0.1 to 25, more preferably between 2 and
20, most
preferably between 5 and 10. Preferably, the alkoxylated preferably
ethoxylated alcohol has an
average alkyl chain length of between 8 and 18, more preferably between 10 and
16, most
preferably 12 and 15. Preferably, the alkyl chain of the alkoxylated alcohol
is linear, branched
or a mixture thereof, wherein the branched alkyloxylated alcohol is a branched
primary
alkoxylated alcohol, a branched secondary alkoxylated alcohol, or a mixture
thereof, preferably
a branched primary alkoxylated alcohol. Preferably, the weight average degree
of branching of
the alkoxylated alcohol is from 0% to 100% preferably from 0% to 95%, more
preferably 0% to
60%, most preferably from 0% to 20%. The branching can be on the 2-alkyl
position, or
alternatively further down the alkyl chain, or can be multi-branched with
individual branches
spread over the alkyl chain. Preferably, the synthetically derived material
comprises oxo-
synthesized material, Ziegler-synthesized material, Guerbet-synthesized
material, Fischer-
Tropsch ¨ synthesized material, iso-alkyl branched materials, or mixtures
thereof, preferably
oxo-synthesised material. Preferably, the liquid laundry detergent composition
comprises
between 0.5% and 20%, preferably between 1% and 15%, more preferably between
3% and
12% by weight of the liquid laundry detergent composition of the non-ionic
surfactant, preferably
wherein the nonionic surfactant consists of the alkoxylated alcohol. Without
wishing to be
bound by theory, non-ionic surfactants, especially alkoxylated alcohol non-
ionic surfactants
provide the benefit of excellent body soil cleaning and soil suspension.
[0202] Preferably, the weight ratio of non-soap anionic surfactant to nonionic
is from 1:1 to
20:1, from 1.5:1 to 17.5:1, from 2:1 to 15:1, or from 2.5:1 to 13:1.
[0203] Preferably, the liquid laundry detergent composition comprises a fatty
acid, preferably
a neutralized fatty acid soap, preferably a fatty acid salt, more preferably
an amine neutralized
fatty acid salt, wherein preferably the amine is an alkanolamine more
preferably selected from
monoethanolamine, diethanolamine, triethanolamine or a mixture thereof, more
preferably
monoethanolamine. The liquid detergent composition may comprise between 1.5%
and 20%,
between 2% and 15%, between 3% and 12%, or between 4% and 10% by weight of the
liquid
detergent composition of fatty acid.
51
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
[0204] Preferably, the liquid laundry detergent composition comprises between
1% and 20%,
preferably between 5% and 15% by weight of the liquid laundry detergent
composition of water.
[0205] Preferably, the liquid laundry detergent composition comprises between
10% and 40%,
preferably between 15% and 30% by weight of the liquid laundry detergent
composition of a
non-aqueous solvent, preferably wherein the non-aqueous solvent is selected
from 1,2-
propanediol, dipropylene glycol, tripropyleneglycol, glycerol, sorbitol,
polyethylene glycol or a
mixture thereof.
[0206] Preferably, the liquid laundry detergent composition comprises an
adjunct ingredient
selected from the group comprising builders, perfumes, enzymes, citrate,
bleach, bleach
catalyst, dye, hueing dye, brightener, cleaning polymers including alkoxylated
polyamines and
polyethyleneimines, soil release polymer, fabric care polymers including
cationic hydroxyethyl
celluloses and cationic polyglucans, surfactant, solvent, dye transfer
inhibitors, chelant,
encapsulated perfume, polycarboxylates, structurant, pH trimming agents, anti-
oxidants
including Ralox 35, and mixtures thereof.
[0207] Preferably, the laundry detergent composition comprises a further
enzyme selected
from the group comprising hemicellulases, peroxidases, proteases, cellulases,
xylanases,
lipases, phospholipases, esterases, cutinases, pectinases, keratanases,
reductases, oxidases,
phenoloxidases, lipoxygenases, ligninases, pullulanases, tannases,
pentosanases, malanases,
13-glucanases, arabinosidases, hyaluronidase, chondroitinase, laccase,
xyloglucanases,
mannanases and amylases, nuclease or mixtures thereof, preferably a further
enzyme selected
from the group comprising proteases, amylase, cellulase, lipases,
xyloglucanases,
mannanases, and mixtures thereof. Preferably the further enzyme is a lipase.
[0208] The term lipase as used herein, includes enzymes which catalyze the
hydrolysis of fats
(lipids). Lipases are a sub class of esterases. Lipases suitable in the
present invention include
phospholipases, acyltransf erases or perhydrolases e.g. acyltransferases with
homology to
Candida antarctica lipase A, acyltransf erase from Mycobacterium smegmatis,
perhydrolases
from the CE 7 family, and variants of the M. smegmatis perhydrolase in
particular the S54V
variant used in the commercial product Gentle Power Bleach from Huntsman
Textile Effects Pte
Ltd. Suitable lipases and cutinases include those of bacterial or fungal
origin. Chemically
modified or protein engineered mutant enzymes are included. Examples include
lipase from
Thermomyces, e.g. from T. lanuginosus (previously named Hum/cola lanuginosa),
cutinase from
Hum/cola, e.g. H. insolens, lipase from strains of Pseudomonas (some of these
now renamed to
Burkholderia), e.g. P. alcaligenes or P. pseudoalcaligenes, P. cepacia, P. sp.
strain SD705, P.
wisconsinensis, GDSL-type Streptomyces lipases, cutinase from Magnaporthe
grisea, cutinase
from Pseudomonas mendocina, lipase from Thermobifida fusca, Geobacillus
stearothermophilus lipase, lipase from Bacillus subtilis, and lipase from
Streptomyces griseus
and S. pristinaespiralis. Typically, the lipase enzyme is present in the
composition in an amount
52
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
from 0.001% to 0.03%, preferably from 0.0025% to 0.025% and more preferably
from 0.005% to
0.02% by weight of the composition of enzyme active protein. Without wishing
to be bound by
theory, enzymes are supplied as a preparation comprising the enzyme and other
ingredients.
Enzymes per se are proteins that catalyse reactions. By enzyme active protein
we herein mean
enzyme that can actively catalyse the relevant reaction.
[0209] Preferably, the liquid laundry detergent composition has a pH between 6
and 10, more
preferably between 6.5 and 8.9, most preferably between 7 and 8, wherein the
pH of the
laundry detergent composition is measured as a 10% product concentration in
deionized water
at 20 C.
[0210] The liquid laundry detergent composition may be Newtonian or non-
Newtonian.
Preferably, the liquid laundry detergent composition is non-Newtonian. Without
wishing to be
bound by theory, a non-Newtonian liquid has properties that differ from those
of a Newtonian
liquid, more specifically, the viscosity of non-Newtonian liquids is dependent
on shear rate,
while a Newtonian liquid has a constant viscosity independent of the applied
shear rate. The
decreased viscosity upon shear application for non-Newtonian liquids is
thought to further
facilitate liquid detergent dissolution. The liquid laundry detergent
composition described herein
can have any suitable viscosity depending on factors such as formulated
ingredients and
purpose of the composition.
Automatic dishwashing detergent composition:
[0211] The treatment composition may be an automatic dish washing detergent
composition
comprising an ingredient selected from surfactant, builder, sulfonated /
carboxylated polymer,
silicone suds suppressor, silicate, metal and/or glass care agent, enzyme,
bleach, bleach
activator, bleach catalyst, source of alkalinity, perfume, dye, solvent,
filler and mixtures thereof.
[0212] A preferred surfactant for use in automatic dishwashing detergents is
low foaming by
itself or in combination with other components (e.g. suds suppressers).
Preferred for use herein
are low and high cloud point nonionic surfactants and mixtures thereof
including nonionic
alkoxylated surfactants (especially ethoxylates derived from C6-C18 primary
alcohols),
ethoxylated-propoxylated alcohols (e.g., Olin Corporation's POLY-TERGENT
SLF18), epoxy-
capped poly(oxyalkylated) alcohols (e.g., Olin Corporation's POLY-TERGENT
SLF18B, ether-
capped poly(oxyalkylated) alcohol surfactants, and block polyoxyethylene-
polyoxypropylene
polymeric compounds such as PLURONIC , REVERSED PLURONIC , and TETRONIC
series by the BASF-Wyandotte Corp., Wyandotte, Michigan; amphoteric
surfactants such as the
012-020 alkyl amine oxides (preferred amine oxides for use herein include
lauryldimethyl
amine oxide and hexadecyl dimethyl amine oxide), and alkyl amphocarboxylic
surfactants such
as MIRANOLTM C2M; and zwitterionic surfactants such as the betaines and
sultaines; and
mixtures thereof. Surfactants can be present at a level of from 0.2% to 30% by
weight, more
53
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
preferably from 0.5% to 10% by weight, most preferably from 1% to 5% by weight
of a detergent
composition.
[0213] Builders suitable for use in the detergent composition described herein
include water-
soluble builders, including citrates, carbonates, silicate and polyphosphates,
e.g. sodium
tripolyphosphate and sodium tripolyphosphate hexahydrate, potassium
tripolyphosphate and
mixed sodium and potassium tripolyphosphate salts.
[0214] Enzymes suitable for use in the detergent composition described herein
include
bacterial and fungal cellulases including CAREZYME8 and CELLUZYME (Novo
Nordisk A/S);
peroxidases; lipases including AMANO-P8 (Amano Pharmaceutical Co.), M1 LIPASE
and
LIPOMAX8 (Gist-Brocades) and LIPOLASE8 and LIPOLASE ULTRA (Novo); cutinases;
proteases including ESPERASE , ALCALASE , DURAZYM8 and SAVINASE (Novo) and
MAXATASE8, MAXACAL , PROPERASE8 and MAXAPEM8 (Gist-Brocades); o Hand
Hamylases including PURAFECT OX AM (Genencor) and TERMAMYL , BAN ,
FUNGAMYL8, DURAMYL8, and NATALASE8 (Novo); pectinases; and mixtures thereof.
Enzymes can be added herein as prills, granulates, or cogranulates at levels
typically in the
range from 0.0001% to 2% pure enzyme by weight of the cleaning composition.
[0215] Suds suppressers suitable for use in the detergent composition
described herein
include nonionic surfactants having a low cloud point. "Cloud point" as used
herein, is a well-
known property of nonionic surfactants which is the result of the surfactant
becoming less
soluble with increasing temperature, the temperature at which the appearance
of a second
phase is observable is referred to as the "cloud point." As used herein, a
"low cloud point"
nonionic surfactant is defined as a nonionic surfactant system ingredient
having a cloud point of
less than 30 C, preferably less than about 20 C, and even more preferably
less than about 10
C, and most preferably less than about 7.5 C. Low cloud point nonionic
surfactants can
include nonionic alkoxylated surfactants, especially ethoxylates derived from
primary alcohol,
and polyoxypropylene/polyoxyethylene/polyoxypropylene (PO/E0/P0) reverse block
polymers.
Also, such low cloud point nonionic surfactants can include, for example,
ethoxylated-
propoxylated alcohol (e.g., BASF POLY-TERGENT SLF18) and epoxy-capped
poly(oxyalkylated) alcohols (e.g., BASF POLY-TERGENT SLF18B series of
nonionics.
[0216] Other suitable components for use in the detergent composition
described herein
include cleaning polymers having anti-redeposition, soil release or other
detergency properties.
Anti-redeposition polymers for use herein include acrylic acid containing
polymers such as
SOKALAN8 PA30, PA20, PA15, PA10 and SOKALAN8 CP10 (BASF GmbH), ACUSOL8 45N,
480N, 460N (Rohm and Haas), acrylic acid/maleic acid copolymers such as
SOKALAN CP5,
and acrylic/methacrylic copolymers. Other suitable polymers include amine-
based polymers
such as alkoxylated polyalkyleneimines (e.g., PEI600 E020 and/or
ethoxysulfated
hexamethylene diamine dimethyl quats), which, optionally, may be quaternized.
Soil release
54
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
polymers for use herein include alkyl and hydroxyalkyl celluloses,
polyoxyethylenes,
polyoxypropylenes and copolymers thereof, and nonionic and anionic polymers
based on
terephthalate esters of ethylene glycol, propylene glycol and mixtures
thereof.
[0217] Heavy metal sequestrants and crystal growth inhibitors are also
suitable for use in the
detergent, for example diethylenetriamine penta(methylene phosphonate),
ethylenediannine
tetra(methylene phosphonate) hexamethylenediamine tetra(methylene
phosphonate), ethylene
diphosphonate, hydroxy-ethylene-1,1-diphosphonate, nitrilotriacetate,
ethylenediaminotetracetate, ethylenediamine-N,N'-disuccinate in their salt and
free acid forms.
[0218] Suitable for use in the detergent composition described herein is also
a corrosion
inhibitor, for example organic silver coating agents (especially paraffins
such as WINOGO 70
sold by Wintershall, Salzbergen, Germany), nitrogen-containing corrosion
inhibitor compounds
(for example benzotriazole and benzimadazole and Mn(II) compounds,
particularly Mn(II) salts
of organic ligands.
[0219] Other suitable components for use in the detergent composition herein
include enzyme
stabilizers, for example calcium ion, boric acid and propylene glycol.
[0220] Suitable rinse additives are known in the art. Commercial rinse aids
for dishwashing
typically are mixtures of low-foaming fatty alcohol polyethylene/polypropylene
glycol ethers,
solubilizers (for example cumene sulfonate), organic acids (for example citric
acid) and solvents
(for example ethanol). The function of such rinse aids is to influence the
interfacial tension of the
water in such a way that it is able to drain from the rinsed surfaces in the
form of a thin coherent
film, so that no water droplets, streaks, or films are left after the
subsequent drying process.
Non-household Care Compositions
[0221] The composition for inclusion in the unit dose article can be a non-
household care
composition. For example, a non-household care composition can be selected
from agricultural
compositions, automotive compositions, aviation compositions, food and
nutritive compositions,
industrial compositions, livestock compositions, marine compositions, medical
compositions,
mercantile compositions, military and quasi-military compositions, office
compositions, and
recreational and park compositions, pet compositions, water-treatment
compositions, including
cleaning and detergent compositions applicable to any such use.
[0222] In one type of embodiment, the composition can include an agrochemical,
e.g. one or
more insecticides, fungicides, herbicides, pesticides, miticides, repellants,
attractants,
defoliaments, plant growth regulators, fertilizers, bactericides,
micronutrients, and trace
elements. Suitable agrochemicals and secondary agents are described in U.S.
Patent Nos.
6,204,223 and 4,681,228 and EP 0989803 Al. For example, suitable herbicides
include
paraquat salts (for example paraquat dichloride or paraquat
bis(methylsulphate), diquat salts
(for example diquat dibromide or diquat alginate), and glyphosate or a salt or
ester thereof (such
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
as glyphosate isopropylammonium, glyphosate sesquisodiunn or glyphosate
trimesium, also
known as sulfosate). Incompatible pairs of crop protection chemicals can be
used in separate
chambers, for example as described in U.S. Patent No. 5,558,228. Incompatible
pairs of crop
protection chemicals that can be used include, for example, bensulfuron methyl
and molinate;
2,4-D and thifensulfuron methy1;2,4-D and methyl 2-EN-4-methoxy-6-methy1-1,3,5-
triazine-2-
y1)-N-methylamino]carbonyl]amino]-sulfonyl]benzoate; 2,4-D and metsulfuron
methyl; maneb or
mancozeb and benomyl; glyphosate and metsulfuron methyl; tralomethrin and any
organophosphate such as monocrotophos or dimethoate; bromoxynil and NI[4,6-
dimethoxypyrimidine-2-y1) -amino]carbonyI]-3-(ethylsulfony1)-2-pyridine -
sulfonamide;
bromoxynil and methyl 2-[[[[(4-methy1-6-methoxy)-1,3,5-triazin-2-
yl)amino]carbonyl]amino]sulfonyl]-benzoate; bromoxynil and methyl 2-M[N-(4-
methoxy-6-
methy1-1,3,5-triazin-2-y1)-N-methylamino]carbonyl]amino]-sulfonyl]benzoate. In
another,
related, type of embodiment, the composition can include one or more seeds,
optionally
together with soil, and further optionally together with one or more
additional components
selected from mulch, sand, peat moss, water jelly crystals, and fertilizers,
e.g. including types of
embodiments described in U.S. Patent No. 8,333,033.
[0223] In another type of embodiment, the composition is a water-treatment
agent. Such
agents include aggressive oxidizing chemicals, e.g. as described in U.S.
Patent Application
Publication No. 2014/0110301 and U.S. Patent No. 8,728,593. For example,
sanitizing agents
can include hypochlorite salts such as sodium hypochlorite, calcium
hypochlorite, and lithium
hypochlorite; chlorinated isocyanurates such as dichloroisocyanuric acid (also
referred to as
"dichlor" or dichloro-s-triazinetrione, 1 ,3-dichloro- 1 ,3,5-triazinane-2,4,6-
trione) and
trichloroisocyanuric acid (also referred to as ''trichlor" or1,3,5-trichloro-
1,3,5- triazinane-2,4,6-
trione). Salts and hydrates of the sanitizing compounds are also contemplated.
For example,
dichloroisocyanuric acid may be provided as sodium dichloroisocyanurate,
sodium
dichloroisocyanurate acid dihydrate, among others. Bromine containing
sanitizing agents may
also be suitable for use in unit dose packaging applications, such as1,3-
dibromo-5,5-
dimethylhydantoin (DBDMH), 2,2- dibromo-3-nitrilopropionamide (DBNPA),
dibromocyano
acetic acid amide, 1-bromo- 3-chloro-5,5-dimethylhydantoin; and 2-bromo-2-
nitro- 1,3 -
propanediol, among others. The oxidizing agent can be one described in U.S.
Patent No.
7,476,325, e.g. potassium hydrogen peroxymonosulfate. The composition can be a
pH-
adjusting chemical, e.g. as described in U.S. Patent Application Publication
No. 2008/0185347,
and can include, for example, an acidic component and an alkaline component
such that the
composition is effervescent when contacted with water, and adjusts the water
pH. Suitable
ingredients include sodium bicarbonate, sodium bisulfate, potassium hydroxide,
sulfamic acid,
organic carboxylic acids, sulfonic acids, and potassium dihydrogen phosphate.
A buffer blend
can include boric acid, sodium carbonate, glycolic acid, and oxone
monopersulfate, for
example.
56
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
[0224] A water-treatment agent can be or can include a flocculant, e.g. as
described in U.S.
Patent Application Publication No. 2014/0124454. The flocculant can include a
polymer
flocculant, e.g. polyacrylamide, a polyacrylamide copolymer such as an
acrylamide copolymers
of diallydimethylammonium chloride (DADMAC), dimethylaminoethylacrylate
(DMAEA),
dimethylaminoethylmethacrylate (DMAEM), 3- methylamidepropyltrimethylammonium
chloride
(MAPTAC) or acrylic acid; a cationic polyacrylamide; an anionic
polyacrylamide; a neutral
polyacrylamide; a polyamine; polyvinylamine; polyethylene imine;
polydimethyldiallylammonium
chloride; poly oxyethylene; polyvinyl alcohol; polyvinyl pyrrolidone;
polyacrylic acid;
polyphosphoric acid; polystyrene sulfonic acid; or any combination thereof. A
flocculant can be
selected from chitosan acetate, chitosan lactate, chitosan adipate, chitosan
glutamate, chitosan
succinate, chitosan malate, chitosan citrate, chitosan fumarate, chitosan
hydrochloride, and
combinations thereof. The water-treating composition can include a phosphate
removing
substance, e.g. one or more selected from a zirconium compound, a rare earth
lanthanide salt,
an aluminum compound, an iron compound, or any combination thereof.
[0225] The composition can be a limescale removing composition, e.g. citric or
maleic acid or
a sulphate salt thereof, or any mixture thereof, e.g. as described in U.S.
Patent Application No.
2006/0172910.
[0226] Various other types of compositions are contemplated for use in the
unit dose articles
described herein, including particulates, for example down feathers, e.g. as
described in US
RE29059 E; super absorbent polymers, e.g. as described in U.S. Patent
Application Publication
Nos. 2004/0144682 and 2006/0173430; pigments and tinters, e.g. as described in
U.S. Patent
No. 3,580,390 and U.S. Patent Application Publication No. 2011/0054111;
brazing flux (e.g.
alkali metal fluoroaluminates, alkali metal fluorosilicates and alkali metal
fluorozincates), e.g. as
described in U.S. Patent No. 8,163,104; ingestible and food items (e.g.,
coffee powder or dried
soup) as described in U.S. Patent Application Publication No. 2007/0003719;
and wound
dressings, e.g. as described in U.S. Patent No. 4,466,431.
Active agents
[0227] In another aspect, the composition for use in the unit dose article can
be characterized
by an active agent therein, e.g. a personal care active agent, a beauty
benefit active agent, a
skin care active agent, a hair care active agent, a fabric care active agent,
a dishwashing active
agent, a hard surface active agent, an agricultural active agent, an
ingestible active agent, a
liquid treatment active agent, an industrial active agent, or a combination of
any of the
foregoing.
[0228] "Personal care active agent," as used herein, means an active agent
that may be
applied to mammalian keratinous tissue without undue undesirable effects.
57
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
[0229] "Keratinous tissue," as used herein, means keratin-containing layers
disposed as the
outermost protective covering of mammals and includes, but is not limited to,
skin, hair, scalp
and nails.
[0230] "Beauty benefit," as used herein in reference to mammalian keratinous
tissue includes,
but is not limited to cleansing, sebum inhibition, reducing the oily and/or
shiny appearance of
skin and/or hair, reducing dryness, itchiness and/or flakiness, reducing skin
pore size,
exfoliation, desquamation, improving the appearance of the keratinous tissue,
conditioning,
smoothening, deodorizing skin and/or providing antiperspirant benefits, etc.
[0231] "Beauty benefit active agent," as used herein, refers to an active
agent that can deliver
one or more beauty benefits.
[0232] "Skin care active agent" as used herein, means an active agent that
when applied to
the skin provides a benefit or improvement to the skin. It is to be understood
that skin care
active agents are useful not only for application to skin, but also to hair,
scalp, nails and other
mammalian keratinous tissue.
[0233] "Hair care active agent" as used herein, means an active agent that
when applied to
mammalian hair provides a benefit and/or improvement to the hair. Non-limiting
examples of
benefits and/or improvements to hair include softness, static control, hair
repair, dandruff
removal, dandruff resistance, hair coloring, shape retention, hair retention,
and hair growth.
[0234] "Fabric care active agent" as used herein means an active agent that
when applied to
fabric provides a benefit and/or improvement to the fabric. Non-limiting
examples of benefits
and/or improvements to fabric include cleaning (for example by surfactants),
stain removal,
stain reduction, wrinkle removal, color restoration, static control, wrinkle
resistance, permanent
press, wear reduction, wear resistance, pill removal, pill resistance, soil
removal, soil resistance
(including soil release), shape retention, shrinkage reduction, softness,
fragrance, anti-bacterial,
anti-viral, odor resistance, and odor removal.
[0235] "Dishwashing active agent" as used herein means an active agent that
when applied to
dishware, glassware, pots, pans, utensils, and/or cooking sheets provides a
benefit and/or
improvement to the dishware, glassware, pots, pans and/or cooking sheets. Non-
limiting
example of benefits and/or improvements to the dishware, glassware, pots,
pans, utensils,
and/or cooking sheets include food and/or soil removal, cleaning (for example
by surfactants)
stain removal, stain reduction, grease removal, water spot removal and/or
water spot
prevention, shining, and polishing.
[0236] "Hard surface active agent" as used herein means an active agent when
applied to
floors, countertops, sinks, windows, mirrors, showers, baths, and/or toilets
provides a benefit
and/or improvement to the floors, countertops, sinks, windows, mirrors,
showers, baths, and/or
toilets. Non-limiting example of benefits and/or improvements to the floors,
countertops, sinks,
58
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
windows, mirrors, showers, baths, and/or toilets include food and/or soil
removal, grease
removal, water spot removal and/or water spot prevention, shining, and
polishing.
[0237] "Agricultural active agent" as used herein means an active agent that
when applied to
crops and/or plants provides a benefit and/or improvement to the crops and/or
plants. For
example, insecticides, herbicides, fertilizers, drought resistant agents, are
non-limiting examples
of suitable agricultural active agents that may be present in the compositions
of the present
invention contained in a unit dose article.
[0238] "Ingestible active agent" as used herein means an active agent that is
suitable for
ingestion and/or consuming by an animal, for example a mammal, such as a
human, by way of
mouth, nose, eyes, ears, skin pores, rectum, vagina, or other orifice or wound
(such as
delivering an active agent by wound dressing) in the animal. Non-limiting
examples of ingestible
active agents include feminine hygiene active agents, baby care active agents,
oral care active
agents, medicinal active agents, vitamins, dietary active agents (for example
delivered in a new
food form), pet care active agents, and mixtures thereof.
[0239] "Liquid treatment active agent" as used herein means an active agent
that when
applied to a liquid such as water and/or alcohol, provides a benefit and/or
improvement to the
liquid. For example, chlorine and/or other swimming pool chemicals are non-
limiting examples
of suitable liquid treatment active agents. In another example, water
clarifying and/or water
disinfecting active agents, such as are used in commercial water filtering
and/or water treatment
technologies such as FUR are non-limiting examples of suitable liquid
treatment active agents
that may be present in the compositions of the present invention contained in
a unit dose article.
Further, oil dispersants and/or oil scavenging agents are non-limiting
examples of other suitable
liquid treatment active agents.
[0240] "Industrial active agent" as used herein means an active agent that
provides a benefit
within an article of manufacture. For example, glue and/or adhesive to provide
bonding between
two object, insecticides incorporated into insulation, such as housing
insulation, oxygen
scavenging active agents incorporated into packaging for food and/or
perishable goods, insect
repellants incorporated into articles used by humans to repel insects, and
moisture scavengers
incorporated into desiccants are non-limiting examples of industrial active
agents that may be
present in the compositions of the present invention contained in a unit dose
article.
[0241] Various aspects of the films, unit dose articles, and methods are
described below
using numbered paragraphs.
Aspect A
[0242] Al. A water-soluble film comprising a water-soluble mixture of:
59
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
a polyvinyl alcohol resin present in an amount of about 50 wt% to about 95
wt%,
based on the total weight of the film, the polyvinyl alcohol resin comprising:
less than about 15 wt%, based on the total weight of the polyvinyl alcohol
resin,
of a polyvinyl alcohol copolymer comprising a carboxylated anionic monomer
unit, and
about 85 wt% to about 100 wt%, based on the total weight of the polyvinyl
alcohol resin, of a polyvinyl alcohol homopolymer or a polyvinyl alcohol
homopolymer blend,
wherein the polyvinyl alcohol homopolymer has an 4% aqueous solution viscosity
in deionized water at 20 C of at least 8 mPa.s and less than 12 mPa.s, or
about 9mPa.s to less
than 12 mPa.s, or about lOmPa.s to less than 12 mPa.s, or the polyvinyl
alcohol homopolymer
blend has a weighted average 4% aqueous solution viscosity in deionized water
at 20 'C of at
least 8 mPa.s and less than 12 mPa.s, or about 9mPa.s to less than 12 mPa.s,
or about
lOmPa.s to less than 12 mPa.s.
[0243] A2. The water-soluble film of Al, wherein the polyvinyl alcohol resin
comprises about
85 wt% to about 100 wt%, based on the total weight of the polyvinyl alcohol
resin of a polyvinyl
alcohol homopolymer blend.
[0244] A3. The water-soluble film of Al, wherein the polyvinyl alcohol resin
comprises the
polyvinyl alcohol homopolymer component, comprising a polyvinyl alcohol
homopolymer or
polyvinyl alcohol homopolymer blend, at a concentration in a range of about 90
wt% to about 99
wt%, or about 90 wt% to about 100 wt%, or 100 wt%, based on the polyvinyl
alcohol resin, and
the polyvinyl alcohol copolymer component at a concentration in a range of
about 0 wt% to
about 10 wt%, 1 wt% to about 10 wt%, or 0 wt%, of the polyvinyl alcohol resin.
[0245] A4. The water-soluble film of any one of Al -A3, wherein the polyvinyl
alcohol
homopolymer or the polyvinyl alcohol homopolymer blend has an average 4%
aqueous solution
viscosity in deionized water at 20 C of at least 8 mPa.s to about 11.5 mPa.s,
or at least
8.5mPa.s to about 11.5 mPa.s, or at least 9mPa.s to about 11.5 mPa.s, or about
lOmPa.s to
about 11.5 mPa.s.
[0246] A5. The water-soluble film of any one of Al -A4, wherein the polyvinyl
alcohol
homopolymer blend comprises a first polyvinyl alcohol homopolymer and a second
polyvinyl
alcohol homopolymer, wherein the first polyvinyl alcohol homopolymer and
second polyvinyl
alcohol homopolymer are present in a relative weight ratio in a range of about
90/10 to about
10/90, or about 80/20 to about 20/80, or about 70/30 to about 50/50.
[0247] A6. The water-soluble film of A5, wherein:
the first polyvinyl alcohol homopolymer has an average viscosity in a range of
about 11 mPa.s to about 20 mPa.s, or about 11 mPa.s to about 15 mPa.s,
measured as a 4%
polyvinyl alcohol polymer solution in deionized water at 20 C; and,
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
the second polyvinyl alcohol hornopolymer has an average viscosity in a range
of
about 1 mPa.s to about 10 mPa.s, or about 5 mPa.s to about 10 mPa.s, measured
as a 4%
polyvinyl alcohol polymer solution in deionized water at 20 00.
[0248] A7. The water-soluble film of A5 or A6, wherein the difference in
average viscosities
between the first polyvinyl alcohol homopolymer and the second polyvinyl
alcohol homopolymer
is at least about 1 mPa.s, or in a range of about 2 mPa.s to about 10 mPa.s,
or in a range of
about 3 mPa.s to about 8 mPa.s, measured as a 4% polyvinyl alcohol polymer
solution in
deionized water at 20 00.
[0249] A8. The water-soluble film of any one of A5-A7, wherein each of the
first polyvinyl
alcohol homopolymer and the second polyvinyl alcohol homopolymer has an
average degree of
hydrolysis in a range of about 75% to about 99%, or about 80% to about 95%, or
about 85% to
about 95%.
[0250] A9. The water-soluble film of any one of A5-A8, wherein the film is
characterized
100% modulus values of less than 20 N/rnm2 as measured by the MOD Test at 35%
RH,
optionally less than about 19N/mm2, or less than about 18N/mm2, or less than
about 17N/mm2,
or less than about 16N/mm2, or less than about 15N/mm2, or less than about
14N/mm2, and
optionally at least about 9N/mm2, or at least about 10N/mm2, or at least about
11N/mm2, at least
about 12N/mm2, or at least about 13N/mm2, for example in a range of about
10N/mm2 to about
16N/mm2, or about 11N/mm2 to about 15N/mm2, or about 12N/mm2 to about 14N/mm2.
[0251] A10. The water-soluble film of any one of Al -A9, wherein the
carboxylated anionic
monomer unit is derived from maleic acid, monoalkyl maleate, dialkyl maleate,
maleic
anhydride, or any combination thereof.
[0252] A11. The water-soluble film of A10, wherein the carboxylated anionic
monomer unit is
derived from maleic acid.
[0253] Al2. The water-soluble film of A10, wherein the carboxylated anionic
monomer unit is
derived from a monoalkyl maleate unit optionally selected from the group
consisting of
monomethyl maleate, salts, e.g. alkali metal salts, thereof, and combinations
thereof.
[0254] A13. The water-soluble film of any one of Al -Al2, wherein the
carboxylated anionic
monomer unit is present in the polyvinyl alcohol copolymer in an average
amount of at least 3
mol%, or in a range of about 3 mol% to about 6 mol%, or about 3 mol% to about
5 mol%, or
about 3.5 mol% to about 4.5 mol%, or about 4 mol% to about 4.5mo1%.
[0255] A14. The water-soluble film of any one Al -A13, wherein the polyvinyl
alcohol resin is
present in the water soluble film an amount in a range of about 50 wt% to
about 80 wt%, or
about 60 wt% to about 75 wt%, based on the total weight of the film.
61
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
[0256] A15. The water-soluble film of any one Al -Al 4, further comprising a
surfactant in an
amount of in a range of about 0.1 wt% to about 3.5 wt%, or about 0.5 wt% to
about 2 wt%,
based on the total weight of the film.
[0257] A16. The water-soluble film of any one of A1-A15, wherein the film has
a residual
moisture content of at least 4 wt%, or in a range of about 4 wt% to about 15
wt%, or about 5
wt% to about 10 wt%, based on the total weight of the film, as measured by
Karl Fischer
titration.
[0258] A17. The water-soluble film of any one of Al -A16, further comprising
one or more
components selected from plasticizers, plasticizer compatibilizers,
lubricants, release agents,
fillers, extenders, cross-linking agents, antiblocking agents, antioxidants,
detackifying agents,
antifoams, nanoparticles, bleaching agents, and aversive agents.
[0259] A18. The water-soluble film of A17, comprising a plasticizer in a total
amount in a
range of about 5 wt% to about 50 wt%, or about 10 wt% to about 40 wt%, or
about 20 wt% to
about 30 wt%, based on the total weight of the film.
[0260] A19. The water-soluble film of Al 7 or A18, wherein the plasticizer
comprises glycerol,
diglycerin, ethylene glycol, diethylene glycol, triethyleneglycol,
tetraethylene glycol, polyethylene
glycols up to 400 MW, neopentyl glycol, 1,2-propylene glycol, 1,3-propanediol,
dipropylene
glycol, polypropylene glycol, 2-methyl-1,3-propanediol, trimethylolpropane,
polyether polyols,
isomalt, maltitol, sorbitol, xylitol, erythritol, adonitol, dulcitol,
pentaerythritol, mannitol, or a
mixture of any of the foregoing.
[0261] A20. The water-soluble film of A19, wherein the plasticizer comprises
sorbitol,
glycerol, dipropyleneglycol, polyethyleneglycol, trimethylolpropane, or a
mixture of any of the
foregoing.
[0262] A21. The water-soluble film of any one of Al -A20, wherein the film has
an average
thickness, prior to any deformation, in a range of about 20 to about 150
micron, or about 35 to
about 125 micron, or about 50 to about 110 micron, or about 76 micron.
[0263] A22. The water soluble film of any one of Al -A21, wherein the film is
a solvent-cast
film.
[0264] A23. A water-soluble unit dose article comprising the water-soluble
film of any one of
Al -A22.
[0265] A24. The water-soluble unit dose article of A23, comprising a
compartment and a
composition housed within the compartment.
Aspect B
62
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
[0266] Bl. A water-soluble unit dose article comprising at least two
compartments and
optionally containing a composition housed in at least one of the
compartments, wherein the
unit dose article comprises;
a. a first water-soluble film, wherein the first water-soluble film has a
first side
and a second side, and wherein the first water soluble film comprises a first
PVOH resin wherein the first polyvinyl alcohol resin comprises;
i. a first polyvinyl alcohol polymer comprising carboxylated anionic
monomer units, vinyl alcohol monomer units and optionally vinyl acetate
monomer units, and wherein the carboxylated anionic monomer unit is
derived from a member selected from the group consisting of maleic acid,
monoalkyl maleate, dialkyl maleate, maleic anhydride, and combinations
thereof;
ii.a second PVOH polymer wherein the second PVOH polymer is a
homopolymer wherein the homopolymer consists of vinyl alcohol monomer
units and optionally vinyl acetate monomer units;
b. a second water-soluble film, wherein the second water-soluble film has a
first side and a second side, and wherein the second water-soluble film
comprises a second polyvinyl alcohol resin wherein the second polyvinyl
alcohol resin comprises;
i. less than 15% by weight of the second polyvinyl alcohol resin of a
polyvinyl alcohol polymer comprising carboxylated anionic monomer units,
vinyl alcohol monomer units and optionally vinyl acetate monomer units,
and wherein the carboxylated anionic monomer unit is derived from a
member selected from the group consisting of maleic acid, monoalkyl
maleate, dialkyl maleate, maleic anhydride, and combinations thereof;
ii.about 85% to about 100% by weight of the second polyvinyl alcohol resin
of a polyvinyl alcohol homopolymer or a homopolymer blend, wherein the
homopolymers consist of vinyl alcohol monomer units and optionally vinyl
acetate monomer units;
wherein the second polyvinyl alcohol resin has an average viscosity in a
range of about 8mPa.s to less than 12 mPa.s, or about 9mPa.s to less than 12
mPa.s, or about lOmPa.s to less than 12 mPa.s, measured as a 4% polyvinyl
alcohol polymer solution in deionized water at 20 qC;
c. a third water-soluble film wherein the third water-soluble film has a
first side
and a second side, and wherein the third water soluble film comprises a
63
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
third polyvinyl alcohol resin, wherein the third polyvinyl alcohol resin
optionally comprises:
i. a first PVOH polymer comprising carboxylated anionic monomer units,
vinyl alcohol monomer units and optionally vinyl acetate monomer units,
and wherein the carboxylated anionic monomer unit is derived from a
member selected from the group consisting of maleic acid, monoalkyl
maleate, dialkyl maleate, maleic anhydride, and combinations thereof;
ii.a second PVOH polymer wherein the second PVOH polymer is a
homopolymer wherein the homopolymer consists of vinyl alcohol monomer
units and optionally vinyl acetate monomer units;
wherein the first side of the first water-soluble film is sealed to the second
side of the second water-soluble film to create a first compartment between
the
first water-soluble film and the second water-soluble film, and the first side
of the
second water-soluble film is sealed to the second side of the third water-
soluble
film to create at least a second compartment between the second water-soluble
film and the third water-soluble film, and wherein the second compartment is
positioned above the first compartment;
provided that when the a composition is housed in at least one of the
compartments then the composition is not a household care composition.
[0267] B2. The water-soluble unit dose article according to 61 wherein the
second polyvinyl
alcohol resin comprises about 90% to about 100%, optionally about 100% by
weight of the
second polyvinyl alcohol resin of the polyvinyl alcohol homopolymer or
polyvinyl alcohol
homopolymer blend, and about 0% to about 10%, optionally about 0% by weight of
the second
polyvinyl alcohol resin of the polyvinyl alcohol polymer comprising
carboxylated anionic
monomer units, vinyl alcohol monomer units and optionally vinyl acetate
monomer units,
wherein the carboxylated anionic monomer unit is derived from a member
selected from the
group consisting of maleic acid, nnonoalkyl maleate, dialkyl maleate, maleic
anhydride, and
combinations thereof.
[0268] B3. The water-soluble unit dose article according to any one of B1-63,
wherein the
second polyvinyl alcohol resin comprises a blend of a first polyvinyl alcohol
homopolymer and a
second polyvinyl alcohol homopolymer, optionally wherein the first polyvinyl
alcohol
homopolymer and second polyvinyl alcohol homopolymer are present in a relative
weight ratio
of in a range of about 90/10 to about 10/90, or about 80/20 to about 20/80, or
about 70/30 to
about 50/50.
[0269] B4. The water-soluble unit dose article according to 63, wherein in the
second
polyvinyl alcohol resin;
64
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
a. the first polyvinyl alcohol homopolymer has an average viscosity in a range
of
about 11 mPa.s to about 20 mPa.s, or about 11 mPa.s to about 15 mPa.s,
measured as a 4% polyvinyl alcohol polymer solution in deionized water at 20
00;
b. the second polyvinyl alcohol homopolymer has an average viscosity in a
range of
about 1 mPa.s to about 10 mPa.s, or about 5mPa.s to about 10 mPa.s,
measured as a 4% polyvinyl alcohol polymer solution in deionized water at 20
00;
optionally, wherein the difference in average viscosity of the first polyvinyl
alcohol
polymer and the second polyvinyl alcohol homopolymer is at least 1mPa.s, or
about 2 to
about 10 mPa.s, or about 3 to about 8 mPa.s, measured as a 4% polyvinyl
alcohol
polymer solution in deionized water at 20 C.
[0270] B5. The water-soluble unit dose article according to any one of B3-135,
wherein the
individual polyvinyl alcohol homopolymers independently have an average degree
of hydrolysis
in a range of about 75% to about 99%, or about 80% to about 95%, or about 85%
to about 95%.
[0271] B6. The water-soluble unit dose article according to any one of B1-135,
wherein
a. the first polyvinyl alcohol resin is present in an amount a range of about
50% to
about 95%, or about 50% to about 80%, or about 60% to about 75%, by weight
of the first water-soluble film, or
b. the second polyvinyl alcohol resin is present in an amount in a range of
about
50% to about 95%, or about 50% to about 80%, or about 60% to about 75%, by
weight of the second water-soluble film, or
c. the third polyvinyl alcohol resin is present in an amount in a range of
about 50%
to about 95%, or about 50% to about 80%, or about 60% to about 75%, by
weight of the third water-soluble film; or
d. any combination of features (a) to (c).
[0272] 67. The water-soluble unit dose article according to any one of B1-66,
wherein each
carboxylated anionic monomer unit in the first water-soluble resin and in the
third water-soluble
resin is independently derived from a monoalkyl maleate unit optionally
selected from the group
consisting of monomethyl maleate, salts, e.g. alkali metal salts, thereof, and
combinations
thereof, and optionally wherein each carboxylated anionic monomer unit is
independently
present in each of the first PVOH polymers in an average amount of in a range
of about 3m01.%
to about 6mo1. /0, or about 3mo1. A) to about 5mo1. A), or about 3.5mo1. /0 to
about 4.5mo1.%, or
about 4mo1.% to about 4.5mo1.%.
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
[0273] B8. The water-soluble unit dose article according to any one of B1-137,
wherein the
second polyvinyl alcohol resin comprises a polyvinyl alcohol polymer
comprising a carboxylated
anionic monomer unit derived from a monoalkyl maleate unit optionally selected
from the group
consisting of monomethyl maleate and salts thereof, optionally alkali metal
salts thereof, and
combinations thereof, wherein the caboxylated anionic monomer unit is present
in the polyvinyl
alcohol polymer comprising a carboxylated anionic monomer unit in an average
amount of at
least 3mo1.%, or about 3mo1. /0 to about 6mo1. /0, or about 3mo1.% to about
5mo1. A), or about
3.5m01.% to about 4.5mo1.%, or about 4m01.% to about 4.5m01.%.
[0274] B9. The water-soluble unit dose article according to any one of B1-B8
wherein each
first PVOH polymer in the first water-soluble resin and third water-soluble
resin is independently
characterized by
a. an average viscosity of in a range of about lOmPa.s to about 40mPa.s, or
about lOmPa.s to about 30mPa.s, or about 12mPa.s to about 25mPa.s,
or about 14mPa.s to about 20mPa.s, measured as a 4% polyvinyl alcohol
polymer solution in deionized water at 20 C, or
b. an average degree of hydrolysis in a range of about 60% to about 99%,
or about 80% to about 98%, or about 83% to about 95%, or about 85% to
about 92%, or
c. a combination of any of the foregoing.
and, wherein each second PVOH polymer in the first water-soluble resin and
third water-soluble resin is independently characterized by
ft an average viscosity of in a range of about 3
mPa.s to about 30mPa.s, or
about 7 mPa.s to about 30mPa.s, or about lOmPa.s to about 30mPa.s, or
about 12mPa.s to about 25mPa.s, measured as a 4% polyvinyl alcohol
polymer solution in deionized water at 20 C; or
e. an average degree of hydrolysis in a range of about 60% to about 99%,
or about 80% to about 98%, or about 85% to about 95%, or about 87% to
about 92%; or
f. a combination of any of the foregoing.
[0275] B10. The water-soluble unit dose article according to any one of B1-
139, wherein the
polyvinyl alcohol polymer comprising carboxylated anionic monomer units, vinyl
alcohol
monomer units and optionally vinyl acetate monomer units in the second
polyvinyl alcohol resin
is characterized by;
a. an average viscosity in a range of about lOmPa.s
to about 40mPa.s, or
about lOmPA.s to about 30mPa.s, or about 12mPa.s to about 25mPa.s,
66
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
or about 14rinPa.s to about 20mPa.s, measured as a 4% polyvinyl alcohol
polymer solution in deionized water at 20 C, or
b. an average degree of hydrolysis in a range of about 60% to about 99%,
or about 80% to about 98%, or about 83% to about 95%, or about 85% to
about 92%, or
c. a combination of any of the foregoing.
[0276] B11. The water-soluble unit dose article according to any one of B1-
610, wherein
independently in the first water-soluble film and in the third water-soluble
film, the relative weight
ratio of the first PVOH polymer and second PVOH polymer is in a range of about
90/10 to about
10/90, or about 80/20 to about 20/80, or about 70/30 to about 50/50.
[0277] B12. The water-soluble unit dose article according to any one of B1-
611, wherein the
first water-soluble film, the second water-soluble film and the third water-
soluble film each
independently have a surfactant content in a range of about 0.1% to about
3.5%, or about 0.5%
to about 2% by weight of the respective film.
[0278] B13. The water-soluble unit dose article according to any one of B1-
612, wherein the
first water-soluble film, the second water-soluble film, and the third water-
soluble film each
individually have a residual moisture content of at least 4%, or in a range of
about 4% to about
15%, or about 5% to about 10% by weight of the water-soluble film as measured
by Karl Fischer
titration.
[0279] B14. The water-soluble unit dose article according to any one of B1-
613, wherein
each film independently comprises one or more components selected from the
group consisting
of plasticizers, plasticizer compatibilizers, lubricants, release agents,
fillers, extenders, cross-
linking agents, antiblocking agents, antioxidants, detackifying agents,
antifoams, nanoparticles,
bleaching agents, aversive agents, surfactants, and combinations thereof.
[0280] B15. The water-soluble unit dose article according to B14, wherein each
film
independently comprises one or more plasticizers in an amount in a range of
between 5% to
about 50%, or about 10% to about 40%, or about 20% to about 30% by weight of
the individual
film, optionally wherein the plasticiser is selected from polyols, sugar
alcohols, or a mixture
thereof, optionally wherein the polyols include polyols selected from the
group consisting of
glycerol, diglycerin, ethylene glycol, diethylene glycol, triethyleneglycol,
tetraethylene glycol,
polyethylene glycols up to 400 MW, neopentyl glycol, 1,2-propylene glycol, 1,3-
propanediol,
dipropylene glycol, polypropylene glycol, 2-methyl-1,3-propanediol,
trimethylolpropane and
polyether polyols, or a mixture thereof, wherein sugar alcohols include sugar
alcohols selected
from the group consisting of isomalt, maltitol, sorbitol, xylitol, erythritol,
adoritol, dulcitol,
pentaerythritol and mannitol, or a mixture thereof, optionally wherein the
plasticizer is selected
67
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
from the group consisting of sorbitol, glycerol, dipropyleneglycol,
polyethyleneglycol,
trimethylolpropane, or a mixture thereof.
[0281] B16. The water-soluble unit dose article according to any one of B1-
B15, wherein the
first water-soluble film and the second water-soluble film are sealed via
solvent sealing, heat
sealing or a combination thereof, optionally via solvent sealing, further
optionally wherein the
solvent sealing solution comprises an aqueous solvent, a non-aqueous solvent
or a combination
thereof, still further optionally wherein the solvent sealing solution
comprises water; and/or
wherein the second water-soluble film and the third water-soluble film are
sealed via
solvent sealing, heat sealing or a combination thereof, optionally via solvent
sealing, further
optionally wherein the solvent sealing solution comprises an aqueous solvent,
a non-aqueous
solvent or a combination thereof, still further optionally wherein the solvent
sealing solution
comprises water.
[0282] B17. The water-soluble unit dose article according to any one of B1-
616, wherein the
unit dose article comprises at least a third compartment, optionally at least
a third and a fourth
compartment between the second water-soluble film and the third water-soluble
film, optionally
wherein the second compartment and the third compartment, e.g. the second
compartment, the
third compartment and the fourth compartments are positioned side-by-side to
one another and
wherein the second compartment and the third compartment, optionally the
second
compartment, the third compartment and the fourth compartment are positioned
above the first
compartment.
[0283] B18. The water-soluble unit dose article according to any one of B1-
617, wherein the
package comprises a non-household care composition housed in at least one of
the
compartments.
[0284] B19. The water-soluble unit dose article according to B18, wherein the
package
comprises a non-household care composition housed in each compartment.
[0285] B20. The water-soluble unit dose article according to B18 or B19,
wherein the non-
household care composition is selected from the group consisting of
agricultural compositions,
automotive compositions, aviation compositions, food and nutritive
compositions, industrial
compositions, livestock compositions, marine compositions, medical
compositions, mercantile
compositions, military and quasi-military compositions, office compositions,
and recreational
and park compositions, pet compositions, water-treatment compositions,
compositions
containing one or more active agents selected from agriculture active agents,
ingestible active
agents, liquid treatment active agents, industrial active agents, and
combinations of any of the
foregoing.
[0286] B21. A process of making a water-soluble unit dose article according to
any one of B1-
B20, comprising the steps of;
68
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
a. deforming the first water-soluble film in a mould to create an open cavity,
optionally via thermoforming, vacuum forming, or a combination thereof;
b. filling the open cavity with a composition;
c. separately deforming the third water-soluble film in a mould to create at
least one
open cavity, optionally via thermoforming, vacuum forming, or a combination
thereof;
d. filling the at least one open cavity from step (c) with a composition;
e. closing the open filled cavity from step (d) with the second water-soluble
film;
f. sealing the second water-soluble film to the third water-soluble film to
create a
closed intermediate, optionally wherein the second water-soluble film and the
third water soluble films are sealed via solvent sealing, further optionally
wherein
a solvent sealing solution is applied to the first side of the second water-
soluble
film prior to sealing the films together, the first side being the side facing
the third
water-soluble film;
g. closing the open filled cavity from step (b) with the closed intermediate
from step
(f);
h. sealing the first water-soluble film to the second water-soluble film
create the
water-soluble unit dose article, optionally wherein the first water-soluble
film and
the second water soluble films are sealed via solvent sealing, further
optionally
wherein a solvent sealing solution is applied to the second side of the second
water-soluble film prior to sealing the films together, the second side being
the
side facing the first water-soluble film.
[0287] B22. The process according to B21, wherein the first water-soluble film
in step (a) and
the third water-soluble film in step (c) are the same prior to deforming.
Aspect C
[0288] Cl. A water-soluble unit dose article comprising at least a first
compartment and
optionally a composition housed in the at least first compartment, wherein the
unit dose article
comprises;
a. A first water-soluble film, wherein the first water-
soluble film has a first side
and a second side, and wherein the first water soluble film comprises a first
PVOH resin wherein the first polyvinyl alcohol resin comprises:
i. a first polyvinyl alcohol polymer comprising carboxylated anionic
monomer units, vinyl alcohol monomer units and optionally vinyl acetate
monomer units; and wherein the carboxylated anionic monomer unit is
69
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
derived from a member selected from the group consisting of maleic acid,
monoalkyl maleate, dialkyl maleate, maleic anyhydride, and combinations
thereof;
ii.a second PVOH polymer wherein the second PVOH polymer is a
homopolymer wherein the homopolymer consists of vinyl alcohol monomer
units and optionally vinyl acetate monomer units;
b. A second water-soluble film, wherein the second
water-soluble film has a
first side and a second side, and wherein the second water-soluble film
comprises a second polyvinyl alcohol resin wherein the second polyvinyl
alcohol resin comprises;
I. less than 15% by weight of the second polyvinyl alcohol resin of a
polyvinyl alcohol polymer comprising carboxylated anionic monomer
units, vinyl alcohol monomer units and optionally vinyl acetate monomer
units, and wherein the carboxylated anionic monomer unit is derived from
a member selected from the group consisting of maleic acid, monoalkyl
maleate, dialkyl maleate, maleic anhydride, and combinations thereof;
ii about 85% to about 100% by weight of the second polyvinyl alcohol resin
of a polyvinyl alcohol homopolymer or a homopolymer blend, wherein the
homopolymers consist of vinyl alcohol monomer units and optionally vinyl
acetate monomer units;
wherein the second polyvinyl alcohol resin has an viscosity in a range of
about
8mPa.s to less than 12mPa.sõ or about 9mPa.s to less than 12 mPa.s, or about
lOmPa.s to less than 12 mPa.s, measured as a 4% polyvinyl alcohol polymer
solution
in deionized water at 20 C; and
wherein the first side of the first water-soluble film is sealed to the second
side of
the second water-soluble film to create the at least first compartment between
the first
water-soluble film and the second water-soluble film;
provided that when the article comprises a household care composition housed
in the at least first compartment then the first polyvinyl alcohol resin
comprises the first
polyvinyl alcohol polymer in an amount of at least 65%, or in a range of about
65 wt.%
to about 95wt.%, or 65wt.% to about 90wt.%, or in a range of greater than
65wt.% to
about 95%, or greater than 65wt.% to about 90wt.`)/0, or greater than
65wt.`)/0 to about
85wt. /0, or about 70wt. /0 to about 90wt. /0 based on the weight of the first
polyvinyl
alcohol resin.
[0289] C2. The water-soluble unit dose article according to Cl, wherein the
second polyvinyl
alcohol resin comprises about 90% to 100%, optionally about 100% by weight of
the second
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
polyvinyl alcohol resin of the polyvinyl alcohol homopolymer or polyvinyl
alcohol homopolymer
blend and about 0% to about 10%, optionally about 0% by weight of the second
polyvinyl
alcohol resin of the polyvinyl alcohol polymer comprising carboxylated anionic
monomer units,
vinyl alcohol monomer units and optionally vinyl acetate monomer units,
wherein the
carboxylated anionic monomer unit is derived from a member selected from the
group
consisting of maleic acid, monoalkyl maleate, dialkyl maleate, maleic
anhydride, and
combinations thereof.
[0290] C3. The water-soluble unit dose article according to any one of 01-02,
wherein the
second polyvinyl alcohol resin comprises a blend of a first polyvinyl alcohol
homopolymer and a
second polyvinyl alcohol homopolymer, optionally wherein the first polyvinyl
alcohol
homopolymer and second polyvinyl alcohol homopolymer are present in a relative
weight ratio
of 90/10 to 10/90, or 80/20 to 20/80, or 70/30 to 50/50.
[0291] 04. The water-soluble unit dose article according to C3, wherein in the
second
polyvinyl alcohol resin;
a. the first polyvinyl alcohol homopolymer has an average viscosity in a range
of
about 11 mPa.s to about 20 mPa.s, or about 11 mPa.s to about 15 mPa.s,
measured as a 4% polyvinyl alcohol polymer solution in deionized water at 20
00;
b. the second polyvinyl alcohol homopolymer has an average viscosity in a
range of
about 1 mPa.s to about 10 mPa.s, or about 5mPa.s to about 10 mPa.s,
measured as a 4% polyvinyl alcohol polymer solution in deionized water at 20
00;
optionally, wherein the difference in average viscosity of the first polyvinyl
alcohol
polymer and the second polyvinyl alcohol homopolymer is at least 1mPa.s, or
about 2 to
about 10 mPa.s, or about 3 to about 8 mPa.s, measured as a 4% polyvinyl
alcohol
polymer solution in deionized water at 20 C.
[0292] 05. The water-soluble unit dose article according to any one of 03-04,
wherein the
individual polyvinyl alcohol homopolymers independently have an average degree
of hydrolysis
in a range of about 75% to about 99%, or about 80% to about 95%, or about 85%
to about 95%.
[0293] C6. The water-soluble unit dose article according to any one of C1-05,
wherein
a. the first polyvinyl alcohol resin is present in an amount a range of about
50% to
about 95%, or about 50% to about 80%, or about 60% to about 75%, by weight
of the first water-soluble film, or
71
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
b. the second polyvinyl alcohol resin is present in an amount in a range of
about
50% to about 95%, or about 50% to about 80%, or about 60% to about 75%, by
weight of the second water-soluble film, or
c. any combination thereof.
[0294] C7. The water-soluble unit dose article according to any one of C1-C6,
wherein the
carboxylated anionic monomer unit in the first water-soluble film is derived
from a monoalkyl
maleate unit optionally selected from the group consisting of monomethyl
maleate, salts, e.g.
alkali metal salts, thereof, and combinations thereof, and optionally wherein
the carboxylated
anionic monomer unit is present in the first PVOH polymer in an average amount
of in a range
of about 3m01.% to about 6m01.%, or about 3m01.% to about 5m01.%, or about
3.5m01.% to
about 4.5mo1.%, or about 4mo1. /0 to about 4.5mo1. /0.
[0295] C8. The water-soluble unit dose article according to any one of C1-C7,
wherein the
second polyvinyl alcohol resin comprises a polyvinyl alcohol polymer
comprising a carboxylated
anionic monomer unit derived from a monoalkyl maleate unit optionally selected
from the group
consisting of monomethyl maleate, salts, e.g. alkali metal salts, thereof, and
combinations
thereof, wherein the carboxylated anionic monomer unit is present in the
polyvinyl alcohol
polymer comprising a carboxylated anionic monomer unit in an average amount of
in a range of
about 3mo1.% to about 6mo1.%, or about 3mo1.% to about 5mo1.%, or about
3.5mo1.% to about
4.5m01.%, or about 4m01.% to about 4.5m01.%.
[0296] C9. The water-soluble unit dose article according to any one of C1-C8,
wherein the
first PVOH polymer in the first water soluble resin is characterized by
a. an average viscosity of in a range of about lOmPa.s to about 40mPa.s, or
about
lOmPa.s to about 30mPa.s, or about 12mPa.s to about 25mPa.s, or about
14mPa.s to about 20mPa.s, measured as a 4% polyvinyl alcohol polymer
solution in deionized water at 20 C, or
b. an average degree of hydrolysis in a range of about 60% to about 99%, or
about
80% to about 98%, or about 83% to about 95%, or about 85% to about 92%, or
c. a combination of any of the foregoing,
and wherein the second PVOH polymer in the first water soluble resin is
characterized
by
a. an average viscosity of in a range of about 3 mPa.s to about 30mPa.s, or
about 7
mPa.s to about 30mPa.s, or about lOmPa.s to about 30mPa.s, or about 12mPa.s
to about 25mPa.s, measured as a 4% polyvinyl alcohol polymer solution in
deionized water at 20 C; or
72
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
b. an average degree of hydrolysis in a range of about 60% to about 99%, or
about
80% to about 98%, or about 85% to about 95%, or about 87% to about 92%; or
c. a combination of any of the foregoing.
[0297] C10. The water-soluble unit dose article according to any one of C1-C9,
wherein the
polyvinyl alcohol polymer comprising carboxylated anionic monomer units, vinyl
alcohol
monomer units and optionally vinyl acetate monomer units in the second
polyvinyl alcohol resin
is characterized by;
a. an average viscosity in a range of about lOmPa.s to about 40mPa.s, or about
lOmPA.s to about 30mPa.s, or about 12mPa.s to about 25m Pa.s, or about
14mPa.s to about 20mPa.s, measured as a 4% polyvinyl alcohol polymer
solution in deionized water at 20 C, or
b. an average degree of hydrolysis in a range of about 60% to about 99%, or
about
80% to about 98%, or about 83% to about 95%, or about 85% to about 92%, or
c. a combination of any of the foregoing.
[0298] C11. The water-soluble unit dose article according to any one of C1-
C10, wherein in
the first water-soluble film, the relative weight ratio of the first PVOH
polymer and second PVOH
polymer is in a range of about 90/10 to about 10/90, or about 80/20 to about
20/80, or about
70/30 to about 50/50.
[0299] C12. The water-soluble unit dose article according to any one of C1-
C11, wherein the
first water-soluble film and the second water-soluble film each independently
have a surfactant
content in a range of about 0.1% to about 3.5%, or about 0.5% to about 2% by
weight of the
respective film.
[0300] C13. The water-soluble unit dose article according to any one of C1-
C12, wherein the
first water-soluble film and the second water-soluble film each individually
have a residual
moisture content of at least 4%, or in a range of about 4% to about 15%, or
about 5% to about
10% by weight of the water-soluble film as measured by Karl Fischer titration.
[0301] C14. The water-soluble unit dose article according to any one of C1-
C13, wherein
each film independently comprises one or more components selected from the
group consisting
of plasticizers, plasticizer compatibilizers, lubricants, release agents,
fillers, extenders, cross-
linking agents, antiblocking agents, antioxidants, detackifying agents,
antifoams, nanoparticles,
bleaching agents, aversive agents, surfactants, and combinations thereof.
[0302] C15. The water-soluble unit dose article according to C14, wherein each
film
independently comprises one or more plasticizers in an amount in a range of
between 5% to
about 50%, or about 10% to about 40%, or about 20% to about 30% by weight of
the individual
film, optionally wherein the plasticiser is selected from polyols, sugar
alcohols, or a mixture
73
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
thereof, optionally wherein the polyols include polyols selected from the
group consisting of
glycerol, dig lycerin, ethylene glycol, diethylene glycol, triethyleneglycol,
tetraethylene glycol,
polyethylene glycols up to 400 MW, neopentyl glycol, 1,2-propylene glycol, 1,3-
propanediol,
dipropylene glycol, polypropylene glycol, 2-methyl-1,3-propanediol,
trimethylolpropane and
polyether polyols, or a mixture thereof, wherein sugar alcohols include sugar
alcohols selected
from the group consisting of isomalt, maltitol, sorbitol, xylitol, erythritol,
adonitol, dulcitol,
pentaerythritol and mannitol, or a mixture thereof, optionally wherein the
plasticizer is selected
from the group consisting of sorbitol, glycerol, dipropyleneglycol,
polyethyleneglycol,
trimethylolpropane, or a mixture thereof.
[0303] 016. The water-soluble unit dose article according to any one of C1-
C15, wherein the
first water-soluble film and the second water-soluble film are sealed via
solvent sealing, heat
sealing or a combination thereof, optionally via solvent sealing, further
optionally wherein the
solvent sealing solution comprises an aqueous solvent, a non-aqueous solvent
or a combination
thereof, still further optionally wherein the solvent sealing solution
comprises water.
[0304] 017. The water-soluble unit dose article according to any one of C1-
C16, wherein the
unit dose article comprises at least a second compartment, optionally at least
a third
compartment between the first water-soluble film and the second water-soluble
film, optionally
wherein the first compartment and the second compartment, optionally the first
compartment, the second compartment and the third compartments are positioned
side-by-side
to one another, optionally wherein the water-soluble unit dose article
comprises, three, or even
four, or even five side-by-side compartments.
[0305] 018. The water-soluble unit dose article according to any one of C1-
C17, wherein the
article comprises a household care composition housed in the at least first
compartment.
[0306] C19. The water-soluble unit dose article according to any one of C1-
C18, wherein the
household care composition is selected from the group consisting of light duty
liquid detergents
compositions, heavy duty liquid detergent compositions, hard surface cleaning
compositions,
laundry detergent gels, bleaching compositions, laundry additives, fabric
enhancer
compositions, shampoos, body washes, other personal care compositions, and
mixtures
thereof, e.g. a liquid laundry detergent composition.
[0307] 020. The water-soluble unit dose article according to any one of C1-
C19, wherein the
article comprises a non-household care composition housed in the at least
first compartment
and the non-household care composition optionally is selected from the group
consisting of
agricultural compositions, automotive compositions, aviation compositions,
food and nutritive
compositions, industrial compositions, livestock compositions, marine
compositions, medical
compositions, mercantile compositions, military and quasi-military
compositions, office
compositions, and recreational and park compositions, pet compositions, water-
treatment
74
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
compositions, compositions containing one or more active agents selected from
agriculture
active agents, ingestible active agents, liquid treatment active agents,
industrial active agents,
and combinations of any of the foregoing.
[0308] 021. A process of making a water-soluble unit dose article according to
any one of C1-
020, comprising the steps of:
a. deforming the first water-soluble film in a mould to create an open cavity,
optionally via thermoforming, vacuum forming, or a combination thereof;
b. filling the open cavity formed by the first water-soluble film with a
composition;
c. closing the open filled cavity with the second water-soluble film;
d. sealing the first water-soluble film to the second water-soluble film to
create the
water-soluble unit dose article, optionally wherein the first water-soluble
film and
the second water-soluble film are sealed via solvent sealing, further
optionally
wherein a solvent sealing solution is applied to the second water-soluble film
prior to sealing the films together.
[0309] 022. A process of making a water-soluble unit dose article according to
any one of 01-
020, comprising the steps of:
a. sealing portions of the first water-soluble film to portions of the second
water
soluble film to form an open cavity;
b. filling the open cavity with a composition;
c. sealing an additional portion of the first water-soluble film to an
additional portion
the a second water soluble film to close the open cavity and create the water-
soluble unit dose article;
optionally wherein the first water-soluble film and the second water-soluble
film are
sealed in step (a) via solvent sealing, further optionally wherein a solvent
sealing solution is
applied to the second water-soluble film prior to sealing the portions of the
films together, and
optionally wherein the first water-soluble film and the second water-soluble
film are
sealed in step (c) via solvent sealing, further optionally wherein a solvent
sealing solution is
applied to the second water-soluble film prior to sealing the additional
portions of the films
together.
Aspect D
[0310] Dl. A water-soluble unit dose article comprising at least a first
compartment and
optionally a composition housed in the at least first compartment, wherein the
unit dose article
comprises;
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
a. A first water-soluble film, wherein the first water-soluble film has a
first side and a
second side, and wherein the first water soluble film comprises a first PVOH
resin wherein the first polyvinyl alcohol resin comprises a polyvinyl alcohol
consisting of a polyvinyl alcohol homopolymer, an anionic polyvinyl alcohol
copolymer, or a blend thereof;
b. A second water-soluble film, wherein the second water-soluble film has a
first
side and a second side, and wherein the second water-soluble film comprises a
second polyvinyl alcohol resin wherein the second polyvinyl alcohol resin
comprises;
i. less than 15% by weight of the second polyvinyl alcohol resin of a
polyvinyl alcohol polymer comprising carboxylated anionic monomer units,
vinyl alcohol monomer units and optionally vinyl acetate monomer units,
and wherein the carboxylated anionic monomer unit is derived from a
member selected from the group consisting of maleic acid, monoalkyl
maleate, dialkyl maleate, maleic anhydride, and combinations thereof;
ii.about 85% to about 100% by weight of the second polyvinyl alcohol resin
of a polyvinyl alcohol homopolymer or a homopolymer blend, wherein the
homopolynners consist of vinyl alcohol monomer units and optionally vinyl
acetate monomer units;
wherein the second polyvinyl alcohol resin has an viscosity in a range of
about
8mPa.s to less than 12mPa.s, or about 9mPa.s to less than 12 mPa.s, or about
lOmPa.s
to less than 12 mPa.s, measured as a 4% polyvinyl alcohol polymer solution in
deionized
water at 20 C; and
wherein the first side of the first water-soluble film is sealed to the second
side of
the second water-soluble film to create the at least first compartment between
the first
water-soluble film and the second water-soluble film.
[0311] D2. The water-soluble unit dose article according to D1, wherein the
second polyvinyl
alcohol resin comprises about 90% to 100%, optionally about 100% by weight of
the second
polyvinyl alcohol resin of the polyvinyl alcohol homopolymer or polyvinyl
alcohol homopolymer
blend and about 0% to about 10%, optionally about 0% by weight of the second
polyvinyl
alcohol resin of the polyvinyl alcohol polymer comprising carboxylated anionic
monomer units,
vinyl alcohol monomer units and optionally vinyl acetate monomer units,
wherein the
carboxylated anionic monomer unit is derived from a member selected from the
group
consisting of maleic acid, monoalkyl maleate, dialkyl maleate, maleic
anhydride, and
combinations thereof.
76
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
[0312] D3. The water-soluble unit dose article according to any one of D1-D2,
wherein the
second polyvinyl alcohol resin comprises a blend of a first polyvinyl alcohol
homopolymer and a
second polyvinyl alcohol homopolymer, optionally wherein the first polyvinyl
alcohol
homopolymer and second polyvinyl alcohol homopolymer are present in a relative
weight ratio
in a range of about 90/10 to about 10/90, or about 80/20 to about 20/80, or
about 70/30 to about
50/50.
[0313] D4. The water-soluble unit dose article according to D3, wherein in the
second
polyvinyl alcohol resin;
a. the first polyvinyl alcohol homopolymer has an average viscosity in a range
of
about 11 mPa.s to about 20 mPa.s, or about 11 mPa.s to about 15 mPa.s,
measured as a 4% polyvinyl alcohol polymer solution in deionized water at 20
C;
b. the second polyvinyl alcohol homopolymer has an average viscosity in a
range of
about 1 mPa.s to about 10 mPa.s, or about 5mPa.s to about 10 mPa.s,
measured as a 4% polyvinyl alcohol polymer solution in deionized water at 20
C;
optionally wherein the difference in average viscosity of the first polyvinyl
alcohol
polymer and the second polyvinyl alcohol homopolymer is at least 1mPa.s, or
about 2 to
about 10 mPa.s, or about 3 to about 8 mPa.s, measured as a 4% polyvinyl
alcohol polymer
solution in deionized water at 20 C.
[0314] D5. The water-soluble unit dose article according to any one of D3-D4,
wherein the
individual polyvinyl alcohol homopolymers independently have an average degree
of hydrolysis
in a range of about 75% to about 99%, or about 80% to about 95%, or about 85%
to about 95%.
[0315] D6. The water-soluble unit dose article according to any one of Dl-D5,
wherein
a. the first polyvinyl alcohol resin is present in an amount a range of about
50% to
about 95%, or about 50% to about 80%, or about 60% to about 75%, by weight
of the first water-soluble film, or
b. the second polyvinyl alcohol resin is present in an amount in a range of
about
50% to about 95%, or about 50% to about 80%, or about 60% to about 75%, by
weight of the second water-soluble film, or
c. any combination thereof.
[0316] D7. The water-soluble unit dose article according to any one of D1-D6,
wherein the
carboxylated anionic monomer unit in the first water-soluble film is derived
from a monoalkyl
maleate unit optionally selected from the group consisting of monomethyl
maleate, salts, e.g.
alkali metal salts, thereof, and combinations thereof, and optionally wherein
the carboxylated
77
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
anionic monomer unit is present in the first PVOH polymer in an average amount
of in a range
of about 3mo1. /0 to about 6mo1. /0, or about 3mo1. /0 to about 5mo1.%, or
about 3.5mo1. /0 to
about 4.5m01.%, or about 4m01.% to about 4.5m01.%.
[0317] D8. The water-soluble unit dose article according to any one of D1-D7,
wherein the
second polyvinyl alcohol resin comprises a polyvinyl alcohol polymer
comprising a carboxylated
anionic monomer unit derived from a monoalkyl maleate unit optionally selected
from the group
consisting of monomethyl maleate, salts, e.g. alkali metal salts, thereof, and
combinations
thereof, wherein the carboxylated anionic monomer unit is present in the
polyvinyl alcohol
polymer comprising a carboxylated anionic monomer unit in an average amount of
in a range of
about 3mo1.% to about 6mo1.%, or about 3mo1. /0 to about 5mo1. /0, or about
3.5mo1.% to about
4.5mol.%, or about 4mo1.% to about 4.5mo1. /0.
[0318] D9. The water-soluble unit dose article according to any one of D1-D8,
wherein the
first PVOH polymer in the first water soluble film is characterized by
a. an average viscosity of in a range of about lOmPa.s to about 40mPa.s, or
about
lOmPa.s to about 30mPa.s, or about 12mPa.s to about 25mPa.s, or about
14mPa.s to about 20mPa.s, measured as a 4% polyvinyl alcohol polymer
solution in deionized water at 20 C, or
b. an average degree of hydrolysis in a range of about 60% to about 99%, or
about
80% to about 98%, or about 83% to about 95%, or about 85% to about 92%, or
c. a combination of any of the foregoing,
and wherein the second PVOH polymer in the first water soluble film is
characterized by
a. an average viscosity of in a range of about 3 mPa.s to about 30mPa.s, or
about 7
mPa.s to about 30mPa.s, or about lOmPa.s to about 30mPa.s, or about 12mPa.s
to about 25mPa.s, measured as a 4% polyvinyl alcohol polymer solution in
deionized water at 20 C; or
b. an average degree of hydrolysis in a range of about 60% to about 99%, or
about
80% to about 98%, or about 85% to about 95%, or about 87% to about 92%; or
c. a combination of any of the foregoing.
[0319] D10. The water-soluble unit dose article according to any one of D1-D9,
wherein the
polyvinyl alcohol polymer comprising carboxylated anionic monomer units, vinyl
alcohol
monomer units and optionally vinyl acetate monomer units in the second
polyvinyl alcohol resin
is characterized by;
a. an average viscosity in a range of about lOmPa.s to about 40mPa.s, or about
10m PA.s to about 30mPa.s, or about 12mPa.s to about 25mPa.s, or about
78
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
14mPa.s to about 20mPa.s, measured as a 4% polyvinyl alcohol polymer
solution in deionized water at 20 C, or
b. an average degree of hydrolysis in a range of about 60% to about 99%, or
about
80% to about 98%, or about 83% to about 95%, or about 85% to about 92%, or
c. a combination of any of the foregoing.
[0320] D11. The water-soluble unit dose article according to any one of D1-
D10, wherein in
the first water-soluble film, the relative weight ratio of the first PVOH
polymer and second PVOH
polymer is in a range of about 90/10 to about 10/90, or about 80/20 to about
20/80, or about
70/30 to about 50/50.
[0321] D12. The water-soluble unit dose article according to any one of D1-
D11, wherein the
first water-soluble film and the second water-soluble film each independently
have a surfactant
content in a range of about 0.1% to about 3.5%, or about 0.5% to about 2% by
weight of the
respective film.
[0322] D13. The water-soluble unit dose article according to any one of Dl-
D12, wherein the
first water-soluble film and the second water-soluble film each individually
have a residual
moisture content of at least 4%, or in a range of about 4% to about 15%, or
about 5% to about
10% by weight of the water-soluble film as measured by Karl Fischer titration.
[0323] D14. The water-soluble unit dose article according to any one of D1-
D13, wherein
each film independently comprises one or more components selected from the
group consisting
of plasticizers, plasticizer compatibilizers, lubricants, release agents,
fillers, extenders, cross-
linking agents, antiblocking agents, antioxidants, detackifying agents,
antifoams, nanoparticles,
bleaching agents, aversive agents, surfactants, and combinations thereof.
[0324] D15. The water-soluble unit dose article according to D14, wherein each
film
independently comprises one or more plasticizers in an amount in a range of
between 5% to
about 50%, or about 10% to about 40%, or about 20% to about 30% by weight of
the individual
film, optionally wherein the plasticiser is selected from polyols, sugar
alcohols, or a mixture
thereof, optionally wherein the polyols include polyols selected from the
group consisting of
glycerol, diglycerin, ethylene glycol, diethylene glycol, triethyleneglycol,
tetraethylene glycol,
polyethylene glycols up to 400 MW, neopentyl glycol, 1,2-propylene glycol, 1,3-
propanediol,
dipropylene glycol, polypropylene glycol, 2-methyl-1,3-propanediol,
trimethylolpropane and
polyether polyols, or a mixture thereof, wherein sugar alcohols include sugar
alcohols selected
from the group consisting of isomalt, maltitol, sorbitol, xylitol, erythritol,
adonitol, dulcitol,
pentaerythritol and mannitol, or a mixture thereof, optionally wherein the
plasticizer is selected
from the group consisting of sorbitol, glycerol, dipropyleneglycol,
polyethyleneglycol,
trimethylolpropane, or a mixture thereof.
79
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
[0325] D16. The water-soluble unit dose article according to any one of D1-D5,
wherein the
first water-soluble film and the second water-soluble film are sealed via
solvent sealing, heat
sealing or a combination thereof, optionally via solvent sealing, further
optionally wherein the
solvent sealing solution comprises an aqueous solvent, a non-aqueous solvent
or a combination
thereof, still further optionally wherein the solvent sealing solution
comprises water.
[0326] D17. The water-soluble unit dose article according to any one of Dl-
D16, wherein the
unit dose article comprises at least a second compartment, optionally at least
a third
compartment between the first water-soluble film and the second water-soluble
film, optionally
wherein the first compartment and the second compartment, optionally the first
compartment, the second compartment and the third compartments are positioned
side-by-side
to one another, optionally wherein the water-soluble unit dose article
comprises, three, or even
four, or even five side-by-side compartments.
[0327] D18. The water-soluble unit dose article according to any one of Dl-
D17, wherein the
article comprises a household care composition housed in the at least first
compartment.
[0328] D19. The water-soluble unit dose article according to any one of Dl-
D18, wherein the
household care composition is selected from the group consisting of light duty
liquid detergents
compositions, heavy duty liquid detergent compositions, hard surface cleaning
compositions,
laundry detergent gels, bleaching compositions, laundry additives, fabric
enhancer
compositions, shampoos, body washes, other personal care compositions, and
mixtures
thereof, e.g. a liquid laundry detergent composition.
[0329] D20. The water-soluble unit dose article according to any one of Dl-
D19, wherein the
article comprises a non-household care composition housed in the at least
first compartment
and the non-household care composition optionally is selected from the group
consisting of
agricultural compositions, automotive compositions, aviation compositions,
food and nutritive
compositions, industrial compositions, livestock compositions, marine
compositions, medical
compositions, mercantile compositions, military and quasi-military
compositions, office
compositions, and recreational and park compositions, pet compositions, water-
treatment
compositions, compositions containing one or more active agents selected from
agriculture
active agents, ingestible active agents, liquid treatment active agents,
industrial active agents,
and combinations of any of the foregoing.
[0330] D21. A process of making a water-soluble unit dose article according to
any one of D1-
D20, comprising the steps of:
a. deforming the first water-soluble film in a mould to create an open cavity,
optionally via thermoforming, vacuum forming, or a combination thereof;
b. filling the open cavity formed by the first water-soluble film with a
composition;
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
c. closing the open filled cavity with the second water-soluble film;
d. sealing the first water-soluble film to the second water-soluble film to
create the
water-soluble unit dose article, optionally wherein the first water-soluble
film and the
second water-soluble film are sealed via solvent sealing, further optionally
wherein a
solvent sealing solution is applied to the second water-soluble film prior to
sealing
the films together.
[0331] D22. A process of making a water-soluble unit dose article according to
any one of D1-
D20, comprising the steps of:
a. sealing portions of the first water-soluble film to portions of the second
water
soluble film to form an open cavity;
b. filling the open cavity with a composition;
c. sealing an additional portion of the first water-soluble film to an
additional portion
the a second water soluble film to close the open cavity and create the water-
soluble
unit dose article;
optionally wherein the first water-soluble film and the second water-soluble
film
are sealed in step (a) via solvent sealing, further optionally wherein a
solvent
sealing solution is applied to the second water-soluble film prior to sealing
the
portions of the films together, and
optionally wherein the first water-soluble film and the second water-soluble
film
are sealed in step (c) via solvent sealing, further optionally wherein a
solvent
sealing solution is applied to the second water-soluble film prior to sealing
the
additional portions of the films together.
Aspect E
[0332] El. A water-soluble unit dose article comprising at least two
compartments and
optionally a composition housed in at least one of the compartments, wherein
the unit dose
article comprises;
a. a first water-soluble film, wherein the first water-soluble film has a
first side and a
second side, and wherein the first water soluble film comprises a first PV0H
resin wherein the first polyvinyl alcohol resin comprises a polyvinyl alcohol
consisting of a polyvinyl alcohol homopolymer, an anionic polyvinyl alcohol
copolymer, or a blend thereof;
b. a second water-soluble film, wherein the second water-soluble film has a
first
side and a second side, and wherein the second water-soluble film comprises a
81
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
second polyvinyl alcohol resin wherein the second polyvinyl alcohol resin
comprises;
i. less than 15% by weight of the second polyvinyl alcohol resin of a
polyvinyl alcohol polymer comprising carboxylated anionic monomer
units, vinyl alcohol monomer units and optionally vinyl acetate monomer
units, and wherein the carboxylated anionic monomer unit is derived from
a member selected from the group consisting of maleic acid, monoalkyl
maleate, dialkyl maleate, maleic anhydride, and combinations thereof;
ii.about 85% to 100% by weight of the second polyvinyl alcohol resin of a
polyvinyl alcohol homopolymer or a homopolymer blend, wherein the
homopolymers consist of vinyl alcohol monomer units and optionally vinyl
acetate monomer units;
wherein the second polyvinyl alcohol resin has an average
viscosity in a range of about 8mPa.s and less than 12 mPa.s, or about
9nnPa.s to less than 12 mPa.s, or about lOmPa.s to less than 12 mPa.s
measured as a 4% polyvinyl alcohol polymer solution in deionized water
at 20 C;
c. a third water-soluble film wherein the third water-soluble film has a first
side
and a second side, and wherein the third water soluble film comprises a third
PVOH resin, wherein the third polyvinyl alcohol resin optionally comprises a
polyvinyl alcohol consisting of a polyvinyl alcohol homopolymer, an anionic
polyvinyl alcohol copolymer, or a blend thereof ;
wherein the first side of the first water-soluble film is sealed to the second
side of the second water-soluble film to create a first compartment between
the
first water-soluble film and the second water-soluble film, and the first side
of the
second water-soluble film is sealed to the second side of the third water-
soluble
film to create at least a second compartment between the second water-soluble
film and the third water-soluble film, and wherein the second compartment is
positioned above the first compartment;
provided that when the article contains a composition housed within a
compartment, then the composition is not a laundry composition and is not an
automatic dish washing composition.
[0333] E2. The water-soluble unit dose article according to El, wherein the
second polyvinyl
alcohol resin comprises about 90% to 100%, optionally about 100% by weight of
the second
polyvinyl alcohol resin of the polyvinyl alcohol homopolymer or polyvinyl
alcohol homopolymer
blend and about 0% to about 10%, optionally about 0% by weight of the second
polyvinyl
alcohol resin of the polyvinyl alcohol polymer comprising carboxylated anionic
monomer units,
82
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
vinyl alcohol monomer units and optionally vinyl acetate monomer units,
wherein the
carboxylated anionic monomer unit is derived from a member selected from the
group
consisting of maleic acid, monoalkyl maleate, dialkyl maleate, maleic
anhydride, and
combinations thereof.
[0334] E3. The water-soluble unit dose article according to any one of E1-E2,
wherein the
second polyvinyl alcohol resin comprises a blend of a first polyvinyl alcohol
homopolymer and a
second polyvinyl alcohol homopolymer, optionally wherein the first polyvinyl
alcohol
homopolymer and second polyvinyl alcohol homopolymer are present in a relative
weight ratio
in a range of about 90/10 to about 10/90, or about 80/20 to about 20/80, or
about 70/30 to about
50/50.
[0335] E4. The water-soluble unit dose article according to E3, wherein in the
second
polyvinyl alcohol resin;
a. the first polyvinyl alcohol homopolymer has an average viscosity in a range
of
about 11 mPa.s to about 20 mPa.s, or about 11 mPa.s to about 15 mPa.s,
measured as a 4% polyvinyl alcohol polymer solution in deionized water at 20
C;
b. the second polyvinyl alcohol homopolymer has an average viscosity in a
range of
about 1 mPa.s to about 10 mPa.s, or about 5mPa.s to about 10 mPa.s,
measured as a 4% polyvinyl alcohol polymer solution in deionized water at 20
C;
optionally wherein the difference in average viscosity of the first polyvinyl
alcohol
polymer and the second polyvinyl alcohol homopolymer is at least 1mPa.s, or
about 2 to about 10 mPa.s, or about 3 to about 8 mPa.s, measured as a 4%
polyvinyl alcohol polymer solution in deionized water at 20 C.
[0336] E5. The water-soluble unit dose article according to any one of E3-E4,
wherein the
individual polyvinyl alcohol homopolymers independently have an average degree
of hydrolysis
in a range of about 75% to about 99%, or about 80% to about 95%, or about 85%
to about 95%.
[0337] E6. The water-soluble unit dose article according to any one of El-ES,
wherein
a. the first polyvinyl alcohol resin is present in an amount a range of about
50% to
about 95%, or about 50% to about 80%, or about 60% to about 75%, by weight
of the first water-soluble film, or
b. the second polyvinyl alcohol resin is present in an amount in a range of
about
50% to about 95%, or about 50% to about 80%, or about 60% to about 75%, by
weight of the second water-soluble film, or, or
83
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
c. the third polyvinyl alcohol resin is present in an amount a range of about
50% to
about 95%, or about 50% to about 80%, or about 60% to about 75%, by weight
of the third water-soluble film; or
d. any combination of the foregoing.
[0338] E7. The water-soluble unit dose article according to any one of El -E6,
wherein the
first water-soluble film, the third water-soluble film, or both independently
comprise a blend of
polyvinyl alcohol homopolymers and/or anionic polyvinyl alcohol copolymers,
optionally wherein
the first water-soluble film, the third water-soluble film, or both
independently comprise a blend
of a polyvinyl alcohol homopolymer and an anionic polyvinyl alcohol copolymer,
wherein the
polyvinyl alcohol homopolymer and the anionic polyvinyl alcohol copolymer are
present in a
relative weight ratio in a range of about 90/10 to about 10/90, or about 80/20
to about 20/80, or
about 70/30 to about 50/50.
[0339] E8. The water-soluble unit dose article according to E7, wherein the
anionic polyvinyl
alcohol copolymer comprises an anionic monomer unit, optionally wherein the
anionic monomer
unit is present in the anionic polyvinyl alcohol copolymer in an average
amount in a range of
about 1 mol.% to about 10 mol. /0, or about 2 mol. /0 to about 5 mol /0.
[0340] E9. The water-soluble unit dose article according to any one of E7-E8,
wherein the
anionic polyvinyl alcohol copolymer is selected from sulphonated and
carboxylated anionic
polyvinyl alcohol copolymers, e.g. carboxylated anionic polyvinyl alcohol
copolymers, optionally
wherein the first water-soluble film and the third water-soluble film
independently comprise a
blend of a polyvinyl alcohol homopolymer and a carboxylated anionic polyvinyl
alcohol
copolymer, optionally wherein the carboxylate is selected from an acrylate, a
methacrylate, a
maleate, or a mixture thereof, e.g. a maleate.
[0341] El O. The water-soluble unit dose article according to any one of El -
E9, wherein the
second polyvinyl alcohol resin comprises a polyvinyl alcohol polymer
comprising a carboxylated
anionic monomer unit derived from a monoalkyl maleate unit optionally selected
from the group
consisting of monomethyl maleate, salts, e.g. alkali metal salts, thereof, and
combinations
thereof, wherein the carboxylated anionic monomer unit is present in the
polyvinyl alcohol
polymer comprising a carboxylated anionic monomer unit in an average amount of
at least
3mo1.%, or in a range of about 3m01.% to about 6m01.%, or about 3mo1. /0 to
about 5mo1. /0, or
about 3.5mo1.% to about 4.5mo1.%, or about 4mo1. /0 to about 4.5mo1. /0.
[0342] Eli. The water-soluble unit dose article according to any one of El-El
0, wherein the
first water-soluble film, the second water-soluble film and the third water-
soluble film each
independently comprise a surfactant content in a range of about 0.1% to about
3.5%, or about
0.5% to about 2% by weight of the water-soluble film.
84
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
[0343] E12. The water-soluble unit dose article according to any one of El-El
1, wherein the
first water-soluble film, the second water-soluble film, and the third water-
soluble film each
individually have a residual moisture content of at least 4%, or in a range of
about 4% to about
15%, or about 5% to about 10% by weight of the water-soluble film as measured
by Karl Fischer
titration.
[0344] E13. The water-soluble unit dose article according to any one of El-
E12, wherein
each film independently comprises one or more components selected from the
group consisting
of plasticizers, plasticizer compatibilizers, lubricants, release agents,
fillers, extenders, cross-
linking agents, antiblocking agents, antioxidants, detackifying agents,
antifoams, nanoparticles,
bleaching agents, aversive agents, surfactants, and combinations thereof.
[0345] E14. The water-soluble unit dose article according to E13, wherein each
film
independently comprises one or more plasticizers in an amount in a range of
between 5% to
about 50%, or about 10% to about 40%, or about 20% to about 30% by weight of
the individual
film, optionally wherein the plasticiser is selected from polyols, sugar
alcohols, or a mixture
thereof, optionally wherein the polyols include polyols selected from the
group consisting of
glycerol, diglycerin, ethylene glycol, diethylene glycol, triethyleneglycol,
tetraethylene glycol,
polyethylene glycols up to 400 MW, neopentyl glycol, 1,2-propylene glycol, 1,3-
propanediol,
dipropylene glycol, polypropylene glycol, 2-methyl-1,3-propanediol,
trimethylolpropane and
polyether polyols, or a mixture thereof, wherein sugar alcohols include sugar
alcohols selected
from the group consisting of isomalt, maltitol, sorbitol, xylitol, erythritol,
adonitol, dulcitol,
pentaerythritol and mannitol, or a mixture thereof, optionally wherein the
plasticizer is selected
from the group consisting of sorbitol, glycerol, dipropyleneglycol,
polyethyleneglycol,
trimethylolpropane, or a mixture thereof.
[0346] E15. The water-soluble unit dose article according to any one of El-El
4, wherein the
first water-soluble film and the second water-soluble film are sealed via
solvent sealing, heat
sealing or a combination thereof, optionally via solvent sealing, optionally
wherein the solvent
sealing solution comprises an aqueous solvent, a non-aqueous solvent or a
mixture thereof,
e.g. wherein the solvent sealing solution comprises water; and
wherein the second water-soluble film and the third water-soluble film are
sealed via
solvent sealing, heat sealing or a mixture thereof, optionally via solvent
sealing, optionally
wherein the solvent sealing solution comprises an aqueous solvent, a non-
aqueous solvent
or a mixture thereof, e.g. wherein the solvent sealing solution comprises
water.
[0347] E16. The water-soluble unit dose article according to any one of E1-
E15, wherein the
unit dose article comprises at least a third compartment, optionally at least
a third and a fourth
compartment between the second water-soluble film and the third water-soluble
film, optionally
wherein the second compartment and the third compartment, e.g. the second
compartment, the
third compartment and the fourth compartments are positioned side-by-side to
one another and
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
wherein the second compartment and the third compartment, optionally the
second
compartment, the third compartment and the fourth compartment are positioned
above the first
compartment.
[0348] E17. The water-soluble unit dose article according to any one of El-El
5, wherein the
composition is selected from the group consisting of light duty liquid
detergent compositions,
heavy duty liquid detergent compositions, hard surface cleaning compositions,
bleaching
compositions, shampoos, body washes, other personal care compositions, and
mixtures
thereof.
[0349] E18. The water-soluble unit dose article according to any one of E1-
E17, wherein the
article comprises a non-household care composition housed in the at least
first compartment
and the non-household care composition optionally is selected from the group
consisting of
agricultural compositions, automotive compositions, aviation compositions,
food and nutritive
compositions, industrial compositions, livestock compositions, marine
compositions, medical
compositions, mercantile compositions, military and quasi-military
compositions, office
compositions, and recreational and park compositions, pet compositions, water-
treatment
compositions, compositions containing one or more active agents selected from
agriculture
active agents, ingestible active agents, liquid treatment active agents,
industrial active agents,
and combinations of any of the foregoing.
[0350] E19. A process of making a water-soluble unit dose article according to
any one of El -
E18, comprising the steps of;
a. deforming the first water-soluble film in a mould to create an open cavity
via
thermoforming, vacuum forming, or a combination thereof;
b. filling the open cavity with the composition;
c. separately deforming the third water-soluble film in a mould to create at
least one
open cavity via thermoforming, vacuum forming, or a combination thereof
d. filling the at least one open cavity from step (c) with a composition;
e. closing the open filled cavity from step (c) with the second water-soluble
film;
f. sealing the second water-soluble film and the third water-soluble film to
create a
closed intermediate, optionally wherein the second water-soluble film and the
third water-soluble films are sealed via solvent sealing, optionally wherein a
solvent sealing solution is applied to the first side of the second water-
soluble film
ahead of sealing the films together, the first side being the side facing the
third
water-soluble film;
g. closing the open filled cavity from step (b) with the closed intermediate
from step
(f);
86
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
h. sealing the first water-soluble film to the second water-soluble film to
create the
water-soluble unit dose article, optionally wherein the first water-soluble
film and
the second water-soluble film are sealed via solvent sealing, further
optionally
wherein a solvent sealing solution is applied to the second side of the second
water-soluble film ahead of sealing the films together, the second side being
the
side facing the first water-soluble film.
[0351] E20. The process according to E19, wherein the first water-soluble film
in step (a) and
the third water-soluble film in step (c) are the same prior to deforming.
Aspect F
[0352] Fl. A method of making a water-soluble unit dose article, comprising:
a. deforming a first water-soluble film in a mould to create an open cavity,
the first
water soluble film comprising a first PVOH resin;
b. filling the open cavity formed by the first water-soluble film with a
composition;
c. closing the open filled cavity from step (b) with a second water-soluble
film, the
second water soluble film comprising a second polyvinyl alcohol resin wherein
the second polyvinyl alcohol resin comprises;
i. less than 15% by weight of the second polyvinyl alcohol resin of a
polyvinyl alcohol polymer comprising carboxylated anionic monomer
units, vinyl alcohol monomer units and optionally vinyl acetate monomer
units, and wherein the carboxylated anionic monomer unit is derived from
a member selected from the group consisting of maleic acid, monoalkyl
maleate, dialkyl maleate, maleic anhydride, and combinations thereof;
ii 85% about 100% by weight of the second polyvinyl alcohol resin of a
polyvinyl alcohol homopolymer or a homopolymer blend, wherein the
homopolymers consist of vinyl alcohol monomer units and optionally vinyl
acetate monomer units;
and wherein the second polyvinyl alcohol resin has an average 4% solution
viscosity in deionized water at 20 C in a range of 8mPa.s to less than 12
mPa.s, or
about 9mPa.s to less than 12 mPa.s, or about lOmPa.s to less than 12 mPa.s;
d. sealing the second water-soluble film to the first water-soluble film to
create the
water-soluble unit dose article, optionally via solvent sealing.
[0353] F2. The method of Fl, wherein the first water-soluble film has a first
side and a
second side, and the second water-soluble film has a first side and a second
side, and further
comprising prior to step (c),
87
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
e. separately deforming a third water-soluble film in a mould to create at
least one
open cavity, the third water soluble film comprising a third PVOH resin same
or
different from the first and second PVOH resins;
f. filling the at least one open cavity from step (e) with a composition;
g. closing the open filled cavity from step (f) with the second water-soluble
film;
h. sealing the second water-soluble film to the third water-soluble film to
create a
closed intermediate, optionally via solvent sealing;
i. wherein the closing the open filled cavity from step (b) with the second
water-
soluble film according to step (c) comprises closing the open filled cavity
from
step (b) with the closed intermediate from step (h); and
j. wherein the sealing of the second water-soluble film and the first water-
soluble
film to create the water-soluble unit dose article of step (d) comprises
sealing
the first water-soluble film to the second water-soluble film of the closed
intermediate of step (h) to create the water-soluble unit dose article.
[0354] F3. The method according to F2, wherein the first side of the first
water-soluble film is
sealed to the second side of the second water-soluble film to create a first
compartment
between the first water-soluble film and the second water-soluble film, and
the first side of the
second water-soluble film is sealed to the second side of the third water-
soluble film to create at
least a second compartment between the second water-soluble film and the third
water-soluble
film, and wherein the second compartment is positioned above the first
compartment.
[0355] F4. The method according to any one of F1-F3, wherein the sealing of
the first film to
the second film, or the sealing of the third film to the second film, or both
the sealing of the first
film to the second film and the sealing of the third film to the second film
comprises solvent
sealing.
[0356] F5. The method according to F4, comprising applying the sealing
solution to the film
via a contact method.
[0357] F6. The method according to any one of F4-F5, comprising applying the
sealing
solution using an absorbent member.
[0358] F7. The method according to any one of F4-F6, comprising applying the
sealing
solution in a continuous process.
[0359] F8. The method according to any one of F1-F7, wherein the first
polyvinyl alcohol
resin comprises a first polyvinyl alcohol polymer comprising carboxylated
anionic monomer
units, vinyl alcohol monomer units and optionally vinyl acetate monomer units,
and wherein the
carboxylated anionic monomer units are derived from a member selected from the
group
88
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
consisting of maleic acid, monoalkyl maleate, dialkyl maleate, maleic
anyhydride, and
combinations thereof.
[0360] F9. The method according to F8, wherein the first polyvinyl alcohol
resin further
comprises a second PVOH polymer wherein the second PVOH polymer is a
homopolymer
wherein the homopolymer consists of vinyl alcohol monomer units and optionally
vinyl acetate
monomer units.
[0361] F10. The method according to any one of F2-F9, wherein the third
polyvinyl alcohol
resin comprises a first polyvinyl alcohol polymer comprising carboxylated
anionic monomer
units, vinyl alcohol monomer units and optionally vinyl acetate monomer units,
and wherein the
carboxylated anionic monomer unit is derived from a member selected from the
group
consisting of maleic acid, monoalkyl maleate, dialkyl maleate, maleic
anyhydride, and
combinations thereof.
[0362] F11. The method according to Fl 0, wherein the third polyvinyl alcohol
resin further
comprises a second PVOH polymer wherein the second PVOH polymer is a
homopolymer
wherein the homopolymer consists of vinyl alcohol monomer units and optionally
vinyl acetate
monomer units.
[0363] F12. The method according to any one of F2-F11, wherein the first water-
soluble film
in step (a) and the third water-soluble film in step (e) are identical prior
to deforming.
[0364] F13. The method according to any one of Fl -F12, wherein the second
polyvinyl
alcohol resin comprises the polyvinyl alcohol homopolymer or polyvinyl alcohol
homopolymer
blend in an amount in a range of about 90% to about 100%, or about 100% by
weight of the
second polyvinyl alcohol resin.
[0365] F14. The method according to any one of Fl -F13, wherein the second
polyvinyl
alcohol resin comprises a blend of a first polyvinyl alcohol homopolymer and a
second polyvinyl
alcohol homopolymer, optionally wherein the first polyvinyl alcohol
homopolymer and second
polyvinyl alcohol homopolymer are present in a relative weight ratio of in a
range of 90/10 to
about 10/90, or about 80/20 to about 20/80, or about 70/30 to about 50/50.
[0366] F15. The method according to F14, wherein in the second polyvinyl
alcohol resin;
a. the first polyvinyl alcohol homopolymer has an average viscosity in a range
of
about 11 mPa.s to about 20 mPa.s, or about 11 mPa.s to about 15 mPa.s,
measured as a 4% polyvinyl alcohol polymer solution in deionized water at 20
00;
b. the second polyvinyl alcohol homopolymer has an average viscosity in a
range of
in a range of about 1 mPa.s to about 10 mPa.s, or about 5mPa.s to about 10
89
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
mPa.s, measured as a 4% polyvinyl alcohol polymer solution in deionized water
at 20 C;
optionally wherein the difference in average viscosity between the first
polyvinyl
alcohol homopolymer and the second polyvinyl alcohol homopolymer is at least
about
1mPa.s, or in a range of about 2 to about 10 mPa.s, or about 3 mPa.s to about
8 mPa.s,
measured as a 4% polyvinyl alcohol polymer solution in deionized water at 20
C.
[0367] F16. The method according to any one of F14-F15, wherein the individual
polyvinyl
alcohol homopolymers in the second polyvinyl alcohol resin independently have
an average
degree of hydrolysis in a range of about 75% to about 99%, or about 80% to
about 95%, or
about 85% to about 95%.
[0368] F17. The method according to any one of F2-F16, wherein
a. the first polyvinyl alcohol resin is present in a range of about 50% to
about 95%,
or about 50% to about 80%, or about 60% to about 75%, by weight of the first
water-soluble film, or
b. the second polyvinyl alcohol resin is present in a range of about 50% to
about
95%, or about 50% to about 80%, or about 60% to about 75%, by weight of the
second water-soluble film, or
c. the third polyvinyl alcohol resin is present in a range of about 50% to
about 95%,
or about 50% to about 80%, or about 60% to about 75%, by weight of the third
water-soluble film; or
d. any combination of the foregoing.
[0369] F18. The method according to any one of F4-F17, wherein each
carboxylated anionic
monomer unit in the first water-soluble resin and in the third water-soluble
resin is independently
derived from a monoalkyl maleate unit selected from the group consisting of
monomethyl
maleate, salts, optionally alkali metal salts, thereof, and combinations
thereof, and optionally
wherein each carboxylated anionic monomer unit is independently present in
each of the first
PVOH polymers in an average amount in a range of about 3mo1. /0 to about 6mo1.
/0, or about
3m01.% to about 5m01.%, or about 3.5mo1. /0 to about 4.5mo1. /0, or about
4m01.% to about
4.5mol.%.
[0370] F19. The method according to any one of F1-F18, wherein the second
polyvinyl
alcohol resin comprises polyvinyl alcohol comprising a carboxylated anionic
monomer unit
derived from a member selected from the group consisting of maleic acid,
monoalkyl maleate,
dialkyl maleate, maleic anhydride, and combinations thereof and the
carboxylated anionic
monomer unit is present in the polyvinyl alcohol polymer in an average amount
of at least
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
3mo1.%, or in a range of about 3nno1.% to about 6mo1.%, or about 3mo1.% to
about 5mo1.%, or
about 3.5mo1.% to about 4.5mo1.%, or about 4mol.`)/0 to about 4.5mol.`)/0,
[0371] F20. The method according to F19, wherein the carboxylated anionic
monomer unit is
derived from a monoalkyl maleate selected from the group consisting of
monomethyl maleate,
salts thereof, optionally alkali metal salts thereof, and combinations of any
of the foregoing.
[0372] F21. The method according to any one of F4-F20, wherein each first PVOH
polymer
in the first water-soluble resin and third water-soluble resin is
independently characterized by
a. an average viscosity in a range of about lOmPa.s to about 40mPa.s, or about
lOmPa.s to about 30mPa.s, or about 12mPa.s to about 25mPa.s, or about
14mPa.s to about 20mPa.s, measured as a 4% polyvinyl alcohol polymer
solution in deionized water at 20oC, or
b. an average degree of hydrolysis in a range of about 60% to about 99%, or
about
80% to about 98%, or about 83% to about 95%, or about 85% to about 92%, or
c. a combination thereof.
and, wherein each second PVOH polymer in the first water-soluble resin
and third water-soluble resin is independently characterized by
c. an average viscosity in a range of about 3 mPa.s to about 30mPa.s, or in a
range
of about 7 mPa.s to about 30mPa.s, or in a range of about lOmPa.s to about
30mPa.s, or in a range of about 12mPa.s to about 25mPa.s, measured as a 4%
polyvinyl alcohol polymer solution in deionized water at 20oC, or
d. an average degree of hydrolysis in a range of about 60% to about 99%, or
about
80% to about 98%, or about 85% to about 95%, or about 87% to about 92%, or
e. a combination of any of the foregoing.
[0373] F22. The method according to any one of F4-F21, wherein the polyvinyl
alcohol
comprising a carboxylated anionic monomer unit in the second polyvinyl alcohol
resin is
characterized by;
a. an average 4% solution viscosity at 20 C in a range of about lOmPa.s to
less
than 12mPa.s, or
b. an average degree of hydrolysis of in a range of about 60% to about 99%, or
about 80% to about 98%, or about 83% to about 95%, or about 85% to about
92%, or
c. a combination of any of the foregoing thereof.
[0374] F23. The method according to any one of F4-F22, wherein independently
in the first
water-soluble resin and in the third water-soluble resin, the relative weight
ratio of the first
91
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
PVOH polymer and second PVOH polymer is in a range of about 90/10 to about
10/90, or about
80/20 to about 20/80, or about 70/30 to about 50/50.
[0375] F24. The method according to any one of F4-F23, wherein the first water-
soluble film,
the second water-soluble film and third water-soluble film each independently
have a surfactant
content in a range of about 0.1% to about 3.5%, or about 0.5% to about 2% by
weight of the
respective film.
[0376] F25. The method according to any one of F4-F24, wherein the first water-
soluble film,
the second water-soluble film, and the third water-soluble film each
individually have a residual
moisture content of at least 4%, or in a range of about 4% to about 15%, or
about 5% to about
10% by weight of the water-soluble film as measured by Karl Fischer titration.
[0377] F26. The method according to any one of Fl -F25, wherein each film
independently
comprises one or more components in the group of plasticizers, plasticizer
compatibilizers,
lubricants, release agents, fillers, extenders, cross-linking agents,
antiblocking agents,
antioxidants, detackifying agents, antifoams, nanoparticles, bleaching agents,
aversive agents,
surfactants, and combinations thereof.
[0378] F27. The method according to F26, wherein each film independently
comprises one or
more plasticizers in an amount in a range of about 5% to about 50%, or about
10% to about
40%, or about 20% to about 30% by weight of the individual film, optionally
wherein the
plasticizer is selected from polyols, sugar alcohols, or a mixture thereof,
optionally wherein the
polyols include polyols selected from the group consisting of glycerol,
diglycerin, ethylene
glycol, diethylene glycol, triethyleneglycol, tetraethylene glycol,
polyethylene glycols up to 400
MW, neopentyl glycol, 1,2-propylene glycol, 1,3-propanediol, dipropylene
glycol, polypropylene
glycol, 2-methyl-1,3-propanediol, trimethylolpropane and polyether polyols, or
a mixture thereof,
wherein sugar alcohols include sugar alcohols selected from the group
consisting of isomalt,
maltitol, sorbitol, xylitol, erythritol, adonitol, dulcitol, pentaerythritol
and mannitol, or a mixture
thereof.
[0379] F28. The method according to any one of F4-F27, further comprising
forming at least
a third compartment, optionally at least a third and a fourth compartment
between the second
water-soluble film and the third water-soluble film, optionally;
wherein the second compartment and the third compartment, optionally the
second compartment, the third compartment and the fourth compartments are
positioned side-by-side to one another and wherein the second compartment and
the third compartment, optionally the second compartment, the third
compartment and the fourth compartment are positioned above the first
compartment.
92
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
[0380] F29. The method according to any one of Fl -F28, wherein the
composition comprises
a household care composition which is not a laundry or automatic dishwashing
composition,
provided that the first-water soluble film and the third water-soluble film do
not comprise a
polyvinyl alcohol resin comprising;
i. a first polyvinyl alcohol polymer comprising carboxylated anionic
monomer units, vinyl alcohol monomer units and optionally vinyl acetate
monomer units, and wherein the carboxylated anionic monomer unit is
derived from a member selected from the group consisting of maleic acid,
monoalkyl maleate, dialkyl maleate, maleic anhydride, and combinations
thereof;
ii.a second PVOH polymer wherein the second PVOH polymer is a
homopolymer wherein the homopolymer consists of vinyl alcohol
monomer units and optionally vinyl acetate monomer units.
[0381] F30. The method according to F29, wherein the composition is selected
from the
group consisting of light duty liquid detergent compositions, heavy duty
liquid detergent
compositions, hard surface cleaning compositions, bleaching compositions,
shampoos, body
washes, other personal care compositions, and mixtures thereof.
[0382] F31. The method according to any one Fl -F28, wherein the composition
comprises a
non-household care composition.
[0383] F32. The method according to F31, wherein the non-household care
composition is
selected from the group consisting of agricultural compositions, automotive
compositions,
aviation compositions, food and nutritive compositions, industrial
compositions, livestock
compositions, marine compositions, medical compositions, mercantile
compositions, military and quasi-military compositions, office compositions,
and recreational
and park compositions, pet compositions, water-treatment compositions,
compositions
containing one or more active agents selected from agriculture active agents,
ingestible active
agents, liquid treatment active agents, industrial active agents, and
combinations of any of the
foregoing.
[0384] The dimensions and values disclosed herein are not to be understood as
being strictly
limited to the exact numerical values recited. Instead, unless otherwise
specified, each such
dimension is intended to describe in the alternative the recited value, and a
functionally
equivalent range surrounding that value. For example, a dimension disclosed as
"about 40 mm"
is an implicit disclosure of "40 mm" which is hereby made explicit.
[0385] Throughout this specification and the claims which follow, unless the
context requires
otherwise, the word "comprise" and variations such as "comprises" and
"comprising" will be
93
CA 03196098 2023- 4- 18
understood to imply the inclusion of a stated integer or step or group of
integers or steps but not
the exclusion of any other integer or step or group of integers or steps.
[0386] Throughout the specification, where compositions are described as
including
components or materials, it is contemplated that the compositions can also
consist essentially
of, or consist of, any combination of the recited components or materials,
unless described
otherwise. Likewise, where methods are described as including particular
steps, it is
contemplated that the methods can also consist essentially of, or consist of,
any combination of
the recited steps, unless described otherwise. The invention illustratively
disclosed herein
suitably may be practiced in the absence of any element or step which is not
specifically
disclosed herein.
[0387] The practice of a method disclosed herein, and individual steps
thereof, can be
performed manually and/or with the aid of or automation provided by electronic
equipment.
Although processes have been described with reference to particular
embodiments, a person of
ordinary skill in the art will readily appreciate that other ways of
performing the acts associated
with the methods may be used. For example, the order of various of the steps
may be changed
without departing from the scope or spirit of the method, unless described
otherwise. In
addition, some of the individual steps can be combined, omitted, or further
subdivided into
additional steps.
[0388] Intentionally left blank.
EXAMPLES
[0389] The effect of presence versus absence of an anionic polyvinyl alcohol
copolymer, as
well as the effect of varying average molecular weight, expressed as a 4%
viscosity of an
aqueous polymer solution (deionized water), of a polyvinyl alcohol homopolymer
blend within a
polyvinyl alcohol blend comprising polymer resin, has been studied on 1) the
sensitivity of the
corresponding water-soluble film to create a foam layer at the film surface
upon sealing solvent
application, 2) the resulting seal and unit dose article strength, as well as
3) the film / unit dose
article dissolution profile.
Test materials:
Water-soluble films:
[0390] Solvent-casted water-soluble test films single variably differing on
polyvinyl alcohol
type selection were provided by MonoSol LLC. The test films comprised 65% of a
water-soluble
polyvinyl alcohol resin, the remainder being water, plasticizer, surfactant,
and other materials
typically present inside water-soluble films. The test films were sealed in a
manner
94
Date Recue/Date Received 2023-09-15
WO 2022/132853
PCT/US2021/063431
contemplated for the "second water soluble film" described as part of the
invention above,
creating structures in a manner contemplated for the "second water soluble
film" described as
part of the invention above. Accordingly, the test films falling outside the
scope of the "second
water soluble film" described above are characterized as comparative examples.
[0391] Comparative examples 1 to 4 comprised 15% to 30% by weight of the
polyvinyl alcohol
polymer resin of an anionic copolymer in the middle film (Film 2). Comparative
examples 5 to 7
comprised a polyvinyl alcohol homopolymer blend with different average
viscosities.
[0392] Example 1 comprises a polyvinyl alcohol homopolymer blend with an
average viscosity
according to the invention. Additional resins are described below:
= anionic polyvinyl alcohol copolymer comprising resins (%'s by weight of
polyvinyl alcohol
polymeric resin) :
o Comparative Example 1 : polyvinyl alcohol blend comprising 70% polyvinyl
alcohol homopolymer (13 mPa.s, dH 86%) ¨ 30% methyl maleate based anionic
polyvinyl alcohol copolymer (4% anionic substitution, 18 mPa.s, 90% dH)
o Comparative Example 2 : polyvinyl alcohol blend comprising 85% polyvinyl
alcohol homopolymer (13 mPa.s, dH 86%) ¨ 15% methyl maleate based anionic
polyvinyl alcohol copolymer (4% anionic substitution, 18 mPa.s, 90% dH)
o Comparative Example 3 : polyvinyl alcohol blend comprising 85% polyvinyl
alcohol homopolymer (8 mPa.s, dH 88%) ¨ 15% methyl maleate based anionic
polyvinyl alcohol copolymer (4% anionic substitution, 18 mPa.s, 90% dH)
o Comparative Example 4 : polyvinyl alcohol blend comprising 85% polyvinyl
alcohol homopolymer (18 mPa.s, dH 88%) ¨ 15% methyl maleate based anionic
polyvinyl alcohol copolymer (4% anionic substitution, 18 mPa.s, 90% dH)
= resins consisting of polyvinyl alcohol homopolymer (%'s by weight of
polyvinyl alcohol
polymeric resin):
o Comparative Example 5 : 100% polyvinyl alcohol homopolymer (13 mPa.s, dH
86%)
o Comparative Example 6 : polyvinyl alcohol homopolymer blend comprising
80%
polyvinyl alcohol homopolymer (13 mPa.s, dH 86%) ¨ 20% polyvinyl alcohol
homopolymer (8 mPa.s, dH 88%) ¨ average viscosity: 12 mPa.s
o Comparative Example 7 : polyvinyl alcohol homopolymer blend comprising
80%
polyvinyl alcohol homopolymer (13 mPa.s, dH 86%) ¨ 20% polyvinyl alcohol
homopolymer (18 mPa.s, dH 88%) ¨ average viscosity : 14 mPa.s
o Inventive Example 1 : polyvinyl alcohol homopolymer blend comprising 60%
polyvinyl alcohol homopolymer (13 mPa.s, dH 86%) ¨40% polyvinyl alcohol
homopolymer (8 mPa.s, dH 88%) ¨ average viscosity: 11 mPa.s
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
Water-soluble unit dose articles:
[0393] These water-soluble test films were used to create water soluble unit
dose articles. A
first water-soluble film comprising a polyvinyl alcohol blend comprising 60%
polyvinyl alcohol
homopolymer (23 mPa.s, dH 87%) and 40% methyl maleate based anionic polyvinyl
alcohol
copolymer (4% anionic substitution, 18 mPa.s, 90% dH), as provided by the
MonoSol company,
was drawn into a mould comprising 2 side-by-side cavities under influence of
vacuum to create
open compartments. A detergent composition was dosed inside of these open
compartments,
followed by closing the open filled compartments with each of above test
films, individually. The
side-by-side configuration represents the top compartment configuration, as
displayed in Figure
I. The two films were sealed together with water, the sealing water being pre-
applied on the test
films through a pre-wetted felt roll on the surface of the test film facing
the first water-soluble
film. Target sealing water coat weight was 9 gram of water per square meter of
water-soluble
film. A third water-soluble film of the same composition as the first water-
soluble film was drawn
into a separate mold comprising a single cavity under influence of vacuum in
order to create an
open compartment. A detergent composition was dosed inside the open
compartment prior to
closing the open compartment with the side-by-side compartment unit dose
article created as
described above, in order to create a water-soluble unit dose article having
the appearance as
displayed in Figure 1 and as sold under the Fairy NonBio brand by the Procter
and Gamble
company in the UK in July 2020, yet with different films. Sealing water was
pre-applied on the
test films through a pre-wetted felt roll on the surface of the test films
facing the third water-
soluble film. Target sealing water coat weight was 13 gram of water per square
meter of water-
soluble film. All water soluble films used had a starting thickness prior to
article creation, e.g.
prior to deformation, of 76 micron.
Liquid laundry detergent compositions:
[0394] The respective liquid laundry detergent compositions, as added into the
individual
compartments described in the water-soluble unit dose article section above,
are summarized in
Table 1. Liquid laundry detergent compositions were prepared through mixing of
the individual
components in a batch process.
Table 1 : Liquid laundry detergent formulations
100% active Bottom Top compartment Top
compartment
compartment 1 2
Neodol 24/7 3.2 1.8 1.5
ethoxylated alcohol
nonionic surfactant
96
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
Linear alkylbenzene 27.6 21.8
17.7
sulphonic acid
MEA-A24E3S 7.9 11.5
8.9
Citric acid 0.7 0.6
0.5
Fatty acid 11.4 4.7
3.7
Ethoxylated 1.6 1.4
1.1
polyethyleneimine*
Zwitterionic polyamine 1.6 1.6
1.3
HEDP 0.7 2.0
1.6
Texcare SRA300 4.4
Polyquaternium 10***
7.8
FWA 49 0.3 0.1
0.1
Antifoam (AF8017) 0.3
1,2-propanediol 15.6 24.1
23.2
Glycerol 5.3 7.6
3.3
PPG (MW 400)
12.7
Monoethanolamine 9.6 9.5
7.3
(pH trimming agent)
K2S03 0.5 0.4
0.4
MgCl2 0.1 0.3
0.2
Water 1.9 8.6
8.7
Acusol 880
0.6
Hydrogenated castor 0.1 0.8
0.2
oil
Minors (perfume, Balance to 100% Balance to 100% Balance
to 100%
dyes, antioxidant,...)
pH (as 10% aqueous 7.4 7.4
7.4
solution)
*ethoxylated polyethyleneimine having an average degree of ethoxylation of 20
per EO chain
and a polyethyleneimine backbone with MW of about 600
** Lutensit Z96 : partially sulfate polyethoxylated hexamethylenediamine, as
available from the
BASF company
premix composition : 37wV/0 cationic hydroxyethyl cellulose, 60wt% PPG400,
3wV/0 Acusol
880 - premix components reflected in above formula composition
97
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
Test results :
Presence versus absence of foam layer:
[0395] Water-soluble test films have been visually assessed for the presence
versus absence
of a foam layer created at the water-soluble film surface after the sealing
water application step
on the test film prior to contacting the third water-soluble film as described
above. The results
summarized in Table 2 below clearly show that water-soluble films comprising a
water-soluble
resin comprising 15 to 30% of an anionic copolymer (comparative examples 1 to
4) are
sensitive to creating a foaming layer at the surface of the water-soluble film
upon sealing water
application, contrary to water-soluble films comprising a polymeric resin
consisting of a polyvinyl
alcohol homopolymer (blend) (inventive example 1 and comparative examples 5 to
7). This
foam layer is believed to drive an inhomogeneous spreading of the sealing
water, leading to an
inferior seal quality and the presence of weakly sealed spots accordingly.
Table 2 : Presence versus absence of foam layer
Compara Compara Compara Compara Compara Compara Compara Invent
tive tive tive tive tive tive tive
ive
Example Example Example Example Example Example Example Exam
1 2 3 4 5 6 7
ple 1
Foa present present present present absent absent absent absen
laye
% Pouch strength pass rate and % seal failure:
Test method:
[0396] This test method describes the practice for determining the A, pouch
strength pass rate
and % seal failure using a Mark-10 testing instrument ESM750SLCE (j.j. bos
b.v., Marconistraat
1, NL-2809 PH Gouda, The Netherlands) with a load cell of maximum 100 kN (kilo
Newton).
Under the effect of external compression force, a pouch deforms, building
stress on both the
film and the seal area. The internal pressure in the pouch depends on the
outside applied force
on the overall pouch surface area. Pouch strength (in Newtons) is defined as
the maximum
compression force required by two parallel plates to increase the internal
pressure of the pouch
up to the point of burst. Pouches bursting at the seal area are reported as
"seal failures" used in
the calculation of the % Seal Failure rate (Seal Failure = 1, No Seal Failure
= 0) across 18
replicates. Pouches bursting at a pressure equivalent to the one generated by
compression with
98
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
less than 300N are reported as "failures" used in the calculation of the %
Pouch Strength Pass
rate (Failure = 0, Pass = 1) across 18 replicates.
[0397] The % pouch strength pass rate and % seal failure were measured after
having stored
the water-soluble pouches for 7 days at ambient conditions, and pre-
conditioned for 16-24h at
23 C / 50% RH. The method is performed in a room environment between 40-50%
relative
humidity (RH) and at a temperature between 22-24 C. Water-soluble pouches are
tested within
one hour of taking them out of the pre-conditioning.
[0398] FIG. 2 shows a schematic illustration of the basic configuration of the
A, pouch
strength pass rate and % seal failure test. To measure % pouch strength pass
rate and % seal
failure, a pouch 510 is enclosed in a plastic bag 500 and subsequently sealed
(150 mm by 124
mm with closure, 60 micron thick - e.g. Raja grip RGP6B) to prevent
contamination of the
working environment upon pouch rupture. The pouch 510 is centered in the bag
500, and
placed between two compression plates 520, 530 of the instrument. The pouch
510 is placed in
an upright position, so that the width seal dimension 540 (e.g. smallest
dimension within a
defined rectangular plane just encompassing the seal area, 41mm in actual
pouches tested) is
between the compression plates (x-direction) such that the stress will be
applied on the width
seal. Herefore the diameter of the compression plates needs to be big enough
in order not to
pinch the pouch as it deforms (here D=116mm). For the compression, the speed
of decreasing
the distance between the plates 520 and 530 is set at 200 mm/min. 18
replicates are
conducted per test leg, and % pouch strength pass rate and % seal failure data
across those 18
replicates are reported.
Test results:
[0399] The results summarized in Table 3 clearly show that water-soluble unit-
dose articles
comprising a water-soluble test film comprising a polyvinyl alcohol based
polymer resin
consisting of a homopolymer blend with an average viscosity profile according
to the invention
(Inventive Example 1) have a superior pouch strength and seal failure profile
compared to
water-soluble unit-dose articles comprising a water-soluble test film
comprising a polyvinyl
alcohol based polymer resin consisting of a homopolymer blend with an average
viscosity
profiles according to comparative examples 5 to 7, as well as when compared to
water-soluble
unit-dose articles comprising a water-soluble test film comprising a polyvinyl
alcohol based
polymer resin comprising from 15% to 30% of an anionic polyvinyl alcohol
copolymer
(comparative examples 1 to 4).
Table 3 : Pouch strength and seal failure
Connpar Connpar Connpar Connpar Connpar Connpar Connpar Invent
ative ative ative ative ative ative ative
ive
99
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
Example Example Example Example Example Example Example Exam
1 2 3 4 5 6 7
ple 1
0 13 100 42 46 54 63
100
Pouc
Stren
gth
Pass
rate*
100 96 4 75 67 63 63
0
seal
failur
e**
*The higher the better
**The lower the better
Water-soluble unit dose article dissolution - film residue
Test method:
[0400] This test method describes a water-soluble unit dose article
dissolution test, in which
the amount of undissolved water-soluble film residues is assessed. More
particularly, this
method is designed to assess the relative dissolution properties of laundry
water-soluble unit
dose articles under stressed washing machine conditions. For this method,
Electrolux
Programmable Washing machines type W565H comprising a ballast load with a mix
of Cotton
and Polycotton pieces (from Calderon Textiles, LLC 6131 W 80th Street
Indianapolis, IN 46278)
were used. Ballast loads are comprised of cotton and polycotton knit, double-
ply swatches
approximately 50x50cm in size.
[0401] Orange pouches: Brand new Cotton white ballast load from Calderon
50X50cm was
divided into portions of max 3.0kg (-25 items of ballast cotton) and prepared
to be colored into
orange through a washing-machine dying process, using commercially available
dying
solutions. To color the load, any standard household washing machine can be
used, employing
a standard cotton cycle at 40 C. 350g of the Dylon fresh orange machine dye
all-in-one are
added to the drum of the washing machine. Salt may be added, depending on the
dye package
instructions.
[0402] The drum was consequently manually moved to the left and the right
until the dye was
not visible anymore. 25 items of cotton ballast (size of 50cm x 50cm), were
consequently evenly
distributed over the drum without folding of the items. A standard cotton
cycle at 40 C was run
100
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
at a water hardness of 15gpg. After completion of the cycle 75g of Ariel
Professional powder
was added into the dispenser and a normal cotton cycle at 40 C was run at a
water hardness of
15gpg (grains per gallon). After completion of this cycle, 2 additional normal
cotton cycles at
40 C without any detergent were run at a water hardness of 15gpg, followed by
line-drying the
items.
[0403] To note: Brand new Calderon load must be desized before coloring them
by adding 25
items into a front loading Miele washing machine and running 2 short cotton
cycles at 60 C
(approximate duration of 1 hour 30 minutes) with 50g of Ariel sensitive powder
and a water
hardness of 15gpg, followed by running 2 more short cotton cycles at 60 C
(approximate
duration of 1 hour 30 minutes) with no detergent and a water hardness of
15gpg, followed by
tumble drying.
[0404] The orange fabrics are then cut into 48X48cm pieces, folded in half,
cut in half and
sewn at the sides into 4 equivalent pouches of 22X22cm with the top side open.
One test
product of a pre-conditioned water-soluble unit dose article is placed at the
bottom right corner
of the orange pouch, and the pouch is stitched closed. The water-soluble unit
dose article must
be pre-conditioned for a minimum of 2 weeks at 23 C, 50%rH before testing.
[0405] Load: 4 loads of 3 kg of mixed cotton (13 pieces) and polycotton (10
pieces) were de-
sized before use by washing in a short cotton cycle at 60 C with 79g of Ariel
Professional
detergent at a water hardness of 15gpg, followed by another short cotton cycle
at 60 C without
any detergent at a water hardness of 15gpg, and finally tumble-dried. Each
load of 3.0kg is pre-
treated 2 times by washing with 4 Ariel pods in the "prewet" cycle, followed
by a wash without
detergent in the "dissolution program" described below, and finally tumble-
dried.
[0406] The Electrolux W565 programmable washing machines were programmed with
2
programs. The first program was designed to equally wet the load (pre-wet
program). The
second program (dissolution program) was utilized to simulate 15min of a
Western Europe
stressed cycle setting, followed by pumping out the water and starting a spin
of 3min at
110Orpm.
Pre-wet program
Dissolution program
Time 5min 15min
Motor rotation 49rpm 59rpm
Water intake 12L 13.4L
Heating 20 C 20 C
Wash
Water Hardness 15gpg 15gpg
Motor action time 28s 20s
clockwise
Motor resting time 12s 20s
101
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
Motor action time 28s 28s
Counterclockwise
Draining time 1min 20s
Drain
Motor rotation 20rpm 49rpm
Time 30s 3min
Extraction
Motor rotation 900rpm 1100rpm
[0407] A load consisting of 13 pieces of 50X50cm of cotton and 10 pieces of
27X27cm of
polycotton (weighed at 3.0 +/- 0.15kg) was evenly introduced in the Electrolux
W565 washing
machine and the pre-wet program was run 2 times.
[0408] After the pre-wet program, the wet ballast was taken out of the drum
and 4 orange
pouches containing each a different test leg water soluble unit dose article
were aligned at the
bottom of the drum, hence 4 different test products are tested at once in the
same washing
machine in order to render the testing environment as reproducible as possible
across the test
legs. 10g of suds suppressor (Dowsil GP-4314 silicone suds suppressor,
commercially available
from the Dow Corning company) was added in the dispenser, and the wet load was
placed on
top of the orange pouches, without allowing the drum to move. The dissolution
program was
initiated. At the end of the full program, the orange pouches were transferred
to a grading room
(equipped with D65 lighting conditions) to be assessed for residues by expert
graders.
[0409] The orange pouches are cut and graded visually, within 30min after the
end of each
run, according a scale of 0 to 7 (0= No film residue, 7=Full pouch
residue).The final score is
calculated as the average of 4 external replicates, i.e. 4 different washing
machine runs, and
repeated 2 times (average of 8 scores).
Test results:
[0410] The results summarized in Table 4 clearly show that water-soluble unit-
dose articles
comprising a water-soluble test film comprising a polyvinyl alcohol based
polymer resin
consisting of a homopolymer blend with an average viscosity profile according
to the invention
(Inventive Example 1), despite having a superior pouch strength, do not
demonstrate a
dissolution compromise compared to tested water-soluble unit-dose articles
comprising a water-
soluble test film comprising a polyvinyl alcohol based polymer resin
consisting of a polyvinyl
alcohol hornopolymer blend with an average viscosity profile according to
comparative
examples 5 to 6, as well as compared to tested water-soluble unit-dose
articles comprising a
water-soluble test film comprising a polyvinyl alcohol based polymer resin
comprising 15% of an
anionic polyvinyl alcohol copolymer comparative examples 2 to 3.
Table 4 : Film residue grading
102
CA 03196098 2023- 4- 18
WO 2022/132853
PCT/US2021/063431
Comparative Comparative Comparative Comparative Inventive
Example 2 Example 3 Example 5 Example 6
Example 1
Average 1.44 1.50 1.91 1.72
1.38
film residue
grading*
*The lower the better
Test Method:
[0411] This methodology is used to determine the tensile force required to
peel solution-
sealed water-soluble films (seal peel strength). An INSTRON tensile testing
apparatus or
equivalent is used for the collection of film data. An ESIPROOF proofing
apparatus or
equivalent with an anilox roller 140/10 is used to secure two sheets of film
with deionized
water. A minimum of three test specimens, each cut with reliable cutting tools
to ensure
dimensional stability and reproducibility, are tested in the machine direction
(MD) for each
measurement. Tests are conducted in the standard laboratory atmosphere of 23
2.0 C. and
35 5% relative humidity.
[0412] For seal peel strength determination, test specimens are prepared by
cutting two
4"x12" (10.2 cm x30.5 cm) film sheets with the 12" (30.5 cm) dimension in the
machine direction
(MD) (where applicable). For one sheet, the four corners are taped to a
surface with the film
matte surface facing upward. The other sheet is overlaid on top of one of the
taped sheet so
that the matte surfaces are in contact.. A 4-inch (10.2 cm) end of the top
sheet is taped to
secure it to the bottom sheet. The loose end of the top sheet is threaded
through the
ESIPROOF proofing roller using the 140/10 anilox roller. An amount of 0.5 mL
of the test
sealing solution (water) is applied to the doctor blade. The roller is pulled
at a constant speed
(3", 7.6 cm per second) to coat the upper film and to secure it to the lower
sheet. The film is
allowed to weld for a period of about 10 minutes to 15 minutes, thereby
forming a seal but
leaving two unsealed (free) film flaps on one end of the test specimen for
subsequent peel testing. The sealed sample is then transferred to the INSTRON
tensile testing
machine to proceed with testing while minimizing exposure in the 35% relative
humidity
environment. The tensile testing machine is prepared according to the
manufacturer
instructions, equipped with a 500 N load cell, and calibrated. The correct
grips and faces are
fitted.
[0413] For the peel test, there is a 0.50" (1.27 cm) separation between the
rubber grips, all
four of which are flat and square. Three (or more) 1"-wide (2.54 cm) samples
are cut in the
machine direction (MD). The unsealed flaps of each sample are placed in the
grips of the
testing machine, taking care to ensure that the specimen is aligned with the
grips and parallel to
them, and that the specimen is not pulled too tightly in the tester's jaws.
The load is balanced
103
CA 03196098 2023- 4- 18
WO 2022/132853 PCT/US2021/063431
and the test is initiated according to the instructions of the equipment
manufacturer. At the end
of the test, the Absolute Positive Force (APF in Newtons) required to tear or
separate the layers
is recorded as the seal peel strength.
Additional Test Materials:
[0414] Solvent-casted water-soluble test films were prepared with various
levels of copolymer
content. The test films comprised about 65%-77% of one or more water-soluble
polyvinyl
alcohol resins in the ratios described below, the remainder being water,
plasticizer, surfactant,
and other materials typically present inside water-soluble films. The test
films of Comparative
Examples 8-9 were sealed in a manner contemplated for the "first water soluble
film" or "third
water soluble films" described as part of the invention above, with either the
film of Comparative
Example 1, or Inventive Example 1 as the "second water soluble film".
o Comparative Example 8 : polyvinyl alcohol blend comprising 60% polyvinyl
alcohol homopolymer (23 mPa.s, dH 87%) ¨ 40% methyl maleate based anionic
polyvinyl alcohol copolymer (4% anionic substitution, 18 mPa.s, dH 90%).
o Comparative Example 9 : polyvinyl alcohol blend comprising 32% polyvinyl
alcohol homopolymer (23 mPa.s, dH 88%) ¨ 68% methyl maleate based anionic
polyvinyl alcohol copolymer (4% anionic substitution, 18 mPa.s, dH 90%).
o Comparative Example 10 : polyvinyl alcohol homopolymer blend comprising
75%
polyvinyl alcohol homopolymer (23 mPa.s, dH 88%) ¨ 25% polyvinyl alcohol
homopolymer (6 mPa.s, dH BB%)
o Comparative Example 11 : 100% methyl maleate based anionic polyvinyl
alcohol
copolymer (4% anionic substitution, 18 mPa.s, dH 90%).
Test results :
[0415] Results for the peel strengths of each of Comparative Examples 8-11
sealed to
Comparative Example 1, and for each of Comparative Examples 8-11 sealed to
Inventive
Example Film 1, are shown in the table below. In each case, the film of
Inventive Example 1
demonstrated higher mean seal peel strength, i.e. regardless of whether the
first water soluble
film contained no anionic polyvinyl alcohol copolymer content, 100% anionic
polyvinyl alcohol
copolymer content, or an intermediate amount of anionic polyvinyl alcohol
copolymer content.
Film 1: C. Ex. 8 C. Ex. 9 C. Ex. 10
C. Ex. 11
Film 2: C. Ex. 1 I. Ex. 1 C. Ex. 1 I. Ex. 1 C.
Ex. 1 I. Ex. 1 C. Ex. 1 I. Ex. 1
Mean APE
10.261 11.931 10.853 13.033 5.108 5.799 9.418 10.880
(Std.Dev)
in Newtons (0.813) (1.185) (0.611) (1.184) (0.956)
(0.788) (0.404) (0.224)
104
CA 03196098 2023- 4- 18
Overall Conclusion:
[0416] From the data summarized across the different examples it is clear that
water-soluble
films comprising a polyvinyl alcohol based resin consisting of a polyvinyl
alcohol homopolymer
blend according to the invention, comprising a viscosity profile according to
the invention do not
form a foam layer upon sealing solvent application, leading into a superior
seal and pouch
strength profile while not leading to a dissolution compromise, compared to
comparative water-
soluble film compositions outside the scope of the invention.
[0417] Some of the embodiments disclosed in the present description are
provided in the
following items:
1. A
water-soluble unit dose article comprising at least two compartments, wherein
the unit
dose article comprises:
a. a first water-soluble film, wherein the first water-soluble film has a
first side and a
second side, and wherein the first water soluble film comprises a first
polyvinyl alcohol resin,
wherein the first polyvinyl alcohol resin comprises a polyvinyl alcohol
consisting of a polyvinyl
alcohol homopolymer, an anionic polyvinyl alcohol copolymer, or a blend
thereof;
b. a second water-soluble film, wherein the second water-soluble film has a
first side
and a second side, and wherein the second water-soluble film comprises a
second polyvinyl
alcohol resin, wherein the second polyvinyl alcohol resin comprises;
i. 0% to 15% by weight of the second polyvinyl alcohol resin of a polyvinyl
alcohol polymer comprising carboxylated anionic monomer units and vinyl
alcohol monomer
units, and wherein the carboxylated anionic monomer unit is derived from a
member selected
from the group consisting of maleic acid, monoalkyl maleate, dialkyl maleate,
maleic anhydride,
and combinations thereof; and
ii. 85% to 100% by weight of the second polyvinyl alcohol resin of a polyvinyl
alcohol homopolymer or a homopolymer blend comprising a first polyvinyl
alcohol homopolymer
and a second polyvinyl alcohol homopolymer;
wherein the second polyvinyl alcohol resin has an average viscosity in a range
of
8 mPa.s to up to but not including 12 mPa.s, or 9 mPa.s to up to but not
including 12 mPa.s, or
mPa.s to up to but not including 12 mPa.s, the first polyvinyl alcohol
homopolymer has an
average viscosity in a range of 11 mPa.s to 20 mPa.s, or 11 mPa.s to 15 mPa.s,
and the
second polyvinyl alcohol homopolymer has an average viscosity in a range of 1
mPa.s to 10
mPa.s, or 5 mPa.s to 10 mPa.s, the viscosities measured as a 4% polyvinyl
alcohol polymer
solution in deionized water at 20 C using a Brookfield LV type viscometer
with UL adapter as
described in British Standard EN ISO 15023-2:2006 Annex E Brookfield Test
method; and
c. a third water-soluble film, wherein the third water-soluble film has a
first side and a
second side, and wherein the third water soluble film comprises a third
polyvinyl alcohol resin;
wherein the first side of the first water-soluble film is sealed to the second
side of the
105
Date Recue/Date Received 2023-09-15
second water-soluble film to create a first compartment between the first
water-soluble film and
the second water-soluble film, and the first side of the second water-soluble
film is sealed to the
second side of the third water-soluble film to create at least a second
compartment between the
second water-soluble film and the third water-soluble film, and wherein the
second compartment
is positioned above the first compartment;
wherein when the article contains a composition housed within a compartment,
then the
composition is not a laundry composition and is not an automatic dish washing
composition.
2. The water-soluble film according to item 1, wherein the third polyvinyl
alcohol resin
comprises a polyvinyl alcohol consisting of a polyvinyl alcohol homopolymer,
an anionic
polyvinyl alcohol copolymer, or a blend thereof.
3. The water-soluble unit dose article according to item 1 or 2, wherein
the second
polyvinyl alcohol resin comprises 90% to 100% by weight of the second
polyvinyl alcohol resin
of the polyvinyl alcohol homopolymer or polyvinyl alcohol homopolymer blend
and 0% to 10%
by weight of the second polyvinyl alcohol resin of the polyvinyl alcohol
polymer comprising
carboxylated anionic monomer units and vinyl alcohol monomer units, wherein
the carboxylated
anionic monomer unit is derived from a member selected from the group
consisting of maleic
acid, monoalkyl maleate, dialkyl maleate, maleic anhydride, and combinations
thereof.
4. The water-soluble unit dose article according to any one of items 1-3,
wherein the
second polyvinyl alcohol resin comprises a blend of the first polyvinyl
alcohol homopolymer and
the second polyvinyl alcohol homopolymer.
5. The water-soluble film according to item 4, wherein the first polyvinyl
alcohol
homopolymer and second polyvinyl alcohol homopolymer are present in a relative
weight ratio
in a range of 90/10 to 10/90, or 80/20 to 20/80, or 70/30 to 50/50.
6. The water-soluble film according to item 5, wherein the relative weight
ratio is in a range
of 70/30 to 50/50.
7. The water-soluble unit dose article according to any one of items 4-6,
wherein in the
second polyvinyl alcohol resin the difference in average viscosity of the
first polyvinyl alcohol
homopolymer and the second polyvinyl alcohol homopolymer is at least 1 mPa.s,
or 2 to 10
mPa.s, or 3 to 8 mPa.s, measured as a 4% polyvinyl alcohol polymer solution in
deionized
water at 20 C using a Brookfield LV type viscometer with UL adapter as
described in British
Standard EN ISO 15023-2:2006 Annex E Brookfield Test method.
8. The water-soluble unit dose article according to any one of items 4-7,
wherein the
individual polyvinyl alcohol homopolymers independently have an average degree
of hydrolysis
in a range of 75% to 99%, or 80% to 95%, or 85% to 95%.
106
Date Recue/Date Received 2023-09-15
9. The water-soluble unit dose article according to any one of items 1-
8, wherein
a. the first polyvinyl alcohol resin is present in an amount a range of 50% to
95%, or
50% to 80%, or 60% to 75%, by weight of the first water-soluble film, or
b. the second polyvinyl alcohol resin is present in an amount in a range of
50% to 95%,
or 50% to 80%, or 60% to 75%, by weight of the second water-soluble film, or
c. the third polyvinyl alcohol resin is present in an amount a range of 50% to
95%, or
50% to 80%, or 60% to 75%, by weight of the third water-soluble film; or
d. any combinations thereof.
10. The water-soluble unit dose article according to any one of items 1-
9, wherein the first
water-soluble film, the third water-soluble film, or both independently
comprise a blend of
polyvinyl alcohol homopolymers and/or anionic polyvinyl alcohol copolymers.
11. The water-soluble film according to item 10, wherein the first water-
soluble film, the third
water-soluble film, or both independently comprise a blend of a polyvinyl
alcohol homopolymer
and an anionic polyvinyl alcohol copolymer, wherein the polyvinyl alcohol
homopolymer and the
anionic polyvinyl alcohol copolymer are present in a relative weight ratio in
a range of 90/10 to
10/90, or 80/20 to 20/80, or 70/30 to 50/50.
12. The water-soluble unit dose article according to item 10 or 11,
wherein the anionic
polyvinyl alcohol copolymer comprises an anionic monomer unit.
13. The water-soluble film according to item 12, wherein the anionic
monomer unit is present
in the anionic polyvinyl alcohol copolymer in an average amount in a range of
2 mol.% to 5
mol.%.
14. The water-soluble unit dose article according to any one of items 10-
13, wherein the
anionic polyvinyl alcohol copolymer is selected from the group consisting of
sulphonated and
carboxylated anionic polyvinyl alcohol copolymers.
15. The water-soluble film according to item 14, wherein the carboxylate
is selected from the
group consisting of an acrylate, a methacrylate, a maleate, and a mixture
thereof.
16. The water-soluble unit dose article according to any one of items 1-
15, wherein the
second polyvinyl alcohol resin comprises a polyvinyl alcohol polymer
comprising a carboxylated
anionic monomer unit derived from a monoalkyl maleate unit, wherein the
carboxylated anionic
monomer unit is present in the polyvinyl alcohol polymer comprising a
carboxylated anionic
monomer unit in an average amount of at least 3m01.%, or in a range of 3m01.%
to 6m01.%, or
3mo1.% to 5m01.%, or 3.5mo1.% to 4.5mo1.%, or 4m01.% to 4.5m01.%.
107
Date Recue/Date Received 2023-09-15
17. The water-soluble unit dose article according to any one of items 1-16,
wherein the first
water-soluble film, the second water-soluble film and the third water-soluble
film each
independently comprise a surfactant content in a range of 0.1% to 3.5%, or
0.5% to 2% by
weight of the water-soluble film.
18. The water-soluble unit dose article according to any one of items 1-17,
wherein the first
water-soluble film, the second water-soluble film, and the third water-soluble
film each
individually have a residual moisture content of at least 4%, or in a range of
4% to 15%, or 5%
to 10% by weight of the water-soluble film as measured by Karl Fischer
titration.
19. The water-soluble unit dose article according to any one of items 1-18,
wherein each
film independently comprises one or more components selected from the group
consisting of
plasticizers, plasticizer compatibilizers, lubricants, release agents,
fillers, extenders, cross-
linking agents, antiblocking agents, antioxidants, detackifying agents,
antifoams, nanoparticles,
bleaching agents, aversive agents, surfactants, and combinations thereof.
20. The water-soluble unit dose article according to item 19, wherein each
film
independently comprises one or more plasticizers in an amount in a range of
between 5% to
50%, 0110% to 40%, or 20% to 30% by weight of the individual film.
21. The water-soluble unit dose article according to item 20, wherein the
plasticizer is
selected from the group consisting of one or more polyols, one or more sugar
alcohols, and
mixtures thereof, wherein the polyols are selected from the group consisting
of glycerol,
diglycerin, ethylene glycol, diethylene glycol, triethyleneglycol,
tetraethylene glycol, polyethylene
glycols up to 400 MW, neopentyl glycol, 1,2-propylene glycol, 1,3-propanediol,
dipropylene
glycol, polypropylene glycol, 2-methyl-1,3-propanediol, trimethylolpropane,
polyether polyols,
and mixtures thereof, and wherein sugar alcohols are selected from the group
consisting of
isomalt, maltitol, sorbitol, xylitol, erythritol, adonitol, dulcitol,
pentaerythritol, mannitol, and
mixtures thereof.
22. The water-soluble unit dose article according to item 21, wherein the
plasticizer is
selected from the group consisting of sorbitol, glycerol, dipropyleneglycol,
polyethyleneglycol,
trimethylolpropane, and mixtures thereof.
23. The water-soluble unit dose article according to any one of items 1-22,
wherein the first
water-soluble film and the second water-soluble film are sealed via solvent
sealing, heat sealing
or a combination thereof; and
wherein the second water-soluble film and the third water-soluble film are
sealed via
solvent sealing, heat sealing or a mixture thereof.
108
Date Recue/Date Received 2023-09-15
24. The water-soluble unit dose article according to item 23, wherein the
first water-soluble
film and the second water-soluble film are sealed via solvent sealing, and
wherein a solvent
sealing solution comprising an aqueous solvent, a non-aqueous solvent, or a
mixture thereof is
applied to the first water-soluble film and/or the second water-soluble film.
25. The water-soluble unit dose article according to item 23, wherein the
second water-
soluble film and the third water-soluble film are sealed via solvent sealing,
and wherein a
solvent sealing solution comprising an aqueous solvent, a non-aqueous solvent,
or a mixture
thereof is applied to the second water-soluble film and/or the third water-
soluble film.
26. The water-soluble unit dose article according to any one of items 1-25,
wherein the unit
dose article comprises a third compartment, wherein the second compartment and
the third
compartment are positioned side-by-side to one another and wherein the second
compartment
and the third compartment are positioned above the first compartment.
27. The water-soluble unit dose article according to any one of items 1-26,
wherein the
composition is selected from the group consisting of light duty liquid
detergent compositions,
heavy duty liquid detergent compositions, hard surface cleaning compositions,
bleaching
compositions, shampoos, body washes, other personal care compositions, and
mixtures
thereof.
28. The water-soluble unit dose article according to any one of items 1-27,
wherein the
article comprises a non-household care composition housed in the at least
first compartment
and the non-household care composition is selected from the group consisting
of agricultural
compositions, automotive compositions, aviation compositions, food and
nutritive compositions,
industrial compositions, livestock compositions, marine compositions, medical
compositions,
mercantile compositions, military and quasi-military compositions, office
compositions, and
recreational and park compositions, pet compositions, water-treatment
compositions,
compositions containing one or more active agents selected from the group
consisting of
agriculture active agents, ingestible active agents, liquid treatment active
agents, industrial
active agents, and any combinations thereof.
29. The water-soluble unit dose article according to any one of items 1-28,
wherein the first polyvinyl alcohol resin comprises a polyvinyl alcohol
consisting of a
blend of a polyvinyl alcohol homopolymer and a monomethyl maleate-modified
polyvinyl alcohol
copolymer, wherein the polyvinyl alcohol homopolymer and the monomethyl
maleate-modified
polyvinyl alcohol copolymer are present in a relative weight ratio of from
70/30 to 50/50, wherein
the polyvinyl alcohol homopolymer has an average viscosity in a range of 12 to
25 mPa.s
wherein the monomethyl maleate-modified polyvinyl alcohol copolymer has an
average
viscosity in a range of 12 to 25 mPa.s,
109
Date Recue/Date Received 2023-09-15
wherein the third polyvinyl alcohol resin comprises a polyvinyl alcohol
consisting of a
blend of a polyvinyl alcohol homopolymer and a monomethyl maleate-modified
polyvinyl alcohol
copolymer, wherein the polyvinyl alcohol homopolymer and the monomethyl
maleate-modified
polyvinyl alcohol copolymer are present in a relative weight ratio of from
70/30 to 50/50, wherein
the polyvinyl alcohol homopolymer has an average viscosity in a range of 12 to
25 mPa.s
wherein the monomethyl maleate-modified polyvinyl alcohol copolymer has an
average
viscosity in a range of 12 to 25 mPa.s, and
wherein average viscosity is measured as a 4% polyvinyl alcohol polymer
solution in
deionized water at 20 C using a Brookfield LV type viscometer with UL adapter
as described in
British Standard EN ISO 15023-2:2006 Annex E Brookfield Test method.
30. A process of making the water-soluble unit dose article according to
any one of items 1-
29, comprising the steps of:
a. deforming the first water-soluble film in a mould to create an open cavity
via
thermoforming, vacuum forming, or a combination thereof;
b. filling the open cavity with the composition;
c. separately deforming the third water-soluble film in a mould to create at
least one
open cavity via thermoforming, vacuum forming, or a combination thereof;
d. filling the at least one open cavity from step (c) with a composition;
e. closing the open filled cavity from step (d) with the second water-soluble
film;
f. sealing the second water-soluble film to the third water-soluble film to
create a closed
intermediate;
g. closing the open filled cavity from step (b) with the closed intermediate
from step (f);
and
h. sealing the first water-soluble film to the second water-soluble film to
create the
water-soluble unit dose article.
31. The process according to item 30, wherein the second water-soluble film
is sealed to the
third water-soluble films via solvent sealing.
32. The process according to item 31, wherein a solvent sealing solution is
applied to a first
side of the second water-soluble film ahead of sealing the second water-
soluble film to the third
water-soluble film, the first side of the second water-soluble film being the
side facing the third
water-soluble film.
33. The process according to any one of items 30-32, wherein the first
water-soluble film in
step (a) and the third water-soluble film in step (c) are the same prior to
deforming.
110
Date Recue/Date Received 2023-09-15