Note: Descriptions are shown in the official language in which they were submitted.
WO 2022/093878
PCT/US2021/056726
MEMBRANE WITH GUIDE SURFACE
CROSS-REFERENCE TO RELATED APPLICATION
[0001] The present application claims priority to United States Provisional
Application
Serial No. 63/106,701, filed October 28, 2020, entitled "Membrane with Guide
Surface", the
entire disclosure of which is hereby incorporated by reference in its
entirety.
BACKGROUND OF THE INVENTION
Field of the Disclosure
[0002] The present disclosure relates generally to a membrane for a closed
system transfer
device.
Description of the Related Art
[0003] Health care providers reconstituting, transporting, and administering
hazardous
drugs, such as cancer treatments, can put health care providers at risk of
exposure to these
medications and present a hazard in -the health care environment.
Unintentional chemotherapy
exposure can affect the nervous system, impair the reproductive system, and
bring an increased
risk of developing blood cancers in the future. Some drugs must be dissolved
or diluted before
they are administered, which involves transferring a solvent from one
container to a sealed vial
containing the drug in powder or liquid form, by means of a needle. Drugs may
be
inadvertently released into the atmosphere in gas form or by way of
aerosolization, during the
withdrawal of the needle from the vial and while the needle is inside the vial
if any pressure
differential between the interior of the vial and the surrounding atmosphere
exists. In order to
reduce the risk of health care providers being exposed to toxic drugs, the
transfer of these drugs
is accomplished utilizing a closed system transfer device or system.
[0004] Closed system transfer devices or systems may utilize membranes to
ensure the safe
transfer of fluid between components. For example, a syringe adapter may
include a membrane
that contacts a membrane of a mating component, such as a patient connector,
IV bag spike, or
vial adapter.
SUMMARY OF THE INVENTION
[0005] In one aspect or embodiment, a membrane for a closed system transfer
device
includes a body having a first end and a second end positioned opposite the
first end, the body
defining a passageway extending from the first end of the body to a position
intermediate the
first end of the body and the second end of the body, and a guide member
positioned within
CA 03196492 2023- 4- 21
WO 2022/093878
PCT/US2021/056726
the passageway of the body. The guide member having a first end and a second
end positioned
opposite the first end, with the guide member having a guide surface
configured to engage a
needle positioned within the passageway of the body.
[0006] The guide member may be formed integrally with the body. The guide
member may
be formed as a projection extending from the body in a radially inward
direction. The guide
member may be. a plurality of equally-spaced projections extending from the
body in a radially
inward direction. The guide surface may be concave. The guide member may be
positioned
intermediate the first and second ends of the body. The guide member may
extend at least 25
percent of the length of the body. The body may define a slit extending from
the passageway
to the second end of the body. The passageway may include a tapered terminal
end. The guide
surface may be convex.
[0007] In a further aspect or embodiment, a syringe adapter includes a housing
having a
connector configured to be secured to a syringe, a needle received within the
housing and in
fluid communication with the. connector, with the. needle having a first end
and a second end
positioned opposite the first end, and the membrane of any of the aspects or
embodiments
discussed above. The membrane is moveable from a first position within the
housing where
the second end of the needle is received within the passageway of the membrane
to a second
position where the second end of the needle is positioned outside of the
passageway of the
membrane. The needle is engaged with the guide member when the membrane moves
from
the first position to the second position.
[0008] The syringe adapter may further include a collet defining an opening,
with the
membrane received within the opening of the collet. An outer diameter of the
needle may taper
from the first end of the needle to the second end of the needle. The tapered
terminal end of
the passageway of the. membrane may correspond to the shape of the second end
of the needle.
BRIEF DESCRIPTION OF THE DRAWINGS
[0009] The above-mentioned and other features and advantages of this
disclosure, and the
manner of attaining them, will become more apparent and the disclosure itself
will be better
understood by reference to the following descriptions of aspects of the
disclosure taken in
conjunction with the accompanying drawings, wherein:
[0010] FIG. 1 is a top perspective view of a membrane according to one aspect
or
embodiment of the present application.
[0011] FIG, 2 is a bottom perspective view of the membrane of FIG. 1.
[0012] FIG. 3 is a front view of the membrane of FIG. 1.
55Q4600.DOCX
CA 03196492 2023- 4- 21
WO 2022/093878
PCT/US2021/056726
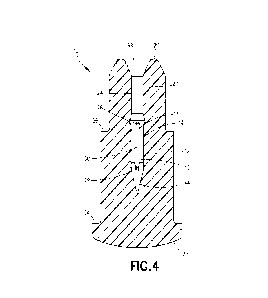
[0013] FIG. 4 is a cross-sectional view of the membrane taken along line A-A
in FIG. 3
according to one aspect or embodiment of the present application.
[00141 HG. 5 is an enlarged cross-sectional view of the area shown in FIG. 4.
[0015] FIG. 6 is a cross-sectional view of the membrane taken along line A-A
according to
a second aspect or embodiment of the present application.
[0016] FIG. 7 is a cross-sectional view of the membrane taken along line A-A
according to
a third aspect or embodiment of the present application.
[0017] HG. 8 is an enlarged cross-sectional view of the area shown in FIG. 7.
[0018] FIG. 9 is a cross-sectional view of the membrane taken along line A-A
according to
a fourth aspect or embodiment of the. present application
[0019] FIG. 10 is an enlarged cross-sectional view of the area shown in FIG.
9.
[0020] FIG. 11 is a perspective view of a system according to one aspect or
embodiment of
the present application.
[0021] FIG. 12 is a cross-sectional view of the system of FIG. 11.
[0022] FIG. 13 is a cross-sectional view of the system of FIG_ 11, showing a
syringe adapter
connected to a patient connector.
[0023] FIG. 14 is a perspective view of a needle according to one aspect or
embodiment of
the present application.
[0024] FIG. 15 is a side view of the needle of FIG. 14.
[0025] Corresponding reference characters indicate corresponding parts
throughout the
several views. The exemplifications set out herein illustrate exemplary
aspects of the
disclosure, and such exemplifications are not to be construed as limiting the
scope of the
disclosure in any manner.
DETAILED DESCRIPTION
[0026] The following description is provided to enable those skilled in the
art to make and
use the described aspects contemplated for carrying out the invention. Various
modifications,
equivalents, variations, and alternatives, however, will remain readily
apparent to those skilled
in the art. Any and all such modifications, variations, equivalents, and
alternatives are intended
to fall within the spirit and scope of the present invention.
[0027] For purposes of the description hereinafter, the terms "upper",
"lower", "right",
"left", "vertical", "horizontal", "top", "bottom", "lateral", "longitudinal",
and derivatives
thereof shall relate to the invention as it is oriented in the drawing
figures. However, it is to be
understood that the invention may assume various alternative variations,
except where
expressly specified to the contrary. It is also to be understood that the
specific devices
55Q4600.DOCX 3
CA 03196492 2023- 4- 21
WO 2022/093878
PCT/US2021/056726
illustrated in the attached drawings, and described in the following
specification, are simply
exemplary aspects of the invention. Hence, specific dimensions and other
physical
characteristics related to the aspects disclosed herein are not to be
considered as limiting.
[0028] Referring to FIGS. 1-5 and 11-13, a membrane 10 for a closed system
transfer device
according to one aspect or embodiment of the present application includes a
body 12 and a
guide member 14. As shown in FIG. 12, the membrane 10 is shown in connection
with a
syringe adapter 46, which is utilized to connect a syringe barrel (not shown)
to another
component of a closed system transfer device or system, such as a patient
connector 18, vial
adapter, IV hag spike, etc. For example, the syringe adapter 16 may be used to
facilitate the
transfer of fluid from one container, such as a syringe barrel, to another
container or line, such
as an intravenous line, IV bag, or other component. The membrane 10 may be
utilized in any
component of a closed system transfer device or system. The syringe adapter 16
may be the
same and operate in the same manner as the syringe adapter shown and described
in United
States Patent Application Publication No. 201510297454. which is hereby
incorporated by
reference in its entirety.
[0029] Referring to FIGS. 1-5, the body 12 has a first end 20 and a second end
22 positioned
opposite the first end 20, with the body 12 defining a passageway 24 extending
from the first
end 20 of the body 12 to a position intermediate the first end 20 of the body
12 and the second
end 22 of the body 12. The guide member 14 is positioned within the passageway
24 of the
body 12, with the guide member 14 having a first end 26 and a second end 28
positioned
opposite the first end 26. The guide member 14 has a guide surface 30
configured to engage a
needle 32 positioned within the passageway 24 of the body 12. In one aspect or
embodiment,
the guide surface 30 of the guide member 14 is configured to guide movement of
the needle 32
passing through the passageway 24 and through the membrane 10 to ensure the
needle 32
passes through the same location during use of the syringe adapter 16.
[0030] Referring to FIGS. 1-4, the second end 22 of the membrane 10 includes a
convex
surface and a first flange 34 extending radially outward from the body 12. The
convex surface
is configured to engage a membrane of a mating component, such as the patient
connector 18,
as discussed in more detail below. The membrane 10 also includes a second
flange 36
positioned intermediate the first and second ends 20, 22 of the membrane 10.
The first end 20
of the membrane 10 is tapered radially inward. The passageway 24 includes a
conical portion
38 at the first end 20 of the membrane 10, a first cylindrical portion 40
extending from the
conical portion 38, a second cylindrical portion 42 extending from the first
cylindrical portion
40, and a tapered terminal end 44 extending from the second cylindrical
portion 42. The first
55Q4600.DOCX 4
CA 03196492 2023- 4- 21
WO 2022/093878
PCT/US2021/056726
cylindrical portion 40 is narrower than the second cylindrical portion 42. The
body 12 is
formed from an elastomeric material, although other suitable materials may be
utilized.
[0031] Referring to FIGS. 4 and 5, in one aspect or embodiment, the guide
member 14 is
formed integrally with the body 12, although the guide member 14 may be formed
separately
and secured to the body 12. The guide member 14 is a projection extending from
the body 12
in a radially inward direction. As shown in FIG. 5, the guide member 14 is
formed as a plurality
of equally-spaced projections extending from the body 12 in a radially inward
direction,
although one or more projections may be provided. The guide surface 30 of the
guide member
14 is concave and configured to receive an outside surface 46 of the needle 32
of the syringe
adapter 16. The guide member 14 is positioned intermediate the first and
second ends 20, 2.2
of the body 12. More specifically, the guide member 14 is positioned within
the second
cylindrical portion 42 of the passageway 24. In one aspect or embodiment, the
guide member
14 extends at least 25% of the length of the body 12, although the guide
member 14 may extend
at least 10%, 15%, 20%, 30%, 35%, 40%, 45%, or 50% of the length of the body
12.
[0032] Referring to FIG. 6, in one aspect or embodiment, the body 12 of the
membrane 10
defines a slit 52 extending from the tapered terminal end 44 to the second end
22 of the body
12. The slit 52 is positioned co-axially with the passageway 24, with the slit
52 configured to
receive the needle 32 of the syringe adapter 16 during use of the syringe
adapter 16. In one
aspect or embodiment, the slit 52 is smaller than an outer diameter of the
needle 32.
[0033] Referring to FIGS. 7 and 8, a membrane 60 for a closed system transfer
device
according to a second aspect or embodiment of the present application is
shown. The
membrane 60 is similar to the membrane 10 shown in FIGS. 1-5 and discussed
above, except
the guide surface 30 of the guide member 14 is convex. The membrane 10
functions in the
same manner as the membrane 60 of FIGS. 1-5 with the convex shape of the guide
surface 30
configured to engage and guide the needle 32 of the syringe adapter 16. In one
aspect or
embodiment, the convex shape of the guide surface 30 of the guide member 14
has less surface
area contact with the needle 32 compared to the guide surface 30 shown in
FIGS. 4 and 5.
[0034] Referring to FIGS. 9 and 10, a membrane 70 for a closed system transfer
device
according to a third aspect or embodiment of the present application is shown.
The membrane
70 is similar to the membrane 60 shown in FIGS. 7 and 8, except the guide
member 14 is
narrower than the guide member 14 of FIGS. 7 and 8. The size, shape, and
configuration of
the guide member 14 may be optimized to ensure the needle 32 of the syringe
adapter 16 is
guided through the same spot of the membrane 10, 60, 70 during use of the
syringe adapter 16.
hi one aspect or embodiment, the guide member 14 of FIGS. 9 and 10 applies
less compression
55Q4600.DOCX 5
CA 03196492 2023- 4- 21
WO 2022/093878
PCT/US2021/056726
to the needle 32 compared to the guide member 14 of FIGS. 7 and 8, but reduces
friction
between the guide member 14 and the needle 32 thereby reducing the force
required to move
the needle 32 through the membrane 70.
100351 Referring to FIGS. 11-13, a system according to one aspect or
embodiment includes
the syringe adapter and the patient connector 18 or other suitable mating
connector, such as an
IV bag spike, vial adapter, etc. The syringe adapter 16 includes a housing 80
having a
connector 82 configured to be secured to a syringe, the needle 32 received
within the housing
80 and in fluid communication with the connector 82, with the needle 32 having
a first end 84
and a second end 86 positioned opposite the first end 84, and the membrane 10,
60, 70 as shown
in any of FIGS. 1-8. The membrane 10, 60, 70 is moveable from a first position
within the
housing 80 where the second end 86 of the needle 32 is received within the
passageway 24 of
the membrane 10, 60, 70 to a second position where the second end 86 of the
needle 32 is
positioned outside of the passageway 24 of the membrane 10, 60, 70. The needle
32 is engaged
with the guide member 14 when the membrane 10, 60, 70 moves from the first
position to the
second position. The patient connector 18 includes a body 92, a membrane 94,
and a connector
96. The connector 96 is male luer connector, although other suitable
connectors may be
[O36] In one aspect or embodiment, the membrane 10, 60, 70 is received by an
opening 88
defined by a collet 90. The first flange 34 and second flange 36 of the
membrane 10, 60, 70
correspond to the opening 88 of the collet 90 and are configured to secure the
membrane 10,
60, 70 within the collet 90. The collet 90 and the membrane 10, 60, 70 are
moveable between
the first and second positions when a mating connector, such as the patient
connector 18, is
received by the housing 80 and the collet 90 to move the collet 90 and the
membrane 10, 60,
70 from the first position (shown in FIG. 12) to the second position (shown in
FIG. 13) such
that the needle 32 is placed in fluid communication with the patient connector
18. As shown
in FIG. 13, the membrane 94 of the patient connector 18 engages the membrane
10, 60, 70 of
the syringe adapter 16, which ensures a drip-free closed connections as the
needle 3.2 passes
through the membrane 10, 60, 70 of the syringe adapter and the membrane 94 of
the patient
connector 18. The collet 90 is configured to engage a portion of the body 92
of the patient
connector 18 to secure the collet 90 to the patient connector 18 when the
collet 90 moves from
the first position to the second position. The syringe adapter 16 may include
a spring 98 to bias
the collet 90 and the membrane 10, 60, 70 to the first position. The tapered
terminal end 44 of
the passageway 24 of the membrane 10, 60, 70 corresponds to the shape of the
second end 86
of the needle 32, which facilitates, along with the guide member 14, the
movement of the needle
55Q4600.DOCX 6
CA 03196492 2023- 4- 21
WO 2022/093878
PCT/US2021/056726
32 of the syringe adapter 16 through the same spot of the membrane 10, 60, 70.
The operation
of the syringe adapter 16 is described in United States Patent Application
Publication No.
2015/0297454,
[00371 Referring to FIGS. 14 and 15, in one aspect or embodiment of the
present application,
an outer diameter of the needle 32 tapers from the first end 84 of the needle
32 to the second
end 86 of the needle 32. When the membrane 10, 60, 70 moves from the first
position to the
second position, the larger diameter portion of the needle 32 passes by the
guide member 14 to
provide a needle feeding effect to further ensure a consistent trajectory path
of the needle 32
during use of the syringe adapter 16. The tapering of the needle 32 provides a
taper lock with
the tapered internal geometry of the membrane 10, 60, 70. Further, the taper
of the needle 32
shown in FIGS. 14 and 15 allows for smaller frictional forces to be overcome,
while still
providing diametrical compression to aid in centering of the needle 32. A
tapered needle also
minimizes coring and maximizes the self-sealing capability of the membrane.
The self-sealing
capability of the membrane helps minimize vapor and/or liquid leakage onto the
surface.
[0038] While this disclosure has been described as having exemplary designs,
the present
disclosure can be further modified within the spirit and scope of this
disclosure. This
application is therefore intended to cover any variations, uses, or
adaptations of the disclosure
using its general principles. Further, this application is intended to cover
such departures from
the present disclosure as come within known or customary practice in the art
to which this
disclosure pertains and which fall within the limits of the appended claims.
To the extent
possible, one or more features of any aspect or embodiment discussed above can
be combined
with one or more features of any other aspect or embodiment.
7
CA 03196492 2023- 4- 21