Note: Descriptions are shown in the official language in which they were submitted.
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
CAST ALUMINUM ALLOYS COMPRISING CALCIUM AND RELATED
PROCESSES
PRIORITY
.. [0001] This application claims priority to US Provisional Application No.
63/199,806, filed
January 26, 2021, the entire contents and disclosure of which are incorporated
herein.
FIELD
[0002] The present disclosure relates to the fields of metallurgy, aluminum
alloys, aluminum
fabrication, and related fields. In particular, the present disclosure
provides cast aluminum
alloy compositions which include calcium, and processes for forming the cast
aluminum alloys
and aluminum alloy articles.
BACKGROUND
[0003] Aluminum (Al) alloys are increasingly replacing steel and other metals
in multiple
applications, such as automotive, transportation, industrial, or electronics-
related applications.
In some applications, such alloys may need to exhibit high strength, high
formability, corrosion
resistance, and/or low weight. However, producing alloys having the
aforementioned
properties is a challenge, as conventional methods and compositions may not
achieve the
necessary requirements, specifications, and/or performances required for the
different
applications when produced via established methods. For example, aluminum
alloys with a
high solute content, including copper (Cu), magnesium (Mg), and zinc (Zn), can
exhibit
cracking when cast, as well as other surface imperfections.
[0004] One known method for addressing such surface imperfections is to scalp
the surface of
the ingot, which involves machining off a surface layer of the ingot. Another
known method
for addressing surface imperfections is to include beryllium in the alloy.
Although beryllium
was effective at controlling surface defects in aluminum cast ingots, it is no
longer allowed in
food or beverage packaging and is a health concern for factory workers.
SUMMARY
[0005] Covered embodiments of the present disclosure are defined by the
claims, not this
summary. This summary is a high-level overview of various aspects of the
invention and
introduces some of the concepts that are further described in the Detailed
Description section
below. This summary is not intended to identify key or essential features of
the claimed subject
matter, nor is it intended to be used in isolation to determine the scope of
the claimed subject
-1-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
matter. The subject matter should be understood by reference to appropriate
portions of the
entire specification, any or all drawings and each claim.
[0006] Provided herein are aluminum alloys that exhibit high strength and high
formability,
and that do not exhibit cracking and have reduced surface defects during
and/or after casting,
along with methods of making and processing the alloys. The alloys can be used
in automotive,
transportation, aerospace, industrial, and electronics applications, to name a
few.
[0007] In some examples, a process of producing an aluminum alloy product
comprises
continuously casting an aluminum alloy to form a slab, wherein the aluminum
alloy comprises
at least 2.0 % by weight Mg and, in molten form, the alloy comprises from 30
ppm to 500 ppm
calcium (Ca). In some cases, the cast slab does not exhibit cracking during
and/or after casting.
In some cases, the slab has reduced surface defects as compared to a slab
without the calcium
addition.
[0008] Also provided herein are aluminum alloy articles prepared according to
the methods
described herein. The aluminum alloy product can be an aluminum alloy sheet,
an aluminum
alloy plate, or an aluminum alloy shate, having an improved surface. The
surface may be
viewed visually, through microscopy, to view the size and amount of exudates,
as well as the
shine of the surface. The aluminum alloy articles prepared according to the
methods herein
have a more uniform surface with less open porosity than aluminum alloy
articles prepared
without calcium addition. Additionally, the intermetallic particles are small
and well
.. distributed. Depending on the aluminum alloy, such as for 7xxx allowy, the
aluminum alloy
product can comprise a long traverse tensile yield strength of at least 560
MPa when in a T6
temper. Optionally, the aluminum alloy product can comprise a bend angle of
from
approximately 80 to approximately 120' when in a T6 temper, such as in alloys
other than
5xxx alloys. Optionally, the aluminum alloy product can comprise a yield
strength of from
approximately 500 MPa to approximately 650 MPa when in a T4 temper and after
paint baking.
The aluminum alloy product can optionally be an automotive body part, a motor
vehicle part,
a transportation body part, an aerospace body part, or an electronics housing.
[0009] Other objects and advantages of the invention will be apparent from the
following
detailed description of embodiments of the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
[0010] FIG. 1 is a digital image showing the surface of an aluminum alloy
according to an
example described herein.
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
[00111 FIG. 2. is a digital image showing the surface of an aluminum alloy
according to an
example described herein.
[0012] FIG. 3 is a digital image showing the surface of aluminum alloys
according to an
example described herein.
[0013] FIG. 4 is a micrograph image showing the surface of aluminum alloys
according to an
example described herein.
[0014] FIG. 5 is a digital image showing the surface of an aluminum alloy
during processing
according to an example described herein.
[0015] FIG. 6 is a digital image showing the surface of an aluminum alloy
during processing
according to an example described herein.
[0016] FIG. 7 is a digital image showing the surface of an aluminum alloy
during processing
according to an example described herein.
[0017] FIG. 8 is a digital image showing the surface of an aluminum alloy
during processing
according to an example described herein.
[0018] FIG. 9 is a digital image showing the surface of aluminum alloys
according to an
example described herein.
[0019] FIG. 10 is a micrograph image showing the surface of an aluminum alloy
according to
an example described herein.
[0020] FIG. 11 is a micrograph image showing the surface of an aluminum alloy
according to
an example described herein.
[0021] FIG. 12 is a composite micrograph image showing the cross-section of an
aluminum
alloy according to an example described herein.
[0022] FIG. 13 is a micrograph image showing the surface of an aluminum alloy
according to
an example described herein.
[0023] FIG. 14 is a micrograph image showing the surface of an aluminum alloy
according to
an example described herein.
[0024] FIG. 15 is a composite micrograph image showing the cross-section of an
aluminum
alloy according to an example described herein.
[0025] FIG. 16 is a micrograph image showing the surface of an aluminum alloy
according to
an example described herein.
[0026] FIG. 17 is a micrograph image showing the surface of an aluminum alloy
according to
an example described herein.
[0027] FIG. 18 is a composite micrograph image showing the cross-section of an
aluminum
alloy according to an example described herein.
-3-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
[0028] FIG. 19 is an XRD of extracted particles of an aluminum alloy according
to an example
described herein.
DETAILED DESCRIPTION
[0029] Described herein are aluminum alloys comprising magnesium, wherein when
the
aluminum alloy is in molten form, calcium is added to the alloy prior to
continuous casting. In
some cases, aluminum alloys comprising magnesium can be difficult to cast
using conventional
casting processes due to their magnesium content. The disclosed processes can
permit the
casting of aluminum alloys comprising magnesium described herein in thin
gauges (c.a.,
aluminum alloy bodies with a thickness of from approximately 5 mm to
approximately 50 mm),
free from cracking during and/or after casting as determined by visual
inspection (e.g., there
are fewer cracks per square meter in the slab prepared according to methods
described herein
than in a direct chill cast ingot). Additionally, the alloys have less surface
defects than those
formed by processes without calcium addition. In some examples, the aluminum
alloys can be
continuously cast according to processes as described herein.
Definitions and Descriptions
[0030] As used herein, the terms "invention," "the invention," "this
invention" and "the present
invention" are intended to refer broadly to all of the subject matter of this
patent application
and the claims below. Statements containing these terms should be understood
not to limit the
subject matter described herein or to limit the meaning or scope of the patent
claims below.
[0031] As used herein, the meaning of "metals" includes pure metals, alloys
and metal solid
solutions unless the context clearly dictates otherwise.
[0032] In this description, reference is made to alloys identified by aluminum
industry
designations, such as -series" or -5xxx." For an understanding of the number
designation
system most commonly used in naming and identifying aluminum and its alloys,
see
"International Alloy Designations and Chemical Composition Limits for Wrought
Aluminum
and Wrought Aluminum Alloys," or "Registration Record of Aluminum Association
Alloy
Designations and Chemical Compositions Limits for Aluminum Alloys in the Form
of Castings
and Ingot," both published by The Aluminum Association.
[0033] As used herein, the meaning of "a," "an," or "the" includes singular
and plural
references unless the context clearly dictates otherwise.
[0034] As used herein, a plate generally has a thickness of greater than
approximately 15 mm.
For example, a plate may refer to an aluminum product having a thickness of
greater than
-4-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
approximately 15 mm, greater than approximately 20 mm, greater than
approximately 25 mm,
greater than approximately 30 min, greater than approximately 35 mm, greater
than
approximately 40 mm, greater than approximately 45 mm, greater than
approximately 50 mm,
or greater than approximately 100 mm.
[0035] As used herein, a shate (also referred to as a sheet plate) generally
has a thickness of
from approximately 4 mm to approximately 15 mm. For example, a shate may have
a thickness
of approximately 4 mm, approximately 5 mm, approximately 6 mm, approximately 7
mm,
approximately 8 mm, approximately 9 mm, approximately 10 mm, approximately 11
mm,
approximately 12 mm, approximately 13 mm, approximately 14 mm, or
approximately 15 mm.
[0036] As used herein, a sheet generally refers to an aluminum product having
a thickness of
less than approximately 4 mm (e.g., less than 3 mm, less than 2 mm, less than
1 mm, less than
0.5 min, less than 0.3 mm, or less than 0.1 mm). For example, a sheet may have
a thickness of
approximately 0.1 mm, approximately 0.2 mm, approximately 0.3 mm,
approximately 0.4 mm,
approximately 0.5, approximately 0.6 mm, approximately 0.7 mm, approximately
0.8 mm,
approximately 0.9 mm, approximately 1 mm, approximately 1.1 mm, approximately
1.2 mm,
approximately 1.3 mm, approximately 1.4 mm, approximately 1.5 mm,
approximately 1.6 mm,
approximately 1.7 mm, approximately 1.8 mm, approximately 1.9 mm,
approximately 2 mm,
approximately 2.1 mm, approximately 2.2 mm, approximately 2.3 mm,
approximately 2.4 mm,
approximately 2.5 mm, approximately 2.6 mm, approximately 2.7 mm,
approximately 2.8 mm,
approximately 2.9 mm, approximately 3 mm, approximately 3.1 mm, approximately
3.2 mm,
approximately 3.3 mm, approximately 3.4 mm, approximately 3.5 mm,
approximately 3.6 mm,
approximately 3.7 mm, approximately 3.8 mm, or approximately 3.9 mm.
[0037] As used herein, formability refers to the ability of a material to
undergo deformation
into a desired shape without fracturing, tearing-off, necking, earing, or
shaping errors such as
wrinkling, spring-back, or galling occurring. In some cases, formability may
be classified
according to deformation modes. Examples of deformation modes include drawing,
stretching,
bending, and stretch-flanging.
[0038] Reference may be made in this application to alloy temper or condition.
For an
understanding of the alloy temper descriptions most commonly used, see
"American National
Standards (ANSI) H35 on Alloy and Temper Designation Systems." An F condition
or temper
refers to an aluminum alloy as fabricated. An 0 condition or temper refers to
an aluminum
alloy after annealing. An Hxx condition or temper, also referred to herein as
an H temper,
refers to a non-heat treatable aluminum alloy after cold rolling with or
without thermal
treatment (e.g., annealing). Suitable H tempers include HX1, HX2, HX3 HX4,
HX5, HX6,
-5-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
HX7, HX8, or HX9 tempers. A Ti condition or temper refers to an aluminum alloy
cooled
from hot working and naturally aged (e.g., at room temperature). A T2
condition or temper
refers to an aluminum alloy cooled from hot working, cold worked and naturally
aged. A T3
condition or temper refers to an aluminum alloy solution heat treated, cold
worked, and
.. naturally aged. A T4 condition or temper refers to an aluminum alloy
solution heat treated and
naturally aged. AT5 condition or temper refers to an aluminum alloy cooled
from hot working
and artificially aged (at elevated temperatures). A T6 condition or temper
refers to an
aluminum alloy solution heat treated and artificially aged. A 17 condition or
temper refers to
an aluminum alloy solution heat treated and artificially overaged. A T8
condition or temper
refers to an aluminum alloy solution heat treated, cold worked, and
artificially aged. A T9
condition or temper refers to an aluminum alloy solution heat treated,
artificially aged, and cold
worked. A W condition or temper refers to an aluminum alloy after solution
heat treatment.
[00391 As used herein, the "forming temper- refers to a temper in which the
aluminum alloy
can be deformed to a greater extent than a high strength temper. For example,
a 6xxx series
aluminum alloy can be deformed to a greater extent in a 14 temper than a16
temper; thus, the
14 temper can be referred to as a forming temper in this example.
[00401 As used herein, the "high strength temper- refers to a temper in which
the aluminum
alloy is artificially aged to peak age strength. For example, a 6xxx series
aluminum alloy can
be solution heat treated and artificially aged to a 16 temper to obtain a peak
age strength.
Additionally, exemplary high strength tempers can include 16, 17, 18, or 19
tempers.
[0041] As used herein, the meaning of "room temperature" can include a
temperature of from
about 15 C to about 30 C, for example about 15 CC, about 16 C, about 17 CC,
about 18 C,
about 19 C, about 20 C, about 21 C, about 22 C, about 23 C, about 24 C,
about 25 C,
about 26 C, about 27 C, about 28 C, about 29 C, or about 30 'C.
[00421 All ranges disclosed herein are to be understood to encompass both
endpoints and any
and all subranges subsumed therein. For example, a stated range of "1 to 10-
should be
considered to include any and all subranges between (and inclusive of) the
minimum value of
1 and the maximum value of 10; that is, all subranges beginning with a minimum
value of 1 or
more, e.g. Ito 6.1, and ending with a maximum value of 10 or less, e.g., 5.5
to 10. All values
modified by "approximately" include the exact value as well.
-6-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
Aluminum Alloys and Articles
[0043] Aluminum alloy properties are partially determined by the composition
of the
aluminum alloys. In certain aspects, the alloy composition may influence or
even determine
whether the alloy will have properties adequate for a desired forming
application.
[0044] The aluminum alloy articles described herein can be made of any
suitable aluminum
alloy, so long as the alloy contains Ma or is modified to contain Ma,
including 5xxx series
aluminum alloys or 7x.-xx series aluminum alloys.
[0045] Suitable 5xxx series aluminum alloys include, for example, AA5017,
AA5018,
AA5018A, AA5018B, AA5019, AA5019A, AA5119, AA5119A, AA5021, AA5022, AA5023,
AA5024, AA5026, AA5027, AA5028, AA5041, AA5052õkA5049, AA5149, AA5249,
AA5349, AA5449, AA5449A, AA5051, AA5051A, AA5151, AA5251, AA5251A, AA5351,
AA5451, AA5052, AA5252, AA5352, AA5154, AA5154A, AA5154B, AA5154C, AA5254,
AA5354, AA5454, AA5554, AA5454õkA5454A, AA5754, AA5854, AA5954õkA5056,
AA5356õkA5356AõkA5456, AA5456A, AA5456B, AA5556, AA5556A, AA5556B,
AA5556C, AA5058, AA5059, AA5070, AA5180, AA5180A, AA5082, AA5182, AA5083,
AA5183, AA5183A, AA5283, AA5283A, AA5283B, AA5383, AA5483, AA5086, AA6186,
AA6087, AA5187, and AA5088.
[0046] Suitable 7xxx series aluminum alloys include, for example, AA7004,
AA7204,
AA7009, AA7010, AA7012, AA7014, AA7015, AA7017, AA7019, AA7019A, AA7022,
AA7122, AA7023, AA7028, AA7029õkA7129, AA7229, AA7032, AA7033õkA7034,
AA7035, AA7035A, AA7036, AA7136, AA7037, AA7039, AA7040, AA7140, AA7041,
AA7042, AA7049, AA7049A, AA7349, AA7449, AA7050, AA7050A, AA7150, AA7055,
AA7155, AA7255, AA7056, AA7060, AA7160, AA7064, AA7068, AA7168, AA7075,
AA7I75, AA7475õkA7076, AA7178, AA7278, AA7278AõkA7081, AA7181, AA7090,
AA7093, AA7095, AA7097, AA7099, and AA7199.
[0047] In addition to the above alloy which comprise Ma in an amount of
approximately 2 %
by weight or greater, any alloy may be used so long as Mg is added to the
alloy, e.g., by adding
Mg to the alloy when the alloy is in molten form.
[0048] In some cases, the aluminum alloy includes a non-heat treatable alloy.
For example, the
alloy can include a lxxx series aluminum alloy, a 3xxx series aluminum alloy,
a 4xxx series
aluminum alloy, or a 5xxx series aluminum alloy other than those described
above. The lxxx,
3xxx, 4xxx, or 5xxx series aluminum alloys can be modified to include an
amount of Mg as
described above.
-7-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
[0049] Suitable lxxx series aluminum alloys include, for example, AA1050,
AAI060,
AA1070, AA1100, AA1100A, AA1200, AA1200A, AA1300, AA1110, AA1120, AA1230,
AA1230AõkA1235, AA1435, AA1145, AA1345, AA1445õkA1150, AA1350, AA1350A,
AA1450, AA1370, AAI275, AA1185, AA1285, AA1385, AAI188, AA1190, AA1290,
AA1193, AA1198, and AA1199.
[0050] Suitable 3xxx series aluminum alloys include, for example,
AA3002õkA3102,
AA3003, AA3103, AA3103A, AA3103BõkA3203, AA3403, AA3004, AA3004AõkA3104,
AA3204, AA3304, AA3005, AA3005A, AA3105, AA3105A, AA3105B, AA3007, AA3107,
AA3207, AA3207A, AA3307, AA3009, AA3010, AA3110, AA3011, AA3012, AA3012A,
AA3013, AA3014, AA3015, AA3016, AA3017, AA3019õkA3020, AA3021, AA3025,
AA3026, AA3030, AA3130, and AA3065.
[0051] Suitable 4xxx series aluminum alloys include, for example, AA4004,
AA4104,
AA4006, AA4007, AA4008, AA4009õkA4010, AA4013, AA4014, AA4015, AA4015A,
AA4115, AA4016, AA4017, AA4018, AA4019, AA4020, AA4021, AA4026, AA4032,
AA4043, AA4043A, AA4143, AA4343, AA4643, AA4943, AA4044, AA4045, AA4145,
AA4145A, AA4046, AA4047, AA4047A, and AA4147.
[0052] In some cases, the aluminum alloy includes a heat treatable alloy. For
example, the
alloy can include a 6xxx series aluminum alloy or a 7xxx series aluminum alloy
other than
those described above. The 6xxx, or 7xxx series aluminum alloys can be
modified to include
an amount of Ma as described above.
[0053] Suitable 6xxx series aluminum alloys include, for
exampleõkA6101õkA6101A,
AA6101B, AA6201, AA620 IA, AA6401, AA6501, AA6002, AA6003, AA6103, AA6005,
AA6005A, AA6005B, AA6005C, AA6105, AA6205, AA6305, AA6006, AA6106, AA6206,
AA6306, AA6008õkA6009, AA6010, AA6110, AA6110AõkA6011, AA6111, AA6012,
AA6012AõkA6013, AA6113, AA6014, AA6015, AA6016, AA6016A, AA6116, AA6018,
AA6019, AA6020, AA6021, AA6022, AA6023, AA6024, AA6025, AA6026, AA6027,
AA6028, AA6031, AA6032, AA6033, AA6040, AA6041, AA6042, AA6043, AA6151,
AA6351, AA6351A, AA6451, AA6951õkA6053, AA6055, AA6056, AA6156õkA6060,
AA6160, AA6260, AA6360, zkA6460, AA6460B, AA6560, AA6660, AA6061õkA6061A,
AA6261, AA6361, AA6162, AA6262, AA6262A, AA6063, AA6063A, AA6463, AA6463A,
AA6763, A6963, AA6064, AA6064A, AA6065, AA6066õkA6068, AA6069, AA6070,
AA6081, AA6181, AA6181A, AA6082õkA6082A, AA6182, AA6091, and AA6092.
[0054] In some cases, the properties of the alloys can be achieved at least in
part due to the
elemental composition of the alloys. In some embodiments, the aluminum alloys
can be heat
-8-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
treatable, age hardenable alloys. Optionally, the aluminum alloys can be
aluminum alloys
classified as 5xxx series aluminum alloys (e.g., wherein Mg is the predominant
alloying
element) or 7xxx series aluminum alloys (e.g., wherein zinc is a predominant
alloying
element). In some cases, the aluminum alloys can be modified lxxx series, 2xxx
series, 3xxx
series, 4xxx series, 5xxx series, 6xxx series, or 7xxx series aluminum alloys.
In some specific
aspects, the aluminum alloy is a 5xxx series aluminum alloy or a 7xxx series
aluminum alloy
comprising at least 2 % by weight Mg. As used herein, the term -modified" as
related to a
series of aluminum alloys refers to an alloy composition that would typically
be classified
within a particular series, but the modification of one or more elements
(types or amounts)
results in a different predominant alloying element, e.g., magnesium.
[0055] In some embodiments, the composition of an aluminum alloy may affect
its response
to the continuous casting processes. For example, the strength during or after
continuous
casting may be affected by an amount of Mg present in the alloy.
[0056] Aluminum alloy articles, formed by the calcium addition process
described herein,
surprisingly and unexpectedly had less exudates, smaller exudates, or both,
than articles formed
without such calcium addition. Additionally, the alloys had a more uniform
surface with less
open porosity that is associated with exudates. The intermetallic particles
were also small and
well dispersed. In some aspects, the articles formed by the processes
described herein resulted
in at least a 10 % reduction in the number of exudates, e.g., at least 20 %,
at least 30%, at least
40 %, at least 50 %, at least 60%, or at least 70 %, when compared to articles
formed by the
same process except for the Ca addition. Similarly, in some aspects, the
articles formed by the
processes described herein resulted in at least a 10 % reduction in the size
of exudates, e.g., at
least 20 ,4>, at least 30 ,4>, at least 40 ,4>, at least 50 %, at least
60%, or at least 70 %, when
compared to articles formed by the same process except for the Ca addition.
Exeinplary Aluminum Alloys
[0057] In some embodiments, the aluminum alloy articles described herein can
be made from
1 xxx series, 2xxx series, 3xxx series, 4xxx series, 5xxx series, 6xxx series,
or 7xxx series
aluminum alloys. In certain aspects, the alloys exhibit high strength, high
formability, and
corrosion resistance.
[0058] In some aspects, the aluminum alloy (as modified) includes Mg in an
amount from
approximately 0.3 % to approximately 10 %, from approximately 0.5 % to
approximately 10
A, from approximately 0.7 `',/,') to 10 %, from approximately 1.0 'A to
approximately 10 A, from
approximately 2.0 % to approximately 10 % (e.g., from 2.25 % to 10 %, from 2.5
% to 10 %,
from 2.5 % to 9 %, from 2.5 % to 8 %, from 2.5 % to 7.5 %, or from 2.5 % to 7
%) based on
-9-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
the total weight of the alloy. For example, the alloy can include
approximately 0.3 %,
approximately 0.4 "A, approximately 0.5%, approximately 0.6 %, approximately
0.7 %,
approximately 0.8 %, approximately 0.9 %, approximately 1.0 %, approximately
1.1 %,
approximately 1.2 %, approximately 1.3 %, approximately 1.4 %, approximately
1.5 %,
approximately 1.6 %, approximately 1.7%, approximately 1.8 4%, approximately
1.9 %,
approximately 2 %, approximately 2.1 %, approximately 2.2 %, approximately 2.3
%,
approximately 2.4 %, approximately 2.5 %, approximately 2.6 A, approximately
2.7 %,
approximately 2.8 %, approximately 2.9 %, approximately 3 A), approximately
3.1 %,
approximately 3.2 A, approximately 3.3 4%, approximately 3.4 %, approximately
3.5 %,
approximately 3.6 A, approximately 3.7 A, approximately 3.8 %, approximately
3.9 %,
approximately 4 %, approximately 4.1 'A, approximately 4.2 A, approximately
4.3 %,
approximately 4.4 'A, approximately 4.5 %, approximately 4.6 ,4>,
approximately 4.7 %,
approximately 4.8 'A, approximately 4.9 %, approximately 5 A, approximately
5.1 %,
approximately 5.2 %, approximately 5.3 %, approximately 5.4 %, approximately
5.5 %,
approximately 5.6 A), approximately 5.7 %, approximately 5.8 %, approximately
5.9 %,
approximately 6 %, approximately 6.1 %, approximately 6.2 %, approximately 6.3
%,
approximately 6.4 A, approximately 6.5 A, approximately 6.6 'A,
approximately 6.7 %,
approximately 6.8 %, approximately 6.9 A, approximately 7 %, approximately
7.1 %,
approximately 7.2 %, approximately 7.3 %, approximately 7.4 %, approximately
7.5 %,
approximately 7.6 %, approximately 7.7 %, approximately 7.8 %, approximately
7.9 /0,
approximately 8 %, approximately 8.1 %, approximately 8.2 %, approximately 8.3
%,
approximately 8.4 %, approximately 8.5 'A, approximately 8.6 %, approximately
8.7 %,
approximately 8.8 %, approximately 8.9 A, approximately 9 %, approximately
9.1 %,
approximately 9.2 %, approximately 9.3 %, approximately 9.4 A, approximately
9.5 %,
approximately 9.6 /,'), approximately 9.7 A, approximately 9.8 %,
approximately 9.9 A, or
approximately 10 'A Mg. All expressed in wt. %.
[0059] In some aspects, the aluminum alloy includes manganese (Mn) in an
amount from 0 %
to approximately 2% (e.g., from 0.01 A to 2%, from 0.05% to 1.75%, from 0.1 %
to 1.5%,
or from 0.25 % to 1 %) based on the total weight of the alloy. For example,
the alloy can
include 0 %, approximately 0.05 'A, approximately 0.1 %, approximately 0.15 %,
approximately 0.2 %, approximately 0.25 A, approximately 0.3 %, approximately
0.35 A,
approximately 0.4 %, approximately 0.45 %, approximately 0.5 %, approximately
0.55 %,
approximately 0.6 %, approximately 0.65 %, approximately 0.7 %, approximately
0.75 %,
approximately 0.8 %, approximately 0.85 %, approximately 0.9 %, approximately
0.95 %,
-10-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
approximately 1 'A, approximately 1.05 %, approximately 1.1 %, approximately
1.15 %,
approximately 1.2 %, approximately 1.25 %, approximately 1.3 'A, approximately
1.35 %,
approximately 1.4 A, approximately 1.45 %, approximately 1.5 %, approximately
1.55 %,
approximately 1.6 %, approximately 1.65 %, approximately 1.7 %, approximately
1.75 %,
approximately 1.8 'A, approximately 1.85 %, approximately 1.9 ?A,
approximately 1.95 %, or
approximately 2 % Mn. In certain aspects, Mn is not present in the alloy
(i.e., 0 %). All
expressed in wt. %.
[0060] In some aspects, the aluminum alloy includes chromium (Cr) in an amount
from 0 %
to approximately 2 % (e.g., from 0.01 % to 2 %, from 0.05 % to 1.75%, from 0.1
'A to 1.5%,
or from 0.15 % to 1 %) based on the total weight of the alloy. For example,
the alloy can
include 0 %, approximately 0.05 %, approximately 0.1 %, approximately 0.15 %,
approximately 0.2 %, approximately 0.25 %, approximately 0.3 %, approximately
0.35 %,
approximately 0.4 %, approximately 0.45 A, approximately 0.5 A,
approximately 0.55 e/o,
approximately 0.6 %, approximately 0.65 %, approximately 0.7 'A, approximately
0.75 %,
approximately 0.8 %, approximately 0.85 %, approximately 0.9 A, approximately
0.95 %,
approximately 1 %, approximately 1.05 ?A, approximately 1.1 ?A, approximately
1.15 %,
approximately 1.2 %, approximately 1.25 A, approximately 1.3 `,Y0,
approximately 1.35 %,
approximately 1.4 %, approximately 1.45 %, approximately 1.5 %, approximately
1.55 %,
approximately 1.6 %, approximately 1.65 %, approximately 1.7 %, approximately
1.75 %,
approximately 1.8 A, approximately 1.85 A, approximately 1.9 %,
approximately 1.95 A, or
approximately 2% Cr. In certain aspects, Cr is not present in the alloy (i.e.,
0 %). All expressed
in wt. %.
[0061] In some aspects, the aluminum alloy includes copper (Cu) in an amount
from 0 ?A to
approximately 2.5 % (e.g., from 0.01 ',./0 to 2.25 %, from 0.02 % to 2 %, from
0.03 % to 1.5%,
or from 0.04 % to 1 A) based on the total weight of the alloy. For example,
the alloy can
include 0 %, approximately 0.01 %, approximately 0.02 %, approximately 0.03 %,
approximately 0.04 ',A, approximately 0.05 ?A, approximately 0.06 'A,
approximately 0.07 %,
approximately 0.08 %, approximately 0.09 %, approximately 0.1 %, approximately
0.15 A,
approximately 0.2 %, approximately 0.25 %, approximately 0.3 'A, approximately
0.35 %,
approximately 0.4 %, approximately 0.45 %, approximately 0.5 'A, approximately
0.55 %,
approximately 0.6 'A, approximately 0.65 %, approximately 0.70 %,
approximately 0.75 %,
approximately 0.8 %, approximately 0.85 %, approximately 0.9 %, approximately
0.95 %,
approximately 1 %, approximately 1.05 %, approximately 1.1 %, approximately
1.15 %,
approximately 1.2 %, approximately 1.25 %, approximately 1.3 %, approximately
1.35 %,
-I1-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
approximately 1.4 %, approximately 1.45 %, approximately 1.5 %, approximately
1.55 %,
approximately 1.6 %, approximately 1.65 %, approximately 1.7 'A, approximately
1.75 %,
approximately 1.8 A, approximately 1.85 %, approximately 1.9 %, approximately
1.95 %,
approximately 2 %, approximately 2.05 %, approximately 2.1, approximately 2.15
%,
approximately 2.2 %, approximately 2.25 %, approximately 2.3 'A, approximately
2.35 %,
approximately 2.4 %, approximately 2.45 %, or approximately 2.5 % Cu. In
certain aspects,
Cu is not present in the alloy (i.e., 0 %). All expressed in wt. %.
[0062] In some aspects, the aluminum alloy includes silicon (Si) in an amount
from 0 % to
approximately 2 % (e.g., from 0.01 % to 2 %, from 0.05 % to 1.75%, from 0.1 %
to 1.5%, or
from 0.15 % to 1 %) based on the total weight of the alloy. For example, the
alloy can include
0 'A, approximately 0.05 %, approximately 0.1 'A, approximately 0.15 %,
approximately 0.2
%, approximately 0.25 'A, approximately 0.3 %, approximately 0.35 %,
approximately 0.4 %,
approximately 0.45 %, approximately 0.5 %, approximately 0.55 %, approximately
0.6 %,
approximately 0.65 A, approximately 0.7 %, approximately 0.75 %,
approximately 0.8 %,
approximately 0.85 'A, approximately 0.9 %, approximately 0.95 %,
approximately 1 %,
approximately 1.05 "/0, approximately 1.1 %, approximately 1.15 %,
approximately 1.2 'A,
approximately 1.25 'A, approximately 1.3 %, approximately 1.35 A,
approximately 1.4 %,
approximately 1.45 approximately 1.5 %, approximately 1.55 A,
approximately 1.6 %,
approximately 1.65 %, approximately 1.7 %, approximately 1.75 %, approximately
1.8 %,
approximately 1.85 A, approximately 1.9 %, approximately 1.95 %, or
approximately 2 % Si.
In certain aspects, Si is not present in the alloy (i.e., 0 `',/). All
expressed in wt. %.
[0063] In some aspects, the aluminum alloy includes iron (Fe) in an amount
from 0 % to
approximately 2 % (e.g., from 0.01 % to 2 %, from 0.05 % to 1.75%, from 0.1 %
to 1.5%, or
from 0.15 % to 1 %) based on the total weight of the alloy. For example, the
alloy can include
0 %, approximately 0.05 %, approximately 0.1 %, approximately 0.15 %,
approximately 0.2
%, approximately 0.25 'A, approximately 0.3 %, approximately 0.35 %,
approximately 0.4 %,
approximately 0.45 %, approximately 0.5 %, approximately 0.55 %, approximately
0.6 %,
approximately 0.65 %, approximately 0.7 %, approximately 0.75 %, approximately
0.8 %,
approximately 0.85 %, approximately 0.9 %, approximately 0.95 %, approximately
1 %,
approximately 1.05 A, approximately 1.1 %, approximately 1.15 %,
approximately 1.2 'A,
approximately 1.25 %, approximately 1.3 %, approximately 1.35 %, approximately
1.4 %,
approximately 1.45 approximately 1.5 %, approximately 1.55 %, approximately
1.6 %,
approximately 1.65 %, approximately 1.7 %, approximately 1.75 %, approximately
1.8 %,
-12-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
approximately 1.85 %, approximately 1.9 %, approximately 1.95 %, or
approximately 2 % Fe.
In certain aspects, Fe is not present in the alloy (i.e., 0 %). All expressed
in wt. %.
[0064] In some aspects, the aluminum alloy includes zinc (Zn) in an amount
from 0 'A to
approximately 10 % (e.g., from 0.01 % to 10 %, from 0.05 % to 9%, from 0.1 %
to 9 %, or
from 0.15 % to 9 A) based on the total weight of the alloy. For example, the
alloy can include
0 A, approximately 0.01 A, approximately 0.02 A, approximately 0.03 A,
approximately 0.04
%, approximately 0.05 'A, approximately 0.06 %, approximately 0.07 A,
approximately 0.08
"Yo, approximately 0.09 ?A, approximately 0.1 %, approximately 0.15 A,
approximately 0.2 %,
approximately 0.25 A, approximately 0.3 %, approximately 0.35 %,
approximately 0.4 %,
approximately 0.45 %, approximately 0.5 A, approximately 0.55 %,
approximately 0.6 %,
approximately 0.65 A, approximately 0.70 A, approximately 0.75 %,
approximately 0.8 %,
approximately 0.85 %, approximately 0.9 %, approximately 0.95 %, approximately
1 %,
approximately 1.1 %, approximately 1.2 %, approximately 1.3 %, approximately
1.4 A,
approximately 1.5 %, approximately 1.6 %, approximately 1.7 %, approximately
1.8 %,
approximately 1.9 %, approximately 2 %, approximately 2.1 %, approximately 2.2
%,
approximately 2.3 %, approximately 2.4 %, approximately 2.5 %, approximately
2.6 %,
approximately 2.7 %, approximately 2.8 %, approximately 2.9 %, approximately 3
%,
approximately 3.1 %, approximately 3.2 %, approximately 3.3 %, approximately
3.4 A,
approximately 3.5 %, approximately 3.6 %, approximately 3.7 %, approximately
3.8 %,
approximately 3.9 %, approximately 4 %, approximately 4.1 A, approximately
4.2 %,
approximately 4.3 %, approximately 4.4 %, approximately 4.5 %, approximately
4.6 %,
approximately 4.7 %, approximately 4.8 %, approximately 4.9 %, approximately 5
%,
approximately 5.1 %, approximately 5.2 %, approximately 5.3 %, approximately
5.4 %,
approximately 5.5 A, approximately 5.6 %, approximately 5.7 %, approximately
5.8 %,
approximately 5.9 approximately 6 %, approximately 6.1 %, approximately 6.2
%,
approximately 6.3 %, approximately 6.4 %, approximately 6.5 %, approximately
6.6 %,
approximately 6.7 %, approximately 6.8 %, approximately 6.9 %, approximately 7
%,
approximately 7.1 %, approximately 7.2 %, approximately 7.3 %, approximately
7.4 A,
approximately 7.5 %, approximately 7.6 %, approximately 7.7 %, approximately
7.8 %,
approximately 7.9 %, approximately 8 %, approximately 8.1 %, approximately 8.2
%,
approximately 8.3 %, approximately 8.4 %, approximately 8.5 %, approximately
8.6 %,
approximately 8.7 %, approximately 8.8 'A, approximately 8.9 %, approximately
9 %,
approximately 9.1 %, approximately 9.2 %, approximately 9.3 %, approximately
9.4 %,
approximately 9.5 %, approximately 9.6 %, approximately 9.7 ,4>,
approximately 9.8 %,
-13-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
approximately 9.9 %, or approximately 10 % Zn. In certain aspects, Zn is not
present in the
alloy (i.e., 0 'N). All expressed in wt. %.
[0065] In some aspects, the aluminum alloy includes zirconium (Zr) in an
amount from 0 'A to
approximately 0.5 % (e.g., from 0 % to 0.45 %, from 0.01 % to 0.4 %, from 0.01
% to 0.35 %,
from 0.01 'A to 0.2 %, or from 0.02 % to 0.1 A) based on the total weight of
the alloy. For
example, the alloy can include 0%, approximately 0.001 %, approximately 0.002
%,
approximately 0.003 A, approximately 0.004 %, approximately 0.005 A,
approximately 0.006
%, approximately 0.007 %, approximately 0.008 %, approximately 0.009 %,
approximately
0.01 %, approximately 0.02 %, approximately 0.03 %, approximately 0.04 'A,
approximately
0.05 A, approximately 0.06 %, approximately 0.07 %, approximately 0.08 %,
approximately
0.09 A, approximately 0.1 %, approximately 0.11 %, approximately 0.12 %,
approximately
0.13 'A, approximately 0.14 A, approximately 0.15 %, approximately 0.16 %,
approximately
0.17 %, approximately 0.18 %, approximately 0.19 %, approximately 0.20 A,
approximately
0.21 %, approximately 0.22
approximately 0.23 A, approximately 0.24 'A, approximately
0.25 ?A, approximately 0.26 %, approximately 0.27 %, approximately 0.28 %,
approximately
0.29 %, approximately 0.30 ?4), approximately 0.31 %, approximately 0.32 %,
approximately
0.33 A, approximately 0.34 %, approximately 0.35 %, approximately 0.36 %,
approximately
0.37 %, approximately 0.38 A, approximately 0.39 %, approximately 0.40 %,
approximately
0.41 'A, approximately 0.42 %, approximately 0.43 %, approximately 0.44 %,
approximately
0.45 %, approximately 0.46 %, approximately 0.47 %, approximately 0.48 A,
approximately
0.49 %, or approximately 0.50 'A Zr. All expressed in wt. %.
[0066] In some aspects, the aluminum alloy includes nickel (Ni) in an amount
up to
approximately 0.5 % (e.g., from 0 % to approximately 0.5 %, from approximately
0.01 'A to
approximately 0.4 %, from approximately 0.01 % to approximately 0.35 %, from
approximately 0.01 `',/,') to approximately 0.2 %, or from approximately 0.02
`',/,') to approximately
0.1 %) based on the total weight of the alloy. For example, the alloy can
include approximately
0.001 %, approximately 0.002 %, approximately 0.003 %, approximately 0.004 %,
approximately 0.005 %, approximately 0.006 %, approximately 0.007 %,
approximately 0.008
%, approximately 0.009 %, approximately 0.01 %, approximately 0.02 A,
approximately 0.03
A, approximately 0.04 'A, approximately 0.05 %, approximately 0.06 %,
approximately 0.07
%, approximately 0.08 %, approximately 0.09 %, approximately 0.1 %,
approximately 0.11 A,
approximately 0.12 %, approximately 0.13 A, approximately 0.14 %,
approximately 0.15 %,
approximately 0.16 %, approximately 0.17 %, approximately 0.18 %,
approximately 0.19 %,
approximately 0.20 %, approximately 0.21 %, approximately 0.22 'A,
approximately 0.23 %,
-14-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
approximately 0.24 %, approximately 0.25 %, approximately 0.26 ?/0,
approximately 0.27 %,
approximately 0.28 %, approximately 0.29 %, approximately 0.30 %,
approximately 0.31 %,
approximately 0.32
approximately 0.33 %, approximately 0.34 'A, approximately 0.35 %,
approximately 0.36 %, approximately 0.37 'A, approximately 0.38 %,
approximately 0.39 %,
approximately 0.40 %, approximately 0.41 %, approximately 0.42 'A,
approximately 0.43 %,
approximately 0.44 %, approximately 0.45 %, approximately 0.46 %,
approximately 0.47 %,
approximately 0.48 %, approximately 0.49 %, or approximately 0.50 % Ni. All
expressed in
wt. %.
[0067] In
certain aspects, the aluminum alloy includes tin (Sn) in an amount up to
.. approximately 0.25 % (e.g., from 0 % to approximately 0.25 %, from 0 % to
approximately 0.2
%, from 0 A to approximately 0.05 %, from approximately 0.01 % to
approximately 0.15 %,
or from approximately 0.01 A to approximately 0.1 %) based on the total
weight of the alloy.
For example, the alloy can include approximately 0.001 A, approximately 0.002
A,
approximately 0.003 %, approximately 0.004 %, approximately 0.005 %,
approximately 0.006
'A, approximately 0.007 %, approximately 0.008 %, approximately 0.009 %,
approximately
0.01 %, approximately 0.02 %, approximately 0.03 %, approximately 0.04 'A,
approximately
0.05 A, approximately 0.06 %, approximately 0.07 %, approximately 0.08 %,
approximately
0.09 A, approximately 0.1 A, approximately 0.11 A, approximately 0.12 A,
approximately
0.13 'A, approximately 0.14 %, approximately 0.15 %, approximately 0.16 ?/0,
approximately
0.17 %, approximately 0.18 %, approximately 0.19 %, approximately 0.20 A,
approximately
0.21 'A, approximately 0.22 %, approximately 0.23 'A, approximately 0.24%, or
approximately
0.25 %, Sn. All expressed in wt. %.
[0068] In
certain aspects, the aluminum alloy includes titanium (Ti) in an amount up to
approximately 0.1 % (e.g., from 0.01 ',./0 to 0.1 %,) based on the total
weight of the alloy. For
example, the alloy can include approximately 0.001 A, approximately 0.002 %,
approximately
0.003 %, approximately 0.004 %, approximately 0.005 %, approximately 0.006 %,
approximately 0.007 %, approximately 0.008 A, approximately 0.009 'A,
approximately 0.01
%, approximately 0.011 %, approximately 0.012 %, approximately 0.013 A,
approximately
0.014 %, approximately 0.015
approximately 0.016 %, approximately 0.017 %,
approximately 0.018 'A, approximately 0.019 %, approximately 0.02 %,
approximately 0.021
%, approximately 0.022 %, approximately 0.023 %, approximately 0.024 %,
approximately
0.025 %, approximately 0.026 %, approximately 0.027 %, approximately 0.028 %,
approximately 0.029 ?/0, approximately 0.03 %, approximately 0.031 %,
approximately 0.032
%, approximately 0.033 A, approximately 0.034 A, approximately 0.035 %,
approximately
-15-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
0.036 %, approximately 0.037 %, approximately 0.038 %, approximately 0.039 %,
approximately 0.04 %, approximately 0.05 %, approximately 0.051 'A,
approximately 0.052
%, approximately 0.053 A, approximately 0.054 A, approximately 0.055 %,
approximately
0.056 %, approximately 0.057 %, approximately 0.058 %, approximately 0.059 %,
approximately 0.06 %, approximately 0.07 %, approximately 0.08 'A,
approximately 0.09 %,
approximately 0.099 A, or approximately 0.1 % Ti. All expressed in wt. A.
[0069] Optionally, the aluminum alloy compositions can further include other
minor elements,
sometimes referred to as impurities, in amounts of approximately 0.05 % or
below,
approximately 0.04 'A or below, approximately 0.03 % or below, approximately
0.02 A or
below, or approximately 0.01 % or below each. These impurities may include,
but are not
limited to, V, Ga, Hf, Sr, or combinations thereof. Accordingly, V, Ga, Hf, or
Sr may be present
in an alloy in amounts of approximately 0.05 % or below, approximately 0.04 %
or below,
approximately 0.03 A or below, approximately 0.02 A or below, or
approximately 0.01 A or
below. In certain aspects, the sum of all impurities does not exceed
approximately 0.15 %
(e.g., approximately 0.1 %). All expressed in wt. %. In certain aspects, the
remaining
percentage of the alloy is aluminum.
[0070] The aluminum alloy may be substantially free of beryllium (Be), e.g.,
contains
approximately 0.01 % Be or below, approximately 0.009 A, approximately 0.008
%,
approximately 0.007 %, approximately 0.006 'A, approximately 0.005 %,
approximately 0.004
%, approximately 0.003 %, approximately 0.002 %, approximately 0.001 A,
approximately
0.0009 approximately 0.0008%, approximately 0.0007 %, approximately
0.0006 %,
approximately 0.0005 %, approximately 0.0004 %, approximately 0.0003 %,
approximately
0.002 %, approximately 0.0001 %, or 0 % Be.
[0071] As described below, once the aluminum alloy is in molten form, calcium
(Ca) is added
to the molten alloy. In molten form, the aluminum alloy includes Ca in an
amount up to
approximately 500 ppm (e.g., from 30 ppm to 500 ppm, from 40 ppm to 500 ppm,
from 50
ppm to 500 ppm, from 50 ppm to 400 ppm, or from 50 ppm to 250 ppm) based on
the total
weight of the alloy. For example, the alloy can include approximately 30 ppm,
approximately
ppm, approximately 40 ppm, approximately 45 ppm, approximately 50 ppm,
approximately
30 55 ppm, approximately 60 ppm, approximately 65 ppm, approximately 70
ppm, approximately
75 ppm, approximately 80 ppm, approximately 85 ppm, approximately 90 ppm,
approximately
95 ppm, approximately 100 ppm, approximately 105 ppm, approximately 110 ppm,
approximately 115 ppm, approximately 120 ppm, approximately 125 ppm,
approximately 130
ppm, approximately 135 ppm, approximately 140 ppm, approximately 145 ppm,
approximately
-16-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
150 ppm, approximately 155 ppm, approximately 160 ppm, approximately 165 ppm,
approximately 170 ppm, approximately 175 ppm, approximately 180 ppm,
approximately 185
ppm, approximately 190 ppm, approximately 195 ppm, approximately 200 ppm,
approximately
205 ppm, approximately 210 ppm, approximately 215 ppm, approximately 220 ppm,
approximately 225 ppm, approximately 230 ppm, approximately 235 ppm,
approximately 240
ppm, approximately 245 ppm, approximately 250 ppm, approximately 255 ppm,
approximately
260 ppm, approximately 265 ppm, approximately 270 ppm, approximately 275 ppm,
approximately 280 ppm, approximately 285 ppm, approximately 290 ppm,
approximately 295
ppm, approximately 300 ppm, approximately 305 ppm, approximately 310 ppm,
approximately
315 ppm, approximately 320 ppm, approximately 325 ppm, approximately 330 ppm,
approximately 335 ppm, approximately 340 ppm, approximately 345 ppm,
approximately 350
ppm, approximately 355 ppm, approximately 360 ppm, approximately 365 ppm,
approximately
370 ppm, approximately 375 ppm, approximately 380 ppm, approximately 385 ppm,
approximately 390 ppm, approximately 395 ppm, approximately 400 ppm,
approximately 405
ppm, approximately 410 ppm, approximately 415 ppm, approximately 420 ppm,
approximately
425 ppm, approximately 430 ppm, approximately 435 ppm, approximately 440 ppm,
approximately 445 ppm, approximately 450 ppm, approximately 455 ppm,
approximately 460
ppm, approximately 465 ppm, approximately 470 ppm, approximately 475 ppm,
approximately
480 ppm, approximately 485 ppm, approximately 490 ppm, approximately 495 ppm,
or
approximately 500 ppm Ca. All expressed in ppm by weight.
Methods of Making
[0072] Methods of producing an aluminum article are also described herein. The
aluminum
alloy can be cast and then further processing steps may be performed. In some
examples, the
processing steps include an optional quenching step, a pre-heating and/or a
homogenizing step,
a hot rolling step, a solutionizing step, an artificial aging step, an
optional coating step and an
optional paint baking step.
[0073] In some examples, the method comprises casting a slab; hot rolling the
slab to produce
a hot rolled aluminum alloy in a form of a sheet, shate or plate;
solutionizing the aluminum
sheet, shate or plate; and aging the aluminum sheet, shate or plate. In some
examples, the slabs
are thermally quenched upon exit from the continuous caster. In some further
examples, the
slabs are coiled upon exit from the continuous caster. In some cases, the
coiled slabs are cooled
in air. In some instances, the method further includes preheating the coiled
slabs. In some
instances, the method further includes coating the aged aluminum sheet, shate,
or plate. In
-17-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
some further instances, the method further includes paint baking the coated
aluminum sheet,
shate, or plate. The method steps are further described below.
Casting
[0074] The alloys described herein can be cast into slabs using a continuous
casting (CC)
process. As described above, the aluminum alloy is melted and when in molten
form, Ca is
added to the alloy. If necessary, Ma may also be added to the alloy in molten
form. In some
aspects, the Mg is added within 5 hours of casting to reduce oxidation of the
Mg. The Ca may
be added to the molten alloy at any point of the process prior to casting,
including when feeding
the molten alloy to the casting device, i.e., in the trough.
[0075] The continuous casting device can be any suitable continuous casting
device. The
continuous casting process can include, but is not limited to, the use of
block casters, twin roll
casters or twin belt casters. The continuous casting can be performed at rates
up to
approximately 35 meters/minute (m/min).
[0076] The resulting cast aluminum alloy (slab) can have a thickness of
approximately 1 mm
to approximately 50 mm (e.g., from approximately 10 mm to approximately 45 mm,
from
approximately 15 mm to approximately 40 mm, or from approximately 20 mm to
approximately 35 mm), such as approximately 10 mm. For example, the resulting
slab can be
1 mm, 2 mm, 3 mm, 4 mm, 5 mm, 6 mm, 7 mm, 8 mm, 9 mm, 10 mm, 11 mm, 12 mm, 13
mm,
14 mm, 15 mm, 16 mm, 17 mm, 18 mm, 19 mm, 20 mm, 21 mm, 22 mm, 23 mm, 24 mm,
25
mm, 26 mm, 27 mm, 28 mm, 29 mm, 30 mm, 31 mm, 32 min, 33 mm, 34 mm, 35 mm, 36
mm,
37 mm, 38 mm, 39 mm, 40 mm, 41 mm, 42 mm, 43 mm, 44 mm, 45 mm, 46 mm, 47 mm,
48
mm, 49 mm, or 50 mm thick.
Quenching
[0077] The resulting slabs can optionally be thermally quenched upon exit from
the continuous
caster. In some examples, the quench is performed with water. Optionally, the
water
quenching step can be performed at a rate of up to approximately 200 cC/s (for
example, from
10 C/s to 190 'Cis, from 25 C/s to 175 C/s, from 50 'Cis to 150 C/s, from
75 C/s to 125
or from 10 C/s to 50 C/s). The water temperature can be from approximately
20 C to
approximately 75 CC (e.g., approximately 25 C, approximately 30 C,
approximately 35 C,
approximately 40 C, approximately 45 C, approximately 50 C, approximately
55 C,
approximately 60 C, approximately 65 C, approximately 70 C, or
approximately 75 C).
Optionally, the resulting slabs can be coiled upon exit from the continuous
caster. The resulting
intennediate coil can be cooled in air. The air cooling step can be performed
at a rate of
approximately 1 C/s to approximately 300 'Clday.
-18-
CA 03204304 2023-06-05
WO 2022/165492 PCT/US2022/070364
[0078] In some examples, water quenching the slab upon exit from the
continuous caster
results in an aluminum alloy slab in a T4-temper condition. After the optional
water quenching,
the slab in T4-temper can then be optionally coiled into an intermediate coil
and stored for a
time period of up to 24 hours. The defect count and the formation of exudates
(e.g., eruptions
of iron rich material) is decreased as compared to continuously cast slabs
without the addition
of the above described amounts of Ca. In some aspects, the cast aluminum alloy
comprises an
improved surface, as quantified by number or exudates, exudate size, and/or -
streaks" in the
article. Without being bound by theory, it is believed that by adding Ca to
the molten alloy,
during casting an oxide surface layer is formed, which reduces surface defects
and exudate
growth. The oxide layer thickness may be quantified using Scanning Electron
microscopy by
comparing the A1-0 ratio to standards of known oxide thickness. Direct
measurement by
Transmission Electron microscopy may also be viable. Without being bound by
theory, it is
also believed that the Ca addition may also help the slab to self-repair of
the oxide, such as
during hot rolling.
Coiling
[0079] Optionally, the slab can be coiled into an intermediate coil upon exit
from the
continuous caster. In some examples, the slab is coiled into an intermediate
coil upon exit from
the continuous caster resulting in F-temper. In some further examples, the
coil is cooled in air.
In some still further examples, the air cooled coil is stored for a period of
time. In some
examples, the intermediate coils are maintained at a temperature of
approximately 100 C to
approximately 350 C (for example, approximately 200 C or approximately 300
C). In some
further examples, the intermediate coils are maintained in cold storage to
prevent natural aging
resulting in F-temper.
Pre-Heating and/or Homogenizing
.. [0080] When stored, the intermediate coils can be optionally reheated in a
pre-heating step. In
some examples, the reheating step can include pre-heating the intermediate
coils for a hot
rolling step. In some further examples, the reheating step can include pre-
heating the
intermediate coils at a rate of up to approximately 150 C/h (for example,
approximately 10
'eh or approximately 50 C/h). The intermediate coils can be heated to a
temperature of
approximately 350 C to approximately 580 C. (e.g., approximately 375 C to
approximately
570 C, approximately 400 C to approximately 550 C, approximately 425 C to
approximately 500 C, or approximately 500 C to approximately 580 CC). The
intermediate
coils can soak for approximately 1 minute to approximately 120 minutes,
preferably
approximately 60 minutes.
-19-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
[0081] Optionally, the intermediate coils after storage and/or pre-heating of
the coils or the
slab upon exit from the caster can be homogenized. The homogenization step can
include
heating the slab or intermediate coil to attain a peak metal temperature (PMT)
of about, or at
least about, 450 CC (e.g., at least 460 'V, at least 470 C, at least 480 C,
at least 490 C, at
least 500 C, at least 510 C, at least 520 C, at least 530 C, at least 540
C, at least 550 CC,
at least 560 C, at least 570 C, or at least 580 C). For example, the cast
aluminum alloy
product can be heated to a temperature of from about 450 C to about 580 C,
from about 460
CC to about 575 C, from about 470 C. to about 570 T., from about 480 C to
about 565 C,
from about 490 C to about 555 oC, or from about 500 OC to about 550 C. In
some cases, the
heating rate to the PMT can be about 100 C/hour or less, 75 C/hour or less,
50 C/hour or
less, 40 5 cC/hour or less, 30 C/hour or less, 25 cC/hour or less, 20 C/hour
or less, or 15
C/hour or less. In other cases, the heating rate to the PMT can be from about
10 C/min to
about 100 C/min (e.g., from about 10 C/min to about 90 C/min, from about 10
C/min to
about 70 C/min, from about 10 C/min to about 60 C/min, from about 20 C/min
to about 90
C/min, from about 30 C/min to about 80 C/min, from about 40 C/min to about
70 C/min,
or from about 50 'C/min to about 60 C/min).
[0082] The cast aluminum alloy product is then allowed to soak (i.e., held at
the indicated
temperature) for a period of time. In some cases, the cast aluminum alloy
product is allowed to
soak for at least 30 minutes at a peak metal temperature as described above.
According to one
non-limiting example, the cast aluminum alloy product is allowed to soak for
up to about 36
hours (e.g., from about 30 minutes to about 36 hours, inclusively). For
example, the cast
aluminum alloy product can be soaked at the peak metal temperature for 30
minutes, 1 hour, 2
hours, 3 hours, 4 hours, 5 hours, 6 hours, 7 hours, 8 hours, 9 hours, 10
hours, 11 hours, 12
hours, 13 hours, 14 hours, 15 hours, 16 hours, 17 hours, 18 hours, 19 hours,
20 hours, 21 hours,
22 hours, 23 hours, 24 hours, 25 hours, 26 hours, 27 hours, 28 hours, 29
hours, 30 hours, 31
hours, 32 hours, 33 hours, 34 hours, 35 hours, 36 hours, or anywhere in
between.
Hot Rolling and Coiling
[0083] Following the pre-heating and/or homogenization step, a hot rolling
step can be
performed. The hot rolling step can include a hot reversing mill operation
and/or a hot tandem
mill operation. The hot rolling step can be performed at a temperature ranging
from about 250
C to about 550 C (e.g., from about 300 C to about 500 C or from about 350
C to about
450 C). In certain cases, the cast aluminum alloy product can be hot rolled
to an about 4 mm
to about 15 mm thick gauge (e.g., from about 5 mm to about 12 mm thick gauge),
which is
referred to as a shate. For example, the cast aluminum alloy product can be
hot rolled to an
-20-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
about 4 mm thick gauge, about 5 mm thick gauge, about 6 mm thick gauge, about
7 mm thick
gauge, about 8 mm thick gauge, about 9 mm thick gauge, about 10 mm thick
gauge, about 11
mm thick gauge, about 12 mm thick gauge, about 13 mm thick gauge, about 14 mm
thick
gauge, or about 15 mm thick gauge. In certain cases, the cast aluminum alloy
product can be
hot rolled to a gauge greater than 15 mm thick (i.e., a plate). In other
cases, the cast aluminum
alloy product can be hot rolled to a gauge less than 4 mm (i.e., a sheet).
[0084] At the end of the hot rolling step, optionally within the single stand
mill or a multi-stand
mill, the hot rolled product can be rolled up as a coil. The coiling
temperature can be at least
285 C and may range from about 285 C to about 450 oC (e.g., from about 285
OC to about
400 C, from about 285 C. to about 350 C, from about 300 C to about 350 C
or from about
310 C to about 330 C).
Cold Rolling
[0085] A cold rolling step can optionally be applied to the alloy to form a
final gauge product.
For example, the cast aluminum alloy product can be cold rolled to a thickness
of less than
about 4 mm. In some examples, a sheet may have a thickness of less than 4 mm,
less than 3
mm, less than 2 mm, less than 1 mm, less than 0.9 mm, less than 0.8 mm, less
than 0.7 mm,
less than 0.6 mm, less than 0.5 mm, less than 0.4 mm, less than 0.3 mm, less
than 0.2 mm, or
less than 0.1 mm. The temper of the as-rolled sheets is referred to as F-
temper.
Worming
[0086] The process described herein can optionally include at least one
deforming step applied
to the final gauge product. The term -deforming," as used herein, includes
cutting, stamping,
pressing, press-forming, drawing, shaping, straining or other processes that
can create two- or
three-dimensional shapes as known to one of ordinary skill in the art. The
deforming step can
be performed on an aluminum alloy sheet, plate, or shate that has a
temperature of about room
temperature (e.g., from about 15 C to about 30 C) (referred to as cold
forming) or that has
been heated to an elevated temperature (referred to as a warm forming process
or a hot forming
process). In some examples, a warm forming procedure can be applied to form an
aluminum
alloy product. In these examples, the warm forming can include heating the
aluminum alloy
product to a temperature of about 40 C to less than about 100 'C. In other
examples, a hot
forming procedure can be applied to form an aluminum alloy article. In these
examples, the hot
forming can include heating the aluminum alloy product to temperatures of
about 100 C. to
about 600 T., at a heating rate of about 3 cC/second to about 90 C/second,
deforming the
aluminum alloy product to form an aluminum alloy article, optionally repeating
the deforming
step and cooling the article. In some examples, a cryogenic forming procedure
can be applied
-21-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
to form an aluminum alloy product. In these examples, the cryogenic forming
can include
cooling the aluminum alloy product to a temperature of about 0 C to about -
200 'C.
[0087] The method of producing the aluminum alloy products described herein
can exclude
heat treatment steps. In some examples, the method of producing an aluminum
product
.. excludes a paint baking step. In some examples, the method of producing an
aluminum product
excludes an artificial aging step. In some examples, the method of producing
an aluminum
product excludes an annealing step.
Aging
[0088] Optionally, the hot rolled metal is subjected to an artificial aging
step. The artificial
aging step develops the high strength property of the alloys and optimizes
other desirable
properties in the alloys. The mechanical properties of the final product can
be controlled by
various aging conditions depending on the desired use. In some cases, the
metal product
described herein can be delivered to customers in a Tx temper (a Ti temper, a
14 temper, a T5
temper, a T6 temper, a 17 temper, or a T8 temper, for example), a W temper, an
0 temper, or
an F temper. In some examples, an artificial aging step can be performed. The
artificial aging
step can be performed at a temperature from approximately 100 C. to
approximately 140 C
(e.g., at approximately 120 C or at approximately 125 C). The artificial
aging step can be
performed for a period of time from approximately 12 hours to approximately 36
hours (e.g.,
for approximately 18 hours or for approximately 24 hours). In some examples,
the artificial
.. aging step can be performed at 125 C for 24 hours to result in a T6
temper. In some still
further examples, the alloys are subjected to a natural aging step. The
natural aging step can
result in a T4 temper.
Coating and/or Paint Baking
[00891 Optionally, the metal product is subjected to a coating step.
Optionally, the coating
step can include zinc phosphating (Zn-phosphating) and electrocoating (E-
coating). The Zn-
phosphating and E-coating are performed according to standards commonly used
in the
aluminum industry as known to one of skill in the art. Optionally, the coating
step can be
followed by a paint baking step. The paint baking step can be performed at a
temperature of
approximately 150 C to approximately 230 C (e.g., at approximately 180 C or
at
approximately 210 C). The paint baking step can be performed for a time
period of
approximately 10 minutes to approximately 60 minutes (e.g., approximately 30
minutes or
approximately 45 minutes).
-22-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
Properties
[0090] The resulting metal product as described herein has a combination of
desired properties,
including high strength and high formability under a variety of temper
conditions, including
Tx temper conditions (where Tx tempers can include Ti. T4, T5, T6, T7, or T8
tempers), W
temper, 0 temper, or F temper. In some examples, such as for 7xxx alloys, the
resulting metal
product has a yield strength of from approximately 400 MPa to 650 MPa (e.g.,
from 450 MPa
to 625 MPa, from 475 MPa to 600 MPa, or from 500 MPa to 575 MPa). For example,
the yield
strength can be approximately 400 MPa, 410 MPa, 420 MPa, 430 MPa, 440 MPa, 450
MPa,
460 MPa, 470 MPa, 480 MPa, 490 MPa, 500 MPa, 510 MPa, 520 MPa, 530 MPa, 540
MPa,
550 MPa, 560 MPa, 570 MPa, 580 MPa, 590 MPa, 600 MPa, 610 MPa, 620 MPa, 630
MPa,
640 MPa, or 650 MPa. Optionally, the metal product having a yield strength of
from
approximately 400 MPa to 650 MPa can be in the T6 temper. In some examples,
the resulting
metal product has a maximum yield strength of from approximately 560 MPa to
650 MPa. For
example, the maximum yield strength of the metal product can be approximately
560 MPa, 570
MPa, 580 MPa, 590 MPa, 600 MPa, 610 MPa, 620 MPa, 630 MPa, 640 MPa, or 650
MPa.
Optionally, the metal product having a maximum yield strength of from
approximately 560
MPa to 650 MPa can be in the T6 temper. Optionally, the metal product can have
a yield
strength of from approximately 500 MPa to approximately 650 MPa after paint
baking the
metal product in the T4 temper (i.e., without any artificial aging). For 5xxx
alloys, such as in
0-temper, the yield strength may be at least 100 MPA and in the H19 temper,
the yield strength
may be at least 300 MPa.
[0091] In some examples, the resulting metal product has an ultimate tensile
strength of from
approximately 500 MPa to 650 MPa (e.g., from 550 MPa to 625 MPa or from 575
MPa to 600
MPa). For example, the ultimate tensile strength can be approximately 500 MPa,
510 MPa,
520 MPa, 530 MPa, 540 MPa, 550 MPa, 560 MPa, 570 MPa, 580 MPa, 590 MPa, 600
MPa,
610 MPa, 620 MPa, 630 MPa, 640 MPa, or 650 MPa. Optionally, the metal product
having an
ultimate tensile strength of from approximately 500 MPa to 650 MPa is in the
T6 temper.
[0092] In some examples, the resulting metal product has an interior bend
angle of from
approximately 1000 to 160 (e.g., from approximately 1100 to 155 or from
approximately
120' to 150'). For example, the bend angle of the resulting metal product can
be approximately
1000, 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 ,
113 , 114 , 115 ,
1160, 117 , 118 , 119 , 120 , 1210, 122 , 123 , 124 , 125 , 126 , 127 , 128 ,
129 , 130 , 131 ,
132', 133', 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 ,
145 , 146 , 147 ,
148', 149', 150', 151 , 152 , 153 , 154 , 1550, 1560, 1570, 158 , 159 , or 160
. Optionally,
-23-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
the metal product having a bend angle of from approximately 100' to 160' can
be in the T6
temper.
Methods of Use
[0093] The alloys and methods described herein can be used in automotive
and/or
transportation applications, including motor vehicle, aircraft, and railway
applications, or any
other desired application. In some examples, the alloys and methods can be
used to prepare
motor vehicle body part products, such as bumpers, side beams, roof beams,
cross beams, pillar
reinforcements (e.g., A-pillars, B-pillars, and C-pillars), inner panels,
outer panels, side panels,
inner hoods, outer hoods, or trunk lid panels. The aluminum alloys and methods
described
herein can also be used in aircraft or railway vehicle applications, to
prepare, for example,
external and internal panels.
[0094] The alloys and methods described herein can also be used in electronics
applications.
For example, the alloys and methods described herein can also be used to
prepare housings for
electronic devices, including mobile phones and tablet computers. In some
examples, the
alloys can be used to prepare housings for the outer casing of mobile phones
(e.g., smart
phones) and tablet bottom chassis.
[0095] In some cases, the alloys and methods described herein can be used in
industrial
applications. For example, the alloys and methods described herein can be used
to prepare
products for the general distribution market.
EXAMPLES
Example 1
[0096] Eight samples were prepared based on AA5182 with the composition shown
below.
Sample A was a control, with no Ca addition (8 ppm Ca was present in the
alloy), while in
Sample B, Ca was added. Similarly, Samples C and E were controls while Sample
D had Ca
added. Each sample had a gauge of 12.6 mm. Samples A-B were cast at a speed of
3 inimin
and Samples C-E were cast at 4 nilmin. Samples AA, BB, and CC were prepared as
described
above and were cast as 3 m/min. Sample AA had 36 ppm Ca, and Samples BB and CC
were
prepared by using the composition of Sample AA and then adding Ca to provide
samples with
72 ppm Ca, and 199 ppm Ca, respectively. The Ca was added by adding a short
piece of Ca
containing rod at specified intervals until the desired Ca concentration was
reached.
-24-
CA 03204304 2023-06-05
WO 2022/165492 PCT/US2022/070364
Sample Si Fe Cu Mn Mg Ti Ca (ppm)
Comparative A 0.09 0.24 0.003 0.32 4.49 0.01 8
0.09 0.24 0.003 0.32 4.49 0.01 83
Comparative C 0.056 0.201 0.001 0.306 4.571 0.013
1
0.057 0.199 0.001 0.306 4.547 0.014 62
Comparative E 0.057 0.200 0.001 0.305 4.579 0.014
Comparative AA 0.08 0.22 0.02 0.36 4.3 0.014 36
BB 0.08 0.22 0.02 0.36 4.3 0.014 72
CC 0.08 0.22 0.02 0.36 4.3 0.014 199
[0097] Following casting, a 200 mm wide slab was then collected and
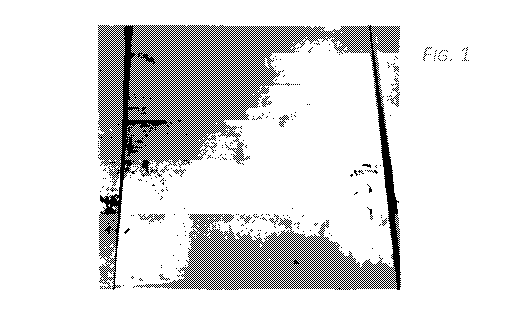
photographed. A
photograph of Sample A is shown in FIG. 1 and a photograph of Sample B is
shown in FIG. 2.
As evident from visual inspection, Sample A had a shinier surface than Sample
B, indicating a
positive effect from Ca addition. Photographs of Samples C-E are shown in FIG.
3. As shown
in FIG. 3, the exudates appeared smaller with Ca addition. FIG. 4 shows a
zoomed in
photograph of Samples C and D, with Sample C shown on the left and Sample D
shown on the
right. Sample D had more uniform porosity associated with the exudates and had
smaller
exudates. The intennetallic particles were small in both Samples C and D,
though smaller in
Sample D.
[0098] Intennetallic particle content, including AlmFeMn, a-Al(FeMn)Si, Al3Fe,
and Mg,Si
(e.g., iron-based intermetallic particles (Fe-IMCs)), was evaluated with
respect to Ca content
in Samples AA, BB, and CC. FIG. 10 shows a number of pores 1010 which were
formed due
to a lack of proper degassing. FIG. 11 shows the presence of Fe-IMCs in the
AA5182 aluminum
alloy. Additionally, FIG. 12 is a cross-sectional composite view of the AA5182
aluminum
alloy. FIG. 12 shows that the pores 1010 formed throughout the bulk of the
AA5182 aluminum
alloy, due to a lack of proper degassing.
Example 2
[0099] Four samples were prepared based on AA6XXX series aluminum alloys
(e.g., X615)
with the composition shown below. Samples F, H, I, J and L were controls, with
no Ca addition
(6 - 7 ppm Ca was present in the alloy), while in Samples G and K, Ca was
added as described
in Example 1 above. Each sample had a gauge of 12.6 mm. Samples F-I were cast
at a speed
of 3 m/min and Samples J-L were cast at a speed of 4 m/min. Sample F was
produced having
a hydrogen content of 0.16 ppm, Sample G was produced having a hydrogen
content of 0.22
ppm, Sample H was produced having a hydrogen content of 0.19 ppm, and Sample I
was
-25-
CA 03204304 2023-06-05
WO 2022/165492 PCT/US2022/070364
produced having a hydrogen content of 0.30 ppm. Samples J-L were produced
having the same
hydrogen content.
Sample Si Fe Cu Mn Mg Ti Cr Ca (ppm)
Comparative F 0.57 0.25 0.52 0.20 0.71 0.02 0.04
6
0.59 0.26 0.53 0.20 0.73 0.02 0.04 61
Comparative H 0.59 0.25 0.53 0.20 0.73 0.02 0.04
7
Comparative I 0.58 0.25 0.52 0.20 0.72 0.02 0.04
7
Comparative J 0.581 0.226 0.525
0.200 0.688 0.026 0.001 2
0.579 0.225 0.521 0.198 0.683 0.025 0.001 51
Comparative L 0.579 0.225 0.522
0.199 0.684 0.026 0.001
Comparative DD 0.52 0.22 0.52 0.19 0.67 0.02 0.03 36
EE 0.52 0.22 0.52 0.19 0.67 0.02 0.03
72
[0100] Again, each sample was photographed. A photograph of Sample F is shown
in FIG. 5,
a photograph of Sample G is shown in FIG. 6, a photograph of Sample H is shown
in FIG. 7,
and a photograph of Sample I is shown in FIG. 8. From a visual inspection,
Sample I had the
best surface appearance. With all else being the same, increased hydrogen
content deteriorated
the surface appearance. A photograph of Sample J is shown on the far left in
FIG. 9, a
photograph of Sample K is shown in the middle of FIG. 9, and a photograph of
Sample L is
shown on the far fight in FIG. 9. As seen by visual inspection, Sample K,
including Ca addition,
had a superior surface appearance, indicating less surface defects.
[0101] Samples DD and EE were prepared as described above, including 36 ppm Ca
and 72
ppm Ca, respectively. Intermetallic particle content, including dendritic a-
Al(FeMn)Si,
platelet 13-AlFeSi, Q-Al5Cu2MgsSi6(e.a., Fe-IMCs), and Al2Cu, was evaluated
with respect to
Ca content. The pores 1010 that formed as shown in FIG. 13 were due to a lack
of proper
degassing. FIG. 14 shows the presence of Fe-I1VICs and Al2Cu in the X615
aluminum alloy.
Additionally, FIG. 15 is a cross-sectional composite view of the X615 aluminum
alloy. FIG.
15 shows that the pores 1010 formed throughout the bulk of the X615 aluminum
alloy, due to
a lack of proper degassing. As shown in FIG. 15, larger Fe-IMCs formed near
the center of the
bulk of the X615 aluminum alloy, which is attributed to centerline segregation
occurring while
producing the X615 aluminum alloy.
Example 3
[0102] Two samples were prepared based on an AA3104 aluminum alloy, with the
composition shown below. In Samples GG, Ca was added as described in Example 1
above.
-26-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
Sample Si Fe Cu Mn Mg Ti Ca (ppm)
Comparative FF 0.21 0.45 0.18 0.95 1.04 0.01 29
GG 0.21 0.45 0.18 0.95 1.04 0.01 107
[0103] Samples FF and GG were prepared as described above, including 29 ppm Ca
and then
addition of Ca so that Sample GG had 107 ppm Ca. Intennetallic particle
content, including
a-A1(FeMn)Siõk16FeMn (e.g., Fe-IMCs), and Mg2Si, was evaluated with respect to
Ca content.
.. Ca addition in Sample GG increased the amount of a-Al(FeMn)Si allowed to
form and
decreased the amount of Al6FeMn allowed to form, as shown in FIG. 19. In
Samples FF and
GG. the relative proportion of intennetallic phases in the extracted
particles, quantified using
XRD, is shown below.
Sample sa-Al(FeMn)Si Al6FeMn Mg ,Si
t57
Comparative FF 95.59 4.4 0.01
GG 99.88 0.1 0.02
[0104] As shown in the above table, adding Ca to increase the Ca content
resulted in an
increased in the proportion of a-Al(FeMn)Si and a decrease in Al6FeMn. Such a
proportion of
alpha may lead to decreased homogenization times during downstream processing
and
improved intermetallic distribution in the final product, thus improve the
final sheet properties.
Pores 1010 formed as shown in FIG. 16 due to a lack of proper degassing. FIG.
17 shows the
presence of Fe-IMCs and Mg2Si in the AA3104 aluminum alloy. Additionally, FIG.
18 is a
cross-sectional composite view of the AA3104 aluminum alloy. FIG. 18 shows
that the pores
1010 formed throughout the bulk of the AA3104 aluminum alloy due to a lack of
proper
degassing.
[0105] Reference has been made in detail to various examples of the disclosed
subject matter,
one or more examples of which were set forth above. In fact, it will be
apparent to those skilled
in the art that various modifications and variations may be made in the
present subject matter
without departing from the scope or spirit of the disclosure. For instance,
features illustrated
or described as part of one embodiment may be used with another embodiment to
yield a still
further embodiment.
ILLUSTRATIONS OF SUITABLE METHODS AND ALLOY PRODUCTS
[0106] Illustration 1 is a process for casting an aluminum alloy, comprising:
melting an
aluminum alloy to form a molten aluminum alloy, wherein the molten aluminum
alloy
-27-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
comprises Mg; adding at least 30 ppm Ca to the molten aluminum alloy; and
continuously
casting the molten aluminum alloy to form a cast aluminum alloy.
[0107] Illustration 2 is the process of any preceding or subsequent
illustration, wherein the
aluminum alloy does not comprise Mg, and wherein the process further comprises
adding Mg
to the molten aluminum alloy.
[0108] Illustration 3 is the process of any preceding or subsequent
illustration, wherein the
aluminum alloy article is a 5xxx series aluminum alloy, a 6xxx series aluminum
alloy, or a
7xxx series aluminum alloy.
[0109] Illustration 4 is the process of any preceding or subsequent
illustration, wherein the Ca
is added to the molten aluminum alloy in an amount from 50 ppm to 500 ppm.
[0110] Illustration 5 is the process of any preceding or subsequent
illustration, wherein the Ca
is added to the molten aluminum alloy in an amount from 50 ppm to 400 ppm.
[0111] Illustration 6 is the process of any preceding or subsequent
illustration, wherein the Ca
is added to the molten aluminum alloy in an amount from 50 ppm to 250 ppm.
[0112] Illustration 7 is the process of any preceding or subsequent
illustration, wherein the Ca
is added to the molten aluminum alloy in a trough of a continuous caster.
[0113] Illustration 8 is the process of any preceding or subsequent
illustration, wherein the
molten aluminum alloy is substantially free of Be.
[0114] Illustration 9 is the process of any preceding or subsequent
illustration, wherein the cast
aluminum alloy comprises at least 0.3 % by weight Mg.
[0115] Illustration 10 is the process of any preceding or subsequent
illustration, wherein the
cast aluminum alloy comprises an oxide surface layer.
[0116] Illustration 11 is the process of any preceding or subsequent
illustration, further
comprising cooling the cast aluminum alloy upon exit from a continuous caster.
[0117] Illustration 12 is the process of any preceding or subsequent
illustration, wherein the
cooling step comprises water quenching the cast aluminum alloy.
[0118] Illustration 13 is the process of any preceding or subsequent
illustration, wherein the
cast aluminum alloy is coiled.
[0119] Illustration 14 is the process of any preceding or subsequent
illustration, further
comprising: solutionizing the aluminum alloy article; quenching the aluminum
alloy article;
and aging the aluminum alloy article.
[0120] Illustration 15 is the process of any preceding or subsequent
illustration, wherein a cold
rolling step is performed.
-28-
CA 03204304 2023-06-05
WO 2022/165492
PCT/US2022/070364
[0121] Illustration 16 is an aluminum alloy article prepared according to the
process of any
preceding or subsequent illustration.
[0122] Illustration 17 is the aluminum alloy of any preceding or subsequent
illustration,
wherein the aluminum alloy article is an aluminum alloy sheet, an aluminum
alloy plate, or an
aluminum alloy shate.
[0123] Illustration 18 is the aluminum alloy of any preceding or subsequent
illustration,
wherein the aluminum alloy article is an automotive body part, a motor vehicle
part, a
transportation body part, an aerospace body part, or an electronics housing.
[0124] Illustration 19 is the aluminum alloy of any preceding illustration,
wherein the surface
of the product has at least 10% less surface defects than a product formed
without addition of
calcium.
[0125] All patents, publications, and abstracts cited above are incorporated
herein by reference
in their entireties. Various embodiments of the invention have been described
in fulfillment of
the various objectives of the invention. It should be recognized that these
embodiments are
merely illustrative of the principles of the present invention. Numerous
modifications and
adaptions thereof will be readily apparent to those skilled in the art without
departing from the
spirit and scope of the present invention as defined in the following claims.
-29-