Note: Descriptions are shown in the official language in which they were submitted.
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
Modeling-guided Light therapy for Adjusting Circadian Rhythm
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application is claims priority benefit of US Provisional
Application Serial Number,
63/124096, filed, December 11, 2020, entitled, "MODELING-GUIDED LIGHT THERAPY
FOR
ADJUSTING CIRCADIAN RHYTHM."
[0002] This application is also a continuation-in-part of US Patent
Application Serial Number
16/803,961, entitled, "Adjustable Mask" filed February 27, 2020 (the '961
Application).
[0003] This application is also a continuation-in-part of US Patent
Application Serial Number
16/810,800, entitled "Circadian Rhythm Adjustment System," filed March 5, 2020
(the '800
Application).
[0004] The '961 Application and the '800 Application both claim the benefit of
US provisional
Application Serial Number 62/835,473, entitled "Circadian Rhythm Adjustment
System," filed
April 17, 2019, US provisional Application Serial Number 62/812,683, entitled
"ADJUSTABLE
MASK," filed March 1, 2019, and US provisional Application Serial Number
62/814,257, entitled
"Circadian Rhythm Adjustment System," filed March 5, 2019.
[0005] All of the above applications are incorporated herein by reference.
FIELD OF THE INVENTION
[0006] This specification relates to the field of methods and devices for
treating and/or adjusting
circadian rhythms.
BACKGROUND
[0007] The subject matter discussed in the background section should not be
assumed to be prior
art merely as a result of its mention in the background section. Similarly, a
problem mentioned in
the background section or associated with the subject matter of the background
section should not
be assumed to have been previously recognized in the prior art. The subject
matter in the
background section merely represents different approaches, which in and of
themselves may also
be inventions.
[0008] Circadian rhythms are physiological and behavioral oscillations that
are normally
synchronized with the natural light-dark cycle of the day. Circadian rhythm
disorders happen
when circadian rhythms are out of synchronization with the actual sleep-wake
schedules.
Disruptions in the circadian rhythm or misalignments of the circadian rhythm
with a user's
schedule, such as due to traveling or night shift work requirement can cause
difficulty falling
asleep, frequent waking up during sleep, and difficulty remaining asleep
throughout the intended
1
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
sleep time. In addition, while awake, circadian rhythm misalignment can lead
to impaired
cognitive and physiologic performance as well as lethargy, drowsiness,
fatigue, and
gastrointestinal distress. The circadian rhythms in humans and other mammals
are affected by the
exposure of the retina to light. Accordingly, it may be desirable to develop
device and methods to
more effectively treat circadian rhythm disorders by exposing the eyes to
light, for example.
SUMMARY
[0009] The following description presents a simplified summary in order to
provide a basic
understanding of some aspects described herein. This summary is not an
extensive overview of
the claimed subject matter. It is intended to neither identify key or critical
elements of the claimed
subject matter nor delineate the scope thereof.
[0010] In some embodiments, a system for adjusting a user's circadian rhythm
may be provided.
In some embodiments, the system may include one or more input modules for
collecting
information relating to a user's sleep and/or wakefulness. The system may also
include a light
source, a processor system including one or more processors that control the
light source, and a
memory system storing one or more machine instructions. In some embodiments,
the system may
be configured to obtain information relating to the user's present circadian
rhythm. The system
may also be configured to obtain information relating to one or more
anticipated times of sleep
and/or wakefulness, for the user, on one or more days. Based on at least the
information relating
to the user's present circadian rhythm, the system may generate a model for
estimating the user's
circadian rhythm over one or more days. The estimates of the user's circadian
rhythm may be
configured to be adjusted in response to application, or anticipated
application, of light by the light
source. Based on at least the one or more anticipated times of sleep and/or
wakefulness, the system
may be configured to generate a model for estimating the user's homeostatic
sleep drive over one
or more days. The estimates of the user's homeostatic sleep drive may be
configured to be adjusted
in response to changes in the user's sleep and wakefulness times. Based on at
least the model for
estimating the user's circadian rhythm and the model for estimating the user's
homeostatic sleep
drive, the system may be configured to generate instructions for activating
the light source to adjust
the user's circadian rhythm.
[0011] In some embodiments, a method for adjusting a user's circadian rhythm
may be provided.
In some embodiments, the method may include one or a combination of the
following steps:
(i) obtaining information relating to the user's present circadian rhythm;
(ii) obtaining information
relating to one or more anticipated times of sleep and/or wakefulness, for the
user, on one or more
days; (iii) based on at least the information relating to the user's circadian
rhythm, generating a
model for estimating the user's circadian rhythm over one or more days, the
estimates of the user's
circadian rhythm being configured to be adjusted in response to application,
or anticipated
application, of light by the light source; (iv) based on at least the one or
more anticipated times of
sleep and/or wakefulness, generating a model for estimating the user's
homeostatic sleep drive
2
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
over one or more days, the estimates of the user's homeostatic sleep drive
being configured to be
adjusted in response to changes in the user's sleep and wakefulness times; and
(v) based on at least
the model for estimating the user's circadian rhythm and the model for
estimating the user's
homeostatic sleep drive, generating instructions for activating the light
source to adjust the user's
circadian rhythm.
[0012] Further variations encompassed within the systems and methods are
described in the
detailed description of the invention below.
BRIEF DESCRIPTION OF THE DRAWINGS
[0013] In the following drawings like reference numbers are used to refer to
like elements.
Although the following figures depict various examples of the invention, the
invention is not
limited to the examples depicted in the figures.
[0014] FIG 1 shows an example of the phase response curve (PRC) for estimating
how the
circadian phase may be shifted upon light treatment.
[0015] FIG. 2 shows how sleep/wake cycle is regulated by Process C, Process S,
and their
interactions.
[0016] FIG. 3A shows a block diagram of an embodiment of the circadian rhythm
therapy system.
[0017] FIG. 3B shows a block diagram of an embodiment of the circadian rhythm
therapy system
with an environmental light sensing system.
[0018] FIG 4A shows a diagram of an embodiment of the eye mask that delivers
the circadian
rhythm treatment programs.
[0019] FIG. 4B shows a diagram of an embodiment of the eye mask prior to
placing the hardware
into the mask.
[0020] FIG 4C shows a diagram of an embodiment of the mask of FIG. 4B after
the hardware has
been installed.
[0021] FIG. 4D shows an embodiment of a cross section of the mask with the
electronic insert
inside the pocket and one light pipe for delivering the light generated to the
user.
[0022] FIG. 4E shows a top view of the embodiment of the electronic insert of
FIG. 4D.
[0023] FIG. 4F shows the angle and dimension design of the electronic insert
of FIG. 4D.
[0024] FIG. 5A shows a diagram of an embodiment of a mask illustrating the
strap.
3
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
[0025] FIG. 5B shows an illustration of how a user would an embodiment of the
mask.
[0026] FIG. 6 shows an embodiment in which the circadian rhythm treatment
hardware is built
into a lighting system of the room of a building, for example.
[0027] FIG. 7 shows a block diagram of an embodiment of the circadian rhythm
therapy system.
[0028] FIG. 8 shows a block diagram of an embodiment of the environmental
sensing system
[0029] FIG. 9 shows a flowchart for a method in which the system of FIG. 3A
and 3B may operate.
[0030] FIG. 10 shows a flowchart for how the circadian rhythm adjustment
algorithm and
treatment program are generated.
[0031] FIG. 11 shows a diagram of how elements of the algorithm interact.
[0032] FIG. 12 shows a diagram of how elements of the algorithm interact and
what the
circadian rhythm adjustment algorithm is optimized for.
[0033] FIG. 13 shows an example of one or more blocks of light flashes to
illustrate the light
flashes parameters.
[0034] FIG. 14 shows a diagram of how the circadian rhythm treatment program
may be adjusted
throughout the course of the treatment based on senser input.
[0035] FIG 15 shows an exemplary method for adjusting a user's circadian
rhythm.
[0036] FIG 16 shows an exemplary method for adjusting a user's circadian
rhythm using input
from sensors.
[0037] FIG 17 shows an exemplary method for selecting light treatment
parameters.
DETAILED DESCRIPTION
[0038] Although various embodiments of the invention may have been motivated
by various
deficiencies with the prior art, which may be discussed or alluded to in one
or more places in the
specification, the embodiments of the invention do not necessarily address any
of these
deficiencies. In other words, different embodiments of the invention may
address different
deficiencies that may be discussed in the specification. Some embodiments may
only partially
address some deficiencies or just one deficiency that may be discussed in the
specification, and
some embodiments may not address any of these deficiencies.
Technical Terminology
4
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
[0039] Some concepts discussed in this specification are as follows,
A. Circadian rhythms: refers to physical, mental, and behavioral changes
that follow a 24-
hour cycle.
B. Circadian pacemaker: refers to a small group of nerve cells located in
the hypothalamus
that controls the circadian cycles and influences many physiological and
behavioral rhythms
occurring over a 24-hour period, including the sleep/wake cycle.
C. Homeostatic sleep drive: also referred as sleep drive or sleep pressure,
which refers to the
drive to sleep that is influenced by the duration of wakefulness. Homeostatic
sleep drive/pressure
can be understood as the hunger for sleep.
D. Process C: refers to a process controlled by the circadian pacemaker in
sleep regulation.
E. Process S: refers to a sleep regulation process that represents the
homeostatic sleep drive.
F. Advance the sleep phase: refers to shifting the wake up time to earlier
in the morning or an
earlier time.
G. Delay the sleep phase: refers to shifting the wake up time to later in
the morning or a later
time.
H. Zeitgeber: refers to physical and social events which entrain the
circadian clock. Light is
an entrainer.
K. Entrainment: refers to the synchronization or alignment of the internal
biological
clock rhythm, including its phase and period, to external time cues, such as
the natural dark-light
cycle.
Overview
[0040] Embodiment of a circadian rhythm adjustment system have been disclosed
in US patent
application 16/810,800, which is incorporated herein by reference in its
entirety. The circadian
rhythm adjustment system described in this specification is an improvement of
the system
described in US patent application 16/810,800. Embodiments of the system
described in this
specification may include: (1) data collection mechanisms including software,
hardware, and/or
lab assays, (2) logic (e.g., software or hardware) for adjusting the circadian
rhythm that is based
on the prediction of not only the circadian phases but also the homeostatic
sleep drive, both of
which are guided by mathematical models, user inputs, and environmental data,
and data obtained
from lab studies (3) circadian rhythm adjustment hardware devices, which may
incorporate logic
modules, and optionally processors, sensors, light sources and other
components, (4) form factor
(e.g. a sleep mask) that delivers the circadian rhythm adjustment treatment.
In order to generate a
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
circadian-shifting treatment program, embodiments of the system described in
this specification
may estimate the current circadian phase and the target circadian phase by
implementing one or
more factors for sleep regulation that affects both the circadian rhythms and
the homeostatic sleep
drive as part of its circadian-adjustment algorithms. Data collected from user
input, sensors in
device, lab assays, and/or third party sources may be used as both input of
the circadian clock
adjustment models and/or a basis for modifying the models (e.g.,
automatically). A device
separating from the circadian rhythm adjustment device, for example, an
environmental light
sensor that may or may not be wearable, may be used to collect data from the
user or the
environment, which may provide input for the circadian rhythm adjustment
models. Based on at
least one algorithm that predicts opportune times for applying therapy,
circadian rhythm
adjustment programs that include light therapy parameters (e.g. using light
flashes or other forms
of light) with or without other stimulation or behavioral suggestions may be
generated by
computation, which may then be executed through a circadian rhythm adjustment
hardware device
(for example, a sleep mask with electronics that delivers light flashes to
shift circadian clocks).
[0041] In one embodiment, a system is designed to treat/prevent circadian
rhythm misalignment
by actively shifting the circadian phase of the user during sleep at night or
during the daytime. In
an embodiment, the user may use light flashes while the user is sleeping, to
adjust the user's
circadian rhythm. As a result of using light pulses, and as a result of light
pulses being less invasive
and less disruptive to sleep, the system may shift circadian rhythm while the
users remain sleeping.
Compared to other light therapy devices that may shift the circadian rhythm,
the use of light pulses
is less disruptive to users' day-time activities, also, and is therefore
potentially more convenient.
More importantly, due to retinal physiology and the sensitivity of the
circadian adjustment system
in the brain, light flash stimulation at night may be more effective in
shifting circadian rhythm than
continuous light stimulation during the day.
[0042] In some embodiments, the circadian rhythm light program may be
generated based on a
treatment regimen, which may be controlled by a software program. The system
may include
software, hardware, a light source, such as an LED, and/or user interface, for
example. The system
may include a light calculation module, which implements light calculation
logic, which
determines a pattern of light to apply to the user. Form factors may include
sleep masks, and/or
different sizes and shapes of other things worn (or that may accompany of a
person while sleeping).
The system may be integrated into a variety of devices of systems of a variety
of sizes and shapes.
[0043] In one embodiment of the circadian rhythm adjustment systems, the light
calculation logic
is based on the phase response curve (PRC) and light flashes are applied
during the windows in
which circadian clocks can be advanced or delayed by light stimulation. FIG 1
is an illustration
of a PRC (202) in response to light stimulation, and its respective time
windows to delay (204) or
advance (206) the circadian phase. When sleep consistency is good, meaning
that the user has
relatively consistent sleep schedules, it is relatively straight forward to
estimate the phase-response
curve, and therefore relatively straight forward to estimate the treatment
windows for phase shifts.
6
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
In at least one embodiment, the light therapy program may be generated by
estimating the
treatment window based on simple circadian-estimating models that consider
input variables such
as current and desired sleep schedules as well as chronotypes. The simple
circadian rhythm
adjustment usually works quite well. However, predicting the PRC and the
corresponding advance
or delay windows becomes difficult with irregular schedules. The light therapy
treatment windows
specified by the circadian rhythm adjustment model based on the PRC alone may
be less effective
or even counterproductive if part of the light therapy occurs in the delay
window and the other part
in advance. Moreover, the circadian phase may be shifted in the opposite
direction when the
advance or delay windows are predicted incorrectly in a circadian rhythm
adjustment system when
using the circadian rhythm adjustment model based only on estimation of
circadian shifts by
comparing bedtime or wake time changes and/or time zone changes, which was not
previously
recognized but later observed in real-life user experience. Additionally, in
the case of frequent
travel or frequent night shift changes (e.g., for a shift worker) when sleep
schedules are subject to
irregularity or full entrainment may be challenging to achieved within a
desired time, and at times,
in some situations, it may be potentially unreliable to use the simple
circadian-estimating models
to estimate the advance and delay windows. In some circadian rhythm
misalignment scenarios,
partial or complete sleep deprivation often occurs due to schedule constraints
and/or incomplete
entrainment, which may further impact how the circadian clocks respond to
light and other
Zeitgeber input. In additional to circadian rhythm misalignment, there are
also times when
performances during certain time frames may need to be optimized and
prioritized, and operations
are highly limited by schedule constraints, for example, in a competition or
in a battlefield, which
are stressful situations that further add to the complexity of predicting the
PRC. Instead of shifting
circadian phases to achieve the maximal possible shift, it now recognized that
an alternative
solution is to shift the circadian phases so the local maximal wakefulness
falls in the window when
high performances are desired, or the local minimal wakefulness falls out of
the window when
high performances are desired. At least some embodiments of the system in this
disclosure
addresses at least some of the above-mentioned circadian rhythm misalignment
scenarios in which
the effect may be limited by merely calculating circadian phase changes.
[0044] The regulation of the sleep-wake cycle is determined by many factors,
including genetics,
circadian rhythms, homeostatic sleep drive, sleep environment, conscious
decisions and behaviors,
and so on. The factors that determine the regulation of sleep may play a part
in the circadian
rhythm misalignment cases described above. Therefore, in at least one
embodiment of the
circadian rhythm adjustment system, to account for the circadian rhythm
misalignment scenarios
described above, instead of the circadian rhythm adjustment algorithm only
considering circadian
rhythm misalignment based only on the estimated PRC based on the current and
target sleep times
and wake times, a model that includes multiple factors that regulate sleep and
wake cycles, such
as the circadian rhythm (Process C) and the homeostatic sleep drive (Process
S), and based on the
combination of multiple factors, the windows of time during which to apply a
light treatment in
order to shift the circadian rhythm are determined.
7
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
[0045] The suprachiasmatic nucleus (SCN) is the central circadian pacemaker in
the brain. SCN
regulates a number of markers for the circadian clock (C), including
peripheral oscillators, such as
melatonin and core body temperature. Process C refers to a process controlled
by the circadian
pacemaker in sleep regulation. The homeostatic sleep drive (S) builds up
during wakefulness and
declines monotonically during sleep. The interaction of Process C and Process
S regulates sleep.
[0046] FIG. 2 shows the two-process model of sleep regulation, and how the
circadian rhythms
and the homeostatic sleep drive together regulate the sleep wake cycle. The
lower solid, oscillating
line (102) represents Process C, and the upper dash line with sharp angles
(104) represents Process
S. Sleep switches on when the distance between S and C reaches maximum and
switches off when
the distance reaches minimum. Sleep gate 108 represents when sleep switches
on. The area 106
represents sleep. In some embodiments, a circadian rhythm optimization system
may use not only
information for circadian rhythm estimation, but also information to calculate
the homeostatic
sleep drive when computing the sleep and wake cycles for the users.
[0047] A circadian rhythm adjustment system may be optimized for at least one
of two outcomes,
or both if possible: (1) maximizing circadian shifts to achieve the largest
overlap between the
"sleep" phase of the circadian clock with desired sleep time, and the largest
overlap between the
"awake" phase of the circadian clock with desired windows of wakefulness; and
(2) shifting the
circadian phase so the window of high alertness can be best overlapped with
desired window when
peak performances are required or so the window of low alertness can be best
avoided from when
peak performances are required. Within each prioritization, the model may have
different
algorithms to handle different tiers of complexity, including but not limited
to: (1) considering
circadian rhythm misalignment only; (2) considering the additive effect
between circadian rhythm
misalignment and the changes of homeostatic sleep pressure with good sleep
consistency and/or
simple schedule changes that are predictable, regular, or lasts for the amount
of time for possible
full entrainment to occur with the light therapy; (3) considering the additive
effect between
circadian rhythm misalignment and the changes of homeostatic sleep pressure
with irregular sleep
consistency or complicated schedule changes that are changing, unpredictable,
or significant over
a short amount of time; (4) considering not only the additive effect between
circadian rhythm
misalignment and the changes of homeostatic sleep pressure, but also the
interaction between the
two and how one process affects the other when responding to regulatory
signals (regulatory
signals are signals that regulate the circadian phase and/or the homeostatic
sleep drive); (5) besides
the circadian rhythm misalignment and homeostatic sleep drive from the
schedule changes, also
considering other sleep-regulating factors (such as genetic components) that
affect the natural
circadian clocks or sleep-wake cycles.
[0048] Embodiments of the system described in this specification may be useful
for treatment of
acute circadian rhythm misalignment, for example, during travel or night shift
work - or the
combination of both. The systems described in this specification may be
especially useful for
scenarios when a substantial circadian phase shift is required and full
entrainment may not be
8
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
feasible, or when performances during certain time frame need to be optimized
and prioritized, for
example, for competition or battlefield (however, the system is not limited to
such uses and some
embodiments may even preclude such uses). Embodiments of this specification
may also be useful
for treating and preventing chronic circadian rhythm misalignment in night
owls, teenagers, and
elderly. The circadian rhythm treatment system can be used before, during,
and/or after the
occurrence of the change of sleep schedules or time zones or anytime when the
user would like to
improve sleep through optimizing circadian rhythms and alertness.
[0049] In this specification, the word "program" may refer to a computer
program or other
software that the processor runs to turn on and off the light and deliver the
treatment plan to the
user to adjust and/or regulate the circadian rhythm. The word "program" may
also (or alternatively)
refer to the treatment plan for adjusting the circadian rhythm (which may be
implemented, via a
software program). The treatment plan may be implemented by software running
on a processor,
embedded software, firmware, middleware, and/or hardware. The program used for
treating the
circadian rhythm disorder may be referred to as a light program, when light is
used for treating the
circadian rhythm disorder or more generally as a circadian rhythm disorder
treatment program. In
this specification, "generate" may mean create, design, produce from
scratches, or modify from
existing version of algorithm, model, or program.
[0050] System
[0051] Embodiments of the system described in this specification may include:
(1) data collection
mechanisms including software, hardware, and/or lab assays, (2) logic (e.g.,
software or hardware)
for adjusting the circadian rhythm that is based on the prediction of not only
the circadian phases
but also the homeostatic sleep drive, both of which are guided by mathematical
models, user inputs,
and environmental data, and data obtained from lab studies (3) circadian
rhythm adjustment
hardware device, which may incorporate logic modules, and optionally
processors, sensors, and
other components, (4) form factor (e.g. a sleep mask) that delivers the
circadian rhythm adjustment
treatment.
[0052] FIG. 3A shows a block diagram of an embodiment of a computing system
300. Computing
system 300 may include computing device 301, an application 302 and display
306. Computing
system 300 may also include cloud 308 and/or circadian rhythm adjustment
device 310.
Computing device 301 may be a mobile device, Personal Computer (PC), laptop,
smart phone,
and/or other computing device. Application 302 may reconfigure a computing
device 301 to
function as a circadian rhythm adjustment apparatus and/or may interface with
a circadian rhythm
adjustment apparatus. Display 306 may be a touch screen or other display via
which the user may
view setting and pages generated by application 302 and enter input for
configuring the computing
device 301 and/or system 300 for adjusting a circadian rhythm. Cloud 308 may
include any
combination of wide area networks and/or local area networks. Cloud 308 may
include one or
more servers, computing devices, and/or devices on which an algorithm for
determining a
9
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
circadian rhythm therapy and/or controlling sleep therapy system may reside.
Besides storing the
data collected from the mobile application 302 and/or from circadian rhythm
adjustment device
310, the cloud 308 may run application 302 and/or may run an algorithm based
on input from
application 302 (which is running of computing device 301), and cloud may send
the sleep therapy
program to computing device 301 and/or circadian rhythm adjustment device 310.
Computing
device 301 may communicate with circadian rhythm adjustment device 310, via
cloud 308. An
example of a circadian rhythm treatment apparatus is a light pulse delivery
apparatus, such as a
mobile sleep mask, goggles, bedroom lights, and/or other circadian rhythm
treatment apparatus
(which may deliver light pulses). In one embodiment, user data input,
environmental data, and
data from the lab may be collected via user interface or set in the backend of
the app 302. In another
embodiment, in addition to user input or manufacturer's preset via app 302,
data used for circadian
rhythm calculation may be collected via sensors on the circadian rhythm
adjustment device 310.
The software may be stored on and run on a mobile device (e.g., a smart
phone), a computational
device (e.g., a computer), an/or delivered from a server, such as, as a result
of the user interacting
with a webpage. The software may be located in a light-emitting device (e.g.,
the sleep mask or a
lamp in a bedroom), and the light emitting device may run the program
producing the pattern of
light used to treat the user. Treatment logic including the mathematical
models and the light
program algorithms may be implemented on the software within the app 302 on
the computing
device 300, or the cloud 308, or the combination of both. In one embodiment,
part of the light
program treatment logic may be implemented in the firmware on the circadian
rhythm adjustment
device 310. Additionally or alternatively, the light emitting device may
respond to signals from a
device running the program to produce the pattern of light for treating the
user, and thereby the
light emitting device may act as the circadian rhythm treatment apparatus. The
light emitting
device may communicate via a wide area network, such as the Internet, with a
server (and/or other
devices), and the light emitting device may receive signals, from a server
(and/or other devices),
directing the light emitting device to flash in a desired pattern to adjust a
circadian rhythm.
Optionally, the circadian rhythm treatment apparatus may have an Application
Program Interface
(API), via which another device may control or interface with the circadian
rhythm treatment
apparatus, allowing the user to adjust the user's circadian clocks, via the
API. The circadian
rhythm treatment device may communicate with other devices and/or programs,
via the API
associated with those other devices and/or programs. The circadian rhythm
treatment apparatus
may interface with a wearable device and/or software applications, or through
a program and/or
hardware device that controls the collection of Internet of Things (IoT)
devices (e.g., a smart home
device or a virtual intelligent personal assistant). Alternatively, or
additionally, circadian rhythm
treatment apparatus may be incorporated into a wearable device and/or software
application.
[0053] In one embodiment, data collection mechanism may include additional
hardware such as a
wearable environmental light sensor. FIG. 3B shows a block diagram of an
embodiment of the
circadian rhythm therapy system 350 that includes both the light sensing
system 351 and the
circadian rhythm adjustment system 353. The light-sensing system include a
light sensor 352 that
may or may not be wearable, and the light sensor computing device 354. The
circadian rhythm
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
adjustment system includes a computing system 356 to calculate the light
program based on the
user's circadian clocks and sleep/work schedules, and a device to deliver the
treatment light
program 355. The light sensor computing device and the circadian rhythm
adjustment computing
device can be one 357 (e.g. one mobile application) or separate computing
devices (e.g. separate
mobile applications). The light sensor captures the ambient light data, stores
on the device,
preliminary processes the data and sends the data to the light sensor
computing device. The light
sensor computing device then further processes the data and send it to the
circadian rhythm
adjustment device as the mathematical model input to calculate the user's
circadian phase. The
mathematical model then output the necessary light program to optimize the
user's circadian
clocks. Cloud 358 may include any combination of wide area networks and/or
local area networks.
Cloud may include one or more servers, computing devices, and/or devices on
which an algorithm
for determining a circadian rhythm therapy and/or controlling sleep therapy
system may reside.
Circadian rhythm adjustment device may be controlled directly by the circadian
rhythm adjustment
computing system and/or a device on cloud. Besides storing the data collected
from the mobile
application and/or from the light sensor and the circadian rhythm adjustment
device, the cloud may
run application and/or may run an algorithm based on input from application
(which is running of
computing device), and cloud may send the sleep therapy program to computing
device and/or
circadian rhythm adjustment device. Computing device may communicate with
circadian rhythm
adjustment device, via cloud. In one embodiment, the light sensor may have its
own computing
device 354. In another embodiment, the light sensor computing device 354 and
the computing
device for the circadian rhythm adjustment device 356 are different parts of
the same computing
device 357. Treatment logic including the mathematical models and the light
program algorithms
may be implemented on the software on the computing device 356 or 357, or the
cloud 358, or the
combination of both. Part of the light program treatment logic may also be
implemented in the
firmware on the wearable light sensor 352 or 354 and the circadian rhythm
adjustment device 355
or 356. In one embodiment, the light sensor 352 and the circadian rhythm
adjustment device 355
may be different parts of the same hardware device. The treatment may be a
pattern of light pulses
and/or other changes in lighting that are delivered to the user, via the
circadian rhythm treatment
apparatus that delivers the treatment (e.g., via a program that controls the
lighting, such as by
controlling pulses of light delivered to the user) to effectively treat or
prevent circadian rhythm
disorders. The treatment may be applied while the subject is asleep and/or
awake in the middle of
sleep or within 1-2 hours before or after sleep. In at least one embodiment,
the program may
recommend nap time or caffeine intake to adjust the homeostatic sleep drive
and its impact on the
sensitivity of circadian system in response to light. There may be an audio
component to the
circadian rhythm disorder treatment program, which may produce therapeutic
sounds, relaxing
sounds, and/or pleasant sounds that are synchronized with, and played together
with, or
independent of, the treatment, such as the pattern of light produced.
[0054] In an embodiment, multiple users may use the same device at different
times. Each user
may have his/her own account with past programs, biological, sleep,
homeostasis sleep load
profiles, and behavioral profiles. Based on the information collected
throughout the time that
11
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
user uses the circadian rhythm treatment apparatus, the software adjusts and
improves itself to
provide personalized circadian rhythm disorder treatment program (e.g., light
program).
[0055] Form factor
[0056] Different form factors (e.g., different types of systems of different
sizes and shapes and/or
using different patterns of light pulses) may be used to deliver the light
programs and adjust
circadian rhythms. The circadian treatment device may be mobile, may be a
sleep mask or a goggle,
a bedside lamp, a hood, a screen, or may not be mobile, such as in-room
lighting. In at least one
embodiment of the circadian rhythm treatment, the circadian rhythm treatment
apparatus can be
an eye mask that the user wears while sleeping.
[0057] For a smart sleep mask that contains electronic components, it may be
desirable to design
the mask so that the mask not only has the ability to hold the electronics and
to deliver the circadian
rhythm disorder treatment program (e.g., a light program), but also the mask
should remain in its
position on the user's head while in use, as well as being worn in bed
comfortably in all, nearly
all, or a large variety of sleeping positions, even when incorporating rigid
electronics components
in the mask. In various embodiments of this specification (but not all
embodiments), the system
of the mask may be built so as to meet a balance of six features (in addition
to other criteria: 1) the
mask should is soft enough not to apply an uncomfortable pressure against the
user's face when
sleeping on the side or face-down; 2) the mask provides enough cushion around
the rigid
electronics to protect the electronics from environmental damage and to
protect the face from
pressure from the electronics; 3) while providing enough cushion with soft
materials, the mask
still breathes (e.g., to help ensure that the electronics cools properly and
does not over heat); 4) the
eye regions of the mask is be recessed enough and away from the eyes to
prevent rubbing against
the user's eyes; 5) the mask effectively blocks environmental light; 6) the
mask is adjustable to
different head sizes and shapes; 7) the mask stays in place to ensure that
light programs are
received properly by the user throughout the treatment process.
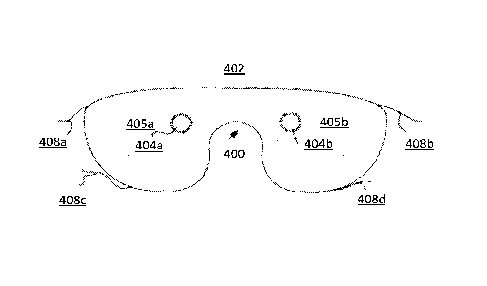
[0058] FIG. 4A shows an embodiment of system in which the circadian rhythm
treatment
hardware is built into a sleep mask 400. Mask 400 covers the user's eyes and
blocks environmental
light from shining on the eyes of the user. Mask 400 may be fashioned from a
soft and/or resilient
material. Cupped regions 405a and 405b are regions that form cups away from
the user's eyes, to
ensure that the mask does not cause discomfort by pressing against the user's
eyes and/or to ensure
that the lights that generate the flashes are a predetermined distance from
the eyes to ensure proper
illumination of the eyes during a light flash.
[0059] In one embodiment, the electronics which are responsible for delivering
light programs can
be inserted into the mask and removed as needed, for example, when the mask
needs to be cleaned.
FIG. 4B shows a diagram of an embodiment of the mask 400 with empty
electronics pocket 402
and light pipe holes 404, prior to placing the hardware into the electronics
pocket 402 and the light
pipes into light pipe holes 404 of the mask 400. FIG 4C shows a diagram of an
embodiment of
12
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
the mask of FIG. 4B after the hardware, electronics 406 and light pipes 408
have been installed.
Light pipe holes 404 are regions where lights and/or light pipes are placed
for illuminating the
user's eyes with light flashes, prior to placing the hardware into the
electronics pocket 402 and the
light pipes into light pipe holes 404 of the mask 400. FIG. 4D shows that
light pipe 408 may be
connected to an LED (or other light source 410) and/or other electronics 412
on one side of the
mask and light pipe for delivering the light generated to the user. The light
pipe 408 has an
enlarged part at the end in the shape of a button that goes through the light
pipe hole on the inner
side of the eye mask to hold the electronics and the mask together tightly in
the right place.
Electronics 406, light pipes 408, light source 410 and/or other electronics
412 may be held between
outer foam 414 and inner foam 416. In an embodiment, the electronic 406, light
pipes 408, light
source 410 and/or other electronics 412 are on one single rigid Printed
Circuit Board (PCB) and
held in a single rigid plastic enclosure housing 420.
[0060] FIG. 4E shows a top view of housing 420, within in which electronics
406, light sources
410, and/or electronics 412 are enclosed. Housing 420 may be made from a hard
resilient plastic
and/or may include (e.g., made from) softer materials. Charging port 422 may
be used for charging
the battery that powers electronics 406 and/or electronics 412. Electronics
412 (e.g. a PCBA) and
light sources 410a and b are shown in dashed lines, because electronics 412
and light sources 410a
and b are hidden from view. FIG. 4F shows that the two light pipes are spaced
apart by a distance
d, and an angle a or a' is made between a line connecting the center of the
light pipes and the
center of the user's eyes, and line perpendicular to the line connecting the
two light pipes,
representing the angle between the center of the light pipe and the center of
the user's eye pointing
from the pupil. FIG. 4F shows different possible locations of the light pipes
408a and 408b. Mask
portions 454a and 454b are portions of mask 400. For clarity, the rest of the
mask 400 is not
shown. As a reference for the direction of the angles nose location 456 and
ear locations 458a and
458b are labeled. Light pipes 460a-c show possible locations for light pipe
408a for different
embodiments mask 400. Similarly, light pipes 460d-f show possible locations
for light pipe 408b
for different embodiments mask 400. Although light pipes 460b and 460e are
drawn in solid lines
and light pipes 460a, 460c, 460d, and 460f are drawn in dotted lines, each of
light pipes 460b and
460e and light pipes 460a-460f represent possible positions of light pipes
408a and 408b.
Although there may be more than two light pipes, since only two are needed
(one for the left eye
and one for the right eye), only two were drawn in solid lines. In FIG. 4E and
4F, B is the distance
between the surface of the eye and the light pipe, the angle a is the angle of
a diagonal line may
between the user's eye 424a or 424b and the light pipe 460c and 460d when the
light pipe is
between the eye 424a or 424b and the nose location 454, respectively. The
angle a' is the angle
of a diagonal line may between the user's eye 424a or 424b and the light pipe
460a and 460f when
the light pipe is between the eye 424a or 424b and the ear locations 458a and
458b, respectively.
[0061] The light pipes direct the lights from the light source to the user's
eyes. In one embodiment,
the light pipes are connected directly on the PCB over the location of the
light sources (e.g. LED)
(FIG 4D-F). The light pipes, and light sources, are separated by a distance d
of 30 - 150 mm apart
13
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
from each other, and located within a distance B of 5-25 mm away from the
surface of the user's
eyes. The direction of the light pipes points at an angle a = 0-72 degrees
from the direction that is
perpendicular to the pupil opening of the user's eyes if the light pipes are
located closer to the nose
than the ears, or at an angle a'= 0-86 degrees from the direction that is
perpendicular to the pupil
opening of the user's eyes if the light pipes are located closer to the ears
than the nose (FIG 4E
and FIG 4F). In one embodiment, a and a' are both 0 degree. In one embodiment,
the light pipes
may include a diffuser, a scattering material, and/or a diverging lens to
spread the light more evenly
across the user's eyes. In one embodiment, the light pipes may be clear. In
one embodiment,
there may be multiple the light pipes within one cupped eye socket, with at
least one pointing
directly to the pupil of the user's eyes within the angle a and a' and
distance B and d described
above. In one embodiment, there may be reflective materials inside the eye
sockets to redirect the
lights coming out of the light pipe into the user's eyes. In an embodiment,
the light pipes may be
flexible or adjustable to adapt to different facial structures such as
pupillary distance.
[0062] FIG. 5A shows a diagram of an embodiment of a mask illustrating the
strap. The strap
may be comprised of one strap at each side (e.g., strap 502 having upper strap
502a and lower strap
502b and strap 504 having upper strap 504a and lower strap 504b). The straps
502 and 504 each
goes through a tunnel (e.g., tunnels 506 and 508) or slot, forming a double-
strap structure that
allows the adjustment of the length of the upper segment (upper straps 502a
and/or 504a) and lower
segment (lower straps 502b and/or 504b). The strap from each side of the mask
forms a loop that
goes through the tunnel 506 or 508 one pad. One part of the loop (the upper
strap 502a and 504a)
spans above the user's ear, and another part (the lower strap 502b and 504b)
spans under the user's
ear, with the third part going through the tunnel the pad (506 or 508). The
double strap design
enables the mask to be worn with the two straps across the upper and lower rim
of the ears,
individually, to keep the mask in place on the user's head without stretching
on the back of the
ears. The tunnel/loop structure (e.g., tunnels 506 and 508) and the relative
movement of the strap
through the tunnel/loop allows the adjustment of the upper and lower segment
of the strap, so the
mask can be adjusted according to different ear sizes, head sizes, and
positions.
[0063] FIG. 5A also shows a diagram of an embodiment of a mask illustrating
the adjustable strap.
For example, the fastener 510 having pads 512 and 514, which may be made from
hook and loop
material 516 and/or 518, such as Velcro g. In an embodiment, hook and loop
material 516 is on
both sides of pad 512 and hook and loop material 518 is on both sides of pad
514. In an
embodiment, hook and loop material 516 is on at least one side of pad 512
and/or hook and loop
material 518 is on at least side of pad 514. The hook and loop material piece
enables the adjustment
of the circumference, so the mask 400 can be adjusted to different head sizes.
In other
embodiments other straps, pads, and/or fasteners may be used. FIG. 5B shows a
diagram of am
embodiment of a mask illustrating how the straps would be worn by a user. The
front of the face
402 and the back of the head 406 are shown. The mask 400 covers the region
around the user's
eyes. Upper strap 502a/504a spans over the top of the ear while the lower
strap 502b/504b spans
around the bottom of the ear.
14
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
[0064] In addition to a sleep mask, the light programs can be delivered in
glasses or goggles.
Another embodiment of the circadian rhythm treatment apparatus may include in-
room lighting
(controlled by a controller) that works with a mobile application that
generates light programs
based on circadian modeling algorithms to treat or prevent circadian rhythm
disorders. FIG. 6
shows an embodiment circadian rhythm treatment hardware that is built into a
lighting system of
the room of a building (e.g., a bedroom). Ceiling lights 602a and b are light
that are attached to, or
that hang from, the ceiling and/or wall. Ceiling lights 602a and b may be used
to deliver a circadian
rhythm treatment (and to illuminate the room). Although two ceiling lights are
shown FIG. 6,
there may be one ceiling light - any number of ceiling lights may be used.
Table lamps 604a and
b are lights that may rest on the furniture or floor, and may be used to
deliver a circadian rhythm
treatment (and to illuminate the room). Furniture 606 (e.g. a bed) and blanket
608 and/or pillows
610a and b may be used by the user while receiving the circadian rhythm
treatment. In an
embodiment, the user may receive the circadian rhythm treatment (e.g. light
flashes) while
sleeping or relaxing on bed 606 (or in a chair), via ceiling lamp 602a,
ceiling lamp 602b, table
lamp 604a, table lamp 604b and/or other lamps or lights. In an embodiment, the
user may receive
circadian rhythm treatment while awake and performing other activities within
1-2 hours before
or after sleep, or in the middle of sleep in case the user wakes up, via
ceiling lamp 602a, ceiling
lamp 602b, table lamp 604a, table lamp 604b, and/or other lights, in addition
to and/or instead of
receiving the treatment while sleeping or relaxing. Another embodiment of the
circadian rhythm
treatment apparatus may be built into, part of, and/or include a lamp or a set
of lamps (controlled
by a controller), that works with a mobile application and uses a personalized
circadian rhythm
disorder treatment program (e.g., light pulse program) to treat or prevent
circadian rhythm
disorders. Motion sensors may be embedded in 602, 604, 606, 608, and/or 610 to
collect sleep
data. Environmental sensors, such as light sensors may be embedded in 602,
604, 606, 608, and/or
610 to capture data input required for the mathematical models to compute
circadian rhythm and
general light treatment programs. The circadian rhythm treatment program may
also work through
accessing the API of a program and/or hardware device (an example of such a
hardware device is
discussed below in conjunction with FIG. 5) that controls the collection of
Internet of Things (IoT)
devices including the ceiling lamp 602a, ceiling lamp 602b, table lamp 604a,
table lamp 604b
and/or other lamps or lights.
[0065] The circadian rhythm treatment system may also deliver treatment via a
computer display,
television display, game console display, or mobile device display, which may
be used for
illuminating a confined area while the user is sleeping in involved in another
activity (not related
to the computer display, television display, game console display, or mobile
device display. In an
embodiment, the pulses of light used for circadian rhythm treatment system are
too bright to be
comfortable if applied while directly watching screen providing the light.
However, the user is
free to use the circadian rhythm treatment while the person is working on the
computer or using
the mobile device, watching television, and/or playing a video game, anyways,
should the use
choose to do so. The circadian rhythm treatment program may run on a mobile
device (e.g., a cell
phone or laptop) that is left on while the user is sleeping or engaged in
other activities (but no
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
looking directly at the screen). The circadian rhythm treatment system may be
built into any
lighting system, such as in any part of a home, television, vehicle, and/or
outdoor area, in which
the circadian rhythm treatment hardware is built into a lighting system of the
room of a building
(e.g., a bedroom). The circadian rhythm treatment system may be built into any
lighting system,
such as in any part of a home, television, vehicle, and/or outdoor area.
[0066] Hardware
[0067] The system may obtain data about the user through sensors in the
circadian rhythm
treatment apparatus or in the mobile phone or through an APIs of other
software applications or
wearables. FIG. 7 shows a block diagram of the system, which may include
various hardware or
software devices as possible sources from where the system can obtain data.
System 700a may
include power 702, near field communications 704, environment sensors 706,
sleep sensors 708a.
System 700 may include light system 710. System 700 includes microprocessor
716a, pulse
modulator 716b, treatment logic 716c, brightness control 716d, sound system
718, which includes
an optional microphone 720 and speaker 722, other communications 724, and/or
other components
726. The sleep sensors 708a may include brain wave detector 708h such as dry-
electrode
Electroencephalography (EEG) sensors, body motion sensors 708e such as 3-6
axis accelerometers
and a gyroscope, a light and body motion sensor combination such as an
activity-monitoring
actigraphy, a heart rate sensor 708c, and/or a respiration sensor 708f. There
may also be sensors
for eye movements 708d, muscle tones 708i, and/or body temperature 708b. The
system may also
obtain data through API of other software applications such as calendar 728
and GPS 732, or
software that contains data collected by other wearable devices. The data may
be used to establish,
modify, or choose the most applicable algorithm to use, and/or as input for
the algorithm to
generate the circadian shifting program, and/or as feedback to evaluate the
effect of the program
and make necessary adjustments to the program. The environmental sensors may
include
temperature sensors, light sensor, sound sensor, and/or humidity sensors.
Environment light sensor
collects ambient light information, which is important for modeling circadian
rhythms. The details
will be discussed below. The sleep sensors may be used to determine the
sleep/wake and/or the
sleep stages and quality of the user's sleep, which may be used in the
circadian rhythm adjustment
algorithm, for example, to estimate the homeostatic sleep drive. The
sleep/wake and sleep stage
information may also be used in the circadian rhythm adjustment algorithm to
determine the timing
and/or intensity of the light emission. Additionally, information of light
programs and sleep stages
may also be used to correlate with the outcome of circadian rhythm adjustment,
to understand
whether the effect of circadian rhythm adjustment changes as lights bring
given at different sleep
stages.
[0068] Alternative or in additional to user input of profiles and schedules,
ambient light
information can be used directly as circadian rhythm mathematical model input.
In FIG 7, one of
the environment sensors 706 can be an ambient light sensor that collects light
data as input for the
circadian rhythm adjustment system. In one embodiment, the ambient light
sensor is a sensing
16
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
device separate from the circadian rhythm treatment device. In one embodiment,
the sensing
device may be wearable. In one embodiment, the sensing device may be a
wearable light sensor
in the form factor of a pin, a button, a brooch, a necklace, a chocker, or an
earring, or some form
factor that can be worn around the neck/chest area. The light-capturing part
of the hardware
generally faces the same direction as the user's face, to maximally mimic the
amount of light
received in the user's eyes. The wearable light sensor records the amount of
light that the user's
eyes are exposed to throughout the day. FIG 8 shows a block diagram of the
light sensor as
possible sources from where the system can obtain data. The light sensor has
multiple components:
the electronic components including a printed circuit board assembly, an
enclosure to house the
electronic components, and mechanisms that enables the sensor to be worn on
the user. System
800 may include power 802, near field communications 804 such as Bluetooth for
data
transmission, microprocessor 806 with data-processing logic 808, and memory
810 to store the
raw data and post-process data. System 800 include environment sensors such as
light sensor 812a,
motion sensors 812d, temperature sensor 812b, microphone 812c, and other
sensors 812e. System
800 may also include system status indicator 814 and other communication such
as USB 816.
[0069] Model input
[0070] A circadian rhythm adjusting system may begin by obtaining information
relating to the
user's circadian rhythm (e.g., information relating to times of sleep and/or
wakefulness). The
device may work with the mobile application with a user interface to take
input and generate
personalized circadian rhythm disorder treatment programs to treat or prevent
circadian rhythm
misalignment. The input variables of the model may be collected, via user
input from a mobile
application interface, information collected by sensors in the hardware,
and/or via APIs of other
software or hardware such as a wearable tracker, a central software data
source such as Apple
Health, a calendar system or a work scheduling system. The inputs for the
model may include
any one of, or any combination of, age, sex, eye colors, pupillometry, light
sensitivity, usual bed
time, usual wake time, current bed time, current wake time, usual sleep time,
desired shift/sleep
schedules, including desired bed time, desired wake time, desired sleep time,
travel schedules,
night shift schedules, and/or schedule constraints. In one embodiment, user
input may also
include nap times, day-time activities, food intake, exercise, caffeine
intake, other potential
factors influencing, and/or indicative of, wake and sleep. In an embodiment,
the user can input
multiple schedules at one time. The program then may automatically detect and
automatically
generate a program to accommodate all the schedules to implement at a given
time based on
calendar information input into a calendar and/or GPS information.
[0071] FIG 9 shows an exemplary process by which a circadian rhythm adjusting
system may
operate. As shown in FIG 9, the system takes input and creates a biological
profile for the user in
step 902, and creates the user's sleep profile in step 904. Both the
biological profile and the user's
sleep profile are stored as part of the user's long-term profile in step 906.
The biological profile
may include the user's age, sex, eye color, pupillometry, weight, height,
amount of exercise, light
17
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
sensitivity, etc. Information about the user's sleep profile may include the
user's typical bedtime
and wake time, the typical sleep efficiency, the number of hours the user
ordinarily sleeps, the
number of times the user gets up in the night, whether the user has a form of
insomnia, sleep apnea,
or other sleep disorder. The sleep profile may also include the chronotype of
family members.
Alternative or additionally, in one embodiment, the system may connect to the
API of other third-
party hardware to retrieve long-term sleep data. In one embodiment, the long-
term profile also
includes genetic information obtained from laboratory assays on sample
collected from the user
and/or third-party sources containing genetic testing information about the
user, for example,
personal genomics companies such as 23andMe. In one embodiment, the long-term
profile may
also include natural circadian rhythm baseline established by bio-samples
collected from the user
or from wearables with sensors such as actigraphy. In step 908, the
application then receives input
(e.g., entered by the user) that indicates the user's current and
target/desired sleep schedules, travel
information, work shift information, sleep constraints (e.g. from work and
social activities) and/or
other information to understand the circadian phase shift needs. For example,
the system may
adjust the circadian rhythm based on the user's current sleep schedules as
compared to the desired
sleep schedules, or based on the user's desired work and/or travel schedules,
where the user may
input information indicating the change in the sleep pattern desired, via a
graphical user interface.
[0072] In at least one embodiment, the inputs for the model may include sleep
data specifically
about the user, collected, or via sensors in the wearable devices and/or other
device, via an APIs
of software applications and/or via a user interface of an application and/or
through hardware in a
sleep mask or devices from which the light flashes are delivered. In this
specification, "sleep data"
may refer to data relating to sleep or which is probative of sleep and can be
used, alone or with
other data, to assess a user's sleep. For example, sleep data may include data
relating to one or
more of sleep schedules, wakefulness schedules, work schedules, flight
schedules, feasible sleep
windows, sleep constraints, circadian rhythms, sleep environment, light
exposure, sleep
disruptions, sleep quality, alertness level, physiological parameters relating
to sleep, including
movements, respiration, heart rate, brainwave, eye movement, muscle tones, and
so on. One
example of sleep data is ambient light data from a wearable light sensor in
the form factor of a pin,
a button, a brooch, a necklace, or an earring, or some form factor that can be
worn around the
neck/chest area facing generally the same direction as the user's face to
mimic the amount of light
received in the user's eyes throughout the day. The user will wear the light
sensor as much as
possible, except when showering and sleeping. When the user is sleeping, the
light sensor may still
be put facing upward next to the user's head to record the environmental light
intensity in the
bedroom during the time when the user is sleeping.
[0073] In at least one embodiment, an adjusted sleep treatment program is
implemented. The
system may generate an initial program based on the initial input. As the
sleep program is running
and new information is received, the treatment program is adjusted based on
information received.
The input may include data collected from sensors, for example, the ambient
light exposure data
or the sleep data, and user's feedback after using the device, for example,
if/when they use the
18
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
device on previous day(s). Based on new information, an updated program may be
generated to fit
the most current circadian clock of the user. The circadian rhythm treatment
system can be used
before, during, and/or after the occurrence of the change of sleep schedules
or time zones or
anytime when the user would like to improve sleep through optimizing circadian
rhythms and
alertness. Different programs may be established, presented, or started when
the user starts the
program depending on the time when the user open the circadian rhythm
application. The
adjustment to the treatment program may occur in the middle of a treatment
program and/or may
be applied to a future treatment.
[0074] In at least one embodiment, the inputs for the model may include data
collected from look-
alike demographics in laboratorial studies, or studies conducted by other
organizations. For
example, benchmark biological markers may include any one of, or any
combination of
pupillometry, iris color, salivary melatonin, core body temperature, plasma
cortisol, plasma
melatonin, and urinary melatonin that can be collected in appropriately
controlled inpatient
environment and used to estimate properties of the central circadian
pacemaker. In one
embodiment, the salivary Dim Light Melatonin Onset (DLMO) data may be obtained
in lab studies
upon light treatment. In one embodiment, salivary or urinary melatonin may be
collected and
analyzed in the lab or by an analysis kit operated by the user. In one
embodiment, ambient light
exposure data, sleep diaries, and other self-reported survey data may be
obtained in lab studies.
These data obtained for certain population and/or certain light treatment
programs may be used as
input variables to model Process C. Similarly, benchmark polysomnography
(PSG), EEG, EMG,
movements, and other biological markers can be collected and fed into the
mathematical mode to
estimate sleep/wake and the Process S and/or calculate the light treatment
program. For example,
movement data may be used to estimate how long the user has been awake for,
which may then be
used to calculate Process S. During the night, the body goes through several
sleep cycles (e.g. of
90 minutes to 120 minutes) with different stages. Each of the stages of sleep
has a different
brainwave frequency and amplitude: waking state/REM sleep: high-frequency (15-
60 Hz), low-
amplitude activity (-30 [tV); Ni: decreased frequency (4-8 Hz), increasing
amplitude (50-100
[tV); N2: (10-15 Hz, 50-150 [tV) spindles; N3: 0.5-4 Hz (100-200 [tV). EEG
sensors on the head
(which may be included in the mask or other elsewhere) can be used to detect
the distinct
brainwave signature in amplitude and frequency during different stages of
sleep. The EEG may
detect brainwaves during sleep, through which the system may be analyzed, to
determine
sleep/wake and/or stages of the sleep of the user. Other physiological markers
such as reduced
body movement and eye movement, slowed down heart rate and breathing,
decreased body
temperature, decreased blood pressure may also be used to determine sleep/wake
and/or stages of
the sleep of the user. During nocturnal awakenings (e.g. a result of the
flashes of light being too
bright), body movement increases, accompanied by a different eye movement
pattern from that in
N1-N3 sleep or REM sleep. The system may then adjust the circadian clock
adjustment program
based on the real-time input. For example, the light flash program may be
paused or reduced
temporarily if nocturnal awakenings were detected. Similar parameter changes
can be captured
by sensors in the system, used independently or in combination, to help inform
and adjust the light
19
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
program.
[0075] In one embodiment, genetics information correlated with circadian
rhythms, chronotypes,
sleep conditions or disorders may be used as part of the model, or be used to
define or refine
parameters of the model. As part of the circadian rhythm adjustment system,
there may be sample
collection devices to collect the saliva, urine, blood, or other bio-samples
from the user as part of
profile establishment or as part of each treatment. The samples may be sent to
the lab for analysis,
the results of which may be used in the model to estimate Process C and/or
Process S, or other
factors that may affect the circadian rhythm treatment program. For example,
genetic variants of
PER], PER2, PER3, or CLOCK may be used to inform the user's natural circadian
tendency.
Additionally, genetic variants of COMT, ADA, ADORA2A, DEC2, or ABCC9 may be
used to
inform the user's natural tendency on daytime sleepiness, brain wave patterns,
usual sleep duration
and estimate their homeostatic sleep drive. Related, but separately, in one
embodiment,
biomarkers such as melatonin may be collected from the saliva, urine, blood,
or other samples of
the user to establish a baseline for circadian rhythm. The biomarkers may be
used for users who
potentially have a natural circadian clock substantially different from 24
hours. For example, by
sampling and measuring salivary melatonin continuously over a period of time,
the system may
determine the natural circadian rhythm of the user. If the user has a
circadian cycle that is 28 hours
instead of the typical 24 hours, the period T used in the model will be
replaced by the actual period
obtained from melatonin monitoring rather than a pre-assumed value of 24 hours
from the general
population or lab-generated data on look-alike demographic. As a similar
example, if mutation in
CRY] which is associated with delayed sleep phase disorder is detected by
genetic testing, then
the model may assume that the user has a naturally delayed circadian phase and
use the information
to adjust the circadian rhythm estimation model.
[0076] Model
[0077] The regulation of the sleep-wake cycle is determined by many factors,
including genetics,
circadian rhythms, homeostatic sleep drive, sleep environment, conscious
decisions and behaviors,
and so on. The factors that determine the regulation of sleep may play a part
in the circadian
rhythm misalignment cases described above. Therefore, in at least one
embodiment of the
circadian rhythm adjustment system, to account for the circadian rhythm
misalignment scenarios
such as night shifts, instead of the circadian rhythm adjustment algorithm
only considering
circadian rhythm misalignment based only on the estimation of the PRC based on
current and
target sleep schedules, a model that includes multiple factors that regulate
sleep and wake cycles,
such as the circadian rhythm (Process C) and the homeostatic sleep drive
(Process S), and based
on the combination of multiple factors (e.g. sleep schedule constraints and
conscious choices), the
windows of time during which to apply a light treatment in order to shift the
circadian rhythm are
determined. As shown in the flow diagram in FIG. 9, after taking model inputs,
a circadian rhythm
adjusting algorithm 912 may compute both Process C in step 910a and Process S
in step 910b and
their interactions in regulating sleep/wake cycles to form a circadian rhythm
adjusting program.
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
The output of circadian rhythm adjusting algorithm 912 may then be
incorporated into a light
therapy algorithm to generate the treatment program in step 914 for the
specific user and the
specific schedules. The treatment program may then be sent to the circadian
rhythm adjustment
device in step 916 to deliver the light program. The model may be calibrated
anytime during the
process using additional information such as data collected from sensors in
step 918 and/or user
feedback in step 920.
[0078] FIG. 10 shows a block diagram of a circadian rhythm adjusting system
with model input,
computation, and model output, in which the computation module calculates
sleep wake cycle via
analyzing Process C and Process S. Model input 1001 may include external
conditions 1002,
Zeitgeber 1004, markers for peripheral oscillators 1008, and schedules
determined by conscious
decisions 1010. Zeitgeber 1004 refers to physical and social events which
entrain the circadian
clock, for example, light from the environment. The suprachiasmatic nucleus
(SCN) 1006 is the
central circadian pacemaker in the brain. SCN regulates a number of markers
for the circadian
clock, including peripheral oscillators, such as melatonin and core body
temperature. In one
embodiment, signals from SCN are included as model input. The computation
module or the
modeling module 1003 include computation of Process C 1012a for the circadian
rhythm (C) and
Process S 1012b for the homeostatic sleep drive (S). The homeostatic sleep
drive is a function of
how long a person has been awake. It builds up during wakefulness and declines
monotonically
during sleep. Together, Process C and Process S regulate sleep. As shown in
the sleep wake cycle
1012c, sleep switches on when the distance between S and C reaches maximum and
switches off
when the distance reaches minimum. The model may estimate sleep and
wakefulness 1012c by
calculating both Process C 1012a and Process S 1012b, and generate a predicted
PRC and the
optimal way to shift circadian phases. Based on the model output, a circadian
rhythm therapy
programs (e.g., treatment regimen) 1014 may be generated to shift circadian
phases. The light
program generated from the algorithm will then be sent to the circadian rhythm
adjustment device
1016. In at least one embodiment, the main outputs for the model may be
suggested timings for
light therapy based on the PRC. Programs generated by the system to delay
circadian phases
happens during hour 15 to hour 24 (CT15-CT24), advance during hour 24 to hour
8 (CT24-CT8)
of the subject's effective circadian time, in which CTO is defined as the
cross-over point of the
human phase-response curve. The cross-over point may be defined as the time of
the fitted
minimum of unmasked core body temperature. The system may predict the time
that corresponds
to CTO based on the information described before (the inputs to the C and/or S
models), such as
the user's biological profile, sleep schedules, travel or shift schedules,
physiological data obtained
from sensors, and/or other information obtained in lab studies. In one
embodiment, the system
may suggest specific light stimulation timing to delay or advance circadian
clocks, together with
other light flash parameters. The light timing will be incorporated into the
circadian adjusting
algorithm 1014.
[0079] The computational module may be designed to optimize for at least one
of two outcomes,
or both if possible: (1) maximizing circadian shifts to achieve the largest
overlap between the
21
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
"sleep" phase of the circadian clock with desired sleep time, and the largest
overlap between the
"awake" phase of the circadian clock with desired windows of wakefulness; and
(2) instead of
maximizing the amount of circadian phase shift, calculating the circadian
phase shift so the
window of high alertness can be best overlapped with desired window when peak
performances
are required or so the window of low alertness can be best avoided from when
peak performances
are required. Depending on the applications, the model may use different
algorithms to handle
different tiers of complexity, including (but not limited to) the following
scenarios: (1) When sleep
schedule change is simple, the model may only consider the difference in
circadian phases and
maximize circadian shifts to achieve most alignment. (2) When sleep schedule
(and/or geographic
location) changes are predictable, regular, with good sleep consistency that
lasts for long enough
to allow full entrainment by light therapy, the model may consider the
additive effect between
circadian rhythms and homeostatic sleep drive to maximize circadian shifts,
with the assumption
that these two processes are independent. (3) When sleep schedules are
irregular, changing,
unpredictable, or significant over a short amount of time that may be
accompanied with prolong
wakefulness and/or naps, the model may optimize for maximal overlap of
circadian phase with
desired sleep-wake schedule, take into account of both circadian rhythms and
homeostatic sleep
drive, with the assumption that these two processes are independent.
Alternatively, the model may
consider the nonadditive relationship caused by the interaction between these
two processes and
how one process affects the other when responding to regulatory signals. (4)
Instead of optimizing
for the maximal circadian phase shift, the model may optimize for performance
by overlapping the
maximal alertness with the windows when peak performance is required. The
model may consider
both circadian rhythms and homeostatic sleep drive, with the assumption of
circadian rhythms and
homeostatic sleep drive being essentially independent. (5) In cases with
prolong wakefulness
and/or naps, the model may optimize for performance during the windows when
peak performance
is required, while taking into account the interdependency between circadian
rhythms and
homeostatic sleep drive. (6) In the presence of other sleep-regulating factors
(e.g. genetic
components) that may significantly affect circadian rhythms and homeostatic
sleep drive directly
or indirectly, the model may take them into consideration and give them
corresponding weight in
the calculation.
[0080] To model Process C, one or the combination of any of at least three
modeling approaches
may be used, which may include (1) modeling the physiological components of
the system; (2)
using mathematical models to match the dynamical properties of the system; (3)
data-driven
modeling or statistical fitting of the data. For example, each of these three
approaches may be
different aspects of the same model. The Process C can be generally be
considered as (or at least
approximated by) a sinusoid function or a skewed sine wave of f (t) = a
sin(cot + c), with
amplitude a, angular frequency co, period ¨27, and phase c (and/or another
harmonic function),
while t is time, Similarly, the function f (t) = a cos(cox + c) , f (t) = a
e+i(ca+c) and/or a
combination of the three (for example) could be used instead (however, the
value of c depends on
the choice or functions). Similarly, since the above functions can be expanded
in terms of any set
22
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
of orthogonal functions, other periodic functions may be used, such as Bessel
functions. In at least
one embodiment, f (t) is a sum of harmonic functions (e.g., a sum of sine
and/or cosine functions
and/or a Fourier series). Multiple physiological parameters display periodic
level changes, which
can be used to determine the phase of Process C directly. Data collected from
the circadian rhythm
adjustment system may also be used to determine the phase of Process C or the
parameters in the
equation to model Process C. For example, biological markers of the circadian
clock collected in
appropriately controlled inpatient environment from the user or look-alike
populations may be
used to model circadian rhythms. These biological markers include a
combination of one or more
of core body temperature, plasma cortisol, plasma melatonin, and salivary
melatonin, and so on.
Process C can be expressed as the oscillation of core body temperature, where
the lowest point of
the circadian wakefulness signal overlaps with the temperature nadir, CTO (the
time of the fitted
minimum of unmasked core body temperature). Once CTO is set from core body
temperature
measurement or calculation based on other physiological parameters such as
melatonin level or
other data such as sleep schedules. Based on CTO, the PRC may be calculated
(FIG 1102), based
on which the light treatment window for delaying circadian phase can then be
calculated as CT15-
CT24 (FIG 1 104), whereas light treatment window for advance circadian phase
can then be
calculated as CT24-CT8 (FIG 1106). (note that at cot + c = CTO, f (t) reaches
its minimum value,
and so the argument cot + c = ¨7r/2, and thus if t is the time on the clock, c
is a measure of time
duration between midnight and the time at which CTO occurs). In some people
with normal sleep
habits and circadian rhythms, CTO may occur between about 3:00 am and 7:00 am,
but may occur
at other times also, depending on the individual. In at least one embodiment,
value used for the
period T or angular frequency co (w= ¨27) is based on an average value, which
may be determined
by measuring co randomized cross section of different people. In at least one
embodiment, the
period is set as 24 or 24.15 hours for general purpose circadian modeling, and
other specific
numbers may be used when modeling for specific populations (and/or
individuals). For example,
a given population or individual may have a circadian clock period of 28 hours
instead of 24 hours.
In at least one embodiment, the circadian clock period may be set based on the
biological sex of
the user, with 24.19 for males and 24.09 for females. In at least one
embodiment, the circadian
clock period and amplitude may be set to demographic baseline according to the
age of the user.
In at least another embodiment, the period is determined based on emprical
data of certain
demographics which may be slightly larger or smaller than one day 24 hours,
for example, in
teenagers, and it would seem that co may vary between individuals. In an
embodiment, the
circadian clock baseline may be established by continues melatonin measurement
from the user's
saliva sample or sleep-wake cycle recording with actigraphy data. In at least
one embodiment,
biological markers obtained from sleep study participants in strictly
controlled lab environment
and/or empirical data aggregated from many individuals may first be used to
establish the models.
The parameters of the model may then be further calibrated by data collected
outside the lab from
each individual, for example, via sensors in the mask, sensors in the ambient
light sensing device,
or other wearable device(s). In at least one embodiment, the biological
markers or the model
generated based on the biological markers may first be correlated with model
inputs that can be
23
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
collected from a non-laboratorial environment. The model may then be applied
to estimate the
circadian phases of users in a non-laboratorial environment, based on user
inputs collected from
the mobile application interface or sensors from the hardware, or via API from
third party
applications. Examples of empirical data collected by the system based on the
user's individual
experiences/behaviors may include: bed time and wake time, core body
temperature, heart rate,
melatonin, cortisol, and so on. Depending on the similarity of the user with
the experiment
participants, adjustments on the model may be applied when estimating the
circadian phase of each
individual user. In at least one embodiment, as the user uses the system and
more data is collected
about the user, the model is adjusted to the user's individual body and
situation, and after sufficient
use, the model may be entirely based on the user's individual body and
situation. User feedback
may be collected via user input or sensors in the hardware after the treatment
sessions, to further
calibrate the model for each user and/or to further perfect the model for
handling users for which
no data has been collected yet. For data collected outside of an appropriately
controlled lab
environment, variability introduced by noncircadian factors such as behaviors
or schedule
constraints may need to be compensated for. In some embodiments, the model may
be matched
to the oscillations behaviors of the circadian system. Stochastic models may
also be used to
account for the noise and randomness of the system, depending on the purpose
of modeling. In
some embodiments, data-driven modeling or statistical fitting may be used to
model circadian
rhythms. For example, Process C can be estimated by the following equation:
[0081] C = A{0.97 sin[w(t ¨ to] + 0.22sin[2w(t ¨ to] + 0.07sin[3w(t ¨ to] +
0.03sin[4w(t ¨ to] + 0.001sin[5w(t ¨ toll
[0082] w = ¨27
[0083] In which: C=Process C (note capital C is different than the phase,
which is represented by
the lower case c), which is independent of Process S; A=amplitude of skewed
sine wave; t=time;
r=period of C; to defines the circadian phase at the beginning of the
stimulation.
[0084] The constants of this sinusoidal curve can be fitted using regression
analysis to the
melatonin or core body temperature data collected in the lab in strictly
controlled environment that
share various levels of similarity with the end user of the circadian rhythm
adjustment system.
When using biological marker such as melatonin and core body temperature, some
rhythmic
properties of the circadian system, such as the period, amplitude, and phase,
can be extracted via
statistical models. Once the model parameters are determined, it can be used
as a base model for
look-alike populations which may be further calibrated with user feedback or
data captured as the
user uses the device. More generally, C = faisin[w(t ¨ to] + a2sin[2w(t ¨
to] +
a3sin[3w(t ¨ to] + a4sin[4w(t ¨ to] + a5sin[5w(t ¨ top} , where the set of
amplitudes,
a2, a3, a4, as), may be determine empirically, and/or first assumed to be from
the literature or
lab studies on look-alike subjects, and then adjusted over time to values
determined based on
sensor input and user input to the system about the user. In at least an
embodiment, higher order
24
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
terms may be added (e.g., for someone without a regular sleep time, but that
is forced to have
periods of napping and being wake through the day and night). In at least one
embodiment, each
term may have a different value for co and/or to.
[0085] The model may also estimate Process S and the linear (e.g. additive)
relationship between
Process C and Process S, with the assumption that Process C and Process S are
independent
without interfering with each other. As shown in FIG 10 1012b, the sleep drive
builds up during
wakefulness and declines monotonically during sleep. The rate of increase and
decrease of S may
be estimated using an exponential model. For example, with a rise factor of r
and a delay factor
of d, Process S may be modeled using the following equation:
-At
[0086] Sleep: St = dSt_i; d = e td
-At
[0087] Wake: St = 1 ¨ r(St_i); r = e Tr
[0088] In which, S=Process S; d=decay factor of S; r=rise factor of S; Td and
Tr = time constants;
At= time step (which may be 30 min or other values as modeling requires); t &
t ¨ 1= time indices
between time step.
[0089] The function d, Td, and/or rrmay be obtained through literature or
empirical data from
look-alike subjects in lab studies and/or data collected the end user of the
circadian rhythm
adjustment system. For example, in laboratorial studies, the decay rate of S
may be calculated
from EEG measurements on experimental subjects and used in the model for look-
alike subjects
outside of the lab. Alternatively, in at least one embodiment, brainwave
signals may be captured
from EEG sensors implemented on the circadian-adjusting hardware (for example,
a sleep mask)
through contacts around forehead. In at least one embodiment, the sleep
pressure may be estimated
using a default r value (for example, from literature or laboratorial studies)
and then adjusted when
more data is collected from the user by sensors in the circadian-adjusting
hardware or other
wearables. The homeostatic sleep pressure may also be measured by the length
of time staying
awake that can be deferred from the sleep/work schedules or length of time
that is required for one
to fall asleep. The data input for this estimation may be captured by sensors,
such as an EEG,
accelerometer (e.g., or 3-6 axis accelerometers), a gyroscope, an activity-
monitoring actigraphy,
an optical pulse sensor, a respiration sensor, from third-party wearables,
applications (e.g.,
downloadable apps), estimated by the sleep onset latency from sleep diaries,
and/or other user
input. As mentioned above, there may be a different accelerometer and/or
gyroscope for or more
of three different, non-parallel axes, where the three axes optionally maybe
perpendicular to one
another. Alternatively, the sleep pressure may also be inferred from measuring
one's alertness,
the tendency for the user to dose off unintentionally, the changes in the
tendency of the user to
make mistakes (e.g., the number of typographical mistakes made), or user
input. Alternatively or
additionally, the alertness may be determined from the number of typographical
mistakes made
can be measured by monitoring the user's interactions with the user's cell
phone and/or computer
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
(or other electronic device) and counting spelling errors (or other types of
clerical errors)
measuring speed of data entry, and/or analysis of voice input during normal
usage (the speed of
ones speech and speech errors may change depending on how alert one is).
Alternatively or
additionally, the user may be asked to copy text during the different times of
the day and speed
and accuracy can be measured.
[0090] When modeling the additive relationship between Process C and Process
S, a model in Fig
11 may be used. In the embodiment of FIG 11, Process C 1102 is considered as a
sin wave curve
that has a higher threshold H and a lower threshold L (in other embodiments a
different curve may
be used for the C Process. Process S 1104 builds up during wakefulness and
dissipates during
sleep. Sleep 1101 switches on when S reaches the higher threshold H, and
switches off when S
reaches the lower threshold L. The frequency of sleep-wake alternations
depends at least in part
on the interval between H and L and on the rate of buildup and breakdown of S.
Quantitative
estimates of the C threshold variations can be derived by results from prior
studies, or by measuring
either the tendency to go to sleep during wakefulness, the tendency to wake up
during sleep, and/or
from the circadian oscillation from the S level at the time of awakening,
which can be calculated
directly from the duration of sleep in subjects waking up at different times
of the day. The
instantaneous breakdown rate of S can be calculated using an exponential model
(in other
embodiments other functions that increase with increases in time may be used,
such as a
polynomial). Sleep initiates when S > H + C, and terminates when S <L + C. The
model may
start with the parameters commonly used to estimate spontaneous sleep
termination and onset,
which may be taken as A = 0.12; x = 24 h; L = 0.17; H = 0.67; At =0.5h. Based
on more
information such as travel and/or night shift schedules, the parameters may be
updated. The
circadian rhythm adjustment system may also update the parameters based on
other information,
such as user input on their biological profiles and normal sleep schedules, or
data collected on
wearables in the system or through the third party, such as actigraphy and/or
EEG readings. After
being used for an extended period of time, the system may also refine the
parameters based on user
feedback or biofeedback on the sleep or the circadian rhythm as a result of
each schedule change
and corresponding treatment. In cases where the user takes a nap during the
period of wakefulness,
the homeostatic sleep drive changes, which also may be accountable for.
Process S may be
dissipated with naps whereas be accumulated with prolonged wakefulness.
Therefore, the timing
and duration of naps and/or prolong wakefulness may be part of the model input
when applicable.
The timing and duration of naps and/or prolong wakefulness may also be part of
the model output
as schedule suggestions for the user.
[0091] In the presence of substantial circadian phase misalignment such as
night shifts or military
deployment, full entrainment may not be physiologically feasible, but
performances during a
certain time frame may still be optimized and prioritized. In scenarios where
full entrainment is
not feasible, the primary parameter to optimize is the period of alertness
when peak performance
is desired. FIG 12 shows how the wake propensity is regulated by Process C and
Process S. In
the plot, upward pointing arrows 1202 indicate the circadian signal for
alertness, which promotes
26
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
propensity to wake. Downward pointing arrows 1204 reprent the sleep
homeostatic drive, which
promotes propensity to sleep. The larger the arrow, the greater the
propensity. A wake propensity
curve 1206 is plotted, which is computed from the homeostatic sleep load and
the circadian
oscillation. The the sleep period is represented as 1208, while the wake
period is represented as
1210. While the homeostatic sleep pressure starts growing and keeps building
up when the user
is awake, late in the afternoon, the circadian signal for wakefulness kicks in
and may override the
homeostatic drive for sleep. The circadian alerting signal continues to
increase into the night and
peaks around 9 pm, offsetting the build-up of homeostatic pressure and
allowing us to stay awake
well into the evening and thereby achieve our human pattern of consolidated
sleep and
wakefulness. There is often a dip (shown as box 1203) in the late afternoon,
when the homeostatic
drive has been building for hours, but the circadian signal has not yet kicked
in yet (that is the
circadian signal has not yet become strong enough so that the person does not
feel sleepy ¨ but
instead the person feels sleepy). If the application prioritizes performance
during a certain time
window while the user is awake, instead of shifting the circadian phase to a
maximal magnitude,
the model may shift the circadian phase, so that a time window with a local
peak wake propensity,
as shown in box 1201 and box 1205 in Fig 12, are overlapped with the desired
window when peak
performance is requried. Alternatively, the model may shift the circadian
phase so that the time
with the local lowest wake propensity, as shown in box 1203, may be moved out
of the desired
window when peak performance is requried. In addition to suggesting the light
therapy parameters
for circadian phase shifts, the model may also suggest timing for naps or
caffeine intake during
wakefulness, among other factors that influence S, to achieve performance
optimization.
[0092] In some circadian rhythm misalignment scenarios, especially during
night shifts, during
periods of partial sleep restriction or complete sleep restriction are
involved, Process S and Process
C are considered inter-dependent in the model. For example, scheduling of
sleep and waking may
alter the timing of light exposure and thereby affect the phase, amplitude,
and/or period of C.
Additionally, circadian rhythm adjustment in response to Zeitgeber may be less
effective when the
homeostatic sleep drive is high, which may change the circadian waveform
between cycles when
there is a partial or complete sleep restriction. Therefore, the impact of
Process S on the shape of
Process C needs to be considered. Meanwhile, where prolonged waking occurs,
the circadian
phase may modify the level of subsequent slow wave activity, so that the
success of predicting the
level of S may depend upon the time of day and not be simply a function of
previous wake duration.
In these scenarios, adjustments may be made in the model in order to
compensate for the
interaction. In an embodiment, the period (T), phase (c), and/or the
amplitudes (a), may be
functions of S. In at least one embodiment, an interaction between the C
process and the S process
and an interaction term may be added to C and/or S, which may have the forms
of a * F (Co, So),
where a is a proportionality constant, "F(,)" represents an operation ¨ which
may be addition,
convolution, correlation, and/or other operation, and Co and So are the
independent models, or
alternatively the interaction term may have another form). For example, may be
So and Co are two
different fucntions of time with different parameters). So for example, a
*F(S,C) might be given
by a *F(Co,So)= a *So(t) * Co(t) (where "*" is just a simple mulitplicaiton)
or it might be that
27
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
F(Co,So) = So(t)/Co(t) or it might be that F(Co,So) = f S(t)C(t)dt.
Similarly, if
a *F(Co,So)= a *So(t)* Co(t), the full model may be repesented as f(t)=
Co(t)+So(t)+ a F(Co,So) or
f(t) = Co(t)+So(t)+ a Co(t)*So(t).
[0093] In at least one of the embodiments, in use cases when light treatment
is applied on multiple
days, the circadian phase shift caused by light (Process L) may be introduced
to model the new
phase response curve after each treatment cycle either before or in the middle
of the multi-day
treatment program. The phase shift caused by the light programs Ac may be
modeled based on
empirical data on the phase shifts caused by similar light treatment program
that were measured
physiological data collected from the lab using the circadian rhythm
adjustment system or
comparable treatment programs, or inferred by sleep/wake data and/or other
data collected by the
circadian rhythm adjustment system. In one embodiment, different light
treatment program may
be used throughout the course of the circadian rhythm adjustment, and
different Ac or different
PRC may be used in the model based on the light treatment program utilized in
the system on
different days.
[0094] As an alternative to regression-based methods, time series for
circadian markers can be
analyzed using spectral methods such as Fourier analysis, periodograms,
spectrograms, wavelet-
based methods, autocorrelation, and Hilbert transforms. Other methods such as
Bayesian spectral
analysis and detrended fluctuation analysis may also be used to analyze and/or
model circadian
data. Depending on the input, different mathematical models may be chosen to
generate the
circadian rhythm adjustment program based on the magnitude of circadian rhythm
misalignment,
the time required for entrainment, and the sleep schedule constraints that may
affect when the
device may be used, and if and how sleep deprivation may affect the
homeostatic sleep drive.
[0095] Model output
[0096] Light therapy programs will be generated in the software (e.g., a
mobile, desktop, or web-
based application) based on the mathematical model outputs. In at least one
embodiment, the main
model output may include a prediction of circadian PRC based on the user's
circadian clock on
any point between the start and the end of the circadian rhythm adjustment,
the type of light
treatment used, and the expected circadian phase shifts caused by the light
treatment. Based on
the PRC, the CTO and the treatment windows in which light flashes can be
delivered to advance
or delay circadian phases will be determined. Based on the treatment windows
and the user's sleep
schedule constraints, a proposed sleep schedules will be generated. The light
treatment program
will be generated based on the timing of light pulse treatment and the other
light flash setting such
as intensity, duration, frequency, and wavelength, etc. In at least one
embodiment, programs
generated by the system to delay circadian phases happens during hour 15 to
hour 24 (CT15-CT24),
advance during hour 24 to hour 8 (CT24-CT8) of the subject's effective
circadian time, in which
CTO is defined as the cross-over point of the human phase-response curve. The
cross-over point
may be defined as the time of the fitted minimum of unmasked core body
temperature. The system
28
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
may predict the time that corresponds to CTO based on the information
described before (the inputs
to the C and/or S models), such as the user's biological profile, sleep
schedules, travel or shift
schedules, physiological data obtained from sensors, and/or other information
obtained in lab
studies.
[0097] In one embodiment, the system may generate a set of recommended
treatment schedules
to achieve the most optimized result and get feedback from the user about the
feasibility of the
schedule, based on which a final, negotiated schedule may be generated, and
parameters of the
mathematical model may be adjusted accordingly. In one embodiment, the system
may generate
a set of general recommended treatment schedules based on universal
assumptions, and then adjust
according to the user's specific variables that may impact their specific
circadian rhythm
computation. The mathematical model generated light treatment schedules may
then be
incorporated into a light program algorithm. A set of light therapy parameters
of the treatment
program may then be sent to the circadian rhythm adjustment hardware that
delivers the light
therapy, and the light therapy program (e.g., the treatment regimen) will be
delivered to the user
to shift circadian phases. The treatment may be a pattern of light pulses
and/or other changes in
lighting that are delivered to the user, via the circadian rhythm treatment
apparatus that delivers
the treatment (e.g., via a program that controls the lighting, such as by
controlling pulses of light
delivered to the user) to effectively treat or prevent circadian rhythm
disorders. The treatment may
be applied while the subject is asleep and/or awake in the middle of sleep or
within 1-2 hours
before or after sleep.
[0098] Alternatively, the model may output a basic function to estimate the
circadian phase, the
parameters of which may be data that can be captured from one or any of the
combination of user
input, sensors in the circadian treatment system, third party software, and/or
third party hardware.
Once the variables are input through the user interface, hardware sensors such
as the ambient light
sensor, and results from the lab studies, the model may generate an estimated
PRC and windows
in which light flashes or other light programs can be delivered to advance or
delay circadian phases.
In one embodiment, the model output may include recommendation for bedtime,
wake time, light
exposure, timings for meals, timings for exercise, and/or caffeine intake.
[0099] Based on the model output, light therapy programs including specific
light therapy
parameters may be generated and executed through the hardware in the circadian
rhythm
adjustment system. The light program may include different light pulse
settings such as
wavelength of the light, duration of pulses of light, interval of time during
which pulses of light
are delivered, the frequency of the pulses, the timing, and duration of the
circadian rhythm disorder
treatment program (e.g., via the light program). The light program may also
include the timing,
intensity, and wavelength of continuous light that works together or
independently of the light
flash program. The circadian rhythm treatment apparatus may deliver the
treatment automatically
and/or when activated. The user may have the option to adjust the parameters
within the ranges
that the manufacturer allows. For example, the user can choose different
wavelengths of the light,
29
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
different timings, and different durations of treatment administered by the
mobile interface in the
circadian rhythm disorder treatment program.
[0100] In at least one embodiment, as the user uses the circadian therapy
program, more data on
the changes and response to circadian rhythm shift may be gathered to
establish a more accurate
baseline (and improve information about the user's homeostasis sleep load),
and the application
may incorporate the data into computation of the circadian rhythm disorder
treatment program
(e.g., light program). In an embodiment, the light therapy program is dynamic
and can be
improved based on additional information collected each day from the user, the
circadian rhythm
treatment apparatus, or sensors in additional hardware. For example, there may
be sleep sensors
in the mask that track the sleep schedule and sleep/wake or stages of sleep of
the user, such as by
detecting the baseline of relevant physiological parameters, whether the
user's eyes are closed,
the user's head and body movements, the user's heart rate, and/or breathing
pattern. The sensors
may also collect information related to how alert the user is during a portion
of the day. In one
embodiment, a wearable light sensor that records ambient light exposure may be
used as input to
model/calibrate the circadian clock of the user each day. In one embodiment,
the user may be
asked about when and for how long they used the circadian rhythm treatment
apparatus from the
day before. In one embodiment, the information about circadian rhythm
treatment device usage
may be collected by a capacitive touch sensor that distinguish whether the
device is in contact
with the user's skin. The information may be used to calibrate the PRC
estimation. In one
embodiment, the system may contain a sample collecting and testing kit to
measure physiological
biomarkers for circadian rhythms, such as melatonin level in saliva, blood, or
urine. The level of
these biomarkers may be detected via assays run in real time, the results of
which may be used to
establish the baseline or to refine the model. The model may first establish a
baseline shift
program based on the average shift that can be achieved on a look-alike
populational level, and
then the application may adjust itself based on the sleep time of the user
once the program starts.
For example, if the mask (and/or system) determines that the user has achieved
the entire shift in
the user's circadian rhythm earlier than the populational average time for
achieving the same shift
in circadian rhythm, then the program may stop. The user can also stop the
program manually
based on how adjusted the user feels.
[0101] There may be a feedback mechanism to further calibrate the model or
improve the circadian
rhythm adjustment program throughout the treatment process. In at least one
embodiment, the
light therapy program may be dynamic and may be improved based on additional
information
collected each day from the user or from sensors on the circadian rhythm
treatment apparatus. As
the user uses the circadian therapy program, more data on the changes and
response to circadian
rhythm shift may be gathered to establish a more accurate estimate on the
user's circadian rhythm
and homeostasis sleep pressure. The data may be fed into the model and adjust
the circadian phase
shift program accordingly. For example, in at least one embodiment, the system
may record sleep
information from a prior day (or other prior time period) or from real time
information to know
how much of a shift has been generated in a user, and the predicted PRC may be
refined. Then
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
based on one or more of the refined model, the refined model output, and the
refined predicted
PRC, the light therapy instructions may be adjusted to improve treatment
efficacy. In at least one
embodiment, the circadian rhythm adjustment system uses data captured from a
wearable ambient
light sensor to adjust the circadian rhythm treatment program on a daily
basis. As shown in flow
chart 1400 of FIG 14, step 1402 illustrates the process of collecting the
ambient light sensor
reading and using it as part of the model input. Based on the ambient light
data, a current circadian
phase of the user may be computed, and a circadian rhythm adjustment program
may be proposed
based on the user's target circadian phase. As shown in step 1404, a similar
process of data
collection may also be performed on day 2. Based on the ambient light data
input, together with
other user feedback such as when the user slept and used the mask the date
before, an updated
circadian rhythm adjustment program may be proposed based on the user's
circadian phase on day
2 and the target circadian phase. The process may repeat until the end of the
treatment regimen.
Step 1406 is an example of process of data collection that leads to light
treatment program update
on day N. In step 1408, if the circadian phase shift has not been achieved to
the desire level on
the target day of shift or performance based on user feedback or sensor data,
a similar program
adjustment process as steps 1402-1406 may be initiated. In one embodiment,
different light
treatment program may be used throughout the course of the circadian rhythm
adjustment, and
different Ac or different PRC may be used in the model based on the light
treatment program
utilized in the system on different days.
[0102] FIGS 15, 16, and 17 show an exemplary methods for adjusting a user's
circadian rhythm.
In some embodiments, the methods shown in FIGS 15, 16, and 17 may be performed
using any of
the systems described above with respect to FIG 1-14. In FIGS 15, 16, and 17,
dashed lines
indicate optional steps. Steps shown in solid lines may also be omitted in
some embodiments.
[0103] FIG 15 shows an exemplary method 1500 which may generally include steps
of collecting
input, generating a circadian rhythm adjustment program, and delivering
treatment in accordance
with the generated instructions. In step 1502, the system may collect
information related to the
user's present circadian rhythm. Such information may include the user's
biological profile and
sleep profiles, schedules about sleep and/or wakefulness, environmental light
exposure, and
chronotype. The data may be collected via a user interface on a software, from
sensors in device,
from lab assays, and/or other software or hardware such as a wearable tracker,
a central software
data source such as Apple Health, a calendar system, or a work scheduling
system. In at least one
embodiment, the biological profile may include the user's age, sex, eye color,
pupillometry, weight,
height, amount of exercise, light sensitivity, etc. In at least one
embodiment, information about
the user's sleep profile may include the user's typical bedtime and wake time,
the typical sleep
efficiency, the number of hours the user ordinarily sleeps, the number of
times the user gets up in
the night, whether the user has a form of insomnia, sleep apnea, or other
sleep disorder. In one
embodiment, the sleep profile may also include the chronotype of family
members. In at least one
embodiment, the inputs for the model may include data specifically about the
user, collected via
wearable devices and/or other device, via an APIs of software applications,
via a user interface of
31
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
an application, and/or through hardware in a sleep mask or devices from which
the light flashes
are delivered. In at least one embodiment, the information may include data
collected sensors in
the circadian rhythm treatment device, or sensors from other wearables such as
actigraphy. In at
least one embodiment, the data may include ambient light data from a wearable
light sensor in the
form factor of a pin, a button, a brooch, a necklace, or an earring, or some
form factor that can be
worn around the neck/chest area facing generally the same direction as the
user's face to mimic
the amount of light received in the user's eyes throughout the day. In at
least one embodiment,
the inputs for the model may include data collected from look-alike
demographics in laboratorial
studies, or studies conducted by other organizations. For example, benchmark
biological markers
may include any one of, or any combination of pupillometry, iris color,
salivary melatonin, core
body temperature, plasma cortisol, plasma melatonin, and urinary melatonin
that can be collected
in appropriately controlled inpatient environment and used to estimate
properties of the central
circadian pacemaker. In one embodiment, the salivary Dim Light Melatonin Onset
(DLMO) data
may be obtained in lab studies upon light treatment. In one embodiment,
salivary or urinary
melatonin may be collected and analyzed in the lab or by an analysis kit
operated by the user. In
one embodiment, ambient light exposure data, sleep diaries, and other self-
reported survey data
may be obtained in lab studies. In one embodiment, the information includes
genetics information
correlated with circadian rhythms, chronotypes, sleep conditions or disorders,
which may be
obtained from laboratory assays on sample collected from the user and/or third-
party sources
containing genetic testing information about the user, for example, personal
genomics companies
such as 23andMe. In at least an embodiment, multiple users may use the same
device at different
times. Each user may have his/her own account with past programs, biological,
sleep, homeostasis
sleep load profiles, and behavioral profiles. Based on the information
collected throughout the
time that user uses the circadian rhythm treatment apparatus, the software
adjusts and improves
itself to provide personalized circadian rhythm disorder treatment program
(e.g., light program).
[0104] In optional step 1504, the system may collect information relating to
one or more
anticipated times of sleep and/or wakefulness, for the user, on one or more
days. In some
embodiments, this step collects any one of, or any combination of, the user's
current and target
sleep schedules, travel information, time zone information, work shift
schedule, sleep constraints
(e.g. from work and social activities), sleep schedule preferences, daylight
saving information,
and/or other information to understand the circadian phase shift needs. In at
least one embodiment,
the information may include past and/or present times of sleep and/or
wakefulness. In at least one
embodiment, the information may be collected from user input via a software
interface. In at least
one embodiment, the information may be collected from a GPS location or a
location-specification
system. In at least one embodiment, the information may be collected via a
calendar system, a
flight-scheduling system, or a work-scheduling system. In at least one
embodiment, the
information may be collected from time zone information, latitude information,
sunrise/sunset
times information via a GIS system. In at least one embodiment, the
information may be deduced
from past activities or schedule templates derived from past activities. In
one embodiment, the
difference between current and target sleep schedules may be collected in a
format of certain
32
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
magnitude of circadian phase misalignment (e.g. 3 hours of circadian phase
misalignment). In at
least one embodiment, the information to estimate current and/or target
circadian rhythm phase
may be collected by an ambient light sensor. The circadian rhythm treatment
system can be used
before, during, and/or after the occurrence of the change of sleep schedules
or time zones or
anytime when the user would like to improve sleep through optimizing circadian
rhythms and
alertness. In at least one embodiment, the user may input the travel
schedules, work schedules, or
any change of sleep and wake schedules that have started before entering the
program. In one
embodiment, the input information may include timing and length of naps, the
intake of caffeine
and other substances that may impact sleep and wakefulness. In at least one
embodiment, the user
may have the option of setting up step 1504 via modifying a preexisting
template or a template
created from a past schedule change. For example, the system may display in a
user interface
proposed times for sleeping and/or waking. The user may provide feedback,
which may include
accepting proposed times, rejecting proposed, and/or adjusting proposed times.
Based on the user
feedback, new proposed times for sleeping and/or waking may be recommended. In
at least one
embodiment, the user may have the option of turning any of their current or
past step 1504 settings
into a template and then repeat it with modifications as necessary.
[0105] Based on at least the information relating to the user's circadian
rhythm, in step 1506, the
system may generate a model for estimating the user's circadian rhythm over
one or more days in
response to the application or anticipated application. The estimates of the
user's circadian rhythm
may be configured to be adjusted in response to changes in the user's sleep
and wakefulness times.
The estimation of circadian rhythm over one or more days may be computed via
estimation of
PRC with certain treatment plan delivered via specific light patterns (for
example, light flashes
with certain intensity and frequency). In at least one embodiment, the
estimation of circadian
rhythm (Process C) may be modeled based on the magnitude of circadian rhythm
misalignment,
the number of days to adjust, and an estimated PRC for the user. In one
embodiment, the PRC
may be estimated based on human-subject study data (for example, light
exposure data and
melatonin data) using treatment programs determined to be representative for
the user's treatment
program (e.g., where both the user's treatment and the representative
treatment use light flash
treatment with intensity, duration, and frequency values within a specified
range). In one
embodiment, the PRC may be proximally estimated based on data from human-
subject studies
with other light treatment programs, and then calibrated based on additional
information such as
user feedback on how well the device works, sleep measurement, alertness
measurement, and/or
via data collected from sensors. In one embodiment, a proximal PRC based on
data from human-
subject studies with other light treatment programs may be used without
further calibration.
[0106] Additionally and selectively, concurrently or subsequently, optional
step 1508 may
generate a model for estimating the user's homeostatic sleep drive (Process S)
over one or more
days, based on at least one or more current, past, and anticipated times of
sleep and/or wakefulness.
The estimates of the user's homeostatic sleep drive may be configured to be
adjusted in response
to changes in the user's sleep and wakefulness times. Based on information
collected in step 1502,
33
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
in one embodiment, the estimated of the user's homeostatic sleep drive may be
based on the time
of wakefulness in the past days. In one embodiment, the estimate of Process S
is based on the
anticipated time of wakefulness in one or more days. In at least one
embodiment, the system may
have the choice to skip calculating Process S depending the user's information
input from step
1502 and 1504. In one embodiment, the system may calculate Process S using
information such
as the timing and duration of naps, or intake of caffeine or other substances,
or other factors that
may impact sleep and wakefulness. In one embodiment, the system may recommend
sleep and
wake schedules and/or nap schedules during the period of wakefulness as part
of the treatment
program.
[0107] After computing Process C and Process S, the system may then generate
instructions to
activate the light source to adjust the user's circadian rhythm, as shown in
step 1510. Optionally,
the step of generating instructions 1510 may be performed based on the models
generated in step
1506 (circadian rhythm model) and optional step 1508 (homeostatic sleep drive
model). For
example, a user's PRC may indicate that to shift forward or backward the
user's sleep schedule a
desired amount, light flashes should be applied at a certain point during the
user's circadian rhythm.
The system may thus use the modeled estimate for the user's circadian rhythm
to apply the light
flashes during the correct time period to achieve the desired result. A model
of homeostatic sleep
drive may likewise be considered in specifying instructions for light flash
treatment. For example,
if the user has work shifts at irregular times, the user may need to be awake
or asleep at times that
deviate from what would ordinarily be expected, and using only a PRC based on
circadian rhythm
may not fully predict how timed light flashes will shift the user's circadian
rhythm and times of
wakefulness and sleep. By taking into account the user's estimated homeostatic
sleep drive, both
at present and over one or more days in the future, the timing of applied
light flash treatment may
better aligned with the times needed to achieve a desired shift to the user's
circadian rhythm.
[0108] The instructions may include the timing of the light treatment program.
In at least one
embodiment, the treatment program may be a pattern of light pulses and/or
other changes in
lighting that are delivered to the user without waking up the user. In at
least one embodiment, the
treatment program may be a pattern of light pulses with period(s) of
continuous light. Optionally,
the user may use the continuous light program, by itself, without the
circadian rhythm treatment
program. For example, the continuous light program may be used for morning
wake up, or may be
supportive of an audio for meditation, which may be used with or without a
circadian rhythm
program in which a pattern of light pulses is delivered. In at least one
embodiment, the light pulses
may be designed with settings such as wavelength of the light, duration of
pulses of light, interval
of time during which pulses of light are delivered, the frequency of the
pulses, intensity of the light
applied during the pulses, the timing, and duration of the circadian rhythm
disorder treatment
program (e.g., via the light program). In at least one embodiment, the
treatment program may be
represented as blocks or trains of light flashes with various intensity,
duration, frequency, and
wavelength that start and stop at different times during sleep. Each light
program may have two
treatment windows as the result of the computation of Process C and/or Process
S: the advance
34
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
window is a time period in which the occurrence of light flashes may advance
the circadian phase,
and the delay window is a time period in which the occurrence of light flashes
may delay the
circadian phase. Within each window there may be one or multiple light flash
treatment blocks,
which is a period of time when light flashes happen. The treatment blocks may
be adjacent to each
other, or separated by a block of time when no light flashes occur. In one
embodiment, one of more
treatment blocks may be continuous light. In another embodiment, one or more
of the treatment
blocks may contain no lights.
[0109] In some embodiments, default settings of light patterns may be
provided. For example,
default settings may include the timing of blocks and the intensity, duration,
and frequency of the
light flashes within the blocks. The timing of blocks may be based on the
models for circadian
rhythm and/or homeostatic sleep drive. In some embodiments, the instructions
may specify for
light flashes to be applied that have an intensity between 25 and 5,000 lux
and a duration between
1 picosecond and 500 milliseconds. The instructions may also specify that the
light flashes should
be applied at a frequency between once per 5 seconds and once per 120 seconds.
[0110] In at least one embodiment, the manufacturer may provide a default
setting for the light
flash parameters and/or provide the user the ability to adjust the settings.
For example, in at least
one embodiment, the manufacturer may provide a default intensity of 100 lux or
even 50 lux at
eyelid level before eyelid penetration for user with combination of certain
biological traits that
result in high light sensitivity, and a default intensity of 3000 lux or even
5000 lux eyelid level
before eyelid penetration for user with combination of certain biological
traits that result in low
light sensitivity, and values in between for users with combination of certain
biological traits that
result in intermediate light sensitivity. Similarly, in at least one
embodiment, the manufacturer
may suggest initial light flash frequency settings based on the user's
biological profile (such as
age and/or eye color) and self-reported light sensitivity and how heavy a
sleeper the user is, and
based on human-subject studies on light flash frequency and sleep disruptions.
For example, the
manufacturer may provide a default light flash frequency of once per minute
for user with
combination of certain biological traits that result in high light
sensitivity, and a default light flash
frequency of once per 8 seconds for user with combination of certain
biological traits that result in
low light sensitivity, and values in between for users with combination of
certain biological traits
that result in intermediate light sensitivity. In at least one embodiment,
different light flash
intensity or frequencies are used at different times of the sleep to achieve
the most substantial
circadian phase shift without causing sleep disruptions. For example, a lower
light flash frequency
or lower intensity, or the combination of both, are used for the same user in
the later part of the
sleep when the homeostatic sleep pressure dissipates and therefore easier for
the user to wake up.
[0111] The instructions to activate light program for circadian rhythm
misalignment treatment in
step 1510 may applied by a circadian rhythm adjustment apparatus to deliver
the treatment
program, which is shown in optional step 1512. Based on the instructions, the
circadian rhythm
adjustment apparatus may activate the light source (e.g. an LED) during a
treatment window to
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
adjust the user's circadian rhythm. In at least one embodiment, the treatment
program may be
delivered via a sleep mask. For example, the sleep mask may emit light flashes
through a
removable insert with electronics. In another embodiment, the light programs
can be delivered in
glasses or goggles. In one embodiment, the circadian rhythm treatment
apparatus may include in-
room lighting (controlled by a controller) that works with a mobile
application that generates light
programs based on circadian modeling algorithms to treat or prevent circadian
rhythm disorders.
In at least one embodiment, the light pulses use light sources that produces a
wavelength between
380 to 750 nm. In at least one embodiment, a subset of the wavelength 380 to
750 nm for a specific
segment of the light treatment, so different treatment segments may have
different color. For
example, a wavelength 380 to 750 nm may be used for the circadian rhythm
adjustment program
during sleep, a wavelength 600 to 750 nm may be used for the treatment towards
the end of sleep,
and wavelengths 380 to 550 nm may be used for treatment before bed, or a
combination of different
subsets of the color spectrum within one treatment segment.
[0112] Optional step 1514 describes a feedback collection mechanism to obtain
updated
information to improve the system, including information relating to circadian
rhythm, user
feedback, device usage, and/or the efficacy of the instructions for activating
the light source during
at least the treatment window. The data may be used to establish, modify, or
choose the most
applicable algorithm to use, and/or as input for the algorithm to generate the
circadian shifting
program, updated circadian rhythm alignment needs, and/or as feedback to
evaluate the effect of
the program and make necessary adjustments to the program. In one embodiment,
the light
treatment may be applied during an initial treatment window. After the initial
treatment window,
the user may be asked to answer questions about their experience using the
device, and/or their
sleep and alertness condition, based on the answers of which the system makes
the adjustment in
the instructions to activate the light source. In one embodiment, the user may
have the option to
update any changes in their schedules for sleep and wakefulness. In one
embodiment, a wearable
light sensor that records ambient light exposure may be used as input to
model/calibrate the
circadian clock of the user each day. In one embodiment, the user may be asked
about when and
for how long they used the circadian rhythm treatment apparatus from the day
before. In one
embodiment, the information about circadian rhythm treatment device usage may
be collected by
a capacitive touch sensor that distinguish whether the device is in contact
with the user's skin. The
information may be used to calibrate the PRC estimation. In one embodiment,
the system may
contain a sample collecting and testing kit to measure physiological
biomarkers for circadian
rhythms, such as melatonin level in saliva, blood, or urine. The level of
these biomarkers may be
detected via assays run in real time, the results of which may be used to
establish the baseline or
to refine the model. In one embodiment, the user may have the option to adjust
light intensity,
frequency, or other light program parameters based on their experience using
the device. In one
embodiment, the system may evaluate the level of sleepiness or alertness of
the user via
neurocognitive measurements for, including any one of, or any combination of,
reaction times,
processing speed, memory, laguage skills, coordination and motor skills,
executive function, and
emotional stability, and so on. In at least one embodiment, the feedback
includes information
36
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
captured from sensors that monitor sleep and wakefulness during the treatment
process, for
example, motion sensor, brainwave sensor, breathing sensor, pulse sensor,
microphone, and so on.
For example, the system may record sleep information from a prior day (or
other prior time period)
or from real time information to know how much of a shift has been generated
in a user, and the
predicted PRC may be refined. Then based on the refined model and the refined
model output,
the light therapy algorithm may be adjusted accordingly. For example, updated
instructions may
be generated and, based on those updated instructions, light treatment may be
applied during a
second treatment window. Further information may be acquired after the second
and subsequent
treatment windows, and any number of adjustments and modified instructions may
be generated
through any number of cycles of treatment and feedback.
[0113] Based on information collected from step 1514, in optional step 1516,
the system may
adjust the instructions as necessary for activating the light source to adjust
the user's circadian
rhythm. The system may recalculate any one of, or any combination of, the
user's current circadian
rhythm, target circadian rhythm, circadian rhythm realignment needs,
recommended sleep
schedules, light program parameters such as intensity, frequency, duration,
wavelength, and timing,
based on updated information. In at least one embodiment, the system may
reduce the light
intensity, pulse duration, and/or frequency, or the combination of both, if
the system detects or the
user reports sleep disruptions during the light program. In at least one
embodiment, the system
may recalculate the PRC if the user forgot to use the device or did not use
the device according to
the treatment protocol in prior day(s). In at least one embodiment, the system
may recalculate the
sleep and/or treatment window if the user reports updated sleep or wake
schedules, time zones,
flight information, or sleep time constraints. In at least one embodiment, the
system may infer the
efficacy of circadian rhythm alignment for a specific user based on the level
of sleepiness or
alertness of the user, or the sleep information collected from the sensors. In
at least one
embodiment, the system may increase the light intensity, pulse duration,
and/or frequency if the
user reports excess sleepiness at a time indicating that the expected shift to
the user's circadian
rhythm has not been achieved. Based on these data, the system may establish a
baseline, as part of
the user's long-term profile, of how sensitive this user's circadian rhythm is
to light treatment
programs, and use this information as model input to update the algorithm that
generates the
circadian rhythm treatment instructions in steps 1506, 1508, and 1510. In some
embodiments,
step 1516 may be performed using any of the details described below with
respect to step 1712.
[0114] Optionally, in at least one embodiment, most of the information
relating to the user's
present circadian rhythms may be collected via sensors. FIG 16 shows an
exemplary method 1600
for adjusting a user's circadian rhythm using input from sensors. In some
embodiments, method
1600 can be performed using any of the systems described above with respect to
FIG 1-14. In step
1602, the system may obtain information relating to times of sleep and/or
wakefulness using a
sensor. In at least one embodiment, the sensor may be an environment light
sensor to collect light
exposure to infer the user's present circadian rhythm. In at least one
embodiment, the light sensor
may be a wearable light sensor in the form factor of a pin, a button, a
brooch, a necklace, a chocker,
37
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
or an earring, or some form factor that can be worn around the neck/chest
area. The light-capturing
part of the hardware generally faces the same direction as the user's face, to
maximally mimic the
amount of light received in the user's eyes. The user may wear the light
sensor for as much as
possible throughout the day except when showering and sleeping. When the user
is sleeping, the
light sensor may still be put facing upward next to the user's head to record
the environmental
light intensity in the bedroom during the time when the user is sleeping. The
wearable light sensor
records the amount of light that the user's eyes are exposed to throughout the
day. In at least one
embodiment, the sensor may be motion-based to estimating the timing and
quality of sleep for the
user, such as 3-6 axis accelerometers and a gyroscope. In at least one
embodiment, a capacitive
touch sensor may be used to determine whether a device is being worn by the
user based on data
obtained from the capacitive sensor.
[0115] In optional step 1604, the system may collect information relating to
one or more
anticipated times of sleep and/or wakefulness, for the user, on one or more
days. For example, any
of the information described above with respect to step 1504 may be collected
in step 1604. Based
on at least the information relating to the user's circadian rhythm, in step
1606, the system may
then generate a model for estimating the user's circadian rhythm over one or
more days in response
to the application or anticipated application. The estimates of the user's
circadian rhythm may be
configured to be adjusted in response to changes in the user's sleep and
wakefulness times. For
example, step 1606 may be performed as described above with respect to step
1506. In step 1608,
the system may then generate instructions to activate the light source to
adjust the user's circadian
rhythm. Step 1608 may be performed generally as described above with respect
to step 1510. In
step optional 1610, based on the generated instructions, the system may
activate the light source
in the circadian rhythm treatment device during a treatment window to adjust
the user's circadian
rhythm. In at least one embodiment, the treatment program may be delivered via
a sleep mask that
emits light flashes through a removable insert with electronics. In another
embodiment, the light
programs can be delivered in glasses or goggles. In one embodiment, the
circadian rhythm
treatment apparatus may include in-room lighting (controlled by a controller)
that works with a
mobile application that generates light programs based on circadian modeling
algorithms to treat
or prevent circadian rhythm disorders. Step 1610 may be performed as described
above with
respect to step 1512.
[0116] In optional step 1612, the system may obtain updated information
relating to circadian
rhythm via user feedback and/or sensors throughout the treatment process. The
data may be used
to establish, modify, or choose the most applicable algorithm to use, and/or
as input for the
algorithm to generate the circadian shifting program, updated circadian rhythm
alignment needs,
and/or as feedback to evaluate the effect of the program and make necessary
adjustments to the
program. In at least one embodiment, the sensor may be an environment light
sensor to collect
light exposure to infer the user's present circadian rhythm. In at least one
embodiment, the light
sensor may be a wearable light sensor. In at least one embodiment, the sensor
may be motion-
based to estimating the timing and quality of sleep for the user, such as 3-6
axis accelerometers
38
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
and a gyroscope. In at least one embodiment, a capacitive touch sensor may be
used to determine
whether a device is being worn by the user based on data obtained from the
capacitive sensor to
infer when the user uses the device in prior days. In at least one embodiment,
the user may be
asked to answer questions about their experience using the device, and/or
their sleep and alertness
condition, based on the answers of which the system makes the adjustment in
the instructions to
activate the light source. In one embodiment, the user may have the option to
update any changes
in their schedules for sleep and wakefulness. In one embodiment, the feedback
may also include
neurocognitive measurements to evaluate the user's sleepiness and alertness
level. The
information collected in step 1612 may also be collected in step 1514,
described above with respect
to FIG 15. Based on information collected in step 1612, in step optional 1614,
the system may
adjust the instructions for activating the light source to adjust the user's
circadian rhythm. This
step may be performed as described above with respect to step 1516.
[0117] Light settings
[0118] The circadian rhythm model output, for example, timing of the treatment
windows to
advance and delay circadian rhythm, will be integrated into a light program
algorithm that
generates the light program for circadian rhythm adjustment treatment. In one
embodiment, the
circadian rhythm treatment systems use light pulse to treat circadian rhythm
misalignment. The
light pulses may be designed with settings such as wavelength of the light,
duration of pulses of
light, interval of time during which pulses of light are delivered, the
frequency of the pulses, the
timing, and duration of the circadian rhythm disorder treatment program (e.g.,
via the light
program). Depending on the light sensitivity of the user, light pulses of
different intensity may be
delivered to effectively treat the user (treating or preventing circadian
rhythm disorder) without
waking up the user. Due to retinal physiology and the sensitivity of the
circadian adjustment
system in the brain, light flash stimulation at night can potentially be more
effective in shifting
circadian rhythm than continuous light stimulation during the day. In any of
the embodiments, the
light flashes can be received either through the eyelids (of closed eyes) when
the user is sleeping
or resting, or directly into open eyes when the user is awake during the night
or within 1-2 hours
before or after sleep. Some users may, at times, sleep with their eyes open,
some users may wake
up in the middle of sleeping, and some users may at times rest their eyes in
the middle of the day
without going to sleep. The intensity of the lights may be adjusted based on
whether or not the
light needs to penetrate through the eyelids.
[0119] In at least one embodiment, the light therapy parameters may be sent to
the hardware to
execute the light program that uses light flashes to shift circadian phases.
In at least one
embodiment, a light-pulse-program may be used alone or in combination with a
continuous light
program before sleep, towards the end of sleep, and/or after waking up. The
continuous light
program may accompany and/or complement a circadian rhythm program, which may
have
different uses, settings, such as wavelength of the light, the timing of the
light, and the duration of
the light treatment. Optionally, the user may use the continuous light
program, by itself, without
39
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
the circadian rhythm treatment program. For example, the continuous light
program may be used
for morning wake up, or may be supportive of an audio for meditation, which
may be used with
or without a circadian rhythm program in which a pattern of light pulses is
delivered. The morning
or evening continuous light that is independent of the circadian rhythm-
adjusting light flashes may
have a different wavelength range than the white flashes.
[0120] The manufacturers may implement light therapy regimen (for example, a
light flash
program) with specific intensity, frequency, duration, wavelength, timing and
reoccurring patterns.
The manufacturer may provide a default setting for the light flash parameters
and/or provide the
user the ability to adjust the settings. The light settings provided by
manufacturer may be based on
results from lab studies or other human-subject studies. For example, the
manufacture may use
white light emitted from LED that covers the full spectrum or a subset of the
full spectrum of the
visible light (380 nm to 750 nm), the spectrum of colors used to make up the
white light may
imitate the spectrum of light emitted by the Sun at midday, Sunrise, or
Sunset. The manufacturers
may also choose a subset of the wavelength 380 to 750 nm for a specific
segment of the light
treatment, so different treatment segments may have different color. For
example, the
manufacturer may choose wavelength 380 to 750 nm for the circadian rhythm
adjustment program
during sleep, wavelength 600 to 750 nm for the treatment towards the end of
sleep, and
wavelengths 380 to 550 nm for treatment before bed, or a combination of
different subsets of the
color spectrum within one treatment segment.
[0121] The light flash programs to treat circadian rhythm misalignment may
follow specific
designed multi-block patterns. Each light program has two treatment windows:
the advance
window is a time period in which the occurrence of light flashes may advance
the circadian phase,
and the delay window is a time period in which the occurrence of light flashes
may delay the
circadian phase. Within each window there may be one or multiple light flash
treatment blocks,
which is a period of time when light flashes happen. FIG. 13 shows an example
of a treatment
regimen 914 for treating circadian rhythm, which may include two treatment
windows Ti and T2.
In at least one embodiment, Ti and T2 are outputs of the circadian rhythm-
estimating
mathematical model. In at least one embodiment, Ti occurs during hour 15 to
hour 24 (CT15-
CT24) cause the user to wake later and T2 occurs during hour 24 to hour 8
(CT24-CT8) of the
subject's effective circadian time cause the user to wake earlier. The time
CTO is defined as the
cross-over point of the human phase-response curve or is defined as the time
of the fitted minimum
of unmasked core body temperature, deducted based on information, such as the
user's biological
profile, sleep schedules or other information such as core body temperature.
In the notation CTxx
(where xx is a one-two digit number, as in CT12), CT stands for Circadian
Time, and CTxx is
defined as the time of activity onset in a free-running human.
[0122] The advance and delay treatment windows Ti and T2 may each contain
blocks of flashes.
FIG. 13 shows an example of one or more blocks of light flashes within the
treatment windows.
Each filled bar 1301 represents one light flash or continuous light for a
period of time. Ti may
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
contain multiple light flash blocks (B1 and B2 in FIG 13), and T2 main contain
multiple treatment
blocks (B3, B4, and B5 in FIG 13). In this specification, the term "block," as
in a block of flashes,
refers to a "train" of flashes. The terms a "block of flashes" and a "train of
flashes" are used
interchangeably throughout this specification, and may be substituted one for
another to obtain
different embodiments. Each treatment block may have the same light flashes
with one set of light
parameters such as light intensity I, flash duration d which represents how
long each light flash
lasts, and the light flash frequency f. The treatment blocks may be adjacent
to each other, or
separated by a block of time when no light flashes occur. In one embodiment,
one of more
treatment blocks may be continuous light. In another embodiment, one or more
of the treatment
blocks may contain no lights. The period P is the time between a particular
point in the cycle of
the turning the flash on and then off or off and then on (e.g., the start of
one flash) and the point
on the next cycle (e.g., the start of the subsequent flash). The frequency of
the flashes may be
computed from the period, P, according to the formula f = 1/P. In each
treatment window there
may be multiple blocks, the duration of the whole block is represented as
width W. Each block of
flashes may have one width that are the same or different from another block.
However, the term
"block" may also refer to a block without any flashes.
[0123] The manufacturer may provide a default setting of light patterns,
including the timing of
blocks and the intensity, duration, and frequency of the light flashes within
the blocks. The timing
of blocks may come from the output of the mathematical model. The flash
patterns over a certain
chosen period of time may be pre-determined by the manufacture based on the
mathematical model
output, and may be adjusted based on user input and/or data collected from
sensors or other
wearables or applications. In an embodiment, the total treatment period is at
least 5 minutes long.
In an embodiment, the treatment period is 240 minutes long or less. In one
embodiment, the
manufacturer may set the number of blocks with their timing and width (for
example, any number
of minutes between 5 minutes and 240 minutes). The manufacturer may also
provide a default
setting for the light flashes within each block. The specific treatment time
within above time
periods for adjusting the circadian rhythm may be further refined based on the
impact the user's
homeostatic sleep drive. The user may have the options to adjust the treatment
windows or to
provide feedback on how the treatment windows may be adjusted. The treatment
windows may
also be adjusted based on data collected from sensors in the system and/or
other wearables or
software.
[0124] Light flash intensity within block is chosen so as to be high enough to
achieve efficacy, but
low enough so as to not be uncomfortable or disruptive of user's sleep. Light
intensity may be
initially set to default values based on an intensity that an average
individual finds comfortable
and still results in an effective treatment. Alternatively or additionally,
the initial setting may be
determined according study results on human subjects with similar biological
traits or light
sensitivity who report sleep disruptions with different levels of light
intensity, as higher intensity
may lead to more substantial circadian phase shift, but could also increase
the risk of sleep
disruption. The manufacturer may suggest initial light intensity settings
based on the user's
41
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
biological profile (such as age and/or eye color) and self-reported light
sensitivity and how heavy
a sleeper the user is. The initial setting of light intensity may come from
human-subject studies
on light flash intensity and sleep disruptions. The user may later adjust the
settings to what works
best for that individual. Alternatively or additionally, the light intensity
values may be adjusted
automatically based on sensor reading, for example, on sleep disruptions. For
example, the
manufacturer may provide a default setting of how bright a flash of between
about 100
microseconds -1 milliseconds. The flash may have a default intensity of 3000
lux at eyelid level
before eyelid penetration set by the manufacturer, with an adjustable range of
50-4000 lux at eyelid
level before eyelid penetration. In an embodiment in which the flash duration
is between about
100-500 microseconds, the intensity of the light flashes may be 100- 3000 lux
as measured at the
level of eyelid before penetrating through the eyelid. The manufacturer may
provide a default
intensity of 100 lux or even 50 lux at eyelid level before eyelid penetration
for user with
combination of certain biological traits that result in high light
sensitivity, and a default intensity
of 3000 lux or even 5000 lux eyelid level before eyelid penetration for user
with combination of
certain biological traits that result in low light sensitivity, and values in
between for users with
combination of certain biological traits that result in intermediate light
sensitivity. The user may
adjust the light intensity setting to anywhere between 300, 600, 1000, or even
3000 lux at eyelid
level before penetration based on system feedback or the user's own
experiencing using the device.
In an embodiment in which the flash duration is between about 1-5 milliseconds
(e.g., 3
millisecond), the intensity of the flash may be 750-3000 lux as measured at
the level of eyelid
before penetrating through the eyelid. The manufacturers may provide a default
setting of light
intensity based on the user's self-reported light sensitivity. In an
embodiment in which the flash
duration is less than 1 microsecond, the intensity may be 1000 lux or higher,
such as, as high as
4000 or 5000 lux, measured at the level of eyelid before penetrating through
the eyelid. In an
embodiment in which the user is sleeping or resting with closed eyes or awake
(or sleeping) with
open eyes, the intensity may be at least 25 lux, 100-1000 lux , or 50-500 lux,
or 100-5000 lux
measured at the level of the open eye or at the level of eyelid before
penetrating through the eyelids
so the user has the flexibility of adjusting based on their sleep/wake
situation. In the embodiments
which apply when the user is sleeping or resting with closed eyes, the light
intensity of flashes is
determined partially based on the eyelid attenuation assumption used in some
sleep research, in
which on average about 10% full-spectrum light penetrates through the eyelid.
Compared to using
sensors to measure attenuation and then to determine the flash intensity, this
assumption simplifies
the device design, avoid components that are potentially uncomfortable for the
user's eyes, and
prolongs the battery life of the device.
[0125] The manufacturer may provide a default setting of how frequently a
flash is repeated over
a period of time. Higher flash frequency may lead to more substantial
circadian phase shift, but
could also increase the risk of sleep disruption. The manufacturer may provide
initial frequency
settings based on results of human-subject studies with look-alike populations
to investigate the
effect of light flash frequency and sleep disruption, together with user input
and/or sensor data.
The user may be provided the option to choose the frequency. For example, the
light flash may
42
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
be set to be any numbers between about .2 Hz to about 8.3 millHz (once every 5
seconds to once
every 120 seconds) based on the effect of light flash frequency on circadian
phase shifts. The
manufacturer may suggest initial light flash frequency settings based on the
user's biological
profile (such as age and/or eye color) and self-reported light sensitivity and
how heavy a sleeper
the user is. The initial setting of light flash frequency may come from human-
subject studies on
light flash intensity and sleep disruptions. For example, the manufacturer may
provide a default
light flash frequency of once per minute for user with combination of certain
biological traits that
result in high light sensitivity, and a default light flash frequency of once
per 8 seconds for user
with combination of certain biological traits that result in low light
sensitivity, and values in
between for users with combination of certain biological traits that result in
intermediate light
sensitivity. The manufacturer may adjust the light flash frequency based on
user feedback, or
provide the settings that allow the user to adjust the frequency. The
manufacturer may provide a
set of default light flash frequencies used at different times of the sleep to
achieve the most
substantial circadian phase shift without causing sleep disruptions. For
example, the manufacturer
may decide to use a lower light flash frequency or lower intensity, or the
combination of both, for
the same user in the later part of the sleep when the homeostatic sleep
pressure dissipates and
therefore easier for the user to wake up.
[0126] In an embodiment, the frequency of the flash is limited to once every 6
seconds to once per
120 seconds. In one embodiment, the frequency of the flash is set at once
every 6 to 12 seconds,
once every 12 to 15 seconds, once every 15 to 20 seconds, or once every 20 -30
seconds. In one
embodiment, the frequency of flashes may be adjusted based on sensor reading
on the sleep/wake
status or the sleep stage information from the user. The flash frequency may
be a constant number
throughout the course of treatment, or can change throughout the course of the
treatment as part
of a pre-designed treatment protocol, or responding to the feedback from the
users via user input
or sensors in the device. For example, in one embodiment, the system may cause
a light flash in
block B1 to be once every 7 seconds, followed by a block B2 with light flashes
of once every 60
seconds, which will then followed by a block B3 of once every 12 seconds.
Alternatively or
additionally, there may be one or more blocks with no flash. In one
embodiment, one or more
blocks may contain continuous light instead of light flashes with certain
frequency. In an
embodiment, any of the frequencies disclosed in this specification may be
used, and may be
applied for 30 minutes off, 30 minutes on, and then 30 minutes off or for
another number of
minutes on and off. In an embodiment, when turning the flashes on for a period
and then turning
the flashes off for another period of time, the total number of minutes to
treatment (the sum of Ws
of all the blocks B with light flashes) may be limited to 5 minutes to 3.5
hours. In an embodiment,
the on/off periods are not necessarily equal.
[0127] In an embodiment, the frequency is automatically varied (to create
"dynamic" frequency
treatment) during the treatment period, based on sleep/wake and/or sleep
stages computed based
on theoretical analysis and/or using data collected from sensor readings.
According to how the
user sleeps at different times, there are an unlimited number of combinations
of frequencies during
43
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
different periods and the durations of each period have any given frequency.
Optionally, the length
of periods (blocks) with light flashes and without light flashes and the flash
frequency within each
block can be dynamically changed, determined in real time while the user is
sleeping and receiving
the circadian adjustment treatment, based on data captured by the sensors. For
example, a block
of light flashes may be canceled temporarily if nocturnal awakenings were
detected.
[0128] The manufacturer may provide a default setting of light flash
durations. Since light flash
duration is unlikely to affect the magnitude of circadian phase shift, the
manufacturer may provide
a default value of the shortest light flash duration that can be achieved by
the hardware design (e.g.
between 1 nanosecond to 1 microsecond, or between 1 microsecond to 100
microseconds). In one
embodiment, the light flash duration may be set by settings that result in a
light flash of between
about 100 microseconds and 500 microseconds. In another embodiment, the light
flash duration
may be set by settings that result in a light flash of between 500
microseconds to 1 millisecond. In
another embodiment, the light flash duration is set a value between 1
millisecond to 5 milliseconds.
In an embodiment, the flash duration may be 10 microseconds to 100
microseconds, 100
microseconds to 1 millisecond, 500 microseconds to 3 milliseconds, or 10
microseconds to 10
milliseconds. In an embodiment, the flash duration maybe 1 nanosecond to 1
microsecond. In
one embodiment, the manufacturer may provide settings for the user to adjust
the flash duration.
Any frequency or range of frequencies may be used with any intensity or range
of intensities, any
color or range of wave length of color, any flash duration or range of flash
duration, treatment
period or range of treatment periods, every treatment pattern of changes in
frequency and/or on/off
periods may be used together with one another. The manufacturer may provide a
default
combination of light flash parameters based on the user's biological profile.
For example, for users
with the combination of certain biological traits that result in high light
sensitivity, the
manufacturer may provide an initial setting of light flashes of 100-500
microseconds at 100 lux
(or 50 lux) at eye level before eyelid penetration at once per 30 seconds in
frequency. For users
with the combination of certain biological traits that result in low light
sensitivity, the manufacturer
may provide an initial setting of light flashes of 100-500 microseconds at
3000 lux (or 5000 lux)
at eye level before eyelid penetration at once per 7 seconds in frequency. For
users with biological
profile that indicates intermediate light sensitivity, the manufacturer may
provide an initial setting
of light flashes of 100-500 microseconds with intensity and frequency between
the previous two
groups. In an embodiment, the values of parameters have a tolerance of 10% of
the value specified.
[0129] FIG 17 shows an exemplary method 1700 for selecting light treatment
parameters. For
example, it may be desirable for a system to select light treatment parameters
that achieve a desired
amount of circadian phase shifts while mitigating the risk of sleep
disruptions. Method 1700 may
be combined with any of the methods described herein, including those
described above with
reference to Figures 14, 15, and 16. By combining method 1700 with those
described in Figures
14, 15, and 16, treatment efficacy may advantageously be improved, while
minimizing sleep
disruptions that could reduce compliance with a proscribed treatment regimen.
44
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
[0130] Higher light pulse intensity, duration, and/or frequency may desirably
increase an amount
of phase shift, but may undesirably increase the risk of sleep disruption.
Thus, it may be desirable
to determine parameters for light intensity, duration, and frequency that are
simultaneously
sufficiently high to achieve substantial phase shift and sufficiently low to
avoid disrupting the
user's sleep. In step 1702, the system obtains data that may indicate or
impact the user's sensitivity
to light during sleep. The inputs for the model may include any one of, or any
combination of,
age, sex, eye colors, pupillometry, light sensitivity, self-rated how light
sleeper the user is, typical
sleep quality, typical hours of sleep, typical sleep efficiency, current sleep
problems such as
insomnia or environmental disruptions. In at least one embodiment, the system
may ask the user
to provided self-rated light sensitivity in predetermined categories. In one
embodiment, the system
may provide a hardware and/or software user-interface to measure the user's
light sensitivity. In
one embodiment, the user may be asked to answer questions that may provide
information about
the user's light sensitivities, for example, how they typically respond to
different levels of light
during sleep or wakefulness.
[0131] In step 1706, the system may compare the user's light sensitivity data
against calibration
data. In some embodiments, the calibration data may indicated relationships
between light
sensitivity and one or more values for light flash duration, light flash
frequency, and/or light flash
intensity determined to present an acceptably low likelihood of disrupting
sleep. In some
embodiments, the system may interpret the light sensitivity data collected
from step 1702. The
system may compare the user's data against existing data for calibration, data
that may indicate
the user's light sensitivity, and/or data that directly or indirectly suggest
the likelihood of sleep
disruption with certain light treatment programs. For example, in at least one
embodiment, the
system may suggest a light sensitivity category for the user based on the
user's biological profile
(such as age and/or eye color) and self-reported light sensitivity and how
heavy a sleeper the user
is. In one embodiment, the data and interpretation may be based on results
from lab studies or other
human-subject studies with look-alike populations to investigate the effect of
light flash frequency
and sleep disruption. In one embodiment, the data may be collected from the
feedback provided
by the pool of look-alike demographic of users using the circadian rhythm
adjustment system. In
one embodiment, the initial interpretation of light sensitivity may be based
on hypothesis, based
on which a set of light program parameters are chosen. The light settings may
then be adjusted
based on the user's feedback. The system may assign a value (for example, 1-5
to indicate high
sensitivity to low sensitivity) to indicate the user's light sensitivity level
as part of the user's long-
term profile.
[0132] Step 1706 may also include determining one or more values for the light
programs,
including light flash duration, light flash frequency, and/or light flash
intensity to present an
acceptably low likelihood of disrupting sleep while maintaining the potential
of causing substantial
circadian phase shifts for the user according to the user's light sensitivity
value, to achieve the
balance of substantial circadian phase shifts without increasing the risk of
sleep disruptions. The
instructions to activate the light source may specify the light intensity,
duration, wavelength,
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
frequency, and timing that likely works with users with certain assigned light
sensitivity value. In
one embodiment, the data may be based on results from lab studies or other
human-subject studies
with look-alike populations to investigate the effect of light flash frequency
and sleep disruption.
For example, a database accumulated through human-subject studies may indicate
that, for users
with certain biological or demographic indicators (e.g., age, sex, eye color,
self-reports on how
heavily the user sleeps), light flashes of specified intensity, duration,
and/or frequency values may
present an acceptably low risk of disrupting the user's sleep. In another
embodiment, the values of
the light program parameters may be directly specified, or through some linear
or nonlinear
conversions. For example, in at least one embodiment, the system may provide a
default light
flash frequency of once per minute for user with combination of certain
biological traits that result
in high light sensitivity, and a default light flash frequency of once per 8
seconds for user with
combination of certain biological traits that result in low light sensitivity,
and values in between
for users with combination of certain biological traits that result in
intermediate light sensitivity.
In at least one embodiment, the system may provide a default intensity of 100
lux or even 50 lux
at eyelid level before eyelid penetration for user with combination of certain
biological traits that
result in high light sensitivity, and a default intensity of 3000 lux or even
5000 lux eyelid level
before eyelid penetration for user with combination of certain biological
traits that result in low
light sensitivity, and values in between for users with combination of certain
biological traits that
result in intermediate light sensitivity. The user may adjust the light
intensity setting to anywhere
between 300, 600, 1000, or even 3000 lux at eyelid level before penetration
based on system
feedback or the user's own experiencing using the device. In one embodiment,
the system may
use a lower light flash frequency or lower intensity, or the combination of
both, for users with
higher sensitivity to light during sleep, and a higher light flash frequency
or higher intensity, or
the combination of both, for users with lower sensitivity to light during
sleep. In one embodiment,
the system may provide a set of default light flash frequencies used at
different times of the sleep
for the same user to achieve the most substantial circadian phase shift
without causing sleep
disruptions. For example, the manufacturer may decide to use a lower light
flash frequency or
lower intensity, or the combination of both, for the same user in the later
part of the sleep when
the homeostatic sleep pressure dissipates and it is therefore easier for the
user to wake up. In at
least one embodiment, the light program parameters to achieve the balance of
substantial circadian
phase shifts without increasing the risk of sleep disruptions may be
determined based on the user's
self-reported sleep challenges. For example, the timing of light program may
be delayed if the
user reports significantly longer sleep onset latency compared to populational
baseline data. In at
least one embodiment, the light program parameters to achieve the balance of
substantial circadian
phase shifts without increasing the risk of sleep disruptions may be
determined based on the sleep
and wake data collected via sensors in the circadian rhythm adjustment system
or other wearables
or sleep trackers.
[0133] In step 1708, based on at least the light sensitivity data and the
calibration data, the system
may then generate instructions for activating a light source to adjust the
user's circadian rhythm
using a processor system comprising one or more processors. For example, the
system may
46
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
generate instructions that specify one or more windows of light flashes having
intensity, duration,
and/or frequency values that are determined, based on the calibration data, to
present an acceptably
low of disrupting the user's sleep. The values may also be determined, based
on the calibration
data, to present an acceptably high likelihood of achieving a desired level of
circadian phase shift.
The instructions may also be based on the data sources and models described
above with reference
to Figures 14, 15, and 16. For example, the instructions may also be based on
one or more models
for estimating circadian rhythm and/or homeostatic sleep drive. In some
embodiments, the
instructions may specify for light flashes to be applied that have an
intensity between 25 and 5,000
lux and a duration between 1 picosecond and 500 milliseconds. The instructions
may also specify
that the light flashes should be applied at a frequency between once per 5
seconds and once per
120 seconds. The instructions may specify that the light flashes should be
applied during one or
more treatment windows selected to achieve a desired shift to the user's
circadian rhythm. This
selection may be made based on, e.g., estimates for the user's PRC, circadian
rhythm, and/or
homeostatic sleep drive.
[0134] The instructions to activate light program for circadian rhythm
misalignment treatment in
step 1708 may be sent to the circadian rhythm adjustment apparatus to deliver
the treatment
program, which is shown in optional step 1710. Based on the instructions, the
circadian rhythm
adjustment apparatus may activate the light source (e.g. an LED) during a
treatment window to
adjust the user's circadian rhythm. In at least one embodiment, the treatment
program may be
delivered via a sleep mask that emits light flashes through a removable insert
with electronics. In
another embodiment, the light programs can be delivered in glasses or goggles.
In one embodiment,
the circadian rhythm treatment apparatus may include in-room lighting
(controlled by a controller)
that works with a mobile application that generates light programs based on
circadian modeling
algorithms to treat or prevent circadian rhythm disorders. In at least one
embodiment, the light
pulses use light sources that produces a wavelength between 380 to 750 nm. In
at least one
embodiment, a subset of the wavelength 380 to 750 nm for a specific segment of
the light treatment,
so different treatment segments may have different color. For example, a
wavelength 380 to 750
nm may be used for the circadian rhythm adjustment program during sleep, a
wavelength 600 to
750 nm may be used for the treatment towards the end of sleep, and wavelengths
380 to 550 nm
may be used for treatment before bed, or a combination of different subsets of
the color spectrum
within one treatment segment.
[0135] Step 1712 describe an optional adjustment step, in which based on
updated information,
light program parameters may be adjusted to improve circadian rhythm alignment
efficacy and/or
to further reduce the risk of sleep disruptions. The system may recalculate
any one of, or any
combination of, the user's light program parameters such as intensity,
frequency, duration,
wavelength, and timing, based on updated information. In at least one
embodiment, the system
may reduce the light intensity, frequency, or duration, or the combination of
all, if the system
detects or the user reports sleep disruptions during the light program. In at
least one embodiment,
the system may increase the intensity, frequency, or duration, or the
combination of all, if the
47
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
system detects good sleep quality or alertness from sleep-monitoring sensors,
neurocognitive
testing, and/or from self-reported survey answers. In at least one embodiment,
the system may
increase the light intensity, pulse duration, and/or frequency if the user
reports excess sleepiness
at a time indicating that the expected shift to the user's circadian rhythm
has not been achieved.
In at least one embodiment, the user may be provided with settings that may
increase or decrease
the light intensity, frequency, or duration, or the combination of all,
according to the data collected
in the feedback step and or how they feel throughout the treatment program.
Optional step 1712
may be performed using any of the exemplary details described above with
respect to step 1516.
[0136] Numbered Embodiments
Al. A system for adjusting a user's circadian rhythm, the system
comprising:
one or more input modules for collecting information relating to a user's
sleep and/or wakefulness;
a light source;
a processor system including one or more processors that control the light
source;
a memory system storing one or more machine instructions, wherein the system
is configured to:
obtain information relating to the user's present circadian rhythm;
obtain information relating to one or more anticipated times of sleep and/or
wakefulness, for the
user, on one or more days;
based on at least the information relating to the user's present circadian
rhythm, generate a model
for estimating the user's circadian rhythm over one or more days, the
estimates of the user's
circadian rhythm being configured to be adjusted in response to application,
or anticipated
application, of light by the light source;
based on at least the one or more anticipated times of sleep and/or
wakefulness, generate a model
for estimating the user's homeostatic sleep drive over one or more days, the
estimates of the user's
homeostatic sleep drive being configured to be adjusted in response to changes
in the user's sleep
and wakefulness times; and
based on at least the model for estimating the user's circadian rhythm and the
model for estimating
the user's homeostatic sleep drive, generate instructions for activating the
light source to adjust
the user's circadian rhythm.
A2. The system of embodiment Al, wherein the information relating to the
user's sleep and/or
wakefulness is collected, at least in part, using a sensor.
48
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
A3. The system of embodiment A2, wherein the sensor is a light sensor, the
light sensor being
configured to sense the user's exposure to environmental light.
A4. The system of embodiment A2, wherein the sensor is a motion sensor, the
system being
configured to estimate whether a user is sleeping or awake based on data
obtained from the motion
sensor.
A5. The system of embodiment A2, wherein the sensor is a capacitive sensor,
the system being
configured to determine whether a device is being worn by the user based on
data obtained from
the capacitive sensor.
A6. The system of any of embodiments A1-A5, wherein the system is further
configured to:
based on the generated instructions for activating the light source, activate
the light source during
a treatment window while the user sleeps to adjust the user's circadian
rhythm;
after applying the light source during the treatment window, obtain subsequent
information; and
based on the subsequent information, modify the instructions for activating
the light source.
A7. The system of embodiment A6, wherein the subsequent information is user
feedback
relating to information about updated sleep and/or wakefulness schedules,
device usage in previous
days/nights, and/or the efficacy of the light application during at least the
treatment window.
A8. The system of embodiment A6, wherein the subsequent information is
obtained by at least
one of a light sensor, a motion sensor, and a capacitive sensor.
A9. The system of any of embodiments A1-A8, wherein the system is further
configured to:
determine recommended sleep times, the recommended sleep times being selected
to achieve a
desired shift to the user's circadian rhythm;
49
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
display to the user the recommended sleep times;
receive, from the user, feedback relating to the recommended sleep times; and
based on the feedback, adjust the recommended sleep times.
A10. The system of any of embodiments Al -A9, wherein the system comprises a
sleep mask,
the light being applied during the user's sleep via the sleep mask.
All. The system of any of embodiments Al -A10, wherein the instructions for
activating the
light source comprise instructions to activate the light source in one or more
pulses having an
intensity between 25 and 5,000 lux and a duration between 1 picosecond and 500
milliseconds.
Bl. A method for adjusting a user's circadian rhythm, the method
comprising:
obtaining information relating to the user's present circadian rhythm;
obtaining information relating to one or more anticipated times of sleep
and/or wakefulness, for
the user, on one or more days;
based on at least the information relating to the user's circadian rhythm,
generating a model for
estimating the user's circadian rhythm over one or more days, the estimates of
the user's circadian
rhythm being configured to be adjusted in response to application, or
anticipated application, of
light by the light source;
based on at least the one or more anticipated times of sleep and/or
wakefulness, generating a model
for estimating the user's homeostatic sleep drive over one or more days, the
estimates of the user's
homeostatic sleep drive being configured to be adjusted in response to changes
in the user's sleep
and wakefulness times; and
based on at least the model for estimating the user's circadian rhythm and the
model for estimating
the user's homeostatic sleep drive, generating instructions for activating the
light source to adjust
the user's circadian rhythm.
B2. The method of embodiment Bl, wherein the information relating to the
user's sleep and/or
wakefulness is collected, at least in part, using a sensor.
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
B3. The method of embodiment B2, wherein the sensor is a light sensor, the
light sensor being
configured to sense the user's exposure to environmental light.
B4. The method of embodiment B2, wherein the sensor is a motion sensor, the
method further
comprising estimating whether the user is sleeping or awake based on data
obtained from the
motion sensor.
B5. The method of embodiment B2, wherein the sensor is a capacitive sensor,
the method
further comprising determining whether a device is being worn by the user
based on data obtained
from the capacitive sensor.
B6. The method of any of embodiments B1-B5, the method further comprising:
based on the generated instructions for activating the light source,
activating the light source during
a treatment window while the user sleeps to adjust the user's circadian
rhythm;
after applying the light source during the treatment window, obtaining
subsequent information;
and
based on the subsequent information, modifying the instructions for activating
the light source.
B7. The method of embodiment B6, wherein the subsequent information is user
feedback
relating to information about updated sleep and/or wakefulness schedules,
device usage in
previous days/nights, and/or the efficacy of the light application during at
least the treatment
window.
B8. The method of embodiment B6, wherein the subsequent information is
obtained by at least
one of a light sensor, a motion sensor, and a capacitive sensor.
B9. The method of any of embodiments Bl-B8, wherein the method further
comprises:
51
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
determining recommended sleep times, the recommended sleep times being
selected to achieve a
desired shift to the user's circadian rhythm;
displaying to the user the recommended sleep times;
receiving, from the user, feedback relating to the recommended sleep times;
and
based on the feedback, adjusting the recommended sleep times.
B10. The method of any of embodiments B1-B9, wherein the method further
comprises applying
light in accordance with the generated instructions via a sleep mask while the
user sleeps.
B11. The method of any of embodiments Bl-B10, wherein the instructions for
activating the
light source comprise instructions to activate the light source in one or more
pulses having an
intensity between 25 and 5,000 lux and a duration between 1 picosecond and 500
milliseconds.
Cl. A system for adjusting a user's circadian rhythm, the system
comprising:
one or more input modules for collecting sleep data;
a light source;
a sensor, the sensor being configured to detect information relating to a
user's sleep and/or
wakefulness;
a processor system including one or more processors that controls the light
source;
a memory system storing one or more machine instructions, wherein the system
is configured to:
using the sensor, obtain information relating to times of sleep and/or
wakefulness;
based on at least the information relating to the user's times of sleep and/or
wakefulness, generate
a model for estimating the user's circadian rhythm over one or more days, the
estimates of the
user's circadian rhythm being configured to be adjusted in response to
application, or anticipated
application, of light by the light source; and
based on at least the model for estimating the user's circadian rhythm,
generate instructions for
activating the light source to adjust the user's circadian rhythm.
52
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
C2. The system of embodiment Cl, wherein the instructions for activating
the light source are
also based, at least in part, on a model for estimating the user's homeostatic
sleep drive over one
or more days, the estimates of the user's homeostatic sleep drive being
configured to be adjusted
in response to changes in the user's sleep and wakefulness times.
C3. The system of any of embodiments C1-C2, wherein the sensor is a light
sensor, the light
sensor being configured to sense the user's exposure to environmental light.
C4. The system of any of embodiment C1-C2, wherein the sensor is a motion
sensor, the system
being configured to estimate whether a user is sleeping or awake based on data
obtained from the
motion sensor.
C5. The system of any of embodiments C1-C2, wherein the sensor is a
capacitive sensor, the
system being configured to determine whether a device is being worn by the
user based on data
obtained from the capacitive sensor.
C6. The system of any of embodiments C1-05, wherein the system is further
configured to:
based on the generated instructions for activating the light source, activate
the light source during
a treatment window while the user sleeps to adjust the user's circadian
rhythm;
after applying the light source during the treatment window, obtain subsequent
information; and
based on the subsequent information, modify the instructions for activating
the light source.
C7. The system of embodiment C6, wherein the subsequent information is user
feedback
relating to information about device usage in previous days/nights and/or the
efficacy of the light
application during at least the treatment window.
C8. The system of embodiment C6, wherein the subsequent information is
obtained by at least
53
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
one of a light sensor, a motion sensor, and a capacitive sensor.
C9. The system of any of embodiments C1-C8, wherein the system is further
configured to:
determine recommended sleep times, the recommended sleep times being selected
to achieve a
desired shift to the user's circadian rhythm;
display to the user the recommended sleep times;
receive, from the user, feedback relating to the recommended sleep times; and
based on the feedback, adjust the recommended sleep times.
C10. The system of any of embodiments Cl -C9, wherein the system comprises a
sleep mask,
the light being applied during the user's sleep via the sleep mask.
C11. The system of any of embodiments Cl-C10, wherein the instructions for
activating the
light source comprise instructions to activate the light source in one or more
pulses having an
intensity between 25 and 5,000 lux and a duration between 1 picosecond and 500
milliseconds.
Dl. A method for adjusting a user's circadian rhythm, the method
comprising:
using a sensor, obtain information relating to times of sleep and/or
wakefulness;
based on at least the information relating to the user's times of sleep and/or
wakefulness, generate
a model for estimating the user's circadian rhythm over one or more days, the
estimates of the
user's circadian rhythm being configured to be adjusted in response to
application, or anticipated
application, of light by the light source; and
based on at least the model for estimating the user's circadian rhythm,
generate instructions for
activating the light source to adjust the user's circadian rhythm.
D2. The method of embodiment D1, wherein the instructions for activating
the light source are
also based, at least in part, on a model for estimating the user's homeostatic
sleep drive over one
or more days, the estimates of the user's homeostatic sleep drive being
configured to be adjusted
54
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
in response to changes in the user's sleep and wakefulness times.
D3. The method of any of embodiments D1-D2, wherein the sensor is a light
sensor, the light
sensor being configured to sense the user's exposure to environmental light.
D4. The method of any of embodiment D 1 -D2, wherein the sensor is a motion
sensor, the
method further comprising estimating whether the user is sleeping or awake
based on data obtained
from the motion sensor.
D5. The method of any of embodiments D1-D2, wherein the sensor is a
capacitive sensor, the
method further comprising determining whether a device is being worn by the
user based on data
obtained from the capacitive sensor.
D6. The method of any of embodiments D1-D5, wherein the method further
comprises:
based on the generated instructions for activating the light source,
activating the light source during
a treatment window while the user sleeps to adjust the user's circadian
rhythm;
after applying the light source during the treatment window, obtaining
subsequent information;
and
based on the subsequent information, modifying the instructions for activating
the light source.
D7. The method of embodiment D6, wherein the subsequent information is user
feedback
relating to information about device usage in previous days/nights and/or the
efficacy of the light
application during at least the treatment window.
D8. The method of embodiment D6, wherein the subsequent information is
obtained by at least
one of a light sensor, a motion sensor, and a capacitive sensor.
D9. The method of any of embodiments D1-D8, wherein the method further
comprises:
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
determining recommended sleep times, the recommended sleep times being
selected to achieve a
desired shift to the user's circadian rhythm;
displaying to the user the recommended sleep times;
receiving, from the user, feedback relating to the recommended sleep times;
and
based on the feedback, adjusting the recommended sleep times.
D10. The method of any of embodiments Dl-D9, wherein the method comprises
applying light
in accordance with the generated instructions via a sleep mask while the user
sleeps.
D11. The method of any of embodiments D 1-D 10, wherein the instructions for
activating the
light source comprise instructions to activate the light source in one or more
pulses having an
intensity between 25 and 5,000 lux and a duration between 1 picosecond and 500
milliseconds.
El. A system for adjusting a user's circadian rhythm, the system
comprising:
one or more input modules for collecting sleep data;
a light source;
a processor system including one or more processors that controls the light
source;
a memory system storing one or more machine instructions, wherein the system
is configured to:
obtain information relating to times of sleep and/or wakefulness for the user;
based on at least the information relating to the user's times of sleep and/or
wakefulness, generate
a model for estimating the user's circadian rhythm over one or more days, the
estimates of the
user's circadian rhythm being configured to be adjusted in response to
application, or anticipated
application, of light by the light source;
based on at least the model for estimating the user's circadian rhythm,
generate instructions for
activating the light source to adjust the user's circadian rhythm;
using the instructions, activate the light source to adjust the user's
circadian rhythm;
obtain efficacy information relating to an efficacy of the instructions for
activating the light source
56
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
to adjust the user's circadian rhythm; and
based on at least the efficacy information, adjusting the instructions for
activating the light source
to adjust the user's circadian rhythm.
E2. The system of embodiment El, wherein the information relating to the
user's sleep and/or
wakefulness is collected, at least in part, using a sensor.
E3. The system of embodiment E2, wherein the sensor is a light sensor, the
light sensor being
configured to sense the user's exposure to environmental light.
E4. The system of embodiment E2, wherein the sensor is a motion sensor, the
system being
configured to estimate whether a user is sleeping or awake based on data
obtained from the motion
sensor.
E5. The system of embodiment E2, wherein the sensor is a capacitive sensor,
the system being
configured to determine whether a device is being worn by the user based on
data obtained from
the capacitive sensor.
E6. The system of any of embodiments El-E5, wherein the instructions for
activating the light
source are also based, at least in part, on a model for estimating the user's
homeostatic sleep drive
over one or more days, the estimates of the user's homeostatic sleep drive
being configured to be
adjusted in response to changes in the user's sleep and wakefulness times.
E7. The system of any of embodiments El-E6, wherein the efficacy
information is user
feedback relating to the efficacy of the light application during at least the
treatment window.
E8. The system of any of embodiments El-E6, wherein the efficacy
information is obtained by
at least one of a light sensor, a motion sensor, and a capacitive sensor.
57
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
E9. The system of any of embodiments El-E8, wherein the system is further
configured to:
determine recommended sleep times, the recommended sleep times being selected
to achieve a
desired shift to the user's circadian rhythm;
display to the user the recommended sleep times;
receive, from the user, feedback relating to the recommended sleep times; and
based on the feedback, adjust the recommended sleep times.
E10. The system of any of embodiments El-E9, wherein the system comprises a
sleep mask, the
light being applied during the user's sleep via the sleep mask.
Eli. The system of any of embodiments El -E 1 0, wherein the instructions for
activating the light
source comprise instructions to activate the light source in one or more
pulses having an intensity
between 25 and 5,000 lux and a duration between 1 picosecond and 500
milliseconds.
Fl. A method for adjusting a user's circadian rhythm, the method
comprising:
obtaining information relating to times of sleep and/or wakefulness for the
user;
based on at least the information relating to the user's times of sleep and/or
wakefulness,
generating a model for estimating the user's circadian rhythm over one or more
days, the estimates
of the user's circadian rhythm being configured to be adjusted in response to
application, or
anticipated application, of light by the light source;
based on at least the model for estimating the user's circadian rhythm,
generating instructions for
activating the light source to adjust the user's circadian rhythm;
using the instructions, activating the light source during a treatment window
to adjust the user's
circadian rhythm;
obtain efficacy information relating to an efficacy of the instructions for
activating the light source
during at least the treatment window; and
based on at least the efficacy information, adjusting the instructions for
activating the light source
58
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
to adjust the user's circadian rhythm.
F2. The method of embodiment Fl, wherein the information relating to the
user's sleep and/or
wakefulness is collected, at least in part, using a sensor.
F3. The method of embodiment F2, wherein the sensor is a light sensor, the
light sensor being
configured to sense the user's exposure to environmental light.
F4. The method of embodiment F2, wherein the sensor is a motion sensor, the
method further
comprising estimating whether the user is sleeping or awake based on data
obtained from the
motion sensor.
F5. The method of embodiment F2, wherein the sensor is a capacitive sensor,
the method
further comprising determining whether a device is being worn by the user
based on data obtained
from the capacitive sensor.
F6. The method of any of embodiments F1-F5, wherein the instructions for
activating the light
source are also based, at least in part, on a model for estimating the user's
homeostatic sleep drive
over one or more days, the estimates of the user's homeostatic sleep drive
being configured to be
adjusted in response to changes in the user's sleep and wakefulness times.
F7. The method of any of embodiments Fl-F6, wherein the efficacy
information is user
feedback relating to the efficacy of the light application during at least the
treatment window.
F8. The method of any of embodiments Fl-F6, wherein the efficacy
information is obtained
by at least one of a light sensor, a motion sensor, and a capacitive sensor.
F9. The method of any of embodiments Fl-F8, wherein the method further
comprises:
59
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
determining recommended sleep times, the recommended sleep times being
selected to achieve a
desired shift to the user's circadian rhythm;
displaying to the user the recommended sleep times;
receiving, from the user, feedback relating to the recommended sleep times;
and
based on the feedback, adjusting the recommended sleep times.
F10. The method of any of embodiments Fl-F9, wherein the method comprises
applying light
in accordance with the generated instructions via a sleep mask while the user
sleeps.
F11. The method of any of embodiments F 1-F 10, wherein the instructions for
activating the
light source comprise instructions to activate the light source in one or more
pulses having an
intensity between 25 and 5,000 lux and a duration between 1 picosecond and 500
milliseconds.
Gl. A system for adjusting a user's circadian rhythm, the system
comprising:
one or more input modules for collecting data;
a light source;
a processor system including one or more processors that controls the light
source;
a memory system storing one or more machine instructions, wherein the system
is configured to:
obtain light sensitivity data for a user, the light sensitivity data being
probative of a user's light
sensitivity during sleep;
compare the user's light sensitivity data against calibration data, the
calibration data indicating
relationships between light sensitivity and one or more values for light flash
duration, light flash
frequency, and/or light flash intensity determined to present an acceptably
low likelihood of
disrupting sleep;
based on at least the light sensitivity data and the calibration data,
generate instructions for
activating the light source to adjust the user's circadian rhythm, the
activations of the light source
applying flashes of light having a duration, frequency, and/or intensity
values determined to
present an acceptably low likelihood of disrupting the user's sleep.
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
G2. The system of embodiment Gl, wherein the light sensitivity data
comprises at least one of
age, eye color, self-reported light sensitivity, and how heavily the user
reports sleeping.
G3. The system of any of embodiments G1-G2, wherein the system is further
configured to:
based on the generated instructions for activating the light source, activate
the light source during
a treatment window while the user sleeps to adjust the user's circadian
rhythm;
after applying the light source during the treatment window, obtain subsequent
information; and
based on the subsequent information, modifying the instructions for activate
the light source.
G4. The system of embodiment G3, wherein the subsequent information is user
feedback
relating to information about device usage in previous days/nights and/or the
efficacy of the light
application during at least the treatment window.
G5. The system of embodiment G3, wherein the subsequent information is
obtained by at least
one of a light sensor, a motion sensor, and a capacitive sensor.
G6. The system of any of embodiments G1-G5, wherein the instructions for
activating the light
source comprise instructions to activate the light source in one or more
pulses having an intensity
between 25 and 5,000 lux and a duration between 1 picosecond and 500
milliseconds.
G7. The system of any of embodiments G1-G6, wherein the instructions for
activating the light
source comprise instructions to activate the light source using pulses having
a frequency between
once per 5 seconds and once per 120 seconds.
G8. The system of any of embodiments G1-G7, wherein the calibration data is
based on human-
subject studies.
61
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
G9. The system of any of embodiments G1-G8, wherein the generated
instructions for
activating the light source specify that a treatment regimen for a night
includes at least one higher-
treatment phase and at least one lower-treatment phase, at least one of light
flash intensity,
frequency, and/or duration being greater during the higher-treatment phase
than during the lower-
treatment phase.
G10. The system of any of embodiments Gl-G9, wherein the higher-treatment
phase is applied
before the lower-treatment phase, such that the user's homeostatic sleep drive
is higher when the
higher-treatment phase is applied than when the lower-treatment phase is
applied.
G11. The system of any of embodiments Gl-G10, wherein the system comprises a
sleep mask,
the light being applied during the user's sleep via the sleep mask.
Hl. A method for adjusting a user's circadian rhythm, the method
comprising:
obtaining, via one or more input modules, light sensitivity data for a user,
the light sensitivity data
being probative of a user's light sensitivity during sleep;
comparing the user's light sensitivity data against calibration data, the
calibration data indicating
relationships between light sensitivity and one or more values for light flash
duration, light flash
frequency, and/or light flash intensity determined to present an acceptably
low likelihood of
disrupting sleep;
based on at least the light sensitivity data and the calibration data,
generate, using a processor
system comprising one or more processors, instructions for activating a light
source to adjust the
user's circadian rhythm; and
based on the generated instructions, apply, using the light source, flashes of
light having duration,
frequency, and/or intensity values determined to present an acceptably low
likelihood of disrupting
the user's sleep.
H2. The method of embodiment H1, wherein the light sensitivity data
comprises at least one of
age, eye color, self-reported light sensitivity, and how heavily the user
reports sleeping.
62
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
H3. The method of any of embodiments Hl-H2, wherein the method further
comprises:
based on the generated instructions for activating the light source,
activating the light source during
a treatment window while the user sleeps to adjust the user's circadian
rhythm;
after applying the light source during the treatment window, obtain subsequent
information; and
based on the subsequent information, modifying the instructions for activate
the light source.
H4. The method of embodiment H3, wherein the subsequent information is user
feedback
relating to information about device usage in previous days/nights and/or the
efficacy of the light
application during at least the treatment window.
H5. The method of embodiment H3, wherein the subsequent information is
obtained by at least
one of a light sensor, a motion sensor, and a capacitive sensor.
H6. The method of any of embodiments Hl-H5, wherein the instructions for
activating the light
source comprise instructions to activate the light source in one or more
pulses having an intensity
between 25 and 5,000 lux and a duration between 1 picosecond and 500
milliseconds.
H7. The method of any of embodiments Hl-H6, wherein the instructions for
activating the light
source comprise instructions to activate the light source using pulses having
a frequency between
once per 5 seconds and once per 120 seconds.
H8. The method of any of embodiments Hl-H7, wherein the calibration data is
based on
human-subject studies.
H9. The method of any of embodiments Hl-H8, wherein the generated
instructions for
activating the light source specify that a treatment regimen for a night
includes at least one higher-
treatment phase and at least one lower-treatment phase, at least one of light
flash intensity,
frequency, and/or duration being greater during the higher-treatment phase
than during the lower-
treatment phase.
63
CA 03204589 2023-06-07
WO 2022/125899 PCT/US2021/062810
H10. The method of any of embodiments Hl-H9, wherein the higher-treatment
phase is applied
before the lower-treatment phase, such that the user's homeostatic sleep drive
is higher when the
higher-treatment phase is applied than when the lower-treatment phase is
applied.
H11. The method of any of embodiments Hl-H10, wherein the step of applying the
light flashes
is performed via a sleep mask.
ALTERNATIVES AND EXTENSIONS
[0137] Each embodiment disclosed herein may be used or otherwise combined with
any of the
other embodiments disclosed. Any element of any embodiment may be used in any
embodiment.
[0138] Although the invention has been described with reference to specific
embodiments, it will
be understood by those skilled in the art that various changes may be made and
equivalents may
be substituted for elements thereof without departing from the true spirit and
scope of the
invention. In addition, modifications may be made without departing from the
essential teachings
of the invention.
64