Note: Descriptions are shown in the official language in which they were submitted.
WO 2022/164466
PCT/US2021/027270
ANTIVIRAL COMPOSITIONS AND DEVICES AND METHODS OF USE THEREOF
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority to US Provisional
Application No.
63/143,370, filed January 29, 2021, the contents of which are entirely
incorporated by
reference herein.
FIELD
[0002] The present disclosure relates to antiviral
compositions, systems,
methods, and devices. More specifically, the disclosure relates to silicon
nitride
compositions, devices, and coatings for the inactivation of viruses.
BACKGROUND
[0003] The need for safe and reliable inactivation or
removal of viruses is
universal. There is a broad need to control the pathogens that affect human
health. Not
only is there a need for materials that possess antiviral properties for human
medicinal
therapies, but also for use as surface coatings and/or composites for various
medical
devices or equipment, examination tables, clothing, filters, masks, gloves,
catheters,
endoscopic instruments, and the like.
[0004] Severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2),
which is responsible for the COVID-19 pandemic, remains viable and therefore
potentially infectious on several materials. One strategy to discourage the
fom ite-
mediated spread of COVID-19 is the development of materials whose surface
chemistry
can spontaneously inactivate SARS-CoV-2.
[0005] Therefore, there is a need for safe and reliable
methods to
inactivate and kill viruses that may be applied to medical devices, equipment,
clothing,
or other systems which may have prolonged contact with the human body.
1
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
SUMMARY
[0006] Provided herein are embodiments of an antiviral
composition
comprising silicon nitride at a concentration of about 1 wt.% to about 15
wt.%, where the
silicon nitride inactivates a virus in contact with the composition.
[0007] In some aspects, the virus may be in contact with
the silicon nitride
for a duration of at least 1 minute or at least 30 minutes. For example, the
virus may be
at least 85% inactivated after contact with the silicon nitride for at least 1
minute. The
silicon nitride may be present at a concentration of less than or equal to 10
wt.%. The
silicon nitride may be a-Si3N4, p-Si3N4, SiYAION, p-SiYAION, SiYON, or SiAION.
The
virus may be Influenza A or SARS-CoV-2. In some aspects, the composition is a
slurry,
suspension, gel, spray, paint, or toothpaste.
[0008] Further provided herein are embodiments of an
antiviral apparatus
comprising silicon nitride at a concentration of about 1 wt.% to about 15
wt.%, wherein
the silicon nitride inactivates a virus in contact with the composition.
[0009] In some aspects, the virus may be in contact with
the silicon nitride
for a duration of at least 1 minute or at least 30 minutes. For example, the
virus may be
at least 85% inactivated after contact with the silicon nitride for at least 1
minute. The
silicon nitride may be present at a concentration of less than or equal to 10
wt.%. The
silicon nitride may be a-Si3N4, p-Si3N4, SiYAION, p-SiYAION, SiYON, or SiAION.
The
virus may be Influenza A or SARS-CoV-2. In some aspects, the apparatus may be
a
medical device, medical equipment, examination table, filters, masks, gloves,
catheters,
endoscopic instruments, or commonly-touched surfaces. The apparatus may be
metallic, polymeric, and/or ceramic and the silicon nitride may be coated on
or
embedded in a surface of the apparatus.
[0010] Also provided herein are embodiments of a method of
preventing
transmission of a virus comprising: contacting an antiviral apparatus with the
virus,
where the apparatus comprises silicon nitride at a concentration of about 1
wt.% to
about 15 wt.%.
[0011] In some aspects, the virus may be in contact with
the silicon nitride
for a duration of at least 1 minute or at least 30 minutes. For example, the
virus may be
at least 85% inactivated after contact with the silicon nitride for at least 1
minute. The
2
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
silicon nitride may be present at a concentration of less than or equal to 10
wt.%. The
silicon nitride may be a-Si3N4, (3-Si3N4, SiYAION, (3-SiYAION, SiYON, or
SiAION. The
virus may be Influenza A or SARS-CoV-2.
[0012] Other aspects and iterations of the invention are
described more
thoroughly below.
BRIEF DESCRIPTION OF THE DRAWINGS
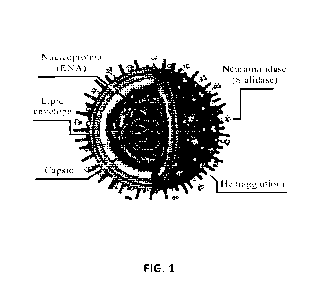
[0013] FIG. 1 is an illustration of the Influenza A virus.
[0014] FIG. 2A is an illustration of a virus exposed to 0
wt.%, 7.5 wt.%, 15
wt.%, and 30 wt.% Si3N4 for 10 minutes.
[0015] FIG. 2B is an illustration of methods used to
determine viability of
cells inoculated with a virus exposed to Si3N4 according to FIG. 2A.
[0016] FIG. 3A is an illustration of a virus exposed to 15
wt.% Si3N4 for 1,
5, 10, and 30 minutes.
[0017] FIG. 3B is an illustration of methods used to
determine viability of a
virus after exposure to Si3N4 according to FIG. 3A.
[0018] FIG. 4A is a graph of PFU/100 pl for Influenza A
exposed to 0 wt.%,
7.5 wt.%, 15 wt.%, and 30 wt.% Si3N4 for 10 minutes according to FIG. 2A.
[0019] FIG. 4B is a graph of cell survivability of cells
inoculated with
Influenza A exposed to 7.5 wt.%, 15 wt.%, and 30 wt.% Si3N4 for 10 minutes
according
to FIG. 2B.
[0020] FIG. 5 includes photographs of cells inoculated with
different ratios
of virus to slurry that had been exposed to various concentrations of Si3N4.
[0021] FIG. 6A shows a fluorescence microscopy image of
MDCK cells
before inoculation.
[0022] FIG. 6B shows a fluorescence microscopy image of
MDCK cells
after inoculation with a virus exposed to the control.
[0023] FIG. 6C shows a fluorescence microscopy image of
MDCK cells
after inoculation with a virus exposed to 30 wt.% Si3N4.
[0024] FIG. 7A is a graph of PFU/100 pl for Influenza A
exposed to 15
wt.% Si3N4 for 1 minute, 5 minutes, 10 minutes, or 30 minutes at room
temperature.
3
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
[0025] FIG. 7B is a graph of cell survivability of cells
inoculated with
Influenza A exposed to 15 wt.% Si3N4 for 1 minute, 5 minutes, 10 minutes, or
30
minutes at room temperature.
[0026] FIG. 8A is a graph of PFU/100 pl for Influenza A
exposed to 15
wt.% Si3N4 for 1 minute, 5 minutes, 10 minutes, or 30 minutes at 4 C.
[0027] FIG. 8B is a graph of cell survivability of cells
inoculated with
Influenza A exposed to 15 wt.% Si3N4 for 1 minute, 5 minutes, 10 minutes, or
30
minutes at 4 C.
[0028] FIG. 9A shows the Raman spectrum of Influenza A
virus before
inactivation.
[0029] FIG. 9B shows changes in the Raman spectrum of the
Influenza A
virus relevant to chemical modifications in RNA and hemagglutinin after
inactivation
after 1 minute of exposure.
[0030] FIG. 10 shows NH3 inactivates Influenza A virus by
the mechanism
of alkaline transesterification.
[0031] FIG. 11 shows O-P-0 stretching in pentacoordinate
phosphate
group after inactivation.
[0032] FIG. 12A shows vibrational modes of methionine in
the
hemagglutinin structure.
[0033] FIG. 12B shows methionine's structural change in the
presence of
ammonia.
[0034] FIG. 13 shows C-S stretching methionine to
homocysteine after
inactivation.
[0035] FIG. 14A is a graph of PFU/100 pl for Feline
calicivirus exposed to
15 wt.% or 30 wt.% Si3N4 for 1 minute, 10 minutes, or 30 minutes.
[0036] FIG. 14B is a graph of cell survivability of cells
inoculated with
Feline calicivirus exposed to 30 wt.% Si3N4 for 1 minute, 10 minutes, 30
minutes, or 60
minutes.
[0037] FIG. 15A shows the Hi Hi Influenza A virus
(nucleoprotein, NP)
stained red after 10 minutes of exposure to a slurry of 15 wt.% silicon
nitride and after
4
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
its inoculation into a biogenic medium containing MDCK cells stained green for
the
presence of filamentous actin (F-actin) proteins.
[0038] FIG. 15B shows the NP stained Hi Hi Influenza A
virus from FIG.
15A.
[0039] FIG. 15C shows the F-actin stained MDCK cells from
FIG. 15A.
[0040] FIG. 16A shows the Hi Hi Influenza A virus
(nucleoprotein, NP)
stained red without exposure to silicon nitride and after its inoculation into
a biogenic
medium containing MDCK cells stained green for the presence of filamentous
actin (F-
actin) proteins.
[0041] FIG. 16B shows the NP stained Hi Hi Influenza A
virus from FIG.
16A.
[0042] FIG. 16C shows the F-actin stained MDCK cells from
FIG. 16A.
[0043] FIG. 17 shows a trimodal distribution of silicon
nitride powder.
[0044] FIG. 18 shows the viability of the MDCK cells as
function of r3-Si3N4
concentration (wt.%/mL).
[0045] FIG. 19 shows a direct comparison of the viral
titers before and
after exposure of Influenza A to the Si3N4 powder for 30 minutes.
[0046] FIG. 20 shows the viability of the MDCK cells as
function of a-Si3N4
concentration (wt.%/mL).
[0047] FIG. 21 shows a comparison of the viral titers
before and after
exposure of Influenza A to the a-Si3N4 powder for 30 minutes.
[0048] FIG. 22 shows a trimodal particle size distribution
of silicon nitride
powder.
[0049] FIG. 23 is an overview of the antiviral testing
method.
[0050] FIG. 24A shows Vero cell viability measured at 24
hours post-
exposure to silicon nitride at concentrations of either 5, 10, 15 or 20
wt.%/vol (n=4)
incubated with cell culture media for 1, 5, and 10m.
[0051] FIG. 24B shows Vero cell viability measured at 48
hours post-
exposure to silicon nitride at concentrations of either 5, 10, 15 or 20 wt.
/0/vol (n=4)
incubated with cell culture media for 1, 5, and 10m.
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
[0052] FIG. 25A shows titers of silicon nitride at
concentrations of 5, 10,
15, and 20 wt.%/vol incubated with SARS-CoV-2 virus diluted in cell culture
media for 1,
5, and 10m expressed as PFU/mL.
[0053] FIG. 25B shows titers of silicon nitride at
concentrations of 5, 10,
15, and 20 wt.%/vol incubated with SARS-CoV-2 virus diluted in cell culture
media for 1,
5, and 10m expressed as % inhibition.
DETAILED DESCRIPTION
[0054] Various embodiments of the disclosure are discussed
in detail
below. While specific implementations are discussed, it should be understood
that this
is done for illustration purposes only. A person skilled in the relevant art
will recognize
that other components and configurations may be used without parting from the
spirit
and scope of the disclosure. Thus, the following description and drawings are
illustrative and are not to be construed as limiting. Numerous specific
details are
described to provide a thorough understanding of the disclosure. However, in
certain
instances, well-known or conventional details are not described in order to
avoid
obscuring the description.
[0055] Several definitions that apply throughout this
disclosure will now be
presented. Reference to "one embodiment" or "an embodiment" means that a
particular
feature, structure, or characteristic described in connection with the
embodiment is
included in at least one embodiment of the disclosure. The appearances of the
phrase
"in one embodiment" in various places in the specification are not necessarily
all
referring to the same embodiment, nor are separate or alternative embodiments
mutually exclusive of other embodiments. Moreover, various features are
described
which may be exhibited by some embodiments and not by others.
[0056] As used herein, the terms "comprising," "having,"
and "including"
are used in their open, non-limiting sense. The terms "a," "an," and "the" are
understood to encompass the plural as well as the singular. Thus, the term "a
mixture
thereof" also relates to "mixtures thereof."
[0057] As used herein, "about" refers to numeric values,
including whole
numbers, fractions, percentages, etc., whether or not explicitly indicated.
The term
6
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
"about" generally refers to a range of numerical values, for instance, 0.5-
1%, 1-5%
or 5-10% of the recited value, that one would consider equivalent to the
recited value,
for example, having the same function or result.
[0058] As used herein, the term "silicon nitride" includes
a-Si3N4, 3-Si3N4,
SiYAION, 13-SiYAION, SiYON, SiAION, or combinations thereof.
[0059] As used herein, "inactivate" or "inactivation"
refers to viral
inactivation in which the virus is stopped from contaminating the product or
subject
either by removing virus completely or rendering them non-infectious.
[0060] The terms "apparatus" or "component" as used herein
include
materials, compositions, devices, surface coatings, and/or composites. In some
examples the apparatus may include various medical devices or equipment,
examination tables, clothing, filters, personal protective equipment such as
masks and
gloves, catheters, endoscopic instruments, commonly-touched surfaces where
viral
persistence may encourage the spread of disease, and the like. The apparatus
may be
metallic, polymeric, and/or ceramic (ex. silicon nitride and/or other ceramic
materials).
[0061] As used herein, "contact" means in physical contact
or within close
enough proximity to a composition or apparatus to be affected by the
composition or
apparatus.
[0062] The terms used in this specification generally have
their ordinary
meanings in the art, within the context of the disclosure, and in the specific
context
where each term is used. Alternative language and synonyms may be used for any
one
or more of the terms discussed herein, and no special significance should be
placed
upon whether or not a term is elaborated or discussed herein. In some cases,
synonyms for certain terms are provided. A recital of one or more synonyms
does not
exclude the use of other synonyms. The use of examples anywhere in this
specification
including examples of any terms discussed herein is illustrative only, and is
not intended
to further limit the scope and meaning of the disclosure or of any example
term.
Likewise, the disclosure is not limited to various embodiments given in this
specification.
[0063] Additional features and advantages of the disclosure
will be set
forth in the description which follows, and in part will be obvious from the
description, or
can be learned by practice of the herein disclosed principles. The features
and
7
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
advantages of the disclosure can be realized and obtained by means of the
instruments
and combinations particularly pointed out in the appended claims. These and
other
features of the disclosure will become more fully apparent from the following
description
and appended claims, or can be learned by the practice of the principles set
forth
herein.
[0064] Provided herein are antiviral devices, compositions,
and
apparatuses that include silicon nitride (Si3N4) for the inactivation of
viruses. Silicon
nitride possesses a unique surface chemistry which is biocompatible and
provides a
number of biomedical applications including 1) concurrent osteogenesis,
osteoinduction,
osteoconduction, and bacteriostasis, such as in spinal and dental implants; 2)
killing of
both gram-positive and gram-negative bacteria according to different
mechanisms; 3)
inactivation of human and animal viruses, bacteria, and fungi; and 4) polymer-
or metal-
matrix composites, natural or manmade fibers, polymers, or metals containing
silicon
nitride powder retain key silicon nitride bone restorative, bacteriostatic,
antiviral, and
antifungal properties.
[0065] In an embodiment, an antiviral composition may
include silicon
nitride. For example, the antiviral composition may an apparatus that includes
silicon
nitride powder. In some embodiments, the antiviral apparatus may be a
monolithic
component comprising up to 100% silicon nitride. Such a component can be fully
dense
possessing no internal porosity, or it may be porous, having a porosity that
ranges from
about 1% to about 80%. The monolithic component may be used as a medical
device or
may be used in an apparatus in which the inactivation of a virus may be
desired. In
another embodiment, an antiviral composition may be incorporated within a
device or in
a coating to inactivate viruses on or within the device. In some embodiments,
the
antiviral composition may be a slurry comprising silicon nitride powder.
[0066] In some embodiments, the antiviral composition may
inactivate or
decrease the transmission of human viruses. Non-limiting examples of viruses
that may
be inactivated by the antipathogenic composition include coronaviruses (e.g.
SARS-
CoV-2), Influenza A, Hi Ni, enterovirus, and Feline calicivirus. For example,
a silicon
nitride composition may be effective in the inactivation of the Influenza A
virus. In other
8
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
examples, a silicon nitride composition may be effective in the inactivation
of the SARS-
CoV-2 virus.
[0067] Silicon nitride may be antipathogenic due to release
of nitrogen
containing species when in contact with an aqueous medium, or biologic fluids
and
tissues. The surface chemistry of silicon nitride may be shown as follows:
Si3N4 + 6H20 3Si02 + 4NH3
SiO2 + 2H20 Si(OH)4
[0068] Nitrogen elutes faster (within minutes) than silicon
because surface
silanols are relatively stable. For viruses, it was surprisingly found that
silicon nitride
may provide for RNA cleavage via alkaline transesterification which leads to
loss in
genome integrity and virus inactivation. This may also reduce the activity of
hemagglutinin. The elution of ammonia, along with an attendant increase in pH,
inactivates viruses, bacteria, and fungi. As shown in the examples, it was
surprisingly
found that each of silicon nitride inactivates a coronavirus and Influenza A.
[0069] In an embodiment, the antipathogenic composition may
exhibit
elution kinetics that show: (i) a slow but continuous elution of ammonia from
the solid
state rather than from the usual gas state; (ii) no damage or negative effect
to
mammalian cells; and (iii) an intelligent elution that increases with
decreasing pH.
[0070] The use of copper (Cu), a historically-recognized
viricidal agent, is
limited by its cell toxicity. In contrast to Cu, ceramic devices or
apparatuses made of
Si3N4 are biocompatible and not toxic to the human body. An advantage of Si3N4
is the
versatility of the material; thus Si3N4 may be incorporated into polymers,
bioactive
glasses, and even other ceramics to create composites and coatings that retain
the
favorable biocompatible and antiviral properties of Si3N4.
[0071] An antiviral device or apparatus may include a
silicon nitride
composition on at least a portion of a surface of the device for antiviral,
antibacterial, or
antifungal action. In an embodiment, an antiviral device may include a silicon
nitride
coating on at least a portion of a surface of the device. The silicon nitride
coating may
be applied to the surface of the device the device as a powder. In some
examples, the
silicon nitride powder may be filled, imbedded, or impregnated in at least a
portion of the
device. In some embodiments, the powder may be micrometric or nanometer in
size.
9
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
The average particle size may range from about 100 nm to about 5 pm, from
about 300
nm to about 1.5 pm, or from about 0.6 pm to about 1.0 pm. In other
embodiments, the
silicon nitride may be incorporated into the device. For example, a device may
incorporate silicon nitride powder within the body of the device. In one
embodiment, the
device may be made of silicon nitride. In another embodiment, the composition
can
comprise a slurry or suspension of nitride particles.
[0072] The silicon nitride coating may be present on the
surface of an
apparatus or within the apparatus in a concentration of about 1 wt.% to about
100 wt%.
In various embodiments, the coating may include about 1 wt.%, 2 wt.%, 5 wt.%,
7.5
wt.%, 8.3 wt.%, 10 wt.%, 15 wt.%, 16.7 wt.%, 20 wt.%, 25 wt.%, 30 wt.%, 33.3
wt.%, 35
wt.%, or 40 wt.% silicon nitride powder. In some examples, the coating may
include
about 10 wt.% to about 20 wt.% silicon nitride. In at least one example, the
coating
includes about 15 wt.% silicon nitride. In some embodiments, silicon nitride
may be
embedded in (as a filler) or on the surface of a device or apparatus in a
concentration of
about 1 wt.% to about 100 wt.%. In various embodiments, a device or apparatus
may
include about 1 wt.%, 2 wt.%, 5 wt.%, 7.5 wt.%, 8.3 wt.%, 10 wt.%, 15 wt.%,
16.7 wt.%,
20 wt.%, 25 wt.%, 30 wt.%, 33.3 wt.%, 35 wt.%, 40 wt.%, 50 wt.%, 60 wt.%, 70
wt. %,
80 wt.%, 90 wt.%, to 100 wt.% silicon nitride. In some examples, the silicon
nitride may
be on the surface of the apparatus at a concentration of about 10 wt.% to
about 20
wt.%. In at least one example, the silicon nitride may be on the surface of
the apparatus
at a concentration of about 15 wt.% silicon nitride.
[0073] In some embodiments, the antiviral composition may
be a
monolithic component consisting of the silicon nitride. Such a component can
be fully
dense possessing no internal porosity, or it may be porous, having a porosity
that
ranges from about 1% to about 80%. The monolithic component may be used as a
medical device or may be used in an apparatus in which the inactivation of a
virus may
be desired.
[0074] In various embodiments, a device or apparatus that
includes silicon
nitride for antiviral properties may be a medical device. Non-limiting
examples of
medical devices or apparatuses include orthopedic implants, spinal implants,
pedicle
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
screws, dental implants, in-dwelling catheters, endotracheal tubes,
colonoscopy scopes,
and other similar devices.
[0075] In some embodiments, silicon nitride may be
incorporated within or
applied as a coating to materials or apparatuses for antiviral properties such
as
polymers and fabrics, surgical gowns, tubing, clothing, air filters and water
filters,
masks, tables such as hospital exam and surgical tables, desks, fixtures,
handles,
knobs, toys, and filters such as air conditioner filters, or toothbrushes. In
some
examples, the filters may be within filtration devices of anesthesia machines,
ventilators,
or CPAP machines such that an antimicrobial surface layer in the filter can
trap
pulmonary pathogens, as air moves in and out of infected lungs.
[0076] In other embodiments, silicon nitride powder may be
incorporated
into compositions including, but not limited to slurries, suspensions, gels,
sprays, paint,
or toothpaste. For example, the addition of silicon nitride to a slurry, such
as paint, that
is then applied to a surface may provide an antibacterial, antifungal, and
antiviral
surface. In other embodiments, silicon nitride may be mixed with water along
with any
appropriate dispersants and slurry stabilization agents, and thereafter
applied by
spraying the slurry onto various surfaces. An example dispersant is Dolapix
A88.
[0077] In some embodiments, silicon nitride may be included
in an antiviral
composition at a concentration of about 5 wt.% to about 20 wt.%. In at least
one
example, the composition may include about 15 wt.% silicon nitride.
Alternatively, in
some embodiments, silicon nitride may be included in an antiviral composition
at a
concentration of about 5 wt.% to about 20 wt.%. In at least one example, the
composition may include about 15 wt.% silicon nitride. In an example, the
antiviral
composition may be a slurry of silicon nitride powder and water. Silicon
nitride may be
combined with water to form an aqueous slurry at concentrations of about 0.1
wt.% up
to about 70 wt.%. In some embodiments, the silicon nitride powder may be
present in
the slurry in a concentration of about 0.1 wt.% to about 55 wt.%. In other
embodiments,
silicon nitride may be incorporated within organic suspensions, gels, sprays,
and/or
paints at concentrations of about 0.1 wt.% up to about 70 wt.% or about 0.1
wt.% up to
about 55 wt.%. In various embodiments, the slurry, organic suspension, gel,
spray,
and/or paint may include about 0.1 wt.%, 0.5 wt.%, 1 wt.%, 1.5 wt.%, 2 wt.%, 5
wt.%, 10
11
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
wt.%, 15 wt.%, 20 wt.%, 25 wt.%, 30 wt.%, 35 wt.%, 40 wt.%, 45 wt.%, 50 wt.%,
or 55
wt.% silicon nitride.
[0078] In some embodiments, the composition comprises a
toothpaste and
the silicon nitride is in the form of a powder that is directly substituted
for silicon dioxide
powder found in standard toothpaste. The silicon nitride powder may be
substituted for
silicon dioxide powder in toothpaste at concentrations of about 1 wt.% to
about 30 wt.%.
In some examples, silicon nitride may be present within a toothpaste at a
concentration
of about 1 wt.%, 5 wt.%, 10 wt.%, 15 wt.%, 20 wt.%, The silicon nitride not
only serves
to be an antiviral and antibacterial agent in the toothpaste, but it also may
serve as a
polishing agent similar to silicon dioxide.
[0079] Further provided herein is a method of inactivating
a pathogen by
contacting a virus with an antiviral composition comprising silicon nitride.
In an
embodiment, the method may include coating a device or apparatus with silicon
nitride
and contacting the coated apparatus with the virus. Coating the apparatus may
include
applying a silicon nitride powder to a surface of the apparatus. In other
embodiments,
the silicon nitride powder may be filled, incorporated, or impregnated within
the device
or apparatus.
[0080] Without being limited to a particular theory, the
antiviral composition
may decrease viral action by alkaline transesterification and reduce the
activity of
hemagglutinin. It was surprisingly found that silicon nitride powder (i)
remarkably
decreases viral action by alkaline transesterification through the breakage of
RNA
internucleotide linkages and (ii) markedly reduced the activity of
hemagglutinin thus
disrupting host cell recognition by denaturing protein structures on viral
surfaces leading
to the inactivation of viruses regardless of the presence of a viral envelope.
[0081] In an embodiment, the antipathogenic composition may
exhibit
elution kinetics that show: (i) a slow but continuous elution of ammonia from
the solid
state rather than from the usual gas state; (ii) no damage or negative effect
to
mammalian cells; and (iii) an intelligent elution which increases with
decreasing pH.
Moreover, the inorganic nature of silicon nitride may be more beneficial than
the use of
petrochemical or organometallic bactericides, virucides, and fungicides which
are
12
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
known to harm mammalian cells or have residual effects in soil, on plants, and
in
vegetables or fruit.
[0082] Also provided herein is a method of treating or
preventing a
pathogen at a location in a human patient. For example, the pathogen may be a
virus.
The method may include contacting the patient with a device, apparatus, or
composition
comprising silicon nitride. Without being limited to any one theory, the
silicon nitride
inactivates the virus (for example, a coronavirus, such as SARS-CoV-2, or
Influenza A).
The device, apparatus, or composition may include about 1 wt.% to about 100
wt.%
silicon nitride. In some examples, the device or apparatus may include about 1
wt.% to
about 100 wt.% silicon nitride on the surface of the device or apparatus. In
an
embodiment, the device or apparatus may be a monolithic silicon nitride
ceramic. In
another embodiment, the device or apparatus may include a silicon nitride
coating, such
as a silicon nitride powder coating. In another embodiment, the device or
apparatus
may incorporate silicon nitride into the body of the device. For example,
silicon nitride
powder may be incorporated or impregnated into the body of the device or
apparatus
using methods known in the art.
[0083] In some embodiments, the composition or apparatus
may be
contacted with the patient or user for at least 1 minute, at least 5 minutes,
at least 30
minutes, at least 1 hour, at least 2 hours, at least 5 hours, or at least 1
day. In at least
one example, the device or apparatus may be permanently implanted in the
patient. In
at least one example, the device or apparatus may be worn externally by a
user. In
another example, the apparatus may be a high contact surface. In further
examples, the
apparatus may be in continuous or sustained contact with a body fluid of a
patient. The
body fluid may be blood or gas (inhalation or exhalation gas).
[0084] In some embodiments, the virus is at least 70%
inactivated, at least
75% inactivated, at least 80% inactivated, at least 85% inactivated, at least
90%
inactivated, at least 95% inactivated, or at least 99% inactivated after
contact with the
silicon nitride in the composition or apparatus for at least 1 minute, at
least 5 minutes, or
at least 30 minutes. In at least one example, the virus is at least 85%
inactivated after
contact with the silicon nitride in the composition or apparatus for at least
1 minute. In
13
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
another example, the virus is at least 99% inactivated after contact with the
silicon
nitride in the composition or apparatus for at least 30 minutes.
EXAMPLES
Example 1: Effect of silicon nitride concentration on virus inactivation
[0085] To show the effect of silicon nitride concentration
on the inactivation
of viruses, Influenza A was exposed to various concentrations of Si3N4 powder.
To
prepare the silicon nitride, a specific weight of silicon nitride powder mixed
with pure
distilled water. For instance, 7.5 g of silicon nitride was dispersed in 92.5
g of pure
distilled water. The virus was added to this mixture in concentrations of 1:1,
1:10 and
1:100 virus/mixture, respectively. These mixtures were then allowed to
incubate under
gentle agitation for 10 minutes at 4 C. Influenza A was exposed to 0 wt.%, 7.5
wt.%, 15
wt.%, and 30 wt.% Si3N4 for 10 minutes at 4 C, as illustrated in FIG. 2A. The
mixtures
were then filtered to remove the silicon nitride powder.
[0086] Influenza A virus-inoculated Madin-Darby canine
kidney (MDCK)
cells were then observed for the effectiveness of Si3N4 in inactivating the
Influenza A.
The remaining mixtures were then inoculated into Petri dishes containing
living MDCK
cells within a biogenic medium. The amount of living MDCK cells were
subsequently
counted using staining methods after 3 days exposure. The viability of MDCK
cells was
determined after inoculating the cells for 3 days with Influenza A exposed to
Si3N4
according to FIG. 2B.
[0087] FIG. 4A is a graph of PFU/100 pl for Influenza A
exposed to 0
wt.%, 7.5 wt.%, 15 wt.%, and 30 wt.% Si3N4 for 10 minutes. FIG. 4B is a graph
of cell
survivability of cells inoculated with Influenza A exposed to 7.5 wt.%, 15
wt.%, and 30
wt.% Si3N4 for 10 minutes.
Example 2: Effect of exposure time and temperature on virus inactivation
[0088] To show the effect of silicon nitride on the
inactivation of viruses,
Influenza A was exposed to a fixed concentration of Si3N4 powder (15 wt.%) for
various
times and temperatures. The mixture was then allowed to incubate under gentle
agitation for 1-30 minutes at room temperature and at 4 C. For example,
Influenza A
14
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
was exposed to 15 wt.% Si3N4 for 1, 5, 10, or 30 minutes at room temperature
or 4 C,
as illustrated in FIG. 3A. Influenza A virus-inoculated Madin-Darby canine
kidney
(MDCK) cells were then observed for the effectiveness of Si3N4 in inactivating
the
Influenza A. The viability of MDCK cells was determined after inoculating the
cells for 3
days with Influenza A exposed to Si3N4 according to FIG. 3B.
[0089] FIG. 7A is a graph of PFU/100 pl for Influenza A
exposed to 15
wt.% Si3N4 for 1 minute, 5 minutes, 10 minutes, 0r30 minutes at room
temperature.
FIG. 7B is a graph of cell survivability of MDCK cells inoculated with
Influenza A
exposed to 15 wt.% Si3N4 for 1 minute, 5 minutes, 10 minutes, or 30 minutes at
room
temperature.
[0090] FIG. 8A is a graph of PFU/100 pl for Influenza A
exposed to 15
wt.% Si3N4 for 1 minute, 5 minutes, 10 minutes, or 30 minutes at 4 C. FIG. 8B
is a
graph of MDCK cell survivability inoculated with Influenza A exposed to 15
wt.% Si3N4
for 1 minute, 5 minutes, 10 minutes, or 30 minutes at 4 C.
Example 3: Effect of silicon nitride on H1H1 Influenza A inactivation
[0091] To show the effect of silicon nitride on the
inactivation of viruses,
Influenza A was exposed to a slurry of 15 wt.% silicon nitride for 10 minutes.
[0092] FIGS. 15A-15C show the Hi Hi Influenza A virus
(A/Puerto
Rico/8/1934 H1N1 (PR8)) stained red (nucleoprotein, NP) after its inoculation
into a
biogenic medium containing MDCK cells stained green for the presence of
filamentous
actin (F-actin) proteins which are found in all eukaryotic cells. FIGS. 16A-
16C shows the
effect of the virus on the MDCK cells without the presence of silicon nitride.
Example 4: Evaluation of Influenza A viricidal activity by silicon nitride in
MDCK cells
[0093] This study was designed to examine the antiviral
capabilities of
beta-silicon nitride (p-Si3N4) powder versus Influenza A at an incubation time-
point of 30
minutes and a concentration of 15 wt.%/wt. A 15 wt.% suspension was prepared
in 1.5
mL of virus diluted in DMEM with no additives.
[0094] A plaque assay methodology was utilized. To
adequately quantify
the plaque assay, the viability of Madin Darby Canine Kidney Cells (MDCK) were
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
assessed as a function of exposure to various concentrations of Si3N4 for
incubation
periods ranging from 30 minutes to 72 hours. The results demonstrated that
Si3N4 was
completely viricidal to Influenza A with a reduction of > 99.98% in viral load
at the
preselected conditions. The viability of the MDCK cells was found to be time-
and dose-
dependent. Essentially no loss in viability was observed for Si3N4
concentrations up to
15 wt. /0/wt. Changes in viability were only noted for the 15 wt.%
concentration at 24,
48, and 72 hours (i.e., 83.3%, 59.7%, and 44.0% viable, respectively).
[0095] The Si3N4 powder used in this study had a nominal
composition of
90 wt.% a-Si3N4, 6 wt.% yttria (Y203), and 4 wt.% alumina (A1203). It was
prepared by
aqueous mixing and spray-drying of the inorganic constituents, followed by
sintering of
the spray-dried granules (-1700 C for -3 h), hot-isostatic pressing (-1600 C,
2 h, 140
MPa in N2), aqueous-based comminution, and freeze-drying. The resulting powder
had
a trimodal distribution with an average particle size of 0.8 1.0 pm as shown
in FIG. 17.
Doping Si3N4 with Y203 and A1203 is useful to densify the ceramic and convert
it from its
a- to 13-phase during sintering. The mechanism of densification is via
dissolution of a-
phase and subsequent precipitation of 13-phase grains facilitated by the
formation of a
transient intergranular liquid that solidifies during cooling. 13-Si3N4 is
therefore a
composite composed of about 10 wt.% intergranular glass phase (IGP) and 90
wt.%
crystalline 6-Si3N4 grains.
[0096] Three sequential assays were conducted in this
study: (1) An
MDCK viability test; (2) An influenza A supernatant titration test with and
without
centrifugation and filtration; and (3) A viral titration using 15 wt.%/wt
Si3N4 as the viral
inhibitor for an incubation period of 30m.
[0097] In FIG. 18, the viability of the MDCK cells is shown
as function of 13-
Si3N4 concentration (wt.%/mL). Starting at 15 wt.%, serial dilutions were
conducted to
arrive at 0.047 wt.%. At the lower concentrations, cell viability was
generally > 80% for
all timepoints up to 72 h. Note also that cell viability generally increased
with exposure
time for all concentrations except 15 wt.%. At 15 wt.% and 30-minutes exposure
the cell
viability was -94.5%.
[0098] Following the determination of MDCK cell viability,
twenty-four
hours prior to the addition of the virus and sample to the cells, MDCK cells
were plated
16
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
in a 6-well plate at a density of 1 x 106 cells/well in a volume of 2 mL in
Dulbecco's
Minimum Essential Medium (DMEM) supplemented with 10% fetal bovine serum
(FBS).
On the day of the assay, triplicate samples of 15 wt.% of silicon nitride in
virus diluted in
DMEM with no additives at 1 x 104 PFU/mL was incubated for 30 minutes at room
temperature with shaking. Following incubation, the samples were centrifuged
for two
minutes at 4 C and 12,000 rpm, and further filtered through a 0.2-micron
polyvinylidene
difluoride (PVDF) filter. The samples were then serially diluted 1:5 and 7
concentrations
were added to cells that had been washed 2 times with Dulbecco's Phosphate
Buffered
Saline (DPBS) in triplicate in a volume of 400 pL. The samples were incubated
for 1
hour at 37 C with rocking every 15 to 20 minutes. Following incubation, 2 mL
of the
plaque assay media was added to the wells and the cultures were incubated for
48
hours at 35 C/5% CO2. After incubation, the cells were stained with crystal
violet and
the plaques were enumerated visually.
[0099] On the day of staining, the plaguing media was
removed, and the
monolayers were washed two times with DPBS. The cells were then fixed with 70%
ethanol for 10 minutes at room temperature. The ethanol was removed, and 0.3%
crystal violet solution was added to each well for 10 minutes at room
temperature.
Following this incubation, the crystal violet was removed, and the monolayers
were
washed two times with DPBS to remove residual crystal violet. The monolayers
were
air-dried overnight prior to counting the plaques.
[0100] The viricidal test was conducted at a concentration
of 15 wt.%/vol
and at 30 min. The process steps of centrifugation and filtration only reduced
the viral
load by about 0.25 logio. Given this result, a subsequent titration was then
conducted
without and with the exposure of the virus to Si3N4 for 30 minutes. The
concentration for
the titration without Si3N4 was a priori selected to be 4.4 x 103 pfu/m I
based on ISO
21702 (Measurement of antiviral activity on plastics and other non-porous
surfaces).
After 30 minutes of exposure to Si3N4, no plaques formed on the MDCK cells.
Si3N4 was
deemed to be 100% effective in inactivating Influenza A. A direct comparison
of the viral
titers before and after exposure to the Si3N4 powder for 30m is provided in
FIG. 19. The
data clearly demonstrate > 3.510gio reduction in viral load after exposure to
Si3N4 (i.e.,
>99.98%).
17
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
[0101] In summary, these tests demonstrated that exposure
of Si3N4 to
MDCK cells had no adverse viability effects at concentrations less than 15
wt.%/vol or
time periods of 30 minutes. At antiviral test conditions of 15 wt. /0/vol
Si3N4 at 30
minutes exposure at a viral load of 4.4 x 103 pfu/m I, Si3N4 inactivated
essentially 100%
of the exposed virions. Si3N4 was found to be viricidal to Influenza A under
these
conditions.
Example 5: Effect of a-Si3N4 Powder on MDCK Cells and Influenza A
[0102] a-Si3N4 powder was first evaluated for toxicity to
MDCK cells
following exposure for 30 minutes, 24 hours, 48 hours and 72 hours. A 15
weight %
(wt.%) suspension was prepared in 1.5 mL of Dulbecco's Modified Eagle Medium
(DMEM) supplemented with 2% fetal bovine serum (FBS).
[0103] Twenty-four hours prior to the addition of the
sample to the cells,
the a-Si3N4 powder suspension prepared as described above was incubated for 30
minutes at room temperature with shaking. Following the incubation, the
suspension
was centrifuged for two minutes at 4 C at 12,000 rpm. The supernatant was
further
filtered through a 0.2-micron polyvinylidene difluoride (PVDF) filter and then
serially
diluted in 1 /2-logarithmic increments. Six (6) concentrations were added to
the pre-
plated cells in triplicate in a volume of 200 pL. The plates were incubated
for 30
minutes, 24, 48, and 72 hours at which time the cells were evaluated for
cellular toxicity
using the tetrazolium dye XTT (2,3-bis(2-methoxy-4-nitro-5-sulfophenyI)-5-
[(phenylamino)carbony1]-2H-tetrazolium hydroxide), as described below.
[0104] TC50 values for the test materials were derived by
measuring the
reduction of the tetrazolium dye XTT. XTT in metabolically active cells is
metabolized by
the mitochondrial enzyme NADPH oxidase to a soluble formazan product. XTT
solution
was prepared daily as a stock of 1 mg/mL in DMEM without additives. Phenazine
methosulfate (PMS) solution was prepared at 0.15 mg/mL in Dulbecco's Phosphate
Buffered Saline (DPBS) and stored in the dark at -20 C. XTT/PMS stock was
prepared
immediately before use by adding 40 pL of PMS per mL of XTT solution. Fifty pL
(50 4)
of XTT/PMS was added to each well of the plate and the plate incubated for 4
hours at
37 C. The 4-hour incubation has been empirically determined to be within the
linear
18
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
response range for XTT dye reduction with the indicated numbers of cells for
each
assay. The plates were sealed and inverted several times to mix the soluble
formazan
product and the plate was read at 450 nm (650 nm reference wavelength) with a
Molecular Devices SpectraMax Plus 384 96 well plate format spectrophotometer.
[0105] MDCK cells were treated with 6 concentrations of the
a-Si3N4
powder ranging from 15 wt.% to 0.047 wt. % for 30 minutes, 24 hours, 48 hours
and 72
hours. In FIG. 20, the viability of the MDCK cells is shown as function of a-
Si3N4
concentration (wt.%/mL). Following 30 minutes of exposure cells treated with
all
concentrations had viability greater than 90% except for cells treated with
4.7 wt.% and
15 wt.% which had 89% and 83% viability, respectively. At 24 hours viability
of cell
treated with each concentration remained above 92%. At 48 hours viability
dropped
below 90% in cells treated with 1.5 wt.%, 4.7 wt.% and 15 wt.% (89.1%, 88.7%
and
74.0%, respectively) but at 72 hours only cells treated with 15 wt. `)/0 had
viability below
90% (87.5 %).
[0106] a-Si3N4 powder at 15 wt.% was then evaluated for
virucidal activity
against Influenza A strain NPR/8/34 in MDCK cells. A 15 wt.% suspension was
prepared in 1.5 mL of virus diluted in DMEM with no additives.
[0107] Twenty-four hours prior to the addition of the virus
and sample to
the cells, MDCK cells were plated in a 6-well plate at a density of 1 x 106
cells/well in a
volume of 2 mL in Dulbecco's Minimum Essential Medium (DMEM) supplemented with
10% fetal bovine serum (FBS). On the day of the assay, triplicate samples of
15 wt.% of
a-Si3N4 in virus diluted in DMEM with no additives at 1 x 104 PFU/mL was
incubated for
30 minutes at room temperature with shaking. Following incubation, the samples
were
centrifuged for two minutes at 4 C and 12,000 rpm, and further filtered
through a 0.2-
micron polyvinylidene difluoride (PVDF) filter. The samples were then serially
diluted 1:5
and 7 concentrations were added to cells that had been washed 2 times with
Dulbecco's Phosphate Buffered Saline (DPBS) in triplicate in a volume of 400
mL. The
samples were incubated for 1 hour at 37 C with rocking every 15 to 20 minutes.
Following incubation, 2 mL of the plaque assay media was added to the wells
and the
cultures were incubated for 48 hours at 35 C/5% CO2. After incubation, the
cells were
stained with crystal violet and the plaques were enumerated visually.
19
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
[0108] On the day of staining, the plaguing media was
removed, and the
monolayers were washed two times with DPBS. The cells were then fixed with 70%
ethanol for 10 minutes at room temperature. The ethanol was removed, and 0.3%
crystal violet solution was added to each well for 10 minutes at room
temperature.
Following this incubation, the crystal violet was removed, and the monolayers
were
washed two times with DPBS to remove residual crystal violet. The monolayers
were
air-dried overnight prior to counting the plaques.
[0109] The virucidal activity of 15 wt.% a-Si3N4 powder was
evaluated
against Influenza virus A strain A/PR8/34 in MDCK cells. The target virus
titer was 1 x
104 PFU/mL and the actual individual replicates were 3.1 x 103, 3.8 x 103, and
4.7 x 103
PFU/mL yielding a mean titer (and standard deviation) of 3.9 x 103 0.8 x 103
P FU/m L.
This actual titer is within two-fold of the targeted PFU/mL. The a-Si3N4
powder treated
samples had one well with a single plaque which resulted in a PFU/mL of 4.1.
[0110] The log reduction was 2.98 and was calculated using
the following
equation: logio(A/B) where A is untreated virus and B is treated virus. The
percent
reduction was 99.89% and was calculated using the following equation: (A-B) x
100/A
where A is untreated virus and B is treated virus. A comparison of the viral
titers before
and after exposure to the a-Si3N4 powder for 30m is provided in FIG. 21.
Therefore, the
a-Si3N4 powder at 15 wt.% was virucidal to influenza A virus strain NPR/8/34
following
a 30-minute exposure.
Example 6: Influenza A Virucidal Activity by two forms of Si3N4 powder in MDCK
Cells
[0111] A 5 and 10 wt.% suspension of a-Si3N4 and 13-Si3N4
powder was
prepared in 1.5 mL of virus diluted in DMEM with no additives.
[0112] Twenty-four hours prior to the addition of the virus
and sample to
the cells, MDCK cells were plated in a 6-well plate at a density of 1 x 106
cells/well in a
volume of 2 mL in Dulbecco's Minimum Essential Medium (DMEM) supplemented with
10% fetal bovine serum (FBS). On the day of the assay, triplicate samples of
10 and 5
wt.% of a-Si3N4 and 6-Si3N4 powders in virus diluted in DMEM with no additives
at 1 x
104 PFU/mL were incubated for 30 minutes at room temperature with shaking.
Following incubation, the samples were centrifuged for two minutes at 4 C and
12,000
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
rpm, and further filtered through a 0.2-micron polyvinylidene difluoride
(PVDF) filter. The
samples were then serially diluted 1:5 and 7 concentrations were added to
cells that
had been washed 2 times with Dulbecco's Phosphate Buffered Saline (DPBS) in
triplicate in a volume of 400 pL. The samples were incubated for 1 hour at 37
C with
rocking every 15 to 20 minutes. Following incubation, 2 mL of the plaque assay
media
was added to the wells and the cultures were incubated for 48 hours at 35 C/5%
CO2.
After incubation, the cells were stained with crystal violet and the plaques
were
enumerated visually.
[0113] On the day of staining, the plaguing media was
removed, and the
monolayers were washed two times with DPBS. The cells were then fixed with 70%
ethanol for 10 minutes at room temperature. The ethanol was removed, and 0.3%
crystal violet solution was added to each well for 10 minutes at room
temperature.
Following this incubation, the crystal violet was removed, and the monolayers
were
washed two times with DPBS to remove residual crystal violet. The monolayers
were
air-dried overnight prior to counting the plaques.
[0114] The virucidal activity of 5 and 10 wt.% of a-Si3N4
and 6-Si3N4
powder was evaluated against Influenza virus A strain AIPR8/34 in MDCK cells.
This
was performed in four individual experiments. The target virus titer was 1 x
104 PFU/mL.
[0115] In the first experiment the individual replicates
for the untreated
virus samples were 5.3 x 103, 5.9 x 103, and 4.1 x 103 PFU/mL yielding a mean
titer
(and standard deviation) of 5.1 x 103 0.9 x 103 PFU/mL. Virus treated with 5
wt.% and
wt.% of 6-Si3N4 for 10 minutes resulted in a PFU/mL of < 21 for the virus
treated with
10 wt.% and a PFU/mL of 21 (1 plaque formed) in virus treated with 5 wt.%. In
this
sample, the log reduction was 2.4 and was calculated using the following
equation:
log10(NB) where A is untreated virus and B is treated virus. The percent
reduction was
99.5 % and was calculated using the following equation: (A-B) x 100/A where A
is
untreated virus and B is treated virus.
[0116] In the second experiment the individual replicates
for the untreated
virus samples were 7.5 x 103, 7.2 x 103, and 5.0 x 103 PFU/mL yielding a mean
titer
(and standard deviation) of 6.6 x 103 1.4 x 103 PFU/mL. Virus treated with 5
wt.% and
10 wt.% of (3-Si3N4 for 5 minutes resulted in a PFU/mL of <21 for both.
21
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
[0117] In the third experiment the individual replicates
were 6.9 x 103, 7.8 x
103, and 5.0 x 103 PFU/mL yielding a mean titer (and standard deviation) of
6.6 x 103
1.4 x 103 PFU/mL. Virus treated with 5 wt.% and 10 wt.% of a-Si3N4 for 10
minutes
resulted in a PFU/mL of < 21 for both.
[0118] In the fourth experiment the individual replicates
were 8.8 x 103, 1.0
x 104, and 7.5 x 103 PFU/mL yielding a mean titer (and standard deviation) of
8.8 x 103
1.3 x 103 PFU/mL. Virus treated with 5 wt.% and 10 wt.% of a-Si3N4 for 5
minutes
resulted in a PFU/mL of < 21 for both.
[0119] In each of the experiments the actual titer
determined for the
untreated virus control was with-in two-fold of the targeted PFU/mL. Virus
treated with
both a-Si3N4 and p-Si3N4 powder at 5 and 10 wt. % for 5 and 10 minutes
resulted in a
PFU/mL of < 1 (no plaques observed) with the exception of the p-Si3N4 powder
treated
sample at 5 wt.% for 10 minutes which had one well with a single plaque
resulting in a
PFU/mL of 21.
Example 7: Silicon Nitride inactivation of SARS-CoV-2 in vitro
[0120] A doped Si3N4 powder (r3-SiYAION) with a nominal
composition of
90 wt.% a-Si3N4, 6 wt.% yttria (Y203), and 4 wt.% alumina (A1203) was prepared
by
aqueous mixing and spray-drying of the inorganic constituents, followed by
sintering of
the spray-dried granules (-1700 C for -3 h), hot-isostatic pressing (-1600 C,
2 h, 140
MPa in N2), aqueous-based comminution, and freeze-drying. The resulting powder
had
a trimodal distribution with an average particle size of 0.8 1.0 pm as shown
in FIG. 22.
Doping Si3N4 with Y203 and A1203 densified the ceramic and converted it from
its a- to
R-phase during sintering. The mechanism of densification is via dissolution of
a-phase
and subsequent precipitation of /3-phase grains facilitated by the formation
of a transient
intergranular liquid that solidifies during cooling. f3-Si3N4 is therefore a
composite
composed of about 10 wt.% intergranular glass phase (IGP) and 90 wt.%
crystalline /3-
Si3N4 grains.
[0121] Vero green African monkey kidney epithelial cells
were chosen for
this analysis due to their ability to support high levels of SARS-CoV-2
replication and
their use in antiviral testing. These cells were cultured in DMEM supplemented
with
22
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
10% FBS, 1% L-glutamine, and 1% penicillin/streptomycin. Cells were maintained
at
37 C and 5% CO2. SARS-CoV-2 isolate USA-WA1/2020 was obtained from BEI
Resources. Vero cells were inoculated with SARS-CoV-2 (M010.1) to generate
viral
stocks. Cell-free supernatants were collected at 72 hours post-infection and
clarified via
centrifugation at 10,000 rpm for 10 minutes and filtered through a 0.2 pm
filter. Stock
virus was titered according to the plaque assay protocol detailed below.
[0122] The Si3N4 powder was suspended in 1 mL DMEM growth
media in
microcentrifuge tubes. Tubes were vortexed for 30 seconds to ensure adequate
contact
and then placed on a tube revolver for either 1, 5, or 10 minutes. At each
time point, the
samples were centrifuged, and the supernatant was collected and filtered
through a 0.2
pm filter. Clarified supernatants were added to cells for either 24 or 48
hours. Untreated
cells were maintained alongside as controls. Cells were tested at each time
point using
CellTiter Glo, which measures ATP production, to determine cell viability.
[0123] SARS-CoV-2 was diluted in DMEM growth media to a
concentration of 2 x 104 PFU/m L. Four mL of the diluted virus was added to
tubes
containing silicon nitride at 20, 15, 10, and 5% (w/v). The virus without
Si3N4was
processed in parallel as a control. Tubes were vortexed for 30 seconds to
ensure
adequate contact and then placed on a tube revolver for either 1, 5, or 10
minutes, while
a virus only control was incubated for the maximum 10 minutes. At each time
point, the
samples were centrifuged, and the supernatant was collected and filtered
through a 0.2
pm filter. The remaining infectious virus in the clarified supernatant was
quantitated by
plaque assay. An overview of the antiviral testing method is provided in FIG.
23. In step
1, SARS-CoV-2 virus was diluted in media. In step 2, 4 mL of diluted virus was
added to
tubes containing silicon nitride at 20, 15, 10, or 5% (w/v). In step 3, tubes
were vortexed
for 30 s to ensure adequate contact and the placed on a tube revolver for
either 1 m, 5
m, or 10 m (virus only control was incubated for the maximum 10 m). In step 4,
at each
time point, the samples were centrifuged, and the supernatant was collected
and filtered
through a 0.2 pm filter. In step 5, clarified supernatant was used to perform
plaque
assays. Samples were serially diluted (10-fold) and added to fresh Vero for 1
h
incubation, ricking every 15 min before adding an agarose medium overlay and
23
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
incubating for 48 h. After 48 h incubation, cells were fixed with 10% FA and
stained with
Crystal Violet for counting.
[0124] Vero cells were plated at 2 x 105 cells/well in a 12-
well plate on the
day before the plaque assay. Clarified supernatants from the antiviral testing
were
serially diluted (10-fold) and 200 pL was added to Vero cells which were
incubated for 1
hour at 37 C, 5% CO2. Plates were rocked every 15 minutes to ensure adequate
coverage and at 1 hour, a 1:1 ratio of 0.6% agarose and 2X EMEM supplemented
with
5% FBS, 2% penicillin/streptomycin, 1% non-essential amino acids (VWR, Cat #
45000-
700), 1% sodium pyruvate, and 1% L-glutamine was added to the cells before
incubating for 48 hours at 37 C, 5% CO2. After incubation, the cells were
fixed with 10%
formaldehyde and stained with 2% crystal violet in 20% ethanol for counting.
[0125] The impact of Si3N4 on eukaryotic cell viability was
tested. Si3N4
was resuspended in cell culture media at 5, 10, 15, and 20% (w/v). Samples
were
collected at 1, 5, and 10 minutes and added to Vero cells. Vero cell viability
was
measured at 24 and 48 hours post-exposure (FIG. 24A and 24B). No significant
decrease in cell viability was observed at either 24 or 48 hours post-exposure
with 5%,
10%, or 15% silicon nitride. A small impact on cell viability (-10% decrease)
was
observed at 48 hours in cells exposed to 20% Si3N4. Interestingly, a -10%
increase in
Vero cell viability was observed at 48 hours with the 5% - 10 minute and 10% -
10
minute samples (FIG. 24B), suggesting that Si3N4 may be stimulating cell
growth or
cellular metabolism under these conditions. These data indicated that Si3N4
has minimal
impact on Vero cell health and viability up to 20 wt.%/vol.
[0126] Given that 5, 10, 15, and 20% Si3N4 were non-toxic
to Vero cells,
antiviral testing at these concentrations was performed. SARS-CoV-2 virions
were
exposed to Si3N4 at these concentrations for 1, 5, or 10 minutes. Following
Si3N4
exposure, the infectious virus remaining in each solution was determined
through
plaque assay. At each timepoint, the samples were centrifuged, and the
supernatant
was collected and filtered through a 0.2um filter. The clarified supernatant
was used to
perform plaque assay in duplicate. Virus processed in parallel but only
exposed to cell
culture media contained 4.2 x 103 PFU/mL. SARS-CoV-2 titers were reduced when
exposed to all concentrations of Si3N4 tested (FIGS. 25A and 25B). The
inhibition was
24
CA 03204665 2023-7- 10
WO 2022/164466
PCT/US2021/027270
dose-dependent with SARS-CoV-2 exposed for 1 minute and 5% Si3N4 having
reduced
viral titers by -0.8 logio, 10% Si3N4 by -1.2 logio, 15% Si3N4 by 1.4 logio,
and 20% Si3N4
by 1.7 logio (FIG. 25A). Similar results were observed with the 5 and 10
minute
samples. This reduction in viral titers corresponded to 85% viral inhibition
at 5% Si3N4,
93% at 10% Si3N4, 96% at 15% Si3N4, and 98% viral inhibition at 20% Si3N4
(FIG. 25B).
Higher Si3N4 concentrations for longer times resulted in increased inhibition -
leading to
99.6% viral inhibition at 20% Si3N4 and 10 minute exposure (FIG. 25B). These
data
indicate that Si3N4 has a strong antiviral effect against SARS-CoV-2.
[0127] The surprising finding was that a one-minute
exposure to a 5%
solution of Si3N4 resulted in 85% inactivation of SARS-CoV-2, while Vero cell
viability
was minimally impacted even after a 48 hour exposure to a 20% concentration of
the
same material.
[0128] Having described several embodiments, it will be
recognized by
those skilled in the art that various modifications, alternative
constructions, and
equivalents may be used without departing from the spirit of the invention.
Additionally,
a number of well-known processes and elements have not been described in order
to
avoid unnecessarily obscuring the present invention. Accordingly, the above
description should not be taken as limiting the scope of the invention.
[0129] Those skilled in the art will appreciate that the
presently disclosed
embodiments teach by way of example and not by limitation. Therefore, the
matter
contained in the above description or shown in the accompanying drawings
should be
interpreted as illustrative and not in a limiting sense. The following claims
are intended
to cover all generic and specific features described herein, as well as all
statements of
the scope of the present method and system, which, as a matter of language,
might be
said to fall therebetween.
CA 03204665 2023-7- 10