Note: Descriptions are shown in the official language in which they were submitted.
MG-AL MAGNESIUM ALLOY, PREPARATION METHOD FOR TUBE MADE OF
MG-AL MAGNESIUM ALLOY, APPLICATION OF MG-AL MAGNESIUM ALLOY
Cross-reference to Related Applications
The disclosure claims priority to Chinese patent application No. CN
202110040804.4 filed in Chinese Patent Office on J anuary 13, 2021, named "Mg-
Al
based magnesium alloy and tube preparation method and application thereof",
the
entire content of which is incorporated here by reference.
Technical Field
The present disclosure relates to a Mg-Al based magnesium alloy, and a
preparation
method of a tube of the magnesium alloy, and an application of the magnesium
alloy,
and belongs to the technical field of alloy materials.
Background Art
Magnesium alloys are by far the lightest metal structural material, their
density is
only 2/3 of that of aluminum and 1/4 of that of steel, and they have high
specific
strength and specific stiffness. In addition, magnesium alloys also have many
excellent
properties such as good damping, cutting machinability and thermal
conductivity, as
well as easy recovering and regeneration, making their application fields
increasingly
expanded.
Magnesium alloys mainly include Mg-Al based and Mg-Zn-Zr based magnesium
alloys, and Mg-Al based magnesium alloys have been widely used because of
their
lower preparation costs and simpler preparation methods. However, the
traditional Mg-
Al based alloys have poor elongation, and are prone to fracture when subjected
to
external impact deformation or cyclic loading. In addition, magnesium alloys
are
generally connected to each other by welding during application, and
traditional Mg-
Al based alloys have a large welding loss rate after welding, which not only
causes a
lot of waste of resources, but also affects the welding firmness and aesthetic
appearance.
Summary
Objection of the disclosure: in view of the problems of the existing Mg-Al
based
magnesium alloys, the disclosure provides a Mg-Al based magnesium alloy with
high
elongation and low welding loss rate and provides a preparation method of a
tube of
the Mg-Al based magnesium alloy; in addition, an application of the Mg-Al
based
magnesium alloy in the fields of vehicle equipment and medical equipment is
also
provided.
The technical solution: the Mg-Al based magnesium alloy of the present
disclosure
includes, by weight percentage, 7.0-8.6% Al, 0.8-2.0% RE, 0.2-0.8% Mn, and a
balance of Mg, and the magnesium alloy has an elongation of 15-22%.
Optionally, the elongation of the Mg-Al based magnesium alloy is 17-21.6%.
CA 03205147 2023- 7- 13
1
Optionally, the Mg-Al based magnesium alloy has a welding loss rate of less
than
6%.
Optionally, the Mg-Al based magnesium alloy has a yield strength of 182-235
MPa
and a tensile strength of 306-342 MPa.
Preferably, in the Mg-Al based magnesium alloy, the weight percentage of Al is
7.0-
8.2%, the weight percentage of RE is 1.1-2.0%, and the weight percentage of Mn
is
0.4-0.8%. The magnesium alloys with components within the above parameter
range
can achieve lower welding loss rate (less than 5.50%), higher elongation, and
higher
strength.
More preferably, in the Mg-Al based magnesium alloy, the weight percentage of
Al
is 7.8-8.2%, the weight percentage of RE is 1.3-1.9%, and the weight
percentage of
Mn is 0.5-0.8%; and in RE, the weight percentage of Y is 0.8-1.6%, and the
mass
percentage of Ce is 0-0.8%. In this case, the obtained magnesium alloy has an
elongation of 17.4-21.6%, a welding loss rate of less than 5%, a yield
strength of 220-
235 MPa, and a tensile strength of 320-342 MPa.
Even more preferably, in the Mg-Al based magnesium alloy, the weight
percentage
of Al is 7.8-8.2%, the weight percentage of RE is 1.5-1.9%, and the weight
percentage
of Mn is 0.5-0.8%; and in RE, the weight percentage of Y is 0.8%, and the mass
percentage of Ce is 0.5-0.8%. In this case, the obtained magnesium alloy has a
welding loss rate of less than or equal to 4.3%.
Optionally, in the magnesium alloy above, RE includes at least one of La, Ce,
Nd,
Y, Gd, Ho, Dy, and Er. RE includes mainly Y and Ce, and other rare earth
elements
are in trace amounts.
The preparation method of a tube of the Mg-Al based magnesium alloy according
to the present disclosure comprises steps of:
mixing An Al source, a RE source, a Mn source, and a Mg source in element
weight
percentage contents of 7.0-8.6% Al, 0.8-2.0% RE, 0.2-0.8% Mn, and a balance of
Mg,
and smelting the mixture to give a liquid mixed metal;
casting the liquid mixed metal into a bar through semi-continuous casting;
performing homogenization heat treatment on the bar at 360-400 C for 6-10h;
and
performing extrusion-forming on the heat-treated bar to obtain a magnesium
alloy
tube.
The application of the Mg-Al based magnesium alloy of the present disclosure
is
use of the Mg-Al based magnesium alloy in the fields of vehicle equipment and
medical
equipment.
Beneficial effects: compared with the prior art, the advantages of the present
disclosure includes: the Mg-Al based magnesium alloy of the present disclosure
has
high elongation, and the elongation of the tube formed using the same can
reach 15-
CA 03205147 2023- 7- 13
2
22%, so that the magnesium alloy can withstand large plastic deformation.
Meanwhile,
this Mg-Al based magnesium alloy has a very low welding loss rate of less than
6%,
which greatly reduces the strength loss of magnesium alloy profiles after
welding, and
ensures the strength of magnesium alloy profiles after welding. In addition,
the Mg-Al
based magnesium alloy of the present disclosure also has high strength, its
yield
strength reaches 182-232 MPa, and its tensile strength reaches 306-340 MPa.
Brief Description of Drawings
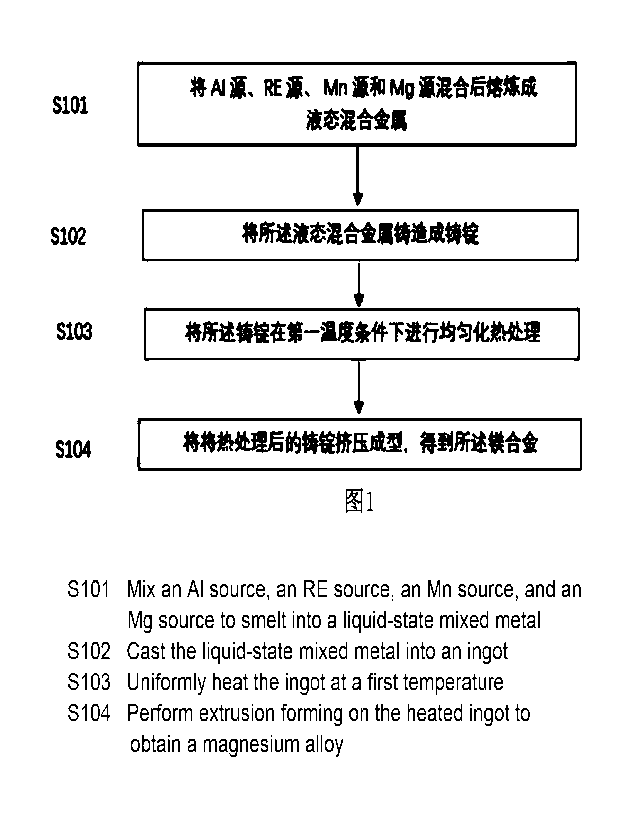
FIG. 1 is a flow chart of a preparation process of the Mg-Al based magnesium
alloy
of the present disclosure.
Detailed Description of the Embodiments
The technical solutions of the present disclosure will be further described
below with
reference to the accompanying drawings and examples.
A Mg-Al based magnesium alloy of the present disclosure includes, by weight
percentage, 7.0-8.6% Al, 0.8-2.0% RE, 0.2-0.8% Mn, and a balance of Mg.
Specifically, in the magnesium alloy of the present disclosure, RE (rare earth
element) and Mn are added to a Mg-Al based alloy with components in a certain
proportion, thereby improving the plasticity and strength of the magnesium
alloy and
reducing the welding loss rate of the alloy.
Addition of Mn allows removing the impurity element Fe introduced during semi-
continuous casting, which is advantageous to welding performance and
mechanical
properties, thereby reducing the welding loss rate. Meanwhile, Mn does not
form a
compound in magnesium, and can be used as heterogeneous nucleation particles
to
refine grains. When the alloy is extruded into a tube, Mn promotes dynamic
recrystallization, refines grains, and weakens texture, thereby improving
strength and
plasticity.
The addition of RE can refine the grain size of the magnesium alloy, improve
the
morphology of the 13 strengthening phase of the magnesium alloy, and enhance
the
strength and plasticity of the magnesium alloy. The strength of the magnesium
alloy
can be reflected by the yield strength and tensile strength. After the Mg-Al
based
magnesium alloy provided by the present disclosure is formed into a tube, the
range
of the yield strength of the tube is 182-235 MPa, and preferably the range of
the yield
strength of the tube is 220-235 MPa. Meanwhile, the tensile strength of the Mg-
Al
based magnesium alloy tube ranges from 306 to 342 MPa, preferably 320 to 340
MPa.
The elongation has a direct correlation to the plasticity of the magnesium
alloy. After
the Mg-Al based magnesium alloy provided by the present disclosure is formed
into a
tube, the elongation of the tube can reach 15-22%, and preferably the
elongation of
the Mg-Al based magnesium alloy tube is 17-21.6%. A high elongation allows the
CA 03205147 2023- 7- 13
3
magnesium alloy to withstand large plastic deformation and improves the
application
range of the magnesium alloy.
The welding strength loss rate is the strength loss rate of the welded sample
compared to the original profile sample after the magnesium alloy profile is
welded.
The welding strength loss rate of the Mg-Al based magnesium alloy provided by
the
present disclosure is less than 6%, preferably, the welding strength loss rate
is less
than 5%, and more preferably, the welding strength loss rate is less than
4.3%. In the
magnesium alloy provided by the examples of the present disclosure, due to the
addition of RE element, Al-RE high-temperature stable phase is formed during
high
temperature welding, and the high-temperature stable phase is pinned at the
grain
boundary, which hinders the growth of magnesium alloy grains during the
welding
process. Furthermore, the RE element can greatly reduce/refine the size of the
13
strengthening phase in the magnesium alloy, and avoid the growth of the 13
strengthening phase in the high temperature welding process, thereby reducing
the
strength loss of the magnesium alloy profile after welding, and ensuring the
strength
of the magnesium alloy profile after welding.
Optionally, the range of the weight percentage of Al in the Mg-Al based
magnesium
alloy of the present disclosure is 7.0-8.6%, preferably the range of the
weight
percentage of Al in the Mg-Al based magnesium alloy is 7.0-8.2%, and more
preferably,
the range of the weight percentage of Al is 7.8-8.2%.
Specifically, when the weight percentage of Al in the Mg-Al based magnesium
alloy
is controlled within a certain range, the combination of Al and Mg elements
has a
second-phase strengthening effect, and during the formation process of the
magnesium alloy, the 13 strengthening phase can achieve the optimum state
(moderate
volume fraction, morphology, and size), thereby improving the strength of
magnesium
alloys. Meanwhile, the Al element as a solid solution part in the magnesium
matrix can
play a role in solid solution strengthening and improving plasticity. When the
weight
percentage of Al in the Mg-Al based magnesium alloy is extremely high, for
example,
the weight percentage of Al in the Mg-Al based magnesium alloy is greater than
8.6%,
due to the precipitation of the coarse eutectic 13 phase, on the one hand,
after welding,
the interface bonding ability between the precipitated phase and the matrix is
weakened, and microscopic pores are easily formed at the interface between the
matrix and the 13 phase, which increase the welding loss rate; and on the
other hand,
the coarse 13 phase may cause, in the course of service, stress concentration,
advance
occurrence of plastic instability and reduced elongation. When the weight
percentage
of Al in the magnesium alloy is extremely low, for example, less than 7%, the
reduction
of the Al element in the crystal is not conducive to improving the plasticity,
and
meanwhile, the amount of precipitated phase is less, and the degree of
refinement of
grains is reduced, causing the second phase strengthening effect not to be
exhibited,
which is not conducive to the improvement of the strength of the magnesium
alloy. In
addition, for the alloy containing less precipitated phase after welding, the
grain growth
is more obvious, thus causing the welding loss rate to increase.
CA 03205147 2023- 7- 13
4
Optionally, the range of the weight percentage of RE in the Mg-Al based
magnesium
alloy of the present disclosure is 0.8-2.0%, preferably, the range of the
weight
percentage of RE in the Mg-Al based magnesium alloy is 1.1-2.0%, and more
preferably, the range of the weight percentage of RE is 1.3-1.9%.
Specifically, after
RE is added to the Mg-Al based magnesium alloy, since the RE element has a
unique
electronic arrangement structure and chemical characteristics, addition of an
appropriate amount of rare earth elements to the magnesium alloy can enhance
the
interatomic bonding force, reduce the diffusion rate of magnesium atoms,
increase the
recrystallization temperature of the magnesium alloy, slow down the
recrystallization
growth rate, and significantly improve the formability and corrosion
resistance of the
magnesium alloy. Further, RE is generally distributed in the grain boundaries
and can
reduce the grain size of the magnesium alloy and improve coordination ability
between
the grains of the magnesium alloy. RE can also form a thermally stable 13
strengthening
phase during the formation process of the magnesium alloy, which improves the
strength and plasticity of the magnesium alloy.
RE may include at least one of La, Ce, Nd, Y, Gd, Ho, Dy, and Er.
Specifically, the
RE elements in the Mg-Al based magnesium alloy of the present disclosure are
mainly
Y and Ce. The weight percentage of Y ranges from 0.8% to 1.6%, and the weight
percentage of Ce ranges from 0 to 0.8%.
In FIG. 1, the present disclosure provides a preparation method of the Mg-Al
based
magnesium alloy, comprising steps of:
S101, mixing an Al source, a RE source, a Mn source, and a Mg source in
element
weight percentage contents of 7.0-8.6% Al, 0.8-2.0% RE, 0.2-0.8% Mn and a
balance
of Mg, and smelting the mixture to give a liquid mixed metal;
S102, casting the liquid mixed metal into an ingot;
S103, performing homogenization heat treatment on the ingot at a first
temperature;
and
S104, performing extrusion-forming on the heat-treated ingot to obtain the Mg-
Al
based magnesium alloy of the present disclosure.
Specifically, the casting process in S102 can be implemented by a semi-
continuous
casting process. With the semi-continuous process, due to rapid water cooling,
the
size of obtained grains is small, and the fine grains can improve both the
strength and
the elongation of the alloy. In S103, the first temperature ranges from 360 C
to 400 C,
and the heat treatment time is 6-10h. The heat treatment process before
extrusion can
increase the content of Al element in the matrix, increase the slip system,
and improve
the elongation of the alloy.
When preparing the Mg-Al based magnesium alloy tube, in step S102, the ingot
is
cast into a bar, that is, the liquid mixed metal is cast into a bar; and in
step S104, the
heat-treated bar is subjected to back extrusion forming to obtain a Mg-Al
based
magnesium alloy tube. The process parameters of back extrusion forming include
CA 03205147 2023- 7- 13
extrusion temperature, extrusion ratio, and extrusion speed, among which the
extrusion temperature ranges from 280 C to 330 C, the extrusion ratio is 49:1,
and
the extrusion speed ranges 8mm/s to 15mm/s.
Taking the preparation of Mg-Al based magnesium alloy tubes as an example, the
magnesium alloy provided by the present disclosure will be described in detail
through
the following specific examples and comparative examples. The magnesium alloy
tubes obtained by the preparation method provided in the examples of the
present
disclosure have a large elongation and can withstand large plastic
deformation, and
the magnesium alloy tubes have a low welding loss rate, and these properties
improve
the application range of the magnesium alloy. Also, the magnesium alloy has
higher
yield strength and tensile strength.
Example 1
A Mg-Al based magnesium alloy included: 7g Al, 0.8g Y, 0.5g Mn, and 91.7g Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Example 2
A Mg-Al based magnesium alloy included: 7.4g Al, 0.8g Y, 0.5g Mn, and 91.3g
Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 360 C for a treatment duration of 10h; and
S104, the heat-treated bar was back-extruded at a speed of 8mm/s and an
extrusion
temperature of 280 C in an extrusion ratio of 49:1 to obtain the magnesium
alloy tube.
Example 3
A Mg-Al based magnesium alloy included: 7.8g Al, 0.8g Y, 0.5g Mn, and 91.9g
Mg.
CA 03205147 2023- 7- 13
6
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Example 4
A Mg-Al based magnesium alloy included: 8.2g Al, 0.8g Y, 0.5g Mn, and 90.5g
Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 380 C for a treatment duration of 6h; and
S104, the heat-treated bar was back-extruded at a speed of lOmm/s and an
extrusion temperature of 330 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Example 5
A Mg-Al based magnesium alloy included: 8.6g Al, 0.8g Y, 0.5g Mn, and 90.1g
Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Example 6
CA 03205147 2023- 7- 13
7
A Mg-Al based magnesium alloy included: 7.8g Al, 1.2g Y, 0.5g Mn, and 90.5g
Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Example 7
A Mg-Al based magnesium alloy included: 7.8g Al, 1.6g Y, 0.5g Mn, and 90.1g
Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Example 8
A Mg-Al based magnesium alloy included: 7.8g Al, 0.8g Y, 0.3g Ce (RE 1.1%),
0.5g
Mn, and 90.6g Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
CA 03205147 2023- 7- 13
8
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Example 9
A Mg-Al based magnesium alloy included: 7.8g Al, 1.2g Y, 0.3g Ce (RE 1.5%),
0.5g
Mn, and 90.2g Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Example 10
A Mg-Al based magnesium alloy included: 7.8g Al, 0.8g Y, 0.5g Ce (RE 1.3%),
0.5g
Mn, and 90.4g Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Example 11
A Mg-Al based magnesium alloy included: 7.8g Al, 0.8g Y, 0.8g Ce (RE 1.6%),
0.5g
Mn, and 90.1g Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
CA 03205147 2023- 7- 13
9
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Example 12
A Mg-Al based magnesium alloy included: 7.8g Al, 0.8g Y, 0.5g Ce, 0.1g La (RE
1.4%), 0.5g Mn, and 90.3g Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Example 13
A Mg-Al based magnesium alloy included: 7.8g Al, 0.8g Y, 0.5g Ce, 0.1g La,
0.1g
Nd (RE 1.5%), 0.5g Mn, and 90.2g Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Example 14
CA 03205147 2023- 7- 13
A Mg-Al based magnesium alloy included: 7.8g Al, 0.8g Y, 0.5g Ce, 0.1g La,
0.1g
Nd, 0.1g Gd (RE 1.6%), 0.5g Mn, and 90.1g Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Example 15
A Mg-Al based magnesium alloy included: 7.8g Al, 0.8g Y, 0.5g Ce, 0.1g La,
0.1g
Nd, 0.1g Gd, 0.1 Ho (RE 1.7%), 0.5g Mn, and 90.1g Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Example 16
A Mg-Al based magnesium alloy included: 7.8g Al, 0.8g Y, 0.5g Ce, 0.1g La,
0.1g
Nd, 0.1g Gd, 0.1 Ho, 0.1 Dy (RE 1.8%), 0.5g Mn, and 90.0g Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
CA 03205147 2023- 7- 13
11
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Example 17
A Mg-Al based magnesium alloy included: 7.8g Al, 0.8g Y, 0.5g Ce, 0.1g La,
0.1g
Nd, 0.1g Gd, 0.1 Ho, 0.1 Dy, 0.1 Er (RE 1.9%), 0.5g Mn, and 89.9g Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Example 18
A Mg-Al based magnesium alloy included: 8.0g Al, 0.8g Y, 0.5g Ce (RE 1.3%),
0.5g
Mn, and 90.4g Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Example 19
A Mg-Al based magnesium alloy included: 8.0g Al, 0.8g Y, 0.5g Ce, 0.1g La,
0.1g
Nd, 0.1g Gd, 0.1 Ho, 0.1 Dy, 0.1 Er (RE 1.9%), 0.5g Mn, and 89.6g Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
CA 03205147 2023- 7- 13
12
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Example 20
A Mg-Al based magnesium alloy included: 8.2g Al, 0.8g Y, 0.5g Ce, 0.1g La,
0.1g
Nd, 0.1g Gd (RE 1.6%), 0.5g Mn, and 89.7g Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Example 21
A Mg-Al based magnesium alloy included: 7.8g Al, 0.8g Y, 0.5g Ce (RE 1.3%),
0.2g
Mn, and 90.7g Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Example 22
CA 03205147 2023- 7- 13
13
A Mg-Al based magnesium alloy included: 7.8g Al, 0.8g Y, 0.5g Ce (RE 1.3%),
0.4g
Mn, and 90.5g Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Example 23
A Mg-Al based magnesium alloy included: 7.8g Al, 0.8g Y, 0.5g Ce (RE 1.3%),
0.8g
Mn, and 90.1g Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Comparative Example 1
A Mg-Al based magnesium alloy included: 6.5g Al, 0.8g Y, 0.5g Mn, and 92.2g
Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
CA 03205147 2023- 7- 13
14
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Comparative Example 2
A Mg-Al based magnesium alloy included: 9.6g Al, 0.8g Y, 0.5g Mn, and 89.1g
Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Comparative Example 3
A Mg-Al based magnesium alloy included: 7g Al, 0.5g Y, 0.5g Mn, and 92.0g Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Comparative Example 4
A Mg-Al based magnesium alloy included: 7g Al, 2.3g Y, 0.5g Mn, and 90.2g Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
CA 03205147 2023- 7- 13
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Comparative Example 5
A Mg-Al based magnesium alloy included: 7g Al, 0.8g Y, and 92.2g Mg.
The Mg-Al based magnesium alloy was obtained by the following preparation
method specifically including:
S101, an Al source, a Y source, a Mn source, and a Mg source were mixed
thoroughly and smelted into a liquid mixed metal;
S102, the liquid mixed metal was cast into a bar through a semi-continuous
casting
process;
S103, the bar was heat-treated at 400 C for a treatment duration of 8h; and
S104, the heat-treated bar was back-extruded at a speed of 12mm/s and an
extrusion temperature of 300 C in an extrusion ratio of 49:1 to obtain the
magnesium
alloy tube.
Table 1 Performance parameters of Mg-Al based magnesium alloys of Examples 1-
20 and Comparative Examples 1-5
Example Yield strength Tensile Elongation Welding
(Mpa) strength (%) strength
loss
(Mpa) rate (%)
Example 1 182 306 18.2 4.7
Example 2 191 319 16.9 5
Example 3 197 322 15.5 5.3
Example 4 202 320 15.2 5.4
Example 5 220 325 15 5.8
Example 6 219 325 16.3 4.8
Example 7 221 329 17.6 4.6
Example 8 224 326 19.6 5.3
Example 9 225 328 18.8 4.9
Example 10 226 325 19.2 4.5
Example 11 227 329 17.4 4.3
Example 12 223 324 19.5 4.6
CA 03205147 2023- 7- 13
16
Example 13 227 328 19.7 4.2
Example 14 230 330 20.1 4.1
Example 15 228 333 20.3 3.8
Example 16 232 338 20.8 3.6
Example 17 230 340 21.6 3.5
Example 18 230 334 19.1 4.6
Example 19 235 342 20 3.7
Example 20 231 339 19.8 4.2
Example 21 218 326 18.2 5.6
Example 22 220 316 16.9 5
Example 23 230 327 19 3.5
Comparative
165 287 15.2 4.9
Example 1
Comparative
226 340 12.7 7.3
Example 2
Comparative 173
294 13.9 5.8
Example 3
Comparative 185
312 12.8 5.3
Example 4
Comparative
179 289 14.3 6.2
Example 5
It can be seen from Table 1 that the yield strengths of the magnesium alloy
tubes of
Examples 1-23 can all reach 182MPa or greater, and the yield strength of the
magnesium alloy tube of Example 19 reached 235MPa; the tensile strengths of
them
can all reach 306 MPa or greater, and the tensile strength of the magnesium
alloy tube
of Example 19 reached 342Mpa; the elongations of them were all greater than
15%,
and the elongation of the magnesium alloy tube of Example 17 reached 21.6%;
and
the welding loss rates of the magnesium alloy tubes of Examples 1-23 were all
less
than 6%, and the welding loss rates of the magnesium alloy tubes of Examples
15-17,
Examples 19-20, and Example 23 were less than or equal to 4%, and can be as
low
as 3.5%.
Comparing Example 1 with Comparative Examples 1 and 2, the magnesium alloy in
Comparative Example 1, because of the low content of Al added, has low yield
CA 03205147 2023- 7- 13
17
strength and tensile strength, which are as low as 165Mpa and 287Mpa
respectively,
and an increased welding loss rate; and the magnesium alloy in Comparative
Example
2, because of the excessively high content of Al added, has deteriorated
plasticity, and
an elongation decreased to 12.7%, and meanwhile, the welding loss rate
increases
significantly to 7.3%.
Comparing Example 1 with Comparative Examples 3 and 4, the magnesium alloy in
Comparative Example 3, because of the low content of RE added, has low yield
strength and tensile strength, poor plasticity, and an elongation of only
13.9%, and
meanwhile, the welding loss rate increases; and for the magnesium alloy in
Comparative Example 4, in which the content of RE added is too high, although
the
yield strength and tensile strength of the magnesium alloy are improved, the
plasticity
is significantly deteriorated, the elongation is only 12.8%, and the welding
loss rate
also increases.
Comparing Example 1 with Comparative Example 5, in Comparative Example 5,
since Mn was not added, the overall performance of the magnesium alloy
decreases,
where the elongation is significantly reduced, and the welding loss rate is
significantly
increased to over 6%.
The Mg-Al based magnesium alloy of the present disclosure can be applied to
the
fields of vehicle equipment and medical equipment. For example, the Mg-Al
based
magnesium alloy is formed into a bar, and a plurality of magnesium alloy bars
can be
used, after welded, as a load-bearing member or support member for equipment
such
as a wheelchair, a stretcher, a bicycle, a mountain bike. The Mg-Al based
magnesium
alloy can reduce the weight of the equipment above while ensuring the strength
and
stability of the equipment above.
CA 03205147 2023- 7- 13
18