Note: Descriptions are shown in the official language in which they were submitted.
CA 03207232 2023-07-04
WO 2022/119470 PCT/RU2020/000661
(2S)-2-AMINOPENTANETHIOIC S-ACID FOR USE AS MEDICAMENT AND IN THERAPY OF
AMYOTROPHIC LATERAL SCLEROSIS
Field of the Invention
[0001] The present invention relates to (25)-2-aminopentanethioic S-acid or a
pharmaceutically acceptable salt thereof for use as a medicament and in the
therapy of
amyotrophic lateral sclerosis.
Background of the invention
[0002] 2-Aminopentanethioic S-acid, having a chemical formula
CH3CH2CH2CH(NH2)C(0)SH, is known from the art as a chemical reagent that has
been
used in the synthesis of organic antibiotics. Cricchio R et al., Eur. J. Med.
Chem. ¨ Chimica
Therapeutica, 1981, 16(4):301-306. Due to the presence of chiral carbon, 2-
aminopentanethioic S-acid may exist as individual enantiomers (25)-2-
aminopentanethioic S-acid and (2R)-2-aminopentanethioic S-acid, or a racemic
mixture
thereof. It should be noted that (S)- or (R)-configuration of the substance
has not been
specified by Cricchio et al. in the above publication. The database SciFinder
contains
mentioning about (S)-configuration of 2-aminopentanethioic S-acid based on
citing the
original publication of Cricchio et al. (1981). But such the mention is an
incorrect citation
and an error in the database, since the original publication of Cricchio et
al. (1981) does
not indicate any configuration of 2-aminopentanethioic S-acid or a
configuration raw
materials used for preparing 2-aminopentanethioic S-acid. Therefore,
individual (5)- and
(R)- enantiomers of 2-aminopentanethioic S-acid have never been disclosed in
the art. In
addition, nothing is known from the art about biological properties of 2-
aminopentanethioic S-acid per se, or its individual enantiomers or racemic
mixture
thereof. Nothing is known from the art about the use of (2S)-2-
aminopentanethioic 5-
acid taken either as the individual substance or as component of the racemic
mixture in
medicine.
[0003] Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease
characterized
by progressive degeneration of lower motor neurons, as well as neurons in the
cortex
and brainstem, which leads to paralysis and premature death. Al-Chalabi A et
al.
Amyotrophic lateral sclerosis: moving towards a new classification system.
Lancet
CA 03207232 2023-07-04
WO 2022/119470 PCT/RU2020/000661
2
Neurol. 2016; 15:1182-1194. The etiology of ALS remains unclear both in the
familial
forms and sporadic cases of ALS. Among the known genetic causes that give rise
to ALS,
the mutation of the fused in sarcoma protein (FUS) is the second most frequent
among
the familial forms of ALS. Blair IP et al. FUS mutations in amyotrophic
lateral sclerosis:
clinical, pathological, neurophysiological and genetic analysis. 1 Neural
Neurosurg
Psychiatry. 2010; 81:639-645. Current ALS treatments can slow the progression
of
symptoms and prevent complications but cannot reverse the neuronal cells
damage. The
Food and Drug Administration has approved two drugs for treating ALS. The
first one,
the oral drug Riluzole (Rilutek) has been shown to increase life expectancy by
three to six
months. The second one, the intravenous drug Edaravone (Radicava) has been
shown to
reduce the decline in daily functioning, while its effect on life span is not
yet known.
Thus, there is the critical unmet medical need for new, effective treatments
for ALS.
[0004] Nothing is known from the art about (2S)-2-Aminopentanethioic S-acid
for use in
the treatment of ALS.
Summary of the invention
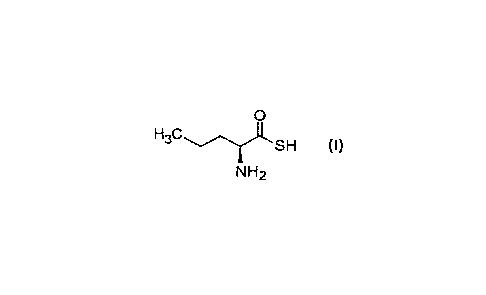
[0005] A first aspect of the invention relates to a substance of formula (I):
0
SH (I)
NH2
or a pharmaceutically acceptable salt thereof for use as a medicament.
[0006] According to another aspect, the invention relates to a substance of
formula (I):
0
H3CASH (I)
NH2
or a pharmaceutically acceptable salt thereof for use in the therapy of
amyotrophic
lateral sclerosis.
[0007] According to another aspect, the invention relates to a method of
treating
amyotrophic lateral sclerosis comprising administering to a subject in need
thereof an
effective amount of a substance of formula (I):
CA 03207232 2023-07-04
WO 2022/119470
PCT/RU2020/000661
3
0
SH (I)
NH2
or a pharmaceutically acceptable salt thereof.
Brief Description of the Drawings
[0008] Figure 1 shows the effects of chronic administration of the substance
of formula
(I) as well as Vehicle (Veh), Riluzole (Ril), and the substance of formula
(IX) on the
occurrence of ALS motor symptoms in FUS-tg mice (FUS), specifically on
percentage of
mice having signs of paralysis on the 95th day (*p<0.05 vs. Veh, Fisher test).
[0009] Figure 2 shows the effects of chronic administration of the substance
of formula
(I) as well as Vehicle (Veh), Riluzole (Ril), and the substance of formula
(IX) on the latency
to fall in the rotarod test in FUS-tg mice (FUS) compared to non-treated wild-
type
littermates (WT). Data are presented as mean SEMs (*p<0.05, vs. WT, #p<0.05
vs. Veh,
one-way ANOVA and Tukey's test).
[0010] Figure 3 shows the effects of chronic administration of the substance
of formula
(I) on the concentrations of malondialdehyde (MDA), a marker of oxidative
stress, in the
spinal cord of FUS-tg mice (FUS) compared to non-treated wild-type littermates
(WT)
and vehicle-treated FUS-tg mice (Veh). Data are presented as mean SEMs
(*p<0.05 vs.
WT group, #p<0.05 vs. Veh group; one-way ANOVA and Tukey's test).
Detailed Description of the Invention
[0011] All terms and definitions explained throughout the text of
specification of the
invention relate to all aspects and embodiments of the invention, unless
otherwise
specified.
[0012] A first aspect of the invention relates to a substance of formula (I):
0
SH (I)
NH2
or a pharmaceutically acceptable salt thereof for use as a medicament.
CA 03207232 2023-07-04
WO 2022/119470 PCT/RU2020/000661
4
[0013] The substance of formula (I) has a chemical name (25)-2-
aminopentanethioic S-
acid and a chemical formula C5HIINOS.
[0014] The present invention relates to all tautomeric forms of the substance
of formula
(I), including S-acid form (I), 0-acid form (II), inner salt form (III), and
pharmaceutically
acceptable salts thereof prepared by reaction with an appropriate acid, where
X is an
anion (IV), or by reaction with an appropriate base, where Y is a cation (V):
0 0
OH
NH2 NH2 (i)NH3
(I) ((I1) (III)
0 0
H3C H3C vo
¨NH3 X NH2
(IV) (V)
wherein non-exclusive examples of X include chloride, bromide, sulfate,
phosphate,
mesylate, acetate, pyruvate, lactate, citrate, succinate, fumarate, malate,
and
ketoglutarate; and wherein non-exclusive examples of Y include lithium,
sodium,
potassium, magnesium, calcium, ammonium, and an alkylammonium.
[0015] The present invention relates to all hydrates, solvates, and
isotopologues of the
substance of formula (I).
[0016] The term "isotopologue", as used herein, refers to molecules that
differ only in
their isotopic composition. Non-exclusive examples of isotopes are 1H and 2H
isotopes of
hydrogen, uct 12C, 13",
and "C isotopes of carbon, 150, 160, 17^,
and 180 isotopes of
oxygen, 13N, "N, and 15N isotopes of nitrogen, and 325, 335, "S, and 36S
isotopes of sulfur.
[0017] The present invention relates to all prodrugs of the substance of
formula (I). The
term "prodrug", as used herein, refers to a substance that being administered
to a
subject in need thereof, will result in a therapeutic effect through the
intermediate
formation of the substance of formula (I).
CA 03207232 2023-07-04
WO 2022/119470
PCT/RU2020/000661
[0018] The term "medicament", as used herein, refers to both, human medicines
and
veterinary medicines.
5 [0019] According to another aspect, the invention relates to a substance
of formula (I):
0
SH (I)
NH2
or a pharmaceutically acceptable salt thereof for use in the therapy of
amyotrophic
lateral sclerosis.
[0020] The term "amyotrophic lateral sclerosis", as used herein, refers to the
neurodegenerative neuromuscular disease classified in the International
Classification of
Diseases, Tenth Revision (ICD-10-CM), under the code G12.21.
[0021] The term "therapy", as used herein, refers to any treatment which is
designed to
cure, alleviate, remove or lessen the symptoms of, or prevent or reduce the
possibility of
malfunction of the subject body.
[0022] According to another aspect, the invention relates to a method of
treating
amyotrophic lateral sclerosis comprising administering to a subject in need
thereof an
effective amount of a substance of formula (I):
0
SH (I)
NH2
or a pharmaceutically acceptable salt thereof.
[0023] The term "treating" or "treatment" refers to the administration of a
medicament
to a patient to cure, alleviate, remove or lessen the symptoms of, or prevent
or reduce
the possibility of malfunction of the subject body.
[0024] In practicing the invention, the substance of formula (I) can be
administered by
different routes, e.g. orally, parenterally, by inhalation spray, buccally,
sublingually,
CA 03207232 2023-07-04
WO 2022/119470 PCT/RU2020/000661
6
nasally, rectally, or parenterally, with oral route being preferred. The term
"parenteral",
as used herein, includes subcutaneous, intracutaneous, intravenous,
intramuscular,
intrathecal, intralesional and intracranial injection or infusion techniques.
[0025] The term "an effective amount", as used herein, refers to the amount of
the
active agent that is required to confer the intended therapeutic effect in the
subject.
Effective amounts may vary, as recognized by those skilled in the art,
depending on
route of administration, excipient usage, medical conditions, and the
possibility of co-
usage with other agents.
[0026] In practicing the invention, the effective amounts of the substance of
formula (I)
or a pharmaceutically acceptable salt thereof may vary from 0.1 to 100 mg per
kg body
weight of the subject.
[0027] The term "subject", as used herein, refers to a mammal, preferably
human.
[0028] In practicing the method of the invention, the effective amounts of the
substance
of formula (I) or a pharmaceutically acceptable salt thereof can be
administered to the
same subject one time or multiple times.
[0029] In practicing the method of the invention, the effective amounts of the
substance
of formula (I) or a pharmaceutically acceptable salt thereof can be
administered to the
same subject for one day or longer.
[0030] In practicing the invention, the substance of formula (I) or a
pharmaceutically
acceptable salt thereof can be administered as an active pharmaceutical
ingredient of a
pharmaceutical composition. In some embodiments, the composition can contain
from
0.01 to 99.99 per cent of the substance of formula (I). These compositions can
contain
some optional ingredients. Such optional ingredients generally are used
individually at
levels from about 0.01 to about 99.99 per cent by weight of the composition.
Examples
of suitable optional ingredients include, but are not limited to, excipients,
carriers,
minerals, carbohydrates, lipids, vitamins, co-factors, buffers, flavors and
sweeteners,
CA 03207232 2023-07-04
WO 2022/119470
PCT/RU2020/000661
7
inorganic salts, cations and anions, taste modifying and/or masking agents,
amino acids,
organic acids, antioxidants, preservatives, colorants, and other suitable
ingredients.
[0031] In some embodiments, the compositions can be formulated in different
dosage
forms. Nonexclusive examples of such forms include an orally acceptable dosage
form,
an injectable dosage form, an inhalation and an intranasal dosage forms, and a
rectal
dosage form.
[0032] The oral composition can be any orally acceptable dosage form
including, but not
limited to, capsules, tablets, powders, aqueous solutions, suspensions,
emulsions, and
dispersions. Non-exclusive examples of carriers for tablets include lactose
and corn
starch. Non-exclusive examples of lubricating agents include magnesium
stearate. Non-
exclusive examples of diluents for oral administration in a capsule form
include lactose
and dried corn starch. When aqueous suspensions or emulsions are administered
orally,
the active ingredient can be suspended or dissolved in an oily phase combined
with
suspending or emulsifying agents.
[0033] The sterile injectable composition can be any acceptable dosage form
including,
but not limited to, a sterile injectable solution or suspension in a
parenterally acceptable
diluent or solvent, for example, as a solution in pharmaceutical water,
propylene glycol,
nnannitol, Ringer's solution, isotonic sodium chloride solution. In addition,
sterile oils
such as synthetic mono- or diglycerides, fatty acids, such as oleic acid and
its glyceride
derivatives, can be conventionally used as a solvent or suspending medium.
Such oil
solutions or suspensions can also contain a long-chain alcohol or
carboxymethyl cellulose
as a diluent or dispersant.
[0034] The inhalation and intranasal composition can be any acceptable dosage
form
including, but not limited to, solutions and dispersions in water or saline
that may
contain suitable preservatives, absorption promoters to enhance
bioavailability, and/or
other solubilizing agents known in the art.
[0035] The rectal composition can be prepared according to procedures well
known in
the art in form of suppositories comprising the active ingredient and base
capable to be
CA 03207232 2023-07-04
WO 2022/119470
PCT/RU2020/000661
8
melted or dissolved at a human body temperature to release the active
ingredient. Non-
exclusive examples of such bases include cacao butter, polyethylene glycol,
and
synthetic esters of glycerol like Witepsol bases.
[0036] In practicing the present invention, the composition of the invention
can be
prepared by procedures well-known from the art. Such procedures include, but
are not
limited to, mixing the substance of formula (I) or a pharmaceutical salt
thereof with
other ingredients of the composition in conventional manner. Guidance for the
preparation of compositions of the invention can be found in "Remington: The
science
and practice of pharmacy" 20th ed. Mack Publishing, Easton PA, 2000 ISBN 0-
912734-04-
3 and "Encyclopaedia of Pharmaceutical Technology", edited by Swarbrick, J. &
J. C.
Boylan, Marcel Dekker, Inc., New York, 1988 ISBN 0-8247-2800-9 or a newer
edition.
[0037] In practicing the invention, the substance of formula (I) can be
prepared as
described in Cricchio R et al., Eur. J. Med. Chem. ¨ Chimica Therapeutica,
1981,
16(4):301-306, starting from (2S)-enantiomer of 2-aminopentanoic acid.
[0038] Also, the substance of formula (I) can be prepared according to the
process
shown in the scheme below, starting from the boc-derivative of (25)-2-
aminopentanoic
acid (VI):
0 0 0
I-13c õ,,,,y11,0H H3C
0
1. CDI 1.1 2 I-1,S CF3COOH
>r
SH ______________________________________________________ 3. SH
I 8 NH
0 I 0
(VII) (I)
[0039] In the first step, N-(tert-butoxycarbony1)-(25)-2-aminopentanoic acid
(VI) is
reacted with carbonyl diimidazole (CDI) in a suitable solvent, for example
methylene
chloride, at a temperature of 0-4 C. In the second step, substance (VII) is
treated with
hydrogen sulfide or a salt thereof, such as sodium hydrogen sulfide, at a
temperature of
0-20 C to obtain N-(tert-butoxycarbony1)-(25)-2-aminopentanethioic S-acid
(VIII), which
deprotection with trifluoroacetic acid results in the substance of formula
(I).
CA 03207232 2023-07-04
WO 2022/119470 PCT/RU2020/000661
9
[0040] In one embodiment of the invention, the substance of formula (VIII) can
be used
as a prod rug of the substance of formula (I).
[0041] The following examples are presented to demonstrate the invention. The
examples are illustrative only and are not intended to limit the scope of the
invention in
any way.
Example 1
[0042] This example illustrates a process for preparing (2S)-2-
aminopentanethioic S-acid,
the substance of formula (I).
[0043] Carbonyl diimidazole (7.96 g; 49.12 mmol; CDI) was added to a solution
of N-(tert-
butoxycarbony1)-(25)-2-aminopentanoic acid (9.69 g; 44.65 mmol; Sigma-Aldrich
15556)
(VI) in methylene chloride (60 ml) with stirring and cooling in an ice bath to
produce the
substance (VII). Then, the mixture was stirred for 1 hour at room temperature,
and an
excessive amount of H2S was passed into the resulting solution for 3 hours
while stirring
and cooling in an ice bath. At the end, 15 ml of 1M aqueous HCI was added, the
mixture
was concentrated under reduced pressure, diluted with ethyl acetate and cooled
to 0 C,
and adjusted to pH 3. The organic layer was separated, washed with aqueous
NaCI, dried
with MgSO4, filtered and concentrated under reduced pressure. Then,
trifluoroacetic
acid (20 ml, 262 mmol) was added to the resulting solution of N-(tert-
butoxycarbonyI)-
(25)-2-aminopentanethioic S-acid (VIII), stirred at room temperature, and
concentrated
under reduced pressure. The white solid was filtered and dried to provide (2S)-
2-
aminopentanethioic acid (I), the substance of formula (I):
H3C0
SH (I),
NH2
yield 47% based on the starting N-(tert-butoxycarbonyI)-(25)-2-aminopentanoic
acid (VI).
(25)-2-Aminopentanethioic S-acid NMR (DMSO-
d6), 6-scale: 0.82 (3H, t); 1.30 (2H,
m); 1.61 (1H, m); 1.81 (1H, m); 3.42 (1H, m); 7.74 (3H, br s). Elemental
analysis for
C5H11NOS (molecular weight 133.21): calculated (%) C 45.08; H 8.32; N 10.51; S
24.07;
found (%) C 44.86; H 8.19; N 10.46; S 23.90.
Example 2
CA 03207232 2023-07-04
WO 2022/119470 PCT/RU2020/000661
[0044] This example illustrates a process for preparing (2R)-2-
aminopentanethioic S-acid,
the substance of formula (IX).
[0045] Carbonyl diimidazole (4.00 g; 24.60 mmol; CDI) was added to a solution
of N-(tert-
5 butoxycarbonyI)-(2R)-2-aminopentanoic acid (4.85 g; 22.33 mmol; Sigma-
Aldrich 12688)
in methylene chloride (30 ml) with stirring and cooling in an ice bath. The
mixture was
stirred for 1 hour at room temperature and an excessive amount of H2S was
passed into
the resulting solution for 3 hours while stirring and cooling in an ice bath.
At the end, 7
ml of 1M aqueous HCl was added, the mixture was concentrated under reduced
10 pressure, diluted with ethyl acetate and cooled to 0 C, then adjusted to
pH 3. The
organic layer was separated, washed with NaCI water solution, dried with
MgSO4,
filtered and concentrated under reduced pressure. Next, trifluoroacetic acid
(10 ml, 131
mmol) was added to the resulting solution of N-(tert-butoxycarbonyI)-(2R)-2-
aminopentanethioic acid, stirred at room temperature, and concentrated under
reduced
pressure. The white solid was filtered and dried to provide (2R)-2-
aminopentanethioic S-
acid (IX) the substance of formula (IX):
0
SH (IX),
yield 39% based on the starting N-(tert-butoxycarbonyI)-(2R)-2-aminopentanoic
acid.
(2R)-2-Aminopentanethioic S-acid (IX): 1H NMR (DMSO-d6), 6-scale: 0.81 (3H,
t); L29
(2H, m); 1.60 (1H, m); 1.80 (1H, m); 3.42 (1H, m); 7.72 (3H, br s). Elemental
analysis for
C5H11NOS (molecular weight 133.21): calculated (%) C 45.08; H 8.32; N 10.51; S
24.07;
found (%) C 44.89; H 8.25; N 10.42; S 23.91.
Example 3
[0046] This example illustrates substance of formula (I) for use in the method
of treating
amyotrophic lateral sclerosis.
[0047] A transgenic FUS[1-359]-tg mouse line (FUS-tg) was used to test the
efficacy of
the substance of formula (I) in the therapy of amyotrophic lateral sclerosis
according to
the guidelines set for ALS preclinical studies. Ludolph AC et al. Guidelines
for preclinical
animal research in ALS/MND: A consensus meeting. Amyotroph Lateral Scler.
2010;
11:38-45. Mutations in DNA/RNA-binding factor (fused-in-sarcoma) FUS cause ALS
in
humans. The FUS-tg mouse model recapitulates core hallmarks of human ALS in
the
CA 03207232 2023-07-04
WO 2022/119470 PCT/RU2020/000661
11
spinal cord, including neuroinflammation and neurodegeneration, ensuing muscle
atrophy and paralysis. Shelkovnikova TA et al. Fused in sarcoma (FUS) protein
lacking
nuclear localization signal (NLS) and major RNA binding motifs triggers
proteinopathy
and severe motor phenotype in transgenic mice. J Biol Chem. 2013; 288:25266-
25274.
Lutz C. Mouse models of ALS: Past, present and future. Brain Res. 2018; 1693:1-
10. de
Munter J et al. Neuro-Cells therapy improves motor outcomes and suppresses
inflammation during experimental syndrome of amyotrophic lateral sclerosis in
mice. CNS
Neurosci Ther. 2020; 26(5):504-517.
[0048] At the age of nine weeks, FUS-tg male mice or their wild type
littermates (WT)
start to receive either vehicle, or the substance of formula (I) (50
mg/kg/day), or the
substance of formula (IX) (50mg/kg/day) as a substance of comparison, or the
reference
anti-ALS drug Riluzole (8 mg/kg) for six weeks, via drinking water. The
substance (I) and
the substance (IX) represent (25)- and (2R)-enantiomers of 2-
aminopentanethioic S-acid,
respectively. The (2R)-enantiomer (IX) was included to the study to clarify
importance of
(5)- and (R)-configurations of 2-aminopentanethioic S-acid during the therapy
of ALS.
[0049] The onset of paralysis, a core ALS symptom, was recorded for each FUS-
tg mouse.
The percentage of FUS-tg mice with signs of paralysis on day 95 was found to
be 49% in
the vehicle group, 38% in the Riluzole group, 43% in the substance (IX) group,
and 16% in
the substance (I) group (Figure 1). The percentage of mice with signs of
paralysis on the
95th day was significantly lower in FUS-tg mice treated with the substance of
formula (I)
in comparison with FUS-tg mice that received vehicle (p<0.05, Fisher test). At
this time
point, no other treatment has produced a significant effect on this measure.
This result
indicates that (2S)-configuration of 2-aminopentanethioic S-acid is critical
for the
achievement of the therapeutic effect of 2-aminopentanethioic S-acid. Thus,
the
substance of formula (I) was effective in ameliorating the ALS symptoms.
[0050] The motor coordination, strength and balance was recorded for each FUS-
tg
mouse in the rotarod test. During the period of the observations, motor
behavior of FUS-
tg mice was gradually declining, as reflected by the significant decrease in
the latency to
fall in the rotarod test in FUS-tg vehicle-treated mice in comparison with WT
control on
the week 5. FUS-tg mice treated with the substance (I) had significantly
longer latency to
CA 03207232 2023-07-04
WO 2022/119470 PCT/RU2020/000661
12
fall than vehicle-treated FUS-tg mice, while there was no significant
difference between
FUS-tg mice treated with Riluzole and vehicle-treated FUS-tg mice as well as
between
FUS-tg mice treated with substance (IX) and vehicle-treated FUS-tg mice.
Results are
presented in Figure 2 as mean SEMs (*p<0.05, vs. WT, #p<0.05 vs. Veh, one-
way
ANOVA and Tukey's test). Thus, the substance of formula (I) was effective in
ameliorating motor dysfunctions in the ALS model.
[0051] The concentration of malondialdehyde (MDA), a marker of oxidative
stress, was
measured in the spinal cord of FUS-tg mice treated with vehicle or substance
of formula
(I) in comparison with wild type controls. Figure 3 shows that there was a
significant
difference between groups (p<0.05, one-way ANOVA and Tukey's test). MDA levels
were
significantly increased in FUS-tg mice as compared to WT. There was a
significant
reduction of the MDA levels in FUS-tg mice treated with the substance of
formula (I)
compared to vehicle-treated FUS-tg mice (Figure 3; p<0.05, one-way ANOVA and
Tukey's
test). Thus, the substance of formula (I) was effective in ameliorating
oxidative stress in
spinal cord of mice with ALS symptoms.
[0052] Collectively, results of testing of the substance of formula (I) in FUS-
tg ALS model,
which reproduces characteristic signs of human ALS, demonstrate that the
substance of
formula (I) is effective in the therapy of ALS by diminishing multiple
symptoms of ALS.
30