Note: Descriptions are shown in the official language in which they were submitted.
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
SYSTEMS AND METHODS FOR HEALTHCARE INTEROPERABILITY
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of and priority to U.S.
Patent
Application No. 17/149,703 entitled "SYSTEM AND METHOD FOR HEALTHCARE
SECURITY AND INTEROPERABILITY," filed January 14, 2021, which is a
continuation-in-part of U.S. Patent Application No. 16/703,848 entitled
"SYSTEM
AND METHOD FOR HEALTHCARE SECURITY AND INTEROPERABILITY,"
filed December 4, 2019 (now U.S. Patent No .10,931,437), which is a
continuation of
U.S. Patent Application No. 16/251,980, entitled "SYSTEM AND METHOD FOR
HEALTHCARE SECURITY AND INTEROPERABILITY," filed January 18, 2019
(now U.S. Patent No. 10,541,807). The above-identified applications are hereby
incorporated by reference herein in their entireties.
FIELD
[0002] The subject matter disclosed herein relates to healthcare
system security
and interoperability, while facilitating treatment selection and transparency
in cost
determination.
BACKGROUND
[0003] Healthcare information systems face compliance challenges that
limit
interoperability. For example, stored information may be subject to various
privacy
regulations such as Health Insurance Portability and Accountability Act
(HIPAA).
Privacy rules under HIPAA establish national standards to protect individual
medical
records and other personal health information when health care transactions
are
conducted electronically. These regulations may cover privacy (e.g. which
entities have
access to information), content (what information an authorized entity may
access),
security (how the information is protected from unauthorized access when
stored and
during electronic communication) and integrity (the accuracy and authenticity
of
information). In addition, commercially valuable information may be protected
under an
organizational policy that may limit sharing of the information with third
parties (e.g. as
trade secrets, and/or for business or commercial reasons). Regulations such as
the
European Union (EU) General Data Protection Regulation (GDPR) and state
regulations
may also impact information collection, storage, sharing, and communication.
These
1
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
regulations have affected the information available to healthcare marketplace
participants and led to the creation of organizational "data silos," where
information
available to an entity is isolated, even when it could be useful systemically
(e.g. to
another non-competitive entity). Such compartmentalization of information has
led to
increased systemic costs (e.g. by a patient or medical provider considering
the costs of
treatment alternatives, treatment locations, etc.), raised patient risk (e.g.
from drug
interactions, prescription abuse etc.), and limited the efficacy of outcome
based
approaches to medical treatment or remediation (e.g. making it more difficult
and
expensive to determine when a desired outcome has been achieved or compare
metrics
in approaches that achieve similar outcomes). Systems and techniques to
address one or
more of the above issues that would help facilitate healthcare information
security while
promoting interoperability between marketplace participants are therefore
desirable.
SUMMARY
[0004] In some embodiments, a processor-implemented method may
comprise:
receiving at least one encrypted first Electronic Health Record (EHR) sub-
block
decryptable by the first entity, wherein the at least one EHR sub-block
comprises patient
medical coverage information for a patient and one or more first treatments;
transmitting, in response to the first EHR sub-block, at least one encrypted
first Drug /
Device Information (DIR) sub-block decryptable by at least one corresponding
second
entity, wherein the at least one first DIR sub-block comprises, for each of
the one or
more first treatments, a corresponding first treatment class, one or more
corresponding
first treatment class members associated with each corresponding first
treatment class,
and for each first treatment class member, corresponding first treatment class
member
cost information; receiving, in response to the first DIR sub-block, an
encrypted second
EHR sub-block decryptable by the first entity, wherein the second EHR sub-
block
comprises one or more second treatments, wherein each of the one or more
second
treatments is associated with a corresponding first treatment and each second
treatment
is selected from the corresponding first treatment class members associated
with the
corresponding first treatment; and augmenting, in response to a transaction
confirmation, a multi-dimensional blockchain, wherein the multi-dimensional
blockchain is augmented with a multi-dimensional block formed by linking: a
DIR
block comprising information associated with the one or more second
treatments, an
2
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
EHR block comprising information associated with the second EHR sub-block, and
a
transaction block.
[0005] In another aspect, a computing device for a first entity may
comprise: a
memory, a communications interface, and a processor coupled to the memory and
the
communications interface. In some embodiments, the processor may be configured
to:
receive at least one encrypted first Electronic Health Record (EHR) sub-block
decryptable by the first entity, wherein the at least one EHR sub-block
comprises patient
medical coverage information for a patient and one or more first treatments;
transmit, in
response to the first EHR sub-block, at least one encrypted first Drug /
Device
Information (DIR) sub-block decryptable by at least one corresponding second
entity,
wherein the at least one first DIR sub-block comprises, for each of the one or
more first
treatments, a corresponding first treatment class, one or more corresponding
first
treatment class members associated with each corresponding first treatment
class, and
for each first treatment class member, corresponding first treatment class
member cost
information; receive, in response to the first DIR sub-block, an encrypted
second EHR
sub-block decryptable by the first entity, wherein the second EHR sub-block
comprises
one or more second treatments, wherein each of the one or more second
treatments is
associated with a corresponding first treatment and each second treatment is
selected
from the corresponding first treatment class members associated with the
corresponding
first treatment; and augment, in response to a transaction confirmation, a
multi-
dimensional blockchain, wherein the multi-dimensional blockchain is augmented
with a
multi-dimensional block formed by linking: a DIR block comprising information
associated with the one or more second treatments, an EHR block comprising
information associated with the second EHR sub-block, and a transaction block.
[0006] In a further aspect, an apparatus may comprise: means for
receiving at
least one encrypted first Electronic Health Record (EHR) sub-block decryptable
by a
first entity, wherein the at least one EHR sub-block comprises patient medical
coverage
information for a patient and one or more first treatments; means for
transmitting, in
response to the first EHR sub-block, at least one encrypted first Drug /
Device
Information (DIR) sub-block decryptable by at least one corresponding second
entity,
wherein the at least one first DIR sub-block comprises, for each of the one or
more first
treatments, a corresponding first treatment class, one or more corresponding
first
3
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
treatment class members associated with each corresponding first treatment
class, and
for each first treatment class member, corresponding first treatment class
member cost
information; means for receiving, in response to the first DIR sub-block, an
encrypted
second EHR sub-block decryptable by the first entity, wherein the second EHR
sub-
block comprises one or more second treatments, wherein each of the one or more
second treatments is associated with a corresponding first treatment and each
second
treatment is selected from the corresponding first treatment class members
associated
with the corresponding first treatment; and means for augmenting, in response
to a
transaction confirmation, a multi-dimensional blockchain, wherein the multi-
dimensional blockchain is augmented with a multi-dimensional block formed by
linking: a DIR block comprising information associated with the one or more
second
treatments, an EHR block comprising information associated with the second EHR
sub-
block, and a transaction block.
[0007] In some embodiments, a non-transitory computer-readable medium
may
comprise executable instructions to configure a processor to: receive at least
one
encrypted first Electronic Health Record (EHR) sub-block decryptable by the
first
entity, wherein the at least one EHR sub-block comprises patient medical
coverage
information for a patient and one or more first treatments; transmit, in
response to the
first EHR sub-block, at least one encrypted first Drug / Device Information
(DIR)
sub-block decryptable by at least one corresponding second entity, wherein the
at least
one first DIR sub-block comprises, for each of the one or more first
treatments, a
corresponding first treatment class, one or more corresponding first treatment
class
members associated with each corresponding first treatment class, and for each
first
treatment class member, corresponding first treatment class member cost
information;
receive, in response to the first DIR sub-block, an encrypted second EHR sub-
block
decryptable by the first entity, wherein the second EHR sub-block comprises
one or
more second treatments, wherein each of the one or more second treatments is
associated with a corresponding first treatment and each second treatment is
selected
from the corresponding first treatment class members associated with the
corresponding
first treatment; and augment, in response to a transaction confirmation, a
multi-
dimensional blockchain, wherein the multi-dimensional blockchain is augmented
with a
multi-dimensional block formed by linking: a DIR block comprising information
4
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
associated with the one or more second treatments, an EHR block comprising
information associated with the second EHR sub-block, and a transaction block.
[0008] Further, in another aspect, a processor-implemented method may
comprise: obtaining, from at least one encrypted first Health Transaction
Record (HTR)
sub-block decryptable by the first entity, a first set of first treatments for
the patient over
a time period, wherein each first treatment comprises a first diagnosis code
and a first
treatment code; obtaining, based on the first set: (a) one or more second sets
of second
treatments, wherein each corresponding second treatment is associated with: a
distinct
first treatment in the first set, and (b) a corresponding number of
recurrences for each
second treatment; obtaining a set of available insurance plans available to
the patient
and corresponding coverage related information for each available insurance
plan;
obtaining, based on the set of second treatments and corresponding coverage
related
information for each insurance plan, corresponding estimated plan-specific
cost metrics
for the patient; and transmitting, a first encrypted Drug / Device Information
Record
(DIR) sub-block decryptable by at least one second entity, wherein the DIR sub-
block
comprises the plan-specific cost metrics for the patient.
[0009] In some embodiments, a computing device for a first entity may
comprise: a memory, a communications interface, and a processor coupled to the
memory and the communications interface. In some embodiments, the processor
may be
configured to: obtain, from at least one encrypted first Health Transaction
Record
(HTR) sub-block decryptable by the first entity, a first set of first
treatments for the
patient over a time period, wherein each first treatment comprises a first
diagnosis code
and a first treatment code; obtain, based on the first set: (a) one or more
second sets of
second treatments, wherein each corresponding second treatment is associated
with: a
distinct first treatment in the first set, and (b) a corresponding number of
recurrences for
each second treatment; obtain a set of available insurance plans available to
the patient
and corresponding coverage related information for each available insurance
plan;
obtain, based on the set of second treatments and corresponding coverage
related
information for each insurance plan, corresponding estimated plan-specific
cost metrics
for the patient; and transmit, a first encrypted Drug / Device Information
Record (DIR)
sub-block decryptable by at least one second entity, wherein the DIR sub-block
comprises the plan-specific cost metrics for the patient.
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
[0010] In a further aspect, an apparatus may comprise: means for
obtaining,
from at least one encrypted first Health Transaction Record (HTR) sub-block
decryptable by the first entity, a first set of first treatments for the
patient over a time
period, wherein each first treatment comprises a first diagnosis code and a
first
treatment code; means for obtaining, based on the first set: (a) one or more
second sets
of second treatments, wherein each corresponding second treatment is
associated with: a
distinct first treatment in the first set, and (b) a corresponding number of
recurrences for
each second treatment; means for obtaining a set of available insurance plans
available
to the patient and corresponding coverage related information for each
available
insurance plan; means for obtaining, based on the set of second treatments and
corresponding coverage related information for each insurance plan,
corresponding
estimated plan-specific cost metrics for the patient; and means for
transmitting, a first
encrypted Drug / Device Information Record (DIR) sub-block decryptable by at
least
one second entity, wherein the DIR sub-block comprises the plan-specific cost
metrics
for the patient.
[0011] In some embodiments, a non-transitory computer-readable medium
may
comprise executable instructions to configure a processor to: obtain, from at
least one
encrypted first Health Transaction Record (HTR) sub-block decryptable by the
first
entity, a first set of first treatments for the patient over a time period,
wherein each first
treatment comprises a first diagnosis code and a first treatment code; obtain,
based on
the first set: (a) one or more second sets of second treatments, wherein each
corresponding second treatment is associated with: a distinct first treatment
in the first
set, and (b) a corresponding number of recurrences for each second treatment;
obtain a
set of available insurance plans available to the patient and corresponding
coverage
related information for each available insurance plan; obtain, based on the
set of second
treatments and corresponding coverage related information for each insurance
plan,
corresponding estimated plan-specific cost metrics for the patient; and
transmit, a first
encrypted Drug / Device Information Record (DIR) sub-block decryptable by at
least
one second entity, wherein the DIR sub-block comprises the plan-specific cost
metrics
for the patient.
6
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
[0012] The methods disclosed may be performed by one or more of mobile
computing devices, computers, including servers, cloud-based systems, etc.
using
computer-readable media or computer-readable memory.
BRIEF DESCRIPTION OF THE DRAWINGS
[0013] Embodiments of the invention will be described, by way of
example
only, with reference to the drawings.
[0014] FIG. 1 shows a schematic block diagram illustrating
interactions
between some entities associated with a conventional healthcare information
system.
[0015] FIG. 2 shows an exemplary Electronic Health Record illustrating
some
exemplary data fields in a record.
[0016] FIGs. 3A and 3B show portions of an exemplary Drug / Device
Information Record (DIR) for a drug.
[0017] FIG. 3C shows an example data flow from an initial proposed
prescription to alternatives that may be presented to a patient and/or an HCP
along with
cost information.
[0018] FIG. 4 shows a portion of exemplary Pharmacy Benefits Record
(PBR),
which may be maintained by a pharmacy benefits manager.
[0019] FIG. 5 shows example Pharmacy Prescription Record, which may be
maintained by an entity such as a ppharmacy.
[0020] FIG. 6 shows an example Health Transaction Record
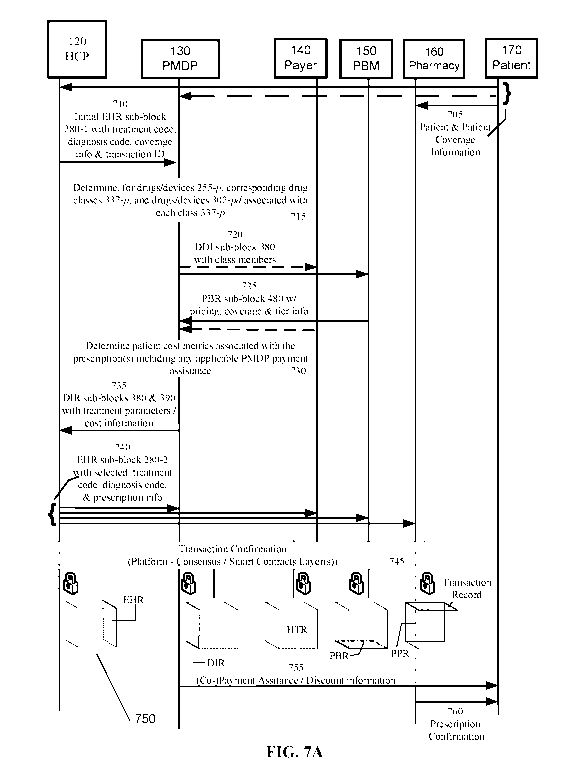
[0021] FIG. 7A shows a flow diagram illustrating an example process
flow to
facilitate healthcare cost determination, healthcare information security and
facilitate
interoperability between a plurality of entities.
[0022] FIG. 7B illustrates entities and layers associated with an
example
platform to facilitate healthcare system security and interoperability.
7
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
[0023] FIG. 8 shows a flowchart of an exemplary method to facilitate
healthcare
information security and interoperability while facilitating patient treatment
selection
and transparency in treatment costs.
[0024] FIG. 9 shows a flow diagram illustrating an example process
flow to
facilitate healthcare insurance selection and cost determination, while
maintaining
healthcare information security and facilitating interoperability between a
plurality of
entities.
[0025] FIGs. 10A and 10B show a flowchart of an exemplary method to
facilitate healthcare insurance selection and cost determination, while
maintaining
healthcare information security and facilitate interoperability between a
plurality of
entities.
[0026] FIG. 11 shows a schematic of an exemplary computer or computing
device capable of facilitating healthcare system security and promoting
interoperability.
[0027] In the figures, like reference numbers and symbols in the
various figures,
which depict certain example embodiments indicate like elements. For example,
sub-blocks with similar information are referred to with the same reference
numbers. In
addition, multiple instances of an element may be indicated by following a
first number
for the element with a letter or with a hyphen and a second number. For
example,
multiple instances of an element 280 may be indicated as 280-1, 280-2, etc.
When
referring to such an element using only the first number, any instance of the
element is
to be understood (e.g. element 280 in the previous example would refer to
elements
280-1, 280-2 . . . and/or 280-N). Further, in the figures below, an asterisk
symbol ("*")
associated with a reference number indicates that the element (or some portion
thereof)
may be repeated (e.g. for each instance of the element). For example, a
prescription may
include several drug instances, and a dosage field may be repeated for each
drug
instance.
[0028] The figures below also show hierarchies of records, which may
comprise
fields and sub-fields. Records include any fields (and some or all parts of
sub-blocks)
that are part of the record. Similarly, a field includes any sub-fields. Sub-
fields may
include additional sub-fields.
8
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
DETAILED DESCRIPTION
[0029] Disclosed embodiments facilitate healthcare system security
while
promoting healthcare system integrity, interoperability, treatment selection,
and cost
transparency.
[0030] FIG. 1 shows a schematic block diagram illustrating
interactions
between some entities associated with a conventional healthcare information
system
100. Healthcare transactions may involve several entities, where each entity
may have
some transaction-relevant information, which may be used to complete the
transaction.
Thus, in conventional systems, some limited information (e.g. limited by
regulation,
contract, privacy considerations, confidentiality, and/or for competitive
reasons) may be
exchanged between the transacting entities in order to complete a transaction.
The term
"entity," as used herein, may refer to an individual (such as a patient or
groups of
patients) or an organization or that participates in a healthcare marketplace
and/or to
computing and information systems (e.g. hardware and/or software) associated
with that
individual/group/organization, which may participate in the healthcare
marketplace on
behalf of the individual/group/organization. For example, the computing
systems
associated with one entity may process and/or exchange information with
computing
systems associated with other entities. The exchange of information between
entities
may occur over secure communication networks and/or in a secure manner (e.g.
using
encryption) over the Internet and/or on an electronic platform (e.g. such as
an
information exchange and/or transaction exchange).
[0031] An entity such as a patient (not shown in FIG. 1) may seek
treatment
from another entity such as Healthcare Provider (HCP) 120 for a medical
condition
afflicting the patient. HCP 120 may determine patient treatment information
and patient
insurance using health information (HI) database 125, which may be maintained
by
HCP 120 and include patient coverage related information 124. Further, HCP 120
may
propose a treatment for the patient's medical condition. When proposing a
treatment to
be prescribed, HCP 120 may send treatment information 123 to Pharmaceutical
Provider and/or Medical Device Provider (PMDP) 130. Treatment information 123
sent
to PMDP 130 may not include any patient personally identifiable information
(PII).
[0032] Personally identifiable information (PII) is any data that
could potentially
be used to identify specific individuals. The term "non-Ph" is used herein to
qualify
9
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
information that does not include PIT. For example, a patient data record
(with PIT) may
include a patient's name, full address, other identifiers (e.g. insurance
identifier, driver's
license number, social security number, and/or other identifying information)
along with
health related information. PIT in the patient's data record may be removed to
obtain a
non-PIT patient data record (without name, full address, and other identifying
information). For example, the non-PIT patient data record may include age,
sex,
medical history (e.g. medical conditions afflicting the patient, other
medication being
used by the patient, etc.), treatment(s), and optionally (e.g. when
appropriate) a city,
state, and/or zip code, and may include full address of HCP 120 (where the
care was
delivered).
[0033] In response, PMDP 130 may send Drug / Device profile and safety
information 133 to HCP 120 based on information in Drug / Device Information
Record
(DIR) database 135. DIR database 135 may include drug/device profile and
safety
information 133. Drug/device profile and safety information 133 may include
information about drug characteristics such as dosage, mode of administration,
absorption, metabolism, duration of action, toxicity, and interactions with
foods or other
medications. Upon receiving drug / device profile and safety information 133,
HCP may
prescribe one or more drugs and/or medical devices and/or procedures, or may
revise
the prescription based on the received Drug / Device profile and safety
information 133.
[0034] As shown in FIG. 1, HCP 120 may also securely send stored
patient
insurance and treatment (e.g. medical procedure related) information 124 to
Payer /
Insurer (hereinafter "Payer") 140 and prescription information 127 to Pharmacy
160.
Prescription information 132 may include patient ID and treatment (e.g. drug
and/or
device) information. Insurance related and treatment information 124 may
include
patient identification (ID) information, insurance plan information, group ID
information, proposed treatment information (e.g. one or more of: medical
procedure
related information, drug related information, medical device related
information, etc.).
While insurance related and treatment information 124 may include some
personally
identifiable information (PIT), the PIT information shared may be limited. For
example,
patient insurance and treatment information 124 may not include patient family
history
and/or other patient information (which may be part of HI database 125) but
may not be
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
relevant to coverage determination, and/or cost determination, and/or
precluded (e.g. by
law / regulations) from being shared with Payer 140.
[0035] Pharmacy 160 may enter and/or update Patient-Prescription
information
(PPI) database 155 using the received patient prescription information 127 and
may
send patient insurance coverage and treatment related information 163 to
Pharmacy
Benefit Manager (PBM) 150.
[0036] PBMs 150 are entities that play a role in patient access to
pharmaceutical
products (drugs and/or devices). PBMs 150 determine pharmaceutical prices
based
PBMs 150 may set or determine retail prices for pharmaceutical products,
obtain
payments or rebates from manufacturers based on the sales volumes of a
particular
product or a basket of products, and, in some instances, may also obtain post-
sale price
concessions and payments from pharmacies on "formularies" for Payers 140.
Formularies may determine: the drugs available to a patient based on their
coverage and
the distribution of drug costs (e.g. proportion of payment to be provided by a
patient and
the proportion to be paid by Payer 140 for each covered drug). Further,
formularies
typically assign drugs to one of a plurality of cost-sharing tiers. The drug
prescribed and
the drug's cost-sharing tier (which may both depend on a patient's health or
prescription
coverage) may determine the patient's out-of-pocket cost at the pharmacy. For
example,
drugs in a preferred tier may have lower cost-sharing requirements (e.g. lower
co-
insurance).
[0037] Thus, the patient's eventual share of a drug's cost at the
pharmacy may
depend on: (a) the plan's deductible (some specified amount to be paid each
plan term
by the patient before the payer cost shares); (b) co-payment (fixed out of
pocket
payment for each transaction as specified by the patient's health plan), and
(c) co-
insurance (percentage of a drug's cost to be borne by the patient after the
deductible and
co-payment requirements have been met). In some instances, based on patient
qualification information, Pharmaceutical / Medical Device Provide (PMDP) 130
may
provide upfront payment assistance to the patient. Patient qualification
information may
include one or more of: health insurance coverage information, patient out-of-
pocket
cost (which may include one or more of copayment, coinsurance, and deductibles
for an
individual prescription or over some time period), and demographic information
associated with the patient (e.g. one or more of patient location, age,
disability
11
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
information, income, prescription costs, etc.). Conventionally, the patient
payment
assistance may be provided as a physical patient-specific discount card that
is applied to
the patient's out-of-pocket cost. The patient payment assistance may be
provided by
PMDP 130 through a patient access program, which, in some instances, may be
administered separately in accordance with applicable laws and regulations.
Partly
because of the complexity of cost determination due to the number of entities
involved
and data compartmentalization by entities, patient cost information is
typically not
available to HCP 120 and/or to a patient at the time of prescription by the
HCP (and
may only be available after Pharmacy 160 and/or PBM 150 determine patient cost
share).
[0038] For medical procedures, payer 140 may compare the received
patient
coverage related information 124 with information in Plan/Coverage database
145 to
determine coverage for the patient. Based on the coverage information, Payer
140 may
update transaction information database 147 and reply with a confirmation
and/or
additional/updated patient coverage related information 124 to HCP 120.
Patient
coverage related information 124 may include approval/denial information,
coverage
information related to the proposed treatment, and cost and payment related
information
such as patient co-pays, billing codes, etc. If the Payer withholds approval
or the
coverage for the proposed treatment is inadequate and/or does not meet the
patient's cost
criteria, HCP 120 may propose revisions, which may lead to further exchange of
information between HCP 120 and Payer 140. The interactions between HCP 120,
Payer 140, and PMDP 130 may continue until HCP 120 finalizes a treatment plan
that:
(a) is acceptable to the patient, (b) meets safety and efficacy
considerations, and (c) may
be covered and/or approved by Payer 140.
[0039] For drugs and/or devices, PBM 150 may use coverage related
information received from the patient (or HCP) with information in Pharmacy
Benefit
Information (PBI) database 155 to determine patient coverage, whether the
prescribed
drug(s) are covered, the out-of-pocket patient drug cost, insurance share of
drug cost,
etc. When the treatment (e.g. drugs and/or devices) are covered, PBM 150 may
send
cost information 153 to Pharmacy 160. Cost information 153 (which may include
one or
more of patient out-of-pocket cost, insurance cost, cost breakdown, and/or
other
information) may be sent to Pharmacy 160, which may use the received cost
12
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
information 153 to update PPI database 155. If the patient later wishes to
change the
prescription (e.g. to different or lower cost drug based on out-of-pocket cost
information
obtained by the patient from the pharmacy 160) the process may need to be
repeated,
which may lead to further exchange of information between the patient, HCP
120,
Pharmacy 160, and/or PBM150. Because cost information is not conventionally
available to HCP 120 and/or to a patient at the time of prescription by the
HCP, patients
and/or HCPs 120 may be unaware of the cost-related aspects of prescriptions
until out-
of-pocket costs are determined by Pharmacy 160 (e.g. based on information
provided by
PBM 150). Thus, a prescribed treatment may need to be changed (or may go
unfulfilled)
if the cost is not acceptable to the patient thereby leading to systemic
inefficiencies and
poor resource utilization.
[0040] In addition, there may be other exchanges of information, for
example,
between Payer 140 and PBM 150 to exchange/verify patient coverage (e.g.
whether the
patient is currently covered), payment information (e.g. remaining patient
deductible
etc.) . PBM 150 may send patient coverage information 142 to Payer 150 and
receive
confirmation on whether coverage is current and deductible related
information, which
may be used by PBM to determine out-of-pocket patient cost. Further, PMDP 130
and
PBM may exchange sales and rebate related information 132, while PMDP 130 and
Payer 140 may exchange contract related information and/or drug / device
pricing
related information 137.
[0041] Thus, conventional healthcare information systems suffer from
several
drawbacks. While each entity obtains and maintains information that may be
relevant
for operating its business, very little of that information may be shared
(e.g. due to legal,
privacy, and/or business considerations) and when information is shared, it is
often
piecemeal, devoid of context, untimely, and may not be useful for decision
making /
planning purposes. For example, HCP 120 and/or patient may not have cost
information
related to a treatment at the time of prescription. As another example,
treatment
alternatives and the costs of the various treatment alternatives may not be
available to
the patient or HCP 120 at the time when a prescription or treatment plan is
being
developed.
[0042] As another example, HCP 120 may not provide details of adverse
drug
effects that may be reported by the patient to PMDP 130 (e.g. due to privacy
concerns).
13
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
As another example, when adverse drug effect information is provided to PMDP
130 by
HCP 120, the information may not include non-PIT demographic information (e.g.
age,
location, medical condition, etc. for a patient ¨ again out of privacy
concerns) so the
information may be of limited value to PMDP 130. In addition, in some
instances, when
adverse drug effects are reported by an entity (e.g. a patient), validation of
adverse drug
effects may often be performed by another entity to determine if the adverse
event can
be attributed to a prescribed drug. Validation, which may involve additional
entities, can
introduce additional complexities that can further delay reporting and/or
create
additional silos thereby further limiting the utility of the information (e.g.
to PMDP
130).
[0043] Further, because the information exchanged may be
compartmentalized
and provided on an ad-hoc basis, aggregating the received information with
information
stored by the receiving entity may be cumbersome. Moreover, because each
entity may
index the information differently, it may be difficult or impossible for the
receiving
entity (or the sending entity) to tie received (or sent) information to an
information
record stored by the sending entity (or stored by the receiving entity). For
example, if
HCP 120 provides adverse drug effect information to a PMDP 130 at some point
in
time, it may be difficult for HCP 120 and/or PMDP 130 to obtain additional
patient or
patient medical condition information pertaining to the adverse drug effect ¨
even when
that information may be legally shared. For example, compartmentalization of
information may prevent or limit access by PMDP 130 to aggregate demographic
information that may be of use in tailoring drug utilization. As a further
example, it may
be difficult for HCPs 120 and/or Payer's 140 to determine prescription abuse
by
patients.
[0044] Many modern machine learning (ML) and other artificial
intelligence
(AI) systems can process large amounts of data to determine hazards, identify
patterns
that may lead to desired outcomes, etc., which may lead to increased
efficiencies, lower
costs, and/or better outcomes. The siloing and compartmentalization of
information also
limits the applicability of such ML and AT techniques thereby contributing to
additional
inefficiencies.
[0045] Some attempts to introduce efficiencies into healthcare
delivery focus on
outcome based approaches. Payers 140 may tie reimbursement to the achievement
of
14
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
some agreed upon outcomes. For example, an outcome based contract may specify
that
HCP 120 will be reimbursed at some agreed upon rate based on a lowering of a
patient's
blood pressure to some defined range within some time period. Tracking and
managing
such outcome based contracts can be notoriously difficult in conventional
systems
because several exchanges of information may need to occur between HCP 120 and
Payer 140 where each exchange is in compliance with legal, regulatory, privacy
and
business related guidelines.
[0046] While the use of blockchains to store health related
information can
facilitate ensuring the integrity and authenticity of the stored information,
conventional
techniques do not address issues raised by data sharing between entities in an
environment of increasing transactional and regulatory complexity ¨ such as
maintaining data privacy while decreasing information compartmentalization.
Moreover, conventional techniques also do not ensure timely information
availability
(e.g. to one or more entities in a manner compliant with legal and regulatory
obligations) to facilitate transaction finalization. Further, conventional
techniques do not
ensure that entities have a coherent and consistent view of completed
transactions. The
lack of a coherent and consistent view of completed transactions across
entities can
constrain interoperability, data utilization, and the quest for increased
operational
efficiencies. In addition, the constraints on interoperability often limit
organizational
ability to maintain a customer-centric focus because customer (e.g. patient)
requests for
information to aid or enhance decision-making (e.g. independently or in
coordination
with HCPs) may be difficult to accommodate.
[0047] Disclosed embodiments facilitate healthcare system security
while
promoting healthcare system integrity, interoperability, and facilitate
healthcare cost
transparency. Some disclosed techniques facilitate timely exchange (e.g. at
the time of a
transaction) of appropriate data (e.g. compliant with legal, privacy, and
business
guidelines) to appropriate entities (e.g. authorized entities associated with
a transaction),
while facilitating a consistent and coherent view of the information across
healthcare
marketplace entities.
[0048] Interoperability is facilitated in part because multiple
entities associated
with a transaction may be able to tie appropriate relevant information shared
during a
transaction to the completed transaction using an agreed upon reference.
Consistency
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
and coherency are facilitated because locally recorded data (e.g. locally at
an entity)
may correspond to reference data (e.g. maintained on a shared platform) and
each
entity's view of the reference data (or portions of the reference data
viewable by the
entity) may be consistent with another authorized entity's view of the data
(and/or with
the other authorized entity's locally recorded data). In some embodiments, the
reference
data may be based on and/or take the form of a decentralized ledger. In some
embodiments, the decentralized ledger may be accessible to authorized entities
and each
entity's view of the decentralized ledger may be compliant with legal,
privacy, business,
and/or contractual obligations. Further, efficiency is promoted because
information
relevant to decision-making is made available to entities early in the
transaction cycle to
facilitate early consideration of alternatives thereby decreasing the
likelihood of and
inefficiencies associated with transaction finalization and decreasing
decision
revisitation.
[0049] In some
embodiments, a first entity (e.g. PMDP 130) may receive at least
one encrypted first Electronic Health Record (EHR) sub-block (e.g. for a
patient) that is
decryptable by the first entity. The first EHR sub-block(s) may comprise
patient
medical coverage information for a patient and one or more first treatments
(e.g.
proposed drugs and/or devices, denoted by F_p,1<p P. The encrypted first EHR
sub-block may be received (e.g. by PMDP 130) with a transaction ID for a
current
transaction and, in some instances, may not include PII information for the
patient. In
other instances, (e.g. when authorized and/or when PMDP 130 determines or uses
patient eligibility for a payment assistance program administered by PMDP
130),
encrypted first EHR sub-block may be received (e.g. by PMDP 130) with a
transaction
ID for a current transaction and may further include PII information for the
patient. In
situations where patient PII information is received (e.g. by PMDP 130), then
PMDP
130 may administer and fund the payment assistance program and may use a
locally
maintained blockchain consistent with disclosed embodiments to maintain
patient
privacy on behalf of PMDP so that access to patient PII information is
restricted to
program administrators and/or authorized personnel. Authorization to provide
PII
information may be obtained (e.g. in advance by HCP 120 or PMDP 130 or another
entity) from the patient. In some embodiments, the first EHR sub-block may
further
indicate that the patient has provided assent to use of information in the
first EHR sub-
block to determine clinical trial participation eligibility.
16
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
[0050] The first entity (e.g. PMDP 130) may transmit, in response to
the first
EHR sub-block, at least one encrypted first Device Drug Information (DIR) sub-
block
that is decryptable by at least one corresponding second entity (e.g. HCP
120), wherein
the DIR sub-block(s) comprise, for each of the one or more first treatments (F
_p , 1 < p
< P): a corresponding first treatment class (e.g. K _p representing drugs /
devices that are
in the same class as F _p). Each treatment class K _p may include one or more
corresponding first treatment class members (individual drugs / devices
denoted by
C _pd , (1 < p < P, 1 < d < D_p), and each first treatment class member (C
_pd) may be
associated with corresponding first treatment class member cost information (M
_pd).
The first treatment class member cost information (M _pd) for a corresponding
first
treatment class member (C _pd) may include out of pocket costs, a total cost
over an
expected treatment duration, treatment, cost over some specified time period,
etc.). D _p
may represent the number of class members in treatment class K _p .
[0051] For example, upon receiving the first EHR sub-block, the first
entity (e.g.
PMDP 130) may determine, for each of the one or more first treatments (F _p),
the
corresponding first treatment class (K _p). Further, in some embodiments, the
first EHR
sub-block may comprise patient-specific parameters and the corresponding first
treatment class may be determined based on the patient-specific parameters.
The
patient-specific parameters may comprise one or more of: patient co-morbidity
information, route of administration information, safety and efficacy
information, and/or
patient location information. Thus, members C_pd in a corresponding first
treatment
class (K _p - corresponding to a treatment F _p) may be determined (e.g. by
the first
entity) based on the patient-specific parameters.
[0052] Each first treatment class (K _p) may comprise the one or more
corresponding first treatment class members (C _pd). Further, the first entity
may
determine, for each of the one or more first treatment class members (C _pd),
one or
more corresponding first treatment class member cost information (M_pd). The
first
entity (e.g. PMDP 130) may then transmit the encrypted first DIR sub-block(s)
decryptable by at least one corresponding second entity (e.g. HCP 120).
[0053] The one or more corresponding first treatment class member cost
metrics
(M_pd) include one or more of: a patient specific out-of-pocket cost
corresponding to
each corresponding first treatment class member (C _pd) for a corresponding
treatment
17
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
unit; or a patient-specific estimated total out-of-pocket cost corresponding
to each
corresponding first treatment class member for a typical or prescribed
treatment
duration; or a patient-specific estimated total out-of-pocket cost
corresponding to each
corresponding first treatment class member (C _pd) for the remaining duration
of a
medical coverage plan associated with the patient, or a combination thereof
[0054] The first entity (e.g. PMDP 130) may receive, in response to
the
transmitted first DIR sub-block(s), an encrypted second EHR sub-block
decryptable by
the first entity, wherein the second EHR sub-block comprises one or more
second
treatments (e.g. one or more selected drugs / devices, S_p,1 < p < P), wherein
each of
the one or more second treatments S _p is selected from corresponding first
treatment
class members (C _pd) associated with a corresponding first treatment class (K
_p). For
example, the set of second treatments S may comprise, for each first treatment
class
(K _p corresponding to a first treatment F _p) one selected corresponding
first treatment
class member (C _pd). For example, the second EHR sub-block may comprise one
or
more selected devices / drugs, where each selected device / drug S _p is
associated with
a distinct corresponding class K _p . As an example, the first EHR sub-block
may include
first treatments (Fl, F_2, F_3) while the second EHR block may include second
treatments G 1, F_2, H_4} where G 1 and F 1 are both in treatment class K 1,
F_2 is
in treatment class K_2, and F_3 and H_4 are in treatment class K_3. As
outlined above,
each treatment classes may include one or more members. In some embodiments,
the
selection of the one or more second treatments (S _p) may be based on patient-
specific
cost metrics.
[0055] In some embodiments, the first entity (e.g. PMDP 130) may then
augment a multi-dimensional blockchain, wherein the multi-dimensional
blockchain is
augmented with a multi-dimensional block formed by linking: a DD1 block
comprising
information associated with the one or more second treatments, an EHR block
comprising information associated with the second EHR sub-block, and a
transaction
block, which may comprise a transaction ID.
[0056] In some embodiments, upon receiving the second EHR sub-block,
the
first entity (e.g. PMDP 130) may transmit the transaction block with the
transaction
confirmation wherein the transaction block comprises at least one prescription
for the
one or more second treatments at one or more corresponding specified second
treatment
18
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
locations. In some embodiments, the transaction block may comprise a
prescription for
the second treatment at the specified location at a specified patient cost. In
some
embodiments, the first entity may further transmit a confirmation of the
transaction
along with the at least one prescription to the patient (e.g. associated with
the first EHR
sub-block and second EHR sub-block).
[0057] The term
sub-block indicates a portion of a data record or a block. which
(when encrypted) may be decryptable by some specific entity or entities (but
not by
other entities). Information in sub-block(s) decrypted by a specific entity
(or entities)
may be incorporated into data records (or data blocks in blockchains) that are
being
maintained by that specific entity (or entities) when a transaction is
finalized. For
example, a transaction with a transaction ID U may involve entities A, B, C,
and D,
which may be owners of data records W, X, Y, and Z, respectively, in a multi-
dimensional blockchain. In the example above, a data record W (owned by A) and
related to transaction U may be encrypted and readable by the owning entity A
(but not
by other entities). However, the data record W may include (in addition to
transaction
ID U) a portion of the information in other data records (e.g. data records X,
Y, and/or
Z, which may not be readable by entity A). For example, data from the one or
more
other data records (e.g. data records X, Y, and/or Z) that is present in W may
have been
received (e.g. by entity A) prior to transaction finalization as decryptable
sub-blocks.
Similarly, the other entities B through D may also include (in addition to
transaction ID
U) transaction related information present in a non-owned data records. For
example,
for entity B, information present in one or more of data records W, Y and/or
Z, may
have been received in the form of sub-blocks decryptable by entity B prior to
transaction finalization. Thus, each entity A, B, C, and D may maintain
distinct
independent blockchains that include (for transaction U) data records W, X, Y,
and Z,
respectively, as blocks in their respective blockchains. The data records (or
blocks) W,
X, Y, and Z, associated with transaction Umay also collectively form a multi-
dimensional block in a multi-dimensional blockchain. Thus, a coherent and
consistent
view of the transaction is available to all marketplace entities that may be
associated
with a transaction while maintaining compliance with legal, privacy and/or
other
regulations / business considerations, and promoting data integrity.
19
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
[0058] The term "blockchain" as used herein, refers to a growable list
of records
or "information blocks" or "blocks," where the blocks are linked using
cryptographic
techniques. Each block includes a cryptographic hash of the previous block, a
timestamp, and transaction data. A current block being added to the blockchain
is also
termed the head of the blockchain. A cryptographic hash function maps data of
arbitrary
size to a bit string of a fixed size, which is termed a "hash." Hash functions
can be
deterministic (the same input will produce the same output) and may be one-way
functions that are infeasible to invert (i.e. determine the original data
input from the
hash value). The transaction data for a block may be represented as a Merkle
tree root
hash. The term "Merkle tree" or "hash tree" is used to refer to a tree, where
every leaf
node is labeled with a hash of the transaction data and each non-leaf node is
labeled
with the cryptographic hash of the labels associated with its child nodes. A
block header
for a block to be added to the blockchain may include a hash reference to the
previous
block header and a hash reference to the root of the Merkle tree that contains
the
transaction data. Blockchains promote data integrity because alterations to
data in the
blockchain results in inconsistencies in one or more of the hash references.
The term
record or data record is also used to indicate non-final data that is to be
added to a
blockchain. Once a data record has been validated and finalized the data
record may be
added to the blockchain and form a block in the blockchain.
[0059] The term "multi-dimensional blockchain" is used to refer to a
sequence
of multi-dimensional records (also referred to as multi-dimensional blocks),
where each
multi-dimensional record includes two or more data records. In some instances,
each of
the data records that may be viewed as forming a dimension of the multi-
dimensional
blockchain may also form blocks in a distinct blockchain associated with some
entity.
Thus, in some embodiments, a multi-dimensional block may comprise a data
record in
each dimension, where the data record corresponding to a dimension may form a
block
in a distinct conventional blockchain associated with a corresponding entity.
For
example, a multi-dimensional block may include an EHR data record as one
dimension,
a DIR data record as another dimension, and a Transaction data record as a
third
dimension. Further, in some instances, the EHR data record associated with a
multi-
dimensional block (in the multi-dimensional blockchain) may separately form a
block in
a distinct EHR blockchain (i.e. distinct from the multi-dimensional
blockchain).
Similarly, in some instances, the DIR data record and Transaction data record
associated
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
with a multi-dimensional block may each form a block in a distinct DIR
blockchain
(e.g. associated with PMDP 130) , and a Transaction record blockchain (e.g.
associated
with Payer 140), respectively. Thus, in some instances, a data record in the
context of
the multi-dimensional blockchain may correspond to a block in a distinct
conventional
blockchain. In some instances, each data record (e.g. associated with a
dimension) in the
multi-dimensional block may correspond to, form part of, and/or or be derived
from
corresponding blocks in distinct conventional blockchains. The multi-
dimensional block
may include a cryptographic hash of a previous multi-dimensional block, a
timestamp,
and data. The data for the multi-dimensional block may include hashes of the
individual
data records that make up the multi-dimensional block. In some embodiments, a
consensus mechanism between the entities may be used to confirm correctness of
data
in a proposed multi-dimensional block before that multi-dimensional block is
committed and locked.
[0060] Thus, the multi-dimensional block may comprise two or more
encrypted
data records, where each encrypted data record may be associated with a
distinct entity
(e.g. in the healthcare marketplace). As outlined above, the data records in a
multi-dimensional block may separately form blocks in distinct blockchains,
where each
of the blockchains may be associated with a distinct entity. Each encrypted
data record
may be decrypted by the corresponding associated entity (e.g. the data record
owner).
Further, an encrypted data record may include portions (termed "sub-blocks")
with data
that may have been decrypted by at least one other specific entity in addition
to the
encrypted data record owner. For example, the sub-block may have been
decrypted by
at least one other distinct entity (in addition to the data record owner) at
the time the
corresponding multi-dimensional block was formed. In some embodiments, at or
prior
to the time of multi-dimensional block formation, the sub-blocks may have been
separately encrypted and made available to another entity along with
information to
decrypt the sub-blocks. Accordingly, a multi-dimensional block may facilitate
availability of transaction data to a plurality of entities associated with a
healthcare
marketplace, while providing a coherent and consistent view of the data to
authorized
marketplace entities, complying with privacy and/or data sharing regulations,
business
guidelines, and/or contractual obligations, and promoting data integrity.
Entities may
also ensure data correlation (e.g. of a record associated with a dimension of
a multi-
dimensional block in the multi-dimensional blockchain) with a corresponding
block in a
21
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
locally maintained blockchain. In embodiments, when information is exchanged
between two entities using sub-blocks, the information exchanged via the
decryptable
sub-blocks may be based on an informational interface between the two
entities. In
some embodiments, when exchanging information (e.g. at the time of multi-
dimensional
block formation), each entity may encrypt blocks associated with a local
blockchain
maintained by the entity while generating sub-blocks that are decryptable by
the other
entity. The informational interface may be based on a smart contract
associated with the
blockchain.
[0061] The term "smart contract" is used to refer program code or
logic, which,
in some instances, may be associated with a blockchain or a blockchain
platform. The
"smart contract" may encode rules or agreement between two or more entities in
relation
to data sharing, transactions, access, contract fulfillment, etc. The smart
contract may be
based on a contract between two or more entities and/or agreements related to
the multi-
dimensional blockchain platform. For example, "smart contract" program code
associated with the multi-dimensional blockchain may process transaction
requests and
determine the validity of transactions based on program logic.
[0062] FIG. 2 shows an EHR 200 illustrating some exemplary data fields
in a
record. In some embodiments, EHR 200 may include information about a patient
and
may be owned and maintained by HCP 120 (e.g. in HI database 125). In some
embodiments, data fields in EHR 200 may be populated and/or updated by HCP 120
based, in part, on information received from a patient. EHR 200 may also
include data
received (e.g. as sub-blocks) from other entities. The fields shown in EHR 200
are
merely exemplary, and EHR 200 may comprise various other additional fields
based on
laws, standards, HCP practice, and/or industry practice, etc. An EHR may
comprise
fields different from (fewer or greater than) those shown in relation to
exemplary EHR
200.
[0063] EHR 200 may be associated with HCP Profile field 295, which may
store
information pertaining to HCP 120 (e.g. HCP identification, registration
and/or
licensing information, address, associated medical professionals, medical
professional
identification/registration information, etc.). In some embodiments, some or
all of the
information in HCP Profile 295 may be shared with other entities in connection
with a
transaction. For example, a portion of HCP Profile 295 may be sent to one or
more
22
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
entities such as PMDP 120, Payer 140, PBM 150, and/or Pharmacy 160 (e.g. as
part of
an encrypted sub-block, which may be decryptable by the designated receiving
entity).
100641 For example, as shown in FIG.2, EHR 200 may comprise basic
profile
information 230 about a patient, which may change relatively infrequently.
Basic
Profile information 230 may include Patient Name, Patient ID, Family History
205,
Date of Birth (DOB) 220, Blood Type 225, etc. Family History 205 may include
Maternal History 210 and Paternal History 215. Basic Profile Information 230
may also
medical history 232, which may include information about other prior or
current
medical conditions associated with the patient.
[0065] EHR 200 may further comprise other data fields such as
Diagnosis 235
(e.g. for a current ailment), Diagnosis Code 240, which may be a standardized
code for
the diagnosis (such as an International Classification of Diseases (ICD)
code),
Treatment Code 245, which may be a standardized code to describe the treatment
(e.g.
such as a Current Procedural Terminology (CPT) code), Prescription Code 250
for each
prescription, which may serve to uniquely identify each prescription.
Prescription code
250 is also referred to herein as prescription 250.
[0066] In some embodiments, prescription code 250 (for a prescription)
may
include further include (e.g. for each drug/device being prescribed in the
prescription)
one or more subfields such as: a Prescribed Drug field 255-p, 1 <p < P (e.g.
drug ID
and/or a Classification Product Code (CPC) for medical devices), Dosage 260-p
(strength and frequency), and Duration 265-p (length of time over which the
drug is to
be taken). In some instances, EHR 200 may also comprise other fields and/or
sub-fields
such as an indication of whether a prescription is a new prescription, or a
refill. EHR
200 may also include Medical Device Report (MDR) adverse event codes, or other
(e.g.
ICD-10) codes to document adverse drug effects. EHR 200 may also include
Patient
Criteria 297, which may specify one or more criteria for drug/device selection
and/or
cost metric determination that may be provided by a patient. For example,
Patient
Criteria 297 may specify a preferred method of administration (e.g. topical,
ingested,
inhaled, etc.), preferred pharmacies, an area or location where prescribed
drugs/devices
are to be obtained, types of cost metrics desired, etc.
23
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
[0067] In some embodiments, EHR 200 for a patient may be stored as a
blockchain, for example, by HCP 120 and each transaction between HCP 120 and
the
patient and/or another entity may form part of an EHR information block in the
EHR
blockchain. When an EHR block associated with a transaction is stored in a
blockchain,
the stored information may, in some instances, be associated with Transaction
ID 695,
which may serve to uniquely identify the transaction. In some embodiments,
Transaction ID 695 may be common to entities associated with a transaction
(e.g., all
entities associated with a transaction may use the same transaction ID). In
some
embodiments, a transaction initiator and/or components of a permissioned
blockchain
platform may provide transaction information such as Transaction ID 695 to one
or
more entities associated with a transaction. Accordingly, sub-blocks sent or
received by
entities may be identified as being associated with a transaction and
processed
appropriately. In some embodiments, the format and content of Transaction ID
695 may
be determined and/or agreed to in advance by entities associated with the
transaction
platform and/or provided by the transaction platform. Other transaction
information
related fields (not shown in FIG. 2) may include a transaction type, which may
be used
by an entity to determine and process information in transmitted and/or
received
sub-blocks and determine an appropriate response. For example, a transaction
type may
indicate that sub-block 280 is being provided to obtain cost-metrics
associated with a
prescription.
[0068] In the description below, when an EHR is maintained as a
blockchain
(e.g. by HCP 120), then EHR information record 200 may also be referred to as
EHR
block 200. EHR block 200 may thus form a block in an EHR blockchain. When an
EHR
block 200 is to be added to an EHR blockchain, some of the data in EHR block
200
being added to the EHR blockchain may depend on other entities. For example, a
treatment (e.g. specified in treatment code 245 for a diagnosis described by
diagnosis
code 240) may be subject to approval by Payer 140 and/or PBM 150 (not shown in
FIG.
2) and may be included as part of an EHR upon approval. As another example, a
drug
warning label (not shown in FIG. 2), which may form part of EHR block 200 may
use
input from PMDP 130 to complete, validate, and/or update information in the
warning
label prior to EHR block 200 being added to the EHR blockchain.
24
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
[0069] In some embodiments, Patient Criteria 297, Diagnosis 235,
Diagnosis
Code 240, Treatment Code 245, Prescription Code 250 along with data fields
Prescribed
Drug/Device 255-p, Dosage 260-p, and Duration 265-p, 1 <p <P, may be used to
form
sub-block 280. In some embodiments, sub-block 280 may further include one or
more
of: basic profile information 230, patient coverage related information 272
and/or plan
ID 270, which may serve to identify an insurance plan (e.g. health insurance
plan,
pharmacy benefits plan, prescription coverage, etc.) subscribed to by the
patient. In
some embodiments, (depending on context, applicable laws, and/or patient
authorization) sub-block 280 may also include some or all of the information
in
sub-block 282 and/or 284 described herein. Some or all of the information in
sub-block
280 may be shared with one or more other entities and may form part of other
records/blocks associated with the transaction.
[0070] Prescription 250 may comprise one or more prescribed
drugs/devices
255-p, 1 <p <P, where P represents the number of drugs associated with a
prescription
250. Thus, in some embodiments, sub-block 280 may comprise a record for each
prescribed drug/device 255-p in prescription 250. Sub-block 280 is merely an
example
that illustrates some information that may be shared with another specific
entity. For
example, sub-block 280 may be encrypted and sent to PMDP 130 (e.g. to obtain
equivalent classes of drugs, devices, and/or treatments and corresponding cost
metrics)
and may be decryptable by PMDP 130.
[0071] In general, information in any appropriate field or combination
of fields
in a data record or block may be aggregated into a sub-block, which may be
encrypted
(e.g. by a first entity), so that the sub-block is decryptable by some other
specific entity
or entities (e.g. one or more second entities). The encrypted sub-block may be
sent (e.g.
by the first entity) to the other specified entity or entities (e.g. the one
or more second
entities), which may decrypt and act as appropriate (e.g. based on the
transaction type)
on the received information. The information that is used to form sub-blocks
from a
data record or a block in a locally maintained blockchain (e.g. an EHR
blockchain) by a
first entity and shared with another (second) entity may depend on regulations
(e.g.
healthcare and/or privacy), laws governing information sharing (e.g. which may
determine information that can or cannot be shared between specific entities),
business
guidelines (e.g. which may govern sharing/protection of trade secret or
sensitive
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
information) and/or contractual obligations (e.g. between or related to the
entities
sharing information) and/or agreements (e.g. as part of a subscription to an
electronic
transaction platform).
[0072] As another example, information in sub-block 282 may include
coverage related information 272, Plan ID 270, Co-payment information 275,
etc. Some
information in sub-block 282 may have been obtained directly from patient,
while other
coverage related information may have been obtained from and/or confirmed by
Payer
140 and/or PBM 150 and decrypted by HCP 120 (e.g. based on one or more
encrypted
sub-blocks received from the appropriate entity and decrypted by HCP 120 - not
shown
in FIG 2) in connection with a transaction (e.g. to determine a cost-effective
treatment)
specified by Transaction ID 695. For example, a transaction type may indicate
that sub-
block 282 is being provided to obtain, confirm, or complete coverage related
information 272.
[0073] Further, in some contexts, a portion of EHR information 200
such as
some portion of Basic Profile information 230, DOB 220, Blood Type 225, and
Medical
History 232 may be encrypted by HCP 120 as sub-block 284 and sent to PMDP 130,
which may then decrypt sub-block 284 in connection with a transaction (e.g. to
determine a level of payment assistance) specified by Transaction ID 695.As a
further
example, the portion of basic profile information 230 included in sub-block
284 may be
non-PII (e.g. exclude name, identification number, etc.) related to a patient.
Thus,
medical information may be shared (e.g. to determine adverse reactions,
medical device
malfunctions, etc.) with another specific entity securely without compromising
patient
privacy. In another instance, where disclosure of PII information is permitted
and
authorized (e.g. by the patient), a sub-block may include PII information
(e.g. for PMDP
130 to determine patient eligibility for payment assistance, or in relation to
a
prescription (for pharmacy 160 to establish patient identity). For example,
(depending
on context, transaction type, applicable laws, and/or patient authorizations)
some or all
of the information present in sub-blocks 282 and/or 284 may also be
incorporated into
sub-block 280.
[0074] As a further example, data in a sub-block 280 may be shared by
an entity
such as HCP 120 with another healthcare marketplace entity such as Payer 140
to
complete a transaction. However, patient profile information (e.g. family
history 205,
26
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
maternal history 210, and/or paternal history 215) associated with Basic
Profile
Information 230 may be deemed private (e.g. based on patient
instructions/privacy,
legal, and/or business guidelines) and the first entity (e.g. HCP 120) may not
share
family history 205, or may limit the portion of Basic Profile Information 230
that is
shared.
[0075] Accordingly, in some embodiments, data used to form sub-blocks
sent by
an entity or received from another entity may depend both on the entity and
the context
(e.g. type or nature of transaction) in which the data is being shared. In
some
embodiments, the informational interface between any two entities may depend
on the
transaction type and/or context. In some embodiments, one or more protocols
(e.g.
agreed to/prearranged by entities and/or set up by a permissioned blockchain
platform
and/or based on a standard) may specify transaction types and the information
to be
present in sub-blocks sent/received by an entity for each transaction type. In
some
embodiments, the data fields included in a sub-block shared between entities
in
connection with a transaction type may be indicated (e.g. by the protocol
and/or agreed
upon standard) as mandatory, conditional, optional, on request, etc.
[0076] Data in a sub-block (e.g. sub-block 280) may be separately
encrypted
and may be decryptable only by an authorized entity. In some embodiments,
encryption
of data that forms a sub-block (e.g. .sub-block 280) may be based on any
appropriate
cryptographic method, including symmetric key encryption techniques (where the
entities, such as HCP 120 and Payer 140 share a secret key) such as Advanced
Encryption Standard (AES) based techniques or variations thereof Sub-block 280
may
be encrypted (e.g. by HCP 120) prior to being shared with the other entity
(e.g. Payer
140). The other entity (e.g. Payer 140) may be able to decrypt sub-block 280,
for
example, using the shared key.
[0077] Further, the data in EHR 200 may also be separately encrypted
by HCP
120 using any secure encryption technique to form EHR block 200 prior to being
added
to a blockchain (e.g. maintained by HCP 120 and/or maintained by an electronic
transaction platform). For example, the data in EHR block 200 may be
separately
encrypted using a private key (e.g. private to HCP 120), so that it is
decryptable by and
available to HCP 120 but unavailable to and/or not viewable any other entity.
27
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
[0078] FIGs. 3A and 3B show portions of example Drug / Device
Information
Record (DIR) 300 for a drug, which may be stored in DIR database 135. In some
embodiments, DIR 300 may include information about a treatment (e.g. drug
and/or
medical device) such as a drug, device, and/or procedure. The fields shown in
DIR 300
are merely exemplary, and DIR 300 may comprise various other fields based on
laws,
standards, industry practice, etc. In addition, a DIR may comprise fields
different from
(fewer or greater than) those shown in relation to exemplary DIR 300.
[0079] DIR 300 may be associated with PMDP Profile field 395, which
may
store information pertaining to PMDP 120 (e.g. PMDP identification, contact
information, address, etc.). In some embodiments, some or all of the
information in
PMDP Profile 395 may be optionally shared with other entities in connection
with a
transaction. For example, a portion of PMDP Profile 395 may be sent to one or
more
entities such as Payer 140, PBM 150, and/or Pharmacy 160 (e.g. as part of an
encrypted
sub-block, which may be decryptable by the designated receiving entity).
[0080] Referring to FIG. 3A, PMDP 130 may use information in
prescribed
drug/device field 255-p (e.g. received as part of sub-block 280) to determine
(e.g. for
each drug/device 255-p associated with prescription code 250) a corresponding
drug/device class 337-p. Drug/Device Class 337 field may specify one or more
drug/device classes associated with drugs/devices 255. In some embodiments,
sub-block
280 may also include some or all of the information in sub-blocks 282 and/or
284.
[0081] A drug/device class may be a set of drugs/devices that may be
used to
treat the same medical condition. Drugs in a drug class may have: similar
chemical
structures, and/or similar or related mechanisms of action (e.g. may act on or
bind to the
same target), and/or related modes of action (induce similar biological or
functional
effects), and/or may be used to treat the same disease. For example, a drug
device class
may also include name brand drugs/devices and generic drugs/devices, and/or
branded
biological products and biosimilars, and/or different dosages of the same
treatment, etc.
In FIGs. 3A-3C, drugs within an individual drugs/device class 337-p are
indicated by
drug/device 302-pd, where 1 < d < D_p, where D_p is the number of drugs in
drug/device class 33'7-p.
28
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
[0082] In some embodiments, Drug/Device Class 33'7-p field may
indicate one
or more of: an organ or biological system that the drug device treats, and/or
a
therapeutic area, which may be specified by Therapeutic Area field 360-pd
comprising
repeating Indications sub-fields 362-pd], 362-pd2 . . ; repeating Dosage sub-
fields
364-pd], 364-pd2 . . . for a corresponding indication; mechanism of action
and/or mode
of action, which may be specified by MOA field 340-pd; general chemical
properties of
a drug, which may be specified by Chemical Data field 350-pd, which may
further
include sub-fields Molecular Formula field 353-pd describing the drug's
chemical
components and Chemical Structure sub-field 355-pd.
[0083] Referring to FIG. 3A, DIR 300 may comprise various other data
fields
including Drug Name(s) 304-pd for a drug/device ID 302-pd, Efficacy 327-pd
(which
may be a measure of therapeutic effect for a medical condition), Route of
Administration 335-pd (e.g. topical, oral, intravenous, etc.), Safety 330-pd
(e.g. drug
interactions, toxicity, contraindications, etc.), which may include Side
Effects sub-field
345 (e.g. secondary effects), and. Adverse Events sub-field 333-pd (e.g.
adverse events
reported for the drug). DIR 300 may also include various other fields related
to the
drug/device.
[0084] In some embodiments, Drug/Device ID 302-pd, Drug/Device Name
304-pd, Safety 330-pd (and sub-fields), and Efficacy 327-pd, Drug/Device class
337-p,
Therapeutic Area 360-p, Indications 362-pdh (e.g. Indications 362-pd],
Indications 362-
pd2, etc.), and corresponding Dosages 364-pdh (e.g. Dosage 364-pd], Dosage 364-
pd2,
etc.), may form part of sub-block 380. The information in sub-block 380 (and
any other
sub-block) may be pre-arranged between the entities, determined by protocol,
and/or
specified by the platform (e.g. based on laws, regulations, authorizations,
and/or
contractual obligations). Thus, information in sub-block 380 (and any other
sub-block)
may be more or less than that shown for an example sub-block (e.g. example sub-
block
380) and may also depend transaction type, transaction context, and the
interacting
entities. Sub-block 380 may further optionally include Route of Administration
335-pd,
MOA 340-pd, Chemical Data 350-pd, and other related fields / sub-fields. In
FIG 3A,
fields associated with example sub-block 380 are shown enclosed within a
dashed
perimeter.
29
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
[0085] DIR sub-block 380 may be provided to another entity (e.g. HCP
120) by
PMDP 130 in connection with a transaction (e.g. identified by Transaction ID
695). In
some embodiments, DIR sub-block 380 may comprise information for a plurality
of
drugs/devices that form part of Drug/Device class 337-p or share one or more
characteristics (e.g. treat the same medical condition, and/or have similar
mechanisms
or modes of action, and/or have similar chemical structures). In some
embodiments,
information in sub-block 380 may be associated with a transaction (e.g.
identified by
Transaction ID 695 and a transaction type) and may be encrypted and
transmitted by
PMDP 120 to another entity (e.g. HCP 120) during the transaction and/or prior
to
transaction finalization.
[0086] In some embodiments, a DIR record 300 may be associated with a
transaction (e.g. specified by transaction ID 695) and may be stored upon
transaction
finalization as a DIR block a DIR blockchain by an entity such as PMDP 130. In
the
description below, when DIR record 300 forms part of a DIR blockchain, then
DIR
information record 300 may also be referred to as DIR block 300. DIR block 300
may
thus form a block in a DIR blockchain.
[0087] A transaction (e.g. specified by transaction ID 695) between
two or more
entities (e.g. patient, HCP 120, PMDP 130, Payer 140, PBM 150 and/or Pharmacy
160)
may involve information (e.g. related to a drug/device) that is maintained (or
owned) by
another entity. For example, HCP 120 may request information from PMDP 130.
Prior
to transaction confirmation, some of the data (e.g. maintained by PMDP 120 in
a DIR
information block 300), which is being integrated into records maintained by
other
entities (i.e. other the PMDP 130) may depend on input from, validation by,
and/or
confirmation by PMDP 130. Conversely, PMDP 130 may receive input from another
entity (e.g. HCP 120 and/or Payer 140 and/or PBM 150) in the form of sub-
blocks.
Some or all of the received information may be incorporated into DIR record
300 and/or
used to determine information in DIR record 300.
[0088] For example, HCP 120 may send sub-block 280 to PMDP 130. PMDP
130 may use information in sub-block 280 (e.g. a Prescribed Drug 255-p) to
determine a
corresponding Drug/Device class 33'7-p and obtain the information in sub-block
380,
which may be sent to Payer 140 and/or PBM 150. In addition, PMDP 130 may store
some of the information in a current (e.g. last received) sub-block 280 prior
to
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
transaction finalization as part of DIR record 300. For example, information
pertaining
to the diagnosis (e.g. Diagnosis code 240), dosage (e.g. Dose 260) etc. may be
stored as
part DIR record 300 and associated with corresponding Drug/Device IDs 302-p
upon
confirmation (e.g. from HCP 120) that the drug in Drug/Device ID field is
being
prescribed (e.g. as may be indicated by the value of Prescribed Drug field 255
in
sub-block 280 at the time of transaction confirmation).
[0089] Accordingly, when information (e.g. related to the drug/device)
pertinent
to a transaction is requested, the owner (e.g. PMDP 130) may respond by
sending the
information (e.g. .in sub-block 380) to the requesting entity (e.g. HCP 120).
The
information (e.g. in sub-block 380) may be encrypted (e.g. by PMDP 130) prior
to
sending and may be decryptable by the requesting entity (e.g. HCP 120) so that
the
information in sub-block 380 is private between the requesting (HCP 120) and
sending
(PMDP 130) entities. In addition, information in the sub-blocks exchanged
between
entities may be restricted so that the information shared is compliant with
existing
regulations, privacy laws, contractual obligations, and/or authorizations
received from a
designated owner of the information (e.g. a patient).
[0090] FIG. 3B shows example DIR record 300 with some additional
information that may be included in DIR record 300 in connection with a
transaction. In
FIG. 3B, sub-blocks 280 and 380 are shown by blocks with dashed outlines. For
simplicity and ease of description, information in sub-blocks 280 and 380 is
not shown.
As outlined previously, some or all of the information in sub-block 280 may
form part
of DIR record 300.
[0091] As shown in FIG. 3B, DIR record 300 may further include
Formulary
405, which may include a list of approved prescription drugs (e.g. generic and
brand
name) related to a therapeutic class. The drug formulary may be used by to
identify
drugs and/or devices covered by a drug plan and/or an insurance plan. The
formulary
may be updated periodically and/or published. For example, laws, regulators,
health
insurance exchanges, and/or private employers may specify that formularies for
available / approved insurance plans be published and updated at specified
times.
Accordingly, in some instances, formulary information for various plans may be
publicly available, and/or available to plan subscribers (e.g. an organization
subscribing
31
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
to the plan on behalf of its members/employees) and/or available from other
entities
(e.g. regulators, health exchanges, etc.).
[0092] Formularies may distinguish between products by dividing
treatments
into "tiers." Each tier may have a different level of patient cost sharing.
For example, for
drugs/devices in a preferred tiers, patient cost sharing may be $0 or limited
to
co-payments specified in the patient's insurance plan (subject to
deductibles).
Drugs/devices in non-preferred tiers may be subject to additional patient cost
sharing,
often referred to as "coinsurance." For example, a patient prescribed a non-
preferred
drug may pay some percentage of the drug price (e.g. 30%) as coinsurance in
addition
to copayments. The amount of coinsurance may depend on the tier. Similar
"tiers" may
also apply to devices and/or other medical treatments.
[0093] Accordingly, Formulary 405 may include repeating Payer-i field
410-i,
(1 < i < n) each with information about a corresponding payer i. Further,
Formulary 405
may include, for each Payer-i 410-i, information about corresponding drug
tiers, which
may appear in repeating Tier-j field 410-j, (1 <j < T i) , where T i is the
number of tiers
associated with Payer-i. The tier associated with a drug may depend on the
indication
(e.g. as listed in Indication field 420-k) for which the drug is being used
and Payer-i
410-i. For example, Formulary 405 in DIR record 300 for a drug may specify
(e.g. for
Payer 1 410-1 and some Indication 320-k) that a first generic drug is a Tier 1
415-1,
while a more expensive second generic drug is a Tier 2 415-2 drug, and a brand
name
drug is a Tier 3 415-3 drug. Further, each Tier-j field 410-j may include
repeating
Indication-k field 420-k, (1 < k < s). The Indication-k field 420-k under a
tier may
specify medical conditions (indications) for which the formulary has been
approved
(e.g. by Payer 1 410-1 and/or PBM 150) as treatment.
[0094] Thus, patient out-of-pocket costs (e.g. coinsurance) may vary
depending
on one or more of: medical treatment guidelines for a medical condition;
available
treatment options; the treatment regimen ultimately selected by a patient or
HCP 120;
the Tier 415 associated with the selected treatment; the Indication 420 for
the selected
treatment; and Payer 140 and/or PBM 150. However, such out-of-pocket cost
information is often difficult to obtain or unavailable to the patient and/or
HCP 120 at
the time of prescription, which can result in higher ongoing costs to the
patient (e.g. if
cheaper treatment options are available), and/or may result additional
transactions such
32
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
as a prescription/treatment change, and/ or a revisit of the treatment
decision by HCP
120 and the patient. Further, when viewed in aggregate, the sub-optimal
decisions
and/or transactional overhead detrimentally impact overall systemic costs and
efficiencies.
100951 As shown in FIG. 3B, DIR 300 may comprise various other data
fields
including Price 425-pd, which may list a price at which a treatment (e.g. drug
or device)
is being made available. Price 425-pd may be a Payer 140 or PBM 150 provided
estimate of patient cost (as seen by Payer 140 / PBM 150 based on current
patient
coverage information). Patient cost (as estimated by Payer 140 / PBM 150) may
be a
function of any unmet deductible, patient copay, coinsurance (e.g. based on
Tier 415),
cost share (e.g. from Payer 140), and the negotiated price of the drug/device
(e.g.
between Payer 140/PBM 150 and PMDP 120/Pharmacy 160). As outlined above, for a
specific payer and treatment, Tier 415 may depend on indication 420 associated
with the
treatment. As another example, Price 425-pd may reflect the negotiated price
(e.g. with
PMDP 120 and/or Pharmacy 160 and/or another entity), and additional cost
breakdown
(.g. copay 421, deductible 423, information may be provided in relation to
patient out-
of-pocket cost.
[0096] In some situations, formulary 405 (including sub-fields) and
related
information may be obtained (e.g. from Payer 140 and/or PBM 150 and/or another
entity such as a health exchange) as formulary sub-block 480. In some
embodiments, a
portion of sub-block 480 such as Price 425, Co-pay 421, Deductible 423, and
Coinsurance 429 may be obtained (e.g. from Payer 140 and/or PBM 150) along
with
updated formulary 405 during a transaction and/or prior to transaction
finalization.
Thus, information in sub-block 480 may be current (or made current/updated) at
transaction time. Data fields Price 425, Co-pay 421, Deductible 423, and
Coinsurance
429 (e.g. which may be part of one or more sub-blocks 480) may be provided for
corresponding drugs/devices 302 based on coverage related information 272
and/or plan
ID 270 received from HCP 120 (e.g. as part of sub-block 280).
[0097] In some instances (e.g. when sub-block 280 does not include
coverage
related information 272 and/or plan ID 270), Payer 140 and/or PBM 150 and
Payer 140
and/or PBM 150 may determine Price 425, Co-pay 421, Deductible 423, and
Coinsurance 429 and provide the information to PMDP 130 in sub-block 480. For
33
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
example, Payer 140 and/or PBM 150 may determine Price 425, Co-pay 421,
Deductible
423, and Coinsurance 429, and/or other information in sub-block 480 by using
transaction ID 695 and transaction type to correlate the request for
information from
PMDP 120 with prior information (e.g. in sub-blocks 280 and/or 284) received
from
HCP 120.
[0098] In some embodiments, PMDP 130 may use information in block 480
including Price 425-pd, Co-pay 421-pd, Deductible 423-pd, and Coinsurance 429-
pd to
determine Payment Assistance 391-pd for a drug/device (e.g. specified by
Drug/Device
ID field 302-pd). PMDPs 130 may often provide Payment Assistance 391--pd (e.g.
for a
drug/device manufactured and/or supplied by PMDP 130) to increase
affordability
and/or to offset patient out-of-pocket costs. The payment assistance provided
by PMDP
130 (or on behalf of PMDP 130) may be based on various patient eligibility
criteria.
Accordingly, an updated patient cost breakdown may be provided along with Cost
Metrics 395 for the prescription. The updated patient cost breakdown may
include for a
corresponding drug/device 302-pd: (a) a patient out of pocket cost Out of
Pocket
392-pd; (b) Payment Assistance 391-pd, (c) Pharmacy ID 3944 at which the out
of
pocket cost in (a) above is applicable, and (d) one or more other optional
fields.
[0099] In some instances (e.g. when a patient wants to avail of
payment
assistance), upon patient authorization, HCP 120 may provide PII information
for a
patient along with coverage information (e.g. in sub-block 280), which may be
correlated with patient insurance plan information provided by Payer 140
and/or PBM
150 (e.g. in sub-block 480) and patient application information (e.g. which
may have
been separately provided by the patient to PMDP 130 and/or affiliated entities
acting on
behalf of PMDP 130) to determine eligibility and Payment Assistance 391--pd
for a
drug/device. Since prescription 250 may comprise multiple drugs/devices,
Payment
Assistance 391-pd may be determined for each corresponding Drug/Device 302-pd
in
prescription 250 for which payment assistance is applicable.
[00100] In some embodiments, a PMDP 130 and/or another entity, and/or a
user
(e.g. patient) application (e.g. running on a mobile device such as a smart
phone,
handheld computing device, tablet, etc.) may obtain payment assistance
information
from a plurality of PMDPs 130 to determine cost information 395 associated
with each
drug/device 302 in a prescription identified by Prescription code 250. For
example, the
34
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
application may aggregate any Payment Assistance 391-pd received from one or
more
PMDPs 130 (or entities affiliated with the one or more PMDPs 130) to determine
cost
metrics 395. In instances where an entity payment assistance programs may be
coordinated and/or administered by an entity other than PMDP 130 (i.e. by an
affiliate,
a patient access program provider/coordinator, community agency, non-profit,
government, or other entity), then payment assistance information may be
provided (e.g.
via the application on the patient's mobile computing device) by the
administering entity
based on information received from each PMDP 130 (e.g. as reflected in sub-
block
390). In some embodiments, the application on the patient's mobile computing
device
may be authorized by an entity (e.g. HCP 120 and/or a patient access program
coordinator and/or a Health Exchange and/or PMDP 130) associated with the
permissioned blockchain platform and may communicate with the entity
authorizing/providing the application via a secure channel to exchange
information with
entities associated with the permissioned blockchain platform.
[00101] Cost information 395 may be based on Patient Criteria 297,
which may
specify criteria or preferences used to determine cost information 395 (such
as locations
where the prescription will be filled, preferred pharmacies, etc.). Cost
metrics 395
maybe at a drug level indicated by 395_pd (e.g. cost breakdowns for a specific
drug/device 302-pd; lowest cost pharmacy at which a specific drug/device 302-
pd may
be obtained, lowest cost drug 302-pd in a class 33'7-p over a specified period
such as the
duration of treatment and/or the duration of coverage), and/or at a
prescription level
indicated by 395_p (e.g. lowest cost to fill a prescription when one or more
specific
drugs/devices 302 are selected; sets of drugs/devices 302, which, if selected,
would
provide the lowest cost to fill the prescription; set of drugs/devices 302
that would
provide the lowest cost over the duration of treatment for the prescription;
set of
drugs/devices 302 that would provide the lowest cost over a specified period
such as the
current insurance/benefits coverage period, etc.)
[00102] FIG. 3C shows an example data flow from an initial proposed
prescription 250, (which may be part of a first EHR sub-block 280) to
alternatives (e.g.
in DIR sub-blocks 380 and/or 390) that may be presented to a patient/HCP 120
along
with cost information 395 to facilitate treatment selection.
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
[00103] As outlined above, in some embodiments, information pertaining
to
initial proposed prescription 250 may be obtained (e.g. by PMDP 130 and/or
another
entity) via a first EHR sub-block 280. EHR sub-block 280 may also include
other
information (e.g. patient medical coverage information). As shown in FIG 3C,
example
proposed prescription 250 may comprise a plurality of first treatments (e.g.
proposed
drugs/devices 255-1, (1 < i < N). Sub-block 280 may be encrypted and
decryptable by
PMDP 130 and/or another authorized entity.
[00104] In some embodiments, a drug/device class 33'7-p (1 <p < P) may
be
determined corresponding to one or more drugs/devices 255-p, (1 <p < P) in
proposed
prescription 250. As shown in FIG. 3C, drugs/devices 302_pd (1 < d<D_p, where
D_p
is the number of selected drugs/devices in class p) corresponding to each
drug/device
class 337-p may be determined.
[00105] For each drug/device 302_pd in a drug/device class 337-p,
patient out of
pocket cost 392_pd, Payment Assistance 391_pd, Cost Breakdown 397_pd, and
Pharmacy information (e.g. Pharmacy ID 394_i at which the patient Out of
Pocket cost
392_pd is applicable, etc.), and Cost information 395 pd (at the level of drug
302_pd)
may be determined. Cost information 395 pd may be determined based on patient
coverage information and/or information received from PBM 150 and/or Payer 140
and/or another entity. In some embodiments, Pharmacy ID 394i may be determined
based on patient criteria 297 (e.g. provided in block 280). The Pharmacy ID
394_i may
include and/or be used to look up pharmacy related information. In some
embodiments,
the information above may be provided to HCP 120, a patient, and/or another
entity. For
example, the information above may be encrypted in sub-block 390 and may be
decrypted and read by an authorized receiving entity. As one example, HCP 120
and/or
a patient may receive the above information in an application running on a
smart phone
or other mobile computing device.
[00106] In some embodiments, the cost information for drugs/devices
302_pd
may be aggregated based on based on one or more user (e.g. patient) specified
criteria to
determine cost metrics 395_p (at the level of prescription 255_p). As one
example, the
(potential) cost information 395-p at the prescription level may be based, for
example,
by assuming that a drug/device 302-pd and/or one or more drugs/devices 302 may
be
selected.
36
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
[00107] As another example, the cost information associated with a
potential
equivalent prescription may be analyzed to determine cost metrics 395_p such
as the
lowest total cost to satisfy the potentially equivalent prescription at a
single pharmacy
within some threshold distance of a location (e.g. current or provided
location). As a
further example, the cost information associated with a potential equivalent
prescription
may be analyzed to determine cost metrics 395_p such as the lowest total cost
for the
potential prescription within some threshold distance of a location (e.g. a
current
location or provided location such as a zip code or address) without regard to
the
number of pharmacies used to fulfill the prescription. As an additional
example, the cost
information associated with a potentially equivalent prescription may be
analyzed to
determine cost metrics 395_p so that the total cost of the prescription within
some
threshold distance of a location (e.g. a current location or provided location
such as a
zip code or address) is within some user-specified threshold of the lowest
cost
prescription while limiting the number of pharmacies used fulfill the
prescription. As
another example, the cost information associated with a prescription may be
analyzed to
determine cost metrics 395_p such as the total cost of treatment over an
expected
treatment duration and/or over some specified time period (e.g. for a current
or
remaining insurance term). Patient criteria 297 may also specify other
considerations
such as a preferred pharmacy, mail order options, etc., which may be used when
determining cost-metrics, out of pocket costs, etc. For example, if a user
specifies that
mail order is acceptable, then sub-block may include costs associated with
mail order
pharmacies in addition to pharmacies 160 within a region.
[00108] When DIR block 300 is to be added to a DIR blockchain, some of
the
data in a DIR information block 300 may depend on input from, validation by,
and/or
confirmation by other entities. For example, information in formulary 405
related to a
payer (e.g. payer 140 specified in Payer i 410-i) may form part of DIR block
300 and
may depend on validation by the payer (e.g. Payer 140). DIR block 300 may be
added
to the DIR blockchain once validation has been provided and a transaction
confirmation
has been received. The validation may be obtained in the form of a sub-block
(e.g. sub-
block 480) from Payer 140. The information in sub-block 480 may be specific to
the
transaction (e.g. as identified by transaction ID 695), encrypted by Payer
140, and may
be decryptable by PMDP 120. In some instances, encrypted sub-block 480 (or
37
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
information present in sub-block 480) may be intended specifically for PMDP
120 and
may not be decryptable by an entity other than PMDP 120.
[00109] In some embodiments, for a transaction at a point in time,
information in
data fields Formulary 405, Payer 1 140-1, tier information in each of the Tier-
j fields
415-j associated with the payer in Payer 1 140-1, information in each of the
Indication-k
fields 410-k associated with each Tier-j field may be part of a sub-block 480
received
from Payer 1 140-1. However, information related to any other payers may not
form
part of sub-block 480 because these payers may not be party to the
transaction. Thus, in
transactions involving a single payer (e.g. Payer 1 140-1), DIR record 300 may
include
information (e.g. in sub-block 480) for that specific payer.
[00110] When multiple payers are involved in a transaction, then
multiple
encrypted sub-blocks (e.g. each from a corresponding payer 140-i) may be
received by
PMDP 130 and integrated by PMDP 130 into DIR record 300. The information in
each
sub-block received from Payer-i may be private between PMDP 130 and the
corresponding Payer-i. Sub-block 480 is merely an example that illustrates
some
information that may be shared with another specific entity. In general, the
information
used to form sub-blocks from a data record or block in a locally maintained
blockchain
(e.g. DIR 300) may depend on regulations (e.g. healthcare and/or privacy),
laws
governing information sharing (e.g. determining information that can or cannot
be
shared by entities), business guidelines (e.g. trade secret or sensitive
information) and/or
contractual obligations (e.g. between or related to the entities sharing
information).
[00111] Accordingly, in some embodiments, data in a sub-block sent to
PMDP
130 may be separately encrypted by the corresponding sending entity (e.g.
Payer-i
140-i) and may be decryptable by PMDP 130 and unavailable to unauthorized
entities.
In some embodiments, encryption of data in a sub-block (e.g. received by PMDP
130)
may be based on any appropriate encryption technique including symmetric key
cryptography. The encryption may be based, for example, on a secret key shared
between the entities (e.g. Payer 140-i, identified in Payer 1 140-1 field and
PMDP 130).
The receiving entity (e.g. PMDP 130) may be able to decrypt the received sub-
block,
for example, based on the secret shared key.
38
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
[00112] Further, prior to being added to a blockchain, data in DIR
block 300 may
also be separately encrypted by a first entity (e.g. PMDP 130) using any
secure
encryption technique, so that it is decryptable by and available to the first
entity (e.g.
PMDP 130) but is unavailable and cannot be viewed by other entities (e.g. HCP
120
and/or Payer 140).
[00113] FIG. 4 shows a portion of exemplary Pharmacy Benefits Record
(PBR)
400, which may be maintained by PBM 150. The term PBM is used to refer to any
entity that serves to administer a portion of benefits (e.g. pharmaceutical
and/or medical
device prescriptions) on behalf of a payer (e.g. Payer 140). In some
instances, PBMs
may be affiliated with insurers/payers. In some instances, insurers/payers may
administer drug/device benefits directly so that payer 140 and PBM 150 may be
viewed
as a single entity and portions of the description below may also be
applicable to the
single (Payer+PBM) entity.
[00114] PBR 400 may include information pertaining to payers and
associated
benefit plans, available benefits, patient/subscriber information, payer
drug/device tiers,
cost sharing, etc. and may be owned and maintained by PBM 150 (e.g. in PBI
database
155). In some embodiments, data fields in PBR 400 may be populated and/or
updated
by PBM 150 based, in part, on information received from patient/HCP 120 and/or
Payer
140 and/or other entities. PBR 400 may also include data received (e.g. as sub-
blocks)
from other entities. The fields shown in PBR 400 are merely exemplary, and PBR
400
may comprise various other additional fields based on laws, standards, PBM
practice,
and/or industry practice, etc. A PBR may comprise fields different from (fewer
or
greater than) those shown in relation to exemplary PBR 400.
[00115] PBMs may determine drug tiers for prescribed drugs, which may
determine coinsurance amounts. PBMs may also set or determine retail prices
for
pharmaceutical products, obtain payments or rebates from manufacturers based
on the
sales volumes of a particular product or a basket of products, and, in some
instances,
may also obtain post-sale price concessions and payments from pharmacies on
formularies. Accordingly, PBR 400 may include Sales Information field 497,
which
may store transaction and other sales information for each drug. PBR 400 may
also
include various other fields (not shown in FIG. 4) to track aggregate payer-
specific and
pharmacy-specific sales volumes for drugs/devices, discount/rebate
information, etc.
39
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
PBM 150 may exchange information (e.g. via encrypted sub-blocks with HCP 120,
Payer 140 and/or Pharmacy 160 and/or PMDP 130).
[00116] In some embodiments, PBR 400 may be associated with PBM Profile
field 495, which may store information pertaining to PBM 150 (e.g. PBM
identification,
contact information, address, etc.). In some embodiments, some or all of the
information
in PBM Profile 495 may be optionally shared with other entities in connection
with a
transaction. For example, a portion of PBM Profile 495 may be sent to one or
more
entities such as PMDP 130, Payer 140, and/or Pharmacy 160 (e.g. as part of an
encrypted sub-block, which may be decryptable by the designated receiving
entity).
[00117] As shown in FIG. 4, PBR 400 may include a portion the
information in
sub-block 280 (shown enclosed in a dashed boundary). In some embodiments, some
the
information in sub-block 280 may be received from HCP120 and updated and/or
validated by PBM 150. In some embodiments, PBM 150 may receive some or all of
the
information in sub-block 280 (e.g. indirectly) from PMDP 130. In some
embodiments,
sub-block 280 may include some or all of the information in sub-blocks 284
and/or 284.
[00118] Accordingly, as shown in FIG. 4, in some embodiments, the one
or more
encrypted sub-blocks 280, may include coverage related information 272 (e.g.
based on
information initially obtained by HCP 120), diagnosis 235, diagnosis code 240,
indication 242, diagnosis code 240, treatment code 245, and prescription code
250.
Further, PBR 400 may also include one or more of: actual prescribed
drugs/devices
255-ps (e.g. corresponding to selected drug/devices 302-pd from drug/device
classes
33'7-p), dose 260-ps, and/or duration 265-ps along with transaction ID 695 to
PBM 150,
where 1 < p < P, and for each drug/device class p, 1 < s < D_p. Prescription
code in sub-
block 280 and associated prescribed drugs/devices 255-ps, dose 260-ps, and/or
duration
265-ps , etc. may represent a selection of drugs/device (e.g. selected by a
patient and/or
prescribed by HCP 120) after evaluation of the drugs/devices 302-pd. For
example,
HCP 120 and/or a patient (in consultation with HCP 120) may select one
drug/device
302-ps 255-ps from each drug/device class 337-p and the selected drugs/devices
255-ps may be associated with a final/selected prescription 250-s. PBM 150 may
incorporate some or all of the information in sub-block 280 into PBR 400 (e.g.
at the
time of transaction confirmation/finalization). Transaction ID 695, which may
uniquely
identify transactions between entities, may facilitate information exchange
and
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
coordination between entities. For example, a unique transaction ID 695 shared
between
entities that are parties to a transaction may be used to correlate,
aggregate, validate, and
process information received from one or more entities with locally stored
information
and/or with information received from other entities. In some embodiments, the
finalized prescription 250-s (e.g. in a second sub-block 280 from HCP 120) may
be sent
subsequent to DIR sub-blocks 380 (with drugs/devices 302-pd).
[00119] PBR 400 may also include a portion the information in sub-block
380,
which may be received from PMDP 130 along with transaction ID 695. For
example, in
some embodiments, PMDP 130 may send (e.g. in one or more encrypted sub-blocks
380) drug/devices 302-pd corresponding to drug/device classes 337-p (where
each
drug/device class 337-p may be associated with at least one prescribed drug
255-p (e.g.
in an initial prescription 250 sent to PMDP 130). PBR 400 may further include
drug/device Name 304-pd (which may be received as part of sub-block 380)
corresponding to drugs/devices 302-pd. PBM 150 may process the information in
sub-
block 380 and return a list of approved drugs, tiers, costs, etc. (e.g. in sub-
block 480)
[00120] In some embodiments, Transaction ID 695 may be used by PBM 150
to
determine that sub-blocks 282 and 380 are associated with the same
transaction. PDM
150 may then use coverage related information 272 in sub-block 280 to
determine the
information (e.g. Formulary, Triers, etc.) in in sub-block 480. In some
embodiments,
sub-block 380 may also include Plan ID 270 and Coverage related information
272 (e.g.
when provided to PMDP 120), which may be used by PBM 150 determine information
in sub-block 480. For example, PBM 150 may use coverage related information
272
(for a patient and a payer 140-i) along with Additional Patient Coverage
information
495 (which may be locally maintained and/or obtained from Payer i 410-i) to
determine
information in sub-block 480.
[00121] As shown in FIG. 4, sub-block 280 in PBR 400 may include for
Payer
140-i (e.g. determined based on coverage information 272 and/or Plan ID 270)
and
drugs/devices 302-pd (e.g. determined based on sub-block 380): formulary 405,
including tier information 415-j, and indications 420-k. In some embodiments,
sub-
block 480 may further include one or more of: diagnosis 235, diagnosis code
240,
indication 242, treatment code 245, prescription code 250, prescribed
drugs/devices
255, corresponding doses 260, and/or durations 265 (e.g. determined based on
sub-
41
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
block 280). PBR 400 may also include transaction ID 695. PBM 150 may
incorporate
some portion of the information in sub-blocks 280 and/or 380 into PBR 400
(e.g. at the
time of transaction confirmation/finalization).
[00122] In some embodiments, PBM 150 may further include (e.g. as part
of
sub-block 480) various other data fields including Price 425, which may list a
price at
which a treatment (e.g. drug or device 302-pd) is being made available (per
plan, tier,
and indication information). Price 425-pd may be a Payer 140 or PBM 150
provided
estimate of patient cost (as seen by Payer 140 / PBM 150 for a drug/device 302-
pd, or
the negotiated price (e.g. as negotiated with PMDP 120 and/or Pharmacy 160
and/or
another entity). In some embodiments, an additional cost breakdown (.g. copay
421-pd,
deductible 423-pd, coinsurance 427-pd, patient out-of-pocket cost for the
drug/device
302-pd may be provided as part of sub-block 480.
[00123] As outlined above, information in sub-blocks received or sent
by PBM
150 may be encrypted. The received sub-blocks may be decrypted by PBM 150,
when
intended for PBM 150, while sub-blocks transmitted by PBM 150 may be
decryptable
by the intended recipient.
[00124] FIG. 5 shows example Pharmacy Prescription Record (PPR) 500,
which
may be maintained by an entity such as Pharmacy 160. Pharmacies refer to any
entity
(physical, virtual, or mail order) that fulfills prescriptions written by a
medical provider
such as HCP 120. Pharmacies may receive prescriptions from HCPs 120, validate
patient coverage information with Payers 140 and/or PBMs 150, and interact
with
patients to fulfill prescriptions and receive payment from the patient (e.g.
based on
coverage by collecting one or more of: negotiated prices for drugs/devices,
copayments,
deductibles, coinsuranceõ etc.) in accordance with any contract and/or payment
arrangements with Payer 140 / PBM 150.
[00125] In some embodiments, PPR 500 may be owned and maintained by
Pharmacy 160 (e.g. in PPI database 165). In some embodiments, data fields in
PPR 500
may be populated and/or updated by Pharmacy 160 based, in part, on information
received from patient/HCP 120 and/or Payer 140 and/or other entities. PPR 500
may
also include data received (e.g. as sub-blocks) from other entities. The
fields shown in
PPR 500 are merely exemplary, and PPR 500 may comprise various other
additional
42
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
fields based on laws, standards, pharmacy practice, and/or industry practice,
etc. A PPR
may comprise fields different from (fewer or greater than) those shown in
relation to
exemplary PPR 500.
[00126] In some embodiments, PPR 500 may include Pharmacy Profile field
595,
which may store information pertaining to pharmacy 160 (e.g. pharmacy
identification,
contact information, address, etc.). In some embodiments, some or all of the
information
in pharmacy profile 595 may be optionally shared with other entities in
connection with
a transaction. For example, a portion of pharmacy profile 595 may be sent to
one or
more entities such as PMDP 130, Payer 140, and/or Pharmacy 160 (e.g. as part
of an
encrypted sub-block, which may be decryptable by the designated receiving
entity)
and/or shared with a patient.
[00127] In some embodiments, PPR 500 may include Patient Information
field
510, which may include sub-fields Patient Name 204, DOB 220, Patient Coverage
Information 572, PBM Information 582, and Patient Copayment 421-pd. PPR 500
may
also include Prescription Code 250 for a prescription 255-p, HCP profile 295
(e.g. a
health care provider ID, registration number, contact information etc.)
associated with
the prescribing entity for prescription 255-p, prescribed drugs/devices 255-ps
associated
with prescription 255-p, duration 265-ps, and dosage 260-ps. In some
embodiments,
PPR 500 may include various other fields (not shown in FIG. 5) such as
prescription
date(s), fulfillment status (e.g. whether ready for pickup, picked up and/or
delivered to
patient, etc.).
[00128] In some embodiments, Patient Information 510 (including sub-
fields
Patient Name 204, DOB 206, portions of Patient Coverage Information 572,
Prescription Code 250, Prescribed drugs/devices 255-ps, duration, dosage, and
HCP
profile information may be received by Pharmacy 160 from HCP 120 as encrypted
sub-block 290 decryptable by Pharmacy 160 upon finalization of a prescription.
For
example, HCP 120 and/or patient may select one drug/device 255-ps form each
drug/device class 337-p, and provide the selected drugs/devices 255-ps to
pharmacy 160
as part of sub-block 290.
[00129] In some embodiments, upon receiving and decrypting sub-block
290,
Pharmacy 160 may use patient coverage related information (e.g. coverage
related
43
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
information 272, which may be received as part of sub-block 290) to determine
a PBM
and/or Payer associated with the prescription.
[00130] In some embodiments, pharmacy may send some or all of the
information in sub-block 290 to Payer 140 and/or PBM 150 (e.g. in accordance
with any
prevailing regulations). The information sent by Pharmacy 160 to Payer 140
and/or
PBM 150 may be in the form of an encrypted sub-block (not shown in FIG. 5)
decryptable by the designated entity (e.g. Payer 140 and/or PBM 150). In
response,
Pharmacy 160 may receive and decrypt encrypted sub-block 490 from Payer 140
and/or
PBM 150. Sub-block 490 may include validation information (e.g. in relation to
patient
coverage), which may be used by Pharmacy 160 to validate and update patient
coverage
information 572, and to update PBM information 582. In some embodiments, PBM
150
and/or Payer 140 may further include co-payment information 421-ps for
prescribed
drugs/devices 255-ps associated with prescription 250-s.
[00131] In some embodiments, PPR 500 may further include Transaction
information 515, which may store information pertaining the to the transaction
such as
patient cost 515 (such as coinsurance 421-ps - as seen by Pharmacy 160), Payer
cost
525 (e.g. amount billed/to be billed to Payer 140 and/or PBM 150), and modes
of
payment (e.g. used by the patient). For example, in some instances, where PMDP
120
provides patient assistance (e.g. as part of a patient assistance program)
with
co-payments (or other out-of-pocket patient costs), PMDP 120 (or an entity
acting on
behalf of PMDP 120) may provide a discount card, discount code, or prepaid
card,
which may be presented to pharmacy 160 as payment and recorded in mode of
payment
field 530.
[00132] FIG. 6 shows an exemplary Health Transaction record (HTR) 600.
As
shown in FIG. 6, HTR 600 may include treatment and cost related information
for a
transaction associated with a patient. The fields shown in HTR 600 are merely
exemplary, and HTR 600 may comprise various other fields based on laws,
standards,
industry practice, etc. In addition, an HTR may comprise fields different from
(fewer or
greater than) those shown in relation to exemplary HTR 600. In some
embodiments,
HTR 600 may be owned and maintained by entity such as Payer 140 (e.g. health
insurer
and form part of Transaction Information database 147), which may be
responsible for
44
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
transaction approval and/or payments to one or more entities associated with a
transaction.
1001331 HTR 600 may comprise various data fields including information
about
entities associated with and/or authorized to transact with Payer 140
including Patient
profile 195, HCP profile 295, PMDP profile 395, PBM profile 495, and Pharmacy
profile 595. The stored profiles may include information to effectuate
transactions
between the entities (e.g. payments, rebates, validations of coverage,
authorizations for
treatments/prescriptions, etc.)
[00134] HTR 600 may include Cost information 605, which may include
Payer's
view of costs associated with the transaction. For example, Cost information
605 may
include Payer cost 610, which may record the net cost to Payer 140 for the
transaction.
Payer cost 610 may be a function of one or more of: PBM cost 612, Pharmacy
Cost 615
(e.g. price charged by Pharmacy 160 to Payer 140), PMDP Cost 620 (e.g.
negotiated
price between Payer 140 and PMDP 120), rebates 622 (e.g. received by Payer 140
from
PMDP 120), HCP treatment cost 625 (e.g. cost for treatment provided by HCP 120
related to the diagnosis), and Patient cost 630 (e.g. patient out of pocket
costs 635 as
seen by Payer 140 under the plan, which may include patient copayments 632 for
the
treatment and prescription, deductibles 637, and coinsurance 639). Some of the
information associated with Cost information 605 may be available to Payer 140
(e.g.
based on contracts etc.), while some cost information 605 may be provided by
other
entities (e.g. via encrypted sub-blocks decryptable by Payer 140) prior to
transaction
finalization. For example, PBM cost information 612 may be determined from by
decrypting information included in an encrypted sub-block 480, which may be
sent by
PBM 150 to Payer 140.
[00135] As another example, HCP 120 may send encrypted sub-block 282
with
coverage related information 272, Plan ID 270 and/or HCP profile 295 to Payer
140.
Payer 140 may decrypt the information sub-block 282 and validate patient
coverage
using additional patient coverage information 698. Validation of patient
coverage may
be provided to HCP 120 by sending to HCP 120 an encrypted sub-block,
decryptable by
HCP 120, with the validation information. Some or all of Additional Patient
Coverage
Information 698 may also be sent (e.g. as an encrypted sub-block, decryptable
by PBM
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
150) to PBM 150 to facilitate validation of patient coverage (e.g. in relation
to
prescriptions) by PBM 150.
[00136] As a further example, encrypted sub-block 280 (which, in some
instances, may include information in sub-block 282) may be sent by HCP 120
(e.g.
once a prescription has been finalized) to Payer 140 for validation and
transaction
approval. In some embodiments, encrypted sub-block 280 may be decrypted by
Payer
140 and Payer 140 may approve the transaction by sending one or more
confirmation
messages (e.g. to HCP 120 and/or to other entities).
[00137] In some embodiments, upon transaction finalization, EHR 600 may
be
stored as part of a local blockchain maintained by and accessible to Payer
140. EHR
600, in encrypted form (and decryptable by Payer 140) may also form part of a
multi-
dimensional block in a multi-dimensional block. Information not in the EHR
block 600
of the multi-dimensional blockchain may be encrypted by other entities and may
not be
decryptable by Payer 140. Conversely, EHR block 600 in the multi-dimensional
block
may not be decryptable by other entities associated with Payer 140 and/or the
platform.
Thus, while each entity has a consistent view of the transaction, which is
recorded as a
multi-dimensional block in a multi-dimensional blockchain, an entity cannot
view
information in blocks (stored as part of a multi-dimensional block) that are
owned by
other entities. Accordingly, information in a block (stored as part of a multi-
dimensional
block) owned a first entity is securely shielded from view by other second
entities.
[00138] FIG. 7A shows a flow diagram illustrating process flow 700 to
facilitate
healthcare cost determination, healthcare information security and facilitate
interoperability between a plurality of entities. In some embodiments,
portions of
process flow 700 may occur on a permissioned blockchain platform, which may be
made available to subscribing and/or authorized entities. In some embodiments,
some or
all of flow 700 may be implemented using applications running on computing
devices
associated with the entities. In flow diagram 700 some routine messages (e.g.
patient
coverage validation, etc.) have not been shown for ease of description.
[00139] As one example, patient 170 may use an application running on a
mobile
computing device (e.g. smartphone, tablet, laptop, etc.) to initiate a
transaction. In some
embodiments, the application may be provided and/or authorized by an entity
associated
46
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
with the permissioned blockchain platform. For example, the application (e.g.
running
on a mobile computing device associated with patient 170) may have been
provided by
a first entity (e.g. HCP 120, PMDP 130, Health Exchange, and/or a patient
access
program (not shown in FIG. 7)), and use an Application Programming Interface
(API)
and/or other network and communication protocols to communicate with the first
entity.
[00140] In some embodiments, at 705, a patient 170 may provide profile
and
coverage information to one or more of HCP 120, Pharmacy 160, and optionally
to
PMDP 130, and/or one or more other entities. For example, patient 170 may
provide (or
may have previously provided) patient profile and coverage related information
to HCP
120 at or prior to the time of obtaining treatment, and/or to Pharmacy 160 at
or prior to
the time of fulfilling a prescription. In some instances, Patient 170 may have
provided
patient profile and coverage information to PMDP 130 to participate in and/or
to obtain
payment assistance. As outlined above, patient profile and coverage
information may be
provided (or have been) using an application on a mobile device that interacts
with
and/or is associated with an entity authorized to transact on the permissioned
blockchain
platform.
[00141] In some embodiments, HCP 120 may provide coverage related
information 272 and Plan ID 270 to Payer 140 and/or PBM 150 in encrypted sub-
blocks
282 (not shown in FIG. 7A), which may be decryptable by the respective
receiving
entity. Payer 140 and/or PBM 150 may respond with additional encrypted sub-
blocks
(not shown in FIG. 7A) decryptable by HCP 120 validating patient coverage.
[00142] In some embodiments, at 710, HCP 120 may determine diagnosis
235
and may initially provide a corresponding diagnosis code 240 and indication(s)
242,
treatment code 245 associated with a treatment for the diagnosed condition,
and
prescription 250 (e.g. drugs/devices 255-p, corresponding doses 260-p each
associated
with a corresponding duration 265-p). In some embodiments, HCP may further
obtain
or determine a transaction ID 695 (as the initiator of the transaction). In
some
embodiments, the information outlined above along with patient criteria 297,
HCP
profile 295, plan ID 270, and, optionally, coverage information 272 may be
sent to
PMDP 130 as encrypted EHR sub-block decryptable by PMDP 130. For example,
coverage information 272 may be sent to PMDP 130 (in EHR sub-block 280), when
47
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
authorized by patient (e.g. when patient is participating or wants to apply to
a payment
assistance program associated with PMDP 130).
[00143] In some embodiments, in step 715, PMDP 130 may determine, for
each
drug/device 255-p, in prescription 250, a corresponding drug class 33'7-p.
Further,
PMDP 130 may determine one or more drugs/devices 302-pd associated with each
class
[00144] At 720, PMDP 130 may send encrypted DIR sub-block 380 (or a
portion
thereof) with class members drugs/devices 302-pd, along with diagnosis 235
(and
related fields such as corresponding diagnosis codes 240 and indication(s)
242,
treatment code(s) 245) and transaction ID 695 to PBM 150. DIR sub-block 380
may
further include information about each drug/device 302-pd (e.g. as described
above in
relation to FIGs. 3A-3C). In some situations (e.g. when Payer 140 performs
functions of
PBM 150 and/or no PBM is used), DIR sub-block 380 may be sent to Payer 140.
DIR
sub-block may be decryptable by the specified receiving entity.
[00145] At 725, PBM 150 (or Payer 140) may respond to PMDP 130 with
encrypted PBR sub-block 480, which may include information about formulary 405
for
Payer 140, tiers 415-j associated with each drug/device 302-pd, pricing
information for
each drug/device 302-pd, including one or more of: copay 421-pd, coinsurance
423-pd,
deductible 423-pd, and price 425-pd. Sub-block 480 may reference transaction
ID 695.
For example, PBM 150 (or Payer 140) may determine information in sub-block 480
based on plan information (Plan 1D 270 and/or coverage related information
272) in
DIR sub-block 380 and/or by using transaction ID 695 and the transaction type
to look
up coverage information associated with patient 170. PBR sub-block 480 may be
decryptable by PMDP 130.
[00146] In step 730, PMDP 130 may decrypt PBR sub-block 480 and use
tier
415-j and pricing information (e.g. one or more of: copay 421-pd, coinsurance
423-pd,
deductible 423-pd, and price 425-pd) for each drug 302-pd along with patient
criteria
(e.g. location, number of pharmacies, time period, etc.) to determine cost
information
395 associated with the prescription. The cost information 395 may take into
account
any payment assistance to be received by patient (when eligible) through a
payment
48
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
assistance program. In some embodiments, cost metrics 395 may include the cost
breakdown 307-pd, which may patient out-of-pocket cost for each drug/device
302-pd.
[00147] In 735 PMDP 130 may send encrypted DIR sub-blocks 380 and 390
with
cost information, to HCP 120. DIR sub-blocks 380 and/or 390 may include
transaction
ID 695, prescription code 250, drug/device classes 337-p corresponding to each
drug/device 255-p in the initial prescription (e.g. at 710), drugs/devices 302-
pd
corresponding to each drug/device class 33'7-p.
[00148] At 740, HCP 120 may send encrypted sub-blocks 280-2 with
transaction
ID 695 with a transaction type indicating HCP approval of the prescription to
one or
more of HCP 120, PBM 130, and/or Payer 140. Encrypted sub-blocks 280-2 may
further include selected drugs devices 255-ps, where 1 < p < P, and for each
drug/device
class p, l< s < D_p. For example, one drug/device 255-ps may be selected from
each
drug/device class 33'7-p. In some embodiments, encrypted EHR sub-block 280-2.
Each
encrypted EHR sub-block 280-2 may be decrypted by the designated receiving
entity.
The information in encrypted sub-blocks 280-2 may be similar to sub-block 280
(e.g. in
FIG. 2).
[00149] At 745, upon approval by Payer 140 and/or PBM 150, the platform
(e.g.
private permissioned blockchain platform) may send transaction ID 695 and a
transaction type indicating transaction confirmation to entities associated
with the
transaction. Upon receiving the confirmation each entity may add its
respective
encrypted record (e.g. EHR 200 for HCP 120, DIR record for PMDP 120, PBR form
PBM 140, PPR for Pharmacy 160, and HTR for Payer 140) to a local blockchain.
In
addition, two or more of the above encrypted records may form part of a
multi-dimensional block 750 in a multi-dimensional blockchain (not shown in
FIG. 7A).
An encrypted block (e.g. EHR 200 for HCP 120, DIR 300 for PMDP 120, PBR 400
for
PBM 140, PPR 500 for Pharmacy 160, and HTR 600 for Payer 140) in the multi-
dimensional block may be decrypted by the entity encrypting the block and may
not be
decrypted by any other entity. Thus, while each block represents an entity's
view of the
transaction, the view is consistent with the views of other entities in
relation to the same
transaction because each block includes (via received sub-blocks) approved
information
from other related entities at the time of transaction finalization. In
addition, while each
multi-dimensional block in the multi-dimensional blockchain represents a
snapshot of a
49
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
finalized transaction as seen by entities that are party to the transaction,
information in
any single encrypted block (e.g. one of EHR 200, DIR 300, PBR 400, PPR 500, or
HTR
600 in FIG. 7A) that is owned and encrypted by a specific entity (e.g. one of
HCP 120,
PMDP 120, PBM 140, Pharmacy 160, or Payer 140, respectively, in FIG. 7A) is
shielded from non-owning entities. As shown in FIG. 7A, multi-dimensional
block 750
is visualized as a cube (a multi-dimensional volume) with each face of the
cube
representing a block associated with an entity that is party to the
transaction. In the
event that the transaction is not approved by PBM 150 and/or Payer 140 and/or
another
entity, one or more of steps 715 through 740 may be repeated.
[00150] At 755, PMDP 130 or the platform may send payment assistance
information to patient 170 (e.g. via the application on a mobile computing
device
associated with the patient). The payment assistance information may take the
form of
an electronic credit, code, or any other payment type (e.g. virtual debit /
credit card,
physical debit or credit card, etc.) that may be used to cover some or all of
the patient's
out-of-pocket costs (e.g. for one one or more specific drugs/devices 255-ps in
the
prescription and/or the prescription as a whole).
[00151] In 760, Pharmacy 160 send a prescription confirmation to
patient 170
(e.g. via the application and/or any other permissible communication such as a
secure
text message, secure e-mail, etc.) indicating receipt and/or availability of
the
prescription. In some embodiments, when obtaining the prescription (e.g. in
person,
online, etc.), patient 170 may present the payment assistance information to
reduce out-
of-pocket
[00152] FIG. 7B depicts entities and layers associated with an example
platform
to facilitate healthcare system security and interoperability. In some
embodiments, the
various entities HCP 120, PMDP 130, Payer 140 etc. may form part of a
permissioned
blockchain platform. In a permissioned blockchain platform, trusted entities
may form a
platform and invite other trusted entities to join the network. In some
embodiments, the
permissioned blockchain platform may also be private (e.g. to invited and/or
authorized
entities). In some embodiments, the permissioned blockchain platform may
support
multi-dimensional blockchains. Rules pertaining to access and adding blocks to
the
multi-dimensional blockchain, program code to determine contracts between the
entities
(e.g. smart contracts), applications that leverage platform functionality
(e.g. on behalf of
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
a patient 170), validation of updates, etc. may be determined and/or
authorized by
entities associated with the permissioned blockchain platform.
[00153] In some embodiments, the permissioned blockchain platform may
take
the form of a cloud-based system. A cloud-based system refers to
infrastructure,
applications, services, and/ or other resources (including hardware resources)
that may
be made available over a network (e.g. the Internet). Cloud-based systems may
be based
on underlying hardware and software resources and may be public (e.g.
available on a
fee basis to all), private (e.g. limited to an organization), or a hybrid
(using some
combination of public and private clouds). In some embodiments, the entities
associated
with the platform may be represented by servers (hardware and/or software),
which, in
some instances, may be cloud based. For example, HCP 120, PMDP 130, and/or
Payer
140 may include agents running on servers, and/or agents running on cloud-
based
platforms including Virtual Machines (VMs).
[00154] In some embodiments, access to functionality afforded by the
permissioned blockchain platform may be facilitated through a layer or an API
associated with the platform. For example, patients 170 and/or another
authorized entity
acting on behalf of a patient (e.g. an entity facilitating access to payment
assistance
programs offered by PMDPs 130) may provide a mobile application (e.g. running
on a
smartphone or other mobile computing device) that interacts with the cloud
based
permissioned blockchain platform to facilitate: (a) determination of patient
choices and
associated cost metrics associated with an initial prescription (e.g. as
indicated by data
in sub-block 280-1 in FIG. 7A) for a patient 170 based on patient criteria 297
and (b
prescription finalization (e.g. as indicated by data in sub-block 280-2 in
FIG. 7A) in
conjunction with the delivery of payment assistance to patient 170.
[00155] As shown in FIG. 7B, multi-dimensional block 750 includes EHR
information block 200, DIR information block 300, PBR information block 400,
PPR
information block 500, and HTR information block 600. In some embodiments,
multi-
dimensional block 750 may form part of a multi-dimensional blockchain. In
multi-
dimensional block 750, EHR information block 200, DIR information block 300,
PBR
information block 400, PPR information block 500, and HTR information block
600
may be encrypted and decryptable by HCP 120, PMDP 130, PBM 150, Pharmacy 160,
and Payer 140, respectively.
51
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
[00156] Further, EHR information block 200, DIR information block 300,
PBR
information block 400, PPR information block 500, and HTR information block
600
may also form blocks in EHR blockchain 772, DIR blockchain 773, PBR blockchain
764, PPR blockchain 775, and HTR blockchain 766, respectively. As shown in
FIG. 7B,
EHR blockchain 772, DIR blockchain 773, PBR blockchain 764, PPR blockchain
775,
and HTR blockchain 766 may be owned and locally maintained by HCP 120, PMDP
130, PBM 150, Pharmacy 160, and Payer 140, respectively. FIG. 7B also depicts
sub-blocks 280 (which forms part of EHR block 200), sub-blocks 380 and 390
(which
form part of DIR block 300) and sub-block 480 (which forms part of PBR block
400).
As outlined above, information in the sub-locks may have been shared between
some of
the entities associated with a transaction prior to transaction finalization
and (at the time
of transaction finalization) may be consistent across a plurality of blocks
that form part
of multi-dimensional block 750. In some embodiments, each field associated
with
information blocks 200, 300, 400, 500 and/or 600 may have a unique global
field id,
which may uniquely identify the field within the multi-dimensional blockchain
system
and/or to relevant entities, when information pertaining to that field is
shared between
entities. Multi-dimensional blocks may include data and a timestamp.
Timestamps may
determine the order in which multi-dimensional blocks (once finalized) are
linked.
[00157] As shown in FIG. 7B, HCP 120, PMDP 130, PBM 150, Pharmacy 160,
and Payer 140 may interact with authentication layer 770. Authentication layer
770,
may include functionality for identification and management (adding,
registering,
authorizing, and deleting) of system entities and/or applications (e.g. mobile
applications) that use (or request use to) functionality provided by the
permissioned
blockchain platform. In addition, authentication layer may include
functionality to
validate permissions related to operations involving: (a) the multi-
dimensional
blockchain (adding new blocks, creating links, etc.); (b) transaction types
(e.g. whether
an entity may initiate or participate in a specified transaction type), etc.
Authentication
layer 770 may interact with consensus layer 780, which may include
functionality to
determine the ordering of transactions and validate correctness of a set of
transactions
related to multi-dimensional block 750.
[00158] In some embodiments, consensus layer 780 may confirm the
correctness
of transactions that constitute the multi-dimensional block. At the time of
transaction
52
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
finalization, a consensus technique applied by consensus layer 780 may confirm
the
correctness of transactions (including shared data between entities) that
constitute the
multi-dimensional block. In some embodiments, consensus techniques such as
Byzantine Fault Tolerance (BFT) or variations thereof such as Redundant BFT,
Fast
Byzantine Consensus, Dynamic Quorums, or some other voting-based consensus
technique may be used to determine if a multi-dimensional block 750 may be
formed
using component blocks (e.g. EHR information block 200, DIR information block
300,
PBR information block 400, PPR information block 500, and HTR information
block
600). When an authorized entity (e.g. Payer 140 or a designated authoritative
entity for
a transaction/transaction type) or some specified number (e.g. all or a
majority) of
entities validates a transaction or block, then consensus is achieved.
Determination of
consensus or transaction validation may vary depending on the transaction
type.
1001591 If the transaction is confirmed as correct by the consensus
technique,
then a first instance of an unlocked multi-dimensional block 750 may be
formed. As
shown in FIG. 7B, unlocked multi-dimensional block 750 may be locked and added
to
the multi-dimensional block when the transaction is finalized. In some
embodiments,
blocks that form part of multi-dimensional block 750 (e.g. EHR information
block 200,
DIR information block 300, PBR information block 400, PPR information block
500,
and HTR information block 600) may also be added as blocks to respective local
blockchains (e.g. EHR blockchain 772, DIR blockchain 773, PBR blockchain 764,
PPR
blockchain 775, and HTR blockchain 766, respectively) at the time of
transaction
finalization and upon locking of multi-dimensional block 750. Thus,
information in the
locally stored block (e.g. EHR information block 200, DIR information block
300, PBR
information block 400, PPR information block 500, and HTR information block
600) is
also consistent with information in multi-dimensional block 750.
[00160] On the other hand, if, for example, a patient identified in
Patient ID 425
in sub-block 480 does not match a Patient ID (e.g. in sub-block 280), the
transaction
may be deemed incorrect and the block addition request may be rejected. In
some
embodiments, the platform, or each entity may maintain a log of rejected
transactions
for traceability and debugging purposes. The log may indicate reasons or codes
associated with transaction rejection.
53
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
[00161] In some embodiments, consensus layer 780 may apply consensus
techniques and may interact with a smart contracts layer 790 to establish
transaction
correctness and/or validity and initiate further actions. Smart Contracts
layer 790 may
comprise program code that implements logic related to a blockchain. For
example,
"smart contract" program code associated with the multi-dimensional blockchain
may
process transaction requests and determine the validity of transactions based
on program
logic. The logic may depend on rules agreed to by the entities for
transactions related to
the blockchain. For example, Smart Contracts layer 790 may reject a
transaction (e.g.
from HCP 120) because of incompatibility between two or more drugs prescribed
to a
patient. Smart contracts may operate at validation time and at commit time
before a
block is locked and/or committed. In some embodiments, smart contracts layer
790 may
encode rules or agreement between two or more entities in relation to data
sharing,
transactions, etc., which may be based on a real-world contract between the
entities. In
some embodiments, each update on a traditional blockchain (e.g. one of EHR
blockchain 772, DIR blockchain 773, PBR blockchain 764, PPR blockchain 775, or
HTR blockchain 766) may be validated by smart contract program code associated
with
the platform. The smart contract program code may reflect agreements between
the
entities in relation to data sharing, authentication, payments, etc. The smart
contract
layer may be viewed as an automation tool that facilitates interaction between
entities
without manual intervention. In some embodiments, the smart contract layer may
initiate actions based on rules associated with one or more contracts when
those rules
have been satisfied. Each update to the multi-dimensional blockchain, and/or
the
passage of time, and/or other events and/or specific requests related to a
contract (e.g.
identified by a contract ID) may trigger actions by the smart contract layer.
[00162] The linking of updated records (e.g. updated record 520 and
updated
record 540) may be performed based on pre-defined rules agreed upon by the
entities
(e.g. HCP 120 and Payer 140). In some embodiments, the linking of blocks (e.g.
updated block 520 and updated block 540) may be performed based on smart
contract(s)
associated with the multi-dimensional blockchain. After linking updated block
520 and
updated blocks 540 may be rehashed. As outlined above, the links may allow an
entity
to correlate information in its blockchain with information in a blockchain
maintained
by another entity. In addition, the entities may be able to determine a
transaction or
transactions associated with information in a specific block maintained by
that entity.
54
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
Accordingly, two or more entities may have a coherent and consistent view of
transactions associated with blocks in distinct blockchains. During the
process of
formation multi-dimensional block 750 may be (at least initially) not fully
formed (or a
multi-dimensional block in progress) ¨ so that blocks received from other
entities may
transform the current in progress (not fully formed) multi-dimensional block
by adding
another dimension. For example, finalized HTR 200 (with a prescription from
HCP
120) may be added to current in progress multi-dimensional block as a
dimension.
Another dimension may then be added to the in progress multi-dimensional block
¨ for
example, a dimension with PPR 500 (with the prescription information). The
process
may continue until the multi-dimensional block is fully formed (e.g. includes
dimensions from all relevant parties to a transaction).
[00163] Multi-dimensional block (and its component records) may be
locked
upon transaction finalization to prevent further modifications and ensure a
consistent
view. Any subsequent modifications may result in a new multi-dimensional block
being
added to the multi-dimensional blockchain. For example, a new multi-
dimensional
block may be formed with updates to a data record for a single dimension while
substantive information associated with the other dimensions may remain
unchanged.
For example, drug related information (e.g. new contraindications) associated
with a
drug prescribed to a patient may be updated (e.g. by PMDP 130) in a new multi-
dimensional block without updates to other records.
[00164] The multi-dimensional block may take the form of a Merkle tree
associated with a multi-dimensional block chain that includes component data
records
(e.g. EHR 200, DIR record 300, PBR 400, PPR 500 and HTR 600). As outlined
earlier,
the data records may also be associated with distinct individual blockchains.
[00165] Accordingly, cryptographic hashes of individual records (e.g.
separate
cryptographic hashes (or "hash") of EHR 200, DIR record 300, PBR 400, PPR 500
and
HTR 600,) in the distinct individual blockchains (772, 773, 764, 775, and 766,
respectively) may be obtained using distinct cryptographic hash functions so
that
records owned by an entity are not decryptable or visible to other entities.
Separate
cryptographic functions (e.g. which may be known to entities associated with
the
permissioned blockchain platform) may be used to obtain cryptographic hashes
of
combinations of records so that a top hash is associated with the multi-
dimensional
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
block as a whole. In some embodiments, each multi-dimensional block may
include a
block header with a timestamp, top hash, information related to the previous
block, a
pointer to the root of Merkle tree, and other appropriate information. The
hash
references may take the form of uniform resource locator (URL) on the private
permissioned blockchain platform and/or local (entity specific) addresses.
[00166] FIG. 7B shows a committed and locked multi-dimensional block
750,
where information from sub-blocks 480, 280, 380, and 390 has been shared
between
corresponding authorized relevant entities. In addition, multi-dimensional
block 750
includes linkages between individual component blocks. Multi-dimensional block
750
may represent a holistic view of transaction at a point in time, in part,
because it may
include real world physical states associated with a drug (dosage, effects,
etc.), a patient
(medical condition, treatment, effect, etc.), and cost at that point in time.
Multi-
dimensional block 750 may also include links to a previous block in the
blockchain.
Validated and finalized multi-dimensional block 750 may include finalized data
records
200, 300, 400, 500 and 600, which may correspond to finalized information
blocks 200,
300, 400, 500, and 600, respectively, in corresponding distinct local
blockchains 772,
773, 764, 775, and 766, respectively.
[00167] FIG. 8 shows a flowchart of an exemplary method 800 to
facilitate
healthcare information security and interoperability while facilitating
patient treatment
selection and transparency in treatment costs. In some embodiments, method 800
may
use multi-dimensional blockchains, which may be based on distinct blockchains
maintained by the individual entities in a system. In some embodiments, method
800
may be performed (at least in part) on a private permissioned blockchain
platform,
which, in some instances, may take the form of a cloud-based system. Method
800 may
also be performed by a processor, computer or networks of computers such as
distributed computing systems, servers (hardware and software), including
application
servers, mobile computing devices (e.g. smartphones, smart wearable devices,
handheld
computers, tablets, laptops, etc.), as well as cloud-based systems.
[00168] In some embodiments, method 800 may be performed at a first
entity.
For example, the first entity may comprise at least one server or a computer
system
associated with at least one of a pharmaceutical provider or a medical device
provider
such as PMDP 130. In some embodiments, the first entity may interact with one
or more
56
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
second entities. The second entities may include one or more servers or
computer
systems associated with healthcare providers such as HCP 120, PBMs 150,
Pharmacies
160, or insurance providers such as Payer 140, or patients 170. In some
embodiments,
the first entity and the one or more second entities may form computing nodes
in a
distributed computing system and the multi-dimensional blockchain may form
part of a
permissioned private blockchain platform such as permissioned private
blockchain
platform.
[00169] In some embodiments, method 700 may be invoked when an entity
such
as the first entity initiates a transaction (e.g. with a transaction ID and/or
transaction
type) to add a block to locally maintained blockchain. The addition of the
block to the
local blockchain may involve inputs from one or more other entities and the
permissioned private blockchain platform may invoke method 800.
[00170] In some embodiments, in step 810, a first entity may receive at
least one
encrypted first Electronic Health Record (EHR) sub-block (e.g. sub-block 280-
1)
decryptable by the first entity, wherein the at least one EHR sub-block
comprises patient
medical coverage information for a patient and one or more first treatments
(prescription 250 with drugs/devices (first treatments) 255-p, 1.<p < P). For
example,
the one or more first treatments may include drugs Dl, D2, D3, and D4.
[00171] In step 820, the first entity may transmit, in response to the
first EHR
sub-block, at least one encrypted first Device Drug Information (DIR) sub-
block (e.g.
DIR sub-block 390) decryptable by at least one corresponding second entity
(e.g. HCP
120) , wherein the at least one first DIR sub-block comprises, for each of the
one or
more first treatments (255-p), a corresponding first treatment class (33'7-p),
one or more
corresponding first treatment class members (302-pd, 1 < d< D_p) each
associated with
the corresponding first treatment class, and for each first treatment class
member
(302-pd), corresponding first treatment class member cost information (395,
397-pd).
[00172] For example, the first treatment class may comprise classes Cl,
C2, C3,
and C4 corresponding to drugs D1, D2, D3, and D4, respectively. Further, each
class
may include members as outlined below: Cl = W11, D121; C2 = {D21}; C3 = 1D31,
D32, D33, D341; and C4 = 1D41, D42, D431, where D1 c Cl, D2 c C2, D3 c C3, and
57
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
D4 c C4. Cost information may be provided for each class member (e.g.
individually as
in cost breakdown 397-pd and in the aggregate as in cost metrics 395).
[00173] In step 830, the first entity may receive, in response to the
first DIR
sub-block, an encrypted second EHR sub-block (e.g. sub-block 280-2)
decryptable by
the first entity, wherein the second EHR sub-block comprises one or more
second
treatments (e.g. treatments 255-ps selected from treatments 302-pd), wherein
each of the
one or more second treatments (255-ps) is associated with a corresponding
first
treatment (302-pd) and each second treatment (255-ps) is selected from the
corresponding first treatment class members (337-pd) associated with the
corresponding
first treatment (255-p); For example, the second treatments may include second
treatments D12, D21, D31, and D43 selected from classes Cl, C2, C3, and C4,
respectively, where second treatments D12, D21, D31, and D43 are associated
(e.g. via
classes Cl, C2, C3, and C4, respectively ) with corresponding first treatment
class
members (D1, D2, D3, and D4, respectively) in the first treatment (e.g.
initial
prescription). In some embodiments, the one or more second treatments in the
second
EHR block may be based on patient-specific cost metrics.
[00174] In block 840, the first entity may augment a multi-dimensional
blockchain, wherein the multi-dimensional blockchain is augmented with a multi-
dimensional block (e.g. multi-dimensional block 750) formed by linking: a DIR
block
(e.g. DIR block 300) comprising information associated with the one or more
second
treatments, an EHR block (e.g. EHR block 200) comprising information
associated with
the second EHR sub-block (e.g. sub-block 280-2), and a transaction block (e.g.
HTR
600). In some embodiments, the transaction block may comprise a prescription
for the
second treatment at the specified location at a specified patient cost.
[00175] In some embodiments, upon receiving the first EHR sub-block
(e.g. in
step 810) , the first entity may: determine, for each of the one or more first
treatments
(255-p - e.g. D1, D2, D3, and D4), the corresponding first treatment class
(33'7-p - e.g.
classes Cl, C2, C3, and C4, respectively), wherein each corresponding first
treatment
class comprises one or more corresponding first treatment class members (302-
pd -
members of Cl, C2, C3, and C4, which include at least D1, D2, D3, and D4,
respectively). Further, the first entity may determine, for each corresponding
first
treatment class (33'7-p - e.g. Cl, C2, C3, and C4), one or more corresponding
first
58
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
treatment class member cost information((e.g. individually as in cost
breakdown 397-pd
for each drug in Cl, C2, C3, and C4 and/or in the aggregate as in cost metrics
395) for
the one or more corresponding first treatment class members associated with
the first
treatment class.
[00176] In some embodiments, the first EHR sub-block (e.g. 280-1) may
comprise patient-specific parameters (e.g. including patient criteria 297) and
the at least
one treatment class is determined based on the patient-specific parameters.
The patient-
specific parameters may include one or more of: patient co-morbidity
information;
.route of administration information (335-pd), safety (330-pd) and efficacy
(337 -pd)
information; and/or patient location information.
[00177] In some embodiments, for each first treatment (255-p), the
corresponding
first treatment class (33'7-p) and the corresponding first treatment class
member cost
information (395, 397-pd) for each treatment class member (302-pd) may be
determined
based on the patient medical coverage information. For example, the cost
information
may be determined based further on one or more of: (a) providers (e.g.
pharmacies 160)
located within a specified distance of a patient location, wherein location
information
for the patient is comprised in the first EHR sub-block (e.g. coverage related
information 272 and/or a portion of patient profile 230 in sub-block 280,
which may
include information in sub-blocks 282 and/or 284); and/or (b) a patient
specific out-of-
pocket cost (corresponding to each corresponding first treatment class member
(302-pd)
for a corresponding treatment unit (e.g. dose and/or duration); and/or (c) a
patient-
specific estimated total out-of-pocket cost (392-pd) corresponding to each
corresponding first treatment class member (302-pd) for a typical treatment
duration;
and/or (d) a patient-specific estimated total out-of-pocket cost corresponding
to each
corresponding first treatment class member (302-pd) for the remaining duration
(e.g. till
the date that current patient coverage ends) of a medical coverage plan
associated with
the patient.
[00178] In some embodiments, upon receiving the second EHR sub-block
(e.g.
in step 830), the first entity may determine payment assistance information
for at least
one of the one or more second treatments; and transmit the payment assistance
information with a transaction confirmation to a patient. In some embodiments,
upon
receiving the second EHR sub-block (e.g. in step 830), the first entity may
transmit a
59
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
transaction confirmation comprising at least one prescription for the one or
more second
treatments along with payment assistance information to the patient.
[00179] The payment assistance information may take the form of an
electronic
credit, code, or any other payment type (e.g. virtual debit / credit card,
physical debit or
credit card, etc.) that may be used to cover some or all of the patient's out-
of-pocket
costs (e.g. for one or more specific drugs/devices 255-ps in the prescription
and/or the
prescription as a whole). Thus, in some instances, the payment assistance
information
may include an actual monetary payment to patient 170 and/or to pharmacy 160.
For
example, in some instances, the payment assistance may specify a drug and/or
pharmacy (or a pharmacy company), where the payment assistance may be used.
[00180] FIG. 9 shows a flow diagram illustrating process flow 900 to
facilitate
healthcare insurance selection and cost determination, while maintaining
healthcare
information security and facilitating interoperability between a plurality of
entities. In
some embodiments, portions of process flow 900 may occur on a permissioned
blockchain platform, which may be made available to subscribing and/or
authorized
entities. In some embodiments, some or all of flow 700 may be implemented
using
applications running on computing devices associated with the entities. In
flow diagram
900 some routine messages have not been shown for ease of description.
[00181] As one example, patient 170 may use an application running on a
mobile
computing device (e.g. smartphone, tablet, laptop, etc.) to initiate a
transaction. In some
embodiments, the application may be provided and/or authorized by an entity
associated
with the permissioned blockchain platform. For example, the application (e.g.
running
on a mobile computing device associated with patient 170) may have been
provided by
a first entity (e.g. HCP 120, PMDP 130, and/or a patient access program (not
shown in
FIG. 9)), and use an Application Programming Interface (API) and/or other
network and
communication protocols to communicate with the first entity. In some
embodiments,
patient 170 may invoke an application that communicates with an entity such as
PMDP
130 (as shown in FIG 9), or a patient assistance program, patient access
program, etc.
associated with the permissioned blockchain platform. Thus, in some
embodiments, the
application may act on behalf of an entity (e.g. PMDP 130) and valid and
authorized
transactions, communications etc. related to the application may appear as if
the entity
(e.g. PMDP 130) was performing the transaction or may be forwarded (after
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
authorization, validation, and appropriate processing/encoding) by the entity
(e.g.
PMDP 130).
[00182] For the purposes of the description below, PMDP 130 has been
used as
an example. However, process flow 900 may also be practiced by other entities
such as
a patient assistance program, and/or patient access program, which may assist
patients
170 to obtain health coverage and/or qualify for patient assistance. As
outlined above,
the application (e.g. used by patient 170) may act on behalf of an entity in a
manner
transparent to patient 170. Thus, in some instances, actions indicated as
being
performed by patient 170 in FIG. 9 may occur through and/or be attributed to
another
entity (e.g. PMDP 130). In some embodiments, a private entity that offers a
choice of
several insurance plans may offer an application implementing process flow 900
to
potential subscribers (e.g. shown as patients 170 in FIG. 9) to facilitate
selection of
appropriate coverage.
[00183] In many situations, patients often need or use treatments
(drugs, devices,
and/or procedures) recurrently. The term recurring treatment is used herein to
refer to
medications, devices, and/or procedures that have been prescribed to a patient
for a
medical condition that is present (ongoing), and/or chronic, and/or likely to
occur,
and/or will recur/manifest itself (e.g. without the recommended treatment)
over an
extended period (e.g. several weeks, months, or years). For example, various
conditions
(e.g. diabetes, blood pressure, heart disease, kidney disease requiring
dialysis, etc.
and/or other chronic conditions) may require extended periods over which a
treatment is
applied or prescribed. In these situations, a drug (e.g. medications), device
(e.g. drug
delivery device), or treatment (e.g. physiotherapy, dialysis, etc.) may be
used and/or
prescribed repeatedly over an extended period. However, at the time of
insurance plan
selection, patients typically do not have information about how total medical
costs
(premiums paid to the plan, deductibles paid, copayments paid, coinsurance
paid, less
any payment assistance received) are affected by a plan selection and/or
drug/device
selection. In some embodiments, some disclosed embodiments, which may utilize
process flow 900, can facilitate intelligent plan selection to lower costs
while
maintaining appropriate levels of treatment as described herein.
[00184] At 905, patient 170 may initiate the transmission of one or
more of:
patient recurring treatment information (e.g. drugs/devices that are being
used by patient
61
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
170 on an ongoing/recurring basis) and/or coverage related information 272.
For
example, patient 170 may indicate (e.g. using an application on the mobile
computing
device) that stored coverage related information 272 and stored prescription
information
related to some (e.g. selected by patient 170) or all prescriptions be sent to
an entity
such as PMDP 130 and/or (e.g. stored either locally on the device and/or in
the cloud,
and/or stored by an entity). As one example, a patient application, which may
track and
store patient prescriptions and coverage information may send or initiate the
sending of
local or cloud-based coverage and prescription information to PMDP 130 upon
authorization by patient 170. In some embodiments, the application, which may
take the
form of a health management application, may include one or more other
functionalities: such as tracking patient prescriptions, indicate when premium
payments
/renewals are due, make payments, indicate when a prescribed treatment is to
be taken,
provide reminders for refills, automatically reorder prescriptions/refills,
track costs,
schedule and provide reminders for appointments, etc. When an application
sends
coverage related information 272 and prescription information to PMDP 130,
events
910 and 915 may not occur.
[00185] In some embodiments, the transmission of coverage related
information
272 and/or recurring treatment information may also be initiated by providing
an
authorization (e.g. to Payer 140 and/or PBM 150 and/or to one or more entities
that may
store patient health related information) to send prescription data to PMDP
130. In
instances, when an authorization is provided and coverage related information
272
and/or recurring treatment information is provided by one or more external
entities
(such as Payer 140 and/or PBM 150), then, at 910, PMDP 130 may transmit a HTR
request with the patient authorization to obtain coverage related information
272 and/or
recurring treatment information to one or more Payer(s) 140-v (1 < v < V)
and/or PBMs
150-u. (1 < u < U). At 915, Payer(s) 140-v (1 < v < V) and/or PBMs 150-u. (1 <
u < U)
may respond (e.g. to PMDP 130) with the requested information pertaining to
patient
170.
[00186] In block 920, PMDP 130 may determine recurring treatment being
used
by the patient. The term recurring treatment is used to refer to medications,
devices,
and/or treatments that have been prescribed to a patient for a medical
condition that is
likely to occur or will be present over an extended time (e.g. several days,
weeks, or
62
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
months) during the period of coverage. Recurring treatment differs from one
time
treatment (e.g. for an isolated medical condition such as a one-time/brief
antibiotic
regimen to cure an opportunistic / difficult to predict infection). In some
embodiments,
information in HTR sub-blocks may be analyzed to determine recurring patient
treatments (e.g. via treatment code(s) 245, prescribed drugs/devices 255-p,
doses 260-p,
and durations 265-p) contained in the HTR sub-blocks. In instances, where
patient
treatment information is available (e.g. via the application used by patient
170), the
patient treatment may be provided by the application and PMDP 130 may
determine
recurring patient treatments (e.g. in step 920) based on the provided
information.
[00187] In some embodiments, at 925 PMDP 130 may send a request for
available plan information to Health Exchange (HEX) 180. HEX 180 may be any
entity
that offers plans for consumer subscription. For example, HEX 180 may be a
government authorized entity that offers plans under the Affordable Care Act
(ACA), or
a department of or contractor affiliated with a private employer that offers
multiple
plans to employees for selection/enrollment (e.g. during an open enrollment
period).
The request for plan information may not include PII information pertaining to
patient
170 and may specify the type of information requested. In some embodiments,
request
for plan information may request a list of payers 140-v and PBMs 150-u
offering plans
in a location specified by patient 170, and plan identification (e.g. Plan ID
and/or Group
ID) for each offered plan. In some instances, where the list of payers 140-v
and PBMs
150-u and plan identification information is publicly available (e.g. via
published
information) then, the published information may be accessed by PMDP 130 to
obtain
the information without the PMDP 130 request (at 925) and HEX 180 response (at
930).
[00188] In some embodiments, at 930, HEX 180 may respond with a list of
payers 140-v and PBMs 150-u offering plans in a location specified by patient
170, and
plan identification (e.g. Plan ID and/or Group ID) for each offered plan (or,
alternatively, the above information may be determined by PMDP 130 using
publicly
available information as outlined above).
[00189] In some embodiments, in step 933, PMDP 130 may determine, for
each
recurring treatment (drug, device and/or procedure) 255-r associated with
patient 170, a
treatment class 337-r, and treatment class members 302-rd, where 1 < d < Dr,
where
Dr is the number of class members in treatment class 337-r. Drugs/devices 302-
rd in a
63
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
treatment class 337-r may: (a) treat a similar therapeutic area 360-r, and/or
(b) have
similar modes/methods of action 340-r, and/or (c) have similar chemical
structure
350-r. In addition, drugs/devices 302-rd in a treatment class 337-r may also
share routes
of administration 335-rd and meet other patient criteria 297. For medical
procedures,
members 302-rd of a treatment class 337-r may: (a) treat a similar indication
and/or (b)
specify alternate facilities (e.g. in some location) where the same/similar
treatment may
be obtained (although potentially at a different price point).
[00190] In step 935 PMDP 130 may send DIR sub-block (e.g. DIR sub-block
380
including treatment code 245 and/or other information in block 280) along with
plan
identification information (e.g. Plan ID and/or Group ID) to one or more PBMs
150-u
and/or Payers 140-v as part of a request for pricing and coverage information
(e.g.
whether the treatment is covered by the corresponding plan (e.g. available to
patient
170).
[00191] At 940, in some embodiments, PBMs 150-u and/or Payers 140-v may
respond with formulary information for each plan (e.g. as in sub-block 480)
and/or
authorized providers (e.g. for a procedure).
[00192] Alternatively, in some embodiments. when formulary information
for
each plan (e.g. as in sub-block 480) and/or authorized providers (e.g. for a
procedure)
are publicly available (and/or available through HEX 180), then, the publicly
available
information may be used and/or the information obtained from HEX 180. For
example,
regulations may require plans (payers 140 and/or PBMs 150) to publish and
update
formulary and other coverage information, so, the most recent published
information or
update may be used to determine formulary information for each plan (e.g. as
in
sub-block 480) and/or authorized providers (e.g. for a procedure).
Accordingly, in some
embodiments, events 935 and 940 may not form part of process flow 900 and PMDP
130 may determine formulary information for each plan (e.g. as in sub-block
480)
and/or authorized providers (e.g. for a procedure) as part of step 933, as a
separate
intermediate step between step 933 and 945, and/or as part of step 945.
[00193] In some embodiments, in step 945, PMDP 130 may determine
overall
patient-specific cost information associated with each plan. For example, a
cost C hs
may be assigned to each plan h and based on selecting one treatment 302-rs
from each
64
CA 03208087 2023-07-11
WO 2022/152695 PCT/EP2022/050440
treatment class 337-r. For example, for a plan h, where the recurring
treatments (e.g.
.determined in step 920) are associated with four treatment classes with one
or more
treatments in each treatment class 337-r, then, one treatment 302-rs from each
of the
four treatment classes may be selected to determine a patient specific cost C
hs
associated with the plan and selected treatments. As one example,
C hs = (Premia over period) + (Copayments) + (Coinsurance)
+ (Deductibles) - (Payment Assistance over period)
In some embodiments, a patient specific cost C hs may be provided for various
selections of treatments in each plan h. Thus, patient 170 and/or HCP 120 may
be able
to determine, prior to plan selection, a potential or likely cost associated
with the plan.
The payment assistance may be provided by PMDPs 130 and/or by other entities
(e.g.
by the government toward insurance premiums under the ACA, or by a non-profit,
by
an organization/program affiliated with PMDP 130, and/or by tax rebates,
etc.).
[00194] In some embodiments, the information may be provided in the
form of a
table (or database) as shown in Table 1 below. The table may comprise
information
about a Plan h, Selected Treatment Set (s), Cost C hs, and Providers for each
selected
treatment 302-rs (e.g. pharmacy, location, etc.). In some embodiments, Cost C
hs may
further include a cost breakdown (copayment, coinsurance, etc.) for each
selected
treatment 302-rs in a treatment set.
Plan h Treatment Set (s) Cost C hs Providers
H1 (h = 1) s=1 C 11
A
H1 (h =1) s =2 . . . C12 . . .
CA 03208087 2023-07-11
WO 2022/152695 PCT/EP2022/050440
H2 (h =2) s = 1 . . . C21 . . .
. . . . . . . . . = = =
Table 1
[00195] In some embodiments, at 950, cost information C hs and other
cost
metrics may be sent to patient 170 either directly or indirectly (e.g. via HEX
180, as
shown by the dashed lines). In some embodiments, patient 170 may authorize
PMDP
130 to share the treatment and cost information be shared with HCP 120 and/or
patient
170 may optionally share and discuss the treatment information with HCP 120.
[00196] At 955, HCP 120 may recommend one of the treatment sets as
appropriate and patient 170 may make a plan selection (e.g. by selecting the
plan shown
on a mobile device screen) and confirming the selection. The plan selected by
patient
170 may be the same as the one recommended by HCP 120 and/or any other
available
plan. Upon confirmation, at 955, the plan selection may be sent to HEX 180.
[00197] At 960, upon approval by Payer 140 and/or PBM 150, the platform
(e.g.
private permissioned blockchain platform) may indicate transaction
confirmation to
entities associated with the transaction. Upon receiving the confirmation each
entity
may add its respective encrypted record (e.g. PBR for PBM 140, HTR for Payer
140
and a corresponding HEX record (not shown in FIG. 9)) to a local blockchain.
In some
embodiments, when authorized, transaction confirmation may also be sent to
PMDP
130 to facilitate participation and payment of benefits from a payment
assistance
program. In some embodiments, at 960, the platform (e.g. private permissioned
blockchain platform) may indicate transaction confirmation by sending a plan
subscription confirmation to patient 170. In addition, two or more of the
above
encrypted records may form part of a multi-dimensional block 965 thereby
augmenting
a multi-dimensional blockchain.
[00198] FIGs. 10A and 10B show a flowchart of an exemplary method 1000
to
facilitate healthcare insurance selection and cost determination, while
maintaining
healthcare information security and facilitate interoperability between a
plurality of
entities. In some embodiments, method 1000 may use multi-dimensional
blockchains,
which may be based on distinct blockchains maintained by the individual
entities in a
66
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
system. In some embodiments, method 1000 may be performed (at least in part)
on a
private permissioned blockchain platform, which, in some instances, may take
the form
of a cloud-based system. Method 1000 may also be performed by a processor,
computer
or networks of computers such as distributed computing systems, servers
(hardware and
software), including application servers, mobile computing devices (e.g.
smartphones,
smart wearable devices, handheld computers, tablets, laptops, etc.), as well
as cloud-
based systems.
[00199] In some embodiments, method 1000 may be performed at a first
entity.
For example, the first entity may comprise at least one server or a computer
system
associated with at least one of a pharmaceutical provider such as PMDP 130
and/or an
application (e.g. provided by PMDP 130 or another entity) used by a patient
(that may
interact and utilize functionality provided by the permissioned blockchain
platform
when authorized on behalf of PMDP 130 and/or the other entity). In some
embodiments, the first entity may interact with one or more second entities.
The second
entities may include one or more servers or computer systems associated with
healthcare exchanges such as HEX 180, pharmacy benefit managers such as PBMs
150-u, insurance providers such as Payers 140-v, or patients 170. In some
embodiments,
the first entity and the one or more second entities may form computing nodes
in a
distributed computing system and the multi-dimensional blockchain may form
part of a
permissioned private blockchain platform.
[00200] In some embodiments, method 1000 may be invoked when an entity
such
as the first entity initiates a transaction (e.g. with a transaction ID and/or
transaction
type) to add a block to locally maintained blockchain. The addition of the
block to the
local blockchain may involve inputs from one or more other entities and the
permissioned private blockchain platform may invoke method 1000.
[00201] Referring to FIG. 10A, in step 1010, a first entity (e.g. PMDP
130 and/or
an application running a patient computing device such as a smartphone and/or
another
entity) may obtain from at least one encrypted first Health Transaction Record
(HTR)
sub-block (e.g. sub-block 280 and/or other treatment related information in
HTR 600)
decryptable by the first entity. In some embodiments, the HTR sub-block may be
received in response to a request comprising a patient authorization. The
first entity may
obtain (e.g. based on information in the at least one first HTR sub-block) a
first set of
67
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
first treatments for the patient over a time period, wherein each first
treatment comprises
a first diagnosis code (e.g. diagnosis code 240) and a first treatment code
(e.g. treatment
code 245). In some embodiments, the first EHR sub-blocks may include treatment
code(s) 245, prescribed drugs/devices 255-p, doses 260-p, and durations 265-p
associated with the first (recurring) treatments 255-p. In some embodiments,
the time
period may include several prior coverage periods and include multiple Payers
140-v,
which may facilitate determination of recurrent and ongoing treatments.
[00202] In some embodiments, the first set of first treatments may be
determined
based on one or more of: treatment code(s) 245-p, prescribed drugs/devices 255-
p,
doses 260-p, and durations 265-p contained in the HTR sub-blocks. The first
treatments
may comprise recurring treatments such as drugs, devices, and/or procedures
255-p that
may have been prescribed and/or are likely to be prescribed over the specified
time
period. In some embodiments, information in the at least one first HTR sub-
block (e.g.
received by the first entity) may be analyzed (e.g. mathematically and/or
statistically) to
determine the first set of first treatments (e.g. recurring patient
treatments). For
example, a monthly prescription may be projected as continuing until the end
of the
specified time period (e.g. based on duration 265-p, a determined or projected
frequency). In some embodiments, projections and/or determined recurring
treatments
may be subject to patient confirmation. In some embodiments, locally stored
data (e.g.
as part of a health management application) may be used to obtain the first
set of first
treatments.
[00203] In some embodiments, treatments associated with certain medical
conditions (e.g. discernible via diagnosis code 240) known to be chronic or
long lasting
may be automatically identified as recurring treatments. In other instances,
treatments
that occur repeatedly over the time period may be determined to be recurring
treatments.
As another example, a treatment may be determined to be recurring based, in
part, on
additional information such as a patient's calendar (e.g. as part of a health
management
application) indicating a pattern of scheduled appointments for a specific
diagnosis code
with a provider. In instances, where an application (e.g. health management or
other
application) stores patient treatment and/or appointment information, the
recurring
patient treatment information may be determined based on the stored patient
treatment
and/or appointment information. The first set of first treatments may be
obtained from
68
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
HTRs 600 maintained by Payer 140 and/or PBRs 400 PBM 150, which may include
records for treatments over a long period. In some embodiments, a plurality of
Payers
140-v and/or PBMs 150-u used by the patient (e.g. over some specified prior
period)
may be contacted to obtain information in the first HTR sub-block(s).
[00204] In some embodiments, in step 1015, the first entity (e.g. PMDP
130) may
determine, based on the first set: (a) one or more second sets, wherein each
second set
comprises corresponding second treatments, wherein each corresponding second
treatment is associated with: a distinct first treatment in the first set, and
(b) a
corresponding number of recurrences (e.g. over the specified time period such
as the
intended duration of coverage). The number of recurrences may be used to
determine
cost (e.g. by multiplying the cost per treatment with a pro-rated or
normalized number
of recurrences over a time period). In some embodiments, an application (e.g.
running a
patient computing device such as a smartphone) may obtain the one or more
second
sets, corresponding second treatments, and recurrences corresponding to each
second
treatment, from PMDP 130 (e.g. via a secure API).
[00205] As one example, each first treatment (255-p) in the set of
first treatments
may be associated with a corresponding first treatment class (33'7-p). Each
treatment
class (337-p) may comprise a one or more treatment class members (302-pd,
1 < d < D _p) . Each second set may comprise corresponding second treatments
(302-pd)
associated with a distinct first treatment (255-p) via the first treatment
class (33'7-p).
Thus, as one example, the corresponding second set may comprise one treatment
class
member (302-pd) from each treatment class (33'7-p) (that corresponds to a
first
treatment 255-p). Step 1015 may be viewed as expanding a set of currently
prescribed
recurring treatments for patient 170 to similar classes of available
treatments.
[00206] In step 1020, a set of available insurance plans (e.g. Plan ID,
Group ID,
etc.) available to the patient and corresponding coverage related information
(e.g.
premiums, deductibles, copayments, coinsurance, formulary information, etc.)
for each
available insurance plan may be obtained (e.g. by PMDP 130 and/or an
application
running a patient computing device such as a smartphone). The available
insurance
plans and coverage related information may be obtained from HEX 180, and/or
Payers
140-v, and/or PBMs 150-u, and/or an employer (e.g. when the plans are employer
sponsored) and/or from publicly available information (e.g. by published
information
69
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
available from government or other public sources). The set of available
insurance plans
and corresponding coverage related information may be obtained based on non-
personal
information (e.g. without providing patient PII) associated with the patient
such as
location (country, state, county, city, zip code), age, etc. In some
embodiments, an
application (e.g. running a patient computing device such as a smartphone) may
obtain
the set of available insurance plans and corresponding coverage related
information by
using published information and/or from PMDP 130 (e.g. via an API).
[00207] In step 1025, for one or more available insurance plans,
corresponding
plan-specific cost information for the patient may be determined based on: (a)
the one or
more sets of second treatments, and (b) corresponding coverage related
information. In
some embodiments, the corresponding plan-specific cost information for the
patient
may reflect applicable payment assistance each second set. For example, for
available
plans, plan-specific cost information may be determined for patient 170 based
on the
recurring treatments in each second set. In some embodiments, the cost
information may
be ranked (e.g. from highest projected cost to lowest projected cost). In some
embodiments, step 1015 may be skipped, and, in block 1025, plan-specific cost
information may be determined for the patient based on the first set of first
treatments
thereby providing plan-specific cost estimates for existing treatments used by
the
patient. In some embodiments, an application (e.g. on a smartphone or other
mobile
computing device associated with patient 170) may determine the plan specific
cost
information and provide a list of plan options, which may be ranked by cost or
other
patient criteria 297 to patient 170. In some embodiments, the plan specific
cost
information for the patient may be received by the application from PMDP 130
(e.g. via
a secure API).
[00208] In some embodiments, optionally in step 1030, when determined
by
PMDP 130, the plan specific cost information may optionally be transmitted
(e.g. by
PMDP 130 in a DIR sub-block comprising information in DIR sub-block 380 and
390
and/or other information in DIR record 300) to a second entity (e.g. to an HCP
120
authorized by the patient 170). In some embodiments, plan specific cost
information
(e.g.in the DIR sub-blocks) may also be provided to an application (e.g. on a
smartphone or other mobile computing device associated with patient 170) via a
secure
API.
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
[00209] For example, the cost information may include aggregate cost
metrics
395-p (e.g. aggregate plan specific cost information associated with
treatments 302-pr
in the corresponding second set). For example, (as shown in Table 1), DIR sub-
blocks
(e.g. 380 and/or 390) may comprise information about a Plan h, Selected
Treatment Set
(s), Cost C hs, and Providers for each selected treatment 302-rs (e.g.
pharmacy,
location, etc.) in the corresponding second set. In some embodiments, Cost C
hs may
further include a cost breakdown (copayment, coinsurance, etc.) for each
selected
treatment 302-rs in a treatment set. In some embodiments, an application (e.g.
on a
smartphone or other mobile computing device associated with patient 170) may
determine the plan specific cost information and provide a list of plan
options, which
may be ranked by cost or other patient criteria 297 to patient 170.
[00210] Referring to FIG. 10B, in step1035, based on the plan-specific
cost
information for the patient, at least one available insurance plan from the
set of
available insurance plans may be obtained. For example, a patient may select
at least
one available insurance plan from the set of available insurance plans. As
another
example, PMDP 130 may receive the at least one selected available insurance
plan from
an application (e.g. on a smartphone or other mobile computing device
associated with
patient 170) via a secure API.
[00211] In optional step 1040, PMDP 130 may further receive a second
encrypted
Health Transaction Record (HTR) sub-block decryptable by PMDP 130, wherein the
HTR sub-block comprises the selected insurance plan selected from the set of
available
insurance plans.
[00212] In optional step 1045 (which may be performed when PMDP 130
receives HTR sub-blocks in step 1040 and/or is deemed to be a party to the
transaction),
then PMDP 130 may augment a multi-dimensional blockchain with a multi-
dimensional
block formed by linking: an HTR block comprising information associated with
the
second HTR sub-block and a DIR block comprising second treatment information,
plan-specific cost metrics for the selected insurance plan, and the
transaction block. In
some embodiments, the multi-dimensional blockchain may be augmented in
response to
a received transaction block (e.g. from HEX 180 and/or Payer 140 and/or PBM
150)
with a transaction confirmation comprising an insurance plan selection.
71
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
[00213] In block 1050, the application (e.g. on a smartphone or other
mobile
computing device associated with patient 170) may receive a confirmation of
enrollment in the at least one selected plan (e.g. via a secure message from
HEX 180,
and/or Payer 140 and/or PBM 150 and/or from PMDP 130 via a secure API).
[00214] FIG. 11 shows a schematic of an exemplary computer 1100 or
computing device capable of facilitating healthcare system security and
promoting
interoperability. In some embodiments, computer 1100 may host and/or interact
with a
permissioned private blockchain platform. Computer 110 may be a computing
device
associated with an entity (e.g. HCP 120, PMDP 130, Payer 140, PBM 150,
Pharmacy
160, and/or HEX 180) and/or patient 170 and/or may be used to implement the
permissioned blockchain platform. Computer 130 is merely an example, and
several
computers may be used in a networked and/or distributed fashion to implement
methods
and process flows disclosed herein.
[00215] In some embodiments, exemplary computer 1100 may be include
(physical) servers or run servers (e.g. application servers) for one or more
entities (such
as HCP 120, PMDP 130, Payer 140, PBM 150, Pharmacy 160, and/or HEX 180). In
some embodiments, one computer 1100 may implement some or all of the process
flows
and/or methods and/or other techniques disclosed herein including those in
FIGs. 7-10.
In some embodiments, computer 1100 may form part of a distributed computing
system,
which may implement the permissioned private blockchain platform. In some
embodiments, the distributed computing system and/or computer 1100 may be
cloud-
based. In some embodiments, computer 1100 may be a mobile computing device
such
as a smartphone, tablet, handheld, notebook, and/or laptop, which may run
applications
to interact with other computers 1100 and/or other applications to implement
techniques
disclosed herein.
[00216] In some embodiments, computer 1100 / processor(s) 1150 may be
able to
process transaction requests, including requests related to the addition of
blocks to a
blockchain, including multi-dimensional blockchains. Further, computer 1100 /
processor(s) 1150 may be able to run encryption and/or decryption algorithms,
obtain
hashes of information blocks, verify hashes, perform digital signing, and may
be
capable of executing and/or support various methods to promote security and
authentication. Authentication may refer to both the verification of the
integrity of
72
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
stored information (e.g. in a block in a blockchain to determine any
unauthorized
alterations) and ensuring that entities accessing the permissioned private
blockchain
platform are trustworthy and have permissions to perform any requested
transactions. In
some embodiments, computer 1100 / processor(s) 1150 may also augment (create
or
add to) blockchains with new blocks (including augmenting multi-dimensional
blockchains with multi-dimensional blocks).
[00217] In some embodiments, computer 1100 / processor(s) 1150 may also
store
and execute smart contracts associated with blockchains to implement
agreements
related to privacy, information sharing, contractual execution, etc. between
entities (e.g.
HCP 120, PMDP 130, Payer 140, and/or patient(s)).
[00218] In some embodiments, computer 1100 / processor(s) 1150 may be
capable of mathematically analyzing and statistically compiling health related
data
including determining treatment classes (e.g. drug classes, device classes,
and/or
procedural classes) associated with a treatment, determining cost metrics,
individually,
and in the aggregate, associated with treatments from the treatment classes
and using
patient criteria to filter and/or narrow selections. In some embodiments,
computer 1100
/ processor(s) 1150 may also be able to initiate the display (e.g. on display
1170) of the
information on a smartphone (e.g. using a Graphical User Interface (GUI).
Computer
1100 / processor(s) 1150 may also be capable of using machine learning
techniques to
determine relationships between various health parameters. For example,
computer
1100 / processor(s) 1150 may comprise one or more neural network processor(s),
and/or
distributed processors capable of being configured as a neural network, and/or
be
capable of executing software to model and/or simulate neural networks, which
may be
used to implement machine learning. For example, a PMDP 130 may use machine
learning techniques based RWE information available through the multi-
dimensional
blocks (e.g. demographic information, side effects, drugs used in combination
with a
specified drug of interest, treatment outcomes etc.) to tailor usage of drug.
For example,
machine learning may be used to determine an effective dosage, target drugs
based on
demographics, improve drug interaction information, increase safety, determine
the
relative efficacy of various modes of administration, etc.
[00219] In some embodiments, computer 1100 may be coupled to other
computers using communications/network interface 1102, which may include wired
73
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
(e.g. Ethernet including Gigabit Ethernet) and wireless interfaces. Wireless
interfaces
may be based on: Wireless Wide Area Network (WWAN) standards such as cellular
standards including 3G, 4G, and 5G standards; IEEE 802.11x standards popularly
known as Wi-Fi. and /or Wireless Personal Area Networks (e.g. Bluetooth, Near
Field
Communication (NFC), etc.). In some embodiments, computer 110 may include
Global
Positioning System and/or location determination units to automatically
determine
location (e.g. of a patient), which may be used in conjunction with the
techniques
disclosed in FIGs. 7-10.
[00220] Computer 1100 may include memory 1104, which may include one or
more of: Read Only Memory (ROM), Programmable Read Only Memory (PROM),
Random Access Memory (RAM) of various types, Non-Volatile RAM, etc. Memory
1104 may be implemented within processor(s) 1150 or external to processor(s)
1150. As
used herein, the term "memory" refers to any type of long term, short term,
volatile,
nonvolatile, or other memory and is not to be limited to any particular type
of memory
or number of memories, or type of media upon which memory is stored.
[00221] Memory may comprise cache memory, primary memory, and secondary
memory. Secondary memory may include computer-readable media 1120.
Computer-readable media may include magnetic and/or optical media, which, in
some
instances, may be removable media. Removable media may comprise optical disks
such
as compact-discs (CDs), laser discs, digital video discs (DVDs), blu-ray
discs, and other
optical media and further include USB drives, flash drives, solid state
drives, memory
cards etc. Computer 1100 may further include storage 1160, which may include
hard
drives, solid state drives (SSDs), flash memory, other non-volatile storage,
and
cloud-based storage.
[00222] Communications / Network interface 1102, storage 1160, memory
1104,
and computer readable media 1120 may be coupled to processor(s) 1150 using
connections 1106, which may take the form of a buses, lines, fibers, links,
etc.
[00223] The methodologies described herein may be implemented by
various
means depending upon the application. For example, these methodologies may be
implemented in hardware, firmware, software, or any combination thereof For a
hardware implementation, the processor(s) 1150 may be implemented within one
or
74
CA 03208087 2023-07-11
WO 2022/152695
PCT/EP2022/050440
more application specific integrated circuits (ASICs), digital signal
processors (DSPs),
neural network processors (NNPs), digital signal processing devices (DSPDs),
programmable logic devices (PLDs), field programmable gate arrays (FPGAs),
processors, controllers, micro-controllers, microprocessors, electronic
devices, other
electronic units designed to perform the functions described herein, or a
combination
thereof
[00224] For a firmware and/or software implementation, the
methodologies may
be implemented with microcode, procedures, functions, and so on that perform
the
functions described herein. Any machine-readable medium tangibly embodying
instructions may be used in implementing the methodologies described herein.
For
example, software may be stored in storage 1160 and/or on removable computer-
readable media. Program code may be resident on computer readable media 1120,
storage 1160, and/or memory 1104 and may be read and executed by processor(s)
1150.
[00225] If implemented in firmware and/or software, the functions may
also be
stored as one or more instructions or code computer-readable medium 1120,
storage
1160, and/or memory 1104. Examples include computer-readable media encoded
with
data structures and computer programs. For example, computer-readable medium
1120
may include program code stored thereon may include program code to support
methods to facilitate healthcare system security and promote system
interoperability,
including by supporting multi-dimensional blockchains, smart contracts,
consensus
determination and performing other function associated with a permissioned
private
blockchain platform as described herein.
[00226] Processor(s) 1150 may be implemented using a combination of
hardware, firmware, and software. In some embodiments, computer 1100 may be
coupled to a display to facilitate viewing of GUIs and interaction with
administrators
and other users.
[00227] Although the present disclosure is described in connection with
specific
embodiments for instructional purposes, the disclosure is not limited thereto.
Various
adaptations and modifications may be made to the disclosure without departing
from the
scope. Therefore, the spirit and scope of the appended claims should not be
limited to
the foregoing description.