Note: Descriptions are shown in the official language in which they were submitted.
WO 2022/182643
PCT/US2022/017281
STRUCTURE AM) METHODS FOR DETECTION OF SAMPLE ANALYTES
CROSS-REFERENCE TO RELATED APPLICATIONS
[00011 The present application claims priority to and the benefit of U.S.
Provisional Application
No. 63/152,607, filed February 23, 2021, and entitled STRUCTURE AND METHODS
FOR
DETECTION OF SAMPLE ANALYTES, the disclosure of which is hereby incorporated
by
reference herein in its entirety.
BACKGROUND
[00021 The current state of personalized healthcare is overwhelmingly genome-
centric,
predominantly focused on quantifying the genes present within an individual.
While such an
approach has proven to be extremely poweiful, it does not provide a clinician
with the complete
picture of an individual's health. This is because genes are the "blueprints"
of an individual and it
merely informs the likelihood of developing an ailment. Within an individual
these "blueprints"
first need to be transcribed into RNA and then translated into various protein
molecules, the real
"actors" in the cell, in order to have any effect on the health of an
individual.
100031 The concentration of proteins, the interaction between the proteins
(protein-protein
interactions or PPI), as well as the interaction between proteins and other
molecules, are intricately
linked to the health of different organs, homeostatic regulatory mechanism as
well as the
interaction of these systems with the external environment. Hence,
quantitative information about
proteins and protein interactions such as PPis is vital to create a complete
picture of an individual's
health at a given time point as well as to predict any emerging health issues.
For instance, the
amount of stress experienced by cardiac muscles (e.g. during a heart attack)
can be inferred by
measuring the concentration of troponin I/II and myosin light chain present
within peripheral
blood. Similar protein biomarkers have also been identified, validated and are
deployed for a wide
variety of organ dysfunctions (e.g. liver disease and thyroid disorders),
specific cancers (e.g.
colorectal or prostate cancer), and infectious diseases (e.g. HIV and Zika).
The interaction between
these proteins are also essential for drug development and are increasingly
becoming a highly
sought-after dataset. The ability to detect and quantify proteins and protein
interaction with other
molecules within a given sample of bodily fluids is an integral component of
such healthcare
development.
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
SUMMARY
[0004] The present disclosure generally relates to systems, structures and
methods for detection
and quantification of analyte molecules in a sample.
[0005] Provided herein, in some embodiments, is a method for detecting an
analyte molecule
present in a sample, the method comprising: a) providing a supramolecular
structure comprising: a
core structure comprising a plurality of core molecules and a capture molecule
linked to the core
structure at a first location, b) contacting the sample with the
supramolecular structure and c)
providing a detector molecule assembly; and d) detecting the analyte molecule
based on a signal
provided by the detector molecule assembly and an associated signal provided
by the
supramolecular structure.
[0006] In some embodiments, any method disclosed herein further comprising
quantifying the
concentration of the analyte molecule in the sample. In some embodiments, any
method disclosed
herein further comprising identifying the detected analyte molecule. In some
embodiments, any
method disclosed herein further comprising detecting the analyte molecule
based on the signal
when the analyte molecule is present in the sample at a count of a single
molecule or higher. In
some embodiments, for any method disclosed herein, the sample comprises a
complex biological
sample and the method provides for single-molecule sensitivity thereby
increasing a dynamic
range and quantitative capture of a range of molecular concentrations within
the complex
biological sample. In some embodiments, for any method disclosed herein, the
analyte molecule
comprises a protein, a peptide, a peptide fragment, a lipid, a DNA, a RNA, an
organic molecule, an
inorganic molecule, complexes thereof, or any combinations thereof. In some
embodiments, for
any method disclosed herein, each supramolecular structure is a nanostructure.
[0007] In some embodiments, for any method disclosed herein, each core
structure is a
nanostnicture. In some embodiments, for any method disclosed herein, the
plurality of core
molecules for each core structure are arranged into a pre-defined shape and/or
have a prescribed
molecular weight. In some embodiments, the pre-defined shape is configured to
limit or prevent
cross-reactivity with another supramolecular structure. In some embodiments,
for any method
disclosed herein, the plurality of core molecules for each core structure
comprises one or more
nucleic acid strands, one or more branched nucleic acids, one or more
peptides, one or more small
molecules, or combinations thereof. In some embodiments, for any method
disclosed herein, each
core structure independently comprises a scaffolded deoxyribonucleic acid
(DNA) origami, a
-2-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
scaffolded ribonucleic acid (RNA) origami, a scaffolded hybrid DNA:RNA
origami, a single-
stranded DNA tile structure, a multi-stranded DNA tile structure, a single-
stranded RNA origami,
a multi-stranded RNA tile structure, hierarchically composed DNA or RNA
origami with multiple
scaffolds, a peptide structure, or combinations thereof
[00081 In some embodiments, for any method disclosed herein, the respective
analyte molecule
is 1) bound to the capture molecule of the respective supramolecular structure
through a chemical
bond and/or 2) bound to the detector molecule of the detector molecule
assembly through a
chemical bond. In some embodiments, for any method disclosed herein, the
capture molecule and
detector molecule independently comprise a protein, a peptide, an antibody, an
aptamer (RNA and
DNA), a fluorophore, a darpin, a catalyst, a polymerization initiator, a
polymer like PEG, or
combinations thereof. In some embodiments, for any method disclosed herein,
wherein for each
supramolecular structure: a) the capture molecule is linked to the core
structure through a capture
barcode, wherein the capture barcode comprises a first capture linker, a
second capture linker, and
a capture bridge disposed between the first and second capture linkers,
wherein the first capture
linker is bound to a first core linker that is bound to the first location on
the core structure, wherein
the capture molecule and the second capture linker are linked together through
binding to a third
capture linker, and b) the detector molecule assembly includes a detector
barcode, wherein the
detector barcode comprises one or more linkers. In some embodiments, the
capture bridge and
detector molecule assembly independently comprise a polymer core. In some
embodiments, the
polymer core of the capture bridge and the polymer core of the detector
molecule assembly
independently comprise a nucleic acid (DNA or RNA) of specific sequence or a
polymer like PEG.
[00091 In some embodiments, for any method disclosed herein, each
supramolecular structure
further comprises an anchor molecule linked to the core structure. In some
embodiments, the
anchor molecule is linked to the core structure via an anchor barcode, wherein
the anchor barcode
comprises a first anchor linker, a second anchor linker, and an anchor bridge
disposed between the
first and second anchor linkers, wherein the first anchor linker is bound to a
third core linker that is
bound to a third location on the core structure, wherein the anchor molecule
is linked to the second
anchor linker. In some embodiments, the anchor molecule comprises an amine, a
thiol, a DBCO, a
maleinxide, biotin, an azide, an acrydite, a NHS-ester, a single stranded
nucleic acid (RNA or
DNA) of specific sequence, one or more polymers like PEG or polymerization
initiators, or
combinations thereof. In some embodiments, the anchor bridge comprises a
polymer core. In sonic
-3-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
embodiments, the polymer core of the anchor bridge comprises a nucleic acid
(DNA. or RNA) of
specific sequence or a polymer like PEG. In some embodiments, the third core
linker, first anchor
linker, second anchor linker, and anchor molecule independently comprise an
anchor reactive
molecule or DNA sequence domain. In some embodiments, each anchor reactive
molecule
independently comprises an amine, a end., a DBCO, a maleimide, biotin, an
azide, an acrydite, a
NHS-ester, a single stranded nucleic acid (RNA or DNA) of specific sequence,
one or more
polymers like PEG or polymerization initiators, or combinations thereof. In
some embodiments,
the anchor molecule is linked to the second anchor linker through a chemical
bond. In some
embodiments, the anchor molecule is covalently bonded to the second anchor
linker.
[000101 In some embodiments, for any method disclosed herein, the signal
comprises the detector
barcode, the capture barcode, or combinations thereof, corresponding to a
supramolecular structure
that is bound to an analyte that in turn is bound to a detector molecule
assembly. In some
embodiments, each detector barcode provides a DNA signal corresponding to the
detector
molecule and providing information indicative of its specificity for an
analyte molecule bound to
the respective detector molecule. In some embodiments, the detector barcodes
are analyzed using
genotyping, qPCR, sequencing, or combinations thereof. In some embodiments, a
plurality of
analyte molecules in the sample are detected simultaneously through
multiplexing. In some
embodiments, for any method disclosed herein, the capture and detector
molecules for each
supramolecular structure is configured for binding to one or more specific
types of analyte
molecules.
[000111 In some embodiments, for any method comprising using a plurality of
supramolecular
structures disclosed herein, each core structure of the plurality of
supramolecular structures are
identical to each other. In some embodiments, each supramolecular structure
comprises a
prescribed shape, size, molecular weight, or combinations thereof, so as to
reduce or eliminate
cross-reactions between a plurality of supramolecular structures. In some
embodiments, each
supramolecular structure comprises a plurality of capture molecules. In some
embodiments, each
suprarnolecular structure comprises a prescribed stoichiornetry of the capture
and detector
molecules so as to reduce or eliminate cross-reactions between the plurality
of supramolecular
structures.
[000121 In some embodiments, one or more supramolecular structures are
attached to a
hydrogelporous matrix. In some embodiments, each supramolecular structure is
co-polymerized
-4-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
with the hydrogel through a corresponding anchor molecule linked to the
respective core structure
of the corresponding supramolecular structure. In some embodiments, the one or
more
supramolecular structures are embedded within the hydrogel. In some
embodiments, a plurality of
supramolecular structures are disposed on a substrate, such as a shaped or
planar substrate,
wherein the substrate comprises a plurality of binding sites, wherein each
binding site is
configured to link with a corresponding supramolecular structure. In some
embodiments, the
plurality of supramolecular structures are configured to detect the same
analyte molecule. In some
embodiments, for any method comprising using a substrate, the method further
comprises
providing a plurality of signaling elements linked with the detector
molecules. In some
embodiments, each signaling element comprises a fluorescent molecule or
microbead, a
fluorescent polymer, highly charged nanoparticles or polymer. In some
embodiments, at least one
supramolecular structure of the plurality of supramolecular structures is
configured to detect a
different analyte molecule from the other supramolecular structures.
1900131 in some embodiments, for any method comprising using a planar
substrate, further
comprising barcoding each supramolecular structure so as to identify the
location of each
supramolecular structure on the planar substrate. In some embodiments, for any
method
comprising using a planar substrate, the method comprises providing a
plurality of signaling
elements as provided herein that are configured to link with the detector
molecules.
10001.41 In some embodiments, for any method disclosed herein, the sample
comprises a
biological particle or a biomolecule. In some embodiments, for any method
disclosed herein, the
sample comprises an aqueous solution comprising a protein, a peptide, a
fragment of a peptide, a
lipid, DNA, RNA, an organic molecule, a viral particle, an exosome, an
organelle, or any
complexes thereof. In. some embodiments, for any method disclosed herein, the
sample comprises
a tissue biopsy, blood, blood plasma, Urine, Saliva, Tear, Cerebrospinal
fluid, extracellular fluid,
cultures cells, culture media, discarded tissue, plant matter, a synthetic
protein, prions, a bacterial
and/or viral sample or fungal tissue, or combinations thereof. The sample may
be processed to
release the analytes from cells or to otherwise prepare the sample for
analysis prior to contacting
the sample with the supramolecular structures provided herein. The sample may
be an
environmental sample, such as a wastewater or soil sample. The sample may also
be a
nonbiological sample. In an embodiment, the sample may be a sample from a
chemical process
step, a sample of food or nutritional components, or packaging components.
-5-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
[00015] Provided herein, in some embodiments, is a substrate for detecting one
or more analyte
molecules in a sample, the substrate comprising a plurality of supramolecular
structures, each
supramolecular structure comprising: a) a core structure comprising a
plurality of core molecules,
and b) a capture molecule linked to the supramolecular core.
[00016] In some embodiments, each core structure of the plurality of
supramolecular structures is
identical to each other. In some embodiments, the substrate comprises a solid
support, solid
substrate, a polymer matrix, or a molecular condensate. In some embodiments,
the sample
comprises a complex biological sample and the method provides for single-
molecule sensitivity
thereby increasing a dynamic range and quantitative capture of a range of
molecular concentrations
within the complex biological sample. In some embodiments, the one or more
analyte molecules
comprises a protein, a peptide, a peptide fragment, a lipid, a DNA, a RNA, an
organic molecule, an
inorganic molecule, complexes thereof, or any combinations thereof. In some
embodiments, each
supramolecular structure is a nanostructure. In some embodiments, each core
structure is a
nanostructure. In some embodiments, the plurality of core molecules for each
core structure are
arranged into a pre-defined shape and/or have a prescribed molecular weight.
In some
embodiments, the pre-defined shape is configured to limit or prevent cross-
reactivity with another
supramolecular structure. In some embodiments, the plurality of core molecules
for each core
structure comprises one or more nucleic acid strands, one or more branched
nucleic acids, one or
more peptides, one or more small molecules, or combinations thereof In some
embodiments, each
core structure independently comprises a scaffolded deoxyribonucleic acid
(DNA.) origami, a
seal-I-bided ribonucleic acid (RNA) origami, a scaffolded hybrid DNA:RNA
origami, a single-
stranded DNA tile structure, a multi-stranded DNA tile structure, a single-
stranded RNA origami,
a multi-stranded RNA. tile structure, hierarchically composed DNA or RNA
origami with multiple
scaffolds, a peptide structure, or combinations thereof. In some embodiments,
the respective
analyte molecule is I) bound to the capture molecule through a chemical bond
and/or 2) bound to
the detector molecule through a chemical bond. In some embodiments, the
capture molecule and
detector molecule independently comprise a protein, a peptide, an antibody, an
aptamer (RNA and
DNA), a fluorophore, a darpin, a catalyst, a polymerization initiator, a
polymer like PEG, or
combinations thereof.
[00017] In some embodiments, wherein for each supramolecular structure of the
substrate: a) the
capture molecule is linked to the core structure through a capture barcode,
wherein the capture
-6-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
barcode comprises a first capture linker, a second capture linker, and a
capture bridge disposed
between the first and second capture linkers, wherein the first capture linker
is bound to a first core
linker that is bound to the first location on the core structure, wherein the
capture molecule and the
second capture linker are linked together through binding to a third capture
linker. The capture
molecule binds to an analyte that in turn binds to a detector molecule of a
detector molecule
assembly. In an embodiment, the detector molecule assembly includes a separate
supramolecular
structure that is not linked to the capture molecule and that is not
immobilized on the substrate.
[000181 In some embodiments, the supramolecular structure that includes the
capture molecule
directly interacts with a substrate material to immobilize the supramolecular
structure on the
substrate. In some embodiments, the supramolecular structure that includes the
capture molecule
further comprises an anchor molecule, as provided herein, linked to the core
structure, and the
anchor molecule is linked to the substrate to immobilize the supramolecular
structure on the
substrate.
1000191 In some embodiments, the signal read or detected from the substrate
comprises the
detector barcode, the capture barcode, or combinations thereof that may be
analyzed using optical
sensors, magnetic sensors, and/or electrical sensors. Detection techniques
include electrochemical
sensing, genotyping, qPCR, sequencing, or combinations thereof. In some
embodiments, one or
more supramolecular structures are configured for multiplexing the sample,
wherein a plurality of
analyte molecules in the sample are detected simultaneously. In some
embodiments, the capture
molecules for each supramolecular structure are configured for binding to one
or more specific
types of analyte molecules.
[000201 In some embodiments, the sample comprises a complex biological sample
and the
method provides for single-molecule sensitivity thereby increasing a dynamic
range and
quantitative capture of a range of molecular concentrations within the complex
biological sample.
In some embodiments, the analyte molecule comprises a protein, a peptide, a
peptide fragment, a
lipid, a DNA, a RNA, an organic molecule, an inorganic molecule, complexes
thereof, or any
combinations thereof. In some embodiments, the supramolecular structure is a
nanostructure. In
some embodiments, the core structure is a nanostructure. In some embodiments,
the
supramolecular structure comprises a prescribed shape, size, molecular weight,
or combinations
thereof, so as to reduce or eliminate cross-reactions with another
supramolecular structure. In some
embodiments, the supramolecular structure comprises a plurality of capture
molecules.
-7-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
[00021] In some embodiments, the plurality of core molecules for the core
structure are arranged
into a pre-defined shape and/or have a prescribed molecular weight. In some
embodiments, the
pre-defined shape is configured to limit or prevent cross-reactivity with
another supramolecular
structure. In some embodiments, the plurality of core molecules for each core
structure comprises
one or more nucleic acid strands, one or more branched nucleic acids, one or
more peptides, one or
more small molecules, or combinations thereof. In some embodiments, the core
structure
independently comprises a scaffolded deoxyribonucleic acid (DNA) origami, a
scaffolded
ribonucleic acid (RNA) origami, a scaffolded hybrid DNA:RNA origami, a single-
stranded DNA
tile structure, a multi-stranded DNA tile structure, a single-stranded RNA
origami, a multi-
stranded RNA tile structure, hierarchically composed DNA or RNA origami with
multiple
scaffolds, a peptide structure, or combinations thereof
BRIEF DESCRIPTION OF THE DRAWINGS
[00022] Specific embodiments of the disclosed devices, delivery systems, or
methods will now be
described with reference to the drawings. Nothing in this detailed description
is intended to imply
that any particular component, feature, or step is essential to the invention.
[00023) FIG. 1 shows a supramolecular structure and the related subcomponents
according to
embodiments of the disclosure.
[00024] FIG. 2 shows a supramolecular structure with an associated analyte and
detector
molecule assembly according to embodiments of the disclosure.
[00025] FIG. 3 shows the supramolecular structure of FIG. 1 with an associated
analyte and
detector molecule assembly and the related subcomponents according to
embodiments of the
disclosure.
[00026] FIG. 4 shows a supramolecular structure with an associated analyte and
detector
molecule assembly that includes a detector core structure according to
embodiments of the
disclosure.
[00027] FIG. 5 shows specific binding of a supramolecular structure and a
detector molecule
assembly to an analyte according to embodiments of the disclosure.
[00028] FIG. 6 shows an analyte with no specific binding to a supramolecular
structure according
to embodiments of the disclosure.
[00029] FIG. 7 shows a workflow for analyte and detector binding to a
supramolecular structure
array according to embodiments of the disclosure.
-8-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
[00030] FIG. 8 shows an example capture molecule identification used in
conjunction with
analyte detection according to embodiments of the disclosure.
[00031] FIG. 9 shows an example of analyte detection according to embodiments
of the
disclosure.
[00032] FIG. 10 shows an example of analyte detection according to embodiments
of the
disclosure.
[00033] FIG. 11 shows an example of a supramolecular structure array that may
be used in
analyte detection according to embodiments of the disclosure.
[00034] FIG. 12 shows an example of a supramolecular structure array that may
be used in
analyte detection according to embodiments of the disclosure.
[00035] FIG. 13 shows an example of a supramolecular structure array that may
be used in
analyte detection according to embodiments of the disclosure.
[00036] FIG. 14 shows an example of a supramolecular structure array that may
be used in
antigen detection according to embodiments of the disclosure.
[00037] FIG. 15 shows an example porous matrix supramolecular structure array
that may be used
in analyte detection according to embodiments of the disclosure.
[00038] FIG. 16 shows a block diagram of an example analyte detection system
according to
embodiments of the disclosure.
DETAILED _DESCRIPTION
[00039] Disclosed herein are structures and methods for detecting one or more
analyte molecules
present in a sample. In some embodiments, the one or more analyte molecules
are detected based
on capture by one or more supramolecular structures. In some embodiments, the
one or more
supramolecular structures include or are linked to a capture molecule that
specifically binds to an.
analyte present in a sample. The bound analyte in turn interacts with a
detector molecule of a
detector molecule assembly that has a detectable moiety, such as a unique
identifier (e.g., a nucleic
acid sequence, a peptide, a polysaccharide, an acrydite) and/or a molecule
that includes, interacts
with, or that can be used to dock other molecules that can be detected (e.g.,
optically, electrically,
magnetically). In some embodiments, the detector molecule assembly generates a
DNA signal,
such that detection and quantification of binding of an analyte molecule to
the capture molecule
comprises converting the presence of the analyte molecule into a DNA signal
through
-9-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
amplification of the unique identifier of the supramolecular structure. In
some embodiments, the
detector molecule assembly is linked to an enzyme that converts a substrate to
an optically
detectable signal. In an embodiment, the supramolecular structure is a nucleic
acid origami that is
linked to or immobilized on a substrate. In an embodiment, the supramolecular
structure carries a
capture molecule via a barcode that include the unique identifier for the
capture molecule and that
links the capture molecule to a scaffold of the supramolecular structure.
[00040] In some embodiments, the disclosed techniques provide a single
molecule enzyme-linked
immunosorbent assay (ELISA) in which the supramolecular structure and
associated capture
molecule operate as a capture entity and the detector molecule assembly
operates as the detection
entity that is used to generate a detection signal (e.g., when reacted with
appropriate detection
reagents that are sensed using sensors of a detection system). Use of the
supramolecular structure
as the capture entity permits specific identification and, in embodiments,
location mapping of each
individual capture molecule immobilized on a substrate. Further, the
supramolecular structures are
configured to be organized on a substrate or within a porous material to
permit single molecule
binding.
[00041] Thus, as provided herein, detectable analyte binding can be associated
with an individual
capture molecule among many different capture molecules on the substrate to
generate assay
results in which binding characteristics of an analyte pool of multiple
different analytes are
characterized. This in turn permits a sample having an uncharacterized
composition of analytes to
analyzed for the presence and/or concentration of particular analytes of
interest. For example, a
human sample can be characterized to determine a presence and/or concentration
of antibodies
with binding specificity to particular antigens in a panel of antigen capture
molecules, such that the
capture molecules represent a known infectious disease antigen panel. The
assay results may show
positive binding results associated with a particular antigen, which is
indicative of the presence of
antibodies in the subject providing the sample. In another embodiment, the
identity of analytes in
the sample may be at least partially known, but their binding affinity may not
be characterized for
a particular pool of capture molecules. For example, the capture molecules can
be a set of
candidate drugs, and the analytes can be molecules in human blood. Binding of
a drug candidate
to such a protein can be used to assess bioavai lability or potential off-
target binding. The assay
results may show positive binding results associated with a particular drug
candidate that can in
-10-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
turn be mapped to a particular analyte, which is based on identification of
particular detector
binding (e.g., identifying binding by barcode identification in a detector
molecule assembly that
includes an antibody specific for the analyte).
100042j While conventional ELISA protocols may include detectable fluorescent
signals
generated by enzymes linked to detection antibodies as an indicator of
binding, the disclosed
techniques may additionally or alternatively provide amplified nucleic acid
signals from a unique
identifier of the detector molecule assembly and from which sequence
information can be
determined or from which an optically detectable signal is released that
corresponds to
amplification (e.g., qPCR using a primer/probe set specific for the unique
identifier). Thus, unique
identity information for the detector molecule assembly permits specific
identification of the
particular detector molecule that is linked to a capture molecule via the
bound analyte in certain
embodiments. However, in embodiments, the detector molecule assembly may not
carry a unique
identifier. Other detection techniques may include optical, magnetic, and or
electrical detection
techniques
Sample
1000431 Disclosed embodiments relate to analyte detection in which the
analytes are present in a
sample, such as a biological sample. In some embodiments, the sample comprises
an aqueous
solution comprising protein, peptides, peptide fragments, lipids, DNA, RNA,
organic molecules,
inorganic molecules, complexes thereof, or any combinations thereof. In some
embodiments, the
analyte molecules in the sample comprise protein, peptides, peptide fragments,
lipids, DNA, RNA,
organic molecules, inorganic molecules, complexes thereof, or any combinations
thereof In some
embodiments, the analyte molecules comprise comprises intact proteins,
denatured proteins,
partially or fully degraded proteins, peptide fragments, denatured nucleic
acids, degraded nucleic
acid fragments, complexes thereof, or combinations thereof. In some
embodiments, the sample is
obtained from tissue, cells, the environment of tissues and/or cells, or
combinations thereof In
some embodiments, the sample comprises tissue biopsy, blood, blood plasma,
urine, saliva, a tear,
cerebrospinal fluid, extracellular fluid, cultures cells, culture media,
discarded tissue, plant matter,
synthetic proteins, bacterial, viral samples, fungal tissue, or combinations
thereof In some
embodiments, the sample is isolated from a primary source such as cells,
tissue, bodily fluids (e.g.,
blood), environmental samples, or combinations thereof, with or without
purification. In some
-11 -
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
embodiments, the cells are lysed using a mechanical process or other cell
lysis methods (e.g., lysis
buffer). In some embodiments, the sample is filtered using a mechanical
process (e.g.,
centrifugation), micron filtration, chromatography columns, other filtration
methods, or
combinations thereof. In some embodiments, the sample is treated with one or
more enzymes to
remove one or more nucleic acids or one or more proteins. In some embodiments,
the sample
comprises intact proteins, denatured proteins, partially or fully degraded
proteins, peptide
fragments, denatured nucleic acids or degraded nucleic acid fragments. In some
embodiments, the
sample is collected from one or more individual persons, one or more animals,
one or more plants,
or combinations thereof. In some embodiments, the sample is collected from an
individual person,
animal and/or plant having a disease or disorder that comprises an infectious
disease, an immune
disorder, a cancer, a genetic disease, a degenerative disease, a lifestyle
disease, an injury, a rare
disease, an age-related disease, or combinations thereof.
Su pra molecular Structure
1000441 In some embodiments, the supramolecular structure is a programmable
structure that can
spatially organize molecules. In some embodiments, the supramolecular
structure comprises a
plurality of molecules linked together. In some embodiments, the plurality of
molecules of the
supramolecular structure interact with at least some of each other. In some
embodiments, the
supramolecular structure comprises a specific shape. In some embodiments, the
supramolecular
nanostructure comprises a prescribed molecular weight based on the plurality
of molecules of the
supramolecular structure. In some embodiments, the supramolecular structure is
a nanostructure.
In some embodiments the plurality of molecules are linked together through a
bond, a chemical
bond, a physical attachment, or combinations thereof. In some embodiments, the
supramolecular
structure comprises a large molecular entity, of specific shape and molecular
weight, formed from
a well-defined number of smaller molecules interacting specifically with each
other. In some
embodiments, the structural, chemical, and physical properties of the
supram.olecular structure are
explicitly designed. In some embodiments, the supramolecular structure
comprises a plurality of
subcomponents that are spaced apart according to a prescribed distance. In
some embodiments, at
least a portion of the supramolecular structure is rigid. In some embodiments,
at least a portion of
the supramolecular structure is semi-rigid. In some embodiments, at least a
portion of the
supramolecular structure is flexible.
-12-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
[00045] FIG. 1 provides an exemplary embodiment of a supramolecular structure
40 comprising a
core structure 13, a capture molecule 2, and an anchor molecule 18. In some
embodiments, the
supramolecular structure comprises one or more capture molecules 2and,
optionally, one or more
anchor molecules 18. In some embodiments, the supramolecular structure does
not comprise an
anchor molecule. In some embodiments, the supramolecular structure is a
polynucleotide structure.
[00046] in some embodiments, the core structure 13 comprises one or more core
molecules linked
together. In some embodiments, the one or more core molecules comprise 2, 3,
4, 5, 6, 7, 8, 9, 10,
20, 50, 100, 200 or 500 unique molecules that are linked together. In some
embodiments, the one
or more core molecules comprises from about 2 unique molecules to about 1000
unique molecules.
In some embodiments, the one or more core molecules interact with each other
and define the
specific shape of the supramolecular structure. In some embodiments, the
plurality of core
molecules interact with each other through reversible non-covalent
interactions.
[00047] In some embodiments, the specific shape of the core structure is a
three-dimensional (3D)
configuration. In some embodiments, the one or more core molecules provide a
specific molecular
weight. In some embodiments, the core structure 13 is a nanostructure. In some
cases, the one or
more core molecules comprise one or more nucleic acid strands (e.g., DNA, RNA,
unnatural
nucleic acids), one or more branched nucleic acids, one or more peptides, one
or more small
molecules, or combinations thereof. In some embodiments, the core structure
comprises a
polynucleotide structure. In some embodiments, at least a portion of the core
structure is rigid. In
some embodiments, at least a portion of the core structure is semi-rigid. In
some embodiments, at
least a portion of the core structure is flexible. In some embodiments, the
core structure comprises
a scaffolded deoxyribonucleic acid (DNA) origami, a scaffolded ribonucleic
acid (RNA) origami,
a scaffolded hybrid DNA / RN.A origami, a single-stranded DNA tile structure,
a multi-stranded
DNA tile structure, a single-stranded DNA origami, a single-stranded RNA
origami, a single-
stranded RNA tile structure, a multi-stranded RNA tile structures, a
hierarchically composed DNA
and/or RNA origami with multiple scaffolds, a peptide structure, or
combinations thereof. In some
embodiments, the DNA origami is scaffolded. In some embodiments, the RNA
origami is
scaffolded. In some embodiments, the hybrid DNAJRNA origami is scaffblded. In
some
embodiments, the core structure comprising a DNA origami, RNA origami, or
hybrid DNA/RNA
origami that comprises a prescribed two-dimensional (2D) or 3D shape.
-13-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
[00048] In an embodiment, the nucleic acid origami has at least one lateral
dimension between
about 50nin to about i.t. In an embodiment, the nucleic acid origami has at
least one lateral
dimension between about 50nm to about 200:nm, about 50nm to about 400nm, about
50nin to
about 600nm, about 50nm to about 800nm, about 100nm to about 200nm, about
100mn to about
300nm, about 100nm to about 400nrn, about 100nm to about 500nm, about 200nm to
about 400nm
by way of example. In an embodiment, the nucleic acid origami has at least a
first lateral
dimension between about 50nm to about lit and a second lateral dimension,
orthogonal to the first,
between about 50nm to about In an embodiment, the nucleic acid origami
has a planar
footprint having an area of about 200nm2 to about 1112.
[000491 As shown in FIG 1, in some embodiments, the core structure 13 is
configured to be
linked to a capture molecule 2an anchor molecule 18, or combinations thereof.
In some
embodiments, the capture molecule 2and/or anchor molecule IS are immobilized
with respect to
the core nanostructure 13 when linked thereto. In some embodiments, any number
of the one or
more core molecules comprises one or more core linkers 12, 14 configured to
form a linkage with
a capture molecule 2 and/or an anchor molecule 18. In some embodiments, any
number of the one
or more core molecules are configured to be linked with one or more core
linkers 12,14 that are
configured to form a linkage with a capture molecule 2and/or an anchor
molecule 18. In some
embodiments, one or more core linkers are linked to one or more core molecules
through a
chemical bond. In some embodiments, at least one of the one or more core
linkers comprises a core
reactive molecule. In some embodiments, each core reactive molecule
independently comprises an
amine, a thiol, a DBCO, a NHS ester, a maleimide, biotin, an azide, an
acrydite, a single stranded
nucleic acid (e.g., RNA or DNA) of specific sequence, or a polymer (e.g.,
polyethylene glycol
(PEG) or one or more polymerization initiators). In some embodiments, at least
one of the one or
more core linkers comprises a DNA sequence domain.
[000501 In some embodiments, the core structure 13 is linked to 1) a capture
molecule 2 at a
prescribed location on the core structure, and optionally 2) an anchor
molecule 18 at a prescribed
different location on the core structure.
[000511 In some embodiments, a specified first core linker 12 is disposed at
the first location on
the core structure. In some embodiments, one or more core molecules at the
first location are
modified to form a linkage with the first core linker 12. In some embodiments,
the first core linker
12 is an extension of the core structure 13.
-14-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
[00052] In some embodiments, a specified third core linker 14 is disposed at
the third location on
the core structure 13. In some embodiments, one or more core molecules at the
third location is
modified to form a linkage with the third core linker 14. In some embodiments,
the third core
linker 14 is an extension of the core structure 13. In some embodiments, the
first and second
locations are disposed on a first side of the core structure 13, and the
optional third location is
disposed on a second side of the core structure 13.
[000531 In some embodiments, the capture molecule 2 comprises a protein, a
peptide, an
antibody, an aptamers (RNA and DNA), a fluorophore, a nanobody, a darpin, a
catalyst, a
polymerization initiator, a polymer like PEG, an organic molecule, or
combinations thereof. In
some embodiments, the anchor molecule comprises a reactive molecule. In some
embodiments, the
anchor molecule 18 comprises a reactive molecule. In some embodiments, the
anchor molecule 18
comprises a DNA strand comprising a reactive molecule. In some embodiments,
the anchor
molecule 18 comprises an amine, a thiol, a DBCO, a NHS ester, a maleimide,
biotin, an azide, an
acrydite, a single stranded nucleic acid (e.g., RNA or DNA) of specific
sequence, or a polymer
(e.g., polyethylene glycol (PEG) or one or more polymerization initiators). In
some embodiments,
the anchor molecule 18 comprises a protein, a peptide, an antibody, an
aptamers (RNA and DNA),
a fluorophore, a nanobody, a darpin, a catalyst, a polymerization initiator, a
polymer like PEG, an
organic molecule or combinations thereof. In some embodiments, a single
capture molecule 2 is
linked to the core structure 13. In some embodiments, a plurality of capture
molecules 2 are linked
to a core structure 13. In some embodiments, the plurality of pairs of capture
molecules 2 are
spaced apart from each other to minimize cross-talk.. For example, different
capture molecules 2
on a same core structure 13 may represent different binding sites for a same
analyte molecule or
may bind different analyte molecules. In another example, multiple same
capture molecules 2 may
be present on a core structure 13.
100054j In some embodiments, each component of the supramolecular structure
may be
independently modified or tuned. In some embodiments, modifying one or more of
the
components of the supramolecular structure may modify the 2D and 3D geometry
of the
supramolecular structure itself In some embodiments, modifying one or more of
the components
of the supramolecular structure may modify the 2D and 3D geometry of the core
structure. In some
embodiments, such capability for independently modifying the components of the
supramolecular
nanostruct-ure enables precise control over the organization of one or more
supramolecular
-15-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
structures on solid surfaces (e.g., planar surfaces or microparticles) and 3D
volumes (e.g., within a
hydrogel matrix).
Capture ',arcade
[000551 As shown in FIG-. 1, in some embodiments, the capture molecule 2 is
linked to the core
structure 13 through a capture barcode 20. In some embodiments, the capture
barcode 20 forms a
linkage with the capture molecule 2, and the capture barcode 20 forms a
linkage with the core
structure 13. In some embodiments, the capture barcode 20 comprises a first
capture linker 11, a
second capture linker 6, and a capture bridge 7. In some embodiments, the
first capture linker 11
comprises a reactive molecule. In some embodiments, the first capture linker
11 comprises a
reactive molecule comprising an amine, a thiol, a DBCO, a NI-IS ester, a
maleimide, an azide, an
acrydite, a single stranded nucleic acid (e.g., RNA or DNA) of specific
sequence, or a polymer
(e.g., polyethylene glycol (PEG) or one or more polymerization initiators). In
some embodiments,
the first capture linker 11 comprises a DNA sequence domain. In some
embodiments, the second
capture linker 6 comprises a reactive molecule. In some embodiments, the
second capture linker 6
comprises a reactive molecule comprising an amine, a thiol, a DBCO, a NHS
ester, biotin, a
maleimide, an azide, an acrydite, a single stranded nucleic acid (e.g., RNA or
DNA) of specific
sequence, or a polymer (e.g., polyethylene glycol (PEG) or one or more
polymerization initiators).
In some embodiments, the second capture linker comprises a DNA sequence
domain. In some
embodiments, the capture bridge 7 comprises a polymer. In some embodiments,
the capture bridge
7 comprises a polymer that comprises a nucleic acid (e.g., DNA. or RNA.) of a
specific sequence. In
some embodiments, the capture bridge 7 comprises a polymer such as PEG. In
some embodiments,
the first capture linker 11 is attached to the capture bridge 7 at a first
terminal end thereof, and the
second capture linker 6 is attached to the capture bridge 7 at a second
terminal end thereof. In
some embodiments, the first capture linker 11 is attached to the capture
bridge 7 via a chemical
bond. In. some embodiments, the second capture linker 6 is attached to the
capture bridge 7 via a
chemical bond. In some embodiments, the first capture linker 11 is attached to
the capture bridge 7
via a physical attachment In some embodiments, the second capture linker 6 is
attached to the
capture bridge 7 via a physical attachment.
[000561 In some embodiments, the capture barcode 20 is linked to the core
structure 13 through a
linkage between the first capture linker 11 and the first core linker 12. In
some embodiments, as
-16-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
described herein, the first core linker 12 is disposed at a first location on
the core structure 13. In
some embodiments, the first capture linker 11 and first core linker 12 are
linked together through a
chemical bond. In some embodiments, the first capture linker 11 and first core
linker 12 are linked
together through a covalent bond.
[000571 In some embodiments, the capture barcode 20 is linked to the capture
molecule 2 through
a linkage between the second capture linker 6 and a third capture linker 5
that is bound to the
capture molecule 2. In some embodiments, the third capture linker 5 comprises
a reactive
molecule. In some embodiments, the third capture linker 5 comprises a reactive
molecule
comprising an amine, a thiol, a DBCO, a NHS ester, a maleimide, biotin, an
azide, an acrydite, a
single stranded nucleic acid (e.g., RNA or DNA) of specific sequence, or a
polymer (e.g.,
polyethylene glycol (PEG) or one or more polymerization initiators). In some
embodiments, the
third capture linker 5 comprises a DNA sequence domain. In some embodiments,
the capture
molecule 2 is bound to the third capture linker 5 through a chemical bond. In
some embodiments,
the capture molecule 2 is bound to the third capture linker 5 through a
covalent bond. In some
embodiments, the second capture linker 6 and third capture linker 5 are linked
together through a
chemical bond. In some embodiments, the second linker 6 and third capture
linker 5 are linked
together through a covalent bond.
Anchor Barcode
1000581 As shown in FIG. 1, in some embodiments, the anchor molecule 18 is
linked to the core
structure 13 through an anchor barcode. In some embodiments, the anchor
barcode forms a linkage
with the anchor molecule 18, and the anchor barcode forms a linkage with the
core structure 13. In
some embodiments, the anchor barcode comprises a first anchor linker 15, a
second anchor linker
17, and an anchor bridge 16. In some embodiments, the first anchor linker 15
comprises a reactive
molecule. In some embodiments, the first anchor linker 15 comprises a reactive
molecule
comprising an amine, a thiol, a DBCO, a MIS ester, a maleimide, biotin, an
azide, an acrydite, a
single stranded nucleic acid (e.g., RNA or DNA) of specific sequence, or a
polymer (e.g.,
polyethylene glycol (PEG) or one or more polymerization initiators). In some
embodiments, the
first anchor linker 15 comprises a DNA sequence domain. In some embodiments,
the second
anchor linker 17 comprises a reactive molecule. In some embodiments, the
second anchor linker
17 comprises a reactive molecule comprising an amine, a thiol, a DBCO, a NHS
ester, a
maleimide, biotin, an azide, an acrydite, a single stranded nucleic acid
(e.g., RNA or DNA) of
-17-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
specific sequence, or a polymer (e.g., polyethylene glycol (PEG) or one or
more polymerization
initiators). In some embodiments, the second anchor linker 17 comprises a DNA
sequence domain.
In some embodiments, the anchor bridge 16 comprises a polymer. In some
embodiments, the
anchor bridge 16 comprises a polymer that comprises a nucleic acid (DNA or
RNA) of a specific
sequence. In some embodiments, the anchor bridge 16 comprises a polymer such
as PEG. In some
embodiments, the first anchor linker 15 is attached to the anchor bridge 16 at
a first terminal end
thereof, and the second anchor linker 17 is attached to the anchor bridge 16
at a second terminal
end thereof. In some embodiments, the first anchor linker 15 is attached to
the anchor bridge 16 via
a chemical bond. In some embodiments, the second anchor linker 17 is attached
to the anchor
bridge 16 via a physical attachment. In some embodiments, the first anchor
linker 15 is attached to
the anchor bridge 16 via a chemical bond. In some embodiments, the second
anchor linker 17 is
attached to the anchor bridge 16 via a physical attachment
[000591 In some embodiments, the anchor barcode is linked to the core
structure 13 through a
linkage between the first anchor linker 15 and the third core linker 14. In
some embodiments, as
described herein, the third core linker 14 is disposed at a third location on
the core structure 13. In
some embodiments, the first anchor linker 15 and third core linker 14 are
linked together through a
chemical bond. In some embodiments, the first anchor linker 15 and third core
linker 14 are linked
together through a covalent bond.
[000601 In some embodiments, the anchor barcode is linked to the anchor
molecule 1.8 through a
linkage between the second anchor linker 17 and the anchor molecule 18. As
disclosed herein, in
some embodiments, the anchor molecule comprises a reactive molecule, a
reactive molecule, a
DNA sequence domain, a DNA sequence domain comprising a reactive molecule, or
combinations
thereof. In some embodiments, the anchor molecule 18 is bound to the second
anchor linker 17
through a chemical bond. In some embodiments, the anchor molecule 18 is bound
to the second
anchor linker 17 through a covalent bond.
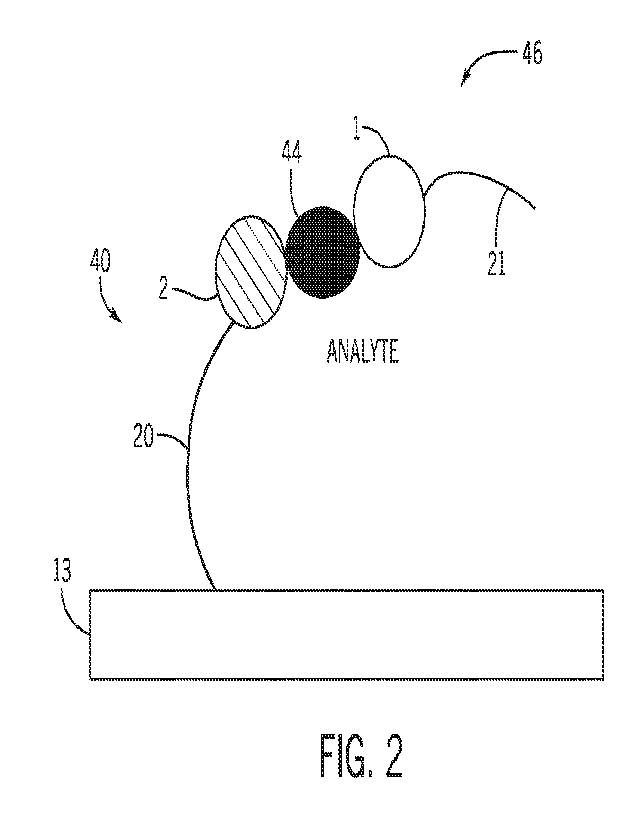
[000611 FIG. 2 is a schematic illustration of the supramolecular structure 40
bound to a
corresponding analyte molecule 44 having binding specificity for the capture
molecule 2. The
supramolecular structure 40 is capable of binding to one or more analyte
molecules 44 as a
function of the particular capture molecule 2 associated with the
supramolecular structure 40. The
analyte molecule 44 is also capable of binding to a detector molecule assembly
46. The detector
molecule assembly 46 includes a detector molecule 1 that binds with
specificity to the analyte
-18-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
molecule 44. FIG. 2 shows a sandwich-type binding arrangement in which the
capture molecule 2
and the detector molecule I bind to different sites on the analyte molecule
44. In some
embodiments, the detector molecule 1 comprises a protein, a peptide, an
antibody, an aptamers
(RNA and DNA), a fluorophore, a nanobody, a darpin, a catalyst, a
polymerization initiator, a
polymer like PEG, an organic molecule, or combinations thereof As shown in
FIG. 2, in some
embodiments, the detector molecule 1 is linked to a detector barcode 21. In
some embodiments,
the detector barcode 21 forms a linkage with the detector molecule 1, and may
include one or more
intervening components. In other embodiments, the detector molecule I is
linked to a detectable
tail or linker, but does not carry unique barcode information.
[000621 FIG. 3 shows an example arrangement of the detector barcode 21. In
some embodiments,
the detector barcode comprises one or more detector linkers including a first
detector linker 4. The
detector barcode 21 may include a dock 8 that serves as an attachment or
extension/amplification
site to facilitate detection. In some embodiments, the linker 4 comprises a
reactive molecule. In
some embodiments, the linker 4 comprises a reactive molecule comprising an
amine, a thiol, a
DBCO, a NHS ester, a maleimide, biotin, an azide, an acrydite, a single
stranded nucleic acid (e.g.,
RNA or DNA) of specific sequence, or a polymer (e.g., polyethylene glycol
(PEG) or one or more
polymerization initiators). In some embodiments, the linker 4 comprises a DNA
sequence domain.
In some embodiments, the dock 8comprises a polymer. In some embodiments, the
dock 8
comprises a polymer that comprises a nucleic acid (DNA or RNA.) of a specific
sequence, e.g., a
single-stranded or double-stranded nucleic acid. In some embodiments, the dock
8 comprises a
polymer such as PEG. In some embodiments, the linker 4 is attached to the dock
8 at a terminal
end thereof, and another detector linker 4 is attached to the dock 8 at a
second terminal end
thereof. The attachments may be via a chemical bond or a physical attachment.
[000631 In some embodiments, the detector barcode 21 is linked to the detector
molecule 1
through a linkage between a plurality of linkers, shown here as the detector
linker 4 and a second
detector linker 3 bound to the detector molecule 1. In some embodiments, the
second detector
linker 3 comprises a reactive molecule. In some embodiments, the second
detector linker 3
comprises a reactive molecule comprising an amine, a thiol, a DBCO, a NHS
ester, a maleimide,
biotin, an azide, an acrydite, a single stranded nucleic acid (e.g., RNA or
DNA) of specific
sequence, or a polymer (e.g., polyethylene glycol (PEG) or one or more
polymerization initiators).
In some embodiments, the second detector linker 3 comprises a DNA sequence
domain. En some
-19-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
embodiments, the detector molecule 1 is bound to the second detector linker 3
through a chemical
bond. In some embodiments, the detector molecule 1 is bound to the second
detector I hiker 3
through a covalent bond. In some embodiments, the second detector linker 4 and
second detector
linker 3 are linked together through a chemical bond. In some embodiments, the
second detector
linker 4 and second detector linker 3 are linked together through a covalent
bond.
100064i As provided herein, a capture molecule assembly includes a
supramolecular structure 40
having the capture molecule 1 and the core structure 13. In certain
embodiments, the detector
molecule assembly 46 is linked to or coupled to a core structure 13, such that
the detector molecule
assembly is a supramolecular structure as provided herein as well. Thus, as
shown in FIG. 4,
detector molecule binding to the captured analyte molecule 44 associated with
the capture
molecule assembly supramolecular structure 40 creates a supramolecular
structure sandwich.
Generally, only one of the supramolecular structure 40 of the capture molecule
assembly or the
detector molecule assembly 46 is immobilized to permit flow of capture or
detection entities. In an
embodiment, the capture molecule assembly is immobilized on a surface or in a
porous material.
[000651 The supramolecular structure 40 and/or the detector molecule assembly
46 may include a
DNA origami. In some embodiments, the subcomponents of the core structure 13
of the
supramolecular structure 40 and/or the detector molecule assembly 46 comprises
a DNA origami
as well as one or more extending nucleic acid strands. In some embodiments,
the core structure 13
of the supramolecular structure 40 and/or the detector molecule assembly 46
comprises a
scaffolded DNA origami, wherein a circular ssDNA molecule, called "scaffold"
strand, is folded
into a predefined 2D or 3D shape by interacting with 2 or more short ssDNA,
called "staple"
strands, which interact with specific sub-sections of the ssDNA "scaffold"
strand.
[00066] As described herein, in some embodiments, one or more supramolecular
structures enable
the detection of one or more analyte molecules in a sample. As shown in a
schematic illustration of
FIG. 5, the supramolecular structure 40, with an associated capture molecule,
is exposed to an
analyte molecule 44 and a detector molecule assembly 46. When the individual
analyte molecule
44 and individual capture molecule 2 have binding specificity for one another,
the analyte
molecule 44 associates with the capture molecule 2. In turn, the detector
molecule assembly 46,
which has binding specificity for the analyte molecule 44, associates with the
analyte molecule 44
to create a bound detection structure 50.
-20-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
[00067] As shown in FIG. 6, analyte molecules 44 with no binding specificity
for the capture
molecule 2 do not associate with the suprainolecular structure 40. In turn,
the detector molecule
assembly 46 also does not associate with the supramolecular structure 40, and
no bound detection
structure 50 is created. For substrates that include an immobilized array of
supramolecular
structures 40, each individual site may have a binding reaction to create the
bound detection
structure 50 when the sample includes an appropriate analyte molecule 44 with
binding specificity
for the capture molecule 2
[000681 While the depicted embodiment shows the detector molecule assembly 46
and the analyte
molecule 44 contacted with the supramolecular structure 40 in one step, it
should be understood
that the analyte molecule 44 and detector molecule assembly 46 may be added in
separate steps as
provided herein and such that any unbound analyte molecule 44 is removed
before addition of the
detector molecule assembly 46. FIG. 7 shows an example method workflow in
which a pool of
analyte molecules 44 is added to a group or array of capture molecule
assemblies implemented as
supramolecular structures 40. The analyte molecules 44 may represent different
analytes present
in a sample, such that different analyte molecules 44 in the pool have
different degrees of binding
specificity to the array of available capture molecules 2.
[00069] The reaction conditions permit binding of the analyte molecules 44 to
specific capture
molecules 2. As provided herein, binding specificity may refer to an
interaction between the
analyte molecule 44 and the capture molecule 2 that remains intact under the
reaction conditions
and after washing or removal steps for unbound reagents. Binding specificity
may include
formation of a covalent or non-covalent bonds, ionic bonds, dipole
interactions, hydrophilic or
hydrophobic interactions, complementary nucleic acid binding, etc. Specific
binding may refer to
binding to an analyte molecule 44 that binds only to a particular capture
molecule 2 and not to
other capture molecules 2. Thus, certain capture molecules 2 of the array bind
to analyte molecules
44 (e.g., the capture molecule 2a) while other capture molecules have no
available binding partners
in a given sample (e.g., capture molecule 2b) and, therefore, do not bind to
any analyte molecule
44 with specificity. Any unbound analytes can be removed from the capture
molecule assemblies,
which are immobilized as provided herein.
[00070] The detector molecule 1 and the analyte molecule 44 may also have
binding specificity to
one another. The array is subsequently contacted with detector molecule
assemblies 46, which
may all be the same or different, as disclosed in various embodiments. Any
unbound detector
-21 -
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
molecule assemblies 46 are removed, e.g., by washing. After these workflow
steps, various bound
detector structures 50 remain on the array, each bound to respective analytes
and detector molecule
assemblies 46 and may be subjected to various detection protocols to associate
the analyte to a
particular supramolecular structure identity, which in turn is associated with
a known capture
molecule 2. Thus, detection permits characterization of analyte-capture
molecule binding.
[000711 FIG. 8 shows an example detection step in which different unique
capture barcodes
(illustrated as capture barcodes 20a, 20b, 20c, and 20d) of the bound detector
structures 50 are
assessed to associate a particular capture barcode with a binding event. In
some embodiments, the
supramolecular structure converts information about the presence of a given
analyte molecule in a
sample to a DNA signal. In some embodiments, the DNA signal corresponds to
sequence data for
a capture barcode and/or detector barcode, wherein the capture molecule and
detector molecule are
simultaneously linked to (e.g., bound to) the analyte molecule (e.g., sandwich
formation).
[000721 In some embodiments, detecting the presence of an analyte molecule, as
described herein,
comprises controllably releasing a single, or multiple, unique nucleic acid
molecules into the
solution to be used to identify as well as quantify properties of the analyte
molecule from the
sample. In some embodiments, said unique nucleic acid molecules are provided
by capture
barcodes 20 of the respective supramolecular structures. In some embodiments,
detecting the
presence of an analyte molecule, as described herein, comprises creating an
optical or electrical
signal connected to the state change that can be counted to quantify the
concentration of the
analyte molecule in solution.
[000731 In some embodiments, a plurality of analyte molecules are
simultaneously detected in a
sample through multiplexing, wherein a plurality of supramolecular structures
provide a plurality
of signals (e.g., detector barcode, capture barcode) for sequencing and
analyte identification. In
some embodiments, methods described herein for detecting analytes in a sample
provide a high-
throughput and high-multiplexing capability by using a plurality of
supramolecular structures. In
some embodiments, the high-throughput and high-multiplexing capability
provides high accuracy
for analyte molecule detection and quantification. In some embodiments,
methods described herein
for detecting analytes in a sample are configured to characterize and/or
identify biopolymers,
including proteins molecules, quickly and at high sensitivity and
reproducibility. In some
embodiments, the plurality of supramolecular structures are configured to
limit cross-reactivity
associated errors. In some embodiments, such cross-reactivity associated
errors comprise capture
-22-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
and/or detector molecules of a supramolecular structure interacting with
capture and/or detector
molecules of another supramolecular structure (e.g., intermolecular
interactions). In some
embodiments, each core structure of the plurality of supramolecular structures
is identical to one
another. In some embodiments, the structural, chemical, and physical property
of each
supramolecular structure is explicitly designed. In some embodiments,
identical core structures
have a prescribed shape, size, molecular weight, prescribed number of capture
and detector
molecules, predetermined distance between corresponding capture and detector
molecules (as
described herein), prescribed stoichiometry between corresponding capture and
detector
molecules, or combinations thereof, so as to limit the cross-reactivity
between supramolecular
structures. In some embodiments, the molecular weight of every core structure
is identical and
precise up to the purity of the core molecules. In some embodiments, each core
structure has at
least one capture molecule.
[000741 In some embodiments, the plurality of supramolecular structures might
share structural
similarities due to certain subcomponents being the same, however the
interaction between an
analyte molecule from the sample and supramolecular structure is defined by
the corresponding
capture molecule and detector molecule. In some embodiments, each bound
detector and capture
molecules on a given bound detection structure 50 may specifically interact
with a particular
analyte molecule in the sample. In some embodiments, each supramolecular
structure comprises
unique DNA barcodes corresponding to the associated capture molecule. In some
embodiments, a
capture molecules is designed to interact with more than one analyte molecule
in the sample.
[000751 As provided herein, the capture barcode 20 can be used to uniquely
identify individual
supramolecular structures 40. In turn, each supramolecular structure 40 is
assembled so that the
capture molecule 2 may be associated with the capture barcode 20, e.g., stored
in a lookup table of
an analyte detection system (see FIG. 16). Thus, when the capture barcode 20
is identified, the
identity of the capture molecule 2 is also accessible.
[000761 In some embodiments, each supramolecular structure is configured for
single-molecule
sensitivity to ensure the highest possible dynamic range needed to
quantitatively capture the wide
range of molecular concentrations within a typical complex biological sample.
In some
embodiments, the plurality of supramolecular structures limit or eliminate the
manipulation of the
sample needed to reduce non-specific interaction as well as any user induced
errors.
-23-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
[00077] FIG. 9 shows an example analyte detection technique in which different
the locations of
suprainolecular structures 40 with respective different capture molecules 2,
together with unique
capture barcode information, can be used to characterize analyte binding. A
sample including a
pool of different analyte molecules 44 is contacted with immobilized
supramolecular structures 40
with respective different capture molecules 2. Analyte molecules 44 and
capture molecules 2 with
binding specificity for one another are contacted under conditions to allow
the interaction to occur.
Detector molecule assemblies 46 are permitted to bind to analyte molecules 44
that are associated
with the supramolecular structures. The bound detector structures can be
characterized based on 1)
the capture barcode 20 and 2) a signal generated by a bound detector molecule
assembly that
corresponds to a location map of a particular capture barcode 20. In an
embodiment, the locations
of the supramolecular structures 40, together with capture barcode
information, can be determined
before analyte binding. That is, the array may be provided pre-mapped, or the
mapping can be a
separate step. The mapping may include a step of detecting the capture barcode
as generally
provided herein, such as detecting a unique optical, electrical, and/or
magnetic pattern. In an
embodiment, the detection includes sequencing a nucleotide sequence of the
capture barcode. In
an embodiment, the detection includes amplification and quantitation of the
amplified product,
e.g., detection of a signal associated with a probe via qPCR.
[00078] Serology tests look for antibodies in a patient's blood to identify a
past infection with a
pathogen. In one example, COVID-I9 serology assays detect the presence of IgG
or IgNI
antibodies against spike protein or nucleocapsid. The capture molecules 2 pull
down antibody
analytes 44 in the patient sample, which are also bound by detector molecule
assemblies 46. In an
embodiment, the detector molecule assemblies 46 may be all of a same type
and/or all have a same
detector moleculel . The detector molecule I can be an anti-human antibody
that binds to any
human antibody, regardless of the antibody-antigen specificity. Thus, the
detector molecule 1 is
capable of binding a range of different analytes 44 associated with respective
antigen capture
molecules 2. The analytes 44 that represent a positive binding event via a
detectable signal from
the detector molecule assemblies 46 can be linked to a particular
supramolecular structure 40
based on the particular barcode 20 to identify the positive antibody result.
The disclosed techniques may be used to create an assay for one or more
infectious diseases, such
as COVID-19, Influenza, RSV, and Pneumonia. In an embodiment, the capture
molecules 2
include a pool of different antigens of different infectious diseases and
respectively associated with
-24-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
different supramolecular structures 40. Additionally or alternatively, the
assay may include
multiple isoforms and multiple potential antigens of an infectious disease as
well as the antigens
from other respiratory pathogens including but not limited to Influenza, RSV,
and Pneumonia. In
an embodiment, the assay may permit differentiation between natural immunity
and gained
immunity from a vaccine through the specific addition of vaccine protein
targets. The assay may
also include the differentiation between IgG, IgM and IgA specificity. This
assay can be updated
or modified seasonally as new infections arises to appropriately interrogate
the current pathogen
climate. The improvement made by this assay to the current workflow will give
greater insight to
the patient's humoral immune system, as well as help inform vaccine
development. For example,
a patient's antibody response or circulating antibody population can be
assessed for binding to
various candidate antigens.
100079] The detector molecule assemblies 46 may be all of a same type or may
be detected using
a same detection modality. The detected signal may not include any unique
barcode information.
In one example, the detector tail may include a reactive molecule that
generates the signal. In an
embodiment, the detector molecule assemblies 46 are detected based on enzyme
conversion of a
substrate to an optically detectable product. The optical detection is
associated with a spot on the
array of supramolecular structures 40. In an embodiment, the detector molecule
1 of each of the
detector molecule assemblies 46 may be all of a same type and/or have a same
binding specificity.
In one example, the analyte molecules 44 detected are all human antibodies,
and the detector
molecule is an anti-human antibody with general binding specificity to a wide
range of human
antibodies, regardless of antigen specificity. Other embodiments are also
contemplated. For
example, the analyte molecules 44 may undergo a tagging or processing step to
add a tag (e.g.,
biotin) that permits binding to streptavidin detector molecules 1. In the
depicted embodiment, the
step of providing the detector molecule assemblies 46 may be less complex,
because the pool of
detector molecule assemblies 46 is not diverse, and the detector molecules I
and associated tail or
linkers may also be all of the same type. Further, the detection step may also
be less complex,
because no sequence or barcode information from the detector side is obtained.
Thus, in an
embodiment, the unique identification information is the capture barcode 20
that is used together
with location information for each capture barcode and a detector-generated
signal location.
Correlation of the detector signal with location of particular barcodes is
used to characterize
analyte binding. It should be understood that the method of analyte detection
FIG. 9 may also be
-25-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
performed using a diverse pool of detector molecule assemblies having unique
barcodes as well as
reactive molecules that generate the detector signal.
1000801 FIG. 10 shows an embodiment that is similar to the analyte detection
method of FIG. 9,
but in which the pool of detector molecule assemblies 46 is diverse. The
diverse pool of detector
molecule assemblies 46 carries different respective detector molecules 1 and
detector barcodes 21
(shown as detector barcode 21a, 21b, 21c, and 21d). The bound detector
structures 50 include both
a unique capture barcode 20 associated with the capture molecule 2 specific
for a particular analyte
molecule 44 as well as a unique detector barcode 21 specific for the analyte
molecule 44. One or
both of the unique capture barcode 20 or the unique detector barcode 21 can be
assessed to
characterize analyte binding. Detection of the unique detector barcode 21 may
be performed using
techniques discussed with reference to the unique capture barcode 20,
including optical, electrical,
and/or magnetic detection. Detection may include generating sequence data or
amplification data
of the unique detector barcode 21.
1000811 In some embodiments, the sample, comprising one or more analytes is
contacted with the
one or more supramolecular structures 40. In some embodiments, as described
herein, the plurality
of supramolecular structures are provided as being attached to one or more
solid substrates. FIGS.
11-14 provide examples of supramolecular structures attached to a patterned
solid substrate. FIG.
15 shows an example of supramolecular structures incorporated into a porous
hydrogel matrix.
The disclosed techniques may be performed in conjunction with a patterned
substrate including
binding sites distributed on or in the binding site. In an embodiment, each
binding site
accommodates a single supramolecular structure 40 with differential chemistry.
The patterned
substrate may be fabricated through lithography processes. Further,
embodiments of the disclosed
techniques may include one or more regeneration steps that remove a bound or
"used" bound
detector structure 50 from the substrate to incorporate a new supramolecular
structure 40.
100082j FIG. 11 provides an exemplary illustration of a method for detecting
analyte molecules in
a sample using a surface based assay that uses supramolecular structures 40,
as described herein,
for single-molecule counting of analytes in the sample (i.e. detecting analyte
molecules in the
sample at a single molecule resolution). In some embodiments, the
supramolecular structures
comprise a core structure comprising a DNA origami core. In some embodiments,
a planar
substrate 60 is provided comprising (a) Fiduciary markers 62 that serves as a
reference
coordinates for all the features on the substrate 60; (b) A defined set of
micropatterned binding
-26-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
sites 66 where individual core structures (e.g., DNA origami) may be
immobilized; (c) background
passivation 64 that minimizes or prevents interaction between the surface of
the substrate 60 and
the supramolecular structure (including capture molecules, core structure
molecules). In some
embodiments, the fiduciary markers comprise geometric features defined on a
surface to be used as
reference features for other features on the substrate. In some embodiments,
the fiduciary markers
62 are coated with a polymer or self-assembled monolayer that does not
interact with a core
structure or other molecules of the supramolecular structure (e.g., DNA
origami). In some
embodiments, the background passivation 64 minimizes or prevents interaction
between the
surface of the substrate 60 and analyte molecules of the sample. In some
embodiments, the planar
substrate 60 comprises optical or electrical devices like FET, ring
resonators, photonic crystals or
microelectrode, to be defined prior to the formation of the binding sites 66.
In some embodiments,
the binding sites 66 are rnicropattemed on the planar substrate 60. In some
embodiments, the
binding sites 66 on the surface are in a periodic pattern. In some
embodiments, the binding sites 66
on the surface are in a non-periodic pattern (e.g., random). In some
embodiments, a minimum
distance is specified between any two binding sites 66. In some embodiments,
the minimum
distance between any two binding sites 66 is at least about 200 nrn. In some
embodiments, the
minimum distance between any two binding sites 66 is from at least about 40 nm
to about 5000
nm. In some embodiments, the geometric shape of the binding sites 66 comprises
a circle, square,
triangle or other polygon shapes. In some embodiments, the chemical groups
that are used for
passivation 64 comprise neutrally charged molecules like a Tr-methyl silyl
(TMS), an uncharged
polymer like PEG a zwitterionic polymer like, or combinations thereof. In some
embodiments, the
chemical group used to define the binding site 66 comprises a silanol group,
carboxyl group, thiol,
other groups, or combinations thereof.
[000831 In some embodiments, a single supramolecular structure 40 is attached
to a respective
binding site 66 (Step 1). Reference character 70 provides a depiction of the
components of the
supramolecular structure 40, individually and as assembled and arranged on the
planar substrate
(components are as described herein, e.g., FIGS. 1-4). In some embodiments,
the supramolecular
structure 40 comprises a core structure 13 comprising a DNA origami, wherein
the supramolecular
structures 40 is attached onto each of the binding sites using DNA origami
placement technique
(step 1). In some embodiments, the supramolecular structure 40 is assembled
prior to being
attached to a respective binding site 66. In some embodiments, the DNA origami
comprises a
-27-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
unique shape and dimension, so as to facilitate binding to a binding site
using the DNA origami
placement technique. In some embodiments, DNA origami placement comprises a
directed self-
assembly technique for organizing individual DNA origami (e.g., a core
structure) on a surface
(e.g., micropatterned surface). In some embodiments, alternatively to the DNA
origami placement,
a reactive group of the supramolecular nanostructure 40 is bound to a DNA
origami that has been
pre-organized on the binding site. In some embodiments, both of these methods
for binding a
supramolecular nanostructure to a corresponding binding site rely on the
ability to organize one or
more molecules on a micropattemed binding site using the DNA origami placement
technique. In
some embodiments, the planar substrate could be stored for a significant
period after this step, in a
clean environment.
1000841 With continued reference to FIG. 11, in some embodiments, a sample (as
described
herein) comprising analyte molecules is contacted with the planar substrate
(step 2) in an analyte
capture step. In some embodiments, the sample is contacted with the planar
substrate using a flow-
cell. In some embodiments, the sample is incubated on the planar substrate
with the
supramolecular structures attached to the binding sites 66. In some
embodiments, the incubation
period may be from about 30 seconds to about 24 hours. In some embodiments,
the incubation
period may be from about 30 seconds to about 1 minute, from about 1 minute to
about 5 minutes,
from about 5 minutes to about 30 minutes, from about 30 minutes to about 1 hr,
from about lhr to
about 5 hours, from about 5 hours to about 12 hours, from about 12 hours to
about 24 hours, from
about 24 hours to about 48 hours.
[000851 In some embodiments, the analyte molecules 44, in the sample, interact
with the
supramolecular structures 40 on the planar surface 60. In some embodiments, a
single copy of a
specific analyte molecule 44 binds to a capture molecule. With continued
reference to FIG. 11, at
step 3, detector molecule assemblies 46 are contacted with the captured
analytes. The detector
molecular assemblies are detected to generate a detectable signal at step 4.
For example, the
detector barcode 21 is used as a binding site for a signaling element 76 that
is contacted with the
bound detector molecule assemblies 46. In some embodiments, the signaling
element 76 comprises
a fluorescent molecule or microbead, a fluorescent polymer, highly charged
nanoparticles or
polymer. In some embodiments, one or more signaling elements 76 are allowed to
interact with the
supramolecular structures on the planar structure. In some embodiments, the
signaling elements 76
are introduced into the flow-cell containing the planar substrates. In some
embodiments, the
-28-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
detector barcode is amplified, as shown in the depicted embodiment. For
example, the detector
barcode is used as a polymerization initiator for growth of highly fluorescent
polymer in a process
such as rolling circle amplification or hybridization chain reaction. In some
embodiments, the
detectable signal as described with step 4 leads to a surface in which every
individual analyte
capture event leads to a signaling element 76 being present at the location of
the respective analyte
(as linked with the capture and detector molecules).
[000861 In some embodiments, the signaling element 76 is optically active and
can be measured
using a microscope or integrated optically sensor within the planar substrate
60. In some
embodiments, the signaling element is electrically active and may be measured
using an integrated
electrical sensor. In some embodiments, the signaling element 76 is
magnetically active and may
be measured using an integrated magnetic sensor. In some embodiments, each
signal event is
associated with the capture of the same type of analyte molecule (a single
copy of the same type of
analyte molecule), determined by the corresponding detector and capture
molecule, thus counting
the number of locations where the signaling element 76 is present gives the
quantification of the
analyte molecule in the sample
100087) FIG. 12 shows an arrangement having a similar planar substrate 60 with
binding sites 66
as in FIG. 11. The planar substrate 60 has assembled supramolecular structures
40 immobilized at
respective binding sites 66 (step 1). After analyte capture (step 2), detector
molecule assemblies
are contacted with the captured analyte molecules 44. Here, each detector
molecule assembly 46
includes a bound core structure (e.g., core structure 13, see FIG. 1), such as
DNA origami, that
operates as an integral signaling element 76. FIG. 13 shows an arrangement in
which the planar
substrate 60 (which may be formed as described with respect to FIG. 11) has
assembled
supramolecular structures 40 immobilized at respective binding sites 66 (step
1). A separate
process associates analytes 44 with respective detector molecule assemblies
46. In an
embodiment, each detector molecule assembly 46 carries a signal element 76,
such as a
supramolecular core structure. The associated analytes and detector molecule
assemblies 46 are
incubated with the planar substrate 60 and immobilized supramolecular
structures 40. The
associated analytes and detector molecule assemblies 46 bind to supramolecular
structures having
capture molecules with binding specificity for the analyte, and the bound
analytes can be
characterized e.g., via detecting signals generated from a signaling element
(which may be part of
-29-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
the detector molecule assembly 46 or which may be added after association of
the analytes and
detector molecule assemblies 46 with the capture molecules).
1000881 In FIG. 14, a patterned substrate 60 with multiple binding sites 66
can be functionalized
with or linked to individual supramolecular structures 40. In the depicted
embodiment, the
supramolecular structure 40 is assembled at step 80 and then arranged on the
substrate 60 before
an antigen capture molecule 2 is linked. For example, a DNA origami containing
a specific
barcode and capture strand specific to a particular antigen is flowed over the
substrate 60 and
placed in a single molecule army, e.g., on DNA binding features. Antigens are
conjugated to
include a complementary strand, shown as capture molecules 2, which can anneal
to the DNA
origami barcode 20 of the supramolecular structures at step 82. Alternatively,
the capture molecule
is associated with the supramolecular structure 40 during assembly (step 80)
and is applied to the
substrate 60 together with the supramolecular structure 40. The barcode 20 of
each
supramolecular structure is read out either via sequencing, amplification, or
via hybridization
assay. A map of antigen capture molecules 2 with the spatial locations on the
substrate 60 can be
obtained prior to performing the assay and performed as quality control of
substrate 60. The map
can be stored in an analyte detection system (see FIG. 16) as provided herein
and used to generate
a report of positive binding events to provide diagnostic information.
[000891 Appropriate blocking conditions added and a sample including patient
antibodies (e.g.,
serum) is then applied to the substrate 60 and allowed to incubate to allow
antibody analytes 44 in
the sample to form antibody-antigen complexes at step 84. The sample is then
washed off and a
detector molecular assembly 46 including a detector molecule 1 that is a
secondary anti-human
antibody and a label for detection is added at step 86. The detection (step
88) of bound detector
structures 50 can be based on detection of the antibody label, which can be
DNA-based for
amplification either through rolling circle amplification, or hybrid chain
reaction: alternatively, the
label can be a DNA nanoparticle, or fluorescent polymer.
[000901 In an embodiment, the assay includes antigens of one or more of
Adenovirus,
Coronavirus 229E, Coronavirus HICU I , Coronavirus B.1.1.7, Coronavirus
B.1.351, Coronavirus
P.1 Coronavirus NL63, Coronavirus 0C43, Human metapneumovirus, Human
rhinovirus/enterovirus, Influenza A, subtypes 2009111N I, 111, 113, Influenza
B, Parainfluenza virus
types I, 2, 3 and 4, Respiratory Sy ncytial Virus, Chlainydophila pneumoniae,
Mycoplasma
pneumoniae, and Bordetella Pertussis. In addition, the assay may include
vaccine targets for the
-30-
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
seasonal flu, as well as the covid vaccines targets for one or more commercial
vaccines (Modema,
Pfizer, Astra Zeneca, Novavax, and Johnson and Johnson). The addition of these
vaccine targets
allows for the classification of the immunity to determine whether a patient
has immunity from
natural infection and/or gained immunity from the vaccine. Multiple antigens
for each pathogen
may be present to define specificity of the immunity.
[00091i Detection may include anti-species for specific antibodies for 1gG,
1gM and/or IgA
determination. Specific detection of subtype aids in further understanding
maturity of immunity.
1000921 FIG. 15 provides an exemplary embodiment for forming a hydrogel matrix
100, wherein
in addition to combining one or more monomers 122 and one or more crosslinking
molecules 124
to form a hydrogel, one or more supramolecular structures 40 are introduced.
In some
embodiments, the one or more supramolecular structures 40 co-polymerizes with
the hydrogel
matrix, forming the matrix 120. In some embodiments, each respective anchor
molecule 18 of the
one or more supramolecular structures 40 co-polymerizes with the hydrogel
matrix 120. In some
embodiments, the one or more monomers 122 comprise an acrylamide. In some
embodiments, the
one or more cross-linkers comprise a bis-acrylamide.
100093) Embodiments of the present disclosure include one or more computer-
implemented
detection systems configured to perform certain methods of the disclosed
embodiments. FIG. 16
shows an analyte detection system 1000 that includes a controller 1001. The
controller 1001
includes processor 1002 and a memory 1004 storing instructions configured to
be executed by the
processor 1002. The controller 1001 includes a user interface 1006 and
communication circuitry,
e.g., to facilitate communication over the intemet 1010 and/or over a wireless
or wired network.
The user interface 1006 facilitates user interaction with characterized
analyte detection results as
provided herein.
[00094] The processor 1002 is programmed to receive analyte detection data and
characterize the
detected analytes. In one embodiment, the processor generates a report of
detected analytes in a
sample after incubation with an array of supramolecular structures and
detection of detector
molecule assemblies. The report may include data of generated optical signals
at various binding
sites that corresponds to a detected analyte binding event. The report may
include processed data,
such as a list of detected analytes or positive/negative binding results. The
report may include a
list of available capture molecules of an array that is indicative of the
analyte detection
capabilities.
-31 -
CA 03208701 2023-8- 16
WO 2022/182643
PCT/US2022/017281
[00095] The system 1000 also includes an analyte detector 1020 that operates
to detect analyte
binding via detection of one or more components of the supramolecular
structure. The analyte
detector 1020 includes a detection system having one or more sensors 1022. The
analyte detector
1020 may also include a reaction controller 1024 that controls sample
incubation and appropriate
release of reaction reagents and detector molecule assemblies at appropriate
time points. The
sensor 1022 may be one or more of an optical sensor (e.g., a fluorescent
sensor, an infrared
sensor), an image sensor, an electrical sensor, or a magnetic sensor. In an
embodiment, the sensor
102 is a metal-oxide semiconductor image sensor device.
[000961 While preferred embodiments of the present invention have been shown
and described
herein, it will be obvious to those skilled in the art that such embodiments
are provided by way of
example only. Numerous variations, changes, and substitutions will now occur
to those skilled in
the art without departing from the invention. It should be understood that
various alternatives to
the embodiments of the invention described herein may be employed in
practicing the invention. It
is intended that the following claims define the scope of the invention and
that methods and
structures within the scope of these claims and their equivalents be covered
thereby.
-3 2 -
CA 03208701 2023-8- 16