Note: Descriptions are shown in the official language in which they were submitted.
SOLID PHASE PEPTIDE SYNTHESIS PROCESSES
FIELD
The present disclosure relates to processes for deprotecting a protected amino
acid
(e.g., as a step in solid phase peptide synthesis, also referred to herein as
SPPS, including
deprotecting and coupling steps).
BACKGROUND
Since its inception in 1963, solid phase peptide synthesis (SPPS) has been a
major
enabling tool for peptide synthesis. SPPS dramatically simplified the
production of peptides
compared to liquid phase peptide synthesis (LPPS) by allowing straightforward
isolation of
products by simple filtration at each step as opposed to more tedious
extraction processes
after each deprotection and coupling step. However, compared to LPPS, SPPS
results in
significant waste production from successive washing steps between each
deprotection and
coupling step. Historically, about 5 washes were needed between each step
resulting in 80 ¨
90% of the total waste being generated from washing.
The use of microwave energy and heating in general was initially applied to
SPPS for
accelerating synthesis times and improving purity by driving reaction steps
toward
completion. While successful in this regard, these initial efforts did not
fundamentally
eliminate the need for washing in the process. Later developments introduced a
microwave-
assisted high efficiency SPPS process for 9-fluorenylmethyloxycarbonyl (Fmoc)
SPPS that
eliminated washing after each coupling step. This was based on the insight
that residual
activated amino ester is quickly scavenged by the deprotection base before an
insertion could
occur. This approach eliminated half of the washing and reduced the overall
cycle time of the
process. It was later further demonstrated that deprotection base could be
added directly to
the post-coupling solution without any draining. This "one-pot" deprotection
and coupling
process allows for reuse of both solvent and heat from the coupling solution
to facilitate the
deprotection process resulting in further solvent, energy, and time savings.
Despite the foregoing, SPPS processes still generally requires significant
washing
during each amino acid addition cycle, primarily due to the need to remove
deprotection
reagents that cause undesirable insertion and deletion impurities to form
during the next
coupling step. Typically, if residual base from deprotection contaminates the
next coupling
step, it will remove the Fmoc protecting group on the next amino acid leading
to the
undesirable insertion of an additional amino acid onto the growing chain.
Furthermore,
-1-
Date Recue/Date Received 2023-08-25
residual base can react with and consume activated amino ester before it
reacts with the
peptide terminus. The result is the generation of both insertions and
deletions of the next
amino acid, which can lead to impurities that are difficult to separate (e.g.,
by reverse-phase
HPLC). Thus, washing after the deprotection step has been considered
unavoidable.
SUMMARY
The present disclosure relates to processes for deprotecting a protected amino
acid
(e.g., as a step in solid phase peptide synthesis, also referred to herein as
SPPS, including
deprotecting and coupling steps). The deprotection reaction removes a
protecting group of
the protected amino acid (deprotects the amino acid) to prepare the amino acid
for a coupling
reaction with a second amino acid.
In contrast to prior deprotection steps of a SPPS process, the present
disclosure can
help reduce the amount of solvent used for washing step(s) after deprotection
or can help
eliminate washing step(s) after deprotection. This in turn can, in some
embodiments, reduce
or eliminate washing step(s) (e.g., reduce or eliminate all post-coupling
washing steps and
reduce or eliminate all post deprotection washing steps) to provide
significant reduction in
waste (e.g., up to 95% reduction in overall waste) and associated cost and
time savings.
The process for deprotecting a protected amino acid (e.g., during solid phase
peptide
synthesis) includes a step of removing a protecting group of a protected amino
acid in a
reaction vessel (e.g., in a batch-type SPPS reaction vessel) using a
deprotecting base in an
amount of about 5 vol% or less, based on the total volume (100 vol%) of a
deprotection
reaction mixture (e.g., a deprotection reaction solution) in the reaction
vessel.
In some embodiments, the deprotecting base may be present in the reaction
vessel in
an amount from about 1 vol% to about 5 vol%, for example from about 2 vol% to
about 5
vol%, for example from about 2 vol% to about 4.5 vol%, for example from about
3 vol% to
about 4.5 vol%, and as another example from about 3.5 vol% to about 4.5 vol%,
based on the
total volume (100 vol%) of a deprotection reaction mixture (e.g., a
deprotection reaction
solution) in the reaction vessel. In some embodiments, the deprotecting base
may be present
in the reaction vessel in an amount greater than zero to about 4.5 vol%, for
example about 2
vol% to about 4.5 vol%, based on the total volume of the deprotection reaction
mixture (e.g.,
the deprotection reaction solution). In some embodiments, the deprotecting
base may be
present in the reaction vessel in an amount greater than zero to about 4 vol%,
for example
about 2 vol% to about 4 vol%, based on the total volume of the deprotection
reaction mixture
-2-
Date Recue/Date Received 2023-08-25
(e.g., the deprotection reaction solution). In some embodiments, the
deprotecting base may
be present in the reaction vessel in an amount greater than zero to about 3.5
vol%, for example
about 2 vol% to about 3.5 vol%, based on the total volume of the deprotection
reaction
mixture (e.g., the deprotection reaction solution). The amount of deprotecting
base may be
any value within the ranges described herein, including end points (e.g., any
value within a
range of greater than zero to about 5 vol%) and all subranges within the range
are also
disclosed.
At least a portion of the deprotecting base evaporates (volatizes) during the
removing
step (e.g., at least a portion of the deprotecting base evaporates into an
upper interior portion
of the reaction vessel during the removing step). The process for deprotecting
a protected
amino acid in accordance with the present disclosure also includes directing
(e.g.,
continuously and/or intermittently directing) inert gas through the reaction
vessel to assist in
removing (e.g., to assist in flushing, venting, discharging, displacing,
replacing, purging, etc.,
e.g., to remove, flush, vent, discharge, displace, replace, purge, etc.)
evaporated (volatized)
deprotecting base from the interior of the reaction vessel (e.g., from the
headspace of the
reaction vessel) during the step of removing the protecting group.
In some embodiments, the directing step can include introducing inert gas into
an
upper interior portion of the reaction vessel through a first opening located
in an upper
portion of the reaction vessel; and venting (flushing) inert gas and
evaporated deprotecting
base from the upper interior portion of the reaction vessel (e.g., from the
headspace of the
reaction vessel) through a second opening also located in the upper portion of
the reaction
vessel.
In some embodiments, the directing step can include introducing the inert gas
into a
lower interior portion of the reaction vessel through an opening located in a
lower portion of
the reaction vessel; and venting (flushing) the inert gas and evaporated
deprotecting base
from an upper interior portion of the reaction vessel (e.g., from the
headspace of the reaction
vessel) through an opening located in an upper portion of the reaction vessel.
In some embodiments, the directing step can include introducing inert gas into
an
upper interior portion of the reaction vessel through a first opening located
in an upper
portion of the reaction vessel and also introducing inert gas into a lower
interior portion of the
reaction vessel through a second opening located in a lower portion of the
reaction vessel;
and venting inert gas and evaporated deprotecting base from the upper interior
portion of the
-3 -
Date Recue/Date Received 2023-08-25
reaction vessel (e.g., from the headspace of the reaction vessel) through a
third opening
located in the upper portion of the reaction vessel. In this embodiment, inert
gas introduced
into the lower interior portion of the reaction vessel may serve to agitate
(bubble, stir, etc.)
materials (e.g., the deprotection reaction mixture, e.g., the deprotection
reaction solution) in
the lower interior portion of the reaction vessel and/or to participate in
headspace venting
(flushing, purging, etc.) of evaporated deprotecting base as described herein.
In some embodiments, the process can further include heating the protected
amino
acid and the deprotecting base during the step of removing the protecting
group from the
protected amino acid. The heating step may be conducted, for example, at a
temperature
from about 40 C to about 120 C, as another example about 60 C to about 120
C, and as
another example about 90 C to about 120 C. The heating step may be conducted
using
microwave radiation.
In exemplary embodiments of the deprotection processes disclosed herein, the
deprotection processes can use (e.g., protected amino acid and/or protected
peptide can be
attached to) a solid resin support having a resin substitution of less than or
about 0.35mmo1/g
(e.g., < 0.35mmo1/g), for example less than or about 0.30mmo1/g (e.g., <
0.30mmo1/g). In
some embodiments, the deprotection processes can use a solid resin support
with resin
substitution from 0.10mmol/g to 0.35mmo1/g, for example 0.15mmol/g to
0.35mmo1/g, for
example 0.10mmol/g to 0.34mmo1/g, for example 0.15mmol/g to 0.34mmo1/g, for
example
0.20mmo1/g to 0.35mmol/g, for example 0.20mmo1/g to 0.34mmo1/g, for example
0.20mmo1/g to 0.33mmol/g. The amount of resin substitution may be any value
within the
ranges described herein, including end points and all subranges within the
range are also
disclosed. In exemplary embodiments, the resin may be PEG-PS (polyethylene
glycol-
polystyrene) resin (e.g., PEG-PS based resin (Pro-Tide), PS (polystyrene)
resin, etc.
The present disclosure also relates to a process for solid phase peptide
synthesis
(SPPS). The SPPS process may include deprotecting a first protected amino acid
to provide a
deprotected amino acid, as described herein; and coupling a second amino acid
to the
deprotected amino acid to form a peptide from the first and second amino
acids.
In some embodiments, the process does not include a washing step after a
deprotecting step and before a (next, successive) coupling step of a SPPS
cycle.
In some embodiments, the process may include a washing step after a
deprotection
step and before a (next, successive) coupling step of a SPPS cycle using a
washing
-4-
Date Recue/Date Received 2023-08-25
composition (e.g., solvent). In some embodiments, the washing step may include
washing
the interior of the reaction vessel using a total volume of solvent that is 2
times or less of a
bed volume of resin present in the reaction vessel (e.g., a solid resin
support present in the
reaction vessel), for example, 1 time or less of a bed volume of resin present
in the reaction
vessel.
BRIEF DESCRIPTION OF THE DRAWINGS
The present invention is described hereinafter with reference to the
accompanying
drawings in which embodiments of the present invention are shown and in which
like
reference numbers may indicate the same or similar elements. The drawings are
provided as
examples, may be schematic, and are not intended to be drawn to scale. The
present inventive
aspects may be embodied in many different forms and should not be construed as
limited to
the examples depicted in the drawings. For purposes of clarity, not every
component is
labeled in every figure, nor is every component of each embodiment of the
invention shown
where illustration is not necessary to allow those of ordinary skill in the
art to understand the
invention.
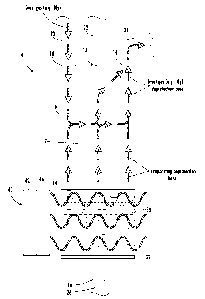
Figure 1A is a cross-sectional view of an exemplary reaction vessel and
schematically
depicts a process of deprotecting a protected amino acid in accordance with
embodiments of
the present disclosure;
Figure 1B is a cross-sectional view of another exemplary reaction vessel and
schematically depicts a process of deprotecting a protected amino acid in
accordance with
other embodiments of the present disclosure;
Figure 2A is a schematic flow diagram depicting selected portions of an
exemplary
peptide synthesis system in accordance with embodiments of the present
disclosure;
Figure 2B is a schematic flow diagram depicting selected portions of an
exemplary
peptide synthesis system in accordance with other embodiments of the present
disclosure;
Figure 3 is a flow chart schematically depicting the steps of a cycle of
conventional
solid phase peptide synthesis (SPPS) processes; and
Figures 4A and 4B are flow charts schematically depicting the steps of a cycle
of
SPPS processes in accordance with the present disclosure.
-5-
Date Recue/Date Received 2023-08-25
DETAILED DESCRIPTION
Examples of embodiments are disclosed in the following. The above and other
aspects, features, and advantages of the present invention will become
apparent from the
detailed description of the following embodiments. The present invention may,
however, be
embodied in many different forms and should not be construed as limited to the
embodiments
set forth herein. For example, features disclosed as part of one embodiment or
example can
be used in the context of another embodiment or example to yield a further
embodiment or
example. The embodiments are provided for complete disclosure and to provide
thorough
understanding of the present invention by those skilled in the art. Sometimes
well-known
aspects are not described in detail to avoid unnecessarily obscuring the
present invention.
This detailed description is thus not to be taken in a limiting sense, and it
is intended that
other embodiments are within the spirit and scope of the present invention.
The scope of the
present invention should be defined only by the appended claims.
Embodiments of the present disclosure relate to processes and systems for
deprotecting a protected amino acid (e.g., deprotecting a protected amino
acid, deprotecting a
protected amino acid-derived unit of a peptide, also referred to herein as
deprotecting a
protected peptide, wherein the protected peptide refers to a peptide including
a unit derived
from a protected amino acid) as a step in peptide synthesis (e.g., solid phase
peptide
synthesis). In exemplary embodiments, the processes and systems are batch-
based processes
and systems.
The skilled artisan will understand the meaning of the term amino acid. As
used
herein, the term amino acid in its broadest sense refers to organic compounds
that contain
both amine and carboxylic acid functional groups, and in some instances also a
side chain.
The skilled artisan will also understand that amino acids include natural
amino acids
(proteinogenic amino acids) and/or non-proteinogenic amino acids, and will
also understand
the single letter designations used to identify the same.
The processes of the present disclosure may be useful in the production of
peptides
and/or proteins. The terms peptide and/or protein will also be understood by
the skilled
artisan. For example, as used herein, the term peptide and/or protein can
refer to amides
derived from two or more amino acids (the same or different) by bonding the
carbonyl carbon
of one amino acid to the nitrogen atom of another amino acid. As understood by
the skilled
artisan, peptides and proteins may be distinguished by chain length (e.g.,
peptides have a
-6-
Date Recue/Date Received 2023-08-25
shorter chain length of amino acids linked by chemical bonds (fewer amino
acids), as
compared to proteins). For ease of discussion, however, the term peptide will
used
consistently throughout, and the present disclosure is not limited to the
production of peptides
(e.g., the processes described herein may be applicable to the production of
peptides and/or
proteins).
For ease of reference, the present disclosure refers to processes for
deprotecting a
protected amino acid (e.g., removing a protecting group of a protected amino
acid). The
skilled artisan will understand that the protected amino acid may be part of a
peptide and that
discussions herein to processes for deprotecting a protected amino acid
include processes for
deprotecting a protected peptide (e.g., removing a protecting group of a
protected amino acid-
derived unit of a peptide).
Figure 1A is a schematic cross-sectional view of a reaction vessel suitable
for use in
the amino acid deprotecting and peptide synthesis processes and systems in
accordance with
embodiments of the present disclosure. Figure 1A also schematically depicts a
process
according to an embodiment of the present disclosure for deprotecting a
protected amino
acid.
Figure 1B is a schematic cross-sectional view of another reaction vessel
suitable for
use in the amino acid deprotecting and peptide synthesis processes and systems
in accordance
with embodiments of the present disclosure. Figure 1B also schematically
depicts a process
according to an embodiment of the present disclosure for deprotecting a
protected amino
acid.
Except where indicated otherwise, elements illustrated in Figure 1A will carry
the
same reference numerals as in the other Figures, including Figure 1B.
As shown in Figures 1A and 1B, a reaction vessel 4 includes at least one side
wall 6
extending around an interior 7 (e.g., cavity) of reaction vessel 4 (the
interior 7 of the reaction
vessel also referred to herein as an outer interior space). The specific size
and shape of
reaction vessel 4 are not limited. Reaction vessels suitable for use in solid
phase peptide
synthesis are well known in the art and are commercially available.
The size (interior volume) of the reaction vessel is not limited. Exemplary
reaction
vessel sizes can range from less than 1 liter up to 40 liters, or more, for
example, 10 ml, 30
ml, 125 ml, 1 liter, 3 liters, 5 liters, 8 liters, 10 liters, 15 liters, etc.
up to 40 liters, or more,
without limitation.
-7-
Date Recue/Date Received 2023-08-25
Reaction vessel 4 further includes one or more openings. As a non-limiting
example,
Figure 1A depicts openings 10, 12, and 14 located in an upper portion (e.g.,
in a top wall) of
reaction vessel 4, and an opening 16 located in a lower portion (e.g., a
bottom wall) of
reaction vessel 4. As another non-limiting example, Figure 1B depicts openings
200, 202,
204, 206, and 208 located in an upper portion (e.g., in a top wall) of
reaction vessel 4, and an
opening 16 located in a lower portion (e.g., a bottom wall) of reaction vessel
4. The
openings (e.g., inlets, outlets, ports, etc.) allow the introduction and/or
removal of fluids
and/or solids, such as reactants, solvents, gases, products (peptides),
byproducts, excess
(residual) reactants, and the like as discussed in more detail herein.
The skilled artisan will understand that the reaction vessel 4 is not limited
to the
number and/or locations of openings depicted in Figures 1A and 1B and that
other reaction
vessel designs and configurations having fewer or more openings and/or
different locations
thereof can be used (e.g., the reaction vessel may include fewer or more
openings located in a
top wall and/or a bottom wall and/or a side wall, etc.).
Fluids and/or solids can be introduced (e.g., moved, transported, directed,
flushed,
purged, evacuated, vented, etc.) into and out of reaction vessel 4 via one or
more flow paths
(e.g., lines, passageways, tubes, manifolds, etc.), such as flow paths 20, 22,
and 24 in fluid
communication with openings 10, 12, and 14, respectively, and flow path 26 in
fluid
communication with opening 16, as depicted in Figure 1A.
Other non-limiting examples of flow paths are depicted in Figure 1B as flow
paths
210, 212, 214, 216, and 218 in fluid communication with openings 200, 202,
204, 206, and
208, respectively, and flow path 26 in fluid communication with opening 16.
In some embodiments, reaction vessel 4 can include at least one spray head
(e.g.,
spray nozzle) or equivalent structure located in the interior of the reaction
vessel for adding
(e.g., directing, supplying, spraying, etc.) fluid (e.g., solvent, reactant,
and/or inert gas) into
the reaction vessel. The spray head can be a part (e.g., component, element,
etc.) that is
separate from the reaction vessel (e.g., can be installed in and removed from
the reaction
vessel) or can be an integrated part of the reaction vessel.
As a non-limiting example, Figure 1B schematically depicts an embodiment
including
a spray head 220 located in the interior space 7 (also referred to herein as
the outer interior
space) of reaction vessel 4. Spray head 220 includes at least one side wall
220a positioned in
the interior space 7 (also referred to as the outer interior space) of
reaction vessel 4 and
-8-
Date Recue/Date Received 2023-08-25
extending around an inner interior space. Spray head 220 includes a first
portion (end) 220b
proximate opening 208 in fluid communication with opening 208 (and flow path
218) and a
second portion (end) 220c distal opening 208. A plurality of holes 222 (e.g.,
ports, etc.)
extend at least partially around the inner interior space and can be, for
example, defined in
side wall 220a. The inner interior space and the outer interior space are in
fluid
communication with one another by way of holes of the plurality of holes.
Spray head 220 is configured so that fluid (such as inert gas, solvent, etc.
as discussed
herein) directed into the inner interior space of spray head 220 via flow path
218 and opening
208 passes (e.g., is sprayed, directed, supplied, etc.) through holes (e.g.,
through one, more
than one, a majority, or all) of the plurality of holes 222 into the outer
interior space 7 of
reaction vessel 4. In some embodiments, spray head 220 can be configured so
that fluid
exiting at least one or more holes of the plurality of holes is directed
(sprayed) towards side
wall 6 of the reaction vessel. An exemplary spray pattern is schematically
depicted in Figure
1B by dashed lines 224 (e.g., in an approximate manner), in which fluid
exiting holes of the
plurality of holes 222 is directed at a generally downward angle towards side
wall 6.
The present disclosure is not limited with respect to a specific spray head
structure,
location in the reaction vessel, spray pattern and/or direction (angle) of
fluid exiting holes of
the spray head, such as illustrated in Figure 1B, and other spray head
structures, locations,
spray patterns, and/or spray angles, etc., can be used. Spray heads suitable
for use in SPPS
processes and systems are known in the art and can be used in the present
disclosure.
The present disclosure is not limited to a specific number and/or locations of
openings
and flow paths and the reaction vessel can accordingly have one, two, three,
four, or more
openings and associated flow paths as appropriate. Further, any series of flow
paths and
associated valves that serve to direct, allow, and/or block (e.g., close,
restrict, etc.) the flow of
fluids and/or solids can be used.
Reaction vessel 4 includes a mixture of components, designated generally at 30
in
Figs. 1A and 1B, located in a lower portion of reaction vessel 4. The mixture
of components
30 can rest on a filter 32 located in the lower portion of reaction vessel 4.
Filter 32 can also
prevent solid materials present in the mixture (such as solid resin support
linked to a growing
peptide chain as discussed in more detail herein) from entering flow path 26.
For example, mixture 30 includes a protected amino acid, i.e., an amino acid
including at least one protecting group attached to a functional group, such
as a terminal
-9-
Date Recue/Date Received 2023-08-25
amine group, to protect from unwanted reactions of the functional group. The
protected
amino acid may be part of a growing peptide chain linked to a solid resin
support, as
understood and known in the art and as discussed in more detail herein.
Suitable protecting groups for use in the processes of the present disclosure
are well-
known in the art. An example of a protecting group suitable for protection of
amine or N-
terminus includes without limitation a fluorenylmethyloxycarbonyl (Fmoc)
protecting group.
See, for example, Chan and White, Fmoc solid phase peptide synthesis, a
practical approach,
Oxford University Press (2000).
The amino acid can also include side-chain protecting groups. Examples of side-
chain
protecting groups can include without limitation trityl, t-butyl, and/or
2,2,4,6,7-
pentamethyldihydrobenzofuran-5-sulfonyl (Pbf) protecting groups, and the like.
When the
desired peptide chain length has been obtained, the side-chain protecting
groups can be
removed.
The protected amino acid can be directly or indirectly attached to a solid
support as
known in the art. For example, the carboxy terminus of the protected amino
acid can be
attached to the solid support via a suitable linker. As another example, the
carboxy terminus
of the protected amino acid can be indirectly attached to the solid support,
for example, the
carboxy terminus of the protected amino acid can be coupled to the amine or N-
terminus of
another amino acid (e.g., to the amine or N-terminus of a single amino acid or
to the amine or
N-terminus of an amino acid that is part of a growing peptide chain) that is
in turn linked to
the solid support via a suitable linker.
Solid supports known in the art may be used in the processes of the present
disclosure.
In exemplary embodiments, the solid support is a solid resin support. Examples
of solid resin
support materials may include without limitation polystyrene (e.g., in resin
form such as
microporous polystyrene resin, mesoporous polystyrene resin, macroporous
polystyrene
resin), glass, polysaccharides (e.g., cellulose, agarose), polyacrylamide
resins, polyethylene
glycol, and/or copolymer resins (e.g., comprising polyethylene glycol,
polystyrene, etc.).
In exemplary embodiments of the deprotection processes disclosed herein, the
protected amino acid can be attached to a solid resin support having a resin
substitution of
less than or about 0.35mmo1/g (e.g., < 0.35mmol/g), for example less than or
about
0.30mmo1/g (e.g., < 0.30mmol/g). In some embodiments, the deprotection
processes can use
resin with resin substitution from 0.10mmol/g to 0.35mmo1/g, for example
0.15mmol/g to
-10-
Date Recue/Date Received 2023-08-25
0.35mmo1/g, for example 0.10mmol/g to 0.34mmol/g, for example 0.15mmol/g to
0.34mmo1/g, for example 0.20mmol/g to 0.35mmo1/g, for example 0.20mmol/g to
0.34mmo1/g, for example 0.20mmol/g to 0.33mmo1/g, for example 0.21mmol/g to
0.35mmo1/g, for example 0.21mmol/g to 0.34mmo1/g, for example 0.21mmol/g to
0.33mmo1/g, for example 0.22mmo1/g to 0.35mmo1/g, for example 0.22mmo1/g to
0.34mmo1/g, for example 0.22mmo1/g to 0.33mmo1/g. The amount of resin
substitution may
be any value within the ranges described herein, including end points and all
subranges
within the range are also disclosed. In exemplary embodiments, the resin may
be PEG-PS
(polyethylene glycol-polystyrene) resin (e.g., PEG-PS based resin (Pro-Tide),
PS
(polystyrene) resin, etc. In exemplary embodiments, the solid support is a
solid PEG-PS
(polyethylene glycol-polystyrene) resin support (e.g., PEG-PS based resin (Pro-
Tide) at
moderate substitution (e.g., 0.2-0.3mmol/g, 0.20-0.25mmo1/g, 0.21-0.3mmol/g,
0.21-
0.25mmol/g, 0.22-0.3mmol/g, 0.22-0.25mmo1/g, etc.).
The present disclosure is not limited to solid support materials described
herein, and
other types of support or carrier materials known in the art may also be used
in the
deprotecting processes during peptide synthesis including deprotecting and
coupling steps,
SPPS processes, etc. described herein.
The solid support may have any suitable form. For example, the solid support
can be
in the form of beads, particles, fibers, and/or in any other suitable form.
The skilled artisan will understand how link (couple) an amino acid to a solid
support
(e.g., a solid resin support). Accordingly, a detailed discussion of methods
known in the art
for linking (coupling) an amino acid to a solid support is not provided.
Turning again to Figs. 1A and 1B, mixture 30 also includes a deprotection
reaction
mixture (e.g., a deprotection reaction solution). The deprotection reaction
mixture (e.g., the
deprotection reaction solution) may include a mixture (e.g., may include the
total volume of
liquid in the reaction vessel) including a deprotection solution including a
deprotecting base
(e.g., a deprotection solution including a deprotecting base added to the
reaction vessel) and a
coupling solution from a preceding coupling step (e.g., an undrained post-
coupling solution
that remains in the reaction vessel from a preceding (previous) coupling
step). In some
embodiments, the deprotection reaction mixture (e.g., the deprotection
reaction solution) may
include additional liquid(s) (e.g., additional solvent added to the reaction
vessel).
In this regard, as discussed in more detail herein, the process for
deprotecting a
-11-
Date Recue/Date Received 2023-08-25
protected amino acid in accordance with the present disclosure may include
adding a
deprotection solution that includes the deprotecting base to the reaction
vessel. The amount
of the deprotection solution including the deprotecting base added to the
reaction vessel
corresponds to the deprotection solution including a deprotecting base of the
deprotection
reaction mixture (e.g., the deprotection reaction solution).
Also in this regard, the process for deprotecting a protected amino acid in
accordance
with the present disclosure may include a preceding coupling step (e.g., a
coupling step prior
to the step of removing the protecting group). As understood in the art, the
reaction vessel
may include a coupling solution after completion of the preceding coupling
step. In
exemplary embodiments, the process of the present disclosure does not require
draining
step(s) (and/or washing step(s)) after the preceding coupling step and before
the next (e.g.,
successive) deprotecting step (e.g., the deprotecting step of the next SPPS
cycle) to remove
(e.g., drain) the coupling solution from the reaction vessel. When there is no
draining step
between a preceding coupling step and a successive deprotecting step, the
deprotection
reaction mixture (e.g., the deprotection reaction solution) in the reaction
vessel includes the
coupling solution remaining in the reaction vessel from the preceding coupling
step. Thus,
the reaction vessel may include various components after completion of the
coupling step.
The components in the reaction vessel post-coupling are referred to herein
generally as a
post-coupling mixture.
The post-coupling mixture may include a post-coupling solution (also referred
to
herein as a coupling solution). For example, the reaction vessel may include a
coupling
solution (e.g., undrained post-coupling solution) that remains in the reaction
vessel from the
previous coupling step. The components of the coupling solution (e.g.,
undrained post-
coupling solution) will be understood by the skilled artisan. For example, the
coupling
solution (e.g., the undrained post-coupling solution) may include solvent and
residual
(excess) coupling reagents and/or coupling reagent byproducts from the
preceding coupling
reaction. Examples of residual (excess) coupling reagents and/or byproducts
thereof that may
be present in the coupling solution include without limitation residual
(excess) activated
amino acid, residual (excess) amino acid activator (e.g., DIC, etc., as
described herein),
residual (excess) amino acid activator additive (e.g., Oxyma, etc., as
described herein), other
residual (excess) coupling additives, non-activated protected amino acids,
and/or byproducts
thereof, without limitation. The skilled artisan will understand the meaning
of the term
-12-
Date Recue/Date Received 2023-08-25
coupling and/or post-coupling solution as used herein and the definition
thereof is not
necessarily limited to the components described herein.
As another example, the skilled artisan will also understand that the post-
coupling
mixture in the reaction vessel may include solids, such as post-coupling
reaction products
(e.g., a growing peptide chain(s) including two or more amino acids coupled to
a solid
support resin) that are not part of the coupling solution (e.g., the undrained
post-coupling
solution that remains in the reaction vessel from the previous coupling step).
Again, the
skilled artisan will understand the meaning of the term post-coupling reaction
products as
used herein and the definition thereof is not necessarily limited to the
components described
herein.
In exemplary embodiments, the process of the present disclosure does not
include a
draining step between the preceding coupling step and the next deprotecting
step (e.g., the
deprotecting step of the next SPPS cycle) to remove (drain) the coupling
solution from the
reaction vessel. When there is no draining step between a preceding coupling
step and a
successive deprotecting step, the coupling solution remaining in the vessel
from the
preceding coupling step corresponds to the coupling solution of the
deprotection reaction
mixture (e.g., the deprotection reaction solution).
In exemplary embodiments, the process of the present disclosure may include a
draining step between the preceding coupling step and the next deprotecting
step (e.g., the
deprotecting step of the next SPPS cycle). When there is a draining step
between a preceding
coupling step and a successive deprotecting step, some of (e.g., less than
half of, half of,
substantially all, all of, etc.) the coupling solution from the preceding
coupling step may be
drained from the reaction vessel and, in some embodiments, the deprotection
reaction mixture
(e.g., the deprotection reaction solution) may include minimal, if any,
coupling solution (e.g.,
undrained post-coupling solution) from the preceding coupling step, depending
on the
amount of post-coupling solution drained from the reaction vessel. When post-
coupling
solution is drained from the reaction vessel between the preceding coupling
step and the next
deprotecting step, the volume percent deprotecting base in the reaction vessel
may still be the
same as described in more detail herein (e.g., an amount greater than zero to
about 5 vol%
based on the total volume (100 vol%) of the deprotection reaction mixture
(e.g., the
deprotection reaction solution)). In some embodiments, much of the total
volume of the
deprotection reaction mixture (e.g., the deprotection reaction solution)
(e.g., half of,
substantially of, all of, etc.) may correspond substantially to the volume of
deprotection
-13-
Date Recue/Date Received 2023-08-25
solution added to the reaction vessel, alone or in combination with a volume
of additional
liquid (e.g., solvent) added to the reaction vessel. For example, in some
embodiments in
which substantially all (e.g., all) of a post-coupling solution is drained
from the reaction
vessel prior to the next deprotecting step, a deprotection solution including
greater than zero
to about 5 vol% deprotecting agent, and optionally additional solvent, may be
added to the
drained reaction vessel to provide the deprotecting base in the reaction
vessel in an amount of
greater than zero to about 5 vol%, based on the total volume (100 vol%) of the
deprotection
reaction mixture (e.g., the deprotection reaction solution) (which in this
case, the volume of
deprotection reaction mixture (e.g., the deprotection reaction solution) may
substantially
correspond to the total volume of the added deprotection solution and
additional solvent
when present) in the reaction vessel. As another example, in some embodiments
in which
substantially all (e.g., all) of a post-coupling solution is drained from the
reaction vessel prior
to the next deprotecting step, a deprotection solution including deprotecting
agent in a vol%
greater than about 5vo1% and additional solvent may be added to the drained
reaction vessel
to provide the deprotecting base in the reaction vessel in an amount of
greater than zero to
about 5 vol%, based on the total volume (100 vol%) of the deprotection
reaction mixture
(e.g., the deprotection reaction solution) (which in this case, the volume of
deprotection
reaction mixture (e.g., the deprotection reaction solution) may substantially
correspond to the
total volume of the added deprotection solution and the added solvent) in the
reaction vessel.
The process for deprotecting the protected amino acid in accordance with the
present
disclosure includes removing the protecting group of the protected amino acid
in a reaction
vessel such as reaction vessel 4 with the deprotecting base, wherein the
deprotecting base is
present in the reaction vessel in an amount greater than zero to about 5 vol%,
based on the
total volume (100 vol%) of the deprotection reaction mixture (e.g., the total
volume of the
deprotection reaction solution, total volume of liquid) in the reaction
vessel. The
deprotecting base reacts with the protected amino acid to remove the
protecting group and to
make the previously protected functional group (e.g., a terminal amine group)
available for
reaction (e.g., with one, two, or more, successive amino acid to form a
peptide chain).
The deprotection reaction mixture (e.g., the deprotection reaction solution)
may
include a mixture (e.g., a total volume of liquid in the reaction vessel)
including a
deprotection solution including the deprotecting base (e.g., a deprotection
solution including
the deprotecting base added to the reaction vessel) and a coupling solution
from a preceding
coupling step (e.g., an undrained post-coupling solution remaining in the
reaction vessel
-14-
Date Recue/Date Received 2023-08-25
from a preceding coupling step). In some embodiments, the deprotection
reaction mixture
(e.g., the deprotection reaction solution) may also include additional liquid
(e.g., solvent)
added to the reaction vessel. In some embodiments, the vol% deprotecting base
of the
deprotection reaction mixture (e.g., the deprotection reaction solution) may
be based on the
total volume of liquid in the reaction vessel (e.g., the deprotection reaction
mixture or
solution may include the total volume of liquid in the reaction vessel). For
example, in some
embodiments, when the coupling solution from a preceding coupling step is not
drained, the
vol% deprotecting base may be based on the total volume of liquid in the
reaction vessel
including the volume of a coupling solution from a preceding coupling step
(e.g., the volume
of an undrained post-coupling solution remaining in the reaction vessel from a
preceding
coupling step, not including solids, for example not including a growing
peptide chain
attached to a solid support) and the volume of the added deprotection solution
including the
deprotecting base (and volume of additional solvent when added to the reaction
vessel). In
some other embodiments, when the coupling solution from a preceding coupling
step is
partially or substantially completely drained (e.g., less than half of, half
of, substantially all
of, or all of coupling solution is drained), the vol% deprotecting base may be
based on the
total volume of liquid in the reaction vessel including the volume of the
added deprotection
solution including the deprotecting base (and volume of additional solvent
when added to the
reaction vessel) and any remaining (e.g., greater than half of, half of,
substantially no or
minimal (residual) or no) volume coupling solution (e.g., from the preceding
coupling step).
In some embodiments, the deprotecting base may be present in the reaction
vessel in
an amount of about 1 vol% to about 5 vol%, for example about 2 vol% to about 5
vol%, for
example from about 2 vol% to about 4.5 vol%, for example from about 3 vol% to
about 4.5
vol%, and as another example from about 3.5 vol% to about 4.5 vol%, based on
the total
volume (100 vol%) of the deprotection reaction mixture (e.g., the total volume
of the
deprotection reaction solution, total volume of liquid) in the reaction
vessel. In some
embodiments, the deprotecting base may be present in the reaction vessel in an
amount
greater than zero to about 4.5 vol%, for example about 2 vol% to about 4.5
vol%, based on
the total volume of the deprotection reaction mixture (e.g., the total volume
of the
deprotection reaction solution, total volume of liquid) in the reaction
vessel. In some
embodiments, the deprotecting base may be present in the reaction vessel in an
amount
greater than zero to about 4 vol%, for example about 2 vol% to about 4 vol%,
based on the
total volume of the deprotection reaction mixture (e.g., the total volume of
the deprotection
-15-
Date Recue/Date Received 2023-08-25
reaction solution, total volume of liquid) in the reaction vessel. In some
embodiments, the
deprotecting base may be present in the reaction vessel in an amount greater
than zero to
about 3.5 vol%, for example about 2 vol% to about 3.5 vol%, based on the total
volume of
the deprotection reaction mixture (e.g., the total volume of the deprotection
reaction solution,
total volume of liquid) in the reaction vessel. The amount of deprotecting
base may be any
value within the ranges described herein, including end points (e.g., any
value within a range
of greater than zero to about 5 vol%) and all subranges within the range are
also disclosed.
Deprotecting bases used in SPPS processes are typically liquid at room
temperature.
Thus, the deprotecting base is typically added to the reaction vessel as a
part of a deprotection
solution that includes the deprotecting base and a suitable solvent.
Alternatively, in some
embodiments, the deprotecting base may be added neat to the reaction vessel.
In exemplary embodiments, the process of the present disclosure includes
adding the
deprotection solution including the deprotecting base (alone or in combination
with a volume
of additional solvent) to the reaction vessel under conditions sufficient to
provide a desired
amount (e.g., volume) of the deprotecting base in the reaction vessel to be
available for
removing the protecting group of the protected amino acid (e.g., to provide
greater than zero
to about 5 vol% deprotecting base in the reaction vessel, based on the total
volume (100
vol%) of the deprotection reaction mixture (e.g., total volume of deprotection
reaction
solution, total volume of liquid) in the reaction vessel). In exemplary
embodiments, this may
include adding the deprotection solution (and optionally additional solvent)
to the reaction
vessel in an amount (volume) sufficient and/or adding deprotection solution
having a
concentration of the deprotecting base sufficient to provide the desired
amount (volume) of
the deprotecting base in the reaction vessel available for removing the
protecting group of the
protected amino acid (e.g., to provide greater than zero to about 5 vol%
deprotecting base in
the reaction vessel, based on the total volume (100 vol%) of the deprotection
reaction mixture
(e.g., deprotection reaction solution, liquid) in the reaction vessel). The
deprotection solution
added to the reaction vessel may include the deprotecting base in a higher
concentration than
the resultant concentration of the deprotecting base in the reaction vessel
after the
deprotection solution is added to the reaction vessel.
As a non-limiting example, following completion of a small-scale coupling
reaction, a
reaction vessel may include about 3.5 mL post-coupling solution (including
solvent, residual
(excess) activated amino acid, residual (excess) amino acid activator such as
DIC, and/or
residual (excess) amino acid activator additive such as Oxyma). A deprotecting
step may be
-16-
Date Recue/Date Received 2023-08-25
initiated by adding 0.75 mL of a 17% v/v pyrrolidine/DMF deprotecting solution
(0.1275 mL of pyrrolidine) directly to the undrained post-coupling solution to
give a total
volume of 4.25 mL deprotection reaction mixture (e.g., deprotection reaction
solution)
in the reaction vessel, including about 3 vol% pyn-olidine based on the total
volume
of the deprotection reaction mixture (e.g., the deprotection reaction
solution).
The deprotection solution to be added to the reaction vessel can have a higher
concentration of the deprotecting base, as compared to the concentration of
the deprotecting
base in the deprotection reaction mixture (e.g., the deprotection reaction
solution) after
deprotection solution is added to the reaction vessel. Thus, a relatively
small amount
(volume) of the deprotection solution may be added to the reaction vessel to
provide the
desired deprotecting base concentration of the deprotection reaction mixture
(e.g., the
deprotection reaction solution).
In some embodiments, as used herein, a deprotection solution added to the
reaction
vessel having a "high" or "higher" concentration of deprotecting base may
include a
deprotection solution including solvent and about 10 vol% or higher
deprotecting base, about
15 vol% or higher deprotecting base, about 20 vol% or higher deprotecting
base, about 25
vol% or higher deprotecting base, about 30 vol% or higher deprotecting base,
about 40 vol%
or higher deprotecting base, and less than 50 vol% deprotecting base, based on
the total
volume of the deprotection solution. In some embodiments, the deprotection
solution added
to the reaction vessel may include the deprotecting base in an amount from
about 10 vol% to
about 40 vol%, for example from about 10 vol% to about 30 vol%, and as another
example
from about 10 vol% to about 25 vol%, based on the total volume of the
deprotection solution.
In some embodiments, the deprotection solution added to the reaction vessel
may include the
deprotecting base in an amount of about 10, 11, 12, 13, 14, 15, 16, 17, 18,
19, 20, 21, 22, 23,
24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42,
43, 44, 45, 46, 47, 48,
or 49 vol%, based on the total vol (100 vol%) of the deprotection solution.
Further,
according to some embodiments, the deprotecting base can be present in an
amount of from
about any of the foregoing amounts to about any other of the foregoing
amounts. In some
other embodiments, a deprotection solution added to the reaction vessel having
a "high" or
"higher" concentration of deprotecting base may include a deprotection
solution including
solvent and deprotecting base in an amount of about 50, 51, 52, 53, 54, 55,
56, 57, 58, 59, 60,
61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79,
80, 81, 82, 83, 84, 85,
86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, or 100 vol%, based on
the total vol (100
-17-
Date Recue/Date Received 2023-08-25
vol%) of the deprotection solution. Further, according to some embodiments,
the
deprotecting base can be present in an amount of from about any of the
foregoing amounts to
about any other of the foregoing amounts.
The present disclosure, however, is not limited to the use of a deprotection
solution
having relatively high concentrations of deprotecting base, and the process
may also include
adding a deprotection solution to the reaction vessel having a concentration
of deprotecting
base that is less than about 10 vol% (e.g., the deprotection solution added to
the reaction
vessel may include solvent and the deprotecting base, wherein the deprotecting
base is
present in an amount greater than zero vol% to about 10 vol%, for example
greater than zero
vol% to about 5 vol%, based on the total volume of the deprotection solution),
so long as the
amount of the deprotection solution added to the reaction vessel is selected
to provide the
desired amount (greater than zero to about 5 vol%) of deprotecting base,
relative to the total
volume of the deprotection reaction mixture (e.g., the deprotection reaction
solution) as
defined herein. In some embodiments, the deprotection solution added to the
reaction vessel
may include the deprotecting base in an amount of about 1, 2, 3, 4, 5, 6, 7,
8, 9, or 10 vol%,
based on the total vol (100 vol%) of the deprotection solution. Further,
according to some
embodiments, the deprotecting base can be present in an amount of from about
any of the
foregoing amounts to about any other of the foregoing amounts.
The deprotecting base can be an organic base. In some embodiments, the
deprotecting base can have a boiling point of less than or about 107 C. In
some
embodiments, the deprotecting base can have a boiling point of less than or
about 107 C
and/or a difference between a deprotection reaction temperature such as
discussed herein and
a boiling point of the deprotecting base may be less than or about 50 C, for
example less
than or about 25 C, for example less than or about 15 C, for example the
difference
between the deprotection reaction temperature and the boiling point of the
deprotecting base
may range from about 1 C to about 50 C, for example the difference between
the
deprotection reaction temperature and the boiling point of the deprotecting
base may range
from about 15 C to about 50 C, for example the difference between the
deprotection
reaction temperature and the boiling point of the deprotecting base may range
from about 1
C to about 35 C, and as another example the difference between the
deprotection reaction
temperature and the boiling point of the deprotecting base may range from
about 1 C to
about 25 C. In some embodiments, the deprotecting base can have a boiling
point of less
than or about 107 C and/or the difference between the deprotection reaction
temperature and
-18-
Date Recue/Date Received 2023-08-25
boiling point of the deprotecting base may be about 1, 2, 3,4, 5, 6, 7, 8, 9,
10, 11, 12, 13, 14,
15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33,
34, 35, 36, 37, 38, 39,
40, 41, 42, 43, 44, 45, 46, 47, 48, 49, or 50 C. The difference between the
deprotection
reaction temperature and boiling point of the deprotecting base may be any
value within the
ranges described herein, including end points (e.g., any value within a range
of about 1 C to
about 50 C) and all subranges within the range are also disclosed. In some
embodiments,
the deprotecting base can have a boiling point of less than or about 107 C
and/or the
deprotection step may be performed at a temperature of no less than about 35 C
below the
boiling point of the deprotection base used. Examples of an organic base
suitable for use as a
deprotecting base may include without limitation piperidine and/or
pyrrolidine. Other
organic bases that provide the deprotection function without otherwise
interfering with the
other steps in the process, the growing peptide chain, or the system, can be
appropriate as
well.
Examples of solvents that can be part of the deprotection solution (and/or may
be
added separately to a reaction vessel) may include without limitation
dimethylformamide
(DMF), dimethylacetamide (DMA), N-methylpyrrolidinone (NMP), green solvents
and/or
non-reprotoxin solvents, and the like, and combinations and/or mixtures
thereof. Examples
of green and/or non-reprotoxin solvents may include without limitation N-
formylmorpholine
(NFM), N-butylpyrrolidinone (NBP), alkoxybenzene-based solvents (e.g.,
anisole,
dimethoxybenzene-based solvents such as 1,3-dimethoxoybenzene, etc.), and the
like, and
combinations and/or mixtures thereof.
Referring again to Figs. 1A and 1B, mixture 30 is typically initially present
in a lower
interior portion of a reaction vessel. At least a portion of the deprotecting
base present in
mixture 30 evaporates during the removing step (e.g., at least a portion of
the deprotecting
base evaporates into an upper interior portion of the reaction vessel during
the removing
step). For example, the deprotecting base can have a lower boiling point
relative to the
deprotection reaction temperature / temperature of the reaction vessel during
deprotection
and/or a lower boiling point relative to the boiling point of solvent(s) that
may be present in
the reaction vessel (e.g., solvents present in the deprotection reaction
mixture (e.g., the
deprotection reaction solution)), such as dimethylformamide (DMF) and N-
methylpyrrolidinone (NMP). Accordingly, the deprotecting base can volatize or
evaporate
during the deprotection process.
-19-
Date Recue/Date Received 2023-08-25
In some embodiments, the deprotecting step (e.g., the deprotection reaction
step) can
be conducted without heat (e.g., can be conducted at room temperature), so
long as the
deprotecting conditions (type of base, time, etc.) are selected to promote
evaporation of the
deprotecting base (e.g., into the headspace of the reaction vessel).
More typically, in some embodiments, the deprotecting step/process may be
conducted with heat (e.g., the deprotecting step/process may further include
the step of
heating the protected amino acid and/or the deprotecting base (e.g., heating
the deprotection
solution including the deprotecting base) and/or other liquids (e.g.,
additional added solvent,
residual coupling solution, etc.) and/or the reaction vessel, etc.). The
protected amino acid
and/or the deprotecting base and/or other liquids and/or the reaction vessel,
etc. may be
heated before and/or during the step of removing the protecting group from the
protected
amino acid. For example, the process may include heating the protected amino
acid and/or
the deprotecting base and/or other liquids such as added solvent before and/or
during delivery
into the reaction vessel 4 and/or heating the protected amino acid and/or the
deprotecting base
and/or other liquids in the reaction vessel 4 before and/or during the
deprotection reaction
(e.g., by heating the reaction vessel before and/or during the step of
removing the protecting
group from the protected amino acid). Heating during solid phase peptide
synthesis can be
useful, for example, to accelerate the rate of deprotection and thereby reduce
the amount of
time required for peptide synthesis.
Heating temperatures of the heating step may vary. In some embodiments, the
heating step (e.g., heating during the deprotecting step/process) can be
conducted at a
temperature from about 40 C to about 120 C, for example from about 50 C to
about 120
C, as another example from about 60 C to about 120 C, as another example
from about 70
C to about 120 C, as another example from about 80 C to about 120 C, as
another
example from about 90 C to about 120 C, as another example from about 80 C
to about
110 C, and as another example from about 90 C to about 110 C, without
limitation. In
certain embodiments, the heating step (e.g., heating during the deprotecting
step/process) can
be conducted at a temperature from about 60 C to about 120 C, for example
from about 90
C to about 120 C, and as another example from about 90 C to about 110 C,
without
limitation. The temperature may be any value within the ranges described
herein, including
end points (e.g., any value within a range of from about 40 C to about 120
C) and all
subranges within the range are also disclosed.
-20-
Date Recue/Date Received 2023-08-25
Figures 1A and 1B schematically depict a heating step wherein a heat source 40
heats
reaction vessel 4 and mixture 30. In certain embodiments, heat source 40
includes a
microwave source 42 positioned to direct microwave radiation 44 through a
waveguide 46
attached to a microwave cavity (not illustrated) containing reaction vessel 4.
Microwave
power may be regulated as known in the art to provide reaction temperatures
and/or reaction
times (e.g., without limitation, to provide deprotection temperatures as
described herein and
to provide deprotection reaction times ranging from about 10 sec to about 15
minutes, as
another non-limiting example from about 40 sec to about 8 minutes).
In embodiments utilizing microwave energy to heat the reactants, reaction
vessel 4
can be formed of a material that is transparent to microwave radiation, such
as but not limited
to glass, Teflon, and/or polypropylene.
Microwave sources are well known in the art and can include, for example,
magnetrons, klystrons, and/or solid-state diodes. Microwave sources,
waveguides and
microwave cavities suitable for solid phase peptide synthesis processes and
systems are well
known in the art and also are commercially available (e.g., systems
commercially available
from CEM Corporation such as discussed herein). Accordingly, the skilled
artisan will
understand how to use the same in solid phase peptide synthesis processes and
systems
without undue experimentation.
The present disclosure, however, is not limited to the use of microwave
sources as the
heat source, and other types of heat sources known in the art for solid phase
peptide synthesis
can used.
Despite the benefits of heating, elevated temperatures during the deprotecting
step can
present various challenges for peptide synthesis.
For example, organic amines used in deprotection reactions can have relatively
low
boiling points, as compared to the boiling point of a solvent used in a
deprotection reaction
and/or the temperature of the deprotecting step. Piperidine has a boiling
point of about 106
C and pyrrolidine has a boiling point of about 87 C. In contrast, the solvent
dimethylformamide (DMF) has a boiling point of about 153 C and the solvent N-
methylpyrrolidinone (NMP) has a boiling point of about 200 C. Also in
contrast, as noted
herein, deprotecting reactions can be conducted at elevated temperatures, for
example up to
about 120 C, for example about 90 C to about 120 C, and as another example
about 90 C
to about 110 C, without limitation.
-21-
Date Recue/Date Received 2023-08-25
A reaction vessel can exhibit a temperature continuum during processing,
wherein an
upper portion thereof can be at a lower temperature than lower portions.
Because the
deprotecting base can have a boiling point lower than the boiling point of
other components
present in the reaction vessel such as solvents and/or lower than reaction
temperatures, the
deprotecting base can volatize (evaporate) into the upper portion of the
reaction vessel (e.g.,
the headspace) and then condense on upper portions of the reaction wall(s)
and/or on a top
wall of the reaction vessel. The rate/amount of volatilization (evaporation)
can also increase,
for example, when the reactants are bubbled during deprotection (e.g., using
an inert gas such
as nitrogen) to help mix the reactants.
Volatilization of a deprotecting base can be especially problematic using
pyrrolidine.
Pyrrolidine would be desirable as a deprotecting base because pyrrolidine can
provide faster
deprotection than piperidine. As a 5-membered ring (versus a 6-membered
piperidine ring),
the carbon atoms of pyrrolidine are bent back more from the nitrogen atom,
which facilitates
an easier attack for deprotection. Because pyrrolidine has a lower boiling
point than
piperidine, however, significant evaporation followed by condensation can
occur during
deprotection processes, thereby limiting its use, including without example in
the synthesis of
long peptides.
In the processes of the present disclosure, the heating step can volatize
(evaporate) the
deprotecting base (e.g., pyrrolidine) in the deprotection reaction mixture
(e.g., the
deprotection reaction solution) from the lower portion of reaction vessel 4
upwardly into the
upper portion (e.g., into the headspace above mixture 30) of reaction vessel
4.
Residual deprotecting base remaining in a reaction vessel (e.g., residual
deprotecting
base condensed on upper portions of the reaction wall(s) and/or on a top wall
of the reaction
vessel) during subsequent solid phase peptide synthesis steps (e.g., a
subsequent coupling
step) can be problematic. Residual deprotecting base can, for example,
prematurely remove a
protecting group from an amino acid to be coupled to the already deprotected
amino acid.
This can result in undesirable insertions into the peptide chain. Residual
deprotecting base
can also reduce activated amino acid by reacting with the amino acid, which
can result in
deletions in the peptide chain.
Accordingly, conventional SPPS processes required washing steps after
deprotection
and before coupling (e.g., to help remove residual deprotecting base to
minimize or prevent
-22-
Date Recue/Date Received 2023-08-25
participation thereof in subsequent solid phase peptide synthesis steps such
as a subsequent
coupling step).
In contrast to conventional SPPS processes, the processes of the present
disclosure
can help eliminate washing step(s) between deprotecting and coupling steps
and/or reduce the
amount of solvent required for a washing step(s) between deprotecting and
coupling steps of
a SPPS process.
To help eliminate washing step(s) between deprotecting and coupling steps
and/or
reduce the amount of solvent required for a washing step(s) between
deprotecting and
coupling steps of a SPPS process, the process of the present disclosure uses
small amounts of
deprotecting base as described herein and/or directs (e.g., continuously
and/or intermittently
directs) an inert gas through the interior of the reaction vessel (e.g.,
directs inert gas through
the upper interior portion, or headspace, of the reaction vessel) during the
deprotection step to
assist in removing (e.g., to assist in flushing, venting, discharging,
displacing, replacing,
purging, etc., e.g., to remove, flush, vent, discharge, displace, replace,
purge, etc.) evaporated
(volatized) deprotecting base from the interior of the reaction vessel (e.g.,
from the reaction
vessel headspace).
The processes of the present disclosure may also generally facilitate the
production of
peptides having acceptable purity levels for downstream applications.
In some embodiments, the step of directing an inert gas through the interior
of the
reaction vessel may include directing (introducing, supplying, flowing, etc.)
inert gas into an
upper interior portion of the reaction vessel via one or more openings (entry
ports) located in
an upper portion of the reaction vessel so that inert gas flows through the
upper interior
portion of the reaction vessel and out of the upper interior portion of the
reaction vessel
through one or more other openings (exit ports) located in the upper portion
of the reaction
vessel. In this manner, inert gas may flow through the upper interior portion
of the reaction
vessel including evaporated deprotecting base (e.g., through the vessel
headspace) and assist
in removing (e.g., assist in flushing, venting, discharging, displacing,
replacing, purging, etc.,
e.g., to remove, flush, vent, discharge, displace, replace, purge, etc.)
evaporated deprotecting
base from the upper interior portion (e.g., from the headspace above mixture
30) of the
reaction vessel through one or more other openings (exit ports) located in the
upper portion of
the reaction vessel.
-23-
Date Recue/Date Received 2023-08-25
In some embodiments, the step of directing an inert gas through the interior
of the
reaction vessel may include directing (introducing, supplying, flowing, etc.)
inert gas into a
lower interior portion of the reaction vessel via one or more openings (entry
ports) located in
a lower portion of the reaction vessel so that inert gas flows upwardly from
the lower interior
portion of the reaction vessel into/through the upper interior portion of the
reaction vessel and
out of the upper interior portion of the reaction vessel through one or more
other openings
(exit ports) located in the upper portion of the reaction vessel. In this
manner, inert gas may
flow upwardly from the lower interior portion of the reaction vessel
into/through the upper
interior portion of the reaction vessel including evaporated deprotecting base
(e.g., through
the vessel headspace) and assist in removing (e.g., assist in flushing,
venting, discharging,
displacing, replacing, purging, etc., e.g., to remove, flush, vent, discharge,
displace, replace,
purge, etc.) evaporated deprotecting base from the upper interior portion
(e.g., from the
headspace above mixture 30) of the reaction vessel through one or more other
openings (exit
ports) located in the upper portion of the reaction vessel.
In some embodiments, the step of directing an inert gas through the interior
of the
reaction vessel may include directing (directing, supplying, flowing, etc.)
inert gas into both
an upper interior portion and a lower interior portion of the reaction vessel
via one or more
openings (entry ports) located in an upper portion and a lower portion of the
reaction vessel,
respectively so that inert gas flows through the upper interior portion of the
reaction vessel
(including optionally upwardly from the lower interior portion of the reaction
vessel
into/through the upper interior portion of the reaction vessel) and out of the
upper interior
portion of the reaction vessel through one or more other openings (exit ports)
located in the
upper portion of the reaction vessel. In this manner, inert gas may flow
through the upper
interior portion of the reaction vessel including evaporated deprotecting base
(e.g., through
the vessel headspace) and may also optionally flow upwardly from the lower
interior portion
of the reaction vessel into/through the upper interior portion of the reaction
vessel including
evaporated deprotecting base (e.g., through the vessel headspace) and assist
in removing
(e.g., assist in flushing, venting, discharging, displacing, replacing,
purging, etc., e.g., to
remove, flush, vent, discharge, displace, replace, purge, etc.) evaporated
(volatized)
deprotecting base from the upper interior portion (e.g., from the headspace
above mixture 30)
of the reaction vessel through one or more other openings (exit ports) located
in the upper
portion of the reaction vessel.
-24-
Date Recue/Date Received 2023-08-25
In some embodiments, the inert gas can be continuously directed through the
reaction
vessel as a continuous flow. In some embodiments, the inert gas can be
directed through the
reaction vessel as an intermittent (e.g., pulsed) flow.
In some embodiments, inert gas directed into and/or flowing through the
reaction
vessel may have a pressure of about 1 psi to about 25 psi. In some
embodiments, inert gas
directed into and/or flowing through the reaction vessel (e.g., flowing
through the headspace
of the reaction vessel including volatized deprotecting base; and/or bubbling
through mixture
30 and/or flowing upwardly through mixture 30 of the reaction vessel) may have
a pressure
of about 1,2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20,
21, 22, 23, 24, or 25
psi. In some embodiments, inert gas directed into and/or flowing through the
reaction vessel
may have a pressure in a range from about any of the foregoing pressure values
to about any
other of the foregoing pressure values. The pressure of the inert gas,
including inert gas
directed into and/or flowing through an upper interior portion of the reaction
vessel and/or
directed into and/or flowing through a lower interior portion of the reaction
vessel (including
directed into and/or flowing through mixture 30, e.g., bubbling through and/or
flowing
generally upwardly through mixture 30 into the upper interior portion of the
reaction vessel),
may be any value within the ranges described herein, including end points
(e.g., any value
within a range of from about 1 to about 25 psi) and all subranges within the
range are also
disclosed.
In some embodiments, the pressure of inert gas directed into and/or flowing
through
the reaction vessel may be higher than about 25 psi. As a non-limiting
example, in some
large-scale production methods (e.g., large scale microwave peptide
synthesizer production
methods) including deprotection step(s) according to the present disclosure
(e.g., including
one or more deprotecting steps as described herein using a deprotecting base
in an amount
from greater than zero to about 5 vol%, based on the total volume of the
deprotection reaction
mixture (e.g., the deprotection reaction solution) and/or using an inert gas
to flush volatized
deprotecting base from the reaction vessel (e.g., from the head space of the
interior of the
reaction vessel), also as described in more detail herein), the inert gas may
have a pressure
from about 1 psi to about 100 psi, for example from greater than or about 25
psi to about 100
psi, for example from about 50 psi to about 95 psi, and as another example
from about 75 psi
to about 95 psi. In some embodiments, a large scale production method
including
deprotection step(s) according to the present disclosure may employ a reaction
vessel having
a size (e.g., interior volume) of 3 liters or larger (e.g., 3 liters, 8
liters, 10 liters, 15 liters, etc.,
-25-
Date Recue/Date Received 2023-08-25
up to 40 liters or larger), and/or may have a synthesis scale of about 25 mmol
or higher (e.g.,
about 25 mmol or higher, about 50 mmol or higher, about 100 mmol or higher,
about 200
mmol or higher, about 250 mmol or higher, about 500 mmol or higher., etc.),
and/or may
provide peptide quantities per batch of up to about 500 grams or higher (e.g.,
about 500
grams or higher, about 1 kg or higher, etc.), and/or have varying deprotection
cycle times
(e.g., from about 8 minutes to about 15 minutes, about 10 minutes, etc.).
In some embodiments in which inert gas is introduced into the reaction vessel
both via
a first opening located in an upper portion of the reaction vessel (the upper
opening) and a
second opening located in a lower portion of the reaction vessel (the lower
opening), inert gas
flowing into and/or through the upper opening and/or upper interior portion of
the reaction
vessel may have a higher psi than inert gas flowing into and/or through the
lower opening
and/or lower interior portion of the reaction vessel.
The inert gas may also be directed through the interior (e.g., through an
upper interior
portion, or headspace) of the reaction vessel during the deprotection step at
a flow rate based
on a time rate within which the inert gas substantially replaces (displaces)
the headspace gas
volume. More specifically, the inert gas flow rate may be an amount (volume)
of inert gas
that allows for (results in) substantial replacement (displacement) of the
volume of gas in the
headspace area of the reaction vessel with (by) the inert gas (e.g., that
results in substantial
replacement of the volume of volatized deprotecting base in the headspace area
of the
reaction vessel with the inert gas) within a selected time period (time rate).
For example, the
inert gas flow rate may be an amount (volume) of inert gas that results in
(allows for) the
substantial replacement (displacement) of the volume of gas in the headspace
area (e.g., the
volume of volatized deprotecting base in the headspace area) of the reaction
vessel about
every one (1) to twenty (20) seconds, for example, about every five (5) to ten
(10) seconds.
The skilled artisan will understand how to determine and calculate suitable
inert gas flow
rates to replace (displace) a volume of headspace gas (volatized deprotecting
base) in a
reaction vessel within a time frame (time rate) without undue experimentation.
Although not wishing to be bound by any explanation or theory, it is currently
believed that directing a source of inert gas into and/or through the
headspace during
deprotection can cause a large air exchange rate in the gas above the
deprotection reaction
mixture (e.g., the deprotection reaction solution) and/or other reactants,
products, etc. in a
lower interior portion of the reaction vessel (e.g., in the headspace gas
including the volatized
deprotecting base) such that the inert gas displaces the volatized
deprotecting base from the
-26-
Date Recue/Date Received 2023-08-25
reaction vessel. This can reduce residence time of the volatized deprotecting
base in the
reaction vessel and the volatized deprotecting base can be more quickly
removed with less
condensation on the side and/or top walls of the vessel. This in turn can help
reduce the
amount of residual deprotecting base remaining in the reaction vessel after
the deprotecting
step is completed. The inert gas can also provide downward force on droplets
(e.g.,
condensed deprotecting base) on a side wall of reaction vessel 4 and can
thereby blow the
droplets toward mixture 30 in the lower portion of reaction vessel 4.
Because the deprotecting process uses a low amount of deprotecting base (less
than or
about 5 vol% deprotecting base, based on the total volume of deprotection
reaction mixture
(e.g., the deprotection reaction solution)), the deprotecting base (e.g.,
pyrrolidine) may be
essentially completely removed from the reaction vessel upon completion of the
deprotection
step. For example, without being bound by any theory or explanation, in such
embodiments,
it is currently believed that the deprotecting base may substantially
completely evaporate
during a heating step and/or volatized deprotecting base may be substantially
completely
removed from the headspace using inert gas flushing, each as described herein.
Also without
being bound by any theory or explanation, in such embodiments, it is currently
believed that
any residual amount of deprotecting base remaining after completion of the
deprotection step
is small enough to minimize issues associated with the presence of residual
deprotecting base
in the next coupling step, even without a washing step after the deprotection
step and/or with
a washing step after the deprotection step using amounts of washing liquid
(e.g. solvent) also
as described in more detail herein.
Thus, in exemplary embodiments, the deprotecting processes described herein
can
remove a significant portion of the deprotecting base used for the
deprotection step from the
reaction vessel (e.g., by evaporation). As used herein, a "significant portion
of the
deprotecting base" can include without limitation at least a majority (e.g.,
more than half), at
least about 60%, at least about 70%, at least about 80%, at least about 90%,
at least about
95%, at least about 99%, or more, of the deprotecting base used for the
deprotection step
from the reaction vessel. For example, in some embodiments, the deprotecting
processes
described herein can remove at least about 51, 52, 53, 54, 55, 56, 57, 58, 59,
60, 61, 62, 63,
64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82,
83, 84, 85, 86, 87, 88,
89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, or 100% of the deprotecting base
used for the
deprotection step from the reaction vessel. Further, according to some
embodiments, the
-27-
Date Recue/Date Received 2023-08-25
amount of deprotecting base removed from the reaction vessel can be an amount
of from
about any of the foregoing amounts to about any other of the foregoing
amounts.
Because the amount of residual deprotecting base can be reduced, in contrast
to
conventional approaches, the process of the present disclosure may also
facilitate the
production of peptides having acceptable purity levels for downstream
applications.
The inert gas can be nitrogen. The present disclosure is not limited to the
use of
nitrogen as the inert gas and other inert gases, such as the noble gases, with
limited or no
interference chemically with solid phase peptide synthesis reactions and with
the solid phase
peptide synthesis system can be used.
In certain embodiments, as depicted in Figure 1A, the process can include
providing
pressurized inert gas from an inert gas source (such as inert gas source
designated as 100 in
Figure 2A) through flow path 20 and directing the pressurized inert gas (e.g.,
generally
downwardly directing the pressurized inert gas) into the upper portion of the
interior of the
reaction vessel 4 through opening 10 and into the headspace above mixture 30
(including the
deprotection reaction mixture (e.g., the deprotection reaction solution) and
protected amino
acids) located in a lower interior portion of the reaction vessel. As the
pressurized inert gas
flows through the headspace from the upper portion of the interior of the
reaction vessel 4,
the inert gas purges (e.g., removes, flushes, displaces, replaces, vents,
discharges, etc.)
volatized deprotecting base out of the interior of the reaction vessel 4
through opening 14 into
flow path 24. In this manner, the pressurized inert gas in effect displaces
the volatized
deprotecting base from the headspace of the reaction vessel. This can reduce
residence time
and minimize condensation of the deprotecting base on the walls of the
reaction vessel.
Gas flow (movement) in reaction vessel 4, including upward flow of volatized
deprotecting base (e.g., pyrrolidine) from mixture 30 in the lower interior
portion of reaction
vessel 4 into the headspace above the mixture (e.g., into the upper interior
portion of reaction
vessel 4), the flow of inert gas downwardly into and through the headspace
(upper interior
portion) of reaction vessel 4, and purging (e.g., removing, flushing,
displacement, venting,
discharging, replacing, etc.) of volatized deprotecting base and inert gas
from the headspace
(upper interior portion) of reaction vessel 4, is schematically depicted by
the arrows in Figure
1A.
In certain embodiments, as depicted in Figure 1B, the process can include
providing
(e.g., directing) pressurized inert gas from an inert gas source (such as
inert gas source
-28-
Date Recue/Date Received 2023-08-25
designated as 100 in Figure 2B) through flow path 218, opening 208, the inner
interior space
of spray head 220, and out openings 222 into outer interior space 7 of
reaction vessel 4 (e.g.,
into the headspace above mixture 30). Figure 1B also depicts embodiments
wherein the
spray head 220 directs (e.g., sprays) inert gas through openings 222 at an
angle (e.g., spray
pattern) schematically depicted by dashed lines 224 towards side wall 6 of the
reaction vessel
4. This can facilitate a washing effect, wherein the inert gas can contact the
side wall and
"wash" condensed deprotecting base toward reactants in the lower portion of
the interior of
the reaction vessel 4.
As the pressurized inert gas flows through the headspace of reaction vessel 4,
the inert
gas purges (e.g., removes, flushes, displaces, replaces, vents, discharges,
etc.) volatized
deprotecting base out of reaction vessel 4 through opening 206 into flow path
216. Again,
the pressurized inert gas in effect displaces the volatized deprotecting base
from the
headspace of the reaction vessel, which may reduce residence time and minimize
condensation of the deprotecting base on the walls of the reaction vessel.
Gas flow (movement) in reaction vessel 4, including upward flow of volatized
deprotecting base (e.g., pyrrolidine) from mixture 30 in the lower interior
portion of reaction
vessel 4 into the headspace above the mixture (e.g., into the upper interior
portion of reaction
vessel 4), flow of inert gas from spray head 220 through openings 222 (e.g.,
angled flow
towards side wall 6) into and through the headspace (e.g., the upper interior
portion) of the
reaction vessel, and purging (e.g., flushing, displacing, venting, removing,
discharging, etc.)
of volatized deprotecting base and inert gas from the headspace (e.g., the
upper interior
portion) of reaction vessel 4, is schematically depicted by the arrows and
dashed lines in
Figure 1B.
In certain embodiments, as discussed herein, the process may include
introducing
(directing, etc.) an inert gas into a lower interior portion of reaction
vessel 4, in addition to or
as an alternative to introducing (directing, etc.) an inert gas into an upper
interior portion
(e.g., into the headspace) of the reaction vessel. For example, referring to
Figures 1A and 1B,
the process can include directing a pressurized inert gas from an inert gas
source (which can
be the same as or different from an inert gas source of an inert gas
introduced into the upper
interior portion of the reaction vessels when present) through flow path 26
and into the lower
interior portion of reaction vessel 4 through opening 16. The pressurized
inert gas may flow
upwardly from the lower interior portion of the reaction vessel (e.g.,
generally upwardly
through mixture 30) and into/through the upper interior portion (e.g.,
headspace) of the
-29-
Date Recue/Date Received 2023-08-25
reaction vessel 4 including volatized (evaporated) deprotecting base. As the
inert gas flows
upwardly (e.g., through the headspace), the inert gas may purge (e.g., flush,
displace, replace,
vent, discharge, remove, etc.) volatized deprotecting base from the upper
interior portion
(e.g., the headspace) of the reaction vessel 4 through opening 14 into flow
path 24. Again, in
this manner, the pressurized inert gas may displace the volatized deprotecting
base from the
headspace of the reaction vessel, which may reduce residence time and minimize
condensation of the deprotecting base on the walls of the reaction vessel.
The pressurized inert gas introduced into the lower interior portion of the
reaction
vessel may in addition, or alternatively, agitate (mix, bubble, etc.) mixture
30.
In some embodiments, the process may include introducing (directing) both a
first
pressurized inert gas into an upper interior portion (e.g., the headspace) of
the reaction vessel
including evaporated deprotecting base and a second pressurized inert gas into
a lower
interior portion of a reaction vessel. As non-limiting examples, referring to
Figs. 1A and 1B,
the process may include directing the first pressurized inert gas into the
upper interior portion
of reaction vessel 4 through a first opening located in an upper portion of
the reaction vessel,
such as opening 10 of Fig. 1A or opening 208 and openings 222 of Fig. 1B, and
directing the
second pressurized inert gas into the lower interior portion of the reaction
vessel 4 through a
second opening located in a lower portion of the reaction vessel such as
opening 16 of Figs.
1A and 1B. The first pressurized inert gas may flow through the upper interior
portion (e.g.,
through the headspace) of the reaction vessel including evaporated
deprotecting base. The
second pressurized inert gas may flow through the mixture 30 to agitate (stir,
bubble, etc.) the
mixture 30 and/or flow generally upwardly from the lower interior portion of
the reaction
vessel through mixture 30 and into/through the upper interior portion (e.g.,
the headspace) of
the reaction vessel including evaporated deprotecting base. The first
pressurized inert gas
and optionally the second pressurized inert gas may purge (e.g., flush,
displace, replace, vent,
discharge, remove, etc.) volatized deprotecting base from the upper interior
portion (e.g.,
from the headspace) of the reaction vessel, for example, through a third
opening located in an
upper portion of the reaction vessel, such as opening 14 or 206 of Figs. 1A
and 1B,
respectively.
The first inert gas (also referred to herein as the overhead inert gas)
directed into
and/or flowing through the upper interior portion (e.g., the headspace above
mixture 30) of
reaction vessel 4 (e.g., the first inert gas directed through a first opening
located in an upper
portion of the reaction vessel, such as opening 10 of Fig. 1A or opening 208
and openings
-30-
Date Recue/Date Received 2023-08-25
222 of Fig. 1B) may have a higher pressure than the second inert gas directed
into and/or
flowing through the lower interior portion (and optionally upwardly into the
headspace) of
reaction vessel 4. As a non-limiting example, the first (overhead) inert gas
directed into
and/or flowing through the upper interior portion (e.g., flowing through the
headspace) of
reaction vessel 4 can have a pressure from about 1 psi to about 25 psi. In
some embodiments,
the first (overhead) inert gas directed into and/or flowing through the upper
interior portion of
reaction vessel 4 can have a pressure of about 1, 2, 3, 4, 5, 6, 7, 8, 9, 10,
11, 12, 13, 14, 15,
16, 17, 18, 19, 20, 21, 22, 23, 24, or 25 psi. Further, according to some
embodiments, the
first (overhead) inert gas directed into and/or flowing through the upper
interior portion of the
reaction vessel 4 can have a pressure in a range from about any of the
foregoing pressure
values to about any other of the foregoing pressure values.
As a non-limiting example, the second inert gas directed into and/or flowing
through
the lower interior portion of the reaction vessel 4 (and optionally upwardly
into the
headspace) can have a pressure that is less than the pressure of the first
(overhead) inert gas
directed into and/or flowing through the upper interior portion of the
reaction vessel 4. For
example, the second inert gas may have a pressure from about 1 psi to about 25
psi, so long
as the pressure of the second inert gas is less than the pressure of the first
inert gas. In
embodiments wherein an inert gas is introduced only into the lower interior
portion of the
reaction vessel (there is no inert gas introduced into an upper interior
portion of the reaction
vessel), the inert gas may also have a pressure from about 1 psi to about 25
psi.
In a non-limiting example, the first (overhead) inert gas directed into and/or
flowing
through the upper interior portion (e.g., head space) of reaction vessel 4 can
have a pressure
of about 15 psi and the second inert gas introduced into and/or flowing
through the lower
portion of the reaction vessel 4 (and optionally upwardly into the headspace)
can have a
pressure that is less than the pressure of the first (overhead) inert gas
directed into and/or
flowing through the upper portion of the reaction vessel 4, such as a pressure
of about 5 psi.
The process accordingly may allow the use of deprotecting bases with
relatively lower
boiling points at higher temperatures to accelerate reaction times, while
minimizing
(reducing) adverse effects associated with using a low boiling point, readily
volatized
reactant.
After deprotecting is completed, the inert gas flow may be stopped and a
coupling
step may be conducted using processes as known in the art.
-31-
Date Recue/Date Received 2023-08-25
The present disclosure also relates to solid phase peptide synthesis processes
including one or more deprotecting steps as described in more detail herein
(e.g., including
one or more deprotecting steps as described herein using a deprotecting base
in an amount of
about 5 vol% or less, based on the total volume of the deprotection reaction
mixture (e.g., the
deprotection reaction solution), and/or using an inert gas to flush volatized
deprotecting base
from the reaction vessel (e.g., from the head space of the interior of the
reaction vessel), also
as described in more detail herein). The solid phase peptide synthesis process
of the present
disclosure further includes one or more coupling steps (e.g., may include one
or more
deprotecting-coupling cycles). Solid phase peptide synthesis coupling steps
and systems for
conducting the same are generally known in the art and accordingly are not
described in
detail herein.
Conventional SPPS processes require multiple washing steps between
deprotection
and coupling steps (e.g., after deprotection and before coupling) to remove
residual
deprotecting base. In some embodiments of the present disclosure (e.g., SPPS
processes
including a deprotection step as described herein), a washing liquid (e.g., a
solvent such as
but not limited to dimethylformamide (DMF), methanol and/or isopropanol) can
be added to
the reaction vessel for a washing step after deprotection. The washing step
may include a
single washing step or the washing step may be carried out repetitively (e.g.,
with two, three,
four, five, etc., repetitions).
Washing steps, however, can require the use of large amounts of solvent,
necessitate
solvent recovery and disposal, etc. This can increase material costs and
peptide synthesis
times, decrease efficiencies, etc. In addition, multiple washing steps may be
less effective in
preventing unwanted reactions and reducing impurities, particularly as peptide
length
increases, which can make it difficult to synthesize peptides with purities
acceptable for
downstream applications.
In contrast to conventional processes, in some embodiments, the present
disclosure is
directed to a SPPS process including a deprotecting step followed by a
coupling step, wherein
the SPPS process does not include a washing step after the deprotecting step
and before the
associated coupling step. Stated differently, the process of the present
disclosure may
eliminate one or more washing steps (e.g., may eliminate all washing steps)
between a
deprotection step and its associated coupling step (i.e., the coupling step
immediately
following the deprotection step). This can provide benefits such as improved
process
-32-
Date Recue/Date Received 2023-08-25
efficiencies, energy savings, reduced amounts of solvent required in the SPPS
process,
reduced material costs, reduced solvent disposal issues, etc.
For example, the SPPS process of the present disclosure may include a series
of
deprotection-coupling cycles, wherein one or more (e.g., all) washing step(s)
are eliminated
(e.g., there is no washing step) between the deprotection step and the
coupling step of at least
one of the deprotection-coupling cycles of the SPPS process. In other
examples, the SPPS
process of the present disclosure may include a series of deprotection-
coupling cycles,
wherein one or more (e.g., all) washing step(s) are eliminated (e.g., there is
no washing step)
between the deprotection step and the coupling step of more than one of the
deprotection-
coupling cycles, for example for half of the deprotection-coupling cycles, for
example for a
majority of the deprotection-coupling cycles, and as another example for all
of deprotection-
coupling cycles, of the SPPS process.
In yet other embodiments, the present disclosure is directed to a SPPS process
including a deprotecting step followed by a coupling step, wherein the SPPS
process includes
one or more washing steps (e.g., one, two, three, four, five, etc. washing
steps) using a
washing composition (e.g., a solvent) after the deprotecting step and before
the associated
coupling step. In contrast to conventional washing steps, however, the washing
step(s) of this
embodiment may use reduced amounts of solvent as compared to conventional SPPS
processes.
In some embodiments, the washing step(s) after deprotection and before
coupling may
include washing the interior of the reaction vessel using a washing
composition (e.g., a
solvent) in an amount (volume) that is less than the total amount (total
volume) of the
deprotection reaction mixture (e.g., the deprotection reaction solution). In
some
embodiments, the process can include washing the interior of the reaction
vessel one or more
times (e.g., one, two, three, four, five, etc. times) using a washing
composition (e.g., a
solvent) in an amount (total volume) that is 2 times or less of a bed volume
of a resin present
in the reaction vessel (e.g., a solid resin support present in the reaction
vessel), for example, 1
time or less of a bed volume of a resin present in the reaction vessel. The
skilled artisan will
understand that the term "bed volume" refers to the area of the reaction
vessel taken up
(occupied) by a resin present in the reaction vessel (e.g., the solid support
resin as described
herein present in the reaction vessel) and that a total volume of solvent that
is 2 times or less
of the bed volume of the resin present in the reaction vessel, for example, 1
time or less of the
bed volume of the resin present in the reaction vessel, refers to a volume of
liquid (e.g.,
-33-
Date Recue/Date Received 2023-08-25
solvent) that fills up this same area (e.g., fills up 2 times or less of the
bed volume of the resin
present in the reaction vessel (e.g., the solid support resin as described
herein present in the
reaction vessel), for example fills up 1 times or less of the bed volume of
the resin present in
the reaction vessel (e.g., the solid support resin as described herein present
in the reaction
vessel), etc.). In addition, the SPPS process may include a series of
deprotection-coupling
cycles, wherein one or more of (e.g., half of, a majority of, or all of) the
deprotection-
coupling cycles include one or more washing steps (e.g., one, two, three,
four, five, etc.
washing steps) between the deprotection step and the coupling step, and
wherein the washing
step(s) uses a washing composition (e.g., solvent) in an amount (total volume)
that is 2 times
or less of the bed volume of the resin present in the reaction vessel, for
example, 1 times or
less of the bed volume of the resin present in the reaction vessel.
When used, the washing composition (washing liquid) can include a solvent such
as
but not limited to dimethylformamide (DMF), methanol and/or isopropanol.
When a washing step is used, in some embodiments, the washing liquid (e.g.,
solvent)
can be introduced into the reaction vessel via a suitable opening into an
upper interior portion
of the reaction vessel, such as opening 10 of Fig. 1A, and/or using a
different spray head or
the same spray head (e.g., spray head 220 of Figure 1B) used to introduce the
inert gas into
the reaction vessel during the deprotecting step described herein. As a non-
limiting example,
as depicted in Figure 1B, the process can include providing (e.g., directing)
solvent from a
solvent source (not illustrated in Figure 1B) through flow path 218, opening
208, the inner
interior space of spray head 220, and out openings 222 into the outer interior
space 7 of
reaction vessel 4. As also schematically depicted in Figure 1B, in some
embodiments, the
spray head 220 can direct (e.g., spray) solvent through openings 222 at an
angle (e.g., spray
pattern) schematically depicted by dashed lines 224 towards side wall 6 of the
reaction vessel
4, which can facilitate washing deprotecting base condensed on the side wall
downwardly
toward the lower interior portion of reaction vessel 4.
When a washing step is included, the washing solution can then be removed in a
draining step, after which a coupling step can be initiated in accordance with
known
processes.
In some embodiments, the solid phase peptide synthesis process can include:
deprotecting a first amino acid (e.g., removing a protecting group of a first
protected amino
acid) to form a deprotected amino acid; coupling a second amino acid to the
deprotected
-34-
Date Recue/Date Received 2023-08-25
amino acid to form a peptide from the first and second amino acids; and
repeating the
deprotecting and coupling steps to form a peptide comprising the first,
second, and successive
plurality of amino acids,
wherein the deprotecting and coupling steps take place in a reaction vessel
(e.g., in a
reaction vessel such as a batch-type reaction vessel 4 described in more
detail herein),
wherein one or more of the deprotecting steps employ a deprotecting base in an
amount of about 5 vol% or less, based on the total volume of a deprotection
reaction mixture
(e.g., the deprotection reaction solution), as described in more detail
herein, and/or
wherein one or more of the deprotecting steps employ an inert gas purging
(e.g.,
headspace flushing) step to assist in removing (e.g., to remove) evaporated
deprotecting base
from the interior (e. g., from the headspace) of the reaction vessel, also as
described in more
detail herein.
In the SPPS processes including a deprotecting step as described herein, the
solid
phase peptide synthesis process may not include a washing step between the
deprotection
step and the coupling step of one or more of the deprotection-coupling cycles
of the SPPS
process. In other SPPS processes including a deprotecting step as described
herein, the solid
phase peptide synthesis process may include one or more washing steps between
the
deprotection step and the coupling step of one or more of the deprotection-
coupling cycles of
the SPPS process, the washing step using amounts of washing liquid (e.g.,
solvent) as
described in more detail herein (e.g., using solvent in an amount (e.g., total
volume) that is 2
times or less of the bed volume of the resin present in the reaction vessel,
for example, 1 time
or less of the bed volume of the resin present in the reaction vessel). For
example, the solid
phase peptide synthesis process may omit one or more (e.g., all) washing steps
between the
deprotection step and the coupling step of one or more of the deprotection-
coupling cycles of
the SPPS process. As another example, the solid phase peptide synthesis
process may
include one or more washing steps using reduced amounts of washing liquid
(e.g., solvent) as
described in more detail herein (e.g., using solvent in an amount (e.g., total
volume) that is 2
times or less of the bed volume of the resin present in the reaction vessel,
for example, 1
times or less of the bed volume of the resin present in the reaction vessel)
between the
deprotection step and the coupling step of one or more of the deprotection-
coupling cycles of
the SPPS process.
-35-
Date Recue/Date Received 2023-08-25
The solid phase peptide synthesis process can further include, prior to
coupling,
activating chemical group(s) on the second amino acid (and successive amino
acid(s)) using
processes and agents known in the art to prepare the second (and successive)
amino acid(s)
for coupling with the first (and sequential) amino acid(s).
An amino acid activating agent (amino acid activator) may be used to activate
the
amino acid (e.g., convert the acid group of the amino acid into an activated
form) prior to a
coupling step. Any suitable amino acid activating agent may be used. Examples
of an amino
acid activating agent include without limitation carbodiimides and/or onium
salt activating
agents. The amino acid activating agent comprises, in some embodiments, a
carbodiimide,
such as but not limited to N,N-diisopropylcarbodiimide (DIC), N,N'-
dicyclohexylcarbodiimide (DCC), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
(EDC),
and the like and combinations thereof. In certain embodiments, the amino acid
activating
agent comprises an onium activating agent, such as but not limited to
benzotriazol-1-
yloxytripyrrolidinophosphonium hexafluorophosphate (PyBOP), 0-(benzotriazol-1-
y1)-
N,N,N,N-tetramethyluronium hexafluorophosphate (HBTU), 2-(7-aza-1H-
benzotriazole-1-
y1)-1,1,3,3-tetramethyluronium hexafluorophosphate (HATU), 1-[(1-(cyano-2-
ethoxy-2-
oxoethylideneaminooxy) dimethylaminomorpholino)] uronium hexafluorophosphate
(COMU), and the like and combinations thereof.
An amino acid activating agent additive (amino acid activator additive) may
also be
used to activate the amino acid prior to a coupling step. Any suitable amino
acid activator
additive may be used. Examples of amino acid activator additives include
without limitation
benzotriazole additives, such as 1-hydroxybenzotriazole (HOBt), 1-hydroxy-7-
azabenzotriazole (HOAt), and 6-chloro-1-hydroxybenzotriazole (6-C1-HOBt);
ethyl
(hydroxyimino)cyanoacetate (Oxyma); 1-hydroxy-2,5-pyrrolidinedione (NHS), and
the like
and combinations thereof.
Still further, in exemplary embodiments, the solid phase peptide synthesis
process can
include applying microwave energy during one or more of the solid phase
peptide synthesis
steps, for example, during the deprotecting and/or coupling steps.
In exemplary embodiments, the solid phase peptide synthesis process can
further
include cleaving the peptide from the solid phase resin after the
deprotecting, optional
washing, and/or coupling steps.
-36-
Date Recue/Date Received 2023-08-25
The skilled artisan will understand how to join or couple amino acids to form
a chain.
Processes and agents for cleaving a peptide from a solid phase resin are also
well known in
the art. Accordingly, a detailed discussion of processes known in the art for
joining amino
acids to form a peptide and/or cleaving a peptide from a solid phase resin is
not provided.
The scale of solid phase peptide synthesis including deprotection processes
disclosed
herein is not limited and may include, for example, research and/or production
(e.g., large)
scale solid phase peptide synthesis.
In exemplary embodiments, the deprotection processes disclosed herein (e.g.,
including one or more deprotecting steps as described herein using a
deprotecting base in an
amount from greater than zero to about 5 vol%, based on the total volume of
the deprotection
reaction mixture (e.g., the deprotection reaction solution) and/or using an
inert gas to flush
volatized deprotecting base from the reaction vessel (e.g., from the head
space of the interior
of the reaction vessel), also as described in more detail herein) may be used
on large scale
systems that incorporate the ability to heat the reaction solution (e.g., a
large scale Liberty
PRO system available from CEM Corporation at 25mmo1, which is around about 125
grams
of resin at 0.2mmo1/g.) In such embodiments, the amount of base (e.g.,
pyrrolidine) may be
used in very low concentrations much less than typically used - amount of 2.5%
vol (standard
is 20%), which can facilitate reducing and/or eliminating washing. Also in
some of these
embodiments, deprotection conditions may be conducted for variable times and
temperatures
to facilitate more complete deprotection and base removal to a satisfactory
removal. One
example of this is 10 minutes at 90 C.
In other exemplary embodiments, the deprotection processes disclosed herein
may
include:
a process for deprotecting a protected amino acid during solid phase peptide
synthesis
(SPPS) including deprotecting steps and coupling steps, the deprotecting
process comprising:
removing a protecting group of a protected amino acid in a reaction vessel
with a
deprotecting base, wherein at least a portion of the deprotecting base
evaporates into an upper
interior portion of the reaction vessel during the removing step; and
directing an inert gas through the reaction vessel to assist in removing
evaporated
deprotecting base from the interior of the reaction vessel during the step of
removing the
protecting group,
-37-
Date Recue/Date Received 2023-08-25
wherein the deprotecting process may be conducted under one or more of the
following conditions:
(a) the deprotecting process can use (e.g., a protected amino acid can be
attached to) a
solid resin support having a resin substitution of less than or about
0.35mmo1/g, for example,
less than or about 0.30mmol/g, for example 0.10mmol/g to 0.35mmol/g, for
example
0.15mmol/g to 0.35mmol/g, for example 0.20mmo1/g to 0.35mmol/g, for example
0.10mmol/g to 0.34mmol/g, for example 0.15mmol/g to 0.34mmo1/g, for example
0.20mmo1/g to 0.34mmol/g, for example 0.20mmo1/g to 0.33mmol/g; and/or
(b) the deprotecting process may use a deprotecting base (e.g., pyrrolidine)
in an
amount greater than zero to about 5 vol%, for example greater than zero to
about or less than
3.5 vol%, based on the total volume (100 vol%) of a deprotection reaction
mixture (e.g., a
deprotection reaction solution) in the reaction vessel as defined herein;
and/or
(c) the deprotecting process may be conducted at a temperature above 30 C,
for
example, above 50 C, as another example above 70 C, as another example at a
temperature
from 30 C to 120 C; and/or
(d) the directing step may include directing the inert gas into the reaction
vessel
through a first opening and out of a second opening located in an upper
portion of the
reaction vessel to assist in removing evaporated deprotecting base from the
interior of the
reaction vessel (e.g., to purge, vent, etc. evaporated deprotecting base from
the headspace);
and/or
(e) the deprotecting process optionally can include washing the interior of
the reaction
vessel after the deprotecting step using a total volume of solvent that is 2
times or less of the
bed volume of the resin present in the reaction vessel, for example, 1 time or
less of the bed
volume of the resin present in the reaction vessel; and/or
(0 the total time for the deprotection reaction (e.g., the total time for a
deprotection
reaction of one deprotection-coupling cycle of a SPPS process) may be within
1.5 hours or
less, for example, from about 30 seconds to about an hour, for example from
about 30
seconds to about 10 minutes, and as another example from about 10 minutes to
about an
hour; and/or
(g) the boiling point of the deprotecting base is less than 107 C; and/or
(h) a difference between the deprotection reaction temperature and boiling
point of
the deprotecting base may be less than or about 50 C, for example less than or
about 25 C,
for example less than or about 15 C, for example the difference between the
deprotection
-38-
Date Recue/Date Received 2023-08-25
reaction temperature and the boiling point of the deprotecting base may range
from 15 C to
50 C; and/or
(i) the deprotection may be performed at a temperature that is no less than 35
C below
the boiling point of the deprotecting base and/or for a time of 1.5 hours or
less.
In exemplary embodiments, the process includes: heating the protected amino
acid
and the deprotecting base during the step of removing the protecting group
from the protected
amino acid; the deprotecting base is pyrrolidine; the protected amino acid is
attached directly
or indirectly to a solid PEG-PS (polyethylene glycol-polystyrene) resin
support, wherein the
resin can have a resin substitution of from 0.2mmo1/g to 0.3mmo1/g, for
example from
0.20mmo1/g to 0.25mmol/g; the protecting group of the protected amino acid is
a 9-
fluorenylmethyloxycarbonyl (Fmoc) protecting group; and the heating step is
conducted at a
temperature of about 60 C or higher.
The present disclosure also relates to a system for solid phase peptide
synthesis.
Figure 2A is a schematic flow diagram depicting selected portions of an
exemplary solid
phase peptide synthesis system in accordance with embodiments of the present
disclosure.
Figure 2B is a schematic flow diagram depicting selected portions of another
exemplary
peptide synthesis system in accordance with other embodiments of the present
disclosure
Generally, the elements illustrated in both Figure 1A and Figure 2A will carry
the
same reference numerals. Similarly, generally the elements illustrated in both
Figure 1B and
Figure 2B will carry the same reference numerals. Also, except where indicated
otherwise,
elements illustrated in both Figure 2A and Figure 2B will carry the same
reference numerals.
The peptide synthesis system of Figures 2A and 2B is designated generally as
2.
Peptide synthesis system 2 includes reaction vessel 4 as discussed herein.
Peptide synthesis
system 2 also includes a plurality of reagent containers located in a position
upstream of
reaction vessel 4 in fluid communication with reaction vessel 4.
For example, as depicted in Figures 2A and 2B, system 2 can include a
plurality of
solid support containers 50a, 50b, and 50c in fluid communication with a flow
path 52 fluidly
connecting the solid support containers and reaction vessel 4 for delivering a
solid support
(e.g., a solid resin having a protected amino acid linked thereto) from the
solid support
container(s) to reaction vessel 4. Flow paths 51a, 51b, and 51c fluidly
connect solid support
containers 50a, 50b, and 50c, respectively, and flow path 52.
-39-
Date Recue/Date Received 2023-08-25
In certain embodiments, flow path 52 can be in direct fluid communication with
reaction vessel 4, e.g., via opening 12 or 204 of Figures 1A or 1B,
respectively. In certain
embodiments, as depicted schematically in Figure 2A, the system can include a
rotary valve
140 that can rotate among multiple positions (e.g., two positions) to fluidly
connect reaction
vessel 4 through opening 12, flow path 22, and flow path 52 with solid support
containers
50a, 50b, and 50c. In certain other embodiments, as depicted schematically in
Figure 2B,
rotary valve 140 can rotate among multiple positions (e.g., two positions, or
more) to fluidly
connect reaction vessel 4 through opening 204, flow path 214, and flow path 52
with solid
support containers 50a, 50b, and 50c. During the deprotecting step described
herein, as
schematically depicted in Figs. 2A and 2B, rotary valve 140 may be closed with
respect to
flow path 52 (and flow path 152).
As another example, in certain embodiments, as depicted schematically in
Figures 2A
and 2B, system 2 can include a plurality of amino acid containers 60a, 60b,
and 60c in fluid
communication with flow path 62 (Figure 2A) or flow path 212 (Figure 2B)
fluidly
connecting the amino acid containers and reaction vessel 4 for delivering
protected amino
acid from the amino acid container(s) to reaction vessel 4. Flow paths 61a,
61b, and 61c
fluidly connect amino acid containers 60a, 60b, and 60c, respectively, with
flow path 62
(Figure 2A) or flow path 212 (Figure 2B). In some embodiments, flow path 62
and/or flow
path 212 can be in direct fluid communication with reaction vessel 4, e.g.,
via opening 10 or
202 of Figures 1A or 1B, respectively. In some embodiments, the system can
include one or
more additional valves and/or flow paths, as schematically depicted in Figures
2A and 2B
and as described in more detail below. During the deprotecting step described
herein, opening
or 202 may be closed with respect to flow path 62 or flow path 212 of Figs. 2A
and 2B,
respectively.
As another example, as schematically depicted in Figures 2A and 2B, system 2
can
include a deprotecting base container 70 in fluid communication with a flow
path 72 fluidly
connecting the deprotecting base container and reaction vessel 4 for
delivering deprotecting
base (for example, as part of a deprotection solution as described herein)
from the
deprotecting base container to reaction vessel 4. In some embodiments, flow
path 72 can be
in direct fluid communication with reaction vessel 4, e.g., via opening 16 of
Figures 1A and
1B. In some embodiments, the system can include one or more additional valves
and/or flow
paths, as schematically depicted in Figures 2A and 2B and as described in more
detail below.
-40-
Date Recue/Date Received 2023-08-25
During the deprotecting step described herein, opening 16 may be closed with
respect to flow
path 72 of Figs. 2A and 2B, respectively.
As yet another example, as schematically depicted in Figures 2A and 2B, system
2
can include a solvent container 80 in fluid communication with a flow path 82
fluidly
connecting the solvent container and reaction vessel 4 for delivering solvent
from the solvent
container to reaction vessel 4. In some embodiments, flow path 82 can be in
direct fluid
communication with reaction vessel 4, e.g., via opening 10 or 208 of Figures
1A or 1B,
respectively. In some embodiments, the system can include one or more
additional valves
and/or flow paths, as schematically depicted in Figures 2A and 2B and as
described in more
detail below. Figure 2B also schematically depicts embodiments wherein solvent
can be
introduced into reaction vessel 4 using at least a portion of the same flow
path used to
introduce an inert gas, e.g., via flow path 218, opening 208, spray head 220
and plurality of
openings 222, as described in more detail herein. During the deprotecting step
described
herein, opening 10 or 208 may be closed with respect to flow path 82 of Figs.
2A and 2B,
respectively.
As yet another example, as schematically depicted in Figures 2A and 2B, system
2
can include an additional reagent container 90, which can be for example an
activating agent
container, in fluid communication with a flow path 92 (Figure 2A) or flow path
210 (Figure
2B) fluidly connecting the additional reagent container and reaction vessel 4
for delivering an
additional reagent such as an activating agent from the additional reagent
container to
reaction vessel 4. In some embodiments, flow path 92 or 210 can be in direct
fluid
communication with reaction vessel 4, e.g., via opening 10 or 200 of Figures
1A and 1B,
respectively. In some embodiments, the system can include one or more
additional valves
and/or flow paths, as schematically depicted in Figures 2A and 2B and as
described in more
detail below. During the deprotecting step described herein, opening 10 or 200
may be
closed with respect to flow path 92 or flow path 210 of Figs. 2A and 2B,
respectively.
The skilled artisan will appreciate that the number of reaction vessels, solid
support
containers, amino acid containers, deprotecting base containers, solvent
containers, and/or
other reagent containers and associated flow paths, as well as the manner in
which these
elements are connected, can vary and is not limited to the depiction thereof
in Figure 2A and
Figure 2B. The skilled artisan will also understand that the peptide synthesis
system can
include various subsystems associated with the aforementioned containers, flow
paths, and/or
reaction vessel(s), including for example flow paths, valves, filters, gauges,
monitors,
-41-
Date Recue/Date Received 2023-08-25
controllers, etc., to direct the flow of materials into and/or out of
containers and/or the
reaction vessel(s) at appropriate stages of the solid phase peptide synthesis
process. Such
subsystems are well known in the art and will not be described in detail
herein.
System 2 is also associated with a heating source (not shown) such as a
microwave
source, and associated elements such as microwave guides and/or microwave
cavities, for
heating reaction vessel 4, as described herein. Heating sources, including
microwave heating
sources, and associated elements such as microwave guides and/or microwave
cavities, and
the use thereof in solid phase peptide synthesis processes and systems, are
also well known in
the art and are not described in more detail herein.
System 2 is schematically depicted as operating in an amino acid deprotecting
step of
a solid phase peptide synthesis, such as described herein with reference to
Figure 1A and
Figure 1B. In this operational state, reactants including a protected amino
acid and a
deprotecting base have already been delivered to reaction vessel 4 (and/or are
already present
in reaction vessel 4). The reaction vessel also may include a coupling
solution from a
preceding coupling step, which together with the deprotection solution forms
the deprotection
reaction mixture (e.g., the deprotection reaction solution), all also as
described herein.
The protected amino acid can be attached to a solid support. In some
embodiments,
the protected amino acid can be directly attached to the solid support (e.g.,
can be a protected
amino acid directly attached to a solid support delivered to reaction vessel 4
via flow path 52
from one or more of solid support containers 50a, 50b, and 50c). In some
embodiments (e.g.,
after a preceding coupling step), the protected amino acid can be directly or
indirectly
attached to a solid support (e.g., can be attached to another amino acid or to
a growing
peptide chain, wherein the other amino acid or peptide is attached to the
solid support).
System 2 further includes an inert gas source 100 located in a position
upstream of
reaction vessel 4 in fluid communication with reaction vessel 4. In certain
embodiments, as
depicted schematically in Figures 2A and 2B, a flow path 102 fluidly connects
inert gas
source 100 and reaction vessel 4 and delivers inert gas supplied from inert
gas source 100
into the upper interior portion of reaction vessel 4 via opening 10 (Figure
1A) or opening 208
(Figure 1B) described herein. In certain embodiments, system 2 includes a
valve 104 in fluid
communication with flow path 102 located between inert gas source 100 and
opening 10
(Figure 1A) or opening 208 (Figure 1B), wherein valve 104 has an open position
and a closed
position relative to flow path 102.
-42-
Date Recue/Date Received 2023-08-25
Valve 104 is shown in an open position in Figure 2A and Figure 2B relative to
flow
path 102. In the open position, valve 104 allows flow of pressurized inert gas
from inert gas
source 100 through flow path 102, flow path 20 (Figure 1A) or flow path 218
(Figure 1B),
and opening 10 (Figure 1A) or opening 208 and spray head 220 (Figure 1B), and
into the
interior upper portion of reaction vessel 4 (e.g., into the reaction vessel
headspace). In this
manner, the system can continuously and/or intermittently direct an overhead
source of
pressurized inert gas into the upper portion of the interior of the reaction
vessel during the
heating/deprotecting step to purge volatized deprotecting base out of the
reactor headspace, in
accordance with the process described herein.
In contrast, when valve 104 is in a closed position relative to flow path 102,
valve 104
can prevent the flow of pressurized inert gas from the inert gas source
through flow path 102,
flow path 20 (Figure 1A) or flow path 218 (Figure 1B), and opening 10 (Figure
1A) or
opening 208 and spray head 220 (Figure 1B).
The system can also include a flow path 106 fluidly connecting inert gas
source 100
and opening 16 in the lower portion of reaction vessel 4. The system can also
include a valve
108 in fluid communication with flow path 106 located between inert gas source
100 and
opening 16, wherein valve 108 has an open position and a closed position
relative to flow
path 106.
When in the open position relative to flow path 106 (such as illustrated in
Figs. 2A
and 2B), valve 108 allows flow of pressurized gas from inert gas source 100
through flow
path 106, flow path 26, and opening 16 into the interior lower portion of
reaction vessel 4.
As described herein, in the deprotecting process (e.g., when valve 108 is
optionally open), in
this manner pressurized inert gas can be directed into the lower portion of
the reaction vessel
to agitate (e.g., stir, bubble) the reactants and/or flush evaporated
deprotecting base from the
headspace of the reaction vessel. The closed position of second valve 108
relative to flow
path 106 prevents flow of pressurized inert gas from inert gas source 100
through flow path
106, flow path 26, and opening 16 into the interior lower portion of reaction
vessel 4.
In certain embodiments, flow paths 102 and 106 can be fluidly connected via a
pressure regulator, designated in Figure 2A and Figure 2B as 110, to a single
inert gas source
100. Alternatively, flow paths 102 and 106 can fluidly connect the reaction
vessel to at least
two different inert gas sources.
-43-
Date Recue/Date Received 2023-08-25
When present, pressure regulator 110 can be located in a downstream position
from
inert gas source 100 and an upstream position from valves 104 and 108. In
these
embodiments, pressure source 100 directs inert gas to pressure regulator 110,
which supplies
pressurized inert gas to flow path 102 having a higher pressure (the "high
pressure" inert gas)
than inert gas supplied to flow path 106 (the "low pressure" inert gas). For
example, without
being limited thereto, pressure regulator 110 can supply "high pressure" inert
gas having a
pressure of about 1 psi to about 25 psi to flow path 102. Pressure regulator
110 can also
supply "low pressure" inert gas having a pressure that is less than the
pressure of the "high
pressure" inert gas supplied to flow path 102.
Pressure regulators are also well known in the art and the skilled artisan
will
understand how to use the same in system 2 to provide a high pressure inert
gas and a low
pressure inert gas as discussed herein.
As also schematically depicted in Figures 2A and 2B (and Figures 1A and 1B),
in
exemplary embodiments, the system can include flow path 24 (Figures 1A and 2A)
or flow
path 216 (Figures 1B and 2B) located in a downstream position from opening 14
(Figure 1A)
or opening 206 (Figure 1B) of reaction vessel 4. Flow paths 24 and 216
function as a
gaseous waste flow path to allow the purging of gaseous waste (e.g., volatized
deprotecting
base, inert gas, etc.) during the deprotecting process described herein from
the upper interior
portion (headspace) of reaction vessel 4.
In certain embodiments, the system includes a valve 120 in fluid communication
with
flow path 24 or flow path 216. Valve 120 has an open position and a closed
position relative
to flow path 24 or flow path 216. The open position of valve 120 allows flow
of gas from the
upper interior portion of reaction vessel 4 through opening 14 or opening 206
and flow path
24 or flow path 216 to a waste recovery zone, such as a vent and/or waste
container (not
shown) to permit purging/venting of gas from reaction vessel 4. The closed
position of valve
120 prevents flow of gas out of the upper interior portion of the reaction
vessel through
opening 14 or opening 206.
Figures 2A and 2B illustrate certain embodiments of the system in an
operational state
wherein both valve 104 and valve 120 are in an open position with respect to
flow path 102,
flow path 20 or 218, and flow path 24 or flow path 216, respectively. This
corresponds to the
positions that would be used during the (heated) deprotecting process
described herein. The
simultaneously open positions of valve 104 and valve 120 relative to flow path
102, flow path
-44-
Date Recue/Date Received 2023-08-25
20 or 218, and flow path 24 or flow path 216, respectively, allow an overhead
supply of
pressurized inert gas to flow continuously and/or intermittently through
reaction vessel 4 (for
example, flow into vessel 4 through opening 10 in the vessel headspace and out
of vessel 4
through a separate opening 14 or as another example flow into vessel 4 through
opening 208,
spray head 220 and openings 222 toward side wall 6 and into the vessel
headspace and out of
vessel 4 through a separate opening 206) to purge (flush) volatized reactants
present in the
headspace during the (heated) deprotecting step.
In certain embodiments, system 2 can include a valve 122 in series with valve
104 and
a flow path 124 positioned (disposed) between and fluidly connecting valve 104
and valve
122. Valve 122 is in fluid communication with flow path 102 and has an open
position and a
closed position relative to flow path 102. When valve 122 is present and valve
122 and valve
104 are in an open position relative to flow path 102, such as depicted in
Figure 2A and
Figure 2B, inert gas source 100, pressure regulator 110, flow path 102, valve
104, flow path
124, valve 122, flow path 20 (Figure 2A) or flow path 218 (Figure 2B), opening
10 (Figure
1A/2A) or opening 208 and spray head 220 (Figure 1B/2B), and reaction vessel 4
can be
fluidly connected (in fluid communication).
In certain embodiments, valve 104 can be a rotary valve which can rotate
between
multiple positions to fluidly connect one flow path selected from a plurality
of flow paths
with reaction vessel 4. As a non-limiting example, Figure 2A depicts rotary
valve 104
capable of rotating between four positions to fluidly communicate with flow
path 102, 62, 82,
or 92, depending on the open or closed position of the valve. For example,
Figure 2A
schematically depicts rotary valve 104 in an open position with respect to
flow path 102 but
in a closed position with respect to flow paths 62, 82, and 92. As another non-
limiting
example, Figure 2B depicts rotary valve 104 capable of rotating between two
positions to
fluidly communicate with flow path 102 or 82, depending on the open or closed
position of
the valve. For example, Figure 2B schematically depicts rotary valve 104 in an
open position
with respect to flow path 102 but in a closed position with respect to flow
path 82. The
open/closed positions of rotary valve 104 relative to the different flow paths
can be selected
depending on the stage of the peptide synthesis process and the reactants,
etc. to be delivered
to reaction vessel 4. Rotary valve 104 (and other valves discussed herein) can
be operated as
known in the art.
Other valves of the system can also be rotary valves. For example, as noted
herein, in
certain embodiments, system 2 can include a flow path 124 positioned
(disposed) between
-45-
Date Recue/Date Received 2023-08-25
and fluidly connecting valve 104 and valve 122. In this embodiment, as
depicted
schematically in Figure 2A and Figure 2B, valve 122 in series with valve 104
can rotate
among multiple positions (e.g., valve 122 between two positions in Figures 2A
and 2B and
valve 104 between four positions as schematically depicted in Figure 2A and
between two
positions as schematically depicted in Figure 2B) to fluidly connect, for
example, inert gas
source 100 and reaction vessel 4 via flow path 102, flow path 124, flow path
20 or flow path
218, and opening 10 or opening 208. Alternatively, as shown in Figure 2A,
valve 122 in
series with valve 104 can rotate among multiple positions (e.g., two positions
and four
positions, respectively) to fluidly connect, for example, one or more amino
acid containers
60a, 60b, and 60c and reaction vessel 4 via flow path 62, flow path 124, flow
path 20, and
opening 10; solvent container 80 and reaction vessel 4 via flow path 82, flow
path 124, flow
path 20, and opening 10; or reagent container 90 and reaction vessel 4 via
flow path 92, flow
path 124, flow path 20, and opening 10. Also in some embodiments, as shown in
Figure 2B,
valve 122 in series with valve 104 can rotate among multiple positions (e.g.,
two positions) to
fluidly connect, for example, solvent container 80 and reaction vessel 4 via
flow path 82,
flow path 124, flow path 218, and opening 208 and spray head 222.
As another example, in certain embodiments, as depicted schematically in
Figure 2A
and Figure 2B, valve 108 can be a rotary valve rotating among multiple
positions (e.g., three
positions) to fluidly connect reaction vessel 4 through opening 16, flow path
26, and flow
path 72, flow path 106 or a flow path 132 with deprotecting base container 70,
inert gas
source 100, or a waste container 130, respectively, depending on the position
of valve 108.
For example, Figure 2A and Figure 2B schematically depict rotary valve 108 in
an open
position with respect to flow path 106 but in a closed position with respect
to flow paths 72
and 132. This can be the position of rotary valve 108 during the heating
and/or deprotecting
process described herein, wherein low pressure inert gas is directed (bubbled)
into a lower
portion of reaction vessel 4 to stir the reactants and/or flush evaporated
deprotecting base
from the headspace of the reaction vessel.
As another example, as discussed herein, in certain embodiments, as also
depicted
schematically in Figure 2A, the system can include a rotary valve 140 that can
rotate among
multiple positions (e.g., two positions) to fluidly connect reaction vessel 4
through opening
12, flow path 22, and flow path 52 with solid support containers 50a, 50b, and
50c.
Alternately, rotary valve 140 can rotate among multiple positions (e.g., two
positions) to
fluidly connect reaction vessel 4 through opening 12, flow path 22, and a flow
path 152,
-46-
Date Recue/Date Received 2023-08-25
wherein flow path 152 is in fluid communication with a plurality of flow paths
151a, 15 lb,
and 151c, which in turn fluidly connect flow path 152 with a plurality of
product containers
150a, 150b, and 150c, respectively. This can allow the passage of product
(e.g., peptide
and/or peptide linked to a solid support) from reaction vessel 4 into
container(s) 150a, 150b,
and 150c. Again, the skilled artisan will appreciate that the number of
product containers
150a, 150b, and 150c, and corresponding flow paths 151a, 151b, and 151c, can
vary and is
not limited to the number depicted in Figure 2A. Figure 2A schematically
depicts rotary
valve 140 in a closed position relative to flow paths 52 and 152 (e.g., in a
closed position
relative to flow paths 52 and 152 during a deprotecting step as described
herein).
As another example, as discussed herein, in certain embodiments, as also
depicted
schematically in Figure 2B, the system can include a rotary valve 140 that can
rotate among
multiple positions (e.g., two positions) to fluidly connect reaction vessel 4
through opening
204, flow path 214, and flow path 52 with solid support containers 50a, 50b,
and 50c.
Alternately, rotary valve 140 can rotate among multiple positions (e.g., two
positions) to
fluidly connect reaction vessel 4 through opening 204, flow path 214, and flow
path 152,
wherein again flow path 152 is in fluid communication with a plurality of flow
paths 151a,
151b, and 151c, which in turn fluidly connect flow path 152 with a plurality
of product
containers 150a, 150b, and 150c, respectively. Again, this can allow the
passage of product
(e.g., peptide and/or peptide linked to a solid support) from reaction vessel
4 into container(s)
150a, 150b, and 150c. Also again, the skilled artisan will appreciate that the
number of
product containers 150a, 150b, and 150c, and corresponding flow paths 151a,
151b, and
151c, can vary and is not limited to the number depicted in Figure 2B. Figure
2B
schematically depicts rotary valve 140 in a closed position relative to flow
paths 52 and 152
(e.g., in a closed position relative to flow paths 52 and 152 during a
deprotecting step as
described herein).
Peptide synthesis system 2 can also include one or more flow paths, vents,
containers,
valves, controllers, and the like, for example, for the removal of waste
(e.g., excess reactants,
solvents, etc.) from the peptide synthesis system. The waste can be in gas,
liquid and/or solid
form and the skilled artisan will appreciate appropriate types of flow paths
and containers for
removing the same from the peptide synthesis system. For example, in certain
embodiments,
as discussed herein, waste container 130 can be fluidly connected to reaction
vessel 4 via
flow path 132 and rotary valve 108 when in the appropriate open position to
allow the
passage of waste products from reaction vessel 4 to waste container 130.
Figures 2A and 2B
-47-
Date Recue/Date Received 2023-08-25
schematically depict rotary valve 108 in a closed position relative to flow
path 132 (e.g., in a
closed position relative to flow path 132 during a deprotecting step as
described herein). As
another example, in certain embodiments, as discussed herein, the open
position of valve 120
can allow flow of gas from the upper interior portion (headspace) of reaction
vessel 4 through
opening 14 or opening 206 and flow path 24 or flow path 216 to a waste
recovery zone, such
as a vent and/or waste container (not shown) to permit purging/venting of gas
from reaction
vessel 4 (e.g., during a deprotecting step as described herein). Figures 2A
and 2B
schematically depict valve 120 in an open position relative to flow path 24 or
216 (e.g., in an
open position relative to flow path 24 or 216 during a deprotecting step as
described herein).
Thus, generally, Figures 2A and 2B illustrate an exemplary system for the
delivery of
solvents, reactants (amino acids, deprotecting bases, activators, etc.), solid
phase resins,
gases, etc. from their respective sources to reaction vessel 4 and the further
delivery of
products and by-products (peptides, gas, liquid, and/or solid waste, etc.)
from reaction vessel
4 to their respective destinations. It will be understood that the particular
flow paths and valve
locations illustrate, rather than limit, the present disclosure.
At least partially reiterating from above, the peptide synthesis system
typically
includes at least one controller operatively associated with, for example,
numerous electrical
components of the system (e.g., the microwave source, sensors, and solenoid
and/or other
motor-operated valves). The at least one controller can include one or more
computers,
computer data storage devices, programmable logic devices (PLDs) and/or
application-
specific integrated circuits (ASIC). A suitable computer can include one or
more of each of a
central processing unit or processor, computer hardware integrated circuits or
memory, user
interface, peripheral or equipment interface for interfacing with other
electrical components
of the system, and/or any other suitable features. The controller(s) can
respectively
communicate with electrical components of the system by way of suitable signal
communication paths. In Figures 2A and 2B, representative signal communication
paths
associated with a controller are schematically depicted and designated by
numerals *2 (signal
communication paths) and *1 (controller), respectively. Processes of this
disclosure can be
controlled (e.g., at least partially controlled) in response to the execution
of computer-based
algorithms operatively associated with the at least one controller *1.
Solid phase peptide synthesis processes, including batch-based processes, are
known
and thus the present disclosure does not provide detailed information on the
same. Reference
is made, for example, to the pioneering work R. B. Merrifield (1963) "Solid
Phase Peptide
-48-
Date Recue/Date Received 2023-08-25
Synthesis I, The Synthesis of a Tetrapeptide," J. Am. Chem. Soc. 85 (14), 2149-
2154).
Accordingly, a detailed discussion of solid phase peptide synthesis processes
is not provided.
Systems suitable for conducting solid phase peptide synthesis, including batch-
based
processes, are also known. Exemplary systems for conducting solid phase
peptide synthesis
include, for example, the LIBERTY line of instruments commercially available
from CEM
Corporation of Matthews N.C.
Reference is also made to exemplary US patents dealing with the subject of
solid
phase peptide synthesis (including exemplary systems and/or processes)
including without
limitation U.S. Pat. Nos. 7,393,920; 7,550,560; 7,563,865; 7,939,628;
7,902,488; 7,582,728;
8,153,761; 8,058,393; 8,426,560; 8,846,862; 9,211,522; 9,669,380; 10,052,607;
10,308,677;
10,125,163; 10,858,390; and 10,239,914. The contents of each of these are
incorporated
entirely herein by reference.
The deprotecting processes and/or SPPS processes of the present disclosure may
be
used as part of a SPPS process that do not include (that eliminate) washing
and/or draining
after each coupling step and/or that add deprotection base directly to a
coupling solution from
a preceding coupling step without any draining after coupling, such as
disclosed in, for
example, U.S. Patent Nos. 10,308,677; 10,125,163; 10,858,390; and 10,239,914.
Such SPPS
processes may be referred to generally as "High Efficiency SPPS (HE-SPPS)."
Figure 3 is a flow chart schematically depicting the steps of a cycle of a
conventional
solid phase peptide synthesis (SPPS) process broadly designated at 300. A
deprotection step
302 is carried out in a reaction vessel as known in the art by adding a
deprotection solution
including a deprotecting base to the reaction vessel. The deprotection
solution is then drained
(step 304) following which a washing liquid (e.g., methanol or isopropanol) is
added to the
vessel for a washing step 306 carried out repetitively with five repetitions
being typical. The
washing solution is then removed in a second draining step 308 and thereafter
a coupling step
310 takes place in the reaction vessel. A coupling solution is then removed in
a third draining
step 312 followed by a second washing step 314, again typically repeated five
times,
followed by a fourth draining step 316.
Thus, Figure 3 illustrates draining and washing steps following each of a
deprotecting
step and a coupling step of a conventional SPPS cycle. It will be understood
that Figure 3 is
schematic, and that there are many details about one SPPS cycle that could be
added, but
-49-
Date Recue/Date Received 2023-08-25
that Figure 3 illustrates the concept sufficiently for the skilled person to
understand both it
and the present invention.
In contrast, Figures 4A and 4B are flow charts schematically depicting the
steps of a
cycle of SPPS processes in accordance with exemplary embodiments of the
present
disclosure, broadly designated at 400a and 400b, respectively, each as
described in more
detail herein.
Figure 4A schematically depicts a deprotecting step/a deprotecting-coupling
cycle of
a SPPS process in accordance with exemplary embodiments of the present
disclosure
described in more detail herein, in which a deprotection solution including a
deprotecting
base is added to the coupling solution from the preceding coupling cycle,
without any
washing or draining steps between the coupling step of the previous cycle and
the addition of
the deprotection solution of the deprotecting step of the successive cycle.
Figure 4A
schematically depicts optionally draining liquid remaining in the vessel post-
deprotection
(e.g., solvent, residual deprotecting agent, if any, etc.). Figure 4A further
schematically
depicts that washing steps after each deprotecting step (before the next
coupling step) in
accordance with exemplary embodiments of the present disclosure described
herein can be
eliminated. Figure 4A thus schematically depicts an exemplary embodiment of
the present
disclosure in which washing steps following a deprotecting step (and also
washing steps
and/or draining steps following a coupling step) of a SPPS cycle may be
eliminated.
Figure 4B schematically depicts a deprotecting step/a deprotecting-coupling
cycle of a
SPPS process in accordance with other exemplary embodiments of the present
disclosure
described in more detail herein, in which a deprotection solution including a
deprotecting
base is added to the coupling solution from the preceding coupling cycle,
without any
washing or draining steps between the coupling step of the previous cycle and
the addition of
the deprotection solution of the deprotecting step of the successive cycle.
Figure 4B
schematically depicts optionally draining liquid remaining in the vessel post-
deprotection
(e.g., solvent, residual deprotecting agent, if any, etc.). Figure 4B further
schematically
depicts one or more washing steps after each deprotecting step and before the
next coupling
step, followed by one or more additional optional draining step(s). In
contrast to
conventional SPPS processes such as schematically depicted in Figure 3,
however, Figure 4B
depicts that any post-deprotecting washing steps may use reduced amounts of
solvent in
accordance with other exemplary embodiments described herein. Figure 4B thus
schematically depicts an exemplary embodiment of the present disclosure in
which the
-50-
Date Recue/Date Received 2023-08-25
volume of a washing liquid (e.g., solvent) may be reduced, relative to the
volume of solvent
used post-deprotection in conventional SPPS processes.
The following examples are provided for illustration only and are not to be in
any way
construed as limiting the present invention. The examples demonstrate that
even using small
amounts of deprotecting base may result in essentially complete deprotection
and scavenging
of the protecting group (e.g., Fmoc protecting group) with only residual base
left, which may
be small enough to minimize issues for the next coupling step.
Example 1
Analysis of One-Pot Synthesis of JR 10 mer Using Low Base Concentrations, With
and
Without Post-Deprotection Washing and With and Without Headspace Flushing
JR 10 mer is synthesized using solid phase peptide synthesis using a
commercially
available automated microwave peptide synthesizer (e.g., from the Liberty line
of microwave
peptide synthesizers commercially available from CEM Corporation, Matthews,
NC, such as
Liberty PRIME 2.0) at a 0.1 mmol scale. PEG-PS resin (e.g., Rink Amide ProTide
Resin LL
commercially available from CEM Corporation) or PS resin (e.g., Fmoc-Rink
Amide MBHA
PS commercially available from CEM Corporation) is used as the solid phase
resin support,
and coupling reactions are performed in the presence of Fmoc-protected amino
acids (AA).
Deprotection reactions are performed by adding a pyrrolidine/dimethylformamide
(DMF) deprotection reagent (the deprotection solution) to an undrained post-
coupling
mixture (the coupling solution). The concentration of pyrrolidine (e.g.,
volume percent
pyrrolidine in the reaction vessel based on the total volume of a deprotection
reaction mixture
(e.g., a deprotection reaction solution) including the added pyrrolidine/DMF
deprotection
solution and the undrained coupling solution from the preceding coupling
reaction) is noted
in Table 1 below. Microwave power is regulated to provide a deprotection
temperature of
110 C and a deprotection reaction time as also noted in Table 1 below.
Table 1 further indicates whether post-deprotection washing and/or headspace
flushing is used. For samples in Table 1 wherein "Headspace Flushing" is
indicated as "ON,"
a nitrogen gas stream is directed through the headspace of the reaction vessel
to purge
headspace gas from the reaction vessel in accordance with embodiments of the
present
disclosure described herein (e.g., directing a pressurized nitrogen gas stream
into the reaction
vessel through an entry port such as shown in Figs. 1A and 1B, through the
headspace, and
out of the reaction vessel through an exit port such as a vent port such as
shown in Figs. 1A
and 1B). For examples in Table 1 wherein "Headspace Flushing" is indicated as
"OFF,"
-51-
Date Recue/Date Received 2023-08-25
headspace flushing as described herein is not used. For examples in Table 1
wherein "Post-
Deprotection Washing" is used, the washing step includes washing two times
using 4 mL of
DMF after each deprotection step.
Following completion of synthesis of the JR 10 mer, the JR 10 mer is cleaved
from
the solid phase and crude purity of the resultant JR 10 mer is analyzed. The
results are also
reported in Table 1 below.
Table 1
Deprotection Deprotection Time Post-Deprotection Headspace
Entry Crude
Purity
(% pyrrolidine) (sec) Washing Flushing
1 4.5 40 2 x 4mL DMF OFF 77%
2 4.5 40 2x 4mLDMF ON 79%
3 4.5 40 None OFF 16%
4 4.5 40 None ON 69%
4.5 80 None OFF 20%
6 4.5 80 None ON 68%
7 3.5 80 None OFF 34%
8 3.5 80 None ON 86%
9 3 80 None OFF 56%
3 80 None ON 84%
11 3 80 None ON 73%
12 3 40 None ON 73%
13 2 80 None OFF 36%
14 2 80 None ON 65%
3 80 2x 4mLDMF ON 81%
16 4.5 40 2 x 4 mL DMF ON 74%
17 3 80 None ON 65%
Notes:
1. Fmoc-Rink Amide ProTideTm LL resin (0.20 meq/g substitution) is used for
all experiments except Entry
11 that uses Fmoc-Rink Amide MBHA PS resin (0.33 meq/g substitution).
2. N-butylpyrrolidinone (NBP) is used in place of DMF for Entries 16 and 17.
-52-
Date Recue/Date Received 2023-08-25
The results for the JR peptide synthesis show that a high purity result can be
obtained
without any washing when using the headspace flushing as described herein with
each
deprotection step. For example, without being bound by any explanation or
theory and
without limiting the scope of the invention, it is currently believed that
directing an inert gas
(nitrogen gas) through the reaction vessel (e.g., into the reaction vessel
through an entry port,
through the headspace, and out of the reaction vessel through an exit port (a
vent port) such
as shown in Figs. 1A and 1B) may result in both a higher gas exchange rate
above the
deprotection solution and a top down directional flow which pushes
condensation back into
the reaction vessel where it is then reheated
In addition, also without being bound by any explanation or theory and without
limiting the scope of the invention, it is currently believed that Example 1
demonstrates that
pyrrolidine base, which has a lower boiling point (87 C) compared to
piperidine (106 C),
may be significantly evaporated in an Fmoc removal step and that even using
small amounts
thereof (as low as 2 vol%) may result in essentially complete deprotection and
scavenging of
the Fmoc group with only residual base left, which may be small enough to
minimize issues
for the next coupling step. It is also currently believed that the processes
can allow high
purity synthesis of JR with a complete cycle waste of only 4.25 mL per amino
acid and a
total cycle time of approximately 3.5 minutes at the common 0.1mmol research
scale.
In recent years, greener solvent replacements have been explored for SPPS.
There
above experiments also assessed the processes of the present disclosure using
a green solvent
alternative. To evaluate this, JR sequence is synthesized with N-
butylpyrrolidinone (NBP)
completely replacing DMF under both control conditions using post-deprotection
washing
and with the wash-free conditions (Table 1, entries 16 and 17). While NBP
shows a
reduction in purity compared to DMF, it is still successful in producing the
target in
relatively high purity with both the wash-based and wash-free conditions. This
result
indicates that the processes disclosed herein may also work with solvent
alternatives to
DMF.
Example 2
Analysis of One-Pot Synthesis of 65-74ACP, Liraglutide, and '213-amyloid
Sequences
Using Protecting Composition Having a Low Base Concentration Without Post-
Deprotection Washing and With Headspace Flushing
Other well-known difficult sequences, namely, the 65-74ACP (acyl carrier
protein), 1-4213_
amyloid and liraglutide (Entries 1, 2, and 3 in Table 2 below), are next
investigated. The
-53-
Date Recue/Date Received 2023-08-25
sequences are synthesized using solid phase peptide synthesis with a
commercially available
automated microwave peptide synthesizer (e.g., from the Liberty line of
microwave peptide
synthesizers commercially available from CEM Corporation, Matthews, NC, such
as Liberty
PRIME 2.0) on a 0.1 mmol scale. 65-74ACP and Liraglutide are synthesized on
Fmoc-Gly-
Wang-ProTide resin (0.24 meq/g substitution) and "213-Amyloid is synthesized
on Fmoc-Ala-
Wang-ProTide resin (0.23 meq/g substitution). Coupling reactions are performed
in the
presence of Fmoc-protected amino acids (AA).
Liraglutide synthesis is further investigated in a simulated run at 0.1 mmol
scale in a 35
mL reaction vessel using deprotection and coupling conditions for a large-
scale (25 mmol)
production method previously developed in a 15 liter reaction vessel (Entry 4
in Table 2 below).
This method employs a lower temperature deprotection and coupling method that
is limited to
80 C. To test the robustness of this process for production scales the
deprotection step is
extended to 8 min at 80 C. As reported in Table 2, this method yields
Liraglutide with crude
purity that matches previous results obtained with wash methods.
Deprotection reactions are performed by adding a pyrrolidine/dimethylformamide
(DMF) deprotection reagent (the deprotection solution) to an undrained post-
coupling
mixture (the coupling solution). The concentration of pyrrolidine (e.g.,
volume percent
pyrrolidine in the reaction vessel based on the total volume of a deprotection
reaction mixture
(e.g., a deprotection reaction solution) including the added pyrrolidine/DMF
deprotection
solution and the undrained coupling solution from the preceding coupling
reaction) is 3vo1%.
A 3 vol% pyrrolidine concentration is chosen as a middle value utilization of
process toward
the synthesis of these sequences. Microwave power is regulated to provide a
deprotection
temperature and a deprotection reaction time as also noted in Table 2 below.
Following completion of synthesis of the sequences, the sequences are cleaved
from
the solid phase and crude purity of the resultant sequences is analyzed. The
results are
reported in Table 2 below. Specifically, the column of Table 2 labeled "Crude
Purity (Wash-
free)" reports crude purity of peptides produced using a deprotection process
in accordance
with embodiments of the present disclosure described herein (including head
space flushing
and no post-deprotection washing). For comparison., the column of Table 2
labeled "Crude
Purity (Wash based)" reports crude purity of peptides produced using a wash-
based
deprotection process (without head space flushing and with post-deprotection
washing).
-54-
Date Recue/Date Received 2023-08-25
Table 2
E
Deprotection Deprotection Crude Purity Crude Purity
ntry Peptide
Temp. Time (VVash-
free) (Wash based)
1 65-74Acp 110 C 80 sec 91% 91%
2 14213-amyloid 110 C 80 sec 67% 69%
3 Liraglutide 110 C 80 sec 73% 68%
4 Liraglutide 80 C 8 min 75% --
The results for the 65-74ACP, 14213_amyloid and Liraglutide sequences also
show that a
high purity result can be obtained without any washing when using the
headspace flushing as
described herein in each deprotection step.
The foregoing Examples 1 and 2 demonstrate that embodiments of the present
disclosure including a deprotection step described herein can provide an
improved process
that can eliminate one or more (e.g., all) washing steps for solid phase
peptide synthesis
(e.g., can eliminate post-deprotection washing steps). In some embodiments,
the process
may use low amounts of deprotecting base (e.g., about 3-4 vol% pyrrolidine)
for Fmoc
removal; and/or heating (e.g., microwave heating at 80 ¨ 110 C); and/or base
removal from
the deprotection solution with elevated temperatures and/or nitrogen purging
(headspace
flushing), which may result in a low enough remaining base so as to eliminate
the need for
washing before the next amino acid is added. Examples 1 and 2 demonstrate
significant
robustness even on longer, more difficult sequences such as Liraglutide. Thus,
the processes
may provide a major savings in solvent and time.
Example 3
Wash-Free Production Scale Synthesis
In preparation for testing the wash-free method at a production scale, 25 mmol
scale
deprotection and coupling conditions are investigated by synthesizing
liraglutide at research
scale (0.1 mmol) in a 35 mL reaction vessel, as discussed in Example 2 above;
this method
employs lower temperature conditions for deprotection (8 min at 80 C) and
coupling (5 min
at 80 C). Also as reported in Example 2 above, this method yields liraglutide
with crude purity
that matches previous results obtained with wash-based methods at research and
production
scales.
-55-
Date Recue/Date Received 2023-08-25
Having established the high enantiomeric and chromatographic purities using
large-
scale reaction conditions for 0.1 mmol scale synthesis, the 25 mmol wash-free
synthesis of
liraglutide is conducted on the Liberty PRO large-scale microwave peptide
synthesizer. Based
on results from an initial optimization experiment involving 6 amino acid
couplings, exemplary
conditions for a 25 mmol wash-free synthesis may include without limitation
(i) 2.5%
pyrrolidine concentration in the reaction vessel, (ii) 10 min at 90 C
deprotection and 5 min at
80 C coupling, and (iii) 85 psi nitrogen pressure of directed headspace
flushing during each
deprotection. 4 equivalent excess of regular amino acids is used and only 2
equivalents of
Fmoc-Lys(palmitoyl-Glu-OtBu)-OH for coupling. Under these conditions, the 25
mmol wash-
free synthesis of liraglutide generates a total waste of 28.4 L as compared to
139.7 L from the
wash-based 25 mmol run which implies an overall waste reduction of
approximately 80%.
Liraglutide samples from wash-based and wash-free 25 mmol syntheses show very
similar (77-
78%) crude purities. These results confirm the general applicability of wash-
free methodology
for research scale as well as production scale synthesis of peptides. Future
use of this technique
in large-scale peptide drug production would be helpful in reducing enormous
amounts of
waste generated from SPPS.
The potential for epimerization with this new wash-free method is evaluated by
measuring the occurrence of D-amino acids in the liraglutide samples.
Liraglutide samples
synthesized by both the research and production scale wash-free methods are
analyzed using
an established method (C.A.T. GmbH). See Gerhardt, J.; Nicholson, G. J.,
Validation of a GC-MS
Method for Determination of the Optical Purity of Peptides. GmbH, C. A. T.,
Ed. The results from
the crude and corresponding purified samples are then compared to a commercial
sample of
liraglutide (Victoza()) as shown in Table 3. The results show very low levels
of epimerization
with well over 99.5% control of stereochemistry for each amino acid in the
liraglutide
sequence using the production method. This demonstrates that all epimerization
related
impurities are below the critical 0.5% limit for any new specified peptide-
related impurity.
Meeting this limit is required for an application of a synthetic peptide as a
substitute for an
approved peptide drug of recombinant deoxyribonucleic acid (rDNA) origin with
an
abbreviated new drug application (ANDA). The results demonstrate that the
processes of the
present disclosure may be used not only in research and development but also
production
processes that require stringent purity standards.
-56-
Date Recue/Date Received 2023-08-25
Table 3
Epimerization data for liraglutide samples
D-Enantiomer
Residue Table 2, Purified Table 2, Purified VICTOZA
Entry 3
0.1mmol Entry 4
Purified 25 mmol Lot
(Crude)
Research (Crude)
0.1 mmol Production #FS61B71
Run
0.1mmol Method 0.1mmol Production
Method
Research Production
Method Method
Alanine 0.15% 0.10% 0.12% 0.10% 0.10% <0.10%
Valine <0.10% <0.10% <0.10% <0.10% <0.10% <0.10%
Threonine <0.10% <0.10% <0.10% <0.10% <0.10% <0.10%
< 0.10% D- < 0.10% D- <0.10% D- <0.10% D- < 0.10% < 0.10% D-
allo allo allo allo D-allo allo
<0.10% L- < 0.10% L- <0.10% L- <0.10% L- < 0.10% L- <0.10% L-
allo allo allo allo allo allo
Isoleucine <0.10% <0.10% <0.10% <0.10% <0.10% <0.10%
0.11% D- <0.10% D- <0.10% D- <0.12% D- < 0.12% <0.10% D-
allo allo allo allo D-allo allo
<0.10% L- <0.10% L- < 0.10% L- < 0.13% L- <0.14% L- <0.11% L-
allo allo allo allo allo allo
Leucine 0.12% 0.16% 0.14% 0.15% 0.13% 0.13%
Serine 0.31% 0.10% 0.21% <0.10% <0.10% 0.38%
Aspartic Acid 0.36% 0.38% 0.17% 0.33% 0.18% 0.12%
Phenylalanine 0.19% 0.22% 0.15% 0.22% 0.14% 0.13%
Glutamic 0.23% 0.37% 0.23% 0.30% 0.25% 0.16%
Acid
Tyrosine 0.15% 0.10% <0.10% 0.14% <0.10% 0.18%
Lysine 0.13% <0.10% 0.15% 0.11% 0.13% 0.11%
Arginine 0.23% 0.11% 0.24% 0.16% 0.10% 0.13%
Tryptophan 0.23% <0.10% 0.22% 0.16% not 0.25%
determined
Histidine 0.72% 0.72% 0.40% 0.34% 0.47% 0.57%
Example 4
Protein Synthesis
-57-
Date Recue/Date Received 2023-08-25
The capabilities of this process are then tested further by the synthesis of
two proteins
with sequence lengths >80 amino acids, proinsulin and barstar. Linear
synthesis of long
sequences by SPPS is challenging due to the iterative accumulation of
impurities and increased
susceptibility for aggregation to occur. The proinsulin 86-mer and barstar 89-
mer sequences
are chosen for synthesis as they were previously synthesized using a fast flow
methodology at
1% and 2% overall yield, respectively. The fast flow approach advantageously
provides a very
fast synthesis time of only ¨2.5 minutes per amino acid cycle at small
synthesis scales (0.035
mmol for proinsulin; 0.027 mmol for barstar). However, the process requires a
large excess of
amino acid (-100 equivalents) and wash solvent (¨ 90 mL per amino acid).
To account for the potential increased synthesis difficulty of these longer
sequences, a
higher coupling concentration is used with 10 equivalents of amino acids and
the deprotection
time (2 minutes) and coupling time (4.5 minutes) are extended. The pyrrolidine
concentration
is also increased (3.8% for wash-free method and 6.8% with a more conservative
3 x 4 mL
wash method) to quench the larger excess of activated amino acid. These
conditions result in a
cycle time of ¨ 7.3 minutes per amino acid, and a total waste of 5.5 mL per
amino acid at 0.1
mmol synthesis scale. Using this wash-free method, both proteins are obtained
with similar
crude purity as when washing is utilized. The crude proinsulin and barstar
samples are then
purified by reversed-phase HPLC which results in 2.4% and 3.4% overall yield,
respectively.
Purified samples of proteins are identified by deconvoluted mass spectra
showing 9395 Da and
10210 Da for proinsulin and barstar, respectively. These protein synthesis
examples
demonstrate that the processes of the present disclosure can be robust for
generating high purity
results even for long and challenging sequences.
The foregoing demonstrates wash-free processes for solid phase synthesis of
peptides
and proteins. The processes of the present disclosure may not negatively
impact peptide
purity versus controls with washing and can provide high purity and rapid
synthesis times, for
example when combined with elevated temperature reaction conditions. Compared
to
traditional SPPS, the processes according to the present disclosure can
provide up to a 95%
reduction in waste generated.
The foregoing also demonstrates successful application of the processes of the
present
disclosure at 25 mmol scale on a large-scale microwave peptide synthesizer and
that the
processes of the present disclosure may be readily scalable. This may allow
for larger
reaction vessel sizes (e.g., up to 15 liters) at which the processes of the
present disclosure
may be applied for synthesis scales above 200 mmol per batch. At these larger
scales, many
-58-
Date Recue/Date Received 2023-08-25
hundreds of liters of solvent can be saved per batch of peptide synthesized.
Additionally, by
largely reducing solvent as a material cost, the processes of the present
disclosure may
provide a significant boost toward overcoming cost barriers associated with
the use of more
expensive and potentially less efficient green solvents for SPPS.
Methods
Synthesis
(a) Peptides: All peptides are synthesized using automated microwave synthesis
conditions on a CEM Liberty PRIME 2.0 system at 0.1 mmol scale using the one-
pot
coupling/deprotection methodology. See, e.g., U.S. Patent 10,239,914; Singh,
S. K.; Collins, J. M.,
New Developments in Microwave¨Assisted Solid Phase Peptide Synthesis. In
Peptide Synthesis:
Methods and Protocols, Hussein, W. M.; Skwarczynski, M.; Toth, I., Eds.
Springer US: New York,
NY, 2020; 95-109. Method details involving reaction time, temperature,
concentration of
deprotection reagent etc. are described in Tables 1 and 2. Couplings are
performed for 30
seconds at room temperature followed by 60 seconds at 105 C using Fmoc-amino
acid (1.0
mL, 0.5 M in DMF, 5 equivalents), DIC (1.0 mL, 0.75 M in DMF, 7.5 equivalents)
and
Oxyma (1.5 mL, 0.26 M in DMF, 4 equivalents). Fmoc deprotection step(s) using
3vo1%
pyrrolidine (e.g., see Example 2) is initiated by adding 0.75 mL of
pyrrolidine/DMF
(17% v/v) directly to the undrained post-coupling solution (optimization
experiments
are performed by adding 0.75 mL of 11.3-25% v/v pyrrolidine/DMF as described
in
Table 1). Headspace flushing pressure of 15 psi is used during the
deprotection step.
The wash-based method uses 2 x 4 mL DMF post-deprotection washings. The cycle
involving deprotection-coupling (for wash-free) or deprotection-washing-
coupling
(for wash-based) runs is automatically performed for all amino acid residues
in the
peptide sequence. JR-10 mer is synthesized on Fmoc-Rink Amide ProTidelm LL
resin (0.20
meq/g substitution) or Fmoc-Rink Amide MBHA PS resin (0.33 meq/g
substitution). 65-
74ACP and Liraglutide are synthesized on Fmoc-Gly-Wang-ProTide resin (0.24
meq/g
substitution) and 'ft-Amyloid is synthesized on Fmoc-Ala-Wang-Portside resin
(0.23 meq/g
substitution).
(b) Wash-Free Production Scale Liraglutide Synthesis: Liraglutide is
synthesized at
25 mmol scale using Fmoc-Gly-Wang-ProTide resin (0.24 meq/g substitution) in a
3 L
reaction vessel on the Liberty PRO microwave peptide synthesizer. Couplings
are performed
for 5 min at 80 C using Fmoc-amino acid (200 mL, 0.5 M in DMF), DIC (50 mL, 4
M in
DMF) and Oxyma (225 mL, 0.33 M in DMF). After draining the post-coupling
mixture,
-59-
Date Recue/Date Received 2023-08-25
Fmoc deprotection step is performed for 10 min at 90 C by adding 50 mL of
pyrrolidine/DMF (15% v/v) followed by additional DMF (250 mL) to obtain a
final
concentration of 2.5% pyrrolidine in the reaction vessel. Nitrogen pressure at
85 psi is
directed through a spray head in the top of the reaction vessel to facilitate
directed flushing of
the headspace gas during each deprotection step. Fmoc-Lys(palmitoyl-Glu-OtBu)-
OH is
coupled using 2 equivalent excess with a wash-based coupling cycle, while all
other amino
acid residues in the sequence use no washings after the deprotection and
coupling steps.
Fmoc-His(Boc)-OH is coupled by using 2 x 30 min at 40 C method. Wash-based
cycles at
25 mmol scale use 4 x 650 mL DMF and 1 x 800 mL DMF for post-deprotection
washings.
The cycles involving deprotection-coupling (for wash-free) or deprotection-
washing-coupling
(for wash-based) runs are automatically performed for all amino acid residues
in the peptide
sequence.
(c) Proteins: Proteins are synthesized using automated microwave synthesis
conditions on a CEM Liberty PRIME 2.0 system at 0.10 mmol scale using the one-
pot
coupling/deprotection methodology. See, e.g., U.S. Patent 10,239,914; Singh,
S. K.; Collins,
J. M., New Developments in Microwave¨Assisted Solid Phase Peptide Synthesis.
In Peptide
Synthesis: Methods and Protocols, Hussein, W. M.; Skwarczynski, M.; Toth, I.,
Eds.
Springer US: New York, NY, 2020; 95-109. Couplings are performed with Fmoc-
amino acid
(2.0 mL, 0.5 M in DMF), DIC (1.0 mL, 2.0 M in DMF) and Oxyma (1.75 mL, 0.50 M
in
DMF) for 30 seconds at room temperature followed by 4 minutes at 90 C. Fmoc
deprotection step is performed for 2 minutes at 110 C and initiated by adding
0.75 mL of
pyrrolidine/DMF (28% v/v) directly to the undrained post-coupling solution.
Headspace
flushing pressure of 15 psi is used during the deprotection step. The wash-
based method uses
3 x 4 mL DMF post-deprotection washings. The cycles involving deprotection-
coupling (for
wash-free) or deprotection-washing-coupling (for wash-based) runs are
automatically
performed for all amino acid residues in the peptide sequence. Proinsulin 86-
mer and Barstar
89-mer proteins are synthesized on Fmoc-Rink Amide ProTideTM LL resin (0.18
meq/g
substitution).
Resin Cleavage
(a) Peptides: The peptidyl resin is washed with DCM (3 x 5 mL) after
synthesis.
Cleavage is performed for 30 min at 38 C using 5 mL of a freshly prepared
cleavage
cocktail [TFA/TIS/H20/DODT (92.5/2.5/2.5/2.5)1. The TFA solution is collected
by filtration
-60-
Date Recue/Date Received 2023-08-25
and ice cold ethyl ether is added followed by centrifugation at 3500 rpm for 5
minutes to
obtain the crude peptide as white pellet.
(b) Proteins: The peptidyl resin is washed with DCM (3 x 15 mL) after
synthesis.
Cleavage is performed for 5 hours at room temperature using a slow cleavage
method by
adding 7.5 mL of [TFA/TIS/H20/DODT (6/0.5/0.5/0.5)1 followed by gradual
addition of 4 mL
TFA every hour for 3 hours. After the third addition (final conc.
TFA/TIS/H20/DODT
(18/0.5/0.5/0.5) the cleavage is allowed to react for an additional 2 hours.
The TFA solution is
collected by filtration and ice cold ethyl ether is added followed by
centrifugation at 3900 rpm
for 3 minutes to obtain the crude peptide as a white pellet.
Analysis
All peptides are lyophilized overnight after dissolving the pellet in 10%
acetic
acid/deionized water. A lyophilized aliquot of the peptide is taken in
deionized water (-2
mg/mL peptide concentration) and a clear solution is obtained by addition of
acetonitrile,
ammonium hydroxide (up to 1%), or acetic acid (up to 9%) followed by
sonication. Protein
samples (barstar and proinsulin) are dissolved by sonicating in a solution of
H20/ACN/AcOH
(8:1:1) for 1 hour. The peptide/protein solution are analyzed on a Vanquish
UHPLC system
(Thermo Fisher; Waltham, MA, USA) with a Waters ACQUITY UPLC BEH C8 reversed-
phase column (100 x 2.1 mm i.d., 1.7 gm, 130 A; Waters Corporation, Milford,
MA, USA)
coupled to an ExactiveTM Plus OrbitrapTM mass spectrometer (Thermo Fisher;
Waltham, MA,
USA) via an ESI source (operated in positive polarity mode). Deconvoluted mass
spectra for
proteins are obtained using UniDec (Universal Deconvolution) Version 6Ø1
developed by
Marty et al. Marty, M. T.; Baldwin, A. J.; Marklund, E. G.; Hoshberg, G. K.
A.; Benesch, J. L.
P.; Robinson, C. V., Bayesian Deconvolution of Mass and Ion Mobility Spectra:
From Binary
Interactions to Polydisperse Ensembles. Anal. Chem. 2015, 87(8), 4370-4376.
Analytical runs
are performed at a flow rate of 0.5 mL/min with gradient elution of 10-70 % B
using 0.05%
trifluoroacetic acid in water (A) and 0.05% trifluoroacetic acid in
acetonitrile (B). The column
and autosampler are maintained at 40 and 24 C, respectively for all peptides
except 1-4213-
amyloid. 1-42n
p_ amyloid is analyzed on a Waters ACQUITY UPLC BEH C8 reversed-phase
column (100 x 2.1 mm i.d., 1.7 gm, 130 A; Waters Corporation, Milford, MA,
USA) with a
flow rate of 0.6 mL/min at 70 C column temperature on a Waters Acquity RP-
UPLC system
with PDA detector coupled to a 3100 Single Quad mass spectrometer.
Purification
-61 -
Date Recue/Date Received 2023-08-25
Lyophilized protein samples (barstar and proinsulin) are dissolved (barstar:
6.4
mg/mL in water with 0.2 % ammonium hydroxide and 10 mM DTT; proinsulin: 8
mg/mL in
6 M GdnHC1 with 0.1% ammonium hydroxide and 100 mM DTT through sonication for
1
hour at 40 C. Lyophilized liraglutide is dissolved (8.1 mg/mL) in 20 %
acetonitrile.
Samples are filtered with a 0.45 gin regenerated cellulose syringe filter
(Phenomenex;
Torrance, CA, USA) prior to purification.
Purifications are completed on a CEM Prodigy HPLC System, which includes an
integrated heating system (column oven and mobile phase heater) to enable high-
efficiency
elevated temperature operation. Barstar and proinsulin purifications are
performed at 60 C
using a Waters Protein XBridge C4 column (19 x 150 mm, 5 gm, 300 A; Waters
Corporation,
Milford, MA, USA) with mobile phases consisting of 0.1 % trifluoroacetic acid
in water (A)
and 0.1 % trifluoroacetic acid in acetonitrile (B). Liraglutide purifications
are performed
using the same conditions, but with a Waters )(Bridge C8 column (19 x 150 mm,
5 gm, 130
A).
Optimized gradient conditions are determined by first injecting ¨10 mg of
crude
sample on a 10-70 % B screening gradient (20 min gradient; ¨3 % B/CV) with a
flow rate of
27 mL/min. The target peak retention time is then used to calculate (using CEM
Focused
Gradient Calculator software, version 1.1.673.1159) optimized focused
gradients for each
purification. The protein samples are purified using focused gradients over 25
min
(proinsulin: 27-39 % B; barstar: 39-51 % B), while liraglutide is purified
using a focused
gradient over 18 min. (46-55 % B).
In the foregoing, examples of embodiments have been disclosed. The present
invention is not limited to such exemplary embodiments. In the foregoing,
descriptions of
sequences of steps or other actions are described for purposes of providing
examples, and not
for the purpose of limiting the scope of this disclosure (e.g., where
appropriate, steps or
actions may be performed in different sequences than described above, and
steps and actions
may be omitted and/or added). The figures are schematic representations and so
are not
necessarily drawn to scale. Unless otherwise noted, specific terms have been
used in a
generic and descriptive sense and not for purposes of limitation.
Numerical values provided throughout this disclosure can be approximate, and
for
each range specified in this disclosure, all values within the range
(including end points) and
all subranges within the range are also disclosed. Those of ordinary skill in
the art will also
readily understand that, in different implementations of the features of this
disclosure,
-62-
Date Recue/Date Received 2023-08-25
reasonably different engineering tolerances, precision, and/or accuracy (for
example with
respect to numerical value(s)) may be applicable and suitable for obtaining
the desired result.
Those of ordinary skill will accordingly readily understand the meaning,
usage, etc. herein of
terms such as "substantially," "about," "approximately," and the like. As non-
limiting
examples, the term "about" can indicate that a numeric value can vary by plus
or minus 25%,
for example plus or minus 20%, for example plus or minus 15%, for example plus
or minus
10%, for example plus or minus 5%, for example plus or minus 4%, for example
plus or
minus 3%, for example plus or minus 2%, for example plus or minus 1%, for
example plus or
minus less than 1%, for example plus or minus 0.5%, for example less than plus
or minus
0.5%, including all values and subranges therebetween for each of the above
ranges.
As used herein, the phrase "and/or" includes any and all combinations of one
or more
of the associated listed items (e.g., can refer to elements that are
conjunctively present in
some embodiments and elements that are disjunctively present in other
embodiments), and in
some embodiments optionally in combination with other elements not
specifically identified
by the "and/or" phrase. As non-limiting examples, "A and/or B" can refer in
some
embodiments to A without B; in some embodiments to B without A; in some
embodiments to
both A and B; etc.
As used herein, the phrase "at least one" in reference to a list of one or
more elements
can refer to at least one element selected from any one or more of the
elements in the list of
elements, but not necessarily including at least one of each and every element
specifically
listed within the list of elements and not excluding any combinations of
elements in the list of
elements. In some embodiments, elements may be optionally present other than
the elements
specifically identified within the list of elements to which the phrase "at
least one" refers,
whether related or unrelated to those elements specifically identified. As non-
limiting
examples, "at least one of A and B"; "at least one of A or B"; and/or "at
least one of A and/or
B" can refer in some embodiments to at least one, optionally including more
than one, A,
with no B present (and optionally including elements other than B); in some
embodiments to
at least one, optionally including more than more one, B, with no A present
(and optionally
including elements other than A); in some embodiments to at least one,
optionally including
more than one, A, and at least one, optionally including more than one, B (and
optionally
including other elements); etc.
As used herein, indefinite articles "a" and "an" refer to at least one ("a"
and "an" can
refer to singular and/or plural element(s)).
-63-
Date Recue/Date Received 2023-08-25