Note: Descriptions are shown in the official language in which they were submitted.
METHOD FOR PRODUCING AQUEOUS SOLUTION CONTAINING NICKEL OR
COBALT
TECHNICAL FIELD
[0001] The present invention relates to a method for producing an aqueous
solution containing
nickel or cobalt. More specifically, the present invention relates to a method
for producing an
aqueous solution containing nickel or cobalt for recovering nickel and cobalt
from a raw material
and then producing an aqueous solution containing nickel or cobalt that can be
used for
producing a cathode active material of a lithium ion secondary battery.
BACKGROUND
[0002] A one-stage atmospheric pressure heating reaction leaching process, or
a two-stage
leaching process consisting of an atmospheric pressure heating reaction and a
pressurizing
heating reaction has been mainly used to ionize nickel and cobalt from a mixed
hydroxide
precipitate (MHP) cake raw material containing nickel/cobalt mixed hydroxide.
[0003] However, in the case of the one-stage atmospheric pressure heating
reaction leaching
process, a problem occurs in that the recovery rate of Ni and Co decreases. In
the case of the
two-stage leaching process consisting of an atmospheric pressure heating
reaction and a
pressurizing heating reaction, a problem is posed in that the range of
material selection is
reduced due to erosion, corrosion, damage, etc. of pipe and reactor materials,
and the
competitiveness is lowered due to energy costs.
[0004] In addition, nickel and cobalt were selectively recovered from the
ionized aqueous of
nickel/cobalt solution using a solvent extractant such as Ion quest 801,
Cyanex 272, Versatic
Acid 10, or LIX 841. However, there is a risk of fire and explosion due to the
use of an organic
solvent in a solvent extraction process. The high unit price of the solvent
extractant increases
the cost of producing high-purity nickel sulfate and cobalt, thereby reducing
competitiveness in
terms of price.
1
Date Regue/Date Received 2024-03-06
SUMMARY
[0005] An object of the present invention is to produce a high-purity aqueous
solution by
recovering nickel and cobalt from an MHP cake raw material containing
nickel/cobalt mixed
hydroxide.
[0006] In addition, it is an object of the present invention to improve a
nickel/cobalt recovery
rate and reduce energy consumption by ionizing nickel and cobalt using a two-
stage atmospheric
pressure heating leaching step.
[0007] In addition, it is an object of the present invention to separate
magnesium and calcium
using sodium fluoride (NaF) as a precipitant in an aqueous solution containing
high-purity nickel
production step, separate magnesium and manganese through a solubility
difference using
sodium hydrogen sulfide (NaSH) in an aqueous solution containing high-purity
cobalt
production step, and additionally separate impurities such as copper,
magnesium, and manganese
through the use of sodium hydrogen sulfide (NaSH) and sodium fluoride (NaF),
thereby
reducing the solvent extraction step.
[0008] Since the solvent extraction step has a risk of fire and explosion due
to the use of an
organic solvent, it is an object of the present invention to minimize the
solvent extraction step,
thereby improving the operating environment and reducing the production cost
of a final
product.
[0009] According to one aspect of the present invention, there is provided a
method for
producing an aqueous solution containing nickel or cobalt, including: (A) a
leaching step, which
includes a first atmospheric pressure heating leaching step and a second
atmospheric pressure
heating leaching step, in which a raw material is heated and leached under an
atmospheric
pressure to form a leachate solution containing nickel, cobalt, and
impurities; (B) a first
extraction step of separating the leachate solution into a first filtrate
containing nickel and
impurities and a first organic layer containing cobalt and impurities by
adding a first solvent
extractant to the leachate solution; (C-i) a precipitation removal step of
precipitating and
removing impurities including magnesium, calcium, or a mixture thereof by
adding a
precipitating agent to the first filtrate; and (D-i) a target material
precipitation step of selectively
2
Date Regue/Date Received 2024-03-06
precipitating a nickel cake containing nickel by adding a neutralizing agent
to the first filtrate
from which the impurities are precipitated and removed.
[0010] According to another aspect of the present invention, there is provided
a method for
producing an aqueous solution containing nickel or cobalt, including: (A) a
leaching step, which
includes a first atmospheric pressure heating leaching step and a second
atmospheric pressure
heating leaching step, in which a raw material is heated and leached under an
atmospheric
pressure to fonii a leachate solution containing nickel, cobalt, and
impurities; (B) a first
extraction step of separating the leachate solution into a first filtrate
containing nickel and
impurities and a first organic layer containing cobalt and impurities by
adding a first solvent
extractant to the leachate solution; and (C-ii) a purification step of
removing impurities including
magnesium, manganese, zinc, copper, or mixtures thereof by adding a sulfuric
acid solution to
the first organic layer to produce a second filtrate, and adding sulfide to
the second filtrate to
precipitate and recover a cobalt precipitate.
100111 In an embodiment of the present invention, a pH of the filtrate
obtained in the second
atmospheric pressure heating leaching step may be lower than a pH of the
filtrate obtained in the
first atmospheric pressure heating leaching step.
[0012] In an embodiment of the present invention, the filtrate obtained in the
second
atmospheric pressure heating leaching step may be fed to the first atmospheric
pressure heating
leaching step.
[0013] In an embodiment of the present invention, the first solvent extractant
may be bis (2,4,4-
trimethylpentyl) phosphinic acid.
[0014] In an embodiment of the present invention, the first extraction step
may be carried out at
a temperature of 40 degrees C and a pH of greater than 5.0 and less than 5.4.
[0015] In an embodiment of the present invention, the precipitating agent may
be sodium
fluoride.
[0016] In an embodiment of the present invention, the precipitating agent may
be added in an
amount of more than 2.0 equivalents and less than 2.4 equivalents of an amount
of the
magnesium, calcium, or a mixture thereof.
3
Date Regue/Date Received 2024-03-06
[0017] In an embodiment of the present invention, the neutralizing agent may
be a basic material
containing sodium.
[0018] In an embodiment of the present invention, after the neutralizing agent
is added, the pH
of the first filtrate may be 8 or more at a temperature of 85 degrees C.
[0019] In an embodiment of the present invention, the method may further
include: (E-i) a
washing step of washing the nickel cake with pure water.
[0020] In an embodiment of the present invention, the sulfide may be sodium
hydrogen sulfide
(NaSH).
[0021] In an embodiment of the present invention, the sulfide may be added in
an amount of
more than 1.0 equivalents and less than 1.6 equivalents of an amount of the
cobalt and zinc.
[0022] In an embodiment of the present invention, the method may further
include: (D-ii) a
copper removal step of dissolving the cobalt precipitate in a sulfuric acid
solution and then
removing copper.
[0023] In an embodiment of the present invention, the copper removal step may
be perfoimed by
adding sodium hydrogen sulfide (NaSH) in an amount greater than 4.5
equivalents and less than
5.5 equivalents of copper content.
[0024] In an embodiment of the present invention, the method may further
include: (E-ii) a
second extraction step of separating the copper-removed aqueous solution into
a third filtrate
containing cobalt and impurities and a second organic layer containing zinc
and impurities by
adding a second solvent extractant to the copper-removed aqueous solution.
[0025] In an embodiment of the present invention, the second solvent
extractant may be
D2EHPA (di-(2-ethylhexyl) phosphoric acid).
[0026] In an embodiment of the present invention, the second extraction step
may be carried out
at a pH of greater than 2.4 and less than 3.2 at a temperature of 40 degrees
C.
[0027] In an embodiment of the present invention, the method may further
include: (F) a
precipitation removal step of precipitating and removing impurities including
magnesium by
adding a precipitating agent to the third filtrate.
[0028] In an embodiment of the present invention, the method may further
include: (G) a target
4
Date Regue/Date Received 2024-03-06
material precipitation step of selectively precipitating a cobalt cake
containing cobalt by adding a
neutralizing agent to the third filtrate from which the impurities are
precipitated and removed.
[0029] In an embodiment of the present invention, after the neutralizing agent
is added, the pH
of the third filtrate may be 8 or more at a temperature of 85 degrees C.
[0030] In an embodiment of the present invention, the method may further
include: (H) a
washing step of washing the cobalt cake with pure water.
[0031] According to the present invention, it is possible to improve the
nickel/cobalt recovery
rate and reduce the energy consumption by using a two-stage atmospheric
pressure heating step.
[0032] In addition, by minimizing the solvent extraction step having a risk of
fire and explosion
due to the use of an organic solvent in the impurity removal step, it is
possible to improve the
operating environment and reduce the production cost of a final product.
BRIEF DESCRIPTION OF THE DRAWINGS
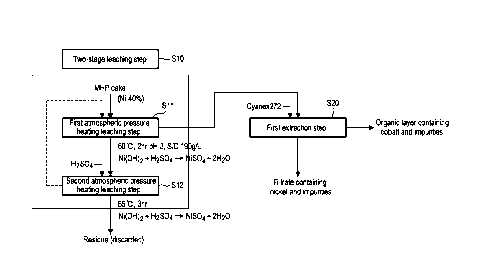
[0033] FIG. 1 is a diagram showing a two-stage leaching step and a first
extraction step for
producing an aqueous solution containing nickel or cobalt according to one
embodiment of the
present invention.
[0034] FIG. 2 is a diagram showing a precipitation removal step and a target
material
precipitation step for producing an aqueous solution containing nickel
according to one
embodiment of the present invention.
[0035] FIG. 3 is a diagram showing an impurity removal step, a copper removal
step, a second
extraction step, a precipitation removal step, and a target material
precipitation step for
producing an aqueous solution containing cobalt according to one embodiment of
the present
invention.
DETAILED DESCRIPTION
[0036] Embodiments of the present disclosure are illustrated for describing
the technical spirit of
the present disclosure. The scope of the claims according to the present
disclosure is not
limited to the embodiments described below or to the detailed descriptions of
these
Date Regue/Date Received 2024-03-06
embodiments.
[0037] The present invention will now be described with reference to the
drawings.
[0038] FIG. 1 is a diagram showing a two-stage leaching step S10 and a first
extraction step S20
for producing an aqueous solution containing nickel or cobalt according to one
embodiment of
the present invention. FIG. 2 is a diagram showing a precipitation removal
step S31 and a
target material precipitation step S32 for producing an aqueous solution
containing nickel
according to one embodiment of the present invention. FIG. 3 is a diagram
showing an
impurity removal step S32 and S42, a copper removal step S62, a second
extraction step S72, a
precipitation removal step S82, and a target material precipitation step S92
for producing an
aqueous solution containing cobalt according to one embodiment of the present
invention.
[0039] Referring to FIGS. 1 to 3, there may be provided a method for producing
an aqueous
solution containing nickel or cobalt that can be used for manufacturing a
cathode active material
of a lithium secondary battery from a mixed hydroxide precipitate (MHP cake)
through a series
of steps. According to this method, it is possible to improve the operation
stability and the
purity, and reduce the production cost. Hereinafter, the respective steps will
be described in
detail with reference to the drawings.
[0040] Firstly, referring to FIG. 1, a leaching step S10 of forming a leachate
by performing two-
stage atmospheric pressure heating leaching steps on an MHP cake, and a first
extraction step
S20 of separating the leachate into a first filtrate containing nickel and
impurities and a first
organic layer containing cobalt and impurities may be performed.
100411 Leaching Step S10
100421 The leaching step S10 is a step of forming a leachate by dissolving an
MHP cake in the
Rain of hydroxide in an acid solution such as sulfuric acid to ionize the MHP
cake. The
leaching step S10 includes a first atmospheric pressure heating leaching step
Si! and a second
atmospheric pressure heating leaching step S12. The atmospheric pressure
heating leaching
step is a step of producing an acid solution in an open reactor at a
temperature of 100 degrees C
or less, introducing a raw material into the reactor, and leaching valuable
metals by a reaction
represented by the following Reaction Formula 1. The raw material introduced
here may be an
6
Date Regue/Date Received 2024-03-06
MHP cake in the form of hydroxide containing nickel in an amount of 40% by
weight.
[0043] Reaction Foimula 1]
[0044] M(OH)2 + H2SO4 MS04 + 2H20 (M is metal such as Ni, Co, or Mg)
[0045] The first atmospheric pressure heating leaching step Sll and the second
atmospheric
pressure heating leaching step S12 may be separately perfoinied in two-stage
apparatuses, or
may be performed in one pressurization apparatus by changing only the process
conditions (e.g.,
temperature, pressure, or acidity).
[0046] Valuable metals may be leached from the raw material through the first
atmospheric
pressure heating leaching step S11. For example, valuable metals such as
nickel, cobalt, and
manganese in the raw material may be leached. In addition, elements such as
iron, copper,
aluminum, zinc, magnesium, and the like in the raw material may also be
leached. The first
atmospheric pressure heating leaching step Sll may be performed for about 2
hours at a
temperature in the range of 50 degrees C to 70 degrees C and a pH in the range
of 2.7 to 3.3.
By satisfying the temperature and the pH, high leaching efficiency can be
obtained under optimal
conditions.
[0047] In the first atmospheric pressure heating leaching step S11, the solid
density of the raw
material introduced into the reactor may be 100 g/L or more. For example, the
solid density of
the raw material may be in the range of 100 g/L to 200 g/L. As used herein,
the term "solid
density" is defined as the ratio of the mass of the raw material introduced
into the pressurization
apparatus to the volume of the acid solution previously introduced into the
reactor. In other
words, the solid density may be the ratio of the mass of the raw material
introduced per unit
solvent, and may be the mass of the raw material per 1 L of a solvent.
[0048] The filtrate obtained in the first atmospheric pressure heating
leaching step S1 1 may be
introduced into the first extraction step S20, and the residue may be
subsequently treated in the
second atmospheric pressure heating leaching step S12.
[0049] In the second atmospheric pressure heating leaching step S12, the
leaching residue
obtained in the first atmospheric pressure heating leaching step Sll may be
leached at a
temperature in the range of 80 degrees C to 100 degrees C for about 3 hours.
Other conditions
7
Date Regue/Date Received 2024-03-06
of the second atmospheric pressure heating leaching step S12 may be the same
as those of the
first atmospheric pressure heating leaching step S11.
[0050] The pH of the filtrate obtained in the second atmospheric pressure
heating leaching step
S12 may be lower than the pH of the filtrate obtained in the first atmospheric
pressure heating
leaching step S11. By controlling the pH, the leaching rate in each
atmospheric pressure
heating leaching step can be increased. Accordingly, the entire amount of the
valuable metals
contained in the raw material can be leached out. For example, the entire
amounts of nickel,
cobalt, manganese, iron, copper, aluminum, zinc, and magnesium contained in
the raw material
can be leached out.
[0051] In one embodiment, the filtrate formed in the second atmospheric
pressure heating
leaching step S12 may be fed to the first atmospheric pressure heating
leaching step S11 as
shown in FIG. 1.
[0052] In the case of a commonly used atmospheric pressure leaching method,
the reaction time
has to be continued for 18 hours or longer to increase the leaching rate of
valuable metals. In
this case, additional costs are incurred due to the increased use of fuel and
steam. The
productivity is low because the amount of raw material processed per day is
small. However,
according to one embodiment of the present invention, by using the two-stage
atmospheric
pressure heating leaching method, it is possible to improve the recovery rate
of nickel and cobalt
and reduce the energy consumption. Thus, it is possible to reduce the
production cost and
improve the productivity.
[0053] The residue generated in the second atmospheric pressure heating
leaching step S12 may
be discarded as shown in FIG. 1.
[0054] First Extraction step S20
[0055] The first extraction step S20 is a step of selectively separating or
extracting nickel from
the aqueous solution containing nickel/cobalt (leachate solution) ionized
through the two-stage
atmospheric pressure heating leaching step by using a first solvent
extractant.
[0056] In this regard, the first solvent extractant is not particularly
limited as long as the loading
rate of nickel is low, and may be, for example, bis(2,4,4-trimethylpentyl)
phosphinic acid
8
Date Regue/Date Received 2024-03-06
(Cyanex272).
[0057] When the first solvent extractant is added to the leachate, nickel may
be not loaded into
the first solvent extractant and may be distributed to the first filtrate
(Raffinate). Cobalt and
other impurities (Mg, Mn, Zn, etc.) may be separated or extracted by being
distributed to an
organic layer together with the first solvent extractant. This separation or
extraction can occur
according to the reactions represented by Reaction Formulae 2 and 3 below. In
this regard, the
reaction of Reaction Formula 3 is a reaction for maintaining the pH by
neutralizing H2SO4
formed by the reaction of Reaction Formula 2.
[0058] [Reaction Founula 2]
[0059] 2HR(org.) + MS04(aq.) MR2(org.) + H2SO4(aq.) (R is Ni etc., and M is
Co, Mg, Mn
etc.)
[0060] [Reaction Foimula 3]
[0061] H2SO4 + Na2CO3 Na2SO4+ H20 + CO2
[0062] The first extraction step may be perfonned at a temperature of 40
degrees C and a pH of
greater than 5.0 and less than 5.4. By satisfying the temperature and the pH,
it is possible to
increase the loading rate of cobalt and impurities, and efficiently separate
nickel into the filtrate.
[0063] In one embodiment, the ratio of the first solvent extractant (0) to the
aqueous solution
(A) can be controlled according to the concentration of the component to be
extracted from the
solution. For example, the ratio (0:A) of the first solvent extractant (0) to
the aqueous solution
(A) may range from 0.5:1 to 2:1. For example, the 0:A may be 1.5:1.
[0064] Referring next to FIG. 2, a precipitation removal step S31 of
precipitating and removing
impurities from the first filtrate that has passed through the first
extraction step S20, a target
material precipitation step S41 of selectively precipitating a nickel cake
containing high-purity
nickel, and a final leaching step of producing an aqueous solution containing
high-purity nickel
may be performed.
[0065] Precipitation Removal Step S31
[0066] The precipitation removal step S31 may be performed to remove
impurities such as
magnesium, calcium, or a mixture thereof remaining in the first filtrate.
After the precipitation
9
Date Regue/Date Received 2024-03-06
removal step S31, the first filtrate may be fed to the target material
precipitation step S41.
[0067] For example, in the precipitation removal step S31, a removing agent
may be introduced
into the solution. The removing agent is not particularly limited as long as
it can react with
magnesium or calcium to form a precipitate. The removing agent may be, for
example, sodium
fluoride (NaF).
[0068] For example, magnesium and calcium may be precipitated as magnesium
fluoride or
calcium fluoride through a reaction represented by Reaction Foimula 4 below.
[0069] For example, by performing the precipitation removal step S31 for about
2 hours or more
at a reaction temperature in the range of 50 degrees C to 70 degrees C, only
magnesium and
calcium can be separated through selective precipitation while reducing nickel
precipitation in
the filtrate.
[0070] [Reaction Foimula 4]
[0071] MS04 + 2NaF = MF2 + Na2SO4 (M is Mg or Ca)
[0072] In one embodiment, sodium fluoride may be added in an amount greater
than 2.0
equivalents (eq) of magnesium, calcium or a mixture thereof. In another
embodiment, sodium
fluoride may be added in an amount less than 2.4 equivalents (eq) of
magnesium, calcium or a
mixture thereof.
[0073] Target Material Precipitation Step S41
[0074] In the target material precipitation step S41, a neutralizing agent may
be added to the first
filtrate after the precipitation removal step S31.
[0075] For example, the neutralizing agent may be a basic material containing
sodium. For
example, the neutralizing agent may be sodium carbonate (Na2CO3).
[0076] After removing impurities such as magnesium and calcium, in the target
material
precipitation step S41, nickel may be precipitated in the form of a cake
through a reaction
indicated by Reaction Formula 5 below.
[0077] [Reaction Foimula 5]
[0078] 3NiSO4 + 3Na2CO3 +2H20 = NiCO3=2Ni(OH)2 + 3Na2SO4 +3CO2
[0079] The target material precipitation step S41 may be performed for 4 hours
or more at a pH
Date Regue/Date Received 2024-03-06
of 8 or higher and a temperature in the range of 80 degrees C to 90 degrees C.
[0080] Since nickel can be recovered through the target material precipitation
step S41, it is
possible to reduce the use of expensive organic solvents that have a risk of
explosion and fire.
Thus, it is possible to improve the operational stability and the
productivity, and reduce the
production costs.
[0081] Although not specifically shown in the drawings, some sodium components
may be
present in the precipitated nickel cake. Therefore, the water-soluble sodium
components can be
removed by a washing step using pure water at the rear stage. In this case,
the production cost
can be reduced by reusing the removed sodium components in producing sodium
carbonate
(Na2CO3), which is a neutralizing agent.
[0082] Final Leaching Step S51
[0083] The final leaching step S51 is a step of producing an aqueous solution
containing high-
purity nickel by removing components such as sodium and the like through
washing and
dissolving the nickel cake in a sulfuric acid solution.
[0084] In the final leaching step S51, the nickel cake may be added to a
solution obtained by
mixing pure water with sulfuric acid at an acidity of 150 g/L to 200 g/L.
Nickel, cobalt, and
trace impurities contained in the nickel cake can be dissolved in the sulfuric
acid solution. The
sulfuric acid solution and the nickel cake are reacted until the pH becomes
2Ø According to
one embodiment, in the final leaching step S51, the reaction may be performed
for 4 hours or
more at a pH in the range of 1.0 to 3.0 and a temperature in the range of 50
degrees C to 70
degrees C.
[0085] Referring next to FIG. 3, a second filtrate production step S32 of
adding a sulfuric acid
solution to the first organic layer that has passed through the first
extraction step S20, a
purification step S42 of precipitating and recovering a cobalt precipitate
from the second filtrate
and purifying impurities, a step S52 of dissolving the cobalt precipitate in
the sulfuric acid
solution again, a copper removal step S62 of removing copper by adding sodium
hydrogen
sulfide, a second extraction step S72 of separating the aqueous solution from
which copper is
removed into a third filtrate containing cobalt and impurities and a second
organic layer
11
Date Regue/Date Received 2024-03-06
containing zinc and impurities, a precipitation removal step S82 of
precipitating and removing
impurities from the third filtrate, a target material precipitation step S92
of selectively
precipitating a cobalt cake containing high-purity cobalt, and a final
leaching step of producing
an aqueous solution containing high-purity cobalt S102 may be performed.
[0086] Second Filtrate Production Step S32
[0087] The second filtrate production step S32 is a step of stripping cobalt
into the sulfuric acid
solution by adding a sulfuric acid solution to the first organic layer
containing cobalt and
impurities.
[0088] Stripping is a process in which a stripping filtrate containing cobalt
is produced by
reacting sulfuric acid with loaded cobalt, and loaded impurities are recovered
with an aqueous
solution.
[0089] Purification Step S42
[0090] The purification step S42 is a step of selectively recovering only
cobalt from the stripped
second filtrate. What is different from the precipitation removal step S31 is
that in the
purification step S42, cobalt, which is the target material, may be recovered
in the form of a
precipitate.
[0091] For example, in the purification step S42, a cobalt precipitate may be
generated by
adding sulfide into the solution. For example, the sulfide may be sodium
hydrogen sulfide
(NaSH). Cobalt may be precipitated and recovered in the form of sulfide by the
reactions
represented by the following Reaction Formulae 6 and 7.
[0092] [Reaction Fonnula 61
[0093] CoSO4 + 2NaSH 2CoS + Na2SO4 + H2SO4
[0094] [Reaction Fonnula 71
[0095] H2SO4 + Na2CO3 ¨> Na2SO4 + H20 + CO2
[0096] For example, while maintaining a pH of 4.5 to 5.0 at a reaction
temperature in the range
of 70 degrees C to 90 degrees C, the purification step S42 may be performed
for about 3 hours or
more.
[0097] In the above pH range, the solubility of CoS and ZnS is very low, 0.1
mg/L or less, and
12
Date Regue/Date Received 2024-03-06
the solubility of MgS and MnS is high. Therefore, only cobalt and zinc can be
separated
through selective precipitation by controlling the pH range, thereby purifying
magnesium and
manganese.
[0098] In one embodiment, sulfide may be added in an amount greater than 1.0
equivalents and
less than 1.6 equivalents of an amount of the cobalt and zinc.
[0099] Sulfuric Acid Solution Production Step S52
[0100] In the sulfuric acid solution production step S52, an aqueous solution
is produced by
dissolving a precipitate containing cobalt in a sulfuric acid solution.
[0101] For example, the sulfuric acid solution production step S52 may be
performed by a
reaction represented by the following Reaction Foimula 8.
[0102] [Reaction Formula 81
[0103] CoS + H2SO4 + 1/202 ¨> CoSat + H20 + S
[0104] For example, in the sulfuric acid solution production step S52, the
solid density (S/D) of
the precipitate in the sulfuric acid solution during the production of the
aqueous solution may be
100 g/L or more.
[0105] For example, the sulfuric acid solution production step S52 may be
perfoimed at a
reaction temperature in the range of 80 degrees C to 100 degrees C for about
20 hours or more.
[0106] Copper Removal Step S62
[0107] The copper removal step S62 is a step of removing copper (Cu) from the
solution by
adding sodium hydrogen sulfide (NaSH) to the solution. Copper may be
precipitated as a
copper sulfide (CuS) compound through a reaction indicated by the following
Reaction Formula
9.
[0108] [Reaction Fonnula 91
[0109] 2CuSO4 + 2NaSH = 2CuS + Na2SO4 +H2SO4
[0110] Copper sulfide (CuS) can be precipitated at a pH of 1.0 or more. To
this end, in the
copper removal step S62, the pH of the solution may be maintained at 1.0 to
2.5. In one
embodiment, the pH of the solution in the copper removal step S62 may be
maintained at 1.0 to
1.5. When the pH in the solution is less than 1.0, it is difficult to remove
copper from the
13
Date Regue/Date Received 2024-03-06
solution at 20 mg/L or less. When the pH is greater than 2.5, the solubility
of cobalt in sulfuric
acid is lowered, and cobalt may be lost.
[0111] On the other hand, sodium hydrogen sulfide (NaSH) may be added slowly
so that the pH
in the solution does not change rapidly. For example, sodium hydrogen sulfide
(NaSH) may be
added over about 3 hours while stirring the leachate. Accordingly, it is
possible to prevent an
increase in the cobalt loss rate due to a rapid increase in pH in some regions
of the solution.
[0112] In one embodiment, sodium hydrogen sulfide (NaSH) may be added in an
amount greater
than 4.5 equivalents (eq) and less than 5.5 equivalents (eq) of copper
content. When the
addition amount of sodium hydrogen sulfide (NaSH) is 4.5 equivalents (eq) or
less, it is difficult
to sufficiently remove copper from the solution because the copper removal
rate is 95% or less.
When the addition amount of sodium hydrogen sulfide (NaSH) is 5.5 equivalents
(eq) or more,
the cobalt recovery rate may decrease because the cobalt removal rate is 0.05%
or more.
[0113] By performing the copper removal step S62 for 3 hours or more at a
reaction temperature
in the range of 50 degrees C to 70 degrees C, it is possible to reduce cobalt
precipitation in the
filtrate and separate only copper through selective precipitation. Sodium
hydrogen sulfide may
be a product having a concentration of 30wt% to 70wt%.
[0114] Second Extraction step S72
[0115] The second extraction step S72 is a step of adding a second solvent
extractant to the
solution and separating the solution into a third filtrate containing cobalt
and impurities and a
second organic layer containing zinc and impurities.
[0116] In this regard, the second solvent extractant is not particularly
limited as long as the
cobalt loading rate thereof is low. For example, the second solvent extractant
may be D2EHPA
(di-(2-ethylhexyl)phosphoric acid).
[0117] When the second solvent extractant is added to the solution, cobalt may
be not loaded
into the second solvent extractant and may be distributed to the third
filtrate (Raffinate), and
zinc, magnesium, manganese, etc. are distributed and separated or extracted to
the organic layer
together with the second solvent extractant. This separation or extraction may
occur through a
reaction represented by the following Reaction Formula 10.
14
Date Regue/Date Received 2024-03-06
[0118] [Reaction Formula 10]
[0119] 2HR(org.) + ZnSO4(aq.) ¨> ZnR2(org.) + H2SO4(aq.) (R is Co, etc.)
[0120] The second extraction step S72 may be performed for about 10 minutes or
longer at a pH
of greater than 2.4 and less than 3.2 and a temperature of 40 degrees C. By
satisfying the pH
range under the temperature condition, it is possible to increase the loading
rate of impurities
such as zinc and the like, lower the loading rate of cobalt, and efficiently
separate cobalt into the
filtrate.
[0121] In one embodiment, the ratio of the second solvent extractant (0) to
the aqueous solution
(A) can be controlled according to the concentration of the component to be
extracted in the
solution. For example, the ratio (0:A) of the second solvent extractant (0) to
the aqueous
solution (A) may range from 0.5:1 to 2:1. For example, the 0:A can be 1.5:1.
[0122] Precipitation Removal Step S82
[0123] The precipitation removal step S82 may be performed to remove
impurities such as
magnesium and the like remaining in the third filtrate. After the
precipitation removal step S82,
the third filtrate may be fed to the target material precipitation step S92.
[0124] Details of the precipitation removal step S82 may be understood by
referring to the
description of the precipitation removal step S31.
[0125] Target Material Precipitation Step 592
[0126] In the target material precipitation step S92, a neutralizing agent may
be added to the
third filtrate after the precipitation removal step S82.
[0127] For example, the neutralizing agent may be a basic material containing
sodium. For
example, the neutralizing agent may be sodium carbonate (Na2CO3).
[0128] After removing impurities such as magnesium and the like, nickel may be
precipitated in
the form of a cake through a reaction represented by the following Reaction
Formula 11 in the
target material precipitation step S41.
[0129] [Reaction Foimula 11]
[0130] 3CoSO4 + 3Na2CO3 +2H20 = CoCO3-2Co(OH)2 + 3Na2SO4 +3CO2
[0131] The target material precipitation step S92 may be performed for 4 hours
or more at a pH
Date Regue/Date Received 2024-03-06
of 8 or higher and a temperature in the range of 80 degrees C to 90 degrees C.
[0132] Since cobalt can be recovered through the target material precipitation
step S92, it is
possible to reduce the use of expensive organic solvents that have a risk of
explosion and fire.
Thus, it is possible to improve the operational stability and the
productivity, and reduce the
production costs.
[0133] Although not specifically shown in the drawings, some sodium components
may be
present in the precipitated cobalt cake. Therefore, the water-soluble sodium
components can be
removed by a washing step using pure water at the rear stage. In this case,
the production cost
can be reduced by reusing the removed sodium components in producing sodium
carbonate
(Na2CO3), which is a neutralizing agent.
[0134] Final Leaching Step S102
[0135] The final leaching step S102 is a step of producing an aqueous solution
containing high-
purity cobalt by removing components such as sodium and the like through
washing and
dissolving the cobalt cake in a sulfuric acid solution.
[0136] Details of the final leaching step S102 may be understood by referring
to the above
description of the final leaching step S51.
[0137] Experimental Example
[0138] (1) Quality of the MHP cake raw material used in the experiment
[0139] [Table 1]
Ni Co Mg Mn Zn
Content (wt%) 38.0 3.80 1.40 6.15 0.90
[0140] * The content not shown in the table is impurities (mostly present with
hydroxyl groups
attached thereto) (2) Metal content in the leaching filtrate after the
leaching step including the
two-stage atmospheric pressure heating leaching step
[0141] [Table 21
Ni Co Mg Mn Zn
Content (g/L) 100.1 10.3 5.11 9.87 2.33
[0142] Comparing Tables 1 and 2, it can be seen that the nickel and cobalt
contents in the
16
Date Regue/Date Received 2024-03-06
leaching filtrate subjected to the two-stage atmospheric pressure heating
leaching step are
increased. (3) Comparison of cobalt contents in the organic layer according to
the pH
conditions in the extraction step
[0143] Loading rates of the respective components in the organic layer at pH
5.0, 5.2, and 5.4
were compared in order to examine the optimal pH conditions for separating
nickel into an
aqueous filtrate (Raffinate) by loading cobalt and impurities (Mg, Mn, Zn,
etc.) into the organic
layer using 30%Cyanex272. The reaction was carried out at 40 degrees C for 10
minutes, and
the ratio of the organic layer to the aqueous solution was 1.5: 1. The loading
rate was expressed
as a relative ratio of the content of each component present in the organic
layer based on the
content of each component present in the leachate.
[0144] [Table 31
pH 5.0
Ni Co Mg Mn Zn
Loading rate (%) 1.18 94.8 68.2 96.3 99.9
pH 5.2
Ni Co Mg Mn Zn
Loading rate (%) 1.20 97.5 70.0 99.0 99.9
pH 5.4
Ni Co Mg Mn Zn
Loading rate (%) 1.55 97.8 70.5 99.2 99.9
[0145] Referring to Table 3, it can be seen that the difference between the
contents of Co and Ni
loaded in the organic layer is largest at pH 5.2, and the separation of Co and
Ni occurs best at the
pH of 5.2.
[0146] In the case of pH 5.0, the content of Ni loaded in the organic layer
was small, but the Co
loading was relatively poor. In the case of pH 5.4, the Co loading was
excellent, but the
separation of Ni was relatively poor.
[0147] (4) Comparison of the content of impurities (Mg and Ca) in the filtrate
according to the
addition amount of sodium fluoride in the precipitation removal step
17
Date Regue/Date Received 2024-03-06
[0148] In order to examine the optimal addition amount of sodium fluoride
(NaF) for
precipitating and removing impurities (Mg and Ca) in the filtrate, the sodium
fluoride (NaF) was
added at 2.0, 2.2, and 2.4 equivalents of Mg and Ca contents in the filtrate.
The contents of
impurities (Mg and Ca) in the filtrate were compared. The reaction was carried
out for 2 hours
at a temperature of 60 degrees C.
[0149] [Table 41
Filtrate 2.0 Equivalent 2.2 Equivalent 2.4
Equivalent
Added Added Added
Mg Ca Mg Ca Mg Ca Mg Ca
Content mg/L 1,024 184 252.2 68.2 204.8 55.1 205.0
54.9
[0150] Referring to Table 4, it can be seen that the sum of the contents of Mg
and Ca in the
filtrate is smallest when sodium fluoride (NaF) is added at 2.2 equivalents.
(5) Comparison of
metal contents (Co, Cu, Zn, Mn, and Mg) in the cobalt cake according to the
addition amount of
sodium hydrogen sulfide (NaSH) in the impurity removal step
[0151] In order to examine the optimal conditions for stripping cobalt loaded
by Cyanex272 in
the organic layer with a sulfuric acid solution and then precipitating and
recovering cobalt in the
form of sulfide using sodium hydrogen sulfide (NaSH), the sodium hydrogen
sulfide (NaSH)
was added at 1.0, 1.3, and 1.6 equivalents of cobalt and zinc contents, and
the precipitated cake
contents were compared. The reaction was carried out for 3 hours at a
temperature of 85
degrees C and a pH of 4.5 to 5Ø
[0152] [Table 51
1.0 Equivalent Added
Co Cu Zn Mn Mg
Content (%) 35.2 0.08 7.76 2.82 0.12
1.3 Equivalent Added
Co Cu Zn Mn Mg
Content (%) 37.0 0.04 8.76 2.59 0.05
18
Date Regue/Date Received 2024-03-06
1.6 Equivalent Added
Co Cu Zn Mn Mg
Content (%) 37.0 0.05 8.72 2.52 0.03
[0153] Referring to Table 5, it can be seen that the cobalt content in the
cake is high when NaSH
is added at 1.3 equivalents, and the cobalt content does not increase any more
even when NaSH
is added in excess of 1.3 equivalents.
[0154] (6) Comparison of copper removal rates according to the addition amount
of sodium
hydrogen sulfide (NaSH) in the copper removal step
[0155] In order to examine the optimal conditions for precipitating and
removing copper in the
form of CuS by adding NaSH to the aqueous solution containing cobalt after
dissolving the
cobalt precipitate in a sulfuric acid solution, NaSH was added at 4.5, 5.0,
and 5.5 equivalents of
the copper content, and the removal rates of copper were compared. The
reaction was carried
out for 3 hours at a temperature of 60 degrees C and a pH of 1Ø The removal
rates were
indicated by comparing the contents of respective components present in the
aqueous solution
before and after adding NaSH.
[0156] [Table 61
4.5 Equivalent Added
Co Cu Zn Mn Mg
Removal rate (%) 0.04 94.2 0.03 1.00 1.92
5.0 Equivalent Added
Co Cu Zn Mn Mg
Removal rate (%) 0.04 96.5 0.01 1.01 2.00
5.5 Equivalent Added
Co Cu Zn Mn Mg
Removal rate (%) 0.05 96.3 0.02 1.08 2.21
[0157] Referring to Table 6, it can be seen that the removal rate of Cu is
highest when NaSH is
added at 5.0 equivalents.
19
Date Regue/Date Received 2024-03-06
[0158] (7) Comparison of cobalt contents in the organic layer according to pH
conditions in the
extraction step
[0159] In order to examine the optimal pH conditions for loading zinc in the
aqueous solution
into the organic layer and separating zinc by using 30% D2EHPA as a solvent
extractant for the
aqueous solution containing cobalt from which cobalt has been precipitated and
removed, the
loading rates of the respective components at pHs of 2.4, 2.8, and 3.2 were
compared. The
reaction was carried out at a temperature of 40 degrees C for 10 minutes. The
ratio of the
organic layer to the aqueous solution was 1.5: 1.
[0160] [Table 71
pH 2.4
Co Zn Mn Mg
Loading rate (%) 0.02 99.6 68.2 30.8
pH 2.8
Co Zn Mn Mg
Loading rate (%) 0.10 100 75.0 40.0
pH 3.2
Co Zn Mn Mg
Loading rate (%) 0.38 100 80.2 43.3
[0161] Referring to Table 7, it can be seen that the difference between the
contents of Zn and Co
loaded in the organic layer is largest under the pH 2.8 condition, and the
separation of Co and Zn
occurs best under the pH 2.8 condition.
[0162] In the case of pH 2.4, the content of Co loaded in the organic layer
was small, but the Zn
loading was relatively poor. In the case of pH 3.2, the a loading was
excellent, but the
separation of Co was relatively poor.
[0163] (8) Metal contents in the aqueous solution containing nickel/cobalt
that have undergone
the final washing step to remove impurities
[0164] [Table 81
Metal Content in Final Aqueous Solution Containing Nickel After Impurity
Removal
Date Regue/Date Received 2024-03-06
Ni(g/L) Co Mg Mn Zn
Content (mg/L) 131.3 17.9 18.9 2.87 2.88
[0165] [Table 9]
Metal Content in Final Aqueous Solution Containing Cobalt After Impurity
Removal
Co(g/L) Cu Zn Mn Mg
Content (mg/L) 110.6 3.63 3.38 12.0 11.4
[0166] Comparing Table 2 and Tables 8 and 9 together, it can be confirmed that
the purity of the
aqueous solution containing nickel/cobalt subjected to the steps of the
present invention is
increased. Although the embodiments of the present disclosure have been
described with
reference to the accompanying drawings, those skilled in the art to which the
present disclosure
pertains will be able to understand that the embodiments can be implemented in
other specific
forms without changing the technical spirit or essential features of the
present disclosure.
[0167] Therefore, it should be understood that the embodiments described above
are exemplary
and not limitative in all respects. The scope of the present disclosure is
defined by the claims
rather than the detailed description. It should be construed that all changes
or modified forms
derived from the meaning and scope of the claims and equivalent concepts
thereof are included
in the scope of the present disclosure.
21
Date Regue/Date Received 2024-03-06