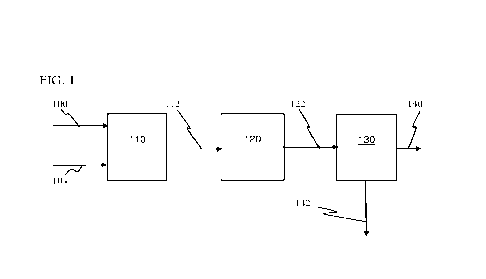Note: Descriptions are shown in the official language in which they were submitted.
WO 2022/214897
PCT/1B2022/052614
1
METHODS OF REMOVING CHLORIDE FROM GYPSUM HAVING HIGH LEVEL OF
CHLORIDE SALT
FIELD OF THE INVENTION
[0001] This application claims priority from US non-provisional
patent application no.
17/643,523 filed December 9, 2021, which claims the benefit of U.S.
Provisional Application
No. 63/171,624 filed April 7, 2021, which is herein incorporated by reference.
[0002] The present invention relates to a method for removing
chloride from synthetic
gypsum and other gypsum sources having high chloride salt concentrations by
treating the
synthetic gypsum and other gypsum sources having high chloride salt
concentrations with beads,
prior to using the gypsum source to form the board core layer, to improve
adhesion of the board
core layer (gypsum core) to a back cover sheet relative to a gypsum board that
is the same except
that it lacks the beads. The present invention also provides a wall system for
employing the
gypsum board.
BACKGROUND OF THE INVENTION
[0003] In the construction of buildings, one of the more common building
elements for
construction and remodeling is gypsum wallboard, often known as drywall,
gypsum boards,
gypsum panels, gypsum paneling, and ceiling tiles. In chemical terms, gypsum
is calcium sulfate
dihydrate (CaSO4=2H20).
[0004] Set gypsum is a well-known material that is used in such
products. Panels containing
set gypsum are often referred to as gypsum boards, which contain a board core
layer (set gypsum
core) sandwiched between two cover sheets, particularly paper cover sheets.
Such panels are
commonly used in drywall construction of the interior walls and ceilings of
buildings. One or
more denser regions, often referred to as "skim coats," may be included as
layers on either face
of the board core layer, usually at an interface (bond surface) between the
board core layer and
an inner surface of a cover sheet. The denser regions may be contiguous with a
less dense region
of the gypsum core following setting of the gypsum.
[0005] During manufacture of a gypsum board, stucco (containing
calcium sulfate
hemihydrate), water, and other ingredients as appropriate may be mixed,
typically in a mixer to
form an aqueous gypsum slurry. The terms of art aqueous gypsum slurry or
aqueous slurry or
CA 03214292 2023- 10- 3
WO 2022/214897 PCT/1B2022/052614
2
gypsum slurry are typically employed for the slurry both before and after the
calcium sulfate
hemihydrate converts to calcium sulfate dihydrate. The gypsum slurry is formed
and discharged
from the mixer onto a moving conveyor carrying a first cover sheet, optionally
bearing a skim
coat. If present, the skim coat is applied upstream from the location where
the gypsum slurry is
discharged onto the first cover sheet. After applying the gypsum slurry to the
first cover sheet, a
second cover sheet, again optionally bearing a skim coat, is applied onto the
gypsum slurry to
form a sandwich assembly having a desired thickness. A forming plate, roller
or the like may aid
in setting the desired thickness. The gypsum slurry is then allowed to harden
by forming set
(i.e., rehydrated) gypsum through a reaction between the calcined gypsum and
water to form a
matrix of crystalline hydrated gypsum (i.e., calcium sulfate dihydrate, also
known as set
gypsum). The desired hydration of the calcined gypsum promotes formation of an
interlocking
matrix of set gypsum crystals, thereby imparting strength to the gypsum board.
Heat may be
applied (e.g., using a kiln) to drive off the remaining free (i.e., unreacted)
water to yield a dry
product. Then the set gypsum product is cut to form gypsum boards of desired
length.
[0006] Gypsum (calcium sulfate dihydrate and any impurities) suitable for
use in wallboard
may be obtained from both natural and synthetic sources, followed by further
processing.
[0007] Natural gypsum may be used by calcining its calcium sulfate
dihydrate to produce the
hemihydrate form. Gypsum from natural sources is a naturally occurring mineral
and can be
mined in rock form. Naturally occurring gypsum is a mineral that is typically
found in old salt-
lake beds, volcanic deposits, and clay beds. When it is mined, raw gypsum is
generally found in
the dihydrate form. Gypsum is also known as calcium sulfate dihydrate, terra
alba or landplaster.
In gypsum, there are approximately two water molecules of water associated
with each molecule
of calcium sulfate.
[0008] Plaster of Paris is also known as calcined gypsum, stucco,
calcium sulfate
hemihydrate, or calcium sulfate half-hydrate.
[0009] When calcium sulfate dihydrate from either source is heated
sufficiently, in a process
called calcining or calcination, the water of hydration is at least partially
driven off and there can
be formed either calcium sulfate hemihydrate (CaS040/2H20) (typically provided
in the material
commonly referred to as "stucco") or calcium sulfate anhydrite (CaSO4)
depending on the
CA 03214292 2023- 10- 3
WO 2022/214897 PCT/1B2022/052614
3
temperature and duration of exposure. As used herein, the terms "stucco" and
"calcined
gypsum" refer to both the hemihydrate and anhydrite forms of calcium sulfate
that may be
contained therein. Calcination of the gypsum to produce the hemihydrate form
takes place by
the following equation:
CaSO4-2H20¨>CaSO4-0.51120 1.5H20
[0010] Calcined gypsum is capable of reacting with water to form
calcium sulfate dihydrate,
which is a rigid product and is referred to herein as "set gypsum."
[0011] Gypsum may also be obtained synthetically (referred to as
"syngyp",
desulphurization gypsum or desulphogyspum or DSG in the art) as a by-product
of industrial
processes such as flue gas desulfurization from power plants, for example.
Natural or synthetic
gypsum can be calcined at high temperatures, typically above 150 C, to form
stucco (i.e.,
calcined gypsum in the form of calcium sulfate hemihydrate and/or calcium
sulfate anhydrite),
which may undergo subsequent rehydration to form set gypsum in a desired
shape, such as a
board.
[0012] Synthetic gypsum obtained from power plants is usually suitable for
use in gypsum
panels intended for construction projects. In particular, flue gas including
sulfur dioxide is wet
scrubbed with lime or limestone, which produces calcium sulfite in the
following reaction.
CaCO3+S02¨>CaS03+CO2
The calcium sulfite is then converted to calcium sulfate in the following
reaction.
CaS03+2H20+1/202¨>CaSO4=2H20
The hemihydrate form may then be produced by calcination in a similar manner
to that used for
natural gypsum.
[0013] However, many conventional coal-fired power plants are being
shut down in favor of
more environmentally friendly sources of energy. The shutdown of coal-fired
power plants has
created a growing shortage of synthetic gypsum suitable for producing gypsum
panels. Lower
quality synthetic gypsum is available from power plants and other sources, but
this alternatively
sourced gypsum often contains fairly high concentrations of extraneous salts,
particularly
magnesium or sodium salts, more particularly magnesium chloride and sodium
chloride. Small
amounts of potassium chloride and calcium chloride may also be present in
alternatively sourced
CA 03214292 2023- 10- 3
WO 2022/214897 PCT/1B2022/052614
4
synthetic gypsum. The extraneous salts can be problematic due to their
tendency to decrease
adhesion between the board core and the cover sheets, particularly a back
paper cover sheet.
[0014] US 2020/0055278 to Li et al discloses gypsum boards formed
from synthetic gypsum
and other gypsum sources having high chloride salt concentrations. Gypsum
boards include a
board core including set gypsum. A total concentration of the chloride anion
in the board core
ranges from about 500 ppm to about 3000 ppm, typically about 1000 ppm to about
3000 ppm,
based on weight of the calcium sulfate hemihydrate. An inner surface of a
front paper cover
sheet contacts a first face of the board core. An inner surface of a back
paper cover sheet
contacts a second face of the board core. A starch layer coats the inner
surface of at least one of
the front and back cover sheet. Methods of making the gypsum board, and a wall
system for
employing the gypsum boards, are also provided.
[0015] US 2020/0055277 to Hemphill et al discloses gypsum boards
formed from synthetic
gypsum and other gypsum sources having high chloride salt concentrations. The
gypsum boards
include a set gypsum board core layer between a front and back paper cover
sheets. The back
paper cover sheet has a plurality of perforations extending therethrough.
Methods of making the
gypsum boards, and a wall system for employing the gypsum boards, are also
provided. The
concentration of the chloride anion in aqueous gypsum slurry used to make the
set gypsum board
core layer and to perform the methods of the invention may range from about
500 ppm to about
3000 ppm by weight calcium sulfate hemihydrate, typically from about 500 ppm
to about 2000
ppm by weight calcium sulfate hemihydrate, more typically from about 500 ppm
to about 1500
ppm by weight calcium sulfate hemihydrate.
[0016] WO 2020/224120 Al discloses a high impurity ion content
desulfurized gypsum
paper-faced gypsum board and a manufacturing method therefor. The paper-faced
gypsum board
comprises a board core and protective paper outside the board core. Raw
materials of the board
core comprise 100 parts by weight of a desulfurized gypsum raw material and
0.5-10 parts by
weight of zeolite. The desulfurized gypsum raw material is selected from any
one or more of a
high-sodium desulfurized gypsum raw material, a high-magnesium desulfurized
gypsum raw
material, a high-potassium desulfurized gypsum raw material, and a high-
chlorine desulfurized
gypsum raw material. The zeolite is a modified zeolite; or, an adsorption
material is provided on
CA 03214292 2023- 10- 3
WO 2022/214897 PCT/1B2022/052614
one side of the protective paper in contact with the board core. Paragraph
[0014] discloses that
the adsorption material is selected from any one or more of zeolite,
diatomaceous earth, fly ash,
attapulgite clay, and bentonite. The desulfurized gypsum having high impurity
ion content is
used as a raw material of the paper-faced gypsum board. Paragraph [0013]
discloses that the
5 chloride ion content in the high-chloride desulfurization gypsum raw
material is w, 421 mg/kg <
w < 8000 mg/kg (421 ppm < w < 8000 ppm). The board core of the manufactured
paper-faced
gypsum board is asserted to not be stripped from the protective paper, and the
bonding effect is
asserted to be good.
[0017] High-salt is especially a problem for employing synthetic
gypsum from sources such
as waste from power plant flue gas desulfurization systems.
[0018] It will be appreciated that this background description has
been created by the
inventors to aid the reader, and is neither a reference to prior art nor an
indication that any of the
indicated problems were themselves appreciated in the art. While the described
principles can,
in some regards and embodiments, alleviate the problems inherent in other
systems, it will be
appreciated that the scope of the protected innovation is defined by the
attached claims, and not
by the ability of the claimed invention to solve any specific problem noted
herein.
BRIEF SUMMARY OF THE INVENTION
[0019] In one or more aspects of the invention, the invention
provides methods for preparing
a gypsum board from gypsum sources having significant quantities of one or
more extraneous
salts.
[0020] The present invention relates to methods for treating salt-
containing gypsum sources
containing calcium sulfate dihydrate and salts, particularly low-quality
synthetic gypsum, to
reduce the salt concentration. The invention particularly relates to methods
for pretreating salt-
containing gypsum sources containing appreciable quantities of extraneous
salts, particularly
chloride salts, and more particularly NaC1, KC1, MgC12 and/or CaCl2. The
method removes salts
from salt-containing gypsum sources containing calcium sulfate dihydrate and
salts by treating
the salt-containing gypsum sources with salt removing beads prior to calcining
the gypsum
source. The calcining converts the calcium sulfate dihydrate of the gypsum
source into calcium
sulfate dihydrate to produce a stucco which contains the calcium sulfate
hemihydrate.
CA 03214292 2023- 10- 3
WO 2022/214897 PCT/1B2022/052614
6
[0021] The invention mixes high salt gypsum (containing calcium
sulfate dihydrate) powder
particles, as received, which naturally contain a small amount (e.g. 5-30
wt.%) of free moisture
with beads which are dry (have an absence of moisture, in other words 0%
water) or may have
up to 30 wt.% free moisture, preferably 10 wt. % to 20 wt.% free moisture. By
"as received- is
meant the high salt gypsum as received from a power plant (in the instance of
syngyp) or other
source. The beads are supplied dry but preferably extra water is added to
increase its moisture
percentage before use in the method of the invention. This makes the method of
the invention
more efficient to absorb salt from the high salt gypsum powder particles. The
mix ratio of the
beads to the high salt gypsum powder particles is in the range of 5 to 50
parts by weight beads
(including their free moisture) to 100 parts by weight high salt gypsum powder
particles
(including its free moisture).
[0022] In the context of beads, an as received condition (also
termed "as-received beads)
means beads exposed to ambient conditions. The beads typically employed in the
present
invention will absorb water molecules when they are exposed in the ambient
condition.
Depending on the exposed ambient condition, the free moisture in the beads
will vary. In
general, the beads contain <1% free moisture when kept in 75 F/30-40%RH. As
received beads
can be directly used for the present invention or they can be exposed to
humidified conditions or
otherwise wetted prior to use.
[0023] The invention uses beads made from inorganic materials such
as activated alumina
beads, zeolite beads, and/or silica gel beads to absorb chloride salts, for
example. The sizes of the
individual beads and the individual particles of gypsum powder do not overlap.
In particular, the
beads are bigger than the particles of gypsum powder. After mixing for a short
time, the mixture
is dried, and the treated gypsum powder particles are separated from the
mixture by using a sieve
or other physical separation apparatus. This results in separated amounts of
the treated gypsum
powder and the salt-laden beads. The separated treated gypsum may then be used
for wallboard
production. The separated beads are regenerated by washing for reuse in the
method of the
invention. The separated beads may be washed for example with water. The salt
adsorption
method can be repeated multiple times.
CA 03214292 2023- 10- 3
WO 2022/214897
PCT/1B2022/052614
7
[0024] It was found that the beads remove a large amount of
chloride salts from the high-salt
gypsum and produce a low-salt gypsum useful for gypsum wallboard production.
[0025] The invention reduces the gypsum salt concentration from a
high level, for example,
greater than 3000 ppm chloride salt, to a lower salt concentration, for
example less than 600 ppm
chloride salt, preferably less than 300 ppm chloride salt, to be suitable for
gypsum wallboard
manufacture.
[0026] Thus, the invention provides a method of treating a salt-
containing gypsum source
comprising salt-containing gypsum powder particles, wherein the treating of
the salt-containing
gypsum powder particles comprises the steps of:
mixing chloride salt absorbing beads, which have an absence of moisture or up
to 30%
free moisture, typically 5-30 wt.% free moisture, preferably 10% - 20% free
moisture, with salt-
containing gypsum powder particles, which contain 5-30 wt.% of free moisture,
typically 10
wt.% to 20 wt. % free moisture, preferably 15 wt.% to 20 wt.% free moisture,
for a time in a
range of 5 minutes to 5 hours, preferably 30 minutes to 2 hours, at a mix
ratio of the chloride salt
absorbing beads to the high salt gypsum powder particles in a range 5 to 50
parts by weight
beads to 100 parts by weight high salt gypsum particles on a moisture
inclusive basis, to transfer
chloride salt from the salt-containing gypsum powder particles to the chloride
salt absorbing
beads to produce a mixture of' salt laden chloride salt absorbing beads and
treated gypsum
powder particles, wherein the salt laden chloride salt absorbing beads are all
larger in particle
size than the treated gypsum powder particles;
wherein the salt-containing gypsum powder particles comprise at least 80 wt.
%,
preferably at least 90 wt. %, calcium sulfate dihydrate on a dry basis,
wherein the salt-containing gypsum powder particles comprise greater than 300
parts by
weight chloride anion, typically about 500 parts by weight to about 3000 parts
by weight
chloride anion, per 1,000,000 parts by weight said salt-containing gypsum
powder particles on a
dry basis,
wherein the salt-containing gypsum powder particles have a D50 median particle
size of
10 to 100 microns, preferably median particle size of 30 to 50 microns,
CA 03214292 2023- 10- 3
WO 2022/214897 PCT/1B2022/052614
8
wherein the chloride salt absorbing beads comprise inorganic material selected
from
activated alumina, zeolite, and/or silica gel,
wherein the chloride salt absorbing beads have a D50 median particle size of
0.5 mm to 5
mm, preferably 1 to 4 mm or 2 to 4 mm, and
after said mixing, drying the mixture of the salt laden chloride salt
absorbing beads and
the treated gypsum powder particles and separating the treated gypsum powder
particles from the
salt laden chloride salt absorbing beads to recover the treated gypsum powder
particles and
recover the salt laden chloride salt absorbing beads.
[0027] The term bead in the context of the present invention, may
be in the form of balls,
extruded pieces or the like. Beads may be rounded. The beads generally have a
length to
diameter ratio of 1-3: I. The beads generally have a volume mean diameter, or
a mean length
(largest dimension when it is not spherical), of particle size of 0.5 mm to 5
mm, preferably 1-4 or
more preferably 2-4 mm. Each bead can be made of thousands of small particles.
These small
particles can themselves be porous or nonporous. These small particles are
bonded to form a
"porous" bead. The term porous bead is meant to be a bead having a "porous"
structure created
from void spaces between the small particles and, if the small particles are
also porous, the pores
of the small particles themselves. The interstitial surface area of the voids
and pores of the beads
contributes to the specific surface area of the beads.
[0028] Specific surface area (SSA) is a property of solids defined
as the total surface area of
a material per unit of mass (5), (with units of m2/kg or m2/g) or solid or
bulk volume (Si) (units
of m2/m3 or in-1). The specific surface area based on the solid volume is
denoted by So.
Typically the beads employed in the present invention have a specific surface
area (S) of >20
m2/g, more typically >50 m2/g, furthermore typically >100 m2/g, preferably
>200 m2/g. A
number of international standards exist for the measurement of specific
surface area, including
ISO standard 9277 which is suitable for measuring specific surface area of
beads of the present
invention.
[0029] Fine materials will exhibit much greater specific surface
area than will coarse
materials. Some fine porous materials contain an enormous specific surface
area. For example
the specific surface area of sandstone may be in the order of 1500 cm2/cm3.
The specific surface
CA 03214292 2023- 10- 3
WO 2022/214897 PCT/1B2022/052614
9
area of a porous material is affected by porosity, by mode of packing, by the
grain size and by
the shape of the grains. For example, disc shaped particles will exhibit a
much larger specific
area than will spherical ones.
[0030] The concentration of the chloride anion in the gypsum source
(salt-containing
gypsum powder particles) treated in the methods of the invention may range
from greater than
300 parts by weight chloride anion, typically about 500 parts by weight to
about 3000 parts by
weight, per 1,000,000 parts by weight of the salt-containing gypsum powder
particles on a dry
basis. The concentration of the chloride anion in the gypsum source is more
typically from about
800 parts by weight to about 2000 parts by weight, and further typically from
about 1000 parts
by weight to about 1500 parts by weight, per 1,000,000 parts by weight of the
salt-containing
gypsum powder particles on a dry basis. Gypsum having about 500 parts by
weight to about
3000 parts by weight chloride anions on a dry basis means about 500 parts by
weight to about
3000 parts by weight chloride anions without free moisture or any other water
per 1,000,000
parts by weight gypsum without free moisture or any other water.
[0031] The chloride anion in the gypsum source used for methods and
products of the
invention may arise from any source. The gypsum source may be a synthetic
gypsum source,
particularly a low-quality synthetic gypsum obtained from a power plant flue
gas stream.
Typically, the one or more chloride salts are any of NaC1, KC1, MgCl2, CaCl2,
or any
combination thereof
[0032] The salt adsorption method can be repeated multiple times to remove
successively
more salt from the gypsum particles. Thus, all or a portion of the separated
treated gypsum
particles can be recycled for additional treating with additional salt-
absorbing beads. Also, the
separated salt-absorbing beads may be regenerated by washing for reuse in the
method of the
present invention. The separated salt-absorbing beads may be washed for
example with water.
[0033] The separated treated gypsum may then be calcined into stucco. The
stucco may be
used to make gypsum board by mixing with water to form an aqueous gypsum
slurry and then
forming the slurry into the shape of a board while allowing it to set such
that the calcium sulfate
hemihydrate converts to calcium sulfate dihydrate of the formed gypsum board.
CA 03214292 2023- 10- 3
WO 2022/214897
PCT/1B2022/052614
[0034] In the present specification, a moisture inclusive basis
means including free moisture.
Free moisture is water that adheres to the gypsum structure or the chloride
salt absorbing bead
but is not chemically bound in the gypsum structure or the chloride salt
absorbing bead. This free
moisture can be removed by air-drying at temperature lower than 110 F. Free
moisture is
5 generally water that is naturally present in or on the salt-containing
gypsum powder particles or
the chloride salt absorbing beads. Typically the moisture from the salt-
containing synthetic
gypsum powder particles is from the flue gas desulfurization process from
which they originated.
However, the humidity present in the air of the surrounding natural atmosphere
may contribute.
The moisture from the chloride salt absorbing beads is due to the humidity
present in the air of
10 the surrounding natural atmosphere. However, the salt-containing gypsum
powder particles or
the chloride salt absorbing beads can be wetted, for example by spraying, with
added water to
each achieve up to 30 wt.% moisture. For example, if salt-containing gypsum
powder particles
have 30 wt.% moisture then, for 100 parts by weight of the salt-containing
gypsum powder
particles, 70 parts by weight is gypsum on a dry (water free) basis and 30
parts by weight is
water. For example, if the chloride salt absorbing beads are dry (has 0 wt.%
moisture) then, for
100 parts by weight of the chloride salt absorbing beads, 100 parts by weight
is chloride salt
absorbing beads on a dry (water free) basis and 0 parts by weight is water.
[0035] Unless specified otherwise, when the specification indicates
a dry basis this is a water
free basis. Thus, a dry basis is also a moisture free basis.
[0036] All average molecular weights, percentages and ratios used herein,
are by weight (i.e.,
wt. %) unless otherwise indicated. When the specification indicates D50 it is
Dn50 which is
number D50. As is known in the art Dn50 is known as number median, it
physically represents
that each number of particles greater or smaller than such value takes account
of 50% of the total
particles number.
[0037] Advantages of the present invention may become apparent to those
having ordinary
skill in the art from a review of the following detailed description, taken in
conj unction with the
examples, and the appended claims. It should be noted, however, that while the
invention is
susceptible of various forms, the present disclosure is intended as
illustrative, and is not intended
to limit the invention.
CA 03214292 2023- 10- 3
WO 2022/214897
PCT/1B2022/052614
11
BRIEF DESCRIPTION OF THE DRAWINGS
[0038] FIG. 1 shows a process flow diagram of the present method.
DETAILED DESCRIPTION OF THE INVENTION
[0039] The present invention provides the ability to treat chloride laden
gypsum to remove at
least a portion of the chloride and produce a treated gypsum having a lower
level of chloride than
prior to treatment.
[0040] The treated gypsum may be used in the board core layer of a
gypsum board. Under
ordinary circumstances, high salt concentrations in the board core layer may
result in insufficient
adhesion between the board core layer and at least one of the front cover
sheet and the back
cover sheet, particularly the back cover sheet. Treating the gypsum according
to the invention to
remove the chloride containing salt assists to solve this problem.
METHOD OF REMOVING CHLORIDE FROM GYPSUM
[0041] FIG. 1 shows a process flow diagram of a method of the present
invention. A stream
of salt-containing gypsum powder particles 100 and a stream of chloride salt
absorbing beads
104 feed a mixer 110, such as a shaker, in which they are mixed dry. Mixing
dry means the
gypsum powder particles and the beads are mixed with at most the free moisture
water adhering
to the gypsum powder particles and to the beads. They are not mixed in a
liquid medium. For
example, they are not mixed in an aqueous or non-aqueous slurry. Typically the
gypsum powder
particles and the beads are mixed for a time in a range of 5 minutes to 5
hours, preferably 30
minutes to 2 hours. During mixing a portion of chloride salt on the gypsum
particles transfers to
the beads. Then the mixed gypsum particles and beads discharge from the mixer
110 as a stream
of a mixture of treated gypsum particles and chloride laden beads 112.
[0042] The stream of the mixture of treated gypsum particles and chloride
laden beads 112
feeds a dryer 120 that removes any water adhering to the gypsum particles and
the beads. The
dryer 120 may be a kiln, an oven, hot air dryer, or other dryer. Then a stream
of dried gypsum
particles and the beads 122 discharges from the dryer 120 and feeds a physical
separation device
CA 03214292 2023- 10- 3
WO 2022/214897
PCT/1B2022/052614
12
130. A typical physical separation device 130 comprises a sieve. However, an
air classifier or
other physical separation device 130 may be employed. In the physical
separation device 130
the treated gypsum particles, which are smaller than the salt-laden beads, are
separated from the
beads. This produces a salt laden bead stream 140 and a treated gypsum
particles stream 142.
[0043] The salt adsorption method can be repeated multiple times. Thus, all
or a portion of
the separated treated gypsum 142 can be recycled for additional treating with
additional salt-
absorbing beads by the method of FIG. 1.
[0044] After separation the salt laden beads 140 may then be
regenerated by washing (not
shown) to remove the chloride, optionally dried, and then recycled for reuse
in the method of
FIG. 1 to treat gypsum particles. The salt laden salt-absorbing beads 140 may
be washed, for
example, by mixing with water, or otherwise cleaned, to remove the chloride
salt.
[0045] BEADS
[0046] The beads may be any one or more of activated alumina,
zeolites, and silica gel. The
beads typically have a D50 median particle size of 0.5 ¨ 5 mm, preferably 1-4
mm or 2-4 mm.
The beads typically have a specific surface area of >20 m2/g, more typically
>50 m2/g,
furthermore typically >100 m2/g or preferably >200 m2/g. As mentioned above,
each bead can
be made of thousands of small particles. These small particles can be
nonporous or porous
materials. Activated alumina and silica gel particles are nonporous, but
zeolite particles are
porous.
The chloride salt absorbing beads fed to the method are all larger in particle
size than the salt-
containing gypsum powder particles fed to the method. Also, after mixing the
chloride salt
absorbing beads and the salt-containing gypsum powder particles, the resulting
salt laden
chloride salt absorbing beads are all larger in particle size than the treated
gypsum powder
particles. Thus, the particle size of each bead is larger than the particle
size of each gypsum
powder particle.
SILICA GEL BEADS
[0047] Silica gel is a granular, vitreous, porous form of silicon
dioxide made synthetically
from sodium silicate. Silica gel contains a nano-porous silica micro-structure
suspended in a
CA 03214292 2023- 10- 3
WO 2022/214897
PCT/1B2022/052614
13
liquid. Silica gel beads, commonly used for removing moisture from packaging
containers, may
be calibrated with a coating of mineral salts to absorb or release humidity in
various RH ranges,
providing a buffering effect on relative humidity. This action referred to as
two-way humidity
control. Porous silica has a sponge structure, from which results a very high
specific surface area,
that varies greatly with pore size (from 20 to 750 m2/g). Typical silica gels
have surface area >
100 m2/g.
[0048] ACTIVATED ALUMINA BEADS
[0049] Activated alumina is a highly porous form of aluminum oxide.
Activated aluminate
beads have a high specific surface area due to the many "tunnel like" pores
that they have. Any
suitable, activated alumina may be used. Suitable activated alumina is
characterized as workable,
or dehydrated with a loss on ignition (LOI) characteristic of preferably less
than or equal to 20,
and most preferably, an LOT of less than or equal to 10. The activated alumina
may be
manufactured by any process that produces a very large surface area on each
particle of alumina,
and the large surface area may be manifested by a very rough surface
characterized by small pits,
voids, and other surface irregularities. These surface irregularities may be
effective at capturing
small particles that impinge on the surface. Preferably, the activated alumina
may be
manufactured in such a way that the surface has a net negative electrical
charge, thereby
allowing positively charged ions, such as certain metals, to attach themselves
to the activated
alumina.
[0050] Activated alumina has a large specific surface area and is
active in a reaction such as
decomposition, isomerization, hydrogenation, dehydrogenation and dehydration.
It is, therefore,
generally used as a catalyst or catalyst support. Activated alumina is
generally prepared by
extracting alumina from an alumina-rich mineral such as bauxite, kaolin, acid
white clay and
colloidal clay; converting the alumina into alumina hydrate by hydrolysis or
neutralization; and
then activating the hydrate. Activated alumina contains 0 to 0.5 moles of
water per one mole of
A1203. The content varies depending on a process temperature during heating
and dehydrating
alumina trihydrate which is a starting material of the activated alumina.
CA 03214292 2023- 10- 3
WO 2022/214897
PCT/1B2022/052614
14
[0051] In a Bayer process, the most typical process of the
industrial preparation processes for
alumina, bauxite as a starting material is ground and the resultant powder is
treated with a hot
solution of sodium hydroxide to elute alumina as sodium aluminate, during
which the
substantially whole amounts of impurities such as iron oxides, silica and
titanium oxide are
separated as an insoluble residue. If there exists alkali-soluble silica, it
reacts with alkali and
alumina to form an alkali aluminosilicate hydrate which is insoluble. The
residue is removed by
filtration and the filtrate, a sodium aluminate solution, is appropriately
diluted. To the solution, a
seed of gibbsite, which is crystalline alumina trihydrate, is added at an
appropriate temperature.
While stirring the mixture, aluminum hydroxide is precipitated. The
precipitate is collected by
filtration, washed with water and dried to give sodium-rich alumina trihydrate
(gibbsite). The
alumina trihydrate can be heated and dehydrated to give various activated
aluminas containing 0
to 0.5 moles of water per 1 mole of A1203. In the course of conversion into a-
alumina as
anhydrous alumina by dehydration, there exist seven types of metastable
aluminas, generally
called activated alumina structures, including kappa-, theta-, delta, eta-,
chi- and rho-alumina
structures in addition to a typical gamma-alumina structure (See, for example,
Publication
Department, Kaken Research Center Management Development Center "Novel High
Performance Adsorbents (Experimental Data Collection)", p. 361, published on
Apr. 5, 1976).
[0052] A specific surface area in activated alumina is generally
about 100 to 400 m2/g. In
case that an average pore radius is 2.5 nm to 8.0 nm both inclusive, for
example, a pore volume
range is 0.125 to 0.4 mL/g when a specific surface area is 100 m2/g, and a
pore volume range is
0.5 to 1.6 mL/g when a specific surface area is 400 m2/g. Activated alumina in
which a specific
surface area and a pore volume are within these ranges may be suitably used in
the present
invention.
[0053] ZEOLITE BEADS
[0054] Typical zeolites suitable for the invention are commercially
available 5A and 13X
zeolite beads with the size of 2-4 mm. However, the invention can also use
other types of zeolite
as long as they are "bead" shape. The zeolite particle itself has a rigid, 3-
dimensional crystalline
CA 03214292 2023- 10- 3
WO 2022/214897
PCT/1B2022/052614
structure (similar to a honeycomb) having a network of interconnected tunnels
and cages. These
tunnels and cages are nearly uniform, allowing the crystal to act as a
molecular sieve.
[0055] Typical zeolite may comprise one or more zeolites such as
"type-A zeolite", FAU
zeolites (LSX, MSX, X, Y), LTA zeolites, CHA zeolites (chabazite), offretite,
erionite,
5 mordenite, gmelinite, mazzite, }MU zeolites (clinoptilolite), ZSM-3, EMT,
EMC-2, ZSM-18,
ZK5, ZSM-5, ZSM-11, Zeolite Beta, Zeolite type L, and mixtures of two or more
of them, and
more preferably from LSX, MSX, X, and X zeolites, and mixtures of two or more
of them.
Typical zeolite may comprise typically 12X, 3A, 4A and 5A zeolites, and
mixtures of two or
more of them. The various types of zeolites present in the zeolite are
determined by XRD. The
10 amount of zeolites is also measured by XRD and is expressed as % by
weight relative to the total
weight of the zeolite adsorbent material.
[0056] In the present description, the term "type-A zeolite"
denotes an LTA zeolite. Typical
zeolite may comprise type-A zeolite chosen from 3A, 4A and 5A zeolites. The
term "3A" is
intended to mean a zeolite of which the pore opening is equal to approximately
3 angstroms; the
15 term "4A" is intended to mean a zeolite of which the pore opening is
equal to approximately 4
angstroms; and the term "5A" is intended to mean a zeolite of which the pore
opening is equal to
approximately 5 angstroms.
[0057] The zeolite may comprise at least one cation chosen from the
ions of groups IA, IIA,
IIIA, IB, JIB and IIIB of the periodic table, the trivalent ions of the
lanthanide or rare earth
series, the zinc (II) ion, the silver (I) ion, the cupric (II) ion, the
chromium (III) ion, the ferric
(III) ion, the ammonium ion and/or the hydronium ion, the preferred ions being
calcium, lithium,
sodium, potassium, barium, cesium, strontium, zinc and rare-earth ions. The
zeolite that can be
used in the context of the present invention may comprise at least one alkali
or alkaline-earth
metal chosen from sodium, calcium, lithium, and mixtures of two or three of
them in any
proportion.
[0058] The agglomerated and formed zeolite adsorbent materials
prepared according to any
techniques known to those skilled in the art, such as extrusion, compacting,
agglomeration on a
granulating plate or granulating drum, atomization and the like. The
proportions of
CA 03214292 2023- 10- 3
WO 2022/214897
PCT/1B2022/052614
16
agglomeration binder and of zeolites used are typically those of the prior
art, that is to say
between 5 parts and 30 parts by weight of binder per 95 parts to 70 parts by
weight of zeolite.
[0059] The zeolite that can be used in the context of the present
invention, whether it is in the
form of balls, extruded pieces or the like, generally has a volume mean
diameter, or a mean
length (largest dimension when it is not spherical), of particle size of 0.5
mm to 5 mm, preferably
1-4 or more preferably 2-4 mm. Typically the zeolite beads have a specific
surface area of >50
1112/g, more typically >100 m2/g.
[0060] CHLORIDE SALTS
[0061] The gypsum to be treated contains chloride anions. The chloride
anions may arise
from one or more chloride salts from any source. Generally, the one or more
chloride salts are
present in the gypsum source from which the gypsum particles were obtained.
The gypsum
source may be a synthetic gypsum source, particularly a low-quality synthetic
gypsum obtained
from a power plant flue gas stream. Such a low-quality gypsum source may not
otherwise be
suitable for forming a wall board without using at least one starch layer,
according to the present
invention.
[0062] The concentration of the chloride anion in the gypsum of
gypsum feed stream 104
(FIG. 1) may range from greater than 300 parts by weight chloride anion,
typically about 500
parts by weight to about 3000 parts by weight, more typically from about 800
parts by weight to
about 2000 parts by weight, and further typically from about 1000 parts by
weight to about 1500
parts by weight per 1,000,000 parts by weight said salt-containing gypsum
powder particles on a
dry basis. Gypsum having about 500 parts by weight to about 3000 parts by
weight chloride
anions on a dry basis means about 500 parts by weight to about 3000 parts by
weight chloride
anions for 1,000,000 parts by weight gypsum without free moisture or any other
water.
[0063] Chloride salts are any salts which contain chloride. Thus, they
include monovalent
salts of chloride anion and a monovalent cation, such as sodium or potassium.
Thus, they include
divalent salts of chloride anions and a divalent cation, such as calcium or
magnesium. Other
chloride salts, are also contemplated, such as trivalent salts of chloride
anions and a trivalent
CA 03214292 2023- 10- 3
WO 2022/214897
PCT/1B2022/052614
17
cation. Generally, the one or more chloride salts may be selected from the
group consisting of
NaCl, KC1, MgCl2, CaC12 and any combination thereof.
[0064] Typically the method removes a sufficient amount of these
chloride salts to produce
treated gypsum particles having a chloride anion concentration which removes
at least 25 wt. %,
for example 50 to 99 wt% of the chloride anion from the gypsum particles fed
to the method.
Preferably the method removes at least 70 wt. %, for example, 75 to 95% wt.%
of the chloride
anion from the gypsum particles fed to the method.
[0065] In particular, the method removes at least 25 wt. %,
typically 25 to 99 wt.%, for
example 50 to 99 wt% or 25 to 50 wt.%, of the chloride anion from the gypsum
particles fed to
the method per pass through the method. By recycling all or a portion of the
treated gypsum to
be retreated, for instance one or two more times, according to the method of
the invention, this
can reduce chloride anion concentration in the gypsum by over 75% relative to
the chloride
anion concentration in the original gypsum prior to any treating according to
the invention.
Typically the method is run as a batch mode.
[0066] USES OF THE TREATED GYPSUM
[0067] The synthetic gypsum and other gypsum particles that have
been treated to reduce
their high chloride salt concentrations according to the present invention may
be calcined to
convert the calcium sulfate dihydrate in the treated gypsum into stucco. This
stucco may be
employed in methods for preparing a gypsum board comprising mixing the stucco
with water to
make an aqueous gypsum slurry containing the calcium sulfate hemihydrate, and
then depositing
the aqueous gypsum slurry onto a gypsum board manufacturing line and allowing
the deposited
aqueous gypsum slurry to set to produce a core layer of the gypsum board.
[0068] In the manufacture of wallboard, stucco can be first mixed
with dry additives such as
perlite, starch, fiberglass, vermiculite or other additives known in the art.
This dry mix can be
combined with water, soap foam, accelerators and shredded paper, or pulpwood
in a mixer at the
head of a board forming line. The slurry is then spread between 2 paper sheets
that serve as a
mold. The edges of the paper can be scored, and sometimes chamfered, to allow
precise folding
of the paper to form the edges of the board. As the wet board travels the
length of a conveying
CA 03214292 2023- 10- 3
WO 2022/214897
PCT/1B2022/052614
18
line, the calcium sulfate hemihydrate combines with the water in the slurry to
form solid calcium
sulfate dihydrate, or gypsum, resulting in rigid board. The board is typically
rough-cut to length,
and it typically enters a multideck kiln dryer, where it is dried. The dried
board is typically
conveyed to a board end sawing area and trimmed and bundled for shipment
[0069] The calcium sulfate hemihydrate is present in the deposited aqueous
slurry in
amounts of at least 60 wt. % of the dry (water-free) materials of the aqueous
slurry. Preferably
the calcium sulfate hemihydrate is at least 70 wt. % of the dry (water-free)
materials of the
aqueous slurry, more preferably at least 80 wt. % of the dry (water-free)
materials of the aqueous
slurry. In typical wallboard formulations of the invention the dry (water-
free) materials of the
aqueous slurry have at least 90 wt. % or at least 95 wt. % calcium sulfate
hemihydrate. Use of
calcium sulfate anhydrite is also contemplated, although it is preferably used
in small amounts of
less than 20 wt. % of the dry (water-free) materials of the aqueous slurry.
[0070] Typically, the aqueous gypsum slurry has less than 10 wt. %,
more typically an
absence, of Portland cement or other hydraulic cement on a dry (water-free)
basis. Typically, the
aqueous gypsum slurry has less than 10 wt. %, more typically an absence, of
fly ash on a dry
(water-free) basis. Typically, the aqueous gypsum slurry has less than 10 wt.
%, more typically
an absence, of calcium carbonate on a dry (water-free) basis. For purposes of
this disclosure a
dry basis is a water-free basis.
[0071] The typical gypsum boards comprise a board core layer
comprising:
a board core layer comprising set gypsum;
a front paper cover sheet having an outer surface and an inner surface, the
inner surface
contacting a first face of the board core layer; and
a back paper cover sheet having an outer surface and an inner surface, the
inner surface
contacting a second face of the board core layer;
wherein the board core layer is disposed between the front paper cover sheet
and the back
paper cover sheet; and
wherein the board core layer resulted from setting an aqueous slurry
comprising water
and stucco between the first cover sheet and the second cover sheet, wherein
the stucco
comprises calcium sulfate hemihydrate, and the aqueous slurry comprises at
least 60 weight
CA 03214292 2023- 10- 3
WO 2022/214897
PCT/1B2022/052614
19
percent said calcium sulfate hemihydrate on a dry (water free) basis, and the
water at a weight
ratio of water to the calcium sulfate hemihydrate of 0.2:1 to 1.2:1.
[0072] One or both of the cover sheets may be paper cover sheets,
which may be the same or
different paper materials. Optionally, various additives known in the art may
be present in the
board core layer or a gypsum slurry used to form the board core layer. The
board core layer may
further comprise one or more high-density regions (layers) in contact with the
inner surface of
the front cover sheet or the back cover sheet and coated thereon. The one or
more high-density
regions may be in contact with a low-density interior of the board core layer.
[0073] CLAUSES OF THE INVENTION
[0074] The following clauses disclose various aspects of the
invention.
[0075] Clause 1. A method of treating a salt-containing gypsum
source comprising salt-
containing gypsum powder particles, comprising:
mixing chloride salt absorbing beads, which have an absence of moisture or up
to 30%
free moisture, typically 5-30 wt.% free moisture, preferably 10% - 20% free
moisture, with salt-
containing gypsum powder particles, which contain 5-30 wt.% of free moisture,
typically 10
wt.% to 20 wt. % free moisture, preferably 15 wt.% to 20 wt.% free moisture,
for a time in a
range of 5 minutes to 5 hours, preferably 30 minutes to 2 hours, at a mix
ratio of the chloride salt
absorbing beads to the high salt gypsum powder particles in a range 5 to 50
parts by weight
beads to 100 parts by weight high salt gypsum particles on a moisture
inclusive basis, to transfer
chloride salt from the salt-containing gypsum powder particles to the chloride
salt absorbing
beads to produce a mixture of salt laden chloride salt absorbing beads and
treated gypsum
powder particles, wherein the salt laden chloride salt absorbing beads are all
larger in particle
size than the treated gypsum powder particles;
wherein the salt-containing gypsum powder particles comprise at least 80 wt.
%%,
preferably at least 90 wt. %, calcium sulfate dihydrate on a dry basis,
wherein the salt-containing gypsum powder particles comprise greater than 300
parts by
weight chloride anion, typically about 500 parts by weight to about 3000 parts
by weight
CA 03214292 2023- 10- 3
WO 2022/214897
PCT/1B2022/052614
chloride anion, per 1,000,000 parts by weight said salt-containing gypsum
powder particles on a
dry basis,
wherein the salt-containing gypsum powder particles have a D50 median particle
size of
10 to 100 microns, preferably D50 median particle size of 30 to 50 microns,
5 wherein the chloride salt absorbing beads comprise inorganic material
selected from
activated alumina, zeolite, and/or silica gel,
wherein the chloride salt absorbing beads have a D50 median particle size of
0.5 mm to 5
mm, preferably 1 to 4 mm or 2 to 4 mm, and
after said mixing, drying the mixture of the salt laden chloride salt
absorbing beads and
10 the treated gypsum powder particles and separating the treated gypsum
powder particles from the
salt laden chloride salt absorbing beads to recover the treated gypsum powder
particles and
recover the salt laden chloride salt absorbing beads.
[0076] Clause 2. The method of clause 1, wherein the beads have a
surface area of >20
15 m2/g, more typically >50 m2/g, furthermore typically >100 m2/g,
preferably >200 m2/g.
[0077] Clause 3. The method of any of the preceding clauses,
wherein the salt-containing
gypsum powder particles have about 500 parts by weight to about 3000 parts by
weight said
chloride anion per 1,000,000 parts by weight of said gypsum particles fed to
the mixer.
[0078] Clause 4. The method of any of the preceding clauses,
wherein the free moisture in
20 the beads fed to the mixer is in the range between 10 and 20% by weight.
[0079] Clause 5. The method of any of the preceding clauses,
wherein the free moisture in
the gypsum fed to the mixer is in the range between 15 and 20% by weight.
[0080] Clause 6. The method of any of the preceding clauses,
wherein the beads and the
gypsum particles are mixed at a mix ratio of the beads to the gypsum particles
in a range 10 to 50
parts by weight beads (including their free moisture) to 100 parts by weight
gypsum particles
(including their free moisture).
[0081] Clause 7. The method of any of the preceding clauses,
further comprising contacting
the chloride laden beads with water to remove chloride from the chloride laden
beads to produce
cleaned beads.
CA 03214292 2023- 10- 3
WO 2022/214897
PCT/1B2022/052614
21
[0082] Clause 8. The method of any of clauses 1 to 7, wherein the
beads comprise activated
alumina.
[0083] Clause 9. The method of any of clauses 1 to 7, wherein the
beads comprise silica
gel.
[0084] Clause 10. The method of any of clauses 1 to 7, wherein the beads
comprise zeolite.
[0085] Clause 11. The method of any of the preceding clauses,
wherein the chloride salt
absorbing beads and the treated gypsum powder particles are separated by
passing the mixture of
the salt laden chloride salt absorbing beads through a sieve.
[0086] Clause 12. The method of any of clauses 7 to 11, further
comprising recycling the
cleaned beads as bead feed to the mixer.
[0087] Clause 13. The method of any of the preceding clauses,
further comprising recycling
the treated gypsum particles as gypsum particles feed to the mixer.
[0088] Clause 14. The method of clause 10, wherein the zeolite is
chosen from zeolites type
X, zeolites type A, zeolites type Y, FAU zeolites (LSX, MSX, X, Y), LTA
zeolites, CHA
zeolites (chabazite), offretite, erionite, mordenite, gmelinite, mazzite, HEU
zeolites
(clinoptilolite), ZSM-3, EMT, EMC-2, ZSM-18, ZK5, ZSM-5, ZSM-11, Zeolite Beta,
Zeolite
type L, and mixtures of two or more of them.
[0089] Clause 15. The method of clause 10, wherein the beads
comprise zeolite 13X or
zeolite 5A.
[0090] Clause 16. The method of any of the preceding clauses, wherein the
gypsum is
formed from synthetic gypsum comprising one or more chloride salts and said
one or more
chloride salts provide at least a portion of said chloride anions.
[0091] The following examples further illustrate the invention but,
of course, should not be
construed as in any way limiting its scope.
[0092] EXAMPLES
[0093] Raw Materials:
CA 03214292 2023- 10- 3
WO 2022/214897
PCT/1B2022/052614
22
[0094] Four types of porous materials are tested. 1) Activated
Alumina (AA) Beads: 2-5 mm
bead size with surface are of >300 m2/g surface area; 2) Silica gel (SiO2)
beads: 3 mm bead size
with surface area of 500 m2/g surface; 3) Zeolite 13X beads: 2-4 mm bead size
with surface area
of >400 m2/g; 4) Zeolite 5A: 2-4 mm bead size with surface area >300 m2/g.
[0095] High-Salt Synthetic Gypsum: (Syngyp) has chemical and physical
compositions
listed in TABLE 1.
[0096]
TABLE 1
Sample Description High Salt Syngyp
Potassium (K+) 67.4 ppm
Sodium (Na+) 206.3ppm
Magnesium (Mg2+) 518.6 ppm
Chloride (Cl-) 2045.2 ppm
Free Moisture % 11 . 5%
Purity % 97.1%
[0097] Analytical Test Methods:
[0098] 1. Pretreatment of beads:
[0099] All of the beads are dried at 350 F for 4 hours prior to the
use. "Wet- beads can be
made by keeping them in a humidified condition, such as in a 90 F and 90%
relative humidity
(RH) room, or directly spraying water on them. Although not used in the
examples, beads could
have been be used in the as-received form as well. The examples showed that
the dried beads and
the "wetted- beads achieved beneficial results. The as-received beads are in
between, in
moisture content, "dried" and "wetted" beads. Thus, the inventors predict the
as-received beads
would also achieve beneficial results.
[00100] 2. Pretreatment of High-salt Syngyp powder:
[00101] In all the examples, As-received High-Salt Syngyp powder (which may
also be
termed "As-is High-Salt Syngyp") contains 11.5 wt.% of free moisture. It can
be directly mixed
with the beads, or a small amount of extra water is added to increase its free
moisture percentage
before mixing with the beads.
CA 03214292 2023- 10- 3
WO 2022/214897
PCT/1B2022/052614
23
[00102] 3. Mixing in Gyro Mixer
[00103] The weight ratio of the beads and the High-salt Syngyp powder is
between 10% and
30%, and the mixing time is between 5 and 30 minutes. The gyroscopic mixing
simultaneously
spins containers which contain the high-salt Syngyp particles and the beads
vertically and
horizontally to accomplish the mixing.
[00104] 4. Drying and Separation
[00105] The mixtures of beads and the High-salt Syngyp powder are dried at 110
F
overnight, then the beads and the Syngyp powder are separated by using No. 20
Mesh sieve.
[00106] 5. Chloride Measurement
[00107] Chloride test strips (available from HACH Company, Loveland, Colorado)
are used
to measure chloride levels before and after the absorption treatment.
[00108] Results:
[00109] 1. Effect of types of beads on chloride reduction. TABLE 2
lists the effect of
different beads on chloride reduction. It is shown that all the beads absorb
the chloride from the
as-received High-Salt Syngyp powder and reduce the chloride level in the High-
Salt Syngyp
powder. AA beads seem most effective among all the beads.
[00110]
TABLE 2: Effect of different types of beads on the chloride reduction of High-
salt
Syngyp powder
As-received
Dried Bead Beads Mixing Time Chloride in
High-salt
Types Weight (g) Syngyp (g) (min) Syngyp (ppm)
150 2020
AA 30 150 5 1422
SiO2 30 150 5 1437
Zeolite 13X 30 150 5 1487
Zeolite 5A 30 150 5 1510
[00111] 2. Effect of "wet" AA beads on chloride reduction. TABLE 3 lists the
effect of
moisture percentage of AA beads on the chloride reduction. It is shown that
when the dried AA
beads become the "wet" beads either by absorbing moisture in 90 F/90%RH
condition or simply
mixing them with water, the wet beads absorb more chloride than the dried
beads. The optimized
CA 03214292 2023- 10- 3
WO 2022/214897
PCT/1B2022/052614
24
H20% in AA beads is in the range between 10% and 20%. Note that in TABLE 3
Sample ID is
listed with wt.% moisture. For example in Table 3, 9.9% AA means activated
alumina with 9.9
wt. % moisture. This nomenclature is also used in other examples of this
specification. In all the
Examples, % is wt. % unless otherwise indicated.
[00112]
TABLE 3: Effect of moisture wt.% of AA beads on chloride reduction
Dried Wet AA in Wet AA by H20% Mixing
[Cl]
Sample ID
AA (g) 90F/90%RH (g) spray (g)
to AA Time (min) (ppm)
AS-received
High-Salt 30 - - - -
2020
Syngyp
0% AA 30 30 - 0.0 5
1422
9.9% AA 30 30 - 9.9 5
1219
10.5% AA 30 33.53 - 10.5 5
1000
15.7% AA 30 35.57 - 15.7 5
925
16.6% AA 30 35.99 - 16.6 5
925
27.1% AA 30 41.13 - 27.1 5
1129
10% AA 30 - 33 10 5
1129
15% AA 30 - 34.5 15 5
999
20% AA 30 - 36 20 5
1045
[00113] 3. Effect of mixing time on chloride reduction. TABLE 4 lists the
effect of mixing
time on chloride reduction. It is shown that the chloride level in the High-
salt Syngyp is reduced
with increasing the mixing time.
CA 03214292 2023- 10- 3
WO 2022/214897
PCT/1B2022/052614
[00114]
TABLE 4: Effect of mixing time on the chloride reduction
As-received
S Dried Sprayed H20% H h-Salt Mixing
[C1]
ample ID ig
AA (g) H20 (g) to AA Time (min)
(ppm)
Syngyp (g)
5 min -
4.5 15% 150 5 999
15% AA
10 min -
30 4.5 15% 150 10
894
15% AA
20 min -
30 4.5 15% 150 20
718
15% AA
30 min -
30 4.5 15% 150 30
575
15% AA
[00115] 5. Effect of mix ratio of AA to high-salt Syngyp on chloride
reduction. TABLE 5
lists the effect of the mix ratio of AA beads to the High-Salt Syngyp on
chloride reduction. It is
5 shown that the chloride concentration in the High-Salt Syngyp reduces
when the mix ratio of the
beads to the powder is increased.
[00116]
TABLE 5: Effect of mix ratio of AA to Syngyp on chloride reduction
As-received High- Mixing Time
Sample ID Dried AA (g)
[Cl] (ppm)
Salt Syngyp (g) (min)
10% AA 15 150 5 1662
20% AA 30 150 5 1422
30% AA 45 150 5 1045
[00117] 6. Effect of "wet" High-salt Syngyp on chloride reduction. TABLE 6
lists the effect
10 of free moisture percentage of the High-salt Syngyp on chloride
reduction. It is shown that when
the high-salt Syngyp contains 16-18% free moisture, more chloride can be
absorbed than the As-
received High-Salt Syngyp (11.5% free moisture). If the -wet" AA beads are
mixed with the
"wet" High-salt Syngyp, such as 16% High-salt Syngyp-15%AA, more chloride can
be further
absorbed than the dried AA beads mixed with the wet High-salt Syngyp, such as
16% High-salt
15 Syngyp-O%AA.
CA 03214292 2023- 10- 3
WO 2022/214897
PCT/1B2022/052614
26
[00118]
TABLE 6. Effect of Free moisture of Syngyp on the chloride reduction
H20 As- H20
Dried AA received added 1120% Mixing
[C1-]
added [C1 ]
AA
Sample ID AA . H20 High-Salt in in Time
reduction
in (PPm)
(g) % Syngyp
Syngyp Syngyp (min)
(g) (g) (g)
As-is
High-salt 150 2061
Syngyp
As
received
High-salt 30 0 0 150 0 11.5% 5 1422 31.0%
Syngyp -
0%AA
As-
received
High-salt 30 4.5 15 150 0 11.5% 5 999 51.5%
Syngyp -
15%AA
16% High-
salt
30 0 0 150 8.04 16.0% 5 960 53.4%
Syngyp -
0%AA
16% High-
salt
30 4.5 15 150 8.04 16.0% 5 847 58.9%
Syngyp -
15%AA
18% High-
salt 30 0 0 150
11.9 18.0% 5 847 58.9%
Syngyp
[00119] 7. Effect of AA absorption cycles on the chloride reduction. TABLE 7
lists the
effect of three cycles on the chloride reduction. After lst cycle of AA
absorption, the chloride
concentration is reduced from 2020 to 920 ppm. After 2nd cycle, the chloride
concentration is
CA 03214292 2023- 10- 3
WO 2022/214897
PCT/1B2022/052614
27
reduced from 920 to 478 ppm. After 3rd cycle, the chloride concentration is
reduced from 478 to
276 ppm, lower than 300 ppm.
[00120]
TABLE 7. Effect of AA absorption cycles on the chloride reduction
H20
added
H20 High-
Dried AA in High- Mixing Cl
Sample added salt
AA H20 High- salt time
[Cl]
me
reduction
ID in AA Syngyp (ppm)
(g) %wt salt Syngyp (min)
(g) (g) Syngyp H20%
(g)
As
received
High- 0 0 0% 150 0 0 2020
Salt
Syngyp
1st Cycle 30 4.5 15% 150 8 16% 5 847
58.9%
2nd 133 (1St
30 4.5 15% Cycle 25 16% 5 478 76.3%
Cycle
powder)
3rd 133 (2nd 16%
30 4.5 15% Cycle 25 5 276 86.3%
Cycle
powder)
*Containing 11.5 wt.% free moisture
[00121] Variations of the specifically disclosed invention may become apparent
to those of
ordinary skill in the art upon reading the foregoing description. The
inventors expect skilled
artisans to employ such variations as appropriate, and the inventors intend
for the invention to be
practiced otherwise than as specifically described herein. Accordingly, this
invention includes
all modifications and equivalents of the subject matter recited in the claims
appended hereto as
permitted by applicable law. Moreover, any combination of the above-described
elements in all
possible variations thereof is encompassed by the invention unless otherwise
indicated herein or
otherwise clearly contradicted by context.
CA 03214292 2023- 10- 3
WO 2022/214897
PCT/1B2022/052614
28
[00122] All references cited herein are hereby incorporated by reference to
the same extent as
if each reference were individually and specifically indicated to be
incorporated by reference and
were set forth in its entirety herein.
[00123] The use of the terms "a- and "an- and "the- and "at least one- and
similar referents in
the context of describing the invention (especially in the context of the
following claims) are to
be construed to cover both the singular and the plural, unless otherwise
indicated herein or
clearly contradicted by context. The use of the term "at least one" followed
by a list of one or
more items (for example, "at least one of A and B") is to be construed to mean
one item selected
from the listed items (A or B) or any combination of two or more of the listed
items (A and B),
unless otherwise indicated herein or clearly contradicted by context. "Bonding
relation" does not
mean that two layers are in direct contact. The terms "comprising," "having,"
"including," and
containing" are to be construed as open-ended terms (i.e., meaning "including,
but not limited
to,") unless otherwise noted. Recitation of ranges of values herein are
intended to serve as a
shorthand method of referring individually to each separate value falling
within the range, unless
otherwise indicated herein, and each separate value is incorporated into the
specification as if it
were individually recited herein. All methods described herein can be
performed in any suitable
order unless otherwise indicated herein or otherwise clearly contradicted by
context. The use of
any and all examples, or exemplary language (e.g., "such as") provided herein,
is intended
merely to better illuminate the invention and does not pose a limitation on
the scope of the
invention unless otherwise claimed. No language in the specification should be
construed as
indicating any non-claimed element as essential to the practice of the
invention.
[00124] The following claims also constitute disclosure of the
present invention.
CA 03214292 2023- 10- 3