Note: Descriptions are shown in the official language in which they were submitted.
CA 03214315 2023-09-20
I 0 e
.
=
DESCRIPTION
METHOD FOR PRODUCING IRON ORE PELLETS
[TECHNICAL FIELD]
[0001]
The present invention relates to a method for producing iron ore pellets.
[BACKGROUND ART]
[0002]
As a blast furnace operation, a method is well-known in which pig iron is
produced
by: alternately stacking, in a blast furnace, a first layer containing an iron
ore material, and a
second layer containing coke; and injecting an auxiliary reductant into the
blast furnace from
a tuyere and melting the iron ore material by using resulting hot blasts. In
this method for
producing pig iron, the iron ore material, being supplied as iron ore pellets,
is reduced,
whereby the pig iron is produced. At this time, the coke functions as a
reduction agent and
serves as a spacer to secure gas permeability.
[0003]
The iron ore pellets need to have high reducibility in order to improve
production
efficiency of pig iron. As iron ore pellets having improved reducibility, for
example, iron ore
pellets obtained by adding dolomite to make a CaO/SiO2 mass ratio greater than
or equal to 0.8
and a MgO/SiO2 mass ratio greater than or equal to 0.4 are known (see Japanese
Unexamined
Patent Application Publication No. H1-136936). The aforementioned publication
further
discloses that increasing porosity of the iron ore pellets can improve
reducibility.
[PRIOR ART DOCUMENTS]
[PATENT DOCUMENTS]
[0004]
Patent Document 1: Japanese Unexamined Patent Application Publication No. H1-
136936
[SUMMARY OF THE INVENTION]
[PROBLEMS TO BE SOLVED BY THE INVENTION]
[0005]
In light of a recent increase in awareness of the environmental problems, a
reduction
in emission of CO2 as the greenhouse gas, specifically an operation with a low
reduction agent
ratio, is required also in a blast furnace operation. In this case, since
pulverization of the iron
ore pellets in the blast furnace and the like leads to lowered gas
permeability, a large amount of
coke as a spacer for ensuring gas permeability needs to be charged. An
increased charged rate
of coke as a reduction agent increases the reduction agent ratio, whereby an
operation with a
low reduction agent ratio is difficult. Therefore, in order to carry out an
operation with a low
reduction agent ratio, the iron ore pellets need to have a high crushing
strength so as not to be
pulverized.
1
CA 03214315 2023-09-20
1 if (
* 1
[0006]
However, adding dolomite tends to lower the crushing strength. In
addition,
increasing the porosity of the iron ore pellets necessarily lowers the
crushing strength.
[0007]
The present invention was made in view of the foregoing circumstances, and an
objective thereof is to provide a method for producing iron ore pellets
superior in reducibility
and high in crushing strength.
[MEANS FOR SOLVING THE PROBLEMS]
[0008]
The present inventors have thoroughly investigated iron ore pellets obtained
by adding
dolomite to increase reducibility, and found that adding dolomite treated to
be present in a
miniaturized state in a pellet structure prior to firing increases crushing
strength. Although an
exact reason is not clear, the present inventors infer that, by subjecting
dolomite to a
predetermined treatment, MgO derived from the dolomite is present in a
miniaturized state in
the iron ore pellets, whereby an effect of increasing a bonding strength of
the pellet structure of
the iron ore pellets is produced during firing. In other words, the bonding
strength of the pellet
structure is considered to be increased due to the fact that: MgO being
miniaturized increases
reactivity of MgO and facilitates generation of a magnesioferrite compound,
thus contributing
to bonding of the pellet structure; and/or MgO having a low bonding strength
that may be an
origin of fracture of the pellet is miniaturized and less likely to be the
origin of fracture.
[0009]
In other words, according to an aspect of the present invention, a method for
producing iron ore pellets used for operation of a blast furnace and in which
a CaO/SiO2 mass
ratio is greater than or equal to 0.8 and a MgO/SiO2 mass ratio is greater
than or equal to 0.4
includes: balling green pellets by adding, to an iron ore material and
dolomite, water for use
in the balling; and firing the green pellets, in which the dolomite has a
characteristic of being
present in a miniaturized state in a structure of the green pellets.
[0010]
The method for producing iron ore pellets enables increasing crushing strength
of the
iron ore pellets to be produced, by adding dolomite that is present in a
miniaturized state in a
structure of the green pellets prior to firing and produces an effect of
increasing the bonding
strength of the pellet structure of the iron ore pellets. In addition, in the
iron ore pellets
produced by the method for producing iron ore pellets, a CaO/SiO2 mass ratio
is greater than
or equal to 0.8 and a MgO/SiO2 mass ratio is greater than or equal to 0.4,
resulting in high
reducibility.
[0011]
It is preferred that the method for producing iron ore pellets further
includes preparing
the dolomite, in which in the preparing, the dolomite is pulverized such that
a Blaine specific
2
CA 03214315 2023-09-20
) (
surface area is greater than or equal to 4,000 cm2/g. Due to the Blaine
specific surface area of
the dolomite being greater than or equal to the lower limit, the dolomite is
miniaturized and
integrated into the pellet structure. As a result, reactivity of dolomite can
be increased, and
MgO can be inhibited from functioning as an origin of fracture in the iron ore
pellets to be
produced. Therefore, the bonding strength of the pellet structure of the iron
ore pellets is
increased, whereby the crushing strength of the iron ore pellets can be
increased. As used
herein, a "Blaine specific surface area" means a value obtained by measurement
in accordance
with JIS-R-5201:2015, and, in a case in which a target object is composed of a
plurality of
powders, indicates a minimum value for an individual powder.
[0012]
It is preferred that the method for producing iron ore pellets further
includes preparing
the dolomite, wherein the dolomite is calcined at a temperature greater than
or equal to 900 C
in the preparing. As used herein, "calcination" means a heat treatment process
of heating a
solid such as ore to cause thermal decomposition and phase transition, and to
remove volatile
components. Dolomite is a carbonate mineral and represented by CaMg(CO3)2.
When
dolomite is calcined, the following reaction takes place
CaCO3 -> CaO + CO2, MgCO3 -> MgO + CO2
and dolomite is thermally decomposed. At a phase of balling, water is added to
Mg0
generated by the calcination, resulting in a transformation into Mg(OH)2 and
miniaturization
(dolomite having a large grain size is reduced). As a result, reactivity of
dolomite can be
increased, and MgO which is generated in the firing and can function as an
origin of fracture
in the iron ore pellets to be produced can be miniaturized. Therefore, the
bonding strength
of the pellet structure of the iron ore pellets to be produced is increased,
whereby the crushing
strength of the iron ore pellets can be increased.
[0013]
The firing temperature in the firing preferably higher than or equal to 1,250
C.
Due to the firing temperature in the firing being higher than or equal to the
aforementioned
lower limit, the crushing strength can further be increased.
[EFFECTS OF THE INVENTION]
[0014]
As explained in the foregoing, by employing the method for producing iron ore
pellets according to the present invention, iron ore pellets superior in
reducibility and having
high crushing strength can be produced.
[BRIEF DESCRIPTION OF THE DRAWINGS]
[0015]
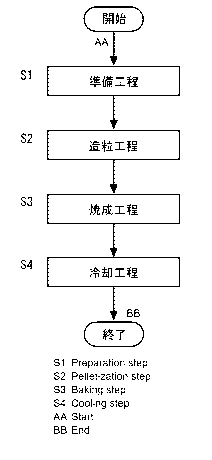
FIG. 1 is a flow chart illustrating a method for producing iron ore pellets
according to
an embodiment of the present invention.
3
CA 03214315 2023-09-20
1 oll e
,
,
FIG. 2 is a schematic view illustrating a structure of a production apparatus
used in
the method for producing iron ore pellets illustrated in FIG. 1.
FIG. 3 is a graph showing a grain size distribution of dolomite before and
after the
calcination.
FIG. 4 is a graph showing a relationship between the Blaine specific surface
area and
the crushing strength in EXAMPLES.
FIG. 5 is a graph showing a relationship between a rate of dolomite particles
having a
grain size of less than or equal to 20 1.1m and the crushing strength in
EXAMPLES.
[DESCRIPTION OF EMBODIMENTS]
[0016]
Hereinafter, the method for producing pig iron according to each embodiment of
the
present invention will be described.
[0017]
First Embodiment
The method for producing iron ore pellets illustrated in FIG. 1 includes a
preparing
step Si, a balling step S2, a firing step S3, and a cooling step S4. For
example, as illustrated
in FIG. 2, the method for producing iron ore pellets is used for operation of
a blast furnace, and
can produce iron ore pellets 1 in which a CaO/SiO2 mass ratio is greater than
or equal to 0.8
and a MgO/SiO2 mass ratio is greater than or equal to 0.4, by using a
production apparatus with
a grate kiln system (hereinafter, may be also merely referred to as
"production apparatus 2").
The production apparatus 2 includes: a pan pelletizer 3; a traveling grate
furnace 4; a kiln 5;
and an annular cooler 6.
[0018]
<Iron Ore Pellets>
The iron ore pellets 1 are obtained by balling and firing finely pulverized
ore to form
agglomerated ore having a great strength. Regarding production of the iron ore
pellets 1, it is
known that adding a CaO-containing compound such as limestone to an iron ore
material to
increase a CaO/SiO2 mass ratio in the iron ore pellets 1 improves reducibility
of the iron ore
pellets 1 (see Patent Document 1). On the basis of this finding, the present
method for
producing iron ore pellets produces the iron ore pellets 1 having the CaO/SiO2
mass ratio of
greater than or equal to 0.8.
[0019]
In a case in which the raw materials are iron ore (iron oxide) and limestone
(CaO-
containing compound), a calcium ferrite compound is generated by a solid phase
reaction
between CaO generated by the thermal decomposition and iron oxide in the
firing, and is
simultaneously bound through solid phase diffusion bonding at an interface
thereof. Since the
bonding is local, fine pores which were present prior to the firing are
retained even after the
firing, whereby the iron ore pellets I are porous bodies in which fine pores
are present relatively
4
CA 03214315 2023-09-20
A.
uniformly.
[0020]
During the blast furnace operation, a reducing gas enters the fine pores
diffusively,
whereby a reduction reaction proceeds from an outer surface to an inner
portion of the iron ore
pellets 1. Due to removal of oxygen from the iron oxide by the reduction
reaction, the existing
fine pores are enlarged and new fine pores are generated, while metallic iron
is generated. In
a process of shrinkage of an external shape of the iron ore pellets 1 due to
aggregation of the
metallic iron, the fine pores start to decrease. As a result, diffusion of the
reduction gas into
the iron ore pellets 1 is suppressed, whereby the reduction is likely to
stagnate.
[0021]
For suppressing this stagnation of the reduction, addition of a high-melting
point
component which suppresses loss of the fine pores during an aggregation
process of the metallic
iron is effective. It is known that particularly adding dolomite as a source
of MgO, which is
the high-melting point component, to increase a MgO/SiO2 mass ratio in the
iron ore pellets 1
enables obtaining a powerful effect of suppressing stagnation of the reduction
(see Patent
Document 1). On the basis of this finding, in the present method for producing
iron ore pellets,
the iron ore pellets 1 are produced having the MgO/SiO2 mass ratio of greater
than or equal to
0.4.
[0022]
It is preferred that the iron ore pellets to be produced are self-fluxing. Due
to the iron
ore pellets 1 being self-fluxing, melting down of reduced iron is likely to be
accelerated. Note
that the self-fluxing property of the iron ore pellets 1 is determined by an
auxiliary material
and/or the like.
[0023]
<Preparing Step>
In the preparing step Si, dolomite is prepared. In the present method for
producing
iron ore pellets, the dolomite has a characteristic of being present in a
miniaturized state in a
structure of green pellets P to be balled in the balling step S2 described
later. In the preparing
step Si, this characteristic is imparted to the dolomite. Specifically, in the
preparing step Si,
the dolomite is pulverized such that a Blaine specific surface area is greater
than or equal to a
predetermined value. Note that the pulverization can be carried out by using a
known
pulverizer.
[0024]
The predetermined value is preferably 4,000 cm2/g, and more preferably 6,000
cm2/g.
Increasing the specific surface area is considered to be substantially the
same as miniaturizing
the dolomite. Due to the miniaturization, reactivity of dolomite can be
increased, and MgO
can be inhibited from functioning as an origin of fracture in the iron ore
pellets 1 to be produced.
Therefore, the bonding strength of the pellet structure of the iron ore
pellets 1 to be produced is
CA 03214315 2023-09-20
, a f
,
1
increased, whereby the crushing strength of the iron ore pellets 1 can be
increased. Note that
an upper limit of the Blaine specific surface area of the pulverized dolomite
is not particularly
limited, but in view of production cost and the like, the Blaine specific
surface area of the
pulverized dolomite is less than or equal to 10,000 cm2/g.
[0025]
A lower limit of a percentage of particles having a grain size of less than or
equal to
20 gm in the pulverized dolomite is preferably 35% by volume, more preferably
45% by volume,
and further preferably 55% by volume. The percentage of particles having a
grain size of less
than or equal to 20 gm being greater than or equal to the lower limit
facilitates an increase in
the crushing strength of the iron ore pellets 1. Note that the "percentage of
particles having a
grain size of less than or equal to 20 gm" indicates a value obtained from a
grain size distribution
measured by a grain size distribution measurement apparatus (Microtrac).
[0026]
An upper limit of a D50 grain size of the pulverized dolomite is preferably 50
gm and
more preferably 20 gm. The D50 grain size of the dolomite being less than or
equal to the
upper limit facilitates an increase in the crushing strength of the iron ore
pellets 1. Note that
the "D50 grain size" indicates a value obtained from a grain size distribution
measured by a
grain size distribution measurement apparatus (Microtrac).
[0027]
<Balling Step>
In the balling step S2, green pellets P are balled by adding water for use in
the balling
to an iron ore material and the dolomite. As described above, an auxiliary
material such as
limestone may be added to obtain the CaO/SiO2 mass ratio of greater than or
equal to 0.8. The
MgO/SiO2 mass ratio can be adjusted mainly by the dolomite.
[0028]
Specifically, in the balling step S2, the water is added to the iron ore
material and the
dolomite, and then this water-containing mixture (the iron ore material and
the dolomite
containing the water) is charged into the pan pelletizer 3, serving as the
pelletizer, and rolled to
produce the green pellets P, having a ball shape.
[0029]
The iron ore material is a main material of the iron ore pellets 1, and
composed of
powder of the iron ore (for example, powder of which at least 90% by mass of
the total has a
grain size of less than or equal to 0.5 mm). Although surface characteristics
of the iron ore
vary greatly depending upon a mining region and a pulverizing/transporting
method, the surface
characteristics of the iron ore are not particularly limited in the present
method for producing
iron ore pellets.
[0030]
The water constitutes bridges between particles of the iron ore material.
Strength of
6
CA 03214315 2023-09-20
3 I ,
,
,
the green pellets P balled in the balling step S2 is maintained due to an
adhesion force acting
between the particles, resulting from this bridging. In other words, a bond
between the
particles is expressed by means of surface tension of the water between the
particles, and the
adhesion force between the particles is ensured by a value obtained by
multiplying the surface
tension by the number of points of contact between the particles.
[0031]
<Firing Step>
In the firing step S3, the green pellets P are fired. In the firing step S3,
the traveling
grate furnace 4 and the kiln 5 are used.
[0032]
Traveling grate furnace
As shown in FIG. 2, the traveling grate furnace 4 has: a traveling grate 41; a
drying
chamber 42; a dehydrating chamber 43: and a preheating chamber 44.
[0033]
The traveling grate 41 is configured to be endless, and the green pellets P
placed on
this traveling grate 41 can be transferred to the drying chamber 42, the
dehydrating chamber
43, and the preheating chamber 44, in this order.
[0034]
In the drying chamber 42, the dehydrating chamber 43, and the preheating
chamber 44,
the green pellets P are subjected to: drying by a heating gas Gl; dehydrating;
and preheating,
whereby preheated pellets H are obtained having strength, imparted to the
green pellets P,
sufficient to resist the rotation in the kiln 5.
[0035]
Specifically, the following procedure is followed. First, in the drying
chamber 42,
the green pellets P are dried at an atmospheric temperature of about 250 C.
Next, in the
dehydrating chamber 43, the green pellets P after the drying are heated to
about 450 C in order
to mainly decompose and remove combined water in the iron ore. Furthermore, in
the
preheating chamber 44, the green pellets P are heated to about 1,100 C,
whereby carbonate
contained in limestone, dolomite, and/or the like is degraded to remove carbon
dioxide, and
magnetite in the iron ore is oxidized. Accordingly, the preheated pellets H
are obtained.
[0036]
As shown in FIG. 2, the heating gas G1 used in the dehydrating chamber 43 is
reused
as the heating gas G1 in the drying chamber 42. Similarly, the heating gas G1
in the preheating
chamber 44 is reused as the heating gas G1 in the dehydrating chamber 43, and
a combustion
exhaust gas G2 used in the kiln 5 is reused as the heating gas G1 in the
preheating chamber 44.
By thus reusing the heating gas Gl, which is on the downstream side and has a
high temperature,
and the combustion exhaust gas G2, heating cost of the heating gas G1 can be
decreased. It is
to be noted that burner(s) may be provided in each chamber to control the
temperature of the
7
CA 03214315 2023-09-20
heating gas G1 . In FIG. 2, burners 45 are provided in the dehydrating chamber
43 and the
preheating chamber 44. Furthermore, the heating gas G1 used in the drying
chamber 42 is
finally discharged from a smokestack C.
[0037]
Kiln
The kiln 5 is directly connected to the traveling grate furnace 4, and is a
rotary furnace
having a sloped cylindrical shape. The kiln 5 fires the preheated pellets H
which are
discharged from the preheating chamber 44 of the traveling grate furnace 4.
Specifically, the
preheated pellets H are fired by combustion with a kiln burner (not shown in
the figure)
provided on an outlet side of the kiln 5. Accordingly, high-temperature iron
ore pellets 1 are
obtained.
[0038]
A lower limit of the firing temperature for firing the preheated pellets H is
preferably
1,250 C, and more preferably 1,300 C. Due to the firing temperature being
higher than or
equal to the aforementioned lower limit, the crushing strength can further be
increased. On
the other hand, the upper limit of the firing temperature is not particularly
limited, and may
be, for example, 1,500 C. When the firing temperature is higher than the
upper limit, the
effect of increasing the crushing temperature tends to be saturated and the
effect may be
insufficient with respect to the increase in the production cost. In addition,
in light of
reduction in a cohesion amount of the iron ore pellets 1 according to a rise
in temperature, the
upper limit is more preferably 1400 C.
[0039]
In the kiln 5, as air for combustion, an atmosphere serving as a cooling gas
G3 used in
the annular cooler 6 is used. Furthermore, the high-temperature combustion
exhaust gas G2
used for firing the preheated pellets H is sent to the preheating chamber 44
as the heating gas
Gl.
[0040]
<Cooling Step>
In the cooling step S4, the high-temperature iron ore pellets 1 obtained in
the firing
step S3 are cooled. In the cooling step S4, the annular cooler 6 is used. The
iron ore pellets
1 cooled in the cooling step S4 are accumulated and used in the blast furnace
operation.
[0041]
In the annular cooler 6, the iron ore pellets 1 can be cooled by blowing the
atmosphere
serving as the cooling gas G3 by using a blowing apparatus 61, while
transferring the high-
temperature iron ore pellets 1 discharged from the kiln 5.
[0042]
It is to be noted that the cooling gas G3, which was used in the annular
cooler 6,
resulting in an increase in temperature, is sent to the kiln 5 and used as the
air for combustion.
8
CA 03214315 2023-09-20
, 4 i
,
[0043]
<Advantages>
In the method for producing iron ore pellets, dolomite, being present in a
miniaturized state in a structure of the iron ore pellets 1 and producing an
effect of increasing
the bonding strength of the pellet structure of the iron ore pellets 1, is
added. Specifically,
due to the Blaine specific surface area of the dolomite being greater than or
equal to 4,000
cm2/g, the dolomite is miniaturized and integrated into the pellet structure.
As a result,
reactivity of dolomite can be increased, and MgO can be inhibited from
functioning as an
origin of fracture in the iron ore pellets 1 to be produced. Therefore, the
bonding strength of
the pellet structure of the iron ore pellets 1 is increased, whereby the
crushing strength of the
iron ore pellets 1 can be increased. In addition, in the iron ore pellets 1
produced by the
method for producing iron ore pellets, a CaO/SiO2 mass ratio is greater than
or equal to 0.8
and a MgO/SiO2 mass ratio is greater than or equal to 0.4, resulting in high
reducibility.
[0044]
Second Embodiment
According to another embodiment of the present invention, a method for
producing
iron ore pellets used for operation of a blast furnace and in which a CaO/SiO2
mass ratio is
greater than or equal to 0.8 and a MgO/Si02 mass ratio is greater than or
equal to 0.4, includes,
as illustrated in FIG. 1: a preparing step Si of preparing dolomite; a balling
step S2 of balling
green pellets by adding, to an iron ore material and the dolomite, water for
use in the balling; a
firing step S3 of firing the green pellets; and a cooling step S4 of cooling
the high-temperature
iron ore pellets obtained in the firing step S3. In addition, the dolomite has
a characteristic of
being present in a miniaturized state in a structure of the green pellets.
[0045]
In the method for producing iron ore pellets, the steps except for the
preparing step Si
are the same as the corresponding steps in the method for producing iron ore
pellets according
to the first embodiment. Hereinafter, the preparing step S1 is described and
description for
the other steps is omitted.
[0046]
<Preparing Step>
In the preparing step Si in the method for producing iron ore pellets, the
dolomite is
calcined at a temperature greater than or equal to a predetermined value. The
present inventors
have found that this treatment imparts to the dolomite a characteristic of
being present in a
miniaturized state in a structure of the green pellets, whereby the crushing
strength of the iron
ore pellets to be produced can be increased.
[0047]
The predetermined value is preferably 900 C, and more preferably 1,100 C.
Note
that an upper limit of a calcination temperature is not particularly limited,
but in view of
9
CA 03214315 2023-09-20
a
production cost and the like, the calcination temperature is less than or
equal to 1,500 C.
[0048]
The effect of enabling an increase in the crushing strength of the iron ore
pellets
produced by the calcination is discussed. Dolomite is a carbonate mineral and
represented by
CaMg(CO3)2. When dolomite is calcined, the following reaction takes place
CaCO3 -> CaO + CO2, MgCO3 -> MgO + CO2
and dolomite is thermally decomposed. At a phase of the balling step S3, water
is added to
MgO generated by the calcination, resulting in the following hydration
reaction
MgO + H20 -> Mg (OH)
to give magnesium hydroxide.
[0049]
The present inventors found that miniaturization of the dolomite proceeds in
the
calcined dolomite due to the hydration reaction. FIG. 3 shows results of
measurement of the
grain size distribution of the calcined dolomite by a Microtrac before and
after the hydration
reaction. As shown in FIG. 3, before the hydration reaction, no significant
change in grain
size is observed between the grain size distribution after the calcination and
that of non-
calcined dolomite after the hydration reaction; however, it can be observed
that the hydration
reaction causes a change in grain size, which is considered to be due to a
change in crystal
structure, and a reduction of large grain-size particles having, for example,
a grain size of
greater than 20 gm, in other words miniaturization, proceeds. Due to the
miniaturization,
reactivity of dolomite can be increased, and MgO which is generated in the
firing step and can
function as an origin of fracture in the iron ore pellets to be produced can
be miniaturized.
Therefore, the bonding strength of the pellet structure of the iron ore
pellets to be produced is
increased, whereby the crushing strength of the iron ore pellets can be
increased.
[0050]
A lower limit of a treatment time of the calcination is preferably 20 minutes,
more
preferably 50 minutes, and still more preferably 100 minutes. Meanwhile, the
upper limit of
the treatment time of the calcination is preferably 200 minutes and more
preferably 150
minutes. When the treatment time of the calcination is less than the lower
limit, thermal
decomposition may not sufficiently proceed and the improvement in the crushing
strength of
the iron ore pellets may be insufficient. To the contrary, when the treatment
time of the
calcination is greater than the upper limit, the effect of increasing the
crushing temperature
tends to be saturated and the effect may be insufficient with respect to the
increase in the
production cost.
[0051]
A lower limit of a percentage of particles having a grain size of less than or
equal to
20 p.m in the dolomite after the hydration reaction (after the balling step
S3) is preferably 45%
by volume, and more preferably 55% by volume. The percentage of particles
having a grain
CA 03214315 2023-09-20
I 44 i
size of less than or equal to 20 p.m being greater than or equal to the lower
limit facilitates an
increase in the crushing strength of the iron ore pellets.
[0052]
<Advantages>
In the method for producing iron ore pellets, due to calcining the dolomite at
a
temperature greater than or equal to the predetermined value in the preparing
step Si, the
dolomite is present in a miniaturized state in a pellet structure prior to
firing, and an effect of
increasing the bonding strength of the pellet structure of the iron ore
pellets is produced. The
crushing strength of the iron ore pellets to be produced can thus be
increased. In addition, in
the iron ore pellets produced by the method for producing iron ore pellets, a
CaO/SiO2 mass
ratio is greater than or equal to 0.8 and a MgO/SiO2 mass ratio is greater
than or equal to 0.4,
resulting in high reducibility.
[0053]
[Other Embodiments]
It is to be noted that the present invention is not limited to the above-
described
embodiments.
[0054]
In the first embodiment, only the method of pulverizing the dolomite in the
preparing
step such that the Blaine specific surface area is greater than or equal to
the predetermined value
has been described, and in the second embodiment, only the method of calcining
the dolomite
at a temperature of greater than or equal to the predetermined value in the
preparing step has
been described; however, these methods may be employed in combination.
[0055]
In the first embodiment, the method of pulverizing the dolomite in the
preparing step
has been described; however, dolomite having the Blaine specific surface area
greater than or
equal to the predetermined value may be prepared in advance. Similarly, in the
second
embodiment, calcined dolomite may be prepared. In this case, the preparing
step may be
omitted.
[0056]
In addition, it is considered that, due to the dolomite being present in a
miniaturized
state in a structure of the green pellets prior to the firing, the crushing
strength of the iron ore
pellets to be produced can be increased as described above. Therefore, the
treatment in the
preparing step is not limited to those of the aforementioned embodiments, and
the dolomite
may be subjected to another treatment to be present in a miniaturized state in
the pellet structure
prior to the firing.
[0057]
In the aforementioned embodiments, the method of producing iron ore pellets by
using
the production apparatus with the grate kiln system has been described;
however, the iron ore
11
CA 03214315 2023-09-20
, 4 I
pellets may also be produced by using a production apparatus with a straight
grate system. In
the production apparatus with the straight grate system, the grate furnace
includes a traveling
grate, a drying chamber, a dehydrating chamber, a preheating chamber, and a
firing chamber,
and the firing step is completed only in the grate furnace. Specifically, the
green pellets are
dried, dehydrated, and preheated by a heating gas in the drying chamber, the
dehydrating
chamber, and the preheating chamber, and finally fired in the firing chamber.
[EXAMPLES]
[0058]
Hereinafter, the present invention is explained in further detail by way of
Examples,
but the present invention is not in any way limited to these Examples.
[0059]
[Experiment 1]
Iron ore pellets in which a CaO/SiO2 mass ratio was 1.4 and a MgO/SiO2 mass
ratio
was 0.8 were produced by the procedure illustrated in FIG. 1. In the preparing
step, the Blaine
specific surface area was changed by pulverization of the dolomite. Note that
the firing
temperature was 1,230 C or 1,250 C.
[0060]
The crushing strength of each of the iron ore pellets thus produced was
measured.
The results are shown in FIG. 4.
[0061]
The graph in FIG. 4 shows that the Blaine specific surface area of the
dolomite being
greater than or equal to 4,000 cm2/g can increase the crushing strength. It is
concluded that,
particularly in the case of the firing temperature being 1,250 C, the Blaine
specific surface area
of the dolomite being greater than or equal to 4,000 cm2/g enables production
of the iron ore
pellets having a high crushing strength of greater than or equal to 270 kg/P.
[0062]
Note that although the CaO/SiO2 mass ratio was 1.4 and the MgO/SiO2 mass ratio
was
0.8 in the present experiment in the present experiment, it is inferred that
since the CaO/SiO2
mass ratio of 0.8 and the MgO/SiO2 mass ratio of 0.4, for example, increase
the crushing
strength, the Blaine specific surface area of the dolomite being greater than
or equal to 4,000
cm2/g gives the crushing strength of greater than or equal to 270 kg/P even in
the case in which
the firing temperature is 1,230 C, by reducing the CaO/SiO2 mass ratio and
the MgO/SiO2
mass ratio.
[0063]
[Experiment 2]
Iron ore pellets in which a CaO/Si02 mass ratio was 1.40 and a MgO/SiO2 mass
ratio
was 0.83 were produced by the procedure illustrated in FIG. 1. In the
preparing step, the
dolomite was calcined while changing the calcination condition within ranges
of temperature
12
CA 03214315 2023-09-20
I
from 900 C to 1,110 C and of the treatment time from 80 minutes to 200
minutes. Note that
the firing temperature was 1,230 C or 1,250 C.
[0064]
Regarding each of the iron ore pellets thus produced, measurements were
performed
on: a percentage of particles having a grain size of less than or equal to 20
tm in the dolomite
after the hydration reaction in the balling step; and the crushing strength.
The results are
shown in FIG. 5.
[0065]
The graph in FIG. 5 shows that the calcination at a temperature of greater
than or equal
to 900 C can increase the crushing strength. It is concluded that,
particularly in the case of
the firing temperature being 1,250 C, the percentage of particles having a
grain size of less
than or equal to 20 um in the dolomite after the hydration reaction being
greater than or equal
to 45% by volume enables production of the iron ore pellets having a high
crushing strength of
greater than or equal to 270 kg/P. In addition, it is inferred that also in
the case of the firing
temperature being 1,230 C, the percentage of particles having a grain size of
less than or equal
to 20 m being greater than or equal to 45% by volume gives the crushing
strength of greater
than or equal to 270 kg/P by reducing the CaO/SiO2 mass ratio and the MgO/SiO2
mass ratio.
[INDUSTRIAL APPLICABILITY]
[0066]
By employing the method for producing iron ore pellets according to the
present
invention, iron ore pellets superior in reducibility and having high crushing
strength can be
produced. Therefore, the iron ore pellets produced by the present method for
producing iron
ore pellets can be used in a blast furnace operated with a low reduction agent
ratio.
[Explanation of the Reference Symbols]
[0067]
1 Iron ore pellets
2 Production apparatus
3 Pan pelletizer
4 Traveling grate furnace
41 Traveling grate
42 Drying chamber
43 Dehydrating chamber
44 Preheating chamber
45 Burner
Kiln
6 Annular cooler
61 Blowing apparatus
P Green pellet
13
CA 03214315 2023-09-20
I
H Preheated pellet
G1 Heating gas
G2 Combustion exhaust gas
G3 Cooling gas
C Smokestack
14