Note: Descriptions are shown in the official language in which they were submitted.
WO 2022/221458
PCT/US2022/024694
COMPOSITIONS AND METHODS FOR TREATING CHRONIC ACTIVE WHITE MATTER
LESIONS / RADIOLOGICALLY ISOLATED SYNDROME
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims the benefit of priority to U.S. Provisional
Application No.
63/174399, filed on April 13, 2021, U.S. Provisional Application No.
63/320655, filed on March
16, 2022, French Provisional Application No. FR2103793, filed on April 13,
2021, and
International Application No. PCT/US2022/024450 filed on April 12, 2022.
BACKGROUND
100011 Multiple sclerosis (MS) is a chronic, inflammatory,
demyelinating disease of the central
nervous system typically characterized by focal lesions in the white matter,
and generally presents
with a spectrum of clinical phenotypes ranging from relapsing (RMS) and
relapsing-remitting
(RRMS) to increasingly progressive forms. The phenotypic categorization of MS
into relapsing
and primary progressive multiple sclerosis (PPMS) and other forms resulted
from definitions put
forth in 1996 by the US National Multiple Sclerosis Society (NMSS) Advisory
Committee on
Clinical Trials in Multiple Sclerosis. Notably, however, the distinctions
drawn were not made on
the basis of a fundamental, well-established, and well-understood scientific
characterization of the
pathophysiology of the various forms. To the contrary, at the time they were
proposed the
committee acknowledged that the phenotypic descriptors were consensus
subjective views of
experts in the field and were not supported by objective biological findings.
100021 As clinicians have more recently come to appreciate, the
differences between these
various clinical phenotypes are relative rather than absolute. For example,
while some evidence
suggests that primary progressive multiple sclerosis (PPMS) represents a
distinct, non-
inflammatory or at least less inflammatory pathologic form of MS than
secondary progressive
multiple sclerosis (SPMS), abundant clinical, imaging, and genetic data
suggest that PPMS is a
part of the spectrum of progressive MS phenotypes, and analyses of natural
history cohorts
demonstrate that worsening proceeds at a similar rate in both SPMS and PPMS.
Lublin, Fred D.,
et at. Defining the Clinical Course of Multiple Sclerosis: The 2013 Revisions.
Neurology. 2014
- 1 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
Jul 15; 83(3): 278-86. Accordingly, there is growing awareness in the field
that there is substantial
overlap of the subtypes with regard to clinical subtypes and likely
pathophysiology.
100031 Given this emerging understanding as well as the inherent
subjectivity of clinical
phenotyping in general, attention has gradually turned to various
pathophysiological
characterizations based on the underlying biological presentation of the
disease. There is a
common view that the underlying pathology of MS involves both inflammation and
neurodegeneration. Lublin el al. Neurology The 2013 clinical course
descriptors for multiple
sclerosis: A clarification 2020:94:1-5. doi.10.1212/WNL.0000000000009636. The
relationship
between the clinical evolution of the disease and these mechanisms is complex,
however, and in
need of further characterization. Id.
100041 One facet of this relates to the prevalence and expansion of
white matter lesions based
on magnetic resonance imaging (MRI). MRI is the most sensitive imaging
technique for detecting
MS lesions in vivo, and lesion load measurements based on conventional T2-
weighted MRI are
widely used to monitor treatment effects in therapeutic trials. Notably,
however, there is only a
modest correlation between the lesion load on conventional MRI and the
clinical disability of
patients with MS, a phenomenon referred to as clinicoradiologic dissociation.
Seewan et al., Arch
Neurol. 2009;66(5):601-609. doi:10.1001/archneuro1.2009.57. As such, more
accurate and
informative radiological methods are still clearly needed to inform
appropriate treatment decisions
for patients.
100051 Individuals with radiologically isolated syndrome (RIS) have
incidental MRI
abnormalities suggestive of MS. Recent studies using susceptibility-based
imaging have shown
that a subgroup of chronic active white matter lesions (CAWMLs) have a rim of
paramagnetic
susceptibility-associated signal loss at the lesion edge, the paramagnetic rim
sign (PRS), that is
associated with the presence of iron inside phagocytes, which indicates
chronic, active
demyelination. Suthiphosuwan et at., JA1I/IA Neuro. Paramagnetic Rim Sign in
Radiologically
Isolated Syndrome March 9, 2020 doi:10.1001/jamaneuro1.20200124. As such,
these patients may
indeed benefit from the more aggressive disease-modifying treatment options
normally reserved
for relapsing and progressive forms of MS. Unfortunately, however, given the
historical emphasis
on subjective clinical phenotypes rather than pathophysiology in the
determination and approval
- 2 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
of available treatments, the RIS patient population in particular has few
treatment options and the
disease is typically left to exacerbate until clinical manifestations become
more apparent.
100061 Ty sab ri (natalizumab) is an anti -very late antigen (VLA)-4
humanized monoclonal
IgG4 antibody that inhibits the migration of lymphocytes throughout the blood-
brain barrier by
blocking VLA-4 interactions with vascular cell adhesion molecules (VCAM)-1 and
reducing
inflammatory lesions. Natalizumab is a biotherapeutic approved for treating
relapsing and
progressive forms of multiple sclerosis. Under U.S. clinical practice,
natalizumab is only used in
patients who have had at least one relapse event, as determined by their
clinician, while in Europe
natalizumab treatment requires the diagnosis of at least one relapse event
along with the
identification of at least one acute lesion (typically defined as an increase
in lesion load/size in a
T2 or a Ti gadolinium-enhanced lesion on MRI). To date, in view of attendant
treatment risks as
well as the long-established reliance on clinical phenotypes to drive
treatment decisions, there has
been no consensus on its potential use in earlier stage disease.
SUMMARY
100071 The present disclosure provides methods for treating and/or
reducing chronic white
matter lesion activity (CWMLA), also referred to as chronic lesion activity
(CLA), in patients in
need thereof, including Radiologically Isolated Syndrome (RIS) patients, with
appropriate disease-
modifying therapies, e.g. anti-VLA-4 antibodies. In particular, the present
invention demonstrates
that certain presentations of CWMLA visible with specific magnetic resonance
imaging techniques
correlate with disease progression, and thus patients having this confluence
of radiological markers
can be effectively treated with more aggressive disease-modifying antibody
therapies even in the
absence of the clinical manifestations (e.g., relapses) conventionally used to
justify such therapies.
Accordingly, new methods of treating earlier-stage and/or asymptomatic (e.g.,
pre-first episode of
relapse) patients such as RIS patients based on the presence of new
radiological characterizations
of CWMLA are described and exemplified herein.
100081 In one aspect, the disclosure provides methods for treating
Radiologically Isolated
Syndrome (RIS) in a patient in need thereof comprising administering a
therapeutically effective
amount of a disease-modifying antibody therapy to said patient, wherein said
patient has CWMLA
as defined by at least one phase rim lesion (PRL) in at least one
susceptibility-weighted magnetic
- 3 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
resonance image (MRI). In some embodiments, the patient has CWMLA as defined
by at least
one slowly expanding lesion (SEL). In some embodiments, the patient has CWMLA
as defined
by at least one SEL that is detected using single time-point non-contrast Ti-
and T2-weighted
MRI.
[0009] In some embodiments, the patient has CWMLA as defined by at
least one SEL that co-
localizes with at least one PRL, or vice-versa. In some embodiments, the at
least one SEL is
detected using single time-point non-contrast Ti- and T2-weighted MRI.
100101 In some embodiments, the SEL is detected using a machine-
learning based classifier
that discriminates acute from chronic MS lesions, and/or SEL from non-SEL,
using unenhanced
Tl/T2 information from a single MRI scan. Advantageously, then, a patient
suspected of having
RIS, or a patient previously identified as having RIS and at further risk of
developing MS, may be
referred for an MRI scan of the brain at a single time point, and without
agent contrast. The scan
may then be input into the classifier algorithm, which may identify and
distinguish between acute
and chronic lesions present on the brain scan, and/or between SEL and non-SEL.
Based on that
identification and distinction, an appropriate disease-modifying antibody
therapy can be
administered that is suitable to the particular patient and disease state.
100111 In some embodiments, one or more features having predictive
value with respect to the
classification of a lesion as either acute or chronic and/or as SELs are
utilized. In some
embodiments, said features are selected from the group comprising or
consisting of: features that
quantify the first order intensity of the core region of a lesion as it
appears on a T2-weighted scan
image; features that quantify the amount of signals appearing as low-gray
around the periphery of
a lesion as it appears on a Ti-weighted scan image; features that quantify the
amount of high-gray
signals that are present in the periphery and/or core of a lesion as it
appears on a Ti-weighted scan
image; features that relate to the inhomogeneity present in the images;
features that relate to the
structure of the image, as relating to the presence of repeating patterns; and
features that relate to
the texture of the images.
[0012] In some embodiments, the disease-modifying antibody therapy
is selected from
natalizumab, BI1B107 and ocrelizumab. In some embodiments, the disease-
modifying antibody
therapy is an anti-VLA-4 antibody, e.g. natalizumab or BIIB107.
- 4 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
100131 In some embodiments, the anti-VLA-4 antibody is natalizumab
administered in a
biphasic dosing regimen, wherein the biphasic regimen comprises an induction
phase comprising
administration of natalizumab once a month for about 10 to about 14 months
followed by a chronic
phase comprising administration of natalizumab once every 5, 6, 7, or 8 weeks.
In some
embodiments, the induction phase comprises administration of natalizumab once
a month for about
months, about 11 months, about 12 months, about 13 months, about 14 months, or
longer than
about 14 months. In some embodiments, at least one phase of a biphasic dosing
regimen comprises
subcutaneous (SC) administration. In some embodiments, both the induction
phase and chronic
phase of a biphasic dosing regimen comprises SC injection. In some
embodiments, the induction
phase of a biphasic dosing regimen comprises SC injection. In some
embodiments, the chronic
phase of a biphasic dosing regimen comprises SC injection. In some
embodiments, surprisingly,
the SC dosing and amount of natalizumab can be consistent with IV dosing. In
some
embodiments, the therapeutically effective amount administered during the
induction phase and
the chronic phase are the same, and the therapeutically effective amount is
between 250 ¨ 450 mg
(e.g. 250 mg, 300 mg, 350 mg, 400 mg, or 450 mg), more preferably about 300
mg, still more
preferably 300 mg. In some embodiments, the therapeutically effective amount
administered SC
during the chronic phase is between 300 - 500 mg (e.g., 300 mg, 350 mg, 400
mg, 450 mg, or 500
mg). In some embodiments, the therapeutically effective amount is between
about 250 ¨ about
450 mg (e.g., about 250 mg, about 300 mg, about 350 mg, about 400 mg, or about
450 mg), more
preferably about 300 mg, still more preferably 300 mg. In some embodiments,
the therapeutically
effective amount administered SC during the chronic phase is between about 300
- about 500 mg
(e.g., about 300 mg, about 350 mg, about 400 mg, about 450 mg, or about 500
mg), more preferably
about 300 mg, still more preferably 300 mg.
100141 In some embodiments, the anti-VLA-4 antibody is natalizumab
administered in a
chronic dose regimen, wherein the chronic dosing regimen comprises
administration of
natalizumab at a fixed interval of every 4 weeks. In some embodiments, the
chronic dosing
regimen is a fixed, non-weight based amount of natalizumab. In some
embodiments, a
therapeutically effective amount of natalizumab is between about 250 ¨ 450 mg,
or about 300 mg.
In some embodiments, a therapeutically effective amount of natalizumab is a
fixed, non-weight
based dose of 300 mg. In further embodiments, the chronic dosing regimen is
every 4 weeks for
a period of about 4 months, about 5 months, about 6 months, about 7 months,
about 8 months,
- 5 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
about 9 months, about 10 months, about 11 months, about 12 months, or longer
than about 12
months. In some embodiments, the chronic dosing regimen comprises SC
injection. In some
embodiments, the chronic dosing regimen comprises IV administration.
[0015] In some embodiments, treatment with anti-VLA-4 therapy is
initiated when at least 1%,
5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, or 75% of
the
patient's total T2 hyperintense lesion volume and/or number is identified as
PRL. In some
embodiments, treatment with anti-VLA-4 therapy is initiated when at least 1%,
5%, 10%, 15%,
20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, or 75% of the patient's
total T2
hyperintense lesion volume and/or number is identified as SEL. In some
embodiments, treatment
with anti-VLA-4 therapy is initiated when at least 5%, 10%, 15%, 20%, 25%,
30%, 35%, 40%,
45%, 50%, 55%, 60%, 65%, 70%, or 75% of the patient's SELs co-localize with
their PRLs. In
some embodiments, treatment with anti-VLA-4 therapy is initiated when at least
5%, 10%, 15%,
20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, or 75% of the patient's
PRLs co-
localize with their SELs.
[0016] In another aspect, the invention provides methods of reducing
and/or treating chronic
active white matter lesions in an asymptomatic and/or early-stage MS patient
(e.g. having no
diagnosed relapses, or fulfilling consensus diagnostic criteria, e.g. Thompson
AJ et al. Lancet
Neurol. 2018; 17:162-173) comprising administering a therapeutically effective
amount of a
disease-modifying antibody therapy to said patient, wherein said patient has
CWMLA as defined
by at least one phase rim lesion (PRL) in at least one susceptibility-weighted
magnetic resonance
image (MRI). In some embodiments, the patient has CWMLA as defined by at least
one slowly
expanding lesion (SEL). In some embodiments, the patient has CWMLA as defined
by at least one
SEL that is detected using single time-point non-contrast Ti- and T2-weighted
MRI.
[0017] In some embodiments, the patient has CWMLA as defined by at
least one SEL that co-
localizes with at least one PRL, or vice-versa. In some embodiments, the at
least one SEL is
detected using single time-point non-contrast Ti- and 12-weighted MRI.
[0018] In some embodiments, the SEL is detected using a machine-
learning based classifier
that discriminates acute from chronic MS lesions and/or SEL from non-SEL using
unenhanced
T1/T2 information from a single MRI scan. Advantageously, then, an
asymptomatic and/or early
stage patient suspected of having a brain ailment such as MS, or an
asymptomatic and/or early
- 6 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
stage patient at risk of developing MS, may be referred for an MRI scan of the
brain at a single
time point, and without agent contrast. The scan may then be input into the
classifier algorithm,
which may identify and distinguish between acute and chronic lesions present
on the brain scan,
and/or between SEL and non-SEL. Based on that identification and distinction,
an appropriate
disease-modifying antibody therapy can be administered that is suitable to the
particular patient
and disease state.
100191 In some embodiments, one or more features having predictive
value with respect to
the classification of a lesion as either acute or chronic and/or as SELs are
utilized. In some
embodiments, said features are selected from the group comprising or
consisting of: features that
quantify the first order intensity of the core region of a lesion as it
appears on a T2-weighted scan
image; features that quantify the amount of signals appearing as low-gray
around the periphery of
a lesion as it appears on a Ti-weighted scan image; features that quantify the
amount of high-gray
signals that are present in the periphery and/or core of a lesion as it
appears on a Ti-weighted scan
image; features that relate to the inhomogeneity present in the images;
features that relate to the
structure of the image, as relating to the presence of repeating patterns; and
features that relate to
the texture of the images.
100201 In some embodiments, the disease-modifying antibody therapy
is selected from
natalizumab, BIIB107 and ocrelizumab. In some embodiments, the disease-
modifying antibody
therapy is an anti-VLA-4 antibody, e.g. natalizumab or BIIB107. In some
embodiments, the anti-
VLA-4 antibody is natalizumab administered in a biphasic dosing regimen,
wherein the biphasic
regimen comprises an induction phase comprising administration of natalizumab
once a month for
about 10 to about 14 months, followed by a chronic phase comprising
administration of
natalizumab once every 5, 6, 7 or 8 weeks. In some embodiments, the induction
phase comprises
administration of natalizumab once a month for about 10 months, about 11
months, about 12
months, about 13 months, about 14 months, or longer than about 14 months. In
some
embodiments, at least one phase of a biphasic protocol comprises subcutaneous
(SC)
administration. In some embodiments, both the induction phase and chronic
phase of a biphasic
dosing regimen comprises SC injection. In some embodiments, the induction
phase of a biphasic
dosing regimen comprises SC injection. In some embodiments, the chronic phase
of a biphasic
dosing regimen comprises SC injection. In some embodiments, surprisingly, the
SC dosing and
amount of natalizumab can be consistent with IV dosing. In some embodiments,
the
- 7 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
therapeutically effective amount administered during the induction phase and
the chronic phase
are the same, and the therapeutically effective amount is between 250 - 450 mg
(e.g. 250 mg, 300
mg, 350 mg, 400 mg, or 450 mg), more preferably about 300 mg, still more
preferably 300 mg. In
some embodiments, the therapeutically effective amount administered SC during
the chronic phase
is between 300 - 500 mg (e.g., 300 mg, 350 mg, 400 mg, 450 mg, or 500 mg). In
some
embodiments, the therapeutically effective amount is between about 250 - about
450 mg (e.g.,
about 250 mg, about 300 mg, about 350 mg, about 400 mg, or about 450 mg), more
preferably
about 300 mg, still more preferably 300 mg. In some embodiments, the
therapeutically effective
amount administered SC during the chronic phase is between about 300 - about
500 mg (e.g., about
300 mg, about 350 mg, about 400 mg, about 450 mg, or about 500 mg), more
preferably about 300
mg, still more preferably 300 mg
[0021] In some embodiments, the anti-VLA-4 antibody is natalizumab
administered in a
chronic dose regimen, wherein the chronic dosing regimen comprises
administration of
natalizumab at a fixed interval of every 4 weeks. In some embodiments, the
chronic dosing
regimen is a fixed, non-weight based amount of natalizumab. In some
embodiments, a
therapeutically effective amount of natalizumab is between about 250 - 450 mg,
or about 300 mg.
In some embodiments, a therapeutically effective amount of natalizumab is a
fixed, non-weight
based dose of 300 mg Tn further embodiments, the chronic dosing regimen is
every 4 weeks for
a period of about 4 months, about 5 months, about 6 months, about 7 months,
about 8 months,
about 9 months, about 10 months, about 11 months, about 12 months, or longer
than about 12
months. In some embodiments, the chronic dosing regimen comprises SC
injection. In some
embodiments, the chronic dosing regimen comprises IV administration.
[0022] In some embodiments, treatment with anti-VLA-4 therapy is
initiated when at least 1%,
5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, or 75% of
the
patient's total T2 hyperintense lesion volume and/or number is identified as
PRL. In some
embodiments, treatment with anti-VLA-4 therapy is initiated when at least 1%,
5%, 10%, 15%,
20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, or 75% of the patient's
total T2
hyperintense lesion volume and/or number is identified as SEL. In some
embodiments, treatment
with anti-VLA-4 therapy is initiated when at least 5%, 10%, 15%, 20%, 25%,
30%, 35%, 40%,
45%, 50%, 55%, 60%, 65%, 70%, or 75% of the patient's SELs co-localize with
their PRLs. In
some embodiments, treatment with anti-VLA-4 therapy is initiated when at least
5%, 10%, 15%,
- 8 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, or 75% of the patient's
PRLs co-
localize with their SELs.
100231
In another aspect, methods for reducing and/or treating chronic white
matter lesion
activity in an asymptomatic and/or early-stage MS patient (e.g. having no
diagnosed relapse
events) are provided comprising a) identifying at least one phase rim lesion
(PRL) in at least one
susceptibility-weighted magnetic resonance image from a patient known or
suspected of having
chronic active white matter lesions, b) identifying at one slowly-expanding
lesion (SEL) in at least
one Ti-weighted/T2-weighted MRI from said patient; c) determining if the at
least one PRL co-
localizes with the at least one SEL in said patient, and/or vice-versa, and d)
in the event of co-
localization initiating treatment with a disease-modifying antibody therapy.
In some
embodiments, the at least one SEL is detected using single time-point non-
contrast Ti- and T2-
weighted MRI. In some embodiments, the disease-modifying antibody therapy is
selected from
natalizumab, BIII3107 and ocrelizumab. In some embodiments, the disease-
modifying antibody
therapy is an anti-VLA-4 antibody, e.g. natalizumab or BI113107.
100241
In some embodiments, the SEL is detected using a machine-learning based
classifier
that discriminate acute from chronic MS lesions and/or SEL from non-SEL using
unenhanced
Ti/T2 information from a single MRI scan. Advantageously, then, an
asymptomatic and/or early
stage patient suspected of having a brain ailment such as MS, or an
asymptomatic and/or early
stage patient at risk of developing MS, may be referred for an MRI scan of the
brain at a single
time point, and without agent contrast. The scan may then be input into the
classifier algorithm,
which may identify and distinguish between acute and chronic lesions present
on the brain scan,
and/or between SEL and non-SEL. Based on that identification and distinction,
an appropriate
disease-modifying antibody therapy can be administered that is suitable to the
particular patient
and disease state.
100251
In some embodiments, one or more features having predictive value with
respect to the
classification of a lesion as either acute or chronic and/or as SELs are
utilized. In some
embodiments, said features are selected from the group comprising or
consisting of: features that
quantify the first order intensity of the core region of a lesion as it
appears on a T2-weighted scan
image; features that quantify the amount of signals appearing as low-gray
around the periphery of
a lesion as it appears on a Ti-weighted scan image; features that quantify the
amount of high-gray
- 9 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
signals that are present in the periphery and/or core of a lesion as it
appears on a Ti-weighted scan
image; features that relate to the inhomogeneity present in the images;
features that relate to the
structure of the image, as relating to the presence of repeating patterns; and
features that relate to
the texture of the images.
100261 In some embodiments, the method further comprises
administering to said patient a
therapeutically effective amount of natalizumab in a biphasic dosing regimen,
wherein the biphasic
dosing regimen comprises an induction phase comprising administration of the
anti-VLA-4
antibody once every 2 weeks, about once very 2 weeks, once every 4 weeks,
about once every 4
weeks, once every 30 days, about once every 30 days, once a month or about
once a month for at
least 6 months, for at least 8 months, for at least 10 months, or for at least
12 months, followed by
a chronic phase comprising administration of the anti-VLA-4 antibody once
every 5 to 10 weeks,
or once every 5, 6, 7 or 8 weeks. In some embodiments, the induction phase is
from 6 to 18
months, from 8 to 16 months, from 10 to 14 months, or is 11 months, is 12
months or is 13 months.
In some embodiments, the induction phase is 12 months, and the chronic phase
comprises
administration of natalizumab every 5 weeks, about every 5 weeks, every 6
weeks, about every 6
weeks, every 7 weeks or about every 7 weeks. In some embodiments, the
induction phase is 12
months and the chronic phase comprises administration of natalizumab every 6
weeks.
100271 In some embodiments, treatment with anti-VLA-4 therapy is
initiated when at least 5%,
10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, or 75% of the
patient's
SELs co-localize with their PRLs. In some embodiments, treatment with anti-VLA-
4 therapy is
initiated when at least 5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%,
60%, 65%,
70%, or 75% of the patient's PRLs co-localize with their SELs.
BRIEF DESCRIPTION OF THE DRAWINGS
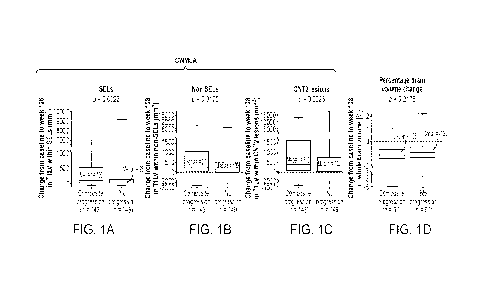
100281 Figure 1: Association between CWMLA or whole brain volume
loss and composite
disability progression in the placebo arm. Change from baseline to week 108 in
T1LV in (A) SELs,
(B) non-SELs, and (C) CNT2 lesions was significantly associated with composite
disability
progression in SPMS patients. No difference in percentage change from baseline
to week 108 in
(D) whole brain volume was observed in SPMS patients with composite disability
progression
compared with those with no progression. Composite progression was confirmed
at 24 weeks and
- 10 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
end of study on one or more of the EDSS, Timed 25-Foot Walk, or 9-Hole Peg
Test. In these box-
and-whisker representations, the box spans the interquartile range, the median
is marked by the
horizontal line inside the box, and the whiskers are the two lines outside the
box that extend to the
highest and lowest observations. p values by Van Elteren test; stratified by
baseline EDSS score
(<5.5 or >6.0) and baseline T2 lesion volume category based on tertiles
(16908.79 mm3, >6908.79
18818.49 mm3, and >18818.49 mm3). CNT2 = chronic nonenhancing T2; CWMLA =
chronic
white matter lesion activity; EDSS = Expanded Disability Status Scale; SEL =
slowly expanding
lesion; SPMS = secondary progressive multiple sclerosis; T1LV = T1 -
hypointense lesion volume.
100291 Figure 2: Association between CWMLA and EDSS progression.
Increase from
baseline to week 108 in T1LV within (A) SELs and (C) CNT2 lesions but not (B)
non-SELs was
associated with EDSS progression in SPMS patients treated with placebo.
(However, a consistent
directional trend was observed in non-SELs.) EDSS progression was confirmed at
24 weeks and
end of study. In these box-and-whisker representations, the box spans the
interquartile range, the
median is marked by the horizontal line inside the box, and the whiskers are
the two lines outside
the box that extend to the highest and lowest observations. p values by Van
Elteren test; stratified
by baseline EDSS score (<5.5 or >6.0) and by baseline 12 lesion volume
category based on tertiles
(<6908.79 mm3, >6908.79-18818.49 mm3, and >18818.49 mm3). CNT2 = chronic
nonenhancing
T2; CWMLA = chronic white matter lesion activity; EDSS = Expanded Disability
Status Scale;
SEL = slowly expanding lesion; SPMS = secondary progressive multiple
sclerosis; T1LV = T1-
hypointense lesion volume.
100301 Figure 3: Association between CWMLA and 9HPT progression.
Change from
baseline to week 108 in T1LV within (A) SELs and (C) CNT2 lesions but not (B)
non-SELs was
associated with 9HPT progression in SPMS patients treated with placebo.
(However, a consistent
directional trend was observed in non-SELs.) 91-IPT progression was confirmed
at 24 weeks and
end of study. In these box-and-whisker representations, the box spans the
interquartile range, the
median is marked by the horizontal line inside the box, and the whiskers are
the two lines outside
the box that extend to the highest and lowest observations. p values by Van
Elteren test; stratified
by baseline EDSS score (<5.5 or >6.0) and by baseline 12 lesion volume
category based on tertiles
(<6908.79 mm3, >6908.79-18818.49 mm3, and >18818.49 mm3). 9HPT = 9-Hole Peg
Test; CNT2
= chronic nonenhancing T2; CWMLA = chronic white matter lesion activity; EDSS
= Expanded
- 11 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
Disability Status Scale; SEL = slowly expanding lesion; SPMS = secondary
progressive multiple
sclerosis; T1LV = T1 -hypointense lesion volume.
100311 Figure 4: Association between CWMLA and T25FW progression.
Change in T1LV
from baseline to week 108 within (B) non-SELs and (C) CNT2 lesions but not (A)
SELs was
associated with T25FW progression in SPMS patients treated with placebo.
(However, a consistent
directional trend was observed in SELs.) T25FW progression was confirmed at 24
weeks and end
of study. In these box-and-whisker representations, the box spans the
interquartile range, the
median is marked by the horizontal line inside the box, and the whiskers are
the two lines outside
the box that extend to the highest and lowest observations. p values by Van
Elteren test; stratified
by baseline EDSS score (<5.5 or >6.0) and by baseline T2 lesion volume
category based on tertiles
(<6908.79 mm3, >6908.79-18818.49 mm3, and >18818.49 mm3). CNT2 = chronic
nonenhancing
T2; CWMLA = chronic white matter lesion activity; EDSS = Expanded Disability
Status Scale;
SEL = slowly expanding lesion; SPMS = secondary progressive multiple
sclerosis; T1LV = T1-
hypointense lesion volume; T25FW = Timed 25-Foot Walk.
100321 Figure 5: Association between CWMLA and composite disability
progression in the
absence of AWMLA. CWMLA in (B) non-SELs and (C) CNT2 lesions but not (A) SELs
remained
associated with composite disability progression in the absence of AWMLA in
SPMS patients
treated with placebo. (However a consistent directional trend was observed in
SELs.) Absence of
acute lesion activity was defined as no baseline and postbaseline Ti
gadolinium-enhancing and no
postbaseline new/enlarging T2 lesions. Composite progression was confirmed at
24 weeks and end
of study on one or more of the EDSS, Timed 25-Foot Walk, or 9-Hole Peg Test.
In these box-and-
whisker representations, the box spans the interquartile range, the median is
marked by the
horizontal line inside the box, and the whiskers are the two lines outside the
box that extend to the
highest and lowest observations. p values by Van Elteren test; stratified by
baseline EDSS score
(<5.5 or >6.0) and baseline T2 lesion volume category based on tertil es
(<6908.79 mm3, >6908.79-
18818.49 mm3, and >18818.49 mm3). AWMLA = acute white matter lesion activity,
also referred
to as acute lesion activity (ALA); CNT2 = chronic nonenhancing T2; CWMLA =
chronic white
matter lesion activity; EDSS = Expanded Disability Status Scale; SEL = slowly
expanding lesion;
SPMS = secondary progressive multiple sclerosis; T1LV = T 1 -hypointense
lesion volume.
- 12 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
100331 Figure 6: Prevalence of SELs and frequency distribution of
SEL severity in the
presence versus absence of AWMLA. SEL (A) number, (B) absolute volume, and (C)
relative
volume (percentage of baseline nonenhancing T2LV) was greater in SPMS patients
treated with
placebo who had AWMLA compared to those with no AWMLA. (D) The frequency
distribution
of patients by range of SEL prevalence indicates that patients with AWMLA had
a greater
percentage of their total T2 lesion burden identified as SELs compared with
patients with no
AWMLA. In these box-and-whisker representations, the box spans the
interquartile range, the
median is marked by the horizontal line inside the box, and the whiskers are
the two lines outside
the box that extend to the highest and lowest observations. No acute lesion
activity was defined as
no baseline or postbaseline Gd+ Ti lesions and no postbaseline new/enlarging
T2 lesions in weeks
24, 48, 72, 96, and 108. Acute lesions were defined as baseline Gd+ Ti lesions
and postbaseline
Gd+ Ti lesions and new/enlarging T2 lesions in weeks 24, 48, 72, 96, and 108.
p values by Van
Elteren test: stratified by baseline EDSS score (<5.5 or >6.0) and baseline
T2LV category based
on tertiles (<6908.79 mm3, >6908.79-18818.49 mm3, and >18818.49 mm3). AWMLA =
acute
white matter lesion activity; BL = baseline; EDSS = Expanded Disability Status
Scale; Gd+ =
gadolinium enhancing; SEL = slowly expanding lesion; SPMS = secondary
progressive multiple
sclerosis; T2LV = T2-hyperintense lesion volume.
100341 Figure 7. Effect of natalizumab on SET, prevalence
Natalizumab reduced the (A)
number, (B) absolute volume, and (C) relative volume (percentage of baseline
nonenhancing
T2LV) of SELs in SPMS patients. Box-and-whisker representations, the box spans
the
interquartile range, the median is marked by the horizontal line inside the
box, and the whiskers
are the two lines outside the box that extend to the highest and lowest
observations. p values by
Van Elteren test; stratified by baseline Expanded Disability Status Scale
score (<5.5 or >6.0) and
baseline T2LV category based on tertiles (<6908.79 mm3, >6908.79-18818.49 mm3,
and
>18818.49 mm3). SEL = slowly expanding lesion; SPMS = secondary progressive
multiple
sclerosis; T2LV = T2-hyperintense lesion volume; T2w = T2 weighted.
100351 Figure 8: Change in CWMLA with natalizumab versus placebo.
Natalizumab reduced
CWMLA as measured by both (A, B) absolute increase and (C, D) percentage
increase in TILV
in SELs and non-SELs compared with placebo in SPMS patients. Distribution-free
quantile
confidence limits are displayed. p values by Van Elteren test; stratified by
baseline EDSS score
(<5.5 or >6.0) and baseline T2 lesion volume category based on tertiles
(<6908.79 mm3, >6908.79-
- 13 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
18818.49 mm3, and >18818.49 mm3). BL = baseline; CI = confidence interval;
CWMLA = chronic
white matter lesion activity; EDSS = Expanded Disability Status Scale; SPMS =
secondary
progressive multiple sclerosis; T1LV = Ti-hypointense lesion volume.
[0036] Figures 9A-9E: Overlap of SELs and PRLs. SELs identified
based on change within
pre-existing lesion from screening to week 72, outlined on Tlw images at (A)
screening and (B)
week 72. (C) Phase rim (PRL) annotations outlined on a co-registered frequency
map at week 72.
(D) T2-lesions associated with the PRLs, corresponding to the area within the
rims in (C), overlaid
on the FLAIR image at week 72. (E) Voxel-wise overlap between the SELs and T2-
lesions
associated with the PRLs (arrows denote voxels that overlap. Also shown are
voxels only present
in SEL, and voxels only in T2-lesions associated with PRLs).
[0037] Figure 10: Correlation between number of SELs versus number
of PRLs. Number of
PRLs is depicted on the x-axis, and number of SELs is depicted on the y-axis.
[0038] Figures HA-11B: Comparisons of lesions across types. PRL size
with and without
SEL co-localization (A), and SEL size with and without PRL co-localization
(B).
[0039] Figures 12A-12B: Evolution of tissue damage within SEL/PRL
lesions. Comparison
of normalized magnetization transfer ratio (nMTR) trajectories, PRL with and
without SEL
colocalization (A) and SEL with and without PRL colocalization (B). In (A)
nonPRL; PRL, SEL;
PRL, non-SEL. In (B) Non-SEL; SEL,PRL; SEL, nonPRL. In (A) and (B), computed
as weighted
means over samples (PRL or SEL). Shaded areas represent 95% CI of mean.
[0040] Figures 13A-13B: Evolution of tissue damage within SEL/PRL
lesions. Comparison
of radial diffusicity trajectories. PRL with and without SEL colocalization
(A) and SEL with and
without PRL colocalization (B). In (A) nonPRL; PRL, SEL; PRL, non-SEL. In (B)
non-SEL;
SEL,PRL; SEL, nonPRL. In (A) and (B), computed as weighted means over samples
(PRL or
SEL). Shaded areas represent 95% CI of mean.
[0041] Figure 14: Selection of two patches (one SEL, one non-SEL)
extracted from chronic
unenhancing MS leasions of a brain T2 MRI scan.
100421 Figures 15A-15B: Non-SEL patch extracted from baseline T2 MRI
scan showing the
core and periphery regions. (A) unhighlighted, (B) core (solid line) and
periphery (dashed line).
- 14 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
100431 Figures 16A-16C: Illustrations of lesion volume matching
between SEL and Non-SEL
patches (top, SEL; bottom, non-SEL). Each of (A), (B), and (C) correspond to a
volume-matched
pair.
100441 Figure 17: Prevalence of each of the fifteen radiomic
features selected for
discriminating SEL from non-SEL patches, with abbreviations as follows: tip:
Ti-weighted MRI
pre-contrast (i.e., feature was extracted from non-contrast Ti-weighted MRI
image); t2w: T2-
weighted MRI (i.e., feature was extracted from T2-weighted MRI image);
core/periphery:
specifies whether feature was computed within core or periphery region; glrlm:
Gray-level Run-
Length Matrix; glcm: Gray-level Cooccurence Matrix; glszm: Gray-level Size
Zone Matrix.
Notably, first-order statistics including the mean, median and 90th percentile
of Ti intensities in
the core of the patch were identified as relevant, consistent with prior
studies reporting that SELs
exhibit a higher degree of Ti hypo-intensity relative to non-SELs at baseline.
100451 Figures 18A-18D: Confusion matrices showing the performance
of the classification
model for patch-level SEL versus non-SEL discrimination on the training,
validation, and
independent testing sets. (A) ADVANCE training set (balanced accuracy: 73.0%),
(B)
ADVANCE validation set (balanced accuracy: 66.8%), (C) ASCEND test set
(balanced accuracy:
65.7%), (D) SYNERGY test set (blanaced accuracy: 68.5%).
100461 Figures 19A-19D: Confusion matrices showing the performance
of the classification
model for patch-level SEL versus non-SEL discrimination on the training,
validation, and
independent testing sets for volume-matched patches.
DETAILED DESCRIPTION
100471 Natalizumab, sold under the trade name TYSABRI (BIOGEN ,
MA), is an integrin
receptor antagonist approved by the U.S. Food and Drug administration (FDA)
for treatment of
multiple sclerosis and Crohn's disease. The FDA approved standard dosing
regimen is 300
milligrams (mg) infused intravenously over approximately one hour, every four
weeks. Among
the population of patients who have received natalizumab therapy, there is a
small subpopulation
of patients who have developed progressive multi focal leukoencephalopathy
(PML) (Plavina, T.
et al. Ann Neural 2014;76:802-12). Substantial efforts have been made to
identify and minimize
- 15 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
this risk, including the development of a wide range of patient monitoring and
alternative dosing
protocols. Nevertheless, in view of these risks as well as costs and other
considerations, there is
general reluctance in the field to use natalizumab or other more aggressive
disease-modifying
antibody therapies with earlier stage disease, including in particular in
asymptomatic patients who
have yet to exhibit any of the clinical manifestations of MS.
100481 The availability of magnetic resonance imaging has led to an
increase in the detection
of abnormal brain findings even in cases when there are no outward symptoms.
When the MRI
findings are similar to those seen in MS patients, but the patient is
asymptomatic of the typical
physical or neurological symptoms associated with MS, e.g., relapses, this is
known as
radiologically isolated syndrome (RIS). Although there is a strong association
between RIS and
MS, an RIS diagnosis does not always progress to an MS diagnosis. Indeed, when
followed over
a two year period, only about one third of patients with RIS develop a
neurological event and are
diagnosed with MS, while one third develop a new finding on MRI without any
symptoms, and
the last third show no change. What is needed, then, are improved methods for
identifying those
RIS patients more likely to progress to MS, so that more effective treatment
decisions can be made
earlier in the disease process.
100491 Chronic active lesions, also known as smoldering plaques, are
a neuropathologic
hallmark of chronic inflammation in multiple sclerosis (Elliott et at.
Patterning chronic active
demyelinati on in slowly expanding/evolving white matter MS legions. AJNR Am J
Neuroradiol
dx.doi.org/10.3174/ajnr.A6742). The chronic active lesions are generally
surrounded by a rim of
activated microglia and/or macrophages that may contain iron or zinc. These
paramagnetic rim
lesions (PRL) are considered a promising pathological biomarker of iron/zinc
accumulation in
chronic active lesions and are identified using susceptibility-weighted
imaging. They have altered
morphology, sparse T- and B-cells at the core, and a slow rate of ongoing
demyelination and axonal
loss. Detection of PRL presently requires susceptibility-weighted imaging, as
is known in the art.
Haller et al., Susceptibility-weighted imaging: technical essentials and
clinical neurologic
applications Radiology 2021; 299:3-26.
100501 Detection of slowly expanding/evolving lesions (SELs) on
conventional T1-
weighted/T2-weighted brain MRI provides an alternative readout of smoldering
or chronic active
plaques (Elliott et al. Slowly expanding/evolving lesions as a magnetic
resonance imaging marker
- 16 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
of chronic active multiple sclerosis lesions. Mult Scler J 2019; 25:1915-
1925). Slowly expanding
lesions have been described in the literature (Elliott et at. Chronic white
matter lesion activity
predicts clinical progression in primary progressive multiple sclerosis, Brain
2019; 142:2787-
2799). SELs on conventional brain MRI are contiguous regions of T2 lesions
showing constant
and concentric local expansion as assessed by the Jacobian determinant of the
non-linear
deformation between the reference and follow-up scans. Generally, SELs are
devoid of Ti
gadolinium (Gd)-enhancement, have a lower mean Ti signal intensity at baseline
and exhibit a
progressive decrease in Ti intensity over time, compared to non-SEL areas of
pre-existing lesions
(Elliott et at. Ocrelizumab may reduce tissue damage in chronic active lesions
as measured by
change in Ti hypointensity of slowly evolving lesions in patients with primary
progressive
multiple sclerosis Poster presented at AAN; Poster 376, April 24, 2018; Los
Angeles, CA).
100511 As described and exemplified herein, the combination of these
two radiological
markers is informative of disease progression in earlier-stage MS patients,
including patients
known or suspected of having RIS, thereby enabling the identification of those
patients likely to
benefit from more aggressive disease-modifying antibody therapies, including
anti-VLA-4
antibody therapies, earlier in the disease process, and consequent treatment
initiation.
100521 Moreover, these same earlier-stage patients may also benefit
from the use of machine-
learning based classifiers that can accurately and reproducibly discriminate
acute from chronic MS
lesions using unenhanced Ti/T2 information from a single MRI scan, as
described in Provisional
Application Serial No. FR2103793, also filed on April 13, 2021, and in co-
pending International
Application No. PCT/US2022/024450, the disclosures of which are expressly
incorporated by
reference herein. Accordingly, in some embodiments, the identification or one
or more chronic
active white matter lesions, and/or one or more SELs, in a single unenhanced
MRI scan is also
informative of disease progression in earlier-stage MS patients, including
patients known or
suspected of having RIS, thereby enabling the identification of those patients
likely to benefit from
more aggressive disease-modifying antibody therapies, including anti-VLA-4
antibody therapies,
earlier in the disease process, and consequent treatment initiation.
Definitions
- 17 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
100531 A subject, as provided herein, is typically a male or female
human subject (patient)
who is undergoing or who will undergo treatment for a particular condition.
The condition may
be an autoimmune condition or an inflammatory condition. Often, autoimmune
conditions are
considered inflammatory conditions and vice versa, thus, in some embodiments
the subject has an
autoimmune condition and/or inflammatory condition. An autoimmune condition is
a condition
in which a subject's immune system attacks the subject's own cells/tissues.
Non-limiting
examples of the autoimmune conditions contemplated by the present invention
include
Radiologically Isolated Syndrome (RIS), and asymptomatic and/or early-stage
multiple sclerosis
(MS) (e.g., those having no diagnosed relapses).
[0054] Relapses in the context of MS occur in the absence of fever
or infection and are not
linked to environmental and systemic triggers; they denote acute inflammation
in the CNS
characterized by breach of integrity of the blood-brain barrier (BBB). In the
radiological domain,
the criteria for relapses are defined as an increase in lesion load/size on T2
imaging or Ti
gadolinium enhancement of lesions on magnetic resonance imaging (MRI) in the
brain, spinal cord
or both. In the clinical domain, patients may present with "mild" symptoms
such as e.g. pins and
needles sensations that are fleeting and/or spasms that persist for a few
seconds or minutes;
alternatively or additionally, more severe exacerbations may include e.g. the
occurrence of ataxia,
visual deficits, diplopia, fatigue, cognitive impairment, bowel/bladder
dysfunction, or motor
weakness of a limb, which interfere with the patient's mobility, dexterity,
ambulation, safety, or
overall ability to function. The latter symptoms are more likely to result in
a finding of relapse.
[0055] As used herein, "about" refers to within 0.1% to 5% of the
given value (e.g., within
5%, 3%, 2%, 1%, 0.5%, 0.1% above or below the given value). Where amounts and
other
designated values are provided herein, the allowable deviation is within
pharmaceutically
acceptable standards.
[0056] The indefinite articles "a" and "an," as used herein in the
specification and in the
claims, unless clearly indicated to the contrary, should be understood to mean
-at least one."
[0057] It should also be understood that, unless clearly indicated
to the contrary, in any
methods claimed herein that include more than one step or act, the order of
the steps or acts of the
method is not necessarily limited to the order in which the steps or acts of
the method are recited.
- 18 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
100581
In the claims, as well as in the specification above, all transitional
phrases such as
"comprising," "including," "carrying," "having," "containing," "involving,"
"holding,"
"composed of," and the like are to be understood to be open-ended, i.e., to
mean including but not
limited to. Only the transitional phrases "consisting of' and "consisting
essentially of' shall be
closed or semi-closed transitional phrases, respectively, as set forth in the
United States Patent
Office Manual of Patent Examining Procedures, Section 2111.03.
100591
The terms "about" and "substantially" preceding a numerical value mean
10% of the
recited numerical value.
100601
Where a range of values is provided, each value between the upper and
lower ends of
the range are specifically contemplated and described herein. A
"pharmaceutically effective
amount" or "therapeutically effective amount," used interchangeably, is an
amount sufficient to
cure or at least partially arrest the symptoms of a disease and/or the
complications of a disease.
100611
A "disease-modifying antibody therapy" as contemplated herein for the
treatment of
CWMLA includes anti-VLA-4 antibodies, e.g., natalizumab and BI113107, as well
as anti-CD20
antibodies such as ocrelizumab. An "anti-VLA-4 antibody" is an anti-very late
antigen (VLA)-4
monoclonal antibody, a humanized, a human, or a chimeric anti-VLA-4 monoclonal
antibody.
Anti-VLA-4 antibodies have been described in the art. They include, but are
not limited to
natalizumab and B1113107, a monoclonal antibody that targets alpha-4 integrins
and is currently
under clinical investigation
(Clinical Trial s.gov no. NCT04593121). See also
PCT/US2011/032641 and PCT/US2019/034962, the disclosures of which are
expressly
incorporated by reference herein.
Radiological Determinations
100621
With the foregoing background in mind, in some embodiments the
invention teaches
the simultaneous or sequential acquisition of a combination of at least one Ti-
and T2-weighted
image from a patient, for the identification of at least one SEL, and at least
one susceptibility-
weighted magnetic resonance image, for the identification of at least one PRL,
and determining
the extent of co-localization between the two, e.g. the percent of SEL that co-
localize with PRLs,
and vice-versa. In some embodiments, treatment with anti-VLA-4 therapy is
initiated when at
least 5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, or
75% of
the patient's SELs co-localize with their PRLs. In some embodiments, treatment
with anti-VLA-
- 19 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
4 therapy is initiated when at least 5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%,
45%, 50%, 55%,
60%, 65%, 70%, or 75% of the patient's PRLs co-localize with their SELs.
Greater detail
regarding specific embodiments of the invention is provided herein below.
100631 In some embodiments, treatment with anti-VLA-4 therapy is
initiated when at least 1%,
5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, or 75% of
the
patient's total T2 hyperintense lesion volume and/or number is identified as
PRL. In some
embodiments, treatment with anti-VLA-4 therapy is initiated when at least 1%,
5%, 10%, 15%,
20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, or 75% of the patient's
total T2
hyperintense lesion volume and/or number is identified as SEL. In some
embodiments, the SEL
is detected using single time-point non-contrast Ti- and T2-weighted MRI.
100641 In various embodiments, the invention teaches a method for
producing a series/set of
brain images utilizing magnetic resonance imaging. In some embodiments, the
method includes
utilizing an MRI machine to apply a standardized 3-Tesla, 3D-isotropic multi-
echo, gradient echo
MRI to identify any PRLs in said patient, and a Ti- and T2 weighted MRI to
identify any SELs.
The 'co-localization' between SELs and PRLs can be based on heuristic
thresholds set from visual
experience of the observer (radiologist) of the corresponding segmented
volumes or alternatively
rely on an automated processing pipeline determining the exact percent of SELs
volume co-
localizing with PRLs, and vice-versa.
100651 One of skill in the art would readily appreciate that several
different types of imaging
systems could be used to perform the inventive methods described herein,
including all of the types
of imaging systems described in the examples and experiments set forth herein,
as well as similar
systems.
Machine Learning Classification
100661 In some embodiments the invention employs machine-learning
based classifiers to
classify MS lesions using unenhanced Ti/T2 information from a single MTH scan,
as described in
Provisional Application Serial No. FR2103793 and co-pending International
Application No.
PCT/US2022/024450, the disclosures of which are expressly incorporated by
reference herein for
all purposes. Use of these classifiers may be able to effectively increase the
sensitivity of single
time-point acute MS lesion detection, and may be able to replicate, approach,
or exceed the
sensitivity of traditional detection of hyperintensities identified on a Ti-
weighted scan with
- 20 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
gadolinium enhancement and/or of new hyperintense lesions on a T2-weighted
scan in comparison
with a prior reference scan, which may be reflective of new local
inflammation.
100671 In some embodiments, suitable methods of classifying brain
lesions based on single
point in time imaging can include: accessing patient image data from a single
point in time;
providing the patient image data as an input to a brain lesion classification
model; generating a
classification for each of one or more lesions identified in the patient image
data; and providing
the classification for each of the one or more lesions for display on one or
more display devices,
wherein the brain lesion classification model is trained using subject image
data for a plurality of
subjects, the subject image data being captured at two or more points in time.
In some
embodiments, the patient image data from the single point in time includes
data from two or more
image scan sequences. In some embodiments, the data from two or more image
scan sequences
include unenhanced MRI data, wherein the two or more image scan sequences do
not include
administration of paramagnetic contrast agents. In some embodiments, the
classification for each
of one or more lesions identified in the patient image data is selected to be
one of acute or chronic,
or SEL or non-SEL.
100681 In some embodiments, certain radiomic features having
predictive value with respect
to the classification of a lesion as either acute or chronic and/or as SELs
are utilized including e.g.
the following exemplary embodiments:
= Features that quantify the first order intensity of the core region of a
lesion as it appears on
a T2-weighted scan image. Such features account for acute lesions tending to
be more
intense than chronic lesions and more uniformly hyperintense, whereas chronic
lesions
may contain less hyperintense voxels.
= Features that quantify the amount of signals appearing as low-gray around
the periphery of
a lesion as it appears on a Ti-weighted scan image.
= Features that quantify the amount of high-gray signals that are present
in the periphery
and/or core of a lesion as it appears on a Ti-weighted scan image.
= Features that relate to the inhomogeneity present in the images. For
example, features may
quantify the complexity of the image (the image is non-uniform and may include
rapid
- 21 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
changes in the gray levels), the variance of the gray levels with respect to a
mean gray
level, or the existence of homogenous patterns in the images.
= Features that relate to the structure of the image, as relating to the
presence of repeating
patterns. For example, an image with more repeating patterns may be considered
to be
more "structured- than one with fewer observable intensity patterns.
= Features that relate to the texture of the images, such as the coarseness
or fineness of an
image.
100691 In some embodiments, the radiomic features for discriminating
SEL from non-SEL are
selected from the group comprising or consisting of the radiomic features
listed in Figure 17:
= Ti-weighted-MRI pre-contrast (extracted from non-contrast Ti weighted MRI
image),
computed within the core region, gray-level Run-Length-Matrix (quantifies gray
level
runs), run length non-uniformity (measures similarity of run lengths
throughout the image,
with a lower value indicating more homogeneity among run lengths in the image)
= T1-weighted-MRI pre-contrast, computed within the core region, first
order features
(describe the distribution of voxel intensities within the image region
defined by the mask
through commonly used and basic metrics), 90th percentile of voxels in the
patch
= T2-weighted MRI (extracted from T2 weighted MRI image) computed within
the periphery
region, first order features, uniformity (a measure of the sum of the squares
of each
intensity value; a measure of homogeneity of the image array; greater
uniformity implies a
greater homogeneity or a smaller range of discrete intensity values)
= Ti-weighted-MRI pre-contrast, computed within the core region, first
order features, mean
(average gray level intensity within the patch)
= Ti-weighted-MRI pre-contrast, computed within the periphery region, first
order features,
robust mean absolute deviation (the mean distance of all intensity values from
the Mean
Value calculated on the subset of image array with gray levels in between, or
equal to the
10th and 90th percentile)
= T1-weighted-MRI pre-contrast, computed within the core region, first
order features,
median gray level intensity within the patch
- 22 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
= T1-weighted-MRI pre-contrast, computed within the core region, gray-level
Co-
occurrence Matrix, sum entropy (sum of neighborhood intensity value
differences)
= T2-weighted MRI computed within the periphery region, first order values,
root mean
squared (the square-root of the mean of all the squared intensity values,
another measure
of the magnitude of the image values)
= T2-weighted MRI computed within the periphery region, first order values,
maximum gray
level intensity within the patch
= T1-weighted-MRI pre-contrast, computed within the core region, Gray-level
Size Zone
Matrix, zone entropy (measures the uncertainty/randomness in the distribution
of zone
sizes and gray levels, higher values indicate more heterogeneneity in the
texture patterns)
= T1-weighted-MRI pre-contrast, computed within the periphery region, gray-
level Run-
Length-Matrix, run length nonuniformity (measures the similarity of run
lengths
throughout the image, a lower value indicates more homogeneity among run
lengths in the
image)
= T1-weighted-MRI pre-contrast, computed within the core region, gray-level
Run-Length-
Matrix, run entropy (measures the uncertainty/randomness in the distribution
of run lengths
and gray levels; higher values indicate more heterogeneity in the texture
patterns)
= T2-weighted MRI, computed within the periphery region, first order
values, skewness
(measures asymmetry of distribution of values about the mean value; can be
positive or
negative)
= T2-weighted MRI computed within the core region, first order values, mean
(average gray
level intensity within the patch)
= T2-weighted MRI computed within the periphery region, first order values,
90th percentile
of voxels in the patch
100701 More generally, the machine learning classifier may employ
one or more machine
learning systems, methods, and/or models. A machine learning model may be
considered as a
model configured to receive input, and to apply one or more of a weight, bias,
classification, or
analysis on the input to generate an output. The output may include, for
example, a classification
- 23 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
of the input, an analysis based on the input, a design, process, prediction,
or recommendation
associated with the input, or any other suitable type of output. A machine
learning model
generally is trained using training data, e.g., experiential data and/or
samples of input data, such
as the types of training data described elsewhere herein, which are fed into
the model in order to
establish, tune, or modify one or more aspects of the model, e.g., the
weights, biases, criteria for
forming classifications or clusters, or the like. Aspects of a machine
learning model may operate
on an input linearly, in parallel, via a network (e.g., a neural network), or
via any suitable
configuration.
100711 Training sets (e.g. subject image data captured at two or
more points in time) may be
used as inputs to train the machine learning classifier, may facilitate a
selection or combination of
machine learning methods, directed toward creating an optimal combination of
such methods. One
goal of such selection is the creation of an optimal subset of features to
provide separation on a
reduced imaging biomarker space between lesion types (e.g., acute versus
chronic lesions or SEL
versus non-SEL) or amount or degree of progression. In an embodiment, linear
and non-linear
feature-to-class correlation tests may be used to identify the features that
account for the highest
variance between the classifications.
100721 This evaluation and classification may employ an initial
feature ranking, such as shown
in Figure 17, and an initial feature selection that may, for example, identify
a number of features
with the strongest individual correlation. In an embodiment, there may be 50
such features selected
as a feature subspace. From those features, embedded selection methods can
leverage tree-based
classifiers and sparse linear models including a least absolute shrinkage and
selection operator
(LASSO).
100731 In this manner, starting from a feature subspace as just
mentioned, it is possible to
conduct a study, in the course of which a number of features in the feature
subspace may be
decremented by removing a feature, so as to eliminate the least useful feature
at each step. In this
fashion, an ensemble classifier can be optimized at each step. The process can
proceed recursively,
cycling between optimization and feature removal, to arrive at a ranking in
which each
decremented combination of radiomic features is associated with the lesion
classification objective
(e.g. active versus chronic or SEL versus non-SEL).
- 24 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
100741 The outcome of this ensemble classification mechanism may be
a selected subset of
classification methods that may involve linear classification methods (e.g.,
logistic regression,
support vector machines) and/or non-linear classification methods (e.g.,
perceptron, deep
convolutional neural networks or other types of neural networks) which act to
optimize separability
between the two classes (active and chronic).
[0075] In an embodiment, a pool of machine learning models may
undergo hyperparameter
tuning via an extensive randomized grid search, which may be followed by a k-
fold cross-
validation on the classification task of interest. This tuning may then lead
to a performance
benchmark that can select the highest performing models, for example, the n
top-performing
models. These models may then be combined under a stacking or a winner takes
all or a
probabilistic importance sampling ensemble strategy.
[0076] Implementing a machine learning model may include deployment
of one or more
machine learning techniques, including various types of neural networks, and
statistical techniques
such as linear regression, logistical regression, random forest, or gradient
boosted machine (GBM).
Depending on the embodiment, training of the machine learning model may be
supervised, or
unsupervised, or both. Supervised learning may include providing training data
and labels
corresponding to the training data. Unsupervised training may include
clustering, classification, or
the like. Different types of clustering, or combinations of clustering, also
may be used, and these
may be supervised or unsupervised.
[0077] In an embodiment, a machine-learning based classifier may
include one or more of a
plurality of types of neural networks, including convolutional neural networks
(CNN), deep or
fully convolutional neural networks (DCNN, FCNN), deep learning neural
networks (DNN), deep
belief networks (DBN), and others with which ordinarily skilled artisans will
be familiar.
[0078] A machine learning system which may be part of a machine
learning model may
include one or more processors, one or more storage devices intended for non-
volatile non-
transitory storage, and one or more memory devices, which may be volatile
memory for transitory
storage, but which al so may include non-volatile memory for non-transitory
storage. A plurality
of machine learning methods, implemented by one or more machine learning
systems, may be
employed as part of the ensemble classification process. In an embodiment, the
processors in a
- 25 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
machine learning system may be graphics processing units (GPUs) or central
processing units
(CPUs), which can lend themselves to neural network structures or other
learning frameworks.
[0079]
Some literature distinguishes among machine learning, deep learning,
artificial
intelligence, and multiple instance learning in various ways. For purposes of
the present
discussion, any or all of these approaches may provide the necessary structure
and functionality to
accomplish one or more inventive goals as described herein.
Dosing Regimens
[0080]
The present disclosure also provides biphasic dosing regimens for
reducing
pathological inflammation with natalizumab, wherein the dosing regimens
comprise an induction
phase employing standard interval dosing (SID) followed by a chronic phase
employing extended
interval dosing (BID). In some embodiments, at least one treatment phase
employs subcutaneous
administration.
In some embodiments, both treatment phases employ subcutaneous
administration. In some embodiments, the same dose administered during the SID
phase can be
administered during the EID phase, and in some embodiments the same dose
administered IV can
be administered SC.
[0081]
The biphasic dosing regimen contemplated herein refers to the
administration of
natalizumab in at least two phases, e.g., an induction phase and a chronic
phase. In some
embodiments, the induction phase comprises administration of natalizumab on an
SlD schedule
and the chronic phase comprises administration of natalizumab on an EID
schedule. In some
embodiments, the induction phase comprises administration of natalizumab once
every 2 weeks,
about once every 2 weeks, once every 3 weeks, about once every 3 weeks, once
every 4 weeks,
about once every 4 weeks, once every 30 days, about once every 30 days, once a
month or about
once a month for at least 6 months, for at least 8 months, for at least 10
months, or for at least 12
months. In some embodiments, the induction phase is from 6 to 18 months, from
8 to 16 months,
from 10 to 14 months, is 11 months, is 12 months, or is 13 months. In some
embodiments, the
chronic phase comprises administration of natalizumab once every 5 to 10
weeks. In some
embodiments, the chronic phase comprises administration of natalizumab every 5
weeks, about
every 5 weeks, every 6 weeks, about every 6 weeks, every 7 weeks, about every
7 weeks, every 8
weeks, or about every 8 weeks. In some embodiments, both the induction phase
and the chronic
- 26 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
phase comprise SC administration. In some embodiments, the induction phase and
the chronic
phase are solely SC administration. In some embodiments, surprisingly, the SC
dosing and
amount of natalizumab can be consistent with IV dosing.
100821 In some embodiments, the therapeutically effective amount
administered during the
induction phase and the chronic phase are the same, and the therapeutically
effective amount is
between 250 ¨ 450 mg (e.g., 250 mg, 300 mg, 350 mg, 400 mg, or 450 mg), more
preferably about
300 mg, still more preferably 300 mg. In some embodiments, the therapeutically
effective amount
administered SC during the chronic phase is between 300 - 500 mg (e.g., 300
mg, 350 mg, 400
mg, 450 mg, or 500 mg). In some embodiments, the therapeutically effective
amount is between
about 250 ¨ about 450 mg (e.g., about 250 mg, about 300 mg, about 350 mg,
about 400 mg, or
about 450 mg), more preferably about 300 mg, still more preferably 300 mg. In
some
embodiments, the therapeutically effective amount administered SC during the
chronic phase is
between about 300 - about 500 mg (e.g., about 300 mg, about 350 mg, about 400
mg, about 450
mg, or about 500 mg), more preferably about 300 mg, still more preferably 300
mg. Representative
biphasic dosing regimens are disclosed in U.S. Provisional Application Serial
Nos. 63/113,864
(filed November 14, 2020), 63/113,865 (filed November 14, 2020), 63/142,968
(filed January 28,
2021), 63/142,970 (filed January 28, 2021), and co-pending International
Application No.
PCT/1JS2021/059266, the disclosures of which are expressly incorporated by
reference herein
100831 The present disclosure also provides a chronic dosing regimen
for reducing
pathological inflammation with natalizumab. In some embodiments, natalizumab
is administered
in a chronic dose regimen, wherein the chronic dosing regimen comprises
administration of
natalizumab at a fixed interval of every 4 weeks. In some embodiments, the
chronic dosing
regimen is a fixed, non-weight based amount of natalizumab. In some
embodiments, a
therapeutically effective amount of natalizumab is between about 250 ¨ 450 mg,
more preferably
about 300 mg, still more preferably a fixed, non-weight based dose of 300 mg.
In further
embodiments, the chronic dosing regimen is every 4 weeks for a period of about
4 months, about
months, about 6 months, about 7 months, about 8 months, about 9 months, about
10 months,
about 11 months, about 12 months, or longer than about 12 months.
Representative chronic dosing
regimens are disclosed in WO 2003/072040, the disclosure of which is expressly
incorporated by
reference herein.
- 27 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
100841 In some embodiments, the chronic dosing regimen comprises SC
injection. In some
embodiments, the chronic dosing regimen comprises IV administration.
Representative SC
administration formulations are disclosed in WO 2008/157356, the disclosure of
which is
expressly incorporated by reference herein.
100851 Relevant biomarkers for determining and/or monitoring
efficacy of the treatment
protocols provided herein include, e.g., sVCAM and/or Nf-L. Without being
bound by theory,
increased saturation and/or occupancy by natalizumab of its target a4 integrin
on the surface of
circulating lymphocytes leads to decreased surface expression of a4-integrin
on lymphocytes, as
well as decreased serum concentration of sVCAM. Correspondingly, sVCAM
provides an
effective surrogate biomarker for a4-integrin receptor saturation, and for
immune surveillance
activity in general, see, e.g. Plavina et at., Neurology (2017) 89(15):1584-
1593. Neurofilament
proteins such as Nf-L, in contrast, provide an indication of axonal damage and
neuronal death, and
serve as effective surrogate biomarkers for ongoing disease activity in MS
patients in particular.
See, e.g., Kuhle et at. Mult Scler. (2013) 19:1597-603; Varhaug et at., Front
Neurol (2019) 10:
338.
100861 These biphasic dosing regimens are provided for increasing
the safety of natalizumab
therapy. In some embodiments, the biphasic dosing regimens are provided for
increasing the
safety of chronic natalizumab therapy. Safety may be increased by reducing the
risk of an adverse
event, e.g. PML. In some cases, the biphasic regimen reduces the risk of PML,
reduces the risk of
inducing generation of anti-natalizumab antibodies, reduces the risk of
patient sensitization to
natalizumab, or a combination thereof In some cases, the biphasic regimen
reduces the risk of
loss of efficacy of natalizumab treatment due to the generation of anti-
idiotypic antibodies to
natalizumab in the patient.
100871 Patients who are seropositive for anti-JCV antibodies are at
a particularly high risk of
PML. In some embodiments, a PML risk subject has an anti-JCV antibody index
level (e.g., a
mean index level) of greater than 1.5. In some embodiments, a low PML risk
subject is a subject
who has an anti-JCV antibody index level (e.g., a mean index level) of less
than or equal to 0.9.
Anti-JC virus index values are calculated from a two-step ELISA antibody assay
of serum/plasma
(STRATIFY JCVTM Antibody (with Index) with Reflex to Inhibition Assay; see,
e.g., Lee, P. et
J of Clin Virol, 2013;57(2):141-146, incorporated herein by reference).
Antibody index level,
- 28 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
assays for assessing index level, and the use of such index levels and assays,
for determining PML
risk are described in, e.g., WO 2012/166971 and WO 2014/193804.
100881 A subject may be considered a high PML risk if the subject
tested seropositive for anti-
JCV antibodies prior to commencement of natalizumab therapy, or if the subject
switches from a
seronegative anti-JCV antibody status to a seropositive anti-JCV antibody
status during
natalizumab therapy. In some embodiments, a subject is considered a high PML
risk if the subject
has an anti-JCV antibody index level of greater than 1.5 prior to commencement
of natalizumab
therapy, or if the subject switches from a lower anti-JCV antibody index level
of less than or equal
to 0.9 to a higher anti-JCV antibody index level of greater than 1.5 during
natalizumab therapy.
For example, prior to starting natalizumab therapy, a subject may be tested
for the presence or
absence of anti-JCV antibodies. If the test results indicate that the subject
is a low PML risk
subject (seronegative for anti-JCV antibodies, or having an anti-JCV antibody
index level of less
than or equal to 0.9), then the subject may be identified as a subject for
natalizumab therapy on a
SID schedule of 4-week intervals. During the course of the natalizumab therapy
on a SID schedule,
the subject may be re-tested for the presence or absence of anti-JCV
antibodies (e.g., tested every
month or every 2, 3, 4, 5 or 6 months, or every year). If upon re-testing the
subject has switched
from seronegative to seropositive for anti-JCV antibodies, or from having an
anti-JCV antibody
index level of less than or equal to 0 9 to having an anti-JCV antibody index
level of greater than
1.5, then the subject may be identified as a subject for natalizumab therapy
on an ElD schedule of
at least 5-week intervals.
EXAMPLES
Example 1: Association between chronic white matter lesion activity and
disability
progression in SPMS patients with or without acute inflammation
100891 Objective: Slowly expanding lesions (SELs), a subgroup of
white matter lesions that
gradually expand over time, have been shown to predict disability accumulation
in primary
progressive multiple sclerosis (MS) disease. The relationships between SELs,
acute white matter
lesion activity (AWMLA), chronic white matter lesion activity (CWMLA), and
disability
progression are not well understood. This study assessed CWMLA and acute
lesion activity
(AWMLA) in the brain white matter of a secondary progressive MS population.
- 29 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
100901 CWMLA was measured in this study by the change in TILV from
baseline to week
108 in SELs, non-SELs, and total pre-existing chronic nonenhancing T2 (CNT2)
lesions. AWMLA
was defined by having either 1) gadolinium-enhancing (Gd+) Ti lesions at any
time point in the
trial up to week 108, including baseline, or 2) any postbaseline new or
enlarging T2 lesions. The
following was examined in a secondary progressive MS population treated with
placebo: 1) the
association between T1-weighted (Tlw) MRI features of CWMLA in SELs and non-
SELs and
confirmed disability progression, 2) the association between CWMLA and
confirmed disability
progression in the absence of AWMLA, and 3) the association between CWMLA and
AWMLA.
In this study, we also examined the ASCEND phase 3 clinical trial
(ClinicalTrials.gov no.
NCT01416181), which compared natalizumab with placebo in secondary progressive
MS (SPMS).
Materials and Methods
Trial Design, Patients, and MRI
100911 The ASCEND study (ClinicalTrials.gov no. NCT01416181) was a
two-part,
multicenter, randomized, double-blind, placebo-controlled phase 3 study in
patients with SPMS to
assess the efficacy and safety of natalizumab. Details of the study design and
outcomes have
previously been described in detail. Kapoor et at. Lancet Neurol 2018; 17:405-
415. Axial 11W
(3D Spoiled gradient echo: TR=28-35 ms; TE=4-11 ms; flip angle=27 -30 ;
resolution
0.98><0.98x3 mm) and Axial T2W (2D Fast Spin Echo: TR=4000-7400 ms; TE=58-95
ms;
resoluti on=0.98 x 0.98 x3 mm) were acquired at baseline, week 24, week 48,
week 72, week 96,
and week 108. The SEL analysis population represents the subset of the
intention-to-treat
population that had available Tlw and T2-weighted (T2w) images at all time
points from baseline
to week 108 (including weeks 24, 48, 72, and 96).
Clinical Measures of Disability Progression
100921 Expanded Disability Status Scale (EDSS), Timed 25-Foot Walk
(T25FW), and 9-Hole
Peg Test (9HPT) assessments were performed at baseline and every 12 weeks
through week 108.
Composite confirmed disability progression was defined as meeting one or more
of the following
three criteria: an increase of >1.0 point from a baseline EDSS score <5.5 or
an increase of >0.5
point from a baseline score >6.0, an increase of >20% from baseline in T25FW
time, and/or an
increase of >20% from baseline in 9HPT time (on either hand). Progression was
confirmed at a
subsequent visit >6 months after the possible start of progression and at the
end of the trial. To
- 30 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
minimize the possibility of capturing disability progression due to clinical
relapses, included
confirmed disability progression events could not have started or been
confirmed <74 days after
onset of an independent neurology evaluation committee¨confirmed clinical
relapse. Absence of
acute lesion activity was defined as no baseline or postbaseline Ti gadolinium-
enhancing (Gd+)
lesions and no postbaseline new or enlarging T2-hyperintense lesions.
Identification of SELs, AWMLA, and overall CWMLA
100931 The process of SEL identification has been described in
detail elsewhere. Elliott et al.
Brain 2019; 142:2787-2799 and Elliott el al. Mult Scler 2019; 25:1915-1925.
Briefly, SELs are
contiguous regions of preexisting T2 lesions showing constant and concentric
local expansion
from baseline to week 108. Prior to SEL detection, T2 lesions were identified
in baseline scans
using a semi-automated method in which a fully automated segmentation of T2
lesions was
subsequently manually reviewed and corrected by a single trained MRI reader.
Francis SJ. In:
McGill University DoN, ed., 2005. In the first stage of SEL detection, SEL
candidates are
identified as contiguous regions of >10 voxels in the baseline T2 lesion mask
that a) are not Gd+
and b) show a minimum local volumetric expansion, as determined by the
Jacobian determinant
of the nonlinear deformation between the baseline and week 108 scans. The
second stage of SEL
detection scores each SEL candidate in turn on the basis of the concentricity
and constancy of
expansion across time. Considering local expansion between baseline and each
intermediate time
point (weeks 24, 48, 72, and 96) allows for the identification of SEL
candidates undergoing
constant and gradual expansion across time, while measuring concentricity
allows for the
identification of SEL candidates exhibiting inside-out radial expansion. Each
SEL candidate is
assigned a SEL score, calculated as the sum of the mean normalized measures
for constancy and
concentricity.
100941 SELs were identified in SPMS patients from the ASCEND phase 3
clinical trial
(ClinicalTrials.gov no. NCT01416181). Non-SELs, defined as the portion of the
nonenhancing
baseline T2 lesion mask not identified as SELs, were also assessed, as were
the totality of
nonenhancing T2 lesions. The ASCEND SEL analysis population (placebo, n = 292;
natalizumab,
n = 308) represents the subset of patients who had available Tlw and T2w
images at all time points
from baseline to week 108. Results are presented for SELs with SEL score >0.
The distribution
-3i -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
of SELs and non-SELs by sex, baseline EDSS, age, and disease duration are
provided in Tables 1-
3.
Table 1: SEL Count
Baseline Age, Years <=45 >45-52 >52
n 210 206 184
Mean 7.5429 8.0825 7.5435
Standard deviation 8.83991 11.39396 10.69489
Median 4.0000 4.0000 3.5000
25%, 75% percentile 1.0000, 12.0000 1.0000, 10.0000 1.0000,
11.0000
Min, Max 0.000, 42.000 0.000, 78.000 0.000, 61.000
Years since First <=8 >8-15 >15
MS symptoms
n 206 213 177
Mean 7.0437 8.0282 8.113
Standard deviation 9.28324 10.38364 11.37675
Median 3.0000 4.0000 4.0000
25%, 75% percentile 1.0000, 9.0000 1.0000, 12.0000 1.0000,
9.0000
Min, Max 0.000, 42.000 0.000, 61.000 0.000, 78.000
- 32 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
Baseline EDSS High EDSS > 6.0 Low EDSS <6.0
361 239
Mean 8.5014 6.5607
Standard deviation 11.05972 9.01466
Median 4.0000 3.0000
25%, 75% percentile 1.0000, 12.0000 1.0000, 8.0000
Min, Max 0.000, 61.000 0.000, 78.000
Gender Female Male
381 219
Mean 8.2572 6.8082
Standard deviation 10.65324 9.69677
Median 4.0000 3.0000
25%, 75% percentile 1.0000, 11.0000 1.0000, 8.0000
Min, Max 0.000, 78.000 0.000, 59.000
Table 2: SEL Volume, Ti-weighted
Baseline Age, Years <=45 >45-52 >52
210 206 184
Mean, mm3 688.81 748.97 799.79
- 33 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
Standard deviation, 1472.156 1552.809 2127.059
mm3
Median, mm3 117.1 200.29 111.44
25%, 75% 5.72, 738.20 5.72, 586.55 2.86, 708.16
percentile, mm3
Min, Max, mm3 0.0, 14131.7 0.0, 10809.8 0.0, 23708.3
Years since First <=8 >8-15 >15
MS symptoms
206 213 177
Mean, mm3 750.11 680.26 812.86
Standard deviation, 1748.405 1178.017 2198.383
nnrn3
Median, mm3 101.00 145.92 145.92
25%, 75% 0.00, 595.14 5.72, 726.76 31.47, 515.02
percentile, mm3
Min, Max, mm3 0.0, 14131.7 0.0, 8123.1 0.0, 23708.3
Baseline EDSS High EDSS > 6.0 Low EDSS <6.0
361 239
Mean, mm3 848.52 584.86
- 34 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
Standard deviation, 1944.682 1304.472
mm3
Median, mm3 151.65 105.87
25%, 75% 8.58, 738.20 2.86, 517.88
percentile, mm3
Min, Max, mm3 0.0, 23708.3 0.0, 14131.7
Gender Female Male
381 219
Mean, mm3 791.37 660.22
Standard deviation, 1631.066 1870.948
mm3
Median, mm3 151.65 103.00
25%, 75% 14.31, 663.81 0.00, 595.14
percentile, mm3
Min, Max, mm3 0.0, 14131.7 0.0, 23708.3
Table 3: non-SEL Volume, Ti-weighted
Baseline Age, Years <=45 >45-52 >52
210 206 184
Mean, mm3 4703.94 4785.52 4949.89
- 35 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
Standard deviation, 5107.976 5174.203 6318.338
mm3
Median, mm3 2658.10 3107.31 2778.27
25%, 75% 1084.41, 7027.21 1178.83, 6878.43 816.89,
5945.66
percentile, mm3
Min, Max, mm3 34.3, 27333.5 8.6, 31751.2 0.0, 45313.5
Years since First <=8 >8-15 >15
MS symptoms
206 213 177
Mean, mm3 4184.01 4811.28 5563.19
Standard deviation, 5141.369 5246.273 6203.788
mm3
Median, mm3 2123.04 2969.97 3525.05
25%, 75% 603.72, 5808.32 1010.02, 6712.48 1599.43,
7264.70
percentile, mm3
Min, Max, mm3 8.6, 27851.3 0.0, 31751.2 68.7, 45313.5
Baseline EDSS High EDSS > 6.0 Low EDSS <6.0
361 239
Mean, mm3 4938.26 4609.67
- 36 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
Standard deviation, 5491.272 5569.331
mm3
Median, mm3 3061.53 2509.31
25%, 75% 1078.69, 6878.43 875.54, 6434.93
percentile, mm3
Min, Max, mm3 0.0, 45313.5 8.6, 30189.0
Gender Female Male
381 219
Mean, mm3 5049.94 4385.38
Standard deviation, 5860.391 4856.158
mm3
Median, mm3 3061.53 2615.18
25%, 75% 841.21, 7138.80 1101.58, 5851.24
percentile, mm3
Min, Max, mm3 22.9, 45313.5 0.0, 27851.3
100951 AWMLA was defined by having either 1) Gd+ Ti lesions at any
time point in the trial
up to week 108, including baseline, or 2) any postbaseline new or enlarging T2
lesions. Gd+ Ti
lesions were determined as a consensus of 2 fully manual identifications by 2
trained MRI readers,
where any discrepancies were adjudicated by a third independent reader. New or
enlarging T2
lesions were determined by comparing T2 lesion masks at successive timepoints
and automatically
identifying focal areas of new T2 lesions, which were not present at the
previous timepoint and
showed a minimum increase in T2-weighted intensity. These focal areas of new
T2 lesions could
- 37 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
be entirely in NAWM (new) or adjacent to pre-existing T2 lesions (enlarging).
All automatically
identified new or enlarging T2 lesions were manually reviewed and corrected
where necessary.
100961 It is important to appreciate that the SEL approach to the
detection of lesion expansion
is fundamentally different from that used for the detection of so-called new
'T2 enlarging lesions',
commonly reported in counts of 'new or enlarging T2 lesions' in clinical
trials. Arnold et al. Mull
Scler 2021; 27(11): 1681-3. Methods for the detection of 'enlarging' lesions
in the context of 'new
or enlarging T2 lesion counts' (which vary from laboratory to laboratory) have
been designed to
detect what are essentially new foci of acute white matter lesion activity
that are connected by
adjacency to areas of pre-existing T2-signal abnormality and therefore may not
qualify as `de
novo' new lesions (which by definition have to be surrounded by normal-
appearing WM).
CWMLA was measured by the change in T1 -hypointense lesion volume (T1LV) from
baseline to
week 108 in SELs, non-SELs, and total pre-existing chronic nonenhancing T2
(CNT2) lesions in
SPMS patients in the placebo arm. T 1-hypointense lesions were defined as
areas of T2-lesion not
showing gadolinium enhancement and with TI-weighted intensity less than or
equal to median
Ti-weighted intensity of gray matter.
100971 Whole brain atrophy was measured via Jacobian integration.
Nakamura et al.
Neuroimage Clin 2014; 4: 10-17.
Statistical Analyses
100981 The statistical analysis of SEL data was exploratory and
included all patients from
ASCEND with no missing or nonevaluable Tlw and T2w scans at any time point
(baseline to
week 108; SEL analysis population). No imputation of missing data was
performed.
100991 A two-sample proportion test was applied to compare baseline
Gd+ Ti lesions between
the two treatment groups. CWMLA was compared between progressors and
nonprogressors using
the Van Elteren test, stratified for progression status, baseline EDSS score
(<5.5 or >6.0), and
baseline T2-hyperintense lesion volume (T2LV) category based on tertiles
(<6908.79 mm3,
>6908.79-18818.49 mm3, and >18,818.49 mm3). Analyses of the association
between AWMLA
and SEL prevalence were based on the Van Elteren test, stratified for AWMLA,
baseline EDSS
score (<5.5 or >6.0), and baseline T2LV category based on tertiles (<6908.79
mm3, >6908.79-
18818.49 mm3, and >18818.49 mm3). Comparisons of CWMLA between placebo and
natalizumab
were based on the Van Elteren test, stratified for treatment, baseline EDSS
score (<5.5 or
- 38 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
and baseline T2LV category based on tertiles (<6908.79 mm3, >6908.79-18818.49
mm3, and
>18818.49 mm3). Statistical tests were two-sided and conducted at the 5%
significance level
without adjustment for multiplicity.
Results
Baseline Demographics and Brain MM Characteristics of the SPMS Analysis
Population
1001001 The baseline demographics and brain MRI characteristics of the
analysis population
available for SEL detection and the intention-to-treat population from the
ASCEND study dataset
are presented in Table 4. Age and gender were distributed similarly across the
SEL analysis and
intention-to-treat populations and between treatment arms. In the SEL analysis
population, a
greater percentage of natalizumab- than placebo-treated individuals had >1 Gd+
Ti lesion at
baseline (28% vs 19%), but the difference was not statistically significant In
the SEL analysis
population, the natalizumab- and placebo-treated groups had a similar mean
T2LV at baseline
(18.1 vs 16.5 cm3). Mean normalized brain volume at baseline was also similar
in the two treatment
groups.
TABLE 4. Baseline Characteristics of ASCEND Analysis Population
SEL Analysis Population ITT Population
Placebo Natalizumab Placebo
Natalizumab
Baseline Characteristics (n = 292) (n = 308) (n = 448)
(n = 439)
Age, mean (SD) 47.8 (7.6) 47.4 (7.2) 47.2 (7.8)
47.3 (7.4)
Female, % 65 62 63 62
Patients with >1 Gd+ Ti lesion, % 19 28 22 26
Mean (SD) T2LV, cm3 16.5 (16.7) 18.1 (1R.5)
16.2 (16.4) 17.4 (17.6)
Normalized brain volume, mean (SD), cm3 1429 8 (81 64) 1422 0 (81 75)
1425 8 (83 1) 1420.9 (82 8)
ASCEND SEL analysis population represents the subset of the ITT population
that had available T1- and T2-weighted images at all time
points from baseline to week 108 (including weeks 24, 48, 72, and 96).
Gd+ = gadolinium enhancing; ITT = intention to treat; SD = standard deviation;
SEL = slowly expanding lesion; T2LV = T2-
hyperintense lesion volume.
1001011 Disability Progression in SPMS Was Associated With Greater CWMLA in
the Placebo
Arm
101001 CWMLA was measured by the change in T1LV from baseline to
week 108 in SELs,
non-SELs, and total preexisting CNT2 lesions in SPMS patients treated with
placebo. The
- 39 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
analysis was restricted to the placebo group to avoid treatment effects, as
natalizumab decreases
AWMLA. CWMLA was compared in patients with composite disability progression (n
= 143)
and patients who remained progression free (n = 149). SPMS patients with
confirmed composite
disability progression had significantly more severe CWMLA than those who were
progression
free, as measured by T1LV change within SELs, non-SELs, and CNT2 lesions
(median increase
[Q1, Q3] progressors vs non-progressors: 100 [3, 524] vs 23 [0, 155] mm3,
p=0.0023; 231 [17,
1090] vs 109 [-29, 538] mm3, p=0.0170; and 372 [26, 1662] vs 160 [-23, 770]
mm3, p = 0.0026,
respectively; Fig 1A-C). In contrast, the brain atrophy rate as measured by
whole brain volume
change from baseline to week 108 did not differ significantly between SPMS
patients with and
without composite confirmed disability progression (p = 0.2176; Fig 1D).
101011 When confirmed disability progression was based only on EDSS
score, CWMLA as
measured by T1LV increase was significantly greater in progressors than
nonprogressors within
SELs (median increase [Q1, Q3] progressors vs non-progressors: 142 [6, 815] vs
39 [0, 258]
mm3, p = 0.0135; Fig 2A) and CNT2 lesions (median increase [Q1, Q31
progressors vs non-
progressors: 577 [66, 2529] vs 246 [0, 1090] mm3, p = 0.0375; Fig 2C), with a
consistent trend in
the same direction in non-SELs (median increase [Q1, Q3] progressors vs non-
progressors: 292
[26, 13021 vs 159 [-9, 7101 mm3, p = 0.1156; Fig 2B). Similarly, when
confirmed disability
progression was based only on 9T-TPT score, CWMLA as measured by T1T,V
increase was
significantly greater in progressors than nonprogressors within SELs (median
increase [Q1, Q3]
progressors vs non-progressors: 112 [0, 629] vs 37 [0, 258] mm3, p = 0.0051;
Fig 3A) and CNT2
lesions (median increase [Q1, Q3] progressors vs non-progressors: 549 [66,
1995] vs 197 [0,
1139] mm3, p = 0.0075; Fig 3C) and numerically greater in progressors than
nonprogressors
within non-SELs (median increase [Q1, Q3] progressors vs non-progressors: 240
[-9, 1097] vs
143 [-3, 6611 mm3, p = 0.0772; Fig 3B). Finally, when confirmed disability
progression was
based only on T25FW, CWMLA as measured by T1LV increase was significantly
greater in
progressors than nonprogressors within non-SELs (median increase [Q1, Q3]
progressors vs non-
progressors: 229 [20, 1133] vs 141 [-25, 609] mm3, p = 0.0230; Fig 4B) and
CNT2 lesions
(median increase [Q1, Q3] progressors vs non-progressors: 371 [23, 1634] vs
205 [-6, 954]
mm3, p = 0.0214; Fig 4C) and numerically greater in progressors than
nonprogressors within
SELs (median increase [Q1, Q3] progressors vs non-progressors: 100 13, 426] vs
29 [0, 220]
mm3, p = 0.0873; Fig 4A).
- 40 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
101021 Disability Progression in SPMS Remained Associated With
Greater CWMLA in the
Complete Absence of AWMLA in the Placebo Arm
101031 From baseline to week 108, 95 of 292 placebo SPMS patients
had no AWMLA,
defined as no baseline or postbaseline Gd+ Ti lesions and no postbaseline
new/enlarging T2
lesions. CWMLA in SPMS patients with no AWMLA was compared in patients with
composite
confirmed disability progression (n = 40) and patients who remained
progression free (n = 55).
Patients exhibiting composite progression had more severe CWMLA than those who
were
progression free, as shown by a significant difference in T1LV change within
non-SELs (p =
0.0045; Fig 5B) and CNT2 lesions (p = 0.0103; Fig 5C), and a trend in the same
direction was
observed in SELs (p = 0.2332, Fig 5A).
101041 SEL Prevalence in SPMS Patients Treated With Placebo Was
Lower in the Absence
of AWMLA
101051 Placebo SPMS patients with AWMLA had a higher SEL prevalence
as measured by
SEL number and volume (based on T2w borders of SELs at baseline) and a higher
proportion of
preexisting baseline T2LV identified as SELs than patients with no AWMLA (Fig
6A¨C). The
proportion of patients with at least one SEL was also higher in patients with
AWMLA (89%,
n=197) compared to those with no AWMLA (71%, n=95). Analysis of the
differences in SEL
prevalence between SPMS patients with no AWMLA (n = 95) and those with AWMLA
at
baseline only (n = 28) or post baseline (n = 169) confirmed that both baseline
and postbaseline
AWMLA were associated with a higher SEL prevalence, though the sample size of
patients with
AWMLA at baseline only was too small for the associated SEL prevalence to
reach significance
with respect to SEL number and relative volume (Fig 6A and C). An analysis of
the frequency
distribution of patients by range of SEL prevalence also showed that 19% of
patients with
AWMLA had >20% of their total T2 lesion burden identified as SELs, compared
with only 5%
of patients with no AWMLA (Fig 6D).
101061 Natalizumab Versus Placebo Effect on SELs and CWMLA in SPMS
Patients
101071 The placebo- and natalizumab-treated arms had similar
percentages of patients with
>1 SEL detected from baseline to week 108 (83% vs 79%).
- 41 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
101081 Natalizumab was associated with lower SEL prevalence than
placebo, as indicated by
a lower number of SELs (median number, 3 vs 4; p < 0.0001; Fig 7A) and a lower
absolute SEL
volume (median T2w SEL volume at baseline, 288 vs 561 mm3; p < 0.0001; Fig
7B).
Accordingly, the proportion of total baseline nonenhancing T2LV longitudinally
identified as
SELs was significantly lower in natalizumab- than placebo-treated patients
(median proportion,
2.7% vs 5.0%; p <O.000; Fig 7C).
101091 Brain tissue loss associated with CWMLA as measured by
absolute and relative
T1LV accumulation was lower in natalizumab- than placebo-treated patients
within both SELs
(Fig 8A and C) and non-SELs (Fig 8B and D).
Discussion
101101 SELs represent a subgroup of MS chronic white matter lesions
with continual
expansion and tissue destruction and predict clinical progression in
progressive-onset MS.
Elliott et al. Brain 2019; 142:2787-2799, Elliott et al. Mult Scler 2019;
25:1915-1925 and Elliott
et al. AJNR Am J Neuroradiol 2020; 41:1584-1591. In SELs, there is an
accumulation over time
of T1LV and changes in imaging metrics reflective of gradual microstructural
tissue alteration,
including a decrease in magnetization transfer ratio and an increase in
diffusion tensor imaging
radial diffusivity. Elliott et al. AJNR Am J Neuroradiol 2020; 411584-1591.
These findings
suggest chronic demyelinating processes and continual axonal/neuronal
destruction (Elliott et al.
AJNR Am J Neuroradiol 2020; 41:1584-1591.), which have been seen in pathology
studies of
chronic active lesions. Frischer et al. Ann Neurol 2015; 78:710-721 and
Luchetti et al. Acta
Neuropathol 2018; 135:511-528. However, it was recently reported that anti-
inflammatory
DMTs such as ocrelizumab (Elliott et al. Brain 2019; 142:2787-2799) and
natalizumab (Preziosa
et al. Mult Scler 2020: 1352458520969105) may have a modest effect on MRI
measures of brain
tissue loss in SELs.
101111 In this study, SELs were identified in most patients with
SPMS, and confirmed
disability progression was associated with more severe CWMLA as measured by
T1LV
accumulation in SELs but also in non-SELs. These findings are consistent with
previously
reported findings in relapsing MS patients in the OPERA I and II dataset and
primary
progressive MS patients in the ORATORIO dataset. Elliott et al. Brain 2019;
142:2787-2799 and
Elliott et al. Mult Scler 2019; 25:1915-1925). SPMS patients who progressed in
a natural history
- 42 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
setting had greater SEL prevalence and more severe CWMLA as measured by T1LV
increase
within SELs, non-SELs, and total baseline nonenhancing T2 lesions. In the
absence of AWMLA
in SPMS patients, SEL prevalence (as shown by number and volume of SELs) was
reduced,
though >70% of patients had >1 SEL. Importantly, the association of confirmed
disability
progression with more severe CWMLA in SPMS patients remained significant in
the absence of
AWMLA. Consistent findings were reported in placebo-treated primary
progressive MS patients
in the ORATORIO dataset, in whom AWMLA, CWMLA, and whole brain atrophy were
measured and only CWMLA in SELs and non-SELs predicted confirmed disability
progression
over time. Elliott et al. Brain 2019; 142:2787-2799. This indicates that brain
tissue loss
associated with CWMLA may be an important driver of disability progression
independent of
AWMLA in MS.
101121 Compared with placebo, natalizumab treatment significantly
reduced the number and
volume of SELs and the proportion of baseline nonenhancing T2 lesions
identified as SELs.
Natalizumab reduced CWMLA as measured by T1LV increase in both SELs and non-
SELs. The
significant association between AWMLA and SEL prevalence suggests that the
effect of
natalizumab on CWMLA in progressive MS patients could be related to its high
efficacy in
suppressing acute inflammation. Prior studies demonstrating the effects of
natalizumab and
depletion of CD20-expressing cells further support this finding Kappos et al
JAMA Neurol
2020; 77:1132-1140, Montalban et al. N Engl J Med 2017; 376:209-220, Polman et
al. N Engl J
Med 2006; 354:899-910, Butzkueven et al. J Neurol Neurosurg Psychiatry 2014;
85:1190-1197,
and Hauser et al. N Engl J Med 2017; 376:221-234. The effects of natalizumab
on chronic active
lesions have also previously been demonstrated in positron emission tomography
studies
measuring activated microglia. Kaunzner et al. Mult Scler Relat Disord 2017;
15:27-33 and
Sucksdorff et al. Neurol Neuroimmunol Neuroinflamm 2019; 6:e574. In these
studies,
natalizumab decreased PK11195 uptake, reflective of activated microglia and
macrophages, in
nonenhancing lesions (Kaunzner et al. Mult Scler Relat Disord 2017; 15:27-33)
and more
specifically at the rim of chronic active lesions (Sucksdorff et al. Neurol
Neuroimmunol
Neuroinflamm 2019; 6:e574).
101131 It is plausible that the smoldering inflammation that
contributes to CWMLA and
disability progression may be influenced by acute inflammation in MS. The
recently published
long-term brain MRI follow-up of primary progressive MS patients continuously
treated with
- 43 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
ocrelizumab over 6.5 years in the ORATORIO study (Wolinsky et al. Lancet
Neurol 2020;
10:998-1009) demonstrated an annual increase in T1LV of approximately 3-6%
(reaching a total
increase of 37% from baseline at approximately 6 years), which may be
attributable to CWMLA
in SELs and non-SELs independent of AWMLA, as those patients were shown to be
devoid of
AWMLA from week 48 onward.
101141 Without wishing to be bound by any one particular theory, the
effects of natalizumab
and/or ocrelizumab on CWMLA (in SELs and non-SELs) and/or disability
progression in
progressive forms of MS may be principally explained by their capacity to
silence AWMLA. For
example, an extended approach using Bayesian inference for a principal stratum
estimand can be
used to assess the treatment effect in subgroups characterized by AWMLA
covariate thresholds
as a postrandomization event occurrence. Magnusson et al. Stat Med 2019;
38(23): 4761-4771.
101151 Efforts to further refine MS lesion histopathological
terminology are ongoing
(Kuhlmann et al. Acta Neuropathol 2017; 133:13-24), but understanding of the
natural history of
chronic active lesion phenotypes remains elusive, as their lifespan could
begin decades before
specimens become available. Dal-Bianco et al. Brain 2021; 144:833-847
doi.org/10.1093/brain/awaa436. Molecular studies of the lesion rim of chronic
active or mixed
active/inactive lesions show a predominance of Ml-polarized macrophages and
activated
microglia. Jackie et al. Brain 2020; 143:2073-2088. Innate and adaptive immune
system
interaction can bidirectionally influence polarization and perpetuate the
disease process.
Strachan et al. J Interferon Cytokine Res 2014; 34:615-622. Phase rims
detected on
susceptibility-weighted imaging are thought to represent an imaging biomarker
of rims of iron-
laden microglia/macrophages and have been linked to disease severity and brain
atrophy. SELs
(Kaunzner et al. Brain 2019; 142:133-145, Absinta et al. J Clin Invest 2016;
126:2597-2609,
Dal-Bianco et al. Acta Neuropathol 2017; 133:25-42, and Absinta et al. JAMA
Neurol 2019;
76:1474-1483) and phase rim lesions (PRLs) may both reflect chronic active
lesions (Preziosa et
al. Mult Scler 2020:1352458520969105). Recent work has demonstrated a partial
concordance
between SELs and PRLs, as a substantial proportion of PRLs do not appear to
expand over time
and some SELs appear to be devoid of phase rims entirely (Elliott et al.
Neurology 2021; 96: (15
Supplement) 4101). As demonstrated herein, elucidating the complementarity of
SEL, non-SEL,
and PRL measures of CWMLA can expand the characterization of longitudinal
tissue alteration
properties within distinct MRI phenotypes of chronic active lesions.
- 44 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
101161 In conclusion, CWMLA in SELs and non-SELs is an imaging
biomarker of chronic
active lesions and/or secondary axonal degeneration that reflects ongoing
inflammation and is
associated with clinical disability progression. We demonstrate that the
presence of AWMLA,
whether new Gd+ lesions or new T2-hyperintense lesions, is associated with a
higher SEL
prevalence in SPMS patients. This indirect evidence for the influence of AWMLA
on CWMLA
highlights the importance of limiting acute inflammation with highly effective
disease-modifying
therapies. However, even in the absence of AWMLA, the association between
CWMLA and
confirmed disability progression persists, albeit to a more limited extent,
with the greater
increase in Tlw lesion volume in progressors than in nonprogressors seen
primarily within non-
SEL tissue of T2w nonenhancing lesions.
101171 The onset of the effect of natalizumab on CWMLA was rapid in
this study, with a
statistically significant reduction in Tlw lesion volume increase in SELs and
non-SELs seen
starting at 24 weeks of treatment. Taken together, these results highlight the
need to continue to
develop therapies that impact AWMLA but also more specifically target CWMLA,
with the hope
of blocking smoldering inflammation and neurodegenerative pathways, which may
both
contribute to CWMLA.
101181 Patients with complete imaging datasets between baseline and
week 108 (N = 600)
were analyzed for SEL prevalence (the number and volume of SELs), disability
progression,
AWMLA (assessed by gadolinium-enhancing lesions and new T2-hyperintense
lesions), and
CWMLA (assessed by Ti-hypointense lesion volume increase within baseline T2-
nonenhancing
lesions identified as SELs and non-SELs).
101191 Results: CWMLA in both SELs and non-SELs was greater in SPMS
patients with
confirmed disability progression than in those with no progression. In the
complete absence of
AWMLA at baseline and on study, SEL prevalence was significantly lower, while
CWMLA
within non-SELs remained associated with disability progression. Natalizumab
decreased SEL
prevalence and CWMLA in SELs and non-SELs compared with placebo.
Example 2: MRI characteristics of phase rim lesions in chronic and recent
acute MS lesions
- 45 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
101201 Objective: To determine the prevalence of phase rim lesions
(PRLs), their
association with acute new T2-lesions, and to quantify normalized Ti-weighted
intensity (nT1),
normalized magnetization transfer ratio (nMTR) and diffusion tensor imaging
radial diffusivity
(DTI-RD) in T2-lesions with (PRL+) and without (PRL-) phase rims, in a
relapsing multiple
sclerosis(RMS) population.
101211 Background: Chronic active lesions are a subset of MS lesions
thought to be
represented by phase rim signals in susceptibility-weighted MR images.
101221 Design/Methods: PRL data was collected at follow-up weeks 72
and 96 in a subset
of RMS patients from the AFFINITY trial [NCT03222973] (N=44) using
standardized 3-Tesla,
3D isotropic multi-echo, gradient echo MRI.
101231 Results: 27 of 44 (61.4%) patients had at least 1 PRL at week
72 follow-up, and 11
of 44 (25.0%) had at least 4 PRLs. Approximately 10% of PRLs identified at
week 72 derived
from acute new lesions formed between baseline and week 72. Acute new T2
lesions that were
PRL+ were larger at first detection (median size of 392 vs 52 mm 3) and had
lower nMTR on
average at detection and recovery stages compared to those acute new T2
lesions that were
rimless. Chronic PRLs detected within T2 lesions pre-existing at baseline
showed lower nMTR
and higher RD at baseline. These lesions also showed a trend of decrease in
nMTR and increase
in RD from baseline to week 72 compared with chronic lesions without phase
rims.
101241 Conclusions: These data suggest that chronic T2 lesions with
phase rims have more
severe tissue injury than rimless chronic lesions. The minority of new T2
lesions that developed
persistent phase rims were larger in size and also associated with more severe
acute tissue
damage as measured by nMTR decrease.
Example 3: MRI characteristics of chronic MS lesions by phase rim detection
and/or slowly
expanding properties
101251 Objective: To evaluate the co-localization of phase rim
lesions (PRLs) and slowly
expanding lesions (SELs) and compare normalized magnetization transfer ratio
(nMTR) and
diffusion tensor imaging radial diffusivity (DTI-RD) in PRLs and SELs in
patients with
relapsing multiple sclerosis (RMS).
- 46 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
101261 Background: PRLs, as detected on susceptibility-weighted
phase images, have been
associated with chronic active MS lesions (Absinta et al. J Clin Invest 2016;
126:2597-2609).
SELs have been posited as a marker of chronic active MS lesions that can be
assessed using only
conventional MRI sequences (Elliot et al. Multiple Sclerosis Journal 2018;
DOT:
10.1177/1352458518814117). These lesions types may be relevant biomarkers, as
SELs have
been associated with ongoing tissue damage within lesions that is predictive
of disability
progression in progressive-onset MS (Elliott et al. Brain 2019; 142:2787-2799,
and Elliott et al.
AJNR Am J Neuroradiol 2020; 41(9): 1584-1591), and patients with > 4 PRLs
demonstrated
faster accumulation of disability Absinta et al. JAMA Neurol 2019; 76:1474-
1483). However,
the degree of overlap of SELs with PRLs is unknown.
101271 Design/Methods: Study Design: Brain MRI data were acquired in
AFFINITY
[NCT03222973], a phase II trial of opicinumab in relapsing MS with an intial
blinded, placebo
controlled portion followed by an open label extension study. Patients stable
on disease
modifying therapies (DMTs) (interferon, natalizumab, or dimethyl fumarate)
were randomized to
receive 750 mg opicinumab every 4 weeks of placebo in addition to their
background DMT.
101281 Imaging: The imaging protocol included Ti-weighted scans pre-
/post gadolinium,
T2-weighted FLAIR, PD-weighted and T2-weighted spin echo images, 2 spoiled
gradient-
recalled echo images with/without an MT pulse for calculating magnetization
transfer ratio
(MTR), and diffusion-weighted imaging using 32 directions. In Part 2 only, the
protocol also
included susceptibility weighted imaging (SWI) using standardized 3T Siemens
3D isotropic
multi-echo spoiled gradient T2*. PRLs were detected from SWI phase images at
Week 72 or
Part 2/Day1. SELs were detected as areas of Part 1 baseline T2 lesions that
showed constant and
concentric expansion from baseline to Week 72, using longitudinal Ti- and T2-
weighted images.
101291 Results: 41 of the patients who participated in the advanced
MRI sub-study of
AFFINITY Part 2 had SWI available at week 72. Patient characteristics are
shown in Table 5.
Table 5: Baseline Characteristics (n=41)
Age, mean (sd), years 38.7 (9.49)
Disease duration, mean (sd), years 6.9 (5.16)
EDSS, median (IQR) 2.5 (2.5)
T2 lesion volume, mean (sd), ml 8.8 (10.61)
- 47 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
Normalized brain volume, mean (sd), ml 1457.3 (117.7)
Proportion with Gd-enhancing lesions 0.15
[0130] Cumulatively, over the 41 patients analyzed, more than twice
as many SELs (267)
were detected as PRLs (119). Most SELs and PRLs were non-overlapping (Fig 9e).
39.5%of
PRLs (47/119) co-localized with SELs while 17.2 % (46/267) of SELs co-
localized with PRLs.
Moderate correlation of SEL and PRL counts across patients was observed
(r=0.67) (Fig 10).
Lesions with SEL/PRL co-localizations were larger than SEL-only or PRL-only
lesions. PRLs
colocalizing with SELs were larger in size than those that did not (Figs 11A-
11B and Table 6).
Table 6: Comparison of Lesion Sizes
PRL w/ SEL PRL non SEL SEL w PRL SEL
non PRL
Mean (sd) size 17L7 (124.5) 124.5 (156.6) 327.8 (629.5)
134.0 (168.1)
(mm)
Median Size 118.9 75.0 120.4 87.8
(mm)
[0131] Chronic lesions that were detected as both PRL+/SEL+ had
lowest normalized
magnetization transfer ratio (nMTR) (Figs 12A-12B) and higher diffusion tensor
imaging radial
diffusivity
101321 (DTI-RD), compared to PRLs with no SEL properties and SELs
with no associated
PRLs (Figs 13A-13B).
[0133] Conclusions: White matter lesions defined as SELs and PRLs
show only partial
correspondence and only a minority of SELs are associated with phase rims and
vice versa. SELs
and PRLs that co-localized may represent the most severe subset of chronic
active white matter
lesions. Ongoing investigations of SELs and PRLs may help to clarify MRI
lesion subtypes and
lead to more sensitive markers of MS disease progression.
- 48 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
Example 4: MRI characteristics of chronic MS lesions by phase rim detection
and/or slowly
expanding properties
101341 Objective: To identify a low-dimensional signature of
radiomics textural biomarkers
discriminative of SEL versus non-SEL MS lesion activity, and to develop a ML-
based classifier
discriminating SELs from non-SELs in chronic non-enhancing white matter MS
lesions, from
cross-sectional Ti- and T2-weighted brain MRI.
101351 Background: Chronic active lesions are thought to play a role
in the progressive
biology of MS through insidious damage to myelin and axons by chronic
inflammatory
processes occurring within pre-existing MS lesions (Luchetti et al. Acta
Neuropathol.
2018;135(4):511-528). Some chronic active lesions can be detected as slowly
expanding lesions
(SELs) identifiable on MRI as contiguous regions of existing T2 lesions
showing gradual
concentric expansion (Elliott C, et al. Mult Scler. 2019;25(14):1915-1925).
Machine learning
and texture analysis techniques may allow for the discrimination of MS lesion
subtypes on
conventional MRI imaging. In multiple sclerosis, disease activity is
traditionally classified into
two forms: relapsing MS or progressive MS. While conventional MRI provides
reliable bio-
markers of acute disease activity associated with the relapsing form of the
disease, there exists
comparatively fewer established biomarkers for the detection of tissue states
characterizing the
progressive phase of MS.
101361 In this context, it is generally believed that chronic active
lesions, which are
pathologically characterized by a rim of activated microglia and macrophages,
may play an
important role in the progressive biology of MS. Across the class of chronic
active lesions, some
can be detected as SELs. These lesions are identifiable on Ti-weighted MRI as
contiguous
regions of existing T2 lesions showing gradual concentric expansion. Current
methods for SEL
detection thus rely on at least 3 longitudinal scans acquired over a period of
1 to 2 years of
follow-up. The requirement for longitudinal data results in delayed
quantification of SEL
activity, which is in part relevant in the context of clinical trials for DMTs
targeting progressive
MS. Therefore, the aim of this Example is to detect SELs in the cross-
sectional setting. The
algorithm presented in this Example leverages techniques of image processing
from the fields of
radiomics analysis combined with machine learning. As such, the solution is
designed to identify
- 49 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
a signature of textural biomarkers associated with SEL activity, and to use
this signature to
discriminate SELs from non-SELs, within the bounds of white matter
hyperintensities.
101371 Design/Methods: Ti-weighted and T2-weighted MRIs were
retrospectively analysed
(ADVANCE ¨ 1512 patients with relapse-remitting MS; ASCEND ¨ 886 patients with
secondary progressive MS; SYNERGY ¨ 419 patients with relapse remitting
MS/secondary
progressive MS). Ground truth SELs were detected in each baseline scan using a
Jacobian
integration-based method (Elliott C, el al. Mult Scler. 2019,25(14).1915-1925)
leveraging
longitudinal MRI data spanning 1 to 2 years of follow-up. Briefly, ground
truth SELs were
detected in each baseline scan using a Jacobian-based method analysing the
evolution in Ti
intensity across a series of longitudinal scans.
101381 Cubic patches of 15x15x15 mm were extracted from the SEL and
non-SEL tissue of
each baseline scan (Fig 14). Specifically, cubic patches of 15x15x 15 mm were
sampled
randomly from SELs and non-SELs across all available baseline scans. For each
patch, texture-
based radiomic features were extracted separately from the core and periphery
of the patch, as
shown at Fig 15B in green and red, respectively. In more details, referring to
Fig 15B, the "core"
region contains all lesion voxels located less than 4 mm away from the central
voxel of the patch.
The "periphery" region contains voxels within a 3-mm margin outside the edge
of the core
region. For each patch, a set of 372 radiomic features was extracted from T1-
and T2-weighted
MRI data.
101391 The feature selection algorithm evaluated the discriminative
value of each feature
Using selected features, a pool of ML models were benchmarked. The 5 top-
performing models
were ensembled using a stacking strategy. A recursive loop further eliminated
noninformative
features.Briefly, patients from ADVANCE were split 80:20 into training and
validation sets,
respectively. The training set was used as input to a feature selection and
ensemble classification
pipeline. The feature selection pipeline evaluated the predictive value of
each one of the 372
features, using an ensemble of correlation tests evaluating the association of
each feature with
the label of the patch, both in the univariate and in the multivariate
setting. This approach
produced a ranking of the radiomic features from most useful to least useful.
In the input space
defined by the 50 most useful features, a pool of standard machine learning
models were
- 50 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
benchmarked via 10-fold cross-validation using patient-level splits. The 5 top-
performing
models were combined under a stacking ensemble strategy.
101401 The dimensionality of the radiomics feature space was further
compressed by
recursively eliminating non-informative features one-by-one. This led to a
compact signature of
only 15 radiomics variables, along with an optimal classifier able to
interpret this signature to
discriminate SELs from non-SELs. This optimal classifier was subsequently
tested on both the
validation set of patches from ADVANCE patients, and the independent sets of
patches from the
ASCEND and SYNERGY patients.
101411 We additionally trained a new classifier to discriminate SELs
from non-SELs under
strict matching of the volume of lesion contained in SEL versus non-SEL
patches (Figs 16A-
16C). This ensures that volume-independent textural biomarkers are detected
101421 This entire pipeline was applied twice: once under random
sampling of SEL and non-
SEL patches, and a second time under strict matching of the volume of lesion
found in SEL
versus non-SEL patches. This volume matching experiment allows us to evaluate
our ability to
discriminate SELs from non-SELs based purely on textural biomarkers and
independently of
geometry.
101431 To clarify, although both experiments were restricted to
radiomic features quantifying
texture, it is important to recognize that some of these features, such as the
entropy and the
energy, are volume-confounded. Therefore, a strict volume matching paradigm
can ensure a
volume-independent classification.
101441 Results: The 15 radiomic features selected via our recursive
elimination pipeline
define a compact signature discriminative of SEL versus non-SEL activity. This
signature
primarily contains information from Ti-weighted MRI signals in the core of the
patch. We
observed that first-order statistics including the mean, median and 90th
percentile of Ti
intensities in the core of the patch were identified as relevant, which is
consistent with prior
studies reporting that SELs exhibit a higher degree of Ti hypo-intensity
relative to non-SELs at
baseline. Radiomics signature of the 15 features included 10 from Ti-weighted
MRI, 5 from T2-
weighted MRI, 8 from the "core" of patches, and 7 from the "periphery" of
patches. Prevalence
of each of the 15 radiomic features selected for discriminating SEL from non-
SEL patches is
shown in Fig 17.
-51 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
101451 When discriminating SEL from non-SEL patches, our optimal
classification model
achieved 67% balanced accuracy on the ADVANCE validation set, 66% on the
ASCEND test
set and 69% on the SYNERGY test set. Importantly, we achieved a high
sensitivity and a low
specificity when testing on the SPMS ASCEND population, while in contrast we
observed a low
sensitivity and a high specificity when testing on the SYNERGY population
(Table 7).
Table 7: Summary statistics for the performance of the classification model
for patch-
level SEL versus non-SEL discrimination on the validation and independent
testing sets
Dataset Balanced Precision Sensitivity Specificity
ROC AUC
accuracy
ADVANCE 67% 66% 70% 64% 73%
(validation)
ASCEND 66% 63% 76% 56% 71%
(testing)
SYNERGY 69% 67% 68% 71% 74%
(testing)
101461 Figs 18A-18D are confusion matrices showing the performance
of the classification
model for patch-level SEL versus non-SEL discrimination on the training,
validation, and
independent testing sets.
101471 In the volume-balanced setting, the resulting ensemble model
achieved 62% balanced
accuracy on the ADVANCE validation set, as well as on the SYNERGY and ASCEND
test sets
(Table 8). The small drop in performance reported in this volume-balanced
experiment relative
to the results reported in the previous table indicate that our initial
experiment did capture
volumetric differences between SELs and non-SELs, beyond textural information.
Table 8: Summary statistics for the performance of the classification model
for patch-
level SEL versus non-SEL discrimination on the validation and independent
testing sets
for volume-matched patches.
Dataset Balanced Precision Sensitivity Specificity
ROC AUC
accuracy
ADVANCE 62% 62% 63% 61% 66%
(validation)
- 52 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
ASCEND 62% 61% 68% 57% 66%
(testing)
SYNERGY 62% 63% 55% 68% 67%
(testing)
101481 Figs 19A-19D are confusion matrices showing the performance
of the classification
model for patch-level SEL versus non-SEL discrimination on the training,
validation, and
independent testing sets for volume-matched patches.
101491 Conclusions: A machine learning classifier was developed that
is able to
discriminate SELs from non-SELs using single-timepoint non-contrast
conventional Ti- and T2-
weighted MRI, with classification accuracy ranging from 66% to 69% under
random patch
sampling, versus 62% under strict lesion volume matching. This indicates that
SELs may express
patterns of conventional non-enhancing MRI signals detectable using ML
techniques in the
cross-sectional setting. The single-timepoint detection of SELs may alleviate
the need for
longitudinal analysis and enable baseline quantification of chronic MS lesion
subtypes.
Applications of the algorithm could include population enrichment in clinical
trials and improved
patient prognostication in the clinical setting. Future work can incorporate
chronic active
leasions detectable by paramagnetic rim identification into the classification
algorithm.
* * *
101501 Preferred embodiments of this application are described
herein, including the best
mode known to the inventors for carrying out the application. Variations on
those preferred
embodiments will become apparent to those of ordinary skill in the art upon
reading the
foregoing description. It is contemplated that skilled artisans can employ
such variations as
appropriate, and the application can be practiced otherwise than specifically
described herein.
Accordingly, many embodiments of this application include all modifications
and equivalents of
the subject matter recited in the claims appended hereto as permitted by
applicable law.
Moreover, any combination of the above-described elements in all possible
variations thereof is
encompassed by the application unless otherwise indicated herein or otherwise
clearly
contradicted by context.
- 53 -
CA 03215371 2023- 10- 12
WO 2022/221458
PCT/US2022/024694
101511 All patents, patent applications, publications of patent
applications, and other
material, such as articles, books, specifications, publications, documents,
things, and/or the like,
referenced herein are hereby incorporated herein by this reference in their
entirety for all
purposes, excepting any prosecution file history associated with same, any of
same that is
inconsistent with or in conflict with the present document, or any of same
that may have a
limiting affect as to the broadest scope of the claims now or later associated
with the present
document. By way of example, should there be any inconsistency or conflict
between the
description, definition, and/or the use of a term associated with any of the
incorporated material
and that associated with the present document, the description, definition,
and/or the use of the
term in the present document shall prevail.
101521 In closing, it is to be understood that the embodiments of
the application disclosed
herein are illustrative of the principles of the embodiments of the
application. Other
modifications that can be employed can be within the scope of the application.
Thus, by way of
example, but not of limitation, alternative configurations of the embodiments
of the application
can be utilized in accordance with the teachings herein. Accordingly,
embodiments of the present
application are not limited to that precisely as shown and described.
- 54 -
CA 03215371 2023- 10- 12