Note: Descriptions are shown in the official language in which they were submitted.
CA 03216360 2023-10-10
Specification
RHABDOVIRUS-NEGATIVE SPODOPTERA FRUGIPERDA INSECT CELL
LINE, AND SCREENING, IDENTIFICATION AND APPLICATION
THEREOF
Technical Field
The invention pertains to the technical fields of genetic engineering and cell
engineering, and relates to a new spodoptera frugiperda insect cell line, and
in
particular relates to a rhabdovirus-negative spodoptera frugiperda insect cell
line and
screening, identification and application thereof.
Background of the Related Art
Insect cell expression system has been widely used in the production of
recombinant protein and has many advantages as compared to other expression
systems. For example, as insect baculovirus is parasitic only on
invertebrates, the
expression system has high safety and high expression level of recombinant
protein,
and can correctly fold and modify the recombinant protein after translation to
obtain a
protein with biological activity, and the insect cell expression system is
adapted to the
complex design of multi-gene expression such as virus-like particles and can
be
applied to large-scale serum-free culture and the like.
At present, multiple recombinant protein vaccines produced by the insect cell
expression system have demonstrated good effects and safety, and have been
approved for listing worldwide, including Cervarix cervical cancer vaccine
produced
by GSK, Provenge prostate cancer vaccine produced by Dendreon, and FluBlok
influenza vaccine produced by ProteinSciences. Moreover, many recombinant
protein
vaccines in the preclinical experimental stage have also demonstrated good
prospects
for application.
Sf9 cell (spodoptera frugiperda cell) is the most commonly used insect cell in
the
insect baculovirus expression system to express and produce foreign proteins,
including antibodies, vaccines and recombinant proteins. SD cells are derived
from an
IPLBSF-21 cell line (also called Sf21), which is originated from the ovarian
tissue of
autumn fly worm (spodoptera frugiperda) pupae isolated and cultured in 1977.
1
Date Recue/Date Received 2023-10-10
CA 03216360 2023-10-10
Sf-rhabdovirus is a new negative-strand RNA virus discovered by FDA
researchers in
2014 in the spodoptera frugiperda cell line SO and its parent cell line Sf21,
including
gene information of structural proteins of N, P, M, G and L. In addition, an
extra gene
sequence X with unknown function between G and L was tested. Although
Sf-rhabdovirus is not integrated into the genome of host cells, it has a
complete
genome and may be packaged into a complete virus particle, which has potential
risks
to the production and use of recombinant proteins such as vaccines obtained
based on
the baculovirus expression system of SO cells.
Brief Summary of the Invention
According to the invention, a rhabdovirus-negative spodoptera frugiperda
insect
cell line WSK-Sf9(abbreviated as WSK-50) is obtained through screening and
identification, and the cell line can be used for the production of
recombinant proteins
and recombinant protein vaccines based on a baculovirus expression system.
An objective of the invention is to provide a rhabdovirus-negative spodoptera
frugiperda insect cell line WSK-50, with a CCTCC accession number C202246. The
cell line was collected with a name of spodoptera frugiperda Sf9-derived cell
line
WSK-50 on February 16, 2022. The Collection Center is China Center for Type
Culture Collection (CCTCC) and its address is Wuhan University Collection
Center,
No.299 Bayi Road, Wuchang District, Wuhan City, Hubei Province, with a zip
code
of 430072.
The rhabdovirus-negative spodoptera frugiperda insect cell line WSK-50 of the
invention is characterized in that a monoclonal cell is screened by using a
limited
dilution method, verified through various high-sensitivity test methods such
as nested
PCR, transcriptome next-generation sequencing, realtime fluorescence
quantitative
PCR and Taqman probe-based real-time PCR, and then obtained the
rhabdovirus-negative spodoptera frugiperda insect cell line WSK-SO. The cell
is
tested for sterility, mycoplasma, exogenous virus and tumorigenicity according
to
pharmacopoeial requirements, and the results show that all indicators satisfy
the
requirements, and the cell can be used as a cell matrix for production.
Another objective of the invention is to provide an application of the
rhabdovirus-negative spodoptera frugiperda insect cell line WSK-Sf9 in
production of
a recombinant protein based on a baculovirus expression system.
The application includes an application of production of a recombinant protein
2
Date Recue/Date Received 2023-10-10
CA 03216360 2023-10-10
medicine or vaccine based on the baculovirus expression system.
The recombinant protein medicine includes a cytokine, a hormone, a
recombinant enzyme or an antibody.
The cytokine includes a recombinant human interleukin, a recombinant human
epidermal growth factor, a recombinant human interferon, a recombinant human
fibroblast growth factor, a recombinant human erythropoietin or a recombinant
human
granulocyte macrophage stimulating factor.
The hormone includes a recombinant human growth hormone, a recombinant
human insulin, an insulin analog or a recombinant human follicle maturing
hormone.
The recombinant enzyme includes a recombinant human alpha-glucosidase or a
recombinant human prourokinase.
The antibody includes a monoclonal antibody, a Fab antibody, a scFv antibody
or
a nanobody.
The vaccine includes a recombinant protein vaccine or a virus-like particle
vaccine.
The recombinant protein vaccine includes a SARS-CoV-2 protein vaccine, an
influenza virus protein vaccine, a syncytial virus recombinant protein
vaccine, a
hepatitis B virus protein vaccine or a rabies virus protein vaccine, and
preferably the
SARS-CoV-2 protein vaccine.
The virus-like particle vaccine includes a SARS-CoV-2 virus-like particle
vaccine (SARS-CoV-2-VLP), a human papillomavirus-like particle vaccine
(HPV-VLP), an influenza virus-like particle vaccine (HA-VLP), a poliovirus-
like
particle vaccine (PV-VLP), a respiratory syncytial virus-like particle vaccine
(RSV-VLP) or a hand-foot-mouth disease virus-like particle vaccine (EV71-VLP).
Preferably, the virus-like particle vaccine is the SARS-CoV-2 virus-like
particle
vaccine.
A technical solution for realizing an application of WSK-Sf9 in the production
of
recombinant protein or vaccine based on the baculovirus expression system is
that: A
Bac-to-Bac insect baculovirus expression system is used to package and produce
baculovirus and infect WSK-SP9 cells to express a target protein, so that a
target
recombinant protein is obtained by an affinity purification technology.
The beneficial effect of the invention is that: A monoclonal cell is screened
by
using a limited dilution method, verified through various high-sensitivity
test methods
such as nested PCR, transcriptome next-generation sequencing, realtime
fluorescence
3
Date Recue/Date Received 2023-10-10
CA 03216360 2023-10-10
quantitative PCR and Taqman probe-based real-time PCR, and then obtained the
rhabdovirus-negative spodoptera frugiperda insect cell line WSK-Sf9. The cell
is
tested for sterility, mycoplasma, exogenous virus and tumorigenicity according
to
pharmacopoeial requirements, and the results show that all indicators satisfy
the
requirements, and the cell can be used for production of recombinant protein
products
such as protein vaccines for clinical use. In addition, according to the
invention, the
Bac-to-Bac insect baculovirus expression system is used to package and produce
baculovirus and infect WSK-Sf9 cells to express the target protein, so that
the target
recombinant protein is obtained by the affinity purification technology or
other
technical ways.
According to the invention, the rhabdovirus-negative spodoptera frugiperda
insect cell line WSK-Sf9 obtained through screening and identification has a
CCTCC
accession number C202246. The cell line was collected on February 16, 2022.
The
Collection Center is China Center for Type Culture Collection (CCTCC) and its
address is Wuhan University Collection Center, No.299 Bayi Road, Wuchang
District,
Wuhan City, Hubei Province, with a zip code of 430072. The name and
identification
characteristics of the collected culture are spodoptera frugiperda Sf9-derived
cell line
WSK-Sf9.
Description of the Drawin2s
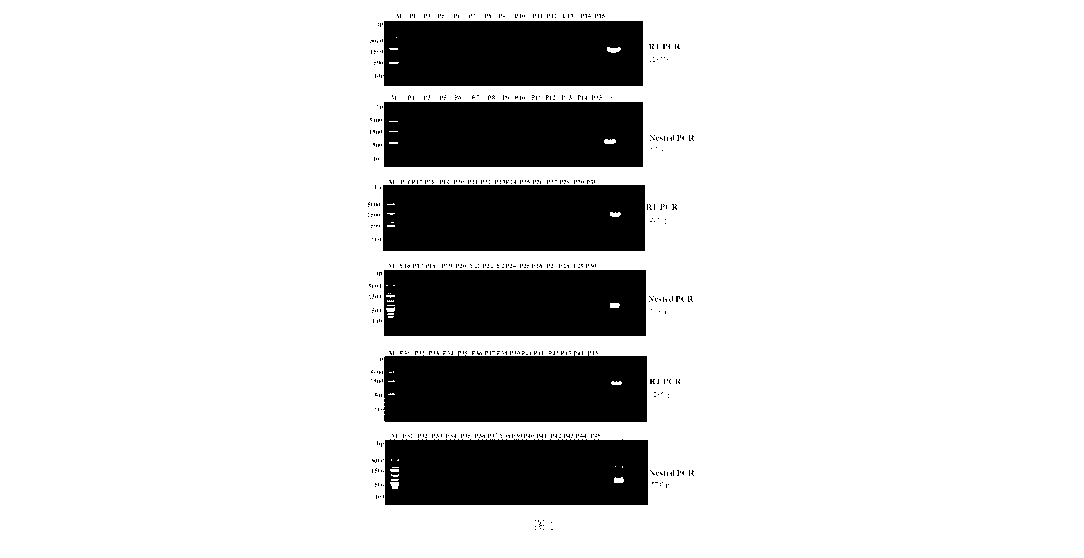
FIG. 1 shows the result of nested PCR agarose gel electrophoresis for
identification of WSK-Sf9 cells according to the invention;
FIG. 2 shows the morphological characteristics of the rhabdovirus-negative
spodoptera frugiperda insect cell line WSK-519 obtained through screening
according
to the invention;
FIG. 3 shows the karyotype analysis of the rhabdovirus-negative spodoptera
frugiperda insect cell line WSK-Sf9 obtained through screening according to
the
invention;
FIG. 4 shows a growth curve of the rhabdovirus-negative spodoptera frugiperda
insect cell line WSK-519 obtained through screening according to the
invention;
FIG. 5 shows a continuous subculturing curve of the rhabdovirus-negative
spodoptera frugiperda insect cell line WSK-519 obtained through screening
according
to the invention;
FIG. 6 shows a time gradient test of an exogenous recombinant protein
expressed
4
Date Recue/Date Received 2023-10-10
CA 03216360 2023-10-10
by the rhabdovirus-negative spodoptera frugiperda insect cell line WSK-S0
obtained
through screening according to the invention.
Detailed Description of the Ebodiments of the Invention
The following will explain the solutions of the present invention with
reference
to specific embodiments. A person skilled in the art will understand that the
following
embodiments are intended to illustrate the invention only and should not be
considered as limiting the scope of the invention. In case that specific
technologies or
conditions are not specified in the embodiments, technologies or conditions
described
in the literature in the art or the product specification shall prevail. If a
manufacturer
is not indicated in reagents or instruments used, they are all conventional
products that
can be purchased from the commercial reagent companies.
The screening, identification and application of the rhabdovirus-negative
spodoptera frugiperda insect cell line WSK-S0 will be further illustrated in
the
following embodiments, and the invention will be further described with
reference to
the attached drawings.
Embodiment 1: Screening and identification of Sf-rhabdovirus-negative
spodoptera frugiperda insect cell line WSK-5f9
The only commercially available Sf-rhabdovirus-negative Sf-RVN cells
(Sf-rhabdovirus-negative Sf9) were screened from Sf9 by Prof. Jarvis' team
through a
limited dilution method combined with antiviral drug treatment (Maghodia AB,
et al.
Protein expression and purification. 2016;122:45-55.), and verified to be
Sf-rhabdovirus-negative by nested PCR for an L gene, and the cells are
attributed to
GlycoBac (http://www.glycobac.com/sf-rvn-cells) which has entered into a
partnership with Millipore/Sigma Inc. to entrust Millipore/Sigma to sell them.
According to non-patent references: "Ma H, Nandakumar S, Bae EH, Chin PJ,
Khan AS, the Spodoptera frugiperda SO cell line is a heterogeneous population
of
rhabdovirus-infected and virus-negative cells: Isolation and characterization
of cell
clones containing rhabdovirus X-gene variants and virus-negative cell clones.
According to Virology 2019;536:125-33", spodoptera frugiperda SO cell line is
a
heterogeneous cell population, which includes two types of cell populations:
Sf-
rhabdovirus-infected cells and Sf-rhabdovirus-negative cells. A single Sf-
rhabdovirus-negative cell population can be obtained by the limited dilution
method.
According to the invention, a monoclonal cell is selected by the limited
dilution
Date Recue/Date Received 2023-10-10
CA 03216360 2023-10-10
method, and then Sf-rhabdovirus-negative cells are screened and identified by
methods such as nested PCR, transcriptome next-generation sequencing, realtime
fluorescence quantitative PCR and Taqman probe-based real-time PCR and the
like.
1) The parental cell line of SO was purchased from ThermoFisher Company (Lot
No.: 2043331), and the purchased cells were defined as passage zero (P0) . The
passage used in this screening experiment was passage 4 (P4), and the cell
culture
medium was SIM SF serum-free medium (Sino Biological, Inc., MSF1). The
suspended SO cells were diluted in a 10-times gradient and plated onto well
plates,
and the cells were observed every a few days. When distinct monoclonal cells
became
noticeable, they were transferred to a new well plate for expansion. Then,
appropriate
cells were collected, total RNA was extracted, and candidate cells were
preliminarily
identified by a nested PCR primer (Table 1) for Sf-rhabdovirus specific M
gene.
Table 1. Nested PCR primer sequence information for Sf-rhabdovirus specific M
gene
Primer
Primer name Primer sequence (5' to 3') amplification
zone nt
Sf-M-NEST-01-for AGGAGAACTCCAAAGACTCAGC (SEQ ID NO.!)
Sf-M-NEST-01-for AAAAGGAGTCCCCACTCAGC (SEQ ID NO.2) 3142-4426
Sf-M-NEST-02-for CCACATCTCCGCTATCACCA ( SEQ ID NO.3)
Sf-M-NEST-02-rev AGGAGAAGGAGCGGTTGGA ( SEQ ID NO.4) 3240-3813
2) The candidate Sf-rhabdovirus-negative cells were continuously subcultured,
and cell samples were collected and frozen at -80 C for later use. After 45
passages of
continuous culture, the nested PCR technology was used to detect the cell
samples,
and the results showed that Sf-rhabdovirus M genes in P1-P45 of the WSK-5f9
were
consistently negative (FIG. 1).
3) Transcriptome next-generation sequencing: 5*106 parental SO cells and 5*106
WSK-Sf9 cells were collected seperately for next-generation sequencing, and
the
results showed that the RNA gene information of Sf-Rhabdovirus was detected in
the
transcriptome of the parental Sf9 cells (GenBank: KF947078.1), but not in that
of
WSK-Sf9 cells.
6
Date Recue/Date Received 2023-10-10
CA 03216360 2023-10-10
4) Realtime fluorescence quantitative PCR: Two pairs of quantitative PCR
primers for Sf-rhabdovirus M genes were designed (Table 2), a Bio-Rad
SsoFastEvaGreensupermix kit was used for fluorescence quantitative PCR test,
and
the results showed that no fluorescence signal was detected in WSK-S0 P3
generation and P28 generation, proving that the WSK-S0 cells were Sf-
rhabdovirus-negative (Table 3).
Table 2. Specific Q-PCR primer for Sf- rhabdovirus M gene
Primer amplification
Primer name Primer sequence (5' to 3')
zone nt
SFV-M1-qPCR-for TGAAAACCTTCGCACAGCAC (SEQ ID NO.5)
CGAGACCCCTTTGGACCTTT (SEQ ID
SFV-M1-qPCR-rev NO.6) 3566-3752
GGATTGCACGGAGCCTATCA (SEQ ID
SFV-M2-qPCR-for NO.7)
TGCCCAAGCTAAGGAAAGGG (SEQ ID
SFV-M2-qPCR-rev NO.8) 3104-3291
Table 3. Q-PCR experimental data
Ct value
WSK-5f9 P3 WSK-5f9 P28 Sf9 (+) 1120(-)
M1 N.D. N.D. 19.76 N.D.
M1 N.D. N.D. 19.74 N.D.
M1 N.D. N.D. 19.75 N.D.
M2 N.D. N.D. 19.12 N.D.
M2 N.D. N.D. 19.23 N.D.
M2 N.D. N.D. 19.13 N.D.
Note: the data in the table 3 represents the results of three replicate
samples.
5) Quantitative PCR by probe method: A taqMan probe for Sf-rhabdovirus M
genes was designed (Table 4), and the results of quantitative PCR showed that
no
fluorescence signal was tested in a main cell bank (MCB) and a working cell
bank
7
Date Recue/Date Received 2023-10-10
CA 03216360 2023-10-10
(WCB) of WSK-Sf9, proving that the WSK-Sf9 cells were Sf-rhabdovirus-negative
(Table 5).
Table 4. Specific taqMan probe primer for Sf-rhabdovirus M gene
Primer
Primer
Primer sequence (5' to 3') Modified
amplification
name
zone nt
Sf-taqM-F TGACATGTGGTCTCCAACCG (SEQ ID NO.9)
Sf-taqM-R GTATGCAGGTGGTTGAGGCT (SEQ ID NO. 10) -
Sf-taqM-pro CTCCTTCTCCTCCACCCACAT (SEQ ID NO.11) 5'-FAM
3'-MGB 3783-3887
Table 5. TaqMan Q-PCR experimental data
Ct value
WSK-5f9 MCB WSK-5f9 WCB SP9 (+) 1120(-)
N.D. N.D. 20.96 N.D.
N.D. N.D. 20.53 N.D.
N.D. N.D. 20.77 N.D.
Note: the data in the table 5 represents the results of three replicate
samples.
Embodiment 2: Growth characteristics of Sf-rhabdovirus-negative
spodoptera frugiperda insect cell line WSK-Sf9
1) Culture characteristics of WSK-Sf9: The cell could grow in a serum-free
medium at 27 C in a way of adherence or suspension, with an average diameter
in
suspension growth of 16.28 0.34 gm and morphological characteristics of WSK-
Sf9
as shown in FIG. 2.
2) Karyotype analysis of WSK-Sf9: A karyotype analysis was carried out on the
parental SO cells, and the results showed that among 60 metaphase cells, the
number
of chromosomes was mainly distributed between 180-250, with an average of 215
chromosomes per cell; Meanwhile, karyotype analysis was also carried out on
WSK-Sf9 cells, and the results showed that among 60 metaphase cells, the
number of
chromosomes was mainly distributed between 191-538, with an average of 299
chromosomes per cell (FIG. 3). This also means that the Sf-rhabdovirus-
negative cell
line WSK-Sf9 is different from its parental SO cells.
8
Date Recue/Date Received 2023-10-10
CA 03216360 2023-10-10
3) WSK-Sf9 cell growth curve: WSK-Sf9 cells in logarithmic growth phase were
diluted to approximately 1 x 106 cells/ml and passaged into 250 ml vented
shake flasks.
The cultivation volume was 100 ml, and the flasks were placed in a constant
temperature shaker at 27 C for continuous cultivation. Cell density and
viability were
counted every 24 hours. After three different batches of cultivation, the
growth curve
and viability are shown in Figure 4. The cell density reached approximately
1x10
cells/ml after 96 hours and remained at this level for 6 days. The highest
cell density
approached 1.2 x107 cells/ml, and the cell viability gradually decreased from
the
11th day, and the average doubling time was around 23 hours.
4) Continuous subculturing curve of WSK-Sf9 cells: WSK-Sf9 cells in
logarithmic growth phase were diluted to approximately 1 x106 cells/ml and
passaged
into 250 ml vented shake flasks. The cultivation volume was 100 ml, and the
flasks
were placed in a constant temperature shaker at 27 C for continuous
cultivation. Cell
density and viability were counted every 3 days, and passaging was performed.
Generally, after 3 days of cultivation, the cell density could reach 6-8x 106
cells/ml,
with a viability of over 98%. The
continuous subculturing growth curve and
viability areshown in FIG. 5. After 100 consecutive passages, the cell growth
characteristics remained stable.
Embodiment 3: Safety test of Sf-rhabdovirus-negative spodoptera
frugiperda insect cell line WSK-Sf9
The following tests were conducted on WSK-Sf9 cells according to Part III of
China Pharmacopoeia (Edition 2020).
1) Species identification: A DNA Barcoding method was used to detect the
WSK-Sf9 cells, and the results showed that the WSK-5F9 cells were from the
spodoptera frugiperda;
2) Aseptic test: A membrane filtration method was adopted to carry out the
aseptic test, and the results showed that the growth of the WSK-5f9 cells was
aseptic;
3) Mycobacterium test: A culture method was adopted to test mycobacterium,
and the results showed that mycobacterium was negative;
4) Mycoplasma test: A culture method, an indicator cell culture method and a
Touchdown PCR method were adopted to test mycoplasma, and the results showed
that mycoplasma was negative;
5) Spiroplasma test: A fluorescence PCR method was adopted to test
spiroplasma,
and the results showed that spiroplasma was negative;
9
Date Recue/Date Received 2023-10-10
CA 03216360 2023-10-10
6) Exogenous virus test:
(1) An in vitro cell observation method, an erythrocyte adsorption test and
hemagglutination inhibition test were adopted to test subculturing of
different cells of
monkey-derived Vero cells, human MRC-5 cells, SO cells, BHK-21 cells,
mosquitoe-derived cells Aedes and Drosophila-derived cells D.Mel, and all the
cells
showed normal morphology and tested negative.
(2) Suckling mice, adult mice and chicken embryos (5-6 days old chicken
embryos and 9-11 days old chicken embryos) were inoculated by the in vivo
method,
and the results showed that they all satisfied the requirements.
7) Retrovirus test: No virus-like particles were found by a transmission
electron
microscope, a HEI(293 cell inoculation subculturing infection test and
chemical
reagent induced virus test, and the results showed that they all satisfied the
requirements;
8) The cells in bovine-derived virus test and pig-derived virus test were
negative;
9) Other specific viruses were tested, including Baculoviruses, T.ni flock
house
virus variant (FHVvar), Rhabdoviruses, Reoviridae, Togaviruses, Flaviviruses,
Bunyaviruses, Asfaviruses, Ascoviridae, Iridoviridae, Poxviridae,
Baculoviridae,
Poldnaviridae, Parvoviridae, Bimaviridae, Reoviridae, Picomaviralses,
Dicistrovitidae,
Nodaviridae and Tetraviridae; and the results showed that they were all
negative and
satisfied the requirements.
10) Tumorigenicity test: Experimental mice were inoculated with the WSK-Sf9
cells, and the results showed that the cells were not tumorigenic and could
satisfy the
requirements.
In conclusion, the Sf-rhabdovirus-negative spodoptera frugiperda insect cell
line
WSK-519 satisfies the requirements for all safety tests and the requirements
for
cellular matrices for the production of biologics.
Embodiment 4: Exogenous recombination protein expressed by
Sf-rhabdovirus-negative spodoptera frugiperda insect cell line WSK-Sf9
A baculovirus expression vector containing an RBD structural domain of
SARS-CoV-2 was constructed, and WSK-S0 cells were used to package the
recombinant protein-expressing baculovirus. SO and WSK-S0 cells were cultured
separately; when the density reached 2.5 x 106/ml, they were infected
separately with
viruses at a ratio of MOI=0.5.Supernatants were collected before infection (0
hr) and
Date Recue/Date Received 2023-10-10
CA 03216360 2023-10-10
after infection (241h hr, 48th hr, 72nd hr, and 961h hr), which were detected
by
western-blot using antibodies against the His tag, and the results showed that
the
expression level of the recombinant protein in the WSK-Sf9 cells was up-
regulated as
compared to that in the Sf9 cells. After production and purification in a GMP
workshop and subsequent adjuvant formulation, the recombinant protein can be
used
to prevent infection of SARS-CoV-2. This demonstrates that the
Sf-rhabdovirus-negative spodoptera frugiperda insect cell line WSK-Sf9 can be
used
to express and produce exogenous recombinant proteins such as protein
vaccines, and
the expression level is higher than the Sf9 cells.
11
Date Recue/Date Received 2023-10-10