Note: Descriptions are shown in the official language in which they were submitted.
WO 2022/241219
PCT/US2022/029198
Processes Producing Alkali Hydroxides, Alkali Carbonates, Alkali Bicarbonates,
and/or
Alkaline Earth Sulfates
CROSS-REFERENCE TO RELATED APPLICATIONS
[00001] For PCT purposes the present application claims
priority to U.S. Provisional
Application No. 63/188,275 filed May 13, 2021 which application is
incorporated herein by
reference. The present application also claims priority to U.S. Application
No. 17/590,483 filed
February 1, 2022 and U.S. Application No. 17/732,808 filed April 29, 2022.
[00002] For U.S purposes the present application is a
continuation-in-part of U.S.
Application No. 17/732,808 filed April 29, 2022 which application is a
continuation-in-part of
U.S. Application No. 17/590,483 filed February 1, 2022 which application is a
continuation of
U.S. Application No. 17/243,714 filed April 29, 2021 issued as U.S. Patent No.
11,236,033
which application is a continuation-in-part of U.S. Application No. 16/944,850
filed July 31,
2020 issued as U.S. Patent No. 11,034,619 which application claims priority
from U.S.
Provisional Application No. 62/895,557 filed September 4, 2019 and U.S.
Provisional
Application No. 63/042,397 filed June 22, 2020 and U.S. Provisional
Application No.
62/890,254 filed August 22, 2019. The present application also claims priority
to U.S.
Provisional Application No. 63/188,275 filed May 13, 2021.
[00003] The above described continuation which is U.S.
Application No. 17/590,483 filed
February 1, 2022 also claims priority to U.S. Provisional Application No.
63/147,286 filed
February 9, 2021; U.S. Provisional Application No. 63/153,461 filed February
25, 2021; U.S.
Provisional Application No. 63/157,847 filed March 8, 2021; U.S. Provisional
Application No.
63/163,993 filed March 22, 2021; and U.S. Provisional Application No.
63/179,822 filed April
26, 2021. All of the above applications are incorporated herein by reference.
BACKGROUND OF THE INVENTION
[00004] Sodium hydroxide production is generally produced using
the chlor-alkali process,
which is energy intensive, requires rare metal anodes and cathodes, and
produces hydrochloric
acid, which has a limited market and cannot be discharged into the
environment. Commercial
applications of hydrochloric acid often involve employing hydrochloric acid in
a reaction with a
carbonate salt, which may result in the release of CO2 and may counter any CO2
emissions
reduction benefit. Additionally, if hydrochloric acid is released into the
environment, it will react
with carbonate or bicarbonate salts present in the environment, emitting
carbon dioxide and
acidifying water bodies, such as the ocean. There is a significant need for a
low energy
consumption, low CO2 emissions, environmentally friendly process for producing
sodium
hydroxide.
1
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
SUMMARY OF THE INVENTION
[00005] Some embodiments of the present invention may pertain
to low carbon emissions,
or low energy consumption, or carbon negative production of sodium hydroxide,
or sodium
carbonate, or sodium bicarbonate, or sodium sulfite, or sodium bisulfite, or
gypsum, or alkaline-
earth sulfate, or alkali hydroxide, or alkali carbonate, or alkali
bicarbonate, or alkali sulfite. Some
embodiments of the present invention may enable ultra-low CO2 emissions
production of sodium
hydroxide with calcium sulfate as the side product. Calcium sulfate comprises
a solid, is
minimally soluble in water, is non-toxic, is not dangerous for the
environment, and has a multi-
billion metric ton per year market in gypsum wallboard, concrete aggregates,
fireproofing,
plaster, building materials, and other applications. Some embodiments of the
present invention
may be capable of scaling to greater than 1 billion ton per year CO2 emissions
reduction, or
carbon removal, or a combination thereof Additionally some embodiments may
lower the
required cost and energy consumption of alkali hydroxides, alkali carbonates,
and alkali
bicarbonates. Some embodiments may be employ equipment comprising abundant and
recyclable
materials.
[00006] Advantages of some embodiments include lower energy
consumption, lower cost,
or lower CO2 emissions, CO2 emissions negative outputs, or application in
carbon dioxide
removal, or no strong acid products, or abundant materials, or global
scalability.
BRIEF FIGURE DESCRIPTIONS
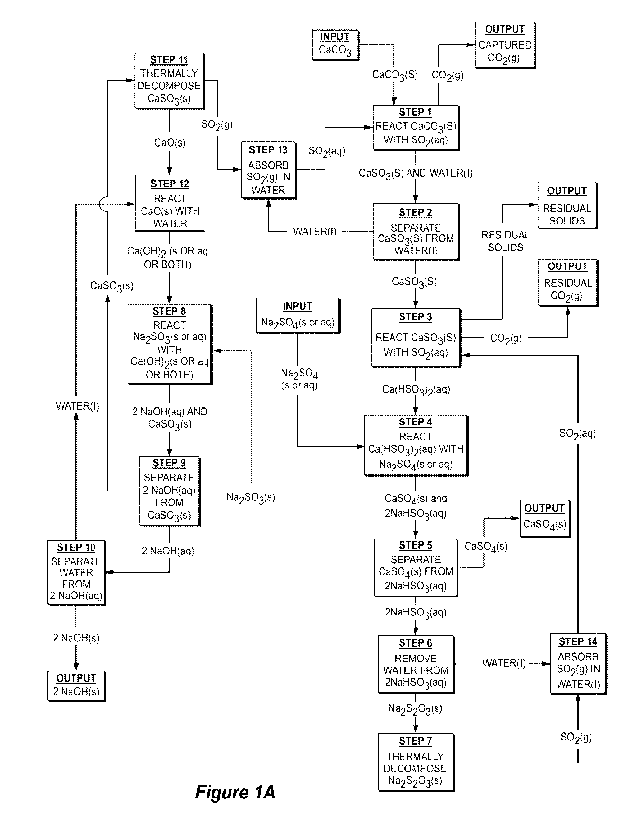
[00007] Figure 1A: Process for Producing Sodium Hydroxide,
Calcium Sulfate, and
Captured Carbon Dioxide with Inputs Comprising Calcium Carbonate and Sodium
Sulfate and
Intermediates Comprising Sulfur Dioxide and Alkaline Earth Intermediates
[00008] Figure 1B: Process for Producing Sodium Hydroxide,
Calcium Sulfate, and
Captured Carbon Dioxide with Inputs Comprising Calcium Carbonate and Sodium
Sulfate and
Intermediates Comprising Sulfur Dioxide and Alkaline Earth Intermediates
[00009] Figure 1C: Process for Producing Sodium Hydroxide,
Calcium Sulfate, and
Captured Carbon Dioxide with Inputs Comprising Calcium Carbonate and Sodium
Sulfate and
Intermediates Comprising Sulfur Dioxide and Alkaline Earth Intermediates
[00010] Figure 1D: Process for Producing Alkali Hydroxide,
Alkaline Earth Sulfate, and
Weak Acid Derivative with Inputs Comprising Alkaline Earth Cation ¨ Weak Acid
Anion salt
and Alkali Sulfate and Intermediates Comprising Sulfur Dioxide, Alkaline Earth
Oxides, and
Alkaline Earth Hydroxides
[00011] Figure 1E: Process for Producing Alkali Hydroxide,
Alkaline Earth Sulfate, and
Weak Acid Derivative with Inputs Comprising Alkaline Earth Cation ¨ Weak Acid
Anion salt
2
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
and Alkali Sulfate and Intermediates Comprising Sulfur Dioxide, Alkaline Earth
Oxides, and
Alkaline Earth Hydroxides
[00012] Figure 2A: Process for Producing Sodium Hydroxide,
Calcium Sulfate, and
Captured Carbon Dioxide with Inputs Comprising Calcium Carbonate and Sodium
Sulfate and
Intermediates Comprising Sulfur Dioxide and Intermediates Comprising Sulfur
Dioxide,
Alkaline Earth Oxides, and Alkaline Earth Hydroxides
[00013] Figure 2B: Process for Producing Sodium Hydroxide,
Calcium Sulfate, and
Captured Carbon Dioxide with Inputs Comprising Calcium ¨ Weak Acid Anion and
Sodium
Sulfate and Intermediates Comprising Sulfur Dioxide, Alkaline Earth Oxides,
and Alkaline Earth
Hydroxides
[00014] Figure 2C: Process for Producing Alkali Hydroxide,
Alkaline Earth Sulfate, and
Weak Acid Derivative with Inputs Comprising Alkaline Earth Cation ¨ Weak Acid
Anion salt
and Alkali Sulfate and Intermediates Comprising Sulfur Dioxide, Alkaline Earth
Oxides, and
Alkaline Earth Hydroxides
[00015] Figure 2D: Process for Producing Alkali Hydroxide,
Alkaline Earth Sulfate, and
Weak Acid Derivative with Inputs Comprising Alkaline Earth Cation ¨ Weak Acid
Anion salt
and Alkali Sulfate and Intermediates Comprising Sulfur Dioxide, Alkaline Earth
Oxides, and
Alkaline Earth Hydroxides
[00016] Figure 2E: Process for Producing Alkali Hydroxide,
Alkaline Earth Sulfate, and
Weak Acid Derivative with Inputs Comprising Alkaline Earth Cation ¨ Weak Acid
Anion salt
and Alkali Sulfate and Intermediates Comprising Sulfur Dioxide, Alkaline Earth
Oxides, and
Alkaline Earth Hydroxides
[00017] Figure 3: Process for Producing Sodium Carbonate or
Sodium Bicarbonate from
Sodium Sulfate
[00018] Figure 4: Process for Producing Sodium Carbonate or
Sodium Bicarbonate from
Sodium Sulfate
[00019] Figure 5: Process for Producing Sodium Carbonate or
Sodium Bicarbonate from
Sodium Sulfate
[00020] Figure 6: Process for Producing Sodium Carbonate and
Alkaline Earth Sulfate
from Sodium Sulfate Employing the Thermal Transformation of Sodium Sulfite
[00021] Figure 7A: Process for Producing Sodium Carbonate or
Sodium Bicarbonate
from Sodium Sulfate and Calcium ¨ Weak Acid Input using an Ammonia
Intermediate
3
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
[00022] Figure 7B: Process for Producing Alkali Carbonate or
Alkali Bicarbonate from
Alkali Sulfate and Alkaline Earth ¨ Weak Acid Input using an Ammonia
Intermediate
[00023] Figure SA: Process for Producing Sodium Carbonate or
Sodium Bicarbonate
from Sodium Sulfate and Calcium ¨ Weak Acid Input using an Ammonia
Intermediate
[00024] Figure SB: Process for Producing Alkali Carbonate or
Alkali Bicarbonate from
Alkali Sulfate and Alkaline Earth ¨ Weak Acid Input using an Ammonia
Intermediate
DETAILED DESCRIPTION OF THE INVENTION
1000251 The present invention may pertain to a process or
system for the production of an
alkali hydroxide, or alkali carbonate, or alkali carbonate, or alkaline earth
sulfate, or captured
carbon dioxide, or aggregate, or silicon dioxide, or any combination thereof.
Some embodiments
may employ ammonia or ammonium intermediate reactants or intermediates. The
present
invention may pertain to a process or system for the production of alkali
bisulfite, or alkali
metabisulfite, or alkali sulfite, or alkaline earth sulfate, or captured
carbon dioxide, or silicon
dioxide, or aggregate, or weak acid, or any combination thereof from alkali
sulfate and alkaline
earth carbonate, or alkaline earth silicate, or alkaline earth - weak acid
anion, or any combination
thereof The present invention may pertain to a process or system for the
production of sodium
bisulfite, or sodium metabisulfite, or sodium sulfite, or calcium sulfate, or
captured carbon
dioxide, or silicon dioxide, or aggregate, or any combination thereof from
sodium sulfate and
calcium carbonate, or calcium silicate, or calcium-weak acid anion, or any
combination thereof
Some embodiments may comprise producing an alkali hydroxide using inputs
comprising an
alkaline earth cation ¨ weak acid anion salt, or an alkali sulfate, or water,
or any combination
thereof Some embodiments may comprise producing an alkali carbonate or alkali
bicarbonate or
alkali sesquicarbonate using inputs comprising an alkaline earth cation ¨ weak
acid anion salt, or
an alkali sulfate, or water, or carbon dioxide, or any combination thereof
Some embodiments
may employ intermediates comprising sulfur dioxide, or calcium, or water, or
any combination
thereof In some embodiments, one or more intermediates may comprise inputs or
outputs.
EXAMPLE CHEMISTRY
[00026] Example 1: Production of Sodium Hydroxide and Weak Acid
or Weak Acid
Anion Derivative
(1) CaCO3 + 502(aq) 4 CaS03(s) + CO2(g)
(2) CaS03(s) + H20(aq) + 502(aq) 4 Ca(HS03)2(aq)
(3) Ca(HS03)2(aq) + Na2SO4(aq or s) 4 2 NaHS03(aq) + CaSO4(s)
(4) 2 NaHS03(aq) 4 Na2S03(aq) + S02(g)
(6) Na2S03(aq) + Ca(OH)2(s or aq or suspension) 4 2 Na0H(aq) + CaS03(s)
Note: In some embodiments, CaO(s) may be reacted with Na2S03(aq).
4
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: CaS03(s) may be separated from 2 NaOH(aq) using a solid-liquid
separation.
(7) CaS03(s) + Heat 4 CaO(s) + S02(g)
(8) 2 NaOH(aq) 4 2 NaOH(s) + Water(solvent)
Note: In some embodiments, it may be desirable for 2 NaOH to remain at an
aqueous phase. For example, NaOH may be sold or transferred or used at an
aqueous phase
(9) CaO(s) + H20 (1 or g) 4 Ca(OH)2(s or aq or suspension)
(10) S02(g) + Water(solvent) 4 S02(aq)
Or
(1) CaCO3 + S02(aq) 4 CaS03(s) + CO2(g)
(2) CaS03(s) + H20(aq) + S02(aq) 4 Ca(HS03)2(aq)
(3) Ca(HS03)2(aq) + Na2SO4(aq or s) 4 2 NaHS03(aq) + CaSO4(s)
(4) May Comprise one or more or any combination of the following:
= 2 NaHS03(aq) 4 Na2S205(s) + H20(1 or g) + Water(solvent)
= 2 NaHS03(aq) 4 Na2S205(s) + Water(solvent)
(5) Na2S205(s) + Heat 4 Na2S03(s) + S02(g)
(6) Na2S03(s or aq) + Ca(OH)2(s or aq or suspension) 4 2 NaOH(aq) + CaS03(s)
(7) CaS03(s) + Heat 4 CaO(s) + S02(g)
(8) 2 NaOH(aq) 4 2 NaOH(s) + Water(solvent)
(9) CaO(s) + H20 (1 or g) 4 Ca(OH)2(s or aq or suspension)
(10) S02(g) + Water(solvent) 4 S02(aq)
[00027] Example 2: Production of Sodium Hydroxide and Weak Acid
or Weak Acid
Anion Derivative
(1) CaCO3 + 2 S02(aq) + H20(aq) 4 Ca(HS03)2(aq) + CO2(g)
(2) Ca(HS03)2(aq) + Na2SO4(aq or s) 4 2 NaHS03(aq) + CaSO4(s)
(3) 2 NaHS03(aq) 4 Na2S03(aq) S02(g)
(4) Na2S03(s or aq) + Ca(OH)2(s or aq or suspension) 4 2 NaOH(aq) + CaS03(s)
(5) CaS03(s) + Heat 4 CaO(s) + S02(g)
(6) 2 NaOH(aq) 4 2 NaOH(s) + Water(solvent)
(7) 2 S02(g) + Water(solvent) 4 2 S02(aq)
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
(8) CaO(s) + H20 (1 or g) 4 Ca(OH)2(s or aq or suspension)
Or
(1) CaCO3 + 2 S02(aq) + H20(aq) 4 Ca(HS03)2(aq) + CO2(g)
(2) Ca(HS03)2(aq) + Na2SO4(aq or s) 4 2 NaHS03(aq) + CaSO4(s)
(3) 2 NaHS03(aq) 4 Na2S205(s) + Water(solvent)
(4) Na2S205(s) + Heat 4 Na2S03(s) + S02(g)
(5) Na2S03(s or aq) + Ca(OH)2(s or aq or suspension) 4 2 NaOH(aq) + CaS03(s)
(6) CaS03(s) + Heat 4 CaO(s) + S02(g)
(7) 2 NaOH(aq) 4 2 NaOH(s) + Water(solvent)
(8) 2 S02(g) + Water(solvent) 4 2 S02(aq)
(9) CaO(s) + H20 (1 or g) 4 Ca(OH)2(s or aq or suspension)
1000281 Example 3: Production of Sodium Hydroxide and Weak Acid
or Weak Acid
Anion Derivative
(1) Calcium Silicate(s) + 2 S02(aq) + H20(aq) 4 Ca(HS03)2(aq) + Silicon
Dioxide(s)
(2) Ca(HS03)2(aq) + Na2SO4(aq or s) 4 2 NaHS03(aq) + CaSO4(s)
(3) 2 NaHS03(aq) 4 Na2S03(aq) + S02(g)
(4) Na2S03(s or aq) + Ca(OH)2(s or aq or suspension) 4 2 NaOH(aq) + CaS03(s)
(5) CaS03(s) + Heat 4 CaO(s) + S02(g)
(6) 2 NaOH(aq) 4 2 NaOH(s) + Water(solvent)
(7) 2 S02(g) + Water(solvent) 4 2 502(aq)
(8) CaO(s) + H20 (1 or g) 4 Ca(OH)2(s or aq or suspension)
Or
(1) Calcium Silicate(s) + 2 S02(aq) + H20(aq) 4 Ca(HS03)2(aq) + Silicon
Dioxide(s)
(2) Ca(HS03)2(aq) + Na2SO4(aq or s) 4 2 NaHS03(aq) + CaSO4(s)
(3) 2 NaHS03(aq) 4 Na2S205(s) + Water(solvent)
(4) Na2S205(s) + Heat 4 Na2S03(s) + S02(g)
(5) Na2S03(s or aq) + Ca(OH)2(s or aq or suspension) 4 2 NaOH(aq) + CaS03(s)
(6) CaS03(s) + Heat 4 CaO(s) + S02(g)
(7) 2 NaOH(aq) 4 2 NaOH(s) Water(solvent)
(8) 2 S02(g) + Water(solvent) 4 2 502(aq)
(9) CaO(s) + H20 (1 or g) 4 Ca(OH)2(s or aq or suspension)
6
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
[00029] Example 4: Production of Sodium Hydroxide and Weak Acid
or Weak Acid
Anion Derivative
(1) Calcium(Weak Acid Anion) + 2 S02(aq) + H20(aq) 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(2) Ca(HS03)2(aq) + Na2SO4(aq or s) 4 2 NaHS03(aq) + CaSO4(s)
(3) 2 NaHS03(aq) 4 Na2S03(aq) + S02(g)
(4) Na2S03(s or aq) + Ca(OH)2(s or aq or suspension) 4 2 NaOH(aq) + CaS03(s)
(5) CaS03(s) + Heat 4 CaO(s) + S02(g)
(6) 2 NaOH(aq) 4 2 NaOH(s) + Water(solvent)
(7) 2 502(g) + Water(solvent) 4 2 S02(aq)
(8) CaO(s) H20 (1 or g) 4 Ca(OH)2(s or aq or suspension)
Or
(1) Calcium(Weak Acid Anion) + 2 S02(aq) + H20(aq) 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(2) Ca(HS03)2(aq) + Na2SO4(aq or s) 4 2 NaHS03(aq) + CaSO4(s)
(3) 2 NaHS03(aq) 4 Na2S205(s) + Water(solvent)
(4) Na2S205(s) + Heat 4 Na2S03(s) + S02(g)
(5) Na2S03(s or aq) + Ca(OH)2(s or aq or suspension) 4 2 NaOH(aq) + CaS03(s)
(6) CaS03(s) + Heat 4 CaO(s) + S02(g)
(7) 2 NaOH(aq) 4 2 NaOH(s) + Water(solvent)
(8) 2 S02(g) + Water(solvent) 4 2 S02(aq)
(9) Ca0(s) + H20 (1 or g) 4 Ca(OH)2(s or aq or suspension)
[00030] Example 5: Production of Sodium Hydroxide and Weak Acid
or Weak Acid
Anion Derivative
(1) Calcium(Weak Acid Anion) + 2 S02(aq) + H20(aq) 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(2) Ca(HS03)2(aq) + Na2SO4(aq or s) 4 2 NaHS03(aq) + CaSO4(s)
(3) 2 NaHS03(aq) 4 Na2S03(aq) + S02(g)
(4) Na2S03(s or aq) + Ca(OH)2(s or aq or suspension) 4 2 NaOH(aq) + CaS03(s)
(5) CaS03(s) + Heat 4 CaO(s) + S02(g)
(6) One or more or any combination of the following:
= 2 NaOH(aq) 4 2 NaOH(s) + Water(solvent)
= 2 NaOH(aq)
= 2 NaOH(aq) or 2 NaOH(s) added to seawater or body of water
7
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
(7) 2 S02(g) + Water(solvent) 4 2 S02(aq)
(8) CaO(s) + H20 (1 or g) 4 Ca(OH)2(s or aq or suspension)
Or
(1) Calcium(Weak Acid Anion) + 2 S02(aq) + H20(aq) 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(2) Ca(HS03)2(aq) + Na2SO4(aq or s) 4 2 NaHS03(aq) + CaSO4(s)
(3) 2 NaHS03(aq) 4 Na2S205(s) + Water(solvent)
Note: NaHS03(aq) may generally exist at an aqueous phase. Upon precipitation
or
crystallization, NaHS03(aq) precipitates or crystalizes as Na2S205(s).
Na2S205(s) may be
considered anhydrous.
(4) Na2S205(s) + Heat 4 Na2S03(s) + S02(g)
(5) Na2S03(s or aq) + Ca(OH)2(s or aq or suspension) 4 2 NaOH(aq) + CaS03(s)
(6) CaS03(s) + Heat 4 CaO(s) + S02(g)
(7) One or more or any combination of the following:
= 2 NaOH(aq) 4 2 NaOH(s) + Water(solvent)
= 2 NaOH(aq)
= 2 NaOH(aq) or 2 NaOH(s) added to seawater or body of water
(8) 2 S02(g) + Water(solvent) 4 2 S02(aq)
(9) CaO(s) + H20 (1 or g) 4 Ca(OH)2(s or aq or suspension)
[00031] Example 6: Production of Sodium Hydroxide and Weak Acid
or Weak Acid
Anion Derivative
(1) Calcium(Weak Acid Anion) + 2 S02(aq) + H20(aq) 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(2) Ca(HS03)2(aq) + Na2SO4(aq or s) 4 2 NaHS03(aq) + CaSO4(s)
(3) 2 NaHS03(aq) 4 Na2S03(aq) H20(aq) + S02(g)
(4) Na2S03(s or aq) + Ca(OH)2(s or aq or suspension) 4 2 NaOH(aq) + CaS03(s)
(5) CaS03(s) + Heat 4 CaO(s) + S02(g)
(6) One or more or any combination of the following:
= 2 NaOH(aq) 4 2 NaOH(s) + Water(solvent)
= 2 NaOH(aq)
= 2 NaOH(aq) or 2 NaOH(s) added to seawater or body of water
(7) 2 S02(g) + Water(solvent) 4 2 S02(aq)
(8) CaO(s) + H20 (1 or g) 4 Ca(OH)2(s or aq or suspension)
[00032] Example 7: Production of Sodium Carbonate or Sodium
Bicarbonate
8
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
(1) One or more or any combination of the following:
= CaCO3 + 2 S02(aq) + H20(aq) 4 Ca(HS03)2(aq) + CO2(g)
= CaS + 2 S02(aq) + H20(aq) 4 Ca(HS03)2(aq) + H2S(g)
= Calcium Silicate(s) + 2 S02(aq) H20(aq) 4 Ca(HS03)2(aq) + Silicon
Dioxide(s)
= Calcium(Weak Acid Anion) + 2 502(aq) + H20(aq) 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(2) Ca(HS03)2(aq) + Na2SO4(aq or s) 4 2 NaHS03(aq) + CaSO4(s)
(3) NaHS03(aq) 4 NaHS03(aq) + Water(solvent)
Note: In some embodiments, residual sodium bicarbonate or sodium carbonate or
sodium
¨ carbon dioxide may be present in solution. In some embodiments, residual
sodium
bicarbonate or sodium carbonate or sodium ¨ carbon dioxide may decompose and /
or
form carbon dioxide gas. Said carbon dioxide gas may be recirculated and / or
employed
in '(6)'.
(4) 2NaHS03(aq) 4 Na2S03(aq) + H20(aq) + S02(g)
(5) Na2S03(aq) + CO2(g) + 2 H20(aq) 4 NaHS03(aq) + NaHCO3(aq or s)
Note: In some embodiments, the aqueous solution may be concentrated, or
cooled, or
both to promote the further precipitation of sodium bicarbonate or further
separation of
sodium bicarbonate. For example, in some embodiments, an aqueous solution
comprising
2 NaHS03(aq) + 2 NaHCO3(aq) may be concentrated using, for example, mechanical
vapor compression distillation, or distillation, or desorption, and the
precipitation of 2
NaHCO3(s) may be facilitated due to, for example, the concentrating beyond
solubility
limits and / or lower temperature and / or extraction and / or other
separation system or
method.
(6) Remaining NaHS03(aq) in `(5)' may be transferred to '(3)'
Or
(1) One or more or any combination of the following:
= CaCO3 + 2 S02(aq) + H20(aq) 4 Ca(HS03)2(aq) + CO2(g)
= CaS + 2 S02(aq) + H20(aq) 4 Ca(HS03)2(aq) + H2S(g)
= Calcium Silicate(s) + 2 502(aq) + H20(aq) 4 Ca(H503)2(aq) + Silicon
Dioxide(s)
= Calcium(Weak Acid Anion) + 2 S02(aq) + H20(aq) 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
9
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
(2) Ca(HS03)2(aq) + Na2SO4(aq or s) 4 2 NaHS03(aq) + CaSO4(s)
(3) 2 NaHS03(aq) 4 Na2S205(s) + Water(solvent)
Note: In some embodiments, residual sodium bicarbonate or sodium carbonate or
sodium
¨ carbon dioxide may be present in solution. In some embodiments, residual
sodium
bicarbonate or sodium carbonate or sodium ¨ carbon dioxide may decompose and /
or
form carbon dioxide gas. Said carbon dioxide gas may be recirculated and / or
employed
in `(6)'.
(4) Na2S205(s) + Heat 4 Na2S03(s) + S02(g)
(5) Na2S03(s) + Water (Solvent) 4 Na2S03(aq)
(6) Na2S03(aq) + CO2(g) + 2 H20(aq) 4 NaHS03(aq) + NaHCO3(aq or s)
Note: In some embodiments, the aqueous solution may be concentrated, or
cooled, or
both to promote the further precipitation of sodium bicarbonate or further
separation of
sodium bicarbonate. For example, in some embodiments, an aqueous solution
comprising
2 NaHS03(aq) + 2 NaHCO3(aq) may be concentrated using, for example, mechanical
vapor compression distillation, or distillation, or desorption, and the
precipitation of 2
NaHCO3(s) may be facilitated due to, for example, the concentrating beyond
solubility
limits and / or lower temperature and / or extraction and / or other
separation system or
method.
(7) Remaining NaHS 03(aq) in `(6)' may be transferred to `(3)'
[00033] Example 8: Production of Sodium Carbonate or Sodium
Bicarbonate
(1) One or more or any combination of the following:
= CaCO3 + 2 S02(aq) + H20(aq) 4 Ca(HS03)2(aq) + CO2(g)
= Calcium Silicate(s) + 2 S02(aq) + H20(aq) 4 Ca(HS03)2(aq) + Silicon
Dioxide(s)
= Calcium(Weak Acid Anion) + 2 S02(aq) + H20(aq) 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(2) Ca(HS03)2(aq) + Na2SO4(aq or s) 4 2 NaHS03(aq) + CaSO4(s)
(3) 2 Naf1S03(aq) 4 Na2S03(aq) + H20(aq) + S02(g)
(4) Na2S03(aq) + CO2(g) 4 NaHS03(aq) + NaHCO3(aq or s)
(5) Remaining NaHS03(aq) in `(4)' may be transferred to (3)'
[00034] Example 9: Production of Sodium Hydroxide for Air
Capture and Producing
Sodium Carbonate or Sodium Bicarbonate from CO2 in the Air or Other CO2 or CO2
in the
Ocean
(1) Calcium(Weak Acid Anion) + 2 S02(aq) + H20(aq) 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
(2) Ca(HS03)2(aq) + Na2SO4(aq or s) 4 2 NaHS03(aq) + CaSO4(s)
(3) May comprise one or more or any combination of the following:
= 2 NaHS03(aq) 4 Na2S03(aq) + S02(g)
= 2 NaHS03(aq) 4 Na2S205(s) + Water(solvent)
= Na2S205(s) + Heat 4 Na2S03(s) + S02(g)
= Na2S03(s) + Water (Solvent) 4 Na2S03(aq)
(4) Na2S03(s or aq) + Ca(OH)2(s or aq or suspension) 4 2 NaOH(aq) + CaS03(s)
(5) CaS03(s) + Heat 4 CaO(s) + S02(g)
(6) 2 NaOH(aq) + CO2(g) 4 Na2CO3(aq) + H20(aq)
Note: CO2(g) for this reaction may, if desired, comprise dilute sources, which
may
include, but are not limited to, air or emissions gases. Na0H(aq or s) is
capable of
reacting with very low concentrations of CO2 and forming sodium carbonate.
Alternatively, the NaOH may be added to seawater, where it will add ocean
alkalinity and
naturally absorb carbon dioxide from the air or ocean, increasing the ocean's
capacity to
sequester carbon dioxide, or react with CO2, or bicarbonate, or carbonate, or
anions, or
any combination thereof in the ocean, or increase the pH of the ocean, or any
combination
thereof.
(7) One or more or any combination of the following:
= Na2CO3(aq) 4 Na2CO3(s) + Water (solvent)
= Na2CO3(aq) + CO2(g) + H20(aq) 4 2 NaHCO3(aq)
= Na2CO3(aq) or Na2CO3(s) added to seawater or a body of water
(8) 2 502(g) + Water(solvent) 4 2 S02(aq)
(9) CaO(s) + H20 (1 or g) 4 Ca(OH)2(s or aq or suspension)
1000351 Example 10: Decomposition of Sodium Sulfite in the
presence of water vapor to
sodium hydroxide and sulfur dioxide
(1) Calcium(Weak Acid Anion) + 2 S02(aq) + H20(aq) 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(2) Ca(HS03)2(aq) + Na2SO4(aq or s) 4 2 NaHS03(aq) + CaSO4(s)
(3) 2 NaHS03(aq) 4 Na2S205(s) + Water(solvent)
(4) Na2S205(s) + Heat 4 Na2S03(s) + S02(g)
(5) Na2S03(s) + H20(g) + Heat 4 2 NaOH(s) + 502(g)
Note: May comprise a high temperature water vapor atmosphere.
(6) 2 S02(g) + Water (solvent) 4 2 S02(aq)
[00036] Example 11: Production of Sodium Sulfite, Gypsum, and
Weak Acid or Weak
Acid Anion Derivative
11
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
(1) Calcium(Weak Acid Anion) + 2 S02(aq) + H20(aq) 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(2) Ca(HS03)2(aq) + Na2SO4(aq or s) 4 2 NaHS03(aq) + CaSO4(s)
(3) May comprise one or more or any combination of the following:
= 2 NaHS03(aq) ¨> Na2S03(aq) + S02(g)
= 2 NaHS03(aq) 4 Na2S205(s) + Water(solvent)
= Na2S205(s) + Heat 4 Na2S03(s) + S02(g)
= Na2S03(s) + Water (Solvent) 4 Na2S03(aq)
(4) S02(g) + water (solvent) 4 S02(aq)
[00037] Example 12: Production of Sodium Bisulfite or Sodium
Metabisulfite and Weak
Acid or Weak Acid Anion Derivative
(1) Calcium(Weak Acid Anion) + 2 502(aq) + H20(aq) 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(2) Ca(HS03)2(aq) + Na2SO4(aq or s) 4 2 NaHS03(aq) + CaSO4(s)
(3) 2 NaHS03(aq) 4 Na2S205(s) + H20(1 or g) + Water(solvent)
[00038] Example 13: Production of Sodium Carbonate or Sodium
Bicarbonate and
Hydrogen Sulfide or Sulfur or Hydrogen or Sulfur Dioxide or Sulfurous Acid or
Sulfuric Acid
from Sodium Sulfate and Calcium Salt
(1) Calcium(Weak Acid Anion) + 8 502(aq) + H20(aq) 4 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(2) 4 Ca(HS03)2(aq) + 4 Na2SO4(aq or s) 4 8 NaHS03(aq) + 4 CaSO4(s)
(3) 8 NaHS03(aq) 4 4 Na2S205(s) + Water(solvent)
(4) 4 Na2S205(s) + Heat 4 4 Na2S03(s) + 4 S02(g)
(5) 4 Na2S03(s) + Heat 4 3 Na2SO4(s) + Na2S(s)
Note: Reaction `(5). may be exothermic.
(6) 3 Na2SO4(s) + Na2S(s) + Water (solvent) 4 3 Na2SO4(aq or s) + Na2S(aq)
(7) 3 Na2SO4(aq or s) + Na2S(aq) + CO2(aq or g) 4 3 Na2SO4(aq or s) +
Na2CO3(aq or s)
+ H2S(aq or g)
(8) 3 Na2SO4(aq or s) + Na2CO3(aq or s) + H2S(aq or g) 4 3 Na2SO4(aq) +
Na2CO3(aq or
s) + H2S(g)
(9) Sodium Sulfate, or Sodium Sulfide, or Sodium Carbonate, or Sodium
Bicarbonate
Separation. Said separation may occur, for example, before or during or after
`(5)', or
`(6)', or `(7)', or `(8)' and / or may comprise, including, but not limited
to, one or more or
any combination of the following:
12
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: Sodium sulfate may be known to possess a solubility curve in water which
sharply increases with temperature between 0 ¨ 35 C. For example, the
solubility
of sodium sulfate in water may be 4.76 g/100 ml at 0 C, or may be 49.7g/100mL
at 32.4 degrees Celsius. Sodium carbonate may be known to possess a solubility
curve in water which increases with temperature between 0 ¨ 35 C, although
slightly less sharply than sodium sulfate. The solubility of sodium carbonate
in
water at 0 C may be 7g/100mL, or at 15 C may be 16.4g/100mL, or at 27.8 C
may be 34.07g/100mL, or at 34.8 C may be 48.69g/100mL. Sodium bicarbonate
may be known to possess a solubility curve in water which increases with
temperature between 0 ¨ 35 C, although not as sharply as sodium sulfate or
sodium carbonate. The solubility of sodium bicarbonate in water at 0 C may be
6.9g/100mL, or 8.2 g/100 ml (10 C), or 9.6 g/100 ml (20 'V), or 10 g/100 ml
(25
C), or 11.1 g/100 ml (30 C) , or 12.7 g/100 ml (40 C).
= Option 1: Na2SO4(s) and Na2S(s) may possess significantly different
densities. For
example, Na2SO4(s) (anhydrous) may possess a density of 2.20 g/cm3 and / or
Na2S(s)
(anhydrous) may possess a density of 1.856 g/cna'. 3 Na2SO4(s) and Na2S(s) may
be at
least partially separated by a centrifuge or other density driven separation
system or
method.
= Option 2: Dissolve 3 Na2SO4(s) + Na2S(s) in cold water (for example 0 ¨
15 C) or
cold aqueous solution (for example -50 ¨ 15 C). For example, a cold aqueous
solution
may comprise, including, but not limited to, one or more or any combination of
the
following: ethylene glycol ¨ water, or propylene glycol ¨ water, or glycerol ¨
water, or
alcohol ¨ water, or urea ¨ water, or any combination thereof The 3 Na2SO4(s) +
Na2S(s)
may comprise more Na2SO4(s) than Na2S(s). The solubility of Na2SO4(s) may be
significantly lower in cold water or cold aqueous solution than Na2S(s), which
may result
in Na2S(s) dissolving in the water, while most of the Na2SO4(s) not dissolving
in the
water or remaining a solid.
= Option 3: Contact or mix 3 Na2SO4(s) + Na2S(s) with an organic solvent,
or a
mixture of water and an organic solvent, or any combination thereof Na2S(s)
may be
soluble in ethanol, or methanol, or propanol, or 2-propanol, or 2-methyl-1-
propanol, or
benzyl alcohol, or ethylene glycol, or propylene glycol, or aqueous organic
solvent
solutions, or any combination thereof Na2S(s) may have a solubility of 3.1
g/100 g in
ethanol and 5.1 g/100 g in methanol. Na2SO4(s) may be practically insoluble in
most
organic solvents. 3 Na2SO4(s) + Na2S(s) may be mixed with an organic solvent,
which
may include, but is not limited to, methanol. The Na2S(s) may dissolve, while
the 3
13
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Na2SO4(s) may remain at a solid phase. The 3 Na2SO4(s) may be separated from
the
Sodium Sulfide organic solvent solution using a solid-liquid separation. The
present
option may occur, for example, after step '(5)'.
= Option 4: In some embodiments, the concentration of sodium sulfate may be
greater than the concentration of sodium sulfide during and / or after '(6)'.
The solution
comprising "3 Na2SO4(aq) + Na2S(aq) may be cooled to precipitate a portion of
3
Na2SO4(aq) or may already be cool, which may prevent the dissolution of a
portion of 3
Na2SO4(s) during `(6)'.
= Option 5: In some embodiments, the concentration of sodium sulfate may be
greater than the concentration of sodium carbonate during and / or after
`(7)'. At least a
portion of Na2SO4 may be precipitated from the solution comprising "3
Na2SO4(aq) +
Na2CO3(aq) + H2S(aq)." It may be desirable to perform the presently described
step after
`(7)' and / or before or during `(8)'.
= Option 6: In some embodiments, additional CO2 may be added to the
solution
comprising Na2SO4(aq) + Na2CO3(aq) to produce sodium bicarbonate, which may
result
in the precipitation of at least a portion of sodium bicarbonate from
solution. It may be
desirable to perform the presently described step after the desorption or
removal or
conversion of hydrogen sulfide from solution.
= Option 7: Separations, which may include, but are not limited to, one or
more or
any combination of the following: Distillation, or Vapor Compression
Distillation, or
Solventing Out, or Solvent Induced Precipitation, or Cooling Precipitation, or
Cryodesalination, or Freezing Desalination, or Evaporation, or Membrane Based
Process,
or Reverse Osmosis, or Forward Osmosis, or Membrane Distillation, or
Osmotically
Assisted Reverse Osmosis, or any combination thereof.
(10) Hydrogen sulfide may be sold or transferred to an external application or
may be
converted in one or more or any combination of the following:
= H2S(g) + 1.5 02(g) 4 S02(g or aq) + H20(g or 1), the SO2
= H2S(g) to elemental sulfur
= H2S(g) to hydrogen and sulfur or sulfur dioxide or sulfuric acid
= H2S(g) input to the Claus Process
= H2S(g) to reduced metal species, such as reduced iron or iron sulfide
= II2S(g) to reduced metal species, such as reduced iron or iron sulfide,
then
produce hydrogen using the reduced metal species
= H25(g) to sulfuric acid
14
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
= Heat from conversion or combustion of H2S(g) to S02(g or aq) or Sulfur or
sulfuric acid may be employed to provide heat to one or more or any
combination of
reactions or separations, which may include, but are not limited to, one or
more or any
combination of the following: -4 Na2S203(s) + Heat 4 4 Na2S03(s) + 4 S02(g)",
or
distillation of organic solvent, or distillation of water, or supplemental
heat for "4
Na2S03(s) + Heat 4 3 Na2SO4(s) + Na2S(s)-, or supplemental heat for "CaS03(s)
+ Heat
4 CaO(s) + S02(g)".
(11) 8 S02(g) + water (solvent) 4 8 S02(aq)
[00039] Example 14: At some temperature, some embodiments may
comprise
decomposition of Sodium Sulfite to Sodium Oxide and Sulfur Dioxide:
(1) Ca1cium(Weak Acid Anion) + 2 S02(aq) + H20(aq) 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(2) Ca(HS03)2(aq) + Na2SO4(aq or s) 4 2 NaHS03(aq) + CaSO4(s)
(3) 2 NaHS03(aq) 4 Na2S205(s) + H20(1 or g) + Water(solvent)
(4) Na2S205(s) + Heat 4 Na2S03(s) + S02(g)
(5) Na2S03(s) I heat 4 Na2O(s) I S02(g)
[00040] Example 15: Decomposition of Sodium Sulfite to Sodium
Sulfate and Sodium
Sulfide and Production of Sodium Hydroxide
(1) Calcium(Weak Acid Anion) + 8 S02(aq) + H20(aq) 4 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(2) 4 Ca(HS03)2(aq) + 4 Na2SO4(aq or s) 4 8 NaHS03(aq) + 4 CaSO4(s)
(3) 8 NaHS03(aq) 4 4 Na2S205(s) + 4 H20(1 or g) + Water(solvent)
(4) 4 Na2S205(s) + Heat 4 4 Na2S03(s) + 4 S02(g)
(5) 4 Na2S03(s) + Heat 4 3 Na2SO4(s) + Na2S(s)
(6) 3 Na2SO4(s) + Na2S(s) + Water (solvent) 4 3 Na2SO4(aq) + Na2S(aq)
(7) 3 Na2SO4(aq) + Na2S(aq) + Ca(OH)2 (aq or s or both) 4 3 Na2SO4(aq) + 2
NaOH(aq)
+ CaS(s)
(8) Separate 3 Na2SO4(aq) + 2 Na0H(aq), which may comprise.
= 3 Na2SO4(aq) + 2 NaOH(aq) 4 2 NaOH(aq) + 3 Na2SO4(s)
(9) CaS(s) + H20(aq or 1) + 502(aq or g) 4 CaS03(s) + H2S (g or aq)
(10) Comprises one or more or any combination of the following:
= H2S(g) + 1.5 02(g) 4 S02(g or aq) + H20(g or 1)
= H2S(g) to elemental sulfur
= H2S(g) to hydrogen and sulfur or sulfur dioxide or sulfuric acid
= H2S(g) to reduced metal species, such as reduced iron or iron sulfide
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
= H2S(g) to sulfuric acid
(11) CaS03(s) + Heat 4 CaO(s) + 502(g)
(12) S02(g) + Water (solvent) 4 S02(aq)
(13) CaO(s) + H20 4 Ca(OH)2 + Heat
1000411 Example 16: Production of Hydrogen Sulfide or Sulfur or
Hydrogen or Sulfur
Dioxide or Sulfurous Acid or Sulfuric Acid from Sodium Sulfate and Calcium
Salt
(1) Calcium(Weak Acid Anion) + 8 S02(aq) + H20(aq) 4 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(2) 4 Ca(HS03)2(aq) + 4 Na2SO4(aq or s) 4 8 NaHS03(aq) + 4 CaSO4(s)
(3) 8 NaHS03(aq) 4 4 Na2S205(s) + Water(solvent)
(4) 4 Na2S205(s) + Heat 4 4 Na2S03(s) + 4 S02(g)
(5) 4 Na2S03(s) + Elevated Temperature 4 3 Na2SO4(s) + Na2S(s)
(6) 3 Na2SO4(s) + Na2S(s) + Water (Solvent) 4 3 Na2SO4(aq) + Na2S(aq)
(7) 3 Na2SO4(aq) + Na2S(aq) + S02(aq or g) 4 3 Na2SO4(aq) + Na2S03(aq) +
H2S(aq or
(8) 3 Na2SO4(aq) I Na2S03(aq) I II2S(aq or g) 4 3 Na2SO4(aq) I Na2S03(aq) I
II2S(g)
Note: -3 Na2SO4(aq) + Na2S03(aq)" may comprise the solution transferred to
`(2)' and /
or '(2)' Na2S03(aq) may be present during '(2)'
(9) Comprises one or more or any combination of the following:
= H2S(g) + 1.5 02(g) 5 S02(g or aq) + H20(g or 1)
= H25(g) to elemental sulfur
= H25(g) to hydrogen and sulfur or sulfur dioxide or sulfuric acid
= H2S(g) to reduced metal species, such as reduced iron or iron sulfide
= H25(g) to reduced metal species, such as reduced iron or iron sulfide,
then
produce hydrogen using the reduced metal species
= H2S(g) to sulfuric acid
(10) 4 502(g) + water (solvent) 4 4 S02(aq)
[00042] Example 17: Production of Sodium Carbonate and Gypsum
(1) Calcium(Weak Acid Anion) + 8 502(aq) + H20(aq) 4 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(2) 4 Ca(HS03)2(aq) + 4 Na2SO4(aq or s) 4 8 NaHS03(aq) + 4 CaSO4(s)
(3) 8 NaHS03(aq) 4 4 Na2S205(s) + Water(solvent)
(4) 4 Na2S205(s) + Heat 4 4 Na2S03(s) + 4 S02(g)
(5) 4 Na2S03(s) + Elevated Temperature 4 3 Na2SO4(s) + Na2S(s)
Note: Reaction `(5). may be exothermic.
16
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
(6) 3 Na2SO4(s) + Na2S(s) + Methanol (solvent) 4 3 Na2SO4(s) + Na2S(dissolved)
Note: Alternatively, in some embodiments, after solid-liquid separation of 3
Na2SO4(s),
Na2S(dissolved) may be removed or recovered by distillation of the solvent and
crystallization of Na2S(s). The Na2S(s) may be transferred to an environment
with
sufficient water and carbon dioxide present for the reaction of Na2S with CO2
and H20 to
form Na2CO3 and H2S.
(7) Na2S(dissolved) + CO2(g) + H20(dissolved) 4 Na2CO3(s) + H2S(dissolved or
g)
Note: In some embodiments, just enough H20 may be added to enable the
production of
Na2CO3(s).
Note: In some embodiments, water may be present in the methanol or organic
solvent
solution or inorganic solvent solution or methanol solution in both `(6)' and
`(7)'.
Note: Na2CO3(s) may be separated using a solid-liquid separation.
Note: H2S may be at least partially stripped or desorbed or removed during our
after '(7)'
to, for example, produce H2S and / or regenerate the organic solvent or
methanol for '(6)'.
Note: Na2CO3(s) may be slightly soluble in methanol and a portion of
Na2CO3(dissolved)
may remaining in the organic solvent solution as a residual.
(8) Hydrogen sulfide may be sold or transferred to an external application or
may be
converted in one or more or any combination of the following:
= H2S(g) + 1.5 02(g) 4 S02(g or aq) + H20(g or 1), the S02
= H2S(g) to elemental sulfur
= H2S(g) to hydrogen and sulfur or sulfur dioxide or sulfuric acid
= H2S(g) input to the Claus Process
= H2S(g) to reduced metal species, such as reduced iron or iron sulfide
= H2S(g) to reduced metal species, such as reduced iron or iron sulfide,
then
produce hydrogen using the reduced metal species
= H2S(g) to sulfuric acid
= Heat from conversion or combustion of H2S(g) to S02(g or aq) or Sulfur or
sulfuric acid may be employed to provide heat to one or more or any
combination of
reactions or separations, which may include, but are not limited to, one or
more or any
combination of the following: "4 Na2S205(s) + Heat 4 4 Na2S03(s) + 4 S02(g)",
or
distillation of organic solvent, or distillation of water, or supplemental
heat for "4
Na2S03(s) + Heat 4 3 Na2SO4(s) + Na2S(s)", or supplemental heat for "CaS03(s)
+ Heat
4 Ca0(s) + S02(g)".
(9) 8 S02(g) + water (solvent) 4 8 S02(aq)
17
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: In some embodiments, the weight percent of water in organic solvent, or
methanol,
or ethanol, or inorganic solvent, or ammonia, or any combination thereof may
be less
than, or greater than, or equal to one or more or any combination of the
following: 0.01%,
or 0.1%, or 0.5%, or 1%, or 2%, or 3%, or 4%, or 5%, or 6%, or 7%, or 8%, or
9%, or
10%, or 11%, or 12%, or 13%, or 14%, or 15%, or 16%, or 17%, or 18%, or 19%,
or
20%, or 25%, or 30%, or 35%, or 40%, or 45%, or 50%, or 55%, or 60%, or 65%,
or
70%, or 75%, or 80%, or 85%, or 90%, or 95%, or 99%.
1000431 Example 18: Production of Sodium Carbonate and Gypsum
(1) Calcium(Weak Acid Anion) + 8 S02(aq) + H20(aq) 4 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(2) 4 Ca(HS03)2(aq) + 4 Na2SO4(aq or s) 4 8 Na1-1S03(aq) + 4 CaSO4(s)
(3) 8 NaHS03(aq) 4 4 Na2S205(s) + Water(solvent)
(4) 4 Na2S205(s) + Heat 4 4 Na2S03(s) + 4 S02(g)
(5) 4 Na2S03(s) + Elevated Temperature 4 3 Na2SO4(s) + Na2S(s)
Note: Reaction `(5)' may be exothermic.
(6) 3 Na2SO4(s) + Na2S(s) + Water (Solvent) 4 3 Na2SO4(s or aq) + Na2S(aq)
(7) 3 Na2SO4(aq) + Na2S(aq) + CaCO3(s or aq) 4 3 Na2SO4(aq) + Na2CO3(aq) +
CaS(s)
Note: CaS(s) may be separated from the solution using solid-liquid separation.
3
Na2SO4(aq) + Na2CO3(aq) may be separated during or after separation of CaS(s).
(8) CaS(s) + H20(1 or aq or g) + CO2(aq or g) 4 CaCO3(s or aq) + H2S(g or aq)
(9) Hydrogen sulfide may be sold or transferred to an external application or
may be
converted in one or more or any combination of the following:
= H2S(g) + 1.5 02(g) -} S02(g or aq) + H20(g or 1), the S02
= H2S(g) to elemental sulfur
= H2S(g) to hydrogen and sulfur or sulfur dioxide or sulfuric acid
= H2S(g) input to the Claus Process
= H2S(g) to reduced metal species, such as reduced iron or iron sulfide
= H2S(g) to reduced metal species, such as reduced iron or iron sulfide,
then
produce hydrogen using the reduced metal species
= H2S(g) to sulfuric acid
= Heat from conversion or combustion of H2S(g) to S02(g or aq) or Sulfur or
sulfuric acid may be employed to provide heat to one or more or any
combination of
reactions or separations, which may include, but are not limited to, one or
more or any
combination of the following: "4 Na2S205(s) + Heat 4 4 Na2S03(s) + 4 S02(g)",
or
distillation of organic solvent, or distillation of water, or supplemental
heat for -4
18
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Na2S03(s) + Heat ¨} 3 Na2SO4(s) + Na2S(s)", or supplemental heat for "CaS03(s)
+ Heat
4 CaO(s) + S02(g)".
(10) 8 S02(g) + water (solvent) 4 8 S02(aq)
[00044] Example 19: Production of Sodium Carbonate and Gypsum
(1) Calcium(Weak Acid Anion) + 8 502(aq) + H20(aq) 4 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(2) 4 Ca(HS03)2(aq) + 4 Na2SO4(aq or s) 4 8 NaHS03(aq) + 4 CaSO4(s)
(3) 8 NaHS03(aq) 4 4 Na2S205(s) + Water(solvent)
(4) 4 Na2S205(s) + Heat 4 4 Na2S03(s) + 4 S02(g)
(5) 4 Na2S03(s) + Elevated Temperature 4 3 Na2SO4(s) + Na2S(s)
Note: Reaction `(5)' may be exothermic.
(6) 3 Na2SO4(s) + Na2S(s) + Methanol (solvent) 4 3 Na2SO4(s) + Na2S(dissolved)
Note: In some embodiments, after solid-liquid separation of 3 Na2SO4(s),
Na2S(dissolved) may be removed or recovered by distillation of the solvent and
crystallization of Na2S(s). The Na2S(s) may be transferred to an environment
with
sufficient water and carbon dioxide present for the reaction of Na2S with CO2
and H20 to
form Na2CO3 and H2S.
(7) Na2S(dissolved) 4 Na2S(s) + Methanol(Solvent)
(8) Na2S(s or aq) + H20(1 or aq or g) + CO2(aq or g) 4 Na2CO3(s or aq) + H2S(g
or aq)
(9) Hydrogen sulfide may be sold or transferred to an external application or
may be
converted in one or more or any combination of the following:
= H2S(g) + 1.5 02(g) 4 S02(g or aq) + H20(g or 1), the S02
= H2S(g) to elemental sulfur
= H25(g) to hydrogen and sulfur or sulfur dioxide or sulfuric acid
= H2S(g) input to the Claus Process
= H2S(g) to reduced metal species, such as reduced iron or iron sulfide
= H2S(g) to reduced metal species, such as reduced iron or iron sulfide,
then
produce hydrogen using the reduced metal species
= H2S(g) to sulfuric acid
= Heat from conversion or combustion of H2S(g) to S02(g or aq) or Sulfur or
sulfuric acid may be employed to provide heat to one or more or any
combination of
reactions or separations, which may include, but are not limited to, one or
more or any
combination of the following: "4 Na2S205(s) + Heat 4 4 Na2S03(s) + 4 S02(g)",
or
distillation of organic solvent, or distillation of water, or supplemental
heat for "4
19
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Na2S03(s) + Heat -} 3 Na2SO4(s) + Na2S(s)-, or supplemental heat for "CaS03(s)
+ Heat
4 CaO(s) + S02(g)".
(10) 8 S02(g) + water (solvent) 4 8 S02(aq)
[00045] Example 20: Production of Sodium Carbonate and Gypsum
(1) Calcium(Weak Acid Anion) + 8 502(aq) + H20(aq) 4 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(2) 4 Ca(HS03)2(aq) + 4 Na2SO4(aq or s) 4 8 NaHS03(aq) + 4 CaSO4(s)
(3) 8 NaHS03(aq) 4 4 Na2S205(s) + Water(solvent)
(4) 4 Na2S205(s) + Heat 4 4 Na2S03(s) + 4 S02(g)
(5) 4 Na2S03(s) + Elevated Temperature 4 3 Na2SO4(s) + Na2S(s)
Note: Reaction `(5)' may be exothermic.
(6) 3 Na2SO4(s) + Na2S(s) + Methanol (solvent) 4 3 Na2SO4(s) + Na2S(dissolved)
Note: In some embodiments, after solid-liquid separation of 3 Na2SO4(s),
Na2S(dissolved) may be removed or recovered by distillation of the solvent and
crystallization of Na2S(s). The Na2S(s) may be transferred to an environment
with
sufficient water and carbon dioxide present for the reaction of Na2S with CO2
and H20 to
form Na2CO3 and H2S.
(7) Na2S(dissolved) 4 Na2S(s) + Methanol(Solvent)
(8) Na2S(s or aq) + Water(solvent) + CaCO3(aq or s) 4 Na2CO3(s or aq) + CaS(s)
(9) CaS(s) + H20(g or 1 or aq) + CO2(g or aq) 4 CaCO3(aq or s) + H2S(g or aq)
(10) Hydrogen sulfide may be sold or transferred to an external application or
may be
converted in one or more or any combination of the following:
= H2S(g) + 1.5 02(g) -} S02(g or aq) + H20(g or 1), the S02
= H2S(g) to elemental sulfur
= H25(g) to hydrogen and sulfur or sulfur dioxide or sulfuric acid
= H2S(g) input to the Claus Process
= H2S(g) to reduced metal species, such as reduced iron or iron sulfide
= H25(g) to reduced metal species, such as reduced iron or iron sulfide,
then
produce hydrogen using the reduced metal species
= H2S(g) to sulfuric acid
= Heat from conversion or combustion of H2S(g) to 502(g or aq) or Sulfur or
sulfuric acid may be employed to provide heat to one or more or any
combination of
reactions or separations, which may include, but are not limited to, one or
more or any
combination of the following: "4 Na2S205(s) + Heat 4 4 Na2S03(s) + 4 S02(g)",
or
distillation of organic solvent, or distillation of water, or supplemental
heat for -4
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Na2S03(s) + Heat ¨} 3 Na2SO4(s) + Na2S(s)", or supplemental heat for "CaS03(s)
+ Heat
4 CaO(s) + S02(g)".
(10) 8 S02(g) + water (solvent) 4 8 S02(aq)
[00046] Example 21: Production of Sodium Hydroxide and Gypsum
(1) Calcium(Weak Acid Anion) + 8 502(aq) + H20(aq) 4 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(2) 4 Ca(HS03)2(aq) + 4 Na2SO4(aq or s) 4 8 NaHS03(aq) + 4 CaSO4(s)
(3) 8 NaHS03(aq) 4 4 Na2S205(s) + Water(solvent)
(4) 4 Na2S205(s) + Heat 4 4 Na2S03(s) + 4 S02(g)
(5) 4 Na2S03(s) + Elevated Temperature 4 3 Na2SO4(s) + Na2S(s)
Note: Reaction `(5)' may be exothermic.
(6) 3 Na2SO4(s) + Na2S(s) + Methanol (solvent) 4 3 Na2SO4(s) + Na2S(dissolved)
Note: In some embodiments, after solid-liquid separation of 3 Na2SO4(s),
Na2S(dissolved) may be removed or recovered by distillation of the solvent and
crystallization of Na2S(s). The Na2S(s) may be transferred to an environment
with
sufficient water and carbon dioxide present for the reaction of Na2S with CO2
and H20 to
form Na2CO3 and H2S.
(7) Na2S(dissolved) 4 Na2S(s) + Methanol(Solvent)
(8) Na2S(s or aq) + Ca(OH)2(aq or s) 4 2 Na0H(s or aq) + CaS(s)
(9) CaS(s) + H20(g or 1 or aq) + S02(g or aq) 4 CaS03(s) + H2S(g or aq)
(10) CaS03(s) + Heat 4 CaO(s) + S02(g)
(11) CaO(s) + H20(1 or g) 4 Ca(OH)2(s or aq)
(12) Hydrogen sulfide may be sold or transferred to an external application or
may be
converted in one or more or any combination of the following:
= H2S(g) + 1.5 02(g) 4 S02(g or aq) + H20(g or 1), the SO2
= H2S(g) to elemental sulfur
= H2S(g) to hydrogen and sulfur or sulfur dioxide or sulfuric acid
= H2S(g) input to the Claus Process
= H2S(g) to reduced metal species, such as reduced iron or iron sulfide
= H2S(g) to reduced metal species, such as reduced iron or iron sulfide,
then
produce hydrogen using the reduced metal species
= H2S(g) to sulfuric acid
= Heat from conversion or combustion of H2S(g) to 502(g or aq) or Sulfur or
sulfuric acid may be employed to provide heat to one or more or any
combination of
reactions or separations, which may include, but are not limited to, one or
more or any
21
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
combination of the following: "4 Na2S205(s) + Heat 4 4 Na2S03(s) + 4 S02(g)",
or
distillation of organic solvent, or distillation of water, or supplemental
heat for "4
Na2S03(s) + Heat 4 3 Na2SO4(s) + Na2S(s)", or supplemental heat for "CaS03(s)
+ Heat
4 Ca0(s) + S02(g)".
(13) 8 S02(g) + water (solvent) 4 8 502(aq)
[00047] Example 22: Production of Sodium Hydroxide and Gypsum
(1) Calcium(Weak Acid Anion) + 8 S02(aq) + H20(aq) 4 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(2) 4 Ca(HS03)2(aq) + 4 Na2SO4(aq or s) 4 8 NaHS03(aq) + 4 CaSO4(s)
(3) 8 NaHS03(aq) 4 4 Na2S205(s) + Water(solvent)
(4) 4 Na2S205(s) + Heat 4 4 Na2S03(s) + 4 S02(g)
(5) 4 Na2S03(s) + Elevated Temperature 4 3 Na2SO4(s) + Na2S(s)
Note: Reaction `(5)' may be exothermic.
(6) 3 Na2SO4(s) + Na2S(s) + Methanol (solvent) 4 3 Na2SO4(s) + Na2S(dissolved)
Note: In some embodiments, after solid-liquid separation of 3 Na2SO4(s),
Na2S(dissolved) may be removed or recovered by distillation of the solvent and
crystallization of Na2S(s). The Na2S(s) may be transferred to an environment
with
sufficient water and carbon dioxide present for the reaction of Na2S with CO2
and H20 to
form Na2CO3 and H2S.
(7) Na2S(dissolved) 4 Na2S(s) + Methanol(Solvent)
(8) Na2S(s or aq) + Ca(OH)2(aq or s) 4 2 Na0H(s or aq) + CaS(s)
(9) CaS(s) + H20(g or 1 or aq) + CO2(g or aq) 4 CaCO3(s) + H2S(g or aq)
(10) CaCO3(s) + Heat 4 Ca0(s) + CO2(g)
Note: `(10)' may be conducted in a manner wherein CO2(g) produced is captured.
(11) Ca0(s) + H20(1 or g) 4 Ca(OH)2(s or aq)
(12) Hydrogen sulfide may be sold or transferred to an external application or
may be
converted in one or more or any combination of the following:
= H25(g) + 1.5 02(g) 4 502(g or aq) + H20(g or 1), the SO2
= H2S(g) to elemental sulfur
= H2S(g) to hydrogen and sulfur or sulfur dioxide or sulfuric acid
= H2S(g) input to the Claus Process
= H2S(g) to reduced metal species, such as reduced iron or iron sulfide
= H2S(g) to reduced metal species, such as reduced iron or iron sulfide,
then
produce hydrogen using the reduced metal species
= H2S(g) to sulfuric acid
22
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
= Heat from conversion or combustion of H2S(g) to S02(g or aq) or Sulfur or
sulfuric acid may be employed to provide heat to one or more or any
combination of
reactions or separations, which may include, but are not limited to, one or
more or any
combination of the following: -4 Na2S205(s) + Heat 4 4 Na2S03(s) + 4 S02(g)",
or
distillation of organic solvent, or distillation of water, or supplemental
heat for "4
Na2S03(s) + Heat 4 3 Na2SO4(s) + Na2S(s)", or supplemental heat for "CaS03(s)
+ Heat
4 CaO(s) + S02(g)".
(13) 8 S02(g) + water (solvent) 4 8 S02(aq)
1000481 Example 23: Production of Sodium Bicarbonate or Sodium
Carbonate and
Gypsum with Ammonia Intermediate
(1) May comprise any combination of the following:
= Na2SO4(s) + Water 4 Na2SO4(aq)
= 2 NH4HCO3(s) + Water 4 2 NH4HCO3(aq)
= Na2SO4(s or aq) + 2 NH4FIC03(aq) 4 (NF-14)2504(aq) + 2 NaHCO3(s)
= Na2SO4(s or aq) + 2 (NH4)2CO3(aq) 4 (NH4)2SO4(aq) + Na2CO3(s)
(2) Calcium(Weak Acid Anion) + 2 S02(g or aq) + H20(aq) 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(3) Ca(HS03)2(aq) + (NI14)2SO4(aq or s) 4 2 NH4FIS03(aq) + CaSO4(s)
(4) 4 NH4HS03(aq) + Heat 4 2 (NH4)2503(aq) + 2 S02(g)
(5) 2 (NH4)2503(aq) + CO2(g or aq) + H20(aq) 4 2 NH4HS03(aq) + 2 NH4HCO3(s)
Or
(1) May comprise any combination of the following:
= Na2SO4(s) + Water 4 Na2SO4(aq)
= 2 NH4HCO3(s) + Water 4 2 NH4HCO3(aq)
= Na2SO4(s or aq) + 2 N11411CO3(aq) 4 (NI-14)2SO4(aq) + 2 NaHCO3(s)
= Na2SO4(S Or aq) + 2 (NH4)2CO3(aq) 4 (NH4)2SO4(aq) + Na2CO3(s)
(2) Calcium(Weak Acid Anion) + 2 S02(g or aq) + H20(aq) 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(3) Ca(HS03)2(aq) + (NH4)2504(aq or s) 4 2 NH4HS03(aq) + CaSO4(s)
(4) 2 NRIHS03(aq) + 2 NRIHS03(s or aq) + Heat 4 2 (NH4)2S03(aq) + 2 S02(g)
(5) 2 (NH4)2S03(aq) + 2 CO2(g or aq) + 2 H20(aq) 4 2 NH4HS03(aq) + 2
NH4HCO3(s)
(6) 2 NH4FIS03(aq) 4 2 NH4FIS03(s) + Water
Or
(1) May comprise any combination of the following:
23
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
= Na2SO4(S) + Water 4 Na2SO4(aq)
= 2 NH4HCO3(s) + Water 4 2 NH4HCO3(aq)
= 2 NH4HCO3(aq) + Heat 4 (NH4)2CO3(aq) + CO2 + H20
= 2 NH4HCO3(s or aq) + Heat 4 (NH4)2CO3(s or aq) + CO2 + H20
= Na2SO4(s Or aq) + 2 NH4HCO3(aq) 4 (NH4)2SO4(aq) + 2 NaHCO3(s)
= Na2SO4(s or aq) + (NH4)2CO3(aq) 4 (NH4)2SO4(aq) + Na2CO3(s)
(2) Calcium(Weak Acid Anion) + 2 S02(g or aq) + H20(aq) 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(3) Ca(HS03)2(aq) + (NH4)2SO4(aq or s) 4 2 NH4HS03(aq) + CaSO4(s)
(4) 2 NH4HS03(aq) + (NH4)2S03(s) + Heat 4 2 (NH4)2S03(aq) + S02(g)
(5) 2 (NH4)2S03(aq) + 2 CO2(g or aq) + 2 H20(aq) 4 2 NH4HS03(aq) + 2
NH4HCO3(s)
(6) 2 NH4HS03(aq) 4 (NH4)2S03(s) + S02(g) + Water
(7) 2 NH4HCO3(s or aq) + Heat 4 (NH4)2CO3(s or aq) + CO2 + H20
Note: `(7)' may be desirable in embodiments producing sodium carbonate.
Note: CO2 produced by `(7)' may be recirculated comprise a portion of the CO2
input
'(5)'.
Or
(1) May comprise any combination of the following:
= Na2SO4(s) + Water 4 Na2SO4(aq)
= 2 NH4HCO3(s) + Water 4 2 NH4HCO3(aq)
= 2 NH4HCO3(aq) + Heat 4 (NH4)2CO3(aq) + CO2 + H20
= 2 NH4HCO3(s or aq) + Heat ¨) (NH4)2CO3(s or aq) + CO2 + H20
= Na2SO4(s Or aq) 2 NH4HCO3(aq) 4 (NH4)2SO4(aq) + 2 NaHCO3(s)
= Na2SO4(s or aq) + (NH4)2CO3(aq) 4 (NH4)2SO4(aq) + Na2CO3(s)
Note: In some embodiments, Na2CO3 or 2 NaHCO3 may be precipitated or may be
separated from (NH4)2SO4(aq) by utilizing the significant difference in
solubility between
Na2CO3 or 2 NaHCO3 and (NH4)2SO4(aq). In some embodiments, Na2CO3 or 2 NaHCO3
may be precipitated or may be separated by one, or more, or any combination of
the
following: cooling precipitation, or distillation, or solventing out, or
cryodesalination, or
evaporation, or mechanical vapor compression distillation, or solubility
properties, or by
supersaturation, or forward osmosis, or membrane based process, or reverse
osmosis, or
membrane distillation, or zero liquid discharge processes, or crystallization.
(2) Calcium(Weak Acid Anion) + 2 S02(g or aq) + H20(aq) + Water 4
Ca(HS03)2(aq) +
Weak Acid(s or aqueous or gas or liquid)
24
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
(3) Ca(HS03)2(aq) + (NH4)2SO4(aq or s) 4 2 NH4HS03(aq) + CaSO4(s)
Note: In some embodiments, `(2)' and (3)' may be combined into a single
process or a
single step or may otherwise be combined. For example, Calcium(Weak Acid
Anion)
and/or 2 S02 and/or H20 may be added to or mixed with (NH4)2SO4(aq) to form,
for
example, 2 NI-141-1S03(aq) + CaSO4(s) and / or Weak Acid(s or aqueous or gas
or liquid).
Said Weak Acid(s or aqueous or gas or liquid) may comprise, including, but not
limited
to, one or more or any combination of the following: carbon dioxide, or
carbonic acid, or
carbonate, or bicarbonate, or sesquicarbonate, or carbamate, or hydrogen
sulfide, or
sulfurous acid, or silicic acid, or orthosilicic acid, or silicon acid
derivatives, or silicon
minerals, or silicon acids, or aluminates, or ferrates, or other weak acids
described herein.
(4) 2 NH4f1S03(aq) + Heat 4 (NH4)2S03(aq) + S02(g) + H20(aq)
Note: A portion of water may be removed or distillated from '2 (NH4)2S03(aq)'
before
'(5)'. It may be desirable for the concentration of '2 (NH4)2S03(aq)' to be
sufficiently
high such that at least a portion of 2 NH4HCO3(s) may precipitate during `(5)'
or upon
cooling the solution during or after (5)'.
(5) (NH4)2S03(aq) + (NH4)2S03(s or aq) + 2 CO2(g or aq) + 2 H20(aq) 4
2 NH4HS03(aq) + 2 NH4HCO3(s)
(6) 2 NH4HS03(aq) 4 (NH4)2S03(s) + S02(g) + Water
Note: (NH4)2S03(s) may be transferred to '(5)' and may comprise a portion of
(NH4)2S03(s) in '(5)'.
(7) 2 NH4HCO3(s or aq) + Heat 4 (NH4)2CO3(s or aq) + CO2 + H20
Note: `(7)' may be desirable in embodiments producing sodium carbonate.
Note: CO2 produced by '(7)' may be recirculated comprise a portion of the CO2
input
[00049] Example 24: Production of Ammonia and Gypsum from
Ammonium Sulfate
(1) Calcium(Weak Acid Anion) + 2 S02(g or aq) + H20(aq) 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(2) Ca(FIS03)2(aq) + (NH4)2SO4(aq or s) 4 2 NH4HS03(aq) + CaSO4(s)
(3) 2 NH4HS03(aq) + (NH4)2S03(s) + Heat 4 2 (NH4)2S03(aq) + S02(g)
(4) 2 (NH4)2S03(aq) + 2 CO2(g or aq) + 2 H20(aq) 4 2 NH4HS03(aq) + 2
NH4HCO3(s)
(5) 2 NH4HS03(aq) 4 (NH4)2S03(s) + S02(g) + Water
(6) 2 NH4HCO4s) + Heat 4 NH3(g) + CO2(g) + H20(g or 1)
[00050] Example 25: Producing Sodium Bicarbonate and Gypsum or
Magnesium Sulfate
with Ammonia Intermediate
(1) May comprise any combination of the following:
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
= Na2SO4(S) + Water 4 Na2SO4(aq)
= 2 NH4HCO3(s) + Water 4 2 NH4HCO3(aq)
= 2 NH4HCO3(aq) + Heat 4 (NH4)2CO3(aq) CO2 + H20
= 2 NH4HCO3(s or aq) + Heat 4 (NH4)2CO3(s or aq) + CO2 + H20
= Na2SO4(s or aq) + 2 NH4HCO3(aq) 4 (NH4)2SO4(aq) + 2 NaHCO3(s)
= Na2SO4(s or aq) + (NH4)2CO3(aq) 4 (NH4)2SO4(aq) + Na2CO3(s)
Note: In some embodiments, Na2CO3 or 2 NaHCO3 may be precipitated or may be
separated
from (NH4)2SO4(aq) by utilizing the significant difference in solubility
between Na2CO3 or 2
NaHCO3 and (NH4)2SO4(aq). In some embodiments, Na2CO3 or 2 NaHCO3 may be
precipitated
or may be separated by one, or more, or any combination of the following:
cooling precipitation,
or distillation, or solventing out, or cryodesalination, or evaporation, or
mechanical vapor
compression distillation, or solubility properties, or by supersaturation, or
forward osmosis, or
membrane based process, or reverse osmosis, or membrane distillation, or zero
liquid discharge
processes, or crystallization.
Note: Ammonium bicarbonate may be decomposed into ammonium carbonate and
carbon
dioxide. The ammonium carbonate may be reacted with sodium sulfate to form
sodium carbonate
and ammonium sulfate. Carbon dioxide formed may be transferred to and / or
employed in one or
more process steps or reactions which require carbon dioxide.
(2) Calcium(Weak Acid Anion) + 2 S02(g or aq) + H20(aq) + Water 4
Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(3) Ca(HS03)2(aq) + (NH4)2SO4(aq or s) 4 2 NH4HS03(aq) + CaSO4(s)
Note: In some embodiments, '(2)' and '(3)' may be combined into a single
process or a single
step or may otherwise be combined. For example, Calcium(Weak Acid Anion)
and/or 2 SO2
and/or H20 may be added to or mixed with (NH4)2SO4(aq) to form, for example, 2
NH4HS03(aq)
+ CaSO4(s) and / or Weak Acid(s or aqueous or gas or liquid). Said Weak Acid(s
or aqueous or
gas or liquid) may comprise, including, but not limited to, one or more or any
combination of the
following: carbon dioxide, or carbonic acid, or carbonate, or bicarbonate, or
sesquicarbonate, or
carbamate, or hydrogen sulfide, or sulfurous acid, or silicic acid, or
orthosilicic acid, or silicon
acid derivatives, or silicon minerals, or silicon acids, or aluminates, or
ferrates, or other weak
acids described herein.
(4) 2 NH4HS03(aq) + Heat 4 (NH4)2S03(aq) + S02(g) + H20(aq)
Note: A portion of water may be removed or distillated from '2 (Nt14)2S03(aq)'
before '(5)'. It
may be desirable for the concentration of '2 (NH4)2S03(aq)' to be sufficiently
high such that at
least a portion of 2 NII4FIC03(s) may precipitate during '(5)' or upon cooling
the solution during
or after `(5)'.
26
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
(5) (NH4)2S03(aq) + (NH4)2S03(S Of aq) + 2 CO2(g or aq) + 2 H20(aq) 4
2 NH4f1S03(aq) + 2 NH4HCO3(s)
(6) 2 NH4HS03(aq) 4 (NH4)2S03(s) + S02(g) + Water
Note: In some embodiments, residual aqueous ammonia ¨ carbon dioxide may be
present in the
solution comprising NH4HS03(aq). A portion of ammonia ¨ carbon dioxide may
decompose into
carbon dioxide gas, which may be transferred or recirculated to reactions
employing carbon
dioxide within the process, or to other applications, or any combination
thereof
[00051] Example 26: Production of Sodium Hydroxide and Weak
Acid or Weak Acid
Anion Derivative
(1) One or more or any combination of the following:
= MgCO3 + S02(aq) 4 MgS03(aq) + CO2(g)
= MgS + S02(aq) + H20(aq) 4 MgS03(aq) + H2S(g)
= MgCa(CO3)2(s) + 502(aq) 4 MgS03(aq) + CaS03(s) + CO2(g)
= MgCa(WA)(s) + 502(aq) 4 MgS03(aq) + CaS03(s) + WA(s)
= MgCa(WA)(s) + 502(aq) 4 MgS03(aq) + Ca(WA)(s) + WA(s)
= Magnesium Silicate(s) + 502(aq) + H20(aq) 4 MgS03(aq) + Silicon
Dioxide(s)
= Magnesium(Weak Acid Anion) + 502(aq) + H20(aq) 4 MgS03(aq) + Weak
Acid(s or aqueous or gas or liquid)
Note: In some embodiments, MgS03(aq) may be separated from at least a portion
of
water to form, for example, MgS03(s). For example, a portion of MgS03(aq) may
be
precipitated as MgS03(s) by cooling precipitation. For example, in some
embodiments,
MgS03(aq) may be cooled to precipitate at least a portion of MgS03(s), then
the
MgS03(s) may be separated from the solution using a solid-liquid separation,
then the
remaining solution may be heated and / or the MgS03(aq) concentrated using
reverse
osmosis, or other membrane based process, or electrodialysis. For example, in
some
embodiments, MgS03(aq) may be cooled to precipitate at least a portion of
MgS03(s),
then the MgS03(s) may be separated from the solution using a solid-liquid
separation,
then the remaining solution may be mixed with new MgS03(aq), and / or heated,
and / or
the MgS03(aq) solution may be concentrated using reverse osmosis, or other
membrane
based process, or electrodialysis. In some embodiments, the concentrated and /
or heated
MgS03(aq) solution may be cooled to precipitate MgS03(s) and the MgS03(s) may
be
separated by a solid-liquid separation. In some embodiments, MgS03 may be
separated
from, for example, water by other separation systems and / or methods
described herein,
or known in the art, or any combination thereof
27
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: The magnesium ¨ `WA' input may comprise a mixture of calcium and
magnesium,
or calcium and magnesium carbonate, or calcium and magnesium sulfide, or
calcium and
magnesium silicate, or any combination thereof.
(2) MgS03(aq or s) + Na2SO4(aq or s) 4 Na2S03(aq or s) + MgSO4(aq or s)
Note: It may be desirable to separate Na2S03(aq or s) from MgSO4(aq or s).
Separating
Na2S03(aq or s) from MgSO4(aq or s) may comprise utilizing the difference in
solubility
properties between Na2S03(aq or s) from MgSO4(aq or s), or concentration, or
electrical
properties, or electrodialysis, or ion exchange, or water removal, or any
combination
thereof In some embodiments, Na2S03(aq or s) from MgSO4(aq or s) may be
separated
by solventing out or selective precipitation of a salt by the addition and /
or dissolution of
a solvent, such as an organic or inorganic solvent, which may result in the
selective or
relative greater precipitation of one salt relative to the other salt. In some
embodiments,
said organic or inorganic solvent may be regenerated or recovered by, for
example,
distillation, or other separation system or method described herein, or other
separation
system or method known in the art, or any combination thereof. In some
embodiments,
separating Na2S03(aq or s) from MgSO4(aq or s) may comprise including, but not
limited
to, one or more or any combination of the following: precipitation, or cooling
induced
precipitation, or concentration induced precipitation, or distillation, or
crystallization, or
cryodesalination, or extraction, or membrane based process, or reverse
osmosis, or other
separation systems or methods described herein, or other separation systems or
methods
known in the art.
Note: The solubility of magnesium sulfite decreases significantly in liquid or
supercritical
water in a temperature between 140 ¨ 220 degrees Celsius. In some embodiments,
a
solution of Na2S03(aq) + MgSO4(aq) may be heated above 140 degrees Celsius to
facilitate the precipitation of MgSO4(aq) as MgS 04(s ).
Note: It may be desirable to add MgS03, or Na2SO4, or any combination thereof
as a
solid to, for example, maximize the concentration of the solution and / or
minimize water
removal requirements or water removal energy consumption.
(4) Na2S03(s or aq) + Ca(OH)2(s or aq or suspension) 4 2 NaOH(aq) + CaS03(s)
(5) CaS03(s) + Heat 4 CaO(s) + S02(g)
(6) One or more or any combination of the following:
= 2 NaOH(aq) 4 2 NaOH(s) + Water(solvent)
= 2 NaOH(aq)
= 2 NaOH(aq) or 2 NaOH(s) added to seawater or body of water
(7) S02(g) + Water(solvent) 4 S02(aq)
2g
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
(8) Ca0(s) + H20 (1 or g) 4 Ca(OH)2(s or aq or suspension)
1000521 Example 27: Production of Sodium Carbonate or Sodium
Bicarbonate and Weak
Acid or Weak Acid Anion Derivative
(1) One or more or any combination of the following:
= MgCO3 + S02(aq) + 4 MgS03(aq) + CO2(g)
= MgS + S02(aq) + H20(aq) 4 MgS03(aq) + H2S(g)
= MgCa(CO3)2(s) + S02(aq) 4 MgS03(aq) + CaS03(s) + CO2(g)
= MgCa(WA)(s) + S02(aq) 4 MgS03(aq) + CaS03(s) + WA(s)
= MgCa(WA)(s) + S02(aq) 4 MgS03(aq) + Ca(WA)(s) + WA(s)
= Magnesium Silicate(s) + S02(aq) + H20(aq) 4 MgS03(aq) + Silicon
Dioxide(s)
= Magnesium(Weak Acid Anion) + S02(aq) + H20(aq) 4 MgS03(aq) + Weak
Acid(s or aqueous or gas or liquid)
Note: In some embodiments, MgS03(aq) may be separated from at least a portion
of
water to form, for example, MgS03(s). For example, a portion of MgS03(aq) may
be
precipitated as MgS03(s) by cooling precipitation. For example, in some
embodiments,
MgS03(aq) may be cooled to precipitate at least a portion of MgS03(s), then
the
MgS03(s) may be separated from the solution using a solid-liquid separation,
then the
remaining solution may be heated and / or the MgS03(aq) concentrated using
reverse
osmosis, or other membrane based process, or electrodialysis. For example, in
some
embodiments, MgS03(aq) may be cooled to precipitate at least a portion of
MgS03(s),
then the MgS03(s) may be separated from the solution using a solid-liquid
separation,
then the remaining solution may be mixed with new MgS03(aq), and / or heated,
and / or
the MgS03(aq) solution may be concentrated using reverse osmosis, or other
membrane
based process, or electrodialysis. In some embodiments, the concentrated and /
or heated
MgS03(aq) solution may be cooled to precipitate MgS03(s) and the MgS03(s) may
be
separated by a solid-liquid separation. In some embodiments, MgS0 3 may be
separated
from, for example, water by other separation systems and / or methods
described herein,
or known in the art, or any combination thereof.
Note: The magnesium ¨ 'WA' input may comprise a mixture of calcium and
magnesium,
or calcium and magnesium carbonate, or calcium and magnesium sulfide, or
calcium and
magnesium silicate, or any combination thereof.
(2) MgS03(aq or s) + Na2SO4(aq or s) 4 Na2S03(aq or s) + MgSO4(aq or s)
Note: It may be desirable to separate Na2S03(aq or s) from MgSO4(aq or s).
Separating
Na2S03(aq or s) from MgSO4(aq or s) may comprise utilizing the difference in
solubility
properties between Na2S03(aq or s) from MgSO4(aq or s), or concentration, or
electrical
29
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
properties, or electrodialysis, or ion exchange, or water removal, or any
combination
thereof. Separating Na2S03(aq or s) from MgSO4(aq or s) may comprise
including, but
not limited to, one or more or any combination of the following:
precipitation, or cooling
induced precipitation, or concentration induced precipitation, or
distillation, or
crystallization, or cryodesalination, or extraction, or membrane based
process, or reverse
osmosis, or electrodialysis, or electrodialysis reversal, or other separation
systems or
methods described herein, or other separation systems or methods known in the
art.
Note: In some embodiments, it may be desirable to concentrate magnesium
sulfite with
electrodialysis, or electrodialysis reversal instead of, or in addition to,
reverse osmosis. In
some embodiments, it may be desirable to separate water from magnesium sulfite
using
electrodialysis, or electrodialysis reversal instead of, or in addition to,
reverse osmosis.
Note: The solubility of magnesium sulfite decreases significantly in liquid or
supercritical
water in a temperature between 140 ¨ 220 degrees Celsius. In some embodiments,
a
solution of Na2S03(aq) + MgSO4(aq) may be heated above 140 degrees Celsius to
facilitate the precipitation of MgSO4(aq) as MgSO4(s).
Note: It may be desirable to add MgS03, or Na2SO4, or any combination thereof
as a
solid to, for example, maximize the concentration of the solution and / or
minimize water
removal requirements or water removal energy consumption.
(3) Na2S03(aq or s) + Na2S03(s) + CO2 + 2 H20 4 2 NaHS03(aq or s) + 2
NaHCO3(aq
or s)
Note: 2 NaHCO3(aq or s) may be separated from NaHS03(aq or s) by
precipitation, or
concentrating, or solubility properties, or cooling precipitation, or water
removal systems
or methods described herein, or separation systems or methods described
herein, or water
removal systems or methods described in the art, or separation systems or
methods
described in the art, or any combination thereof
(4) 2 NaHS03(aq) 4 Na2S205(s) + Water(solvent)
Note: NaHS03(aq) may generally exist at an aqueous phase. Upon precipitation
or
crystallization, NaHS03(aq) precipitates or crystalizes as Na2S205(s).
Na2S205(s) may be
considered anhydrous.
(5) Na2S205(s) + Heat 4 Na2S03(s) + S02(g)
(6) S02(g) + Water(solvent) 4 S02(aq)
[00053] Example 28: Production of Sodium Hydroxide and Weak
Acid or Weak Acid
Anion Derivative
(1) One or more or any combination of the following:
= MgCO3 + S02(aq) 4 MgS03(aq) + CO2(g)
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
= MgS + S02(aq) + H20(aq) 4 MgS03(aq) + H2S(g)
= MgCa(CO3)2(s) + S02(aq) 4 MgS03(aq) + CaS03(s) + CO2(g)
= MgCa(WA)(s) + S02(aq) 4 MgS03(aq) + CaS03(s) + WA(s)
= MgCa(WA)(s) + S02(aq) 4 MgS03(aq) + Ca(WA)(s) + WA(s)
= Magnesium Silicate(s) + 502(aq) + H20(aq) 4 MgS03(aq) + Silicon
Dioxide(s)
= Magnesium(Weak Acid Anion) + S02(aq) + H20(aq) 4 MgS03(aq) + Weak
Acid(s or aqueous or gas or liquid)
Note: In some embodiments, MgS03(aq) may be separated from at least a portion
of
water to form, for example, MgS03(s). For example, a portion of MgS03(aq) may
be
precipitated as MgS03(s) by cooling precipitation. For example, in some
embodiments,
MgS03(aq) may be cooled to precipitate at least a portion of MgS03(s), then
the
MgS03(s) may be separated from the solution using a solid-liquid separation,
then the
remaining solution may be heated and / or the MgS03(aq) concentrated using
reverse
osmosis, or other membrane based process, or electrodialysis. For example, in
some
embodiments, MgS03(aq) may be cooled to precipitate at least a portion of
MgS03(s),
then the MgS03(s) may be separated from the solution using a solid-liquid
separation,
then the remaining solution may be mixed with new MgS03(aq), and / or heated,
and / or
the MgS03(aq) solution may be concentrated using reverse osmosis, or other
membrane
based process, or electrodialysis. In some embodiments, the concentrated and /
or heated
MgS03(aq) solution may be cooled to precipitate MgS03(s) and the MgS03(s) may
be
separated by a solid-liquid separation. In some embodiments, MgS03 may be
separated
from, for example, water by other separation systems and / or methods
described herein,
or known in the art, or any combination thereof
Note: The magnesium ¨ 'WA' input may comprise a mixture of calcium and
magnesium,
or calcium and magnesium carbonate, or calcium and magnesium sulfide, or
calcium and
magnesium silicate, or any combination thereof.
(2) MgS03(aq or s) + Na2SO4(aq or s) 4 Na2S03(aq or s) + MgSO4(aq or s)
Note: It may be desirable to separate Na2S03(aq or s) from MgSO4(aq or s).
Separating
Na2S03(aq or s) from MgSO4(aq or s) may comprise utilizing the difference in
solubility
properties between Na2S03(aq or s) from MgSO4(aq or s), or concentration, or
electrical
properties, or electrodialysis, or ion exchange, or water removal, or any
combination
thereof In some embodiments, Na2S03(aq or s) from MgSO4(aq or s) may be
separated
by solventing out or selective precipitation of a salt by the addition and /
or dissolution of
a solvent, such as an organic or inorganic solvent, which may result in the
selective or
relative greater precipitation of one salt relative to the other salt. In some
embodiments,
31
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
said organic or inorganic solvent may be regenerated or recovered by, for
example,
distillation, or other separation system or method described herein, or other
separation
system or method known in the art, or any combination thereof. In some
embodiments,
separating Na2S03(aq or s) from MgSO4(aq or s) may comprise including, but not
limited
to, one or more or any combination of the following: precipitation, or cooling
induced
precipitation, or concentration induced precipitation, or distillation, or
crystallization, or
cryodesalination, or extraction, or membrane based process, or reverse
osmosis, or other
separation systems or methods described herein, or other separation systems or
methods
known in the art.
Note: The solubility of magnesium sulfite decreases significantly in liquid or
supercritical
water in a temperature between 140 ¨ 220 degrees Celsius. In some embodiments,
a
solution of Na2S03(aq) + MgSO4(aq) may be heated above 140 degrees Celsius to
facilitate the precipitation of MgSO4(aq) as MgSO4(s).
Note: It may be desirable to add MgS03, or Na2SO4, or any combination thereof
as a
solid to, for example, maximize the concentration of the solution and / or
minimize water
removal requirements or water removal energy consumption.
(4) Na2S03(s or aq) + Mg(OH)2(s or aq or suspension) 4 2 NaOH(aq) + MgS03(aq
or s)
Note: MgS03(aq) may be separated into MgS03(s) using systems or methods
described
herein, or separating systems and methods described in the art, or any
combination
thereof
Note: 2 NaOH(aq) may be transformed into a concentrated solution comprising 2
NaOH(aq) or into a solid comprising Na0H(s). Said separation may comprise
systems or
methods described herein, or separating systems and methods described in the
art, or any
combination thereof At least a portion of MgS03 may be separated from NaOH
during
said transforming.
(5) MgS03(s) + Heat 4 Mg0(s) + S02(g)
(6) One or more or any combination of the following:
= 2 NaOH(aq) 4 2 NaOH(s) + Water(solvent)
= 2 NaOH(aq)
= 2 NaOH(aq) or 2 NaOH(s) added to seawater or body of water
(7) S02(g) + Water(solvent) 4 S02(aq)
(8) MgO(s) + H20 (1 or g) 4 Mg(OH)2(s or aq or suspension)
[00054] Example 29: Production of Sodium Hydroxide and Weak
Acid or Weak Acid
Anion Derivative
32
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
(1) Calcium(Weak Acid Anion) + 2 S02(aq) + H20(aq) 4 Ca(HS03)2(aq) + Weak
Acid(s or aqueous or gas or liquid)
(2) Ca(HS03)2(aq) + Na2SO4(aq or s) 4 2 NaHS03(aq) + CaSO4(s)
(3) 2 NaHS03(aq) 4 Na2S205(s) + Water(solvent)
Note: NaHS03(aq) may generally exist at an aqueous phase. Upon precipitation
or
crystallization, NaHS03(aq) precipitates or crystalizes as Na2S205(s).
Na2S205(s) may be
considered anhydrous.
(4) Na2S205(s) + Heat 4 Na2S03(s) + S02(g)
(5) Na2S03(s or aq) + Mg(OH)2(s or aq or suspension) 4 2 NaOH(aq) + MgS03(s or
aq)
Note: MgS03(aq) may be separated into MgS03(s) using systems or methods
described
herein, or separating systems and methods described in the art, or any
combination
thereof
Note: 2 NaOH(aq) may be transformed into a concentrated solution comprising 2
NaOH(aq) or into a solid comprising Na0H(s). Said separation may comprise
systems or
methods described herein, or separating systems and methods described in the
art, or any
combination thereof At least a portion of MgS03 may be separated from NaOH
during
said transforming.
(6) MgS03(s) + Heat 4 MgO(s) + S02(g)
(7) One or more or any combination of the following:
= 2 NaOH(aq) 4 2 NaOH(s) + Water(solvent)
= 2 NaOH(aq)
= 2 NaOH(aq) or 2 NaOH(s) added to seawater or body of water
(8) 2 S02(g) + Water(solvent) 4 2 502(aq)
(9) MgO(s) + H20 (1 or g) 4 Mg(OH)2(s or aq or suspension)
Note: Calcium may be provided as an example alkaline earth. Alkaline earths in
addition
to or instead of calcium may be employed where calcium is provided as an
example.
Note: Magnesium may be provided as an example alkaline earth. Alkaline earths
in
addition to or instead of magnesium may be employed where magnesium is
provided as
an example.
Note: In some embodiments, the reaction of magnesium oxide with water may
generate
heat. Said heat may be employed where useful within the process, or outside
the process,
or any combination thereof
33
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Example Sodium Bicarbonate and / or Sodium Carbonate Production with Ammonia
Intermediate Step-by-Step Descriptions
[00055] (1) Sodium sulfate, or potassium sulfate, or an alkali
sulfate, may be mixed with
ammonium bicarbonate, or ammonium carbonate, or ammonium carbamate, or any
combination
thereof, which may result in the formation of sodium bicarbonate, or sodium
carbonate, or any
combination thereof and / or ammonium sulfate. Sodium sulfate may be mixed
with ammonium
bicarbonate, which may result in the formation of sodium bicarbonate and
ammonium sulfate. In
some embodiments, the reaction of ammonium bicarbonate with sodium sulfate may
be
conducted at an aqueous state, wherein ammonium bicarbonate may be dissolved
in water and /
or sodium sulfate may be dissolved in water. In some embodiments, it may be
desirable for `(1)'
to be conducted in multiple steps or stages. For example, in some embodiments,
sodium sulfate
and ammonium bicarbonate may be mixed in a solution at a temperature where
sodium sulfate is
more soluble in water, such as at a temperature greater than 10 C, or 15 C, or
20 C, or 25 C, or
30 C, or 35 C, or 40 C, to, for example, facilitate the reaction and / or
prevent the precipitation of
sodium sulfate and / or promote the dissolution of sodium sulfate (if, for
example, sodium sulfate
is added at a solid phase). For example, in some embodiments, after the mixing
of sodium sulfate
and ammonium bicarbonate, in some embodiments, the combined solution may be
cooled to
facilitate the precipitation of at least a portion of sodium bicarbonate,
while, for example,
ammonium sulfate may remain dissolved. In some embodiments, the `(1)' may be a
continuous
process, which may involve, for example, mixing of sodium sulfate and ammonium
bicarbonate
and precipitation of sodium bicarbonate due to, for example, supersaturation
or the resulting
formation of sodium bicarbonate exceeding the solubility limits of sodium
bicarbonate in the
solution. In some embodiments, it may be desirable to remove or separate
sodium bicarbonate
from ammonium sulfate. For example, in some embodiments, temperature and / or
concentration
induced precipitation may separate the sodium bicarbonate from the aqueous
ammonium sulfate
or ammonium sulfate. For example, in some embodiments, separation may include,
but is not
limited to, one or more or any combination of the following: precipitation, or
cooling induced
precipitation, or concentration induced precipitation, or distillation, or
reverse osmosis, or
membrane distillation, or membrane based process, or forward osmosis, or
crystallization, or
cryodesalination, or extraction, or other separation systems or methods
described herein, or other
separation systems or methods known in the art. In some embodiments, sodium
bicarbonate solid
may be separated from an aqueous solution using a solid-liquid separation
process. Sodium
bicarbonate may comprise a valuable byproduct from the process, and / or may
be, for example,
further processed, or converted to sodium carbonate, or transferred, or sold,
or employed in other
34
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
systems or processes. The ammonium sulfate, which may exit the process as an
aqueous solution,
as a solid, or any combination thereof, may be transferred to `(3)'.
[00056] (2) Calcium ¨ weak acid salt, or magnesium ¨ weak acid
salt, or alkaline-earth
weak acid salt may be mixed with sulfur dioxide, or aqueous sulfur dioxide, or
sulfurous acid, or
water, or any combination thereof, which may result in the formation of
calcium bisulfite, or
magnesium bisulfite, or alkaline earth bisulfite, or any combination thereof
and / or a weak acid
or weak acid derivative. Calcium ¨ weak acid salt may be mixed with sulfur
dioxide, or aqueous
sulfur dioxide, or sulfurous acid, or water, or any combination thereof, which
may result in the
formation of calcium bisulfite and / or a weak acid or weak acid derivative.
Said weak acid may
comprise a gas, such as carbon dioxide gas or hydrogen sulfide gas. Said weak
acid may
comprise a solid, such as silicon dioxide, or iron oxide, or aluminum oxide,
or manganese oxide,
or transition metal oxide, or zinc oxide. In some embodiments, said weak acid
or weak acid
derivative may comprise a byproduct and may be removed from the process. In
some
embodiments, said weak acid or weak acid derivative may be employed elsewhere
in the process.
For example, if said weak acid or weak acid derivative comprises carbon
dioxide, said carbon
dioxide may be employed as a portion of the input carbon dioxide employed in
the production of
ammonium carbonate, or ammonium bicarbonate, or ammonium carbamate, or any
combination
thereof within the process. In some embodiments, said a solid weak acid or
weak acid derivative
may be separated from the bisulfite aqueous solution by a solid-liquid
separation process.
Calcium bisulfite aqueous solution may be transferred to '(3)'.
[00057] (3) Calcium bisulfite, which may comprise an aqueous
solution, may be mixed
with ammonium sulfate, which may comprise an aqueous solution, or solid, or
any combination
thereof, which may result in the formation of calcium sulfate and ammonium
bisulfite. Calcium
sulfate may form as a precipitated due to its low solubility in water and / or
due to calcium
sulfate possessing a solubility in water significantly lower than ammonium
bisulfite. The
solubility of calcium sulfate in water may be about 0.26g/100m1 and the
solubility of ammonium
bisulfite in water may be greater than 100g/100mL. Calcium sulfate or gypsum
may be separated
from the aqueous solution or the aqueous ammonium bisulfite solution by a
solid-liquid
separation system and / or method. Calcium sulfate or gypsum may comprise a
product or output.
The aqueous ammonium bisulfite solution may be transferred to '(4)'.
[00058] (4) Ammonium bisulfite or an aqueous solution
comprising ammonium bisulfite
may be heated, or depressurized, or may have its pressure reduced, or may have
its temperature
increased, or may have its pressure increased, or may have its temperature
reduced, or any
combination thereof, which may result in the desorption of sulfur dioxide and
/ or the formation
of aqueous ammonium sulfite.
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: It may be desirable for the concentration of aqueous ammonium sulfite to
be sufficiently high to enable in the precipitation of at least a portion of
ammonium bicarbonate in '(5)'. In some embodiments, a portion of concentrating
or distillation may be desired.
[00059] (5) Ammonium sulfite, which may comprise an aqueous
solution comprising
ammonium sulfite, may be contacted with or reacted with carbon dioxide, which
may result in
the formation of ammonium bisulfite and ammonium bicarbonate.
Note: In some embodiments, at least a portion of ammonium bicarbonate may be
separated from the ammonium bisulfite. For example, in some embodiments,
ammonium bicarbonate may be separated from ammonium bisulfite by
precipitation. For example, in some embodiments, ammonium bicarbonate may be
separated from ammonium bisulfite by electrodialysis. For example, in some
embodiments, separation may include, but is not limited to, one or more or any
combination of the following: precipitation, or cooling induced precipitation,
or
concentration induced precipitation, or distillation, or crystallization, or
cryodesalination, or extraction, or reverse osmosis, or membrane based
process, or
membrane distillation, or other separation systems or methods described
herein, or
other separation systems or methods known in the art. In some embodiments,
ammonium bicarbonate solid may be separated from an aqueous solution using a
solid-liquid separation process.
Note: It may be desirable for the partial pressure of carbon dioxide added to
be
sufficient to enable the formation of ammonium bicarbonate and / or sufficient
or
desirable absorption kinetics and / or sufficient or desirable reaction
kinetics. For
example, it may be desirable for the partial pressure of CO2(g) reactant to be
greater than or equal to one or more or any combination of the following: 0.01
Bar, or 0.05 bar, or 0.1 Bar, or 0.2 Bar, or 0.3 Bar, or 0.4 Bar, or 0.5 Bar,
or 0.6
Bar, or 0.7 Bar, or 0.8 Bar, or 0.9 Bar, or 1.0 Bar. For example, it may be
desirable for the concentration of CO2(g) reactant to be greater than or equal
to
one or more or any combination of the following: 1%, or 5%, or 10%, or 20%, or
30%, or 40%, or 50%, or 60%, or 70%, or 80%, or 90%, or 95%.
[00060] (6) An aqueous ammonium bisulfite solution may be
transformed into ammonium
sulfite, or sulfur dioxide, or water, or any combination thereof In some
embodiments, the
resulting ammonium sulfite may comprise a concentrated aqueous solution, or a
solid, or any
combination thereof In some embodiments, residual ammonium bicarbonate or
ammonium
carbonate or ammonium ¨ carbon dioxide may be present in solution. In some
embodiments,
36
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
residual ammonium bicarbonate or ammonium carbonate or ammonium ¨ carbon
dioxide may
decompose and / or otherwise form carbon dioxide gas. Said carbon dioxide gas
may be
recirculated and / or employed in `(5)'.
Or
[00061] (1) Sodium sulfate, or potassium sulfate, or an alkali
sulfate, may be mixed with
ammonium bicarbonate, or ammonium carbonate, or ammonium carbamate, or any
combination
thereof, which may result in the formation of sodium bicarbonate, or sodium
carbonate, or any
combination thereof and / or ammonium sulfate. Sodium sulfate may be mixed
with ammonium
carbonate, which may result in the formation of sodium carbonate and ammonium
sulfate. In
some embodiments, the reaction of ammonium carbonate with sodium sulfate may
be conducted
at an aqueous state, wherein ammonium carbonate may be dissolved in water and
/ or sodium
sulfate may be dissolved in water. In some embodiments, it may be desirable
for `(1)' to be
conducted in multiple steps or stages. For example, in some embodiments,
sodium sulfate and
ammonium carbonate may be mixed in a solution at a temperature where sodium
sulfate is more
soluble in water, such as at a temperature greater than 10 C, or 15 C, or 20
C, or 25 C, or 30 C,
or 35 C, or 40 C, to, for example, facilitate the reaction and / or prevent
the precipitation of
sodium sulfate and / or promote the dissolution of sodium sulfate (if, for
example, sodium sulfate
is added at a solid phase). For example, in some embodiments, after the mixing
of sodium sulfate
and ammonium carbonate, in some embodiments, the combined solution may be
cooled to
facilitate the precipitation of at least a portion of sodium carbonate, while,
for example,
ammonium sulfate may remain dissolved. In some embodiments, the `(1)= may be a
continuous
process, which may involve, for example, mixing of sodium sulfate and ammonium
carbonate
and precipitation of sodium carbonate due to, for example, supersaturation or
the resulting
formation of sodium bicarbonate exceeding the solubility limits of sodium
carbonate in the
solution. In some embodiments, it may be desirable to remove or separate
sodium carbonate from
ammonium sulfate. For example, in some embodiments, temperature and / or
concentration
induced precipitation may separate the sodium bicarbonate from the aqueous
ammonium sulfate
or ammonium sulfate. For example, in some embodiments, separation may include,
but is not
limited to, one or more or any combination of the following: precipitation, or
cooling induced
precipitation, or concentration induced precipitation, or distillation, or
crystallization, or
cryodesalination, or extraction, or other separation systems or methods
described herein, or other
separation systems or methods known in the art. In some embodiments, sodium
carbonate solid
may be separated from an aqueous solution using a solid-liquid separation
process. Sodium
carbonate may comprise a valuable byproduct from the process, and / or may be,
for example,
further processed, or transferred, or sold, or employed in other systems or
processes. The
37
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
ammonium sulfate, which may exit the process as an aqueous solution, as a
solid, or any
combination thereof, may be transferred to `(3)'.
[00062] (2) Calcium ¨ weak acid salt, or magnesium ¨ weak acid
salt, or alkaline-earth
weak acid salt may be mixed with sulfur dioxide, or aqueous sulfur dioxide, or
sulfurous acid, or
water, or any combination thereof, which may result in the formation of
calcium bisulfite, or
magnesium bisulfite, or alkaline earth bisulfite, or any combination thereof
and / or a weak acid
or weak acid derivative. Calcium ¨ weak acid salt may be mixed with sulfur
dioxide, or aqueous
sulfur dioxide, or sulfurous acid, or water, or any combination thereof, which
may result in the
formation of calcium bisulfite and / or a weak acid or weak acid derivative.
Said weak acid may
comprise a gas, such as carbon dioxide gas or hydrogen sulfide gas. Said weak
acid may
comprise a solid, such as silicon dioxide, or iron oxide, or aluminum oxide,
or manganese oxide,
or transition metal oxide, or zinc oxide. In some embodiments, said weak acid
or weak acid
derivative may comprise a byproduct and may be removed from the process. In
some
embodiments, said weak acid or weak acid derivative may be employed elsewhere
in the process.
For example, if said weak acid or weak acid derivative comprises carbon
dioxide, said carbon
dioxide may be employed as a portion of the input carbon dioxide employed in
the production of
ammonium carbonate, or ammonium bicarbonate, or ammonium carbamate, or any
combination
thereof within the process. In some embodiments, said a solid weak acid or
weak acid derivative
may be separated from the bisulfite aqueous solution by a solid-liquid
separation process.
Calcium bisulfite aqueous solution may be transferred to '(3)'.
[00063] (3) Calcium bisulfite, which may comprise an aqueous
solution, may be mixed
with ammonium sulfate, which may comprise an aqueous solution, or solid, or
any combination
thereof, which may result in the formation of calcium sulfate and ammonium
bisulfite. Calcium
sulfate may form as a precipitated due to its low solubility in water and / or
due to calcium
sulfate possessing a solubility in water significantly lower than ammonium
bisulfite. The
solubility of calcium sulfate in water may be about 0.26g/1 00m1 and the
solubility of ammonium
bisulfite in water may be greater than 100g/100mL. Calcium sulfate or gypsum
may be separated
from the aqueous solution or the aqueous ammonium bisulfite solution by a
solid-liquid
separation system and / or method. Calcium sulfate or gypsum may comprise a
product or output.
The aqueous ammonium bisulfite solution may be transferred to '(4)'.
[00064] (4) Ammonium bisulfite or an aqueous solution
comprising ammonium bisulfite
may be heated, or depressurized, or may have its pressure reduced, or may have
its temperature
increased, or may have its pressure increased, or may have its temperature
reduced, or any
combination thereof, which may result in the desorption of sulfur dioxide and
/ or the formation
of aqueous ammonium sulfite.
3s
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: It may be desirable for the concentration of aqueous ammonium sulfite to
be sufficiently high to enable in the precipitation of at least a portion of
ammonium bicarbonate in '(5)'. In some embodiments, a portion of concentrating
or distillation may be desired.
[00065] (5) Ammonium sulfite, which may comprise an aqueous
solution comprising
ammonium sulfite, may be contacted with or reacted with carbon dioxide, which
may result in
the formation of ammonium bisulfite and ammonium bicarbonate.
Note: In some embodiments, at least a portion of ammonium bicarbonate may be
separated from the ammonium bisulfite. For example, in some embodiments,
ammonium bicarbonate may be separated from ammonium bisulfite by
precipitation. For example, in some embodiments, ammonium bicarbonate may be
separated from ammonium bisulfite by electrodialysis. For example, in some
embodiments, separation may include, but is not limited to, one or more or any
combination of the following: precipitation, or cooling induced precipitation,
or
concentration induced precipitation, or distillation, or crystallization, or
cryodesalination, or extraction, or other separation systems or methods
described
herein, or other separation systems or methods known in the art. In some
embodiments, ammonium bicarbonate solid may be separated from an aqueous
solution using a solid-liquid separation process.
Note: It may be desirable for the partial pressure of carbon dioxide added to
be
sufficient to enable the formation of ammonium bicarbonate and / or sufficient
or
desirable absorption kinetics and / or sufficient or desirable reaction
kinetics. For
example, it may be desirable for the partial pressure of CO2(g) reactant to be
greater than or equal to one or more or any combination of the following: 0.01
Bar, or 0.05 bar, or 0.1 Bar, or 0.2 Bar, or 0.3 Bar, or 0.4 Bar. or 0.5 Bar,
or 0.6
Bar, or 0.7 Bar, or 0.8 Bar, or 0.9 Bar, or 1.0 Bar. For example, it may be
desirable for the concentration of CO2(g) reactant to be greater than or equal
to
one or more or any combination of the following: 1%, or 5%, or 10%, or 20%, or
30%, or 40%, or 50%, or 60%, or 70%, or 80%, or 90%, or 95%.
[00066] (6) An aqueous ammonium bisulfite solution may be
transformed into ammonium
sulfite, or sulfur dioxide, or water, or any combination thereof In some
embodiments, the
resulting ammonium sulfite may comprise a concentrated aqueous solution, or a
solid, or any
combination thereof In some embodiments, residual ammonium bicarbonate or
ammonium
carbonate or ammonium ¨ carbon dioxide may be present in solution. In some
embodiments,
residual ammonium bicarbonate or ammonium carbonate or ammonium ¨ carbon
dioxide may
39
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
decompose and / or otherwise form carbon dioxide gas. Said carbon dioxide gas
may be
recirculated and / or employed in `(5)'.
[00067] (7) Ammonium bicarbonate may be transformed into
ammonium carbonate and /
or carbon dioxide and / or water. Ammonium bicarbonate, which may comprise a
solid, or an
aqueous solution, or any combination thereof, may be decomposed into ammonium
carbonate
and / or carbon dioxide and / or water. In some embodiments, ammonium
carbonate may be
transferred to `(1)'. In some embodiments, said carbon dioxide and / or water
may be transferred
to `(5)'.
Example Sodium Hydroxide Production Step-by-Step Descriptions
Example (Two Aqueous Sulfur Dioxide Reaction Steps)
1000681 (1) Calcium carbonate, or magnesium carbonate, or any
combination thereof may
be reacted with a solution comprising aqueous sulfur dioxide (which may be
from step '(10)'),
which may form a gas comprising carbon dioxide, and a solid comprising calcium
sulfite, or
magnesium sulfite, or any combination thereof
Note: Solid calcium sulfite may be separated from a solution comprising water
using a solid-liquid separation process, if desired.
Note: Gaseous carbon dioxide may comprise high partial pressure, or high
purity
carbon dioxide.
Note: In some embodiments, calcium may further comprise magnesium, or a
mixture of magnesium and calcium, or magnesium. In some embodiments,
magnesium sulfite may form, which may be soluble in water. After, for example,
solid-liquid separation, the remaining liquid solution may comprise aqueous
magnesium sulfite. In some embodiments, it may be desirable to recirculate
said
aqueous magnesium sulfite as the absorption solution to absorb sulfur dioxide.
In
some embodiments, it may be desirable for the aqueous sulfur dioxide to
comprise
a portion of aqueous magnesium sulfite. In some embodiments, it may be
desirable to separate at least a portion of said magnesium sulfite from water
or the
aqueous solution using, for example, including, but not limited to, one or
more or
any combination of the following: cooling precipitation, or reverse osmosis,
or
membrane based process, or concentrating, or evaporation, or distillation, or
membrane distillation, or forward osmosis, or solventing out, or addition of a
soluble solvent to precipitate at least a portion of magnesium sulfite, or
other
separation systems and methods described herein, or other separation systems
and
methods described in the art.
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
[00069] (2) Solid calcium sulfite (which may be from step
'(1)') may be reacted with a
solution comprising aqueous sulfur dioxide (which may be from step `(11)'),
which may form a
solution comprising aqueous calcium bisulfite.
Note: '(2)= may be conducted at an elevated temperature and / or over an
extended
residence time. For example, the present step may be conducted at a
temperature
greater than 20 C, or 30 C, or 40 C, or 50 C, or 60 C, or 70 C, or 80 C, or 90
C,
or 100 C, or any combination thereof For example, the present step may be
conducted over a residence time period greater than 2 minutes, or 5 minutes,
or 10
minutes, or 15 minutes, or 30 minutes, or 45 minutes, or 1 hour, or 1.5 hours,
or 2
hours, or 2.5 hours, or any combination thereof. For example, the present step
may
be conducted in the presence of mixing. In some embodiments, sulfur dioxide
may
be added continuously during the reaction or dissolution of calcium bisulfite.
In
some embodiments, the present reaction may be continuous, or semi-continuous,
or cascading, or multi-stage, or multi-tank, or batch, or any combination
thereof
[00070] (3) The solution comprising aqueous calcium bisulfite
(which may be from step
'(2)') may be mixed with a solid or an aqueous solution comprising sodium
sulfate, which may
form an aqueous solution comprising sodium bisulfite and a solid comprising
calcium sulfate.
Note: A solid comprising calcium sulfate may be separated form an aqueous
solution comprising sodium bisulfite using a solid-liquid separation process.
[00071] (4) In some embodiments, an aqueous solution comprising
sodium bisulfite
(which may be from step '(3)') may be decomposed to form sulfur dioxide gas
and aqueous
sodium sulfite. In some embodiments, an aqueous solution comprising sodium
bisulfite may be
heated to desorb sulfur dioxide gas and form aqueous sodium sulfite. In some
embodiments, an
aqueous solution comprising sodium bisulfite (which may be from step '(3).)
may be
transformed into solid comprising sodium metabisulfite and a liquid comprising
water. An
aqueous solution comprising sodium bisulfite (which may be from step '(3)')
may be
transformed into solid comprising sodium metabisulfite, or a liquid comprising
water, or any
combination thereof For example, an aqueous solution comprising sodium
bisulfite may
undergo a water removal process, or a distillation process, or a precipitation
process, or a
combination thereof, which may result in the formation of at least a portion
of solid sodium
metabisulfite and at least a portion of water.
Note: When sodium bisulfite is precipitated or otherwise removed from water,
the
solid form generally comprises sodium metabisulfite.
Note: In some embodiments, a solvent, such as an organic or inorganic solvent,
may be added to aqueous solution comprising sodium bisulfite to solvent out,
or
41
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
solvent-out, or solvent dissolution induced precipitation, or adding a water
soluble
solvent to induce precipitation, or precipitate at least a portion of the
aqueous
sodium ¨ sulfur dioxide salt or aqueous sodium bisulfite as a solid comprising
sodium metabisulfite solid. Said solvent may comprise an organic solvent, or
an
inorganic solvent, or any combination thereof. In some embodiments, after
solvent
addition dissolution inducted sodium metabisulfite precipitation, the added
solvent
may be recovered or regenerated by distillation, or a separation system or
method
described herein, or separation system or method described in the art, or any
combination thereof
[00072] (5) Solid sodium metabisulfite (which may be from step
`(4)') may be thermally
decomposed into a solid comprising sodium sulfite and a gas comprising sulfur
dioxide.
Note: In some embodiments, said gas comprising sulfur dioxide in '(5)' may
comprise a relatively high partial pressure sulfur dioxide or sulfur dioxide
with a
partial pressure greater than 0.2 atm, or 0.3 atm, or 0.4 atm, or 0.5 atm, or
0.6 atm,
or 0.7 atm, or 0.8 atm, or 0.9 atm, or 1 atm, or 1.5 atm, or 2 atm, or any
combination thereof In some embodiments, the concentration of aqueous sulfur
dioxide in the resulting solution comprising aqueous sulfur dioxide may be
suitable to form aqueous calcium bisulfite from at least a portion of solid
calcium
sulfite or calcium salt under suitable conditions with sufficient residence
time.
[00073] (6) Sodium sulfite (which may be from step '(4)' or
'(5)') may be mixed with a
calcium oxide (which may be from step '(7)') or calcium hydroxide (which may
be in part from
step `(9)), which may form a solution comprising aqueous sodium hydroxide and
a solid
comprising calcium sulfite. Said calcium hydroxide may comprise one or more or
any
combination of the following: a solid, or an aqueous solution, or a slurry, or
a suspension, or milk
of lime.
Note: A solid comprising calcium sulfite may be separated form a solution
comprising aqueous sodium hydroxide using a solid-liquid separation process.
[00074] (7) A solid comprising calcium sulfite (which may be
from step `(6)') may be
thermally decomposed to form a solid comprising calcium oxide and a gas
comprising sulfur
dioxide.
Note: Said thermal decomposing may be conducted in a kiln or calciner.
Note: Said calcium sulfite may be dried or dehydrated before or during or both
said thermal decomposing into a solid comprising calcium oxide and a gas
comprising sulfur dioxide.
42
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: In some embodiments, said gas comprising sulfur dioxide in '(5)= may
comprise a relatively low partial pressure sulfur dioxide or sulfur dioxide
with a
partial pressure lower than 0.2 atm, or 0.3 atm, or 0.4 atm, or 0.5 atm, or
0.6 atm,
or 0.7 atm, or 0.8 atm, or 0.9 atm, or 1 atm, or 1.5 atm, or 2 atm, or any
combination thereof. In some embodiments, the concentration of aqueous sulfur
dioxide in the resulting solution comprising aqueous sulfur dioxide may be
suitable to form or facilitate the formation of aqueous calcium bisulfite from
at
least a portion of solid calcium sulfite or calcium salt under suitable
conditions
with sufficient residence time.
[00075] (8) A solution comprising aqueous sodium hydroxide
(which may be from step
`(6)') may be converted into a solid comprising sodium hydroxide and a liquid
comprising water.
For example, an aqueous solution comprising sodium hydroxide may undergo a
water removal
process, or a distillation process, or a precipitation process, or a
combination thereof, which may
result in the formation of at least a portion of solid sodium hydroxide and at
least a portion of
water. In some embodiments, the solution comprising aqueous sodium hydroxide
may be a
valuable product, and / or it may be desired for the sodium hydroxide to
remain at an aqueous
phase. In some embodiments, it may be desirable to concentrate the sodium
hydroxide solution
such that the concentration of sodium hydroxide is greater while remaining at
an aqueous state
and then selling or otherwise using the concentrated sodium hydroxide
solution.
[00076] (9) Calcium oxide (which may be from step '(7)') may be
reacted with water
from step '(8)", forming a material comprising calcium hydroxide. Said
material comprising
calcium hydroxide may comprise one or more or any combination of the
following: a solid, or an
aqueous solution, or a slurry, or a suspension, or milk of lime. Heat
generated from the reaction
of calcium oxide and water may be employed in one or more other process steps,
which may
include, but is not limited to, one or more or any combination of the
following: separation steps,
or calcining steps, or heating steps, or distillation steps, or drying steps,
or any combination
thereof
Note: In some embodiments, calcium oxide may be reacted directly with a
solution comprising sodium sulfite, which may result in the formation of
calcium
sulfite solid and aqueous sodium hydroxide.
[00077] (10) A gas comprising sulfur dioxide (which may be from
step '(7)') may be
absorbed into a solution comprising water (which may be from step '(1)'),
which may form a
solution comprising aqueous sulfur dioxide.
Note: In some embodiments, said gas comprising sulfur dioxide may comprise
sulfur dioxide mixed with other gases. In some embodiments, the concentration
of
43
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
aqueous sulfur dioxide in the resulting solution comprising aqueous sulfur
dioxide
may be suitable to form calcium sulfite and carbon dioxide in step -(1)'.
Note: For example, in some embodiments, it may be desirable for the
concentration or partial pressure of the sulfur dioxide produced from the
decomposition or calcining of calcium sulfite to be lower than the
concentration or
partial pressure of sulfur dioxide from the decomposition or calcining of
sodium
metabisulfite. For example, in some embodiments, when the S02(g) is absorbed
in
`(10)', it may be desirable for the concentration of S02(aq) in the resulting
solution to be sufficiently high to form calcium sulfite and carbon dioxide in
`(1)',
however to be sufficiently low such that the vapor pressure of sulfur dioxide
minimally contaminates the formed carbon dioxide.
[00078] (11) A gas comprising sulfur dioxide (which may be from
step `(5)') may be
absorbed into a solution comprising water (which may be from step `(4)'),
which may form a
solution comprising aqueous sulfur dioxide.
Note: In some embodiments, said gas comprising sulfur dioxide in `(11)' may
comprise a relatively high partial pressure sulfur dioxide or sulfur dioxide
with a
partial pressure greater than 0.2 atm, or 0.3 atm, or 0.4 atm, or 0.5 atm, or
0.6 atm,
or 0.7 atm, or 0.8 atm, or 0.9 atm, or 1 atm, or any combination thereof. In
some
embodiments, the concentration of aqueous sulfur dioxide in the resulting
solution
comprising aqueous sulfur dioxide may be suitable to form aqueous calcium
bisulfite from at least a portion of solid calcium sulfite or calcium salt
under
suitable conditions with sufficient residence time.
Example (One Aqueous Sulfur Dioxide Reaction Steps)
[00079] (1) A material comprising calcium or magnesium and / or
a salt comprising
calcium ¨ weak acid or magnesium ¨ weak acid or alkaline earth ¨ weak acid may
be reacted
with a solution comprising aqueous sulfur dioxide (which may be from step
'(10)'), which may
form a weak acid byproduct, such as a solid comprising silicon dioxide or a
gas comprising
carbon dioxide, and a solution comprising aqueous calcium bisulfite, or
aqueous magnesium
bisulfite, or any combination thereof
Note: In some embodiments, gaseous carbon dioxide may comprise high partial
pressure carbon dioxide.
Note: Gaseous carbon dioxide may form earlier in the residence time. In some
embodiments, at least a portion of gaseous carbon dioxide formation may occur
before most of the formation of aqueous calcium bisulfite.
44
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: In some embodiments, `(1)' may be conducted in two stages. For example,
some embodiments may involve a first stage wherein carbon dioxide gas may be
formed, and a second stage wherein at least a portion of calcium sulfite
dissolved
to form aqueous calcium bisulfite.
Note: At least a portion of `(1)' may be conducted at an elevated temperature
and
/ or over an extended residence time. For example, the present step may be
conducted at a temperature greater than 20 C, or 30 C, or 40 C, or 50 C, or 60
C,
or 70 C, or 80 C, or 90 C, or 100 C, or any combination thereof For example,
the
present step may be conducted over a residence time period greater than 2
minutes, or 5 minutes, or 10 minutes, or 15 minutes, or 30 minutes, or 45
minutes,
or 1 hour, or 1.5 hours, or 2 hours, or 2.5 hours, or any combination thereof
For
example, the present step may be conducted in the presence of mixing.
[00080] (2) The solution comprising aqueous calcium bisulfite
(which may be from step
`(1)') may be mixed with a solid or an aqueous solution comprising sodium
sulfate, which may
form an aqueous solution comprising sodium bisulfite and a solid comprising
calcium sulfate.
Note: A solid comprising calcium sulfate may be separated form an aqueous
solution comprising sodium bisulfite using a solid-liquid separation process.
[00081] (3) In some embodiments, an aqueous solution comprising
sodium bisulfite
(which may be from step `(2)') may be decomposed to form sulfur dioxide gas
and aqueous
sodium sulfite. In some embodiments, an aqueous solution comprising sodium
bisulfite may be
heated to desorb sulfur dioxide gas and form aqueous sodium sulfite. In some
embodiments, an
aqueous solution comprising sodium bisulfite (which may be from step `(2)')
may be
transformed into a solid comprising sodium metabisulfite and a liquid
comprising water. For
example, an aqueous solution comprising sodium bisulfite may undergo a water
removal process,
or a distillation process, or a precipitation process, or a combination
thereof, which may result in
the formation of at least a portion of solid sodium metabisulfite and at least
a portion of water.
Note: When sodium bisulfite is precipitated or otherwise removed from water,
the
solid form may generally comprise sodium metabisulfite.
[00082] (4) Solid sodium metabisulfite (which may be from step
`(3)') may be thermally
decomposed into a solid comprising sodium sulfite and a gas comprising sulfur
dioxide.
[00083] (5) Sodium sulfite (which may be from step `(3)' or
`(4)') may be mixed with
calcium oxide or calcium hydroxide (which may be from step `(6)' or `(8)'),
which may form a
solution comprising aqueous sodium hydroxide and a solid comprising calcium
sulfite. Calcium
hydroxide may comprise one or more or any combination of the following: a
solid, or an aqueous
solution, or a slurry, or a suspension, or milk of lime.
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: A solid comprising calcium sulfite may be separated form a solution
comprising aqueous sodium hydroxide using a solid-liquid separation process.
[00084] (6) A solid comprising calcium sulfite (which may be
from step '(5)') may be
thermally decomposed to form a solid comprising calcium oxide and a gas
comprising sulfur
dioxide.
Note: Said thermal decomposing may be conducted in a kiln or calciner.
Note: Said calcium sulfite may be dried or dehydrated before or during or both
said thermal decomposing into a solid comprising calcium oxide and a gas
comprising sulfur dioxide.
[00085] (7) A solution comprising aqueous sodium hydroxide
(which may be from step
`(5)') may be converted into a solid comprising sodium hydroxide and a liquid
comprising water.
For example, an aqueous solution comprising sodium hydroxide may undergo a
water removal
process, or a distillation process, or a precipitation process, or a
combination thereof, which may
result in the formation of at least a portion of solid sodium hydroxide and at
least a portion of
water. Alternatively, or additionally, a solution comprising aqueous sodium
hydroxide may
comprise a valuable product, and / or may be further concentrated to produce a
concentrated
sodium hydroxide solution, which may comprise a valuable product.
[00086] (9) Calcium oxide (which may be from step '(6).) may be
reacted with water
from step '(7)', forming calcium hydroxide. Said calcium hydroxide may
comprise one or more
or any combination of the following: a solid, or an aqueous solution, or a
slurry, or a suspension,
or milk of lime. Heat generated from the reaction of calcium oxide and water
to form calcium
hydroxide may be employed in one or more other process steps, which may
include, but is not
limited to, one or more or any combination of the following: separation steps,
or calcining steps,
or heating steps, or distillation steps, or drying steps, or any combination
thereof
[00087] (10) A gas comprising sulfur dioxide (which may be from
step '(6)' and / or step
'(4)') may be absorbed into a solution comprising water (which may be from
step '(3)'), which
may form a solution comprising aqueous sulfur dioxide.
Figure Keys
Figure 1D Key
Label Description
1 '1' may comprise an input material comprising an alkaline earth metal
salt. '1' may
comprise an input material comprising a calcium salt. '1' may comprise an
input
material comprising a calcium ¨ weak acid salt. '1' may comprise an input
material
comprising a salt of calcium and a weak acid with an acidity less than
sulfurous
acid. '1' may comprise an input material comprising calcium carbonate. '1' may
comprise a solid. '1' may comprise limestone. '1' may comprise, including, but
not
limited to, one or more or a combination of the following: an alkaline-earth
metal
salt, a carbonate, a silicate, or silicon derivative, a carboxylic acid salt,
a ferrate
46
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Figure ID Key
Label Description
salt, an aluminate salt, a zincate salt, an iron derivative salt, an manganese
derivative salt, a zinc derivative salt, or an aluminum derivative salt, or
any
combination thereof
2 Same as '1'.
3 '3' may comprise a reaction between an input material
with a solution comprising
aqueous sulfur dioxide or sulfurous acid. In some embodiments, '3' may
comprise
a reaction between a solid material comprising calcium carbonate and sulfur
dioxide or a liquid comprising aqueous sulfur dioxide to form solid calcium
sulfite
and gaseous carbon dioxide. In some embodiments, '3' may comprise a reaction
between a material comprising calcium carbonate and a solution comprising
aqueous sulfur dioxide, which may form a solid comprising calcium sulfite and
an
aqueous solution comprising water. in some embodiments, '3' may comprise a
reaction between a material comprising calcium carbonate and a solution
comprising a rich concentration of aqueous sulfur dioxide, which may form a
solid
comprising calcium sulfite and an aqueous solution comprising a lean
concentration of aqueous sulfur dioxide. '3' may comprise a reactor or mixer
or
any combination thereof '3' may be configured to allow the pressurization of
gaseous carbon dioxide. '3' may be configured to enable at least a portion of
carbon dioxide formed to comprise a high partial pressure, or high purity, or
a
combination thereof In some embodiments, it may be desirable for the reaction
to
be conducted under conditions to form calcium sulfite and minimize or prevent
the
formation of calcium bisulfite.
4 '4' may comprise a gas comprising carbon dioxide. '4' may
comprise an output. '4'
may comprise a gas comprising carbon dioxide, which may undergo further
treatment, or compression, or both. '4' may comprise a gas comprising a high
partial pressure and / or concentration of carbon dioxide. '4' may comprise at
least
a portion of carbon dioxide produced from a reaction of sulfur dioxide or
sulfurous
acid or both with a carbonate salt.
Same as '4'.
6 '6' may comprise a solid-liquid mixture. '6' may comprise
a mixture of a solid
phase comprising at least a portion of calcium sulfite and a liquid phase
comprising
at least a portion of water.
7 '7' may comprise a process for solid-liquid separation.
'7' may involve separating a
solid comprising at least a portion of calcium sulfite from a liquid
comprising water
using a solid-liquid separation process.
8 '8' may comprise a material comprising at least a portion
of calcium sulfite. In
some embodiments, '8' may comprise at least a portion of a solid comprising
calcium sulfite. In some embodiments, '8' may comprise at least a portion of a
solid comprising greater than 90 weight percent calcium sulfite. In some
embodiments, '8' may comprise at least a portion of a solid comprising greater
than
50 weight percent calcium sulfite. In some embodiments, '8' may comprise at
least
a portion of a solid comprising greater than 30 weight percent calcium
sulfite.
9 '9' may comprise a reactor or mixer. '9' may comprise a
solid-liquid reaction
which may result in the dissolution of at least a portion of a solid phase and
/ or the
formation of a gaseous product. '9' may comprise a reaction between a material
comprising at least a portion of calcium sulfite with an aqueous sulfur
dioxide
solution, which may form at least a portion of dissolved or aqueous calcium
bisulfite. In some embodiments, said material comprising at least a portion of
calcium sulfite may further comprise residual calcium carbonate, which may
react
with at least a portion of the sulfur dioxide and form gaseous carbon dioxide.
'9'
47
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Figure ID Key
Label Description
may be conducted under conditions which may facilitate the dissolution of
calcium
sulfite and / or the formation of aqueous calcium bisulfite. For example, '9'
may
involve, including, but not limited to, high concentration of sulfur dioxide,
or a
concentration of aqueous sulfur dioxide entering '9' greater than 3 weight
percent
aqueous sulfur dioxide, or an elevated temperature, or a temperature greater
than 20
degrees Celsius, or a sufficient residence time, or a residence time greater
than 30
minutes, or any combination thereof
zts
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Sodium Bicarbonate Production
Example Inputs and Outputs
Inputs Outputs
CaCO3(s) or CaSiO3(s) or 1/2 Ca2SiO4(s) or CaSO4
Ca(WA)(s) or a combination thereof
Na2SO4 NaHCO3 or Na2CO3
CO2 (g or! or s) SiO2 or 'WA'
H20
Energy (Heat and / or Electricity)
Example Reaction Steps
[00088] (la) CaCO3 or MgCO3 Input Version:
= (1a1) CaCO3(s) + SO2 (aq or! or g) 4 CaS03(s) + CO2(g)
= (1a2) Separating CaS03(s) from remaining liquid (if any) using, for
example, a
liquid-solid separation process
= (1a3) CaS03(s) + S02(aq) + H20(1) 4 Ca(HS03)2(aq)
Or
(lb) Calcium Silicate, or Magnesium Silicate, or Other Silicate, or Other
Calcium ¨ Weak Acid,
or Magnesium ¨ Weak Acid, or a Combination Thereof Salt Input Version:
= (1b1) CaSiO3(s) + 2 S02(aq) + H20(1) 4 Ca(HS03)2(aq) + SiO2
Or
= (1b10pt2) 1/2 Ca2SiO4(s) + 2 S02(aq) + H20(1) 4 Ca(HS03)2(aq) + 1/2 SiO2
Or
= (1b1opt3) Ca(WA)(s) + 2 S02(aq) + H20(1) 4 Ca(HS03)2(aq) + WA
[00089] (2) Ca(HS03)2(aq) + Na2SO4(aq) 4 2 NaHS03(aq) +
CaSO4(s)
1000901 (3a) 2 Na1-IS03(aq) + 2 Na1-IS03(aq) + Heat 4 2
Na2S03(aq) + 2 S02(g)
[00091] (4) 2 Na2S03(aq) + 2 CO2(g) + 2 H20(1) 4 2 NaHS03(aq) +
2 NaHCO3(s)
Note: In some embodiments, the aqueous solution may be concentrated, or
cooled, or both to promote the precipitation of sodium bicarbonate. For
example,
in some embodiments, an aqueous solution comprising 2 NaHS03(aq) + 2
NaHCO3(aq) may be concentrated using, for example, mechanical vapor
compression distillation, or distillation, or desorption, and the
precipitation of 2
NaHCO3(s) may be facilitated due to, for example, the concentrating beyond
solubility limits and / or lower temperature.
[00092] (5) - in some embodiments with sodium carbonate
production
NaHCO3(s) + Heat 4 Na2CO3(s) + CO2(g) + H20(g or 1)
49
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
= Note: 'WA' or 'Weak Acid Anion' or 'Weak Acid' may comprise a weak acid
or
weak acid anion, which may include, but not limited to, carbon dioxide, or
carbonic
acid, or carbonate, or bicarbonate, or sesquicarbonate, or carbamate, or
hydrogen
sulfide, or sulfurous acid, or silicic acid, or orthosilicic acid, or silicon
acid
derivatives, or silicon minerals, or silicon acids, or aluminates, or
ferrates, or any
combination thereof
= Note: Concentration of NaHS03 produced from step '(4)' may be increased
to match
concentration of NaHS03 from step '(2)' by, for example, distillation, or
membrane
based process, or evaporation, or other separation process, or other
concentrating
process, or a combination thereof
= Note: In step `(3)', one of the two '2 NaHS03(aq)' is from step `(2)' and
the other of
the two '2 NaHS03(aq)' is from step '(4)'.
= Note: Step `(4)' may require pure CO2(g) or high partial pressure CO2(g)
or CO2(1) or
CO2(g).
o At least a portion of CO2 input to step '(4)' may be sourced from step
'(1)' if step
'(1)' employs a carbonate input, such as, for example, step `(1a)'.
o At least a portion of CO2 input to step `(4)' may be sourced from step
`(5)' in
some embodiments employing a step '(5)'
o At least a portion of CO2 input to step '(4)' may be sourced from CO2
captured
from a combustion source, or a combustion source employed to produce heat, or
emissions source, or air, or geological CO2 source, or natural CO2 source, or
a
combination thereof
= Note: Some embodiments may be designed to operate as a low temperature
process,
where the solutions and / or solid reagents in thermal desorption or
decomposition
may undergo or operate thermal desorption or decomposition at less than 150 C,
or
less than 200 C, or less than 250 C, or less than 300 C, or less than 350 C.
CALCIUM OXIDE PRODUCTION EXAMPLE EMBODIMENTS
= Example Process Steps:
[00093] (1) React a material comprising a silicate of calcium
with aqueous sulfur dioxide
or sulfurous acid, which may produce a solution comprising at least a portion
dissolved calcium
bisulfite and a solid phase comprising at least a portion silicon or silica or
silicon dioxide or a
derivative of silicon. Said material comprising a silicate of calcium may
comprise an input to the
process.
[00094] (2) Separate solid silicon or silica or silicon dioxide
or a derivative of silicon or
other solids from a liquid solution comprising aqueous calcium bisulfite.
`(2)' may involve one
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
or more or a combination of solid-liquid separation processes. Said liquid
solution comprising
aqueous calcium bisulfite may be transferred to step `(3)'.
[00095] (3) Desorb or separate sulfur dioxide from a solution
comprising calcium
bisulfite, which may produce solid calcium sulfite and liquid solution
comprising water, or lean
aqueous sulfur dioxide, or lean calcium sulfite, or lean calcium bisulfite, or
a combination
thereof Desorption may require heat input, or depressurization, or vacuum, or
vapor
compression, or stripping gas, or a combination thereof Desorbed sulfur
dioxide may be
transferred to step '(6)'. Solid calcium sulfite and liquid solution may be
transferred to step '(4)'.
In some embodiments, '(3)' and '(4)' may be conducted in the same step.
[00096] (4) Separate solid calcium sulfite from a liquid
solution comprising water, or lean
aqueous sulfur dioxide, or lean calcium sulfite, or lean calcium bisulfite, or
a combination
thereof `(4)' may involve one or more or a combination of solid-liquid
separation processes.
Solid calcium sulfite may be transferred to step `(5)' and liquid solution
comprising water, or
lean aqueous sulfur dioxide, or lean calcium sulfite, or lean calcium
bisulfite, or a combination
thereof may be transferred to step `(6)'.
[00097] (5) Decompose solid calcium sulfite into calcium oxide
and sulfur dioxide.
Calcium oxide may comprise an output of the process. Sulfur dioxide may be
transferred
[00098] (6) Absorb sulfur dioxide into a liquid solution
comprising water, or lean aqueous
sulfur dioxide, or lean calcium sulfite, or lean calcium bisulfite, or a
combination thereof to form
a sulfur dioxide rich solution, or a sulfurous acid solution, or a combination
thereof. It may be
desirable for the concentration of sulfurous acid or sulfur dioxide in said
formed liquid solution
to be stoichiometrically at a molar ratio greater than or equal to 1:1
relative to the calcium input
in step 1 to, for example, enable the formation of soluble calcium bisulfite.
Said sulfur dioxide
rich solution, or a sulfurous acid solution, or a combination thereof may be
transferred to, for
example, step 1.
= Example Chemistry Steps:
[00099] (1) Calcium Silicate, or Magnesium Silicate, or Other
Silicate, or cement, or
concrete, or Other Calcium ¨ Weak Acid, or Magnesium ¨ Weak Acid, or a
Combination
Thereof Salt Input Version:
= (la) CaSiO3(s) + 2 H2S03(aq) 4 Ca(1-1S03)2(aq) + SiO2(s) + H20(1)
= Or
= (1b) 1/2 Ca2SiO4(s) + 2 S02(aq) + H20(1) 4 Ca(HS03)2(aq) + 1/2 SiO2
= Or
= (1c) Ca(WA)(s) + 2 S02(aq) + H20(1) 4 Ca(HS03)2(aq) + WA
[00100] (2) Ca(HS03)2(aq) + Heat 4 CaS03(s) + H20(1) + S02(g)
51
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
[00101] (3) CaS03(s) + Heat 4 Ca0(s) + S02(g)
1001021 (4) 2S02(g) + 2H20(1) 4 2 H2S03(aq)
ADDITIONAL DESCRIPTION
Example Chemistry
Example Summary of Inputs and Outputs
Inputs Outputs
CaCO3(s) or CaSiO3(s) or 1/2 Ca2SiO4(s) or CaSO4
Ca(WA)(s) or a combination thereof
Na2SO4 NaHCO3 or Na2CO3
CO2 (g or 1 or s) Si02 or 'WA'
H20
Energy (Heat and / or Electricity)
Summary of Example Reactions
1001031 (la) CaCO3 or MgCO3 Input Version:
= (laloptl) CaCO3(s) + SO2 (aq or 1 or g) 4 CaS03(s) + CO2(g)
= (1a2) Separating CaS03(s) from remaining liquid (if any) using, for
example, a liquid-
solid separation process
= (1a3) CaS03(s) + S02(aq) + H20(1) 4 Ca(HS03)2(aq)
= Or
= (lal opt2) CaCO3(s) + 2 SO2 (aq or 1 or g) + H20(1) 4 Ca(HS03)2 (aq) +
CO2(g)
And / Or
[00104] (lb) Calcium Silicate, or Magnesium Silicate, or Other
Silicate, or Other Calcium
¨ Weak Acid, or Magnesium ¨ Weak Acid, or a Combination Thereof Salt Input
Version:
= (1b1) CaSiO3(s) + 2 S02(aq) + H20(1) 4 Ca(HS03)2(aq) + SiO2
Or
= (1b1opt2) 1/2 Ca2SiO4(s) + 2 S02(aq) + H20(1) 4 Ca(HS03)2(aq) + 1/2 SiO2
Or
= (1b1opt3) Ca(WA)(s) + 2 502(aq) + H20(1) 4 Ca(H503)2(aq) + WA
1001051 (2) Ca(HS03)2(aq) + Na2SO4(aq) 4 2 NaHS03(aq) +
CaSO4(s)
[00106] (3optA) 2 NaHS03(aq) + 2 NaHS03(aq or s) + Heat 4 2
Na2S03(aq) + 2 S02(g)
+ 2 H20(1)
[00107] (4optA) 2 Na2S03(aq) + 2 CO2(g) + 2 H20(1) 4 2
NaHS03(aq) + 2 Nal-IC03(s)
[00108] (5optA) 2 S02(g) + 2 H20(1) 4 2 H2503(aq)
[00109] (6optA) - in some embodiments with sodium carbonate
production
NaHCO3(s) + Heat 4 Na2CO3(s) + CO2(g) + H20(g or 1)
And/Or
52
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
[00110] (3optB) 2 NaHS03(aq) + Separation 4 Na2S205(s) + H20 (g
or 1)
Note: A portion of CO2 may be generated from the decomposition of residual
sodium bicarbonate. May be desirable to decompose the residual sodium
bicarbonate at a lower temperature than the sodium bisulfite. May be desirable
to
precipitate residual sodium bicarbonate or sodium carbonate by concentrating
and
/ or cooling precipitation before or while separating or concentrating the
sodium
bisulfite solution.
[00111] (4optB) Na2S205(s) + Na2S205(s) + Heat 4 2 Na2S03(s) +
2 S02(g)
Note: A portion of CO2 may be generated from, for example, the decomposition
of residual sodium bicarbonate. May be desirable to decompose the residual
sodium bicarbonate at a lower temperature than the sodium bisulfite.
[00112] (5optB) 2 Na2S03(aq) + 2 CO2(g) + 2 H20(1) 4 2
NaHS03(aq) + 2 NaHCO3(s)
[00113] (6optB) 2 NaHS03(aq) + Separation 4 Na2S205(s) + H20 (g
or 1)
Note: In some embodiments, a portion of CO2 may be generated from the
decomposition of residual sodium bicarbonate, if any. In some embodiments, it
may be desirable to decompose the residual sodium bicarbonate at a lower
temperature than the sodium bisulfite. May be conducted in the same step as
(3optB). For example, before or during or after one or more separation
processes,
2 NaHS03(aq) in (6optB) may be mixed with 2 NaHS03(aq) in (3optB) to form a
combined solution and said combined solution may undergo one or more
separation processes to form 4 NaHS03(s).
[00114] (7optB) 2 S02(g) + 2 H20(1) 4 2 H2S03(aq)
[00115] (8optB) - in some embodiments with sodium carbonate
production
NafIC03(s) + Heat 4 Na2CO3(s) + CO2(g) + H20(g or 1)
Note: H2 S 03 (aq)' or '2 502(aq) + H20(1)' may be employed interchangeably.
DETAILED DESCRIPTION OF EACH REACTION
Reaction lalopl:
CaCO3(s) + S02(aq or 1 or g) CaS03(s) + CO2(g)
AH = ¨24.67 kJ /mol
[00116] Description: The present reaction may involve reacting
calcium carbonate with
sulfur dioxide or sulfurous acid to produce calcium sulfite and carbon
dioxide. Calcium
carbonate may comprise limestone. Calcium carbonate may comprise magnesium
carbonate
instead of or in addition to calcium carbonate. Sulfur dioxide or sulfurous
acid may be a gas or a
liquid or a solution or an aqueous solution. It may be desirable to conduct
the present reaction to
facilitate the formation of carbon dioxide with minimal gaseous impurities, or
at a high partial
53
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
pressure, or both. For example, the present reaction may be conducted with a
dilute solution of
sulfur dioxide to minimize sulfur dioxide vapor.
[00117] Conditions: In some embodiments, the present reaction
may be conducted at
ambient temperature or may be cooled or both to, for example, minimize the
vapor pressure of
sulfur dioxide. It may be desirable to conduct the present reaction in a low
diatomic oxygen
environment or low diatomic oxygen atmosphere to, for example, prevent
oxidation of sulfur
dioxide or calcium sulfite to sulfuric acid or sulfate and / or to increase
the purity of carbon
dioxide produced. It may be desirable to conduct the present reaction under
conditions to
minimize the formation of calcium bisul rite or dissolved calcium bisulfite
to, for example, enable
calcium sulfite to be separated from liquid as a solid using a solid-liquid
separation process and
transferred to subsequent steps. For example, a CO2 desorption step or the
present step may
possess relatively fast kinetics and may be conducted at a relatively low
temperature and low
concentration of sulfur dioxide. For example, a CO2 desorption step or the
present step may be
conducted using a lower concentration of sulfur dioxide or sulfurous acid,
which may be
produced by recovering harder to separate, or less valuable, or lower
concentration sources of
sulfur dioxide or may be produced by smaller size, or less complex, or lower
energy
consumption, or lower cost equipment. For example, subsequent steps involving
the formation of
dissolved calcium bisulfite may possess relatively slower kinetics and may
benefit from being
conducted with a higher sulfurous acid concentration and / or at higher
temperatures. For
example, it may be desirable to conduct subsequent steps involving the
formation of dissolved
calcium bisulfite with a higher concentration of sulfurous acid or with the
formation of higher
concentration of calcium bisulfite to accelerate the reaction kinetics in
subsequent steps and / or
minimize or reduce water removal or water separation energy consumption in
subsequent steps.
Reaction 1a3:
CaS03(s) + S02(ag or 1 or g) + H20(1) Ca(HS03)2(ag)
[00118] Description: The present reaction may involve reacting
calcium sulfite with a
sulfurous acid solution to produce an aqueous solution of calcium bisulfite.
The present reaction
may be conducted under conditions to accelerate reaction rate or accelerate
the formation of
dissolve calcium bicarbonate. For example, the present reaction may be
conducted with excess
sulfur dioxide or excess sulfurous acid, or the present reaction may be
conducted wherein the
molar ratio of sulfurous acid to calcium sulfite(s) is greater than 1. For
example, the present
reaction may be conducted at temperatures at or above room temperature. For
example, the
present reaction may be conducted such that heat generated by the reaction
remains at least a
portion in the reaction, which may enable at least a portion of adiabatic
temperature rise, which
may facilitate reaction kinetics. For example, the present reaction may be
conducted with mild
54
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
heating or heat recovery to, for example, accelerate reaction kinetics. For
example, the present
reaction may be conducted such that product solution comprising calcium
bisulfite, which may
have experienced at least a portion of adiabatic temperature rise, is heat
exchanged with at least a
portion of the input reactants, which may raise the reaction temperature or
enable higher
temperature reaction operation, or operating at a reaction temperature above
room temperature
with less or minimal external heating or without the need for external
heating. In some
embodiments, heat may be recovered from the present reaction and / or employed
in other steps
of the present invention or for other applications. In some embodiments, it
may be desirable to
maximize the concentration of dissolved calcium bisulfite or reach near
maximum feasible
concentration of dissolved calcium bisulfite or both to, for example, minimize
water removal or
water separation which may be required in later steps. For example, it may be
desirable for
dissolve calcium bisulfite concentration in the product solution following the
present reaction
step to be greater than one or more of the following: 2.5wt%, or 5wt%, or
7.5wt%, or lOwt%, or
12.5wt%, or 15wt%, or 17.5wt%, or 20wt%, or 22.5wt%, or 25wt%, or 27.5wt%, or
30wt%.
Reaction lalop2:
CaCO3(s) + 2 S02(aq or 1 or g) + 1120(1) Ca(HS03)2(aq) + CO2(g)
CaSiO3(s) + 2 S02(aq or 1 or g) + H20(1) = Ca(HS03)2(aq) + SiO2(s)
And/or
1 1
¨2 Ca2SiO4(s) + 2 502(aq or 1 or g) + H20(1) Ca(H503)2(aq) + ¨25102(s)
And/or
Ca(WA)(s) + 2 S02(aq or 1 or g) + H20(1) Ca(HS03)2(aq) + WA(s)
1001191 Description: The present reaction may involve reacting
a calcium or alkali metal ¨
weak acid salt with sulfur dioxide or sulfurous acid or aqueous sulfurous acid
or excess aqueous
sulfurous acid to produce dissolved calcium bisulfite and weak acid. The weak
acid produce may
comprise a solid, which may be separated from the liquid aqueous calcium
bisulfite solution by
means of, for example, a solid-liquid separation process. The weak acid
produced may comprise
a gas, which may be separated from the liquid aqueous calcium bisulfite
solution by means of,
for example, removal of headspace gases, or depressurization, or vacuum, or
heat, or a gas-liquid
separation process, and / or may be further separated from residual sulfur
dioxide gas. The weak
acid produced may comprise a solid, which may be separated from the liquid
aqueous calcium
bisulfite solution by means of, for example, a solid-liquid separation
process. For example, the
present reaction may involve reacting a calcium silicate material with a
sulfurous acid solution to
form a solution comprising calcium bisulfite and a solid comprising a silicate
or derivative of
silicon. It may be desirable to separate said solid comprising a silicate or
derivative of silicon
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
from said solution comprising calcium bisulfite by means of a solid-liquid
separation process. It
may be desirable to react the calcium silicate material with sulfurous acid at
a molar ratio equal
to or greater than the sulfur to calcium molar ratio in calcium bisulfite.
Example Mass, Heat, and Power Flows (Figure 3)
Example Mass, Heat, and Power Flows for an Example Embodiment of Figure 3
ID Description
1 1 mole CaSiO3; or 691.4 kg of CaSiO3 per metric ton of
sodium bicarbonate produced
2 A mixing or reacting process. May employ thermal management, such as
cooling or
heating. May require some electricity or other power for, for example, pumping
or
mixing.
3 1 mole Ca(HS03)2(aq) and / or 1 mole Si02(s); or 1203.7 kg of
Ca(HS03)2(aq) per
metric ton of sodium bicarbonate produced and / or 357.6 kg of SiO2(s) per
metric ton
of sodium bicarbonate produced
Solvent: 100 moles of H20; or 10,714.3 kg of H20 per metric ton of sodium
bicarbonate produced
4 A solid-liquid separation process. May require some thermal management,
such as
cooling or heating. May require some electricity or other power for, for
example,
pumping or mixing.
5 1 mole Si02(s); or 357.6 kg of SiO2(s) per metric ton of sodium
bicarbonate produced
Note: May comprise other materials instead of or in addition to silicon
dioxide.
6 1 mole Ca(HS03)9(aq); or 1203.7 kg of Ca(HS03)9(aq) per metric ton of
sodium
bicarbonate produced
Solvent: 100 moles of H20; 10,714.3 kg of H20 per metric ton of sodium
bicarbonate
produced
7 1 mole Na2SO4(s); or 845.18 kg of Na2SO4(s) per metric ton of sodium
bicarbonate
produced
8 A mixing or reacting process. May employ thermal management, such as
cooling or
heating. May require some electricity or other power for, for example, pumping
or
mixing.
9 2 moles NaHS03(aq) and / or 1 mole CaSO4(s); or 1,238.02 kg of NaHS03(aq)
per
metric ton of sodium bicarbonate produced and / or 810.31 kg of CaSO4(s) per
metric
ton of sodium bicarbonate produced
Solvent: 100 moles of H20; 10,713.6 kg of H20 per metric ton of sodium
bicarbonate
produced
10 A solid-liquid separation process. May require some thermal management,
such as
cooling or heating. May require some electricity or other power for, for
example,
pumping or mixing.
11 1 mole CaSO4(s); or 810.31 kg of CaSO4(s) per metric ton of sodium
bicarbonate
produced
12 2 moles NaHS03(aq); or 1,238.02 kg of Na1-1S03(aq) per metric ton of
sodium
bicarbonate produced
Solvent: 100 moles of H20; 10,713.6 kg of H20 per metric ton of sodium
bicarbonate
produced
13 '13' may comprise a process for distillation, or a water removal, or a
drying, or a
separation, or a crystallization or a combination thereof. For the present
example, '13'
may employ mechanical vapor compression distillation employing electricity as
the
energy input to power the process.
Separation may require the removal of 100 moles of H20 (solvent) and 1 mole
H20
(part of sodium bisulfite dissolved, although practically part of the solvent
due to
properties of sodium bisulfite and sodium metabisulfite), which means 101
moles of
56
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Example Mass, Heat, and Power Flows for an Example Embodiment of Figure 3
ID Description
H20 needs to be removed or distilled; or about 10,820.7 kg of H20 per metric
ton of
sodium bicarbonate produced.
Estimated Mechanical Vapor Compression (MVC) Distillation for 'ZLD' Energy
Consumption: 15 kWh per m3 of water
To remove 101 moles of H20 using MVC: 0.0273 kWh
To remove 10,820.7 kg of H20 using MVC: 162.31 kWh
Note: Residual sulfur dioxide may be separated or may vaporize during
distillation. If
desired, residual sulfur dioxide may be condensed with the separated water.
Condensing the sulfur dioxide with the separated water or condensing water may
be
desirable as the water may be transferred to a sulfur dioxide absorption step.
14 101 moles of H20; or 10,820.7 kg of H20 per metric ton of
sodium bicarbonate
produced
15 May comprise sulfur dioxide. It is important to note
residual or excess sulfur dioxide
may be condensed with or within the water, which may comprise '14'.
16 1 mole Na2S205(s); or 1,130.88 kg of Na2S205(s) per metric
ton of sodium
bicarbonate produced
17 '17' may comprise a calcination, or a thermal
decomposition, or a desorption, or
decomposition, or a combination thereof process. For the present example, '17'
may
employ a calciner employing heat as an energy input. Heat may be sourced from
combustion or electricity or heat pump or steam or waste heat or thermal
storage or
solar thermal or other energy source, or a combination thereof.
Enthalpy of Decomposition of 2 Na2S205(s) to 2 Na2S03 and 2 S02(g) is: 87
kJ/mol
S02 produced at greater than or equal to about 150 C
To thermally decompose 2 moles of Na2S205(s) to 2 moles of 2 Na2S03 and 2
moles
of S02(g) is: 174 kJ heat
To thermally decompose 2,261.9 kg of Na2S205(s) to 1,500 kg of 2 Na2S03 and
761.9
kg of S02(g) is: 1.036 GJ heat
18 2 moles S02(g); or 761.9 kg of S02(g) per metric ton of
sodium bicarbonate produced
19 '19' may comprise an absorption process. '19' may comprise
a process for dissolving
sulfur dioxide in water. '19' may comprise a process for producing sulfurous
acid
from sulfur dioxide and a solution comprising water. May require some thermal
management, such as cooling or heating. May require some electricity or other
power
for, for example, pumping or mixing.
20 2 moles of S02(aq), 1 mole of H20: or 761.9 kg of S02 and
107.1 kg of H20 per
metric ton of sodium bicarbonate produced
Solvent: 100 moles of H20; or 10,713.6 kg of H20 per metric ton of sodium
bicarbonate produced
Note: Reactants may be dissolved in solvent. S02 is provided in the present
example
in a molar ratio to calcium based on the molar ratio in calcium bisulfite. In
some
embodiments, S02 may be in excess of or greater than the molar ratio of sulfur
to
calcium in calcium bisulfite. In some embodiments, S02 may be in less than the
molar
ratio of sulfur to calcium in calcium bisulfite.
21 2 moles Na2S03(s); or 1,500 kg ofNa2S03(s) per metric ton
of sodium bicarbonate
produced
22 '22' may comprise a mixing and / or dissolution process.
'22' may comprise a process
for dissolving sodium sulfite in water to form an aqueous sodium sulfite
solution.
23 2 moles Na2S03(aq); or 1,500 kg of Na2S03(aq) per metric
ton of sodium bicarbonate
produced
Solvent: 56 moles of H20; 5,999.6 kg of H20 per metric ton of sodium
bicarbonate
produced
57
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Example Mass, Heat, and Power Flows for an Example Embodiment of Figure 3
ID Description
24 2 moles CO2(g); or 523.77 kg of CO2(g) per metric ton of
sodium bicarbonate
produced
25 '25' may comprise a gas-liquid contactor. '25' may comprise
a gas-liquid contactor,
or an absorber, or a reactor, or a precipitator, or a combination thereof
process. May
require some thermal management, such as cooling or heating. May require some
electricity or other power for, for example, pumping or mixing.
26 2 moles NaHCO3(s and/or aq), 2 moles NaHS03(aq); or 1,000
kg of NaHCO3(s and/or
aq) and 1,239.2 kg of NaHS03(aq) per metric ton of sodium bicarbonate produced
Solvent: 54 moles of H20; or 5,785 kg of H20 per metric ton of sodium
bicarbonate
produced
27 '27' may comprise a solid-liquid separation process. May
require some thermal
management, such as cooling or heating. May require some electricity or other
power
for, for example, pumping or mixing.
28 0.935 mole NaHCO3(s); or 467.5 kg of NaHCO3(s) per metric
ton of sodium
bicarbonate produced in total
Note: Based on solubility per 100g water. Actual results may vary.
29 1.064 moles NaHCO3(aq), 2 moles NaHS03(aq); or 532.5 kg of
NaHCO3(s and/or aq)
and 1,239.2 kg of NaHS03(aq) per metric ton of sodium bicarbonate produced
Solvent: 54 moles of H20; or 5,785 kg of H20 per metric ton of sodium
bicarbonate
produced
30 '30' may comprise a process for distillation, or a water
removal, or a drying, or a
separation, or a crystallization, or cooling crystallization, or heating
concentrating, or
cooling concentration, or a combination thereof For the present example, '30'
may
employ mechanical vapor compression distillation employing electricity as the
energy
input to power the process. '30' may involve precipitating or crystalizing
remaining or
residual sodium bicarbonate or sodium carbonate or both before precipitating
or
crystalizing remaining or residual sodium bisulfite or sodium metabisulfite.
Separation may require the removal of 54 moles of H20 (solvent) and 1 mole H20
(part of sodium bisulfite dissolved, although practically part of the solvent
due to
properties of sodium bisulfite and sodium metabisulfite), which means 55 moles
of
H20 needs to be removed or distilled; or about kg of H20 per metric ton of
sodium
bicarbonate produced.
Estimated Mechanical Vapor Compression (MVC) Distillation for 'ZLD' Energy
Consumption: 15 kWh per in3 of water
To remove 55 moles of H20 using MVC: 0.01485 kWh
To remove 5,892.5 kg of H20 using MVC: 88.39 kWh
31 55 moles H20; or 5,892.5 kg of H20 per metric ton of sodium
bicarbonate produced
total.
Makeup water comprising, for example 1 mole of H20 per every 55 moles of
water,
may be added to, for example, makeup for water lost in the sodium bicarbonate
product. Some H20 in the sodium bicarbonate product may be recovered if sodium
bicarbonate is converted to sodium carbonate and / or water in subsequent
treatment
or processing.
32 1.064 moles NaHCO3(s); or 532.5 kg of NaHCO3(s) per metric
ton of sodium
bicarbonate produced total
33 1 mole Na2S205(s); or 1,130.88 kg of Na2S205(s) per metric
ton of sodium
bicarbonate produced total
58
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Example Heat Input Requirements (Figure 3)
Summary of Example Heat and Power Requirements Figure 3
1.036 G3 heat per metric ton of sodium
bicarbonate produced, heat may be at a
Heat
temperature greater than or equal to 150 C.
Heat may be supplied to, for example, 13.
250.7 kWh per metric ton of sodium
bicarbonate produced, assumes process for
Electricity dewatering or removing water comprises a
mechanical vapor compression distillation
process or similar electricity powered process.
Example CO2 Balance (Figure 3)
Example CO2 Net Balance from Example Heat, and Power Flows Figure 3
(Assumes Heat is from Natural Gas and Power is Electricity and Electricity is
USA Grid
Electricity with Average USA Electricity Carbon Intensity)
Heat (Natural Gas
0.05116 metric tons CO2
CO2 Emissions per Metric Combustion)
Ton of Sodium Electric Power (based on
Bicarbonate (Direct and USA average electric grid
0.11282 metric tons CO2
Indirect Emissions) carbon intensity
of 450kg CO2
per MWh)
CO2 Consumed in Produced
Net CO2 Consumption -0.52381 metric tons CO2
Sodium Bicarbonate Output
Net CO2 Balance (Negative Values are Good)
-0.35983 metric tons CO2
Example CO2 Net Balance from Example Heat, and Power Flows Figure 3
(Assumes Heat is from Natural Gas and Power is Electricity and Electricity is
CO2
Emissions Free)
Heat (Natural Gas
CO2 Emissions per Metric 0.05116 metric tons CO2
Combustion)
Ton of Sodium
Electric Power (based on
Bicarbonate (Direct and
Indirect Emissions) hydropower or
renewables or 0 metric tons CO2
nuclear sourced electricity)
CO2 Consumed in Produced
Net CO2 Consumption -0.52381 metric tons CO2
Sodium Bicarbonate Output
Net CO2 Balance (Negative Values are Good)
-0.47265 metric tons CO2
Example CO2 Net Balance from Example Heat, and Power Flows Figure 3
(Assumes Heat is from Electricity and Power is from Electricity and
Electricity is CO2
Emissions Free)
Heat (based on hydropower or
CO2 Emissions per Metric renewables or nuclear sourced 0 metric tons CO2
Ton of Sodium electricity)
Bicarbonate (Direct and Electric Power (based on
Indirect Emissions) hydropower or renewables or 0 metric tons CO2
nuclear sourced electricity)
CO2 Consumed in Produced
Net CO2 Consumption -0.52381 metric tons CO2
Sodium Bicarbonate Output
59
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Net CO2 Balance (Negative Values are Good)
-0.52381 metric tons CO2
Example Cost of Inputs, Value of Outputs, and Operating Profit (Figure 3)
Example Inputs and Cost of Inputs (Figure 3)
Cost per Metric Ton of
Cost per Standard Unit of
Input Measure
m Sodiu Bicarbonate
Produced
Calcium Silicate or
Free (if concrete waste)
Magnesium Silicate or Zinc
$10 ¨ 80 per metric ton if co-
Silicate or Iron Silicate or Free (if
concrete waste)
located to silicate ore mineral
other similar composition
resource
or a combination thereof
Sodium Sulfate $80 per metric ton
$67.61
Free (if co-located with CO2
source requiring offtake)
$10 ¨ 35 per metric ton on
commodity market ($20 for
Carbon Dioxide simplicity)
$10.48
(Note: May be paid to offtake
and convert CO2¨ not
included to ensure
conservative estimate)
Water $0.40 per metric ton
$0.04
Heat
$3.00 per MMBtu
$2.95
(Natural Gas Combustion)
Electricity $0.06 per kWh $15.04
Total Cost of Inputs per Metric Ton Sodium Bicarbonate
$96.12
Produced
Example Outputs and Value of Outputs (Figure 3)
Value per Metric Ton of
Value per Standard Unit of
Output Measure
Sodium Bicarbonate
Produced
Silicon Dioxide None
None
$120 per metric ton
Calcium Sulfate (high purity because it is
$97.24
precipitated gypsum)
Sodium Bicarbonate $200 per metric ton
$200
Total Value of Outputs per Metric Ton Sodium
$297.24
Bicarbonate Produced
Inputs Cost, Value of Outputs, and Net Operating Profit (Figure 3)
Inputs Cost $96.12
Value of Outputs $297.24
Net Profit per Metric Ton of Sodium
$201.12
Bicarbonate Produced
Example Mass, Heat, and Power Flows (Figure 4)
Example Mass, Heat, and Power Flows for an Example Embodiment of Figure 4
ID Description
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
1 1 mole CaCO3; or 595.7 kg of CaCO3 per metric ton of sodium
bicarbonate produced
2 A mixing or reacting process. May employ thermal
management, such as cooling or
heating. May require some electricity or other power for, for example, pumping
or
mixing.
3 1 mole Ca(HS03)2(aq) and / or 1 mole CO2(g); or 1203.7 kg
of Ca(HS03)2(aq) per
metric ton of sodium bicarbonate produced and / or 261.9 kg of CO2(g) per
metric ton
of sodium bicarbonate produced
Solvent: 100 moles of H20; or 10,714.3 kg of H20 per metric ton of sodium
bicarbonate produced
4 A solid-liquid separation process. May require some thermal
management, such as
cooling or heating. May require some electricity or other power for, for
example,
pumping or mixing.
Residual solids. Residual solids may include, but is not limited to,
impurities, silicon
dioxide, or unreacted reagents, or a combination thereof
6 1 mole Ca(HS03)2(aq); or 1203.7 kg of Ca(HS03)2(aq) per
metric ton of sodium
bicarbonate produced
Solvent: 100 moles of H20; 10,714.3 kg of H20 per metric ton of sodium
bicarbonate
produced
7 1 mole Na2SO4(s); or 845.18 kg of Na2SO4(s) per metric ton
of sodium bicarbonate
produced
8 A mixing or reacting process. May employ thermal
management, such as cooling or
heating. May require some electricity or other power for, for example, pumping
or
mixing.
9 2 moles NaHS03(aq) and / or 1 mole CaSO4(s); or 1,238.02 kg
of NaHS03(aq) per
metric ton of sodium bicarbonate produced and / or 810.31 kg of CaSO4(s) per
metric
ton of sodium bicarbonate produced
Solvent: 100 moles of H2O; 10,713.6 kg of H20 per metric ton of sodium
bicarbonate
produced
A solid-liquid separation process. May require some thermal management, such
as
cooling or heating. May require some electricity or other power for, for
example,
pumping or mixing.
11 1 mole CaSO4(s); or 810.31 kg of CaSO4(s) per metric ton of
sodium bicarbonate
produced
12 2 moles NaHS03(aq); or 1,238.02 kg of NaHS03(aq) per metric
ton of sodium
bicarbonate produced
Solvent: 100 moles of H20; 10,713.6 kg of H20 per metric ton of sodium
bicarbonate
produced
13 '13' may comprise a process for distillation, or a water
removal, or a drying, or a
separation, or a crystallization or a combination thereof For the present
example, '13'
may employ mechanical vapor compression distillation employing electricity as
the
energy input to power the process.
Separation may require the removal of 100 moles of H20 (solvent) and 1 mole
H20
(part of sodium bisulfite dissolved, although practically part of the solvent
due to
properties of sodium bisulfite and sodium metabisulfite), which means 101
moles of
H20 needs to be removed or distilled; or about 10,820.7 kg of H20 per metric
ton of
sodium bicarbonate produced.
Estimated Mechanical Vapor Compression (MVC) Distillation for `ZLD' Energy
Consumption: 15 kWh per m3 of water
To remove 101 moles of H20 using MVC: 0.0273 kWh
To remove 10,820.7 kg of H20 using MVC: 162.31 kWh
Note: Residual sulfur dioxide may be separated or may vaporize during
distillation. If
desired, residual sulfur dioxide may be condensed with the separated water.
61
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Condensing the sulfur dioxide with the separated water or condensing water may
be
desirable as the water may be transferred to a sulfur dioxide absorption step.
14 101 moles of H20; or 10,820.7 kg of H20 per metric ton of
sodium bicarbonate
produced
15 May comprise sulfur dioxide. It is important to note
residual or excess sulfur dioxide
may be condensed with or within the water, which may comprise '14'.
16 1 mole Na2S205(s); or 1,130.88 kg of Na2S205(s) per metric
ton of sodium
bicarbonate produced
17 '17' may comprise a calcination, or a thermal
decomposition, or a desorption, or
decomposition, or a combination thereof process. For the present example, '17'
may
employ a calciner employing heat as an energy input. Heat may be sourced from
combustion or electricity or heat pump or steam or waste heat or thermal
storage or
solar thermal or other energy source, or a combination thereof.
Enthalpy of Decomposition of 2 Na2S205(s) to 2 Na2S03 and 2 S02(g) is: 87
kJ/mol
S02 produced at greater than or equal to about 150 C
To thermally decompose 2 moles of Na2S205(s) to 2 moles of 2 Na2S03 and 2
moles
of S02(g) is: 174 kJ heat
To thermally decompose 2,261.9 kg of Na2S205(s) to 1,500 kg of 2 Na2S03 and
761.9
kg of S02(g) is: 1.036 GJ heat
18 2 moles S02(g); or 761.9 kg of S02(g) per metric ton of
sodium bicarbonate produced
19 '19' may comprise an absorption process. '19' may comprise
a process for dissolving
sulfur dioxide in water. '19' may comprise a process for producing sulfurous
acid
from sulfur dioxide and a solution comprising water. May require some thermal
management, such as cooling or heating. May require some electricity or other
power
for, for example, pumping or mixing.
20 2 moles of S02(aq), 1 mole of H20; or 761.9 kg of SO2 and
107.1 kg of H20 per
metric ton of sodium bicarbonate produced
Solvent: 100 moles of H20; or 10,713.6 kg of H20 per metric ton of sodium
bicarbonate produced
Note: Reactants may be dissolved in solvent. SO2 is provided in the present
example
in a molar ratio to calcium based on the molar ratio in calcium bisulfite. In
some
embodiments, SO2 may be in excess of or greater than the molar ratio of sulfur
to
calcium in calcium bisulfite. In some embodiments, SO2 may be in less than the
molar
ratio of sulfur to calcium in calcium bisulfite.
21 2 moles Na2S03(s); or 1,500 kg of Na2S03(s) per metric ton
of sodium bicarbonate
produced
22 '22' may comprise a mixing and / or dissolution process.
'22' may comprise a process
for dissolving sodium sulfite in water to form an aqueous sodium sulfite
solution.
23 2 moles Na2S03(aq); or 1,500 kg of Na2S03(aq) per metric
ton of sodium bicarbonate
produced
Solvent: 56 moles of H20; 5,999.6 kg of H20 per metric ton of sodium
bicarbonate
produced
24 1 mole CO2(g); or 261.9 kg of CO2(g) per metric ton of
sodium bicarbonate produced
25 '25' may comprise a gas-liquid contactor. '25' may comprise
a gas-liquid contactor,
or an absorber, or a reactor, or a precipitator, or a combination thereof
process. May
require some thermal management, such as cooling or heating. May require some
electricity or other power for, for example, pumping or mixing.
26 2 moles NaHCO3(s and/or aq), 2 moles NaHS03(aq); or 1,000
kg of NaHCO3(s and/or
aq) and 1,239.2 kg of NaHS03(aq) per metric ton of sodium bicarbonate produced
Solvent: 54 moles of H20; or 5,785 kg of H20 per metric ton of sodium
bicarbonate
produced
62
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
27 '27' may comprise a solid-liquid separation process. May
require some thermal
management, such as cooling or heating. May require some electricity or other
power
for, for example, pumping or mixing.
28 0.935 mole NaHCO3(s); or 467.5 kg of NaHCO3(s) per metric
ton of sodium
bicarbonate produced in total
Note: Based on solubility per 100g water. Actual results may vary.
29 1.064 moles NaHCO3(aq), 2 moles NaHS03(aq); or 532.5 kg of
NaHCO3(s and/or aq)
and 1,239.2 kg of NaHS03(aq) per metric ton of sodium bicarbonate produced
Solvent: 54 moles of H20; or 5,785 kg of H20 per metric ton of sodium
bicarbonate
produced
30 '30' may comprise a process for distillation, or a water
removal, or a drying, or a
separation, or a crystallization, or cooling crystallization, or heating
concentrating, or
cooling concentration, or a combination thereof For the present example, '30'
may
employ mechanical vapor compression distillation employing electricity as the
energy
input to power the process. '30' may involve precipitating or crystalizing
remaining or
residual sodium bicarbonate or sodium carbonate or both before precipitating
or
crystalizing remaining or residual sodium bisulfite or sodium metabisulfite.
Separation may require the removal of 54 moles of H20 (solvent) and 1 mole H20
(part of sodium bisulfite dissolved, although practically part of the solvent
due to
properties of sodium bisulfite and sodium metabisulfite), which means 55 moles
of
H20 needs to be removed or distilled: or about kg of H20 per metric ton of
sodium
bicarbonate produced.
Estimated Mechanical Vapor Compression (MVC) Distillation for 'ZLD' Energy
Consumption: 15 kWh per M3 of water
To remove 55 moles of H20 using MVC: 0.01485 kWh
To remove 5,892.5 kg of H20 using MVC: 88.39 kWh
31 55 moles H20; or 5,892.5 kg of H20 per metric ton of sodium
bicarbonate produced
total.
Makeup water comprising, for example 1 mole of H20 per every 55 moles of
water,
may be added to, for example, makeup for water lost in the sodium bicarbonate
product. Some H20 in the sodium bicarbonate product may be recovered if sodium
bicarbonate is converted to sodium carbonate and / or water in subsequent
treatment
or processing.
32 1.064 moles NaHCO3(s); or 532.5 kg of NaHCO3(s) per metric
ton of sodium
bicarbonate produced total
33 1 mole Na2S205(s); or 1,130.88 kg of Na2S205(s) per metric
ton of sodium
bicarbonate produced total
34 1 mole CO2(g); or 261.9 kg of CO2(g) per metric ton of
sodium bicarbonate produced
63
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Example Heat Input Requirements (Figure 4)
Summary of Example Heat and Power Requirements Figure 4
1.036 G3 heat per metric ton of sodium
bicarbonate produced, heat may be at a
Heat
temperature greater than or equal to 150 C.
Heat may be supplied to, for example, 13.
250.7 kWh per metric ton of sodium
bicarbonate produced, assumes process for
Electricity dewatering or removing water
comprises a
mechanical vapor compression distillation
process or similar electricity powered process.
Example CO2 Balance (Figure 4)
Example CO2 Net Balance from Example Heat, and Power Flows Figure 4
(Assumes Heat is from Natural Gas and Power is Electricity and Electricity is
USA Grid
Electricity with Average USA Electricity Carbon Intensity)
Heat (Natural Gas
0.05116 metric tons CO2
Combustion)
Electric Power (based on
USA average electric grid
0.11282 metric tons CO2
CO2 Emissions per Metric carbon intensity of 450kg CO2
Ton of Sodium per MWh)
Bicarbonate (Direct and High Purity CO2 produced
Indirect Emissions) by Sulfurous Acid +
Carbonate Reaction
0.2619 metric tons CO2
(transferred to sodium
bicarbonate production step in
Figure 4)
CO2 Consumed in Produced
Net CO2 Consumption -0.52381 metric tons CO2
Sodium Bicarbonate Output
Net CO2 Balance (Negative Values are Good)
-0.09793 metric tons CO2
Example CO2 Net Balance from Example Heat, and Power Flows Figure 4
(Assumes Heat is from Natural Gas and Power is Electricity and Electricity is
CO2
Emissions Free)
Heat (Natural Gas
0.05116 metric tons CO2
Combustion)
Electric Power (based on
hydropower or renewables or 0 metric
tons CO2
CO2 Emissions per Metric
nuclear sourced electricity)
Ton of Sodium
High Purity CO2 produced
Bicarbonate (Direct and
b
Indirect Emissions) y Sulfurous Acid +
Carbonate Reaction
0.2619 metric tons CO2
(transferred to sodium
bicarbonate production step in
Figure 4)
CO2 Consumed in Produced
Net CO2 Consumption -0.52381 metric tons CO2
Sodium Bicarbonate Output
Net CO2 Balance (Negative Values are Good)
-0.21075 metric tons CO2
64
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Example CO2 Net Balance from Example Heat, and Power Flows Figure 4
(Assumes Heat is from Electricity and Power is from Electricity and
Electricity is CO2
Emissions Free)
Heat (based on hydropower or
renewables or nuclear sourced 0 metric
tons CO2
electricity)
Electric Power (based on
CO2 Emissions per Metric hydropower or renewables or 0 metric
tons CO2
Ton of Sodium nuclear sourced electricity)
Bicarbonate (Direct and High Purity CO2 produced
Indirect Emissions) by Sulfurous Acid +
Carbonate Reaction
0.2619 metric tons CO2
(transferred to sodium
bicarbonate production step in
Figure 4)
CO2 Consumed in Produced
Net CO2 Consumption -0.52381 metric tons CO2
Sodium Bicarbonate Output
Net CO2 Balance (Negative Values are Good)
-0_26191 metric tons CO2
Example Cost of Inputs, Value of Outputs, and Operating Profit (Figure 4)
Example Inputs and Cost of Inputs (Figure 4)
Cost per Metric Ton of
Cost per Standard Unit of
Input Sodium
Bicarbonate
Measure
Produced
Calcium Carbonate or
Magnesium Carbonate or Crushed limestone:
$20.25
Limestone or a $30-$38 per metric ton
Combination Thereof
Sodium Sulfate $80 per metric ton
$67.61
Free (if co-located with CO2
source requiring offtake)
$10 ¨ 35 per metric ton on
commodity market ($20 for
Carbon Dioxide simplicity)
$5.24
(Note: May be paid to offtake
and convert CO2¨ not
included to ensure
conservative estimate)
Water $0.40 per metric ton
$0.04
Heat
$3.00 per MMBtu
$2.95
(Natural Gas Combustion)
Electricity $0.06 per kWh
$15.04
Total Cost of Inputs per Metric Ton Sodium Bicarbonate
$111.13
Produced
Example Outputs and Value of Outputs (Figure 4)
Value per Metric Ton of
Value per Standard Unit of
Output Sodium
Bicarbonate
Measure
Produced
Silicon Dioxide None
None
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
$120 per metric ton
Calcium Sulfate (high purity because it is $97.24
precipitated gypsum)
Sodium Bicarbonate $200 per metric ton $200
Total Value of Outputs per Metric Ton Sodium
$297.24
Bicarbonate Produced
Inputs Cost, Value of Outputs, and Net Operating Profit (Figure 4)
Inputs Cost $111.13
Value of Outputs $297.24
Net Profit per Metric Ton of Sodium
$186.11
Bicarbonate Produced
Example Mass, Heat, and Power Flows (Figure 5)
Example Mass, Heat, and Power Flows for an Example Embodiment of Figure 5
ID Description
1 1 mole CaCO3; or 595.7 kg of CaCO3 per metric ton of sodium
bicarbonate produced
2 A mixing or reacting process. May employ thermal management, such as
cooling or
heating. May require some electricity or other power for, for example, pumping
or
mixing.
3 1 mole Ca(HS03)2(aq) and / or 1 mole Si02(s); or 1203.7 kg of
Ca(HS03)2(aq) per
metric ton of sodium bicarbonate produced and / or 357.6 kg of SiO2(s) per
metric ton
of sodium bicarbonate produced
Solvent: 100 moles of H20; or 10,714.3 kg of H20 per metric ton of sodium
bicarbonate produced
4 A solid-liquid separation process. May require some thermal management,
such as
cooling or heating. May require some electricity or other power for, for
example,
pumping or mixing.
5 Residual solids. Residual solids may include, but is not limited to,
impurities, silicon
dioxide, or unreacted reagents, or a combination thereof
6 1 mole Ca(HS03)2(aq); or 1203.7 kg of Ca(HS03)2(aq) per metric ton of
sodium
bicarbonate produced
Solvent: 100 moles of H20; 10,714.3 kg of H20 per metric ton of sodium
bicarbonate
produced
7 1 mole Na2SO4(s); or 845.18 kg of Na2SO4(s) per metric ton of sodium
bicarbonate
produced
8 A mixing or reacting process. May employ thermal management, such as
cooling or
heating. May require some electricity or other power for, for example, pumping
or
mixing.
9 2 moles NaHS03(aq) and / or 1 mole CaSO4(s); or 1,238.02 kg of NaHS03(aq)
per
metric ton of sodium bicarbonate produced and / or 810.31 kg of CaSO4(s) per
metric
ton of sodium bicarbonate produced
Solvent: 100 moles of H20; 10,713.6 kg of H20 per metric ton of sodium
bicarbonate
produced
10 A solid-liquid separation process. May require some thermal management,
such as
cooling or heating. May require some electricity or other power for, for
example,
pumping or mixing.
11 1 mole CaSO4(s); or 810.31 kg of CaSO4(s) per metric ton of sodium
bicarbonate
produced
12 2 moles NaHS03(aq); or 1,238.02 kg of NaHS03(aq) per metric ton of
sodium
bicarbonate produced
66
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Example Mass, Heat, and Power Flows for an Example Embodiment of Figure 5
ID Description
Solvent: 100 moles of H20; 10,713.6 kg of H20 per metric ton of sodium
bicarbonate
produced
13 '13' may comprise a process for distillation, or a water
removal, or a drying, or a
separation, or a crystallization or a combination thereof For the present
example, `13'
may employ mechanical vapor compression distillation employing electricity as
the
energy input to power the process.
Separation may require the removal of 100 moles of H20 (solvent) and 1 mole
H20
(part of sodium bisulfite dissolved, although practically part of the solvent
due to
properties of sodium bisulfite and sodium metabisulfite), which means 101
moles of
H20 needs to be removed or distilled; or about 10,820.7 kg of H20 per metric
ton of
sodium bicarbonate produced.
Estimated Mechanical Vapor Compression (MVC) Distillation for `ZLD' Energy
Consumption: 15 kWh per rn3 of water
To remove 101 moles of H20 using MVC: 0.0273 kWh
To remove 10,820.7 kg of H20 using MVC: 162.31 kWh
Note: Residual sulfur dioxide may be separated or may vaporize during
distillation. If
desired, residual sulfur dioxide may be condensed with the separated water.
Condensing the sulfur dioxide with the separated water or condensing water may
be
desirable as the water may be transferred to a sulfur dioxide absorption step.
14 101 moles of H20; or 10,820.7 kg of H20 per metric ton of
sodium bicarbonate
produced
15 May comprise sulfur dioxide. It is important to note
residual or excess sulfur dioxide
may be condensed with or within the water, which may comprise '14'.
16 1 mole Na2S205(s); or 1,130.88 kg of Na2S205(s) per metric
ton of sodium
bicarbonate produced
17 '17' may comprise a calcination, or a thermal
decomposition, or a desorption, or
decomposition, or a combination thereof process. For the present example, `17'
may
employ a calciner employing heat as an energy input. Heat may be sourced from
combustion or electricity or heat pump or steam or waste heat or thermal
storage or
solar thermal or other energy source, or a combination thereof.
Enthalpy of Decomposition of 2 Na2S205(s) to 2 Na2S03 and 2 S02(g) is: 87
kJ/mol
S02 produced at greater than or equal to about 150 C
To thermally decompose 2 moles of Na2S205(s) to 2 moles of 2 Na2S03 and 2
moles
of S02(g) is: 174 kJ heat
To thermally decompose 2,261.9 kg of Na2S205(s) to 1,500 kg of 2 Na2S03 and
761.9
kg of S02(g) is: 1.036 GJ heat
18 2 moles S02(g); or 761.9 kg of S02(g) per metric ton of
sodium bicarbonate produced
19 '19' may comprise an absorption process. `19' may comprise
a process for dissolving
sulfur dioxide in water. `19' may comprise a process for producing sulfurous
acid
from sulfur dioxide and a solution comprising water. May require some thermal
management, such as cooling or heating. May require some electricity or other
power
for, for example, pumping or mixing.
20 2 moles of S02(aq), 1 mole of H20; or 761.9 kg of S02 and
107.1 kg of H20 per
metric ton of sodium bicarbonate produced
Solvent: 100 moles of H20; or 10,713.6 kg of H20 per metric ton of sodium
bicarbonate produced
Note: Reactants may be dissolved in solvent. S02 is provided in the present
example
in a molar ratio to calcium based on the molar ratio in calcium bisulfite. In
some
embodiments, S02 may be in excess of or greater than the molar ratio of sulfur
to
67
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Example Mass, Heat, and Power Flows for an Example Embodiment of Figure 5
ID Description
calcium in calcium bisulfite. In some embodiments, S02 may be in less than the
molar
ratio of sulfur to calcium in calcium bisulfite.
21 2 moles Na2S03(s); or 1,500 kg of Na2S03(s) per metric ton
of sodium bicarbonate
produced
22 '22' may comprise a mixing and / or dissolution process.
'22' may comprise a process
for dissolving sodium sulfite in water to form an aqueous sodium sulfite
solution.
23 2 moles Na2S03(aq); or 1,500 kg of Na2S03(aq) per metric
ton of sodium bicarbonate
produced
Solvent: 56 moles of H20; 5,999.6 kg of H20 per metric ton of sodium
bicarbonate
produced
24 2 moles CO2(g); or 523.77 kg of CO2(g) per metric ton of
sodium bicarbonate
produced
25 '25' may comprise a gas-liquid contactor. '25' may comprise
a gas-liquid contactor,
or an absorber, or a reactor, or a precipitator, or a combination thereof
process. May
require some thermal management, such as cooling or heating. May require some
electricity or other power for, for example, pumping or mixing.
26 2 moles NaHCO3(s and/or aq), 2 moles NaHS03(aq); or 1,000
kg of NaHCO3(s and/or
aq) and 1,239.2 kg of NaHS03(aq) per metric ton of sodium bicarbonate produced
Solvent: 54 moles of H20; or 5,785 kg of H20 per metric ton of sodium
bicarbonate
produced
27 '27' may comprise a solid-liquid separation process. May
require some thermal
management, such as cooling or heating. May require some electricity or other
power
for, for example, pumping or mixing.
28 0.935 mole NaHCO3(s); or 467.5 kg of NaHCO3(s) per metric
ton of sodium
bicarbonate produced in total
Note: Based on solubility per 100g water. Actual results may vary.
29 1.064 moles NaHCO3(aq), 2 moles NaHS03(aq); or 532.5 kg of
NaHCO3(s and/or aq)
and 1,239.2 kg of NaHS03(aq) per metric ton of sodium bicarbonate produced
Solvent: 54 moles of H20; or 5,785 kg of H20 per metric ton of sodium
bicarbonate
produced
30 '30' may comprise a process for distillation, or a water
removal, or a drying, or a
separation, or a crystallization, or cooling crystallization, or heating
concentrating, or
cooling concentration, or a combination thereof For the present example, '30'
may
employ mechanical vapor compression distillation employing electricity as the
energy
input to power the process. '30' may involve precipitating or crystalizing
remaining or
residual sodium bicarbonate or sodium carbonate or both before precipitating
or
crystalizing remaining or residual sodium bisulfite or sodium metabisulfite.
Separation may require the removal of 54 moles of H20 (solvent) and 1 mole H20
(part of sodium bisulfite dissolved, although practically part of the solvent
due to
properties of sodium bisulfite and sodium metabisulfite), which means 55 moles
of
H20 needs to be removed or distilled; or about 5892.5 kg of H20 per metric ton
of
sodium bicarbonate produced.
Estimated Mechanical Vapor Compression (MVC) Distillation for 'ZLD' Energy
Consumption: 15 kWh per m3 of water
To remove 55 moles of H20 using MVC: 0.01485 kWh
To remove 5,892.5 kg of H20 using MVC: 88.39 kWh
31 55 moles H20; or 5,892.5 kg of H20 per metric ton of sodium
bicarbonate produced
total.
68
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Example Mass, Heat, and Power Flows for an Example Embodiment of Figure 5
ID Description
Makeup water comprising, for example 1 mole of H20 per every 55 moles of
water,
may be added to, for example, makeup for water lost in the sodium bicarbonate
product. Some H20 in the sodium bicarbonate product may be recovered if sodium
bicarbonate is converted to sodium carbonate and / or water in subsequent
treatment
or processing.
32 1.064 moles NaHCO3(s); or 532.5 kg of NaHCO3(s) per metric
ton of sodium
bicarbonate produced total
33 1 mole Na2S205(s); or L130.88 kg of Na2S205(s) per metric
ton of sodium
bicarbonate produced total
35 1 mole CO2(g); or 261.9 kg of CO2(g) per metric ton of
sodium bicarbonate produced
Example Heat Input Requirements (Figure 5)
Summary of Example Heat and Power Requirements Figure 5
1.036 GJ heat per metric ton of sodium
H bicarbonate produced, heat
may be at a
eat
temperature greater than or equal to 150 C.
Heat may be supplied to, for example, 13.
250.7 kWh per metric ton of sodium
bicarbonate produced, assumes process for
Electricity dewatering or removing water
comprises a
mechanical vapor compression distillation
process or similar electricity powered process.
Example CO2 Balance (Figure 5)
Example CO2 Net Balance from Example Heat, and Power Flows Figure 5
(Assumes Heat is from Natural Gas and Power is Electricity and Electricity is
USA Grid
Electricity with Average USA Electricity Carbon Intensity)
Heat (Natural Gas
0.05116 metric tons CO2
Combustion)
Electric Power (based on
USA average electric grid
0.11282 metric tons CO2
CO2 Emissions per Metric carbon intensity of 450kg CO2
Ton of Sodium per MWh)
Bicarbonate (Direct and High Purity CO2 produced
Indirect Emissions) by Sulfurous Acid +
Carbonate Reaction
0
(transferred to CO2 utilization,
or conversion, or sequestration
in Figure 5)
CO2 Consumed in Produced
Net CO2 Consumption -0.52381 metric tons CO2
Sodium Bicarbonate Output
Net CO2 Balance (Negative Values are Good)
-0.35983 metric tons CO2
Example CO2 Net Balance from Example Heat, and Power Flows Figure 5
(Assumes Heat is from Natural Gas and Power is Electricity and Electricity is
CO2
Emissions Free)
69
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Heat (Natural Gas
0.05116 metric tons CO2
Combustion)
Electric Power (based on
hydropower or renewables or 0 metric tons CO2
CO2 Emissions per Metric
nuclear sourced electricity)
Ton of Sodium
Bicarbonate (Direct and High Purity CO2
produced
b
Indirect Emissions) y Sulfurous Acid +
Carbonate Reaction
0
(transferred to CO2 utilization,
or conversion, or sequestration
in Figure 5)
CO2 Consumed in Produced
Net CO2 Consumption
-0.52381 metric tons C07
Sodium Bicarbonate Output
Net CO2 Balance (Negative Values are Good)
-0.47265 metric tons CO2
Example CO2 Net Balance from Example Heat, and Power Flows Figure 5
(Assumes Heat is from Electricity and Power is from Electricity and
Electricity is CO2
Emissions Free)
Heat (based on hydropower or
renewables or nuclear sourced 0 metric tons CO2
electricity)
Electric Power (based on
CO2 Emissions per Metric hydropower or renewables or 0 metric tons CO2
Ton of Sodium nuclear sourced electricity)
Bicarbonate (Direct and High Purity CO2
produced
Indirect Emissions) by Sulfurous Acid +
Carbonate Reaction
0
(transferred to CO2 utilization,
or conversion, or sequestration
in Figure 5)
CO2 Consumed in Produced
Net CO2 Consumption
-0.52381 metric tons CO2
Sodium Bicarbonate Output
Net CO2 Balance (Negative Values are Good)
-0.52381 metric tons CO2
Example Cost of Inputs, Value of Outputs, and Operating Profit (Figure 5)
Example Inputs and Cost of Inputs (Figure 5)
Cost per Metric Ton of
Cost per Standard Unit of
Input Measure Sodium
Bicarbonate
Produced
Calcium Carbonate or
Magnesium Carbonate or Crushed limestone:
$20.25
Limestone or a $30-$38 per metric ton
Combination Thereof
Sodium Sulfate $80 per metric ton
$67.61
Free (CO2 Emissions or CO2
Carbon Dioxide $0.00
Emissions Gas Mixture)
Water $0,40 per metric ton $0,04
Heat
$3.00 per MMBtu $2.95
(Natural Gas Combustion)
Electricity $0.06 per kWh
$15.04
CA 03218484 2023- 11- 8
WO 2022/241219 PCT/US2022/029198
Example Inputs and Cost of Inputs (Figure 5)
Cost per Metric Ton of
Cost per Standard Unit of
Input Sodium
Bicarbonate
Measure
Produced
Total Cost of Inputs per Metric Ton Sodium Bicarbonate
$105.89
Produced
Example Outputs and Value of Outputs (Figure 5)
Value per Metric Ton of
Value per Standard Unit of
Output Sodium
Bicarbonate
Measure
Produced
S10 ¨ 35 per ton ($20 for
High Purity CO2 $5.24
simpl i city)
$120 per metric ton
Calcium Sulfate (high purity because it is $97.24
precipitated gypsum)
Sodium Bicarbonate $200 per metric ton $200
Total Value of Outputs per Metric Ton Sodium
$302.48
Bicarbonate Produced
Inputs Cost, Value of Outputs, and Net Operating Profit (Figure 5)
Inputs Cost $111.13
Value of Outputs $302.48
Net Profit per Metric Ton of Sodium
$191.35
Bicarbonate Produced
Example Figure Keys
Figure 3 Key
ID Description
1 An input material comprising a salt of silicate, or
carbonate, or bicarbonate, or a salt
of a weaker acid than sulfurous acid, or a salt an acid with a higher pKa than
sulfurous
acid, or a combination thereof An input material comprising calcium silicate,
or
magnesium silicate, or calcium carbonate, or magnesium carbonate, or a calcium
salt
comprising silicon, or a magnesium salt comprising silicon, or a calcium ¨
weak acid
anion salt, or a magnesium ¨ weak acid anion salt, or an alkaline earth ¨ weak
acid
anion salt, or a mineral thereof, or a derivative thereof, or a combination
thereof
2 '2' may comprise a process for mixing or reacting or both
an input material (such as,
for example, '1') with sulfurous acid or a solution comprising dissolved
sulfur
dioxide. '2' may involve mixing sulfurous acid with a calcium or magnesium ¨
weak
acid salt. '2' may involve mixing sulfurous acid with a calcium or magnesium ¨
weak
acid salt to form calcium or magnesium sulfite or bisulfite. In the present
embodiment,
it may be desirable for the molar ratio of sulfur in the sulfurous acid
reactant to the
calcium and / or magnesium in the input material reactant to be about the same
or
greater than the molar ratio of sulfur to calcium or magnesium in dissolved
calcium
bisulfite or magnesium bisulfite. Sulfurous acid reactant in excess of the
molar ratio
than the molar ratio of sulfur to calcium or magnesium in dissolved calcium
bisulfite
or magnesium bisulfite may comprise 'excess' sulfurous acid. In some
embodiments,
'excess' sulfurous acid may be desirable in '2' to, for example, improve
reaction
kinetics or otherwise facilitate the reaction to form calcium bisulfite and /
or
magnesium bisulfite. '2' may form dissolved calcium bisulfite and / or
magnesium
bisulfite and a weak acid product. Said weak acid product may comprise a
solid, or a
71
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Figure 3 Key
ID Description
liquid, or a gas, or a combination thereof, which may be separated from the
calcium
bisulfite and / or magnesium bisulfite within '2' or in a separate step. For
example,
said weak acid product may comprise a solid comprising silicon dioxide or a
silicon
derivative.
3 '3' may comprise the products from '2'. '3' may involve transferring the
products
from '2' to a separation step. For example, in some embodiments, the products
from
'2' may comprise a solid-liquid slurry comprising an aqueous liquid phase
solution of
calcium bisulfite and / or magnesium bisulfite and a solid phase comprising
silicon
dioxide or a silicon derivative or a combination thereof In some embodiments,
the
products from '2' may comprise at least a portion residual sulfurous acid or
residual
excess sulfurous acid, which may, if desired, remain at a liquid phase with
the liquid
solution comprising calcium bisulfite and / or magnesium bisulfite in '3'.
4 '4' may comprise a phase separation process. For example, '4' may
comprise a
process designed to separate at least a portion of the aqueous liquid phase
solution
comprising calcium bisulfite and / or magnesium bisulfite from at least a
portion of
the weak acid product in '3'. For example, '4' may comprise a solid-liquid
separation
process. For example, '4' may comprise a process designed to separate at least
a
portion of the aqueous liquid phase solution comprising calcium bisulfite and
/ or
magnesium bisulfite from at least a portion of a solid weak acid product
comprising
silicon dioxide or a derivative of silicon.
5 '5' may comprise separated weak acid product. '5' may comprise separated
solid
phase weak acid product comprising silicon dioxide or a derivative of silicon.
'5' may
comprise an output. '5' may undergo further separation, treatment, or use, or
a
combination thereof
6 '6' may comprise separated aqueous liquid phase solution comprising
calcium
bisulfite and / or magnesium bisulfite. '6' may comprise separated aqueous
liquid
phase solution comprising calcium bisulfite and / or magnesium bisulfite
transferred
from a solid-liquid separation process to a reaction with sodium sulfate.
7 '7' may comprise an input material comprising sodium sulfate. '7' may be
at a solid
phase, a liquid phase, or both.
8 '8' may comprise a process for mixing or reacting or both an input
material, such as
'7', with a separated aqueous liquid phase solution comprising calcium
bisulfite and /
or magnesium bisulfite, such as '6'. Aqueous solution comprising calcium
bisulfite or
magnesium bisulfite may react with sodium sulfate to form an aqueous solution
comprising sodium bisulfite and a solid phase comprising calcium sulfate or
magnesium sulfate. Residual dissolved calcium sulfate or magnesium sulfate may
remain present in the aqueous solution comprising sodium bisulfite, although
it is
important to note the appreciably lower solubility of calcium sulfate or
magnesium
sulfate in water than sodium bisulfite.
9 '9' may comprise the products from '8'. '9' may involve transferring the
products
from '8' to a separation step. For example, in some embodiments, the products
from
'8' may comprise a solid-liquid slurry comprising an aqueous liquid phase
solution of
sodium bisulfite and a solid phase comprising calcium sulfate or magnesium
sulfate.
In some embodiments, the products from '8' may comprise at least a portion
residual
sulfurous acid or residual excess sulfurous acid, which may, if desired,
remain at a
liquid phase with the liquid solution comprising sodium bisulfite in '9'.
10 '10' may comprise a phase separation process. For example, '10' may
comprise a
process designed to separate at least a portion of the aqueous liquid phase
solution
comprising sodium bisulfite from at least a portion of the solid calcium
sulfate or
magnesium sulfate in '9'. For example, '10' may comprise a solid-liquid
separation
72
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Figure 3 Key
ID Description
process. For example, '10' may comprise a process designed to separate at
least a
portion of the aqueous liquid phase solution comprising sodium sulfite from at
least a
portion of a solid comprising calcium sulfate or magnesium sulfate.
11 ' 1 1 ' may comprise a separated solid phase. '11' may
comprise separated calcium
sulfate, or magnesium sulfate, or both. '11 may comprise an output. It is
important to
note the separated calcium sulfate, or magnesium sulfate, or both may be of
sufficiently high purity for commercial uses of gypsum. For some applications,
'11'
may be in an appropriate form of use or sale. For some applications, '11' may
require
additional treatment, or dehydration, or drying, or refining, or pulverizing,
or a
combination thereof
12 '12' may comprise separated aqueous liquid phase solution
comprising sodium
bisulfite. '12' may comprise separated aqueous liquid phase solution
comprising
sodium bisulfite transferred from a solid-liquid separation process to a
distillation, or a
water removal, or a drying, or a separation, or a crystallization or a
combination
thereof step.
13 '13' may comprise a process for distillation, or a water
removal, or a drying, or a
separation, or a crystallization or a combination thereof '13' may comprise a
process
employed to separate a salt solution into at least a portion water and at
least a portion
solid salt. '13' may comprise one or more or a combination of separation
processes
described herein. '13' may comprise, for example, MVC, or MED, or MSF, or
membrane-based process, or a combination thereof '13' may comprise a process
for
separating an aqueous solution comprising sodium bisulfite, such as '12', into
at least
a portion of water and at least a portion of solid sodium metabisulfite. '13'
may
comprise a process for separating an aqueous solution comprising sodium
bisulfite,
such as '12', into at least a portion of water, at least a portion of solid
sodium
metabisulfite, and at least a portion of residual sulfur dioxide. Said at
least a portion of
residual sulfur dioxide may comprise dissolved sulfur dioxide or sulfurous
acid in the
at least a portion of water, or may comprise gas phase sulfur dioxide, or may
comprise
liquid phase sulfur dioxide, or may comprise a combination thereof
14 '14. may comprise water. '14' may comprise water and
residual dissolved sulfur
dioxide. '14' may comprise water transferred from a water removal or water
separation process to an absorption process.
15 '15' may comprise at least a portion of gaseous sulfur
dioxide. In embodiments
employing excess sulfur dioxide and / or embodiments employing thermal or gas-
liquid phase transition separation for water removal, gaseous sulfur dioxide
may be
produced during a water removal step, such as '14'.
16 '16' may comprise a separated solid. '16' may comprise
solid sodium metabisulfite
separated from water. '16' may comprise solid sodium metabisulfite separated
from a
solution comprising sodium bisulfite. '16' may comprise a solid comprising
sodium
metabisulfite, or sodium sulfite, or a combination thereof. '16' may comprise
a solid
transferred from a separation process or water removal process to a
calcination or a
thermal decomposition or a thermal desorption process.
17 '17' may comprise a calcination, or a thermal
decomposition, or a desorption, or
decomposition, or a combination thereof process. '17' may involve thermally
decomposing or calcining sodium metabisulfite into solid sodium sulfite and
gaseous
sulfur dioxide. '17' may employ one or more processes described herein, or
known in
the art, or a combination thereof for calcination, or a thermal decomposition,
or a
desorption, or decomposition, or a combination thereof
18 '18' may comprise gaseous sulfur dioxide produced from a
process for calcination, or
a thermal decomposition, or a desorption, or decomposition, or a combination
thereof
73
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Figure 3 Key
ID Description
'18' may comprise gaseous sulfur dioxide transferred to a sulfur dioxide
absorption
process or a process for producing sulfurous acid.
19 '19' may comprise an absorption process. '19' may comprise
a process for dissolving
sulfur dioxide in water. '19' may comprise a process for producing sulfurous
acid
from sulfur dioxide and a solution comprising water. '19' may comprise a
process for
producing concentrated or 'rich' sulfurous acid from sulfur dioxide and a
solution
comprising water.
20 '20' may comprise a solution comprising sulfur dioxide.
'20' may comprise an
aqueous sulfurous acid solution, or a concentrated sulfurous acid solution, or
a
combination thereof '20' may comprise an aqueous sulfurous acid solution
transferred from a sulfur dioxide absorption step to a sulfurous acid reaction
step.
21 '21' may comprise a solid comprising at least a portion of
sodium sulfite. '21' may
comprise sodium sulfite transferred from a calcination step to a dissolution
step.
22 '22' may comprise a mixing and / or dissolution process.
'22' may comprise a process
for dissolving sodium sulfite in water to form an aqueous sodium sulfite
solution.
23 '23' may comprise a solution comprising sodium sulfite.
'23' may comprise an
aqueous sodium sulfite solution or a solution comprising dissolved sodium
sulfite.
'23' may comprise an aqueous sodium sulfite solution transferred from a
dissolution
step to an absorber, or gas-liquid contactor, or reactor, or a precipitator,
or a
combination thereof process.
24 '24' may comprise carbon dioxide '24' may comprise input
carbon dioxide '24' may
comprise a gas stream comprising carbon dioxide. '24' may comprise carbon
dioxide
in a pure gas stream, for example, a gas stream with greater than 93% carbon
dioxide.
'24' may comprise carbon dioxide in a mixture with other gases, which may
include,
but is not limited to, one or more or a combination of the following: flue
gas, carbon
dioxide in a gas mixture with air, air, biogas, stripped carbon dioxide,
stripping gas
comprising carbon dioxide, sour gas, natural gas, or other gas mixture
comprising
carbon dioxide. '24' may comprise carbon dioxide transferred to an absorber or
reactor or both, wherein, for example, carbon dioxide may be reacted or
absorbed.
25 '25' may comprise a gas-liquid contactor. '25' may comprise
a gas-liquid contactor,
or an absorber, or a reactor, or a precipitator, or a combination thereof
process. '25'
may comprise a process for reacting carbon dioxide with a solution comprising
sodium sulfite to form sodium bicarbonate and sodium bisulfite. '25' may
comprise a
process for reacting carbon dioxide with a solution comprising sodium sulfite
to form
sodium bicarbonate, or sodium carbonate, or a combination thereof and sodium
bisulfite. In some embodiments, '25' may be heated or allowed to increase in
temperature during absorption to minimize sodium bicarbonate or sodium
carbonate
precipitation during absorption, then the solution may be cooled to produce
sodium
bicarbonate or sodium carbonate precipitate. Alternatively, or additionally,
in some
embodiments, '25' may be cooled to facilitate the precipitation of sodium
bicarbonate
or sodium carbonate. Alternatively, or additionally, in some embodiments, '25'
may
be cooled to facilitate the precipitation of sodium bicarbonate or sodium
carbonate
during the absorption of carbon dioxide.
26 '26' may comprise products of a reaction. '26' may comprise
a solid-liquid mixture
comprising sodium bicarbonate solid and an aqueous solution comprising sodium
bisulfite. '26' may undergo further cooling to facilitate the precipitation of
sodium
bicarbonate, or sodium carbonate, or both. '26' may comprise solid-liquid
mixture
transferred from an absorber or reactor step to a solid-liquid separation
step.
27 '27' may comprise a solid-liquid separation process. '27'
may comprise a process for
separating at least a portion of solid phase sodium bicarbonate, or sodium
carbonate,
74
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Figure 3 Key
ID Description
or a combination thereof from at least a portion of liquid phase solution
comprising
aqueous sodium bisulfite.
28 '28' may comprise a solid separated by a solid-liquid
separation process. '28' may
comprise a solid comprising sodium bicarbonate, or sodium carbonate, or a
combination thereof. In some embodiments, said sodium bicarbonate, or sodium
carbonate, or a combination thereof may be transferred or used in an
application. In
some embodiments, said sodium bicarbonate, or sodium carbonate, or a
combination
thereof may undergo further treatment in some embodiments. For example, in
some
embodiments, said sodium bicarbonate, or sodium carbonate, or a combination
thereof
may undergo drying, or calcining, or further purification, or a combination
thereof
before use in one or more applications.
29 '29' may comprise a liquid solution separated from a solid
following a solid-liquid
separation process. '29' may comprise an aqueous solution comprising sodium
bisulfite. '29' may comprise residual sodium bicarbonate, or sodium carbonate,
or a
combination thereof '29' may comprise an aqueous solution comprising sodium
bisulfite and residual dissolved sodium bicarbonate, or sodium carbonate, or a
combination thereof '29' may be transferred from a solid-liquid separation
process to
a process for distillation, or a water removal, or a drying, or a separation,
or a
crystallization or a combination thereof
30 '30' may comprise a process for distillation, or a water
removal, or a drying, or a
separation, or a crystallization or a combination thereof '30' may comprise a
process
for separating a solution comprising sodium bisulfite and / or residual sodium
bicarbonate, or sodium carbonate, or a combination thereof into liquid, or
solid
sodium bicarbonate, or solid sodium carbonate, or solid sodium metabisulfite,
or solid
sodium sulfite, or a combination thereof In some embodiments, '30' may involve
removing or distilling at least a portion of water with subsequent or
simultaneous
precipitation of lower solubility salts, such as residual sodium bicarbonate,
or residual
sodium carbonate, or a combination thereof In some embodiments, '30' may
involve
removing or distilling at least a portion of water with subsequent or
simultaneous
precipitation of sodium metabisulfite, or sodium sulfite, or a combination
thereof In
some embodiments, a portion of carbon dioxide, or sulfur dioxide, or a
combination
thereof may be produced in `30'.
31 '31' may comprise at least a portion of water separated
during a solid-liquid
separation process. '31' may comprise water transferred from a process for
distillation, or a water removal, or a drying, or a separation, or a
crystallization or a
combination thereof to a dissolution process.
32 '32' may comprise a solid comprising sodium bicarbonate, or
sodium carbonate, or a
combination thereof In some embodiments, said sodium bicarbonate, or sodium
carbonate, or a combination thereof may be transferred or used in an
application. In
some embodiments, said sodium bicarbonate, or sodium carbonate, or a
combination
thereof may undergo further treatment in some embodiments. For example, in
some
embodiments, said sodium bicarbonate, or sodium carbonate, or a combination
thereof
may undergo drying, or calcining. or further purification, or a combination
thereof
before use in one or more applications.
33 '33' may comprise a solid comprising sodium metabisulfite,
or sodium sulfite, or a
combination thereof '33' may comprise a solid transferred from a separation
process
or water removal process to a calcination or a thermal decomposition or a
thermal
desorption process.
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Figure 4 Key
ID Description
1 An input material comprising a salt of carbonate, or
silicate, or bicarbonate, or a salt
of a weaker acid than sulfurous acid, or a salt an acid with a higher pKa than
sulfurous
acid, or a combination thereof. An input material comprising calcium
carbonate, or
magnesium carbonate, or calcium silicate, or magnesium silicate, or a calcium
salt
comprising carbon, or a magnesium salt comprising carbon, or a calcium ¨ weak
acid
anion salt, or a magnesium ¨ weak acid anion salt, or an alkaline earth ¨ weak
acid
anion salt, or a mineral thereof, or a derivative thereof, or a combination
thereof
2 '2' may comprise a process for mixing or reacting or both
an input material (such as,
for example, '1') with sulfurous acid or a solution comprising dissolved
sulfur
dioxide. '2' may involve mixing sulfurous acid with a calcium or magnesium ¨
weak
acid salt. '2' may involve mixing sulfurous acid with a calcium or magnesium ¨
weak
acid salt to form calcium or magnesium sulfite or bisulfite. In the present
embodiment,
it may be desirable for the molar ratio of sulfur in the sulfurous acid
reactant to the
calcium and / or magnesium in the input material reactant to be about the same
or
greater than the molar ratio of sulfur to calcium or magnesium in dissolved
calcium
bisulfite or magnesium bisulfite. Sulfurous acid reactant in excess of the
molar ratio
than the molar ratio of sulfur to calcium or magnesium in dissolved calcium
bisulfite
or magnesium bisulfite may comprise 'excess' sulfurous acid. In some
embodiments,
'excess' sulfurous acid may be desirable in '2' to, for example, improve
reaction
kinetics or otherwise facilitate the reaction to form calcium bisulfite and /
or
magnesium bisulfite. '2' may form dissolved calcium bisulfite and / or
magnesium
bisulfite and a weak acid product. Said weak acid product may comprise a
solid, or a
liquid, or a gas, or a combination thereof, which may be separated from the
calcium
bisulfite and / or magnesium bisulfite within '2' or in a separate step. For
example,
said weak acid product may comprise gaseous carbon dioxide, which may be
employed as a valuable byproduct or employed internally or a combination
thereof.
3 '3' may comprise the liquid and / or solid products from
'2'. '3' may involve
transferring the products from '2' to a separation step. For example, in some
embodiments, the products from '2' may comprise a solid-liquid slurry
comprising an
aqueous liquid phase solution of calcium bisulfite and / or magnesium
bisulfite and a
solid phase comprising one or more or a combination of the following:
unreacted
material, or silicon dioxide, or a silicon derivative, or a combination
thereof In some
embodiments, the products from '2' may comprise at least a portion residual
sulfurous
acid or residual excess sulfurous acid, which may, if desired, remain at a
liquid phase
with the liquid solution comprising calcium bisulfite and / or magnesium
bisulfite.
4 '4' may comprise a phase separation process. For example,
'4' may comprise a
process designed to separate at least a portion of the aqueous liquid phase
solution
comprising calcium bisulfite and / or magnesium bisulfite from solid phase
material.
For example, '4' may comprise a solid-liquid separation process. For example,
'4'
may comprise a process designed to separate at least a portion of the aqueous
liquid
phase solution comprising calcium bisulfite and / or magnesium bisulfite from
at least
a portion of a solid phase comprising, for example, one or more or a
combination of
the following: unreacted material, or silicon dioxide, or a silicon
derivative, or a
combination thereof
'5' may comprise separated solid phase. For example, '5' may comprise a solid
phase
comprising, for example, including, but not limited to, one or more or a
combination
of the following: unreacted material, or silicon dioxide, or a silicon
derivative, or a
combination thereof '5' may comprise an output. '5' may undergo further
separation,
treatment, or use, or a combination thereof
76
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Figure 4 Key
ID Description
6 '6' may comprise separated aqueous liquid phase solution comprising
calcium
bisulfite and / or magnesium bisulfite. '6' may comprise separated aqueous
liquid
phase solution comprising calcium bisulfite and / or magnesium bisulfite
transferred
from a solid-liquid separation process to a reaction with sodium sulfate.
7 '7' may comprise an input material comprising sodium sulfate. '7' may be
at a solid
phase, a liquid phase, or both.
8 '8' may comprise a process for mixing or reacting or both an input
material, such as
'7', with a separated aqueous liquid phase solution comprising calcium
bisulfite and /
or magnesium bisulfite, such as '6'. Aqueous solution comprising calcium
bisulfite or
magnesium bisulfite may react with sodium sulfate to form an aqueous solution
comprising sodium bisulfite and a solid phase comprising calcium sulfate or
magnesium sulfate. Residual dissolved calcium sulfate or magnesium sulfate may
remain present in the aqueous solution comprising sodium bisulfite, although
it is
important to note the appreciably lower solubility of calcium sulfate or
magnesium
sulfate in water than sodium bisulfite.
9 '9' may comprise the products from '8-. '9' may involve transferring the
products
from '8' to a separation step. For example, in some embodiments, the products
from
'8' may comprise a solid-liquid slurry comprising an aqueous liquid phase
solution of
sodium bisulfite and a solid phase comprising calcium sulfate or magnesium
sulfate.
In some embodiments, the products from '8' may comprise at least a portion
residual
sulfurous acid or residual excess sulfurous acid, which may, if desired,
remain at a
liquid phase with the liquid solution comprising sodium bisulfite in '9'.
10 '10' may comprise a phase separation process. For example, -10' may
comprise a
process designed to separate at least a portion of the aqueous liquid phase
solution
comprising sodium bisulfite from at least a portion of the solid calcium
sulfate or
magnesium sulfate in '9'. For example, '10' may comprise a solid-liquid
separation
process. For example, '10' may comprise a process designed to separate at
least a
portion of the aqueous liquid phase solution comprising sodium sulfite from at
least a
portion of a solid comprising calcium sulfate or magnesium sulfate.
11 '11' may comprise a separated solid phase. '11' may comprise separated
calcium
sulfate, or magnesium sulfate, or both. '11' may comprise an output. It is
important to
note the separated calcium sulfate, or magnesium sulfate, or both may be of
sufficiently high purity for commercial uses of gypsum. For some applications,
'11'
may be in an appropriate form of use or sale. For some applications, `11' may
require
additional treatment, or dehydration, or drying, or refining, or pulverizing,
or a
combination thereof
12 '12' may comprise separated aqueous liquid phase solution comprising
sodium
bisulfite. '12' may comprise separated aqueous liquid phase solution
comprising
sodium bisulfite transferred from a solid-liquid separation process to a
distillation, or a
water removal, or a drying, or a separation, or a crystallization or a
combination
thereof step.
13 '13' may comprise a process for distillation, or a water removal, or a
drying, or a
separation, or a crystallization or a combination thereof '13' may comprise a
process
employed to separate a salt solution into at least a portion water and at
least a portion
solid salt. '13' may comprise one or more or a combination of separation
processes
described herein. '13' may comprise, for example, MVC, or MED, or MSF, or
membrane-based process, or a combination thereof '13' may comprise a process
for
separating an aqueous solution comprising sodium bisulfite, such as '12', into
at least
a portion of water and at least a portion of solid sodium metabisulfite. '13'
may
comprise a process for separating an aqueous solution comprising sodium
bisulfite,
77
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Figure 4 Key
ID Description
such as '12', into at least a portion of water, at least a portion of solid
sodium
metabisulfite, and at least a portion of residual sulfur dioxide. Said at
least a portion of
residual sulfur dioxide may comprise dissolved sulfur dioxide or sulfurous
acid in the
at least a portion of water, or may comprise gas phase sulfur dioxide, or may
comprise
liquid phase sulfur dioxide, or may comprise a combination thereof
14 '14' may comprise water. '14' may comprise water and
residual dissolved sulfur
dioxide. '14' may comprise water transferred from a water removal or water
separation process to an absorption process.
15 '15' may comprise at least a portion of gaseous sulfur
dioxide. In embodiments
employing excess sulfur dioxide and / or embodiments employing thermal or gas-
liquid phase transition separation for water removal, gaseous sulfur dioxide
may be
produced during a water removal step, such as '14'.
16 '16' may comprise a separated solid. '16' may comprise
solid sodium metabisulfite
separated from water. '16' may comprise solid sodium metabisulfite separated
from a
solution comprising sodium bisulfite. '16' may comprise a solid comprising
sodium
metabisulfite, or sodium sulfite, or a combination thereof '16' may comprise a
solid
transferred from a separation process or water removal process to a
calcination or a
thermal decomposition or a thermal desorption process.
17 '17' may comprise a calcination, or a thermal
decomposition, or a desorption, or
decomposition, or a combination thereof process. '17' may involve thermally
decomposing or calcining sodium metabisulfite into solid sodium sulfite and
gaseous
sulfur dioxide. '17' may employ one or more processes described herein, or
known in
the art, or a combination thereof for calcination, or a thermal decomposition,
or a
desorption, or decomposition, or a combination thereof
18 '18' may comprise gaseous sulfur dioxide produced from a
process for calcination, or
a thermal decomposition, or a desorption, or decomposition, or a combination
thereof
'18' may comprise gaseous sulfur dioxide transferred to a sulfur dioxide
absorption
process or a process for producing sulfurous acid.
19 '19' may comprise an absorption process. '19' may comprise
a process for dissolving
sulfur dioxide in water. '19' may comprise a process for producing sulfurous
acid
from sulfur dioxide and a solution comprising water. '19' may comprise a
process for
producing concentrated or 'rich' sulfurous acid from sulfur dioxide and a
solution
comprising water.
20 '20' may comprise a solution comprising sulfur dioxide.
'20' may comprise an
aqueous sulfurous acid solution, or a concentrated sulfurous acid solution, or
a
combination thereof '20' may comprise an aqueous sulfurous acid solution
transferred from a sulfur dioxide absorption step to a sulfurous acid reaction
step.
21 '21' may comprise a solid comprising at least a portion of
sodium sulfite. '21' may
comprise sodium sulfite transferred from a calcination step to a dissolution
step.
22 '22' may comprise a mixing and / or dissolution process.
'22' may comprise a process
for dissolving sodium sulfite in water to form an aqueous sodium sulfite
solution.
23 '23' may comprise a solution comprising sodium sulfite.
'23' may comprise an
aqueous sodium sulfite solution or a solution comprising dissolved sodium
sulfite.
'23' may comprise an aqueous sodium sulfite solution transferred from a
dissolution
step to an absorber, or gas-liquid contactor, or reactor, or a precipitator,
or a
combination thereof process.
24 '24' may comprise carbon dioxide. '24' may comprise input
carbon dioxide. '24' may
comprise a gas stream comprising carbon dioxide. '24' may comprise carbon
dioxide
in a pure gas stream, for example, a gas stream with greater than 93% carbon
dioxide.
'24' may comprise carbon dioxide in a mixture with other gases, which may
include,
78
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Figure 4 Key
ID Description
but is not limited to, one or more or a combination of the following: flue
gas, carbon
dioxide in a gas mixture with air, air, biogas, stripped carbon dioxide,
stripping gas
comprising carbon dioxide, sour gas, natural gas, or other gas mixture
comprising
carbon dioxide. '24' may comprise carbon dioxide transferred to an absorber or
reactor or both, wherein, for example, carbon dioxide may be reacted or
absorbed.
25 '25' may comprise a gas-liquid contactor. '25' may comprise
a gas-liquid contactor,
or an absorber, or a reactor, or a precipitator, or a combination thereof
process. '25'
may comprise a process for reacting carbon dioxide with a solution comprising
sodium sulfite to form sodium bicarbonate and sodium bisulfite. '25' may
comprise a
process for reacting carbon dioxide with a solution comprising sodium sulfite
to form
sodium bicarbonate, or sodium carbonate, or a combination thereof and sodium
bisulfite. In some embodiments, '25' may be heated or allowed to increase in
temperature during absorption to minimize sodium bicarbonate or sodium
carbonate
precipitation during absorption, then the solution may be cooled to produce
sodium
bicarbonate or sodium carbonate precipitate. Alternatively, or additionally,
in some
embodiments, '25' may be cooled to facilitate the precipitation of sodium
bicarbonate
or sodium carbonate. Alternatively, or additionally, in some embodiments, '25'
may
be cooled to facilitate the precipitation of sodium bicarbonate or sodium
carbonate
during the absorption of carbon dioxide.
26 '26' may comprise products of a reaction. '26' may comprise
a solid-liquid mixture
comprising sodium bicarbonate solid and an aqueous solution comprising sodium
bisulfite. '26' may undergo further cooling to facilitate the precipitation of
sodium
bicarbonate, or sodium carbonate, or both. '26' may comprise solid-liquid
mixture
transferred from an absorber or reactor step to a solid-liquid separation
step.
27 '27' may comprise a solid-liquid separation process. '27'
may comprise a process for
separating at least a portion of solid phase sodium bicarbonate, or sodium
carbonate,
or a combination thereof from at least a portion of liquid phase solution
comprising
aqueous sodium bisulfite.
28 '28' may comprise a solid separated by a solid-liquid
separation process. '28' may
comprise a solid comprising sodium bicarbonate, or sodium carbonate, or a
combination thereof In some embodiments, said sodium bicarbonate, or sodium
carbonate, or a combination thereof may be transferred or used in an
application. In
some embodiments, said sodium bicarbonate, or sodium carbonate, or a
combination
thereof may undergo further treatment in some embodiments. For example, in
some
embodiments, said sodium bicarbonate, or sodium carbonate, or a combination
thereof
may undergo drying, or calcining, or further purification, or a combination
thereof
before use in one or more applications.
29 '29' may comprise a liquid solution separated from a solid
following a solid-liquid
separation process. '29' may comprise an aqueous solution comprising sodium
bisulfite. '29' may comprise residual sodium bicarbonate, or sodium carbonate,
or a
combination thereof '29' may comprise an aqueous solution comprising sodium
bisulfite and residual dissolved sodium bicarbonate, or sodium carbonate, or a
combination thereof '29' may be transferred from a solid-liquid separation
process to
a process for distillation, or a water removal, or a drying, or a separation,
or a
crystallization or a combination thereof.
30 '30' may comprise a process for distillation, or a water
removal, or a drying, or a
separation, or a crystallization or a combination thereof. '30' may comprise a
process
for separating a solution comprising sodium bisulfite and / or residual sodium
bicarbonate, or sodium carbonate, or a combination thereof into liquid, or
solid
sodium bicarbonate, or solid sodium carbonate, or solid sodium metabisulfite,
or solid
79
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Figure 4 Key
ID Description
sodium sulfite, or a combination thereof In some embodiments, -30' may involve
removing or distilling at least a portion of water with subsequent or
simultaneous
precipitation of lower solubility salts, such as residual sodium bicarbonate,
or residual
sodium carbonate, or a combination thereof In some embodiments, '30' may
involve
removing or distilling at least a portion of water with subsequent or
simultaneous
precipitation of sodium metabisulfite, or sodium sulfite, or a combination
thereof In
some embodiments, a portion of carbon dioxide, or sulfur dioxide, or a
combination
thereof may be produced in '30'.
31 '31' may comprise at least a portion of water separated during a solid-
liquid
separation process. '31' may comprise water transferred from a process for
distillation, or a water removal, or a drying, or a separation, or a
crystallization or a
combination thereof to a dissolution process.
32 '32' may comprise a solid comprising sodium bicarbonate, or sodium
carbonate, or a
combination thereof In some embodiments, said sodium bicarbonate, or sodium
carbonate, or a combination thereof may be transferred or used in an
application. In
some embodiments, said sodium bicarbonate, or sodium carbonate, or a
combination
thereof may undergo further treatment in some embodiments. For example, in
some
embodiments, said sodium bicarbonate, or sodium carbonate, or a combination
thereof
may undergo drying, or calcining, or further purification, or a combination
thereof
before use in one or more applications.
33 '33' may comprise a solid comprising sodium metabisulfite, or sodium
sulfite, or a
combination thereof '33' may comprise a solid transferred from a separation
process
or water removal process to a calcination or a thermal decomposition or a
thermal
desorption process.
34 '34' may comprise gaseous carbon dioxide. '34' may comprise gaseous
carbon
dioxide produced from the reaction of sulfurous acid with a carbonate or
bicarbonate
salt in '2'. '34' may comprise high concentration, or high purity, or high
partial
pressure carbon dioxide. '34' may be reacted with at least a portion sodium
sulfite to
produce sodium bicarbonate and / or sodium bisulfite.
Figure 5 Key
ID Description
1 An input material comprising a salt of carbonate, or
silicate, or bicarbonate, or a salt
of a weaker acid than sulfurous acid, or a salt an acid with a higher pKa than
sulfurous
acid, or a combination thereof An input material comprising calcium carbonate,
or
magnesium carbonate, or calcium silicate, or magnesium silicate, or a calcium
salt
comprising carbon, or a magnesium salt comprising carbon, or a mineral
thereof, or a
derivative thereof, or a calcium ¨ weak acid anion salt, or a magnesium ¨ weak
acid
anion salt, or an alkaline earth ¨ weak acid anion salt, or a combination
thereof
2 '2' may comprise a process for mixing or reacting or both an input
material (such as,
for example, '1') with sulfurous acid or a solution comprising dissolved
sulfur
dioxide. '2' may involve mixing sulfurous acid with a calcium or magnesium ¨
weak
acid salt. '2' may involve mixing sulfurous acid with a calcium or magnesium ¨
weak
acid salt to form calcium or magnesium sulfite or bisulfite. In the present
embodiment,
it may be desirable for the molar ratio of sulfur in the sulfurous acid
reactant to the
calcium and / or magnesium in the input material reactant to be about the same
or
greater than the molar ratio of sulfur to calcium or magnesium in dissolved
calcium
bisulfite or magnesium bisulfite. Sulfurous acid reactant in excess of the
molar ratio
than the molar ratio of sulfur to calcium or magnesium in dissolved calcium
bisulfite
or magnesium bisulfite may comprise 'excess' sulfurous acid. In some
embodiments,
so
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Figure 5 Key
ID Description
'excess' sulfurous acid may be desirable in '2' to, for example, improve
reaction
kinetics or otherwise facilitate the reaction to form calcium bisulfite and /
or
magnesium bisulfite. '2' may form dissolved calcium bisulfite and / or
magnesium
bisulfite and a weak acid product. Said weak acid product may comprise a
solid, or a
liquid, or a gas, or a combination thereof, which may be separated from the
calcium
bisulfite and / or magnesium bisulfite within '2' or in a separate step. For
example,
said weak acid product may comprise gaseous carbon dioxide, which may be
employed as a valuable byproduct or employed internally or a combination
thereof
3 '3' may comprise the liquid and / or solid products from '2'. '3' may
involve
transferring the products from '2' to a separation step. For example, in some
embodiments, the products from '2' may comprise a solid-liquid slurry
comprising an
aqueous liquid phase solution of calcium bisulfite and / or magnesium
bisulfite and a
solid phase comprising one or more or a combination of the following:
unreacted
material, or silicon dioxide, or a silicon derivative, or a combination
thereof In some
embodiments, the products from '2' may comprise at least a portion residual
sulfurous
acid or residual excess sulfurous acid, which may, if desired, remain at a
liquid phase
with the liquid solution comprising calcium bisulfite and / or magnesium
bisulfite.
4 '4' may comprise a phase separation process. For example, '4' may
comprise a
process designed to separate at least a portion of the aqueous liquid phase
solution
comprising calcium bisulfite and / or magnesium bisulfite from solid phase
material.
For example, '4' may comprise a solid-liquid separation process. For example,
'4'
may comprise a process designed to separate at least a portion of the aqueous
liquid
phase solution comprising calcium bisulfite and / or magnesium bisulfite from
at least
a portion of a solid phase comprising, for example, one or more or a
combination of
the following: unreacted material, or silicon dioxide, or a silicon
derivative, or a
combination thereof
5 '5' may comprise separated solid phase. For example, '5' may comprise a
solid phase
comprising, for example, including, but not limited to, one or more or a
combination
of the following: unreacted material, or silicon dioxide, or a silicon
derivative, or a
combination thereof '5' may comprise an output. '5' may undergo further
separation,
treatment, or use, or a combination thereof
6 '6' may comprise separated aqueous liquid phase solution comprising
calcium
bisulfite and / or magnesium bisulfite. '6' may comprise separated aqueous
liquid
phase solution comprising calcium bisulfite and / or magnesium bisulfite
transferred
from a solid-liquid separation process to a reaction with sodium sulfate.
7 '7' may comprise an input material comprising sodium sulfate. '7' may be
at a solid
phase, a liquid phase, or both.
8 '8' may comprise a process for mixing or reacting or both an input
material, such as
'7', with a separated aqueous liquid phase solution comprising calcium
bisulfite and /
or magnesium bisulfite, such as '6'. Aqueous solution comprising calcium
bisulfite or
magnesium bisulfite may react with sodium sulfate to form an aqueous solution
comprising sodium bisulfite and a solid phase comprising calcium sulfate or
magnesium sulfate. Residual dissolved calcium sulfate or magnesium sulfate may
remain present in the aqueous solution comprising sodium bisulfite, although
it is
important to note the appreciably lower solubility of calcium sulfate or
magnesium
sulfate in water than sodium bisulfite.
9 '9' may comprise the products from '8'. '9' may involve transferring the
products
from '8' to a separation step. For example, in some embodiments, the products
from
'8' may comprise a solid-liquid slurry comprising an aqueous liquid phase
solution of
sodium bisulfite and a solid phase comprising calcium sulfate or magnesium
sulfate.
Si
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Figure 5 Key
ID Description
In some embodiments, the products from '8' may comprise at least a portion
residual
sulfurous acid or residual excess sulfurous acid, which may, if desired,
remain at a
liquid phase with the liquid solution comprising sodium bisulfite in '9'.
'10' may comprise a phase separation process. For example, '10' may comprise a
process designed to separate at least a portion of the aqueous liquid phase
solution
comprising sodium bisulfite from at least a portion of the solid calcium
sulfate or
magnesium sulfate in '9'. For example, '10' may comprise a solid-liquid
separation
process. For example, '10' may comprise a process designed to separate at
least a
portion of the aqueous liquid phase solution comprising sodium sulfite from at
least a
portion of a solid comprising calcium sulfate or magnesium sulfate.
11 '11' may comprise a separated solid phase. '11' may
comprise separated calcium
sulfate, or magnesium sulfate, or both. '11' may comprise an output. It is
important to
note the separated calcium sulfate, or magnesium sulfate, or both may be of
sufficiently high purity for commercial uses of gypsum. For some applications,
'11'
may be in an appropriate form of use or sale. For some applications, '11' may
require
additional treatment, or dehydration, or drying, or refining, or pulverizing,
or a
combination thereof.
12 '12' may comprise separated aqueous liquid phase solution
comprising sodium
bisulfite. '12' may comprise separated aqueous liquid phase solution
comprising
sodium bisulfite transferred from a solid-liquid separation process to a
distillation, or a
water removal, or a drying, or a separation, or a crystallization or a
combination
thereof step.
13 '13' may comprise a process for distillation, or a water
removal, or a drying, or a
separation, or a crystallization or a combination thereof '13' may comprise a
process
employed to separate a salt solution into at least a portion water and at
least a portion
solid salt. '13' may comprise one or more or a combination of separation
processes
described herein. '13' may comprise, for example, MVC, or MED. or MSF, or
membrane-based process, or a combination thereof '13' may comprise a process
for
separating an aqueous solution comprising sodium bisulfite, such as '12', into
at least
a portion of water and at least a portion of solid sodium metabisulfite. '13.
may
comprise a process for separating an aqueous solution comprising sodium
bisulfite,
such as '12', into at least a portion of water, at least a portion of solid
sodium
metabisulfite, and at least a portion of residual sulfur dioxide. Said at
least a portion of
residual sulfur dioxide may comprise dissolved sulfur dioxide or sulfurous
acid in the
at least a portion of water, or may comprise gas phase sulfur dioxide, or may
comprise
liquid phase sulfur dioxide, or may comprise a combination thereof
14 '14' may comprise water. '14' may comprise water and
residual dissolved sulfur
dioxide. '14' may comprise water transferred from a water removal or water
separation process to an absorption process.
'15' may comprise at least a portion of gaseous sulfur dioxide. In embodiments
employing excess sulfur dioxide and / or embodiments employing thermal or gas-
liquid phase transition separation for water removal, gaseous sulfur dioxide
may be
produced during a water removal step, such as '14'.
16 '16' may comprise a separated solid. '16' may comprise
solid sodium metabisulfite
separated from water. '16. may comprise solid sodium metabisulfite separated
from a
solution comprising sodium bisulfite. '16' may comprise a solid comprising
sodium
metabisulfite, or sodium sulfite, or a combination thereof '16' may comprise a
solid
transferred from a separation process or water removal process to a
calcination or a
thermal decomposition or a thermal desorption process.
g2
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Figure 5 Key
ID Description
17 '17' may comprise a calcination, or a thermal
decomposition, or a desorption, or
decomposition, or a combination thereof process. '17' may involve thermally
decomposing or calcining sodium metabisulfite into solid sodium sulfite and
gaseous
sulfur dioxide. '17' may employ one or more processes described herein, or
known in
the art, or a combination thereof for calcination, or a thermal decomposition,
or a
desorption, or decomposition, or a combination thereof
18 '18' may comprise gaseous sulfur dioxide produced from a
process for calcination, or
a thermal decomposition, or a desorption, or decomposition, or a combination
thereof.
'18' may comprise gaseous sulfur dioxide transferred to a sulfur dioxide
absorption
process or a process for producing sulfurous acid.
19 '19' may comprise an absorption process. '19' may comprise
a process for dissolving
sulfur dioxide in water. '19' may comprise a process for producing sulfurous
acid
from sulfur dioxide and a solution comprising water. '19' may comprise a
process for
producing concentrated or 'rich' sulfurous acid from sulfur dioxide and a
solution
comprising water.
20 '20' may comprise a solution comprising sulfur dioxide.
'20' may comprise an
aqueous sulfurous acid solution, or a concentrated sulfurous acid solution, or
a
combination thereof. '20' may comprise an aqueous sulfurous acid solution
transferred from a sulfur dioxide absorption step to a sulfurous acid reaction
step.
21 '21' may comprise a solid comprising at least a portion of
sodium sulfite. '21' may
comprise sodium sulfite transferred from a calcination step to a dissolution
step.
22 '22' may comprise a mixing and / or dissolution process.
'22' may comprise a process
for dissolving sodium sulfite in water to form an aqueous sodium sulfite
solution.
23 '23' may comprise a solution comprising sodium sulfite.
'23' may comprise an
aqueous sodium sulfite solution or a solution comprising dissolved sodium
sulfite.
'23' may comprise an aqueous sodium sulfite solution transferred from a
dissolution
step to an absorber, or gas-liquid contactor, or reactor, or a precipitator,
or a
combination thereof process.
24 '24. may comprise carbon dioxide. '24' may comprise input
carbon dioxide. '24. may
comprise a gas stream comprising carbon dioxide. '24' may comprise carbon
dioxide
in a pure gas stream, for example, a gas stream with greater than 93% carbon
dioxide.
'24' may comprise carbon dioxide in a mixture with other gases, which may
include,
but is not limited to, one or more or a combination of the following: flue
gas, carbon
dioxide in a gas mixture with air, air, biogas, stripped carbon dioxide,
stripping gas
comprising carbon dioxide, sour gas, natural gas, or other gas mixture
comprising
carbon dioxide. '24' may comprise carbon dioxide transferred to an absorber or
reactor or both, wherein, for example, carbon dioxide may be reacted or
absorbed.
25 '25' may comprise a gas-liquid contactor. '25' may comprise
a gas-liquid contactor,
or an absorber, or a reactor, or a precipitator, or a combination thereof
process. '25'
may comprise a process for reacting carbon dioxide with a solution comprising
sodium sulfite to form sodium bicarbonate and sodium bisulfite. '25' may
comprise a
process for reacting carbon dioxide with a solution comprising sodium sulfite
to form
sodium bicarbonate, or sodium carbonate, or a combination thereof and sodium
bisulfite. In some embodiments, '25' may be heated or allowed to increase in
temperature during absorption to minimize sodium bicarbonate or sodium
carbonate
precipitation during absorption, then the solution may be cooled to produce
sodium
bicarbonate or sodium carbonate precipitate. Alternatively, or additionally,
in some
embodiments, '25' may be cooled to facilitate the precipitation of sodium
bicarbonate
or sodium carbonate. Alternatively, or additionally, in some embodiments, '25'
may
83
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Figure 5 Key
ID Description
be cooled to facilitate the precipitation of sodium bicarbonate or sodium
carbonate
during the absorption of carbon dioxide.
26 '26' may comprise products of a reaction. '26' may comprise
a solid-liquid mixture
comprising sodium bicarbonate solid and an aqueous solution comprising sodium
bisulfite. '26' may undergo further cooling to facilitate the precipitation of
sodium
bicarbonate, or sodium carbonate, or both. '26' may comprise solid-liquid
mixture
transferred from an absorber or reactor step to a solid-liquid separation
step.
27 '27' may comprise a solid-liquid separation process. '27'
may comprise a process for
separating at least a portion of solid phase sodium bicarbonate, or sodium
carbonate,
or a combination thereof from at least a portion of liquid phase solution
comprising
aqueous sodium bisulfite.
28 '28' may comprise a solid separated by a solid-liquid
separation process. '28' may
comprise a solid comprising sodium bicarbonate, or sodium carbonate, or a
combination thereof In some embodiments, said sodium bicarbonate, or sodium
carbonate, or a combination thereof may be transferred or used in an
application. In
some embodiments, said sodium bicarbonate, or sodium carbonate, or a
combination
thereof may undergo further treatment in some embodiments. For example, in
some
embodiments, said sodium bicarbonate, or sodium carbonate, or a combination
thereof
may undergo drying, or calcining, or further purification, or a combination
thereof
before use in one or more applications.
29 '29' may comprise a liquid solution separated from a solid
following a solid-liquid
separation process. '29' may comprise an aqueous solution comprising sodium
bisulfite. '29' may comprise residual sodium bicarbonate, or sodium carbonate,
or a
combination thereof '29' may comprise an aqueous solution comprising sodium
bisulfite and residual dissolved sodium bicarbonate, or sodium carbonate, or a
combination thereof '29' may be transferred from a solid-liquid separation
process to
a process for distillation, or a water removal, or a drying, or a separation,
or a
crystallization or a combination thereof
30 '30' may comprise a process for distillation, or a water
removal, or a drying, or a
separation, or a crystallization or a combination thereof. '30' may comprise a
process
for separating a solution comprising sodium bisulfite and / or residual sodium
bicarbonate, or sodium carbonate, or a combination thereof into liquid, or
solid
sodium bicarbonate, or solid sodium carbonate, or solid sodium metabisulfite,
or solid
sodium sulfite, or a combination thereof In some embodiments, -30' may involve
removing or distilling at least a portion of water with subsequent or
simultaneous
precipitation of lower solubility salts, such as residual sodium bicarbonate,
or residual
sodium carbonate, or a combination thereof In some embodiments, '30' may
involve
removing or distilling at least a portion of water with subsequent or
simultaneous
precipitation of sodium metabisulfite, or sodium sulfite, or a combination
thereof. In
some embodiments, a portion of carbon dioxide, or sulfur dioxide, or a
combination
thereof may be produced in '30'.
31 '31' may comprise at least a portion of water separated
during a solid-liquid
separation process. '31' may comprise water transferred from a process for
distillation, or a water removal, or a drying, or a separation, or a
crystallization or a
combination thereof to a dissolution process.
32 '32' may comprise a solid comprising sodium bicarbonate, or
sodium carbonate, or a
combination thereof In some embodiments, said sodium bicarbonate, or sodium
carbonate, or a combination thereof may be transferred or used in an
application. In
some embodiments, said sodium bicarbonate, or sodium carbonate, or a
combination
thereof may undergo further treatment in some embodiments. For example, in
some
84
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Figure 5 Key
ID Description
embodiments, said sodium bicarbonate, or sodium carbonate, or a combination
thereof
may undergo drying, or calcining, or further purification, or a combination
thereof
before use in one or more applications.
33 '33' may comprise a solid comprising sodium metabisulfite,
or sodium sulfite, or a
combination thereof. '33' may comprise a solid transferred from a separation
process
or water removal process to a calcination or a thermal decomposition or a
thermal
desorption process.
35 '35' may comprise gaseous carbon dioxide. '35' may comprise
gaseous carbon
dioxide produced from the reaction of sulfurous acid with a carbonate or
bicarbonate
salt in '2'. '35' may comprise high concentration, or high purity, or high
partial
pressure carbon dioxide. '35' may undergo further separation, or treatment, or
compression, or phase change into a supercritical fluid, or phase change into
a liquid,
or a combination thereof. '35' may comprise a valuable byproduct.
Description of an Example Embodiment
[00120] (1) Use reaction of Calcium Bisulfite with Sodium
Sulfate to produce Calcium
Sulfate (Gypsum) and Sodium Bisulfite (aqueous).
[00121] (2) Decompose Sodium Bisulfite (Sodium
Metabisulfite) into Sodium Sulfite.
[00122] (3) Absorb CO2 into Sodium Sulfite solution to
produce Sodium Bicarbonate
(at least a portion of which may be precipitated or otherwise separated) and
Sodium Bisulfite
(aqueous). Recirculate a portion of the remaining Sodium Bisulfite aqueous
solution to step 2
(above)
[00123] Notes
Note: `WA' may comprise a weak acid, which may include, but not limited to,
silicic acid, or
orthosilicic acid, or silicon acid derivatives, or silicon minerals, or
silicon acids, or
aluminates, or fen-ates, or a combination thereof
Note: Calcium or magnesium ¨ weak acid input may comprise, for example,
including, but
not limited to, one or more or a combination of the following: carbonates, or
bicarbonates, or
silicates, or silicate derivatives, or minerals, or concrete, or cement, or
waste concrete, or
waste cement, or steel slag, or fly ash, or ash, or limestone, or rock.
Note: Concentration of NaHS03 produced from one step may be increased to match
concentration of NaHS03 from another step by, for example, distillation, or
membrane based
process, or evaporation, or other separation process, or other concentrating
process, or a
combination thereof.
o Note: A portion SO2 may desorb during some concentrating processes
o Note: CO2 may desorb during some concentration processes
o Water produced from some separation processes may employed to absorb SO2
in to form
H2S03 or aqueous sulfurous acid or sulfurous acid.
g5
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: In some embodiments, higher partial pressure CO2, or higher
concentration CO2, or pure
CO2(g), or high partial pressure CO2(g), or CO2(1), or CO2(g), may be employed
to facilitate
formation of bicarbonate salts. For example, in some embodiments, one or more
or a
combination of the following may be employed:
o At least a portion of CO2 input may be sourced from a reaction of calcium
carbonate
with sulfurous acid
o At least a portion of CO2 input may be sources from CO2 sources produced
within the
process, or other CO2 sources, or a combination thereof
o At least a portion of CO2 input may be sourced from CO2 captured from a
combustion
source, or a combustion source employed to produce heat, or emissions source,
or air,
or geological CO2 source, or natural CO2 source, or a combination thereof.
Note: CO2 sources include, but are not limited to, one or more or any
combination of the
following: Air, or combustion, or emissions gases, or refinery gases, or Power
Plant (Natural
gas, coal, oil, petcoke, biofuel, municipal waste), Cement production,
chemical production,
Waste Water Treatment, Landfill gas, Air, Metal production/refining (such as
Iron, Steel,
Aluminum, etc.), Glass production, Oil refineries, LNG liquification, HVAC,
Transportation
vehicles (ships, boats, cars, buses, trains, trucks, airplanes), Natural Gas,
Biogas, Alcohol
fermentation, Volcanic Activity, Decomposing leaves/biomass, Septic tank,
Respiration,
Manufacturing facilities, Fertilizer production, or Geothermal processes where
CO2(g)
[00124] Additional Notes:
Note: Some embodiments may be designed to operate as a low temperature
process, where
the solutions and / or solid reagents in thermal desorption or decomposition
may undergo or
operate thermal desorption or decomposition at less than 150 C, or less than
200 C, or less
than 250 C, or less than 300 C, or less than 350 C.
Note: In some embodiments, at least a portion of heat may be supplied by a
heat pump, or a
refrigeration cycle, or a combination thereof. A heat pump may comprise,
including, but not
limited to, a mechanical, or absorption, or a combination thereof process. A
heat pump may
be powered by, including, but not limited to, electricity, or heat, or
photons, or chemical
reaction, or radiation, or mechanical work, or pneumatic process, or hydraulic
process, or
expansion, or compression, or evaporation, or absorption, or vapor pressure
differences, or
osmotic pressure differences, or temperature differences, or pressure
differences, or a
combination thereof
Note: Heat greater than or equal to 150 C can be supplied by heat pumps known
in the art.
Heat pumps may reduce the total energy consumption required to supply heat.
g6
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: In some embodiments, at least a portion of CO2 may be supplied by a gas
stream
comprising CO2 and at least one other gas. For example, said gas stream may
comprise,
including, but not limited to, one or more or a combination of the following:
air, flue gas,
waste gases, sour gas, or fermentation gases, purge gases, or a combination
thereof
o For example, Na2S03(aq) or sodium sulfite may be contacted with a gas
mixture
comprising CO2, such as flue gas, and absorb CO2 from said gas to form, for
example,
at least a portion of Na2CO3(aq), or NaHCO3, or a combination thereof In some
embodiments, a subsequent step may involve further reacting with higher
partial
pressure CO2 to enable, for example, maximum conversion efficiency to NaHCO3.
o It may be desirable to contact Na2S03 and / or NaHS03 and / or other
sulfites and / or
bisulfites exclusively with gases or fluids comprising low, or minimal, or
practically
no presence of oxygen to, for example, prevent the formation of sulfates and /
or
bisulfates.
Note: In some embodiments, sulfides and / or hydrogen sulfide may comprise a
weak acid or
weak acid anion.
Note: Sources of low cost sodium sulfate may possess a higher purity or
require less
treatment to produce high purity sodium sulfite than common sources of sodium
chloride,
such as sodium chloride brines. The use of relatively high purity sodium
sulfate input may
result in lower pre-treatment or purification costs, especially compared to
some sodium
chloride input sources.
Note: Sodium sulfate may be employed in the reaction with calcium bisulfite
because
calcium sulfate is minimally soluble or practically insoluble in water,
especially relative to
sodium bisulfite reaction product. The bulk of calcium sulfate may be
separated by, for
example, one or more processes for solid-liquid separation.
[00125] Additional Notes:
Note: Dehydrating sodium bicarbonate or sodium carbonate can be energy
intensive and may
be unnecessary in embodiments where the end application of sodium bicarbonate
or sodium
carbonate can employ wet or hydrates sodium bicarbonate or sodium carbonate.
For example,
in embodiments producing sodium bicarbonate or sodium carbonate for
applications which
are or may be conducted at an aqueous or wet state, it may be desirable to
allow the sodium
bicarbonate to remain at a hydrated state. Applications which are or may be
conducted at an
aqueous or wet state may include, but are not limited to, one or more or a
combination of the
following: water treatment, or water processing, or waste water treatment, or
pH balancing,
or alkalinization, or sulfur dioxide scrubbing, or nitrogen oxide scrubbing,
or acid scrubbing,
g7
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
or addition to ocean water or other water body to increase alkalinity or
enable effective CO2
sequestration.
Note: Advantageously, unlike sodium bicarbonate or sodium carbonate, sodium
bisulfite
precipitates or solidifies or crystalizes at a non-hydrated state. Sodium
bisulfite is not known
to exist in a solid form. The solid form of sodium bisulfite may be sodium
metabisulfite,
which comprises sodium bisulfite without the water molecule. When a solution
comprising
sodium bisulfite undergoes precipitation or crystallization, the solid salt
which forms may
comprise sodium metabisulfite. Advantageously, calcining sodium metabisulfite
or
'precipitated sodium bisul lite' to, for example, sodium sulfite, may not
require dehydrating
sodium metabisulfite, which may enable lower energy consumption. For
comparison,
decomposing wet sodium bicarbonate to sodium carbonate at a hydrated state and
carbon
dioxide requires 0.92 GJ per ton of sodium carbonate product, while
decomposing wet
sodium bicarbonate to sodium carbonate at a de-hydrated state requires 3.7 GJ
per ton of
sodium carbonate.
Note: Separations for recovering water, or concentrating, or crystalizing, or
precipitating, or
separating, or a combination thereof may include, but are not limited to, one
or more or a
combination of the following: mechanical vapor compression (MVC), or
mechanical vapor
recompression, or multi-effect distillation (MED), or multi-stage flash
distillation (MSF), or
vapor compression (VC) distillation, or vacuum vapor compression (VVC), or
membrane
distillation, or evaporation, or distillation, or forward osmosis, or reverse
osmosis, or
nanofiltration, or hot nanofiltration, or hot reverse osmosis, or hot
concentrating followed by
cooling precipitation, or hot concentrating followed by cooling precipitation
and solid-liquid
separation, or heating precipitation, centrifuge, settling, or filter, or
rotary filter, or calcining,
or desorption, or absorption, or coalescing, or decanting, or aggregation, or
coagulation, or
frothing, or density based methods, or surface tension based methods, or
foaming separation.
emulsification, or de-emulsification, or flocculation, solventing out, or
salting out, or cooling
precipitation, or heating, or cryodesalination, or freeze desalination, or
zero liquid discharge
processes, or crystallization processes, or electrodialysis reversal (EDR), or
electrodialysis
process.
[00126] Additional Notes:
Note: Technologies for transforming salt brines into water and crystalized
salt, which may be
considered 'zero-liquid discharge' technologies, generally require 15 to 40
kWh per m3 of
water recovered.
The process in Figure 4 may be the same as the process in Figure 3, except
Figure 4 may
involve a process employing a calcium carbonate or magnesium carbonate input.
Figure 4
SS
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
may involve producing CO2(g) in the production of calcium sulfite or calcium
bisulfite or
both. The produced CO2(g) may be reacted in a later step to produce sodium
bicarbonate or
sodium carbonate or both. In some embodiments, the produced CO2 may be reacted
or
absorbed or both in a concentrated or almost pure or pure form. In some
embodiments, the
produced CO2 may be mixed with a CO2 gas mixture, such as flue gas, increasing
the
concentration of said CO2 gas mixture before employing the CO2 gas mixture to
produce
sodium bicarbonate, or sodium carbonate, or a combination thereof
Note: Advantageously, some embodiments of the present invention may produce
sodium
bicarbonate or sodium carbonate without requiring the presence of ammonia or
an ammonia
catalyst.
Note: Some embodiments may employ an inert gas, such as nitrogen or argon, or
a gas other
than diatomic oxygen, such as CO2, or a combination thereof in the headspace
to prevent, for
example, oxidation of or reaction of oxygen with sulfite, metabisulfite,
bisulfite, sulfur
dioxide, sulfurous acid, or a combination thereof
[00127] Additional Notes:
Note: Potassium or other alkali or alkali salts may be employed instead of or
in addition to
sodium. Alternatively, or additionally, ammonia, or ammonium, or amine, or a
combination
thereof may be employed instead of or in addition to sodium. Alternatively, or
additionally,
zinc may be employed instead of or in addition to sodium. Zinc can form
sulfites and / or
metabisulfites.
Note: Magnesium or other alkaline earth or alkaline earth salts may be
employed instead of
or in addition to calcium. Alternatively, or additionally, zinc or other metal
cation may be
employed instead of or in addition to sodium. Zinc can form sulfites and / or
metabisulfites.
Note: Concrete waste is produced in excess of 600 million tons annually in the
USA alone,
which is more than twice the amount of generated municipal solid waste.
Note: At least a portion of sulfur dioxide may be lost in one or more or a
combination of
steps. Alternatively, or additionally, sulfur dioxide may be exit the process
as a, for example,
a residual, in one or more outputs. Sulfur dioxide or `make-up sulfur dioxide'
may be added
to the process. In some embodiments, sulfur dioxide may be stored on site and
added as
desired or needed to the process. In some embodiments, elemental sulfur, or
hydrogen
sulfide, or a salt comprising sulfur, or sulfide salt, or sulfite salt, or
sulfate salt, or a
combination thereof may be a source of sulfur dioxide or sulfurous acid, by,
for example,
including, but not limited to, one or more or a combination thereof:
combustion, or acid-base
reaction, or reaction with an acid, or carbothermic reduction, or thermal or
decomposition, or
electrolysis, or electrodialysis, or electrochemical reaction.
g9
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
[00128] Additional Notes:
Note: At least a portion of residual calcium sulfate may be removed. For
example, a portion
of residual dissolved calcium sulfate may precipitate and may be removed by,
for example,
including, but not limited to, solid-liquid separation, or removal of calcium
sulfate scaling, or
a combination thereof.
Note: One or more or a combination of steps in one or more embodiments may
require
heating and / or cooling. For example, a reaction of sulfurous acid with a
calcium - weak acid
or magnesium ¨ weak acid may require or may be facilitated by cooling or
heating. For
example, a reaction of sodium sulfate with aqueous calcium bisulfite or
magnesium bisulfite
may require or may be facilitated by cooling or heating. Alternatively, or
additionally, heat or
heating or cooling or a combination thereof may be recovered from one or more
or a
combination of reaction steps. In some embodiments, heat or heating or cooling
or a
combination thereof may be recovered and said recovered heat or heating or
cooling or a
combination thereof may be transferred or employed in one or more other steps,
or in the
same step, or in other applications.
Note: Losses may occur during the process. Makeup streams of one or more or a
combination
of reagents may be added.
Note: Contaminants may exist or accumulate in the process. If desired, one or
more
contaminants may be at least partially removed periodically, or continuously,
or as desired, or
a combination thereof
[00129] Additional Notes:
Note: List of example Silicate Minerals which may be employed may include, but
are not
limited to, silicate minerals or minerals described in the following
reference:
o Daval, D. Carbon dioxide sequestration through silicate degradation and
carbon
mineralisation: promises and uncertainties. npj Mater Degrad 2, 11 (2018).
Note: 80-100% lower emissions than virgin concrete or virgin calcium oxide.
Exact same
product.
Note: Some embodiments may employ waste concrete, or steel slag, or fly ash,
or olivine, or
any combination thereof as an input.
Note: If non-calcium of non-magnesium metals dissolve or react with SO2 or
sulfurous acid,
said metals or metal salts may be separated before or after separation of
calcium sulfite or
magnesium sulfite or both. If said non-calcium of non-magnesium metal salts
are still
dissolved, said non-calcium of non-magnesium salts may be separated by
precipitation, or
systems and / or methods for zero liquid discharge, or a combination thereof
[00130] Additional Notes:
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: In some embodiments, steam may be employed as a stripping or carrier
gas. Steam can
be condensed after decomposition of one or more reagents. If steam is
employed, it must be
contacted at a temperature greater than the decomposition temperature of
calcium hydroxide.
Contact calcium oxide with steam to form calcium hydroxide may enable the
reaction of
calcium oxide and water to generate higher temperature and / or higher quality
heat, which
may be employed within one or more reaction steps or may be employed in a
different
application.
Note: Kiln with cryogenic separation of SO2 from the flue gas or off gases.
The separated
SO2 may be employed in one or more or any combination of reaction steps.
Note: React calcium oxide or calcium hydroxide or both with sodium sulfite or
sodium
sulfite to produce precipitated calcium sulfate (precipitated, clean gypsum,
which can be
sold) or precipitate calcium sulfide and sodium hydroxide solution.
Note: Sodium hydroxide solution may be crystallized from solution and sold.
Note: Sodium hydroxide solution may be sold
Note: Sodium hydroxide solution may be added to the ocean to increase ocean
alkalinity and
permanently remove CO2 from the atmosphere (two moles of CO2 for each mole of
original
calcium oxide)
Note: Sodium hydroxide solution may be reacted with flue gas and other CO2
emissions, and
then sold as sodium carbonate or bicarbonate
Note: Sodium hydroxide solution may be reacted with CO2 in the air, producing
Sodium
carbonate. Sodium carbonate may be sold as a valuable product, or added to the
ocean to
increase ocean alkalinity and permanently remove more CO2 from the atmosphere
/ ocean, or
a combination thereof.
Note: Thermally decompose Calcium sulfite in an electric kilns
Note: Thermally decompose calcium sulfite in a natural gas, or coal, or waste
incinerator, or
biofuel, or biomass, or electricity, or oil, or petcoke, or fossil fuel, or
charcoal, or solar
thermal, or thermal, or any combination thereof powered kiln.
Note: Thermally decompose calcium sulfite using a hydrogen fuels system. If
hydrogen is
used for heat, there will be no CO2 emissions in the end to end process. Also,
green hydrogen
can be produced from solar energy and stored, eliminating the challenge of
solar
intermittency. Alternatively or additionally, hydrogen may be blue hydrogen,
or hydrogen
from natural gas, where the carbon or CO2 is removed from the natural gas to
produce
hydrogen before hydrogen is burned. Alternatively, a process may employ a
combination of
blue hydrogen (during the night) and solar electricity (during the day).
[00131] Additional Notes:
91
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: Recovery heat form hydrating calcium oxide to calcium hydroxide to
provide heat or
steam or both for applications requiring heat (e.g. separating SO2 from
calcium bisulfite
solution may require heat)
Note: Remaining flue gas after most or all SO2 is removed or recovered may
comprise at
least a portion CO2.
Note: Flue gas CO2 may be concentrated with pressure swing absorption or
pressure swing
adsorption or gas membrane or both, then the flue gas with higher
concentrations of CO2
may be employed as a feedstock for the production of sodium bicarbonate or
sodium
carbonate.
Note: CO2 may be cryogenically separated from this remaining flue gas.
Note: Convert calcium silicate from the Pidgeon process
May employ calcium, or magnesium, or alkaline earth, or a combination thereof
Calcium or
magnesium or alkaline earth may be substituted.
Note: 'H2S03(aq)' or '2 S02(aq) + H20(1)' or 'S02(aq)' may be used
interchangeably.
Note: The weight percent concentration of S02 in one or more aqueous sulfurous
acid
solutions may be greater than or equal to one or more or a combination of the
following:
0.001%, or 0.1%, or 1%, or 2%, or 3%, or 4%, or 5%, or 6%, or 7%, or 8%, or
9%, or 10%,
or 11%, or 12%, or 13%, or 14%, or 15%, or 16%, or 17%, or 18%, or 19%, or
20%, or 21%,
or 22%, or 23%, or 24%, or 25%, or 26%, or 27%, or 28%, or 29%, or 30%, or
31%, or 32%,
or 33%, or 34%, or 35%, or 36%, or 37%, or 38%, or 39%, or 40%, or 41%, or
42%, or 43%,
or 44%, or 45%, or 46%, or 47%, or 48%, or 49%, or 50%, or 51%, or 52%, or
53%, or 54%,
or 55%, or 56%, or 57%, or 58%, or 59%, or 60%, or 61%, or 62%, or 63%, or
64%, or 65%,
or 66%, or 67%, or 68%, or 69%, or 70%, or 71%, or 72%, or 73%, or 74%, or
75%, or 76%,
or 77%, or 78%, or 79%, or 80%, or 81%, or 82%, or 83%, or 84%, or 85%, or
86%, or 87%,
or 88%, or 89%, or 90%, or 90.5%, or 91%, or 91.5%, or 92%, or 92.5%, or 93%,
or 93.5%,
or 94%, or 94.5%, or 95%, or 95.5%, or 96%, or 96.5%, or 97%, or 97.5%, or
98%, or
98.5%, or 99%, or 99.5%, or 99.9%, or less than or equal to 100%.
[00132] Additional Notes:
Note: The volume% concentration of 02 in the headspace gases may be less than
or equal to
one or more or a combination of the following: 0.001%, or 0.1%, or 1%, or 2%,
or 3%, or
4%, or 5%, or 6%, or 7%, or 8%, or 9%, or 10%, or 11%, or 12%, or 13%, or 14%,
or 15%,
or 16%, or 17%, or 18%, or 19%, or 20%, or 21%, or 22%, or 23%, or 24%, or
25%, or 26%,
or 27%, or 28%, or 29%, or 30%, or 31%, or 32%, or 33%, or 34%, or 35%, or
36%, or 37%,
or 38%, or 39%, or 40%, or 41%, or 42%, or 43%, or 44%, or 45%, or 46%, or
47%, or 48%,
or 49%, or 50%, or 51%, or 52%, or 53%, or 54%, or 55%, or 56%, or 57%, or
58%, or 59%,
92
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
or 60%, or 61%, or 62%, or 63%, or 64%, or 65%, or 66%, or 67%, or 68%, or
69%, or 70%,
or 71%, or 72%, or 73%, or 74%, or 75%, or 76%, or 77%, or 78%, or 79%, or
80%, or 81%,
or 82%, or 83%, or 84%, or 85%, or 86%, or 87%, or 88%, or 89%, or 90%, or
90.5%, or
91%, or 91.5%, or 92%, or 92.5%, or 93%, or 93.5%, or 94%, or 94.5%, or 95%,
or 95.5%,
or 96%, or 96.5%, or 97%, or 97.5%, or 98%, or 98.5%, or 99%, or 99.5%, or
99.9%, or less
than or equal to 100%.
Note: `WA' may comprise a weak acid, which may include, but not limited to,
silicic acid, or
orthosilicic acid, or silicon acid derivatives, or silicon minerals, or
silicon acids, or
aluminates, or ferrates, or a combination thereof
Note: Some embodiments may involve reacting calcium silicate or a material
comprising
silicon directly with sulfur dioxide, or liquid sulfur dioxide, or sulfur
dioxide in an non-
aqueous solution, or any combination thereof
Note: In some embodiments, contaminants or impurities may dissolve in a
solution
comprising sulfur dioxide, or due to the presence of sulfuric acid, or a
combination thereof
Contaminants or impurities may include, but are not limited to, one or more or
a combination
of the following: iron, or aluminum, or alkali metals, or transition metals,
or other non-
bisulfite soluble salts, or non-alkaline earth bisulfite salts, or a
combination thereof In some
embodiments, dissolved contaminants may be present after solid-liquid
separation, and / or
after calcium sulfite precipitation. In some embodiments, at least a portion
of contaminants
may be separated periodically or continuously. Contaminants may be separated
by, including,
but not limited to, precipitation, or membrane based process, or cooling, or
heating, or
crystallization, or cryodesalination, or a separation process described
herein, or a separation
process in the art, or a combination thereof.
Note: 'Calcium. may also refer to magnesium and / or other alkaline earth
metals.
Note: NaHS03(aq) may generally exist at an aqueous phase. Upon precipitation
or
crystallization, NaHS03(aq) precipitates or crystalizes as Na2S205(s).
Na2S205(s) may be
considered anhydrous.
[00133] Additional Notes:
Note: In some embodiments, sulfur dioxide may be sourced from the roasting of
sulfide ores,
which generally may produce sulfur dioxide. In some embodiments, sulfur
dioxide may be
sourced from the combustion of sulfur, or hydrogen sulfide, or fuels, or any
combination
thereof
Note: In some embodiments, it may be desirable for the partial pressure of
CO2(g) reactant to
be greater than or equal to one or more or any combination of the following:
0.01 Bar, or 0.05
bar, or 0.1 Bar, or 0.2 Bar, or 0.3 Bar, or 0.4 Bar, or 0.5 Bar, or 0.6 Bar,
or 0.7 Bar, or 0.8
93
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Bar, or 0.9 Bar, or 1.0 Bar. For example, it may be desirable for the
concentration of CO2(g)
reactant to be greater than or equal to one or more or any combination of the
following: 1%,
or 5%, or 10%, or 20%, or 30%, or 40%, or 50%, or 60%, or 70%, or 80%, or 90%,
or 95%.
In some embodiments, it may be desirable for the partial pressure of CO2(g)
reactant to
facilitate or enable the formation of bicarbonate to be greater than or equal
to one or more or
any combination of the following: 0.01 Bar, or 0.05 bar, or 0.1 Bar, or 0.2
Bar, or 0.3 Bar, or
0.4 Bar, or 0.5 Bar, or 0.6 Bar, or 0.7 Bar, or 0.8 Bar, or 0.9 Bar, or 1.0
Bar. For example, it
may be desirable for the concentration of CO2(g) reactant to facilitate or
enable the formation
of bicarbonate to be greater than or equal to one or more or any combination
of the following:
1%, or 5%, or 10%, or 20%, or 30%, or 40%, or 50%, or 60%, or 70%, or 80%, or
90%, or
95%.
Note: In some embodiments, magnesium sulfite may form an aqueous solution
comprising
aqueous magnesium sulfite. In some embodiments, magnesium sulfite may be
separated from
at least a portion of calcium sulfite, or calcium carbonate, or magnesium
carbonate, or other
practically insoluble materials. Calcium sulfite is practically insoluble in
water, with a
solubility of 0.043 grams per liter at 18 C. Magnesium sulfite is soluble in
water, with a
solubility of 5.2 grams per liter at 25 C. The reaction of a material
comprising calcium and
magnesium with aqueous sulfur dioxide may result in the formation of at least
a portion of a
solid phase comprising calcium and at least a portion of an aqueous phase
comprising
magnesium sulfite.
Note: Recovering magnesium sulfite from an aqueous solution comprising
magnesium sulfite
may be conducted using one or more or a combination of methods from separating
a
dissolved salt from an aqueous solution. Some properties of aqueous magnesium
sulfite may
enable simple, or low energy, or high throughput, or a combination thereof
separation of solid
magnesium sulfite from a solution comprising aqueous magnesium sulfite. For
example, the
solubility of magnesium sulfite increases with temperature ¨ according to
Solubilities of
magnesium sulfite hydrates by Sohnel, et al, the solubility of magnesium
sulfite or
magnesium sulfite hexahydrate is 11.04 grams per liter at 43.0 C, or 14.19
grams per liter at
51.4 C, or 19.30 grams per liter at 61.4 C, or 28.87 grams per liter at 71.5
C, or 40.17 grams
per liter at 79.0 C, or 53.73 grams per liter at 84.1 C, or 71.21 grams per
liter at 88.0 C, or
95.19 grams per liter at 94.0 C. In some embodiments, the reaction of aqueous
sulfur dioxide
with a material comprising calcium and / or magnesium may be conducted at an
elevated
temperature, such as, for example, greater than room temperature, or greater
than ambient air
temperature, or greater than 25 C, or greater than 35 C, or greater than 45 C,
or greater than
55 C, or greater than 65 C, or greater than 75 C, or greater than 85 C, or
greater than 95 C,
94
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
or greater than 100 C, or less than the boiling point of the solution at the
pressure of the
reactor, or greater than 105 C. By conducting at an elevated temperature, the
concentration of
magnesium sulfite in the aqueous magnesium sulfite may be greater, or the rate
of reaction
may be greater, or a combination thereof.
[00134] Additional Notes:
= Regardless of the temperature of the reaction of aqueous sulfur dioxide
with a material
comprising calcium and / or magnesium, it may be desirable to concentrate the
aqueous
magnesium sulfite before or during the precipitation of aqueous magnesium
sulfite. It may be
desirable for at least a portion of said solution to be concentrated. It may
be desirable for at
least a portion of said solution to be concentrated using distillation. It may
be desirable for at
least a portion of said solution to be concentrated using a membrane based
process at an
elevated temperature. It may be desirable for at least a portion of said
solution to be
concentrated using forward osmosis at an elevated temperature. It may be
desirable for at
least a portion of said solution to be concentrated using a reverse osmosis at
an elevated
temperature. It may be desirable for at least a portion of said solution to be
heated before or
during concentrating. It may be desirable for at least a portion of said
solution to be heated
before or during concentrating, to, for example, enable greater solubility of
aqueous
magnesium sulfite. It may be desirable for said aqueous magnesium sulfite to
be treated to
prevent scaling during concentrating, or to remove at least a portion of non-
magnesium
sulfite impurities, or a combination thereof. It may be desirable for at least
a portion of said
solution to be concentrated using a membrane-based process. For example, said
aqueous
magnesium sulfite solution may comprise a feed solution to a reverse osmosis
process,
wherein the reverse osmosis process separates said aqueous magnesium sulfite
solution into a
permeate comprising water and a concentrate comprising a greater concentration
of aqueous
magnesium sulfite. Said permeate comprising water may be transferred to a
countercurrent
heat exchanger for heat recovery and / or to a sulfur dioxide absorption
process and / or to
another step within the process requiring water or water solvent. It may be
desirable for the
reverse osmosis process to concentrate magnesium sulfite and / or other salts
to a
concentration lower than their solubility limits at the temperature of the
solution to prevent or
minimize membrane scaling. It may be desirable for the solution to be at an
elevation
temperature during the reverse osmosis process due to the greater solubility
limit of
magnesium sulfite with higher temperature and / or to prevent scaling or
precipitation during
reverse osmosis concentrating. Said concentrate comprising aqueous magnesium
sulfite may
be cooled to precipitate at least a portion of magnesium sulfite solid, due
to, for example, the
lesser solubility of magnesium sulfite in water with decreasing temperature,
and / or said
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
magnesium sulfite solid may be separated using a solid-liquid separation
process. The
remaining solution after separating magnesium sulfite solid using a solid-
liquid separation
process may comprise residual dissolved magnesium sulfite and / or dissolved
non-
magnesium sulfite salts or chemicals, and / or may undergo further treatment.
For example,
said remaining solution after separating magnesium sulfite solid using a solid-
liquid separate
process may be heated and / or transferred or mixed with additional new
aqueous magnesium
sulfite solution and / or may comprise at portion the feed solution to the
reverse osmosis
process. For example, said remaining solution after separating magnesium
sulfite solid using
a solid-liquid separate process may undergo further reverse osmosis steps. For
example, said
remaining solution after separating magnesium sulfite solid using a solid-
liquid separate
process may be heated and / or transferred to another membrane-based process.
For example,
said remaining solution after separating magnesium sulfite solid using a solid-
liquid separate
process may be distilled and / or crystalized, which may further separate
water from
dissolved chemicals and / or separate magnesium sulfite from other salts or
chemicals. For
example, said remaining solution after separating magnesium sulfite solid
using a solid-liquid
separate process may be mixed with solution transferred to a sulfur dioxide
absorption
process. For example, said remaining solution after separating magnesium
sulfite solid using
a solid-liquid separate process may be further treated with, including, but
not limited to, one
or more or a combination of the following: ion exchange, or resins, or
filters, or chemical
treatments, or chemical reactions, or membrane based process, or distillation,
or multi-effect
distillation, or mechanical vapor recompression distillation, or mechanical
vapor compression
distillation, or multistage flash distillation, or membrane distillation, or
cooling, or heating, or
freezing, or crydesalination, or solventing-out, or solvent induced
precipitation, or salting-
out, or other treatment. One or more solutions comprising water may be
transferred to a
sulfur dioxide absorption step, or mixed with a solution transferred to a
sulfur dioxide
absorption step, or a combination thereof.
[00135] Additional Notes:
= In some embodiments, the material comprising magnesium and calcium may
further
comprise impurities. In some embodiments, the material comprising magnesium
carbonate
and calcium carbonate may further comprise impurities. For example, the
material
comprising magnesium carbonate and calcium carbonate may further comprise
magnesium
sulfate, or calcium sulfate, or sodium salts, or potassium salts, or iron
salts, or manganese
salts, or silicon chemicals, or silicon salts, or aluminum salts, or zinc
salts, or other salts.
Additionally, the aqueous solution comprising magnesium sulfite may be exposed
to diatomic
oxygen or inadvertently exposed to diatomic oxygen, which may result in a
portion of the
96
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
magnesium sulfite converting to magnesium sulfate. In some embodiments,
impurities in the
solution comprising aqueous magnesium sulfite may comprise dissolved salts or
other
chemicals other than magnesium sulfite. In some embodiments, although certain
chemicals
may be classified as 'impurities', some 'impurities' may comprise valuable
products. For
example, impurities comprising calcium sulfate and / or magnesium sulfate may
be separated
and may comprise valuable products. In some embodiments, at least a portion of
impurities
may be separated from an aqueous solution comprising magnesium sulfite before,
or during,
or after magnesium sulfite concentrating and / or precipitation of magnesium
sulfite. In some
embodiments, potential impurities may be practically insoluble in the aqueous
magnesium
sulfite solution. For example, iron sulfite, or manganese sulfite may be
practically insoluble
in water if the formation of bisulfite salts is avoided or minimized by
employing
stoichiometric concentrations of aqueous sulfur dioxide, and / or minimizing
residence time.
Calcium sulfite solid may comprise other chemicals than calcium sulfite, which
may include,
but are not limited to, non-calcium sulfite salts described herein.
= Calcium sulfite produced from a reaction with aqueous sulfur dioxide may
comprise wet
calcium sulfite. Wet calcium sulfite may be physically wetted, as in wet
calcium sulfite may
contain water on the surface of the solid or embedded within the solid. Wet
calcium sulfite
may comprise hydrated calcium sulfite, which contains a chemically reacted
hydrate or
wherein water is reacted or part of the calcium sulfite solid. Dry calcium
sulfite may
comprise calcium sulfite solid which has minimal or no water on its surface or
is not
physically wetted. Dry calcium sulfite may comprise calcium sulfite solid
which is
anhydrous. In some embodiments, dry calcium sulfite may comprise calcium
sulfite solid
may comprise calcium sulfite solid which is partially hydrated, which means it
may comprise
hydrates of calcium sulfite, although is less hydrated than the potential full
hydrate capacity
of the calcium sulfite. Transforming wet calcium sulfite to dry calcium
sulfite may require
energy. Transforming wet calcium sulfite to dry calcium sulfite may comprise
'drying'. Some
embodiments may involve employing wet calcium sulfite as an input to a
calcining process to
produce calcium oxide. Employing wet calcium sulfite as an input to a
calcining process to
produce calcium oxide may require more energy than employing dry calcium
sulfite.
Additionally, the amount and / or quality of energy required to calcine wet
calcium sulfite
may greater than if the wet calcium sulfite is dried into dry calcium sulfite
before calcining.
One or more or a combination of systems and methods may be employed to dry or
dehydrate
calcium sulfite. For example, calcium sulfite may be dried by heating the wet
calcium sulfite
to liberate water as a liquid or a vapor or both and separating said liberated
water. If heating
is employed, it may be desirable for the temperature of the heat employed to
be less than the
97
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
temperature of calcining calcium sulfite, or for the energy consumed to
provide said heat to
be less expensive or less carbon emission intensive than the energy consumed
to calcine
calcium sulfite, or a combination thereof. For example, said heat may be
provided by,
including, but not limited to, solar thermal, or heat pump, or waste heat, or
steam, or low
pressure steam, or stored heat, or process heat, or geothermal heat, or
nuclear heat, or co-gen
heat, or any combination thereof For example, calcium sulfite may be dried by
a carrier gas
or stripping gas. For example, calcium sulfite may be dried by a recirculating
carrier gas. For
example, said recirculating carrier gas may comprise a gas or gas mixture with
a diatomic
oxygen concentration lower than 1 percent, or 2 percent, or 3 percent, or 4
percent, or 5
percent, or 6 percent, or 7 percent, or S percent, or 9 percent, or 10
percent, or 11 percent, or
12 percent, or 13 percent, or 14 percent, or 15 percent, or 16 percent, or 17
percent, or 18
percent, or 19 percent, or 20 percent, or 21 percent, or 22 percent, or any
combination thereof
by volume. For example, calcium sulfite may be dried by a recirculating
carrier gas, wherein
at least a portion of water vapor in the recirculating carrier gas is removed
by a regenerable
liquid desiccant. For example, calcium sulfite may be dried by a recirculating
carrier gas,
wherein at least a portion of water vapor in the recirculating carrier gas is
removed by a
regenerable liquid desiccant, which may include, but is not limited to, a
glycol liquid
desiccant, or glycol dehydration system, or a salt brine, or lithium bromide,
or calcium
chloride, or a liquid-liquid phase transition liquid desiccant, or a liquid
desiccant regenerated
by heat, or a liquid desiccant regenerated by vaporization of water, or a
liquid desiccant
regenerated by freezing desalination, or a liquid desiccant regenerated by a
liquid-liquid
phase transition into a water-rich phase and a water-lean phase, or a
combination thereof. For
example, calcium sulfite may be dried by a recirculating carrier gas, wherein
at least a portion
of water vapor in the recirculating carrier gas is removed by a regenerable
solid desiccant,
which may include, but is not limited to, an adsorbent. or gypsum, or
silicate, or silica gel, or
calcium oxide ¨ calcium hydroxide, or a combination thereof. For example,
calcium sulfite
may be dried by a liquid desiccant, or a solid desiccant, or a combination
thereof. For
example, calcium sulfite may be dried by a recirculating carrier gas, wherein
at least a portion
of water vapor in the recirculating carrier gas is removed by a non-
regenerated desiccant,
which may comprise a solid or a liquid. For example, a non-regenerated
desiccant may
comprise a material which reacts with water to form a product, which may be
removed from
the process as a valuable product, or is disposed. For example, an example non-
regenerated
solid desiccant may comprise calcium oxide reacted with water or water vapor
to form
calcium hydroxide. For example, in some embodiments, calcium oxide produced by
the
process may be reacted with water vapor in said carrier gas, removing at least
a portion of
98
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
said water vapor while forming calcium hydroxide. Said calcium hydroxide may
comprise a
valuable product, or may be further reacted with water, or may be converted
into other
derivatives of calcium hydroxide. In some embodiments, at least a portion of
heat generated
from forming calcium hydroxide, from, for example, calcium oxide, may be
employed to
power at least a portion of the energy required to dry the wet calcium sulfite
solid.
[00136] Additional Notes:
= Magnesium sulfite solid produced in one or more steps of the process may
comprise wet
magnesium sulfite. Wet magnesium sulfite may be physically wetted, as in wet
magnesium
sulfite may contain water on the surface of the solid or embedded within the
solid. Wet
magnesium sulfite may comprise hydrated magnesium sulfite solid, which
contains a
chemically reacted hydrate or wherein water is reacted or part of the
magnesium sulfite solid.
Dry magnesium sulfite may comprise magnesium sulfite solid which has minimal
or no water
on its surface or is not physically wetted. Dry magnesium sulfite may comprise
magnesium
sulfite solid which is anhydrous. In some embodiments, dry magnesium sulfite
may comprise
magnesium sulfite solid and / or may comprise magnesium sulfite solid which is
partially
hydrated, which means it may comprise hydrates of magnesium sulfite, although
is less
hydrated than the potential full hydrate capacity of the magnesium sulfite.
Transforming wet
magnesium sulfite into dry magnesium sulfite may require energy. Transforming
wet
magnesium sulfite to dry magnesium sulfite may comprise 'drying'. Some
embodiments may
involve employing wet magnesium sulfite as an input to a calcining process to
produce
magnesium oxide. Employing wet magnesium sulfite as an input to a calcining
process to
produce magnesium oxide may require more energy than employing dry magnesium
sulfite.
Additionally, the amount and / or quality of energy required to calcine wet
magnesium sulfite
may greater than if the wet magnesium sulfite is dried into dry magnesium
sulfite before
calcining. One or more or a combination of systems and methods may be employed
to dry or
dehydrate magnesium sulfite. For example, magnesium sulfite may be dried by
heating the
wet magnesium sulfite to liberate water as a liquid or a vapor or both and
separating said
liberated water. If heating is employed, it may be desirable for the
temperature of the heat
employed to be less than the temperature of calcining magnesium sulfite, or
for the energy
consumed to provide said heat to be less expensive or less carbon emission
intensive than the
energy consumed to calcine magnesium sulfite, or a combination thereof For
example, said
heat may be provided by, including, but not limited to, solar thermal, or heat
pump, or waste
heat, or steam, or low pressure steam, or stored heat, or process heat, or
geothermal heat, or
nuclear heat, or co-gen heat, or any combination thereof. For example,
magnesium sulfite
may be dried by a carrier gas or stripping gas. For example, magnesium sulfite
may be dried
99
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
by a recirculating carrier gas. For example, said recirculating carrier gas
may comprise a gas
or gas mixture with a diatomic oxygen concentration less than 2 percent by
volume. For
example, magnesium sulfite may be dried by a recirculating carrier gas,
wherein at least a
portion of water vapor in the recirculating carrier gas is removed by a
regenerable liquid
desiccant. For example, magnesium sulfite may be dried by a recirculating
carrier gas,
wherein at least a portion of water vapor in the recirculating carrier gas is
removed by a
regenerable liquid desiccant, which may include, but is not limited to, a
glycol liquid
desiccant, or glycol dehydration system, or a salt brine, or lithium bromide,
or calcium
chloride, or a liquid-liquid phase transition liquid desiccant, or a liquid
desiccant regenerated
by heat, or a liquid desiccant regenerated by vaporization of water, or a
liquid desiccant
regenerated by freezing desalination, or a liquid desiccant regenerated by a
liquid-liquid
phase transition into a water-rich phase and a water-lean phase, or a
combination thereof. For
example, magnesium sulfite may be dried by a recirculating carrier gas,
wherein at least a
portion of water vapor in the recirculating carrier gas is removed by a
regenerable solid
desiccant, which may include, but is not limited to, an adsorbent, or gypsum,
or silicate, or
silica gel, or calcium oxide calcium hydroxide, or an acid, or a combination
thereof. For
example, magnesium sulfite may be dried by a liquid desiccant, or a solid
desiccant, or a
combination thereof. For example, magnesium sulfite may be dried by a
recirculating carrier
gas, wherein at least a portion of water vapor in the recirculating carrier
gas is removed by a
non-regenerated solid desiccant. For example, a non-regenerated solid
desiccant may
comprise a material which reacts with water to form a product, which may be
removed from
the process as a valuable product, or may be disposed, or both. For example,
an example non-
regenerated solid desiccant may comprise calcium oxide reacted with water or
water vapor to
form calcium hydroxide. For example, in some embodiments, calcium oxide
produced by the
process may be reacted with water vapor in said carrier gas, removing at least
a portion of
said water vapor while forming calcium hydroxide. Said calcium hydroxide may
comprise a
valuable product, or may be further reacted with water, or may be converted
into other
derivatives of calcium hydroxide. In some embodiments, at least a portion of
heat generated
from forming calcium hydroxide, from, for example, calcium oxide, may be
employed to
power at least a portion of the energy required to dry the wet magnesium
sulfite solid.
[00137] Notes:
Note: Excess water may be removed from system. Similarly, water may be added
to the
system if desired. Water removal may be conducted by for example, including,
but not
limited to, one or more or a combination of the following: forward osmosis,
decanter,
separatory funnel, coalescer, centrifuge, filter, switchable solvent, cyclone,
semi-permeable
100
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
membrane, nanofiltration, organic solvent nanofiltration, reverse osmosis,
ultrafiltration,
microfiltration, hot nanofiltration, hot ultrafiltration, distillation,
membrane distillation, flash
distillation, multi-effect distillation, mechanical vapor compression
distillation, or hybrid
systems.
Note: Sodium Bicarbonate may be decomposed to form Sodium Carbonate, Sodium
hydroxide, Sodium Sesquicarbonate, or a combination thereof, or other
sodium¨carbon
dioxide or sodium bicarbonate derivative chemicals.
Note: Separation devices, or systems, or methods, or any combination thereof
may include,
but are not limited to, one or more or a combination of the following:
decanter, separatory
funnel, coalescer, centrifuge, filter, switchable solvent, cyclone, semi-
permeable membrane,
nanofiltration, organic solvent nanofiltration, reverse osmosis,
ultrafiltration, microfiltration,
hot nanofiltration, hot ultrafiltration, distillation, membrane distillation,
flash distillation,
multi-effect distillation, mechanical vapor compression distillation, or
hybrid systems
Note: The temperature of recovered heat or ambient heat source may be
increased using a
heat pump or a refrigeration cycle, if, for example, higher temperature heat
is required for one
or more process steps or one or more applications. For example, if recovered
heat is in the
form of steam, said steam may be compressed to a greater pressure, which may
enable said
steam to condense at a higher temperature and/or supply higher temperature
heat.
1001381 Additional Notes:
Note: Heat sources may include, but are not limited to, one or more or a
combination of the
following: flare gas heat, or combustion, or biofuel, or fossil fuel, or
slaking lime, or natural
gas combustion, nuclear heat, Waste Heat, Ambient Temperature Changes, or
ambient heat,
Diurnal Temperature Variation, 'Thermocline liquid body, thermocline solid
body,
thermocline gaseous body, Thermocline of a water body, halocline, heat pump,
solar thermal,
solar thermal pond, light, electricity, steam, combustion, compression,
pressure increase,
geothermal, radiative heat, condensation, exothermic dissolution, exothermic
precipitation,
exothermic formation of more liquid phases, exothermic formation of less
liquid phases,
exothermic phase change, or other heat sources described herein, or other heat
sources known
in the art.
Note: Systems and methods described herein may be batch, semi-batch, or
continuous, or a
combination thereof.
Note: Other metals or metal ions or cations which may be present or may be
employed, may
include, but are not limited to, one or more or a combination of the
following: iron, lead,
copper, cobalt, nickel, manganese, chromium, silver, scandium, vanadium,
titanium,
aluminum, magnesium, calcium, sodium, potassium, Yttrium, Zirconium, Niobium,
101
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Molybdenum Technetium, Ruthenium, Rhodium, Palladium, Silver, Cadmium,
Hafnium,
Tantalum, Tungsten, Rhenium, Osmium, Iridium, Platinum, Gold, Mercury,
Rutherfordium,
Dubnium, Seaborgium, Bohrium, Hassium, Meitnerium, Ununnilium, Unununium, or
Ununbium.
Note: Reactions or systems and methods, steps, or a combination thereof herein
may
comprise a batch, semi-batch, semi-continuous, continuous stirred reactor
(CSTR),
continuous, or a combination thereof
Note: Depending on the operating conditions, phases of inputs, concentrations,
or a
combination thereof, heating or cooling or separating or any combination
thereof may be
required in one or more or a combination of the steps or parts of one or more
or a
combination of embodiments.
1001391 Additional Notes:
Note: Some embodiments may employ equipment comprising materials compatible
with one
or more or a combination of the following: 502, CO2, or H20, or sulfur, or
sulfur derivatives
or one or more of the fuels (if any) employed in heating and/or their
combustion products. It
may be desirable for said materials to be compatible at temperature ranges of
operation.
Note: In some embodiments, it may be desirable for the CaCO3 or SO2 or CaS03
or CaO or
a combination thereof in an oxygen-free or very low oxygen environment. An
oxygen-free or
very low oxygen environment may, for example, prevent the oxidation of SO2 or
CaS03 or
other S03 salt into a SO4 salt.
Note: The present invention may be employed to regenerate CaO from CaCO3 or
similar
carbonate or bicarbonate molecules in a CO2 capture process. For example, the
present
invention may be employed in a device to capture CO2 from the air.
Note: The SO2 may be substituted with nitric acid (HNO3). Ca(NO3)2 (which may
be a
resulting byproduct) can be thermally decomposed in a similar manner to CaS03
to form
CaO and NOx or 02 or NO2 or NO or a combination thereof. NOx, NO2, or NO may
be
converted back into nitric acid through reaction with water in, for example,
the NOx+02 and
NOx+H20 reaction steps of the Ostwald process, regenerating the nitric acid in
the present
embodiment. Advantageously, Ca(NO3)2 does not oxidize in the presence of 02,
which may
enable the process to operate in an environment with the presence of 02, if
desired.
Note: The carrier gas may comprise a reactive gas if desired. For example,
steam may be
employed as a carrier gas. Advantageously, steam may condense following
calcination and
the heat generated may be recoverable and the heat generated may exceed
initial heat input to
generate steam due to, for example, the exothermic dissolution of SO2 in the
condensed steam
(water) and/or the exothermic reaction of H20 with Ca0 to produce calcium
hydroxide.
102
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: Excess water may be removed from system. Similarly, water may be added
to the
system if desired. Water removal may be conducted by for example, including,
but not
limited to, one or more or a combination of the following: forward osmosis,
decanter_
separatory funnel, coalescer, centrifuge, filter, switchable solvent, cyclone,
semi-permeable
membrane, nanofiltration, organic solvent nanofiltration, reverse osmosis,
ultrafiltration,
microfiltration, hot nanofiltration, hot ultrafiltration, distillation,
membrane distillation, flash
distillation, multi-effect distillation, mechanical vapor compression
distillation, or hybrid
systems.
[00140] Additional Notes:
Note: Sodium bicarbonate may be thermally decomposed into at least a portion
carbon
dioxide to, for example, produce sodium carbonate or sodium sesquicarbonate.
Said carbon
dioxide may be recycled internally, for example, to a carbon dioxide
absorption step. Said
carbon dioxide, may improve absorption characteristics including, but not
limited to, one or
more or a combination of the following: absorption rate, maximum carbon
dioxide loading,
absorption capacity, solution carrying capacity, sodium bicarbonate recovery
yield, sodium
bicarbonate recovery rate, or sodium bicarbonate recovery rate per a unit
volume or mass of
solution. Said carbon dioxide may increase the concentration of carbon dioxide
in one or
more or a combination of parts of the system, for example, which may be
related, including,
but not limited to, one or more or a combination of the following: carbon
dioxide solutions,
carbon dioxide gases, carbon dioxide absorption, bicarbonate salts, salts.
Note: Solutions may be passed or cycled or recycled or recirculated through a
step more than
once. Said 'passed or cycled or recycled or recirculated' may be conducted
before, for
example, proceeding to a next step. Said solutions may comprise, for example,
absorption
solutions or solutions undergoing precipitation or distillation solutions or
solution undergoing
treatment or concentrating with a membrane based process.
Note: One or more or a combination of the embodiments described herein may be
employed
as a net carbon dioxide emission negative method for permanently or semi-
permanently
sequestering carbon dioxide. For example, the sodium bicarbonate, or sodium
sesquicarbonate, or sodium carbonate or sodium hydroxide or a combination
thereof
produced by one or more embodiments may be dissolved in the ocean. Adding net
carbon
dioxide emission negative sodium bicarbonate, or sodium sesquicarbonate, or
sodium
carbonate or a combination thereof to the ocean may have multiple benefits,
which may
include, but are not limited to, one or more or a combination of the
following: permanent or
semi-permanent sequestration of carbon dioxide in the ocean; increasing the pH
of ocean
water; increasing the concentration of carbonate ions in the ocean; buffering
ocean
103
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
acidification, restoring coral reefs; restoring marine life; local
rejuvenation of marine life;
local rejuvenation of coraL rejuvenation of coral.
Note: Cooling and/or heating may be conducted at additional or different
temperatures and/or
at additional or different locations than described herein.
Note: One or more or a combination of embodiments of the present invention may
comprise
a retrofit to pre-existing processes for producing sodium bicarbonate or
sodium carbonate or
sodium hydroxide, or other alkali hydroxide, or carbonate, or bicarbonate
salts.
1001411 Additional Notes:
Note: One or more or a combination of embodiments of the present invention may
require
solid handling or solid transfer or solid storage. Solid transfer may include,
but is not limited
to, conveyor belts, screw conveyors, bucket elevators, belt conveyors,
pneumatic conveyors,
or a combination thereof Solid storage or transport or a combination thereof
may include, but
is not limited to, bin, or silo, hopper cars, bulk sacks, or other solids
shipping containers, or a
combination thereof
Note: Temperatures in one or more parts of one or more embodiments may
include, but are
not limited to, greater than, equal to, or less than one or more or a
combination of the
following in degrees Celsius: -50, -40, -30, -20, -10, 0, 5, 10, 15, 20, 25,
30 35, 40, 45, 50,
55, 60, 65, 70, 75, 80, 85, 90, 95, 100, 110, 120, 130, 140, 150, 160, 170,
180, 190, 200, 210,
220, 230, 240, 250, 260, 270, 280, 290, 300, 310, 320, 330, 340, 350, 360,
370, 380, 390,
400, 410, 420, 430, 440, 450, 460, 470, 480, 490, 500, 510, 520, 530, 540,
550, 560, 570,
580, 590, 600, 610, 620, 630, 640, 650, 660, 670, 680, 690, 700, 710, 720,
730, 740, 750,
760, 770, 780, 790, 800, 810, 820, 830, 840, 850, 860, 870, 880, 890, 900,
910, 920, 930,
940, 950, 960, 970, 980, 990, 1000, 1010, 1020, 1030, 1040, 1050, 1060, 1070,
1080, 1090,
1100, 1150, 1200, 1250, 1300, 1350, 1400, 1450, 1500, 1600, 1700, 1800, 1900,
2000, 2250,
2500, 2750, 3000
Note: Sodium may be provided as an example alkali. Other alkali metal salts or
cations may
be employed instead of or in addition to sodium. For example, potassium or
lithium or
rubidium or cesium or a combination thereof may be employed. For example,
alkali-like
cations or salts, such as ammonia or ammonium, may be employed.
Note: Ammonia may be provided as an example weak base or alkali-like cation
derivative.
Other weak bases or weak base gases may be employed instead of or in addition
to ammonia.
For example, said other weak bases may include, but are not limited to, one or
more or a
combination of the following: amines, ammonia derivatives, imines, azines, CO2
capture
absorbent cations, CO2 capture absorbents, or a combination thereof, or other
weak bases, or
other weak gases.
104
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: CO2 sources may include, but are not limited to, one or more or a
combination of the
following: Power Plant (Natural gas, coal, oil, petcoke, biofuel, municipal
waste), Cement
production, chemical production, Waste Water Treatment, Landfill gas, Air,
Metal
production/refining (such as Iron, Steel, Aluminum, etc.), Glass production,
Oil refineries,
LNG liquification, HVAC, Transportation vehicles (ships, boats, cars, buses,
trains, trucks,
airplanes), Natural Gas, Biogas, Alcohol fermentation, Volcanic Activity,
Decomposing
leaves/biomass, Septic tank, Respiration, Manufacturing facilities, Fertilizer
production, or
Geothermal processes where CO2(g) releases from a well or wells.
[00142] Additional Notes:
Note: Input CO2 vol % concentration may be greater than or equal to one or
more or a
combination of the following volume percent concentrations: 0%, or 0.001%, or
0.1%, or
0.5%, or 1%, or 1.5%, or 2%, or 2.5%, or 3%, or 3.5%, or 4%, or 4.5%, 5%, or
5.5%, or 6%,
or 6.5%, or 7%, or 7.5%, or 8%, or 8.5%, or 9%, or 9.5%, or 10%, or 10.5%, or
11%, or
11.5%, or 12%, or 12.5%, or 13%, or 13.5%, or 14%, or 14.5%, or 15%, or 20%,
or 30%, or
40%, or 50%, or 60%, or 70%, or 80%, or 90%, or 100%.
Note: A gas stream comprising CO2 may be concentrated to a greater
concentration of CO2 or
a greater partial pressure of CO2 before being absorbed or reacted in one or
more or a
combination of embodiments of the present invention. Said concentrating may be
conducted
using including, but not limited to, one or more or a combination of the
following: gas
membrane, or absorption/desorption CO2 capture, or adsorption/desorption CO2
capture, or
recirculated CO2, or desorption CO2, or CO2 from one or more or a combination
of higher
concentration CO2 sources, or condensation of non-0O2 gas, or cooling, or
heating, or
deposition, or deposition/sublimination, or cryogenic separation, or
compression, or
pressurization, electrochemical process, or ion exchange, or electrodialysis,
or fuel cell, or a
combination thereof.
Note: A gas stream comprising SO2 may be concentrated to a greater
concentration of SO2 or
a greater partial pressure of SO2 before being absorbed or reacted in one or
more or a
combination of embodiments of the present invention. Said concentrating may be
conducted
using including, but not limited to, one or more or a combination of the
following: gas
membrane, or membrane based process, or absorption/desorption SO2 capture, or
adsorption/desorption SO2 capture, or recirculated S02, or desorption S02, or
SO2 from one or
more or a combination of higher concentration SO2 sources, or condensation of
non-S02 gas,
or cooling, or heating, or deposition, or deposition/sublimination, or
cryogenic separation, or
compression, or pressurization, electrochemical process, or ion exchange, or
electrodialysis,
or fuel cell, or a combination thereof.
105
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: Absorption of a gas into a solution containing ammonia and/or absorption
of ammonia
into a solution may result in the formation of a residual or remaining gas
stream comprising
residual ammonia. Said residual or remaining gas stream may comprise, for
example,
remaining unabsorbed gases or inert gases. One or more or a combination of
embodiments
herein may employ an ammonia recovery or ammonia abatement cycle or system.
Alternately
or additionally, ammonia may be removed to ultra-low concentrations (e.g.
single or double
digit PPM concentrations) using hydrochloric acid (which may be produced by
some
embodiments herein), and/or ammonia or hydrochloric acid or both may be
recovered from
the resulting ammonium chloride using one or more or a combination of
embodiments herein.
[00143] Additional Notes:
Note: Ammonia losses may occur within one or more or a combination of
embodiments
described herein. Makeup ammonia may be provided, for example, as needed or as
desired.
Note: SO2 losses may occur within one or more or a combination of embodiments
described
herein. Makeup SO2 may be provided, for example, as needed or as desired.
Note: Losses may occur within one or more or a combination of embodiments
described
herein. Makeup reagents may be provided, for example, as needed or as desired.
Note: In some embodiments, ammonia may form at elevated temperatures. In some
embodiments, if oxygen is present, some ammonia may undergo combustion.
Ammonia
combustion products, even at residual or low concentrations, may be present in
one or more
gases or liquids or solids or a combination thereof in one or more or a
combination of
embodiments. Said ammonia combustion products may comprise, including, but not
limited
to, nitrogen oxides, or nitrogen, or nitric acid, or a derivative thereof, or
a combination
thereof Systems and methods for detecting, treating, removing, economically
using,
recovering, or a combination thereof said ammonia combustion products may be
employed.
Note: Filling, or reacting, or emptying, or a combination thereof may be
conducted
simultaneously if desired.
[00144] Notes
Note: Example alkalis may include, but are not limited to, one or more or any
combination of
the following: lithium (Li), or sodium (Na), or potassium (K), or rubidium
(Rb), or cesium
(Cs)
Note: Example alkaline earths may include, but are not limited to, one or more
or any
combination of the following: beryllium (Be), or magnesium (Mg), or calcium
(Ca), or
strontium (Sr), or barium (Ba), or radium (Ra).
Note: Calcium may comprise an example alkaline earth. Other alkaline earths
may be
employed in addition to or instead of calcium where calcium is described
herein. For
106
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
example, in some embodiments, calcium may comprise mixtures of calcium and
magnesium,
or calcium may instead comprise magnesium.
[00145] Additional Notes:
Note: Sodium may comprise an example alkali. Other alkalis may be employed in
addition to
or instead of sodium where sodium is described herein. For example, in some
embodiments,
Sodium may comprise mixtures of sodium and potassium, or sodium and lithium,
or
potassium, or lithium, or any combination thereof
Note: An alkaline earth cation ¨ weak acid anion salt may include, but is not
limited to,
alkaline earth cation salts with one or more or any combination of the
following anions:
carbonate, or bicarbonate, or sulfite, or sulfide, or silicate, or ferrate, or
aluminate, or ferrite,
or a silicate, or silicon derivative, or a carboxylic acid salt, or a ferrate
salt, or an aluminate
salt, or a zincate salt, or an iron derivative salt, or a manganese derivative
salt, or a zinc
derivative salt, or an aluminum derivative salt, or transition metal oxide
anion, or metal oxide
anion, or organic acid, or carboxylic acid, or phosphor acid, or anion of an
acid weaker than
sulfuric acid, or anion of an acid weaker than nitric acid, or an anion of an
acid weaker than
sulfurous acid.
Note: Heat produced from the reaction of calcium oxide with water to form
calcium
hydroxide may be utilized. For example, said heat may be employed within
separation steps,
or distillation steps, or drying steps, or calcining steps, or decomposition
steps, or gas
liberating steps, or any combination thereof within the invention. For
example, said heat may
be utilized in an external application.
Note: Heat produced from the combustion or conversion of hydrogen sulfide, or
the
production of sulfuric acid, or exothermic reactions comprising sulfur
chemicals, or any
combination thereof may be utilized. For example, said heat may be employed
within
separation steps, or distillation steps, or drying steps, or calcining steps,
or decomposition
steps, or gas liberating steps, or any combination thereof within the
invention. For example,
said heat may be utilized in an external application.
Note: In some embodiments, sodium sulfate may be produced by the reaction of
sodium
chloride with sulfuric acid or sulfur dioxide or oxygen or any combination
thereof, which
may produce hydrochloric acid and sodium sulfate.
o 2 NaCl + H2SO4 ¨> 2 HCl + Na2SO4
o 4 NaCl + 2 SO2 + 02+2 H20 ¨> 4 HC1+ 2 Na2SO4
Note: Sodium sulfate may be produced by mining of sodium sulfate deposits or
extraction of
sodium sulfate from natural resources.
[00146] Additional Notes:
107
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: In some embodiments, sodium hydroxide, or sodium carbonate, or sodium
sesquicarbonate, or sodium bicarbonate, or any combination thereof may be
added to an
ocean or sea to, for example_ including, but not limited to, one or more or
any combination of
the following: increase the pH, or increase the local pH, or provide a high
quality mechanism
to permanently absorb carbon dioxide from the air, or to increase the local pH
to improve
health of marine ecosystems and corals, or improve biomass production, or
improve
productivity of a fishery, or facilitate tourism, or grow a local economy, or
to improve the
health of the ocean, or the prevent or combat algae blooms or cyanobacteria
blooms, or any
combination thereof.
Note: Sodium sulfate may be a byproduct in the production of, including, but
not limited to,
lithium carbonate, or chelating agents, or resorcinol, or ascorbic acid, or
silica pigments, or
nitric acid, or phenol, or any combination thereof
Note: The present invention may comprise a process for recycling sodium or
sodium
carbonate in the production of lithium or lithium carbonate.
Note: In some embodiments, sodium sulfate may be added directly to the aqueous
calcium
bisulfite as solid sodium sulfate
Note: In some embodiments, sodium sulfate may be dissolved in at least a
portion of the
water and the resulting aqueous solution comprising aqueous sodium sulfate is
mixed with an
aqueous solution comprising calcium bisulfite
Note: A portion of gaseous sulfur dioxide may form during the formation of
sodium
metabisulfite from aqueous sodium bisulfite. For example, excess aqueous
sulfur dioxide
may be present in the aqueous sodium bisulfite solution and a portion of said
aqueous sulfur
dioxide may desorb during the formation of sodium metabisulfite solid. For
example, a
portion of bisulfite may decompose to form gaseous sulfur dioxide during the
formation of
sodium metabisulfite.
Note: Forming sodium metabisulfite from an aqueous solution comprising sodium
bisulfite
may involve, including, but not limited to, one or more or a combination of
the following:
removing water, or precipitation, or crystallization, or cryodesalination, or
freezing
desalination, or distillation, or membrane based process, or forward osmosis,
or reverse
osmosis, or multi effect distillation, or mechanical vapor compression
distillation, or
multistage flash distillation, or membrane distillation, or heat recovery
distillation, or zero
liquid discharge
1001471 Additional Notes:
1 Og
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: Use raw minerals of sodium sulfate as the input material, rather than
processed sodium
sulfate, because the price of these materials are practically free. These
minerals are listed
below:
o haps://en.m, wikipecii a. orpjwiki/Mirabiiite
o ht-tns://en.m.wilciodia.ongwikifThenardite
Note: Alternatively, "Sodium sulfate is produced on a very large scale as a by-
product of
several important industrial processes. In many cases, disposal of this
material is difficult".
In some embodiments, the present invention may be co-located with a process
where sodium
sulfate is produced as a product, or byproduct, or waste product.
Note: Sodium sulfate is known to be a very significant waste product of the
lithium
production industry. In some lithium production applications, sodium hydroxide
is reacted
with lithium sulfate produced from a roasting process to recover lithium,
which may result in
the production of a sodium sulfate product. Some embodiments of the present
invention may
enable recycling of sodium sulfate into sodium hydroxide or sodium carbonate.
Note: Sodium hydroxide may facilitate hydrogen production.
Note: Some embodiments may involve reacting a material comprising calcium and
/ or
magnesium with supercritical, or liquid, or gaseous, or any combination
thereof sulfur
dioxide to form calcium sulfite and / or magnesium sulfite, then contacting at
least a portion
of the formed calcium sulfite and / or magnesium sulfite with water to form at
least a portion
of dissolve magnesium sulfite.
Note: MgCa(CO3)2(s) may comprise a solid comprising a mixture of calcium and
magnesium
salts. MgCa(CO3)2(s) may comprise, for example, including, but not limited to,
limestone or
dolomite. Alternatively, or additionally. MgCa(CO3)2(s) may comprise a portion
of
magnesium silicate or magnesium aluminate or magnesium ferrate. Alternatively,
or
additionally, MgCa(CO3)2(s) may comprise a portion of calcium silicate or
calcium aluminate
or calcium ferrate.
Note: In some embodiments, a solvent other than or in addition to water may be
employed.
For example, an organic solvent or inorganic solvent may be present in
solution. For
example, a glycol, or an alcohol, or a sugar alcohol may be present. For
example, an organic
solvent or a solvent other than water. For example, ammonia or urea may be
present in
solution.
Note: Concentration of aqueous magnesium sulfite in a solution comprising
aqueous
magnesium sulfite may be greater than or equal to one or more or a combination
of the
following: 0.025 g/L, or 0.05 g/L, or 0.1 g/L, or 0.2 g/L, or 0.3 g/L, 0.4
g/L, or 0.5 g/L, or 0.6
109
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
g/L, or 0.7 g/L, or 0.8 g/L, or 0.9 g/L, or 1.0 g/L, or 1.1 g/L, or 1.2 g/L,
or 1.3 g/L, or 1.4 g/L,
or 1.5 g/L. or 1.6 g/L, or 1.7 g/L, or 1.8 g/L, or 1.9 g/L, or 2 g/L
[00148] Additional Notes:
Note: `g/L= may comprise grams of solute per liter of solution. For example, 1
g/L of
magnesium sulfate may comprise a solution with 1 gram of dissolved magnesium
sulfite per
liter of total solution.
Note: Temperature of at least a portion of concentrating with reverse osmosis
or forward
osmosis or both may be greater than or equal to one or more or a combination
of the
following: 0 C, or 5 C, or 10 C, or 15 C, or 20 C, or 25 C, or 30 C, or 35 C,
or 40 C, or
45 C, or 50 C, or 55 C, or 60 C, or 65 C, or 70 C, or 75 C, or 80 C, or 85
C, or 90 C, or
95 C, or 100 C, or 105 C, or 110 C, or 115 C
Note: Temperature of calcining at least a portion of calcium sulfite, or
magnesium sulfite, or
both may be greater than or equal to one or more or a combination of the
following: 500 C,
or 550 C, or 600 C, or 650 C, or 700 C, or 750 C, or 775 C, or 800 C or 825
C, or 850
C, or 875 C, or 900 C.
Note: Temperature of drying or dehydrating or both may be less than or equal
to one or more
or a combination of the following: 800 C, or 750 C, or 700 C, or 650 C, or
600 C, or 550
C, or 500 C, or 450 C, or 400 C, or 350 C, or 300 C, or 250 C, or 200 C, or
150 C, or
100 C
Note: The partial pressure of captured carbon dioxide produced by one or more
or a
combination of embodiments may be greater than or equal to one or more or a
combination of
the following: 0.05 atm, or 0.1 atm, or 0.2 atm, or 0.3 atm, or 0.4 atm, or
0.5 atm, or 0.6 atm,
or 0.7 atm, or 0.8 atm, or 0.9 atm, or 1 atm, or 1.1 atm, or 1.2 atm, or 1.3
atm, or 1.4 atm, or
1.5 atm, or 1.6 atm, or 1.7 atm, or 1.8 atm, or 1.9 atm, or 2.0 atm, or 2.25
atm, or 2.5 atm, or
2.75 atm, or 3 atm, or 4 atm, or 5 atm, or 6 atm, or 7 atm, or 8 atm, or 9
atm. or 10 atm, or
12.5 atm, or 15 atm, or 17.5 atm, or 20 atm, or 25 atm, or 30 atm, or 35 atm,
or 40 atm, or 45
atm, or 50 atm
Note: The concentration of captured carbon dioxide produced by the process may
comprise a
volume percent concentration of carbon dioxide which may include, but is not
limited to,
greater than, or equal to, one or more or a combination of the following: 50%,
or 50.5%, or
51%, or 51.5%, or 52%, or 52.5%, or 53%, or 53.5%, or 54%, or 54.5%, or 55%,
or 55.5%,
or 56%, or 56.5%, or 57%, or 57.5%, or 58%, or 58.5%, or 59%, or 59.5%, or
60%, or
60.5%, or 61%, or 61.5%, or 62%, or 62.5%, or 63%, or 63.5%, or 64%, or 64.5%,
or 65%,
or 65.5%, or 66%, or 66.5%, or 67%, or 67.5%, or 68%, or 68.5%, or 69%, or
69.5%, or
70%, or 70.5%, or 71%, or 71.5%, or 72%, or 72.5%, or 73%, or 73.5%, or 74%,
or 74.5%,
110
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
or 75%, or 75.5%, or 76%, or 76.5%, or 77%, or 77.5%, or 78%, or 78.5%, or
79%, or
79.5%, or 80%, or 80.5%, or 81%, or 81.5%, or 82%, or 82.5%, or 83%, or 83.5%,
or 84%,
or 84.5%, or 85%, or 85.5%, or 86%, or 86.5%, or 87%, or 87.5%, or 88%, or
88.5%, or
89%, or 89.5%, or 90%, or 90.5%, or 91%, or 91.5%, or 92%, or 92.5%, or 93%,
or 93.5%,
or 94%, or 94.5%, or 95%, or 95.5%, or 96%, or 96.5%, or 97%, or 97.5%, or
98%, or
98.5%, or 99%, or 99.5%
[00149] Additional Notes:
Note: The concentration of captured carbon dioxide produced by the process may
comprise a
volume percent concentration of carbon dioxide which may include, greater
than, or equal to,
one or more or a combination of the following: 0.5%, or 1%, or 1.5%, or 2%, or
2.5%, or 3%,
or 3.5%, or 4%, or 4.5%, or 5%, or 5.5%, or 6%, or 6.5%, or 7%, or 7.5%, or
8%, or 8.5%, or
9%, or 9.5%, or 10%, or 10.5%, or 11%, or 11.5%, or 12%, or 12.5%, or 13%, or
13.5%, or
14%, or 14.5%, or 15%, or 15.5%, or 16%, or 16.5%, or 17%, or 17.5%, or 18%,
or 18.5%,
or 19%, or 19.5%, or 20%, or 20.5%, or 21%, or 21.5%, or 22%, or 22.5%, or
23%, or
23.5%, or 24%, or 24.5%, or 25%, or 25.5%, or 26%, or 26.5%, or 27%, or 27.5%,
or 28%,
or 28.5%, or 29%, or 29.5%, or 30%, or 30.5%, or 31%, or 31.5%, or 32%, or
32.5%, or
33%, or 33.5%, or 34%, or 34.5%, or 35%, or 35.5%, or 36%, or 36.5%, or 37%,
or 37.5%,
or 38%, or 38.5%, or 39%, or 39.5%, or 40%, or 40.5%, or 41%, or 41.5%, or
42%, or
42.5%, or 43%, or 43.5%, or 44%, or 44.5%, or 45%, or 45.5%, or 46%, or 46.5%,
or 47%,
or 47.5%, or 48%, or 48.5%, or 49%, or 49.5%, or 50%, or 50.5%, or 51%, or
51.5%, or
52%, or 52.5%, or 53%, or 53.5%, or 54%, or 54.5%, or 55%, or 55.5%, or 56%,
or 56.5%,
or 57%, or 57.5%, or 58%, or 58.5%, or 59%, or 59.5%, or 60%, or 60.5%, or
61%, or
61.5%, or 62%, or 62.5%, or 63%, or 63.5%, or 64%, or 64.5%, or 65%, or 65.5%,
or 66%,
or 66.5%, or 67%, or 67.5%, or 68%, or 68.5%, or 69%, or 69.5%, or 70%, or
70.5%, or
71%, or 71.5%, or 72%, or 72.5%, or 73%, or 73.5%, or 74%, or 74.5%, or 75%,
or 75.5%,
or 76%, or 76.5%, or 77%, or 77.5%, or 78%, or 78.5%, or 79%, or 79.5%, or
80%, or
80.5%, or 81%, or 81.5%, or 82%, or 82.5%, or 83%, or 83.5%, or 84%, or 84.5%,
or 85%,
or 85.5%, or 86%, or 86.5%, or 87%, or 87.5%, or 88%, or 88.5%, or 89%, or
89.5%, or
90%, or 90.5%, or 91%, or 91.5%, or 92%, or 92.5%, or 93%, or 93.5%, or 94%,
or 94.5%,
or 95%, or 95.5%, or 96%, or 96.5%, or 97%, or 97.5%, or 98%, or 98.5%, or
99%, or
99.5%, or 100%
[00150] Additional Notes:
Note: 'A portion': A portion may comprise a part of a stream or material, or
all of a stream or
material. A portion may include, but is not limited to, less than, or greater
than, or equal to,
one or more or a combination of the following: 0%, or 0.5%, or 1%, or 1.5%, or
2%, or 2.5%,
111
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
or 3%, or 3.5%, or 4%, or 4.5%, or 5%, or 5.5%, or 6%, or 6.5%, or 7%, or
7.5%, or 8%, or
8.5%, or 9%, or 9.5%, or 10%, or 10.5%, or 11%, or 11.5%, or 12%, or 12.5%, or
13%, or
13.5%, or 14%, or 14.5%, or 15%, or 15.5%, or 16%, or 16.5%, or 17%, or 17.5%,
or 18%,
or 18.5%, or 19%, or 19.5%, or 20%, or 20.5%, or 21%, or 21.5%, or 22%, or
22.5%, or
23%, or 23.5%, or 24%, or 24.5%, or 25%, or 25.5%, or 26%, or 26.5%, or 27%,
or 27.5%,
or 28%, or 28.5%, or 29%, or 29.5%, or 30%, or 30.5%, or 31%, or 31.5%, or
32%, or
32.5%, or 33%, or 33.5%, or 34%, or 34.5%, or 35%, or 35.5%, or 36%, or 36.5%,
or 37%,
or 37.5%, or 38%, or 38.5%, or 39%, or 39.5%, or 40%, or 40.5%, or 41%, or
41.5%, or
42%, or 42.5%, or 43%, or 43.5%, or 44%, or 44.5%, or 45%, or 45.5%, or 46%,
or 46.5%,
or 47%, or 47.5%, or 48%, or 48.5%, or 49%, or 49.5%, or 50%, or 50.5%, or
51%, or
51.5%, or 52%, or 52.5%, or 53%, or 53.5%, or 54%, or 54.5%, or 55%, or 55.5%,
or 56%,
or 56.5%, or 57%, or 57.5%, or 58%, or 58.5%, or 59%, or 59.5%, or 60%, or
60.5%, or
61%, or 61.5%, or 62%, or 62.5%, or 63%, or 63.5%, or 64%, or 64.5%, or 65%,
or 65.5%,
or 66%, or 66.5%, or 67%, or 67.5%, or 68%, or 68.5%, or 69%, or 69.5%, or
70%, or
70.5%, or 71%, or 71.5%, or 72%, or 72.5%, or 73%, or 73.5%, or 74%, or 74.5%,
or 75%,
or 75.5%, or 76%, or 76.5%, or 77%, or 77.5%, or 78%, or 78.5%, or 79%, or
79.5%, or
80%, or 80.5%, or 81%, or 81.5%, or 82%, or 82.5%, or 83%, or 83.5%, or 84%,
or 84.5%,
or 85%, or 85.5%, or 86%, or 86.5%, or 87%, or 87.5%, or 88%, or 88.5%, or
89%, or
89.5%, or 90%, or 90.5%, or 91%, or 91.5%, or 92%, or 92.5%, or 93%, or 93.5%,
or 94%,
or 94.5%, or 95%, or 95.5%, or 96%, or 96.5%, or 97%, or 97.5%, or 98%, or
98.5%, or
99%, or 99.5%, or 100%
Note: Calcining may involve thermally decomposing calcium sulfite and / or
magnesium
sulfite into calcium oxide and / or magnesium oxide. Calcining may involve
thermally
decomposing calcium carbonate and / or magnesium carbonate into calcium oxide
and / or
magnesium oxide.
Note: In some embodiments, calcium sulfite and magnesium sulfite may be
calcined
separately. For example, in some embodiments, calcium sulfite may be calcined
in a separate
kiln than magnesium sulfite. For example, in some embodiments, calcium sulfite
may be
calcined in the same kiln as magnesium sulfite, although in different
locations within the
same kiln. For example, in some embodiments, calcium sulfite may be calcined
in the same
kiln as magnesium sulfite, although at different times.
Note: In some embodiments, calcium sulfite and magnesium sulfite may be
calcined in the
same kiln. For example, a material may comprise both calcium sulfite and
magnesium sulfite,
and said material comprising both calcium sulfite and magnesium sulfite may be
calcined.
112
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
For example, a separate calcium sulfite and magnesium sulfite may be mixed and
may be
calcined in the same kiln as a mixture.
Note: Some embodiments may involve using an input material comprising a salt
of calcium
and / or magnesium and a weak acid, wherein said weak acid comprises a weak
acid anion
other than a carbon dioxide derivative, or other than a carbonate. For
example, said weak acid
anion other than a carbon dioxide derivative may comprise, including, but not
limited to, one
or more or a combination of the following: a sulfide, or silicon derivative,
or silicate, or
aluminate. or ferrate, or ferrite, or iron, or zinc, or aluminum, or
manganese, or copper, or a
combination thereof.
[00151] Additional Notes:
Note: In some embodiments, a material comprising calcium and / or magnesium
may
comprise calcium silicate or magnesium silicate or both. In some embodiments,
a material
comprising calcium and / or magnesium may comprise, for example, including,
but not
limited to, cement, or concrete, or waste concrete, or steel slag, or iron
slag, or slag, or a
combination thereof
Note: If non-calcium of non-magnesium metals dissolve or react with SO2 or
sulfurous acid,
these minerals may be separated before or after separation of calcium sulfite
or magnesium
sulfite or both. If these non-calcium of non-magnesium metal salts are still
dissolved, they
may be separated by precipitation, or systems and / or methods for zero liquid
discharge, or a
combination thereof
Note: Employ the calcium oxide produced by the present invention as an input
to the Solvay
to make reduced CO2 emissions sodium carbonate and sodium bicarbonate. Calcium
oxide is
used in the Solvay process to remove chloride from ammonium chloride.
Note: Some embodiments may employ high temperature steam in the calcination
process. In
some embodiments, it may be desirable for the temperature of the steam to be
greater than the
decomposition temperature or decomposition temperature range of calcium
hydroxide. At
least a portion of the steam may be condensed after forming a mixture with
sulfur dioxide. If
steam is employed, it must be contacted at a temperature greater than the
decomposition
temperature of calcium hydroxide.
Note: Sulfur dioxide may be separated or recovered by cryogenic separation, or
freezing
separation, or liquification separation, or condensing separation, or
deposition separation, or a
combination thereof The resulting liquid or solid or supercritical SO2 may be
added to water
or sulfurous acid solution to form or maintain concentrated or 'excess'
sulfurous acid.
Alternatively or additionally, the resulting liquid or solid or supercritical
SO2 may be reacted
with directly with the material comprising calcium and / or magnesium.
113
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
[00152] Additional Notes:
Note: Thermally decompose calcium sulfite in an electric kiln
Note: Thermally decompose calcium sulfite in a natural gas or coal or both
kiln.
Note: Thermally decompose calcium sulfite using a hydrogen fuels system. If
hydrogen is
used for heat, there may be no CO2 emissions in the end to end process. Also,
green
hydrogen can be produced from solar energy and stored, eliminating the
challenge of solar
intermittency. Alternatively or additionally, hydrogen may be blue hydrogen,
or hydrogen
from natural gas, where the carbon or CO2 is removed from the natural gas to
produce
hydrogen before hydrogen is burned. Alternatively, a process may employ a
combination of
blue hydrogen (during the night) and solar electricity (during the day).
Note: Some embodiments may employ a hydrogen powered kiln. In some
embodiments, the
resulting water vapor may be condensed to form sulfurous acid. In some
embodiments where
combustion is employed to power the calcining and said combustion forms water,
it may be
desirable for a portion of said water to be condensed to form at least a
portion of aqueous
sulfur dioxide.
Note: 'Aqueous sulfur dioxide' and 'sulfurous acid' may be employed
interchangeably
Note: Recovery heat form hydrating calcium oxide to calcium hydroxide to
provide heat or
steam or both for applications requiring heat
Note: Remaining flue gas after most or all SO2 is removed or recovered may
comprise at
least a portion CO2.
Note: Flue gas or CO2 from the flue gas may be employed as a CO2 input or CO2
source for
a Solvay process to produce Sodium Bicarbonate or Sodium Carbonate. The Solvay
process
calcium oxide will be sourced from the present invention.
Note: Flue gas CO2 may be concentrated with pressure swing absorption or
pressure swing
adsorption or gas membrane or both, then the flue gas with higher
concentrations of CO2
may be employed as a feedstock for the production of sodium bicarbonate or
sodium
carbonate.
[00153] Additional Notes:
Note: Convert calcium silicate from the Pidgeon process to calcium oxide or
recovery
calcium oxide from the Pidgeon process
Note: a process for enabling full conversion of calcium carbonate or calcium
silicate or a
combination thereof to calcium oxide: CO2 production process from calcium
carbonate,
where the first step is to react Calcium Carbonate with equal to or less than
stoichiometric
amounts of sulfurous acid or with low vapor pressure sulfurous acid. The
calcium sulfite
solid is separated from this solution using solid-liquid separation. Then the
resulting solid
114
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
calcium sulfite (which may still comprise at least a portion calcium
carbonate) is transferred
to a step where it is dissolved in excess concentrated sulfurous acid, forming
dissolve calcium
bisulfate and CO2 from any unreacted calcium carbonate. Remaining CO2 is
separated from
the SO2 gas atmosphere by, for example, condensation of at least a portion of
SO2 and/or a
combination of other systems and / or methods. Any non-calcium sulfite or
calcium carbonate
(e.g. calcium sulfate or silica or other mostly insoluble chemicals) may
remain as a solid and
may be separated from the calcium bisulfite solution via liquid-solid
separation.
Note: May employ calcium, or magnesium, or alkaline earth, or a combination
thereof
Calcium or magnesium or alkaline earth may be substituted.
Note: In some embodiments, gas comprising sulfur dioxide may be compressed
prior to or
during absorption of sulfur dioxide in one or more or a combination of process
steps
described herein.
Note: In some embodiments, it may be desirable to avoid the formation of
dissolved calcium
bisulfite. In some embodiments, the formation of calcium bisulfite may be
prevented by
employing an organic solvent, or a non-water solvent, or both instead of or in
addition to
water as a solvent to absorb sulfur dioxide and / or react sulfur dioxide with
calcium
carbonate, or magnesium carbonate, or calcium silicate, or magnesium silicate,
or a calcium-
'WA' salt, or a magnesium-'WA' salt, or a combination thereof. The absence of
water, or a
lower concentration of water, or the presence of other solvents than water, or
a combination
thereof may inhibit the formation of dissolved calcium bisulfite in, for
example, some
embodiments where it is desired.
Note: 'WA' may comprise a weak acid. For example, 'WA' may comprise an acid
with
acidity less than or equal to sulfurous acid.
1001541 Additional Notes:
Note: A calcium silicate, or magnesium silicate, or both may comprise at least
a portion
calcium carbonate in some embodiments.
Note: One or more or a combination of reagents, or process steps, or a
combination thereof
may be heated, or cooled, or a combination thereof
Note: Calcium silicate may comprise a material comprising silicate. A material
comprising
an impure limestone comprising at a portion a silicate material. For example,
a material
comprising silicate may comprise clay, or silicon dioxide, or alumino-
silicate, or ferrite, or a
combination thereof.
Note: Calcining of calcium sulfite may be conducted in the presence of clay,
or silicon
dioxide, or shale, or sand, or iron ore, or bauxite, or fly ash, and or slag
or other materials
115
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
employed to, for example, produce or facilitate the production of cement, or
cement clinker,
or a combination thereof
Note: In some embodiments, it may be desirable to operate the calcination of
calcium sulfite
and / or cement manufacturing inputs in the presence of diatomic oxygen. For
example, in
some embodiments, diatomic oxygen present in a flue gas stream, or in hot
gases entering or
within a kiln, or a combination thereof may react or oxidize sulfur dioxide,
or calcium sulfite,
or derivatives thereof to form materials or chemicals which may be facilitate
the
manufacturing of cement or clinker or may enable advantageous properties in
the cement or
clinker. For example, in some embodiments, diatomic oxygen present in a flue
gas stream, or
in hot gases entering or within a kiln, or a combination thereof may react or
oxidize sulfur
dioxide, or calcium sulfite, or derivatives thereof to form calcium sulfate
and / or derivatives
thereof, which may be an advantageous ingredient or component of some cement
or clinker
compositions. For example, in some embodiments, diatomic oxygen present in a
flue gas
stream, or in hot gases entering or within a kiln, or a combination thereof
may react or
oxidize sulfur dioxide, or calcium sulfite, or derivatives thereof to form
compounds or
materials comprising sulfur with superior strength, or chemical resistance, or
longevity, or
pressure, or compressive strength, or water resistance, or temperature
resilience, or other
resilience, or cost, or adhesive properties, or chemical compatibility, or a
combination
thereof For example, in some embodiments, diatomic oxygen present in a flue
gas stream, or
in hot gases entering or within a kiln, or a combination thereof may react
with or oxidize
sulfur dioxide, or calcium sulfite, or derivatives thereof to form compounds
or materials with
superior strength, or chemical resistance, or longevity, or pressure, or
compressive strength,
or water resistance, or temperature resilience, or other resilience, or cost,
or adhesive
properties, or chemical compatibility, or a combination thereof For example,
in some
embodiments, diatomic oxygen present in a flue gas stream, or in hot gases
entering or within
a kiln, or a combination thereof may react with or oxidize sulfur dioxide, or
calcium sulfite,
or derivatives thereof to produce heat, which may reduce energy requirements
or increase the
energy efficiency of calcining.
Note: In some embodiments, the use of calcium sulfite as an input material for
the production
of cement may enable cement with superior properties, which may include, but
are not
limited to, superior strength, or chemical resistance, or longevity, or
pressure, or compressive
strength, or water resistance, or temperature resilience, or other resilience,
or cost, or
adhesive properties, or chemical compatibility, or a combination thereof
[00155] Additional Notes:
116
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: In some embodiments, calcium silicate may comprise cement manufacturing
inputs. In
some embodiments, cement manufacturing inputs may comprise calcium silicate.
In some
embodiments, cement manufacturing inputs may comprise calcium sulfite_ or
calcium oxide,
or a combination thereof In some embodiments, cement manufacturing inputs may
comprise
calcium bisulfite.
Note: Weak acids and weak acid anions may include, but are not limited to, one
or more or a
combination of the following: silicates, or carbonates, or aluminates, or
aluminoferrites, or
aluminum oxides, or zinc oxides, or iron oxides, or A1206, or Al2Fe201o.
Note: In some embodiments, at least a portion of the gases produced during or
from the
calcination of calcium sulfite may comprise water or water vapor. For example,
if hydrogen,
or natural gas, or ammonia, or a hydrocarbon, or other combustion, or steam,
or a
combination thereof is / are employed to provide heat for calcination, water
vapor may be
generated. In some embodiments, at least a portion of said gases produced
during or from the
calcination of calcium sulfite may be condensed to form an aqueous solution
comprising
aqueous sulfur dioxide or sulfurous acid. In some embodiments, said aqueous
solution
comprising aqueous sulfur dioxide or sulfurous acid may be employed as an
aqueous sulfur
dioxide solution or sulfurous acid solution in one or more process steps. In
some
embodiments, said aqueous solution comprising aqueous sulfur dioxide or
sulfurous acid may
undergo further concentrating, or diluting, or treating, or a combination
thereof before being
employed as an aqueous sulfur dioxide solution or sulfurous acid solution in
one or more
process steps.
Note: In some embodiments, nitrogen gas may be added to air before combustion
with said
air to reduce the concentration of oxygen before said air may be employed in
the combustion
of fuel for calcining calcium sulfite. For example, a nitrogen concentrating
process may be
employed. For example, an oxygen concentrating or oxygen removal process may
be
employed.
[00156] Additional Notes:
Note: In some embodiments, at least a portion of oxygen may be removed from
air before
combustion with said air to reduce the concentration of oxygen before said air
may be
employed in the combustion of fuel for calcining calcium sulfite. For example,
a nitrogen
concentrating process may be employed. For example, an oxygen concentrating or
oxygen
removal process may be employed.
Note: In some embodiments, a portion of gases after combustion and after
sulfur dioxide
removal may be added to air to reduce the concentration of diatomic oxygen
before said air
may be employed in the combustion of fuel for calcining calcium sulfite.
117
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: In some embodiments, sulfur dioxide or carbon dioxide or both may be
added to air to
reduce the concentration of diatomic oxygen before said air may be employed in
the
combustion of fuel for calcining calcium sulfite.
Note: It may be desirable to calcine the calcium sulfite under conditions
where the
temperature is sufficiently low to prevent produced CaO crystallites from
fusing. It may be
desirable to calcine calcium sulfite under conditions and temperatures where
the specific
surface of the calcium oxide remains intact. It may be desirable to produce
CaO with non-
fused crystals, or where the specific surface of the calcium oxide remains
intact, or a
combination thereof for applications, which may include, but are not limited
to, the steel
industry.
=Note: It may be desirable to calcine the calcium sulfite under conditions
where the
temperature is sufficiently high to facilitate the production of fused CaO
crystallites. It may
be desirable to calcine calcium sulfite under conditions and temperatures
which reduce the
specific surface of the calcium oxide. It may be desirable to produce CaO with
fused crystals,
or where the specific surface of the calcium oxide is reduced, or a
combination thereof for
applications, which may include, but are not limited to, the production of
aerated concrete, or
sand lime bricks, or a combination thereof
Note: Heat sources may include, but are not limited to, one or more or a
combination of the
following: combustion of a fuel, hydrogen, ammonia, natural gas, heavy fuel
oil, pulverized
coal, liquefied gas, off-gas from steel-making process, wood dust, waste oil,
biomass, biofuel,
electricity, heat pump, solar thermal, chemical reaction, sulfur, sulfurous
fuel, sulfuric acid
production, salt production, waste heat, waste gases, nuclear heat,
geothermal, quicklime,
hydration reaction, oxidation.
1001571 Additional Notes:
Note: One or more of the present embodiments may produce strongly carbon
dioxide
negative or negative emissions calcium oxide
Note: In some embodiments, produced calcium oxide may be reacted with carbon
dioxide
originating from the air or separated from the air. For example, calcium oxide
may be reacted
with sodium carbonate or potassium carbonate or sodium carbonate or potassium
carbonate
solution to produce sodium hydroxide or potassium hydroxide solution and
calcium
carbonate, which may be a permanent sequestration byproduct. Said sodium
hydroxide or
potassium hydroxide solution may then be contacted with air or CO2 originating
from air to
produce a solution comprising sodium carbonate, or potassium carbonate, or a
combination
thereof
11s
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: A portion of the calcium oxide produced may be converted to calcium
carbonate by
reaction, with, for example, carbon dioxide in the air, or carbon dioxide
originating from the
air, or an air capture process, or regenerating an alkali-carbonate to an
alkali-oxide in an
absorption loop, or regenerating an alkali-carbonate to an alkali-oxide in an
absorption or
separation process, or a combination thereof.
Note: A portion of the cement produced may be employed in the production of
non-hydraulic
cement, or cement employing at least a portion of CO2 input, or a combination
thereof to
increase the net CO2 removal or emissions reduction.
Note: Magnesium and calcium may be present in the same input material. For
example,
slags, or waste concrete, or minerals may comprise at least a portion of
magnesium. For
example, dolomite may comprise a portion of magnesium. In some embodiments, at
least a
portion of magnesium sulfite and / or magnesium oxide and / or magnesium
hydroxide may
be produced separately from calcium sulfite and / or calcium oxide and / or
calcium
hydroxide. For example, the separation of calcium and magnesium may be
conducted by
including, but not limited to, the significant difference in solubility in
water between
magnesium sulfite and calcium sulfite and / or the significant temperature
dependent
solubility of magnesium sulfite.
[00158] Additional Notes:
Note: The concentration of sulfur dioxide in aqueous sulfur dioxide may be
greater than or
equal to one or more of the following weight percent concentrations: 0.0001%,
or 0.5%, or
1%, or 1.5%, or 2%, or 2.5%, or 3%, or 3.5%, or 4%, or 4.5%, or 5%, or 5.5%,
or 6%, or
6.5%, or 7%, or 7.5%, or 8%, or 8.5%, or 9%, or 9.5%, or 10%, or 10.5%, or
11%, or 11.5%,
or 12%, or 12.5%, or 13%, or 13.5%, or 14%, or 14.5%, or 15%, or 15.5%, or
16%, or
16.5%, or 17%, or 17.5%, or 18%, or 18.5%, or 19%, or 19.5%, or 20%, or 20.5%,
or 21%,
or 21.5%, or 22%, or 22.5%, or 23%, or 23.5%, or 24%, or 24.5%, or 25%, or
25.5%, or
26%, or 26.5%, or 27%, or 27.5%, or 28%, or 28.5%, or 29%, or 29.5%, or 30%,
or 30.5%,
or 31%, or 31.5%, or 32%, or 32.5%, or 33%, or 33.5%, or 34%, or 34.5%, or
35%, or
35.5%, or 36%, or 36.5%, or 37%, or 37.5%, or 38%, or 38.5%, or 39%, or 39.5%,
or 40%,
or 40.5%, or 41%, or 41.5%, or 42%, or 42.5%, or 43%, or 43.5%, or 44%, or
44.5%, or
45%, or 45.5%, or 46%, or 46.5%, or 47%, or 47.5%, or 48%, or 48.5%, or 49%,
or 49.5%,
or 50%, or 50.5%, or 51%, or 51.5%, or 52%, or 52.5%, or 53%, or 53.5%, or
54%, or
54.5%, or 55%, or 55.5%, or 56%, or 56.5%, or 57%, or 57.5%, or 58%, or 58.5%,
or 59%,
or 59.5%, or 60%, or 60.5%, or 61%, or 61.5%, or 62%, or 62.5%, or 63%, or
63.5%, or
64%, or 64.5%, or 65%, or 65.5%, or 66%, or 66.5%, or 67%, or 67.5%, or 68%,
or 68.5%,
or 69%, or 69.5%, or 70%, or 70.5%, or 71%, or 71.5%, or 72%, or 72.5%, or
73%, or
119
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
73.5%, or 74%, or 74.5%, or 75%, or 75.5%, or 76%, or 76.5%, or 77%, or 77.5%,
or 78%,
or 78.5%, or 79%, or 79.5%, or 80%, or 80.5%, or 81%, or 81.5%, or 82%, or
82.5%, or
83%, or 83.5%, or 84%, or 84.5%, or 85%, or 85.5%, or 86%, or 86.5%, or 87%,
or 87.5%,
or 88%, or 88.5%, or 89%, or 89.5%, or 90%, or 90.5%, or 91%, or 91.5%, or
92%, or
92.5%, or 93%, or 93.5%, or 94%, or 94.5%, or 95%, or 95.5%, or 96%, or 96.5%,
or 97%,
or 97.5%, or 98%, or 98.5%, or 99%, or 99.5%, or 99.999%
Note: The concentration of sulfur dioxide gas in a gas comprising sulfur
dioxide may be
greater than or equal to one or more of the following volume percent
concentrations:
0.0001%, or 0.5%, or 1%, or 1.5%, or 2%, or 2.5%, or 3%, or 3.5%, or 4%, or
4.5%, or 5%,
or 5.5%, or 6%, or 6.5%, or 7%, or 7.5%, or 8%, or 8.5%, or 9%, or 9.5%, or
10%, or 10.5%,
or 11%, or 11.5%, or 12%, or 12.5%, or 13%, or 13.5%, or 14%, or 14.5%, or
15%, or
15.5%, or 16%, or 16.5%, or 17%, or 17.5%, or 18%, or 18.5%, or 19%, or 19.5%,
or 20%,
or 20.5%, or 21%, or 21.5%, or 22%, or 22.5%, or 23%, or 23.5%, or 24%, or
24.5%, or
25%, or 25.5%, or 26%, or 26.5%, or 27%, or 27.5%, or 28%, or 28.5%, or 29%,
or 29.5%,
or 30%, or 30.5%, or 31%, or 31.5%, or 32%, or 32.5%, or 33%, or 33.5%, or
34%, or
34.5%, or 35%, or 35.5%, or 36%, or 36.5%, or 37%, or 37.5%, or 38%, or 38.5%,
or 39%,
or 39.5%, or 40%, or 40.5%, or 41%, or 41.5%, or 42%, or 42.5%, or 43%, or
43.5%, or
44%, or 44.5%, or 45%, or 45.5%, or 46%, or 46.5%, or 47%, or 47.5%, or 48%,
or 48.5%,
or 49%, or 49.5%, or 50%, or 50.5%, or 51%, or 51.5%, or 52%, or 52.5%, or
53%, or
53.5%, or 54%, or 54.5%, or 55%, or 55.5%, or 56%, or 56.5%, or 57%, or 57.5%,
or 58%,
or 58.5%, or 59%, or 59.5%, or 60%, or 60.5%, or 61%, or 61.5%, or 62%, or
62.5%, or
63%, or 63.5%, or 64%, or 64.5%, or 65%, or 65.5%, or 66%, or 66.5%, or 67%,
or 67.5%,
or 68%, or 68.5%, or 69%, or 69.5%, or 70%, or 70.5%, or 71%, or 71.5%, or
72%, or
72.5%, or 73%, or 73.5%, or 74%, or 74.5%, or 75%, or 75.5%, or 76%, or 76.5%,
or 77%,
or 77.5%, or 78%, or 78.5%, or 79%, or 79.5%, or 80%, or 80.5%, or 81%, or
81.5%, or
82%, or 82.5%, or 83%, or 83.5%, or 84%, or 84.5%, or 85%, or 85.5%, or 86%,
or 86.5%,
or 87%, or 87.5%, or 88%, or 88.5%, or 89%, or 89.5%, or 90%, or 90.5%, or
91%, or
91.5%, or 92%, or 92.5%, or 93%, or 93.5%, or 94%, or 94.5%, or 95%, or 95.5%,
or 96%,
or 96.5%, or 97%, or 97.5%, or 98%, or 98.5%, or 99%, or 99.5%, or 99.999%
[00159] Additional Notes:
Note: The concentration of magnesium oxide in the output comprising magnesium
oxide may
be greater than or equal to one or more of the following weight percent
concentrations:
0.0001%, or 0.5%, or 1%, or 1.5%, or 2%, or 2.5%, or 3%, or 3.5%, or 4%, or
4.5%, or 5%,
or 5.5%, or 6%, or 6.5%, or 7%, or 7.5%, or 8%, or 8.5%, or 9%, or 9.5%, or
10%, or 10.5%,
or 11%, or 11.5%, or 12%, or 12.5%, or 13%, or 13.5%, or 14%, or 14.5%, or
15%, or
120
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
15.5%, or 16%, or 16.5%, or 17%, or 17.5%, or 18%, or 18.5%, or 19%, or 19.5%,
or 20%,
or 20.5%, or 21%, or 21.5%, or 22%, or 22.5%, or 23%, or 23.5%, or 24%, or
24.5%, or
25%, or 25.5%, or 26%, or 26.5%, or 27%, or 27.5%, or 28%, or 28.5%, or 29%,
or 29.5%,
or 30%, or 30.5%, or 31%, or 31.5%, or 32%, or 32.5%, or 33%, or 33.5%, or
34%, or
34.5%, or 35%, or 35.5%, or 36%, or 36.5%, or 37%, or 37.5%, or 38%, or 38.5%,
or 39%,
or 39.5%, or 40%, or 40.5%, or 41%, or 41.5%, or 42%, or 42.5%, or 43%, or
43.5%, or
44%, or 44.5%, or 45%, or 45.5%, or 46%, or 46.5%, or 47%, or 47.5%, or 48%,
or 48.5%,
or 49%, or 49.5%, or 50%, or 50.5%, or 51%, or 51.5%, or 52%, or 52.5%, or
53%, or
53.5%, or 54%, or 54.5%, or 55%, or 55.5%, or 56%, or 56.5%, or 57%, or 57.5%,
or 58%,
or 58.5%, or 59%, or 59.5%, or 60%, or 60.5%, or 61%, or 61.5%, or 62%, or
62.5%, or
63%, or 63.5%, or 64%, or 64.5%, or 65%, or 65.5%, or 66%, or 66.5%, or 67%,
or 67.5%,
or 68%, or 68.5%, or 69%, or 69.5%, or 70%, or 70.5%, or 71%, or 71.5%, or
72%, or
72.5%, or 73%, or 73.5%, or 74%, or 74.5%, or 75%, or 75.5%, or 76%, or 76.5%,
or 77%,
or 77.5%, or 78%, or 78.5%, or 79%, or 79.5%, or 80%, or 80.5%, or 81%, or
81.5%, or
82%, or 82.5%, or 83%, or 83.5%, or 84%, or 84.5%, or 85%, or 85.5%, or 86%,
or 86.5%,
or 87%, or 87.5%, or 88%, or 88.5%, or 89%, or 89.5%, or 90%, or 90.5%, or
91%, or
91.5%, or 92%, or 92.5%, or 93%, or 93.5%, or 94%, or 94.5%, or 95%, or 95.5%,
or 96%,
or 96.5%, or 97%, or 97.5%, or 98%, or 98.5%, or 99%, or 99.5%, or 99.999%
1001601 Additional Notes:
Note: The concentration of calcium oxide in the output comprising magnesium
oxide may be
greater than or equal to one or more of the following weight percent
concentrations:
0.0001%, or 0.5%, or 1%, or 1.5%, or 2%, or 2.5%, or 3%, or 3.5%, or 4%, or
4.5%, or 5%,
or 5.5%, or 6%, or 6.5%, or 7%, or 7.5%, or 8%, or 8.5%, or 9%, or 9.5%, or
10%, or 10.5%,
or 11%, or 11.5%, or 12%, or 12.5%, or 13%, or 13.5%, or 14%, or 14.5%, or
15%, or
15.5%, or 16%, or 16.5%, or 17%, or 17.5%, or 18%, or 18.5%, or 19%, or 19.5%,
or 20%,
or 20.5%, or 21%, or 21.5%, or 22%, or 22.5%, or 23%, or 23.5%, or 24%, or
24.5%, or
25%, or 25.5%, or 26%, or 26.5%, or 27%, or 27.5%, or 28%, or 28.5%, or 29%,
or 29.5%,
or 30%, or 30.5%, or 31%, or 31.5%, or 32%, or 32.5%, or 33%, or 33.5%, or
34%, or
34.5%, or 35%, or 35.5%, or 36%, or 36.5%, or 37%, or 37.5%, or 38%, or 38.5%,
or 39%,
or 39.5%, or 40%, or 40.5%, or 41%, or 41.5%, or 42%, or 42.5%, or 43%, or
43.5%, or
44%, or 44.5%, or 45%, or 45.5%, or 46%, or 46.5%, or 47%, or 47.5%, or 48%,
or 48.5%,
or 49%, or 49.5%, or 50%, or 50.5%, or 51%, or 51.5%, or 52%, or 52.5%, or
53%, or
53.5%, or 54%, or 54.5%, or 55%, or 55.5%, or 56%, or 56.5%, or 57%, or 57.5%,
or 58%,
or 58.5%, or 59%, or 59.5%, or 60%, or 60.5%, or 61%, or 61.5%, or 62%, or
62.5%, or
63%, or 63.5%, or 64%, or 64.5%, or 65%, or 65.5%, or 66%, or 66.5%, or 67%,
or 67.5%,
121
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
or 68%, or 68.5%, or 69%, or 69.5%, or 70%, or 70.5%, or 71%, or 71.5%, or
72%, or
72.5%, or 73%, or 73.5%, or 74%, or 74.5%, or 75%, or 75.5%, or 76%, or 76.5%,
or 77%,
or 77.5%, or 78%, or 78.5%, or 79%, or 79.5%, or 80%, or 80.5%, or 81%, or
81.5%, or
82%, or 82.5%, or 83%, or 83.5%, or 84%, or 84.5%, or 85%, or 85.5%, or 86%,
or 86.5%,
or 87%, or 87.5%, or 88%, or 88.5%, or 89%, or 89.5%, or 90%, or 90.5%, or
91%, or
91.5%, or 92%, or 92.5%, or 93%, or 93.5%, or 94%, or 94.5%, or 95%, or 95.5%,
or 96%,
or 96.5%, or 97%, or 97.5%, or 98%, or 98.5%, or 99%, or 99.5%, or 99.999%
Note: The concentration of oxygen gas or diatomic oxygen in a head space or a
reactor may
be lower than or equal to one or more of the following volume percent
concentrations:
0.0001%, or 0.5%, or 1%, or 1.5%, or 2%, or 2.5%, or 3%, or 3.5%, or 4%, or
4.5%, or 5%,
or 5.5%, or 6%, or 6.5%, or 7%, or 7.5%, or 8%, or 8.5%, or 9%, or 9.5%, or
10%, or 10.5%,
or 11%, or 11.5%, or 12%, or 12.5%, or 13%, or 13.5%, or 14%, or 14.5%, or
15%, or
15.5%, or 16%, or 16.5%, or 17%, or 17.5%, or 18%, or 18.5%, or 19%, or 19.5%,
or 20%,
or 20.5%, or 21%, or 21.5%, or 22%, or 22.5%, or 23%, or 23.5%, or 24%, or
24.5%, or
25%, or 25.5%, or 26%, or 26.5%, or 27%, or 27.5%, or 28%, or 28.5%, or 29%,
or 29.5%,
or 30%, or 30.5%, or 31%, or 31.5%, or 32%, or 32.5%, or 33%, or 33.5%, or
34%, or
34.5%, or 35%, or 35.5%, or 36%, or 36.5%, or 37%, or 37.5%, or 38%, or 38.5%,
or 39%,
or 39.5%, or 40%, or 40.5%, or 41%, or 41.5%, or 42%, or 42.5%, or 43%, or
43.5%, or
44%, or 44.5%, or 45%, or 45.5%, or 46%, or 46.5%, or 47%, or 47.5%, or 48%,
or 48.5%,
or 49%, or 49.5%, or 50%, or 50.5%, or 51%, or 51.5%, or 52%, or 52.5%, or
53%, or
53.5%, or 54%, or 54.5%, or 55%, or 55.5%, or 56%, or 56.5%, or 57%, or 57.5%,
or 58%,
or 58.5%, or 59%, or 59.5%, or 60%, or 60.5%, or 61%, or 61.5%, or 62%, or
62.5%, or
63%, or 63.5%, or 64%, or 64.5%, or 65%, or 65.5%, or 66%, or 66.5%, or 67%,
or 67.5%,
or 68%, or 68.5%, or 69%, or 69.5%, or 70%, or 70.5%, or 71%, or 71.5%, or
72%, or
72.5%, or 73%, or 73.5%, or 74%, or 74.5%, or 75%, or 75.5%, or 76%, or 76.5%,
or 77%,
or 77.5%, or 78%, or 78.5%, or 79%, or 79.5%, or 80%, or 80.5%, or 81%, or
81.5%, or
82%, or 82.5%, or 83%, or 83.5%, or 84%, or 84.5%, or 85%, or 85.5%, or 86%,
or 86.5%,
or 87%, or 87.5%, or 88%, or 88.5%, or 89%, or 89.5%, or 90%, or 90.5%, or
91%, or
91.5%, or 92%, or 92.5%, or 93%, or 93.5%, or 94%, or 94.5%, or 95%, or 95.5%,
or 96%,
or 96.5%, or 97%, or 97.5%, or 98%, or 98.5%, or 99%, or 99.5%, or 99.999%
[00161] Additional Notes:
Note: cH2S03(ag)' or '2 S02(aq) + H20(1)' may be employed interchangeably.
Note: The weight percent concentration of S02 in one or more aqueous sulfurous
acid
solutions or one or more solutions comprising sulfur dioxide may be greater
than or equal to
one or more or a combination of the following: 0.001%, or 0.1%, or 1%, or 2%,
or 3%, or
122
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
4%, or 5%, or 6%, or 7%, or 8%, or 9%, or 10%, or 11%, or 12%, or 13%, or 14%,
or 15%,
or 16%, or 17%, or 18%, or 19%, or 20%, or 21%, or 22%, or 23%, or 24%, or
25%, or 26%,
or 27%, or 28%, or 29%, or 30%, or 31%, or 32%, or 33%, or 34%, or 35%, or
36%, or 37%,
or 38%, or 39%, or 40%, or 41%, or 42%, or 43%, or 44%, or 45%, or 46%, or
47%, or 48%,
or 49%, or 50%, or 51%, or 52%, or 53%, or 54%, or 55%, or 56%, or 57%, or
58%, or 59%,
or 60%, or 61%, or 62%, or 63%, or 64%, or 65%, or 66%, or 67%, or 68%, or
69%, or 70%,
or 71%, or 72%, or 73%, or 74%, or 75%, or 76%, or 77%, or 78%, or 79%, or
80%, or 81%,
or 82%, or 83%, or 84%, or 85%, or 86%, or 87%, or 88%, or 89%, or 90%, or
90.5%, or
91%, or 91.5%, or 92%, or 92.5%, or 93%, or 93.5%, or 94%, or 94.5%, or 95%,
or 95.5%,
or 96%, or 96.5%, or 97%, or 97.5%, or 98%, or 98.5%, or 99%, or 99.5%, or
99.9%, or less
than or equal to 100%.
Note: The volume percent concentration of S02 in one or more gases described
herein may be
greater than or equal to one or more or a combination of the following:
0.001%, or 0.1%, or
1%, or 2%, or 3%, or 4%, or 5%, or 6%, or 7%, or 8%, or 9%, or 10%, or 11%, or
12%, or
13%, or 14%, or 15%, or 16%, or 17%, or 18%, or 19%, or 20%, or 21%, or 22%,
or 23%, or
24%, or 25%, or 26%, or 27%, or 28%, or 29%, or 30%, or 31%, or 32%, or 33%,
or 34%, or
35%, or 36%, or 37%, or 38%, or 39%, or 40%, or 41%, or 42%, or 43%, or 44%,
or 45%, or
46%, or 47%, or 48%, or 49%, or 50%, or 51%, or 52%, or 53%, or 54%, or 55%,
or 56%, or
57%, or 58%, or 59%, or 60%, or 61%, or 62%, or 63%, or 64%, or 65%, or 66%,
or 67%, or
68%, or 69%, or 70%, or 71%, or 72%, or 73%, or 74%, or 75%, or 76%, or 77%,
or 78%, or
79%, or 80%, or 81%, or 82%, or 83%, or 84%, or 85%, or 86%, or 87%, or 88%,
or 89%, or
90%, or 90.5%, or 91%, or 91.5%, or 92%, or 92.5%, or 93%, or 93.5%, or 94%,
or 94.5%,
or 95%, or 95.5%, or 96%, or 96.5%, or 97%, or 97.5%, or 98%, or 98.5%, or
99%, or
99.5%, or 99.9%, or less than or equal to 100%.
1001621 Additional Notes:
Note: 'WA' may comprise a weak acid, which may include, but not limited to,
silicic acid, or
orthosilicic acid, or silicon acid derivatives, or silicon minerals, or
silicon acids, or
aluminates, or ferrates, or a combination thereof
Note: Some embodiments may involve reacting calcium silicate or a material
comprising
silicon directly with sulfur dioxide or sulfur dioxide in an non-aqueous
solution or a
combination thereof.
Note: In some embodiments, contaminants or impurities may dissolve in a
solution
comprising sulfur dioxide, or due to the presence of sulfuric acid, or a
combination thereof
Contaminants or impurities may include, but are not limited to, one or more or
a combination
of the following: iron, or aluminum, or alkali metals, or transition metals,
or other non-
123
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
bisulfite soluble salts, or non-alkaline earth bisulfite salts, or a
combination thereof In some
embodiments, dissolved contaminants may be present after solid-liquid
separation, and / or
after calcium sulfite precipitation. In some embodiments, at least a portion
of contaminants
may be separated periodically or continuously. Contaminants may be separated
by, including,
but not limited to, precipitation, or membrane based process, or cooling, or
heating, or
crystallization, or cryodesalination, or a separation process described
herein, or a separation
process in the art, or a combination thereof.
Note: 'Calcium' may also refer to magnesium and / or other alkaline earth
metals.
Note: In some embodiments, one absorption column, or absorption step, or
absorption
process, or a combination thereof may be employed to absorb sulfur dioxide gas
and form
aqueous sulfur dioxide or a sulfurous acid solution. In some embodiments, more
than one
absorption column, or absorption step, or absorption process, or a combination
thereof may
be employed to absorb sulfur dioxide gas and form aqueous sulfur dioxide or a
sulfurous acid
solution.
Note: Calcining kilns may include, but are not limited to, one or more or a
combination of the
following: Shaft kilns, or Counter-current shaft kilns, or Regenerative kilns,
or Annular kilns,
or Rotary kilns.
[00163] Additional Notes:
Note: 'WA' may comprise a weak acid, which may include, but not limited to,
silicic acid, or
orthosilicic acid, or silicon acid derivatives, or silicon minerals, or
silicon acids, or
aluminates, or ferrates, or a combination thereof
Note: Calcium or magnesium ¨ weak acid input may comprise, for example,
including, but
not limited to, one or more or a combination of the following: carbonates, or
bicarbonates, or
silicates, or silicate derivatives, or minerals, or concrete, or cement, or
waste concrete, or
waste cement. or steel slag, or fly ash, or ash, or limestone, or rock.
Note: In some embodiments, higher partial pressure CO2, or higher
concentration CO2, or
pure CO2(g), or high partial pressure CO2(g), or CO2(1), or CO2(g), may be
employed to
facilitate formation of bicarbonate salts. For example, in some embodiments,
one or more or
a combination of the following may be employed:
At least a portion of CO2 input may be sourced from a reaction of calcium
carbonate with
sulfurous acid
At least a portion of CO2 input may be sources from CO2 sources produced
within the
process, or other CO2 sources, or a combination thereof
124
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
At least a portion of CO2 input may be sourced from CO2 captured from a
combustion source,
or a combustion source employed to produce heat, or emissions source, or air,
or geological
CO2 source, or natural CO2 source, or a combination thereof.
Note: CO2 sources include, but are not limited to,
Note: Some embodiments may be designed to operate as a low temperature
process, where
the solutions and / or solid reagents in thermal desorption or decomposition
may undergo or
operate thermal desorption or decomposition at less than 150 C, or less than
200 C, or less
than 250 C, or less than 300 C, or less than 350 C.
Note: In some embodiments, at least a portion of heat may be supplied by a
heat pump, or a
refrigeration cycle, or a combination thereof. A heat pump may comprise,
including, but not
limited to, a mechanical, or thermal, or absorption, or a combination thereof
process. A heat
pump may be powered by, including, but not limited to, electricity, or heat,
or photons, or
chemical reaction, or radiation, or mechanical work, or pneumatic process, or
hydraulic
process, or expansion, or compression, or evaporation, or absorption, or vapor
pressure
differences, or osmotic pressure differences, or temperature differences, or
pressure
differences, or a combination thereof
[00164] Additional Notes:
Note: In some embodiments, sulfides and / or hydrogen sulfide may comprise a
weak acid or
weak acid anion.
Note: Separations for removing accumulating water, or removing water, or
recovering water,
or concentrating, or crystalizing, or precipitating, or separating, or
removing, or a
combination thereof may include, but are not limited to, one or more or a
combination of the
following: falling film evaporator, mechanical vapor compression (MVC), or
mechanical
vapor recompression, or multi-effect distillation (MED), or multi-stage flash
distillation
(MSF), or vapor compression (VC) distillation, or vacuum vapor compression
(VVC), or
membrane distillation, or evaporation, or distillation, or forward osmosis, or
reverse osmosis,
or nanofiltration, or hot nanofiltration, or hot reverse osmosis, or hot
concentrating followed
by cooling precipitation, or hot concentrating followed by cooling
precipitation and solid-
liquid separation, or heating precipitation, centrifuge, settling, or filter,
or rotary filter, or
calcining, or desorption, or absorption, or coalescing, or decanting, or
aggregation, or
coagulation, or frothing, or density based methods, or surface tension based
methods, or
foaming separation, emulsification, or de-emulsification, or flocculation,
solventing out, or
salting out, or cooling precipitation, or heating. or cryodesalination, or
zero liquid discharge
processes, or crystallization processes, or electrodialysis reversal (EDR), or
electrodialysis
process, or magnetic separation, or eddy currents, or electromagnetic
induction, or filtration,
125
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
or activated carbon, or ion exchange, or ion exchange membrane, or
precipitation process, or
cryodesalination, or cooling desalination, or cooling, or heating, or salting-
out, or solventing-
out, or adding a solvent to precipitate a solid and then removing the added
solvent, or a
combination thereof
Note: Some embodiments may employ an inert gas, such as nitrogen or argon, or
a gas other
than diatomic oxygen, such as CO2, or a combination thereof in the headspace
to prevent, for
example, oxidation of or reaction of oxygen with sulfite, metabisulfite,
bisulfite, sulfur
dioxide, sulfurous acid, or a combination thereof
[00165] Additional Notes:
Note: Magnesium or other alkaline earth or alkaline earth salts may be
employed instead of
or in addition to calcium.
Note: Concrete waste may be produced in excess of 600 million tons annually in
the USA
alone, which is more than twice the amount of generated municipal solid waste.
Note: At least a portion of sulfur dioxide may be lost in one or more or a
combination of
steps. Alternatively, or additionally, sulfur dioxide may be exit the process
as a, for example,
a residual, in one or more outputs. Sulfur dioxide or 'make-up sulfur dioxide'
may be added
to the process. In some embodiments, sulfur dioxide may be stored on site and
added as
desired or needed to the process. In some embodiments, elemental sulfur, or
hydrogen
sulfide, or a salt comprising sulfur, or sulfide salt, or sulfite salt, or
sulfate salt, or a
combination thereof may be a source of sulfur dioxide or sulfurous acid, by,
for example,
including, but not limited to, one or more or a combination thereof:
combustion, or acid-base
reaction, or reaction with an acid, or carbothermic reduction, or thermal or
decomposition, or
electrolysis, or electrodialysis, or electrochemical reaction.
Note: In some embodiments, at least a portion of calcium sulfate may be
removed. For
example, a portion of residual dissolved calcium sulfate may precipitate and
may be removed
by, for example, including, but not limited to, solid-liquid separation, or
removal of calcium
sulfate scaling, or a combination thereof
Note: One or more or a combination of steps in one or more embodiments may
require
heating and / or cooling. For example, a reaction of sulfurous acid with a
calcium - weak acid
or magnesium ¨ weak acid may require or may be facilitated by cooling or
heating.
Alternatively, or additionally, heat or heating or cooling or a combination
thereof may be
recovered from one or more or a combination of reaction steps. In some
embodiments, heat
or heating or cooling or a combination thereof may be recovered and said
recovered heat or
heating or cooling or a combination thereof may be transferred or employed in
one or more
other steps, or in the same step, or in other applications.
126
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: Losses may occur during the process. Makeup streams of one or more or a
combination
of reagents may be added.
[00166] Additional Notes:
Note: Contaminants may exist or accumulate in the process. If desired, one or
more
contaminants may be at least partially removed periodically, or continuously,
or as desired, or
a combination thereof
Note: Other acid gases may be employed instead of or in addition to sulfur
dioxide, which
may include, but are not limited to, nitrogen oxides, or nitrogen dioxide, or
nitrogen
monoxide, or dinitrogen tetroxide, or nitric acid, or carbon dioxide, or
carbonic acid, or
hydrogen sulfuric, or sulfonic acid, or hydrosulfuric acid, or organo-
sulfurous compounds, or
hydrochloric acid, or hydrobromic acid, or hydroiodic acid, or hydrogen
cyanide, or sulfuric
acid, or perchloric acid, or nitrous acid, or hydrofluoric acid, or nitrogen
derivative acids, or
halogen derivative acids, or derivatives thereof, or a combination thereof
Note: At least a portion of heat may be provided from the reaction of calcium
oxide with
water to form calcium hydroxide or a calcium hydroxide solution.
Note: Calcium weak acid or magnesium weak acid salts or 'WA' may include, but
are not
limited, salts of organic acids. Organic acids, or carboxylic acids, or
organic acid anions, or a
combination thereof may include, but are not limited to, one or more or a
combination of the
following: citric acid, or aconitates, or citrates, or isocitrates, or
alloisocitrate, or oxalic acid,
or acetic acid, or carboxylic acids, or lactic acid, or aconitic acid, or
formic acid, or uric acid,
or malic acid, or tartaric acid, methanoic acid, or hydroxymethanoic acid, or
ethanoic acid, or
2-hydroxyethanoic acid, or oxoethanoic acid, or ethanedioic acid, or propanoic
acid, or
propenoic acid, or propynoic acid, or 2-hydroxypropanoic acid, or 3-
hydroxypropanoic acid,
or 2,3-dihydroxypropanoic acid, or 2-oxopropanoic acid, or 3-oxopropanoic
acid, or 2,3-
dioxopropanoic acid, or propanedioic acid, or 2-hydroxypropanedioic acid, or
2,2-
dihydroxypropanedioic acid, or oxopropanedioic acid, or oxirane-2-carboxylic
acid, or
butanoic acid, or 2-methylpropanoic acid, or (E)-but-2-enoic acid, or (Z)-but-
2-enoic acid, or
2-methylpropenoic acid, or but-3-enoic acid, or but-2-ynoic acid, or 2-
hydroxybutanoic acid,
or 3-hydroxybutanoic acid, or 4-hydroxybutanoic acid, or 2-oxobutanoic acid,
or 3-
oxobutanoic acid, or 4-oxobutanoic acid, or butanedioic acid, or 2-
methylpropanedioic acid,
or (E)-butenedioic acid, or (Z)-butenedioic acid, or butynedioic acid, or
hydroxybutanedioic
acid, or 2,3-dihydroxybutanedioic acid, or oxobutanedioic acid, or
dioxobutanedioic acid, or
pentanoic acid, or 3-methylbutanoic acid, or 2-methylbutanoic acid, or 2,2-
dimethylpropanoic acid, or 3-hydroxypentanoic acid, or 4-hydroxypentanoic
acid, or 3-
hydroxy-3-methylbutanoic acid, or pentanedioic acid, or 2-oxopentanedioic
acid, or 3-
127
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
oxopentanedioic acid, or furan-2-carboxylic acid, or tetrahydrofuran-2-
carboxylic acid, or
hexanoic acid, or hexanedioic acid, or 2,3-dimethylbutanoic acid. or 3,3-
dimethylbutanoic
acid, or 2-hydroxypropane-1,2,3-tricarboxylic acid, or prop-1-ene-1,2,3-
tricarboxylic acid, or
1-hydroxypropane-1,2,3-tricarboxylic acid, or (2E,4E)-hexa-2,4-dienoic acid,
or heptanoic
acid, or heptanedioic acid, or cyclohexanecarboxylic acid, or
benzenecarboxylic acid, or 2-
hydroxybenzoic acid, or 4-carboxybenzoic acid, or 2,2-dimethylpentanoic acid,
or 2,3-
dimethylpentanoic acid, or 2,4-dimethylpentanoic acid, or 3,3-
dimethylpentanoic acid, or 2-
ethylpentanoic acid, or 3-ethylpentanoic acid, or 2-methylhexanoic acid, or 3-
methylhexanoic
acid, or 2,2,3-trimethylbutanoic acid, or 2-ethyl-2-methylbutanoic acid, or 2-
ethyl-3-, or
methylbutanoic acid, or octanoic acid, or benzene-1,2-dicarboxylic acid, or 2-
methylheptanoic acid, or 3-methylheptanoic acid, or 4-methylheptanoic acid, or
5-
methylheptanoic acid, or 6-methylheptanoic acid, or 2,2-dimethylhexanoic acid,
or 2,3-
dimethylhexanoic acid, or 2,4-dimethylhexanoic acid, or 2,5-dimethylhexanoic
acid, or 3,3-
dimethylhexanoic acid, or 3,4-dimethylhexanoic acid, or 3,5-dimethylhexanoic
acid, or 4,4-
dimethylhexanoic acid, or 4,5-dimethylhexanoic acid, or 5,5-dimethylhexanoic
acid, or 2-
ethanehexanoic acid, or 3-ethanehexanoic acid, or 4-ethanehexanoic acid, or 5-
ethanehexanoic acid, or 2-octenoic acid, or 3-octenoic acid, or 4-octenoic
acid, or 5-octenoic
acid, or 6-octenoic acid, or 7-octenoic acid, or nonanoic acid, or benzene-
1,3,5-tricarboxylic
acid, or (E)-3-phenylprop-2-enoic acid, or decanoic acid, or decanedioic acid,
or undecanoic
acid, or dodecanoic acid, or benzene-1,2,3,4,5,6-hexacarboxylic acid, or
tridecanoic acid, or
tetradecanoic acid, or pentadecanoic acid, or hexadecanoic acid, or
heptadecanoic acid, or
octadecanoic acid, or (9Z)-octadec-9-enoic acid, or (9Z,12Z)-octadeca-9,12-
dienoic acid, or
(9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid, or (6Z,9Z,12Z)-octadeca-6,9,12-
trienoic acid,
or (6Z,9Z,12Z,15Z)-octadeca-6,9,12,15-tetraenoic acid, or nonadecanoic acid,
or eicosanoic
acid, or (5Z,8Z,11Z)-eicosa-5,8,11-trienoic acid, or (5Z.8Z,11Z,14Z)-eicosa-
5,8,11,14-
tetraenoic acid, or (5Z,8Z,11Z,14Z,17Z)-eicosa-5,8,11,14-pentaenoic acid, or
heneicosanoic
acid, or docosanoic acid, or (4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-
hexaenoic
acid, or tricosanoic acid, or tetracosanoic acid, or pentacosanoic acid, or
hexacosanoic acid,
or amino acids, or glutamate, or glutamic acid.
Note: Weak acids or organic acids may include, but are not limited to, one or
more or a
combination of the following: carboxylic acids, or sulfonic acids, or
alcohols, or thiols, or
enols, or phenols, or carbonic acid
1001671 Additional Notes:
Note: Calcium citrate may be in the form of tri-calcium citrate tetrahydrate.
12g
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: Separated citric acid may be in the form of an aqueous solution, or
citric acid
monohydrate, or anhydrous citric acid, or a combination thereof
Note: Remaining solution after removal of citric acid may be treated with
activated carbon,
or passed through cation or anion exchangers, or an anion exchange resin, or a
cation
exchanger resin, or a combination thereof.
Note: Citric acid, or other carboxylic acid, or organic acid, or a combination
thereof may be
separated from or recovered from including, but not limited to, one or more or
a combination
of the following: fermentation broth, or sugar broths, or sugars, or raw
sugars, or raw
agricultural feedstocks, or agricultural byproducts, or sugar refining
liquids, or mold
produced citric acid, or juices, or fungi produced acids, or liquids or acids
produced by
Aspergillus niger, or sucrose broth, or dextrose broth, or glucose broth, or
corn steep liquor,
or molasses, or hydrolyzed corn starch, or citrus fruits, or fruit juices.
Said separating or
recovering may involve reaction or contacting with calcium hydroxide or
aqueous calcium
hydroxide solution.
Note: Heat may be recovered in one or more process steps and may employed in
one or more
other process steps, or within the same process step, or both.
Note: Calcium citrate may be provided as an example organic acid salt of
calcium.
Note: Calcium may be provided as an example alkaline earth metal or alkaline
earth metal
cation or alkaline earth metal cation salt.
Note: Calcium silicate may be provided as an example weak acid salt of calcium
or an
example reagent representing a wide array of compositions or minerals
comprising calcium,
magnesium, silicon, oxygen, and derivatives thereof.
Note: Calcium oxide produced may be reacted with water to produce calcium
hydroxide or a
solution comprising aqueous calcium hydroxide. The aqueous calcium hydroxide
may be
reacted with CO2. such as a gas comprising CO2, to produce precipitate calcium
carbonate
and water. For example, aqueous sodium hydroxide may be reacted with flue gas,
or raw gas,
or air, or gases produced from fuel combusted to power the calciner, or
remaining gases after
absorption of sulfur dioxide, or other gas comprising at least a portion CO2,
or a combination
thereof Some embodiments of the present invention may be employed to produce
CO2-
emissions neutral or negative precipitated calcium carbonate. Some embodiments
of the
present invention may involve producing CO2-emissions neutral or negative
precipitated
calcium carbonate using CO2 from the air or captured from the air using the
presently
described process.
[00168] Additional Notes:
129
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Note: Calcium oxide produced may be reacted with water to produce calcium
hydroxide or a
solution comprising aqueous calcium hydroxide. The aqueous calcium hydroxide
may be
reacted sodium carbonate, such as an aqueous solution of sodium carbonate, to
produce
precipitate calcium carbonate and aqueous sodium hydroxide. The precipitated
calcium
carbonate may be separated from the aqueous sodium hydroxide and may comprise
a
valuable byproduct. The aqueous sodium hydroxide may be reacted with a gas
comprising
carbon dioxide to produce aqueous sodium carbonate. For example, aqueous
sodium
hydroxide may be reacted with flue gas, or raw gas, or air, or gases produced
from fuel
combusted to power the calciner, or remaining gases after absorption of sulfur
dioxide, or
other gas comprising at least a portion CO2, or a combination thereof. Some
embodiments of
the present invention may be employed to produce CO2-emissions neutral or
negative
precipitated calcium carbonate. Some embodiments of the present invention may
involve
producing CO2-emissions neutral or negative precipitated calcium carbonate
using CO2 from
the air or captured from the air using the presently described process. Some
embodiments of
the present invention may involve producing CO2-emissions neutral or negative
precipitated
calcium carbonate using CO2 from emissions sources, or air, or both using the
presently
described process.
Note: The weight percent concentration of one or more or a combination of
reagents may
include, but is not limited to, less than, or equal to, or greater than one or
more or a
combination of the following: 0%, or 0.5%, or 1%, or 1.5%, or 2%, or 2.5%, or
3%, or 3.5%,
or 4%, or 4.5%, or 5%, or 5.5%, or 6%, or 6.5%, or 7%, or 7.5%, or 8%, or
8.5%, or 9%, or
9.5%, or 10%, or 10.5%, or 11%, or 11.5%, or 12%, or 12.5%, or 13%, or 13.5%,
or 14%, or
14.5%, or 15%, or 15.5%, or 16%, or 16.5%, or 17%, or 17.5%, or 18%, or 18.5%,
or 19%,
or 19.5%, or 20%, or 20.5%, or 21%, or 21.5%, or 22%, or 22.5%, or 23%, or
23.5%, or
24%, or 24.5%, or 25%, or 25.5%, or 26%, or 26.5%, or 27%, or 27.5%, or 28%,
or 28.5%,
or 29%, or 29.5%, or 30%, or 30.5%, or 31%, or 31.5%, or 32%, or 32.5%, or
33%, or
33.5%, or 34%, or 34.5%, or 35%, or 35.5%, or 36%, or 36.5%, or 37%, or 37.5%,
or 38%,
or 38.5%, or 39%, or 39.5%, or 40%, or 40.5%, or 41%, or 41.5%, or 42%, or
42.5%, or
43%, or 43.5%, or 44%, or 44.5%, or 45%, or 45.5%, or 46%, or 46.5%, or 47%,
or 47.5%,
or 48%, or 48.5%, or 49%, or 49.5%, or 50%, or 50.5%, or 51%, or 51.5%, or
52%, or
52.5%, or 53%, or 53.5%, or 54%, or 54.5%, or 55%, or 55.5%, or 56%, or 56.5%,
or 57%,
or 57.5%, or 58%, or 58.5%, or 59%, or 59.5%, or 60%, or 60.5%, or 61%, or
61.5%, or
62%, or 62.5%, or 63%, or 63.5%, or 64%, or 64.5%, or 65%, or 65.5%, or 66%,
or 66.5%,
or 67%, or 67.5%, or 68%, or 68.5%, or 69%, or 69.5%, or 70%, or 70.5%, or
71%, or
71.5%, or 72%, or 72.5%, or 73%, or 73.5%, or 74%, or 74.5%, or 75%, or 75.5%,
or 76%,
130
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
or 76.5%, or 77%, or 77.5%, or 78%, or 78.5%, or 79%, or 79.5%, or 80%, or
80.5%, or
81%, or 81.5%, or 82%, or 82.5%, or 83%, or 83.5%, or 84%, or 84.5%, or 85%,
or 85.5%,
or 86%, or 86.5%, or 87%, or 87.5%, or 88%, or 88.5%, or 89%, or 89.5%, or
90%, or
90.5%, or 91%, or 91.5%, or 92%, or 92.5%, or 93%, or 93.5%, or 94%, or 94.5%,
or 95%,
or 95.5%, or 96%, or 96.5%, or 97%, or 97.5%, or 98%, or 98.5%, or 99%, or
99.5%, or
100%
1001691 Additional Notes:
Note: Calcium silicate input or magnesium silicate input may comprise a slag.
For example,
global iron slag production is estimated to be 320 million to 384 million tons
annually and
steel stag is estimated to be between 190 million to 280 million tons
annually. Other slags
may include, but are not limited to, slags from magnesium production. Slags
are generally
produced when calcium oxide or magnesium oxide are added to a metal production
process to
remove impurities, or facilitate certain conditions or properties, or a
combination thereof. The
present invention may convert said slags into calcium oxide or magnesium oxide
or other
alkaline earth oxide. The present invention may enable a circular economy in
the iron-
marking, or steel-making, or other metal production industries because calcium
oxide and / or
magnesium oxide are used as the inputs which result in the production of slag.
If iron or steel
makers can recycle at least a portion of slag into calcium oxide or magnesium
oxide, iron or
steel makers may greatly reduce or eliminate their need to purchase calcium
oxide or
magnesium oxide, significantly reducing operating costs. If at least a portion
of iron or steel
stag is recycled into calcium oxide or magnesium oxide, iron and/or steel
production lifecycle
carbon dioxide emissions will be greatly reduced.
Note: Calcium sulfite and / or magnesium sulfite may comprise hydrates. For
example,
magnesium sulfite may form a hexahydrate, or a trihydrate, or may be
anhydrous. For
example, calcium sulfite may form a tetrahydrate, or a hemihydrate, or may be
anhydrous. It
may be desirable to dehydrate at least a portion of the hydrate of calcium
sulfite, or
magnesium sulfite, or both before or during calcining of a sulfite into an
oxide and sulfur
dioxide. It may be desirable to dehydrate at least a portion of the hydrate of
calcium sulfite,
or magnesium sulfite, or both before calcining of a sulfite into an oxide and
sulfur dioxide.
For example, magnesium sulfite hexahydrate may be heated to above 40 C, where
magnesium sulfite hexahydrate may decompose or dehydrate into magnesium
sulfite
trihydrate. For example, calcium sulfite tetrahydrate may be heated to
decompose or
dehydrate into calcium sulfite hemihydrate. For example, calcium and / or
magnesium
hydrates may be decomposed or dehydrated into anhydrous forms. For example,
calcium
sulfite hydrate and / or magnesium sulfite hydrate may be heated to decompose
or dehydrate
131
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
into anhydrous forms. Dehydrating hydrates may require heat or other energy.
It may be
desirable to supply said heat or other energy for dehydrating hydrates from
lower cost, or
lower quality heat sources, such as, including, but not limited to, one or
more or a
combination of the following: waste heat, or heat from other process steps, or
low quality
steam, or medium quality steam, or high quality step, or combustion of one or
more fuels, or
solar thermal, or slacking lime, or hydrating a oxide to a hydroxide, or other
heat source.
[00170] Additional Notes:
Note: Systems and methods may be employed to remove impurities, or prevent or
minimize
accumulation of impurities, or a combination thereof For example, input
materials may
comprise impurities other than desired reagents. In some instances, said
impurities or
contaminants may accumulate, or may result in the formation of other
impurities, or a
combination thereof In some instances, impurities may dissolve in one or more
solutions in
the process. Impurities may be removed, or treated, or separated, by,
including, but not
limited to, one or more or a combination of the following: chemical reaction,
or
electrodialysis, or ion-exchanger, or precipitation, or cooling, or heating,
or distillation, or
membrane-based process, or solventing-out, or salting out.
Note: At least a portion of the weak acid product, or undissolved materials,
or a combination
thereof are employed as a concrete aggregate.
Note: At least a portion of the weak acid product, or undissolved materials,
or a combination
thereof may be disposed of or may comprise a waste product.
Note: A material comprising calcium and / or magnesium may comprise a material
comprising an alkaline-earth. Alkaline-earths may include one or more or a
combination of
the following: beryllium (Be), or magnesium (Mg), or calcium (Ca), or
strontium (Sr), or
barium (Ba), or radium (Ra)
Note: In some embodiments, a material comprising calcium and / or magnesium
may further
comprise one or more or a combination of the following: iron oxides, or iron,
or manganese
oxide, or manganese, may include, but are not limited to, one or more or a
combination of the
following: iron (II), or iron (II,III), or iron (III), or iron (II) oxide, or
iron (II,II) oxide, or iron
(III) oxide, or iron sulfite, or iron sulfate, or iron sulfide, or iron, or
ferrites, or ferrates, or
calcium-iron salts, or magnesium iron salts, or iron silicate salts, or iron
silicon salts, or iron
carbon salts, or manganese salts, or manganese ¨3, or manganese ¨2, or
manganese ¨1, or
manganese 0, or manganese +1, or manganese +2, or manganese, or manganese +3,
or
manganese +4, or manganese +5, or manganese +6, or manganese +7, or manganese
sulfite,
or manganese oxide, or manganese carbonate, or manganese ¨ iron, or calcium ¨
manganese,
132
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
or calcium ¨ manganese salts, or magnesium ¨ manganese, or magnesium ¨
manganese salts,
or manganese silicon, or manganese carbon, or manganese
[00171] Additional Notes:
Note: The properties of iron and manganese may be similar. Manganese may be
present in
some materials which may comprise iron, such as some slags, or concretes, or
minerals. In
some embodiments, iron and manganese may be used interchangeably.
Note: Solutions comprising salts of metals lead, or copper, or gold, or
silver, or zinc, or
aluminum, or chromium, or cobalt, or manganese, or rare-earth metals, or iron,
or
molybdenum, or cadmium, or nickel, or silver, or cobalt, or zinc, or gold, or
platinum, or
platinum group metals, or a combination thereof may undergo a separations and
/ or refining
process. For example, one or more or a combination of said metals may be
separated or
produced from solution or from a separated state or both by means of, for
example, including,
but not limited to, one or more or a combination of the following:
electrolytic refining, or
electrowinning, or electroextraction, or electrodeposition. For example, a
solution comprising
aqueous iron bisulfite, or manganese bisulfite, or iron sulfate, or manganese
bisulfate, or iron
chloride, or magnesium chloride may undergo electroextraction to produce
manganese, iron,
or a combination thereof In some embodiments, one or more or a combination of
the
aforementioned metals may be separated by reaction with hydrogen sulfide or
sulfur to
produce a sulfide or an insoluble sulfide, then said sulfide may be converted
into a form for
use as in input to an electroextraction process.
Note: Separation of at least a portion of iron sulfite solid from at least a
portion of calcium
sulfite solid, or separation of iron from calcium or magnesium, or a
combination thereof may
be conducted by, including, but not limited to, one or more or a combination
of the following:
density based separation, or floatation and sinking separation using a dense
liquid, or
separation using a dense liquid, or separation using a liquid with a lower
density than iron
sulfite and a greater density than calcium sulfite, or magnetic separation, or
magnetic
separation of iron from calcium, or oxidation of iron, or reaction of solution
comprising
dissolved iron with hydrogen sulfide to produce iron sulfide solid
precipitate, or reaction of
solution comprising calcium with sulfuric acid to form calcium sulfate
precipitate, or
frothing, or floatation, or solid separation, or centrifuge, or grinding, or
pulverization, or
reaction of iron sulfite and calcium sulfite solids with sulfuric acid to form
dissolved or
aqueous iron sulfate and calcium sulfate solid, or reaction of a mixture of
calcium oxide and
iron oxide with water to form calcium hydroxide dissolved or aqueous and iron
oxide solid,
or precipitation of iron sulfite before calcium sulfite, or precipitation of
calcium sulfite before
iron sulfite, or electrodialysis, or electrodialysis reversal, or ion
exchange, or iron exchange
133
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
resin, or iron reaction, or double-salt reaction, or precipitation reaction,
or temperature driven
precipitation, or concentration driven precipitation
[00172] Additional Notes:
Note: Oxygen or 'oxide' or 'hydroxide' or a combination thereof may be
considered weak
acids or 'weak acid anions' or a combination thereof.
Note: Desorption of sulfur dioxide form a solution comprising bisulfite may be
conducted by,
for example, including, but not limited to, one or more or a combination of
the following:
thermal desorption, or steam stripping, or a combination thereof A solution
comprising
bisulfite may include, but is not limited to, a solution comprising one or
more or a
combination of the following: alkaline earth bisulfite, or magnesium
bisulfite, or calcium
bisulfite, or iron bisulfite, or manganese bisulfite, or zinc bisulfite, or
sulfur dioxide, or water,
or sulfurous acid.
Note: Separations may include, but are not limited to, one or more or a
combination of the
following: Separation by density, or Separation by magnetism, or Separation by
frothing or
surface tension, or Separation by residual solubility differences, or
Separation by oxidation,
or Separation by ion exchange, or Separation by reaction with an alkali
hydroxide solution, or
Separation by reaction with hydrogen sulfide, or Separation by reaction with
aqueous sulfuric
acid, or Separation by density using a high density liquid with a density less
than at least one
salt and a density greater than one salt, or Separation by density using a
high density liquid
with a density less than iron sulfite and a density greater than calcium
sulfite, or Separation
by density using a centrifuge, or Separation by a magnetic field using a
mixing and an
externally applied magnetic field, or Separation by reaction with and / or
dissolution in water,
or Grinding or pulverization, or Separation by froth flotation, or Other solid
¨ solid separation
method, or Other method for separating iron from calcium, or Other separation
method
[00173] Additional Notes:
Note: In some embodiments, remaining solution after separating magnesium
sulfite solid
using a solid-liquid separate process may be further treated with, including,
but not limited to,
one or more or a combination of the following: ion exchange, or resins, or
filters, or chemical
treatments, or chemical reactions, or membrane based process, or distillation,
or cooling, or
heating, or freezing, or cryodesalination, or solventing-out, or solvent
induced precipitation,
or salting-out, or other treatment. One or more solutions comprising water may
be transferred
to a sulfur dioxide absorption step, or mixed with a solution transferred to a
sulfur dioxide
absorption step, or a combination thereof
134
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Example Embodiments Sodium Hydroxide Production using Calcium or Magnesium
Input
and Sulfur Dioxide Intermediate
1. A process comprising:
reacting a material comprising calcium carbonate with a solution comprising
aqueous
sulfur dioxide to form a gas comprising carbon dioxide and a solid comprising
calcium sulfite;
reacting the solid comprising calcium sulfite with water and sulfur dioxide to
form a
solution comprising aqueous calcium bisulfite;
reacting the solution comprising aqueous calcium bisulfite with sodium sulfate
to form an
aqueous solution comprising sodium bisulfite and a solid comprising calcium
sulfate;
decomposing said aqueous sodium bisulfite to form sodium sulfite and sulfur
dioxide gas;
reacting said sodium sulfite with calcium hydroxide to form an aqueous
solution
comprising sodium hydroxide and a solid comprising calcium sulfite;
decomposing said calcium sulfite to form calcium oxide and sulfur dioxide;
2. The process of example embodiment 1 further comprising reacting calcium
oxide with
water to form calcium hydroxide.
3. The process of example embodiment 1 wherein said decomposing of an aqueous
solution comprising sodium bisulfite comprises desorbing sulfur dioxide gas
from said aqueous
solution to form aqueous sodium sulfite.
4. The process of example embodiment 1 wherein said decomposing of an aqueous
solution comprising sodium bisulfite comprises:
Removing water from said aqueous sodium bisulfite to form sodium metabisulfite
solid;
and
Thermally decomposing said sodium metabisulfite to form solid sodium sulfite
and sulfur
dioxide.
5. The process of example embodiment 1 further comprising capturing at least a
portion of the
carbon dioxide.
6. The process of example embodiment 5 wherein the captured carbon dioxide
comprises a
concentration greater than 70 percent or a partial pressure greater than 0.7
Bar.
7. The process of example embodiment 1 further comprising absorbing the sulfur
dioxide formed
from the decomposition of calcium sulfite into an aqueous solution to form
aqueous sulfurous
acid.
135
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
8. The process of example embodiment 7 further comprising reacting aqueous
sulfurous acid
with calcium carbonate to form a gas comprising carbon dioxide and a solid
comprising calcium
sulfite.
9. The process of example embodiment 1 further comprising absorbing the sulfur
dioxide formed
from the decomposition of sodium bisulfite into an aqueous solution to form
aqueous sulfurous
acid.
10. The process of example embodiment 9 further comprising reacting said
aqueous sulfurous
acid with calcium sulfite to form a solution comprising aqueous calcium
bisulfite.
11. The process of example embodiment 1 further comprising absorbing the
sulfur dioxide
formed from the decomposition of sodium bisulfite into an aqueous solution in
the presence of
calcium sulfite to form a solution comprising aqueous calcium bisulfite.
12. The process of example embodiment 1 wherein said calcium hydroxide
comprises milk of
lime.
13. The process of example embodiment 2 wherein the reaction of calcium oxide
and water
produces heat; and
wherein at least a portion of said heat is employed to dry a calcium sulfite
before a
calcination.
14. The process of example embodiment 1 wherein the reaction of calcium oxide
and water
produces heat; and
wherein at least a portion of said heat is employed in decomposing said sodium
bisulfite
to sodium sulfite and sulfur dioxide.
15. The process of example embodiment 1 wherein said material comprising
calcium carbonate
further comprises magnesium; and
wherein said reacting a material comprising calcium carbonate with a solution
comprising
aqueous sulfur dioxide to form a gas comprising carbon dioxide and a solid
comprising calcium
sulfite solid further comprises forming a solution comprising aqueous
magnesium sulfite.
136
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
16. The process of example embodiment 15 further comprising:
cooling the solution comprising aqueous magnesium sulfite to precipitate at
least a
portion of magnesium sulfite;
removing at least a portion of precipitated magnesium sulfite from the
solution
comprising aqueous magnesium sulfite to form a second solution comprising less
magnesium
sulfite; ;
heating said second solution;
mixing said second heated solution with a third solution comprising magnesium
sulfite to
form a fourth solution; and
separating said fourth solution at a higher temperature using reverse osmosis
to form a
retentate comprising concentrated aqueous magnesium sulfite and a permeate
comprising water.
17. The process of example embodiment 1 wherein the partial pressure of sulfur
dioxide gas
formed from the decomposing of calcium sulfite is lower than the partial
pressure of sulfur
dioxide gas formed from the decomposing of sodium bisulfite.
18. A process comprising:
reacting a material comprising alkaline earth cation - weak acid anion with
sulfur dioxide
and an aqueous solution to form a weak acid derivative and an aqueous solution
comprising
alkaline earth bisulfite;
separating said weak acid derivative from said aqueous solution comprising
alkaline earth
bisulfite;
reacting aqueous alkaline earth bisulfite with sodium sulfate to form an
aqueous solution
comprising sodium bisulfite and a solid comprising an alkaline earth sulfate;
separating said solid comprising an alkaline earth sulfate from said aqueous
solution
comprising sodium bisulfite;
decomposing the aqueous solution comprising sodium bisulfite to form sodium
sulfite
and sulfur dioxide gas;
reacting said sodium sulfite with calcium hydroxide to form an aqueous
solution
comprising sodium hydroxide and a solid comprising calcium sulfite;
separating said solid comprising calcium sulfite from said aqueous solution
comprising
sodium hydroxide;
decomposing said solid comprising calcium sulfite to form calcium oxide and
sulfur
dioxide gas;
reacting the calcium oxide with water to form calcium hydroxide.
137
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
19. The process of example embodiment 18 wherein the alkaline earth cation
comprises one or
more or any combination of the following cations: beryllium (Be), or magnesium
(Mg), or
calcium (Ca), or strontium (Sr), or barium (Ba), or radium (Ra).
20. The process of example embodiment 18 wherein said weak acid derivative
comprises a
derivative of an acid with an acid strength lower than or less acidic than
sulfurous acid.
21. The process of example embodiment 18 wherein said weak acid derivative
comprises carbon
dioxide, or hydrogen sulfide, or silicon dioxide, or iron oxide, or manganese
oxide, or aluminum
oxide, or any mixture thereof
22. The process of example embodiment 18 wherein said decomposing of an
aqueous
solution comprising sodium bisulfite comprises desorbing sulfur dioxide gas
from said aqueous
solution to form aqueous sodium sulfite.
23. The process of example embodiment 18 wherein said decomposing of an
aqueous
solution comprising sodium bisulfite comprises:
Removing water from said aqueous sodium bisulfite to form sodium metabisulfite
solid;
and
Thermally decomposing said sodium metabisulfite to form solid sodium sulfite
and sulfur
dioxide.
24. The process of example embodiment 18 wherein said sulfur dioxide and an
aqueous solution
comprises reacting sulfur dioxide gas with an aqueous solution in the presence
of alkaline earth
sulfite to facilitate the formation of aqueous alkaline earth bisulfite.
25. The process of example embodiment 18 further comprising absorbing at least
a portion of
sulfur dioxide gas into an aqueous solution to form aqueous sulfurous acid.
26. A process comprising:
reacting a material comprising magnesium - weak acid with a solution
comprising
aqueous sulfur dioxide to form a weak acid derivative and an aqueous solution
comprising
magnesium sulfite;
138
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
separating said weak acid derivative from said aqueous solution comprising
magnesium
sulfite;
reacting magnesium sulfite with sodium sulfate to form sodium sulfite and
magnesium
sulfate;
separating at least a portion of said sodium sulfite from said magnesium
sulfate;
reacting said sodium sulfite with calcium hydroxide to form an aqueous
solution
comprising sodium hydroxide and a solid comprising calcium sulfite;
separating said solid comprising calcium sulfite from said aqueous solution
comprising
sodium hydroxide;
decomposing calcium sulfite to form calcium oxide and sulfur dioxide; and
reacting calcium oxide with water to form calcium hydroxide.
27. The process of example embodiment 26 further comprising separating the
aqueous solution
comprising magnesium sulfite to form water and a magnesium sulfite solid.
28. The process of example embodiment 26 further comprising:
cooling the aqueous solution comprising magnesium sulfite to precipitate at
least a
portion of magnesium sulfite;
separating at least a portion of precipitated magnesium sulfite to form a
second solution
comprising less magnesium sulfite:
heating said second solution comprising less magnesium sulfite; and
separating said heated second solution at a higher temperature using reverse
osmosis to
form a retentate comprising concentrated aqueous magnesium sulfite and a
permeate comprising
water.
29. The process of example embodiment 26 further comprising:
precipitating a portion of magnesium sulfite from a first solution comprising
concentrated
aqueous magnesium sulfite by cooling;
separating magnesium sulfite solid precipitate from the remaining solution
comprising a
second solution comprising aqueous magnesium sulfite;
heating said second solution comprising aqueous magnesium sulfite to a higher
temperature;
mixing said second solution comprising aqueous magnesium sulfite at a higher
temperature with a third solution comprising magnesium sulfite to form a
fourth solution; and
139
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
separating said fourth solution at a higher temperature using reverse osmosis
into a
retentate comprising a first solution comprising concentrated aqueous
magnesium sulfite and a
permeate comprising water.
30. A process comprising:
reacting a material comprising alkaline earth cation - weak acid anion with
sulfur dioxide
and an aqueous solution to form a weak acid derivative and an aqueous solution
comprising
alkaline earth bisulfite;
separating said weak acid derivative from said aqueous solution comprising
alkaline earth
bisulfite;
reacting the aqueous solution comprising alkaline earth bisulfite with sodium
sulfate to
form an aqueous solution comprising sodium bisulfite and a solid comprising an
alkaline earth
sulfate;
separating said solid comprising the alkaline earth sulfate from said aqueous
solution
comprising sodium bisulfite;
decomposing said aqueous solution comprising sodium bisulfite to form sodium
sulfite
and sulfur dioxide gas;
reacting said sodium sulfite with alkaline earth hydroxide to form sodium
hydroxide and
an alkaline earth sulfite;
separating said alkaline earth sulfite from said aqueous solution comprising
sodium
hydroxide;
decomposing alkaline earth sulfite to form alkaline earth oxide and sulfur
dioxide; and
reacting alkaline earth oxide with water to form alkaline earth hydroxide.
[00174] 1. A process for producing sodium hydroxide and gypsum
from a material
comprising calcium wherein the process comprises:
reacting a material comprising calcium carbonate with a solution comprising
aqueous
sulfur dioxide to form a gas comprising carbon dioxide and a solid comprising
calcium sulfite;
reacting the solid comprising calcium sulfite with water and sulfur dioxide to
form a
solution comprising aqueous calcium bisulfite;
reacting the aqueous solution comprising aqueous calcium bisulfite with sodium
sulfate to
form aqueous sodium bisulfite and a solid comprising calcium sulfate;
separating sodium metabisulfite from said aqueous solution comprising sodium
bisulfite;
decomposing said sodium metabisulfite to form sodium sulfite and sulfur
dioxide gas;
140
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
reacting said sodium sulfite with calcium hydroxide to form an aqueous
solution
comprising sodium hydroxide and a solid comprising calcium sulfite;
decomposing said calcium sulfite to form calcium oxide and sulfur dioxide;
reacting calcium oxide with water to form calcium hydroxide.
[00175] 2. The process of example embodiment 1 wherein the
carbon dioxide formed from
the reaction of calcium carbonate with aqueous sulfur dioxide may comprise
captured carbon
dioxide.
1001761 3. The process of example embodiment 2 wherein the
carbon dioxide formed
comprises a concentration greater than 70 percent or a partial pressure
greater than 0.7 Bar.
[00177] 4. The process of example embodiment 1 wherein the
sulfur dioxide formed from
the decomposition of calcium sulfite is absorbed into an aqueous solution to
form aqueous
sulfurous acid.
[00178] 5. The process of example embodiment 4 wherein said
formed aqueous sulfur
dioxide is reacted with calcium carbonate to form a gas comprising carbon
dioxide and a solid
comprising calcium sulfite.
[00179] 6. The process of example embodiment 1 wherein the
sulfur dioxide formed from
the decomposition of sodium metabisulfite is absorbed into an aqueous solution
to form aqueous
sulfurous acid.
[00180] 7. The process of example embodiment 6 wherein said
formed aqueous sulfur
dioxide is reacted with calcium sulfite to form a solution comprising aqueous
calcium bisulfite.
[00181] 8. The process of example embodiment 1 wherein the
sulfur dioxide formed from
the decomposition of sodium metabisulfite is absorbed into an aqueous solution
in the presence
of calcium sulfite to form a solution comprising aqueous calcium bisulfite.
[00182] 9. The process of example embodiment 1 wherein said
calcium hydroxide
comprise milk of lime.
[00183] 10. The process of example embodiment 1 wherein the
reaction of calcium oxide
and water produces heat; and
wherein at least a portion of said heat is employed to dry calcium sulfite
before
calcination.
[00184] 11. The process of example embodiment 1 wherein the
reaction of calcium oxide
and water produces heat; and
wherein at least a portion of said heat is employed to facilitate the
decomposition of
sodium metabisulfite to sodium sulfite and sulfur dioxide.
[00185] 12. The process of example embodiment 1 wherein said
separating sodium
metabisulfite from said aqueous solution comprising sodium bisulfite comprises
removing water,
141
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
or precipitation of sodium metabisulfite, or distillation, or cooling, or
freeze desalination, or
solvent addition precipitation.
[00186] 13. The process of example embodiment 1 wherein said
calcium carbonate further
comprises magnesium; and
wherein said reaction of calcium carbonate with aqueous sulfur dioxide to form
calcium
sulfite solid further comprises forming a solution comprising aqueous
magnesium sulfite.
[00187] 14. The process of example embodiment 13 wherein
magnesium sulfite solid is
separated from said aqueous magnesium sulfite by cooling precipitation.
[00188] 15. The process of example embodiment 13 comprising:
Precipitating a portion of magnesium sulfite from a first solution comprising
concentrated
aqueous magnesium sulfite by cooling; and
Separating magnesium sulfite solid precipitate from the remaining solution
comprising a
second solution comprising aqueous magnesium sulfite; and
Heating said second solution comprising aqueous magnesium sulfite to a higher
temperature; and
Mixing said second solution comprising aqueous magnesium sulfite at a higher
temperature with a third solution comprising magnesium sulfite introduced from
the process to
form a fourth solution; and
Separating said fourth solution at a higher temperature using reverse osmosis
into a
retentate comprising a first solution comprising concentrated aqueous
magnesium sulfite and a
permeate comprising water.
[00189] 16. The process of example embodiment 1 wherein the
partial pressure of sulfur
dioxide gas formed from the decomposing of calcium sulfite is lower than 0.3
atm.
[00190] 17. The process of example embodiment 1 wherein the
partial pressure of sulfur
dioxide gas formed from the decomposing of sodium metabisulfite is greater
than 1 atm.
[00191] 18. A process for producing sodium hydroxide and gypsum
from a material
comprising alkaline earth wherein the process comprises:
reacting a material comprising alkaline earth cation - weak acid anion with
sulfur dioxide
and an aqueous solution to form a weak acid derivative and an aqueous solution
comprising
alkaline earth bisulfite;
separating said weak acid derivative from said aqueous solution comprising
alkaline earth
bisulfite;
reacting aqueous alkaline earth bisulfite with sodium sulfate to form aqueous
solution
comprising sodium bisulfite and a solid comprising an alkaline earth sulfate;
142
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
separating said solid comprising an alkaline earth sulfate from said aqueous
solution
comprising sodium bisulfite;
removing water from said aqueous solution comprising sodium bisulfite to form
sodium
metabisulfite;
decomposing sodium metabisulfite to form sodium sulfite and sulfur dioxide
gas;
reacting said sodium sulfite with calcium hydroxide to form an aqueous
solution
comprising sodium hydroxide and a solid comprising calcium sulfite;
separating said solid comprising calcium sulfite from said aqueous solution
comprising
sodium hydroxide;
decomposing calcium sulfite to form calcium oxide and sulfur dioxide;
reacting calcium oxide with water to form calcium hydroxide.
[00192] 19. The process of example embodiment 18 wherein the
alkaline earth comprises
one or more or any combination of the following: beryllium (Be), or magnesium
(Mg), or
calcium (Ca), or strontium (Sr), or barium (Ba), or radium (Ra).
[00193] 20. The process of example embodiment 18 wherein said
weak acid derivative
comprises a derivative of an acid with an acid strength lower than or less
acidic than sulfurous
acid.
[00194] 21. The process of example embodiment 18 wherein said
weak acid derivative
comprises a gas selected from carbon dioxide, or hydrogen sulfide, or a
mixture thereof
[00195] 22. The process of example embodiment 18 wherein said
weak acid derivative
comprises silicon dioxide, or iron oxide, or manganese oxide, or aluminum
oxide, or a mixture
thereof.
[00196] 23. The process of example embodiment 18 wherein said
sulfur dioxide and an
aqueous solution comprises aqueous sulfur dioxide.
[00197] 24. The process of example embodiment 18 wherein said
sulfur dioxide and an
aqueous solution comprises reacting sulfur dioxide gas with an aqueous
solution in the presence
of alkaline earth sulfite to facilitate the formation of aqueous alkaline
earth bisulfite.
[00198] 25. The process of example embodiment 18 further
comprising absorbing sulfur
dioxide from the decomposition of calcium sulfite and decomposition of sodium
metabisulfite
into an aqueous solution to form aqueous sulfurous acid.
[00199] 26. A process for producing sodium hydroxide and
magnesium sulfate from a
material comprising magnesium wherein the process comprises:
reacting a material comprising magnesium - weak acid with a solution
comprising
aqueous sulfur dioxide to form a weak acid derivative and an aqueous solution
comprising
magnesium sulfite;
143
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
separating said weak acid derivative from said aqueous solution comprising
magnesium
sulfite;
reacting magnesium sulfite with sodium sulfate to form sodium sulfite and
magnesium
sulfate;
separating at least a portion of said sodium sulfite from said magnesium
sulfate;
reacting said sodium sulfite with calcium hydroxide to form an aqueous
solution
comprising sodium hydroxide and a solid comprising calcium sulfite;
separating said solid comprising calcium sulfite from said aqueous solution
comprising
sodium hydroxide;
decomposing calcium sulfite to form calcium oxide and sulfur dioxide;
reacting calcium oxide with water to form calcium hydroxide.
[00200] 27. The process of example embodiment 26 further
comprising separating the
aqueous magnesium sulfite into water and magnesium sulfite solid.
[00201] 28. The process of example embodiment 27 comprising:
Precipitating a portion of magnesium sulfite from a first solution comprising
concentrated
aqueous magnesium sulfite by cooling; and
Separating magnesium sulfite solid precipitate from the remaining solution
comprising a
second solution comprising aqueous magnesium sulfite; and
Mixing said second solution comprising aqueous magnesium sulfite with a third
solution
comprising magnesium sulfite introduced from the process to form a fourth
solution; and
Heating said fourth solution to a higher temperature; and
Separating said fourth solution at a higher temperature using reverse osmosis
into a
retentate comprising a first solution comprising concentrated aqueous
magnesium sulfite and a
permeate comprising water.
[00202] 29. The process of example embodiment 27 comprising:
Precipitating a portion of magnesium sulfite from a first solution comprising
concentrated
aqueous magnesium sulfite by cooling; and
Separating magnesium sulfite solid precipitate from the remaining solution
comprising a
second solution comprising aqueous magnesium sulfite; and
Heating said second solution comprising aqueous magnesium sulfite to a higher
temperature; and
Mixing said second solution comprising aqueous magnesium sulfite at a higher
temperature with a third solution comprising magnesium sulfite introduced from
the process to
form a fourth solution; and
144
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
Separating said fourth solution at a higher temperature using reverse osmosis
into a
retentate comprising a first solution comprising concentrated aqueous
magnesium sulfite and a
permeate comprising water.
[00203] 30. A process for producing sodium hydroxide and gypsum
from a material
comprising alkaline earth wherein the process comprises:
reacting a material comprising alkaline earth cation - weak acid anion with
sulfur dioxide
and an aqueous solution to form a weak acid derivative and an aqueous solution
comprising
alkaline earth bisulfite;
separating said weak acid derivative from said aqueous solution comprising
alkaline earth
bisulfite;
reacting aqueous alkaline earth bisulfite with sodium sulfate to form aqueous
solution
comprising sodium bisulfite and a solid comprising an alkaline earth sulfate;
separating said solid comprising an alkaline earth sulfate from said aqueous
solution
comprising sodium bisulfite;
removing water from said aqueous solution comprising sodium bisulfite to form
sodium
metabisulfite;
decomposing sodium metabisulfite to form sodium sulfite and sulfur dioxide
gas;
reacting said sodium sulfite with alkaline earth hydroxide to form sodium
hydroxide and
an alkaline earth sulfite;
separating said alkaline earth sulfite from said aqueous solution comprising
sodium
hydroxide;
decomposing alkaline earth sulfite to form alkaline earth oxide and sulfur
dioxide;
reacting alkaline earth oxide with water to form alkaline earth hydroxide.
Additional Example Embodiments:
[00204] 1. A process for producing sodium hydroxide and gypsum
from a material
comprising calcium wherein the process comprises:
reacting a material comprising calcium carbonate with a solution comprising
aqueous
sulfur dioxide to form a gas comprising CO2 and a solid comprising calcium
sulfite;
separating said solid comprising calcium sulfite;
reacting the calcium sulfite solid with a solution comprising aqueous sulfur
dioxide to
form aqueous calcium bisulfite;
reacting aqueous calcium bisulfite with sodium sulfate to form aqueous sodium
bisulfite
and a solid comprising calcium sulfate;
separating said solid comprising calcium sulfate from said aqueous solution
comprising
sodium bisulfite;
145
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
removing water from said aqueous solution comprising sodium bisulfite to form
sodium
metabisulfite;
decomposing said sodium metabisulfite to form sodium sulfite and sulfur
dioxide gas;
reacting said sodium sulfite with calcium hydroxide to form an aqueous
solution
comprising sodium hydroxide and a solid comprising calcium sulfite;
separating said solid comprising calcium sulfite from said aqueous solution
comprising
sodium hydroxide;
decomposing calcium sulfite to form calcium oxide and sulfur dioxide;
reacting calcium oxide with water to form calcium hydroxide.
[00205] 1. A process for producing sodium hydroxide from a
material comprising alkaline
earth wherein the process comprises:
reacting a material comprising alkaline earth cation - weak acid anion with a
solution
comprising aqueous sulfur dioxide to form a weak acid derivative and a solid
comprising alkaline
earth sulfite;
separating said solid comprising alkaline earth sulfite and said weak acid
derivative;
reacting the alkaline earth sulfite with a solution comprising aqueous sulfur
dioxide to
form aqueous alkaline earth bisulfite;
reacting aqueous alkaline earth bisulfite with sodium sulfate to form aqueous
sodium
bisulfite and a solid comprising alkaline earth sulfate;
separating said solid comprising alkaline earth sulfate from said aqueous
solution
comprising sodium bisulfite;
removing water from said aqueous solution comprising sodium bisulfite to form
sodium
metabisulfite;
decomposing said sodium metabisulfite to form sodium sulfite and sulfur
dioxide gas;
reacting said sodium sulfite with calcium hydroxide to form an aqueous
solution
comprising sodium hydroxide and a solid comprising calcium sulfite;
separating said solid comprising calcium sulfite from said aqueous solution
comprising
sodium hydroxide;
decomposing calcium sulfite to form calcium oxide and sulfur dioxide;
reacting calcium oxide with water to form calcium hydroxide.
[00206] 1. A process for producing sodium hydroxide and gypsum
from a material
comprising alkaline earth wherein the process comprises:
reacting a material comprising alkaline earth cation - weak acid anion with a
solution
comprising aqueous sulfur dioxide to form a weak acid derivative and an
aqueous solution
comprising alkaline earth sulfite;
146
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
separating said weak acid derivative from said aqueous solution comprising
alkaline earth
sulfite;
reacting aqueous alkaline earth sulfite with sodium sulfate to form aqueous
sodium sulfite
and a solid comprising an alkaline earth sulfate;
separating said solid comprising an alkaline earth sulfate from said aqueous
solution
comprising sodium sulfite;
reacting said sodium sulfite with calcium hydroxide to form an aqueous
solution
comprising sodium hydroxide and a solid comprising calcium sulfite;
separating said solid comprising calcium sulfite from said aqueous solution
comprising
sodium hydroxide;
decomposing calcium sulfite to form calcium oxide and sulfur dioxide;
reacting calcium oxide with water to form calcium hydroxide.
[00207] 1. A process for producing sodium hydroxide and
magnesium sulfate from a
material comprising magnesium wherein the process comprises:
reacting a material comprising magnesium - weak acid with a solution
comprising
aqueous sulfur dioxide to form a weak acid derivative and an aqueous solution
comprising
magnesium sulfite;
separating said weak acid derivative from said aqueous solution comprising
magnesium
sulfite;
reacting aqueous magnesium sulfite with sodium sulfate to form aqueous sodium
sulfite
and magnesium sulfate;
separating at least a portion of said magnesium sulfate from said sodium
sulfite;
reacting said sodium sulfite with calcium hydroxide to form an aqueous
solution
comprising sodium hydroxide and a solid comprising calcium sulfite;
separating said solid comprising calcium sulfite from said aqueous solution
comprising
sodium hydroxide;
decomposing calcium sulfite to form calcium oxide and sulfur dioxide;
reacting calcium oxide with water to form calcium hydroxide.
[00208] 1. A process for producing sodium hydroxide and
magnesium sulfate from a
material comprising magnesium wherein the process comprises:
reacting a material comprising magnesium - weak acid with a solution
comprising
aqueous sulfur dioxide to form a weak acid derivative and an aqueous solution
comprising
magnesium sulfite;
separating said weak acid derivative from said aqueous solution comprising
magnesium
sulfite;
147
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
reacting aqueous magnesium sulfite with sodium sulfate to form aqueous sodium
sulfite
and magnesium sulfate;
separating at least a portion of said sodium sulfite from said magnesium
sulfate;
reacting said sodium sulfite with calcium hydroxide to form an aqueous
solution
comprising sodium hydroxide and a solid comprising calcium sulfite;
separating said solid comprising calcium sulfite from said aqueous solution
comprising
sodium hydroxide;
decomposing calcium sulfite to form calcium oxide and sulfur dioxide;
reacting calcium oxide with water to form calcium hydroxide.
[00209] 1. A process for producing sodium hydroxide and gypsum
from a material
comprising alkaline earth wherein the process comprises:
reacting a material comprising alkaline earth cation - weak acid anion with a
solution
comprising aqueous sulfur dioxide to form a weak acid derivative and an
aqueous solution
comprising alkaline earth bisulfite;
separating said weak acid derivative from said aqueous solution comprising
alkaline earth
bisulfite;
reacting aqueous alkaline earth bisulfite with sodium sulfate to form aqueous
solution
comprising sodium bisulfite and a solid comprising an alkaline earth sulfate;
separating said solid comprising an alkaline earth sulfate from said aqueous
solution
comprising sodium bisulfite;
removing water from said aqueous solution comprising sodium sulfite or sodium
bisulfite
to form sodium metabisulfite;
decomposing sodium metabisulfite to form sodium sulfite and sulfur dioxide
gas;
reacting said sodium sulfite with calcium hydroxide to form an aqueous
solution
comprising sodium hydroxide and a solid comprising calcium sulfite;
separating said solid comprising calcium sulfite from said aqueous solution
comprising
sodium hydroxide;
decomposing calcium sulfite to form calcium oxide and sulfur dioxide;
reacting calcium oxide with water to form calcium hydroxide.
[00210] 1. A process for producing sodium hydroxide and gypsum
from a material
comprising alkaline earth wherein the process comprises:
reacting a material comprising alkaline earth cation - weak acid anion with a
solution
comprising aqueous sulfur dioxide to form a weak acid derivative and an
aqueous solution
comprising alkaline earth sulfite or bisulfite;
14g
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
separating said weak acid derivative from said aqueous solution comprising
alkaline earth
sulfite or bisulfite;
reacting aqueous alkaline earth sulfite or bisulfite with sodium sulfate to
form aqueous
sodium sulfite or bisulfite and a solid comprising an alkaline earth sulfate;
separating said solid comprising an alkaline earth sulfate from said aqueous
solution
comprising sodium sulfite or sodium bisulfite;
removing water from said aqueous solution comprising sodium sulfite or sodium
bisulfite
to form sodium sulfite or sodium metabisulfite;
decomposing sodium metabisulfite to form sodium sulfite and sulfur dioxide
gas;
reacting said sodium sulfite with calcium hydroxide to form an aqueous
solution
comprising sodium hydroxide and a solid comprising calcium sulfite;
separating said solid comprising calcium sulfite from said aqueous solution
comprising
sodium hydroxide;
decomposing calcium sulfite to form calcium oxide and sulfur dioxide;
reacting calcium oxide with water to form calcium hydroxide.
[00211] 2. The process of example embodiment 1 wherein the weak
acid derivative
comprises carbon dioxide.
[00212] 3. The process of example embodiment 1 wherein the weak
acid derivative
comprising silicon dioxide.
[00213] 4. The process of example embodiment 1 wherein the
alkaline earth comprises
one or more or any combination of the following: beryllium (Be), or magnesium
(Mg), or
calcium (Ca), or strontium (Sr), or barium (Ba), or radium.
[00214] 5. The process of example embodiment 1 wherein said
water removal comprises
precipitation, or crystallization, or cryodesalination, or freezing
desalination, or distillation, or
membrane based process, or forward osmosis, or reverse osmosis, or multi
effect distillation, or
mechanical vapor compression distillation, or multistage flash distillation,
or membrane
distillation, or heat recovery distillation, or zero liquid discharge.
[00215] 6. The process of example embodiment 1 wherein the heat
from the reaction of
calcium oxide with water to form calcium hydroxide is used to thy calcium
sulfite before
decomposing calcium sulfite.
[00216] 7. The process of example embodiment 1 wherein the heat
from the reaction of
calcium oxide with water to form calcium hydroxide is used to facilitate the
removal of water
from the aqueous solution comprising sodium sulfite or sodium bisulfite.
[00217] . The process of example embodiment wherein magnesium
sulfite solid is
separated from said aqueous magnesium sulfite by cooling precipitation.
149
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
[00218]
. The process of example embodiment wherein water is separated from said
aqueous magnesium sulfite by reverse osmosis.
Example Embodiments Sodium Bicarbonate and / or Sodium Carbonate Production
using
Calcium or Magnesium Input with an Ammonia Intermediate
[00219] 1. A process for producing sodium carbonate and gypsum
from a material
comprising an alkaline earth wherein the process comprises:
reacting ammonium carbonate with a solution comprising aqueous sodium sulfate
to form
ammonium sulfate and sodium carbonate;
reacting a material comprising alkaline earth cation - weak acid anion with
sulfur dioxide
and an aqueous solution to form a weak acid derivative and an aqueous solution
comprising
alkaline earth bisulfite;
reacting the aqueous solution comprising alkaline earth bisulfite with
ammonium sulfate
to form aqueous ammonium bisulfite and an alkaline earth sulfate;
desorbing sulfur dioxide from said aqueous ammonium bisulfite to form ammonium
sulfite and sulfur dioxide gas;
reacting said ammonium sulfite with carbon dioxide to form ammonium bisulfite
and
ammonium bicarbonate;
decomposing said ammonium bicarbonate to form ammonium carbonate and carbon
dioxide gas.
[00220] 2. The process of example embodiment 1 wherein said
formed carbon dioxide gas
is employed in the reaction of ammonium sulfite and carbon dioxide.
[00221] 3. The process of example embodiment 1 wherein aqueous
ammonium bisulfite is
transformed into ammonium sulfite solid, water, and sulfur dioxide gas.
[00222] 4. The process of example embodiment 3 wherein said
transforming comprises
thermal desorption or distillation.
[00223] 5. The process of example embodiment 3 wherein said
ammonium sulfite is
employed in the reaction of ammonium sulfite with carbon dioxide.
[00224] 6. The process of example embodiment 3 wherein residual
ammonium
bicarbonate is present; and wherein said residual ammonium bicarbonate is
decomposed to
produce carbon dioxide.
[00225] 7. The process of example embodiment 6 wherein said
carbon dioxide is
employed in the reaction of ammonium sulfite with carbon dioxide.
[00226] 8. The process of example embodiment 1 wherein the
alkaline earth comprises
one or more or any combination of the following: beryllium (Be), or magnesium
(Mg), or
calcium (Ca), or strontium (Sr), or barium (Ba), or radium (Ra).
150
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
[00227] 9. The process of example embodiment 1 wherein said
weak acid derivative
comprises a derivative of an acid with an acid strength lower than or less
acidic than sulfurous
acid.
[00228] 10. The process of example embodiment 1 wherein said
weak acid derivative
comprises a gas selected from carbon dioxide, or hydrogen sulfide, or a
mixture thereof.
[00229] 11. The process of example embodiment 1 wherein said
weak acid derivative
comprises silicon dioxide, or iron oxide, or manganese oxide, or aluminum
oxide, or a mixture
thereof
[00230] 12. The process of example embodiment 1 wherein said
sulfur dioxide and an
aqueous solution comprises aqueous sulfur dioxide.
[00231] 13. The process of example embodiment 1 wherein
Na2CO3is separated from
(NH4)2SO4(aq) using the significant solubility difference in water between
Na2CO3 and
(NH4)2SO4(aq).
[00232] 14. The process of example embodiment 1 wherein the
alkaline earth sulfate is
separated from the aqueous ammonium bisulfite as a solid precipitate.
[00233] 15. The process of example embodiment 1 wherein
ammonium bicarbonate is
separated from ammonium bisulfite as a solid precipitate.
[00234] 16. A process for producing sodium bicarbonate and
gypsum from a material
comprising an alkaline earth wherein the process comprises:
reacting ammonium bicarbonate with a solution comprising aqueous sodium
sulfate to
form ammonium sulfate and sodium bicarbonate;
reacting a material comprising alkaline earth cation - weak acid anion with
sulfur dioxide
and an aqueous solution to form a weak acid derivative and an aqueous solution
comprising
alkaline earth bisulfite;
reacting the aqueous solution comprising alkaline earth bisulfite with
ammonium sulfate
to form aqueous ammonium bisulfite and an alkaline earth sulfate;
desorbing sulfur dioxide from said aqueous ammonium bisulfite to form ammonium
sulfite and sulfur dioxide gas;
reacting said ammonium sulfite with carbon dioxide to form ammonium bisulfite
and
ammonium bicarbonate.
[00235] 17. The process of example embodiment 1 wherein aqueous
ammonium bisulfite
is transformed into ammonium sulfite solid, water, and sulfur dioxide gas.
[00236] 18. The process of example embodiment 17 wherein said
transforming comprises
thermal desorption or distillation.
151
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
[00237] 19. The process of example embodiment 17 wherein said
ammonium sulfite is
employed in the reaction of ammonium sulfite with carbon dioxide.
[00238] 20. The process of example embodiment 17 wherein
residual ammonium
bicarbonate is present; and wherein said residual ammonium bicarbonate is
decomposed to
produce carbon dioxide.
[00239] 21. The process of example embodiment 20 wherein said
carbon dioxide is
employed in the reaction of ammonium sulfite with carbon dioxide.
1002401 22. The process of example embodiment 16 wherein the
alkaline earth comprises
one or more or any combination of the following: beryllium (Be), or magnesium
(Mg), or
calcium (Ca), or strontium (Sr), or barium (Ba), or radium (Ra).
[00241] 23. The process of example embodiment 16 wherein said
weak acid derivative
comprises a derivative of an acid with an acid strength lower than or less
acidic than sulfurous
acid.
[00242] 24. The process of example embodiment 16 wherein said
weak acid derivative
comprises a gas selected from carbon dioxide, or hydrogen sulfide, or a
mixture thereof
[00243] 25. The process of example embodiment 16 wherein said
weak acid derivative
comprises silicon dioxide, or iron oxide, or manganese oxide, or aluminum
oxide, or a mixture
thereof.
[00244] 26. The process of example embodiment 16 wherein said
sulfur dioxide and an
aqueous solution comprises aqueous sulfur dioxide.
[00245] 27. The process of example embodiment 16 wherein
NaHCO3is separated from
(NH4)2SO4(aq) using the significant solubility difference in water between
NaHCO3 and
(NH4)2SO4(aq).
[00246] 28. The process of example embodiment 16 wherein the
alkaline earth sulfate is
separated from the aqueous ammonium bisulfite as a solid precipitate.
[00247] 29. The process of example embodiment 16 wherein
ammonium bicarbonate is
separated from ammonium bisulfite as a solid precipitate.
Example Embodiments Sodium Bicarbonate and / or Sodium Carbonate Production
using
Calcium or Magnesium Input
[00248] 1. A process for producing sodium bicarbonate and
gypsum from a material
comprising an alkaline earth wherein the process comprises:
reacting a material comprising alkaline earth cation - weak acid anion with
sulfur dioxide
and an aqueous solution to form a weak acid derivative and an aqueous solution
comprising
alkaline earth bisulfite;
152
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
reacting the aqueous solution comprising alkaline earth bisulfite with sodium
sulfate to
form aqueous sodium bisulfite and an alkaline earth sulfate;
separating sodium metabisulfite from said aqueous solution comprising sodium
bisulfite;
decomposing said sodium metabisulfite to form sodium sulfite and sulfur
dioxide gas;
reacting said sodium sulfite with carbon dioxide to form sodium bisulfite and
sodium
bicarbonate;
decomposing said ammonium bicarbonate to form ammonium carbonate and carbon
dioxide gas.
[00249] 2. The process of example embodiment 1 wherein sodium
bicarbonate is separated
from sodium bisulfite due to the difference in solubility between sodium
bicarbonate and sodium
bisulfite in water.
[00250] 3. The process of example embodiment 1 wherein said
separating sodium
metabisulfite from said aqueous solution comprising sodium bisulfite comprises
precipitating
sodium metabisulfite.
[00251] 4. The process of example embodiment 1 wherein said
separating sodium
metabisulfite from said aqueous solution comprising sodium bisulfite comprises
removing water
using one or more or any combination of the following: multistage flash
distillation, or multi-
effect distillation, or mechanical vapor compression distillation, or
electrodialysis, or
electrodialysis reversal, or forward osmosis, or membrane distillation, or
evaporation, or
crystallization, or solventing out.
[00252] 5. The process of example embodiment 1 wherein
remaining aqueous sodium
bisulfite from the reaction of sodium sulfite with carbon dioxide is separated
into solid sodium
metabisulfite.
[00253] 6. The process of example embodiment 1 wherein residual
sodium bicarbonate is
present in the sodium metabisulfite; and
Wherein said residual sodium bicarbonate decomposes into carbon dioxide.
[00254] 7. The process of example embodiment 6 wherein said
carbon dioxide is
employed as a portion of the carbon dioxide in the reaction of sodium sulfite
with carbon
dioxide.
[00255] 8. The process of example embodiment 1 wherein the
partial pressure of sulfur
dioxide gas formed from the decomposing of sodium metabisulfite is greater
than 0.5 atm.
[00256] 9. The process of example embodiment 1 wherein the
alkaline earth comprises
one or more or any combination of the following: beryllium (Be), or magnesium
(Mg), or
calcium (Ca), or strontium (Sr), or barium (Ba), or radium (Ra).
153
CA 03218484 2023- 11- 8
WO 2022/241219
PCT/US2022/029198
[00257] 10. The process of example embodiment 1 wherein said
weak acid derivative
comprises a derivative of an acid with an acid strength lower than or less
acidic than sulfurous
acid.
[00258] 12. The process of example embodiment 1 wherein said
weak acid derivative
comprises a gas selected from carbon dioxide, or hydrogen sulfide, or a
mixture thereof.
[00259] 13. The process of example embodiment 1 wherein said
weak acid derivative
comprises silicon dioxide, or iron oxide, or manganese oxide, or aluminum
oxide, or a mixture
thereof
[00260] 14. The process of example embodiment 1 wherein said
sulfur dioxide and an
aqueous solution comprises aqueous sulfur dioxide.
[00261] 15. The process of example embodiment 1 wherein said
sodium bicarbonate is
decomposed to form sodium carbonate and carbon dioxide.
[00262] 16. The process of example embodiment 15 wherein said
formed carbon dioxide
is employed in the reaction of sodium sulfite and carbon dioxide.
[00263] 17. The process of example embodiment 11 wherein said
sulfur dioxide and an
aqueous solution comprises reacting sulfur dioxide gas with an aqueous
solution in the presence
of alkaline earth-'weak acid' to facilitate the formation of aqueous alkaline
earth bisulfite.
[00264] 18. The process of example embodiment 1 wherein the
weak acid derivative is
separated from the aqueous solution using a solid-liquid separation method.
154
CA 03218484 2023- 11- 8