Note: Descriptions are shown in the official language in which they were submitted.
WO 2022/240964
PCT/US2022/028733
PHARMACEUTICAL COMPOSITION AND METHOD FOR TREATMENT OF
ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) IN CORONAVIRUS
DISEASE (COVID-19)
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority to and the benefit of U.S.
Provisional Application No.
63/187,077 filed on May 11, 2021, the disclosure of which is incorporated
herein by reference in
its entirety.
FIELD
[0002] The aspects of the disclosed embodiments relates to methods
and compositions for
reduction of edema in the lungs, improvement in ratio of arterial partial
pressure of oxygen to
fraction of inspired oxygen (Pa02/Fi02 or Sp02/Fi02), blood oxygen saturation
(Sp02),
improvement in Ordinal Scale of COVID-19, normalization in respiratory rate,
reduction in lung
infiltration, improvement in ARDS score, MODS and better blood flow and
oxygenation of tissues,
thereby treating or preventing acute respiratory distress syndrome (ARDS),
multiple end organ
failure and shock symptoms in coronavirus disease (COVID-19) and other
diseases causing
ARDS. Thus far, the only treatment found to improve survival in ARDS is a
mechanical ventilation
strategy using low tidal volumes (6 ml_./kg based upon ideal body weight), In
particular, the present
disclosure discloses a method and pharmaceutical composition comprising
centhaquine in a
predefined amount and its analogues and/or administering antiviral therapies,
convalescent
plasma, stem cells or their exosomes, immunomodulation and cytokine-targeted
therapies, blood
purification systems, oxygen concentrator and generator, plasminogen
supplementation,
1
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
plasminogen activators, anticoagulants, steroids, inhaled synthetic
surfactant, antibody to
endotoxi n, interferon-beta-1a, IV prostaglandin El, neutrophil elastase
inhibitors, nitric oxide for
treating ARDS, multiple end organ failure and shock symptoms caused by
coronaviruses infection,
in particular SARS-CoV-2, MERS-CoV and SARS-CoV.
BACKGROUND
[0003] Human coronavirus (CoV) infections have traditionally caused
a low percentage of
annual respiratory infections There are HCoV-0C43, HCoV-229E, HCoV-NL63 and
HCoV-
HKU1, which cause mild respiratory illness (1, 2). Over the past two decades,
two novel
coronaviruses, severe acute respiratory syndrome CoV (SARS-CoV) and Middle
East respiratory
syndrome CoV (MERS-CoV), have emerged and caused severe human diseases (3, 4).
The 2019-
nCoV infection is of clustering onset and is more likely to affect older males
with comorbidities
and can result in severe and even fatal respiratory diseases such as acute
respiratory distress
syndrome (ARDS)
[0004] Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
infection leading to
coronavirus disease 2019 (COVED-19) has become a global pandemic. COVID-19
illness can
manifest from mild disease to severe life-threatening stage involving severe
pneumonia and acute
respiratory distress syndrome (ARDS) requiring admission in the intensive care
unit (ICU). In a
review by the WHO-China Joint Mission of 55,924 laboratory-confirmed cases in
China 6.1%
were classified as critical and 13.8% as severe. Critical stage is when there
is respiratory failure,
shock, and multiple organ dysfunction or failure. Severe disease is defined as
dyspnea, respiratory
2
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
frequency >30 breaths per minute, blood oxygen saturation (Sp02) <93%, ratio
of arterial partial
pressure of oxygen to fraction of inspired oxygen (Pa02/Fi02) <300, or lung
infiltrates >50%
within 24 to 48 hours. Thus, a total of about 20% of patients were in life
threatening situation.
About 25% of severe and critical cases required mechanical ventilation while
the remaining 75%
needed only oxygen supplementation.
[00051 An increasing incidence of COVID-19 illness is challenging
healthcare providers to
come up with appropriate treatment decisions. At present there is no widely
accepted standard of
care regarding pharmacotherapy of patients with COVID-19. It is an unmet
medical need and it is
critical that potential treatment strategies are identified on a priority
basis. There are numerous
novel agents that are either in clinical trials or are available through
emergency or compassionate
use. Most of these agents are repurposed antiviral agents and immune
modulating therapies (5).
On March 31st 2020 the U.S. Food and Drug Administration in a news release
mentioned that
there many therapeutic areas being evaluated, including antiviral drugs like
remdesivir that might
treat the specific virus, as well as host targets, such as interleukin-6 (IL-
6) receptor inhibitors that
may be helpful in reducing lung inflammation and improving lung function in
COVID-19 patients.
FDA is also interested in examining whether therapies such as convalescent
plasma and
hyperimmune globulin, antibody-rich blood products that are taken from blood
donated by people
who have recovered from the virus, could shorten the length or lessen the
severity of the illness.
FDA is also working to evaluate whether existing therapies such as chloroquine
and
hydroxychloroquine help treat patients with COVID-19. In addition,
pharmaceutical and biotech
companies in China have been gearing up to repurpose existing drugs as
treatments for the
3
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
coronavirus outbreak.
[00061 Coronavirus disease (COVID-19), which appeared in December
2019, presents a
global challenge, particularly in the rapid increase of critically ill
patients with pneumonia and
absence of definitive treatment As of March 19, 2020, over 241,000 cases have
been confirmed,
with over 9980 deaths. The mortality appears to be around 3-4%; early
published data indicate
25.9% with SARS-CoV-2 pneumonia required ICU admission and 20.1% developed
ARDS (6).
[00071 Vaccines
[00081 Several companies are working on the idea of using
formulations of RNA or DNA that
when injected into the body will initiate cells making a protein used by SARS-
CoV-2 (7). A DNA
vaccine candidate, INO-4800, designed to prevent COVID-19 infection is being
developed by
INOVIO Pharmaceuticals, Inc. An open-label trial to evaluate the safety,
tolerability and
immunological profile of INO-4800 administered by intradermal injection
followed by
electroporation in healthy adult volunteers is in progress (NCT04336410).
Moderna Therapeutics
and CureVac are moving fast with DNA and RNA vaccines against COVID-19 in
human testing.
Moderna' s mRNA-1273 is a novel lipid nanoparticle (LNP)-encapsulated mRNA-
based vaccine
that encodes for a full-length, prefusion stabilized spike (S) protein of SARS-
CoV-2. A phase I
study of mRNA-1273 sponsored by National Institute of Allergy and Infectious
Diseases (NIAID)
has begun at Emory University in Atlanta. (NCT04283461). BioNTech (partnered
with Pfizer)
and CureVac are set to start humans testing, of vaccines developed using
messenger RNA, within
the coming weeks. BioNTech will manufacture its vaccine, BNT162, at its
European mRNA
4
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
manufacturing facilities with the support of its CDMO partner Polymun.
However, no mRNA
vaccine is in the market, making this approach more of an unknown. Vaccines
using the synthetic
biology approach contain synthetic strands of RNA or DNA that code for protein
molecules on the
surface of the virus. The Bill and Melinda Gates Foundation and the National
Institute of Health
(NTH) are betting on synthetic biology to engineer new vaccines against the
COVID-19 virus. A
single-center, open-label, dose-escalating phase I clinical trial in healthy
subjects is being
conducted to assess the safety, reactogenicity and immunogenicity of
recombinant novel
coronavirus vaccine (Adenovirus Type 5 Vector (Ad5-nCoV)) manufactured by
Beijing Institute
of Biotechnology and CanSino Biologics Inc. (NCT04313127). A randomized,
double-blinded and
placebo-controlled trial in healthy adults (500 subjects) to evaluate the
immunogenicity and safety
of Ad5-nCoV which encodes for a full-length spike (S) protein of SARS-CoV-2 is
ongoing
(NCT04341389). Symvivo Corporation is evaluating the safety, tolerability and
immunogenicity
of bacTRL-Spike vaccine for prevention of COVID-19 (NCT04334980). Johnson &
Johnson
revealed a lead COVID-19 vaccine candidate that is being developed in
partnership with the U.S.
Biomedical Advanced Research and Development Authority.
[0009] The race to find and produce a safe and effective vaccine is
on and optimistically one
could be available in 12-18 months. It is possible that multiple vaccines
against COVID-19 could
be available on a limited basis by second quarter of next year. However, the
warnings are that it
may not be possible to produce enough vaccine to accommodate the demand (7).
[0010] Antiviral agents
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
[00111 Antiviral therapies for SARS-CoV-2 infection are under
intense investigation.
Remdesivir and chloroquine have been shown to effectively inhibit SARS-CoV-2
in vitro (8).
Remdesivir, a nucleotide analogue prodrug that inhibits viral RNA polymerases,
has shown in
vitro activity against SARS-CoV-2 (9, 10). A phase III randomized study in
2400 patients to
evaluate the safety and antiviral activity of remdesivir with severe COVID-19
is ongoing
(NCT04292899). Gilead Sciences is also conducting a trial in 1600 patients
with the primary
objective to evaluate the efficacy of two regimens of remdesivir compared to
standard of care,
where clinical status assessment will be done on 11th day of treatment in
moderate COVID-19
patients (NCT04292730). In addition, the U.S. National Institute of Allergy
and Infectious
Diseases has initiated a phase II adaptive, randomized, double-blind, placebo-
controlled trial to
evaluate remdesivir as a potential treatment for hospitalized adult patients
diagnosed with COVID-
19 (NCT04280705). Gilead provided remdesivir on a compassionate-use basis to
patients
hospitalized with confirmed COVID-19 and clinical improvement was observed in
36 of 53
patients (68%) and 7 of the 53 patients (13%) died (11).
[0012] Chloroquine is approved as an antimalarial and autoimmune
disease drug, however, in
vitro testing showed that chloroquine acts as an endosomal acidification
fusion inhibitor and
blocked infection of a clinical isolate of SARS-CoV-2. Results showing
promising in vitro activity
of chloroquine against SARS-CoV-2 (8), promoted pilot clinical study to
determine efficacy of
this drug in COVID-19 patients with different levels of severity. A study
conducted in France
where confirmed COVID-19 patients were included in a single arm protocol to
receive 600 mg of
hydroxychloroquine daily and their viral load in nasopharyngeal swabs was
tested daily in a
6
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
hospital setting. Twenty cases were treated in this study and showed a
significant reduction of the
viral carriage at day 6 post inclusion compared to controls (12). Although
these results appear
positive, but the study excluded six patients in the hydroxychloroquine arm
because they did not
complete the study: one patient died, three were transferred to the ICU, and
two withdrew. On the
other hand, none of the 16 patients in the control group died, withdrew, or
needed care in an ICU.
An exploratory study is to be conducted in 400 patients to evaluate the
efficacy of
hydroxychloroquine and azithromycin to treat moderate to severe COVID-19
pneumonia
(NCT04329572). A randomized double-blind placebo-controlled clinical trial to
determine
hydroxychloroquine for chemoprophylaxis in healthcare workers exposed to COVID-
19 is being
conducted (NCT04328285). A double blind randomized clinical trial has been
designed to evaluate
the efficacy of hydroxychloroquine as treatment for COVID-19. The
investigators hypothesize that
a 400 mg per day dose of hydroxychloroquine for 10 days will reduce all-cause
hospital mortality
in patients with severe respiratory COVID-19 disease (NCT04315896). A triple
blinded, phase III
randomized controlled trial with parallel groups (200 mg of hydroxychloroquine
per day vs.
placebo) is aiming to prove safety and efficacy of hydroxychloroquine as
prophylaxis treatment
for healthcare personnel exposed to COVID-19 patients (NCT04318015). Sanofi
has sponsored a
clinical study to assess the effect of hydroxychloroquine versus placebo on
nasopharyngeal SARS-
CoV-2 viral load in outpatient adults with COVID-19 (NCT04333654). A study to
evaluate the
effectiveness and safety of hydroxychloroquine combined with azithromycin
compared to
hydroxychloroquine monotherapy in patients hospitalized with pneumonia by SARS-
CoV2 virus
(NCT04321278) is ongoing and another study to determine efficacy of
hydroxychloroquine and
7
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
azytromicyn for COVID-19 infection in hospitalized but noncritical patients
(NCT04322123) is in
progress.
[00131 The US Food and Drug Administration has authorized clinicians
to prescribe
chloroquine and hydroxychloroquine for patients admitted to hospital with
covid-19 In the
emergency use authorization issued on March 28th, 2020 the agency acknowledged
that the
approval was based on "limited in-vitro and anecdotal clinical data." Under
this emergency use
authorization chloroquine and hydroxychloroquine can only be used in a
hospital setting to treat
COVID-19 in adults weighing at least 50 kg. France is another country that
permits use of
chloroquine and hydroxychloroquine for COVID-19 patients. However, there is
opposition from
European Medicines Agency and WHO
[00141 A study which aims to assess the efficacy of a daily single
dose of tenofovir disoproxil
fumarate (TDF) (245 mg)/ Emtricitabine (FTC) (200 mg), a daily single dose of
hydroxychloroquine (HC) (200 mg), a daily single dose of TDF (245 mg)/FTC (200
mg) plus HC
(200 mg) versus placebo, during 12 weeks in (1) reducing the incidence of
symptomatic disease
and (2) reducing clinical severity COVID-19 among hospital healthcare workers
aged 18 to 65
years in public and private hospitals will be conducted in Spain
(NCT04334928). AbbVie has
sponsored a study where lopinavir/ritonavir will be administered 400 mg/100 mg
orally (or weight-
based dose adjustment for children) for a 14-day course, or until discharge
from hospital,
whichever occurs first. This study will have 2-arms in a 1:1 ratio
randomization to either the
control arm, consisting of standard of care supportive treatment for COVID-19,
or the
investigational product, lopinavir/ritonavir plus standard of care
(NCT04330690). Ascletis Pharma
8
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
has applied to the Chinese authorities to test two HIV protease inhibitors
(ritonavir and ASC09) to
treat COVID-19. They are conducting a randomized, open-label, multi-center
trial to evaluate the
safety and efficiency of A SC09/ritonayi r and lopinayir/ritonavir in
pneumonia caused by COVID-
19 (NCT04261907). Favipiravir, also known as T-705 or Avigan, is a pyrazine
derivative that acts
as an inhibitor of viral RNA-dependent RNA polymerase (13). It has
demonstrated activity against
influenza viruses and has been approved in Japan and China for the treatment
of novel influenza
virus infections and is therefore an attractive candidate for study in
patients with COVID-19.
Danoprevir, an oral Hepatitis C virus protease inhibitor, approved in China in
June 2018 is being
investigated to evaluate its efficacy and safety in hospitalized patients
infected with SARS-CoV-
2 (NCT04291729).
[0015] SARS-CoV-2 uses the receptor angiotensin-converting enzyme
(ACE) 2 for entry into
target cells (Hoffmann et al., 2020) and that both ACEI and ARB could
significantly increase
mRNA expression of cardiac ACE2 (14). The use of ACEIs/ARBs in patients with
COVID-19 or
at risk of COVID-19 infection is currently a subject of intense debate. A
multicenter, double-blind,
placebo-controlled phase II randomized clinical trial of starting losartan in
patients with COVID-
19 in outpatient settings (NCT04311177) and in inpatient settings
(NCT04312009) is currently
being planned. In addition, Apeiron Biologics is starting a study using
recombinant human
angotensin-converting enzyme 2 (rhACE2) as a treatment for patients with COVID-
19 to block
viral entry and decrease viral replication (NCT04335136).
[0016] Jiangxi Qingfeng Pharmaceutical Co. Ltd. Xiyanping injection
has anti-inflammatory
and immune regulation effects. A randomized, parallel controlled clinical
study to treat patients
9
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
with COVID-19 infection is in progress at multiple centers to determine the
efficacy and safety of
Xiyanping (NCT04295551). The study design has two groups having
lopinavir/ritonavir tablets
with (experimental) or without (control) Xiyanping. Another clinical trial is
also planned to
determine safety and efficacy of Xiyanping in patients with coronavirus
infection pneumonia. In
this study lopinavir/ritonavir, alpha-interferon nebulization is the
comparator group, while
experimental group will receive lopinavir/ritonavir, alpha-interferon
inhalation plus Xiyanping
injection (NCT04275388). Ansun BioPharma of San Diego, California is
developing lead
candidate Fludase (DAS181), which has shown potential for the treatment of
parainfluenza,
influenza and other viruses is being tried in severe COVID-19 patients on
compassionate use basis
(NCT04324489). Eicosapentaenoic acid free fatty acid (EPA-FFA), an omega-3
fatty acid, is being
developed by S.L.A. Pharma AG and planning to conduct a randomized controlled
study to treat
hospitalized subjects with confirmed SARS-CoV-2 (NCT04335032).
[0017] Nitric oxide has inhibitory effects on a variety of viral
infections and its inhalation has
been shown to be safe. University of British Columbia in collaboration with
Mallinckrodt is
conducting a study using inhaled gaseous nitric oxide antimicrobial treatment
of COVID-19
infections (NCT03331445). Sanotize Research and Development Corp. in
collaboration with the
Emmes Company, LLC are planning to conduct a multicenter, randomized,
controlled study to
determine the efficacy of nitric oxide releasing solution treatment on the
prevention and treatment
of COVID-19 in healthcare workers and individuals at risk of infection
(NCT04337918). PUL-
042 is an inhalation solution consisting of a combination of two toll-like
receptor ligands:
Pam2CSK4 acetate, an agonist of TLR2 and TLR6, and a TLR9 agonist
oligodeoxynucleotide
1()
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
with potential immunostimulating activity. Pulmotect, Inc. is conducting two
clinical studies to
evaluate the efficacy and safety of PUL-042 Inhalation Solution in reducing
the severity of
COVID-19 (NCT04312997; NCT04313023).
[0018] Convalescent plasma
[0019] Convalescent plasma from patients who have recovered has been
suggested to be safe
and effective in SARS-CoV-2-infected patients. In an uncontrolled case series
of 5 critically ill
patients with COVID-19 and ARDS, convalescent plasma having neutralizing
antibody showed
an improvement in clinical status (15). A study conducted in two patients of
COVID-19 with
severe pneumonia and ARDS treated with convalescent plasma infusion showed
favorable
outcome (16). US Food and Drug Administration (FDA) announced on March 24th,
2020 that it
is facilitating access to convalescent plasma, antibody-rich blood products
that are taken from
blood donated by people who have recovered from the COV1D-19 virus, could
shorten the length,
or lessen the severity, of illness in COVID-19 patients. The classification of
convalescent plasma
as an investigational new drug by the FDA permits conducting clinical trials
and compassionate
use to treat patients with serious or life-threatening COV1D-19 infections
(17) via an emergency
investigational new drug application (eIND). Red Cross has been asked by FDA
to help identify
prospective donors and manage the distribution of these products to hospitals
that are treating
COV1D-19 patients.
[0020] Stem Cells
[0021] Experimental studies have demonstrated that mesenchymal stem
cells (MSCs) or their
11
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
exosomes (MSCs-Exo) significantly reduced lung inflammation and pathological
impairment
resulting from different types of lung injury. A pilot clinical study is being
conducted using aerosol
inhalation of the exosomes derived from all ogeni c adipose mesenchymal stem
cells in the
treatment of severe patients with novel coronavirus pneumonia (NCT04276987).
Tianhe Stem Cell
Biotechnologies Inc. has developed Stem Cell Educator (SCE) technology to
reverse autoimmune
response using human multipotent cord blood stem cells. SCE therapy is an
attempt to restore
immune balance and correct the overreaction of immune responses, the
investigators therefore plan
to treat COVID-19 patients with SCE therapy (NCT04299152). CAR-T (Shanghai)
Biotechnology
Co., Ltd. is conducting a clinical study to treat novel coronavirus induced
severe pneumonia by
dental pulp mesenchymal stem cells via an open, single center, single arm in
24 subjects
(NCT04302519). A mesenchymal stem cell therapy produced by Cellavita is to
assess its efficacy
as an add-on therapy to standard treatment to treat patients with severe COVID-
19 pneumonia
(NCT04315987).
[0022] Inflammatory response
[0023] COV1D-19 patients with certain risk factors seem to die by an
overwhelming reaction
of the immune system to the virus, causing a cytokine storm with features of
cytokine-release
syndrome (CRS) and macrophage activation syndrome (MAS) and ARDS. There is
evidence that
cytokine-targeted therapies can improve outcomes in CRS or MAS. Neutralization
of the
inflammatory pathway induced by TL-6 may reduce mortality in patients with
severe COVID-19
prone to CRS and ARID S. Tocilizumab (developed by Genentech, Roche), an anti-
IL-6R biological
therapy, has been approved for the treatment of CRS and is used in patients
with MAS. It is
12
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
hypothesized that it can reduce mortality in patients with severe COVID-19
prone to CRS and
ARDS. The overall purpose of this study is to evaluate whether treatment with
tocilizumab reduces
the severity and mortality in patients with COVID-19. A multicenter, double-
blind, randomized
controlled phase II trial to determine the efficacy and safety of tocilizumab
in the treatment of
COVID-19 is being conducted in 100 patients (NCT04335071). Another randomized,
double-
blind, placebo-controlled, multicenter study to evaluate the safety and
efficacy of tocilizumab in
patients with severe COVID-19 pneumonia is in progress (NCT04320615).
[0024] Sarilumab, is being jointly developed by Regeneron and
Sanofi, it is a fully human,
monoclonal antibody that inhibits the IL-6 pathway by binding and blocking the
IL-6 receptors.
IL-6 may play a key role in driving the inflammatory response that leads to
morbidity and mortality
and patients with COVID-19 who develop ARDS. An adaptive phase 111111,
randomized, double-
blind, placebo-controlled study assessing efficacy and safety of sarilumab for
hospitalized patients
with COVID-19 is in progress of enrolling 400 patients (NCT04315298). Another
study is in
progress with the primary objective of evaluating the efficacy of sarilumab
relative to the control
arm in adult patients hospitalized with severe COVID-19 (NCT04327388).
AstraZeneca would
start a new clinical trial of acalabrutinib aimed at assessing it as a
treatment for COV1D-19.
Acalabrutinib belongs to a class of drugs called Bruton's tyrosine kinase
(BTK) inhibitors which
can suppress autoimmune diseases and the trial will be to determine if it can
prevent over reaction
of the immune system producing cytokine storm in patients with COVID-19.
[0025] Piclidenoson is an anti-inflammatory agent that induces a
robust anti-inflammatory
effect, hence a trial has been proposed where hospitalized patients with COVID-
19 will be
13
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
randomized 1:1 to receive piclidenoson with standard care (intervention arm)
or standard care
alone (control arm) (NCT04333472). Tradipitant is an NK-1R antagonist being
developed by
Vanda Pharmaceuticals. A randomized, double-blind placebo-controlled trial to
investigate the
efficacy and safety of tradipitant to treat inflammatory lung injury
associated with severe or critical
COVID-19 infection is being planned (NCT04326426). OncoImmune, Inc. is
conducting a phase
III trial to determine efficacy of CD24Fc as a non-antiviral immunomodulator
in COVID-19
treatment (NCT04317040). This trial will involve 230 patients randomized into
blinded placebo
and CD24Fc arms, with time to clinical improvement from severe to mild symptom
as the primary
endpoint. Swedish Orphan Biovitrum is sponsoring a phase
randomized, open-label, parallel
group, 3-arm, multicenter study investigating the efficacy and safety of
intravenous
administrations of emapalumab, an anti-interferon gamma (Anti-IFNy) monoclonal
antibody, and
anakinra, an interleukin-1(IL-1) receptor antagonist, versus standard of care,
in reducing hyper-
inflammation and respiratory distress in patients with SARS-CoV-2 infection
(NCT04324021). I-
Mab Biopharma Co. Ltd. is conducting a randomized, double-blind, placebo-
controlled, multi-
center trial to evaluate the safety and efficacy of TJ003234 (anti-GM-CSF
monoclonal antibody)
administered as an intravenous infusion in subjects with severe COVID-19 under
supportive care,
and to assess its effect on cytokines levels (NCT04341116). The potential for
anti-viral activity by
calpain inhibition in animal bleomycin lung injury models demonstrated that
BLD-2660 (Blade
Therapeutics) normalized tissue IL-6 levels. Therefore, a study to evaluate
BLD-2660 as an add-
on therapy to standard of care in hospitalized subjects with COVID-19 has been
planned in 120
patients (NCT04334460).
14
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
[0026] The U.S. Food and Drug Administration on April 10th, 2020
issued an emergency use
authorization for a blood purification system to treat patients with COVID-19
admitted to the ICU
with confirmed or imminent respiratory failure. Spectra Optia Apheresis System
and Depuro
D2000 Adsorption Cartridge developed by Terumo BCT Inc. and Marker
Therapeutics AG work
by reducing the amount of cytokines and other inflammatory mediators
associated with cytokine
storm in the bloodstream by filtering the blood and returning the filtered
blood to the patient.
[0027] Other therapies
[0028] Corticosteroids have been tried in different scenarios of
ARDS, including viral
pneumonia, and the early use of dexamethasone appears to reduce the duration
of mechanical
ventilation in ARDS patients. A study to evaluate the effectiveness of
dexamethasone compared
to control (no corticosteroids) in ventilator-free days in patients with
moderate and severe ARDS
due to COV1D-19 (NCT04327401).
[0029] A clinical study has been initiated by Tasly Pharmaceuticals,
Inc. This is an open-label,
randomized, blank-controlled treatment clinical study with an objective to
investigate the effect of
T89 (dantonic) on improving oxygen saturation and clinical symptoms in
patients with non-critical
type of COVID-19 pneumonia. The primary efficacy parameters include the time
to oxygen
saturation recovery to normal level (>97%), the proportion of patients with
normal level of oxygen
saturation after treatment, and the total duration of oxygen inhalation
(NCT04285190).
[0030] A study that will evaluate the efficacy and safety of
Hydrogen-Oxygen Generator with
Nebulizer (model AMS-H-03) developed by Shanghai Asclepius Meditech Co., Ltd.
as an adjuvant
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
therapy for the patients with COVID-19 infected pneumonia is recruiting
patients
(NCT04336462). This is an attempt to determine whether this device improves
the clinical
symptoms and reduces the incidence of severe pneumonia, as compared with the
reference device
of EverFlo Oxygen Concentrator (registration certificate No.: NMPA
Registration Standard:
20162542389) manufactured by Respironics, Inc. USA.
[00311 Plasminogen has been reported to significantly increase in
patients with ARDS and is
important in degrading core components of the extracellular matrix including
fibrin (18, 19).
Intravenous plasminogen supplementation was effective in reducing premature
infant ARDS and
death (20-22). Since lungs from patients with COVID-19 have shown typical
signs of ARDS, and
hyaline membrane formation is mainly composed of fibrin, a study was conducted
in 13 patients
to determine whether plasminogen supplementation may be effective in treating
lung lesions and
hypoxemia during COVID-19 infections. Inhalation of plasminogen (10 mg
dissolved in 2 ml
sterile water) was given twice daily for severe and once daily for moderate
COVID-19 patients. It
was found that 5 patients showed improvement in density of 'ground glass'
opacity and 6 patients
showed improved oxygen saturation. This study has major limitation of lack of
proper control
group, however, it indicates a possible hope of combating critically ill
patients with COVID-19
(23).
[0032] The coagulation and fibrinolytic systems have been considered
to improve ARDS (24,
25). Plasminogen activators have been indicated in preclini cal studies to
attenuate ARDS
progression and death (26, 27). It has been proposed that administration of
tPA, as a compassionate
approach, deserves merit because of high mortality in patients with COVID-19
suffering from
16
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
ARDS (28). In a report of 3 cases where off-label intravenous administration
of tPA (Alteplase)
was carried out in patients with COVID-19 suffering from ARDS and respiratory
failure. An
improvement in Pa02/Fi02 (P/F) or Sp02/Fi02 (S/F) ratio was observed in all 3
patients ranging
from 38% to 100%. However, improvements were transient and lost in all 3
patients after
completion of tPA infusion (29). Moreover, a high risk of catastrophic
bleeding from tPA must be
carefully considered despite high mortality in COVID-19 patients with ARDS.
[0033] There exists a need to develop a method and pharmaceutical
composition comprising
centhaquine in a predefined amount and its analogues, and/or antiviral drugs,
and/or supportive
therapies to reduce fever, and/or anticoagulants for reduction of edema in the
lungs, improvement
in ratio of arterial partial pressure of oxygen to fraction of inspired oxygen
(Pa02/Fi02 or
Sp02/Fi02), blood oxygen saturation (Sp02), normalization in respiratory rate,
reduction in lung
infiltration, improvement in ARDS score, MODS and better blood flow and
oxygenation of tissues
to treat ARDS, multiple end organ failure and shock symptoms caused by
coronaviruses infection,
in particular SARS-CoV-2, MERS-CoV and SARS-CoV.
SUMMARY
[0034] The present disclosure is envisioned towards a method and
pharmaceutical composition
comprising of centhaquine with or without antiviral therapies, convalescent
plasma, stem cells or
their exosomes, immunom odul ati on and cytokine-targeted therapies, blood
purification systems,
oxygen concentrator and generator, plasminogen supplementation, plasminogen
activators,
anticoagulants, steroids for improvement in ratio of arterial partial pressure
of oxygen to fraction
of inspired oxygen (Pa02/Fi02 or 5p02/Fi 02), blood oxygen saturation (Sp02),
normalization in
17
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
respiratory rate, reduction in lung infiltration, improvement in ARDS score,
MODS and better
blood flow and oxygenation of tissues to prevent or treat ARDS, multiple end
organ failure and
shock symptoms caused by coronaviruses infection, in particular SARS-CoV-2,
MERS-CoV and
SARS-CoV.
BRIEF DESCRIPTION OF DRAWINGS
[0035] These and other features, aspects, and advantages of the
present disclosure will become
better understood with regard to the following description, appended claims,
and accompanying
drawings where:
[0036] Figure 1 illustrates a proposal to use centhaquine as an add-
on treatment to provide
hemodynamic stability, improve acute respiratory distress syndrome (ARDS),
multiple organ
dysfunction score (MODS) and reduce mortality.
[0037] Figure 2 illustrates a graphical representation of
significant improvement in oxygen
saturation (Sp02) of COVID-19 patients by intravenous administration of
centhaquine in the dose
of 0.01 mg/kg was observed;
[0038] Figure 3 illustrates a graphical representation of
Centhaquine improved Sp02/Fi02 in
all 10 patients irrespective of age of the patient. Basal Sp02/Fi02 was found
to be slightly poor in
aged patients and the slope was -1.062, however, treatment with centhaquine
started flattening the
slope to -0.5905 at 2 hours and -0.2718 at 4 hours of treatment with
centhaquine;
[0039] Figure 4 illustrates a graphical representation of
Centhaquine improved Sp02/Fi02 in
COVID-19 patients. Sp02/Fi02 was found to improve following administration of
centhaquine
18
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
by 34.48 units within 2 hours and by 41.42 units in 4 hours;
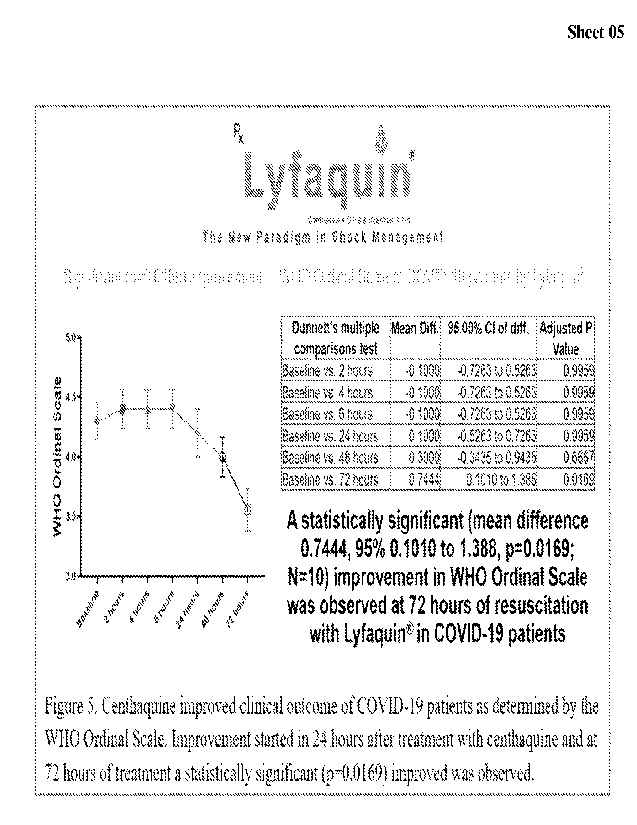
[0040] Figure 5 illustrates a graphical representation of
Centhaquine improved clinical
outcome of COVID-19 patients as determined by the WHO Ordinal Scale.
Improvement started
in 24 hours after treatment with centhaquine and at 72 hours of treatment a
statistically significant
(p=0.0169) improved was observed.
DETAILED DESCRIPTION OF THE DISCLOSED EMBODIMENTS
[0041] As used herein, the term "an amount sufficient to" refers to
amount that enables the
achievement of the intended effect. Such an amount may be determined through
various assays
known in the art based on the intended effect. As used herein, the terms
"applying" or
"administering" refer to all means of introducing the specified agent,
composition, or force to the
specified legion or subject. "Administration" or "application" can be effected
in one dose,
continuously or intermittently throughout the course of treatment. Methods of
determining the
most effective means and dosage of administration are known to those of skill
in the art and will
vary with the composition used for therapy, the purpose of the therapy, the
target cell being treated,
and the subject being treated. Single or multiple administrations can be
carried out with the dose
level and pattern being selected by the treating physician. Suitable dosage
formulations and
methods of administering the agents are known in the art Route of
administration can also be
determined and method of determining the most effective route of
administration are known to
those of skill in the art and will vary with the composition used for
treatment, the purpose of the
treatment, the health condition or disease stage of the subject being treated,
and target cell or tissue.
19
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
Non-limiting examples of route of administration include oral administration,
nasal administration,
inhalation, injection, and topical application. Administration can be for use
in industrial as well as
therapeutic applications. As used herein, the term "biodegradable" is used
herein to describe
substances, such as polymers, compositions, and formulations, intended to
degrade during use.
Biodegradable substances may also be "biocompatible,- i.e. not harmful to
living tissue.
[00421 As used herein, the term "therapeutically effective amount"
refers to a quantity
sufficient to achieve a desired effect. In the context of therapeutic
applications, the effective
amount will depend on the type and severity of the condition at issue and the
characteristics of the
individual subject, such as general health, age, sex, body weight, and
tolerance to pharmaceutical
compositions. The skilled artisan will be able to determine appropriate
amounts depending on
these and other factors. In the case of an in vitro application, in some
embodiments the effective
amount will depend on the size and nature of the application in question. It
will also depend on the
nature and sensitivity of the in vitro target and the methods in use. The
skilled artisan will be able
to determine the effective amount based on these and other considerations. The
effective amount
may comprise one or more administrations of a composition depending on the
embodiment. The
dose range of centhaquine could be from 0.00001 to about 1 mg/kg and may be
administered once
or multiple times in a day or in weeks or in months.
[00431 As used herein, the term -treating" or "treatment" includes
preventing a disease,
disorder or condition from occurring in a subject predisposed to or having a
disease, disorder
and/or condition; inhibiting the disease, disorder or condition, e.g.,
impeding its progress; and
relieving or reversing the disease, disorder, or condition, e.g., causing
regression of the disease,
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
disorder and/or condition. Treating a disease or condition may also include
ameliorating at least
one symptom of the particular disease or condition.
[00441 The term "ARDS" refers to Acute respiratory distress syndrome
(ARDS) is a type of
respiratory failure characterized by rapid onset of widespread inflammation in
the lungs (30) The
signs and symptoms of ARDS often begin within two hours of an inciting event
but can occur after
1-3 days. Signs and symptoms may include shortness of breath, fast breathing,
and a low oxygen
level in the blood due to abnormal ventilation (31) Other common symptoms
include muscle
fatigue and general weakness, low blood pressure, a dry, hacking cough, and
fever (31).
[0045] In some embodiments, the basic composition, may be combined
with remdesivir or
lopinavir or ritonavir or arbidol or favipiravir or ribavirin or interferon
beta-1B or alpha-interferon
or mesenchymal stem cells or their exosomes or chloroquine or chloroquine
phosphate or
hydroxychloroquine or pirfenidone or antibodies like REGN3048 and REGN3051 or
mRNA-1273
or bevacizumab or bromhexine or fingolimod or T89 or eculizumab or carrimycin
or oxygen
treatment or corticosteroids or methylprednisolone or inhaled nitric oxide gas
or losartan or
darunavir or tocilizumab or tetrandrine or aviptadil or thalidomide or
sarilumab or vitamin C or
plasma therapy.
[00461 Preclinical and clinical studies have demonstrated that
centhaquine effectively
addresses the major challenges associated with COVID-19. First, studies in
swine model of shock
centhaquine significantly reduced pulmonary edema and improved The Horowitz
index the
Pa02/Fi02 ratio. Second, improved tissue blood perfusion by centhaquine can
rapidly clear
21
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
inflammatory cytokines and prevent oxidative and apoptotic damage. Third, in
phase III clinical
trial centhaquine was effective in reducing ARDS and MODS. Fourth, in clinical
studies
centhaquine statistically significantly reduce mortality of patients.
[0047] Centhaquine) is a first-in-class resuscitative agent that is
final stages of approval in
India. Centhaquine acts through a unique mechanism of action that is
completely different from
any of the existing resuscitative agents. It increases blood pressure and
cardiac output by
augmenting venous blood return to the heart (venous alpha2B-adrenergic
receptor stimulation)
(32-36). It also produces arterial dilation by acting on central a2A-
adrenergic receptors to reduce
sympathetic activity and systemic vascular resistance (37). A significant
number of patients with
COVID-19 are admitted to the ICU and many of them are intubated and kept on
positive pressure
ventilation. A very high mortality is associated with patients who are on
ventilator support. About
30% of patients encounter life-threatening hypotension due a decrease in
venous return to the heart
following endotracheal intubation and/or positive pressure ventilation (38,
39). As a result of its
unique mechanism of action, centhaquine is expected to attenuate positive
pressure ventilation
induced decrease in venous return to the heart and prevent life-threatening
hypotension.
Centhaquine is likely to provide hemodynamic stability, improve tissue
oxygenation, reduce
pulmonary edema, reduce ARDS, reduce MODS and decrease mortality in COVID-19
patients.
[0048] Recently, guidelines on the management of critically ill
adults with COVID-19 were
published (40, 41). These guidelines authored by 36 experts from 12 countries
were developed by
The Surviving Sepsis Campaign (SSC). They have been grouped in four
categories: (1) infection
control and testing; (2) hemodynamic support; (3) ventilatory support and (4)
therapy. It is
22
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
recommended that acute resuscitation of adults with shock be done with a
conservative fluid
administration and preferring crystalloids over colloids. Norepinephrine has
been suggested as the
first-line vasoactive, adding vasopressin as a second-line agent is suggested
if the target (60-65
mmHg) mean arterial pressure cannot be achieved by norepinephrine alone (40,
41). Acute
hypoxemic respiratory failure despite conventional oxygen therapy requires
close monitoring, and
if worsening occurs an early intubation along with positive pressure
ventilation is recommended.
A mortality rate in the intensive care unit (ICU) of COVID-19 patients has
been reported to be
more than 79% (42). It has been found that using centhaquine as a
resuscitative agent in shock
(hypovolemic) significantly reduced 28-day all-cause mortality from 11.76% in
patients receiving
standard treatment to 2.94% in patients that received centhaquine (P=0.0742).
In a metanalysis of
phase II and III trials of centhaquine in hypovolemic shock mortality was
reduced from 10.71% to
2.20% (Odds ratio 5.340, 95% CI 1.27-26.50, p=0.0271). It is quite likely that
centhaquine as a
resuscitative agent will help patients with COVID-19 and reduce mortality.
[0049] The outbreak of COVID-19 disease which is evolving and
expanding at a rapid pace
has created major challenges to resuscitation efforts. Critically ill COVID-19
patients are managed
for ARDS and continued intensive care management. Patients with COVID-19
usually have
hypovolemia and fluids are administered with caution keeping in mind pre-load
responsiveness.
A high incidence of myocardial dysfunction has been reported in COVID-19
patients (43-45).
Using centhaquine as a resuscitative agent can be beneficial because it has
also been shown to be
highly effective in a swine model of in hospital cardiac arrest (46).
[0050] An improved blood perfusion will enhance the clearance of
toxic cytokines produced
23
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
as a result of overactive immune reaction in patients with COVID-19. Plasma
cytokine levels
depend on several factors: the intensity of production, the number of cell
receptors availability, the
clearance of cytokines, the affinity of the receptors for cytokines (47).
Centhaquine can help and
promote rapid clearance of these cytokines. It will be particularly useful
when centhaquine is
combined with various agents that are either available or being developed to
counter the
overwhelming reaction of the immune system to the virus, causing a cytokine
storm. Blood
purification systems to remove cytokines such as high-volume continuous
hemofiltration or
cytokine and/or endotoxin removal have been suggested but with little success
(47). There are
several methods being developed to remove cytokines from blood circulation
using devices such
as Cytosorb (extracorporeal cytokine removal), Hemofeel (continuous venovenous
hemodiafiltration) and EMiC2 (continuous venovenous hemodialysis) (48, 49).
Most of these
devices are extremely expensive, complicated to operate, and are available
only at a limited
number of institutions. Centhaquine increases stroke volume, cardiac output
(32, 36, 50, 51) and
blood flow to the vital organs, prevents organ failure and improves survival
in rat, rabbit and swine
models of hypovolemic shock (32, 36, 50, 51). Enhancing tissue perfusion is a
significant
advantage in reducing the volume of resuscitation and preventing extravasation
of fluid and
adverse effect of lung edema. Centhaquine does not act on beta-adrenergic
receptors, and therefore
the risk of arrhythmias is alleviated. Centhaquine has several advantages
because improved tissue
blood perfusion will not only remove toxic cytokines but also provide
oxygenation and nutrition
to the tissues. Since there are limited therapeutic options for this life-
threatening condition,
centhaquine may fulfil the unmet need for serious, life-threatening condition
of COVID-19 during
24
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
this pandemic outbreak. Centhaquine is likely to restore the immune balance
and correct the
overreaction of immune responses in patients with COVID-19 that develop
cytokine storm.
[0051] Studies in a swine model of shock showed that, centhaquine
significantly reduced
pulmonary edema and improved Horowitz index (ratio of partial pressure of
oxygen in blood and
the fraction of oxygen in the inhaled air (36).
[0052] Improvement in ARDS and MODS: In randomized, controlled,
multicentric clinical
trial patients (N=155) with hypovolemic shock, centhaquine significantly
improved ARDS scores
and MODS score (MODS). In a phase 3 study of hypovolemic shock, ARDS and MODS
were
secondary endpoints and they were both achieved with a significant p-value
with centhaquine (33,
52).
[0053] ARDS in Shock Patients (N=105): Acute Respiratory Distress
Syndrome (ARDS) was
compared between day 1 (before resuscitation) and day 3 of resuscitation. In
control patients
receiving standard treatment the difference between means was 0.04839 1
0.05696 (P=0.4023).
On the other hand, in centhaquine treated group the ARDS difference between
means was 0.1065
0.04464 (P=0.0202). These results indicate that centhaquine treatment
significantly improved
ARDS following resuscitation, whereas in control group there was insignificant
improvement.
[0054] MODS in Shock Patients (N=105): Multiple Organ Dysfunction
Score (MODS) was
compared between day 3 and day 7 of resuscitation. There was no improvement in
MODS in the
control group and the difference between means was 0.00 0.2697 (P>0.999),
whereas in
centhaquine group the difference between means was 0.9091 0.1964 (P=0.0001).
Centhaquine
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
treatment significantly decreased MODS whereas in control the improvement was
not significant.
[0055] Centhaquine has been evaluated for its safety, sensitivity
and toxicity in various species
for single and multiple doses and acute as well as chronic exposure (33).
Centhaquine has been
found to be safe and well tolerated in preclinical and clinical studies Its
safety has also been
demonstrated in a Phase I study (NCT02408731) in 25 human subjects (53, 54).
There were NO
adverse events related to centhaquine reported in phase II (NCT04056065) and
phase III
(NCT04045327) clinical studies.
[0056] Results of clinical phase II study (CTRI/2017/03/008184;
NCT04056065) indicate that,
centhaquine is a novel, first-in-class, highly effective resuscitative agent
for hypovolemic shock
as it demonstrated highly significant efficacy in improving blood pressure
(p<0.0001), lactate
levels (p=0.0012), base-deficit (p<0.0001), reduction in use of vasopressors
and reduced mortality
(33, 55-57). In a 105-patient randomized, blinded, multicenter study
(CTRI/2019/01/017196;
NCT04045327) a total of 34 (22 male and 12 female) patients in control and 68
(41 male and 27
female) patients in centhaquine groups completed the study. Blood lactate
levels at day 3 of
resuscitation were found to be significantly lower in centhaquine group
compared to control group
receiving standard treatment (P=0.046). Base-deficit improved in patients
treated with
centhaquine by 1.430 1.047 mmol/L compared to control patients receiving
standard treatment
(33). In total 180 human subjects have been studied (combined phase I, II and
III), out of which
155 were patients with hypovolemic shock. Centhaquine reduced the mortality
from 9.68% in
patients receiving standard treatment to 2.15% in patients that received
centhaquine (odds ratio
4.875; 95% CI 1.162-24.18; P=0.0190).
26
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
[00571 Results of phase II and phase III clinical studies indicate
that, centhaquine treatment
can provide hemodynamic stability and prove to be beneficial in improving
ARDS, MODS and
shock symptoms in patients infected with COVID-19. Centhaquine can reduce
morbidity and
mortality in COVID-19 by reduction of edema in the lungs, improved ARDS scores
and better
oxygenation of tissues.
[00581 Centhaquine has been evaluated for its safety, sensitivity
and toxicity in various species
for single and multiple doses and acute as well as chronic exposure (33)
Centhaquine was found
to be safe and well tolerated in healthy human subjects (53, 54). Safety and
efficacy of centhaquine
is established (Phase I, phase II and phase III clinical studies)
[00591 Centhaquine has shown efficacy in improving ARDS, MODS and
survival in serious
and life-threatening condition of hypovolemic shock and it has the potential
to improve morbidity
and mortality in patients with COVID-19. Preclinical and clinical studies have
demonstrated that
centhaquine effectively addresses the major challenges associated with COVID-
19.
[00601 First, studies in swine model of shock centhaquine
significantly reduced pulmonary
edema and improved The Horowitz index the Pa02/Fi02 ratio. Second, improved
tissue blood
perfusion by centhaquine can rapidly clear inflammatory cytokines and prevent
oxidative and
apoptotic damage. Third, in phase III clinical trial centhaquine was effective
in reducing ARDS
and MODS. Fourth, in clinical studies centhaquine statistically significantly
reduce mortality of
patients.
[0061] Centhaquine has shown efficacy in improving ARDS, MODS, and
survival in a serious
27
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
life-threatening condition of hypovolemic shock; hence, it can improve
morbidity and mortality in
patients with COVID-19. Preclinical and clinical studies have demonstrated
that centhaquine
effectively addresses the major challenges associated with COVID-19. Studies
in the swine model
of shock, centhaquine significantly reduced pulmonary edema and improved The
Horowitz index
(Pa02/Fi02 ratio). Improved tissue blood perfusion by centhaquine can rapidly
clear inflammatory
cytokines and prevent oxidative and apoptotic damage. In phase III clinical
trial, centhaquine was
effective in reducing ARDS and MODS. In clinical studies, centhaquine
statistically significantly
reduce the mortality of patients. We propose using centhaquine at a dose of
0.01 mg/kg, along with
the standard of care, to be administered to patients meeting the eligibility
criteria. There will be no
change in the current standard of care of critically ill COVID-19 patients.
Patients will continue
receiving standard of care, and centhaquine will be an add-on treatment to
provide hemodynamic
stability and improve ARDS, MODS scores and reduce mortality.
[0062] The effect of centhaquine (Lyfaquin ) was determined COVID-19
patients. A
significant improvement in oxygen saturation (Sp02) of COVID-19 patients by
intravenous
administration of centhaquine in the dose of 0.01 mg/kg was observed (Figure
2). An improvement
of Sp02 improved by 12.40 units within 2 hours of administration of
centhaquine and further
treatment with centhaquine led to an improvement of Sp02 by about 20 units.
Four out of 10
patients did not even need oxygen therapy at 72 hours of treatment with
centhaquine.
[0063] We also determined the effect of age on the improvement in
ratio of Sp02 and Fi02
(Sp02/Fi02) after administration of centhaquine (Figure 3). We found that
centhaquine improved
Sp02/Fi02 in all patients irrespective of age of the patient. Basal Sp02/Fi02
was found to be
28
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
slightly poor in aged patients and the slope was -1.062, however, treatment
with centhaquine
started flattening the slope to -0.5905 at 2 hours and -0.2718 at 4 hours of
treatment with
centhaquine (Figure 3). Centhaquine (Lyfaquie) improved Sp02/Fi02 in COVID-19
patients.
Sp02/Fi02 was found to improve following administration of centhaquine by
34.48 units within
2 hours and by 41.42 units in 4 hours (Figure 4).
[00641 WHO Ordinal Scale was used to determine whether centhaquine
(Lyfaquie) improved
the outcome of patients with COVID-19 (Figure 5). A statistically significant
(mean difference
0.7444, 95% 0.1010 to 1.388, p=0.0169; N=10) improvement in WHO Ordinal Scale
was observed
at 72 hours of resuscitation with centhaquin (Lyfaquin ) in COVID-19 patients.
[00651 While the disclosed embodiments have been described with
reference to illustrative
embodiments, this description is not intended to be construed in a limiting
sense. Various
modifications and combinations of the illustrative embodiments as well as
other embodiments of
the present disclosure, will be apparent to persons skilled in the art upon
reference to the
description. It is, therefore, intended that the appended claims encompass any
such modifications
or embodiment.
29
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
REFERENCES:
1. Channappanavar R, Zhao J, Perlman S. T cell-mediated immune response to
respiratory
coronaviruses. Immunol Res. 2014;59(1-3):118-28. Epub 2014/05/23. doi:
10.1007/s12026-014-
8534-z. PubMed PMID: 24845462; PMCID: PMC4125530.
2. Zumla A, Chan JF, Azhar El, Hui DS, Yuen KY. Coronaviruses - drug
discovery and
therapeutic options. Nat Rev Drug Discov. 2016;15(5):327-47. Epub 2016/02/13.
doi:
10.1038/nrd.2015.37. PubMed PMID: 26868298; PMCID: PMC7097181.
3. Chan JF, Lau SK, To KK, Cheng VC, Woo PC, Yuen KY. Middle East
respiratory
syndrome coronavirus. another zoonotic betacoronavirus causing SARS-like
disease. Clin
Microbiol Rev. 2015;28(2).465-522. Epub 2015/03/27. doi: 10.1128/CMR.00102-14.
PubMed
PMID: 25810418; PMCID: PMC4402954.
4. Cheng VC, Lau SK, Woo PC, Yuen KY. Severe acute respiratory syndrome
coronavirus
as an agent of emerging and reemerging infection. Clin Microbiol Rev.
2007;20(4):660-94. Epub
2007/10/16. doi: 10.1128/CMR.00023-07. PubMed PMID: 17934078; PMCID:
PMC2176051.
5. Barlow A, Landolf KM, Barlow B, Yeung SYA, Heavner JJ, Claassen CW,
Heavner MS.
Review of Emerging Pharmacotherapy for the Treatment of Coronavirus Disease
2019.
Pharmacotherapy. 2020;40(5):416-37. Epub 2020/04/08. doi: 10.1002/phar. 2398.
PubMed PMID:
32259313; PMCID: PMC7262196,
6. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory
syndrome
coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The
epidemic and the
challenges. Int J Antimicrob Agents. 2020;55(3):105924. Epub 2020/02/23. doi:
10.1016/j .ij antimicag.2020.105924. PubMed PMID: 32081636; PMCID: PMC7127800.
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
7. Khamsi R. If a coronavirus vaccine arrives, can the world make enough?
Nature.
2020;580(7805):578-80. Epub 2020/04/11. doi: 10.1038/d41586-020-01063-8.
PubMed PMID:
32273621.
8. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao
G.
Remdesivir and chloroquine effectively inhibit the recently emerged novel
coronavirus (2019-
nCoV) in vitro. Cell Res. 2020;30(3):269-71. Epub 2020/02/06. doi:
10.1038/s41422-020-0282-0.
PubMed PMID: 32020029; PMCID: PMC7054408.
9. de Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, Scott D,
Cihlar T,
Feldmann H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the
rhesus macaque
model of MERS-CoV infection. Proc Natl Acad Sci U S A. 2020;117(12):6771-6.
Epub
2020/02/15. doi: 10.1073/pnas.1922083117. PubMed PMID: 32054787; PMCID:
PMC7104368.
10. Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB,
Leist SR, Pyrc
K, Feng JY, Trantcheva I. Broad-spectrum antiviral GS-5734 inhibits both
epidemic and zoonotic
coronaviruses. Science translational medicine. 2017;9(396).
11. Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T,
Green G, Green
ML, Lescure FX, Nicastri E, Oda R, Yo K, Quiros-Roldan E, Studemeister A,
Redinski J, Ahmed
S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, D'Arminio
Monforte A,
Ismail S, Kato H, Lapadula G, L'Her E, Maeno T, Majumder S, Massari M, Mora-
Rillo M, Mutoh
Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, DeZure A, Zhao Y, Zhong L,
Chokkalingam
A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK,
Winterbourne L, Desai
P, Mera R, Gaggar A, Myers RP, Brainard DM, Childs R, Flanigan T.
Compassionate Use of
Remdesivir for Patients with Severe Covid-19. N Engl J Med. 2020;382(24):2327-
36. Epub
2020/04/11. doi: 10.1056/NEJMoa2007016. PubMed PMID: 32275812; PMCID:
PMC7169476.
12. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier
B, Courj on J,
Giordanengo V, Vieira VE, Tissot Dupont H, Honore S, Colson P, Chabriere E, La
Scola B, Rolain
31
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
JIM, Brouqui P, Raoult D. Hydroxychloroquine and azithromycin as a treatment
of COVID-19:
results of an open-label non-randomized clinical trial. Int J Antimicrob
Agents.
2020;56(1):105949. Epub 2020/03/25. doi: 10.1016/j.ijantimicag.2020.105949.
PubMed PMID:
32205204; PMCID: PMC7102549.
13. Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum
inhibitor of viral
RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(7):449-63. Epub
2017/08/05. doi:
10.2183/pjab.93.027. PubMed PMID: 28769016; PMCID: PMC5713175.
14. Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant
EA, Diz DI,
Gallagher PE. Effect of angiotensin-converting enzyme inhibition and
angiotensin II receptor
blockers on cardiac angiotensin-converting enzyme 2. Circulation.
2005;111(20):2605-10. Epub
2005/05/18. doi: 10.1161/CIRCULATIONAHA.104.510461. PubMed PMID: 15897343.
15. Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F, Li D, Yang M,
Xing L, Wei J,
Xiao H, Yang Y, Qu J, Qing L, Chen L, Xu Z, Peng L, Li Y, Zheng H, Chen F,
Huang K, Jiang
Y, Liu D, Zhang Z, Liu Y, Liu L. Treatment of 5 Critically Ill Patients With
COVID-19 With
Convalescent Plasma. JAMA. 2020;323(16):1582-9. Epub 2020/03/29. doi:
10.1001/j ama.2020.4783. PubMed PMID: 32219428; PMCID: PMC7101507.
16. Yoo JH. Convalescent Plasma Therapy for Corona Virus Disease 2019: a
Long Way to Go
but Worth Trying. J Korean Med Sci. 2020;35(14):e150. Epub 2020/04/14. doi:
10.3346/jkms.2020.35.e150. PubMed PMID: 32281318; PMCID: PMC7152529.
17. Maxmen A. How blood from coronavirus survivors might save lives.
Nature.
2020;580(7801):16-7. Epub 2020/03/28. doi: 10.1038/d41586-020-00895-8. PubMed
PMID:
32214238.
18. Guo Y, Li J, Hagstrom E, Ny T. Beneficial and detrimental effects of
plasmin(ogen) during
infection and sepsis in mice. PLoS One. 2011;6(9):e24774. Epub 2011/09/21.
doi:
10.1371/j ournal.pone.0024774. PubMed PMID: 21931850; PMCID: PMC3171470.
32
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
19. Shen Y, Guo Y, Mikus P, Sulniute R, Wilczynska M, Ny T, Li J.
Plasminogen is a key
proinflammatory regulator that accelerates the healing of acute and diabetic
wounds. Blood.
2012;119(24):5879-87. Epub 2012/05/09. doi: 10.1182/blood-2012-01-407825.
PubMed PMID:
22563086.
20. Ambrus CM, Weintraub DH, Choi TS, Eisenberg B, Staub HP, Courey NG,
Foote RJ,
Goplerud D, Moesch RV, Ray M, Irwin, Bross DJ, Jung OS, Mink TB, Ambrus JL.
Plasminogen
in the prevention of hyaline membrane disease. Res Commun Chem Pathol
Pharmacol.
1973;6(1):341-4. Epub 1973/07/01. PubMed PMID: 4582209.
21. Ambrus CM, Choi TS, Cunnanan E, Eisenberg B, Staub HP, Weintraub DH,
Courey NG,
Patterson RJ, Jockin H, Pickren JW, Bross ID, Jung OS, Ambrus it. Prevention
of hyaline
membrane disease with plasminogen. A cooperative study. JAMA.
1977;237(17):1837-41. Epub
1977/04/25. PubMed PMID: 321823.
22. Ambrus CM, Choi TS, Weintraub DH, Eisenberg B, Staub HP, Courey NG,
Foote RJ,
Goplerud D, Moesch RV, Ray M, editors. Studies on the prevention of
respiratory distress
syndrome of infants due to hyaline membrane disease with plasminogen. Seminars
in thrombosis
and hemostasis; 1975: Copyright 1975 by Thieme Medical Publishers, Inc.
23. Wu Y, Wang T, Guo C, Zhang D, Ge X, Huang Z, Zhou X, Li Y, Peng Q, Li
J. Plasminogen
improves lung lesions and hypoxemia in patients with COVID-19. QJM.
2020;113(8):539-45.
Epub 2020/04/11. doi: 10.1093/qjmed/hcaa121. PubMed PMID: 32275753; PMCID:
PMC7184376.
24. Laterre PF, Wittebole X, Dhainaut IF. Anticoagulant therapy in acute
lung injury. Crit Care
Med. 2003;31(4 Suppl): S329-36. Epub 2003/04/12.
doi:
10.1097/01.CCM.0000057912.71499.A5. PubMed PMID: 12682461.
33
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
25. MacLaren R, Stringer KA. Emerging role of anticoagulants and
fibrinolytics in the
treatment of acute respiratory distress syndrome. Pharmacotherapy.
2007;27(6):860-73. Epub
2007/06/05. doi: 10.1592/phco.27.6.860. PubMed PMID: 17542769; PMCID:
PMC2515375.
26. Liu C, Ma Y, Su Z, Zhao R, Zhao X, Nie HG, Xu P, Zhu L, Zhang M, Li X,
Zhang X,
Matthay MA, Ji EL. Meta-Analysis of Preclinical Studies of Fibrinolytic
Therapy for Acute Lung
Injury. Front Immunol. 2018;9:1898. Epub 2018/09/05. doi:
10.3389/fimmu.2018.01898. PubMed
PMID: 30177934; PMCID: PMC6110197.
27. Stringer KA, Hybertson BM, Cho OJ, Cohen Z, Repine JE. Tissue
plasminogen activator
(tPA) inhibits interleukin-1 induced acute lung leak. Free Radic Biol Med.
1998;25(2):184-8. Epub
1998/07/17. doi: 10.1016/s0891-5849(98)00047-1. PubMed PMID: 9667494.
28. Moore HB, Barrett CD, Moore EE, McIntyre RC, Moore PK, Talmor DS, Moore
FA, Yaffe
MB. Is there a role for tissue plasminogen activator as a novel treatment for
refractory COVID-19
associated acute respiratory distress syndrome? J Trauma Acute Care Surg.
2020;88(6):713-4.
Epub 2020/04/14. doi: 10.1097/TA.0000000000002694. PubMed PMID: 32281766;
PMCID:
PMC7147395.
29. Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, Yaffe
MB, Moore
HB, Barrett CD. Tissue plasminogen activator (tPA) treatment for COVID-19
associated acute
respiratory distress syndrome (ARDS): A case series. J Thromb Haemost.
2020;18(7):1752-5.
Epub 2020/04/09. doi: 10.1111/jth.14828. PubMed PMID: 32267998; PMCID:
PMC7262152.
30. Fan E, Brodie D, Slutsky AS. Acute Respiratory Distress Syndrome:
Advances in
Diagnosis and Treatment. JA_MA. 2018;319(7):698-710. Epub 2018/02/22. doi:
10.1001/j ama.2017.21907. PubMed PMID: 29466596.
31. Bakowitz M, Bruns B, McCunn M. Acute lung injury and the acute
respiratory distress
syndrome in the injured patient. Scand J Trauma Resusc Emerg Med. 2012;20:54.
Epub
2012/08/14. doi: 10.1186/1757-7241-20-54. PubMed PMID: 22883052; PMCID:
PMC3518173.
34
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
32. Gulati A, Lavhale MS, Garcia DJ, Havalad S. Centhaquin improves
resuscitative effect of
hypertonic saline in hemorrhaged rats. The Journal of surgical research.
2012;178(1)415-23. Epub
2012/04/11. doi: 10.1016/j.jss.2012.02.005. PubMed PMID: 22487389.
33. Gulati A, Lavhale M, Gin i R, Andurkar S, Xanthos T. Centhaquine
citrate. Alpha2B-
Adrenoceptor ligand, Resuscitative agent for hypovolemic shock. Drugs Fut.
2020;45(3): 153 -63 .
34. Gulati A, Voshtina E, Zhang Z, Murphy A. Alpha Adrenergic Receptors
Mediate
Resuscitative Effect of Centhaquin in Hemorrhaged Rats. Critical Care
Medicine.
2012;40(12):U162-U3. PubMed PMID: WO S:000312045700549.
35. Gulati A, Zhang Z, Arshad K. Centhaquin Decreases the Requirement of
Norepinephrine,
Maintains Blood Pressure and Improves Survival Following Resuscitation of
Hemorrhaged Rats.
Critical Care Medicine. 2011;39(12):114.
36. Kontouli Z, Staikou C, Iacovidou N, Mamais I, Kouskouni E, Papalois A,
Papapanagiotou
P, Gulati A, Chalkias A, Xanthos T. Resuscitation with centhaquin and 6%
hydroxyethyl starch
130/0.4 improves survival in a swine model of hemorrhagic shock: a randomized
experimental
study. European Journal of Trauma and Emergency Surgery. 2019;45(6):1077-85.
37. Srimal RC, Gulati K, Nityanand S, Dhawan BN. Pharmacological studies on
2-(2-(4-(3-
methylpheny1)-1-piperazinyl)ethyl) quinoline (centhaquin). I. Hypotensive
activity. Pharmacol
Res. 1990;22(3):319-29. Epub 1990/05/01. doi: 10.1016/1043-6618(90)90729-w.
PubMed PMID:
2367281.
38. Franklin C, Samuel J, Hu TC. Life-threatening hypotension associated
with emergency
intubation and the initiation of mechanical ventilation. The American journal
of emergency
medicine. 1994; 12(4):425-8. Epub 1994/07/01. doi: 10.1016/0735-6757(94)90053-
1. PubMed
PMID: 8031425.
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
39. Manthous CA. Avoiding circulatory complications during endotracheal
intubation and
initiation of positive pressure ventilation. J Emerg Med. 2010;38(5):622-31.
Epub 2009/05/26. doi:
10.1016/j.jemermed.2009.01.018. PubMed PMID: 19464138.
40. Alhazzani W, Moller MB, Arabi YM, Loeb M, Gong MN, Fan E, Oczkowski S,
Levy MM,
Derde L, Dzierba A, Du B, Aboodi M, Wunsch H, Cecconi M, Koh Y, Chertow DS,
Maitland K,
Alshamsi F, Belley-Cote E, Greco M, Laundy M, Morgan JS, Kesecioglu J, McGeer
A, Mermel
L, Mammen MJ, Alexander PE, Arrington A, Centofanti JE, Citerio G, Baw B,
Memish ZA,
Hammond N, Hayden FO, Evans L, Rhodes A Surviving Sepsis Campaign: guidelines
on the
management of critically ill adults with Coronavinls Disease 2019 (COVID-19).
Intensive Care
Med. 2020;46(5):854-87. Epub 2020/03/31. doi: 10.1007/s00134-020-06022-5.
PubMed PMID:
32222812; PMCID: PMC7101866.
41. Poston JT, Patel BK, Davis AM. Management of Critically Ill Adults With
COVID-19.
JAMA. 2020;323(18):1839-41. Epub 2020/03/28. doi: 10.1001/jama.2020.4914.
PubMed PMID:
32215647.
42. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M,
Yu T, Wang Y,
Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill
patients with SARS-
CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective,
observational study. Lancet
Respir Med. 2020;8(5):475-81. Epub 2020/02/28. doi: 10.1016/S2213-
2600(20)30079-5. PubMed
PMID: 32105632; PMCID: PMC7102538.
43. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X,
Cheng Z, Yu
T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J,
Wang G, Jiang
R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with
2019 novel coronavirus
in Wuhan, China. Lancet. 2020;395(10223):497-506. Epub 2020/01/28. doi:
10.1016/S0140-
6736(20)30183-5. PubMed P1V11D: 31986264; PMCID: PMC7159299.
36
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
44. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of
mortality due to COVID-
19 based on an analysis of data of 150 patients from Wuhan, China. Intensive
care medicine.
2020:1-3.
45. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z,
Xiong Y, Zhao
Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients
With 2019 Novel
Coronavirus-Infected Pneumonia in Wuhan, China. JA_MA. 2020;323(11):1061-9.
Epub
2020/02/08. doi: 10.1001/j ama.2020.1585. PubMed PMID: 32031570; PMCID:
PMC7042881.
46. Papalexopoulou K, Chalkias A, Pliatsika P, Papalois A, Papapanagiotou
P. Papadopoulos
G, Arnaoutoglou E, Petrou A, Gulati A, Xanthos T. Centhaquin Effects in a
Swine Model of
Ventricular Fibrillation: Centhaquin and Cardiac Arrest. Heart Lung Circ.
2017;26(8):856-63.
Epub 2017/04/08. doi: 10.1016/j .h1c.2016.11.008. PubMed PMID: 28385449.
47. Honore PM, Hoste E, Molnar Z, Jacobs R, Joannes-Boyau 0, Malbrain M,
Forni LG.
Cytokine removal in human septic shock: Where are we and where are we going?
Ann Intensive
Care. 2019;9(1):56. Epub 2019/05/16. doi: 10.1186/s13613-019-0530-y. PubMed
PMID:
31089920; PMCID: PMC6517449.
48. Harm S, Schildbock C, Hartmann J. Cytokine Removal in Extracorporeal
Blood
Purification: An in vitro Study. Blood Purif. 2020;49(1-2):33-43. Epub
2019/09/12. doi:
10.1159/000502680. PubMed PMID: 31509822.
49. Hawchar F, Laszlo I, Oveges N, Trasy D, Ondrik Z, Molnar Z.
Extracorporeal cytokine
adsorption in septic shock: A proof of concept randomized, controlled pilot
study. J Crit Care.
2019;49:172-8. Epub 2018/H/19. doi: 10.1016/j.jcrc.2018.11.003. PubMed PMID:
30448517.
50. Lavhale MS, Havalad S, Gulati A. Resuscitative effect of centhaquin
after hemorrhagic
shock in rats. The Journal of surgical research. 2013;179(1):115-24. Epub
2012/09/12. doi:
10.1016/j.jss.2012.08.042. PubMed PMID: 22964270.
37
CA 03218643 2023- 11- 9
WO 2022/240964
PCT/US2022/028733
51. Papapanagiotou P, Xanthos T, Gulati A, Chalkias A, Papalois A, Kontouli
Z, Alegakis A,
Iacovidou N. Centhaquin improves survival in a swine model of hemorrhagic
shock. The Journal
of surgical research. 2016;200(1):227-35. Epub 2015/07/29. doi: 10.1016/j .j
ss.2015.06.056.
PubMed PMID: 26216751.
52. Gulati A, Choudhuri R, Gupta A, Singh S, Ali SKN, Sidhu GK, Hague PD,
Rahate P,
Bothra AR, Singh GP, Maheshwari S, Jeswani D, Haven i S, Agarwal A, Agrawal
NR. A
Multicentric, Randomized, Controlled Phase III Study of Centhaquine
(Lyfaquin((R))) as a
Resuscitative Agent in Hypovolemic Shock Patients. Drugs. 2021;81(9):1079-100.
Epub
2021/06/02. doi: 10.1007/s40265-021-01547-5. PubMed PMID: 34061314; PMCID:
PMC8167383
53. Goyal AO, Lavhale MS, Gulati A. Safety and Efficacy of Centhaquin as a
Novel
Resuscitative Agent for Hypovolemic Shock. Circulation. 2015;132 (Suppl
3):A17521-A.
54. Gulati A, Goyal AO, Lavhale MS, Gulati S, Scheetz M. Human
Pharmacokinetics of
Centhaquin Citrate, a Novel Resuscitative Agent. Circulation. 2016;134 (Suppl
1):A16607-A.
55. Gulati A, Jain D, Agrawal N, Rahate P, Das 5, Chowdhuri R, Dhibar D,
Prabhu M, Haveni
S, Agarwal R. Clinical Phase II Results Of PMZ-2010 (centhaquin) As A
Resuscitative Agent For
Hypovolemic Shock. Critical Care Medicine. 2019;47(1):12.
56. Gulati A, Jain D, Agrawal N, Rahate P, Das S, Chowdhuri R, Prabhu M,
Haven i S, Dhibar
D, Agarwal R. Evaluation of Centhaquine, a Novel Resuscitative Agent, in
Hemorrhagic Shock
Patients. Circulation. 2019;140(Suppl 1):A16250-A.
57. Gulati A, Jain D, Agrawal N, Rahate P, Das S, Chowdhuri R, Prabhu M,
Haven i S, Dhibar
D, Lavhale M. A phase II multicentric randomized controlled study of
centhaquine in hemorrhagic
shock patients. Critical Care Medicine. 2020;48(1):840.
38
CA 03218643 2023- 11- 9