Note: Descriptions are shown in the official language in which they were submitted.
WO 2022/253469
PCT/EP2022/025262
READY-MIXED DRYING-TYPE JOINT COMPOUNDS CONTAINING pH
BUFFER SYSTEMS
FIELD OF THE INVENTION
[0001] This invention relates generally to ready-mixed drying-type joint
compounds comprising a pH buffer system of a weak acid and its
conjugate base to maintain a pH of 8-12, preferably 9-11, for a significant
length of time, typically 120 days or more.
lo BACKGROUND OF THE INVENTION
[0002] In the construction of buildings, one of the most common
building
elements is gypsum wallboard, often known as drywall or gypsum paneling, used
in the construction of walls and/or ceilings. The board may be composed of any
of
a variety of materials, including but not limited to, cementitious materials
such as,
for example, cement or gypsum. Walls made from gypsum wallboard are
traditionally constructed by affixing the panels to wood studs or metal
framing,
and treating the joints between adjoining panels with a specially prepared
adhesive called a joint compound. Gypsum panels easily accommodate walls that
are unusual in size and can be shaped around structural elements such as
beams or pipes. The side edges of the drywall panels are tapered, thus
allowing
the joint compound to be applied to the seam, between adjoining panels, in
such
a way that a monolithic surface is created when finished.
[0003] Ready mixed, drying-type joint compounds (referred to as
"joint
compounds" or "ready-mixed joint compounds" in this specification) are pre-
mixed
with water during manufacturing and require little or no addition of water at
the job
site. Drying-type joint compositions can also be dry powders that are mixed
with
water at the job site. Drying-type joint compounds harden when the water
evaporates and the compound dries. Drying-type joint compounds substantially
contain a filler component. Prior to use (generally during manufacturing), the
filler,
a binder, a thickener, optionally a dedusting agent, and optionally several
other
ingredients are mixed for a specific time with water to produce the drying-
type
joint compound. In the case of ready-mixed compounds, water and other liquid
1
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
additives are added during manufacture. Such a composition has a high ionic
content and basic pH. Once the drying-type joint compound is applied to the
wallboard panels, the composition dries (i.e., water evaporates) and a dry,
relatively hard cementitious material remains.
[0004] US Patent 4,853,085 (incorporated herein by reference) to United
States Gypsum Company teaches buffer systems used in gypsum wallboard in an
amount from about 0.25 to about 10%, capable of maintaining the pH of the
paper
stock at a value of at least 7 and preferably 7 to 7.8. The buffer material
may be
any of a number of compounds which are salts of a cation of a strong base and
an anion of a weak acid. Although a number of materials may be utilized such
as
sodium carbonate and sodium bicarbonate. The preferred filler is calcium
carbonate.
[0005] US Patent 7,066,996 to Hercules Incorporated discloses
ready-mixed
joint compounds that have pH modifiers to increase the alkalinity of the
composition to achieve the desired pH values of 8 to 10. US Patent 7,066,996
to
Hercules Incorporated also discloses that depending on local preferences,
other
ingredients may be used in the joint compound formulation. These include air
entraining agents, surfactants, humectants, pH buffering salts, and defoamers.
[0006] US Patent Application Publication No. 2005/0020743 to
Ruh!mann
teaches buffer-effect dispersants for paint bases and pigmentary compositions
comprising the combination of a partially or totally neutralized water-soluble
dispersing agent which is a homopolymer of acrylic or methacrylic acid with at
least one other unsaturated ethylenic monomer with a compound having specific
buffer properties.
[0007] Typically oxides and hydroxides of group IA, I IA metals in the
periodic
table, or ammonia/ammonium compounds and/or certain salts of weak acids are
used to adjust pH in joint compounds. The pH is raised initially, over a very
brief
span of time, relative to the shelf life of such joint compound. However, the
pH of
the joint compounds stabilized with the metal hydroxides drops and reaches a
steady state below the target pH. Many additives used in ready-mixed joint
compounds are pH sensitive.
2
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
SUMMARY OF THE INVENTION
[0008] This invention relates generally to ready-mixed, drying-
type joint
compounds comprising an alkali sensitive component and comprising a pH buffer
system to impart a stable pH. The invention may maintain the pH of the ready-
mixed, drying-type joint compounds in a range having a lower limit of pH of 8
or
9. The invention may maintain the pH of the ready-mixed, drying-type joint
compounds in a range having an upper limit of pH of 11 or 12. Typically the
invention maintains the pH of the ready-mixed, drying-type joint compounds in
a
range of 8-12, more preferably 9-11.
[0009] The invention provides a more cost effective and better performing
joint compound, which incorporates components, typically additives, which are
"alkali sensitive" (also known as "alkali activated/dependent"). For purposes
of
this disclosure, a component of a joint compound that is "alkali sensitive"
(also
known as "activated/dependent") is a component that requires an alkaline pH
(pH
above 7, or typically a pH of 8 to 12, preferably 9 to 11) to function
optimally for its
intended purpose. Components such as rheology modifiers (including but not
limited to cellulosics (for example hydroxyethyl cellulose, hydroxy propyl
methyl
cellulose), acrylics, clays (for example bentonites, kaolites), binders, some
preservatives (for example triazine) and many mineral additives can be alkali
sensitive and require a pH above 7 to perform optimally. At lower pH, the
effectiveness of alkali sensitive components can drop. This results in sub-
optimal
usage rates for these materials with potential cost and ecological impacts.
For example, an alkali sensitive rheology modifier would not maintain the
targeted
viscosity when pH falls below an alkaline pH. The pH drift both up and down is
minimized with a buffer system.
[0010] Providing a stable alkali pH, for example at or about 10,
ensures proper
activation and effectiveness of the alkali sensitive components. Employing pH
buffer system in the product at the point of manufacture ensures a stable
alkali
pH and, as a result, helps to ensure consistent product performance over the
shelf life of the ready-mixed joint compound.
3
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
[0011] The use of the pH buffer system may also result in the need
for less
biocide (for example, fungicides, bactericides) in a joint compound than in a
joint
compound without a pH buffer system.
[0012] Selection of the buffer can be made by selecting a weak
acid with a
Log P function of the acid dissociation constant (Ka) at or near the target pH
for
the joint compound. The preferred buffer system includes a weak acid and its
conjugate base and function due to the counter ion effect or Le Chatelier's
principle.
[0013] The joint compound may be a ready-mixed drying-type joint
compound
comprising a mixture of joint compound components and water;
the joint compound components comprise:
a filler at about 50 wt.% to about 98 wt.% (preferably about 50 wt.% to
about 93 wt.%, more preferably about 65 wt.% to about 93 wt.%) of the joint
compound on a dry basis;
a binder at up to about 15 wt. % of the joint compound on a dry basis;
a polymeric thickener at up to about 3 wt. % of the joint compound on a
dry basis;
a pH buffer system comprising a weak acid and a conjugate base of
the weak acid, preferably at about 0.01 to about 1.0 wt. %, at about 0.01 wt.
to about 0.25 wt. (Yo, at about 0.025 to about 0.5 wt. (Yo, typically about
0.025
to about 0.15 wt. %, at about 0.05 to about 0.25 wt. %, more typically about
0.05 to about 0.10 wt. %, of the joint compound on a dry basis; and
an additive up to about 10 wt. % of the joint compound on a dry basis,
wherein optionally the additive comprises an accelerator and/or a rheology
modifier;
wherein weight ratio of the water to the dry joint compound
components is about 1:6 to about 3:1,
wherein at least one of the joint compound components is alkali sensitive.
[0014] The joint compound of the invention may be used by being applied
to boards, joint tape, and/or another layer of the joint compound.
4
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
[0015] The joint compound of the invention may be made by
combining
the calcium dihydrate, the filler (if present), the binder, the polymeric
thickener, the pH buffer system, and the other additives.
[0016] A preferred method of evaluating the stability of the pH of
the ready-
mixed, drying-type joint compound exposes the joint compound in a tightly
sealed container to 75 F when the joint compound is prepared and/or
manufactured and placed in the sealed container. The container is periodically
opened for a pH measurement and then resealed and maintained at 75 F.
Relative humidity is not relevant because the container is sealed and
therefore
the joint compound will reach equilibrium in the container. Advantageously,
the
joint compound of the invention can maintain a pH of 8-12, preferably 9-11,
when
kept for 14 days or more at 75 F in a sealed container under this pH stability
evaluation method. Typically, the joint compound of the invention can maintain
the pH of 8-12, preferably 9-11, when kept for 90 days or more at 75 F in the
sealed container under this pH stability evaluation method. More typically,
the
joint compound of the invention can maintain the pH of 8-12, preferably 9-11,
when kept for 120 days or more at 75 F in the sealed container under this pH
stability evaluation method.
[0017] In the present description the term "dry basis" means a
water-free
basis and the term "wet basis" means a water-inclusive basis.
[0018] Other advantages, benefits and aspects of the invention are
discussed
below, are illustrated in the accompanying figures, and will be understood by
those
of skill in the art from the more detailed disclosure below. All percentages,
ratios
and proportions herein are by weight, unless otherwise specified.
[0019] As used in the present specification at the very least, and not as
an
attempt to limit the application of the doctrine of equivalents to the scope
of the
claim, each numerical parameter modified by the term "about" should at least
be
construed in light of the number of reported significant digits and by
applying
ordinary rounding techniques.
BRIEF DESCRIPTION OF THE DRAWINGS
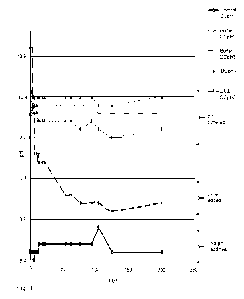
[0020] FIG. 1 is a plot showing pH of buffered coatings.
5
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
DETAILED DESCRIPTION OF THE INVENTION
[0021] All percentages and ratios used herein, unless otherwise
specified,
are by weight (i.e., wt. % as denoted as %(wt/wt)) unless otherwise indicated.
[0022] The present invention provides ready-mixed, drying-type joint
compounds comprising a pH buffer system comprising a weak acid and a
conjugate base of the weak acid, (sometimes also listed as a conjugate base of
a weak acid and the weak acid) for example: sodium carbonate and sodium
bicarbonate, tribasic phosphate and dibasic phosphate, or ammonium chloride
and ammonium hydroxide, preferably at about 0.01 to about 1.0 wt. %, at about
0.01 wt. to about 0.25 wt. %, at about 0.025 to about 0.5 wt. %, typically
about
0.025 to about 0.15 wt. %, at about 0.05 to about 0.25 wt. %, typically about
0.05 to about 0.10 wt. %, more typically 0.05 wt. % to 0.15 wt. %, wherein at
least one of the components of the joint compound is alkali sensitive and the
joint compound has a pH of 8-12, preferably 9-11, for example 9.5-10.5, for 14
days or more, typically 90 days or more, more typically 120 days or more, at
75 F in a sealed container. The pH buffer systems around 9.5-11.5 may be a
weak acid and a conjugate base of (for example): carbonates, borates, 2-am ino-
2-methyl-1-propanol ("AMP"), glycine, ammonia, methylamine, piperazine,
phosphate and piperidine.
[0023] Without being limited to mechanism, a pH buffer system,
using a weak
acid as well as the conjugate base of the weak acid, offers greater pH
stability
due to le Chatelier's principle or the counterion effect. Common buffer
systems
in or about the pH range of 9.5-10.5 include carbonate/bicarbonate buffers and
tribasic phosphate/dibasic phosphate buffers.
[0024] The contemplated buffer system, or any other buffer system
can be
incorporated into a ready-mixed, drying-type joint compound to impart a stable
pH. Selection of the buffer can be made by selecting a weak acid with a Log P
function of the acid dissociation constant (Ka) at or near the target pH for
the joint
compound. The preferred buffer system includes a weak acid and its conjugate
base.
6
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
[0025] The joint compound may be a ready-mixed drying type joint
compound comprising a mixture of joint compound components and water,
the joint compound components comprising:
calcium carbonate and/or calcium sulfate dehydrate at about 20 weight
percent (wt. %) to about 98 wt. % of the joint compound on a dry basis,
a binder at up to about 15 wt. % of the joint compound on a dry basis;
a polymeric thickener at up to about 3 wt. "Yo of the joint compound on a
dry basis;
a pH buffer system comprising a weak acid and a conjugate base of
the weak acid, preferably at 0.2-1.0 wt. % of the joint compound on a dry
basis; and
other additives up to about 10 wt. % of the joint compound on a dry
basis, wherein optionally the additive comprises an accelerator and/or a
rheology modifier;
wherein weight ratio of the water to the dry joint compound
components is about 1:6 to about 3:1,
wherein at least one of the joint compound components is alkali sensitive.
[0026] The joint compound may be a ready-mixed drying-type joint
compound
comprising a mixture of joint compound components and water;
the joint compound components comprise:
a filler at about 50 wt. % to about 93 wt. % of the joint compound on a
dry basis;
a binder at up to about 8 wt. % of the joint compound on a dry basis;
a polymeric thickener at up to about 2 wt. % of the joint compound on a
dry basis;
a pH buffer system comprising a weak acid and a conjugate base of
the weak acid, at about 0.025 wt. to about 0.15 wt. % of the joint compound
on a dry basis; and
an additive up to about 10 wt. % of the joint compound on a dry basis,
wherein optionally the additive comprises an accelerator and/or a rheology
modifier;
7
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
wherein weight ratio of the water to the dry joint compound
components is about 1:6 to about 3:1,
wherein at least one of the joint compound components is alkali sensitive.
[0027] The joint compound may be a ready-mixed drying-type joint compound
comprising a mixture of joint compound components and water;
the joint compound components comprise:
a filler at about 65 wt. % to about 93 wt. % of the joint compound on a
dry basis;
a binder at up to about 4 wt. % of the joint compound on a dry basis;
a polymeric thickener at about 0.1 wt. % to about 2 wt. % of the joint
compound on a dry basis;
a pH buffer system comprising a weak acid and a conjugate base of
the weak acid, at about 0.05 wt. to about 0.10 wt. % of the joint compound on
a dry basis; and
an additive about 0.1 to about 10 wt. % of the joint compound on a dry
basis, wherein optionally the additive comprises an accelerator and/or a
rheology modifier;
wherein weight ratio of the water to the dry joint compound
components is about 1:3 to about 1:1, more preferably about 1:3 to about 2:1,
wherein at least one of the joint compound components is alkali sensitive.
[0028] As mentioned above, advantageously the joint compounds of
the
invention can maintain a pH of 8-12, preferably 9-11, for example 9.5-10.5,
when
kept for 14 days or more, typically 90 days or more, more typically 120 days
or
more, at 75 F in a sealed container.
[0029] pH Testing
The pH of a joint compound can be easily tested with a two or three-point
calibrated hydronium ion-selective electrode with meter. The pH of the
material is
determined when a stable reading on the pH meter is achieved. The following
steps are followed:
8
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
= Calibrate the pH meter daily. Ensure calibration includes the expected
pH range of the test material and calibration is completed with pH standards
covering the tested pH range.
= Prepare the material to be tested following customer practice and/or
manufacturer's recommendations.
= Rinse and dry the calibrated ion-selective electrode and insert into the
compound following electrode/meter recommendations provided by the
equipment manufacturer.
= Low-viscosity materials, such as paints, can be tested by immersion of
the electrode into the test sample which is under slow, constant mixing (for
example with a magnetic stir bar).
= High-viscosity materials, such a joint compounds, can be tested by
immersion of the electrode into the test sample and the electrode is used to
gently stir the material to provide a slow, constant flow of material for the
electrode.
= Record the sample reading and rinse and dry electrode before the next
sample reading.
[0030] The pH may also tested as set forth in ISO 19396-2
Determination of
pH Value.
[0031] Table 1 provides examples of the ready-mixed, drying-type
joint
compound formulations of the present invention. Typically values in a single
column of the table are used together. However, a value for a component from a
column can be substituted for a value of that component in another column
where
mathematically permitted.
Table 1: Ready-mixed, drying-type joint compound formulations
Component Useable range Preferred Most
range Preferred
Range
9
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
Filler (wt.% on a dry basis) about 50 - about about 50 ¨ about
65 ¨
98 about 93 about 93
Binder (wt.% on a dry basis) up to about 15 up to about 8 up to
about 4
Polymeric Thickener (wt.% on up to about 3 up to about 2 about 0.1
to
a dry basis) about 2
pH buffer system (wt.% on a 0.01-1.0 0.025-0.5 0.05-0.25
dry basis)
Other Additives (wt.% on a dry up to about 10 up to about 10 about
0.1 to
basis) about 10
Water (weight ratio of water to about 1:6 to about 1:6 to about
1:3 to
joint compound components) about 3:1 about 3:1 about 1:1
[0032] The joint compound of the invention may be used by being
applied
to boards, joint tape, and/or another layer of the joint compound.
[0033] The joint compound of the invention may be made by
combining
the calcium dihydrate, the filler (if present), the binder, the polymeric
thickener, the pH buffer system, and the other additives.
[0034] pH Buffer system
[0035] The pH buffer system comprises a weak acid and a conjugate base of
the weak acid, preferably at about 0.01 to about 0.25 wt.%, typically about
0.025
to about 0.15 wt. %, more typically about 0.05 to about 0.10 wt. %, of the
joint
compound on a dry basis. The pH buffer system maintains the joint compound at
a pH of 8-12, preferably 9-11. Embodiments of pH buffer systems include but
are
not limited to a tribasic phosphate and dibasic phosphate, such as sodium
phosphate dibasic (Na2HPO4) and sodium phosphate tribasic (Na3PO4) or
potassium phosphate dibasic (K2HPO4) and potassium phosphate tribasic
(K3PO4), or sodium carbonate and sodium bicarbonate. Another potential buffer
pair is ammonia and ammonium chloride. The preferred pH buffer system
comprises sodium carbonate and sodium bicarbonate.
[0036] Fillers
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
[0037] The joint compounds of the invention include a filler.
Examples of fillers
for joint compounds of the invention include, but are not limited to, calcium
carbonate (or limestone), calcium sulfate dihydrate, talc, glass micro
bubbles,
mica, perlite, pyrophyllite, silica, calcium sulfate anhydrite, diatomaceous
earth,
clay (e.g., attapulgite, sepiolite and kaolin), resin microspheres, and
mixtures
thereof.
[0038] Drying-type (DT) joint compounds preferably include a
primary DT filler
and optionally a secondary DT filler. Examples of primary DT fillers include
calcium carbonate, calcium sulfate dihydrate, talc, and mixtures thereof.
[0039] The primary DT filler can preferably be included at about 50 wt. %
to
about 98 wt. % on a dry basis of the joint compound, and more preferably about
50 wt. % to about 93 wt. % on a dry basis. For example, calcium carbonate as
the
primary DT filler can preferably be included in a drying-type joint compound
at
about 65 wt. % to about 93 wt. % on a dry basis. In another example, calcium
sulfate dihydrate as the primary DT filler can preferably be included in a
drying-
type joint compound at about 50 wt. % to about 93 wt. % on a dry basis, and
more preferably at about 55 wt. % to about 75 wt. % on a dry basis.
[0040] Examples of secondary DT fillers include, but are not
limited to, glass
micro bubbles, mica, perlite, pyrophyllite, silica, calcium sulfate anhydrite,
diatomaceous earth, clay (e.g., attapulgite, sepiolite and kaolin), resin
microspheres, and mixtures thereof. Secondary DT fillers may be useful as
fillers
and used to impart specific properties to the joint compounds. For example,
mica
aids in reduced cracking of the joint compound as it dries, and is preferred
in
amounts of up to 25 wt. % on a dry basis. It is also preferred to add clay in
amounts of up to about 10 wt. % on a dry basis to improve the body and
workability of the joint compound, and as a rheology modifier.
For drying-type joint compounds, the secondary DT filler can be included at up
to
about 25 wt.% on a dry basis of the joint compound, preferably about 3 wt.% to
about 25 wt.% on a dry basis, and more preferably about 4 wt.% to about 25
wt.%
on a dry basis.
11
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
[0041] Typically the joint compound has an absence of calcium
sulfate
hem ihydrate. The joint compound may have an absence of calcium sulfate
dihydrate or less than 10 wt. % calcium sulfate dihydrate.
[0042] Fillers may be useful as fillers and also as functional
fillers used to
impart specific properties to the joint compounds. For example, mica aids in
reduced cracking of the joint compound as it dries, and is preferred in
amounts of
up to 25 wt. % on a dry basis. It is also preferred to add clay in amounts of
up to
about 10 wt. % on a dry basis to improve the body and workability of the joint
compound, and as a rheology modifier.
[0043] The perlite can be uncoated perlite or coated perlite. Coated
perlite is
perlite coated with a hydrophobic coating, for example, a coating containing
siloxane or silane. The perlite can be a mixture of coated perlite and
uncoated
perlite. Perlite or expanded perlite is a lightweight filler that may be used
where
the joint compound is preferably lightweight. Use of expanded perlite in a
lightweight joint compound is taught in U.S. Pat. No. 4,454,267, which is
herein
incorporated by reference. Expanded perlite is a very lightweight material
that
contains many cracks and fissures. The perlite may be treated with a
hydrophobic coating, for example a hydrophobic coating of silane or siloxane.
For example, the perlite can be treated according to the teachings of U.S.
Pat.
No. 4,525,388 to Rehder et al, which is hereby incorporated by reference, so
that the material does not increase in weight due to water absorbed by
capillary
action. The treated, expanded perlite, when used, is typically present in
concentrations of at least 5 wt. % on a dry basis of the joint compound.
[0044] Any joint compound of the present invention optionally
includes resin
microspheres as a filler to be used in place of or in addition to expanded
perlite in
lightweight formulations. Preferred shell resins suitable for use in the
present
invention are homopolymers, copolymers, or blends of homopolymers and/or
copolymers formed one or more of acrylonitrile ("ACN"), vinylidene chloride
("VDC"), or methyl nnethacrylate ("MMA") monomers. Particularly preferred
resins
are polyacrylonitrile ("PACN"), polyvinylidene chloride ("PVDC"), copolymers
formed from ACN and VDC, and copolymers found from ACN, VDC, and MMA.
The microspheres demonstrate high resiliency to compression without collapse
12
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
(non-friable) and are able to withstand the exerted shear stress (shear-
stability) of
a typical joint treatment manufacturing process and subsequent customer
preparation.
[0045] Binders
[0046] Any binder that is suitable for use in a joint compound is
appropriate
for use in the present invention. The binder can enhance the adhesion of the
joint compound to its substrate, typically drywall.
[0047] Examples of binders include, but are not limited to,
polyvinyl acetate,
polyvinyl alcohol, ethylene vinyl acetate co-polymer, vinyl chlorides, vinyl
acrylic
co-polymer, styrene acrylics, styrene butadiene, polyacrylamide,
polyvinylacrylic, latex emulsions, natural and synthetic starch, dextrin,
casein,
and mixtures thereof. There may also be an absence of vinyl acetate.
[0048] For drying-type joint compounds, binders can be included up
to about
wt. %, up to about 8 wt. %, or up to about 4 wt. %.% on a dry basis of the
joint
15 compound. For example, at about 1 wt.% to about 15 wt.%, preferably
about 1
wt.% to about 10 wt.%, and most preferably about 1 wt.% to about 8 wt.% on a
dry basis.
[0049] For example, latex emulsion binders are often used in joint
compounds and may be included in joint compounds of the invention. Examples
include polyvinyl acetate, ethylene vinyl acetate and vinyl acrylic emulsions.
The
amount used may range from about 1.5 wt. % to about 7 wt. % on a dry basis of
the joint compound, preferably about 2 wt. % to about 5.5 wt. % on a dry
basis.
[0050] The weight ratio of total fillers to total binders is
preferably in the
range of from about 25:1 to about 5:1, more preferably 15:1 to about 5:1.
[0051] The present invention may employ one or more latexes, for example
one latex as the sole latex with an absence of additional latex, or a
combination of latexes wherein their respective glass transition temperatures
may be the same or different. Each of the one or more latexes typically has a
glass transition temperature in the range of less than 40 C, or less than 30
C, or less than 20 C, or less than 15 C, or less than 10 C, or less than 0
C, or less than -15 C. Each of the one or more latexes typically has a glass
transition temperature of greater than about -100 C, greater than about -80 C,
13
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
greater than about -40 C, or greater than 10 C, for example about 15 C to
less than 40 C. Compositions of the present invention may include mixtures
comprising a first binder and a second. For example, the first binder may
comprise a first polymer having a glass transition temperature that is equal
to
or greater than about -10 C. The second binder may comprise a second
polymer having a glass transition temperature in the range of about -80 C to
about 10 C. In the case of these two binders the glass transition temperature
of the first binder is at least about 5 C greater than the glass transition
temperature of the second binder, and the first and second polymers have the
same chemistry. The glass transition temperature may be measured by any
typical method. The resulting glass transition temperature of each test method
is within plus or minus 5 C. The most common tests are differential scanning
calorimeter (DSC), or dynamic mechanical analyzer (DMA). See for example,
ASTM D6604-00 Standard Practice of Glass Transition Temperatures of
Hydrocarbon Method by DSC.
[0052] Polymeric Thickeners
[0053] Polymeric thickeners are added to the joint compound of the
present
invention. After water is added to the composition, the thickener becomes
hydrated and swells, thereby thickening the joint compound. Thickeners are
useful, for example, in helping to create the body and flow properties
commonly
associated with joint compounds. Preferably, the thickener is selected so that
it
substantially hydrates during the mixing process after water is added to the
composition, with little or no hydration of the thickener occurring after
mixing is
completed, to prevent formation of lumps in the joint compound.
[0054] Examples of polymeric thickeners include, but are not limited to,
ethylhydroxy ethylcellulose, hydroxypropyl methylcellulose,
methylhydroxypropyl
cellulose, hydroxyethyl cellulose, cellulose-based gums (e.g., xanthan gum,
gum
Arabic, alginate, pectin, and guar gums), and mixtures thereof.
[0055] For drying-type joint compounds, polymer thickeners can be
included
at about 0.05 wt. % to about 3 wt. % on a dry basis of the joint compound,
preferably about 0.1 wt. % to about 3 wt. A on a dry basis, more preferably
14
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
about 0.1 wt. % to about 2 wt. % on a dry basis, and most preferably about 0.5
wt. % to about 2 wt. % on a dry basis.
[0056] Other Additives
[0057] Other additives that can optionally be included in joint
compounds
include, but are not limited to, rheology modifier, preservatives, biocides,
scents,
wetting agents, acid-base indicators, fungicides, bactericides, dyes,
pigments,
defoaming agents, glycols, humectants, dedusting agents, and mixtures thereof.
Other additives can be included at up to about 10 wt. % on a dry basis of the
joint compound, and preferably about 0.1 wt. % to about 10 wt. % on a dry
basis.
[0058] Other additives that can optionally be included in joint
compounds
include, but are not limited to, rheology modifiers which can include
surfactants,
thickeners, dispersing aids, and/or dedusting agents, such as a wax, oil,
and/or
polyethylene glycol. The plasticizers and the additional dedusting agents may
act
together to lower airborne dust generation during sanding. When included, the
amount of wax, oil and/or polyethylene glycol used in a joint compound of the
invention is preferably in a range of about 0.1 wt.% to about 1 wt.%, more
preferably 0.1 wt.% to 0.5 wt.% on a dry basis of the joint compound.
[0059] Defoamers reduce or hinder the formation of air bubbles,
which may
form especially when mixing. Examples of defoamers include, but are not
limited to, hydrocarbon-based, silicon-based defoamer, and mixtures thereof.
[0060] A glycol can be used in a joint compound to provide
functional
properties to the joint compound such as wet edge, open time, controlling
drying
time, and freeze/thaw stability. Examples of glycols include, but are not
limited
to, diethyl glycol, ethylene glycol, propylene glycol, and mixtures thereof.
When
included, the amount of glycol used in a joint compound of the invention is
preferably in a range of about 0.1 wt.% to about 1 wt.% or 0.1 wt.% to 0.5
wt.%
or 0.1 wt.% to 0.25 wt.% on a dry basis of the joint compound.
[0061] Methods
[0062] The joint compounds described herein can be applied to a surface
(e.g., a gypsum board) and allowed to dry and/or set. The dried/set joint
compound can then be dry sanded, or wet sanded or sponged. Alternatively,
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
because the joint compounds described herein have improved smoothness when
dried/set, the joint compounds can be wet sanded after drying and/or setting.
[0063] Dry sanding is generally done by rubbing with dry
sandpaper, optionally
with a vacuum attachment (e.g., using a drywall vacuum sander). Wet sandpaper
is done for example by rubbing with wet sandpaper. Sponging is rubbing the
applied joint compound with a sponge wetted with water to be damp. One or more
of these can be performed with a vacuum attachment (e.g., using a drywall
vacuum sander) to collect any dust formed. Alternatively or additionally, one
or
more of these methods can be performed in a negative pressure enclosure (e.g.,
a plastic enclosure with a fan to create negative pressure in the enclosure).
EXAM P LES
[0064] In the examples herein, as mentioned above, percentages of
compositions or product formulae are in weight percentages, unless otherwise
expressly stated. The reported measurements also in approximate amounts
unless expressly stated, for example, approximate percentages, weights,
ternperatures, distances or other properties.
[0065] Example 1
[0066] Ready-mixed joint compounds were produced according to
Table 2.
The dosing levels of the pH buffer systems are shown in Table 3. The control
formulation (DCpH1) had no pH buffer system. DCpH5 had only lime added to
boost the pH as is typical in the industry and is for comparison.
16
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
Table 2.
Compound Kg
Calcium Carbonate 865
Clay 39.3
Perlite 133
Hydroxy Ethyl Cellulose 6.4
Sodium Nitrite 0.8
Starch 5
Polyvinyl Alcohol 5
Hydroxy propyl methyl cellulose 2.2
Bleach Solution 1.5
Latex 48.4
Preservative 4.4
Plasticizer 4.35
PEG 750 26.22
Water 968.5
Total 2110.07
Table 3 Dosing Level for Buffer
DC pH1 DC pH2 DC pH3 DC pH4 DC pH5
(Control)
Compound (g) (at 511.23 497.6 520.9 503.37
505.17
about 50% solids)
Na2CO3*10H20 (g) 0 1.74 3.48 5.13 0
NaHCO3 (g) 0 0.36 0.69 0.99 0
Lime (g) 0 0 0 0 1.25
[0067] DC pH 2, DC pH3, and DC pH4 had the pH buffer system
(Na2CO3*10H20 and NaHCO3) as shown in Fig. 1, maintained its pH over time,
unlike the control with no pH buffer system and the formulation with lime. The
storage conditions were 75 F in a sealed container.
[0068] Table 4 reflects the values in Fig. 1.
17
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
Table 4 pH of Joint Compounds
Days DCpH1 DCpH2 DCpH3 DCpH4 DCpH5
0 8.5 9.9 10.1 10.2 11.0
3 8.5 10.3 10.4 10.5 11.0
4 8.5 10.3 10.4 10.4 10.2
8.4 10.3 10.4 10.4 10.3
6 8.4 10.3 10.4 10.4 10.3
7 8.5 10.2 10.3 10.3 9.7
8.5 10.2 10.3 10.3 9.7
11 8.5 10.2 10.3 10.3 9.7
12 8.5 10.1 10.3 10.4 9.6
13 8.6 10.1 10.3 10.4 9.7
14 8.6 10.1 10.3 10.4 9.6
19 8.6 10.1 10.3 10.4 9.6
22 8.6 10.1 10.3 10.4 9.6
54 8.6 10.1 10.3 10.4 9.2
63 8.6 10.1 10.3 10.4 9.2
77 8.6 10 10.3 10.3 9.1
94 8.6 10.1 10.3 10.4 9.1
105 8.8 10 10.2 10.3 9.1
125 8.5 9.9 10.2 10.3 9
201 8.5 10.0 10.3 10.4 9.1
[0069] Example 2
5 [0070] Ready-mixed joint compounds were produced with the amount of
preservatives varied for comparison in bacterial and fungal resistance tests.
[0071] B292, B345, B348, B373, B376, B389, and B 392 had 100% of
the
typical amount of triazine for a joint compound with an initial pH of 10.1.
B293,
B343, B346, B371, B374, B387 and B390 had 50% of the typical amount of
10 triazine for a joint compound with an initial pH of 10.1. B294, B344,
B347, B372,
18
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
B375, B388 and B391 B 375 had 75% of the typical amount of triazine for a
joint
compound with an initial pH of 10.2.
[0072] To evaluate the microbial state of the samples, swabs of
each well-
mixed sample were applied to potato dextrose agar (PDA) and tryptic soy agar
(TSA). PDA plates were incubated at 22C for 5-7 days, while TSA plates were
incubated at 35C for at 48 hours. After incubation, the degree of
contamination
along the streak lines was visually estimated for coverage and reported from
"0"
(no growth) to "4" indicating heavy growth. Growth on PDA and TSA plates was
then identified as either bacteria or fungi based on colony morphology and
microscopy, where necessary.
[0073] The microbial resistance of the samples was determined
through
bacterial and fungal challenge testing. Wet-state bacterial challenge testing
was
performed by inoculating the samples with -106 CFU/g of a mixed bacterial
culture using the following bacterial organisms: Alcaligenes faecalis (ATCC
#25094), Enterobacter Aerogenes (ATCC #13048), Escherichia coli (ATCC
#11229), and Pseudomonas aeruginosa (ATCC #101045). Contamination was
monitored at the indicated intervals. Contamination was monitored by streaking
the challenged material on TSA plates, incubating, and then rating the growth
along the streak liones from "0" (no growth) to "4" (>60% coverage). To
evaluate the ability of the formulation to resist repeated challenge,
inoculation
was repeated in the same manner, as indicated. Results were measured in
CFU. "CFU" stands for colony forming unit, as known in the art.
[0074] Wet-state fungal challenge testing was performed by
inoculating the
samples with -106 CFU/g of a mixed fungal culture using these fungal
organisms: Penicillium polonicum (ATCC #12667), Aspergillus Niger (ATCC
#6275). Contamination was monitored at the indicated intervals. Contamination
was monitored by streaking the challenged material on PDA plates, incubating,
and then rating the growth along the streak lines from "0" (no growth) to "4"
(>60% coverage). To evaluate the ability of the formulation to resist repeated
challenge, inoculation was repeated in the same manner, as indicated.
[0075] All samples arrived free of detectable microbial growth and
with pH
values between 10.1-10.2 The samples all resisted two inoculations with a
19
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
standard bacteria inoculum. Analytical results showed varying levels of
triazine
in each sample but within expected levels.
[0076] The samples were tested at day 1, 2, and 7 after the
microbial
challenge over four challenges and no fungal nor bacterial growth was
recorded.
After the 7 days, the sample was presented with an additional challenge of
biological loading and the result is read 48 hours after the re-inoculation
(denoted as 48 HR (re) in the tables below.
[0077] Material was aged at 50 C for 2 weeks to simulate long-term
aging.
Material came in sterile and resisted 2 series of microbial challenge.
Table 5 Microbial In-can Preservation Resistance TSA platings
P. aeruginosa, A. faecalis, E. aerogenes, E. coli
sterility 24 hours 48 hours 7 days 48 HR (re)
ID Rating Rating Rating Rating Rating
B292 0 0 0 0 0
B293 0 0 0 0 0
B294 0 0 0 0 0
Table 6 Microbial In-can Preservation Resistance PDA platings
A. niger, Penicillium sp.
sterility 24 hours 48 hours 7 days 48 HR (re)
ID Rating Rating Rating Rating Rating
B292 0 0 0 0 0
B293 0 0 0 0 0
B294 0 0 0 0 0
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
Table 7 Ratings for Tables 5, 6, and 8-13
Rating Average Colonies
0 0
1 <10
2 11-39
3 40-100
4 >100
Table 8 Microbial In-can Preservation Resistance TSA platings
P. aeruginosa, A. faecalis, E. aerogenes, E. coli
sterility 24 hours 48 hours 7 days 48 HR (re)
ID Rating Rating Rating Rating Rating
B343 0 0 0 0 0
B344 0 0 0 0 0
B345 0 0 0 0 0
B346 0 0 0 0 0
B347 0 0 0 0 0
B348 0 0 0 0 0
Table 9 Microbial In-can Preservation Resistance PDA platings
A. niger, Penicillium sp.
sterility 24 hours 48 hours 7 days 48 HR (re)
ID Rating Rating Rating Rating Rating
B343 0 0 0 0 0
B344 0 0 0 0 0
B345 0 0 0 0 0
B346 0 0 0 0 0
B347 0 0 0 0 0
B348 0 0 0 0 0
21
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
Table 10 Microbial In-can Preservation Resistance TSA platings
P. aeruginosa, A. faecalis, E. aerogenes, E. coli
sterility 24 hours 48 hours 7 days 48 HR (re)
ID Rating Rating Rating Rating Rating
B371 0 0 0 0 0
B372 0 0 0 0 0
B373 0 0 0 0 0
B374 0 0 0 0 0
B375 0 0 0 0 0
B376 0 0 0 0 0
Table 11 Microbial In-can Preservation Resistance PDA platings
A. niger, Penicillium sp.
sterility 24 hours 48 hours 7 days 48 HR (re)
ID Rating Rating Rating Rating Rating
B371 0 0 0 0 0
B372 0 0 0 0 0
B373 0 0 0 0 0
B374 0 0 0 0 0
B375 0 0 0 0 0
B376 0 0 0 0 0
22
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
Table 12 Microbial In-can Resistance TSA platings
P. aeruginosa, A. faecalis, E. aerogenes, E. coli
sterility 24 hours 48 hours 7 days 48 HR (re)
ID Rating Rating Rating Rating Rating
B387 0 0 0 0 0
B388 0 0 0 0 0
B389 0 0 0 0 0
B390 0 0 0 0 0
B391 0 0 0 0 0
B392 0 0 0 0 0
Table 13 Microbial In-can Preservation Resistance PDA platings
A. niger, Penicillium sp.
sterility 24 hours 48 hours 7 days 48 HR (re)
ID Rating Rating Rating Rating Rating
B387 0 0 0 0 0
B388 0 0 0 0 0
B389 0 0 0 0 0
B390 0 0 0 0 0
B391 0 0 0 0 0
B392 0 0 0 0 0
23
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
[0078] CLAUSES DESCRIBING VARIOUS CHARACTERISTICS OF
COMPOSITIONS AND METHODS OF THE INVENTION
[0079] Clause 1. A ready-mixed, drying-type joint compound
comprising a
mixture of joint compound components and water;
the joint compound components comprising:
a filler at about 50 wt. % to about 98 wt. % of the joint compound on a
dry basis,
a binder at up to about 15 wt. % of the joint compound on a dry basis;
a polymeric thickener at up to about 3 wt. % of the joint compound on a
dry basis;
a pH buffer system comprising a weak acid and a conjugate base of
the weak acid, at about 0.01 wt.% to about 1.0 wt. %, at about 0.01 wt. % to
about 0.25 wt. (Yo, at about 0.025 wt.% to about 0.5 wt. (Yo, at about 0.025
to
about 0.15 wt. %, at about 0.05 wt. % to about 0.25 wt. %,at about 0.05 wt. %
to about 0.15 wt. %, at about 0.05 wt. % to about 0.10 wt. % of the joint
compound on a dry basis; and
an additive up to about 10 wt. % of the joint compound on a dry basis,
wherein optionally the additive comprises an accelerator and/or a rheology
modifier;
wherein weight ratio of the water to the dry joint compound
components is about 1:6 to about 3:1,
wherein at least one of the joint compound components is alkali sensitive.
[0080] Clause 2. The joint compound of clause 1, wherein the pH
buffer
system comprises sodium carbonate and sodium bicarbonate.
[0081] Clause 3. The joint compound of clause 1, wherein the pH buffer
system comprises K2HPO4 and K3PO4.
[0082] Clause 4. The joint compound of any of the preceding
clauses,
wherein the pH is 9-11.
[0083] Clause 5. The joint compound of clause 1, wherein the pH
buffer
system comprises ammonia and ammonia chloride.
24
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
[0084] Clause 6. The joint compound of any of the preceding
clauses,
wherein the alkali sensitive component comprises one or more of the rheology
modifier, the binder, and the additive.
[0085] Clause 7. The joint compound of clause 6, wherein the
alkali
sensitive component comprises the rheology modifier.
[0086] Clause 8. The joint compound of clause 6, wherein the
alkali
sensitive component comprises the binder.
[0087] Clause 9. The joint compound of clause 6, wherein the
alkali
sensitive component comprises the additive.
[0088] Clause 10. The joint compound of any of the preceding clauses,
wherein:
the filler is at about 50 wt. % to about 93 wt. % of the joint compound on a
dry basis;
the binder is up to about 8 wt. % of the joint compound on a dry basis;
the polymeric thickener is up to about 2 wt. % of the joint compound on a
dry basis;
the pH buffer system is at about 0.025 wt. to about 0.15 wt. % of the joint
compound on a dry basis; and
the additive is up to about 10 wt. % of the joint compound on a dry basis
wherein optionally the additive comprises an accelerator and/or a rheology
modifier;
wherein the weight ratio of the water to the dry joint compound
components about 1:6 to about 3:1.
[0089] Clause 11. The joint compound of clause 10, wherein:
the filler is at about 65 wt. % to about 93 wt. % of the joint compound on a
dry basis;
the binder is up to about 4 wt. % of the joint compound on a dry basis;
the polymeric thickener is about 0.1 wt. % to about 2 wt. % of the joint
compound on a dry basis;
the pH buffer system is at about 0.05 wt. to about 0.10 wt. % of the joint
compound on a dry basis;
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
the additive is about 0.1 wt. % to about 10 wt. % of the joint compound on
a dry basis, wherein optionally the additive comprises an accelerator and/or a
rheology modifier; and
wherein the weight ratio of the water to the dry joint compound
components is about 1:3 to about 1:1.
[0090] Clause 12. The joint compound of any of the preceding
clauses,
wherein the filler comprises calcium carbonate, calcium sulfate dihydrate,
talc,
glass micro bubbles, mica, perlite, pyrophyllite, silica, calcium sulfate
anhydrite, diatomaceous earth, clay, resin microspheres, or mixtures thereof.
[0091] Clause 13. The joint compound of any of clauses 1 to 12, wherein
the joint compound has a pH of 8-12, preferably 9-11, for 120 days or more at
75 F (23.9 C) in a sealed container.
[0092] Clause 14. The joint compound of any of the preceding
clauses,
further comprising up to 0.1 wt. `)/0 biocide.
[0093] Clause 15. The joint compound of any of the preceding clauses,
comprising:
Calcium carbonate,
Clay,
Perlite,
Hydroxyl ethylcellulose,
Sodium nitrite,
Starch,
Polyvinyl alcohol,
hydroxyl propyl methyl cellulose,
Bleach solution,
latex,
preservative,
plasticizer,
PEG 750,
Water and
the pH buffer system.
26
CA 03219509 2023- 11- 17
WO 2022/253469
PCT/EP2022/025262
[0094] Clause 16. The joint compound of clause 15, wherein the pH
buffer
system comprises Na2CO3*10H20 and NaHCO3.
[0095] Clause 17.The joint compound of any of clauses 1-16,
wherein the
pH buffer system is at about 0.01 wt.% to about 0.25 wt. % of the joint
compound on a dry basis.
[0096] Clause 18. The joint compound of any of clauses 1-16,
wherein the
pH buffer system is at about 0.025 wt.% to about 0.5 wt. % of the joint
compound on a dry basis.
[0097] Clause 19. A method of using the joint compound of any of
clauses
1 to 18, comprising applying the joint compound to one or more of the group
consisting of boards, joint tape, and another layer of the joint compound.
[0098] Clause 20. A method of making the joint compound of any of
clauses 1 to 18, comprising combining the at least one filler, the binder, the
polymeric thickener, the pH buffer system, and the additive.
[0099] While particular versions of the invention have been shown and
described, it will be appreciated by those skilled in the art that changes and
modifications may be made thereto without departing from the invention in its
broader aspects and as set forth in the following claims.
27
CA 03219509 2023- 11- 17