Note: Descriptions are shown in the official language in which they were submitted.
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
SYNTHETIC PROTEIN FOR INDUCING IMMUNE TOLERANCE
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims priority to U.S. Provisional Application No.
63/189,359 filed
-- on May 17, 2021, the contents of which are incorporated by reference in
their entireties.
SEQUENCE LISTING
A Sequence Listing accompanies this application and is submitted as an ASCII
text
file of the sequence listing named "960296 04290 5T25.txt" which is 114,175
bytes in size
and was created on April 29, 2022. The sequence listing is electronically
submitted via EFS-
Web with the application and is incorporated herein by reference in its
entirety.
BACKGROUND
Transplant rejection occurs when the recipient's immune system attacks the
donated
graft and begins destroying the transplanted tissue or organ. Currently,
chronic systemic
immunosuppression is the only clinical strategy available to prevent the
rejection of allogenic
transplants (1). Despite significant improvements in post-transplant
immunosuppressive
therapies, long-term inhibition of the host immune response still causes
serious adverse
effects such as opportunistic infections, cardiac and renal toxicity, and
increased risk of
malignancies (2). Both these adverse effects and the severe shortage of
cadaver-derived cells
and tissues are major obstacles preventing the broad adaptation of allogenic
transplant
therapies as treatments for several end-stage human diseases (1, 3-5). For
example, islet
transplantation is a promising therapy for treatment of type-1 diabetes
(T1D)(6-8). But,
unfortunately, the majority of islet allograft recipients lose graft function
and insulin
independence within 3-5 years post-transplant (9). Further, an
immunosuppressive regimen
that prevents the rejection of xenogeneic transplants has never been
established. Thus, there
remains a critical and unmet need for a safer and more effective means of
inducing immune
tolerance to allogeneic or xenogeneic grafts.
SUMMARY
The present invention provides engineered fusion polypeptides that are based
on the
inventor's fusion protein, referred to herein as PIDO. The fusion proteins
comprise from N-
terminus to C-terminus: (a) a PD-Li peptide comprising at least a portion of
the extracellular
domain of a PD-Li protein, (b) a transmembrane domain, and (c) an IDO peptide
comprising
at least a portion of an IDO protein. In some embodiments, the PD-Li peptide
is capable of
binding to PD-1 and the IDO peptide is catalytically active.
1
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
In a second aspect, the present invention provides nucleic acid constructs
comprising
a polynucleotide encoding the fusion proteins described herein operably linked
to a promoter.
In a third aspect, the present invention provides cells comprising the nucleic
acid
construct described herein. Under suitable conditions, the cells express the
fusion proteins
described herein.
In a fourth aspect, the present invention provides methods of transplanting
the cell
described herein into a subject.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 demonstrates that the PIDO fusion protein is expressed in transduced
cells.
(A) Schematic depiction of the experiment. Lentivirus was used to transduce
pancreatic islets
for PIDO expression. (B) Schematic of the PIDO expression construct (top) and
the PIDO
protein sequence (SEQ ID NO:1; bottom). (C) Predicted 3D structure of PIDO.
(D)
Lentivirus transduction efficiency in A375 human melanoma cells, detected as
expression of
the indicated fluorescent reporters. DNA was counterstained with DAPI (blue).
C57BL/6
mouse islets were transduced by lentiviruses expressing PD-L1, IDO, or PIDO
followed by
enzymatic dispersion. The transduced cells were analyzed by (E) flow cytometry
(i.e., to
measure extracellular PD-Li expression) and (F) western blot (representative
of 3) of extracts
from PIDO-expressing mouse or pig islets using an anti-IDO antibody (i.e., to
measure
intracellular IDO expression). (G) Schematic of the predicted subcellular
localization of the
PIDO constituent proteins. PD-Li is displayed on the cell membrane while IDO
is tethered to
cytoplasmic tail of PD-Li in the cytoplasm. (H) Kynurenine ELISA to detect IDO
catalytic
activity (n = 4). (I) Mouse islets transduced with constructs for the
expression of PD-L1,
IDO, or PIDO were compared to unmodified islets in a glucose-stimulated
insulin secretion
assay after 48 hours of in vitro culture. These results show the insulation
secretion at low
(2.8G) and high (16.7G) glucose concentration. Data are presented as mean
SEM.
(*p<005 **P <0.01,***P <0.001).
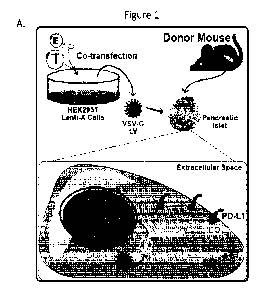
Figure 2 demonstrates that PIDO-expressing allogeneic islets reverse pre-
existing
chemically induced diabetes in mice. (A) Schematic depiction of the
experiment. Diabetes
was included with streptozotocin (STZ) and PIDO-expressing allogeneic C57BL/6
mouse
islets were transplanted into BALB/c mice. (B) Representative sections of
transplanted islet
allografts under the kidney capsule (bright field, left, 4X magnification)
were stained for
insulin (green) and actin (red). DNA was counterstained with DAPI (blue). The
original
magnification was 20X. (C) Blood glucose measurements taken before and after
STZ
treatment and after transplantation with engineered allogeneic islets. Five
groups were
2
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
studied: (1) non-diabetic mice without a transplant ("No STZ/Txp"; no STZ, no
transplant; n
= 3; dotted line), (2) diabetic mice transplanted with control islets
("Isletsc"1"; +STZ, EGFP-
expressing transplant; 400 islets; n = 4; red), (3) diabetic mice transplanted
with PD-L1-
expressing islets ("IsletsPD-L1"; +STZ, PD-Li-expressing transplant; 400
islets; n = 5;
diamond symbols, broken line, (4) diabetic mice transplanted with IDO-
expressing islets
("Isletsm"; +STZ, IDO-expressing transplant; 400 islets; n = 5; hexagon
symbols, broken
line), and (5) diabetic mice transplanted with islets that co-express PD-Li
and IDO
individually (IsletsPL -13 1+1130,,; +STZ, PIDO-expressing transplant; 400
islets; n = 5; blue).
(D) Blood glucose measurements taken before and after STZ treatment and after
transplantation with engineered allogeneic islets, in both the fasted (right)
and random-fed
(left) state. Three groups were studied: (1) mice without a transplant ("No
Txp"; no STZ, no
transplant; n = 3; dotted line), (2) diabetic mice transplanted with control
islets ("Isletsc"1";
+STZ, EGFP-expressing transplant; 400 islets; n = 4; red), and (3) diabetic
mice transplanted
with PIDO-expressing islets ("IsletsPID "; +STZ, PIDO-expressing transplant;
400 islets; n =
5; blue). (E) Glucose tolerance test (GTT) performed 2 weeks and 10 weeks
after transplant.
Three groups were studied: (1) mice without a transplant ("No Txp"; no STZ, no
transplant; n
= 3; 2Wk and 10Wk, black), (2) diabetic mice transplanted with control islets
("Isletsc"1";
+STZ, EGFP-expressing transplant; n = 5; 2Wk and 10Wk, red), and (3) diabetic
mice
transplanted with PIDO-expressing islets ("IsletsPID +"; +STZ, PIDO-expressing
transplant; n
= 5; 2Wk and 10Wk, blue). Lower panel: area under the curve (AUC)
quantification of GTT
data. (F) In vivo glucose-stimulated insulin secretion (GSIS) assay performed
2 and 10 weeks
after transplantation. Three groups were studied: (1) mice without a
transplant ("No Txp"; no
STZ, no transplant; n = 3; 2Wk and 10Wk, black), (2) diabetic mice
transplanted with control
islets ("Islets"; +STZ, EGFP-expressing transplant; n = 4; 2Wk and 10Wk, red),
and (3)
diabetic mice transplanted with PIDO-expressing islets "Islets'""; +STZ, PIDO-
expressing
transplant; n = 5; 2Wk and 10Wk, blue). Data are presented as mean SEM. (*P
< 0.05,
**P < 0.01, ***P < 0.001).
Figure 3 demonstrates that PIDO-expressing islet allografts improve
hyperglycemia
in diabetic NOD mice. (A) Schematic depiction of the experiment. PIDO-
expressing
allogeneic C57BL/6 mouse islets were transplanted into diabetic NOD mice. (B)
Fed blood
glucose measurements in NOD mice after transplantation with naïve or PIDO-
expressing
allogeneic islets. Three groups were studied: (1) normoglycemic mice without a
transplant
("Non-diabetic/No Txp"; n = 4; black), (2) diabetic mice transplanted with
control islets
("Islets"; EGFP-expressing transplant; 400 islets; n = 4; red), and (3)
diabetic mice
3
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
transplanted with PIDO-expressing islets ("Isletsm +"; PIDO-expressing
transplant; 400
islets; n = 5; blue). Animals that died of diabetes complications
(hypoinsulinemia) or that had
relapsing diabetes were removed from the analysis at the observed time of
death/relapse and
are marked on the plot with an * and , respectively. (C) Stairstep graph
showing diabetes
relapse incidence in PIDO+ allogeneic islet-transplanted and naive allogeneic
islet-
transplanted NOD mice. Diabetes relapse (blood glucose >250 mg/dL) was used as
the
terminal event.
Figure 4 demonstrates that PIDO does not confer acquired immune tolerance
against
naive allogeneic islets. (A) Schematic of the experiment. Diabetes was induced
with
streptozotocin (STZ) and PIDO-expressing allogeneic C57BL/6 mouse islets were
transplanted into BALB/c mouse recipients. The recipients were then
rechallenged with STZ
or nephrectomy and a second subrenal transplantation in the contralateral
kidney was
performed. (B) Blood glucose measurements taken before and after STZ
treatment, after
transplantation with allogeneic islets, after rechallenge with STZ, and after
the second
transplantation with naive allogeneic islets. Three groups were studied: (1)
mice without a
transplant ("No Txp"; no STZ, no transplant; n = 3; dotted line), (2) diabetic
mice
transplanted with control islets ("Islets'; +STZ, EGFP-expressing transplant;
400 islets; n
= 4; red), and (3) diabetic mice transplanted with PIDO-expressing islets
("IsletsPD +";
+STZ, PIDO-expressing transplant; 400 islets; n = 5; blue). (C) Blood glucose
measurements
taken before and after STZ treatment, after transplantation with engineered
allogeneic islets,
after rechallenge via nephrectomy, and after the second transplantation with
naive allogeneic
islets. Three groups were studied: (1) mice without a transplant ("No Txp"; no
STZ, no
transplant; n = 3; dotted line), (2) diabetic mice transplanted with control
islets ("Isletsc"1";
+STZ, EGFP-expressing transplant; 400 islets; n = 5; red), and (3) diabetic
mice transplanted
with PIDO-expressing islets ("IsletsPID +"; +STZ, PIDO-expressing transplant;
400 islets; n =
5; blue). Data are presented as mean SD. (*P < 0.05, **P <0.01, ***P
<0.001).
Figure 5 demonstrates that PIDO-induced immune evasion of engineered islet
allografts requires CD4 expression. (A) Schematic of the experiment. PIDO-
expressing
BALB/c mouse allogeneic islets were transplanted in diabetic CD4-deficient
mice. (B) Blood
glucose measurements taken before and after STZ treatment and after
transplantation of
allogeneic islets. Three groups were studied: (1) mice without a transplant
("No Txp"; no
STZ, no transplant; black), (2) diabetic mice transplanted with control islets
("Islets";
+STZ, EGFP-expressing transplant; red), and (3) diabetic mice transplanted
with PIDO-
4
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
expressing islets ("IsletsPID +"; +STZ, PIDO-expressing transplant; blue).
Data are presented
as mean SEM.
Figure 6 demonstrates that PIDO-expressing xenogeneic islets survive in
immunocompetent murine and canine recipients. (A) Schematic depiction of the
experiment.
-- PIDO-expressing porcine islets were transplanted into normoglycemic C57BL/6
mice and
dogs. (B) Porcine insulin measurements in normoglycemic immunocompetent
C57BL/6 mice
after renal subcapsular transplantation with engineered pig islets. Three
groups were studied:
(1) mice without a transplant ("No Txp"; n = 3; black), (2) mice transplanted
with control
islets ("Islets'', EGFP Txp; 400 islets; n = 4; red), and (3) mice
transplanted with PIDO-
expressing islets ("IsletsPID "; PIDO Txp; 400 islets; n = 5; blue). (C)
Porcine C-peptide
measurements after intravenous glucose tolerance test (GTT) in a normoglycemic
beagle dog
at 3-, 6-, 10-, 15-, and 20-weeks post-transplantation in epaxial muscle.
Figure 7 shows a representative western blot comparing IDO expression and
abundance in samples from A375 cells that were transduced to express the
indicated proteins.
Figure 8 shows plasmid maps of lentiviral vectors encoding the PIDO fusion
protein.
(A) Plasmid map of the lentiviral vector comprising an enhanced green
fluorescent protein
(EGFP) reporter that was used in the Examples. (B) Plasmid map of a lentiviral
vector
designed for use in a transplant therapy.
DETAILED DESCRIPTION
A more effective means of inducing immune tolerance would address a critical
unmet
need to improve the safety of transplantation therapies. To address this unmet
need, the
inventors created a novel fusion protein, referred to herein as PIDO (PD-Li
and IDO). PIDO
comprises peptides derived from two immunoregulatory proteins: programmed
death ligand-
1 (PD-L1) and indolamine 2,3-dioxygenase (IDO). PD-Li and IDO are known to
induce
-- distinct immune tolerance mechanisms, which are discussed below.
In the Examples, the inventors generate cells that express PIDO and confirm
that the
components of this fusion protein each localize to the appropriate subcellular
compartments
(Figure 1): PD-Li spans the cell membrane, while IDO is anchored
intracellularly via a
flexible linker. Further, they confirm that IDO, which usually moves freely
throughout the
cytoplasm, remains catalytically active when tethered to the membrane as part
of this fusion
protein (Figure 1). To test whether the expression of PIDO induces local
immune tolerance,
the inventors engineered murine pancreatic islets to express this fusion
protein and
transplanted them into diabetic mice. Following transplantation, the modified
islet grafts
survived, produced insulin, and reversed the diabetes of these mice (Figure 2,
Figure 3).
5
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
Further, the inventors showed that PIDO-expressing porcine islet xenografts
remain
functional in murine and canine recipients for more than 20 weeks (Figure 6).
Thus, the
inventors have demonstrated that expression of the PIDO fusion protein may be
used to
improve the outcomes of both allogenic and xenogeneic transplant.
The methods of transplanting cells described herein offer multiple advantages
over
current transplant methods that rely on immune suppression. First, because
PIDO remains
anchored in the cell membrane, this fusion protein provides immune suppression
that is
locally restricted. Therefore, the use of PIDO would avoid the undesirable
side effects
associated with pharmacological immune suppression regimens, which can cause
off-target
immune suppression and toxicity. Second, the peptide components of PIDO can be
matched
to the species of the subject for greater compatibility and reduced risk of
antigenicity. Third,
because nearly all cell types can be modified to express PIDO, this fusion
protein can be used
with a wide variety of transplantation therapies.
Fusion proteins:
In a first aspect, the present invention provides fusion proteins based on the
PIDO
fusion protein. The fusion proteins comprise, from N-terminus to C-terminus:
(a) a PD-Li
peptide comprising at least a portion of the extracellular domain of a PD-Li
protein, (b) a
transmembrane domain, and (c) an IDO peptide comprising at least a portion of
an IDO
protein. Ideally, within the fusion proteins, the PD-Li peptide is capable of
binding to PD-1
and the IDO peptide is catalytically active.
As used herein, the term "fusion protein" refers to a single polypeptide
comprising at
least two peptide components, e.g., a PD-Li component and an IDO component.
Each
peptide component may comprise a synthetic peptide or a naturally occurring
peptide. The
peptide components may comprise a full-length protein or a fragment thereof,
and they may
comprise mutations or other modifications relative to the wild-type version of
the protein
from which they are derived.
Programmed death ligand-1 (PD-Li; also known as cluster of differentiation 274
(CD274)) is a transmembrane protein that plays a major role in suppressing the
adaptive
immune system. This protein is constitutively expressed by a wide variety of
immune cells
and can also be expressed by non-immune cells such as pancreatic islets (13,
14). The
cognate receptor for this protein, i.e., the programmed cell death-1 (PD-1)
receptor, is
expressed on the surface of T cells and other immune cells (12). PD-1/PD-L1
binding inhibits
effector T cell function and stimulates regulatory T cell function (15, 16) .
Thus, the PD-
6
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
1/PD-L1 interaction forms an immune checkpoint that protects normal tissues
from
inflammation and plays a critical role in the maintenance of immune tolerance.
The PD-Li peptide used with the present invention must comprise a portion of
the
extracellular domain of a PD-Li protein that is capable of binding to PD-1. An
"extracellular
domain" is a protein domain that localizes to the extracellular space when the
protein is
expressed by a cell. The amino acid residues within PD-Li that are necessary
for PD-1
binding were recently mapped by Zak et at. (Structure 25(8):1163-1174, 2017),
which is
incorporated by reference in its entirety. The key residues for PD-1 binding
include A121,
D122, Y123, K124, and R125 (i.e., the ADYKR sequence). Thus, the PD-Li peptide
used
with the present invention should comprise these key amino acid residues. The
ability of a
PD-Li peptide to bind to PD-1 may be assessed using a PD 1/PD-L1 binding assay
or any
protein-protein binding assay, including those that utilize surface plasmon
resonance, co-
immunoprecipitation, or fluorescence resonance energy transfer (FRET).
Alternatively, the
ability of a PD-Li peptide to bind to PD-1 may be assessed using in sit/co
modeling.
The PD-Li peptide may be a portion of a PD-Li protein from any vertebrate
animal.
Suitable sources of PD-Li peptides include, but are not limited to, humans,
non-human
primates, cows, cats, dogs, pigs, and rodents. In some embodiments, the PD-Li
peptide has at
least 95% identity to the extracellular domain of the mouse PD-Li protein (SEQ
ID NO:3;
amino acids 19-239 of SEQ ID NO: 2). In other embodiments, the PD-Li peptide
has at least
95% identity to the extracellular domain of the human PD-Li protein (SEQ ID
NO:7).
In some embodiments, the PD-Li peptide further comprises a PD-Li signal
peptide.
The PD-Li signal peptide is a membrane localization signal that is cleaved off
in the mature
PD-Li protein. While the inclusion of a signal peptide is required for proper
membrane
localization, comparable localization could be achieved by substituting the
native PD-Li
signal peptide for the signal peptide of another membrane bound protein or a
synthetic signal
peptide. In some embodiments, the PD-Li signal peptide is the signal peptide
of the mouse
PD-Li protein (SEQ ID NO:4; amino acids 1-18 of SEQ ID NO: 2). In other
embodiments,
the PD-Li signal peptide is the signal peptide of the human PD-Li protein (SEQ
ID NO:8).
A "transmembrane domain" is a protein domain that spans the cell membrane when
the protein is expressed by a cell. Transmembrane domains consist
predominantly of
hydrophobic amino acids. The transmembrane domain of the fusion protein may be
any
transmembrane domain that does not disrupt the ability of the PD-Li peptide to
bind to PD-1
or the catalytic activity of the IDO protein. In the Examples, the inventors
utilized a full-
length PD-Li protein in their PIDO fusion protein, such that both the
extracellular domain
7
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
and the transmembrane domain of the fusion protein were provided by PD-Li.
Thus, in some
embodiments, the transmembrane domain comprises at least a portion of the
transmembrane
domain of a PD-Li protein. In some embodiments, the transmembrane domain has
at least
95% identity to the transmembrane domain of the mouse PD-Li protein (SEQ ID
NO:5). In
other embodiments, the transmembrane domain has at least 95% identity to the
transmembrane domain of the human PD-Li protein (SEQ ID NO:9).
Indolamine 2,3-dioxygenase (IDO) is an intracellular, heme-containing enzyme
that
catalyzes the oxidation of tryptophan. This enzyme performs the initial, rate-
limiting step
necessary to degrade tryptophan via the kynurenine pathway. Tryptophan
degradation and the
products of this process (i.e., kynurenine derivatives and 02 free radicals)
suppress innate and
adaptive immunity by several mechanisms, including apoptosis, inhibition of
activated T
cells, and activation of resting regulatory T cells (19). IDO can be expressed
in a variety of
human tissues when its expression is induced by inflammatory cytokines, and it
is known to
be expressed in chronic inflammatory conditions such as cancers, infections,
autoimmune and
allergic diseases, and transplant rejection (20). Further, recent reports
suggest that subsets of
human myeloid dendritic cells and cancer cells constitutively express IDO to
suppress
allogeneic T-cell immune responses (21, 22).
The IDO peptide used with the present invention must comprise a catalytically
active
portion of an IDO protein, i.e., a portion that can catalyze 1-tryptophan
oxidation. Sugimoto,
et at. (Proc Natl Acad Sci USA (2006), 103(8): 2611-2616) have determined that
amino acid
residues F226, F227, and R231 of IDO are essential for its catalytic activity.
Thus, the IDO
peptide used with the present invention should comprise these key residues.
The catalytic
activity of the IDO peptide may be assessed by measuring conversion of
tryptophan to
kynurenine, for example, by kynurenine ELISA.
The IDO peptide may be a portion of an IDO protein from any vertebrate animal.
Suitable animals include, but are not limited to, humans, non-human primates,
cows, cats,
dogs, pigs, and rodents. In some embodiments, the IDO peptide has at least 95%
identity to
the full-length human IDO protein (SEQ D NO:10).
In some embodiments, the transmembrane domain is linked to the IDO peptide by
a
.. linker peptide. As used herein, the term "linker peptide" refers to a
peptide that connects two
peptide components within a fusion protein. The linker may be flexible such
that it has no
fixed structure in solution and the adjacent peptide components are free to
move relative to
one another. The flexible linker comprises 1 or more amino acid residues,
preferably 1, 2, 3,
4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, or 20 or more
residues. The linker may
8
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
be an existing sequence provided by a protein included in the fusion protein
or it may be
provided by insertion of one or more amino acid residues between the peptide
components of
the fusion protein. The linker may comprise any amino acid sequence that does
not
substantially hinder the function of the peptide components (i.e., PD-Ll's
ability to bind PD-
1 and IDO' s catalytic activity). Preferred amino acid residues for flexible
linker sequences
include glycine, alanine, serine, threonine, lysine, arginine, glutamine, and
glutamic acid, but
are not limited thereto. In some embodiments, the linker peptide is a glycine-
serine linker
(i.e., a linker consisting of serine and glycine). In specific embodiments,
the glycine-serine
linker is a 3X GGGS linker (SEQ ID NO: ii).
In some embodiments, the fusion protein comprises the mouse PIDO fusion
protein
described in the Examples (SEQ ID NO:1; encoded by SEQ ID NO:12), which
comprises the
full-length mouse PD-Li protein (SEQ ID NO:2) linked to the full-length human
DO protein
(SEQ ID NO: i0) via a 3X GGGS linker (SEQ ID NO: ii). In other embodiments,
the fusion
protein comprises the human PIDO fusion protein (SEQ ID NO: i4; encoded by SEQ
ID
NO:15), which comprises the full-length human PD-Li protein (SEQ ID NO:6)
linked to the
full-length human IDO protein (SEQ ID NO:10) via a 3X GGGS linker (SEQ ID
NO:11). In
other embodiments, the fusion protein comprises the canine PIDO fusion protein
(SEQ ID
NO:17; encoded by SEQ ID NO:18), which comprises the full-length canine PD-Li
protein
(SEQ ID NO:23) linked to the full-length human DO protein (SEQ ID NO: i0) via
a 3X
GGGS linker (SEQ ID NO: ii). In other embodiments, the fusion protein
comprises the feline
PIDO fusion protein (SEQ ID NO:20; encoded by SEQ ID NO:21), which comprises
the full-
length feline PD-Li protein (SEQ ID NO:24) linked to the full-length feline DO
protein
(SEQ ID NO: i0) via a 3X GGGS linker (SEQ ID NO: ii).
Nucleic acid constructs:
The present invention provides nucleic acid constructs comprising a
polynucleotide
encoding the fusion proteins described herein operably linked to a promoter.
The terms "polynucleotide," "oligonucleotide," and "nucleic acid" are used
interchangeably to refer a polymer of DNA or RNA. A polynucleotide may be
single-
stranded or double-stranded and may represent the sense or the antisense
strand. A
polynucleotide may be synthesized or obtained from a natural source. A
polynucleotide may
contain natural, non-natural, or altered nucleotides, as well as natural, non-
natural, or altered
internucleotide linkages. The term polynucleotide encompasses constructs,
plasmids, vectors,
and the like.
9
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
As used herein, the term "construct" or "nucleic acid construct" refers a to
recombinant polynucleotide, i.e., a polynucleotide that was formed by
combining at least two
polynucleotide components from different sources, natural or synthetic. For
example, a
construct may comprise the coding region of one gene operably linked to a
promoter that is
(1) associated with another gene found within the same genome, (2) from the
genome of a
different species, or (3) is synthetic. Constructs can be generated using
conventional
recombinant DNA methods.
In some embodiments, the nucleic acid construct is a viral vector. As used
herein, a
"viral vector" is a recombinant viral nucleic acid that has been engineered to
express a
heterologous polypeptide (e.g., the fusions proteins of the present
invention). Viral vectors
include cis-acting elements that drive the expression of the encoded
heterologous
polypeptide. Suitable viral vectors are known in the art and include, but are
not limited to,
adenovirus vectors; adeno-associated virus vectors, pox virus vectors (e.g.,
fowlpox virus
vectors), alpha virus vectors, baculoviral vectors, herpes virus vectors,
retrovirus vectors
(e.g., lentivirus vectors), Modified Vaccinia virus Ankara vectors, Ross River
virus vectors,
Sindbis virus vectors, Semliki Forest virus vectors, and Venezuelan Equine
Encephalitis virus
vectors. In a preferred embodiment, the viral vector is a lentiviral vector.
As used herein, the term "promoter" refers to a DNA sequence that regulates
the
expression of a gene. Typically, a promoter is a regulatory region that is
capable of binding
RNA polymerase and initiating transcription of a downstream (3' direction)
sequence.
However, a promoter may be located at the 5' or 3' end, within a coding
region, or within an
intron of a gene that it regulates. Promoters may be derived in their entirety
from a native
gene, may be composed of elements derived from multiple regulatory sequences
found in
nature, or may comprise synthetic DNA. A promoter is "operably linked" to a
polynucleotide
if the promoter is connected to the polynucleotide such that it can affect
transcription of the
polynucleotide. It is understood by those skilled in the art that different
promoters may direct
the expression of a gene in different tissues or cell types, at different
stages of development,
or in response to different environmental conditions. Suitable promoters for
use with the
present invention include, but are not limited to, constitutive, inducible,
temporally regulated,
developmentally regulated, chemically regulated, tissue-preferred, and tissue-
specific
promoters. In some embodiments, the promoter is an elongation factor la short
(EFS)
promoter or a hybrid CMV enhancer/chicken 13-actin (CBA) promoter. The EF-la
promoter
is known to be one of the strongest promoters for driving expression in
various mammalian
cell lines. The CBA promoter is commonly used for gene transfer because it
provides robust,
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
long-term expression in all cell types. Those of skill in the art will
understand how to select
an appropriate promoter to drive expression of the fusion proteins disclosed
herein for a
particular application.
In some embodiments, the nucleic acid construct is SEQ ID NO:13, i.e., a
lentiviral
vector encoding the PIDO fusion protein comprising mouse PD-Li (SEQ ID NO: 1).
In some
embodiments, the nucleic acid construct is SEQ ID NO: i6, i.e., a lentiviral
vector encoding
the PIDO fusion protein comprising human PD-Li (SEQ ID NO: i4). In some
embodiments,
the nucleic acid construct is SEQ ID NO: i9, i.e., a lentiviral vector
encoding the PIDO fusion
protein comprising canine PD-Li (SEQ ID NO: i7). In some embodiments, the
nucleic acid
construct is SEQ ID NO:22, i.e., a lentiviral vector encoding the PIDO fusion
protein
comprising feline PD-Li (SEQ ID NO:20).
Cells:
The present invention provides cells comprising the nucleic acid construct
described
herein. Under suitable conditions, the cells express the fusion proteins
described herein.
A "cell" is the basic unit from which all living things are composed. Every
cell
consists of cytoplasm (i.e., gelatinous liquid that fills the inside of the
cell) enclosed within a
membrane. The space outside of the cell membrane is referred to as the
"extracellular space".
Any cell type may be used with the present invention. In some embodiments, the
cell
is useful for transplantation. For example, in some embodiments, the cell is
an induced
pluripotent stem cell, embryonic stem cell, retinal pigment epithelial cell,
dopaminergic
neuron, stromal cell, or cardiomyocyte. In certain embodiments, the cell is a
hematopoietic
stem cell or mesenchymal stem cell. In the Examples, the inventors generated
islets that
express the PIDO fusion protein. Thus, in preferred embodiments, the cells are
islets i.e.,
pancreatic cells that produces hormones (e.g., insulin and glucagon) that are
secreted into the
bloodstream.
In some embodiments, the nucleic acid construct is a viral vector, and the
nucleic acid
construct is introduced to the cell by viral infection. In other embodiments,
the nucleic acid
construct is introduced to the cell using plasmid DNA, transposons, CRISPR-
based gene
editing, or chromosome transfer.
The inventors designed the PIDO fusion protein such that (1) the PD-Li
extracellular
domain would localize to the extracellular space where it can interact with PD-
1 receptors on
the surface of activated T cells, and (2) the DO protein would localize to the
cytoplasm
where it can function in the kynurenine pathway. Thus, in some embodiments, at
least a
portion of the fusion protein is expressed on the surface of the cell. In
preferred
11
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
embodiments, the PD-Li peptide is localized in the extracellular space and the
DO peptide is
localized in the cytoplasm of the cell.
Any method of protein detection may be used to test whether a cell expresses a
fusion
protein disclosed herein. Suitable methods for detecting proteins include,
without limitation,
enzyme-linked immunoassay (ELISA), dot blotting, western blotting, flow
cytometry, mass
spectrometry, and chromatographic methods. In the Examples, PD-Li was detected
at the cell
surface via flow cytometry using an anti-CD274 antibody, whereas DO was
detected
intracellularly via western blot (Figure 1). Thus, in certain embodiments, the
fusion protein is
detected using flow cytometry or western blot.
Methods:
The present invention provides methods of transplanting a cell described
herein into a
subject. As used herein, the term "transplanting" refers to a procedure in
which cells from a
donor are placed in the body of a recipient. The transplant may be allogeneic,
i.e., from a
different individual of the same species, or xenogeneic, i.e., from an
individual of a different
species. The methods may involve any transplant techniques known in the art.
The
transplanted cells may be individual cells. Alternatively, the transplanted
cells may be part of
an organ, tissue, organoid, or cellular aggregate. Importantly, these methods
will allow
treatments that rely upon cells that are in limited supply (e.g., islets from
human cadavers) to
be replaced with treatments that utilize cells from a renewable source (e.g.,
embryonic stem
cells).
The transplanted cells may be from any suitable donor. Suitable donor animals
include, but are not limited to, humans, non-human primates, cows, cats, dogs,
pigs, and
rodents. The donor cells may be from an allogenic or xenogeneic source. For
example, for a
human recipient, the donor cells may from another human (i.e., an allogenic
source) or a pig
(i.e., a xenogeneic source). Suitable xenogeneic sources for transplant into
humans include
mammalian sources such as pigs, sheep, cows, horses, and non-human primates.
Because
humans are known to respond to pig insulin, pigs are a promising source of
pancreatic islets
for transplantation into type I diabetics. Thus, in some embodiments, the
transplanted cells
are from a pig.
The "subject" (i.e., recipient) may be any animal that could reasonably
receive
transplant cells from the donor. Suitable subjects include, but are not
limited to, humans, non-
human primates, cows, cats, dogs, pigs, and rodents. In some embodiments, the
subject is a
human. In some embodiments, the subject is in need of a functional cell or
tissue. For
example, in some embodiments, the subject has diabetes and is in need of
functional islets.
12
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
Advantageously, the fusion protein, particularly the extracellular PD-Li
peptide
portion, is matched to the species of the subject for greater compatibility
and reduced risk of
antigenicity. However, those of skill in the art will understand that matching
the species is
less critical for proteins that are highly conserved (e.g., DO) as compared to
those that are
less conserved (e.g., PD-L1).
In the absence of immunosuppression, allogenic and xenogeneic transplants are
destroyed by the recipient's immune system, which attacks the transplants as a
foreign
substance. However, in the Examples, the inventors demonstrate that expression
of the PIDO
fusion protein by transplanted cells locally suppresses the immune system.
Specifically, they
demonstrate that PIDO-expressing murine islets transplanted into mice (i.e.,
an allogenic
graft; see Figure 2) and PIDO-expressing porcine islets transplanted into mice
and dogs (i.e.,
a xenogeneic graft; see Figure 6) survive and are functional in the recipient
animal. Thus, in
some embodiments, the transplanted cell is tolerated by the immune system in
the absence of
immunosuppression. A transplanted cell is "tolerated" when the immune system
of the
recipient is unresponsive or minimally responsive to it. Immune tolerance can
be assessed by
monitoring the survival or function of the transplanted cells. For example,
the inventors
showed that the transplanted PIDO-expressing porcine islets survived longer
than naive
porcine islets (i.e., islets that were not engineered to express PIDO) and
remained functional
(i.e., produced insulin) in recipients for more than 20 weeks. Thus, in some
embodiments, the
transplanted cells may exhibit prolonged survival relative to a transplanted
control cell
lacking the nucleic acid construct encoding the fusion protein. Alternatively,
immune
tolerance may be inferred by a lack of immune rejection (i.e., by quantifying
the number of
reactive immune cells that co-localize with PIDO-expressing grafts) or by the
presence of
regulatory T cells, which mediate immune tolerance.
As used herein, the term "immunosuppression" refers to the partial or complete
suppression of the immune response of a subject. Immunosuppression may be
deliberately
induced in a subject using drugs to help transplanted donor cells survive.
Examples of
immunosuppressive drugs that are used to reduce the risk of transplant
rejection include,
without limitation, tacrolimus, cyclosporine, mycophenolate mofetil,
azathioprine,
everolimus, sirolimus, and glucocorticoids (steroids).
The cells that are transplanted in the methods of the present invention may be
of any
cell type that is amenable to ex vivo transplantation. In some embodiments,
the transplanted
cell performs its native function (e.g., an islet produces insulin).
13
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
In the Examples, the inventors engineered allogeneic islets to express the
PIDO fusion
protein and transplanted them into immune competent diabetic mice. Thus, in
some
embodiments, the subject is diabetic, and the cell is an islet. Diabetes
mellitus, commonly
known as diabetes, is a group of metabolic disorders that is characterized by
a high blood
sugar level (hyperglycemia) over a prolonged period. There are three main
types of diabetes:
type 1 diabetes, type 2 diabetes, and gestational diabetes. Type 1 diabetes
results from the
failure of the pancreas to produce enough insulin due to the destruction of
insulin-producing
pancreatic beta cells by a beta cell-specific autoimmune process. Type 2
diabetes is caused by
insulin resistance, a condition in which cells fail to respond to insulin
properly. Type 2
-- diabetes primarily occurs as a result of obesity and lack of exercise.
Gestational diabetes
occurs when pregnant women without a previous history of diabetes develop high
blood
sugar levels.
Ideally, the diabetic subject treated by the present methods will produce
insulin post-
transplantation with PIDO-expressing islets. Insulin secretion can be
measured, for example,
-- using the glucose-stimulated insulin secretion (GSIS) test. In the GSIS
test, blood is sampled
at specific time points to measure plasma insulin levels in the basal (fasted)
state and after
induction of hyperglycemia via administration of a glucose bolus.
Alternatively, insulin
secretion can be measured indirectly via detection of C-peptide, a protein
that is produced
and secreted along with insulin. C-peptide tests are commonly used by doctors
to diagnose
-- type I diabetes.
Additionally, diabetic subjects treated by the present methods may demonstrate
improved glucose tolerance post-transplantation as compared to pre-
transplantation. Glucose
tolerance can be measured using any glucose tolerance test known in the art.
Alternatively,
glycosylated hemoglobin (HbAlc) may be measured as an indicator of long-term
glycemic
control.
In some embodiments, the subject becomes normoglycemic post-transplantation.
As
used herein the term "normoglycemic" refers to the presence a normal
concentration of
glucose in the blood. The concentration of glucose in the blood can be
measured using any
blood glucose test. A blood glucose level of less than 140 mg/dL is considered
normal in
humans, whereas, in mice, a blood glucose level of less than 100 mg/dL is
considered
normal. However, fed mice with less than 200 mg/dL blood glucose are also
considered non-
diabetic or normoglycemic. In some embodiments, the subject remains
normoglycemic for at
least 50 weeks post-transplantation.
14
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
In other embodiments, the cells used in the methods of the present invention
are
derived from stem cells. Suitable stem cells for use with the present
invention include,
without limitation, embryonic stem cells (ESC), induced pluripotent stem cells
(iPSC),
hematopoietic stem cells (HSC), and mesenchymal stem cells (MSC). In certain
embodiments, the cell is the differentiated progeny of a hematopoietic stem
cell, which give
rise to myeloid, lymphoid, and monocytic cell types. The stem cells may be
transplanted into
the animal in an undifferentiated state or may be differentiated in vitro
prior to
transplantation. Stem cells may be obtained from established stem cell lines
or may be
obtained directly from primary tissue.
The inventors also envision that the fusion proteins of the present invention
could be
used to generate genetically modified transplant donor animals. For example,
pigs could be
genetically engineered to express the PIDO fusion protein throughout their
bodies, such that
they produce whole organs and tissues that could be used as xenogeneic
transplants for
humans. Suitable organs for transplantation include, without limitation,
kidney, heart, liver,
lungs, pancreas, intestine, thymus, and uterus. Suitable tissues for
transplantation include, for
example, bones, tendons, corneae, skin, heart valves, nerves, and veins.
The present disclosure is not limited to the specific details of construction,
arrangement of components, or method steps set forth herein. The compositions
and methods
disclosed herein are capable of being made, practiced, used, carried out
and/or formed in
various ways that will be apparent to one of skill in the art in light of the
disclosure that
follows. The phraseology and terminology used herein is for the purpose of
description only
and should not be regarded as limiting to the scope of the claims. Ordinal
indicators, such as
first, second, and third, as used in the description and the claims to refer
to various structures
or method steps, are not meant to be construed to indicate any specific
structures or steps, or
any particular order or configuration to such structures or steps. All methods
described herein
can be performed in any suitable order unless otherwise indicated herein or
otherwise clearly
contradicted by context. The use of any and all examples, or exemplary
language (e.g., "such
as") provided herein, is intended merely to facilitate the disclosure and does
not imply any
limitation on the scope of the disclosure unless otherwise claimed. No
language in the
specification, and no structures shown in the drawings, should be construed as
indicating that
any non-claimed element is essential to the practice of the disclosed subject
matter. The use
herein of the terms "including," "comprising," or "having," and variations
thereof, is meant
to encompass the elements listed thereafter and equivalents thereof, as well
as additional
elements. Embodiments recited as "including," "comprising," or "having"
certain elements
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
are also contemplated as "consisting essentially of' and "consisting of' those
certain
elements.
Recitation of ranges of values herein are merely intended to serve as a
shorthand
method of referring individually to each separate value falling within the
range, unless
otherwise indicated herein, and each separate value is incorporated into the
specification as if
it were individually recited herein. For example, if a concentration range is
stated as 1% to
50%, it is intended that values such as 2% to 40%, 10% to 30%, or 1% to 3%,
etc., are
expressly enumerated in this specification. These are only examples of what is
specifically
intended, and all possible combinations of numerical values between and
including the lowest
value and the highest value enumerated are to be considered to be expressly
stated in this
disclosure. Use of the word "about" to describe a particular recited amount or
range of
amounts is meant to indicate that values very near to the recited amount are
included in that
amount, such as values that could or naturally would be accounted for due to
manufacturing
tolerances, instrument and human error in forming measurements, and the like.
All
percentages referring to amounts are by weight unless indicated otherwise.
Percent identity (% sequence identity or % identity). Refers to the percentage
of
residue matches between at least two amino acid sequences aligned using a
standardized
algorithm. Methods of amino acid sequence alignment are well known in the art.
Some
alignment methods take into account conservative amino acid substitutions.
Such
conservative substitutions, explained in more detail below, generally preserve
the charge and
hydrophobicity at the site of substitution, thus preserving the structure (and
therefore
function) of the polypeptide. Percent identity for amino acid sequences may be
determined as
understood in the art. (See, e.g., U.S. Patent No. 7,396,664, which is
incorporated herein by
reference in its entirety). A suite of commonly used and freely available
sequence comparison
algorithms is provided by the National Center for Biotechnology Information
(NCBI) Basic
Local Alignment Search Tool (BLAST), which is available from several sources,
including
the NCBI, Bethesda, Md., at its website. The BLAST software suite includes
various
sequence analysis programs including "blastp," that is used to align a known
amino acid
sequence with other amino acids sequences from a variety of databases.
Polypeptide
sequence identity may be measured over the length of an entire defined
polypeptide
sequence, for example, as defined by a particular SEQ ID number, or may be
measured over
a shorter length, for example, over the length of a fragment taken from a
larger, defined
polypeptide sequence, for instance, a fragment of at least 10, at least 15, at
least 20, or more
contiguous residues. Such lengths are exemplary only, and it is understood
that any fragment
16
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
length supported by the sequences shown herein, in the tables, figures or
Sequence Listing,
may be used to describe a length over which percentage identity may be
measured.
No admission is made that any reference, including any non-patent or patent
document cited in this specification, constitutes prior art. In particular, it
will be understood
that, unless otherwise stated, reference to any document herein does not
constitute an
admission that any of these documents forms part of the common general
knowledge in the
art in the United States or in any other country. Any discussion of the
references states what
their authors assert, and the applicant reserves the right to challenge the
accuracy and
pertinence of any of the documents cited herein. All references cited herein
are fully
incorporated by reference, unless explicitly indicated otherwise. The present
disclosure shall
control in the event there are any disparities between any definitions and/or
description found
in the cited references.
The following examples are meant only to be illustrative and are not meant as
limitations on the scope of the invention or of the appended claims.
EXAMPLES
Allogeneic islet transplantation is a promising experimental therapy for
poorly
controlled diabetes but is limited by the adverse effects of chronic
immunosuppression.
Induction of immune tolerance against allogeneic antigens is necessary to
prevent allograft
rejection and to obviate the need for immunosuppressant drugs. However, the
need for an
effective means to induce immune tolerance remains unmet.
In the following example, the inventors describe a novel fusion protein that
was
created by combining two biochemically distinct proteins: programmed death
ligand-1 (PD-
L1) and indoleamine 2,3-dioxygenase (IDO). PD-Li is a transmembrane protein
that is
known to play a major role in suppressing the adaptive immune system. IDO is
an
intracellular, monomeric, heme-containing enzyme that regulates the breakdown
of
tryptophan in the kynurenine pathway. IDO affects immune tolerance by
regulating the
function of natural killers (NK), T cells, T regulatory cells (Tregs) and
myeloid-derived
suppressor cells (MDSC) via tryptophan depletion. Thus, the inventors' fusion
portion, which
is referred to herein as PIDO (PD-Li + IDO), offers two distinct tolerogenic
mechanisms for
the prevention of transplant rejection.
The inventors have demonstrated that PIDO is robustly expressed in and
displayed on
the surface of mammalian cells, including mouse and pig islets. When
allogeneic PIDO-
expressing islets are transplanted into hyperglycemic mice, the islet grafts
survive and reverse
both streptozotocin-induced and autoimmune diabetes for more than 50 weeks and
10 weeks,
17
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
respectively. Further, PIDO-expressing porcine islet xenografts exhibit
glucose-responsive
insulin secretion for up to 30 weeks in euglycemic dogs. The survival of these
PIDO-
expressing allografts and xenografts suggests that this fusion protein may be
a means to
achieve local immunomodulation and allow for improved transplant outcomes in
the absence
of chronic immunosuppression.
Materials and Methods:
Study design. The objective of this study was to generate allogenic PIDO-
expressing
islets and transplant them into mice with preexisting diabetes to test the
ability of the PIDO
fusion protein to induce immune tolerance to the allogeneic islets. We used
lentiviral delivery
to genetically engineer islets derived from allogeneic or xenogeneic donors.
We transplanted
PIDO-expressing islets and naïve islets (i.e., islets that were not engineered
to express PIDO)
into 15 and 9 streptozotocin (STZ)-treated diabetic mice, respectively. STZ-
treated diabetic
and nondiabetic mice without transplants served as transplantation controls.
Mouse groups
were assigned randomly, and the study was not blinded. Transplanted mice were
monitored
through blood glucose measurements and blood plasma collection and were then
euthanized
for ex vivo analysis. Nephrectomy surgery was performed on PIDO + islet
transplanted mice to
confirm that the transplanted islets were the source of the glucose tolerance
and nondiabetic
blood glucose concentrations observed in these mice. Data collection was
stopped at
predetermined, arbitrary times. Mice that did not develop diabetes post-STZ
administration
and mice that died pre- or pen-transplant surgery were excluded from the
study.
Enzymatic activity of IDOL Kynurenine levels were analyzed in conditioned
media
collected from mesenchymal stromal cells (positive control) or islets via
enzyme-linked
immunosorbent assay (ELISA) using the Kynurenine ELISA kit (#F56401, LSBio,
USA) 48
hours after the cells were transduced with a PIDO-encoding lentiviral vector.
Glucose-stimulated insulin secretion (GSIS). To assess static GSIS,
approximately 50
size-matched islets were transduced with lentiviral vectors encoding either
PIDO or EGFP
(control) in 48-well plates. Islets were washed with KRB buffer and were then
pre-incubated
in glucose-free KRB buffer for 30 minutes. Static insulin secretion was
measured by
incubating islets in media with basal (2.8 mM or 2.8 G) or stimulatory (16.7
mM or 16.7 G)
glucose for 2 hours each. The supernatant was collected for use in an insulin
assay. To
perform intracellular insulin detection, the islets were harvested, rinsed
with PBS,
resuspended in 300pL acid ethanol, and homogenized by ultrasonic disruption of
the cell
membrane. Insulin was measured using a mouse insulin ELISA kit (#10-1247-01,
Mercodia,
Uppsala, Sweden) according to the manufacturer's protocol.
18
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
Immunocytochemical staining and imaging. Intact mouse islets were transduced
with
lentivirus vectors that delivered various transgenes (EGFP, PD-Li :EGFP,
IDO:mCherry, and
PIDO:EGFP) and were stained with nuclear counterstain Hoechst 33342 (Cat#
H1399,
ThermoFisher, USA). Formalin-fixed paraffin embedded kidney sections from
recipient mice
were either stained with hematoxylin-eosin (H&E) for visualization of islet
microscopic
anatomy or with anti-insulin antibody (1:1000; Immunostar, USA) and actin
(Acti-Stain 555
Phalloidin, Cat # PHDH1) for detection of transplanted, insulin-positive
islets by
immunofluorescence (IF) microscopy and imaging. Nuclei were counterstained
with
ProLongTM Diamond Antifade Mountant (#P36970, ThermoFisher, USA). H&E images
were
acquired using a Zeiss AX10 inverted microscope equipped with a Zeiss Axiocam
305 color
camera. IF images were acquired using a laser-scanning microscope (MR; Nikon,
USA).
Islet cell flow cytometry. Islets expressing the PIDO fusion protein, PD-L1
only, or
EGFP (control) were washed in 2 mmo1/1 EDTA/PBS, incubated for 5 minutes at
ambient
temperature in Ca2+-free PBS supplemented with 0.025% trypsin, and dissociated
into a
.. single-cell suspension by gentle pipetting. Dissociated islets were stained
with viability dye
(Ghost Dye Red 780, Cat# 13-0865, Tonbo Biosciences, USA) for 30 minutes, and
were then
stained for CD274 (PD-L1) to detect PD-L1 expression on the cell membrane. PD-
L1 and
EGFP stained cells were used to set the gates. All samples were FSC-H and SSC-
H gated and
then FSC-A/FSC-H gated to select single cells. Live cells were gated based on
Ghost Red
780. Flow cytometry plots for PD-L1 expression are shown as histograms.
Islet isolation and culture. Juvenile porcine islets were isolated from the
pancreata of
8- to 15-day-old, pre-weaned Yorkshire piglets and were cultured as described
previously
(52). Mouse islets were isolated from male 12- to 16-week-old C57BL/6J mice
(Jackson
Laboratory, USA) as described previously (53). Islets were cultured (37 C, 5%
CO2) in
RPMI-1640 medium (Corning, USA) with 10% FBS (Gibco, USA) and 1% antibiotic-
antimycotic (ThermoFisher, #15240096) for the indicated duration or overnight
before they
were co-cultured with pluripotent stem cells (PSCs) in a 1:1 mix of complete
RPMI and
DMEM F-12 (RDmix) media.
Lentiviral transduction of mouse and pig pancreatic islets. After islet
viability was
assessed using dithiazone, islets were cultured in RPMI medium overnight. The
next day,
islets were partially disrupted by mild enzymatic dissociation. Briefly,
islets were incubated
for two minutes in pre-warmed Accutase (2.5 ul/islet, StemCell technologies)
and washed
with Ca/Mg-free HBSS. Purified viruses were added to the islets in an ultra-
low attachment
plate or dish (Costar, Corning) and were incubated with viral supernatant for
6 hours or
19
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
overnight. For the default transduction condition, a vesicular stomatitis
virus glycoprotein
(VSV-G)-pseudotyped cytomegalovirus-green fluorescent protein (CMV-GFP) vector
was
used at a multiplicity of infection (MOI) of 10, and transduction was
performed in serum-free
medium supplemented with 0.1% bovine albumin, lx Insulin-Transferrin-Selenium
(ITS)
-- (Sigma Aldrich), and 8 ug/ml polybrene. The transduction volume was kept
uniform
throughout all experiments. The volume of the growth area of the well/dish was
135.5 .1/cm2
and a minimum of 50% of the transduction volume consisted of fresh medium.
Islets were
cultured in RPMI medium supplemented with 10% FBS for 48 hours, and the
transduction
efficiency was evaluated prior to transplant.
Mouse transplants. Mice were randomly designated for the STZ treatment and
transplantation groups. The number of mice per group (i.e., 9 and 15) was
selected to allow
for statistical significance. Surgical procedures and follow-up studies were
performed by
unblinded individuals. Male ¨8-week-old BALB/c, C57BL6/j, and CD44- (B6.12952-
Cd4'IJ, Strain #002663) mice were purchased from the Jackson Laboratory and
were
rendered diabetic via injection of STZ (45 mg/kg; R&D systems) for 5 days.
Diabetes was
confirmed after 7 days. Spontaneously diabetic female NOD mice (-12-16 weeks
old) with
blood glucose levels higher than 350 mg/di were transplanted with islets
harvested from
euglycemic 8-week-old C57BL/6J donor mice. Anaesthetized mice were
transplanted with
¨400 handpicked, mixed size islets (PIDO-expressing or control transduced), or
saline under
the kidney capsule. Animals were monitored for up to 50 weeks. Blood glucose
was
measured with a Contour Blood Glucose Monitoring System (Bayer). Glucose
tolerance and
in vivo GSIS assays were performed by fasting mice for 4 hours and then
injecting them with
glucose (2 g/kg). Serum hormones were quantified using ELISA kits for insulin
(mouse #10-
1247-01, porcine # 10-1200-01) and porcine C-peptide (#10-1256-01) following
the
-- manufacturer's instructions (Mercodia, Uppsala, Sweden). Twenty weeks after
transplantation, transplant recipient mice were rechallenged, either by a
second STZ injection
or by live nephrectomy, which was performed on five anaesthetized mice from
each group.
Dog transplants. An intact male beagle (10 kg) was used in this study. The dog
was
sedated and anesthetized using approved agents. Anesthesia was maintained by
inhalation of
isoflurane (0.75-1.75%) in oxygen. Carprofen (4.4 mg/kg; Rimadylg, Zoetis,
Parsippany,
NJ) was given subcutaneously at the time of anesthesia and on the day after
implantation of
cells to provide analgesia. The skin overlying the epaxial musculature of the
back was
prepared for aseptic surgery removing the hair and scrubbing with
chlorhexidine from the
thirteenth rib to the cranial limit of the ileal crest. A small (5 mm) stab
incision was made in
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
the skin 2 cm caudal to the thirteenth rib. An 18 ga 6-inch spinal needle
(Becton Dickinson,
Franklin Lakes, NJ) that was preloaded with porcine pancreatic islets (30,000
IEQ/kg; total
volume of 2.0 ml) was inserted through the skin incision into the epaxial
musculature to a
distance of 10 cm deep. 0.5 ml of the islet suspension was instilled, and the
needle was
withdrawn in 1.5 cm increments such that four total injections were made, each
2.5 cm from
the previous injection site. The needle was withdrawn from the site of
insertion, and the skin
was sealed with tissue glue (Vetbond Tissue AdhesiveTM, 3M, Minneapolis, MN).
Glucose
tolerance tests were performed starting 3 weeks after cell implantation and
were repeated at
3-5 week intervals for 28 weeks post-transplantation. An 18 ga intravenous
catheter was
placed in a cephalic vein. At time 0, sterile 50% glucose in water (500 mg/ml;
total dose of
500 mg/kg) was given intravenously over 1-2 minutes. A 1 ml blood sample was
collected
prior to intravenous administration of glucose and 5, 10, 20, 60, 90, and 120
minutes after the
instillation of glucose. A drop of blood was tested for glucose concentration
using a
glucometer (AlphaTrak, Abbott, Chicago, IL), and the remainder of the blood
was placed in a
tube containing EDTA. Tubes were placed on ice, and the plasma was separated
by cold
centrifugation at 1100xg for 10 minutes (Sorvall, ThermoScientific, Waltham,
MA). Plasma
was stored at -80 C until it was tested for concentrations of C-peptide.
Western blot. Protein samples for western blotting were isolated from murine
or
porcine islets via homogenization with lysis buffer (#9803, CST, USA). The
samples were
boiled in laemmli buffer (#161-0737, BioRad, USA) for 5 minutes and were
resolved on a 4-
12% gradient SDS-PAGE gel and blotted to PVDF membrane. Following an overnight
incubation with primary antibodies against IDO (1:1000; #86630, CST, USA) and
beta-actin
(1:1000, # NB600-503, Novus Biologicals, USA), detection was performed using
HRP-
conjugated IgG. Bands were visualized using an Azure 300 chemiluminescent
imaging
.. system (Azure Biosystems, USA).
Statistical analysis. Statistical analysis was performed using GraphPad Prism.
One-
and two-sided unpaired and paired t tests and one- and two-way ANOVA with
Tukey's or
Dunnett's tests were used for datasets with a normal distribution. P < 0.05
was considered
statistically significant. Data are shown as means SEM unless otherwise
noted. The sample
size, n, indicates the total number of biological replicates.
Results:
PIDO retains structural and functional characteristics of its constituent
domains and does
not alter islet function
21
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
We created a synthetic gene containing sequences encoding the full-length
mouse PD-
Li protein and full-length human IDO1 protein separated by a 3X GGGS linker.
This
synthetic gene was subcloned in-frame with the PD-Li membrane localization
signal in the
pLV-EXP/CMV-EGFP lentiviral vector (Figure 5). The resulting PIDO cDNA encodes
a
single polypeptide chain of 708 amino acids with a predicted non-glycosylated
molecular
weight of about 80 kDa (Figure 1B). The in-silico 3D structure of PIDO was
predicted and
constructed using I-TASSER and webserver Phyre2 (29, 3 0)(Figure 1C). The
expression
vector was packaged in lentiviral particles.
Next, we engineered A375 human melanoma cells and C57BL6/J mouse islets to
express PIDO via transduction with the lentiviral particles. The expression,
sub-cellular
localization, and biological activity of PIDO fusion protein was verified by
immunofluorescence staining, flow cytometry, western blot, and ELISA. We
detected robust
expression of PD-L1, IDO, and the PIDO fusion protein in mouse islets via
fluorescent
protein tags (Figure 1D). To investigate the sub-cellular localization of the
chimeric PIDO
protein, we assessed surface expression of the PD-Li component in dispersed
islet cells by
flow cytometry. Our data show that almost twice as many PIDO-expressing mouse
islet cells
displayed surface PD-Li expression compared to islet cells that express PD-Li
alone (65%
vs 24%), suggesting that the PIDO fusion protein allows for a higher cell
surface density of
PD-Li than that afforded by ectopic expression of PD-Li on its own (Figure
1E).
Denaturing immunoblotting performed on 'DO- or PIDO-expressing mouse or pig
islets
showed that the fusion protein was highly expressed and migrated at a
molecular weight of
about 90 kDa (Figure 1F). Our data also show that, when normalized to input
protein, the
abundance of the PIDO fusion protein was significantly higher than the
abundance of IDO
expressed alone or co-expressed with PD-Li (Figure 7). Together, these data
suggest that
PIDO-expressing islets display PD-Li on the membrane and express IDO in the
cytoplasm
tethered to the C-terminus of cytoplasmic tail of PD-L1, as depicted
schematically in Figure
1G.
The activity of IDO was assessed via detection of extracellular kynurenine
produced
from its catalysis of tryptophan present in the culture media. As shown in
Figure 111,
kynurenine levels increased significantly in the conditioned media of both DO-
and PIDO-
expressing islets, comparable the levels in the media of IFNy-treated
mesenchymal stromal
cells (positive control). Interestingly, mouse islets that were dual
transduced to co-express
PD-Li and IDO as separate proteins displayed lower IDO activity, as
demonstrated by lower
22
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
kynurenine levels in the conditioned media of these islets. This suggests that
the effect of co-
expression of PD-Li and IDO is not equivalent to that of the PIDO fusion
protein.
Islet 13-cells are known to augment their surface expression of PD-Li during
the
development of insulitis (31), potentially as a defense mechanism against
autoreactive T
cells. This increase in PD-Li expression may initiate stress pathways in 13-
cells. Further, IDO
is not naturally expressed in islets and the effects of IDO-driven tryptophan
depletion and
kynurenine production on 13-cell function are undefined. Thus, to understand
the effect of
increased PD-Li surface expression and ectopic IDO catabolic activity on these
cells, we
cultured islets expressing PD-L1, IDO, or PIDO for 48 hours and then subjected
them to
glucose-stimulated insulin secretion (GSIS) assays. The GSIS data showed no
difference in
insulin secretion as a function of transgene expression (Figure 1I).
Together, these data show that the PIDO fusion protein is more stable than its
protein
constituents, that it is expressed robustly on the cell surface, that its IDO
component retains
its catalytic activity in the context of the fusion protein, and that
constitutive expression of
PIDO does not interfere with islet GSIS.
PIDO-expressing islet allografts reverse hyperglycemia in diabetic mice
To assess the potential of PIDO-expressing allogeneic islets for use in
transplantation
therapies, we transplanted ¨450 handpicked and size-matched lentivirus-
transduced C57BL/6
mouse islets under the left kidney capsule of BALB/c mice that were previously
rendered
diabetic by streptozotocin (STZ) injection, which depletes endogenous islets
(Figure 2A).
Three mice that were transplanted with islets that had been transduced with
control lentivirus
died spontaneously, one at 12 weeks and two others at 24 weeks, likely due to
their diabetes.
At 20 weeks post-transplantation, the PIDO + islet allografts were detected
under the kidney
capsule and stained positive for insulin (Figure 2B). To understand whether
expression of
PD-L1, IDO, or both of these proteins is sufficient to reverse diabetes in
mice, we also
transduced C57BL/6 mouse islets with PD-Li alone, IDO alone, or both PD-Li and
IDO as
individual proteins. As is shown in Figure 2C, islets expressing PD-Li and/or
IDO failed to
reverse the preexisting hyperglycemic diabetes in mice. While the allograft
recipients
transplanted with islets that co-express PD-Li and IDO showed some initial
recovery (-3
weeks post-transplantation), they never achieved normoglycemia, and by about 5
weeks post-
transplantation, their initial glycemic improvement was lost. This observation
further
strengthens the notion that the activity of the PIDO fusion protein is
superior to the combined
activities of PD-Li and IDO. Next, we tracked the blood glucose of mice with
preexisting
STZ-induced diabetes that were transplanted with control or PIDO-expressing
islets. In
23
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
PIDO + islet transplanted mice, the blood glucose dropped to less than 200
mg/di within three
weeks (Figure 2D) and became completely normoglycemic by 10 weeks (no
difference from
healthy, non-transplanted mice). These PIDO + allograft recipients remained
normoglycemic
for the entire duration of study with an average blood glucose concentration
of 87 7 mg/di
(fasting, Figure 2D, right) or 109 12 mg/di (random-fed, Figure 2D, left).
We performed
glucose tolerance tests 2 weeks and 10 weeks post-transplantation. Mice
transplanted with
PIDO + islets demonstrated improved glucose tolerance as compared to control
islet
transplanted mice as early as 2 weeks post-transplantation (Figure 2E). For
the 50-week
observation period, only PIDO + islet transplanted mice achieved and
maintained
normoglycemic blood glucose levels, as the mice transplanted with control
islet allografts did
not show any glycemic recovery. Serum was collected from all groups of mice at
2- and 10-
weeks post-transplantation and was assayed for insulin. Figure 2F shows that
the PIDO + islet
transplantation groups had detectable insulin at 2 weeks (0.64 0.38 ng/ml)
and by 10 weeks,
their insulin levels were comparable to normoglycemic, non-transplanted mice
(0.9 0.17
.. ng/ml).
Finally, we sought to test the effect of PIDO expression on the survival of
allogeneic
islets in NOD mice. Control or PIDO + allogeneic (C57BL/6J) islets were
transplanted into
diabetic female NOD mice, and the mice were monitored for eight weeks. As is
shown in
Figure 3, control islet recipients showed variable and transient improvements
in blood
.. glucose, but eventually rejected their grafts. The mean survival time of
these grafts was 8
days (n = 4). In contrast, PIDO + islet-recipients (n= 5) showed glycemic
improvement within
a week, remained normoglycemic for the duration of the study (8 weeks), and
showed a
reversal of preexisting autoimmune diabetes (Figure 3B, C). The relapse (blood
glucose >
250mg/dL) incidence rate was 100% in the control islet recipient group and was
20% in
PIDO + islet recipient group. All recipients were presumed to be non-diabetic
for the ease of
data visualization (Figure 3C).
Cumulatively, these data demonstrate that constitutive PIDO expression allows
islet
allografts to evade immune rejection and to reverse preexisting diabetes
(i.e., both chemically
induced and autoimmune diabetes) in immunocompetent mice. In addition, these
data also
support the hypothesis that the PIDO fusion protein possesses biochemical and
functional
characteristics that are distinct from those of its constituent proteins.
PIDO-induced graft immune evasion does not lead to acquired immunologic
tolerance to
allogeneic islets
24
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
Reversal of preexisting diabetes in PIDO+ islet allograft transplanted BALB/c
or NOD
mice is consistent with immune evasion. To test whether acquired immune
tolerance of the
BALB/c recipients contributes to the sustained survival of the C57BL/6 islet
allografts, we
destroyed/removed the PIDO+ islet allografts from the BALB/c recipients via
STZ treatment
or nephrectomy. Thereafter, we retransplanted these mice, which were once
again diabetic,
with naive C57BL/6 islets (Figure 4A). Specifically, we injected first set of
BALB/c mice (n
= 5) with a second dose of STZ to destroy 13-cells (i.e., the PIDO+ C57BL/6
islet allografts)
20 weeks post-transplantation. All recipients developed hyperglycemia within
two weeks
(Figure 4B, C). Two weeks following the destruction of the primary PIDO+
C57BL/6 islet
allografts and re-induction of diabetes, these BALB/c mice were transplanted
with a second
set of naive C57BL/6 islets under the capsule of their contralateral kidney.
The naive
allografts effected only a partial and transient recovery (Figure 4B), as
these mice quickly
(i.e., within 3 weeks) developed hyperglycemia, indicating a loss of the naive
allografts.
Streptozotocin (STZ) is a toxic glucose analog (i.e., a DNA alkylating agent)
that
accumulates in islet 13-cells via selective uptake by the GLUT2 glucose
transporter, resulting
in their destruction. While STZ is broadly used to produce a mouse model of
diabetes, its
efficacy in the pancreas and kidney capsule may not be equivalent due to
inherent differences
in the vascularization of these tissues. Thus, we hypothesized that the
partial and transient
glycemic recovery produced by the naive islets could be attributed to an
incomplete effect of
STZ on islets under the kidney capsule. Therefore, we also tested for the
existence of
acquired tolerance using an independent metric. In a second set of allograft
recipients (n = 5),
we removed the host kidney containing the PIDO+ islets. These recipients
developed
hyperglycemia swiftly (within 1 week). Two weeks post-nephrectomy, we
transplanted these
BALB/c mice with naive C57BL/6 islet allografts under the contralateral kidney
capsule. All
recipients became hyperglycemic within one week (Figure 4C). Thus, the
secondary naive
C57BL/6 allografts failed to reverse diabetes. These data demonstrate that the
mice that had
initially received PIDO+ islets and were "cured" did not acquire immunologic
tolerance to the
allogeneic islets. Instead, the allograft tolerance achieved via PIDO
expression must be
mediated by immune evasion.
PIDO-mediated immune evasion requires host CD4 T-cell competence
It has been established that alloreactive tissue rejection is primarily
mediated by CD8+
T cells (32) whereas allo-tolerance is mediated by host CD4+ T cells with Treg
competency
(33). To determine if the tolerogenic host cells that mediate PIDO-induced
immune evasion
are CD4+ T cells, we tested the therapeutic efficacy of PIDO+ islet allografts
in CD4m/mak
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
(CD4-/-) recipients that had been rendered diabetic by STZ treatment (Figure
5A). The PIDO+
islet allografts were rapidly rejected in these CD4-/- mice (Figure 5B),
indicating that the
responsible tolerogenic host cells are indeed CD4+ T-cells.
PIDO-expressing porcine pancreatic islets are immune evasive in xenogeneic
murine and
canine recipients
While the use of gene editing methods has improved tolerance to porcine
xenografts
(34), immunosuppression remains necessary to prevent immune rejection of islet
xenografts
in non-human primates (35, 36). In view of the successful reversal of diabetes
by PIDO+
allografts in our murine model of allotransplantation, we next wanted to test
the ability of
PIDO to induce cross-species xenogeneic islet tolerance. We, therefore,
created two islet
xenotransplant models: a porcine-to-murine model and a porcine-to-canine model
(Figure
6A). In both models, in vitro matured juvenile porcine islets were engineered
to express
PIDO and were transplanted either under the kidney capsule (porcine-to-murine)
or into the
epaxial muscle (porcine-to-canine).
We detected porcine insulin in recipient immunocompetent hyperglycemic C57BL/6
mice up to 16 weeks post transplantation (Figure 6B). The data show that naive
pig islet
xenografts are quickly rejected and that only PIDO + pig islets survive and
remain functional
in the diabetic mice. However, the impact of these xenografts on clinical
diabetes could not
be tested using this model because porcine insulin is not compatible with the
rodent insulin
receptor and is, therefore, unable to regulate glucose homeostasis in mice and
rats (37).
However, porcine insulin is indistinguishable from canine insulin. Thus, we
also
transplanted PIDO-expressing porcine islets into a normoglycemic,
immunocompetent, non-
diabetic beagle dog. Specifically, we tested whether a muscle implant of PIDO
+ porcine islets
would preserve glucose homeostasis and the normal response to glucose
challenge. Notably,
a previous report has shown that naive pig islets lose function quickly in
diabetic canine
recipients (38). The C-peptide (connecting peptide) is a short polypeptide
that connects
insulin's A- and B-chains in the proinsulin molecule. C-peptide is a marker of
insulin
secretion, as it is cleaved during mature insulin production and secreted
along with insulin.
Thus, to determine the effect of the muscle implant on insulin secretion, we
measured the
porcine C-peptide in canine plasma. (Note: This is feasible because the
porcine C-peptide has
negligible cross-reactivity with the canine C-peptide.) We first performed an
intravenous
glucose tolerance test (ivGTT) to invoke a response in the euglycemic dog,
which would
otherwise not recruit the porcine islets due to its fully competent endogenous
islet mass. We
then detected porcine C-peptide for 20 weeks in dog plasma in response to the
glucose
26
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
stimulus (Figure 6C). These data strongly suggests that the porcine islet
xenograft survived.
Interestingly, we also observed a progressive decline in C-peptide response to
GTT over
time. This decline, however, cannot be attributed solely to a loss of
xenograft due to immune
rejection as the duration of detectable graft function extended well beyond
the known period
of immune rejection in the canine recipients (38).
Discussion:
Allogeneic pancreatic islet transplantation is a potentially life-saving
therapy for
poorly controlled diabetes mellitus. However, adverse effects of systemic
chronic
pharmacological immunosuppression significantly limit the benefits and
consequently the
adaptation of this therapy (4). Strategies for enabling pharmacopeia-free
durable allogeneic
islet immune evasion are needed (39).
Knowledge gained from the field of cancer immunotherapy, which is focused on
eliminating immune evasion, provides insights as to how to achieve allogeneic
tissue
tolerance. Malignancies often exploit several immunosuppressive pathways to
evade an
immune response. The PD-1:PD-L1 (40) and IDO (41) pathways are both implicated
in such
microenvironments and have been recognized as important immune checkpoints.
Thus,
oncology researchers view these pathways as potential therapeutic targets and
have attempted
to block them and have shown the biological potency of immunological escape
for select
malignant disorders with high mutation burden. For example, researchers
recently tested the
utility of constitutive expression of PD-Li by human islet-like organoids as a
means to evade
xenorejection in mice (27). In analogous work, PD-Li-expressing islets on a
microgel/biomaterial platform bypassed the need for genetic modification of
the graft
cell/tissue (24, 42) by transient expression of PD-Li. However, this failed to
provide
sustained protection of islets against alloreactive responses and
contemporaneous
pharmacological immunosuppression was necessary. This work demonstrated the
need for an
approach that can provide specific, localized, and durable immune evasion
while
circumventing the need for immunosuppression.
In the present study, we generated a novel chimeric fusion protein comprising
PD-Li
and IDO. By harnessing the immune evasive potential of both the PD-1 :PD-L1
and IDO
pathways, we sought to modulate the alloreactive immune response against
pancreatic islet
allografts in murine recipients. PD-Li and IDO have not been used together
before as an
immune blockade therapeutic. The observations made herein suggest that
tethering IDO to
the cytoplasmic tail of PD-Li produces beneficial gain-of-function properties
that are not
achieved via simultaneous independent expression of these proteins. We
observed that both
27
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
immortalized cell lines and primary islet cells that express PIDO displayed PD-
Li on the
surface and enzymatically active IDO in the cytoplasm. The PIDO fusion protein
not only
retained the biological functions of both constituent proteins, but it also
granted its
constituent proteins enhanced stability. We tested the efficacy of PIDO in
immunoprotection
.. of allografts by generating PIDO-expressing islets which, after
transplantation, reversed
preexisting diabetes in STZ-diabetic mice and established sustained euglycemia
for more
than 50 weeks without immunosuppression.
In agreement with some previous studies, we observed that stable expression of
PD-
Li or IDO individually did not meaningfully improve graft survival.
Interestingly, we also
observed that co-expression of PD-Li and IDO only delayed the immune rejection
of islet
allografts temporarily. These observations reveal that while PD-Li and IDO are
insufficient
individually, the PIDO fusion protein can establish and maintain an immune
evasive
microenvironment that protects allografts from rejection long term.
Non-specific, off-target effects are always a concern with ectopic expression
of
immunomodulatory proteins. However, we did not observe any effect of PIDO
expression on
features of bona fide mature islet 13-cells such as robust dynamic function or
diabetes reversal
upon transplantation. Similarly, the absence of meaningful cellular
proliferation (data not
shown) in homeostatic conditions in mature, terminally differentiated islet 13-
cells (43)
remained uninfluenced by PIDO expression.
Type I (i.e., autoimmune) diabetes will be the primary application for any
treatment
that enables immune evasion for islet allografts. This prompted us to
determine the
therapeutic effects of PIDO-expressing islets in the NOD mouse T1D model. In
this model,
we showed that blood glucose levels of PIDO + islet recipients decreased to
¨230 mg/di (as
compared to ¨450 mg/di in the control diabetic NOD mice) within three weeks
after
transplantation and continued to improve further. Death of mice in the control
islet group also
suggested that there would be significant difference in survival. These data
indicate that
PIDO-mediated immune evasion protects islet allografts from autoimmune
destruction and
consequently reverses diabetes in NOD mice. Previous studies (44, 45) have
revealed that
induced expansion or differentiation of Tregs prolongs survival in diabetic
NOD mice. Since
both the PD-Li and IDO pathways converge on Treg induction, we hypothesized
that the
impact of PIDO on allograft survival may be related to host CD4 T-cell
competency. Our
observation that PIDO expression by allogeneic islets elicits an endogenous
CD4-dependent
immune evasive response is consistent with the central role of host acquired T-
cell drivers of
tolerance. Among various traditional and novel tolerogenic approaches to
prevent graft
28
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
rejection, allo- and autoreactive T cell suppression via Treg cell therapy has
shown
feasibility, tolerability, and potential efficacy in transplantation settings
(46-49). However,
these approaches (including Treg enhancing drugs and antigen-specific Treg
cell therapies)
have demonstrated only modest and limited efficacy in T1D and transplant
rejection in
clinical settings (50, 51). Islet-restricted, constitutive PIDO expression and
its attendant host
CD4-dependent immune evasion may address the shortcomings of Treg adoptive
cell
therapies via continuous in vivo solicitation of endogenous regulatory CD4+
cells.
We observed that PIDO expression led to significantly improved,
immunosuppression-free, long-term engraftment of allogeneic islets and
consequently,
-- reversal of diabetes and maintenance of euglycemia for more than 50 weeks
in murine
recipients. This led us to hypothesize that PIDO was leading to the
development of acquired
tolerance for alloantigens in the murine hosts. However, using two different
models of
rechallenge, we determined that long-term (20-weeks) localized PIDO expression
did not
establish acquired memory tolerance, as the hosts rejected naive islet
allografts promptly (-3
weeks) after re-transplantation. These results demonstrate that PIDO must be
constitutively
expressed by islets to confer immune evasive qualities.
Success in xenogeneic tissue transplantation has remained elusive. Rare and
moderate
improvements have been reported among the few attempts that have been made
towards
achieving xenograft tolerance (35, 37, 38), and there are virtually no
published reports of
-- immunosuppressant-free survival of xenografts in immune sufficient
mammalian recipients.
To test the efficacy of PIDO in this application, we transplanted porcine
islet xenografts into
immunocompetent mice and dogs. We observed a substantial prolongation of
xenograft
survival in both the murine (-16 weeks) and canine (-20 weeks) recipients.
However, there
are several important limitations to these xenograft survival experiments.
First, our results
were obtained from normoglycemic recipients, which prevented us from testing
the ability of
the islet xenografts to reverse pathological hyperglycemia. Second, to glean
as much
information possible from as few experimental canines as possible, we studied
porcine islet
xenograft in only a singular canine recipient. While the results of these
experiments are
promising, differences in functional integration of ectopic islet grafts are
unknown, especially
under metabolic stress conditions. Thus, further in-depth studies of more
animals and
different models is warranted to better understand the PIDO-mediated
improvement in
xenograft islet survival and function. Lastly, the murine model of diabetes
that we utilized for
xenogeneic transplant is representative of drug induced (STZ) islet
insufficiency and
secondary diabetes mellitus. Though this model system mirrors clinical
diabetes caused by
29
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
non-immune pancreatic insufficiency or pancreatectomy, it does not reflect the
pathology of
autoimmune islet destruction typically seen in type I diabetes. However, our
data generated in
diabetic NOD mice suggest that PIDO enables immune evasion in the context of
autoimmune
diabetes as well.
Taken together, our data support the use of PIDO as a novel immune evasive
blockade therapeutic that effectively prevents islet allograft rejection in
immunocompetent
recipients and successfully circumvents the need for immunosuppressive
therapy. While the
mechanism by which PIDO establishes non-memory immune evasion remains to be
elucidated, we hypothesize that it involves evasion of both innate and
adaptive immune
.. responses. In conclusion, expression of the PIDO fusion protein may allow
off-the-shelf islet
transplants to be used as a standard therapy for treating poorly controlled
insulin-dependent
diabetes.
References:
1. Justiz Vaillant AA, Misra S, Fitzgerald BM. Acute Transplantation
Rejection. In.
StatPearls. Treasure Island (FL), 2021.
2. Molnar AO, Fergusson D, Tsampalieros AK, Bennett A, Fergusson N, Ramsay
T et
al. Generic immunosuppression in solid organ transplantation: systematic
review and meta-
analysis. BMJ 2015;350:h3163.
3. Kawase T, Matsuo K, Kashiwase K, Inoko H, Saji H, Ogawa S et al. HLA
mismatch
.. combinations associated with decreased risk of relapse: implications for
the molecular
mechanism. Blood 2009;113(12):2851-2858.
4. Wood KJ, Goto R. Mechanisms of rejection: current perspectives.
Transplantation
2012;93(1):1-10.
5. Calne R. Clinical transplantation: current problems, possible solutions.
Philos Trans R
Soc Lond B Biol Sci 2005;360(1461):1797-1801.
6. Barton FB, Rickels MR, Alejandro R, Hering BJ, Wease S, Naziruddin B et
al.
Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes
Care
2012;35(7):1436-1445.
7. Robertson RP. Islet transplantation as a treatment for diabetes - a work
in progress. N
Engl J Med 2004;350(7):694-705.
8. Rother KI, Harlan DM. Challenges facing islet transplantation for the
treatment of
type 1 diabetes mellitus. J Clin Invest 2004;114(7):877-883.
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
9. Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson
RP et al.
International trial of the Edmonton protocol for islet transplantation. N Engl
J Med
2006;355(13):1318-1330.
10. Bluestone JA, Anderson M. Tolerance in the Age of Immunotherapy. N Engl
J Med
2020;383(12):1156-1166.
11. Jiang Y, Chen M, Nie H, Yuan Y. PD-1 and PD-Li in cancer immunotherapy:
clinical
implications and future considerations. Hum Vaccin Immunother 2019;15(5):1111-
1122.
12. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in
tolerance and
immunity. Annu Rev Immunol 2008;26:677-704.
13. Colli ML, Hill JLE, Marroqui L, Chaffey J, Dos Santos RS, Leete P et
al. PDL1 is
expressed in the islets of people with type 1 diabetes and is up-regulated by
interferons-alpha
and-gamma via IRF1 induction. EBioMedicine 2018;36:367-375.
14. Ishida M, Iwai Y, Tanaka Y, Okazaki T, Freeman GJ, Minato N et al.
Differential
expression of PD-Li and PD-L2, ligands for an inhibitory receptor PD-1, in the
cells of
lymphohematopoietic tissues. Immunol Lett 2002;84(1):57-62.
15. de Filette JMK, Pen JJ, Decoster L, Vissers T, Bravenboer B, Van der
Auwera BJ et
al. Immune checkpoint inhibitors and type 1 diabetes mellitus: a case report
and systematic
review. Eur J Endocrinol 2019;181(3):363-374.
16. Cai J, Wang D, Zhang G, Guo X. The Role Of PD-1/PD-L1 Axis In Treg
Development And Function: Implications For Cancer Immunotherapy. Onco Targets
Ther
2019;12:8437-8445.
17. Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment
of NOD-
Pdcd1-/- mice as an efficient animal model of type I diabetes. Proc Nat! Acad
Sci U S A
2005;102(33):11823-11828.
18. Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B et al.
Prevention of allogeneic fetal rejection by tryptophan catabolism. Science
1998;281(5380):1191-1193.
19. Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D et al. GCN2
kinase
in T cells mediates proliferative arrest and anergy induction in response to
indoleamine 2,3-
dioxygenase. Immunity 2005;22(5):633-642.
20. Huang L, Baban B, Johnson BA, 3rd, Mellor AL. Dendritic cells,
indoleamine 2,3
dioxygenase and acquired immune privilege. Int Rev Immunol 2010;29(2):133-155.
21. Munn DH, Mellor AL, Rossi M, Young JW. Dendritic cells have the option
to express
IDO-mediated suppression or not. Blood 2005;105(6):2618.
31
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
22. Litzenburger UM, Opitz CA, Sahm F, Rauschenbach KJ, Trump S, Winter M
et al.
Constitutive IDO expression in human cancer is sustained by an autocrine
signaling loop
involving IL-6, STAT3 and the AHR. Oncotarget 2014;5(4):1038-1051.
23. McGrath MM, Najafian N. The role of coinhibitory signaling pathways in
transplantation and tolerance. Front Immunol 2012;3:47.
24. Batra L, Shrestha P, Zhao H, Woodward KB, Togay A, Tan M et al.
Localized
Immunomodulation with PD-Li Results in Sustained Survival and Function of
Allogeneic
Islets without Chronic Immunosuppression. J Immunol 2020.
25. Liu H, Liu L, Fletcher BS, Visner GA. Sleeping Beauty-based gene
therapy with
indoleamine 2,3-dioxygenase inhibits lung allograft fibrosis. FASEB J
2006;20(13):2384-
2386.
26. Jalili RB, Forouzandeh F, Rezakhanlou AM, Hartwell R, Medina A, Warnock
GL et
al. Local expression of indoleamine 2,3 dioxygenase in syngeneic fibroblasts
significantly
prolongs survival of an engineered three-dimensional islet allograft. Diabetes
2010;59(9):2219-2227.
27. Yoshihara E, O'Connor C, Gasser E, Wei Z, Oh TG, Tseng TW et al. Immune-
evasive
human islet-like organoids ameliorate diabetes. Nature 2020;586(7830):606-611.
28. Li R, Lee J, Kim MS, Liu V, Moulik M, Li H et al. PD-Li-driven
tolerance protects
neurogenin3-induced islet neogenesis to reverse established type 1 diabetes in
NOD mice.
Diabetes 2015;64(2):529-540.
29. Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. The Phyre2 web
portal
for protein modeling, prediction and analysis. Nat Protoc 2015;10(6):845-858.
30. Yang J, Zhang Y. Protein Structure and Function Prediction Using I-
TASSER. Curr
Protoc Bioinformatics 2015;52:5 8 1-5 8 15.
31. Osum KC, Burrack AL, Martinov T, Sahli NIL, Mitchell JS, Tucker CG et
al.
Interferon-gamma drives programmed death-ligand 1 expression on islet beta
cells to limit T
cell function during autoimmune diabetes. Sci Rep 2018;8(1):8295.
32. Marino J, Paster J, Benichou G. Allorecognition by T Lymphocytes and
Allograft
Rejection. Front Immunol 2016;7:582.
33. Walsh PT, Taylor DK, Turka LA. Tregs and transplantation tolerance. J
Clin Invest
2004;114(10):1398-1403.
34. Cowan PJ. The use of CRISPR/Cas associated technologies for cell
transplant
applications. Curr Opin Organ Transplant 2016;21(5):461-466.
32
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
35. Hering BJ, Wijkstrom M, Graham ML, Hardstedt M, Aasheim TC, Jie T et
al.
Prolonged diabetes reversal after intraportal xenotransplantation of wild-type
porcine islets in
immunosuppressed nonhuman primates. Nat Med 2006;12(3):301-303.
36. Shin JS, Kim JIM, Kim JS, Min BH, Kim YH, Kim HJ et al. Long-term
control of
diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of
adult
porcine islets. Am J Transplant 2015;15(11):2837-2850.
37. Pepper AR, Gall C, Mazzuca DM, Melling CW, White DJ. Diabetic rats and
mice are
resistant to porcine and human insulin: flawed experimental models for testing
islet
xenografts. Xenotransplantation 2009;16(6):502-510.
38. Kin T, Iwata H, Aomatsu Y, Ohyama T, Kanehiro H, Hisanaga M et al.
Xenotransplantation of pig islets in diabetic dogs with use of a microcapsule
composed of
agarose and polystyrene sulfonic acid mixed gel. Pancreas 2002;25(1):94-100.
39. Melton D. The promise of stem cell-derived islet replacement
therapy. Diabetologia
2021;64(5):1030-1036.
40. Dermani FK, Samadi P, Rahmani G, Kohlan AK, Najafi R. PD-1/PD-L1 immune
checkpoint: Potential target for cancer therapy. J Cell Physiol
2019;234(2):1313-1325.
41. Zhai L, Bell A, Ladomersky E, Lauing KL, Bollu L, Sosman JA et al.
Immunosuppressive IDO in Cancer: Mechanisms of Action, Animal Models, and
Targeting
Strategies. Front Immunol 2020;11:1185.
42. Coronel MM, Martin KE, Hunckler MD, Barber G, O'Neill EB, Medina JD et
al.
Immunotherapy via PD-Li-presenting biomaterials leads to long-term islet graft
survival. Sci
Adv 2020;6(35):eaba5573.
43. Paul PK, Rabaglia ME, Wang CY, Stapleton DS, Leng N, Kendziorski C et
al.
Histone chaperone ASF1B promotes human beta-cell proliferation via recruitment
of hi stone
H3.3. Cell Cycle 2016;15(23):3191-3202.
44. Lin JR, Huang SH, Wu CH, Chen YW, Hong ZJ, Cheng CP et al. Valproic
Acid
Suppresses Autoimmune Recurrence and Allograft Rejection in Islet
Transplantation through
Induction of the Differentiation of Regulatory T Cells and Can Be Used in Cell
Therapy for
Type 1 Diabetes. Pharmaceuticals (Basel) 2021;14(5).
45. Shi Q, Lees JR, Scott DW, Farber DL, Bartlett ST. Endogenous expansion
of
regulatory T cells leads to long-term islet graft survival in diabetic NOD
mice. Am J
Transplant 2012;12(5):1124-1132.
33
CA 03220007 2023-11-13
WO 2022/245841
PCT/US2022/029653
46. Bruder D, Westendorf AM, Hansen W, Prettin S, Gruber AD, Qian Y et al.
On the
edge of autoimmunity: T-cell stimulation by steady-state dendritic cells
prevents autoimmune
diabetes. Diabetes 2005;54(12):3395-3401.
47. Mukhopadhaya A, Hanafusa T, Jarchum I, Chen YG, Iwai Y, Serreze DV et
al.
Selective delivery of beta cell antigen to dendritic cells in vivo leads to
deletion and tolerance
of autoreactive CD8+ T cells in NOD mice. Proc Nat! Acad Sci U S A
2008;105(17):6374-
6379.
48. Ryba-Stanislawowska M, Sakowska J, Zielinski M, Lawrynowicz U,
Trzonkowski P.
Regulatory T cells: the future of autoimmune disease treatment. Expert Rev
Clin Immunol
2019;15(7):777-789.
49. Sharabi A, Tsokos MG, Ding Y, Malek TR, Klatzmann D, Tsokos GC.
Regulatory T
cells in the treatment of disease. Nat Rev Drug Discov 2018;17(11):823-844.
50. Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK
et al.
Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl
Med
2015;7(315):315ra189.
51. MacDonald KG, Hoeppli RE, Huang Q, Gillies J, Luciani DS, Orban PC et
al.
Alloantigen-specific regulatory T cells generated with a chimeric antigen
receptor. J Clin
Invest 2016;126(4):1413-1424.
52. Vanderschelden R, Sathialingam M, Alexander M, Lakey JRT. Cost and
Scalability
Analysis of Porcine Islet Isolation for Islet Transplantation: Comparison of
Juvenile,
Neonatal and Adult Pigs. Cell Transplant 2019;28(7):967-972.
53. de Souza AH, Santos LRB, Roma LP, Bensellam M, Carpinelli AR, Jonas JC.
NADPH oxidase-2 does not contribute to beta-cell glucotoxicity in cultured
pancreatic islets
from C57BL/6J mice. Mol Cell Endocrinol 2017;439:354-362.
34