Note: Descriptions are shown in the official language in which they were submitted.
WO 2023/274974
PCT/EP2022/067603
COMBINATION THERAPY USING ANTIBODY-DRUG CONJUGATES
Earlier applications
This application claims priority from United Kingdom application numbers
GB2109373.7,
filed 29 June 2021; GB2109375.2, filed 29 June 2021; and GB2109377.8, filed 29
June
2021. The priority applications are hereby incorporated by reference in their
entirety and
for any and all purposes as if fully set forth herein.
FIELD
The present disclosure relates to combination therapies for the treatment of
pathological
conditions, such as cancer. In particular, the present disclosure relates to
combination
therapies comprising treatment with an anti-CD19 Antibody Drug Conjugate (anti-
CD19
ADC) and an anti-CD79b agent.
BACKGROUND
Antibody Therapy
Antibody therapy has been established for the targeted treatment of subjects
with cancer,
immunological and angiogenic disorders. The use of antibody-drug conjugates
(ADC), i.e.
immunoconjugates, for the local delivery of cytotoxic or cytostatic agents,
i.e. drugs to kill
or inhibit tumour cells in the treatment of cancer, targets delivery of the
drug moiety to
tumours, and intracellular accumulation therein, whereas systemic
administration of these
unconjugated drug agents may result in unacceptable levels of toxicity to
normal cells.
CD19
CD19 is a 95 kDa membrane receptor that is expressed early in B cell
differentiation and
continues to be expressed until the B cells are triggered to terminally
differentiate. The
CD19 extracellular domain contains two C2-type immunoglobulin (IG)-like
domains
separated by a smaller potentially disulfide-linked domain. The CD19
cytoplasmic domain
is structurally unique, but highly conserved between human, mouse, and guinea
pig. CD19
is part of a protein complex found on the cell surface of B-lymphocytes. The
protein complex
includes CD19, CD21 (complement receptor, type 2), CD81 (TAPA-1), and CD225
(Leu-13)
CD19 is an important regulator of transmennbrane signals in B cells. An
increase or
decrease in the cell surface density of CD19 affects B cell development and
function,
resulting in diseases such as autoimmunity or hypogammaglobulinemia. The CD19
complex potentiates the response of B cells to antigen in vivo through cross-
linking of two
separate signal transduction complexes found on B cell membranes. The two
signal
transduction complexes, associated with membrane IgM and CD19, activate
phospholipase C (PLC) by different mechanisms. CD19 and B cell receptor cross-
linking
reduces the number of IgM molecules required to activate PLC. CD19 also
functions as a
specialized adapter protein for the amplification of Arc family kinases.
CD19 binding has been shown to both enhance and inhibit B-cell activation and
proliferation, depending on the amount of cross-linking that occurs. CD19 is
expressed on
1
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
greater than 90% of B-cell lymphomas and has been predicted to affect growth
of
lymphomas in vitro and in vivo.
Therapeutic uses of anti-CD19 ADCs
The efficacy of an Antibody Drug Conjugate comprising an anti-CD19 antibody
(an
anti-CD19-ADC) in the treatment of, for example, cancer has been established ¨
see, for
example, W02014/057117 and W02016/166298.
Research continues to further improve the efficacy, tolerability, and clinical
utility of anti-
CD19 ADCs. To this end, the present authors have identified clinically
advantageous
combination therapies in which an anti-CD19 ADC is administered in combination
with at
least one anti-CD79b agent.
SUMMARY
The present authors have determined that the administration of a combination
of an
anti-CD19 ADC and an anti-CD79b agent to an individual leads to unexpected
clinical
advantages. The present authors have further determined that administration of
an anti-
CD19 ADC to an individual that has either been treated with, or is being
treated with, and
an anti-CD79b agent leads to a synergistic increase in treatment efficacy.
Accordingly, in a first aspect the present disclosure provides a method of
selecting an
individual as suitable for treatment with an anti-CD19 ADC, wherein the
individual is
selected for treatment with the anti-CD19 ADC if the individual has been
treated, or is being
treated, with an anti-CD79b agent. The individual may be selected for
treatment if the
individual is refractory to treatment, or further treatment, with the anti-
CD79b agent.
In another aspect, the present disclosure provides a method for treating a
disorder in an
individual, the method comprising selecting an individual as suitable for
treatment by a
method of the first aspect, and then administering to the individual an
effective amount of
the anti-CD19 ADC. The method of treatment may further comprise administering
an anti-
CD79b agent in combination with the anti-CD19 ADC.
In another aspect the disclosure provides a method for treating a disorder in
an individual,
the method comprising administering to the individual an effective amount of
an anti-CD19
ADC and an anti-CD79b agent. The individual may be selected for treatment
according to
a method according of the first aspect.
The disorder may be a proliferative disease, for example a cancer such as non-
Hodgkin's
Lymphoma, including diffuse large B-cell lymphoma (DLBCL), follicular
lymphoma, (FL),
Burkitt lymphoma, Mantle Cell lymphoma (MCL), chronic lymphatic lymphoma
(CLL),
Waldenstrom Macroglobulinemia (WM), and Marginal Zone B-cell lymphoma (MZBL),
and
leukemias such as Hairy cell leukemia (HCL), Hairy cell leukemia variant (HCL-
v), chronic
lymphocytic leukemia (CLL) including Richter syndrome, and Acute Lymphoblastic
Leukaemia (ALL) such as Philadelphia chromosome-positive ALL (Ph+ALL) or
Philadelphia
chromosome-negative ALL (Ph-ALL).
2
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
The anti-CD19 ADC may be Loncastuximab tesirine.
The anti-CD19 ADC may be ADCx19 described herein.
The anti-CD79b agent may be Polatuzumab vedotin.
The individual may be human. The individual may have cancer, or may have been
determined to have cancer. The individual may have, or have been determined to
have, a
CD19+ cancer or CD19+ tumour-associated non-tumour cells, such as CD19+
infiltrating
B-cells.
In the disclosed methods the anti-CD19 ADC may be administered before the anti-
CD79b
agent, simultaneous with the anti-CD79b agent, or after the anti-CD79b agent.
The
disclosed methods may comprise administering a further chemotherapeutic agent
to the
individual.
In another aspect, the present disclosure provides an anti-CD19 ADC, or a
composition
comprising an anti-CD19 ADC, for use in a method of treatment as described
herein.
In one aspect, the present disclosure provides an anti-CD79b agent, or a
composition
comprising an anti-CD79b agent, for use in a method of treatment as described
herein.
In a further aspect, the present disclosure provides for the use of an anti-
CD19 ADC or an
anti-CD79b agent in the manufacture of a medicament for treating a disorder in
an
individual, wherein the treatment comprises a method of treatment as described
herein.
In another aspect, the disclosure provides a first composition comprising an
anti-CD19 ADC
for use in a method of treating a disorder in an individual, wherein the
treatment comprises
administration of the first composition in combination with a second
composition comprising
an anti-CD79b agent.
Also provided by this aspect is a first composition comprising an anti-CD79b
agent for use
in a method of treating a disorder in an individual, wherein the treatment
comprises
administration of the first composition in combination with a second
composition comprising
an anti-CD19 ADC.
The disorder may be a proliferative disease, for example a cancer such as non-
Hodgkin's
Lymphoma, including diffuse large B-cell lymphoma (DLBCL), follicular
lymphoma, (FL),
Burkitt lymphoma, Mantle Cell lymphoma (MCL), chronic lymphatic lymphoma
(CLL),
Waldenstrom Macroglobulinemia (WM), and Marginal Zone B-cell lymphoma (MZBL),
and
leukemias such as Hairy cell leukemia (HCL), Hairy cell leukemia variant (HCL-
v), chronic
lymphocytic leukemia (CLL) including Richter syndrome, and Acute Lymphoblastic
Leukaemia (ALL) such as Philadelphia chromosome-positive ALL (Ph+ALL) or
Philadelphia
chromosome-negative ALL (Ph-ALL).
3
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
The anti-0D19 ADC may be Loncastuximab tesirine.
The anti-CD19 ADC may be ADCx19 described herein.
The anti-CD79b agent may be Polatuzunnab vedotin.
The individual may be human. The individual may have cancer, or may have been
determined to have cancer. The individual may have, or have been determined to
have, a
0D19+ cancer or 0D19+ tumour-associated non-tumour cells, such as 0D19+
infiltrating
B-cells.
The first composition may be administered before the second composition,
simultaneous
with the second composition, or after the second composition. The treatment
may comprise
administering a further chemotherapeutic agent to the individual.
In a further aspect, the disclosure provides the use of an anti-CD19 ADC in
the manufacture
of a medicament for treating a disorder in an individual, wherein the
medicament comprises
an anti-CD19 ADC, and wherein the treatment comprises administration of the
medicament
in combination with a composition comprising anti-CD79b agent.
Also provided by this aspect is the use of anti-CD79b agent in the manufacture
of a
medicament for treating a disorder in an individual, wherein the medicament
comprises an
anti-CD79b agent, and wherein the treatment comprises administration of the
medicament
in combination with a composition comprising an anti-CD19 ADC.
The disorder may be a proliferative disease, for example a cancer such as non-
Hodgkin's
Lymphoma, including diffuse large B-cell lymphoma (DLBCL), follicular
lymphoma, (FL),
Burkitt lymphoma, Mantle Cell lymphoma (MCL), chronic lymphatic lymphoma
(CLL),
Waldenstrom Macroglobulinemia (WM), and Marginal Zone B-cell lymphoma (MZBL),
and
leukemias such as Hairy cell leukemia (HCL), Hairy cell leukemia variant (HCL-
v), chronic
lymphocytic leukemia (CLL) including Richter syndrome, and Acute Lymphoblastic
Leukaemia (ALL) such as Philadelphia chromosome-positive ALL (Ph-FALL) or
Philadelphia
chromosome-negative ALL (Ph-ALL).
The anti-0D19 ADC may be Loncastuximab tesirine.
The anti-0D19 ADC may be ADCx19 described herein.
The anti-CD79b agent may be Polatuzumab vedotin.
The individual may be human. The individual may have cancer, or may have been
determined to have cancer. The individual may have, or have been determined to
have, a
4
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
CD19+ cancer or CD19+ tumour-associated non-tumour cells, such as CD19+
infiltrating
B-cells.
The medicament may be administered before the composition, simultaneous with
the
composition, or after the composition. The treatment may comprise
administering a further
chemotherapeutic agent to the individual.
Another aspect of the disclosure provides a kit comprising:
a first medicament comprising an anti-CD19 ADC;
a package insert comprising instructions for administration of the first
medicament
according to a method of treatment as disclosed herein. The kit may further
comprise a
second medicament comprising an anti-CD79b agent.
Another aspect of the disclosure provides a kit comprising:
a first medicament comprising an anti-CD19 ADC;
a second medicament comprising an anti-CD79b agent; and, optionally,
a package insert comprising instructions for administration of the first
medicament
to an individual in combination with the second medicament for the treatment
of a disorder.
Also provided by this aspect is a kit comprising a medicament comprising an
anti-CD19
ADC and a package insert comprising instructions for administration of the
medicament to
an individual in combination with a composition comprising an anti-CD79b agent
for the
treatment of a disorder.
Further provided by this aspect is a kit comprising a medicament comprising an
anti-CD79b
agent and a package insert comprising instructions for administration of the
medicament to
an individual in combination with a composition comprising an anti-CD19 ADC
for the
treatment of a disorder.
The disorder may be a proliferative disease, for example a cancer such as non-
Hodgkin's
Lymphoma, including diffuse large B-cell lymphoma (DLBCL), follicular
lymphoma, (FL),
Burkitt lymphoma, Mantle Cell lymphoma (MCL), chronic lymphatic lymphoma
(CLL),
Waldenstrom Macroglobulinemia (WM), and Marginal Zone B-cell lymphoma (MZBL),
and
leukemias such as Hairy cell leukemia (HCL), Hairy cell leukemia variant (HCL-
v), chronic
lymphocytic leukemia (CLL) including Richter syndrome, and Acute Lymphoblastic
Leukaemia (ALL) such as Philadelphia chromosome-positive ALL (Ph+ALL) or
Philadelphia
chromosome-negative ALL (Ph-ALL).
The anti-CD19 ADC may be Loncastuximab tesirine.
The anti-CD19 ADC may be ADCx19 described herein.
The anti-CD79b agent may be Polatuzumab vedotin.
5
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
The individual may be human. The individual may have cancer, or may have been
determined to have cancer. The individual may have, or have been determined to
have, a
CD19+ cancer or CD19+ tumour-associated non-tumour cells, such as CD19+
infiltrating
B-cells.
The medicament or composition comprising the anti-0D19 ADC may be administered
before the medicament or composition comprising the anti-CD79b agent,
simultaneous
with the medicament or composition comprising the anti-CD79b agent, or after
the
medicament or composition comprising the anti-CD79b agent. The treatment may
comprise
administering a further chemotherapeutic agent to the individual.
In a yet further aspect, the disclosure provides a composition comprising an
anti-0D19
ADC and an anti-CD79b agent, along with the use of such composition in the
methods
disclosed herein.
Also provided in this aspect of the disclosure is a method of treating a
disorder in an
individual, the method comprising administering to the individual an effective
amount of the
composition comprising an anti-0D19 ADC and an anti-CD79b agent.
Also provided in this aspect of the disclosure is a composition comprising an
anti-0D19
ADC and an anti-CD79b agent for use in a method of treating a disorder in an
individual.
Also provided in this aspect of the disclosure is the use of a composition
comprising an
anti-CD19 ADC and an anti-CD79b agent in the manufacture of a medicament for
treating
a disorder in an individual.
Also provided in this aspect of the disclosure is a kit comprising composition
comprising an
anti-CD19 ADC and an anti-CD79b agent and a set of instructions for
administration of the
medicament to an individual for the treatment of a disorder.
The disorder may be a proliferative disease, for example a cancer such as non-
Hodgkin's
Lymphoma, including diffuse large B-cell lymphoma (DLBCL), follicular
lymphoma, (FL),
Burkitt lymphoma, Mantle Cell lymphoma (MCL), chronic lymphatic lymphoma
(CLL),
Waldenstrbm Macroglobulinemia (WM), and Marginal Zone B-cell lymphoma (MZBL),
and
leukemias such as Hairy cell leukemia (HCL), Hairy cell leukemia variant (HCL-
v), chronic
lymphocytic leukemia (CLL) including Richter syndrome, and Acute Lymphoblastic
Leukaemia (ALL) such as Philadelphia chromosome-positive ALL (Ph+ALL) or
Philadelphia
chromosome-negative ALL (Ph-ALL).
The anti-CD19-ADC may be ADCX19 as described herein.
The anti-CD79b agent may be Polatuzumab vedotin.
6
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
The individual may be human. The individual may have cancer, or may have been
determined to have cancer. The individual may have, or have been determined to
have, a
CD19+ cancer or CD19+ tumour-associated non-tumour cells, such as CD19+
infiltrating
B-cells.
The treatment may comprise administering a further chemotherapeutic agent to
the
individual.
The present disclosure also relates more generally to a combination of an
antibody drug
conjugate that comprises a PBD dimer as the warhead (such as the PBDs
described
herein) and an antibody drug conjugate that comprises monomethyl auristatin E
as the
warhead, and therapeutics methods as described herein which comprise
administering a
combination of the two antibody drug conjugates. The antibodies for each of
the individual
agents are selected to bind to a different target molecule. One example of an
antibody
drug conjugate that comprises monomethyl auristatin E is where the antibody
targets anti-
CD79b. Examples of antibody drug conjugates that comprise a PBD dimer, are
those that
target CD19 as described above as well as those that target CD25 or CD22, as
described
below.
Accordingly, in a further aspect the present disclosure provides a combination
of (i) an
antibody drug conjugate that comprises a PBD dimer; and (ii) an antibody drug
conjugate
that comprises monomethyl auristatin E, wherein the antibody of (i) binds to a
different
target molecule to the antibody of (ii) (e.g. different cell surface molecule
such as proteins,
receptors and the like).
In a related aspect the present disclosure provides a method of treating an
individual
suffering from a proliferative disorder by administering to the individual a
combination of (i)
an antibody drug conjugate that comprises a PBD dimer; and (ii) an antibody
drug
conjugate that comprises monomethyl auristatin E, wherein the antibody of (i)
binds to a
different target molecule to the antibody of (ii) (e.g. different cell surface
molecule such as
proteins, receptors and the like). Such administration may be at the same time
or
separately.
It will be appreciated by a person skilled in the art that further embodiments
described
below in detail for anti-CD19 ADCs and anti-CD79b ADCs, such as the selection
of PBD,
disorders to be treated, patient selection and administration, can be applied
mutatis
mutandis to the ADCs of (i) and (ii) that bind to different targets.
Particular examples of
other targets are 0D25 and 0D22 which are described in more detail below.
CD25
The type I transmembrane protein CD25 is present on activated T- and B- cells,
some
thymocytes, myeloid precursors, and oligodendrocytes. On activated T-cells, it
forms
heterodinners with the beta- and gamma subunits (CD122 and CD132), thus
comprising the
high-affinity receptor for IL-2. This ligand represents a survival factor for
activated T-cells,
as removal of IL-2 leads to immediate death of these cells.
7
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
In case of B-cells, CD25 is physiologically expressed in early developmental
stages of late
pro-B and pre-B cells. Malignancies arising from this stage of B-cell
differentiation may thus
also express 0D25. Mast cell lesions are also positive for 0D25 which is thus
considered
as a key diagnostic criterion for determination of systemic mastocytosis. In
Hodgkin
lymphomas, CD25 is reported to be not expressed in Hodgkin-/Reed-Sternberg
cells in
nodular lymphocyte predominance Hodgkin lymphoma (NLPHL), whereas the same
cell
type expresses CD25 at varying levels in classical Hodgkin' lymphomas of mixed
cellularity
type. The general expression levels are reported to be lower than in tumor
infiltrating
lymphocytes (TILs), which may result in problems demonstrating 0D25 tumor
cells in these
cases (Levi et al., Merz et al, 1995).
Expression of the target antigen has also been reported for several B- and T-
cell-derived
subtypes of non-Hodgkin-lymphomas, i.e. B-cell chronic lymphatic leukemia,
hairy cell
leukemia, small cell lymphocytic lymphoma/chronic lymphocytic leukemia as well
as adult
T-cell leukemia/lymphoma and anaplastic large cell lymphoma.
CD25 may be localised to the membrane, with some expression observed in the
cytoplasm.
Soluble CD25 may also be observed outside of cells, such as in serum.
Therapeutic uses of anti-CD25 ADCs
The efficacy of an Antibody Drug Conjugate comprising an anti-0D25 antibody
(an anti
CD25-ADC) in the treatment of, for example, cancer has been established ¨ see,
for
example, W02014/057119, W02016/083468, W02016/166341, and W02019/224275.
Research continues to further improve the efficacy, tolerability, and clinical
utility of anti-
CD25 ADCs. To this end, the present authors have identified clinically
advantageous
combination therapies in which an anti-CD25 ADC is administered in combination
with at
least one anti-CD79b agent.
Thus, in other aspects of the invention, instead of an anti-CD19-ADC in
combination with
an Anti-CD76b agent, an anti-CD25-ADC is used in combination with an anti-
CD79b agent.
Therefore all references to "anti-CD19-ADC" in the above embodiments can in
additional
embodiments be replaced with "anti-CD25-ADC".
CD22
0D22 is a 135-kDa type I transmembrane sialoglycoprotein of the immunoglobulin
(Ig)
superfamily. CD22 expression is specific to B cells and is developmentally
regulated so
that expression is limited in pro-B and pre-B cells. As B-cells mature,
expression increases
and localization of CD22 shifts to the cell surface. CD22 is strongly
expressed on follicular,
mantle and marginal-zone B cells, but is weakly present in germinal B cells.
CD22 is an
inhibitory co-receptor that down modulates B-cell receptor (BCR) signalling by
setting a
signalling threshold that prevents overstimulation of B cells.
Antibodies against CD22, such as epratuzumab (hLL2), have been used for
treatment of a
variety of cancers and autoimmune diseases, including but not limited to acute
lymphoblastic leukemia, chronic lymphocytic leukemia, non-Hodgkin's lymphoma,
follicular
lymphoma, diffuse large B-cell lymphoma, mantle cell lymphoma, systemic lupus
8
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
erythematosus, and primary Sjogren's syndrome. A phase III clinical trial of
epratuzumab
in systemic lupus erythematosus is currently in progress (see, e.g.,
ClinicalTrials.gov,
"Study of Epratuzunnab versus Placebo in Subjects with Moderate to Severe
General
Systemic Lupus Erythematosus (EMBODY 1)"). Because 0D22 regulates B-cell
functions
and survival, it is an important link for modulating humoral immunity and
proliferation of B-
cell lymphomas and a target for therapeutic antibodies in cancer and
autoimnnune disease.
Therapeutic uses of anti-CD22 ADCs
The efficacy of an Antibody Drug Conjugate comprising an anti-CD22 antibody
(an
anti-CD22-ADC) in the treatment of, for example, cancer has been established ¨
see, for
example, W02014/057122 and W02016/166307, or as described in Kantarjian et
al.,
(2016, New Eng J Med).
Research continues to further improve the efficacy, tolerability, and clinical
utility of anti-
CD22 ADCs. To this end, the present authors have identified clinically
advantageous
combination therapies in which an anti-CD22 ADC is administered in combination
with at
least one anti-CD79b agent.
Thus, in other aspects of the invention, instead of an anti-CD19-ADC in
combination with
an anti-CD79b agent, an anti-CD22-ADC is used in combination with an anti-
CD79b agent.
Therefore all references to "anti-CD19-ADC" in the above embodiments can in
additional
embodiments be replaced with "anti-CD22-ADC".
DETAILED DESCRIPTION
Antibody Drug Conjugates (ADCs)
The present disclosure relates to the improved efficacy of combinations of an
ADC and an
anti-CD79b agent.
The ADC can deliver a drug to a target location. The target location is
preferably a
proliferative cell population. The antibody is an antibody for an antigen
present on a
proliferative cell population. In one aspect the antigen is absent or present
at a reduced
level in a non-proliferative cell population compared to the amount of antigen
present in the
proliferative cell population, for example a tumour cell population.
The ADC may comprise a linker which may be cleaved so as to release the drug
at the
target location. The drug may be a compound selected from RelA, RelB, ReIC,
RelD or
RelE. Thus, the conjugate may be used to selectively provide a compound RelA,
RelB,
Rel C, RelD or RelE to the target location.
The linker may be cleaved by an enzyme present at the target location.
9
CA 03220935 2023- 11- 30
WO 2023/274974 PCT/EP2022/067603
The disclosure particularly relates to treatment with an anti-CD19 ADC
disclosed in
W02014/057117, and as herein described.
Anti-CD19 ADCs
As used herein, the terms "anti-CD19 ADC" or "CD19-ADC" refers to an ADC in
which the
antibody component is an anti-CD19 antibody. The term "PBD-ADC" refers to an
ADC in
which the drug component is a pyrrolobenzodiazepine (PBD) warhead. The term
"anti-
CD19-ADC" refers to an ADC in which the antibody component is an anti-CD19
antibody,
and the drug component is a PBD warhead.
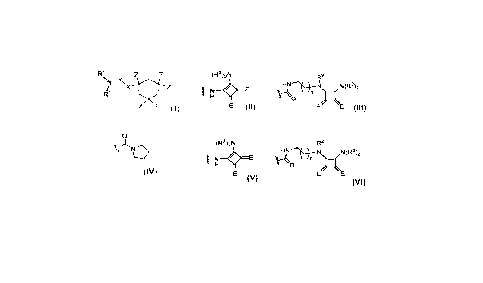
The ADC may comprise a conjugate of formula L - (DL),, where DL is of formula
I or II:
9, 9 RI-1'
21 R R
Ifj2
Y'
R11 a
C2' . N R7' R7 N C2
2
R1 R2
R6 0 C.3
_30 9, 10
R31I R R9 RI R11
;28-11-1ta
NR"
R7' R7
R22
R12 --
Rs'
Rs 0/
wherein:
15 L is an antibody (Ab) which is an antibody that binds to CD19;
when there is a double bond present between C2' and C3', R12 is selected from
the group consisting of:
(ia) C5-10 aryl group, optionally substituted by one or more substituents
selected from the
group comprising: halo, nitro, cyano, ether, carboxy, ester, C1-7 alkyl, 03-7
heterocyclyl and
20 bis-oxy-C1_3 alkylene;
(ib) C1_5 saturated aliphatic alkyl;
(ic) C3-6 saturated cycloalkyl;
R22
*y- r`-1,R23
,21
(id) , wherein each of R21, R22 and R23 are
independently selected from H,
01_3 saturated alkyl, 02_3 alkenyl, C2_3 alkynyl and cyclopropyl, where the
total number of
carbon atoms in the R12 group is no more than 5;
R25b
*
(ie) , wherein one of R25a and R25b is H and the other
is selected from:
phenyl, which phenyl is optionally substituted by a group selected from halo,
methyl,
methoxy; pyridyl; and thiophenyl; and
CA 03220935 2023-11-30
WO 2023/274974
PCT/EP2022/067603
24
(if) R, where R24 is selected from: H; C1_3 saturated
alkyl; 02-3 alkenyl; 02-3
alkynyl; cyclopropyl; phenyl, which phenyl is optionally substituted by a
group selected
from halo, methyl, methoxy; pyridyl; and thiophenyl;
when there is a single bond present between 02' and C3',
126b
Ri2 is where R26a and R26b are independently selected from H, F, C1_4
saturated alkyl, 02-3 alkenyl, which alkyl and alkenyl groups are optionally
substituted by a
group selected from C1_4 alkyl amido and C1_4 alkyl ester; or, when one of
R26a and R2613 is
H, the other is selected from nitrile and a C1_4 alkyl ester;
R6 and R9 are independently selected from H, R, OH, OR, SH, SR, NH2, NHR,
NRR',
nitro, Me3Sn and halo;
where R and R' are independently selected from optionally substituted 01-12
alkyl, 03-20
heterocyclyl and 06-20 aryl groups;
R7 is selected from H, R, OH, OR, SH, SR, NH2, NHR, NHRR', nitro, Me3Sn and
halo;
R" is a 03-12 alkylene group, which chain may be interrupted by one or more
heteroatoms,
e.g. 0, S, NR^12 (where R^12 is H or 01_4 alkyl), and/or aromatic rings, e.g.
benzene or
pyridine;
Y and Y' are selected from 0, S, or NH;
R6', R7', R9' are selected from the same groups as R6, R7 and R9 respectively;
[Formula 11
R1-1' is a linker for connection to the antibody (Ab);
R11a is selected from OH, ORA, where RA is 01_4 alkyl, and SOzM, where z is 2
or 3 and M
is a monovalent pharmaceutically acceptable cation;
R29 and R21 either together form a double bond between the nitrogen and carbon
atoms
to which they are bound or;
R29 is selected from H and Rc, where Rc is a capping group;
R21 is selected from OH, ORA and SON;
when there is a double bond present between C2 and C3, R2 is selected from the
group
consisting of:
(ia) 05_10 aryl group, optionally substituted by one or more substituents
selected from the
group comprising: halo, nitro, cyano, ether, carboxy, ester, C1.7 alkyl, C3-7
heterocyclyl and
bis-oxy-Ci_3 alkylene;
(ib) C1-6 saturated aliphatic alkyl;
(ic) 03-6 saturated cycloalkyl;
R12
R13
(id) R , wherein each of R11, R12 and R13 are
independently selected from H,
C1-3 saturated alkyl, C2-3 alkenyl, C2-3 alkynyl and cyclopropyl, where the
total number of
carbon atoms in the R2 group is no more than 5;
11
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
R15b
R15a
(ie)
7wherein one of R15a and R15b is H and the other is selected from:
phenyl, which phenyl is optionally substituted by a group selected from halo,
methyl,
methoxy; pyridyl; and thiophenyl; and
14
(if) R 7where R14 is selected from: H; C1_3 saturated
alkyl; 02-3 alkenyl; 02-3
alkynyl; cyclopropyl; phenyl, which phenyl is optionally substituted by a
group selected
from halo, methyl, methoxy; pyridyl; and thiophenyl;
when there is a single bond present between C2 and 03,
R16a
I 16b
R2 is R , where R16a and R16b are independently selected
from H, F, C1_4
saturated alkyl, 02-3 alkenyl, which alkyl and alkenyl groups are optionally
substituted by a
group selected from C1_4 alkyl amido and C1_4 alkyl ester; or, when one of R16
and R161) is
H, the other is selected from nitrile and a 01_4 alkyl ester;
[Formula Ill
R22 is of formula IIla, formula IIlb or formula 111c:
2..X IIla
(a) Q Q
where A is a 05-7 aryl group, and either
(i) Q1 is a single bond, and Q2 is selected from a single bond and -Z-(CH2)-,
where Z is
selected from a single bond, 07S and NH and n is from 1 to 3; or
(ii) Q1 is -CH=CH-, and Q2 is a single bond;
RC2
IIlb
c3x
(b) R R
where;
Rci7Rc2 and .-,C3
are independently selected from H and unsubstituted 01-2 alkyl;
IIIc
(c)
where Q is selected from 0-R1_2', S- RI-2' and NRN-R1-2', and RN is selected
from H, methyl
and ethyl
X is selected from the group comprising: 0-R1-2', S-R1-2', 002-R1-2', CO-R1-
2', NH-C(=0)-R1-2',
1 _____________________________________ \ N¨RL2' N/--\N¨RL2'
NHNH-RI-2', CONHNH-RI-2', ,
wherein RN is
selected from the group comprising H and 01-4 alkyl;
R1-2' is a linker for connection to the antibody (Ab);
R1 and R11 either together form a double bond between the nitrogen and carbon
atoms
to which they are bound or;
12
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
R1 is H and R" is selected from OH, ORA and SON;
R3 and R31 either together form a double bond between the nitrogen and carbon
atoms
to which they are bound or;
R3 is H and R31 is selected from OH, ORA and SOzM.
In some embodiments L-RL1' or L-R'2' is a group:
L *
'`1_2
0
where the asterisk indicates the point of attachment to the PBD, Ab is the
antibody, L1 is a cleavable linker, A is a connecting group connecting L1 to
the antibody,
L2 is a covalent bond or together with -0C(=0)- forms a self-immolative
linker.
In some of these embodiments, L1 is enzyme cleavable.
In one embodiment, the PBD is of formula (III):
RLL
0 H
0 0
0 0
0 0
wherein RLL is a linker for connection to Ab.
In another embodiment, the PBD is of formula (IV):
LLA LL
HO 0 H
N
0 0
wherein RLL is a linker for connection to Ab and RLLA is a linker for
connection to Ab
or capping group Rc.
It has previously been shown that such ADCs are useful in the treatment of
CD19
expressing cancers (see, for example, W02014/057117, W02018/193105, and
W02018/229222 which are incorporated by reference herein in their entirety).
The term anti-CD19-ADC may include any embodiment described in W02014/057117.
In
particular, in preferred embodiments the ADC may have the chemical structure:
13
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
ON
No
H
8
of H
oo
N w N-Tõ.õ..,1
0 0
where the Ab is a CD19 antibody, and the DAR is between 1 and 8.
The antibody may comprise a VH domain having the sequence according to any one
of
SEQ ID NOs. 1, 2, 3, 4, 5 or 6, optionally further comprising a VL domain
having the
sequence according to any one of SEQ ID NOs. 7, 8, 9, 10, 11 or 12.
In some aspects the antibody component of the anti-CD19-ADC is an antibody
comprising:
VH and VL domains respectively having the sequences of: SEQ ID NO. 1 and SEQ
ID NO.
7, SEQ ID NO. 2 and SEQ ID NO. 8, SEQ ID NO. 3 and SEQ ID NO. 9, SEQ ID NO. 4
and
SEQ ID NO. 10, SEQ ID NO. 5 and SEQ ID NO. 11, or SEQ ID NO. 6 and SEQ ID NO.
12.
In some embodiments the antibody has a VH domain comprising a VH CDR1, a VH
CDR2,
and a VH CDR3, wherein the antibody comprises the CDR sequences of the VH
domain
having the sequence according to SEQ ID NO: 2. In some embodiments the
antibody has
a VL domain comprising a VL CDR1, a VL CDR2, and a VL CDR3, wherein the
antibody
comprises the CDR sequences of the VL domain having the sequence according to
SEQ
ID NO: 8.
In preferred embodiments the antibody comprises a VH domain and a VL domain,
the VH domain comprising a VH CDR1, a VH CDR2, and a VH CDR3, wherein the
antibody comprises the CDR sequences of the VH domain having the sequence
according
to SEQ ID NO: 2; and
the VL domain comprising a VL CDR1, a VL CDR2, and a VL CDR3, wherein the
antibody comprises the CDR sequences of the VL domain having the sequence
according
to SEQ ID NO: 8.
The CDRs of antibody variable domains described herein may be identified by
any suitable
method known in the art, for example using any suitable antibody numbering
scheme. The
CDRs may be identified using any of the Kabat numbering scheme (Kabat et al.,
U.S.
Department of Health and Human Services, 1991), the Chothia numbering scheme
(Chothia C, Lesk A M. J Mol Biol. (1987) 196:901-17), or the IMGT numbering
scheme
(Giudicelli V, et al. Nucleic Acids Res. (1997) 25:206-11; Lefranc MP. Immunol
Today
(1997) 18:509). The skilled person will appreciate that these different CDR
labelling
systems can give slightly different results, but in each case the CDRs can be
easily
identified by the skilled person.
14
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
In preferred embodiments the antibody comprises a VH domain having the
sequence
according to SEQ ID NO. 2. In preferred embodiments the antibody comprises a
VL domain
having the sequence according to SEQ ID NO. 8.
In preferred embodiments the antibody comprises a VH domain and a VL domain,
the VH
and domain having the sequence of SEQ ID NO. 2 and the VL domain having the
sequences of SEQ ID NO. 8.
The VH and VL domain(s) may pair so as to form an antibody antigen binding
site that
binds CD19.
In some embodiments the antibody is an intact antibody comprising a VH domain
and a VL
domain, the VH and VL domains having sequences of SEQ ID NO. 2 and SEQ ID NO.
8.
In some embodiments the antibody is an antibody comprising a heavy chain
having
sequences of SEQ ID NO. 13 and a light chain having the sequences of SEQ ID
NO. 14.
In some embodiments the antibody is a fully human monoclonal IgG1 antibody,
preferably
IgG1,k.
In some embodiments the antibody is the RB4v1.2 antibody described in
W02014/057117.
In an aspect the antibody is an antibody as described herein which has been
modified (or
further modified) as described below. In some embodiments the antibody is a
humanised,
deimmunised or resurfaced version of an antibody disclosed herein.
The most preferred anti-CD19-ADC for use with the aspects of the present
disclosure is
ADCx19, as described herein below.
A second preferred anti-CD19-ADC for use with the aspects of the present
disclosure is
ADCT-402.
ADCx19
ADCx19 is an antibody drug conjugate composed of a humanized antibody against
human
CD19 attached to a pyrrolobenzodiazepine (PBD) warhead via a cleavable linker.
The
mechanism of action of ADCX19 depends on CD19 binding. The CD19 specific
antibody
targets the antibody drug conjugate (ADC) to cells expressing CD19. Upon
binding, the
ADC internalizes and is transported to the lysosome, where the protease
sensitive linker is
cleaved and free PBD dimer is released inside the target cell. The released
PBD dimer
inhibits transcription in a sequence-selective manner, due either to direct
inhibition of RNA
polymerase or inhibition of the interaction of associated transcription
factors. The PBD
dinner produces covalent crosslinks that do not distort the DNA double helix
and which are
not recognized by nucleotide excision repair factors, allowing for a longer
effective period
(Hartley 2011).
15
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
It has the chemical structure:
N
\ 0
H
N
cElljH
0 0 'pi 0,õ0
r 0 H
N
0 0
Ab represents Antibody RB4v1.2 (antibody with the VH and VL sequences SEQ ID
NO. 2
and SEQ ID NO. 8, respectively). It is synthesised as described in
W02014/057117
(RB4v1.2-E) and typically has a DAR (Drug to Antibody Ratio) of 2 +/- 0.5,
such as +/- 0.3.
CD19 binding
As used herein, "binds CD19" is used to mean the antibody binds CD19 with a
higher affinity
than a non-specific partner such as Bovine Serum Albumin (BSA, Genbank
accession no.
CAA76847, version no. CAA76847.1 GI:3336842, record update date: Jan 7,2011
02:30
PM). In some embodiments the antibody binds CD19 with an association constant
(Ka) at
least 2, 3, 4, 5, 10, 20, 50, 100, 200, 500, 1000, 2000, 5000, 104, 105 or 106-
fold higher than
the antibody's association constant for BSA, when measured at physiological
conditions.
The antibodies of the invention can bind CD19 with a high affinity. For
example, in some
embodiments the antibody can bind CD19 with a KD equal to or less than about
10-6 M,
such as 1 x 10-6, 10-7, 10-8, 10-9,10-10, I0h1, 10-12, 10_13 or 10-14.
In some embodiments, CD19 polypeptide corresponds to Genbank accession no.
NP_001171569, version no. NP_001171569.1 GI:296010921, record update date: Sep
10,
2012 12:43 AM. In one embodiment, the nucleic acid encoding CD19 polypeptide
corresponds to Genbank accession no NM_001178098, version no. NM_001178098.1
GI:296010920, record update date: Sep 10, 2012 12:43 AM.In some embodiments,
CD19
polypeptide corresponds to Uniprot/Swiss-Prot accession No. P15391.
The present disclosure also relates more generally to a combination of an
antibody drug
conjugate that comprises a PBD dimer as the warhead (such the PBDs described
herein)
and an antibody drug conjugate that comprises monomethyl auristatin E as the
warhead,
and therapeutics methods as described herein which comprise administered a
combination
of the two antibody drug conjugates. The antibodies for each of the individual
agents are
selected to bind to a different target molecule.
One example of an antibody drug conjugate that comprises monomethyl auristatin
E is
where the antibody targets anti-CD79b.
Examples of antibody drug conjugates that comprises a PBD dimer, are those
that target
CD19 as described above as well as those that target CD25 or CD22, as
described below.
16
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
It will be appreciated by a person skilled in the art that further embodiments
described
throughout in detail for anti-CD19 ADCs and anti-CD79b, such as the selection
of PBD,
disorders to be treated, patient selection and administration, can be applied
mutatis
mutandis to other ADCs described herein that bind to different targets.
Particular examples
of other targets are 0D25 and 0D22 which are described in more detail below.
Anti-CD25-ADCs
The disclosure also relates to treatment with an anti-0D25 ADC disclosed in
W02014/057119, and as herein described.
As used herein, the terms "anti-CD25 ADC" or "CD25-ADC" refers to an ADC in
which the
antibody component is an anti-0D25 antibody. The term "PBD-ADC" refers to an
ADC in
which the drug component is a pyrrolobenzodiazepine (PBD) warhead. The term
"anti-
0D25-ADC" refers to an ADC in which the antibody component is an anti-0D25
antibody,
and the drug component is a PBD warhead. The ADC component is as defined above
for
anti-CD19-ADCs.
In some embodiments the antibody has a VH domain comprising a VH CDR1, a VH
CDR2,
and a VH CDR3, wherein the antibody comprises the CDR sequences of the VH
domain
having the sequence according to SEQ ID NO: 25. In some embodiments the
antibody has
a VL domain comprising a VL CDR1, a VL CDR2, and a VL CDR3, wherein the
antibody
comprises the CDR sequences of the VL domain having the sequence according to
SEQ
ID NO: 25.
In preferred embodiments the antibody comprises a VH domain and a VL domain,
the VH domain comprising a VH CDR1, a VH CDR2, and a VH CDR3, wherein the
antibody comprises the CDR sequences of the VH domain having the sequence
according
to SEQ ID NO: 25; and
the VL domain comprising a VL CDR1, a VL CDR2, and a VL CDR3, wherein the
antibody comprises the CDR sequences of the VL domain having the sequence
according
to SEQ ID NO: 26.
The antibody may comprise a VH domain comprising a VH CDR1 with the amino acid
sequence of SEQ ID NO.27, a VH CDR2 with the amino acid sequence of SEQ ID
NO.28,
and a VH CDR3 with the amino acid sequence of SEQ ID NO.29.
In some aspects the antibody component of the anti-CD25-ADC is an antibody
comprising:
a VH domain comprising a VH CDR1 with the amino acid sequence of SEQ ID NO.27,
a
VH CDR2 with the amino acid sequence of SEQ ID NO.28, and a VH CDR3 with the
amino
acid sequence of SEQ ID NO.29. In some embodiments the antibody comprises a VH
domain having the sequence according to SEQ ID NO. 25.
The antibody may further comprise: a VL domain comprising a VL CDR1 with the
amino
acid sequence of SEQ ID NO.30, a VL CDR2 with the amino acid sequence of SEQ
ID
NO.31, and a VL CDR3 with the amino acid sequence of SEQ ID NO.32. In some
17
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
embodiments the antibody further comprises a VL domain having the sequence
according
to SEQ ID NO. 26.
In some embodiments the antibody comprises a VH domain and a VL domain, the VH
and
VL domains having the sequences of SEQ ID NO. 25 paired with SEQ ID NO. 26.
The VH and VL domain(s) may pair so as to form an antibody antigen binding
site that
binds CD25.
In preferred embodiments the antibody is an intact antibody comprising a VH
domain and
a VL domain, the VH and VL domains having sequences of SEQ ID NO. 25 and SEQ
ID
NO. 26.
In some embodiments the antibody is a fully human monoclonal IgG1 antibody,
preferably
IgG1,k.
In some embodiments the antibody is the AB12 antibody described in WO
2004/045512
(Genmab A/S).
In an aspect the antibody is an antibody as described herein which has been
modified (or
further modified) as described below. In some embodiments the antibody is a
humanised,
deimmunised or resurfaced version of an antibody disclosed herein.
A preferred anti-CD25-ADC for use with the aspects of the present disclosure
is ADCX25,
as described herein below.
Another preferred anti-CD25-ADC for use with the aspects of the present
disclosure is
camidanlumab tesirine.
ADCx25
ADCx25 is an antibody drug conjugate composed of a human antibody against
human
CD25 attached to a pyrrolobenzodiazepine (PBD) warhead via a cleavable linker.
The
mechanism of action of ADCX25 depends on CD25 binding. The CD25 specific
antibody
targets the antibody drug conjugate (ADC) to cells expressing CD25. Upon
binding, the
ADC internalizes and is transported to the lysosome, where the protease
sensitive linker is
cleaved and free PBD dimer is released inside the target cell. The released
PBD dimer
inhibits transcription in a sequence-selective manner, due either to direct
inhibition of RNA
polynnerase or inhibition of the interaction of associated transcription
factors. The PBD
dimer produces covalent crosslinks that do not distort the DNA double helix
and which are
not recognized by nucleotide excision repair factors, allowing for a longer
effective period
(Hartley 2011).
It has the chemical structure:
18
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
0
\ 0
H
8
8 'I 0 MI 0 0 H
o
0 0
Ab represents Antibody AB12 (fully human monoclonal IgG1, K antibody with the
VH and
VL sequences SEQ ID NO. 25 and SEQ ID NO. 26, respectively, also known as
HuMax-
TAC). It is synthesised as described in WO 2014/057119 (Conj AB12-E) and
typically has
a DAR (Drug to Antibody Ratio) of 2.0+/-0.3.
0D25 binding
As used herein, "binds CD25" is used to mean the antibody binds CD25 with a
higher affinity
than a non-specific partner such as Bovine Serum Albumin (BSA, Genbank
accession no.
CAA76847, version no. CAA76847.1 GI:3336842, record update date: Jan 7,2011
02:30
PM). In some embodiments the antibody binds 0D25 with an association constant
(Ka) at
least 2, 3, 4, 5, 10, 20, 50, 100, 200, 500, 1000, 2000, 5000, 104, 105 or 106-
fold higher than
the antibody's association constant for BSA, when measured at physiological
conditions.
The antibodies of the disclosure can bind 0D25 with a high affinity. For
example, in some
embodiments the antibody can bind 0D25 with a KD equal to or less than about
10-6 M,
such as equal to or less than one of 1 x 10-6, 10-7, 10-8, 10-6,10-10, 10-11,
10-12, 10-13 or 10-14.
In some embodiments, CD25 polypeptide corresponds to Genbank accession no.
NP_000408, version no. NP_000408.1 GI:4557667, record update date: Sep 09,
2012
04:59 PM. In one embodiment, the nucleic acid encoding CD25 polypeptide
corresponds
to Genbank accession no. NM_000417, version no. NM_000417.2 GI:269973860,
record
update date: Sep 09, 2012 04:59 PM. In some embodiments, CD25 polypeptide
corresponds to Uniprot/Swiss-Prot accession No. P01589.
Anti-CD22-ADCs
The disclosure also relates to treatment with an anti-0D22 ADC disclosed in
W02014/057122, and as herein described.
As used herein, the terms "anti-0D22 ADC" or "0D22-ADC" refers to an ADC in
which the
antibody component is an anti-0D22 antibody. The term "PBD-ADC" refers to an
ADC in
which the drug component is a pyrrolobenzodiazepine (PBD) warhead. The term
"anti-
0D22-ADC" refers to an ADC in which the antibody component is an anti-0D22
antibody,
and the drug component is a PBD warhead. The ADC component is as defined above
for
anti-CD19-ADCs.
19
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
The antibody component of the anti-CD22 ADC
The antibody may comprise an amino acid substitution of an interchain cysteine
residue by
an amino acid that is not cysteine, wherein the conjugation of the drug moiety
to the
antibody is at an interchain cysteine residue
The antibody preferably comprises: (i) a heavy chain having an amino acid
substitution of
each of the interchain cysteine residues HC226 and H0229 according to the EU
index as
set forth in Kabat; (ii) a light chain having an amino acid substitution of
the interchain
cysteine residue KLC214 or ALC213 according to the EU index as set forth in
Kabat; and
(iii) a heavy chain retaining the unsubstituted interchain cysteine HC220
according to the
EU index as set forth in Kabat.
Preferably the drug moiety is conjugated to the unsubstituted interchain
cysteine HC220.
The interchain cysteine residues HC226 and HC229 may each be substituted for
valine.
The interchain cysteine residues KLC214 or ALC213 may be substituted for
serine.
In some embodiments, the antibody of the conjugates described herein comprises
a light
chain comprising the amino acid sequence of SEQ ID NO. 45, or fragment
thereof, wherein
the cysteine at position 105, if present, is substituted by an amino acid that
is not cysteine.
For example, SEQ ID NO. 46 discloses a light chain comprising the amino acid
sequence
of SEQ ID NO. 45 wherein the cysteine at position 105 is substituted by a
serine residue.
In some embodiments, the antibody of the conjugates described herein comprises
a light
chain comprising the amino acid sequence of SEQ ID NO. 47, or fragment
thereof, wherein
the cysteine at position 102, if present, is substituted by an amino acid that
is not cysteine.
For example, SEQ ID NO. 48 discloses a light chain comprising the amino acid
sequence
of SEQ ID NO. 47 wherein the cysteine at position 102 is substituted by a
serine residue.
In some embodiments the antibody comprises:
(i) a heavy chain having an amino acid substitution of each of the interchain
cysteine
residues HC226 and HC229 according to the EU index as set forth in Kabat,
optionally
wherein H0226 and H0229 is each substituted for valine;
(ii) a light chain having an amino acid substitution of the interchain
cysteine residue
KLC214 or ALC213 according to the EU index as set forth in Kabat, optionally
wherein
KLC214 or ALC213 is substituted for serine;
(iii) a heavy chain retaining the unsubstituted interchain cysteine HC220
according
to the EU index as set forth in Kabat, optionally wherein the drug moiety is
conjugated to
the cysteine at HC220. In these embodiments, the antibody preferably further
comprises a
VH domain and VL domain as defined herein below. The light chain may comprise
the
amino acid sequence of: (i) SEQ ID NO. 45, or fragment thereof, wherein the
cysteine at
position 105, if present, is substituted by an amino acid that is not cysteine
(such as in SEQ
ID NO. 46); or SEQ ID NO. 47, or fragment thereof, wherein the cysteine at
position 102, if
present, is substituted by an amino acid that is not cysteine (such as in SEQ
ID NO. 48).
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
The antibody may comprise a heavy chain comprising the amino acid sequence of
SEQ ID
NO.43, and a light chain comprising the amino acid sequence of SEQ ID NO. 45
or SEQ
ID NO. 47;
wherein each of the cysteines at positions 109 and 112 in SEQ ID NO: 43 is
substituted by an amino acid that is not cysteine;
and wherein the cysteine at position 105 in SEQ ID NO: 45 or the cysteine at
position 102 in SEQ ID NO: 47, is substituted by an amino acid that is not
cysteine.
Preferably the drug moiety is conjugated to the cysteine at position 103 of
SEQ ID NO.43.
In some embodiments the cysteines at positions 109 and 112 in SEQ ID NO: 43
are
substituted for valine, such as in SEQ ID NO: 44. In some embodiments the
cysteine at
position 105 in SEQ ID NO: 45 or the cysteine at position 102 in SEQ ID NO: 47
is
substituted by serine such as in SEQ ID NOs: 46 and 48.
In some embodiments the antibody is a fully human monoclonal IgG1 antibody,
preferably
IgG1,K.
The VH and VL domains
In some embodiments the antibody has a VH domain comprising a VH CDR1, a VH
CDR2,
and a VH CDR3, wherein the antibody comprises the CDR sequences of the VH
domain
having the sequence according to SEQ ID NO: 1. In some embodiments the
antibody has
a VL domain comprising a VL CDR1, a VL CDR2, and a VL CDR3, wherein the
antibody
comprises the CDR sequences of the VL domain having the sequence according to
SEQ
ID NO: 34.
In preferred embodiments the antibody comprises a VH domain and a VL domain,
the VH domain comprising a VH CDR1, a VH CDR2, and a VH CDR3, wherein the
antibody comprises the CDR sequences of the VH domain having the sequence
according
to SEQ ID NO: 33; and
the VL domain comprising a VL CDR1, a VL CDR2, and a VL CDR3, wherein the
antibody comprises the CDR sequences of the VL domain having the sequence
according
to SEQ ID NO: 34.
The antibody may comprise a VH domain comprising a VH CDR1 with the amino acid
sequence of SEQ ID NO.35, a VH CDR2 with the amino acid sequence of SEQ ID
NO.36,
and a VH CDR3 with the amino acid sequence of SEQ ID NO.37.
In some aspects the antibody component of the anti-CD22-ADC is an antibody
comprising:
a VH domain comprising a VH CDR1 with the amino acid sequence of SEQ ID NO.35,
a
VH CDR2 with the amino acid sequence of SEQ ID NO.36, and a VH CDR3 with the
amino
acid sequence of SEQ ID NO.37. In some embodiments the antibody comprises a VH
domain having the sequence according to SEQ ID NO. 22.
The antibody may further comprise: a VL domain comprising a VL CDR1 with the
amino
acid sequence of SEQ ID NO.38, a VL CDR2 with the amino acid sequence of SEQ
ID
NO.39, and a VL CDR3 with the amino acid sequence of SEQ ID NO.40. In some
21
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
embodiments the antibody further comprises a VL domain having the sequence
according
to SEQ ID NO. 34.
In some embodiments the antibody comprises a VH domain and a VL domain, the VH
and
VL domains having the sequences of SEQ ID NO. 33 paired with SEQ ID NO. 34.
The VH and VL domain(s) may pair so as to form an antibody antigen binding
site that
binds CD22.
In some aspects the antibody component of the anti-CD22-ADC is an antibody
comprising:
a VH domain having the sequence according to SEQ ID NO. 33.
The antibody may further comprise a VL domain having the sequence according to
SEQ
ID NO. 34.
In some embodiments the antibody comprises a VH domain and VL domain as
described
herein below. In some embodiments, the antibody comprises:
a heavy chain having the sequence according to SEQ ID NO: 44;
a light chain having the sequence according to SEQ ID NO: 46;
a VH domain having the sequence according to SEQ ID NO. 33; and
a VL domain having the sequence according to SEQ ID NO. 32.
Preferably the drug moiety is conjugated to the cysteine at position 103 of
SEQ ID NO.44.
In some embodiments the antibody is the epratuzumab antibody described in
W02014/057122.
In come embodiments the antibody comprises a heavy chain having the sequence
according to SEQ ID NO. 47 and a light chain having the sequence according to
SEQ ID
NO. 48. Preferably the drug moiety is conjugated to the cysteine at position
219 of SEQ ID
NO.47.
In an aspect the antibody is an antibody as described herein which has been
modified (or
further modified) as described below. In some embodiments the antibody is a
humanised,
deimmunised or resurfaced version of an antibody disclosed herein.
The most preferred anti-CD22-ADC for use with the aspects of the present
disclosure is
ADCx22, as described herein below.
ADCx22
ADCx22 is an antibody drug conjugate composed of a human antibody against
human
CD22 attached to a pyrrolobenzodiazepine (PBD) warhead via a cleavable linker.
The
mechanism of action of ADCX22 depends on CD22 binding. The CD22 specific
antibody
targets the antibody drug conjugate (ADC) to cells expressing CO22. Upon
binding, the
ADC internalizes and is transported to the lysosome, where the protease
sensitive linker is
cleaved and free PBD dimer is released inside the target cell. The released
PBD dimer
22
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
inhibits transcription in a sequence-selective manner, due either to direct
inhibition of RNA
polymerase or inhibition of the interaction of associated transcription
factors. The PBD
dinner produces covalent crosslinks that do not distort the DNA double helix
and which are
not recognized by nucleotide excision repair factors, allowing for a longer
effective period.
It has the chemical structure:
ON
41)
H
0000
H
OH
</.
N N
0 0
wherein Ab represents an antibody comprising a VH domain having the sequence
of SEQ
ID NO. 33 and a VL domain having the sequence of SEQ ID NO. 34. Typically the
antibody
further comprises: (i) a heavy chain having an amino acid substitution of each
of the
interchain cysteine residues HC226 and HC229 according to the EU index as set
forth in
Kabat (for example, to valine); (ii) a light chain having an amino acid
substitution of the
interchain cysteine residue KLC214 according to the EU index as set forth in
Kabat (for
example, to serine); and (iii) a heavy chain retaining the unsubstituted
interchain cysteine
HC220 according to the EU index as set forth in Kabat. Typically the drug
moiety is
conjugated to the cysteine at HC220.
Accordingly, the antibody typically comprises a heavy chain having the
sequence according
to SEQ ID NO. 41 and a light chain having the sequence according to SEQ ID NO.
42.
Linkage to the drug occurs on Heavy Chain interchain cysteine Cys220 (EU
numbering).
HC220 corresponds to position 219 of SEQ ID NO.41.
It is noted that "having the sequence" has the same meaning as "comprising the
sequence";
in particular, in some embodiments the heavy chain of ADCx22 is expressed with
an
additional terminal 'K' residue (so, ending ...SPGIS), with the terminal K
being optionally
removed post-translationally to improve the homogeneity of the final
therapeutic ADC
product.
CD22 binding
As used herein, "binds CD22" is used to mean the antibody binds CD22 with a
higher affinity
than a non-specific partner such as Bovine Serum Albumin (BSA, Genbank
accession no.
0AA76847, version no. CAA76847.1 GI:3336842, record update date: Jan 7,2011
02:30
PM). In some embodiments the antibody binds 0D22 with an association constant
(Ka) at
least 2, 3, 4, 5, 10, 20, 50, 100, 200, 500, 1000, 2000, 5000, 104, 105 or 106-
fold higher than
the antibody's association constant for BSA, when measured at physiological
conditions.
The antibodies of the invention can bind CD22 with a high affinity. For
example, in some
embodiments the antibody can bind CD22 with KD equal to or less than about 10-
6 M, such
as 1 x 10-6, 10-7, 10-8, 10-9,10-10, 10-1 1, 10-12, 10-1301 10-14.
23
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
In some embodiments, CD22 polypeptide corresponds to Genbank accession no.
BAB15489, version no. BAB15489.1 GI:10439338, record update date: Sep 11, 2006
11:24 PM. In one embodiment, the nucleic acid encoding CD22 polypeptide
corresponds
to Genbank accession no AK026467, version no. AK026467.1 GI:10439337, record
update date: Sep 11, 2006 11:24 PM.
Anti-CD79b agents
CD79b
CD79 (composed of subunits CD79a and CD79b) is a heterodimeric signal-
transduction
component of the B-cell receptor. CD79b membrane expression is restricted to
the B-cell
compartment and is ubiquitously expressed in mature B-cell lymphomas and
placed on the
cell surface by the earliest committed B-cell progenitors before expression of
immunoglobulin p. Antibodies to CD79b induce negative cell signals and
suppress
response to 1-cell-dependent antigens (Nakamura et al., Int J Hematol. 1996;
64: 39-46).
However, unconjugated anti-CD79b antibodies induce modest B-cell depletion and
show
moderate antibody-dependent and complement-dependent cellular cytotoxicity, if
any (Fuh
et al., Br J Pharmacol. 2017; 174: 628-640). Conversely, anti-CD79b ADCs are
trafficked
to a lysosomal-like compartment of B cells as part of antigen presentation,
(Poison et al.,
Blood. 2007; 110: 616-623) and induce a prolonged and sustained depletion of
proliferating
B cells (Fuh et al., ibid.).
Anti-CD79b agents
`Anti-CD79b agent' is used herein to mean any agent that specifically binds to
CD79b
and/or a cell that expresses CD79b. Preferably the agent induces B-cell
depeletion.
As used herein, "specifically binds CD79b" is used to mean the agent binds
CD79b with a
higher affinity than a non-specific partner such as Bovine Serum Albumin (BSA,
Genbank
accession no. CAA76847, version no. CAA76847.1 GI:3336842, record update date:
Jan
7, 2011 02:30 PM). In some embodiments the agent binds CD79b with an
association
constant (K.) at least 2, 3, 4, 5, 10, 20, 50, 100, 200, 500, 1000, 2000,
5000, 104, 105 or
106-fold higher than the agent's association constant for BSA, when measured
at
physiological conditions. The agents may bind CD79b with a high affinity. For
example, in
some embodiments the agent can bind CD79b with a KD equal to or less than
about 10-6
M, such as 1 x 10-8, 10-7, 10-8, 10-9,10-10, 10-11, 10-12, 10-13 or 10-14.
Anti-CD79b ADCs
Anti-CD79b ADC is used herein to mean a conjugate comprising a moiety that
specifically
binds to CD79b conjugated to a payload.
In some embodiments the moiety that specifically binds to CD79b is an
antibody. In some
cases the antibody is Polatuzumab.
24
CA 03220935 2023- 11- 30
WO 2023/274974 PCT/EP2022/067603
In some embodiments the payload comprises a drug, such as a cytotoxic drug. In
some
cases the cytotoxic drug is an auristatin, such as a monomethyl auristatin. In
some cases
the cytotoxic drug is nnonomethyl auristatin (MMAE).
In some cases the payload comprises a linker moiety through which the payload
I
conjugated to the moiety that specifically binds to CD79b. In some embodiments
the
payload is a drug-linker, wherein the drug-linker comprises a drug moiety and
a linker
moiety. The linker moiety may be cleavable by an enzyme such as a protease.
For
example, in some embodiments the linker is a dipeptide such as Valine-
Citrulline (val-cit,
or vc).
In some embodiments the ADC has the structure:
A ,L2. Oy,"
0
wherein the asterisk indicates the point of attachment to the drug moiety, Ab
is the
antibody, L1 is a cleavable linker, A is a connecting group connecting L1 to
the antibody,
L2 is a covalent bond or together with -0C(=0)- forms a self-immolative
linker. In some of
these embodiments, L1 is enzyme cleavable.
In preferred embodiments the ADC may be a conjugate of formula (I):
Ab ¨ (DL)p (I)
wherein:
Ab is an antibody that binds to CD79b;
DL is
0
0 J
0
NH
0
Et ,,
, N 0
0
H = H
0 0
and p is between 1 and 8, such as between 3 and 4, for example about 3.5.
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
In some embodiments the antibody has a VH domain comprising a VH CDR1, a VH
CDR2,
and a VH CDR3, wherein the antibody comprises the CDR sequences of the VH
domain
having the sequence according to SEQ ID NO: 17. In some embodiments the
antibody has
a VL domain comprising a VL CDR1, a VL CDR2, and a VL CDR3, wherein the
antibody
comprises the CDR sequences of the VL domain having the sequence according to
SEQ
ID NO: 18.
In preferred embodiments the antibody comprises a VH domain and a VL domain,
the VH domain comprising a VH CDR1, a VH CDR2, and a VH CDR3, wherein the
antibody comprises the CDR sequences of the VH domain having the sequence
according
to SEQ ID NO: 17; and
the VL domain comprising a VL CDR1, a VL CDR2, and a VL CDR3, wherein the
antibody comprises the CDR sequences of the VL domain having the sequence
according
to SEQ ID NO: 18.
In some embodiments the antibody comprises a VH domain having a VH CDR3 with
the
amino acid sequence of SEQ ID NO.21. In some embodiments the VH domain further
comprises a VH CDR2 with the amino acid sequence of SEQ ID NO.20, and/or a VH
CDR1
with the amino acid sequence of SEQ ID NO.19. In some embodiments the antibody
comprises a VH domain having a VH CDR1 with the amino acid sequence of SEQ ID
NO.19, a VH CDR2 with the amino acid sequence of SEQ ID N0.20, and a VH CDR3
with
the amino acid sequence of SEQ ID NO.21. In some embodiments the antibody a VH
domain comprising a VH CDR1, a VH CDR2, and a VH CDR3, wherein the antibody
comprises the CDR sequences of the VH domain having the sequence of SEQ ID NO:
17.
In preferred embodiments the antibody comprises a VH domain having the
sequence
according to SEQ ID NO. 17.
The antibody may further comprise a VL domain. In some embodiments the
antibody
comprises a VL domain having a VL CDR3 with the amino acid sequence of SEQ ID
NO.24.
In some embodiments the VL domain further comprises a VL CDR2 with the amino
acid
sequence of SEQ ID NO.23, and/or a VL CDR1 with the amino acid sequence of SEQ
ID
NO.22. In some embodiments the antibody comprises a VL domain having a VL CDR1
with
the amino acid sequence of SEQ ID NO.22, a VL CDR2 with the amino acid
sequence of
SEQ ID NO.23, and a VL CDR3 with the amino acid sequence of SEQ ID NO.24. In
some
embodiments the antibody a VL domain comprising a VL CDR1, a VL CDR2, and a VL
CDR3, wherein the antibody comprises the CDR sequences of the VL domain having
the
sequence of SEQ ID NO: 18. In preferred embodiments the antibody comprises a
VL
domain having the sequence according to SEQ ID NO. 18.
The CDRs of antibody variable domains described herein may be identified by
any suitable
method known in the art, for example using any suitable antibody numbering
scheme. The
CDRs may be identified using any of the Kabat numbering scheme (Kabat et al.,
U.S.
Department of Health and Human Services, 1991), the Chothia numbering scheme
(Chothia C, Lesk A M. J Mol Biol. (1987) 196:901-17), or the IMGT numbering
scheme
(Giudicelli V, et al. Nucleic Acids Res. (1997) 25:206-11; Lefranc MP. Immunol
Today
(1997) 18:509). The skilled person will appreciate that these different CDR
labelling
26
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
systems can give slightly different results, but in each case the CDRs can be
easily
identified by the skilled person.
The VH and VL domain(s) may form an antibody antigen binding site that binds
CD79b.
In some embodiments the antibody is an intact antibody comprising a VH domain
and a VL
domain, the VH and VL domains having sequences of SEQ ID NO.17 paired with SEQ
ID
NO.18.
In some embodiments the antibody is a fully human monoclonal IgG1 antibody,
preferably
IgG1,k.
In an aspect the antibody is an antibody as described herein which has been
modified (or
further modified) as described below. In some embodiments the antibody is a
humanised,
deimmunised or resurfaced version of an antibody disclosed herein.
The most preferred anti-CD79b agent is Polatuzumab vedotin.
Polatuzumab vedotin
Polatuzumab vedotin (Polivy, Roche) is a CD79b-directed antibody-drug
conjugate
comprised of a humanized IgG1 anti-CD79b mAb conjugated to monomethyl
auristatin E
(MMAE) via the protease-cleavable linker vc (Val-Cit).
It has the chemical structure:
0
N H2
H
0 NY
0
0 L., 0
N H
0
Et ''''' 0 0
0
0 H
0 0 0 411111
27
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
Ab represents antibody Polatuzumab (antibody with the VH and VL sequences SEQ
ID
NO. 17 and SEQ ID NO. 18, respectively). It typically has a DAR (Drug to
Antibody Ratio)
of 3.5.
Advantageous properties of the described combinations
Both the anti-CD19 ADC (or anti-0D25 ADC or anti-0D22 ADC) and anti-CD79b
agent
when used as a single agent in isolation have demonstrated clinical utility ¨
for example, in
the treatment of cancer. However, as described herein, combination of the anti-
0D19 ADC
and anti-CD79b agent is expected to provide one or more of the following
advantages over
treatment with either anti-CD19 ADC or anti-CD79b agent alone:
1) effective treatment of a broader range of cancers;
2) effective treatment of resistant or refractory forms of disorders such as
cancer, and individuals with disorders such as cancer who have relapsed
after a period of remission;
3) increased response rate to treatment; and / or
4) Increased durability of treatment.
Effective treatment of a broader range of cancers as used herein means that
following
treatment with the combination a complete response is observed with a greater
range of
recognised cancer types. That is, a complete response is seen from cancer
types not
previously reported to completely respond to either anti-CD19 ADC or anti-
CD79b agent
alone.
Without wishing to be bound by theory, in embodiments where the anti-CD19 ADC
and
anti-CD79b agent comprise different classes of cytotoxic drug (for example
where the anti-
CD19 ADC is Loncastuximab tesirine and the anti-CD79b agent is Pola-V) that
have
different mode of actions (PBD dimers are DNA cross-linking agents while MMAE
are
tubulin inhibitor), the cellular toxicity acting through two distinct pathways
is believed to
contribute to the additive or synergistic cytotoxicity. Moreover, the fact the
two agents target
two different cell surface antigens means that they are not competing for
binding to the
same antigen on the cell surface. This facilitates delivery of the cytotoxic
drugs into the
target cell.
Effective treatment of a resistant, refractory, or relapsed forms as used
herein means that
following treatment with the combination a complete response is observed in
individuals
that are either partially or completely resistant or refractory to treatment
with either
anti-CD19 ADC or anti-CD79b agent alone (for example, individuals who show no
response
or only partial response following treatment with either agent alone, or those
with relapsed
disorder). In some embodiments, a complete response following treatment with
the
anti-CD19 ADC / anti-CD79b agent combination is observed at least 10% of
individuals
that are either partially or completely resistant or refractory to treatment
with either
anti-CD19 ADC or anti-CD79b agent alone. In some embodiments, a complete
response
following treatment with the anti-CD19 ADC / anti-CD79b agent combination is
observed
28
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
at least 20%, at least 30%, at least 40%, at least 50%, at least 60%, at least
70%, at least
80%, at least 90%, at least 95%, at least 98%, or at least 99% of individuals
that are either
partially or completely resistant or refractory to treatment with either anti-
CD19 ADC or anti-
CD79b agent alone.
Increased response rate to treatment as used herein means that following
treatment with
the combination a complete response is observed in a greater proportion of
individuals than
is observed following treatment with either anti-CD19 ADC or anti-CD79b agent
alone. In
some embodiments, a complete response following treatment with the anti-CD19
ADC /
anti-CD79b agent combination is observed at least 10% of treated individuals.
In some
embodiments, a complete response following treatment with the anti-CD19 ADC /
anti-
CD79b agent combination is observed at least 20%, at least 30%, at least 40%,
at least
50%, at least 60%, at least 70%, at least 80%, at least 90%, at least 95%, at
least 98%, or
at least 99% of treated individuals.
Increased durability of treatment as used herein means that average duration
of complete
response in individuals treated with the combination is longer than in
individuals who
achieve complete response following treatment with either anti-CD19 ADC or
anti-CD79b
agent alone. In some embodiments, the average duration of a complete response
following
treatment with the anti-CD19 ADC / anti-CD79b agent combination is at least 6
months. In
some embodiments, the average duration of a complete response following
treatment with
the anti-CD19 ADC / anti-CD79b agent combination is at least 12 months, at
least 18
months, at least 24 months, at least 3 years, at least 4 years, at least 5
years, at least 6
years, at least 7 years, at least 8 years, at least 9 years, at least 10
years, at least 15 years,
or at least 20 years.
'Complete response' is used herein to mean the absence of any clinical
evidence of disease
in an individual. Evidence may be assessed using the appropriate methodology
in the art,
for example CT or PET scanning, or biopsy where appropriate. The number of
doses
required to achieve complete response may be one, two, three, four, five, ten
or more. In
some embodiments the individuals achieve complete response no more than a year
after
administration of the first dose, such as no more than 6 months, no more than
3 months,
no more than a month, no more than a fortnight, or no more than a week after
administration
of the first dose.
References to anti-CD19 in this section and in all the following sections can
in other
embodiments be replaced with anti-CD22 and ant-CD25, unless specifically
indicated,
mutatis mutandis.
Treated disorders
The combined therapies described herein include those with utility for
anticancer activity.
In particular, in certain aspects the therapies include an antibody
conjugated, i.e. covalently
attached by a linker, to a PBD drug moiety, i.e. toxin. When the drug is not
conjugated to
an antibody, the PBD drug has a cytotoxic effect. The biological activity of
the PBD drug
moiety is thus modulated by conjugation to an antibody. The antibody-drug
conjugates
29
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
(ADC) of the disclosure selectively deliver an effective dose of a cytotoxic
agent to tumor
tissue whereby greater selectivity, i.e. a lower efficacious dose, may be
achieved.
Thus, in one aspect, the present disclosure provides combined therapies
comprising
administering an anti-CD19 ADC which binds CD19 for use in therapy, wherein
the method
comprises selecting a subject based on expression of the target protein.
In one aspect, the present disclosure provides a combined therapy with a label
that
specifies that the therapy is suitable for use with a subject determined to be
suitable for
such use. The label may specify that the therapy is suitable for use in a
subject has
expression of CD19, such as overexpression of CD19. The label may specify that
the
subject has a particular type of cancer.
The cancer may be lymphoma, such as non-Hodgkins lymphoma. The label may
specify
that the subject has a CD19+ lymphoma.
In a further aspect there is also provided a combined therapy as described
herein for use
in the treatment of a proliferative disease. Another aspect of the present
disclosure
provides the use of a conjugate compound in the manufacture of a medicament
for treating
a proliferative disease.
One of ordinary skill in the art is readily able to determine whether or not a
candidate
combined therapy treats a proliferative condition for any particular cell
type. For example,
assays which may conveniently be used to assess the activity offered by a
particular
compound are described below.
The combined therapies described herein may be used to treat a proliferative
disease. The
term "proliferative disease" pertains to an unwanted or uncontrolled cellular
proliferation of
excessive or abnormal cells which is undesired, such as, neoplastic or
hyperplastic growth,
whether in vitro or in vivo.
Examples of proliferative conditions include, but are not limited to, benign,
pre-malignant,
and malignant cellular proliferation, including but not limited to, neoplasms
and tumours
(e.g. histocytoma, glioma, astrocyoma, osteoma), cancers (e.g. lung cancer,
small cell lung
cancer, gastrointestinal cancer, bowel cancer, colon cancer, breast carinoma,
ovarian
carcinoma, prostate cancer, testicular cancer, liver cancer, kidney cancer,
bladder cancer,
pancreas cancer, brain cancer, sarcoma, osteosarcoma, Kaposi's sarcoma,
melanoma),
lymphomas, leukemias, psoriasis, bone diseases, fibroproliferative disorders
(e.g. of
connective tissues), and atherosclerosis. Cancers of interest include, but are
not limited
to, leukemias and ovarian cancers.
Any type of cell may be treated, including but not limited to, lung,
gastrointestinal (including,
e.g. bowel, colon), breast (mammary), ovarian, prostate, liver (hepatic),
kidney (renal),
bladder, pancreas, brain, and skin.
30
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
Proliferative disorders of particular interest include, but are not limited
to, non-Hodgkin's
Lymphoma, including diffuse large B-cell lymphoma (DLBCL), follicular
lymphoma, (FL),
Burkitt lymphoma, Mantle Cell lymphoma (MCL), chronic lymphatic lymphoma
(CLL),
Waldenstrbm Macroglobulinemia (WM), and Marginal Zone B-cell lymphoma (MZBL),
and
leukemias such as Hairy cell leukemia (HCL), Hairy cell leukemia variant (HCL-
v), chronic
lynnphocytic leukemia (CLL) including Richter syndrome, and Acute
Lynnphoblastic
Leukaemia (ALL) such as Philadelphia chromosome-positive ALL (Ph+ALL) or
Philadelphia
chromosome-negative ALL (Ph-ALL). [Fielding A., Haematologica. 2010 Jan;
95(1): 8-12].
It is contemplated that the combined therapies of the present disclosure may
be used to
treat various diseases or disorders, e.g. characterized by the overexpression
of a tumor
antigen. Exemplary conditions or hyperproliferative disorders include benign
or malignant
tumors; leukemia, haematological, and lymphoid malignancies. Others include
neuronal,
glial, astrocytal, hypothalamic, glandular, macrophagal, epithelial, stromal,
blastocoelic,
inflammatory, angiogenic and immunologic, including autoimmune disorders and
graft-
versus-host disease (GVHD).
Generally, the disease or disorder to be treated is a hyperproliferative
disease such as
cancer. Examples of cancer to be treated herein include, but are not limited
to, carcinoma,
lymphoma, blastoma, sarcoma, and leukemia or lymphoid malignancies. More
particular
examples of such cancers include squamous cell cancer (e.g. epithelial
squamous cell
cancer), lung cancer including small-cell lung cancer, non-small cell lung
cancer,
adenocarcinoma of the lung and squamous carcinoma of the lung, cancer of the
peritoneum, hepatocellular cancer, gastric or stomach cancer including
gastrointestinal
cancer, pancreatic cancer, glioblastoma, cervical cancer, ovarian cancer,
liver cancer,
bladder cancer, hepatoma, breast cancer, colon cancer, rectal cancer,
colorectal cancer,
endometrial or uterine carcinoma, salivary gland carcinoma, kidney or renal
cancer,
prostate cancer, vulval cancer, thyroid cancer, hepatic carcinoma, anal
carcinoma, penile
carcinoma, as well as head and neck cancer.
Autoimmune diseases for which the combined therapies may be used in treatment
include
rheumatologic disorders (such as, for example, rheumatoid arthritis, Sjogren's
syndrome,
scleroderma, lupus such as SLE and lupus nephritis,
polymyositis/dermatomyositis,
cryoglobulinemia, anti-phospholipid antibody syndrome, and psoriatic
arthritis),
osteoarthritis, autoimmune gastrointestinal and liver disorders (such as, for
example,
inflammatory bowel diseases (e.g. ulcerative colitis and Crohn's disease),
autoimmune
gastritis and pernicious anemia, autoimmune hepatitis, primary biliary
cirrhosis, primary
sclerosing cholangitis, and celiac disease), vasculitis (such as, for example,
ANCA-
associated vasculitis, including Churg-Strauss vasculitis, Wegener's
granulomatosis, and
polyarteriitis), autoimmune neurological disorders (such as, for example,
multiple sclerosis,
opsoclonus myoclonus syndrome, myasthenia gravis, neuromyelitis optica,
Parkinson's
disease, Alzheimer's disease, and autoimmune polyneuropathies), renal
disorders (such
as, for example, glonnerulonephritis, Goodpasture's syndrome, and Berger's
disease),
autoimmune dermatologic disorders (such as, for example, psoriasis, urticaria,
hives,
pemphigus vulgaris, bullous pemphigoid, and cutaneous lupus erythematosus),
hematologic disorders (such as, for example, thrombocytopenic purpura,
thrombotic
31
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
thrombocytopenic purpura, post-transfusion purpura, and autoimmune hemolytic
anemia),
atherosclerosis, uveitis, autoimmune hearing diseases (such as, for example,
inner ear
disease and hearing loss), Behcet's disease, Raynaud's syndrome, organ
transplant, graft-
versus-host disease (GVHD), and autoimmune endocrine disorders (such as, for
example,
diabetic-related autoimmune diseases such as insulin-dependent diabetes
mellitus (IDDM),
Addison's disease, and autoimmune thyroid disease (e.g. Graves' disease and
thyroiditis)). More preferred such diseases include, for example, rheumatoid
arthritis,
ulcerative colitis, ANCA-associated vasculitis, lupus, multiple sclerosis,
SjOgren's
syndrome, Graves' disease, IDDM, pernicious anemia, thyroiditis, and
glomerulonephritis.
In some aspects, the subject has a proliferative disorder selected from non-
Hodgkin's
Lymphoma, including diffuse large B-cell lymphoma (DLBCL), follicular
lymphoma, (FL),
Burkitt lymphoma, Mantle Cell lymphoma (MCL), chronic lymphatic lymphoma
(CLL),
Waldenstribm Macroglobulinemia (WM), and Marginal Zone B-cell lymphoma (MZBL),
and
leukemias such as Hairy cell leukemia (HCL), Hairy cell leukemia variant (HCL-
v), chronic
lymphocytic leukemia (CLL) including Richter syndrome, and Acute Lymphoblastic
Leukaemia (ALL) such as Philadelphia chromosome-positive ALL (Ph-i-ALL) or
Philadelphia
chromosome-negative ALL (Ph-ALL) .
CD25
The proliferative disease may be characterised by the presence of a neoplasm
comprising
both CD25+ve and CD25-ve cells. The proliferative disease may be characterised
by the
presence of a neoplasm composed of CD25-ve neoplastic cells, optionally
wherein the
CD25-ve neoplastic cells are associated with CD25+ve non-neoplastic cells such
as
CD25+ve T-cells. The target neoplasm or neoplastic cells may be all or part of
a solid
tumour.
"Solid tumor" herein will be understood to include solid haematological
cancers such as
lymphomas (Hodgkin's lymphoma or non-Hodgkin's B- and T-cell lymphoma) which
are
discussed in more detail herein.
Solid tumors may be neoplasms, including non-haematological cancers,
comprising or
composed of CD25+ve neoplastic cells. Solid tumors may be neoplasms, including
non-
haematological cancers, infiltrated with CD25+ve cells, such as CD25+ve T-
cells; such
solid tumours may lack expression of CD25 (that is, comprise or be composed of
CD25-ve
neoplastic cells).
For example, the solid tumour may be a tumour with high levels of infiltrating
T-cells, such
as infiltrating regulatory T-cells (Treg; Menetrier-Caux, C., et al., Targ
Oncol (2012) 7:15-
28; Arce Vargas et al., 2017, Immunity 46, 1-10; Tanaka, A., et al., Cell Res.
2017
Jan;27(1):109-118). Accordingly, the solid tumour may be pancreatic cancer,
breast
cancer, colorectal cancer, gastric and oesophageal cancer, leukemia and
lymphoma,
melanoma, non-small cell lung cancer, ovarian cancer, hepatocellular
carcinoma, renal cell
carcinoma, and head and neck cancer.
32
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
Patient Selection
In certain aspects, the individuals are selected as suitable for treatment
with the combined
treatments before the treatments are administered.
As used herein, individuals who are considered suitable for treatment are
those individuals
who are expected to benefit from, or respond to, the treatment. Individuals
may have, or
be suspected of having, or be at risk of having cancer. Individuals may have
received a
diagnosis of cancer. In particular, individuals may have, or be suspected of
having, or be
at risk of having, lymphoma. In some cases, individuals may have, or be
suspected of
having, or be at risk of having, a solid cancer that has tumour associated non-
tumor cells
that express CD19, such as infiltrating cells that express CD19.
In some aspects, individuals are selected on the basis of the amount or
pattern of
expression of CD19. In some aspects, the selection is based on expression of
CD19 at
the cell surface.
In certain aspects, the target is a CD79b. In some aspects, the selection is
based on
expression of a CD79b.
In some aspects, the selection is based on levels of both CD19 and CD79b at
the cell
surface.
In some cases, expression of the target in a particular tissue of interest is
determined. For
example, in a sample of lymphoid tissue or tumor tissue.
In some cases, systemic
expression of the target is determined. For example, in a sample of
circulating fluid such
as blood, plasma, serum or lymph.
In some aspects, the individual is selected as suitable for treatment due to
the presence of
target expression in a sample. In those cases, individuals without target
expression may
be considered not suitable for treatment.
In other aspects, the level of target expression is used to select a
individual as suitable for
treatment. Where the level of expression of the target is above a threshold
level, the
individual is determined to be suitable for treatment.
In some aspects, the presence of CD19 and/or in cells in the sample indicates
that the
individual is suitable for treatment with a combination comprising an anti-
CD19 ADC and
an anti-CD79b agent. In other aspects, the amount of CD19 and/or expression
must be
above a threshold level to indicate that the individual is suitable for
treatment. In some
aspects, the observation that CD19 and/or localisation is altered in the
sample as compared
to a control indicates that the individual is suitable for treatment.
In some aspects, an individual is indicated as suitable for treatment if cells
obtained from
lymph node or extra nodal sites react with antibodies against CD19 and/or as
determined
by IHC.
33
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
In some aspects, a patient is determined to be suitable for treatment if at
least 5%, 10%,
15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%
or more of all cells in the sample express CD19. In some aspects disclosed
herein, a
patient is determined to be suitable for treatment if at least at least 10% of
the cells in the
sample express CD19.
In some aspects, a patient is determined to be suitable for treatment if at
least 5%, 10%,
15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%
or more of all cells in the sample express. In some aspects disclosed herein,
a patient is
determined to be suitable for treatment if at least at least 10% of the cells
in the sample
express.
In some aspects, the individual is selected as suitable for treatment based on
their current
or previous treatment regime. In some embodiments the individual is selected
for treatment
with the anti-CD19 ADC if the individual has been treated with an anti-CD79b
agent. In
some embodiments the individual is selected for treatment with the anti-CD19
ADC if the
individual is being treated with an anti-CD79b agent. In some cases the
individual is
selected for treatment if they are refractory to treatment (or further
treatment) with the anti-
CD79b agent. In some cases the anti-CD79b agent may be Polatuzumab vedotin. In
embodiments where the individual is undergoing, or has undergone, treatment
with an anti-
CD79b agent, the anti-CD19 ADC may be administered in combination with an anti-
CD79b
agent, or without continued administration of the anti-CD79b agent.
In some embodiments the anti-CD19 ADC is administered to the selected
individual in
combination with an anti-CD79b agent. In some embodiments the anti-CD19 ADC is
administered to the selected individual without continued administration of an
anti-CD79b
agent. The anti-CD79b agent is preferably Polatuzumab vedotin.
The term 'refractory to treatment (or further treatment) with the anti-CD79b
agent' is used
herein to mean that the disorder (such as cancer) does not respond, or has
ceased to
respond, to administration of the anti-CD79b agent when administered as a
monotherapy.
In some embodiments, individuals with refractory NHL are identified using the
response
criteria disclosed in Cheson at al., 2014 (South Asian J Cancer. 2014 Jan-Mar;
3(1): 66-
70). In that document, non-responders are defined as individuals where there
is either (i) a
>50% increase from nadir in the sum product of diameters of any previously
identified
abnormal node, or (ii) an appearance of any new lesion during or at the end of
therapy. In
some embodiments, individuals with refractory leukaemia are identified as
individuals with
either stable or progressive disease who have completed one complete treatment
cycle, or
individual achieving partial response after two or more complete treatment
cycles.
CD25
In some aspects, subjects are selected on the basis they have a neoplasm
comprising both
CD25+ve and CD25-ve cells. The neoplasm may be composed of CD25-ve neoplastic
cells, optionally wherein the CD25-ve neoplastic cells are associated with
CD25+ve non-
neoplastic cells such as 0D25+ve Tregs. The neoplasm or neoplastic cells may
be all or
part of a solid tumour. The solid tumour may be partially or wholly CD25-ve,
and may be
34
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
infiltrated with CD25+ve cells, such as CD25+ve Tregs. In preferred aspects,
the solid
tumour is associated with high-levels of 0D25+ve infiltrating cells, such as
Treg cells. In
some aspects, the solid tumour is associated with low-levels of 0D25+ve
infiltrating cells,
such as Treg cells. In some aspects, the solid tumour is not associated with
0D25+ve
infiltrating cells, such as Treg cells; for example, the levels of CD25+ve
cells may be below
the detection limit.
Samples
The sample may comprise or may be derived from: a quantity of blood; a
quantity of serum
derived from the individual's blood which may comprise the fluid portion of
the blood
obtained after removal of the fibrin clot and blood cells; a quantity of
pancreatic juice; a
tissue sample or biopsy; or cells isolated from said individual.
A sample may be taken from any tissue or bodily fluid. In certain aspects, the
sample may
include or may be derived from a tissue sample, biopsy, resection or isolated
cells from
said individual.
In certain aspects, the sample is a tissue sample. The sample may be a sample
of tumor
tissue, such as cancerous tumor tissue. The sample may have been obtained by a
tumor
biopsy. In some aspects, the sample is a lymphoid tissue sample, such as a
lymphoid
lesion sample or lymph node biopsy. In some cases, the sample is a skin
biopsy.
In some aspects the sample is taken from a bodily fluid, more preferably one
that circulates
through the body. Accordingly, the sample may be a blood sample or lymph
sample. In
some cases, the sample is a urine sample or a saliva sample.
In some cases, the sample is a blood sample or blood-derived sample. The blood
derived
sample may be a selected fraction of a individual's blood, e.g. a selected
cell-containing
fraction or a plasma or serum fraction.
A selected cell-containing fraction may contain cell types of interest which
may include
white blood cells (WBC), particularly peripheral blood mononuclear cells (PBC)
and/or
granulocytes, and/or red blood cells (RBC). Accordingly, methods according to
the present
disclosure may involve detection of CD19 protein or nucleic acid in the blood,
in white blood
cells, peripheral blood mononuclear cells, granulocytes and/or red blood
cells.
The sample may be fresh or archival. For example, archival tissue may be from
the first
diagnosis of an individual, or a biopsy at a relapse. In certain aspects, the
sample is a fresh
biopsy.
Individual status
The individual may be an animal, mammal, a placental mammal, a marsupial
(e.g.,
kangaroo, wombat), a monotrenne (e.g., duckbilled platypus), a rodent (e.g., a
guinea pig,
a hamster, a rat, a mouse), murine (e.g., a mouse), a lagomorph (e.g., a
rabbit), avian (e.g.,
a bird), canine (e.g., a dog), feline (e.g., a cat), equine (e.g., a horse),
porcine (e.g., a pig),
ovine (e.g., a sheep), bovine (e.g., a cow), a primate, simian (e.g., a monkey
or ape), a
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
monkey (e.g., marmoset, baboon), an ape (e.g., gorilla, chimpanzee,
orangutang, gibbon),
or a human.
Furthermore, the individual may be any of its forms of development, for
example, a foetus.
In one preferred embodiment, the individual is a human. The terms "subject",
"patient" and
"individual" are used interchangeably herein.
In some aspects disclosed herein, an individual has, or is suspected as
having, or has been
identified as being at risk of, cancer. In some aspects disclosed herein, the
individual has
already received a diagnosis of cancer. The individual may have received a
diagnosis of
non-Hodgkin's Lymphoma, including diffuse large B-cell lymphoma (DLBCL),
follicular
lymphoma, (FL), Burkitt lymphoma, Mantle Cell lymphoma (MCL), chronic
lymphatic
lymphoma (CLL), Waldenstrom Macroglobulinemia (WM), and Marginal Zone B-cell
lymphoma (MZBL), and leukemias such as Hairy cell leukemia (HCL), Hairy cell
leukemia
variant (HCL-v), chronic lymphocytic leukemia (CLL) including Richter
syndrome, and
Acute Lymphoblastic Leukaemia (ALL) such as Philadelphia chromosome-positive
ALL
(Ph+ALL) or Philadelphia chromosome-negative ALL (Ph-ALL) .
In some cases, the individual has received a diagnosis of non-Hodgkin's
Lymphoma,
including diffuse large B-cell lymphoma (DLBCL), follicular lymphoma, (FL),
Burkitt
lymphoma, Mantle Cell lymphoma (MCL), chronic lymphatic lymphoma (CLL),
Waldenstrom Macroglobulinemia (WM), and Marginal Zone B-cell lymphoma (MZBL),
and
leukemias such as Hairy cell leukemia (HCL), Hairy cell leukemia variant (HCL-
v), chronic
lymphocytic leukemia (CLL) including Richter syndrome, and Acute Lymphoblastic
Leukaemia (ALL) such as Philadelphia chromosome-positive ALL (Ph+ALL) or
Philadelphia
chromosome-negative ALL (Ph-ALL) [Fielding A., Haematologica. 2010 Jan; 95(1):
8-12].
In some cases, the individual has received a diagnosis of a solid cancer
containing CD19+
expressing infiltrating cells.
The Individual may be undergoing, or have undergone, a therapeutic treatment
for that
cancer. The subject may, or may not, have previously received ADCX19. In some
cases
the cancer is lymphoma, including non-Hodgkins lymphoma.
The Individual may be undergoing, or have undergone, treatment with an anti-
CD79b
agent. In some cases the individual may be refractory to treatment (or further
treatment)
with the anti-CD79b agent. In some cases the anti-CD79b agent may be
Polatuzumab
vedotin. In embodiments where the individual is undergoing, or has undergone,
treatment
with an anti-CD79b agent, the anti-CD19 ADC may be administered in combination
with an
anti-CD79b agent, or without continued administration of the anti-CD79b agent.
Controls
In some aspects, target expression in the individual is compared to target
expression in a
control. Controls are useful to support the validity of staining, and to
identify experimental
artefacts.
36
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
In some cases, the control may be a reference sample or reference dataset. The
reference
may be a sample that has been previously obtained from a individual with a
known degree
of suitability. The reference may be a dataset obtained from analyzing a
reference sample.
Controls may be positive controls in which the target molecule is known to be
present, or
expressed at high level, or negative controls in which the target molecule is
known to be
absent or expressed at low level.
Controls may be samples of tissue that are from individuals who are known to
benefit from
the treatment. The tissue may be of the same type as the sample being tested.
For
example, a sample of tumor tissue from a individual may be compared to a
control sample
of tumor tissue from a individual who is known to be suitable for the
treatment, such as a
individual who has previously responded to the treatment.
In some cases the control may be a sample obtained from the same individual as
the test
sample, but from a tissue known to be healthy. Thus, a sample of cancerous
tissue from
a individual may be compared to a non-cancerous tissue sample.
In some cases, the control is a cell culture sample.
In some cases, a test sample is analyzed prior to incubation with an antibody
to determine
the level of background staining inherent to that sample.
In some cases an isotype control is used. Isotype controls use an antibody of
the same
class as the target specific antibody, but are not immunoreactive with the
sample. Such
controls are useful for distinguishing non-specific interactions of the target
specific
antibody.
The methods may include hematopathologist interpretation of morphology and
immunohistochemistry, to ensure accurate interpretation of test results. The
method may
involve confirmation that the pattern of expression correlates with the
expected pattern.
For example, where the amount of CD19 and/or CD79b expression is analyzed, the
method
may involve confirmation that in the test sample the expression is observed as
membrane
staining, with a cytoplasmic component. The method may involve confirmation
that the
ratio of target signal to noise is above a threshold level, thereby allowing
clear
discrimination between specific and non-specific background signals.
Methods of Treatment
The term "treatment," as used herein in the context of treating a condition,
pertains
generally to treatment and therapy, whether of a human or an animal (e.g., in
veterinary
applications), in which some desired therapeutic effect is achieved, for
example, the
inhibition of the progress of the condition, and includes a reduction in the
rate of progress,
a halt in the rate of progress, regression of the condition, amelioration of
the condition, and
cure of the condition. Treatment as a prophylactic measure (i.e., prophylaxis,
prevention)
is also included.
37
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
The term "therapeutically-effective amount" or "effective amount" as used
herein, pertains
to that amount of an active compound, or a material, composition or dosage
from
comprising an active compound, which is effective for producing some desired
therapeutic
effect, commensurate with a reasonable benefit/risk ratio, when administered
in
accordance with a desired treatment regimen.
Similarly, the term "prophylactically-effective amount," as used herein,
pertains to that
amount of an active compound, or a material, composition or dosage from
comprising an
active compound, which is effective for producing some desired prophylactic
effect,
commensurate with a reasonable benefit/risk ratio, when administered in
accordance with
a desired treatment regimen.
Disclosed herein are methods of therapy. Also provided is a method of
treatment,
comprising administering to a subject in need of treatment a therapeutically-
effective
amount of an anti-0D19 ADC and an anti-CD79b agent. The term "therapeutically
effective
amount" is an amount sufficient to show benefit to a subject. Such benefit may
be at least
amelioration of at least one symptom. The actual amount administered, and rate
and time-
course of administration, will depend on the nature and severity of what is
being treated.
Prescription of treatment, e.g. decisions on dosage, is within the
responsibility of general
practitioners and other medical doctors. The subject may have been tested to
determine
their eligibility to receive the treatment according to the methods disclosed
herein. The
method of treatment may comprise a step of determining whether a subject is
eligible for
treatment, using a method disclosed herein.
The anti-CD19 ADC comprises an anti-0D19 antibody. The anti-CD19 antibody may
be
RB4v1.2 antibody. The ADC may comprise a drug which is a PBD dimer. The ADC
may
be ADCx19. The ADC may be an ADC disclosed in W02014/057117.
The anti-0D25 ADC comprises an anti-0D25 antibody. The anti-0D25 antibody may
be
HuMax-TACTm. The ADC may comprise a drug which is a PBD dimer. The ADC may be
an anti-CD25-ADC, and in particular, ADCX25 or camidanlumab tesirine. The ADC
may
be an ADC disclosed in W02014/057119.
The anti-CD22 ADC comprises an anti-CD22 antibody. The anti-CD22 antibody may
be
EMabC220. The ADC may comprise a drug which is a PBD dimer. The ADC may be an
anti-CD22-ADC such as ADCT-602 or ADCx22. The ADC may be an ADC disclosed in
W02014/057122 or W02016/166307.
Typically the an individual to which the treatment disclosed herein is
administered is in need
of said treatment, or has been identified or diagnosed as in need of the
treatment.
The anti-CD79b agent may be Polatuzunnab vedotin.
38
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
The treatment may involve administration of the anti-CD19 ADC / anti-CD79b
agent
combination alone or in further combination with other treatments, either
simultaneously or
sequentially dependent upon the condition to be treated.
An example method of treatment involves:
(1) identifying an individual has been treated with, or is being treated with
an anti-
CD79b agent, such as Polatuzumab vedotin;
(2) administering to the individual an anti-CD19 ADC, such as ADCx19; and,
optionally
(3) administering to the individual an anti-CD79b agent, such as Polatuzumab
vedotin in combination with the anti-CD19 ADC (for example, at the same time
as the ADC,
or after the ADC).
Examples of treatments and therapies include, but are not limited to,
chemotherapy (the
administration of active agents, including, e.g. drugs, such as
chemotherapeutics); surgery;
and radiation therapy.
A "chemotherapeutic agent" is a chemical compound useful in the treatment of
cancer,
regardless of mechanism of action. Classes of chemotherapeutic agents include,
but are
not limited to: alkylating agents, antimetabolites, spindle poison plant
alkaloids,
cytotoxic/antitumor antibiotics, topoisomerase inhibitors, antibodies,
photosensitizers, and
kinase inhibitors. Chemotherapeutic agents include compounds used in "targeted
therapy"
and conventional chemotherapy.
Examples of chemotherapeutic agents include: Lenalidomide (REVLIMID ,
Celgene),
Vorinostat (ZOLINZA , Merck), Panobinostat (FARYDAKO, Novartis), Mocetinostat
(MGCD0103), Everolimus (ZORTRESS , CERTICAN , Novartis), Bendamustine
(TREAKISYM , RIBOMUSTIN , LEVACT , TREANDA , Mundipharma International),
erlotinib (TARCEVA , Genentech/OSI Pharm.), docetaxel (TA)(OTERE , Sanofi-
Aventis),
5-FU (fluorouracil, 5-fluorouracil, CAS No. 51-21-8), gemcitabine (GEMZAR ,
Lilly), PD-
0325901 (CAS No. 391210-10-9, Pfizer), cisplatin (cis-diamine,
dichloroplatinum(II), CAS
No. 15663-27-1), carboplatin (CAS No. 41575-94-4), paclitaxel (TAXOL , Bristol-
Myers
Squibb Oncology, Princeton, N.J.), trastuzumab (HERCEPTIN , Genentech),
temozolomide (4-methyl-5-oxo- 2,3,4,6,8-pentazabicyclo [4.3.0] nona-2,7,9-
triene- 9-
carboxamide, CAS No. 85622-93-1, TEMODAR , TEMODAL , Schering Plough),
tamoxifen
((Z)-2-[4-(1,2-diphenylbut-1-enyl)phenoxy]-N,N-dimethylethanamine,
NOLVADEX , ISTUBAL , VALODEX0), and doxorubicin (ADRIAMYCINO), Akti-1/2,
HPPD, and rapamycin.
More examples of chemotherapeutic agents include: oxaliplatin (ELOXATIN ,
Sanofi),
bortezomib (VELCADE , Millennium Pharm.), sutent (SUNITINIB , SU11248,
Pfizer),
letrozole (FEMARA , Novartis), imatinib mesylate (GLEEVEC , Novartis), XL-518
(Mek
inhibitor, Exelixis, WO 2007/044515), ARRY-886 (Mek inhibitor, AZD6244, Array
BioPharma, Astra Zeneca), SF-1126 (anti-CD79b agent, Semafore
Pharmaceuticals),
BEZ-235 (anti-CD79b agent, Novartis), XL-147 (anti-CD79b agent, Exelixis),
PTK787/ZK
222584 (Novartis), fulvestrant (FASLODEX , AstraZeneca), leucovorin (folinic
acid),
39
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
rapamycin (sirolimus, RAPAMUNE , Wyeth), lapatinib (TYKERB , GSK572016, Glaxo
Smith Kline), lonafarnib (SARASARTM, SCH 66336, Schering Plough), sorafenib
(NEXAVAR , BAY43-9006, Bayer Labs), gefitinib (IRESSA , AstraZeneca),
irinotecan
(CAMPTOSAR , CPT-11, Pfizer), tipifarnib (ZARNESTRATm, Johnson & Johnson),
ABRAXANETM (Cremophor-free), albumin-engineered nanoparticle formulations of
paclitaxel (American Pharmaceutical Partners, Schaunnberg, II), vandetanib
(rINN,
ZD6474, ZACTIMA , AstraZeneca), chloranmbucil, AG1478, AG1571 (SU 5271;
Sugen),
temsirolimus (TORISEL , Wyeth), pazopanib (GlaxoSmithKline), canfosfamide
(TELCYTA , Telik), thiotepa and cyclosphosphamide (CYTOXAN , NEOSARO); alkyl
sulfonates such as busulfan, improsulfan and piposulfan; aziridines such as
benzodopa,
carboquone, nneturedopa, and uredopa; ethylenimines and methylamelamines
including
altretamine, triethylenemelamine, triethylenephosphoramide,
triethylenethio
phosphoramide and trimethylomelamine; acetogenins (especially bullatacin and
bullatacinone); a camptothecin (including the synthetic analog topotecan);
bryostatin;
callystatin; CC-1065 (including its adozelesin, carzelesin and bizelesin
synthetic analogs);
cryptophycins (particularly cryptophycin 1 and cryptophycin 8); dolastatin;
duocarmycin
(including the synthetic analogs, KW-2189 and CBI-TM1); eleutherobin;
pancratistatin; a
sarcodictyin; spongistatin; nitrogen mustards such as chlorambucil,
chlornaphazine,
chlorophosphamide, estramustine, ifosfamide, mechlorethamine, mechlorethamine
oxide
hydrochloride, melphalan, novembichin, phenesterine, prednimustine,
trofosfamide, uracil
mustard; nitrosoureas such as carmustine, chlorozotocin, fotemustine,
lomustine,
nimustine, and ranimnustine; antibiotics such as the enediyne antibiotics
(e.g.
calicheamicin, calicheamicin gammal I, calicheamicin omegal1 (Angew Chem.
Intl. Ed.
Engl. (1994) 33:183-186); dynemicin, dynemicin A; bisphosphonates, such as
clodronate;
an esperamicin; as well as neocarzinostatin chromophore and related
chromoprotein
enediyne antibiotic chromophores), aclacinomysins, actinomycin, authramycin,
azaserine,
bleomycins, cactinomycin, carabicin, carminomycin, carzinophilin,
chromomycinis,
dactinomycin, daunorubicin, detorubicin, 6-diazo-5-oxo-L-norleucine,
morpholino-
doxorubicin, cyanomorpholino-doxorubicin, 2-pyrrolino-
doxorubicin and
deoxydoxorubicin), epirubicin, esorubicin, idarubicin, nemorubicin,
marcellomycin,
mitomycins such as mitomycin C, mycophenolic acid, nogalamycin, olivomycins,
peplomycin, porfiromycin, puromycin, quelamycin, rodorubicin, streptonigrin,
streptozocin,
tubercidin, ubenimex, zinostatin, zorubicin; anti-metabolites such as
methotrexate and 5-
fluorouracil (5-FU); folic acid analogs such as denopterin, methotrexate,
pteropterin,
trimetrexate; purine analogs such as fludarabine, 6-mercaptopurine,
thiamiprine,
thioguanine; pyrimidine analogs such as ancitabine, azacitidine, 6-azauridine,
carmofur,
cytarabine, dideoxyuridine, doxifluridine, enocitabine, floxuridine; androgens
such as
calusterone, dromostanolone propionate, epitiostanol, nnepitiostane,
testolactone; anti-
adrenals such as aminoglutethimide, mitotane, trilostane; folic acid
replenisher such as
frolinic acid; aceglatone; aldophosphamide glycoside; aminolevulinic acid;
eniluracil;
amsacrine; bestrabucil; bisantrene; edatraxate; defofamine; demecolcine;
diaziquone;
elfornithine; elliptinium acetate; an epothilone; etoglucid; gallium nitrate;
hydroxyurea;
lentinan; lonidainine; nnaytansinoids such as nnaytansine and ansannitocins;
nnitoguazone;
mitoxantrone; mopidanmol; nitraerine; pentostatin; phenamet; pirarubicin;
losoxantrone;
podophyllinic acid; 2-ethylhydrazide; procarbazine; PSK polysaccharide
complex (JHS
Natural Products, Eugene, OR); razoxane; rhizoxin; sizofiran; spirogermanium;
tenuazonic
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
acid; triaziquone; 2,2',2"-trichlorotriethylamine; trichothecenes
(especially T-2 toxin,
verracurin A, roridin A and anguidine); urethan; vindesine; dacarbazine;
mannomustine;
nnitobronitol; nnitolactol; pipobronnan; gacytosine;
arabinoside ("Ara-C");
cyclophosphamide; thiotepa; 6-thioguanine; mercaptopurine; methotrexate;
platinum
analogs such as cisplatin and carboplatin; vinblastine; etoposide (VP-16);
ifosfamide;
nnitoxantrone; vincristine; vinorelbine (NAVELBINE8); novantrone; teniposide;
edatrexate;
daunomycin; aminopterin; capecitabine (XELODA , Roche); ibandronate; CPT-11;
topoisomerase inhibitor RFS 2000; difluoromethylornithine (DMF0); retinoids
such as
retinoic acid; and pharmaceutically acceptable salts, acids and derivatives of
any of the
above. Combinations of agents may be used, such as CHP (doxorubicin,
prednisone,
cyclophosphamide), or CHOP (doxorubicin, prednisone, cyclophopsphamide,
vincristine).
Also included in the definition of "chemotherapeutic agent" are: (i) anti-
hormonal agents
that act to regulate or inhibit hormone action on tumors such as anti-
estrogens and
selective estrogen receptor modulators (SERMs), including, for example,
tamoxifen
(including NOLVADEXO; tamoxifen citrate), raloxifene, droloxifene, 4-
hydroxytamoxifen,
trioxifene, keoxifene, LY117018, onapristone, and FARESTON (toremifine
citrate); (ii)
aromatase inhibitors that inhibit the enzyme aromatase, which regulates
estrogen
production in the adrenal glands, such as, for example, 4(5)-imidazoles,
aminoglutethimide,
MEGASE (megestrol acetate), AROMASINO (exemestane; Pfizer), formestanie,
fadrozole, RIVISOR (vorozole), FEMARA (letrozole; Novartis), and ARIMIDEX
(anastrozole; AstraZeneca); (iii) anti-androgens such as flutamide,
nilutamide,
bicalutamide, leuprolide, and goserelin; as well as troxacitabine (a 1,3-
dioxolane
nucleoside cytosine analog); (iv) protein kinase inhibitors such as MEK
inhibitors (WO
2007/044515); (v) lipid kinase inhibitors; (vi) antisense oligonucleotides,
particularly those
which inhibit expression of genes in signaling pathways implicated in aberrant
cell
proliferation, for example, PKC-alpha, Raf and H-Ras, such as oblimersen
(GENASENSE , Genta Inc.); (vii) ribozymes such as VEGF expression inhibitors
(e.g.,
ANGIOZYMEO) and HER2 expression inhibitors; (viii) vaccines such as gene
therapy
vaccines, for example, ALLOVECTIN , LEUVECTIN , and VAXIDO; PROLEUKIN rIL-
2; topoisomerase 1 inhibitors such as LURTOTECANO; ABARELIX rmRH; (ix) anti-
angiogenic agents such as bevacizumab (AVASTIN , Genentech); and
pharmaceutically
acceptable salts, acids and derivatives of any of the above.
Also included in the definition of "chemotherapeutic agent" are therapeutic
antibodies such
as alemtuzumab (Campath), bevacizumab (AVASTIN , Genentech); cetuximab
(ERBITUX , Imclone); panitumumab (VECTIBIX , Amgen), pertuzumab (PERJETATm,
OMNITARGTm, 2C4, Genentech), trastuzumab (HERCEPTIN , Genentech), MDX-060
(Medarex) and the antibody drug conjugate, gemtuzumab ozogannicin (MYLOTARG ,
Wyeth).
Humanized monoclonal antibodies with therapeutic potential as chemotherapeutic
agents
in combination with the conjugates of the disclosure include: alenntuzunnab,
apolizunnab,
aselizumab, atlizumab, bapineuzumab, bevacizumab, bivatuzumab mertansine,
cantuzumab mertansine, cedelizumab, certolizumab pegol, cidfusituzumab,
cidtuzumab,
daclizumab, eculizumab, efalizumab, epratuzumab, erlizumab, felvizumab,
fontolizumab,
41
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
gemtuzumab ozogamicin, inotuzumab ozogamicin, ipilimumab, labetuzumab,
lintuzumab,
matuzumab, mepolizumab, motavizumab, motovizumab, natalizumab, nimotuzumab,
nolovizunnab, nunnavizunnab, onnalizunnab, palivizunnab, pascolizunnab,
pecfusituzunnab,
pectuzumab, pertuzumab, pexelizumab, ralivizumab, ranibizumab, reslivizumab,
reslizumab, resyvizumab, rovelizumab, ruplizumab, sibrotuzumab, siplizumab,
sontuzunnab, tacatuzunnab tetraxetan, tadocizunnab, talizunnab, tefibazunnab,
tocilizunnab,
toralizumab, trastuzumab, tucotuzumab celmoleukin, tucusituzumab, umavizumab,
urtoxazumab, and visilizumab.
Compositions according to the present disclosure are preferably pharmaceutical
compositions. Pharmaceutical compositions according to the present disclosure,
and for
use in accordance with the present disclosure, may comprise, in addition to
the active
ingredient, i.e. a conjugate compound, a pharmaceutically acceptable
excipient, carrier,
buffer, stabiliser or other materials well known to those skilled in the art.
Such materials
should be non-toxic and should not interfere with the efficacy of the active
ingredient. The
precise nature of the carrier or other material will depend on the route of
administration,
which may be oral, or by injection, e.g cutaneous, subcutaneous, or
intravenous.
Pharmaceutical compositions for oral administration may be in tablet, capsule,
powder or
liquid form. A tablet may comprise a solid carrier or an adjuvant. Liquid
pharmaceutical
compositions generally comprise a liquid carrier such as water, petroleum,
animal or
vegetable oils, mineral oil or synthetic oil. Physiological saline solution,
dextrose or other
saccharide solution or glycols such as ethylene glycol, propylene glycol or
polyethylene
glycol may be included. A capsule may comprise a solid carrier such a gelatin.
For intravenous, cutaneous or subcutaneous injection, or injection at the site
of affliction,
the active ingredient will be in the form of a parenterally acceptable aqueous
solution which
is pyrogen-free and has suitable pH, isotonicity and stability. Those of
relevant skill in the
art are well able to prepare suitable solutions using, for example, isotonic
vehicles such as
Sodium Chloride Injection, Ringer's Injection, Lactated Ringer's Injection.
Preservatives,
stabilisers, buffers, antioxidants and/or other additives may be included, as
required.
Dosage
It will be appreciated by one of skill in the art that appropriate dosages of
the anti-CD19
ADC and/or the anti-CD79b agent, and compositions comprising these active
elements,
can vary from subject to subject. Determining the optimal dosage will
generally involve the
balancing of the level of therapeutic benefit against any risk or deleterious
side effects. The
selected dosage level will depend on a variety of factors including, but not
limited to, the
activity of the particular compound, the route of administration, the time of
administration,
the rate of excretion of the compound, the duration of the treatment, other
drugs,
compounds, and/or materials used in combination, the severity of the
condition, and the
species, sex, age, weight, condition, general health, and prior medical
history of the subject.
The amount of compound and route of administration will ultimately be at the
discretion of
the physician, veterinarian, or clinician, although generally the dosage will
be selected to
achieve local concentrations at the site of action which achieve the desired
effect without
causing substantial harmful or deleterious side-effects.
42
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
In certain aspects, the dosage of anti-0D19 ADC is determined by the
expression of 0D19
observed in a sample obtained from the subject. Thus, the level or
localisation of
expression of 0D19 in the sample may be indicative that a higher or lower dose
of
anti-CD19 ADC is required. For example, a high expression level of CD19 may
indicate
that a higher dose of anti-CD19 ADC would be suitable. In some cases, a high
expression
level of CD19 may indicate the need for administration of another agent in
addition to the
anti-CD19 ADC. For example, administration of the anti-CD19 ADC in conjunction
with a
chemotherapeutic agent. A high expression level of CD19 may indicate a more
aggressive
therapy.
In certain aspects, the dosage of the anti-CD79b agent is determined by the
expression of
observed in a sample obtained from the subject. Thus, the level or
localisation of
expression of in the sample may be indicative that a higher or lower dose of
anti-CD79b
agent is required. For example, a high expression level of CD79b may indicate
that a
higher dose of anti-CD79b agent would be suitable. In some cases, a high
expression
level of CD79b may indicate the need for administration of another agent in
addition to the
anti-CD79b agent. For example, administration of the anti-CD79b agent in
conjunction with
a chemotherapeutic agent. A high expression level of CD79b may indicate a more
aggressive therapy.
Administration can be effected in one dose, continuously or intermittently
(e.g., in divided
doses at appropriate intervals) throughout the course of treatment. Methods of
determining
the most effective means and dosage of administration are well known to those
of skill in
the art and will vary with the formulation used for therapy, the purpose of
the therapy, the
target cell(s) being treated, and the subject being treated. Single or
multiple administrations
can be carried out with the dose level and pattern being selected by the
treating physician,
veterinarian, or clinician.
In general, a suitable dose of each active compound is in the range of about
100 ng to
about 25 mg (more typically about 1 pg to about 10 mg) per kilogram body
weight of the
subject per day. Where the active compound is a salt, an ester, an amide, a
prodrug, or
the like, the amount administered is calculated on the basis of the parent
compound and
so the actual weight to be used is increased proportionately.
In one embodiment, each active compound is administered to a human subject
according
to the following dosage regime: about 100 mg, 3 times daily.
In one embodiment, each active compound is administered to a human subject
according
to the following dosage regime: about 150 mg, 2 times daily.
In one embodiment, each active compound is administered to a human subject
according
to the following dosage regime: about 200 mg, 2 times daily.
43
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
However in one embodiment, each conjugate compound is administered to a human
subject according to the following dosage regime: about 50 or about 75 mg, 3
or 4 times
daily.
In one embodiment, each conjugate compound is administered to a human subject
according to the following dosage regime: about 100 or about 125 mg, 2 times
daily.
For the anti-CD19 ADC, where it is a PBD bearing ADC, the dosage amounts
described
above may apply to the conjugate (including the PBD moiety and the linker to
the antibody)
or to the effective amount of PBD compound provided, for example the amount of
compound that is releasable after cleavage of the linker.
The anti-CD19 ADC comprises an anti-CD19 antibody. The anti-CD19 antibody may
be
RB4v1.2 antibody. The ADC may comprise a drug which is a PBD dimer. The anti-
CD19-
ADC may be ADCx19. The anti-CD19 ADC may be Loncastuximab tesirine. The ADC
may
be an ADC disclosed in W02014/057117.
The anti-CD79b agent may be Polatuzumab vedotin.
Antibodies
The term "antibody" herein is used in the broadest sense and specifically
covers
monoclonal antibodies, polyclonal antibodies, dimers, multimers, multispecific
antibodies
(e.g., bispecific antibodies), intact antibodies (also described as "full-
length" antibodies)
and antibody fragments, so long as they exhibit the desired biological
activity, for example,
the ability to bind CD19 (Miller et al (2003) Jour. of Immunology 170:4854-
4861).
Antibodies may be murine, human, humanized, chimeric, or derived from other
species
such as rabbit, goat, sheep, horse or camel.
Brief Description of the Figures
Embodiments and experiments illustrating the principles of the disclosure will
now be
discussed with reference to the accompanying figures in which:
Figure 1. Plot of median tumour volume showing in vivo efficacy of a ADCx19
and
Pola-V combination
The disclosure includes the combination of the aspects and preferred features
described
except where such a combination is clearly impermissible or expressly avoided.
The section headings used herein are for organizational purposes only and are
not to be
construed as limiting the subject matter described.
Aspects and embodiments of the present disclosure will now be illustrated, by
way of
example, with reference to the accompanying figures. Further aspects and
embodiments
44
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
will be apparent to those skilled in the art. All documents mentioned in this
text are
incorporated herein by reference.
Throughout this specification, including the claims which follow, unless the
context requires
otherwise, the word "comprise," and variations such as "comprises" and
"comprising," will
be understood to imply the inclusion of a stated integer or step or group of
integers or steps
but not the exclusion of any other integer or step or group of integers or
steps.
As used in the specification and the appended claims, the singular forms "a,"
"an," and "the"
include plural referents unless the context clearly dictates otherwise. Ranges
may be
expressed herein as from "about" one particular value, and/or to "about"
another particular
value. When such a range is expressed, another embodiment includes from the
one
particular value and/or to the other particular value. Similarly, when values
are expressed
as approximations, by the use of the antecedent "about," it will be understood
that the
particular value forms another embodiment.
STATEMENTS OF INVENTION
1. A method for treating a disorder in an individual, the method comprising
administering to the individual an effective amount of an anti-CD19 ADC (or
anti-CD22 ADC
or anti-CD25 ADC) and an anti-CD79b agent.
2. The method according to statement 1, wherein the individual is selected
for
treatment.
3. The method according to statement 2, wherein the individual is selected
for
treatment with an anti-CD19 ADC (or anti-CD22 ADC or anti-0D25 ADC) if the
individual
has been treated with an anti-CD79b agent.
4. The method according to statement 2, where the individual is selected
for treatment
with an anti-CD19 ADC (or anti-CD22 ADC or anti-CD25 ADC) if the individual is
being
treated with an anti-CD79b agent.
5. The method according to any one of the preceding
statements, wherein the
individual is selected for treatment if the individual is refractory to
treatment, or further
treatment, with the anti-CD79b agent.
6. A method for treating a disorder in an individual, the
method comprising:
(i) selecting an individual as suitable for treatment by a method according to
any
one of statements 3 to 5; and
(ii) administering to the individual an effective amount of an anti-CD19 ADC
(or anti-
CD22 ADC or anti-CD25 ADC).
7. The method according to statement 6, further comprising
administering an anti-
CD79b agent in combination with the anti-CD19 ADC (or anti-CD22 ADC or anti-
CD25
ADC).
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
8. The method according to any one of statements 1 to 5 or 7, wherein the
treatment
comprises administering the anti-CD19 ADC (or anti-0D22 ADC or anti-CD25 ADC)
before
the anti-CD79b agent, simultaneous with the anti-CD79b agent, or after the
anti-CD79b
agent.
9. The method according to any preceding statement, wherein the treatment
further
comprises administering a chemotherapeutic agent.
10. The method
according to any preceding statement, wherein the individual is human.
11.
The method according to any preceding statement, wherein the individual
has a
disorder or has been determined to have a disorder.
12. The method
according to statement 11, wherein the individual has, or has been
determined to have, a cancer which expresses CD19 (or CD22 or 0D25) or CD19+ve
(or
CD22+ve or CD25+ve) tumour-associated non-tumour cells, such as CD19+ve (or
CD22+ve or CD25+ve) infiltrating cells.
13. The method
according to any preceding statement, wherein the individual is
undergoing treatment with an anti-CD79b agent.
14. The method according to any preceding statement, wherein the individual
has
undergone treatment with an anti-CD79b agent.
15. The method according to any preceding statement, wherein the individual
is
refractory to treatment, or further treatment, with the anti-CD79b agent.
16. The method according to any one of the preceding statements, wherein
the
treatment has increased efficacy as compared to monotherapy with either an
anti-CD19
ADC (or anti-CD22 ADC or anti-CD25 ADC) or an anti-CD79b agent alone.
17. The method according to any preceding statement, wherein the disorder
is a
proliferative disease.
18. The method of statement 17, wherein the disorder is characterised by
the presence
of a neoplasm comprising CD19+ve cells (or CD22+ve cells or CD25+ve cells).
19. The method of statement 17, wherein the individual has, or has been has
been
determined to have, a disorder characterised by the presence of a neoplasm
comprising
both CD25+ve and CD25-ve cells.
20. The method of statement 17, wherein the individual has, or has been has
been
determined to have, a disorder characterised by the presence of a neoplasm
comprising,
or composed of, CD25-ve neoplastic cells.
46
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
21. The method according to any of statements 18 to 20, wherein the
neoplasm is all
or part of a solid tumour.
22. The method of statement 21, wherein the solid tumour is associated with
CD25+ve infiltrating cells;
optionally wherein the solid tumour is associated with high levels of 0D25+ve
infiltrating cells.
23. The method of statement 21 or 122, wherein the solid tumour is selected
from the
group consisting of pancreatic cancer, breast cancer (including triple
negative breast
cancer), colorectal cancer, gastric and oesophageal cancer, melanoma, non-
small cell
lung cancer, ovarian cancer, hepatocellular carcinoma, renal cell carcinoma,
bladder, and
head and neck cancer.
24. The method of any preceding statement, wherein the disorder is cancer.
25. The method of any preceding statement , wherein the disorder is
selected from the
group comprising: non-Hodgkin's Lymphoma, including diffuse large B-cell
lymphoma
(DLBCL), follicular lymphoma, (FL), Burkitt lymphoma, Mantle Cell lymphoma
(MCL),
chronic lymphatic lymphoma (CLL), Waldenstrbm Macroglobulinemia (WM), and
Marginal
Zone B-cell lymphoma (MZBL), and leukemias such as Hairy cell leukemia (HCL),
Hairy
cell leukemia variant (HCL-v), chronic lymphocytic leukemia (CLL) including
Richter
syndrome, and Acute Lynnphoblastic Leukaemia (ALL) such as Philadelphia
chromosome-
positive ALL (Ph+ALL) or Philadelphia chromosome-negative ALL (Ph-ALL).
26. The method of any preceding statement, wherein the anti-CD79b agent is
an anti-
CD79b ADC
27. The method of statement 26, wherein the CD79b ADC is a conjugate of
formula (I):
Ab ¨ (DL) p (I)
wherein:
Ab is an antibody that binds to CD79b;
DL is
47
CA 03220935 2023- 11- 30
WO 2023/274974 PCT/EP2022/067603
o
yN H2
0
N H
Et , ,,,
0
= H
0 00
and p is between 1 and 8, such as between 3 and 4, for example about 3.5.
28. The method of any preceding statement, wherein the anti-CD79b agent or
ADC
comprises an antibody having a VH domain comprising a VH CDR1, a VH CDR2, and
a
VH CDR3, wherein the antibody comprises the CDR sequences of the VH domain
having
the sequence according to SEQ ID NO: 17.
29. The method of statement 28, wherein the antibody further comprises a VL
domain
comprising a VL CDR1, a VL CDR2, and a VL CDR3, wherein the antibody comprises
the
CDR sequences of the VL domain having the sequence according to SEQ ID NO: 18.
30. The method of any one of statements 1 to 27, wherein the anti-CD79b
agent or
ADC comprises an antibody having a VH domain and a VL domain,
the VH domain having a VH CDR1 with the amino acid sequence of SEQ ID NO.19,
a VH CDR2 with the amino acid sequence of SEQ ID NO.20, and a VH CDR3 with the
amino acid sequence of SEQ ID NO.21; and
the VL domain having a VL CDR1 with the amino acid sequence of SEQ ID NO.22,
a VL CDR2 with the amino acid sequence of SEQ ID NO.23, and a VL CDR3 with the
amino
acid sequence of SEQ ID NO.24.
31. The method of any one of statements 1 to 27, wherein the anti-CD79b
agent or
ADC comprises an antibody having a VH domain having the sequence according to
SEQ
ID NO: 17, and a VL domain having the sequence according to SEQ ID NO: 18.
32. The method of any one of statements 1 to 27, wherein the anti-CD79b
agent is
polatuzumab vedotin.
48
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
33. The method according to any preceding statement, wherein
the anti-CD19 ADC
(or anti-0D22 ADC or anti-0D25 ADC) comprises a conjugate of formula L -
(DL)p, where
DL is of formula I or II:
0
21 R2 R9
R9 RI L1'
R11 a
Rucz N R7'
R7 N C2 2
C3' 0 R6'
R6 0 C3
9, 10
R31 R R9 RI R11
N
'R"
N I R7' R7
R22
II
C3' 0 R6'
R6 0
wherein:
L is an antibody (Ab) which is an antibody that binds to 0D19 (or 0D22 or
0D25);
when there is a double bond present between C2' and 03', R12 is selected from
the group consisting of:
(ia) 05_10 aryl group, optionally substituted by one or more substituents
selected from the
group comprising: halo, nitro, cyano, ether, carboxy, ester, 01.7 alkyl, 03-7
heterocycly1 and
bis-oxy-01_3 alkylene;
(ib) 01_5 saturated aliphatic alkyl;
(ic) 03_6 saturated cycloalkyl;
R22
,21
(id) r` , wherein each of R21, R22 and R23 are independently selected
from H,
01_3 saturated alkyl, 02-3 alkenyl, C2_3 alkynyl and cyclopropyl, where the
total number of
carbon atoms in the R12 group is no more than 5;
R25b
--R25a
(ie) , wherein one of R25a and R25b is H and the other
is selected from:
phenyl, which phenyl is optionally substituted by a group selected from halo,
methyl,
methoxy; pyridyl; and thiophenyl; and
(if) R24 , where R24 is selected from: H; C13 saturated
alkyl; C2_3 alkenyl; C2_3
alkynyl; cyclopropyl; phenyl, which phenyl is optionally substituted by a
group selected
from halo, methyl, methoxy; pyridyl; and thiophenyl;
when there is a single bond present between 02' and 03',
49
CA 03220935 2023- 11- 30
WO 2023/274974 PCT/EP2022/067603
R12 is R26b , where R26a and R26b are independently
selected from H, F, C1-4
saturated alkyl, 02-3 alkenyl, which alkyl and alkenyl groups are optionally
substituted by a
group selected from C1-4 alkyl amido and C1_4 alkyl ester; or, when one of R26
and R2613 is
H, the other is selected from nitrile and a C1-4 alkyl ester;
R6 and R9 are independently selected from H, R, OH, OR, SH, SR, NH2, NHR,
NRR',
nitro, Me3Sn and halo;
where R and R' are independently selected from optionally substituted 01-12
alkyl, 03-20
heterocyclyl and 06-20 aryl groups;
R7 is selected from H, R, OH, OR, SH, SR, NH2, NHR, NHRR', nitro, Me3Sn and
halo;
R" is a C3-12 alkylene group, which chain may be interrupted by one or more
heteroatoms,
e.g. 0, S, NRN2 (where RN2 is H or C1-4 alkyl), and/or aromatic rings, e.g.
benzene or
pyridine;
Y and Y' are selected from 0, S, or NH;
R6', R7', R9' are selected from the same groups as R6, R7 and R9 respectively;
[Formula 11
RI-1' is a linker for connection to the antibody (Ab);
R11a is selected from OH, ORA, where RA is 01_4 alkyl, and SOzM, where z is 2
or 3 and M
is a monovalent pharmaceutically acceptable cation;
R29 and R21 either together form a double bond between the nitrogen and carbon
atoms
to which they are bound or;
R29 is selected from H and Rc, where Rc is a capping group;
R21 is selected from OH, ORA and SOLNA;
when there is a double bond present between 02 and 03, R2 is selected from the
group
consisting of:
(ia) C6-10 aryl group, optionally substituted by one or more substituents
selected from the
group comprising: halo, nitro, cyano, ether, carboxy, ester, 01.7 alkyl, 03-7
heterocyclyl and
bis-oxy-C1_3 alkylene;
(ib) 01_5 saturated aliphatic alkyl;
(ic) 03-6 saturated cycloalkyl;
R12
R13
11
(id) R , wherein each of R1i, R12 and R13 are independently selected
from H,
01_3 saturated alkyl, 02_3 alkenyl, 02_3 alkynyl and cyclopropyl, where the
total number of
carbon atoms in the R2 group is no more than 5;
R15b
R15a
(ie) , wherein one of R15a and R151a is H and the
other is selected from:
phenyl, which phenyl is optionally substituted by a group selected from halo,
methyl,
methoxy; pyridyl; and thiophenyl; and
CA 03220935 2023-11-30
WO 2023/274974
PCT/EP2022/067603
(if) 'sR14 7 where R14 is selected from: H; 01_3 saturated alkyl; 02_3
alkenyl; 02_3
alkynyl; cyclopropyl; phenyl, which phenyl is optionally substituted by a
group selected
from halo, methyl, methoxy; pyridyl; and thiophenyl;
when there is a single bond present between 02 and 03,
1µR16a
116b
R2 is R 7 where R16a and R16b are independently selected from H7 F7 C1_4
saturated alkyl, 02_3 alkenyl, which alkyl and alkenyl groups are optionally
substituted by a
group selected from 01_4 alkyl amido and 01_4 alkyl ester; or, when one of
R16a and R166 is
H, the other is selected from nitrile and a C1-4 alkyl ester;
[Formula H]
R22 is of formula Illa, formula Illb or formula 111c:
2..X Illa
(a) Q Q
where A is a 05_7 aryl group, and either
(i) Q1 is a single bond, and Q2 is selected from a single bond and -Z-(CH2),-,
where Z is
selected from a single bond, 0, S and NH and n is from 1 to 3; or
(ii) Q1 is -CH=CH-7 and Q2 is a single bond;
C2
f
Illb (fc:Irc3X
(b) R R
where;
Rc17 RC2 and Rc3 are independently selected from H and unsubstituted 01_2
alkyl;
IIIc
(c)
where Q is selected from 0-R1_2', S-R'2' and NRN-R1-2', and RN is selected
from H7 methyl
and ethyl
X is selected from the group comprising: 0-R1-2', S-R1-2', 002-R1-2',
NH-C(=0)-R1-2',
\N RL2' 1¨N/¨\N¨R1-2
NHNH-R1-2', CONHNH-R1-2',
, NRNR1-2', wherein RN is
selected from the group comprising H and C1-4 alkyl;
R1-2' is a linker for connection to the antibody (Ab);
R1 and R11 either together form a double bond between the nitrogen and carbon
atoms
to which they are bound or;
R1 is H and R11 is selected from OH, ORA and SON;
R3 and R31 either together form a double bond between the nitrogen and carbon
atoms
to which they are bound or;
R3 is H and R31 is selected from OH, ORA and SOzM.
51
CA 03220935 2023- 11- 30
WO 2023/274974 PCT/EP2022/067603
34. The method according to statement 33, wherein the anti-CD19 ADC (or anti-
CD22 ADC
or anti-0D25 ADC) has the chemical structure:
ON
H
(01
N
8 ),N
z H
Of OH
HJ N
N
O 0
, where Ab is the antibody that binds to CD19
(or CD22 or CD25), and p is between 1 and 8.
35. The method of any preceding statement, wherein the anti-CD19 ADC
comprises an
antibody having a VH domain comprising a VH CDR1, a VH CDR2, and a VH CDR3,
wherein the antibody comprises the CDR sequences of the VH domain having the
sequence according to SEQ ID NO: 2.
36. The method of statement 35, wherein the antibody further comprises a VL
domain
comprising a VL CDR1, a VL CDR2, and a VL CDR3, wherein the antibody comprises
the
CDR sequences of the VL domain having the sequence according to SEQ ID NO: 8.
37. The
method of any preceding statement, wherein the anti-CD19 ADC comprises an
antibody having a VH domain having the sequence according to SEQ ID NO: 2.
38. The method of statement 37, wherein the anti-CD19 ADC further comprises
an
antibody having a VH domain having the sequence according to SEQ ID NO: 8.
39. The method according to any previous statement, wherein the anti-CD19
ADC
comprises a heavy chain having the amino acid sequence of SEQ ID NO.13 and/or
a light
chain having the amino acid sequence of SEQ ID NO.14.
40. The
method according to any preceding statement, wherein the anti-CD19 ADC is
ADCx19.
41. The method according to any one of statements Ito 39, wherein the anti-
CD19
ADC is Loncastuximab tesirine
42. An anti-CD19 ADC (or anti-CD22 ADC or anti-CD25 ADC) for use in a
method of
treatment according to any preceding statement.
43. A composition comprising an anti-CD19 ADC (or anti-CD22 ADC or anti-
CD25
ADC), for use in a method of treatment according to any preceding statement.
52
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
44. A composition comprising an anti-CD19 ADC (or anti-0D22 ADC or anti-
CD25
ADC) and an anti-CD79b agent.
45. The anti-CD19 ADC (or anti-0D22 ADC or anti-0D25 ADC) for use according
to
statement 42, the composition for use according to statement 43, or the
composition
according to statement 44, wherein the anti-CD19 ADC is as defined in any one
of
statements 33 to 39 and/or the anti-CD79b agent is as defined in any one of
statements 27
to 32.
46. An anti-
CD79b agent for use in a method of treatment according to any preceding
statement.
47. A composition comprising an anti-CD79b agent, for use in a method of
treatment
according to any preceding statement.
48. The anti-CD79b agent for use according to statement 46, or the
composition for use
according to statement 42, wherein the anti-CD79b agent is polatuzumab vedotin
49. Use of an anti-0D19 ADC (or anti-0D22 ADC or anti-0D25 ADC) in the
manufacture of a medicament for treating a disorder in an individual, wherein
the treatment
comprises the method of any preceding statement.
50. The use of an anti-CD19 ADC according to statement 49, wherein the anti-
0D19
ADC is as defined in any one of statements 33 to 39.
51. Use of an anti-CD79b agent in the manufacture of a medicament for
treating a
disorder in an individual, wherein the treatment comprises the method of any
preceding
statement.
52. The use of
the anti-CD79b agent according to statement 51, wherein the anti-
CD79b agent is polatuzumab vedotin.
53. A kit comprising:
a first medicament comprising an anti-CD19 ADC (or anti-0D22 ADC or anti-0D25
ADC);
a package insert comprising instructions for administration of the first
medicament
according to the method of any one of statements 1 to 41.
54. The kit according to statement 53, wherein the anti-CD19 ADC is as
defined in any
one of statements 28 to 36.
55. The kit according to statements 53 or 54, further comprising:
a second medicament comprising an anti-CD79b agent.
56. The kit
according to statement 55, wherein the anti-CD79b agent is polatuzumab
vedotin.
53
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
EXAMPLES
Example 1: in vitro efficacy of a ADCx19 and Pola-V combination
Methods
MTT proliferation assay on cell lines exposed (120h) to increasing ADCx19 and
Pola-
V concentrations. Synergy at 120h was assessed by Chou-Talalay combination
index
(Cl) (synergism 01<0.9, additive 0I=0.9-1.1, antagonism/no benefit CI> 1.1) on
an
activated B cell like (ABC) DLBCL cell line (TMD8), a germinal center (GCB)
DLBCL
(WSU-DLCL2) cell line, and a Burkitt lymphoma (Ramos) cell line.
Results
ADCx19 was combined with the anti-CD79b agent, Polatuzumab vedotin (Pola-V) in
GCB- and ABC- DLBCL cell lines, as well as a Burkitt lymphoma cell line.
Synergism
was achieved in Ramos and TMD8 cell lines combining ADCx19 with Pola-V (median
Cl 0.74 and 0.764, respectively), with WSU-DLCL2 showing additive efficacy
(median
Cl 0.96).
Data are shown in the tables below (Fa = fraction affected).
Table 1. Cell line: Ramos
RRID cell accession identifier: CVCL_0597
ADCx19 Pola-V ADCx19 Pola-V
Fa Cl Fa Cl
(PM) (ng/ml) (PM) (ng/ml)
0.01 250.0 0.85 0.79 0.20 3.9 0.26
1.07
0.01 62.5 0.67 0.73 0.20 1.0 0.21
0.96
0.01 15.6 0.18 2.75 0.20 0.2 0.26
0.71
0.01 3.9 0.05 3.94 0.78 250.0 0.98
0.17
0.01 1.0 0.05 1.34 0.78 62.5 0.93
0.32
0.01 0.2 0.07 0.28 0.78 15.6 0.87
0.44
0.05 250.0 0.85 0.85 0.78 3.9 0.87
0.43
0.05 62.5 0.64 0.89 0.78 1.0 0.86
0.42
0.05 15.6 0.16 3.74 0.78 0.2 0.88
0.38
0.05 3.9 0.09 2.42 3.13 250.0 0.97
0.59
0.05 1.0 0.05 1.58 3.13 62.5 0.95
0.82
0.05 0.2 0.10 0.45 3.13 15.6 0.96
0.75
0.20 250.0 0.92 0.38 3.13 3.9 0.96
0.75
0.20 62.5 0.72 0.72 3.13 1.0 0.96
0.70
0.20 15.6 0.28 2.02 3.13 0.2 0.97
0.60
54
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
Table 2. Cell line: TMD8
RRID cell accession identifier: CVCL_A442
Reference: Tohda et al., Leuk. Res. 30:1385-1390(2006)
ADCx19 Pola-V ADCx19 Pola-V
Fa CI Fa CI
(PM) (ng/ml) (pM) (ng/ml)
0.025 222 0.91 0.70 6.25 8 0.17
1.09
0.025 74 0.70 0.66 6.25 3 0.12
1.32
0.025 25 0.08 2.32 25 222 0.97
0.27
0.025 8 0.01 3.87 25 74 0.93
0.21
0.025 3 0.01 1.52 25 25 0.80
0.21
0.1 222 0.90 0.73 25 8 0.61 0.30
0.1 74 0.68 0.71 25 3 0.48 0.45
0.1 25 0.05 3.42 25 222 0.99 0.38
0.1 8 0.01 3.45 25 74 0.93 0.48
0.1 3 0.01 1.74 25 25 0.77 0.75
0.39 222 0.84 1.09 25 8 0.63
1.06
0.39 74 0.62 0.86 25 3 0.57
1.22
0.39 25 0.07 2.93 50 222 0.99
0.31
0.39 8 0.01 5.67 50 74 0.95
0.48
0.39 3 0.01 3.17 50 25 0.86
0.80
1.56 222 0.90 0.74 50 8 0.76
1.17
1.56 74 0.66 0.76 50 3 0.72
1.37
1.56 25 0.17 1.48 100 222 0.98
0.23
1.56 8 0.05 2.23 100 74 0.89
0.37
1.56 3 0.02 3.51 100 25 0.88
0.22
3 222 0.93 0.97 100 8 0.89 0.14
3 74 0.73 0.82 100 3 0.86 0.16
3 25 0.31 1.04 100 222 1.00 0.25
3 8 0.07 2.28 100 74 0.98 0.31
3 3 0.03 4.17 100 25 0.93 0.71
6 222 0.96 0.73 100 8 0.86 1.30
6 74 0.80 0.70 100 3 0.84 1.44
6 25 0.53 0.74 200 222 0.99 0.49
6 8 0.19 1.60 200 74 0.99 0.35
6 3 0.12 2.19 200 25 0.98 0.39
6.25 222 0.95 0.46 200 8 0.96
0.83
6.25 74 0.84 0.37 200 3 0.95
0.90
6.25 25 0.49 0.50
55
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
Table 3. Cell line: WSU-DLCL2
RRID cell accession identifier: CVCL_1902
ADCx19 Pola-V ADCx19 Pola-V
Fa CI Fa CI
(PM) (ng/ml) (PM) (ng/ml)
0.0064 48.8 0.01 10.82 0.8 12.3 0.34 0.59
0.0064 37.0 0.28 0.53 0.8 12.2 0.51 0.38
0.0064 12.3 0.00 15.78 0.8 4.1 0.31 0.60
0.0064 12.2 0.01 2.93 0.8 3.1 0.43 0.41
0.0064 4.1 0.01 2.40 0.8 1.4 0.29 0.63
0.0064 3.1 0.01 0.96 4 48.8 0.87 0.30
0.0064 1.4 0.01 0.88 4 37.0 0.83 0.23
0.032 48_8 0.01 1204. 4 12.3 0.80
0.24
0.032 37.0 0.18 0.95 4 12.2 0.83
0.31
0.032 12.3 0.03 2.27 4 4.1 0.76
0.30
0.032 12.2 0.01 4.16 4 3.1 0.80
0.33
0.032 4.1 0.01 3.33 4 1.4 0.74
0.32
0.032 3.1 0.01 2.18 20 48.8 0.88
0.96
0.032 1.4 0.01 2.24 20 37.0 0.88
0.56
0.16 48.8 0.02 9.15 20 12.3 0.87
0.64
0.16 37.0 0.12 1.87 20 12.2 0.86
1.07
0.16 12.3 0.01 13.39 20 4.1 0.85
0.74
0.16 12.2 0.01 10.26 20 3.1 0.87
0.95
0.16 4.1 0.01 10.13 20 1.4 0.84
0.82
0.16 3.1 0.01 8.29 100 37.0 0.91
1.95
0.16 1.4 0.01 9.04 100 12.3 0.90
2.21
0.8 48.8 0.66 0.41 100 4.1 0.89 2.36
0.8 37.0 0.50 0.45 100 1.4 0.88 2.56
Example 2: in vivo efficacy of a ADCx19 and Pola-V combination
Methods
Female severe combined immunodeficient mice (Fox Chase SCIDe, CB17/1cr-
Prkdcscid/IcrIcoCrl, Charles River) were nine weeks old with a body weight
(BVV) range
of 17.3 to 24.1 g on Day 1 of the study.
On the day of implant, 1 x 107 WSU-DLCL2 cells (0.1 mL suspension) were
subcutaneously implanted into the right flank of each test animal and tumors
were
monitored as their volumes approached the target range.
56
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
Ten days after tumor implantation, designated as Day 1 of the study, the
animals were
sorted into groups (n=10) with individual tumor volumes of 88 to 126 mm3 and
group
mean tumor volumes of 109 to 111 mm3.
All vehicle and ADC doses were administered i.v. via tail vein injection once
on Day 1
(qd x 1). The dosing volume was 0.2 mL per 20 grams of body weight (10 mL/kg)
and
was scaled to the body weight of each individual animal.
Tumors were measured using calipers twice per week, and each animal was
euthanized when its tumor reached the endpoint volume of 1000 mm3 or at the
end of
the study, whichever came first. The study ended on Day 62.
Tumors were measured in two dimensions using calipers, and volume was
calculated
using the formula
Tumor Volume (mm3) = w2 x1/2, where w = width and I = length, in mm, of the
tumor.
Tumor weight may be estimated with the assumption that 1 mg is equivalent to 1
mm3
of tumor volume.
Results
In this study, Pola-V is the CD79bxADC used and Loncastuximab tesirine is the
CD19xADC used.
Figure 1 shows a plot of median tumour volume. The tables below sets out the
study
response summary, where PR = partial response, CR = complete response, TFS =
tumour free survivor.
Table 4
Response summary PR CR TFS
Vehicle 0 0 0
CD79bxADC, 1 mg/kg 3 3 1
CD19xADC, 0.25 mg/kg 3 0 0
CD19xADC, 0.5 mg/kg 2 5 3
CD79bxADC, 1 mg/kg + CD19xADC, 0.25 mg/kg 2 3 1
CD79bxADC, 1 mg/kg + CD19xADC, 0.5 mg/kg 1 8 6
Example 3: in vivo efficacy of a ADCx19 and Pola-V combination
A further in vivo study was conducted, similar to Example 2 but using a Ramos
xenograft model.
57
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
Methods
Female severe combined immunodeficient mice (Fox Chase SCID , CB17/1cr-
Prkdcscid/IcrIcoCrl, Charles River) were eight weeks old with a body weight
(BW) range
of 15.6 to 22.9 g on Day 1 of the study.
On the day of implant, 1 x 107 Ramos cells (0.1 mL suspension) were
subcutaneously
implanted into the right flank of each test animal and tumors were monitored
as their
volumes approached the target range. All vehicle and ADC doses were
administered
i.v. via tail vein injection once on Day 1 (qd x 1). The dosing volume was 0.2
mL per
grams of body weight (10 mL/kg) and was scaled to the body weight of each
individual animal.
Fourteen days after tumor implantation, designated as Day 1 of the study, the
animals
15 were sorted into groups (n=10) with individual tumor volumes of 108 to
144 mm3 and
group mean tumor volumes of 130 to 134 mm3.
Tumors were measured using calipers twice per week, and each animal was
euthanized when its tumor reached the endpoint volume of 1000 mm3 or at the
end of
20 the study, whichever came first. The study ended on Day 62.
Results
Table 5
Response summary PR CR TFS
Vehicle 0 0 0
CD79bxADC, 2 mg/kg 0 0 0
CD19xADC, 0.3 mg/kg 0 0 0
CD19xADC, 0.6 mg/kg 0 7 7
CD79bxADC, 2 mg/kg + CD19xADC, 0.3 mg/kg 1 0 0
CD79bxADC, 2 mg/kg + CD19xADC, 0.6 mg/kg 0 10 9
In vivo, combination of CD19xADC with CD79bxADC resulted in improved anti-
tumor
activity and better response rates in the WSU-DLCL2 and Ramos xenog raft
models . The
combination was acceptably well tolerated in both models. Translation of these
pre-clinical
data in the clinic is currently being investigated in a phase I trial
evaluating the safety and
tolerability of loncastuximab tesirine in combination with polatuzumab vedotin
in patients
with r/r NHL (N0T04970901).
58
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
Example 4: in vitro efficacy of SG3199 (PBD) and MMAE combination
We tested a combination of the warheads alone used in CD19xADC (PBD SG3199)
and
CD79bxADC (monomethyl auristatin E (MMAE)). This was performed essentially as
per
Example 1, in TMD8 cells. Cells were seeded at a density of 50,000 cells/mL.
We used
SG3199 and MMAE with the following titration: SG3199 - a 7 point 1 in 4 serial
titration
from 230pM to 0.06pM; MMAE: a 7 point 1 to 3 serial titration from 50nM to
0.08nM.
Cells were incubated for 24 hours and then viability was measured with
CellTiterGlo assay.
For analysis of the results, data were first blank corrected (average of blank
of the 3
replicates was subtracted from each data value). For GraphPad analysis, each
replicate
was normalized over the untreated cells (average of the 3 replicates). Data
were analysed
by GraphPad Prism to generate a dose response curve with Log drug
concentration on
X-axis and cell viability (%) on Y-axis. For CalcuSyn analysis, the average of
the 3
replicates was corrected over the untreated cells.
The median Chou-Talalay combination index (Cl) was 0.85 (<0.9 indicates
synergy).
Table 6
SG3199 MMAE SG3199 MMAE
(PM) (ng/ml) Fa Cl (pM) (ng/ml) Fa Cl
0.06 0 0.12 N/A 0.06 0.08 0.12 1.65
0.23 0 0.16 N/A 0.23 0.08 0.05 434.19
0.90 0 0.15 N/A 0.90 0.08 0.19 0.58
3.6 0 0.15 N/A 3.6 0.08 0.16 5.16
14.4 0 0.19 N/A 14.4 0.08 0.17 12.16
57.5 0 0.23 N/A 57.5 0.08 0.34 0.22
230 0 0.46 N/A 230 0.08 0.47 0.03
O 0.08 0.11 N/A 0.06 0.2
0.13 2.06
O 0.2 0.17 N/A 0.23 0.2
0.14 2.22
O 0.6 0.27 N/A 0.90 0.2
0.16 2.08
O 1.9 0.29 N/A 3.6 0.2
0.11 69.07
O 5.6 0.38 N/A 14.4 0.2
0.16 18.45
57.5 0.2 0.33
0.39
230 0.2 0.51
0.02
59
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
SG3199 MMAE SG3199 MMAE
Fa CI Fa CI
(PM) (ng/ml) (pM) (nglml)
0.06 0.6 0.27 0.53 0.06 5.6 0.38
1.23
0.23 0.6 0.29 0.40 0.23 5.6 0.40
0.98
0.90 0.6 0.31 0.29 0.90 5.6 0.41
0.85
3.6 0.6 0.23 1.26 3.6 5.6 0.34 1.93
14.4 0.6 0.29 0.60 14.4 5.6 0.39
1.12
57.5 0.6 0.37 0.23 57.5 5.6 0.49
0.37
230 0.6 0.54 0.03 230 5.6 0.62
0.09
0.06 1.9 0.32 0.79
0.23 1.9 0.29 1.18
0.90 1.9 0.32 0.83
3.6 1.9 0.28 1.36
14.4 1.9 0.29 1.32
57.5 1.9 0.42 0.30
230 1.9 0.59 0.04
Example 5: in vitro efficacy of an ADCx25 and Pola-V combination
Methods
MTT proliferation assay on cell lines to be exposed (120h) to increasing
ADCx25 and
Pola-V concentrations. Synergy at 120h will be assessed by Chou-Talalay
combination index (Cl) (synergism Cl<0.9, additive CI=0.9-1.1, antagonism/no
benefit
CI> 1.1).
Example 6: in vivo efficacy of an ADCx25 and Pola-V combination
Methods
Female severe combined immunodeficient mice (Fox Chase SCID , CB17/1cr-
Prkdcscid/IcrIcoCrl, Charles River) will be nine weeks old with a body weight
(BVV) range
of 17.3 to 24.1 g on Day 1 of the study.
The study will be further conducted as per Examples 2 and 3.
Example 7: in vitro efficacy of an ADCx22 and Pola-V combination
Methods
MTT proliferation assay on cell lines to be exposed (120h) to increasing
ADCx22 and
Pola-V concentrations. Synergy at 120h will be assessed by Chou-Talalay
combination index (Cl) (synergism Cl<0.9, additive CI=0.9-1.1, antagonism/no
benefit
CI> 1.1) on a germinal center (GCB) DLBCL (WSU-DLCL2) cell line and a Burkitt
lymphoma (Ramos) cell line.
60
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
Example 8: in vivo efficacy of an ADCx22 and Pola-V combination
Methods
Female severe combined immunodeficient mice (Fox Chase SCIDe, CB17/1cr-
Prkdcscicl/IcrIcoCrl, Charles River) will be subcutaneously implanted into the
right flank
of each test animal with either WSU-DLCL2 or Ramos cells and tumors will be
monitored as their volumes approached the target range.
The study will be further conducted as per Examples 2 and 3.
********
A number of publications are cited above to more fully describe and disclose
the
disclosures and the state of the art to which inventions herein may pertain.
The entirety
of each of the references mentioned in this disclosure is hereby incorporated
by
reference.
SEQUENCE LISTING PART OF THE DESCRIPTION
SEQ ID NO. 1 (RB4v1.0 VH):
QVQLVQPGAEVVKPGASVKLSCKTSGYTFTSNWMHVVVKQRPGQGLEWIGEIDPSDSYTNYNQN
FKGKAKLTVDKSTSTAYMEVSSLRSDDTAVYYCARGSNPYYYAMDYWGQGTSVTVS
SEQ ID NO. 2 (RB4v1.2 VH):
QVQLVQPGAEVVKPGASVKLSCKTSGYTFTSNWMHVVVKQAPGQGLEWIGEIDPSDSYTNYNQN
FQGKAKLTVDKSTSTAYMEVSSLRSDDTAVYYCARGSNPYYYAMDYWGQGTSVTVS
SEQ ID NO. 3 (643 VH):
QVQLLESGAELVRPGSSVKISCKASGYAFSSYWMNVVVKQRPGQGLEWIGQIWPGDGDTNYNGK
FKGKATLTADESSSTAYMQLSSLRSEDSAVYSCARRETTTVGRYYYAMDYWGQGTTVT
SEQ ID NO. 4 (HD37 VH):
QVQLQQSGAELVRPGSSVKISCKASGYAFSSYWMNVVVKQRPGQGLEWIGQIWPGDGDTNYNG
KFKGKATLTADESSSTAYMQLSSLASEDSAVYFCARRETTTVGRYYYAMDYWGQGTSVTVS
SEQ ID NO. 5 (4G7 VH):
EVQLQQSGPELIKPGASVKMSCKASGYTFTSYVMHWVKQKPGQGLEWIGYINPYNDGTKYNEKF
KGKATLTSDKSSSTAYMELSSLTSEDSAVYYCARGTYYYGSRVFDYWGQGTTLTVS
SEQ ID NO. 6 (FMC63 VH):
EVKLQESGPGLVAPSQSLSVTCTVSGVSLPDYGVSWIRQPPRKGLEWLGVIWGSETTYYNSALK
SRLTIIKDNSKSQVFLKMNSLQTDDTAIYYCAKHYYYGGSYAMDYWGQGTSVTVS
SEQ ID NO.7 (RB4v1.0 VK):
EIVLTQSPAIMSASPGERVTMTCSASSGVNYMHVVYQQKPGTSPRRWIYDTSKLASGVPARFSGS
GSGTSYSLTISSMEPEDAATYYCHQRGSYTFGGGTKLEIK
SEQ ID NO. 8 (RB4v1.2 VK):
61
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
EIVLTQSPAIMSASPGERVTMTCSASSGVNYMHVVYQQKPGTSPRRWIYDTSKLASGVPARFSGS
GSGTSYSLTISSMEPEDAATYYCHQRGSYTFGGGTKLEIK
SEQ ID NO. 9 (B43 VK):
ELVLTQSPASLAVSLGQRATISCKASQSVDYDGDSYLNVVYQQIPGQPPKLLIYDASNLVSGIPPRF
SGSGSGTDFTLN IH PVEKVDAATYHCQQSTEDPVVTFGGGTKLEIK
SEQ ID NO. 10 (HD37 VK):
ILLTQTPASLAVSLGQRATISCKASQSVDYDGDSYLNWYQQ1PGQPPKLLIYDASNLVSG IPP RFS
GSGSGTDFTLN IH PVEKVDAATYHCQQSTEDPVVTFGGGTKLEIK
SEQ ID NO. 11 (4G7 VK):
D IVMTQAAPSIPVTPGESVSISCRSS KSLLNSNGNTYLYWFLQRPGQSPQLLIYRMSNLASGVPDR
FSGSGSGTAFTLRISRVEAEDVGVYYCMQHLEYPFTFGAGTKLELK
SEQ ID NO. 12 (FMC63 VK):
D IQMTQTTSSLSASLGDRVTISCRASQD ISKYLNWYQQKPDGTVKLLIYHTSRLHSGVPSRFSGSG
SGTDYSLTISN LEO ED IATYFCQQG NTLPYTFGGGTKLE IT
SEQ ID NO. 13 (R134v1.2-HC):
QVQLVQPGAEVVKPGASVKLSCKTSGYTFTSNWMHWVKQAPGQGLEWIGE I D PSDSYTNYNQN
FQGKAKLTVDKSTSTAYMEVSSLRSDDTAVYYCARGSNPYYYAMDYWGQGTSVTVSSASTKGP
SVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTV
PSSSLGTQTYICNVNH KPSNTKVDKKVEPKSCD KTHTCPPCPAPELLGGPSVFLFPPKP KDTLM IS
RTPEVTCVVVDVSH EDPEVKFNVVYVDGVEVH NAKTKPREEQYNSTYRVVSVLTVLHQDWLNGK
EYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTCLVKGFYPSDIAVEWES
NGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMH EALHN HYTQKSLSLSPG
SEQ ID NO. 14 (RB4v1.2-LC):
EIVLTQSPAIMSASPGERVTMTCSASSGVNYMHWYQQKPGTSPRRWIYDTSKLASGVPARFSGS
GSGTSYSLTISSM EPEDAATYYC HQRGSYTFGGGTKLE I KRTVAAPSVFI FPPSD EQLKSGTASVV
CLLNNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKH KVYACEVT
HQGLSSPVTKSFN RGEC
SEQ ID NO. 15 (evi-5 POLA.VH-hl .HC):
EVQLVESGGGLVQPGGSLRLSCAASGYTFSSYWIEVVVRQAPG KG LEWIG E ILPGGGDTNYN E IF
KG RATFSADTSKNTAYLQM NSLRAEDTAVYYCTRRVP I RLDYWGQGTLVTVSSASTKGPSVFPLA
PSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLG
TQTYICNVN HKPSNTKVDKKVEPKSCDKTHTCPPCPAP ELLGGPSVFLFPPKPKDTLM ISRTPEVT
CVVVDVSH ED PEVKFNWYVDGVEVH NAKTKPREEQYNSTYRVVSVLTVLHQDWLNG KEYKCKV
SNKALPAPIEKTISKAKGQPREPQVYTLPPSREEMTKNQVSLTC LVKGFYPS DIAVEWESNGQPE
N NYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMH EALH N HYTQKSLSLSPGK
SEQ ID NO. 16 (evi-5 POLA.VL-hk.LC):
D IQLTQSPSSLSASVGDRVTITCKASQSVDYEGDSFLNVVYQQKPGKAPKLLIYAASNLESGVPSRF
SGSGSGTDFTLTISSLQPEDFATYYCQQSNEDPLTFGQGTKVEIKRTVAAPSVFIFPPSDEQLKSG
TASVVCLLNNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKHKVY
ACEVTHQGLSSPVTKSF
62
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
SEQ ID NO. 17 (evi-5 POLA.VH):
EVQLVESGGGLVQPGGSLRLSCAASGYTFSSYWIEVVVRQAPGKGLEWIGEILPGGGDTNYNEIF
KGRATFSADTSKNTAYLQMNSLRAEDTAVYYCTRRVPIRLDYWGQGTLVTVSS
SEQ ID NO. 18 (evi-5 POLA.VL):
DIQLTQSPSSLSASVGDRVTITCKASQSVDYEGDSFLNVVYQQKPGKAPKLLIYAASNLESGVPSRF
SGSGSGTDFTLTISSLQPEDFATYYCQQSNEDPLTFGQGTKVEIK
SEQ ID NO. 19 (evi-5 POLA.VH.CDR1):
SYWIE
SEQ ID NO. 20 (evi-5 POLA.VH.CDR2):
EILPGGGDTNYNEIFKG
SEQ ID NO. 21 (evi-5 POLA.VH.CDR3):
RVPIRLDY
SEQ ID NO. 22 (evi-5 POLA.VL.CDR1):
KASQSVDYEGDSFLN
SEQ ID NO. 23 (evi-5 POLA.VL.CDR2):
AASNLES
SEQ ID NO. 24 (evi-5 POLA.VL.CDR3):
QQSNEDPLT
SEQ ID NO. 25 (AB12 VH):
QVQLVQSGAEVKKPGSSVKVSCKASGGTFSRYIINWVRQAPGQGLEWMGRIIPILGVENYAQKFQ
GRVTITADKSTSTAYMELSSLRSEDTAVYYCARKDWFDYWGQGTLVTVSSASTKGPSVFPLA
SEQ ID NO. 26 (AB12 VL):
EIVLTQSPGTLSLSPGERATLSCRASQSVSSYLAVVYQQKPGQAPRLLIYGASSRATGIPDRFSGS
GSGTDFTLTISRLEPEDFAVYYCQQYGSSPLTFGGGTKVEIKRTVAAPSVFIFP
SEQ ID NO. 27 (VH CDR1):
RYIIN
SEQ ID NO. 28 (VH CDR2):
RIIPILGVENYAQKFQG
SEQ ID NO. 29 (VH CDR3):
KDWFDY
SEQ ID NO. 30 (VL CDR1):
RASQSVSSYLA
SEQ ID NO. 31 (VL CDR2):
GASSRAT
63
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
SEQ ID NO. 32 (VL CDR3):
QQYGSSPLT
SEQ ID NO. 33 (Epratuzumab VH):
QVQLVQSGAEVKKPGSSVKVSCKASGYTFTSYWLHWVRQAPGQGLEWIGYINPRNDYTEYNQN
FKDKATITADESTNTAYMELSSLRSEDTAFYFCARRDITTFYWGQG
SEQ ID NO. 34 (Epratuzumab VL):
DIQLTQSPSSLSASVGDRVTMSCKSSQSVLYSANHKNYLAWYQQKPGKAPKLLIYWASTRESGV
PSRFSGSGSGTDFTFTISSLQPEDIATYYCHQYLSSVVTFGQG
SEQ ID NO. 35 (Epratuzumab.VH.CDR1):
YTFTSYWLH
SEQ ID NO. 36 (Epratuzumab.VH.CDR2):
WIGYINPRNDYTEY
SEQ ID NO. 37 (Epratuzumab.VH.CDR3):
RRDITTFY
SEQ ID NO. 38 (Epratuzumab.VL.CDR1):
QSVLYSANHKNYLA
SEQ ID NO. 39 (Epratuzumab.VL.CDR2):
LLIYWASTRES
SEQ ID NO. 40 (Epratuzumab.VL.CDR3):
HQYLSSW
SEQ ID NO. 41 (EMabC220¨HC):
QVQLVQSGAEVKKPGSSVKVSCKASGYTFTSYWLHWVRQAPGQGLEWIGYINPRNDYTEYNQN
FKDKATITADESTNTAYMELSSLRSEDTAFYFCARRDITTFYWGQGTLVTVSSASTKGPSVFPLAP
SSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGT
QTYICNVNHKPSNTKVDKKVEPKSCDKTHTVPPVPAPELLGGPSVFLFPPKPKDTLMISRTPEVTC
VVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVS
NKALPAPIEKTISKAKGQPREPQVYTLPPSRDELTKNQVSLICLVKGFYPSDIAVEWESNGQPENN
YKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPG
SEQ ID NO. 42 (EMabC220-LC):
DIQLTQSPSSLSASVGDRVTMSCKSSQSVLYSANHKNYLAVVYQQKPGKAPKLLIYWASTRESGV
PSRFSGSGSGTDFTFTISSLQPEDIATYYCHQYLSSWTFGQGTKVEIKRTVAAPSVFIFPPSDEQL
KSGTASVVCLLNNFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTYSLSSTLTLSKADYEKH
KVYACEVTHQGLSSPVTKSFNRGES
SEQ ID NO. 43 (IgG1 HC constant region)
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSL
SSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKP
KDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQ
DWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRDELTKNQVSLTCLVKGFYPSDI
64
CA 03220935 2023- 11- 30
WO 2023/274974
PCT/EP2022/067603
AVEWESNGQPEN NYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMH EALH NHYTQKS
LSLSPG
SEQ ID NO. 44 (IgG1 HC constant region, BJ C-V)
ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYFPEPVTVSWNSGALTSGVHTFPAVLQSSGLYSL
SSVVTVPSSSLGTQTYICNVNHKPSNTKVDKKVEPKSCDKTHTVPPVPAPELLGGPSVFLFPPKPK
DTLM ISRTPEVTCVVVDVSH ED PEVKFNWYVDGVEVH NAKTKPREEQYNSTYRVVSVLTVLHQD
WLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRDELTKNQVSLTCLVKGFYPSD IA
VEVVESNGQPEN NYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVM H EALHN HYTQKSL
SLSPG
SEQ ID NO. 45 (KLC constant region)
VAAPSVFIFPPSDEQLKSGTASVVCLLN NFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTY
SLSSTLTLSKADYEKH KVYACEVTHQG LSSPVTKS FN RG EC
SEQ ID NO. 46 (KLC constant region, C105S)
VAAPSVFIFPPSDEQLKSGTASVVCLLN NFYPREAKVQWKVDNALQSGNSQESVTEQDSKDSTY
SLSSTLTLSKADYEKH KVYACEVTHQG LSSPVTKS FN RG ES
SEQ ID NO. 47 (ALC constant region)
KAAPSVTLFPPSSEELQAN KATLVC LISDFYPGAVTVAWKADSSPVKAGVETTTPSKQSN NKYAA
SSYLSLTPEQWKSHRSYSCQVTH EGSTVEKTVAPTECS
SEQ ID NO. 48 (ALC constant region, C102S)
KAAPSVTLFPPSSEELQAN KATLVC LISDFYPGAVTVAWKADSSPVKAGVETTTPSKQSN NKYAA
SSYLSLTPEQWKSHRSYSCQVTH EGSTVEKTVAPTESS
CA 03220935 2023- 11- 30