Note: Descriptions are shown in the official language in which they were submitted.
IMPLANTABLE ACCOMODATING INTRAOCULAR LENSES, IOL IMPLANTS, AND
RELATED SYSTEMS AND METHODS
TECHNICAL FIELD
[0001] This document relates to implantable accommodating intraocular
lens (IOLs),
IOL implants, and related systems and methods.
BACKGROUND
[0002] The following paragraphs are not an admission that anything
discussed in
them is prior art or part of the knowledge of persons skilled in the art.
[0003] Multifocal or monofocal intraocular lenses (IOLs) may be inserted
in the
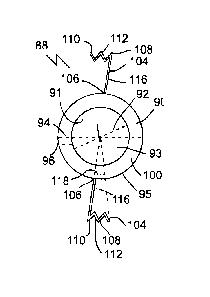
capsular lens bag of the eye to provide improved vision at a variety or a
single focal distance.
Accommodating lenses such as the CrystalensTM fit within the capsular lens bag
via haptics.
The dioptric power range of the lens is inherently limited by the degree the
lens can move or
adjust.
SUMMARY
[0004] An accommodation-facilitating intraocular implant is disclosed
comprising: a
ring sized to fit within a capsular lens bag of an eye; and a plurality of
haptics angularly
spaced around and radially extended from the ring.
[0005] An accommodating intraocular lens (IOL) assembly is disclosed
comprising:
an implantable accommodating intraocular lens (IOL) within a capsular bag of
an eye, the
implantable accommodating IOL having an optic lens and a plurality of IOL
haptics
angularly spaced around and radially extended from the optic lens; and an
accommodation-
facilitating intraocular implant comprising a ring fitted within the capsular
lens bag of an
eye, anterior to and in contact with the optic lens.
[0006] A method comprising inserting the accommodation-facilitating
intraocular
implant into a capsular lens bag of an eye.
[0007] A method comprising: inserting an accommodation-facilitating
intraocular
implant into a capsular lens bag of an eye, into contact with and anterior to
an intraocular
1
Date Recue/Date Received 2023-12-12
lens (TOL) that is also within the capsular lens bag, in which the
accommodation-facilitating
intraocular implant is inserted through an incision in an anterior portion of
the capsular lens
bag, to position the accommodation-facilitating intraocular implant such that:
a plurality of
haptics of the accommodation-facilitating intraocular implant are inserted
into and follow a
circumferential groove of the sulcus to grip the sulcus; and under contraction
and expansion
of ciliary muscles of the eye, the plurality of haptics move to adjust the
ring along an optical
axis of the eye to adjust a dioptric power of the TOL to accommodate a focal
power of the
eye.
[0008] An implantable accommodating intraocular lens (TOL) comprising:
an optic
lens sized to fit within a capsular lens bag of an eye; a plurality of haptics
angularly spaced
around and radially extended from the curved optic lens, with each haptic
being structured to
move, under contraction and expansion of the ciliary muscles of the eye, to
adjust the optic
lens to accommodate a focal power of the eye; in which: the optic lens defines
a posterior
face and an anterior face; the posterior face has a concave profile; and the
anterior face has a
convex profile.
[0009] A method comprising inserting the accommodation-facilitating
intraocular
implant into a capsular lens bag of an eye, through an incision in the
capsular lens bag, such
that the arcuate sulcus gripping parts of the plurality of haptics insert into
and follow the
circumferential groove of the sulcus to grip the sulcus.
[0010] In various embodiments, there may be included any one or more of
the
following features: An inner annular edge of the ring defines an open void
center. A portion
of the ring defined between inner and outer annular edges of the ring has a
rectangular cross-
sectional shape defined in a plane parallel with a central axis defined by the
ring. A radial
width, of an intraocular-lens-contacting annular posterior face opposite an
annular anterior
face of the ring, is greater than an axial thickness defined between the
intraocular-lens-
contacting annular posterior face and the annular anterior face. A radial
width is four or more
times greater than axial thickness. A radial width, of an intraocular-lens-
contacting annular
posterior face opposite an annular anterior face of the ring, is smaller than
a radius of an
open void center defined by the inner annular edge of the ring. Outer and
inner annular edges
of the ring have a circular shape. A material of the ring is rigid or
resilient. A ring
2
Date Recue/Date Received 2023-12-12
comprising one or more of poly(methyl methacrylate) (PMMA) or stainless steel.
A ring
comprises ultraviolet (A and B) absorbing material. Each haptic: has a tongue
that forms an
arcuate sulcus gripping part that, in use within the capsular lens bag,
inserts into and follows
a circumferential groove of the sulcus to restrict circumferential sliding of
the tongue around
the sulcus; and is structured to move, under contraction and expansion of the
ciliary muscles
of the eye, to adjust the ring along an optical axis of the eye. Each haptic
is attached to an
annular anterior face of the ring. A plurality of implant haptics angularly
spaced around and
radially extended from the ring. A plurality of implant haptics are inserted
into the
circumferential grove of the sulcus anterior relative to the plurality of IOL
haptics. A
plurality of implant haptics are configured to bias the ring in a posterior
direction under
contraction of the ciliary muscles of the eye to press upon the IOL and
thereby increase a
dioptric power of the IOL. An inner diameter of the ring is smaller than an
outer diameter of
the IOL; and an outer diameter of the ring is larger than the outer diameter
of the IOL. A
convex profile of the anterior face has a greater degree of curvature than the
concave profile
of the posterior face. A plurality of haptics is attached to the anterior face
of the IOL. A
plurality of haptics are configured to bias the IOL in a posterior direction
under contraction
of the ciliary muscles of the eye to increase a dioptric power of the IOL. An
IOL comprises a
material of a high refractive index. An implantable accommodating IOL being
positioned
such that an outer edge of the implantable accommodating IOL is posterior an
iris of the eye
of the user. An IOL is positioned relatively more anterior decreases a night
glare experienced
be the user. A concave curvature of the posterior face increases a dioptric
power when the
IOL is moved in a backward direction. A convex curvature of the anterior face
increasing the
dioptric power of the IOL and increases a force of the papillary constriction
in order to move
the IOL in the backward direction.
[0011] The
foregoing summary is not intended to summarize each potential
embodiment or every aspect of the subject matter of the present disclosure.
These and other
aspects of the device and method are set out in the claims.
BRIEF DESCRIPTION OF THE FIGURES
3
Date Recue/Date Received 2023-12-12
[0012] Embodiments will now be described with reference to the figures,
in which
like reference characters denote like elements, by way of example, and in
which:
[0013] Fig. 1 is a front elevation view of an accommodation facilitating
secondary
implant (AFSI), having a ring and two opposed haptic arms, with a plurality of
protrusions
along respective sulcus gripping parts.
[0014] Fig. 2 is a front elevation view, partially in section of the
AFSI of Fig.1
positioned in a human eye, anterior to an intraocular lens (TOL) identified in
dashed lines,
with the ring haptics gripping the sulcus.
[0015] Fig. 3 is a front elevation view of a second embodiment of an
AFSI, having a
ring and two opposed arcuate haptic arms with a plurality of protrusions along
respective
arcuate sulcus gripping parts.
[0016] Fig. 4 is a front elevation view of a third embodiment of an
AFSI, having a
ring and two opposed haptic arm assemblies, each formed of dual arms that form
a haptic
bridge, with a plurality of convex protrusions along respective sulcus
gripping parts.
[0017] Fig. 5 is a front elevation view of a fourth embodiment of an
AFSI, having a
ring and four haptic arm assemblies from Fig. 4, with a plurality of convex
protrusions along
respective sulcus gripping parts.
[0018] Fig. 6 is a side elevation view of an AFSI.
[0019] Fig. 7 is a cross-sectional side view of an embodiment of an AFSI
positioned
in a human eye after the removal of the natural crystalline lens and the
addition of an
implantable accommodating IOL, in which the ciliary muscles are shown relaxed,
with the
lens zonules taut, such that the IOL is accommodating for far-sighted viewing
of a distant
focal point, with focal lines of light illustrated with dashed lines and
travelling from the focal
point and through the AFSI and IOL combination.
[0020] Fig. 8 is a cross-sectional side view of the AFSI, IOL and eye
combination of
Fig. 7 in which the ciliary muscles are contracted and the lens zonules are
slacked, such that
the lens is accommodating for near-sighted viewing of a nearby focal point
(relative to the
focal point in Fig. 7), with focal lines of light illustrated with dashed
lines and travelling
from the focal point and through the AFSI and IOL combination. In Fig. 8 the
AFSI and IOL
combination are moved forward relative to the position the AFSI and IOL adopts
in Fig. 7.
4
Date Recue/Date Received 2023-12-12
[0021] Fig. 9 is a cross-sectional side view of an embodiment of an AFSI
positioned
in a simplified human eye after the removal of the natural crystalline lens
and the addition of
an implantable accommodating IOL, in which the ciliary muscles are shown
relaxed, such
that the lens is accommodating for far-sighted viewing of a distant focal
point, with focal
lines of light illustrated with dashed lines and travelling from the focal
point and through the
AFSI and IOL combination.
[0022] Fig. 10 is a cross-sectional side view of the AFSI, IOL and eye
combination
of Fig. 9, in which the ciliary muscles are contracted, such that the lens is
accommodating
for near-sighted viewing of a nearby focal point (relative to the focal point
in Fig. 9), with
focal lines of light illustrated with dashed lines and travelling from the
focal point and
through the AFSI and IOL combination. In Fig. 9 the AFSI and IOL combination
are moved
forward relative to the position the AFSI and IOL adopts in Fig. 9.
[0023] Fig. 11 is front elevation view of a mixed curvature lens implant
(MCLI)
having an optic lens and two opposed haptic arm assemblies each forming a
haptic bridge,
with a plurality of protrusions along respective sulcus gripping parts.
[0024] Fig. 12 is a front elevation view of a second embodiment of an
MCLI, having
an optic lens and four haptic arm assemblies, with a plurality of protrusions
along respective
sulcus gripping parts.
[0025] Fig. 13 is a side elevation view of a third embodiment of an MCLI
positioned
within a capsular lens bag of a human eye, having an optic lens and two
arcuate haptic arms
with a plurality of protrusions along respective sulcus gripping parts.
[0026] Fig. 14 is a side elevation view of a fourth embodiment of an
MCLI, having
an optic lens and two opposed haptic arms with a plurality of protrusions
along respective
sulcus gripping parts.
[0027] Fig. 15 is a cross-sectional side view of an embodiment of an
MCLI
positioned in a human eye after the removal of the natural crystalline lens,
in which the
ciliary muscles are shown relaxed, with the lens zonules taut, such that the
lens is
accommodating for far-sighted viewing of a distant focal point, with focal
lines of light
illustrated with dashed lines and travelling from the focal point and through
the MCLI.
Date Recue/Date Received 2023-12-12
[0028] Fig. 16 is a cross-sectional side view of the MCLI and eye
combination of
Fig. 15 in which the ciliary muscles are contracted and the lens zonules are
slacked, such that
the lens is accommodating for near-sighted viewing of a nearby focal point
(relative to the
focal point in Fig. 15), with focal lines of light illustrated with dashed
lines and travelling
from the focal point and through the MCLI. In Fig. 16 the MCLI is moved
forward relative
to the position the MCLI adopts in Fig. 15.
DETAILED DESCRIPTION
[0029] Immaterial modifications may be made to the embodiments described
here
without departing from what is covered by the claims.
[0030] Problems with vision may take numerous forms. These include
myopia
(nearsightedness), hyperopia (farsightedness) as well as cataracts
(opacification of the lens).
Contact lenses and glasses containing refractive lenses are commonly used for
basic
correction of myopia, hyperopia, and astigmatism. Both contact lenses and
glasses represent
non-permanent solutions that are susceptible to loss, breakage and require
cleaning in order
to maintain efficacy.
[0031] An implantable intraocular contact lens, known as an IOL, is a
surgical
implantation used to permanently improve an eyesight condition, such as
myopia, hyperopia
or cataracts. An IOL incorporates a corrective lens tailored and structured to
the degree of
vision impairment desired to be corrected. An IOL solution may be a viable
option for a
patient who has a condition that would otherwise disqualify them from
alternative laser-
assisted in situ keratomileusis (LASIK) treatment such as: thin corneas, dry
eyes or
astigmatism (imperfection in the curvature of the lens). An IOL may be
considered and used
as a permanent vision correction solution, but may be removed or adjusted to
address any
change in efficacy or in a patient's vision deficit.
[0032] Two types of IOL solutions exists - phakic and pseudophakic. With
a phakic
solution ('phakic' meaning "having a lens") the eye's natural lens is left
untouched.
Intraocular lenses that are implanted into eyes after the eye's natural lens
has been removed
during cataract surgery are known as pseudophakic. Phakic intraocular lenses
are indicated
for patients with high refractive errors when the usual laser options for
surgical correction
6
Date Recue/Date Received 2023-12-12
(LASIK and PRK) are contraindicated. Phakic IOLs may be designed to correct
high myopia
ranging from ¨5 to ¨20 D if the patient has enough anterior chamber depth
(ACD) of at least
3 mm. The most common type of IOL is the pseudophakic IOL, which may be
implanted
after the eye's natural lens has been removed. The pseudophakic IOL provides
the same light
focusing function as the natural crystalline lens. A pseudophakic IOL may be
available as:
monofocal (focus on only one distance), multifocal (for example bifocal), or
accommodating
(permits focus changing).
[0033] An IOL may contain non-optic side struts known as haptics. A
haptic may be
the part of an IOL responsible for its attachment to the ciliary muscles or
suspensory
ligaments called lens zonules, which are connected to both the ciliary muscle
and natural
crystalline lens within the capsular lens bag of the eye. Haptics may use
hinges at its ends to
aid in attaching to the ciliary muscles or zonules. In any given IOL, the
haptics may vary in
number and shape, including having loops or hooks, for example having loops to
sew into
the ciliary sulcus of the eye.
[0034] Accommodation is how an eye may change optical power to maintain
a clear
image as the eye focuses on objects at different distances. When the eye
focuses on an object
that is relatively far away, the ciliary muscles may relax, leading to the
lens zonules
becoming taut, leading to a flattening of the natural crystalline lens. When
the eye focuses on
an object that is relatively near an individual, the ciliary muscles may
contract, leading to the
lens zonules slackening, reducing tension upon the natural crystalline lens,
making the lens
more convex.
[0035] An IOL may be designed to use non-optical elements known as
haptics to
connect to the ciliary muscles or zonules of the eye, allowing for
accommodation to occur.
With an accommodating IOL, the accommodation process may occur as a result of
one or
more of a change in the shape of the lens or a change in the position of the
lens relative to the
lens capsule. In the case of the former (change in lens shape causing
accommodation),
similar to the natural crystalline lens, when viewing an object that is
relatively nearby, the
ciliary muscles may contract, resulting in reduced tension on the haptics,
resulting in the lens
becoming convex in shape. As well, when viewing an object that is relatively
far away, the
ciliary muscles relax, increasing the tension on the haptics and flattening
the natural
7
Date Recue/Date Received 2023-12-12
crystalline lens. In the case of the latter (movement of lens causing
accommodation),
accommodation may occur through the haptics changing the position of the lens
anterior or
posterior relative to the lens capsule. When viewing an object that is
relatively nearby, for
example a book held at arm's length, under the tension of the contracted
ciliary muscles the
haptics may push the lens in an anterior direction, moving the lens relatively
closer to the
pupil. When viewing an object that is far away, the ciliary muscles may relax,
resulting in
the haptics pushing the lens in a posterior direction, moving the lens
relatively further from
the pupil. It is through such anterior-posterior movement of the lens that
accommodation
may be achieved in a manner analogous to that of the natural eye.
[0036] In contrast to accommodation, a static or non-accommodating IOL
may be
used, for example with a monofocal or multifocal lens. A monofocal lens may
only focus at
a single distance, for example a distance over 20 meters to correct only
distance vision. A
multifocal IOL may have plural regions that each focus at different relative
distances, for
example two or three focal regions spaced throughout the lens simultaneously
based on the
position of the pupil. In some cases, the central part of the lens may be
designed for focusing
on nearby objects, while the outer regions of the lens may be structured for
focusing on far
away objects. When viewing a nearby object, the pupil of the eye may constrict
and the
central region of the IOL may be used, while for far away viewing the pupil
dilates and an
outer IOL focal region may be used.
[0037] Some newer lens designs attempt to allow the eye to regain some
partial
focusing ability in order to change focus from distance to near via
accommodation.
However, many accommodating IOLs used today only achieve a very limited
improvements
in near vision which reduced over time. Accommodative IOLs may also have a
slightly
higher risk of developing posterior capsule opacification (PCO), though there
is some
uncertainty around this finding. PCO is a common side-effect of many cataract
surgeries and
is easily treatable with a one-time laser capsulotomy procedure. Accommodating
IOLs
interact with ciliary muscles and zonules, using hinges at both ends to latch
on and move
forward and backward inside the eye using the same mechanism as normal
accommodation.
8
Date Recue/Date Received 2023-12-12
The haptic hinges may be made of an advanced silicone called BioSil that has
been
thoroughly tested to make sure it is capable of unlimited flexing in the eye.
[0038] An IOL may be implanted in a surgical procedure. A surgeon may
use drugs
to dilate the pupil of the patient. A cut may be made into the cornea and
anterior capsular
lens bag of the eye, where the natural crystalline lens is contained, to
facilitate the insertion
of surgical tools and an IOL. The natural crystalline lens may be destroyed in
what is known
as a pseudophakic procedure, by a suitable technique such as the use of a
laser or ultrasound.
In some cases, it may be unnecessary to destroy the crystalline lens, such as
where the
crystalline lens has already been removed or destroyed in a previous
procedure, as might be
the case where an IOL is being replaced or upgraded. Alternatively, in a
phakic procedure
the natural crystalline lens may be kept intact. An IOL may then be inserted
into the capsular
lens bag. Insertion may be achieved by folding the IOL and inserting it
through the cut made
in the anterior lens capsule lens, assuming that the IOL is made with flexible
material. The
non-optic haptics may contact the sulcus of the eye.
[0039] There may be various problems with IOLs. Stiff haptics may impair
the
ability of the ciliary muscles to change the shape of the lens. Stiff haptics
may further make
it difficult to remove an IOL if a patient elects to do so after having a
phakic procedure. Lens
zonules may drive accommodation as opposed to the ciliary muscles, reducing
the eye's
ability to accommodate as following the initial cut into the anterior lens
capsule the zonular
system may not perform as efficiently as pre-surgery to change the shape of
the lens. IOLs
may need to be tailored in size to a patient's eye, and thus would not be
considered to be
one-size-fits-all. As the sulcus shape to which the IOL must match cannot be
accurately
measured, a surgeon may implant either too large of an optic lens, which will
resist ciliary
muscle action, or too small of an optic lens, in which the ciliary muscle may
not
accommodate properly or at all.
[0040] Referring to Figs. 1-10, an accommodation-facilitating
intraocular implant 88
(which may be referred elsewhere in this document as an AFSI), may be used to
assist in the
accommodation of an accommodating intraocular lens (IOL). The accommodation-
facilitating intraocular implant 88 may comprise a ring 90. The implant 88 may
also have a
9
Date Recue/Date Received 2023-12-12
plurality of haptics 104. The ring 90 may be sized to fit within a capsular
lens bag 24 of an
eye 10. The plurality of haptics 104 may be angularly spaced around (for
example around an
optical axis 11, which is discussed herein as defined by the eye, but may also
for
convenience of discussion be referred to as being defined by the ring 90
and/or the IOL 50)
and radially extended from the ring 90. The implant 88 may be used with an
accommodating
or non-accommodating IOL 50, and with mono or multi-focal IOLs. The implant 88
may
have no dioptric power. The use of the ring 90 pushes the lens 54 backward
toward the retina
in use, allowing a user to experience relatively improved near vision. The
lens may have a
fixed dioptric power, or in the case of flexible IOLs such as made of foldable
silicon, may
slightly increase the dioptric power of the lens in use. In some cases, the
IOL may comprise
flexible or pliable material, such as a liquid, solid, or gel.
[0041] Referring to Figs. 2 and 7-10, the accommodation-facilitating
intraocular
implant 88 may form part of an accommodating IOL assembly 126. An
accommodating IOL
assembly 126 may include an implant 88 and the IOL 50. The implant 88 may be
referred to
as a secondary implant, as such may be inserted to assist an existing IOL 50.
The
accommodating IOL assembly 126 may comprise an implantable accommodating IOL
50,
which may comprise an optic lens 54. The IOL 50 may also comprise a plurality
of IOL
haptics 52 angularly spaced around (for example around optical axis 11 defined
by the IOL
50 and/or eye and/or ring 90) and radially extended from the optic lens 54.
The ring 90 may
be fitted within the capsular lens bag 24 of the eye 10 in use, for example
anterior to and in
contact with the optic lens 54.
[0042] Referring to Figs. 7-10, the accommodation-facilitating
intraocular implant 88
may be inserted within the capsular lens bag 24 of an eye 10 in a method of
use. Once the
intraocular implant 88 is within the lens bag 24, the implant 88 may be placed
anterior to and
in contact, for example direct or indirect, with the lens 54 of the IOL 50
that is also with in
the lens bag 24. The accommodation-facilitating intraocular implant 88 may be
inserted
during surgery through an incision in an anterior portion of the capsular lens
bag 24, to
position the accommodation-facilitating intraocular implant 88. During the
positioning of the
intraocular implant 88, the plurality of haptics 104 of the accommodation-
facilitating
intraocular implant 88 are inserted into and may follow a circumferential
groove 33 of the
Date Recue/Date Received 2023-12-12
sulcus 32 to grip the sulcus 32. Once the haptics 104 of the intraocular
implant 88 are
positioned in the sulcus 32, under contraction and expansion of ciliary
muscles 28 of the eye,
the plurality of haptics 104 may be biased by the ciliary muscles 28 to adjust
the ring 90
along an optical axis 11 of the eye 10 to adjust a dioptric power of the IOL
50 to
accommodate a focal power of the eye 10.
[0043] Referring to Figs. 1-10, the accommodation-facilitating
intraocular implant 88
may have a suitable structure. The ring 90 may comprise any suitable shape,
such as a
circular shape, elliptical shape, or an oblong shape. The ring 90 of the
implant 88 may define
an inner annular edge 91, an outer annular edge 95, an annular anterior face
100, and an
intraocular-lens-contacting annular posterior face 102. The inner annular edge
91 may define
an open void center 93 of the ring 90. The void center 93 may allow the light
entering 78 the
eye 10 to pass through unhindered toward the lens 54 of the IOL 50. Referring
to Figs. 6-10,
a portion of the ring 90 defined between the inner annular edge 91 and the
outer annular edge
95 may have a rectangular cross-sectional shape, for example defined in a
plane (the plane is
understood as being the plane of the page in Figs. 6-10) parallel with a
central axis 11
defined by the ring and/or eye. The rectangular shape may include rounded
corners, and in
some cases nominal curvature of sides. In some cases, the cross-sectional
shape of the ring
may follow a circle, oval, ellipse, or other shape. The outer annular edge 95
of the ring 90
may allow the ring 90 to define an outer diameter 94. The ring 90 may define a
variety of
dimensions, such as inner and outer diameters 92 and 94 defined by the inner
and outer
annular peripheral edges 91 and 95, respectively. A radial width 96, which may
be defined
between a radius 94A defined by the outer annular edge 95 and a radius 93A
defined by the
inner annular edge 91. The radial width 96 may be greater than an axial ring
thickness 98,
which may be defined between the annular faces 100 and 102. The radial width
96 may be
four or more times greater than the axial thickness 98. The radial width 96
may be smaller
than a radius 93A of the open void center 93.
[0044] Referring to Figs. 1-10, the accommodation-facilitating
intraocular implant 88
may be formed of suitable material. The intraocular implant 88, for example
the ring 90, may
comprise a rigid or resilient material. The material of the ring 90 being
rigid or resilient may
allow the ring 90 to act on the lens 54 of the IOL 50 as intended, for example
by pressing
11
Date Recue/Date Received 2023-12-12
against and deforming and/or moving the lens 54 to a greater extent than if
the implant 88
was not present. The implant 88 may be made of a material that is relatively
more rigid than
the material of the lens 54. The ring 90 may comprise any suitable rigid
material, such as one
or more of poly(methyl methacrylate) (PMMA) or stainless steel. PMMA is
already
approved for implantation in the human eye 10. The ring 90 may comprise
ultraviolet (A and
B) absorbing material, for example transparent or opaque to visual light,
and/or may be
tinted.
[0045] Referring to Figs. 1-6, as above, the accommodation-facilitating
intraocular
implant 88 may have a plurality of implant haptics 104 angularly spaced around
and radially
extended from the ring 90. The haptics 104 may define an anchor end 106 and a
sulcus end
110. Each haptic 104 may have a tongue 108, which may form a suitable sulcus
gripping part
120, such as an arcuate sulcus gripping part. The sulcus gripping part 120 may
be present at
the sulcus end 110 of the haptics 104. Each haptic 104 may comprise an arm
116, which
may connect the anchor end 106 and the sulcus end 110. The sulcus end 110 of
the haptics
104 may define a suitable gripping profile, such as an indented profile 112,
for example with
scallops, protrusions, contours, textures, or other physical or chemical
structures that
increase the friction between the sulcus 32 and the part 120. The anchor end
106 of the
haptic 104 may connect the haptic 104 to the ring 90, for example as shown
where the
anchor end 106 attaches each haptic to the annular anterior face 100 of the
ring 90. In some
cases, the haptics 104 may be attached to the posterior face or edge of the
ring 90.
[0046] Referring to Figs 1-6, the haptics 104 of the ring 90 may take a
variety of
forms. The haptics 104 may form cantilevers, for example made up of single
arms 116 (Figs.
1-3), dual arms 116 forming bridges (Figs. 4-6), and other designs. Referring
to Figs. 1-3, the
haptics 104 with a single arm 116 may have an indented profile 112 at the
sulcus end 110
extend in one lateral (partially circumferential) direction (Figs. 1-2), or in
two lateral
directions (Fig. 3), or more. The gripping part 120, for example the indented
profile 112 of
same, may extend laterally an arc length of suitable distance, for example
defined by an
angle 118 referring to an angular distance partially around the optical axis
11. An indented
profile 112 or part 120 that is extended in opposing lateral directions about
the arm stem may
define a greater arc angle 118 than in the case of extension in one lateral
direction. Referring
12
Date Recue/Date Received 2023-12-12
to Figs. 4-5, the arms 116 of multi-armed haptics 104 may be connected at the
sulcus end
110, forming a haptic bridge. The double armed 116 haptic 104 may be anchored
to the ring
90 at the anchor end 106 of both arms 116. The ring 90, double arms 116 and
the indented
profile 112 of the haptic may define a cutout or gap 124, which may be defined
by the inner
edge 122 of the haptic 104. The plurality of haptics 104 may be angularly
spaced about axis
11 at suitable intervals around the ring 90. In the examples shown in Figs. 1-
3, a pair of the
single arm 116 haptics 104 are shown directly across the ring 90 from each
other, while in
Fig. 4, a pair of the double armed 116 haptics 104 are shown directly across
the ring 90 from
each other, and in Fig. 5 four of the double armed 116 haptics 104 are shown
evenly spaced
around the ring 90. The arrangement of the haptics 104 is not limited to the
embodiments
shown, for example, single arm 116 haptics 104 are able to be arranged in a
similar fashion
as the double armed 116 haptics 104. In the example shown, the haptics 104 are
shown
spaced evenly around the ring 90, however, the spacing of the haptics 104 may
vary, and the
haptics 104 do not have to be evenly spaced around the ring 90.
[0047] Referring to Figs. 1-6, the accommodation-facilitating
intraocular implant 88
may have a suitable number and arrangement of haptics 104. For example, in
Fig. 1, the
embodiment has two haptics 104, opposed from one another. In Fig. 5 the
implant 88 has
four haptics 104. A relatively increased number of haptics 104 may help
distribute the force
of the ciliary muscles 28 evenly across the ring 90, thereby leading to more
effective use of
the implant 88 as opposed to only two haptics 104. Any suitable number of
haptics 104 may
be used. In some cases, the accommodation-facilitating intraocular implant 88
may have
between two and eight haptics 104.
[0048] Referring to Figs. 7-10, when the implant 88 is inserted in to
the lens bag 24
of the eye 10, the haptics 104 may be inserted into and follow a
circumferential groove 33 of
the sulcus 32 to restrict circumferential sliding of the tongue 108 around the
sulcus 32. The
haptics 104 may be structured to be biased, for example move, under
contraction and
expansion of the ciliary muscles 28 of the eye, to press against and adjust
the ring 90 along
an optical axis 11 of the eye 10. The plurality of haptics 104 of the implant
88 may be
inserted into the circumferential groove 33 of the sulcus 32 anterior relative
to the plurality
of haptics 52 of the IOL 50. The haptics 104 of the implant 88 may be
configured to cause
13
Date Recue/Date Received 2023-12-12
the ring 90 to move or be biased in a posterior direction under contraction of
the ciliary
muscles 28. The biasing or movement of the ring 90 in the posterior direction
may cause the
ring 90 to press upon the IOL 50, and thereby increase a dioptric power of the
IOL 50.
[0049] Referring to Figs. 2 and 7-10, the ring 90 may be appropriately
sized to act
upon the lens 54 of the IOL 50. The inner diameter 92 of the ring 90 may be
smaller than an
outer diameter 55 of the lens 54 of the IOL 50. The outer diameter 94 of the
ring 90 may be
larger than the outer diameter 55 of the lens 54 of the IOL 50. The inner
diameter 92 and the
outer diameter 94 of the ring 90 may be structured to allow for the posterior
face 102 of the
ring 90 to contact, and in at least projection, overlap the edge 56 of the
lens 54 of the IOL 50.
The overlap of the ring 90 onto the outer edge 56 of the lens 54 may allow the
ring 90 to
push the IOL 50 in a backward direction when a ciliary muscle 28 contracts.
[0050] Referring to Figs. 7-10, the accommodation-facilitating
intraocular implant 88
may be implanted via a suitable method. Prior to inserting the accommodation-
facilitating
intraocular implant 88, an incision may be formed into the cornea 12 and
anterior chamber
16 of the eye 10. This method may not need to be performed if a patient has a
pre-existing
incision in the anterior chamber 16, for example if they previously had phakic
or
pseudophakic surgery and were simply adding the accommodation-facilitating
intraocular
implant 88 to an already existing implantable accommodation IOL 50. A surgeon
may use a
suitable tool to make the incision, for example lasers. The anterior chamber
16 incision may
be used for the insertion of the accommodation-facilitating intraocular
implant 88.
[0051] Referring to Figs. 7-10, a suitable implantation method may
incorporate the
destruction or removal of the natural crystalline lens of the eye 10. Such a
method may be
performed in a pseudophakic surgery, such as for the removal of a cataract,
where the natural
crystalline lens of the eye 10 must be destroyed and/or removed. The natural
crystalline lens
of the eye 10 may be destroyed using a suitable technique, such as using
ultrasound waves
through phacoemulsification. The process of phacoemulsification may use an
ultrasonic
probe to break up and emulsify the natural crystalline lens into liquid using
the energy of
ultrasound waves. The resulting emulsion may be vacuumed out through the use
of surgical
tools. Alternatively, a laser may be used to soften the natural crystalline
lens as it is broken
up before phacoemulsification is performed to remove it. Using lasers to
soften followed by
14
Date Recue/Date Received 2023-12-12
ultrasound to break up may help to ensure there are no sharp-edged debris
formed as a result
of the destruction of the natural lens, as such debris could otherwise cause
damage to the eye
during the removal of the natural lens from the capsular lens bag 24.
[0052] Referring to Figs. 7-10, an implantation method may involve
inserting the
intraocular implant 88 into a capsular lens bag 24 of an eye 10, into contact
with and anterior
to the IOL 50 that is also within the capsular lens bag 24. The implant 88 may
be inserted
coaxial with the IOL 50, for example coaxial with optical axis 11 defined by
the eye. The
accommodation-facilitating intraocular implant 88 may be inserted through an
incision in an
anterior portion of the capsular lens bag 24. The intraocular implant 88 may
also be inserted
into the eye 10 as part of the IOL assembly 126, or independently if the
implant 88 is being
inserted into an eye 10 that already has an IOL 50 installed. The
accommodation-facilitating
intraocular implant 88 may be positioned such that the plurality of haptics
104 of the
accommodation-facilitating intraocular implant 88 are inserted into and follow
the
circumferential groove 33 of the sulcus 32 to grip the sulcus 32. During under
contraction
and expansion of ciliary muscles 28 of the eye 10, the plurality of haptics
104 may move to
adjust the ring 90 along an optical axis 11 of the eye 10 to adjust a dioptric
power of the IOL
50 to accommodate a focal power of the eye 10.
[0053] Referring to Figs. 11-16 a variation of an IOL 50 is shown, which
may be
referred to as a mixed curved implantable accommodating intraocular lens (IOL)
50. The
IOL 50 may have a mixed curved optic lens 54. The optic lens 54 may define a
posterior face
74, which may have a concave profile, such as a concave cross-sectional
profile 75 defined
in a plane parallel with the optical axis 11. The optic lens 54 may define an
anterior face 72,
which may have a convex profile, such as a convex cross-sectional profile 73
defined in a
plane parallel with the optical axis 11 (the plane is understood as referring
to the plane of the
page in the embodiments). The faces 72 and 74 may have a suitable convex and
concave,
respectively, profile shape, for example a semi-circular, arcuate, bell,
conical, or other shape
including complex shapes. The posterior face 74 may have another suitable
shape, such as a
flat or convex shape in some cases.
[0054] Referring to Figs. 11-14, the lens 54 of the IOL 50 may have a
suitable shape.
As above, the anterior face 72 of the lens 54 may have a convex curvature. The
posterior
Date Recue/Date Received 2023-12-12
face 74 of the lens 54 may have a convex curvature. The convex cross-sectional
profile 73 of
the anterior face 72 may have a greater degree of curvature than the concave
cross-sectional
profile 75 of the posterior face 74. Other curvature relationships are
possible, including ones
where the profile 75 has more or the same curvature as profile 73. The lens 54
may be a
suitable size, and may define a diameter 55 across the lens 54. The curvature
of the anterior
face 72 may depend on the curvature of the posterior face 74, for example, the
curvatures
may be selected to provide a desired range of dioptric power, using the
formula of the sum of
the curvature of the anterior face 72 in diopters and the curvature of the
posterior face 74 in
diopters, to equal the dioptric power of the lens 54. Example dioptric power
desired in an
IOL may be from 15-25 diopters, although larger or smaller power levels may be
used.
Referring to Figs. 13 and 14, an outer edge 56 of the lens 54 may be shaped
with a non-zero
curvature. The curvature of the outer edge 56 of the lens 54 may provide a
smooth transition
from the convex curvature of the anterior face 72 to the concave curvature of
the posterior
face 74. The anterior curvature of the IOL 50 may be increased such that the
lens 54 is in
contact in use with the iris so that when the pupil contracts the iris can
push the lens 54
backward, resulting in a greater degree of focusing on a near object. With
more curvature,
the dioptric power is increased, imitating normal near vision without reading
glasses. The
IOL 50 may be configured to ensure that an outer edge is relatively hidden by
the iris when
the pupil dilates in the relative darkness at night, thus diminishing or
eliminating night glare.
In some cases, while using a conventional IOL, while driving at night, the
pupil 14 may be
relatively wide and the edge of the implanted lens 54 may be exposed to
incoming light, such
as light coming from an approaching vehicle or street lights. Such may cause
an annoying
glare to the user and my make driving at night difficult. Thus, by making a
lens 54
sufficiently wide to hide the edge of the lens 54 behind the pupillary edge of
a dilated pupil
14, the glare otherwise experienced at night by the user may be reduced or
avoided. The
concave curvature of the posterior of the IOL 50 may provide additional
dioptric power
when the lens is pushed backward. The anterior curvature of the lens 50 may be
increased
because the posterior curve is made concave, keeping desired dioptric power
the same as
present conventional lenses, with increased curvature increasing the force of
the papillary
constriction to push the lens posterior. The decreased curve of the posterior
surface of the
16
Date Recue/Date Received 2023-12-12
lens may decrease chance of damage to the back surface of the lens during
laser capsulotomy
of the posterior capsule, or during other procedures where the IOL 50 remains
in the eye.
[0055] Referring to Figs. 11-16, the IOL 50 may have a plurality of
haptics 52 with
suitable characteristics. In some cases, the haptics 52 may have one, or more,
or all of the
features of haptics 104, and vice versa. The optic lens 54 may be sized to fit
within a
capsular lens bag 24 of an eye 10. The plurality of haptics 52 may be
angularly spaced
around and radially extended from the optic lens 54. Referring to Figs. 13-14,
the haptics 52
may be attached anteriorly to the optic lens 54, for example anterior, or more
anterior than
posterior, of a central profile 53 of the lens 54 defined perpendicular to the
axis 11, and/or
attached to the anterior face 72. Referring to Figs. 15-16 anterior attachment
may assist the
implantable accommodating IOL 50 to move forward during ciliary muscle
contraction, and
may assist with implantation by facilitating the ability of the IOL 50 to
assume a compact,
folded state for implantation. By attaching the haptics to the anterior
surface, the force is
directed to push the lens backward, and the constricting pupil also pushes the
lens backward,
in use. In some cases, the haptics may be attached to the posterior surface or
edge of the
IOL. Referring to Figs. 15 and 16, during implantation, the implantable
accommodating IOL
50 may be inserted into the capsular lens bag 24 of an eye 10 with or without
the removal of
the natural crystalline lens in what is known as a pseudophakic or phakic
surgical procedure,
respectively. Each haptic 52 may be structured to move or be biased, under
contraction and
expansion of ciliary muscles 28 of the eye 10, to adjust one or more of a
position or shape of
the optic lens to accommodate a focal power of the eye 10. Inserting the IOL
50 into the
capsular lens bag 24 of the eye, may be done through an incision in an
anterior portion of the
capsular lens bag 24. The IOL 50 may be positioned so that an arcuate sulcus
gripping parts
120 of the plurality of haptics 52 are inserted into and follow a
circumferential groove 33 of
the sulcus 32 to grip the sulcus 32.
[0056] Referring to Figs. 11-14, the haptics 52 may have suitable
characteristics,
including one or more or all of the characteristics of haptics 52. The
implantable
accommodating IOL 50 may have haptics 52 with an arm 64 that defines a first,
anchor end
58, for example that originates at the optic lens 54, and a second, sulcus end
61 that may
define the tongue 60. The anchor end 58 of the haptic 52 may be directly
attached to the
17
Date Recue/Date Received 2023-12-12
optic lens 54, for example the haptic 52 may be attached to the anterior face
72 of the lens 54
of the IOL 50. The sulcus end 61 may form the arcuate sulcus gripping part 66
to aid in
attachment to the sulcus 32 of the eye 10. Referring to Figs. 13-14, each arm
64 may be a
single arm, and referring to Figs. 11-12, each haptic 52 may be formed of two
or more arms
64, for example forming a haptic bridge as shown. The implantable
accommodating IOL 50
may have a suitable number and arrangement of haptics 52. For example, in Fig.
11, the
embodiment has two haptics 52, opposed from one another. In Fig. 12 the IOL 50
has four
haptics 52. A relatively increased number of haptics 52 may help distribute
the force of the
ciliary muscles 28 evenly across the optic lens 54, thereby leading to more
effective
accommodation of the IOL 50 as opposed to only two haptics 52. Any suitable
number of
haptics 52 may be used. In some cases, the implantable accommodating IOL 50
may have
between two and eight haptics.
[0057] Referring to Figs. 11-16, each haptic 52 may have a tongue 60
that forms an
arcuate sulcus gripping part 66. In use within the capsular lens bag 24, the
gripping part 66
may insert into and follow a circumferential groove 33 of the sulcus 32 to
restrict
circumferential sliding of the tongue 60 around the sulcus 32. A plurality of
haptics 52 may
contain tongues 60 that insert into and follow the circumferential groove 33
of the sulcus 32
of the eye 10. The use of sulcus gripping parts may prevent rotation of the
lens 54 about an
optical axis 11 of the eye 10. An optical axis 11 may be defined as a line
that passes through
a center of the eye 10 and a center of the pupil 14. In use, the implantable
accommodating
IOL 50 may be fitted within a capsular lens bag 24 of an eye 10, with the
respective tongues
60 of the plurality of haptics 52 inserted into and following the
circumferential groove 33 of
the sulcus 32 of the eye 10. The haptics 52 may be configured to bias or move
the IOL 50 in
a posterior direction under contraction of the ciliary muscles 28 of the eye
10 to increase a
dioptric power of the IOL 50.
[0058] Referring to Figs 11-14, the haptics 52 of the IOL 50 may take a
variety of
forms. The haptics 52 may have a single or double arm 64, although only double
arm
versions are shown in these figures. Haptics 52 with a single arm 64 may have
the indented
profile 62 at the sulcus end 61 extend in one lateral direction or in two
lateral directions.
Referring to Figs. 11-12, the arms 64 of the double armed 64 haptics 52, may
be connected
18
Date Recue/Date Received 2023-12-12
at the sulcus end 61 by the indented profile 62 of the haptic 52, forming a
haptic bridge. The
double armed 64 haptic 52 may be anchored to the lens 54 of the IOL 50 at the
anchor end
58 of both arms 64. The lens 54, double arms 64 and the indented profile 62 of
the haptic
may define a cutout or gap 70, which may be defined from the inner edge 68 of
the haptic
52. The plurality of haptics 52 may be spaced out at various intervals around
the lens 54.
[0059] Referring to Figs. 11-14, the IOL 50 may comprise a suitable
material. The
lens 54 may be made of material with a high refractive index. Materials with a
high
refractive index, such as 1.7 or higher, may allow the lens 54 to be made
thinner relative to
lower refractive index materials. Materials with a high refractive index may
allow the
posterior face 74 of the lens 54 to be increased in curvature, compared to if
a material of a
lower refractive index is used. Materials with a higher refractive index can
bend incoming
light more than material with a lower refractive index. Since material with a
higher refractive
index can bend light more readily, less material is need to achieve the same
effect as material
with a lower refractive index. One example of a high refractive index material
is PMMA.
Plural materials may be combined in one IOL, for example PMMA may be used for
an
exterior shell, with a silicon core.
[0060] Referring to Figs. 15-16, the implantable accommodating IOL 50
may be
implanted via a suitable method. In a suitable method an incision may be made
in the eye 10
unless such incision is already present. Prior to inserting the implantable
accommodating
IOL 50, an incision may be formed into the cornea 12 and anterior chamber 16
of the eye 10.
This method may not need to be performed if a patient has a pre-existing
incision in the
anterior chamber 16, for example if they previously had phakic or pseudophakic
surgery and
were simply replacing the implantable accommodation IOL 50. A surgeon may use
a
suitable tool to make the incision, for example lasers. The anterior chamber
16 incision may
be used for the insertion of the implantable accommodating IOL 50. As above, a
suitable
implantation method may incorporate the destruction or removal of the natural
crystalline
lens of the eye 10. Referring to Figs. 15-16, an implantation method may
involve inserting
the IOL 50 into the capsular lens bag of an eye 10 via a suitable method.
Insertion may be
carried out through the incision made prior in an anterior chamber 16 of the
capsular lens
bag 24 of an eye 10, such that the arcuate sulcus gripping parts 66 of the
plurality of haptics
19
Date Recue/Date Received 2023-12-12
52 insert into and follow the circumferential groove 33 of the sulcus 32. The
IOL 50 may be
inserted in a compact, such as a folded, configuration, into the capsular lens
bag 24, which
may be advantageous to retain the IOL 50 in the bag 24 and also to reduce the
size of
incision required. The IOL 50 may be positioned more anterior when compared to
the
placement of other IOL's. The positioning of the IOL 50 being more anterior
may allow the
outer edge 56 of the implantable accommodating IOL 50 to be posterior an iris
18 of the eye
of the user. The IOL 50 being partially behind the iris 18 may allow decrease
or eliminate
night glare. which is a phenomenon in which a source of light does not help
the user see
better, but instead interferes with the user's vision.
[0061] In the claims, the word "comprising" is used in its inclusive
sense and does
not exclude other elements being present. The indefinite articles "a" and "an"
before a claim
feature do not exclude more than one of the feature being present. Each one of
the individual
features described here may be used in one or more embodiments and is not, by
virtue only
of being described here, to be construed as essential to all embodiments as
defined by the
claims.
Date Recue/Date Received 2023-12-12
IMPLANTABLE ACCOMODATING INTRAOCULAR LENSES, IOL IMPLANTS, AND
RELATED SYSTEMS AND METHODS
TECHNICAL FIELD
[0001] This document relates to implantable accommodating intraocular
lens (IOLs),
IOL implants, and related systems and methods.
BACKGROUND
[0002] The following paragraphs are not an admission that anything
discussed in
them is prior art or part of the knowledge of persons skilled in the art.
[0003] Multifocal or monofocal intraocular lenses (IOLs) may be inserted
in the
capsular lens bag of the eye to provide improved vision at a variety or a
single focal distance.
Accommodating lenses such as the CrystalensTM fit within the capsular lens bag
via haptics.
The dioptric power range of the lens is inherently limited by the degree the
lens can move or
adjust.
SUMMARY
[0004] An accommodation-facilitating intraocular implant is disclosed
comprising: a
ring sized to fit within a capsular lens bag of an eye; and a plurality of
haptics angularly
spaced around and radially extended from the ring.
[0005] An accommodating intraocular lens (IOL) assembly is disclosed
comprising:
an implantable accommodating intraocular lens (IOL) within a capsular bag of
an eye, the
implantable accommodating IOL having an optic lens and a plurality of IOL
haptics
angularly spaced around and radially extended from the optic lens; and an
accommodation-
facilitating intraocular implant comprising a ring fitted within the capsular
lens bag of an
eye, anterior to and in contact with the optic lens.
[0006] A method comprising inserting the accommodation-facilitating
intraocular
implant into a capsular lens bag of an eye.
[0007] A method comprising: inserting an accommodation-facilitating
intraocular
implant into a capsular lens bag of an eye, into contact with and anterior to
an intraocular
1
Date Recue/Date Received 2023-12-12
lens (TOL) that is also within the capsular lens bag, in which the
accommodation-facilitating
intraocular implant is inserted through an incision in an anterior portion of
the capsular lens
bag, to position the accommodation-facilitating intraocular implant such that:
a plurality of
haptics of the accommodation-facilitating intraocular implant are inserted
into and follow a
circumferential groove of the sulcus to grip the sulcus; and under contraction
and expansion
of ciliary muscles of the eye, the plurality of haptics move to adjust the
ring along an optical
axis of the eye to adjust a dioptric power of the TOL to accommodate a focal
power of the
eye.
[0008] An implantable accommodating intraocular lens (TOL) comprising:
an optic
lens sized to fit within a capsular lens bag of an eye; a plurality of haptics
angularly spaced
around and radially extended from the curved optic lens, with each haptic
being structured to
move, under contraction and expansion of the ciliary muscles of the eye, to
adjust the optic
lens to accommodate a focal power of the eye; in which: the optic lens defines
a posterior
face and an anterior face; the posterior face has a concave profile; and the
anterior face has a
convex profile.
[0009] A method comprising inserting the accommodation-facilitating
intraocular
implant into a capsular lens bag of an eye, through an incision in the
capsular lens bag, such
that the arcuate sulcus gripping parts of the plurality of haptics insert into
and follow the
circumferential groove of the sulcus to grip the sulcus.
[0010] In various embodiments, there may be included any one or more of
the
following features: An inner annular edge of the ring defines an open void
center. A portion
of the ring defined between inner and outer annular edges of the ring has a
rectangular cross-
sectional shape defined in a plane parallel with a central axis defined by the
ring. A radial
width, of an intraocular-lens-contacting annular posterior face opposite an
annular anterior
face of the ring, is greater than an axial thickness defined between the
intraocular-lens-
contacting annular posterior face and the annular anterior face. A radial
width is four or more
times greater than axial thickness. A radial width, of an intraocular-lens-
contacting annular
posterior face opposite an annular anterior face of the ring, is smaller than
a radius of an
open void center defined by the inner annular edge of the ring. Outer and
inner annular edges
of the ring have a circular shape. A material of the ring is rigid or
resilient. A ring
2
Date Recue/Date Received 2023-12-12
comprising one or more of poly(methyl methacrylate) (PMMA) or stainless steel.
A ring
comprises ultraviolet (A and B) absorbing material. Each haptic: has a tongue
that forms an
arcuate sulcus gripping part that, in use within the capsular lens bag,
inserts into and follows
a circumferential groove of the sulcus to restrict circumferential sliding of
the tongue around
the sulcus; and is structured to move, under contraction and expansion of the
ciliary muscles
of the eye, to adjust the ring along an optical axis of the eye. Each haptic
is attached to an
annular anterior face of the ring. A plurality of implant haptics angularly
spaced around and
radially extended from the ring. A plurality of implant haptics are inserted
into the
circumferential grove of the sulcus anterior relative to the plurality of IOL
haptics. A
plurality of implant haptics are configured to bias the ring in a posterior
direction under
contraction of the ciliary muscles of the eye to press upon the IOL and
thereby increase a
dioptric power of the IOL. An inner diameter of the ring is smaller than an
outer diameter of
the IOL; and an outer diameter of the ring is larger than the outer diameter
of the IOL. A
convex profile of the anterior face has a greater degree of curvature than the
concave profile
of the posterior face. A plurality of haptics is attached to the anterior face
of the IOL. A
plurality of haptics are configured to bias the IOL in a posterior direction
under contraction
of the ciliary muscles of the eye to increase a dioptric power of the IOL. An
IOL comprises a
material of a high refractive index. An implantable accommodating IOL being
positioned
such that an outer edge of the implantable accommodating IOL is posterior an
iris of the eye
of the user. An IOL is positioned relatively more anterior decreases a night
glare experienced
be the user. A concave curvature of the posterior face increases a dioptric
power when the
IOL is moved in a backward direction. A convex curvature of the anterior face
increasing the
dioptric power of the IOL and increases a force of the papillary constriction
in order to move
the IOL in the backward direction.
[0011] The
foregoing summary is not intended to summarize each potential
embodiment or every aspect of the subject matter of the present disclosure.
These and other
aspects of the device and method are set out in the claims.
BRIEF DESCRIPTION OF THE FIGURES
3
Date Recue/Date Received 2023-12-12
[0012] Embodiments will now be described with reference to the figures,
in which
like reference characters denote like elements, by way of example, and in
which:
[0013] Fig. 1 is a front elevation view of an accommodation facilitating
secondary
implant (AFSI), having a ring and two opposed haptic arms, with a plurality of
protrusions
along respective sulcus gripping parts.
[0014] Fig. 2 is a front elevation view, partially in section of the
AFSI of Fig.1
positioned in a human eye, anterior to an intraocular lens (TOL) identified in
dashed lines,
with the ring haptics gripping the sulcus.
[0015] Fig. 3 is a front elevation view of a second embodiment of an
AFSI, having a
ring and two opposed arcuate haptic arms with a plurality of protrusions along
respective
arcuate sulcus gripping parts.
[0016] Fig. 4 is a front elevation view of a third embodiment of an
AFSI, having a
ring and two opposed haptic arm assemblies, each formed of dual arms that form
a haptic
bridge, with a plurality of convex protrusions along respective sulcus
gripping parts.
[0017] Fig. 5 is a front elevation view of a fourth embodiment of an
AFSI, having a
ring and four haptic arm assemblies from Fig. 4, with a plurality of convex
protrusions along
respective sulcus gripping parts.
[0018] Fig. 6 is a side elevation view of an AFSI.
[0019] Fig. 7 is a cross-sectional side view of an embodiment of an AFSI
positioned
in a human eye after the removal of the natural crystalline lens and the
addition of an
implantable accommodating IOL, in which the ciliary muscles are shown relaxed,
with the
lens zonules taut, such that the IOL is accommodating for far-sighted viewing
of a distant
focal point, with focal lines of light illustrated with dashed lines and
travelling from the focal
point and through the AFSI and IOL combination.
[0020] Fig. 8 is a cross-sectional side view of the AFSI, IOL and eye
combination of
Fig. 7 in which the ciliary muscles are contracted and the lens zonules are
slacked, such that
the lens is accommodating for near-sighted viewing of a nearby focal point
(relative to the
focal point in Fig. 7), with focal lines of light illustrated with dashed
lines and travelling
from the focal point and through the AFSI and IOL combination. In Fig. 8 the
AFSI and IOL
combination are moved forward relative to the position the AFSI and IOL adopts
in Fig. 7.
4
Date Recue/Date Received 2023-12-12
[0021] Fig. 9 is a cross-sectional side view of an embodiment of an AFSI
positioned
in a simplified human eye after the removal of the natural crystalline lens
and the addition of
an implantable accommodating IOL, in which the ciliary muscles are shown
relaxed, such
that the lens is accommodating for far-sighted viewing of a distant focal
point, with focal
lines of light illustrated with dashed lines and travelling from the focal
point and through the
AFSI and IOL combination.
[0022] Fig. 10 is a cross-sectional side view of the AFSI, IOL and eye
combination
of Fig. 9, in which the ciliary muscles are contracted, such that the lens is
accommodating
for near-sighted viewing of a nearby focal point (relative to the focal point
in Fig. 9), with
focal lines of light illustrated with dashed lines and travelling from the
focal point and
through the AFSI and IOL combination. In Fig. 9 the AFSI and IOL combination
are moved
forward relative to the position the AFSI and IOL adopts in Fig. 9.
[0023] Fig. 11 is front elevation view of a mixed curvature lens implant
(MCLI)
having an optic lens and two opposed haptic arm assemblies each forming a
haptic bridge,
with a plurality of protrusions along respective sulcus gripping parts.
[0024] Fig. 12 is a front elevation view of a second embodiment of an
MCLI, having
an optic lens and four haptic arm assemblies, with a plurality of protrusions
along respective
sulcus gripping parts.
[0025] Fig. 13 is a side elevation view of a third embodiment of an MCLI
positioned
within a capsular lens bag of a human eye, having an optic lens and two
arcuate haptic arms
with a plurality of protrusions along respective sulcus gripping parts.
[0026] Fig. 14 is a side elevation view of a fourth embodiment of an
MCLI, having
an optic lens and two opposed haptic arms with a plurality of protrusions
along respective
sulcus gripping parts.
[0027] Fig. 15 is a cross-sectional side view of an embodiment of an
MCLI
positioned in a human eye after the removal of the natural crystalline lens,
in which the
ciliary muscles are shown relaxed, with the lens zonules taut, such that the
lens is
accommodating for far-sighted viewing of a distant focal point, with focal
lines of light
illustrated with dashed lines and travelling from the focal point and through
the MCLI.
Date Recue/Date Received 2023-12-12
[0028] Fig. 16 is a cross-sectional side view of the MCLI and eye
combination of
Fig. 15 in which the ciliary muscles are contracted and the lens zonules are
slacked, such that
the lens is accommodating for near-sighted viewing of a nearby focal point
(relative to the
focal point in Fig. 15), with focal lines of light illustrated with dashed
lines and travelling
from the focal point and through the MCLI. In Fig. 16 the MCLI is moved
forward relative
to the position the MCLI adopts in Fig. 15.
DETAILED DESCRIPTION
[0029] Immaterial modifications may be made to the embodiments described
here
without departing from what is covered by the claims.
[0030] Problems with vision may take numerous forms. These include
myopia
(nearsightedness), hyperopia (farsightedness) as well as cataracts
(opacification of the lens).
Contact lenses and glasses containing refractive lenses are commonly used for
basic
correction of myopia, hyperopia, and astigmatism. Both contact lenses and
glasses represent
non-permanent solutions that are susceptible to loss, breakage and require
cleaning in order
to maintain efficacy.
[0031] An implantable intraocular contact lens, known as an IOL, is a
surgical
implantation used to permanently improve an eyesight condition, such as
myopia, hyperopia
or cataracts. An IOL incorporates a corrective lens tailored and structured to
the degree of
vision impairment desired to be corrected. An IOL solution may be a viable
option for a
patient who has a condition that would otherwise disqualify them from
alternative laser-
assisted in situ keratomileusis (LASIK) treatment such as: thin corneas, dry
eyes or
astigmatism (imperfection in the curvature of the lens). An IOL may be
considered and used
as a permanent vision correction solution, but may be removed or adjusted to
address any
change in efficacy or in a patient's vision deficit.
[0032] Two types of IOL solutions exists - phakic and pseudophakic. With
a phakic
solution ('phakic' meaning "having a lens") the eye's natural lens is left
untouched.
Intraocular lenses that are implanted into eyes after the eye's natural lens
has been removed
during cataract surgery are known as pseudophakic. Phakic intraocular lenses
are indicated
for patients with high refractive errors when the usual laser options for
surgical correction
6
Date Recue/Date Received 2023-12-12
(LASIK and PRK) are contraindicated. Phakic IOLs may be designed to correct
high myopia
ranging from ¨5 to ¨20 D if the patient has enough anterior chamber depth
(ACD) of at least
3 mm. The most common type of IOL is the pseudophakic IOL, which may be
implanted
after the eye's natural lens has been removed. The pseudophakic IOL provides
the same light
focusing function as the natural crystalline lens. A pseudophakic IOL may be
available as:
monofocal (focus on only one distance), multifocal (for example bifocal), or
accommodating
(permits focus changing).
[0033] An IOL may contain non-optic side struts known as haptics. A
haptic may be
the part of an IOL responsible for its attachment to the ciliary muscles or
suspensory
ligaments called lens zonules, which are connected to both the ciliary muscle
and natural
crystalline lens within the capsular lens bag of the eye. Haptics may use
hinges at its ends to
aid in attaching to the ciliary muscles or zonules. In any given IOL, the
haptics may vary in
number and shape, including having loops or hooks, for example having loops to
sew into
the ciliary sulcus of the eye.
[0034] Accommodation is how an eye may change optical power to maintain
a clear
image as the eye focuses on objects at different distances. When the eye
focuses on an object
that is relatively far away, the ciliary muscles may relax, leading to the
lens zonules
becoming taut, leading to a flattening of the natural crystalline lens. When
the eye focuses on
an object that is relatively near an individual, the ciliary muscles may
contract, leading to the
lens zonules slackening, reducing tension upon the natural crystalline lens,
making the lens
more convex.
[0035] An IOL may be designed to use non-optical elements known as
haptics to
connect to the ciliary muscles or zonules of the eye, allowing for
accommodation to occur.
With an accommodating IOL, the accommodation process may occur as a result of
one or
more of a change in the shape of the lens or a change in the position of the
lens relative to the
lens capsule. In the case of the former (change in lens shape causing
accommodation),
similar to the natural crystalline lens, when viewing an object that is
relatively nearby, the
ciliary muscles may contract, resulting in reduced tension on the haptics,
resulting in the lens
becoming convex in shape. As well, when viewing an object that is relatively
far away, the
ciliary muscles relax, increasing the tension on the haptics and flattening
the natural
7
Date Recue/Date Received 2023-12-12
crystalline lens. In the case of the latter (movement of lens causing
accommodation),
accommodation may occur through the haptics changing the position of the lens
anterior or
posterior relative to the lens capsule. When viewing an object that is
relatively nearby, for
example a book held at arm's length, under the tension of the contracted
ciliary muscles the
haptics may push the lens in an anterior direction, moving the lens relatively
closer to the
pupil. When viewing an object that is far away, the ciliary muscles may relax,
resulting in
the haptics pushing the lens in a posterior direction, moving the lens
relatively further from
the pupil. It is through such anterior-posterior movement of the lens that
accommodation
may be achieved in a manner analogous to that of the natural eye.
[0036] In contrast to accommodation, a static or non-accommodating IOL
may be
used, for example with a monofocal or multifocal lens. A monofocal lens may
only focus at
a single distance, for example a distance over 20 meters to correct only
distance vision. A
multifocal IOL may have plural regions that each focus at different relative
distances, for
example two or three focal regions spaced throughout the lens simultaneously
based on the
position of the pupil. In some cases, the central part of the lens may be
designed for focusing
on nearby objects, while the outer regions of the lens may be structured for
focusing on far
away objects. When viewing a nearby object, the pupil of the eye may constrict
and the
central region of the IOL may be used, while for far away viewing the pupil
dilates and an
outer IOL focal region may be used.
[0037] Some newer lens designs attempt to allow the eye to regain some
partial
focusing ability in order to change focus from distance to near via
accommodation.
However, many accommodating IOLs used today only achieve a very limited
improvements
in near vision which reduced over time. Accommodative IOLs may also have a
slightly
higher risk of developing posterior capsule opacification (PCO), though there
is some
uncertainty around this finding. PCO is a common side-effect of many cataract
surgeries and
is easily treatable with a one-time laser capsulotomy procedure. Accommodating
IOLs
interact with ciliary muscles and zonules, using hinges at both ends to latch
on and move
forward and backward inside the eye using the same mechanism as normal
accommodation.
8
Date Recue/Date Received 2023-12-12
The haptic hinges may be made of an advanced silicone called BioSil that has
been
thoroughly tested to make sure it is capable of unlimited flexing in the eye.
[0038] An IOL may be implanted in a surgical procedure. A surgeon may
use drugs
to dilate the pupil of the patient. A cut may be made into the cornea and
anterior capsular
lens bag of the eye, where the natural crystalline lens is contained, to
facilitate the insertion
of surgical tools and an IOL. The natural crystalline lens may be destroyed in
what is known
as a pseudophakic procedure, by a suitable technique such as the use of a
laser or ultrasound.
In some cases, it may be unnecessary to destroy the crystalline lens, such as
where the
crystalline lens has already been removed or destroyed in a previous
procedure, as might be
the case where an IOL is being replaced or upgraded. Alternatively, in a
phakic procedure
the natural crystalline lens may be kept intact. An IOL may then be inserted
into the capsular
lens bag. Insertion may be achieved by folding the IOL and inserting it
through the cut made
in the anterior lens capsule lens, assuming that the IOL is made with flexible
material. The
non-optic haptics may contact the sulcus of the eye.
[0039] There may be various problems with IOLs. Stiff haptics may impair
the
ability of the ciliary muscles to change the shape of the lens. Stiff haptics
may further make
it difficult to remove an IOL if a patient elects to do so after having a
phakic procedure. Lens
zonules may drive accommodation as opposed to the ciliary muscles, reducing
the eye's
ability to accommodate as following the initial cut into the anterior lens
capsule the zonular
system may not perform as efficiently as pre-surgery to change the shape of
the lens. IOLs
may need to be tailored in size to a patient's eye, and thus would not be
considered to be
one-size-fits-all. As the sulcus shape to which the IOL must match cannot be
accurately
measured, a surgeon may implant either too large of an optic lens, which will
resist ciliary
muscle action, or too small of an optic lens, in which the ciliary muscle may
not
accommodate properly or at all.
[0040] Referring to Figs. 1-10, an accommodation-facilitating
intraocular implant 88
(which may be referred elsewhere in this document as an AFSI), may be used to
assist in the
accommodation of an accommodating intraocular lens (IOL). The accommodation-
facilitating intraocular implant 88 may comprise a ring 90. The implant 88 may
also have a
9
Date Recue/Date Received 2023-12-12
plurality of haptics 104. The ring 90 may be sized to fit within a capsular
lens bag 24 of an
eye 10. The plurality of haptics 104 may be angularly spaced around (for
example around an
optical axis 11, which is discussed herein as defined by the eye, but may also
for
convenience of discussion be referred to as being defined by the ring 90
and/or the IOL 50)
and radially extended from the ring 90. The implant 88 may be used with an
accommodating
or non-accommodating IOL 50, and with mono or multi-focal IOLs. The implant 88
may
have no dioptric power. The use of the ring 90 pushes the lens 54 backward
toward the retina
in use, allowing a user to experience relatively improved near vision. The
lens may have a
fixed dioptric power, or in the case of flexible IOLs such as made of foldable
silicon, may
slightly increase the dioptric power of the lens in use. In some cases, the
IOL may comprise
flexible or pliable material, such as a liquid, solid, or gel.
[0041] Referring to Figs. 2 and 7-10, the accommodation-facilitating
intraocular
implant 88 may form part of an accommodating IOL assembly 126. An
accommodating IOL
assembly 126 may include an implant 88 and the IOL 50. The implant 88 may be
referred to
as a secondary implant, as such may be inserted to assist an existing IOL 50.
The
accommodating IOL assembly 126 may comprise an implantable accommodating IOL
50,
which may comprise an optic lens 54. The IOL 50 may also comprise a plurality
of IOL
haptics 52 angularly spaced around (for example around optical axis 11 defined
by the IOL
50 and/or eye and/or ring 90) and radially extended from the optic lens 54.
The ring 90 may
be fitted within the capsular lens bag 24 of the eye 10 in use, for example
anterior to and in
contact with the optic lens 54.
[0042] Referring to Figs. 7-10, the accommodation-facilitating
intraocular implant 88
may be inserted within the capsular lens bag 24 of an eye 10 in a method of
use. Once the
intraocular implant 88 is within the lens bag 24, the implant 88 may be placed
anterior to and
in contact, for example direct or indirect, with the lens 54 of the IOL 50
that is also with in
the lens bag 24. The accommodation-facilitating intraocular implant 88 may be
inserted
during surgery through an incision in an anterior portion of the capsular lens
bag 24, to
position the accommodation-facilitating intraocular implant 88. During the
positioning of the
intraocular implant 88, the plurality of haptics 104 of the accommodation-
facilitating
intraocular implant 88 are inserted into and may follow a circumferential
groove 33 of the
Date Recue/Date Received 2023-12-12
sulcus 32 to grip the sulcus 32. Once the haptics 104 of the intraocular
implant 88 are
positioned in the sulcus 32, under contraction and expansion of ciliary
muscles 28 of the eye,
the plurality of haptics 104 may be biased by the ciliary muscles 28 to adjust
the ring 90
along an optical axis 11 of the eye 10 to adjust a dioptric power of the IOL
50 to
accommodate a focal power of the eye 10.
[0043] Referring to Figs. 1-10, the accommodation-facilitating
intraocular implant 88
may have a suitable structure. The ring 90 may comprise any suitable shape,
such as a
circular shape, elliptical shape, or an oblong shape. The ring 90 of the
implant 88 may define
an inner annular edge 91, an outer annular edge 95, an annular anterior face
100, and an
intraocular-lens-contacting annular posterior face 102. The inner annular edge
91 may define
an open void center 93 of the ring 90. The void center 93 may allow the light
entering 78 the
eye 10 to pass through unhindered toward the lens 54 of the IOL 50. Referring
to Figs. 6-10,
a portion of the ring 90 defined between the inner annular edge 91 and the
outer annular edge
95 may have a rectangular cross-sectional shape, for example defined in a
plane (the plane is
understood as being the plane of the page in Figs. 6-10) parallel with a
central axis 11
defined by the ring and/or eye. The rectangular shape may include rounded
corners, and in
some cases nominal curvature of sides. In some cases, the cross-sectional
shape of the ring
may follow a circle, oval, ellipse, or other shape. The outer annular edge 95
of the ring 90
may allow the ring 90 to define an outer diameter 94. The ring 90 may define a
variety of
dimensions, such as inner and outer diameters 92 and 94 defined by the inner
and outer
annular peripheral edges 91 and 95, respectively. A radial width 96, which may
be defined
between a radius 94A defined by the outer annular edge 95 and a radius 93A
defined by the
inner annular edge 91. The radial width 96 may be greater than an axial ring
thickness 98,
which may be defined between the annular faces 100 and 102. The radial width
96 may be
four or more times greater than the axial thickness 98. The radial width 96
may be smaller
than a radius 93A of the open void center 93.
[0044] Referring to Figs. 1-10, the accommodation-facilitating
intraocular implant 88
may be formed of suitable material. The intraocular implant 88, for example
the ring 90, may
comprise a rigid or resilient material. The material of the ring 90 being
rigid or resilient may
allow the ring 90 to act on the lens 54 of the IOL 50 as intended, for example
by pressing
11
Date Recue/Date Received 2023-12-12
against and deforming and/or moving the lens 54 to a greater extent than if
the implant 88
was not present. The implant 88 may be made of a material that is relatively
more rigid than
the material of the lens 54. The ring 90 may comprise any suitable rigid
material, such as one
or more of poly(methyl methacrylate) (PMMA) or stainless steel. PMMA is
already
approved for implantation in the human eye 10. The ring 90 may comprise
ultraviolet (A and
B) absorbing material, for example transparent or opaque to visual light,
and/or may be
tinted.
[0045] Referring to Figs. 1-6, as above, the accommodation-facilitating
intraocular
implant 88 may have a plurality of implant haptics 104 angularly spaced around
and radially
extended from the ring 90. The haptics 104 may define an anchor end 106 and a
sulcus end
110. Each haptic 104 may have a tongue 108, which may form a suitable sulcus
gripping part
120, such as an arcuate sulcus gripping part. The sulcus gripping part 120 may
be present at
the sulcus end 110 of the haptics 104. Each haptic 104 may comprise an arm
116, which
may connect the anchor end 106 and the sulcus end 110. The sulcus end 110 of
the haptics
104 may define a suitable gripping profile, such as an indented profile 112,
for example with
scallops, protrusions, contours, textures, or other physical or chemical
structures that
increase the friction between the sulcus 32 and the part 120. The anchor end
106 of the
haptic 104 may connect the haptic 104 to the ring 90, for example as shown
where the
anchor end 106 attaches each haptic to the annular anterior face 100 of the
ring 90. In some
cases, the haptics 104 may be attached to the posterior face or edge of the
ring 90.
[0046] Referring to Figs 1-6, the haptics 104 of the ring 90 may take a
variety of
forms. The haptics 104 may form cantilevers, for example made up of single
arms 116 (Figs.
1-3), dual arms 116 forming bridges (Figs. 4-6), and other designs. Referring
to Figs. 1-3, the
haptics 104 with a single arm 116 may have an indented profile 112 at the
sulcus end 110
extend in one lateral (partially circumferential) direction (Figs. 1-2), or in
two lateral
directions (Fig. 3), or more. The gripping part 120, for example the indented
profile 112 of
same, may extend laterally an arc length of suitable distance, for example
defined by an
angle 118 referring to an angular distance partially around the optical axis
11. An indented
profile 112 or part 120 that is extended in opposing lateral directions about
the arm stem may
define a greater arc angle 118 than in the case of extension in one lateral
direction. Referring
12
Date Recue/Date Received 2023-12-12
to Figs. 4-5, the arms 116 of multi-armed haptics 104 may be connected at the
sulcus end
110, forming a haptic bridge. The double armed 116 haptic 104 may be anchored
to the ring
90 at the anchor end 106 of both arms 116. The ring 90, double arms 116 and
the indented
profile 112 of the haptic may define a cutout or gap 124, which may be defined
by the inner
edge 122 of the haptic 104. The plurality of haptics 104 may be angularly
spaced about axis
11 at suitable intervals around the ring 90. In the examples shown in Figs. 1-
3, a pair of the
single arm 116 haptics 104 are shown directly across the ring 90 from each
other, while in
Fig. 4, a pair of the double armed 116 haptics 104 are shown directly across
the ring 90 from
each other, and in Fig. 5 four of the double armed 116 haptics 104 are shown
evenly spaced
around the ring 90. The arrangement of the haptics 104 is not limited to the
embodiments
shown, for example, single arm 116 haptics 104 are able to be arranged in a
similar fashion
as the double armed 116 haptics 104. In the example shown, the haptics 104 are
shown
spaced evenly around the ring 90, however, the spacing of the haptics 104 may
vary, and the
haptics 104 do not have to be evenly spaced around the ring 90.
[0047] Referring to Figs. 1-6, the accommodation-facilitating
intraocular implant 88
may have a suitable number and arrangement of haptics 104. For example, in
Fig. 1, the
embodiment has two haptics 104, opposed from one another. In Fig. 5 the
implant 88 has
four haptics 104. A relatively increased number of haptics 104 may help
distribute the force
of the ciliary muscles 28 evenly across the ring 90, thereby leading to more
effective use of
the implant 88 as opposed to only two haptics 104. Any suitable number of
haptics 104 may
be used. In some cases, the accommodation-facilitating intraocular implant 88
may have
between two and eight haptics 104.
[0048] Referring to Figs. 7-10, when the implant 88 is inserted in to
the lens bag 24
of the eye 10, the haptics 104 may be inserted into and follow a
circumferential groove 33 of
the sulcus 32 to restrict circumferential sliding of the tongue 108 around the
sulcus 32. The
haptics 104 may be structured to be biased, for example move, under
contraction and
expansion of the ciliary muscles 28 of the eye, to press against and adjust
the ring 90 along
an optical axis 11 of the eye 10. The plurality of haptics 104 of the implant
88 may be
inserted into the circumferential groove 33 of the sulcus 32 anterior relative
to the plurality
of haptics 52 of the IOL 50. The haptics 104 of the implant 88 may be
configured to cause
13
Date Recue/Date Received 2023-12-12
the ring 90 to move or be biased in a posterior direction under contraction of
the ciliary
muscles 28. The biasing or movement of the ring 90 in the posterior direction
may cause the
ring 90 to press upon the IOL 50, and thereby increase a dioptric power of the
IOL 50.
[0049] Referring to Figs. 2 and 7-10, the ring 90 may be appropriately
sized to act
upon the lens 54 of the IOL 50. The inner diameter 92 of the ring 90 may be
smaller than an
outer diameter 55 of the lens 54 of the IOL 50. The outer diameter 94 of the
ring 90 may be
larger than the outer diameter 55 of the lens 54 of the IOL 50. The inner
diameter 92 and the
outer diameter 94 of the ring 90 may be structured to allow for the posterior
face 102 of the
ring 90 to contact, and in at least projection, overlap the edge 56 of the
lens 54 of the IOL 50.
The overlap of the ring 90 onto the outer edge 56 of the lens 54 may allow the
ring 90 to
push the IOL 50 in a backward direction when a ciliary muscle 28 contracts.
[0050] Referring to Figs. 7-10, the accommodation-facilitating
intraocular implant 88
may be implanted via a suitable method. Prior to inserting the accommodation-
facilitating
intraocular implant 88, an incision may be formed into the cornea 12 and
anterior chamber
16 of the eye 10. This method may not need to be performed if a patient has a
pre-existing
incision in the anterior chamber 16, for example if they previously had phakic
or
pseudophakic surgery and were simply adding the accommodation-facilitating
intraocular
implant 88 to an already existing implantable accommodation IOL 50. A surgeon
may use a
suitable tool to make the incision, for example lasers. The anterior chamber
16 incision may
be used for the insertion of the accommodation-facilitating intraocular
implant 88.
[0051] Referring to Figs. 7-10, a suitable implantation method may
incorporate the
destruction or removal of the natural crystalline lens of the eye 10. Such a
method may be
performed in a pseudophakic surgery, such as for the removal of a cataract,
where the natural
crystalline lens of the eye 10 must be destroyed and/or removed. The natural
crystalline lens
of the eye 10 may be destroyed using a suitable technique, such as using
ultrasound waves
through phacoemulsification. The process of phacoemulsification may use an
ultrasonic
probe to break up and emulsify the natural crystalline lens into liquid using
the energy of
ultrasound waves. The resulting emulsion may be vacuumed out through the use
of surgical
tools. Alternatively, a laser may be used to soften the natural crystalline
lens as it is broken
up before phacoemulsification is performed to remove it. Using lasers to
soften followed by
14
Date Recue/Date Received 2023-12-12
ultrasound to break up may help to ensure there are no sharp-edged debris
formed as a result
of the destruction of the natural lens, as such debris could otherwise cause
damage to the eye
during the removal of the natural lens from the capsular lens bag 24.
[0052] Referring to Figs. 7-10, an implantation method may involve
inserting the
intraocular implant 88 into a capsular lens bag 24 of an eye 10, into contact
with and anterior
to the IOL 50 that is also within the capsular lens bag 24. The implant 88 may
be inserted
coaxial with the IOL 50, for example coaxial with optical axis 11 defined by
the eye. The
accommodation-facilitating intraocular implant 88 may be inserted through an
incision in an
anterior portion of the capsular lens bag 24. The intraocular implant 88 may
also be inserted
into the eye 10 as part of the IOL assembly 126, or independently if the
implant 88 is being
inserted into an eye 10 that already has an IOL 50 installed. The
accommodation-facilitating
intraocular implant 88 may be positioned such that the plurality of haptics
104 of the
accommodation-facilitating intraocular implant 88 are inserted into and follow
the
circumferential groove 33 of the sulcus 32 to grip the sulcus 32. During under
contraction
and expansion of ciliary muscles 28 of the eye 10, the plurality of haptics
104 may move to
adjust the ring 90 along an optical axis 11 of the eye 10 to adjust a dioptric
power of the IOL
50 to accommodate a focal power of the eye 10.
[0053] Referring to Figs. 11-16 a variation of an IOL 50 is shown, which
may be
referred to as a mixed curved implantable accommodating intraocular lens (IOL)
50. The
IOL 50 may have a mixed curved optic lens 54. The optic lens 54 may define a
posterior face
74, which may have a concave profile, such as a concave cross-sectional
profile 75 defined
in a plane parallel with the optical axis 11. The optic lens 54 may define an
anterior face 72,
which may have a convex profile, such as a convex cross-sectional profile 73
defined in a
plane parallel with the optical axis 11 (the plane is understood as referring
to the plane of the
page in the embodiments). The faces 72 and 74 may have a suitable convex and
concave,
respectively, profile shape, for example a semi-circular, arcuate, bell,
conical, or other shape
including complex shapes. The posterior face 74 may have another suitable
shape, such as a
flat or convex shape in some cases.
[0054] Referring to Figs. 11-14, the lens 54 of the IOL 50 may have a
suitable shape.
As above, the anterior face 72 of the lens 54 may have a convex curvature. The
posterior
Date Recue/Date Received 2023-12-12
face 74 of the lens 54 may have a convex curvature. The convex cross-sectional
profile 73 of
the anterior face 72 may have a greater degree of curvature than the concave
cross-sectional
profile 75 of the posterior face 74. Other curvature relationships are
possible, including ones
where the profile 75 has more or the same curvature as profile 73. The lens 54
may be a
suitable size, and may define a diameter 55 across the lens 54. The curvature
of the anterior
face 72 may depend on the curvature of the posterior face 74, for example, the
curvatures
may be selected to provide a desired range of dioptric power, using the
formula of the sum of
the curvature of the anterior face 72 in diopters and the curvature of the
posterior face 74 in
diopters, to equal the dioptric power of the lens 54. Example dioptric power
desired in an
IOL may be from 15-25 diopters, although larger or smaller power levels may be
used.
Referring to Figs. 13 and 14, an outer edge 56 of the lens 54 may be shaped
with a non-zero
curvature. The curvature of the outer edge 56 of the lens 54 may provide a
smooth transition
from the convex curvature of the anterior face 72 to the concave curvature of
the posterior
face 74. The anterior curvature of the IOL 50 may be increased such that the
lens 54 is in
contact in use with the iris so that when the pupil contracts the iris can
push the lens 54
backward, resulting in a greater degree of focusing on a near object. With
more curvature,
the dioptric power is increased, imitating normal near vision without reading
glasses. The
IOL 50 may be configured to ensure that an outer edge is relatively hidden by
the iris when
the pupil dilates in the relative darkness at night, thus diminishing or
eliminating night glare.
In some cases, while using a conventional IOL, while driving at night, the
pupil 14 may be
relatively wide and the edge of the implanted lens 54 may be exposed to
incoming light, such
as light coming from an approaching vehicle or street lights. Such may cause
an annoying
glare to the user and my make driving at night difficult. Thus, by making a
lens 54
sufficiently wide to hide the edge of the lens 54 behind the pupillary edge of
a dilated pupil
14, the glare otherwise experienced at night by the user may be reduced or
avoided. The
concave curvature of the posterior of the IOL 50 may provide additional
dioptric power
when the lens is pushed backward. The anterior curvature of the lens 50 may be
increased
because the posterior curve is made concave, keeping desired dioptric power
the same as
present conventional lenses, with increased curvature increasing the force of
the papillary
constriction to push the lens posterior. The decreased curve of the posterior
surface of the
16
Date Recue/Date Received 2023-12-12
lens may decrease chance of damage to the back surface of the lens during
laser capsulotomy
of the posterior capsule, or during other procedures where the IOL 50 remains
in the eye.
[0055] Referring to Figs. 11-16, the IOL 50 may have a plurality of
haptics 52 with
suitable characteristics. In some cases, the haptics 52 may have one, or more,
or all of the
features of haptics 104, and vice versa. The optic lens 54 may be sized to fit
within a
capsular lens bag 24 of an eye 10. The plurality of haptics 52 may be
angularly spaced
around and radially extended from the optic lens 54. Referring to Figs. 13-14,
the haptics 52
may be attached anteriorly to the optic lens 54, for example anterior, or more
anterior than
posterior, of a central profile 53 of the lens 54 defined perpendicular to the
axis 11, and/or
attached to the anterior face 72. Referring to Figs. 15-16 anterior attachment
may assist the
implantable accommodating IOL 50 to move forward during ciliary muscle
contraction, and
may assist with implantation by facilitating the ability of the IOL 50 to
assume a compact,
folded state for implantation. By attaching the haptics to the anterior
surface, the force is
directed to push the lens backward, and the constricting pupil also pushes the
lens backward,
in use. In some cases, the haptics may be attached to the posterior surface or
edge of the
IOL. Referring to Figs. 15 and 16, during implantation, the implantable
accommodating IOL
50 may be inserted into the capsular lens bag 24 of an eye 10 with or without
the removal of
the natural crystalline lens in what is known as a pseudophakic or phakic
surgical procedure,
respectively. Each haptic 52 may be structured to move or be biased, under
contraction and
expansion of ciliary muscles 28 of the eye 10, to adjust one or more of a
position or shape of
the optic lens to accommodate a focal power of the eye 10. Inserting the IOL
50 into the
capsular lens bag 24 of the eye, may be done through an incision in an
anterior portion of the
capsular lens bag 24. The IOL 50 may be positioned so that an arcuate sulcus
gripping parts
120 of the plurality of haptics 52 are inserted into and follow a
circumferential groove 33 of
the sulcus 32 to grip the sulcus 32.
[0056] Referring to Figs. 11-14, the haptics 52 may have suitable
characteristics,
including one or more or all of the characteristics of haptics 52. The
implantable
accommodating IOL 50 may have haptics 52 with an arm 64 that defines a first,
anchor end
58, for example that originates at the optic lens 54, and a second, sulcus end
61 that may
define the tongue 60. The anchor end 58 of the haptic 52 may be directly
attached to the
17
Date Recue/Date Received 2023-12-12
optic lens 54, for example the haptic 52 may be attached to the anterior face
72 of the lens 54
of the IOL 50. The sulcus end 61 may form the arcuate sulcus gripping part 66
to aid in
attachment to the sulcus 32 of the eye 10. Referring to Figs. 13-14, each arm
64 may be a
single arm, and referring to Figs. 11-12, each haptic 52 may be formed of two
or more arms
64, for example forming a haptic bridge as shown. The implantable
accommodating IOL 50
may have a suitable number and arrangement of haptics 52. For example, in Fig.
11, the
embodiment has two haptics 52, opposed from one another. In Fig. 12 the IOL 50
has four
haptics 52. A relatively increased number of haptics 52 may help distribute
the force of the
ciliary muscles 28 evenly across the optic lens 54, thereby leading to more
effective
accommodation of the IOL 50 as opposed to only two haptics 52. Any suitable
number of
haptics 52 may be used. In some cases, the implantable accommodating IOL 50
may have
between two and eight haptics.
[0057] Referring to Figs. 11-16, each haptic 52 may have a tongue 60
that forms an
arcuate sulcus gripping part 66. In use within the capsular lens bag 24, the
gripping part 66
may insert into and follow a circumferential groove 33 of the sulcus 32 to
restrict
circumferential sliding of the tongue 60 around the sulcus 32. A plurality of
haptics 52 may
contain tongues 60 that insert into and follow the circumferential groove 33
of the sulcus 32
of the eye 10. The use of sulcus gripping parts may prevent rotation of the
lens 54 about an
optical axis 11 of the eye 10. An optical axis 11 may be defined as a line
that passes through
a center of the eye 10 and a center of the pupil 14. In use, the implantable
accommodating
IOL 50 may be fitted within a capsular lens bag 24 of an eye 10, with the
respective tongues
60 of the plurality of haptics 52 inserted into and following the
circumferential groove 33 of
the sulcus 32 of the eye 10. The haptics 52 may be configured to bias or move
the IOL 50 in
a posterior direction under contraction of the ciliary muscles 28 of the eye
10 to increase a
dioptric power of the IOL 50.
[0058] Referring to Figs 11-14, the haptics 52 of the IOL 50 may take a
variety of
forms. The haptics 52 may have a single or double arm 64, although only double
arm
versions are shown in these figures. Haptics 52 with a single arm 64 may have
the indented
profile 62 at the sulcus end 61 extend in one lateral direction or in two
lateral directions.
Referring to Figs. 11-12, the arms 64 of the double armed 64 haptics 52, may
be connected
18
Date Recue/Date Received 2023-12-12
at the sulcus end 61 by the indented profile 62 of the haptic 52, forming a
haptic bridge. The
double armed 64 haptic 52 may be anchored to the lens 54 of the IOL 50 at the
anchor end
58 of both arms 64. The lens 54, double arms 64 and the indented profile 62 of
the haptic
may define a cutout or gap 70, which may be defined from the inner edge 68 of
the haptic
52. The plurality of haptics 52 may be spaced out at various intervals around
the lens 54.
[0059] Referring to Figs. 11-14, the IOL 50 may comprise a suitable
material. The
lens 54 may be made of material with a high refractive index. Materials with a
high
refractive index, such as 1.7 or higher, may allow the lens 54 to be made
thinner relative to
lower refractive index materials. Materials with a high refractive index may
allow the
posterior face 74 of the lens 54 to be increased in curvature, compared to if
a material of a
lower refractive index is used. Materials with a higher refractive index can
bend incoming
light more than material with a lower refractive index. Since material with a
higher refractive
index can bend light more readily, less material is need to achieve the same
effect as material
with a lower refractive index. One example of a high refractive index material
is PMMA.
Plural materials may be combined in one IOL, for example PMMA may be used for
an
exterior shell, with a silicon core.
[0060] Referring to Figs. 15-16, the implantable accommodating IOL 50
may be
implanted via a suitable method. In a suitable method an incision may be made
in the eye 10
unless such incision is already present. Prior to inserting the implantable
accommodating
IOL 50, an incision may be formed into the cornea 12 and anterior chamber 16
of the eye 10.
This method may not need to be performed if a patient has a pre-existing
incision in the
anterior chamber 16, for example if they previously had phakic or pseudophakic
surgery and
were simply replacing the implantable accommodation IOL 50. A surgeon may use
a
suitable tool to make the incision, for example lasers. The anterior chamber
16 incision may
be used for the insertion of the implantable accommodating IOL 50. As above, a
suitable
implantation method may incorporate the destruction or removal of the natural
crystalline
lens of the eye 10. Referring to Figs. 15-16, an implantation method may
involve inserting
the IOL 50 into the capsular lens bag of an eye 10 via a suitable method.
Insertion may be
carried out through the incision made prior in an anterior chamber 16 of the
capsular lens
bag 24 of an eye 10, such that the arcuate sulcus gripping parts 66 of the
plurality of haptics
19
Date Recue/Date Received 2023-12-12
52 insert into and follow the circumferential groove 33 of the sulcus 32. The
IOL 50 may be
inserted in a compact, such as a folded, configuration, into the capsular lens
bag 24, which
may be advantageous to retain the IOL 50 in the bag 24 and also to reduce the
size of
incision required. The IOL 50 may be positioned more anterior when compared to
the
placement of other IOL's. The positioning of the IOL 50 being more anterior
may allow the
outer edge 56 of the implantable accommodating IOL 50 to be posterior an iris
18 of the eye
of the user. The IOL 50 being partially behind the iris 18 may allow decrease
or eliminate
night glare. which is a phenomenon in which a source of light does not help
the user see
better, but instead interferes with the user's vision.
[0061] In the claims, the word "comprising" is used in its inclusive
sense and does
not exclude other elements being present. The indefinite articles "a" and "an"
before a claim
feature do not exclude more than one of the feature being present. Each one of
the individual
features described here may be used in one or more embodiments and is not, by
virtue only
of being described here, to be construed as essential to all embodiments as
defined by the
claims.
Date Recue/Date Received 2023-12-12
IMPLANTABLE ACCOMODATING INTRAOCULAR LENSES, IOL IMPLANTS, AND
RELATED SYSTEMS AND METHODS
TECHNICAL FIELD
[0001] This document relates to implantable accommodating intraocular
lens (IOLs),
IOL implants, and related systems and methods.
BACKGROUND
[0002] The following paragraphs are not an admission that anything
discussed in
them is prior art or part of the knowledge of persons skilled in the art.
[0003] Multifocal or monofocal intraocular lenses (IOLs) may be inserted
in the
capsular lens bag of the eye to provide improved vision at a variety or a
single focal distance.
Accommodating lenses such as the CrystalensTM fit within the capsular lens bag
via haptics.
The dioptric power range of the lens is inherently limited by the degree the
lens can move or
adjust.
SUMMARY
[0004] An accommodation-facilitating intraocular implant is disclosed
comprising: a
ring sized to fit within a capsular lens bag of an eye; and a plurality of
haptics angularly
spaced around and radially extended from the ring.
[0005] An accommodating intraocular lens (IOL) assembly is disclosed
comprising:
an implantable accommodating intraocular lens (IOL) within a capsular bag of
an eye, the
implantable accommodating IOL having an optic lens and a plurality of IOL
haptics
angularly spaced around and radially extended from the optic lens; and an
accommodation-
facilitating intraocular implant comprising a ring fitted within the capsular
lens bag of an
eye, anterior to and in contact with the optic lens.
[0006] A method comprising inserting the accommodation-facilitating
intraocular
implant into a capsular lens bag of an eye.
[0007] A method comprising: inserting an accommodation-facilitating
intraocular
implant into a capsular lens bag of an eye, into contact with and anterior to
an intraocular
1
Date Recue/Date Received 2023-12-12
lens (TOL) that is also within the capsular lens bag, in which the
accommodation-facilitating
intraocular implant is inserted through an incision in an anterior portion of
the capsular lens
bag, to position the accommodation-facilitating intraocular implant such that:
a plurality of
haptics of the accommodation-facilitating intraocular implant are inserted
into and follow a
circumferential groove of the sulcus to grip the sulcus; and under contraction
and expansion
of ciliary muscles of the eye, the plurality of haptics move to adjust the
ring along an optical
axis of the eye to adjust a dioptric power of the TOL to accommodate a focal
power of the
eye.
[0008] An implantable accommodating intraocular lens (TOL) comprising:
an optic
lens sized to fit within a capsular lens bag of an eye; a plurality of haptics
angularly spaced
around and radially extended from the curved optic lens, with each haptic
being structured to
move, under contraction and expansion of the ciliary muscles of the eye, to
adjust the optic
lens to accommodate a focal power of the eye; in which: the optic lens defines
a posterior
face and an anterior face; the posterior face has a concave profile; and the
anterior face has a
convex profile.
[0009] A method comprising inserting the accommodation-facilitating
intraocular
implant into a capsular lens bag of an eye, through an incision in the
capsular lens bag, such
that the arcuate sulcus gripping parts of the plurality of haptics insert into
and follow the
circumferential groove of the sulcus to grip the sulcus.
[0010] In various embodiments, there may be included any one or more of
the
following features: An inner annular edge of the ring defines an open void
center. A portion
of the ring defined between inner and outer annular edges of the ring has a
rectangular cross-
sectional shape defined in a plane parallel with a central axis defined by the
ring. A radial
width, of an intraocular-lens-contacting annular posterior face opposite an
annular anterior
face of the ring, is greater than an axial thickness defined between the
intraocular-lens-
contacting annular posterior face and the annular anterior face. A radial
width is four or more
times greater than axial thickness. A radial width, of an intraocular-lens-
contacting annular
posterior face opposite an annular anterior face of the ring, is smaller than
a radius of an
open void center defined by the inner annular edge of the ring. Outer and
inner annular edges
of the ring have a circular shape. A material of the ring is rigid or
resilient. A ring
2
Date Recue/Date Received 2023-12-12
comprising one or more of poly(methyl methacrylate) (PMMA) or stainless steel.
A ring
comprises ultraviolet (A and B) absorbing material. Each haptic: has a tongue
that forms an
arcuate sulcus gripping part that, in use within the capsular lens bag,
inserts into and follows
a circumferential groove of the sulcus to restrict circumferential sliding of
the tongue around
the sulcus; and is structured to move, under contraction and expansion of the
ciliary muscles
of the eye, to adjust the ring along an optical axis of the eye. Each haptic
is attached to an
annular anterior face of the ring. A plurality of implant haptics angularly
spaced around and
radially extended from the ring. A plurality of implant haptics are inserted
into the
circumferential grove of the sulcus anterior relative to the plurality of IOL
haptics. A
plurality of implant haptics are configured to bias the ring in a posterior
direction under
contraction of the ciliary muscles of the eye to press upon the IOL and
thereby increase a
dioptric power of the IOL. An inner diameter of the ring is smaller than an
outer diameter of
the IOL; and an outer diameter of the ring is larger than the outer diameter
of the IOL. A
convex profile of the anterior face has a greater degree of curvature than the
concave profile
of the posterior face. A plurality of haptics is attached to the anterior face
of the IOL. A
plurality of haptics are configured to bias the IOL in a posterior direction
under contraction
of the ciliary muscles of the eye to increase a dioptric power of the IOL. An
IOL comprises a
material of a high refractive index. An implantable accommodating IOL being
positioned
such that an outer edge of the implantable accommodating IOL is posterior an
iris of the eye
of the user. An IOL is positioned relatively more anterior decreases a night
glare experienced
be the user. A concave curvature of the posterior face increases a dioptric
power when the
IOL is moved in a backward direction. A convex curvature of the anterior face
increasing the
dioptric power of the IOL and increases a force of the papillary constriction
in order to move
the IOL in the backward direction.
[0011] The
foregoing summary is not intended to summarize each potential
embodiment or every aspect of the subject matter of the present disclosure.
These and other
aspects of the device and method are set out in the claims.
BRIEF DESCRIPTION OF THE FIGURES
3
Date Recue/Date Received 2023-12-12
[0012] Embodiments will now be described with reference to the figures,
in which
like reference characters denote like elements, by way of example, and in
which:
[0013] Fig. 1 is a front elevation view of an accommodation facilitating
secondary
implant (AFSI), having a ring and two opposed haptic arms, with a plurality of
protrusions
along respective sulcus gripping parts.
[0014] Fig. 2 is a front elevation view, partially in section of the
AFSI of Fig.1
positioned in a human eye, anterior to an intraocular lens (TOL) identified in
dashed lines,
with the ring haptics gripping the sulcus.
[0015] Fig. 3 is a front elevation view of a second embodiment of an
AFSI, having a
ring and two opposed arcuate haptic arms with a plurality of protrusions along
respective
arcuate sulcus gripping parts.
[0016] Fig. 4 is a front elevation view of a third embodiment of an
AFSI, having a
ring and two opposed haptic arm assemblies, each formed of dual arms that form
a haptic
bridge, with a plurality of convex protrusions along respective sulcus
gripping parts.
[0017] Fig. 5 is a front elevation view of a fourth embodiment of an
AFSI, having a
ring and four haptic arm assemblies from Fig. 4, with a plurality of convex
protrusions along
respective sulcus gripping parts.
[0018] Fig. 6 is a side elevation view of an AFSI.
[0019] Fig. 7 is a cross-sectional side view of an embodiment of an AFSI
positioned
in a human eye after the removal of the natural crystalline lens and the
addition of an
implantable accommodating IOL, in which the ciliary muscles are shown relaxed,
with the
lens zonules taut, such that the IOL is accommodating for far-sighted viewing
of a distant
focal point, with focal lines of light illustrated with dashed lines and
travelling from the focal
point and through the AFSI and IOL combination.
[0020] Fig. 8 is a cross-sectional side view of the AFSI, IOL and eye
combination of
Fig. 7 in which the ciliary muscles are contracted and the lens zonules are
slacked, such that
the lens is accommodating for near-sighted viewing of a nearby focal point
(relative to the
focal point in Fig. 7), with focal lines of light illustrated with dashed
lines and travelling
from the focal point and through the AFSI and IOL combination. In Fig. 8 the
AFSI and IOL
combination are moved forward relative to the position the AFSI and IOL adopts
in Fig. 7.
4
Date Recue/Date Received 2023-12-12
[0021] Fig. 9 is a cross-sectional side view of an embodiment of an AFSI
positioned
in a simplified human eye after the removal of the natural crystalline lens
and the addition of
an implantable accommodating IOL, in which the ciliary muscles are shown
relaxed, such
that the lens is accommodating for far-sighted viewing of a distant focal
point, with focal
lines of light illustrated with dashed lines and travelling from the focal
point and through the
AFSI and IOL combination.
[0022] Fig. 10 is a cross-sectional side view of the AFSI, IOL and eye
combination
of Fig. 9, in which the ciliary muscles are contracted, such that the lens is
accommodating
for near-sighted viewing of a nearby focal point (relative to the focal point
in Fig. 9), with
focal lines of light illustrated with dashed lines and travelling from the
focal point and
through the AFSI and IOL combination. In Fig. 9 the AFSI and IOL combination
are moved
forward relative to the position the AFSI and IOL adopts in Fig. 9.
[0023] Fig. 11 is front elevation view of a mixed curvature lens implant
(MCLI)
having an optic lens and two opposed haptic arm assemblies each forming a
haptic bridge,
with a plurality of protrusions along respective sulcus gripping parts.
[0024] Fig. 12 is a front elevation view of a second embodiment of an
MCLI, having
an optic lens and four haptic arm assemblies, with a plurality of protrusions
along respective
sulcus gripping parts.
[0025] Fig. 13 is a side elevation view of a third embodiment of an MCLI
positioned
within a capsular lens bag of a human eye, having an optic lens and two
arcuate haptic arms
with a plurality of protrusions along respective sulcus gripping parts.
[0026] Fig. 14 is a side elevation view of a fourth embodiment of an
MCLI, having
an optic lens and two opposed haptic arms with a plurality of protrusions
along respective
sulcus gripping parts.
[0027] Fig. 15 is a cross-sectional side view of an embodiment of an
MCLI
positioned in a human eye after the removal of the natural crystalline lens,
in which the
ciliary muscles are shown relaxed, with the lens zonules taut, such that the
lens is
accommodating for far-sighted viewing of a distant focal point, with focal
lines of light
illustrated with dashed lines and travelling from the focal point and through
the MCLI.
Date Recue/Date Received 2023-12-12
[0028] Fig. 16 is a cross-sectional side view of the MCLI and eye
combination of
Fig. 15 in which the ciliary muscles are contracted and the lens zonules are
slacked, such that
the lens is accommodating for near-sighted viewing of a nearby focal point
(relative to the
focal point in Fig. 15), with focal lines of light illustrated with dashed
lines and travelling
from the focal point and through the MCLI. In Fig. 16 the MCLI is moved
forward relative
to the position the MCLI adopts in Fig. 15.
DETAILED DESCRIPTION
[0029] Immaterial modifications may be made to the embodiments described
here
without departing from what is covered by the claims.
[0030] Problems with vision may take numerous forms. These include
myopia
(nearsightedness), hyperopia (farsightedness) as well as cataracts
(opacification of the lens).
Contact lenses and glasses containing refractive lenses are commonly used for
basic
correction of myopia, hyperopia, and astigmatism. Both contact lenses and
glasses represent
non-permanent solutions that are susceptible to loss, breakage and require
cleaning in order
to maintain efficacy.
[0031] An implantable intraocular contact lens, known as an IOL, is a
surgical
implantation used to permanently improve an eyesight condition, such as
myopia, hyperopia
or cataracts. An IOL incorporates a corrective lens tailored and structured to
the degree of
vision impairment desired to be corrected. An IOL solution may be a viable
option for a
patient who has a condition that would otherwise disqualify them from
alternative laser-
assisted in situ keratomileusis (LASIK) treatment such as: thin corneas, dry
eyes or
astigmatism (imperfection in the curvature of the lens). An IOL may be
considered and used
as a permanent vision correction solution, but may be removed or adjusted to
address any
change in efficacy or in a patient's vision deficit.
[0032] Two types of IOL solutions exists - phakic and pseudophakic. With
a phakic
solution ('phakic' meaning "having a lens") the eye's natural lens is left
untouched.
Intraocular lenses that are implanted into eyes after the eye's natural lens
has been removed
during cataract surgery are known as pseudophakic. Phakic intraocular lenses
are indicated
for patients with high refractive errors when the usual laser options for
surgical correction
6
Date Recue/Date Received 2023-12-12
(LASIK and PRK) are contraindicated. Phakic IOLs may be designed to correct
high myopia
ranging from ¨5 to ¨20 D if the patient has enough anterior chamber depth
(ACD) of at least
3 mm. The most common type of IOL is the pseudophakic IOL, which may be
implanted
after the eye's natural lens has been removed. The pseudophakic IOL provides
the same light
focusing function as the natural crystalline lens. A pseudophakic IOL may be
available as:
monofocal (focus on only one distance), multifocal (for example bifocal), or
accommodating
(permits focus changing).
[0033] An IOL may contain non-optic side struts known as haptics. A
haptic may be
the part of an IOL responsible for its attachment to the ciliary muscles or
suspensory
ligaments called lens zonules, which are connected to both the ciliary muscle
and natural
crystalline lens within the capsular lens bag of the eye. Haptics may use
hinges at its ends to
aid in attaching to the ciliary muscles or zonules. In any given IOL, the
haptics may vary in
number and shape, including having loops or hooks, for example having loops to
sew into
the ciliary sulcus of the eye.
[0034] Accommodation is how an eye may change optical power to maintain
a clear
image as the eye focuses on objects at different distances. When the eye
focuses on an object
that is relatively far away, the ciliary muscles may relax, leading to the
lens zonules
becoming taut, leading to a flattening of the natural crystalline lens. When
the eye focuses on
an object that is relatively near an individual, the ciliary muscles may
contract, leading to the
lens zonules slackening, reducing tension upon the natural crystalline lens,
making the lens
more convex.
[0035] An IOL may be designed to use non-optical elements known as
haptics to
connect to the ciliary muscles or zonules of the eye, allowing for
accommodation to occur.
With an accommodating IOL, the accommodation process may occur as a result of
one or
more of a change in the shape of the lens or a change in the position of the
lens relative to the
lens capsule. In the case of the former (change in lens shape causing
accommodation),
similar to the natural crystalline lens, when viewing an object that is
relatively nearby, the
ciliary muscles may contract, resulting in reduced tension on the haptics,
resulting in the lens
becoming convex in shape. As well, when viewing an object that is relatively
far away, the
ciliary muscles relax, increasing the tension on the haptics and flattening
the natural
7
Date Recue/Date Received 2023-12-12
crystalline lens. In the case of the latter (movement of lens causing
accommodation),
accommodation may occur through the haptics changing the position of the lens
anterior or
posterior relative to the lens capsule. When viewing an object that is
relatively nearby, for
example a book held at arm's length, under the tension of the contracted
ciliary muscles the
haptics may push the lens in an anterior direction, moving the lens relatively
closer to the
pupil. When viewing an object that is far away, the ciliary muscles may relax,
resulting in
the haptics pushing the lens in a posterior direction, moving the lens
relatively further from
the pupil. It is through such anterior-posterior movement of the lens that
accommodation
may be achieved in a manner analogous to that of the natural eye.
[0036] In contrast to accommodation, a static or non-accommodating IOL
may be
used, for example with a monofocal or multifocal lens. A monofocal lens may
only focus at
a single distance, for example a distance over 20 meters to correct only
distance vision. A
multifocal IOL may have plural regions that each focus at different relative
distances, for
example two or three focal regions spaced throughout the lens simultaneously
based on the
position of the pupil. In some cases, the central part of the lens may be
designed for focusing
on nearby objects, while the outer regions of the lens may be structured for
focusing on far
away objects. When viewing a nearby object, the pupil of the eye may constrict
and the
central region of the IOL may be used, while for far away viewing the pupil
dilates and an
outer IOL focal region may be used.
[0037] Some newer lens designs attempt to allow the eye to regain some
partial
focusing ability in order to change focus from distance to near via
accommodation.
However, many accommodating IOLs used today only achieve a very limited
improvements
in near vision which reduced over time. Accommodative IOLs may also have a
slightly
higher risk of developing posterior capsule opacification (PCO), though there
is some
uncertainty around this finding. PCO is a common side-effect of many cataract
surgeries and
is easily treatable with a one-time laser capsulotomy procedure. Accommodating
IOLs
interact with ciliary muscles and zonules, using hinges at both ends to latch
on and move
forward and backward inside the eye using the same mechanism as normal
accommodation.
8
Date Recue/Date Received 2023-12-12
The haptic hinges may be made of an advanced silicone called BioSil that has
been
thoroughly tested to make sure it is capable of unlimited flexing in the eye.
[0038] An IOL may be implanted in a surgical procedure. A surgeon may
use drugs
to dilate the pupil of the patient. A cut may be made into the cornea and
anterior capsular
lens bag of the eye, where the natural crystalline lens is contained, to
facilitate the insertion
of surgical tools and an IOL. The natural crystalline lens may be destroyed in
what is known
as a pseudophakic procedure, by a suitable technique such as the use of a
laser or ultrasound.
In some cases, it may be unnecessary to destroy the crystalline lens, such as
where the
crystalline lens has already been removed or destroyed in a previous
procedure, as might be
the case where an IOL is being replaced or upgraded. Alternatively, in a
phakic procedure
the natural crystalline lens may be kept intact. An IOL may then be inserted
into the capsular
lens bag. Insertion may be achieved by folding the IOL and inserting it
through the cut made
in the anterior lens capsule lens, assuming that the IOL is made with flexible
material. The
non-optic haptics may contact the sulcus of the eye.
[0039] There may be various problems with IOLs. Stiff haptics may impair
the
ability of the ciliary muscles to change the shape of the lens. Stiff haptics
may further make
it difficult to remove an IOL if a patient elects to do so after having a
phakic procedure. Lens
zonules may drive accommodation as opposed to the ciliary muscles, reducing
the eye's
ability to accommodate as following the initial cut into the anterior lens
capsule the zonular
system may not perform as efficiently as pre-surgery to change the shape of
the lens. IOLs
may need to be tailored in size to a patient's eye, and thus would not be
considered to be
one-size-fits-all. As the sulcus shape to which the IOL must match cannot be
accurately
measured, a surgeon may implant either too large of an optic lens, which will
resist ciliary
muscle action, or too small of an optic lens, in which the ciliary muscle may
not
accommodate properly or at all.
[0040] Referring to Figs. 1-10, an accommodation-facilitating
intraocular implant 88
(which may be referred elsewhere in this document as an AFSI), may be used to
assist in the
accommodation of an accommodating intraocular lens (IOL). The accommodation-
facilitating intraocular implant 88 may comprise a ring 90. The implant 88 may
also have a
9
Date Recue/Date Received 2023-12-12
plurality of haptics 104. The ring 90 may be sized to fit within a capsular
lens bag 24 of an
eye 10. The plurality of haptics 104 may be angularly spaced around (for
example around an
optical axis 11, which is discussed herein as defined by the eye, but may also
for
convenience of discussion be referred to as being defined by the ring 90
and/or the IOL 50)
and radially extended from the ring 90. The implant 88 may be used with an
accommodating
or non-accommodating IOL 50, and with mono or multi-focal IOLs. The implant 88
may
have no dioptric power. The use of the ring 90 pushes the lens 54 backward
toward the retina
in use, allowing a user to experience relatively improved near vision. The
lens may have a
fixed dioptric power, or in the case of flexible IOLs such as made of foldable
silicon, may
slightly increase the dioptric power of the lens in use. In some cases, the
IOL may comprise
flexible or pliable material, such as a liquid, solid, or gel.
[0041] Referring to Figs. 2 and 7-10, the accommodation-facilitating
intraocular
implant 88 may form part of an accommodating IOL assembly 126. An
accommodating IOL
assembly 126 may include an implant 88 and the IOL 50. The implant 88 may be
referred to
as a secondary implant, as such may be inserted to assist an existing IOL 50.
The
accommodating IOL assembly 126 may comprise an implantable accommodating IOL
50,
which may comprise an optic lens 54. The IOL 50 may also comprise a plurality
of IOL
haptics 52 angularly spaced around (for example around optical axis 11 defined
by the IOL
50 and/or eye and/or ring 90) and radially extended from the optic lens 54.
The ring 90 may
be fitted within the capsular lens bag 24 of the eye 10 in use, for example
anterior to and in
contact with the optic lens 54.
[0042] Referring to Figs. 7-10, the accommodation-facilitating
intraocular implant 88
may be inserted within the capsular lens bag 24 of an eye 10 in a method of
use. Once the
intraocular implant 88 is within the lens bag 24, the implant 88 may be placed
anterior to and
in contact, for example direct or indirect, with the lens 54 of the IOL 50
that is also with in
the lens bag 24. The accommodation-facilitating intraocular implant 88 may be
inserted
during surgery through an incision in an anterior portion of the capsular lens
bag 24, to
position the accommodation-facilitating intraocular implant 88. During the
positioning of the
intraocular implant 88, the plurality of haptics 104 of the accommodation-
facilitating
intraocular implant 88 are inserted into and may follow a circumferential
groove 33 of the
Date Recue/Date Received 2023-12-12
sulcus 32 to grip the sulcus 32. Once the haptics 104 of the intraocular
implant 88 are
positioned in the sulcus 32, under contraction and expansion of ciliary
muscles 28 of the eye,
the plurality of haptics 104 may be biased by the ciliary muscles 28 to adjust
the ring 90
along an optical axis 11 of the eye 10 to adjust a dioptric power of the IOL
50 to
accommodate a focal power of the eye 10.
[0043] Referring to Figs. 1-10, the accommodation-facilitating
intraocular implant 88
may have a suitable structure. The ring 90 may comprise any suitable shape,
such as a
circular shape, elliptical shape, or an oblong shape. The ring 90 of the
implant 88 may define
an inner annular edge 91, an outer annular edge 95, an annular anterior face
100, and an
intraocular-lens-contacting annular posterior face 102. The inner annular edge
91 may define
an open void center 93 of the ring 90. The void center 93 may allow the light
entering 78 the
eye 10 to pass through unhindered toward the lens 54 of the IOL 50. Referring
to Figs. 6-10,
a portion of the ring 90 defined between the inner annular edge 91 and the
outer annular edge
95 may have a rectangular cross-sectional shape, for example defined in a
plane (the plane is
understood as being the plane of the page in Figs. 6-10) parallel with a
central axis 11
defined by the ring and/or eye. The rectangular shape may include rounded
corners, and in
some cases nominal curvature of sides. In some cases, the cross-sectional
shape of the ring
may follow a circle, oval, ellipse, or other shape. The outer annular edge 95
of the ring 90
may allow the ring 90 to define an outer diameter 94. The ring 90 may define a
variety of
dimensions, such as inner and outer diameters 92 and 94 defined by the inner
and outer
annular peripheral edges 91 and 95, respectively. A radial width 96, which may
be defined
between a radius 94A defined by the outer annular edge 95 and a radius 93A
defined by the
inner annular edge 91. The radial width 96 may be greater than an axial ring
thickness 98,
which may be defined between the annular faces 100 and 102. The radial width
96 may be
four or more times greater than the axial thickness 98. The radial width 96
may be smaller
than a radius 93A of the open void center 93.
[0044] Referring to Figs. 1-10, the accommodation-facilitating
intraocular implant 88
may be formed of suitable material. The intraocular implant 88, for example
the ring 90, may
comprise a rigid or resilient material. The material of the ring 90 being
rigid or resilient may
allow the ring 90 to act on the lens 54 of the IOL 50 as intended, for example
by pressing
11
Date Recue/Date Received 2023-12-12
against and deforming and/or moving the lens 54 to a greater extent than if
the implant 88
was not present. The implant 88 may be made of a material that is relatively
more rigid than
the material of the lens 54. The ring 90 may comprise any suitable rigid
material, such as one
or more of poly(methyl methacrylate) (PMMA) or stainless steel. PMMA is
already
approved for implantation in the human eye 10. The ring 90 may comprise
ultraviolet (A and
B) absorbing material, for example transparent or opaque to visual light,
and/or may be
tinted.
[0045] Referring to Figs. 1-6, as above, the accommodation-facilitating
intraocular
implant 88 may have a plurality of implant haptics 104 angularly spaced around
and radially
extended from the ring 90. The haptics 104 may define an anchor end 106 and a
sulcus end
110. Each haptic 104 may have a tongue 108, which may form a suitable sulcus
gripping part
120, such as an arcuate sulcus gripping part. The sulcus gripping part 120 may
be present at
the sulcus end 110 of the haptics 104. Each haptic 104 may comprise an arm
116, which
may connect the anchor end 106 and the sulcus end 110. The sulcus end 110 of
the haptics
104 may define a suitable gripping profile, such as an indented profile 112,
for example with
scallops, protrusions, contours, textures, or other physical or chemical
structures that
increase the friction between the sulcus 32 and the part 120. The anchor end
106 of the
haptic 104 may connect the haptic 104 to the ring 90, for example as shown
where the
anchor end 106 attaches each haptic to the annular anterior face 100 of the
ring 90. In some
cases, the haptics 104 may be attached to the posterior face or edge of the
ring 90.
[0046] Referring to Figs 1-6, the haptics 104 of the ring 90 may take a
variety of
forms. The haptics 104 may form cantilevers, for example made up of single
arms 116 (Figs.
1-3), dual arms 116 forming bridges (Figs. 4-6), and other designs. Referring
to Figs. 1-3, the
haptics 104 with a single arm 116 may have an indented profile 112 at the
sulcus end 110
extend in one lateral (partially circumferential) direction (Figs. 1-2), or in
two lateral
directions (Fig. 3), or more. The gripping part 120, for example the indented
profile 112 of
same, may extend laterally an arc length of suitable distance, for example
defined by an
angle 118 referring to an angular distance partially around the optical axis
11. An indented
profile 112 or part 120 that is extended in opposing lateral directions about
the arm stem may
define a greater arc angle 118 than in the case of extension in one lateral
direction. Referring
12
Date Recue/Date Received 2023-12-12
to Figs. 4-5, the arms 116 of multi-armed haptics 104 may be connected at the
sulcus end
110, forming a haptic bridge. The double armed 116 haptic 104 may be anchored
to the ring
90 at the anchor end 106 of both arms 116. The ring 90, double arms 116 and
the indented
profile 112 of the haptic may define a cutout or gap 124, which may be defined
by the inner
edge 122 of the haptic 104. The plurality of haptics 104 may be angularly
spaced about axis
11 at suitable intervals around the ring 90. In the examples shown in Figs. 1-
3, a pair of the
single arm 116 haptics 104 are shown directly across the ring 90 from each
other, while in
Fig. 4, a pair of the double armed 116 haptics 104 are shown directly across
the ring 90 from
each other, and in Fig. 5 four of the double armed 116 haptics 104 are shown
evenly spaced
around the ring 90. The arrangement of the haptics 104 is not limited to the
embodiments
shown, for example, single arm 116 haptics 104 are able to be arranged in a
similar fashion
as the double armed 116 haptics 104. In the example shown, the haptics 104 are
shown
spaced evenly around the ring 90, however, the spacing of the haptics 104 may
vary, and the
haptics 104 do not have to be evenly spaced around the ring 90.
[0047] Referring to Figs. 1-6, the accommodation-facilitating
intraocular implant 88
may have a suitable number and arrangement of haptics 104. For example, in
Fig. 1, the
embodiment has two haptics 104, opposed from one another. In Fig. 5 the
implant 88 has
four haptics 104. A relatively increased number of haptics 104 may help
distribute the force
of the ciliary muscles 28 evenly across the ring 90, thereby leading to more
effective use of
the implant 88 as opposed to only two haptics 104. Any suitable number of
haptics 104 may
be used. In some cases, the accommodation-facilitating intraocular implant 88
may have
between two and eight haptics 104.
[0048] Referring to Figs. 7-10, when the implant 88 is inserted in to
the lens bag 24
of the eye 10, the haptics 104 may be inserted into and follow a
circumferential groove 33 of
the sulcus 32 to restrict circumferential sliding of the tongue 108 around the
sulcus 32. The
haptics 104 may be structured to be biased, for example move, under
contraction and
expansion of the ciliary muscles 28 of the eye, to press against and adjust
the ring 90 along
an optical axis 11 of the eye 10. The plurality of haptics 104 of the implant
88 may be
inserted into the circumferential groove 33 of the sulcus 32 anterior relative
to the plurality
of haptics 52 of the IOL 50. The haptics 104 of the implant 88 may be
configured to cause
13
Date Recue/Date Received 2023-12-12
the ring 90 to move or be biased in a posterior direction under contraction of
the ciliary
muscles 28. The biasing or movement of the ring 90 in the posterior direction
may cause the
ring 90 to press upon the IOL 50, and thereby increase a dioptric power of the
IOL 50.
[0049] Referring to Figs. 2 and 7-10, the ring 90 may be appropriately
sized to act
upon the lens 54 of the IOL 50. The inner diameter 92 of the ring 90 may be
smaller than an
outer diameter 55 of the lens 54 of the IOL 50. The outer diameter 94 of the
ring 90 may be
larger than the outer diameter 55 of the lens 54 of the IOL 50. The inner
diameter 92 and the
outer diameter 94 of the ring 90 may be structured to allow for the posterior
face 102 of the
ring 90 to contact, and in at least projection, overlap the edge 56 of the
lens 54 of the IOL 50.
The overlap of the ring 90 onto the outer edge 56 of the lens 54 may allow the
ring 90 to
push the IOL 50 in a backward direction when a ciliary muscle 28 contracts.
[0050] Referring to Figs. 7-10, the accommodation-facilitating
intraocular implant 88
may be implanted via a suitable method. Prior to inserting the accommodation-
facilitating
intraocular implant 88, an incision may be formed into the cornea 12 and
anterior chamber
16 of the eye 10. This method may not need to be performed if a patient has a
pre-existing
incision in the anterior chamber 16, for example if they previously had phakic
or
pseudophakic surgery and were simply adding the accommodation-facilitating
intraocular
implant 88 to an already existing implantable accommodation IOL 50. A surgeon
may use a
suitable tool to make the incision, for example lasers. The anterior chamber
16 incision may
be used for the insertion of the accommodation-facilitating intraocular
implant 88.
[0051] Referring to Figs. 7-10, a suitable implantation method may
incorporate the
destruction or removal of the natural crystalline lens of the eye 10. Such a
method may be
performed in a pseudophakic surgery, such as for the removal of a cataract,
where the natural
crystalline lens of the eye 10 must be destroyed and/or removed. The natural
crystalline lens
of the eye 10 may be destroyed using a suitable technique, such as using
ultrasound waves
through phacoemulsification. The process of phacoemulsification may use an
ultrasonic
probe to break up and emulsify the natural crystalline lens into liquid using
the energy of
ultrasound waves. The resulting emulsion may be vacuumed out through the use
of surgical
tools. Alternatively, a laser may be used to soften the natural crystalline
lens as it is broken
up before phacoemulsification is performed to remove it. Using lasers to
soften followed by
14
Date Recue/Date Received 2023-12-12
ultrasound to break up may help to ensure there are no sharp-edged debris
formed as a result
of the destruction of the natural lens, as such debris could otherwise cause
damage to the eye
during the removal of the natural lens from the capsular lens bag 24.
[0052] Referring to Figs. 7-10, an implantation method may involve
inserting the
intraocular implant 88 into a capsular lens bag 24 of an eye 10, into contact
with and anterior
to the IOL 50 that is also within the capsular lens bag 24. The implant 88 may
be inserted
coaxial with the IOL 50, for example coaxial with optical axis 11 defined by
the eye. The
accommodation-facilitating intraocular implant 88 may be inserted through an
incision in an
anterior portion of the capsular lens bag 24. The intraocular implant 88 may
also be inserted
into the eye 10 as part of the IOL assembly 126, or independently if the
implant 88 is being
inserted into an eye 10 that already has an IOL 50 installed. The
accommodation-facilitating
intraocular implant 88 may be positioned such that the plurality of haptics
104 of the
accommodation-facilitating intraocular implant 88 are inserted into and follow
the
circumferential groove 33 of the sulcus 32 to grip the sulcus 32. During under
contraction
and expansion of ciliary muscles 28 of the eye 10, the plurality of haptics
104 may move to
adjust the ring 90 along an optical axis 11 of the eye 10 to adjust a dioptric
power of the IOL
50 to accommodate a focal power of the eye 10.
[0053] Referring to Figs. 11-16 a variation of an IOL 50 is shown, which
may be
referred to as a mixed curved implantable accommodating intraocular lens (IOL)
50. The
IOL 50 may have a mixed curved optic lens 54. The optic lens 54 may define a
posterior face
74, which may have a concave profile, such as a concave cross-sectional
profile 75 defined
in a plane parallel with the optical axis 11. The optic lens 54 may define an
anterior face 72,
which may have a convex profile, such as a convex cross-sectional profile 73
defined in a
plane parallel with the optical axis 11 (the plane is understood as referring
to the plane of the
page in the embodiments). The faces 72 and 74 may have a suitable convex and
concave,
respectively, profile shape, for example a semi-circular, arcuate, bell,
conical, or other shape
including complex shapes. The posterior face 74 may have another suitable
shape, such as a
flat or convex shape in some cases.
[0054] Referring to Figs. 11-14, the lens 54 of the IOL 50 may have a
suitable shape.
As above, the anterior face 72 of the lens 54 may have a convex curvature. The
posterior
Date Recue/Date Received 2023-12-12
face 74 of the lens 54 may have a convex curvature. The convex cross-sectional
profile 73 of
the anterior face 72 may have a greater degree of curvature than the concave
cross-sectional
profile 75 of the posterior face 74. Other curvature relationships are
possible, including ones
where the profile 75 has more or the same curvature as profile 73. The lens 54
may be a
suitable size, and may define a diameter 55 across the lens 54. The curvature
of the anterior
face 72 may depend on the curvature of the posterior face 74, for example, the
curvatures
may be selected to provide a desired range of dioptric power, using the
formula of the sum of
the curvature of the anterior face 72 in diopters and the curvature of the
posterior face 74 in
diopters, to equal the dioptric power of the lens 54. Example dioptric power
desired in an
IOL may be from 15-25 diopters, although larger or smaller power levels may be
used.
Referring to Figs. 13 and 14, an outer edge 56 of the lens 54 may be shaped
with a non-zero
curvature. The curvature of the outer edge 56 of the lens 54 may provide a
smooth transition
from the convex curvature of the anterior face 72 to the concave curvature of
the posterior
face 74. The anterior curvature of the IOL 50 may be increased such that the
lens 54 is in
contact in use with the iris so that when the pupil contracts the iris can
push the lens 54
backward, resulting in a greater degree of focusing on a near object. With
more curvature,
the dioptric power is increased, imitating normal near vision without reading
glasses. The
IOL 50 may be configured to ensure that an outer edge is relatively hidden by
the iris when
the pupil dilates in the relative darkness at night, thus diminishing or
eliminating night glare.
In some cases, while using a conventional IOL, while driving at night, the
pupil 14 may be
relatively wide and the edge of the implanted lens 54 may be exposed to
incoming light, such
as light coming from an approaching vehicle or street lights. Such may cause
an annoying
glare to the user and my make driving at night difficult. Thus, by making a
lens 54
sufficiently wide to hide the edge of the lens 54 behind the pupillary edge of
a dilated pupil
14, the glare otherwise experienced at night by the user may be reduced or
avoided. The
concave curvature of the posterior of the IOL 50 may provide additional
dioptric power
when the lens is pushed backward. The anterior curvature of the lens 50 may be
increased
because the posterior curve is made concave, keeping desired dioptric power
the same as
present conventional lenses, with increased curvature increasing the force of
the papillary
constriction to push the lens posterior. The decreased curve of the posterior
surface of the
16
Date Recue/Date Received 2023-12-12
lens may decrease chance of damage to the back surface of the lens during
laser capsulotomy
of the posterior capsule, or during other procedures where the IOL 50 remains
in the eye.
[0055] Referring to Figs. 11-16, the IOL 50 may have a plurality of
haptics 52 with
suitable characteristics. In some cases, the haptics 52 may have one, or more,
or all of the
features of haptics 104, and vice versa. The optic lens 54 may be sized to fit
within a
capsular lens bag 24 of an eye 10. The plurality of haptics 52 may be
angularly spaced
around and radially extended from the optic lens 54. Referring to Figs. 13-14,
the haptics 52
may be attached anteriorly to the optic lens 54, for example anterior, or more
anterior than
posterior, of a central profile 53 of the lens 54 defined perpendicular to the
axis 11, and/or
attached to the anterior face 72. Referring to Figs. 15-16 anterior attachment
may assist the
implantable accommodating IOL 50 to move forward during ciliary muscle
contraction, and
may assist with implantation by facilitating the ability of the IOL 50 to
assume a compact,
folded state for implantation. By attaching the haptics to the anterior
surface, the force is
directed to push the lens backward, and the constricting pupil also pushes the
lens backward,
in use. In some cases, the haptics may be attached to the posterior surface or
edge of the
IOL. Referring to Figs. 15 and 16, during implantation, the implantable
accommodating IOL
50 may be inserted into the capsular lens bag 24 of an eye 10 with or without
the removal of
the natural crystalline lens in what is known as a pseudophakic or phakic
surgical procedure,
respectively. Each haptic 52 may be structured to move or be biased, under
contraction and
expansion of ciliary muscles 28 of the eye 10, to adjust one or more of a
position or shape of
the optic lens to accommodate a focal power of the eye 10. Inserting the IOL
50 into the
capsular lens bag 24 of the eye, may be done through an incision in an
anterior portion of the
capsular lens bag 24. The IOL 50 may be positioned so that an arcuate sulcus
gripping parts
120 of the plurality of haptics 52 are inserted into and follow a
circumferential groove 33 of
the sulcus 32 to grip the sulcus 32.
[0056] Referring to Figs. 11-14, the haptics 52 may have suitable
characteristics,
including one or more or all of the characteristics of haptics 52. The
implantable
accommodating IOL 50 may have haptics 52 with an arm 64 that defines a first,
anchor end
58, for example that originates at the optic lens 54, and a second, sulcus end
61 that may
define the tongue 60. The anchor end 58 of the haptic 52 may be directly
attached to the
17
Date Recue/Date Received 2023-12-12
optic lens 54, for example the haptic 52 may be attached to the anterior face
72 of the lens 54
of the IOL 50. The sulcus end 61 may form the arcuate sulcus gripping part 66
to aid in
attachment to the sulcus 32 of the eye 10. Referring to Figs. 13-14, each arm
64 may be a
single arm, and referring to Figs. 11-12, each haptic 52 may be formed of two
or more arms
64, for example forming a haptic bridge as shown. The implantable
accommodating IOL 50
may have a suitable number and arrangement of haptics 52. For example, in Fig.
11, the
embodiment has two haptics 52, opposed from one another. In Fig. 12 the IOL 50
has four
haptics 52. A relatively increased number of haptics 52 may help distribute
the force of the
ciliary muscles 28 evenly across the optic lens 54, thereby leading to more
effective
accommodation of the IOL 50 as opposed to only two haptics 52. Any suitable
number of
haptics 52 may be used. In some cases, the implantable accommodating IOL 50
may have
between two and eight haptics.
[0057] Referring to Figs. 11-16, each haptic 52 may have a tongue 60
that forms an
arcuate sulcus gripping part 66. In use within the capsular lens bag 24, the
gripping part 66
may insert into and follow a circumferential groove 33 of the sulcus 32 to
restrict
circumferential sliding of the tongue 60 around the sulcus 32. A plurality of
haptics 52 may
contain tongues 60 that insert into and follow the circumferential groove 33
of the sulcus 32
of the eye 10. The use of sulcus gripping parts may prevent rotation of the
lens 54 about an
optical axis 11 of the eye 10. An optical axis 11 may be defined as a line
that passes through
a center of the eye 10 and a center of the pupil 14. In use, the implantable
accommodating
IOL 50 may be fitted within a capsular lens bag 24 of an eye 10, with the
respective tongues
60 of the plurality of haptics 52 inserted into and following the
circumferential groove 33 of
the sulcus 32 of the eye 10. The haptics 52 may be configured to bias or move
the IOL 50 in
a posterior direction under contraction of the ciliary muscles 28 of the eye
10 to increase a
dioptric power of the IOL 50.
[0058] Referring to Figs 11-14, the haptics 52 of the IOL 50 may take a
variety of
forms. The haptics 52 may have a single or double arm 64, although only double
arm
versions are shown in these figures. Haptics 52 with a single arm 64 may have
the indented
profile 62 at the sulcus end 61 extend in one lateral direction or in two
lateral directions.
Referring to Figs. 11-12, the arms 64 of the double armed 64 haptics 52, may
be connected
18
Date Recue/Date Received 2023-12-12
at the sulcus end 61 by the indented profile 62 of the haptic 52, forming a
haptic bridge. The
double armed 64 haptic 52 may be anchored to the lens 54 of the IOL 50 at the
anchor end
58 of both arms 64. The lens 54, double arms 64 and the indented profile 62 of
the haptic
may define a cutout or gap 70, which may be defined from the inner edge 68 of
the haptic
52. The plurality of haptics 52 may be spaced out at various intervals around
the lens 54.
[0059] Referring to Figs. 11-14, the IOL 50 may comprise a suitable
material. The
lens 54 may be made of material with a high refractive index. Materials with a
high
refractive index, such as 1.7 or higher, may allow the lens 54 to be made
thinner relative to
lower refractive index materials. Materials with a high refractive index may
allow the
posterior face 74 of the lens 54 to be increased in curvature, compared to if
a material of a
lower refractive index is used. Materials with a higher refractive index can
bend incoming
light more than material with a lower refractive index. Since material with a
higher refractive
index can bend light more readily, less material is need to achieve the same
effect as material
with a lower refractive index. One example of a high refractive index material
is PMMA.
Plural materials may be combined in one IOL, for example PMMA may be used for
an
exterior shell, with a silicon core.
[0060] Referring to Figs. 15-16, the implantable accommodating IOL 50
may be
implanted via a suitable method. In a suitable method an incision may be made
in the eye 10
unless such incision is already present. Prior to inserting the implantable
accommodating
IOL 50, an incision may be formed into the cornea 12 and anterior chamber 16
of the eye 10.
This method may not need to be performed if a patient has a pre-existing
incision in the
anterior chamber 16, for example if they previously had phakic or pseudophakic
surgery and
were simply replacing the implantable accommodation IOL 50. A surgeon may use
a
suitable tool to make the incision, for example lasers. The anterior chamber
16 incision may
be used for the insertion of the implantable accommodating IOL 50. As above, a
suitable
implantation method may incorporate the destruction or removal of the natural
crystalline
lens of the eye 10. Referring to Figs. 15-16, an implantation method may
involve inserting
the IOL 50 into the capsular lens bag of an eye 10 via a suitable method.
Insertion may be
carried out through the incision made prior in an anterior chamber 16 of the
capsular lens
bag 24 of an eye 10, such that the arcuate sulcus gripping parts 66 of the
plurality of haptics
19
Date Recue/Date Received 2023-12-12
52 insert into and follow the circumferential groove 33 of the sulcus 32. The
IOL 50 may be
inserted in a compact, such as a folded, configuration, into the capsular lens
bag 24, which
may be advantageous to retain the IOL 50 in the bag 24 and also to reduce the
size of
incision required. The IOL 50 may be positioned more anterior when compared to
the
placement of other IOL's. The positioning of the IOL 50 being more anterior
may allow the
outer edge 56 of the implantable accommodating IOL 50 to be posterior an iris
18 of the eye
of the user. The IOL 50 being partially behind the iris 18 may allow decrease
or eliminate
night glare. which is a phenomenon in which a source of light does not help
the user see
better, but instead interferes with the user's vision.
[0061] In the claims, the word "comprising" is used in its inclusive
sense and does
not exclude other elements being present. The indefinite articles "a" and "an"
before a claim
feature do not exclude more than one of the feature being present. Each one of
the individual
features described here may be used in one or more embodiments and is not, by
virtue only
of being described here, to be construed as essential to all embodiments as
defined by the
claims.
Date Recue/Date Received 2023-12-12