Note: Descriptions are shown in the official language in which they were submitted.
ELECTROLYTE BALANCING STRATEGIES FOR FLOW BATTERIES
10001]
TECHNICAL FIELD
100021 The present invention relates to redox flow batteries and methods for
operating
the same.
BACKGROUND
100031 Flow batteries are electrochemical energy storage systems in which
electrochemical reactants, typically redox active compounds, are dissolved in
liquid electrolytes,
which are individually contained in negative electrolyte or negolyte and
positive electrolyte or
posolyte loops and circulated through reaction cells, where electrical energy
is either converted
to or extracted from chemical potential energy in the reactants by way of
reduction and oxidation
reactions. Optimal performance of the flow battery relies on the ability to
maintain balance
between the posolyte and negolyte, both in terms of pH and state of charge.
Upon extended
cycling, flow batteries typically develop an imbalance in both proton and
electron content
between the posolyte and negolyte due to the presence of parasitic
electrochemical side
reactions. One reaction is the evolution of hydrogen gas from water at the
negative electrode,
which results in an imbalance in both the electron (state-of-charge) and
proton content between
the posolyte and negolyte. This imbalance, if left uncorrected, subsequently
results in a decrease
in system performance. An imbalanced state may be corrected by processing
either the posolyte,
negolyte, or both in a balancing cell.
100041 Various methods have been described for balancing flow battery
electrolytes.
These methods primarily address balancing the electron (state-of-charge)
content between the
posolyte and negolyte. No methods have been described that adequately address
the
simultaneous balancing of both the electron and proton contents of these
electrolytes The present
invention is aimed at addressing at least this deficiency.
- 1 -
Date Recue/Date Received 2023-12-22
SUMMARY
[0005] The present invention is directed to flow batteries, and methods of
operating the
same, said redox flow batteries comprising at least one electrochemical cell
in fluid
communication with at least one balancing cell, each balancing cell
comprising:
a first and second half-cell chamber,
wherein the first half-cell chamber comprises a first electrode in contact
with a
first aqueous electrolyte of the redox flow battery; and
wherein the second half-cell chamber comprises a second electrode in contact
with a second aqueous electrolyte, said second electrode comprising a catalyst
for the
generation of 02. In some of these embodiments, the pH of the second
electrolyte is at
least 2. In other embodiments, there is no added second electrolyte.
[0006] In another embodiment, the flow battery balancing cell comprises a
first half-
cell chamber with a first electrode in contact with a first aqueous
electrolyte of the redox flow
battery; the second half-cell chamber comprises a second electrode in contact
with a second
aqueous electrolyte, said second electrode comprising a sacrificial carbon
electrode material for
the generation of 02 and/or CO2. The two half-cell chambers are separated by
an ion exchange
ionomer membrane.
[0007] In another embodiment, the flow battery balancing cell comprises a
first half-
cell chamber with a first electrode in contact with a first aqueous
electrolyte of the redox flow
battery; the second half-cell chamber comprises a second electrode comprising
a catalyst for the
generation of 02 but said electrode is not in contact with a second aqueous
electrolyte. The two
half-cell chambers are separated by an ion exchange ionomer membrane.
[0008] In certain embodiments, the first aqueous electrolyte comprises a
negative
working electrolyte ("negolyte") of the redox flow battery. In other
embodiments, the second
aqueous electrolyte has a pH of at least 2 or higher. In still other
embodiments, the balancing
cell comprises composite, multiple, or bipolar membranes between the
electrochemical cell and
balancing cell.
BRIEF DESCRIPTION OF THE DRAWINGS
100091 The present application is further understood when read in conjunction
with the
appended drawings. For the purpose of illustration, there are shown in the
drawings exemplary
embodiments of the subject matter; however, the presently disclosed subject
matter is not limited
to the specific methods, devices, and systems disclosed. In addition, the
drawings are not
necessarily drawn to scale. In the drawings:
- 2 -
Date Recue/Date Received 2023-12-22
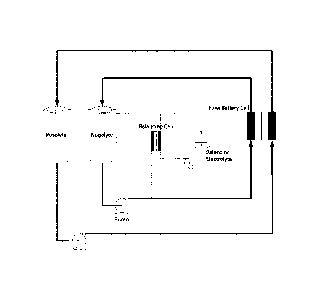
[0010] FIG. 1 provides a schematic of one embodiment of a flow battery of the
present
invention, including placement of the balancing cell in-line with the negolyte
line of the flow
battery.
[0011] FIG. 2 provides a schematic of one embodiment of an electron balancing
and
pH correction cell of the present invention. Mn may be the charged form of the
positive
electrolyte or the discharged form of the negative electrolyte in the flow
battery.
[0012] FIG. 3 provides data for flow battery cycle charge capacity and pH of
negative
electrolyte before and after initiation of the flow battery balancing cell.
The vertical dashed line
indicates the time at which the balancing cell is initiated (-125 hrs).
[0010] FIGs. 4A-B provide balancing cell data for voltage and current (FIG.
4A) and
pH of negative electrolyte (FIG. 4B) for long-term operation of the balancing
cell (>1600h)
[0011] FIG. 5 provides voltage and Amp-hour data for a balancing cell that
comprises a
N117 membrane in combination with a modified Aquivion E87 membrane.
[0012] FIG. 6 provides voltage and Amp-hour data for a balancing cell that
uses a
bipolar membrane. In this balancing cell configuration, 02 evolution is
performed under alkaline
conditions.
[0013] FIG. 7 provides voltage and current data for two balancing cells that
use semi-
sacrificial carbon electrodes in the second half-chamber. Cell A utilizes a
carbon cloth electrode
and Cell B utilizes a graphite felt electrode. In the Figure, Cell Voltage B
is the top curve, Cell
Voltage A is the middle curve, and Cell Current is ca. 25 mA/cm2.
DETAILED DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS
[0013] The present invention relates to redox flow batteries and methods and
apparatuses for monitoring the compositions of the electrolytes (posolyte or
negolyte or both)
therein. In particular, the present invention relates to methods and
configurations for balancing
the pH and state-of-charge of an electrolyte stream of a flow battery.
[0014] The present invention may be understood more readily by reference to
the
following description taken in connection with the accompanying Figures and
Examples, all of
which form a part of this disclosure. It is to be understood that this
invention is not limited to the
specific products, methods, conditions or parameters described and / or shown
herein, and that
the terminology used herein is for the purpose of describing particular
embodiments by way of
example only and is not intended to be limiting of any claimed invention.
Similarly, unless
specifically otherwise stated, any description as to a possible mechanism or
mode of action or
reason for improvement is meant to be illustrative only, and the invention
herein is not to be
- 3 -
Date Recue/Date Received 2023-12-22
constrained by the correctness or incorrectness of any such suggested
mechanism or mode of
action or reason for improvement. Throughout this text, it is recognized that
the descriptions
refer to apparatuses and methods of using said apparatuses. That is, where the
disclosure
describes and/or claims a feature or embodiment associated with a system or
apparatus or a
method of making or using a system or apparatus, it is appreciated that such a
description and/or
claim is intended to extend these features or embodiment to embodiments in
each of these
contexts (i.e., system, apparatus, and methods of using).
[0015] It is to be appreciated that certain features of the invention which
are, for clarity,
described herein in the context of separate embodiments, may also be provided
in combination in
a single embodiment. That is, unless obviously incompatible or specifically
excluded, each
individual embodiment is deemed to be combinable with any other embodiment(s)
and such a
combination is considered to be another embodiment. Conversely, various
features of the
invention that are, for brevity, described in the context of a single
embodiment, may also be
provided separately or in any sub-combination. Finally, while an embodiment
may be described
as part of a series of steps or part of a more general structure, each said
step may also be
considered an independent embodiment in itself, combinable with others.
[0016] In but one example of the divisibility of the embodiments, while this
specification describes embodiments of the invention in terms of "A redox flow
battery or other
electrochemical device comprising at least one electrochemical cell in fluid
communication with
a balancing cell, said balancing cell comprising. . ." it should be apparent
that the balancing cell
provides its own stand-alone embodiments as described herein. That is, each
description of the
redox flow battery or other electrochemical device also includes those
embodiments which may
be described only in terms of the balancing cell.
[0017] When a list is presented, unless stated otherwise, it is to be
understood that each
individual element of that list, and every combination of that list, is a
separate embodiment. For
example, a list of embodiments presented as "A, B, or C" is to be interpreted
as including the
embodiments, "A," "B," "C," "A or B," "A or C," "B or C," or "A, B, or C."
[0018] The present invention is directed to, inter alit', flow battery
configurations
where a balancing cell operates in fluid communication with an electrolyte,
especially the
negative electrolyte ("negolyte"), of a flow battery or other electrochemical
device, so as to
provide a device capable of correcting excursions of pH and state-of-charge
within the
electrolyte. It is desirable to configure and operate the balancing cell such
that impurities are not
introduced into the flow battery electrolytes. Particular impurities to be
avoided are those which
can accumulate onto the negative electrode of the flow battery, catalyze the
evolution of Hz, and
- 4 -
Date Recue/Date Received 2023-12-22
further catalyze the flow battery state-of-charge imbalance. In particular, it
will be preferred to
operate the balancing cell, wherein its positive electrode comprises an 02
evolution catalyst that
is highly resistant to corrosion and its negative electrode comprises current
collector that is
highly resistant to corrosion. Furthermore, it is desirable to prevent cross-
over of active materials
from the negolyte compartment into the second half-chamber, as such cross-over
can deteriorate
performance by either catalyst fouling or by formation of deposits inside the
membrane leading
to higher membrane resistance. Finally, it may be desirable to prevent large
pH gradients across
the membrane in the balancing cell. 02 evolution is often performed under
strongly acidic
conditions, which may not be compatible with flow battery technologies in
which the electrolytes
are formulated with neutral or alkaline pH: the resulting pH gradient may
eventually lead to pH
equilibration, causing electrolytes in the main batteries to acidify. As such,
it may be desirable to
perform 02 evolution at a pH value as similar as possible to the pH of
electrolytes in the main
battery, which may require careful selection of membrane and catalyst
materials.
[0019] Accordingly, certain embodiments of the present invention provide redox
flow
batteries comprising at least one electrochemical cell in fluid communication
with an
electrochemical balancing cell, each cell comprising:
a first and second half-cell chamber,
wherein the first half-cell chamber comprises a first electrode in contact
with a
first aqueous (working) electrolyte of the redox flow battery; and
wherein the second half-cell chamber comprises a second electrode in contact
with a second aqueous electrolyte, said second electrode comprising a catalyst
for the
generation of 02.
In some of these embodiments, the pH of the second electrolyte is at least 2.
In other
embodiments, there is no added second electrolyte. Corresponding embodiments
corresponding
to the balancing cell alone are also within the scope of this disclosure.
[0020] In certain embodiments, the balancing cell may also be characterized as
electrochemical rebalancing cell. See FIG. 1. In either case, the purpose of
the balancing cell is
to generate electrons and protons, for delivery to the working electrolyte via
an appropriate ion
exchange membrane, with the concomitant generation of oxygen. That is, the
electrochemistry
associated with the second half-cell of the balancing cell at acidic or
neutral pH values may be
described in terms of Equation (1):
2H20 ¨> 202 + 4H+ + 4e- (1)
- 5 -
Date Recue/Date Received 2023-12-22
At more basic pH values, the electrochemistry associated with the second half-
cell of the
balancing cell may be described in terms of Equation (2):
4 OH- 2H20 + 02 + 4e- (2)
[0021] The corresponding electrochemical reactions associated with the first
half-cell
of the pH correction may be described in Equation (3):
M" + (3)
where Mn and M"-1 represent the redox active species in the negolyte. Note
that the transport of
protons through the membrane from the second to first half-cell of the pH
correction cell
provides a charge balance to the negolyte. See FIG. 2.
[0022] The balancing cell may be configured to be in fluid communication with
either
the positive or negative electrolytes of the flow battery, but in preferred
embodiments, it is
configured to be in fluid communication with the negative working electrolyte
of the redox flow
battery (i.e., the first aqueous electrolyte of the pH control cell is
compositionally the same as the
negolyte of the working flow battery). In such an arrangement, the balancing
cell may directly
correct any imbalance both the proton and electron inventories of the flow
battery due to
parasitic hydrogen evolution at the negative electrode of the main flow
battery.
[0023] The membranes of the balancing cell should be those which
preferentially
conduct protons, to the virtual exclusion of other soluble materials.
Alternatively, or
additionally, the membranes may be matched with the redox active materials to
as to further
exclude the latter, for example by size, charge, equivalent weight, or
chemical functionality.
Suitable membranes may be composed of an ionomeric polymer. Such polymers may
comprise
perfluorosulphonic acid, (e.g. Nafion). Other membranes types are described
herein.
[0024] In some embodiments, the second aqueous electrolyte may comprise an
aqueous
solution with a certain ionic strength, which may be selected so as to control
the transport of
water across the membrane of the balancing cell. The ionic strength of the
second aqueous
electrolyte may be tuned to influence the activity of water in the second
aqueous electrolyte and,
therein, control the osmotic flux of water across the membrane. It may be
preferred for water to
migrate from the second aqueous electrolyte to the negolyte or from the
negolyte to the second
aqueous electrolyte. The ionic strength may be selected such that the osmotic
flux matches the
rate at which water is consumed in the production of 02. In some embodiments,
the ionic
- 6 -
Date Recue/Date Received 2023-12-22
strength of the second aqueous electrolyte may be selected to yield an osmotic
flux that is
essentially zero.
[0025] As described above, the second electrode comprises a catalyst for the
generation
of 02. In certain of these embodiments, the second electrode comprises a metal
oxide catalyst,
said metal oxide catalyst being suitable for the electrochemical generation of
02 from water. In
addition to the ability to generate 02, these oxidation catalysts preferably
resist corrosion under
the pHs considered in this application, are poor catalysts for the reduction
of water to hydrogen,
or both. Catalysts which corrode under the acidic or basic oxidizing
conditions of the operating
second aqueous electrolyte of the pH correction cell have the potential to
cross-over to the first
pH correction half-cell, interfering with either the intended effect of the pH
correction cell or,
worse, with the operation of the flow battery. If such cross-over catalysts
are further efficient
catalysts for the generation of hydrogen under the reducing conditions of the
first half-cell, one
can envision scenarios where the evolution of hydrogen in the first half-cell
or at the negative
electrode of the working flow battery causes safety concerns. Accordingly, the
present invention
contemplates the preferred use oxides of cobalt, iridium, iron, manganese,
nickel, ruthenium, tin,
or a combination thereof for use in the second electrode. Iridium oxide is
especially preferred,
because of its good catalytic activity toward 02 evolution and its high
corrosion resistance. In
case the second half-chamber comprises an alkaline electrolyte, catalysts such
as nickel oxide or
nickel-iron oxide are especially preferred because of their good catalytic
activity toward 02
evolution and their high corrosion resistance in base.
[0026] In some embodiments, the first electrode of the balancing cell
comprises carbon.
Such electrodes are well known in the art and include graphitic carbon, glassy
carbon,
amorphous carbon, carbon doped with boron or nitrogen, diamond-like carbon,
carbon onion,
carbon nanotubes, carbon felt, carbon paper, and graphene. Carbon materials
are capable of
evolving 02, albeit at rather high overpotentials, but it is inevitable that
the carbon electrode
itself will be oxidized into CO2. As such, the carbon electrodes are semi-
sacrificial of nature.
[0027] In some embodiments, the balancing cell is used to balance pH and SOC
for a
flow battery comprising metal-ligand coordination compounds as redox-active
materials.
Traditional flow batteries (e.g. all-Vanadium, iron-chrome, etc.) often
operate under strongly
acidic conditions, but flow batteries based on metal-ligand coordination
compounds may operate
under neutral or alkaline pH conditions. Each coordination compound exhibits
optimal
electrochemical reversibility, solubility, and chemical stability at a
specific pH value, hence the
optimal pH window of operation is different for each coordination compound-
based flow battery,
depending what active materials are being used. A number of different
considerations have to be
- 7 -
Date Recue/Date Received 2023-12-22
taken into account when designing a balancing cell aimed at balancing a
coordination compound
flow battery (CCFB) that operates at weakly acidic, neutral or alkaline pH.
[0028] First all, pH control is more important in CCFB's than in traditional,
strongly
acidic, flow batteries. The latter flow batteries typically operate in 1-5M
strong acid (e.g. H2SO4,
wherein the pH of the electrolytes is 1 or below), so that small imbalances in
proton
concentration will not significantly alter the pH of the main flow battery. In
contrast, when a
CCFB is operated at, for instance, pH 11, a relatively small build-up or
depletion of protons may
lead to significant pH changes, potentially affecting the battery performance
by reduced
electrochemical reversibility, solubility or chemical degradation of the
coordination compounds.
[0029] Secondly, it may be preferred to avoid the use of strongly acidic
electrolyte in
the second half-chamber to prevent a large pH gradient across the membrane of
the balancing
cell. This is especially relevant for CCFB's that are operated at neutral or
alkaline pH. The
presence of large pH gradients may eventually lead to pH equilibration,
effectively causing
acidification of the electrolyte in the first half-chamber, which may be
highly undesirable from
the point of view of stable operation, as mentioned above. Hence, operation of
the main battery
at e.g. pH 11 may require the electrolyte in the second half-chamber of the
balancing cell to be
alkaline for long-term stable operation, impacting the selection of the water
oxidation catalyst
and the ionomer material for the membrane separator. In such circumstances it
is necessary to
operate any associated balancing cell at pH values more in line with those of
the electrolytes. In
certain embodiments of the present invention(s), then, the second aqueous
electrolyte has a pH of
at least 2. Other embodiments provide that the pH of this second aqueous
electrolyte is in a
range of from about 2 to about 3, from about 3 to about 4, from about 4 to
about 5, from about 5
to about 6, from about 6 to about 7, from about 7 to about 8, from about 8 to
about 9, from about
9 to about 10, from about 10 to about 11, from about 11 to about 12, from
about 12 to about 13,
from about 13 to about 14, or a combination of these ranges. Alternatively,
the pH of the second
aqueous electrolyte may be defined in terms of its difference from the working
electrolyte that it
is balancing (i.e., typically the negolyte). In such embodiments, this
difference is less than about
8, less than about 7, less than about 6, less than about 5, less than about 4,
less than about 3, less
than about 2, or less than about 1 pH unit. In certain preferred embodiments,
the working
electrolyte has a pH in a range of from about 9 to about 13. Indeed, in many
circumstances, the
use of alkaline working electrolytes is preferred. In such embodiments, the pH
of the second
aqueous electrolyte may be in a range of from about 3, 4, 5, 6, 7, 8, or 9 to
about 14, for example.
[0030] Thirdly, active material cross-over in the balancing may reduce long-
term
performance of the main battery. In case anionic coordination complexes are
used in the main
- 8 -
Date Recue/Date Received 2023-12-22
battery, these molecules may be transported from the first half-chamber of the
balancing cell to
the second half-chamber by means of migration. One side-effect of this
unintended cross-over of
anions is that, in order to fulfill charge balancing, fewer protons need to be
transported from the
second to the first half-chamber of the balancing cell, compromising the pH
balancing function
of the balancing cell. A second side effect of cross-over of coordination
compounds is that these
molecules may deposit within the membrane, increasing the membrane resistance.
A third side-
effect of cross-over of coordination compounds is that these molecules may be
oxidized at the
catalyst in the second half-chamber, and the oxidation products of this
reaction may foul and/or
deactivate the catalyst. Hence, prevention of cross-over of coordination
compound active
materials may be essential in certain embodiments.
[0031] Cross-over of active material may be minimized by intelligently
selecting the
configuration and/or composition of the separator material. Various membranes
or membrane
combinations may be selected for the balancing cell to address these potential
issues. In some
embodiments, standard membranes based on perfluorosulphonic acid or sulfonated
polymers or
co-polymers of tetrafluoroethylene, optionally comprising perfluorovinyl
ethers may be used.
Other exemplary perfluorinated membrane materials include copolymers of
tetrafluoroethylene
and one or more fluorinated, acid-functional co-monomers, which are
commercially available as
NAFIONTM perfluorinated polymer electrolytes from E.I. du Pont de Nemours and
Company,
Wilmington Del.. Other useful perfluorinated electrolytes comprise copolymers
of
tetrafluoroethylene (TFE) and FS02¨CF2CF2CF2CF2-0-CF=CF2.
[0032] In certain embodiments, however, membranes with a higher selectivity
may be
required. In some embodiments, it is helpful to precipitate metals, metal
oxides, organometallic
material, polymeric material, or a combination thereof. Such methods and
materials are known
in the art to improve membrane selectivity, for example by acting as a barrier
for ions having
large volumes (such as coordination compounds).
[0033] In other embodiments, a membrane specifically modified to suppress
cross-over
may be utilized. One attractive class of such membranes includes ionomer
membranes,
especially melt-extruded ionomer membranes based on the unique Short Side
Chain (SSC)
copolymer of Tetrafluoroethylene and a Sulfonyl Fluoride Vinyl Ether (SFVE)
F2C¨CF-0-
CF2CF2-S02F of low molecular weight, commercially available as AquivionTM
PFSA.
AquivionTM membranes with low equivalent weight (980EW, 870EW, or lower) are
especially
preferred. These membranes, modified or as provided, can be used on their own,
or it can be
combined with more traditional membranes (e.g. N117, see Example 3).
- 9 -
Date Recue/Date Received 2023-12-22
[0034] In one other preferred embodiment, membranes that are typically used in
the
chlor-alkali industry may be applied. These membranes are bi-membranes
consisting of one
sulfonated and one carboxylated layer. In a chlorine-producing electrochemical
cell, the
carboxylated side of the membrane faces the negative half-chamber comprising a
NaOH or KOH
solution. The carboxylated membrane is highly effective at suppressing cross-
over of anionic
hydroxide ions from the negative to the positive half-chamber, which is a
highly undesirable
process during electrochemical chlorine production. It is expected that
carboxylated membranes
will also be effective at suppressing cross-over of anionic coordination
complexes, given the
much larger size of typical coordination complexes relative to OH- ions.
[0035] In certain embodiments, it may be desirable to avoid a pH gradient
across the
membrane of the balancing cell in order to prevent acidification of the
electrolytes in the main
battery. One approach to omitting pH gradients is to perform water oxidation
under basic
conditions (Equation 2, see above). In this scenario, charge balancing would
have to be achieved
by transport of hydroxide ions into the second half-chamber (as opposed to
proton transport from
the second to the first half-chamber when water oxidation is performed under
acidic conditions).
At the same time, protons still have to be injected into the first half-
chamber to compensate for
'lost' protons due to H2 evolution in the main battery. These requirements can
be met by a
bipolar membrane, which is a bi-membrane consisting of one cation exchange and
one anion
exchange ionomer membrane. Between these two layers, a metal oxide film is
present that
facilitates water dissociation. When a sufficiently high voltage is applied
across this composite
membrane, water is dissociated at the metal oxide layer, and as-generated
protons migrate to the
negative electrode whereas as-generated hydroxide ions migrate to the positive
electrode. Using
a bipolar membrane, the balancing cell can be operated while deploying a basic
electrolyte in the
second half-chamber (see Example 4). Besides the advantage of the absence of a
significant pH
gradient across the membrane in flow batteries that operate with basic
electrolytes, additional
advantages include the availability of more stable water oxidation catalysts
(e.g. NiO, NiFe0,
etc.) and an expected suppression of cross-over of active materials because of
the presence of
both anion and cation exchange ionomer material in the bipolar membrane.
[0036] In embodiments where the balancing cell is used to correct for H2
evolution in
the main flow battery, protons are transferred from the second half-chamber in
the balancing cell
to the first half chamber. In certain situations, especially when the
balancing cell is operated at
high current densities, the flux of protons into the electrolyte in the first
half-chamber is high,
potentially causing local pH drops in regions in the first half-chamber
adjacent to the membrane.
Local pH drops may result in local decomposition and/or precipitation of
coordination
- 10 -
Date Recue/Date Received 2023-12-22
compounds, potentially leading to obstruction of the flow field and/or cross-
over the
decomposition products (e.g. the dissociated ligand). In standard
configurations of the balancing
cell, the first half-chamber comprises a carbon paper electrode supported by
plate made of a
graphite / vinyl-ester composite material, in which an interdigitated flow
field is machined. In
this configuration, convective flow of electrolyte only occurs within the
channels and close to
ribs of the flow field. In contrast, in the region where most of the
electrochemistry takes place
(i.e. in the region adjacent to the membrane) there is no convective flow but
active material and
protons have to migrate in and out of that region by means of diffusion. Local
pH drops due to
proton injection may be prevented by configuring the first half-chamber in
such a way that
injected protons mix more effectively with the bulk electrolyte. Possible
configurations include a
non-conductive high-porosity medium (e.g. a polyester or polypropylene felt,
porosity at least
about 80% of total volume) that may be inserted between the membrane and the
carbon paper or
cloth (i.e., materials typically having lower porosities, e.g., on the order
of about 70-80 volume
%, based on the total volume of the material). Alternatively, the machined
flow field in the plate
can be omitted altogether by adopting an open flow field design where the
electrolyte flows
through a high-porosity electrode (e.g. a felt, or mesh, porosity at least 80
vol%). Both
configurations should lead to more convective flow in regions adjacent to the
membrane,
potentially preventing local pH drops in the first half-chamber.
[0037] The balancing cell may be operated in a flow-through or batchwise
arrangement.
In preferred embodiments, at least the first half-cell chamber and optionally
the second half-cell
chamber is configured as a flow-through cell.
[0038] This disclosure also provides embodiments in which the second half-
chamber of
the balancing cell does not contain any aqueous electrolyte at all. In this
configuration, the
water required for the 02 evolution reaction is provided by water from the
aqueous electrolyte in
the first half-chamber that is transported across the membrane. To avoid the
situation of mass
transport limitations, the water transport across the membrane needs to be
faster than the
consumption of water at the metal oxide catalyst. The membrane on the side of
the second half-
chamber is coated with a metal oxide 02 evolution catalyst (e.g. IrOx) as a
result of which water
that is transported from the first half-chamber across the membrane is
directly oxidized into
molecular oxygen and protons. This configuration may greatly simplify the
design of the
balancing cell. For instance, the metal oxide catalyst on the membrane can be
directly interfaced
with the titanium endplate, omitting the need for the titanium meshes that act
as a flow field for
the second aqueous electrolyte in examples 1-3. Furthermore, the balance of
plant would be
significantly simplified because the pump, tubing, and flow meters associated
with the second
- 11 -
Date Recue/Date Received 2023-12-22
half chamber can be omitted. The only additional design feature would be a
vent for the
molecular oxygen that is evolved at the metal oxide catalyst. Furthermore,
water would have to
be added periodically to the negolyte electrolyte tank to compensate for water
that is consumed
in the 02 evolution reaction. Optionally, this make-up water can be produced
in-situ by
combining the evolved 02 from the second half-chamber with the H2 evolved in
the second half-
chamber of the balancing cell and in the negolyte compartment of the main
cell. This water
production process may be catalyzed by a noble metal catalyst (e.g. Pt, Pd,
etc).
[0039] To this point, the invention has been described in terms of redox flow
batteries
in fluid communication with at least one electrochemical balancing cell.
However, the invention
also contemplates the operation of such cells. Accordingly, additional
embodiments provide
methods of operating any of the flow batteries described herein, each method
comprising
applying an electric potential across said first and second electrodes of the
pH correction cell. In
specific embodiments, the potential across these electrodes is maintained
within about 500 mV
of the overpotential voltage of the second aqueous electrolyte. In other
independent
embodiments, the potential across these electrodes is maintained within about
100 mV, about
250 mV, about 500 mV, or about 750 mV of the overpotential voltage of the
second aqueous
electrolyte.
[0040] In further embodiments, the balancing cell devices may be incorporated
into
electrochemical devices, including fuel cells and flow batteries, which
themselves are
incorporated into larger systems, for example, including cell stacks, storage
tanks and pipings for
containing and transporting the electrolytes, control hardware and software
(which may include
safety systems), and at least one power conditioning unit as part of an energy
storage system. In
such systems, the storage tanks contain the electroactive materials. The
control software,
hardware, and optional safety systems include all sensors, mitigation
equipment and
electronic/hardware controls and safeguards to ensure safe, autonomous, and
efficient operation
of the flow battery or other energy storage system.
[0041] Such storage systems may also include a power conditioning unit at the
front
end of the energy storage system to convert incoming and outgoing power to a
voltage and
current that is optimal for the energy storage system or the application. For
the example of an
energy storage system connected to an electrical grid, in a charging cycle the
power conditioning
unit would convert incoming AC electricity into DC electricity at an
appropriate voltage and
current for the electrochemical stack. In a discharging cycle the stack
produces DC electrical
power and the power conditioning unit converts to AC electrical power at the
appropriate voltage
and frequency for grid applications. Such energy storage systems of the
present invention are
- 12 -
Date Recue/Date Received 2023-12-22
well suited to sustained charge or discharge cycles of several hour durations.
As such, the
systems of the present invention are suited to smooth energy supply/demand
profiles and provide
a mechanism for stabilizing intermittent power generation assets (e.g. from
renewable energy
sources). It should be appreciated, then, that various embodiments of the
present invention
include those electrical energy storage applications where such long charge or
discharge
durations are valuable. For example, non-limiting examples of such
applications include those
where systems of the present invention are connected to an electrical grid
include renewables
integration, peak load shifting, grid firming, baseload power generation /
consumption, energy
arbitrage, transmission and distribution asset deferral, weak grid support,
and/or frequency
regulation. Additionally the devices or systems of the present invention can
be used to provide
stable power for applications that are not connected to a grid, or a micro-
grid, for example as
power sources for remote camps, forward operating bases, off-grid
telecommunications, or
remote sensors.
[0042] Terms
[0043] Throughout this specification, words are to be afforded their normal
meaning, as
would be understood by those skilled in the relevant art. However, so as to
avoid
misunderstanding, the meanings of certain terms will be specifically defined
or clarified.
[0044] In the present disclosure the singular forms "a," "an," and "the"
include the
plural reference, and reference to a particular numerical value includes at
least that particular
value, unless the context clearly indicates otherwise. Thus, for example, a
reference to "a
material" is a reference to at least one of such materials and equivalents
thereof known to those
skilled in the art, and so forth.
[0045] When a value is expressed as an approximation by use of the descriptor
"about,"
it will be understood that the particular value forms another embodiment. In
general, use of the
term "about" indicates approximations that can vary depending on the desired
properties sought
to be obtained by the disclosed subject matter and is to be interpreted in the
specific context in
which it is used, based on its function. The person skilled in the art will be
able to interpret this
as a matter of routine. In some cases, the number of significant figures used
for a particular
value may be one non-limiting method of determining the extent of the word
"about." In other
cases, the gradations used in a series of values may be used to determine the
intended range
available to the term "about" for each value. Where present, all ranges are
inclusive and
combinable. That is, references to values stated in ranges include every value
within that range.
[0046] As used herein, the term "redox couple" is a term of the art generally
recognized
by the skilled electrochemist and refers to the oxidized (electron acceptor)
and the reduced
- 13 -
Date Recue/Date Received 2023-12-22
(electron donor) forms of the species of a given redox reaction. The pair
Fe(CN)63f / Fe(CN)64H
is but one, non-limiting, example of a redox couple. Similarly, the term
"redox active metal ion"
is intended to connote that the metal undergoes a change in oxidation state
under the conditions
of use. As used herein, the term "redox couple" may refer to pairs of organic
or inorganic
materials. As described herein, inorganic materials may include "metal ligand
coordination
compounds" or simply "coordination compounds" which are also known to those
skilled in the
art of electrochemistry and inorganic chemistry. A (metal ligand) coordination
compound may
comprise a metal ion bonded to an atom or molecule. The bonded atom or
molecule is referred to
as a "ligand". In certain non-limiting embodiments, the ligand may comprise a
molecule
comprising C, H, N, and/or 0 atoms. In other words, the ligand may comprise an
organic
molecule. In some embodiments of the present inventions, the coordination
compounds comprise
at least one ligand that is not water, hydroxide, or a halide (F-, Cl-, Br-, I-
), though the invention
is not limited to these embodiments. Additional embodiments include those
metal ligand
coordination compounds described in U.S. Patent Application Ser. No.
13/948,497, filed July 23,
2013, which is incorporated by reference herein in its entirety at least for
its teaching of
coordination compounds
[0047] Unless otherwise specified, the term "aqueous" refers to a solvent
system
comprising at least about 98% by weight of water, relative to total weight of
the solvent. In
some applications, soluble, miscible, or partially miscible (emulsified with
surfactants or
otherwise) co-solvents may also be usefully present which, for example, extend
the range of
water's liquidity (e.g., alcohols / glycols). When specified, additional
independent embodiments
include those where the "aqueous" solvent system comprises at least about 55%,
at least about 60
wt%, at least about 70 wt%, at least about 75 wt%, at least about 80%, at
least about 85 wt%, at
least about 90 wt%, at least about 95 wt%, or at least about 98 wt% water,
relative to the total
solvent. It some situations, the aqueous solvent may consist essentially of
water, and be
substantially free or entirely free of co-solvents or other species. The
solvent system may be at
least about 90 wt%, at least about 95 wt%, or at least about 98 wt% water,
and, in some
embodiments, be free of co-solvents or other species. Unless otherwise
specified, the term
"non-aqueous" refers to a solvent system comprising less than 10% by weight of
water, generally
comprising at least one organic solvent. Additional independent embodiments
include those
where the "non-aqueous" solvent system comprises less than 50%, less than 40
wt%, less than 30
wt%, less than 20 wt%, less than 10%, less than 5 wt%, or less than 2 wt%
water, relative to the
total solvent.
- 14 -
Date Recue/Date Received 2023-12-22
[0048] The term "aqueous electrolyte" is intended to connote an
aqueous solvent
system comprising at least one material, typically ionic, whose electrical
conductivity is higher
than the solvent system without the material. In addition to the redox active
materials, an
aqueous electrolyte may contain additional buffering agents, supporting
electrolytes, viscosity
modifiers, wetting agents, and the like.
[0049] As used herein, the terms "negative electrode" and "positive electrode"
are
electrodes defined with respect to one another, such that the negative
electrode operates or is
designed or intended to operate at a potential more negative than the positive
electrode (and vice
versa), independent of the actual potentials at which they operate, in both
charging and
discharging cycles. The negative electrode may or may not actually operate or
be designed or
intended to operate at a negative potential relative to the reversible
hydrogen electrode.
[0050] In the present invention, the negative electrode associated with the
first aqueous
electrolyte of the balancing cell may comprise the same or different materials
than the negative
electrode of the operating flow batteries, although they share a common
electrolyte. By contrast,
the positive electrode associated with the second aqueous electrolyte of the
balancing cell will
almost certainly comprise different materials than the positive electrode of
the operating flow
battery; in this case, the positive electrolyte of the flow battery will
almost certainly be
compositionally different, and physically separated from, the second
electrolyte of the balancing
cell.
[0051] As used herein, an "ionomer," refers to a polymer comprising both
electrically
neutral and a fraction of ionized repeating units, wherein the ionized units
are pendant and
covalently bonded to the polymer backbone. The fraction of ionized units may
range from about
1 mole percent to about 90 mole percent, but may be categorized according to
their ionized unit
content. For example, in certain cases, the content of ionized units are less
than about 15 mole
percent; in other cases, the ionic content is higher, typically greater than
about 80 mole percent.
In still other cases, the ionic content is defined by an intermediate range,
for example in a range
of about 15 to about 80 mole percent.
[0052] The terms "negolyte" and "posolyte," generally refer to the
electrolytes
associated with the negative electrode and positive electrodes, respectively.
As used herein,
however, the terms "negolyte" and "posolyte" are reserved for the respective
electrolytes of the
flow battery. As contemplated herein, the negative working electrolyte
(negolyte) of the flow
battery comprises a coordination compounds or metal-ligand coordination
compounds. Metal
ligand coordination compounds may comprise at least one "redox active metal
ion," at least one
"redox inert metal ion," or both. The term "redox active metal ion" is
intended to connote that
- 15 -
Date Recue/Date Received 2023-12-22
the metal undergoes a change in oxidation state under the conditions of use.
In specific
embodiments, the negolyte comprises a metal ligand coordination complex having
a formula
comprising
M(L1)õ(L2)y(L3)zm, where
M is Al, Ca , Ce, Co, Cr, Fe, Mg, Mn, Mo, Si, Sn, Ti, V, W, Zn, or Zr;
Li, L2, and L3 are each independently ascorbate, a catecholate, citrate, a
glycolate or
polyol (including ligands derived from ethylene glycol, propylene glycol, or
glycerol), gluconate,
glycinate, ct-hydroxyalkanoate (e.g., ct-hydroxyacetate, or from glycolic
acid),
flhydroxyalkanoate, y-hydroxyalkanoate, malate, maleate, a phthalate, a
pyrogallate, sarcosinate,
salicylate, or lactate;
x, y, and z are independently 0, 1, 2, or 3, and 1 < x + y + z < 3;
and m is +1,0, -1, -2, -3, -4, or -5.
Related and independent embodiments provide that (a) x = 3, y = z = 0; (b) x =
2, y = 1, z = 0;
(c) x = 1, y = 1, z= 1; (d) x= 2, y = 1, z= 0; (e) x = 2, y = z =0; or (f) x =
1, y =z = 0. In
individual preferred embodiments, M is Al, Cr, Fe, or Ti and x + y + z = 3. In
more preferred
embodiments, the negolyte comprises a metal-ligand coordination compound of
titanium.
[0053] As used herein, unless otherwise specified, the term "substantially
reversible
couples" refers to those redox pairs wherein the voltage difference between
the anodic and
cathodic peaks is less than about 0.3 V, as measured by cyclic voltammetry,
using an ex-situ
apparatus comprising a flat glassy carbon disc electrode and recording at 100
mV/s. However,
additional embodiments provide that this term may also refer to those redox
pairs wherein the
voltage difference between the anodic and cathodic peaks is less than about
0.2 V, less than
about 0.1 V, less than about 0.075 V, or less than about 0.059 V, under these
same testing
conditions. The term "quasi-reversible couple" refers to a redox pair where
the corresponding
voltage difference between the anodic and cathodic peaks is in a range of from
0.3 V to about 1
V. Other embodiments provide that "substantially reversible couples" are
defined as having
substantially invariant (less than 10% change) peak separation with respect to
scan rate.
[0054] The term "stack" or "cell stack" or "electrochemical cell stack" refers
to a
collection of individual electrochemical cells that are in electrically
connection. The cells may be
electrically connected in series or in parallel. The cells may or may not be
fluidly connected.
[0055] The term "state of charge" (SOC) is well understood by those skilled in
the art
of electrochemistry, energy storage, and batteries. The SOC is determined from
the
concentration ratio of reduced to oxidized species at an electrode (Xred X.).
For example, in the
case of an individual half-cell, when Xred = X.,, such that Xred / X.x = 1,
the half-cell is at 50%
- 16 -
Date Recue/Date Received 2023-12-22
SOC, and the half-cell potential equals the standard Nernstian value, E . When
the concentration
ratio at the electrode surface corresponds to Xred X0x = 0.25 or Xred X0x =
0.75, the half-cell is
at 25% and 75% SOC respectively. The SOC for a full cell depends on the SOCs
of the
individual half-cells and in certain embodiments the SOC is the same for both
positive and
negative electrodes. Measurement of the cell potential for a battery at its
open circuit potential,
and using Equations 2 and 3 the ratio of Xmd X0, at each electrode can be
determined, and
therefore the SOC for the battery system.
[0056] ADDITIONAL ENUMERATED EMBODIMENTS
[0057] The following embodiments are intended to complement, rather than
supplant,
those embodiments already described.
[0058] Embodiment 1. A redox flow battery or other electrochemical device
comprising at least one electrochemical cell in fluid communication with a
balancing cell, said
balancing cell comprising:
a first and second half-cell chamber separated by a membrane,
wherein the first half-cell chamber comprises a first electrode in contact
with a
first aqueous electrolyte of the redox flow battery; and
wherein the second half-cell chamber comprises a second electrode in contact
with a second aqueous electrolyte, said second electrode comprising a catalyst
for the
generation of 02.
[0059] Embodiment 2. A redox flow battery comprising at least one
electrochemical
cell in fluid communication with a balancing cell, said balancing cell
comprising:
a first and second half-cell chamber separated by a membrane,
wherein the first half-cell chamber comprises a first electrode in contact
with a
first aqueous electrolyte of the redox flow battery; and
wherein the second half-cell chamber comprises a second electrode comprising a
catalyst for the generation of 02; and wherein
the second half-cell chamber does not contain (is free of) an aqueous
electrolyte.
[0060] Embodiment 3. A redox flow battery or other electrochemical device
comprising at least one electrochemical cell in fluid communication with a
balancing cell, said
balancing cell comprising:
- 17 -
Date Recue/Date Received 2023-12-22
a first and second half-cell chamber separated by a membrane,
wherein the first half-cell chamber comprises a first electrode in contact
with a
first aqueous electrolyte of the redox flow battery; and
wherein the second half-cell chamber comprises a second electrode in contact
with a second aqueous electrolyte, said second electrode comprising a catalyst
for the
generation of 02; and wherein the membrane comprises:
(1) a negatively charged ionomer, preferably a first and second type of a
negatively charged ionomer, for example a sulfonated perfluorinated polymer
or co-polymer;
(2) a positively charged ionomer;
(3) a bipolar membrane; or
(4) a combination of (1) to (3).
[0061] Embodiment 4. A balancing cell comprising:
a first and second half-cell chamber separated by a membrane,
wherein the first half-cell chamber comprises a first electrode in contact
with a
first aqueous electrolyte of an electrochemical device; and
wherein the second half-cell chamber comprises a second electrode in contact
with a second aqueous electrolyte, said second electrode comprising a catalyst
for the
generation of 02,
[0062] Embodiment 5. A working balancing cell comprising:
a first and second half-cell chamber separated by a membrane,
wherein the first half-cell chamber comprises a first electrode in contact
with a
first aqueous electrolyte of an electrochemical device; and
wherein the second half-cell chamber is free of added aqueous electrolyte.
[0063] Embodiment 6. The flow battery of any one of Embodiments 1 to 3 or the
balancing cell of Embodiment 4 or 5, wherein the second aqueous electrolyte
has a pH of at least
2, preferably greater than about 7, more preferably in a range of about 9 to
about 14.
[0064] Embodiment 7. The flow battery of any one of Embodiments 1 to 3,
wherein the
first aqueous electrolyte comprises a negative working electrolyte of the
redox flow battery.
[0065] Embodiment 8. The flow battery or balancing cell of any one of
Embodiments 1
to 7, wherein the first aqueous electrolyte has a pH in a range of from about
9 to about 14.
- 18 -
Date Recue/Date Received 2023-12-22
[0066] Embodiment 9. The flow battery of any one of Embodiments 1 to 8,
wherein the
first and second aqueous electrolytes each have a pH whose difference is less
than about 8, 7, 6,
5, 4, 3, 2, or 1.
[0067] Embodiment 10. The flow battery or balancing cell of any one of
Embodiments
1 to 9, further comprising a high porosity medium located near or adjacent to
the membrane in
the first chamber, the high porosity medium providing enhanced convection in
that region,
leading to neutralization of protons that are injected into the first half-
chamber.
[0068] Embodiment 11. The flow battery or balancing cell of any one of
Embodiments
1 to 10, wherein the membrane comprises a sulfonated perfluorinated polymer or
co-polymer
[0069] Embodiment 12. The flow battery or balancing cell of any one of
Embodiments
1 to 11, wherein the membrane comprises a sulfonated perfluorinated polymer or
co-polymer of
tetrafluoroethylene, optionally comprising perfluorovinyl ether moieties.
[0070] Embodiment 13. The flow battery or balancing cell of any one of
Embodiments
1 to 12, wherein the membrane comprises an ionomer membrane.
100711 Embodiment 14. The flow battery or balancing cell of any one of
Embodiments
1 to 13, wherein the membrane comprises an ionomer membrane characterized as a
short side
chain (SSC) copolymer of tetrafluoroethylene and a sulfonyl fluoride vinyl
ether (SFVE)
F2C¨CF-0-CF2CF2-S02F of low molecular weight.
[0072] Embodiment 15. The flow battery or balancing cell of any one of
Embodiments
1 to 14, wherein the membrane comprises an ionomer membrane characterized as a
short side
chain (SSC) copolymer of tetrafluoroethylene and a sulfonyl fluoride vinyl
ether (SFVE)
F2C=CF-0-CF2CF2-S02F of low molecular weight, in which the membrane is
modified by
precipitating particles therewithin , the particles comprising a metal, a
metal oxide, an insoluble
or poorly soluble metalloorganic material, a polymer, or a combination
thereof.
[0073] Embodiment 16. The flow battery or balancing cell of any one of
Embodiments
1 to 15, wherein the membrane comprises bipolar membrane.
[0074] Embodiment 17. The flow battery or balancing cell of any one of
Embodiments
1 to 16, wherein the membrane comprises a bipolar membrane, the bipolar
membrane
comprising at least one cation exchange ionomer membrane and one anion
exchange ionomer
membrane.
[0075] Embodiment 18. The flow battery or balancing cell of any one of
Embodiments
1 to 17, wherein the membrane comprises a bipolar membrane, the bipolar
membrane
comprising at least one cation exchange ionomer membrane and one anion
exchange ionomer
membrane and having a metal oxide film sandwiched therebetween, said metal
oxide film
- 19 -
Date Recue/Date Received 2023-12-22
capable of catalyzing the dissociation of water upon the application of an
electric potentiaal
thereto.
[0076] Embodiment 19. The flow battery or balancing cell of any one of
Embodiments
1 to 18, wherein the second electrode comprises a catalyst suitable for the
electrochemical
generation of oxygen from water.
[0077] Embodiment 20. The flow battery or balancing cell of any one of
Embodiments
1 to 19, wherein the second electrode comprises a metal oxide catalyst, said
metal oxide catalyst
suitable for the electrochemical generation of oxygen from water.
[0078] Embodiment 21. The flow battery or balancing cell of any one of
Embodiments
1 to 20, wherein the second electrode comprises an oxide of cobalt, iridium,
iron, manganese,
nickel, ruthenium, indium, tin, or a combination thereof, the oxide being
optionally doped with
fluorine (e.g., fluorine-doped tin oxide).
[0079] Embodiment 22. The flow battery or balancing cell of any one of
Embodiments
1 to 21, wherein the second electrode comprises an oxide of iridium or an
oxide of nickel.
[0080] Embodiment 23. The flow battery or balancing cell of any one of
Embodiments
Ito 19, wherein the second electrode comprises an allotrope of carbon, for
example carbon
black, diamond, glassy carbon, graphite, amorphous carbon, graphene,
fullerenes, carbon
nanotubes, or a combination thereof.
[0081] Embodiment 24. The flow battery of any one of Embodiments 7 to 23, as
applied
to flow batteries, wherein the negative working electrolyte of the flow
battery comprises a
compound comprising Al, Ca, Ce, Co, Cr, Fe, Mg, Mn, Mo, Si, Sn, Ti, V, W, Zn,
or Zr.
[0082] Embodiment 25. The flow battery Embodiment 24, wherein the negative
working electrolyte of the flow battery comprises a coordination compound of
titanium.
[0083] Embodiment 26. The flow battery or balancing cell of any one of
Embodiments
1 to 25, wherein at least the half-cell chamber and optionally the second half-
cell chamber is
configured as a flow-through cell.
[0084] Embodiment 27. A energy storage system comprising the flow battery or
balancing cell of any one of Embodiments 1 to 26.
[0085] Embodiment 28. A method of operating a flow battery or balancing cell
of any
one of Embodiments 1 to 26 or a system of Embodiment 27, said method
comprising applying an
electric potential across said first and second electrodes.
[0086] Embodiment 29. The method of Embodiment 28, wherein the potential is
maintained within 500 mV of the overpotential voltage of the second aqueous
electrolyte.
- 20 -
Date Recue/Date Received 2023-12-22
100871 EXAMPLES
[0088] The following Examples are provided to illustrate some of the concepts
described within this disclosure. While each Example is considered to provide
specific
individual embodiments of composition, methods of preparation and use, none of
the Examples
should be considered to limit the more general embodiments described herein.
[0089] Example 1: A balancing electrochemical cell was constructed with a
NafionTM
117 membrane (produced by E. I. du Pont de Nemours and Company, Wilmington,
Delaware),
and an iridium oxide catalyst on the positive side with a metal oxide loading
of not less than 1
mg/cm2. The positive side of the membrane was supported with commercial
titanium meshes
(1.4 mm thick) and negative side was supported with a carbon paper (MGL 370,
350 microns
thick), produced by Avcarb Material Products, Lowell, Massachusetts. The
carbon paper was
supported by a flow field machined on commercially available graphite vinyl-
ester composite.
The active area of the cell was 25 cm2, and the overall cell area was 64 cm2.
A flow rate of
approximately 50 cc/min of de-ionized water was maintained on the positive
side. A flow rate of
approximately 200 cc/min of negative flow battery electrolyte was maintained
on the negative
side. The balancing cell was operated at a current density of about 25 mA/cm2,
and a cell voltage
of about 2.7 V. FIG. 3 illustrates the cycling capacity in Amp-hours (Ah) and
the pH of the
negative electrolyte as a function of operating time with and without a
balancing cell. The target
Ah for the battery system was about 30 Ah and the target negative electrolyte
pH was about 11.5.
At the beginning of the experiment, the system exhibits a state-of-charge and
pH imbalance as
illustrated by the pH of ¨12 and the low charge capacity of ¨22 Ah. As the
system is operated,
the imbalance continues as pH of the negative electrolyte continues to rise
and the charge
capacity continues to fall. The imbalance is corrected through initiation of
the balancing cell at
¨125 hrs (vertical dashed line in FIG. 3); the pH is seen to decrease toward
the target value of
11.5 and the charge capacity of the negative electrolyte recovers to the
target 30 Ah.
[0090] Example 2: A balancing electrochemical cell was constructed with a
NafionTM
117 membrane (produced by E. I. du Pont de Nemours and Company, Wilmington,
Delaware),
and an iridium oxide catalyst deposited on the positive side of the membrane
with a metal oxide
loading of not less than 1 mg/cm2. The positive side of the membrane was
supported with
commercial titanium meshes (1.4 mm thick) and negative side was supported with
a carbon
paper (MGL 370, 350 microns thick), produced by Avcarb Material Products,
Lowell,
Massachusetts. The carbon paper was supported by a flow field machined on
commercially
available graphite vinyl-ester composite. The active area of the cell was 25
cm2, and the overall
cell area was 64 cm2. A flow rate of approximately 50 cc/min of de-ionized
water was
-21 -
Date Recue/Date Received 2023-12-22
maintained on the positive side. A flow rate of approximately 200 ce/min of
negative flow
battery electrolyte was maintained on the negative side. The balancing cell
was operated in
conjunction with a typical Coordination Compound Flow Battery (CCFB) as
described in U.S.
Patent US 2014/0028260 Al. In this example, the negolyte electrolyte material
was circulated
through both the flow battery and the balancing cell, hence pH and SOC control
was directed at
the ngative active material. FIG. 4A illustrates that in this particular
example, the balancing cell
operated at a stable voltage (¨ 2.8V) for prolonged periods of time (> 1600h).
Small fluctuations
in the voltage of the balancing cell can be explained by higher set points of
the current density,
which is displayed as the bottom curve in FIG. 4A. As a result of even small
amounts of 1-I2
evolution on the negative electrode of the main flow battery, operation of the
flow battery for
more than 1600 hr would inevitably lead an pH increase of the negolyte
electrolyte. FIG. 4B
illustrates that the pH of the negolyte was kept relatively constant, which is
a direct consequence
of the pH control effected by the balancing cell.
[0042] Example 3. A balancing electrochemical cell was constructed with a
NafionTM
117 membrane (produced by E. I. du Pont de Nemours and Company, Wilmington,
Delaware),
and an iridium oxide catalyst deposited on the positive side of the membrane
with a metal oxide
loading of not less than 1 mg/cm2. The positive side of the membrane was
supported with
commercial titanium meshes (1.4 mm thick) and negative side was supported with
a carbon
paper (MGL 370, 350 microns thick), produced by Avcarb Material Products,
Lowell,
Massachusetts. The carbon paper was supported by a flow field machined on
commercially
available graphite vinyl-ester composite. Between the carbon paper negative
electrode and the
N117 membrane, an additional membrane was added to prevent cross-over of
active species
across the N117 membrane. This membrane consisted of a Solvay Aquiviong E87
membrane.
The active area of the cell was 25 cm2, and the overall cell area was 64 cm2.
A flow rate of
approximately 50 cc/min of de-ionized water was maintained on the positive
side. A flow rate of
approximately 200 cc/min of negative flow battery electrolyte was maintained
on the negative
side. The balancing cell was operated in conjunction with a typical
Coordination Compound
Flow Battery (CCFB) as described in U.S. Patent US 2014/0028260 Al. In this
example, the
negolyte electrolyte material was circulated through both the flow battery and
the balancing cell,
hence pH and SOC control was directed at the negative active material. FIG. 5
shows that the as-
configured balancing cell is capable of balancing SOC and pH for hundreds of
hours (current
density: 10 mA/cm2). The voltage of this cell is only minimally higher
compared to balancing
cells without the additional membrane. The slightly higher voltage observed
(caused by the
resistance of the added membrane) may be acceptable when suppression of active
material cross-
- 22 -
Date Recue/Date Received 2023-12-22
over is affected. During the duration of this test, no coloration of the DI
water (anolyte) was
observed, indicating that cross-over of active material is minimal.
[0043] Example 4. A balancing electrochemical cell was constructed with a
Fumasep
FBM bipolar membrane (produced by Fumatech GmbH, Germany). The positive side
of the
membrane was supported with commercial nickel meshes (1.4 mm thick) and the
negative side
was supported with a carbon paper (MGL 370, 350 microns thick), produced by
Avcarb
Material Products, Lowell, Massachusetts. The carbon paper was supported by a
flow field
machined on commercially available graphite vinyl-ester composite. A flow rate
of
approximately 50 cc/min of 1M NaOH solution was maintained on the positive
side. A flow rate
of approximately 200 cc/min of negative flow battery electrolyte was
maintained on the negative
side. FIG. 6 shows the voltage-time data for this balancing cell for operation
at 25 mA/cm2. As
can be seen in FIG. 6, the voltage required to sustain a 25 mA/cm2 current
density is higher
(-3.3V) compared to the balancing cells that utilized a IrOx catalyst (-2.8V,
FIGs. 4-5), which is
a consequence of the higher membrane resistance of bipolar membranes. This
increased voltage
may be acceptable when a pH gradient across the membrane in the balancing cell
is to be
avoided and/or when cross-over of active material across the bipolar membrane
is significantly
lower compared to more traditional ionomer membranes.
[0044] Example 5. Two balancing electrochemical cells were constructed with
carbonaceous materials as positive electrodes. Both cells were constructed
with a NafionTM 117
membrane (produced by E. I. du Pont de Nemours and Company, Wilmington,
Delaware). The
first cell (cell A) utilized a BMCTm composite graphite plate in combination
with an AvCarbTm
1071HCB carbon cloth (0.356 mm thickness) as positive electrode. The carbon
cloth was
supported by a flow field machined into the BMCTm composite material. The
second cell (cell B)
utilized an Eisenhuth composite graphite plate in combination with a MorganTM
graphite felt
(2.8 mm thickness) as the positive electrode. The negative side of the
membrane comprised a
carbon paper (AvcarbTM MGL 370, 0.35 mm thick), which was supported by a flow
field
machined into the BMCTm composite material. The active area of both cells was
25 cm2, and the
overall cell area was 64 cm2. A flow rate of approximately 50 cc/min of de-
ionized water was
maintained on the positive side. A flow rate of approximately 200 cc/min of
negative flow
battery electrolyte was maintained on the negative side. The balancing cells
were operated at a
current density of 25 mA/cm2, and exhibited cell voltages of about 3.3-3.7V.
As can be seen in
FIG. 7, the voltage required to sustain a 25 mA/cm2 current density was
significantly higher
compared to the balancing cells that utilized a IrO, catalyst (FIGs. 4-5),
clearly illustrating that
catalyzing the water oxidation reaction results in balancing cells with lower
voltages. For both
- 23 -
Date Recue/Date Received 2023-12-22
cells A and B, the voltage required to drive 25 mA/cm2 slowly increased over
the timescale of
¨15h. Without intending to be bound by the correctness of any particular
theory, it is believed
that this voltage increase was due to the two electrochemical reactions
occurring at the positive
electrode, i.e. water oxidation and carbon oxidation. Hence, these carbon
positive electrodes
were truly sacrificial and needed to be replaced periodically. The advantage
of this concept,
however, is that no precious metal water oxidation catalyst was present in the
balancing,
reducing the risk corrosion and migration of these catalyst to the first half-
chamber, where these
metals would exacerbate H2 evolution.
[0091] As those skilled in the art will appreciate, numerous modifications and
variations of the present invention are possible in light of these teachings,
and all such are
contemplated hereby. For example, in addition to the embodiments described
herein, the present
invention contemplates and claims those inventions resulting from the
combination of features of
the invention cited herein and those of the cited prior art references which
complement the
features of the present invention. Similarly, it will be appreciated that any
described material,
feature, or article may be used in combination with any other material,
feature, or article, and
such combinations are considered within the scope of this invention.
[0092] The disclosures of each patent, patent application, and publication
cited or
described in this document are hereby incorporated herein by reference, each
in its entirety, for
all purposes.
- 24 -
Date Recue/Date Received 2023-12-22