Note: Descriptions are shown in the official language in which they were submitted.
WO 2023/288022 PCT/US2022/037213
DRUG DELIVERY SYSTEMS, DEVICES, AND METHODS
INCORPORATION BY REFERENCE TO ANY PRIORITY APPLICATIONS
[0001] .. This application is a continuation-in-part of U.S. Application No.
17/650,783, filed February 11, 2022. This application claims the benefit of
U.S. Provisional
Application Nos. 63/263,863, filed November 10, 2021, 63/261,638, filed
September 24,
2021, and 63/203,324, filed July 16, 2021. Each of the above-listed
applications is
incorporated by reference herein in its entirety. All applications for which a
foreign or
domestic priority claim is identified in the Application Data Sheet as filed
with the present
application are hereby incorporated by reference under 37 CFR 1.57.
BACKGROUND
Field of the Invention
[0002] This disclosure relates to systems, devices, and methods for
delivering
a substance, or a combination of substances, for a medical or therapeutic
purpose in
accordance with a treatment program. More particularly, this invention relates
to systems,
devices, and methods for delivering substances in a monitored and controlled
treatment
program, that is individually tailored for a user and can be dynamically
changed based on
day-to-day sensed characteristics of the user's actions and progress in the
treatment
program.
Description of the Related Art
[0003] One or more medicants are often provided to a user for a medical or
therapeutic purpose. Drugs are typically administered by a user through the
user's mouth
(e.g., as pills, chewables, and lozenges, etc.), skin (e.g., via gel, cream,
spray, and a patch)
or through the user's nose (e.g., via an inhaler). In all of these
administration programs, the
user is often responsible to properly follow a drug administrative program and
track their
compliance with the program to ensure the treatment is effective and safe, and
thus the
proper administration of drugs using such methods can be highly unreliable.
Although for
some treatments strict adherence to the program may not be needed, for other
programs
adherence is absolutely necessary for effective and safe treatment.
1
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
[0004] Patients with certain additions can be helped
through certain treatment
programs. Addictions can cause physical and mental harm. As an example,
smoking causes
many diseases including cancer, heart disease, stroke, lung diseases,
diabetes, emphysema
and chronic bronchitis. Smoking is also known to increase a person's risk for
tuberculosis,
certain eye diseases, and problems of the immune system, including rheumatoid
arthritis.
smoking is one of the nic.-ist prevalent sources of preventable death
worldwide. PecTile who
don't smoke but are near a person who is smoking may also be afflicted with
one of these
diseases through secondary smoke. Smoking and vaping (which is, for ease of
reference,
are both generally referred to herein as "smoking" unless context or specific
language
indicates otherwise) are highly addictive, which makes quitting smoking
difficult. Many
aids have been developed to quit smoking. For example, nicotine patches and
nicotine gum
may help a person to quit smoking. Certain devices (e.g., electronic
cigarettes) have been
developed as an aid to quit smoking, or at least somewhat lower health risks,
by providing
a less harmful source of inhaled nicotine.
[0005] Current devices that are used in programs for
quitting smoking,
addressing other addictions, and other treatment programs are generally used
in a similar
manner for everyone, even though different people have different biological
factors and
psychological factors (e.g., related to smoking). For example, aerosol
producing devices
typically cannot dynamically provide tailored aerosol mixtures based as
required by a
sophisticated individual treatment program. In addition, such devices cannot
adequately
monitor the use of the device and provide feedback to the system controlling
the treatment
program to ensure medicants are accurately provided and their use is tracked.
Furthermore,
in treatment programs for smoking and other addictions, such devices do not
allow a user
to address an overwhelming addiction urge, where it is automatically monitored
and
dynamically changes the treatment the treatment program. Accordingly, there is
a need for
an improved process for administering drugs in controlled, trackable treatment
programs,
e.g., to help a user quit smoking, or stop another addiction.
SUMMARY
[0006] The systems, methods, and devices described herein
each have several
aspects, no single one of which is solely responsible for its desirable
attributes. Without
limiting the scope of this disclosure, several non-limiting features will now
be discussed
briefly. The methods and techniques described herein relate to systems,
devices, and
methods for controlled delivery of a drug, medicant, or an active
pharmaceutical ingredient
2
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
(API) to a user as part of a treatment program. In some specific examples,
methods and
techniques described herein relate to systems, devices, and methods for a
controlled
delivery of an aerosol mixture to a user during a treatment program.
[0007] In one innovation, a delivery system for providing
an aerosol mixture
in a treatment program for quitting smoking, comprise, a delivery device
including a
housing; a channel in the housing, the channel structured to receive air from
an opening in
the housing and communicate air to an aerosolizer pod coupled to the delivery
device; a
flow sensor positioned to sense air flowing through the channel; a first,
second, and third
aerosolizer driver each having an electrical connection configured to
electrically couple to
a first, second, and third aerosolizer, respectively, of an aerosolizer pod
coupled to the
delivery device; a rescue button configured to, when actuated by a user,
provide a signal to
provide an additional dose of the aerosol mixture according to the treatment
program; a
power source; and a controller circuit coupled to the power source, the
controller circuit
comprising a hardware controller electrically coupled to the first, second,
and third
aerosolizer drivers, the flow sensor, and the rescue button, the hardware
controller
including a hardware processor and a non-transitory computer readable medium
in
communication with the hardware controller, the computer readable medium
configured to
store treatment program information, and to store executable instructions
that, when
executed, configure the hardware controller to individually control the three
aerosolizer
drivers to provide aerosol generation signals to the first, second and third
aerosolizers,
respectively, of a pod coupled to the delivery device, to generate an aerosol
mixture based
at least in part on the stored treatment program and information that is
received from the
flow sensor and the rescue button.
[0008] Various implementations can include one or more
additional aspects. In
some implementations, the housing comprises an aperture to receive an
aerosolizer pod
therein, the housing configured to at least partially surround the aerosolizer
pod when the
aerosolizer pod in positioned in the aperture and coupled to the delivery
device. In some
implementations, the housing comprises a distal end and a proximal end, and
wherein the
aperture to receive the aerosolizer pod is on the proximal end of the housing.
In some
implementations, further comprising the aerosolizer pod, wherein the
aerosolizer pod is
structured to be removably coupleable to the delivery device. In some
implementations, the
aerosolizer pod further comprises an ID chip, and wherein the delivery device
further
comprises an aerosolizer pod interface configured to sense the ID chip and
communicate
the ID chip to the hardware controller to identify the aerosolizer pod and the
substances
3
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
contained therein. In some implementations, the aerosolizer pod comprises an
aerosolizer
system including the first, second, and third aerosolizers. In some
implementations, each
of the first, second, and third aerosolizers includes an electrical connection
configured to
electrically couple to one of the first aerosolizer, second aerosolizer, and
third aerosolizer
drivers of the delivery device. In some implementations, wherein the first,
second, and third
aerosolizers are thermal aerosolizers. In some implementations, the first,
second, and third
aerosolizers are mechanical aerosolizers. In some implementations, the
aerosolizer pod
includes a first container holding a first substance, a second container
holding a second
substance, and a third container holding a third substance, the first, second
and third
containers structured to provide the first, second and third substances to the
first, second,
and third aerosolizers, respectively. In some implementations, the first
substance comprises
freebase nicotine, the second substance comprises monoprotonated nicotine, and
the third
substance is a flavorant.
[0009] In some implementations, the system further
comprises the aerosolizer
pod, the aerosolizer pod structured to be removably coupleable to the delivery
device, the
aerosolizer pod comprising: a distal end and a proximal end; an intake port on
the distal
end for receiving air flowing through the channel; a mixing chamber; an
exhaust port on
the proximal end for communicating the aerosol mixture from the mixing chamber
out of
the aerosolizer pod; an aerosolizer system comprising the first, second, and
third
aerosolizer, the first, second, and third aerosolizers each having an
electrical connection
that electrically couples to the first, second, and third aerosolizer drivers,
respectively,
when the aerosolizer pod is coupled to the delivery device. In some
implementations, the
mixing chamber includes intake openings in fluid communication with the first,
second and
third aerosolizer and an exhaust opening in fluid communication with the
exhaust port such
that aerosol generated by the first, second and third aerosolizer can enter
the mixing
chamber via the intake openings, mix together, and be communicated out of the
pod via the
pod exhaust port. In some implementations, the first aerosolizer includes a
first container
for holding a first substance, a first thermal aerosolizer configured to
generate aerosol from
the first substance based on signals received from the first aerosolizer
driver, and a first
passage in fluid communication with the thermal aerosolizer and the mixing
chamber for
communicating aerosol generated by the first thermal aerosolizer to the mixing
chamber;
the second aerosolizer includes a second container for holding a second
substance, a second
thermal aerosolizer configured to generate aerosol from the second substance
based on
signals received from the second aerosolizer driver, and a second passage in
fluid
4
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
communication with thermal aerosolizer and the mixing chamber for
communicating
aerosol generated by the second thermal aerosolizer to the mixing chamber; and
the third
aerosolizer includes a third container for holding a third substance, a third
thermal
aerosolizer configured to generate aerosol from the third substance based on
signals
received from the third aerosolizer driver, and a third passage in fluid
communication with
the third thermal aerosolizer and the mixing chamber for communicating aerosol
generated
by the third thermal aerosolizer to the mixing chamber. In some
implementations, the first
substance comprises a nicotine-based substance therein, and the second
substance
comprises a nicotine-based substance therein. In some implementations, the
third substance
comprises a flay orant. In some implementations, the controller circuit
further comprises a
transceiver, wherein the controller circuit is configured to receive treatment
program
information using the transceiver, and wherein the controller circuit is
configured to
provide signals to generate an aerosol mixture that includes a portion of a
first substance, a
second substance, and a third substance contained in the aerosolizer pod,
based on the
received treatment program information In some implementations, the controller
circuit is
further configured to provide signals to generate an aerosol mixture having a
certain aerosol
droplet size, from each of the first, second, and third aerosolizers based on
the received
treatment program information.
[0010] In some implementations, the controller circuit is
further configured to
provide signals to generate an aerosol mixture having droplets of less than or
equal to a
first diameter from each of the first, second, and third aerosolizers for a
first portion of the
treatment program, and to provide signals to generate an aerosol mixture
having droplets
of greater than or equal to a second diameter for a second portion of the
treatment program,
based on the received treatment program information. In some implementations,
the
controller circuit is further configured to provide signals to generate an
aerosol mixture
having a majority of droplets of less than or equal to a first diameter from
each of the first,
second, and third aerosolizers for a first portion of the treatment program,
and to provide
signals to generate an aerosol mixture having a majority of droplets of
greater than or equal
to a second diameter for a second portion of the treatment program, based on
the received
treatment program information. In some implementations, the second portion of
the
treatment program is subsequent to the first portion of the treatment program,
and wherein
the first diameter is smaller than the second diameter. In some
implementations, the first
diameter is less than or equal to 1 p.m, and the second diameter is greater
than or equal to
p.m. In some implementations, the system further comprises a button in
electrical
5
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
communication with the controller circuit, the controller circuit configured
to activate the
delivery device to provide an aerosol mixture when the button is actuated. In
some
implementations, the button comprises a fingerprint sensor, and wherein the
controller
circuit is configured to activate the delivery device to provide an aerosol
mixture if
fingerprint information sensed by the fingerprint sensor matches the
fingerprint sensor of a
specific user.
[0011] Another innovation includes a system, comprising a
delivery device
including a housing; a channel in the housing, the channel structured to
receive air from an
opening in the housing and communicate air to an aerosolizer pod coupled to
the delivery
device; a flow sensor positioned to sense air flowing through the channel; a
first, second,
and third aerosolizer driver each having an electrical connection configured
to electrically
couple to a first, second, and third aerosolizer, respectively, of an
aerosolizer pod coupled
to the delivery device; and a controller circuit coupled to a power source,
the controller
circuit comprising a transceiver and a hardware controller electrically
coupled to the first,
second, and third aerosolizer drivers, and the fl ow sensor, the controller
circuit configured
to individually control the first, second, and third aerosolizer drivers to
provide aerosol
generation signals to a first, second and third aerosolizer to generate an
aerosol mixture
based at least in part on a treatment program received using the transceiver;
and a user
computing device comprising an application in communication with the delivery
system
via the transceiver. In some implementations, the system further comprises a
server system
configured with a hardware processor and non-transitory computer readable
storage media
encoded with a treatment program including instructions executable by an
operating system to control the generation of the aerosol mixtures over time
according to
the treatment program, and provide treatment program information to the
delivery system
to control the generation of the aerosol mixture by the delivery system. In
some
implementations, the treatment program information provided to the delivery
system
includes information to individually control the first, second, and third
aerosolizer drivers
to provide signals to a first, second and third aerosolizer coupled to the
first, second and
third aerosolizer drivers, respectively, to generate a desired aerosol mixture
of a first aerosol
generated from a first substance, a second aerosol generated by a second
substance, and a
third aerosol generated by a third substance. In some implementations, the
treatment
program information provided to the delivery system includes information to
individually
control the first, second, and third aerosolizer drivers to provide signals to
a first, second
and third aerosolizer coupled to the first, second and third aerosolizer
drivers to generate
6
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
the first, second and third aerosol having an aerosol droplet of a certain
diameter. In some
implementations, the treatment program information provided to the delivery
system
includes information to individually control the first, second, and third
aerosolizer drivers
to provide signals to a first, second and third aerosolizer coupled to the
first, second and
third aerosolizer drivers to generate the first, second and third aerosol
having an aerosol
droplet of a first diameter for a first portion of time and an aerosol droplet
of a second
diameter for a second portion of time.
100121 Another innovation includes a method for smoking
cessation, the
method comprising providing a delivery system including a delivery device
including a
housing; a channel in the housing, the channel structured to receive air from
an opening in
the housing and communicate air to an aerosolizer pod coupled to the delivery
device; a
flow sensor positioned to sense air flowing through the channel; a first,
second, and third
aerosolizer driver configured to electrically couple to a first, second, and
third aerosolizer,
respectively, of an aerosolizer pod coupled to the delivery device; a rescue
button
configured to, when actuated by a user, provide a signal indicative of the
user's need for an
additional dose of an aerosol mixture; a power source; and a controller
circuit coupled to
the power source, the controller circuit comprising a hardware controller
electrically
coupled to the first, second, and third aerosolizer drivers, the flow sensor,
and the rescue
button, the hardware controller including a hardware processor and a non-
transitory
computer readable medium in communication with the hardware controller, the
computer
readable medium configured to store treatment program information, and to
store
executable instructions that, when executed, configure the hardware controller
to
individually control the three aerosolizer drivers to provide aerosol
generation signals to
first, second and third aerosolizers, respectively, of a pod coupled to the
delivery device to
generate an aerosol mixture based at least in part on the stored treatment
program for
quitting smoking, and information that is received from the flow sensor and
the rescue
button; and an aerosolizer pod, the aerosolizer pod comprising an aerosolizer
system
including the first, second, and third aerosolizers, wherein the pod is
structured to be
removably coupleable to the delivery device, each of the first, second, and
third aerosolizers
having an electrical connection that electrically couples to one of the first
aerosolizer,
second aerosolizer, and third aerosolizer drivers of the delivery device; a
first container
holding a first substance, a second container holding a second substance, and
a third
container holding a third substance, the first, second and third containers
structured to
provide the first, second and third substances to the first, second, and third
aerosolizers,
7
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
respectively, wherein the first substance is freebase nicotine and the second
substance is
monoprotonated nicotine; and
generating aerosol mixtures in accordance with a
smoking cessation treatment program to generate aerosol mixtures that are
dynamically
changed over a period of time to have different aerosol drop sizes and
different
concentrations of the first, second and third substances based at least in
part on received
signals from the flow sensor and the rescue button, and on smoking cessation
treatment
program information stored in the non-transitory computer readable medium.
[0013]
Another innovation includes a method for smoking cessation, the
method comprising providing signals, from a hardware controller in a hand-held
delivery
device, to a first, second and third aerosolizer driver in the delivery device
to dynamically
control a first, second and third aerosolizer in a pod coupled to the delivery
device, to
generate aerosol mixtures that are dynamically changed over a period of time
to have
different aerosol droplet sizes and different concentration of a first, second
and third
substance in containers of the pod based at least in part on received input
signals from one
or more of a flow sensor, a rescue button, and on smoking cessation treatment
program
information stored in a non-transitory computer readable medium coupled to the
hardware
controller, wherein the method is executed by the controller executing
computer executable
instructions stored on the non-transitory computer readable medium, wherein
the
executable instructions when executed cause the hardware controller to
providing signal to
the first, second, and third aerosolizer drivers according to the smoking
cessation program.
[0014]
Another innovation includes a delivery system for providing an aerosol
mixture in a treatment program for quitting smoking, comprising a pod
comprising an
aerosolizer system including a first, second, and third aerosolizers, and a
first, second and
third container in communication with the first, second, and third
aerosolizer, respectively,
each container holding a substance that is used to generate an aerosol mixture
according to
the treatment program, a delivery device, wherein the pod is removably
coupleable to the
delivery device, the delivery device including a housing; a channel structured
to receive air
from an opening in the housing and communicate air to an aerosolizer pod
coupled to the
delivery device; a flow sensor positioned to sense air flowing through the
channel; a first,
second, and third aerosolizer driver configured to electrically couple to the
first, second,
and third aerosolizer, respectively, of the pod when the pod is coupled to the
delivery
device; a rescue button configured to, when actuated by a user, provide a
signal indicative
of the user's need for an additional dose of an aerosol mixture; a power
source; and a
controller circuit coupled to the power source, the controller circuit
comprising a hardware
8
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
controller electrically coupled to the first, second, and third aerosolizer
drivers, the flow
sensor, and the rescue button, the hardware controller including a hardware
processor and
a non-transitory computer readable medium in communication with the hardware
controller, the computer readable medium configured to store treatment program
information, and to store executable instructions that, when executed,
configure the
hardware controller to individually control the three aerosolizer drivers to
provide aerosol
generation signals to the first, second and third aerosolizers, respectively,
to generate an
aerosol mixture based at least in part on the stored treatment program, and
information that
is received from the flow sensor and the rescue button. In some
implementations, the three
aerosolizers are thermal aerosolizers. In some implementations, the three
aerosolizers are
mechanical aerosolizers. In some implementations, the first container contains
freebase
nicotine, and the second container contains monoprotonated nicotine.
100151 Another innovation includes a computer-implemented
method for
providing a treatment program for smoking cessation, the method comprising
generating a
smoking cessation treatment program that includes a plurality of treatment
periods based
on received patient information, the patient information including a nicotine
metabolic rate;
[0016] communicating aerosol mixture information, based on
the treatment
program, to a handheld delivery system that includes three substances which
are used to
generate an aerosol mixture that is provided to the patient, the aerosol
mixture information
indicating, for each of the plurality of treatment periods, an amount of each
of the three
substances to be included in the aerosol mixture and the droplet size of the
aerosol droplets
in the aerosol mixture, wherein the method is performed by one or more
computer hardware
processors executing a plurality of computer readable instructions stored on a
non-
transitory computer memory. In some implementations, the method further
includes
generating, on the delivery system, the aerosol mixture based on the aerosol
mixture
information. In some implementations, the method further includes receiving
usage
information from the delivery system, and communicating to the delivery system
updated
aerosol mixture information, based at least in part on the usage information.
[0017] Another innovation is a method of operating a
smoking or vaping
cessation system, the method comprising providing a smoking cessation system
(for
example, in any of the embodiments and having features described herein) and
controlling
aerosol produced by each of the three aerosolizers to form an aerosol mixture
in an aerosol
mixing chamber, based on the cessation program, and based on information
received from
one or more sensors.
9
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
[0018] Another innovation includes a method of operating a
handheld smoking
or vaping cessation system, the method comprising controlling aerosol
generated by each
of the three or more aerosolizers of an aerosolizer system to form an aerosol
mixture in an
aerosol mixing chamber, the aerosol mixing chamber being in fluid
communication with
an exhaust opening for providing the aerosol mixture to a user.
[0019] Another innovation is a non-transitory computer
readable medium
having instructions stored thereon, that when executed by a computer hardware
processor
cause the computer hardware processor to perform a portion of, or all of, any
of the methods
described herein.
[0020] In an example, an innovation includes a non-
transitory computer
readable medium for operating a smoking cessation system, the computer
readable medium
having program instructions for causing a hardware processor to perform a
method of
providing signals, from a hardware controller in a hand-held cessation device,
to a first,
second and third aerosolizer driver in the hand-held cessation device to
dynamically and
individually control a first, second and third aerosolizer, in an aerosolizer
pod coupled to
the hand-held cessation device, to generate aerosol mixtures that are
dynamically changed
over a period of time. In another example, an innovation includes anon-
transitory computer
readable medium for operating a smoking cessation system, the computer
readable medium
having program instructions for causing a hardware processor to perform a
method of
providing signals, from a hardware controller in a hand-held cessation device,
to a first,
second and third aerosolizer driver in the hand-held cessation device to
dynamically and
individually control a first, second and third aerosolizer, in an aerosolizer
pod coupled to
the hand-held cessation device, to generate aerosol mixtures that are
dynamically changed
over a period of time, including controlling the aerosol mixture having to
have one or more
of different drop sizes and different concentration of a first, second and
third substance
contained in the first, second, and third aerosolizer, respectively, based at
least in part the
cessation program, and which can also be based on received input signals from
one or more
sensors, and on a smoking cessation program information stored in the non-
transitory
computer readable medium coupled to the hardware controller.
100211 Additional embodiments of the disclosure are
described below in
reference to the appended claims, which may serve as an additional summary of
the
disclosure.
[0022] In various embodiments, systems disclosed that a
computer readable
storage medium having program instructions embodied therewith, and one or more
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
processors configured to execute the program instructions to cause the one or
more
processors to perform operations comprising one or more aspects of the above-
and/or
below-described embodiments (including one or more aspects of the appended
claims). The
cessation program can be included on server system, or can be included on an
application-specific integrated circuit (ASIC) or other integrated circuit
chips that are
customized to include data flow processing and classifying, and such ASIC's or
other
integrated circuit chips can be included in a network or network element.
100231 In various embodiments, computer--implemented
methods are disclosed
in which, by one or more processors executing program instructions, one or
more aspects
of the above- and/or below-described embodiments (including one or more
aspects of the
appended claims) are implemented and/or performed.
[0024] In various embodiments, computer program products
comprising a
non-transitory computer readable storage medium are disclosed, wherein the
computer
readable storage medium has program instructions embodied therewith, the
program
instructions executable by one or more processors to cause the one or more
processors to
perform operations comprising one or more aspects of the above-described
and/or
below-described embodiments (including one or more aspects of the appended
claims).
BRIEF DESCRIPTION OF THE DRAWINGS
[0025] The features and advantages of the systems and
methods described
herein will become more fully apparent from the following description and
appended
claims, taken in conjunction with the accompanying drawings. These drawings
depict only
several embodiments in accordance with the disclosure and are not to be
considered
limiting of its scope. In the drawings, similar reference numbers or symbols
typically
identify similar components, unless context dictates otherwise. The drawings
are not
intended to depict every feature, structure, and/or component of actual
embodiments of the
systems and components illustrated, are they intended to depict relative
dimensions of the
illustrated elements, and the drawings may not be drawn to scale.
[0026] Figure 1 illustrates an overview of an addiction
cessation system, for
example, a smoking or vaping cessation system ("cessation system").
[0027] Figure 2 is a schematic of an example of a
cessation system that includes
a housing and an aerosolizer system (or "aerosolizer pod") that can be
removably coupled
to the housing.
11
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
[0028] Figure 3A is a schematic of an example of a
cessation system,
illustrating the aerosolizer system coupled to the housing.
[0029] Figure 3B is a schematic of the housing of the
cessation system
illustrated in Figure 3A.
[0030] Figure 3C is a schematic of the aerosolizer system
of the cessation
system illustrated in Figure 3A.
[0031] Figure 3D is a schematic of the case and the
cessation device containing
the components illustrated in Figure 3A.
[0032] Figure 4 is a schematic of an example of a
controller that can be used in
a cessation system, showing communication lines between a hardware processor
and other
components of the cessation system.
[0033] Figure 5 is an example of a computer system that
may be used to
implement the functionality described herein.
[0034] Figure 6A is a diagram of a system for developing
and implementing a
smoking cessation program, according to one embodiment.
[0035] Figure 6B is a flowchart illustrating an example of
a smoking cessation
process, according to one embodiment.
[0036] Figure 6C is a flowchart illustrating another
example of a smoking
cessation process, according to one embodiment.
[0037] Figure 7A is a diagram illustrating an example of
intersecting
personalization domains utilized by the smoking cessation system.
[0038] Figures 7B-1 and 7B-2 is a table illustrating
personalization parameters
that influence how the body processes nicotine.
[0039] Figure 7C is a diagram illustrating an example of
primary, secondary',
and other focused personalization domains utilized by the smoking cessation
system.
100401 Figure 7D is a diagram illustrating an example of
how nicotine is
deposited within the body, according to one embodiment.
[0041] Figure 7E is a diagram illustrating an example of
how nicotine input and
output are measured, according to one embodiment.
100421 Figure 7F is a diagram illustrating an example of
four key
personalization parameters and their effect on nicotine deposited in the body.
[0043] Figure 7G is a diagram illustrating an example of
how the four key
personalization parameters determine three critical variables.
12
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
[0044] Figure 7H is a diagram illustrating the cascaded D-
2ADME
personalization model.
[0045] Figure 71 is a diagram illustrating the
personalized parameters and
device managed variables used in the smoking cessation system.
[0046] Figure 7J is a diagram illustrating the
psychosocial co-factors when a
user is being compelled to initiate a smoking experience.
[0047] Figure 7K is a diagram illustrating how
personalization parameters and
clustered persona characteristics are used to map cessation liquid parameters.
[0048] Figure 7L is a diagram illustrating concentration
levels of nicotine
mapped to persona profile and metabolism rate.
[0049] Figure 7M is a diagram illustrating bioavailability
mapped to persona
and metabolism rate.
100501 Figure 7N is a diagram illustrating aerosol droplet
size mapped to
persona profile and metabolism rate.
[0051] Figure 70 is a diagram illustrating a starting
combination of cessation
liquid variables defined for a unique user.
[0052] Figure 7P is a diagram illustrating variables that
can be applied to
uniquely tailor a cessation program.
[0053] Figure 7Q is a diagram illustrating a five step
process that can be
implemented for a cessation program.
[0054] Figure 8A is a block diagram illustrating aspects
of an onboarding
process for a smoking cessation system, which can be the first portion of a
process for
helping a user quit smoking, an example of other subsequent portions of such a
process are
illustrated in Figures 10A ¨ 10L.
[0055] Figure 8B is a diagram illustrating aspects of data
communications
between the server system, the user device (mobile platform), and the delivery
system, for
example, the server system, user device, and the delivery system illustrated
in Figure 1.
[0056] Figures 8C and 8D illustrate examples of user
interfaces that are
displayed on the user device during an on-boarding process according to some
embodiments, where questions are presented to the user, input is received from
the user
relating to their smoking habits and personal information, and the input is
used to tailor the
treatment program (in this example, a smoking cessation program).
13
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
[0057] Figure 8E illustrates examples of user interfaces
that are displayed on
the user device during a device personalization process (calibration process),
according to
some embodiments.
[0058] 8F illustrates examples of user interfaces thar are
displayed on the user
device during the treatment program, according to some embodiments.
[0059] Figure 9A illustrates an example of a delivery
device with the pod
capable of being coupled to the delivery device.
[0060] Figure 9B illustrates the delivery device coupled
to pod shown in Figure
9A.
[0061] Figure 9C further illustrates the device without
the pod that is show in
Figure 9B
[0062] Figure 9D illustrates a pod that utilizes non-
thermal aerosolizers for a
drug delivery device.
[0063] Figure 9E is a schematic of a non-thermal example
circuit that can be
used in a drug delivery system, similar to the smoking cessation device
illustrated in Figure
3A
[0064] Figure 9F is a flowchart illustrating an example of
a drug dosage and
delivery process, according to one embodiment.
[0065] Figure 10A is a graph illustrating the quit journey
period and the taper
parameters implemented in a cessation program.
[0066] Figure 10B illustrates dosage graphs, and Figures
10B-1, 10B-2, 10B-3
refer to portions of Figure 10B. Figure 10B-1 is a graph illustrating the
dosage map
specification of monoprotonated nicotine.
[0067] Figure 10B-2 is a graph illustrating the dosage map
specification of
freebase nicotine.
100681 Figure 10B-3 is a graph illustrating the dosage map
specification of
dimensionless values for freebase nicotine ratio and enantiomeric ratio.
[0069] Figure 10C is a graph illustrating the hypothetical
dosage map
specification of monoprotonated nicotine dose.
100701 Figure 10D is a graph illustrating the hypothetical
dosage map
specification of freebase nicotine dose (FND).
[0071] Figure 10E is a graph illustrating the hypothetical
dosage map
specification of total nicotine dose (TND) (sum of monoprotonated and
freebase).
14
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
[0072] Figure IOF is a graph illustrating the hypothetical
dosage map
specification of freebase nicotine ratio (FNR) (ratio of (free dose)/ (total
dose)).
[0073] Figure 10G is a graph illustrating the hypothetical
dosage map
specification of enantiomeric ratio (ratio of (S-nicotine)/(R-nicotine)).
[0074] Figure 10H is a graph illustrating the hypothetical
dosage map
specification of variable aerosol droplet size (ADS).
[0075] Figure 101 is a graph illustrating the hypothetical
dosage map
specification of combined previous 6 taper variables shown as a function of
the quit journey
period, based on initial values of TND, FND and FNR, plus taper TND reduction
targets.
[0076] Figure 10J is a graph illustrating an example of a
representative dosage
map specification of a cigarette-smoking patient (Patient 1- Marlboro Red @
2.6mg/cig
with FNR=0.11, Taper regime TEND(FNR constant)).
100771 Figure 10K is a graph illustrating an example of a
representative dosage
map specification of a vaping patient (Patient 2- Vapors' XROS & Zen-Haus e-
Liq (0_,)
17mg/mL with FNR=0.84, Taper regime TFND(FNR constant)).
[0078] Figure 10L is a graph illustrating an example of a
representative dosage
map specification of a cigarette-smoking patient (Patient 3- Winston Blue A
1.7mg/cig
with FNR=0.05, Taper regime TFND initial (FNR constant) TrNR(FND constant)).
DETAILED DESCRIPTION OF CERTAIN INVENTIVE ASPECTS
[0079] The detailed description of various exemplary
embodiments below, in
relation to the drawings, is intended as a description of various aspects of
the various
exemplary embodiments, components, and methods implemented with a system for
delivering substances to a user in accordance with an individually tailored
treatment
program, and is not intended to represent the only aspects in which the
various exemplary
embodiments described herein may be practiced. The detailed description
includes specific
details for the purpose of providing a thorough understanding of the various
exemplary
embodiments of the present invention. However, it will be apparent to those
skilled in the
art that some aspects of the various exemplary embodiments of the present
invention may
be practiced without these specific details. In some instances, well-known
structures and
components are shown in block diagram form in order to avoid obscuring various
examples
of various embodiments. It will be understood that unless a term is expressly
defined in this
disclosure to possess a described meaning, there is no intent to limit the
meaning of such
term, either expressly or indirectly, beyond its plain or ordinary meaning.
Any element in
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
a claim that does not explicitly state "means for" performing a specified
function, or "step
for" performing a specific function is not to be interpreted as a "means or
"step" clause as
specified in 35 U.S.C. 112.
[0080] This disclosure relates to systems and methods for
delivering substances
to a user in accordance with an individually tailored treatment program. For
ease of
reference, unless specifically indicated otherwise, the terms substance, drug,
medicant, or
active pharmaceutical ingredient (API) are used herein interchangeably unless
indicated
otherwise, explicitly or by context, and all may be referred to as a
"substance." As a specific
illustrative example, systems and methods are described are for delivering
substances to a
user for a smoking cessation treatment program. The disclosed systems, devices
and
processes can be used for many other treatment programs, as well, for example
but not
limited to, hormone replacement programs, programs for cessation of other
addictions, etc.
where it is desired to educate the patient, control administration of a
plurality of drugs to
the patient (e.g., substances, medicants, API's, etc.), and automatically
monitor the drugs
provided for precise tracking of the patient in the treatment program.
[0081] Accordingly, as an illustrative example, in some
implementations, the
systems, devices, and methods are employed to help a user stop smoking or
vaping. For
ease of reference, as used herein "smoking- is used in reference to smoking
and/or vaping.
Reference to a "smoking cessation device," a "smoking cessation system," or -a
method
for smoking cessation," or similar phrases, refer to either, or both, a
device, system, or a
method that can be used to facilitate a user to stop smoking, or to stop
vaping. For example,
a device, system, or a method that can be implemented in a cloud-based (or
server-based)
system for helping a user quit smoking or vaping, such as illustrated in
Figure 1, and
described in further details in the subsequent figures. As mentioned above,
the cessation
systems, devices, and processes can be implemented in a smoking cessation
program, or a
vaping cessation program, the systems, devices, and processes can also be used
to address,
and quit, many other types of addictions. For example, the systems, devices,
and processes
can be used in many types of an addiction quitting program that benefits from
having an
individually tailored program based on user physiological and psychological
characteristics, administering mixtures of multiple substances to a user via
an inhalation
device, daily automatic monitoring of the user's progress in the quitting
program, and
providing nearly instantaneous feedback to the user throughout each day of the
quitting
program, as needed. Accordingly, although many of the examples herein may
relate to a
smoking cessation program or a vaping cessation program, the uses of the
disclosed
16
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
systems, devices, and processes are not limited to these applications. For
ease of reference,
"smoking" is used herein to refer to either and both smoking and vaping unless
otherwise
indicated explicitly or by the context of the disclosure, such that a "smoking
cessation
program" refers to a smoking and/or a vaping cessation program. In addition,
smoking is
not limited to a tobacco product, but instead the applies to any substance or
material that
can be smoked, atomized, aerosolized, or sprayed, and inhaled by a user.
[0082] The difficulty of quitting smoking is well-known.
The likelihood a
smoker's attempt to quit smoking will be successful is greatly increased when
both
physiological and psychological aspects of smoking are addressed. To date, the
physiological and psychological aspects of quitting smoking are generally
addressed at
least somewhat separately. Therefore, many smokers attempt to quit smoking or
vaping
using one or the other. Additionally, the lack of integration between the
physiological and
psychological aids also reduces the effectiveness of an attempt to quit
smoking. In addition,
during many, if not all, smoking cessation programs monitoring of the user's
smoking
behavior is based on user provided information and is not objectively
collected, and there
has been no way to accurately monitor and track the user's daily behavior.
User provided
information can be inconsistent and inaccurate at least for the reasons that
as it may be
unreliably collected and even falsely provided.
[0083] The systems, devices, and methods disclosed herein
address these
problems, and others. They advantageously can objectively monitor a user's
activities,
collecting accurate and detailed information of a user's use of a smoking
cessation device
as the user progresses through a cessation program, the collected information
being relating
to use characteristics of the cessation device that are impossible for a user
to collect
themselves. In the disclosed embodiments of a cessation program, is
"onboarded" where a
cessation program is individually generated based on user individual
characteristics that
may be genetic, determined from a user interview, and/or testing. For example,
for
smoking, one or more of nicotine dependence, strength of urges, perceived
sensation of
smoking, sex, race/ethnicity, nicotine metabolite rate (NMR), environmental
(e.g., air
quality, pollution index, work environment), age, co-morbidities, body mass
index (BMI),
adipose tissue proportion, nicotine consumption, puff topology, medications,
oral
contraceptives use, menopausal status, sleep pattern, exercise profile, and
diet, nutrition,
and meal pattern. These factors and others are illustrated in Figures 7A-7P,
which disclose
aspects of personalization of a smoking cessation treatment program, behavior
modification objectives, and steps of the treatment program. For example,
disclosing an
17
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
exemplary smoking cessation process that includes on-boarding a user into the
cessation
program, cigarette taper, nicotine taper, placebo usage, and relapse
protection (see, for
example, Figure 7Q). Information relating to onboarding is also described at
least in
reference to Figure 8A.
[0084] Accordingly, in one implementation, a treatment
system can provide an
individually tailored dynamically controlled smoking cessation program to help
a user quit
smoking. The system can be a server-based system (e.g., cloud-based system),
which is
running at least a portion of the treatment program. The system can also
include a user
device (computer) that communicates with the server-based system. The user
device
includes a display that can provide information relating to the treatment
program to the
user. The system can also include a delivery device (e.g., an inhalation
device) that
administers an aerosol mixture to the user based on the treatment program. The
delivery
device can include components, a (computer) hardware controller and one or
more sensors
for providing the aerosol mixture to the user and monitoring the user of the
delivery device.
Signals from the sensors are used to monitor the user's use of the delivery
device which
can then be used to determine a user's progress through the treatment program
and to
dynamically tune the treatment program, as needed. For example, the hardware
controller
in the delivery device may monitor and record the number of "puffs- a user
takes from the
delivery device, determine a flow rate of air provided to the user during a
puff, determine
a duration of each puff (e.g., the length of time of a certain amount of
airflow), determine
a total inhaled time (e.g., cumulative), and/or determine an amount of
substances ingested
by the user based on its control of individual aerosolizers of the delivery
device.
Information indicative of the change in the rate of airflow during a puff can
also be
determined, and used to determine when to generate an aerosol mixture for most
effective
inhalation of aerosol mixture. Information is sensed by sensors of the
delivery device (e.g.,
flow sensor, ambient temperature, and/or ambient pressure, and the like) can
also be
communicated to the user device and server system, and used to change (e.g.,
optimize) the
treatment program. Information generated from the delivery device can be
communicated
to the user device (e.g., via a Bluetooth link), and certain information
relating to the user's
use of the delivery system may be displayed on the user device. Information
received by
the user device can also be communicated to the server-system to be used in
the treatment
application,
[0085] The systems described herein can include a delivery
device that is
configured to control an aerosolizer system which can have a plurality of
aerosolizers. The
18
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
aerosolizer system can be incorporated in a pod (sometimes referred to herein
a an
"aerosolizer pod"). In some examples, a pod includes an aerosolizer system
having two
aerosolizers, each aerosolizer having a corresponding container holding a
substance (e.g.,
a fluid) that is provided to the aerosolizer for use in generating aerosol. In
some
implementations, an aerosolizer system has two or more containers
corresponding to each
aerosolizer, such that the fluids held in the two or more containers are
provided to a single
aerosolizer. Each container can hold a substance (e.g., drug, medicant, etc.)
that is needed
to provided to the user according to the treatment program. In some examples,
the
containers hold the same substance, but typically they hold different
substances to allow
aerosol mixtures of the different substances to be administered to the user.
In an example
of a delivery device used in a smoking cessation treatment program, the
delivery device
includes two aerosolizers and two containers (a container corresponding to
each
aerosolizer). The first container can hold a first fluid comprising
monoprotonated nicotine.
The first fluid can also include a flavorant (e.g., any substance that
provides a perceived
flavor to the user when inhaled as an aerosol). The second container can hold
a second fluid
comprising freebase nicotine. The second fluid can also include a flavorant
(e.g., any
substance that provides a perceived flavor to the user when inhaled as an
aerosol). In some
examples, including examples illustrated in Figures 3A, 3B, 3C, and 4, an
aerosolizer
system includes three aerosolizers. In some examples, the aerosolizer system
can include
four or more aerosolizers. Each aerosolizer is associated with a substance and
generates an
aerosol from its associated substance. The delivery device is configured to
control each
aerosolizer individually such that the plurality of aerosolizers generate
aerosol mixtures
based on an individually tailored treatment program (e.g., a smoking cessation
treatment
program). That is, the delivery device is configured to control the
aerosolizers, in
accordance with the treatment program, to produce an aerosol mixture that
includes an
amount of two or more substances. The delivery device is configured to control
each
aerosolizer individually, in accordance with the treatment program, to produce
an aerosol
mixture that includes an amount of two or more substances. In addition, in
some
embodiments (e.g., where the aerosolizers are thermal-based aerosolizers) the
delivery
device can control each aerosolizer individually, in accordance with the
treatment program,
to generate aerosol having a desired droplet size such that the resulting
aerosol mixture
comprises droplets of a desired size, which affect where the aerosol is
deposited in the
mouth, throat, and/or lungs of the user. Additional details and certain
illustrative
19
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
embodiments of the treatment system, the delivery system, the user device, and
related
methods are described hereinbelow.
[0086] Although particular aspects various exemplary
embodiments are
described herein, numerous variations, combinations and permutations of these
aspects fall
within the scope of the disclosure. Although some benefits and advantages of
certain
aspects are mentioned, the scope of the disclosure is not intended to be
limited to particular
benefits, uses or objectives.
List of Certain Components
[0087] For ease of reference, below is a list of certain
components that are
described and enumerated in this disclosure in reference to the above-listed
figures of a
cessation system. Other components not listed below may also be included in a
drug-
delivery treatment system. Any aspect of the items in the list below, or
illustrated and/or
described in the figures and description, whether or not named out separately
herein, can
form a portion of various embodiments of the invention and may provide basis
for claim
limitation relating to such aspects, with or without additional description.
Certain
enumerated items of the figures include:
- treatment system (e.g., smoking cessation system)
- user device (e.g., mobile device / smart phone / computer)
17 - sensor(s) (e.g., wearable, patch, etc.)
- network
- server system (e.g., cloud-based server system)
- user
- advisor(s) / medical practitioner(s)
- communication link between delivery device and user device
41 - communication link between mobile device and network
42 - optional communication link between cessation device and network
43 - communication link between network and advisor(s) / medical
practitioner(s)
44 - communication link between server system and network
46 - communication link between sensor and user device
47 - communication link between sensor and delivery device
100 - delivery system (e.g., delivery device 109 and pod 150)
101 - distal end of housing
102 - housing
103 - proximal end of housing
104 - channel
105 - opening in housing for receiving aerosolizer system
106 - opening for receiving air
107 - proximal end of channel
108 - opening on proximal end of channel for providing air to aerosolizer
system
109 - delivery device (or -pen")
110 - aerosolizer driver
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
111 - electrical connection(s)
112 - flow sensor
113 - circuit
114 - power source (e.g., battery)
116 - rescue button
118 - fingerprint sensor
119 - carbon dioxide sensor / oxygen sensor
120 - antenna
121 - carbon dioxide sensor
122 - distal end of channel
130 - controller
140 - cavity for receiving aerosolizer system
141 - cessation device exhaust port
145 - substance (in aerosolizer container)
150 - aerosolizer system (pod)
151 - distal end aerosolizer system
152 - heating element
153 - proximal end aerosolizer system
154 - intake (opening) of aerosolizer
155 - proximal end passage
156 - passage
157 - distal end of passage
158 - temperature sensor
159 - aerosolizer container
160 - density sensor
161 - aerosolizer (unit)
162 - aerosol mixing chamber
163 - pod ID chip
164 - exhaust port (opening) of mixing chamber for providing aerosol mixture
165 - case
166 - walls of mixing chamber
167 - mixing space (volume) in mixing chamber
167 - charging connection to pen
168 - case battery
169 - charging port
170 - mixing chamber intake opening
171 -sensing port
172 ¨ exhaust port sensor(s)
405 - ambient temperature sensor
407 - ambient pressure sensor
410 - flash memory
415 - LEDs
420 - Battery Manager
425 - case data interface
430 - case charge interface
465 - pod ID chip interface
500 - computer system
502 - communication bus
504 - hardware processor
506 - non-transitory memory (component)
21
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
510 - storage device (e.g., solid-state memory)
512 - display
514 - input controls
518 - communication interface
601 -puff data
602 - usage
603 - profile
604 - data input
605 - progress
606 - output
607 - cessation schedule
608 - cessation application
801 - on-boarding phase
802 - cigarette taper phase
803 - nicotine taper phase
804 - placebo usage phase
805 - software support phase
850 - treatment program phase 1 (after onboarding)
851 - treatment program phase 2
852 - treatment program phase n-1
853 - treatment program phase n
900 - drug delivery system
901 - distal end
902 - housing
903 - proximal end
904 - channel
905 - opening in housing for receiving pod
906 - opening for receiving air
907 - ambient temperature sensor
908 - opening
909 - drug delivery device
910 - aerosolizer drivers
911 - electrical connections
912 - flow sensor
913 - ambient pressure sensor
914 - power source (e.g., battery, capacitor(s))
916 - rescue button
918 - fingerprint sensor
920 - antenna
930 - controller circuit
940 - cavity for receiving pod
950 - pod
951 - distal end of pod
952 - non-thermal aerosol generating component
953 - proximal end of pod
954 - container (for holding drug)
955 - distal end passage
956 - passage
957 - proximal end passage
959 - drug container
22
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
960 - sensor (e.g., density, temperature)
961 - non-thermal aerosolizer assembly
962 - mixing chamber
963 - pod ID chip
964 - opening, exhaust port where aerosol exits pod
966 - walls
967 - mixing space (cavity)
Illustrative Example of Treatment System for Smoking Cessation
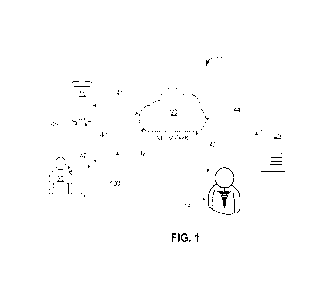
100881 Figure 1 illustrates an example of system 10 that
can be used to
precisely and dynamically administer substances in accordance with a treatment
program,
monitor the patient's use of the substances throughout the treatment program,
and provide
feedback to the patient and others on the patient's progress in the treatment
program. For
ease of reference, a provided or administered -substance," as used herein, is
a broad term
that refers to drugs, medicants, API's, or another material that is provided
to a user by a
delivery device as part of a treatment program. As an illustrative embodiment,
a system,
delivery devices, and methods are described for a smoking cessation treatment
program.
Smoking cessation treatment programs include cessation of any type of smoking,
or
inhaling any type of tobacco or non-tobacco product, including vaping, using
an electronic
device. Although the illustrative embodiment is for a smoking cessation
treatment program,
the described system, devices and methods are not limited to smoking cessation
but instead
can be used for other types of treatment programs where substances are
administered to a
patient over a duration of time. In particular, treatment programs where a
combination of
substances are delivered to a patient over a period of time, and the amount of
the substances
is dynamically changed over time based on the treatment program. Examples of
other
treatment programs can include treatment programs related to hormones or
hormone
replacements, drug addiction, allergies, pain mitigation, and the like.
[0089] Specifically, Figure 1 illustrates examples of
components and
communication links that can exist between the components of a treatment
system
(-system") 10. In this example, the system 10 includes a server system 25, a
delivery system
100 used by a user 30, and a user device 15. One or more advisors or medical
practitioners
35 can also receive information relating to the treatment program and the
user's progress
in the cessation program, and provide input to the cessation program or to the
user 30. In
some embodiments, the system 10 can also include a sensor 17, which can be in
communication with the delivery system 100 and/or the user device 15.
23
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
[0090] The components of the system 10 can communicate via
a network 20,
and one or more communication links. This example includes a communication
link 40
between the delivery system 100 and the user device 15, a communication link
41 between
the user device 15 and the network 20, a communication link 43 between the
advisors 35
and network 20, a communication link 44 between the server 25 and the network
20. In
embodiments having a sensor 17, a communication link 46 can exist between the
sensor 17
and the user device 15, and/or a communication link 47 can exist between the
sensor 17
and the delivery system 100. One or more portions of the network 20 and
communication
links 40-44, 46, 47 can be a wired or wireless communication link, and can
include Wi-Fi,
Bluetooth, cellular, or any suitable communication link. The network 20 can
be, for
example the Internet, or another large area network (LAN), or a wide area
network (WAN).
In some examples, the delivery system 100 can include directly to the network
20 via a
communication link 42 (e.g., a wireless communication link).
[0091] In this example, the server 25 is configured with a
smoking cessation
treatment program which is tailored to an individual user. As described below
(for example
in reference to Figures 7A-7Q, 8) at the beginning of the treatment program
the user is
"onboarded" and an individually tailored program is generated using factors
specific to the
user (see, for example, one or more of factors as shown in Figures 7A, 7B-1,
and 7B-2).
The server 25 provides information to the user device 15 to operate the
treatment program,
and the user device 15 provides information to the delivery system 100, which
may include
software updates, revised treatment program information and control
parameters, and the
like. During a treatment program, the use of the delivery system 100 is
monitored by the
delivery system 100 using one or more sensors incorporated into the delivery
system 100.
Figures 3A, 3B, and 4 illustrated examples of sensors that may be included on
the delivery
system 100. Information related to the treatment program can be communicated
from the
delivery system 100 to the user device 15, and then to the server 25. The
information
received by the server 25 can be used by the server 25 to monitor and/or
revise the cessation
program. The information received by the sever 25 can also be used to provide
reports to
advisors 35. In addition, the information received by the server 25 for each
user can may
be used as information to change overall parameters of the treatment program
for other
users. For example, information from hundreds, thousands, tens of thousands or
more of
users can be used to increase the efficiency and the effectivity of the
treatment program for
current users and/or new users. In some examples, machine learning processes
can be used
24
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
with a dataset of information of numerous users to determine parameters of the
treatment
program.
[0092] The user device 15 can be a smart phone, a tablet
computer a laptop
computer, or another mobile computing device. The user device 15 can also be a
desktop
computer, a specialized computer in a medical practitioner's facility, or
another suitable
computer resource. In some preferred embodiments, the user device is a mobile
computing
device that a user can conveniently have with them at all times, or most of
the time (e.g., a
smart phone). The user device 15 can provide treatment program information to
the
cessation (or "delivery") device 100, including information which is used to
control
generation of an aerosol mixture that the delivery system 100 controls the
generation of,
from a plurality of aerosolizers. The aerosolizers can be dynamically
controlled to provide
desired aerosol mixtures as required by the cessation program. In some
embodiments, the
user device 15 can run at least a part of the cessation program, for example,
through an app
running on the user device 15. The user device 15 includes a display, and
provides certain
cessation program information to the user 30 on various graphical user
displays (GIJI's) of
the display based on the received information, for example, information
relating the user's
progress, or encouraging information for the user to adhere to the cessation
program. The
user device 15 also receives information from the delivery system 100 relating
to the user's
use of the device (including information from sensors on the delivery system
100), and can
communicate some or all of the received information to the server 25. As
indicated above,
the user device 15 can communicate changes/revisions of the cessation program
and related
information, including revisions to software or new software, to the cessation
system 100,
including information the user device 15 receives from the server 25.
[0093] In this embodiment, the delivery system 100 is an
aerosol generating
inhalation-type device used by the user 30 to help the user 30 stop smoking or
vaping. The
delivery system 100 can include a delivery device 109 and an aerosolizer pod
109 (e.g.,
illustrated in Figure 2). In some embodiments, the delivery device includes a
plurality of
aerosolizers and the pod includes containers that hold substances (e.g.,
fluids) that are
provided to the aerosolizers. In the embodiment illustrated in Figures 2, 3A-
3C, the delivery
device 109 includes a controller, one or more sensors, and other components
that are used
to control the generation of an aerosol mixture, and the pod 150 includes a
plurality of
aerosolizers and a plurality of containers each containing a substance that is
provided to the
aerosolizers.
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
[0094] The delivery device 109 and the pod 150 are
configured to be coupled
together, such that the delivery device 109 can provide control signals to the
pod 150 and
control aerosolizers in the pod 150 to generate an aerosol mixture in
accordance with the
treatment program. An example of a delivery device 109 and an aerosolizer pod
150 having
three aerosolizers is illustrated in Figures 3A-3C. The delivery device 109
can include
structure and a variety of sensors and components that are used to monitor use
of the
cessation device and implement the treatment program including revisions to
the cessation
program when needed. In an example, a delivery device 109 can include a
housing having
a distal end and a proximal end, a channel having an opening on a portion of
the housing
(e.g., a distal end of the housing or in a center portion of the housing) for
receiving air, the
cessation device can also have an opening on a proximal end of the channel to
communicate
air to an aerosolizer pod coupled to the housing.
100951 The delivery device 109 can also include an
aperture (e.g., on the
proximal end of the housing) configured to receive an aerosolizer pod therein
(e.g., pod
150 Figure 2). The housing can be configured to at least partially surround
the pod when
the pod in positioned in the housing. The cessation device can include a
plurality of sensors.
The sensors can include, for example, one or more of a flow sensor positioned
to sense air
flowing through the channel, one or more density sensors for sensing density
of aerosol
generated by one or more aerosolizers, one or more temperature sensors
configured to sense
a temperature of aerosol generated by the one or more aerosolizers
(respectively), an
ambient temperature sensor, an ambient pressure sensor, a fingerprint sensor,
a carbon
dioxide sensor, and/or an oxygen sensor.
[0096] The delivery system 100 can also include a
plurality aerosolizer driver
("driver") configured to electrically couple to and drive aerosolizers in a
pod. In some
embodiments, the delivery system 100 includes two or more drivers to control
two or more
aerosolizers in a pod. In some embodiments, the delivery system 100 includes
three drivers
to control three aerosolizers in a pod. In some embodiments, the delivery
device 100
includes four or more drivers to control four or more aerosolizers in a pod. A
controller
circuit of the delivery system 100 can include a hardware controller coupled
to the flow
sensor, other sensors, the aerosolizer drivers, the rescue button and the
fingerprint sensor.
The hardware controller can include a hardware processor and a non-transitory
computer
readable medium in communication with the hardware controller, the computer
readable
medium configured to store smoking cessation program information, and to store
executable instructions that, when executed, configure the hardware controller
to perform
26
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
a smoking cessation program that includes receiving input signals from the
flow sensor,
other sensors, and the rescue button, and individually controlling the
aerosolizer drivers to
provide aerosolizer generation signals to control the multiple aerosolizers of
the aerosolizer
pod to generate an aerosol mixture based at least on the received input
signals and the
smoking cessation program information. These, and other components, are
described in
more detail below, for example, in Figures 2-5. Figure 1 illustrates a
simplified view of the
treatment system 10 that includes only one delivery system 100 associated with
one user
30. In operation, the system 10 can include a plurality of delivery systems
100 each
associated with a different user, and the server 25 can include a treatment
program that is
configured to control each of the plurality of delivery systems in accordance
with its
associated user their individual treatment program.
[0097] Sensor(s) 17 can be optionally included in the
system. Sensor 17 can
include one or more sensors that sense a characteristic of the user, and
communicates
information of the sensed characteristic to the delivery system 100 and/or the
user device
15. In various embodiments, the sensor 17 can include a patch, a wearable, or
any other
sensor that can sense a characteristic of the user. As anon-limiting example,
the sensor may
be configured to sense a characteristic in the blood, sweat, urine, or saliva
of the user (e.g.,
sugar level, nicotine level, pH, level of a drug/medicant/hormone, and the
like).
[0098] Figure 2 is a schematic of an example a delivery
device 109 and an
aerosolizer system (or "pod") 150 that can be used in the cessation system 10.
As described
in more detail in reference to Figure 3A, the delivery device 109 includes
components to
perform a treatment program including components to control multiple
aerosolizers in the
pod 150 to generate an aerosol mixture in accordance with the treatment
program, which is
then inhaled by a user. The pod 150 is configured to be removably coupled to
the delivery
device 109. The pod 150 can be a consumable item. During a treatment program,
a series
of pods maybe provided to the user to in the delivery device. Each of the pods
can include
a substance, or multiple substances. As dictated by the treatment program, the
substances
in the series of pods provided to the user may be the same in each of the
pods, in some of
the pods, or in none of the pods.
100991 In this example, the pod 109 is at least partially
inserted into the delivery
device 109 to couple them together. Also in this example, when the pod 150 is
coupled to
the delivery device 109, the aerosolizer drivers in the delivery device
electrically connect
to corresponding aerosolizers in the pod 109. The aerosolizer drivers 110
(Figure 3A) can
independently and separately provide signals to each aerosolizer 152 in the
pod 109, and in
27
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
this way generate desired aerosol mixtures of the different substances in the
multiple
aerosolizers of the pod 150 in accordance with the cessation program. For
example, the
aerosolizers 110 can be independently controlled to generate an aerosol
mixture having
various portions (e.g., percentages) of the various substances in the three
containers 159
(Figure 3A) of the pod 150. Also, the aerosolizers 152 can each be
independently controlled
to generate an aerosol having different droplet sizes. Other configurations
are possible. For
example, in some embodiments, the aerosolizers can be part of the delivery
system 100,
the pod includes containers of substances, and when the pod is inserted into
the delivery
device the substance in each pod is provided to a corresponding aerosolizer in
the delivery
device.
101001 The delivery device 109 includes a distal end 105
having an opening 106
for receiving ambient air into the delivery device 109. The delivery device
109 is
configured with one or more air communication channels such that air received
through the
opening 106 is provided to the pod 150. The pod 109 also can includes one or
more air
communication channels to provide air to each aerosolizer in the pod 150_ The
delivery
device 109 includes a second opening 105 in the housing 102 which is
configured to receive
the pod 150 such that at least a portion of the pod 150 is positioned within
the housing 102
in his coupled to the housing 109. The pod 150 includes an opening 154 on a
distal end 151
through which to receive air passing through the delivery device 109. The pod
150 further
includes, on its distal end 153, an opening 164 for providing an aerosol
mixture to a user.
Examples of certain components that can be included in the delivery device 109
and the
pod 150 are illustrated in Figures 3A-3C, 4, and 5.
101011 An example of a delivery device 109 and a pod 150
that is coupled to
the delivery device 109 and used as the delivery system 100 in the cessation
system 10 are
illustrated in Figures 3A-3D. Specifically, Figure 3A illustrates an example
of the delivery
device 109, with the pod 150 inserted into and coupled to the delivery device
109. Figure
3B further illustrates the delivery device 109 shown in Figure 3A. Figure 3C
further
illustrates the pod 150 of the shown in Figure 3A. Figure 3D illustrates a
case 165 that can
be used to house the delivery device 109 and pod 150. In various embodiments,
the case
165 can include one or more additional components for facilitating use of the
delivery
device 109. Referring to Figure 3A, in this example, the entire pod 150 is
positioned within
the housing 102 when the pod 150 is coupled to the delivery device 109. In
other examples,
a portion of the pod 150 may from the proximal end 103 of the delivery device
109 when
the pod 150 is coupled to the delivery device 109. In this example, the pod
150 is coupled
28
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
to the delivery device 109 such that it is in electrical communication with
the delivery
device 109 and in fluid communication with air flow into and through the
housing 102 (e.g.,
through opening 106, through channel 104, and through opening 108).
[0102] A number of components and structures can be
positioned within the
housing 102 of the delivery device 109, for example but not limited to those
illustrated in
Figures 3A and 4. In this example, the delivery device 109 includes a channel
104 for
providing ambient air to the pod 150, the channel having a distal end 122 at
the distal end
101 of the housing 102. The channel extends from the opening 106 towards a
proximal end
107 of the channel to opening 108. When the pod 150 is in the housing 102, an
intake 154
of the pod 150 is aligned with the opening 108 such that air is communicated
through the
channel 104 to the pod 150. In other examples, instead of a single opening
106, the delivery
device 109 may include one or more openings 106 and/or one or more channels
104 to
receive air into the housing 102 and communicate the air to the pod 150. In
some
embodiments, the opening 106 may be located at a different portion of the
housing instead
of at the distal end 101. In an example, the housing may include one or more
openings 106
on a side surface of the housing 102 instead of, or in addition to, the distal
end 101. In some
embodiments, one or more openings 106 are located in a gap, or near a gap,
between the
housing 102 and the pod 150. The delivery device 109 also includes an opening
105, in the
housing 102 on the proximal end 103 of the delivery device 109, which is
structured to
allow the pod 150 to be placed into the housing 102 through the opening 105.
In this
example, the delivery device 109 includes a cavity 140 which extends from the
proximal
end 103 of the housing 102 into the housing 102. The cavity 140 and walls of
the housing
102 surrounding the cavity are structured to receive and hold the pod 150.
[0103] The delivery device 109 also includes a controller
circuit 130 which is
connected to a power source 114. The controller circuit 130 can include one of
more
hardware processors and non-transitory computer readable medium, for example,
as
described in reference to Figure 5. The power source 114 can include, for
example, a
battery, capacitor, super-capacitors, or another energy storage medium, or a
combination
thereof The power source 114 can be configured to provide electrical power to
the pod 150
when the pod 150 is coupled into the delivery device 109. In some examples,
the pod 150
also includes a power source. The controller circuit 130 is in communication
with one or
more sensors to receive information (e.g., signals) from the sensors that the
controller
circuit 130 can use (at least in part) to operate a treatment program. For
example, the
delivery device 109 can include a flow sensor 112 which is positioned and
configured to
29
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
sense airflow into the delivery device 109 through the channel 104. The
controller circuit
130 is connected to the flow sensor 112 and receives information indicative of
the of air
passing through the channel 104 from the flow sensor 112. Based on information
from the
flow sensor 112, the controller circuit 130 can determine information
indicating the use of
the cessation system by a user. For example, a "puff' (an inhalation of
air/aerosol from the
cessation system by a user) frequency, a puff duration, and/or a puff amount,
can be
determined by the controller circuit 130 based on information from the flow
sensor 112. A
puff profile can also be determined by the controller circuit 130 based on
information from
the flow sensor 112. The "puff profile" at least in part refers to how changes
in the air flow
during the duration of a puff. For example, whether the profile (e.g., airflow
as a function
of time) of the puff is a square wave, trapezoidal-shaped, sinusoidal-shaped,
and the like).
The determined puff frequency, puff duration, puff amount, and/or the puff
profile can be
used by the cessation program to dynamically tailor the cessation program to a
particular
user's needs. Any signals/information the controller circuit 130 receives can
be
communicated one or more of the user device 15 and the server 25, and used to
monitor the
user's progress in the cessation program, and used to modify the cessation
program.
101041 In this example, the delivery device 109 is
configured to be used with a
pod 150 that has three aerosolizer units ("aerosolizers-) 161 (as shown in the
example in
Figure 3C. Each aerosolizer 161a-c can generate an aerosol from a substance
contained in
the pod 150. In this example, each aerosolizer 161a-c includes a heating
element 152a-c
(e.g., a resistive heating element) that can be controlled by the controller
circuit 130 via the
aerosolizer drivers 110a-c to produce aerosol in accordance with the cessation
program.
The aerosol is communicated via passages 156A-c and enters the mixing chamber
162
through the mixing chamber intake openings 170a-c (Figure 3C), where it forms
an aerosol
mixture which can be inhaled by a user. Each aerosolizer driver 110a-c can
interact with a
corresponding aerosolizer 161a-c to cause it to produce some or all of the
aerosol mixture
such that the aerosol mixture can include any proportion of a plurality of
substances in the
aerosolizers. In some embodiments, a temperature value is determined by the
controller
circuit 130 for each aerosolizer 161 by using the heating element 152 as a
temperature
sensor. For example, by sensing a change in a resistance (or impedance) value
of the heating
element as the temperature of the heating element 152 increases and
correlating the
resistance value to a temperature. Other embodiments may sense the temperature
of an
aerosolizer in different ways. For example, in some embodiments, the delivery
device 109
can include temperature sensors 158a-c (shown in dashed lines) that are
configured to sense
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
the temperature of aerosol generated by the three aerosolizers 16 la-c of the
pod 150. The
temperature sensors 158a-c can be positioned in the delivery device 109 such
that when the
pod 150 is coupled to the delivery device 109, each temperature sensor 158a-c
is adjacent
to one of the passages 156a-c that communicate aerosol from heating elements
152a-c to
the mixing chamber 162 of the pod 150. The delivery device 109 can also
include density
sensors 160a-c that are configured to sense the density of aerosol generated
by the three
aerosolizers 161 of the pod 150. The density sensors 160a-c can be positioned
on the
delivery device 109 such that when the pod 150 is coupled to the delivery
device 109, each
density sensor 160a-c is positioned adjacent to one of the passages 156a-c
that
communicate aerosol from heating elements 152a-c of the pod 15010 the mixing
chamber
162 of the pod 150. In some embodiments, the density sensors 160a-c can be
optical
sensors.
101051 The delivery device 109 can also include a
fingerprint sensor 118 which
is connected to the controller circuit 130 and is used to sense a user's
fingerprint to unlock
the delivery device 109. In addition, the delivery device 109 can include an
ambient
temperature sensor 405 in ambient pressure sensor 407, in the controller
circuit 130 can be
configured to use information from the ambient temperature sensor 405 in the
ambient
pressure sensor 407 to control the cessation program provided to the user. For
example, to
control the aerosol mixture generated by pod 150 based at least in part on the
ambient
temperature and/or the ambient pressure.
[0106] In some examples, the delivery device 109
optionally also includes a
carbon dioxide sensor 121 that is coupled to the controller circuit 130 and
provides a signal
to the controller circuit 130 indicative of an amount of carbon dioxide. The
delivery device
109 can also include a control (e.g., a button, or fingerprint sensor 116) to
activate the
carbon dioxide sensor 121. In operation, after activating the sensor, a user
exhales into the
opening 106 to provide a flow of air to the carbon dioxide sensor 121 which
provides a
signal to the controller circuit 130. The information from the carbon dioxide
sensor 121
can be used to determine a carbon dioxide level of the user and used in the
cessation
program, for example, to modify the cessation program.
101071 In some examples, the delivery device 109
optionally also includes a
blood oxygen sensor 119 that is coupled to the controller circuit 130 and
provides a signal
to the controller circuit 130 indicative of an amount of oxygen in the blood.
In an example,
the blood oxygen sensor 119 can be a pulse oximetry sensor. In some examples,
the blood
31
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
oxygen sensor 119 can be incorporated into the fingerprint sensor 116. In some
embodiments, the blood oxygen sensor 119 can be separate from the fingerprint
sensor 116.
[0108] The delivery device 109 can also include a pod ID
chip interface 465,
which can be positioned near the cavity 140 that receives the pod 150. A pod
150
configured to be used with the delivery device 109 can include a pod ID chip
163. In some
embodiments, the pod ID chip 163 can be positioned on a portion of the pod 150
such that
when the pod 150 placed into the delivery device 109, the pod ID chip
interface 465
physically and/or electronically aligns with pod ID chip 163 such that
information may be
communicated from the pod ID chip 163 to the pod ID chip interface 465. The
information
can relate to one or more aspects of the configuration of the pod 150. In an
example, the
information can relate to one or more of the substances in the aerosolizer's
of the pod 109
(e.g., type of substance, amount of substance left). In another example, the
information can
relate to a pod ID which the delivery device 109 can compare to stored data to
determine
information relating to the pod 109 (e.g., information related to the
aerosolizers 152) that
the delivery device 109 may use to properly provide the desired aerosol
mixture to a user.
[0109] In this example, the delivery device 109 also
includes a -rescue" button
116 which can be activated to provide a user an additional (or "rescue") dose
of one or
more of the substances in the pod 150, for example, an additional nicotine
dose in a smoking
cessation program. When the rescue button 116 is used, the controller circuit
130 saves
information relating to its use (e.g., date/time information of each use). The
rescue button
use information can be used by the delivery system to modify the cessation
program. In
some examples, the rescue button use information is communicated by the
delivery device
109 to the user device 15 and/or the server 25, and used to track the user's
progress in the
cessation program and/or dynamically modify the cessation program as a result
of the user
needing a rescue dose. Modifications made to the cessation program, based on
actuation of
the rescue button 116 can be communicated from the server system 25 to the
user device
15, and then communicated to the delivery device 109 from the user device 15.
The delivery
device 109 can include one or more other features, including an antenna 120,
and
communication circuitry in the controller 130, or implemented in a separate
hardware
component in communication with the controller 130, that allows the delivery
device 109
to communicate with the user device 15 or to another computer device either
directly or
indirectly via a network. Certain features of the controller circuit 130, or
components of the
delivery device 109 that are in communication with the controller circuit 130,
are further
illustrated in Figures 4 and 5.
32
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
[0110] As described in further detail in reference Figure
5, the controller circuit
130 includes one or more hardware processors 504 in communication with at
least one
non-transitory memory component 506, 508 that includes executable instructions
that
configure the one or more hardware processors 504 to run a treatment program.
The
delivery device 109 includes an aerosolizer driver 110a-c that corresponds to
each of the
aerosolizers 161 in the pod 150. The controller circuit 130 is connected to
the aerosolizer
drivers 110a-c and controls the aerosolizer drivers 110a-c to operate the
respective
aerosolizers 161a-c to generate aerosol such that a desired aerosol mixture
reduced by the
aerosolizer system provided to a user, as prescribed by the cessation program.
For example,
the controller circuit 130 can control the aerosolizers 161a-c, via the
aerosolizer drivers
110a-c, to each generate a certain amount of aerosol from a substance (a
fluidic mixture of
nicotine) that in each aerosolizer 161a-c, such that the aerosol from each of
the aerosolizers
161a-c is combined in the aerosol mixing chamber 162 of the pod 150 forming a
desired
aerosol mixture is provided to a user.
[0111] The controller circuit 130 can also independently
control the
aerosolizers 161a-c, via the aerosolizer drivers 110a-c, to affect the aerosol
droplet size
(ADS) in the aerosol generated by each of the aerosolizers 161a-c. The ADS of
an
aerosolized substance can determine where it will be absorbed in the user.
Smaller ADS's
generally travel to and are absorbed in the lungs, and larger ADS's generally
travel to and
are absorbed in the mouth or throat. The controller circuit 130 can
independently control
the aerosolizers 161a-c, via the aerosolizer drivers 110a-c, to produce ADS's
of different
sizes based on the treatment program. For example, depending on what portion
or stage of
a treatment program the user is in (for example, as illustrated in Figure
10H). In some
embodiments, the aerosol droplet diameters will be less than or equal to 20
pm. In some
embodiments, the aerosol droplet diameters will be less than or equal to 10
vim. In some
embodiments, the aerosol droplet diameters will be less than 1 vim, or equal
to 1 p,m, 2p.m,
3pm, 4pm, 5pm, 6pm, 7pm, 8pm, 9pm, 10pm, 11pm, 12pm, 13pm, 14pm, 15p.m, 16pm,
17 m, 18pm, 19 m, or 20 m, plus or minus 0.5 vim. In some embodiments, aerosol
droplet
diameters will be less than or equal to 1pm (at least for a portion of the
treatment program).
In some embodiments, aerosol droplet diameters will be greater than or equal
to lOpin (at
least for a portion of the treatment program). In some embodiments, aerosol
droplet
diameters for one portion of the treatment program can be less than or equal
to 3pm and
for another portion of the treatment program are greater than or equal to
8p.m. In some
embodiments, the aerosol droplet diameters for one portion of the treatment
program can
33
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
be less than or equal to lnm and for another portion of the treatment program
are greater
than or equal to 1 Onm. Practically speaking, when the aerosolizers are
controlled to
generate a certain aerosol droplet diameter, the aerosol droplet diameters may
have a range
of diameters but are mostly of the target diameter such that the deposition
site of the aerosol
is effectively the target deposition site (e.g., the mouth, lungs, etc.).
Accordingly, when
referring to certain aerosol diameters, this is understood to indicating that
an effective
amount of aerosol droplets are generated of the specified diameter. In an
example, more
than 30%, 40%, 50%, 60%, 70%, 80%, 90%, or 95% of the aerosol has the
specified
diameter. In another example, more than 50%, 60%, 70%, 80%, 90%, or 95% of the
aerosol
has the specified diameter. In an example, more than 70%, 80%, 90%, or 95% of
the aerosol
has the specified diameter.
101121 As illustrated in the example of Figure 3C, the pod
150 includes three
aerosolizers 161a-c. Electrical connections 111a-c are connected to one of the
aerosolizers
161a-c respectively, and can provide electrical power and/or control
information to the
aerosolizers 161a-c. When the pod 150 is coupled to the delivery device 109,
the electrical
connections 111a-c are coupled to the aerosolizer drivers 110a-c,
respectively. In some
embodiments that include thermal aerosolizers 161. Control of the operation of
the
aerosolizers is achieved by changing the electrical power supplied by the
aerosolizer drivers
110, to each heating element 152 of each aerosolizer 161, via the electrical
connections
111. The controller circuit 130 can individually control the multiple
aerosolizer drivers 110
to supply the electrical power, and correspondingly, can individually control
each
aerosolizer to produce a certain amount of aerosol and to produce aerosol with
a desired
droplet size, according to the smoking cessation treatment program. Each
thermal
aerosolizer 161 can include a heating element 152, a container 159 that is
configured to
hold a substance (e.g., a fluid containing nicotine for the smoking cessation
example), and
a passage 156. In an example of the fluids that can be in the containers 159,
in an example,
a first container 159a contains a fluid comprising monoprotonated nicotine. In
an example,
a second container 159b contains a fluid comprising freebase nicotine. In an
example, a
third container includes a fluid comprising another medicant, a flavored
substance
(flavorant), or a non-nicotine placebo.
101131 In an example, the three containers 159a-c contain
2.5mL of liquid
comprising 5% flavor system, and a propylene glycol (PG) and glycerin (G) mix,
in a 60:40
PG:G ratio. In addition, container 159a contains 60mg/mL synthetic nicotine
plus a pH
adjuster to create a pH of 4.0 thus ensuring that the species in this
container 159a is
34
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
monoprotonated nicotine. Container 159b contains 60mg/mL synthetic nicotine at
pH
10.0 thus ensuring that the nicotine species in this container 159b is
freebase nicotine. 5%
of container 159c' s volume is flavor system, and the remainder is a PG:G in
the ratio 60:40.
Container 159c contains no nicotine. In various examples, the precise ratio of
PG:G, the
amount of flavor system, the amount of pH adjuster, the final dosage algorithm
settings,
and the aerosolization power settings, can be different, and may be based on
characterization of the actual aerosolizer components that are used in an
implementation.
A number of factors can affect aerosol delivered to the user including, but
not limited to,
micro-aerodynamics of droplet collision and condensation change what aerosol
exits the
mouthpiece. Information relating to these factors can be determined during
bench-testing,
and then used to calibrate a delivery device 109 based on the aerosolizers and
the pod
design. Correspondingly, the algorithms can be adjusted to ensure that the
aerosol mixture
delivered to the user is according to the treatment program across the full
aerosol parameter
ranges in a quit journey treatment program, for example, the quit journey
program
illustrated in Figure 7Q.
[0114] Different aerosol droplet sizes are achieved by
changing the power that
is supplied to the surface electrodes. In principle any porous material can be
used with a
conductive electrode - earlier generations used cotton wicks with electrode
coils
surrounding them. In an example of a thermal aerosolizer, each aerosolizer can
use of a
ceramic wick and a surface electrode to permit more precise aerosolization. As
an example,
the amount of aerosol produced by each aerosolizer 161 can be controlled by
the duration
the aerosolizer is supplied electrical power such that a thermal component of
the aerosolizer
reaches a temperature sufficient to generate an aerosol from the liquid in the
respective
container of the aerosolizer. As an example, the size of the aerosol produced
by each
aerosolizer 161 can be controlled by the amount of power the aerosolizer is
supplied
electrical power, changing the temperature of the thermal component which can
correspondingly cause generation of aerosol of different droplet sizes.
[0115] Each passage 156 includes a distal end 155 closest
to the aerosolizer 159
and a proximal end 157 adjacent to the mixing chamber 162. The passage 156
provides a
flowpath for aerosol generated by the heating element 152 to flow to the
mixing chamber
162. The mixing chamber 162 includes walls 166 which enclose a mixing space
167. In the
mixing chamber 162, the individual aerosols generated by each of the aeros ol
zers 161 a-c
mixes together and forms an aerosol mixture, which a user can inhale (ingest)
through
opening 164. In this example, power for the heating elements 152 is provided
by the
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
delivery device 109 via the connections 111. In some embodiments, the pod 150
includes
one or more power sources that can provide electrical power to the heating
elements 152,
or provide power to other electrical components of the pod 150. The controller
circuit 130
can control the aerosolizers 161 to generate an aerosol mixture of a certain
total nicotine
concentration by controlling the aerosol generated by each of the aerosolizers
161 in
accordance with the treatment program.
[0116] The controller circuit 130 can be configured by
treatment program
information (e.g., algorithms) the delivery device 109 has coded in its
firmware and/or that
it receives to drive the aerosolizers to produce the desired aerosol amount
and droplet
diameter size from each of the aerosolizers (e.g., the three aerosolizers).
The process begins
with the target delivery parameters for a puff on the nth day of the Quit
Journey. These
parameters inform a dosage algorithm that determines how much liquid is
required from
each of the three containers 159a-c. Then, given the known microfluidic
performance of
the wick system of the aerosolizers that are implemented in the delivery
device 109, a mass
conversion algorithm determines how much energy must be delivered to each
aerosolization electrode to achieve this. One or more sensors in the delivery
device 109
(e.g., the flow sensor 112) determine the topography of the patient's puff,
and from this
data the algorithm ensures that the correct proportion of the fluid in
containers 159a, 159b,
and 159c is aerosolized, and that energy is provided to the electrodes for the
duration of the
puff to achieve the desired aerosol mixture. The aerosolization "drivers" can
incorporate
data that adjusts parameters to account for condensation losses in the
aerodynamic channel
before mouthpiece exit, and for the aggregation of aerosol droplets in this
channel (for
example, based on previously determined test data and/or based on one or more
sensors in
the delivery device, e.g., an ambient temperature sensor) thus ensuring that
the aerosol
droplet size at mouthpiece exit is what is expected. The pod 150 can
optionally include a
sensor 172 positioned on or near the exhaust port 164 that can sense a
characteristic of a
user when the user is using the delivery system 100. Specifically, when the
sensor 172 is
in contact with, or is adjacent to or near, the user's mouth. In some
examples, the sensor
172 is configured to sense a characteristic of the user's saliva or the user's
breath. The
sensor 172 can include a hardware processor and other hardware components
(e.g., sensor,
transceiver, antenna, etc.) to sense the characteristic and communicate
information about
the sensed characteristic to the delivery device 109 (for subsequent
communication to the
user device 15) or to the user device 15.
36
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
[0117] Control of the aerosolizes 161 can be based on the
smoking treatment
program and based on inputs received from one or more of the sensors (e.g.,
the flow
sensor). Nicotine in e-liquids can exist in two forms: free base (meaning free
from protons),
and monoprotonated (meaning has one proton, also called a -salt"). There is a
correlation
between the pH level of the liquid and the ratio between the two forms. A
common way to
control the pH levels (and free base ratio) in the liquid is by using an
organic acid in certain
amounts to adjust the pH. In some embodiments, the total nicotine
concentration delivered
in the aerosol can range from 0 to 58 mg/mL. This total concentration is the
sum of
monoprotonated nicotine concentration, [NicH ], and free-base nicotine
concentration
[Nic]. Note that nicotine can also exist in a di-protonated state, but this is
practically never
reached in tobacco aerosols because conditions in the aerosol droplets are not
sufficiently
acidic.
101181 The free nicotine ratio ("FNR-) can be calculated
as:
FNR=[Nich[Nicl + [NicH+1)
FNR=1/(1+ 10-PH/Ka)
where Ka is the acidity constant for NicH+ which is 8.01. So given a target
FNR, the
controller circuit 130 (e.g., firmware in the hardware processor of the
controller circuit 130)
may determine the required pH, and the microfluidics mix the high and low pH
solutions
(which contain exactly the same total nicotine concentration) to achieve the
target pH
thereby ensuring that the FNR is the value required by the treatment program.
[0119] An embodiment of a portable charging case 165 is
illustrated in Figure
3D. The delivery device 109 can be stored and charged within the portable
charging case
165. In this embodiment, the case 165 includes a display 166 for the user to
observe both
statistics and settings to which the system is set. The case 165 houses and
can be powered
by a rechargeable power source, for example, battery 168, which can be
accessed via a
charging port 169 located on the case 165, the charging port 169 configured to
receive a
mating plug connector to charge the battery 168. When the delivery device 109
is not in
use, it can be stored in a cavity within the case 165. In some
implementations, power source
114 of the delivery device can be charged from the battery 168 through a wired
or wireless
connection.
[0120] Figure 4 is a schematic of an example of a circuit
113 that can be used
in a delivery device, for example, the delivery device 109 illustrated in
Figures 1 and 3A.
As illustrated in Figure 4 communication lines can connect the controller
circuit 130 and
37
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
other components of the delivery device to form the circuit 113. The
controller circuit 130
can include one or more hardware processors (e.g., hardware processors 504,
Figure 5). An
airflow is created when a user sucks in air through the delivery device. In
this schematic,
the airflow is generally from left to right such that intake of air is sensed
by the flow sensor
112 and is received by the aerosolizers 161. The aerosolizers 161 generate
aerosol in the
airflow, and the airflow then may (in some embodiments) pass by sensors (e.g.,
temperature
sensor 158 and/or density sensor 160), into a mixing chamber, and then out of
the pod to
the user's mouth. Figure 4 illustrates many components that are illustrated in
Figure 3A-3C,
and also illustrates some additional components. For example, in this
embodiment, a flash
memory 410 is in communication with the controller circuit 130. The controller
circuit 130
can include a transceiver or other communication circuitry that is coupled to
an antenna
120 which allows the delivery device 109 to communicate with a smart phone,
another
device, or a network. As illustrated in Figure 4, the circuit 113 can also
include a pod ID
chip interface 465. In such embodiments, when the pod 150 is coupled to the
delivery
device 109, the pod ID chip interface 465 is in communication with a pod ID
chip 163 of
the pod 150 for communicating information between the pod and the delivery
device. The
circuit 113 can also include a case data interface 425 which is in
communication the
controller circuit 130, and a case charge interface 430 which is in
communication with a
battery manager 420 which manages power provided to the controller circuit 130
from a
power source (e.g., battery) 114 to, for example, manage the charging of the
battery 114 by
a case or another power source.
[0121] Figure 5 is an example of a computer system 500
that may be used to
implement the functionality described herein for a treatment system, e.g., a
delivery device,
a user device, and/or a server system. In some embodiments, the computer
system 500 can
be characterized as including all electrical and electronic components of the
treatment
system. In some embodiments, the computer system 500 can be characterized as
being the
system illustrated in Figure 1. In this particular example, the computer
system 500 is
described broadly as including the controller circuit 130 in a delivery device
109, and other
components are in communication with the controller circuit 130. However,
nothing in this
description is intended to limit the computer system 500 to be interpreted as
referring to
only the controller circuit 130 and its components.
[0122] The controller circuit 130 can include a bus 502 or
other communication
mechanism for communicating information between components of a cessation
system,
and a hardware processor, or multiple processors, 504 coupled with bus 502 for
processing
38
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
information. Hardware processor(s) 504 may be, for example, one or more
general purpose
microprocessors. The hardware processor(s) 504 include non-transitory memory
505. In
some examples, the functionality the components illustrated in the controller
circuit 130
can be implemented in a single chip (e.g., an ASIC) and the classification
policy is stored
in memory and/or in circuitry, for example, memory 505.
[0123] Computer system 500 also includes a main memory
506, such as a
random access memory (RAM), cache and/or other dynamic storage devices,
coupled to
bus 502 for storing information and instructions to be executed by processor
504. Main
memory 506 also may be used for storing temporary variables or other
intermediate
information during execution of instructions to be executed by processor 504.
Such
instructions, when stored in storage media accessible to processor 504,
including on
memory 505 integrated on a processor chip, render computer system 500 into a
special-purpose machine that is customized to perform the operations specified
in the
instructions for a smoking or cessation program. Computer system 500 further
includes a
read only memory (ROM) 508 or other static storage device coupled to bus 502
for storing
static information and instructions for processor 504. A storage device 510,
such as a
magnetic disk, optical disk, or USB thumb drive (Flash drive), etc., is
provided and coupled
to bus 502 for storing information and instructions.
[0124] Computer system 500 may be coupled via bus 502 to a
display 512, such
as a LCD or liquid crystal display, and which may include a touchscreen, for
displaying
information to a network operator. An input device 514, including alphanumeric
and other
keys, is coupled to bus 502 for communicating information and command
selections to
processor 504. Another type of user input device is cursor control 516, such
as a mouse, a
trackball, or cursor direction keys for communicating direction information
and command
selections to processor 504 and for controlling cursor movement on display 512
by a
network operator.
[0125] Computing system 500 may include a user interface
module to
implement a GUI that may be stored in a mass storage device as computer
executable
program instructions that are executed by the computing device(s). Computer
system 500
may, as described below, implement the techniques described herein using
customized
hard-wired logic, one or more ASICs or FPGAs, firmware and/or program logic
which in
combination with the computer system causes or programs computer system 500 to
be a
special-purpose machine. According to one embodiment, the techniques herein
are
performed by computer system 500 in response to processor(s) 504 executing one
or more
39
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
sequences of one or more computer readable program instructions contained in
main
memory 506. Such instructions may be read into main memory 506 from another
storage
medium, such as storage device 510. Execution of the sequences of instructions
contained
in main memory 506 causes processor(s) 504 to perform the process steps
described herein.
In alternative embodiments, hard-wired circuitry may be used in place of or in
combination
with software instructions.
[0126] Various forms of computer readable storage media
may be involved in
carrying one or more sequences of one or more computer readable program
instructions to
processor 504 for execution. The instructions can be for operating a cessation
program
using a user device 15 and/or a delivery system 100. For example, the
instructions may
initially be carried on a magnetic disk or solid state drive of a remote
computer (for
example, server 25). The remote computer can load the instructions into its
dynamic
memory and send the instructions over a network. A transceiver in the computer
system
500 and place the data on bus 502. Bus 502 carries the data to main memory
506, from
which processor 504 retrieves and executes the instructions. The instructions
received by
main memory 506 may optionally be stored on storage device 510 either before
or after
execution by processor 504.
[0127] Computer system 500 also includes a communication
interface 518
coupled to bus 502. Communication interface 518 provides a two-way data
communication
coupling to a network link 520 that is connected to a network 20. For example,
communication interface 518 may be an integrated services digital network
(ISDN) card,
cable modem, satellite modem, or a modem to provide a data communication
connection
to a corresponding type of telephone line. As another example, communication
interface
518 may be a local area network (LAN) card to provide a data communication
connection
to a compatible LAN (or WAN component to communicated with a WAN). Wireless
links
may also be implemented. In any such implementation, communication interface
518 sends
and receives electrical, electromagnetic or optical signals that carry digital
data streams
representing various types of information.
[0128] Network links typically provides data communication
through one or
more networks to other data devices. For example, the network link may provide
a
connection through a smartphone 15 to a server 25 via network 20. Computer
system 500
can send messages and receive data, including program code, through the
network(s),
network links and communication interface 518. In an Internet example, a
server 25 might
transmit a requested code for an application program through network 20 and
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
communication interface 518. The received code may be executed by processor
504 as it is
received, and/or stored in storage device 510, or other non-volatile storage
for later
execution.
[0129] In various embodiments certain functionality may be
accessible by a
user through a web-based viewer (such as a web browser), or other suitable
software
program on the user device 15 or another computer. In such implementations,
the user
interface may be generated by the server system 25 and transmitted to the user
device 15.
Alternatively, data (e.g., user interface data) necessary for generating the
user interface may
be downloaded as a separate app to the user device 15. A user may then
interact with the
user interface on the user device 15 through the app to view information
related to the
treatment program and interact with the treatment program.
[0130] Figure 6A is a diagram demonstrating an example of
a high level data
flow logic of a smoking cessation system. A new user 30 seeking to quit
smoking may
perform at least a portion of their onboarding using a cessation application
608. The
cessation application 608 can he implemented on a user device (e g., a smart
phone, a
mobile computer, a desktop computer, a specialized computer in a medical
practitioner's
facility, or another suitable computer resource). For example, user device 15
(Figure 1).
The cessation application 608 can include functionality that runs in-part on a
portable
computer, and functionality that runs in-part on a server system. As part of
the onboarding,
the user 30 may be prompted to enter their profile 603 into the cessation
application 608.
The profile 603 can include characteristics that are relevant to nicotine
withdrawal. This
may include age, sex, height, weight, smoking history, including the number of
cigarettes
per day, the time of day of usage, biological co-factors, psychosocial co-
factors, clinical
co-factors, and any other relevant characteristics. For example, the
application 608 may
request the number of cigarettes smoked per day, the brand of cigarettes,
whether the user
30 smokes e-cigarettes. If the user 30 indicates through the interface that
they smoke
e-cigarettes, the application 608 may additionally request the user provide
how long each
cartridge is used and the amount of nicotine per cartridge.
[0131] Other information may also be entered, including
objective medical
information determined from one or more tests of the user. For example, the
nicotine
metabolic rate (NMR) of the user. Once the data has been entered, the
application 608 may
determine treatment program / smoking cessation schedule 607 based on the
user's 30
individual profile 603 and other provided information. That schedule may then
be output
606 to the user 30 on an app on the user's device 15. The output 606 may be in
the form of
41
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
one or more charts, tables and/or other information related to the treatment
program. The
information may include an indication of the amount of cigarettes or puffs
recommended
for the user 30 for a particular period of time during the program. This may
include the
number of cigarettes recommended for the day, week, or month, with options to
display
different time periods. Additionally, the user 30 may be given different
options to select
the pace of withdrawal from different recommended paces, some being a much
faster drop
from nicotine use.
[0132] The user 30 may seek help from outside advisors 35,
like a physician, to
measure or calculate the information required for the profile 603. The advisor
35 may also
play a role in monitoring the progress 605 of the cessation application 608
which could
lead to changes in the program over use.
[0133] The cessation system 100 receives data from the
cessation application
608 to deliver the intended dose to the user 30. The cessation system 100 also
stores puff
data 601, which may include puff duration, puff interval, and/or puff volume,
in memory
506, to send via the controller circuit 130 back to the cessation application
608 and the
treatment program on the server system 25. This puff data 601 may be monitored
by the
user 30 or the advisor 35, and the treatment program itself to determine
adjustments for the
cessation schedule 607.
[0134] Figure 6B is a flowchart illustrating an example of
a cessation process
600, according to one embodiment. In an example, the cessation process 600 is
for helping
a user quit smoking. In another example, the cessation process 600 is for
helping a user to
quit vaping. In another example, the cessation process 600 is for helping a
user to quit an
addictive behavior. At block 605, the process 600 operates at least a portion
of a treatment
program on a handheld delivery system. In one example, the handheld delivery
system is
the delivery system 100 illustrated in Figure 3A. At block 610, the process
600
independently controls the aerosol generated by each of three or more
aerosolizers in the
delivery system, based on the treatment program, to form an aerosol mixture.
In another
example, the process 600 can independently control the aerosol generated by
each of two
aerosolizers in a delivery system, based on the treatment program, to form an
aerosol
mixture. The aerosol mixture can then be inhaled by a user.
101351 Figure 6C is a flowchart illustrating another
example of a treatment
process 700, for example, for treating a smoking or vaping addiction. At block
705 the
process 700 provides a hand-held drug delivery system, the delivery system
having an
aerosolizer system with three aerosolizers each including a different
substance. In an
42
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
example, the aerosolizer system is similar to aerosolizer system in pod 150
illustrated in
Figure 3C which has three aerosolizers. Even though this disclosure generally
refers to pods
having three aerosolizers, or more than three aerosolizers, in other
embodiments, a pod can
have two aerosolizers and provide aerosol mixtures from the substances
contained in the
two aerosolizers, similar to what is described herein for a three aerosolizer
embodiment.
[0136] At block 710, the process 700 controls the aerosol
produced by each of
the three or more aerosolizer's to form an aerosol mixture in an aerosol
mixing chamber of
the cessation system, the aerosol mixture formed based on the treatment
program and based
on informant from one or more sensors on the hand-held delivery system. For
example, one
or more of the sensors illustrated in Figure 4.
[0137] When the delivery system operates to provide a
cessation program to a
user, a controller circuit executes cessation program instructions which
causes signals to be
provided to a plurality of aerosolizer drivers. The signals provided to the
plurality of
aerosolizer drivers can be based on the treatment program instructions, and
optionally on
information that the controller circuit receives from one or more of the
sensors of the
delivery system. For example, any one or more of (but not limited to) a flow
sensor, an
aerosol density sensor, and aerosol temperature sensor, an ambient temperature
sensor,
ambient pressure sensor, a blood oxygen sensor, and/or a carbon dioxide
sensor. The
treatment program instructions may include instructions and information that
was stored
on the delivery system when it was manufactured or configured, and/or
instructions and
information that were received by the delivery system from a user device 15, a
server
system, and/or another computer system.
[0138] In the embodiments disclosed herein, an aerosolizer
system can include
one, two, three or more than aerosolizers. In some embodiments, pods having a
different
number of aerosolizers may be used for different portions of the cessation
program, and the
(same) delivery device (e.g., delivery device 109) may be configured to work
with pods
having one, two, three or more aerosolizers.
[0139] In the embodiments disclosed herein of a delivery
device used with pod
having a plurality of aerosolizers, the controller circuit can provide signals
to the plurality
of aerosolizer drivers 110, based on the treatment program, to cause the
plurality of
aerosolizers in the pod to produce an aerosol mixture having portions (e.g.,
percentages) of
varying amounts of the substances in the pod. For example, for a pod having
three
aerosolizers in three substances in the pod (one corresponding to each
aerosolizer), the
delivery device can provide signals to the three aerosolizer drivers such that
a first driver
43
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
may drive a first aerosolizer to produce 0, 1,2, 3,4, 5, 6,7, 8, 9, 10, 11,
12, 13, 14, 15,
16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34,
35, 36, 37, 38, 39,
40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58,
59, 60, 61, 62, 63,
64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82,
83, 84, 85, 86, 87,
88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, or 100 percent (plus or minus
0.5%) of an
aerosol mixture (a "resulting" aerosol mixture). The delivery device 109
(e.g., the
controller circuit 130) can also provide signals to the three aerosolizer
drivers such that a
second driver may drive a second aerosolizer to produce 0, 1, 2, 3, 4, 5, 6,
7, 8, 9, 10, 11,
12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25,26, 27, 28, 29, 30, 31,
32, 33, 34, 35,
36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54,
55, 56, 57, 58, 59,
60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78,
79, 80, 81, 82, 83,
84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, or 100 percent
(plus or minus
0.5%) of the aerosol mixture. The delivery device 109 can provide signals to
the three
aerosolizer drivers such that a third driver may drive a third aerosolizer to
produce 0, 1, 2,
3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23,
24, 25,26, 27, 28,
29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47,
48, 49, 50, 51, 52,
53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71,
72, 73, 74, 75, 76,
77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95,
96, 97, 98, 99, or
100 percent (plus or minus 0.5%) of the aerosol mixture. Similarly, in other
embodiments
with two aerosolizers or four or more aerosolizers, a delivery device can
provide signals
to the aerosolizer drivers each aerosolizer correspondingly produces 0-100% of
the
resulting aerosol mixture.
[0140] In addition, the delivery device 109 (e.g., the
controller circuit 130) can
provide signals to the aerosolizer drivers 110 to produce aerosol from the
different
substances of different droplet sizes, in accordance with the treatment
program. For
example, the controller circuit 130 can provide signals to three aerosolizer
drivers such that
the signals drive three corresponding aerosolizers in a pod to produce aerosol
having the
same droplet size, or two or more different droplet sizes. In some
embodiments, the aerosol
droplet diameters will be less than 1 p.m, or equal to 1 pm, 2p.m, 3 m, 411m,
5pm, 6pm,
7pm, 8pm, 9 m, 10pm, llpm, 12pm, 13pm, 141.im, 151,tm, 16pm, 17pm, 18pm, 19pm,
or
20pm, plus or minus 0.5 pm.
[0141] In reference to the illustrative example of a
smoking cessation treatment
program developed to wean an individual from smoking or vaping, certain co-
factors can
be evaluated to create a cessation program or treatment plan for a particular
user, for
44
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
example similar to the program illustrated Figure 7Q. Figures 7A, 7B-1, 7B-2,
and 7C
illustrate how behavior modification and precision medicine can affect
personalization
domains that influence how a body processes nicotine. Figures 7D, 7E, 7F, 7G,
7H, and 71
illustrate the D-ADME2 model that can determine how biological effects are
elements of a
smoking persona. Figure 7J illustrates how psychosocial co-factors may result
in a user
being compelled to initiate a smoking experience. Finally, Figures 7K, 7L, 7M,
7N, 70,
and 7P demonstrate how persona and personalization parameters can be used to
map
cessation liquid variables.
[0142] A treatment program or smoking cessation can be created by a
combination of biological, psychosocial, and clinical co-factors, which can be
referred to
as a smoking persona. This information may be input as parameters that are
used to control
the delivery device to vary concentrations of the substances contained in the
aerosolizer
system at different points in a period of time during a program schedule for a
certain person.
Biological co-factors can be an individual's unique adaptation to nicotine
related to their
individual neurobi logical phenotype. Psychosocial co-factors can be an
individual's
unique conditioned triggers to smoke with respect to personal and
environmental
conditions. Clinical co-factors can be severe psychological or cognitive
challenges.
[0143] Additional parameter considerations may include smoking drivers and
quit inhibitors. Managing smoking drivers and quit inhibitors to wean an
individual off
smoking or vaping can be a combination of a delivery /device system use,
behavioral
software use, and medical consultation. The following table indicates possible
smoking
drivers or quit inhibitors, a description of each, and how the cessation
system, software, or
advisor/medical consultation may help. In this example, and generally herein,
a smoking
cessation delivery device and smoking cessation treatment program may be
referred to a
-NoMore" device/program.
Smoking Driver, Description/Example Assessment Delivery
Behavioral Medical
Quit Inhibitor & Device
Software Consultation
Related Persona ("NoMore")
Element
Nicotine Nicotine dependence Nicotine Metabolite
Dependence
Dependence varies across Ratio (NM R) level sets the
Ts (Manage with smokers. Some FagerstrOm FIND Initial
Nicotine
'En Device) people have severe Question 1.
Concentration
0
withdrawal, others Wisconsin Smoking Sots the
have almost no Withdrawal Scale Nicotine
withdrawal. Nicotine Taper Rate
half-life is 2 hours so
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
Smoking Driver, Description/Example Assessment Delivery
Behavioral Medical
Quit Inhibitor & Device Software
Consultation
Related Persona ("NoMore")
Element
withdrawal may start
1 hour after smoking
Sensory Some people enjoy 1. How much do you
Use desired
Experience the sensations of enjoy smoking?
throat hit to
(Manage with smoking while others 2. How much to like the set
afb to an
Device) do not. feeling of inhaling initial
setting.
People like different cigarette smoke? Then reset
amounts of "throat hit" 3. How much do you afb during
People like different like the feeling of nicotine
taper
flavors "nicotine burn"? when nicotine
4. What flavor do you concentration
like? is low.
Triggers i. External stimuli (the Ask about each Before
the
(Manage with smell of smoke, category of
trigger + quit smoking
Behavioral Tech) movie) give example. Ask how day,
develop
ii. Daily routine (e.g., much does this
trigger a strategy for
after each meal, drive your smoking. managing
driving, phone) each
iii. Places (their chair, category of
outside) trigger. This
iv. Emotions will include
(celebration, anger, the use of
loneliness, boredom) NoMore, and
v. Social situations vi will include
Stressful events other
vii. Alcohol/other strategies as
substances well.
Social Smoking Social smoking can Do you
sometimes Add "Social
(Manage with be difficult to smoke with
others? If Module" to
Behavioral Tech) overcome because it yes, Who do you plan ¨
.ca involves group smoke with? 1. Tell friend
0
identification, social you will use
0
acceptance. This is a NoMore.
bigger problem for 2. Ask friend
0_ younger people, not to
smoke.
3. Stop
seeing
friend.
Ritual Smoking Older smokers in Ask about daily routine Extend
taper Introduce
(Manage with particular, tend to (Part of triggers survey)
to 12 weeks, plan to
Device and show increased ritual will likely replace
Behavioral smoking ¨ smoke the need a long routine
Technology) same number of cigs period after
cigarettes
each day with their taper also. with
NoMore
daily routine (e.g., use
NoMore after
each meal)
Stressful Event Stressful event may 1. Did you ever quit
Use of Mindful use
(Manage with be very small to very smoking before? Rescue
Dose of NoMore,
Device and large. A stressful 2. If yes,
Mindfulness
event is the number 1 what led to relapse? of emotions.
46
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
Smoking Driver, Description/Example Assessment Delivery
Behavioral Medical
Quit Inhibitor & Device Software
Consultation
Related Persona ("NoMore")
Element
Behavioral reason for relapse 3. What
portion of your The dosing Pause,
Technology) back to smoking. cigarettes do you
will be set up recognize
Relapse occurs smoke due to stress? the stressful
because of high 4. How much has situation,
stress in combination stress kept you from recognize
with poor coping quitting? your
skills, response to
the situation.
Decide what
to do.
Attachment Some feel cigarettes "Have you ever thought
Training in Inability to
(Manage with are their "best friend." of smoking as a close
letting go, quit may
Behavioral Tech) They can cut down to friend?" grief
require
1 cig/day, but cannot Also ¨Device check: if counseling
medical
stop. Grieving occurs they are unable to quit
consult if
after stopping smoking after tapering person
is
down cigs or unable to stuck.
stop product after
tapering down
Alcohol/Substances Alcohol/substance 1. On average, how 1. While 3.
If they
(Manage with use is common in many days per
week drinking, can continue to
Behavioral Tech smokers and highly do you drink?
a person use smoke while
and possibly with associated with 2. What is the
most NoMore drinking, and
Medical Consult) relapse. number of drinks you instead of
are unable to
1. Alcohol and will have in a
day? smoking? If cut down, this
smoking can be yes,
requires a
associated as a continue
Medical
routine, drinking as
Consult
2. Increases smoking before
urges. 2. If no, then
3. Alcohol decreases reduce
ability to exert will drinking to 1
power and increases drink per
automatic smoking. day.
Depression Severe depression is PHQ-2 > 3 do PHQ-9 PHQ >4
add PHQ >9
2.? (Manage with highly associated with PHQ-9 > 4 ¨ see B
depression requires
.s Behavioral Tech inability to quit and Technology PHQ-
9 > 9 component medical
and possibly with relapse Get consult to behavioral
consult
Medical Consult) approach
Anxiety Severe anxiety is GAD-2 > 3 do
GAD-7 GAD > 4 ¨ GAD-9 > 9
(Manage with highly associated GAD > 4 ¨
add anxiety requires
Behavioral Tech with inability to quit see Beh.
module to medical
and possibly with and relapse Technology behavioral
consult
Medical Consult) GAD-9 > 9 approach.
Get consult
Low Change People with Ask ¨ do you have a
Requires a
Capacity traumatic brain
history of traumatic medical
(Manage with injury, cognitive
brain injury, cognitive consult
Medical Consult) impairment, impairment,
dementia, late stage dementia, late stage
schizophrenia may schizophrenia, or
smoke as a ritualized similar?
behavior, may
47
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
Smoking Driver, Description/Example Assessment Delivery
Behavioral Medical
Quit Inhibitor & Device Software
Consultation
Related Persona ("NoMore")
Element
smoke more e.g.,
3-4 packs per day.
[0144] In the table above, "PHQ" refers to the commonly
known Patient Health
Questionnaire self-administered version of the PRIM-MD diagnostic instrument
for
common mental disorders. PHQ-9 is the depression module. PHQ-2 inquires about
the
frequency of depressed mood and anhedonia over a time period (e.g., 2 weeks),
and
includes the first two items of the PHQ-9. "GAD" refers to the commonly known
a
generalized anxiety disorder score.
[0145] Figure 7A is a diagram illustrating pharmacological
dosage related
personalization data and behavior modification as two intersecting domains of
cessation
program personalization.
[0146] Figures 7B-1 and 7B-2 are a two-part table
illustrating personalization
parameters that influence how the body processes nicotine and how each may be
quantified.
The personalization parameters can be divided into primary, secondary, and
tertiary/quaternary parameters based on prioritization.
[0147] Figure 7C is a diagram illustrating an example of
primary, secondary,
and other focused personalization domains utilized by the smoking cessation
system which
were previously illustrated in Figure 7A and Figures 7B-1 and 7B-2.
[0148] Figure 7D is a diagram illustrating the basic model
components of
D-ADME2 and how nicotine may be deposited within the body. As the figure
indicates,
nicotine can be absorbed, distributed, and metabolized based on an
individual's genomics
and personal and environmental factors. The byproducts from this process can
be
eliminated and excreted from the body. Figure 7D demonstrates how biological
co-factors
can help define a smoking persona.
[0149] Figure 7E is an example of how calculating nicotine
input and
measuring nicotine output can determine how an individual metabolizes it,
often called the
Nicotine Metabolite Ratio (NMR). In some embodiments, NMR may be calculated
for an
individual to reveal how rapidly an individual biochemically processes
nicotine. This
information can be applied to form an individual's persona and be input
parameters for a
cessation program.
48
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
[0150] Figure 7F illustrates the same diagram as Figure 7D
and how nicotine
is deposited in the body. Additionally, the diagram identifies four key
personalization
parameters that can be configured for use in a cessation program. In some
embodiments,
four parameters include the dose of nicotine received by the user when using a
cessation
device, the site of the nicotine deposition, how quickly the nicotine is
absorbed, and how
rapidly the nicotine is metabolized.
[0151] Figure 7G contains the four key personalization
parameters as
illustrated in Figure 7F, but in this embodiment, shows three critical
variables of the
cessation liquid and aerosol that can affect the personalization parameters.
In some
embodiments, three critical variables include nicotine concentration, aerosol
droplet size,
and free nicotine ratio.
[0152] Figure 7H is an example embodiment of the detailed
cascaded
D-2ADME personalization model illustrating the central compartment and the
peripheral
compartment.
[0153] Figure 71 takes the same cascaded personalization
model of Figure 7H
and illustrates how adding personalized user persona information and
configuration of the
cessation device can affect NMR.
[0154] Figure 7J is a diagram illustrating how
psychosocial co-factors can
affect how nicotine is deposited in the body when a user is compelled to
smoke.
Psychosocial co-factors, as discussed previously, can include triggers,
attachment,
substances, and pathologies.
[0155] Figure 7K is a diagram illustrating how
personalization parameters and
clustered persona characteristics can be used to map the magnitude and range
of associated
cessation liquid parameters. The intersection of biological persona parameters
and
psychosocial persona parameters can create varying levels of cessation liquid
necessary for
a cessation program.
[0156] Figure 7L illustrates the diagram of Figure 7K with
low, moderate, or
high nicotine levels according to persona level. In one embodiment, reduced
biological
persona parameters with mild psychosocial persona parameters would result in a
low
concentration of concentrated nicotine for a cessation program. In an
alternate embodiment,
enhanced biological persona parameters paired with intense psychosocial
persona
parameters may result in a recommended high concentration of nicotine.
[0157] Figure 7M adds to the Figure 7L to illustrate
bioavailability of nicotine
across personas.
49
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
[0158] Figure 7N adds to Figure 7M to illustrate how
aerosol droplet size is
mapped to persona profile and metabolism rate. In one embodiment, a larger
aerosol droplet
may result in a higher concentration at a pulmonary deposition. In another
embodiment, a
smaller aerosol droplet may result in a lower concentration at a buccal
deposition.
[0159] Figure 70 adds to Figure 7N illustrating how
frequency of smoking and
puff topology can add to the uniqueness of the cessation liquid variables for
a user. In one
embodiment, a middle-aged, heavy smoker, typical metabolizer, severe
withdrawal
symptoms, elevated smoking urges, enhanced sensory enjoyment, intense alcohol
use, and
elevated smoking attachment may result in a higher concentration of nicotine
for the user.
[0160] Figure 7P illustrates how the users from Figure 70
can benefit from a
unique cessation program based on cessation start point, age, behavioral
information related
to triggers, stresses, anxiety, depression, alcohol consumption, and social
cue responses. In
one embodiment, the cessation program may create unique taper paths, taper
rates, and
program durations for different users.
[0161] Figure 7Q is a diagram illustrating an example of a
five-step process
can be implemented for a treatment program designed for quitting smoking. In
this
example, the program includes on-boarding 801, cigarette taper 802, nicotine
taper 803,
placebo usage 804, and software support 805 phases.
[0162] The objectives of the on-boarding phase 801 is to
register and enroll a
user and to do preliminary device configuration. This on-boarding phase 801
may begin
with an appointment with a prescribing physician and end with NMR analytical
results.
Some steps for dosage calculation may include obtaining a saliva sample for
analytical
NMR determination, identifying a likely consumable category based on persona,
creating
a preliminary definition of cessation liquid initial values based on persona,
confirmation of
age, a physician interview to assess menopausal status, co-morbidities,
medications,
lifestyle factors, creating a preliminary definition of taper path and taper
rate based on age
and persona, and configuring NMR input upon receipt of laboratory results.
[0163] Some psychosocial and clinical actions included in
the on-boarding
phase 801 may include defining an aspirational definition of cessation process
duration,
qualitative determination of persona elements (e.g., name, gender, age,
occupation,
location, etc.), an overview of the behavioral program in subsequent steps
(education,
identification of triggers, managing urges, managing stress, the smoking
taper, the quit
process), overview of social support, overview of professional support,
physician interview
to identify presence of/ susceptibility to additional behavioral components
(uncontrolled
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
severe stress, generalized anxiety disorder, depression, bipolar disorder,
post traumatic
stress disorder, pre-existing traumatic brain injury, schizophrenia), and to
set a date for
profiling.
[0164] Other actions of an on-boarding phase 801 can
include various
registration activities, subscription to consumables delivery service, user
specification of
flavor system (tobacco, menthol none), or enlist willing onboarded cessation
system users
under the auspices of a -pay it forward- strategy to help two other people
quit once they
have successfully quit. Although the length of time for on-boarding can vary
based on a
number of factors, it typically may be about 5-10 working days.
[0165] In this example, the second phase of the treatment
program is a cigarette
taper phase 802 which may include profiling and product familiarization.
Objectives of this
cigarette taper phase 802 may be to finalize personalization, define start
delivery
parameters, and/or set a quit smoking day. This phase may start immediately
after NMR
results are configured and end with a successful quit smoking day.
[0166] Some steps for dosage calculation may include
setting initial delivery
parameters to match cigarette experience based on persona data. In one
example, set initial
nicotine concentration r = 3% w/w, initial free fraction afb = 0.03, for light
smoker < 1
pack/day with low NMR. In another example, set initial nicotine concentration
r = 5% w/w,
initial free fraction ctfb = 0.07, for heavy smoker > 1 pack/day with elevated
NMR. In yet
another example, set initial aerosol droplet size 6 < 1.0 nm.
[0167] Another dosage calculation action may be setting
initial PRN/rescue
concentration higher than standard but with same afb and 6 according to
persona. For
example, a light smoker nicotine concentration r = 5% w/w and a heavy smoker
nicotine
concentration r = 7% w/w.
[0168] Other dosage calculation actions may include 5 day
and 10 day
parameter ranging evaluation (including systemic increasing and decreasing
around initial
settings and PRN dose settings for nicotine concentration r and initial free
fraction afb with
6 constant to establish final parameter values for the objective of a
satisfying and pleasant
routine dose or maximally satisfying and tolerable PRN dose), recording of
user puff
topology to enable dose calculation, finalize delivery parameters and update
program
configuration, or confirmation or adjustment of cessation taper duration for
subsequent
step.
[0169] Psychosocial and clinical actions in the cigarette
taper phase 802 can
include to define a Quit Smoking Date (QSD) (estimate 2-4 weeks from beginning
of Dual
51
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
Usage phase) comprising: cessation program learning/adaptation period (5-10
days) using
initial delivery parameter values from the on-boarding phase 801, or dual
usage (14-21
days) with cigarette taper (steps below).
= The cigarette taper:
= Techniques for smoking tapering: 1) ad-lib; 2) frequency reduction (only
every
hr 16/day, then only every 2hrs 8/day); 3) scheduled smoking (on waking,
after eating, and one in the evening ¨ 5/day).
= Using selected technique to cut cigarette smoking in half to 10/day in
the first
50% of time to QSD; then again in half to 5/day at 75% to QSD; then zero when
QSD has been reached.
= Replace cigarette reduction with NoMore usage.
101701 Another psychosocial action may be progressive
behavioral training
(education concerning health risks like lung cancer, chronic obstructive
pulmonary disease
(COPD), and heart disease, identification and recording of triggers like
temporal,
association, emotional, location, and alcohol, or mindfulness training for
stress
management), a call with a professional counsellor 2-3 days before Quit
Smoking Day to
enhance chance of behavior change, or calls with former cessation program
users as quit
advisors.
[0171] Other actions in the cigarette taper phase 802 may
include a cessation
program training video to cover inhalation changes with respect to reduced
rate & increased
depth, how the cessation program is different from smoking, function of lights
and buttons,
battery recharging, replacing consumables, or general usage recommendations
for learning
period. Other actions may also be configuration of consumable replenishment
schedule.
One action may cover steps to take if Quit Attempt is unsuccessful: do not
progress to the
nicotine taper phase 803, re-evaluate and assess what went wrong, create a new
strategy
based on what went wrong (if stress, then stress management behavioral
therapy, if
motivation/social, then enhance social support elements, or if technical, then
review
product information/video), engage medical support if simply not engaged, set
new Quit
Day in 2-4 weeks & make plan to prepare for it, or reset taper and start
cigarette taper phase
802 again. Although the length of time for the cigarette taper phase 802 can
vary based on
a number of factors, it typically may be in the range of about 7 to 15 weeks
long.
[0172] In this example, a third phase of the treatment
program for quitting
smoking may be a nicotine taper phase 803. This phase may include the
objective to end
52
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
the use of nicotine. This phase may start after a successful quit smoking day
and end when
cessation taper reaches final dose of nicotine level.
[0173] In some embodiments, the steps for dosage
calculation may include not
changing the previously established PRN/rescue settings, beginning cessation
taper from
previously defined start conditions for nicotine concentration, free fraction,
and aerosol
droplet size, starting taper two weeks after beginning the nicotine taper
phase 803, applying
taper variables and rate according to the previously defined taper parameters,
monitoring
PRN/rescue usage and adjusting taper variables if it is determined that the
taper is too fast,
or not long enough in duration.
= Regular high PRN use >10x on consecutive days (3-4):
= Indicates standard dose insufficient. Re-evaluate taper and consider
adjustment.
= Consult with user concerning dosage increase.
= Re-test to determine threshold for routine dose; reset taper to this new
dosage;
reduce taper rate.
= At final dose likely nicotine concentration p<1% w/w:
= Increase aerosol 8 > 2.0 [trn to reduce absorption rate & peak
= -increase free ratio 0.01 < afb < 0.03 to give throat hit.
[0174] In some embodiments, the psychosocial actions may
include defining a
date for taper to reach final dose nicotine and the end of the nicotine taper
phase 803 (clearly
defined date with total use of cessation program not to exceed 26 weeks).
Other
psychosocial actions may include recording clearly defined date and time, or
monitoring
PRN/rescue dose usage, assisting with recognition of stressful events &
management
without smoking.
= If Lapse occurs (use of 1-2 cigs/day plus cessation program):
= Counselling, evaluation, encouragement to restart.
= Consider taper adjustments.
= If Relapse occurs (return to full smoking at level present starting the
cigarette taper
phase 802):
= Restart at beginning of the cigarette taper phase 802.
= Consider taper adjustments.
= If repeated taper reset, relapsing and failure in the nicotine taper
phase 803:
= Ensure cessation program is not regarded as a recreational product that
can be
used forever.
53
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
= Allow for 2-3 potential taper resets in high-dependence users.
= Allow for possible extension of total Quit Process time on condition of
medical
consult.
= In taper "tail" phase with final dose extremely low, expect phase to last
2-6 weeks:
= NoMore as crutch in this phase.
= PRN/rescue doses still available.
= At final dose, assess user and consider prolonged usage as long as total
usage does
not exceed 26 weeks.
[0175] Other actions in the nicotine taper phase may
include preparing for
transition out of cessation program use or preparing for provision of
potential support for
others in the online community.
[0176] In this example, the fourth phase of the treatment
program may be a
placebo usage phase 804 which may include the objectives of no nicotine
consumption and
no user relapse. This phase may start after successful Quit Day or 26 weeks,
whichever
first. The phase may end with no grieving or withdrawal symptoms. The
psychosocial &
clinical actions may include defining post-quit duration, monitoring to ensure
no physical
withdrawal symptoms, support for potential grief management, ongoing
behavioral
modification support (proactive recognition of stressful events or stress
management
without resorting to smoking), or recording of triggers and cues (uploading
for monitoring
and behavioral support).
[0177] Other actions in the placebo usage phase 804 may
include video training
for potential role as coach and support resource in online cessation program
community.
[0178] In this example, the fifth phase of the cessation
program is a support
phase 805 which may include the objective of stopping the use of the cessation
program.
The phase may end when the user decides. Dosage actions in this phase may
include
cessation system device usage disabled unless intended use is transitioned
from Cessation
to Reduced Exposure & Reduced Risk. One psychosocial action may include
community
reinforcement support. Other actions may include active participation in
Online Support
"pay it forward" community or potential intended use transition to Reduced
Exposure &
Reduced Risk in certain extreme cases.
101791 Figure 8A is a block diagram further illustrating
certain aspects of the
onboarding process for a treatment program for quitting smoking. An onboarding
process
of the treatment program can be run by the user device 15 and the server
system 25 to
54
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
register a user, including providing questions to the user and receiving
information specific
to the user/answers to the questions. One or more test may be performed on the
user and
information from a lab conducting the tests (e.g., determination of NMR) can
be received
by the treatment program, e.g., on the server system 25. Based on user
provided information
and any test information, the treatment program can determine for the user a
smoking
persona profile, an individual profile, a smoking cessation treatment program.
The
treatment program can include various aspects including one or more of a
determination of
a number of steps, a planned duration of each of the steps, initial parameters
that will be
needed to control the delivery device, an expected nicotine consumption of the
program
and portions of the program.
[0180] Figure 8B is a diagram illustrating aspects of data
communications
between a server system, a user device (mobile platform), and a delivery
system performing
a process for a medical treatment. For example, the server system 25, user
device 15, and
the delivery system 100 illustrated in Figure 1 performing processes for a
smoking
cessation program. All of the server system 25, user device 15, the delivery
system 100,
and sensor(s) 17 can include hardware processors and non-transitory computer
readable
medium and that include instructions that when executed, configure the
respective
hardware processors to perform processes for a health treatment program. These
processes
include hardware components (e.g., a transceiver, antenna, etc.) for one or
two-way
communications between the sensor(s) 17, delivery system 100. user device 15,
and server
system 25 to control providing substances to a user by the delivery system 100
according
to a treatment program being run on the server system 25 and the user device
15.
[0181] As discussed in reference to Figure 8A, during
onboarding 801
registration information specific to a user can be communicated from the user
device 15 to
the server system 25, and the treatment program can be based the information,
according
so some embodiments. Figures 8C and 8D illustrate examples of user interfaces
861-872
that can be displayed on the user device during an on-boarding process. In
this process,
questions are presented to the user, input is received from the user relating
to their smoking
habits and personal information, and the input can be used by the user device
and the server
system to tailor the treatment program (in this example, a smoking cessation
program).
101821 Calibration and configuration information is
communicated from the
server system 25 to the user device 15 and then to the delivery system 100
also as a part of
onboarding the user into the treatment program. Figure 8E illustrates examples
of user
interfaces that are displayed on the user device during a device calibration
process,
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
according to some embodiments. User interface (U/I) 873 prompts the user to
unlock the
delivery device using the fingerprint sensor. If fingerprint information has
not yet been
received from the user, it can also be done at this stage. U/I 874-877
personalize puff
information for a user to calibrate the device to the specific user. Using the
U/I's, the
process prompts the user to take a number of puffs using the delivery system
and the
delivery system monitors the puffs using the flow sensor. U/I 876 receives
input from the
user relating to the strength of the aerosol mixture that was received during
the multiple
puffs (e.g., three puffs). Multiple puffs can be used and the data averaged.
The input can be
used to change the way the aerosolizers are driven to tailor the strength of a
"dose" as
perceived by the user.
[0183] As an example, the treatment program can be a
smoking cessation
treatment program as illustrated in Figure 7Q. After onboarding, during phase
1 of the
treatment program 850, program data is communicated from the server system 25
to the
user device 15, and from the user device 15 to the delivery system 100. In an
example, the
program data can include information that the controller circuit 130 uses to
drive the
aerosolizers 161a-c to generate aerosol mixtures comprising monoprotonated
nicotine,
freebased nicotine, and a flavor, and having a particular droplet size, for
example, droplets
having a diameter of less than or equal to 1.0 ium, according to the treatment
program.
During phase 1 of the treatment program 850, use data generated by the
delivery system is
communicated from the delivery device 100 to the user device 15 and then to
the server
system 25. The use data can also include any information that is sensed by the
delivery
system 109 based on the user's use of the delivery system 100 during the
treatment
program. In an example, the use data can include the number of puffs, the
duration of each
puff, sensed data from the delivery system 109 (e.g., data related to: air
flow in/through
delivery system (e.g., from flow sensor), ambient temperature, ambient
pressure,
fingerprint verification data, pod information (e.g., from the pod ID chip),
blood oxygen
sensed information, and/or carbon dioxide sensed information, information
sensed from the
user's saliva, etc.). In some embodiments, the use data can include
information related to
the amount of substances (e.g., fluids) that are in one or more of the
containers of the pod
150. Also, in some embodiments, user data sensed by the sensor(s) 17 is
communicated
from the sensor(s) 17 to the delivery system 100 or the user device 15, and
then it can be
communicated to the server system 25, where it can be used to dynamically
adjust the
treatment program or monitor the patient's progress in the treatment program.
The user data
sensed by the sensor(s) 17 can include information related to any sensed
characteristic of
56
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
the user. For example, temperature, pH, sweat, sugar level in blood, nicotine
level in blood,
another characteristic of the user's blood, information from a pacemaker,
and/or
information related to an embedded sensor in the user. Information relating to
the use of
the delivery system and the user's progress in the treatment program can be
illustrated in a
U/1 of the user device. Figure 8F illustrates examples of user interfaces 878,
879, 880 that
can be displayed on the user device during the treatment program, according to
some
embodiments.
[0184] As shown in Figure 8B, the subsequent phases of the
treatment program
851, 852. = = are indicative of additional phases of a treatment program, for
example, the
cigarette taper 802, nicotine taper 803, placebo taper 804 phases illustrated
in Figure 7Q.
During subsequent phases of the treatment program 851, 852'" additional
program data
related to the particular phase is communicated from the server system 25 to
the user device
15, and from the user device 15 to the delivery system 100, and use data
generated by the
delivery system is communicated from the delivery device 100 to the user
device 15 and
then to the server system 25. In the subsequent phases of the treatment
program 851, 852- - -
the program data can include information that the controller circuit 130 uses
to drive the
aerosolizers 161a-c to generate aerosol mixtures comprising monoprotonated
nicotine,
freebased nicotine, and a flavor, and having a particular droplet diameter,
for example,
droplets having a diameter of between less than or equal to 1.01,tm, to
greater than 101,tm,
according to the treatment program.
[0185] Also during these subsequent phases of the
treatment program 851,
852. - = , use data generated by the delivery system is communicated from the
delivery device
100 to the user device 15 and then to the server system 25. The use data can
include any
information that is sensed by the delivery system 109. In an example, the use
data can
include the number of puffs, the duration of each puff, sensed data from the
delivery system
109 (e.g., data related to: air flow in/through delivery system (e.g., from
flow sensor),
ambient temperature, ambient pressure, fingerprint verification data, pod
information (e.g.,
from the pod ID chip), blood oxygen sensed information, and/or carbon dioxide
sensed
information, information sensed from the user's saliva, etc.). Also, in some
embodiments,
user data sensed by the sensor(s) 17 in the subsequent phases 851, 852. = = is
communicated
from the sensor(s) 17 to the delivery system 100 or the user device 15, and
then it can be
communicated to the server system 25, where it can be used to dynamically
adjust the
treatment program or monitor the patient's progress in the treatment program.
The user data
sensed by the sensor(s) 17 can include information related to any sensed
characteristic of
57
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
the user. For example, temperature, pH, sweat, sugar level in blood, nicotine
level in blood,
another characteristic of the user's blood, information from a pacemaker,
and/or
information related to an embedded sensor in the user.
[0186] After all of the phases for the treatment program
that include
dynamically providing substances to the user in aerosol mixtures, in the final
phase 853,
the used device 15 provides app-based user support. In an example, the app-
based user
support can include positive reinforcement information and motivational
information to
help the user maintain the non-usage of nicotine products. During the final
phase 853,
questions may be provided to the user on the user device 15 to help determine
how the user
is feeling/handling not having nicotine, and information provided by the user
is
communicated back to the server system 25 and used to evaluate the user's
progress in the
treatment program. In some examples, information related to the final phase
853 from
numerous users is collected by the server system and this data is used to make
changes to
the treatment program for future users. Also, in some embodiments, user data
sensed by
the sensor(s) 17 in the subsequent phases in the final phase 853 is
communicated from the
sensor(s) 17 to the delivery system 100 or the user device 15, and then it can
be
communicated to the server system 25, where it can be used to monitor the
patient's
adherence to the treatment program. The user data sensed by the sensor(s) 17
can include
information related to any sensed characteristic of the user. For example,
temperature, pH,
sweat, sugar level in blood, nicotine level in blood, another characteristic
of the user's
blood, information from a pacemaker, and/or information related to an embedded
sensor in
the user.
Non-Thermal Aerosolizers
[0187] Drug delivery devices can include various types of
aerosolizers. For
example, thermal or non-thermal. Examples of non-thermal aerosolizers include
mechanical (e.g., using a vibrating mesh) and jet nebulizers (e.g., using
compressed air).
Mesh nebulizers can use electricity to vibrate a piezo (at approximately ¨128
KHz.)
element that moves liquid formulations through a fine mesh to generate
aerosol. The
diameter of the mesh or aperture determines the size of the particle
generated. Mesh
nebulizers are very efficient and result in minimal residual volume (0.1-0.5
mL). Mesh
nebulizers can utilize two basic mechanisms of action: active vibrating mesh
and passive
mesh. Active vibrating mesh nebulizers have an aperture plate with 1,000-4,000
funnel-
shaped holes vibrated by a piezo-ceramic element that surrounds the aperture
plate. Passive
58
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
mesh nebulizers (aerosolizers) utilize an ultrasonic horn to push fluid
through a mesh. An
adaptive aerosol delivery (AAD) system such as the I-neb has a small, battery-
powered,
lightweight, and silent drug delivery device designed to deliver a precise,
reproducible dose
of drug. The aerosol is created by a passive mesh, and aerosol is injected
into the breath at
the beginning of inhalation. The dosage of the drug is controlled through
specific metering
chambers. The metering chambers can deliver a pre-set volume ranging from 0.25
to 1.7
mL with a residual volume of about 0.1 mL. Some systems use a AAD algorithm
that pulses
medication delivery into 50-80% of each inspiration, based on a rolling
average of the last
three breaths.
[0188] Another type of non-thermal aerosolizer is an
ultrasonic nebulizer that
uses ultrasound to create an aerosol. Ultrasonic nebulizers convert electrical
energy to high-
frequency vibrations using a transducer. These vibrations are transferred to
the surface of
the solution, creating a standing wave that generates aerosol (Figure 10).
Ultrasonic
nebulizers were initially introduced as large-volume nebulizers most commonly
used to
deliver hypertoni c saline for sputum inductions. Small -volume ultrasonic
nehul i zers are
now commercially available for delivery of inhaled bronchodilators but should
not be used
with suspensions such as budesonide. Ultrasonic nebulizers tend to heat
medication. This
raises concerns about disrupting proteins, but that does not affect commonly
inhaled
medications.
[0189] Implementations of the drug delivery systems
described herein (e.g., for
smoking cessation or for delivery of other aerosolized drugs) can use non-
thermal
aerosolizers. Figures 9A-9F illustrate some examples of a drug delivery system
that uses
non-thermal aerosolizers. These drug delivery systems can be used in the
system illustrated
in Figure 1 as the handheld delivery system, or other systems. These systems,
devices, and
methods are employed to properly deliver an uncontaminated dose of aerosolized
medication or active pharmaceutical ingredient (API) to a user's respiratory
system in a
metered dose. Such systems deliver the medications directly to a user's
respiratory system
by aerosolizing a desired dose of the medication in liquid form. The user can
inhale the
aerosolized medication directly into the respiratory system, enabling faster
treatment of
various medical conditions. Delivery of accurate and consistent metered doses
of
aerosolized medication to a user is very important. Additionally, there is a
need for real-
time monitoring of drug delivery to a user that can be tracked and adjusted
outside a
doctor's office or medical setting.
59
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
[0190] The systems, devices, and methods disclosed herein
address many of the
same problems that were previously discussed with the smoking cessation
device, but
instead with respect to drug (medicant, active pharmaceutical ("API"))
delivery. Current
drug delivery systems often provide inconsistent doses by allowing some of the
medication
to remain in a reservoir in liquid form after the aerosolization process is
completed.
Sometimes the aerosolized medication is delivered with too great or too little
force for
substantially all the metered dose to properly enter the user's respiratory
system.
Contamination is also a pressing problem for many aerosolized drug delivery
systems.
Finally, enabling a medical advisor to monitor drug delivery and adjust dosage
or schedule
in real time would enable a user to receive more individualized and immediate
care.
[0191] Due to the nature of many APIs and liquid
medications, heat can have a
destructive effect on the chemical composition. Therefore, turning the liquid
medication
into an aerosol through a non-thermal aerosol generator would be necessary to
preserve the
efficacy of the APIs. Similar to the cessation system discussed in prior
embodiments, a
handheld system can make the aerosolized API available to the user at any time
or place.
The system could be linked to an application and server in order for a medical
professional
to monitor or control the user's intake of API.
[0192] In one embodiment, the liquid medication containing
an API may be
stored in a container in connection with a mesh or membrane. A vibratable
element vibrates
the mesh for a metered amount of time causing aerosolization of the liquid
medication from
the container and subsequent movement of the aerosol down a passage into a
mixing
chamber. The amount of time the mesh is vibrated can change the amount of
liquid
medicant that is aerosolized. If multiple aerosol generators are used in the
device, they can
each be operated individually or in combination to create an aerosol mixture
of multiple
medications.
101931 In another embodiment, a liquid reservoir between
the container with
the medicant and the mesh receives a metered dose of the medicant from the
container. The
mesh is then actuated to vibrate and turn the dose of liquid containing the
API into an
aerosol to be inhaled by the user.
101941 Like the smoking cessation program, a drug dosage
program can
monitor a user's activity, collecting accurate and detailed information of a
user's use of a
drug delivery device as the user progresses through a dosage program. The
collected
information is related to use characteristics of the delivery device and
includes information
that that would be impossible for the user to collect themselves. In one
embodiment, the
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
user is "onboarded" where a dosage program is individually generated based on
user
individual characteristics that may be genetic, determined from a user
interview, and/or
testing. The generated program can include an individually tailored and
dynamic dosage
program for the user to be administered using a delivery system that includes
a server-based
system, which is running the dosage application. The dosage system can also
include a
mobile device, just like the cessation system, that communicates with the
server-based
system and provides information relating to the dosage program and the user's
progress to
the user. The drug delivery system may also include a delivery device, or
inhalation device,
that administers an aerosol mixture containing an API to the user based on the
dosage
program. The delivery device includes a plurality of sensors relating to its
use, and signals
from the sensors are used to monitor the user's progress through the delivery
program, and
the program id dynamically tuned as needed.
101951 The delivery device and medicant containers can
hold a plurality of APIs
to be delivered to the patient. For example, the liquid API may comprise
insulin, asthma
medication, COPD treatments, hormone therapy, vaccines, pain relief treatment,
or other
protein formulations. Additionally, the delivery device may contain one, two,
three, or more
aerosol generators and containers, each holding a unique medication or aerosol
component.
[0196] Referring back to Figure 1, and similar as
described above relating to
thermal aerosolizers, the delivery system 10 may be used for drug dosage
programming for
a user where there is a server system 25, a delivery system 100 used by the
user 30, and a
computer/user device 15. One or more advisors or medical practitioners 35 can
receive
information relating to the drug dosage program and the use of the device.
This system
allows both the user 30 and the medical practitioner 35 to have real-time
feedback on and
control over the delivery of the API in the device. The doctor 35 may make
changes
depending on the user's response to the delivered drug or in response to
changes in the
user's medical status.
[0197] Figure 9A illustrates an example of a delivery
system 900 with
mechanical aerosolizers. In various implementations, the delivery system 900
can include
many or all of the same components as the previously described delivery system
100 that
has thermal aerosolizers. The delivery system 900 includes a delivery device
909 and a pod
950, the pod 950 configured to be removably coupled to the delivery device
909. The
delivery device 909 includes components to perform a treatment program that
includes
delivering multiple substances from the pod 950 in accordance with the
treatment program.
Specifically, the delivery device 909 controls multiple aerosolizers in the
pod 950 to
61
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
generate desired aerosol mixtures to be inhaled by the user, similar to the
previously
disclosed cessation system. When the pod 950 is coupled to the delivery device
909, the
aerosolizer drivers in the delivery device electrically connect to
corresponding aerosolizers
in the pod 950. The aerosolizer drivers can independently and separately
provide signals to
each aerosolizer in the pod 950 and generate desired aerosol mixtures of the
different
substances in the multiple aerosolizers of the pod 950 in accordance with the
treatment
program. All or a portion of the pod 950 is positioned within the housing 902
when the pod
950 is coupled to the delivery device 909. In some examples, a portion of the
pod 950 may
be coupled to the delivery device 909 and extend from the proximal end 903 of
the cessation
device 909. In this example, the pod 950 is coupled to the cessation device
909 such that it
is in electrical communication with the cessation device 909 and in fluid
communication
with air flow into and through the housing 902 (e.g., through opening 906,
through channel
904, and through opening 908).
[0198] Figure 9B illustrates the delivery device 909
coupled to the pod 950.
Figure 9C further illustrates the delivery device 909 (without the pod 950).
These
embodiments may be similar to that of the delivery device 109 and pod 150,
containing
many identical or similar components. However, in order to accommodate a non-
thermal
aerosol generator, the pod 950 does not have heating elements to turn the
liquid into vapor.
Instead, non-thermal aerosolizers are in place of the previous thermal
elements. The non-
thermal aerosolizers can include a vibrating mesh or other non-thermal
aerosolizer
components. In some embodiments, the pod 950 contains the majority of changes
to
accommodate non-thermal aerosolizers. The pod 950 is discussed in more detail
in Figure
9D.
[0199] Figure 9D illustrates a pod 950 that utilizes non-
thermal aerosolizers for
a drug delivery device. Pod 950 may be used within a device such as
illustrated in Figure
9C. As Figure 9C illustrates, pod 950 includes non-thermal aerosolizers 961a-
c. Electrical
connections 911a-c are each connected to one of the aerosolizers 961a-c, and
can provide
electrical power and/or control information to the aerosolizers 961a-c. When
the pod 950
is coupled to the delivery device 909, the electrical connections I ii a-c are
coupled to the
aerosolizer drivers 910a-c, respectively. Each aerosolizer 961 includes
aerosol generating
components 952, for example, a mesh, membrane, a mechanism to vibrate the mesh
or
membrane, or wave generating component. Containers 959a-c are configured to
hold a
substance (e.g., a fluid or powder containing an API) and are adjacent to a
passage 956a-c
having a distal end 955 and a proximal end 957. The passage provides a flow
path for
62
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
aerosol generated by the aerosol generating components 952 to flow to the
mixing chamber
962. The mixing chamber 962 includes walls 966 enclose a mixing space 967. In
the mixing
chamber 962, the aerosol generated by the aerosolizer is readily available to
a user to inhale
through opening 964. In some embodiments, the pod 950 includes one or more
power
sources that can provide electrical power to electrical components of the pod
950. The
controller circuit 930 can control the aerosolizer system to generate an
aerosol as prescribed
by the drug delivery system.
[0200] Figure 9E is a schematic of a non-thermal example
circuit 913 that can
be used in a drug delivery system for a treatment program, which is similar to
the controller
circuit of device 109 illustrated in Figure 4. This circuit 913 shows
communication lines
between a controller circuit 930 and other components of the delivery system.
The
controller circuit 930 can include one or more hardware processors, which can
be the
hardware processor 504 illustrated in Figure 5. In this schematic, the airflow
is from left to
right such that intake of air is sensed by the flow sensor 912 and is received
by the non-
thermal aerosolizers 961. The aerosolizers 961 generate aerosol in the
airflow, and the
airflow then passes pressure sensors 958. Figure 9E illustrates many
components that are
illustrated in Figure 4, but with non-thermal aerosolizers in place of thermal
aerosolizers.
There may be more than one non-thermal aerosolizer present in the device.
Figure 9E also
illustrates some additional components. For example, flash memory 910 is in
communication with the controller circuit 930. The controller circuit 930 in
this example
includes a transceiver or other communication circuitry that is coupled to an
antenna 920
which allows drug dosage circuit 913 to communicate with a smart phone or to
another
device or a network. As illustrated in Figure 9E, the circuit 913 can also
include a pod ID
chip interface 465 (or aerosolizer chip interface) which, when the pod 950 is
coupled to the
delivery device 909, is in communication with a pod ID chip 963 of the pod 950
for
providing signals (e.g., control signals) to the aerosolizer system and/or
receiving
information from the pod 950. The circuit 913 also includes the case data
interface 425
which is in communication the controller circuit 930, and a case charge
interface 430 which
is in communication with a battery manager for 420 which manages power
provided to the
controller circuit 930 into a power source (e.g., battery) 914 to, for
example, manage the
charging of the battery 114.
[0201] Figure 9F is a flowchart illustrating a process 990
for providing aerosol
mixtures of dosages of drugs during a treatment program. At block 991, the
process
implements a treatment program on a handheld delivery system having a
plurality of
63
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
mechanical aerosolizers. In an example the delivery system is the delivery
system 900,
illustrated in Figure 9B, that includes a handheld delivery device 909 and a
pod 950. At
block 992, the process 900 controls the plurality of mechanical aerosolizers
to generate an
aerosol mixture according to the treatment program, and based on signals
received from
one or more sensors of the delivery system. For example, a rescue button
and/or an air flow
sensor. The treatment program is typically over a period of time, and the
period of time
can include portions of time where certain drugs are provided in the aerosol
mixture to a
user. Various pods can be used during each portion of time of the treatment
program to
provide aerosol mixtures of different drugs to a user.
[0202] Figures 10A - 10L illustrate further details of an
example of a smoking
cessation treatment program, such as the treatment program illustrated in
Figure 7Q.
Specifically, Figures 10A - IOL illustrate dosages that can be provided by a
delivery system
as described herein (e.g., as shown in Figure 1) in accordance with a
treatment program,
where dosages of monoprotonated nicotine and freebase nicotine can be varied
during the
different time periods of the treatment program, and illustrate varying the
aerosol droplet
diameter during the treatment program so that the aerosol mixture is absorbed
in the mouth,
throat, or lungs. Using information provided from the user device and/or the
server system,
the delivery system controls the multiple aerosolizers to produce aerosol
mixtures in
accordance with the dosages in the different portions of a treatment program.
For example,
to generate aerosol mixtures having certain amount of each of two or three
substances, and
to generate aerosol mixtures having aerosol droplets of a certain diameter,
during different
portions of a treatment program. The term -dose- or "dosage- refers to the
mass of active
nicotine ingredient per single usage. The term "Dosage Map" refers to a
precision
medicine-based treatment program (e.g., a Predictably Human Therapeutic
Process) that
defines the variation of aerosol parameters during the treatment program, in
an
individualized manner that maximizes cessation efficacy. Variation of aerosol
parameters
permits initial duplication of the nicotine plasma pharmacokinetic (PK)
response
representing the patient's habituated nicotine source (cigarettes or vaping
devices); and
then gradual reduction of nicotine dose in a manner that minimizes triggering
of patient
withdrawal symptoms and urges by precise management of the nicotine plasma
pharmacokinetics that are uniquely associated with a patient's phenotype and
metabolic
response to nicotine.
[0203] The term single usage refers to that of an
"Experience" (cigarette) or a
"Session" (vaping device). In this context, the single usage dose is the sum
of all nicotine
64
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
doses per puff over the total number of puffs taken by a patient, during an
Experience/Session. Specifically, in these examples, the doses discussed in
this application
refer to the maximum possible mass of total nicotine comprised of
monoprotonated nicotine
and freebase nicotine species; that can be delivered during a single
experience/session
defined to consist of a maximum of 20 puffs from the device at some constant
conversion
of liquid mass to aerosol. This notion of dose is, by definition, an upper
limit as to what
will actually be delivered to a patient.
[0204] The actual dose delivered to the patient will be
less than, but unlikely
equal to, the maximum due to: a) aerosol losses that occur in the device due
to condensation
on device surfaces that form the fluid dynamic path of the aerosol, b) aerosol
losses that
occur at the device mouthpiece due to unsynchronized withdrawal of the device
from the
patient's mouth upon completion of a puff, and c) aerosol losses that may
occur when a
patient does not fully inhale the aerosol that was orally presented.
[0205] There are seven Quit Journey Periods during which
the Device delivers
Aerosol to a patient: a) Switching, b) Vigilance, c) Taper Start, d) Taper
Continuation, e)
Taper End, f) Low Nicotine, g) Device-Based Relapse Prevention
[0206] There are six Aerosol Variables:
1. Total Nicotine Dose per Experience/Session ¨ TNDE (mg) = The sum of all
nicotine species dosages over the number of Puffs taken by the Patient.
2. Monoprotonated Nicotine Dose fraction per Experience/Session ¨ MNDE
(mg) = The fraction of Total Nicotine Dose due to Monoprotonated
Nicotine.
3. Freebase Nicotine Dose fraction per Experience/Session ¨ FNDE (mg) ¨
The fraction of Total Nicotine Dose due to Freebase Nicotine.
4. Aerosol Droplet Size per Experience/Session ¨ ADSE = The Mass
Mean Aerodynamic Diameter of aerosol droplets.
5. Freebase Nicotine Ratio per Experience/Session ¨ FNRE (dimensionless) =
The Ratio of Freebase Nicotine Dose to Total Nicotine Dose.
6. Enantiomeric Ratio per Experience/Session ¨ ENME (dimensionless) = The
relative proportion of the nicotine enantiomer S fraction compared to the R
fraction present in the Total Nicotine Dose.
[0207] Figure 10A is a graph illustrating an example the
quit journey period
and the taper parameters implemented in a cessation program. The graph has one
vertical
axis and one horizontal axis. The vertical axis shows the total nicotine dose
er experience
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
in mg. The horizontal axis shows an example of periods for a smoking cessation
treatment
program. In this example there are seven periods ¨ Switching Duration,
Vigilance Duration,
Start Duration, Continuation Duration, End Duration, No Nicotine Duration and
Relapse
Prevention Period, which are briefly described below:
Switching Duration: The start of the Quit Journey is the Switching Duration is
the
time during which patient is switching from the source (smoking cigarettes and
vaping device) to a delivery system (e.g., a delivery system describe herein)
used
in the treatment program.
Vigilance Period: The Vigilance Period is the time during which patient quits
using
the source and continues to use the delivery system in their usual routine.
Start Duration: The Start Duration is the time during which the tapering
starts
using delivery system.
Continuation Duration: The Continuation Duration is the time during which the
patient undergoes tapering continues using delivery device.
End Duration: The End Duration is the time during which the tapering stops
using
the delivery system.
No Nicotine Duration: The No Nicotine Duration is the time during which the
patient maintains a low level of nicotine using delivery system.
Relapse Prevention Duration: The Relapse Prevention Duration is the time
during
which the delivery system is not used any more.
[0208] Still referring to Figure 10A, this graph shows the
starting dose of total
nicotine at the start of the quit journey as TNDsrAwr which is constant till
the end of the
Vigilance Duration. At the end of the Vigilance Duration to the end of the
Start Duration,
the total nicotine dose decreases at a constant rate to RNDEI #1. From the end
of the Start
Duration to the end of the Continuation Duration, the total nicotine dose is
further reduced
at a constant rate to RNDE2#2. From the end of the Continuation Duration to
the end of the
End Duration, the total nicotine dose is reduced at a constant rate to the
targeted low
nicotine dose, E. From the start to the end of the No Nicotine Duration, the
total nicotine
dose is maintained at the target level, E, and at the end of the duration, the
use of nicotine
is completely stopped. Then at the end of the Relapse Prevention Period, the
use of the
delivery system is stopped. This is followed by a software-based relapse
prevention period.
The delivery system is scheduled to be calibrated at different times: once at
the start of the
Switching Duration, once during the Switching Duration, once at the end of the
Vigilance
66
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
Duration, once at the end of the Start Duration, once at the end of the
Continuation
Duration, and once at the end of the End Duration,
[0209] Figure 10B illustrates the axis of the graphs
illustrating an example of
a dosage map specification. Each graph has three vertical axis and one
horizontal axis. The
horizontal axis shows the total cessation process duration periods 2-8
involving the delivery
system. The cessation process duration periods 2-8 involving the delivery
system can be
divided into 7 periods: Switch Duration, Vigilance Duration, T-Start Duration,
T-
Continuation Duration, T-End Duration, Low Nicotine Duration and Relapse
Prevention
Duration. The Switch Duration is the time during which patient starts
switching from
smoking cigarettes and v aping device to delivery system. The Vigilance
Duration is the
time during which patient only uses the delivery system in their usual
routine. The T-Start
Duration is the time during which the tapering starts using delivery system.
The T-
Continuation Duration is the time during which the patient undergoes tapering
continues
using delivery system. The T-End Duration is the time during which the
tapering stops
using delivery system. The Low Nicotine Duration is the time during which the
patient
maintains a low level of nicotine using delivery system. The Relapse
Prevention Duration
is the time during which no more nicotine is given. Figure 10B-1 illustrates
the first axis
on the top left side of the graph indicating the dose of monoprotonated
nicotine in
milligrams. The axis ranges from 3.0 to 0.0 mg. Figure 10B-2 illustrates the
second axis on
the bottom left side of the graph indicating the dose of freebase nicotine in
milligrams. The
axis ranges from 0.0 to 3.0 mg. Figure 10B-3 illustrates the third axis on the
right side of
the graph indicating the dimensionless ratio. This axis reads the
dimensionless values of
the Freebase Nicotine Ratio (FNR) and of the Enantiomeric Ratio. The axis
ranges from a
maximum value of 1.0 to a minimum value of 0Ø
[0210] Figure 10C is a graph illustrating the hypothetical
dosage map
specification of monoprotonated nicotine dose. Both the vertical and
horizontal axis are the
same as described in Figure 10B. In this hypothetical example the line 1001
shows how the
dose of monoprotonated nicotine varies from 2.0 mg to 0.0 mg over the total
cessation
process duration period 2-8 of using the delivery system. From the start of
the switching to
the use of delivery system to the end of the T-Start, the amount of
monoprotonated nicotine
was a constant dose of 2.0 mg. Then from the end of the T-Start to the end of
the T-
Continuation, the amount of monoprotonated nicotine declined at a constant
rate from 2.0
mg to 0.5 mg. From the end of the T-Continuation to the end of the T-End, the
amount of
monoprotonated nicotine was a constant dose of 0.5 mg. Then, from the end of
the T-End
67
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
to the end of the low nicotine period, the amount of monoprotonated nicotine
declined at a
constant rate from 0.5 mg to 0.0 mg. During the relapse prevention period, the
amount of
monoprotonated nicotine remained at 0.0 mg.
[0211] Figure 10D is a graph illustrating the hypothetical
dosage map
specification of freebase nicotine dose (FND). Both the vertical and
horizontal axis are the
same as described in Figure 10B. In this hypothetical example the dashed line
1002 shows
that the dose of freebase nicotine. In this graph, the dose of freebase
nicotine remained at a
constant of 0.8 mg during the entire total cessation process duration period 2-
8 of using the
delivery system.
[0212] Figure 10E is a graph illustrating an example of a
hypothetical dosage
map specification of total nicotine dose (TND) (i.e., the sum of
monoprotonated nicotine
and freebase nicotine). Both the vertical and horizontal axis are the same as
described in
Figure 10B. In this hypothetical example the dotted line 1001 shows the how
the dose of
monoprotonated nicotine varies from 2.0 mg to 0.0 mg and the dashed line 1002
shows
how the dose of freebase nicotine remained at a constant of 0.8 mg over the
total cessation
process of using the delivery system. From the start of the switching to the
use of delivery
system to the end of the T-Start, the TND was a constant dose of 2.8 mg. Then
from the
end of the T-Start to the end of the T-Continuation, the TND declined at a
constant rate
from 2.8 mg to 1.3 mg. From the end of the T-Continuation to the end of the T-
End, the
TND was a constant dose of 1.3 mg. Then, from the end of the T-End to the end
of the low
nicotine period, the TND declined at a constant rate from 1.3 mg to 0.8 mg.
During the
relapse prevention period, the TND remained at 0.8 mg.
[0213] Figure 1OF is a graph illustrating the hypothetical
dosage map
specification of freebase nicotine ratio (FNR) (ratio of (freebase nicotine
dose) / (total
nicotine dose)). This graph can be used to calculate the FNR of the patient
over the
cessation process duration of using the delivery system. Both the vertical and
horizontal
axis are the same as described in Figure 10B. The FNR value can be read from
the vertical
axis on the right side which gives dimensionless ratio. In this hypothetical
example the light
dotted line 1001 shows how the dose of monoprotonated nicotine varies from 2.0
mg to 0.0
mg. The light dashed line 1002 shows how the dose of freebase nicotine
remained at a
constant of 0.8 mg and the large dashed line 1003 shows how FNR varies from
0.29 to 1.0
over the total cessation process duration using the delivery system. During
the start of the
switching to the use of delivery system to the end of the T-Start, the FNR
equals to 0.29.
Then from the end of the T-Start to the end of the T-Continuation, the FNR
increased to
68
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
0.62 at a constant rate. From the end of the T-Continuation to the end of the
T-End, the
FNR remained constant at 0.62. Then, from the end of the T-End to the end of
the low
nicotine period, the increased to 1.0 at a constant rate. During the relapse
prevention period,
the FNR remained constant at 1Ø
[0214] Figure 10G is a graph illustrating the hypothetical
dosage map
specification of enantiomeric ratio (ratio of (S-nicotine) / (R-nicotine)).
This graph can be
used to show the Enantiomeric ratio, i.e., the ratio of the different
enantiomer of the nicotine
used by the patient over the total cessation process duration of using the
delivery system.
Both the vertical and horizontal axis are the same as described in Figure 10B.
The
Enantiomeric ratio value can be read from the vertical axis on the right side
which gives
dimensionless ratio. In this hypothetical example the light dot and dashed
line 1004 shows
how the Enantiomeric ratio remained at a constant of 0.99 over the total
cessation process
duration period of using the delivery system.
[0215] Figure 10H is a graph illustrating the hypothetical
dosage map
specification of variable aerosol droplet size (ADS). Both the vertical and
horizontal axis
are the same as described in Figure 10B. In this hypothetical example the
dotted line 1001
shows how the dose of monoprotonated nicotine varies from 2.0 mg to 0.0 mg and
the
dashed line 1002 shows how the dose of freebase nicotine remained at a
constant of 0.8 mg
over the total cessation process duration period of using the delivery system.
From the start
of the switching to the use of delivery system to the end of the T-
Continuation, the ADS is
less than or equal to 1.0 um as shown in the clear area between line 1001 of
the
monoprotonated and line 1002 of the freebase nicotine dose. Then from the end
of the T-
Continuation to the end of the relapse prevention period, the size of the ADS
increases to
great than or equal to 10.0 um as shown in the yellow area between the line
1001 of the
monoprotonated and line 1002 of the freebase nicotine dose.
102161 Figure 101 is a graph illustrating an example of a
hypothetical dosage
map specification of combined previous six taper variables shown as a function
of the quit
journey period, based on initial values of TND, FND and FNR, plus taper TND
reduction
targets. This is a graph which shows all the variables which are collected and
calculated to
reach the target of the reduced TND. Both the vertical and horizontal axis are
the same as
described in Figure 10B. In this hypothetical example line 1001 shows the
varying dose of
monoprotonated nicotine from 2.0 mg to 0.0 mg, line 1002 shows the constant
dose of
freebase nicotine at 0.8 mg, line 1003 shows varying FNR from 0.29 to 1.0 and
line 1004
shows constant Enantiomeric ratio remains at 0.99 over the total cessation
process duration
69
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
period 2-8 of using the delivery system. This graph indicates that with a
constant FND, as
the dose of monoprotonated nicotine decreases, the TND also decreases, but the
FNR
increases, and the enantiomeric ratio remains constant during the total
cessation process
duration period of using the delivery system.
[0217] Figure 10J is a graph illustrating the
representative dosage map
specification of a cigarette-smoking patient (Patient 1- Marlboro Red @
2.6mg/cig with
FNR=0.11, Taper regime Th.". (FNR constant)). Both the vertical and horizontal
axis are
the same as described in Figure 10B. In this example, in the beginning.
Patient 1 smokes a
typical full-flavor lit end cigarette which has a high dose of monoprotonated
nicotine,
MPDE, of 1.78 mg, relatively low free base nicotine, FNDE, of 0.22 mg and the
ADSE is
less than or equal to 1.0 [tm. For Patient 1, the FNRE has a constant value of
0.11 shown
by line 1003 and the enantiomeric ratio, ENM, has a constant value of 0.99
shown with line
1004, from start of the switching to the use of delivery system to the end of
the Low nicotine
period of using the delivery system. The TND is the area under lines 1001 and
1002. From
the start of the switching to the use of delivery system to the end of the
Vigilance Period,
the TND E is 2.0 mg. From the start of T-Start to the end of Low Nicotine
period, the TND
decreases from 2.0 mg and during the relapse prevention period, the TND is 0.0
mg.
Towards the end cessation process, Patient 1 has very low dose of nicotine,
maintains
freebase ratio for sensory response and the ADS is increased to reduce
absorption.
[0218] Figure 10K is a graph illustrating the
representative dosage map
specification of a vaping patient (Patient 2- Vaporesso XROS & Zen-Haus e-Liq
(4
17mg/mL with FNR=0.84, Taper regime TEND(FNR constant)). Both the vertical and
horizontal axis are the same as described in Figure 10B. In this example, in
the beginning,
Patient 2 smokes a typical vaping device which has a modest dose of
monoprotonated
nicotine, MPDE, of 0.25 mg, high free base nicotine, FNDE, of 0.99 mg and the
ADSE is
greater than or equal to 10.0 p.m. For Patient 2, the FNRE has a constant
value of 0.84 shown
by line 1003 and the enantiomeric ratio, ENM, has a constant value of 0.99
shown by line
1004, from start of the switching to the use of delivery system to the end of
the Low nicotine
period of using the delivery system. The TND is the area under lines 1001 and
1002. From
the start of the switching to the use of delivery system to the end of the
Vigilance Period,
the TNDE is 1.24 mg. From the start of T-Start to the end of Low Nicotine
period, the TND
decreases from 1.24 mg and during the relapse prevention period, the TND is
0.0 mg.
Towards the end cessation process, Patient 2 has very low dose of nicotine,
maintains
freebase ratio for sensory response and the ADS is kept same throughout the
process.
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
[0219] Figure 10L is a graph illustrating the
representative dosage map
specification of a cigarette-smoking patient (Patient 3- Winston Blue A
1.7mg/cig with
FNR=0.05, Taper regime TEND initial (FNR constant) TENR(FND constant)). Both
the
vertical and horizontal axis are the same as described in Figure 10B. In this
example,
initially the FNR is constant and later the FND is constant. In the beginning,
Patient 3
smokes a typical mild lit-end cigarette which has a moderate dose of
monoprotonated
nicotine, MPDE, of 1.78 mg, relatively low free base nicotine, FNDE, of 0.22
mg and the
ADSE is less than or equal to 1.0 p.m. For Patient 3, the enantiomeric ratio,
ENM, has a
constant value of 0.99 from start of the switching to the use of delivery
system to the end
of the Low nicotine period of using the delivery system. The TND is the area
under lines
1001 and 1002. From the start of the switching to the use of delivery system
to the end of
the Vigilance Period, the TNDE is 2.0 mg. From the start of T-Start to the end
of Low
Nicotine period, both monoprotonated and freebase nicotine dose decreases, and
the TND
decreases from 2.0 mg and during the relapse prevention period, the TND is 0.0
mg. The
FNRE has a constant value of 0.11 from start of the switching to the use of
delivery system
to the end of the T-Continue period. Then from the start to the end of the T-
End period,
FNR increases at a constant rate from 0.11 to 1.0 and remains constant from
the start to the
end of the low nicotine period. Towards the end cessation process, Patient 3
has very low
dose of nicotine, maintains freebase ratio for sensory response and for
maximum
bioavailability and the ADS is small for slow absorption.
Further Examples
[0220] This disclosure includes numerous examples of drug
delivery systems.
Such systems can be implemented with thermal or non-thermal aerosolizer
systems. In one
example, a drug delivery system, includes a housing having a distal end and a
proximal
end, a channel in the housing, the channel for receiving air, and an opening
for
communicating air to an aerosolizer pod coupled to the housing; an aperture on
the
proximal end of the housing configured to receive the aerosolizer pod therein.
In some
implementations, the housing configured to at least partially surround the
aerosolizer pod
when the aerosolizer pod is positioned in the housing. The system can include
a flow sensor
positioned to sense air flowing through the channel, a power source, and a
controller circuit
coupled to the power source, the controller circuit comprising a hardware
controller
coupled to the flow sensor, the aerosolizer system, and the rescue button, the
hardware
controller including a hardware processor and a non transitory computer
readable medium
71
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
in communication with the hardware controller, the computer readable medium
configured
to store dosage program information, and to store executable instructions
that, when
executed, configure the hardware controller to perform a dosage program that
includes
receiving input signals from the flow sensor and the rescue button, and
individually
controlling the aerosolizer system to provide aerosolizer generation signals
to control the
aerosol generator of the aerosolizer pod to generate an aerosol mixture based
at least on the
received input signals and the dosage program information. The delivery system
can further
include the aerosolizer pod, wherein the aerosolizer pod has a distal end and
a proximal
end, and includes an intake port on the distal end for receiving air flowing
through the
channel, an exhaust port on the proximal end for communicating the aerosol
mixture out of
the aerosolizer pod, and an aerosolizer system comprising a first, second, and
third
aerosolizer, the first, second, and third aerosolizers including an electrical
connection that
electrically couples to the first, second, and third aerosol drivers,
respectively, when the
aerosolizer pod is received into the housing. The first, second, and third
aerosolizers can
also include a mechanical aerosolizer The mechanical aerosolizer can include a
membrane.
The mechanical aerosolizer can be configured to vibrate the membrane. In some
examples,
the mechanical aerosolizer can include a piezo-electric mechanism to vibrate
the
membrane. The membrane can be positioned between the medicant in the container
and the
channel in the housing. When activated, the mechanical aerosolizer can use the
membrane
to turn a dose of the medicant into an aerosol that is inhalable by a user.
System Implementation
102211 Various embodiments of the present disclosure may
be a system, a
method, and/or a computer program product at any possible technical detail
level of
integration. The computer program product may include a computer readable
storage
medium (or mediums) having computer readable program instructions thereon for
causing
a processor to carry out aspects of the present disclosure. For example, the
functionality
described herein may be performed as software instructions are executed by,
and/or in
response to software instructions being executed by, one or more hardware
processors
and/or any other suitable computing devices. The software instructions and/or
other
executable code may be read from a computer readable storage medium (or
mediums).
Computer readable storage mediums may also be referred to herein as computer
readable
storage or computer readable storage devices.
72
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
[0222] A non-transitory computer readable storage medium
can be a tangible
device that can retain and store data and/or instructions for use by an
instruction execution
device. The computer readable storage medium may be, for example, but is not
limited to,
an electronic storage device, a magnetic storage device, an optical storage
device, an
electromagnetic storage device, a semiconductor storage device, or any
suitable
combination of the foregoing. A non-exhaustive list of more specific examples
of the
computer readable storage medium includes the following: a portable computer
diskette, a
hard disk, a solid state drive, a random access memory (RAM), a read-only
memory
(ROM), an erasable programmable read-only memory (EPROM or Flash memory), a
static
random access memory (SRAM), a portable compact disc read-only memory (CD-
ROM),
a digital versatile disk (DVD), a memory stick, a floppy disk, a mechanically
encoded
device such as punch-cards or raised structures in a groove having
instructions recorded
thereon, and any suitable combination of the foregoing. A computer readable
storage
medium, as used herein, is not to be construed as being transitory signals per
se, such as
radio waves or other freely propagating electromagnetic waves, electromagnetic
waves
propagating through a waveguide or other transmission media (e.g., light
pulses passing
through a fiber-optic cable), or electrical signals transmitted through a
wire.
[0223] Computer readable program instructions described
herein can be
downloaded to respective computing/processing devices from a computer readable
storage
medium or to an external computer or external storage device via a network,
for example,
the Internet, a local area network, a wide area network and/or a wireless
network. The
network may comprise copper transmission cables, optical transmission fibers,
wireless
transmission, routers, firewalls, switches, gateway computers and/or edge
servers. A
network adapter card or network interface in each computing/processing device
receives
computer readable program instructions from the network and forwards the
computer
readable program instructions for storage in a computer readable storage
medium within
the respective computing/processing device.
[0224] Computer readable program instructions (as also
referred to herein as,
for example, -code," -instructions," -module," -application," -software
application,"
and/or the like) for carrying out operations of the present disclosure may be
assembler
instructions, instruction-set-architecture (ISA) instructions, machine
instructions, machine
dependent instructions, microcode, firmware instructions, state-setting data,
configuration
data for integrated circuitry, or either source code or object code written in
any combination
of one or more programming languages, including an object oriented programming
73
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
language such as Java, C++, or the like, and procedural programming languages,
such as
the -C" programming language or similar programming languages. Computer
readable
program instructions may be callable from other instructions or from itself,
and/or may be
invoked in response to detected events or interrupts. Computer readable
program
instructions configured for execution on computing devices may be provided on
a computer
readable storage medium, and/or as a digital download (and may be originally
stored in a
compressed or installable format that requires installation, decompression or
decryption
prior to execution) that may then be stored on a computer readable storage
medium. Such
computer readable program instructions may be stored, partially or fully, on a
memory
device (e.g., a computer readable storage medium) of the executing computing
device, for
execution by the computing device. The computer readable program instructions
may
execute entirely on a user's computer (e.g., the executing computing device),
partly on the
user's computer, as a standalone software package, partly on the user's
computer and partly
on a remote computer or entirely on the remote computer or server. In the
latter scenario,
the remote computer may be connected to the user's computer through any type
of network,
including a local area network (LAN) or a wide area network (WAN), or the
connection
may be made to an external computer (for example, through the Internet using
an Internet
Service Provider). In some embodiments, electronic circuitry including, for
example,
programmable logic circuitry, field-programmable gate arrays (FPGA), or
programmable
logic arrays (PLA) may execute the computer readable program instructions by
utilizing
state information of the computer readable program instructions to personalize
the
electronic circuitry, in order to perform aspects of the present disclosure.
[0225] Aspects of the present disclosure are described
herein with reference to
flowchart illustrations and/or block diagrams of methods, apparatus (systems),
and
computer program products according to embodiments of the disclosure. It will
be
understood that each block of the flowchart illustrations and/or block
diagrams, and
combinations of blocks in the flowchart illustrations and/or block diagrams,
can be
implemented by computer readable program instructions.
[0226] These computer readable program instructions may be
provided to a
processor of a general purpose computer, special purpose computer, or other
programmable
data processing apparatus to produce a machine, such that the instructions,
which execute
via the processor of the computer or other programmable data processing
apparatus, create
means for implementing the functions/acts specified in the flowchart and/or
block diagram
block or blocks. These computer readable program instructions may also be
stored in a
74
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
computer readable storage medium that can direct a computer, a programmable
data
processing apparatus, and/or other devices to function in a particular manner,
such that the
computer readable storage medium having instructions stored therein comprises
an article
of manufacture including instructions which implement aspects of the
function/act
specified in the flowchart(s) and/or block diagram(s) block or blocks.
[0227] The computer readable program instructions may also
be loaded onto a
computer, other programmable data processing apparatus, or other device to
cause a series
of operational steps to be performed on the computer, other programmable
apparatus or
other device to produce a computer implemented process, such that the
instructions which
execute on the computer, other programmable apparatus, or other device
implement the
functions/acts specified in the flowchart and/or block diagram block or
blocks. For
example, the instructions may initially be carried on a magnetic disk or solid
state drive of
a remote computer. The remote computer may load the instructions and/or
modules into its
dynamic memory and send the instructions over a telephone, cable, or optical
line using a
modem. A modem local to a server computing system may receive the data on the
telephone/cable/optical line and use a converter device including the
appropriate circuitry
to place the data on a bus. The bus may carry the data to a memory, from which
a processor
may retrieve and execute the instructions. The instructions received by the
memory may
optionally be stored on a storage device (e.g., a solid state drive) either
before or after
execution by the computer processor.
[0228] The diagrams in the Figures illustrate the
architecture, functionality, and
operation of possible implementations of systems, methods, and computer
program
products according to various embodiments of the present disclosure. In this
regard, each
block in the flowchart or block diagrams may represent a module, segment, or
portion of
instructions, which comprises one or more executable instructions for
implementing the
specified logical function(s). In some alternative implementations, the
functions noted in
the blocks may occur out of the order noted in the Figures. For example, two
blocks shown
in succession may, in fact, be executed substantially concurrently, or the
blocks may
sometimes be executed in the reverse order, depending upon the functionality
involved. In
addition, certain blocks may be omitted in some implementations. The methods
and
processes described herein are also not limited to any particular sequence,
and the blocks
or states relating thereto can be performed in other sequences that are
appropriate.
[0229] It will also be noted that each block of the block
diagrams illustration,
and combinations of blocks in the block diagrams and/or flowchart
illustration, can be
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
implemented by special purpose hardware-based systems that perform the
specified
functions or acts or carry out combinations of special purpose hardware and
computer
instructions. For example, any of the processes, methods, algorithms,
elements, blocks,
applications, or other functionality (or portions of functionality) described
in the preceding
sections may be embodied in, and/or fully or partially automated via,
electronic hardware
such application-specific processors (e.g., application-specific integrated
circuits (ASICs)),
programmable processors (e.g., field programmable gate arrays (FPGAs)),
application-specific circuitry, and/or the like (any of which may also combine
custom
hard-wired logic, logic circuits, ASICs, FPGAs, etc. with custom
programming/execution
of software instructions to accomplish the techniques).
[0230] Any of the above-mentioned processors, and/or
devices incorporating
any of the above-mentioned processors, may be referred to herein as, for
example,
"computers," "computer devices," "computing devices," "hardware computing
devices,"
"hardware processors,- "processing units,- and/or the like. Computing devices
of the
above-embodiments may generally (but not necessarily) be controlled and/or
coordinated
by operating system software, such as Mac OS, i0S, Android, Chrome OS, Windows
OS
(e.g.. Windows XP, Windows Vista, Windows 7, Windows 8, Windows 10, Windows
Server, etc.), Windows CE, Unix, Linux, SunOS, Solaris, Blackberry OS,
VxWorks, or
other suitable operating systems. In other embodiments, the computing devices
may be
controlled by a proprietary operating system. Conventional operating systems
control and
schedule computer processes for execution, perform memory management, provide
file
system, networking, I/O services, and provide a user interface functionality,
such as a
graphical user interface ("GUI"), among other things.
[0231] As described above, in various embodiments certain
functionality may
be accessible by a user through a web-based viewer (such as a web browser), or
other
suitable software program). In such implementations, the user interface may be
generated
by a server computing system and transmitted to a web browser of the user
(e.g., running
on the user's computing system). Alternatively, data (e.g., user interface
data) necessary
for generating the user interface may be provided by the server computing
system to the
browser, where the user interface may be generated (e.g., the user interface
data may be
executed by a browser accessing a web service and may be configured to render
the user
interfaces based on the user interface data). The user may then interact with
the user
interface through the web-browser. User interfaces of certain implementations
may be
accessible through one or more dedicated software applications. In certain
embodiments,
76
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
one or more of the computing devices and/or systems of the disclosure may
include mobile
computing devices, and user interfaces may be accessible through such mobile
computing
devices (for example, smartphones and/or tablets). Many variations and
modifications may
be made to the above-described embodiments, the elements of which are to be
understood
as being among other acceptable examples. All such modifications and
variations are
intended to be included herein within the scope of this disclosure. The
foregoing description
details certain embodiments. It will be appreciated, however, that no matter
how detailed
the foregoing appears in text, the systems and methods can be practiced in
many ways. As
is also stated above, it should be noted that the use of particular
terminology when
describing certain features or aspects of the systems and methods should not
be taken to
imply that the terminology is being re-defined herein to be restricted to
including any
specific characteristics of the features or aspects of the systems and methods
with which
that terminology is associated.
[0232] Conditional language, such as, among others, -can,"
-could," -might,"
or "may," unless specifically stated otherwise, or otherwise understood within
the context
as used, is generally intended to convey that certain embodiments include,
while other
embodiments do not include, certain features, elements, and/or steps. Thus,
such
conditional language is not generally intended to imply that features,
elements and/or steps
are in any way required for one or more embodiments or that one or more
embodiments
necessarily include logic for deciding, with or without user input or
prompting, whether
these features, elements and/or steps are included or are to be performed in
any particular
embodiment.
[0233] The term "substantially" when used in conjunction
with the term
"real-time" forms a phrase that will be readily understood by a person of
ordinary skill in
the art. For example, it is readily understood that such language will include
speeds in
which no or little delay or waiting is discernible, or where such delay is
sufficiently short
so as not to be disruptive, irritating, or otherwise vexing to a user.
[0234] Conjunctive language such as the phrase "at least
one of X, Y, and Z,"
or -at least one of X, Y, or Z," unless specifically stated otherwise, is to
be understood with
the context as used in general to convey that an item, term, etc. may be
either X, Y, or Z,
or a combination thereof For example, the term -or- is used in its inclusive
sense (and not
in its exclusive sense) so that when used, for example, to connect a list of
elements, the
term "or" means one, some, or all of the elements in the list. Thus, such
conjunctive
77
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
language is not generally intended to imply that certain embodiments require
at least one
of X. at least one of Y, and at least one of Z to each be present.
[0235] The term "a" as used herein should be given an
inclusive rather than
exclusive interpretation. For example, unless specifically noted, the term "a"
should not be
understood to mean "exactly one- or -one and only one-; instead, the term "a-
means "one
or more" or "at least one," whether used in the claims or elsewhere in the
specification and
regardless of uses of quantifiers such as "at least one,- "one or more,- or "a
plurality"
elsewhere in the claims or specification.
[0236] The term "comprising" as used herein should be
given an inclusive
rather than exclusive interpretation. For example, a general purpose computer
comprising
one or more processors should not be interpreted as excluding other computer
components,
and may possibly include such components as memory, input/output devices,
and/or
network interfaces, among others.
Example Embodiments
[0237] Embodiment 1: A system, comprising: a delivery
device including a
housing; a channel in the housing, the channel structured to receive air from
an opening in
the housing and communicate air to an aerosolizer pod coupled to the delivery
device; a
flow sensor positioned to sense air flowing through the channel; a first,
second, and third
aerosolizer driver each having an electrical connection configured to
electrically couple to
a first, second, and third aerosolizer, respectively, of an aerosolizer pod
coupled to the
delivery device; and a controller circuit coupled to a power source, the
controller circuit
comprising a transceiver and a hardware controller electrically coupled to the
first, second,
and third aerosolizer drivers, and the flow sensor, the controller circuit
configured to
individually control the first, second, and third aerosolizer drivers to
provide aerosol
generation signals to a first, second and third aerosolizer to generate an
aerosol mixture
based at least in part on a treatment program received using the transceiver;
and a user
computing device comprising an application in communication with the delivery
system
via the transceiver.
[0238] Embodiment 2: The treatment program system of
embodiment 1, further
comprising a server system configured with a hardware processor and non-
transitory
computer readable storage media encoded with a treatment program including
instructions
executable by an operating system to control the generation of the aerosol
mixtures over
78
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
time according to the treatment program, and provide treatment program
information to the
delivery system to control the generation of the aerosol mixture by the
delivery system.
[0239] Embodiment 3: The system of embodiment 2, wherein
the treatment
program information provided to the delivery system includes information to
individually
control the first, second, and third aerosolizer drivers to provide signals to
a first, second
and third aerosolizer coupled to the first, second and third aerosolizer
drivers, respectively,
to generate a desired aerosol mixture of a first aerosol generated from a
first substance, a
second aerosol generated by a second substance, and a third aerosol generated
by a third
substance.
[0240] Embodiment 4: The system of any one of embodiments
1 - 3, wherein
the treatment program information provided to the delivery system includes
information to
individually control the first, second, and third aerosolizer drivers to
provide signals to a
first, second and third aerosolizer coupled to the first, second and third
aerosolizer drivers
to generate the first, second and third aerosol having an aerosol droplet of a
certain
di ameter.
[0241] Embodiment 5: The system of any one of embodiments
1 - 4, wherein
the treatment program information provided to the delivery system includes
information to
individually control the first, second, and third aerosolizer drivers to
provide signals to a
first, second and third aerosolizer coupled to the first, second and third
aerosolizer drivers
to generate the first, second and third aerosol having an aerosol droplet of a
first diameter
for a first portion of time and an aerosol droplet of a second diameter for a
second portion
of time.
[0242] Embodiment 6: A method for smoking cessation, the
method
comprising: providing a delivery system including: a delivery device including
a housing;
a channel in the housing, the channel structured to receive air from an
opening in the
housing and communicate air to an aerosolizer pod coupled to the delivery
device; a flow
sensor positioned to sense air flowing through the channel; a first, second,
and third
aerosolizer driver configured to electrically couple to a first, second, and
third aerosolizer,
respectively, of an aerosolizer pod coupled to the delivery device; a rescue
button
configured to, when actuated by a user, provide a signal indicative of the
user's need for an
additional dose of an aerosol mixture; a power source; and a controller
circuit coupled to
the power source, the controller circuit comprising a hardware controller
electrically
coupled to the first, second, and third aerosolizer drivers, the flow sensor,
and the rescue
button, the hardware controller including a hardware processor and a non-
transitory
79
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
computer readable medium in communication with the hardware controller, the
computer
readable medium configured to store treatment program information, and to
store
executable instructions that, when executed, configure the hardware controller
to
individually control the three aerosolizer drivers to provide aerosol
generation signals to
first, second and third aerosolizers, respectively, of a pod coupled to the
delivery device to
generate an aerosol mixture based at least in part on the stored treatment
program for
quitting smoking, and information that is received from the flow sensor and
the rescue
button; and an aerosolizer pod, the aerosolizer pod comprising: an aerosolizer
system
including the first, second, and third aerosolizers, wherein the pod is
structured to be
removably coupleable to the delivery device, each of the first, second, and
third aerosolizers
having an electrical connection that electrically couples to one of the first
aerosolizer,
second aerosolizer, and third aerosolizer drivers of the delivery device; a
first container
holding a first substance, a second container holding a second substance, and
a third
container holding a third substance, the first, second and third containers
structured to
provide the first, second and third substances to the first, second, and third
aerosolizers,
respectively, wherein the first substance is freebase nicotine and the second
substance is
monoprotonated nicotine; and generating aerosol mixtures in accordance with a
smoking
cessation treatment program to generate aerosol mixtures that are dynamically
changed
over a period of time to have different aerosol drop sizes and different
concentrations of
the first, second and third substances based at least in part on received
signals from the flow
sensor and the rescue button, and on smoking cessation treatment program
information
stored in the non-transitory computer readable medium.
[0243] Embodiment 7: A method for smoking cessation, the
method
comprising: providing signals, from a hardware controller in a hand-held
delivery device,
to a first, second and third aerosolizer driver in the delivery device to
dynamically control
a first, second and third aerosolizer in a pod coupled to the delivery device,
to generate
aerosol mixtures that are dynamically changed over a period of time to have
different
aerosol droplet sizes and different concentration of a first, second and third
substance in
containers of the pod based at least in part on received input signals from
one or more of a
flow sensor and smoking cessation treatment program information stored in a
non-transitory computer readable medium coupled to the hardware controller,
wherein the
method is executed by the controller executing computer executable
instructions stored on
the non-transitory computer readable medium, wherein the executable
instructions when
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
executed cause the hardware controller to providing signal to the first,
second, and third
aerosolizer drivers according to the smoking cessation program.
[0244] Embodiment 8: A delivery system for providing an
aerosol mixture in a
treatment program for quitting smoking, comprising: a pod comprising an
aerosolizer
system including a first, second, and third aerosolizers, and a first, second
and third
container in communication with the first, second, and third aerosolizer,
respectively, each
container holding a substance that is used to generate an aerosol mixture
according to the
treatment program; a delivery device, wherein the pod is removably coupleable
to the
delivery device, the delivery device including a housing; a channel structured
to receive air
from an opening in the housing and communicate air to an aerosolizer pod
coupled to the
delivery device; a flow sensor positioned to sense air flowing through the
channel; a first,
second, and third aerosolizer driver configured to electrically couple to the
first, second,
and third aerosolizer, respectively, of the pod when the pod is coupled to the
delivery
device; a rescue button configured to, when actuated by a user, provide a
signal indicative
of the user's need for an additional dose of an aerosol mixture; a power
source; and a
controller circuit coupled to the power source, the controller circuit
comprising a hardware
controller electrically coupled to the first, second, and third aerosolizer
drivers, the flow
sensor, and the rescue button, the hardware controller including a hardware
processor and
a non-transitory computer readable medium in communication with the hardware
controller, the computer readable medium configured to store treatment program
information, and to store executable instructions that, when executed,
configure the
hardware controller to individually control the three aerosolizer drivers to
provide aerosol
generation signals to the first, second and third aerosolizers, respectively,
to generate an
aerosol mixture based at least in part on the stored treatment program, and
information that
is received from the flow sensor and the rescue button.
102451 Embodiment 9: The delivery system of embodiment 8,
wherein the three
aerosolizers are thermal aerosolizers.
[0246] Embodiment 10: The delivery system of embodiment 8,
wherein the
three aerosolizers are mechanical aerosolizers.
102471 Embodiment 11: The delivery system of embodiment 8,
wherein the first
container contains freebase nicotine, and the second container contains
monoprotonated
nicotine.
[0248] Embodiment 12: A computer-implemented method for
providing a
treatment program for smoking cessation, the method comprising: generating a
smoking
81
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
cessation treatment program that includes a plurality of treatment periods
based on received
patient information, the patient information including a nicotine metabolic
rate;
communicating aerosol mixture information, based on the treatment program, to
a handheld
delivery system that includes three substances which are used to generate an
aerosol
mixture that is provided to the patient, the aerosol mixture information
indicating, for each
of the plurality of treatment periods, an amount of each of the three
substances to be
included in the aerosol mixture and the droplet size of the aerosol droplets
in the aerosol
mixture, wherein the method is performed by one or more computer hardware
processors
executing a plurality of computer readable instructions stored on a non-
transitory computer
memory.
[0249] Embodiment 13: The method of embodiment 12, further
comprising
generating, on the delivery system, the aerosol mixture based on the aerosol
mixture
information.
[0250] Embodiment 14 The method of any one of embodiments
12 or 13,
further comprising receiving usage information from the delivery system, and
communicating to the delivery system updated aerosol mixture information,
based at least
in part on the usage information.
[0251] Embodiment 15: A computer-implemented method for
providing
substances to a user, the method comprising: providing aerosol generation
information to a
hand-held delivery device that includes three aerosolizer drivers, wherein the
aerosol
generation information is based on a treatment program generated based on user
inputs and
test data of the user's nicotine metabolic rate (NMR); and generating
different aerosol
mixtures for inhalation by the user over a period of time by providing drive
signals from
three aerosolizer drivers to control three aerosolizes to produce the aerosol
mixtures having
a composition of three substances and to control the three aerosolizers to
produce aerosol
having a aerosol droplet diameter, according to the treatment program.
[0252] Embodiment 16: The method of embodiment 15, wherein
the aerosol
generation information is provided to the hand-held delivery device from a
user device.
[0253] Embodiment 17: The method of embodiment 16, wherein
the user
device is a mobile computer device.
102541 Embodiment 18. The method of embodiment 16, wherein
the user
device is a smart phone, tablet computer, or a laptop computer.
[0255] While the above detailed description has shown,
described, and pointed
out novel features as applied to various embodiments, it may be understood
that various
82
CA 03225939 2024- 1- 15
WO 2023/288022
PCT/US2022/037213
omissions, substitutions, and changes in the form and details of the devices
or processes
illustrated may be made without departing from the spirit of the disclosure.
As may be
recognized, certain embodiments of the inventions described herein may be
embodied
within a form that does not provide all of the features and benefits set forth
herein, as some
features may be used or practiced separately from others. The scope of certain
inventions
disclosed herein is indicated by the appended embodiments rather than by the
foregoing
description. All changes which come within the meaning and range of
equivalency of the
embodiments are to be embraced within their scope.
83
CA 03225939 2024- 1- 15