Note: Descriptions are shown in the official language in which they were submitted.
WO 2023/028366
PCT/US2022/041886
AUTOMATED SYSTEM FOR IMAGING, IDENTIFICATION, AND ISOLATION OF
ORGANOIDS
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims the benefit of U.S. Provisional Patent Application
Serial No.
63/237,781 filed August 27, 2021, the disclosure of which is incorporated
herein by reference
in its entirety. This application claims the benefit of U.S. Provisional
Patent Application Serial
No. 63/280,224 filed November 17, 2021, the disclosure of which is
incorporated herein by
reference in its entirety.
STATEMENT REGARDING FEDERALLY SPONSORED RESEARCH
This invention was made with government support under grant number
5R44ES032782
awarded by the National Institutes of Health. The government has certain
rights in the
invention.
BACKGROUND
For decades, two-dimensional (2D) cell culture models have been used to study
disease
and advance drug development, as cell lines are typically inexpensive and easy
to culture,
making them convenient for high-throughput analysis. However, establishing
cell lines, which
are often derived from tumors or immortalized, involves extensive genetic and
phenotypic
adaptation to the culture environment, which decreases their relevance to
normal cells and
ultimately reduces their applicability as a model system In addition, 2D
models lack important
spatial arrangement and cell-to-matrix interactions, further limiting their
predictive power.
This lack of translation of 2D cell culture models to in vivo outcomes
significantly impacts the
drug discovery pipeline, where the probability of success has been estimated
at only 13.8%.
While 2D models continue to provide significant value in research and
development programs,
there is considerable need for more advanced cellular models that provide
better physiological
relevance to tissues and organs.
There are several three-dimensional (3D) models that offer increased
complexity, and
subsequent physiological relevance, over 2D models, including co-cultures,
spheroids,
encapsulated cells, and organoids. Organoids are self-organizing three-
dimensional (3D)
structures that are grown from embryonic stem cells, induced pluripotent stem
cells (iPSCs),
- 1 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
or adult stem cells from humans and animal models alike. Organoids derived
from adult stem
cells, which are organ-constrained, and pluripotent stem cells (PSCs) have
seemingly infinite
expansion potential; and when the organoids are differentiated, they exhibit
tissue-specific
physiological and diseased states that make them a more relevant and
attractive in vitro model
than 2D single cell type monolayer cultures for developmental research, drug
discovery,
personalized medicine, and toxicological studies.
For 3D assembly, organoids require a source of extracellular matrix (ECM) to
serve as
a basement membrane. There are several commercially available basement
membrane extract
products, with Matrigel being one of the most commonly used. Traditional
culture methods of
organoids involve cells propagated between layers of or embedded in Matrigel
domes. These
culture methods are highly effective in supporting organoid assembly and
growth but present
several challenges in accurate assessment and throughput. First, standard
culture methods
result in a random arrangement of organoids in all three dimensions, with
multiple structures
per well that are frequently overlapping. This increases the number of focal
planes needed to
capture the population and requires advanced instrumentation and computational
methods for
complex 3D image-based analysis to resolve and assess each organoid. In
addition, while the
source materials for organoids are heterogeneous, which is advantageous in
capturing the
diversity of in vivo cellular material, traditional culture methods fail to
capture the
heterogeneity since responses are often homogenized across the population
within each well.
For these reasons, there is a need for new culture techniques and automated
instrumentation
that can more efficiently and accurately evaluate heterogenous organoid
populations through
single-organoid imaging and recovery. Newer consumable technologies have been
developed
that focus on multiple microwell positions that house individual organoids in
standard tissue
culture consumable formats; however, these technologies still require
extensive, manual
upstream effort and do not offer retri eval capabilities. Some recent technol
ogi es have leveraged
image-guided aspiration for retrieval of individual 3D structures, but these
tools have their own
shortcomings ¨ such as involving physical manipulation forces that can disturb
the structure of
organoids ¨ that limit their utility to destructive endpoint analysis.
One instrument system that enables high throughput imaging and retrieval of
single
cells in 2D cell culture is the CellRaft AIR System (Cell Microsystems, Inc.,
Durham, NC) and
see also, for example, PCT patent application publication no. WO 2018/097950.
The CellRaft
AIR System is a hardware and software system with a cell culture consumable
designed for 2D
- 2 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
adherent cell culture that enables automated identification and isolation of
single cells. The
CellRaft AIR System utilizes a microwell array comprising a formed,
elastomeric grid of
indentations or "wells", where the wells contain a releasable, optically
transparent,
microfabricated element, referred to as a "cellraft". These microarrays enable
the isolation of
cells in a viable and unperturbed state, while simultaneously providing a
culture environment
that replicates standard in vitro conditions in tissue culture dishes. The
micron-sized cellrafts
in the microarrays used in the CellRaft AIR System have a size of 100x100pm or
200x200 m
with three reservoir layouts having the cellrafts in either a single
reservoir, 4 separate
reservoirs, or 24 separate reservoirs. While the microarrays facilitate growth
of a wide range
of adherent and suspension cell types, the growth area for organoids on the
largest 200x20011m
cellraft format is limited. In the CellRaft AIR System, a cell sample of
interest is seeded on
the microwell array where the cells randomly distribute into microwells
following a Poisson-
like distribution. After imaging and identification, individual cells of
interest can be isolated
through a stress-free methodology that utilizes mechanical forces to release
the chosen cellraft
from its microwell without disturbing the attached cell layer and gently
transfers it to a 96-well
tissue culture or PCR plate using a magnetic wand. The process for release of
100pm and
200 p.m cellrafts from their microwells and collection to a separate plate has
been validated to
success rate of >95%. However, organoids are large structures grown in
extracellular matrix
rather than in liquid culture like 2D cells, and this presents challenges to
the automated culture,
image analysis, and collection of organoids.
Therefore, current methods for culture and analysis of organoids face
bottlenecks in
accurate assessment and throughput. The present disclosure provides a system
for automated,
high throughput assembly, growth, image analysis, and isolation of organoids.
SUMMARY
In one embodiment of the present invention, an automated method is provided
for
culturing, monitoring, and retrieving organoids. The method includes loading
an organoid
fragment suspension or a single cell suspension in a cell culture media that
includes a dilute
extracelluar matrix (ECM) at a temperature below the polymerization point of
the ECM into
the microwells of a microarray. The microwells of the microarray include a
releasable,
paramagnetic cellraft at the bottom of the microwell and the organoid
fragments or single cells
settle onto the surface of the cellrafts. The method includes placing the
microarray at a
- 3 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
temperature sufficient to cause the ECM to polymerize, and the organoid
fragments or single
cells become loosely attached to the cellrafts as a result of the ECM
polymerization. The
organoid fragments or single cells are cultured for a desired period to enable
the formation of
organoids. The method includes mounting, at one or more times, the microarray
onto an
instrument assembly of a system. The instrument assembly includes a microscope
objective
having a lens and an optical axis, a motorized release needle, and a motorized
magnetic
collection wand. The needle and the wand are aligned with the microscope
optical axis. The
system comprises: i) an imaging device that includes the microscope objective
and that is
configured for obtaining images of the forming or formed organoids on the
cellrafts within the
microwells of the microarray, ii) an actuator that is configured for
controlling the instrument
assembly to release a selected cellraft having an organoid of interest from
the microwell, and
iii) a computer system that includes at least one processor and memory, the
computer system
programmed for automated imaging of the forming or formed organoids and
release and
transfer of the selected cellraft having the organoid of interest to a
collection plate. The system
affects the automated imaging and release and transfer by: acquiring one or
more images of the
forming or formed organoids on the cellrafts within the microwells of the
microarray, including
in a z-axis, using the imaging device, identifying, by analyzing the one or
more images, one or
more selected cellrafts, and controlling the actuator to release the selected
cellraft from the
microarray by controlling the release needle to apply pushing energy to a
surface opposite the
microwell comprising the selected cellraft, and to deposit the released
cellraft into a mapped
location of a collection plate by controlling the magnetic collection wand.
The method includes
instructing, at one or more times, through a user interface with the computer
system, the
acquisition of one or more images of the forming or formed organoids on the
cellrafts and the
deposit of at least one selected cellraft having the organoid of interest into
the collection plate.
In some embodiments, the collection plate is a U-bottom 96-well plate, PCR
collection
plate, or PCR tube.
The microwells of the microarray, are at least about 75 p.m deep, have a width
of at
least about 400 vim, have cellrafts of at least about 400x400pm, and are
separated by walls
having an average width of at least about 25 p.m. In one embodiment, the
microwells of the
microarray are about 80nm deep, have a width of about 500 p.m, have cellrafts
of about
500x500pm, and are separated by walls having an average width of about 30 nm.
The
microarray can include 46x46 of the microwells in a single reservoir for the
cell culture media.
- 4 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
In one embodiment, the selected cellrafts are transferred to the collection
plate at 90%
efficiency.
In some embodiments, the ECM is Matrigel, UltiMatrix, Basement Membrane
Extract
Type II, or other matrices purified from animal-derived sources. The dilute
ECM can include
an ECM diluted to a final concentration of about 2%, 3%, 4%, 5%, 10%, 20%, or
30% or the
dilute ECM can range from about 0.24, 0.36, 0.48, 0.6, 1.2, 2.4, or about 3.6
mg/ml total
protein.
In one embodiment, the ECM is a xeno-free synthetic hydrogel, not derived from
animal sources. The dilute hydrogel can range from about 0.24, 0.36, 0.48,
0.6, 1.2, 2.4, or
about 3.6 mg/ml total protein.
The microarray can be mounted onto the instrument assembly of the system for
imaging
and/or release and transfer of one or more selected cellrafts containing an
organoid of interest
at one or more times including, but not limited to, 1 day, 2 days, 3 days, 4
days, 5 days, 6 days,
7 days, 2 weeks, 3 weeks, or 4 weeks, or more.
The automated method can include instructing, at one or more times, through a
user
interface with the computer system a calculation of one or both of diameter
and other
phenotypic parameters of the forming or formed organoids in the microarray.
The automated method can include exporting one or more of the acquired images,
wherein the acquired images include one or more z-stack images acquired in the
z-axis.
In some embodiments, one or more clonal organoids having a diameter ranging
from
200 1.im to lmm are formed in the microarray by culturing for the desired
period of time a
single cell of the single cell suspension loaded into one or more of the
microwells.
In some embodiments, the organoid fragment suspension or the single cell
suspension
loaded into the microarray comprises a gene edit or a gene mutation. The gene
edit can be a
CRISPR edit The single cell suspension can he from a patient derived cell or
tissue The patient
derived cell or tissue can have a known mutation.
In one embodiment, the organoid fragment suspension is generated from a parent
organoid, and the parent organoid is subcloned by culturing for the desired
period of time one
or more single fragments of the organoid fragment suspension in one or more of
the microwells
and instructing the acquisition of one or more images of the forming or formed
organoids on
the cellrafts and the deposit of at least one selected cellraft having the
organoid of interest into
the collection plate.
- 5 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
In one embodiment, the organoid of interest deposited into the collection
plate is
derived from a single cell of the single cell suspension loaded into the
microarray, and the
method further includes dissociating the deposited organoid of interest into
an organoid
fragment suspension and repeating the steps of loading, placing, mounting, and
instructing to
form and deposit one or more child organoids of interest into the collection
plate. The organoid
of interest and the one or more child organoids of interest can contain a gene
edit or a known
mutation.
In some embodiments, the single cell of the single cell suspension contains a
gene edit
or a known mutation and each of the deposited organoids of interest have the
gene edit or the
known mutation The gene edit can be a CRISPR edit
In some embodiments, the method further includes screening the forming or
formed
organoids for response to a drug or a molecule for a functional response.
In some embodiments, the method further includes extracting RNA from one or
more
of the forming or formed organoids for downstream gene expression or
transcriptomic analysis.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1A is an illustration of an example three-dimensional microwell array
of the
invention, where each microwell comprises a releasable cellraft for culturing
and retrieval of
organoids showing layout and dimensions of individual cellrafts and PDMS
walls.
Figure 1B is an illustration of an example three-dimensional microwell array
of the
invention, where each microwell comprises a releasable cellraft for culturing
and retrieval of
organoids showing a fully fabricated microarray.
Figure 1C is an illustration of an example three-dimensional microwell array
of the
invention, where each microwell comprises a releasable cellraft for culturing
and retrieval of
organoids showing an image of a cellraft after release from the automated
system of the
invention.
Figure 2A is an overview diagram of an exemplary system of the invention for
imaging
and collection of organoids.
Figure 2B is a block diagram of the computer system 102 shown in Figure 2A.
Figure 2C is a block diagram of the instrument assembly 104 shown in Figure
2A.
Figure 3A is an isometric view of an example implementation of the instrument
assembly 104 depicted in Figure 2C for organoid imaging and collection.
- 6 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
Figure 3B is an isometric view of an example implementation of the instrument
assembly 104 depicted in Figure 2C for organoid imaging and collection with an
inset
illustrating a concentric needle design.
Figure 4A is a series of images acquired using the automated system, where the
software includes automated z-stack acquisition in brightfield and 3-color
fluorescence for
organoids of interest. Mouse hepatic organoids were co-stained with a FITC-
conjugated
antibody for EpCAM and Hoechst 33342 to highlight cell membranes (green) and
nuclei (blue).
Using the automated system, z-stack images were taken every 10[tm through the
full height of
the organoid.
Figure 4B is an image showing one example of the system software of the
invention,
and companion OFF THE AIR data analysis software, which provides a user-
friendly, intuitive
interface to automatically acquire and explore z-stack images of organoids of
interest. After
the user defines the z-stack slice pitch and brightfield and fluorescence
exposures, the software
acquires the images across the full organoid height. After image acquisition,
the user can easily
view each image that was captured within the stack. The software reports the
organoid diameter
(width), allows the user to zoom in and out of each image to visualize the
organoid at single-
cell resolution, and provides tools to modify the relative contrast of each
imaging channel for
composite display. The software also allows the user an area to write in a
"description" of each
organoid of interest that was imaged, providing a complete data catalog of
individual
organoids.
Figure 4C is a series of images showing temporal imaging of mouse pancreatic
organoids on the microarray. Mouse pancreatic organoids were mechanically
dissociated into
small fragments and seeded on the microarray in dilute Matrigel media. Serial
scans of the
array were performed every 24 hours for 10 days to monitor organoid
development.
Figure 5A is a schematic of the system of the present disclosure that enables
automated
isolation and transfer of organoid-containing cellrafts from the microarray,
where first, the
release needle punctures the elastomeric floor of the microarray to dislodge
the cellraft from
its microwell, and next the collection wand, which houses a retractable
magnet, is lowered into
the microarray to collect the released cellraft, which is doped with
paramagnetic iron
nanoparti cl es .
- 7 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
Figure 5B is an illustration a zoomed in portion of Figure 3A, where the
collection
wand is inserted into the designated well of a 96-well plate or PCR tube while
the internal
magnet is retracted, allowing the cellraft to fall into the well.
Figure 5C is an illustration showing the original "off axis" needle of a
previous design
which relies on factory calibration to conduct prescribed "pokes" for cellraft
release.
Figure SD is an illustration showing the design of the automated system of the
present
disclosure where the concentric needle design aligns the release needle with
the objective,
enabling dynamic image-based guidance of the release needle (and magnetic
wand), which
increases the accuracy and speed of dislodging (and collecting) organoid-
containing cellrafts
in extrac el lul ar matrix
Figure SE is a series of images showing release of a cellraft using the
automated system
of the present disclosure
Figure SF is a series of images using the brightfield and fluorescence imaging
capabilities of the system, where mouse pancreatic organoids were assessed for
viability using
the ReadyProbes Blue/Green Cell Viability kit (Invitrogen). Individual
organoids are easily
imaged and assessed for viability, including necrotic cores.
Figure 6A illustrates an image on the left showing a traditional dome culture
method
for organoids that presents challenges in imaging and clonal propagation due
to random
arrangement of organoids in the x, y, and z dimensions in contrast to an image
on the right
showing organoids cultured according to the methods of the present disclosure
where the
organoids are organized in segregated microwells in a single focal plane on
the microarray.
Figure 6B illustrates that organoids cultured using the methods, microarray,
and
automated system of the present disclosure enable clonal propagation and
temporal monitoring
of clonal organoid development, with serial imaging, beginning 4 hours after
cell seeding (Day
0) on the microarray, cellrafts with single cells (green box), or small
clusters of cells (red
boxes), can be easily identified using the system software and tracked over
time
Figure 6C is a series of images of the microwells of the microarray taken over
8 days
illustrating that the system and methods of the present disclosure allows for
a complete data
record that verifies clonality.
Figure 7 is a schematic showing temporal imaging of the development of a mouse
hepatic organoid from a small fragment of cells for 10 days on the microarray,
where on day
- 8 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
10, the organoids on the microarray were stained with a FITC-conjugated
primary antibody for
EpCAM and imaged for phenotypic assessment.
Figure 8A is a schematic of images showing that organoids isolated from the
microarray and system of the present disclosure continue to grow post-
isolation and can be
used for downstream applications, including organoid sub-cloning.
Figure 8B is a schematic of images showing mouse hepatic organoids that were
isolated
as in Figure 6,8A and subsequently dissociated into single cells and seeded
onto a second
microarray and imaged every 24 hours for 8 days to monitor clonal organoid
development.
Specifically, clonal -parent" organoids were isolated into 96-well collection
plates, containing
dilute Matri gel media, using the automated system and allowed to grow for 5
additional days
off-array. In the 96-well collection plate, individual "parent" organoids were
enzymatically
dissociated, and the second-generation "child" cells were seeded onto a second
microarray for
clonal organoid propagation.
Figure 9 is a schematic showing that by using the system of the present
disclosure,
single organoids can be isolated from the microarray for downstream -omics
applications.
Mouse hepatic organoids, grown to various sizes (200-700p.m) on the
microarray, were isolated
into PCR strip tubes containing lysis buffer for RNA isolation. High quality
RNA was purified
from all organoids (RIN > 9.4) and RNA concentration was correlated to
organoid diameter.
Figure 10 is a flow chart of an example method for processing images of cell
rafts
depicting organoids.
Figure 11A is an example image of cell rafts depicting an organoid. Figure 11B
shows
the example image with histograms drawn alongside each of the X and Y axes.
Figure 11C
shows the example image with lines drawn between wall boundaries.
Figure 12A is a series of images of the microwells of the microarray taken
over 14 days
illustrating differentiation of RFP+ iPSCs into choroid plexus organoids
demonstrating that the
system and methods of the present disclosure allows for a complete data record
and phenotypic
monitoring of differentiation from pluripotent stem cells to 3D organoids.
Figure 12B is a series of images of the microwells of the microarray taken
over 12 days
illustrating differentiation of RFP+ iPSCs into kidney organoids demonstrating
that the system
and methods of the present disclosure allows for a complete data record and
phenotypic
monitoring of differentiation from pluripotent stem cells to 3D organoids.
- 9 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
Figure 13 is a series of images of the microwells of the microarray taken over
7 days
illustrating differentiation of RFP+ only, GFP+ only, and both RFP+ GFP+ iPSCs
into choroid
plexus organoids demonstrating that the system and methods of the present
disclosure allows
for co-culture of edited iPSCS and a complete data record that can track and
trace development
of co-cultured pluripotent stem cells to 3D organoids.
Figure 14A is a schematic of images showing that organoids, cultured,
analyzed, and
isolated using the presently disclosed system that are not selected for size
prior to isolation for
downstream compound-induced toxicity assays results in a heterogenous
population for
assessment.
Figure 14B is a schematic of images showing that the methods presently
disclosed for
culturing and CellRaft Cytometry can be used to select organoids for isolation
based on
diameter, which enables customized, single organoid assay development for
downstream
compound-induced toxicity that maintain inter-assay consistency.
Figure 15A is a graph demonstrating the system of the present disclosure can
be used
for growing, analyzing, and isolating single organoids for downstream compound-
induced
toxicity assays. The graph demonstrates that organoids unselected for size,
have large
variability in the relative kinetic viability (CellTox Green) and relative
terminal ATP (CellTiter
Glo) readouts of mouse hepatic organoids treated with a 6-point dose curve (n
= 5) of
acetaminophen (APAP).
Figure 15B is a graph demonstrating the system of the present disclosure can
be used
for building customized, single organoid assays for downstream compound-
induced toxicity
assays. The graph demonstrates that the automated system can be used to select
organoids
based on specific sizes to maintain consistency within the assay, which
translates to reduced
variability in the relative kinetic viability (CellTox Green) and relative
terminal ATP (CellTiter
Glo) readouts of mouse hepatic organoids treated with a 6-point dose curve (n
= 5) of
acetaminophen (AP AP).
Figure 16A is a screen shot of an example user interface.
Figure 16B is a screen shot of another example user interface.
Figure 16C is a flow diagram of an example method for creation of a
population.
Figure 17 shows an example user interface for illustrating population sets.
Figure 18 is a flow diagram of an example method for organoid detection.
- 10 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
Figures 19A ¨ 19D show examples of masks and outputs generated by organoid
segmentation.
DETAILED DESCRIPTION
Embodiments of the present invention now will be described more fully
hereinafter
with reference to the accompanying drawings, in which embodiments of the
invention are
shown. This invention may, however, be embodied in many different forms and
should not be
construed as limited to the embodiments set forth herein. Terminology used
herein is for the
purpose of describing particular embodiments only and is not intended to be
limiting of the
invention. As used herein, the singular forms "a", "an" and "the" are intended
to include the
plural forms as well, unless the context clearly indicates otherwise. It will
be further
understood that the terms "comprises" or "comprising," when used in this
specification, specify
the presence of stated features, steps, operations, elements, or components,
but do not preclude
the presence or addition of one or more other features, steps, operations,
elements, components,
or groups thereof. Additionally, comparative, quantitative terms such as
"above", "below",
"less", "more", are intended to encompass the concept of equality, thus,
"less" can mean not
only "less" in the strictest mathematical sense, but also, "less than or equal
to."
As used herein the term "about" refers to 10%.
The terms "comprises", "comprising", "includes", "including", "having" and
their
conjugates mean "including but not limited to".
The term "consisting of' means "including and limited to".
The term "consisting essentially of' means that the composition, method or
structure
may include additional ingredients, steps and/or parts, but only if the
additional ingredients,
steps and/or parts do not materially alter the basic and novel characteristics
of the claimed
composition m ethod or structure.
Throughout this application, various embodiments of this invention may be
presented
in a range format. It should be understood that the description in range
format is merely for
convenience and brevity and should not be construed as an inflexible
limitation on the scope
of the invention. Accordingly, the description of a range should be considered
to have
specifically disclosed all the possible subranges as well as individual
numerical values within
that range. For example, description of a range such as from 1 to 6 should be
considered to
have specifically disclosed subranges such. as from I. to 3, from I to 4, from
1 to 5, from .2 to
- 11 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
4, from 2 to 6, from 3 to 6 etc., as well as individual numbers within that
range, for example,
1, 2, 3, 4. 5, and 6. This applies regardless of the breadth of the range.
Unless otherwise defined, all terms (including technical and scientific terms)
used
herein have the same meaning as commonly understood by one of ordinary skill
in the art to
which this invention belongs. It will be further understood that terms used
herein should be
interpreted as having a meaning that is consistent with their meaning in the
context of this
specification and the relevant art and will not be interpreted in an idealized
or overly formal
sense unless expressly so defined herein. It will also be understood that when
an element is
referred to as being "connected" or "coupled" to another element, it can be
directly connected
or coupled to the other element or intervening elements may be present.
For the purpose of the specification and claims the terms "microscope optical
axis" and
"microscope imaging axis" are herein used interchangeably.
Traditional organoid culture methods are inadequate because they are low
throughput
and ill-suited for single organoid imaging, phenotypic assessment, and
isolation from
heterogenous organoid populations. In one embodiment the present invention
provides a
microwell array specifically designed for culturing organoids referred to
herein as a
"microarray" along with an instrument hardware and software system designed to
enable
automated imaging, identification, and isolation of individual organoids. The
microwells of
the microarray include a releasable cellraft that enables the automated
release and transfer of
selected organoids present on the cellrafts to a separate collection plate.
Organoids grown on
the microarray can be reliably tracked, imaged, and phenotypically analyzed by
the instrument
system in brightfield and fluorescence as they grow over time, then released
and transferred
fully intact for use in downstream applications. The use of the disclosed
system is
demonstrated using mouse hepatic and mouse pancreatic organoids for single-
organoid
imaging, clonal organoid generation, parent organoid subcloning, and single-
organoid RNA
extraction for downstream gene expression or transcriptomic analysis The
results validate the
ability of the system to facilitate efficient, user-friendly, and automated
workflows broadly
applicable to organoid research by overcoming several common bottlenecks: 1)
single organoid
time-course imaging and phenotypic assessment, 2) establishment of single cell-
derived
organoids, and 3) isolation and retrieval of single organoids for downstream
applications.
Embodiments of the present invention fulfill an unmet need for automated tools
that
are specialized for culture, imaging-based evaluation, and intact isolation of
individual
- 12 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
organoids. In the present invention, a system is provided including a
microarray consumable
that is specifically designed for organoid workflows.
Embodiments of the system of the present invention include a microarray
consumable
for establishing and tracking large, compartmentalized organoids in culture,
software with
capabilities for imaging and analysis of 3D organoid structures, and hardware
that enables fast
and high efficiency single organoid isolation. Extracellular matrix (ECM)
culture methods are
provided that can facilitate a reliable, user-friendly workflow for the
development and
evaluation of hundreds of individual organoids on a single microarray culture
consumable.
Proof-of-principle experiments provided in Examples 1-7 demonstrate the
utility of
embodiments of the invention for high-quality brightfi el d and fluorescence
imaging and
temporal assessment of individual organoids, establishing single-cell derived
organoids, and
isolating individual organoids for downstream applications, such as subcloning
and -omics.
These workflows and their endpoints continue to increase in prevalence and
value in
developmental biology, drug discovery, personalized medicine, and toxicology,
demonstrating
the broad potential of these methods and systems to advance organoid research.
The utility of organoids in bridging the gap between traditional 2D in vitro
assays and
clinical applications is clear due to their ability to recapitulate key
aspects of in vivo organs.
However, traditional methods of organoid culture present challenges in
throughput, phenotypic
assessment via imaging, and recovery of intact, viable organoids for
downstream expansion
and analysis. The system of the present invention can fill the unmet needs of
organoid
workflows by enabling user-friendly organoid protocols that offer greater
throughput, temporal
image acquisition, and data cataloging of individual organoids, while also
providing automated
isolation and transfer of individual organoids to a collection plate.
To that end, the presently disclosed system includes a microarray tissue
culture
consumable that can support large organoid growth and hardware and software
that can allow
for more advanced 3D phenotypic characterization and decision-based automated
isolation. In
addition, methods are provided that can be widely applicable for using
organoids or other 3D
culture models in research and development. The experiments provided herein
including in
Examples 1-7 have demonstrated the use of the automated system including the
microarray for
phenotypic characterization of organoids using high-quality brightfield and
fluorescent
imaging, including z-sectioning, as well as the isolation and transfer of
organoids of interest
- 13 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
into 96-well tissue culture and PCR plates for downstream growth, expansion,
or -omics
applications.
The system provided herein is uniquely suited to address the bottlenecks and
inefficiencies of using standard consumables for organoid culture, including:
1) the manual
manipulation of cellular material in ECM, 2) challenges in image acquisition
and analysis due
to multiple, overlapping structures per well, and 3) the inability to collect
individual organoids
of interest for further analysis.
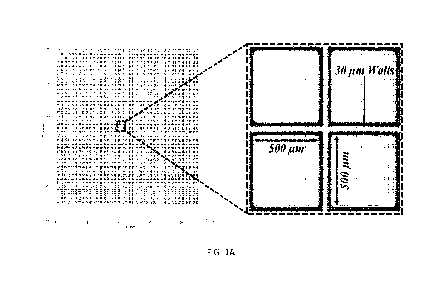
The largest microwell array for use with the CellRaft Air System sold by Cell
Microsystems, Inc. has a cellraft size of 200x200gm The growth area for
organoids on the
200x200gm cellraft format is limited. Therefore, in one embodiment of the
present invention,
a microarray is provided having cellrafts of 500x500gm size to support the
growth of 3D
structures up to lmm in diameter, separated by 301.tm walls to deter
nonspecific binding and
undesired growth of single cells/organoid fragments off the cellraft (Figure
1A). The
microwells of the microarray having cellrafts 500x500pm in size have a width
of about 500
gm and are at least about 75 p.m deep. The microwell array comprises a formed,
elastomeric
grid of wells, where the wells contain a releasable, optically transparent,
microfabricated
element, the cellraft. The microarray enables the isolation of organoids in a
viable and
unperturbed state, while simultaneously providing a culture environment that
replicates
standard in vitro conditions in tissue culture dishes. An exemplary microarray
comprises 46x46
cellrafts of 500x500gm size in a single reservoir, yielding more than 2,100
cellraft positions
for segregated organoid growth and the potential to interrogate, characterize,
and recover as
many organoids from a single microarray consumable as seeding twenty-two 96-
well or six
384-well plates. Fabrication of an exemplary microarray is described in
Example 1.
In another embodiment, the microwells of the microarray are at least about 75
gm deep,
have a width of at least about 400 gm, have cellrafts of at least about
400x400gm size and are
separated by walls having an average width of at least about 25 gm.
The microarray can be attached to a polystyrene cassette that provides
exterior borders
of a contiguous media reservoir that facilitates a highly viable culture
environment (Figure
1B). The polystyrene cassette can be a 65mm-diameter injection molded
polystyrene cassette.
When housed in an adapter plate on the instrument hardware of the automated
system, each
cassette is within ANSI/SLAS tolerance ranges for height, width, and length to
ensure
compatibility with standard microscopy equipment, liquid handlers, and the
automated system.
- 14 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
Because establishment of organoids, and organoid assay times, are generally
extended
compared to 2D culture methods, the present inventors evaluated the integrity
of the
microarrays after 4 weeks of culture time to mimic use-case scenarios. At the
end of the test
period, no leaks or structural disruptions nor negative impact on cellraft
releasability were
observed (Figure IC), validating the robustness of the microarray for 3D
culture.
In one embodiment, the automated system of invention enables z-stack image
acquisition and analysis. In one example, cells are seeded onto the
microarray, and the system
software, and its companion Off The Air data analysis software (Cell
Microsystems, Inc.,
Durham, NC), provide a user-friendly interface to power the automated imaging,
identification,
and isolation of organoid-containing cellrafts. While most of the software
algorithms and code
from the CellRaft AIR System were transferrable to incorporate the larger
microarray format,
the previous software did not include z-stack imaging functionality desired
for 3D workflows,
as its original purpose was to scan and image 2D cells attached to the
cellraft surface. After
performing a standard, single-plane scan of the microarray, the user can now
select organoids
of interest for z-stack acquisition and specify the slice thickness that the
automated system uses
as it acquires brightfield and fluorescence images throughout the full height
of the selected
organoid.
A description of embodiments of the system of the invention is provided with
reference
to Figures 2A-2C and Figures 3A-3B. Figures 2A-2C are diagrams of an example
system 100
for organoid imaging, and collection. The system 100 can be used to identify
and collect
cellrafts having organoids embedded in extracellular matrix that is loosely
attached to the
surface of the cellraft. Figure 2A is an overview diagram of the system 100.
The system 100
includes a computer system 102, an instrument assembly 104, an experimental
environment
106 (e.g., one or more pieces of laboratory equipment such as power supplies
and
environmental control systems), and a user 108 The instrument assembly 104
includes an
optional adapter plate for receiving a microarray 112 and a collection plate
114 for receiving
cellrafts that have been selected and released from the microarray 112. The
collection plate
114 can be organized into a standardized format, e.g., as an SBS collection
plate. Although a
collection plate 114 is shown, the system 100 can alternatively use any
appropriate collection
structure, such as PCR strip tubes.
Typically, the user 108 would load an organoid fragment suspension or a single
cell
suspension in a cell culture media comprising a dilute extracelluar matrix
(ECM) at a
- 15 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
temperature below the polymerization point of the ECM into the microwells of a
microarray
112 and allow the organoid fragments or cells to settle onto individual
cellrafts. After the
organoid fragments or cells settle onto the cellrafts, the microarray is
placed at a temperature
sufficient to cause the ECM to polymerize, and the organoid fragments or
single cells become
loosely attached to the cellrafts as a result of the ECM polymerization. The
organoid fragments
or single cells are cultured for a desired period of time to enable organoid
formation. The
microarray 112 is then placed into the adapter plate 110 of the system 100 for
scanning and
image analysis. The scanning and image analysis can take place at any number
of times over
the entire course of the culture period for organoid formation. The user can
instruct the system
100 to release a cellraft from the microarray 112 by controlling and using an
actuator and to
collect the cellraft with the isolated organoid using a magnet. For example,
the actuator can be
one or more motors configured to move a needle or similar device to release
cellrafts. In some
examples, the system 100 includes multiple actuators, including, possibly
another actuator to
move a magnetic wand, and possibly actuators to move a stage, imaging optics,
and other
mechanical parts of the system.
Figure 2B is a block diagram of the computer system 102. The computer system
102
includes at least one processor 120, memory 122, a controller 124 implemented
as a computer
program using the processor 120 and memory 122, and a graphical user interface
(GUI) 126.
For example, the computer system 102 can be a desktop computer with a monitor
and keyboard
and mouse, or the computer system 102 can be a laptop or tablet computer or
any other
appropriate device. The computer system 102 is operatively coupled to the
instrument
assembly 104, e.g., by universal serial bus (USB) cables.
The controller 124 is programmed for obtaining one or more images of the
forming or
formed organoids on the cellrafts within the microarray 112; identifying, by
analyzing the
images, a selected cellraft having a forming or formed organoid of interest;
and controlling the
instrument assembly 104 to release the selected cellraft carrying the forming
or formed
organoid of interest. The GUI 126 is configured to present various control and
results screens
to the user 108 and to receive input from the user 108.
Figure 2C is a block diagram of the instrument assembly 104. The instrument
assembly 104 can include various components for imaging the individual
cellrafts 130 having
the forming or formed organoids loosely attached on the surface and
selectively releasing the
cellrafts 130 having a forming or formed organoid of interest from the
microarray for
- 16 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
placement into the collection plate 114. For example, the instrument assembly
104 can include
a power breakout board 138 and various control boards for controlling motors
and actuators
(e.g., PS3 control board 132, PS3 XYZ control board 134, and PS3 FILTER
control board 136).
The motor control boards can contain TTL and shutter functions that allow the
controller 124
to control or address various components of the instrument assembly 104.
The instrument assembly 104 can include a digital camera 140 or other
appropriate
imaging device, a communications hub (e.g., USB Hub 142), a fluorescence light
emitting
diode (LED) engine 144, and a light guide adapter 146. The fluorescence LED
engine 144
can include multiple narrow-band LEDs configured to illuminate the microarray
112 by way
of the light guide adapter 146
The instrument assembly 104 includes a microscope system (e.g., an internal
inverted
digital microscope) including a motorized XY stage 148 and an autofocus motor
150
configured for translating a microscope objective 152. Typically, the camera
140 and the
fluorescence LED engine 144 and microscope system are arranged in an epi-
fluorescence
configuration. The
microscope system includes a release needle 154 configured for
individually releasing cellrafts 130 from the microarray 112. The release
needle 154 can be
actuated by the autofocus motor 150.
In some examples, the microscope system supports imaging of a region on a
microarray
having 46 x 46 cellrafts of 500 micron x 500 micron in dimension in a single
reservoir, at a
resolution of less than 2 microns per pixel in a given field of view. The
microscope system
may also support the capture of images using brightfield imaging (i.e. white
light illumination
and white light emission) and the capture of images in one or more fluorescent
emission
channels. In some examples, the instrument assembly 104 is capable of scanning
an entire
microarray in under 20 minutes for all three fluorescent channels and
brightfield assuming an
exposure time 750 ms across all channels
The release needle 154 typically comprises materials resistant to oxidation
when
exposed to saline or cell culture media. In some examples, the release needle
154 is a stainless
steel 100 micron needle. The release needle 154 can have a possible travel
distance of, e.g., at
least 15 mm in the X and Y directions with respect to the center of the
microarray 112.
The instrument assembly 104 includes a gantry assembly including a belt drive
156 for
moving the gantry assembly, a brightfield LED 158 for illuminating the
microarray 112 during
imaging, and a linear actuator 160 configured for moving a magnetic wand 162
to collect
- 17 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
cellrafts carrying a forming or formed organoid after release. The gantry
assembly can
alternatively use a lead screw instead of a belt drive, or any other
appropriate motor. The linear
actuator 160 can be, e.g., a stepper motor configured to raise and lower the
magnetic wand 162
into and out of the microarray 112 and the collection plate 104.
The instrument assembly 104 includes a collection magnet 164 positioned
underneath
the collection plate 114 to collect cellrafts into the collection plate 114
from the magnetic wand
162. The collection magnet 164 can have a polarization opposite that of the
magnetic wand
162 to repel the magnets within the magnetic wand 162 and pull the cellraft to
the bottom of
the collection plate 104. The magnetic wand 162 typically comprises a material
that is capable
of being rendered sterile (e.g., rinsed with ethanol or isopropanol while
removed from the
instrument) so as not to contaminate the released cellraft or the media used
in the collection
plate 104. The material for the magnetic wand 162 is also generally selected
such that contact
with the culture media does not cause any detectable decrement in cell
viability or proliferation,
or in the performance of molecular biology reagents, such as Tag polymerase,
or reverse
transcriptase.
Figures 3A-3B are isometric views of an example apparatus for organoid imaging
and
collection. The apparatus is an example implementation of the instrument
assembly 104
depicted in Figure 2C. As shown in Figure 3A, the adapter plate 110 and the
collection plate
114 are positioned on top of the horizontal XY stage 148. Some components,
such as the
electronic control boards 132, 134, and 136 are located below the XY stage
148. The XY stage
148 is configured to move the microarray 112 and the collection plate 104. The
XY stage 148
is electronically controllable for positioning cellrafts for imaging the
organoid structures
contained on the surface (aligning cellrafts with the microscope objective
152), releasing
selected cellrafts carrying the organoid structures of interest (aligning
cellrafts with the release
needle 154), and depositing the selected cellrafts with organoid cargo
(aligning the magnetic
wand 162 and selected locations of the collection plate 104 over the
collection magnet 164)
The gantry assembly, including the belt drive 156, is positioned vertically
over the XY
stage 148. The gantry assembly is configured to move laterally to position the
brightfield LED
158 for imaging and also to position the magnetic wand 162. The gantry
assembly positions
the magnetic wand 162 over the microarray 112 to collect cellrafts during
release, and then the
gantry assembly positions the magnetic wand 162 over the collection plate 114
to deposit
cellrafts into selected locations of the collection plate 114.
- 18 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
The camera 140 and the autofocus motor 150 are located beneath the XY stage
148,
e.g., so that the autofocus motor 150 can move vertically with respect to the
XY stage 148.
The fluorescence LED engine 144 and liquid light guide ports 146 are located
below the XY
stage 148 and coupled to a fluorescence filter cube 170. The fluorescence
filter cube 170 is
configured for fluorescence imaging, e.g., to allow light from the
fluorescence LED engine 144
to reach the microarray 112 and to block that light from reaching the camera
140.
Figure 3B shows a cut-away view 172 of the microscope objective 152 and the
release
needle 154. As shown, the release needle 154 is located within the field of
view of the
microscope objective 152. Locating the release needle 154 in the field of view
of the
microscope objective 152 enables visualization of the release of a cellraft
with organoid cargo
in real time, which is required to accurately position the release needle for
dislodgement of the
larger cellraft carrying an organoid structure embedded in ECM from the
elastomeric substrate
of the microwell. However, this location of the release needle in alignment
with the microscope
optical axis can require imaging through a window 174 of material, such as
acrylic, that is
transparent and can be machined to mount the release needle 154. This can
reduce the
transmission of the excitation and emission light and require longer
integration times during
scanning. In addition, the magnetic wand 162 is positioned above the target
cellraft during
release to affect more rapid collection of the cellrafts, however, in this
geometry, the lateral
position of the brightfield LED 158 is offset from the microscope objective
152 and the
magnetic wand 162 casts a shadow of its light. To solve these issues, the
assembly comprising
the release needle 154 as illustrated in Figure 3B can include an annular
printed circuit board
176 containing light-emitting diodes 178 and resistors 180 to provide epi-
illumination of the
microarray and cellraft targeted for release. The light from the diodes 178
travels upward
through the microarray 112 and is reflected by the tip of the magnetic wand
162 ¨ positioned
inside the fluid within the reservoir of the microarray 112¨ to create pseudo-
transilluminati on
of the cellraft as it is released.
It can be useful to calibrate the offset between the center of the field of
view of the
microscope objective 152 and the puncture location of the release needle 154
on the microarray
112. Calibration can be performed, e.g., after every needle replacement, or at
the start of every
experiment, or one time during manufacturing. In some examples, the controller
124 of Figure
2B is programmed to perform automated calibration.
- 19 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
For example, the controller 124 can move the microarray 112 to position the
field of
view of the microscope objective 152 with a microarray border, autofocus the
microscope
objective, and then puncture the microarray border with the release needle
154. Then, the
controller 124 acquires an image (e.g., using the brightfield LED 158) and
analyzes the image
to locate the puncture position, e.g., by segmenting the image. The controller
124 can then
calculate an offset. In some examples, the controller 124 repeats the process
a specified number
of times by moving to different locations and determines a calibration
distance based on the
offset positions, e.g., by averaging the offset positions. In some other
examples, the controller
124 can move a microarray 112 that does not contain cellrafts to position the
field of view of
the microscope objective 152 with a microwell in the center of the microarray
112, autofocus
the microscope objective, and then puncture the microarray with the release
needle 154. Then,
the controller 124 acquires an image (e.g., using the brightfield LED 158) and
analyzes the
image to locate the puncture position, e.g. by segmenting the image, or
prompts the user to
locate the puncture position, e.g. by clicking on a display of the image. The
controller 124 can
then calculate an offset. In some examples, the controller 124 repeats the
process a specified
number of times by moving to different microwells and determines a calibration
distance based
on the offset positions, e.g., by averaging the offset positions.
To test the image quality of brightfield and widefield fluorescence z-stacks
acquired by
the automated system, two 200-300pm mouse hepatic organoids were selected on a
microarray
stained with Hoechst and EpCAM (primary antibody conjugated with FITC) for
imaging (as
described in Example 2). Brightfield, blue fluorescence (exposure = 50, 50ms),
and green
fluorescence (exposure = 200, 100ms) images were acquired every 101,tm across
focal ranges
that encompassed the full height of the two organoids (24 and 30 images,
respectively). The
brightfield and false-colored fluorescence images (Figure 4A) demonstrate
excellent image
quality with the ability to visualize individual cells, cell junctions
(green), and substructures
within nuclei (blue), including mitotic figures.
To leverage the phenotypic content in the z-stack images, 1) an intuitive user
interface
within the software is provided for users to visually explore each image stack
through
composite brightfield and fluorescence display (Figure 4B); and 2) automated
algorithms are
provided to measure organoid diameter and other morphologic and phenotypic
parameters to
identify and group organoids based on size or other metrics, whether for
phenotypic assessment
or isolation thresholds. This includes assessment of large organoids (> 500 m)
that overgrow
-20 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
the cellraft footprint and require image processing into neighboring
cellrafts. Z-stack images
can be additionally exported for display and manipulation using external
software. This
combination of expanded software capability allows for automated phenotypic
characterization
of individual organoids that is not possible using standard organoid culture
methods and
imaging platforms, with the option to designate any organoid-containing
cellraft of interest for
isolation from multiple screens within the software.
Embodiments of the automated system of the invention provide image-driven
cellraft
isolation. In one example, after performing automated imaging using the
system, the user can
identify forming or formed organoids of interest and designate cellrafts for
isolation using a
variety of software-guided or manual selection tools. Once selected, a
cellraft is dislodged
from its microwell by a motorized release needle that penetrates the
elastomeric bed of the
microarray (Figure 5A) and, because of paramagnetism in the exemplary case of
a cellraft
doped with iron nanoparticles, collected on the tip of a magnetic wand. The
system then aligns
and inserts the wand into the designated well of a 96-well tissue culture or
PCR plate, while
retracting its internal magnet, to deposit the cellraft carrying the forming
or formed organoid
in the collection plate (Figure 5B). The process is repeated for each cellraft
selected by the
user for isolation, one cellraft (with its attached organoid structure) per
collection well.
With the previous CellRaft AIR System, the process has been validated (>95%
success
rate) to release 1001am and 2001.tm cellrafts in liquid culture media from
their microwells using
a regimented 2-poke pattern and then to collect them with the magnetic wand
positioned up to
5mm away. With the previous CellRaft AIR System, the prescribed poke locations
and large
attraction distance have allowed cellraft isolations to be conducted "off
axis" from the
microscope imaging path (Figure 5C), relying on system calibration between the
microscope,
needle, and wand to align the cellraft of interest for release and collection.
Release of cellrafts larger than about 400ttm, e.g., 5001.tm cellrafts, from
the microarray
and collection through an extracellular matrix are significantly more
challenging than in liquid
cell culture. Release from the microarray requires a more targeted needle poke
to release
cellraft corners that remain engaged with the elastomeric microwell and much
closer proximity
(0-1mm) of the magnetic wand tip to the cellraft to overcome the ECM
viscosity. To address
these challenges, a "concentric" release needle design (Figure 5D) was
developed and
validated to align the release needle and collection wand with the microscope
imaging axis,
which facilitates real-time imaging of cellraft release and three-dimensional
alignment of the
-21 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
collection wand tip to the target cellraft. Imaging data is instantly analyzed
to achieve and
detect cellraft release (Figure 5E) after 1-4 targeted needle pokes and to
dynamically control
the height, lateral position, and dwell time of the magnetic wand tip to
achieve cellraft
collection. The concentric design of the system hardware, paired with the
system software that
performs image-based decisions, yields faster organoid isolation with a higher
success rate than
the previous "off axis" CellRaft AIR System.
In some embodiments of the invention, a number of organoid workflows are
enabled
by the microarray and automated system including clonal identification and
temporal
phenotypic assessment of organoids on the microarray. Traditional culture
methods of
organoids in semi-solid basement membrane extract (BME) dome, such as
Matrigel, result in
random arrangement in the x, y, and z dimensions, making imaging challenging
due to
overlapping 3D structures and multi-focal imaging requirements. In addition,
the random
arrangement of cells in a BME dome does not permit clonal organoid growth or
temporal
growth assessment of individual organoids (Figure 6A). The larger microarray
of the
invention, e.g., the exemplary 500 x 500 pm microarray, which provides
segregated microwell
positions that have unique IDs, and a revised seeding protocol (see Example
2), which
facilitates alignment of organoids onto the predictable z-plane of the
microwells, overcome this
bottleneck of traditional organoid culture methods. The system in combination
with the
exemplary 46X46 cellraft microarray provides an automated solution to
temporally image more
than 2,100 available cellraft, or organoid, positions on a single cell culture
consumable. In one
embodiment, images captured for each cellraft are automatically stored in
system software
providing a complete, easily viewable data record for each organoid.
Using both mouse pancreatic (Figure 4C) and mouse hepatic organoids, a robust
and
reliable method is demonstrated for obtaining high-quality, time-course images
of developing
organoids on the microarray (see Example 3) In addition, the methods described
demonstrate
the ability to monitor differentiation of pluripotent stem cells into
organoids (Figures 12 and
13) of a variety of tissue types, including kidney, choroid plexus, cerebral
(see Example 5). A
dynamic growth record of each organoid can be maintained during the entire
development
process from single cell to isolation. In one example, beginning with an array
scan shortly after
cell seeding (i.e., loading of an organoid fragment or single cell suspension
onto the
microarray) (4 hours), the user can identify cellrafts with single or multiple
cells either
manually or by using a CellRaft Cytometry tool (Cell Microsystems, Inc.,
Durham, NC)
-22 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
(Figure 6B) The ability to reliably image the forming or formed organoid on
each cellraft in
every field of view on the microarray can enable clonal organoid workflows
that are not
currently possible using standard culture methods and imaging tools.
Subsequent serial scans
of the microarray can then be initiated by the user at desired time intervals
to capture temporal
images of organoid development (Figure 6C, Figure 7). The system can acquire a
full array
scan in brightfield and three fluorescent channels in under 15 minutes (under
9 minutes for
brightfield only), providing a rapid solution for multiparametcr phenotypic
and morphologic
screening of hundreds of individual organoids.
In addition to brightfield imaging, the system can perform advanced
fluorescence-based
phenotypic assessment for a variety of applications, including, but not
limited to, live cell
staining, CRISPR editing, and on-array viability assays (Figure 5F) For
example, mouse
hepatic organoids stained with FITC-conjugated antibody for EpCAM demonstrate
detailed
visualization of cell membranes (Figure 4A, Figure 7) and Hoechst-stained
nuclei enable cell
counting throughout the z-stack (Figure 4A). Such temporal brightfield and
fluorescence
imaging that can be applied to hundreds of individual organoids on a single
microarray using
the system represents a significant advancement over current methods that
require dozens of
standard cell culture consumables, expensive imaging platforms, and extensive
manual
upstream and downstream effort by the user.
In various embodiments, organoids are isolated from the microarray for
downstream
assays, growth and subcloning, and -omics applications. In addition to the
issue of multifocal
imaging requirements, traditional organoid culture methods are susceptible to
producing large
variations in organoid size, shape, viability, and growth rate due to
inconsistent starting
material. Standard Matrigel dome culture methods used to evaluate organoid
development,
viability, or molecular-based changes in response to toxicants or therapies
homogenize the
response of many organoids, ignoring phenotypic and genetic heterogeneity.
With the
microarray and system of the invention, a solution is provided for
investigating heterogenous
organoid populations, as well as generating clonally derived organoid
populations. In addition
to image-based phenotypic characterization, the microarray and system permit
isolation and
transfer of individual organoids of interest for downstream applications and
expansion
Using organoid models to understand the dynamics and evolution of intra- and
inter-
tumor heterogeneity on the molecular level is becoming widely used to better
predict drug
efficacy. While studies have been performed using standard culture methods,
largely focused
-23 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
on populations of organoids, the reliability and efficiency of the disclosed
system for enabling
such applications for individual organoids is demonstrated. For example, the
utility of the
disclosed system for downstream organoid growth and subcloning, and nucleic
acid isolation
from individual organoids isolated from the microarray is demonstrated using
both mouse
pancreatic (data not shown) and mouse hepatic organoids (see Example 4).
Specifically, after
isolation from the microarray into 96-well collection plates, organoids remain
viable for
downstream assays and continue to grow in dilute Matrigel growth media (Figure
8A).
The ability to create clonal organoid populations by leveraging the imaging
and
isolation capabilities of the disclosed system is also demonstrated (see
Example 4). For
example, organoids derived from single cells are identified as verified by
temporal imaging on
the microarray and the identified organoids can be isolated into 96-well
collection plates using
the automated system After 5 days of growth off-array, each "parent" organoid
can be
enzymatically dissociated in the 96-well plate into small fragments of cells,
then re-seeded onto
a new microarray to propagate hundreds of second-generation "child" organoids
for further
expansion or evaluation of lineage-based phenotypes (Figure 8B).
In addition to performing single organoid isolations for downstream growth,
assays,
and subcloning, the system can deposit single organoids into PCR strip tubes
or 96-well PCR
plates for nucleic acid isolation, a commonly investigated endpoint for drug
discovery and
toxicology. In one example, mouse pancreatic (data not shown) and mouse
hepatic organoids
are seeded onto microarrays and temporal scans are performed to monitor
organoid
development (see Example 4). Organoids ranging in size, greater than lmm, can
be isolated
directly into a collection plate such as, but not limited to, standard PCR
strip tubes. In one
embodiment, the organoids range in size from 200 to 700 pm. In one embodiment,
organoids
of a desired size range are isolated directly into lysis buffer in standard
PCR strip tubes for
RNA purification. In one embodiment, a size threshold for RNA quality and
concentration is
determined. High-quality RNA (RIN > 9.4) suitable for use in downstream -omi
cs applications
can be obtained. The amount of RNA obtained can be directly correlated with
organoid size
(Figure 9). The data presented demonstrate the flexibility and utility of the
automated system
including the microarray to provide an all-in-one platform that facilitates
efficient, user-
friendly workflows for temporal phenotypic assessment of individual organoids
upstream of
single organoid applications.
-24 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
Figure 10 is a flow chart of an example method 1000 for processing images of
cell rafts
depicting organoids. The method 1000 can be performed by a computer system,
e.g., the
computer system 102 of Figure 2A.
The method 1000 includes acquiring an image of cell rafts, e.g., 500-micron
rafts
(1002). Figure 11A shows an example of an image of cell rafts.
The method 1000 includes inversely thresholding the image to binary (black and
white)
to highlight raft walls and segmenting rafts by identifying distinct white
blobs (1004). The
method 1000 includes determining whether 2 rows of 3 rafts have been
successfully segmented
(1006). If segmentation was successful (YES), then the method 1000 includes
labelling each
raft with the addresses of the raft within the array (1014) and then moving on
to the next image
(1016).
If segmentation was not successful (NO), then oversized organoids caused
segmentation failure due to organoid features creating connected blobs. The
method 1000
includes performing a histogram of a count of white pixels along both X and Y
axes (1008).
The resulting histograms peak along the wall boundaries in each dimensions
(1010). Figure
11B shows the example image from Figure 11A with histograms drawn alongside
each of the
X and Y axes.
The method 1000 includes drawing a black line between each identified wall
boundary
in each axis (1012). Figure 11C shows the example image from Figure 11B with
black lines
drawn between identified wall boundaries. Segmentation is recomputed so that 2
rows of 3
rafts are found. Then, the method 1000 proceeds after successful segmentation,
i.e., by
labelling each raft with the addresses of the raft within the array (1014) and
then moving on to
the next image (1016).
Figures 16A ¨ 16C, 17, 18, and 19A ¨ 19D illustrate an example system that
provides
users the ability to query analysis data retrieved during scanning on the
system 100 The feature
names provided in the examples are shown for purposes of illustration.
In general, the system includes software configured for analyzing data
collected by the
system 100 and a user interface for receiving queries and presenting results.
These queries can
be structured to run analysis on cell morphology and across time if multiple
scans of a single
CytoSort array is taken on the system 100. Analysis on cell morphology is
achieved using a
feature called -Populations." Analysis across time is achieved using a
function called -Venn
Diagram."
-25 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
The system can include a function, which can be called "Single Populations,"
that is
configured for finding cells in the CytoSort array that match a set of
criteria based on the
features extracted during image processing. A single Population will only
focus on a single
scan, meaning it focuses only on a single point in time. This means that the
focus of a
Population is to identify cellrafts that contain specific objects at a single
timepoint.
One example could be a Population that retrieves all the cellrafts containing
single cells
on the first scan taken using the system 100. A second example would be a
Population that
focuses on identifying all cellrafts that contain a colony of cells on the
second scan of a
CytoSort Array taken by the system 100.
Each Population has a set of search criteria defined by the user. These search
criteria
leverage the features retrieved during analysis to filter through the areas of
interest (AOIs)
contained in cellrafts. The definition of which AOIs qualify as "good" is
determined by the
search criteria. After defining the search criteria for a Population, the
software will filter
through all AOIs found during a scan, keep all the AOIs that pass the search
criteria, then return
all cellrafts that contain one of these AOIs that pass the filter set.
The Venn Diagram tool allows the users to then further leverage the
Populations built
in CellRaft Cytometry to conduct analysis across time points. If a CytoSort
Array is scanned
multiple times, Populations with different search criteria can be defined for
each scan. Then,
using the Venn Diagram, the intersection, union, symmetric difference, or
difference can be
retrieved from these populations. For example, the intersection of a
Population of cellrafts
containing single cells on time point 1 and a Population of cellrafts
containing cell colonies on
time point 2 would yield a list of all cellrafts that contain a clonal colony
of cells that started
from single cells.
Display of cellrafts contained in either a Population or the result of using
the Venn
Diagram can be viewed using the GIJI in CellRaft Cytometry. This allows users
to rapidly
review the output of their search criteria and adjust as necessary. Visuals
and readouts on how
the image processing algorithms are performing, how many cellrafts are
retrieved during
querying, and general information on final Venn Diagram set queries can all be
provided to the
user as well.
Figure 16A is a screen shot of an example user interface for the system. The
user
interface can be displayed, for example, on the computer system 102 of Figure
2A.
-26 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
The user interface includes a tab labelled "CellRaft CytometryTM" for
providing input
and output to the system to query analysis data retrieved during scanning. The
user interface
includes buttons for creating a new population and importing a population and
a window for
listing populations. The user interface includes a window for displaying set
members and a
window for displaying images captured during scanning. The user interface
includes a window
for displaying population sets.
Figure 16B is a screen shot of an example user interface for the system. The
user
interface can be displayed, for example, on the computer system 102 of Figure
2A.
The user interface includes a tab labelled -Z Stack Viewer" for displaying
results from
query analysis and corresponding images captured during scanning. The user
interface
provides text fields for descriptions of rafts and descriptions of stacks. The
descriptions can
be used to cause the user interface to display images of corresponding images.
The user
interface can include a user interface element, e.g., a slider, for selecting
a zoom level for the
images.
Figure 16C is a flow diagram of an example method 1600 for creation of a
population,
e.g., using the VennDiagram function. The method 1600 can be performed, e.g.,
by software
executing on the computer system 102 of Figure 2A.
The method 1600 includes receiving a query and accessing a database of
cellraft arrays,
the database including scored scans from multiple time points (1602). The
method 1600
includes determining, for a selected cellraft, whether the corresponding
cellraft data belongs to
a desired timepoint, as specified by a query (1604). The method 1600 includes
determining
whether the cellraft data belongs to a desired reservoir (1606). The method
1600 includes
determining whether the cellraft data belongs to a desired segmentation method
(1608).
The method 1600 includes determining whether the cellraft data passes one or
more
filters, up to N filters As shown in this example, the method 1600 includes
determining
whether the cellraft data passes Filter 1 (1610), Filter 2 (1612) and up to
Filter N (1614).
Examples of filter criteria include the following:
= Timepoint
Reservoir (if applicable)
= Segmentation type
= Area
-27 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
= Debris
= Aspect Ratio
* Solidity
= Circularity
* Fluorescent Intensity (ROB)
= Amplitude
a' Texture
* Mean Intensity
= Fiducial AOIs
Fiducial Rafts
= AOT Count
= Coverage
If the cellraft data belongs as specified by the query, then the cellraft is
added to the
population (1616). Cellrafts are selected and checked until an end condition
is reached and
then the population is chosen.
Figure 17 shows an example user interface 1700 for illustrating population
sets. A user
can create a set from multiple populations using the user interface 1700. A
first user interface
element 1702 illustrates Population A - Members of cellraft Population having
single cells on
day 1 of the experiment. A second user interface element 1704 illustrates
Population B -
Members of cellraft Population exhibiting target Red Fluorescence on either
day 3 or day 4 of
the experiment. A third user interface element 1706 illustrates Population C -
Members of
cellraft Population having of a clonal colony on day 6 of the experiment. A
fourth user interface
element 1708 shows that the set includes only cellrafts that are members of
all three populations
A, B and C.
Figure 18 is a flow diagram of an example method 1800 for organoid detection.
The
system 100 can be configured for detection and selection of organoids of
interest in the
CytoSort Array. The image analysis algorithms can be configured to allow for
users to identify
Organoids of specific size. The features and functionality for identifying
cell rafts can be
applied for organoids, and new parameters can be included for the unique type
of analysis that
would be done on Organoids and 3D tissue structures. Organoid diameter is the
primary feature
used to detect organoids of interest; however, several other features that can
be used to
-28 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
differentiate between 3-dimensional organoid/tissue growth and 2 dimensional
cell growth can
be included in the integration of organoid analysis in the system.
The method 1800 can be performed for organoid brightfield segmentation. The
method
1800 includes inputting a full brightfield image (1802). The method 1800
includes identifying
locations of cellrafts (1804). The method 1800 includes generating a depth map
(1806). The
method 1800 includes identifying areas of high contrast (1808). The method
1800 includes
suppressing noise (1810). The method 1800 includes extracting organoid
contours, locations,
and features (1812). The method 1800 includes returning organoid locations and
features
(1814).
Figures 19A ¨ 19D show examples of masks and outputs generated by organoid
segmentation. Figure 19A is an example image of cell rafts having organoids.
Figure 19B is
an annotated image showing dotted circles around the organoids. Figure 19C is
an example of
a mask used for organoid segmentation. Figure 19D is an example of a different
mask used
for organoid segmentation.
EXAMPLES
Example 1
Microarray Fabrication
The microarrays were manufactured for organoid culture and recovery utilizing
the
following protocol. A SU-8 photoresist master template consisting of 80 gm
tall, 500 x 500
gm pillars separated by 30 gm spaces was fabricated by deep reactive-ion
etching (Alcatel
AMS 100) at the Chapel Hill Analytical and Nanofabrication Laboratory (UNC-
Chapel Hill,
NC). The master was covalently modified through chemical vapor deposition with
octyltrichlorosilane to reduce adhesion to polydimethylsiloxane (PDMS).
Sacrificial rigid
substrates to ensure efficient dip-coating on the microarrays, as well as
minimal PDMS
deformity/sag, were prepared by spin-coating (H6-23 Spin Coater, Laurel],
North Wales, PA)
a thin layer of 7.5% poly(acrylic acid) (PAA) onto glass slides (75 x 50 mm,
Corning, Corning,
NY) at 500 rpm for 10 seconds and then 1500 rpm for 30 seconds. PDMS was
poured onto the
silica master template and degassed for 10 minutes at -710 torr. The master
was then placed
on the spin-coater for 30 seconds at 225 rpm and then cured at 100 C for 60
minutes.
Demolding the glass-backed PDMS from the silanized master template resulted in
a microwell
array (80 gm deep, 500 x 500 gm). Each array was dip-coated in a solution of
20%
-29 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
poly(styrene-co-acrylate) (weight percentage) in gamma butyrolactone (GBL)
containing 1%
yFe203 nanoparticles. Polymer solution was isolated in each individual
microwell through
discontinuous dewetting from the hydrophobic PDMS. Cellrafts were formed after
baking off
the GBL solvent for 18 hours at 100 C. The cellraft array was bonded to an
injection-molded
polystyrene cassette using PDMS glue cured at 70 C for 60 minutes and then
oxygen plasma
treated (Harrick Plasma, Ithaca, NY) for 2 minutes. Sacrificial glass backings
were removed
by soaking the backing in DI water at 70 C for 2 hours to dissolve the PAA.
Each array
underwent 2 additional minutes of oxygen plasma treatment and then were coated
with an anti-
bubble solution for 30 minutes. After this treatment, extra solution was
aspirated, the array
was topped with a polystyrene lid and packaged in a self-sealing sterilization
pouch.
Completed arrays were then gamma sterilized at 10-15 kGy for 130 minutes
(Steris Applied
Sterilization Technologies, Libertyville, IL) before use for cell culture.
Array Manufacturing Materials. Sylgard 184 Polydimethylsiloxane (PDMS) was
prepared from a silicone elastomer kit from Ellsworth Adhesive Co (Germantown,
WI).
Octyltrichlorosilane (97%) and gamma butyrolactone were purchased from Sigma-
Aldrich (St.
Louis, MO). Poly(acrylic acid), 30% solution in water (MW-30 kDa) was
purchased from
PolySciences, Inc. (Warrington, PA). Custom cassettes were injection molded
using
polystyrene material and were purchased from Protolabs (Maple Plain, MN).
Custom dip-
coating solution was prepared at Cell Microsystems, Inc. (Durham, NC).
Example 2
Cell Seeding on the Microctrray and Z-Stack Image Acquisition and Analysis
Mouse pancreatic and hepatic organoid suspensions were prepared for cell
seeding as
fragments from 24-well Matrigel dome culture as described in the
manufacturer's protocols
(Corning Matrigel Growth Factor Reduced (GFR) Basement Membrane Matrix,
Corning, Inc.,
Corning, NY)) using the standard complete media as described herein below.
Mouse hepatic
organoids were enzymatically dissociated for single cell suspension using a
DNase I with
TrypLE solution prepared by mixing 50 1AL of lmg/mL DNase I Solution (cat #
07469,
StemCell Technologies, Inc.) with 5 mL TrypLE Express Enzyme (cat # 12605010,
Gibco
Biosciences). Pelleted fragments were resuspended in lmL of the DNase I with
TrypLE
solution for 10 minutes in a 37 C water bath, mixing the suspension every 2.5
minutes by
pipetting to ensure fragments dissociated into single cells. For single cell-
derived organoid
- 30 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
culture only, complete HepatiCult Growth Medium was supplemented with 10p,M Y-
27632
(cat# ACS-3030, ATCC, Baltimore, MD).
Cells, Media, and 3D culture matrix. Mouse pancreatic organoids and mouse
hepatic
organoids (cat # 70933, cat # 70932, StemCell Technologies, Inc., Vancouver,
BC) were
cultured and maintained in a 37 C, 5% CO2 incubator in PancreaCult Organoid
Growth
Medium (Mouse) or HepatiCult Organoid Growth Medium (Mouse) (cat # 06040, cat
# 06030,
StemCell Technologies, Inc.) supplemented with 1% penicillin/streptomycin (cat
# 15140-122,
Gib co Biosciences, Dublin, Ireland) per the manufactures' guidelines for
growth and expansion
in Matrigel domes (Corning Matrigel Growth Factor Reduced (GFR) Basement
Membrane
Matrix, Phenol Red-free, LDEV-free, cat # 356231, Corning, Inc., Corning, NY).
Cell seeding procedures were adapted from the traditional Matrigel dome
culture
methods to facilitate seeding within the microwells of the microarray and the
release and
collection of the cellrafts for single-organoid recovery. To prepare the array
for cell seeding,
the array was washed three times (3mL each, 3 minutes per wash) with sterile
pre-warmed
(37 C) Ca" Mg" PBS (cat# 10010-023, Gibco Biosciences). After the final wash
was aspirated,
3mL of fresh PBS was added to the reservoir and the array was placed on ice
for 1 hour to cool
the array prior to cell seeding. The organoid fragment or single cell
suspension was prepared
as previously described. Fragment suspensions were counted by light microscope
in n=3 10pL
droplets. Volume needed for seeding the array was calculated using the
following equation:
Desired number of fragments
Volume needed for seeding (4) = _______________________________ (1)
(Average fragment count /10 4)
For single cell suspension, cells were counted using the Countess II Automated
Cell Counter
(Invitrogen, Waltham, MA) and volume needed for seeding was calculated by the
following
equation:
Desired number of cells
Volume needed for seeding (mL) = ___________________________ (2)
Cell concentration (cells /mL)
Cells or cell fragments were seeded at a 1:1 ratio of cells:cellrafts. The
desired volume of cell
suspension was added to a 15 mL conical tube with 1 mL of cold Advanced DMEM/F-
12 (cat#
12634010, Gibco Biosciences) and centrifuged at 300 x g to pellet the cells.
To prepare dilute
Matrigel media for cell seeding, the volume of Matrigel needed to achieve a
final concentration
of 0.24mg/mL was added to 5 mL ice cold complete PancreaCult or HepatiCult
growth media
(1.2 mg Matrigel in total seeding volume). After centrifugation, the
supernatant was carefully
-31 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
removed, and the cell pellet was resuspended in 1 mL of ice cold dilute
Matrigel media. The
microarray was removed from ice, PBS was aspirated off the array. To prepare
the array for
cell seeding, 2mL of dilute Matrigel media is added to the reservoir, followed
by the lmL of
dilute Matrigel media with cell suspension. After cell inoculation, the
remaining 2mL of cold
dilute Matrigel media was slowly added to reach 5 mL total volume within the
array reservoir,
and the array was returned to ice for 20 minutes. This cold incubation is
essential to ensure the
dilute Matrigel and cell suspension successfully wick into the microwells
prior to
polymerization of Matrigel at 37 C. Cells, or clusters of cells, settle into
the microwell
footprint of the microarray in a Poisson-like distribution and the dilute
Matrigel allows for a
loose attachment of developing organoids to the cellrafts. After the cold
incubation, the array
was placed in a 37 C, 5% CO2 incubator. The microarray was scanned in
brightfield using the
system at 4 hours and every 24 hours after seeding to monitor organoid growth
and
development.
Live Cell Staining Orgartoids for phenotypic charucterization. Mouse hepatic
organoids were stained with Hoechst 33342 (cat # R37605, Molecular Probes,
Eugene, OR)
and a directly conjugated (FITC) primary antibody for epithelial cell adhesion
molecule
(EpCAM, cat# 11-5791-82, eBioScience, San Diego, CA). Briefly, a 50% media
exchange
was performed 5 times (2 mL each) using Fluorobrite DMEM (cat# A1896701, Gibco
Biosciences) being careful not to dislodge organoids from the microwell
footprint. A 2X
staining cocktail was prepared as follows: 1.6 mL Fluorobrite DMEM with 400uL
of 10% BSA
in PBS (cat# 37525, ThermoFisher Scientific, Waltham, MA), 1:400 (20 L) of
the EpCAM
primary antibody, 8 drops of Hoeschst 33342. After the final wash with
Fluorobrite DMEM,
media was removed, leaving approximately 2 mL in the reservoir. The total
volume of the
staining cocktail was added to the reservoir for a final concentration of 1%
BSA, 1:200
EpCAM-FITC primary antibody, and 2 drops per mL Hoechst 33342 The array was
placed in
a 37 C, 5% CO2 incubator for 45 minutes, followed by five 50% media changes (2
mL each)
with Fluorobrite DMEM to wash away excess antibody for imaging. Immediately
following
staining, the array was scanned using the system in brightfield, blue
fluorescence (390 /
432nm), and green fluorescence (475 / 522nm).
Two organoid-containing cellrafts were selected for additional z-stack imaging
with the
system, as described herein below. To test the image quality of brightfield
and widefield
fluorescence z-stacks acquired by the system, two 200-300 m mouse hepatic
organoids on a
- 32 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
microarray stained with Hoechst and EpCAM (primary antibody conjugated with
FITC) were
selected for imaging (as described herein below). Brightfield, blue
fluorescence (exposure =
50, 50ms), and green fluorescence (exposure = 200, 100ms) images were acquired
every 10 m
across focal ranges that encompassed the full height of the two organoids (24
and 30 images,
respectively). The brightfield and false-colored fluorescence images (Figure
4A) demonstrate
excellent image quality with the ability to visualize individual cells, cell
junctions (green), and
substructures within nuclei (blue), including mitotic figures. The green
fluorescence intensity
is attenuated toward the top of the organoid, but it is unclear whether that
is due to a reduction
in excitation-emission light transmission through the lower regions of the
organoid, reduction
in EpCAM staining, or a combination.
Example 3
System-Acquired High-Quality, Time-Course Images of Developing Organoids
Using both mouse pancreatic (Figure 4C) and mouse hepatic organoids, a robust
and
reliable method has been demonstrated for obtaining high-quality, time-course
images of
developing organoids on the microarray to maintain a dynamic growth record of
each organoid
during the entire development process from single cell to isolation. Cells
were seeded onto the
microarray and stained as described herein above. Beginning with a microarray
scan shortly
after cell seeding (4 hours), the user can identify cellrafts with single or
multiple cells either
manually or by using a CellRaft Cytometry tool (Cell Microsystems, Inc.,
Durham, NC)
(Figure 6B). Subsequent serial scans of the microarray were then initiated by
the user at
desired time intervals to capture temporal images of organoid development
(Figure 6C, Figure
7). The system was utilized to acquire a full microarray scan in brightfield
and three fluorescent
channels in under 15 minutes (under 9 minutes for brightfield only), providing
a rapid solution
for multiparameter ph en otypi c and morphologic screening of hundreds of in
di vi dual organoids.
In addition to brightfield imaging, the system was used to perform advanced
fluorescence-
based phenotypic assessment, which can be used for a variety of applications,
including live
cell staining, CRISPR editing, and on-array viability assays (Figure 5F).
Mouse hepatic
organoids stained with FITC-conjugated antibody for EpCAM demonstrated
detailed
visualization of cell membranes (Figure 4A, Figure 7) and Hoechst-stained
nuclei enable cell
counting throughout the z-stack (Figure 4A).
- 33 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
Example 4
Isolation of Organoids for Downstream Assays, Growth and Subcloning, and -
Omics
Applications
Using both mouse pancreatic (data not shown) and mouse hepatic organoids, the
utility
of the disclosed system and methods was evaluated for downstream organoid
growth and
subcloning, and nucleic acid isolation from individual organoids isolated form
the microarray.
After isolation from the array into 96-well collection plates, organoids
remained viable for
downstream assays and continued to grow in dilute Matrigel growth media
(Figure 8A).
The ability to create clonal organoid populations was al so demonstrated by
leveraging
the imaging and isolation capabilities of the presently disclosed system.
Organoids derived
from single cells were identified as verified by temporal imaging and isolated
into 96-well
collection plates. After 5 days of growth off-array, each "parent" organoid
was enzymatically
dissociated in the 96-well plate into small fragments of cells, then re-seeded
onto a new
microarray to propagate hundreds of second-generation "child" organoids for
further expansion
or evaluation of lineage-based phenotypes (Figure 8B).
In addition to performing single organoid isolations for downstream growth,
assays,
and subcloning, the presently disclosed system was used to automatically
deposit single
organoids into PCR strip tubes or 96-well PCR plates for nucleic acid
isolation, a commonly
investigated endpoint for drug discovery and toxicology. Mouse pancreatic
(data not shown)
and mouse hepatic organoids were seeded onto microarrays and temporal scans
were
performed to monitor organoid development. Organoids ranging in size from 200
to 700 tim
were isolated directly into lysis buffer in standard PCR strip tubes for RNA
purification to
determine a size threshold for RNA quality and concentration. High-quality RNA
(RIN > 9.4)
was obtained suitable for use in downstream -omics applications (n=8), and the
amount of RNA
obtained was directly correlated with organoid size (Figure 9) The data
presented
demonstrates the flexibility and utility of the disclosed system to provide an
all-in-one platform
that facilitates efficient, user-friendly workflows for temporal phenotypic
assessment of
individual organoids upstream of single organoid applications.
RNA purificalion and quanillation. Individual mouse hepatic organoids ranging
in size
from approximately 200 m to 700p.m were isolated using the disclosed system
for RNA
purification using the Qiagen RNeasy Plus Micro kit (cat# 74034, Qiagen,
Hilden, Germany).
Organoids were released from the microarray and collected using the system's
PCR-style wand
- 34 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
into PCR strip tubes with 25 ttL of RLT Plus Buffer. After isolation was
performed, 325 L
of RLT Plus was added to each sample to reach the final volume of 350 L. RNA
purification
was performed per the manufacturer's guidelines with a final elution volume of
14 L. Purified
RNA was quantified using the Agilent 2100 Bioanalyzer and RNA 6000 Pico Kit
(cat# 5067-
1513, Agilent Technologies, Santa Clara, CA) using the standard protocol.
Example 5
Generation of CRISPR-Edited Organoids, and subsequent Clonal Propagation and
Functional Screening
CRISPR editing is performed on human adult stem cells to introduce a
fluorescent
reporter. A single cell suspension of the gene-edited stem cells is loaded
onto a microarray
according to the methods described in Example 2. The cells are cultured in the
microarray and
monitored at desired time intervals for growth and phenotypic characteristics
using the
automated system as described above in Examples 3 and 4. Fluorescent CRISPR-
edited
organoids can be identified using the imaging capabilities of the disclosed
system. Organoids
of interest are isolated into 96-well collection plates using the automated
system for further
expansion. After 5 days of growth off-array, each organoid is enzymatically
dissociated in the
96-well plate into small fragments of cells, then re-seeded onto a new
microarray to propagate
hundreds of second-generation reporter organoids. The reporter organoids are
screened for
pathway activation, differentiation, or phenotypic response to a drug or other
molecule.
Cells, Media, and 3D culture matrix. Edited human induced pluripotent stem
cells
(iPSCs) that express red fluorescent protein (REP), or green fluorescent
protein (GFP) (cat #
IPSC1028, cat # IPSC1030, Sigma-Aldrich) were cultured and maintained in a 37
C, 5% CO2
incubator in mTESR Plus (cat # 100-0276, StemCell Technologies, Inc.) per the
manufactures'
guidelines for 2D growth and expansion To demonstrate iPSC-derived organoid
workflows,
three commercially available media kits were used to differentiate edited
iPSCs into kidney,
choroid plexus, and cerebral organoids (cat # 05160, cat # 100-0824, and cat #
08570, StemCell
Technologies, Inc.). To grow and monitor phenotypic changes of clonal iPSC-
derived
organoids, iPSCs were dissociated into a single cell suspension and seeded
onto the microarray
as described herein above. Dilute Matrigel media (0.24mg/mL) was made using
the
manufacturer recommended media for forming kidney, choroid plexus, and
cerebral organoids
(data not shown). Microarrays were seeded with one edited iPSC cell line, or a
mixed
- 35 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
population of both RFP and GFP positive cells, to demonstrate the ability to
form single color,
or dual fluorescent organoids. For differentiation, media changes were
performed based on the
manufacturers' guidelines for media formulations and duration.
In addition to the ability to generate clonal organoids from single edited
iPSCs, the
presently disclosed system presents a unique advantage to traditional iPSC-
derived organoid
culture methods because it enables temporal monitoring of phenotypic changes
of individual
organoids throughout the differentiation process. Standard techniques for
generating iPSC-
derived organoids can require moving 3D structures to various culture plate
formats, in addition
to media changes, to achieve cell differentiation and organoid formation.
While they support
organoid differentiation, they do not permit assessment of individual
structures throughout
organoid formation. Using the presently disclosed system, we have demonstrated
the ability
to generate choroid plexus and kidney organoids from single cells, and small
fragments of cells,
derived from the same iPSC suspension. Hundreds of single-cell, or fragment-
derived, choroid
plexus (Figure 12A) and kidney (Figure 12B) organoids can be imaged at desired
timepoints
to closely monitor phenotypic changes throughout the differentiation process.
Using the presently disclosed system, and methods to seed the microarray
described
herein above, we have also demonstrated the ability to form dual fluorescent
choroid plexus
organoids by co-culturing RFP+ and GFP+ iPSCs on the same microarray (Figure
13). Using
the automated system, we can identify cellrafts that contain a single RFP+
iPSC or a single
GFP+ iPSC, as well as cellrafts that contain more than one iPSC, of one or
both fluorescent
reporters. Temporal development of mono- and dual-fluorescent organoids can be
monitored
and phenotypically characterized, and single organoids can be isolated for
further downstream
evaluation, including -omics and drug screening applications, or for clonal
propagation.
Example 6
Clonal Propagation of Human Tissue-Derived Org-anoids for Compound-Induced
Toxicity
Screening
A single cell suspension of tissue-specific cells, from common sites of
compound-
induced toxicity such as liver, kidney, and lung, is loaded onto a microarray
according to the
methods described in Example 2. The cells are cultured in the microarray and
monitored at
desired time intervals for growth and phenotypic characteristics using the
automated system as
described above in Examples 3 and 4. Using the automated system, organoids of
interest
- 36 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
derived from single cells are identified by temporal imaging and isolated into
96-well collection
plates. After 5 days of growth off-array, each "parent" organoid is
enzymatically dissociated
in the 96-well plate into small fragments of cells, then re-seeded onto a new
microarray to
propagate hundreds of second-generation "child" organoids. Second-generation
clonal
organoids, of a desired size range, are isolated using the automated system
into 96-well plates
for assessment in downstream compound toxicity assays. Clonal, tissue-specific
organoids are
screened for toxicity to a drug or other molecule for functional response,
such as viability, or
used for single organoid transcriptomics to reveal cellular mechanisms of
toxicity.
Example 7
Custom Organoid Assay Development for Compound-Induced Toxicity Screening
using Single Organoids
Using a similar approach to that outlined in Example 6, the automated system
described
herein above can be used to culture, analyze, and isolate single organoids
with similar
phenotypic characteristics, including, but not limited to, parameters such as
organoid size and
morphology, and fluorescent marker inclusion, for reproducible compound-
induced toxicity
screening assays. To demonstrate a compound-induced toxicity screening assay
on single,
phenotypically selected organoids, mouse hepatic organoids were grown on the
microarray, as
described in Example 3. Using the CellRaft Cytometry software described it the
presently
disclosed system, the population of mouse hepatic organoids were sorted based
on organoid
diameter. Two populations were selected for isolation; one population for
organoids with
diameters greater than 50pm, and a more selective population of organoids
ranging from 300-
500ttm in diameter. After isolation of cellrafts containing organoids from the
populations
described above, the 96-well collection plates were spiked with 0.24mg/mL
dilute Matrigel
media to support organoid viability and growth post-isolation Single organoids
from each
population were treated with a compound with known toxic effects to
demonstrate a standard
drug-screening assay.
Compounds and Reagents far Toxicity Screening. Mouse hepatic organoids (n = 5
per
dose) were treated with a five-fold, 6-point dose curve of a canonical
hepatotoxicant,
acetaminophen (APAP, 0.0008-2.5mM, cat # A7085, Sigma-Aldrich) in 0.5%
dimethylsulfoxide (DMSO, cat # D12345, Molecular Probes). To measure toxicity
responses,
CellTox Green Cytotoxicity Assay (cat # G8731, Promega Corporation) was used
to measure
- 37 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
kinetic viability for 72 hours, and CellTiter-Glo 3D Cell Viability Assay (cat
# G9681,
Promega Corporation) was used to determine the relative viability, via ATP
quantitation, at the
final 72-hour timepoint.
Using the presently disclosed system, described herein above, we have
demonstrated
the ability to generate reliable, reproducible organoid screening assays.
Representative images
of organoids not selected on size (> 501,1m) have a large variability in size
(Figure 14A),
whereas organoids that were selected on a more limited diameter range (300-
500m) maintain
more consistent size throughout the assay (Figure 14B). The ability to select
organoids based
on size is a critical component in measuring viability readouts, such as Cell
Tox Green and
CellTiter-Glo, because these assays are dependent on cell number. The
variability in organoid
size in the unselected population directly translates to large variability in
replicate doses in both
viability readouts, which prevents the calculation of an ED50, or mean
effective dose (Figure
15A). Using CellRaft Cytometry to select organoids based on size, organoid
size is more
consistent, and the viability readouts can be used to calculate an ED50
(0.6003mM). This
approach can also enable assay consistency across many microarrays and 96-well
collection
plates. In summary, the presently disclosed system presents a unique advantage
over traditional
methods, which rely on pooled readouts of heterogeneous organoid populations,
because
assays can be customized to select single organoids based on size, and other
phenotypic
characteristics, which enables intra- and inter-assay consistency that is
unachievable using
other methodologies.
Example 8
Propagation of Mono- and Co-Cultured Spheroids for Evaluating Anti-Cancer
Therapeutics
Spheroids, or tumor cell aggregates, provide a more physiologically relevant
in vitro
model to study tumor cell responses to genetic manipulations or
pharmacological compound
effects, making them valuable tools for therapeutic discovery and personalized
medicine. A
single cell, or aggregate suspension of tumor cells, from human or animal
tumors, is loaded
onto a microarray according to the methods described in Example 2. The cells
are cultured in
the microarray and monitored at desired time intervals for growth and
phenotypic
characteristics using the automated system as described above in Examples 3
and 4. Anti-
cancer therapeutics can be added to the microarray, and phenotypic evaluation
of the spheroids,
such as spheroid size or viability, can be monitored on the array to identify
the efficacy of the
- 38 -
CA 03230050 2024- 2- 26
WO 2023/028366
PCT/US2022/041886
therapy on the heterogenous spheroid population. Using the automated system,
spheroids of
interest are identified by temporal imaging and isolated into 96-well
collection plates for
downstream assessment, such as transcriptomics, as described in Example 4.
Alternatively,
using the presently disclosed system, spheroids grown on the microarray, as
described herein
above, can be evaluated using automated CellRaft Cytometry, and isolated into
96-well
collection plates for downstream assays of therapeutic agents.
Although specific embodiments have been illustrated and described herein,
those of
ordinary skill in the art appreciate that any arrangement which is calculated
to achieve the same
purpose may be substituted for the specific embodiments shown and that the
invention has
other applications in other environments. This application is intended to
cover any adaptations
or variations of the present invention. The following claims are in no way
intended to limit the
scope of the invention to the specific embodiments described herein.
- 39 -
CA 03230050 2024- 2- 26